UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
FOR THE FISCAL YEAR ENDED
For the transition period from ____________to _____________
Commission File Number:
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of | (I.R.S. Employer Identification Number) | |
| incorporation or organization) |
| (Address of principal executive offices, Zip Code) | ||
| (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| The |
Securities registered pursuant to Section 12(g) of the Act:
| None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act
Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer | ☐ | Accelerated Filer | ☐ |
| |
☒ | Smaller reporting company | |
| Emerging growth company | |
||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark if the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7362(b)) by the registered public accounting firm that prepared or issued its audit report.
Yes ☐ No ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐
The aggregate market value of the voting and non-voting common equity held
by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such
common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter was $
There were shares of the Registrant's $0.0001 par value Class A common stock outstanding as of September 13, 2022.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive proxy statement relating to its 2022 annual meeting of stockholders (the “2022 Proxy Statement”) are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated. The 2022 Proxy Statement will be filed with the U.S. Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
BIOVIE INC.
FORM 10-K INDEX
| PART I | |||
| Item 1. | Business | 1 | |
| Item 1A. | Risk Factors | 13 | |
| Item 1B. | Unresolved Staff Comments | 28 | |
| Item 2. | Properties | 28 | |
| Item 3. | Legal Proceedings | 28 | |
| Item 4. | Mine Safety Disclosures | 28 | |
| PART II | |||
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 29 | |
| Item 6. | [Reserved] | 29 | |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 29 | |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 33 | |
| Item 8. | Financial Statements and Supplementary Data | 33 | |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 33 | |
| Item 9A | Controls and Procedures | 34 | |
| Item 9B. | Other Information | 34 | |
| Item 9C. | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | 34 | |
| PART III | |||
| Item 10. | Directors, Executive Officers and Corporate Governance | 35 | |
| Item 11. | Executive Compensation | 43 | |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 49 | |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 51 | |
| Item 14. | Principal Accountant Fees and Services | 51 | |
| PART IV | |||
| Item 15. | Exhibits and Financial Statement Schedules | 53 | |
| Item 16. | Form 10-K Summary | ||
-i-
BIOVIE INC.
FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, and Section 27A of the Securities Act of 1933. Any statements contained in this report that are not statements of historical fact may be forward-looking statements. When we use the words “intends,” “estimates,” “predicts,” “potential,” “continues,” “anticipates,” “plans,” “expects,” “believes,” “should,” “could,” “may,” “will” or the negative of these terms or other comparable terminology, we are identifying forward-looking statements. Forward-looking statements involve risks and uncertainties, which may cause our actual results, performance or achievements to be materially different from those expressed or implied by forward-looking statements. These factors include our research and development activities, distributor channel; compliance with regulatory impositions; and our capital needs. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
Except as may be required by applicable law, we do not undertake or intend to update or revise our forward-looking statements, and we assume no obligation to update any forward-looking statements contained in this report as a result of new information or future events or developments. Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. You should carefully review and consider the various disclosures we make in this report and our other reports filed with the Securities and Exchange Commission that attempt to advise interested parties of the risks, uncertainties and other factors that may affect our business.
All statements other than statements of historical fact are statements that could be deemed forward-looking statements. The Company assumes no obligation and does not intend to update these forward-looking statements, except as required by law. When used in this report, the terms “BioVie”, “Company”, “we”, “our”, and “us” refer to BioVie, Inc.
-ii-
PART I
| ITEM 1. | BUSINESS |
BioVie Inc. is a clinical-stage company developing innovative drug therapies to overcome unmet medical needs in chronic debilitating conditions.
In liver disease, our Orphan Drug candidate BIV201 (continuous infusion terlipressin) is being developed as a future treatment option for patients suffering from ascites and other life-threatening complications of advanced liver cirrhosis caused by NASH, hepatitis, and alcoholism. The initial target for BIV201 therapy is refractory ascites. These patients suffer from frequent life-threatening complications, generate more than $5 billion in annual treatment costs, and have an estimated 50% mortality rate within 6 to 12 months. The US Food and Drug Administration (FDA) has not approved any drug to treat refractory ascites. A Phase 2a clinical trial of BIV201 was completed in 2019, and a multi-center, randomized 30-patient Phase 2b trial is currently underway. As of June 30, 2022, eleven US study centers had been activated and are actively screening and enrolling patients in the study. Top-line results from this trial are expected in mid calendar year 2023.
The BIV201 development program was initiated by LAT Pharma LLC. On April 11, 2016, the Company acquired LAT Pharma LLC and the rights to its BIV201 development program. The Company currently owns all development and marketing rights to its drug candidate. Pursuant to the Agreement and Plan of Merger entered into on April 11, 2016, between our predecessor entities, LAT Pharma LLC and NanoAntibiotics, Inc., BioVie is obligated to pay a low single digit royalty on net sales of BIV201 (continuous infusion terlipressin) to be shared among LAT Pharma Members, PharmaIn Corporation, and The Barrett Edge, Inc.
In neurodegenerative disease, BioVie acquired the biopharmaceutical assets of NeurMedix, Inc., a related party privately held clinical-stage pharmaceutical company and related party affiliate, in June 2021. The acquired assets include NE3107, a potentially selective inhibitor of inflammatory ERK signaling that, based on animal studies, is believed to reduce neuroinflammation. NE3107is a novel orally administered small molecule that is thought to inhibit inflammation-driven insulin resistance and major pathological inflammatory cascades with a novel mechanism of action. There is emerging scientific consensus that both inflammation and insulin resistance may play fundamental roles in the development of Alzheimer’s and Parkinson’s Disease, and NE3107 could, if approved, represent an entirely new medical approach to treating these devastating conditions affecting an estimated 6 million Americans suffering from Alzheimer’s and 1 million from Parkinson’s. The FDA has authorized a potentially pivotal Phase 3 randomized, double-blind, placebo-controlled, parallel group, multicenter study to evaluate NE3107 in subjects who have mild to moderate Alzheimer’s disease (NCT04669028). We initiated this trial on August 5, 2021 and are targeting primary completion in mid calendar year 2023.
On January 20, 2022, the Company initiated a study by treating the first patient, in its Phase 2 study assessing NE3107’s safety and tolerability and potential pro-motoric impact in Parkinson’s disease patients. The NM201 study (NCT05083260) is a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study in Parkinson’s Disease (PD). Participants will be treated with carbidopa/levodopa and NE3107 or placebo. Forty patients with a defined PD medication “off state” will be randomized 1:1 placebo to active NE3107 20 mg twice daily for 28 days. Safety assessments will look at standard measures of patient health and potential for drug-drug interactions affecting L-dopa pharmacokinetics and activity. Exploratory efficacy assessments will use the Motor Disease Society Unified Parkinson’s Disease Rating (MDS-UPDRS) parts 1-3, ON/OFF Diary, and Non-Motor Symptom Scale. Topline results are expected for the NM201 study by the end of the calendar year 2022.
-1-
Investigator-Initiated Trial in MCI and Mild Alzheimer’s Disease, NCT05227820
The Company provided the financial support and the use of our NE3107 formulated drug product to The Regenesis Project of Dr Sheldon Jordan in an open-label phase 2 study in Dr. Sheldon’s patients with Alzheimer’s disease related dementias. The study received FDA authorization on December 12, 2021and was designed to measure NE3107’s effect on cognition, cerebral spinal fluid (“CSF”) and blood biomarkers, and neuro-imagining endpoints. The study seeks to measure changes in cognition through verbal and visual test procedures and changes in biomarkers of Alzheimer's disease and inflammatory and metabolic parameters that can be measured in the central nervous system with advanced neuroimaging techniques in patients before and after treatment with 20 mg of NE3107 twice daily for 3 months following three months of treatment. Data analysis for the study is expected to be in completed in second half of the calendar year 2022.
Inflammation-driven insulin resistance is believed to be implicated in a broad range of serious diseases, including multiple myeloma and prostate cancer, and we plan to begin exploring these opportunities in the coming months using NE3107 or related compounds acquired in the NeurMedix asset purchase. NE3107 is patented in the United States, Australia, Canada, Europe and South Korea.
Liver Cirrhosis Program
BioVie’s orphan drug candidate BIV201 (continuous infusion terlipressin) represents a novel approach to the treatment of ascites due to chronic liver cirrhosis. BIV201 is based on a drug that is approved in about 40 countries to treat related complications of liver cirrhosis (part of the same disease pathway as ascites), but not yet available in the United States. The active agent in BIV201, terlipressin, is a potent vasoconstrictor and is marketed in multiple foreign countries. The goal of BIV201 therapy is to interrupt the ascites disease pathway, thereby halting the cycle of accelerated fluid generation in ascites patients.
In 2017, we began administering BIV201 to patients at the McGuire Research Institute Inc. in Richmond, VA. In April 2019, we announced top-line results for our Phase 2a clinical trial of BIV201 (continuous infusion terlipressin) in six patients with refractory ascites due to advanced liver cirrhosis. The following results were observed:
| ● | Continuous infusion of terlipressin via portable infusion pump was maintained for 28 days in three patients with refractory ascites, and all patients remained hemodynamically stable during treatment. |
| ● | The steady state plasma concentration data characterized terlipressin pharmacokinetics (PK) within the predicted PK model concentrations. |
| ● | Four of the six patients treated with BIV201 experienced an increase in the number of days between paracenteses ranging from 71% to 414% compared to prior to initiating therapy. |
In June 2019, we met with representatives of the FDA for a Type C Guidance Meeting to plan our next clinical study in ascites. We discussed our clinical development program with the FDA and proposed safety and efficacy endpoints required for future marketing approval. In September 2019, the FDA granted our Type B meeting request and committed to providing feedback in early 2020 for our proposed clinical trial design. In April 2020, we received the FDA’s written response to our Type B meeting questions which required changes to our clinical trial design. Subsequently we received further guidance from the FDA. Based on this guidance, the Company finalized the clinical trial protocol and prepared for a randomized 30-patient Phase 2b study. The IND for this study was submitted and has become effective. The Phase 2b study was initiated in June 2021. As of July 2022, eleven planned US study centers have been activated. We plan to follow this study with a larger potentially pivotal Phase 3 clinical trial expected to begin in 2023. The FDA communicated that pending positive Phase 2 study results, a sufficiently large and well-controlled Phase 3 trial, with supportive trend data from the Phase 2b (statistical significance not required), could potentially yield the clinical data needed to apply for BIV201 marketing approval. The Phase 2b clinical trial protocol is summarized on www.clinicaltrials.gov, trial identifier NCT04112199.
-2-
We have invented a proprietary novel liquid formulation of terlipressin which is currently being studied in the above clinical studies intended to improve convenience for outpatient administration and avoid potential formulation errors when pharmacists reconstitute the powder version. In May 2020, we received CMC division clearance to use the new BIV201 prefilled terlipressin syringe in the current Phase 2b trial subject to conducting certain additional standard analytical testing which has been successfully completed. To date analytical testing results have confirmed room temperature stability of the prefilled syringe in storage for 18 months, with the potential for up two years stability. Room temperature storage presents a key product differentiation versus terlipressin products in countries where the drug is approved. To the best of the Company’s knowledge, all other terlipressin products sold globally must be stored under refrigeration and there is no prefilled syringe format of terlipressin available for treating patients in these countries. BioVie has also filed a Patent Cooperation Treaty (“PCT”) application covering our novel liquid formulations of terlipressin (international patent application PCT/US2020/034269, published as WO2020/237170) and we plan to seek patent protection in at least the United States, Europe, China and Japan.
BIV201 (continuous infusion terlipressin) has the potential to improve the health of thousands of patients suffering from life-threatening complications of liver cirrhosis due to hepatitis, NASH, and alcoholism. The FDA has granted Fast-Track status and Orphan Drug designation for the most common of these complications, ascites, which represents a significant unmet medical need. Patients with cirrhosis and ascites account for an estimated 116,000 U.S. hospital discharges annually, with frequent early readmissions. Those requiring paracentesis (removal of ascites fluid) experience an average hospital stay lasting 8 days incurring over $86,000 in medical costs (HCUP Nationwide Readmissions Database 2016). This translates into a total addressable ascites market size for BIV201 therapy exceeding $650 million based on Company estimates. The FDA has never approved any drug specifically for treating ascites. For patients with refractory ascites the mean one-year survival rate is only 50% (Bureau et al. 2017). BIV201 has also received Orphan Drug designation for hepatorenal syndrome (“HRS”). Patients with refractory ascites often progress to HRS which is the onset of kidney failure and requires emergency hospitalization. About one-half of these patients typically succumb within only 2 to 4 weeks and no drug therapies have been FDA approved specifically to treat HRS.
The BIV201 development program began at LAT Pharma LLC. On April 11, 2016, we acquired LAT Pharma LLC and the rights to its BIV201 development program and currently own all development and marketing rights to the product candidate. We and PharmaIN, LAT Pharma’s former partner focused on the development of new modified product candidates in the same therapeutic field but not including BIV201, have agreed to pay royalties equal to less than 1% of future net sales of each company’s ascites drug development programs, or if such program is licensed to a third party, less than 5% of each company’s net license revenues. On December 24, 2018, we returned our partial ownership rights to the PharmaIN modified terlipressin development program and simultaneously paid the remaining balance due on a related debt. PharmaIN’s rights to our program remain unchanged. Our pending U.S patent (a continuation application related to the ’945 Patent) for the use of BIV201 as a monotherapy for the treatment of patients diagnosed with ascites due to liver cirrhosis in the outpatient setting using ambulatory pump infusion, issued on June 21, 2022 (U.S 11,364,277). Corresponding patent applications are pending in Japan, Europe, China and Hong Kong.
About Ascites and Liver Cirrhosis
Cirrhosis is a leading cause of death in the US. The condition results primarily from hepatitis, alcoholism, and fatty liver disease linked to obesity. Ascites is a common complication of advanced liver cirrhosis, involving kidney dysfunction and the accumulation of large amounts of fluid in the abdominal cavity.
The Need for an Ascites Therapy
With no medications approved by the FDA specifically for treating ascites, an estimated 40% of patients die within two years of diagnosis. Certain drugs approved for other uses such as diuretics may provide initial relief, but patients may fail to respond to treatment as ascites worsens. This represents a critical unmet medical need. U.S. treatment costs for liver cirrhosis, including ascites and other complications, are estimated at more than $5 billion annually.
-3-
The Ascites Development Pathway
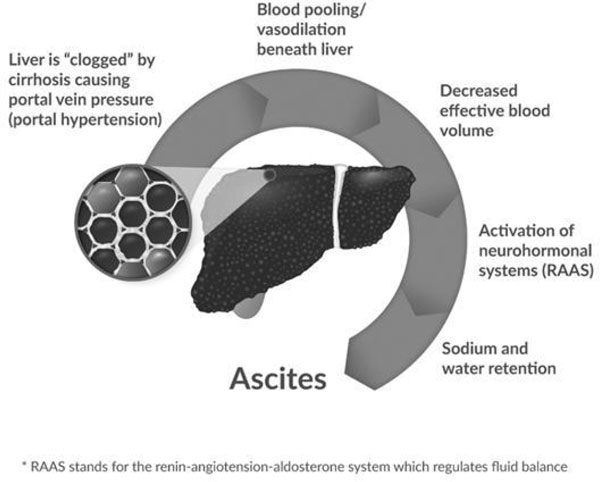
Most experts agree that ascites develops through a sequence of events illustrated by the above diagram. High blood pressure in the vein that supplies blood to the liver, called “portal hypertension,” occurs as increasing liver damage (fibrosis) impedes blood flow through the liver. This causes vasodilation and blood pooling in the central or “splanchnic” region of the body and low blood volume in the arteries. The decrease in effective blood volume activates a signaling pathway (“neurohormonal systems”) which tells the kidneys to retain large amounts of salt and water in an effort to increase blood volume. Ultimately the retention of excess sodium and water leads to the formation of ascites as these substances “weep” from the liver and lymph system and collect in the patient’s abdomen.
The BIV201 Mechanism of Action
BIV201 is being developed with the goal of alleviating the portal hypertension and correcting splanchnic vasodilation, thereby increasing effective blood volume and reducing the signals to the kidneys to retain excess salt and water. If successful, BIV201 could halt the cycle of accelerating fluid generation in ascites patients and reduce the need for the frequent and painful paracentesis procedures many of these patients currently require.
-4-
Future Possible BIV201 Indications
Based on international investigative studies of the active agent in BIV201, terlipressin, our new drug candidate has potential future applications in other life-threatening conditions due to liver cirrhosis, such as those listed below. Securing marketing approvals for any of these new uses will require well-controlled clinical trials to satisfy the FDA and/or other countries’ regulatory requirements, none of which have commenced at this time. The Company may be unable to, or chose not to, pursue the development BIV201 for these indications.
| ● | Bleeding Esophageal Varices (BEV): The bursting of blood vessels lining the esophagus due to high blood pressure (“portal hypertension”) in the vein which supplies blood to the liver resulting as a result of advanced liver cirrhosis. This situation requires emergency treatment to avoid blood loss and death. |
| ● | Hepatorenal Syndrome-Acute Kidney Injury (HR/S-AKI): As liver cirrhosis and ascites progress, the patients’ kidneys may begin to fail, and this deadly condition may set in. It often occurs once a patient no longer responds to (off-label) drugs used to control ascites. Treatment of HRS-AKI requires hospitalization as multiple organ failure and death may occur, typically within 2-4 weeks absent liver transplant. We obtained Orphan Drug designation for BIV201 in the U.S. for the treatment of HRS on November 21, 2018. In May 2021, BioVie submitted a Type B Meeting Package to the FDA seeking to conduct a single pivotal US Phase 3 clinical trial in the treatment of HRS-AKI. In June 2021, we received FDA feedback on the proposed trial design. Agreement was reached on the key elements of a Phase 3 trial in the Preliminary Meeting Comments received from the FDA on April 15, 2022 in response to a subsequent Type C meeting request. |
Neurodegenerative Disease Program
BioVie acquired the biopharmaceutical assets of NeurMedix, Inc., a privately held clinical-stage pharmaceutical company and related party affiliate, in June 2021. The acquired assets include NE3107, a potentially selective inhibitor of inflammatory ERK signaling that, based on animal studies, is believed to reduce neuroinflammation. NE3107 is a novel orally administered small molecule that is thought to inhibit inflammation-driven insulin resistance and major pathological inflammatory cascades with a novel mechanism of action. There is emerging scientific consensus that both inflammation and insulin resistance may play fundamental roles in the development of Alzheimer’s and Parkinson’s Disease, and NE3107 could, if approved, represent an entirely new medical approach to treating these devastating conditions affecting an estimated 6 million Americans suffering from Alzheimer’s and 1 million from Parkinson’s. The FDA has authorized a potentially pivotal Phase 3 randomized, double-blind, placebo-controlled, parallel group, multicenter study to evaluate NE3107 in subjects who have mild to moderate Alzheimer’s disease (NCT04669028). We initiated this trial on August 5, 2021 and are targeting primary completion in mid calendar year of 2023.
Alzheimer’s Disease
Alzheimer’s disease (AD), which affects an estimated 6 million Americans, is a neuroinflammatory and neurodegenerative condition characterized by progressive deterioration of cognitive function and loss of short-term memory and executive function. Cognitive tests quantifying AD severity have been exhaustively developed. Formal diagnosis of AD has historically been dependent on the presence of extraneuronal amyloid beta (Aβ) plaques, which can only be observed at autopsy or with the aid of sophisticated radioimaging techniques. However, diagnostic methods have recently been approved that quantify Aβ in peripheral blood and correlate well with imaging results. Aβ plaques can also be found in people without apparent AD symptoms, which has cast doubt about the role of Aβ as the central mediator of disease pathology.
Scientific investigations in the past twenty years have provided strong evidence that inflammation, type 2 diabetes (T2D), and inflammation-driven insulin resistance (IR) are drivers of AD. The link between these factors and cognitive impairment are described by relatively new terms, type 3 diabetes and metabolic-cognitive syndrome.
-5-
A large body of evidence supports inflammation as a primary driver of pathology in AD. The major inflammation signaling node, NFkB, and the cytokine tumor necrosis factor (TNF) are important initiators of inflammatory signaling in AD pathology. NE3107 is believed to inhibit extracellular signal regulated kinase (ERK)/NFkB activation and TNF production stimulated by inflammatory mediators, such as lipopolysaccharide. Inhibition of NFkB activation and TNF production from this type of stimulation has broad potential implications for reduction of pathological peripheral and central nervous system (CNS) inflammatory signaling in AD, which include reduction of inflammation-driven insulin resistance, decreased inflammatory cell infiltration into the CNS, and decreased microglia activation. Reduction of systemic inflammation and inflammation driven insulin resistance are also predicted to have beneficial effects on hypothalamus-pituitary-adrenal (HPA) axis dysregulation and hippocampal dysregulation of cortisol secretion that are consequences of adipose inflammation and insulin resistance, and known to promote cognitive impairment, and are also forward-feeding for insulin resistance.
Inflammation, insulin resistance, and associated metabolic dysregulation in the brain contribute to Aβ oligomerization and aggregation, phospho-tau formation, reduced neuron survival stimulus, and a forward-feeding cycle of neuronal energy deficit and oxidative stress, causing neuronal dysfunction (cognitive impairment) and neurodegeneration. NE3107’s combination of anti-inflammatory and insulin sensitizing activity has the potential to disrupt this forward-feeding cycle of AD pathology.
Insulin has a major role in metabolic regulation and neuron survival, while insulin resistance and T2D are closely linked to AD pathology. Insulin signaling is involved in synaptic plasticity, learning, and memory. Exogenous insulin enhances cognition in normal and cognitively impaired subjects. Insulin resistance is linked to cognitive impairment.
The multifactorial influence of insulin signaling on neuron survival and cognition suggests that correction of insulin signaling deficits with NE3107 in the target population may provide significant benefits on both cognition and disease progression. Additional rationale for targeting metabolic dysregulation with NE3107 has come from recent work showing peripheral insulin resistance promotes insulin resistance and senescence in the CNS.
There is also an extensive literature on the complex role of adipose tissue inflammation in systemic inflammation, insulin resistance, hypothalamus-pituitary-adrenal axis (HPA) dysregulation and chronic cortisol excess in cognitive impairment in AD. Obesity and inflammation are closely linked in expanding adipose tissue, where the production of inflammatory cytokines and increased cortisol are driven though up-regulation of 11β-hydroxysteroid dehydrogenase type 1 and adipocyte mineralocorticoid receptor activation. Inflamed adipose tissue interacts with the HPA axis and hippocampus to increase systemic cortisol, and promote hippocampal inflammation through chronically elevated cortisol, which freely penetrates the blood-brain barrier. Hyperglycemia (secondary to insulin resistance) exacerbates adrenal cortisol production and promotes forward feeding of inflammation and HPA-hippocampal dysregulation.
Systemic inflammation from inflamed adipose and associated mononuclear cells, promotes CNS inflammation with associated cognitive decline and neurodegeneration. NE3107’s anti-inflammatory activity against systemic/adipose inflammation and factors that dysregulate cortisol secretion, such as hyperglycemia, has the potential to decrease cognitive impairment and neurodegenerative mechanisms that have been linked to cortisol excess.
-6-
Parkinson’s Disease
The Company initiated a study by treating the first patient, in its Phase 2 study assessing NE3107’s safety and tolerability and potential pro-motoric impact in Parkinson’s disease patients on January 20, 2022. The NM201 study (NCT05083260) is a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study in Parkinson’s Disease (PD). Participants will be treated with carbidopa/levodopa and NE3107 or placebo. Forty patients with a defined PD medication “off state” will be randomized 1:1 placebo to active NE3107 20 mg twice daily for 28 days. Safety assessments will look at standard measures of patient health and potential for drug-drug interactions affecting L-dopa pharmacokinetics and activity. Exploratory efficacy assessments will use the Motor Disease Society Unified Parkinson’s Disease Rating (MDS-UPDRS) parts 1-3, ON/OFF Diary, and Non-Motor Symptom Scale.
Neuroinflammation and activation of brain microglia, leading to increased proinflammatory cytokines (particularly TNF) which play a pivotal role in Parkinson’s Disease (PD), which affects an estimated 1 million Americans. Daily administration of levodopa (converted to dopamine in the brain) is the current standard of care treatment for this movement disorder, but prolonged daily administration leads to side effects of uncontrolled movements called levodopa-induced dyskinesia, commonly referred to as LID. Recent evidence demonstrates that daily administration of levodopa further increases neuroinflammation, microglia activation, and TNF inflammatory damage in neurons.
We have shown in a mouse model that PD NE3107 decreases inflammation and TNF in the brain and increases neuron survival (Nicoletti, 2012 Parkinson’s Disease 969418.) In this neurotoxin induced model, NE3107 decreased clinical signs of disease and neuronal death compared to placebo treated mice.
An unpublished study in a neurotoxin induced marmoset model of Parkinson’s disease reported that administration of NE3107 decreased movement abnormalities that are the clinical signs of the disease. In the same study, NE3107 in combination with levodopa had a stronger effect on clinical signs of disease than levodopa or NE3107 alone, while marmosets treated with NE3107 developed less LID. NE3107-treated monkeys also exhibited neuroprotective activity that promoted the survival of twice as many neurons in the substantia nigra (primary region of the brain that degenerates to cause parkinsonism) as monkeys treated with placebo. The results from the marmoset study suggest that NE3107 may decrease clinical signs of disease in humans (improve motor function), which if true could enable a straightforward clinical development strategy to test NE3107 in PD patients needing promotoric therapy.
If approved as a promotoric agent, NE3107 would provide a non-dopaminergic alternative to Parkinson’s patients, and an opportunity to significantly delay the need to start levodopa therapy. This could represent a first step toward supplanting levodopa as the primary PD therapy, and in addition to delaying the emergence of LID, could also imply a slowing of disease progression, the most important and still unmet objective of PD drug development.
Intellectual Property
BioVie relies on a combination of patent, trade secret, other intellectual property laws (such as FDA data exclusivity), nondisclosure agreements, and other measures to protect our proposed products. We require our employees, consultants, and advisors to execute confidentiality agreements and to agree to disclose and assign to us all inventions conceived during the workday, using our property, or which relate to our business. Despite any measures taken to protect our intellectual property (IP), unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary.
BIV201 was awarded Orphan Drug Designations in the U.S. for the treatment of hepatorenal syndrome (received November 21, 2018) and treatment of ascites due to all etiologies except cancer (received September 8, 2016). We also filed a PCT application covering our novel liquid formulations of terlipressin (international patent application PCT/US2020/034269, published as WO2020/237170) and are seeking patent protection in at least the United States, Europe, China, Japan and other jurisdictions. Also, we own U.S. Patent 11,364,277, which is directed to a method of treating ascites with BIV201, and we are pursuing similar patent coverage in Japan, Europe, and China.
-7-
As of August 22, 2022, we have fifteen (15) issued U.S. patents, one (1) pending U.S. patent application, one (1) pending U.S. PCT application and six (6) issued foreign patents directed to protecting NE3107 and related compounds and methods of making and using thereof. The U.S. patents and pending patent applications and their projected expiration dates are provided below.
| Title | Patent Application Number |
Patent Number |
Expiration Date |
| Steroids Having 7-Oxygen and 17-Heteroaryl Substitution | 13/095,528 14/027,825 14/027,842 |
8,569,275 9,102,702 9,115,168 |
2/14/2024 3/28/2024 3/28/2024 |
| Unsaturated Steroid Compounds | 13/030,326 | 8,586,770 | 6/2/2026 |
| Solid State Forms of a Pharmaceutical | 12/418,559 | 8,252,947* | 4/18/2030 |
| Crystalline Anhydrate Forms of a Pharmaceutical | 14/459,528 15/348,107 16/598,694 17/240,728 |
9,555,046 9,850,271 10,995,112 pending |
4/3/2029 4/3/2029 4/3/2029 — |
| Pharmaceutical Solid State Forms | 12/370,510 | 8,518,922 | 9/24/2031 |
| Methods of Preparing Pharmaceutical Solid State Forms | 13/919,593 | 9,314,471 | 6/28/2029 |
| Steroid Tetrol Solid State Forms | 12/272,767 | 8,486,926 | 1/10/2030 |
| Drug Identification and Treatment Method | 11/941,936 | 8,354,396 | 7/7/2031 |
| Method For Preparing Substituted 3,7-Dihydroxy Steroids | 13/664,304 14/886,738 |
9,163,059** 9,994,608 |
6/5/2029 6/5/2029 |
| Treatment Methods Using Pharmaceutical Solid State Forms | 14/459,493 | 9,877,972 | 4/3/2029 |
| Compositions for Treatment of Neurodegenerative Conditions | PCT/US2022/027294 | pending | — |
| * | Foreign counterparts issued in Australia, Canada, Europe and South Korea expire 4/3/2029. |
| ** | Foreign counterparts issued in Europe and Japan expire 6/5/2029. |
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products such as those we are developing. Any pharmaceutical candidate that we develop must be approved by the FDA before it may be legally marketed in the United States and by the appropriate foreign regulatory agency before it may be legally marketed in foreign countries.
United States Drug Development Process
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, or FDCA, and implements regulations. Drugs are also subject to other federal, state and local statutes and regulations. Biologics are subject to regulation by the FDA under the FDCA, the Public Health Service Act, or the PHSA, and related regulations, and other federal, state and local statutes and regulations. Biological products include, among other things, viruses, therapeutic serums, vaccines and most protein products. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable United States requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. FDA sanctions could include refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
-8-
The process required by the FDA before a drug or biological product may be marketed in the United States generally involves the following:
| ● | Completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices or other applicable regulations; |
| ● | Submission to the FDA of an Investigational New Drug Application, or an IND, which must become effective before human clinical trials may begin; | |
| ● | Performance of adequate and well-controlled human clinical trials according to the FDA’s current good clinical practices, or GCPs, to establish the safety and efficacy of the proposed drug or biologic for its intended use; |
| ● | Submission to the FDA of a New Drug Application, or an NDA, for a new drug product, or a Biologics License Application, or a BLA, for a new biological product; |
| ● | Satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the drug or biologic is to be produced to assess compliance with the FDA’s current good manufacturing practice standards, or cGMP, to assure that the facilities, methods and controls are adequate to preserve the drug’s or biologic’s identity, strength, quality and purity; |
| ● | Potential FDA audit of the nonclinical and clinical trial sites that generated the data in support of the NDA or BLA; and |
| ● | FDA review and approval of the NDA or BLA. |
The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources. There can be no certainty that approvals will be granted.
Clinical trials involve the administration of the drug or biological candidate to healthy volunteers or patients having the disease being studied under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety. Each protocol must be submitted to the FDA as part of the IND. Clinical trials must be conducted in accordance with the FDA’s good clinical practices requirements. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until it is completed.
Human clinical trials prior to approval are typically conducted in three sequential phases that may overlap or be combined:
| ● | Phase 1. The drug or biologic is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients having the specific disease. |
| ● | Phase 2. The drug or biologic is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing schedule for patients having the specific disease. |
| ● | Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials, which usually involve more subjects than earlier trials, are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. Generally, at least two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA or BLA. |
-9-
Post-approval studies, or Phase 4 clinical trials, may be conducted after initial marketing approval. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication and may be required by the FDA as part of the approval process.
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and written IND safety reports must be submitted to the FDA by the investigators for serious and unexpected adverse events or any finding from tests in laboratory animals that suggests a significant risk for human subjects. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor or its data safety monitoring board may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug or biologic has been associated with unexpected serious harm to patients.
Concurrent with clinical trials, companies usually complete additional animal studies and develop additional information about the chemistry and physical characteristics of the drug or biologic as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug or biological candidate and, among other things, must include methods for testing the identity, strength, quality and purity of the final drug or biologic. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug or biological candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug or biologic, proposed labeling and other relevant information are submitted to the FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of substantial user fees; a waiver of such fees may be obtained under certain limited circumstances.
The FDA reviews all NDAs and BLAs submitted before it accepts them for filing and may request additional information rather than accepting an NDA or BLA for filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA or BLA.
After the NDA or BLA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA reviews a BLA to determine, among other things, whether the product is safe, pure and potent and the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, purity and potency. In addition to its own review, the FDA may refer applications for novel drug or biological products or drug or biological products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. During the approval process, the FDA also will determine whether a risk evaluation and mitigation strategy, or REMS, is necessary to assure the safe use of the drug or biologic. If the FDA concludes that a REMS is needed, the sponsor of the NDA or BLA must submit a proposed REMS; the FDA will not approve the NDA or BLA without a REMS, if required.
-10-
Before approving an NDA or BLA, the FDA will inspect the facilities at which the product is to be manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA will typically inspect one or more clinical sites to assure compliance with cGMP. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable it will outline the deficiencies in the submission and often will request additional testing or information.
The NDA or BLA review and approval process is lengthy and difficult and the FDA may refuse to approve an NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical data or other data and information. Even if such data and information is submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA will issue a “complete response” letter if the agency decides not to approve the NDA or BLA. The complete response letter usually describes all of the specific deficiencies in the NDA or BLA identified by the FDA. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the NDA or BLA, addressing all of the deficiencies identified in the letter, or withdraw the application.
If a product receives regulatory approval, the approval may be limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may require Phase 4 testing which involves clinical trials designed to further assess a product’s safety and effectiveness and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan product designation must be requested before submitting an NDA or BLA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has Orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. Competitors, however, may receive approval of different products for the indication for which the Orphan product has exclusivity or obtain approval for the same product but for a different indication for which the Orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug or biological product as defined by the FDA or if our drug or biological candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug or biological product designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity. Orphan Drug status in the European Union has similar but not identical benefits in the European Union.
-11-
Expedited Development and Review Programs
The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drug and biological products that meet certain criteria. Specifically, new drug and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. Unique to a Fast Track product, the FDA may consider for review sections of the NDA or BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA or BLA, the FDA agrees to accept sections of the NDA or BLA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA or BLA.
Any product submitted to the FDA for marketing approval, including those submitted to a Fast Track program, may also be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared with marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Drug or biological products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical studies establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA generally requires that a sponsor of a drug or biological product receiving accelerated approval perform adequate and well-controlled post-marketing clinical studies to establish safety and efficacy for the approved indication. Failure to conduct such studies or conducting such studies that do not establish the required safety and efficacy may result in revocation of the original approval. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch or subsequent marketing of the product. Fast Track designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
Post-Approval Requirements
Any drug or biological products for which we receive FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information on an annual basis or as required more frequently for specific events, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements, which include, among others, standards for direct-to-consumer advertising, prohibitions against promoting drugs and biologics for uses or in patient populations that are not described in the drug’s or biologic’s approved labeling (known as “off-label use”), rules for conducting industry-sponsored scientific and educational activities, and promotional activities involving the internet. Failure to comply with FDA requirements can have negative consequences, including the immediate discontinuation of noncomplying materials, adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties. Although physicians may prescribe legally available drugs and biologics for off-label uses, manufacturers may not market or promote such off-label uses.
We will need to rely, on third parties for the production of our product candidates. Manufacturers of our product candidates are required to comply with applicable FDA manufacturing requirements contained in the FDA’s cGMP regulations. cGMP regulations require among other things, quality control and quality assurance as well as the corresponding maintenance of comprehensive records and documentation. Drug and biologic manufacturers and other entities involved in the manufacture and distribution of approved drugs and biologics are also required to register their establishments and list any products made there with the FDA and comply with related requirements in certain states, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance. Discovery of problems with a product after approval may result in serious and extensive restrictions on a product, manufacturer, or holder of an approved NDA or BLA, including suspension of a product until the FDA is assured that quality standards can be met, continuing oversight of manufacturing by the FDA under a “consent decree,” which frequently includes the imposition of costs and continuing inspections over a period of many years, and possible withdrawal of the product from the market. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
-12-
The FDA also may require post-marketing testing, known as Phase 4 testing, risk minimization action plans and surveillance to monitor the effects of an approved product or place conditions on an approval that could otherwise restrict the distribution or use of the product.
Employees
Our business is managed by our officers who consist of Mr. Cuong Do, Chief Executive Officer & President; Dr Joseph M Columbo, Chief Medical Officer, who joined the Company on November 1, 2021; and Wendy Kim, our Chief Financial Officer and Corporate Secretary; along with Penelope Markham, PhD, Executive Vice President - Liver Cirrhosis R&D; Chris Reading, PhD, Executive Vice President - Neuroscience R&D; and Clarence Ahlem, Executive Vice President - Neuroscience Product Development. These individuals devote their full-time efforts to the Company activities. The company has 13 employees which are all full time. We also rely on a team of highly experienced scientific, medical, and regulatory consultants to conduct its product development activities.
| ITEM 1A. | RISK FACTORS |
Our business, financial condition, operating results and prospects are subject to the following risks. Additional risks and uncertainties not presently foreseeable to us may also impair our business operations. If any of the following risks or the risks described elsewhere in this report actually occurs, our business, financial condition or operating results could be materially adversely affected. In such case, the trading price of our common stock could decline, and our stockholders may lose all or part of their investment in the shares of our common stock.
This Form 10-K contains forward-looking statements that involve risks and uncertainties. These statements can be identified by the use of forward-looking terminology such as “believes,” “expects,” “intends,” “plans,” “may,” “will,” “should,” “predict” or “anticipation” or the negative thereof or other variations thereon or comparable terminology. Actual results could differ materially from those discussed in the forward- looking statements as a result of certain factors, including those set forth below and elsewhere in this Form 10-K.
Risks Relating to Our Business and Industry
We have no products approved for commercial sale, have never generated any revenues and may never achieve revenues or profitability, which could cause us to cease operations.
We have no products approved for commercial sale and, to date, we have not generated any revenue. Our ability to generate revenue depends heavily on (a) successful completion of one or more development programs demonstrating in human clinical trials that BIV201 and NE3107, our product candidates, are safe and effective; (b) our ability to seek and obtain regulatory approvals, including, without limitation, with respect to the indications we are seeking; (c) successful commercialization of our product candidates; and (d) market acceptance of our products. There are no assurances that we will achieve any of the forgoing objectives. Furthermore, our product candidates are in the development stage, and have not been fully evaluated in human clinical trials. If we do not successfully develop and commercialize our product candidates we will not achieve revenues or profitability in the foreseeable future, if at all. If we are unable to generate revenues or achieve profitability, we may be unable to continue our operations.
-13-
We are a development stage company with a limited operating history, making it difficult for you to evaluate our business and your investment.
BioVie Inc. was incorporated on April 10, 2013. We are a development stage biopharmaceutical company with potential therapies that have not been fully evaluated in clinical trials, and our operations are subject to all of the risks inherent in the establishment of a new business enterprise, including but not limited to the absence of an operating history, the lack of commercialized products, insufficient capital, expected substantial and continual losses for the foreseeable future, limited experience in dealing with regulatory issues, the lack of manufacturing experience and limited marketing experience, possible reliance on third parties for the development and commercialization of our proposed products, a competitive environment characterized by numerous, well-established and well capitalized competitors and reliance on key personnel.
Since inception, we have not established any revenues or operations that would provide financial stability in the long term, and there can be no assurance that we will realize our plans on our projected timetable in order to reach sustainable or profitable operations.
Investors are subject to all the risks incident to the creation and development of a new business and each investor should be prepared to withstand a complete loss of his, her or its investment. Furthermore, the accompanying financial statements have been prepared assuming that we will continue as a going concern. We have not emerged from the development stage, and may be unable to raise further equity. These factors raise substantial doubt about our ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Because we are subject to these risks, you may have a difficult time evaluating our business and your investment in our Company. Our ability to become profitable depends primarily on our ability to develop drugs, to obtain approval for such drugs, and if approved, to successfully commercialize our drugs, our research and development (“R&D”) efforts, including the timing and cost of clinical trials; and our ability to enter into favorable alliances with third-parties who can provide substantial capabilities in clinical development, regulatory affairs, sales, marketing and distribution.
Even if we successfully develop and market BIV201 and/or NE3107, we may not generate sufficient or sustainable revenue to achieve or sustain profitability, which could cause us to cease operations and cause you to lose all of your investment.
If the FDA or comparable foreign regulatory authorities approve generic versions of any of our product candidates that receive marketing approval, or such authorities do not grant our products sufficient, or any, periods of exclusivity before approving generic versions of our products, the sales of our products could be adversely affected.
Once a new drug application (“NDA”) is approved, the product covered thereby becomes a “reference listed drug” or RLD, in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluations,” commonly known as the Orange Book. Other manufacturers may seek approval of generic versions of reference listed drugs through submission of abbreviated new drug applications (“ANDAs”) in the United States. In support of an ANDA, a generic manufacturer need not conduct clinical trials. Rather, the applicant generally must show that its product has the same active ingredient(s), dosage form, strength, route of administration and conditions of use or labeling as the reference listed drug and that the generic version is bioequivalent to the reference listed drug, meaning it is absorbed in the body at the same rate and to the same extent as the RLD. Generic products may be significantly less costly to bring to market than the reference listed drug and companies that produce generic products are generally able to offer them at lower prices. Moreover, generic versions of RLDs are often automatically substituted for the RLD by pharmacies when dispensing a prescription written for the RLD. Thus, following the introduction of a generic drug, a significant percentage of the sales of any branded product or reference listed drug is typically lost to the generic product.
-14-
The FDA may not approve an ANDA for a generic product until any applicable period of non-patent exclusivity for the reference listed drug has expired. The United States Federal Food, Drug, and Cosmetic Act (“FDCA”) provides a period of five years of non-patent exclusivity for a new drug containing a new chemical entity (“NCE”). An NCE is an active ingredient that has not previously been approved by FDA in any other NDA. Specifically, in cases where such exclusivity has been granted, an ANDA may not be submitted to the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification that a patent covering the reference listed drug is either invalid or will not be infringed by the generic product, in which case the applicant may submit its application four years following approval of the reference listed drug. If an ANDA is submitted to FDA with a Paragraph IV Certification, the generic applicant must also provide a “Paragraph IV Notification” to the holder of the NDA for the RLD and to the owner of the listed patent(s) being challenged by the ANDA applicant, providing a detailed written statement of the basis for the ANDA applicant’s position that the relevant patent(s) is invalid or would not be infringed. If the patent owner brings a patent infringement lawsuit against the ANDA applicant within 45 days of the Paragraph IV Notification, FDA approval of the ANDA will be automatically stayed for 30 months, or until 7-1/2 years after the NDA approval if the generic application was filed between 4 years and 5 years after the NDA approval. Any such stay will be terminated earlier if the court rules that the patent is invalid or would not be infringed.
While we believe that BIV201 contains an active ingredient, terlipressin, that would be treated as an NCE by the FDA and, therefore, if it is the first terlipressin drug product to be approved, should be afforded NCE exclusivity, the FDA may disagree with that conclusion and may approve generic products after a period that is less than five years. If the FDA were to award NCE exclusivity to someone who receives approval of a terlipressin drug product before us, we believe that we could still be awarded a different type of exclusivity protection from generic competition, which is awarded when an NDA or supplemental NDA for a new use of a drug contains reports of new clinical investigations (other than bioavailability studies) conducted or sponsored by an applicant and which FDA deems to have been essential for approval of the application or supplement. Such exclusivity prevents FDA approval of a generic version of the RLD for three years from the date of the RLD approval. Manufacturers may seek to launch generic products following the expiration of any applicable marketing exclusivity period, even if we still have patent protection for our product and no 30-month stay is in effect. If we do not maintain patent protection and regulatory exclusivity for our product candidates, our business may be materially harmed.
Competition that our products may face from generic versions of our products could materially and adversely impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on the investments we have made in those product candidates.
If we fail to obtain or maintain Orphan Drug exclusivity for BIV201, we will have to rely on other potential marketing exclusivity, and on our intellectual property rights, which may reduce the length of time that we can prevent competitors from selling generic versions of BIV201.
We have obtained Orphan Drug Designation for BIV201 (terlipressin) in the U.S. for the treatment of hepatorenal syndrome (received November 21, 2018) and treatment of ascites due to all etiologies except cancer (received September 8, 2016). Under the Orphan Drug Act, the FDA may designate a product as an Orphan Drug if it is a drug intended to treat a rare disease or condition, defined, in part, as a patient population of fewer than 200,000 in the U.S. In the EU, Orphan Drug designation may be granted to drugs intended to treat, diagnose or prevent a life-threatening or chronically debilitating disease having a prevalence of no more than five in 10,000 people in the EU, and which meet other specified criteria. The company that first obtains FDA approval for a designated Orphan Drug for the associated rare disease may receive a seven year period of marketing exclusivity during which time FDA may not approve another application for the same drug for the same orphan disease or condition. Orphan Drug Exclusivity does not prevent FDA approval of another application for the same drug for a different disease or condition, or of an application for a different drug for the same rare disease or condition. Orphan Drug exclusive marketing rights may be lost under several circumstances, including a later determination by the FDA that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug. Similar regulations are available in the EU with a ten-year period of market exclusivity.
-15-
Even though BioVie has obtained two Orphan Drug Designations for its lead product candidate, terlipressin, for treatment of ascites and for treatment of hepatorenal syndrome, and may seek other Orphan Drug Designations for BIV201, and Orphan Drug Designation for other product candidates, there is no assurance that BioVie will be the first to obtain marketing approval for any particular rare indication. Further, even though BioVie has obtained Orphan Drug Designations for its lead product candidate, or even if BioVie obtains Orphan Drug Designation for other potential product candidates, such designation may not effectively protect BioVie from competition because different drugs can be approved for the same condition and the same drug can be approved for different conditions and potentially used off-label in the Orphan indication. Even after an Orphan Drug is approved, the FDA can subsequently approve another competing drug with the same active ingredient for the same condition for several reasons, including, if the FDA concludes that the later drug is clinically superior due to being safer or more effective or because it makes a major contribution to patient care. Orphan Drug Designation neither shortens the development time or regulatory review time of a drug, nor gives the drug any advantage in the regulatory review or approval process.
In addition, other companies have received Orphan Drug designations for terlipressin. Mallinckrodt Hospital Products IP Limited received Orphan Drug designation in 2004 for terlipressin for the treatment of Hepatorenal Syndrome. Mallinckrodt has already filed an NDA for its product, and the FDA convened an advisory committee meeting to discuss that application in 2020. FDA then issued a complete response letter declining to approve the NDA as filed based on safety concerns. Mallinckrodt resubmitted the NDA in November 2021 with additional analysis and received a CRL in February 2022 for a packaging and labelling issue. In their press release Mallinckrodt stated there were no safety or efficacy issues cited in the CRL. In a press release on June 13, 2022 Mallinckrodt announce resubmission of the NDA. PharmaIN Corporation received Orphan Drug Designation in 2012 for PGC-C12E-terlipressin for treatment of ascites due to all etiologies except cancer. In addition, Ferring Pharmaceuticals Inc. received Orphan Drug designation in 1986 for terlipressin for the treatment of bleeding esophageal varices. If one of those or any other company with Orphan Drug Designation for the same drug as ours for the same proposed disease or condition receives FDA approval and Orphan Drug Exclusivity before our product is approved, approval of our drug(s) for the orphan indication may be blocked for seven years by the other company’s Orphan Exclusivity and they may obtain a competitive advantage even after the exclusivity period expires associated with being the first to market.
We will need to raise substantial additional capital in the future to fund our operations and we may be unable to raise such funds when needed and on acceptable terms, which could have a materially adverse effect on our business.
Developing biopharmaceutical products, including conducting pre-clinical studies and clinical trials and establishing manufacturing capabilities, requires substantial funding. Additional financing will be required to fund the research and development of our product candidates. We have not generated any product revenues, and do not expect to generate any revenues until, and only if, we develop, and receive approval to sell our product candidates from the FDA and other regulatory authorities for our product candidates.
We may not have the resources to complete the development and commercialization of any of our proposed product candidates. We will require additional financing to further the clinical development of our product candidates. In the event that we cannot obtain the required financing, we will be unable to complete the development necessary to file an NDA with the FDA for BIV201 or NE3107. This will delay or require termination of research and development programs, preclinical studies and clinical trials, material characterization studies, regulatory processes, the establishment of our own laboratory or a search for third party marketing partners to market our products for us, which could have a materially adverse effect on our business.
-16-
The amount of capital we may need will depend on many factors, including the progress, timing and scope of our research and development programs, the progress, timing and scope of our preclinical studies and clinical trials, the time and cost necessary to obtain regulatory approvals, the time and cost necessary to establish our own marketing capabilities or to seek marketing partners, the time and cost necessary to respond to technological and market developments, changes made or new developments in our existing collaborative, licensing and other commercial relationships, and new collaborative, licensing and other commercial relationships that we may establish.
Until we can generate a sufficient amount of product revenue, if ever, we expect to finance future cash needs through public or private equity offerings, debt financings, or corporate collaboration and licensing arrangements. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available, we may be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or our commercialization efforts. In addition, we could be forced to discontinue product development and reduce or forego attractive business opportunities. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience additional significant dilution, and debt financing, if available, may involve restrictive covenants. To the extent that we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our technologies or our product candidates, or grant licenses on terms that may not be favorable to us. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
Our fixed expenses, such as rent and other contractual commitments, will likely increase in the future, as we may enter into leases for new facilities and capital equipment and/or enter into additional licenses and collaborative agreements. Therefore, if we fail to raise substantial additional capital to fund these expenses, we could be forced to cease operations, which could cause you to lose all of your investment.
We have limited experience in drug development and may not be able to successfully develop any drugs, which would cause us to cease operations.
We have never successfully developed a new drug and brought it to market. Our management and clinical teams have experience in drug development but they may not be able to successfully develop any drugs. Our ability to achieve revenues and profitability in our business will depend on, among other things, our ability to develop products internally or to obtain rights to them from others on favorable terms; complete laboratory testing and human studies; obtain and maintain necessary intellectual property rights to our products; successfully complete regulatory review to obtain requisite governmental agency approvals; enter into arrangements with third parties to manufacture our products on our behalf; and enter into arrangements with third parties to provide sales and marketing functions. If we are unable to achieve these objectives we will be forced to cease operations and you will lose all of your investment.
Development of pharmaceutical products is a time-consuming process, subject to a number of risks, many of which are outside of our control. Consequently, if we are unsuccessful or fail to timely develop new drugs, we could be forced to discontinue our operations.
Our lead product candidate, BIV201 (continuous infusion terlipressin), has been cleared by the FDA to undergo testing in a mid-stage (Phase 2b) clinical trial for treatment of ascites. On June 24, 2021, we announced that the first patient has been enrolled in this study. If our Phase 2b study in ascites fails to generate sufficient evidence of effectiveness, or shows significant safety risks, we may not be able to continue development of the product for that proposed use. As reflected by the FDA’s complete response letter to Malllinckrodt’s new drug application (NDA) for terlipressin dosed as an intermittent IV bolus (1 or 2 mg every 6 hours) to treat hepatorenal syndrome (HRS), terlipressin may cause significant toxicity when administered this way. We believe that our continuous infusion approach to terlipressin treatment may overcome some of those safety concerns, but there can be no assurance that we will be able to demonstrate acceptable safety for BIV201 to the FDA’s satisfaction. On June 23, 2021, we announced that FDA has provided guidance on our planned Phase 3 clinical trial of BIV201 in hepatorenal syndrome-acute kidney syndrome and have since reached agreement on the key elements of the trial design. On April, 15, 2022, we received FDA comments in respons to a subsequent Type C meeting request. We may fail to obtain FDA clearance to proceed with the study in our proposed form.
-17-
Our new drug product candidate NE3107, which we acquired from NeurMedix in 2021, has been cleared by FDA for use in a Phase 3, randomized, double blind, placebo controlled, parallel group, multicenter study in subjects who have mild to moderate Alzheimer’s Disease. Enrollment in that trial began in August 2021, with a planned primary completion in late 2022/early 2023. Alzheimer’s Disease is a complex and still poorly understood disease. In June 2021, FDA approved the drug aducanumab for treatment of Alzheimer’s despite a strong recommendation against approval from an FDA advisory committee. That FDA approval has generated significant medical and political controversy, including a Congressional investigation, announced on June 25, 2021, into the basis for FDA’s approval decision. That investigation, other potential investigations, and negative publicity of FDA’s approval decision could adversely impact the agency’s oversight of our clinical development program, how the agency may view and act upon any NDA we may file for NE3107, and the commercial viability of NE3107 if it were to be approved and marketed.
Further development and extensive testing will be required to determine the technical feasibility and commercial viability of BIV201 and NE3107. Our success will depend on our ability to achieve scientific and technological advances and to translate such advances into reliable, commercially competitive drugs on a timely basis. Drugs that we may develop are not likely to be commercially available, at a minimum, for several years, if ever. The proposed development schedules for our product candidates may be affected by a variety of factors, including technological difficulties, proprietary technology of others, and changes in government regulation, many of which will not be within our control. Any delay in the development, introduction or marketing of our product candidates could result either in such drugs being marketed at a time when their cost and performance characteristics would not be competitive in the marketplace or in the shortening of their commercial lives. In light of the long-term nature of our projects and other risk factors described elsewhere in this document, we may not be able to successfully complete the development or marketing of any drugs, which could cause us to cease operations.
We may fail to successfully develop and commercialize our product candidate(s) if it is found to be unsafe or ineffective in clinical trials; does not receive necessary approval from the FDA or foreign regulatory agencies; fails to conform to a changing standard of care for the disease it seeks to treat; or is less effective or more expensive than current or alternative treatment methods.
Drug development failure can occur at any stage of clinical trials and as a result of many factors, there can be no assurance that we or our collaborators will reach our anticipated clinical targets. Even if we or our collaborators complete our clinical trials, we do not know what the long-term effects of exposure to our product candidates will be. Furthermore, our product candidates may be used in combination with other treatments and there can be no assurance that such use will not lead to unique or unexpected safety issues. Failure to complete clinical trials or to prove that our product candidates are safe and effective would have a material adverse effect on our ability to generate revenue and could require us to reduce the scope of or discontinue our operations, which could cause you to lose all of your investment.
We face business disruption and related risks resulting from the outbreak of the novel coronavirus 2019 (COVID-19) pandemic, which could have a material adverse effect on our business plan.
The continual widespread health emergencies or pandemics such as the coronavirus (“COVID-19”) pandemic (and its related variants), has led to continued regional quarantines, business shutdowns, labor shortages, disruptions to supply chains, and overall economic instability, which could materially and adversely affect the clinical trials, supply chain, financial condition and financial performance of our company. Although some jurisdictions have relaxed these measures, others have not or have reinstated them as COVID-19 cases surge and its variants continue to emerge. The duration and spread of the COVID-19 pandemic and the long-term impact of COVID-19 and its variants on the financial markets and the overall economy are highly uncertain and cannot be predicted at this time. If the financial markets and/or the overall economy are impacted for an extended period, the Company’s ability to raise funds may be materially adversely affected. In addition, the COVID-19 pandemic has created a widespread labor shortage, including a shortage of medical professionals, and has impacted and may continue to impact the potential patient participation in our studies which may adversely impact our ability to continue or complete our clinical trials in the planned timeline.
-18-
We have no manufacturing experience, and the failure to comply with all applicable manufacturing regulations and requirements could have a materially adverse effect on our business.
We have never manufactured products in the highly regulated environment of pharmaceutical manufacturing, and our team has limited experience in the manufacture of drug therapies. There are numerous regulations and requirements that must be maintained to obtain licensure and permitting required prior to the commencement of manufacturing, as well as additional requirements to continue manufacturing pharmaceutical products. We currently do not own or lease facilities that could be used to manufacture any products that might be developed by us, and have contracted with an experienced Contract Manufacturing Organization (“CMO”) to perform the manufacturing of our new product candidates BIV201 and NE 3107. In addition, we do not have the resources at this time to acquire or lease suitable facilities. If we or our CMO fail to comply with regulations, to obtain the necessary licenses and knowhow or to obtain the requisite financing in order to comply with all applicable regulations and to own or lease the required facilities in order to manufacture our products, we could be forced to cease operations, which would cause you to lose all of your investment.
In addition, the FDA and other regulatory authorities require that product candidates and drug products be manufactured according to cGMP. Any failure by our third-party manufacturers to comply with cGMP could lead to a shortage of BIV201 and NE3107. In addition, such failure could be the basis for action by the FDA to withdraw approval, if granted to us, and for other regulatory enforcement action, including Warning Letters, product seizure, injunction or other civil or criminal penalties.
BIV201 and NE3107 and any other product candidates that we develop may have to compete with other products and product candidates for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that are both capable of manufacturing for us and willing to do so. If we need to find another source of drug substance or drug product manufacturing for BIV201 and NE3107, we may not be able to identify, or reach agreement with, commercial-scale manufacturers on commercially reasonably terms, or at all. If we are unable to do so, we will need to develop our own commercial-scale manufacturing capabilities, which would: impact commercialization of BIV201 and NE3107 in the U.S. and other countries where it may be approved; require a capital investment by us that could be quite costly; and increase our operating expenses.
If our existing third-party manufacturers, or the third parties that we engage in the future to manufacture a product for commercial sale or for our clinical trials, should cease to continue to do so for any reason, we likely would experience significant delays in obtaining sufficient quantities of product for us to meet commercial demand or to advance our clinical trials while we identify and qualify replacement suppliers. If for any reason we are unable to obtain adequate supplies of BIV201 or any other product candidate that we develop, or the drug substances used to manufacture it, it will be more difficult for us to compete effectively, generate revenue, and further develop our products. In addition, if we are unable to assure a sufficient quantity of the drug for patients with rare diseases or conditions, we may lose any Orphan Drug exclusivity to which the product otherwise would be entitled.
We do not currently have the sales and marketing personnel necessary to sell products, and the failure to hire and retain such staff could have a materially adverse effect on our business.
We are an early stage development company with limited resources. Even if we had products available for sale, which we currently do not, we have not secured sales and marketing staff at this early stage of operations to sell products. We cannot generate sales without sales or marketing staff and must rely on others to provide any sales or marketing services until such personnel are secured, if ever. If we fail to hire and retain the requisite expertise in order to market and sell our products or fail to raise sufficient capital in order to afford to pay such sales or marketing staff, then we could be forced to cease operations and you could lose all of your investment.
-19-
Even if we were to successfully develop approvable drugs, we will not be able to sell these drugs if we or our third-party manufacturers fail to comply with manufacturing regulations, which could have a materially adverse effect on our business.
If we were to successfully develop approvable drugs, before we can begin selling these drugs, we must obtain regulatory approval of our manufacturing facility and process or the manufacturing facility and process of the third party or parties with whom we may outsource our manufacturing activities. In addition, the manufacture of our products must comply with the FDA’s current Good Manufacturing Practices regulations, commonly known as GMP regulations. The GMP regulations govern quality control and documentation policies and procedures. Our manufacturing facilities, if any in the future, and the manufacturing facilities of our third-party manufacturers will be continually subject to inspection by the FDA and other state, local and foreign regulatory authorities, before and after product approval. We cannot guarantee that we, or any potential third-party manufacturer of our products, will be able to comply with the GMP regulations or other applicable manufacturing regulations. The failure to comply with all necessary regulations would have a materially adverse effect on our business and could force us to cease operations and you could lose all of your investment.
We must comply with significant and complex government regulations, compliance with which may delay or prevent the commercialization of our product candidates, which could have a materially adverse effect on our business.
The R&D, manufacture and marketing of drug product candidates are subject to regulation, primarily by the FDA in the United States and by comparable authorities in other countries. These national agencies and other federal, state, local and foreign entities regulate, among other things, R&D activities (including testing in animals and in humans) and the testing, manufacturing, handling, labeling, storage, record keeping, approval, advertising and promotion of the product that we are developing. Noncompliance with applicable requirements can result in various adverse consequences, including approval delays or refusals to approve drug licenses or other applications, suspension or termination of clinical investigations, revocation of approvals previously granted, warning letters, fines, criminal prosecution, recalls or seizures of products, injunctions against shipping drugs and total or partial suspension of production and/or refusal to allow a company to enter into governmental supply contracts.
The process of obtaining FDA approval is costly and time consuming. Current FDA requirements for a new human drug or biological product to be marketed in the United States include, among other things: (a) the successful conclusion of pre-clinical laboratory and animal tests, if appropriate, to gain preliminary information on the product’s safety; (b) filing with the FDA of an IND application to conduct human clinical trials for drugs or biologics; (c) the successful completion of adequate and well-controlled human clinical investigations to establish the safety and efficacy of the product for its recommended use; and (d) filing by a company and acceptance and approval by the FDA of a NDA for a drug product or a BLA for a biological product to allow commercial distribution of the drug or biologic. A delay in one or more of the procedural steps outlined above could be harmful to us in terms of getting our product candidates through clinical testing and to market, which could have a materially adverse effect on our business.
The FDA, clinical investigators, Data Safety Monitoring Boards, and Institutional Review Boards review the ongoing conduct of, and emerging safety information from, clinical trials and may order the temporary or permanent discontinuation of clinical trials at any time if it believes the product candidate exposes clinical subjects to an unacceptable health risk. Investigational drugs used in clinical studies must be produced in compliance with cGMP rules pursuant to FDA regulations.
Development, approval, and sales outside the United States of products that we develop will also be subject to regulatory requirements governing human clinical trials and marketing for drugs and biological products and devices. The requirements vary widely from country to country, but typically the registration and approval process takes several years and requires significant resources.
If we experience delays or discontinuations of our clinical trials by the FDA or comparable authorities in other countries, or if we fail to obtain registration or other approvals of our products or devices then we could be forced to cease our operations and you will lose all of your investment.
-20-
Even if we are successful in developing BIV201 and NE3107, our product candidates, we have limited experience in conducting or supervising clinical trials that must be performed to obtain data to submit in concert with applications for approval by the FDA. The regulatory process to obtain approval for drugs for commercial sale involves numerous steps. Drugs are subjected to clinical trials that allow development of case studies to examine safety, efficacy, and other issues to ensure that sale of drugs meets the requirements set forth by various governmental agencies, including the FDA. In the event that our protocols do not meet standards set forth by the FDA, or that our data is not sufficient to allow such trials to validate our drugs in the face of such examination, we might not be able to meet the requirements that allow our drugs to be approved for sale which could have a materially adverse effect on our business.
We can provide no assurance that our product candidates will obtain regulatory approval or that the results of clinical studies will be favorable.
The business plan we have developed for the next twenty-four months for the liver disease program is to complete the Phase 2b clinical development program for our lead new product candidate BIV201 for treatment of ascites, conduct a single pivotal Phase 3 trial of BIV201 for ascites, and commence a pivotal Phase 3 trial required for new drug approval of BIV201 for the treatment of hepatorenal syndrome-acute kidney injury (HRS-AKI), and to pursue other key milestones such as additional patent issuances. For NE3107, we have commenced a potentially pivotal 18-month Phase 3 trial in Alzheimer’s Disease, commenced a Phase 2 study of NE3017 in Parkinson’s Disease. Due to our financial constraints, we do not have the resources necessary to complete all of these clinical studies. Subject to FDA guidance, we plan to commence additional Phase 2 and potentially Phase 3 clinical trials upon receipt of a successful capital raise. There is no guarantee the FDA will approve the commencement of a Phase 3 trial for BIV201, and even if they do our financial constraints may prevent us from undertaking clinical trials.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information and disclosure of our trade secrets or proprietary information could compromise any competitive advantage that we have, which could have a materially adverse effect on our business.
Our success depends, in part, on our ability to protect our proprietary rights to the technologies used in our product candidates. We depend heavily upon confidentiality agreements with our officers, employees, consultants and subcontractors to maintain the proprietary nature of our technology. These measures may not afford us complete or even sufficient protection, and may not afford an adequate remedy in the event of an unauthorized disclosure of confidential information. If we fail to protect and/or maintain our intellectual property, third parties may be able to compete more effectively against us, we may lose our technological or competitive advantage, and/or we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property. In addition, others may independently develop technology similar to ours, otherwise avoiding the confidentiality agreements, or produce patents that would materially and adversely affect our business, prospects, financial condition and results of operations, in which event you could lose all of your investment.
We may be unable to obtain or protect intellectual property rights relating to our product candidates, and we may be liable for infringing upon the intellectual property rights of others, which could have a materially adverse effect on our business.
Our ability to compete effectively will depend on our ability to maintain the proprietary nature of our technologies. We cannot assure investors that we will continue to innovate and file new patent applications, or that if filed any future patent applications will result in granted patents with respect to the technology owned by us or licensed to us. Further, we cannot predict how long it will take for such patents to issue, if at all. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated or circumvented.
-21-
BioVie has also filed a PCT (“Patent Cooperation Treaty”) application covering our novel liquid formulations of terlipressin (international patent application PCT/US2020/034269 published as WO2020/237170) and we will seek patent protection in at least the United States, Europe, China and Japan. We also have fifteen (15) issued U.S. patents one (1) pending U.S. application and one (1) pending U.S. provisional application (provisional application filed May 18, 2021) directed to our newly acquired drug candidates, including NE3107. However, there can be no assurance that our pending patent applications will result in issued patents, or that any issued patent claims from pending or future patent applications will be sufficiently broad to protect BIV201, NE3107, or any other product candidates or to provide us with competitive advantages.
Any patents we do obtain may be challenged by re-examination or otherwise invalidated or eventually found unenforceable. Both the patent application process and the process of managing patent disputes can be time consuming and expensive. If we were to initiate legal proceedings against a third party to enforce a patent related to one of our products, the defendant in such litigation could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity and/or unenforceability are commonplace, as are validity challenges by the defendant against the subject patent or other patents before the USPTO. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement, failure to meet the written description requirement, indefiniteness, and/or failure to claim patent eligible subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent intentionally withheld material information from the USPTO, or made a misleading statement, during prosecution. Additional grounds for an unenforceability assertion include an allegation of misuse or anticompetitive use of patent rights, and an allegation of incorrect inventorship with deceptive intent. Third parties may also raise similar claims before the USPTO even outside the context of litigation. The outcome is unpredictable following legal assertions of invalidity and unenforceability. With respect to the validity question, for example, we cannot be certain that no invalidating prior art existed of which we and the patent examiner were unaware during prosecution. These assertions may also be based on information known to us or the Patent Office. If a defendant or third party were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the claims of the challenged patent. Such a loss of patent protection would or could have a material adverse impact on our business.
The standards that the United States Patent and Trademark Office (and foreign countries) use to grant patents are not always applied predictably or uniformly and can change. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents. Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others.
Further, we rely on a combination of trade secrets, know-how, technology and nondisclosure, and other contractual agreements and technical measures to protect our rights in the technology. If any trade secret, know-how or other technology not protected by a patent were to be disclosed to or independently developed by a competitor, our business and financial condition could be materially adversely affected. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the U.S., and we may encounter significant problems in protecting our proprietary rights in these countries.
We do not believe that either BIV201 or NE3107, the product candidates we are currently developing, infringe upon the rights of any third parties nor are they infringed upon by third parties. However, there can be no assurance that our technology will not be found in the future to infringe upon the rights of others or be infringed upon by others. Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our products or product candidates infringe. For example, pending applications may exist that provide support or can be amended to provide support for a claim that results in an issued patent that our product infringes. In such a case, others may assert infringement claims against us, and should we be found to infringe upon their patents, or otherwise impermissibly utilize their intellectual property, we might be forced to pay damages, potentially including treble damages, if we are found to have willfully infringed on such parties’ patent rights. In addition to any damages we might have to pay, we may be required to obtain licenses from the holders of this intellectual property. We may fail to obtain any of these licenses or intellectual property rights on commercially reasonable terms. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products, which could materially harm our business and the third parties owning such intellectual property rights could seek either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation. Conversely, we may not always be able to successfully pursue our claims against others that infringe upon our technology. Thus, the proprietary nature of our technology or technology licensed by us may not provide adequate protection against competitors.
-22-
The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Moreover, the cost to us of any litigation or other proceeding relating to our patents and other intellectual property rights, even if resolved in our favor, could be substantial, and the litigation would divert our management’s efforts. We may not have sufficient resources to bring any such action to a successful conclusion. Uncertainties resulting from the initiation and continuation of any litigation could limit our ability to continue our operations and you could lose all of your investment.
We depend upon our management and their loss or unavailability could put us at a competitive disadvantage which could have a material adverse effect on our business.
We currently depend upon the efforts and abilities of our executive management team of Cuong Do, our Chief Executive Officer & President; Wendy Kim, our Chief Financial Officer; Dr Joseph Palumbo, our Chief Medical Officer; Penelope Markham, Executive Vice President – Cirrhosis R&D; Chris Reading, our Executive Vice President of Neuroscience R&D and Mr. Clarence Ahlem, our Executive Vice President Product Development who all serve the Company the full-time. The loss or unavailability of the services of any of these individuals for any significant period of time could have a material adverse effect on our business, prospects, financial condition and results of operations which may cause you to lose all of your investment. We have not obtained, do not own, nor are we the beneficiary of key-person life insurance.
We may not be able to attract and retain highly skilled personnel, which could have a materially adverse effect on our business.
Our ability to attract and retain highly skilled personnel is critical to our operations and expansion. We face competition for these types of personnel from other pharmaceutical companies and more established organizations, many of which have significantly larger operations and greater financial, technical, human and other resources than us. We may not be successful in attracting and retaining qualified personnel on a timely basis, on competitive terms, or at all. If we are not successful in attracting and retaining these personnel, our business, prospects, financial condition and results of operations will be materially and adversely affected.
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition. We may be unable to compete with enterprises equipped with more substantial resources than us, which could cause us to curtail or cease operations.
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition based primarily on scientific and technological factors. These factors include the availability of patent and other protection for technology and products, the ability to commercialize technological developments and the ability to obtain government approval for testing, manufacturing and marketing.
We compete with biopharmaceutical firms in the United States, Europe and elsewhere, as well as a growing number of large pharmaceutical companies that are applying biotechnology to their operations. Many biopharmaceutical companies have focused their development efforts in the human therapeutics area. Many major pharmaceutical companies have developed or acquired internal biotechnology capabilities or made commercial arrangements with other biopharmaceutical companies. These companies, as well as academic institutions, government agencies and private research organizations, also compete with us in recruiting and retaining highly qualified scientific personnel and consultants. Our ability to compete successfully with other companies in the pharmaceutical field will also depend to a considerable degree on the continuing availability of capital to us.
Although there are not currently any therapies approved by the FDA specifically for the treatment of ascites due to liver cirrhosis, we still face significant competitive and market risk. Other companies, such as Mallinckrodt Inc., are developing therapies for severe complications of advanced liver cirrhosis, which may in the future be developed for the treatment of ascites, and these therapies could compete indirectly or directly with our product candidate. Similarly, other companies, such as Biogen and Eli Lilly, are developing treatments for Alzheimer’s Disease and Parkinson’s Disease, which could compete indirectly or directly with our product candidate. There may be other competitive development programs of which we are unaware. Even if our product candidates are ultimately approved by the FDA, there is no guarantee that once it is on the market doctors will adopt them in favor of current ascites treatment procedures such as diuretics and paracentesis with respect to BIV201 and Alzheimer’s Disease and Parkinson’s Disease with respect to NE3107. These competitive and market risks could have a material adverse effect on our business, prospects, financial condition and results of operations which may cause you to lose all of your investment.
-23-
Our competition will be determined in part by the potential indications for which drugs are developed and ultimately approved by regulatory authorities. Additionally, the timing of the market introduction of some of our potential product candidate or of competitors’ products may be an important competitive factor. Accordingly, the relative speed with which we can develop drugs, complete pre-clinical testing, clinical trials, approval processes and supply commercial quantities to market are important competitive factors. We expect that competition among drugs approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price and patent protection.
The successful development of biopharmaceuticals is highly uncertain. A variety of factors including, pre-clinical study results or regulatory approvals, could cause us to abandon the development of our product candidates.
Successful development of biopharmaceuticals is highly uncertain and is dependent on numerous factors, many of which are beyond our control.
Product candidates that appear promising in the early phases of development may fail to reach the market for several reasons. Pre-clinical study results may show the product candidate to be less effective than desired (e.g., the study failed to meet its primary endpoints) or to have harmful or problematic side effects. Product candidates may fail to receive the necessary regulatory approvals or may be delayed in receiving such approvals. Among other things, such delays may be caused by slow enrollment in clinical studies, length of time to achieve study endpoints, additional time requirements for data analysis or a IND and later NDA, preparation, discussions with the FDA, an FDA request for additional pre-clinical or clinical data or unexpected safety or manufacturing issues; manufacturing costs, pricing or reimbursement issues, or other factors that make the product not economical. Proprietary rights of others and their competing products and technologies may also prevent the product from being commercialized.
Success in pre-clinical and early clinical studies does not ensure that large-scale clinical studies will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. The length of time necessary to complete clinical studies and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly from one product to the next, and may be difficult to predict. There can be no assurance that any of our products will develop successfully, and the failure to develop our products will have a materially adverse effect on our business and will cause you to lose all of your investment.
There may be conflicts of interest among our officers, directors and stockholders.
Certain of our executive officers and directors and their affiliates are engaged in other activities and have interests in other entities on their own behalf or on behalf of other persons. Neither we nor any of our shareholders will have any rights in these ventures or their income or profits. In particular, our executive officers or directors or their affiliates may have an economic interest in or other business relationship with partner companies that invest in us or are engaged in competing drug development. Our executive officers or directors may have conflicting fiduciary duties to us and third parties. The terms of transactions with third parties may not be subject to arm’s length negotiations and therefore may be on terms less favorable to us than those that could be procured through arm’s length negotiations. Although we have established an audit committee comprised solely of independent directors to oversee transactions between us and our insiders, we do not have any formal policies in place to deal with such conflicting fiduciary duties should such a conflict arise.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or detect fraud. Consequently, investors could lose confidence in our financial reporting and this may decrease the trading price of our common stock.
We must maintain effective internal controls to provide reliable financial reports and detect fraud. We have concluded that our disclosure controls and procedures internal controls, as well as internal controls over financial reporting, are effective. Failure to implement changes to our internal controls or any others that we identify as necessary to establish an effective system of internal controls could harm our operating results and cause investors to lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the trading price of our common stock.
-24-
RISKS RELATING TO OUR COMMON STOCK
You may experience future dilution as a result of future equity offerings or if we issue shares subject to options, warrants, stock awards or other arrangements.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock, including under the Sales Agreement (as defined below). We may sell shares or other securities in any other offering at a price per share that is less than the current market price of our securities, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The sale of additional shares of common stock or other securities convertible into or exchangeable for our common stock would dilute all of our stockholders, and if such sales of convertible securities into or exchangeable into our common stock occur at a deemed issuance price that is lower than the current exercise price of our outstanding warrants sold to Acuitas in August 2022, the exercise price for those warrants would adjust downward to the deemed issuance price pursuant to price adjustment protection contained within those warrants.
In addition, as of June 30, 2022, there were warrants outstanding to purchase an aggregate of 510,372 shares of common stock at exercise prices ranging from $1.88 to $75.00 per share and 3,398,764 shares issuable upon exercise of outstanding options at exercise prices ranging from $1.69 to $42.09 per share. Our Loan Agreement entered into on November 30, 2021, contains a conversion feature whereby at the option of lender, up to $5 million of the outstanding loan amount maybe converted to shares of common stock at a conversion price of $6.98 per share. We may grant additional options, warrants or stock awards. To the extent such shares are issued, the interest of holders of our common stock will be diluted.
Moreover, we are obligated to issue shares of common stock upon achievement of certain clinical, regulatory and commercial milestones with respect to certain of our drug candidates (i.e., NE3107, NE3291, NE3413, NE3789) pursuant to the asset purchase agreement, dated April 27, 2021, by and among the Company, NeurMedix, Inc. and Acuitas Group Holdings, LLC, as amended on May 9, 2021. The achievement of these milestones could result in the issuance of up to 18 million shares of our common stock, further diluting the interest of holders of our common stock.
Certain stockholders who are also officers and directors of the Company may have significant control over our management.
As of September 13, 2022 our directors and executive officers currently own an aggregate 23,431,826 shares of our common stock, which currently constitutes 81.8% of our issued and outstanding common stock. As a result, directors and executive officers may have a significant influence on our affairs and management, as well as on all matters requiring member approval, including electing and removing members of our Board of Directors, causing us to engage in transactions with affiliated entities, causing or restricting our sale or merger, and certain other matters. Our Chairman, Mr. Terren Peizer, may be deemed to beneficially own the shares held by Acuitas. Such concentration of ownership and control could have the effect of delaying, deferring or preventing a change in control of us even when such a change of control would be in the best interests of our stockholders.
We may, in the future, issue additional common stock, which would reduce investors’ percent of ownership and may dilute our share value.
As of September 13, 2022, our Articles of Incorporation, as amended, authorize the issuance of 800,000,000 shares of common stock, and we had 30,165,319 shares of common stock outstanding. Accordingly, we may issue up to an additional 758,532,203 shares of common stock. The future issuance of common stock may result in substantial dilution in the percentage of our common stock held by our then existing shareholders. We may value any common stock in the future on an arbitrary basis. The issuance of common stock for future services or acquisitions or other corporate actions may have the effect of diluting the value of the shares held by our investors, might have an adverse effect on any trading market for our common stock and could impair our ability to raise capital in the future through the sale of equity securities.
-25-
The market price and trading volume of our common stock may be volatile.
The market price and trading volume of our common stock has been volatile. We expect that the market price of our common stock will continue to fluctuate significantly for many reasons, including in response to the risk factors described in this prospectus or for reasons unrelated to our specific performance. In recent years, the stock market has experienced extreme price and volume fluctuations. This volatility has affected the market prices of securities issued by many companies for reasons unrelated to their operating performance and may adversely affect the market price and trading volume of our common stock. Prices for our common stock may also be influenced by the depth and liquidity of the market for our common stock, investor perceptions about us and our business, our future financial results, the absence of cash dividends on our common stock and general economic and market conditions. In the past, securities class action litigation has often been instituted against companies following periods of volatility in their stock price. This type of litigation could result in substantial costs and could divert our management and other resources.
We have a large number of restricted shares outstanding, a portion of which may be sold under Rule 144 which may reduce the market price of our shares.
As of September 13, 2022, we had 30,165,319 shares of common stock issued and outstanding, of which 6,733,493 shares are held by non-affiliates and 23,431,826 are owned by affiliates of the Company, consisting of our officers and directors or entities controlled by them. The majority of our common stock, including all of the affiliates’ securities are deemed “restricted securities” within the meaning of Rule 144 as promulgated under the Securities Act.
It is anticipated that all of the “restricted securities” will be eligible for resale under Rule 144. In general, under Rule 144, subject to the satisfaction of certain other conditions, a person, who is not an affiliate (and who has not been an affiliate for a period of at least three months immediately preceding the sale) and who has beneficially owned restricted shares of our common stock for at least six months is permitted to sell such shares without restriction, provided that there is sufficient public information about us as contemplated by Rule 144. An affiliate who has beneficially owned restricted shares of our common stock for a period of at least one year may sell a number of shares equal to one percent of our issued and outstanding common stock approximately every three months.
Any failure to maintain effective internal control over financial reporting could harm us.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. generally accepted accounting principles (“GAAP”). Under standards established by the Public Company Accounting Oversight Board (“PCAOB”), a deficiency in internal control over financial reporting exists when the design or operation of a control does not allow management or personnel, in the normal course of performing their assigned functions, to prevent or detect misstatements on a timely basis. The PCAOB defines a material weakness as a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented, or detected and corrected, on a timely basis.
If we are unable to assert that our internal control over financial reporting is effective, or when required in the future, if our independent registered public accounting firm is unable to express an unqualified opinion as to the effectiveness of our internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our financial reports, the market price of our common stock could be adversely affected and we could become subject to litigation or investigations by the stock exchange on which our securities are listed, the SEC or other regulatory authorities, which could require additional financial and management resources.
-26-
The lack of public company experience of our management team could adversely impact our ability to comply with the reporting requirements of U.S. securities laws, which could have a materially adverse effect on our business.
Our officers have limited public company experience, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our ability to comply with the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which is necessary to maintain our public company status. If we were to fail to fulfill those obligations, our ability to continue as a U.S. public company would be in jeopardy in which event you could lose your entire investment in our Company.
We are considered a smaller reporting company and is exempt from certain disclosure requirements, which could make our stock less attractive to potential investors.
Rule 12b-2 of the Exchange Act defines a “smaller reporting company” as an issuer that is not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent that is not a smaller reporting company and that:
| · | Had a public float of less than $250 million as of the last business day of its most recently completed fiscal quarter, computed by multiplying the aggregate number of worldwide number of shares of its voting and non-voting common equity held by non-affiliates by the price at which the common equity was last sold, or the average of the bid and asked prices of common equity, in the principle market for the common equity; or |
| · | In the case of an initial registration statement under the Securities Act or the Exchange Act for shares of its common equity, had a public float of less than $250 million as of a date within 30 days of the date of the filing of the registration statement, computed by multiplying the aggregate worldwide number of such shares held by non-affiliates before the registration plus, in the case of a Securities Act registration statement, the number of such shares included in the registration statement by the estimated public offering price of the shares; or |
| · | In the case of an issuer who had annual revenue of less than $100 million during the most recently completed fiscal year for which audit financial statements are available, had a public float as calculated under paragraph (1) or (2) of this definition that was either zero or less than $700 million. |
As a “smaller reporting company” we are not required and may not include a Compensation Discussion and Analysis (“CD&A”) section in our proxy statements; we provide only 3 years of business development information; and have other “scaled” disclosure requirements that are less comprehensive than issuers that are not “smaller reporting companies” which could make our stock less attractive to potential investors, which could make it more difficult for you to sell your shares.
We are subject to the periodic reporting requirements of the Exchange Act, which require us to incur audit fees and legal fees in connection with the preparation of such reports. These additional costs will negatively affect our ability to earn a profit.
We are required to file periodic reports with the SEC pursuant to the Exchange Act and the rules and regulations thereunder. In order to comply with such requirements, our independent registered auditors have to review our financial statements on a quarterly basis and audit our financial statements on an annual basis. Moreover, our legal counsel has to review and assist in the preparation of such reports. Factors such as the number and type of transactions that we engage in and the complexity of our reports cannot accurately be determined at this time and may have a major negative effect on the cost and amount of time to be spent by our auditors and attorneys.
-27-
However, the incurrence of such costs is an expense to our operations and thus has a negative effect on our ability to meet our overhead requirements and earn a profit.
Because we do not intend to pay any cash dividends on our common stock, our stockholders will not be able to receive a return on their shares unless they sell them.
We intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless they sell them. There is no assurance that stockholders will be able to sell shares when desired.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
From July 1, 2018 to October 31, 2021, the Company paid monthly rent of $1,000 to Acuitas for its headquarter office at 2120 Colorado Avenue Suite 230, Santa Monica, CA 90404. Effective November 1, 2021, the Company relocated its headquarters to 680 W. Nye Lane, Suite 201, Carson City Nevada 897603. The Company paid annual rent of $2,200 for a 12 month lease term that began November 1, 2021.
On June 1, 2021, the Company assumed a NeurMedix office lease at 6165 Greenwich Drive Suite 150, San Diego, CA 92122 for monthly rent of $8,782 which ended on February 28, 2022. On February 26, 2022 the Company relocated its’ office to 5090 Shoreham Place, Suite 212, San Diego, CA 92122 and entered into a 38 months lease that commenced on March 1, 2022 with a 2 month rent abatement. The monthly base rate payment of $4,175 begins June 1, 2022 with annual three percent increases.
| ITEM 3. | LEGAL PROCEEDINGS |
To our knowledge, neither the Company nor any of our officers or directors is a party to any material legal proceeding or litigation and such persons know of no material legal proceeding or contemplated or threatened litigation, other than as described below. There are no judgments against us or our officers or directors. None of our officers or directors has been convicted of a felony or misdemeanor relating to securities or performance in corporate office.
| ITEM 4. | MINE SAFETY DISCLOSURES |
None.
-28-
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Unregistered Sales of Securities
All sales of unregistered securities during the year ended June 30, 2022 were previously disclosed in a Quarterly Report on Form 10-Q or Current report on Form 8-K.
Issuer Purchases of Common Stock
During the year ended June 30, 2022, there were no issuer repurchases of shares of common stock.
| ITEM 6. | [Reserved] |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion of the Company’s financial condition and the results of operations should be read in conjunction with the Financial Statements and Notes thereto appearing elsewhere in this report.
Overview
BioVie Inc. is a clinical-stage company developing innovative drug therapies to overcome unmet medical needs in chronic debilitating conditions.
In liver disease, our Orphan Drug candidate BIV201 (continuous infusion terlipressin) is being developed as a future treatment option for patients suffering from ascites and other life-threatening complications of advanced liver cirrhosis caused by NASH, hepatitis, and alcoholism. The initial target for BIV201 therapy is refractory ascites. These patients suffer from frequent life-threatening complications, generate more than $5 billion in annual treatment costs, and have an estimated 50% mortality rate within 6 to 12 months. The US Food and Drug Administration (FDA) has not approved any drug to treat refractory ascites. A Phase 2a clinical trial of BIV201 was completed in 2019, and a multi-center, randomized 30-patient Phase 2b trial is currently underway. As of June 30, 2022, eleven US study centers had been activated and are actively screening and enrolling patients in the study. Top-line results from this trial are expected in mid calendar year 2023.
In neurodegenerative disease, BioVie acquired the biopharmaceutical assets of NeurMedix, Inc., a privately held clinical-stage pharmaceutical company and related party affiliate, in June 2021. The acquired assets include NE3107, a potentially selective inhibitor of inflammatory ERK signaling that, based on animal studies, is believed to reduce neuroinflammation. NE3107is a novel orally administered small molecule that is thought to inhibit inflammation-driven insulin resistance and major pathological inflammatory cascades with a novel mechanism of action. There is emerging scientific consensus that both inflammation and insulin resistance may play fundamental roles in the development of Alzheimer’s and Parkinson’s Disease, and NE3107 could, if approved, represent an entirely new medical approach to treating these devastating conditions affecting an estimated 6 million Americans suffering from Alzheimer’s and 1 million from Parkinson’s. The FDA has authorized a potentially pivotal Phase 3 randomized, double-blind, placebo-controlled, parallel group, multicenter study to evaluate NE3107 in subjects who have mild to moderate Alzheimer’s disease (NCT04669028). We initiated this trial on August 5, 2021 and are targeting primary completion in mid calendar year 2023.
-29-
On January 20, 2022, the Company initiated a study by treating the first patient, in its Phase 2 study assessing NE3107’s safety and tolerability and potential pro-motoric impact in Parkinson’s disease patients. The NM201 study (NCT05083260) is a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study in Parkinson’s Disease (PD). Participants will be treated with carbidopa/levodopa and NE3107 or placebo. Forty patients with a defined PD medication “off state” will be randomized 1:1 placebo to active NE3107 20 mg twice daily for 28 days. Safety assessments will look at standard measures of patient health and potential for drug-drug interactions affecting L-dopa pharmacokinetics and activity. Exploratory efficacy assessments will use the Motor Disease Society Unified Parkinson’s Disease Rating (MDS-UPDRS) parts 1-3, ON/OFF Diary, and Non-Motor Symptom Scale. Topline results are expected for the NM201 study by the end of calendar year 2022.
Investigator-Initiated Trial in MCI and Mild Alzheimer’s Disease, NCT05227820
The Company provided the financial support and the use of our NE3107 formulated drug product to The Regenesis Project of Dr Sheldon Jordan in an open-label phase 2 study in Dr. Sheldon’s patients with Alzheimer’s disease related dementias. The Study which received FDA authorization on December 12, 2021; was designed to measure NE3107 effected on cognition, cerebral spinal fluid (“CSF”) and blood biomarkers, and neuro-imagining endpoints. The study seeks to measure changes in cognition through verbal and visual test procedures and changes in biomarkers of Alzheimer's disease and inflammatory and metabolic parameters that can be measured in the central nervous system with advanced neuroimaging techniques in patients before and after treatment with 20 mg of NE3107 twice daily for 3 months following three months of treatment. Data analysis for the study is expected be in completed in the second half of the calendar year 2022.
Results of Operations
Comparison of the Year Ended June 30, 2022 to the Year Ended June 30, 2021
Net loss
The net loss for the year ended June, 2022 was approximately $26.1 million as compared to net loss of $130.2 million for the year ended June 30, 2021. The net decline in net loss of approximately $104.1 million was primarily attributed to the In process research and development (“IPR&D”) purchased in June 2021 of $130.6 million offset by an increase in research and development (“R&D”) expenses and selling, general and administrative (“SG&A”) expenses totaling approximately $19.8 million and the decline in other income, net of approximately $6.6 million.
Total operating expenses for the year ended June 30, 2022 were approximately $27.3 million as compared to $138.1 million for the year ended June 30, 2021. The net decrease of approximately $110.8 million was primarily attributed to the IPR&D purchased in June 2021 of $130.6 million offset by an increase in R&D expenses of approximately $14.7 million and SG&A expenses of approximately $5.1 million.
Research and Development Expenses
Research and development expenses were approximately $17.3 and $2.5 million for the years ended June 30, 2022 and 2021, respectively. The net increase of approximately $14.8 million, was comprised of the Neuroscience clinical operations of approximately $8.1 million for the activities in the Alzheimer pivotal Phase 3 clinical trial, the initiation of the Parkinson’s Phase 2 clinical that launched in January 2022, financial support to the investigator initiated study in MCI and Mild Alzheimer, and other research projects and R&D; approximately $1.7 million of the increase relates to the ongoing Orphan Drug candidate BIV201’s Phase 2b clinical trial and increased expenses for the expanded clinical team for employee compensation and benefit expenses and supporting clinical consultant roles of approximately $4.9 million which included stock based compensation expense of $1.3 million. The Company expanded the clinical team personnel during the fiscal year ended June 30, 2022 and hired our Chief Medical Officer; VP of Clinical Operations, VP Head of CMC, VP Head of Q&A and other clinical team members, increasing the clinical team head count in to eight full-time employees.
-30-
Selling, General and Administrative Expenses
Selling, general and administrative expenses were approximately $9.8 million and $4.6 million for the years ended June 30, 2022 and 2021, respectively. The net increase of approximately $5.2 million was primarily comprised of increased employee compensation and benefit expenses of approximately $3.2 million which included stock based compensation expense of approximately $2.4 million ; increased legal expense of approximately $804,000; increased investor relations and advisory fees totaling approximately $1.0 million and increased expenses related to other consulting fees, accounting and audit, insurance premiums, office and website development expenses totaling approximately $673,000 and offset by approximately $617,000 of directors stock based compensation. The overall increased SG&A was attributed to the expanded operations of the company, the addition of Neuroscience operations which began in June 2021; the hiring of the new CEO who came on board effective April 27, 2021, the addition of a Chief Social Impact Officer and two administrative staff during the fiscal year ended June 30, 2022.
Other income, net
Other income, net; for the year ended June 30, 2022 was approximately $1.2 million compared to $7.8 million for the year ended June 30, 2021. The net decline of approximately $6.6 million was attributed to the change in the fair value of the derivative liabilities of approximately $5.0 million and the increase in interest expense of approximately $1.6 million.
Capital Resources and Liquidity
As of June 30, 2022 the Company had working capital of approximately $14.6 million, cash of approximately $18.6 million, stockholders’ equity of approximately $3.7 million, and an accumulated deficit of approximately $251 million. In addition, the Company has not generated any revenues to date and no revenues are expected in the foreseeable future. The Company’s future operations are dependent on the success of the Company’s ongoing development and commercialization efforts, as well as its ability to secure additional financing as needed.
On August 31, 2022, the Company entered into a Controlled Equity Offering Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. and B. Riley Securities, Inc. (collectively, the “Agents”), pursuant to which the Company may issue and sell from time to time shares of Company’s Class A common stock, par value $0.0001 per share, through the Agents, subject to the terms and conditions of the Sales Agreement.
As of September 12, 2022, the Company has issued 1,544,872 shares under the Sales Agreement for a total net proceeds of $5.9 million after commissions and expenses of approximately $400,000.
On July 15, 2022, the Company, entered into a securities purchase agreement (the “Purchase Agreement”) with Acuitas. pursuant to which Acuitas agreed to purchase from the Company, in a private placement (the “Private Placement”), (i) an aggregate of 3,636,364 shares of the Company’s Class A common stock, par value $0.0001 per share at a price of $1.65 per share, and (ii) a warrant to purchase 7,272,728 shares of Common Stock, at an exercise price of $1.82, with a term of exercise of five years; (collectively, the “Securities”). The aggregate purchase price for the Securities sold in the Private Placement was approximately $6 million. The Private Placement closed on August 15, 2022.
Additionally in November 2021, the Company closed a debt financing, pursuant to which it received a loan in the aggregate principal amount of $15 million and incurred direct financing costs of approximately $390,000.
Although the resulting increase in the Company’s cash balance from the capital raise and debt financing could possibly sustain operations over the next 12 months if measures are taken to delay planned expenditures in our research protocols and slow the progress in the Company’s clinical programs, given the Company’s current planned operations to meet certain goals and objectives, we expect projected cash flows to be depleted within that period of time.
The future viability of the Company is largely dependent upon its ability to raise additional capital to finance its operations. We cannot assure you that our drug candidate will be developed, work, or receive regulatory approval; that we will ever earn revenues sufficient to support our operations or that we will ever be profitable. Furthermore, since we have no committed source of sufficient financing, we cannot assure that we will be able to raise money as and when we need it to continue our operations. If we cannot raise funds as and when we need them, we may be required to severely curtail, or even to cease, our operations.
-31-
Although management continues to pursue its strategic plans, there is no assurance that the Company will be successful in obtaining sufficient financing on terms acceptable to the Company, if at all, to fund continuing operations. Management intends to attempt to secure additional required funding primarily through additional equity or debt financings. We may also seek to secure required funding through sales or out-licensing of intellectual property assets, seeking partnerships with other pharmaceutical companies or third parties to co-develop and fund research and development efforts, or similar transactions. However, there can be no assurance that we will be able to obtain required funding. If we are unsuccessful in securing funding from any of these sources, we will defer, reduce or eliminate certain planned expenditures in our research protocols. If we do not have sufficient funds to continue operations, we could be required to seek bankruptcy protection or other alternatives that could result in our stockholders losing some or all of their investment in us.
The continual widespread health emergencies or pandemics such as the coronavirus (“COVID-19”) pandemic (and its related variants), has lead to continued regional quarantines, business shutdowns, labor shortages, disruptions to supply chains, and overall economic instability. Although some jurisdictions have relaxed these measures, others have not or have reinstated them as COVID-19 cases and its variants continue to emerge The duration and spread of the COVID-19 pandemic and the long-term impact of COVID-19 and its variants on the financial markets and the overall economy, are highly uncertain and cannot be predicted at this time. If the financial markets and/or the overall economy are impacted for an extended period, the Company’s ability to raise funds may be materially adversely affected. In addition, the COVID-19 pandemic has created a widespread labor shortage, including a shortage of medical professionals, and may possibly impact the potential patient participation in our studies of which may adversely impact our ability to continue or complete our clinical trials in the planned timeline.
These circumstances raise substantial doubt on our ability to continue as a going concern. The financial statements included in this report do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts and classification of liabilities that might result from this uncertainty.
Off-Balance Sheet Arrangements
The term “off-balance sheet arrangement” generally means any transaction, agreement or other contractual arrangement to which an entity unconsolidated with the Company is a party, under which the Company has (i) any obligation arising under a guarantee contract, derivative instrument or variable interest; or (ii) a retained or contingent interest in assets transferred to such entity or similar arrangement that serves as credit, liquidity or market risk support for such assets. The Company has no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect or change on the Company’s financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Critical Accounting Policies and Estimates
Accounting for Stock-based Compensation
The Company follows the provision of ASC 718- Stock Compensation, which requires the measurement of compensation expense for all shared – based payment awards made to employees and non-employee director, including employee stock options. Share-based compensation expense is based on the grant date fair value estimated in accordance with the provisions of ASC 718 and is generally recognized as an expense over the requisite service period, net of forfeitures.
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the assets to the future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets and would be charged to earnings.
-32-
Purchase Accounting for Transactions with Related Party
Purchase accounting for transactions with related party, entities under common control, are recorded at the historical carrying cost with no step up in basis to the fair market value of the asset or liability are recognized.
Leases
The Company determines whether an arrangement contains a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, current portion of operating lease liabilities, and net of current portion of operating lease liabilities on our balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent an obligation to make lease payments arising from the lease. Lease ROU assets and lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at the commencement date. As the Company’s leases do not provide an implicit rate, an incremental borrowing rate is used based on the information available at the commencement date in determining the present value of lease payments. The Company does not include options to extend or terminate the lease term unless it is reasonably certain that the Company will exercise any such options. Rent expense is recognized under the operating leases on a straight-line basis. The Company does not recognize right of-use assets or lease liabilities for short-term leases, which have a lease term of twelve months or less, and instead will recognize lease payments as expense on a straight-line basis over the lease term.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value for applicable assets and liabilities, we consider the principal or most advantageous market in which we would transact and we consider assumptions. market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance. This guidance also establishes a fair value hierarchy to prioritize inputs used in measuring fair value as follows:
| ● | Level 1: Observable inputs such as quoted prices in active markets; |
| ● | Level 2: Inputs, other than quoted prices in active markets, that are observable either directly or indirectly; and |
| ● | Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Not applicable.
| ITEM 8. | FINANCIAL STATEMENTS |
Our financial information required to be filed hereunder are indexed under Item 15 of this report and are incorporated herein by reference.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES |
Not applicable.
-33-
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
We have evaluated, with the participation of our principal executive and our principle financial officer, the effectiveness of our disclosure controls and procedures as defined in Rules 13a-15(e) and 15(d)-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our principal executive officer and our principal financial officer have concluded that our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of the effectiveness of internal control to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures may deteriorate. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of June 30, 2022 using the criteria established in Internal Control Integrated Framework (“2013 Framework”) issued by the Committee of Sponsoring Organization of the Treadway Commission (“COSO”). Based on our evaluation using those criteria, our management has concluded that, as of June 30, 2022, our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles for the reasons discussed above.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal controls over financial reporting during quarter ended June 30, 2022, that materially affected, or are reasonably likely to materially affect our internal controls over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not applicable.
-34-
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The following table sets forth certain information regarding our Board of Directors, our executive officers, and some of our key employees, as of September 13, 2022.
| Name | Age | Director Since | Position | |||||
| Cuong Do | 56 | 2016 | CEO & President and Director | |||||
| Terren Peizer | 63 | 2018 | Chairman | |||||
| Joanne Wendy Kim | 67 | -- | Chief Financial Officer | |||||
| Joseph M. Palumbo, MD | 62 | -- | Chief Medical Officer | |||||
| Penelope Markham, PhD | 56 | -- | EVP - Liver Cirrhosis R&D | |||||
| Chris Readings, PhD | 75 | -- | EVP - Neuroscience R&D | |||||
| Clarence Ahlem | 67 | -- | EVP - Neuroscience Product Development | |||||
| Jim Lang | 57 | 2016 | Director | |||||
| Michael Sherman | 63 | 2017 | Director | |||||
| Richard J. Berman | 80 | 2019 | Director | |||||
| Steve Gorlin | 85 | 2020 | Director | |||||
| Robert Hariri, MD, PhD | 63 | 2020 | Director | |||||
| Sigmund Rogich | 78 | 2020 | Director | |||||
According to our Bylaws, the directors shall be elected at the annual meeting of the stockholders and each director shall be elected to serve until his successor shall be elected and shall qualify. A director need not be a stockholder. Directors shall not receive any stated salary for their services as directors or as members of committees, but by resolution of the Board of Directors a fixed fee and expenses of attendance may be allowed for attendance at each meeting. The Bylaws shall not be construed to preclude any director from serving the Company in any other capacity as an officer, agent or otherwise, and receiving compensation therefor.
There are no familial relationships among any of our directors or officers. Mr. Terren Peizer, Chairman of the Board of Directors, is also the founder of Catasys, Inc. a U.S. reporting company listed on Nasdaq on whose board Mr. Sherman also serves. Additionally, Jim Lang currently serves as a director at OptimizeRX, a U.S. reporting company that is listed on the Nasdaq stock exchange. None of our other directors or officers is or has been a Director or has held any form of directorship in any other U.S. reporting companies. None of our directors or officers has been affiliated with any Company that has filed for bankruptcy within the last five years. We are not aware of any proceedings to which any of our officers or directors, or any associate of any such officer or director, is a party that are adverse to the Company. We are also not aware of any material interest of any of our officers or directors that is adverse to our own interests.
Biographical Information
Mr. Cuong Do, has served on the Company’s board of directors since 2016 and effective April 27, 2021 was appointed the Company’s CEO and President. He served as the President, Global Strategy Group, at Samsung from February 2015 to December 2020. Mr. Do helped set the strategic direction for Samsung Group’s diverse business portfolio. He was previously the Chief Strategy Officer for Merck from October 2011 to March 2014, and Tyco Electronics from June 2009 to October 2011, and Lenovo from December 2007 to March 2009. Mr. Do is a former senior partner at McKinsey & Company, where he spent 17 years and helped build the healthcare, high tech and corporate finance practices. He holds a BA from Dartmouth College, and an MBA from the Tuck School of Business at Dartmouth.
We believe Mr. Do’s qualifications to serve on our Board of Directors and as the CEO are primarily based on his decades of experience as an executive in the pharma, biotech, and other high technology industries and his extensive experience in strategy, corporate finance practice and the development of companies in all stages.
-35-
Mr. Terren Peizer, Chairman of the Board of Directors, since July 2018, is an entrepreneur, investor, and financier with a particular interest in healthcare, having founded and successfully commercialized several healthcare companies. Mr. Peizer was the founder of Ontrak (Formerly known as Catasys, Inc.), a leader in behavioral and mental health management services, having served as the Chairman of the Board of Directors since Ontrak’s inception in 2003 through April 11, 2021. Effective April 12, 2021, Mr. Peizer was appointed to serve as Ontrak’s Executive Chairman. He was the founder, Chairman and CEO of NeurMedix, Inc., a biotechnology company with a focus on inflammatory, neurological and neuro-degenerative diseases. Mr. Peizer is also Executive Chairman of Verde, Inc., a company producing 100% plant-based, compostable, and biodegradable plastic. He is the Executive Chairman of the mobility delivery company ZipMo, Inc. He also is the Executive Chairman of the blockchain company, Casper Labs, Inc. Mr. Peizer owns Acuitas Group Holdings, LLC, (Acuitas) his personal holding company that owns his portfolio Company interests. Through Acuitas , Mr. Peizer owns, Acuitas Capital, LLC, an industry leader in investing in micro and small capitalization equities, having invested over $1.5 billion directly into portfolio companies. Mr. Peizer has been the largest beneficial shareholder of, and has held various senior executive positions with several other publicly traded growth companies. He served as Chairman of Cray, Inc., a supercomputer company recently sold to Hewlet Packard, Inc. Mr. Peizer has a background in venture capital, investing, mergers and acquisitions, corporate finance, and previously held senior executive positions with the investment banking firms Goldman Sachs, First Boston, and Drexel Burnham Lambert. He received his B.S.E. in finance from The Wharton School of Finance and Commerce.
We believe Mr. Peizer’s qualifications to serve on our board of directors include his role as an investor and executive positions in several private and public companies, including numerous companies in the healthcare field. He has extensive knowledge and experience in the financial and healthcare industries and provides extensive insight and experience with capital markets and publicly traded companies at all stages of development.
Ms. Joanne Wendy Kim has served as the Company’s Chief Financial Officer since October 2018. Ms. Kim previously served as CFO for several companies throughout her career, previously with Landmark Education Enterprises, and prior to that; other public entities in the entertainment and financial services industry sectors. She provided interim CFO services to various organizations through Group JWK from 2016 to 2018. In her various roles, Ms. Kim oversaw corporate finance and operational groups, closed eight acquisitions, secured bank financings, developed and implemented new business strategies, managed risk and implemented new financial policies and procedures. As a CPA professional, she advised on accounting transactions, SEC reporting matters and other regulatory matters to clients serving as a Director at BDO USA, LLP’s National Office SEC Department and sat the US desk in London for BDO LLP UK Firm in 2008-2016 and as a Senior Manager at KPMG in earlier part of her career. She brings more than 35 years of accounting and finance experience to this position. Ms. Kim earned her BSA in accounting and finance at California State University, Long Beach.
Wendy Kim’s qualifications to serve as our Chief Financial Officer are primarily based on her 35 years of accounting and finance experience both as a CFO and as a CPA in major global accounting and consultancy firms.
Dr. Joseph M. Palumbo has served as our Chief Medical Officer since November 2021. Formerly he served as the CMO at Zynerba Pharmaceuticals from July 2019 to October 2021, responsible for clinical operations, development, regulatory, and medical affairs. Prior to his time at Zynerba, Dr. Palumbo held senior worldwide governance roles at Mitsubishi Tanabe Pharma in both the United States and Japan from April 2012 to June 2019, where he led medical science and translational research across multiple therapeutic areas, and guided successful registrational programs for Radicava® (edaravone) for the treatment of Amyotrophic Lateral Sclerosis. From April 2003 to March 2012, Dr. Palumbo was Global Head and Franchise Medical Leader for Psychiatry, and the Interim Head of Global Neuroscience at Johnson & Johnson, where he led the medical teams who achieved successful global registrations for Risperdal® (risperidone); Concerta® (methylphenidate HCL); and Invega® (paliperidone). He was Head of Psychiatry and Neurology at Pharmanet for from April 2002 to April 2003. Dr Palumbo previously held industry positions in European Pharma with Sanofi-Synthelabo from April 1999 to April 2002, Biotech at Cephalon, from April 1997 to April 1998, and from July 1989 to April 2002, he held senior leadership and hospital administration roles at prestigious academic research institutions including Yale, Cornell, and the University of Pennsylvania. He holds a Bachelor of Arts at the University of Pennsylvania and received his Doctor of Medicine at the George Washington University School of Medicine. He was a Biological Sciences Training Program Fellow of the National Institutes of Health and Chief Resident for the Abraham Ribicoff Clinical Neuroscience Research Unit at Yale University. Dr Palumbo has received Board Certification in Psychiatry and Addiction Psychiatry.
-36-
Dr. Palumbo’s qualifications to serve as our Chief Medical Officer is based on the decades and depth of experiences in the roles he has served in his medical profession and commercial experience in the healthcare industry and biopharma industries.
Dr. Penelope Markham currently serves as our Executive Vice President of Liver Cirrhosis Research and Development. Formerly she served as the Company’s Chief Scientific Officer from November 2018 to June 30, 2021. Dr. Markham served as a Technical Consultant at LAT Pharma for 7 years prior to our acquisition of LAT Pharma. She has spent 15 years in immunology, infectious disease, bacteriology and drug discovery research. Dr. Markham was a co-founder and Research Director for Influx, Inc. involved in antibiotic drug discovery. She has been a member of NIH grant review panels and consulted for several pharmaceutical companies in a variety of therapeutic areas including Orphan Drug development. Dr. Markham has more than 20 publications in peer-reviewed journals and three patents. She holds a BS in Biochemistry from the University College Cork, Ireland, a Masters from Strathclyde University, Scotland, and a PhD from Rush University, Chicago.
Dr. Markham’s qualifications to serve as our EVP-Liver Cirhossis – Research and Development scientist are primarily based on her years of experience with LAT Pharma, as well as having been a member of NIH grant review panels and consulted for several pharmaceutical companies in a variety of therapeutic areas including Orphan Drug development.
Dr. Chris Reading joined the Company on July 1, 2021 and serves as our Executive Vice President of Neuroscience-Research and Development. Formerly, he served as the Chief Scientific Officer, Hollis-Eden Pharmaceuticals and its successor companies from 2000 to 2021. Previously, served as the VP of Product and Process Development for SyStemix/Novartis from 1993 to 1999 From there, he moved to San Diego where he has spent over 20 years on the NE3107 platform development. He received his Ph.D. in Biochemistry from UC Berkeley, performed post-doctoral studies in cancer biology at UC Irvine, and joined MD Anderson Cancer Center and the University of Texas, Graduate School of Biomedical Sciences in Houston for 13 years, where he became Associate Professor of Medicine in the Department of Developmental Therapeutics with a joint appointment in the Department of Tumor Biology.
Dr Reading’s qualifications to serve as our EVP of Neuroscience Research and Development are based on his over 40 years of research and drug development experience, and over 130 peer-reviewed scientific publications, he has also authored numerous patents in the areas of monoclonal antibodies, cell separation technologies, stem cell transplantation, and sterol drug development.
Mr. Clarence Ahlem joined the Company on July 1, 2021 and serves as our Executive Vice President- Product Development. Previously he served as the Vice President of Product Development of Hollis-Eden Pharmaceuticals and successor companies Harbor Biosciences and Harbor Therapeutics from 2000 to 2014, where he led the development effort for NE3107 for its initial clinical application, type 2 diabetes. He previously served as the manager of bioorganic chemistry at Systemix, Inc. in Palo Alto CA from June 1991 to June 1995. He began his career in industry with a six-year term in the Therapeutics Division at Hybritch developing synthetic bifunctional antibodies and their clinical applications. Prior to that worked four years in academic research on the enzymology of DNA replication at the University of California San Diego. He received his MS in microbiology at SDSU in 1981.
Mr. Ahlem’s qualifications to serve as our EVP of Product Development are based on more than 35 years of product-oriented research and product development experience that include protein and cell-based biopharmaceutical development, and responsibility for pharmacological characterization, manufacturing, and regulatory submissions to support pharmaceutical development of novel derivatives of the dehydroepiandrosterone metabolome, including NE3107.
-37-
Mr. Jim Lang has served as the Company’s director since 2016. He is currently CEO of EVERSANA, the leading commercialization services company for the life sciences industry. In five years since he founded EVERSANA, it is now over $1B in revenue, with >7000 employees across 40 global locations. He formerly served as the CEO of Decision Resources Group (DRG), which he transformed into a leading healthcare data and analytics firm. Prior to that, Jim was CEO of IHS Cambridge Energy Research Associates (IHS CERA), a recognized leader in energy industry subscription information products, and formerly the President of Strategic Decisions Group (SDG), a leading global strategy consultancy. Mr. Lang holds a BS summa cum laude in electrical and computer engineering from the University of New Hampshire and an MBA with Distinction from the Tuck School of Business. Jim Lang currently also serves as a Director at OptimizeRX (OPRX), a Nasdaq listed Company.
Jim Lang’s qualifications to serve on our Board of Directors are primarily based on his decades of experience as a strategy consultant, broad industry expertise, and senior-level management experience running several healthcare and information technology companies.
Mr. Michael Sherman JD has served as the Company director since 2017. He retired from his position as a Managing Director at Barclays Plc in 2018, where he had worked since 2008. Previously he was a Managing Director at Lehman Brothers, Inc. He has worked in investment banking for 30 years. Mr. Sherman has significant experience in healthcare finance, most recently assisting on a $450 million convertible transaction for Neurocrine Biosciences. He has worked on successful financial transactions for Teva Pharmaceutical Industries, Amgen Inc., Cubist Pharmaceuticals, Merck & Co., and Cardinal Health, among other companies. After graduating from the University of Pennsylvania, Michael Sherman received his JD, cum laude, from the Harvard Law School.
Michael Sherman’s qualifications to serve on our Board of Directors are primarily based on his decades of finance industry experience and investment banking. Mr. Sherman has significant experience in healthcare finance including having worked on successful financial transactions for several pharmaceutical and healthcare focused companies.
Mr. Richard J. Berman has served as the Company’s director since June 2019. Mr. Berman has over 35 years of venture capital, senior management, and merger & acquisitions experience. He currently is a director of four public companies including; Cryoport Inc., Genius Group, Context Therapeutics, and over the last decade served on the boards of six companies that reached a market capitalization over one billion including Cryoport, Advaxis, EXIDE, Internet Commerce Corporation, Kapitus and Ontrak. From 1998-2000, he was employed by Internet Commerce Corporation (now Easylink Services) as Chairman and CEO and was a director from 1998-2012. Previously, Mr. Berman was Senior Vice President of Bankers Trust Company, where he started the M&A and Leveraged Buyout Departments; created the largest battery company in the world in the 1980’s by merging Prestolite, General Battery and Exide and advised on over $4 billion of M&A transactions (completed over 300 deals). He is a past Director of the Stern School of Business of NYU where he obtained his BS and MBA. He also has US and foreign law degrees from Boston College and The Hague Academy of International Law, respectively.
We believe Richard J. Berman’s qualifications to serve on our board of directors include his experience in the healthcare industry, and his current and past experience in numerous private and publicly traded companies.
Mr. Steven Gorlin has served as the Company’s director since June 2020. He has founded many biopharma companies including Hycor Biomedical, Theragenics, Medicis Pharmaceutical, EntreMed, MRI Interventions, DARA BioSciences, MiMedx, Medivation (sold to Pfizer for $14 billion) and NantKwest. Mr. Gorlin served for many years on the Business Advisory Council to the Johns Hopkins School of Medicine and on The Johns Hopkins BioMedical Engineering Advisory Board. He is currently a member of the Research Institute Advisory Committee (RIAC) of Massachusetts General Hospital. He started The Touch Foundation, a nonprofit organization for the blind, and was a principal contributor to Camp Kudzu for diabetic children.
-38-
Steve Gorlin’s qualifications to serve on our Board of Directors are primarily based on his over 45 years of experience in founding and investing in several biopharma companies, leading multiple NASDAQ AND NYSE companies to their success.
Dr. Robert Hariri MD, PhD, has served as the Company’s director since June 2020. Dr Hariri is the Chairman, founder, and CEO of Celularity, Inc., a leading cellular therapeutics company. He was the founder and CEO of Anthrogenesis Corporation, and after its acquisition served as CEO of Celgene Cellular Therapeutics. Dr. Hariri co-founded the genomic health intelligence company, Human Longevity, Inc. Dr. Hariri pioneered the use of stem cells to treat a range of life-threatening human diseases. He is widely acknowledged for his discovery of pluripotent stem cells and for assisting with discovering the physiological activities of tumor necrosis factor (TNF). He holds over 170 issued and pending patents.
Robert (Bob) Hariri’s qualifications to serve on our Board of Directors are primarily based on his decades of founding and leading several companies in the cellular therapeutic space, as well as pioneering in the use of stem cells to treat a range of life-threatening human diseases and discoveries in the physiological activities of tumor necrosis factor. He has authored over 150 publications and garnered numerous awards for contributions to the fields of biomedicine and aviation.
Mr. Sigmund (Sig) Rogich has served as the Company’s director since June 2020. Sig is the CEO and President of The Rogich Communications Group and serves on the Board of Keep Memory Alive, a philanthropic organization which raises awareness about brain disorders and Alzheimer's disease. Keep Memory Alive funds clinical trials to advance new treatments for patients with Alzheimer’s, Huntington’s and Parkinson’s disease, as well as multiple sclerosis. Mr. Rogich was formerly the US Ambassador to Iceland. He has served as a senior consultant to Presidents Ronald Reagan and George H.W. Bush. Mr. Rogich serves on multiple boards of directors for charitable causes.
We believe Mr. Rogich’s qualifications to serve on our Board of Directors are based on his experience in the Communications sector and philanthropic organization raising awareness about brain disorders. His experience in service as a senior consultant to candidates of the highest office.
Delinquent Section 16(a) Reports
Section 16(a) of the Securities Exchange Act of 1934, as amended (Exchange Act), requires our directors and executive officers, and persons who own more than 10% of our outstanding common stock, to file with the SEC, initial reports of ownership and reports of changes in ownership of our equity securities. Such persons are required by SEC regulations to furnish us with copies of all such reports they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us regarding the filing of required reports, we believe that, except for the reports filed by Jonathan Adams (Form 4s filed on September 16, 2021, October 29, 2021 and February 16, 2022), Clarence Ahlem (Form 3 and Form 4 filed on September 28, 2021), Richard J. Berman (Form 3 and Form 4 filed on September 29, 2021 and Form 4 filed on April 20, 2022), Cuong Do (Form 4s filed on September 16, 2021, January 25, 2022 and July 7, 2022), Steve Gorlin (Form 4 filed on April 20, 2022), Robert J. Hariri (Form 3 and Form 4 filed on September 28, 2021 and Form 4 filed on April 20, 2022), Wendy Kim (Form 4 filed on September 16, 2021), James Lang (Form 4 filed on April 25, 2022), Penelope Markham (Form 4 filed on September 16, 2022), Joseph M Palumbo (Form 4 filed on February 17, 2022), Terren Peizer (Form 3 filed on August 16, 2022 and Form 4 filed on August 26, 2022), Christopher Reading (Form 3 filed on October 8, 2021 and Form 4 filed on October 8, 2021), Sigmund Rogich (Form 4 filed on April 21, 2022) and Michael Sherman (Form 4 filed on April 20, 2022), all Section 16(a) reports applicable to our directors, executive officers and greater-than-ten-percent beneficial owners with respect to fiscal 2022 were timely filed.
-39-
Independence of the Board of Directors
Our common stock is traded on the Nasdaq Capital Market. The Board of Directors has determined that six of the members of the Board of Directors qualify as “independent,” as defined by the listing standards of the Nasdaq. Consistent with these considerations, after review of all relevant transactions and relationships between each director, or any of the director's family members, and the Company, its senior management and its independent auditors, the Board has determined further that Messrs. Lang, Sherman, Berman, Gorlin, Hariri and Rogich are independent under the listing standards of Nasdaq. In making this determination, the Board of Directors considered that there were no new transactions or relationships between its current independent directors and the Company, its senior management and its independent auditors since last making this determination.
2022 Meetings and Attendance
During fiscal year 2021, the Board held four Board of Directors meetings, four Audit Committee meetings, five Compensation Committee meetings and one Nominating and Corporate Governance Committee meeting. All Directors attended at least 75% or more of the aggregate number of meetings of the Board and Board Committees on which they served.
Committees of the Board of Directors
Our Board of Directors has three standing committees: an audit committee, a compensation committee and a nominating and corporate governance committee. Both our audit committee and our compensation committee will be composed solely of independent directors. The audit committee is comprised solely of independent directors, and the compensation committee and the nominating and corporate governance committee are comprised solely of independent directors. Each committee operates under a charter approved by our Board of Directors and have the composition and responsibilities described below. The charter of each committee is available on our website.
Audit Committee
We have established an audit committee of the Board of Directors. The members of our audit committee are Richard Berman, Michael Sherman, Jim Lang and Sigmund Rogich each of which is an independent director within the meaning of the Nasdaq rules. Mr. Berman has served as chairman of the audit committee since October 2020 and qualifies as an “audit committee financial expert” as defined by Item 401(h)(2) of Regulation S-K.
We have adopted an audit committee charter, detailing the principal functions of the audit committee, including:
| · | assisting board oversight of (1) the integrity of our financial statements, (2) our compliance with legal and regulatory requirements, (3) our independent auditor’s qualifications and independence, and (4) the performance of our internal audit function and independent auditors; the appointment, compensation, retention, replacement, and oversight of the work of the independent auditors and any other independent registered public accounting firm engaged by us; |
| · | pre-approving all audit and non-audit services to be provided by the independent auditors or any other registered public accounting firm engaged by us, and establishing pre-approval policies and procedures; reviewing and discussing with the independent auditors all relationships the auditors have with us in order to evaluate their continued independence; |
| · | setting clear policies for audit partner rotation in compliance with applicable laws and regulations; |
-40-
| · | obtaining and reviewing a report, at least annually, from the independent auditors describing (1) the independent auditor’s internal quality-control procedures and (2) any material issues raised by the most recent internal quality-control review, or peer review, of the audit firm, or by any inquiry or investigation by governmental or professional authorities, within the preceding five years respecting one or more independent audits carried out by the firm and any steps taken to deal with such issues; |
| · | meeting to review and discuss our annual audited financial statements and quarterly financial statements with management and the independent auditor, including reviewing our specific disclosures under “Management’s Discussion and Analysis of Financial Condition and Results of Operations”; reviewing and approving any related party transaction required to be disclosed pursuant to Item 404 of Regulation S-K promulgated by the SEC prior to us entering into such transaction; and |
| · | reviewing with management, the independent auditors, and our legal advisors, as appropriate, any legal, regulatory or compliance matters, including any correspondence with regulators or government agencies and any employee complaints or published reports that raise material issues regarding our financial statements or accounting policies and any significant changes in accounting standards or rules promulgated by the Financial Accounting Standards Board, the SEC or other regulatory authorities. |
Compensation Committee
We have established a compensation committee of the Board of Directors. The members of our Compensation Committee are Richard Berman, Michael Sherman and Steve Gorlin. Mr. Sherman has served as chairman of the compensation committee since October 2020.
We have adopted a compensation committee charter, which details the principal functions of the compensation committee, including:
| · | reviewing and approving on an annual basis the corporate goals and objectives relevant to our Chief Executive Officer’s compensation, evaluating our Chief Executive Officer’s performance in light of such goals and objectives and determining and approving the remuneration (if any) of our Chief Executive Officer based on such evaluation; |
| · | reviewing and making recommendations to our Board of Directors with respect to the compensation, and any incentive-compensation and equity-based plans that are subject to board approval of all of our other officers; |
| · | reviewing our executive compensation policies and plans; |
| · | implementing and administering our incentive compensation equity-based remuneration plans; assisting management in complying with our proxy statement and annual report disclosure requirements; |
| · | approving all special perquisites, special cash payments and other special compensation and benefit arrangements for our officers and employees; and |
| · | producing a report on executive compensation to be included in our annual proxy statement; and reviewing, evaluating and recommending changes, if appropriate, to the remuneration for directors. |
The charter also provides that the compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, independent legal counsel or other adviser and will be directly responsible for the appointment, compensation and oversight of the work of any such adviser. However, before engaging or receiving advice from a compensation consultant, external legal counsel or any other adviser, the compensation committee will consider the independence of each such adviser, including the factors required by Nasdaq and the SEC.
-41-
Compensation Committee Interlocks and Insider Participation
None of our officers currently serves, or in the past year has served, as a member of the compensation committee of any entity that has one or more officers serving on our Board of Directors.
Nominating and Corporate Governance Committee
We have established a nominating and corporate governance committee of the Board of Directors. The members of our nominating and corporate governance committee are, Jim Lang, Michael Sherman and Robert Hariri. Mr. Lang has served as chair of the nominating and corporate governance committee since August 2021.
We have adopted a nominating and corporate governance committee charter, which details the purpose and responsibilities of the nominating and corporate governance committee, including:
| · | identifying, screening and reviewing individuals qualified to serve as directors, consistent with criteria approved by the Board of Directors, and recommending to the Board of Directors candidates for nomination for election at the annual meeting of stockholders or to fill vacancies on the Board of Directors; |
| · | developing and recommending to the Board of Directors and overseeing implementation of our corporate governance guidelines; |
| · | coordinating and overseeing the annual self-evaluation of the Board of Directors, its committees, individual directors and management in the governance of the company; and |
| · | reviewing on a regular basis our overall corporate governance and recommending improvements as and when necessary. |
The charter also provides that the nominating and corporate governance committee may, in its sole discretion, retain or obtain the advice of, and terminate, any search firm to be used to identify director candidates, and will be directly responsible for approving the search firm’s fees and other retention terms.
We have not formally established any specific, minimum qualifications that must be met or skills that are necessary for directors to possess. In general, in identifying and evaluating nominees for director, the Board of Directors considers educational background, diversity of professional experience, knowledge of our business, integrity, professional reputation, independence, wisdom, and the ability to represent the best interests of our stockholders. Prior to our initial business combination, holders of our public shares will not have the right to recommend director candidates for nomination to our Board of Directors.
Set forth below is information concerning the gender and demographic background of each of our current directors, as self-identified and reported by each director. This information is being provided in accordance with Nasdaq’s board diversity rules.
-42-
| Board Diversity Matrix (As of September 13, 2022) | |||||||||
| Total Number of Directors: | 8 | ||||||||
| Female | Male | Non- Binary |
Did Not Disclose Gender |
||||||
| Part I: Gender Identity | |||||||||
| Directors | 0 | 8 | 0 | 0 | |||||
| Part II: Demographic Background | |||||||||
| African American or Black | - | - | - | - | |||||
| Alaskan Native or Native American | - | - | - | - | |||||
| Asian | - | 1 | - | - | |||||
| Hispanic or Latinx | - | - | - | - | |||||
| Native Hawaiian or Pacific Islander | - | - | - | - | |||||
| White | - | 4 | - | - | |||||
| Two or More Races or Ethnicities | - | - | - | - | |||||
| LGBTQ+ | - | - | - | - | |||||
| Did Not Disclose Demographic Background | - | 3 | - | - | |||||
Code of Ethics
We have adopted a code of conduct and ethics meeting the requirements of Section 406 of the Sarbanes-Oxley Act of 2002. We believe our code of conduct and ethics is reasonably designed to deter wrongdoing and promote honest and ethical conduct; provide full, fair, accurate, timely and understandable disclosure in public reports; comply with applicable laws; ensure prompt internal reporting of violations; and provide accountability for adherence to the provisions of the code of ethic. Our code of conduct and ethics is available on our website.
A copy of our code of conduct and ethics is filed as an exhibit to this Form 10-K.
Anti-Hedging Policy
We have adopted an insider trading policy that includes a provision restricting trading of any interest or provision relating to the future price of our securities, such as a put, call or short sale.
| ITEM 11. EXECUTIVE COMPENSATION |
Summary Compensation Table
The following table sets forth the total compensation paid during the last two fiscal years ended June 30, 2022 and 2021 to the following executive officers of the Company, who are referred to as our “named executive officers”:
| ● | Cuong Do, our President and Chief Executive Officer |
| ● | Joanne Wendy Kim, our Chief Financial Officer and Corporate Secretary |
| ● | Joseph Palumbo, our Chief Medical Officer |
| ● |
Jonathan Adams, our former President and Chief Operating Officer |
-43-
| Name and Principal Position | Year | Salary | Bonus | Stock Awards (1) | Option Awards (1) | Non-Equity Incentive Plan Compensation | Nonqualified Deferred Compensation Earnings | All Other Compensation | Total | |||||||||||||||||||||||||||
| Cuong Do (2) | ||||||||||||||||||||||||||||||||||||
| Chief Executive Officer and President | 2022 | $ | 300,000 | $ | 400,000 | $ | 210,439 | $ | 3,632,382 | $ | — | $ | — | $ | — | $ | 4,542,821 | |||||||||||||||||||
| 2021 | $ | — | $ | — | $ | 454,794 | $ | — | $ | — | $ | — | $ | — | $ | 454,794 | ||||||||||||||||||||
| Joanne Wendy Kim (3) | ||||||||||||||||||||||||||||||||||||
| Chief Financial Officer, Treasurer and Corporate Secretary | 2022 | $ | 235,000 | $ | 127,656 | $ | — | $ | 582,343 | $ | — | $ | — | $ | — | $ | 944,999 | |||||||||||||||||||
| 2021 | $ | 120,625 | $ | — | $ | — | $ | 4,706 | $ | — | $ | — | $ | — | $ | 125,331 | ||||||||||||||||||||
| Joseph Palumbo (4) | ||||||||||||||||||||||||||||||||||||
| Chief Medical officer | 2022 | $ | 333,333 | $ | 239,167 | $ | — | $ | 244,465 | $ | — | $ | — | $ | — | $ | 816,965 | |||||||||||||||||||
| Jonathan Adams (5) | ||||||||||||||||||||||||||||||||||||
| Former President and Chief Operating Officer | 2021 | $ | 250,000 | $ | — | $ | — | $ | 20,721 | $ | — | $ | — | $ | — | $ | 270,721 | |||||||||||||||||||
| (1) | The aggregate grant date fair value of such awards were computed in accordance with Financial Accounting Standards Board ASC Topic 718, Stock Compensation (ASC Topic 718), and do not take into account estimated forfeitures related to service-based vesting conditions, if any. The valuation assumptions used in calculating these values are discussed in Note 9 of our Notes to Financial Statements included in our Annual Report on Form 10-K for the year ended June 30, 2022. These amounts do not represent actual amounts paid or to be realized. Amounts shown are not necessarily indicative of values to be achieved, which may be more or less than the amounts shown as awards may subject to time-based vesting. The Stock Awards and Stock Option Awards were awarded pursuant to the 2019 Omnibus Incentive Plan, (the “2019 Plan”). |
| (2) | Mr. Do’s salary from April 27, 2021 (date of his appointment as CEO) through December 31, 2021 was paid through a restricted stock unit awards ("RSUs”). The aggregate grant date fair value of the award was $454,794 and the total 58,759 RSUs awarded allows Mr. Do to receive one shares of common stock for each restricted stock unit. |
| (3) | Ms. Kim served as the Chief Financial Officer and Corporate Secretary and Treasure on a full time basis effective July 1, 2021. |
| (4) | Dr. Palumbo joined the Company on November 1, 2021 and served as the Chief Medical Officer. |
| (5) | Mr. Adams served as President and Chief Operating Officer from July 2018 to April 27, 2021. |
-44-
Narrative Disclosures to Summary of Compensation Table
Employment Agreements
All employment arrangements are “at will” agreements.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth all outstanding equity awards held by our named executive officers as of June 30, 2022:
| Option awards | Stock awards | |||||||||||||||||||||||||||||||
| Name | Grant Date | Number of Securities underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Options Exercise Price | Option Expiration Date | Number of shares or units of stock that have not vested | Market Value of shares or units of stock that have not vested | Equity incentive plan awards: number of unearned shares, units or other rights that have not vested | Equity incentive plan award: market or payout value of unearned shares, units or other rights that have not vested | |||||||||||||||||||||||
| Cuong Do | 12/18/16 | 800 | — | $ | 26.25 | 12/18/21 | — | — | — | — | ||||||||||||||||||||||
| 1/19/18 | 800 | — | $ | 12.50 | 1/19/23 | — | — | — | — | |||||||||||||||||||||||
| 1/19/19 | 800 | — | $ | 3.75 | 1/19/24 | — | — | — | — | |||||||||||||||||||||||
| 1/19/20 | 800 | — | $ | 2.80 | 1/29/20 | — | — | — | — | |||||||||||||||||||||||
| 12/18/20 | 24,375 | — | $ | 13.91 | 12/18/25 | — | — | — | — | |||||||||||||||||||||||
| 8/20/21 | 149,000 | 596,000 | $ | 7.74 | 8/20/31 | — | — | — | — | |||||||||||||||||||||||
| 6/21/22 | — | 124,520 | $ | 1.69 | 6/21/27 | 124,520 | $ | 180,554 | — | — | ||||||||||||||||||||||
| Joanne Wendy Kim | 10/01/18 | 800 | — | $ | 8.75 | 10/01/23 | — | — | — | — | ||||||||||||||||||||||
| 10/01/19 | 800 | — | $ | 8.75 | 10/01/24 | — | — | — | — | |||||||||||||||||||||||
| 10/01/20 | 800 | — | $ | 9.54 | 10/01/25 | — | — | — | — | |||||||||||||||||||||||
| 8/20/21 | 24,833 | 99,334 | $ | 7.74 | 8/20/31 | — | — | — | — | |||||||||||||||||||||||
| Joseph M Palumbo, MD | 2/01/22 | 24,833 | 99,334 | $ | 3.20 | 2/01/32 | — | — | — | — | ||||||||||||||||||||||
There were a total 1,117,854 of stock options outstanding to named officers of as of June 30, 2022, with an aggregate grant date fair value of $4,459,190 the last of which vest in 2026.
Potential Payments Upon Termination or Change-In-Control
There are no arrangements with the named executive officers or our equity incentive plan or individual award agreements thereunder providing for certain payments to our named executive officers at or following or in connection with a termination of their employment or a change of control of the Company.
-45-
Director Compensation
There are no arrangements pursuant to which our directors are or will be compensated in the future for any services provided to the Company.
The following table provides information regarding compensation that was earned or paid to the individuals who served as non-employee directors during the year ended June 30, 2022. Except as set forth in the table, during the fiscal year 2022, directors did not earn nor receive cash compensation or compensation in the form of stock awards, options awards or any other form:
Directors’ Compensation Table
| Name | Stock awards | Option awards(1) | Non-equity incentive plan compensation | Change in pension value and nonqualified deferred compensation | All other compensation | Total | ||||||||||||||||||
| Terren Peizer (2) | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Cuong Do (3) | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Jim Lang | $ | — | $ | 417,907 | $ | — | $ | — | $ | — | $ | 417,906.86 | ||||||||||||
| Michael Sherman | $ | — | $ | 425,259 | $ | — | $ | — | $ | — | $ | 425,258.62 | ||||||||||||
| Richard Berman | $ | — | $ | 421,664 | $ | — | $ | — | $ | — | $ | 421,664.42 | ||||||||||||
| Steve Gorlin | $ | — | $ | 399,446 | $ | — | $ | — | $ | — | $ | 399,445.76 | ||||||||||||
| Robert Hariri MD, Phd | $ | — | $ | 399,446 | $ | — | $ | — | $ | — | $ | 399,445.76 | ||||||||||||
| Sigmund Rogich | $ | — | $ | 403,203 | $ | — | $ | — | $ | — | $ | 403,203.33 | ||||||||||||
| (1) | The aggregate grant date fair value of such awards were computed in accordance with Financial Accounting Standards Board ASC Topic 718, Stock Compensation (ASC Topic 718), and do not take into account estimated forfeitures related to service-based vesting conditions, if any. The valuation assumptions used in calculating these values are discussed in Note 9 of our Notes to Financial Statements included in our Annual Report on Form 10-K for the year ended June 30, 2022. These amounts do not represent actual amounts paid or to be realized. Amounts shown are not necessarily indicative of values to be achieved, which may be more or less than the amounts shown as awards may subject to time-based vesting. |
| (2) | Mr. Peizer became our Chief Executive Officer and Chairman in July 2018 and served as the CEO to April 27, 2021 at which time Mr. Do was appointed the Company’s CEO. Mr Peizer did not earn nor was he paid any non-employee director's compensation. |
| (3) | Mr. Do was appointed CEO and President effective April 27, 2021. Mr. Do did not earn nor was he paid any director’s compensation since his appointment as CEO. |
-46-
Our directors are eligible to participate in our equity incentive plans, which are administered by our Compensation Committee under authority delegated by our board of directors. The terms and conditions of option grants to our non-employee directors under our equity incentive plans are and will be determined in the discretion of our Compensation Committee, consistent with the terms of the applicable plan. 2022 compensation to existing board members were granted $7,767,256 worth of the Company’s stock options, and $11,109 for each member of the Audit Committee and $22,219 for the Chairman and $7,351 for each member of the Compensation Committee and $14,703 for the Chairman and $7,351 for each member of the Nominations and Governance Committee and $14,703 for the Chairman, using the Black-Scholes model with the price struck on the date of grant and vested 25% on the grant date and the remaining 75% vest over a 3-year period, on the first, second, and third anniversary of the grant date.
Outstanding equity awards held by non-employee directors as of June 30, 2022 were as follows:
| Name | Grant Date | Number of securities underlying Unexercised options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Options Exercise Price | Option Expiration Date | |||||||||||
| Jim Lang | 12/18/16 | 800 | 26.25 | 12/18/21 | ||||||||||||
| 01/19/18 | 800 | 12.50 | 01/19/23 | |||||||||||||
| 01/19/19 | 800 | 3.75 | 01/19/24 | |||||||||||||
| 01/19/20 | 800 | 2.80 | 01/29/25 | |||||||||||||
| 12/18/20 | 49,500 | 49,500 | 13.91 | 12/18/25 | ||||||||||||
| 04/05/22 | 31,975 | 95,925 | 5.04 | 04/05/27 | ||||||||||||
| Michael Sherman | 10/13/17 | 800 | 25.00 | 10/13/22 | ||||||||||||
| 10/13/18 | 800 | 6.25 | 10/23/23 | |||||||||||||
| 10/13/19 | 800 | 7.50 | 01/13/24 | |||||||||||||
| 10/13/20 | 800 | 9.90 | 01/13/25 | |||||||||||||
| 12/18/20 | 51,550 | 51,550 | 13.91 | 12/18/25 | ||||||||||||
| 04/05/22 | 32,538 | 97,613 | 5.04 | 04/05/27 | ||||||||||||
| Richard J. Berman | 01/19/20 | 800 | 2.80 | 10/19/25 | ||||||||||||
| 12/18/20 | 51,250 | 51,250 | 13.91 | 12/18/25 | ||||||||||||
| 04/05/22 | 32,263 | 96,788 | 5.04 | 04/05/27 | ||||||||||||
| Steve Gorlin | 12/18/20 | 48,150 | 48,150 | 13.91 | 12/18/25 | |||||||||||
| 04/05/22 | 30,563 | 91,688 | 5.04 | 04/05/27 | ||||||||||||
| Robert Hariri | 12/18/20 | 47,950 | 47,950 | 13.91 | 12/18/25 | |||||||||||
| 04/05/22 | 30,563 | 91,688 | 5.04 | 04/05/27 | ||||||||||||
| Sigmund Rogich | 12/18/20 | 48,650 | 48,650 | 13.91 | 12/18/25 | |||||||||||
| 04/05/22 | 30,850 | 92,550 | 5.04 | 04/05/27 | ||||||||||||
There was a total of 1,349,100 stock options outstanding to directors as of June 30, 2022, with an aggregate grant date fair value of $12,858,693 million, the last of which vest in 2025.
-47-
Long-Term Incentive Plans and Awards
Other than the options granted as described above, we do not currently have any long-term incentive plans that provide compensation intended to serve as incentive for performance. Since prior to such grants, no individual grants or agreements regarding future payouts under non-stock price-based plans had been made to any executive officer or any director or any employee or consultant since our inception, no future payouts under non-stock price-based plans or agreements had been granted or entered into or exercised by our officer or director or employees or consultants.
2019 Omnibus Equity Incentive Plan
On April 20, 2019, our Board of Directors and our stockholders approved and adopted the 2019 Plan. The 2019 Plan allows us, under the direction of our Board of Directors or a committee thereof, to make grants of stock options, restricted and unrestricted stock and other stock-based awards to employees, including our executive officers, consultants and directors. The 2019 Plan allows for the issuance of up to 6,540,000 shares of common pursuant to new awards granted under the 2019 Plan and as of June 30, 2022, there were 3,705,157 shares of common stock available for new awards granted under the 2019 Plan.
Equity Compensation Plan Information[1]
The following table provides certain aggregate information with respect to all of the Company’s equity compensation plans in effect as of June 30, 2022:
| Plan Category | (a) Number of securities to be issued upon exercise of outstanding options, warrants and right |
(b) Weighted-average exercise price of outstanding options, warrants and rights | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| Equity compensation plans approved by security holders | 3,467,684 | $ | 7.15 | 3,763,916 | ||||||||
| Equity compensation plans not approved by security holders | — | — | — | |||||||||
| Total | 3,467,684 | 3,763,916 | ||||||||||
___________
(1) We adopted our 2019 Omnibus Equity Incentive Plan (the “2019 Plan”) in 2019.
-48-
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Based solely upon information made available to us, the following table sets forth information as of September 13, 2022 regarding the beneficial ownership of our common stock by:
| ● | each person known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock; | |
| ● | each of our named executive officers and directors; and | |
| ● | all our executive officers and directors as a group. |
The percentage ownership information shown in the table is based upon 30,165,319 shares of common stock outstanding as of September 13, 2022.
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Except as otherwise indicated, each person or entity named in the table has sole voting and investment power with respect to all shares of our capital shown as beneficially owned, subject to applicable community property laws.
In computing the number and percentage of shares beneficially owned by a person as of a particular date, shares that may be acquired by such person (for example, upon the exercise of options or warrants) within 60 days of such date are counted as outstanding, while these shares are not counted as outstanding for computing the percentage ownership of any other person.
The address of each holder listed below, except as otherwise indicated, is c/o BioVie Inc., 680 W Nye Lane, Suite 201, Carson City, Nevada 89703.
-49-
| Name and Address of Beneficial Owner | Number of Common Shares of Beneficial Ownership | Percentage of Beneficial Ownership | ||||||
| Terren Peizer (1) | 30,438,938 | 81.3 | % | |||||
| Cuong Do (2) | 521,241 | 1.7 | % | |||||
| Joanne Wendy Kim (3) | 49,100 | * | ||||||
| Joseph Palumbo (4) | 24,883 | * | ||||||
| Penny Markham (5) | 58,593 | * | ||||||
| Chris Reading (6) | 44,700 | * | ||||||
| Clarence Ahlem (6\) | 44,700 | * | ||||||
| Richard Berman (7) | 85,913 | * | ||||||
| Steve Gorlin (8) | 128,713 | * | ||||||
| Robert Hariri (9) | 78,513 | * | ||||||
| James Lang (10) | 126,252 | * | ||||||
| Sigmund Rogich (11) | 79,500 | * | ||||||
| Michael Sherman (12) | 121,299 | * | ||||||
| All directors and executive officers as a group (13) | 31,802,345 | 82.5 | % | |||||
___________
*Less than 1%
| (1) | Includes warrants to purchase 7,272,728 shares of Common Stock. All shares held of record by Acuitas Group Holdings, LLC, a limited liability company 100% owned by Terren Peizer, and as to which, Mr. Peizer may be deemed to beneficially own or control. Mr. Peizer disclaims beneficial ownership of any such securities. | |
| (2) | Includes warrants to purchase 70,667 shares of Common Stock and options to purchase 294,975 shares of Common Stock, all of which are exercisable within 60 days of September 13, 2022. 167,607 shares of Common Stock, warrants are held of record by Do & Rickles Investments, LLC, a limited liability company 100% owned by Cuong Do and his wife, and as such, Mr. Do may be deemed to beneficially own or control. | |
| (3) | Includes options to purchase 47,100 shares of Common Stock exercisable within 60 days of September 13, 2022. | |
| (4) | Represents options to purchase 24,833 shares of Common Stock exercisable within 60 days of September 13, 2022. | |
| (5) | Includes options to purchase 47,900 shares of Common Stock exercisable within 60 days of September 13, 2022. | |
| (6) | Represents options to purchase 47,700 shares of Common Stock exercisable within 60 days of September 13, 2022. | |
| (7) | Includes options to purchase 84,313 shares of Common Stock, which are exercisable within 60 days of September 13, 2022. | |
| (8) | Includes options to purchase 78,713 shares of common stock, all of which are exercisable within 60 days of September 13, 2022. Common Stock is held by Mr Gorlin’s wife. | |
| (9) | Represents options to purchase 78,513 shares of common stock, all of which are exercisable within 60 days September 13, 2022. | |
| (10) | Includes warrants to purchase 17,333 shares of Common Stock and options to purchase 83,875 shares of Common Stock, all of which are exercisable within 60 days of September 13, 2022. | |
| (11) | Represents options to purchase 79,5005 shares of common stock, all of which are exercisable within 60 days of September 13, 2022. | |
| (12) | Includes warrants to purchase 13,333 shares of Common Stock and options to purchase 87,288 shares of Common Stock, all of which are exercisable within 60 days of September 13, 2022. Common Stock held by Michael Sherman includes 13,333 shares of the Common Stock held of record by Sherman Children’s Trust Brian Krisber, Trustee. All shares of Common Stock, warrants and options are deemed to be beneficially owned or controlled by Michael Sherman. |
-50-
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The following includes a summary of transactions since June 30, 2021, to which we have been a party in which the amount involved exceeded or will exceed the lesser of (i) $120,000 and (ii) one percent (1%) of the average of our total assets at year-end for the prior two fiscal years, and in which any of our directors, executive officers or beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
On July 15, 2022, the Company, entered into a securities purchase agreement (the “Purchase Agreement”) with Acuitas, pursuant to which Acuitas agreed to purchase from the Company, in a private placement (the “Private Placement”), (i) an aggregate of 3,636,364 shares of the Company’s Class A common stock, par value $0.0001 per share at a price of $1.65 per share, and (ii) a warrant to purchase 7,272,728 shares of Common Stock, at an exercise price of $1.82, with a term of exercise of five years; (collectively, the “Securities”). The aggregate purchase price for the Securities sold in the Private Placement was $6 million. The Private Placement closed on August 15, 2022.
Review and Approval of Transactions with Related Persons
Either the audit committee or the Board of Directors approves all related party transactions. The procedure for the review, approval or ratification of related party transactions involves discussing the proposed transaction with management, discussing the proposed transaction with the external auditors, reviewing financial statements and related disclosures, and reviewing the details of major deals and transactions to ensure that they do not involve related party transactions. Members of management have been informed and understand that they are to bring related party transactions to the audit committee or the Board of Directors for pre-approval. These policies and procedures are evidenced in the audit committee charter and our code of ethics.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The following table shows what the auditor billed for the audit and other services for the years ended June 30, 2022 and 2021.
| 2022 | 2021 | |||||||
| Audit Fees | $ | 223,102 | $ | 191,970 | ||||
| Audit - Related Fees | — | — | ||||||
| Tax Fees | — | — | ||||||
| All other Fees | — | — | ||||||
| Total | $ | 223,102 | $ | 191,970 | ||||
Audit Fees—This category includes the audit of the Company’s annual financial statements, review of financial statements included in the Company’s Form 10-Q Quarterly Reports and services that are normally provided by the independent auditors in connection with engagements for those years.
Audit-Related Fees —N/A
Tax Fees—N/A
-51-
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-audit Services of Independent Public Accountant
Consistent with SEC policies regarding auditor independence, the Audit Committee has responsibility for appointing, setting compensation and overseeing the work of our independent registered public accounting firm. In recognition of this responsibility, the Audit Committee has established a policy to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm.
Prior to engagement of an independent registered public accounting firm for the next year’s audit, management will submit an aggregate of services expected to be rendered during that year for each of four categories of services to the Audit Committee for approval.
| 1. | Audit services include audit work performed in the preparation of financial statements, as well as work that generally only an independent registered public accounting firm can reasonably be expected to provide, including comfort letters, statutory audits, and attest services and consultation regarding financial accounting and/or reporting standards. |
| 2. | Audit-Related services are for assurance and related services that are traditionally performed by an independent registered public accounting firm, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements. |
| 3. | Tax services include all services performed by an independent registered public accounting firm’s tax personnel except those services specifically related to the audit of the financial statements, and includes fees in the areas of tax compliance, tax planning, and tax advice. |
| 4. | Other Fees are those associated with services not captured in the other categories. The Company generally does not request such services from our independent registered public accounting firm. |
Prior to engagement, the Audit Committee pre-approves these services by category of service. The fees are budgeted and the Audit Committee requires our independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage our independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the Audit Committee requires specific pre-approval before engaging our independent registered public accounting firm.
The Audit Committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the Audit Committee at its next scheduled meeting.
-52-
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a)(1),(2) Financial Statements
The Financial Statements listed on page F-1 of this document are filed as part of this filing.
(a)(3) Exhibits
The following is a list of exhibits filed as a part of this report:
-53-
-54-
| 31.2 | Rule 13a-14(a) Certification | |
| 32.1 | Certification Pursuant to 18 U.S.C Section 1350, as Adopted Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2 | Certification Pursuant to 18 U.S.C Section 1350, as Adopted Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema Document | |
| 101.CAL | XBRL Taxonomy Calculation Linkbase Document | |
| 101.LAB | XBRL Taxonomy Label Linkbase Document | |
| 101.PRE | XBRL Taxonomy Presentation Linkbase Document | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document |
# Indicates a management contract or compensatory plan or arrangement
-55-
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| BIOVIE INC. | |||
| By: | /s/ Cuong Do | ||
| Name: | Cuong Do | ||
| Title: | Chief Executive Officer (Principal Executive Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons in the capacities and on the dates indicated.
| Person | Capacity | Date | ||
| /s/ Cuong Do | Chief Executive Officer | September 27, 2022 | ||
| Cuong Do | (Principal Executive Officer) | |||
| /s/ Joanne Wendy Kim | Chief Financial Officer | September 27, 2022 | ||
| Joanne Wendy Kim | (Principal Financial Officer) | |||
| /s/ Terren Piezer | Chairman | September 27, 2022 | ||
| Terren Piezer | ||||
| /s/ Jim Lang | Director | September 27, 2022 | ||
| Jim Lang | ||||
| /s/ Michael Sherman | Director | September 27, 2022 | ||
| Michael Sherman | ||||
| /s/ Richard J. Berman | Director | September 27, 2022 | ||
| Richard J. Berman | ||||
| /s/ Steve Gorlin | Director | September 27, 2022 | ||
| Steve Gorlin | ||||
| /s/ Robert Hariri | Director | September 27, 2022 | ||
| Robert Hariri | ||||
| /s/ Sigmund Rogich | Director | September 27, 2022 | ||
| Sigmund Rogich |
-56-
BioVie, Inc.
Index to Financial Statements
| Report of Independent Registered Public Accounting Firm – EisnerAmper LLP | F-2 | ||||
| Financial Statements: | |||||
| Balance Sheets | F-4 | ||||
| Statements of Operations | F-5 | ||||
| Statements of Changes in Stockholders’ Equity (Deficit) | F-6 | ||||
| Statements of Cash Flows | F-7 | ||||
| Notes to Financial Statements | F-8 | ||||
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
BioVie, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of BioVie, Inc. (the “Company”) as of June 30, 2022 and 2021, and the related statements of operations, changes in stockholders’ equity (deficit), and cash flows for each of the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2022 and 2021, and the results of its operations and its cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company’s recurring losses from operations and negative cash flows from operating activities raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
F-2
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Notes Payable
As described in Note 7 to the accompanying financial statements, the Company entered into a Loan and Security Agreement (the “Loan Agreement”) by issuing notes payable to the lender. The Loan Agreement included a conversion option which allows the lender to convert up to $5,000,000 of the principal amount of the notes payable into the Company’s Class A common stock and required the issuance of warrants to purchase 361,002 shares of the Company’s class A common stock by the lender. The carrying value of the notes payable was determined by allocating portions of the outstanding principal of the notes to the fair value of the warrants and the embedded conversion option. The fair values of the warrants and the conversion option of $194,531 and $188,030, respectively as of June 30, 2022, and the change in their fair values for the year ended June 30, 2022 of $3,287,418 were determined using a Black Scholes model which uses inputs such as the closing price of the stock, the option’s exercise price, the term of the option, a risk free interest rate and the volatility of the stock to arrive at the values.
We identified the valuation and the accounting for the notes payable and the related derivative liabilities to be a critical audit matter due to the complexity of their accounting and the subjective judgment required by management in selecting the inputs and assumptions used in determining fair value. This in turn led to a high degree of auditor judgment, subjectivity and effort in applying the procedures related to the accounting and those assumptions.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the financial statements. These procedures include obtaining an understanding and evaluating the design of controls relating the accounting and valuation of these instruments. Our procedures included, among others: reading the terms of the Loan Agreement; reviewing the valuation assumptions used by management; obtaining the valuation calculations from the Company and agreeing the inputs to the source information used by management; determining the mathematical accuracy of the calculations; reviewing the recording of the notes payable and related derivative liabilities; testing the amortization of the discount arising from the derivative liabilities through the end of the year; and confirming the notes payable balances with the lender at the end of the year.
/s/
We have served as the Company’s auditor since 2019.
EISNERAMPER LLP
September 27, 2022
F-3
BioVie Inc.
Balance Sheets
| June 30 | June 30, | |||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | $ | ||||||
| Prepaids and other assets | ||||||||
| Total current assets | ||||||||
| OTHER ASSETS: | ||||||||
| Operating lease right-of-use assets | ||||||||
| Intangible assets, net | ||||||||
| Goodwill | ||||||||
| Other assets, non-current | ||||||||
| Total other assets | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable and accrued expenses | $ | $ | ||||||
| Current portion of other liabilities | ||||||||
| Current portion of operating lease liabilities | ||||||||
| Warrant liabilities | ||||||||
| Embedded derivative liability | ||||||||
| Total current liabilities | ||||||||
| Other liabilities, net of current portion | ||||||||
| Operating lease liabilities, net of current portion | ||||||||
| Note payable net of financing costs and unearned premium and discount ($ | ||||||||
| TOTAL LIABILITIES | ||||||||
| Commitments and contingencies (Note 11) | ||||||||
| STOCKHOLDERS' EQUITY : | ||||||||
| Preferred stock; $ par value; shares authorized; shares issued and outstanding | ||||||||
| Common stock, $ par value; shares authorized at June 30, 2022 and June 30, 2021, respectively; and shares issued and outstanding at June 30, 2022 and June 30, 2021, respectively | ||||||||
| Additional paid in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders' equity | ||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | $ | $ | ||||||
The accompanying notes are an integral part of the financial statements.
F-4
BioVie Inc.
Statements of Operations
| Year ended | Year ended | |||||||
| June 30, 2022 | June 30, 2021 | |||||||
| OPERATING EXPENSES: | ||||||||
| Amortization | $ | $ | ||||||
| Research and development expenses | ||||||||
| In process research and development expenses | ||||||||
| Selling, general and administrative expenses | ||||||||
| TOTAL OPERATING EXPENSES | ||||||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ||||
| OTHER (INCOME) EXPENSE: | ||||||||
| Change in fair value of derivative liabilities | ( | ) | ( | ) | ||||
| Gain on extinguishment of debt | ( | ) | ||||||
| Interest expense | ||||||||
| Interest income | ( | ) | ( | ) | ||||
| TOTAL OTHER INCOME, NET | ( | ) | ( | ) | ||||
| NET LOSS | $ | ( | ) | $ | ( | ) | ||
| Deemed dividends - related party | ||||||||
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS | $ | ( | ) | $ | ( | ) | ||
| NET LOSS PER COMMON SHARE | ||||||||
| - Basic | $ | ( | ) | $ | ( | ) | ||
| - Diluted | $ | ( | ) | $ | ( | ) | ||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING | ||||||||
| - Basic | ||||||||
| - Diluted | ||||||||
The accompanying notes are an integral part of the financial statements.
F-5
BioVie Inc.
Statements of Changes in Stockholders’ Equity
(Deficit)
For the Years Ended June 30, 2022 and 2021
| Total | ||||||||||||||||||||
| Common Stock | Common Stock | Additional Paid in | Accumulated | Stockholders' Equity | ||||||||||||||||
| Shares | Amount | Capital | Deficit | (Deficit) | ||||||||||||||||
| Balance, June 30, 2020 | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||
| Proceeds from issuance of common stock, net of costs of $ | ||||||||||||||||||||
| Redemption of warrants - related party | ||||||||||||||||||||
| Deemed dividend for purchase option - related party | ( | ) | ||||||||||||||||||
| Cashless exercise of options | ||||||||||||||||||||
| Stock-based compensation | — | |||||||||||||||||||
| Proceeds from exercise of warrants | ||||||||||||||||||||
| Issuance of shares for purchase of in process research and development expenses - related party | ||||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||
| Balance, June 30, 2021 | $ | $ | $ | ( | ) | $ | ||||||||||||||
| Stock-based compensation | — | $ | ||||||||||||||||||
| Proceeds from issuance of common stock, net costs of $ | $ | |||||||||||||||||||
| Stock based compensation - restricted stock | $ | |||||||||||||||||||
| Net loss | — | $ | ( | ) | ( | ) | ||||||||||||||
| Balance, June 30, 2022 | $ | $ | $ | ( | ) | $ | ||||||||||||||
The accompanying notes are an integral part of the financial statements.
F-6
BioVie Inc.
Statements of Cash Flows
| Year ended | Year ended | |||||||
| June 30, 2022 | June 30, 2021 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Amortization of intangible assets | ||||||||
| Stock based compensation - restricted stock | ||||||||
| Stock based compensation expense - stock options | ||||||||
| Common shares issued for asset acquisition | ||||||||
| Gain on extinguishment of loan payable | ( | ) | ||||||
| Amortization of financing costs | ||||||||
| Accretion of unearned loan discount | ||||||||
| Accretion of loan premium | ||||||||
| Amortization of operating lease, net | ||||||||
| Change in fair value of derivative liability | ( | ) | ( | ) | ||||
| Changes in operating assets and liabilities: | ||||||||
| Other assets | ( | ) | ||||||
| Accounts payable and accrued expenses | ( | ) | ||||||
| Other liabilities | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Net proceeds from issuance of common stock | ||||||||
| Payment of convertible debenture - related party | ( | ) | ||||||
| Proceeds from convertible debenture - related party | ||||||||
| Proceeds from exercise of warrants | ||||||||
| Proceeds from note payable net of financing costs | ||||||||
| Net cash provided by financing activities | ||||||||
| Net increase in cash | ||||||||
| Cash, beginning of period | ||||||||
| Cash, end of period | $ | $ | ||||||
| SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | $ | $ | ||||||
| Cash paid for taxes | $ | $ | ||||||
| SCHEDULE OF NON-CASH FINANCING AND INVESTING ACTIVITIES: | ||||||||
| Deemed dividends - related party | $ | $ | ||||||
| Right of use assets obtained in exchange for lease obligations | $ | $ | ||||||
The accompanying notes are an integral part of the financial statements.
F-7
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 1. | Background Information |
BioVie Inc. (the “Company” or “we” or “our”) is a clinical-stage company developing innovative drug therapies to treat chronic debilitating conditions including liver disease and neurological and neuro-degenerative disorders and certain cancers.
In liver disease, our Orphan Drug candidate BIV201 (continuous infusion terlipressin) is being developed as a future treatment option for patients suffering from ascites and other life-threatening complications of advanced liver cirrhosis caused by NASH, hepatitis, and alcoholism. The initial target for BIV201 therapy is refractory ascites. These patients suffer from frequent life-threatening complications, generate more than $5 billion in annual treatment costs, and have an estimated 50% mortality rate within 6 to 12 months. The US Food and Drug Administration (FDA) has not approved any drug to treat refractory ascites. A Phase 2a clinical trial of BIV201 was completed in 2019, and a multi-center, randomized 30-patient Phase 2b trial is currently underway. As of June 30 2022, ten US study centers had been activated and are actively screening and enrolling patients in the study. Top-line results from this trial are expected in mid calendar year 2023.
The BIV201 development program was initiated by LAT Pharma LLC. On April 11, 2016, the Company acquired LAT Pharma LLC and the rights to its BIV201 development program. The Company currently owns all development and marketing rights to its drug candidate. Pursuant to the Agreement and Plan of Merger entered into on April 11, 2016, between our predecessor entities, LAT Pharma LLC and NanoAntibiotics, Inc., BioVie is obligated to pay a low single digit royalty on net sales of BIV201 (continuous infusion terlipressin) to be shared among LAT Pharma Members, PharmaIn Corporation, and The Barrett Edge, Inc.
In neurodegenerative disease, BioVie acquired the biopharmaceutical assets of NeurMedix, Inc. (“NeurMedix”), a privately held clinical-stage pharmaceutical company, in June 2021 (See Note 5 Related Party Transactions). The acquired assets included NE3107, a potentially selective inhibitor of inflammatory extracellular single-regulated kinase(“ERK”) signaling that, based on animal studies, is believed to reduce neuroinflammation. NE3107 is a novel orally administered small molecule that is thought to inhibit inflammation-driven insulin resistance and major pathological inflammatory cascades with a novel mechanism of action. There is emerging scientific consensus that both inflammation and insulin resistance may play fundamental roles in the development of Alzheimer’s and Parkinson’s Disease, and NE3107 could, if approved represent an entirely new medical approach to treating these devastating conditions affecting an estimated 6 million Americans suffering from Alzheimer’s and 1 million from Parkinson’s. The FDA has authorized a potentially pivotal Phase 3 randomized, double-blind, placebo-controlled, parallel group, multicenter study to evaluate NE3107 in subjects who have mild to moderate Alzheimer’s disease (NCT04669028). In August 2021, the study was initiated and the Company is anticipating top line results in mid calendar year 2023.
On January 20, 2022, the Company initiated a study by treating the first patient, in it’s Phase 2 study assessing NE3107’s safety and tolerability and potential pro-motoric impact in Parkinson’s disease patients. The NM201 study (NCT05083260) is a double-blind, placebo-controlled, safety, tolerability, and pharmacokinetics study in Parkinson’s Disease (PD). Participants will be treated with carbidopa/levodopa and NE3107 or placebo. Forty patients with a defined PD medication “off state” will be randomized 1:1 placebo to: active NE3107 20 mg twice daily for 28 days. Safety assessments will look at standard measures of patient health and potential for drug-drug interactions affecting L-dopa pharmacokinetics and activity. Exploratory efficacy assessments will use the Motor Disease Society Unified Parkinson’s Disease Rating (MDS-UPDRS) parts 1-3, ON/OFF Diary, and Non-Motor Symptom Scale. Topline results are expected for the NM201 study by the end of the calendar year 2022.
Inflammation-driven insulin resistance is believed to be implicated in a broad range of serious diseases, including multiple myeloma and prostate cancer, and we plan to begin exploring these opportunities in the coming months using NE3107 or related compounds acquired in the NeurMedix asset purchase. NE3107 is patented in the United States, Australia, Canada, Europe and South Korea.
F-8
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 2. | Liquidity and Going Concern |
The Company’s operations are subject to a number of factors
that can affect its operating results and financial conditions. Such factors include, but are not limited to: the results of
clinical testing and trial activities of the Company’s products, the Company’s ability to obtain regulatory approval to
market its products; competition from products manufactured and sold or being developed by other companies; the price of, and demand
for, Company products; the Company’s ability to negotiate favorable licensing or other manufacturing and marketing agreements
for its products; and the Company’s ability to raise capital. The Company’s financial statements have been prepared
assuming the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of
liabilities in the normal course of business. As of June 30, 2022, the Company had working capital of approximately $
The future viability of the Company is largely dependent upon its ability to raise additional capital to finance its operations. Management expects that future sources of funding may include sales of equity, obtaining loans, or other strategic transactions.
The continual widespread health emergencies or pandemics such as the coronavirus (“COVID-19”) pandemic (and its related variants), has led to continued regional quarantines, business shutdowns, labor shortages, disruptions to supply chains, and overall economic instability. Although some jurisdictions have relaxed these measures, others have not or have reinstated them as COVID-19 cases and its variants continue to emerge. The duration and spread of the COVID-19 pandemic and the long-term impact of COVID-19 and its variants on the financial markets and the overall economy are highly uncertain and cannot be predicted at this time. If the financial markets and/or the overall economy are impacted for an extended period, the Company’s ability to raise funds may be materially adversely affected. In addition, the COVID-19 pandemic has created a widespread labor shortage, including a shortage of medical professionals, and has impacted and may continue to impact the potential patient participation in our studies, which may adversely impact our ability to continue or complete our clinical trials in the planned timeline.
Although management continues to pursue the Company’s strategic plans, there is no assurance that the Company will be successful in obtaining sufficient financing on terms acceptable to the Company, if at all, to fund continuing operations. These circumstances raise substantial doubt on the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
F-9
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 3. | Significant Accounting Policies |
Basis of Presentation
The Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and include all adjustments necessary for the fair presentation of the Company’s financial position for the periods presented.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. The Company bases its estimates on historical experience and on various assumptions that are believed to be reasonable under the circumstances. The amounts of assets and liabilities reported in the Company’s balance sheet and the amounts of expenses reported for each of the periods presented are affected by estimates and assumptions, which are used for, but not limited to, accounting for share-based compensation and other equity instruments, accounting for derivatives and accounting for income taxes. Actual results could differ from those estimates.
Reclassifications
Certain prior period amounts have been reclassified for consistency to conform with the current year’s presentation.
Cash
The Company considers all highly liquid instruments with original maturities of three months or less to be cash equivalents. Cash is maintained at two financial institutions, and, at times, balances may exceed federally insured limits. The Company has never experienced any losses related to these balances.
Prepaid and other Assets
Prepaid and other assets consist of prepayments of certain expenses and direct costs related to capital raise which will offset proceeds upon the close.
Other Assets, non-current
Other assets consist of security deposit for the office lease.
Leases
The Company determines whether an arrangement contains a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, current portion of operating lease liabilities, and operating lease liabilities, net of current portion on our balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent an obligation to make lease payments arising from the lease. Lease ROU assets and lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at the commencement date. As the Company’s leases do not provide an implicit rate, an incremental borrowing rate is used based on the information available at the commencement date in determining the present value of lease payments. The Company does not include options to extend or terminate the lease term in its calculation unless it is reasonably certain that the Company will exercise any such options. Rent expense is recognized under the operating leases on a straight-line basis. The Company does not recognize right of-use assets or lease liabilities for short-term leases, which have a lease term of twelve months or less, and instead will recognize lease payments as expense on a straight-line basis over the lease term.
F-10
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 3. | Significant Accounting Policies (continued) |
Fair Value of Financial Instruments
Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value for applicable assets and liabilities, we consider the principal or most advantageous market in which we would transact and we consider assumptions market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance. This guidance also establishes a fair value hierarchy to prioritize inputs used in measuring fair value as follows:
| ● | Level 1: Observable inputs such as quoted prices in active markets; |
| ● | Level 2: Inputs, other than quoted prices in active markets, that are observable either directly or indirectly; and |
| ● | Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions |
The Company’s financial instruments include cash, accounts payable, the carrying value of the operating lease liabilities and notes payable. The carrying amounts of cash and accounts payable approximate their fair value, due to the short-term nature of these items. The carrying amounts of notes payable and operating lease liabilities approximate their fair values since they bear interest at rates which approximate market rates for similar debt instruments.
Research and Development
Research and development expenses consist primarily of costs associated with the preclinical and/ or clinical trials of drug candidates, compensation and other expenses for research and development, personnel, supplies and development materials, costs for consultants and related contract research and facility costs. Expenditures relating to research and development are expensed as incurred. In the fiscal year ended June 30, 2021 the Company recorded the assets acquired totaling approximately $130.6 million from NeurMedix, a controlled affiliate of Acuitas, our majority shareholder, that were under development as research and development expenses in the accompanying Statements of Operations. See Note 1 - Background Information.
Income Taxes
The Company uses the asset and liability method of accounting for deferred income taxes. Deferred income taxes are measured by applying enacted statutory rates to net operating loss carryforwards and to the differences between the financial reporting and tax bases of assets and liabilities. Deferred tax assets are reduced, if necessary, by a valuation allowance if it is more likely than not that some portion or all of the deferred tax assets will not be realized.
The Company recognizes uncertainty in income taxes in the financial statements using a recognition threshold and measurement attribute of a tax position taken or expected to be taken in a tax return. The Company applies the “more-likely-than-not” recognition threshold to all tax positions, commencing at the adoption date of the applicable accounting guidance, which resulted in no unrecognized tax benefits as of such date. Additionally, there have been no unrecognized tax benefits subsequent to adoption. The Company has opted to classify interest and penalties that would accrue, if any, according to the provisions of relevant tax law as general and administrative expenses, in the Statements of Operations. For the years ended June 30, 2022 and 2021, there was no such interest or penalty.
F-11
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 3. | Significant Accounting Policies (continued) |
Basic net loss per common share is computed by dividing the net loss attributable to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted net loss per common share is computed by dividing the net loss attributable to common stockholders by the weighted average number of shares of common stock outstanding and potentially outstanding shares of common stock during the period to reflect the potential dilution that could occur from common shares issuable through stock options, warrants, and convertible debentures. For the year ended June 30, 2022 and 2021, such amounts were excluded from the diluted loss since their effect was considered anti-dilutive due to the net loss for the period.
The table below shows the number of outstanding stock options and warrants as of June 30 2022 and 2021:
| June 30, 2022 | June 30, 2021 | |||||||
| Number of Shares | Number of Shares | |||||||
| Stock Options | ||||||||
| Warrants | ||||||||
| Total | ||||||||
Stock-based Compensation
The Company has accounted for stock-based compensation under the provisions of FASB ASC 718 – “Stock Compensation” which requires the use of the fair-value based method to determine compensation for all arrangements under which employees and others receive shares of stock or equity instruments (stock options and common stock purchase warrants). For employee awards, the fair value of each stock option award is estimated on the date of grant using the Black-Scholes valuation model that uses assumptions for expected volatility, expected dividends, expected term, and the risk-free interest rate. For non-employees, the fair value of each stock option award is estimated on the measurement date using the Black-Scholes valuation model that uses assumptions for expected volatility, expected dividends, expected term, and the risk-free interest rate. For non-employees, the Company utilizes the graded vesting attribution method under which the entity treats each separately vesting portion (tranche) as a separate award and recognizes compensation cost for each tranche over its separate vesting schedule. Expected volatilities are based on historical volatility of peer companies and other factors estimated over the expected term of the stock options. For employee awards, the expected term of options granted is derived using the “simplified method” which computes expected term as the average of the sum of the vesting term plus the contract term. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant for the period of the expected term. The Company recognizes forfeitures as they occur.
Goodwill
Goodwill is recorded when the purchase price paid for an acquisition exceeds the fair value of net identified tangible and intangible assets acquired. The Company performs an annual impairment test of goodwill and further periodic tests to the extent indicators of impairment develop between annual impairment tests. The Company’s impairment review process compares the fair value of the reporting unit to its carrying value, including the goodwill related to the reporting unit. To determine the fair value of the reporting unit, the Company may use various approaches including an asset or cost approach, market approach or income approach or any combination thereof. These approaches may require the Company to make certain estimates and assumptions including future cash flows, revenue and expenses. These estimates and assumptions are reviewed each time the Company tests goodwill for impairment and are typically developed as part of the Company’s routine business planning and forecasting process. While the Company believes its estimates and assumptions are reasonable, variations from those estimates could produce materially different results. The Company did not recognize any goodwill impairments for the years ended June 30, 2022 and 2021.
F-12
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 3. | Significant Accounting Policies (continued) |
Impairment of Long-Lived Assets
Long-lived assets, including intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset.
If the carrying amount of an asset exceeds its undiscounted estimated future cash flows, an impairment review is performed. An impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Generally, fair value is determined using valuation techniques such as expected discounted cash flows or appraisals, as appropriate. Assets to be disposed of would be separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell, and are no longer depreciated or amortized. The assets and liabilities of a disposed group classified as held for sale would be presented separately in the appropriate asset and liability sections of the balance sheets.
Purchase Accounting for Transactions with Related Party
Purchase accounting for transactions with related party, entities under common control, are recorded at the historical carrying cost with no step up in basis to the fair market value of the asset or liability are recognized.
Recent Accounting Pronouncements
The Company considers the applicability and impact of all Accounting Standards Updates (“ASU’s”). There were no recent ASU’s that are expected to have a material impact on our balance sheets or statements of operations.
| 4. | Intangible Assets |
The Company’s intangible assets consist of intellectual property acquired from LAT Pharma, Inc. and are amortized over their estimated useful lives. The following is a summary of the intangible assets as of June 30, 2022 and 2021:
| June 30, 2022 | June 30, 2021 | |||||||
| Intellectual Property | $ | $ | ||||||
| Less Accumulated Amortization | ( | ) | ( | ) | ||||
| Intellectual Property, Net | $ | $ | ||||||
Amortization expense amounted to $
Estimated future amortization expense is as follows:
| Year ending June 30, | |
| 2023 | $ |
| 2024 | |
| 2025 | |
| 2026 | |
| $ |
F-13
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 5. | Related Party Transactions |
Asset Acquisition with NeurMedix
On April 27, 2021, the Company entered into an APA with NeurMedix and Acuitas, which are related party affiliates, pursuant to which the Company acquired certain assets from NeurMedix and assumed certain liabilities of NeurMedix, in exchange for consideration of cash and shares of common stock. The acquired assets include, among others, those related to certain drug candidates being developed by NeurMedix, including NE3107, a small molecule orally administered inhibitor of insulin resistance and the pathological inflammatory cascade, with a novel mechanism of action that has potential applications for treatment against Alzheimer’s Disease and Parkinson’s Disease.
Subject to the terms and conditions of the Asset Purchase Agreement, following the closing, the Company may be obligated to deliver contingent stock consideration to NeurMedix (or its successor). Previously, the Company was obligated to deliver contingent stock consideration to NeurMedix (or its successor) consisting of shares of the Company’s common stock having an aggregate value of up to $3.0 billion, subject to the achievement of certain clinical, regulatory and commercial milestones related to the drug candidates to be acquired by the Company from NeurMedix, and subject to a cap limiting each issuance of shares if such issuance would result in the beneficial ownership of NeurMedix and its affiliates exceeding 89.9999% of the Company’s issued and outstanding common stock. Pursuant to Amendment No. 1 to the APA, dated May 9, 2021, the Company may now be obligated to deliver contingent stock consideration to NeurMedix (or its successor) consisting of up to 18 million shares of BioVie’s common stock, with 4.5 million shares issuable upon the achievement of each of the four milestones set forth in the APA, subject to a cap limiting the issuance of shares if such issuance would result in the beneficial ownership of NeurMedix and its affiliates exceeding 87.5% of the Company’s issued and outstanding common stock.
On June 10, 2021, and pursuant to the Asset Purchase Agreement, the Company issued to Acuitas (as NeurMedix’s assignee) 8,361,308 shares of the Company’s common stock and made a cash payment of approximately $2.3 million, representing NeurMedix’s direct and documented cash expenditures to advance certain programs from March 1, 2021 through the closing date and cash payments to other third parties for expenses totaling approximately $4.0 million for due diligence, legal fees, transaction fees and the fairness opinion. Since the transaction was between entities under common control, there were no fair value adjustments of the purchased assets and the historical cost basis of the purchased assets was zero. The total consideration paid was expensed as in process research and development expense in the accompanying statement of operations for the year ended June 30, 2021.
Equity Transactions with Acuitas
On September 22, 2020, concurrent with the closing of the Company’s registered public offering, (“the Offering’), approximately $1.8 million was paid to Acuitas satisfying all amounts owed on the Debenture due September 24, 2020 held by the Company’s controlling stockholder, Acuitas.
Additionally, in connection with the close of the public offering on September 22, 2020, the Company issued an aggregate of shares of Common Stock to Acuitas, representing (i) 5.4 million shares issuable pursuant to Acuitas’ rights under the Purchase Agreement dated July 3, 2018, as amended on June 24, 2019 and October 9, 2019; and the various extension letters; which resulted in a deemed dividend at the close of the public offering at price of $10 per share, consistent with the Company’s accounting policy; and (ii) the automatic exercise of 1.5 million warrants issued to Acuitas in connection with the Debenture financing at the par value of the Common Stock.
F-14
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 5. | Related Party Transactions (continued) |
During the year ended June 30, 2021, the Company received additional draws under the Debenture totaling $436,000. The total draws as of September 22, 2020 were $1.7 million and the related total number of warrants issuable at $4.00 per share of common stock was 424,750 of which 328,250 warrants had been issued. In accordance with the Debenture agreements, at September 22, 2020 upon the Company’s close of its public offering, all the warrants issued related to the debenture totaling 1,453,250 were mandatorily redeemed along with the additional 96,500 shares common stock issued to Acuitas.
The following paragraphs summarize the background of those financings and arrangements which were settled and redeemed on September 22, 2020.
On July 3, 2018, we entered into a Securities Purchase Agreement (the “Purchase Agreement”) with Acuitas and certain other purchasers identified in the Purchase Agreement (together with Acuitas, the “Purchasers”) pursuant to which (i) the Purchasers agreed to purchase an aggregate of 2,133,332 shares of the our Series A Convertible Preferred Stock (the “Preferred Stock”) at a price per share of $1.50 per share of Preferred Stock (the “Initial Sale”) and (ii) we agreed to issue warrants (the “Warrants”) to purchase 1,706,666 shares of common stock, each subject to the terms and conditions set forth in the Purchase Agreement, for an aggregate consideration of $3.2 million. We received $160,000 of the $3.2 million in April and May 2018 as prepaid equity. Acuitas also received an additional 6,667 Warrants in connection with the payoff of a note issued by us in favor of Acuitas. The Initial Sale and issuance of the Warrants occurred on July 3, 2018. In addition, Acuitas had the option to purchase up to an additional 1,600,000 shares of common stock at a price per share of $1.88, and warrants on the same terms as the Warrants, within two weeks following the one year anniversary of the closing of the Initial Sale (the “Subsequent Sale”) in the event that we did not obtain $3,000,000 of funding through various non-dilutive grants prior to the one year anniversary of the closing of the Initial Sale, less any federal or FDA grant funding received by the Company.
Acuitas is controlled by our Chairman and Chief Executive Officer, Terren Peizer and the Purchasers included James Lang, Cuong Do and Michael Sherman, who are members of our Board; and Jonathan Adam, a former Board member.
The Purchase Agreement contained customary representations and warranties. In connection with the disclosure schedule associated with the representations and warranties, we also disclosed customary information, including the following: (i) the existence of the Mallinckrodt petition before the U.S. Patent Trial and Appeal Board, (ii) our capitalization, (iii) our obligation to pay a low single digit royalty on the net sales of BIV201 (continuous infusion terlipressin) to be shared among LAT Pharma LLC members, PharmaIN Corporation and The Barrett Edge, Inc. pursuant to the Agreement and Plan of Merger, dated April 11, 2016, by and between LAT Pharma LLC and us, (iv) our obligation to pay a low single digit royalty on net sales of all terlipressin products covered by specified patents up to a maximum of $200,000 per year pursuant to the Technology Transfer Agreement, dated July 25, 2016, by and between us and the University of Padova (Italy), and (v) certain recent issuances of common stock by us.
Each share of Preferred Stock automatically converted into 1 share of common stock upon the filing with the Secretary of State of the State of Nevada of a Certificate of Amendment to our Articles of Incorporation (the “Amendment”) on August 13, 2018 that increased the number of authorized shares of common stock to 800,000,000. The Amendment was approved by the written consent of the holders of more than a majority of our issued and outstanding common stock on July 3, 2018 and was filed with the Secretary of State of the State of Nevada 20 calendar days following the distribution of our Definitive Information Statement on Schedule 14 that was filed with the SEC on July 13, 2018.
F-15
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 5. | Related Party Transactions (continued) |
Pursuant to a letter agreement dated June 24, 2019, Acuitas agreed to modify its existing rights under the Purchase Agreement so that:
| - | Acuitas agreed to immediately exchange its existing 1,606,667 Warrants for common stock such that it will have effectively exercised its Warrants in full pursuant to a cashless exercise thereof at an assumed current market price of $45.00 per share and, as a result received an aggregate of 95% of the shares covered thereby, or 1,526,094 shares of common stock; |
| - | Acuitas agreed to (i) waive its rights to a 50% adjustment of the purchase price of the Preferred Stock in the Initial Sale, the exercise price of the Warrants and the price per share in the Subsequent Sale in the event of certain reductions in the useful life of our current intellectual property rights, and (ii) effectively exercise its rights to purchase securities in a Subsequent Sale pursuant to a “cashless purchase” at an assumed current market price of approximately $11.25 per share, conditioned in each case on the listing of our common stock on Nasdaq or the raising of $2.0 million in additional funds in the form of another securities offering, in either case not later than November 30, 2019, which will result Acuitas having irrevocably waived its rights to an adjustment in the purchase price of the Preferred Stock in the Initial Sale and the exercise price of the Warrants and the purchase price of per share in the Subsequent Sale upon the issuance by us of an aggregate of 1,339,958 shares of common stock (the “Subsequent Sale Shares”) to Acuitas, which is expected to occur concurrently with the closing of our potential public offering and listing on Nasdaq; |
| - | Acuitas shall in exchange for the foregoing agreements and waivers have the option to purchase additional shares of common stock and warrants to purchase one share of common stock for each share of common stock purchased during the period from September 1, 2019 to November 30, 2019 at the then-effective purchase price of the Preferred Stock in the Initial Sale (the “Funding Option”), provided that any shares issued pursuant to any exercise of the Funding Option will reduce share-for-share the amount of shares issued pursuant to the deemed exercise of its rights to purchase securities in a Subsequent Sale mentioned above. |
Convertible Debenture Transaction with Acuitas
On September 24, 2019, the Company entered into a Securities Purchase Agreement (the “2019 Purchase Agreement”) with Acuitas pursuant to which (i) Acuitas agreed to purchase a 10% OID Convertible Delayed Draw Debenture due September 24, 2020 for an aggregate commitment amount of up to $2.0 million, and (ii) the Company issued 1,125,000 shares (the “Commitment Shares”) of the Company’s common stock and warrants (the “Commitment Warrants”) to purchase an equal number of shares, each subject to the terms and conditions set forth in the 2019 Purchase Agreement. The Debenture accrues additional principal at the rate of 6% per annum and interest at the rate of 10% per annum, is convertible into shares of common stock at $4.00 per share prior to the completion of the company’s planned public offering of units (the “Public Offering”) or, subsequent to the closing of the Public Offering, the lower of $4.00 or 80% of the offering price per unit to the public in the Public Offering and are mandatorily redeemable upon such closing at 100% of the accrued principal amount and unpaid interest to the date of redemption. The Commitment Warrants are five-year warrants, exercisable upon the earlier of the effectiveness of the Company’s current reverse stock split or December 1, 2019, at an amount equal to the lower of $4.00 or 80% of the offering price per unit to the public in the Public Offering. Upon entering into the 2019 Purchase Agreement, the Company drew an initial $500,000 under the Debenture and in accordance with the 2019 Purchase Agreement, Acuitas received an additional 125,000 warrants (the “Bridge Warrants”) having the same terms as the Commitment Warrants.
F-16
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 5. | Related Party Transactions (continued) |
Any future draws under the Debenture, which may be made from and after October 15, 2019, November 15, 2019 and December 15, 2019 in equal tranches of $500,000 each, will entitle Acuitas to receive additional Bridge Warrants in equal amount upon such funding. In addition, the 2019 Purchase Agreement provides that, should the underwriters in the Public Offering exercise their option to purchase additional securities during the 45 days following closing and the issuance of such securities would result in Acuitas’ beneficial ownership (on a fully diluted basis) of shares of common stock being below 60%, Acuitas shall be issued a number of additional shares of common stock and warrants having the same terms as the Commitment Warrants to result in its beneficial ownership (on a fully diluted basis) of shares of common stock equaling 60%.
The issuance of 1,125,000 shares of the Company’s commons stock and warrants to purchase an equal amount number of shares, to its controlling stockholder for the Bridge Financing was accounted for as a deemed dividend due to its related party nature and $17.1 million representing the excess of the fair value of the consideration given for the financing, net of debt discount; was recorded in accumulated deficit for the year ended June 30, 2020, accordingly. A debt discount of $500,000 against the debenture was recorded which will be amortized over the term of the debenture using the effective interest method.
The Company received draws under the Debenture that totaled approximately $1.3 million during the year ended June 30, 2020. The total interest expense related to the draws under the Debenture was approximately $99,000 for the year ended June 30, 2020. On April 1, 2020, the Company entered an amendment to modify the payment of accrued interest amounts under the original terms of the Debenture to capitalize all such amounts as would otherwise accrue on the Debenture. On January 4, 2020, payment of $13,487 accrued interest due was paid through the issuance of 4,422 shares of the Company’s common stock. Acuitas and the Company continue to discuss the need and timing for some or all the remaining draws under the Debenture Agreement. Subsequent to the initial $500,000 draw on September 24, 2019, the Company received draws that totaled $813,000 as July 13, 2020, and accordingly; the Company issued additional Bridge Warrants to purchase 203,250 shares of common stock to its controlling stockholder under the terms of the Bridge Financing. Accordingly, on April 16, 2020, the Company recorded the warrants to purchase 125,000 common stock related to the second $500,000 draw under the debenture as a derivative warrant liability as of June 30, 2020. The Company recorded the warrants related to the draws totaling $313,000 to purchase 78,250 common shares as derivative liabilities.
Pursuant to the 2019 Purchase Agreement, Acuitas has agreed to further modify its existing rights under the Purchase Agreement dated July 3, 2018 with the Company so that Acuitas’ previous agreement in June 2019 to waive its rights to a 50% adjustment of the purchase price of the Preferred Stock in the July 2018 transaction, the exercise price of the warrants in such transaction and the price per share in a Subsequent Sale in the event of certain reductions in the useful life of our current intellectual property rights, and effectively exercise its rights to purchase securities in a Subsequent Sale pursuant to a “cashless purchase” at an assumed current market price of approximately $11.25 per share, conditioned in each case on the listing of the Company’s common stock on Nasdaq or the raising of $2.0 million in additional funds in the form of another securities offering, in either case not later than November 30, 2019, such that Acuitas will have irrevocably waived its rights to an adjustment in the purchase price of the Preferred Stock in the Initial Sale and the exercise price of the Warrants and the purchase price of per share in the Subsequent Sale upon the issuance by us of an aggregate of 2,679,916 shares of common stock and 2,679,916 warrants having the same terms as the Commitment Warrants to Acuitas, upon the closing of the Public Offering.
F-17
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 5. | Related Party Transactions (continued) |
Pursuant to an amendment to the 2019 Purchase Agreement dated October 9, 2019, Acuitas agreed to modify its existing rights under the 2019 Purchase Agreement so that:
| - | The Commitment Warrants (and related warrants issued upon the first draw under the Debenture) were replaced with warrants having similar terms, but which are automatically exercised upon the closing of the offering at an exercise price equal to the par value of the common stock; |
| - | Acuitas’ existing rights under the Purchase Agreement dated July 3, 2018 with the Company were further amended so that the number of Subsequent Sale Shares would be multiplied by four (in lieu of the changes to the Purchase Agreement originally provided for in the 2019 Purchase Agreement); and |
| - | The provisions of the 2019 Purchase Agreement providing that, should the underwriters in the offering exercise their option to purchase additional securities during the 45 days following closing and the issuance of such securities would result in Acuitas’ beneficial ownership (on a fully diluted basis) of shares of common stock being below 60%, Acuitas will be issued a number of additional shares of common stock and warrants having the same terms as the Commitment Warrants to result in its beneficial ownership (on a fully diluted basis) of shares of common stock equaling 60% have been modified such that, upon the exercise of such option by the underwriters, the Company will issue to Acuitas a number of securities that will result in Acuitas’ fully diluted beneficial ownership after the exercise of such option being the same as prior thereto. |
On July 14, 2020, the Company, entered into a further extension of its letter agreements dated April 8, 2020, that furthered extended its letter agreement dated February 10, 2020 with Acuitas regarding Acuitas’ previous agreement to modify its existing rights under the Purchase Agreement dated July 3, 2018 with the Company so that its June 2019 waiver of its rights to a 50% adjustment of the purchase price applicable to its initial investment in the Company and the exercise price of the warrants received in such transaction and the price per share should it exercise certain rights to purchase additional securities in the event of certain reductions in the useful life of the Company’s intellectual property rights and commitment to purchase such securities upon the closing of the Company’s planned public offering of shares of Class A common stock (the “Common Stock”) as described in its Registration Statement on Form S-1 (File No. 333-231136) and commitment to purchase such additional securities would remain effective until October 31, 2020, and accordingly Acuitas shall be entitled to receive an aggregate of 5,359,832 shares of Common Stock at such closing. In addition, the parties agreed that certain draws under the Company’s current bridge financing with Acuitas were to be made based with respect to the Company’s ongoing capital requirements and current market conditions, notwithstanding certain scheduled availability dates set forth in the 10% OID Convertible Delayed Draw Debenture issued in connection therewith. The letter agreement of July 14, 2020 also confirmed the understanding between the Company and Acuitas regarding certain amounts funded to BioVie that were intended as “partial draws” of credit available under the Debenture which, as of the date hereof aggregated $813,000 in aggregate principal amount in additional to amounts initial funded under the Debenture. Accordingly, such “partial draws” shall accrue additional principal as amounts otherwise funded pursuant to the original schedule of draws included in the Debenture (as modified by the letter agreement between BioVie and Acuitas dated April 1, 2020 regarding the capitalization of interest otherwise payable) and shall entitle Acuitas to receive a pro rata amount of Bridge Warrants.
F-18
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 6. | Other Liabilities |
Other liabilities at June 30, 2022 of $
| 7. | Notes Payable |
On November 30, 2021, (the “Closing Date”) the Company entered
into a Loan and Security Agreement and the Supplement to the Loan and Security Agreement and Promissory Notes (together, the “Loan
Agreement”) with Avenue Venture Opportunities Fund, L.P. (“AVOPI” and Avenue Venture Opportunities Fund II, L.P. (“AVOPII”)
together (“Avenue”) for growth capital loans in an aggregate commitment amount of up to $20 million (the “Loan”).
On the closing date, $15 million funded (“Tranche 1”) and up to $5 million will be made available to the Company on or prior
to September 15, 2022, subject to the Company’s achievement of certain milestones with respect to certain of its ongoing clinical
trials (“Tranche 2”). The Loan bears interest at an annual rate equal to the greater of (a) the sum of
The Loan Agreement requires monthly interest-only payments during the first eighteen months of the term of the Loan, which may be increased up to an additional six months from the end of such eighteen-month period prior to receipt of the Tranche 2 Loan. Following the interest-only period, the Company will make equal monthly payments of principal, plus accrued interest, until the Loan’s maturity date when all remaining principal and accrued interest is due. If the Company prepays the Loan, it will be required to pay (a) a prepayment fee in an amount equal to 3.0% of the principal amount of the Loan that is prepaid during the interest-only period; and (b) a prepayment fee in an amount equal to 1.0% of the principal amount of the Loan that is prepaid after the interest-only period. At the Loan’s maturity date, or on the date of the prepayment of the Loan, a final payment equal to 4.25% of the sum of (a) the Loan commitment amount under Tranche 1 and Tranche 2, plus (b) the aggregate principal amount of additional growth capital loans borrowed under Tranche 3.
The Loan Agreement includes a conversion option to convert up to $5.0 million of the principal amount of the Loan outstanding at the option of the Lenders, into shares of the Company’s Class A common stock at a conversion price of $6.98 per share.
On the Closing Date, the Company issued to the Lenders warrants to purchase 361,002 shares of Class A common stock of the Company (the “Warrants”) at an exercise price per share equal to $5.82, the stock purchase price. The warrants are exercisable until November 30, 2026, the expiration date.
F-19
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 7. | Notes Payable (continued) |
The amount of the carrying value of the notes payable were determined by
allocating portions of the outstanding principal of the notes to the fair value of the warrants of approximately $
The following is a summary of the Note Payable as of June 30, 2022 and June 30, 2021:
| June 30, 2022 | June 30, 2021 | |||||||
| Note Payable | $ | $ | ||||||
| Less debt financing costs | ( | ) | ||||||
| Less unearned discount | ( | ) | ||||||
| Plus accretion of loan premium | ||||||||
| Note Payable, net of financing costs, unearned premiums and discount | $ | $ | ||||||
Estimated future amortization expense and accretion of premium is as follows:
| Unearned Discount | Debt Financing Costs | Loan accretion Premium | ||||||||||||
| Year ending June 30, | ||||||||||||||
| 2023 | $ | $ | $ | |||||||||||
| 2024 | ||||||||||||||
| 2025 | ||||||||||||||
| Total | $ | $ | $ | |||||||||||
F-20
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 8. | Fair Value Measurements |
At June 30, 2022 and 2021, the estimated fair value of derivative liabilities measured on a recurring basis are as follows:
| Fair Value Measurements at | ||||||||||||||||
| June 30, 2022 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Derivative liability - Warrants | $ | $ | $ | $ | ||||||||||||
| Derivative liability -Conversion option on notes payable | ||||||||||||||||
| Total derivatives | $ | $ | $ | $ | ||||||||||||
| Fair Value Measurements at | ||||||||||||||||
| June 30, 2021 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Derivative liability - Warrants | $ | $ | $ | $ | ||||||||||||
| Derivative liability -Conversion option on note payable | ||||||||||||||||
| Total derivatives | $ | $ | $ | $ | ||||||||||||
The following table presents the activity for liabilities measured at fair value using unobservable inputs for the year ended June 30, 2022 and 2021:
| Derivative liabilities - Warrants | Derivative liability - Conversion Option on Convertible Debenture | |||||||
| Beginning balance at July 1, 2020 | $ | $ | ||||||
| Additions to level 3 liabilities | ||||||||
| Change in fair value of level 3 liability | ( | ) | ( | ) | ||||
| Transfer in and/or out of Level 3 | ( | ) | ( | ) | ||||
| Balance at June 30, 2021 | $ | $ | ||||||
| Additions to level 3 liabilities | ||||||||
| Change in fair value of level 3 liability | ( | ) | ( | ) | ||||
| Transfer in and/or out of Level 3 | ||||||||
| Balance at June 30, 2022 | $ | $ | ||||||
F-21
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 8. | Fair Value Measurements (continued) |
On September 22, 2020, concurrent with the closing of the Offering; the warrants related to derivative liabilities were automatically exercised in full and the convertible Debenture was paid off in cash expiring the conversion option. The fair value of the derivative liabilities – warrants and derivative liability – conversion option on convertible Debenture prior to redemption at September 22, 2020 was $13.1 million, and the change in the fair value of $8.3 million from June 30, 2020 was recorded in the accompanying Statements of Operations for the year ended June 30, 2021. At September 22, 2020, the derivative liabilities, both the warrants and expired conversion option totaling $ 13.1 million were then recorded as additional paid in capital upon automatic exercise of the warrants and payoff of the Debenture.
The fair values of derivative liabilities for the warrants and conversion option at June 30, 2022 in the accompanying balance sheet, were approximately $195,000 and approximately $188,000, respectively. The total change in the fair value of the derivative liabilities totaled approximately $3.3 million for the year ended June 30, 2022, and accordingly, was recorded in the accompanying statement of operations. The assumptions used in the Black Scholes model to value the derivative liabilities at June 30, 2022 included the closing stock price of $ per share, and for the warrants the exercise price of $, -year term, risk free rate of% and volatility of %. and for the embedded derivative liability of the conversion option, the conversion price of $; -year term, risk free rate of % and volatility of %.
Derivative liability – Warrants
The Company accounts for stock purchase warrants as either equity instruments or derivative liabilities depending on the specific terms of the warrant agreements. Under applicable accounting guidance, stock warrants that are precluded from being indexed to the Company’s own stock because of full-rachet and anti-dilution provisions or adjustments to the strike price due to an occurrence of a future event; are accounted as derivative financial instruments. The warrants issued on November 30, 2021 in connection with the Avenue loan financing were not considered to be indexed to the Company’s own stock, and accordingly, were recorded as a derivative liability at fair value in the accompany balance sheet at June 30, 2022.
The Black Scholes model was used to calculate the fair value of the warrant derivative to bifurcate the warrant derivative amount from the Avenue loan amount funded. The warrants are recorded at their fair values at the date of issuance and remeasured at June 30, 2022. The assumptions used for the fair value calculation at November 30, 2021 follows: the closing stock price of $per share; the exercise price of $; year term; a risk free rate of % and volatility of %.
Embedded derivative liability – Conversion Option
The embedded derivative represents the optional conversion feature of up to $5.0 million of the outstanding Avenue note amounts meets the definition of a derivative and requires bifurcation from the loan amount.
The Black Scholes model was used to calculate the fair value of the embedded derivative to bifurcate the embedded derivative amount representing the conversion option from the Avenue loan amount funded. The assumption used for the fair value calculation at November 30, 2021 follows: the closing stock price of $ per share; the conversion price of $; year term; risk free rate of % and volatility of %.
F-22
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 9. | Equity Transactions |
Stock Options
The following table summarizes the activity relating to the Company’s stock options for the years ended June 30, 2022 and 2021:
| Options | Weighted-Average Exercise Price | Weighted Remaining Average Contractual Term | Aggregate Intrinsic Value | |||||||||||||
| Outstanding at June 30, 2020 | $ | $ | ||||||||||||||
| Granted | ||||||||||||||||
| Options Exercised or Forfeited | ( | ) | — | |||||||||||||
| Outstanding at June 30, 2021 | ||||||||||||||||
| Granted | ||||||||||||||||
| Options Expired | ( | ) | — | |||||||||||||
| Options Forfeited | ( | ) | ( | ) | — | |||||||||||
| Outstanding at June 30, 2022 | $ | $ | ||||||||||||||
| Exercisable at June 30, 2022 | $ | $ | ||||||||||||||
The fair value of each option grant on the date of grant is estimated using the Black-Scholes Option – Pricing model reflecting the following weighted-average assumptions:
| June 30, 2022 | June 30, 2021 | |||||||
| Expected life of options (In years) | ||||||||
| Expected volatility | % | % | ||||||
| Risk free interest rate | % | % | ||||||
| Dividend yield | % | % | ||||||
F-23
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 9. | Equity Transactions (continued) |
Expected volatility is based on the historical volatilities of three comparable companies of the daily closing price of their respective common stock and the expected life of options is based on historical data with respect to employee exercise periods. The Company accounts for forfeitures as they are incurred.
The Company recorded stock-based compensation expense of approximately $ million and $ million for the years ended June 30, 2022 and 2021, respectively.
The following is a summary of stock options outstanding and exercisable by exercise price as of June 30, 2022:
| Exercise Price | Outstanding | Weighted Average Contract Life | Exercisable | |||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
| $ | ||||||||||||||
Issuance of common stock through exercise of Stock Options and Warrants
On July 28, 2020, the Company issued
On January 27, 2021, the Company issued
On March 23, 2021, the Company issued
F-24
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 9. | Equity Transactions (continued) |
On March 24, 2021, the Company issued
On April 19, 2021, the Company issued
Issuance of common stock for cash
On August 11, 2021, the Company closed a registered public offering issuing
of its Class A common stock at $ per share, resulting in net proceeds to the Company of approximately $
On September 24, 2021, the Company issued of its Class A common
stock at $ per share in connection with the underwriters’ exercise of its over-allotment option in for the August 2021
registered public offering, resulting in net proceeds to the Company of approximately $
Issuance of Shares for Services
On August 20, 2021, the Company awarded
The stock-based compensation expense related to these RSUs totaled $ for the fiscal year ended June 30, 2022.
On June 21, 2022, the Company awarded
The stock-based compensation expense related to these RSUs totaled $ for the fiscal year ended June 30, 2022.
Issuance of Stock Options
On October 1, 2020, the Company granted stock options to purchase shares of common stock at each grant date to the Chief Financial Officer as part of her compensation. The exercise prices of the stock options are $ and are exercisable at any time and expire in years from the date of issuance.
F-25
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 9. | Equity Transactions (continued) |
On October 13, 2020, the Company granted stock options to purchase shares of common stock, at each grant date; to a director as part of his annual director’s compensation. The exercise price of the stock options is $and are exercisable at any time and expire in years from the date of grant.
On December 18, 2020, the Company granted stock options under the Company’s 2019 Omnibus Incentive Compensation Plan to purchase shares of common stock to the members of the board as part of their annual compensation. The first 25% of the stock options vest on the grant date, and the remaining 75% vest over a 3-year period, on the first, second, and third anniversary of the grant date. The stock options were issued at an exercise price of $per share and expire years from the date of grant.
On January 19, 2021, the Company granted stock option to purchase a total of shares of common stock, granting 800 shares each to then Chief Operations Officer, an executive clinical team member and to four of its key consultants as part of their annual compensation. The exercise price of the options is $ per share, are exercisable at any time and expire years from the date of issuance.
On August 20, 2021, the Company granted, under the 2019 Omnibus Plan, stock options to purchase shares of common stock to the executive management team. Twenty percent (20%) of the shares underlying the options awarded vested on the grant date, and the remaining 80% vest equally over a 5-year period, on the first, second, third, fourth and fifth anniversary of the grant date. The exercise price of the options is $ per share, the grant date fair value of the stock, and the options terminate on the earlier of the tenth anniversary of the grant date or the date as of which the options were fully exercised.
On February 1, 2022, the Company granted stock options to purchase shares of common stock to a new employee. Twenty percent (20%) of the shares underlying the options awarded vested on the grant date, and the remaining 80% vest equally over a -year period, on the first, second, third, fourth and fifth anniversary of the grant date. The exercise price is $ per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
On February 1, 2022, the Company granted stock options to purchase shares of common stock to two new employee. Twenty percent (20%) of the shares underlying the options awarded vested on the first grant anniversary date, and the remaining 80% vest in equal monthly installments over 48 months. The exercise price is $ per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
On February 8, 2022, the Company granted stock options to purchase shares of common stock to a new employee. Twenty percent (20%) of the shares underlying the options awarded vest on the one-year anniversary of the grant date, and the remaining 80% vest in equal monthly installments over 48 month. The exercise price is $ per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
On March 1, 2022, the Company granted stock options to purchase shares of common stock to a new employee. Twenty percent (20%) of the shares underlying the options awarded vest on the one-year anniversary of the grant date, and the remaining 80% vest in equal monthly installments over 48 month. options terminate on the tenth anniversary of the grant date or date as of which the options were fulling exercised. The exercise price is $ per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
F-26
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 9. | Equity Transactions (continued) |
On April 5, 2022, the Company granted stock options to purchase shares of common stock to the independent directors of the board as compensation for services at an exercise price of $ per share, the grant date fair value. Twenty-five percent (25%) of the shares underlying the options awarded vested on the grant date, and the remaining 75% vest ratably over three years on the first, second, and third anniversary of the grant date. The options terminate on the earlier of the fifth anniversary of the grant date or the date as of which the options are fully exercised.
On June 3, 2022, the Company granted stock options to purchase shares of common stock to a new employee. Twenty percent (20%) of the shares underlying the options awarded vested on the grant date, and the remaining 80% vest equally over a 5-year period, on the first, second, third, fourth and fifth anniversary of the grant date. The exercise price is $ per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
On June 3, 2022, the Company granted stock options to purchase shares of common stock to a new employee. Twenty percent (20%) of the shares underlying the options awarded vest on the one-year anniversary of the grant date, and the remaining 80% vest in equal monthly installments over 48 month. The exercise price is $ per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
On June 6, 2022, the Company granted stock options to purchase shares of common stock to a new employee. Twenty percent (20%) of the shares underlying the options awarded vest on the one year anniversary of the grant date, and the remaining 80% vest in equal monthly installments over 48 month. The exercise price is $ per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
On June 21, 2022, the Company granted stock options to purchase shares of common stock to the CEO. The options vest in equal annual installments over three years on the anniversary grant date. The exercise price is $ per share, the grant date fair value, and the options terminate on the tenth anniversary of the grant date.
Pursuant to a former employee Separation Agreement, dated April 11, 2022; the Company modified a former employee’s stock option award granted on August 20, 2021 pursuant to the 2019 Omnibus Plan (“2021 Options Grant”). Pursuant to the terms of the Separation Agreement, effective on July 8, 2022, (“the Separation Date”) of the employee; the modification accelerated the vesting of options to purchase 74,500 shares of common stock as deemed vested, (“Accelerated Options”) and after giving effect to the Accelerated Options, extended the exercise period of the total vested outstanding and unexercised options of the 2021 Options Grant as of July 8, 2022 to one year following the Separation Date. The modification was remeasured as of the July 8, 2022 and the incremental difference totaled $181,154, net credit; due to the original exercise price of $7.74 is greater than the stock price of $1.80 on the remeasurement date and accordingly was recognized on July 8, 2022.
Forfeiture of Stock Options
On August 27, 2021, the Chief Executive Officer forfeited unvested stock options to purchase up to shares of common stock that were previously granted to him as compensation as an independent director of the board.
F-27
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 9. | Equity Transactions (continued) |
Stock Warrants
The following table summarizes the warrants activity during the years ended June 30, 2022 and 2021:
| Number of Shares | Weighted Average Exercise Price | Weighted Average Remaining Life (Years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding and exercisable at June 30, 2020 | $ | $ | ||||||||||||||
| Granted | — | |||||||||||||||
| Exercised | ( | ) | — | |||||||||||||
| Exercised - Acuitas | ( | ) | — | |||||||||||||
| Outstanding and exercisable at June 30, 2021 | $ | $ | ||||||||||||||
| Granted | — | |||||||||||||||
| Expired | ( | ) | — | — | ||||||||||||
| Exercised | — | — | ||||||||||||||
| Outstanding and exercisable at June 30, 2022 | $ | $ | ||||||||||||||
Of the above warrants, 4,815 expire in the fiscal year ending June 30, 2023, 2,714 expire in the fiscal year ending June 30, 2025, and 502,843 expire in the fiscal year ending June 30, 2026.
Issuance of warrants
On July 13, 2020, the Company issued Warrants to purchase shares of common stock to its controlling stockholder under the terms of the Bridge Financing. The warrants were exercisable at an exercise price of $ at any time from the date of issuance until years from the date of issuance. (See Note 5 Related Party Transactions.)
On September 22, 2020, the Company issued warrants to purchase shares of common stock to the underwriters of the Offering in connection with the close of the Offering of registered Common Stock The warrants are exercisable at an exercise price of $ at any time from date of issuance until years from the date of issuance.
F-28
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 10. | Leases |
Office Leases
From July 1, 2018 to October 31, 2021, the Company paid monthly rent of $1,000 to Acuitas for its headquarter office at 2120 Colorado Avenue Suite 230, Santa Monica, CA 90404. Effective November 1, 2021, the Company relocated its headquarters to Nevada. The Company paid an annual rent of $2,200 for the address at 680 W Nye Lane, Suite 201, Carson City Nevada 897603. The rental agreement is for a one year term.
On June 1, 2021, the Company assumed a NeurMedix office lease that was extended to February 2022 at 6165 Greenwich Dr Suite 150, San Diego, CA 92122. The lease agreement required monthly payments of $8,782. On February 26, 2022 the Company’s San Diego office relocated to 5090 Shoreham Place, San Diego, CA 92122. (the “New Office”). The New Office lease term for 38 months, commenced on March 1, 2022. The monthly base rate of $4,175 begins June 1, 2022 with annual increases of three percent.
The operating lease cost recognized in in our
statement of operations was approximately $
The following table provides balance sheet information related to leases as of June 30, 2022 and June 30, 2021:
| June 30, 2022 | June 30, 2021 | |||||||
| Assets | ||||||||
| Operating lease, right-of-use asset, net | $ | $ | ||||||
| Liabilities | ||||||||
| Current portion of operating lease liabilities | $ | $ | ||||||
| Operating lease liabilities, net of current portion | ||||||||
| Total operating lease liabilities | $ | $ | ||||||
At June 30, 2022, the future estimated minimum lease payments under non-cancelable operating leases are as follows:
| Year ending June 30: | ||||
| 2023 | $ | |||
| 2024 | ||||
| 2025 | ||||
| Total minimum lease payments | ||||
| Less amount representing interest | ( | ) | ||
| Present value of future minimum lease payments | ||||
| Less current portion of operating lease liabilities | ( | ) | ||
| Operating lease liabilities, net of current portion | $ | |||
The weighted average remaining lease term and discount rate as of June 30, 2022 and 2021 were as follows:
| June 30, 2022 | June 30, 2021 | |||||||
| Weighted average remaining lease term (Years) | ||||||||
| Operating leases | — | |||||||
| Weighted average discount rate | ||||||||
| Operating leases | % | |||||||
F-29
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 11. | Commitments and Contingencies |
Royalty Agreements
Pursuant to the Agreement and Plan of Merger entered into on April 11, 2016, between our predecessor entities, LAT Pharma LLC and NanoAntibiotics, Inc., BioVie is obligated to pay a low single digit royalty on net sales of BIV201 (continuous infusion terlipressin) to be shared among LAT Pharma Members, PharmaIn Corporation, and The Barrett Edge, Inc.
Pursuant to the Technology Transfer Agreement entered into on July 25, 2016 between BioVie and the University of Padova (Italy), BioVie is obligated to pay a low single digit royalty on net sales of all terlipressin products covered by US patent no. 9,655,645 and any future foreign issuances capped at a maximum of $200,000 per year.
| 12. | Employee Benefit Plan |
On August 1, 2021, the Company began sponsoring an employee benefit plan subject to Section 401(K) of the Internal Revenue Service Code (the “401K Plan”) pursuant to which, all employees meeting eligibility requirements are able to participate.
Subject to certain limitations in the Internal Revenue Code, eligible employees
are permitted to make contributions to the 401K Plan on a pre-tax salary reduction basis and the Company will match 5% of the first 5%
of an employee’s contributions to the 401K Plan. For the year ended June 30, 2022, the Company’s contributions to the 401K
Plan totaled approximately $
F-30
BioVie Inc.
Notes to Financial Statements
For the Years Ended June 30, 2022 and 2021
| 13. | Income Taxes |
Significant components of the Company’s deferred tax assets (liabilities) are as follows:
| June 30, 2022 | June 30, 2021 | |||||||
| Deferred tax assets (liabilities): | ||||||||
| Tax loss carryforward | $ | $ | ||||||
| Intangible assets | ( | ) | ( | ) | ||||
| Stock based compensation | ||||||||
| Valuation Allowance | ( | ) | ( | ) | ||||
| Net deferred tax assets | $ | $ | ||||||
At June 30, 2022 and 2021, the Company has recorded a full valuation against
its net deferred tax assets of $
At June 30, 2022, the Company had a Net Operating Loss (“NOL”) carryforward of approximately $23,600,000. NOL’s generated prior to 2018 will expire during the years ranging from 2032 to 2037.
The Company has no current tax expense due to its losses.
Reconciliation of the differences between income tax benefit computed at the federal and state statutory tax rates and the provision for income tax benefit for the years ended June 30, 2022 and 2021 is as follows:
| 2022 | 2021 | |||||||
| Income tax expense at federal statutory rate | % | % | ||||||
| State taxes, net of federal benefit | % | % | ||||||
| Change in valuation allowance | - | % | - | % | ||||
| Effective tax rate | ||||||||
| 14. | Subsequent Events |
Company, entered into a securities purchase agreement (the
“Purchase Agreement”) with Acuitas, pursuant to which Acuitas agreed to purchase from the Company, in a private
placement (the “Private Placement”), (i) an aggregate of
shares of the Company’s Class A common stock, par value $
per share at a price of $
per share, and (ii) a warrant to purchase
On August 31, 2022, the Company entered into a Controlled Equity Offering Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. and B. Riley Securities, Inc. (collectively, the “Agents”), pursuant to which the Company may issue and sell from time to time shares of Company’s Class A common stock, par value $ per share, through the Agents, subject to the terms and conditions of the Sales Agreement.
As of September 12, 2022, the Company has issued shares
under the Sales Agreement for a total net proceeds of $
F-31