
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
| For
the fiscal year ended |
||
| or | ||
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___ to ___
Commission
File Number:

(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
| ||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s
telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol | Name of each exchange on which registered | ||
| The Stock Market LLC | ||||
| The Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements. Yes ☐ No
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). Yes ☐ No ☐
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | |
| Smaller
reporting company | ||
| Emerging
growth company |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes
As
of April 2, 2024, the aggregate market value of the common equity of the registrant held by non-affiliates was $
As of April 2, 2024, there were there were shares of common stock, par value $0.0001 per share, issued and outstanding, and 0 shares of preferred stock, par value $0.0001 per share, of the registrant issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
As used in this Annual Report on Form 10-K, unless otherwise indicated or the context otherwise requires, references to “we,” “us,” “our,” “OneMedNet” and the “Company” refer OneMedNet Corporation (f/k/a Data Knights Acquisition Corp.), a Delaware corporation, and its consolidated subsidiaries following the effective time of the business combination between Data Knights Acquisition Corp. and OneMedNet (the “Business Combination”) pursuant to an agreement and plan of merger, dated April 25, 2022 (the “Merger Agreement”), by and among Company, Data Knights Merger Sub, Inc., a Delaware corporation and a wholly-owned subsidiary of the Company (“Merger Sub”), OneMedNet, Data Knights, LLC, a Delaware limited liability company (“Sponsor” or “Purchaser Representative”) that closed on November 7, 2023.
ONEMEDNET CORPORATION
ANNUAL REPORT ON FORM 10-K FOR THE YEAR ENDED DECEMBER 31, 2023
INDEX
| i |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements that we make from time to time, including statements contained in this Annual Report on Form 10-K constitute “forward-looking statements” within the meaning Private Securities Litigation Reform Act of 1995, and of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements other than statements of historical facts contained in this Annual Report on Form 10-K are forward-looking statements. The forward-looking statements in this Annual Report on Form 10-K are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition, and results of operations. In some cases, you can identify these forward-looking statements by terms such as “anticipate,” “believe,” “continue,” “could,” “depends,” “estimate,” “expects,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms or other similar expressions, although not all forward-looking statements contain those words. We have based these forward-looking statements on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short- and long-term business operations and objectives, and financial needs.
Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or a combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. Forward-looking statements in this Annual Report on Form 10-K include, without limitation, statements reflecting management’s expectations for future financial performance and operating expenditures (including our ability to continue as a going concern, to raise additional capital and to succeed in our future operations), expected growth, profitability and business outlook, and operating expenses.
Forward-looking statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. These factors include, among other things, the unknown risks and uncertainties that we believe could cause actual results to differ from these forward looking statements as set forth under the heading, “Risk Factors” and elsewhere in this Annual Report on Form 10-K. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all of the risks and uncertainties that could have an impact on the forward-looking statements, including without limitation, risks and uncertainties relating to:
| ● | our projected financial position and estimated cash burn rate; | |
| ● | our estimates regarding expenses, future revenues and capital requirements; | |
| ● | our ability to continue as a going concern; | |
| ● | our ability to raise substantial additional capital in sufficient amounts or on acceptable terms to fund our operations and our business plan; | |
| ● | our ability to reverse the recent decline in our revenue and resume growing our revenue; | |
| ● | our ability to compete in the global space industry; | |
| ● | our ability to obtain and maintain intellectual property protection for our current products and services; | |
| ● | our ability to protect our intellectual property rights and the potential for us to incur substantial costs from lawsuits to enforce or protect our intellectual property rights; | |
| ● | the possibility that a third party may claim we have infringed, misappropriated or otherwise violated their intellectual property rights and that we may incur substantial costs and be required to devote substantial time defending against these claims; | |
| ● | our reliance on third-party suppliers and manufacturers; | |
| ● | the success of competing products or services that are or become available; | |
| ● | our ability to expand our organization to accommodate potential growth and our ability to retain and attract key personnel; and | |
| ● | the potential for us to incur substantial costs resulting from lawsuits against us and the potential for these lawsuits to cause us to limit our commercialization of our products and services. |
| ii |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements including those described in the “Risk Factors” section beginning on page 31 and elsewhere in this prospectus.
| iii |
PART I
Item 1. Business
Company Overview
OneMedNet is a global provider of clinical imaging innovation and curator of regulatory-grade Imaging Real-World Data or iRWDTM. OneMedNet’s innovative solutions connect healthcare providers and patients satisfying a crucial need within the Life Sciences field offering direct access to clinical images and the associated contextual patient record. OneMedNet’s innovative technology proved the commercial and regulatory viability of imaging Real-World Data, an emerging market, and provides regulatory-grade image-centric iRWDTM that exactly matches OMN’s Life Science partners Case Selection Protocols and paves the way for Real World Evidence.
OneMedNet was founded to solve a deficiency in how clinical images were shared between healthcare providers. This resulted in OMN’s initial product BEAMTM image exchange that enabled the successful sharing of images for more than a decade with OMN’s largest customer being the Country of Ireland.
OneMedNet continued to innovate by responding to the demand for and utilization of Real-World Data and Real-World Evidence, specifically data that focused on clinical images with its associated contextual clinical record. We were able to leverage internal technological competencies along with OneMedNet’s formidable healthcare provider installed base from its first product with BEAMTM to become the first RWD solution for Life Science companies with its launch of iRWDTM in 2019.
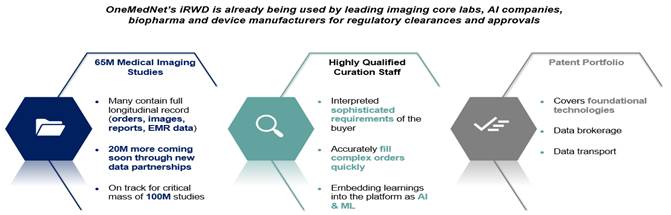
OneMedNet provides innovative solutions that unlock the significant value contained within clinical image archives. With a growing federated network of 95+ healthcare facilities, OneMedNet has the immediate ability to quickly search and extensively curate multi-layer data from a Federated group of healthcare facilities. The term “healthcare facilities” refers specifically to the hospitals, integrated delivery networks (“IDNs”) and imaging centers that provide imaging to OneMedNet, which represent the core source of our data. At present, OneMedNet works with more than 95 facilities who provide regulatory grade imaging to us. OneMedNet has access to these more than 95 facilities because these 95+ contracted facilities have more than 200 locations among them including offices and clinics, which in total generates regulatory grade imaging from more than 200 customers. Among these customers, all are data providers and some are data purchasers.
OneMedNet is ahead of the curve when it comes to providing fast and secure access to curated medical images. Initially, it was all about solving the diverse access needs of patient care providers. This focus systematically evolved to addressing the rapidly growing needs of image analysis and researchers, clinicians, regulators, scientists and more.
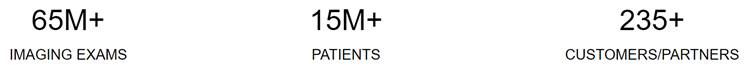
Real-world data is any data that is collected in the context of the routine delivery of care, in contrast to data collected within a clinical trial where study design controls variability in ways that are not representative of real-world care and outcomes.
A key component driving its mission is that OneMedNet believes we have a unique opportunity to affect a material positive impact on the lives of tens of millions of people while improving our customers’ business productivity. First and foremost, OneMedNet’s iRWDTM offering plays a significant role in enabling Life Science companies to bring safer and more effective patient care to market sooner. Using our highly curated de-identified clinical data in our iRWDTM offering in Life Science product development, validation, and regulatory approval processes, they contribute to patient care advancements in more meaningful ways. Moreover, Life Sciences improve their product development and validation processes, which benefits all parties.
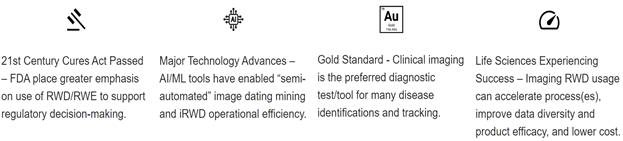
Significant documentation exists that shows that Real-World Data can provide expanded insights across broader and more representative patient populations. For this reason, the Food and Drug Administration (“FDA”) has instituted Real-World Data guidelines for regulatory approvals. Utilization of highly reliable and quality Real-World Data that strictly adheres to all of the very specific data stratification requirements can supplement or supplant clinical trials.
OneMedNet covers the complete value chain in imaging Real-World Data; it begins with our 10+ year federated network of providers and is supported by a multi-faceted data curation process managed by an expert in-house clinical team. Additionally, we work hand-in-hand with our Life Science partners regarding the Case Selection Protocol and when required producing Case Report Forms for regulatory clearance. We are focused on delivering value by supporting Life Science Advancements with OneMedNet’s iRWDTM which holds the key to unlocking boundless patient care advances. We unleash the power of research-grade image-centric iRWDTM that is highly curated to painstakingly meet every cohort requirement and stand up to all of the rigors of prospective clinical trials.
Today, life science companies, including pharmaceutical companies, artificial intelligence (AI) developers, medical device businesses, and clinical research organizations share the same widespread challenge in obtaining insight-rich, high-quality patient data that explicitly matches their precise cohort specifications. A substantial portion of patient diagnosis involves clinical imaging and approximately 90% of healthcare data, by size, is associated with imaging. Historically, much of imaging value has been derived from its initial review and further gains from the image archives have been very limited.
| 1 |
We help providers to “Unlock the Value in Imaging Archives”.TM By utilizing OneMedNet’s iRWDTM offering, providers can greatly improve their research efforts with streamlined data access. Health care providers such as hospitals, clinics, and imaging centers can also accelerate life science patient care innovations by sharing de-identified data in a well-defined and de-identified and secure manner. In return for doing so, income is generated and applied to critical and possibly unfunded provider projects.
The OneMedNet Difference
OneMedNet has been a leader in the business of extracting, securing, and transferring medical data for 12+ years. Doing so requires specialized expertise in:
| ● | Compliancy (HIPAA, GDPR, 21 Part11) | |
| ● | Advanced privacy & security measures | |
| ● | Clinical patient condition(s) and hospital processes | |
| ● | Radiology interpretation | |
| ● | AI/ML technology |
Attaining in-house expertise in all essential elements is quite a challenge and deters many organizations from even attempting such a venture. We take pride in this ambitious achievement – while continually working to maintain state-of-the-art expertise. OneMedNet strictly adheres to the highest level of professional and ethical standards and applicable regulations throughout all interactions and activities.
We believe there is a reason OneMedNet is the leader in an uncrowded field of regulatory-grade imaging RWD curators. Doing so requires specialized expertise in AI/ML technology, data privacy/security, as well as expertise in clinical patient condition(s) and healthcare record keeping. Having, or achieving, expertise in all essential disciplines is a challenging achievement. OneMedNet had a significant head start with our clinical image exchange solution which served to launch the Company nearly a decade ago. All data remains “native” within the federated OneMedNet iRWDTM provider network – meaning all the data remains locally onsite until specific de-identified data is licensed for a particular Life Science research opportunity.
OneMedNet’s Competitive Advantages
We believe that OneMedNet iRWDTM offers the best of advanced technology, clinical expert curation, and service. Medical imaging and associated clinical data is indexed at each network site using state-of-the-art AI/ML technology. This typically includes electronic health records (“EHR”), radiology, cardiology, lab, path and more. Our in-house clinical team performs intensive curation of the data ensuring that results meet the exact specification and requirements of Life Science Data Collection Protocol (“DCP”) – regardless of the complexity.
We believe that OneMedNet unlocks the value in imaging and electronic health records data in the following three principal ways:
| ● | Regulatory Grade — Our imaging results serve as proof of effectiveness for regulatory agencies, meeting requirements for quality & diversity; | |
| ● | On Demand — Our powerful indexing platform access and harmonizes complete patient profiles across fragmented data silos, delivering images and records on-demand; | |
| ● | Expertly Curated — We curate to the most stringent multi-level stratified requirements, providing unmatched data accuracy and completeness. |
| 2 |
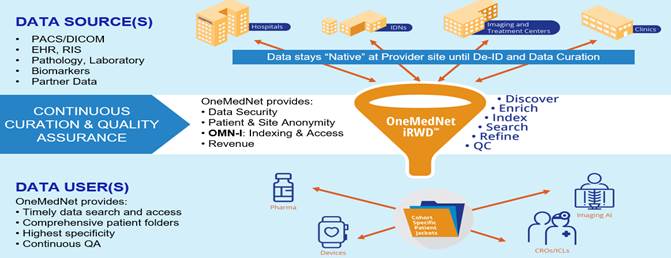
OneMedNet’s data is fully de-identified using a multi-step quality control process and goes beyond PHI to include PII (personally identifiable information), SII (Site Identifiable Information), and more. Importantly, Life Science users receive the data in the exact format that they require. No data sifting or manipulation is needed. The data is simply ready for use. Moreover, OneMedNet has the unique combination of knowledge, tools, and experience to:
| ● | Access and harmonize complete patient profiles across fragmented data silos; | |
| ● | Provide unmatched data accuracy and completeness; | |
| ● | Ensure the security and privacy of patients’ Protected Health Information (PHI)Imaging RWD is our singular passion and focus and no one does it better. |
Finally, OneMedNet has the most experienced and clinically trained data curators in the industry. This team appreciates the complexity and criticality of clinical data and can effectively communicate with both Provider and Life Science specialists.
Industry Background
A 2016 analysis published in the Journal of Health Economics and authored by the Tufts Center for the Study of Drug Development placed the cost of bringing a drug to market, including post-approval research and development, at a staggering $2.87 billion. Meanwhile, a 2018 study from the Tufts Center noted that the timeline for new drug development ranged from 12.8 years for the average drug to 17.2 years for ultra-orphan drugs that only affect several hundred patients. This places the onus on life science organizations to find ways to deliver treatments to patients faster — especially those who cannot wait 17 years for a potentially life-saving treatment. Knowing how a medicinal product is actually used by patients can help stakeholders across the healthcare ecosystem make important and potentially life-saving real-time decisions.
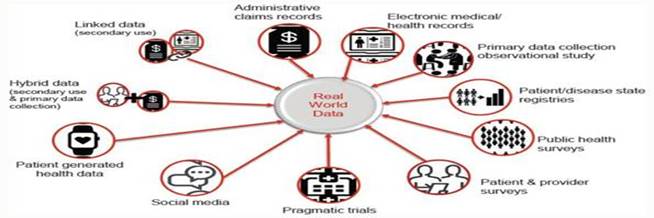
Real-World Data is observational data typically gathered when an approved medical product is on the market and used by “real” patients in real life, as opposed to clinical trials or real world images for real patients. The FDA cites several potential sources of Real-World Data, including electronic health records (“EHRs”), claims, disease and product registries, there are multiple types of data including structured and unstructured data, clinical and billing data, transactional and claims data, patient-generated data, and data gathered from additional sources that can shed light on a patient’s health status and more. As reliance on healthcare data grows exponentially, OneMedNet has observed that the reliance on information has increased coming from multiple additional sources including EHRs, claims, registries, clinical trials, patient and provider surveys, wearable devices and more. These additional sources include the internet of things (“IoT”), social media forums and blogs. Real-World Data has the potential to break down inefficiencies and fill gaps in information silos among stakeholders throughout the healthcare ecosystem of providers, payers, manufacturers, government entities and patients. This information sharing, in turn, enables all parties to derive new insights, support value-based care and deliver better health outcomes.
| 3 |
Commercializing a drug requires its developer to harness various sources of Real-World Data to identify patient populations and refine sales and marketing strategies for those populations among many other undertakings. Historically, this practice involved purchasing large amounts of data from data aggregators or data platforms, if not directly from the source itself, sometimes without much knowledge about the quality of the data. Preparing this data for analysis is both expensive and time-consuming thus many organizations would outsource the process to consultants or third-party vendors; moreover, the process of preparing this data for analysis by untrained consultants can yield a static analysis that is difficult to modify or rerun in response to follow-up questions or potential discrepancies.
Definitions of Real-World Data and Real-World Evidence
Real-World Data has become a powerful tool in the life sciences industry. After decades of relying on clinical data as the gold standard for decision making, industry leaders now recognize how data collected in the real world adds valuable context and insight to their efforts. From identifying unmet medical needs and defining the patient journey, to supporting regulatory submissions, proving value to payers, and shaping market strategies, Real-World Data adds value at every stage of the drug development lifecycle. Real-World Data also sets the foundation for Real World Evidence, and while the terms are often used interchangeably, they are distinct and they are changing health care. Here’s how it happens:
| 1. | First, Real-World Data are data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources. Real-World Data is aggregated and transformed such as through OneMedNet’s robust analytics. Real-World Data are the data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources. There are many different types, sources and uses of Real-World Data, for example: |
| ● | Clinical Data — For example, clinical data from EHRs and case report forms (“eCRF”) including biopsies and other Pathology tests, diagnostic imaging, social determinants of health, cancer organoids, that provide patient demographics, family history, comorbidities, procedure and treatment history, and outcomes. | |
| ● | Patient Generated Data — For example, patient-generated data from patient-reported outcome surveys, which data provide insights directly from the patient, and they help researchers understand what happens outside of clinic visits, procedures, and hospital stays. | |
| ● | Cost and Utilization Data (Qualitative Studies) — For example, cost and utilization data from claims and public datasets, which data provides information regarding healthcare services utilization, population coverage, and prescribing patterns. | |
| ● | Public Health Data — For example, public health data from various government data sources. These add critical information to enable stakeholders to best serve the needs of the populations they serve. |
The availability of medical imaging in Real-World Data such as that provided by OneMedNet is facilitated by the development of digital image analysis to increase the accuracy of diagnostics and conduct passive screening on large databases of medical images using artificial-intelligence (“AI”) algorithms such as those applied by OneMedNet. Algorithms can also help identify additional diagnostic tests of value from medical images with pathology.
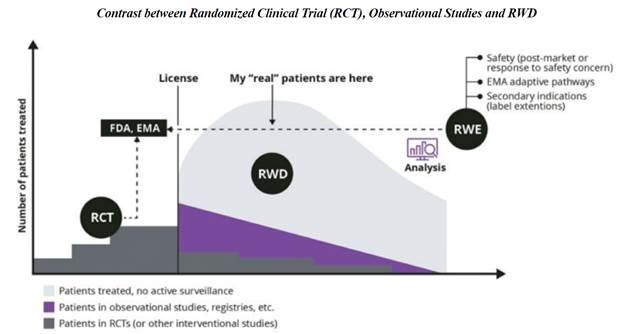
Real-World Evidence is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of Real-World Data, as defined by the Food and Drug Administration. Real-World Evidence can be generated by different study designs or analyses, including but not limited to, randomized trials, including large simple trials, pragmatic trials, and observational studies (prospective and/or retrospective). The difference in Real World Evidence and Real World Data focuses on the end use case. Real World Data can take the form of claims, electronic health records, labs, data etc. Often this insight is used to better understand a patient’s journey or a natural history of a disorder (how does a disease progress if left untreated.)
| 4 |
Real World Evidence in contrast builds upon many of these data sets and prepares them for submission, as part of regulatory review such as to the Food and Drug Administration or the European Medicines Agency, for example, in support of a customer’s clinical trial application. When data and in particular imaging data is submitted to the FDA the agency requires the following:
| ● | Guard against biased — evidence must align with the patient population being study — expectations focus on the similar patient demographics, comorbidities, disease severity, etc.; | |
| ● | Traceability — confirm the chain of custody, the source of the data is known and can be validated if required; and | |
| ● | Go forward basis — regulatory agencies seek evidence that aligns with the trials timeframe and when possible collect evidence that mirrors the clinical trials timeline. |
One area where Real World Evidence has been relief on heavily relates to oncology approvals. Food and Drug Administration’s Oncology Center of Excellence actually presented an analysis of this at American Society of Clinical Oncology in 2021, looking at oncology applications containing Real-World Data and Real-World Evidence. That analysis looked at 94 applications that were submitted from 2011-2020 and showed that inclusion of Real-World Data to support regulatory decision-making has increased dramatically over that period. In 2020 alone, there were 28 submissions for oncology products that contained Real-World Data. Outside of the oncology context, probably the most notable recent example of an approval relying on Real World Evidence is the July 2021 approval of a new indication for Astellas’ drug Program (or tacrolimus) for the prevention of organ rejection in lung transplant patients. The approval there was based on a non-interventional study providing Real-World Evidence of effectiveness. FDA’s press release announcing the approval noted that the approval was “significant because it reflects how a well-designed, non-interventional study relying on fit-for-purpose real-world data, when compared to a suitable control, can be considered adequate and well-controlled under FDA regulations.”
An additional recent approval of note was the December 2021 approval of the supplemental BLA for Orencia to prevent graft versus host disease. The application included data from a randomized clinical trial, with additional evidence of effectiveness provided by a registry-based clinical study that was conducted using real-world data from the Center for International Blood and Marrow Transplant Research. And that registry study analyzed outcomes of 54 patients treated with Orencia for the prevention of graft versus host disease, in combination with standard immunosuppressive drugs, versus 162 patients treated with the standard immunosuppressive drugs alone, and showed efficacy in that indication.
AI is employed in Real-World Data to enhance data anomaly detection, standardization, and quality checking at the pre-processing stage. AI is expected to offer pharma and biotech companies the ability to increase meaningful Real World Evidence output, decrease time to insights, and make the most of the available vast data sources. A Real World Evidence technology platform that delivers smart data processing, analysis, and outcomes offers an unparalleled opportunity to capitalize on these computing advancements.
When used as part of an overall comprehensive Real World Evidence strategy, AI innovations can enhance drug development, improve patient treatment and access, and drive valuable new business opportunities.
In post-marketing studies, adverse events reporting is an area where AI is used, creating greater automation and efficiency in historical data sets. Techniques like natural language processing (“NLP”) enable AI to scan tens of thousands of records and quickly find adverse event details. AI integrated analytics and automation provide access to crucial insights from historical clinical trial Real-World Data and Real World Evidence, expanding end-to-end clinical trial capabilities:
| ● | Data ingestion — publicly/historical available Real-World Data | |
| ● | Text extraction — NLP used to extract key entities from clinical trial documents | |
| ● | Data transformation & standardization — data standardization using pre-built models | |
| ● | AI model deployment — predicting trial design impacts on costs, feasibility, cycle times, and quality risk |
AI is driving ground-breaking leaps in protein structure identification, and advances in regulations are providing healthcare research organizations with access to real-world data to accelerate clinical trial processes. We believe that AI-enabled technologies have unparalleled potential to offer innovative trial design and collection, organizing, and analyzing the increasing amount of data generated by clinical trials. AI has many applications in clinical trials, both short and long-term. AI technologies make possible innovations crucial for transforming clinical trials, such as seamlessly combining Phases I and II, developing novel patient-centered endpoints, and collecting and analyzing Real-World Data.
OneMedNet believes that AI tools also have wider benefits for hospitals and health systems. Professor Alexander Wong, University of Waterloo Canada Research Chair in AI and Medical Imaging, points out that AI benefits include the potential to ease the burden on radiology departments in terms of assessing scans and predicting upcoming demand for general hospital and intensive care beds, and demand for equipment such as respirators and ventilators, medicines, masks, and ventilator mouthpieces, as well as aiding workforce planning.
| 5 |
Across a diverse set of imaging modalities, digital images typically include metadata and/or annotations that may include protected health information (e.g., patient name, date of birth). Although diagnostic images generally do not warrant the same level of privacy concerns as genomic data, researchers must also remove facial characteristics or other features that could identify a patient.
Digital image analysis can be used to support research and development by analyzing large volumes of tissue specimens or other medical images to run molecular screens that model biomarkers and treatment responses by transplanting a portion of a patient’s tumor into humanized mice or 3D tissue cultures derived from stem cells that resemble miniature organs. These models allow researchers to conduct controlled laboratory experiments that can inform treatment approaches and link predicted treatment response to actual clinical outcomes by linking this data to EHR, claims, and other sources of Real-World Data. Similarly, preclinical studies can be informed by safety assessments conducted in animal models or studies of animal molecular biomarkers or anatomic abnormalities to minimize the burden on human study participants. Findings can also inform clinical trial optimization by stratifying participants according to predicted response and determining appropriate eligibility criteria.
| 2. | Second, Real-World Evidence is the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of Real-World Data. Real World Evidence provides clinically-rich insights into what actually happens in everyday practice and why. The FD&C Act defines Real-World Evidence as “data regarding the usage, or the potential benefits or risks, of a drug derived from sources other than traditional clinical trials.” In developing its Real-World Evidence program, FDA believes it is helpful to distinguish between the sources of Real-World Data and the evidence derived from that data. |
Evaluating Real-World Evidence in the context of regulatory decision-making depends not only on the evaluation of the methodologies used to generate the evidence but also on the reliability and relevance of the underlying Real-World Data; these constructs may raise different types of considerations. Real-World Evidence refers to evidence about the risks and benefits of a product derived from analysis of the Real-World Data. For example, the FDA has used Real-World Data and Real-World Evidence, derived from its Sentinel system for monitoring the safety of regulated products, in place of post-marketing studies. It has carried this out for nine potential safety issues involving five products.
Real-World Evidence is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of Real-World Data. Real World Evidence can be generated by different study designs or analysis, including but not limited to, randomized trials, including large simple trials, pragmatic trials, and observational studies (prospective and/or retrospective).
Unlike traditional clinical trials, where necessary data elements can be curated and collection mandated, the creation of Real World Evidence requires assessing, validating and aggregating various, often disparate, sources of data available through routine clinical practice. Real-world evidence is used by different stakeholders in many different ways.
| ● | It gives life sciences companies insight into how their drugs are being used. | |
| ● | It helps providers improve the delivery of care. | |
| ● | It enables regulatory authorities to monitor post-market safety and adverse events. | |
| ● | It helps payers assess outcomes from treatments. |
From Real-World Data to Real World Evidence
The creation of Real World Evidence requires a combination of high-powered analytics, a validated approach and a robust knowledge of available Real-World Data sources (e.g., what data is captured within existing quality registries, what data can be captured through electronic health records and case report forms or claims, which patient organizations capture data on relevant patient cohorts). This process includes several steps, which are summarized here:
| 1. | Defining a study protocol answering relevant clinical questions. | |
| 2. | Defining which data elements can be collected from which Real-World Data sources. | |
| 3. | Establishing data capture arrangements and protocols with existing Real-World Data sources. | |
| 4. | Blending disparate data sources through probabilistic record matching algorithms. | |
| 5. | Validating and supplementing blended data through editable eCRFs. | |
| 6. | Defining and calculating clinically relevant outcomes and measures. | |
| 7. | Appropriately assessing and controlling for variability in data quality, availability and confounding patient factors affecting measured outcomes. | |
| 8. | Real World Evidence can provide a holistic view of patients that in many cases cannot be studied through traditional clinical trials. |
Real World Evidence has been proven to fill a gap between research (what we learn) and everyday practice (what we do) in healthcare, and it creates a difference between what is expected to happen and what really happens. Driving measurable improvements in healthcare requires us all to be rooted in the reality of what actually happens before, during, and after clinical procedures, interventions, and office visits. Real World Evidence fill those gaps and documents the truth by establishing definitively what really happens when doctors treat a wide range of patients that do not look like the homogeneous patient groups in a clinical trial. Because of this, Real World Evidence serves many uses and provides many benefits across the healthcare ecosystem.
| 6 |
As more countries battle to contain healthcare costs, and as the population ages and the number of patients with chronic diseases increases, the need to remove inefficiencies and upgrade the delivery of coordinated care that improves outcomes is more pressing. At the same time, life sciences companies are facing tumultuous times. Industry globalization, the end of the blockbuster era, and an increasingly complex regulatory environment all add to the difficulty of bringing products to market. And across the board, companies are moving toward a patient-centric and outcome-focused model. In this environment, Real World Evidence can be transformative for the industry when Real-World Data is combined with the right technology framework and the regulatory intelligence to make sense of it. As data is consumed across life sciences in different ways and by different stakeholders, it can provide valuable insights and “evidence” across the product life cycle. In addition, stakeholders across the healthcare ecosystem use this new knowledge to support decision-making and improve safety and effectiveness, and ultimately, patient outcomes.
Uses of Real World Evidence in Life Sciences, Among Regulators, Clinicians, Researchers and Healthcare Systems
According to repeated studies by Deloitte, the importance of Real World Evidence continues to rise as it promises to accelerate regulatory decision-making and support the approval of new indications for drugs already on the market. Life Sciences, pharmaceutical and medical device companies are significant consumers of Real World Evidence because it can provide value across the entire product lifecycle from pre-trial design to clinical studies and trials to post-market surveillance. Medical product developers are using Real World Evidence to support clinical trial designs (e.g., large simple trials, pragmatic clinical trials) and observational studies to generate innovative, new treatment approaches.
Real World Evidence can be used to make clinical trials more effective and efficient, for example in patient recruitment or label extension, Real World Evidence gathered from other studies or from currently marketed products in a similar category, for example, can have a positive effect on the product portfolio by exposing positive side effects as new potential indications. The most famous example is Viagra, which was initially studied as a drug to lower blood pressure, but an unexpected side effect led to the drug ultimately being approved for erectile dysfunction.
The benefits of Real World Evidence derived from Real-World Data are increasingly being recognized by regulatory authorities. The FDA released a framework for using Real World Evidence to support the process of drug regulation and submission. This is a major step toward recognizing that clinical trials, while still relevant, are not the only way to assess the efficacy and safety of a product. Indeed, the FDA is soon expected to conduct its first full post-market safety approval using only Real World Evidence.
Real World Evidence is now accepted as a reliable source of information for regulatory decision making in certain circumstances. A primary rationale for the FDA to use Real World Evidence E is to help support the approval of a new or extended use for a drug approved under the FD&C Act and to help support or satisfy post-approval study requirements always with the condition that the data quality is up to the standard required. In a recent statement, the FDA even noted how new tools for capturing data in the post-market period, including more sophisticated use of Real-World Data and Real-World Evidence are providing new approaches to address important questions about the safety and benefits of new drugs in real world settings and that these approaches have the potential to do to so more rapidly and with greater efficiency than traditional methods.
Why Do We Need Real-World Evidence?
There is a gap between research (what we learn) and everyday practice (what we do) in healthcare, and it creates a difference between what is expected to happen and what really happens. But it is what really happens that matters. Driving measurable improvements in healthcare requires us all to be rooted in the reality of what actually happens before, during, and after clinical procedures, interventions, and office visits. Real-World Evidence is here to fill those gaps and root us in truth. It tells us what really happens when doctors treat a wide range of patients that don’t look like the homogeneous patient groups in a clinical trial. Because of this, Real-World Evidence serves many uses and provides many benefits across the healthcare ecosystem.
Uses of Real-World Evidence in Pharmaceutical and Device Companies
Pharmaceutical and medical device companies are major consumers of Real-World Evidence, as it can provide value across the entire product lifecycle. Real-World Evidence plays an important role for research across the product lifecycle for both pharmaceutical and device companies. It can inform pre-trial study design by helping researchers identify potential patients and create proper inclusion criteria for clinical trials. Much of medical innovation is driven by traditional clinical trials, where new pharmaceuticals and devices are rigorously studied and tracked before they can be sold and widely distributed.
Although clinical trials are incredibly important to determine the safety and efficacy of new technologies, when compared to real-world evidence they do have some limitations. For example, traditional clinical trials can have strict inclusion criteria that makes it challenging for providers to accurately extrapolate the results of a clinical trial to a broader population. Clinical trial participation is often limited by who the study administrators are able to recruit, and various demographics are often not able to participate. This again challenges the generalizability of clinical trial results across patient populations. Real-world evidence can help overcome the limitations of clinical trials by providing information about a broader cross-section of society. This can help clinicians, researchers, and industry partners better understand their products and how they work.
| 7 |
Once a product is approved and marketed, Real-World Evidence assists pharmaceutical or medical device company understand their products’ relative safety, effectiveness, value, off-label use and more. This post-market surveillance, or post-marketing surveillance, is valuable to stakeholders across the healthcare industry.
The AI-enabled patient enrichment and recruitment process can improve suitable cohorts and increase clinical trial effectiveness, data management, analysis, and interpretation of multiple Real-World Data sources, including EHRs and medical imaging data. This presents a unique opportunity for NLP to perform the sophisticated analysis necessary to combine genomic data with electronic medical records (“EMRs”) and other patient data, present in various locations, owners, and formats — from handwritten paper copies to digital medical images — to surface biomarkers that lead to endpoints that can be more efficiently measured, and thereby identify and characterize appropriate patient subpopulations. AI-enabled systems can help to improve patient cohort composition and aid with patient recruitment.
AI technologies can help biopharma companies identify target locations, qualified investigators, and priority candidates and collect and collate evidence to satisfy regulators that the trial process complies with good clinical practice (“GMP”) requirements. One of the most important elements of a clinical trial is a selection of high-functioning investigator sites. Site qualities such as resource availability, administrative procedures, and experienced clinicians with in-depth knowledge and understanding of the disease can shape study timelines and data quality, accuracy, completeness, and consistency.
AI integrated clinical trial programs can help monitor and manage patients by automating real-world data capture, sharing data across systems, and digitalizing standard clinical assessments. AI technologies and wearable technologies can help enable continuous patient monitoring and generate real-time insights into the safety and effectiveness of treatment while predicting the possible risk of dropouts, thereby enhancing patient engagement and retention. To comply with trial adherence criteria, patients must keep detailed records of their medication intake and other data points related to their bodily functions, response to medication, and daily protocols. This can be an overwhelming and tedious task, leading to 40% of patients becoming non-adherent after 150 days into a clinical trial. Wearable devices/sensors and video monitoring are used to collect patient data automatically and continuously, thereby relieving the patient of this task. In combination with wearable technology, AI techniques offer new approaches to developing real-time, power-efficient, mobile, and personalized patient monitoring systems.
Among regulators, clinicians, academic researchers and healthcare systems, the reliance on curated Real World Evidence has grown significantly because of the value it can provide, which is unique relative to each parties’ objectives and mandates. It also helps that the FDA has also sharpened its focus on Real-World Data and Real World Evidence. For example, late last year, the FDA published proposed guidance related to data standards for product submissions with Real-World Data and also weighed in on the use of Real-World Data and Real World Evidence to support regulatory decision-making for drugs and biological products with specific advice for data from electronic health records and medical claims. In addition, the FDA uses Real-World Data and Real World Evidence to monitor post-market safety and adverse events and to make regulatory decisions. The health care community is using these data to support coverage decisions and to develop guidelines and decision support tools for use in clinical practice.
AI with deep-learning capability is also helpful in organizing and translating a vast amount of structured and unstructured data to RWE. The human mind can possibly manage 4-5 variables, therefore, AI-enabled data mapping and integration and their normalization into a common data model according to disease pathway and workflow will likely be useful for both quality management in clinical trials and generating meaningful insight for human disease by providing a broader perspective based on real-world data.
| 8 |
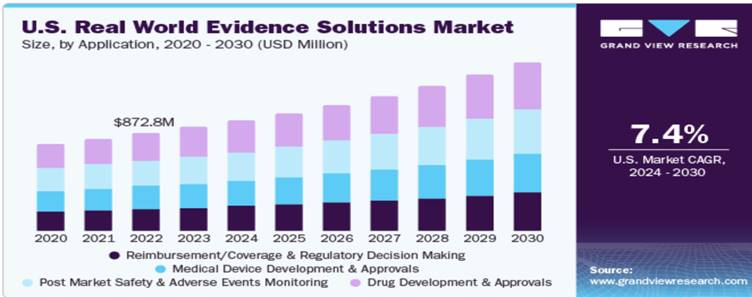
Market Size
The global real world evidence solutions market size was estimated at USD 2.6 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 8.4% from 2024 to 2030. The market growth is driven by rising demand for enhanced Real-World Evidence (RWE) capabilities within the life science industry, reflecting an increasing market shift from volume to value-based care. Advancements in data analytics and real-world evidence (RWE) contribute to supporting regulatory compliance, research, and solution development efforts in medical device and life sciences organizations. For instance, the increased demand for Real-World Evidence solutions is prompting players to introduce new products, fostering market growth. In October 2023, Maxis Clinical Sciences launched Real-World Evidence Solutions, providing diverse real-world data capture and analysis to improve clinical research and care.
Government initiatives supporting Real-World Evidence programs, evolving regulations, and actionable Real-World Data enable organizations to conduct outcomes-based analyses, contributing to the overall market expansion. For instance, in December 2022, the FDA launched the Real-World Evidence Program. This program aims to raise awareness that Real-World Evidence can support regulatory decisions, identify approaches for generating Real-World Evidence to meet post-approval study requirements or effectiveness labeling and develop agency processes that foster consistent decision-making and shared learning regarding Real-World Evidence.
The COVID-19 pandemic further accelerated the adoption of Real-World Evidence solutions, with governments collaborating with market players to implement these solutions. For instance, in June 2021, ConcertAI and the FDA initiated a five-year collaborative research program, Evaluation of Real-World Outcomes and Safety in the Treatment of Cancer. The partnership leverages ConcertAI’s oncology Real-World Data and advanced AI technology solutions to generate Real-World Evidence for various clinical and regulatory use cases.
The global real world evidence solutions market is projected to grow from $16.13 billion in 2023 to $36.24 billion by 2030, at a CAGR of 12.3%. The drug development and approvals segment accounted for the highest revenue share of around 28.9% in 2020. Real-world evidence solutions services allow pharmaceutical companies and healthcare providers as well as payers for efficient management of operations and accelerate the process of drug development and its approval, which fuels market growth. Support from regulatory bodies for using Real World Evidence solutions and an increase in research and development spending are anticipated to boost the market growth.
The RWE solution providers are increasingly forming strategic partnerships with AI solution providers to offer integrated solutions. For instance, in April 2023, ConcertAI, a player in AI SaaS technology and RWE solutions for healthcare and life sciences, partnered with PathAI, an AI-powered pathology provider, to introduce a first-in-class quantitative histopathology and curated clinical Real-World Data solution. This collaboration integrates ConcertAI’s Patient360 and RWD360 products with PathAI’s PathExplore tumor microenvironment panel. Based on end user, the global Real World Evidence solutions market is segmented into pharmaceutical, biotechnology, and medical device companies; healthcare payers; healthcare providers; and other end-users (academic research institutions, patient advocacy groups, regulators, and health technology assessment agencies). The large share of this segment is primarily attributed to the increasing importance of Real World Evidence studies in drug development and approvals and the growing need to avoid costly drug recalls and assess drug performance in real-world settings.
With the growing need for evidence generated from Real-World Data, the increasing importance of epidemiological data in decision making, and a shift from volume to value-based care, there has been an increased focus on patient registries, a rise in the adoption of EMR in hospitals, and exponential growth in mobile health data and social media which have resulted in the generation of huge amounts of medical data. In 2021, the real-world datasets segment is estimated to account for the larger share of 51.2% of the global real-world evidence solutions market. According to Coherent Market Insights, the global Real-World Data market is estimated to be valued at $1.59 billion in 2023 and is expected to exhibit a CAGR of 14.4% during the forecast period (2023-2030).
| 9 |
Our Long-Term Growth Strategies
Our long-term growth strategy is anchored on the following key pillars:
| ● | Increase Global Reach to Meet Demand: Our strategy is to continue growing our global footprint into areas where we expect high demand growth in the global real world evidence solutions market, which is projected to grow from $16.13 billion in 2023 to $36.24 billion by 2030, at a CAGR of 12.3%. There is a rise in emphasis on evidence-based medicine that relies on Real-World Evidence, which comes from Real-World Data. Market players in healthcare industries, including regulators, healthcare providers, and payers are becoming more aware of the importance of using Real-World Data for making informed decisions regarding comparative effectiveness, treatment effectiveness, cost-effectiveness, and safety. As a result, the demand for real world data solutions is increasing rapidly, which is further driving growth of the market. Regulatory agencies such as the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) are making use of real-world evidence in regulatory decision making processes. These regulatory authorities have frameworks and guidelines for using Real-World Evidence and Real-World Data in regulatory submissions, post-market surveillance, and drug approvals. As a result, the demand for real-world data is rising, which in turn is expected to support growth of the market in the coming future.17 The use of Real-World Evidence derived from Real-World Data demonstrates value and cost-effectiveness of medical devices and drugs for healthcare technology assessment agencies and payers. With this Real-World Evidence, market access becomes easier and it also enables reimbursement negotiations. This further facilities the inclusion of new therapies in the coverage of healthcare, which in turn creates major opportunities in the global market. | |
| ● | Innovate Our Commercial Approach to Drive Incremental Market Share: We intend to rapidly expand our sales network across the globe, while simultaneously building out our sales infrastructure. We intend to focus on our target markets, which include (i) Imaging AI; (ii) medical device companies; and (iii) pharmaceutical companies, as summarized here: |

| ● | Enhance and Refine Our Service Offering: Building on our customer-centric mindset throughout our development, curation and commercial processes, we plan to continue expanding and improving our service offering. As we continue to expand into additional geographies globally, we plan to build upon these three pillars |
| ● | Expand Our Product Offering: We plan to continually evaluate the benefits of expanding our portfolio into other high-growth, high-demand Real-World Data and Real-World Evidence solutions in the future. |
| 10 |
Corporate Information
We were originally incorporated in Delaware on February 8, 2021 under the name “Data Knights Acquisition Corp” as a special purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. On November 7, 2023, we held the Closing of the previously announced Merger whereby Merger Sub merged with and into OneMedNet Solutions Corporation (formerly named OneMedNet Corporation), with OneMedNet Solutions Corporation continuing as the surviving entity, which resulted in all of the issued and outstanding capital stock of OneMedNet Solutions Corporation being exchanged for shares of the Company’s Common Stock upon the terms set forth in the Merger Agreement.
The Merger and other transactions that closed on November 7, 2023, pursuant to the Merger Agreement, led to Data Knights changing its name to “OneMedNet Corporation” and the business of the Company became the business of OneMedNet Solutions Corporation. We are located at 6385 Old Shady Oak Road, Suite 250, Eden Prairie, MN 55344 and reachable by telephone on 800-918-7189.
The information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our Common Stock.
OneMedNet Corporation a Delaware corporation (the “Company,” “we,” “us,” or “OneMedNet”) together with its wholly-owned subsidiary OneMedNet Solutions Corporation, a Delaware corporation, founded on October 13, 2009 in the State of Hawaii and later incorporated in the State of Delaware on November 20, 2015 and its wholly-owned subsidiary, OneMedNet Technologies (Canada) Inc., incorporated on October 16, 2015 under the provisions of the Business Corporations Act of British Columbia whose functional currency is the Canadian dollar. All refences in this prospectus to the “Company,” “we,” “us,” or “OneMedNet” include OneMedNet Solutions Corporation and its wholly-owned subsidiary, OneMedNet Technologies (Canada) Inc., incorporated on October 16, 2015 under the provisions of the Business Corporations Act of British Columbia whose functional currency is the Canadian dollar.
Recent Developments
Closing of Business Combination
OneMedNet Corporation, a Delaware corporation (the “Company,” “we,” “us” or “OneMedNet”) together with its wholly-owned subsidiary, OneMedNet Solutions Corporation, a Delaware corporation, and its wholly-owned subsidiary, OneMedNet Technologies (Canada) Inc., incorporated under the provisions of the Business Corporations Act of British Columbia whose functional currency is the Canadian dollar. All references in this prospectus to the “Company,” “we,” “us,” or “OneMedNet” include OneMedNet Corporation and both OneMedNet Solutions Corporation and OneMedNet Technologies (Canada) Inc., except that references to the “Company” “we,” “us,” or “Data Knights” in this Item 7 refer to OneMedNet Corporation f/k/a Data Knights Acquisition Corp.
We were originally incorporated in Delaware on February 8, 2021 under the name “Data Knights Acquisition Corp” as a special purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. On May 11, 2021, we consummated an initial public offering.
On November 7, 2023, following the approval at the special meeting of the shareholders of Data Knights Acquisition Corp., a Delaware corporation held on October 17, 2023 (the “Special Meeting”), Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”) and a wholly-owned subsidiary of Data Knights Acquisition Corp., a Delaware corporation (“Data Knights”), consummated a merger (the “Merger”) with and into OneMedNet Solutions Corporation (formerly named OneMedNet Corporation), a Delaware corporation (“OneMedNet”) pursuant to an agreement and plan of merger, dated as of April 25, 2022 (the “Merger Agreement”), by and among Data Knights, Merger Sub, OneMedNet, Data Knights, LLC, a Delaware limited liability company (“Sponsor” or “Purchaser Representative”) in its capacity as the representative of the stockholders of Data Knights, and Paul Casey in his capacity as the representative of the stockholders of OneMedNet (“Seller Representative”). Accordingly, the Merger Agreement was adopted, and the Merger and other transactions contemplated thereby (collectively, the “Business Combination”) were approved and completed.
At the closing, on November 7, 2023, of the Business Combination pursuant to the Merger Agreement, Merger Sub merged with and into OneMedNet with OneMedNet surviving the Merger, as a wholly-owned subsidiary of Data Knights, and Data Knights changed its name to “OneMedNet Corporation.”
The Business Combination was accounted for as a reverse recapitalization in accordance with U.S. GAAP. Under this method of accounting, Data Knights was treated as the acquired company and OneMedNet Corporation was treated as the acquirer for financial statement reporting purposes.
Lock-up Agreements
Effective April 25, 2022, in connection with the execution of the Merger Agreement, certain stockholders of OneMedNet and certain of OneMedNet’s officers and directors (such stockholders, the “Company Holders”) entered into a lock-up agreement (the “Lock-up Agreement”) pursuant to which the Company Holders will be contractually restricted, during the Lock-up Period (as defined below), from selling or transferring any of (i) their shares of OneMedNet common stock held immediately following the Closing and (ii) any of their shares of OneMedNet common stock that result from converting securities held immediately following the Closing (the “Lock-up Shares”). Effective November 7, 2023, the newly appointed officers and directors of OneMedNet Corporation have entered into a Lock-Up Agreement.
| 11 |
The “Lock-up Period” means the period commencing at Closing and end the earliest of: (a) six months from the Closing, and (b) the date after the Closing on which the Purchaser consummates a liquidation, merger, capital stock exchange, reorganization, or other similar transaction with an unaffiliated third party that results in all of the Purchaser’s stockholders having the right to exchange their shares of the Purchaser Common Stock for cash, securities, or other property: (i) lend, offer, pledge, hypothecate, encumber, donate, assign, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any Restricted Securities, (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the Restricted Securities, or (iii) publicly disclose the intention to do any of the foregoing, whether any such transaction described in clauses (i), (ii), or (iii) above is to be settled by delivery of Restricted Securities or other securities, in cash or otherwise (any of the foregoing described in clauses (i), (ii), or (iii), a “Prohibited Transfer”).
In addition, the Sponsor is subject to a lock-up pursuant to a letter agreement (the “Sponsor Lock-up Agreement”), entered into on May 6, 2021, at the time of the IPO (as defined below), among Data Knights, the Sponsor and each of the individuals who were a member of Data Knights’ board of directors and/or management team (each, an “Insider” and collectively, the “Insiders”), who agreed that it, he or she shall not transfer any founder shares which means the 2,875,000 shares of Data Knights Class B common stock, par value $0.0001 per share, initially held by the Sponsor, or shares of OneMedNet’s Common Stock issuable upon conversion thereof) until the earlier of (A) six months after the date of Data Knights’ initial Business Combination or (B) subsequent to the initial Business Combination, (x) if the reported last sale price of the Common Stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock dividends, right issuances, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after the Company’s initial Business Combination, or (y) the date on which the Company completes a liquidation, merger, capital stock exchange, reorganization or other similar transaction that results in all of our stockholders having the right to exchange their shares of common stock for cash, securities or other property. Further, the Sponsor and each of the Insiders agreed further in the Sponsor Lock-Up Agreement that he, she or it shall not transfer any private placement units, the private placement shares, the private placement warrants or shares of Common Stock issued or issuable upon the exercise of the private placement warrants, until 30 days after the completion of the initial Business Combination.
Registration Rights Agreements
At the Closing of the Business Combination and funding of the PIPE, the PIPE Investors each executed a PIPE Note and a PIPE Warrant in the amount corresponding to each PIPE Investor’s investment amount and in accordance with the terms set forth in the PIPE SPA as well as a registration rights agreement (the “PIPE Registration Rights Agreement”). We are registering the offer and sale of these securities to satisfy the registration rights we have granted in the PIPE Registration Rights Agreement. At the Closing of the Business Combination, OneMedNet, Data Knights and the Sponsor entered into a registration rights agreement (the “Registration Rights Agreement”), pursuant to which, among other things, the Company is obligated to file a registration statement to register the resale of certain securities of the Company held by the holders, as defined in the Registration Rights Agreement and the Sponsor. The Registration Rights Agreement also provides the holders and the Sponsor with “piggy-back” registration rights, subject to certain requirements and customary conditions.
Voting Agreement and Sponsor Support Agreement
In connection with entry into the Merger Agreement, the Company entered into voting agreements (the “Voting Agreements”) with certain stockholders of OneMedNet representing approximately 55% of the outstanding voting power of OneMedNet’s equity securities (the “OneMedNet Stockholders”) pursuant to which OneMedNet Stockholders agreed to vote their securities in favor of the approval of the Merger Agreement and the Business Combination, be bound by certain covenants and agreements related to the Business Combination and to take other customary actions to cause the Business Combination to occur.
In connection with entry into the Merger Agreement, the Company, the Sponsor and OneMedNet entered into a sponsor support agreement (the “Sponsor Support Agreement”) pursuant to which the Sponsor agreed to vote its Data Knights securities in favor of the approval of the Merger Agreement and the Business Combination and to take other customary actions to cause the Business Combination to occur.
Executive Employment Agreements
In connection with the Closing of the Business Combination, the Company has entered into employment agreements (the “Employment Agreements”) with executive officers: Aaron Green (President), Lisa Embree (Chief Financial Officer), and Paul Casey (Chief Executive Officer). The Employment Agreements provide for at-will employment that may be terminated by the Company with or without cause, by the executive with or without good reason, or mutually terminated by the parties.
The Employment Agreement for Mr. Green provides for $350,000 annual salary, eligibility to receive an annual cash performance bonus of $175,000 upon his achievement of the performance goals set by the Company’s CEO and Board of Directors, and eligibility to receive 600,000 of the Company’s outstanding shares at closing, as part of the Company’s Restricted Stock Unit Plan, subject to the approval of the Company’s Board of Directors. In the event that his employment is terminated by the Company without Cause (as defined in the Employment Agreement), or is terminated by Mr. Green for Good Reason (as defined in the Employment Agreement), after six months of employment, and he signs and does not revoke a standard release of claims with the Company in a form reasonably satisfactory to the Company’s Board of Directors (a “Release”), which Release becomes irrevocable no later than sixty (60) days (the “Release Deadline”), after the date of his termination of employment (the “Termination Date”) he will be entitled to the following severance payment, as follows: (a) if the Termination Date is after six (6) months’ of employment, but before he has completed 12 months’ of employment, he will receive three (3) months’ salary; and (b) if the Termination Date is after 12 months’ employment he will receive six (6) months’ salary. If the Release does not become effective and irrevocable by the Release Deadline, he will forfeit any right to severance.
| 12 |
The Employment Agreement for Ms. Embree provides for $225,000 annual salary, eligibility to receive an annual cash performance bonus of twenty-five percent (25%) of her annual salary upon her achievement of the performance goals set by the Company’s CEO and Board of Directors, and eligibility to receive 260,000 of the Company’s outstanding shares, as part of the Company’s Restricted Stock Unit Plan, subject to the approval of the Company’s Board of Directors. In the event that her employment with the Company is terminated by the Company without Cause (as defined in the Employment Agreement) or is terminated by Ms. Embree for Good Reason (as defined in the Employment Agreement) she will receive six (6) months’ salary as a Severance Payment.
The Employment Agreement for Mr. Casey provides for $144,000 annual salary, eligible to receive 147,000 shares of stock upon the successful fundraising of an amount equal to or greater than $5,000,000 and, as part of the Company’s Restricted Stock Unit Plan, further equity will be rewarded to Mr. Casey subject to the approval of the Company’s Board of Directors. In the event that his employment with the Company is terminated by the Company without Cause (as defined in the Employment Agreement) or is terminated by Mr. Casey for Good Reason (as defined in the Employment Agreement) he will receive six (6) months’ salary as a Severance Payment.
Stock Purchase Agreement
On June 28, 2023, the Company and Data Knights entered into a Securities Purchase Agreement (the “PIPE SPA”) with certain investors (collectively referred to herein as the “Purchasers”) for PIPE financing in the aggregate original principal amount of $1,595,744.70 and the purchase price of $1.5 million. Pursuant to the Securities Purchase Agreement, Data Knights will issue and sell to each of the Purchasers, a new series of senior secured convertible notes (the “PIPE Notes”), which are convertible into shares of Common Stock at the Purchasers election at a conversion price equal to the lower of (i) $10.00 per share, and (ii) 92.5% of the lowest volume weighted average trading price for the ten (10) Trading Days immediately preceding the Conversion Date. The Purchasers’ $1.5 million investment in the PIPE Notes closed and funded contemporaneous to the Closing of the Business Combination. Effective immediately prior to the Closing, Data Knights issued the PIPE Notes to the Purchasers pursuant to the private offering rules under the Securities Act of 1933, as amended (the “Securities Act”).
Government Regulation
Many aspects of our businesses are regulated by federal and state laws, rules and regulations. Accordingly, we maintain a robust compliance program aimed at ensuring we operate our business in compliance with all existing legal requirements material to the operation of our businesses. There are, however, occasionally uncertainties involving the application of various legal requirements, the violation of which could result in, among other things, fines or other sanctions. See “Risk Factors” for additional detail.
Regulation of Patient Information. Our information management services relate to the processing of information regarding patient diagnosis and treatment of disease and are, therefore, subject to substantial governmental regulation. In addition, the confidentiality of patient-specific information and the circumstances under which such patient-specific records may be released for inclusion in our databases or used in other aspects of our business is heavily regulated. Federal, state and foreign governments are contemplating or have proposed or adopted additional legislation governing the possession, use and dissemination of personal data, such as personal health information and personal financial data, as well as security breach notification rules for loss or theft of such data. Additional legislation or regulation of this type might, among other things, require us to implement additional security measures and processes or bring within the legislation or regulation deidentified health or other data, each of which may require substantial expenditures or limit our ability to offer some of our services.
In particular, personal health information is recognized as a special, sensitive category of personal information, subject to additional mandatory protections. Violations of data protection regulations are subject to administrative penalties, civil money penalties and criminal prosecution, including corporate fines and personal liability.
Data Privacy
Certain of our operations are subject to regulation under the administrative simplification provisions of the Health Insurance Portability and Accountability Act of 1996, as amended (HIPAA). Federal regulations related to HIPAA contain minimum standards for electronic transactions and code sets and for the privacy and security of protected health information. Patient health information is among the most sensitive of personal information, and it is critically important that information about an individual’s healthcare is properly protected from inappropriate access, use and disclosure. Real world evidence — information that allows us to examine actual practices and outcomes — is essential to increase access to care, improve outcomes, and lower costs.
OneMedNet uses a wide variety of privacy-enhancing technologies and safeguards to protect individual privacy while generating and analyzing information on a scale that helps healthcare stakeholders identify disease patterns and correlate with the precise treatment path and therapy needed for better outcomes. We employ a wide variety of methods to manage privacy requirements, including:
| ● | governance, frameworks, models and training to promote good decision making and accountability; | |
| ● | a layered approach to privacy and security management to avoid a single point of failure; | |
| ● | ongoing evaluation of privacy and security practices to promote continuous improvement; | |
| ● | use of technical, administrative, physical and organizational safeguards and controls; | |
| ● | collaboration with data suppliers and trusted third parties for our syndicated market research and analytics offerings to remove identifiable information or employ effective encryption or other techniques to render information non-identified before data is delivered to us; and | |
| ● | work with leading researchers, policy makers, thought leaders and others in a variety of fields relevant to the application of effective privacy and security practices, including statistical, epidemiological and cryptographic sciences, legal, information security and compliance, and privacy. |
| 13 |
We have relied on expertise in the industry with de-identifying data. Our capabilities allow us to render data non-identified while still maintaining data utility, thus protecting privacy while still advancing innovation. Not only do we make use of de-identification techniques with respect to the data we hold, but we also share our expertise in this area with policymakers, regulators and others to help them understand de-identification methodologies and practical considerations to avoid re-identification risk. We operate in more than 100 countries around the world, many of which have data protection and privacy laws and regulations based on similar core principles (e.g., openness, accountability, security safeguards, etc.). We apply those principles globally and augment our practices to address local laws, contractual obligations and other data privacy requirements.
Our Compliance team, led by our Chief Compliance Officer, is comprised of privacy professionals and privacy law experts who drive our strategy and develop and manage our policies and standards. The Compliance team provides subject matter expertise related to the proper management of all data types. In addition, our Compliance team liaises with our Legal, IT, Information Security and other teams so that privacy requirements are addressed in technology, contracting, offerings and other business activities.
The OneMedNet Privacy Policy (the “Privacy Policy”) is our foundational privacy policy. It explains how, when applicable, we collect, hold, use and disclose personal information, including that of our personnel, consumers, healthcare professionals, patients, medical research subjects, clinical investigators, customers, suppliers, vendors, business partners and investors.
Regulatory Quality Compliance (FDA 21 CFR Part 11)
OneMedNet provides high-quality, de-identified, regulatory-grade imaging and clinical data; as such OneMedNet adheres to all applicable local and Federal regulatory quality requirements, including but not limited to FDA 21 CFR Part 11. OneMedNet maintains a rigorous and ongoing internal quality management system to enable the organization to produce the highest quality regulatory compliant clinical data for our clients and consumers. This program includes:
| ● | Ongoing internal audits, policy reviews, and procedure testing to ensure validation, audit trails, legacy systems, and record handling and retention adhere to the latest regulatory guidelines and best practices. | |
| ● | Regular third-party or client initiated external audits to assess the compliance of OneMedNet to ensure operations are in accordance with, but not limited to the applicable regulations, standards, policies, and standard operation procedures. |
Organizational Structure
The following is a current organizational chart of our Company:
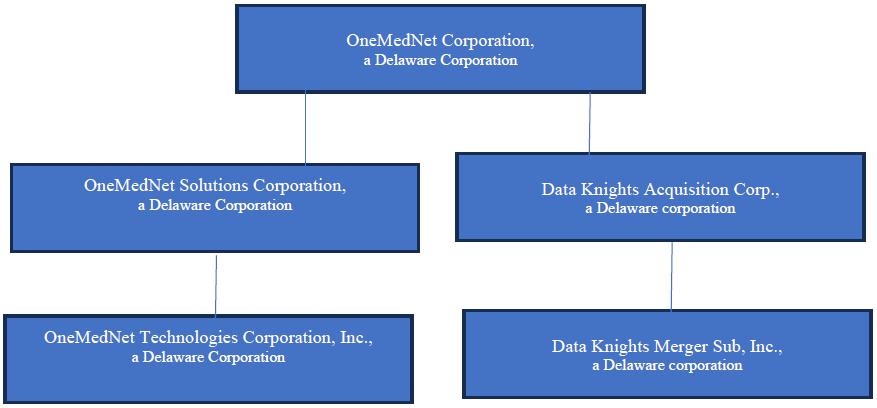
Human Capital Resources
Our workforce is comprised of approximately 20 employees (as of December 31, 2023), including approximately 0 part-time employees (references herein to “employees” include to the employees of our subsidiaries). Our Board of Directors and its committees oversee human capital matters through regular reporting from management and advisors.
| 14 |
Diversity, Equity and Inclusion
We are committed to fostering a culture of inclusion that embraces and supports our patients, colleagues, partners, physicians and communities. Our policies prohibit discrimination on the basis of age, gender, disability, race, color, ancestry, citizenship, religion, pregnancy, sexual orientation, gender identity or expression, national origin, medical condition, marital status, veteran status, payment source or ability, or any other basis prohibited by federal, state or local law.
Compensation and Benefits
We provide competitive compensation and benefits programs to help meet the needs of our employees. In addition to salaries, these programs (which vary by location) include a 2024 Stock Option Plan, a 401(k) Plan, health care and insurance benefits, health savings and flexible spending accounts, paid time off, family leave, family care resources, flexible work schedules, employee assistance programs, tuition and student loan assistance and on-site services, such as cafeterias and fitness centers, among many others.
Facilities
Prior to the closing of the Business Combination, the Company’s executive offices were located at Unit G6, Frome Business Park, Manor Road, Frome, United Kingdom, BA11 4FN and its telephone number was +44 203 833 4000. The Company agreed to pay ARC Group Ltd., an affiliate of the Sponsor, up to an amount of $10,000 per month for office space, secretarial and administrative support. For the nine months ended September 30, 2023 and 2022, we had incurred $60,000 in fees under this agreement, respectively. Upon completion of our Business Combination, the Company ceased paying these monthly fees.
After the closing of the Business Combination, our headquarters is located at 6385 Old Shady Oak Road, Suite 250, Eden Prairie, MN 55344 and our telephone number is (800) 918-7189, where we lease and occupy our office space with an aggregate floor area of approximately 67 square feet from unrelated third parties under operating lease agreements. We believe the current office space is adequate for our current operations and are adequate for our anticipated future needs.
Implications of Being an Emerging Growth Company
As a company with less than $1.235 billion in revenues during our last fiscal year, we qualify as an emerging growth company as defined in the Jumpstart Our Business Startups Act (“JOBS Act”) enacted in 2012. As an emerging growth company, we expect to take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| ● | being permitted to present only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure in this prospectus; | |
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (“Sarbanes-Oxley Act”); | |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and | |
| ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
Item 1A. Risk Factors
An investment in our securities involves a high degree of risk. This prospectus contains a discussion of the risks applicable to an investment in our securities. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these known or unknown risks might cause you to lose all or part of your investment in the offered securities. We may not be successful in preventing the material adverse effects that any of the following risks and uncertainties may cause. You could lose all or a significant portion of your investment due to any of these risks and uncertainties.
You should carefully consider the following risks, as well as the other information contained in this prospectus, including our historical financial statements and related notes included elsewhere in this prospectus before you decide to purchase our securities. Any one of these risks and uncertainties has the potential to cause material adverse effects on our business, prospects, financial condition and operating results which could cause actual results to differ materially from any forward-looking statements expressed by us and a significant decrease in the value of our Common Stock shares and warrants. Refer to “Cautionary Statement Regarding Forward-Looking Statements.”
Risks Related to this Offering and Our Common Stock
Our stock price may be volatile, and purchasers of our Common Stock could incur substantial losses.
The stock market in general has experienced significant price and volume fluctuations that have often been unrelated or disproportionate to operating performance of individual companies, particularly following a public offering of a company with a small public float. There is the potential for rapid and substantial price volatility of our Common Stock following this offering. These broad market factors may seriously harm the market price of our Common Stock, regardless of our actual or expected operating performance and financial condition or prospects, which may make it difficult for investors to assess the rapidly changing value of our Common Stock.
| 15 |
We are currently listed on The Nasdaq Global Market. If we are unable to maintain listing of our securities on Nasdaq or any stock exchange, our stock price could be adversely affected and the liquidity of our stock and our ability to obtain financing could be impaired and it may be more difficult for our stockholders to sell their securities.
Although our Common Stock is currently listed on The Nasdaq Global Market, we may not be able to continue to meet the exchange’s minimum listing requirements or those of any other national exchange. If we are unable to maintain listing on Nasdaq or if a liquid market for our Common Stock does not develop or is sustained, our Common Stock may remain thinly traded.
As previously reported on Form 8-K on February 9, 2024, the Company received written notice (the “Nasdaq Notice”), dated February 7, 2024, from Nasdaq indicating that for the preceding 30 consecutive business days, the market value of the Company’s listed securities (“MVLS”) did not maintain a minimum market value of $50,000,000 (the “Minimum MVLS Requirement”) as required by Nasdaq Listing Rule 5450(b)(2)(A). In accordance with Nasdaq Listing Rule 5810(c)(3)(C), the Company has a compliance period of 180 calendar days, or until August 5, 2024, to regain compliance with the Minimum MVLS Requirement. Compliance may be achieved if the Company’s MVLS closes at $50,000,000 or more for a minimum of ten consecutive business days at any time during the 180-day compliance period, in which case Nasdaq will notify the Company of its compliance and the matter will be closed.
If the Company does not regain compliance with the Minimum MVLS Requirement by August 5, 2024, Nasdaq will provide written notification to the Company that its common stock is subject to delisting. At that time, the Company may appeal the relevant delisting determination to a hearings panel pursuant to the procedures set forth in the applicable Nasdaq Listing Rules. However, there can be no assurance, if the Company does appeal the delisting determination by Nasdaq to the hearings panel, that such appeal would be successful. In such event, the Company may also seek to apply for a transfer to The Nasdaq Capital Market if it meets the requirements for continued listing thereon.
The Nasdaq Notice received have no immediate effect on the Company’s continued listing on the Nasdaq Global Market or the trading of Company’s common stock, subject to the Company’s compliance with the other continued listing requirements. The Company is presently evaluating potential actions to regain compliance with all applicable requirements for continued listing on the Nasdaq Global Market. There can be no assurance that the Company will be successful in maintaining the listing of its common stock on the Nasdaq Global Market.
The listing rules of Nasdaq require listing issuers to comply with certain standards in order to remain listed on its exchange. If, for any reason, we should fail to maintain compliance with these listing standards and Nasdaq should delist our securities from trading on its exchange and we are unable to obtain listing on another national securities exchange, a reduction in some or all of the following may occur, each of which could have a material adverse effect on our stockholders:
| ● | the liquidity of our Common Stock; | |
| ● | the market price of our Common Stock; | |
| ● | our ability to obtain financing for the continuation of our operations; | |
| ● | the number of institutional and general investors that will consider investing in our Common Stock; | |
| ● | the number of investors in general that will consider investing in our Common Stock; | |
| ● | the number of market makers in our Common Stock; | |
| ● | the availability of information concerning the trading prices and volume of our Common Stock; and | |
| ● | the number of broker-dealers willing to execute trades in shares of our Common Stock. |
Our principal stockholders will continue to have significant influence over the election of our board of directors and approval of any significant corporate actions, including any sale of the Company.
Our founders, executive officers, directors, and other principal stockholders, in the aggregate, beneficially own a majority of our outstanding stock. These stockholders currently have, and likely will continue to have, significant influence with respect to the election of our board of directors and approval or disapproval of all significant corporate actions. The concentrated voting power of these stockholders could have the effect of delaying or preventing an acquisition of the Company or another significant corporate transaction.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against companies following a decline in the market price of their securities. In 2020, 22% of securities class action litigation filings were against defendants in the health technology and services sector, which accounted for 22% of new filings. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
| 16 |
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the market price for the shares and trading volume could decline.
The trading market for our Common Stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. If research analysts do not establish and maintain adequate research coverage or if one or more of the analysts who covers us downgrades our Common Stock or publishes inaccurate or unfavorable research about our business, the market price for our Common Stock would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which, in turn, could cause the market price or trading volume for our common stock to decline.
We do not expect to pay dividends in the foreseeable future, and you must rely on price appreciation of your shares of Common Stock for return on your investment.
We have paid no cash dividends on any class of our stock to date, and we do not anticipate paying cash dividends in the near term. For the foreseeable future, we intend to retain any earnings to finance the development and expansion of our business, and we do not anticipate paying any cash dividends on our stock. Accordingly, investors must be prepared to rely on sales of their shares after price appreciation to earn an investment return, which may never occur. Investors seeking cash dividends should not purchase our shares. Any determination to pay dividends in the future will be made at the discretion of our board of directors and will depend on our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board deems relevant.
Future sales of substantial amounts of our Common Stock or securities convertible into or exchangeable or exercisable for shares of Common Stock, either by us or by our existing stockholders, or the possibility that such sales could occur, could adversely affect the market price of our Common Stock.
Future sales in the public market of shares of our Common Stock or securities convertible into or exchangeable or exercisable for shares of Common Stock, shares held by our existing stockholders or shares issued upon exercise of our outstanding stock options or warrants, or the perception by the market that these sales could occur, could lower the market price of our Common Stock or make it difficult for us to raise additional capital.
We are an “emerging growth company,” and the reduced reporting requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act (“the JOBS Act”). For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including exemption from compliance with the auditor attestation requirements of Section 404, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the closing of our initial public offering, (b) in which we have total annual gross revenue of at least $1.235 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock held by non-affiliates exceeds $700 million as of the end of our prior second fiscal quarter, and (2) the date on which we have issued more than $1 billion in non-convertible debt during the prior three-year period.
In addition, under the JOBS Act, emerging growth companies may delay adopting new or revised accounting standards until such time as those standards apply to private companies. We may elect not to avail ourselves of this exemption from new or revised accounting standards and, therefore, may be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our share price may be more volatile.
Anti-takeover provisions contained in our certificate of incorporation and bylaws as well as provisions of Delaware law, could impair a takeover attempt.
Our certificate of incorporation, bylaws and Delaware law contain provisions which could have the effect of rendering more difficult, delaying or preventing an acquisition deemed undesirable by our board of directors. Our corporate governance documents include provisions:
| ● | authorizing “blank check” preferred stock, which could be issued by our board of directors without stockholder approval and may contain voting, liquidation, dividend, and other rights superior to our common stock; | |
| ● | limiting the liability of, and providing indemnification to, our directors and officers; | |
| ● | limiting the ability of our stockholders to call and bring business before special meetings; | |
| ● | requiring advance notice of stockholder proposals for business to be conducted at meetings of our stockholders and for nominations of candidates for election to our board of directors; | |
| ● | controlling the procedures for the conduct and scheduling of board of directors and stockholder meetings; and | |
| ● | providing our board of directors with the express power to postpone previously scheduled annual meetings and to cancel previously scheduled special meetings. |
| 17 |
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. As a Delaware corporation, we are also subject to provisions of Delaware law, including Section 203 of the Delaware General Corporation law, which prevents some stockholders holding more than 15% of our outstanding common stock from engaging in certain business combinations without approval of the holders of substantially all of our outstanding common stock.
Any provision of our certificate of incorporation, bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our Common Stock and could also affect the price that some investors are willing to pay for our Common Stock.
Our Business Risks
We have a history of operating losses and may never achieve profitability in the future.
We have experienced net losses in each annual period since inception. We generated net losses of $23.2 million and $6.2 million for the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023, we had accumulated losses of approximately $55.1 million.
We expect to continue to incur significant losses in the development, marketing, sale and delivery of our services. If we do not grow our revenues or if we lose existing customers, we expect to continue to incur losses from operations for the foreseeable future. Because of the numerous risks and uncertainties associated with the development, marketing, sale and delivery of our imaging real world data (“iRWDTM”) services, we may experience larger than expected future losses and may never become profitable. Moreover, there is a substantial risk that we may not be able to successfully commercialize our iRWDTM services, which would make it unlikely that we would ever achieving profitability.
OneMedNet believes it has demonstrated its quality and responsiveness in clinical imaging and curation of Real-World Data based upon success in compiling one of the largest networks of imaging centers (comprised of hospitals, imaging centers and clinics) throughout the United States covering more than 15 million patients to date. On the global front, OneMedNet works with hospitals and life science companies around the world including Ireland, United Kingdom, Ghana, Denmark and South Korea and growing. We base these claims on our understanding of our competition in the United States and globally. However, if we were to lose these relationships with our network of imaging centers or lose our customers or our competitors’ technology surpasses ours, our competitors could claim a greater market share domestically or abroad, which could reduce our growth and our profits, which could harm our business, financial position, results of operations and prospects.
Two significant customers represented 53% and 52% of our revenues for 2022 and 2023 respectively, and is expected to continue to represent a significant portion of our forecasted revenue for 2024.
Change Healthcare and Siemens Medical Solutions USA, collectively represented 53% and 52% of our revenues in 2023 and 2022, respectively. Change Healthcare is expected to continue to represent a significant portion of our forecasted revenue for 2024. If we fail to maintain and grow our relationships with Change Healthcare, we could lose a significant portion of our revenue for 2023, which would materially adversely affect our results of operations and our business. If OneMedNet were to lose one or more of its significant customers, its revenue may significantly decline. In addition, revenue from significant customers may vary from period to period depending on the timing of renewing existing agreements or entering into new agreements for additional OneMedNet products as well as other unforeseen risks and variables discussed in this proxy statement/prospectus. The loss of one or more of OneMedNet’s significant customers could adversely affect its business, results of operations and financial condition. You should not rely on our historical relationship with these companies as an indication of our future performance.
We may encounter difficulties in managing our attempted growth of our business, which could negatively impact our operations.
As we expand, market, sell and deliver our service offerings, we anticipate that we will need to increase our service development, sales and marketing and administrative headcount. Such an evolution may impact our strategic focus and our deployment and allocation of resources. Our ability to manage our operations and growth effectively depends upon the continual improvement of our procedures, reporting systems and operational, financial and management controls. We may not be able to implement administrative and operational improvements in an efficient or timely manner and may discover deficiencies in existing systems and controls. If we do not meet these challenges, we may be unable to execute our business strategies and may be forced to expend more resources than anticipated addressing these issues.
We may acquire additional technology and complementary businesses in the future. Acquisitions involve many risks, any of which could materially harm our business, including the diversion of management’s attention from core business concerns, failure to effectively exploit acquired technologies, failure to successfully integrate the acquired business or realize expected synergies or the loss of key employees from either our business or the acquired businesses.
| 18 |
We may be unable to execute our business objectives and growth strategies successfully or sustain our growth and, as a result, this could have a material adverse effect on our operating results.
The highly complex nature of our industry requires that we effectively execute and manage our business objectives and growth strategies, such as expanding our marketing and commercialization of our services in the U.S. and internationally, adding new customers, and increasing our service delivery capacity. However, we may not be able to execute on these strategies as effectively as anticipated. Our ability to execute on these strategies depends on a number of factors, including, without limitation:
| ● | our ability to obtain adequate capital resources to complete execute our growth plans; | |
| ● | our ability to hire, train and retain skilled managers and personnel, including quality and production personnel, and marketing and commercial specialists; | |
| ● | our ability to protect our existing and new services by registering and defending our intellectual property rights; and | |
| ● | our ability to successfully add new customers. |
To the extent we are unable to execute on our growth strategies in accordance with our expectations, this could have a material adverse effect on our business, financial condition, and future results of operations.
The real-world data and real-world evidence business market continues to evolve, is highly competitive, and we may not be successful in competing in this industry or establishing and maintaining confidence in our long-term business prospects among current and future partners and customers.
The real-world data and real-world evidence business market in which we compete continues to evolve and is highly competitive. To date, we have focused our efforts on its expertise in clinical imaging innovation solutions that connects healthcare providers and patients and satisfies a crucial need with the life sciences. We offer direct access to clinical images and associated contextual patient record. OneMedNet proved the commercial and regulatory viability of imaging Regulatory Grade Real-World Data (“iRWDTM”), a promising emerging market, that exactly matches OneMedNet’s life science partners’ case selection protocol. OneMedNet has the immediate ability to quickly search and extensively curate multi-layer data from a federated group of healthcare facilities and to provide fast access to curated medical images that has proved the commercial and regulatory viability of imaging RWD and covers the complete value chain in imaging RWD, validated by an increasing federated network of providers. However, real-world data and real-world evidence has been increasingly adopted and our current competitors have, and future competitors may have, greater resources than we do and may also be able to devote greater resources to the development of their current and future technologies. These competitors also may have greater access to customers and may be able to establish cooperative or strategic relationships amongst themselves or with third parties that may further enhance their resources and competitive positioning.
Developments in improvements in real-world data and real-world evidence curation by competitors may materially adversely affect the sales, pricing and gross margins of our business. If a competing technology or process is developed that has superior operational or price performance, our business will be harmed. Similarly, if we fail to accurately predict and ensure that our real-world data and real-world evidence offering can address customers’ changing needs or emerging technological trends, or if our customers fail to achieve the benefits expected from our real-world data and real-world evidence offering, our business will be harmed.
We must continue to commit resources to develop our real-world data and real-world evidence technology in order to establish a competitive position, and these commitments will be made without knowing whether such investments will result in products potential customers will accept. There is no assurance we will successfully identify new customer requirements, develop and bring our real-world data and real-world evidence to market on a timely basis, or that products and technologies developed by others will not render our real-world data and real-world evidence obsolete or noncompetitive, any of which would adversely affect our business and operating results.
If we are unable to attract and retain key employees and qualified personnel, our ability to compete could be harmed.
We depend on the talents and continued efforts of our senior management and key employees. The loss of members of our management or key employees may disrupt our business and harm our results of operations. Further, our ability to manage further expansion will require us to continue to attract, motivate and retain additional qualified personnel. Competition for this type of personnel is intense, and we may not be successful in attracting, integrating and retaining the personnel required to grow and operate our business effectively. There can be no assurance that our current management team or any new members of our management team will be able to successfully execute our business and operating strategies.
Our operations could be damaged or adversely affected as a result of natural disasters and other catastrophic events.
Our operations could be adversely affected by events outside of our control, such as natural disasters, wars, health epidemics such as the ongoing COVID-19 pandemic, and other calamities. We cannot assure you that any backup systems will be adequate to protect us from the effects of fire, floods, typhoons, earthquakes, power loss, telecommunications failures, break-ins, war, riots, terrorist attacks or similar events. Any of the foregoing events may give rise to interruptions, breakdowns, system failures, technology platform failures or internet failures, which could cause the loss or corruption of data or malfunctions of software or hardware as well as adversely affect our ability to provide services.
| 19 |
Any financial or economic crisis, or perceived threat of such a crisis, including a significant decrease in consumer confidence, may materially and adversely affect our business, financial condition, and results of operations.
In recent years, the United States and global economies suffered dramatic downturns as the result of the COVID-19 pandemic, a deterioration in the credit markets and related financial crisis as well as a variety of other factors including, among other things, extreme volatility in security prices, severely diminished liquidity and credit availability, ratings downgrades of certain investments and declining valuations of others. The United States and certain foreign governments have taken unprecedented actions in an attempt to address and rectify these extreme market and economic conditions by providing liquidity and stability to the financial markets. If the actions taken by these governments are not successful, the return of adverse economic conditions may negatively impact the demand for iRWDTM offering and may negatively impact our ability to raise capital, if needed, on a timely basis and on acceptable terms or at all.
Our ability to utilize our net operating loss and tax credit carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Section 382 of the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to use its pre-change net operating loss carryforwards (“NOLs”), to offset future taxable income. The limitations apply if a corporation undergoes an “ownership change,” which is generally defined as a greater than 50 percentage point change (by value) in its equity ownership by certain stockholders over a three-year period. If we have experienced an ownership change at any time since our incorporation, we may already be subject to limitations on our ability to utilize our existing NOLs and other tax attributes to offset taxable income or tax liability. In addition, the Business Combination and future changes in our stock ownership, which may be outside of our control, may trigger an ownership change. Similar provisions of state tax law may also apply to limit our use of accumulated state tax attributes. As a result, even if we earn net taxable income in the future, our ability to use these or our pre-change NOL carryforwards and other tax attributes to offset such taxable income or tax liability may be subject to limitations, which could potentially result in increased future income tax liability to us.
There is also a risk that changes in law or regulatory changes made in response to the need for some jurisdictions to raise additional revenue to help counter the fiscal impact from unforeseen reasons, including suspensions on the use of net operating losses or tax credits, possibly with retroactive effect, may result in our existing net operating losses or tax credits expiring or otherwise being unavailable to offset future income tax liabilities.
We are subject to many hazards and operational risks that can disrupt our business, some of which may not be insured or fully covered by insurance.
Our operations are subject to many hazards and operational risks inherent to our business, including: (a) general business risks; (b) warranty liability; and (c) damage to third parties (e.g., our vendors), our infrastructure or properties caused by fires, floods and other natural disasters, power losses, telecommunications failures, terrorist attacks, riots, cyberattacks, public health crises such as the current COVID-19 pandemic (and other future pandemics or epidemics), human errors and similar events. As a result of the COVID-19 outbreak, or similar pandemics, we have and may in the future experience disruptions that could severely impact our business and the business of our customers.
Our insurance coverage may be inadequate to cover our liabilities related to such hazards or operational risks. For example, we do not currently maintain cybersecurity insurance and our insurance providers may take the position that our coverage, under present circumstances, does not extend to business interruptions as they relate to the COVID-19 pandemic. In addition, we may not be able to maintain adequate insurance in the future at rates we consider reasonable and commercially justifiable, and insurance may not continue to be available on terms as favorable as our current arrangements. The occurrence of a significant uninsured claim or a claim in excess of the insurance coverage limits maintained by us could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Being a Public Company
Our management has limited experience in operating a public company.
Our executive officers have limited experience in the management of a publicly traded company. Our management team may not successfully or effectively manage our transition to a public company that will be subject to significant regulatory oversight and reporting obligations under federal securities laws. Their limited experience in dealing with the increasingly complex laws pertaining to public companies could be a significant disadvantage in that it is likely that an increasing amount of their time may be devoted to these activities which will result in less time being devoted to the management and growth of our Company. We may not have adequate personnel with the appropriate level of knowledge, experience, and training in the accounting policies, practices or internal controls over financial reporting required of public companies in the United States. The development and implementation of the standards and controls necessary for us to achieve the level of accounting standards required of a public company in the United States may require costs greater than expected. It is possible that we will be required to expand our employee base and hire additional employees to support our operations as a public company which will increase our operating costs in future periods.
We will incur significant increased expenses and administrative burdens as a public company, which could have an adverse effect on our business, financial condition and results of operations.
We will face increased legal, accounting, administrative and other costs and expenses as a public company that legacy OneMedNet Corporation did not incur as a private company. The Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), including the requirements of Section 404, as well as rules and regulations subsequently implemented by the SEC, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and the rules and regulations promulgated and to be promulgated thereunder, the PCAOB and the securities exchanges, impose additional reporting and other obligations on public companies. Compliance with public company requirements will increase costs and make certain activities more time-consuming. A number of those requirements will require us to carry out activities we have not done previously. For example, we have created new Board committees and adopted new internal controls and disclosure controls and procedures. In addition, expenses associated with SEC reporting requirements will be incurred. Furthermore, if any issues in complying with those requirements are identified (for example, if the auditors identify a material weakness or significant deficiency in the internal control over financial reporting), we could incur additional costs rectifying those issues, and the existence of those issues could adversely affect our reputation or investor perceptions of it. It may also be more expensive to obtain director and officer liability insurance. Risks associated with our status as a public company may make it more difficult to attract and retain qualified persons to serve on our Board or as executive officers. The additional reporting and other obligations imposed by these rules and regulations will increase legal and financial compliance costs and the costs of related legal, accounting and administrative activities. These increased costs will require us to divert a significant amount of money that could otherwise be used to expand the business and achieve strategic objectives. Advocacy efforts by stockholders and third parties may also prompt additional changes in governance and reporting requirements, which could further increase costs.
| 20 |
If securities or industry analysts do not publish or cease publishing research or reports about us, our business, or the market in which we operate, or if they change their recommendations regarding our securities adversely, the price and trading volume of our securities could decline.
The trading market for our securities will be influenced by the research and reports that industry or securities analysts may publish about us, our business, market or competitors. Securities and industry analysts do not currently, and may never, publish research on us. If no securities or industry analysts commence coverage of us, our share price and trading volume would likely be negatively impacted. If any of the analysts who may cover us change their recommendation regarding our shares of Common Stock adversely, or provide more favorable relative recommendations about our competitors, the price of our shares of Common Stock would likely decline. If any analyst who may cover us were to cease our coverage of us or fail to regularly publish reports on it, we could lose visibility in the financial markets, which in turn could cause our share price or trading volume to decline.
Our Common Stock may be subject to extreme volatility.
The trading price of our Common Stock may be subject to extreme volatility. We cannot predict the magnitude of future fluctuations in the trading price of our Common Stock. The trading price of our Common Stock may be affected by a number of factors, including events described in the risk factors set forth in this prospectus and in our periodic reports filed with the SEC from time to time, as well as our operating results, financial condition and other events or factors. Any of the factors listed below could have a material adverse effect on your investment in our securities. Factors affecting the trading price of our securities may include:
| ● | announcements by us or our competitors regarding technical developments and levels of performance achieved by our or their real-world data and real-world evidence offering; | |
| ● | announcements by us regarding developments in our relationship with existing and future key customers; | |
| ● | our ability to bring our products and technologies to market on a timely basis, or at all; | |
| ● | our operating results or development efforts failing to meet the expectation of securities analysts or investors in a particular period; | |
| ● | Actual or anticipated fluctuations in our quarterly financial results or the quarterly financial results of companies perceived to be similar to it; | |
| ● | changes in the market’s expectations about our operating results or the real-world data and real-world evidence industry; | |
| ● | success of competitors actual or perceived development efforts; | |
| ● | changes in financial estimates and recommendations by securities analysts concerning the Company or the real-world data and real-world evidence industry in general; | |
| ● | operating and share price performance of other companies that investors deem comparable to the Company; | |
| ● | disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain intellectual property protection for our technologies; | |
| ● | changes in laws and regulations affecting our business; | |
| ● | our ability to meet compliance requirements; | |
| ● | commencement of, or involvement in, litigation involving the Company; | |
| ● | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; | |
| ● | the volume of shares of Common Stock available for public sale; | |
| ● | the level of demand for our Common Stock, including the amount of short interest in our stock; | |
| ● | any major change in our Board or management; | |
| ● | sales of substantial amounts of the shares of Common Stock by our directors, executive officers or significant stockholders or the perception that such sales could occur; | |
| ● | the expiration of contractual lock-up agreements with our executive officers, directors and stockholders, which we have entered into and may enter into in the future from time to time; and | |
| ● | general economic and political conditions such as recessions, interest rates, fuel prices, international currency fluctuations and acts of war or terrorism. |
| 21 |
Broad market and industry factors may materially harm the market price of our securities irrespective of our operating performance. The stock market in general, and the Nasdaq in particular, have experienced price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of the particular companies affected. The trading prices and valuations of these stocks, and of our securities, may not be predictable. A loss of investor confidence in the market for retail stocks or the stocks of other companies which investors perceive to be similar to the Company could depress our share price regardless of our business, prospects, financial conditions or results of operations. A decline in the market price of our securities also could adversely affect our ability to issue additional securities and our ability to obtain additional financing in the future.
Following certain periods of volatility in the market price of our securities, we may become subject of securities litigation. We have experienced, and may in the future experience additional litigation following periods of volatility. This type of litigation may result in substantial costs and a diversion of management’s attention and resources.
Our business model is capital-intensive, and we may not be able to raise additional capital on attractive terms, if at all, which could be dilutive to stockholders. If we cannot raise additional capital when needed, our operations and prospects could be materially and adversely affected.
We can be expected to continue to sustain substantial operating expenses without generating sufficient revenues to cover expenditures. Over time, we expect that we will need to raise additional funds, including through the issuance of equity, equity-related or debt securities or through obtaining credit from financial institutions to fund, together with our principal sources of liquidity, ongoing costs, any significant unplanned or accelerated expenses, and new strategic investments. We cannot be certain that additional capital will be available on attractive terms, if at all, when needed, which could be dilutive to stockholders, and our financial condition, results of operations, business and prospects could be materially and adversely affected.
Risks Related to Our Warrants
We may redeem unexpired Warrants prior to their exercise at a time that is disadvantageous to Warrantholders.
Our public Warrants are currently exercisable for one share of Common Stock at a price of $11.50 per share. We have the ability to redeem outstanding Warrants at any time prior to their expiration, at a price of $0.01 per Warrant, provided that the last reported sales price of Common Stock equals or exceeds $18.00 per share for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date we send the notice of redemption to Warrantholders and provided certain other conditions are met. If and when the Warrants become redeemable by us, we may exercise our redemption rights even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws. As a result, we may redeem the Warrants, as set forth above even if the holders are otherwise unable to exercise the Warrants.
Redemption of the outstanding Warrants could force Warrantholders (i) to exercise their Warrants and pay the exercise price therefor at a time when it may be disadvantageous for them to do so, (ii) to sell their Warrants at the then-current market price when they might otherwise wish to hold their Warrants or (iii) to accept the nominal redemption price which, at the time the outstanding Warrants are called for redemption, we expect would be substantially less than the market value of their Warrants. None of the private placement Warrants will be redeemable by us so long as they are held by the Sponsor or its permitted transferees.
Item 1b. Unresolved Staff Comments
None.
Item 1c. Cybersecurity
OneMedNet manages cybersecurity and data protection through a continuously evolving framework. The framework allows us to identify, assess and mitigate the risks we face, and assists us in establishing policies and safeguards to protect our systems and the information of those we serve. Our cybersecurity program is managed by our Director Product Management, Head of Data. The Audit Committee of the Board of Directors has oversight of our cybersecurity program and is responsible for reviewing and assessing the Company’s cybersecurity and data protection policies, procedures and resource commitment, including key risk areas and mitigation strategies. As part of this process, the Audit receives regular updates from the Director Product Management, Head of Data on critical issues related to our information security risks, cybersecurity strategy, supplier risk and business continuity capabilities. The Company’s framework includes an incident management and response program that continuously monitors the Company’s information systems for vulnerabilities, threats and incidents; manages and takes action to contain incidents that occur; remediates vulnerabilities; and communicates the details of threats and incidents to management, including the Director Product Management, Head of Data, as deemed necessary or appropriate. Pursuant to the Company’s incident response plan, any incidents are to be reported to the Audit Committee, appropriate government agencies and other authorities, as deemed necessary or appropriate, considering the actual or potential impact, significance and scope.
| 22 |
We employ an array of data security technologies, processes, and methods across our infrastructure to protect systems and sensitive information from unauthorized access. OneMedNet maintains comprehensive identity and access management practices (e.g., roles and access privileges for each user; multi-factor authentication, privileged user accounts, single sign-on, user lifecycle management) and employs a variety of security information and event management tools. We developed, maintain and utilize a global integrated information security framework to guide our practices, based on relevant industry frameworks and laws, including, but not limited to NIST, GxP, HITRUST, the ISO 27000 family, COBIT, GDPR, and HIPAA.
The framework consists of policies, standards, procedures, work Instructions and documentation. Information is classified into four categories to help individuals apply the right level of controls and safeguards to information, applications and systems. Our cybersecurity program focuses on all areas of our business, including cloud-based environments, data centers, devices used by employees and contractors, facilities, networks, applications, vendors, disaster recovery / business continuity and controls and safeguards enabled through business processes and tools. We continuously monitor for threats and unauthorized access.
We draw on the knowledge and insight of external cybersecurity experts and vendors, and internally employ dedicated, certified, cybersecurity staff, such as but not limited to, CISSP, CISM, CISA, CSSP or other equivalent certifications, that leverage an array of third-party tools to secure OneMedNet information infrastructure and protect systems and information from unauthorized access. Non-technical safeguards also play an important role in our cybersecurity program. We provide various training programs and tools to employees so they can avoid risky practices and help us promptly identify potential or actual issues. We also have global incident response procedures, global service tools to log incidents and issues for investigation, and an ethics line to report concerns and follow-up on matters already reported. The Compliance team, led by our Chief Compliance Officer, develops and implements our strategy, as well as monitors systems and devices for risks and threats.
Item 2. Properties
Our corporate headquarters is in Eden Prairie, Minnesota is leased on a month-to-month basis.
Item 3. Legal Proceedings
We may be subject from time to time to various claims, lawsuits and other legal and administrative proceedings arising in the ordinary course of business. Some of these claims, lawsuits and other proceedings may involve highly complex issues that are subject to substantial uncertainties, and could result in damages, fines, penalties, non-monetary sanctions or relief.
We do not currently expect the results of any of these matters to have a material effect on our business, results of operations, financial condition or cash flows.
We intend to recognize provisions for claims or pending litigation when we determine that an unfavorable outcome is probable, and the amount of loss can be reasonably estimated. Due to the inherent uncertain nature of litigation, the ultimate outcome or actual cost of settlement may materially vary from estimates. See “Risk Factors—Other Risks—Any future litigation against us could be costly and time-consuming to defend.”
Item 4. Mine Safety Disclosures
Not applicable.
| 23 |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information for Common Stock and Warrants
Our Common Stock is traded on The Nasdaq Global Select Market under the symbol “ONMD”. Our Public Warrants, each entitling the holder to purchase one share of our Common Stock are traded on traded on The Nasdaq Global Select Market under the symbol “ONMDW”.
Holders of our Common Stock
As of April 2, 2024, there were approximately 122 holders of record of our Common Stock. Certain shares of our Common Stock are held in “street” name and, accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number. The number of holders of record also does not include beneficial owners of shares that are be held in trust by other entities.
Dividend Policy
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends in the foreseeable future.
| 24 |
Issuer Purchases of Equity Securities
There were no purchases of equity securities by the issuer or affiliated purchasers, as defined in Rule 10b-18(a)(3) the Securities Exchange Act of 1934, during the quarter ended December 31, 2023.
Performance Graph
We are a “smaller reporting company,” as defined by Item 10(f)(1) of Regulation S-K, and therefore are not required to provide the information required by paragraph (e) of Item 201 of Regulation S-K.
Recent Sales of Unregistered Securities
On June 28, 2023, the Company executed a Securities Purchase Agreement for PIPE financing in the aggregate original principal amount of $1,595,744.70 and a purchase price of $1.5 million. Pursuant to the Securities Purchase Agreement, the Company agreed to issue and sell to each of Thomas Kosasa, Dr. Jeffrey Yu, Aaron Green and Steve Kester (the “PIPE Investors”), a new series of senior secured convertible notes (the “PIPE Notes”), which Notes shall be convertible into shares of Common Stock at the PIPE Investors election at the conversion price (rounded to the nearest 1/100th of one cent) which shall be computed as the lesser of:
(a) with respect to a conversion pursuant to Section 4.1 of the Securities Purchase Agreement (discussed below), the lesser of: (i) a price per share equal to the product of (x) 100% less the Discount and (y) the lowest per share purchase price of the Equity Securities issued in the Next Equity Financing; and (ii) $2.50 per share; and
(b) with respect to a conversion pursuant to Section 4.2 (discussed below), (relating to payment at maturity) or Section 4.3, $2.50 per share. The Securities Purchase agreement provided that the PIPE Investors’ $1.5 million investment in the PIPE Notes would close and fund contemporaneous to the Closing of the Business Combination.
Section 4.1 of the Securities Purchase Agreement provides that the principal balance and unpaid accrued interest on each Note will automatically convert into the PIPE Conversion Shares upon the closing of the Next Equity Financing (“Next Equity Financing” means the next sale or series of related sales by the Company of its Common Stock in one or more offerings relying on Section 4(a)(2) of the Securities Act or Regulation D thereunder for exemption from the registration requirements of Section 5 of the Securities Act, from which the Company receives gross proceeds of not less than US$5,000,000 (excluding, for the avoidance of doubt, the aggregate principal amount of the Notes).
Section 4.2 of the Securities Purchase Agreement provides that in the event of a Corporate Transaction or the repayment of such Note, at the closing of a corporate transaction, the holder of each Note may elect that either: (a) the Company will pay the holder of such Note an amount equal to the sum of (x) the outstanding principal balance of such Note, and (y) a premium equal to 20% of the outstanding principal balance of such Note (which premium, is in lieu of all accrued and unpaid interest due on such Note); or (b) such Note will convert into that number of Conversion Shares equal to the quotient (rounded down to the nearest whole share) obtained by dividing (x) the outstanding principal balance and unpaid accrued interest of such Note on a date that is no more than five days prior to the closing of such corporate transaction by (y) the applicable Conversion Price.
Notwithstanding the foregoing, any sale (or series of related sales) of the Company’s Equity Securities to a special purpose acquisition company will not be deemed a “Next Equity Financing. Notwithstanding the foregoing, the Company may, at its option, pay any unpaid accrued interest on each Note in cash at the time of conversion. The number of PIPE Conversion Shares the Company issues upon such conversion will equal the quotient (rounded down to the nearest whole share) obtained by dividing (x) the outstanding principal balance and unpaid accrued interest under each converting Note on a date that is no more than five days prior to the closing of the Next Equity Financing by (y) the applicable Conversion Price. At least five days prior to the closing of the Next Equity Financing, the Company will notify the holder of each Note in writing of the terms of the Equity Securities that are expected to be issued in such financing. The issuance of PIPE Conversion Shares pursuant to the conversion of each Note will be on, and subject to, the same terms and conditions applicable to the Equity Securities issued in the Next Equity Financing.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required by Item 5 of Form 10-K regarding equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report.
Item 6. [Reserved]
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read in conjunction with our consolidated financial statements and notes thereto that appear elsewhere in this Annual Report on Form 10-K. See “Risk Factors” elsewhere in this Annual Report on Form 10-K for a discussion of certain risks associated with our business. The following discussion contains forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. The use of words such as “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. From time to time, we also may provide forward-looking statements in other materials we release to the public. Unless the context otherwise requires, references in this Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations” to “OneMedNet Corporation,” “we,” “us,” “our” and the “Company” are intended to mean the business and operations of OneMedNet Corporation.
| 25 |
Company Overview
Founded in 2009, we provide innovative solutions that unlock the significant value contained within the clinical image archives of healthcare providers. Employing our proven OneMedNet iRWD™ solution, we securely de-identifies, searches, and curates a data archive locally, bringing a wealth of internal and third-party research opportunities to providers. By leveraging this extensive federated provider network, together with industry leading technology and in-house clinical expertise, OneMedNet successfully meets the most rigorous RWD Life Science requirements.
Business Combination
On November 7, 2023, we held the closing of the previously announced merger (the “Merger”) whereby Data Knights Merger Sub, Inc., merged with and into OneMedNet Solutions Corporation (formerly named OneMedNet Corporation), with OneMedNet Solutions Corporation continuing as the surviving entity, which resulted in all of the issued and outstanding capital stock of OneMedNet Solutions Corporation being exchanged for shares of the Company’s Common Stock upon the terms set forth in the Merger Agreement (collectively, the “the Business Combination”). The Merger and other transactions that closed on November 7, 2023, pursuant to the Merger Agreement, led to Data Knights changing its name to “OneMedNet Corporation” and the business of the Company became the business of OneMedNet Solutions Corporation.
Pursuant to the terms of the Merger Agreement, the total consideration for the Business Combination and related transactions (the “Merger Consideration”) was approximately $200 million. In connection with the Special Meeting, certain public holders (the “Redeeming Stockholders”) holding 1,600,741 shares of Common Stock exercised their right to redeem such shares for a pro rata portion of the funds held by Continental Stock Transfer & Trust Company, as trustee (“Continental”) in the trust account established in connection with Data Knights’ initial public offering (the “Trust Account”). Effective November 7, 2023, Data Knights’ units ceased trading, and effective November 8, 2023, OneMedNet’s common stock began trading on the Nasdaq Global Market under the symbol “ONMD” and the warrants began trading on the Nasdaq Global Market under the symbol “ONMDW.”
As a result of the Merger and the Business Combination, holders of Data Knights common stock automatically received common stock of OneMedNet, and holders of Data Knights warrants automatically received warrants of OneMedNet with substantively identical terms. At the Closing of the Business Combination, all shares of Data Knights owned by the Sponsor (consisting of shares of Common Stock and shares of Class B common stock, which we refer to as the founder shares), automatically converted into an equal number of shares of OneMedNet’s Common Stock, and the Private Placement Warrants held by the Sponsor, automatically converted into warrants to purchase one share of OneMedNet Common Stock with substantively identical terms.
Key Components of Consolidated Statements of Operations
Revenue
The Company generates revenue from two streams: (1) iRWD (imaging Real World Data) which provides regulatory grade imaging and clinical data in the Pharmaceutical, Device Manufacturing, CRO’s and AI markets and (2) BEAM which is a Medical Imaging Exchange platform between Hospital/Healthcare Systems, Imaging Centers, Physicians and Patients. iRWD is sold on a fixed fee basis based on the number of data units and the cost per data unit committed to in the customer contract. Revenue is recognized when the data is delivered to the customer. Beam revenue is subscription-based revenue which is recognized ratably over the subscription period committed to by the customer. The Company invoices its Beam customers quarterly or annually in advance with the customer contracts automatically renewing unless the customer issues a cancellation notice.
The Company excludes from revenue taxes collected from a customer that are assessed by a governmental authority and imposed on and concurrent with a specific revenue-producing transaction. The transaction price for the products is the invoiced amount. Advanced billings from contracts are deferred and recognized as revenue when earned. Deferred revenue consists of payments received in advance of performance under the contract. Such amounts are generally recognized as revenue over the contractual period. The Company receives payments from customers based upon contractual billing schedules. Accounts receivable is recorded when the right to consideration becomes unconditional. Payment terms on invoiced amounts typically range from zero to 90 days, with typical terms of 30 days.
Cost of Revenue
Our cost of revenue is composed of our distinct performance obligations of hosting, labor, and data cost.
| 26 |
General, and Administrative
General and administrative functions, includes finance, legal, human resources, and information technology support. These functions include costs for items such as salaries and benefits and other personnel-related costs, maintenance and supplies, professional fees for external legal, accounting, and other consulting services, and depreciation expense.
Operation services
Operations consists primarily of labor cost for our operations team who provides services to our customers.
Research and Development
Costs incurred in the research and development of our products are expensed as incurred. Research and development costs include personnel, contracted services, materials, and indirect costs involved in the design and development of new products and services, as well as hosting expense.
Sales & Marketing
Our sales and marketing costs consist of labor and tradeshow costs.
Interest Expense
Interest incurred on convertible notes and shareholder loans.
Other Expense
Foreign exchange and tax expenses related to the Company’s operations and revenue outside of the United States.
Results of Operations
The following tables set forth our Consolidated Statements of Operations data for the periods presented:
| Year Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Revenue | $ | 1,021,651 | $ | 1,152,738 | ||||
| Cost of Revenue | 1,149,551 | 1,513,428 | ||||||
| Gross Margin | (127,900 | ) | (360,690 | ) | ||||
| Operating Expenses | ||||||||
| General and administrative | 5,273,503 | 8,755,620 | ||||||
| Operations | 226,257 | 398,760 | ||||||
| Sales & Marketing | 1,114,977 | 957,690 | ||||||
| Research and Development | 1,631,613 | 952,701 | ||||||
| Total Operating Expenses | 8,246,350 | 11,064,771 | ||||||
| Operating loss | (8,374,250 | ) | (11,425,461 | ) | ||||
| Other Expense (income) | ||||||||
| Impairment | 10,504,327 | - | ||||||
| Income tax provision | - | 214,850 | ||||||
| Interest expense | 749,213 | 403,307 | ||||||
| Other expense | 52,256 | 46,820 | ||||||
| Change in FV of Warrants | (46,822 | ) | (4,489,110 | ) | ||||
| Stock Expense | 3,572,232 | |||||||
| Unrealized gain or loss | - | (1,371,689 | ) | |||||
| 14,831,206 | $ | (5,195,822 | ) | |||||
| Net loss | $ | (23,205,456 | ) | $ | (6,229,639 | ) | ||
Year Ended December 31, 2023 Compared to the Year Ended December 31, 2022
Revenue
| Year Ended December 31, 2023 | Year Ended December 31, 2022 | % Percentage Change | ||||||||||
| Data Exchange (Beam) | $ | 878,416 | $ | 678,138 | 30 | % | ||||||
| Data Broker (RWD) | $ | 143,235 | $ | 474,600 | -70 | % | ||||||
| Master Reseller Agreement | $ | 1,021,651 | $ | 1,152,738 | -11 | % | ||||||
| 27 |
Our revenue comprises of sales made from our data exchange (BEAM) and from data broker (RWD). For the year ended 2023, overall revenue was down by 11%. The primary driver for exchange revenue increase was delivery of revenue to a significant customer. The primary drive for the decrease in broker revenue was revenue deliveries pushed to Q1 of Fiscal 2024.
Cost of Revenue
| Year Ended December 31, 2023 | Year Ended December 31, 2022 | |||||||
| Cost of Revenue | 1,149,551 | 1,513,428 | ||||||
| As a percentage of Revenue | 113 | % | 131 | % | ||||
In 2023 we were able to reduce our cost of revenue as a percentage of revenue by 24%. In the year ended 2023 our Software cost, iRWD consultants and iRWD Data cost each decreased by $0.2 million. The decrease was partially offset by a $0.2 million increase in payroll expenses.
General and Administrative
Our general and administrative expense increased year over year by $1.8 million from the year ended 2022 compared to the year ended 2023. The increase is primarily due to the additional cost incurred in connection with our Business Combination. We incurred an additional $1.0 million legal cost, $0.7 million on warrants issued to convertible note holders that were converted into share of commons stock, $0.4 million additional employees’ salaries, $0.3 million for investor relations cost, and $0.3 million in additional audit fees. The increase in general and administrative expenses were partially offset by $0.2 million decrease in both recruitment fees and bad debt expense.
Operation
Our operations expense includes payroll and consultant costs. Operations expense decreased year over year by $0.2 million from the year ended 2022 compared with the year ended 2023. This decrease was primarily due to a decrease in headcount.
Sales & Marketing
Our sales & marketing expense increased by $0.15 million year over year from the year ended 2022 compared to the year ended 2023. The increase is due to the addition of an employee and consultant in 2023.
Research and development
Our research and development expense increased by $0.7 million year over year from the year ended 2022 compared to the year ended 2023. The increase is primarily due to the additional cost in salaries for curators, consultants and increased hosting costs, which increased by $0.4 million, $0.2 million and $0.1 million, respectively.
Impairment
The Company recorded goodwill of $10.5 million in connection with the Business Combination. In December 2023, the Company concluded that the entire goodwill was impaired, as such the $10.5 million of goodwill was written-off.
Income tax provision
For the year ended 2023, the Company is in a significant loss, as such we did not record any income tax provision.
Interest Expense
The Company incurred interest expense on Loan extensions associated with the Business Combination, convertible promissory notes, the Pipe Senior Secured Convertible Notes and Loans made from related parties (Management and Directors). Interest expense in the year ended 2023 increased by $0.3 million. The increase was mainly from the Pipe Senior Secured Convertible Notes issued in 2023.
Change in Fair Value of Warrants
The change in Warrant Fair Value was due to the closing of the Business Combination Agreement and the resulting fluctuations of the share market price.
| 28 |
Stock Expense
The Company incurred approximately $3.5 million in common stock issuance expense for the Data Knight shares converted to OneMedNet Corporation shares.
Non-GAAP Financial Measure
In addition to providing financial measurements based on generally accepted accounting principles in the United States of America, or GAAP, we provide an additional financial metric that is not prepared in accordance with GAAP, or non-GAAP financial measure. We use this non-GAAP financial measure, in addition to GAAP financial measures, to understand and compare operating results across accounting periods, for financial and operational decision making, for planning and forecasting purposes, to measure executive compensation, and to evaluate our financial performance. This non-GAAP financial measure is Adjusted EBITDA, as discussed below.
We believe that this non-GAAP financial measure reflects our ongoing business in a manner that allows for meaningful comparisons and analysis of trends in the business, as it facilitates comparing financial results across accounting periods and to those of peer companies. We also believe that this non-GAAP financial measure enables investors to evaluate our operating results and future prospects in the same manner as we do. This non-GAAP financial measure may exclude expenses and gains that may be unusual in nature, infrequent, or not reflective of our ongoing operating results.
The non-GAAP financial measure does not replace the presentation of our GAAP financial measures and should only be used as a supplement to, not as a substitute for, our financial results presented in accordance with GAAP.
We consider Adjusted EBITDA to be an important indicator of the operational strength and performance of our business and a good measure of our historical operating trends. Adjusted EBITDA eliminates items that we do not consider to be part of our core operations. We define Adjusted EBITDA as GAAP net loss excluding the following items: interest income; income taxes; depreciation and amortization of tangible and intangible assets; unit and stock-based compensation; Business Combination transaction expenses; and other non-recurring items that may arise from time to time.
The non-GAAP adjustments, and our basis for excluding them from our non-GAAP financial measure, are outlined below:
| ● | Unit and Stock-based compensation – Although unit and stock-based compensation is an important aspect of the compensation paid to our employees, the grant date fair value varies based on the derived stock price at the time of grant, varying valuation methodologies, subjective assumptions, and the variety of award types. This makes the comparison of our current financial results to previous and future periods difficult to interpret; therefore, we believe it is useful to exclude unit and stock-based compensation from our non-GAAP financial measures in order to highlight the performance of our business and to be consistent with the way many investors evaluate our performance and compare our operating results to peer companies. | |
| ● | Business Combination transaction expenses – Business Combination transaction expenses represent the expenses incurred solely related to the Business Combination, which we completed on June 7, 2022. It primarily includes investment banker fees, legal fees, professional fees for accountants, transaction fees, advisory fees, due diligence costs, certain other professional fees, and other direct costs associated with strategic activities. These amounts are impacted by the timing of the Business Combination. We exclude Business Combination transaction expenses from our non-GAAP financial measures to provide a useful comparison of our operating results to prior periods and to our peer companies because such amounts vary significantly based on the magnitude of the Business Combination transaction and do not reflect our core operations. |
The following table reconciles GAAP net loss to Adjusted EBITDA during the periods presented (in thousands):
| Year Ended December 31, 2023 | Year Ended December 31, 2022 | |||||||
| Net loss | $ | (23,205,456 | ) | $ | (6,229,639 | ) | ||
| Interest Expense | 749,213 | 403,307 | ||||||
| Impairment | 10,504,327 | - | ||||||
| Depreciation and amortization | 27,983 | 24,807 | ||||||
| Unit and Stock-based compensation | 3,572,232 | 45,584 | ||||||
| Business combination transaction expenses | 1,427,73 | 900,152 | ||||||
| Adjusted EBITDA | $ | (6,932,966 | ) | $ | (4,855,789 | ) | ||
Liquidity and Capital Resources
As of December 31, 2023, our principal sources of liquidity were net proceeds received related to the Business Combination and cash received from customers.
| 29 |
The following table shows net cash and cash equivalents provided by (used in) operating activities, net cash and cash equivalents used in investing activities, and net cash and cash equivalents provided by financing activities during the periods presented:
| Year Ended | ||||||||
| December 31, 2023 | December 31, 2022 | |||||||
| Net cash provided by (used in) | ||||||||
| Operating activities | $ | 8,220,910 | $ | 87,239,622 | ||||
| Investing activities | (43,757 | ) | (58,137 | ) | ||||
| Financing Activities | (8,431,875 | ) | (88,032,226 | ) | ||||
Operating Activities
Our net cash and cash equivalents provided by (used in) operating activities consists of net loss adjusted for certain non-cash items, including depreciation and amortization, business combination cost, stock-based compensation expense, cash held in trust account, and as well as changes in operating assets and liabilities. The primary changes in working capital items, such as the changes in accounts receivable and deferred revenue, result from the difference in timing of payments from our customers related to contract performance obligation. This may result in an operating cash flow source or use for the period, depending on the timing of payments received as compared to the fulfillment of the performance obligation.
Net cash used in operating activities was $8.2 million during the year ended December 31, 2023. Net cash used in operating activities was due to our net loss of $23.2 million adjusted for non-cash items of $31.4 million, primarily consisting of the redemption of public shares in connection with the Business Combination causing the withdrawal of $29.0 million of cash held in the trust account, $0.9 million business combination cost, $0.4 million extension loan, and use of cash for operating assets and liabilities of $1.1million due to the timing of cash payments to vendors and cash receipts from customers.
By comparison, the Company’s net cash provided by operating activities was $87.2 million during the year ended December 31, 2022. Net cash provided by operating activities was due to our net loss of $6.2 million adjusted for non-cash items of $93.5 million, primarily consisting of the redemption of public shares in connection with the Business Combination causing the withdrawal of $88.3 million of cash held in trust account $1.6 million of stock-based compensation expense, $2.5 million extension loan, less $0.5 million and use of cash for operating assets and liabilities of $.5 million due to the timing of cash payments to vendors and cash receipts from customers.
Investing Activities
Our investing activities have consisted primarily of property and equipment purchases.
Net cash and cash equivalents used in investing activities during the year ended December 31, 2023 consisted of $44 thousand of purchased property and equipment.
By comparison, the Company’s net cash and cash equivalents used in investing activities during the year ended December 31, 2022 consisted primarily of $58 thousand of purchased property and equipment.
Financing Activities
Net cash flows from financing activities was ($8.4 million) for the year ended December 31, 2023, which primarily consisted of $10.7 million repayment on convertible promissory note payable, $1.5 million proceeds from issuance of PIPE Convertible Notes and Warrants, $0.5 proceeds from related loan, $28.8 million from Common Stock subject to redemption in connection with the Business Combination, $0.5 million underwriting fee related to the Business Combination, $0.3 million decrease in warrant liability, $18.2 million additional paid in capital and $11.6 million retained earning adjustment.
By comparison, the Company’s net cash flows from financing activities was ($88.0 million) for the year ended December 31, 2023, which primarily consisted of $5.5 million proceeds from convertible promissory notes payable, $88.5 million from common stock subject to redemption in connection with the Business Combination, $4.5 million decrease in warrant liability, $2.8 million additional paid in capital and $3.4 million retained earning adjustment.
Contractual Obligations and Commitments and Liquidity Outlook
Currently, management does not believe the cash and cash equivalents is sufficient to meet our foreseeable cash needs for at least the next 12 months. Our foreseeable cash needs, in addition to our recurring operating expenses, include our expected capital expenditures to support the expansion of our infrastructure and workforce, interest expense and minimum contractual obligations. Management hopes to raise cash either through a public offering or private debt and equity offering. Our inability to raise cash would cause to operate as a going concern.
Our future capital requirements will depend on many factors, including our growth rate, the timing and extent of spending to support research and development efforts, the expansion of sales and marketing activities, the introduction of new and enhanced product and service offerings, and the cost of any future acquisitions of technology or businesses. In the event that additional financing is required from outside sources, we may be unable to raise the funds on acceptable terms, if at all.
| 30 |
The following table summarizes our current and long-term material cash requirements as of December 31, 2023:
| Payments due in: | ||||||||||||
| Total | Less than 1 year | 1-3 years | ||||||||||
| Accounts payable and accrued expenses | $ | 4,184,398 | $ | 4,184,398 | $ | - | ||||||
| Excise tax | 113,353 | 113,353 | - | |||||||||
| Income tax payable | 120,017 | 120,017 | - | |||||||||
| PIPE Notes, net of discount including interest | 1,549,820 | 1,549,820 | - | |||||||||
| Loan, related party of OMN including interest | 465,023 | - | 465,023 | |||||||||
| $ | 6,432,610 | $ | 5,967.587 | $ | 465,023 | |||||||
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our consolidated financial statements which have been prepared in accordance with accounting principles generally accepted in the United States of America. In preparing our financial statements, we make estimates, assumptions, and judgments that can have a significant impact on our reported revenue, results of operations, and net income or loss, as well as on the value of certain assets and liabilities on our balance sheet during and as of the reporting periods. These estimates, assumptions, and judgments are necessary because future events and their effects on our results and the value of our assets cannot be determined with certainty and are made based on our historical experience and on other assumptions that we believe to be reasonable under the circumstances. These estimates may change as new events occur or additional information is obtained, and we may periodically be faced with uncertainties, the outcomes of which are not within our control and may not be known for a prolonged period of time. Because the use of estimates is inherent in the financial reporting process, actual results could differ from those estimates.
We believe that the assumptions and estimates associated with the following critical accounting policies involve significant judgment and thus have the most significant potential impact on our Consolidated Financial Statements.
Revenue Recognition
We generate revenue from the sale of products and services. A description of our revenue recognition policies is included in Note 2, Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K.
Although most of our sales agreements contain standard terms and conditions, certain agreements contain multiple performance obligations or non-standard terms and conditions. For customer contracts that contain more than one performance obligation, we allocate the total transaction consideration to each performance obligation based on the relative stand-alone selling price of each performance obligation within the contract. We rely on either observable standalone sales or an expected cost plus a margin approach to determine the standalone selling price of offerings, depending on the nature of the performance obligation.
As we further discuss in Note 2, Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K, for contracts with customers entered into during fiscal years 2023 and 2022, revenue from the sales of our iRWD and BEAM are recognized over time as the asset created by our performance does not have alternative use to us and an enforceable right to payment for performance completed to date is present. We recognize revenue as work progresses, using costs incurred to date relative to total estimated costs at completion. Incurred costs represent work performed, which correspond with and best depict transfer of control to the customer. Contract costs are incurred over a period of time, which can span periods, and the estimation of these costs requires management’s judgment. Due to the nature of the work required to be performed on the iRWD and BEAM and our reliance on the availability the estimation of total revenue and cost at completion is complex, subject to many variables, and requires significant judgment on a contract-by-contract basis. As part of this process, we review information including, but not limited to, any outstanding key contract matters, progress towards completion and the related program schedule, identified risks and opportunities and the related changes in estimates of revenue and costs. The risks and opportunities relate to our judgment about the delays that may or may not be within our control. Risks and opportunities may also relate to supply chain trends and commodity pricing, as well as changes in foreign currencies. Changes in estimates of net sales, cost of sales, and the related impact to operating profit are recognized on a cumulative catch-up basis, which recognizes the cumulative effect of the profit changes on current and prior periods based on a performance obligation’s percentage of completion in the current period. A significant change in one or more of these estimates could affect the profitability of one of more of our performance obligations and could have a material impact on our financial condition and results of operations.
Stock-based Compensation
Prior to the Business Combination, OneMedNet Corporation (now OneMedNet Solutions Corporation) had five authorized classes of membership interests, consisting of a class of common units known as the Class A Common Units (the “Class A Units”), a class of preferred units known as the Series A-2 Preferred Units (the “A-2 Preferred Units”), a class of preferred units known as the Series A-1 Preferred Units (the “A-1 Preferred Units”), Convertible Notes, Stock Options units, known as the Options and Warrants units granted to employees, officers, and directors pursuant to an incentive plan.
| 31 |
Following the Business Combination, the Company has authorized 101,000,000 shares of common stock, including 100,000,000 shares of Common Stock and 1,000,000 shares of Preferred Stock. In addition, the Company has three classes of warrants (i.e., Public Warrants, Private Warrants and PIPE Warrants) issued and outstanding.
As the Business Combination is accounted for as a reverse recapitalization, all periods prior to the Business Combination have been retroactively adjusted using the Exchange Ratio as stipulated by the Merger Agreement for the equivalent number of shares outstanding immediately after the Merger to effect the reverse recapitalization. The Class A Units, A-2 Preferred Units, A-1 Preferred Units, Options and Warrants were converted into Common Stock using an exchange ratio of 1:1, the Convertible Notes were converted into Common Stock using an exchange ratio of 2.5 per share. This is presented within the consolidated statements of changes in redeemable preferred and common units and equity (deficit).
We typically issue restricted stock units (“RSUs”) as stock-based compensation. For RSUs, the fair value is the closing market price of the stock on the date immediately preceding the grant. We recognize compensation expense over the requisite service period for awards expected to vest. We account for forfeitures as they occur, rather than applying an estimated forfeiture rate. The graded-vesting method of expense recognition is applied to all awards with service-only conditions.
Certain RSUs involve stock to be issued upon the achievement of certain performance conditions. Such RSUs become available, subject to time-based vesting conditions if, and to the extent that, financial performance criteria for the applicable period are achieved. Accordingly, the number of RSUs earned will vary based on the level of achievement of financial performance objectives for the applicable period. Until such time that our financial performance can ultimately be determined, each quarter we estimate the number of RSUs to be earned based on an evaluation of the probability of achieving the financial performance objectives. Such estimates are revised, if necessary, in subsequent periods when the underlying factors change our evaluation of the probability of achieving the financial performance objectives. Accordingly, stock-based compensation expense associated with performance-based RSUs may differ significantly from the amount recorded in the current period.
The assumptions used in calculating the fair value of stock-based compensation awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management’s judgment. As a result, if factors change and we use different assumptions, our stock-based compensation expense could be materially different in the future.
Warrant transactions
PIPE Warrants to purchase our shares of Common Stock may be accounted for as either liability or equity instruments depending on the terms of the warrant agreements. The warrants issued by us are accounted for as equity instruments due to our ability to settle the warrants through the issuance of units and the absence of terms which would require liability classification, including the rights of the grantee to require cash settlement. We classify these equity instruments within additional paid-in capital on the consolidated balance sheets.
Private Warrants to purchase units accounted for as liability instruments represent the warrants issued to significant shareholders and related parties.
In order to calculate warrant charges, we used the Black-Scholes pricing model, which required key inputs including volatility and risk-free interest rate and certain unobservable inputs for which there is little or no market data, requiring us to develop our own assumptions. We estimated the fair value of unvested warrants, considered to be probable of vesting, at the time. Based on that estimated fair value, we determined warrant charges, which were recorded as a reduction of the transaction price.
Off-Balance Sheet Arrangements:
As of December 31, 2023, we had no off-balance sheet arrangements as defined in Instruction 8 to Item 303(b) of Regulation S-K.
Recently Adopted Accounting Pronouncements
See Note 2 to the accompanying consolidated financial statements included elsewhere in this Annual Report on Form 10-K for a description of recently adopted accounting standards.
Recently Issued Accounting Pronouncements
See Note 2 to the accompanying consolidated financial statements included elsewhere in this Annual Report on Form 10-K for a description of certain recently issued accounting standards which may impact our financial statements in future reporting periods.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to market risk, including changes to interest rates and foreign currency exchange rates.
| 32 |
Interest Rate Sensitivity
We had cash and cash equivalents totaling $0.05 million and $0.30 million as of December 31, 2023, and December 31, 2022, respectively. Cash and cash equivalents include cash on hand and investments with original maturities of three months or less, are stated at cost, and approximate fair value. Our investment policy and strategy are focused on preservation of capital, supporting our liquidity requirements, and delivering competitive returns subject to prevailing market conditions. We were not exposed to material risks due to changes in market interest rates given the liquidity of the cash and investments with original maturities of three months.
Foreign Currency Risk
Although we are exposed to foreign currency risk from our international operations, we do not consider it to have a material impact. Certain transactions of the Company and its subsidiaries are denominated in currencies other than the functional currency. Foreign currency transaction losses totaled $23,109 for the year ended December 31, 2023, which is up from $13,066 for the year ended December 31, 2022, each of which were recorded within other income, net on the consolidated statements of operations.
Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable.
The Company’s cash and cash equivalents are generally held with large financial institutions. Although the Company’s deposits may exceed federally insured limits, the financial institutions that the Company uses have high investment-grade credit ratings and, as a result, the Company believes that, as of December 31, 2023, its risk relating to deposits exceeding federally insured limits was not significant.
The Company has no significant off-balance sheet risk such as foreign exchange contracts, options contracts, or other hedging arrangements.
The Company believes its credit policies are prudent and reflect normal industry terms and business risk. The Company generally does not require collateral from its customers and generally requires payment from zero to 90 days from the invoice date with typical terms of 30 days. As of December 31, 2023, three customers accounted for over 10% of the Company’s accounts receivable balance, and one customer accounted for more than 10% of the Company’s accounts receivable balance as of December 31, 2022.
| 33 |
Item 8. Financial Statements and Supplementary Data
ONEMEDNET CORPORATION
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 34 |
Report of Independent Registered Public Accounting Firm
To the shareholders and the board of directors of OneMedNet Corporation
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of OneMedNet Corporation as of December 31, 2023 and 2022, the related statements of operations, stockholders' equity (deficit), and cash flows for the years then ended, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations and has a significant accumulated deficit. In addition, the Company continues to experience negative cash flows from operations. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/
BF Borgers CPA PC (PCAOB ID 5041)
We have served as the Company’s auditor since 2022
April 9, 2024
| F-1 |
ONEMEDNET CORPORATION
CONSOLIDATED BALANCE SHEETS
| December 31, 2023 | December 31, 2022 | |||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | |||||||
| Investments held in Trust | ||||||||
| Accounts receivable, net of allowance | ||||||||
| Prepaid expenses and other assets | ||||||||
| Receivable from SPAC | ||||||||
| Total current assets | ||||||||
| Property and Equipment, Net | ||||||||
| Total assets | $ | $ | ||||||
| Current Liabilities | ||||||||
| Accounts payable & accrued expenses | ||||||||
| Loan Amount due to related parties | ||||||||
| Excise tax | ||||||||
| Loan Extensions | ||||||||
| Deferred revenues | ||||||||
| Loan Payable | ||||||||
| Convertible promissory notes | ||||||||
| Canada Emergency Business Loan Act | ||||||||
| Income tax payable | ||||||||
| Franchise tax payable | ||||||||
| Pipe Notes, net of discount including interest | ||||||||
| Deferred underwriter fee payable | ||||||||
| Total current liabilities | ||||||||
| Long Term Liabilities | ||||||||
| Convertible promissory note | ||||||||
| Canada Emergency Business Loan Act | ||||||||
| Accrued interest | ||||||||
| Loan, related party of OMN | ||||||||
| Warrant liabilities | ||||||||
| Deferred underwriter fee payable | ||||||||
| Working capital Loan | ||||||||
| Extension loans | ||||||||
| Total liabilities | ||||||||
| Stockholders’ Equity (Deficit) | ||||||||
| Preferred Series A-2, par value $, shares authorized and, and shares issued and outstanding as of December 31, 2023 and December 31, 2022 | ||||||||
| Preferred Shares A-1, par value $, shares authorized and, and shares issued and outstanding as of December 31, 2023 and December 31, 2022 | ||||||||
| Common Stock, par value $, shares authorized and and shares issued and outstanding as of December 31, 2023 and December 31, 2022 | ||||||||
| Data Knights Acquisition Corp. Class A Common Stock, par value $, shares authorized and, and shares issued and outstanding as of December 31, 2023 and December 31, 2022 | ||||||||
| Data Knights Acquisition Corp. Class A Common Stock, par value $, shares authorized and, and shares issued and outstanding as of December 31, 2023 and December 31, 2022 | ||||||||
| Commitments and contingencies | ||||||||
| Additional paid in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders’ equity (deficit) | ( | ) | ||||||
| Total liabilities and stockholders’ equity (deficit) | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-2 |
ONEMEDNET CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year Ended | ||||||||
| 2023 | 2022 | |||||||
| Revenue | $ | $ | ||||||
| Cost of Revenue | ||||||||
| Gross Margin | ( | ) | ( | ) | ||||
| Operating Expenses | ||||||||
| General and administrative | ||||||||
| Operations | ||||||||
| Sales & Marketing | ||||||||
| Research and development | ||||||||
| Total Operating Expenses | ||||||||
| Operating loss | ( | ) | ( | ) | ||||
| Other Expense (income) | ||||||||
| Impairment | ||||||||
| Income tax provision | ||||||||
| Interest expense | ||||||||
| Other expense | ||||||||
| Change in FV of Warrants | ( | ) | ( | ) | ||||
| Stock Expense | ||||||||
| Unrealized gain or loss | ( | ) | ||||||
| ( | ) | |||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Loss per share of Common Stock:(1) | ||||||||
| Basic and Diluted | $ | ) | N/M | |||||
| Weighted-average shares of Common Stock outstanding: | ||||||||
| Basic and Diluted | N/M | |||||||
| (1) |
The accompanying notes are an integral part of these consolidated financial statements
| F-3 |
ONEMEDNET CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
| Data Knights Acquisition Corp. | Data Knights Acquisition Corp. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A-2 Preferred Stock | Series A-1 Preferred Stock | Class A-Common Stock | Class B-Common Stock | Class A-Common Stock | Additional Paid-in | Accumulated | Stockholders’ | |||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Commitments | Capital | Deficit | Equity | |||||||||||||||||||||||||||||||||||||||||||
| Balances, December 31, 2021 | $ | $ | $ | $ | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common shares in exchange for services | - | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common shares in exchange for cash at $ per share | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of Data Knights Acquisition Corp. Class B Common Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Re-Measurement of Data Knights Acquisition Corp. Class A Common Stock Subject to Possible Redemption | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2022 net loss | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balances, December 31, 2022 | $ | $ | $ | $ | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Preferred Stock to Common Stock | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Convertible Notes to Common Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock Options to Common Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Converting of Warrants to Common Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Private OneMedNet to ONMD Public Shares | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of PIPE Warrants | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Converting Data Knights Common Shares (A and B) to ONMD Public Shares | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock redemption | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance Public Shares | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Retained earnings adjustment | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2023 net loss | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balances, December 31, 2023 | $ | $ | $ | $ | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
ONEMEDNET CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended | ||||||||
| December 31, 2023 | December 31, 2022 | |||||||
| Cash flow from Operating Activities | ||||||||
| Net Loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash flows from operating activities: | ||||||||
| Depreciation and amortization | ||||||||
| Business combination cost | ||||||||
| Stock-based compensation expense | ||||||||
| Cash Held in Trust Account | ||||||||
| Prepaid Expenses | ( | ) | ||||||
| Other current assets | ( | ) | ||||||
| Accounts payable and accrued Expenses | ||||||||
| Accounts receivable, net of allowance | ( | ) | ||||||
| Deferred Revenue & Customer Deposits | ( | ) | ||||||
| Amount due to related party | ( | ) | ||||||
| Exercise tax liability | ||||||||
| Extension loan | ||||||||
| Franchise tax payable | ( | ) | ( | ) | ||||
| Income Tax Payable | ( | ) | ||||||
| Working capital loan | ( | ) | ||||||
| Net cash flows used in operating activities | $ | $ | ||||||
| Cash used for Investing Activities | ||||||||
| Purchase of property and equipment | $ | ( | ) | $ | ( | ) | ||
| Cash flow from Financing Activities | ||||||||
| Class B Common Stock | ||||||||
| Proceeds (repayment) from issuance of convertible promissory note payable | ( | ) | ||||||
| Proceeds from issuance of PIPE Convertible Notes and Warrants | ||||||||
| Proceed from related party loan | ||||||||
| Proceeds from Canada Emergency Business Loan Act | ( | ) | ||||||
| Common Stock Subject to Redemption | ( | ) | ( | ) | ||||
| Deferred underwriting fee | ( | ) | ||||||
| Warrant liability | ( | ) | ( | ) | ||||
| Additional Paid-in Capital | ||||||||
| Class A Common Stock | ( | ) | ||||||
| Class B Common Stock | ( | ) | ||||||
| Retained Earnings adjustment | ( | ) | ||||||
| Net cash flows from financing activities | ( | ) | ( | ) | ||||
| Net change in cash and cash equivalents | ( | ) | ( | ) | ||||
| Cash and Cash Equivalents, Beginning | ||||||||
| Cash and Cash Equivalents, Ending | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
ONEMEDNET CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Operations
OneMedNet Corporation (the “Company”) is a healthcare software company with solutions focused on digital medical image management, exchange, and sharing. The Company was incorporated in Delaware on September 20, 2006. The Company has been solely focused on creating solutions that simplify digital medical image management, exchange, and sharing. The Company has one wholly-owned subsidiary, OneMedNet Technologies (Canada) Inc., incorporated on October 16, 2015 under the provisions of the Business Corporations Act of British Columbia whose functional currency is the Canadian dollar. The Company’s headquarters location is Eden Prairie, Minnesota.
On November 7, 2023, as contemplated by the Company, Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”), and Data Knights, LLC, the Merger Sub’s sponsor merged with and into OneMedNet Corporation, with OneMedNet Corporation surviving the merger. The Business Combination is further described in Note 3, Business Combination.
Data Knights Acquisition Corp Merger
On November 7, 2023, we consummated a merger (the “Merger”) following the approval at the special meeting of the shareholders of Data Knights Acquisition Corp., a Delaware corporation held on October 17, 2023 (the “Special Meeting”), Data Knights Merger Sub, Inc., a Delaware corporation (“Merger Sub”) and a wholly-owned subsidiary of Data Knights Acquisition Corp., a Delaware corporation (“Data Knights”), consummated a merger (the “Merger”) with and into OneMedNet Solutions Corporation (formerly named OneMedNet Corporation), a Delaware corporation (“OneMedNet”) pursuant to an agreement and plan of merger, dated as of April 25, 2022 (the “Merger Agreement”), by and among Data Knights, Merger Sub, OneMedNet, Data Knights, LLC, a Delaware limited liability company (“Sponsor” or “Purchaser Representative”) in its capacity as the representative of the stockholders of Data Knights, and Paul Casey in his capacity as the representative of the stockholders of OneMedNet (“Seller Representative”). Accordingly, the Merger Agreement was adopted, and the Merger and other transactions contemplated thereby (collectively, the “Business Combination”) were approved and completed.
The Business Combination was accounted for as a as a reverse recapitalization with OneMedNet as the accounting acquirer under the accounting principles generally accepted in the United States of America (“U.S. GAAP”). Accordingly, the financial statements of the combined company represent a continuation of the financial statements of OneMedNet.
On
June 28, 2023, the Company and Data Knights entered into a Securities Purchase Agreement (the “SPA”) with certain investors
(collectively referred to herein as the “Purchasers”) for PIPE financing in the aggregate original principal amount of $
Effective immediately prior to the Closing, OneMedNet, Inc. issued the PIPE Notes to the Purchasers under the private offering exemptions under Securities Act of 1933, as amended (the “Securities Act”).
Risks and Uncertainties
The Company is subject to risks common to companies in the markets it serves, including, but not limited to, global economic and financial market conditions, fluctuations in customer demand, acceptance of new products, development by its competitors of new technological innovations, dependence on key personnel, and protection of proprietary technology.
As previously reported on Form 8-K on February 9, 2024, the Company received written notice (the “Nasdaq Notice”), dated February 7, 2024, from the Nasdaq Stock Market (“Nasdaq”) indicating that for the preceding 30 consecutive business days, the market value of the Company’s listed securities (“MVLS”) did not maintain a minimum market value of $50,000,000 (the “Minimum MVLS Requirement”) as required by Nasdaq Listing Rule 5450(b)(2)(A). In accordance with Nasdaq Listing Rule 5810(c)(3)(C), the Company has a compliance period of 180 calendar days, or until August 5, 2024, to regain compliance with the Minimum MVLS Requirement. Compliance may be achieved if the Company’s MVLS closes at $50,000,000 or more for a minimum of ten consecutive business days at any time during the 180-day compliance period, in which case Nasdaq will notify the Company of its compliance and the matter will be closed.
If the Company does not regain compliance with the Minimum MVLS Requirement by August 5, 2024, Nasdaq will provide written notification to the Company that its common stock is subject to delisting. At that time, the Company may appeal the relevant delisting determination to a hearings panel pursuant to the procedures set forth in the applicable Nasdaq Listing Rules. However, there can be no assurance, if the Company does appeal the delisting determination by Nasdaq to the hearings panel, that such appeal would be successful. In such event, the Company may also seek to apply for a transfer to The Nasdaq Global Market if it meets the requirements for continued listing thereon. The Nasdaq Notice received have no immediate effect on the Company’s continued listing on the Nasdaq Global Market or the trading of Company’s common stock, subject to the Company’s compliance with the other continued listing requirements. The Company is presently evaluating potential actions to regain compliance with all applicable requirements for continued listing on the Nasdaq Global Market. There can be no assurance that the Company will be successful in maintaining the listing of its common stock on the Nasdaq Global Market.
| F-6 |
2. Summary of Significant Accounting Policies
Basis of Presentation and Foreign Currency Translation
The consolidated financial statements have been prepared in U.S. dollars, in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The accompanying consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. The consolidated financial statements include 100% of the accounts of wholly-owned subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
Business Combination
We account for business acquisitions under ASC Topic 805, Business Combinations (“ASC Topic 805”). The total purchase consideration for an acquisition is measured as the fair value of the assets given, equity instruments issued, and liabilities assumed at the acquisition date. Costs that are directly attributable to the acquisition are expensed as incurred. Identifiable assets (including intangible assets) and liabilities assumed (including contingent liabilities) are measured initially at their fair values at the acquisition date. We recognize goodwill if the fair value of the total purchase consideration is in excess of the net fair value of the identifiable assets acquired and the liabilities assumed. We recognize a bargain purchase gain within Other income (expense), net, in the consolidated statement of operations if the net fair value of the identifiable assets acquired and the liabilities assumed is in excess of the fair value of the total purchase consideration. We include the results of operations of the acquired business in the consolidated financial statements beginning on the acquisition date.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates, judgments, and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses, and the amounts disclosed in the related notes to the consolidated financial statements. Actual results and outcomes may differ materially from management’s estimates, judgments, and assumptions. Significant estimates, judgments, and assumptions used in these financial statements include, but are not limited to, those related to revenue, useful lives and realizability of long-lived assets, accounting for income taxes and related valuation allowances, and unit and stock-based compensation. Estimates are periodically reviewed in light of changes in circumstances, facts, and experience.
Operating Segments
The Company operates as one operating segment. Operating segments are defined as components of an enterprise for which separate financial information is regularly evaluated by the chief operating decision maker (“CODM”), which is the Company’s Chief Executive Officer, in deciding how to allocate resources and assess performance. The Company’s CODM evaluates the Company’s financial information and resources and assesses the performance of these resources on a consolidated basis. The Company is not organized by market and is managed and operated as one business. A single management team that reports to the chief executive officer comprehensively manages the entire business. Accordingly, the Company does not accumulate discrete financial information with respect to separate divisions and does not have separate operating or reportable segments. Since the Company operates in one operating segment, all required financial segment information can be found in the consolidated financial statements.
Cash and Cash Equivalents
Cash and cash equivalents consist of highly liquid, short-term investments with a maturity of three months or less when purchased. Cash equivalents consist of money market funds and are carried at cost, which approximates fair value. The balances, at times, may exceed FDIC Insured limits. The Company believes that, as of December 31, 2023, its risk relating to deposits exceeding federally insured limits was not significant.
Accounts Receivable
Accounts
receivable are unsecured, recorded at net realizable value, and do not bear interest. Accounts receivable are considered past due if
not paid within the terms established between the Company and the customer. Amounts are only written off after all attempts at collections
have been exhausted. The Company determines the need for an allowance for doubtful accounts based upon factors surrounding the credit
risk of specific customers, historical trends and other information. As of December 31, 2023 and 2022, the Company established allowances
of $
The
Company believes its credit policies are prudent and reflect normal industry terms and business risk. The Company generally does not
require collateral from its customers and generally requires payment from 0 to 90 days from the invoice date. For the year ended December
31, 2023, there was 1 customer that accounted for
| Year Ended | ||||||||
| December 31, 2023 | December 31, 2022 | |||||||
| Customer 1 | % | % | ||||||
| Customer 2 | % | |||||||
| Aggregate Percent of Total Revenue | % | % | ||||||
| F-7 |
As
of December 31, 2023, three customers accounted for more than
| Year Ended | ||||||||
| December 31, 2023 | December 31, 2022 | |||||||
| Customer 1 | % | |||||||
| Customer 2 | % | |||||||
| Customer 3 | % | |||||||
| Customer 4 | % | |||||||
| Customer 5 | % | |||||||
| Aggregate Percent of Total Accounts Receivable | % | % | ||||||
Property and Equipment
Property and equipment are recorded at cost. The straight-line method is used for computing depreciation and amortization. Assets are depreciated over their estimated useful lives ranging from three to five years. Cost of maintenance and repairs are charged to expense when incurred.
Impairment of Long-Lived Assets
The
Company reviews long-lived assets, including property and equipment, for impairment whenever events or changes in business circumstances
indicate that the carrying amount of an asset may not be fully recoverable. An impairment loss would be recognized when the estimated
future undiscounted net cash flows from the use of the asset are less than the carrying amount of that asset. There have been
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. To increase the comparability of fair value measures, the following hierarchy prioritizes the inputs to valuation methodologies used to measure fair value:
Level 1 — Valuations based on quoted prices for identical assets and liabilities in active markets.
Level 2 — Valuations based on observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data.
Level 3 — Valuations based on unobservable inputs reflecting our own assumptions, consistent with reasonably available assumptions made by other market participants. These valuations require significant judgment.
We measure the fair value of money market funds and certain marketable equity securities based on quoted prices in active markets for identical assets or liabilities. Other marketable securities were valued either based on recent trades of securities in inactive markets or based on quoted market prices of similar instruments and other significant inputs derived from or corroborated by observable market data. We did not hold significant amounts of marketable securities categorized as Level 3 assets as of the years ended December 31, 2022 and December 31, 2023.
The Company’s financial instruments consist of cash and cash equivalents, accounts receivable, accounts payable, convertible notes payable and certain privately issued warrants. The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable financial instruments approximate their fair value due to their short-term nature. The Company’s Private Warrants estimated fair values are provided by a third party pricing vendor and are reviewed by the Company’s management. The Private Warrants valuations are based on unobservable inputs reflecting the vendor’s assumptions, consistent with reasonably available assumptions made by other market participants and thus are classified as Level 3.
Revenue Recognition
Revenue is recognized in accordance with the five-step model set forth by Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (“Topic 606”), which involves identification of the contract, identification of performance obligations in the contract, determination of the transaction price, allocation of the transaction price to the previously identified performance obligations, and revenue recognition as the performance obligations are satisfied.
Revenue from all customers is recognized when a performance obligation is satisfied by transferring control of a distinct good or service to a customer. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account under Topic 606. A contract’s transaction price is allocated to each distinct performance obligation in proportion to the standalone selling price for each and recognized as revenue when, or as, the performance obligation is satisfied.
| F-8 |
Individual promised goods and services in a contract are considered a performance obligation and accounted for separately if the good or service is distinct. A good or service is considered distinct if the customer can benefit from the good or service on its own or with other resources that are readily available to the customer and the good or service is separately identifiable from other promises in the arrangement.
The transaction price for the products is the invoiced amount. Advanced billings from contracts are deferred and recognized as revenue when earned. Revenue is recognized only to the extent that it is probable that a significant reversal of revenue will not occur and when collection is considered probable The Company excludes from revenue taxes collected from a customer that are assessed by a governmental authority and imposed on and concurrent with a specific revenue-producing transaction. Deferred revenue consists of payments received in advance of performance under the contract. Such amounts are generally recognized as revenue over the contractual period. The Company receives payments from customers based upon contractual billing schedules. Accounts receivable is recorded when the right to consideration becomes unconditional. Payment terms on invoiced amounts typically range from zero to 90 days, with typical terms of 30 days.
The Company generates revenue from two streams: (1) iRWD (imaging Real World Data) which provides regulatory grade imaging and clinical data in the Pharmaceutical, Device Manufacturing, CRO’s and AI markets and (2) BEAM which is a Medical Imaging Exchange platform between Hospital/Healthcare Systems, Imaging Centers, Physicians and Patients. iRWD is sold on a fixed fee basis based on the number of data units and the cost per data unit committed to in the customer contract. Revenue is recognized when the data is delivered to the customer. Beam revenue is subscription-based revenue which is recognized ratably over the subscription period committed to by the customer. The Company invoices its Beam customers quarterly or annually in advance with the customer contracts automatically renewing unless the customer issues a cancellation notice.
Income Taxes
The Company is subject to U.S. federal, state and local income taxes. The Company accounts for income taxes in accordance with ASC Topic 740, Accounting for Income Taxes (“ASC Topic 740”), which requires the recognition of tax benefits or expenses on temporary differences between the financial reporting and tax bases of its assets and liabilities by applying the enacted tax rates in effect for the year in which the differences are expected to reverse. Such net tax effects on temporary differences are reflected on the Company’s consolidated balance sheets as deferred tax assets and liabilities.
ASC Topic 740 prescribes a two-step approach for the recognition and measurement of tax benefits associated with the positions taken or expected to be taken in a tax return that affects amounts reported in the financial statements. The Company has reviewed and will continue to review the conclusions reached regarding uncertain tax positions, which may be subject to review and adjustment at a later date based on ongoing analyses of tax laws, regulations and interpretations thereof. To the extent that the Company’s assessment of the conclusions reached regarding uncertain tax positions changes as a result of the evaluation of new information, such change in estimate will be recorded in the period in which such determination is made. The Company reports income tax-related interest and penalties relating to uncertain tax positions, if applicable, as a component of income tax expense
Deferred tax assets are reduced by a valuation allowance when the Company believes that it is more-likely-than-not that some portion or all of the deferred tax assets will not be realized. The Company provides deferred taxes at the enacted tax rate that is expected to apply when the temporary differences reverse. The Company has recorded a full valuation allowance against the net deferred tax asset due to the uncertainty of realizing the related benefits.
Patents and Trademarks
Costs associated with the submission of a patent application are expensed as incurred given the uncertainty of the patents resulting in probable future economic benefits to the Company and are included in research and development expenses on the consolidated statements of operations.
Research and Development
The Company account for its research and development cost in accordance with ASC Topic 730, Research and Development (“ASC Topic 730”). ASC Topic 730 requires that all R&D costs be recognized as an expense as incurred. However, some costs associated with R&D activities that have an alternative future use (e.g., materials, equipment, facilities) may be capitalizable. For the years ended December 31, 2023 and December 31, 2022 research and development expenditures were charged to operating expense as incurred..
Stock-based Compensation
The Company has a stock-based compensation plan, which is described in more detail in Note 8. The fair value of stock option and warrant grants are determined on the date of grant using the Black Scholes valuation model. Forfeitures of stock based awards are recorded as the actual forfeitures occur. Stock based compensation expense is recognized over the service period, net of estimated forfeitures, using the straight-line method. The Company converted all unvested stock based compensation awards to common shares in the year ended December 31, 2023.
| F-9 |
General, and Administrative Expenses
General and administrative expenses include all costs that are not directly related to satisfaction of customer contracts. General, and administrative expenses include items for the Company’s selling and administrative functions, such as sales, finance, legal, human resources, and information technology support. These functions include costs for items such as salaries and benefits and other personnel-related costs, maintenance and supplies, professional fees for external legal, accounting, and other consulting services, intangible asset amortization, and depreciation expense.
Emerging Growth Company
The Company is an emerging growth company, as defined in Section 2(a) of the Securities Act of 1933, as amended (the “Securities Act”), as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. The Company has not elected to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company , can adopt the new or revised standard at the time private companies adopt the new or revised standard.
Accounting Pronouncements Not Yet Adopted
In November 2023, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2023-07, Improvements to Reportable Segment Disclosures (Topic 280). This ASU updates reportable segment disclosure requirements by requiring disclosures of significant reportable segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a segment’s profit or loss. This ASU also requires disclosure of the title and position of the individual identified as the CODM and an explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and deciding how to allocate resources. The ASU is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Adoption of the ASU should be applied retrospectively to all prior periods presented in the financial statements. Early adoption is also permitted. This ASU will likely result in us including the additional required disclosures when adopted. We are currently evaluating the provisions of this ASU and expect to adopt them for the year ending December 31, 2024.
In December 2023, the Financial Accounting Standards Board issued an Accounting Standards Update (“ASU”) amending existing income tax disclosure guidance, primarily requiring more detailed disclosure for income taxes paid and the effective tax rate reconciliation. The ASU is effective for annual reporting periods beginning after December 15, 2024, with early adoption permitted and can be applied on either a prospective or retroactive basis. We are currently evaluating the ASU to determine its impact on our income tax disclosures.
Recently adopted accounting pronouncements
In October 2021, the FASB issued ASU No. 2021-08, Accounting for Contract Assets and Contract Liabilities from Contracts with Customers (ASC Topic 805). This ASU requires an acquirer in a business combination to recognize and measure contract assets and contract liabilities (deferred revenue) from acquired contracts using the revenue recognition guidance in Topic 606. At the acquisition date, the acquirer applies the revenue model as if it had originated the acquired contracts. The ASU is effective for annual periods beginning after December 15, 2022, including interim periods within those fiscal years. We adopted this ASU prospectively on January 1, 2023. This ASU has not and is currently not expected to have a material impact on our consolidated financial statements.
3. Business Combination
The Business Combination was accounted for as a reverse recapitalization as OneMedNet Corporation was determined to be the accounting acquirer under Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 805, Business Combinations. This determination was primarily based on OneMedNet Corporation comprising the ongoing operations of the combined entity, OneMedNet Corporation’s senior management comprising of all the senior management of the combined company, and the prior shareholders of OneMedNet owning a majority of the voting power of the combined entity. Accordingly, for accounting purposes, the financial statements of the combined entity upon consummation of the Business Combination represented a continuation of the financial statements of OneMedNet Corporation with the merger being treated as the equivalent of OneMedNet issuing stock for the net assets of Data Knights Inc., accompanied by a recapitalization. Operations prior to the Business Combination are presented as those of OneMedNet Corporation in future reports of the combined entity.
4. Going Concern
The
accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets
and satisfaction of liabilities and commitments in the normal course of business. The Company does not have adequate liquidity to fund
its operations through at least twelve months from the date these financial statements were available for issuance. The Company has an
accumulated deficit
| F-10 |
5. Property and Equipment
Property and equipment are summarized as of December 31:
| 2023 | 2022 | |||||||
| Computers | $ | $ | ||||||
| Furniture and equipment | ||||||||
| Total Property and Equipment | ||||||||
| Less: accumulated depreciation | ( | ) | ( | ) | ||||
| Net Property and Equipment | $ | $ | ||||||
Depreciation
and amortization expense was $
6. Income Taxes
The
Company has generated both federal and state net operating losses (NOL) of approximately $
Components of deferred income taxes are as follows as of December 31:
| 2023 | 2022 | |||||||
| Deferred Tax Assets | ||||||||
| Net operating loss carry forward | $ | $ | ||||||
| Stock Compensation | ||||||||
| Other | ||||||||
| Gross deferred tax assets | ||||||||
| Less valuation allowance | ( | ) | ( | ) | ||||
| Net deferred tax assets | ||||||||
The
change in the valuation allowance was $
As a result of the Business Combination, the Company was appointed as the sole managing member of Data Knights. The Company is subject to U.S. federal income taxes, in addition to state and local income taxes. The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the estimated future tax consequences attributable to temporary differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax base. Deferred tax assets and liabilities are determined on the basis of the differences between the consolidated financial statements and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the temporary differences are expected to be settled or recovered. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes. In assessing the realizability of deferred tax assets, the Company considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. The Company considers the scheduled reversal of deferred tax liabilities, projected future income, and tax planning strategies in making this assessment.
The
Company has established a valuation allowance related deferred tax assets on deductible temporary differences, tax losses, and tax credit
carryforwards. The valuation allowance as of December 31, 2023 was $
| F-11 |
As
of December 31, 2023, the Company had a U.S. federal net operating loss carryforwards of $
7. Convertible Promissory Notes held by Related Party
During
2023, the Company entered various Convertible Promissory Notes (“Note”) with related party investors totaling $
The
principal and unpaid accrued interest on each Note will convert: (i) automatically, upon the Company’s issuance of equity securities
(the “Next Equity Financing”) in a single transaction, or series of related transactions, with aggregate gross proceeds to
the Company of at least $
If a Corporate Transaction occurs before the repayment or conversion of the Notes, the Company will pay at the closing of the Corporate Transaction to each noteholder that elects not to convert its Notes in connection with such Corporate Transaction an amount equal to the outstanding principal amount of such noteholder’s Note plus a 20% premium. “Corporate Transaction” means (a) a sale by the Company of all or substantially all of its assets, (b) a merger of the Company with or into another entity (if after such merger the holders of a majority of the Company’s voting securities immediately prior to the transaction do not hold a majority of the voting securities of the successor entity) or (c) the transfer of more than 50% of the Company’s voting securities to a person or group.
During
November 2019, the Company entered into a Convertible Promissory Note (“Note”) agreement with a related party investor. The
total amount of the Note is $
As
of December 31, 2022 there was $
In
November 2023, the Business Combination between Data Knights and the Company triggered the Notes’ conversion to common stock. Approximately
$
8. Canadian Emergency Business Loan Act (CEBA)
During
December 2020, the Company applied for and received a $
The Company accounted for the loan as debt in accordance with FASB Accounting Standards Codification 470 Debt and accrued interest in accordance with the interest method under FASB ASC 835-30.
| F-12 |
9. Shareholders’ Equity
Series A-2 Preferred Stock
The
Company’s previously issued and outstanding Series A-2 preferred stock included a $ per share annual noncumulative dividend
when and if declared by the board of directors.
In
November 2023, the Business Combination between Data Knights and the Company triggered the
Series A-1 Preferred Stock
The
Company’s previously issued and outstanding Series A-1 preferred stock included a $ per share annual noncumulative dividend
when and if declared by the board of directors.
In
November 2023, the Business Combination between Data Knights and the Company triggered the
Common Stock
In 2023, in connection with services performed by the Board of Directors common shares of (- 2022) were issued at $ per share. These were expensed as general and administrative expenses in the Statement of Operations.
The table below summarizes the Common Stock activities during the year ended December 31, 2023.
| Common Shares | ||||
| Balances, December 31, 2022 | ||||
| Preferred Stock to Common Stock | ||||
| Convertible Notes to Common Stock | ||||
| Stock Options to Common Stock | ||||
| Converting of Warrants to Common Stock | ||||
| Private OneMedNet to ONMD Public Shares | ( | ) | ||
| Converting Data Knights Common Shares (A and B) to ONMD Public Shares | ||||
| Issuance Public Shares | ||||
| Balances, December 31, 2023 | ||||
During 2020, the Company adopted a new equity incentive plan (the Plan), which provides for the granting of incentive and nonqualified stock options to employees, directors, and consultants. As of December 31, 2020, the Company has reserved shares of common stock under the Plan. The Company believes that such awards better align the interests of its employees with those of its stockholders. Option awards are generally granted with an exercise price equal to the fair market value of the Company’s stock at the date of grant; those option awards generally vest with a range of to of continuous service and have ten-year contractual terms. As there is no public data available for the share price valuation, the Company considers the Fair Market Value of $ to be on the conservative side and similar to the exercise price. Certain option awards provide for accelerated vesting if there is a change in control, as defined in the Plan. The Plan also permits the granting of restricted stock and other stock-based awards. Unexercised options are cancelled upon termination of employment and become available under the Plan.
| F-13 |
| Options Outstanding | Weighted- Average Exercise Price | Aggregate Intrinsic Value | ||||||||||
| Outstanding as of December 31, 2020 | $ | $ | ||||||||||
| Granted - under the Plan | ||||||||||||
| Exercised | ||||||||||||
| Cancelled | ( | ) | ||||||||||
| Outstanding as of December 31, 2021 | $ | $ | ||||||||||
| Granted - under the Plan | ||||||||||||
| Exercised | ( | ) | ||||||||||
| Cancelled | ( | ) | ||||||||||
| Outstanding as of December 31, 2022 | $ | $ | ||||||||||
| Options exercisable as of December 31, 2022 | $ | $ | ||||||||||
As of December 31, 2022 and 2021, there were and common stock options outstanding with a weighted average remaining contractual life of years and years, respectively.
As of December 31, 2022 and 2021, there were and common stock options exercisable at a weighted average remaining contractual life of years and years, respectively.
On November 7, 2023, the Company issued shares of common stock for vested options less an exercise price of $.
At the Special Meeting held on October 17, 2023, Data Knights shareholders considered and approved the OneMedNet Corporation 2022 Equity Incentive Plan (the “Plan”) and reserved an amount of shares of . The Plan was approved by the OneMedNet pre-Closing board of directors on October 17, 2023. The Plan became effective immediately upon the Closing of the Business Combination.
Black Scholes Assumptions
The determination of the fair value of stock options using an option valuation model is affected by the Company’s stock price valuation, as well as assumptions regarding a number of complex and subjective variables. The volatility assumption is based on volatilities of similar companies over a period of time equal to the expected term of the stock options. The volatilities of similar companies are used in conjunction with the Company’s historical volatility because of the lack of sufficient relevant history for the Company’s common stock equal to the expected term. The expected term of the employee stock options represents the weighted average period for which the stock options are expected to remain outstanding. The expected term assumption is estimated based primarily on the options’ vesting terms and remaining contractual life and employees’ expected exercise and post- vesting employment termination behavior. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The dividend yield assumption is based on the expectation of no future dividend payouts by the Company.
The fair value of the Company’s previous stock options was estimated assuming no expected dividends and the following weighted average assumptions:
| 2022 | 2021 | |||||||
| Expected life in years | ||||||||
| Risk-free interest rate | % | % | ||||||
| Expected dividend yield | % | % | ||||||
| Expected volatility | % | % | ||||||
The total expense recognized for share-based payments was $ and $ for the years ended December 31, 2022 and 2021, respectively. These costs are included in the statements of operations. As of December 31, 2022, there was $ of unrecognized compensation costs related to stock option grants which will be recognized over the next four years.
During 2023, the Company issued common stock to employees and extinguished all outstanding stock options. The shares outstanding were recorded as stock expense in the Consolidated Statement of Operations.
| F-14 |
11. Stock Warrants
In
2021, there were
As
of December 31, 2023 and December 31, 2022, the Company had
As
of December 31, 2023 and December 31, 2022, the Company had
12. Fair Value Measures
The fair value measurement accounting standards establish a framework for measuring fair value and expand disclosures about fair value measurements. The standard does not require any new fair value measurements; rather, it applies to other accounting pronouncements that require or permit fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market in an orderly transaction between market participants on the measurement date. This pronouncement also establishes a three-level hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability on the measurement date. The three levels are defined as follows:
Level 1—inputs to the valuation methodology are quoted prices (unadjusted) for an identical asset or liability in an active market
Level 2—inputs to the valuation methodology include quoted prices for a similar asset or liability in an active market or model-derived valuations in which all significant inputs are observable for substantially the full term of the asset or liability
Level 3—inputs to the valuation methodology are unobservable and significant to the fair value measurement of the asset or liability
The following table presents the Company’s financial assets measured and recorded at fair value on a recurring basis using the above input categories as of December 31, 2023 and December 31, 2022 (in thousands):
| Year Ended | ||||||||||||||||||||||||||||||||
| December 31, 2023 | December 31, 2022 | |||||||||||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||||||||
| Assets: | ||||||||||||||||||||||||||||||||
| Investments held in Trust | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Total Assets | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
13. Related Party Transactions
Loan Extensions
| F-15 |
Data
Knights closed its initial public offering in May 2021 and had 12 months to complete a business combination. Alternatively, the Data
Knight could extend the period up to two times for an additional three months each time with an extension costing $
PIPE Convertible Notes and Warrants
In
November 2023, the Company entered into a Securities Purchase Agreement (SPA) in which the Company was required to sell senior secured
convertible notes and warrants to Directors of the Company. The SPA stipulates a collateral security agreement between the Company and
the Directors for punctual payment and performance by the Company on its Obligations to the Directors. The Intellectual Property of the
Company serves as the collateral for the Directors. The senior secured convertible notes and warrants were issued through a private issuance
of a public entity (PIPE) transaction, which is a form of debt and equity offering under an exception in the securities law for qualifying
private placements by issuers of publicly traded securities. The Company received a total of $
The
warrants are classified as equity and the total proceeds received from the Directors are allocated based on the relative fair values
of the convertible notes and the warrants at the issued date. The portion allocable to warrants is accounted for as paid-in capital.
The senior secured convertible notes are classified as long term debt in the Consolidated Balance Sheet. The estimate fair value of the
senior secured convertible notes at December 31, 2023 was $
14. Commitments, Contingencies, and Concentrations Operating lease
The
Company has a month-to-month lease for a suite at a cost of $
15. Subsequent Events
The Company has evaluated subsequent events occurring through April 9, 2024, the date the financial statements were available for issuance, for events requiring recording or disclosure in the Company’s financial statements.
During
2024, through to the date of this report, the Company issued
and
shares of Common Stock to EF Hutton LLC and Kingwood Capital Partners, LLC, respectively, as consideration for $
During 2024, through to the date of this report, the Company bought back shares of Common Stock from a convertible note holder.
During
2024, through to the date of this report, the Company received $from a majority shareholder for the purchase
of shares, and an additional $
During
2024, through to the date of this report, the Company entered into a definitive securities purchase agreement with an institutional investor
providing up to $
As previously announced on Form 8-K, on March 28, 2024, OneMedNet Corporation (the “Company”) entered into a definitive securities purchase agreement (the “Securities Purchase Agreement”) with Helena Global Investment Opportunities 1 Ltd., an affiliate of Helena Partners Inc., a Cayman-Islands based advisor and investor providing for up to USD$4.54 million in funding through a private placement for the issuance of senior secured convertible notes (the “Notes”).
As previously announced on Form 8-K, on March 27, 2024, Paul J. Casey, Chief, Chief Executive Officer of the Company, notified the Company of his intention to retire as Chief Executive Officer of the Company effective March 29, 2024. Mr. Casey will continue to serve as a member of the Board of Directors (the “Board”) of the Company. In connection with Mr. Casey’s service on the Advisory Board of the Company, the Board approved a Stock Option Grant (the “Option Grant”) providing for the grant of five-year options exercisable at $ per share adviser to Mr. Casey. Also on March 27, 2024, Scott Holbrook, a member of the Board of the Company and a member of the Company’s Audit Committee, notified the Company of his intention to retire from the Company’s Board effective March 29, 2024.
Effective March 29, 2024, the Board (i) appointed Mr. Aaron Green, to serve as Chief Executive Officer of the Company to fill the vacancy created by the retirement of Paul Casey; (ii) appointed Mr. Aaron Green, to serve as a member of the Board to fill the vacancy created by the retirement of Scott Holbrook; and (iii) appointed Board member, Dr. Thomas Kosasa, to serve on the Company’s Audit Committee, also to fill the vacancy created by the retirement of Scott Holbrook.
| F-16 |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), as of the end of the period covered by this Annual Report on Form 10-K. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2023, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Report on Internal Control Over Financial Reporting
As disclosed elsewhere in this Annual Report on Form 10-K, we completed the Business Combination on November 7, 2023. Prior to the Business Combination Data Knights, our predecessor, was a special purpose acquisition company formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, recapitalization or similar business combination with one or more businesses. As a result, previously existing internal controls are no longer applicable or comprehensive enough as of the assessment date, because Data Knights’ operations prior to the Business Combination were insignificant compared to those of the consolidated entity post-Business Combination. As a result, management was unable, without incurring unreasonable effort or expense, to complete an assessment of our internal control over financial reporting as of December 31, 2023. Accordingly, we are excluding management’s report on internal control over financial reporting pursuant to Section 215.02 of the SEC Division of Corporate Finance’s Regulation S-K Compliance and Disclosure Interpretations.
Changes in Internal Control Over Financial Reporting
No change in our internal control over financial reporting occurred during the quarter ended December 31, 2023 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
| 35 |
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2023.
Item 11. Executive Compensation
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2023.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2023.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2023.
Item 14. Principal Accounting Fees and Services
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2023.
| 36 |
PART IV
Item 15. Exhibits, Financial Statement Schedules
The following documents are filed as a part of this Form 10-K:
(a)(1) Financial Statements
| Index to Financial Statements | Page |
| Consolidated Balance Sheets | F-2 |
| Consolidated Statements of Operations and Comprehensive Income (Loss) | F-3 |
| Consolidated Statements of Changes in Shareholders’ Equity | F-4 |
| Consolidated Statements of Cash Flows | F-5 |
| Notes to Consolidated Financial Statements | F-6 |
(a)(2) Financial Statement Schedules
None.
(a)(3) Exhibits.
These exhibits listed below are filed or incorporated by reference into this Report.
| 37 |
†Schedules and exhibits to this Exhibit omitted pursuant to Regulation S-K Item 601(b)(2). The Registrant agrees to furnish supplementally a copy of any omitted schedule of exhibit to the SEC upon request.
+ Management or compensatory agreement or arrangement.
* The certifications furnished in Exhibit 32.1 and Exhibit 32.2 hereto are deemed to accompany this Annual Report on Form 10-K and will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, except to the extent that the registrant specifically incorporates it by reference.
Item 16. Form 10-K Summary
None.
| 38 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
April 9, 2024
| OneMedNet Corporation | ||
| /s/ Aaron Green | ||
| Name: | Aaron Green | |
| Title: | Chief Executive Officer | |
| (Principal Executive Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Jeffrey Yu, MD | Director and Chairman of the Board | April 9, 2024 | ||
| Jeffrey Yu, MD | ||||
| /s/ Aaron Green | Chief Executive Officer and Director | April 9, 2024 | ||
Aaron Green |
(Principal Executive Officer) | |||
| /s/ Paul J. Casey | Director | April 9, 2024 | ||
| Paul J. Casey | ||||
/s/ Lisa Embree |
Chief Financial Officer and VP of Finance | April 9, 2024 | ||
| Lisa Embree | (Principal Accounting Officer) | |||
| /s/ Erkan Akyuz | Director | April 9, 2024 | ||
Erkan Akyuz |
||||
| /s/ Thomas Kosasa | Director | April 9, 2024 | ||
| Thomas Kosasa | ||||
| /s/ Eric Casaburi | Director | April 9, 2024 | ||
| Eric Casaburi | ||||
| /s/ Sun Joo Huh | Director | April 9, 2024 | ||
| Dr. Julianne (Sun Joo) Huh |
| 39 |