0000927653FY2021falseP1YP3YP1YP1YP5YP1YP1Y00009276532020-04-012021-03-310000927653us-gaap:CommonStockMember2020-04-012021-03-310000927653mck:A0.625notesDue2021Member2020-04-012021-03-310000927653mck:A1.500NotesDue2025Member2020-04-012021-03-310000927653mck:A1.625NotesDue2026Member2020-04-012021-03-310000927653mck:A3.125NotesDue2029Member2020-04-012021-03-31iso4217:USD00009276532020-09-30xbrli:shares00009276532021-04-3000009276532019-04-012020-03-3100009276532018-04-012019-03-31iso4217:USDxbrli:shares00009276532021-03-3100009276532020-03-310000927653us-gaap:CommonStockMember2018-03-310000927653us-gaap:AdditionalPaidInCapitalMember2018-03-310000927653us-gaap:OtherAdditionalCapitalMember2018-03-310000927653us-gaap:RetainedEarningsMember2018-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-03-310000927653us-gaap:TreasuryStockMember2018-03-310000927653us-gaap:NoncontrollingInterestMember2018-03-3100009276532018-03-310000927653us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2018-03-310000927653srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2018-03-310000927653us-gaap:CommonStockMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2018-03-310000927653us-gaap:AdditionalPaidInCapitalMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2018-03-310000927653us-gaap:OtherAdditionalCapitalMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2018-03-310000927653us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2018-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2018-03-310000927653us-gaap:TreasuryStockMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2018-03-310000927653us-gaap:NoncontrollingInterestMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2018-03-310000927653srt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2018-03-310000927653us-gaap:CommonStockMember2018-04-012019-03-310000927653us-gaap:AdditionalPaidInCapitalMember2018-04-012019-03-310000927653us-gaap:TreasuryStockMember2018-04-012019-03-310000927653us-gaap:NoncontrollingInterestMember2018-04-012019-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-04-012019-03-310000927653us-gaap:RetainedEarningsMember2018-04-012019-03-310000927653us-gaap:OtherAdditionalCapitalMember2018-04-012019-03-310000927653us-gaap:CommonStockMember2019-03-310000927653us-gaap:AdditionalPaidInCapitalMember2019-03-310000927653us-gaap:OtherAdditionalCapitalMember2019-03-310000927653us-gaap:RetainedEarningsMember2019-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-03-310000927653us-gaap:TreasuryStockMember2019-03-310000927653us-gaap:NoncontrollingInterestMember2019-03-3100009276532019-03-310000927653us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2019-03-310000927653srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2019-03-310000927653us-gaap:CommonStockMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2019-03-310000927653us-gaap:AdditionalPaidInCapitalMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2019-03-310000927653us-gaap:OtherAdditionalCapitalMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2019-03-310000927653us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2019-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2019-03-310000927653us-gaap:TreasuryStockMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2019-03-310000927653us-gaap:NoncontrollingInterestMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2019-03-310000927653srt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2019-03-310000927653us-gaap:CommonStockMember2019-04-012020-03-310000927653us-gaap:AdditionalPaidInCapitalMember2019-04-012020-03-310000927653us-gaap:TreasuryStockMember2019-04-012020-03-310000927653us-gaap:NoncontrollingInterestMember2019-04-012020-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-04-012020-03-310000927653us-gaap:RetainedEarningsMember2019-04-012020-03-310000927653us-gaap:OtherAdditionalCapitalMember2019-04-012020-03-310000927653us-gaap:CommonStockMember2020-03-310000927653us-gaap:AdditionalPaidInCapitalMember2020-03-310000927653us-gaap:OtherAdditionalCapitalMember2020-03-310000927653us-gaap:RetainedEarningsMember2020-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-03-310000927653us-gaap:TreasuryStockMember2020-03-310000927653us-gaap:NoncontrollingInterestMember2020-03-310000927653us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2020-03-310000927653srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2020-03-310000927653us-gaap:CommonStockMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2020-03-310000927653us-gaap:AdditionalPaidInCapitalMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2020-03-310000927653us-gaap:OtherAdditionalCapitalMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2020-03-310000927653us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2020-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2020-03-310000927653us-gaap:TreasuryStockMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2020-03-310000927653us-gaap:NoncontrollingInterestMembersrt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2020-03-310000927653srt:CumulativeEffectPeriodOfAdoptionAdjustedBalanceMember2020-03-310000927653us-gaap:CommonStockMember2020-04-012021-03-310000927653us-gaap:AdditionalPaidInCapitalMember2020-04-012021-03-310000927653us-gaap:TreasuryStockMember2020-04-012021-03-310000927653us-gaap:NoncontrollingInterestMember2020-04-012021-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-04-012021-03-310000927653us-gaap:RetainedEarningsMember2020-04-012021-03-310000927653us-gaap:CommonStockMember2021-03-310000927653us-gaap:AdditionalPaidInCapitalMember2021-03-310000927653us-gaap:OtherAdditionalCapitalMember2021-03-310000927653us-gaap:RetainedEarningsMember2021-03-310000927653us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-03-310000927653us-gaap:TreasuryStockMember2021-03-310000927653us-gaap:NoncontrollingInterestMember2021-03-31mck:segment00009276532020-07-012021-03-31mck:customer0000927653us-gaap:CustomerConcentrationRiskMemberus-gaap:SalesRevenueNetMember2020-04-012021-03-31xbrli:pure0000927653us-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMember2020-04-012021-03-310000927653mck:LargestCustomerMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:SalesRevenueNetMember2020-04-012021-03-310000927653mck:LargestCustomerMemberus-gaap:AccountsReceivableMemberus-gaap:CustomerConcentrationRiskMember2020-04-012021-03-310000927653mck:MedicalSurgicalSolutionsSegmentMember2020-04-012021-03-310000927653us-gaap:ShippingAndHandlingMember2020-04-012021-03-310000927653us-gaap:ShippingAndHandlingMember2019-04-012020-03-310000927653us-gaap:ShippingAndHandlingMember2018-04-012019-03-310000927653srt:MinimumMemberus-gaap:BuildingAndBuildingImprovementsMember2020-04-012021-03-310000927653us-gaap:BuildingAndBuildingImprovementsMembersrt:MaximumMember2020-04-012021-03-310000927653mck:MachineryEquipmentAndOtherMembersrt:MinimumMember2020-04-012021-03-310000927653mck:MachineryEquipmentAndOtherMembersrt:MaximumMember2020-04-012021-03-310000927653srt:MinimumMember2020-04-012021-03-310000927653srt:MaximumMember2020-04-012021-03-310000927653mck:DistributionandRetailBusinessMemberus-gaap:ProductConcentrationRiskMemberus-gaap:SalesRevenueNetMember2020-04-012021-03-310000927653mck:DistributionandRetailBusinessMemberus-gaap:ProductConcentrationRiskMemberus-gaap:SalesRevenueNetMember2019-04-012020-03-310000927653us-gaap:ProductConcentrationRiskMembermck:ServicesBusinessMemberus-gaap:SalesRevenueNetMember2019-04-012020-03-310000927653us-gaap:ProductConcentrationRiskMembermck:ServicesBusinessMemberus-gaap:SalesRevenueNetMember2020-04-012021-03-310000927653us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccountingStandardsUpdate201613Member2020-04-010000927653us-gaap:CorporateJointVentureMember2017-03-310000927653us-gaap:CorporateJointVentureMembermck:ChangeHealthcareInc.Member2017-03-310000927653mck:ChangeHealthcareInc.Member2019-07-012019-07-010000927653mck:ChangeHealthcareLLCChangeHealthcareMembermck:ChangeHealthcareInc.Member2019-07-012019-07-010000927653us-gaap:IPOMembermck:ChangeHealthcareInc.Member2019-07-010000927653mck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:CorporateJointVentureMember2019-07-010000927653mck:ChangeHealthcareLLCChangeHealthcareMembermck:ChangeHealthcareInc.Member2019-07-010000927653us-gaap:CorporateJointVentureMember2019-07-012019-09-300000927653mck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:CorporateJointVentureMember2019-07-012019-09-300000927653mck:SeparationofChangeHealthcareJVMember2020-03-092020-03-090000927653mck:ChangeHealthcareLLCChangeHealthcareMember2020-03-092020-03-090000927653mck:SeparationofChangeHealthcareJVMember2020-03-100000927653mck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:CorporateJointVentureMember2020-01-012020-03-310000927653us-gaap:RetainedEarningsMemberus-gaap:AccountingStandardsUpdate201409Membersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMembermck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:CorporateJointVentureMember2019-04-010000927653mck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:CorporateJointVentureMember2019-04-012020-03-310000927653mck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:CorporateJointVentureMember2018-04-012019-03-310000927653mck:TransitionServicesAgreementsTSAMemberus-gaap:CorporateJointVentureMember2019-04-012020-03-310000927653mck:TransitionServicesAgreementsTSAMemberus-gaap:CorporateJointVentureMember2018-04-012019-03-310000927653mck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:AllOtherSegmentsMembermck:TaxReceivableAgreementTRAMember2018-04-012019-03-310000927653mck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:AllOtherSegmentsMembermck:TaxReceivableAgreementTRAMember2020-03-310000927653mck:ChangeHealthcareLLCChangeHealthcareMemberus-gaap:AllOtherSegmentsMembermck:TaxReceivableAgreementTRAMember2021-03-310000927653mck:TaxReceivableAgreementTRAMember2021-03-310000927653mck:TaxReceivableAgreementTRAMember2020-03-310000927653us-gaap:DisposalGroupHeldforsaleNotDiscontinuedOperationsMember2021-03-310000927653us-gaap:DisposalGroupHeldforsaleNotDiscontinuedOperationsMember2020-03-310000927653mck:WalgreensBootsAllianceJointVentureMember2020-11-010000927653mck:GermanWholesaleBusinessMember2020-11-010000927653mck:GermanPharmaceuticalWholesaleJointVentureMemberus-gaap:CorporateJointVentureMember2021-03-310000927653mck:GermanWholesaleBusinessMember2020-10-012020-12-310000927653mck:InternationalSegmentMembermck:GermanWholesaleBusinessMember2020-04-012021-03-310000927653mck:InternationalSegmentMembermck:GermanWholesaleBusinessMember2019-04-012020-03-310000927653mck:GermanWholesaleBusinessMemberus-gaap:DisposalGroupHeldforsaleNotDiscontinuedOperationsMember2020-11-010000927653mck:GermanWholesaleBusinessMemberus-gaap:DisposalGroupHeldforsaleNotDiscontinuedOperationsMember2020-03-310000927653mck:GermanWholesaleBusinessMemberus-gaap:DisposalGroupHeldforsaleNotDiscontinuedOperationsMember2019-12-122020-03-310000927653srt:MinimumMemberus-gaap:SubsequentEventMember2021-05-120000927653srt:MaximumMemberus-gaap:SubsequentEventMember2021-05-120000927653srt:MinimumMembercountry:GBmck:InternationalSegmentMember2021-03-310000927653srt:MaximumMembercountry:GBmck:InternationalSegmentMember2021-03-310000927653country:GBmck:InternationalSegmentMember2020-04-012021-03-310000927653mck:StrategicGrowthInitiativePlanAdditionalGlobalReorganizationandBusinessConsolidationProgramsMembermck:EmployeeSeveranceAcceleratedDepreciationandProjectConsultingFeesMember2020-04-012021-03-310000927653mck:StrategicGrowthInitiativePlanAdditionalGlobalReorganizationandBusinessConsolidationProgramsMembermck:EmployeeSeveranceAcceleratedDepreciationandProjectConsultingFeesMember2019-04-012020-03-310000927653mck:StrategicGrowthInitiativePlanAdditionalGlobalReorganizationandBusinessConsolidationProgramsMembermck:EmployeeSeveranceAcceleratedDepreciationandProjectConsultingFeesMember2018-04-012019-03-310000927653mck:StrategicGrowthInitiativePlanRelocationofCorporateHeadquartersMembermck:EmployeeRetentionExpensesAssetImpairmentsandAcceleratedDepreciationMember2020-04-012021-03-310000927653mck:StrategicGrowthInitiativePlanRelocationofCorporateHeadquartersMembermck:EmployeeRetentionExpensesAssetImpairmentsandAcceleratedDepreciationMember2019-04-012020-03-310000927653mck:StrategicGrowthInitiativePlanRelocationofCorporateHeadquartersMembermck:EmployeeRetentionExpensesAssetImpairmentsandAcceleratedDepreciationMember2018-04-012019-03-310000927653mck:OperatingModelAndCostOptimizationProgramsMembermck:EmployeeSeveranceExitrelatedCostsandAssetImpairmentChargesMember2018-04-012019-03-310000927653mck:OperatingModelAndCostOptimizationProgramsMembermck:EmployeeSeveranceExitrelatedCostsandAssetImpairmentChargesMember2019-03-310000927653mck:EmployeeSeveranceExitrelatedCostsandAssetImpairmentChargesMember2019-04-012020-03-310000927653mck:EmployeeSeveranceExitrelatedCostsandAssetImpairmentChargesMember2018-04-012019-03-310000927653us-gaap:OperatingSegmentsMembermck:USPharmaceuticalSegmentMember2020-04-012021-03-310000927653us-gaap:OperatingSegmentsMembermck:InternationalSegmentMember2020-04-012021-03-310000927653mck:MedicalSurgicalSolutionsSegmentMemberus-gaap:OperatingSegmentsMember2020-04-012021-03-310000927653us-gaap:OperatingSegmentsMembermck:PrescriptionTechnologySolutionsSegmentMember2020-04-012021-03-310000927653us-gaap:CorporateNonSegmentMember2020-04-012021-03-310000927653us-gaap:OperatingSegmentsMembermck:USPharmaceuticalSegmentMember2019-04-012020-03-310000927653us-gaap:OperatingSegmentsMembermck:InternationalSegmentMember2019-04-012020-03-310000927653mck:MedicalSurgicalSolutionsSegmentMemberus-gaap:OperatingSegmentsMember2019-04-012020-03-310000927653us-gaap:OperatingSegmentsMembermck:PrescriptionTechnologySolutionsSegmentMember2019-04-012020-03-310000927653us-gaap:CorporateNonSegmentMember2019-04-012020-03-310000927653us-gaap:OperatingSegmentsMembermck:USPharmaceuticalSegmentMember2018-04-012019-03-310000927653us-gaap:OperatingSegmentsMembermck:InternationalSegmentMember2018-04-012019-03-310000927653mck:MedicalSurgicalSolutionsSegmentMemberus-gaap:OperatingSegmentsMember2018-04-012019-03-310000927653us-gaap:OperatingSegmentsMembermck:PrescriptionTechnologySolutionsSegmentMember2018-04-012019-03-310000927653us-gaap:CorporateNonSegmentMember2018-04-012019-03-310000927653us-gaap:OperatingSegmentsMembermck:USPharmaceuticalSegmentMember2019-03-310000927653us-gaap:OperatingSegmentsMembermck:InternationalSegmentMember2019-03-310000927653mck:MedicalSurgicalSolutionsSegmentMemberus-gaap:OperatingSegmentsMember2019-03-310000927653us-gaap:OperatingSegmentsMembermck:PrescriptionTechnologySolutionsSegmentMember2019-03-310000927653us-gaap:CorporateNonSegmentMember2019-03-310000927653us-gaap:OperatingSegmentsMembermck:USPharmaceuticalSegmentMember2020-03-310000927653us-gaap:OperatingSegmentsMembermck:InternationalSegmentMember2020-03-310000927653mck:MedicalSurgicalSolutionsSegmentMemberus-gaap:OperatingSegmentsMember2020-03-310000927653us-gaap:OperatingSegmentsMembermck:PrescriptionTechnologySolutionsSegmentMember2020-03-310000927653us-gaap:CorporateNonSegmentMember2020-03-310000927653us-gaap:OperatingSegmentsMembermck:USPharmaceuticalSegmentMember2021-03-310000927653us-gaap:OperatingSegmentsMembermck:InternationalSegmentMember2021-03-310000927653mck:MedicalSurgicalSolutionsSegmentMemberus-gaap:OperatingSegmentsMember2021-03-310000927653us-gaap:OperatingSegmentsMembermck:PrescriptionTechnologySolutionsSegmentMember2021-03-310000927653us-gaap:CorporateNonSegmentMember2021-03-310000927653us-gaap:AccruedLiabilitiesMember2020-03-310000927653us-gaap:OtherNoncurrentLiabilitiesMember2020-03-310000927653us-gaap:AccruedLiabilitiesMember2021-03-310000927653us-gaap:OtherNoncurrentLiabilitiesMember2021-03-310000927653mck:InternationalSegmentMember2020-04-012021-03-310000927653mck:InternationalSegmentMembermck:RetailPharmacyReportingUnitMember2019-04-012020-03-310000927653mck:InternationalSegmentMembermck:RexallHealthMember2019-04-012020-03-310000927653mck:InternationalSegmentMembermck:RetailPharmacyReportingUnitMember2018-04-012019-03-310000927653us-gaap:CustomerRelationshipsMembermck:IntangibleAssetandStoreAssetsImpairmentMembermck:RexallHealthMember2018-04-012019-03-310000927653mck:MedicalSpecialtiesDistributorsLLCMSDMember2018-06-012018-06-010000927653mck:MedicalSpecialtiesDistributorsLLCMSDMember2019-05-310000927653mck:MedicalSpecialtiesDistributorsLLCMSDMember2018-06-012019-05-310000927653mck:CoverMyMedsLLCMember2017-04-032017-04-030000927653mck:CoverMyMedsLLCMember2017-04-030000927653mck:CoverMyMedsLLCMember2019-05-012019-05-310000927653mck:CoverMyMedsLLCMember2018-05-012018-05-310000927653mck:CoverMyMedsLLCMember2020-03-310000927653us-gaap:RestrictedStockUnitsRSUMember2020-04-012021-03-310000927653us-gaap:RestrictedStockUnitsRSUMember2019-04-012020-03-310000927653us-gaap:RestrictedStockUnitsRSUMember2018-04-012019-03-310000927653us-gaap:StockOptionMember2020-04-012021-03-310000927653us-gaap:StockOptionMember2019-04-012020-03-310000927653us-gaap:StockOptionMember2018-04-012019-03-310000927653us-gaap:EmployeeStockMember2020-04-012021-03-310000927653us-gaap:EmployeeStockMember2019-04-012020-03-310000927653us-gaap:EmployeeStockMember2018-04-012019-03-310000927653mck:A2013StockPlanMember2013-07-310000927653mck:A2013StockPlanMember2021-03-310000927653srt:MinimumMemberus-gaap:RestrictedStockUnitsRSUMember2020-04-012021-03-310000927653srt:MaximumMemberus-gaap:RestrictedStockUnitsRSUMember2020-04-012021-03-310000927653us-gaap:RestrictedStockUnitsRSUMembersrt:DirectorMember2021-03-310000927653mck:PerformancebasedStockUnitsMember2020-04-012021-03-310000927653mck:PeRSUsMember2020-04-012021-03-310000927653mck:RestrictedStockUnitsandPerformancebasedStockUnitsMember2020-04-012021-03-310000927653mck:RestrictedStockUnitsandPerformancebasedStockUnitsMember2019-04-012020-03-310000927653mck:RestrictedStockUnitsandPerformancebasedStockUnitsMember2018-04-012019-03-310000927653mck:RestrictedStockUnitsandPerformancebasedStockUnitsMember2020-03-310000927653mck:RestrictedStockUnitsandPerformancebasedStockUnitsMember2021-03-310000927653mck:RestrictedStockUnitsandPerformancebasedStockUnitsMember2019-03-310000927653us-gaap:EmployeeStockOptionMember2020-04-012021-03-310000927653us-gaap:EmployeeStockOptionMember2018-04-012019-03-310000927653mck:RangeOneMember2020-04-012021-03-310000927653mck:RangeOneMember2021-03-310000927653mck:RangeTwoMember2020-04-012021-03-310000927653mck:RangeTwoMember2021-03-310000927653us-gaap:EmployeeStockOptionMember2019-04-012020-03-310000927653us-gaap:EmployeeStockOptionMember2021-03-310000927653us-gaap:EmployeeStockOptionMember2020-03-310000927653us-gaap:EmployeeStockOptionMember2019-03-310000927653us-gaap:EmployeeStockMember2021-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMembermck:TerminationofU.SDefinedBenefitPensionPlanMember2019-04-012020-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMembersrt:ExecutiveOfficerMembermck:SettlementofExecutiveRetirementBenefitsMember2019-04-012020-03-310000927653mck:PendingAndFutureOpioidRelatedClaimsMember2020-04-012021-03-310000927653mck:InternationalSegmentMember2019-04-012020-03-310000927653mck:InternationalSegmentMember2018-04-012019-03-310000927653us-gaap:DomesticCountryMember2021-03-310000927653us-gaap:StateAndLocalJurisdictionMember2021-03-310000927653us-gaap:ForeignCountryMember2021-03-310000927653us-gaap:ForeignCountryMemberus-gaap:CapitalLossCarryforwardMember2021-03-31iso4217:EURxbrli:shares0000927653mck:MckessonEuropeSubsidiaryMemberus-gaap:SubsequentEventMember2021-04-120000927653mck:MckessonEuropeSubsidiaryMember2021-03-310000927653mck:MckessonEuropeSubsidiaryMember2020-04-012021-03-310000927653mck:MckessonEuropeSubsidiaryMember2019-04-012020-03-310000927653mck:MckessonEuropeSubsidiaryMember2018-04-012019-03-310000927653mck:MckessonEuropeSubsidiaryMemberus-gaap:SubsequentEventMember2021-04-120000927653mck:MckessonEuropeSubsidiaryMember2021-03-310000927653mck:MckessonEuropeSubsidiaryMember2020-03-310000927653mck:MckessonEuropeSubsidiaryMember2018-09-192018-09-190000927653mck:MckessonEuropeSubsidiaryMember2018-09-190000927653mck:VantageandClarusOneSourcingServicesLLCMember2020-04-012021-03-310000927653mck:VantageandClarusOneSourcingServicesLLCMember2019-04-012020-03-310000927653mck:VantageandClarusOneSourcingServicesLLCMember2018-04-012019-03-310000927653mck:RedeemableNoncontrollingInterestMember2018-03-310000927653mck:RedeemableNoncontrollingInterestMember2018-04-012019-03-310000927653mck:RedeemableNoncontrollingInterestMember2019-03-310000927653mck:RedeemableNoncontrollingInterestMember2019-04-012020-03-310000927653mck:RedeemableNoncontrollingInterestMember2020-03-310000927653mck:RedeemableNoncontrollingInterestMember2020-04-012021-03-310000927653mck:RedeemableNoncontrollingInterestMember2021-03-3100009276532019-04-010000927653us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2019-04-010000927653srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2019-04-012019-04-010000927653srt:MinimumMemberus-gaap:BuildingMember2021-03-310000927653srt:MaximumMemberus-gaap:BuildingMember2021-03-310000927653srt:MinimumMemberus-gaap:EquipmentMember2021-03-310000927653srt:MaximumMemberus-gaap:EquipmentMember2021-03-310000927653mck:RealPropertyMember2021-03-310000927653srt:MinimumMember2021-03-310000927653srt:MaximumMember2021-03-310000927653mck:USPharmaceuticalSegmentMember2019-03-310000927653mck:InternationalSegmentMember2019-03-310000927653mck:MedicalSurgicalSolutionsSegmentMember2019-03-310000927653mck:PrescriptionTechnologySolutionsSegmentMember2019-03-310000927653mck:USPharmaceuticalSegmentMember2019-04-012020-03-310000927653mck:MedicalSurgicalSolutionsSegmentMember2019-04-012020-03-310000927653mck:PrescriptionTechnologySolutionsSegmentMember2019-04-012020-03-310000927653mck:USPharmaceuticalSegmentMember2020-03-310000927653mck:InternationalSegmentMember2020-03-310000927653mck:MedicalSurgicalSolutionsSegmentMember2020-03-310000927653mck:PrescriptionTechnologySolutionsSegmentMember2020-03-310000927653mck:USPharmaceuticalSegmentMember2020-04-012021-03-310000927653mck:PrescriptionTechnologySolutionsSegmentMember2020-04-012021-03-310000927653mck:USPharmaceuticalSegmentMember2021-03-310000927653mck:InternationalSegmentMember2021-03-310000927653mck:MedicalSurgicalSolutionsSegmentMember2021-03-310000927653mck:PrescriptionTechnologySolutionsSegmentMember2021-03-3100009276532020-07-012020-09-300000927653mck:InternationalSegmentMembermck:RetailPharmacyReportingUnitMember2020-04-012021-03-310000927653mck:InternationalSegmentMembersrt:EuropeMember2021-03-310000927653mck:InternationalSegmentMembermck:PharmaceuticalDistributionsReportingUnitJune2018OneMember2018-04-012018-06-300000927653mck:InternationalSegmentMembermck:RetailPharmacyReportingUnitMember2018-04-012018-06-300000927653mck:PharmaceuticalDistributionsReportingUnitJune2018TwoMembermck:InternationalSegmentMember2018-04-012018-06-300000927653mck:InternationalSegmentMembermck:RetailPharmacyReportingUnitMember2019-01-012019-03-310000927653mck:InternationalSegmentMembermck:PharmaceuticalDistributionsReportingUnitMember2019-01-012019-03-310000927653mck:InternationalSegmentMembermck:RexallHealthMember2018-10-012018-12-31mck:reportingUnit0000927653mck:EuropeanPharmaceuticalSolutionsMember2018-04-012018-06-300000927653us-gaap:CustomerRelationshipsMember2020-04-012021-03-310000927653us-gaap:CustomerRelationshipsMember2021-03-310000927653us-gaap:CustomerRelationshipsMember2020-03-310000927653us-gaap:ServiceAgreementsMember2020-04-012021-03-310000927653us-gaap:ServiceAgreementsMember2021-03-310000927653us-gaap:ServiceAgreementsMember2020-03-310000927653us-gaap:LicensingAgreementsMember2020-04-012021-03-310000927653us-gaap:LicensingAgreementsMember2021-03-310000927653us-gaap:LicensingAgreementsMember2020-03-310000927653us-gaap:TrademarksAndTradeNamesMember2020-04-012021-03-310000927653us-gaap:TrademarksAndTradeNamesMember2021-03-310000927653us-gaap:TrademarksAndTradeNamesMember2020-03-310000927653us-gaap:TechnologyBasedIntangibleAssetsMember2020-04-012021-03-310000927653us-gaap:TechnologyBasedIntangibleAssetsMember2021-03-310000927653us-gaap:TechnologyBasedIntangibleAssetsMember2020-03-310000927653us-gaap:OtherIntangibleAssetsMember2020-04-012021-03-310000927653us-gaap:OtherIntangibleAssetsMember2021-03-310000927653us-gaap:OtherIntangibleAssetsMember2020-03-310000927653mck:NotesDueNovember302020Memberus-gaap:LoansPayableMember2021-03-310000927653mck:NotesDueNovember302020Memberus-gaap:LoansPayableMember2020-03-310000927653us-gaap:LoansPayableMembermck:FourPointSevenFivePercentNotesDuesMarchOneTwoThousandTwentyOneMember2021-03-310000927653us-gaap:LoansPayableMembermck:FourPointSevenFivePercentNotesDuesMarchOneTwoThousandTwentyOneMember2020-03-310000927653mck:TwoPointSevenZeroPercentNotesDueDecemberFifteenTwoThousandTwentyTwoMemberus-gaap:LoansPayableMember2021-03-310000927653mck:TwoPointSevenZeroPercentNotesDueDecemberFifteenTwoThousandTwentyTwoMemberus-gaap:LoansPayableMember2020-03-310000927653mck:TwoPointEightFivePercentNotesDueMarchFifteenTwoThousandTwentyThreeMemberus-gaap:LoansPayableMember2021-03-310000927653mck:TwoPointEightFivePercentNotesDueMarchFifteenTwoThousandTwentyThreeMemberus-gaap:LoansPayableMember2020-03-310000927653mck:ThreePointEightZeroPercentNotesDueMarchFifteenthTwoThousandTwentyFourMemberus-gaap:LoansPayableMember2021-03-310000927653mck:ThreePointEightZeroPercentNotesDueMarchFifteenthTwoThousandTwentyFourMemberus-gaap:LoansPayableMember2020-03-310000927653us-gaap:LoansPayableMembermck:A090NotesDueDecember32025Member2021-03-310000927653us-gaap:LoansPayableMembermck:A090NotesDueDecember32025Member2020-03-310000927653mck:SevenPointSixFivePercentDebenturesDueMarchOneTwoThousandTwentySevenMemberus-gaap:LoansPayableMember2021-03-310000927653mck:SevenPointSixFivePercentDebenturesDueMarchOneTwoThousandTwentySevenMemberus-gaap:LoansPayableMember2020-03-310000927653mck:ThreePointNineFivePercentNotesDueFebruarySixteenthTwoThousandTwentyEightMemberus-gaap:LoansPayableMember2021-03-310000927653mck:ThreePointNineFivePercentNotesDueFebruarySixteenthTwoThousandTwentyEightMemberus-gaap:LoansPayableMember2020-03-310000927653mck:NotesDueMay302029Memberus-gaap:LoansPayableMember2021-03-310000927653mck:NotesDueMay302029Memberus-gaap:LoansPayableMember2020-03-310000927653mck:SixPercentNotesDueMarchOneTwoThousandFortyOneMemberus-gaap:LoansPayableMember2021-03-310000927653mck:SixPercentNotesDueMarchOneTwoThousandFortyOneMemberus-gaap:LoansPayableMember2020-03-310000927653us-gaap:LoansPayableMembermck:FourPointEightEightPercentNotesDueMarchFifteenthTwoThousandFortyFourMember2021-03-310000927653us-gaap:LoansPayableMembermck:FourPointEightEightPercentNotesDueMarchFifteenthTwoThousandFortyFourMember2020-03-310000927653mck:ZeroPointSixThreePercentEuroNotesDueAugustTwoThousandTwentyOneMemberus-gaap:LoansPayableMember2021-03-310000927653mck:ZeroPointSixThreePercentEuroNotesDueAugustTwoThousandTwentyOneMemberus-gaap:LoansPayableMember2020-03-310000927653mck:OnePointFiveZeroPercentEuroNotesDueNovemberTwoThousandTwentyFiveMemberus-gaap:LoansPayableMember2021-03-310000927653mck:OnePointFiveZeroPercentEuroNotesDueNovemberTwoThousandTwentyFiveMemberus-gaap:LoansPayableMember2020-03-310000927653us-gaap:LoansPayableMembermck:OnePointSixThreePercentEuroNotesDueOctoberTwoThousandTwentySixMember2021-03-310000927653us-gaap:LoansPayableMembermck:OnePointSixThreePercentEuroNotesDueOctoberTwoThousandTwentySixMember2020-03-310000927653us-gaap:LoansPayableMembermck:ThreePointOneThreePercentSterlingNotesDueFebruaryTwoThousandTwentyNineMember2021-03-310000927653us-gaap:LoansPayableMembermck:ThreePointOneThreePercentSterlingNotesDueFebruaryTwoThousandTwentyNineMember2020-03-310000927653mck:LeaseandOtherObligationsMember2021-03-310000927653mck:LeaseandOtherObligationsMember2020-03-310000927653mck:LongtermDebtCurrentMaturitiesMember2021-03-310000927653mck:LongtermDebtCurrentMaturitiesMember2020-03-310000927653us-gaap:LoansPayableMembermck:A090NotesDueDecember32025Member2020-12-030000927653us-gaap:LoansPayableMembermck:A090NotesDueDecember32025Member2020-12-032020-12-030000927653mck:NotesDueNovember302020Memberus-gaap:LoansPayableMember2020-11-300000927653mck:A475NotesDueMarch12021Memberus-gaap:LoansPayableMember2020-12-01iso4217:EUR0000927653mck:FloatingRateEuroNotesDueFebruaryTwelveTwoThousandandTwentyMemberus-gaap:LoansPayableMember2019-04-012020-03-310000927653us-gaap:LoansPayableMembermck:TwoPointTwoEightNotesDueMarchFifteenthTwoThousandNineteenMember2019-03-152019-03-150000927653us-gaap:LoansPayableMembermck:TwoPointTwoEightNotesDueMarchFifteenthTwoThousandNineteenMember2019-03-150000927653us-gaap:NotesPayableOtherPayablesMember2020-04-012021-03-310000927653us-gaap:UnsecuredDebtMemberus-gaap:RevolvingCreditFacilityMembermck:SeniorUnsecuredCreditFacilitythe2020CreditFacilityMember2019-09-300000927653us-gaap:UnsecuredDebtMemberus-gaap:RevolvingCreditFacilityMembermck:SeniorUnsecuredCreditFacilitythe2020CreditFacilityMember2019-09-012019-09-300000927653mck:SeniorUnsecuredCreditFacilitythe2020CreditFacilityCanadianDollarBritishPoundSterlingandEurosSublimitMemberus-gaap:UnsecuredDebtMemberus-gaap:RevolvingCreditFacilityMember2019-09-300000927653us-gaap:UnsecuredDebtMemberus-gaap:RevolvingCreditFacilityMembermck:SeniorUnsecuredCreditFacilitythe2020CreditFacilityMember2020-04-012021-03-310000927653us-gaap:UnsecuredDebtMemberus-gaap:RevolvingCreditFacilityMembermck:SeniorUnsecuredCreditFacilitythe2020CreditFacilityMember2021-03-310000927653mck:GlobalFacilityMember2019-09-300000927653mck:GlobalFacilityMember2019-09-012019-09-300000927653mck:GlobalFacilityMember2019-04-012019-09-300000927653us-gaap:LineOfCreditMember2021-03-310000927653us-gaap:CommercialPaperMember2021-03-310000927653us-gaap:CommercialPaperMember2020-03-310000927653us-gaap:VariableInterestEntityPrimaryBeneficiaryMember2021-03-310000927653us-gaap:VariableInterestEntityPrimaryBeneficiaryMember2020-03-31mck:participant0000927653country:USus-gaap:PensionPlansDefinedBenefitMember2019-06-012019-06-300000927653country:USus-gaap:PensionPlansDefinedBenefitMember2019-04-012019-06-300000927653country:USus-gaap:PensionPlansDefinedBenefitMember2019-07-012019-09-300000927653country:USus-gaap:PensionPlansDefinedBenefitMember2021-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMemberus-gaap:UnfundedPlanMember2019-10-012019-12-310000927653country:USus-gaap:PensionPlansDefinedBenefitMemberus-gaap:UnfundedPlanMember2020-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMemberus-gaap:UnfundedPlanMember2019-03-310000927653mck:GermanWholesaleBusinessMemberus-gaap:DisposalGroupHeldforsaleNotDiscontinuedOperationsMember2020-12-310000927653mck:GermanWholesaleBusinessMemberus-gaap:DisposalGroupHeldforsaleNotDiscontinuedOperationsMember2020-10-012020-12-310000927653country:USus-gaap:PensionPlansDefinedBenefitMember2020-04-012021-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMember2019-04-012020-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMember2018-04-012019-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2020-04-012021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2019-04-012020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2018-04-012019-03-310000927653us-gaap:PensionPlansDefinedBenefitMember2021-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMember2020-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMember2019-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2019-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2021-03-310000927653country:USmck:GermanWholesaleBusinessMemberus-gaap:PensionPlansDefinedBenefitMember2020-03-310000927653country:USmck:GermanWholesaleBusinessMemberus-gaap:PensionPlansDefinedBenefitMember2020-10-012020-12-310000927653country:USmck:TerminationofU.SDefinedBenefitPensionPlanandSettlementofRetirementBenefitsMemberus-gaap:PensionPlansDefinedBenefitMember2019-04-012020-03-310000927653country:USus-gaap:PensionPlansDefinedBenefitMemberus-gaap:UnfundedPlanMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:UnfundedPlanMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:UnfundedPlanMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:USTreasuryAndGovernmentMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:USTreasuryAndGovernmentMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Memberus-gaap:USTreasuryAndGovernmentMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:USTreasuryAndGovernmentMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:USTreasuryAndGovernmentMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:USTreasuryAndGovernmentMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Memberus-gaap:USTreasuryAndGovernmentMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:USTreasuryAndGovernmentMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FixedIncomeFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Membermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMembermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMembermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2021-03-310000927653us-gaap:ForeignPlanMembermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Membermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMembermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMembermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2020-03-310000927653us-gaap:ForeignPlanMembermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel3Member2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PortionAtOtherThanFairValueFairValueDisclosureMemberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PortionAtOtherThanFairValueFairValueDisclosureMemberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMember2020-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PortionAtOtherThanFairValueFairValueDisclosureMembermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PortionAtOtherThanFairValueFairValueDisclosureMembermck:DefinedBenefitPlanOtherFundsMemberus-gaap:PensionPlansDefinedBenefitMember2020-03-310000927653us-gaap:PensionPlansDefinedBenefitMember2020-04-012021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMembermck:NorwegianPublicServicePensionFundSPKMember2021-03-310000927653us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMembermck:NorwegianPublicServicePensionFundSPKMember2020-03-310000927653mck:FirstPartOfPayContributionMember2020-04-012021-03-310000927653mck:SecondPartOfPayContributionMember2020-04-012021-03-310000927653us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-03-310000927653us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2020-03-310000927653us-gaap:LoansPayableMembermck:EuroDenominatedNotesMemberus-gaap:NetInvestmentHedgingMember2020-03-310000927653us-gaap:LoansPayableMembermck:EuroDenominatedNotesMemberus-gaap:NetInvestmentHedgingMember2021-03-310000927653us-gaap:LoansPayableMembermck:EuroDenominatedNotesMember2019-12-31iso4217:GBP0000927653us-gaap:LoansPayableMembermck:BritishPoundSterlingDenominatedNotesMember2019-03-310000927653us-gaap:LoansPayableMembermck:BritishPoundSterlingDenominatedNotesMember2019-09-30iso4217:CAD0000927653us-gaap:DesignatedAsHedgingInstrumentMembermck:November2018CrossCurrencySwapsMemberus-gaap:NetInvestmentHedgingMember2021-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMembermck:November2018CrossCurrencySwapsMemberus-gaap:NetInvestmentHedgingMember2020-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMembermck:November2018CrossCurrencySwapsMemberus-gaap:NetInvestmentHedgingMember2019-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMembermck:November2018CrossCurrencySwapsMemberus-gaap:NetInvestmentHedgingMember2019-04-012020-03-310000927653us-gaap:NetInvestmentHedgingMember2020-04-012021-03-310000927653us-gaap:NetInvestmentHedgingMember2019-04-012020-03-310000927653us-gaap:NetInvestmentHedgingMember2018-04-012019-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMember2021-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMember2020-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CashFlowHedgingMemberus-gaap:InterestRateSwapMember2020-04-270000927653mck:ForwardContractsandCrossCurrencySwapsMember2020-04-012021-03-310000927653mck:ForwardContractsandCrossCurrencySwapsMember2019-04-012020-03-310000927653mck:ForwardContractsandCrossCurrencySwapsMember2018-04-012019-03-310000927653us-gaap:NondesignatedMembercurrency:GBPus-gaap:ForeignExchangeContractMember2021-03-310000927653us-gaap:NondesignatedMembercurrency:GBPus-gaap:ForeignExchangeContractMember2020-03-310000927653mck:ForwardContractstoOffsetImpactofIneffectivenessofNetinvestmentHedgesMemberus-gaap:NondesignatedMember2020-12-310000927653mck:ForwardContractstoOffsetImpactofIneffectivenessofNetinvestmentHedgesMemberus-gaap:NondesignatedMember2019-04-012020-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:CurrencySwapMember2021-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentLiabilitiesMemberus-gaap:CurrencySwapMember2021-03-310000927653mck:PrepaidExpensesandOtherAssetsOtherAccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMember2021-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:CurrencySwapMember2020-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentLiabilitiesMemberus-gaap:CurrencySwapMember2020-03-310000927653mck:PrepaidExpensesandOtherAssetsOtherAccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMember2020-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentAssetsMemberus-gaap:CurrencySwapMember2021-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentLiabilitiesMemberus-gaap:CurrencySwapMember2021-03-310000927653mck:OtherNoncurrentAssetsLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMember2021-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentAssetsMemberus-gaap:CurrencySwapMember2020-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentLiabilitiesMemberus-gaap:CurrencySwapMember2020-03-310000927653mck:OtherNoncurrentAssetsLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMember2020-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentLiabilitiesMemberus-gaap:InterestRateSwapMember2021-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentLiabilitiesMemberus-gaap:InterestRateSwapMember2020-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMember2021-03-310000927653us-gaap:DesignatedAsHedgingInstrumentMember2020-03-310000927653us-gaap:NondesignatedMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeContractMember2021-03-310000927653us-gaap:NondesignatedMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeContractMember2020-03-310000927653us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMemberus-gaap:OtherCurrentLiabilitiesMember2021-03-310000927653us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMemberus-gaap:OtherCurrentLiabilitiesMember2020-03-310000927653us-gaap:NondesignatedMember2021-03-310000927653us-gaap:NondesignatedMember2020-03-3100009276532021-01-012021-03-310000927653us-gaap:FairValueMeasurementsNonrecurringMember2020-03-310000927653us-gaap:FairValueMeasurementsNonrecurringMember2021-03-310000927653us-gaap:CarryingReportedAmountFairValueDisclosureMember2021-03-310000927653us-gaap:EstimateOfFairValueFairValueDisclosureMember2021-03-310000927653us-gaap:CarryingReportedAmountFairValueDisclosureMember2020-03-310000927653us-gaap:EstimateOfFairValueFairValueDisclosureMember2020-03-310000927653srt:MinimumMembermck:GuaranteeObligationsInventoryRepurchaseGuaranteesMember2020-04-012021-03-310000927653srt:MaximumMembermck:GuaranteeObligationsInventoryRepurchaseGuaranteesMember2020-04-012021-03-310000927653srt:MinimumMembermck:GuaranteeObligationsCustomersDebtMember2020-04-012021-03-310000927653srt:MaximumMembermck:GuaranteeObligationsCustomersDebtMember2020-04-012021-03-310000927653mck:GuaranteeObligationsInventoryRepurchaseGuaranteesMember2021-03-310000927653mck:GuaranteeObligationsCustomersDebtMember2021-03-310000927653us-gaap:StandbyLettersOfCreditMember2021-03-31mck:case0000927653mck:InreNationalPrescriptionOpiateLitigationMember2021-03-310000927653us-gaap:PendingLitigationMembermck:InreNationalPrescriptionOpiateLitigationMember2020-01-130000927653mck:InvestigationintoFactorsContributingtoIncreaseinOpioidrelatedHospitalizationsandDeathsMember2021-03-31mck:state0000927653mck:InvestigationintoFactorsContributingtoIncreaseinOpioidrelatedHospitalizationsandDeathsMember2017-12-052021-03-310000927653mck:InvestigationintoFactorsContributingtoIncreaseinOpioidrelatedHospitalizationsandDeathsMembercountry:CA2021-03-31mck:stateAttorneymck:countymck:company0000927653mck:ThreeLargestU.S.PharmaceuticalDistributorsMemberus-gaap:PendingLitigationMembermck:InreNationalPrescriptionOpiateLitigationMember2021-03-310000927653mck:ThreeLargestU.S.PharmaceuticalDistributorsMemberus-gaap:PendingLitigationMembermck:InreNationalPrescriptionOpiateLitigationMember2019-10-212021-03-310000927653us-gaap:PendingLitigationMembermck:InreNationalPrescriptionOpiateLitigationMember2019-10-212021-03-310000927653us-gaap:PendingLitigationMembermck:InreNationalPrescriptionOpiateLitigationMember2021-03-310000927653mck:NationalPrescriptionOpiateLitigationMember2020-04-012021-03-310000927653mck:UnitedStatesexrel.Manchesterv.PurduePharmaL.P.etalMember2019-02-252019-02-25mck:complaint0000927653mck:UnitedStatesofAmericaexrel.CarlKelleyandMichaelMcElligott19cv2233andStateofCaliforniaexrel.CarlKelleyandMichaelMcElligottCGC19576931Member2019-12-31mck:relator0000927653mck:UnitedStatesofAmericaexrel.CarlKelleyandMichaelMcElligott19cv2233andStateofCaliforniaexrel.CarlKelleyandMichaelMcElligottCGC19576931Member2019-12-012019-12-310000927653mck:InsuranceCoverageLitigationMember2020-10-230000927653srt:MinimumMembermck:TrueHealthChiropracticInc.etal.v.McKessonCorporationetalMember2013-05-172013-05-170000927653srt:MaximumMembermck:TrueHealthChiropracticInc.etal.v.McKessonCorporationetalMember2013-05-172013-05-17mck:fax_number0000927653mck:TrueHealthChiropracticInc.etal.v.McKessonCorporationetalMember2019-08-13mck:faxmck:investmentFund0000927653mck:PolygonEuropeanEquityOpportunityMasterFundetal.v.McKessonEuropeHoldingsGmbHCo.KGaAMember2017-12-292017-12-290000927653mck:DavidsonKempnerInternationalBVILtd.etal.v.McKessonEuropeHoldingsGmbHCo.KGaAMember2017-12-302017-12-300000927653mck:UnitedStatesexrel.Piacentilev.AmgenInc.etal.Member2013-04-162013-04-16mck:defendant0000927653mck:UnitedStatesexrel.OmniHealthcareInc.v.McKessonCorporationetal.Member2018-04-032018-04-03mck:city0000927653mck:UnitedStatesexrel.OmniHealthcareInc.v.McKessonCorporationetal.Member2018-04-03mck:officer0000927653mck:EvanstonPolicePensionFundv.McKessonCorporationMember2018-12-120000927653mck:PowellPrescriptionCenteretal.v.SurescriptsLLCetal.andIntergratedPharmaceuticalSolutionsLLCv.SurescriptsLLCMember2019-10-310000927653mck:KennebunkVillagePharmacyInc.v.SureScriptsLLCetal.119cv7445Whitmanv.SureScriptsLLCetal.No.119cv7448BBKGlobalCorp.v.SureScriptsLLCetal.119cv7640Member2019-11-300000927653mck:ConsolidatedActionsvSureScriptsLLCMember2019-12-310000927653mck:UnitedStatesExRelHartVMcKessonCorporationEtAl15Cv00903RAMember2020-07-012020-07-310000927653mck:CivilInvestigativeDemandsbytheU.S.AttorneysOfficefortheEasternDistrictofNewYorkMember2018-08-31mck:softwareProduct0000927653mck:NewYorkOpioidTaxSurchargeMembermck:USPharmaceuticalSegmentMember2020-04-012021-03-310000927653mck:NewYorkOpioidTaxSurchargeMember2020-04-012021-03-31mck:site0000927653mck:EnvironmentalLitigationMember2021-03-310000927653srt:MinimumMembermck:EnvironmentalLitigationMember2020-04-012021-03-310000927653srt:MaximumMembermck:EnvironmentalLitigationMember2020-04-012021-03-310000927653mck:EnvironmentalLitigationMember2020-04-012021-03-31mck:vote00009276532020-04-012020-06-3000009276532020-07-012020-07-3100009276532018-05-310000927653mck:OpenMarketTransactionsMember2018-04-012019-03-310000927653mck:AcceleratedShareRepurchaseMember2018-04-012019-03-310000927653mck:OpenMarketTransactionsMember2019-04-012020-03-310000927653mck:AcceleratedShareRepurchaseMember2019-04-012020-03-3100009276532021-01-310000927653mck:OpenMarketTransactionsMember2020-04-012021-03-310000927653us-gaap:OtherCurrentLiabilitiesMember2021-03-3100009276532020-03-092020-03-090000927653us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2020-04-012021-03-310000927653us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2019-04-012020-03-310000927653us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2018-04-012019-03-310000927653us-gaap:AccumulatedNetInvestmentGainLossIncludingPortionAttributableToNoncontrollingInterestMember2020-04-012021-03-310000927653us-gaap:AccumulatedNetInvestmentGainLossIncludingPortionAttributableToNoncontrollingInterestMember2019-04-012020-03-310000927653us-gaap:AccumulatedNetInvestmentGainLossIncludingPortionAttributableToNoncontrollingInterestMember2018-04-012019-03-310000927653us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2020-04-012021-03-310000927653us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2019-04-012020-03-310000927653us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2018-04-012019-03-310000927653us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2020-04-012021-03-310000927653us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2019-04-012020-03-310000927653us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2018-04-012019-03-310000927653mck:AccumulatedDefinedBenefitPlansAdjustmentNetPriorServiceandNetGainLossIncludingPortionAttributabletoNoncontrollingInterestMember2020-04-012021-03-310000927653mck:AccumulatedDefinedBenefitPlansAdjustmentNetPriorServiceandNetGainLossIncludingPortionAttributabletoNoncontrollingInterestMember2019-04-012020-03-310000927653mck:AccumulatedDefinedBenefitPlansAdjustmentNetPriorServiceandNetGainLossIncludingPortionAttributabletoNoncontrollingInterestMember2018-04-012019-03-310000927653mck:AccumulatedDefinedBenefitPlansAdjustmentAmortizationofActuarialLossPriorServiceCostandTransitionObligationIncludingPortionAttributabletoNoncontrollingInterestMember2020-04-012021-03-310000927653mck:AccumulatedDefinedBenefitPlansAdjustmentAmortizationofActuarialLossPriorServiceCostandTransitionObligationIncludingPortionAttributabletoNoncontrollingInterestMember2019-04-012020-03-310000927653mck:AccumulatedDefinedBenefitPlansAdjustmentAmortizationofActuarialLossPriorServiceCostandTransitionObligationIncludingPortionAttributabletoNoncontrollingInterestMember2018-04-012019-03-310000927653mck:AccumulatedDefinedBenefitPlansForeignCurrencyAdjustmentIncludingPortionAttributabletoNoncontrollingInterestMember2020-04-012021-03-310000927653mck:AccumulatedDefinedBenefitPlansForeignCurrencyAdjustmentIncludingPortionAttributabletoNoncontrollingInterestMember2019-04-012020-03-310000927653mck:AccumulatedDefinedBenefitPlansForeignCurrencyAdjustmentIncludingPortionAttributabletoNoncontrollingInterestMember2018-04-012019-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2020-04-012021-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2019-04-012020-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2018-04-012019-03-310000927653us-gaap:AccumulatedForeignCurrencyAdjustmentAttributableToNoncontrollingInterestMember2020-04-012021-03-310000927653us-gaap:AccumulatedForeignCurrencyAdjustmentAttributableToNoncontrollingInterestMember2019-04-012020-03-310000927653us-gaap:AccumulatedForeignCurrencyAdjustmentAttributableToNoncontrollingInterestMember2018-04-012019-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetGainLossAttributableToNoncontrollingInterestMember2020-04-012021-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetGainLossAttributableToNoncontrollingInterestMember2019-04-012020-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetGainLossAttributableToNoncontrollingInterestMember2018-04-012019-03-310000927653us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2019-03-310000927653us-gaap:AccumulatedNetInvestmentGainLossIncludingPortionAttributableToNoncontrollingInterestMember2019-03-310000927653us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2019-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetUnamortizedGainLossMember2019-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetUnamortizedGainLossMember2019-04-012020-03-310000927653us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2020-03-310000927653us-gaap:AccumulatedNetInvestmentGainLossIncludingPortionAttributableToNoncontrollingInterestMember2020-03-310000927653us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2020-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetUnamortizedGainLossMember2020-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetUnamortizedGainLossMember2020-04-012021-03-310000927653us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2021-03-310000927653us-gaap:AccumulatedNetInvestmentGainLossIncludingPortionAttributableToNoncontrollingInterestMember2021-03-310000927653us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2021-03-310000927653us-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetUnamortizedGainLossMember2021-03-310000927653mck:CaliforniaFoundationMemberus-gaap:OtherCurrentLiabilitiesMember2019-03-310000927653mck:McKessonFoundationMember2020-01-012020-03-310000927653us-gaap:InvesteeMember2020-04-012021-03-310000927653us-gaap:InvesteeMember2019-04-012020-03-310000927653us-gaap:InvesteeMember2018-04-012019-03-310000927653mck:U.S.PharmaceuticalandSpecialtySolutionsMemberus-gaap:InvesteeMember2020-04-012021-03-310000927653mck:U.S.PharmaceuticalandSpecialtySolutionsMemberus-gaap:InvesteeMember2019-04-012020-03-310000927653mck:U.S.PharmaceuticalandSpecialtySolutionsMemberus-gaap:InvesteeMember2018-04-012019-03-31mck:countrymck:numberOfProducts0000927653mck:USPharmaceuticalSegmentMember2018-04-012019-03-310000927653mck:MedicalSurgicalSolutionsSegmentMember2018-04-012019-03-310000927653mck:PrescriptionTechnologySolutionsSegmentMember2018-04-012019-03-310000927653us-gaap:DisposalGroupDisposedOfBySaleNotDiscontinuedOperationsMembermck:InternationalSegmentMembermck:GermanWholesaleBusinessMember2020-04-012021-03-310000927653mck:RexallHealthMembermck:InternationalSegmentMembercountry:CAmck:ThirdPartySellerofRexallHealthMember2018-04-012019-03-310000927653mck:ShareholderDerivativeActionMember2020-04-012021-03-310000927653mck:ForTwoCountiesInreNationalPrescriptionOpiateLitigationMember2019-04-012020-03-310000927653country:US2021-03-310000927653country:US2020-03-310000927653us-gaap:NonUsMember2021-03-310000927653us-gaap:NonUsMember2020-03-3100009276532020-10-012020-12-3100009276532019-04-012019-06-3000009276532019-07-012019-09-3000009276532019-10-012019-12-3100009276532020-01-012020-03-310000927653us-gaap:AllowanceForCreditLossMember2020-03-310000927653us-gaap:AllowanceForCreditLossMember2020-04-012021-03-310000927653us-gaap:AllowanceForCreditLossMember2021-03-310000927653mck:OtherAllowancesMember2020-03-310000927653mck:OtherAllowancesMember2020-04-012021-03-310000927653mck:OtherAllowancesMember2021-03-310000927653us-gaap:AllowanceForCreditLossMember2019-03-310000927653us-gaap:AllowanceForCreditLossMember2019-04-012020-03-310000927653mck:OtherAllowancesMember2019-03-310000927653mck:OtherAllowancesMember2019-04-012020-03-310000927653us-gaap:AllowanceForCreditLossMember2018-03-310000927653us-gaap:AllowanceForCreditLossMember2018-04-012019-03-310000927653mck:OtherAllowancesMember2018-03-310000927653mck:OtherAllowancesMember2018-04-012019-03-310000927653mck:ValuationAllowancesAndReservesDeductionsWrittenOffMember2020-04-012021-03-310000927653mck:ValuationAllowancesAndReservesDeductionsWrittenOffMember2019-04-012020-03-310000927653mck:ValuationAllowancesAndReservesDeductionsWrittenOffMember2018-04-012019-03-310000927653mck:ValuationAllowancesAndReservesDeductionsCreditedToOtherAccountsMember2020-04-012021-03-310000927653mck:ValuationAllowancesAndReservesDeductionsCreditedToOtherAccountsMember2019-04-012020-03-310000927653mck:ValuationAllowancesAndReservesDeductionsCreditedToOtherAccountsMember2018-04-012019-03-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended March 31, 2021
OR
| | | | | |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 1-13252
McKESSON CORPORATION
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | | 94-3207296 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
| | |
| | |
6555 State Hwy 161,
Irving, TX 75039
(Address of principal executive offices, including zip code)
(972) 446-4800
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | |
| (Title of each class) | (Trading Symbol) | (Name of each exchange on which registered) |
| Common stock, $0.01 par value | MCK | New York Stock Exchange |
| 0.625% Notes due 2021 | MCK21A | New York Stock Exchange |
| 1.500% Notes due 2025 | MCK25 | New York Stock Exchange |
| 1.625% Notes due 2026 | MCK26 | New York Stock Exchange |
| 3.125% Notes due 2029 | MCK29 | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically, every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | | ☒ | | Accelerated filer | | ☐ |
| Non-accelerated filer | | ☐ | | Smaller reporting company | | ☐ |
| | | | Emerging growth company | | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, computed by reference to the closing price as of the last business day of the registrant’s most recently completed second fiscal quarter, September 30, 2020, was approximately $23.9 billion.
Number of shares of common stock outstanding on April 30, 2021: 158,186,277
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for its 2021 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
TABLE OF CONTENTS
| | | | | | | | |
| Item | Page |
| | |
| | |
| | |
| 1 | | |
| | |
| 1A. | | |
| | |
| 1B. | | |
| | |
| 2 | | |
| | |
| 3 | | |
| | |
| 4 | | |
| | |
| | |
| | |
| | |
| | |
| 5 | | |
| | |
| 6. | | |
| | |
| 7. | | |
| | |
| 7A. | | |
| | |
| 8 | | |
| | |
| 9 | | |
| | |
| 9A. | | |
| | |
| 9B. | | |
| | |
| | |
| | |
| 10. | | |
| | |
| 11. | | |
| | |
| 12. | | |
| | |
| 13. | | |
| | |
| 14. | | |
| | |
| | |
| | |
| 15. | | |
| | |
| 16. | | |
| | |
| | |
PART I
Item 1. Business.
General
McKesson Corporation (“McKesson,” the “Company,” or “we,” and other similar pronouns), originally founded in 1833, is a global leader in healthcare supply chain management solutions, retail pharmacy, community oncology and specialty care, and healthcare information solutions. McKesson partners with life sciences companies, manufacturers, providers, pharmacies, governments, and other healthcare organizations to help provide the right medicines, medical products, and healthcare services to the right patients at the right time, safely, and cost-effectively.
The Company’s fiscal year begins on April 1 and ends on March 31. Unless otherwise noted, all references in this document to a particular year shall mean the Company’s fiscal year. The Company was incorporated on July 7, 1994 in the State of Delaware.
Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act,”) are available free of charge on the Company’s website (www.mckesson.com under the “Investors — Financials — SEC Filings” caption) as soon as reasonably practicable after such material is electronically filed with, or furnished to, the Securities and Exchange Commission (“SEC” or the “Commission”). The content on any website referred to in this Annual Report on Form 10-K is not incorporated by reference into this report, unless expressly noted otherwise. The SEC maintains a website that contains reports, proxy and information statements, and other information regarding issuers, including the Company, that file electronically with the SEC. The address of the website is www.sec.gov.
Business Segments
Commencing with the second quarter of 2021, the Company operates its business in four reportable segments: U.S. Pharmaceutical, International, Medical-Surgical Solutions, and Prescription Technology Solutions (“RxTS”). The Company’s equity method investment in Change Healthcare LLC (“Change Healthcare JV”), which was split-off from McKesson in the fourth quarter of 2020, has been included in Other for retrospective periods presented.
Our U.S. Pharmaceutical segment distributes branded, generic, specialty, biosimilar and over-the-counter (“OTC”) pharmaceutical drugs, and other healthcare-related products. This segment provides practice management, technology, clinical support, and business solutions to community-based oncology and other specialty practices. In addition, the segment sells financial, operational, and clinical solutions to pharmacies (retail, hospital, alternate site) and provides consulting, outsourcing, technological, and other services.
Our International segment provides distribution and services to wholesale, institutional, and retail customers in 13 European countries and Canada where we own, partner or franchise with retail pharmacies, and support better, safer patient care by delivering vital medicines, supplies, and information technology solutions.
Our Medical-Surgical Solutions segment provides medical-surgical supply distribution, logistics, and other services to healthcare providers, including physician offices, surgery centers, nursing homes, hospital reference labs, and home health care agencies. We offer more than 275,000 national brand medical-surgical products as well as McKesson’s own line of high-quality products through a network of distribution centers within the United States (“U.S.”).
Our RxTS segment brings together CoverMyMeds, RelayHealth, RxCrossroads, and McKesson Prescription Automation, including Multi-Client Central Fill as a Service, to serve our biopharma and life sciences partners and patients. Together, we work across the healthcare delivery system to connect pharmacies, providers, payers, and biopharma for next-generation patient access and adherence solutions that help people get the medicine they need to live healthier lives.
U.S. Pharmaceutical Segment:
Our U.S. Pharmaceutical segment provides distribution and logistics services for branded, generic, specialty, biosimilar, and OTC pharmaceutical drugs along with other healthcare-related products to customers. This business provides solutions and services to pharmacies, hospitals and other providers, pharmaceutical manufacturers, physicians, payers, and patients throughout the U.S. and Puerto Rico. We also source generic pharmaceutical drugs through our joint sourcing entity, ClarusONE Sourcing Services LLP (“ClarusONE”).
Our U.S. Pharmaceutical segment operates and serves customers through a network of 33 distribution centers, as well as a strategic redistribution center, a primary and a secondary redistribution center. We invest in technology and other systems at all of our distribution centers to enhance safety, reliability, and product availability. For example, we offer McKesson ConnectSM, an internet-based ordering system that provides item look-up and real-time inventory availability as well as ordering, purchasing, third-party reconciliation, and account management functionality. We make extensive use of technology as an enabler to ensure customers have the right products at the right time in the right place.
To maximize distribution efficiency and effectiveness, we follow the Six Sigma methodology, which is an analytical approach that emphasizes setting high-quality objectives, collecting data, and analyzing results to a fine degree in order to improve processes, reduce costs, and enhance service accuracy and safety. We provide solutions to our customers including supply management technology, world-class marketing programs, managed care, repackaging products, and services to help them meet their business and quality goals. We continue to implement information systems to help achieve greater consistency and accuracy both internally and for our customers.
We have four primary customer pharmaceutical distribution channels: (i) retail national accounts which include national and regional chains, food and drug combinations, mail order pharmacies, and mass merchandisers, (ii) independent, small, and medium chain retail pharmacies, (iii) institutional healthcare providers such as hospitals, health systems, integrated delivery networks, and long-term care providers, and (iv) provider solutions.
Retail National Accounts: We provide business solutions that help retail national account customers increase revenues and profitability. Solutions include:
•Central FillSM - Prescription refill service that enables pharmacies to more quickly refill prescriptions remotely, more accurately, and at a lower cost, while reducing inventory levels and improving customer service.
•Redistribution Centers - Three facilities totaling over 930,000 square feet that offer access to inventory for single source warehouse purchasing, including pharmaceuticals and biologics. These distribution centers also provide the foundation for a two-tiered distribution network that supports best-in-class direct store delivery.
•McKesson SynerGx® - Generic pharmaceutical purchasing program and inventory management that helps pharmacies maximize their cost savings with a broad selection of generic drugs, competitive pricing, and one-stop shopping.
•Inventory Management - An integrated solution comprising forecasting software and automated replenishment technologies that reduce inventory-carrying costs.
•ExpressRx Track™ - Pharmacy automation solution featuring state-of-the-art robotics, upgraded imaging, and expanded vial capabilities, and industry-leading speed and accuracy in a small footprint.
Independent, Small and Medium Chain Retail Pharmacies: We provide managed care contracting, branding and advertising, merchandising, purchasing, operational efficiency, and automation that help independent pharmacists focus on patient care while improving profitability. Solutions include:
•Health Mart® - Health Mart® is a national network of approximately 5,000 independently-owned pharmacies and is one of the industry’s most comprehensive pharmacy franchise programs. Health Mart® provides franchisees support for managed care contracting, branding and local marketing solutions, the Health Mart private label line of products, merchandising solutions, and programs for enhanced patient support.
•Health Mart Atlas® - Comprehensive managed care and reconciliation assistance services that help independent pharmacies save time, access competitive reimbursement rates, and improve cash flow.
•McKesson Reimbursement AdvantageSM (“MRA”) - MRA is one of the industry’s most comprehensive reimbursement optimization packages, comprising financial services (automated claim resubmission), analytic services, and customer care.
•McKesson OneStop Generics® - Generic pharmaceutical purchasing program that helps pharmacies maximize their cost savings with a broad selection of generic drugs, competitive pricing, and one-stop shopping.
•Sunmark® - Complete line of products that provide retail independent pharmacies with value-priced alternatives to national brands.
•FrontEdge™ - Strategic planning, merchandising, and price maintenance program that helps independent pharmacies maximize store profitability.
•McKesson Sponsored Clinical Services (“SCS”) Network - Access to patient-support services that allow pharmacists to earn service fees and to develop stronger patient relationships.
•McKesson RxOwnership Program - Assist independent pharmacist owners with the opportunity to remain independent via succession planning and business operation loans.
Institutional Healthcare Providers: We provide electronic ordering/purchasing and supply chain management systems that help customers improve financial performance, increase operational efficiencies, and deliver better patient care. Solutions include:
•Fulfill-RxSM - Ordering and inventory management system that empowers hospitals to optimize the often complicated processes related to unit-based cabinet replenishment and inventory management.
•Asset Management - Award-winning inventory optimization and purchasing management program that helps institutional providers lower costs while ensuring product availability.
•SKY Packaging - Blister, Unit of Use, and Unit dose packaging containing the most widely prescribed dosages and strengths in generic oral-solid and liquid medications. SKY Packaging enables acute care, long-term care, and institutional pharmacies to provide cost-effective, uniform packaging.
•McKesson Plasma and Biologics - A full portfolio of plasma-derivatives and biologic products.
•McKesson OneStop Generics® - Described above.
Provider Solutions:
The U.S. Pharmaceutical segment provides a range of solutions to oncology and other specialty practices and offers community specialists (oncologists, rheumatologists, ophthalmologists, urologists, neurologists, and other specialists) an extensive set of customizable products and services designed to strengthen core practice operations, enhance value-based care delivery, and expand their service offering to patients. Community-based physicians in this business have broad flexibility and discretion to select the products and commitment levels that best meet their practice needs. Services in provider solutions include specialty drug distribution, group purchasing organizations (“GPO”) like Onmark®, technology solutions, practice consulting services, and vaccine distribution, including our exclusive distributor relationship with the Centers for Disease Control and Prevention’s (“CDC”) Vaccines for Children program. Additionally, to support the U.S. efforts to fight the coronavirus disease 2019 (“COVID-19”) pandemic, this segment is distributing the COVID-19 vaccines manufactured by ModernaTX, Inc. and Janssen Biotech Inc., a Janssen pharmaceutical company of Johnson & Johnson, at the direction of the U.S. government.
This business provides a variety of solutions, including practice operations, healthcare information technology, revenue cycle management and managed care contracting solutions, evidence-based guidelines, and quality measurements to support U.S. Oncology Network (“USON”), one of the nation’s largest networks of physician-led, integrated, community-based oncology practices dedicated to advancing high-quality, evidence-based cancer care. We also support U.S. Oncology Research, one of the nation’s largest research networks, specializing in oncology clinical trials.
This segment includes our Ontada business, providing software to support the clinical, financial, and operational needs of our oncology practice partners. Ontada also partners with oncology providers and biopharma partners to perform real-world evidence studies, retrospective research, and to provide clinical data insights, advisory solutions and education opportunities.
This segment also offers solutions which enable its customers to drive greater efficiencies in their day to day operations, effectively managing their inventories and complying with complex government regulations. Solutions include McKesson Pharmacy Systems, MacroHelix and Supply Logix, all of which provide innovative software technology and services that support retail pharmacies and hospitals.
When we discuss specialty products or services, we consider the following factors: diseases requiring complex treatment regimens such as cancer and rheumatoid arthritis; plasma and biologics products; ongoing clinical monitoring requirements, high-cost, special handling, storage, and delivery requirements and, in some cases, exclusive distribution arrangements. Our use of the term “specialty” may not be comparable to that used by other industry participants, including our competitors.
International Segment:
Our International segment provides distribution and services to wholesale, institutional, and retail customers in 13 European countries where we own, partner, or franchise with retail pharmacies and operate through two businesses: Pharmaceutical Distribution and Retail Pharmacy. Our operations in Canada, including Rexall retail pharmacies, support better, safer patient care by delivering vital medicines, supplies, and information technology solutions throughout Canada.
Our European Pharmaceutical Distribution business delivers pharmaceutical and other healthcare-related products to pharmacies across Europe. This business functions as a vital link, using technology-enabled management systems at our regional wholesale branches to connect manufacturers to retail pharmacies, supplying medicines and other products sold in pharmacies.
Our European Retail Pharmacy business serves patients and consumers in European countries directly through approximately 2,100 of our own pharmacies and 5,500 participant pharmacies operating under brand partnership arrangements. In addition, this business includes outpatient dispensing, eCommerce and homecare arrangements mainly in the United Kingdom (“U.K.”), and provides traditional prescription pharmaceuticals, non-prescription products and medical services, and operates under the Lloyds pharmacy branding in Belgium, Ireland, Italy, Sweden, and the U.K. In addition, we partner with independent pharmacies under local banner programs.
McKesson Canada is one of the largest pharmaceutical wholesale and retail distributors in Canada. The wholesale business delivers products to retail pharmacies, hospitals, long-term care centers, clinics and institutions in Canada through a national network of distribution centers and provides logistics and distribution services for manufacturers.
Beyond wholesale pharmaceutical logistics and distribution, McKesson Canada provides automation and technology solutions to its retail and hospital customers. Additionally, McKesson Canada provides comprehensive specialty health services to Canadians, including a national network of specialty pharmacies, personalized patient care and support programs, and INVIVA, Canada’s first and largest accredited network of private infusion clinics.
The Canada retail business includes over 2,500 banner pharmacies under the IDA, Guardian, The Medicine Shoppe, Remedy’sRx, Proxim, and Uniprix banners, and more than 400 owned pharmacies under the Rexall brand where we provide patients with greater choice and access, integrated pharmacy care and industry-leading service levels. McKesson Canada also owns and operates Well.ca, a leading Canadian online health and wellness retailer.
Medical-Surgical Solutions Segment:
Our Medical-Surgical Solutions segment delivers medical-supply distribution, logistics, biomedical maintenance, and other services to healthcare providers across the alternate-site spectrum. Our more than 250,000 customers include physician offices, surgery centers, post-acute care facilities, hospital reference labs, and home health agencies. We distribute medical-surgical supplies (such as gloves, needles, syringes and wound care products), infusion pumps, laboratory equipment and pharmaceuticals. Through a network of distribution centers within the U.S., we offer more than 275,000 products from national brand manufacturers and McKesson’s own high-quality product line. Through the right mix of products and services, we help improve efficiencies, profitability and compliance. We also never lose focus on helping customers improve patient and business outcomes. We develop customized plans to address the product, operational, and clinical support needs of our customers, including tackling inventory management, reducing administrative burdens, and training and educating clinical staff. We deliver for our customers, so they can deliver and care for their patients. Additionally, under a contract with the Department of Health and Human Services (“HHS”), McKesson’s Medical-Surgical business leverages its expertise to manage the assembly of supply kits needed to administer COVID-19 vaccines, as well as some of the sourcing of those supplies. The kits are being produced and distributed at the direction of HHS to support the administration of all vaccines approved in the U.S.
Prescription Technology Solutions Segment:
Our Prescription Technology Solutions segment works across the healthcare delivery system to connect pharmacies, providers, payers, and biopharma for next generation patient access and adherence solutions and operates primarily through the following businesses:
•CoverMyMeds – Provides solutions to help patients get the medications they need to live healthy lives by seamlessly connecting the healthcare network to improve medication access; thereby increasing speed to therapy and reducing prescription abandonment. By facilitating appropriate access to medications, the company can help its customers avoid millions of dollars each year in administrative waste and avoidable medical spending caused by prescription abandonment.
•RelayHealth Pharmacy Solutions – Provides workflow solutions to connect key healthcare stakeholders with more than 50,000 U.S. retail pharmacies and processes more than 18 billion pharmacy transactions annually.
•RxCrossroads – Uses deep insights and innovative technology to help biopharma manufacturers thrive throughout the product lifecycle and create flexible, connected solutions that increase access, adherence, and safe use conditions for therapies and interventions.
•McKesson Prescription Automation (“MPA”) – Provides customized pharmacy automation technology that allows our partners to control costs, work faster, offer higher-quality products, and better serve patients.
•Multi-Client Central Fill as a Service – McKesson-owned pharmacy that utilizes MPA dispensing automation to enable low-cost fulfillment of up to 50,000 prescriptions daily for retail and independent pharmacy customers, new digital pharmacies, and manufacturers.
Other:
Change Healthcare: Our equity ownership interest in Change Healthcare JV, a joint venture, has been accounted for using the equity method of accounting. Change Healthcare JV provided software and analytics, network solutions, and technology-enabled services that deliver wide-ranging financial, operational, and clinical benefits to payers, providers and consumers. On March 10, 2020, we completed the separation of our interest in the Change Healthcare JV through a split-off transaction. This transaction reduced our investment in the Change Healthcare JV to zero. Refer to Financial Note 2, “Investment in Change Healthcare Joint Venture,” to the consolidated financial statements appearing in this Annual Report on Form 10-K for additional information related to this transaction.
Restructuring, Business Combinations, Investments, and Divestitures
We have undertaken additional strategic initiatives in recent years designed to further focus on our core healthcare businesses and enhance our competitive position. These initiatives are detailed in Financial Notes 2, 3, 4, and 5, “Investment in Change Healthcare Joint Venture,” “Held for Sale,” “Restructuring, Impairment, and Related Charges,” and “Business Acquisitions and Divestitures,” to the consolidated financial statements appearing in this Annual Report on Form 10-K.
Competition
We face highly competitive global environments. Additionally, in recent years the healthcare industry has been subject to increasing consolidation. In the pharmaceutical distribution environment in which our U.S. Pharmaceutical and International segments operate, we face strong competition from international, national, regional and local full-line, short-line and specialty distributors, service merchandisers, self-warehousing chain drug stores, manufacturers engaged in direct distribution, third-party logistics companies, and large payer organizations. Our retail businesses, which primarily operate in our International segment, face competition from various global, national, regional, and local global retailers, including chain and independent pharmacies. We consider our largest competitors in distribution, wholesaling, and logistics to be AmerisourceBergen Corporation and Cardinal Health, Inc.
Our Medical-Surgical Solutions segment provides medical-surgical supply distribution, logistics, and other services to healthcare providers, including physician offices, surgery centers, nursing homes, hospital reference labs, home health care agencies, and alternative health sites with competition from a wide range of national and regional medical supply and equipment distributors throughout the U.S.
Our RxTS business experiences substantial competition from many companies, including other software services firms, consulting firms, shared service vendors, and internet-based companies with technology applicable to the healthcare industry. Competition in this business varies in size from large to small companies, in geographical coverage, and in scope and breadth of products and services offered.
In addition, we compete with other service providers, pharmaceutical and other healthcare manufacturers, as well as other potential customers of our businesses, which may from time to time decide to develop, for their own internal needs, supply management capabilities that might otherwise be provided by our businesses. We believe that our scale and diversity of product and service offerings are our primary competitive advantages. In all areas, key competitive factors include price, quality of service, breadth of product lines, innovation and, in some cases, convenience to the customer.
Patents, Trademarks, Copyrights, and Licenses
McKesson and its subsidiaries hold patents, copyrights, trademarks, and trade secrets related to McKesson products and services. We pursue patent protection for our innovations and obtain copyright protection for our original works of authorship, when such protection is advantageous. Through these efforts, we have developed a portfolio of patents and copyrights in the U.S. and worldwide. In addition, we have registered or applied to register certain trademarks and service marks in the U.S. and in foreign countries.
We believe that, in the aggregate, McKesson’s confidential information, patents, copyrights, trademarks, and intellectual property licenses are important to its operations and market position, but we do not consider any of our businesses to be dependent upon any one patent, copyright, trademark, or trade secret, or any family or families of the same. We cannot guarantee that our intellectual property portfolio will be sufficient to deter misappropriation, theft, or misuse of our technology, nor that we can successfully enjoin infringers. We periodically receive notices alleging that our products or services infringe on third party patents and other intellectual property rights. These claims may result in McKesson entering settlement agreements, paying damages, discontinuing use or sale of accused products, or ceasing other activities. While the outcome of any litigation or dispute is inherently uncertain, we do not believe that the resolution of any of these infringement notices would have a material adverse impact on our results of operation.
We hold inbound licenses for certain intellectual property that is used internally, and in some cases, utilized in McKesson’s products or services. While it may be necessary in the future to seek or renew licenses relating to various aspects of our products and services, we believe, based upon past experience and industry practice, such licenses generally can be obtained on commercially reasonable terms. We believe our operations and products and services are not materially dependent on any single license or other agreement with any third party.
Human Capital
Our vision for a healthier world begins with our employees, who strive to bring our mission to life every day. As a company, we deliver programs that focus on improving employee health and wellness, creating opportunities for growth and development, and providing an inclusive workplace where our employees can reach their full potential. At March 31, 2021, we had approximately 76,000 employees worldwide, including 17,000 part-time employees and 32,000 employees in the U.S.
Diversity, Equity, and Inclusion (“DEI”): At McKesson, we are committed to making DEI integral to everything we do, because we believe building a healthier future is everyone’s business. We build successful teams by recruiting, developing, and retaining diverse talent and we recognize our culture of inclusion as an important element that drives long-term shareholder value. During 2021, we appointed the newly created role of chief impact officer, who will drive our strategy and execution related to DEI as well as sustainability, environmental, social, and governance (“ESG”), and philanthropy.
At March 31, 2021, women and people of color represented the following:
| | | | | | | | | | | |
| | McKesson
Overall | | McKesson Leadership (2) |
Metric (1) | | | |
Women (3) | 63 | % | | 35 | % |
People of Color (4) (5) | 45 | % | | 21 | % |
(1)The data for our metrics is derived from our voluntary, self-identification process as of March 31, 2021 and therefore represents our best estimate at this time.
(2)Represents our leadership at the vice president level and above.
(3)Represents worldwide employees.
(4)Represents U.S. employees only as the data for Canada and Europe is not available.
(5)People of Color includes the following races and ethnicities: Hispanic or Latino, Black or African American, Asian, Native Hawaiian or Other Pacific Islander, American Indian or Alaska Native, or Two or More Races.
Culture and Leadership: What sets McKesson apart as an exceptional place is our people. Our employees understand that together, unified by our global I2CARE and ILEAD principles, we fulfill our mission of improving care in every setting. Our I2CARE values (Integrity, Inclusion, Customer-First, Accountability, Respect, Excellence) are foundational to all that we do, and who we are as a company. ILEAD (Inspire, Leverage, Execute, Advance, Develop) is our common definition and shared commitment to leadership. By embracing this commitment, we bring out the best in ourselves and position McKesson to continue to drive better health – for our company, our customers, and the patients they serve for years to come. We promote leadership behaviors through culture initiatives that offer practical tips on how to debate, decide, and commit, be open and candid, and maintain an enterprise-first mindset when navigating conversations affecting operations within and across our business segments. These values and behaviors help make McKesson unique.
Investment in Employees: To support employee growth, we provide regular feedback and training, and work to create and maintain inclusive work settings where everyone can bring their authentic self to work and feel welcomed and appreciated, and where their perspectives are sought out, heard, and considered. Through training, we encourage leaders to embrace diverse perspectives and lead inclusively. Employee development programs include training, coaching, and 360-degree assessments, which can support the careers of future leaders and their teams. We offer financial assistance programs for higher education opportunities that support employees’ career growth at the company. To provide compensation that is focused on attracting and retaining talent with the skills and experience necessary for a specific role, our compensation program is built on a set of quantifiable factors defined by our guiding principles of internal fairness, market competitiveness, and pay for performance. We operate in several countries and our benefits offerings vary accordingly. We offer health and wellness benefits to advance the physical, mental, and social well-being of our people, savings programs to help prepare them for retirement, and flexible work arrangements, among other benefits offerings, when possible. In response to the COVID-19 pandemic, we offered extended medical benefits covering COVID-19 related visits, treatment and testing, expanded telehealth options, emergency paid time off (“PTO”), and a platform for employees to donate their regular PTO to co-workers who were more impacted by COVID-19. We also seek employee feedback through an annual employee opinion survey, which assesses our employees’ levels of engagement, commitment, and overall satisfaction using industry benchmarks, and we then design action plans to improve those metrics.
Health and Safety: Our security and safety departments employ systems designed to continually monitor our facilities and work environment to help identify and prevent or mitigate any potential risks. This includes having procedures in place and investing in equipment for both physical and electronic security. We routinely assess facilities to monitor closely adherence to established security and safety standards. If we identify a vulnerability, it is documented, and the facility prepares an action plan. Our employees receive specialized training related to their role, work setting, and equipment used in their work environment. As our processes evolve, we update relevant safety training modules, which may include new employee training programs. In response to the COVID-19 pandemic, our priority has been, and continues to be, protecting the health and safety of our employees. The various responses we put in place to mitigate the impact of COVID-19 on our business operations, including telecommuting and work-from-home policies, restricted travel, and enhanced safety measures, are intended to limit employee exposure to the virus that causes COVID-19 as they perform their jobs while also providing employee support programs and a sense of belonging. For additional information on our response to COVID-19 in the workplace, refer to the COVID-19 section of “Trends and Uncertainties” in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 of Part II included in this Annual Report on Form 10-K.
Government Regulation
McKesson, generally and in many of the highly regulated industries in which it operates, is subject to oversight by various federal, state, and local governmental entities. Failure to comply with laws, regulations, and guidance promulgated by those entities could have a material adverse impact to the Company’s business operations, reputation, results of operations, and financial position.
Controlled Substances: We are subject to the operating and security standards of the Drug Enforcement Administration (“DEA”), the U.S. Food and Drug Administration (“FDA”), various state boards of pharmacy, state health departments, HHS, the Centers for Medicare & Medicaid Services (“CMS”), and other comparable agencies. We have received monetary penalties and/or licensing sanctions pursuant to these requirements and future allegations of noncompliance could result in an inability to obtain, maintain or renew permits, licenses or other regulatory approvals needed for the operation of our businesses.
Additionally, the Company is a defendant in approximately 3,200 cases alleging claims related to the distribution of controlled substances (opioids), as described in Financial Note 19, “Commitments and Contingent Liabilities,” to the consolidated financial statements in this Annual Report on Form 10-K. The plaintiffs in those cases include governmental entities (such as states, provinces, counties and municipalities) as well as businesses, groups and individuals. As a result of ongoing, advanced discussions with state attorneys general and plaintiffs’ representatives regarding a framework to resolve the claims of governmental entities, and our assessment of certain other opioid-related claims, we have reached a stage at which a broad settlement of opioid claims by governmental entities is probable and recorded a charge of $8.1 billion for the year ended March 31, 2021 within “Claims and litigation charges, net” in our Consolidated Statement of Operations in this Annual Report on Form 10-K. Because of the many uncertainties associated with any potential settlement arrangement or other resolution of opioid-related litigation, including the uncertainty of the scope of participation by plaintiffs in any potential settlement, we are not able to reasonably estimate the upper or lower ends of the range of ultimate possible loss for all opioid-related litigation matters. The adverse outcome of legal proceedings might also involve significant expense, management time and distraction, and risk of loss that can be difficult to predict or quantify. In addition to this litigation, legislative or regulatory measures related to the distribution of controlled substances such as prescription opioids could affect our business in ways that we may not be able to predict. For example, some states have passed legislation that could require us to pay taxes or assessments on the distribution of opioid medications in those states and other states have considered similar legislation.
Government Contracts: Our contracts with government entities are subject to unique compliance risks and typically are subject to procurement laws that include socio-economic, employment practices, environmental protection, recordkeeping and accounting, and other requirements. We are subject to government audits, investigations and oversight proceedings. Government agencies routinely review and audit government contractors to determine whether they are complying with contractual and legal requirements. If we fail to comply with these requirements, or we fail an audit, we may be subject to various sanctions such as monetary damages, criminal and civil penalties, termination of contracts and suspension or debarment from government contract work. These requirements complicate our business and increase our compliance burden and material non-compliance could harm our reputation.
Local, state, and federal governments continue to strengthen their position and scrutiny over practices involving or allegedly involving fraud, waste, and abuse affecting Medicare, Medicaid, other government healthcare programs, and government contracts. Our relationships with pharmaceutical and medical surgical product manufacturers and healthcare providers, as well as our provision of products and services to government entities, subject our business to laws, regulation, and government guidance on fraud and abuse. Many of these laws are vague or indefinite and have not been interpreted by the courts and, as such, may be interpreted or applied by a prosecutorial, regulatory, or judicial authority in a manner that could require us to make changes in our operations. Failure to comply with applicable laws, regulations, and government guidance, including but not limited to those involving the regulation of controlled substances, the federal Anti-Kickback Statute, and others, could subject us to federal or state government investigations or qui tam actions, and to liability for damages and civil and criminal penalties, including the loss of licenses or our ability to participate in Medicare, Medicaid and other federal and state healthcare programs, or pursue government contracts.
Healthcare Regulation: In the U.S., the Patient Protection and Affordable Care Act (“ACA”) significantly expanded health insurance coverage to uninsured Americans and changed the way healthcare is financed by both governmental and private payers. There are also further efforts to broaden healthcare coverage. U.S. lawmakers also have explored proposals to reduce drug prices, including requiring price transparency and drug importation measures. Provincial governments in Canada that provide partial funding for the purchase of pharmaceuticals and independently regulate the sale and reimbursement of drugs have sought to reduce the costs of publicly funded health programs. For example, provincial governments have taken steps to reduce consumer prices for generic pharmaceuticals and, in some provinces, change professional allowances paid to pharmacists by generic manufacturers. Many European governments provide or subsidize healthcare to consumers and regulate pharmaceutical prices, patient eligibility and reimbursement levels in order to control government healthcare system costs. Some European governments have implemented or are considering austerity measures to reduce healthcare spending. These measures exert pressure on the pricing and reimbursement timelines for pharmaceuticals and may cause our customers to purchase fewer of our products and services or influence us to reduce prices. There is substantial uncertainty about the likelihood, timing and results of these health reform efforts.
Additionally, there have been increasing efforts by governments to regulate the pharmaceutical distribution system in order to prevent the introduction of counterfeit, adulterated and/or mislabeled drugs into the pharmaceutical distribution system, otherwise known as pedigree tracking. For example, the U.S. Drug Quality and Security Act of 2013 (“DQSA”) requires us to participate in a federal prescription drug track and trace system that preempts state drug pedigree requirements, and the U.S. Food and Drug Administration Amendments Act of 2007 requires the FDA to establish standards and identify and validate effective technologies, such as track and trace or authentication technologies, to secure the pharmaceutical supply chain against counterfeit drugs. We also have record-keeping and other obligations under the E.U. Falsified Medicines Directive. Pedigree tracking laws such as these increase our compliance burden and our pharmaceutical distribution costs.
Data Security and Privacy: We are subject to a variety of privacy and data protection laws that change frequently and have requirements that vary from jurisdiction to jurisdiction. For example, under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) we must maintain administrative, physical and technological safeguards to protect individually identifiable health information (“protected health information”) and ensure the confidentiality, integrity, and availability of electronic protected health information. We are subject to significant compliance obligations under privacy laws such as the General Data Protection Regulation (“GDPR”) in the European Union, the Personal Information Protection and Electronic Documents Act (“PIPEDA”) in Canada, and the California Consumer Protection Act (“CCPA”). Some privacy laws prohibit the transfer of personal information to certain other jurisdictions. We are subject to privacy and data protection compliance audits or investigations by various government agencies. Failure to comply with these laws subjects us to potential regulatory enforcement activity, fines, private litigation including class actions, and other costs. We also have contractual obligations to customers that might be breached if we fail to comply with privacy laws. Our efforts to comply with privacy laws complicate our operations and add to our compliance costs.
We and our external service providers use technology and systems to perform our business operations, such as the secure electronic transmission, processing, storage and hosting of sensitive information, including protected health information and other types of personal information, confidential financial information, proprietary information, and other sensitive information relating to our customers, company and workforce. Despite physical, technical, and administrative security measures that we implement in order to, among other things, address regulatory requirements, our technology systems and operations may continue to be subject to cybersecurity incidents. The risk of cybersecurity incidents may be increased due to a variety of factors, both internal and external. A cybersecurity incident might involve a material data breach or other material impact to the integrity and operations of the technology systems and operations, which might result in litigation or regulatory action.
Environmental Regulation: We are subject to many environmental and hazardous materials regulations, including those relating to radiation-emitting equipment operated at U.S. Oncology Network practices. Additionally, our operations are subject to regulations under various federal, state, local and foreign laws concerning the environment, including laws addressing the discharge of pollutants into the air and water, the management and disposal of hazardous substances and wastes, and the cleanup of contaminated sites, as discussed in more detail below. We could incur substantial costs, including cleanup costs, fines and civil or criminal sanctions, and third-party damage or personal injury claims, if in the future we were to violate or become liable under environmental laws. We are committed to maintaining compliance with all environmental laws applicable to our operations, products and services and to reducing our environmental impact across all aspects of our business. We meet this commitment through an environmental strategy and sustainability program.
We sold our chemical distribution operations in 1987 and retained responsibility for certain environmental obligations. Agreements with the Environmental Protection Agency and certain states may require environmental assessments and cleanups at several closed sites. These matters are described further in Financial Note 19, “Commitments and Contingent Liabilities,” to the consolidated financial statements appearing in this Annual Report on Form 10-K.
The liability for environmental remediation and other environmental costs is accrued when the Company considers it probable and can reasonably estimate the costs. Environmental costs and accruals, including that related to our legacy chemical distribution operations, presently are not material to our operations or financial position. Although there is no assurance that existing or future environmental laws applicable to our operations or products will not have a material adverse impact on our operations or financial condition, we do not currently anticipate material capital expenditures for environmental matters. Other than the expected expenditures that may be required in connection with our legacy chemical distribution operations, we do not anticipate making substantial capital expenditures either for environmental issues or to comply with environmental laws, regulations, or government guidance in the future. The amount of our capital expenditures for environmental compliance was not material in 2021 and is not expected to be material in the next year.
Climate Change Regulation: Governments in the U.S. and abroad are considering new or expanded laws to address climate change. Such laws may include limitations on greenhouse gas (“GHG”) emissions, mandates that companies implement processes to monitor and disclose climate-related matters, additional taxes or offset charges on specified energy sources, and other requirements. Compliance with climate-related laws may be further complicated by disparate regulatory approaches in various jurisdictions. New or expanded climate-related laws could impose substantial costs on us. Until the timing and extent of climate-related laws are clarified, we cannot predict their potential effect on our capital expenditures or our results of operations.
Other Information about the Business
Customers: During 2021, sales to our ten largest customers, including group purchasing organizations (“GPOs”) accounted for approximately 51% of our total consolidated revenues. Sales to our largest customer, CVS Health Corporation (“CVS”), accounted for approximately 21% of our total consolidated revenues in 2021. In May 2019, we extended our pharmaceutical distribution relationship with CVS to June 2023. Our ten largest customers comprised approximately 32%, and CVS was approximately 19%, of total trade accounts receivable at March 31, 2021. We also have agreements with GPOs, each of which functions as a purchasing agent on behalf of member hospitals, pharmacies and other healthcare providers, as well as with government entities and agencies. The accounts receivable balances are with individual members of the GPOs, and therefore no significant concentration of credit risk exists. Substantially all of these revenues and accounts receivable are included in our U.S. Pharmaceutical segment.
Suppliers: We obtain pharmaceutical and other products from manufacturers, none of which accounted for more than 7% of our purchases in 2021. The loss of a supplier could adversely affect our business if alternate sources of supply are unavailable. We believe that our relationships with our suppliers are generally sound. The ten largest suppliers in 2021 accounted for approximately 50% of our purchases.
Some of our distribution arrangements with the manufacturers provides us compensation based on a percentage of our purchases. In addition, we have certain distribution arrangements with pharmaceutical manufacturers that include an inflation-based compensation component whereby we benefit when the manufacturers increase their prices as we sell our existing inventory at the new higher prices. For these manufacturers, a reduction in the frequency and magnitude of price increases, as well as restrictions in the amount of inventory available to us, could have an adverse impact on our gross profit margin.
Research and Development: Research and development (“R&D”) expenses were $74 million, $96 million, and $71 million during 2021, 2020, and 2019, respectively.
Financial Information About Foreign and Domestic Operations: Certain financial information relating to foreign and domestic operations is included in Financial Note 22, “Segments of Business,” to the consolidated financial statements appearing in this Annual Report on Form 10-K. See “Risk Factors” in Item 1A of Part I below for information regarding risks associated with our foreign operations.
Forward-Looking Statements
This Annual Report on Form 10-K, including “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 of Part II of this report and the “Risk Factors” in Item 1A of Part I of this report, contains forward-looking statements within the meaning of section 27A of the Securities Act of 1933 (“Securities Act”) and section 21E of the Securities Exchange Act of 1934, as amended. Some of these statements can be identified by use of forward-looking words such as “believes,” “expects,” “anticipates,” “may,” “will,” “should,” “seeks,” “approximately,” “intends,” “plans,” or “estimates,” or the negative of these words, or other comparable terminology. The discussion of financial trends, strategy, plans, or intentions may also include forward-looking statements. Forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from those projected, anticipated, or implied. Although it is not possible to predict or identify all such risks and uncertainties, they include, but are not limited to, the factors discussed in Item 1A of Part I of this report under “Risk Factors” and in our publicly available SEC filings and press releases. Readers are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date such statements were first made. Except to the extent required by federal securities laws, we undertake no obligation to publicly release the result of any revisions to any forward-looking statements to reflect events or circumstances after the date the statements are made, or to reflect the occurrence of unanticipated events.
Item 1A. Risk Factors
The discussion below identifies certain representative risks that might cause our actual business results to materially differ from our estimates. It is not practical to identify or describe all risks and uncertainties that might materially impact our business operations, reputation, financial position or results of operations. Our business could be materially affected by risks that we have not yet identified or that we currently consider to be immaterial. This is not a complete statement of all potential risks and uncertainties.
Litigation and Regulatory Risks
We experience costly and disruptive legal disputes.
We are routinely named as a defendant in litigation or regulatory proceedings and other legal disputes, which may include asserted class action litigation, such as those described in Financial Note 19, “Commitments and Contingent Liabilities,” to the consolidated financial statements in this report. Regulatory proceedings might involve allegations such as false claims, healthcare fraud and abuse, and antitrust violations. Civil litigation proceedings might involve commercial, employment, environmental, intellectual property, tort and other claims. Despite valid defenses that we assert, legal disputes are often costly, time-consuming, distracting to management and disruptive to normal business operations. The outcome of legal disputes is difficult to predict. Outcomes can occur that are not justified by the evidence or existing law. The uncertainty and expense associated with unresolved legal disputes might harm our business and reputation even if the matter is favorably resolved. Accordingly, any legal dispute might have a materially adverse impact on our reputation, our business operations and our financial position or results of operations.
We might experience losses not covered by insurance.
Our business exposes us to risks that are inherent in the distribution, manufacturing, dispensing and administration of pharmaceuticals and medical-surgical supplies, the provision of ancillary services, the conduct of our payer businesses and the provision of products that assist clinical decision-making and relate to patient medical histories and treatment plans. For example, pharmacy operations are exposed to risks such as improper filling of prescriptions, mislabeling of prescriptions, inadequacy of warnings, unintentional distribution of counterfeit drugs and expiration of drugs. Although we seek to maintain adequate insurance coverage, such as property insurance for inventory and professional and general liability insurance, coverages on acceptable terms might be unavailable, or coverages might not cover our losses. We generally seek to limit our contractual exposure, but limitations of liability or indemnity provisions in our contracts may not be enforceable or adequately protect us from liability. Uninsured losses might have a materially adverse impact on our business operations and our financial position or results of operations.
We experience costly legal disputes, government actions and adverse publicity regarding our role in distributing controlled substances such as opioids.
The Company is a defendant in approximately 3,200 cases alleging claims related to the distribution of controlled substances (opioids), as described in Financial Note 19, “Commitments and Contingent Liabilities,” to the consolidated financial statements in this report. We regularly are named as a defendant in similar, new cases. The plaintiffs in those cases include governmental entities (such as states, provinces, counties and municipalities) as well as businesses, groups and individuals. The cases allege violations of controlled substance laws and other laws, and they make common law claims such as negligence and public nuisance. Many of these cases raise novel theories of liability. Any proceedings can have unexpected outcomes that are not justified by evidence or existing law. All proceedings involve significant expense, management time and distraction, and risk of loss that can be difficult to predict or quantify. It is not uncommon for claims to be resolved over many years. Proceedings can result in monetary damages, penalties and fines, and injunctive or other relief. Although the Company has valid defenses and is vigorously defending itself, some proceedings have been and others may be resolved by negotiated outcome. Our reputation has been and may continue to be impacted by publicity regarding the litigation and related allegations. The adverse outcome of legal proceedings might have a materially adverse impact on our business operations and our financial position or results of operations.
We might experience increased costs to distribute controlled substances such as opioids.
Legislative, regulatory or industry measures related to the distribution of controlled substances such as prescription opioids could affect our business in ways that we may not be able to predict. For example, some states have passed legislation that could require us to pay taxes or assessments on the distribution of opioid medications in those states and other states have considered similar legislation. Liabilities for taxes or assessments or other costs of compliance under any such laws might have a materially adverse impact on our reputation, business operations, and our financial position or results of operations.
We are subject to extensive, complex and challenging healthcare and other laws.
Our industry is highly regulated, and further regulation of our distribution businesses and technology products and services could impose increased costs, negatively impact our profit margins and the profit margins of our customers, delay the introduction or implementation of our new products, or otherwise negatively impact our business and expose the Company to litigation and regulatory investigations. For example, we are subject to many environmental and hazardous materials regulations, including those relating to radiation-emitting equipment operated at U.S. Oncology Network practices. Additionally, we are subject to various routine agency (e.g., Drug Enforcement Administration (“DEA”), the U.S. Food and Drug Administration (“FDA”)) inspections to determine compliance with various federal regulations. Any noncompliance by us with applicable laws or the failure to maintain, renew or obtain necessary permits and licenses could lead to litigation and might have a materially adverse impact on our business operations and our financial position or results of operations.
We are subject to extensive and frequently changing local, state and federal laws and regulations relating to healthcare fraud, waste and abuse.
Local, state and federal governments continue to strengthen their position and scrutiny over practices involving or allegedly involving fraud, waste and abuse affecting Medicare, Medicaid and other government healthcare programs. Our relationships with pharmaceutical and medical surgical product manufacturers and healthcare providers, as well as our provision of products and services to government entities, subject our business to laws and regulations on fraud and abuse, which among other things: (1) prohibit persons from soliciting, offering, receiving or paying any remuneration in order to induce the referral of a patient for treatment or to induce the ordering or purchasing of items or services that are in any way paid for by Medicare, Medicaid or other government-sponsored healthcare programs; (2) impose many restrictions upon referring physicians and providers of designated health services under Medicare and Medicaid programs; and (3) prohibit the knowing submission of a false or fraudulent claim for payment to, and knowing retention of an overpayment by, a federal healthcare program such as Medicare and Medicaid. Many of these laws, regulations, and government guidance, including those relating to marketing incentives, are vague or indefinite and have not been interpreted by the courts. The laws may be interpreted or applied by a prosecutorial, regulatory, or judicial authority in a manner that could require us to make changes in our operations. Failures to comply with applicable laws subject us to federal or state government investigations or qui tam actions, and to liability for damages and civil and criminal penalties, including the loss of licenses or our ability to participate in Medicare, Medicaid and other federal and state healthcare programs. These sanctions might have a materially adverse impact on our business operations and our financial position or results of operations.
We might lose our ability to purchase, compound, store or distribute pharmaceuticals and controlled substances.
We are subject to the operating and security standards of the DEA, the FDA, various state boards of pharmacy, state health departments, Department of Health and Human Services (“HHS”), the Centers for Medicare & Medicaid Services (“CMS”) and other comparable agencies. Certain of our businesses may be required to register for permits and/or licenses with, and comply with operating and security standards of, the DEA, FDA, HHS, CMS, various state boards of pharmacy, state health departments and/or comparable state agencies as well as foreign agencies and certain accrediting bodies, depending upon the type of operations and location of product development, manufacture, distribution, and sale. For example, we are required to hold valid DEA and state-level registrations and licenses, meet various security and operating standards and comply with the Controlled Substances Act and its accompanying regulations governing the sale, marketing, packaging, holding, distribution, and disposal of controlled substances. Noncompliance with these requirements has resulted in monetary penalties and/or licensing sanctions. For example, under a January 2017 agreement with the DEA and Department of Justice we paid $150 million to settle potential administrative and civil claims about our practices for reporting suspicious orders of controlled substances and the DEA suspended, on a staggered basis for limited periods of time, our registrations to distribute certain controlled substances from four distribution centers. As of March 31, 2021, suspensions at the four distribution centers had all expired by their own terms. If we are not able to obtain, maintain or renew permits, licenses or other regulatory approvals needed for the operation of our businesses, it might have a materially adverse impact on our business operations and our financial position or results of operations.
Pedigree tracking laws increase our compliance burden and our pharmaceutical distribution costs.
There have been increasing efforts by governments to regulate the pharmaceutical distribution system in order to prevent the introduction of counterfeit, adulterated and/or mislabeled drugs into the pharmaceutical distribution system, otherwise known as pedigree tracking. For example, the U.S. Drug Quality and Security Act of 2013 (“DQSA”) requires us to participate in a federal prescription drug track and trace system that preempts state drug pedigree requirements, and the U.S. Food and Drug Administration Amendments Act of 2007 requires the FDA to establish standards and identify and validate effective technologies, such as track and trace or authentication technologies, to secure the pharmaceutical supply chain against counterfeit drugs. We also have record-keeping and other obligations under the E.U. Falsified Medicines Directive. Pedigree tracking laws such as these increase our compliance burden and our pharmaceutical distribution costs, and they might have a materially adverse impact on our business operations and our financial position or results of operations.
Privacy and data protection laws increase our compliance burden.
We are subject to a variety of privacy and data protection laws that change frequently and have requirements that vary from jurisdiction to jurisdiction. For example, under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) we must maintain administrative, physical and technological safeguards to protect individually identifiable health information (“protected health information”) and ensure the confidentiality, integrity and availability of electronic protected health information. We are subject to significant compliance obligations under privacy laws such as the General Data Protection Regulation in the European Union (“GDPR”), the Personal Information Protection and Electronic Documents Act (“PIPEDA”) in Canada, and the California Consumer Protection Act (“CCPA”). Some privacy laws prohibit the transfer of personal information to certain other jurisdictions. We are subject to privacy and data protection compliance audits or investigations by various government agencies. Failure to comply with these laws subjects us to potential regulatory enforcement activity, fines, private litigation including class actions, and other costs. We also have contractual obligations to customers that might be breached if we fail to comply with privacy laws. Our efforts to comply with privacy laws complicates our operations and adds to our compliance costs. A significant privacy breach or failure to comply with privacy laws might have a materially adverse impact on our reputation, business operations and our financial position or results of operations.
Anti-bribery and anti-corruption laws increase our compliance burden.
We are subject to laws prohibiting improper payments and bribery, including the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act and similar regulations in foreign jurisdictions. The U.K. Bribery Act, for example, prohibits both domestic and international bribery, as well as bribery across both private and public sectors. An organization that fails to prevent bribery committed by anyone associated with the organization can be charged under the U.K. Bribery Act unless the organization can establish the defense of having implemented adequate procedures to prevent bribery. Our failure to comply with these laws might subject us to civil and criminal penalties that might have a materially adverse impact on our reputation, business operations and our financial position or results of operations.
Company and Operational Risks
We might record significant charges from impairment to goodwill, intangibles and other assets or investments.
We are required under U.S. Generally Accepted Accounting Principles (“GAAP”) to test our goodwill for impairment annually or more frequently if indicators for potential impairment exist. Indicators that are considered include significant changes in performance relative to expected operating results, significant changes in the use of the assets, significant negative industry or economic trends or a significant decline in the Company’s stock price and/or market capitalization for a sustained period of time. In addition, we periodically review our intangible and other long-lived assets for impairment when events or changes in circumstances, such as a divestiture, indicate the carrying value may not be recoverable. Factors that may be considered a change in circumstances indicating that the carrying value of our intangible and other long-lived assets may not be recoverable include slower growth rates, the loss of a significant customer, burdensome new laws or divestiture of a business or asset for less than its carrying value. There are inherent uncertainties in management’s estimates, judgments and assumptions used in assessing recoverability of goodwill, intangible and other long-lived assets. Any material changes in key assumptions, including failure to meet business plans, negative changes in government reimbursement rates, a deterioration in the U.S. and global financial markets, an increase in interest rate or an increase in the cost of equity financing by market participants within the industry or other unanticipated events and circumstances, may decrease the projected cash flows or increase the discount rates and could potentially result in an impairment charge. For example, the COVID-19 pandemic has disrupted the global economy and exacerbated uncertainties inherent in estimates, judgments and assumptions used in our forecasts and impairment assessments. We may be required to record a significant charge to earnings in our consolidated financial statements during the period in which any impairment of our goodwill or intangible and other long-lived assets is determined, which might have a materially adverse impact on our business operations and our financial position or results of operations.
We experience cybersecurity incidents that might significantly compromise our technology systems or might result in material data breaches.
We and our external service providers use technology and systems to perform our business operations, such as the secure electronic transmission, processing, storage and hosting of sensitive information, including protected health information and other types of personal information, confidential financial information, proprietary information, and other sensitive information relating to our customers, company and workforce. Despite physical, technical, and administrative security measures, our technology systems and operations have been, and likely will continue to be, subject to cyberattacks from sources beyond our control. Cybersecurity incidents include actual or attempted unauthorized access, tampering, malware insertion, ransomware attacks or other system integrity events. The risk of cyberattacks may be increased due to a variety of factors, both internal and external. A cybersecurity incident might involve a material data breach or other material impact to the integrity and operations of our technology systems, which might result in litigation or regulatory action, loss of customers or revenue, and increased expense, any of which might have a materially adverse impact on our business operations, reputation, and our financial position or results of operations.
We might experience significant problems with information systems or networks.
We rely on sophisticated information systems and networks to perform our business operations, such as to obtain, rapidly process, analyze and manage data that facilitate the purchase and distribution of thousands of inventory items from distribution centers. If those information systems suffer errors, interruptions, or become unavailable, there might be a materially adverse impact on our business operations, reputation, and our financial position or results of operations.
Our products or services might not conform to specifications or perform as we intend.
We sell and provide services involving complex software and technology that may contain errors, especially when first introduced to market. Healthcare professionals delivering patient care tend to have heightened sensitivity to system and software errors. If our software and technology services are alleged to have contributed to faulty clinical decisions or injury to patients, we might be subject to claims or litigation by users of our software or services or their patients. Errors or failures might damage our reputation and negatively affect future sales. A failure of a system or software to conform to specifications might constitute a breach of warranty that could result in repair costs, contract termination, refunds of amounts previously paid or claims for damages. Any of these types of errors or failures might have a materially adverse impact on our reputation, business operations and our financial position or results of operations.
We might be impeded in providing customers online services and data access.
We provide remote services that involve hosting customer data and operating software on our own or third party systems. Our customers rely on their ability to access the systems and their data as needed. The networks and hosting systems are vulnerable to interruption or damage from sources beyond our control, such as power loss, telecommunications failures, fire, natural disasters, software and hardware failures and cyberattacks. If the timely delivery of medical care or other customer business requirements are impaired by data access, network or systems problems, we could be exposed to significant claims and reputational harm. Any such problems might have a materially adverse impact on our business operations and our financial position or results of operations.
We might not realize expected benefits from business process initiatives.
We may implement restructuring, cost reduction or other business process initiatives that might result in extraordinary charges and expenses, failures to achieve our desired objectives, or unintended consequences such as distraction of our management and employees, business disruption, attrition beyond any planned reduction in workforce, inability to attract or retain key personnel and reduced employee productivity. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be unable to successfully complete or integrate acquisitions or other business combinations.
Our growth strategy includes consummating acquisitions or other business combinations that either expand or complement our business. To fund acquisitions, we may require financing that may not be available on acceptable terms. We may not receive regulatory approvals needed to complete proposed transactions, or such approvals may be subject to delays or conditions that reduce transaction benefits. Achieving the desired outcomes of business combinations involves significant risks including: diverting management’s attention from other business operations; challenges with assimilating the acquired businesses, such as integration of operations and systems; failure or delay in realizing operating synergies; difficulty retaining key acquired company personnel; unanticipated accounting or financial systems issues with the acquired business, which might affect our internal controls over financial reporting; unanticipated compliance issues in the acquired business; challenges retaining customers of the acquired business; unanticipated expenses or charges to earnings, including depreciation and amortization or potential impairment charges; and risks of known and unknown assumed liabilities in the acquired business. Any of these risks could adversely affect our ability to achieve the anticipated benefits of an acquisition and might have a materially adverse impact on our business operations and our financial position or results of operations.
Exclusive forum provisions in our Bylaws could limit our stockholders’ ability to choose their preferred judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated bylaws provide that, unless the Corporation consents in writing to the selection of an alternative forum, the sole and exclusive forum for specified legal actions is the Court of Chancery of the State of Delaware or the United States District Court for the District of Delaware if the Court of Chancery does not have or declines to accept jurisdiction (collectively, “Delaware Courts”). Current and former stockholders are deemed to have consented to the personal jurisdiction of the Delaware Courts in connection with any action to enforce that exclusive forum provision and to service of process in any such action. These provisions of the bylaws are not a waiver of, and do not relieve anyone of duties to comply with, federal securities laws including those specifying the exclusive jurisdiction of federal courts under the Exchange Act and concurrent jurisdiction of federal and state courts under the Securities Act. To the extent that these provisions of the bylaws limit a current or former stockholder’s ability to select a judicial forum other than the Delaware Courts, they might discourage the specified legal actions, might cause current or former stockholders to incur additional litigation-related expenses and might result in outcomes unfavorable to current or former stockholders. A court might determine that these provisions of the bylaws are inapplicable or unenforceable in any particular action, in which case we may incur additional litigation related expenses in such action, and the action may result in outcomes unfavorable to us, which could have a materially adverse impact on our reputation, our business operations and our financial position or results of operations.
We might be adversely impacted by delays or other difficulties with divestitures.
When we decide to sell assets or a business, we may encounter difficulty in finding buyers or exit strategies on acceptable terms or in a timely manner, which could delay the achievement of our strategic objectives. After the disposition, we might experience greater dissynergies than expected, and the impact of the divestiture on our revenue or profit might be larger than we expected. We might have difficulties with pre-closing conditions such as regulatory and governmental approvals, which could delay or prevent the divestiture. We might have financial exposure in a divested business, such as through minority equity ownership, financial or performance guarantees, indemnities or other obligations, such that conditions outside of our control might negate the expected benefits of the disposition. Any of these risks could adversely affect our ability to achieve the anticipated benefits of a divestiture and might have a materially adverse impact on our business operations and our financial position or results of operations.
We might not realize the expected tax treatment from our split-off of Change Healthcare.
On March 10, 2020, the Company completed a separation of its interest in Change Healthcare LLC (“Change Healthcare JV”). The divestiture was effected through the split-off of PF2 SpinCo, Inc. (“SpinCo”), a wholly owned subsidiary of the Company that held all of the Company’s interest in the Change Healthcare JV, to certain of the Company’s stockholders through an exchange offer (the “Exchange Offer”), followed by a merger of SpinCo with and into Change Healthcare Inc. (“Change”), with Change surviving the merger (the “Merger” and, together with the Exchange Offer, the “Transactions”). The Company received an opinion from outside legal counsel to the effect that the Transactions qualified as generally tax-free transactions to the Company and its shareholders for U.S. federal income tax purposes. An opinion of legal counsel is not binding on the Internal Revenue Service (the “IRS”) or the courts, and the IRS or the courts may not agree with the intended tax-free treatment of the Transactions. In addition, the opinion could not be relied upon if certain assumptions, representations and undertakings upon which the opinion was based are materially inaccurate or incomplete, or are violated in any material respect. If the intended tax-free treatment of the Transactions is not sustained, the Company and its stockholders who participated in the Transactions may be required to pay substantial U.S. federal income taxes. In connection with the Transactions, the Company, SpinCo, Change and the Change Healthcare JV entered into the Tax Matters Agreement, which governs their respective rights, responsibilities and obligations with respect to tax liabilities and benefits, tax attributes, tax contests and other tax sharing regarding U.S. federal, state and local, and non-U.S. taxes, other tax matters and related tax returns. Under the Tax Matters Agreement, Change is required to indemnify the Company if the Transactions become taxable as a result of certain actions by Change or SpinCo, or as a result of certain changes in ownership of the stock of Change after the Merger. If Change does not honor its obligations to indemnify the Company, or if the Transactions fail to qualify for the intended tax-free treatment for reasons not related to a disqualifying action by Change or SpinCo, the resulting tax to the Company could have a significant adverse effect on our financial position or results of operations.
We might be adversely impacted by outsourcing or similar third-party relationships.
We rely on third parties to perform certain business and administrative functions for us. We might not adequately develop, implement and monitor these outsourced service providers, and we might not realize expected cost savings or other benefits. Third-party services providers might fail to perform as anticipated, or we might experience unanticipated operational difficulties, compliance requirements or increased costs related to outsourced services. For example, our ability to use outsourcing resources in certain jurisdictions might be limited by legislative action or customer contracts, with the result that the work must be performed at greater expense or we may be subject to sanctions for non-compliance. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We may be unsuccessful in retail pharmacy operations or maintaining profitability.
Our business strategy included expanding our retail pharmacy operations. Our retail pharmacy operations involve numerous risks, such as the following ones. We might encounter difficulties attracting and retaining customers to our retail locations due to their unfamiliarity with our brands or our inexperience with local market preferences. Competition from our retail pharmacy operations might strain relationships with our retail pharmacy customers. Consolidation of retail pharmacies with third party payers, expansion of large retail pharmacy networks, reductions in reimbursement rates, shifts in the mix of branded and generic pharmaceutical sales, and exclusion from preferred pharmacy networks can impair our retail pharmacy sales and profitability. Failure to maintain profitable retail pharmacy operations may result in significant costs, including those associated with site closures and reductions in workforce. If our retail pharmacy operations fail to achieve, or are unable to sustain, acceptable net sales and profitability levels, it might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be harmed by large customer purchase reductions, payment defaults or contract non-renewal.
We derive a significant portion of our revenue from, and have a significant portion of our accounts receivable with, a small number of customers. At March 31, 2021, sales to our largest customer represented approximately 21% of our consolidated revenues and approximately 19% of our trade receivables, and those of our ten largest customers combined accounted for approximately 51% of our consolidated revenues and approximately 32% of our trade receivables. A material default in payment, reduction in purchases, or the loss of business from a large customer might have a materially adverse impact on our business operations and our financial position or results of operations.
Our contracts with government entities involve future funding and compliance risks.
Our contracts with government entities are subject to risks such as lack of funding and compliance with unique requirements. For example, government contract purchase obligations are typically subject to the availability of funding, which may be eliminated or reduced. In addition, the future volume of products or services purchased by a government customer is uncertain. Our government contracts might not be renewed or might be terminated for convenience with little or no prior notice. Government contracts typically expose us to higher potential liability than do other types of contracts. In addition, government contracts typically are subject to procurement laws that include socio-economic, employment practices, environmental protection, recordkeeping and accounting and other requirements. For example, our contracts with the U.S. government generally require us to comply with the Federal Acquisition Regulations, Procurement Integrity Act, Buy American Act, Trade Agreements Act, and other laws and regulations. We are subject to government audits, investigations and oversight proceedings. Government agencies routinely review and audit government contractors to determine whether they are complying with contractual and legal requirements. If we fail to comply with these requirements, or we fail an audit, we are subject to various sanctions such as monetary damages, criminal and civil penalties, termination of contracts and suspension or debarment from government contract work. These requirements complicate our business and increase our compliance burden. The occurrence of any of these risks could harm our reputation and might have a materially adverse impact on our business operations and our financial position or results of operations.
Our participation in vaccination distribution programs may materially affect our operating results, reputation, and business.
We participate as a distributor in government-sponsored vaccination programs, such as the U.S. government’s COVID-19 distribution program (“Federal COVID-19 Response”). We also provide supplies used for vaccine administration in the Federal COVID-19 Response. Our participation in such programs exposes us to various uncertainties. For example, the novel nature of the SARS-CoV‑2 virus and the broad scope of the ongoing COVID-19 vaccine distribution program introduce uncertainty about what volumes of products may become available for distribution by us, the effectiveness of vaccines, and the cost of distribution. Because of such uncertainties, our operating results may be subject to variability. Our participation in such programs also exposes us to various risks, including regulatory compliance, government oversight, dependence on government funding, contractual performance, litigation, security risks, and supply chain challenges. Any significant problems with our participation in such programs might have a materially adverse impact on our reputation and our business. Because of these risks and uncertainties our operating results may be materially higher or lower than our projections.
We might be harmed by changes in our relationships or contracts with suppliers.
We attempt to structure our pharmaceutical distribution agreements with manufacturers to ensure that we are appropriately and predictably compensated for the services we provide. Certain distribution agreements with manufacturers include pharmaceutical price inflation as a component of our compensation, and we cannot control the frequency or magnitude of pharmaceutical price changes. We might be unable to renew pharmaceutical distribution agreements with manufacturers in a timely and favorable manner. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We might infringe intellectual property rights or our intellectual property protections might be inadequate.
We believe that our products and services do not infringe the proprietary rights of third parties, but third parties have asserted infringement claims against us and may do so in the future. If a court were to hold that we infringed other’s rights, we might be required to pay substantial damages, develop non-infringing products or services, obtain a license, stop selling or using the infringing products or services, or incur other sanctions. We rely on trade secret, patent, copyright and trademark laws, nondisclosure obligations and other contractual provisions and technical measures to protect our proprietary rights in our products and solutions. We might initiate costly and time-consuming litigation to protect our trade secrets, to enforce our patent, copyright and trademark rights and to determine the scope and validity of the proprietary rights of others. Our intellectual property protection efforts might be inadequate to protect our rights. Our competitors might develop non-infringing products or services equivalent or superior to ours. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be unable to successfully recruit and retain qualified employees.
Our ability to attract, engage, develop and retain qualified and experienced employees, including key executives and other talent, is essential for us to meet our objectives. We compete with many other businesses to attract and retain employees. Competition among potential employers might result in increased salaries, benefits or other employee-related costs, or in our failure to recruit and retain employees. We may experience sudden loss of key personnel due to a variety of causes, such as illness, and must adequately plan for succession of key management roles. Employees might not successfully transition into new roles. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
Industry and Economic Risks
We might be adversely impacted by healthcare reform such as changes in pricing and reimbursement models.
Many of our products and services are designed and intended to function within the structure of current healthcare financing and reimbursement systems. The healthcare industry and related government programs are changing. Some of these changes increase our risks and create uncertainties for our business.
For example, some changes in reimbursement methodologies (including government rates) for pharmaceuticals, medical treatments and related services reduce profit margins for us and our customers and impose new legal requirements on healthcare providers. Those changes have included cuts in Medicare and Medicaid reimbursement levels, changes in the basis for payments, shifting away from fee-for-service and towards value-based payments and risk-sharing models, and increases in the use of managed care.
In the U.S., the Patient Protection and Affordable Care Act (“ACA”) significantly expanded health insurance coverage to uninsured Americans and changed the way healthcare is financed by both governmental and private payers. There are continued efforts to challenge the ACA. There are also efforts to broaden healthcare coverage. U.S. lawmakers also have explored proposals to reduce drug prices, including requiring price transparency and drug importation measures. These proposals might result in significant changes in the pharmaceutical value chain as manufacturers, PBM, managed care organizations and other industry stakeholders look to implement new transactional flows and adapt their business models.
Provincial governments in Canada that provide partial funding for the purchase of pharmaceuticals and independently regulate the sale and reimbursement of drugs have sought to reduce the costs of publicly funded health programs. For example, provincial governments have taken steps to reduce consumer prices for generic pharmaceuticals and, in some provinces, change professional allowances paid to pharmacists by generic manufacturers.
Many European governments provide or subsidize healthcare to consumers and regulate pharmaceutical prices, patient eligibility and reimbursement levels in order to control government healthcare system costs. Some European governments have implemented or are considering austerity measures to reduce healthcare spending. These measures exert pressure on the pricing and reimbursement timelines for pharmaceuticals and may cause our customers to purchase fewer of our products and services or influence us to reduce prices.
Although there is substantial uncertainty about the likelihood, timing and results of these health reform efforts, their implementation might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be adversely impacted by competition and industry consolidation.
Our businesses face a highly competitive global environment with strong competition from international, national, regional and local full-line, short-line and specialty distributors, service merchandisers, self-warehousing chain drug stores, manufacturers engaged in direct distribution, third-party logistics companies and large payer organizations. In addition, our businesses face competition from various other service providers and from pharmaceutical and other healthcare manufacturers as well as other potential customers, which may from time-to-time decide to develop, for their own internal needs, supply management capabilities that might otherwise be provided by our businesses. Due to consolidation, a few large suppliers control a significant share of the pharmaceuticals market. This concentration reduces our ability to negotiate favorable terms with suppliers and causes us to depend on a smaller number of suppliers. Many of our customers, including healthcare organizations, have consolidated and have greater power to negotiate favorable prices. Consolidation by our customers, suppliers and competitors might reduce the number of market participants and give the remaining enterprises greater bargaining power, which might lead to erosion in our profit margin. Consolidation might increase counter-party credit risk because credit purchases increase for fewer market participants. These competitive pressures and industry consolidation might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be adversely impacted by changes or disruptions in product supply.
Our supply arrangements might be interrupted or adversely affected by a variety of causes over which we have no control, such as export controls or trade sanctions, labor disputes, unavailability of key manufacturing sites, inability to procure raw materials, quality control concerns, ethical sourcing issues, supplier’s financial distress, natural disasters, civil unrest or acts of war. Our inventory might be requisitioned, diverted or allocated by government order such as under emergency, disaster and civil defense declarations. For example, government actions in response to the COVID-19 pandemic affect our supply allocation, and those and our own allocation decisions can impact our customer relationships. Changes in the healthcare industry’s or our suppliers’ pricing, selling, inventory, distribution or supply policies or practices could significantly reduce our revenues and net income. We might experience disruptions in our supply of higher margin pharmaceuticals, including generic pharmaceuticals. Any of these changes or disruptions might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be adversely impacted as a result of our distribution of generic pharmaceuticals.
Our generic pharmaceuticals distribution business is subject to pricing risks. We might be adversely impacted if our ClarusONE generic pharmaceutical sourcing joint venture with Walmart, Inc. is unsuccessful or experiences margins declines. Generic drug manufacturers often offer a generic version of branded pharmaceuticals while they challenge the validity or enforceability branded pharmaceutical patents. The patent holder might assert infringement claims against us for distributing those generic versions and the generic drug manufactures may not fully indemnify us against such claims. These risks, as well as changes in the availability, pricing volatility, reimbursement rates for generic drugs, or significant changes in the nature, frequency or magnitude of generic pharmaceutical launches, might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be adversely impacted by an economic slowdown or recession.
An economic slowdown or recession affecting our businesses in one or more regions could reduce the prices our customers are able or willing to pay for our products and services and the volume of their purchases. This risk is increased by the COVID-19 pandemic. Any economic slowdown or recession might have a materially adverse impact on our business operations and our financial position or results of operations.
Disruption or other changes in capital and credit markets might impede access to credit and increase borrowing costs for us and our customers and suppliers and might impair the financial soundness of our customers and suppliers.
Volatility and disruption in global capital and credit markets, including the bankruptcy or restructuring of certain financial institutions, reduced lending activity by financial institutions, or decreased liquidity and increased costs in the commercial paper market, might adversely affect our borrowing ability and cost of borrowing. We generally sell our products and services under short-term unsecured credit arrangements. An adverse change in general economic conditions or access to capital might cause our customers to reduce their purchases from us, or delay or fail paying amounts owed to us. Suppliers might increase their prices, reduce their output or change their terms of sale due to limited availability of credit. Suppliers might be unable to make payments due to us for fees, returned products or incentives. These risks are increased by the COVID-19 pandemic. Interest rate increases or changes in capital market conditions might impede our or our customers’ or suppliers’ ability or cost to obtain credit. Any of these risks might have a material adverse impact on our business operations and our financial position or results of operations.
We may have difficulties in sourcing or selling products due to a variety of causes.
We might experience difficulties and delays in sourcing and selling products due to a variety of causes, such as: difficulties in complying with the legal requirements for export or import of pharmaceuticals or components; suppliers’ failure to satisfy production demand; manufacturing or supply problems such as inadequate resources; and real or perceived quality issues. Difficulties in product manufacturing or access to raw materials could result in supplier production shutdowns, product shortages and other supply disruptions. The FDA banned certain manufacturers from selling raw materials and drug ingredients in the U.S. due to quality issues. The COVID-19 pandemic adversely affects the availability of some products, resulting in product allocation and delivery delays. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be adversely impacted by tax legislation or challenges to our tax positions.
We are subject to the tax laws in the U.S. at the federal, state and local government levels and to the tax laws of many other jurisdictions in which we operate or sell products or services. Tax laws might change in ways that adversely affect our tax positions, effective tax rate and cash flow. The tax laws are extremely complex and subject to varying interpretations. We are subject to tax examinations in various jurisdictions that might assess additional tax liabilities against us. Our tax reporting positions might be challenged by relevant tax authorities, we might incur significant expense in our efforts to defend those challenges, and we might be unsuccessful in those efforts. Developments in examinations and challenges might materially change our provision for taxes in the affected periods and might differ materially from our historical tax accruals. Any of these risks might have a materially adverse impact on our business operations, our cash flows and our financial position or results of operations.
We might be adversely impacted by the Brexit withdrawal of the United Kingdom from the European Union.
We have operations in the U.K. and the European Union (“E.U.”) and face risks associated with the uncertainty and potential disruptions that might follow the U.K. withdrawing from the European Union (“Brexit”). Brexit could adversely affect political, regulatory, economic or market conditions and contribute to instability in global political institutions, regulatory agencies and financial markets. For example, we might experience volatility in exchange rates and interest rates and changes in laws regulating our U.K. operations. Customers might reduce purchases due to the uncertainty caused by Brexit. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We might be adversely impacted by fluctuations in foreign currency exchange rates.
We conduct our business in various currencies, including the U.S. dollar, euro, British pound sterling and Canadian dollar. Changes in foreign currency exchange rates could reduce our revenues, increase our costs or otherwise adversely affect our financial results reported in U.S. dollars. For example, we are exposed to transactional currency exchange risk due to our import and export of products that are purchased or sold in currencies other than the U.S. dollar. We also have currency exchange risk due to intercompany loans denominated in various currencies. The COVID-19 pandemic has affected and might increase currency exchange rate volatility. We may from time to time enter into foreign currency contracts, foreign currency borrowings or other techniques intended to hedge a portion of our foreign currency exchange rate risks. These hedging activities may not completely offset the adverse financial effects of unfavorable movements in foreign currency exchange rates during the time the hedges are in place. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
General Risk Factors
We might be adversely impacted by events outside of our control, such as widespread public health issues, natural disasters, political events and other catastrophic events.
We might be adversely affected by events outside of our control, including: widespread public health issues, such as epidemic or pandemic infectious diseases; natural disasters such as earthquakes, floods or severe weather; political events such as terrorism, military conflicts and trade wars; and other catastrophic events. These events can disrupt operations for us, our suppliers, our vendors, and our customers. For example, in February 2021, a severe winter storm affecting the United States temporarily impacted our distribution business operations, primarily in Texas. They might affect consumer confidence levels and spending. In response to these events, we might suspend operations, implement extraordinary procedures, seek alternate sources for product supply, or suffer consequences that are unexpected and difficult to mitigate. In particular, the rapid and widespread transmission of the SARS-CoV-2 novel coronavirus beginning in late 2019 impacts us in significant ways. For example, to mitigate the spread of the COVID-19 disease caused by SARS-CoV-2, we implemented travel restrictions and remote working arrangements for most of our employees in order to minimize physical contact, and we implemented additional sanitation and personal protection measures in our warehouse, retail pharmacy and delivery operations. These measures might not fully mitigate COVID-19 risks to our workforce and we could experience unusual levels of absenteeism that might impair operations and delay delivery of products. The COVID-19 pandemic affects product manufacturing, supply and transport availability and cost. The pandemic reduces demand for some products due to delays or cancellations of elective medical procedures, consumer self-isolation and business closures, among other reasons. The COVID-19 pandemic influences shortages of some products, with product allocation resulting in delivery delays for customers. The ongoing impacts of the pandemic might cause a general economic slowdown or recession in one or more markets, disruptions and volatility in global capital markets and other broad and adverse effects on the economy, business conditions, commercial activity and the healthcare industry. The pandemic might impact our business operation, financial position and results of operation in unpredictable ways that depend on highly uncertain future developments, such as determining the effectiveness of current or future government actions to address the public health or economic impacts of the pandemic. Any of these risks might have a materially adverse impact on our business operations and our financial position or results of operations.
We may be adversely affected by global climate change or by legal, regulatory or market responses to such change.
The long-term effects of climate change are difficult to predict and may be widespread. The impacts may include physical risks (such as rising sea levels or frequency and severity of extreme weather conditions), social and human effects (such as population dislocations or harm to health and well-being), compliance costs and transition risks (such as regulatory or technology changes) and other adverse effects. The effects could impair, for example, the availability and cost of certain products, commodities and energy (including utilities), which in turn may impact our ability to procure goods or services required for the operation of our business at the quantities and levels we require. We bear losses incurred as a result of, for example, physical damage to or destruction of our facilities (such as distribution or fulfillment centers), loss or spoilage of inventory, and business interruption due to weather events that may be attributable to climate change. These events and impacts could materially adversely affect our business operations, financial position or results of operation.
We might be adversely impacted by changes in accounting standards.
Our consolidated financial statements are subject to the application of U.S. GAAP, which periodically is revised or reinterpreted. From time to time, we are required to adopt new or revised accounting standards issued by recognized authoritative bodies, including the Financial Accounting Standards Board (“FASB”) and the SEC. It is possible that future accounting standards may require changes to the accounting treatment in our consolidated financial statements and may require us to make significant changes to our financial systems. Such changes might have a materially adverse impact on our financial position or results of operations.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
Because of the nature of our principal businesses, our plant, warehousing, retail pharmacies, office and other facilities are operated in widely dispersed locations, primarily throughout North America and Europe. Retail pharmacies and most warehouses are typically owned or leased on a long-term basis. We consider our operating properties to be in satisfactory condition and adequate to meet our needs for the next several years without making capital expenditures materially higher than historical levels. Information as to material lease commitments is included in Financial Note 11, “Leases,” to the consolidated financial statements appearing in this Annual Report on Form 10-K.
Item 3. Legal Proceedings.
Certain legal proceedings in which we are involved are discussed in Financial Note 19, “Commitments and Contingent Liabilities,” to the consolidated financial statements appearing in this Annual Report on Form 10-K. Disclosure of an environmental proceeding where a governmental agency is a party generally is included only if we expect monetary sanctions in the proceeding to exceed $1 million, unless otherwise material.
Item 4. Mine Safety Disclosures.
Not applicable.
Information about our Executive Officers
The following table sets forth information regarding the executive officers of the Company, including their principal occupations during the past five years. The number of years of service with the Company includes service with predecessor companies.
There are no family relationships between any of the executive officers or directors of the Company. The executive officers are elected on an annual basis generally and their term expires at the first meeting of the Board of Directors (“Board”) following the annual meeting of stockholders, or until their successors are elected and have qualified, or until death, resignation, or removal, whichever is sooner.
| | | | | | | | | | | | | | |
| Name | | Age | | Position with Registrant and Business Experience |
| | | | |
| Brian S. Tyler | | 54 | | Chief Executive Officer since April 2019; President and Chief Operating Officer from August 2018 to March 2019; Chairman of the Management Board of McKesson Europe AG from 2017 to 2018; President and Chief Operating Officer, McKesson Europe from 2016 to 2017; President of North America Distribution and Services from 2015 to 2016; Executive Vice President, Corporate Strategy and Business Development from 2012 to 2015; and a director since April 2019. Service with the Company - 24 years. |
| | | | |
| Britt J. Vitalone | | 52 | | Executive Vice President and Chief Financial Officer since January 2018; Senior Vice President and Chief Financial Officer, U.S. Pharmaceutical from July 2014 to December 2017; Senior Vice President and Chief Financial Officer, U.S. Pharmaceutical and Specialty Health from October 2017 to December 2017; Senior Vice President of Corporate Finance and M&A Finance from March 2012 to June 2014. Service with the Company - 15 years. |
| | | | |
| Tracy Faber | | 51 | | Executive Vice President and Chief Human Resources Officer since October 2019. Previously, Senior Vice President of Human Resources. Service with the Company - 10 years. |
| | | | |
| Nancy Flores | | 54 | | Executive Vice President, Chief Information Officer and Chief Technology Officer since January 2020; Chief Information Officer, Johnson Controls from 2018 to July 2019. Corporate Officer and Vice President of Business and Technology Services, Abbott Laboratories from 1996 to 2018. Service with the Company - 1 year. |
| | | | |
| Tom Rodgers | | 50 | | Executive Vice President, Chief Strategy Officer since June 2020. Previously Senior Vice President and Managing Director of McKesson Ventures from 2014-2020. Service with the Company - 7 years. |
| | | | |
| Lori A. Schechter | | 59 | | Executive Vice President, Chief Legal Officer and General Counsel since June 2014; Associate General Counsel from January 2012 to June 2014; Litigation Partner, Morrison & Foerster LLP from 1995 to December 2011. Service with the Company - 9 years. |
PART II
Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information: The principal market on which our common stock is traded is the New York Stock Exchange (“NYSE”) under the trading symbol of “MCK.”
Holders: The number of record holders of our common stock at March 31, 2021 was approximately 4,841.
Dividends: In July 2020, our quarterly dividend was raised from $0.41 to $0.42 per common share for dividends declared on or after such date by the Board. We declared regular cash dividends of $1.67 and $1.62 per share in the years ended March 31, 2021 and 2020, respectively.
We anticipate that we will continue to pay quarterly cash dividends in the future. However, the payment and amount of future dividends remain within the discretion of the Board and will depend upon our future earnings, financial condition, capital requirements, and other factors.
Securities Authorized for Issuance under Equity Compensation Plans: Information relating to this item is provided under Part III, Item 12, to this Annual Report on Form 10-K.
Share Repurchase Plans: Stock repurchases may be made from time-to-time in open market transactions, privately negotiated transactions, through accelerated share repurchase (“ASR”) programs, or by combinations of such methods, any of which may use pre-arranged trading plans that are designed to meet the requirements of Rule 10b5-1(c) of the Securities Exchange Act of 1934. The timing of any repurchases and the actual number of shares repurchased will depend on a variety of factors, including the Company’s stock price, corporate and regulatory requirements, restrictions under the Company’s debt obligations, and other market and economic conditions.
During the last three years, our share repurchases were transacted through both open market transactions and ASR programs with third-party financial institutions.
| | | | | | | | | | | | | | | | | | | | |
| Share Repurchases (1) |
| (In millions, except price per share data) | | Total Number of Shares Purchased (2) | | Average Price
Paid Per Share | | Approximate
Dollar Value of
Shares that May
Yet Be Purchased
Under the
Programs |
| Balance, March 31, 2018 | | | | | | $ | 1,096 | |
| Shares repurchase plans authorized in May 2018 | | | | | | 4,000 | |
| Shares repurchased - Open market | | 10.4 | | | $ | 132.14 | | | (1,377) | |
| Shares repurchased - ASR | | 2.1 | | | $ | 117.98 | | | (250) | |
| Balance, March 31, 2019 | | | | | | 3,469 | |
| Shares repurchased - Open market | | 9.2 | | | $ | 144.68 | | | (1,334) | |
| Shares repurchased - ASR | | 4.7 | | | $ | 127.68 | | | (600) | |
| Balance, March 31, 2020 | | | | | | 1,535 | |
| Shares repurchase plans authorized in January 2021 | | | | | | 2,000 | |
Shares repurchased - Open market (3) | | 4.7 | | | $ | 160.33 | | | (750) | |
| | | | | | |
| Balance, March 31, 2021 | | | | | | $ | 2,785 | |
(1)This table does not include the value of equity awards surrendered to satisfy tax withholding obligations. It also excludes shares related to our Split-off of the Change Healthcare JV as described in Financial Note 20, “Stockholders' Equity” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K.
(2)The number of shares purchased reflects rounding adjustments.
(3)$8 million was accrued within “Other accrued liabilities” on our Consolidated Balance Sheet as of March 31, 2021 for share repurchases that were executed in late March and settled in early April.
In 2019, we retired 5.0 million or $542 million of our treasury shares previously repurchased. Under the applicable state law, these shares resume the status of authorized and unissued shares upon retirement. In accordance with our accounting policy, we allocate any excess of share repurchase price over par value between additional paid-in capital and retained earnings. Accordingly, our retained earnings and additional paid-in capital were reduced by $472 million and $70 million, respectively, during 2019.
The following table provides information on our share repurchases during the fourth quarter of 2021:
| | | | | | | | | | | | | | | | | | | | | | | |
| Share Repurchases (1) |
| (In millions, except price per share) | Total Number of Shares Purchased | | Average Price Paid per Share | | Total Number of Shares Purchased as Part of Publicly Announced Programs | | Approximate Dollar Value of Shares that May Yet Be Purchased Under the Programs |
| January 1, 2021 - January 31, 2021 | 0.4 | | | $ | 181.50 | | | 0.4 | | | $ | 2,958 | |
| February 1, 2021 - February 28, 2021 | 0.4 | | | 180.56 | | | 0.4 | | | 2,880 | |
| March 1, 2021 - March 31, 2021 | 0.5 | | | 184.68 | | | 0.5 | | | 2,785 | |
Total | 1.3 | | | | | 1.3 | | | |
(1)This table does not include the value of equity awards surrendered to satisfy tax withholding obligations.
Stock Price Performance Graph*: The following graph compares the cumulative total stockholder return on our common stock for the periods indicated with the Standard & Poor’s 500 Index and the S&P 500 Health Care Index. The S&P 500 Health Care Index was selected as a comparator because it is generally available to investors and broadly used by other companies in the same industry.
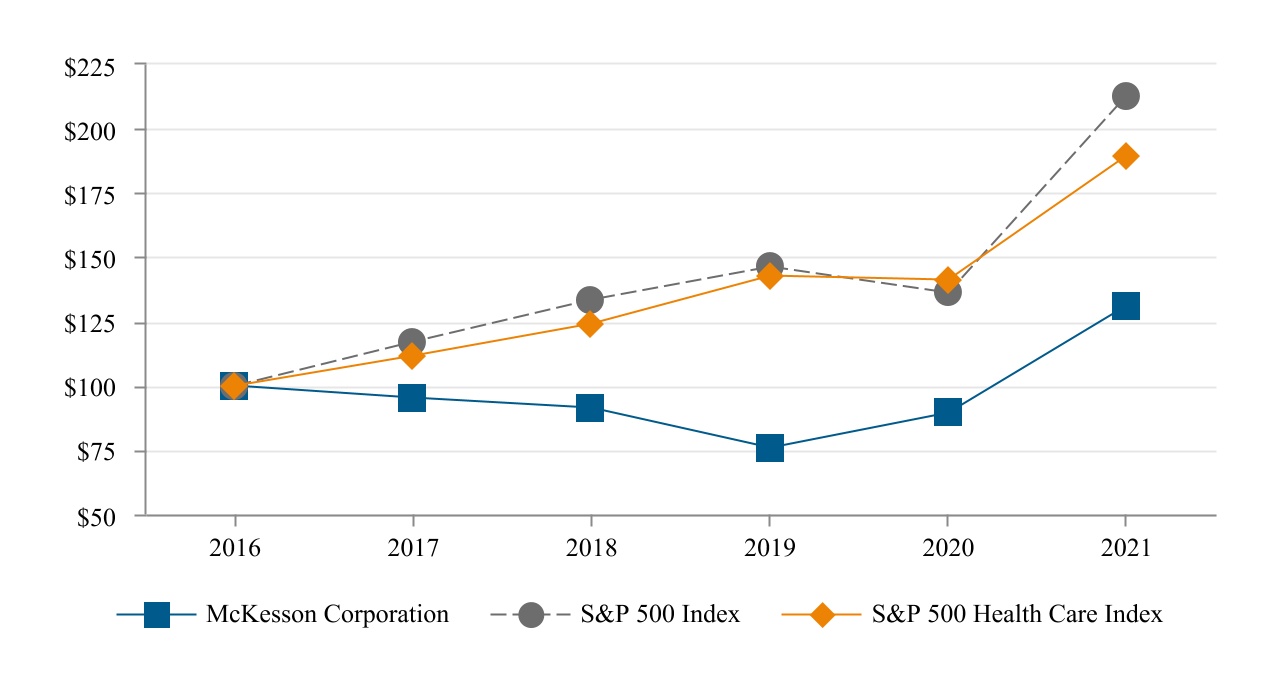
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| March 31, |
| 2016 | | 2017 | | 2018 | | 2019 | | 2020 | | 2021 |
| McKesson Corporation | $ | 100.00 | | | $ | 95.30 | | | $ | 91.37 | | | $ | 75.92 | | | $ | 89.37 | | | $ | 131.03 | |
| S&P 500 Index | $ | 100.00 | | | $ | 117.17 | | | $ | 133.57 | | | $ | 146.25 | | | $ | 136.05 | | | $ | 212.71 | |
S&P 500 Health Care Index | $ | 100.00 | | | $ | 111.59 | | | $ | 124.17 | | | $ | 142.66 | | | $ | 141.21 | | | $ | 189.28 | |
* Assumes $100 invested in McKesson Common Stock and in each index on March 31, 2016 and that all dividends are reinvested. |
Item 6. Reserved.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
GENERAL
Management’s discussion and analysis of financial condition and results of operations, referred to as the “Financial Review,” is intended to assist the reader in the understanding and assessment of significant changes and trends related to the results of operations and financial position of McKesson Corporation together with its subsidiaries (collectively, the “Company,” “McKesson,” “we,” “our,” or “us” and other similar pronouns). This discussion and analysis should be read in conjunction with the consolidated financial statements and accompanying financial notes in Item 8 of Part II of this Annual Report on Form 10-K.
Our fiscal year begins on April 1 and ends on March 31. Unless otherwise noted, all references to a particular year shall mean our fiscal year.
Certain statements in this report constitute forward-looking statements. See Item 1 - Business - Forward-Looking Statements in Part I of this Annual Report on Form 10-K for additional factors relating to these statements and Item 1A - Risk Factors in Part I of this Annual Report on Form 10-K for a list of certain risk factors applicable to our business, financial condition, and results of operations.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Overview of Our Business:
We are a global leader in healthcare supply chain management solutions, retail pharmacy, community oncology and specialty care, and healthcare information solutions. We partner with life sciences companies, manufacturers, providers, pharmacies, governments, and other healthcare organizations to help provide the right medicines, medical products, and healthcare services to the right patients at the right time, safely, and cost-effectively.
We implemented a new segment reporting structure commencing with the second quarter of 2021, which resulted in four reportable segments: U.S. Pharmaceutical, International, Medical-Surgical Solutions, and Prescription Technology Solutions (“RxTS”). Other, for retrospective periods presented, consists of our equity method investment in Change Healthcare LLC (“Change Healthcare JV”), which was split-off from McKesson in the fourth quarter of 2020. All prior segment information has been recast to reflect our new segment structure and current period presentation. Our organizational structure also includes Corporate, which consists of income and expenses associated with administrative functions and projects, and the results of certain investments. The factors for determining the reportable segments include the manner in which management evaluates the performance of the Company combined with the nature of individual business activities. We evaluate the performance of our operating segments on a number of measures, including revenues and operating profit before interest expense and income taxes.
The following summarizes our four reportable segments and the changes made to our reporting structure commencing in the second quarter of 2021. Refer to Financial Note 22, “Segments of Business,” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K for further information regarding our reportable segments.
•U.S. Pharmaceutical, previously the U.S. Pharmaceutical and Specialty Solutions reportable segment, continues to distribute branded, generic, specialty, biosimilar, and over-the-counter pharmaceutical drugs and other healthcare-related products. This segment also provides practice management, technology, clinical support, and business solutions to community-based oncology and other specialty practices. In addition, the segment sells financial, operational, and clinical solutions to pharmacies (retail, hospital, alternate site) and provides consulting, outsourcing, technological, and other services.
•International is a new reportable segment that includes our operations in Europe and Canada, bringing together non-U.S.-based drug distribution services, specialty pharmacy, retail, and infusion care services. McKesson Europe was previously reflected as the European Pharmaceutical Solutions reportable segment and McKesson Canada was previously included in Other.
•Medical-Surgical Solutions provides medical-surgical supply distribution, logistics, and other services to healthcare providers in the United States (“U.S.”) and was unaffected by the segment realignment.
•RxTS is a new reportable segment that brings together existing businesses, including CoverMyMeds, RelayHealth, RxCrossroads, and McKesson Prescription Automation, including Multi-Client Central Fill as a Service, to serve our biopharma and life sciences partners and patients. RxCrossroads was previously included in our former U.S. Pharmaceutical and Specialty Solutions reportable segment and CoverMyMeds, RelayHealth, and McKesson Prescription Automation were previously included in Other.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Executive Summary:
The following summary provides highlights and key factors that impacted our business, operating results, financial condition, and liquidity for the year ended March 31, 2021.
•Coronavirus disease 2019 (“COVID-19”) impacted our results of operations for the year ended March 31, 2021. Following the declaration of COVID-19 as a global pandemic by the World Health Organization (“WHO”) on March 11, 2020, there was a temporary increase in demand for pharmaceuticals across our businesses. Subsequently, pharmaceutical distribution volumes decreased during the first quarter as a result of the weakened and uncertain global economic environment and COVID-19 restrictions, including government shutdowns and shelter-in-place orders. The recovery from the COVID-19 pandemic continued to fluctuate throughout our fiscal year. We benefited from demand for COVID-19 tests, favorable contributions from our vaccine and related ancillary supply kit distribution programs as discussed further below, and savings from reduced travel and meetings throughout 2021;
•We expanded our existing contractual relationship with the Centers for Disease Control and Prevention (“CDC”) through an amendment to our existing Vaccines for Children Program contract to support the U.S. government as a centralized distributor of COVID-19 vaccines and ancillary supplies needed to administer vaccines. We have also partnered with the Department of Health and Human Services (“HHS”) and Pfizer to manage the assembly and distribution of the ancillary supplies needed to administer COVID-19 vaccines;
•In December 2020, we began distributing certain COVID-19 vaccines under the direction of the CDC. Through the end of the fiscal year, we had distributed approximately 100 million of COVID-19 vaccine doses. For a more in-depth discussion of how COVID-19 impacted our business, operations, and outlook, refer to the COVID-19 section of "Trends and Uncertainties" included below;
•Revenues of $238.2 billion, reflects a 3% increase from the prior year primarily in our U.S. Pharmaceutical segment driven by market growth;
•Gross profit increased 1% from the prior year primarily in our Medical-Surgical Solutions segment driven by sales of COVID-19 tests;
•Total operating expenses in 2021 includes the following:
◦a charge of $8.1 billion related to our estimated liability for opioid-related claims as further described in the Opioid-Related Litigation and Claims section of “Trends and Uncertainties” included below; and
◦charges of $115 million to impair certain long-lived assets within our International segment; partially offset by
◦a net gain of $131 million recorded in connection with insurance proceeds received from the settlement of the shareholder derivative action related to our controlled substances monitoring program;
•Other income, net in 2021 includes net gains of $133 million related to our equity investments;
•Diluted loss per common share from continuing operations attributable to McKesson Corporation in 2021 of $28.26 reflects the aforementioned items, net of any respective tax impacts, and a lower share count compared to the prior year driven largely by the separation of our investment in Change Healthcare JV on March 10, 2020;
•On November 1, 2020, we completed the contribution of our German pharmaceutical wholesale business to a newly formed joint venture with Walgreens Boots Alliance (“WBA”) in which we have a 30% ownership interest;
•On December 3, 2020, we completed a public offering of 0.90% Notes due December 3, 2025 (the “2025 Notes”) in a principal amount of $500 million and repaid $1.0 billion of long-term debt in 2021. Refer to Financial Note 13, “Debt and Financing Activities,” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K for more information;
•We returned $1.0 billion of cash to shareholders through $770 million of common stock repurchases, including the value of equity awards surrendered for tax withholding, and $276 million of dividend payments during 2021. On July 29, 2020, we raised our quarterly dividend from $0.41 to $0.42 per common share; and
•In January 2021, our Board of Directors (the “Board”) approved an increase of $2.0 billion for the authorized share repurchase of McKesson’s common stock.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Trends and Uncertainties:
COVID-19
In December 2019, a novel strain of coronavirus, which causes the infectious disease known as COVID-19, was reported in Wuhan, China. The WHO declared COVID-19 a “Public Health Emergency of International Concern” on January 30, 2020 and a global pandemic on March 11, 2020.
We continue to evaluate the nature and extent of the impacts COVID-19 has on our business and operations. The pandemic developed rapidly during our fourth quarter of 2020 and continued to evolve throughout 2021. Infection rates varied throughout our fiscal year, peaking in January 2021. A significant number of new COVID-19 cases continue to be reported, particularly in the U.S. These also include cases from new and emerging COVID-19 variants, which could have the potential to be more severe, spread more easily, require different treatments, or change the effectiveness of current vaccines. However, vaccines which have met the U.S. Food and Drug Administration’s (“FDA’s”) standards for safety, effectiveness, and manufacturing quality needed to support Emergency Use Authorization (“EUA”), are currently being administered across the country, as further discussed below. As of March 31, 2021, nearly 154 million doses of COVID-19 vaccines have been administered in the U.S. according to the CDC. The full extent to which COVID-19 will impact us depends on many factors and future developments, which are described at the end of this COVID-19 section.
In response to the COVID-19 pandemic, federal, state, and local government directives and policies have been put in place in the U.S. to enhance availability of medications and supplies to meet the increased demand, assist front-line healthcare providers, manage public health concerns by creating social distancing, and address the economic impacts, including sharply reduced business activity, increased unemployment, and overall uncertainty presented by this healthcare emergency. Similar governmental actions have occurred in Canada and Europe, the timing of which has varied across geographies. In December 2020, the FDA issued an EUA for the Pfizer-BioNTech COVID-19 vaccine manufactured by Pfizer, Inc. (“Pfizer Vaccine”) and the Moderna COVID-19 vaccine manufactured by ModernaTX, Inc. (“Moderna Vaccine”) to be distributed in the U.S. These authorizations were followed by an EUA for the Janssen COVID-19 vaccine manufactured by Janssen Biotech Inc., a Janssen pharmaceutical company of Johnson & Johnson, (“Janssen Vaccine”) in February 2021. Government-coordinated administrative or allocation decisions at the federal, state, and local levels may cause variability in the timing and volume of COVID-19 vaccine distribution and administration activities. Our role in the distribution of COVID-19 vaccines in the U.S. as well as the assembly and distribution of related ancillary supply kits is discussed further below. Similar COVID-19 vaccine authorizations have occurred in Canada and Europe. McKesson Canada’s corporately owned retail pharmacy chain, Rexall, as well as independent pharmacy banners are supporting Canada’s vaccination efforts. McKesson Europe is also playing a role in helping support governments and public health entities in not only distributing COVID-19 vaccines across several European countries, but administering them in pharmacies as well.
As a global leader in healthcare supply chain management solutions, retail pharmacy, community oncology and specialty care, and healthcare information solutions, we are well positioned to respond to the COVID-19 pandemic in the U.S., Canada, and Europe. We have worked and continue to work closely with national and local governments, agencies, and industry partners to ensure that available supplies, including personal protective equipment (“PPE”), and medicine reach our customers and patients.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Our Response to COVID-19 in the Workplace
During this unprecedented time, we are committed in continuing to supply our customers and protect the safety of our employees. The various responses we put in place to mitigate the impact of COVID-19 on our business operations, including telecommuting and work-from-home policies, restricted travel, employee support programs, and enhanced safety measures, are intended to limit employee exposure to the virus that causes COVID-19. We expanded employee medical benefits covering COVID-19 related visits, treatments, and testing as well as expanded telehealth options to protect employee safety. We provided further support including additional emergency leave and an internal paid time off donation platform for employees impacted by COVID-19. For employees whose roles require presence at our facilities, we enhanced safety by promoting the practice of social distancing, providing reminders to wash or disinfect hands and avoid unnecessary face touching, making face masks available, placing hand sanitizers within our operating environments, and periodically cleaning and disinfecting our facilities. For employees whose roles do not require presence at our facilities, we added technology resources to support their working remotely. These responses were initially put in place during our fourth quarter of 2020. During the second quarter of 2021, we also implemented on-site workplace temperature screening as we continue to adapt our health and safety practices in response to the COVID-19 pandemic. When working in frozen vaccine storage environments, employees are provided with protective gear, including special clothing, gloves, and facial gear. These steps to protect employee safety have resulted in limited disruption from COVID-19 to our normal business operations, productivity trends, and have not materially impacted our operating expenses or operating margins.
We have evaluated the impact of our telecommuting and work-from-home policies on our system of internal controls and we have concluded that these policies did not have a material effect on our internal control over financial reporting during the year ended March 31, 2021. We also took various actions to mitigate the impact of COVID-19 on our results from operations through cost-containment and payroll-related expenses.
Trends in our Business
At the onset of the COVID-19 pandemic late in our fourth quarter of 2020, we experienced higher pharmaceutical distribution volumes and increased retail pharmacy foot traffic as our customers increased supplies on hand in March, which drove unfavorability in our results of operations when comparing 2021 versus 2020.
During the first quarter of 2021, we experienced growth in pharmaceutical distribution and specialty drug volumes at a lower rate in the U.S., while pharmaceutical distribution volumes decreased in Europe and Canada due to the COVID-19 pandemic, as compared to the same prior year period. Specialty drug volumes increased, but were negatively impacted by lower demand for elective specialty drugs, as compared to the same prior year period. We also experienced decreased demand for primary care medical-surgical supplies due to deferrals in elective procedures in hospitals and surgery centers as well as decreased traffic and closures of doctors’ offices, which was partially offset by demand for PPE and COVID-19 tests. Additionally, the decreased traffic in doctors’ offices and general shelter-in-place guidance by governmental authorities negatively impacted retail pharmacy foot traffic in both Europe and Canada.
While we experienced improvements in prescription volumes and primary care patient visits during our second quarter of 2021, the impact and recovery of COVID-19 for the second half of our fiscal year was non-linear and tracked with patient mobility. We also saw increased demand for COVID-19 tests and continued sales of PPE throughout the year in our Medical-Surgical Solutions segment partially offset by the impacts of social distancing measures which resulted in a less severe cough, cold, and flu season, savings for reduced travel and meetings across the enterprise, as well as improvements of retail pharmacy foot traffic in Europe and Canada. The vaccine and related ancillary kit distribution in the U.S. favorably impacted our results in the second half of fiscal 2021 as further discussed below.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Our Role in the Distribution of COVID-19 Vaccines and Ancillary Supply Kits
On August 14, 2020, we expanded our contractual relationship with the CDC through an amendment to our existing Vaccines for Children Program contract for the distribution of certain COVID-19 vaccines. The COVID-19 vaccine distribution agreement with the CDC was finalized during the third quarter of 2021. We support the U.S. government as a centralized distributor of COVID-19 vaccines and ancillary supplies needed to administer vaccines. In the centralized model, the U.S. government directs us on the distribution of the vaccines and related supplies to point-of-care sites across the country. We make no decisions on where products are to be distributed nor how the products are allocated between the various provider sites and ultimately administered to the individuals receiving a vaccine. We utilize our expertise and capabilities to support the CDC’s efforts to vaccinate everyone in the U.S. who wants to receive a COVID-19 vaccine. The CDC and McKesson collaborated similarly in response to the 2009 H1N1 pandemic.
In December 2020, we began distributing the Moderna Vaccine in the U.S. and in March 2021, we began distributing the Janssen Vaccine. We may distribute other future authorized COVID-19 vaccines that are refrigerated or frozen. Ancillary supply kits may be shipped either together with the Moderna Vaccine and Janssen Vaccine or in advance of the vaccines. The results of operations related to our vaccine distribution are reflected in our U.S. Pharmaceutical segment. The Pfizer Vaccine, which is ultra-frozen, is not being distributed by McKesson, although we are providing ancillary supplies needed for its administration.
On September 23, 2020, we announced our contract with the HHS under which our Medical-Surgical Solutions segment manages the assembly and distribution of ancillary supply kits needed to administer COVID-19 vaccines, including sourcing some of those supplies. We also have an agreement with Pfizer to assemble and distribute ancillary supply kits needed to administer that particular COVID-19 vaccine. Ancillary supply kits include alcohol prep pads, face shields, surgical masks, needles and syringes, and other vaccine administration items. For the Pfizer Vaccine, ancillary supply kits also include the diluent needed to administer the ultra-frozen vaccine. We began assembling the ancillary supply kits in September 2020 in preparation for vaccine authorization and subsequent distribution. Ancillary supply kits to administer the Pfizer Vaccine are shipped directly to point-of-care sites, and all other ancillary supply kits are shipped to our dedicated vaccine distribution centers. The results of operations for the kitting and distribution of ancillary supplies are reflected in our Medical-Surgical Solutions segment. The future financial impact of the arrangements with the CDC and HHS depend on numerous uncertainties, which are described at the end of this COVID-19 section.
To manage the COVID-19 vaccine and ancillary supply kit distribution, we have set up special, dedicated vaccine distribution centers that include large-scale, custom freezers and refrigerators to safely store and process millions of vaccine doses. These facilities can scale to meet the demand of increasing volumes of vaccines being manufactured. We have also set up distribution centers for kitting and inventory management as part of our contract with the HHS. We are working with delivery partners to manage the delivery of vaccines and ancillary supply kits from our centralized vaccine distribution centers to point-of-care destinations as directed by the CDC. The capital expenditures we made during 2021 to prepare for vaccine and ancillary supply kit distribution were not material to our financial condition or liquidity.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Impact to our Results of Operations, Financial Condition, and Liquidity
For the year ended March 31, 2021, the demand for COVID-19 tests, the year over year impact from PPE and other related products, net of inventory charges, as well as the kitting and distribution of ancillary supplies for COVID-19 vaccines in our Medical-Surgical Solutions segment contributed approximately 20% in segment revenues and segment operating profit. Additionally, the distribution of COVID-19 vaccines in our U.S. Pharmaceutical segment contributed approximately 2% in segment operating profit for the year ended March 31, 2021. During our fourth quarter of 2020, we experienced a temporary increase in demand for pharmaceuticals. Subsequently, during the first quarter, we had lower pharmaceutical volumes, specialty drug volumes, and patient care visits that negatively impacted our consolidated revenues and income (loss) from continuing operations before income taxes for the year ended March 31, 2021. During the year ended March 31, 2021, selling, distribution, general, and administrative expenses decreased as a result of the pandemic, largely due to savings from restricted travel and decreased meetings. The favorable reduction in selling, distribution, general, and administrative expenses was partially offset by increased costs of transport, costs for enhanced procedures to sanitize operating facilities, and costs of providing PPE and other related products for employee use. Additionally, increased costs for certain PPE compressed our margins. Certain PPE items held for resale were valued in our inventory at costs that were inflated by earlier COVID-19 pandemic demand levels. That inventory valuation, if not supported by market resale prices, may be written down to net realizable value. We may also write-off inventory due to decreased customer demand and excess inventory. During the year ended March 31, 2021, we recorded charges totaling $136 million in cost of sales on certain PPE and other related products due to inventory impairments and excess inventory in our Medical-Surgical Solutions segment. Although market price volatility and changes to anticipated customer demand may require additional write-downs in future periods, we are taking measures to mitigate such risk. Overall, these COVID-19 related items had a net favorable impact on consolidated income (loss) from continuing operations before income taxes for the year ended March 31, 2021 compared to the prior year. Impacts to future periods due to COVID-19 may differ based on future developments, which is described at the end of this COVID-19 section.
We were able to maintain appropriate labor and overall vendor supply levels during the year ended March 31, 2021. Our inventory levels have fluctuated in response to supply availability and customer demand patterns for certain products, with varying inventory level impacts depending on the specific product within our portfolio. We collaborated closely with the federal government and other healthcare stakeholders to source more critical PPE to the U.S. This collaboration expedited the shipment of critical medical supplies to areas hit hardest by COVID-19, as identified by the Federal Emergency Management Agency. As our supply levels improve, and the federal government evolves guidance on the prioritization of providers or geographic markets, we will continue to adapt our distribution policies.
On March 27, 2020, the U.S. government enacted the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”) to address the economic impact of the COVID-19 pandemic. Among other things, the CARES Act provides certain changes to tax laws and includes provisions to provide relief for citizens, companies, healthcare providers and patients, and others. We have deferred certain employer payroll taxes and continue to monitor the potential impact of other tax legislation changes as result of the CARES Act, including refundable payroll tax credits on certain qualified wages. We anticipate changes due to the CARES Act in the timing of certain cash flows, with no material impact to our financial results for the year ended March 31, 2021. On December 27, 2020, the U.S. government enacted the Consolidated Appropriations Act, 2021 (the “CA Act”), which enhances and expands certain provisions of the CARES Act. The CA Act did not have a material impact on our financial condition, results of operations, or liquidity for the year ended March 31, 2021 nor do we currently expect a material impact on our future financial results.
Our consolidated balance sheets and ability to maintain financial liquidity remains strong. We have experienced no material impacts to our liquidity or net working capital due to the COVID-19 pandemic. We are monitoring our customers closely for changes to their timing of payments or ability to pay amounts owed to us as a result of COVID-19 pandemic impacts to their businesses. We remain well-capitalized with access to liquidity from our revolving credit facility. Long-term debt markets and commercial paper markets, our primary sources of capital after cash flow from operations, have remained open and accessible to us during the COVID-19 pandemic. At March 31, 2021, we were in compliance with all debt covenants, and believe we have the ability to continue to meet our debt covenants in the future.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Impact to our Supply Chain
We also continue to monitor the COVID-19 pandemic impacts on our supply chain. Although the availability of various products is dependent on our suppliers, their locations, and the extent to which they are impacted by the COVID-19 pandemic, we are proactively working with manufacturers, industry partners, and government agencies to meet the needs of our customers during the pandemic. We have assembled a Critical Care Drug Task Force, made up of our procurement specialists, clinical health systems pharmacists, and supply chain professionals, that is focused on securing additional product where available, sourcing back-up products, and protecting our operations across all locations and facilities. We are also working with manufacturers through several channels, including our ClarusONE Sourcing Services LLP (“ClarusONE”) and global sourcing teams in London, and our leaders are actively engaged in addressing potential shortages. We have engaged with industry partners and government agencies to gain visibility into supply and demand. Additionally, we have a robust Business Continuity and Disaster Recovery Program (“BCRP”) and we have proactively enhanced our BCRP in response to the COVID-19 pandemic to protect the supply chain to minimize disruption in healthcare, protect our customers, ensure the safety and security of our employees and workplaces, and ensure the continuity of critical business processes.
The situation remains fluid and we are taking a proactive approach to protect inventory during this crisis and ensure our provider partners have needed supplies and medications to help prevent the spread of the disease and treat those that are ill. COVID-19 has put an unprecedented strain on the supply of high-demand PPE, including N95 masks, gloves, as well as disinfecting sprays and wipes. The supply chain has improved over the initial impact of pandemic-related demand, and we continue to closely monitor demand by customer type and certain PPE and infection prevention items are still in short supply. Our allocation approach has helped us avoid stock outs in many critical product categories, allowing us to provide PPE supplies to many more customers for a much longer time. We anticipate these market conditions will remain for the foreseeable future. Our efforts to help the supply chain have included sourcing products from new suppliers all over the world, working closely with the federal government to help with the nation’s response and collaborating with partners to source, develop, and deliver new products to market.
Risks and Forward-Looking Information
The COVID-19 pandemic has disrupted the global economy and exacerbated uncertainties inherent in estimates, judgments, and assumptions used in our forecasts. We face numerous uncertainties in estimating the direct and indirect effects of COVID-19 on our future business operations, financial condition, results of operations, and liquidity. The full extent to which COVID-19 will impact us depends on many factors and future developments, including: the duration and spread of the virus; governmental actions to limit the spread of the virus; potential seasonality of viral outbreaks; potential new strains or variants of the original virus; the amount of COVID-19 vaccines authorized, manufactured, distributed, and administered; the amount of ancillary supply kits assembled and distributed; the effectiveness of COVID-19 vaccines and governmental measures in controlling the spread of the virus; and the effectiveness of treatments of infected individuals. Due to several rapidly changing variables related to the COVID-19 pandemic, estimations of future economic trends and the timing of when stability will return remains challenging. Additionally, we periodically review our intangible and other long-lived assets for impairment when events or changes in circumstances indicate the carrying value may not be recoverable. Key assumptions and estimates about future values in our impairment assessments can be affected by a variety of factors, including the impacts of the global pandemic on industry and economic trends as well as on our business strategy and internal forecasts. Material changes to key assumptions and estimates can decrease the projected cash flows or increase the discount rates and have resulted in impairment charges of certain long-lived assets as disclosed in Financial Note 4, “Restructuring, Impairment, and Related Charges,” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K, and could potentially result in future impairment charges. Refer to Item 1A - Risk Factors in Part I of this Annual Report on Form 10-K for a disclosure of risk factors related to COVID-19.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Opioid-Related Litigation and Claims
We are a defendant in approximately 3,200 legal proceedings asserting claims related to the distribution of controlled substances (opioids) in federal and state courts throughout the U.S., and in Puerto Rico and Canada. Those proceedings include approximately 2,900 federal cases and approximately 300 state court cases throughout the U.S., and cases in Puerto Rico and Canada. We continue to be involved in discussions with the objective of achieving broad resolution of opioid-related claims of states, their political subdivisions and other government entities (“governmental entities”). We are in ongoing, advanced discussions with state attorneys general and plaintiffs’ representatives regarding a framework under which, in order to resolve the claims of governmental entities, the three largest U.S. pharmaceutical distributors would pay up to approximately $21.0 billion over a period of 18 years, with up to approximately $8.0 billion to be paid by us, of which more than 90% is anticipated to be used to remediate the opioids crisis. Most of the remaining amount relates to plaintiffs’ attorneys fees and costs, and would be payable over a shorter time period. In addition, the proposed framework would require the three distributors, including the Company, to adopt changes to anti-diversion programs.
We have concluded that discussions under that framework have reached a stage at which a broad settlement of opioid claims by governmental entities is probable, and the loss related thereto can be reasonably estimated as of March 31, 2021. As a result of that conclusion, and our assessment of certain other opioid-related claims, we recorded a charge of $8.1 billion for the year ended March 31, 2021 within “Claims and litigation charges, net” in our Consolidated Statement of Operations, related to our share of the settlement framework described above, as well as other opioid-related claims. Because of the many uncertainties associated with any potential settlement arrangement or other resolution of opioid-related litigation, including the uncertainty of the scope of participation by plaintiffs in any potential settlement, we are not able to reasonably estimate the upper or lower ends of the range of ultimate possible loss for all opioid-related litigation matters. In light of the uncertainty of the timing of amounts that would be paid with respect to the charge, the charge was recorded in “Long-term litigation liabilities” in our Consolidated Balance Sheet as of March 31, 2021. Moreover, in light of this uncertainty, the amount of any ultimate loss may differ materially from the amount accrued.
While we continue to be involved in discussions regarding a potential broad settlement framework, we also continue to prepare for trial in these pending matters. We believe that we have valid defenses to the claims pending against us and, absent an acceptable settlement, intend to vigorously defend against all such claims. An adverse judgment or negotiated resolution in any of these matters could have a material adverse impact on our financial position, cash flows or liquidity, or results of operations. Refer to Financial Note 19, “Commitments and Contingent Liabilities,” to the accompanying consolidated financial statements included in this Annual Report on Form 10‑K for more information.
State Opioid Statutes
Legislative, regulatory, or industry measures to address the misuse of prescription opioid medications could affect our business in ways that we may not be able to predict. In April 2018, the State of New York adopted the Opioid Stewardship Act (“OSA”) which required the imposition of an annual surcharge on all manufacturers and distributors licensed to sell or distribute opioids in New York. On December 19, 2018, the U.S. District Court for the Southern District of New York found the law unconstitutional and issued an injunction preventing the State of New York from enforcing the law. The State of New York appealed to the U.S. Court of Appeals for the Second Circuit. The State of New York has subsequently adopted an excise tax on sales of opioids in the State, which became effective July 1, 2019. The law adopting the excise tax made clear that the OSA would apply only to opioid sales on or before December 31, 2018. The excise tax applies only to the first sale occurring in New York, and thus may not apply to sales from our distribution centers in New York to New York customers.
On September 14, 2020, a panel of the U.S. Court of Appeals for the Second Circuit reversed the district court’s decision on procedural grounds. The Healthcare Distribution Alliance filed a petition for panel rehearing, or, in the alternative, for rehearing en banc with the U.S. Court of Appeals for the Second Circuit; that petition was denied on December 18, 2020. On February 12, 2021, the U.S. Court of Appeals for the Second Circuit granted a motion by the Healthcare Distribution Alliance to stay its mandate pending the filing and disposition of a petition for writ of certiorari before the U.S. Supreme Court. The due date for filing such a petition is May 17, 2021. Unless the appellate court’s decision is overturned, the OSA will be reinstated for calendar years 2017 and 2018 (but not beyond those years), and, subject to any further legal challenge, we will have to pay our ratable share of the annual surcharge for those two years. During the second quarter of 2021, we reflected an estimated liability of $50 million for the OSA surcharge in our accompanying consolidated financial statements on the assumption that the appellate court’s decision will stand. Refer to Note 19, “Commitments and Contingent Liabilities,” to the accompanying consolidated financial statements included in this Annual Report on Form 10‑K for more information.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
RESULTS OF OPERATIONS
Overview of Consolidated Results:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In millions, except per share data) | Years Ended March 31, | | Change |
| 2021 | | 2020 | | 2019 | | 2021 | 2020 |
| Revenues | $ | 238,228 | | | $ | 231,051 | | | $ | 214,319 | | | 3 | | % | 8 | | % |
| Gross profit | 12,148 | | | 12,023 | | | 11,754 | | | 1 | | | 2 | | |
| Gross profit margin | 5.10 | | % | 5.20 | | % | 5.48 | | % | (10) | | bp | (28) | | bp |
| Total operating expenses | $ | (17,188) | | | $ | (9,534) | | | $ | (10,868) | | | 80 | | % | (12) | | % |
| Total operating expenses as a percentage of revenues | 7.21 | | % | 4.13 | | % | 5.07 | | % | 308 | | bp | (94) | | bp |
| Other income, net | $ | 223 | | | $ | 12 | | | $ | 182 | | | NM | | (93) | | % |
| Equity earnings and charges from investment in Change Healthcare Joint Venture | — | | | (1,108) | | | (194) | | | (100) | | | 471 | | |
| | | | | | | | | | |
| Interest expense | (217) | | | (249) | | | (264) | | | (13) | | | (6) | | |
| Income (loss) from continuing operations before income taxes | (5,034) | | | 1,144 | | | 610 | | | (540) | | | 88 | | |
| Income tax benefit (expense) | 695 | | | (18) | | | (356) | | | NM | | (95) | | |
| Income (loss) from continuing operations | (4,339) | | | 1,126 | | | 254 | | | (485) | | | 343 | | |
| Income (loss) from discontinued operations, net of tax | (1) | | | (6) | | | 1 | | | (83) | | | (700) | | |
| Net income (loss) | (4,340) | | | 1,120 | | | 255 | | | (488) | | | 339 | | |
| Net income attributable to noncontrolling interests | (199) | | | (220) | | | (221) | | | (10) | | | - | |
| Net income (loss) attributable to McKesson Corporation | $ | (4,539) | | | $ | 900 | | | $ | 34 | | | (604) | | % | NM | |
| | | | | | | | | | |
| Diluted earnings (loss) per common share attributable to McKesson Corporation | | | | | | | | | | |
| Continuing operations | $ | (28.26) | | | $ | 4.99 | | | $ | 0.17 | | | (666) | | % | NM | |
| Discontinued operations | — | | | (0.04) | | | — | | | (100) | | | NM | |
| Total | $ | (28.26) | | | $ | 4.95 | | | $ | 0.17 | | | (671) | | % | NM | |
| | | | | | | | | | |
| Weighted-average diluted common shares outstanding | 160.6 | | | 181.6 | | | 197.3 | | | (12) | | % | (8) | | % |
bp - basis points
NM - computation not meaningful
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Revenues
Revenues increased for the years ended March 31, 2021 and 2020 compared to the respective prior years primarily due to market growth, including expanded business with existing customers, within our U.S. Pharmaceutical segment. Market growth includes growing drug utilization, price increases, and newly launched products, partially offset by price deflation associated with branded to generic drug conversion.
Gross Profit
Gross profit increased for the year ended March 31, 2021 compared to the prior year primarily in our Medical-Surgical Solutions segment driven by the demand for COVID-19 tests and the contribution from kitting and distribution of ancillary supplies for COVID-19 vaccines, partially offset by the unfavorable impact from PPE and other related products largely due to inventory charges. Gross profit was favorably impacted by growth of specialty pharmaceuticals and the contribution from our vaccine distribution programs in our U.S. Pharmaceutical segment. Gross profit was unfavorably impacted by the adverse impacts from COVID-19 largely during the first quarter of 2021, including disruptions of doctors’ office operations, deferred or cancelled elective procedures, lower demand for pharmaceuticals, and overall reduction of foot traffic in pharmacies.
Gross profit increased for the year ended March 31, 2020 compared to the prior year primarily due to market growth in our Medical-Surgical Solutions segment, partially offset by unfavorable effects of foreign currency exchange fluctuations. Gross profit and gross profit margin for the year ended March 31, 2020 compared to the prior year were unfavorably impacted by lower gains from antitrust legal settlements, partially offset by higher last-in, first-out (“LIFO”) credits in 2020. The impact from COVID-19 increased gross profit by less than 1% and decreased gross profit margin by less than 10 basis points for the year ended March 31, 2020.
Gross profit for the years ended March 31, 2021, 2020, and 2019 included LIFO inventory credits of $38 million, $252 million, and $210 million, respectively. The lower LIFO credits in 2021 compared to 2020 is primarily due to higher brand inflation and delays of branded off-patent to generic drug launches. The higher LIFO credits in 2020 compared to 2019 is primarily driven by higher generic deflation. Refer to the “Critical Accounting Policies and Estimates” section included in this Financial Review for further information. Gross profit for the years ended March 31, 2021, 2020, and 2019 also included net cash proceeds received of $181 million, $22 million, and $202 million, respectively, representing our share of antitrust legal settlements.
Total Operating Expenses
A summary and description of the components of our total operating expenses for the years ended March 31, 2021, 2020, and 2019 is as follows:
•Selling, distribution, general, and administrative expenses (“SDG&A”): SDG&A consists of personnel costs, transportation costs, depreciation and amortization, lease costs, professional fee expenses, and administrative expenses.
•Claims and litigation charges, net: These charges include adjustments for estimated probable settlements related to our controlled substance monitoring and reporting, and opioid-related claims, as well as any applicable income items or credit adjustments due to subsequent changes in estimates. Legal fees to defend claims, which are expensed as incurred, are included within SDG&A. We have reclassified prior period amounts to conform to the current period presentation.
•Goodwill impairments charges: We perform an impairment test on goodwill balances annually in the third quarter and more frequently if indicators for potential impairment exist. The resulting goodwill impairment charges are reflected within this line item.
•Restructuring, impairment, and related charges: Restructuring charges that are incurred for programs in which we change our operations, the scope of a business undertaken by our business units, or the manner in which that business is conducted as well as long-lived asset impairments.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended March 31, | | Change |
| (Dollars in millions) | 2021 | | 2020 | | 2019 | | 2021 | 2020 |
| Selling, distribution, general, and administrative expenses | $ | 8,849 | | | $ | 9,182 | | | $ | 8,437 | | | (4) | | % | 9 | | % |
| Claims and litigation charges, net | 7,936 | | | 82 | | | 37 | | | NM | | 122 | | |
| Goodwill impairment charges | 69 | | | 2 | | | 1,797 | | | NM | | (100) | | |
| Restructuring, impairment, and related charges, net | 334 | | | 268 | | | 597 | | | 25 | | | (55) | | |
| Total operating expenses | $ | 17,188 | | | $ | 9,534 | | | $ | 10,868 | | | 80 | | % | (12) | | % |
| Percent of revenues | 7.21 | | % | 4.13 | | % | 5.07 | | % | 308 | | bp | (94) | | bp |
bp - basis points
NM - computation not meaningful
Total operating expenses and total operating expenses as a percentage of revenues increased for the year ended March 31, 2021 compared to the prior year, and decreased for the year ended March 31, 2020 compared to the prior year. Total operating expenses for the years ended March 31, 2021, 2020, and 2019 were affected by the following significant items:
2021
•SDG&A includes opioid-related costs of $153 million, primarily related to litigation expenses;
•SDG&A reflects cost savings of $95 million on travel and entertainment due to travel and meeting restrictions associated with COVID-19;
•SDG&A reflects charges of $58 million to remeasure assets and liabilities held for sale to fair value less costs to sell related to the completed contribution of the majority of our German pharmaceutical wholesale business to create a joint venture with WBA in which we have a 30% ownership interest within our International segment. Refer to Financial Note 3, “Held for Sale,” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K for more information;
•SDG&A includes a charge of $50 million related to our estimated liability under the OSA as previously discussed in the “Trends and Uncertainties” section;
•SDG&A also includes lower operating expenses due to the contribution of our German pharmaceutical wholesale business to a joint venture with WBA and a divestiture in our Medical-Surgical Solutions segment that closed in 2020;
•Claims and litigation charges, net includes a charge of $8.1 billion related to our estimated liability for opioid-related claims as previously discussed in the “Trends and Uncertainties” section;
•Claims and litigation charges, net includes a net gain of $131 million reflecting insurance proceeds received, net of attorneys' fees and expenses awarded to plaintiffs' counsel, in connection with the previously reported $175 million settlement of the shareholder derivative action related to our controlled substances monitoring program;
•Goodwill impairment charges of $69 million were recorded in connection with our segment realignment that commenced in the second quarter of 2021. Refer to the “Goodwill Impairment” section below for further details;
•Restructuring, impairment, and related charges, net includes long-lived asset impairment charges of $115 million primarily related to our retail pharmacy businesses in Canada and Europe within our International segment, and the remaining $219 million primarily represents costs associated with our operating model and cost optimization efforts in our corporate headquarters and International segment; and
•Total operating expenses were unfavorably impacted by foreign currency exchange fluctuations.
2020
•SDG&A includes charges of $275 million to remeasure assets and liabilities held for sale to fair value less costs to sell related to the completed contribution of the majority of our German pharmaceutical wholesale business to create a joint venture with WBA;
•SDG&A includes opioid-related costs of $150 million, primarily related to litigation expenses;
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
•Claims and litigation charges, net includes a settlement charge of $82 million recorded in connection with an agreement to settle all opioid-related claims filed by two Ohio counties;
•Restructuring, impairment, and related charges, net includes long-lived asset impairment charges of $112 million, primarily for our United Kingdom (“U.K.”) business (mainly pharmacy licenses) and Rexall Health retail business (“Rexall Health”) (mainly customer relationships) within our International segment, and the remaining $156 million primarily represents employee severance and exit-related costs related to our 2019 restructuring initiatives, as further discussed below; and
•Total operating expenses includes higher SDG&A due to our business acquisitions and to support business growth, as well as our technology initiatives, partially offset by favorable effects of foreign currency exchange fluctuations.
2019
•SDG&A includes opioid-related costs of $114 million, primarily related to litigation expenses, and increased expenses due to our business acquisitions and to support growth, partially offset by a gain from an escrow settlement of $97 million representing certain indemnity and other claims related to our 2017 acquisition of Rexall Health and a credit of $90 million for the derecognition of a liability related to the tax receivable agreement (“TRA”) payable to the shareholders of Change Healthcare, Inc. (“Change”);
•Goodwill impairment charges of $1.8 billion in our European Retail Pharmacy (“European RP”) and European Pharmaceutical Distribution (“European PD”) reporting units within the International segment. Of these impairment charges, $238 million was recognized upon the 2019 first quarter segment changes, which resulted in two new reporting units. The remaining charges primarily were due to declines in the reporting units’ estimated future cash flows and the selection of higher discount rates. These impairment charges generally were not deductible for income tax purposes. The declines in estimated future cash flows primarily were attributed to additional government reimbursement reductions and competitive pressures within the U.K. The risk of successfully achieving certain business initiatives was the primary factor in the use of a higher discount rate. At March 31, 2019, both the European RP and European PD reporting units had no remaining goodwill balances; and
•Restructuring, impairment, and related charges, net primarily includes employee severance and exit-related costs of $352 million for our 2019 restructuring initiatives, as further discussed below and long-lived asset impairment charges of $245 million primarily for our U.K. business (mainly pharmacy licenses) within our International segment driven by additional government reimbursement reductions and competitive pressures in the U.K.
Goodwill Impairments
As discussed in the “Overview of Our Business” section, our operating structure was realigned commencing in the second quarter of 2021 into four reportable segments: U.S. Pharmaceutical, International, Medical-Surgical Solutions, and RxTS. These reportable segments encompass all operating segments of the Company. The second quarter segment realignment resulted in changes in multiple reporting units across the Company. As a result, we were required to perform a goodwill impairment test for these reporting units and recorded a goodwill impairment charge in our European RP reporting unit of $69 million during the second quarter of 2021. At March 31, 2021, the balance of goodwill for our reporting units in Europe was approximately nil and the remaining balance of goodwill in the International segment primarily relates to one of our reporting units in Canada.
We evaluate goodwill for impairment on an annual basis as of October 1, and at an interim date, if indicators of potential impairment exist. The annual impairment testing performed in 2021 did not indicate any impairment of goodwill. As of the testing date, other risks, expenses, and future developments, such as additional government actions, increased regulatory uncertainty, and material changes in key market assumptions limit our ability to estimate projected cash flows, which could adversely affect the fair value of various reporting units in future periods, including our McKesson Canada reporting unit within our International segment and our RxCrossroads reporting unit within our RxTS segment, where the risk of a material goodwill impairment is higher than other reporting units. Refer to “Critical Accounting Policies and Estimates” included in this Financial Review for further information.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
On October 1, 2019, we voluntarily changed our annual goodwill impairment testing date from January 1 to October 1 to better align with the timing of our annual long-term planning process. This change was not material to our consolidated financial statements as it did not delay, accelerate, or avoid any potential goodwill impairment charge. Refer to Note 12, “Goodwill and Intangible Assets, Net,” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K for further information.
Restructuring Initiatives and Long-Lived Asset Impairments
During the first quarter of 2022, we approved an initiative to increase operational efficiencies and flexibility by transitioning to a partial remote work model for certain employees. This initiative primarily led us to rationalize our office space in North America. Where we determine to cease using office space, we plan to exit the portion of the facility no longer used. We also may retain and repurpose certain other office locations. We expect to incur total charges of approximately $180 million to $280 million for this initiative, consisting primarily of exit related costs, accelerated depreciation and amortization of long-lived assets, and asset impairments. This initiative is expected to be completed in 2022.
During the first quarter of 2021, we committed to an initiative within the U.K., which is included in our International segment, to further drive transformational changes in technologies and business processes, operational efficiencies, and cost savings. The initiative includes reducing the number of retail pharmacy stores, decommissioning obsolete technologies and processes, reorganizing and consolidating certain business operations, and related headcount reductions. We expect to incur total charges of approximately $85 million to $90 million, of which $57 million of charges were recorded to date. The initiative is expected to be substantially complete in 2022 and estimated remaining charges primarily consist of accelerated amortization of long-lived assets, facility and other exit costs, and employee-related costs.
In the fourth quarter of 2019, we committed to certain programs to continue our operating model and cost optimization efforts. We continue to implement centralization of certain functions and outsourcing through an expanded arrangement with a third-party vendor to achieve operational efficiency. The programs also include reorganization and consolidation of business operations, related headcount reductions, the further closures of retail pharmacy stores in Europe, and closures of other facilities. Total charges of $297 million were recorded to date. This initiative was substantially complete in 2021 and remaining costs we expect to record under this initiative are not material.
We also committed to certain actions in connection with the previously announced relocation of our corporate headquarters from San Francisco, California to Irving, Texas, which became effective April 1, 2019. Total charges of $105 million were recorded to date. The relocation was substantially complete in January 2021 and remaining costs we expect to record under this initiative, primarily relating to lease costs, are not material.
In the second quarter of 2018, we committed to a restructuring plan, which primarily consisted of the closures of underperforming retail pharmacy stores in the U.K., and a reduction in workforce. The plan was substantially complete in 2020.
On April 25, 2018, we announced a strategic growth initiative intended to drive long-term incremental profit growth and to increase operational efficiency. The initiative consisted of multiple growth priorities and plans to optimize our operating models and cost structures primarily through centralization, cost management, and outsourcing of certain administrative functions. As part of the growth initiative, we committed to implement certain actions including a reduction in workforce, facility consolidation, and store closures. This set of initiatives was substantially complete by the end of 2020.
Restructuring, impairment, and related charges for the years ended March 31, 2021, 2020, and 2019 also includes long-lived asset impairment charges of $115 million, $112 million, and $245 million, respectively, primarily related to our retail pharmacy businesses in Canada and Europe within our International segment. In addition, certain charges related to restructuring initiatives are included under the caption “Cost of sales” in our Consolidated Statements of Operations and were not material for the years ended March 31, 2021, 2020, and 2019.
Refer to Financial Note 4, “Restructuring, Impairment, and Related Charges,” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K for more information.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Other Income, Net
Other income, net for the year ended March 31, 2021 increased compared to the prior year primarily due to net gains recognized from our equity investments of $133 million. This primarily reflects mark-to-market gains on our investments in certain U.S. growth stage companies in the healthcare industry and realized gains on the exit of some of these investments as further described in Financial Note 17, “Fair Value Measurements,” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K. In future periods, fair value adjustments recognized in our operating results for these types of investments may be adversely impacted by market volatility. Other income, net also increased year over year due to pension settlement charges of $122 million recognized in 2020 related to our previously approved termination of the frozen U.S. defined benefit pension plan. In connection with the pension plan termination, we purchased annuity contracts from an insurer that will pay and administer the future pension benefits of the remaining participants.
Other income, net, for the year ended March 31, 2020 decreased compared to the prior year primarily due to the 2020 pension settlement charges described above and higher gains recognized from the sale of investments in 2019, partially offset by higher net settlement gains in 2020 from our derivative contracts.
Equity Earnings and Charges from Investment in Change Healthcare Joint Venture
Until the separation of our investment in Change Healthcare JV on March 10, 2020, we accounted for this investment using the equity method of accounting. Excluding the impairment and transaction-related items described below, our proportionate share of loss from our investment in Change Healthcare JV for the years ended March 31, 2020 and 2019 was $119 million and $194 million, respectively, which primarily includes transaction and integration expenses incurred by the joint venture and basis differences between the joint venture and McKesson including amortization of fair value adjustments. During the first quarter of 2020 and for the year ended March 31, 2019, we owned approximately 70% of this joint venture.
On June 27, 2019, common stock and certain other securities of Change began trading on the NASDAQ (“IPO”). On July 1, 2019, upon the completion of its IPO, Change contributed net cash proceeds it received from its offering of common stock to Change Healthcare JV in exchange for additional membership interests of Change Healthcare JV at the equivalent of its offering price of $13 per share. The proceeds from the concurrent offering of other securities were also used by Change to acquire certain securities of Change Healthcare JV. As a result, McKesson’s equity interest in Change Healthcare JV was reduced from 70% to approximately 58.5%, which was used to recognize our proportionate share in net loss from Change Healthcare JV, commencing in the second quarter of 2020. As a result of the ownership dilution to 58.5% from 70%, we recognized a dilution loss of approximately $246 million in the second quarter of 2020. Additionally, our proportionate share of income or loss from this investment was subsequently reduced as immaterial settlements of stock option exercises occurred after the IPO and further diluted our ownership.
In the second quarter of 2020, we recorded an other-than-temporary-impairment (“OTTI”) charge of $1.2 billion to our investment in Change Healthcare JV, representing the difference between the carrying value of our investment and the fair value derived from the corresponding closing price of Change’s common stock at September 30, 2019. This charge was included within “Equity earnings and charges from investment in Change Healthcare Joint Venture” in our Consolidated Statements of Operations for the year ended March 31, 2020.
On March 10, 2020, we completed the previously announced separation of our interest in Change Healthcare JV, which eliminated our investment in the joint venture. The separation was effected through the split-off of PF2 SpinCo, Inc. (“SpinCo”), a wholly owned subsidiary of the Company that held all of our interest in Change Healthcare JV, to certain of our stockholders through an exchange offer (“Split-off”), followed by the merger of SpinCo with and into Change, with Change surviving the merger (“Merger”).
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
In connection with the exchange offer, on March 9, 2020, we distributed all 176.0 million outstanding shares of common stock of SpinCo to participating holders of the Company’s common stock in exchange for 15.4 million shares of McKesson common stock. Following consummation of the exchange offer, on March 10, 2020, the Merger was consummated, with each share of SpinCo common stock converted into one share of Change common stock, par value $0.001 per share, with cash being paid in lieu of fractional shares of Change common stock. The Split-off and Merger are intended to be generally tax-free transactions to McKesson and its shareholders for U.S. federal income tax purposes. Following the Split-off, we do not beneficially own any of Change’s outstanding securities. In connection with this transaction, we recognized a net gain for financial reporting purposes of $414 million during the fourth quarter of 2020, which was largely driven by the reversal of a related deferred tax liability. Under the agreement with Change Healthcare JV, McKesson, Change, and certain subsidiaries of the Change Healthcare JV, there may be changes in future periods to the amount reversed. Any such change is not expected to be material.
After the separation, Change Healthcare JV is required under the TRA to pay McKesson 85% of the net cash tax savings realized, or deemed to be realized, resulting from depreciation or amortization allocated to Change by McKesson. The receipt of any payments under the TRA is dependent upon Change benefiting from this depreciation or amortization in future tax return filings, which creates uncertainty over the amount, timing and probability of the gain recognized. As such, we accounted for the TRA as a gain contingency, with no receivable recognized as of March 31, 2021 nor 2020.
Interest Expense
Interest expense decreased in 2021 compared to the prior year primarily due to the repayment of $1.0 billion of long-term debt in the third quarter of 2021 and a decrease in the issuance of commercial paper. Interest expense decreased in 2020 compared to the prior year primarily due to a decrease in the issuance of commercial paper, partially offset by a decrease in interest income from our derivative contracts. Interest expense fluctuates based on timing, amounts and interest rates of term debt repaid and new term debt issued, as well as amounts incurred associated with financing fees.
Income Tax (Benefit) Expense
We recorded income tax (benefit) expense of ($695 million), $18 million, and $356 million for the years ended March 31, 2021, 2020, and 2019, respectively. Our reported income tax (benefit) expense rates were (13.8%), 1.6%, and 58.4% in 2021, 2020, and 2019, respectively.
Our reported income tax rate for 2021 was impacted by the charge for opioid-related claims of $8.1 billion ($6.8 billion after-tax).
Our reported income tax expense rate for 2020 was favorably impacted by a net gain on the Change Healthcare JV divestiture of $414 million (pre-tax and after-tax), which was intended to generally be a tax-free split-off for U.S. federal income tax purposes, and unfavorably impacted by charges of $275 million (pre-tax and after-tax) to remeasure assets and liabilities held for sale to fair value less costs to sell related to the completed contribution of the majority of our German pharmaceutical wholesale business to create a joint venture with WBA. Refer to Financial Note 2,“Investment in Change Healthcare Joint Venture” and Note 3,“Held for Sale,” to the accompanying consolidated financial statements included in this Annual Report on Form 10‑K for more information.
Our reported income tax expense rate for 2019 was unfavorably impacted by charges of $1.8 billion (pre-tax and after-tax) to impair the carrying value of goodwill of our European RP and European PD reporting units within the International segment, given that these charges are generally not deductible for tax purposes. Refer to Financial Note 12, “Goodwill and Intangible Assets, Net,” to the accompanying consolidated financial statements included in this Annual Report on Form 10‑K for additional information.
Significant judgments and estimates are required in determining the consolidated income tax provision and evaluating income tax uncertainties. Although our major taxing jurisdictions include the U.S., Canada, and the U.K., we are subject to income taxes in numerous foreign jurisdictions. Our income tax expense, deferred tax assets and liabilities, and uncertain tax liabilities reflect management’s best assessment of estimated current and future taxes to be paid. We believe that we have made adequate provision for all income tax uncertainties.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Income (Loss) from Discontinued Operations, Net of Tax
Income (loss) from discontinued operations, net of tax, was $(1) million, $(6) million, and $1 million for the years ended March 31, 2021, 2020, and 2019, respectively.
Net Income Attributable to Noncontrolling Interests
Net income attributable to noncontrolling interests primarily represents ClarusONE, Vantage Oncology Holdings, LLC, and the accrual of the annual recurring compensation amount of €0.83 per McKesson Europe AG (“McKesson Europe”) share that McKesson is obligated to pay to the noncontrolling shareholders of McKesson Europe under the December 2014 domination and profit and loss transfer agreement (the “Domination Agreement”). Noncontrolling interests with redemption features, such as put rights, that are not solely within our control are considered redeemable noncontrolling interests. Redeemable noncontrolling interests are presented outside of McKesson Corporation stockholders’ equity (deficit) on our consolidated balance sheet. Refer to Financial Note 9, “Redeemable Noncontrolling Interests and Noncontrolling Interests,” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K for additional information.
Net Income (Loss) Attributable to McKesson Corporation
Net income (loss) attributable to McKesson Corporation was $(4.5) billion, $900 million, and $34 million for the years ended March 31, 2021, 2020, and 2019, respectively. Diluted earnings (loss) per common share attributable to McKesson Corporation was $(28.26), $4.95, and $0.17 for the years ended March 31, 2021, 2020, and 2019, respectively. Net loss per diluted share for the year ended March 31, 2021 is calculated by excluding dilutive securities from the denominator due to their antidilutive effects. Additionally, our 2021, 2020, and 2019 diluted earnings (loss) per share reflect the cumulative effects of share repurchases.
Weighted-Average Diluted Common Shares Outstanding
Diluted earnings (loss) per common share was calculated based on a weighted-average number of shares outstanding of 160.6 million, 181.6 million, and 197.3 million for the years ended March 31, 2021, 2020, and 2019, respectively. Weighted-average diluted common shares outstanding is impacted by the exercise and settlement of share-based awards and the cumulative effect of share repurchases, including the impact of shares exchanged as part of the split-off from our investment in Change Healthcare JV, as discussed above.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Overview of Segment Results:
Segment Revenues:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended March 31, | | Change |
| (Dollars in millions) | 2021 | | 2020 | | 2019 | | 2021 | 2020 |
| Segment revenues | | | | | | | | | | |
| U.S. Pharmaceutical | $ | 189,274 | | | $ | 181,700 | | | $ | 166,189 | | | 4 | | % | 9 | | % |
| International | 35,965 | | | 38,341 | | | 38,023 | | | (6) | | | 1 | | |
| Medical-Surgical Solutions | 10,099 | | | 8,305 | | | 7,618 | | | 22 | | | 9 | | |
| Prescription Technology Solutions | 2,890 | | | 2,705 | | | 2,489 | | | 7 | | | 9 | | |
| Total revenues | $ | 238,228 | | | $ | 231,051 | | | $ | 214,319 | | | 3 | | % | 8 | | % |
U.S. Pharmaceutical
2021 vs. 2020
U.S. Pharmaceutical revenues for the year ended March 31, 2021 increased 4% compared to the prior year primarily due to market growth, including branded pharmaceutical price increases, growth in specialty pharmaceuticals, and higher volumes from retail national account customers, partially offset by branded to generic drug conversions. Revenues for this segment were unfavorably impacted by fluctuations in demand for pharmaceuticals in retail pharmacies and institutional healthcare providers due to COVID-19 largely during the onset of the pandemic in late March 2020 and during our first quarter of 2021 combined with the loss of certain customers.
2020 vs. 2019
U.S. Pharmaceutical revenues for the year ended March 31, 2020 increased 9% compared to the prior year primarily due to market growth, including branded pharmaceutical price increases, growth in specialty pharmaceuticals, higher volumes from retail national account customers, partially offset by branded to generic drug conversions.
International
2021 vs. 2020
International revenues for the year ended March 31, 2021 decreased 6% compared to the prior year. Excluding the favorable effects of foreign currency exchange fluctuations, revenues for this segment decreased 9% primarily due to the contribution of our German pharmaceutical wholesale business to a joint venture with WBA and to a lesser extent, the exit of unprofitable customers in our Canadian business. Revenues for this segment were also unfavorably impacted by lower volumes from the adverse impacts from COVID-19 in our pharmaceutical distribution and retail pharmacy businesses within Europe.
2020 vs. 2019
International revenues for the year ended March 31, 2020 increased 1% compared to the prior year. Excluding the unfavorable effects of foreign currency exchange fluctuations, revenues for this segment increased 4% primarily due to market growth in our European pharmaceutical distribution and retail pharmacy businesses.
Medical-Surgical Solutions
2021 vs. 2020
Medical-Surgical Solutions revenues for the year ended March 31, 2021 increased 22% compared to the prior year largely due to sales of COVID-19 tests and PPE.
2020 vs. 2019
Medical-Surgical Solutions revenues for the year ended March 31, 2020 increased 9% compared to the prior year primarily due to market growth in our primary care business.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Prescription Technology Solutions
2021 vs. 2020
RxTS revenues for the year ended March 31, 2021 increased 7% compared to the prior year driven by increased volume with new and existing customers primarily in our CoverMyMeds business.
2020 vs. 2019
RxTS revenues for the year ended March 31, 2020 increased 9% compared to the prior year primarily driven by increased volume with new and existing customers.
Segment Operating Profit (Loss) and Corporate Expenses, Net:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended March 31, | | Change |
| (Dollars in millions) | 2021 | | 2020 | | 2019 | | 2021 | 2020 |
Segment operating profit (loss) (1) | | | | | | | | | | |
U.S. Pharmaceutical (2) | $ | 2,763 | | | $ | 2,745 | | | $ | 2,710 | | | 1 | | % | 1 | | % |
International (3) | (37) | | | (161) | | | (1,903) | | | (77) | | | (92) | | |
Medical-Surgical Solutions (4) | 707 | | | 499 | | | 455 | | | 42 | | | 10 | | |
Prescription Technology Solutions (5) | 395 | | | 396 | | | 355 | | | - | | 12 | | |
Other (6) | — | | | (1,113) | | | (104) | | | (100) | | | 970 | | |
| Subtotal | 3,828 | | | 2,366 | | | 1,513 | | | 62 | | | 56 | | |
Corporate expenses, net (7) | (8,645) | | | (973) | | | (639) | | | 788 | | | 52 | | |
| Interest expense | (217) | | | (249) | | | (264) | | | (13) | | | (6) | | |
| Income (loss) from continuing operations before income taxes | $ | (5,034) | | | $ | 1,144 | | | $ | 610 | | | (540) | | % | 88 | | % |
| | | | | | | | | | |
| Segment operating profit (loss) margin | | | | | | | | | | |
| U.S. Pharmaceutical | 1.46 | | % | 1.51 | | % | 1.63 | | % | (5) | | bp | (12) | | bp |
| International | (0.10) | | | (0.42) | | | (5.00) | | | 32 | | | 458 | | |
| Medical-Surgical Solutions | 7.00 | | | 6.01 | | | 5.97 | | | 99 | | | 4 | | |
| Prescription Technology Solutions | 13.67 | | | 14.64 | | | 14.26 | | | (97) | | | 38 | | |
bp - basis points
(1)Segment operating profit (loss) includes gross profit, net of operating expenses, as well as other income (expense), net, for our reportable segments. For retrospective periods presented, operating loss for Other reflects equity earnings and charges from our equity method investment in Change Healthcare JV, which we split-off in the fourth quarter of 2020.
(2)Operating profit for our U.S. Pharmaceutical segment includes a charge of $50 million for the year ended March 31, 2021 related to our estimated liability under the OSA.
(3)Operating loss for our International segment for the years ended March 31, 2021 and 2020 includes charges of $58 million and $275 million, respectively, to remeasure to fair value the assets and liabilities of our German pharmaceutical wholesale business which was contributed to a joint venture with WBA. This segment’s operating loss for the years ended March 31, 2021 and 2019 includes goodwill impairment charges of $69 million and $1.8 billion, respectively, as well as long-lived asset impairment charges for the years ended March 31, 2021, 2020, and 2019 of $115 million, $112 million, and $245 million, respectively, primarily related to our retail pharmacy businesses in Canada and Europe.
(4)Operating profit for our Medical-Surgical Solutions segment for the year ended March 31, 2021 includes charges totaling $136 million on certain PPE and other related products due to inventory impairments and excess inventory.
(5)Operating profit for our RxTS segment for the year ended March 31, 2019 includes a gain of $56 million from the divestiture of an equity investment.
(6)Operating loss for Other for the year ended March 31, 2020 includes an OTTI charge of $1.2 billion and a dilution loss of $246 million related to our investment in Change Healthcare JV, partially offset by a net gain of $414 million related to the completed separation of our interest in Change Healthcare JV during the fourth quarter of 2020. Operating loss for the year ended March 31, 2019 includes a credit of $90 million for the derecognition of a liability related to the TRA payable to the shareholders of Change.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
(7)Corporate expenses, net for the year ended March 31, 2021 includes a charge of $8.1 billion related to our estimated liability for opioid-related claims, net gains of $133 million from our equity investments, and a net gain of $131 million recorded in connection with insurance proceeds received from the settlement of the shareholder derivative action related to our controlled substances monitoring program. Corporate expenses, net for the year ended March 31, 2020 includes pension settlement charges of $122 million and a settlement charge of $82 million related to opioid claims.
U.S. Pharmaceutical
2021 vs. 2020
Operating profit increased for the year ended March 31, 2021 compared to the prior year primarily due to an increase in net cash proceeds received of $159 million in 2021 compared to 2020 representing our share of antitrust legal settlements, growth in specialty pharmaceuticals, and the contribution from our vaccine distribution programs. This was partially offset by a decrease in LIFO credits of $214 million, a charge of $50 million recorded in 2021 related to our estimated liability under the OSA, net impacts from COVID-19, including a less severe cough, cold, and flu season, as well as increased costs for strategic growth initiatives.
2020 vs. 2019
Operating profit increased for the year ended March 31, 2020 compared to the prior year primarily due to market growth in our specialty business. Operating profit and operating profit margin were favorably impacted by a charge of $61 million related to a customer bankruptcy in 2019 and an increase in LIFO credits of $42 million. Operating profit and operating profit margin were unfavorably impacted by customer mix and a decrease in net cash proceeds received of $180 million representing our share of antitrust legal settlements.
International
2021 vs. 2020
Operating loss and operating loss margin improved for the year ended March 31, 2021 compared to the prior year primarily due to a decrease in the charges recorded to remeasure to fair value the assets and liabilities of our German pharmaceutical wholesale business which was contributed to a joint venture with WBA, of which $58 million and $275 million was reflected for the years ended March 31, 2021 and 2020, respectively. This was partially offset by a goodwill impairment charge of $69 million recorded in the second quarter of 2021 related to our European retail pharmacy business. The impacts from COVID-19 in our pharmaceutical distribution and retail pharmacy businesses within Europe also caused unfavorability in our segment results year over year.
2020 vs. 2019
Operating loss and operating loss margin improved for the year ended March 31, 2020 compared to the prior year primarily due to goodwill impairment charges of $1.8 billion in 2019 and a decrease in long-lived asset impairment charges of $112 million in 2020 compared to $245 million in 2019. This was partially offset by the fair value remeasurement charges in 2020 described above and a gain from an escrow settlement of $97 million in 2019 related to our 2017 acquisition of Rexall Health.
Medical-Surgical Solutions
2021 vs. 2020
Operating profit and operating profit margin increased for the year ended March 31, 2021 compared to prior year primarily due to COVID-19, including demand for COVID-19 tests and PPE, as well as the contribution from kitting and distribution of ancillary supplies for COVID-19 vaccines. This was partially offset by inventory charges on certain PPE and other related products, unfavorability in our primary care business due to customer closures largely during the first quarter of 2021, and a less severe cough, cold, and flu season. Additionally, operating profit was favorable year over year due to lower operating expenses, including a decrease in our provision for bad debts.
2020 vs. 2019
Operating profit and operating profit margin increased for the year ended March 31, 2020 compared to prior year primarily due to market growth in our primary care business and lower restructuring charges, partially offset by the remeasurement of assets and liabilities to fair value related to a divestiture that was completed in 2020 and higher operating expenses, including an increase in our provision for bad debts.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Prescription Technology Solutions
2021 vs. 2020
Operating profit remained relatively flat for the year ended March 31, 2021 compared to prior year primarily due to higher operating expenses to support business growth, offset by increased volume with new and existing customers. Operating profit margin decreased for the year ended March 31, 2021 compared to prior year primarily due to higher operating expenses.
2020 vs. 2019
Operating profit and operating profit margin increased for the year ended March 31, 2020 compared to prior year primarily due to increased volumes with new and existing customers, integration costs incurred in 2019 for our acquisition of RxCrossroads that closed during the fourth quarter of 2018, partially offset by a gain of $56 million from the divestiture of an equity investment in 2019.
Other
Operating loss for Other for the year ended March 31, 2020 includes an OTTI charge of $1.2 billion and a dilution loss of $246 million related to our investment in Change Healthcare JV, partially offset by a net gain of $414 million related to the completed separation of our interest in Change Healthcare JV during the fourth quarter of 2020. Operating loss for Other for the year ended March 31, 2019 includes a credit of $90 million for the derecognition of a liability related to the TRA payable to the shareholders of Change. Operating loss for Other also includes our proportionate share of loss from Change Healthcare JV of $119 million and $194 million for the years ended March 31, 2020 and 2019, respectively.
Corporate
2021 vs. 2020
Corporate expenses, net increased for the year ended March 31, 2021 compared to the prior year due to a charge of $8.1 billion related to our estimated liability for opioid-related claims.
Corporate expenses, net for 2021 also includes net gains recognized from our equity investments of $133 million and a net gain of $131 million recognized in connection with insurance proceeds received from the settlement of the shareholder derivative action related to our controlled substances monitoring program. Corporate expenses, net, for 2020 includes pension settlement charges of $122 million, an opioid claim settlement charge of $82 million, and net settlement gains of $26 million recognized from our derivative contracts.
2020 vs. 2019
Corporate expenses, net, increased for the year ended March 31, 2020 compared to the prior year primarily due to the pension settlement charges and opioid claim settlement charge mentioned above, as well as higher costs for technology initiatives, partially offset by net settlement gains recognized in 2020 from our derivative contracts. Corporate expenses, net, for 2020 also included charitable contribution expenses of approximately $20 million primarily for the McKesson Foundation.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Foreign Operations
Our foreign operations represented approximately 15%, 17%, and 18% of our consolidated revenues in 2021, 2020, and 2019, respectively. Foreign operations are subject to certain risks, including currency fluctuations. We monitor our operations and adopt strategies responsive to changes in the economic and political environment in each of the countries in which we operate. We conduct our business worldwide in local currencies including Euro, British pound sterling, and Canadian dollar. As a result, the comparability of our results reported in U.S. dollars can be affected by changes in foreign currency exchange rates. In discussing our operating results, we may use the term “foreign currency exchange fluctuations,” which refers to the effect of changes in foreign currency exchange rates used to convert the local currency results of our operations in foreign countries where the functional currency is not the U.S. dollar. We present this information to provide a framework for assessing how our business performed excluding the effect of foreign currency exchange rate fluctuations. In computing the foreign currency exchange fluctuations, we translate our current year results of our operations in foreign countries recorded in local currencies into U.S dollars by applying their respective average foreign currency exchange rates of the corresponding prior year periods, and we subsequently compare those results to the previously reported results of the comparable prior year periods reported in U.S. dollars. Additional information regarding our foreign operations is included in Financial Note 22, “Segments of Business,” to the consolidated financial statements appearing in this Annual Report on Form 10-K.
Business Combinations
Refer to Financial Note 5, “Business Acquisitions and Divestitures,” to the consolidated financial statements appearing in this Annual Report on Form 10-K for additional information.
Fiscal 2022 Outlook
Information regarding the Company’s fiscal 2022 outlook is contained in our Form 8-K dated May 6, 2021. That Form 8-K should be read in conjunction with the forward-looking statements in the "Trends and Uncertainties" section of this Financial Review, as well as the cautionary statements in Item 1, "Business - Forward-Looking Statements," and Item 1A, "Risk Factors," in Part I of this Annual Report on Form 10-K.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
We consider an accounting estimate to be critical if the estimate requires us to make assumptions about matters that were uncertain at the time the accounting estimate was made and if different estimates that we reasonably could have used in the current period, or changes in the accounting estimate that are reasonably likely to occur from period to period, could have a material impact on our financial condition or results from operations. Below are the estimates that we believe are critical to the understanding of our operating results and financial condition. Other accounting policies are described in Financial Note 1, “Significant Accounting Policies,” to the consolidated financial statements appearing in this Annual Report on Form 10-K. Because of the uncertainty inherent in such estimates, actual results may differ from these estimates.
Allowance for Doubtful Accounts: We provide short-term credit and other customer financing arrangements to customers who purchase our products and services. Other customer financing primarily relates to guarantees provided to our customers, or their creditors, regarding the repurchase of inventories. We also provide financing to certain customers related to the purchase of pharmacies, which serve as collateral for the loans. We estimate the receivables for which we do not expect full collection based on historical collection rates and specific knowledge regarding the current creditworthiness of our customers and record an allowance in our consolidated financial statements for these amounts.
The Company considers historical experience, the current economic environment, customer credit ratings or bankruptcies, and reasonable and supportable forecasts to develop its allowance for doubtful accounts. Management reviews these factors quarterly to determine if any adjustments are needed to the allowance.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Sales to the Company’s ten largest customers, including group purchasing organizations (“GPOs”), accounted for approximately 51% of total consolidated revenues in 2021 and comprised approximately 32% of total trade accounts receivable at March 31, 2021. Sales to our largest customer, CVS Health Corporation (“CVS”), accounted for approximately 21% of our total consolidated revenues in 2021 and comprised approximately 19% of total trade accounts receivable at March 31, 2021. As a result, our sales and credit concentration is significant. We also have agreements with GPOs, each of which functions as a purchasing agent on behalf of member hospitals, pharmacies and other healthcare providers, as well as with government entities and agencies. The accounts receivables balances are with individual members of the GPOs, and therefore no significant concentration of credit risk exists. A material default in payment, a material reduction in purchases from these or any other large customers, or the loss of a large customer or GPO could have a material adverse impact on our financial position, results of operations, and liquidity.
Reserve methodologies are assessed annually based on historical losses and economic, business and market trends. In addition, reserves are reviewed quarterly and updated if unusual circumstances or trends are present. We believe the reserves maintained and expenses recorded in 2021 are appropriate and consistent in the context of historical methodologies employed, as well as assessment of trends currently available.
At March 31, 2021, trade and notes receivables were $17.5 billion prior to allowances of $211 million. In 2021, 2020 and, 2019, our provision for bad debts was $4 million, $91 million, and $132 million, respectively. At March 31, 2021 and 2020, the allowance as a percentage of trade and notes receivables was 1.2% and 1.4%, respectively. An increase or decrease of a hypothetical 0.1% in the 2021 allowance as a percentage of trade and notes receivables would result in an increase or decrease in the provision for bad debts of approximately $18 million. The selected 0.1% hypothetical change does not reflect what could be considered the best or worst-case scenarios. Additional information concerning our allowance for doubtful accounts may be found in Schedule II included in this Annual Report on Form 10-K.
Inventories: Inventories consist of merchandise held for resale. We report inventories at the lower of cost or net realizable value, except for inventories determined using the LIFO method which are valued at the lower of LIFO cost or market. LIFO method presumes that the most recent inventory purchases are the first items sold and the inventory cost under LIFO approximates market. The majority of the cost of domestic inventories is determined using the LIFO method. The majority of the cost of inventories held in foreign and certain domestic locations is based on the first-in, first-out (“FIFO”) method and weighted-average purchase prices. Rebates, cash discounts and other incentives received from vendors relating to the purchase or distribution of inventory are considered product discounts and are accounted for as a reduction in the cost of inventory and are recognized when the inventory is sold.
At March 31, 2021 and 2020, total inventories, net were $19.2 billion and $16.7 billion, respectively, in our Consolidated Balance Sheets. The LIFO method was used to value approximately 58% and 60% of our inventories at March 31, 2021 and 2020, respectively. If we had used the moving average method of inventory valuation, inventories would have been approximately $406 million and $444 million higher than the amounts reported at March 31, 2021 and 2020, respectively. These amounts are equivalent to our LIFO reserves. The lower LIFO credits in 2021 compared to 2020 is primarily due to higher brand inflation and delays of branded off-patent to generic drug launches. Our LIFO valuation amount includes both pharmaceutical and non-pharmaceutical products. We recognized LIFO credits of $38 million, $252 million, and $210 million, respectively, in 2021, 2020, and 2019 in our Consolidated Statements of Operations. A LIFO charge is recognized when the net effect of price increases on pharmaceutical and non-pharmaceutical products held in inventory exceeds the impact of price declines, including the effect of branded pharmaceutical products that have lost market exclusivity. A LIFO credit is recognized when the net effect of price declines exceeds the impact of price increases on pharmaceutical and non-pharmaceutical products held in inventory. Excluding LIFO reserves, our inventory reserves as of March 31, 2021 and 2020 were $263 million and $96 million, respectively. The increase was primarily due to 2021 charges totaling $136 million on certain PPE and other related products due to inventory impairments and excess inventory within our Medical-Surgical Solutions segment.
We believe that the moving average inventory costing method provides a reasonable estimation of the current cost of replacing inventory (i.e., “market”). As such, our LIFO inventory is valued at the lower of LIFO or market. As of March 31, 2021 and 2020, inventories at LIFO did not exceed market.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
In determining whether an inventory valuation allowance is required, we consider various factors including estimated quantities of slow-moving inventory by reviewing on-hand quantities, outstanding purchase obligations and forecasted sales. Shifts in market trends and conditions, changes in customer preferences due to the introduction of generic drugs or new pharmaceutical products or the loss of one or more significant customers are factors that could affect the value of our inventories. We write down inventories which are considered excess and obsolete as a result of these reviews. These factors could make our estimates of inventory valuation differ from actual results.
Business Combinations: The Company accounts for business combinations using the acquisition method of accounting whereby the identifiable assets and liabilities of the acquired business, as well as any noncontrolling interest in the acquired business, are recorded at their estimated fair values as of the date that the Company obtains control of the acquired business. Any purchase consideration in excess of the estimated fair values of the net assets acquired is recorded as goodwill. Acquisition-related expenses and related restructuring costs are expensed as incurred.
Several valuation methods may be used to determine the fair value of assets acquired and liabilities assumed. For intangible assets, we typically use a method that is a form or variation of the income approach, whereby a forecast of future cash flows attributable to the asset are discounted to present value using a risk-adjusted discount rate. Some of the more significant estimates and assumptions inherent in the income approach include the amount and timing of projected future cash flows, the discount rate selected to measure the risks inherent in the future cash flows and the assessment of the asset’s expected useful life. Refer to Financial Note 5, “Business Acquisitions and Divestitures,” to the consolidated financial statements included in this Annual Report on Form 10-K for additional information regarding our acquisitions.
Goodwill and Long-Lived Assets: As a result of acquiring businesses, we have $9.5 billion and $9.4 billion of goodwill at March 31, 2021 and 2020, respectively, and $2.9 billion and $3.2 billion of intangible assets, net at March 31, 2021 and 2020, respectively. We perform an impairment test on goodwill balances annually in the third quarter and more frequently if indicators for potential impairment exist. Indicators that are considered include significant declines in performance relative to expected operating results, significant changes in the use of the assets, significant negative industry or economic trends, or a significant decline in the Company’s stock price and/or market capitalization for a sustained period of time.
Goodwill impairment testing is conducted at the reporting unit level, which is generally defined as an operating segment or a component, one level below our operating segments, for which discrete financial information is available and segment management regularly reviews the operating results of that reporting unit.
We apply the goodwill impairment test by comparing the estimated fair value of a reporting unit to its carrying value and recording an impairment charge equal to the amount of excess carrying value above the estimated fair value, if any, but not to exceed the amount of goodwill allocated to the reporting unit.
To estimate the fair value of our reporting units, we generally use a combination of the market approach and the income approach. Under the market approach, we estimate fair value by comparing the business to similar businesses, or guideline companies whose securities are actively traded in public markets. Under the income approach, we use a discounted cash flow (“DCF”) model in which cash flows anticipated over several periods, plus a terminal value at the end of that time horizon, are discounted to their present value using an appropriate rate that is commensurate with the risk inherent within the reporting unit. In addition, we compare the aggregate of the reporting units’ fair values to our market capitalization as further corroboration of the fair values.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Estimates of fair value result from a complex series of judgments about future events and uncertainties and rely heavily on estimates and assumptions at a point in time. Judgments made in determining an estimate of fair value may materially impact our results of operations. The valuations are based on information available as of the impairment testing date and are based on expectations and assumptions that have been deemed reasonable by management. Any material changes in key assumptions, including failure to meet business plans, negative changes in government reimbursement rates, deterioration in the U.S. and global financial markets, an increase in interest rates or an increase in the cost of equity financing by market participants within the industry or other unanticipated events and circumstances, may decrease the projected cash flows or increase the discount rates and could potentially result in an impairment charge. Under the market approach, significant estimates and assumptions also include the selection of appropriate guideline companies and the determination of appropriate valuation multiples to apply to the reporting unit. Under the income approach, significant estimates and assumptions also include the determination of discount rates. The discount rates represent the weighted-average cost of capital measuring the reporting unit’s cost of debt and equity financing, which are weighted by the percentage of debt and percentage of equity in a company’s target capital structure. Included in the estimate of the weighted-average cost of capital is the assumption of an unsystematic risk premium to address incremental uncertainty related to the reporting units’ future cash flow projections. An increase in the unsystematic risk premium increases the discount rate.
Based on the 2019 annual goodwill impairment tests, the estimated fair values of our reporting units, excluding the Europe Retail Pharmacy and Europe Pharmaceutical Distribution reporting units in our International segment, exceeded their carrying values. The impairment testing performed in 2020 did not indicate any material impairment of goodwill. The segment change in the second quarter of 2021 prompted changes in multiple reporting units across the Company. As a result, goodwill included in impacted reporting units was reallocated using a relative fair value approach and assessed for impairment both before and after the reallocation. We recorded a goodwill impairment charge of $69 million in 2021 as the estimated fair value of the Europe Retail Pharmacy reporting unit was lower than its reassigned carrying value based on changes in the composition of the Europe Retail Pharmacy reporting unit within the International segment. At March 31, 2021, the balance of goodwill for the reporting units in Europe was approximately nil and the remaining balance of goodwill in the International segment primarily relates to one of our reporting units in Canada.
The estimated fair values of our McKesson Canada reporting unit in our International segment and our RxCrossroads reporting unit in our RxTS segment exceeded the carrying values of these reporting units by 11% and 14%, respectively, in 2021. The goodwill balance of these reporting units was $1.5 billion for McKesson Canada and $312 million for RxCrossroads at March 31, 2021 or approximately 19% of the consolidated goodwill balance. Generally, a decline in estimated future cash flows in excess of 16% for McKesson Canada and 17% for RxCrossroads or an increase in the discount rate in excess of approximately 1.5% could result in an indication of goodwill impairment for these reporting units in future reporting periods. Other risks, expenses and future developments, such as additional government actions, increased regulatory uncertainty, and material changes in key market assumptions that we were unable to anticipate as of the testing date may require us to further revise the projected cash flows, which could adversely affect the fair value of our other reporting units in future periods. Refer to Financial Note 12, “Goodwill and Intangible Assets, Net,” to the consolidated financial statements appearing in this Annual Report on Form 10-K for additional information.
Currently, all of our intangible and other long-lived assets are amortized or depreciated based on the pattern of their economic consumption or on a straight-line basis over their estimated useful lives, ranging from one to 38 years. We review intangible assets for impairment at an asset group level whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Determination of recoverability of intangible assets is based on the lowest level of identifiable estimated future undiscounted cash flows resulting from use of the asset and its eventual disposition. Measurement of any impairment loss is based on the excess of the carrying value of the asset group over its fair value. Assumptions and estimates about future values and remaining useful lives of our purchased intangible assets are complex and subjective. They can be affected by a variety of factors, including external factors such as industry and economic trends, and internal factors such as changes in our business strategy and our internal forecasts.
Our ongoing consideration of all the factors described previously could result in further impairment charges in the future, which could adversely affect our net income. Refer to Financial Note 4, “Restructuring, Impairment, and Related Charges” to the consolidated financial statements appearing in this Annual Report on Form 10-K for additional information.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Valuation of Equity Method Investments: We evaluate our investments for other-than-temporary impairments when circumstances indicate those assets may be impaired. When the decline in value is deemed to be other than temporary, an impairment is recognized to the extent that the fair value is less than the carrying value of the investment. We consider various factors in determining whether a loss in value of an investment is other than temporary including: the length of time and the extent to which the fair value has been below cost, the financial condition of the investees, and our intent and ability to retain the investment for a period of time sufficient to allow for recovery of value. Management makes certain judgments and estimates in its assessment including but not limited to: identifying if circumstances indicate a decline in value is other than temporary, expectations about the business operations of investees, as well as industry, financial, and market factors. Any significant changes in assumptions or judgments in assessing impairments could result in an impairment charge.
Restructuring Charges: We have certain restructuring reserves which require significant estimates related to the timing and amount of future employee severance and other exit-related costs to be incurred when the restructuring actions take place. We generally recognize employee severance costs when payments are probable and amounts are estimable. Costs related to contracts without future benefit or contract termination are recognized at the earlier of the contract termination or the cease-use dates. Other exit-related costs are recognized as incurred. In connection with these restructuring actions, we also assess the recoverability of long-lived assets used in the business, and as a result, we may recognize accelerated depreciation and amortization reflecting shortened useful lives of the underlying assets.
Income Taxes: Our income tax expense and deferred tax assets and liabilities reflect management’s best assessment of estimated current and future taxes to be paid. We are subject to income taxes in the U.S. and numerous foreign jurisdictions. Significant judgments and estimates are required in determining the consolidated income tax provision and in evaluating income tax uncertainties and include those used to conclude on the tax-free nature of the separation of the Change Healthcare JV and the unrecognized tax position related to opioid-related litigation and claims, which remains unfinalized, and which may differ from the actual amounts of tax benefit recognized. We review our tax positions at the end of each quarter and adjust the balances as new information becomes available.
Deferred income taxes arise from temporary differences between the tax and financial statement recognition of revenue and expense. In evaluating our ability to recover our deferred tax assets, we consider all available positive and negative evidence including our past operating results, the existence of cumulative net operating losses in the most recent years, and our forecast of future taxable income. In estimating future taxable income, we develop assumptions including the amount of future federal, state and foreign pre-tax operating income, the reversal of temporary differences, and the implementation of feasible and prudent tax planning strategies. These assumptions require significant judgment about the forecasts of future taxable income and are consistent with the plans and estimates we use to manage the underlying businesses.
Changes in tax laws and rates could also affect recorded deferred tax assets and liabilities in the future. Should tax laws change, our tax expense and cash flows could be materially impacted.
In addition, the calculation of our tax liabilities includes estimates for uncertainties in the application of complex new tax regulations across multiple global jurisdictions where we conduct our operations.
We recognize liabilities for tax and related interest for issues in the U.S. and other tax jurisdictions based on our estimate of whether, and the extent to which, additional taxes and related interest will be due. If our current estimate of tax and interest liabilities is less than the ultimate settlement, an additional charge to income tax expense may result. If our current estimate of tax and interest liabilities is more than the ultimate settlement, a reduction to income tax expense may be recognized.
Loss Contingencies: We are subject to various claims, including claims with customers and vendors, pending and potential legal actions for damages, investigations relating to laws and regulations and other matters arising out of the normal conduct of our business. When a loss is considered probable and reasonably estimable, we record a liability in the amount of our best estimate for the ultimate loss. However, the likelihood of a loss with respect to a particular contingency is often difficult to predict and determining a meaningful estimate of the loss or a range of loss may not be practicable based on the information available and the potential effect of future events and decisions by third parties that will determine the ultimate resolution of the contingency. Moreover, it is not uncommon for such matters to be resolved over many years, during which time relevant developments and new information must be reevaluated at least quarterly to determine both the likelihood of potential loss and whether it is possible to reasonably estimate a range of possible loss. When a material loss is reasonably possible or probable but a reasonable estimate cannot be made, disclosure of the proceeding is provided. Legal fees are recognized as incurred when the legal services are provided.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
We review all contingencies at least quarterly to determine whether the likelihood of loss has changed and to assess whether a reasonable estimate of the potential loss or range of the loss can be made. As discussed above, development of a meaningful estimate of loss or a range of potential loss is complex when the outcome is directly dependent on future negotiations with or decisions by third parties, such as regulatory agencies, the court system and other interested parties. In conjunction with the preparation of the accompanying financial statements, we considered matters related to ongoing controlled substances claims to which we are a party. As a result of ongoing, advanced discussions with state attorneys general and plaintiffs’ representatives regarding a framework to resolve the claims of governmental entities, and our assessment of certain other opioid-related claims, we have reached a stage at which a broad settlement of opioid claims by governmental entities is probable and recorded a charge of $8.1 billion for the year ended March 31, 2021 within “Claims and litigation charges, net” in our Consolidated Statement of Operations in this report. Because of the many uncertainties associated with any potential settlement arrangement or other resolution of opioid-related litigation, including the uncertainty of the scope of participation by plaintiffs in any potential settlement, we are not able to reasonably estimate the upper or lower ends of the range of ultimate possible loss for all opioid-related litigation matters. While we are not able to predict the outcome or reasonably estimate a range of possible losses in these matters, an adverse judgment or negotiated resolution in any of these matters could have a material adverse effect on our results of operations, consolidated financial position, cash flows or liquidity. Refer to Financial Note 19, “Commitments and Contingent Liabilities,” to the consolidated financial statements included in this Annual Report on Form 10-K for additional information.
FINANCIAL CONDITION, LIQUIDITY, AND CAPITAL RESOURCES
We expect our available cash generated from operations and our short-term investment portfolio, together with our existing sources of liquidity from our credit facilities and commercial paper program, will be sufficient to fund our short-term and long-term capital expenditures, working capital, and other cash requirements. As described within the “Trends and Uncertainties” section above, the COVID-19 pandemic continues to develop rapidly. We continue to monitor its impact on demand within parts of our business, as well as trends potentially impacting the timing or ability for some of our customers to pay amounts owed to us. We remain well-capitalized with access to liquidity from our $4.0 billion revolving credit facility. Additionally, long-term debt markets and commercial paper markets, our primary sources of capital after cash flow from operations, have remained open and accessible to us during the COVID-19 pandemic. We have seen continued improvement in conditions in the debt markets and commercial paper markets as the Federal Reserve has taken steps to stabilize the markets. At March 31, 2021, we were in compliance with all debt covenants, and believe we have the ability to continue to meet our debt covenants in the future.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
The following table summarizes the net change in cash, cash equivalents, and restricted cash for the periods shown:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended March 31, | | Change |
| (Dollars in millions) | 2021 | | 2020 | | 2019 | | 2021 | | 2020 |
| Net cash provided by (used in): | | | | | | | | | |
| Operating activities | $ | 4,542 | | | $ | 4,374 | | | $ | 4,036 | | | $ | 168 | | | $ | 338 | |
| Investing activities | (415) | | | (579) | | | (1,381) | | | 164 | | | 802 | |
| Financing activities | (1,693) | | | (2,734) | | | (2,227) | | | 1,041 | | | (507) | |
Effect of exchange rate changes on cash, cash equivalents and restricted cash | (61) | | | (19) | | | (119) | | | (42) | | | 100 | |
| Net change in cash, cash equivalents, and restricted cash | $ | 2,373 | | | $ | 1,042 | | | $ | 309 | | | $ | 1,331 | | | $ | 733 | |
Operating Activities
Net cash provided from operating activities was $4.5 billion, $4.4 billion, and $4.0 billion for the years ended March 31, 2021, 2020, and 2019, respectively. Cash flows from operations can be significantly impacted by factors such as the timing of receipts from customers and payments to vendors. Additionally, working capital is primarily a function of sales and purchase volumes, inventory requirements, and vendor payment terms. Operating activities for the year ended March 31, 2021 were affected by net income adjusted for non-cash items, including the pre-tax $8.1 billion (after-tax $6.8 billion) non-cash charge related to our estimated liability for opioid-related claims, an increase in inventory of $2.3 billion and an increase in drafts and accounts payable of $1.3 billion driven by higher inventory stock levels to meet increased volume demand as part of our inventory management, as well as a decrease in receivables of $1.1 billion driven by timing, higher sales recognized at the end of March 2020, and higher collections in our fourth quarter of 2021. Operating activities for the year ended March 31, 2020 were affected by increases in drafts and accounts payable of $4.0 billion primarily associated with timing, replenishing inventory stocks, and effective working capital management, and an increase in receivables of $2.5 billion primarily due to revenue growth. Operating activities for the year ended March 31, 2019 were affected by increases in drafts and accounts payable of $2.0 billion primarily due to increased inventory purchases and timing of payments, and an increase in receivables of $1.0 billion due to the overall increase in sales volume and timing of receipts.
Other non-cash items within operating activities for the year ended March 31, 2021 primarily includes stock-based compensation of $151 million and fair value remeasurement charges of $58 million related to the contribution of our German pharmaceutical wholesale business to a joint venture with WBA. Other non-cash items for the year ended March 31, 2020 primarily includes fair value remeasurement charges of $275 million described above, pension settlement charges of $122 million, and stock-based compensation of $119 million. Additionally, we made a cash payment of $114 million from the executive benefit retirement plan in 2020. Other non-cash items for the year ended March 31, 2019 primarily includes stock-based compensation of $95 million.
Investing Activities
Net cash used in investing activities was $415 million, $579 million, and $1.4 billion for the years ended March 31, 2021, 2020, and 2019, respectively. Investing activities for the year ended March 31, 2021 include $451 million and $190 million in capital expenditures for property, plant, and equipment and capitalized software, respectively. Investing activities for the year ended March 31, 2021 also includes net cash proceeds of $400 million from sales of businesses and investments, including $286 million in exchange for the contribution of our German pharmaceutical wholesale business to a joint venture with WBA.
Investing activities for the year ended March 31, 2020 include $362 million and $144 million in capital expenditures for property, plant, and equipment and capitalized software, respectively, and $133 million of net cash payments for acquisitions.
Investing activities for the year ended March 31, 2019 include $905 million of net cash payments for acquisitions, including $784 million for our acquisition of Medical Specialties Distributors LLC, $426 million and $131 million in capital expenditures for property, plant, and equipment and capitalized software, respectively, and $101 million of net cash proceeds from sales of businesses and investments.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Financing Activities
Net cash used in financing activities was $1.7 billion, $2.7 billion, and $2.2 billion for the years ended March 31, 2021, 2020, and 2019, respectively. Financing activities for the year ended March 31, 2021 include cash receipts of $6.3 billion and payments of $6.3 billion from short-term borrowings, primarily commercial paper, along with the issuance of the 2025 Notes in a principal amount of $500 million, the retirement of our $700 million total principal amount of notes due on November 30, 2020 at a fixed interest rate of 3.65% upon maturity, and the redemption of our 4.75% $323 million total principal of notes due on March 1, 2021 prior to maturity. The notes were redeemed using cash on hand and proceeds from the 2025 Notes. Financing activities for the year ended March 31, 2021 also include $770 million of cash paid for stock repurchases and $276 million of dividends paid. Cash used for other financing activities generally includes payments to noncontrolling interests and activity from our finance leases. Other financing activities for the year ended March 31, 2021 also include restricted cash net inflow related to funds temporarily held on behalf of unaffiliated medical practice groups and a payment of $49 million to purchase shares of McKesson Europe through exercises of a put right option by noncontrolling shareholders.
Financing activities for the year ended March 31, 2020 include cash receipts of $21.4 billion and payments of $21.4 billion from short-term borrowings, primarily commercial paper. Financing activities for the year ended March 31, 2020 also include $2.0 billion of cash paid for stock repurchases, repayments of long-term debt of $298 million, and $294 million of dividends paid.
Financing activities for the year ended March 31, 2019 include cash receipts of $37.3 billion and payments of $37.3 billion from short-term borrowings, primarily commercial paper. We received cash from long-term debt issuances of $1.1 billion and made repayments on long-term debt of $1.1 billion in 2019. Financing activities for the year ended March 31, 2019 also include $1.6 billion of cash paid for stock repurchases and $292 million of dividends paid.
Share Repurchase Plans
The Board has authorized the repurchase of McKesson’s common stock from time to time in open market transactions, privately negotiated transactions, accelerated share repurchase (“ASR”) programs, or by combinations of such methods, any of which may use pre-arranged trading plans that are designed to meet the requirements of Rule 10b5-1(c) of the Securities Exchange Act of 1934. The timing of any repurchases and the actual number of shares repurchased will depend on a variety of factors, including our stock price, corporate and regulatory requirements, restrictions under our debt obligations, and other market and economic conditions.
Information regarding the share repurchase activity over the last three years is as follows:
| | | | | | | | | | | | | | | | | | | | |
| Share Repurchases (1) |
| (In millions, except price per share data) | | Total Number of Shares Purchased (2) | | Average Price
Paid Per Share | | Approximate
Dollar Value of
Shares that May
Yet Be Purchased
Under the
Programs |
| Balance, March 31, 2018 | | | | | | $ | 1,096 | |
| Shares repurchase plans authorized in May 2018 | | | | | | 4,000 | |
| Shares repurchased - Open market | | 10.4 | | | $ | 132.14 | | | (1,377) | |
| Shares repurchased - ASR | | 2.1 | | | $ | 117.98 | | | (250) | |
| Balance, March 31, 2019 | | | | | | 3,469 | |
| Shares repurchased - Open market | | 9.2 | | | $ | 144.68 | | | (1,334) | |
| Shares repurchased - ASR | | 4.7 | | | $ | 127.68 | | | (600) | |
| Balance, March 31, 2020 | | | | | | 1,535 | |
| Shares repurchase plans authorized in January 2021 | | | | | | 2,000 | |
Shares repurchased - Open market (3) | | 4.7 | | | $ | 160.33 | | | (750) | |
| | | | | | |
| Balance, March 31, 2021 | | | | | | $ | 2,785 | |
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
(1)This table does not include the value of equity awards surrendered to satisfy tax withholding obligations. It also excludes shares related to the Split-off of the Change Healthcare JV as described below.
(2)The number of shares purchased reflects rounding adjustments.
(3)$8 million was accrued within “Other accrued liabilities” on our Consolidated Balance Sheet as of March 31, 2021 for share repurchases that were executed in late March and settled in early April.
During the last three years, our share repurchases were transacted through both open market transactions and ASR programs with third party financial institutions.
In 2019, we retired 5.0 million or $542 million of the Company’s treasury shares previously repurchased. Under the applicable state law, these shares resume the status of authorized and unissued shares upon retirement. In accordance with our accounting policy, we allocate any excess of share repurchase price over par value between additional paid-in capital and retained earnings. Accordingly, our retained earnings and additional paid-in capital were reduced by $472 million and $70 million during 2019, respectively.
On March 9, 2020, we completed the Split-off of our interest in the Change Healthcare JV. In connection with the Split-off, we distributed all 176.0 million outstanding shares of SpinCo common stock, which held all of the Company’s interests in the Change Healthcare JV, to participating holders of the Company’s common stock in exchange for 15.4 million shares of McKesson stock, which are now held as treasury stock on our consolidated balance sheet. Following consummation of the exchange offer, on March 10, 2020, SpinCo merged with and into Change and each share of SpinCo common stock was converted into one share of Change common stock, par value $0.001 per share, with cash being paid in lieu of fractional shares of Change common stock. See Note 2, “Investment in Change Healthcare Joint Venture” to the accompanying consolidated financial statements included in this Annual Report on Form 10-K for more information.
The total authorization outstanding for repurchase of the Company’s common stock was $2.8 billion at March 31, 2021.
We believe that our future operating cash flow, financial assets, and current access to capital and credit markets, including our existing credit facilities, will give us the ability to meet our financing needs for the foreseeable future. However, there can be no assurance that an increase in volatility or disruption in the global capital and credit markets will not impair our liquidity or increase our costs of borrowing. As described within the “Trends and Uncertainties” section above, the COVID-19 pandemic continues to develop rapidly. We continue to monitor its impact on demand within parts of our business, as well as trends potentially impacting the timing or ability for some of our customers to pay amounts owed to us.
Selected Measures of Liquidity and Capital Resources:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| March 31, |
| (Dollars in millions) | 2021 | | 2020 | | 2019 |
| Cash, cash equivalents, and restricted cash | $ | 6,396 | | | | $ | 4,023 | | | | $ | 2,981 | | |
| Working capital | 1,279 | | | | (402) | | | | 839 | | |
Days sales outstanding for: (1) | | | | | | | | |
| Customer receivables | 26 | | | | 26 | | | | 26 | | |
| Inventories | 31 | | | | 27 | | | | 31 | | |
| Drafts and accounts payable | 63 | | | | 61 | | | | 62 | | |
Debt to capital ratio (2) | 83.1 | | % | | 52.1 | | % | | 43.3 | | % |
Return on McKesson stockholders’ equity (deficit) (3) | (142.5) | | % | | 13.3 | | % | | 0.4 | | % |
(1)Based on year-end balances and sales or cost of sales for the last 90 days of the year.
(2)This ratio describes the relationship and changes within our capital resources, and is computed as total debt divided by the sum of total debt and McKesson stockholders’ equity (deficit), which excludes noncontrolling and redeemable noncontrolling interests and accumulated other comprehensive loss.
(3)Ratio is computed as net income (loss) attributable to McKesson Corporation for the last four quarters, divided by a five-quarter average of McKesson stockholders’ equity (deficit), which excludes noncontrolling and redeemable noncontrolling interests.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Cash equivalents, which are readily convertible to known amounts of cash, are carried at fair value. Cash equivalents are primarily invested in AAA-rated U.S. government money market funds and overnight deposits with financial institutions. Deposits with financial institutions are primarily denominated in U.S. dollars and the functional currencies of our foreign subsidiaries, including Euro, British pound sterling, and Canadian dollars. We mitigate the risk of our short-term investment portfolio by depositing funds with reputable financial institutions and monitoring risk profiles and investment strategies of money market funds.
Our cash and cash equivalents balance as of March 31, 2021 and 2020 included approximately $2.3 billion and $1.7 billion of cash held by our subsidiaries outside of the U.S., respectively. Our primary intent is to utilize this cash for foreign operations for an indefinite period of time. Although the vast majority of cash held outside the U.S. is available for repatriation, doing so could subject us to foreign withholding taxes and state income taxes. Following enactment of the 2017 Tax Cuts and Jobs Act, the repatriation of cash to the U.S. is generally no longer taxable for federal income tax purposes.
Working capital primarily includes cash and cash equivalents, receivables, and inventories, net of drafts and accounts payable, short-term borrowings, current portion of long-term debt, and other current liabilities. Our businesses require substantial investments in working capital that are susceptible to large variations during the year as a result of inventory purchase patterns and seasonal demands. Inventory purchase activity is a function of sales activity and other requirements. The COVID-19 pandemic has potential to increase the variations in our working capital, which we continue to monitor closely.
Consolidated working capital improved at March 31, 2021 compared to the prior year primarily due to an increase in cash and cash equivalents and inventory, partially offset by an increase in drafts and accounts payable and a decrease in receivables. Consolidated working capital decreased at March 31, 2020 compared to the prior year primarily due to an increase in drafts and accounts payable and the current portion of long-term debt for term notes due in 2021, partially offset by an increase in receivables and cash and cash equivalents.
Our debt to capital ratio increased for the year ended March 31, 2021 primarily due to a decrease in stockholders’ equity driven by net loss for the year and share repurchases. Our unfavorable return on McKesson’s stockholder’s equity (deficit) as of March 31, 2021 was also driven by net loss for the year. Net loss for the year ended March 31, 2021 includes an after-tax non-cash charge of $6.8 billion related to our estimated liability for opioid-related claims, as discussed in “Trends and Uncertainties” of this Financial Review and Financial Note 19, “Commitments and Contingent Liabilities,” to the accompanying consolidated financial statements included in this Annual Report on Form 10‑K. Our debt to capital ratio increased for 2020 primarily due to a decrease in stockholders’ equity driven by the Split-off of our interest in Change Healthcare JV and share repurchases.
On July 29, 2020, we raised our quarterly dividend from $0.41 to $0.42 per common share for dividends declared on or after such date by the Board. Dividends were $1.67 per share in 2021, $1.62 per share in 2020, and $1.51 per share in 2019. We anticipate that we will continue to pay quarterly cash dividends in the future. However, the payment and amount of future dividends remain within the discretion of the Board and will depend upon our future earnings, financial condition, capital requirements, and other factors. In 2021, 2020, and 2019, we paid total cash dividends of $276 million, $294 million, and $292 million, respectively. Additionally, as required under the Domination Agreement, we are obligated to pay an annual recurring compensation amount of €0.83 per McKesson Europe share (effective January 1, 2015) to the noncontrolling shareholders of McKesson Europe.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
Contractual Obligations:
The table and information below presents our significant financial obligations and commitments at March 31, 2021:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years |
| (In millions) | Total | | Within 1 | | Over 1 to 3 | | Over 3 to 5 | | After 5 |
| On balance sheet | | | | | | | | | |
Total debt (1) | $ | 7,148 | | | $ | 742 | | | $ | 1,970 | | | $ | 1,253 | | | $ | 3,183 | |
Operating lease obligations (2) | 2,505 | | | 433 | | | 733 | | | 516 | | | 823 | |
Other (3) | 250 | | | 30 | | | 53 | | | 51 | | | 116 | |
| | | | | | | | | |
| Off balance sheet | | | | | | | | | |
Interest on borrowings (4) | 1,617 | | | 199 | | | 367 | | | 268 | | | 783 | |
Purchase obligations (5) | 7,354 | | | 7,268 | | | 76 | | | 10 | | | — | |
Other (6) | 472 | | | 268 | | | 59 | | | 26 | | | 119 | |
Total | $ | 19,346 | | | $ | 8,940 | | | $ | 3,258 | | | $ | 2,124 | | | $ | 5,024 | |
(1)Represents maturities of the Company’s long-term obligations, including an immaterial amount of finance lease obligations.
(2)Represents undiscounted minimum operating lease obligations under non-cancelable operating leases having an initial remaining term over one year and is not adjusted for imputed interest. Refer to Financial Note 11, “Leases” to the consolidated financial statements appearing in this Annual Report on Form 10-K for more information.
(3)Includes our estimated benefit payments for the unfunded benefit plans and minimum funding requirements for the pension plans.
(4)Primarily represents interest that will become due on our fixed rate long-term debt obligations.
(5)A purchase obligation is defined as an arrangement to purchase goods or services that is enforceable and legally binding on the Company. These obligations primarily relate to inventory purchases and capital commitments.
(6)Includes agreements under which we have guaranteed the repurchase of our customers’ inventory and our customers’ debt in the event these customers are unable to meet their obligations to those financial institutions.
The contractual obligations table above excludes the following obligations:
At March 31, 2021, the liability recorded for uncertain tax positions, excluding associated interest and penalties, was approximately $738 million. Additionally, any future payments that may be made related to our estimated litigation liability of $8.1 billion for opioid-related claims, as described in the “Trends and Uncertainties” section in this Financial Review and Financial Note 19, “Commitments and Contingent Liabilities,” to the consolidated financial statements included in this Annual Report on Form 10-K, are excluded. The ultimate amount and timing of any future cash settlements related to these items cannot be predicted with reasonable certainty.
Our banks and insurance companies have issued $146 million of standby letters of credit and surety bonds at March 31, 2021. These were issued on our behalf and are mostly related to our customer contracts and to meet the security requirements for statutory licenses and permits, court and fiduciary obligations, and our workers’ compensation and automotive liability programs.
Our redeemable noncontrolling interests primarily relate to our consolidated subsidiary, McKesson Europe. Under the Domination Agreement, the noncontrolling shareholders of McKesson Europe have a right to put (“Put Right”) their shares at €22.99 per share, increased annually for interest in the amount of five percentage points above a base rate published semi-annually by the German Bundesbank, less any compensation amount or guaranteed dividend already paid by McKesson (“Put Amount”). The exercise of the Put Right will reduce the balance of redeemable noncontrolling interests. During 2021, we paid $49 million to purchase 1.8 million shares of McKesson Europe through exercises of the Put Right by the noncontrolling shareholders. During 2020 and 2019, there were no material exercises of the Put Right.
McKESSON CORPORATION
FINANCIAL REVIEW (Continued)
The balance of redeemable noncontrolling interests is reported at the greater of its carrying value or its maximum redemption value at each reporting date. The redemption value is the Put Amount adjusted for exchange rate fluctuations each period. The carrying value of redeemable noncontrolling interests is also adjusted each period for the portion of other comprehensive income attributable to the noncontrolling shareholders, which is primarily due to changes in foreign currency exchange rates. At March 31, 2021 and 2020, the carrying value of redeemable noncontrolling interests related to McKesson Europe of $1.3 billion and $1.4 billion, respectively, exceeded the maximum redemption value of $1.2 billion. In future periods, unfavorable foreign currency exchange rate fluctuations between the Euro and the U.S. dollar could adversely impact the carrying value of our redeemable noncontrolling interests and require an adjustment to increase the balance of our redeemable noncontrolling interests to its maximum redemption value. Such adjustments would be recorded in “Net income attributable to noncontrolling interests” in our consolidated statements of operations.
Additionally, we are obligated to pay an annual recurring compensation of €0.83 per McKesson Europe share (the “Compensation Amount”) to the noncontrolling shareholders of McKesson Europe under the Domination Agreement. The Compensation Amount is recognized ratably during the applicable annual period. The Domination Agreement does not expire, but it may be terminated at the end of any fiscal year by giving at least six month’s advance notice. The Put Amount, Compensation Amount, and the guaranteed dividend were subject to ongoing appraisal proceedings. On April 12, 2021, we received the Stuttgart Court of Appeals’ final ruling confirming the original put value of €22.99 per share and the annual recurring compensation of €0.83 per McKesson Europe share. The Put Right exercise window will expire on June 15, 2021. While the ultimate amount of any future cash payments related to exercises of the Put Right are uncertain, Put Right exercises could result in cash payments of up to approximately $1.3 billion prior to the expiration of the Put Right.
Refer to Financial Note 9, “Redeemable Noncontrolling Interests and Noncontrolling Interests,” to the consolidated financial statements included in this Annual Report on Form 10-K for additional information on redeemable noncontrolling interests.
Credit Resources:
We fund our working capital requirements primarily with cash and cash equivalents as well as short-term borrowings from our credit facilities and commercial paper issuances. Funds necessary for future debt maturities and our other cash requirements are expected to be met by existing cash balances, cash flow from operations, existing credit sources, and other capital market transactions. Detailed information regarding our debt and financing activities is included in Financial Note 13, “Debt and Financing Activities,” to the consolidated financial statements included in this Annual Report on Form 10-K.
RELATED PARTY BALANCES AND TRANSACTIONS
Information regarding our related party balances and transactions is included in Financial Note 2, “Investment in Change Healthcare Joint Venture,” and Financial Note 21, “Related Party Balances and Transactions,” to the consolidated financial statements included in this Annual Report on Form 10-K.
NEW ACCOUNTING PRONOUNCEMENTS
New accounting pronouncements that we have recently adopted, as well as those that have been recently issued but not yet adopted by us, are included in Financial Note 1, “Significant Accounting Policies,” to the consolidated financial statements appearing in this Annual Report on Form 10-K.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest rate risk: Our long-term debt bears interest predominately at fixed rates, whereas our short-term borrowings are at variable interest rates.
Our cash and cash equivalents balances earn interest at variable rates. At March 31, 2021 and 2020, we had $6.3 billion and $4.0 billion, respectively, in cash and cash equivalents. The effect of a hypothetical 50 bp increase in the underlying interest rate on our cash and cash equivalents, net of short-term borrowings and variable rate debt, would have resulted in a favorable impact to earnings in 2021 and 2020 of approximately $17 million and $6 million, respectively.
McKESSON CORPORATION
FINANCIAL REVIEW (Concluded)
Foreign exchange risk: We conduct our business worldwide in U.S. dollars and the functional currencies of our foreign subsidiaries, including Euro, British pound sterling, and Canadian dollar. Changes in foreign currency exchange rates could have a material adverse impact on our financial results that are reported in U.S. dollars. We are also exposed to foreign exchange rate risk related to our foreign subsidiaries, including intercompany loans denominated in non-functional currencies.
We have certain foreign exchange rate risk programs that use foreign currency forward contracts and cross-currency swaps. The forward contracts and cross-currency swaps are intended to reduce the income statement effects from fluctuations in foreign exchange rates and have been designated as cash flow hedges. These programs reduce but do not entirely eliminate foreign exchange risk.
As of March 31, 2021 and 2020, the effect of a hypothetical adverse 10% change in the underlying foreign currency exchange rates would have impacted the fair value of our foreign exchange contracts by approximately $267 million and $435 million, respectively. However, our risk management programs are designed such that the potential loss in value of these risk management portfolios described above would be largely offset by changes in the value of the underlying exposure. Refer to Financial Note 16, “Hedging Activities,” for more information on our foreign currency forward contracts and cross-currency swaps.
The selected hypothetical change in interest rates and foreign currency exchange rates does not reflect what could be considered the best or worst case scenarios.
Item 8. Financial Statements and Supplementary Data.
INDEX TO CONSOLIDATED FINANCIAL INFORMATION
| | | | | |
| Page |
| |
| |
| Consolidated Financial Statements: | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
MANAGEMENT’S ANNUAL REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of McKesson Corporation is responsible for establishing and maintaining an adequate system of internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). With the participation of the Chief Executive Officer and the Chief Financial Officer, our management conducted an assessment of the effectiveness of our internal control over financial reporting based on the framework and criteria established in Internal Control—Integrated Framework (2013), issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, our management has concluded that our internal control over financial reporting was effective as of March 31, 2021.
Deloitte & Touche LLP, an independent registered public accounting firm, audited the financial statements included in this Annual Report on Form 10-K and has also audited the effectiveness of the Company’s internal control over financial reporting as of March 31, 2021. This audit report appears on the following page of this Annual Report on Form 10-K.
May 12, 2021
| | |
|
| /s/ Brian S. Tyler |
| Brian S. Tyler |
| Chief Executive Officer |
| (Principal Executive Officer) |
| | |
|
| /s/ Britt J. Vitalone |
| Britt J. Vitalone |
| Executive Vice President and Chief Financial Officer |
| (Principal Financial Officer) |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of McKesson Corporation
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of McKesson Corporation and subsidiaries (the “Company”) as of March 31, 2021 and 2020, the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity, and cash flows, for each of the three years in the period ended March 31, 2021, and the related notes and the schedule listed in the Index at Item 15 (collectively referred to as the “financial statements”). We also have audited the Company’s internal control over financial reporting as of March 31, 2021, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period ended March 31, 2021, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of March 31, 2021, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
Change in Accounting Principle
As discussed in Note 11 to the financial statements, effective April 1, 2019, the Company adopted the Financial Accounting Standards Board’s (“FASB”) new standard related to leases using the modified retrospective basis.
Basis for Opinions
The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on these financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the financial statements included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures to respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Contingent Liabilities - Broad Settlement of Opioid Claims brought by Governmental Entities - Refer to Note 1 and Note 19 to the financial statements
Critical Audit Matter Description
The Company and its affiliates are defendants in many cases asserting claims related to distribution of controlled substances, including opioids. The Company is named as a defendant along with other pharmaceutical wholesale distributors, pharmaceutical manufacturers and retail pharmacy chains. The plaintiffs in these actions include state attorneys general, county and municipal governments, hospitals, tribal nations, health and welfare funds, third-party payors and individuals. The Company is in ongoing, advanced discussions with state attorneys general and plaintiffs’ representatives, who represent states, their political subdivisions and other government entities (“governmental entities”), regarding a framework under which the three largest U.S. pharmaceutical distributors would pay up to approximately $21.0 billion over a period of 18 years, with up to approximately $8.0 billion to be paid by the Company to resolve the claims brought by governmental entities (“broad settlement of opioid claims”). When a loss is considered probable and reasonably estimable, the Company records a liability in the amount of its estimate for the ultimate loss. The Company reviews all loss contingencies at least quarterly to determine whether the likelihood of loss has changed and to assess whether a reasonable estimate of the loss or range of loss can be made. The Company also performs an assessment of loss contingencies where a loss is reasonably possible. If it is reasonably possible that a loss may have been incurred and the effect on the financial statements could be material, the Company discloses the nature of the loss contingency and an estimate of the possible loss or range of loss or a statement that such an estimate cannot be made within the notes to the financial statements. For the year ended March 31, 2021, management believes that a loss through broad settlement of opioid claims brought by governmental entities is both probable and reasonably estimable, and accordingly, recorded a charge in the amount of $8.0 billion, which represents management’s best estimate of future loss related to these specific matters.
We identified the potential broad settlement of opioid claims as a critical audit matter because of the significant judgment and challenges auditing management’s determination of whether such loss is probable and reasonably estimable. Specifically, auditing management’s determination and disclosure of whether the contingent loss arising from the potential broad settlement of opioid claims is probable, and the related measurement of such loss, is subjective and requires significant judgment given that the potential loss is based upon settlement terms that have not yet been finalized.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the potential broad settlement of opioid claims included the following, among others:
•We tested the effectiveness of internal controls related to the potential broad settlement of opioid claims, and approval of the accounting treatment and related disclosures based on the most recent facts and circumstances.
•We inquired of the Company’s internal and external legal counsel, as well as executives and other members of management, to understand the basis for the Company’s conclusion that a loss related to a potential broad settlement of opioid claims, is probable and reasonably estimable as of March 31, 2021. In addition, we inspected responses to inquiry letters sent to both internal and external legal counsel as it relates to the status of discussions with plaintiffs’ counsel and the Company’s intent regarding the framework for a potential broad settlement of opioid claims.
•We evaluated management’s analysis of the potential broad settlement of opioid claims, including the methodology used by management to determine the probability of such loss. We also evaluated the methodology used by management to estimate the most likely loss to be incurred by the Company as a result of a potential broad settlement of opioid claims.
•We examined Board of Directors meeting minutes, including relevant sub-committee meeting minutes, held inquiries with a director serving on the sub-committee, and compared to internal and external counsel’s written responses to our inquiry letters.
•We performed public domain searches for evidence contrary to management’s analysis.
•With the assistance of our specialists in accounting for loss contingencies, we evaluated the facts, evidence and the Company’s related accounting treatment for the potential broad settlement of opioid claims.
•We evaluated any events subsequent to March 31, 2021 that might impact our evaluation of the potential broad settlement of opioid claims.
•We obtained written representations from executives and internal counsel of the Company.
•We examined proposed terms related to the potential broad settlement framework.
•We evaluated the Company’s related disclosures for consistency with our testing.
Uncertain Tax Position - Broad Settlement of Opioid Claims brought by Governmental Entities - Refer to Note 1 and Note 8 to the financial statements
Critical Audit Matter Description
For the year ended March 31, 2021, the Company recognized $1.3 billion of tax benefit related to a potential broad settlement of opioid claims and had an additional $0.5 billion of potential benefit relating to an uncertain tax position that had not been recognized. Tax benefits from uncertain tax positions are recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. The net amount recognized by management is measured as the largest amount of tax benefit that is greater than 50 percent likely of being realized. The Company uses significant judgment in evaluating the technical tax merits of income tax benefits that qualify for recognition, including the determination of the amount that is more likely than not of being realized for U.S. federal and state income tax purposes.
We identified the Company’s uncertain tax position related to the charge for the potential broad settlement of opioid claims as a critical audit matter because of the challenges in auditing management’s estimate of the amount of income tax benefit that qualifies for recognition. Specifically, auditing management’s uncertain tax position in this area was challenging because the assumptions and estimates involved in management’s analysis required significant judgment as they are based upon the potential terms of a broad settlement, including provisions related to deductibility, that have not yet been finalized. There is also significant judgment associated with the assessment of the technical tax merits of such a settlement, including the related interpretation of applicable tax laws and regulations.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the Company's uncertain tax position associated with a potential broad settlement of opioid claims included the following, among others:
•We tested the effectiveness of internal controls related to the Company’s assessment of the technical merits of its tax position, including the Company’s assessment as to the amount of benefit that is more likely than not to be realized.
•With the assistance of our tax specialists, we evaluated the facts, evidence and the Company’s related income tax analysis for the charge related to the potential broad settlement of opioid claims, including assumptions used by management to measure the related recognized and unrecognized tax benefits.
•We inquired of the Company’s internal and external legal counsel to understand the basis for the Company’s conclusion that a portion of the potential broad settlement of opioid claims would be deductible based on the most recent discussions with plaintiffs’ counsel.
•We held inquiries with the Company’s external income tax advisors and we also read and evaluated management’s documentation of information received from these external advisors, which informed the basis of management’s position related to the uncertain tax position associated with the potential broad settlement of opioid claims.
•We compared management's income tax assessment of this matter to the treatment of other recorded opioid charges to evaluate the consistency of the Company’s judgments related to the uncertain tax position.
•We evaluated any events subsequent to March 31, 2021 that might impact our evaluation of the Company’s uncertain tax position related to the charge for the potential broad settlement of opioid claims.
•We obtained written representations from executives and internal counsel of the Company.
•We examined proposed terms related to the potential broad settlement framework.
•We evaluated the Company’s related disclosures for consistency with our testing and also searched for contradictory evidence by reading disclosures from peer companies, who are also party to the potential broad settlement of opioid claims.
Goodwill - Refer to Note 1 and Note 12 to the financial statements
Critical Audit Matter Description
The Company’s evaluation of goodwill for impairment involves comparing the carrying amount of each reporting unit to its fair value on the first day of the third fiscal quarter or whenever the Company believes a potential indicator of impairment requiring a more frequent assessment has occurred. The Company uses a combination of the income and market approaches to estimate reporting unit fair value. Under the market approach, fair value is estimated by comparing the business to similar businesses, or guideline companies whose securities are actively traded in public markets. Under the income approach, the Company uses a discounted cash flow (“DCF”) model where cash flows anticipated over future periods, plus a terminal value at the end of that time horizon, are discounted to their present value using an appropriate rate that is commensurate with the risk inherent within the reporting unit. The rate used to discount to present value includes an unsystematic risk premium, which is intended to address uncertainty related to the reporting unit’s future cash flow projections. The goodwill balance was $9.5 billion as of March 31, 2021, of which $1.5 billion was allocated to the McKesson Canada reporting unit. The fair value of all reporting units exceeded their respective carrying amounts as of the measurement date and, therefore, no impairment was recognized.
We identified the estimation of the fair value of the McKesson Canada reporting unit used to evaluate the recoverability of goodwill as a critical audit matter because of the challenges auditing significant judgments used in the selection of a discount rate, including the unsystematic risk premium. In particular, the fair value estimate is sensitive to the unsystematic risk premium assumption, which is affected by expected risk of changes in the Canadian business and regulatory environments. Auditing management’s selected discount rate required a high degree of auditor judgment and an increased extent of effort, including the need to involve more senior members of the team and our fair value specialists.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to the Company’s selection of a discount rate, including consideration of the unsystematic risk premium, for the McKesson Canada reporting unit included the following, among others:
•We tested the effectiveness of internal controls related to management’s goodwill impairment evaluation, including those related to the selection of a discount rate and consideration of an unsystematic risk premium.
•We evaluated management’s ability to accurately forecast operating results for the McKesson Canada reporting unit by comparing actual results to management’s historical forecasts, in order to consider the reasonableness and adequacy of management’s selected unsystematic risk premium.
•As part of our assessment of the unsystematic risk premium, we evaluated the reasonableness of strategic plans expected to be implemented during the forecast period by comparing the forecasts to:
•Actual results of historical strategic plans
•Internal communications to management and the Board of Directors
•With the assistance of our fair value specialists, we evaluated the reasonableness of the discount rate, including the unsystematic risk premium, by developing a range of independent estimates, testing the mathematical accuracy of the calculation and comparing to the discount rate selected by management.
| | |
|
| /s/ Deloitte & Touche LLP |
| Dallas, Texas |
| May 12, 2021 |
We have served as the Company’s auditor since 1968.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In millions, except per share amounts)
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| | 2021 | | 2020 | | 2019 |
| Revenues | $ | 238,228 | | | $ | 231,051 | | | $ | 214,319 | |
| Cost of sales | (226,080) | | | (219,028) | | | (202,565) | |
| Gross profit | 12,148 | | | 12,023 | | | 11,754 | |
| Operating expenses | | | | | |
| Selling, distribution, general, and administrative expenses | (8,849) | | | (9,182) | | | (8,437) | |
| Claims and litigation charges, net | (7,936) | | | (82) | | | (37) | |
| Goodwill impairment charges | (69) | | | (2) | | | (1,797) | |
| Restructuring, impairment, and related charges, net | (334) | | | (268) | | | (597) | |
| | | | | |
| | | | | |
| Total operating expenses | (17,188) | | | (9,534) | | | (10,868) | |
| Operating income (loss) | (5,040) | | | 2,489 | | | 886 | |
| Other income, net | 223 | | | 12 | | | 182 | |
Equity earnings and charges from investment in Change Healthcare
Joint Venture | — | | | (1,108) | | | (194) | |
| | | | | |
| Interest expense | (217) | | | (249) | | | (264) | |
| Income (loss) from continuing operations before income taxes | (5,034) | | | 1,144 | | | 610 | |
| Income tax benefit (expense) | 695 | | | (18) | | | (356) | |
| Income (loss) from continuing operations | (4,339) | | | 1,126 | | | 254 | |
| Income (loss) from discontinued operations, net of tax | (1) | | | (6) | | | 1 | |
| Net income (loss) | (4,340) | | | 1,120 | | | 255 | |
| Net income attributable to noncontrolling interests | (199) | | | (220) | | | (221) | |
| Net income (loss) attributable to McKesson Corporation | $ | (4,539) | | | $ | 900 | | | $ | 34 | |
| | | | | |
| Earnings (loss) per common share attributable to McKesson Corporation | | | | | |
| Diluted | | | | | |
| Continuing operations | $ | (28.26) | | | $ | 4.99 | | | $ | 0.17 | |
| Discontinued operations | — | | | (0.04) | | | — | |
| Total | $ | (28.26) | | | $ | 4.95 | | | $ | 0.17 | |
| Basic | | | | | |
| Continuing operations | $ | (28.26) | | | $ | 5.01 | | | $ | 0.17 | |
| Discontinued operations | — | | | (0.03) | | | — | |
| Total | $ | (28.26) | | | $ | 4.98 | | | $ | 0.17 | |
| | | | | |
| Weighted-average common shares outstanding | | | | | |
| Diluted | 160.6 | | | 181.6 | | | 197.3 | |
| Basic | 160.6 | | | 180.6 | | | 196.3 | |
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(In millions)
| | | | | | | | | | | | | | | | | |
| | Years Ended March 31, |
| 2021 | | 2020 | | 2019 |
| Net income (loss) | $ | (4,340) | | | $ | 1,120 | | | $ | 255 | |
| | | | | |
| Other comprehensive income (loss), net of tax | | | | | |
Foreign currency translation adjustments | 184 | | | (66) | | | (190) | |
| Unrealized gains (losses) on cash flow hedges | (36) | | | 86 | | | 24 | |
Changes in retirement-related benefit plans | 22 | | | 129 | | | (32) | |
| Other comprehensive income (loss), net of tax | 170 | | | 149 | | | (198) | |
| | | | | |
| Comprehensive income (loss) | (4,170) | | | 1,269 | | | 57 | |
| Comprehensive income attributable to noncontrolling interests | (146) | | | (223) | | | (155) | |
| Comprehensive income (loss) attributable to McKesson Corporation | $ | (4,316) | | | $ | 1,046 | | | $ | (98) | |
CONSOLIDATED BALANCE SHEETS
(In millions, except per share amounts) | | | | | | | | | | | |
| March 31, |
| 2021 | | 2020 |
| ASSETS | | | |
| Current assets | | | |
| Cash and cash equivalents | $ | 6,278 | | | $ | 4,015 | |
| Receivables, net | 19,181 | | | 19,950 | |
| Inventories, net | 19,246 | | | 16,734 | |
| Assets held for sale | 12 | | | 906 | |
| Prepaid expenses and other | 665 | | | 617 | |
| Total current assets | 45,382 | | | 42,222 | |
| Property, plant, and equipment, net | 2,581 | | | 2,365 | |
| Operating lease right-of-use assets | 2,100 | | | 1,886 | |
| Goodwill | 9,493 | | | 9,360 | |
| Intangible assets, net | 2,878 | | | 3,156 | |
| Other non-current assets | 2,581 | | | 2,258 | |
| Total assets | $ | 65,015 | | | $ | 61,247 | |
| LIABILITIES, REDEEMABLE NONCONTROLLING INTERESTS, AND EQUITY | | | |
| Current liabilities | | | |
| Drafts and accounts payable | $ | 38,975 | | | $ | 37,195 | |
| | | |
| Current portion of long-term debt | 742 | | | 1,052 | |
| Current portion of operating lease liabilities | 390 | | | 354 | |
| Liabilities held for sale | 9 | | | 683 | |
| Other accrued liabilities | 3,987 | | | 3,340 | |
| Total current liabilities | 44,103 | | | 42,624 | |
| Long-term debt | 6,406 | | | 6,335 | |
| Long-term deferred tax liabilities | 1,411 | | | 2,255 | |
| Long-term operating lease liabilities | 1,867 | | | 1,660 | |
| Long-term litigation liabilities | 8,067 | | | — | |
| Other non-current liabilities | 1,715 | | | 1,662 | |
Commitments and contingent liabilities (Note 19) | | | |
| Redeemable noncontrolling interests | 1,271 | | | 1,402 | |
| McKesson Corporation stockholders’ equity (deficit) | | | |
Preferred stock, $0.01 par value, 100 shares authorized, no shares issued or outstanding | — | | | — | |
Common stock, $0.01 par value, 800 shares authorized, 273 and 272 shares issued at March 31, 2021 and 2020, respectively | 2 | | | 2 | |
| Additional paid-in capital | 6,925 | | | 6,663 | |
| Retained earnings | 8,202 | | | 13,022 | |
| Accumulated other comprehensive loss | (1,480) | | | (1,703) | |
| | | |
Treasury shares, at cost, 115 and 110 shares at March 31, 2021 and 2020, respectively | (13,670) | | | (12,892) | |
| Total McKesson Corporation stockholders’ equity (deficit) | (21) | | | 5,092 | |
| Noncontrolling interests | 196 | | | 217 | |
| Total equity | 175 | | | 5,309 | |
| Total liabilities, redeemable noncontrolling interests, and equity | $ | 65,015 | | | $ | 61,247 | |
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In millions, except per share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| McKesson Corporation Stockholders’ Equity | | | | | | |
| Common
Stock | | Additional Paid-in Capital | | Other Capital | | Retained Earnings | | Accumulated Other
Comprehensive
Loss | | Treasury | | Noncontrolling
Interests | | Total
Equity | | |
| Shares | | Amount | | Common Shares | | Amount |
| Balances, March 31, 2018 | 275 | | | $ | 3 | | | $ | 6,188 | | | $ | (1) | | | $ | 12,986 | | | $ | (1,717) | | | (73) | | | $ | (7,655) | | | $ | 253 | | | $ | 10,057 | | | |
| Opening retained earnings adjustments: adoption of new accounting standards | — | | | — | | | — | | | — | | | 154 | | | — | | | — | | | — | | | — | | | 154 | | | |
| Balances, April 1, 2018 | 275 | | | 3 | | | 6,188 | | | (1) | | | 13,140 | | | (1,717) | | | (73) | | | (7,655) | | | 253 | | | 10,211 | | | |
| Issuance of shares under employee plans | 1 | | | — | | | 75 | | | — | | | — | | | — | | | — | | | (12) | | | — | | | 63 | | | |
| Share-based compensation | — | | | — | | | 92 | | | — | | | — | | | — | | | — | | | — | | | — | | | 92 | | | |
| Payments to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (184) | | | (184) | | | |
| Other comprehensive loss | — | | | — | | | — | | | — | | | — | | | (132) | | | — | | | — | | | — | | | (132) | | | |
| Net income | — | | | — | | | — | | | — | | | 34 | | | — | | | — | | | — | | | 176 | | | 210 | | | |
| Repurchase of common stock | — | | | — | | | 150 | | | — | | | — | | | — | | | (13) | | | (1,777) | | | — | | | (1,627) | | | |
| Retirement of common stock | (5) | | | — | | | (70) | | | — | | | (472) | | | — | | | 5 | | | 542 | | | — | | | — | | | |
Cash dividends declared, $1.51 per common share | — | | | — | | | — | | | — | | | (298) | | | — | | | — | | | — | | | — | | | (298) | | | |
| Other | — | | | — | | | — | | | (1) | | | 5 | | | — | | | — | | | — | | | (52) | | | (48) | | | |
| Balances, March 31, 2019 | 271 | | | 3 | | | 6,435 | | | (2) | | | 12,409 | | | (1,849) | | | (81) | | | (8,902) | | | 193 | | | 8,287 | | | |
| Opening retained earnings adjustments: adoption of new accounting standards | — | | | — | | | — | | | — | | | 11 | | | — | | | — | | | — | | | — | | | 11 | | | |
| Balances, April 1, 2019 | 271 | | | 3 | | | 6,435 | | | (2) | | | 12,420 | | | (1,849) | | | (81) | | | (8,902) | | | 193 | | | 8,298 | | | |
| Issuance of shares under employee plans | 1 | | | — | | | 113 | | | — | | | — | | | — | | | — | | | (20) | | | — | | | 93 | | | |
| Share-based compensation | — | | | — | | | 115 | | | — | | | — | | | — | | | — | | | — | | | — | | | 115 | | | |
| Payments to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (154) | | | (154) | | | |
| Other comprehensive income | — | | | — | | | — | | | — | | | — | | | 146 | | | — | | | — | | | — | | | 146 | | | |
| Net income | — | | | — | | | — | | | — | | | 900 | | | — | | | — | | | — | | | 178 | | | 1,078 | | | |
| Repurchase of common stock | — | | | — | | | — | | | — | | | — | | | — | | | (14) | | | (1,934) | | | — | | | (1,934) | | | |
| Change Healthcare share exchange | — | | | — | | | — | | | — | | | — | | | — | | | (15) | | | (2,036) | | | — | | | (2,036) | | | |
Cash dividends declared, $1.62 per common share | — | | | — | | | — | | | — | | | (294) | | | — | | | — | | | — | | | — | | | (294) | | | |
| Other | — | | | (1) | | | — | | | 2 | | | (4) | | | — | | | — | | | — | | | — | | | (3) | | | |
| Balances, March 31, 2020 | 272 | | | 2 | | | 6,663 | | | — | | | 13,022 | | | (1,703) | | | (110) | | | (12,892) | | | 217 | | | 5,309 | | | |
| Opening retained earnings adjustments: adoption of new accounting standard | — | | | — | | | — | | | — | | | (13) | | | — | | | — | | | — | | | — | | | (13) | | | |
| Balances, April 1, 2020 | 272 | | | 2 | | | 6,663 | | | — | | | 13,009 | | | (1,703) | | | (110) | | | (12,892) | | | 217 | | | 5,296 | | | |
| Issuance of shares under employee plans | 1 | | | — | | | 92 | | | — | | | — | | | — | | | — | | | (28) | | | — | | | 64 | | | |
| Share-based compensation | — | | | — | | | 151 | | | — | | | — | | | — | | | — | | | — | | | — | | | 151 | | | |
| Payments to noncontrolling interests | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (177) | | | (177) | | | |
| Other comprehensive income | — | | | — | | | — | | | — | | | — | | | 223 | | | — | | | — | | | — | | | 223 | | | |
| Net income (loss) | — | | | — | | | — | | | — | | | (4,539) | | | — | | | — | | | — | | | 156 | | | (4,383) | | | |
| Exercise of put right by noncontrolling shareholders of McKesson Europe | — | | | — | | | 3 | | | — | | | — | | | — | | | — | | | — | | | — | | | 3 | | | |
| Repurchase of common stock | — | | | — | | | — | | | — | | | — | | | — | | | (5) | | | (750) | | | — | | | (750) | | | |
| | | | | | | | | | | | | | | | | | | | | |
Cash dividends declared, $1.67 per common share | — | | | — | | | — | | | — | | | (270) | | | — | | | — | | | — | | | — | | | (270) | | | |
| Other | — | | | — | | | 16 | | | — | | | 2 | | | — | | | — | | | — | | | — | | | 18 | | | |
| Balances, March 31, 2021 | 273 | | | $ | 2 | | | $ | 6,925 | | | $ | — | | | $ | 8,202 | | | $ | (1,480) | | | (115) | | | $ | (13,670) | | | $ | 196 | | | $ | 175 | | | |
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In millions) | | | | | | | | | | | | | | | | | |
| | Years Ended March 31, |
| | 2021 | | 2020 | | 2019 |
| OPERATING ACTIVITIES | | | | | |
| Net income (loss) | $ | (4,340) | | | $ | 1,120 | | | $ | 255 | |
| Adjustments to reconcile to net cash provided by operating activities: | | | | | |
| Depreciation | 321 | | | 321 | | | 317 | |
| Amortization | 566 | | | 601 | | | 632 | |
| Goodwill and other asset impairment charges | 242 | | | 139 | | | 2,079 | |
| Equity earnings and charges from investment in Change Healthcare Joint Venture | — | | | 1,084 | | | 194 | |
| Deferred taxes | (908) | | | (342) | | | 189 | |
| Credits associated with last-in, first-out inventory method | (38) | | | (252) | | | (210) | |
| Non-cash operating lease expense | 334 | | | 366 | | | — | |
| (Gain) loss from sales of businesses and investments | (9) | | | 33 | | | (86) | |
| Other non-cash items | 188 | | | 615 | | | 52 | |
| Changes in assets and liabilities, net of acquisitions: | | | | | |
| Receivables | 1,145 | | | (2,494) | | | (967) | |
| Inventories | (2,276) | | | (376) | | | (368) | |
| Drafts and accounts payable | 1,267 | | | 3,952 | | | 1,976 | |
| Operating lease liabilities | (362) | | | (377) | | | — | |
| Taxes | (166) | | | (8) | | | (95) | |
| Litigation liabilities | 8,067 | | | — | | | — | |
| Other | 511 | | | (8) | | | 68 | |
| Net cash provided by operating activities | 4,542 | | | 4,374 | | | 4,036 | |
| | | | | |
| INVESTING ACTIVITIES | | | | | |
| Payments for property, plant, and equipment | (451) | | | (362) | | | (426) | |
| Capitalized software expenditures | (190) | | | (144) | | | (131) | |
| Acquisitions, net of cash, cash equivalents, and restricted cash acquired | (35) | | | (133) | | | (905) | |
| Proceeds from sales of businesses and investments, net | 400 | | | 37 | | | 101 | |
| Other | (139) | | | 23 | | | (20) | |
| Net cash used in investing activities | (415) | | | (579) | | | (1,381) | |
| | | | | |
| FINANCING ACTIVITIES | | | | | |
| Proceeds from short-term borrowings | 6,323 | | | 21,437 | | | 37,265 | |
| Repayments of short-term borrowings | (6,323) | | | (21,437) | | | (37,268) | |
| Proceeds from issuances of long-term debt | 500 | | | — | | | 1,099 | |
| Repayments of long-term debt | (1,040) | | | (298) | | | (1,112) | |
| | | | | |
| Common stock transactions: | | | | | |
| Issuances | 92 | | | 113 | | | 75 | |
| Share repurchases, including shares surrendered for tax withholding | (770) | | | (1,954) | | | (1,639) | |
| Dividends paid | (276) | | | (294) | | | (292) | |
| Other | (199) | | | (301) | | | (355) | |
| Net cash used in financing activities | (1,693) | | | (2,734) | | | (2,227) | |
| Effect of exchange rate changes on cash, cash equivalents, and restricted cash | (61) | | | (19) | | | (119) | |
| Net increase in cash, cash equivalents, and restricted cash | 2,373 | | | 1,042 | | | 309 | |
| Cash, cash equivalents, and restricted cash at beginning of year | 4,023 | | | 2,981 | | | 2,672 | |
| Cash, cash equivalents, and restricted cash at end of year | 6,396 | | | 4,023 | | | 2,981 | |
| Less: Restricted cash at end of year included in Prepaid expenses and other | (118) | | | (8) | | | — | |
| | | | | |
| Cash and cash equivalents at end of year | $ | 6,278 | | | $ | 4,015 | | | $ | 2,981 | |
| | | | | |
| SUPPLEMENTAL CASH FLOW INFORMATION | | | | | |
| Cash paid for: | | | | | |
| Interest, net | $ | 220 | | | $ | 235 | | | $ | 383 | |
| Income taxes, net of refunds | 379 | | | 368 | | | 262 | |
McKESSON CORPORATION
FINANCIAL NOTES
1. Significant Accounting Policies
Nature of Operations: McKesson Corporation (“McKesson,” or the “Company,”) is a global provider of healthcare supply chain management solutions, retail pharmacy, community oncology and specialty care, and healthcare information solutions. McKesson partners with pharmaceutical manufacturers, providers, pharmacies, governments, and other organizations in healthcare to help provide the right medicines, medical products, and healthcare services to the right patients at the right time, safely, and cost-effectively. Commencing with the second quarter of 2021, the Company reports its financial results in four reportable segments: U.S. Pharmaceutical, International, Medical-Surgical Solutions, and Prescription Technology Solutions (“RxTS”). All prior segment information has been recast to reflect the Company’s new segment structure and current period presentation. The Company’s equity method investment in Change Healthcare LLC (“Change Healthcare JV”), which was split-off from McKesson in the fourth quarter of 2020, has been included in Other for retrospective periods presented. Refer to Financial Note 22, “Segments of Business,” for more information.
Basis of Presentation: The consolidated financial statements and accompanying notes are prepared in accordance with United States (“U.S.”) generally accepted accounting principles (“GAAP”). The consolidated financial statements of McKesson include the financial statements of all wholly-owned subsidiaries and majority-owned or controlled companies. For those consolidated subsidiaries where the Company’s ownership is less than 100%, the portion of the net income or loss allocable to the noncontrolling interests is reported as “Net income attributable to noncontrolling interests” in the Consolidated Statements of Operations. All significant intercompany balances and transactions have been eliminated in consolidation, including the intercompany portion of transactions with equity method investees.
The Company considers itself to control an entity if it is the majority owner of or has voting control over such entity. The Company also assesses control through means other than voting rights (“variable interest entities” or “VIEs”) and determines which business entity is the primary beneficiary of the VIE. The Company consolidates VIEs when it is determined that it is the primary beneficiary of the VIE. Investments in business entities in which the Company does not have control but has the ability to exercise significant influence over operating and financial policies, are accounted for using the equity method.
Fiscal Period: The Company’s fiscal year begins on April 1 and ends on March 31. Unless otherwise noted, all references to a particular year shall mean the Company’s fiscal year.
Reclassifications: Certain prior year amounts have been reclassified to conform to the current year presentation.
Use of Estimates: The preparation of financial statements in conformity with U.S. GAAP requires that the Company make estimates and assumptions that affect the reported amounts in the consolidated financial statements and accompanying notes. Actual amounts could differ from those estimated amounts. The severity, magnitude and duration, as well as the economic consequences of the coronavirus diseases 2019 (“COVID-19”) pandemic, are uncertain, rapidly changing, and difficult to predict. Therefore, the Company’s accounting estimates and assumptions may change over time in response to COVID-19 and may change materially in future periods.
The Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”) was enacted on March 27, 2020 in the U.S., and includes several provisions related to employment and income taxes, including provisions for the deferral of the employer portion of social security taxes through December 31, 2020. On December 27, 2020, the U.S. government enacted the Consolidated Appropriations Act, 2021, which enhances and expands certain provisions of the CARES Act. These legislative acts are not expected to have a material impact on the Company’s consolidated financial results.
Cash and Cash Equivalents: All highly liquid debt and money market instruments purchased with an original maturity of three months or less at the date of acquisition are included in cash and cash equivalents. Cash equivalents are carried at fair value. Cash equivalents are primarily invested in AAA-rated U.S. government money market funds and overnight deposits with financial institutions. Deposits with financial institutions are primarily denominated in U.S. dollars and the functional currencies of the Company’s foreign subsidiaries, including Euro, British pound sterling, and Canadian dollars. Deposits may exceed the amounts insured by the Federal Deposit Insurance Corporation in the U.S. and similar deposit insurance programs in other jurisdictions. The Company mitigates the risk of its short-term investment portfolio by depositing funds with reputable financial institutions and monitoring risk profiles and investment strategies of money market funds.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Restricted Cash: Cash that is subject to legal restrictions or is unavailable for general operating purposes is classified as restricted cash and is included in “Prepaid expenses and other” and “Other non-current assets” in the Consolidated Balance Sheets. As of March 31, 2021, restricted cash primarily consists of funds temporarily held on behalf of unaffiliated medical practice groups related to their COVID-19 business continuity borrowings. The amounts have been designated as restricted cash due to contractual provisions requiring their segregation from all other funds until utilized by the medical practices for a limited list of qualified activities. Corresponding deposit liabilities associated with these funds have been recorded by the Company within “Other accrued liabilities” on the Company’s Consolidated Balance Sheet as of March 31, 2021.
Marketable Securities Available-for-Sale: The Company’s marketable securities, which are available-for-sale, are carried at fair value and are included in “Prepaid expenses and other” in the Consolidated Balance Sheets. The unrealized gains and losses, net of the related tax effect, computed in marking these securities to market have been reported in stockholders’ equity. At March 31, 2021 and 2020, marketable securities were not material. In determining whether an other-than-temporary decline in market value has occurred, the Company considers the duration that, and extent to which, the fair value of the investment is below its cost, the financial condition and future prospects of the issuer or underlying collateral of a security, and its intent and ability to retain the security in order to allow for an anticipated recovery in fair value. Other-than-temporary declines in fair value from amortized cost for available-for-sale equity securities that the Company intends to sell or would more likely than not be required to sell before the expected recovery of the amortized cost basis are charged to other income (expense), net, in the period in which the loss occurs.
Equity Method Investments: Investments in business entities in which the Company does not have control, but has the ability to exercise significant influence over operating and financial policies, are accounted for using the equity method. The Company evaluates its equity method investments for impairment whenever an event or change in circumstances occurs that may have a significant adverse impact on the carrying value of the investment. If a loss in value has occurred that is deemed to be other-than-temporary, an impairment loss is recorded.
Receivables, Net and Allowances for Doubtful Accounts: The Company’s receivables are presented net of an allowance for doubtful accounts and primarily consist of trade accounts receivables from customers that result from the sale of goods and services. Receivables, net also includes other receivables, which primarily represent amounts due from suppliers.
We are exposed to credit losses on accounts receivable balances. The Company estimates credit losses by considering historical credit losses, the current economic environment, customer credit ratings or bankruptcies, as well as reasonable and supportable forecasts. Management reviews these factors quarterly to determine if any adjustments are needed to the allowance. Trade accounts receivable represent the majority of the Company's financial assets, for which an allowance for credit losses of $198 million and $224 million were included in “Receivables, net” on the Consolidated Balance Sheet as of March 31, 2021 and 2020, respectively. Changes in the allowance were not material for the year ended March 31, 2021.
The following table presents the components of the Company’s receivables as of March 31, 2021 and 2020:
| | | | | | | | | | | |
| March 31, |
| (In millions) | 2021 | | 2020 |
| Customer accounts | $ | 17,106 | | | $ | 17,201 | |
| Other | 2,325 | | | 3,014 | |
| Total receivables | 19,431 | | | 20,215 | |
| Allowances | (250) | | | (265) | |
| Receivables, net | $ | 19,181 | | | $ | 19,950 | |
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Concentrations of Credit Risk and Receivables: The Company’s trade accounts receivable are subject to concentrations of credit risk with customers primarily in its U.S. Pharmaceutical segment. During 2021, sales to the Company’s ten largest customers, including group purchasing organizations (“GPOs”), accounted for approximately 51% of its total consolidated revenues and approximately 32% of total trade accounts receivable at March 31, 2021. Sales to the Company’s largest customer, CVS Health Corporation (“CVS”), accounted for approximately 21% of its total consolidated revenues in 2021 and comprised approximately 19% of total trade accounts receivable at March 31, 2021. As a result, the Company’s sales and credit concentration is significant. The Company has agreements with GPOs, each of which functions as a purchasing agent on behalf of member hospitals, pharmacies and other healthcare providers, as well as with government entities and agencies. The accounts receivables balances are with individual members of the GPOs, and therefore no significant concentration of credit risk exists. A material default in payment, a material reduction in purchases from these or any other large customers, or the loss of a large customer or customer groups could have a material adverse impact on the Company’s financial condition, results of operations, and liquidity. In addition, trade receivables are subject to concentrations of credit risk with customers in the institutional, retail, and healthcare provider sectors, which can be affected by a downturn in the economy and changes in reimbursement policies. This credit risk is mitigated by the size and diversity of the Company’s customer base as well as its geographic dispersion.
Financing Receivables: The Company assesses and monitors credit risk associated with financing receivables, primarily notes receivable, through regular review of its collections experience in determining its allowance for loan losses. On an ongoing basis, the Company also evaluates credit quality of its financing receivables utilizing historical collection rates and write-offs, as well as considering existing economic conditions, to determine if an allowance is required. As of March 31, 2021 and 2020, financing receivables were not material to the Company’s consolidated financial statements. Financing receivables and the related allowances are included in “Receivables, net” and “Other non-current assets” in the Consolidated Balance Sheets.
Inventories: Inventories consist of merchandise held for resale. The Company reports inventories at the lower of cost or net realizable value, except for inventories determined using the last-in, first-out (“LIFO”) method which are valued at the lower of LIFO cost or market. The LIFO method presumes that the most recent inventory purchases are the first items sold and the inventory cost under LIFO approximates market. The majority of the cost of domestic inventories is determined using the LIFO method. The majority of the cost of inventories held in foreign and certain domestic locations is based on the first-in, first-out (“FIFO”) method and weighted-average purchase prices. Rebates, cash discounts, and other incentives received from vendors are recognized in cost of sales upon the sale of the related inventory.
The LIFO method was used to value approximately 58% and 60% of the Company’s inventories at March 31, 2021 and 2020, respectively. If the Company had used the moving average method of inventory valuation, inventories would have been approximately $406 million and $444 million higher than the amounts reported at March 31, 2021 and 2020, respectively. These amounts are equivalent to the Company’s LIFO reserves. The Company’s LIFO valuation amount includes both pharmaceutical and non-pharmaceutical products. The Company recognized LIFO credits of $38 million, $252 million, and $210 million in 2021, 2020, and 2019, respectively, in “Cost of sales” in its Consolidated Statements of Operations. The lower LIFO credits in 2021 compared to 2020 is primarily due to higher brand inflation and delays of branded off-patent to generic drug launches. A LIFO charge is recognized when the net effect of price increases on pharmaceutical and non-pharmaceutical products held in inventory exceeds the impact of price declines, including the effect of branded pharmaceutical products that have lost market exclusivity. A LIFO credit is recognized when the net effect of price declines exceeds the impact of price increases on pharmaceutical and non-pharmaceutical products held in inventory. Excluding LIFO reserves, inventory reserves as of March 31, 2021 and 2020 were $263 million and $96 million, respectively. The increase was primarily due to charges in 2021 totaling $136 million on certain personal protective equipment and other related products due to inventory impairments and excess inventory within our Medical-Surgical Solutions segment. These charges are recorded in "Cost of sales" in the Consolidated Statements of Operations.
The Company believes that the moving average inventory costing method provides a reasonable estimation of the current cost of replacing inventory (i.e., “market”). As such, its LIFO inventory is valued at the lower of LIFO cost or market. As of March 31, 2021 and 2020, inventories at LIFO did not exceed market.
Shipping and Handling Costs: The Company includes costs to pack and deliver inventory to its customers in Selling, distribution, general, and administrative expenses. Shipping and handling costs of $1.0 billion, $1.0 billion, and $951 million were recognized in 2021, 2020, and 2019, respectively.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Held for Sale: Assets and liabilities to be disposed of by sale (“disposal groups”) are reclassified into “held for sale” if their carrying amounts are principally expected to be recovered through a sale transaction rather than through continuing use. The reclassification occurs when the disposal group is available for immediate sale and the sale is highly probable. These criteria are generally met when an agreement to sell exists, or management has committed to a plan to sell the assets within one year. Disposal groups are measured at the lower of carrying amount or fair value less costs to sell and are not depreciated or amortized. When the net realizable value of a disposal group increases during a period, a gain can be recognized to the extent that it does not increase the value of the disposal group beyond its original carrying value when the disposal group was reclassified as held for sale. The fair value of a disposal group, less any costs to sell, is assessed each reporting period it remains classified as held for sale and any remeasurement to the lower of carrying value or fair value less costs to sell is reported as an adjustment to the carrying value of the disposal group. Refer to Financial Note 3, “Held for Sale,” for more information.
Property, Plant, and Equipment, Net: Property, plant, and equipment, net is stated at historical cost and depreciated under the straight-line method over the estimated useful life of each asset, which ranges from 15 to 30 years for building and improvements and 3 to 15 years for machinery, equipment, and other. Leasehold improvements and property, plant, and equipment, net under finance leases are amortized over their respective useful lives or over the term of the lease, whichever is shorter. Depreciation and amortization begins when an asset is placed in service and ready for its intended use. Repairs and maintenance costs are expensed as incurred. When certain events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable, an impairment assessment may be performed on the recoverability of the carrying amounts.
The following table presents the components of the Company’s property, plant, and equipment, net as of March 31, 2021 and 2020:
| | | | | | | | | | | |
| March 31, |
| (In millions) | 2021 | | 2020 |
| Land | $ | 156 | | | $ | 151 | |
| Building and improvements | 1,745 | | | 1,604 | |
| Machinery, equipment, and other | 2,512 | | | 2,308 | |
| Construction in progress | 382 | | | 131 | |
| Total property, plant, and equipment | 4,795 | | | 4,194 | |
| Accumulated depreciation and amortization | (2,214) | | | (1,829) | |
| Property, plant, and equipment, net | $ | 2,581 | | | $ | 2,365 | |
Total depreciation expense for property, plant, and equipment, net and amortization of finance leases was $344 million, $335 million, and $317 million for the years ended March 31, 2021, 2020, and 2019, respectively.
Goodwill: Goodwill is tested for impairment on an annual basis in the third quarter and more frequently if indicators of potential impairment exist. Impairment testing is conducted at the reporting unit level, which is generally defined as an operating segment or one level below an operating segment (also known as a component), for which discrete financial information is available and segment management regularly reviews the operating results.
The Company applies the goodwill impairment test by comparing the estimated fair value of a reporting unit to its carrying value and recording an impairment charge equal to the amount of excess carrying value above estimated fair value, if any, but not to exceed the amount of goodwill allocated to the reporting unit.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
To estimate the fair value of its reporting units, the Company generally uses a combination of the market approach and the income approach. Under the market approach, it estimates fair value by comparing the business to similar businesses, or guideline companies whose securities are actively traded in public markets. Under the income approach, it uses a discounted cash flow (“DCF”) model in which cash flows anticipated over future periods, plus a terminal value at the end of that time horizon, are discounted to their present value using an appropriate rate that is commensurate with the risk inherent within the reporting unit. Other estimates inherent in both the market and income approaches include long-term growth rates, projected revenues, and earnings and cash flow forecasts for the reporting units. In addition, the Company compares the aggregate of the reporting units’ fair values to the Company’s market capitalization as a further corroboration of the fair values. Goodwill testing requires a complex series of assumptions and judgments by management in projecting future operating results, selecting guideline companies for comparisons and assessing risks. The use of alternative assumptions and estimates could affect the fair values and change the impairment determinations.
Intangible Assets: Currently all of the Company’s intangible assets are subject to amortization and are amortized based on the pattern of their economic consumption or on a straight-line basis over their estimated useful lives, ranging from one to 38 years. The Company reviews intangible assets for impairment at an asset group level whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Determination of recoverability is based on the lowest level of identifiable estimated future undiscounted cash flows resulting from use of the asset and its eventual disposition. Measurement of any impairment loss is based on the excess of the carrying value of the asset group over its estimated fair market value.
Capitalized Software Held for Internal Use: The Company capitalizes costs of software held for internal use during the application development stage of a project and amortizes those costs using the straight-line method over their estimated useful lives, not to exceed 10 years. As of March 31, 2021 and 2020, capitalized software held for internal use was $513 million and $400 million, respectively, net of accumulated amortization of $1.4 billion and $1.3 billion, respectively, and is included in “Other non-current assets” in the Consolidated Balance Sheets. Costs incurred during the preliminary project and post-implementation stages are expensed as incurred. Amortization expense for capitalized software held for internal use was $117 million, $129 million, and $137 million for the years ended March 31, 2021, 2020, and 2019, respectively.
Insurance Programs: The Company maintains insurance programs through its wholly-owned captive insurance subsidiaries (“Captives”), from which it obtains coverage for catastrophic exposures, including certain exposures arising from the opioid-related claims of governmental entities against the Company, as discussed in more detail in Financial Note 19, “Commitments and Contingent Liabilities,” as well as those risks required to be insured by law or contract. It is the Company’s policy to retain a significant portion of certain losses, including those related to workers’ compensation and comprehensive general, product, and vehicle liability. Provisions for losses expected under insurance programs are recorded based on the Company’s estimate of the aggregate liability for claims incurred as well as for claims incurred but not yet reported. Such estimates utilize certain actuarial assumptions followed in the insurance industry. The Captives receive direct premiums, which are eliminated on consolidation against the Company’s premium costs within Operating Expenses in the Consolidated Statements of Operations.
Revenue Recognition: Revenue is recognized when an entity satisfies a performance obligation by transferring control of a promised good or service to a customer in an amount that reflects the consideration to which the entity expects to be entitled for that good or service.
Revenues generated from the distribution of pharmaceutical and medical products represent the majority of the Company’s revenues. The Company orders product from the manufacturer, receives and carries the product at its central distribution facilities, and delivers the product directly to its customers’ warehouses, hospitals, or retail pharmacies. The distribution business primarily generates revenue from a contract related to a confirmed purchase order with a customer in a distribution arrangement. Revenue is recognized when control of goods is transferred to the customer which occurs upon the Company’s delivery to the customer or upon customer pick-up. The Company also earns revenues from a variety of other sources including its retail, services, and technology businesses. Retail revenues are recognized at the point of sale. Service revenues, including technology service revenues, are recognized when services are rendered. Revenues derived from distribution and retail business at the point of sale, and revenues derived from services represent approximately 98% and 2% of total revenues for each of the years ended March 31, 2021 and 2020, respectively.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Revenues are recorded gross when the Company is the principal in the transaction, has the ability to direct the use of the goods or services prior to transfer to a customer, is responsible for fulfilling the promise to its customer, has latitude in establishing prices, and controls the relationship with the customer. The Company records its revenues net of sales taxes. Revenues are measured based on the amount of consideration that the Company expects to receive, reduced by estimates for return allowances, discounts, and rebates using historical data. Sales returns from customers were approximately $3.1 billion in each of 2021 and 2020 and $2.9 billion in 2019. Assets for the right to recover products from customers and the associated refund liabilities for return allowances were not material as of March 31, 2021. Shipping and handling costs associated with outbound freight after control over a product has transferred to a customer are accounted for as fulfillment costs. The Company records deferred revenues when payments are received or due in advance of its performance. Deferred revenues are primarily from the Company’s services arrangements and are recognized as revenues over the periods when services are performed.
The Company had no material contract assets, contract liabilities, or deferred contract costs recorded in its Consolidated Balance Sheets as of March 31, 2021 and 2020. The Company generally expenses costs to obtain a contract as incurred when the amortization period is less than one year.
Supplier Incentives: Fees for services and other incentives received from suppliers, relating to the purchase or distribution of inventory, are considered product discounts and are generally reported as a reduction to cost of sales.
Supplier Reserves: The Company establishes reserves against amounts due from suppliers relating to various fees for services and price and rebate incentives, including deductions taken against payments otherwise due to it. These reserve estimates are established based on judgment after considering the status of current outstanding claims, historical experience with the suppliers, the specific incentive programs, and any other pertinent information available. The Company evaluates the amounts due from suppliers on a continual basis and adjusts the reserve estimates when appropriate based on changes in facts and circumstances. Adjustments to supplier reserves are generally included in cost of sales unless consideration from the vendor is in exchange for distinct goods or services or for pass-through rebate purchases. The ultimate outcome of any outstanding claims may be different than the Company’s estimate. The supplier reserves primarily pertain to the Company’s U.S. Pharmaceutical segment.
Income Taxes: The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or the tax returns. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statements and the tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Tax benefits from uncertain tax positions are recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. The amount recognized is measured as the largest amount of tax benefit that is greater than 50 percent likely of being realized upon effective settlement.
Interest Expense: Interest expense primarily includes interest for the Company’s long-term debt obligations, commercial paper, net interest settlements of interest rate swaps, and the amortization of deferred issuance costs and original issue discounts on debt.
Foreign Currency Translation: The reporting currency of the Company and its subsidiaries is the U.S. dollar. Its foreign subsidiaries generally consider their local currency to be their functional currency. Foreign currency-denominated assets and liabilities of these foreign subsidiaries are translated into U.S. dollars at period-end exchange rates, while revenues and expenses are translated at average exchange rates during the corresponding period and stockholders’ equity accounts are primarily translated at historical exchange rates. Foreign currency translation adjustments are included in “Other comprehensive income (loss), net of tax” in the Consolidated Statements of Comprehensive Income (Loss), and the cumulative effect is included in the stockholders’ equity section of the Consolidated Balance Sheets. Realized gains and losses from currency exchange transactions are recorded in “Selling, distribution, general, and administrative expenses” in the Consolidated Statements of Operations and were not material to the Company’s consolidated results of operations in 2021, 2020, or 2019. The Company releases cumulative translation adjustments from stockholders’ equity into earnings as a gain or loss only upon a complete or substantially complete liquidation of a controlling interest in a subsidiary or a group of assets within a foreign entity. It also releases all or a pro rata portion of the cumulative translation adjustments into earnings upon the sale of an equity method investment that is a foreign entity or has a foreign component.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Derivative Financial Instruments: Derivative financial instruments are used principally in the management of foreign currency exchange and interest rate exposures and are recorded in the Consolidated Balance Sheets at fair value. If a derivative is designated as a fair value hedge, the changes in the fair value of the derivative and of the hedged item attributable to the hedged risk are recognized in earnings. The Company uses foreign currency-denominated notes and cross-currency swaps to hedge a portion of its net investment in its foreign subsidiaries. It uses cash flow hedges primarily to reduce the effects of foreign currency exchange rate risk related to intercompany loans denominated in non-functional currencies. If the financial instrument is designated as a cash flow hedge or net investment hedge, the effective portions of changes in the fair value of the derivative are included in “Other comprehensive income (loss), net of tax” in the Consolidated Statements of Comprehensive Income (Loss), and the cumulative effect is included in the stockholders’ equity section of the Consolidated Balance Sheets. The cumulative changes in fair value are reclassified to the same line as the hedged item in the Consolidated Statements of Operations when the hedged item affects earnings. The Company evaluates hedge effectiveness at inception and on an ongoing basis, and ineffective portions of changes in the fair value of cash flow hedges and net investment hedges are recognized in earnings following the date when ineffectiveness was identified. Derivative instruments not designated as hedges are marked-to-market at the end of each accounting period with the change included in earnings.
Comprehensive Income (Loss): Comprehensive income (loss) consists of two components: net income (loss) and other comprehensive income. Other comprehensive income refers to revenue, expenses, and gains and losses that under GAAP are recorded as an element of stockholders’ equity but are excluded from earnings. The Company’s other comprehensive income primarily consists of foreign currency translation adjustments from those subsidiaries where the local currency is the functional currency including gains and losses on net investment hedges, unrealized gains and losses on cash flow hedges, and unrealized gains and losses on retirement-related benefit plans.
Noncontrolling Interests and Redeemable Noncontrolling Interests: Noncontrolling interests represent the portion of profit or loss, net assets, and comprehensive income that is not allocable to McKesson Corporation. Net income attributable to noncontrolling interests includes recurring compensation that McKesson is obligated to pay to the noncontrolling shareholders of McKesson Europe AG (“McKesson Europe”), formerly known as Celesio AG, under the domination and profit and loss transfer agreement. Net income attributable to noncontrolling interests also includes third-party equity interests in the Company’s consolidated entities including Vantage Oncology Holdings, LLC (“Vantage”) and ClarusONE Sourcing Services LLP (“ClarusONE”), which was established between McKesson and Walmart, Inc in 2017. Noncontrolling interests with redemption features, such as put rights, that are not solely within the Company’s control are considered redeemable noncontrolling interests. Redeemable noncontrolling interests are presented outside of stockholders’ equity in the Company’s Consolidated Balance Sheets. Refer to Financial Note 9, “Redeemable Noncontrolling Interests and Noncontrolling Interests,” for more information.
Share-Based Compensation: The Company accounts for all share-based compensation transactions at fair value. The share-based compensation expense, for the portion of the awards that is ultimately expected to vest, is recognized on a straight-line basis over the requisite service period. The share-based compensation expense recognized is classified in the Consolidated Statements of Operations in the same manner as cash compensation paid to the Company’s employees.
Loss Contingencies: The Company is subject to various claims, including, but not limited to, claims with customers and vendors, pending and potential legal actions for damages, investigations relating to governmental laws and regulations, and other matters arising out of the normal conduct of its business. When a loss is considered probable and reasonably estimable, the Company records a liability in the amount of its best estimate for the ultimate loss. However, the likelihood of a loss with respect to a particular contingency is often difficult to predict and determining a meaningful estimate of the loss or a range of loss may not be practicable based on the information available and the potential effect of future events and decisions by third parties that will determine the ultimate resolution of the contingency. Moreover, it is not uncommon for such matters to be resolved over many years, during which time relevant developments and new information must be reevaluated at least quarterly to determine both the likelihood of potential loss and whether it is possible to reasonably estimate the loss or a range of possible loss. When a material loss is reasonably possible or probable, but a reasonable estimate cannot be made, disclosure of the proceeding is provided. The Company recognizes legal fees as incurred when the legal services are provided.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The Company reviews all contingencies at least quarterly to determine whether the likelihood of loss has changed and to assess whether a reasonable estimate of the loss or a range of the loss can be made. As discussed above, development of a meaningful estimate of loss or a range of potential loss is complex when the outcome is directly dependent on negotiations with or decisions by third parties, such as regulatory agencies, the court system, and other interested parties. Refer to Financial Note 19, “Commitments and Contingent Liabilities,” for additional information related to ongoing controlled substances claims to which the Company is a party.
Restructuring Charges: Employee severance costs are generally recognized when payments are probable and amounts are reasonably estimable. Costs related to contracts without future benefit or contract termination are recognized at the earlier of the contract termination or the cease-use dates. Other exit-related costs are recognized as incurred.
Business Combinations: The Company accounts for business combinations using the acquisition method of accounting whereby the identifiable assets and liabilities of the acquired business, as well as any noncontrolling interest in the acquired business, are recorded at their estimated fair values as of the date that the Company obtains control of the acquired business. Any purchase consideration in excess of the estimated fair values of the net assets acquired is recorded as goodwill. Acquisition-related expenses and related restructuring costs are expensed as incurred.
Several valuation methods may be used to determine the fair value of assets acquired and liabilities assumed. For intangible assets, the Company typically uses a method that is a form or variation of the income approach, whereby a forecast of future cash flows attributable to the asset are discounted to present value using a risk-adjusted discount rate. Some of the more significant estimates and assumptions inherent in the income approach include the amount and timing of projected future cash flows, the discount rate selected to measure the risks inherent in the future cash flows, and the assessment of the asset’s expected useful life.
Recently Adopted Accounting Pronouncements
In the first quarter of 2021, the Company prospectively adopted Accounting Standard Update (“ASU”) 2018-15, Intangibles - Goodwill and Other - Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract, which aligns the requirements for capitalizing implementation costs incurred in a cloud computing arrangement that is a service contract with the requirements for capitalizing implementation costs in a cloud computing arrangement that has a software license. As a result, the Company began capitalizing eligible implementation costs for such contracts and recognizing the expense over the service period. The adoption of this amended guidance did not have a material impact on the Company’s consolidated financial statements or disclosures.
In the first quarter of 2021, the Company retrospectively adopted ASU 2018-14, Compensation - Retirement Benefits - Defined Benefit Plans, which requires the Company to disclose the weighted-average interest crediting rates for cash balance plans and other plans with promised interest crediting rates, and an explanation of reasons for significant gains and losses related to changes in the benefit obligation for the period. The amended guidance also requires the Company to remove disclosures on the amounts in accumulated other comprehensive income expected to be recognized as components of net periodic benefit costs over the next fiscal year. The adoption of this amended guidance resulted in changes in disclosures but did not have an impact on the Company’s Consolidated Statements of Operations, Comprehensive Income (Loss), Balance Sheets, or Cash Flows.
In the first quarter of 2021, the Company adopted ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement, to remove, modify, and add disclosure requirements on fair value measurements. Certain requirements were applied prospectively while other changes were applied retrospectively on the effective date. The amended guidance removes disclosure requirements for transfers between Level 1 and Level 2 measurements and valuation processes for Level 3 measurements, but adds new disclosure requirements including changes in unrealized gains or losses in other comprehensive income related to recurring Level 3 measurements and requirements to disclose the range, and weighted-average used to develop significant unobservable inputs for Level 3 fair value measurements. The adoption of this amended guidance resulted in changes in disclosures but did not have an impact on the Company’s Consolidated Statements of Operations, Comprehensive Income (Loss), Balance Sheets, or Cash Flows.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
In the first quarter of 2021, the Company adopted ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, which changed the impairment model for most financial assets from one based on current losses to a forward-looking model based on expected losses. The forward-looking model requires the Company to consider historical experience, current conditions, and reasonable and supportable forecasts that affect the collectability of the reported amount in estimating credit losses. The amended guidance requires financial assets that are measured at amortized cost be presented at the net amount expected to be collected. An allowance for credit losses is established as a valuation account that is deducted from the amortized cost basis of financial assets. The guidance also requires enhanced disclosures. This guidance was adopted on a modified retrospective basis and did not have a material impact on the Company’s consolidated financial statements or disclosures. Upon adoption of the amended guidance in the first quarter of 2021, the Company recorded a cumulative-effect adjustment of $13 million to the opening balance of retained earnings, primarily as a result of adjustments to allowances for trade accounts receivable.
Recently Issued Accounting Pronouncements Not Yet Adopted
In December 2019, ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, was issued with the intent to simplify various aspects related to accounting for income taxes. The guidance eliminates certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences. The guidance also simplifies and clarifies certain other aspects of accounting for income taxes. The guidance is effective for the Company in the first quarter of 2022 and early adoption is permitted. The adoption of this amended guidance is not expected to have a material impact on the Company’s consolidated financial statements or disclosures.
2. Investment in Change Healthcare Joint Venture
In the fourth quarter of 2017, the Company contributed the majority of its McKesson Technology Solutions businesses to form a joint venture, the Change Healthcare JV, under a contribution agreement between McKesson and Change Healthcare Inc. (“Change”) and others, including shareholders of Change. In exchange for the contribution, the Company initially owned approximately 70% of the joint venture, with the remaining equity ownership of approximately 30% held by Change. The Change Healthcare JV was jointly governed by McKesson and shareholders of Change.
On June 27, 2019, common stock and certain other securities of Change began trading on the NASDAQ (“IPO”). Change was a holding company and did not own any material assets or have any operations other than its interest in the Change Healthcare JV. On July 1, 2019, upon the completion of its IPO, Change received net cash proceeds of approximately $888 million. Change contributed the proceeds of $609 million from its offering of common stock to the Change Healthcare JV in exchange for additional membership interests of the Change Healthcare JV (“LLC Units”) at the equivalent of its offering price of $13 per share. The proceeds of $279 million from the concurrent offering of other securities were used by Change to acquire certain securities of the Change Healthcare JV that substantially mirrored the terms of other securities included in the offering by Change. As a result, McKesson’s equity interest in the Change Healthcare JV was diluted from approximately 70% to approximately 58.5% while Change owned approximately 41.5% of the outstanding LLC Units. Accordingly, in the second quarter of 2020, the Company recognized a dilution loss of $246 million, primarily representing the difference between its proportionate share of the IPO proceeds and the dilution effect on the investment’s carrying value. The Company’s proportionate share of income or loss from this investment was subsequently reduced as immaterial settlements of stock option exercises occurred after the IPO. These amounts were included in “Equity earnings and charges from investment in Change Healthcare Joint Venture” in the Company’s Consolidated Statements of Operations for the year ended March 31, 2020.
In the second quarter of 2020, the Company recorded an other-than-temporary impairment (“OTTI”) charge of $1.2 billion to its investment in the Change Healthcare JV, representing the difference between the carrying value of the Company’s investment and the fair value derived from the corresponding closing price of Change’s common stock at September 30, 2019. This charge was included in “Equity earnings and charges from investment in Change Healthcare Joint Venture” in the Company’s Consolidated Statements of Operations for the year ended March 31, 2020.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Separation of the Change Healthcare JV
On March 10, 2020, the Company completed the previously announced separation of its interest in the Change Healthcare JV. The separation was affected through the split-off of PF2 SpinCo, Inc. (“SpinCo”), a wholly-owned subsidiary of the Company that held all of the Company’s interest in the Change Healthcare JV, to certain of the Company’s stockholders through an exchange offer (“Split-off”), followed by the merger of SpinCo with and into Change, with Change surviving the merger (“Merger”).
In connection with the Split-off, on March 9, 2020, the Company distributed all 176.0 million outstanding shares of common stock of SpinCo to participating holders of the Company’s common stock in exchange for 15.4 million shares of McKesson common stock which now are held as treasury stock on the Company’s Consolidated Balance Sheets. Refer to Financial Note 20, “Stockholders' Equity,” for more information. Following consummation of the exchange offer, on March 10, 2020, SpinCo was merged with and into Change Healthcare, and each share of SpinCo common stock converted into one share of Change common stock, par value $0.001 per share, with cash being paid in lieu of fractional shares of Change common stock. The Split-off and the Merger are intended to be generally tax-free transactions for U.S. federal income tax purposes. Following the Split-off, the Company does not beneficially own any of Change’s outstanding securities. In the fourth quarter of 2020, the Company recognized a net gain of $414 million related to the transaction which is included under the caption “Equity earnings and charges from investment in Change Healthcare Joint Venture” in the Company’s Consolidated Statements of Operations for the year ended March 31, 2020. The net gain was calculated as follows:
| | | | | |
| (In millions, except per share data) | |
Fair value of McKesson common stock accepted (15.4 million shares at $131.97 per share on March 9, 2020) | $ | 2,036 | |
| Investment in the Change Healthcare JV at exchange date | (2,096) | |
| Reversal of deferred tax liability | 521 | |
| Release of accumulated other comprehensive attributable to the joint venture | (24) | |
| Less: Transaction costs incurred | (23) | |
| Net gain on split-off of the Change Healthcare JV | $ | 414 | |
Equity Method Investment in the Change Healthcare Joint Venture
The Company’s investment in the joint venture was accounted for using the equity method of accounting on a one-month reporting lag. The Company’s accounting policy has been to disclose any intervening events of the joint venture in the lag period that could materially affect its consolidated financial statements. Effective April 1, 2019, the Change Healthcare JV adopted the amended revenue recognition guidance and, in the first quarter of 2020, the Company recorded its proportionate share of the joint venture’s adoption impact of the amended revenue recognition guidance of approximately $80 million, net of tax, in the Company’s opening retained earnings.
The Company recorded its proportionate share of loss from its investment in the Change Healthcare JV of $119 million and $194 million in 2020 and 2019, respectively. The Company’s proportionate share of income or loss from this investment includes transaction and integration expenses incurred by the Change Healthcare JV and basis differences between the joint venture and McKesson including amortization of fair value adjustments primarily representing incremental intangible amortization and removal of profit associated with the recognition of deferred revenue. These amounts were recorded under the caption “Equity earnings and charges from investment in Change Healthcare Joint Venture” in the Company’s Consolidated Statements of Operations.
Related Party Transactions
In connection with the formation of the Change Healthcare JV, McKesson, the Change Healthcare JV and certain shareholders of Change entered into various ancillary agreements, including transition services agreements (“TSA”), a transaction and advisory fee agreement (“Advisory Agreement”), a tax receivable agreement (“TRA”) and certain other agreements. Fees incurred or earned from the Advisory Agreement were not material for 2020 and 2019. Fees incurred or earned from the TSA were not material in 2021 and were $22 million in 2020 and $60 million in 2019. The Advisory Agreement was terminated in 2020.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
In 2019, the Company renegotiated the terms of the TRA which resulted in the extinguishment and derecognition of the $90 million non-current liability payable to the shareholders of Change. In exchange for the shareholders of Change agreeing to extinguish the liability, the Company agreed to an allocation of certain tax amortization that had the effect of reducing the amount of a distribution from the Change Healthcare JV that would otherwise have been required to be made to the shareholders of Change. As a result of the renegotiation, McKesson was relieved from any potential future obligations associated with the non-current liability and recognized a credit of $90 million in “Selling, distribution, general, and administrative expenses” in its Consolidated Statement of Operations in 2019. At March 31, 2021 and 2020, the Company had no outstanding payable balance to the shareholders of Change under the TRA.
Under the agreement executed in 2019 between the Change Healthcare JV, McKesson, Change, and certain subsidiaries of the Change Healthcare JV, McKesson had the ability to adjust the manner in which certain depreciation or amortization deductions are allocated among Change and McKesson. McKesson exercised its right under the agreement and allocated certain depreciation and amortization deductions to Change for the tax years ended March 31, 2020 and 2019.
After McKesson’s separation of its interest in the Change Healthcare JV, the aforementioned TRA agreement requires the Change Healthcare JV to pay McKesson 85% of the net cash tax savings realized, or deemed to be realized, by Change resulting from the depreciation or amortization allocated to Change by McKesson. The receipt of any payments from the Change Healthcare JV under the TRA is dependent upon Change benefiting from this depreciation or amortization in future tax return filings. This creates uncertainty over the amount, timing, and probability of the gain recognized. As such, the Company accounts for the TRA as a gain contingency, with no receivable recognized as of March 31, 2021 or 2020.
In conjunction with the separation transaction in the fourth quarter of 2020, the Company recorded a reversal of the deferred tax liability related to its investment. Under the agreement with the Change Healthcare JV, McKesson, Change, and certain subsidiaries of the Change Healthcare JV, there may be changes in future periods to the amount reversed as the relevant periods are audited by tax authorities. Any such change is not expected to have a material impact on the Company’s consolidated financial statements.
3. Held for Sale
Assets and liabilities that have met the classification as held for sale were $12 million and $9 million, respectively, as of March 31, 2021 and $906 million and $683 million, respectively, as of March 31, 2020. The amounts at March 31, 2020 primarily consisted of the majority of the Company’s German pharmaceutical wholesale business as described below. This disposal group had been recorded as assets and liabilities held for sale since the third quarter of 2020 through its contribution to a joint venture in the third quarter of 2021. Based on its analysis, the Company determined that the disposal groups classified as held for sale do not meet the criteria for classification as discontinued operations and are not considered to be significant disposals based on its quantitative and qualitative evaluation.
German Wholesale Joint Venture
On November 1, 2020, the Company completed its previously announced transaction with Walgreens Boots Alliance (“WBA”) whereby the majority of its German pharmaceutical wholesale business was contributed to a newly formed joint venture in which McKesson has a 30% noncontrolling interest.
Consideration received included a receivable amount of $41 million, primarily related to working capital and net debt adjustments from WBA, and the 30% interest in the newly formed joint venture. At the transaction date, the carrying value of the equity investment in the joint venture was recorded at its fair value, which was measured using inputs that fell within Level 3 of the fair value hierarchy. The carrying value of the investment in the joint venture was nil as of March 31, 2021. The Company accounts for its interest in the joint venture as an equity method investment within the International segment. The joint venture also assumed a note payable to the Company in the amount of approximately $291 million as of the transaction date, which was paid to the Company in the third quarter of 2021.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
In conjunction with the contribution, the Company recorded losses of $58 million and $275 million (pre-tax and after-tax), respectively, in the years ended March 31, 2021 and 2020, which includes adjustments to remeasure the assets and liabilities held for sale to fair value less costs to sell. These charges were included within “Operating expenses” in the Consolidated Statements of Operations. The Company’s measurement of the fair value of the disposal group was based on estimates of total consideration to be received by the Company as outlined in the contribution agreement between the Company and WBA. As a result of finalization of working capital amounts contributed and other adjustments, the Company may record additional gains or losses in future periods; however, these adjustments are not expected to have a material impact on the Company’s consolidated financial statements.
Following the completion of the transaction on November 1, 2020, there were no assets or liabilities of the German pharmaceutical wholesale joint venture classified as held for sale on the Company’s Consolidated Balance Sheet. The total assets and liabilities of the German pharmaceutical wholesale joint venture that were classified as held for sale on the Company’s Consolidated Balance Sheet as of March 31, 2020, were as follows:
| | | | | |
| (In millions) | March 31, 2020 |
| Assets | |
| Current Assets | |
| Receivables, net | $ | 548 | |
| Inventories, net | 478 | |
| |
| Long-term assets | 88 | |
Remeasurement of assets of business held for sale to fair value less cost to sell (1) | (272) | |
| Total Assets held for sale | $ | 842 | |
| |
| Liabilities | |
| Current Liabilities | |
| Drafts and accounts payable | $ | 450 | |
| Other accrued liabilities | 40 | |
| Long-term liabilities | 166 | |
| Total Liabilities held for sale | $ | 656 | |
(1)Includes the effect of approximately $3 million of favorable cumulative foreign currency translation adjustment.
4. Restructuring, Impairment, and Related Charges
The Company recorded restructuring, impairment, and related charges of $334 million, $268 million and $597 million in 2021, 2020, and 2019, respectively. These charges are included in “Restructuring, impairment, and related charges, net” in the Consolidated Statements of Operations. In addition, charges related to restructuring initiatives are included in “Cost of sales” in the Consolidated Statements of Operations and were not material for the years ended 2021, 2020, and 2019.
Restructuring Initiatives
During the first quarter of 2022, the Company approved an initiative to increase operational efficiencies and flexibility by transitioning to a partial remote work model for certain employees. This initiative primarily includes the rationalization of its office space in North America. Where the Company determines to cease using office space, it plans to exit the portion of the facility no longer used. It also may retain and repurpose certain other office locations. The Company expects to incur total charges of approximately $180 million to $280 million for this initiative, consisting primarily of exit related costs, accelerated depreciation and amortization of long-lived assets, and asset impairments. This initiative is expected to be completed in 2022.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
During the first quarter of 2021, the Company committed to an initiative within the United Kingdom (“U.K.”), which is included in the Company’s International segment, to further drive transformational changes in technologies and business processes, operational efficiencies, and cost savings. The initiative includes reducing the number of retail pharmacy stores, decommissioning obsolete technologies and processes, reorganizing and consolidating certain business operations, and related headcount reductions. Under this initiative, the Company expects to incur total charges of approximately $85 million to $90 million. The Company recorded charges of $57 million in 2021, primarily related to asset impairments and accelerated depreciation expense as well as employee severance and other employee-related costs. The initiative is expected to be substantially complete in 2022 and estimated remaining charges primarily consist of accelerated amortization of long-lived assets, facility and other exit costs, and employee-related costs.
During the fourth quarter of 2019, the Company committed to certain programs to continue its operating model and cost optimization efforts. The Company continues to implement centralization of certain functions and outsourcing through an expanded arrangement with a third-party vendor to achieve operational efficiency. The programs also include reorganization and consolidation of business operations, related headcount reductions, the further closures of retail pharmacy stores in Europe, and closures of other facilities. The Company recorded charges of $62 million, $72 million, and $163 million in 2021, 2020, and 2019, respectively, consisting primarily of employee severance, accelerated depreciation expense, and project consulting fees. This initiative was substantially complete in 2021 and remaining costs the Company expects to record under this initiative are not material.
As previously announced on November 30, 2018, the Company relocated its corporate headquarters, effective April 1, 2019, from San Francisco, California to Irving, Texas to improve efficiency, collaboration, and cost competitiveness. As a result, the Company recorded charges of $28 million, $44 million, and $33 million in 2021, 2020, and 2019, respectively, consisting primarily of employee retention expenses, severance, long-lived asset impairments, and accelerated depreciation. The relocation was substantially complete in January 2021 and remaining costs the Company expects to record under this initiative, primarily relating to lease costs, are not material.
In the second quarter of 2018, the Company committed to a restructuring plan, which primarily consisted of the closures of underperforming retail pharmacy stores in the U.K., included in its International segment, and a reduction in workforce. In 2019, the Company recorded charges of $18 million, consisting primarily of employee severance and lease exit costs, with $92 million of total charges recorded through the end of 2019. The plan was substantially completed in 2020 and additional charges were not material.
On April 25, 2018, the Company announced a strategic growth initiative intended to drive long-term incremental profit growth and to increase operational efficiency. The initiative consisted of multiple growth priorities and plans to optimize the Company’s operating models and cost structures primarily through centralization, cost management, and outsourcing of certain administrative functions. As part of the growth initiative, the Company committed to implement certain actions including a reduction in workforce, facility consolidation, and store closures. This set of initiatives was substantially complete by the end of 2020 and charges in 2021 were not material. The Company recorded charges of $15 million and $135 million in 2020 and 2019, respectively.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Fiscal 2021
Restructuring, impairment, and related charges, net for the year ended March 31, 2021 consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended March 31, 2021 |
| (In millions) | U.S. Pharmaceutical | | International (1) | | Medical-Surgical Solutions | | Prescription Technology Solutions | | Corporate (2) | | Total |
| Severance and employee-related costs, net | $ | 10 | | | $ | 22 | | | $ | (1) | | | $ | 4 | | | $ | 69 | | | $ | 104 | |
Exit and other-related costs (3) | 11 | | | 17 | | | 4 | | | — | | | 27 | | | 59 | |
| Asset impairments and accelerated depreciation | — | | | 46 | | | 1 | | | — | | | 9 | | | 56 | |
| Total | $ | 21 | | | $ | 85 | | | $ | 4 | | | $ | 4 | | | $ | 105 | | | $ | 219 | |
(1)Primarily represents costs associated with the operating model and cost optimization efforts described above.
(2)Represents costs associated with the operating model cost optimization efforts and the relocation of the Company’s headquarters described above in addition to various other initiatives.
(3)Exit and other-related costs primarily include project consulting fees.
Fiscal 2020
Restructuring, impairment, and related charges, net for the year ended March 31, 2020 consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended March 31, 2020 |
| (In millions) | U.S. Pharmaceutical (1) | | International (2) | | Medical-Surgical Solutions (3) | | Prescription Technology Solutions | | Corporate (4) | | Total |
| Severance and employee-related costs, net | $ | 12 | | | $ | 2 | | | $ | 4 | | | $ | (1) | | | $ | 30 | | | $ | 47 | |
Exit and other-related costs (5) | 1 | | | 13 | | | 19 | | | — | | | 46 | | | 79 | |
| Asset impairments and accelerated depreciation | 10 | | | 6 | | | 1 | | | — | | | 13 | | | 30 | |
| Total | $ | 23 | | | $ | 21 | | | $ | 24 | | | $ | (1) | | | $ | 89 | | | $ | 156 | |
(1)Represents costs associated with dispositions and costs related to the relocation of the Company’s corporate headquarters described above.
(2)Primarily represents costs associated with the operating model and cost optimization efforts described above.
(3)Primarily represents costs associated with the growth initiative described above.
(4)Represents costs associated with the growth initiative, operating model cost optimization efforts, and with the relocation of the Company’s corporate headquarters described above.
(5)Exit and other-related costs primarily include project consulting fees.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Fiscal 2019
Restructuring, impairment, and related charges, net for the year ended March 31, 2019 consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended March 31, 2019 |
| (In millions) | U.S. Pharmaceutical (1) | | International (2) | | Medical-Surgical Solutions (3) | | Prescription Technology Solutions | | Corporate (4) | | Total |
| Severance and employee-related costs, net | $ | 46 | | | $ | 51 | | | $ | 18 | | | $ | 3 | | | $ | 36 | | | $ | 154 | |
Exit and other-related costs (5) | 9 | | | 83 | | | 20 | | | — | | | 52 | | | 164 |
| Asset impairments and accelerated depreciation | 6 | | | 24 | | | 3 | | | — | | | 1 | | | 34 | |
| Total | $ | 61 | | | $ | 158 | | | $ | 41 | | | $ | 3 | | | $ | 89 | | | $ | 352 | |
(1)Represents costs associated with the operating model cost optimization efforts and growth initiative described above.
(2)Primarily represents costs associated with the operating model cost optimization efforts and U.K. restructuring initiative focusing on underperforming retail pharmacy stores described above.
(3)Primarily represents costs associated with the growth initiative described above.
(4)Represents costs associated with operating model cost optimization efforts and with the relocation of the Company’s corporate headquarters described above.
(5)Exit and other-related costs primarily include lease and other contract exit costs associated with closures of facilities and retail pharmacy stores as well as project consulting fees.
The following table summarizes the activity related to the restructuring liabilities associated with the Company’s restructuring initiatives for the years ended March 31, 2021 and 2020:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In millions) | U.S. Pharmaceutical | | International | | Medical-Surgical Solutions | | Prescription Technology Solutions | | Corporate | | Total |
| Balance, March 31, 2019 | $ | 35 | | | $ | 129 | | | $ | 26 | | | $ | 3 | | | $ | 44 | | | $ | 237 | |
| Restructuring, impairment, and related charges | 23 | | 21 | | | 24 | | | (1) | | | 89 | | | 156 |
| Non-cash charges | (10) | | | (6) | | | (1) | | | — | | | (13) | | | (30) | |
| Cash payments | (15) | | | (45) | | | (26) | | | (1) | | | (61) | | | (148) | |
| Other | (4) | | | (33) | | | (1) | | | — | | | (20) | | | (58) | |
Balance, March 31, 2020 (1) | 29 | | | 66 | | | 22 | | | 1 | | | 39 | | | 157 | |
| Restructuring, impairment, and related charges | 21 | | | 85 | | | 4 | | | 4 | | | 105 | | | 219 | |
| Non-cash charges | — | | | (46) | | | (1) | | | — | | | (9) | | | (56) | |
| Cash payments | (31) | | | (31) | | | (21) | | | (1) | | | (75) | | | (159) | |
| Other | — | | | (8) | | | (1) | | | — | | | (1) | | | (10) | |
Balance, March 31, 2021 (2) | $ | 19 | | | $ | 66 | | | $ | 3 | | | $ | 4 | | | $ | 59 | | | $ | 151 | |
(1) As of March 31, 2020, the total reserve balance was $157 million of which $118 million was recorded in Other accrued liabilities and $39 million was recorded in Other non-current liabilities.
(2) As of March 31, 2021, the total reserve balance was $151 million of which $99 million was recorded in Other accrued liabilities and $52 million was recorded in Other non-current liabilities.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Long-Lived Asset Impairments
Fiscal 2021
In 2021, the Company recognized charges of $115 million to impair certain long-lived assets within the Company’s International segment. These charges primarily related to long-lived assets associated with the Company’s retail pharmacy businesses in Canada and Europe and were due to declines in estimated future cash flows partially driven by a revised outlook regarding the impacts of COVID-19. The Company used both an income approach (a DCF method) and a market approach to estimate the fair value of the long-lived assets.
Fiscal 2020
In 2020, the Company recognized charges of $82 million to impair certain long-lived and intangible assets for its retail pharmacy business in Europe within the Company’s International segment. These charges related primarily to intangible assets associated with pharmacy licenses within the U.K retail business due to a decline in estimated future cash flows driven by additional U.K. government reimbursement reductions communicated in the third quarter of 2020. The Company used a combination of an income approach (a DCF method) and a market approach to estimate the fair value of the long-lived and intangible assets.
In 2020, the Company performed an interim impairment test of long-lived and intangible assets for its Rexall Health retail business, within the Company's International segment, due to the decline in the estimated future cash flows primarily driven by lower than expected growth in both prescription volume and sales of non-prescription goods. As a result, the Company recognized a charge of $30 million to impair certain long-lived and intangible assets, primarily customer relationships. The Company utilized an income approach (a DCF method) for estimating the fair value of the long-lived and intangible assets.
Fiscal 2019
In 2019, the Company recognized charges of $210 million to impair certain long-lived assets (primarily pharmacy licenses) for its U.K. retail business, within the Company’s International segment, primarily driven by government reimbursement reductions and competitive pressures in the U.K. The Company used an income approach (a DCF method) or a combination of an income approach and a market approach to estimate the fair value of the long-lived assets.
In 2019, the Company recorded charges of $35 million to impair certain intangible assets (primarily customer relationships) for its Rexall Health retail business within the Company’s International segment. The impairments were primarily the result of the decline in estimated future cash flows for this business. The estimated cash flow projections were negatively affected by a lower projected overall growth rate from the ongoing impact of government regulations in 2019. The Company utilized an income approach (a DCF method) for estimating the fair value of long-lived assets.
The fair value of the long-lived and intangible assets described above is considered a Level 3 fair value measurement due to the significance of unobservable inputs developed using company-specific information. Refer to Financial Note 17, “Fair Value Measurements,” for more information on nonrecurring fair value measurements.
5. Business Acquisitions and Divestitures
During 2021 and 2020, the Company did not complete any material acquisitions. During 2021, 2020, and 2019, the Company did not complete any material divestitures other than the contribution of the majority of its German wholesale business to a newly formed joint venture, as described in Financial Note 3, “Held for Sale,” in 2021 and the separation of the Change Healthcare JV, as described in Financial Note 2, “Investment in Change Healthcare Joint Venture,” in 2020.
Acquisitions
Goodwill recognized for business acquisitions is generally not expected to be deductible for tax purposes. However, if the assets of another company are acquired, the goodwill may be deductible for tax purposes.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
2019 Acquisition
Medical Specialties Distributors LLC (“MSD”)
On June 1, 2018, the Company completed its acquisition of MSD for the net purchase consideration of $784 million, which was funded from cash on hand. MSD is a leading national distributor of infusion and medical-surgical supplies as well as a provider of biomedical services to alternate site and home health providers. The financial results of MSD have been included in the Company’s Consolidated Statements of Operations within its Medical-Surgical Solutions segment since the acquisition date.
The fair value of assets acquired and liabilities assumed as of the acquisition date were finalized upon completion of the measurement period in the first quarter of 2020. The final purchase price allocation included acquired identifiable intangibles of $326 million primarily representing customer relationships with a weighted-average life of 18 years.
The following table summarizes the final recording of the fair value of the assets acquired and liabilities assumed for this acquisition as of the acquisition date, including immaterial adjustments made during the measurement period:
| | | | | | | | | | | | | | | |
| (In millions) | | | | | Amounts Recognized as of the Acquisition Date (1) |
| Receivables | | | | | $ | 112 | |
| Other current assets, net of cash and cash equivalents acquired | | | | | 71 | |
| Goodwill | | | | | 388 | |
| Intangible assets | | | | | 326 | |
| Other long-term assets | | | | | 56 | |
| Current liabilities | | | | | (72) | |
| Other long-term liabilities | | | | | (97) | |
| Net assets acquired, net of cash and cash equivalents | | | | | $ | 784 | |
(1)Final amounts as of May 31, 2019.
Other Acquisitions
CoverMyMeds LLC (“CMM”)
On April 3, 2017, the Company completed its acquisition of CMM for the net purchase consideration of $1.3 billion, which was funded from cash on hand. Pursuant to the agreement, McKesson’s purchase consideration was subject to an additional $160 million of contingent consideration based on CMM’s financial performance for 2018 and 2019. Pursuant to the agreement, the Company paid additional contingent consideration of $69 million and $68 million in May 2019 and May 2018, respectively. As of March 31, 2020, the related liability was nil.
During the three years presented, the Company also completed a number of other de minimis acquisitions within its operating segments. Financial results for the Company’s business acquisitions have been included in the Company’s consolidated financial statements since their respective acquisition dates. Purchase prices for business acquisitions have been allocated based on estimated fair values at the respective acquisition dates.
6. Share-Based Compensation
The Company provides share-based compensation to its employees, officers, and non-employee directors, including restricted stock units (“RSUs”), performance-based stock units (“PSUs”, formerly referred to as total shareholder return units or “TSRUs”), performance-based restricted stock units (“PeRSUs”), stock options, and an employee stock purchase plan (“ESPP”) (collectively, “share-based awards”). Most of the share-based awards are granted in the first quarter of each fiscal year.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Compensation expense for the share-based awards is recognized for the portion of awards ultimately expected to vest. The Company estimates the number of share-based awards that will ultimately vest primarily based on historical experience. The estimated forfeiture rate established upon grant is re-assessed throughout the requisite service period and is adjusted when actual forfeitures occur. The actual forfeitures in future reporting periods could be higher or lower than current estimates.
Compensation expense is classified in the Consolidated Statements of Operations in the same manner as cash compensation paid to the Company’s employees.
Impact on Net Income
The components of share-based compensation expense and related tax benefits are as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
Restricted stock unit awards (1) | $ | 137 | | | $ | 104 | | | $ | 75 | |
| Stock options | 4 | | | 7 | | | 12 | |
| Employee stock purchase plan | 10 | | | 8 | | | 8 | |
Share-based compensation expense | 151 | | | 119 | | | 95 | |
Tax benefit for share-based compensation expense (2) | (23) | | | (18) | | | (12) | |
Share-based compensation expense, net of tax | $ | 128 | | | $ | 101 | | | $ | 83 | |
(1)Includes compensation expense recognized for RSUs, PeRSUs, and PSUs.
(2)Income tax benefit is computed using the tax rates of applicable tax jurisdictions. Additionally, a portion of compensation expense is not tax-deductible. Income tax expense for 2021, 2020, and 2019 included discrete income tax expense of $2 million, $2 million, and $4 million, respectively.
Stock Plans
In July 2013, the Company’s stockholders approved the 2013 Stock Plan to replace the 2005 Stock Plan. Under these stock plans, the Company may issue restricted stock, RSUs, PSUs, PeRSUs, stock options, and other share-based awards to selected employees, officers, and non-employee directors. The 2013 Stock Plan reserves 30 million shares plus unused reserved shares under the 2005 Stock Plan. As of March 31, 2021, 20 million shares remain available for future grant under the 2013 Stock Plan.
Restricted Stock Unit Awards
RSUs entitle the holder to receive a specified number of shares of the Company’s common stock which vest over a period of generally three to four years as determined by the Compensation Committee at the time of grant. The fair value of the award is determined based on the market price of the Company’s common stock on the grant date and the related compensation expense is recognized over the vesting period on a straight-line basis.
Non-employee directors receive an annual grant of RSUs, which vest immediately and are expensed upon grant. The director may elect to receive the underlying shares immediately or defer receipt of the shares if they meet director stock ownership guidelines. The shares will be automatically deferred for those directors who do not meet the director stock ownership guidelines. At March 31, 2021, approximately 82,000 RSUs for the Company’s directors are vested.
PSUs are conditional upon the attainment of market and performance objectives over a specified period. The number of vested PSUs is assessed at the end of a three-year performance period upon attainment of meeting certain earnings per share targets, average return on invested capital, and for certain participants, total shareholder return relative to a peer group of companies and, for special PSUs granted in 2019, meeting certain cumulative operating profit metrics. The Company uses the Monte Carlo simulation model to measure the fair value of the total shareholder return portion of the PSUs. The earnings per share portion of the PSUs is measured at the grant date market price. PSUs have a requisite service period of generally three years. Expense is attributed to the requisite service period on a straight-line basis based on the fair value of the PSUs, adjusted for the performance modifier at the end of each reporting period. For PSUs that are designated as equity awards, the fair value is measured at the grant date. For PSUs that are eligible for cash settlement and designated as liability awards, the Company re-measures the fair value at the end of each reporting period and adjusts a corresponding liability in its Consolidated Balance Sheets for changes in fair value.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
PeRSUs are awards for which the number of RSUs awarded is conditional upon the attainment of one or more performance objectives over a specified period. All outstanding PeRSU awards have completed the performance period and are now classified and accounted for as RSUs. The Company did not grant any PeRSUs during the years ended March 31, 2021 and 2020.
The weighted-average assumptions used in the Monte Carlo valuations are as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| 2021 | | 2020 | | 2019 |
| Expected stock price volatility | 36 | % | | 30 | % | | 31 | % |
Expected dividend yield | 1.1 | % | | 1.3 | % | | 0.9 | % |
| Risk-free interest rate | 0.2 | % | | 2.2 | % | | 2.6 | % |
Expected life (in years) | 3 | | 3 | | 3 |
The following table summarizes activity for restricted stock unit awards (RSUs, PSUs, and PeRSUs) during 2021:
| | | | | | | | | | | |
| (In millions, except per share data) | Shares | | Weighted-
Average
Grant Date Fair
Value Per Share |
| Nonvested, March 31, 2020 | 3 | | $ | 135.57 | |
| Granted | 1 | | 155.47 | |
| Cancelled | — | | 133.70 | |
| Vested | (1) | | 147.63 | |
| Nonvested, March 31, 2021 | 3 | | $ | 142.13 | |
The following table provides data related to restricted stock unit award activity:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
| Total fair value of shares vested | $ | 79 | | | $ | 67 | | | $ | 59 | |
Total compensation cost, net of estimated forfeitures, related to nonvested restricted stock unit awards not yet recognized, pre-tax | $ | 147 | | | $ | 155 | | | $ | 119 | |
Weighted-average period in years over which restricted stock unit award cost is expected to be recognized | 2 | | 3 | | 2 |
Stock Options
Stock options are granted with an exercise price at no less than the fair market value and those options granted under the stock plans generally have a contractual term of seven years and follow a four-year vesting schedule.
Compensation expense for stock options is recognized on a straight-line basis over the requisite service period and is based on the grant-date fair value for the portion of the awards that is ultimately expected to vest. The Company uses the Black-Scholes options-pricing model to estimate the fair value of its stock options. Once the fair value of an employee stock option is determined, current accounting practices do not permit it to be changed, even if the estimates used are different from actual.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Weighted-average assumptions used to estimate the fair value of employee stock options were as follows: (1)
| | | | | | | | | | |
| | | | Year Ended
March 31, 2019 |
| | | |
Expected stock price volatility (2) | | | | 26 | % |
Expected dividend yield (3) | | | | 0.9 | % |
Risk-free interest rate (4) | | | | 2.8 | % |
Expected life (in years) (5) | | | | 4.6 |
(1)The Company did not grant any stock options during the years ended March 31, 2021 and 2020.
(2)The computation of expected volatility was based on a combination of the historical volatility of the Company’s common stock and implied market volatility. The Company believes this market-based input provides a reasonable estimate of its future stock price movements and is consistent with employee stock option valuation considerations.
(3)Expected dividend yield is based on historical experience and investors’ current expectations.
(4)The risk-free interest rate for periods within the expected life of the option is based on the constant maturity U.S. Treasury rate in effect at the grant date.
(5)The expected life of the options is based primarily on historical employee stock option exercises and other behavioral data and reflects the impact of changes in the contractual life of current option grants compared to the Company’s historical grants.
The following is a summary of stock options outstanding at March 31, 2021:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Options Outstanding | | Options Exercisable |
Range of Exercise
Prices | | Number of
Options
Outstanding
at Year End
(In millions) | | Weighted-
Average
Remaining
Contractual
Life (Years) | | Weighted-
Average
Exercise Price | | Number of
Options
Exercisable at
Year End
(In millions) | | Weighted-
Average
Exercise Price |
| $ | 118.41 | | – | $183.20 | | 1 | | | 3 | | $ | 166.18 | | | 1 | | | $ | 171.38 | |
| 183.20 | | – | 237.86 | | 1 | | | 1 | | 216.23 | | | 1 | | | 216.23 | |
| | | | 2 | | | | | | | 2 | | | |
The following table summarizes stock option activity during 2021:
| | | | | | | | | | | | | | | | | | | | | | | |
| (In millions, except per share data) | Shares | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contractual
Term (Years) | | Aggregate Intrinsic Value (2) |
| Outstanding, March 31, 2020 | 2 | | $ | 180.48 | | | 3 | | $ | 1 | |
| Granted | — | | — | | | | | |
| Cancelled | — | | 187.12 | | | | | |
| Exercised | — | | 153.51 | | | | | |
| Outstanding, March 31, 2021 | 2 | | $ | 183.29 | | | 2 | | $ | 36 | |
Vested and expected to vest (1) | 2 | | $ | 183.38 | | | 2 | | $ | 35 | |
| Vested and exercisable, March 31, 2021 | 2 | | 189.20 | | | 2 | | 24 | |
(1)The number of options expected to vest takes into account an estimate of expected forfeitures.
(2)The intrinsic value is calculated as the difference between the period-end market price of the Company’s common stock and the exercise price of “in-the-money” options.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The following table provides data related to stock option activity:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions, except per share data) | 2021 | | 2020 | | 2019 |
| Weighted-average grant date fair value per stock option | $ | — | | | $ | — | | | $ | 34.98 | |
| Aggregate intrinsic value on exercise | $ | 5 | | | $ | 17 | | | $ | 16 | |
| Cash received upon exercise | $ | 38 | | | $ | 66 | | | $ | 29 | |
| Tax benefits realized related to exercise | $ | 4 | | | $ | 4 | | | $ | 4 | |
| Total fair value of stock options vested | $ | 10 | | | $ | 16 | | | $ | 16 | |
Total compensation cost, net of estimated forfeitures, related to unvested stock options not yet recognized, pre-tax | $ | 2 | | | $ | 6 | | | $ | 15 | |
Weighted-average period in years over which stock option compensation cost is expected to be recognized | 2 | | 2 | | 2 |
Employee Stock Purchase Plan
The Company has an ESPP under which 21 million shares have been authorized for issuance. The ESPP allows eligible employees to purchase shares of the Company’s common stock through payroll deductions. The deductions occur over three-month purchase periods and the shares are then purchased at 85% of the market price at the end of each purchase period. Employees are allowed to terminate their participation in the ESPP at any time during the purchase period prior to the purchase of the shares. The 15% discount provided to employees on these shares is included in compensation expense. The shares related to funds outstanding at the end of a quarter are included in the calculation of diluted weighted-average shares outstanding. These amounts have not been significant for all the years presented. The Company recognizes costs for employer matching contributions as ESPP expense over the relevant purchase period. Shares issued under the ESPP were not material in 2021, 2020, and 2019. At March 31, 2021, 2 million shares remain available for issuance.
7. Other Income, Net
Other income, net consists of the following:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
| Interest income | $ | 12 | | | $ | 49 | | | $ | 39 | |
Equity in earnings, net (1) | 48 | | | 36 | | | 43 | |
Net gains on investments in equity securities (2) | 133 | | | 17 | | | 23 | |
Actuarial losses from pension plans (3) | — | | | (127) | | | — | |
Gain from sale of equity method investment (4) | — | | | — | | | 56 | |
| Other, net | 30 | | | 37 | | | 21 | |
Total | $ | 223 | | | $ | 12 | | | $ | 182 | |
(1)Primarily recorded within the Company’s International segment.
(2)Represents net realized and unrealized gains on the Company’s investments in equity securities of certain U.S. growth stage companies in the healthcare industry. These gains primarily relate to mark-to-market adjustments for investments which are measured at fair value based on changes in the observable price of the securities and realized gains on disposal of certain of these investments and were included within Corporate expenses, net. Refer to Financial Note 17, “Fair Value Measurements,” and to Financial Note 22, “Segments of Business.”
(3)Includes $116 million from the termination of the U.S. defined benefit pension plan and $11 million related to a settlement from the executive benefit retirement plan for a retired executive. Refer to Financial Note 15, “Pension Benefits.”
(4)Represents a gain from the sale of an equity investment to a third party included in RxTS during 2019.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
8. Income Taxes
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
| Income (loss) from continuing operations before income taxes | | | | | |
| U.S. | $ | (6,019) | | | $ | 216 | | | $ | 1,512 | |
| Foreign | 985 | | | 928 | | | (902) | |
| Income (loss) from continuing operations before income taxes | $ | (5,034) | | | $ | 1,144 | | | $ | 610 | |
Income tax expense (benefit) related to continuing operations consists of the following:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
| Current | | | | | |
| Federal | $ | (15) | | | $ | 170 | | | $ | (20) | |
| State | 47 | | | 48 | | | 35 | |
| Foreign | 181 | | | 142 | | | 152 | |
Total current | 213 | | | 360 | | | 167 | |
| | | | | |
| Deferred | | | | | |
| Federal | (562) | | | (204) | | | 223 | |
| State | (204) | | | (105) | | | 44 | |
| Foreign | (142) | | | (33) | | | (78) | |
Total deferred | (908) | | | (342) | | | 189 | |
Income tax expense (benefit) | $ | (695) | | | $ | 18 | | | $ | 356 | |
The Company reported an income tax benefit rate of 13.8% in 2021. Income tax expense rates were 1.6% and 58.4% in 2020 and 2019, respectively. Fluctuations in the Company’s reported income tax rates are primarily due to the impact of opioid-related claims of $8.1 billion ($6.8 billion after-tax) in 2021, the impact of the Change Healthcare joint venture divestiture in 2020, the impact of nondeductible impairment charges in 2019, and varying proportions of income attributable to foreign countries that have income tax rates different from the U.S. rate.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The reconciliation of income tax expense (benefit) and the amount computed by applying the statutory federal income tax rate of 21% to income before income taxes is as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
| Income tax expense (benefit) at federal statutory rate | $ | (1,057) | | | $ | 240 | | | $ | 128 | |
| State income taxes, net of federal tax benefit | (206) | | | (41) | | | 70 | |
| Tax effect of foreign operations | (77) | | | (81) | | | (86) | |
| Unrecognized tax benefits and settlements | 41 | | | (7) | | | 20 | |
| Non-deductible goodwill | 14 | | | 7 | | | 357 | |
| Opioid-related litigation and claims | 715 | | | — | | | — | |
| Net tax benefit on intellectual property transfer | (105) | | | — | | | (42) | |
Tax-free gain on investment exit (1) | — | | | (87) | | | — | |
| Impact of change in U.S. tax rate on temporary differences | — | | | — | | | (81) | |
| | | | | |
| Capital loss carryback | — | | | (19) | | | — | |
Other, net (2) | (20) | | | 6 | | | (10) | |
Income tax expense (benefit) | $ | (695) | | | $ | 18 | | | $ | 356 | |
(1)Refer to Financial Note 2, “Investment in Change Healthcare Joint Venture,” for additional information regarding the separation of the Change Healthcare JV.
(2)The Company’s effective tax rates were impacted by other favorable U.S. federal permanent differences including research and development credits of $5 million in 2021 and $7 million in each of 2020 and 2019.
The Company’s reported income tax rate for 2021 was impacted by the charge for pending and future opioid-related claims of $8.1 billion ($6.8 billion after-tax), as described further in Financial Note 19, “Commitments and Contingent Liabilities.” The Company recorded a deferred tax benefit of $1.3 billion, which is net of certain non-deductible expenses and an unrecognized tax benefit of $455 million.
During 2021 and 2019, the Company sold intellectual property between wholly-owned legal entities within McKesson that are based in different tax jurisdictions. In both instances, the transferor entity recognized a gain on the sale of assets which was not subject to income tax in its local jurisdiction; such gains were eliminated upon consolidation. The acquiring entities of the intellectual property were entitled to amortize the purchase price of the assets for tax purposes. In accordance with ASU 2016-16, “Intra-Entity Transfers of Assets Other Than Inventory,” discrete tax benefits of $105 million and $42 million were recognized for 2021 and 2019, respectively, with a corresponding increase to a deferred tax assets for the temporary difference arising from the buyer’s excess tax basis.
On March 10, 2020, the Company completed the previously announced separation of its interest in the Change Healthcare JV as described in Financial Note 2, “Investment in Change Healthcare Joint Venture.” The Company’s reported income tax expense rate for 2020 was favorably impacted by this transaction given that it was intended to generally be a tax-free split-off for U.S. federal income tax purposes. In the fourth quarter of 2020, the Company recognized a net gain for financial reporting purposes of $414 million related to the separation transaction.
The Company’s reported income tax expense rate for 2020 was unfavorably impacted by non-cash charges of $275 million to remeasure the carrying value of assets and liabilities held for sale related to the formation of a new German wholesale joint venture within the Company’s International segment. Refer to Financial Note 3, “Held for Sale,” for more information on this transaction which closed in the third quarter of 2021.
The Company’s reported income tax expense rate for 2019 was unfavorably impacted by non-cash charges of $1.8 billion to impair the carrying value of goodwill for its International segment, given that these charges are generally not deductible for tax purposes. Refer to Financial Note 12, “Goodwill and Intangible Assets, Net,” for more information.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Deferred tax balances consisted of the following:
| | | | | | | | | | | |
| March 31, |
| (In millions) | 2021 | | 2020 |
| Assets | | | |
| Receivable allowances | $ | 69 | | | $ | 72 | |
| Opioid-related litigation and claims | 724 | | | — | |
| Compensation and benefit related accruals | 305 | | | 331 | |
| Net operating loss and credit carryforwards | 974 | | | 828 | |
| Lease obligations | 539 | | | 482 | |
| Other | 115 | | | 109 | |
Subtotal | 2,726 | | | 1,822 | |
| Less: valuation allowance | (864) | | | (833) | |
Total assets | 1,862 | | | 989 | |
| Liabilities | | | |
| Inventory valuation and other assets | (1,939) | | | (1,947) | |
| Fixed assets and systems development costs | (196) | | | (202) | |
| Intangibles | (411) | | | (531) | |
| | | |
| Lease right-of-use assets | (505) | | | (449) | |
| Other | (37) | | | (56) | |
Total liabilities | (3,088) | | | (3,185) | |
Net deferred tax liability | $ | (1,226) | | | $ | (2,196) | |
| | | |
| Long-term deferred tax asset | $ | 185 | | | $ | 59 | |
| Long-term deferred tax liability | (1,411) | | | (2,255) | |
Net deferred tax liability | $ | (1,226) | | | $ | (2,196) | |
The Company assesses the available positive and negative evidence to determine whether deferred tax assets are more likely than not to be realized. As a result of this assessment, valuation allowances have been recorded on certain deferred tax assets in various tax jurisdictions. The valuation allowances were approximately $864 million and $833 million in 2021 and 2020, respectively, and primarily relate to net operating and capital losses incurred in certain tax jurisdictions for which no tax benefit was recognized. The increase in the valuation allowance of $31 million in the current year relates primarily to net operating losses incurred and deferred tax movements in certain tax jurisdictions for which no tax benefit was recognized.
The Company has federal, state, and foreign net operating loss carryforwards of $2.4 billion, $3.9 billion, and $2.2 billion at March 31, 2021. Federal and state net operating losses will expire at various dates from 2022 through 2041. Substantially all its foreign net operating losses have indefinite lives. In addition, the Company has foreign capital loss carryforwards of $783 million with indefinite lives.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The following table summarizes the activity related to the Company’s gross unrecognized tax benefits for the last three years:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
| Unrecognized tax benefits at beginning of period | $ | 958 | | | $ | 1,052 | | | $ | 1,183 | |
| Additions based on tax positions related to prior years | 53 | | | 20 | | | 78 | |
| Reductions based on tax positions related to prior years | (5) | | | (168) | | | (234) | |
| Additions based on tax positions related to current year | 755 | | | 82 | | | 68 | |
| Reductions based on settlements | (8) | | | (8) | | | (13) | |
| Reductions based on the lapse of the applicable statutes of limitations | (12) | | | (13) | | | (25) | |
| Exchange rate fluctuations | 13 | | | (7) | | | (5) | |
| Unrecognized tax benefits at end of period | $ | 1,754 | | | $ | 958 | | | $ | 1,052 | |
As of March 31, 2021, the Company had $1.8 billion of unrecognized tax benefits, of which $1.3 billion would reduce income tax expense and the effective tax rate, if recognized. The increase in unrecognized tax benefits in 2021 compared to 2020 is primarily attributable to uncertainty in connection with the deductibility of Opioid-related litigation and claims. Because many uncertainties associated with any potential settlement arrangements or other resolutions of opioid claims including provisions related to deductibility have not been finalized, the actual amount of the tax benefit related to uncertain tax positions may differ from these estimates. Refer to Financial Note 19, “Commitments and Contingent Liabilities,” for more information. The decrease in unrecognized tax benefits in 2020 compared to 2019 is primarily attributable to the favorable resolution of an outstanding California tax refund claim which decreased unrecognized tax benefits by $91 million.
During the next twelve months, it is reasonably possible that the Company’s unrecognized tax benefit may decrease by as much as $93 million due to settlements of tax examinations and statute of limitations expirations in the U.S. federal and state jurisdictions and in foreign jurisdictions. However, this amount may change as the Company continues to have ongoing negotiations with various taxing authorities throughout the year.
The Company reports interest and penalties on income taxes as income tax expense. It recognized income tax expense of $9 million, $23 million, and $33 million in 2021, 2020, and 2019, respectively, representing interest and penalties, in its Consolidated Statements of Operations. As of March 31, 2021 and 2020, it accrued $101 million and $91 million cumulatively in interest and penalties on unrecognized tax benefits.
The Company files income tax returns in the U.S. federal jurisdiction, various U.S. state jurisdictions, and various foreign jurisdictions. The Internal Revenue Service (“IRS”) is currently examining the Company’s U.S. corporation income tax returns for 2018 and 2019. The Company is generally subject to audit by taxing authorities in various U.S. states and in foreign jurisdictions for fiscal years 2013 through the current fiscal year.
Undistributed earnings of the Company’s foreign operations of approximately $6.0 billion were considered indefinitely reinvested. Following enactment of the 2017 Tax Act, the repatriation of cash to the U.S. is generally no longer taxable for federal income tax purposes. However, the repatriation of cash held outside the U.S. could be subject to applicable foreign withholding taxes and state income taxes. The Company may remit foreign earnings to the U.S. to the extent it is tax efficient to do so. It does not expect the tax impact from remitting these earnings to be material.
9. Redeemable Noncontrolling Interests and Noncontrolling Interests
Redeemable Noncontrolling Interests
The Company’s redeemable noncontrolling interests primarily relate to its consolidated subsidiary, McKesson Europe. Under the December 2014 domination and profit and loss transfer agreement (the “Domination Agreement”), the noncontrolling shareholders of McKesson Europe are entitled to receive an annual recurring compensation amount of €0.83 per share. As a result, during 2021, 2020, and 2019, the Company recorded a total attribution of net income to the noncontrolling shareholders of McKesson Europe of $43 million, $42 million, and $45 million, respectively. All amounts were recorded in “Net income attributable to noncontrolling interests” in the Company’s Consolidated Statements of Operations and the corresponding liability balance was recorded in “Other accrued liabilities” in the Company’s Consolidated Balance Sheets.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Under the Domination Agreement, the noncontrolling shareholders of McKesson Europe have a right to put (“Put Right”) their noncontrolling shares at €22.99 per share, increased annually for interest in the amount of five percentage points above a base rate published by the German Bundesbank semi-annually, less any compensation amount or guaranteed dividend already paid by McKesson with respect to the relevant time period (“Put Amount”). The exercise of the Put Right will reduce the balance of redeemable noncontrolling interests. During 2021, the Company paid $49 million to purchase 1.8 million shares of McKesson Europe through exercises of the Put Right by the noncontrolling shareholders. This decreased the carrying value of the noncontrolling interests by $49 million, and the associated effect of the increase in the Company’s ownership interest on its equity of $3 million was recorded as a net increase to McKesson’s stockholders paid-in capital. During 2020 and 2019, there were no material exercises of the Put Right. The balance of the associated liability for Redeemable noncontrolling interests is reported as the greater of its carrying value or its maximum redemption value at each reporting date. The redemption value is the Put Amount adjusted for exchange rate fluctuations each period. The Redeemable noncontrolling interest is also adjusted each period for the proportion of other comprehensive income, primarily due to changes in foreign currency exchange rates, attributable to the noncontrolling shareholders. At March 31, 2021 and 2020, the carrying value of redeemable noncontrolling interests of $1.3 billion and $1.4 billion, respectively, exceeded the maximum redemption value of $1.2 billion and $1.2 billion, respectively. At March 31, 2021 and 2020, the Company owned approximately 78% and 77%, respectively, of McKesson Europe’s outstanding common shares.
Appraisal Proceedings
Subsequent to the Domination Agreement’s registration, certain noncontrolling shareholders of McKesson Europe initiated appraisal proceedings (“Appraisal Proceedings”) with the Stuttgart Regional Court (the “Court”) to challenge the adequacy of the Put Amount, annual recurring compensation amount, and/or the guaranteed dividend. During the pendency of the Appraisal Proceedings, such amount was paid as specified currently in the Domination Agreement. On September 19, 2018, the Court ruled that the Put Amount shall be increased by €0.51 resulting in an adjusted Put Amount of €23.50. The annual recurring compensation amount and/or the guaranteed dividend remained unadjusted. Noncontrolling shareholders of McKesson Europe appealed this decision. McKesson Europe Holdings GmbH & Co. KGaA also appealed the decision. On April 12, 2021, the Company received notice that the Stuttgart Court of Appeals ruled that the Put Amount shall remain €22.99, thereby rejecting the lower court’s increase, and the recurring compensation shall remain €0.83 per share.
Noncontrolling Interests
Noncontrolling interests represent third-party equity interests in the Company’s consolidated entities primarily related to ClarusONE and Vantage, which were $196 million and $217 million at March 31, 2021 and 2020, respectively, in the Company’s Consolidated Balance Sheets. During 2021, 2020, and 2019, respectively, the Company allocated a total of $156 million, $178 million, and $176 million of net income to noncontrolling interests.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Changes in redeemable noncontrolling interests and noncontrolling interests for the years ended March 31, 2021, 2020, and 2019 were as follows:
| | | | | | | | | | | | |
| (In millions) | |
Noncontrolling
Interests | | Redeemable
Noncontrolling
Interests |
| Balance, March 31, 2018 | | $ | 253 | | | $ | 1,459 | |
| Net income attributable to noncontrolling interests | | 176 | | | 45 | |
| Other comprehensive loss | | — | | | (66) | |
| Reclassification of recurring compensation to other accrued liabilities | | — | | | (45) | |
| Payments to noncontrolling interests | | (184) | | | — | |
| Other | | (52) | | | — | |
| Balance, March 31, 2019 | | 193 | | | 1,393 | |
| Net income attributable to noncontrolling interests | | 178 | | | 42 | |
| Other comprehensive income | | — | | | 3 | |
| Reclassification of recurring compensation to other accrued liabilities | | — | | | (42) | |
| Payments to noncontrolling interests | | (154) | | | — | |
| Other | | — | | | 6 | |
| Balance, March 31, 2020 | | 217 | | | 1,402 | |
| Net income attributable to noncontrolling interests | | 156 | | | 43 | |
| Other comprehensive loss | | — | | | (79) | |
| Reclassification of recurring compensation to other accrued liabilities | | — | | | (43) | |
| Payments to noncontrolling interests | | (177) | | | — | |
| Exercises of put right | | — | | | (49) | |
| Other | | — | | | (3) | |
| Balance, March 31, 2021 | | $ | 196 | | | $ | 1,271 | |
10. Earnings per Common Share
Basic earnings (loss) per common share is computed by dividing net income (loss) by the weighted-average number of common shares outstanding during the reporting period. The computation of diluted earnings (loss) per common share is similar to that of basic earnings (loss) per common share, except that the former reflects the potential dilution that could occur if dilutive securities or other obligations to issue common stock were exercised or converted into common stock.
Diluted loss per common share for the year ended March 31, 2021 was calculated by excluding potentially dilutive securities from the denominator of the share computation due to their anti-dilutive effects. Potentially dilutive securities include outstanding stock options, restricted stock units, and performance-based and other restricted stock units. Approximately 2 million and 3 million of potentially dilutive securities for 2020 and 2019, respectively, were excluded from the computations of diluted net earnings per common share, as they were anti-dilutive.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The computations for basic and diluted earnings or loss per common share are as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions, except per share amounts) | 2021 | | 2020 | | 2019 |
| Income (loss) from continuing operations | $ | (4,339) | | | $ | 1,126 | | | $ | 254 | |
| Net income attributable to noncontrolling interests | (199) | | | (220) | | | (221) | |
| Income (loss) from continuing operations attributable to McKesson | (4,538) | | | 906 | | | 33 | |
| Income (loss) from discontinued operations, net of tax | (1) | | | (6) | | | 1 | |
| Net income (loss) attributable to McKesson | $ | (4,539) | | | $ | 900 | | | $ | 34 | |
| | | | | |
| Weighted-average common shares outstanding: | | | | | |
| Basic | 160.6 | | | 180.6 | | | 196.3 | |
| Effect of dilutive securities: | | | | | |
| | | | | |
| Restricted stock units | — | | | 1.0 | | | 1.0 | |
| Diluted | 160.6 | | | 181.6 | | | 197.3 | |
| | | | | |
Earnings (loss) per common share attributable to McKesson: (1) | | | | | |
Diluted | | | | | |
| Continuing operations | $ | (28.26) | | | $ | 4.99 | | | $ | 0.17 | |
| Discontinued operations | — | | | (0.04) | | | — | |
Total | $ | (28.26) | | | $ | 4.95 | | | $ | 0.17 | |
Basic | | | | | |
| Continuing operations | $ | (28.26) | | | $ | 5.01 | | | $ | 0.17 | |
| Discontinued operations | — | | | (0.03) | | | — | |
Total | $ | (28.26) | | | $ | 4.98 | | | $ | 0.17 | |
(1)Certain computations may reflect rounding adjustments.
11. Leases
In the first quarter of 2020, the Company adopted amended guidance for leases using the modified retrospective method. Upon adoption of this amended guidance, the Company recorded $2.2 billion of operating lease liabilities, $2.1 billion of operating lease ROU assets, and a cumulative-effect adjustment of $69 million to opening retained earnings as of April 1, 2019. The adjustment to opening retained earnings included impairment charges of $89 million, net of tax, to the ROU assets primarily related to previously impaired long-lived assets at the retail pharmacies in the Company’s U.K. and Canadian businesses, partially offset by derecognition of existing deferred gain on the Company’s sale-leaseback transaction related to its former corporate headquarters building. The Company also elected to adopt the transition package of practical expedients provided within the amended guidance which eliminated the requirements to reassess lease identification, lease classification, and initial direct costs for leases which commenced before April 1, 2019. The adoption of this guidance did not have a material impact on the Company’s consolidated statements of operations and cash flows.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Lessee
The Company leases facilities and equipment, primarily under operating leases. The Company recognizes lease expense on a straight-line basis over the term of the lease, taking into account, when applicable, lessor incentives for tenant improvements, periods where no rent payment is required, and escalations in rent payments over the term of the lease. As a practical expedient, the Company does not separate lease components from non-lease components such as common area maintenance, utilities, and repairs and maintenance. Remaining terms for facility leases generally range from one to 15 years, while remaining terms for equipment leases generally range from one to five years. Most real property leases contain renewal options (typically for five-year increments). Generally, the renewal option periods are not included within the lease term as the Company is not reasonably certain to exercise that right at lease commencement. The Company’s lease agreements do not contain any material residual value guarantees or material restrictive covenants.
Operating right-of-use (“ROU”) assets and operating lease liabilities are recognized at the lease commencement date. ROU assets represent the Company’s right to use an underlying asset for the lease term and operating lease liabilities represent its obligation to make lease payments arising from the lease. Operating lease liabilities are recognized based on the present value of the future lease payments over the lease term, discounted at the Company’s incremental borrowing rate as the implicit rate in the lease is not readily determinable for most of the Company’s leases. The Company estimates the discount rate as its incremental borrowing rate based on qualitative factors including Company specific credit rating, lease term, general economics, and the interest rate environment. For existing leases that commenced prior to the adoption of the amended leasing guidance, the Company determined the discount rate on April 1, 2019 using the full lease term. Operating lease liabilities are recorded in “Current portion of operating lease liabilities” and “Long-term operating lease liabilities,” and the corresponding lease assets are recorded in “Operating lease right-of-use assets” in the Company’s Consolidated Balance Sheets. Finance lease assets are included in “Property, plant, and equipment, net” and finance lease liabilities are included in “Current portion of long-term debt” and “Long-term debt” in the Company’s Consolidated Balance Sheets. As a practical expedient, short-term leases with an initial term of 12 months or less are excluded from the Consolidated Balance Sheets and charges from these leases are expensed as incurred.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Supplemental balance sheet information related to leases was as follows:
| | | | | | | | | | | | |
| | |
| March 31, | |
| (In millions, except lease term and discount rate) | 2021 | | 2020 | |
| Operating leases | | | | |
| Operating lease right-of-use assets | $ | 2,100 | | | $ | 1,886 | | |
| | | | |
| Current portion of operating lease liabilities | $ | 390 | | | $ | 354 | | |
| Long-term operating lease liabilities | 1,867 | | | 1,660 | | |
| Total operating lease liabilities | $ | 2,257 | | | $ | 2,014 | | |
| | | | |
| Finance leases | | | | |
| Property, plant and equipment, net | $ | 237 | | | $ | 180 | | |
| | | | |
| Current portion of long-term debt | $ | 22 | | | $ | 15 | | |
| Long-term debt | 206 | | | 151 | | |
| Total finance lease liabilities | $ | 228 | | | $ | 166 | | |
| | | | |
| Weighted-average remaining lease term (Years) | | | | |
| Operating leases | 7.8 | | 7.7 | |
| Finance leases | 10.1 | | 12.1 | |
| | | | |
| Weighted-average discount rate | | | | |
| Operating leases | 2.53 | % | | 3.03 | % | |
| Finance leases | 2.71 | % | | 2.86 | % | |
The components of lease cost were as follows:
| | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 |
| Short-term lease cost | $ | 32 | | | $ | 29 | |
| Operating lease cost | 465 | | | 459 | |
| | | |
| Finance lease cost: | | | |
| Amortization of right-of-use assets | 23 | | | 14 | |
| Interest on lease liabilities | 6 | | | 5 | |
| Total finance lease cost | 29 | | | 19 | |
| | | |
Variable lease cost (1) | 125 | | | 125 | |
| Sublease income | (36) | | | (33) | |
Total lease cost (2) | $ | 615 | | | $ | 599 | |
| | | |
| | | |
(1) These amounts include payments for maintenance, taxes, payments affected by the consumer price index, and other similar metrics and payments contingent on usage.
(2) These amounts were primarily recorded in “Selling, distribution, general, and administrative expenses” in the Consolidated Statements of Operations.
Rent expense under operating leases was $576 million in 2019.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Supplemental cash flow information related to leases was as follows: | | | | | | | | | | | | |
| Years Ended March 31, | |
| (In millions) | 2021 | | 2020 | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | |
| Operating cash flows from operating leases | $ | (362) | | | $ | (377) | | |
| Operating cash flows from finance leases | (4) | | | (3) | | |
| Financing cash flows from finance leases | (31) | | | (18) | | |
| Right-of-use assets obtained in exchange for lease obligations: | | | | |
Operating leases (1) | $ | 321 | | | $ | 2,378 | | |
| Finance leases | 75 | | | 166 | | |
| | | | |
| | | | |
(1) The amount for the year ended March 31, 2020 includes the transition adjustment of $2.1 billion for operating lease right-of-use assets recorded as of April 1, 2019 upon adoption of the amended leasing guidance included in ASU 2016-02, Leases.
Maturities of lease liabilities as of March 31, 2021 were as follows:
| | | | | | | | | | | | | | | | | |
| | | | | |
| (In millions) | Operating Leases | | Finance Leases | | Total |
| 2022 | $ | 433 | | | $ | 28 | | | $ | 461 | |
| 2023 | 401 | | | 28 | | | 429 | |
| 2024 | 332 | | | 27 | | | 359 | |
| 2025 | 283 | | | 25 | | | 308 | |
| 2026 | 233 | | | 24 | | | 257 | |
| Thereafter | 823 | | | 130 | | | 953 | |
Total lease payments (1) | 2,505 | | | 262 | | | 2,767 | |
| Less imputed interest | (248) | | | (34) | | | (282) | |
| Present value of lease liabilities | $ | 2,257 | | | $ | 228 | | | $ | 2,485 | |
(1)Total lease payments are not reduced by minimum sublease income of $202 million which are due under future noncancellable subleases.
As of March 31, 2021, the Company entered into additional leases primarily for facilities that have not yet commenced with future lease payments of $217 million that are not reflected in the table above. These operating leases will commence between 2022 and 2024 with noncancellable lease terms of five to 15 years.
Lessor
The Company primarily leases certain owned equipment, that are classified as direct financing or sales-type leases, to physician practices. As of March 31, 2021 and 2020, the total lease receivable was $298 million and $272 million, respectively, with a weighted-average remaining lease term of approximately seven years. Interest income from these leases was not material for the years ended March 31, 2021 and 2020.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
12. Goodwill and Intangible Assets, Net
Goodwill
Changes in the carrying amount of goodwill were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In millions) | U.S. Pharmaceutical | | International | | Medical-Surgical Solutions | | Prescription Technology Solutions | | Total |
| Balance, March 31, 2019 | $ | 3,935 | | | $ | 1,446 | | | $ | 2,451 | | | $ | 1,526 | | | $ | 9,358 | |
| Goodwill acquired | — | | | 62 | | | — | | | 14 | | | 76 | |
| Acquisition accounting, transfers and other adjustments | 1 | | | 4 | | | 7 | | | — | | | 12 | |
| Other changes/disposals | (1) | | | — | | | (5) | | | — | | | (6) | |
| Impairment charges | — | | | (2) | | | — | | | — | | | (2) | |
| Foreign currency translation adjustments, net | (11) | | | (67) | | | — | | | — | | | (78) | |
| Balance, March 31, 2020 | 3,924 | | | 1,443 | | | 2,453 | | | 1,540 | | | 9,360 | |
| Goodwill acquired | — | | | 5 | | | — | | | — | | | 5 | |
| Acquisition accounting, transfers and other adjustments | — | | | — | | | — | | | 2 | | | 2 | |
| Other changes/disposals | (1) | | | — | | | — | | | — | | | (1) | |
| Impairment charges | — | | | (69) | | | — | | | — | | | (69) | |
| Foreign currency translation adjustments, net | 40 | | | 156 | | | — | | | — | | | 196 | |
| Balance, March 31, 2021 | $ | 3,963 | | | $ | 1,535 | | | $ | 2,453 | | | $ | 1,542 | | | $ | 9,493 | |
Goodwill Impairment Charges
The Company evaluates goodwill for impairment on an annual basis each year and at an interim date, if indicators of potential impairment exist. On October 1, 2019, the Company voluntarily changed its annual goodwill impairment testing date from January 1 to October 1 to better align with the timing of the Company’s annual long-term planning process. Accordingly, management determined that the change in accounting principle is preferable under the circumstance. This change has been applied prospectively from October 1, 2019 as retrospective application is deemed impracticable due to the inability to objectively determine the assumptions and significant estimates used in earlier periods without the benefit of hindsight. This change was not material to the Company’s consolidated financial statements as it did not delay, accelerate, or avoid any potential goodwill impairment charge.
Goodwill impairment testing is conducted at the reporting unit level, which is generally defined as an operating segment or one level below an operating segment (also known as a component), for which discrete financial information is available and segment management regularly reviews the operating results of that reporting unit.
The fair value of the reporting units was determined using a combination of an income approach based on a DCF model and a market approach based on appropriate valuation multiples observed for the reporting unit’s guideline public companies. Fair value estimates result from a complex series of judgments about future events and uncertainties and rely heavily on estimates and assumptions that have been deemed reasonable by management as of the measurement date. Any material changes in key assumptions, including failure to improve operations of certain retail pharmacy stores, additional government reimbursement reductions, deterioration in the financial markets, an increase in interest rates or an increase in the cost of equity financing by market participants within the industry, or other unanticipated events and circumstances, may affect such estimates. The discount rates are the weighted-average cost of capital measuring the reporting unit’s cost of debt and equity financing weighted by the percentage of debt and percentage of equity in a company’s target capital. The unsystematic risk premium is an input factor used in calculating the discount rate that specifically addresses uncertainty related to the reporting unit’s future cash flow projections. Fair value assessments of the reporting unit are considered a Level 3 measurement due to the significance of unobservable inputs developed using company-specific information.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Goodwill charges listed below were recorded in “Goodwill impairment charges” in the Consolidated Statements of Operations. Most of the goodwill impairment for these reporting units were generally not deductible for income tax purposes.
Fiscal 2021
In the second quarter of 2021, the Company implemented a new segment reporting structure which resulted in four reportable segments: U.S. Pharmaceutical, International, Medical-Surgical Solutions, and RxTS. These reportable segments encompass all operating segments of the Company. This segment change prompted changes in multiple reporting units across the Company. As a result, goodwill included in impacted reporting units was reallocated using a relative fair value approach and assessed for impairment both before and after the reallocation.
The Company recorded a goodwill impairment charge of $69 million in 2021 as the estimated fair value of the Europe Retail Pharmacy reporting unit was lower than its reassigned carrying value based on changes in the composition of the Europe Retail Pharmacy reporting unit within the International segment. At March 31, 2021, the balance of goodwill for the reporting units in Europe was approximately nil and the remaining balance of goodwill in the International segment primarily relates to one of its reporting units in Canada.
The annual impairment testing performed for 2021 did not indicate any impairment of goodwill.
Fiscal 2020
The impairment testing performed in 2020 did not indicate any material impairment of goodwill.
Fiscal 2019
The impairment testing performed in 2019 resulted in the following impairment charges:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In millions, except rates) | | | | | | | | | |
| Quarter Ended | | Reporting Unit | | Segment (1) | | Discount Rate | | Terminal Growth Rate | | Goodwill Impairment (2) | |
| June 2018 | | Pharmaceutical Distribution | | International | | 8.0 | % | | 1.25 | % | | $ | 238 | | (3) |
| June 2018 | | Retail Pharmacy | | International | | 8.5 | % | | 1.25 | % | | 251 | | (4) |
| June 2018 | | Pharmaceutical Distribution | | International | | 8.0 | % | | 1.25 | % | | 81 | | (4) |
| March 2019 | | Retail Pharmacy | | International | | 10.0 | % | | 1.25 | % | | 465 | | (5) |
| March 2019 | | Pharmaceutical Distribution | | International | | 9.0 | % | | 1.25 | % | | 741 | | (5) |
| | | | Total | | | | | | $ | 1,776 | | |
(1)As described above, the Company implemented its new segment reporting structure in the second quarter of 2021 and its European Pharmaceutical Solutions segment and its Rexall Health business in Canada became part of the International segment. Amounts included herein were previously included within the former European Pharmaceutical Solutions segment.
(2)Represents pre-tax and after-tax amounts, except for an aggregate $20 million of tax charges related to the March 2019 Retail Pharmacy impairment. Total goodwill impairment for 2019 also included $21 million related to the Company’s Rexall Health business, within the International segment, recorded in the third quarter of 2019.
(3)Prior to implementing its new segment reporting structure in the first quarter of 2019, the Company’s European operations were considered a single reporting unit. Following the change in reportable segments, its European Pharmaceutical Solutions segment was divided into two distinct reporting units, Retail Pharmacy (“RP”), formerly Consumer Solutions, and Pharmaceutical Distribution (“PD”), formerly Pharmacy Solutions, for the purposes of goodwill impairment testing. This change required performance of a goodwill impairment test for these two new reporting units which resulted in a goodwill impairment charge as PD’s estimated fair value was lower than its reassigned carrying value.
(4)Both RP and PD projected a decline in the estimated future cash flows primarily triggered by U.K. government actions which were announced on June 29, 2018. An interim goodwill impairment test for these reporting units identified that their carrying values exceeded their estimated fair value and resulted in an impairment charge.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
(5)As a result of the annual goodwill impairment test, the carrying values of the PD and RP reporting units exceeded their estimated fair value which required the Company to record impairment charges for the reporting units. These additional impairments were primarily due to declines in the reporting units’ estimated future cash flows and the selection of higher discount rates. The declines in estimated future cash flows were primarily attributed to additional government reimbursement reductions and competitive pressures within the U.K. The risk of successfully achieving certain business initiatives was the primary factor in the use of a higher discount rate. As of March 31, 2019 the entire remaining goodwill balances of both reporting units were impaired.
Refer to Financial Note 17, “Fair Value Measurements,” for more information on these nonrecurring fair value measurements. As of March 31, 2021 and 2020, accumulated goodwill impairment losses in the Company’s International segment were $3.6 billion and $3.5 billion, respectively.
Intangible Assets
Information regarding intangible assets is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| March 31, 2021 | | March 31, 2020 |
| (Dollars in millions) | Weighted-
Average
Remaining
Amortization
Period
(Years) | | Gross
Carrying
Amount | | Accumulated
Amortization | | Net
Carrying
Amount | | Gross
Carrying
Amount | | Accumulated
Amortization | | Net
Carrying
Amount |
| Customer relationships | 12 | | $ | 3,739 | | | $ | (2,269) | | | $ | 1,470 | | | $ | 3,650 | | | $ | (1,950) | | | $ | 1,700 | |
| Service agreements | 10 | | 1,081 | | | (513) | | | 568 | | | 994 | | | (480) | | | 514 | |
| Pharmacy licenses | 23 | | 497 | | | (244) | | | 253 | | | 492 | | | (232) | | | 260 | |
| Trademarks and trade names | 12 | | 925 | | | (394) | | | 531 | | | 808 | | | (242) | | | 566 | |
| Technology | 4 | | 150 | | | (122) | | | 28 | | | 175 | | | (111) | | | 64 | |
| Other | 6 | | 254 | | | (226) | | | 28 | | | 273 | | | (221) | | | 52 | |
Total | | | $ | 6,646 | | | $ | (3,768) | | | $ | 2,878 | | | $ | 6,392 | | | $ | (3,236) | | | $ | 3,156 | |
Amortization expense of intangible assets was $422 million, $462 million, and $485 million for 2021, 2020, and 2019, respectively. Estimated annual amortization expense of intangible assets is as follows: $370 million, $270 million, $259 million, $253 million, and $220 million for 2022 through 2026, and $1.5 billion thereafter. All intangible assets were subject to amortization as of March 31, 2021 and 2020.
Refer to Financial Note 4, “Restructuring, Impairment, and Related Charges,” for more information on intangible asset impairment charges recorded in 2021, 2020, and 2019.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
13. Debt and Financing Activities
Long-term debt consisted of the following:
| | | | | | | | | | | |
| March 31, |
| (In millions) | 2021 | | 2020 |
U.S. Dollar notes (1) (2) | | | |
3.65% Notes due November 30, 2020 | $ | — | | | $ | 700 | |
4.75% Notes due March 1, 2021 | — | | | 323 | |
2.70% Notes due December 15, 2022 | 400 | | | 400 | |
2.85% Notes due March 15, 2023 | 400 | | | 400 | |
3.80% Notes due March 15, 2024 | 1,100 | | | 1,100 | |
0.90% Notes due December 3, 2025 | 500 | | | — | |
7.65% Debentures due March 1, 2027 | 167 | | | 167 | |
3.95% Notes due February 16, 2028 | 600 | | | 600 | |
4.75% Notes due May 30, 2029 | 400 | | | 400 | |
6.00% Notes due March 1, 2041 | 282 | | | 282 | |
4.88% Notes due March 15, 2044 | 411 | | | 411 | |
Foreign currency notes (1) (3) | | | |
0.63% Euro Notes due August 17, 2021 | 704 | | | 662 | |
1.50% Euro Notes due November 17, 2025 | 700 | | | 659 | |
1.63% Euro Notes due October 30, 2026 | 587 | | | 552 | |
3.13% Sterling Notes due February 17, 2029 | 627 | | | 557 | |
| | | |
| Lease and other obligations | 270 | | | 174 | |
Total debt | 7,148 | | | 7,387 | |
| Less: Current portion | 742 | | | 1,052 | |
Total long-term debt | $ | 6,406 | | | $ | 6,335 | |
(1)These notes are unsecured and unsubordinated obligations of the Company.
(2)Interest on these notes is payable semi-annually.
(3)Interest on these foreign currency notes is payable annually.
Long-Term Debt
The Company’s long-term debt includes both U.S. dollar and foreign currency-denominated borrowings. At March 31, 2021 and 2020, $7.1 billion and $7.4 billion, respectively, of total debt was outstanding, of which $742 million and $1.1 billion, respectively, was included in “Current portion of long-term debt” in the Company’s Consolidated Balance Sheets.
On December 3, 2020, the Company completed a public offering of 0.90% Notes due December 3, 2025 (the “2025 Notes”) in a principal amount of $500 million. Interest on the 2025 Notes is payable semi-annually on June 3rd and December 3rd of each year, commencing on June 3, 2021. Proceeds received from this note issuance, net of discounts and offering expenses, were $496 million.
During the year ended March 31, 2021, the Company retired its 3.65% $700 million total principal of notes due on November 30, 2020 upon maturity. On December 1, 2020, the Company redeemed its 4.75% $323 million total principal of notes due on March 1, 2021 prior to maturity. These notes were redeemed using cash on hand and the proceeds of the notes offering discussed above. In 2020, the Company repaid at maturity its €250 million Floating Rate Euro Notes due February 12, 2020. In 2019, the Company repaid at maturity its $1.1 billion 2.28% notes due March 15, 2019.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Each note, which constitutes a “Series”, is an unsecured and unsubordinated obligation of the Company and ranks equally with all of the Company’s existing and, from time-to-time, future unsecured and unsubordinated indebtedness outstanding. Each Series is governed by materially similar indentures and officers’ certificates. Upon required notice to holders of notes with fixed interest rates, the Company may redeem those notes at any time prior to maturity, in whole or in part, for cash at redemption prices. In the event of the occurrence of both (1) a change of control of the Company and (2) a downgrade of a Series below an investment grade rating by each of Fitch Inc., Moody’s Investors Service, Inc. and Standard & Poor’s Ratings Services within a specified period, an offer must be made to purchase that Series from the holders at a price equal to 101% of the then outstanding principal amount of that Series, plus accrued and unpaid interest to, but not including, the date of repurchase. The indenture and the related officers’ certificate for each Series, subject to the exceptions and in compliance with the conditions as applicable, specify that the Company may not consolidate, merge or sell all or substantially all of its assets, incur liens, or enter into sale-leaseback transactions exceeding specific terms, without the lenders’ consent. The indentures also contain customary events of default provisions.
Other Information
Scheduled principal payments of long-term debt are $742 million in 2022, $838 million in 2023, $1.1 billion in 2024, $34 million in 2025, $1.2 billion in 2026, and $3.2 billion thereafter.
Revolving Credit Facilities
In the second quarter of 2020, the Company entered into a Credit Agreement, dated as of September 25, 2019 (the “2020 Credit Facility”), that provides a syndicated $4.0 billion five-year senior unsecured credit facility with a $3.6 billion aggregate sublimit of availability in Canadian dollars, British pound sterling, and Euro. Borrowings under the 2020 Credit Facility bear interest based upon the London Interbank Offered Rate (“LIBOR”), Canadian Dealer Offered Rate for credit extensions denominated in Canadian dollars, a prime rate, or alternative overnight rates as applicable, plus agreed margins. The 2020 Credit Facility matures in September 2024 and had no borrowings during 2021 and 2020 and no amounts outstanding as of March 31, 2021 and 2020.
On March 31, 2021, the Company entered into Amendment No. 2 to the 2020 Credit Facility, which superseded Amendment No. 1, dated as of February 1, 2021. The 2020 Credit Facility, as amended, contains various customary investment grade covenants, including a financial covenant which obligates the Company to maintain a maximum Total Debt to Consolidated EBITDA ratio, as defined in the amended credit agreement. If the Company does not comply with these covenants, its ability to use the 2020 Credit Facility may be suspended and repayment of any outstanding balances under the 2020 Credit Facility may be required. At March 31, 2021, the Company was in compliance with all covenants. The remaining terms and conditions of the 2020 Credit Facility are substantially similar to those previously in place under the $3.5 billion five-year senior unsecured revolving credit facility (the “Global Facility”), which was scheduled to mature in October 2020. The Global Facility was terminated in connection with the execution of the 2020 Credit Facility in September 2019 and had no borrowings during the six months ended September 30, 2019.
The Company also maintains bilateral credit facilities primarily denominated in Euros with a committed amount of $8 million and an uncommitted amount of $152 million as of March 31, 2021. Borrowings and repayments were not material in 2021 and 2020 and amounts outstanding under these credit lines were not material as of March 31, 2021 and 2020.
Commercial Paper
The Company maintains a commercial paper program to support its working capital requirements and for other general corporate purposes. Under the program, the Company can issue up to $4.0 billion in outstanding commercial paper notes. During 2021 and 2020, it borrowed $6.3 billion and $21.4 billion, respectively, and repaid $6.3 billion and $21.4 billion, respectively, under the program. At March 31, 2021 and 2020, there were no commercial paper notes outstanding.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
14. Variable Interest Entities
The Company evaluates its ownership, contractual, and other interests in entities to determine if they are VIEs, if it has a variable interest in those entities, and the nature and extent of those interests. These evaluations are highly complex and involve management judgment and the use of estimates and assumptions based on available historical information, among other factors. Based on its evaluations, if the Company determines it is the primary beneficiary of such VIEs, it consolidates such entities into its financial statements.
Consolidated Variable Interest Entities
The Company consolidates a VIE when it has the power to direct the activities that most significantly impact the VIE’s economic performance and the obligation to absorb losses or the right to receive benefits of the VIE and, as a result, is considered the primary beneficiary of the VIE. It consolidates certain single-lessee leasing entities where it, as the lessee, has the majority risk of the leased assets due to its minimum lease payment obligations to these leasing entities. As a result of absorbing this risk, the leases provide the Company with the power to direct the operations of the leased properties and the obligation to absorb losses or the right to receive benefits of the entity. Consolidated VIEs do not have a material impact on the Company’s Consolidated Statements of Operations and Cash Flows. Total assets and liabilities included in its Consolidated Balance Sheets for these VIEs were $662 million and $74 million, respectively, at March 31, 2021 and $695 million and $82 million, respectively, at March 31, 2020.
Investments in Unconsolidated Variable Interest Entities
The Company is involved with VIEs which it does not consolidate because it does not have the power to direct the activities that most significantly impact their economic performance and thus is not considered the primary beneficiary of the entities. Its relationships include equity method investments and lending, leasing, contractual or other relationships with the VIEs. The Company’s most significant relationships are with oncology and other specialty practices. Under these practice arrangements, it generally owns or leases all of the real estate and equipment used by the affiliated practices and manages the practices’ administrative functions. It also has relationships with certain pharmacies in Europe with whom it may provide financing, have equity ownership, and/or a supply agreement whereby it supplies the vast majority of the pharmacies’ purchases. The Company’s maximum exposure to loss (regardless of probability) as a result of all unconsolidated VIEs was $1.5 billion at March 31, 2021 and $1.4 billion at March 31, 2020, which primarily represents the value of intangible assets related to service agreements, equity investments, and lease and loan receivables. This amount excludes the customer loan guarantees discussed in Financial Note 18, “Financial Guarantees and Warranties.” The Company believes there is no material loss exposure on these assets or from these relationships.
15. Pension Benefits
The Company maintains a number of qualified and nonqualified defined benefit pension plans and defined contribution plans for eligible employees.
Defined Benefit Pension Plans
The Company has an unfunded nonqualified supplemental defined benefit plan for certain U.S. executives, as well as benefit pension plans for eligible employees outside the U.S.
On May 23, 2018, the Company’s Board of Directors approved the termination of its frozen U.S. defined benefit pension plan (“Plan”). During the first quarter of 2020, the Company offered the option of receiving a lump sum payment to certain participants with vested qualified Plan benefits in lieu of receiving monthly annuity payments. Approximately 1,300 participants elected to receive the settlement, and lump sum payments of approximately $49 million were made from Plan assets to these participants in June 2019. The benefit obligation settled approximated payments to Plan participants and a settlement charge of $17 million was recorded during the first quarter of 2020. During the second quarter of 2020, the Company transferred the remainder of the Plan’s pension obligation to a third-party insurance provider by purchasing annuity contracts for approximately $280 million which was fully funded directly by Plan assets. The third-party insurance provider assumed the obligation to pay future pension benefits and provide administrative services on November 1, 2019 and a pre-tax settlement charge of $105 million was recorded during the second quarter of 2020. Settlement charges were included within “Other income, net,” in the Consolidated Statements of Operations for the year ended March 31, 2020. As of March 31, 2020, this defined benefit pension plan had an accumulated comprehensive loss of approximately nil.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
During the third quarter of 2020, a cash payment of $114 million was made to settle a participant’s liability from the executive benefit retirement plan. As a result, a majority of the remaining recorded unrecognized losses in accumulated other comprehensive loss for this Plan were recognized as expense and a settlement charge of approximately $11 million was recorded in “Other income, net”, in the Consolidated Statements of Operations. As of March 31, 2020 and 2019, this plan had an accumulated comprehensive loss of approximately $1 million and $12 million, respectively.
The Company’s non-U.S. defined benefit pension plans cover eligible employees located predominantly in Norway, the United Kingdom, Germany, and Canada. Benefits for these plans are based primarily on each employee’s final salary, with annual adjustments for inflation. The obligations in Norway are largely related to the state-regulated pension plan which is managed by the Norwegian Public Service Pension Fund (“SPK”). According to the terms of the SPK, the plan assets of state regulated plans in Norway must correspond very closely to the pension obligation calculated using the principles codified in Norwegian law. In the U.K., the Company has subsidiaries that participate in a joint pension plan. The pension obligation in Germany is unfunded with the exception of the contractual trust arrangement used to fund pensions of McKesson Europe’s Management Board.
During the third quarter of 2021, the Company derecognized $187 million of pension liabilities included in liabilities held for sale and $33 million of accumulated other comprehensive loss related to its German pharmaceutical wholesale business contributed to a joint venture, as discussed in more detail in Financial Note 3, “Held for Sale.”
Defined benefit plan assets and obligations are measured as of the Company’s fiscal year-end. The net periodic expense for the Company’s pension plans is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Plans | | Non-U.S. Plans |
| Years Ended March 31, | | Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 | | 2021 | | 2020 | | 2019 |
| Service cost - benefits earned during the year | $ | — | | | $ | — | | | $ | — | | | $ | 15 | | | $ | 16 | | | $ | 15 | |
| Interest cost on projected benefit obligation | — | | | 6 | | | 14 | | | 19 | | | 19 | | | 21 | |
| Expected return on assets | — | | | (4) | | | (16) | | | (20) | | | (22) | | | (23) | |
Amortization of unrecognized actuarial loss and prior service costs | — | | | 2 | | | 5 | | | 5 | | | 6 | | | 4 | |
| Curtailment/settlement loss | — | | | 127 | | | 4 | | | — | | | — | | | 1 | |
| | | | | | | | | | | |
Net periodic pension expense | $ | — | | | $ | 131 | | | $ | 7 | | | $ | 19 | | | $ | 19 | | | $ | 18 | |
The projected unit credit method is utilized in measuring net periodic pension expense over the employees’ service life for the pension plans. Unrecognized actuarial losses exceeding 10% of the greater of the projected benefit obligation or the market value of assets are amortized straight-line over the average remaining future service period of active employees.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Information regarding the changes in benefit obligations and plan assets for the Company’s pension plans is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Plans | | Non-U.S. Plans |
| Years Ended March 31, | | Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2021 | | 2020 |
| Change in benefit obligations | | | | | | | |
Benefit obligation at beginning of period (1) | $ | 10 | | | $ | 439 | | | $ | 896 | | | $ | 990 | |
| Service cost | — | | | — | | | 15 | | | 16 | |
| Interest cost | — | | | 6 | | | 19 | | | 19 | |
| Actuarial loss (gain) | — | | | 20 | | | 89 | | | (36) | |
| Benefits paid | (1) | | | (179) | | | (34) | | | (43) | |
| Annuity Premium Transfer | — | | | (276) | | | — | | | — | |
| | | | | | | |
Divestiture (2) | — | | | — | | | (187) | | | — | |
| Acquisitions | — | | | — | | | — | | | 2 | |
| Foreign exchange impact and other | — | | | — | | | 77 | | | (52) | |
Benefit obligation at end of period (1) | $ | 9 | | | $ | 10 | | | $ | 875 | | | $ | 896 | |
| | | | | | | |
| Change in plan assets | | | | | | | |
| Fair value of plan assets at beginning of period | $ | — | | | $ | 322 | | | $ | 594 | | | $ | 642 | |
| Actual return on plan assets | — | | | 27 | | | 87 | | | 3 | |
| Employer and participant contributions | 1 | | | 116 | | | 27 | | | 28 | |
| Benefits paid | (1) | | | (179) | | | (34) | | | (43) | |
| Annuity Premium Transfer | — | | | (276) | | | — | | | — | |
| | | | | | | |
| | | | | | | |
| Foreign exchange impact and other | — | | | (10) | | | 61 | | | (36) | |
Fair value of plan assets at end of period | $ | — | | | $ | — | | | $ | 735 | | | $ | 594 | |
| | | | | | | |
| Funded status at end of period | $ | (9) | | | $ | (10) | | | $ | (140) | | | $ | (302) | |
| | | | | | | |
| Amounts recognized on the balance sheet | | | | | | | |
| Assets | $ | — | | | $ | — | | | $ | 54 | | | $ | 49 | |
Current liabilities (2) | (1) | | | (1) | | | (9) | | | (162) | |
| Long-term liabilities | (8) | | | (9) | | | (185) | | | (189) | |
Total | $ | (9) | | | $ | (10) | | | $ | (140) | | | $ | (302) | |
(1)The benefit obligation is the projected benefit obligation.
(2)The divestiture relates to the contribution of the Company’s German pharmaceutical wholesale business to a joint venture in 2021 as discussed in more detail in Financial Note 3, “Held for Sale.” These amounts were included within current liabilities and totaled $151 million at March 31, 2020.
The actuarial loss of $89 million in 2021 was primarily attributable to:
•Discount rates ($32 million loss): The weighted average discount rate for Non-U.S. plans decreased from 2.03% as of March 31, 2020 to 1.89% as of March 31, 2021.
•Demographic and assumption changes ($57 million loss): This represents the difference between actual and estimated participant data and demographic factors, including items such as inflation assumption, compensation changes, mortality, and other changes including losses related to the divestiture in 2021.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The actuarial gain of $36 million in 2020 was primarily attributable to:
•Discount rates ($6 million loss): The weighted average discount rate for Non-U.S. plans decreased from 2.13% as of March 31, 2019 to 2.03% as of March 31, 2020.
•Demographic and assumption changes ($42 million gain): This represents the difference between actual and estimated participant data and demographic factors, including items such as inflation assumption, compensation changes mortality, and other changes. The difference between actual inflation and assumed inflation in our U.K. pension plans resulted in a gain of $23 million.
The following table provides the projected benefit obligation, accumulated benefit obligation, and fair value of plan assets for all the Company’s pension plans, including accumulated benefit obligation in excess of plan assets:
| | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Plans | | Non-U.S. Plans |
| March 31, | | March 31, |
| (In millions) | 2021 | | 2020 | | 2021 | | 2020 |
| Projected benefit obligation | $ | 9 | | | $ | 10 | | | $ | 875 | | | $ | 896 | |
| Accumulated benefit obligation | 9 | | | 10 | | | 847 | | | 856 | |
| Fair value of plan assets | — | | | — | | | 735 | | | 594 | |
Amounts recognized in accumulated other comprehensive income consist of:
| | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Plans | | Non-U.S. Plans |
| March 31, | | March 31, |
| (In millions) | 2021 | | 2020 | | 2021 | | 2020 |
| Net actuarial loss | $ | 1 | | | $ | 1 | | | $ | 120 | | | $ | 149 | |
| Prior service credit | — | | | — | | | (2) | | | (3) | |
Total | $ | 1 | | | $ | 1 | | | $ | 118 | | | $ | 146 | |
Other changes in accumulated other comprehensive income were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Plans | | Non-U.S. Plans |
| Years Ended March 31, | | Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 | | 2021 | | 2020 | | 2019 |
| Net actuarial loss (gain) | $ | — | | | $ | (3) | | | $ | 8 | | | $ | (9) | | | $ | (24) | | | $ | 42 | |
| | | | | | | | | | | |
| Amortization of: | | | | | | | | | | | |
Net actuarial loss | — | | | (129) | | | (9) | | | (35) | | | (6) | | | (5) | |
Prior service credit (cost) | — | | | — | | | — | | | 1 | | | — | | | — | |
| Foreign exchange impact and other | — | | | — | | | — | | | 15 | | | (6) | | | (12) | |
Total recognized in other comprehensive loss (income) | $ | — | | | $ | (132) | | | $ | (1) | | | $ | (28) | | | $ | (36) | | | $ | 25 | |
The Company recognized $33 million in actuarial losses for pension plans to stockholders’ equity in 2021 as a result of the contribution of the Company’s German pharmaceutical wholesale business to a joint venture as discussed in more detail in Financial Note 3, “Held for Sale.” The Company recognized $127 million in actuarial losses for the pension plans to stockholders’ equity in 2020 as a result of $116 million from the termination of the U.S. defined benefit pension plan and $11 million from the settlement from the executive benefit retirement plan for a retired executive.
Projected benefit obligations related to the Company’s unfunded U.S. plans were $9 million and $10 million at March 31, 2021 and 2020, respectively. Pension obligations for its unfunded plans are based on the recommendations of independent actuaries. Projected benefit obligations relating to the Company’s unfunded non-U.S. plans were $162 million and $298 million at March 31, 2021 and 2020, respectively. Funding obligations for its non-U.S. plans vary based on the laws of each non-U.S. jurisdiction.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Expected benefit payments for the Company’s pension plans are as follows: $43 million, $36 million, $37 million, $36 million and $38 million for 2022 to 2026 and $202 million for 2027 through 2031. Expected benefit payments are based on the same assumptions used to measure the benefit obligations and include estimated future employee service. Expected contributions to be made for the Company’s pension plans are $24 million for 2022.
Weighted-average assumptions used to estimate the net periodic pension expense and the actuarial present value of benefit obligations were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Plans | | Non-U.S. Plans |
| Years Ended March 31, | | Years Ended March 31, |
| 2021 | | 2020 | | 2019 | | 2021 | | 2020 | | 2019 |
| Net periodic pension expense | | | | | | | | | | | |
| Discount rates | 3.08% | | 3.66% | | 3.83% | | 1.89% | | 2.03% | | 2.35% |
| Rate of increase in compensation | N/A (1) | | N/A (1) | | N/A (1) | | 3.20 | | 2.93 | | 3.13 |
| Expected long-term rate of return on plan assets | N/A | | 4.00 | | 5.25 | | 2.56 | | 3.01 | | 3.71 |
| Benefit obligation | | | | | | | | | | | |
| Discount rates | 2.35% | | 3.08% | | 3.65% | | 1.89% | | 2.03% | | 2.13% |
| Rate of increase in compensation | N/A (1) | | N/A (1) | | N/A (1) | | 3.20 | | 2.93 | | 3.18 |
(1) This assumption is no longer needed in actuarial valuations as U.S. plans are frozen or have fixed benefits for the remaining active participants.
The Company’s defined benefit pension plan liabilities are valued using a discount rate based on a yield curve developed from a portfolio of high quality corporate bonds rated AA or better whose maturities are aligned with the expected benefit payments of its plans. For March 31, 2021, the Company’s U.S. defined benefit liabilities are valued using a weighted-average discount rate of 2.35%, which represents a decrease of 73 basis points from its 2020 weighted-average discount rate of 3.08%. The Company’s non-U.S. defined benefit pension plan liabilities are valued using a weighted-average discount rate of 1.89%, which represents a decrease of 14 basis points from its 2020 weighted-average discount rate of 2.03%.
Plan Assets
Investment Strategy: For non-U.S. plan assets, the investment strategies are subject to local regulations and the asset/liability profiles of the plans in each individual country. Plan assets of the non-U.S. plans are broadly invested in a manner appropriate to the nature and duration of the expected future retirement benefits payable under the plans. Plan assets are primarily invested in high-quality corporate and government bond funds and equity securities. Assets are properly diversified to avoid excessive reliance on any particular asset, issuer, or group of undertakings so as to avoid accumulations of risk in the portfolio as a whole.
The Company develops the expected long-term rate of return assumption based on the projected performance of the asset classes in which plan assets are invested. The target asset allocation was determined based on the liability and risk tolerance characteristics of the plans and at times may be adjusted to achieve overall investment objectives.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Fair Value Measurements: The fair value hierarchy has three levels based on the reliability of the inputs used to determine fair value. Level 1 refers to fair values determined based on unadjusted quoted prices in active markets for identical assets. Level 2 refers to fair values estimated using significant other observable inputs and Level 3 includes fair values estimated using significant unobservable inputs. The following tables represent the Company’s pension plan assets as of March 31, 2021 and 2020, using the fair value hierarchy by asset class:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Non-U.S. Plans |
| March 31, 2021 | | March 31, 2020 |
| (In millions) | Level 1 | | Level 2 | | Level 3 | | Total | | Level 1 | | Level 2 | | Level 3 | | Total |
| Cash and cash equivalents | $ | 5 | | | $ | — | | | $ | — | | | $ | 5 | | | $ | 13 | | | $ | — | | | $ | — | | | $ | 13 | |
| Equity securities: | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
Equity commingled funds | 64 | | | 117 | | | — | | | 181 | | | 53 | | | 75 | | | — | | | 128 | |
| Fixed income securities: | | | | | | | | | | | | | | | |
Government securities | 5 | | | 144 | | | — | | | 149 | | | 6 | | | 139 | | | — | | | 145 | |
Corporate bonds | 6 | | | 30 | | | — | | | 36 | | | 14 | | | 17 | | | — | | | 31 | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
Fixed income commingled funds | 51 | | | 222 | | | 1 | | | 274 | | | 107 | | | 101 | | | — | | | 208 | |
| Other: | | | | | | | | | | | | | | | |
| Real estate funds and Other | 31 | | | 4 | | | 3 | | | 38 | | | 22 | | | 2 | | | 3 | | | 27 | |
Total | $ | 162 | | | $ | 517 | | | $ | 4 | | | $ | 683 | | | $ | 215 | | | $ | 334 | | | $ | 3 | | | $ | 552 | |
| | | | | | | | | | | | | | | |
Assets held at NAV practical expedient (1) | | | | | | | | | | | | | | | |
| Equity commingled funds | | | | | | | 10 | | | | | | | | | 8 | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Other | | | | | | | 42 | | | | | | | | | 34 | |
| Total plan assets | | | | | | | $ | 735 | | | | | | | | | $ | 594 | |
(1) Equity commingled funds, fixed income commingled funds, real estate funds, and other investments for which fair value is measured using the NAV per share as a practical expedient are not leveled within the fair value hierarchy and are included as a reconciling item to total investments.
Cash and cash equivalents - Cash and cash equivalents include short-term investment funds that maintain daily liquidity and aim to have constant unit values of $1.00. The funds invest in short-term fixed income securities and other securities with debt-like characteristics emphasizing short-term maturities and high credit quality. Directly held cash and cash equivalents are classified as Level 1 investments. Cash and cash equivalents include money market funds and other commingled funds, which have daily net asset values derived from the underlying securities; these are classified as Level 1 investments.
Equity commingled funds - Some equity investments are held in commingled funds, which have daily net asset values derived from quoted prices for the underlying securities in active markets; these are classified as Level 1 or Level 2 investments.
Fixed income securities - Government securities consist of bonds and debentures issued by central governments or federal agencies; corporate bonds consist of bonds and debentures issued by corporations. Inputs to the valuation methodology include quoted prices for similar assets in active markets, and inputs that are observable for the asset, either directly or indirectly, for substantially the full term of the asset. Multiple prices and price types are obtained from pricing vendors whenever possible, enabling cross-provider price validations. Fixed income securities are generally classified as Level 1 or Level 2 investments.
Fixed income commingled funds - Some fixed income investments are held in exchange traded or commingled funds, which have daily net asset values derived from the underlying securities; these are classified as Level 1, 2, or 3 investments.
Real estate funds - The value of the real estate funds is reported by the fund manager and is based on a valuation of the underlying properties. Inputs used in the valuation include items such as cost, discounted future cash flows, independent appraisals, and market based comparable data. The real estate funds are classified as Level 1, 2, or 3 investments.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Other - At March 31, 2021 and 2020, this includes $36 million and $29 million, respectively, of plan asset value relating to the SPK. In principle, the SPK is organized as a pay-as-you-go system guaranteed by the Norwegian government as it holds no Company-owned assets to back the pension liabilities. The Company pays a pension premium used to fund the plan, which is paid directly to the Norwegian government who establishes an account for each participating employer to keep track of the financial status of the plan, including managing the contributions and the payments. Further, the investment return credited to this account is determined annually by the SPK based on the performance of long-term government bonds.
The activity attributable to Level 3 plan assets was not material for the years ended March 31, 2021 and 2020.
Multiemployer Plans
The Company contributes to a number of multiemployer pension plans under the terms of collective-bargaining agreements that cover union-represented employees in the U.S. In 2017, it also contributed to the Pensjonsordningen for Apoteketaten (“POA”), a mandatory multiemployer pension scheme for its pharmacy employees in Norway, managed by the association of Norwegian Pharmacies.
The risks of participating in these multiemployer plans are different from single-employer pension plans in the following aspects: (i) assets contributed to the multiemployer plan by one employer may be used to provide benefits to employees of other participating employers; (ii) if a participating employer stops contributing to the plan, the unfunded obligations of the plan may be borne by the remaining participating employers; and (iii) if the Company chooses to stop participating in some of its multiemployer plans, the Company may be required to pay those plans an amount based on the underfunded status of the plan, referred to as a withdrawal liability. Actions taken by other participating employers may lead to adverse changes in the financial condition of a multiemployer benefit plan and the Company’s withdrawal liability and contributions may increase.
Contributions and amounts accrued for U.S. Plans were not material for the years ended March 31, 2021, 2020, and 2019. Contributions to the POA for non-U.S. Plans exceeding 5% of total plan contributions were $22 million, $17 million, and $27 million in 2021, 2020, and 2019, respectively. Based on actuarial calculations, the Company estimates the funded status for its non-U.S. Plans to be approximately 78% as of March 31, 2021. No amounts were accrued for liability associated with the POA as the Company has no intention to withdraw from the plan.
Defined Contribution Plans
The Company has a contributory retirement savings plan (“RSP”) for U.S. eligible employees. Eligible employees may contribute to the RSP up to 75% of their eligible compensation on a pre-tax or post-tax basis not to exceed IRS limits. The Company makes matching contributions in an amount equal to 100% of the employee’s first 3% of pay contributed and 50% for the next 2% of pay contributed. The Company also may make an additional annual matching contribution for each plan year to enable participants to receive a full match based on their annual contribution. The Company also contributed to non-U.S. plans that are available in certain countries. Contribution expenses for the RSP and non-U.S. plans were $102 million, $102 million, and $92 million for the years ended March 31, 2021, 2020, and 2019, respectively.
Postretirement Benefits
The Company maintains a number of postretirement benefits, primarily consisting of healthcare and life insurance (“welfare”) benefits, for certain eligible U.S. employees. Eligible employees consist of those who retired before March 31, 1999 and those who retired after March 31, 1999, but were an active employee as of that date, after meeting other age-related criteria. It also provides postretirement benefits for certain U.S. executives. Defined benefit plan obligations are measured as of the Company’s fiscal year-end. The net periodic (credit) expense for the Company’s postretirement welfare benefits was not material for the years ended March 31, 2021, 2020, and 2019. The benefit obligation at March 31, 2021 and 2020 was $64 million and $65 million, respectively.
16. Hedging Activities
In the normal course of business, the Company is exposed to interest rate and foreign currency exchange rate fluctuations. At times, the Company limits these risks through the use of derivatives such as cross-currency swaps, foreign currency forward contracts, and interest rate swaps. In accordance with the Company’s policy, derivatives are only used for hedging purposes. It does not use derivatives for trading or speculative purposes.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Foreign currency exchange risk
The Company conducts its business worldwide in U.S. dollars and the functional currencies of its foreign subsidiaries, including Euro, British pound sterling, and Canadian dollars. Changes in foreign currency exchange rates could have a material adverse impact on the Company’s financial results that are reported in U.S. dollars. The Company is also exposed to foreign currency exchange rate risk related to its foreign subsidiaries, including intercompany loans denominated in non-functional currencies. The Company has certain foreign currency exchange rate risk programs that use foreign currency forward contracts and cross-currency swaps. These forward contracts and cross-currency swaps are generally used to offset the potential income statement effects from intercompany loans and other obligations denominated in non-functional currencies. These programs reduce but do not entirely eliminate foreign currency exchange rate risk.
Non-Derivative Instruments Designated as Hedges
At March 31, 2021 and 2020, the Company had €1.7 billion of Euro-denominated notes designated as non-derivative net investment hedges. These hedges are utilized to hedge portions of the Company’s net investments in non-U.S. subsidiaries against the effect of exchange rate fluctuations on the translation of foreign currency balances to the U.S. dollar. For all notes that are designated as net investment hedges and meet effectiveness requirements, the changes in carrying value of the notes attributable to the change in spot rates are recorded in foreign currency translation adjustments in “Accumulated other comprehensive loss” in the Consolidated Statements of Stockholders’ Equity where they offset foreign currency translation gains and losses recorded on the Company’s net investments. To the extent foreign currency-denominated notes designated as net investment hedges are ineffective, changes in carrying value attributable to the change in spot rates are recorded in earnings. In December 2019, the Company prospectively de-designated from net investment hedges €250 million of its Euro-denominated notes which matured in February 2020.
At March 31, 2019, the Company also had £450 million British pound sterling-denominated notes designated as non-derivative net investment hedges. On September 30, 2019, the Company de-designated its £450 million British pound sterling-denominated notes prospectively from net investment hedges as the hedging relationship ceased to be effective.
Gains or losses from net investment hedges recorded within Other comprehensive income were losses of $118 million in 2021 and gains of $39 million and $259 million in 2020 and 2019, respectively. Ineffectiveness on the Company’s non-derivative net investment hedges during 2020 resulted in gains of $34 million which were recorded in earnings in “Other income, net” in the Consolidated Statements of Operations. There was no ineffectiveness in the Company’s net investment hedges for the years ended March 31, 2021 and 2019.
Derivatives Designated as Hedges
At March 31, 2021 and 2020, the Company had cross-currency swaps designated as net investment hedges with a total gross notional amount of $500 million and $1.5 billion Canadian dollars, respectively. Under the terms of the cross-currency swap contracts, the Company agrees with third parties to exchange fixed interest payments in one currency for fixed interest payments in another currency at specified intervals and to exchange principal in one currency for principal in another currency, calculated by reference to agreed-upon notional amounts. These swaps are utilized to hedge portions of the Company’s net investments denominated in Canadian dollars against the effect of exchange rate fluctuations on the translation of foreign currency balances to the U.S. dollar. The changes in the fair value of these derivatives attributable to the changes in spot currency exchange rates and differences between spot and forward interest rates are recorded in “Accumulated other comprehensive loss” in the Consolidated Statements of Stockholders’ Equity where they offset foreign currency translation gains and losses recorded on the Company’s net investments denominated in Canadian dollars. To the extent cross-currency swaps designated as hedges are ineffective, changes in carrying value attributable to the change in spot rates are recorded in earnings. There was no ineffectiveness in the Company’s net investment hedges for the years ended March 31, 2021, 2020, and 2019. In 2021, cross-currency swaps with an aggregate gross notional amount of $999 million Canadian dollars matured and the remaining cross-currency swaps will mature in November 2024.
At March 31, 2019, the Company also had cross-currency swaps designated as net investment hedges with a total gross notional amount of £932 million British pound sterling. In 2020, the Company terminated these swaps due to ineffectiveness in its British pound sterling hedging program that arose due to 2019 impairments of goodwill and certain long-lived assets in the U.K. businesses. Proceeds from the termination of these swaps totaled $84 million and resulted in a settlement gain of $34 million in 2020. This gain was recorded in earnings in “Other income, net” in the Consolidated Statements of Operations.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Gains or losses from the Company’s cross-currency swaps designated as net investment hedges recorded in Other comprehensive income were losses of $119 million in 2021 and gains of $76 million and $53 million in 2020 and 2019, respectively. There was no ineffectiveness in the Company’s hedges for the years ended March 31, 2021 and 2019.
On September 30, 2019, the Company entered into a number of cross-currency swaps designated as fair value hedges with total notional amounts of £450 million British pound sterling. Under the terms of the cross-currency swap contracts, the Company agreed with third parties to exchange fixed interest payments in British pound sterling for floating interest payments in U.S. dollars based on three-month LIBOR plus a spread. These swaps are utilized to hedge the changes in the fair value of the underlying £450 million British pound sterling notes resulting from changes in benchmark interest rates and foreign exchange rates. The changes in the fair value of these derivatives, which are designated as fair value hedges, and the offsetting changes in the fair value of the hedged notes are recorded in earnings. Gains from these fair value hedges recorded in earnings were largely offset by the losses recorded in earnings related to these notes. The swaps will mature in February 2023.
From time to time, the Company also enters into cross-currency swaps to hedge intercompany loans denominated in non-functional currencies. For cross-currency swap transactions, the Company agrees with third parties to exchange fixed interest payments in one currency for fixed interest payments in another currency at specified intervals and to exchange principal in one currency for principal in another currency, calculated by reference to agreed-upon notional amounts. These cross-currency swaps are designed to reduce the income statement effects arising from fluctuations in foreign exchange rates and have been designated as cash flow hedges. At March 31, 2021 and 2020, the Company had cross-currency swaps with total gross notional amounts of approximately $2.6 billion and $2.9 billion, respectively, which are designated as cash flow hedges. These swaps will mature between May 2021 and January 2024.
For forward contracts and cross-currency swaps that are designated as cash flow hedges, the effective portion of changes in the fair value of the hedges is recorded in Accumulated other comprehensive loss and reclassified into earnings in the same period in which the hedged transaction affects earnings. Changes in fair values representing hedge ineffectiveness are recognized in current earnings.
On April 27, 2020, the Company entered into forward starting interest rate swaps designated as cash flow hedges, with combined notional amounts of $500 million and €600 million, to hedge the variability of future benchmark interest rates on planned bond issuances. Under the terms of the forward interest rate swap contracts, the Company agreed with third parties to pay fixed interest payments for the $500 million swaps for floating interest payments in U.S. dollars based on three-month LIBOR and to pay fixed interest payments for floating interest payments in Euros based on six-month Euro Interbank Offered Rate (“EURIBOR”) for the €600 million swaps. The $500 million swaps were terminated upon the issuance of the 2025 Notes in November 2020. The settlement loss on the swaps was not material and will be amortized on a straight-line basis as interest expense over the five-year life of the 2025 Notes. Refer to Financial Note 13, “Debt and Financing Activities,” for more information.
Gains or losses from cash flow hedges recorded in Other comprehensive income were losses of $42 million in 2021 and gains of $98 million and $28 million in 2020 and 2019, respectively. Gains or losses reclassified from Accumulated other comprehensive income and recorded in “Selling, distribution, general, and administrative expenses” in the Consolidated Statements of Operations were not material in 2021, 2020, and 2019. There was no ineffectiveness in the Company’s cash flow hedges for the years ended March 31, 2021, 2020, and 2019.
Derivatives Not Designated as Hedges
Derivative instruments not designated as hedges are marked-to-market at the end of each accounting period with the change in value included in earnings.
The Company has a number of forward contracts to hedge the Euro against cash flows denominated in British pound sterling and other European currencies. At March 31, 2021 and 2020, the total gross notional amounts of these contracts were $39 million and $29 million, respectively. These contracts will predominately mature between April 2021 and December 2021 and none of these contracts were designated for hedge accounting. Changes in the fair values for contracts not designated as hedges are recorded directly into earnings in “Selling, distribution, general, and administrative expenses” in the Consolidated Statements of Operations. Changes in the fair values were not material in 2021, 2020, and 2019. Gains or losses from these contracts are largely offset by changes in the value of the underlying intercompany obligations.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
In 2020, the Company also entered into a number of forward contracts and swaps to offset a portion of the earnings impacts from the ineffectiveness of the non-derivative net investment hedges discussed above. These contracts matured through January 2020 and none of these contracts were designated for hedge accounting. In December 2019, the Company entered into a series of forward contracts with a total notional amount of €250 million to offset the earnings impact from its Euro-denominated notes. These contracts and the notes against which they are offsetting matured in February 2020 and were not designated for hedge accounting. Changes in the fair value for contracts not designated as hedges are recorded directly in earnings. In 2020, losses of $44 million were recorded in earnings in “Other income, net” in the Consolidated Statements of Operations, which offset the ineffectiveness on the Company’s non-derivative net investment hedges noted above.
Information regarding the fair value of derivatives on a gross basis is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance Sheet
Caption | March 31, 2021 | | March 31, 2020 |
| Fair Value of
Derivative | U.S. Dollar Notional | | Fair Value of
Derivative | U.S. Dollar Notional |
| (In millions) | Asset | Liability | | Asset | Liability |
| Derivatives designated for hedge accounting | | | | | | | | |
| | | | | | | | |
Cross-currency swaps (current) | Prepaid expenses and other/Other accrued liabilities | $ | 4 | | $ | 47 | | $ | 826 | | | $ | 112 | | $ | 19 | | $ | 1,279 | |
| Cross-currency swaps (non-current) | Other non-current assets/liabilities | 72 | | 92 | | 2,663 | | | 182 | | — | | 3,313 | |
| Forward starting interest rate swaps (current) | Other accrued liabilities | — | | 7 | | 704 | | | — | | — | | — | |
| Total | | $ | 76 | | $ | 146 | | | | $ | 294 | | $ | 19 | | |
| Derivatives not designated for hedge accounting | | | | | | | | |
| Foreign exchange contracts (current) | Prepaid expenses and other | $ | — | | $ | — | | $ | 29 | | | $ | 2 | | $ | — | | $ | 24 | |
| Foreign exchange contracts (current) | Other accrued liabilities | — | | 1 | | 10 | | | — | | — | | 5 | |
| Total | | $ | — | | $ | 1 | | | | $ | 2 | | $ | — | | |
Refer to Financial Note 17, “Fair Value Measurements,” for more information on these recurring fair value measurements.
17. Fair Value Measurements
The Company measures certain assets and liabilities at fair value in accordance with Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurements and Disclosures. The fair value hierarchy consists of three levels of inputs that may be used to measure fair value as follows:
Level 1 - quoted prices in active markets for identical assets or liabilities.
Level 2 - significant other observable market-based inputs.
Level 3 - significant unobservable inputs for which little or no market data exists and requires considerable assumptions that are significant to the fair value measurement.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
Cash and cash equivalents at March 31, 2021 and 2020 included investments in money market funds of $1.6 billion and $2.0 billion, respectively, which are reported at fair value. The fair value of money market funds was determined using quoted prices for identical investments in active markets, which are considered to be Level 1 inputs under the fair value measurements and disclosure guidance. The carrying value of all other cash equivalents approximates their fair value due to their relatively short-term nature. Fair values for the Company’s marketable securities were not material at March 31, 2021 and 2020.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Fair values of the Company’s interest rate swaps and foreign currency forward contracts were determined using observable inputs from available market information, including quoted interest rates, foreign currency exchange rates and other observable inputs from available market information. These inputs are considered Level 2 under the fair value measurements and disclosure guidance, and may not be representative of actual values that could have been realized or that will be realized in the future. Refer to Financial Note 16, “Hedging Activities,” for fair value and other information on the Company’s derivatives including interest rate swaps, forward foreign currency contracts and cross-currency swaps.
The Company holds investments in equity securities of U.S. growth stage companies that address both current and emerging business challenges in the healthcare industry and which had carrying values of $269 million and $170 million at March 31, 2021 and 2020, respectively. These investments primarily consist of equity securities without readily determinable fair values and are included in “Other non-current assets” in the Consolidated Balance Sheets. During 2021, several of the Company’s investments in equity securities without readily determinable fair values experienced transactions which resulted in changes in the observable price of those securities, while others were converted into shares of public common stock through initial public offerings and an acquisition. The Company exited most of its investments in publicly traded shares in the fourth quarter of 2021. Net gains related to the Company’s investments in these equity securities were approximately $133 million for the year ended March 31, 2021. These gains were recorded in “Other income, net” in the Consolidated Statements of Operations. There were no other material changes in the carrying value of these investments during the year ended March 31, 2021. The carrying value of publicly traded investments was determined using quoted prices for identical investments in active markets and are considered to be Level 1 inputs.
Assets and Liabilities Measured at Fair Value on a Nonrecurring Basis
In addition to assets and liabilities that are measured at fair value on a recurring basis, the Company’s assets and liabilities are also subject to nonrecurring fair value measurements. Generally, assets are recorded at fair value on a nonrecurring basis as a result of impairment charges.
At March 31, 2021, assets measured at fair value on a nonrecurring basis included long-lived assets of the Company’s International segment and goodwill of the Company’s Europe Retail Pharmacy reporting unit within the International segment.
At March 31, 2020, assets measured at fair value on a nonrecurring basis included long-lived assets of the Company’s International segment. Refer to Financial Note 4, “Restructuring, Impairment, and Related Charges” and Financial Note 12, “Goodwill and Intangible Assets, Net,” for more information.
The aforementioned investments in equity securities of U.S. growth stage companies include the carrying value of investments without readily determinable fair values, which were determined using a measurement alternative and are recorded at cost less impairment, plus or minus any changes in observable price from orderly transactions of the same or similar security of the same issuer. These inputs are considered Level 2 under the fair value measurements and disclosure guidance and may not be representative of actual values that could have been realized or that will be realized in the future.
There were no liabilities measured at fair value on a nonrecurring basis at March 31, 2021 and 2020.
Other Fair Value Disclosures
At March 31, 2021 and 2020, the carrying amounts of cash, certain cash equivalents, restricted cash, marketable securities, receivables, drafts and accounts payable, short-term borrowings, and other current liabilities approximated their estimated fair values because of the short maturity of these financial instruments.
The Company determines the fair value of commercial paper using quoted prices in active markets for identical instruments, which are considered Level 1 inputs under the fair value measurements and disclosure guidance.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The Company’s long-term debt is also recorded at cost. The carrying value and fair value of the Company’s long-term debt was as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| March 31, 2021 | | March 31, 2020 | | |
| (In millions) | Carrying Value | | Fair Value | | Carrying Value | | Fair Value | | |
| Long-term debt, including current maturities | $ | 7,148 | | | $ | 7,785 | | | $ | 7,387 | | | $ | 7,792 | | | |
The estimated fair value of the Company’s long-term debt was determined using quoted market prices in a less active market and other observable inputs from available market information, which are considered to be Level 2 inputs, and may not be representative of actual values that could have been realized or that will be realized in the future.
Goodwill
Fair value assessments of the reporting unit and the reporting unit's net assets, which are performed for goodwill impairment tests, are considered a Level 3 measurement due to the significance of unobservable inputs developed using company-specific information. The Company considered a market approach as well as an income approach using a DCF model to determine the fair value of the reporting unit.
Refer to Financial Note 12, “Goodwill and Intangible Assets, Net,” for more information regarding goodwill impairment charges recorded for certain reporting units during 2021 and 2019.
Long-lived Assets
The Company utilizes multiple approaches including the DCF model and market approaches for estimating the fair value of intangible assets. The future cash flows used in the analysis are based on internal cash flow projections from its long-range plans and include significant assumptions by management. Accordingly, the fair value assessment of the long-lived assets is considered a Level 3 fair value measurement.
The Company measures certain long-lived and intangible assets at fair value on a nonrecurring basis when events occur that indicate an asset group may not be recoverable. If the carrying amount of an asset group is not recoverable, an impairment charge is recorded to reduce the carrying amount by the excess over its fair value. Refer to Financial Note 4, “Restructuring, Impairment, and Related Charges” under the heading “Long-Lived Assets Impairments” for more information.
18. Financial Guarantees and Warranties
Financial Guarantees
The Company has agreements with certain of its customers’ financial institutions, primarily in its International segment, under which it has guaranteed the repurchase of its customers’ inventory or its customers’ debt in the event these customers are unable to meet their obligations to those financial institutions. For the Company’s inventory repurchase agreements, among other requirements, inventories must be in resalable condition and any repurchase would be at a discount. The inventory repurchase agreements mostly relate to certain Canadian customers and generally range from one to two years. Customers’ debt guarantees generally range from one to ten years and are primarily provided to facilitate financing for certain customers. The majority of the Company’s customers’ debt guarantees are secured by certain assets of the customer. At March 31, 2021, the maximum amounts of inventory repurchase guarantees and customers’ debt guarantees were $329 million and $143 million, respectively, of which the Company has not accrued any material amounts. The expirations of these financial guarantees are as follows: $268 million, $26 million, $33 million, $11 million, and $15 million from 2022 through 2026 and $119 million thereafter.
At March 31, 2021, the Company’s banks and insurance companies have issued $146 million of standby letters of credit and surety bonds, which were issued on the Company’s behalf primarily related to its customer contracts and in order to meet the security requirements for statutory licenses and permits, court and fiduciary obligations, and its workers’ compensation and automotive liability programs.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The Company’s software license agreements generally include certain provisions for indemnifying customers against liabilities if its software products infringe a third party’s intellectual property rights. To date, the Company has not incurred any material costs as a result of such indemnification agreements and has not accrued any liabilities related to such obligations.
In conjunction with certain transactions, primarily divestitures, the Company may provide routine indemnification agreements (such as retention of previously existing environmental, tax, and employee liabilities) whose terms vary in duration and often are not explicitly defined. Where appropriate, obligations for such indemnifications are recorded as liabilities. Because the amounts of these indemnification obligations often are not explicitly stated, the overall maximum amount of these commitments cannot be reasonably estimated. Other than obligations recorded as liabilities at the time of divestiture, the Company has historically not made material payments as a result of these indemnification provisions.
Warranties
In the normal course of business, the Company provides certain warranties and indemnification protection for its products and services. For example, the Company provides warranties that the pharmaceutical and medical-surgical products it distributes are in compliance with the U.S. Food, Drug and Cosmetic Act and other applicable laws and regulations. It has received the same warranties from its suppliers, which customarily are the manufacturers of the products. In addition, the Company has indemnity obligations to its customers for these products, which have also been provided from its suppliers, either through express agreement or by operation of law. Accrued warranty costs were not material to the Consolidated Balance Sheets.
19. Commitments and Contingent Liabilities
In addition to commitments and obligations incurred in the ordinary course of business, the Company is subject to a variety of claims and legal proceedings, including claims from customers and vendors, pending and potential legal actions for damages, governmental investigations, and other matters. The Company and its affiliates are parties to the legal claims and proceedings described below. The Company is vigorously defending itself against those claims and in those proceedings. Significant developments in those matters are described below. If the Company is unsuccessful in defending, or if it determines to settle, any of these matters, it may be required to pay substantial sums, be subject to injunction and/or be forced to change how it operates its business, which could have a material adverse impact on its financial position or results of operations.
Unless otherwise stated, the Company is unable to reasonably estimate the loss or a range of possible loss for the matters described below. Often, the Company is unable to determine that a loss is probable, or to reasonably estimate the amount of loss or a range of loss, for a claim because of the limited information available and the potential effects of future events and decisions by third parties, such as courts and regulators, that will determine the ultimate resolution of the claim. Many of the matters described are at preliminary stages, raise novel theories of liability or seek an indeterminate amount of damages. It is not uncommon for claims to remain unresolved over many years. The Company reviews loss contingencies at least quarterly to determine whether the likelihood of loss has changed and whether it can make a reasonable estimate of the loss or range of loss. When the Company determines that a loss from a claim is probable and reasonably estimable, it records a liability for an estimated amount. The Company also provides disclosure when it is reasonably possible that a loss may be incurred or when it is reasonably possible that the amount of a loss will exceed its recorded liability.
I. Litigation and Claims Involving Distribution of Controlled Substances
The Company and its affiliates are defendants in many cases asserting claims related to distribution of controlled substances. They are named as defendants along with other pharmaceutical wholesale distributors, pharmaceutical manufacturers, and retail pharmacy chains. The plaintiffs in these actions include state attorneys general, county and municipal governments, tribal nations, hospitals, health and welfare funds, third-party payors, and individuals. These actions have been filed in state and federal courts throughout the U.S., and in Puerto Rico and Canada. They seek monetary damages and other forms of relief based on a variety of causes of action, including negligence, public nuisance, unjust enrichment, and civil conspiracy, as well as alleging violations of the Racketeer Influenced and Corrupt Organizations Act (“RICO”), state and federal controlled substances laws, and other statutes.
Since December 5, 2017, nearly all such cases pending in federal district courts have been transferred for consolidated pre-trial proceedings to a multi-district litigation (“MDL”) in the United States District Court for the Northern District of Ohio captioned In re: National Prescription Opiate Litigation, Case No. 17-md-2804. At present, there are approximately 2,900 cases under the jurisdiction of the MDL court.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Three cases involving McKesson that were previously part of the federal MDL have been remanded to other federal courts for discovery and trial. On January 14, 2020, the Judicial Panel on Multidistrict Litigation finalized its Conditional Remand Order, ordering that the cases brought by Cabell County, West Virginia and the City of Huntington, West Virginia be remanded to the U.S. District Court for the Southern District of West Virginia. Trial in that case began on May 3, 2021. These two West Virginia plaintiffs are not expected to participate in any broader multistate resolution of opioid-related claims. On February 5, 2020, the case brought by the City and County of San Francisco was remanded to the U.S. District Court for the Northern District of California; trial has been set for December 6, 2021. Also on February 5, 2020, the case brought by the Cherokee Nation was remanded by the MDL court to the U.S. District Court for the Eastern District of Oklahoma. The Cherokee Nation is not expected to participate in any broader multistate resolution of opioid-related claims.
The Company is also named in approximately 300 similar state court cases pending in 38 states plus Puerto Rico, along with 3 cases in Canada. These include actions filed by 26 state attorneys general, and some by or on behalf of individuals, including wrongful death lawsuits, and putative class action lawsuits brought on behalf of children with neonatal abstinence syndrome due to alleged exposure to opioids in utero. Trial dates have been set in several of these state court cases. For example, trial in the Supreme Court of New York, Suffolk County for a case brought by the New York attorney general and two New York county governments, is scheduled to begin in June 2021, the cases brought by the Ohio and Washington attorneys general are scheduled to go to trial in September 2021, and the case brought by the Alabama attorney general is scheduled to go to trial in November 2021.
The Company continues to be involved in discussions with the objective of achieving broad resolution of opioid-related claims of states, their political subdivisions and other government entities (“governmental entities”). The Company is in ongoing, advanced discussions with state attorneys general and plaintiffs’ representatives regarding a framework under which, in order to resolve claims of governmental entities, the three largest U.S. pharmaceutical distributors would pay up to approximately $21.0 billion over a period of 18 years, with up to approximately $8.0 billion to be paid by the Company, of which more than 90% is anticipated to be used to remediate the opioids crisis. Most of the remaining amount relates to plaintiffs’ attorneys’ fees and costs, and would be payable over a shorter time period. In addition, the proposed framework would require the three distributors, including the Company, to adopt changes to anti-diversion programs.
Under the framework, before the distributors determine whether to enter into any final settlement, they would assess the sufficiency of the scope of settlement, based in part on the number and identities of the governmental entities that would participate in any such settlement. The framework contemplates that if certain governmental entities do not agree to a settlement under the framework, but the distributors nonetheless concluded that there was sufficient participation to warrant the settlement, there would be a corresponding reduction in the amount due from the Company to account for the unresolved claims of the governmental entities that do not participate. Those non-participating governmental entities would be entitled to pursue their claims against the Company and other defendants.
The Company disclosed in its financial statements for the quarter ended December 31, 2020 its determination that discussions under that framework reached a stage at which a broad settlement of opioid claims by governmental entities was probable, and for which the loss could be reasonably estimated.
As a result of that conclusion, and its assessment of certain other opioid-related claims, the Company recorded a charge of $8.1 billion ($6.8 billion after-tax) within “Claims and litigation charges, net” in the Consolidated Statements of Operations related to its share of the settlement framework described above, as well as those certain other opioid-related claims. There was no change to the estimated liability as of March 31, 2021.
In light of the uncertainties, as described below, of the timing of amounts that would be paid with respect to these charges, they were recorded in “Long-term litigation liabilities” in the Company’s Consolidated Balance Sheet as of March 31, 2021. In addition, for the same reasons, the amount of loss that the Company ultimately might incur may differ materially from the amounts accrued.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Discussions with attorneys general and other parties continue. If the negotiating parties agree on terms under the framework for a broad resolution of claims of governmental entities, then those potential terms would need to be agreed to by numerous other state and local governments before an agreement could be accepted by the Company and finalized. In some cases, discovery has been paused during the parties’ discussions. While the Company continues to be involved in discussions regarding a potential broad settlement framework, the Company also continues to prepare for trial in these pending matters. The Company believes that it has valid defenses to the claims pending against it, and it intends to vigorously defend against all such claims if acceptable settlement terms are not achieved.
Although the vast majority of opioid claims have been brought by governmental entities in the U.S., the Company is also a defendant in cases brought in the U.S. by private plaintiffs, such as hospitals, health and welfare funds, third-party payors, and individuals, as well as 3 cases brought by governmental entities in Canada. These claims, and those of private entities generally, are not included in the settlement framework for governmental entities, or in the charges recorded by the Company, described above. The Company believes it has valid legal defenses in these matters and intends to mount a vigorous defense. The Company has not concluded a loss is probable in any of these matters; nor is the amount of any loss reasonably estimable.
Because of the many uncertainties associated with any potential settlement arrangement or other resolution of all of these opioid-related litigation matters, including the uncertain scope of participation by governmental entities in any potential settlement under the framework described above, the Company is not able to reasonably estimate the upper or lower ends of the range of ultimate possible loss for all opioid-related litigation matters. An adverse judgment or negotiated resolution in any of these matters could have a material adverse impact on the Company’s financial position, cash flows or liquidity, or results of operations.
On August 8, 2018, the Company was served with a qui tam complaint pending in the United States District Court for the District of Massachusetts alleging that the Company violated the federal False Claims Act and various state false claims acts due to the alleged failure of the Company and other defendants to report providers who were engaged in diversion of controlled substances. United States ex rel. Manchester v. Purdue Pharma, L.P., et al., Case No. 1-16-cv-10947. On August 22, 2018, the United States filed a motion to dismiss. The relator died, and on February 25, 2019 the court entered an order staying the matter until a proper party can be substituted, and providing that if no party is substituted within 90 days of February 25, 2019, the case would be dismissed. In April 2019, the widow of the relator filed a motion to substitute their daughter as the relator; the United States and defendants opposed this substitution request. The motion remains pending and the case remains stayed.
In December 2019, the Company was served with two qui tam complaints filed by the same two relators alleging violations of the federal False Claims Act, the California False Claims Act, and the California Unfair Business Practices statute based on alleged predicate violations of the Controlled Substances Act and its implementing regulations, United States ex rel. Kelley, 19-cv-2233, and State of California ex rel. Kelley, CGC-19-576931. The complaints seek relief including treble damages, civil penalties, attorney fees, and costs in unspecified amounts. On February 16, 2021, the court in the federal action dismissed the second amended complaint with prejudice, and the relators appealed the dismissal to the U.S. Court of Appeals for the Ninth Circuit.
Insurance Coverage Litigation
Two cases pending in the Northern District of California were filed against McKesson by its liability umbrella insurers about policies they issued to the Company for the period 1999-2017, AIU Insurance Company and National Union Fire Insurance Company of Pittsburgh, Pa. (together "AIG") and ACE Property and Casualty Insurance Company ("ACE"). AIU Insurance Company et al. v. McKesson Corporation, No. 3:20-cv-07469 (N.D. Cal.) was initiated by AIG in the Northern District of California on October 23, 2020. Ace Property and Casualty Insurance Company v. McKesson Corporation et al., No. 3:20-cv-09356 (N.D. Cal.) was brought by ACE in California state court on November 2, 2020, and was removed by McKesson to federal court, transferred to the Northern District of California, and designated as related to the AIU action. AIG and ACE are seeking declarations that they have no duty to defend or indemnify McKesson in the thousands of lawsuits pending in federal and state courts related to opioids. In both actions, McKesson has asserted claims under the AIG and ACE policies seeking declarations and damages for past and future defense and indemnity costs.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
II. Other Litigation and Claims
On May 17, 2013, the Company was served with a complaint filed in the United States District Court for the Northern District of California by True Health Chiropractic Inc., alleging that McKesson sent unsolicited marketing faxes in violation of the Telephone Consumer Protection Act of 1991 (“TCPA”), as amended by the Junk Fax Protection Act of 2005 or JFPA, True Health Chiropractic Inc., et al. v. McKesson Corporation, et al., No. CV-13-02219 (HG). Plaintiffs seek statutory damages from $500 to $1,500 per violation plus injunctive relief. True Health Chiropractic later amended its complaint, adding McLaughlin Chiropractic Associates as an additional named plaintiff and McKesson Technologies Inc. as a defendant. Both plaintiffs alleged that defendants violated the TCPA by sending faxes that did not contain notices regarding how to opt out of receiving the faxes. On July 16, 2015, plaintiffs filed a motion for class certification. On August 22, 2016, the court denied plaintiffs’ motion. On July 17, 2018, the United States Court of Appeals for the Ninth Circuit Court affirmed in part and reversed in part the district court’s denial of class certification and remanded the case to the district court for further proceedings. On August 13, 2019, the court granted plaintiffs’ renewed motion for class certification. After class notice and the opt-out period, 9,490 fax numbers remain in the class, representing 48,769 faxes received. On March 5, 2020, McKesson moved to decertify the class and moved for summary judgment on plaintiffs’ claim for treble damages. Plaintiffs’ moved for summary judgment on the same day. On December 24, 2020, the court declined to decertify the class but modified the class definition to distinguish between physical faxes (kept in the class) versus online or e-fax recipients (removed from the class). On March 19, 2021, the court denied summary judgment for plaintiffs on the issue of liability but found that McKesson’s affirmative defense of prior consent fails as a matter of law and precluded McKesson from presenting individualized evidence of consent at trial. On McKesson’s motion for summary judgment, the court demurred and will let the issue of treble damages go to the jury. Trial has been scheduled for October 18, 2021.
On December 29, 2017, two investment funds holding shares in Celesio AG filed a complaint against McKesson Europe Holdings (formerly known as “Dragonfly GmbH & Co KGaA”), a subsidiary of the Company, in a German court in Stuttgart, Germany, Polygon European Equity Opportunity Master Fund et al. v. McKesson Europe Holdings GmbH & Co. KGaA, No. 18 O 455/17. On December 30, 2017, four investment funds, which had allegedly entered into swap transactions regarding shares in Celesio AG that would have enabled them to decide whether to accept McKesson Europe Holdings’s takeover offer in its acquisition of Celesio AG, filed a complaint, Davidson Kempner International (BVI) Ltd. et al. v. McKesson Europe Holdings GmbH & Co. KGaA, No.16 O 475/17. The complaints allege that the public tender offer document published by McKesson Europe in its acquisition of Celesio AG incorrectly stated that McKesson Europe’s acquisition of convertible bonds would not be treated as a relevant acquisition of shares for the purposes of triggering minimum pricing considerations under Section 4 of the German Takeover Offer Ordinance. On May 11, 2018, the court in Polygon dismissed the claims against McKesson Europe. Plaintiffs appealed this ruling and, on December 19, 2018, the Higher Regional Court (Oberlandesgericht) of Stuttgart confirmed the full dismissal of the Polygon matter. Plaintiffs filed a complaint against denial of leave to appeal with the Federal Court of Justice (Bundesgerichtshof), which was rejected on November 17, 2020, making the dismissal final and binding. With no further right to appeal, Plaintiffs filed an objection against the decision of the Federal Court of Justice on November 27, 2020, claiming their right to be heard had been violated. On March 16, 2021, the Federal Court of Justice (Bundesgerichtshof) issued an order granting the Polygon plaintiffs leave to appeal. On March 15, 2019, the lower court in Davidson similarly dismissed the case. Plaintiffs appealed this ruling and, on October 9, 2019, the Higher Regional Court (Oberlandesgericht) of Stuttgart confirmed the full dismissal of the Davidson matter. On November 13, 2019, plaintiffs filed a complaint against denial of leave to appeal with the Federal Court of Justice (Bundesgerichtshof). On March 16, 2021, the Federal Court of Justice (Bundesgerichtshof) issued an order granting the Davidson plaintiffs leave to appeal.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
On March 5, 2018, the Company’s subsidiary, RxC Acquisition Company (d/b/a RxCrossroads), was served with a qui tam complaint filed in July 2017 in the United States District Court for the Southern District of Illinois by a relator against RxC Acquisition Company, among others, alleging that UCB, Inc. provided illegal “kickbacks” to providers, including nurse educator services and reimbursement assistance services provided through RxC Acquisition Company, in violation of the Anti-Kickback Statute, the False Claims Act, and various state false claims statutes. United States ex rel. CIMZNHCA, LLC v. UCB, Inc., et al., No. 17-cv-00765. The complaint sought treble damages, civil penalties, and further relief. The United States and the states named in the complaint declined to intervene in the suit. On December 17, 2018, the United States filed a motion to dismiss the complaint in its entirety; this motion was denied on April 15, 2019. On June 7, 2019, the court denied the United States’ motion for reconsideration. On July 8, 2019, the United States appealed to the United States Court of Appeals for the Seventh Circuit seeking interlocutory review of the denial of its motion for reconsideration of the denial of the motion to dismiss the complaint. On September 3, 2019, the United States District Court for the Southern District of Illinois stayed the district court proceedings pending the appeal. On August 17, 2020, the Seventh Circuit reversed the district court’s decision on the United States’ motion to dismiss and remanded the case with instructions that the district court enter judgment for the defendants on the relator’s claims under the False Claims Act. The relator sought a re-hearing en banc at the Seventh Circuit, which was denied. The relator’s False Claims Act case was dismissed, with judgment entered in favor of the defendants on September 30, 2020. On February 10, 2021, the relator filed a Petition for Writ of Certiorari at the United States Supreme Court seeking review of the Seventh Circuit’s ruling.
On April 16, 2013, the Company’s subsidiary, U.S. Oncology, Inc. (“USON”), was served with a third amended qui tam complaint filed in the United States District Court for the Eastern District of New York by two relators, purportedly on behalf of the United States, 21 states and the District of Columbia, against USON and five other defendants, alleging that USON solicited and received illegal “kickbacks” from Amgen in violation of the Anti-Kickback Statute, the False Claims Act, and various state false claims statutes, and seeking damages, treble damages, civil penalties, attorneys’ fees and costs of suit, all in unspecified amounts, United States ex rel. Piacentile v. Amgen Inc., et al., CV 04-3983 (SJ). Previously, the United States declined to intervene in the case as to all allegations and defendants except for Amgen. On September 30, 2013, the court granted the United States’ motion to dismiss the claims pled against Amgen. On September 17, 2018, the court granted USON’s motion to dismiss the claims pled against it, with leave to amend. On November 16, 2018, the relators filed a fourth amended complaint.
On June 17, 2014, U.S. Oncology Specialty, LP (“USOS”) was served with a fifth amended qui tam complaint filed in the United States District Court for the Eastern District of New York by a relator alleging that USOS, among others, solicited and received illegal “kickbacks” from Amgen in violation of the Anti-Kickback Statute, the federal False Claims Act, and various state false claims statutes, and seeking damages, treble damages, civil penalties, attorneys’ fees and costs of suit, all in unspecified amounts, United States ex rel. Hanks v. Amgen, Inc., et al., CV-08-03096 (SJ). Previously, the U.S. declined to intervene in the case as to all allegations and defendants except for Amgen. On September 17, 2018, the court granted USOS’s motion to dismiss. Following the relator’s appeal, the United States Court of Appeals for the Second Circuit vacated the district court’s order and remanded the suit to the district court, directing it to consider the question of whether the suit should be dismissed for lack of jurisdiction. The district court granted the relator leave to amend the complaint for a seventh time. The relator filed the seventh amended complaint on November 30, 2020.
On April 3, 2018, a second amended qui tam complaint was filed in the United States District Court for the Eastern District of New York by a relator, purportedly on behalf of the United States, 30 states, the District of Columbia, and two cities against McKesson Corporation, McKesson Specialty Care Distribution Corporation, McKesson Specialty Distribution LLC, McKesson Specialty Care Distribution Joint Venture, L.P., Oncology Therapeutics Network Corporation, Oncology Therapeutics Network Joint Venture, L.P., US Oncology, Inc. and US Oncology Specialty, L.P., alleging that from 2001 through 2010 the defendants repackaged and sold single-dose syringes of oncology medications in a manner that violated the federal False Claims Act and various state and local false claims statutes, and seeking damages, treble damages, civil penalties, attorneys’ fees and costs of suit, all in unspecified amounts, United States ex rel. Omni Healthcare Inc. v. McKesson Corporation, et al., 12-CV-06440 (NG). The United States and the named states have declined to intervene in the case. On October 15, 2018, the Company filed a motion to dismiss the complaint as to all named defendants. On February 4, 2019, the court granted the motion to dismiss in part and denied it in part, leaving the Company and Oncology Therapeutics Network Corporation as the only remaining defendants in the case. On December 9, 2019, the United States District Court for the Eastern District of New York ordered the unsealing of another complaint filed by the same relator, alleging the same misconduct and seeking the same relief with respect to US Oncology, Inc., purportedly on behalf of the same government entities, United States ex rel. Omni Healthcare, Inc. v. US Oncology, Inc., 19-cv-05125. The United States and the named states declined to intervene in the case.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The Company is a defendant in an amended complaint filed on June 15, 2018 in a case pending in the United States District Court for the Southern District of Illinois alleging that the Company’s subsidiary, McKesson Medical-Surgical Inc., among others, violated the Sherman Act by restraining trade in the sale of safety and conventional syringes and safety IV catheters. Marion Diagnostic Center, LLC v. Becton, Dickinson, et al., No. 18:1059. The action is filed on behalf of a purported class of purchasers, and seeks treble damages and further relief, all in unspecified amounts. On July 20, 2018, the defendants filed a motion to dismiss. On November 30, 2018, the district court granted the motion to dismiss, and dismissed the complaint with prejudice. On December 27, 2018, plaintiffs appealed the order to the United States Court of Appeals for the Seventh Circuit. On March 5, 2020, the United States Court of Appeals for the Seventh Circuit vacated the district court’s order, and ruled that dismissal was appropriate on alternative grounds. The case was remanded to the district court to allow the plaintiffs an opportunity to amend their complaint. Plaintiffs filed an amended complaint on August 21, 2020. Defendants filed a motion to dismiss the amended complaint, which the district court granted on March 15, 2021. Plaintiffs appealed the order to the United States Court of Appeals for the Seventh Circuit on March 23, 2021.
On December 30, 2019, a group of independent pharmacies and a hospital filed a class action complaint alleging that the Company and other distributors violated the Sherman Act by colluding with manufacturers to restrain trade in the sale of generic drugs. Reliable Pharmacy, et al. v. Actavis Holdco US, et al., No. 2:19-cv-6044; MDL No. 16-MD-2724. The complaint seeks relief including treble damages, disgorgement, attorney fees, and costs in unspecified amounts.
On December 12, 2018, the Company received a class action complaint in the United States District Court for the Northern District of California, alleging that McKesson and two of its former officers, CEO John Hammergren and CFO James Beer, violated the Securities Exchange Act of 1934 by reporting profits and revenues from 2013 until early 2017 that were false and misleading, due to an alleged undisclosed conspiracy to fix the prices of generic drugs. Evanston Police Pension Fund v. McKesson Corporation, No. 3:18-06525. The complaint seeks relief including damages, attorney fees, and costs in unspecified amounts. On February 8, 2019, the court appointed the Pension Trust Fund for Operating Engineers as the lead plaintiff. On April 10, 2019, the lead plaintiff filed an amended complaint that added insider trading allegations against defendant Hammergren. On April 8, 2021, the court granted plaintiff’s motion for class certification.
In July 2015, The Great Atlantic & Pacific Tea Company (“A&P”), a former customer of the Company, filed for reorganization in bankruptcy under Chapter 11 of the United States Bankruptcy Code in the Bankruptcy Court for the Southern District of New York. In re The Great Atlantic & Pacific Tea Company, Inc., et al., Case No. 15-23007. A suit filed in 2017 against the Company in this bankruptcy case seeks to recover alleged preferential transfers. The Official Committee of Unsecured Creditors on behalf of the bankruptcy estate of The Great Atlantic & Pacific Tea Company, Inc., et al. v. McKesson Corporation d/b/a McKesson Drug Co., Adv. Proc. No. 17-08264.
In October 2019, the Company’s subsidiary NDCHealth Corporation dba RelayHealth (“RelayHealth”) was served with three purported class action complaints filed in the United States District Court for the Northern District of Illinois. The complaints allege that RelayHealth violated the Sherman Act by entering into an agreement with co-defendant Surescripts, LLC not to compete in the electronic prescription routing market, and by conspiring with Surescripts, LLC to monopolize that market, Powell Prescription Center, et al. v. Surescripts, LLC, et al., No. 1:19-cv-06627; Intergrated Pharmaceutical Solutions LLC v. Surescripts, LLC, et al., 1:19-cv-06778; Falconer Pharmacy, Inc. v. Surescripts LLC, et al., No. 1:19-cv-07035. In November 2019, three similar complaints were filed in the United States District Court for the Northern District of Illinois. Kennebunk Village Pharmacy, Inc. v. SureScripts, LLC, et al., 1:19-cv-7445; Whitman v. SureScripts, LLC et al., No. 1:19-cv-7448; BBK Global Corp. v. SureScripts, LLC et al., 1:19-cv-7640. In December 2019, the six actions were consolidated in the Northern District of Illinois. The complaints seek relief including treble damages, attorney fees, and costs. Subject to final court approval, plaintiffs and RelayHealth reached an agreement in June 2020 to resolve the class action lawsuits and RelayHealth paid into escrow an amount not material in the context of the Company’s overall financial results. The settlement does not include any admission of liability, and RelayHealth expressly denies wrongdoing.
In July 2020, the Company was served with a first amended qui tam complaint filed in the United States District Court for the Southern District of New York by a relator on behalf of the U.S., 27 states and the District of Columbia against McKesson Corporation, McKesson Specialty Distribution LLC, and McKesson Specialty Care Distribution Corporation, alleging that defendants violated the Anti-Kickback Statute, federal False Claims Act, and various state false claims statutes by providing certain business analytical tools to oncology practice customers, United States ex rel. Hart v. McKesson Corporation, et al., 15-cv-00903-RA. The U.S. and the named states have declined to intervene in the case. The complaint seeks relief including damages, treble damages, civil penalties, attorney fees, and costs of suit, all in unspecified amounts.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
III. Government Subpoenas and Investigations
From time to time, the Company receives subpoenas or requests for information from various government agencies. The Company generally responds to such subpoenas and requests in a cooperative, thorough and timely manner. These responses sometimes require time and effort and can result in considerable costs being incurred by the Company. Such subpoenas and requests can lead to the assertion of claims or the commencement of civil or criminal legal proceedings against the Company and other members of the health care industry, as well as to settlements of claims against the Company. The Company responds to these requests in the ordinary course of business. The following are examples of the type of subpoenas or requests the Company receives from time to time.
In May 2017 and August 2018, respectively, the Company was served with two separate Civil Investigative Demands by the U.S. Attorney’s Office for the Eastern District of New York relating to the certification the Company obtained for two software products under the U.S. Department of Health and Human Services’ Electronic Health Record Incentive Program.
In April and June 2019, the United States Attorney’s Office for the Eastern District of New York served grand jury subpoenas seeking documents related to the Company’s anti-diversion policies and procedures and its distribution of Schedule II controlled substances. The Company believes the subpoenas are part of a broader investigation by that office into pharmaceutical manufacturers’ and distributors’ compliance with the Controlled Substances Act and related statutes.
On November 12, 2019, the New York Department of Financial Services sent a Notice of Intent to Commence Enforcement Action to McKesson Corporation and PSS World Medical, Inc. for alleged violations of the New York Insurance Law and/or New York Financial Services Law, and seeking civil monetary penalties, in connection with manufacturing and distributing opioids in New York.
In January 2020, the United States Attorney’s Office for the District of Massachusetts served a Civil Investigative Demand on the Company seeking documents related to certain discounts and rebates paid to physician practice customers.
On November 21, 2016, the Belgian Competition Authority carried out inspections at the premises of several Belgian wholesalers, including Belmedis SA, which was subsequently acquired by the Company. Pharma Belgium NV is also part of the investigation. On April 23, 2021, McKesson received correspondence from the Belgian Competition Authority seeking civil penalties.
IV. State Opioid Statutes
Legislative, regulatory or industry measures to address the misuse of prescription opioid medications could affect the Company’s business in ways that it may not be able to predict. For example, in April 2018, the State of New York adopted the Opioid Stewardship Act (the “OSA”) which required the creation of an aggregate $100 million annual surcharge on all manufacturers and distributors licensed to sell or distribute opioids in New York. The initial surcharge payment would have been due on January 1, 2019 for opioids sold or distributed during calendar year 2017. On July 6, 2018, the Healthcare Distribution Alliance filed a lawsuit challenging the constitutionality of the law and seeking an injunction against its enforcement. On December 19, 2018, the U.S. District Court for the Southern District of New York found the law unconstitutional and issued an injunction preventing the State of New York from enforcing the law. The State appealed that decision. On September 14, 2020, a panel of the U.S. Court of Appeals for the Second Circuit reversed the district court’s decision on procedural grounds. The Company has accrued a $50 million pre-tax charge ($37 million after-tax) as its estimated share of the OSA surcharge for calendar years 2017 and 2018. This OSA provision was recognized in “Selling, distribution, general, and administrative expenses” in the Consolidated Statement of Operations for the year ended March 31, 2021 and in “Other accrued liabilities” in the Consolidated Balance Sheet as of March 31, 2021. The State of New York adopted an excise tax on sales of opioids in the State, which became effective July 1, 2019. The law adopting the excise tax made clear that the OSA does not apply to sales or distributions occurring after December 31, 2018. The Healthcare Distribution Alliance filed a petition for panel rehearing, or, in the alternative, for rehearing en banc with the U.S. Court of Appeals for the Second Circuit; that petition was denied on December 18, 2020. On February 12, 2021, the Court of Appeals for the Second Circuit granted a motion by the Healthcare Distribution Alliance to stay its mandate pending the filing and disposition of a petition for writ of certiorari before the U.S. Supreme Court. The due date for filing such a petition is May 17, 2021.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
V. Environmental Matters
Primarily as a result of the operation of the Company’s former chemical businesses, which were fully divested by 1987, the Company is involved in various matters pursuant to environmental laws and regulations. The Company has received claims and demands from governmental agencies relating to investigative and remedial actions purportedly required to address environmental conditions alleged to exist at five sites where it, or entities acquired by it, formerly conducted operations and the Company, by administrative order or otherwise, has agreed to take certain actions at those sites, including soil and groundwater remediation.
Based on a determination by the Company’s environmental staff, in consultation with outside environmental specialists and counsel, the current estimate of the Company’s probable loss associated with the remediation costs for these five sites is $10 million, net of amounts anticipated from third parties. The $10 million is expected to be paid out between April 2021 and March 2051. The Company has accrued for the estimated probable loss for these environmental matters.
The Company has been designated as a Potentially Responsible Party (“PRP”) under the Superfund law for environmental assessment and cleanup costs as the result of its alleged disposal of hazardous substances at 14 sites. With respect to these sites, numerous other PRPs have similarly been designated and while the current state of the law potentially imposes joint and several liabilities upon PRPs, as a practical matter, costs of these sites are typically shared with other PRPs. At one of these sites, the United States Environmental Protection Agency has selected a preferred remedy with an estimated cost of approximately $1.4 billion. It is not certain at this point in time what proportion of this estimated liability will be borne by the Company. Accordingly, the Company’s estimated probable loss at those 14 sites is approximately $28 million, which has been accrued for in the Consolidated Balance Sheets. However, it is possible that the ultimate costs of these matters may exceed or be less than the reserves.
VI. Value Added Tax Assessments
The Company operates in various countries outside the U.S. which collect value added taxes (“VAT”). The determination of the manner in which a VAT applies to the Company’s foreign operations is subject to varying interpretations arising from the complex nature of the tax laws. The Company has received assessments for VAT which are in various stages of appeal. The Company disagrees with these assessments and believes that it has a strong legal argument to defend its tax positions. Certain VAT assessments relate to years covered by an indemnification agreement. Due to the complex nature of the tax laws, it is not possible to estimate the outcome of these matters. However, based on currently available information, the Company believes the ultimate outcome of these matters will not have a material adverse effect on its financial position, cash flows or results of operations.
VII. Antitrust Settlements
Numerous lawsuits have been filed against certain brand pharmaceutical manufacturers alleging that the manufacturer, by itself or in concert with others, took improper actions to delay or prevent generic drugs from entering the market. These lawsuits are typically brought as class actions. The Company has not been named a plaintiff in any of these class action lawsuits, but has been a member of the class of those who purchased directly from the pharmaceutical manufacturers. Some of these class action lawsuits have settled in the past with the Company receiving proceeds, including $181 million, $22 million, and $202 million in 2021, 2020, and 2019, respectively, which were included in “Cost of sales” in the Consolidated Statements of Operations.
VIII. Other Matters
The Company is involved in various other litigation, governmental proceedings and claims, not described above, that arise in the normal course of business. While it is not possible to determine the ultimate outcome or the duration of such litigation, governmental proceedings or claims, the Company believes, based on current knowledge and the advice of counsel, that such litigation, proceedings and claims will not have a material impact on the Company’s financial position or results of operations.
20. Stockholders' Equity
Each share of the Company’s outstanding common stock is permitted one vote on proposals presented to stockholders and is entitled to share equally in any dividends declared by the Company’s Board of Directors (the “Board”).
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
In July 2020, the quarterly dividend was raised from $0.41 to $0.42 per common share for dividends declared on or after such date by the Board. Dividends were $1.67 per share in 2021, $1.62 per share in 2020, and $1.51 per share in 2019. The Company anticipates that it will continue to pay quarterly cash dividends in the future. However, the payment and amount of future dividends remain within the discretion of the Board and will depend upon the Company’s future earnings, financial condition, capital requirements, and other factors.
Share Repurchase Plans
Stock repurchases may be made from time-to-time in open market transactions, privately negotiated transactions, through accelerated share repurchase (“ASR”) programs, or by combinations of such methods, any of which may use pre-arranged trading plans that are designed to meet the requirements of Rule 10b5-1(c) of the Securities Exchange Act of 1934. The timing of any repurchases and the actual number of shares repurchased will depend on a variety of factors, including the Company’s stock price, corporate and regulatory requirements, restrictions under the Company’s debt obligations, and other market and economic conditions.
Information regarding the share repurchase activity over the last three years is as follows:
| | | | | | | | | | | | | | | | | | | | |
| Share Repurchases (1) |
| (In millions, except price per share data) | | Total Number of Shares Purchased (2) | | Average Price
Paid Per Share | | Approximate
Dollar Value of
Shares that May
Yet Be Purchased
Under the
Programs |
| Balance, March 31, 2018 | | | | | | $ | 1,096 | |
| Shares repurchase plans authorized in May 2018 | | | | | | 4,000 | |
| Shares repurchased - Open market | | 10.4 | | | $ | 132.14 | | | (1,377) | |
| Shares repurchased - ASR | | 2.1 | | | $ | 117.98 | | | (250) | |
| Balance, March 31, 2019 | | | | | | 3,469 | |
| Shares repurchased - Open market | | 9.2 | | | $ | 144.68 | | | (1,334) | |
| Shares repurchased - ASR | | 4.7 | | | $ | 127.68 | | | (600) | |
| Balance, March 31, 2020 | | | | | | 1,535 | |
| Shares repurchase plans authorized in January 2021 | | | | | | 2,000 | |
Shares repurchased - Open market (3) | | 4.7 | | | $ | 160.33 | | | (750) | |
| | | | | | |
| Balance, March 31, 2021 | | | | | | $ | 2,785 | |
(1)This table does not include the value of equity awards surrendered to satisfy tax withholding obligations. It also excludes shares related to the Company’s Split-off of the Change Healthcare JV as described below.
(2)The number of shares purchased reflects rounding adjustments.
(3)$8 million was accrued within “Other accrued liabilities” on the Company’s Consolidated Balance Sheet as of March 31, 2021 for share repurchases that were executed in late March and settled in early April.
During the last three years, the Company’s share repurchases were transacted through both open market transactions and ASR programs with third party financial institutions.
In 2019, the Company retired 5.0 million or $542 million of its treasury shares previously repurchased. Under the applicable state law, these shares resume the status of authorized and unissued shares upon retirement. In accordance with the Company’s accounting policy, any excess of share repurchase price over par value is allocated between additional paid-in capital and retained earnings. Accordingly, its retained earnings and additional paid-in capital were reduced by $472 million and $70 million, respectively, during 2019.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
On March 9, 2020, the Company completed the Split-off of its interest in the Change Healthcare JV. In connection with the Split-off, the Company distributed all 176.0 million outstanding shares of SpinCo common stock, which held all of the Company’s interests in the Change Healthcare JV, to participating holders of the Company’s common stock in exchange for 15.4 million shares of McKesson stock, which are now held as treasury stock on the Company’s Consolidated Balance Sheets. Following consummation of the exchange offer, on March 10, 2020, SpinCo merged with and into Change with each share of SpinCo common stock converted into one share of Change common stock, par value $0.001 per share, with cash being paid in lieu of fractional shares of Change common stock. See Note 2, “Investment in Change Healthcare Joint Venture,” for more information.
Other Comprehensive Income (Loss)
Information regarding other comprehensive income (loss) including noncontrolling interests and redeemable noncontrolling interests, net of tax, by component is as follows:
| | | | | | | | | | | | | | | | | |
| Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
Foreign currency translation adjustments: (1) | | | | | |
Foreign currency translation adjustments arising during period, net of income tax expense of nil, nil, and nil (2) | $ | 312 | | | $ | (151) | | | $ | (431) | |
Reclassified to income statement, net of income tax expense of nil, nil, and nil (3) | 47 | | | — | | | — | |
| 359 | | | (151) | | | (431) | |
Unrealized gains (losses) on net investment hedges: (4) | | | | | |
Unrealized gains (losses) on net investment hedges arising during period, net of income tax (expense) benefit of $62, $(30), and $(71) | (175) | | | 85 | | | 241 | |
Reclassified to income statement, net of income tax expense of nil, nil, and nil | — | | | — | | | — | |
| (175) | | | 85 | | | 241 | |
| Unrealized gains (losses) on cash flow hedges: | | | | | |
Unrealized gains (losses) on cash flow hedges arising during period, net of income tax (expense) benefit of $6, $(12), and $(4) | (36) | | | 86 | | | 24 | |
Reclassified to income statement, net of income tax expense of nil, nil, and nil | — | | | — | | | — | |
| (36) | | | 86 | | | 24 | |
| Changes in retirement-related benefit plans: | | | | | |
Net actuarial gain (loss) and prior service credit (cost) arising during the period, net of income tax (expense) benefit of $2, $(8), and $5 (5) | 9 | | | 27 | | | (51) | |
Amortization of actuarial loss, prior service cost and transition obligation, net of income tax benefit of $1, $1, and nil (6) | — | | | 2 | | | 9 | |
Foreign currency translation adjustments and other, net of income tax expense of nil, nil, and nil | (11) | | | 6 | | | 10 | |
Reclassified to income statement, net of income tax expense of $9, $33, and nil (3) (7) | 24 | | | 94 | | | — | |
| 22 | | | 129 | | | (32) | |
| | | | | |
| Other comprehensive income (loss), net of tax | $ | 170 | | | $ | 149 | | | $ | (198) | |
(1)Foreign currency translation adjustments primarily result from the conversion of non-U.S. dollar financial statements of the Company’s foreign subsidiary McKesson Europe, and its operations in Canada into the Company’s reporting currency, U.S. dollars.
(2)2021, 2020, and 2019 include net foreign currency translation adjustments of $(60) million, $1 million, and $(61) million, respectively, attributable to noncontrolling and redeemable noncontrolling interests.
(3)2021 primarily includes adjustments for amounts related to the contribution of the Company’s German pharmaceutical wholesale business to a joint venture, as discussed in more detail in Financial Note 3, “Held for Sale.” These amounts were included in the current and prior periods calculation of charges to remeasure the assets and liabilities held for sale to fair value less costs to sell recorded within Operating expenses in the Consolidated Statements of Operations.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
(4)2021, 2020, and 2019 include foreign currency adjustments of $(118) million, $39 million, and $259 million, respectively, on the net investment hedges from the Euro and British pound sterling-denominated notes. 2021, 2020, and 2019 also include foreign currency adjustments of $(119) million, $76 million, and $53 million, respectively, on the net investment hedges from the cross-currency swaps.
(5)The 2021 and 2020 net actuarial gains of $8 million and $2 million, respectively, and 2019 net actuarial loss of $5 million were attributable to noncontrolling and redeemable noncontrolling interests.
(6)Pre-tax amount was reclassified into “Cost of sales” and “Operating expenses” in the Consolidated Statements of Operations. The related tax expense was reclassified into “Income tax benefit (expense)” in the Consolidated Statements of Operations.
(7)2020 primarily reflects a reclassification of losses in 2020 upon the termination of the Plan from “Accumulated other comprehensive loss” to “Other income, net” in the Company’s Consolidated Statement of Operations.
Accumulated Other Comprehensive Income (Loss)
Information regarding changes in the Company’s accumulated other comprehensive income (loss) by component are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign Currency Translation Adjustments | | | | | | |
| (In millions) | Foreign Currency Translation Adjustments, Net of Tax | | Unrealized Gains (Losses) on Net Investment Hedges,
Net of Tax | | Unrealized Gains (Losses) on Cash Flow Hedges,
Net of Tax | | Unrealized Net Gains (Losses) and Other Components of Benefit Plans, Net of Tax | | Total Accumulated Other Comprehensive Loss |
| Balance at March 31, 2019 | $ | (1,628) | | | $ | 53 | | | $ | (37) | | | $ | (237) | | | $ | (1,849) | |
Other comprehensive income (loss) before reclassifications | (151) | | | 85 | | | 86 | | | 33 | | | 53 | |
Amounts reclassified to earnings and other | — | | | — | | | — | | | 96 | | | 96 | |
| Other comprehensive income (loss) | (151) | | | 85 | | | 86 | | | 129 | | | 149 | |
Less: amounts attributable to noncontrolling and redeemable noncontrolling interests | 1 | | | — | | | — | | | 2 | | | 3 | |
Other comprehensive income (loss) attributable to McKesson | (152) | | | 85 | | | 86 | | | 127 | | | 146 | |
| Balance at March 31, 2020 | (1,780) | | | 138 | | | 49 | | | (110) | | | (1,703) | |
Other comprehensive income (loss) before reclassifications | 312 | | | (175) | | | (36) | | | (2) | | | 99 | |
Amounts reclassified to earnings and other | 47 | | | — | | | — | | | 24 | | | 71 | |
| Other comprehensive income (loss) | 359 | | | (175) | | | (36) | | | 22 | | | 170 | |
Less: amounts attributable to noncontrolling and redeemable noncontrolling interests | (60) | | | (1) | | | — | | | 8 | | | (53) | |
Other comprehensive income (loss) attributable to McKesson | 419 | | | (174) | | | (36) | | | 14 | | | 223 | |
| Balance at March 31, 2021 | $ | (1,361) | | | $ | (36) | | | $ | 13 | | | $ | (96) | | | $ | (1,480) | |
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
21. Related Party Balances and Transactions
During the fourth quarter of 2018, a public benefit California foundation (“Foundation”) was established to provide opioid education to patients, caregivers, and providers, address policy issues, and increase patient access to life-saving treatments. Certain officers of the Company also serve as directors and officers of the Foundation. During 2019, the Company paid cash of $100 million to the Foundation to settle an outstanding pledge it made in 2018. During the fourth quarter of 2020, the Company also contributed $20 million to the McKesson Foundation, which supports the Company’s employees and their community involvement efforts, with a special focus on cancer. A portion of this contribution was directed to an emergency employee assistance fund administered by the Emergency Assistance Foundation, an independent nonprofit organization, to provide support for employees impacted by the COVID-19 pandemic.
McKesson Europe has investments in pharmacies located across Europe that are accounted for under the equity method. McKesson Europe maintains distribution arrangements with these pharmacies for the sale of related goods and services under which revenues of $178 million, $141 million, and $137 million, are included in the Consolidated Statements of Operations for the years ended March 31, 2021, 2020, and 2019, respectively, and receivables related to these transactions included in the Consolidated Balance Sheets were not material as of March 31, 2021 and 2020.
In 2021, 2020, and 2019, the Company’s pharmaceutical sales to one of its equity method investees in the U.S. Pharmaceutical segment totaled $111 million, $60 million, and $34 million, respectively. Trade receivables related to these transactions from this investee were not material as of March 31, 2021 and 2020.
Refer to Financial Note 2, “Investment in Change Healthcare Joint Venture,” for information regarding related party balances and transactions with Change and the Change Healthcare JV.
22. Segments of Business
Commencing with the second quarter of 2021, the Company implemented a new segment reporting structure which resulted in four reportable segments: U.S. Pharmaceutical, International, Medical-Surgical Solutions, and RxTS. Other, for retrospective periods presented, consists of the Company’s equity method investment in the Change Healthcare JV, which was split-off from McKesson in the fourth quarter of 2020. All prior segment information has been recast to reflect the Company’s new segment structure and current period presentation. The organizational structure also includes Corporate, which consists of income and expenses associated with administrative functions and projects, and the results of certain investments. The factors for determining the reportable segments included the manner in which management evaluates the performance of the Company combined with the nature of the individual business activities. The Company evaluates the performance of its operating segments on a number of measures, including revenues and operating profit before interest expense and income taxes. Assets by operating segment are not reviewed by management for the purpose of assessing performance or allocating resources.
The U.S. Pharmaceutical segment distributes branded, generic, specialty, biosimilar, and over-the-counter pharmaceutical drugs and other healthcare-related products. This segment also provides practice management, technology, clinical support, and business solutions to community-based oncology and other specialty practices. In addition, the segment sells financial, operational, and clinical solutions to pharmacies (retail, hospital, alternate site) and provides consulting, outsourcing, technological, and other services.
The International segment includes the Company’s operations in Europe and Canada, bringing together non-U.S.-based drug distribution services, specialty pharmacy, retail, and infusion care services. The Company’s operations in Europe provide distribution and services to wholesale, institutional, and retail customers in 13 European countries where it owns, partners, or franchises with retail pharmacies and operates through two businesses: Pharmaceutical Distribution and Retail Pharmacy. The Company’s Canada operations deliver vital medicines, supplies, and information technology solutions throughout Canada and includes Rexall retail pharmacies. McKesson Europe was previously reflected as the European Pharmaceutical Solutions reportable segment and McKesson Canada was previously included in Other.
The Medical-Surgical Solutions segment provides medical-surgical supply distribution, logistics, and other services to healthcare providers, including physician offices, surgery centers, nursing homes, hospital reference labs, and home health care agencies. This segment offers more than 275,000 national brand medical-surgical products as well as McKesson’s own line of high-quality products through a network of distribution centers within the United States.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
The RxTS segment brings together existing businesses, including CoverMyMeds, RelayHealth, RxCrossroads, and McKesson Prescription Automation, including Multi-Client Central Fill as a Service, to serve the Company’s biopharma and life sciences partners and patients. RxTS works across the healthcare delivery system to connect pharmacies, providers, payers, and biopharma for next-generation patient access and adherence solutions. RxCrossroads was previously included in the former U.S. Pharmaceutical and Specialty Solutions reportable segment and CoverMyMeds, RelayHealth, and McKesson Prescription Automation were previously included in Other.
Other, for retrospective periods presented consists of the Company’s investment in the Change Healthcare JV, which was split-off from the Company in the fourth quarter of 2020.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
Financial information relating to the Company’s reportable operating segments and reconciliations to the consolidated totals is as follows:
| | | | | | | | | | | | | | | | | |
| | Years Ended March 31, |
| (In millions) | 2021 | | 2020 | | 2019 |
Segment revenues (1) | | | | | |
| U.S. Pharmaceutical | $ | 189,274 | | | $ | 181,700 | | | $ | 166,189 | |
| International | 35,965 | | | 38,341 | | | 38,023 | |
| Medical-Surgical Solutions | 10,099 | | | 8,305 | | | 7,618 | |
| Prescription Technology Solutions | 2,890 | | | 2,705 | | | 2,489 | |
| Total revenues | $ | 238,228 | | | $ | 231,051 | | | $ | 214,319 | |
| | | | | |
Segment operating profit (loss) (2) | | | | | |
U.S. Pharmaceutical (3) | $ | 2,763 | | | $ | 2,745 | | | $ | 2,710 | |
International (4) | (37) | | | (161) | | | (1,903) | |
Medical-Surgical Solutions (5) | 707 | | | 499 | | | 455 | |
Prescription Technology Solutions (6) | 395 | | | 396 | | | 355 | |
Other (7) | — | | | (1,113) | | | (104) | |
| Subtotal | 3,828 | | | 2,366 | | | 1,513 | |
Corporate expenses, net (8) | (8,645) | | | (973) | | | (639) | |
| Interest expense | (217) | | | (249) | | | (264) | |
| Income (loss) from continuing operations before income taxes | $ | (5,034) | | | $ | 1,144 | | | $ | 610 | |
| | | | | |
Segment depreciation and amortization (9) | | | | | |
| U.S. Pharmaceutical | $ | 211 | | | $ | 208 | | | $ | 227 | |
| International | 334 | | | 357 | | | 392 | |
| Medical-Surgical Solutions | 130 | | | 136 | | | 118 | |
| Prescription Technology Solutions | 87 | | | 85 | | | 90 | |
| | | | | |
| Corporate | 125 | | | 136 | | | 122 | |
| Total depreciation and amortization | $ | 887 | | | $ | 922 | | | $ | 949 | |
| | | | | |
Segment expenditures for long-lived assets (10) | | | | | |
| U.S. Pharmaceutical | $ | 246 | | | $ | 109 | | | $ | 103 | |
| International | 212 | | | 218 | | | 199 | |
| Medical-Surgical Solutions | 57 | | | 36 | | | 116 | |
| Prescription Technology Solutions | 22 | | | 23 | | | 14 | |
| | | | | |
| Corporate | 104 | | | 120 | | | 125 | |
| Total expenditures for long-lived assets | $ | 641 | | | $ | 506 | | | $ | 557 | |
(1)Revenues from services on a disaggregated basis represent less than 1% of the U.S. Pharmaceutical segment’s total revenues, less than 7% of the International segment’s total revenues, less than 2% of the Medical-Surgical Solutions segment’s total revenues, and approximately 39% of the RxTS segment’s total revenues. The International segment reflects foreign revenues. Revenues for the remaining three reportable segments are domestic.
(2)Segment operating profit (loss) includes gross profit, net of operating expenses, as well as other income (expense), net, for the Company’s reportable segments. For retrospective periods presented, Operating loss for Other reflects equity earnings and charges from the Company’s equity method investment in the Change Healthcare JV, which was split-off from McKesson in the fourth quarter of 2020.
McKESSON CORPORATION
FINANCIAL NOTES (Continued)
(3)The Company’s U.S. Pharmaceutical segment’s operating profit for 2021, 2020, and 2019 includes credits of $38 million, $252 million, and $210 million, respectively, related to the LIFO method of accounting for inventories. Operating profit for 2021, 2020, and 2019 also includes $181 million, $22 million, and $202 million, respectively, of cash receipts for the Company’s share of antitrust legal settlements. In addition, operating profit for 2021 includes a charge of $50 million recorded in connection with the Company’s estimated liability under the State of New York’s OSA, as further discussed in Note 19, “Commitments and Contingent Liabilities,” and operating profit for 2019 includes a charge of $61 million related to a customer bankruptcy.
(4)The Company’s International segment’s operating loss for 2021 and 2020 includes charges of $58 million and $275 million (both pre-tax and after-tax), respectively, to remeasure to fair value the assets and liabilities of the Company’s German pharmaceutical wholesale business which was contributed to a joint venture, as further discussed in Financial Note 3, “Held for Sale.” Operating loss for 2021, 2020, and 2019 includes long-lived asset impairment charges of $115 million, $112 million, and $245 million, respectively, primarily related to retail pharmacy businesses in Canada and Europe, as discussed in more detail in Financial Note 4, “Restructuring, Impairment, and Related Charges.” Operating loss for 2021 and 2019 includes goodwill impairment charges of $69 million and $1.8 billion (both pre-tax and after-tax), respectively, as discussed in more detail in Financial Note 12, “Goodwill and Intangible Assets, Net.” In addition, operating loss for 2019 includes a gain from an escrow settlement of $97 million (pre-tax and after-tax) representing certain indemnity and other claims related to the Company’s 2017 acquisition of Rexall Health.
(5)The Company’s Medical-Surgical Solutions segment’s operating profit for 2021 includes charges totaling $136 million on certain personal protective equipment and other related products due to inventory impairments and excess inventory.
(6)The Company’s RxTS segment’s operating profit for 2019 includes a gain of $56 million recognized from the sale of an equity investment.
(7)Operating loss for Other for 2020 includes an impairment charge of $1.2 billion and a dilution loss of $246 million associated with the Company’s investment in the Change Healthcare JV, partially offset by a net gain of $414 million (pre-tax and after-tax) related to the separation of its interest in the Change Healthcare JV completed during the fourth quarter of 2020. Operating loss for 2019 includes a credit of $90 million for the derecognition of the TRA liability payable to the shareholders of Change. Operating loss for 2020 and 2019 also includes the Company’s proportionate share of loss from the Change Healthcare JV of $119 million and $194 million, respectively.
(8)Corporate expenses, net, for 2021 includes a charge of $8.1 billion related to the estimated liability for opioid-related claims, as discussed in more detail in Financial Note 19, “Commitments and Contingent Liabilities." Corporate expenses, net, for 2021 also includes net gains of $133 million associated with certain of the Company’s equity investments and a net gain of $131 million recorded in connection with insurance proceeds received from the settlement of the shareholder derivative action related to the Company’s controlled substances monitoring program. Corporate expenses, net, for 2020 includes settlement charges of $122 million for the termination of the Company’s defined benefit pension plan and a settlement charge of $82 million related to opioid claims.
(9)Amounts primarily consist of amortization of acquired intangible assets purchased in connection with business acquisitions and capitalized software for internal use as well as depreciation and amortization of property, plant, and equipment, net.
(10)Long-lived assets consist of property, plant, and equipment, net and capitalized software.
Segment assets and long-lived assets by geographic areas were as follows:
| | | | | | | | | | | |
| | March 31, |
| (In millions) | 2021 | | 2020 |
| Segment assets | | | |
| U.S. Pharmaceutical | $ | 35,236 | | | $ | 33,541 | |
| International | 14,987 | | | 14,994 | |
| Medical-Surgical Solutions | 5,986 | | | 5,395 | |
| Prescription Technology Solutions | 3,446 | | | 3,786 | |
| | | |
| Corporate | 5,360 | | | 3,531 | |
| Total assets | $ | 65,015 | | | $ | 61,247 | |
| | | |
Long-lived assets (1) | | | |
| United States | $ | 2,110 | | | $ | 1,873 | |
| Foreign | 984 | | | 892 | |
| Total long-lived assets | $ | 3,094 | | | $ | 2,765 | |
(1)Long-lived assets consist of property, plant, and equipment, net and capitalized software.
McKESSON CORPORATION
FINANCIAL NOTES (Concluded)
23. Quarterly Financial Information (Unaudited)
The quarterly results of operations are not necessarily indicative of the results that may be expected for the entire year. Selected quarterly financial information for the last two years is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| (In millions, except per share amounts) | First
Quarter | | Second
Quarter | | Third
Quarter | | Fourth
Quarter |
| Fiscal 2021 | | | | | | | |
| Revenues | $ | 55,679 | | | $ | 60,808 | | | $ | 62,599 | | | $ | 59,142 | |
| Gross profit | 2,700 | | | 3,000 | | | 3,151 | | | 3,297 | |
| Income (loss) after income taxes: | | | | | | | |
Continuing operations | $ | 495 | | | $ | 627 | | | $ | (6,174) | | | $ | 713 | |
Discontinued operations | (1) | | | — | | | — | | | — | |
| Net income (loss) | $ | 494 | | | $ | 627 | | | $ | (6,174) | | | $ | 713 | |
| Net income (loss) attributable to McKesson Corporation | $ | 444 | | | $ | 577 | | | $ | (6,226) | | | $ | 666 | |
Earnings (loss) per common share attributable to McKesson Corporation (1) | | | | | | | |
Diluted (2) | | | | | | | |
Continuing operations | $ | 2.72 | | | $ | 3.54 | | | $ | (39.03) | | | $ | 4.15 | |
Discontinued operations | — | | | — | | | — | | | — | |
| Total | $ | 2.72 | | | $ | 3.54 | | | $ | (39.03) | | | $ | 4.15 | |
Basic | | | | | | | |
| Continuing operations | $ | 2.74 | | | $ | 3.56 | | | $ | (39.03) | | | $ | 4.19 | |
| Discontinued operations | — | | | — | | | — | | | — | |
| Total | $ | 2.74 | | | $ | 3.56 | | | $ | (39.03) | | | $ | 4.19 | |
| | | | | | | |
| Fiscal 2020 | First
Quarter | | Second
Quarter | | Third
Quarter | | Fourth
Quarter |
| Revenues | $ | 55,728 | | | $ | 57,616 | | | $ | 59,172 | | | $ | 58,535 | |
| Gross profit | 2,787 | | | 2,867 | | | 3,033 | | | 3,336 | |
| Income (loss) after income taxes: | | | | | | | |
| Continuing operations | $ | 483 | | | $ | (676) | | | $ | 247 | | | $ | 1,072 | |
Discontinued operations | (6) | | | (1) | | | (5) | | | 6 | |
| Net income (loss) | $ | 477 | | | $ | (677) | | | $ | 242 | | | $ | 1,078 | |
| Net income (loss) attributable to McKesson Corporation | $ | 423 | | | $ | (730) | | | $ | 186 | | | $ | 1,021 | |
Earnings (loss) per common share attributable to McKesson Corporation (1) | | | | | | | |
Diluted (2) | | | | | | | |
Continuing operations | $ | 2.27 | | | $ | (3.99) | | | $ | 1.06 | | | $ | 5.82 | |
Discontinued operations | (0.03) | | | — | | | (0.03) | | | 0.03 | |
| Total | $ | 2.24 | | | $ | (3.99) | | | $ | 1.03 | | | $ | 5.85 | |
Basic | | | | | | | |
| Continuing operations | $ | 2.28 | | | $ | (3.99) | | | $ | 1.06 | | | $ | 5.86 | |
| Discontinued operations | (0.03) | | | — | | | (0.02) | | | 0.03 | |
| Total | $ | 2.25 | | | $ | (3.99) | | | $ | 1.04 | | | $ | 5.89 | |
(1)Certain computations may reflect rounding adjustments.
(2)As a result of the Company’s reported net loss for the third quarter of 2021 and the second quarter of 2020, potentially dilutive securities were excluded from the per share computations for those quarters due to their antidilutive effect.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Disclosure Controls and Procedures
Our Chief Executive Officer and our Chief Financial Officer, with the participation of other members of the Company’s management, have evaluated the effectiveness of the Company’s “disclosure controls and procedures” (as such term is defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of the end of the period covered by this report and have concluded that our disclosure controls and procedures are effective based on their evaluation of these controls and procedures as required by paragraph (b) of Exchange Act Rules 13a-15 or 15d-15.
Internal Control over Financial Reporting
Management’s report on the Company’s internal control over financial reporting (as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) and the related report of our independent registered public accounting firm are included in this Annual Report on Form 10-K, under the headings, “Management’s Annual Report on Internal Control Over Financial Reporting” and “Report of Independent Registered Public Accounting Firm” and are incorporated herein by reference.
Changes in Internal Controls
There was no change in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred during our fourth quarter of 2021 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Information about our Directors is incorporated by reference from the discussion under Item 1 of our Proxy Statement for the 2021 Annual Meeting of Stockholders (the “Proxy Statement”) under the heading “Election of Directors.” Information about our Executive Officers is incorporated by reference from the discussion in Part I of this report under the heading “Information about our Executive Officers.” Information about our Audit Committee, including the members of the committee and our Audit Committee Financial Expert, is incorporated by reference from the discussion under Item 1 of our Proxy Statement under the headings “The Board, Committees and Meetings,” and “Audit Committee Report.”
Information about the Code of Conduct applicable to all employees, officers and directors can be found on our website, www.mckesson.com, under the caption “Investors - Corporate Governance.” The Company’s Corporate Governance Guidelines and Charters for the Audit, Compensation and Governance Committees can also be found on our website under the same caption.
The Company intends to post on its website required information regarding any amendment to, or waiver from, the Code of Conduct that applies to our Chief Executive Officer, Chief Financial Officer, Controller and persons performing similar functions within four business days after any such amendment or waiver.
Item 11. Executive Compensation.
Information with respect to this item is incorporated by reference from the discussion under the heading “Executive Compensation” in our Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information about security ownership of certain beneficial owners and management is incorporated by reference from the discussion under the heading “Principal Shareholders” in our Proxy Statement.
The following table sets forth information as of March 31, 2021 with respect to the plans under which the Company’s common stock is authorized for issuance:
| | | | | | | | | | | | | | | | | |
Plan Category
(In millions, except per share amounts) | Number of securities
to be issued upon
exercise of
outstanding options,
warrants and rights | | Weighted-average exercise price of outstanding options, warrants and rights (1) | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in the first column) |
Equity compensation plans approved by security holders | 4.2 (2) | | $ | 183.29 | | | 21.9 (3) |
Equity compensation plans not approved by security holders | — | | | $ | — | | | — | |
(1) The weighted-average exercise price set forth in this column is calculated excluding outstanding restricted stock unit (“RSU”) awards, since recipients are not required to pay an exercise price to receive the shares subject to these awards.
(2) Represents option and RSU awards outstanding under the following plans: (i) 1997 Non-Employee Directors’ Equity Compensation and Deferral Plan; (ii) the 2005 Stock Plan; and (iii) the 2013 Stock Plan.
(3) Represents 2.23 million shares available for purchase under the 2000 Employee Stock Purchase Plan and 19.67 million shares available for grant under the 2013 Stock Plan.
The following are descriptions of equity plans that have been approved by the Company’s stockholders. The plans are administered by the Compensation Committee of the Board of Directors, except for the portion of the 2013 Stock Plan and 2005 Stock Plan related to non-employee directors, which is administered by the Board of Directors or its Governance Committee.
2013 Stock Plan: The 2013 Stock Plan was adopted by the Board of Directors on May 22, 2013 and approved by the Company’s stockholders on July 31, 2013. The 2013 Stock Plan permits the grant of awards in the form of stock options, stock appreciation rights, restricted stock (“RS”), restricted stock units (“RSUs”), performance-based restricted stock units (“PeRSUs”), performance shares, and other share-based awards. The number of shares reserved for issuance under the 2013 Stock Plan equals the sum of (i) 30.0 million shares, (ii) the number of shares reserved but unissued under the 2005 Stock Plan as of the effective date of the 2013 Stock Plan, and (iii) the number of shares that become available for reuse under the 2005 Stock Plan following the effective date of the 2013 Stock Plan. For any one share of common stock issued in connection with an RS, RSU, performance share or other full share award, three and one-half shares shall be deducted from the shares available for future grants. Shares of common stock not issued or delivered as a result of the net exercise of a stock option, including in respect of the payment of applicable taxes, or shares repurchased on the open market with proceeds from the exercise of options shall not be returned to the reserve of shares available for issuance under the 2013 Stock Plan. Shares withheld to satisfy tax obligations relating to the vesting of a full-share award shall be returned to the reserve of shares available for issuance under the 2013 Stock Plan.
Stock options are granted at no less than fair market value and those options granted under the 2013 Stock Plan generally have a contractual term of seven years. Options generally become exercisable in four equal annual installments beginning one year after the grant date. The vesting of RS or RSUs is determined by the Compensation Committee at the time of grant. Beginning with awards granted in fiscal year 2021, RS and RSUs generally vest over three years. RSUs granted under the PeRSU program vest three years following the end of the performance period. The Company’s executive officers and other members of senior management are annually granted performance awards called performance stock units (“PSUs”), which have a three-year performance period and are payable in shares without an additional vesting period.
Non-employee directors may be granted an award on the date of each annual meeting of the stockholders for up to 5,000 RSUs, as determined by the Board. Such non-employee director award is fully vested on the date of the grant.
2005 Stock Plan: The 2005 Stock Plan was adopted by the Board of Directors on May 25, 2005 and approved by the Company’s stockholders on July 27, 2005. The 2005 Stock Plan permits the granting of up to 42.5 million shares in the form of stock options, RS, RSUs, PeRSUs, performance shares and other share-based awards. For any one share of common stock issued in connection with an RS, RSU, performance share or other full-share award, two shares shall be deducted from the shares available for future grants. Shares of common stock not issued or delivered as a result of the net exercise of a stock option, shares withheld to satisfy tax obligations relating to the vesting of a full-share award or shares repurchased on the open market with proceeds from the exercise of options shall not be returned to the reserve of shares available for issuance under the 2005 Stock Plan. Stock options were granted at no less than fair market value and options granted under the 2005 Stock Plan generally have a contractual term of seven years.
Following the effectiveness of the 2013 Stock Plan, no further shares were made subject to award under the 2005 Stock Plan. Shares reserved but unissued under the 2005 Stock Plan as of the effective date of the 2013 Stock Plan, and shares that become available for reuse under the 2005 Stock Plan following the effectiveness of the 2013 Stock Plan, will be available for awards under the 2013 Stock Plan.
1997 Non-Employee Directors’ Equity Compensation and Deferral Plan: The 1997 Non-Employee Directors’ Equity Compensation and Deferral Plan was approved by the Company’s stockholders on July 30, 1997; however, stockholder approval of the 2005 Stock Plan on July 27, 2005 had the effect of terminating the 1997 Non-Employee Directors’ Equity Compensation and Deferral Plan such that no new awards would be granted under the 1997 Non-Employee Directors’ Equity Compensation and Deferral Plan.
2000 Employee Stock Purchase Plan (the “ESPP”): The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Internal Revenue Code. In March 2002, the Board amended the ESPP to allow for participation in the plan by employees of certain of the Company’s international and other subsidiaries. Currently, 21.1 million shares have been approved by stockholders for issuance under the ESPP.
The ESPP is implemented through a continuous series of three-month purchase periods (“Purchase Periods”) during which contributions can be made toward the purchase of common stock under the plan. Each eligible employee may elect to authorize regular payroll deductions during the next succeeding Purchase Period, the amount of which may not exceed 15% of a participant’s compensation. At the end of each Purchase Period, the funds withheld by each participant will be used to purchase shares of the Company’s common stock. The purchase price of each share of the Company’s common stock is 85% of the fair market value of each share on the last day of the applicable Purchase Period. In general, the maximum number of shares of common stock that may be purchased by a participant for each calendar year is determined by dividing $25,000 by the fair market value of one share of common stock on the offering date.
There currently are no equity awards outstanding that were granted under equity plans that were not submitted for approval by the Company’s stockholders.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Information with respect to certain transactions with directors and management is incorporated by reference from the Proxy Statement under the heading “Related Party Transactions Policy and Transactions with Related Persons.” Information regarding Director independence is incorporated by reference from the Proxy Statement under the heading “Director Independence.” Additional information regarding certain related party balances and transactions is included in the Financial Review section of this report and Financial Note 21, “Related Party Balances and Transactions” to the consolidated financial statements appearing in this report.
Item 14. Principal Accounting Fees and Services.
Information regarding principal accountant fees and services is set forth under the heading “Ratification of Appointment of Deloitte & Touche LLP as the Company’s Independent Registered Public Accounting Firm for Fiscal 2022” in our Proxy Statement and all such information is incorporated herein by reference.
PART IV
Item 15. Exhibits and Financial Statement Schedule.
| | | | | |
| Page |
| (a)(1) Consolidated Financial Statements | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| (a)(2) Financial Statement Schedule | |
| |
| |
| |
| All other schedules not included have been omitted because of the absence of conditions under which they are required or because the required information, where material, is shown in the financial statements, financial notes or supplementary financial information. | |
| |
| |
| |
SCHEDULE II
SUPPLEMENTARY CONSOLIDATED FINANCIAL STATEMENT SCHEDULE
VALUATION AND QUALIFYING ACCOUNTS
(In millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Additions | | | | |
| Description | Balance at Beginning of Year | | Charged to Costs and Expenses | | Charged to Other Accounts (3) | | Deductions From Allowance Accounts (1) | | Balance at End of Year (2) |
| Year Ended March 31, 2021 | | | | | | | | | |
| Allowances for doubtful accounts | $ | 252 | | | $ | 4 | | | $ | 1 | | | $ | (46) | | | $ | 211 | |
| Other allowances | 30 | | | 11 | | | 9 | | | — | | | 50 | |
| $ | 282 | | | $ | 15 | | | $ | 10 | | | $ | (46) | | | $ | 261 | |
| | | | | | | | | |
| Year Ended March 31, 2020 | | | | | | | | | |
| Allowances for doubtful accounts | $ | 273 | | | $ | 91 | | | $ | (19) | | | $ | (93) | | | $ | 252 | |
| Other allowances | 24 | | | — | | | — | | | 6 | | | 30 | |
| $ | 297 | | | $ | 91 | | | $ | (19) | | | $ | (87) | | | $ | 282 | |
| | | | | | | | | |
| Year Ended March 31, 2019 | | | | | | | | | |
| Allowances for doubtful accounts | $ | 187 | | | $ | 132 | | | $ | (1) | | | $ | (45) | | | $ | 273 | |
| Other allowances | 39 | | | — | | | (15) | | | — | | | 24 | |
| $ | 226 | | | $ | 132 | | | $ | (16) | | | $ | (45) | | | $ | 297 | |
| | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | 2021 | | 2020 | | 2019 |
| (1) | Deductions: | | | | | | |
| Written-off | | $ | (40) | | | $ | (93) | | | $ | (45) | |
| Credited to other accounts and other | | (6) | | | 6 | | | — | |
| Total | | $ | (46) | | | $ | (87) | | | $ | (45) | |
| | | | | | | |
| (2) | Amounts shown as deductions from current and non-current receivables (current allowances were $250 million, $265 million, and $279 million at March 31, 2021, 2020, and 2019, respectively) | | $ | 261 | | | $ | 282 | | | $ | 297 | |
| | | | | | | |
| (3) | Primarily represents reclassifications to other balance sheet accounts. | | | | | | |
EXHIBIT INDEX
The agreements included as exhibits to this report are included to provide information regarding their terms and not intended to provide any other factual or disclosure information about the Company or the other parties to the agreements. The agreements may contain representations and warranties by each of the parties to the applicable agreement that were made solely for the benefit of the other parties to the applicable agreement, and;
•should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate;
•may apply standards of materiality in a way that is different from what may be viewed as material to you or other investors; and
•were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments.
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time.
Exhibits identified under “Incorporated by Reference” in the table below are on file with the Commission and are incorporated by reference as exhibits hereto.
| | | | | | | | | | | | | | | | | |
| | Incorporated by Reference |
| Exhibit Number | Description | Form | File Number | Exhibit | Filing Date |
| 2.1 | Agreement of Contribution and Sale, dated as of June 28, 2016, by and among McKesson Corporation, PF2 NewCo LLC, PF2 NewCo Intermediate Holdings, LLC, PF2 NewCo Holdings, LLC, HCIT Holdings, Inc., Change Healthcare, Inc., Change Aggregator L.P. and H&F Echo Holdings, L.P. | 8-K | 1-13252 | 2.1 | July 5, 2016 |
| 2.2 | Amendment No. 1 to Agreement Contribution and Sale, dated as of March 1, 2017, by and among by and among Change Healthcare LLC, Change Healthcare Intermediate Holdings, LLC, Change Healthcare Holdings, LLC, HCIT Holdings, Inc., Change Healthcare, Inc., a Delaware corporation, for itself and in its capacity as Echo Representative, certain affiliates of The Blackstone Group, L.P., certain affiliates of Hellman & Friedman LLC, and McKesson Corporation, a Delaware corporation. | 8-K | 1-13252 | 2.1 | March 7, 2017 |
| 2.3 | | 8-K | 1-13252 | 2.1 | February 10, 2020 |
| 3.1 | | 8-K | 1-13252 | 3.1 | August 2, 2011 |
| 3.2 | | 8-K | 1-13252 | 3.1 | March 13, 2020 |
| 4.1 | | 10-K | 1-13252 | 4.4 | June 19, 1997 |
| 4.2 | | S-4 | 333-30899 | 4.2 | July 8, 1997 |
| 4.3 | | 8-K | 1-13252 | 4.1 | March 5, 2007 |
| | | | | | | | | | | | | | | | | |
| | Incorporated by Reference |
| Exhibit Number | Description | Form | File Number | Exhibit | Filing Date |
| 4.4 | First Supplemental Indenture, dated as of February 28, 2011, to the Indenture, dated as of March 5, 2007, among the Company, as issuer, the Bank of New York Mellon Trust Company, N.A. (formerly known as The Bank of New York Trust Company, N.A.), and Wells Fargo Bank, National Association, as trustee, and related Form of 2021 Note and Form of 2041 Note. | 8-K | 1-13252 | 4.2 | February 28, 2011 |
| 4.5 | | 8-K | 1-13252 | 4.1 | December 4, 2012 |
| 4.6 | | 8-K | 1-13252 | 4.2 | December 4, 2012 |
| 4.7 | | 8-K | 1-13252 | 4.2 | March 8, 2013 |
| 4.8 | | 8-K | 1-13252 | 4.2 | March 10, 2014 |
| 4.9 | | 8-K | 1-13252 | 4.1 | February 17, 2017 |
| 4.10 | | 8-K | 1-13252 | 4.1 | February 13, 2018 |
| 4.11 | | 8-K | 1-13252 | 4.1 | February 21, 2018 |
| 4.12 | | 8-K | 1-13252 | 4.1 | November 30, 2018 |
| 4.13 | | 8-K | 1-13252 | 4.1 | December 3, 2020 |
| 4.14† | | — | — | — | — |
| 10.1* | | 10-K | 1-13252 | 10.4 | June 10, 2004 |
| 10.2* | | 10-K | 1-13252 | 10.6 | June 6, 2003 |
| 10.3* | | 10-Q | 1-13252 | 10.2 | October 30, 2019 |
| 10.4* | | 10-K | 1-13252 | 10.7 | May 7, 2008 |
| 10.5* | | 10-Q | 1-13252 | 10.1 | October 30, 2019 |
| 10.6* | | 8-K | 1-13252 | 10.1 | January 25, 2010 |
| 10.7* | | 10-K | 1-13252 | 10.11 | May 7, 2013 |
| 10.8* | | 10-K | 1-13252 | 10.8 | May 22, 2020 |
| 10.9* | | 8-K | 1-13252 | 10.1 | July 31, 2015 |
| 10.10* | | 10-Q | 1-13252 | 10.1 | July 29, 2015 |
| | | | | | | | | | | | | | | | | |
| | Incorporated by Reference |
| Exhibit Number | Description | Form | File Number | Exhibit | Filing Date |
| 10.11* | | 10-Q | 1-13252 | 10.1 | October 25, 2018 |
| 10.12* | | 10-K | 1-13252 | 10.14 | May 5, 2016 |
| 10.13* | | 10-Q | 1-13252 | 10.4 | July 30, 2010 |
| 10.14* | | 10-Q | 1-13252 | 10.2 | July 26, 2012 |
| 10.15* | | 8-K | 1-13252 | 10.1 | August 2, 2013 |
| 10.16*† | | — | — | — | — |
| 10.17 | | 8-K | 1-13252 | 10.1 | March 7, 2017 |
| 10.18 | | 10-K | 1-13252 | 10.19 | May 5, 2016 |
| 10.19 | Credit Agreement, dated as of October 22, 2015, among the Company and Certain Subsidiaries, as Borrowers, Bank of America, N.A. as Administrative Agent, Bank of America, N.A. (acting through its Canada Branch), Citibank, N.A. and Barclays Bank PLC, as Swing Line Lenders, Wells Fargo Bank, National Association as L/C Issuer, Barclays Bank PLC, Citibank N.A., Wells Fargo Bank, National Association as Co-Syndication Agents, Goldman Sachs Bank USA, JPMorgan Chase Bank, N.A., The Bank of Tokyo-Mitsubishi UFJ, Ltd. as Co-Documentation Agents, and The Other Lenders Party Thereto, and Merrill Lynch, Pierce, Fenner & Smith Incorporated, Barclays Bank PLC, Citigroup Global Markets Inc., Goldman Sachs Bank USA, J.P. Morgan Securities, LLC, The Bank of Tokyo-Mitsubishi UFJ, Ltd. and Wells Fargo Securities, LLC as Joint Lead Arrangers and Joint Book Runners. | 8-K | 1-13252 | 10.1 | October 23, 2015 |
| 10.20 | Amendment No. 2, dated January 30, 2014, and Amendment No. 1, dated November 15, 2013, to the Credit Agreement and the Credit Agreement dated as of September 23, 2011, among the Company and McKesson Canada Corporation, collectively, the Borrowers, Bank of America, N.A. as Administrative Agent, Bank of America, N.A. (acting through its Canada branch), as Canadian Administrative Agent, JPMorgan Chase Bank, N.A. and Wells Fargo Bank, National Association, as Co-Syndication Agents, Wells Fargo Bank, National Association as L/C Issuer, The Bank of Tokyo-Mitsubishi UFJ, LTD., The Bank of Nova Scotia and U.S. Bank National Association as Co-Documentation Agents, and The Other Lenders Party Thereto, and Merrill Lynch, Pierce, Fenner & Smith Incorporated, Sole Lead Arranger and Sole Book Manager. | 8-K | 1-3252 | 10.1 | February 5, 2014 |
| | | | | | | | | | | | | | | | | |
| | Incorporated by Reference |
| Exhibit Number | Description | Form | File Number | Exhibit | Filing Date |
| 10.21 | Credit Agreement dated as of September 25, 2019, among the Company and certain subsidiaries, as borrowers, Bank of America, N.A., as administrative agent, Barclays Bank PLC, Citibank, N.A., Wells Fargo Bank, National Association, Goldman Sachs Bank USA, JPMorgan Chase Bank, N.A., and HSBC Securities (USA) Inc., as co-syndication agents, the lenders party thereto, the letter of credit issuers party thereto ("2020 Credit Facility"). | 8-K | 1-13252 | 10.1 | September 27, 2019 |
| | 8-K | 1-13252 | 10.1 | April 2, 2021 |
| | 8-K | 1-13252 | 10.2 | April 2, 2021 |
| 10.22* | | 10-K | 1-13252 | 10.27 | May 4, 2010 |
| 10.23 | | 8-K | 1-13252 | — | March 23, 2020 |
| 10.24 | | 8-K | 1-13252 | 10.1 | March 13, 2020 |
| 21† | | — | — | — | — |
| 23† | | — | — | — | — |
| 31.1† | | — | — | — | — |
| 31.2† | | — | — | — | — |
| 32†† | | — | — | — | — |
| 101† | The following materials from the McKesson Corporation Annual Report on Form 10-K for the fiscal year ended March 31, 2021, formatted in Inline Extensible Business Reporting Language (iXBRL): (i) the Consolidated Statements of Operations, (ii) Consolidated Statements of Comprehensive Income, (iii) Consolidated Balance Sheets, (iv) Consolidated Statements of Stockholders' Equity, (v) Consolidated Statements of Cash Flows, and (vi) related Financial Notes. | — | — | — | — |
| 104† | Cover Page Interactive Data File (formatted as iXBRL and contained in Exhibit 101). | — | — | — | — |
________________
* Management contract or compensation plan or arrangement in which directors and/or executive officers are eligible to participate.
† Filed herewith.
†† Furnished herewith.
Registrant agrees to furnish to the Commission upon request a copy of each instrument defining the rights of security holders with respect to issues of long-term debt of the registrant, the authorized principal amount of which does not exceed 10% of the total assets of the registrant.
Item 16. Form 10-K Summary
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| | | MCKESSON CORPORATION |
| | | |
| Date: May 12, 2021 | | /s/ Britt J. Vitalone |
| | | Britt J. Vitalone |
| | | Executive Vice President and Chief Financial Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated:
| | | | | | | | |
| /s/ Brian S. Tyler | | /s/ Marie L. Knowles |
Brian S. Tyler Chief Executive Officer and Director (Principal Executive Officer) | | Marie L. Knowles, Director |
| | |
| /s/ Britt J. Vitalone | | /s/ Bradley E. Lerman |
Britt J. Vitalone Executive Vice President and Chief Financial Officer (Principal Financial Officer) | | Bradley E. Lerman, Director |
| | |
| /s/ Sundeep G. Reddy | | /s/ Linda Mantia |
Sundeep G. Reddy Senior Vice President and Controller (Principal Accounting Officer) | | Linda Mantia, Director |
| | |
| /s/ Dominic J. Caruso | | /s/ Maria Martinez |
| Dominic J. Caruso, Director | | Maria Martinez, Director |
| | |
| /s/ N. Anthony Coles | | /s/ Edward A. Mueller |
N. Anthony Coles, M.D., Director
| | Edward A. Mueller, Director |
| | |
| /s/ M. Christine Jacobs | | /s/ Susan R. Salka |
| M. Christine Jacobs, Director | | Susan R. Salka, Director |
| | |
| /s/ Donald R. Knauss | | /s/ Kenneth E. Washington |
| Donald R. Knauss, Director | | Kenneth E. Washington, Director |
| | |
| Date: May 12, 2021 | | |


