UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number
(Exact name of Registrant as specified in its Charter)
|
|
|
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
|
|
|
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
|
|
|
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ NO ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such
files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
Large accelerated filer |
|
☐ |
|
|
|
☒ |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
Non-accelerated filer |
|
☐ |
|
Smaller reporting company |
|
|
Emerging growth company |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES
The aggregate market value of the Common Stock held by shareholders other than officers, directors or holders of more than 5% of the outstanding stock of the Registrant as of March 31, 2020 was approximately $
The number of shares of Registrant’s Common Stock outstanding as of November 30, 2020 was
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement for the Registrant’s 2021 Annual Meeting of Shareholders are incorporated by reference into Part III.
TABLE OF CONTENTS
|
|
|
Page |
|
|
3 |
|
|
|
|
|
|
Part I |
||
|
|
||
|
Item 1. |
5 |
|
|
|
17 |
|
|
Item 1A. |
19 |
|
|
Item 1B. |
31 |
|
|
Item 2. |
31 |
|
|
Item 3. |
31 |
|
|
Item 4. |
31 |
|
|
|
|
|
|
Part II |
||
|
|
||
|
Item 5. |
32 |
|
|
Item 6. |
34 |
|
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
34 |
|
Item 7A. |
46 |
|
|
Item 8. |
47 |
|
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
81 |
|
Item 9A. |
81 |
|
|
Item 9B. |
81 |
|
|
|
|
|
|
Part III |
||
|
|
||
|
Item 10. |
82 |
|
|
Item 11. |
82 |
|
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
82 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
82 |
|
Item 14. |
82 |
|
|
|
|
|
|
Part IV |
||
|
|
||
|
Item 15. |
83 |
|
|
|
86 |
|
|
|
|
|
Forward-looking Statements
Certain statements contained in this Form 10-K, or in other reports of the Company and other written and oral statements made from time to time by the Company, do not relate strictly to historical or current facts. As such, they are considered “forward-looking statements” that provide current expectations or forecasts of future events. These forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, expectations concerning: the impacts, duration and severity of the global COVID-19 pandemic and the effects of responses to it on healthcare systems, the general economy, our business partners, and our operations; clinical studies, their results and the potential timing of future clinical studies; our growth strategy, including our ability to sign new license agreements, conduct market evaluations, and bring new products to market; the development of future products and their anticipated attributes; regulatory submissions and approvals; the potential impact of FDA communications; the initiation of product evaluation activities; revenue potential related to the potential commercial launch of the SurVeil™ drug-coated balloon (“DCB”); future revenue growth and our future success; future gross margins and operating expenses; estimated future amortization expense; recognition of unrecognized compensation costs; anticipated patent expirations and their potential impacts on our royalties revenue; potential future customer actions; milestone achievements; research and development plans and expenses, including the estimated cost associated with the TRANSCEND clinical trial; future cash flow and sources of funding, and their ability together with existing cash, cash equivalents, and investments to provide liquidity sufficient to meet our cash needs and fund our operations and planned capital expenditures for the next twelve months; future property and equipment investment levels; plans regarding our securities investments; the impact of potential lawsuits or claims; where our manufacturing activities will take place for various categories of products; the impact of potential change in raw material prices, sources of raw materials and our ability to manufacture raw materials ourselves; the impact of Abbott, Medtronic, as well as other significant customers; our ability to recognize the expected benefits of our acquisitions; our strategic transformation to become a provider of whole-product solutions; future income tax (benefit) expense; the future impact of off-balance sheet arrangements and contractual obligations; and the impact of the adoption of new accounting pronouncements. Without limiting the foregoing, words or phrases such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “intend,” “may,” “plan,” “possible,” “project,” “will” and similar terminology, generally identify forward-looking statements. Forward-looking statements may also represent challenging goals for us. These statements, which represent our expectations or beliefs concerning various future events, are based on current expectations that involve a number of risks and uncertainties that could cause actual results to differ materially from those of such forward-looking statements. We caution that undue reliance should not be placed on such forward-looking statements, which speak only as of the date made. Some of the factors which could cause results to differ from those expressed in any forward-looking statement are set forth under “Risk Factors” in Part I, Item 1A of this Annual Report on Form 10-K. We disclaim any intent or obligation to update publicly these forward-looking statements, whether because of new information, future events or otherwise.
Although it is not possible to create a comprehensive list of all factors that may cause actual results to differ from our forward-looking statements, such factors include, among others:
|
• |
the impacts, duration and severity of the global COVID-19 pandemic, which has impacted, and may continue to impact, our revenue, operations, the conduct of clinical studies, and our ability to access healthcare professionals and facilities; |
|
• |
our reliance on a small number of significant customers, including our largest customers, Abbott and Medtronic, which causes our financial results and stock price to be subject to factors affecting those significant customers and their products, the timing of market introduction of their or competing products, product safety or efficacy concerns and intellectual property litigation impacting such customers, which could adversely affect our growth strategy and the royalties revenue we derive; |
|
• |
clinical and regulatory developments relating to the evaluation of risks associated with paclitaxel-coated products, which developments may adversely impact our ability to complete our TRANSCEND clinical trial on any particular time frame, obtain marketing approval (or the timing of any such approval) for our SurVeil DCB and other paclitaxel-coated products, to treat peripheral artery disease in the femoral and/or popliteal arteries; |
|
• |
our ability to successfully develop, obtain regulatory approval for, and commercialize our SurVeil DCB product, including our reliance on clinical research organizations to manage the TRANSCEND clinical trial and uncertainty related to the impacts of any clinical research relative to drug-coated balloons, including our Avess™ DCB, other DCB products and other catheter and balloon-based products, which will impact our ability to receive additional milestone payments under our agreement with Abbott; |
|
• |
general economic conditions that are beyond our control, such as the impact of recession, customer mergers and acquisitions, business investment, changes in consumer confidence, and medical epidemics or pandemics such as the COVID-19 pandemic, which has negatively impacted, and will likely continue to negatively impact, our business and results from operations; |
|
• |
a decrease in our available cash or failure to generate cash flows from operations, which could impact short-term liquidity requirements and expected capital and other expenditures; |
|
• |
our ability to comply with the covenants in our credit facility; |
3
|
• |
the difficulties and uncertainties associated with the lengthy and costly new product development and foreign and domestic regulatory approval processes, such as delays, difficulties or failures in achieving acceptable clinical results or obtaining foreign or United Stated (“U.S.”) Food and Drug Administration (“FDA”) marketing clearances or approvals, which may result in lost market opportunities, failure to bring new products to market or postpone or preclude product commercialization by licensees or ourselves; |
|
• |
whether operating expenses that we incur related to the development and commercialization of new technologies and products are effective; |
|
• |
our ability to successfully perform product development activities, the related R&D expense impact and governmental and regulatory compliance activities, which we have not previously undertaken in any significant manner; |
|
• |
our ability to identify and execute new acquisition opportunities and successfully managing the risks associated with acquisitions, which include the potential inability to integrate acquired operations, personnel, technology, information systems, and internal control systems and products; a lack of understanding of tax, legal and cultural differences for non-U.S. acquisitions; diversion of management’s attention; difficulties and uncertainties in transitioning the customers or other business relationships from the acquired entity to us; the loss of key employees of acquired companies; and potential impacts on cash flows; and |
|
• |
other factors described under “Risk Factors” in Part I, Item 1A of this Annual Report on Form 10-K, which you are encouraged to read carefully. |
Many of these factors are outside our control and knowledge and could result in increased volatility in period-to-period results. Investors are advised not to place undue reliance upon our forward-looking statements and to consult any further disclosures by us on this subject in our filings with the SEC.
4
PART I
ITEM 1. BUSINESS
OVERVIEW
Surmodics, Inc. and subsidiaries (referred to as “Surmodics,” the “Company,” “we,” “us,” “our” and other like terms) is a leading provider of surface modification technologies for intravascular medical devices and chemical components for in vitro diagnostic (“IVD”) immunoassay tests and microarrays. Surmodics is pursuing development and commercialization of highly differentiated medical devices that are designed to address unmet clinical needs and engineered to the most demanding requirements. This key growth strategy leverages the combination of the Company’s expertise in proprietary surface technologies, along with enhanced device design, development, and manufacturing capabilities. The Company mission remains to improve the detection and treatment of disease. Surmodics is headquartered in Eden Prairie, Minnesota.
We operate two reportable segments:
|
• |
Medical Device: Surface modification coating technologies to improve access, deliverability, and predictable deployment of medical devices, as well as drug-delivery coating technologies to provide site-specific drug-delivery from the surface of a medical device, with end markets that include coronary, peripheral, neuro-vascular, and structural heart, among others; and the design, development, and manufacture of interventional medical devices, primarily balloons and catheters, including drug-coated balloons, for peripheral arterial disease treatment and other applications; and |
|
• |
In Vitro Diagnostics (“IVD”): Design, development and manufacture of component products and technologies for diagnostic immunoassay, as well as molecular tests and biomedical research applications, with products that include protein stabilization reagents, substrates, surface coatings and antigens. |
We derive our revenue from three primary sources:
|
• |
Product revenues from the sale of chemical components within our IVD segment, including stabilization products, substrates, surface coatings and antigens to the diagnostic and biomedical research markets; the sale of reagent chemicals to licensees within our Medical Device segment; and the sale of medical devices and related products (such as balloons and catheters) to original equipment manufacturer (“OEM”) suppliers and distributors within our Medical Device segment; |
|
• |
Royalties and license fees from licensing our proprietary surface modification coating and medical device technologies to medical device manufacturers within our Medical Device segment; and |
|
• |
Research and development fees generated from contract coating services within our Medical Device segment and new product development and testing within our Medical Device and IVD segments. |
Revenue fluctuates from quarter to quarter depending on, among other factors: our customers’ success in selling products incorporating our technologies; the occurrence of milestone events under our development contracts; the timing of introductions of licensed products by our customers and proprietary products by us and our distributors; the timing of introductions of products that compete with our, and our customers’, products; the number and activity level associated with customer development projects; the number and terms of new license agreements that are finalized; and the value of reagent chemicals, medical device and diagnostic products sold to our customers.
The information below provides an overview of the principal products, services and markets for each of our two reportable segments. The discussion of other aspects of our business including research and development (“R&D”), intellectual property, marketing and sales, significant customers, competition, manufacturing, government regulation, and our human capital applies to our business in general, and we describe material segment information within these sections where relevant.
We file annual reports, quarterly reports, proxy statements, and other documents with the SEC under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The SEC maintains a website that contains reports, proxy and information statements, and other information regarding issuers, including the Company, that file electronically with the SEC. The public may obtain any documents that we file with the SEC at http://www.sec.gov.
We make available, free of charge, copies of our annual report on Form 10-K, proxy statement, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act on our website, www.surmodics.com, as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the SEC. We are not including the information on our website as a part of, or incorporating it by reference into, our Form 10-K.
5
MEDICAL DEVICE SEGMENT
Our Medical Device segment centers on our proprietary surface modification coating technologies that impart lubricity, pro-healing, biocompatibility characteristics, or drug-delivery capabilities (together, “surface modification coating technologies”) to our customers’ and our own medical devices and delivery systems. Widespread practice of minimally invasive surgical procedures, which often employ catheter-based delivery technologies, has increased the importance of surface modification coating technologies, including drug-delivery coatings.
Advances in medical device technology have helped drive improved device efficacy and patient outcomes. The convergence of the pharmaceutical, biotechnology and medical device industries, often made possible by surface coatings and device drug-delivery technologies, presents an opportunity for major advancements in the healthcare industry. We believe the benefits of combining drugs and biologics with implantable and minimally invasive devices are becoming increasingly valuable in applications in cardiology, peripheral vascular disease, neurology, ophthalmology, orthopedic and other large interventional markets. Positive clinical outcomes and acceptance by patients, physicians and insurance companies of such innovations has helped certain segments of the U.S. medical device industry grow at a faster pace than the economy as a whole. Attractive growth rates for innovative medical products have generated intense competition among the companies participating in the medical device industry.
Historically, we provided our surface modification coating technologies to medical device manufacturers for use in their medical devices and delivery systems, and we continue to do so. In fiscal 2013, we initiated a strategy to develop and manufacture our own proprietary medical device products that combine our surface modification coating technologies with medical devices or delivery systems that we have designed (our “whole-product solutions”). We believe this strategy has increased, and will continue to increase, our relevance in the medical device industry. The strategy is key to our future growth and profitability, providing us with the opportunity to capture more revenue and operating margin with whole-product solutions than we would by licensing our device-enabling technologies. We also continue to develop and commercialize our surface modification coating technologies through license agreements with medical device manufacturers.
Medical Device Segment: Overview of Whole-product Solutions
Our aim is to develop highly differentiated medical devices that address unmet clinical needs, improve patient outcomes and reduce procedure costs. The initial focus of our product development for whole-product solutions has been on the vascular interventional treatment of peripheral artery disease (“PAD”) and other vascular diseases. PAD is a condition that causes a narrowing of the blood vessels supplying the extremities, most often due to plaque buildup in the arterial walls. Left untreated, PAD may lead to symptoms such as large non-healing ulcers, infections, or gangrene, and may require limb amputation or, in extreme cases, result in death. Over 200 million people are living with PAD worldwide, and 12% to 20% of Americans over 60 years old have PAD. The number of people affected by PAD is expected to increase as a result of an aging population, coupled with the increasing prevalence of conditions linked to PAD, such as diabetes and obesity. The interventional PAD market utilizes a variety of access and therapy catheters to treat PAD. These technologies are delivered through a number of access points into the vascular system including femoral (leg), radial (wrist or arm), and pedal (foot).
Drug-coated balloon (“DCB”) products are a natural application for our surface modification coating technologies to medical devices. We acquired Creagh Medical Ltd. (“Creagh Medical”), located in Ballinasloe, Ireland, in fiscal 2016 to establish our angioplasty balloon catheter development and production capabilities. Creagh Medical has state-of-the-art balloon catheter research, development and manufacturing facilities and expertise. Since the acquisition, we have expanded our medical device expertise through investments in R&D personnel and facilities, the acquisition of an early-stage device technology with multiple potential peripheral vascular applications, and the acquisition of an innovative thrombectomy platform technology with potential applications in peripheral vascular and other areas.
We are focused on developing devices that meet the needs of a spectrum of care settings ranging from hospitals, to ambulatory surgery centers, to office-based interventional labs. Our pipeline of medical device products under development and recently commercialized for the treatment of PAD and other vascular diseases includes the following platforms: (1) drug-coated balloons; (2) radial access devices; (3) thrombectomy devices; and (4) specialty catheters.
Drug-coated Balloons
DCBs are currently used in a variety of vascular interventions and may be helpful in preventing restenosis, or the narrowing of vessels, after treatment. DCBs have transformed intravascular therapies by enhancing patient outcomes, while not leaving stents in the vascular system. Surmodics is focused on the development of DCBs to treat PAD.
6
SurVeil™ DCB – The development of our SurVeil DCB to treat the superficial femoral artery over the past several years has been a major component of our whole-product solutions strategy. Our SurVeil DCB is a next-generation device that utilizes best-in-class technology for the treatment of PAD, including a proprietary paclitaxel drug-excipient formulation for a durable balloon coating. It is manufactured using an innovative process to improve coating uniformity.
PREVEIL Early Feasibility Clinical Trial. During fiscal 2016, we initiated PREVEIL, an early feasibility clinical trial of the SurVeil DCB, which is intended to treat PAD in the leg above the knee. Enrollment in PREVEIL was completed in fiscal 2017, and the study met its primary endpoint by demonstrating peak paclitaxel plasma concentrations post-index procedure. Consistent with pre-clinical data, systemic drug levels were low and cleared rapidly. Data from the PREVEIL study continues to demonstrate excellent safety results, with 91.7% of treated patients free of clinically driven target lesion revascularization through 24 months.
TRANCEND Pivotal Clinical Trial. In fiscal 2017, we received an investigational device exemption (“IDE”) from the U.S. Food and Drug Administration (“FDA”) to initiate a pivotal clinical trial of the SurVeil DCB. The TRANSCEND trial will provide the data necessary to evaluate the safety and effectiveness of our SurVeil DCB compared with the Medtronic IN.PACT® Admiral® DCB in treating PAD in the upper leg. The trial enrolled 446 subjects at 65 global sites. The trial’s primary efficacy endpoint is primary patency, defined as a composite of freedom from restenosis and clinically-driven target lesion revascularization through 12 months post-index procedure. All randomized subjects will be followed through 60 months post-index procedure. If successful, the TRANSCEND clinical trial data will be used to support application for regulatory approval and reimbursement for the SurVeil DCB in the U.S. We estimate that the total cost of the TRANSCEND clinical trial will range between $35 million to $40 million from inception to completion. TRANSCEND trial enrollment began in the first quarter of fiscal 2018 and was completed in the fourth quarter of fiscal 2019. Patient 12-month follow-up visits have successfully concluded, and we have achieved a sufficient level of follow-up visits to evaluate the primary endpoints. In fiscal 2021, we expect to submit the final clinical report to the FDA for premarket approval (“PMA”). There is no assurance that the data from the TRANSCEND clinical trial will support regulatory approval, or that any anticipated time frame will be met.
Abbott Agreement. In fiscal 2018, we entered into an agreement with Abbott Vascular, Inc. (“Abbott”) that provided Abbott with exclusive worldwide commercialization rights for the SurVeil DCB (the “Abbott Agreement”). Pursuant to the terms of the Abbott Agreement, the Company has received, as of September 30, 2020, upfront and milestone payments totaling $45.8 million. The Company may receive up to $45 million of additional contingent milestone payments, pursuant to the terms of the Abbott Agreement, consisting of: (i) $15 million upon successful completion of the clinical study report of the TRANSCEND pivotal trial demonstrating safety and clinical non-inferiority with the control device and (ii) $30 million upon PMA of our SurVeil DCB by the FDA. Separately, Abbott also has the option to negotiate an agreement for Surmodics' below-the-knee SundanceTM DCB product.
Surmodics is responsible for conducting all necessary clinical trials and other activities required to achieve U.S. and European Union (“E.U.”) regulatory clearances for the SurVeil DCB, including completion of the ongoing TRANSCEND pivotal clinical trial. Expenses related to these activities are paid by Surmodics. Abbott and Surmodics participate on a joint development committee charged with providing guidance on the Company’s clinical and regulatory activities related to the SurVeil DCB product. Upon commercial launch of the SurVeil DCB by Abbott, Surmodics will be responsible for manufacturing clinical and commercial quantities of the product and will realize revenue from product sales to Abbott, as well as a share of profits resulting from sales to third parties.
Status of Regulatory Approval and Commercialization. In the third quarter of fiscal 2020, we received Conformité Européenne Mark (“CE Mark”) approval, which is a prerequisite for commercialization of the SurVeil DCB in the E.U. The timeline for commercialization of the SurVeil DCB in the E.U. is to be determined at the discretion of Abbott, subject to the terms of the Abbott Agreement. Unless and until FDA regulatory approval has been obtained, our SurVeil DCB is not approved for commercial sale in the U.S.
Paclitaxel Long-term Mortality Signal. On March 15, 2019, the FDA issued a communication (the “FDA communication”) to healthcare providers about the potential for increased long-term mortality after use of paclitaxel-coated balloons and paclitaxel-eluting stents (collectively “paclitaxel-coated products”) to treat PAD in the femoropopliteal artery. The FDA communication updated a previous notification from the FDA on the same topic, which was in response to meta-analysis of randomized trials published in the Journal of the American Heart Association in December 2018. Subsequently, in August 2019, the FDA issued an update on the use of paclitaxel devices to treat PAD that recommended that physicians discuss the risks and benefits of all available treatment options with their patients. The FDA communication and the potential long-term mortality signal related to the use of paclitaxel-coated devices may adversely affect market acceptance of our paclitaxel-coated DCB products or the willingness of Abbott to commercialize the SurVeil DCB.
7
AvessTM DCB – Our paclitaxel-coated Avess DCB is used for the treatment of arteriovenous (“AV”) fistulae commonly associated with hemodialysis in patients with end-stage renal disease (“ESRD”). It is estimated that approximately 800,000 Medicare patients and nearly five million patients worldwide live with ESRD. Our Avess DCB includes a proprietary drug-excipient formulation for the balloon coating and is manufactured using a proprietary process to improve coating uniformity. Pre-clinical data for our Avess DCB has shown a three to five times higher target tissue drug concentration, a more evenly distributed and durable drug effect, and lower incidence of downstream drug concentrations compared to control DCBs. In fiscal 2019, we commenced and completed enrollment in a first in-human, 12-patient clinical study of our Avess DCB. In fiscal 2020, initial study results were received and demonstrated promising early safety data and performance insights, with greater than 90% of treated patients free from revascularization at six months. We plan to further evaluate the initial first-in human study results for our Avess DCB in conjunction with relevant TRANSCEND study data when it is available.
Sundance DCB – Our sirolimus-coated Sundance DCB is used for the treatment of below-the-knee PAD, otherwise known as critical limb ischemia (“CLI”). CLI is estimated to impact between 2.1 million and 3.8 million Americans, a number that could grow to between 2.4 million and 4.7 million by 2030. Rates of amputation and death are significant for CLI patients and there are currently no drug-delivery devices approved to treat the condition in the U.S. In October 2019, the FDA designated the Sundance DCB as a “Breakthrough Device” under the FDA’s Breakthrough Devices Program, which is designed to streamline the market clearance/approval process for products that have the potential to provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. In fiscal 2019, we froze the design of our Sundance DCB and submitted an application for a first in-human study of this device. We commenced the SWING first in-human, 35-patient clinical study in the third quarter of fiscal 2020. We expect to complete enrollment in the SWING clinical study in the second half of fiscal 2021.
Radial Access
We are developing a series of devices designed to provide radial (wrist) access to the peripheral vasculature, our SublimeTM radial access platform. Radial artery access, which has already been widely adopted for coronary interventions, offers many benefits relative to traditional femoral artery access. These benefits include reduced bleeding complications, earlier ambulation, and reduced length of stay and costs. We believe the integration of our catheter and coatings technologies will result in highly differentiated radial access devices intended to capture market share from standard femoral access devices.
Sublime radial access platform – In fiscal 2019, we received FDA 510(k) clearance for our Sublime guide sheath, which enables the performance of lower extremity interventions from the radial artery. In the third quarter of fiscal 2020, we received FDA 510(k) clearance for the Sublime radial-access 0.014” percutaneous transluminal angioplasty (“PTA”) balloon catheter for treatment of lesions in arteries below the knee. An important precursor to commercialization of our radial access platform devices is establishment of product performance experience through physician evaluations in real-world case settings. These evaluations are designed to assess the human use factors and performance of these devices. We are targeting the first half of fiscal 2021 to initiate product evaluation activities for our Sublime guide sheath and 0.014” PTA balloon catheter products.
In the fourth quarter of fiscal 2020, we froze the design of our Sublime 0.018” PTA balloon catheter, and we expect to pursue FDA 510(k) clearance in fiscal 2021. Looking forward, our goal is to continue to expand the Sublime radial access platform through development and regulatory approval of additional complementary products with distinct applications.
Thrombectomy
Acute vascular occlusion, or the blocking of arteries by clots or plaque, is another peripheral vascular condition commonly associated with PAD. Often, these clots require surgical intervention and have proven difficult to remove with currently available medical device technologies. A similar condition in the venous system, known as Venous Thromboembolism (“VTE”), includes both pulmonary embolism and deep vein thrombosis. VTE has a high prevalence in the U.S. and high overall and in-hospital mortality rates, which causes strain on the U.S. healthcare system.
We look to leverage our proprietary Pounce™ thrombectomy platform technology to develop products to treat these conditions in a more effective, cost-efficient manner than currently available treatments. The Pounce technology platform is designed for the non-surgical removal of thrombi and emboli (clots) from the vasculature. The technology offers an innovative design that may reduce the need for the use of thrombolytics. Thrombolytics are often associated with complications, which can include bleeding complications, longer hospital stays and higher cost of treatment. Our goal with this technology is to reduce procedure time and eliminate the need for additional external capital equipment, thereby providing an easy-to-use, on-the-table solution for clinicians.
8
Pounce Thrombus Retrieval System – In the fourth quarter of fiscal 2020, we received FDA 510(k) clearance on our first thrombectomy device, the Pounce Thrombus Retrieval System, intended for the non-surgical removal of thrombi and emboli (clots) from the peripheral arterial vasculature. Depending on the age and magnitude of the occlusion and the viability of the threatened limb, existing treatments for this condition may include catheter directed thrombolysis, surgical embolectomy, and/or percutaneous mechanical thrombectomy. In cases in which the occlusion has caused irreversible damage to the limb, acute limb ischemia can result in the amputation of a lower extremity. The Pounce Thrombus Retrieval System is a mechanical thrombectomy device that facilitates thrombus removal in the peripheral vasculature without the added expense or commitment to any additional, external capital equipment. The device consists of three components: a basket delivery catheter, a basket wire assembly, and a trumpet assembly. After the basket wire assembly is delivered distal to the location of the thrombus, two nitinol self-expanding baskets are deployed to collect and entrain the clot into a trumpet-shaped nitinol wire mesh. With the clot entrained, the trumpet assembly is then collapsed into a guide sheath through which the clot is withdrawn and removed from the body. The Pounce Thrombus Retrieval System is a standalone mechanical thrombus removal system that eliminates the need for capital equipment and may reduce the need for thrombolytics and complex procedures. We expect to initiate product evaluation activities for our Pounce Thrombus Retrieval System in the second half of fiscal 2021 to assess human-use factors and product performance prior to commercialization.
Looking forward, our goal is to expand our thrombectomy development efforts to serve other clinical indications, which may include deep vein thrombosis, pulmonary embolism and ischemic stroke.
Specialty Catheters
Interventional vascular procedures often require one or more devices to provide appropriate access and necessary support for other interventional devices. The integration of our proprietary balloon catheter technology, ultra-thin-walled capabilities, and surface modification coating technologies enable the development of our highly differentiated portfolio of medical devices designed to improve currently available minimally-invasive PAD treatments, or in some cases offer an option for complex cases. Our specialty catheters are designed for high performance in challenging vascular anatomy, providing clinicians enhanced ability to access, cross and treat increasingly complex vascular lesions.
Telemark™ – Telemark is a coronary/peripheral support microcatheter. In fiscal 2019, we executed an agreement with Medtronic plc (“Medtronic”) to distribute our Telemark microcatheter in the U.S. and Europe for coronary applications. Shipment of initial U.S. orders of our Telemark microcatheter commenced early in fiscal 2020. In the third quarter of fiscal 2020, we obtained CE Mark for our Telemark microcatheter and shipped initial European orders.
0.014” and 0.018” low-profile PTA balloon dilation catheters – Our specialty catheter products include PTA balloon catheters for difficult-to-treat lesions. In fiscal 2019, we executed an agreement with Cook Medical for worldwide distribution, excluding Japan, of these products. Shipment of initial orders and the U.S. commercial launch commenced in the third quarter of fiscal 2020.
For all our products under development, as further described under the caption “Government Regulation” below, the expected timing and potential success of regulatory approval and commercialization for the products pending regulatory approval can vary greatly given the significant uncertainty inherent in product development and regulatory approval processes.
Medical Device Segment: Overview of Surface Modification Coating Technologies
Our business model for our surface modification coating technology is to leverage our proprietary product portfolio, as well as unique collaborative research, development and manufacturing capabilities, to enable our customers to improve their existing products or develop entirely new devices using surface modification coating technologies as product differentiators or device enablers. The continuing trend toward minimally invasive surgical procedures, which often employ catheter-based delivery technologies, has increased the demand for hydrophilic (i.e., lubricious or slippery) coatings and other coating technologies, including drug-delivery coatings. For example, stents, particularly drug-eluting stents, have significantly reduced the need for repeat intravascular procedures or more invasive cardiac bypass surgery. Transcatheter heart valve repair or replacement via a minimally invasive catheter-based system has enabled the treatment of patients suffering from heart valve disease who are too ill to undergo open-heart surgery.
Key differentiating characteristics of our coating platforms are their flexibility, durability and ease of use. In terms of flexibility, coatings can be applied to many kinds of surfaces and can immobilize a variety of chemical, pharmaceutical and biological agents. Additionally, the surface modification process can be tailored to provide customers with the ability to improve their devices’ performance by choosing the specific coating properties desired for particular applications. Our surface modification coating technologies can also be combined to deliver multiple surface-enhancing characteristics on the same device.
9
Hydrophilic Coatings
Our proprietary PhotoLinkTM coating technology (“PhotoLink Technology”) is a versatile, easily applied, coating technology that modifies medical device surfaces by creating covalent bonds between device surfaces and a variety of chemical agents. PhotoLink Technology can impart many performance-enhancing characteristics, such as advanced lubricity (slippery) and hemocompatibility (preventing blood clot formation), when bound onto surfaces of medical devices or other biological materials without materially changing the dimensions or other physical properties of devices.
PhotoLink Technology reagents can be applied to a range of substrates. The coating formulations are easily applied to the material surface by a variety of methods including, but not limited to, dipping, spraying, roll-coating or ink-jetting. We continue to expand our proprietary reagent portfolio for use by our customers. These reagents enable our customers to develop novel surface features for their devices, satisfying the expanding healthcare industry requirements. We are also continually working to expand the list of materials that are compatible with our surface modification and device drug-delivery reagents. Additionally, we develop coating processes and coating equipment to meet the device quality, manufacturing throughput, and cost requirements of our customers.
The PhotoLink Technology coating process is relatively simple to use and is easily integrated into the customer’s manufacturing operations. In addition, the process does not subject the coated products to harsh chemical or temperature conditions, produces no hazardous byproducts, and does not require lengthy processing or curing time. Further, coatings incorporating the PhotoLink Technology are generally compatible with accepted sterilization processes, so the surface attributes are not lost when the medical device is sterilized.
The latest generation of our Photolink Technology, our SereneTM hydrophilic coating platform, optimizes lubricity and durability, while significantly reducing particulates generation. This next-generation, PhotoLink Technology-enabled coating has demonstrated excellent lubricity on a wide range of substrates and has been used on FDA-cleared coronary, peripheral and structural heart devices.
Drug-delivery Coatings
Our device drug-delivery coating technologies allow therapeutic drugs to be incorporated within our proprietary polymer matrices to provide controlled, site-specific release of the drug into the surrounding environment. The drug release can be tuned to elute quickly (within minutes to a few days) or slowly (from several months to over a year), illustrating the wide range of release profiles that can be achieved with our coating systems. On a wide range of devices, drug-eluting coatings can help improve device performance, increase patient safety, and enable innovative new treatments. DCBs are a typical example of short-term use drug-delivery devices. An example of longer-term drug-delivery devices is drug eluting stents. We work with companies in the medical device and biotechnology industries to develop specialized coatings that allow for the controlled release of drugs from device surfaces. We see at least three primary areas with strong future potential: (1) improving the function of a device which itself is necessary to treat the medical condition; (2) enabling site-specific drug delivery while limiting systemic exposure; and (3) enhancing the biocompatibility of a medical device to ensure that it continues to function over a long period of time.
Coating Licensing Arrangements
We commercialize our surface modification coating technologies primarily through licensing arrangements with medical device manufacturers. We believe this approach allows us to focus our resources on further developing new technologies and expanding our licensing activities. Many of our technologies have been designed to allow manufacturers to implement them easily into their own manufacturing processes so customers can control production and quality internally without the need to send their products to a contract manufacturer. We generate the largest proportion of our revenue through licensing arrangements. Royalties and license fees represented 42.8%, 48.4% and 43.6% of our total revenue in fiscal 2020, 2019 and 2018, respectively. Revenue from these licensing arrangements typically includes license fees and milestone payments, minimum royalties, and royalties based on a percentage of licensees’ product sales. We also generate revenue from reagent chemical product sales to licensees for use in their coating processes, as well as from providing contract coating services.
The licensing process for our coating technology licenses begins with the customer specifying a desired product feature to be created, such as lubricity or drug delivery. Because each device and coating application is unique, we routinely conduct a feasibility study to qualify each new potential product application, often generating commercial development revenue. Feasibility studies can range in duration from several months to a year. After we complete a feasibility study, our customers cannot market their product until they receive regulatory approval. As further described under the caption “Government Regulation,” the regulatory approval process varies in each country and ranges from several months to four or more years. At any time prior to a customer’s commercial launch, a license agreement may be executed granting the licensee rights to use our technology. We often support our customers by providing coating assistance for parts required in animal tests and human clinical trials. Typically, we complete a technology transfer to most customers which enables those customers to apply the coating at their own facilities.
10
License agreement terms are generally for a specified number of years or our patent’s life, whichever is longer, although a license generally may be terminated by the licensee for any reason with advance written notice. In cases where the royalty obligation extends beyond the life of the applicable patent, it is because the license also includes rights to our know-how or other proprietary rights. Under these circumstances, the royalty obligation typically continues at a reduced royalty rate for a specified number of years, generally tied to the date on which the licensee’s medical device product was first sold.
Our license agreements may include certain license fees and/or milestone payments. Substantially all our licensed coatings technology applications are nonexclusive, allowing us to license each technology to multiple customers. Moreover, even exclusive coatings technology licenses generally are limited to a specific “field of use,” allowing us the opportunity to further license technology to other customers. The royalty rate on a substantial number of the coatings agreements has traditionally been in the 2% to 3% range, but there are certain contracts with lower or higher rates. In certain agreements, our royalty is based on an agreed-upon amount per unit. License fees, milestone payments, and royalty rates are based on various factors, including the licensed product’s or technology’s stage of development, the perceived value of our technology to the customer’s product, the size of the potential market, and whether the arrangement is exclusive or nonexclusive. Our agreements often incorporate a minimum royalty to be paid by the licensee. Royalty payments generally commence one quarter after the customer’s actual product sales occur because of the delay in reporting sales by our licensees. Under the new revenue recognition guidance in effect in fiscal 2020 and 2019, we estimate and recognize sales-based royalties revenue from our coating technology licensees in the same quarter that the underlying customer product sale occurs. In fiscal 2018 under the legacy revenue recognition guidance, we recognized royalties revenue in the quarter that customer royalty payments were due to us.
We have over 150 licensed product classes (customer products utilizing Surmodics technology) already in the market generating royalties and greater than 100 customer product classes incorporating our technology in various stages of pre-commercialization.
Under our coatings technology license agreements, the responsibility for securing regulatory approval for and ultimately commercializing these products rests with our customers. Our reliance on our customers in this regard and the potential risks to our operations as a result are discussed in “Risk Factors” in Part I, Item IA of this Annual Report on Form 10-K. Moreover, we are often contractually obligated to keep the details concerning our customers’ R&D efforts (including the timing of expected regulatory filings, approvals and market introductions) confidential.
Our licensing agreements generally require us to keep our customers’ identities confidential, unless they approve of such disclosure. Licensed customers that allow the use of their name include: Abbott Laboratories and Abbott Vascular, Inc. (together, “Abbott”), Boston Scientific Corporation (“Boston Scientific”), Cook Medical, Cordis Corporation (a subsidiary of Cardinal Health, Inc.), Covidien PLC (a subsidiary of Medtronic), Edwards Lifesciences Corporation, Evalve, Inc. (a subsidiary of Abbott), ev3 Inc. (a subsidiary of Medtronic), Medtronic, OrbusNeich Medical, Inc., and Spectranetics Corporation (a subsidiary of Koninklijke Philips N.V.).
Coating Technology Patents
Medical Device royalties revenue from licensing our proprietary surface coating technology to customers was 30%, 35% and 38% of our total revenue for fiscal 2020, 2019 and 2018, respectively. The most significant source of royalties revenue was derived from our hydrophilic coating technology. The latest generation of our hydrophilic coating technology, our Serene hydrophilic coating, is protected by a family of patents that begin to expire in 2033. We are experiencing growth in revenue associated with our next-generation Serene hydrophilic coating technology driven by customer product launches and resulting market share increases associated with the customer device applications that incorporate this next-generation coating technology.
The family of patents that protected our fourth-generation PhotoLink hydrophilic coating technology expired in the first quarter of fiscal 2020 in all countries where patent coverage existed for the technology, except in Japan, where the relevant patent will expire in the first quarter of fiscal 2021. Medical Device royalties revenue associated with our fourth-generation hydrophilic coating technology was approximately 14%, 21% and 21% of our total revenue for fiscal 2020, 2019 and 2018, respectively. Of the license agreements using our fourth-generation Photolink and early-generation technologies, most continue to generate royalties revenue for know-how and other proprietary rights, at a reduced royalty rate, beyond patent expiration. Refer to caption “Patents and Proprietary Rights” within this section of this Annual Report on Form 10-K for further information on the Company’s patents.
11
IN VITRO DIAGNOSTICS SEGMENT
Our In Vitro Diagnostics segment manufactures and sells components for in vitro diagnostic immunoassay and molecular tests within the diagnostic, biomedical research, and life science markets. Our component products include protein stabilizers, substrates, surface coatings and antigens.
Immunoassay Diagnostics. An immunoassay is a biochemical test that measures the presence or concentration of a target molecule, or analyte, in a biological fluid or sample. Analyte levels are correlated to the patient’s disease state or medical condition to diagnose the presence, absence or severity of disease. Analytes can range from large molecules such as proteins to small molecules such as hormones. Immunoassays are developed and produced using multiple components. The component’s selection and optimization confer the assay quality and performance of the assay in terms of sensitivity and specificity. IVD companies select these critical biochemical and reagent components to meet the assay’s diagnostic specifications. We develop, manufacture and sell high-performing, consistent-quality and stable immunoassay component products to enable our customers’ diagnostic tests to detect the absence or presence of disease.
Molecular Diagnostics – DNA and Protein Immobilization. Both DNA and protein microarrays are useful tools for the pharmaceutical, diagnostic and research industries. During a DNA gene analysis, typically thousands of different probes need to be placed in a pattern on a surface, called a DNA microarray. These microarrays are used by the pharmaceutical industry to screen for new drugs; by genome mappers to sequence human, animal or plant genomes; or by diagnostic companies to search a patient sample for disease-causing bacteria or viruses. However, DNA does not readily adhere to most surfaces. We have developed various surface chemistries for both DNA and protein immobilization. Protein microarrays are used as diagnostic and research tools to determine the presence and/or quantity of proteins in a biological sample. The most common type of protein microarray is the antibody microarray, where antibodies are spotted onto a surface and used as capture molecules for protein detection.
The sales cycle for our IVD products generally begins when an IVD company initiates the process to develop a new, or improve a current, diagnostic test. During product development, these companies seek to source the test’s critical components with reagents that it produces internally or with reagents from a supplier, such as Surmodics.
As IVD tests are developed and various reagents are tested, companies will generally seek to optimize the sensitivity (false negative reductions), specificity (false positive reductions), speed (time from sample to results), convenience (ideally as few steps as possible), and cost effectiveness. Upon regulatory approval or clearance, the customer’s diagnostic test can be sold in the marketplace. It may take several years after approval or clearance for the test to achieve peak market share and optimize Surmodics’ revenue.
IVD Segment: Overview of Diagnostics Products
Protein Stabilizers. We offer a full line of stabilization products for the IVD market. These products increase sensitivity and specificity and reduce false positive and false negative results, while extending the diagnostic test’s shelf life, thereby producing more consistent assay results. Our stabilization products are ready-to-use, eliminating the in-house manufacturing preparation time and cost of producing stabilization and blocking reagents.
Substrates. We provide colorimetric and chemiluminescent substrates to the IVD market under our BioFX® trademark. A substrate is the diagnostic test kit component that detects and signals that a reaction has taken place so that a result can be recorded. Colorimetric substrates signal a positive diagnostic result through a color change. Chemiluminescent substrates signal a positive diagnostic result by emitting light. We believe that our substrates offer a high level of stability, sensitivity and consistency.
Surface Coatings for Molecular Diagnostic Applications. We offer custom coatings for molecular diagnostic applications, including DNA, RNA and protein microarrays. Our TRIDIA™ surface coatings bind molecules to a variety of surfaces and geometries and may be customized for selectivity using passivating polymers and reactive groups. This proprietary technology immobilizes DNA and protein to adhere to testing surfaces. We offer other surface coatings that improve flow characteristics through membranes and microfluidic channels on diagnostic devices, including point-of-care components.
Antigens and Antibodies. Antigens and Antibodies. We are the exclusive distributor in the U.S., Canada and Puerto Rico (and non-exclusive distributor in Japan) of DIARECT GmbH’s (“DIARECT”) line of antigens and antibodies. DIARECT produces the majority of these antigens and antibodies using recombinant technology.
12
OTHER FACTORS IMPACTING OUR OPERATIONS
Research and Development
In fiscal 2020, 2019 and 2018, R&D expenses totaled $50.2 million, $52.9 million and $41.0 million, respectively. R&D expenses primarily consist of research, development, clinical and regulatory activities necessary to design, develop and commercialize our products, as well as costs associated with our research, development and other revenue. We intend to continue investing significantly in R&D to advance our medical device platform technologies, surface modification coating technologies, and in vitro diagnostic technologies, as well as to expand uses for our technology platforms. We anticipate R&D expenses will continue to be significant in fiscal 2021, primarily related to medical device product development, including DCB development and related clinical study activities. In addition, we continue to pursue access to products and technologies developed outside the Company to complement our medical device platforms.
Medical Device Segment
Internal R&D. As treatment technologies become more sophisticated and increasingly leverage minimally invasive techniques, we believe the need for improved medical devices that benefit from surface modification and device drug delivery will continue to grow. We intend to continue our development efforts to expand our proprietary medical device offerings, including advancing our surface modification and device drug-delivery technologies to better meet these needs across multiple medical markets and to capture more of the final product value. Our medical device product development and clinical activities are primarily focused on the peripheral vascular market, where we believe the integration of our surface modification, balloon catheter, thrombectomy and ultra-thin-walled catheter technologies will result in unique devices capable of producing better patient outcomes in complex, difficult-to-treat vascular disease cases. Our product pipeline continues to be bolstered through developing and acquiring medical device technologies and funding development activities, which has included pre-clinical and clinical studies.
In fiscal 2019, the Company acquired an early-stage device technology with multiple potential peripheral vascular applications to complement our pipeline of medical devices for treatment of PAD. In fiscal 2018, we acquired an innovative thrombectomy platform technology with broad potential applications in peripheral vascular and other areas. These acquisitions resulted in acquired in-process R&D charges of $0.8 million and $7.9 million in fiscal 2019 and 2018, respectively. We plan to leverage our design, development and manufacturing capabilities to advance these acquired technology platforms for a variety of peripheral vascular applications as part of our whole-product solutions strategy. These acquisitions, in conjunction with our significant investments in our R&D infrastructure, facilities and personnel over the past several years, reflect our ongoing commitment to strengthen our proprietary product pipeline and broaden our capacity for medical device R&D activities. In fiscal 2018, we completed the build-out of an R&D-focused facility, which we lease in Eden Prairie, Minnesota. This accomplishment brought together the development teams focused on our DCB, radial access, thrombectomy, and specialty catheter technologies, as well as our internal regulatory team, in a state-of-the-art R&D facility in order to provide synergies and development efficiencies. Our facility in Ballinasloe, Ireland is focused on the design and manufacture of balloon-based peripheral vascular devices. This facility’s capabilities include balloon forming, extrusion, coating, braiding and assembly of finished products, with sufficient space for future growth. Our proprietary, whole-product solutions integrate our surface modification coatings, radial access, thrombectomy, catheter and balloon technologies and are being developed with a combined team from our U.S. and Irish facilities. For additional details on our significant product development R&D activities over the past three years, refer to caption “Medical Device Segment: Overview of Whole-product Solutions” within this section of this Annual Report on Form 10-K.
Customer R&D. With respect to revenue-generating R&D activities, we have R&D personnel and facilities specifically dedicated to delivering R&D services to our customers. We work with our customers to integrate the best possible surface modification and device drug-delivery technologies with their products, not only to meet their performance requirements, but also to perform services quickly so that the product may reach the market ahead of the competition. To quickly solve problems that might arise during the development and optimization process, we offer extensive capabilities in analytical chemistry and surface characterization within our R&D organization. Our state-of-the-art instrumentation and extensive experience allow us to test the purity of coating reagents, to monitor the elution rate of drug from coatings, to measure coating thickness and smoothness, and to map the distribution of chemicals throughout coatings. We believe our capabilities in this area exceed those of our competitors. Our R&D staff support our business development staff and business units in performing feasibility studies, as well as providing technical assistance to existing and potential customers. These services, which generate our research, development and other revenue, include optimizing the relevant technologies for specific customer applications; supporting clinical trials; training customers; and integrating our technologies and know-how into customer manufacturing operations.
In Vitro Diagnostics Segment
Our R&D efforts to grow our IVD business segment include identifying and addressing unmet needs that exist in the global IVD marketplace. Our pipeline of IVD products includes components for immunoassay and molecular diagnostic applications, such as new protein stabilizers, detection technologies, accessory reagents and surface coatings that have the potential to add greater sensitivity, specificity, speed, convenience, and lower cost for IVD test manufacturers.
13
Clinical Trials
Our DCB products, which combine a pharmaceutical drug with a medical device, are required to go through clinical studies for us to obtain regulatory approval or clearance to market the product in the U.S. Each clinical trial includes a primary endpoint or endpoints, which measure effectiveness and/or safety of a device based on the product’s ability to achieve a pre-specified outcome or outcomes and is selected based on the proposed intended use of the medical device. A pivotal trial is a definitive study designed to gather evidence to evaluate the safety and effectiveness of a product prior to its marketing. For additional details on our significant clinical trial activities over the past three years, refer to caption “Medical Device Segment: Overview of Whole-product Solutions” within this section of this Annual Report on Form 10-K.
Patents and Proprietary Rights
Patents and other forms of proprietary rights are an essential part of Surmodics’ business. We aggressively pursue patent protection covering the proprietary technologies that we consider strategically important to our business. In addition to seeking patent protection in the U.S., we also generally file patent applications in European countries and, on a selective basis, other foreign countries. We strategically manage our patent portfolio in a manner designed to ensure that we have valid and enforceable patent rights protecting our technological innovations. As of September 30, 2020, Surmodics owned or had exclusive rights to 159 issued U.S. patents and 251 international patents. As of the same date, we also owned or had exclusive rights to 47 pending U.S. patent applications and 108 foreign patent applications.
We have licensed our PhotoLink Technology on a non-exclusive basis to a number of our customers for use in a variety of medical device surface applications, including those described above. In particular, we have 30 issued U.S. patents, eight pending U.S. patent applications, 64 issued international patents, and 24 pending international patent applications protecting various aspects of these technologies, including compositions, methods of manufacture and methods of coating devices. The expiration dates for these patents and anticipated expiration dates of the patent applications range from fiscal 2022 to 2035. These patents and patent applications represent distinct families, with each family generally covering a successive generation of the technology, including improvements that enhance coating performance, manufacturability, or other important features desired by our customers. For additional details, refer to captions “Coating Licensing Arrangements” and “Coating Technology Patents” within this section of this Annual Report on Form 10-K.
We also rely upon trade secrets, trademarks and other un-patented proprietary technologies. We seek to maintain the confidentiality of such information by requiring employees, consultants and other parties to sign confidentiality agreements and by limiting access by parties outside the Company to such information. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of this information, or that others will not be able to independently develop such information. Additionally, there can be no assurance that any agreements regarding confidentiality and non-disclosure will not be breached, or, in the event of any breach, that adequate remedies would be available to us.
Marketing and Sales
Through our whole-product solutions strategy, we utilize our design, development, manufacturing and regulatory capabilities to provide our customers access to highly differentiated products that address important, unmet clinical needs. While medical device product development and manufacturing capability and capacity scale-up have been a significant focus over the past several years, we continue to provide world-class surface modification coating technologies to our medical device customers, and sales of our hydrophilic coating reagents and related sales-based royalties continue to account for the majority of the revenue from our Medical Device segment. For our whole-product solutions, we have focused on negotiating license and distribution agreements with our customers that call for revenue from product sales at a specified transfer price and, in certain cases, license fees and sales-based royalties. As we continue to develop and seek regulatory approval for our proprietary medical device products, we expect the majority of revenue growth in the Medical Device business to come from these products.
Sales and marketing professionals working within our Medical Device business work in concert with our R&D personnel to coordinate commercialization activities for both our surface modification coatings and medical device products. Our sales professionals’ specialization fosters an in-depth knowledge of the issues faced by our customers, such as industry trends, technology changes, biomaterial changes and the regulatory environment. We have signed agreements with third-party distributors to bring our first cleared proprietary medical device products to the market and shipped initial orders in fiscal 2020. Following receipt of 510(k) clearance or CE Mark, we conduct product evaluations of our proprietary medical device products in order to generate data and receive important feedback regarding the attributes and performance of our devices from physicians in real-world case settings. These evaluations allow us to build the value proposition for each of our products to support successful commercialization.
With respect to our diagnostics products, our sales professionals sell directly to IVD kit manufacturers, and we enter into supply agreements with third parties to distribute those products around the world. We also offer diagnostics products for sale through our website.
14
To support our marketing and sales activities, we publish technical literature on our various surface modification, drug delivery, and IVD technologies and products. In addition, we exhibit at major trade shows and technical meetings, advertise in selected trade journals and through our website, and conduct direct mailings to appropriate target markets.
We also offer ongoing customer service and technical support to our customers. This service and support may begin with a feasibility study, and also may include additional services such as assistance in the transfer of the technology to the customer, further optimization, process control and troubleshooting, preparation of product for clinical studies, and assistance with regulatory submissions for product approval. Some of these services are billable to customers, mainly feasibility and optimization activities.
Significant Customers
Revenue from Abbott and Medtronic represented approximately 19% and 14%, respectively, of our consolidated revenue for fiscal 2020. Revenue from these customers was generated from multiple products and fields of use, including revenue from the Abbott Agreement, substantially all of which were recognized in our Medical Device segment. No other customer provided more than 8% of our consolidated revenue in fiscal 2020. One customer in our IVD business accounted for 24% of our IVD segment revenue for fiscal 2020.
Competition
Medical Device Segment
We provide differentiated whole-product solutions that integrate our surface modification, catheter, balloon and other proprietary technologies. This high degree of differentiation is strategically designed to capture market share in a highly competitive, dynamic industry. Our commercialized PTA balloon catheter and microcatheter products compete with larger OEM suppliers, as well as some of our largest medical device customers. Our products in development will compete with the global leaders in the vascular medical device market. We believe our whole-product solutions will be competitive on the basis of their safety and efficacy as a result of the innovative design and differentiated coating and device design technology, which will lead to demonstrated improvements in patient outcomes through reduced invasiveness compared to other devices used for comparable procedures.
We believe that the intense competition within the medical device market creates opportunities for our coating technologies as medical device manufacturers seek to differentiate their products through new enhancements or to remain competitive with enhancements offered by other manufacturers. Because a significant portion of our revenue depends on royalties derived from our customers’ medical device product sales incorporating our surface modification coating technologies, we are also affected by competition within the markets for such devices. As we typically license our surface modification coating technologies on a non-exclusive basis, we benefit by offering our technologies to multiple competing manufacturers of a device. However, competition in the medical device market could also have an adverse effect on us. While we seek to license our coatings products to established manufacturers, in certain cases, our surface modification licensees may compete directly with larger, dominant manufacturers with extensive product lines and greater sales, marketing and distribution capabilities. We also are unable to control other factors that may impact commercialization of our whole-product solutions and licensees with medical devices that utilize our surface modification coatings, such as regulatory approval, marketing and sales efforts of our customers and licensees, or competitive pricing pressures within the particular market. Many of our existing and potential competitors have greater financial, technical and marketing resources than we have.
The ability for surface modification coating technologies to improve the performance of medical devices and drugs and to enable new product categories has resulted in increased competition in these markets. Some of our competitors offer device drug-delivery technologies, while others specialize in lubricious or hemocompatible coating technology. Some of these companies target cardiovascular, peripheral or other medical device applications. In addition, because of the many product possibilities afforded by surface modification coating technologies, many of the large medical device manufacturers have developed, or are engaged in efforts to develop, internal competency in the area of surface modification, including drug-delivery technologies.
We attempt to differentiate ourselves from our competitors by providing what we believe is a high value-added approach to device, drug-delivery and surface modification coating technologies. We believe that the primary factors customers consider in choosing a particular technology include performance (e.g., flexibility, ability to fine tune drug elution profiles, biocompatibility), ease of manufacturing, time-to-market, intellectual property protection, ability to produce multiple products from a single process, compliance with manufacturing regulations, ability to manufacture clinical and commercial products, customer service and total cost of goods (including manufacturing process labor). We believe our technologies deliver exceptional performance in these areas, allowing us to compete favorably with respect to these factors. With respect to our licensed surface modification coating technologies, we believe that the cost and time required to obtain the necessary regulatory approvals significantly reduces the likelihood of a customer changing the manufacturing process it uses once a device or drug has been approved for sale.
15
In Vitro Diagnostics Segment
Competition in the diagnostics market is highly fragmented. In the product lines in which we compete (protein stabilization reagents, substrates, antigens and surface chemistry technologies), we face an array of competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of products. Some of our competitors have substantially more capital resources, marketing experience, R&D resources and production facilities than we do. We believe that our products compete on performance, stability (shelf life), sensitivity (lower levels detected, faster results), consistency and price. We believe that our continued competitive success will depend on our ability to gain market share, to develop or acquire new proprietary products, obtain patent or other protection for our products and successfully market our products directly or through partners.
Manufacturing
We manufacture our surface modification and drug-delivery reagents and our IVD products in one of our Eden Prairie, Minnesota facilities. In certain limited circumstances, we also provide contract manufacturing services for our customers, including, for example, coating their medical devices that are intended for pre-clinical and clinical development (including human clinical trials), and products that are sold for commercial use by our customers. We manufacture PTA balloon catheters and microcatheters in our Ballinasloe, Ireland facility, which offers a suite of capabilities, including balloon forming, extrusion, coating, braiding and assembly of finished products. We plan to manufacture the majority of our whole-product solutions devices in our Ireland facility as the products are launched, including our SurVeil DCB. We plan to maintain manufacturing capacity in our U.S. facilities for certain products and for redundancy. At both of our manufacturing facilities, we perform a limited volume of contract manufacturing of medical devices for our customers.
We attempt to maintain multiple sources of supply for the key raw materials used to manufacture our products. We do, however, purchase some raw materials from single sources, but we believe that additional sources of supply are readily available. Further, to the extent additional sources of supply are not readily available, we believe that we could manufacture such raw materials.
We follow quality management procedures in accordance with applicable regulations and guidance for the development and manufacture of materials and device, biotechnology or combination products that support clinical trials and commercialization. In order to meet our customers’ needs in this area, our manufacturing facility in Eden Prairie, Minnesota is certified to ISO 13485 and ISO 9001. Our manufacturing facility in Ballinasloe, Ireland is certified to ISO 13485. Each of these facilities is registered with the U.S. FDA as a “Contract Manufacturer.”
Government Regulation
Our medical device products and the IVD, third-party device and biotechnology products incorporating our technologies are often required to undergo long, expensive and uncertain regulatory review processes that are governed by the FDA and other international regulatory authorities. Our strategy for our proprietary medical device products is to obtain regulatory clearance in the U.S and E.U. New medical devices can only be marketed in the U.S. after a pre-market notification for 510(k) clearance or a PMA by the FDA. These processes can take anywhere from several months (e.g., for medical device products seeking regulatory approval under the 510(k) clearance process) to several years (e.g., for medical device products seeking regulatory approval under the PMA application process). In the E.U., regulatory approval is signified by the CE Mark, which is generally granted by one of several competent authorities and is based on the submission of a design dossier, a manufacturer validation assessment, a third-party assessment, and review of the design dossier by a “Notified Body.” In 2017, the E.U. authorized a new medical device regulation. The new regulation, which will impose significant additional pre-market and post-market requirements, becomes effective for devices submitted for CE Mark after May 2021. Medical devices granted CE Mark prior to May 2021 will require recertification based on the new requirements within five years after the effective date.
For our customers’ products that incorporate our surface modification coating and IVD technologies, the burden of securing regulatory approval typically rests with the customer, as the medical device manufacturer. For our whole-product solutions, including the SurVeil DCB, our other DCB-platform devices, and any additional medical device products that we develop, the burden of securing regulatory approval will rest on us, unless we contract with other organizations to pursue such approval.
In support of our customers’ and our own regulatory filings, we maintain various confidential Device Master Files with the FDA and provide technical information to other regulatory agencies outside the U.S. regarding the nature, chemical structure and biocompatibility of our reagents. Our licensees generally do not have direct access to these files. However, they may, with our permission, reference these files in their various regulatory submissions to these agencies. This approach allows regulatory agencies to understand the details of our technologies without our having to share this highly confidential information with our customers.
16
U.S. legislation allows companies, prior to obtaining FDA clearance or approval to market a medical product in the U.S., to manufacture medical products in the U.S. and export them for sale in international markets. This generally allows us to realize earned royalties sooner and may result in opportunities to market our whole-product solutions in other countries. However, sales of medical products outside the U.S. are subject to international requirements that vary from country to country. The time required to obtain approval for sale internationally may be longer or shorter than that required by the FDA.
Human Capital
As of September 30, 2020, we had 370 employees, of which 140 were employed outside the U.S., primarily in R&D and manufacturing operations functions. We are not a party to any collective bargaining agreements.
Our success depends upon our ability to retain and attract highly qualified management and technical personnel. Talent management is critical to our ability to execute on our long-term growth strategy. Through our history of technological innovation, we appreciate the importance of retention, growth and development of our employees. We continue to be committed to an inclusive culture which values equality, opportunity, and respect. In support of our inclusive culture, we believe we offer competitive compensation and benefits, including an annual pay gap assessment; provide respectful workplace training to strengthen employee understanding; and strive to recruit a diverse talent pool across all levels of the organization. We are focused on the engagement and empowerment of our employees through demonstration of our foundational values, which we refer to as the five Cs: we have courage to face challenges with determination, honesty and resourcefulness; candor to speak openly and respectfully; collaboration that recognizes teamwork as the key to success; camaraderie that is genuine and supportive; and commitment to our cause.
COVID-19 Health and Safety
During the COVID-19 pandemic, the majority of our employees have continued to work from our facilities, where we have adopted health screening, implemented socially distancing and personal protective equipment requirements, enhanced cleaning and sanitation procedures, and modified workspaces to reduce the potential for disease transmission. In early July 2020, we suspended production for one week in one production work cell in our facility in Eden Prairie, Minnesota when one of the employees in the cell was identified as having COVID-19. The production suspension did not have a material impact on our operations and the cell has since resumed normal operations.
Our employees who do not require access to our facility to perform their work have been working from home during the pandemic, without a significant impact to productivity. We cannot be sure that the measures we have implemented will be effective to prevent an outbreak of COVID-19 in one of our facilities, or a portion thereof. Likewise, we cannot be sure that our employees working from home will continue to be productive. Adverse impacts of the pandemic on our employees could have material adverse effects on our business, results of operations, cash flows, financial condition, and capital investments.
EXECUTIVE OFFICERS OF THE REGISTRANT
As of November 30, 2020, the names, ages and positions of the Company’s executive officers are were follows:
|
Name |
|
Age |
|
Position |
|
Gary R. Maharaj |
|
57 |
|
President and Chief Executive Officer |
|
Timothy J. Arens |
|
53 |
|
Senior Vice President of Finance and Information Technology and Chief Financial Officer |
|
Thomas A. Greaney |
|
54 |
|
Chief Operating Officer of Medical Devices |
|
Charles W. Olson |
|
56 |
|
Senior Vice President of Commercial and Business Development, Medical Devices |
|
Teryl L.W. Sides |
|
51 |
|
Senior Vice President of Product Development and Chief Marketing Officer |
|
Joseph J. Stich |
|
55 |
|
Senior Vice President and General Manager of Human Resources and In Vitro Diagnostics |
|
Nusrath Sultana |
|
46 |
|
Vice President of Clinical Affairs |
|
Gordon S. Weber |
|
57 |
|
Senior Vice President of Legal, General Counsel and Secretary |
17
Gary R. Maharaj joined the Company in December 2010 as President and Chief Executive Officer and was also appointed to the Surmodics Board of Directors at such time. Prior to joining Surmodics, Mr. Maharaj served as President and Chief Executive Officer of Arizant Inc., a provider of patient temperature management systems in hospital operating rooms, from 2006 to 2010. Previously, Mr. Maharaj served in several senior-level management positions for Augustine Medical, Inc. (predecessor to Arizant Inc.) from 1996 to 2006, including Vice President of Marketing, and Vice President of Research and Development. During his 37 years in the medical device industry, Mr. Maharaj has also served in various management and research positions for the orthopedic implant and rehabilitation divisions of Smith & Nephew, PLC.
Timothy J. Arens joined the Company in February 2007 as Director, Business Development and became Senior Director of Financial Planning and Analysis and General Manager, In Vitro Diagnostics in October 2010. He was promoted to Vice President of Finance and Interim Chief Financial Officer in August 2011 and in February 2013 became Vice President Corporate Development and Strategy. In May 2018, Mr. Arens was named interim Vice President of Finance and Chief Financial Officer for a second time and in February 2019 he was named Vice President of Finance and Chief Financial Officer. In April 2020, he was promoted to Senior Vice President of Finance and Information Technology and Chief Financial Officer. Prior to joining Surmodics, Mr. Arens was employed at St. Jude Medical, Inc., a medical technology company, from 2003 to 2007, in positions of increasing responsibility related to business development and strategic planning functions.
Thomas A. Greaney joined the Company in November 2015 as Vice President of Operations and General Manager of Creagh Medical, after we acquired it. In August 2017, Mr. Greaney was promoted to Chief Operating Officer, Medical Devices. Prior to joining Surmodics, he served as Chief Executive Officer for Creagh Medical, from September 2005 to November 2015. Prior to his tenure in Creagh Medical, Mr. Greaney served in a variety of roles with Boston Scientific for 10 years including the worldwide operations responsibility for the Taxus Stent commercialization. From 1989 to 1995, he worked for a number of electronics companies in a variety of engineering and management roles. On September 18, 2020, Mr. Greaney notified the Company of his intention to resign from his positions with the Company. In order to assure an orderly transition of his responsibilities, Mr. Greaney remains an executive officer and has agreed to provide transitional services to the Company through December 30, 2020.
Charles W. Olson joined the Company in July 2001 as Market Development Manager, was promoted in December 2002 to Director, Business Development, named General Manager of the Hydrophilic Technologies business unit in April 2004, and promoted to Vice President and General Manager, Hydrophilic Technologies in October 2004. In April 2005, the position of Vice President, Sales was added to his responsibilities. In November 2008, Mr. Olson was named Vice President of our Cardiovascular business unit, in October 2010, he was named Senior Vice President and General Manager, Medical Device, and in August 2016 he was named Senior Vice President of Commercial and Business Development, Medical Devices. Prior to joining Surmodics, Mr. Olson was employed as General Manager at Minnesota Extrusion from 1998 to 2001 and at Lake Region Manufacturing in project management and technical sales from 1993 to 1998.
Teryl L.W. Sides joined the Company in November 2018 as Senior Vice President and Chief Marketing Officer. In April 2020, Ms. Sides was promoted to Senior Vice President of Product Development and Chief Marketing Officer. Before joining Surmodics, Ms. Sides served as Founder and Chief Executive Officer of Projectory, a consulting firm that provides strategic marketing services to med tech clients, ranging from start-ups to global businesses, from 2011 to 2018. Prior to joining Projectory, Ms. Sides was the Vice President of Marketing and Product Development for Arizant, Inc. from 1998 to 2011.
Joseph J. Stich joined the Company in March 2010 as Vice President of Marketing, Corporate Development and Strategy. In August 2011, Mr. Stich became Vice President, Business Operations and General Manager In Vitro Diagnostics. In September 2013, Mr. Stich’s role was adjusted to Vice President and General Manager, In Vitro Diagnostics. In April 2020, Mr. Stich was promoted to Senior Vice President and General Manager of Human Resources and In Vitro Diagnostics. Prior to joining Surmodics, Mr. Stich was Vice President of Corporate Development for Abraxis BioScience, LLC, a biotechnology company focused on oncology therapeutics, from 2009 to 2010. Prior to joining Abraxis, he was a Vice President for MGI Pharma, Inc., a biopharmaceutical company, from 2005 to 2009. Mr. Stich’s prior experience also includes serving as President/COO of Pharmaceutical Corp. of America (a subsidiary of Publicis Healthcare Specialty Group), and positions of increasing responsibility in sales and marketing at Sanofi-Aventis Pharmaceuticals.
Nusrath Sultana joined the Company in February 2020 as Vice President of Clinical Affairs. From 2015 until joining Surmodics, Ms. Sultana served as the Senior Director of Global Medical Affairs for Edwards Lifesciences, a medical technology company focused on structural heart disease, critical care and surgical monitoring, where she provided leadership, oversight and strategic direction for core medical affairs activities and was responsible for development of medical affairs infrastructure. Prior to joining Edwards Lifesciences, Ms. Sultana held numerous positions of increasing responsibility with St. Jude Medical from 2003 to 2015, most recently Senior Director of Global Clinical Operations. Ms. Sultana’s prior experience also includes serving as a consultant responsible for strategic discussions on pre and post market trial designs, development of clinical evidence reports and coordination with the clinical team in developing dossiers, and FDA submissions.
18
Gordon S. Weber joined the Company in May 2020 as Senior Vice President of Legal, General Counsel and Secretary. Prior to joining Surmodics, Mr. Weber served as the Founder and President of Sapere Aude, LLC, a consulting firm, from 2018 to 2020. From 2017 to 2018, Mr. Weber served as Vice President, General Counsel and Secretary of CHF Solutions, Inc., which manufactures and markets ultrafiltration systems for patients suffering from fluid overload. Mr. Weber served as Vice President, General Counsel and Secretary of Vascular Solutions, Inc., a medical device company focused on products treating coronary and peripheral vascular disease, from 2013 until the company was acquired by Teleflex Incorporated in 2017. Mr. Weber practiced law for 13 years with Faegre & Benson LLP (now Faegre Drinker Biddle & Reath LLP), where he was Partner. Mr. Weber began his career with the accounting firm now known as KPMG and has served as Corporate Controller for Osmonics, Inc., an NYSE-listed manufacturer of fluid filtration equipment.
The executive officers of the Company are elected by and serve at the discretion of the Board of Directors. None of our executive officers are related to any other executive officer or any of our directors.
ITEM 1A. RISK FACTORS.
RISKS RELATING TO OUR BUSINESS, STRATEGY AND INDUSTRY
The loss of, or significant reduction in business from, one or more of our major customers could significantly reduce our revenue, earnings or other operating results.
A significant portion of our revenue is derived from a relatively small number of customers. Two of our customers each provided more than 10% of our revenue in fiscal 2020. Revenue from Abbott and Medtronic represented approximately 19% and 14%, respectively, of our total revenue for fiscal 2020 and was generated from multiple products and fields of use. The loss of Medtronic, Abbott or any of our other largest customers, or reductions in business from them, could have a material adverse effect on our business, financial condition, results of operations, and cash flow. There can be no assurance that revenue from any customer will continue at their historical levels. If we cannot broaden our customer base, we will continue to depend on a small number of customers for a significant portion of our revenue.
The COVID-19 pandemic has had an adverse effect on our business and results of operations and is expected to continue to have further adverse effects, which could be material, on our business, results of operations, financial condition, liquidity, and capital investments.
The COVID-19 pandemic has negatively impacted the global economy, disrupted supply chains and created significant volatility in financial markets. We have implemented business policies intended to protect our employees from the spread of COVID-19. Those policies include employees working from home when possible, but the majority of our employees have continued to work from our facilities, where we have adopted health screening, implemented socially distancing and personal protective equipment requirements, enhanced cleaning and sanitation procedures, and modified workspaces to reduce the potential for disease transmission, which have involved additional costs to us.
On March 18, 2020, the Centers for Medicare & Medicaid Services (“CMS”) released guidance for U.S. healthcare providers to limit non-emergent elective medical procedures other than high acuity treatments in order to conserve personal protective equipment and limit exposure to COVID-19. On April 16, 2020, the White House issued “Guidelines for Opening Up America Again” (the “White House Guidelines”) that described a phased resumption of economic activities with gating conditions for a region or state to move from one phase to another. On June 9, 2020, CMS issued recommendations for regions and states in Phase II of the White House Guidelines that non-emergent, non-COVID-19 care should be offered to patients, as clinically appropriate, in localities or facilities that have the resources to provide such care, as well as the ability to quickly respond to a surge in COVID-19 cases, if necessary.
19
Since the White House Guidelines and related CMS recommendations were issued, rates of COVID-19 have vacillated by region and state, in many cases surging in the fall of 2020. Accordingly, consistent with the CMS recommendations, the degree to which elective medical procedures have been offered varies by region, state, and even between healthcare systems within a state. Where elective procedures have been offered, and even for emergency procedures, some people appear to have avoided healthcare facilities, presumably out of concern for contracting COVID-19. Many of our customers use our licensed technology and purchased materials to manufacture products used in procedures impacted by the guidance and recommendations. Based on the CMS guidance and recommendations, as well as industry data regarding elective procedures volumes, we adjusted the assumptions used in our royalties revenue recognition for the quarters ended March 31, 2020, June 30, 2020 and September 30, 2020, which reduced royalty revenue in these periods relative to the revenue that would have been recognized under our prior assumptions. We currently understand that elective procedures volumes were higher in the quarter ended June 30, 2020 than we originally estimated, resulting in a net favorable impact to revenue recognized in the quarter ended September 30, 2020. In the quarter ended September 30, 2020, our royalty revenue reflected our understanding and assumptions of procedure volumes through the end of the period. We continue to believe that elective procedure volumes were lower in the quarter ended September 30, 2020 than they would have been absent the pandemic. In addition to limiting medical procedures, hospitals and other healthcare providers vary in the degree to which they are permitting access to their facilities during the pandemic.
In early July 2020, we suspended production for one week in one production work cell in our facility in Eden Prairie when one of the employees in the cell was identified as having COVID-19. The production suspension did not have a material impact on our operations and the cell has since resumed normal operations. From time to time, we have had employees test positive for COVID-19. In such instances, we have instructed employees who have tested positive for COVID-19, or who have had recent exposure to another individual with suspected or confirmed COVID-19, to avoid coming into our facilities for a quarantine period recommended by the Centers for Disease Control and Prevention.
We cannot predict the duration or scope of the pandemic, actions that governments and businesses may take in response to the pandemic, or the impacts of the pandemic on healthcare systems. The impacts of the pandemic may include, but not be limited to:
|
• |
Reduced revenues from our customers, including our major customers, whose products are impacted by reductions in the delivery of elective medical procedures or patients’ unwillingness to visit healthcare facilities for medical procedures; |
|
• |
Diminished ability or willingness of third parties to market, distribute and sell products incorporating our coating and device technologies, as well as our whole-product solutions, due to reduced demand from, or lack of access to, healthcare facilities and providers; |
|
• |
Diminished ability, or inability, to complete clinical trials and other activities required to achieve regulatory clearance of our products under development due to lack of access to healthcare facilities, healthcare providers and patients; |
|
• |
Diminished or lost access to third-party service providers that we use in our research and development or marketing efforts; |
|
• |
Loss of manufacturing capacity, which could lead to failures to meet product delivery commitments, or increased operating costs if our facilities were to experience additional incidents of COVID-19; |
|
• |
Reduced cash flow from our operations due to reductions in revenues or collections from our customers and increases in operating costs related to actions we have taken in response to the pandemic; |
|
• |
Reduced business productivity due to inefficiencies in employees working from home or increasing physical distancing and other pandemic response protocols in our production facilities; |
|
• |
Increased susceptibility to the risk of information technology security breaches and other disruptions due to increased volumes of remote access to our information systems from our employees working at home; |
|
• |
Inability to source sufficient components used in our products due to disruptions in supply chains; |
|
• |
Diminished ability to identify, evaluate and acquire, or effectively integrate, complementary businesses, products, materials or technologies due to travel restrictions, physical distancing protocols, and lack of access to third party service providers related to our development activities; |
|
• |
Difficulties in assessing and securing intellectual property rights due to lack of access to, or delayed responsiveness of, third-party service providers or governmental agencies; |
|
• |
Impairment of goodwill or other assets due to reductions in the fair value of our reporting units; |
|
• |
Diminished ability to retain personnel over concerns about workplace exposure to COVID-19, or to hire and effectively train new personnel, due to physical distancing protocols; and |
|
• |
Increased volatility in our stock price due to financial market instability. |
20
These and other factors relating to, or arising from, the pandemic could have material adverse effects on our business, results of operations, cash flows, financial condition, and capital investments. Actual or anticipated adverse effects on our cash flows or financial condition may lead us to seek additional funding. We cannot be certain that additional funding will be available on acceptable terms, if at all. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or otherwise curtail our operations. Any of these events could materially harm our business and operating results.
The long-term success of our business may suffer if we are unable to expand our licensing base.
We intend to continue pursuing a strategy of licensing our coatings technologies to a diverse array of medical device companies, thereby expanding the commercialization opportunities for our technologies. A significant portion of our revenue is derived from customer devices used in connection with procedures in cardiovascular, peripheral vascular, neurovascular, structural heart and other applications. As a result, our business is susceptible to adverse trends in procedures. Further, we may also be subject to adverse trends in specific markets such as the cardiovascular industry, including declines in procedures using our customers’ products as well as declines in average selling prices from which we earn royalties. Our success will depend, in part, on our ability to attract new licensees, to enter into agreements for additional applications with existing licensees, and to develop technologies for use in new applications. There can be no assurance that we will be able to identify, develop and adapt our technologies for new applications in a timely and cost-effective manner; that new license agreements will be executed on terms favorable to us; that new applications will be accepted by customers in our target markets; or that products incorporating newly licensed technology, including new applications, will gain regulatory approval, be commercialized or gain market acceptance. Delays or failures in these efforts could have an adverse effect on our business, financial condition and operating results.
Our success depends on our ability to effectively develop and market our products against those of our competitors.
We operate in a competitive and evolving field, and new developments are expected to continue at a rapid pace. Our success depends, in part, upon our ability to maintain a competitive position in the development of technologies and products in the fields of surface modification, device drug delivery, medical device products and diagnostics. Our surface modification coating technologies compete with technologies developed by a number of other companies. In addition, many medical device manufacturers have developed, or are engaged in efforts to develop, surface modification coating technologies for use on their own products, particularly in the area of drug delivery. With respect to commercialization of our whole-product solutions, we have faced, and expect to continue to face, competitive pricing pressures from larger OEM suppliers, as well as some of our largest medical device partners that have in-house resources that produce similar products. Some of our existing and potential competitors (especially medical device manufacturers pursuing coating solutions through their own R&D efforts) have greater financial and technical resources as well as production and marketing capabilities than us. Further, even if we are successful with respect to our plan to develop new medical device products, the commercialization of these products may be dependent upon a commercial partner to effectively market and sell our products to end users. Competitors may succeed in developing competing technologies or obtaining governmental approval for products before us. Products incorporating our competitors’ technologies may gain market acceptance more rapidly than products using our technologies. Furthermore, there can be no assurance that new products or technologies developed by others, or the emergence of new industry standards, will not render our products or technologies or licensees’ products incorporating our technologies uncompetitive or obsolete. Any new technologies that make our surface modification coating, medical device platforms or In Vitro Diagnostics technologies less competitive or obsolete would have a material adverse effect on our business, financial condition and results of operations. Competition in the diagnostics market is highly fragmented, and in the product lines in which we compete, we face an array of competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of products. Some of our competitors have substantially more capital resources, marketing experience, R&D resources and production facilities than we do.
We may not be successful in implementing our whole-product solutions strategy and related important strategic initiatives.
Since fiscal 2013, we have been focused on a key growth strategy for our Medical Device business by expanding the business to offer whole-product solutions to medical device customers. Our aim is to provide customers with highly differentiated products that address unmet clinical needs. We may seek to market and sell these products to existing customers, through third-party distributors or via other distribution channels.
Successfully implementing our whole-product solutions strategy and related strategic initiatives will place substantial demands on our resources and require, among other things:
|
• |
continued enhancement of our medical device R&D capabilities, including those needed to support the clinical evaluation and regulatory approval for our whole-product solutions; |
21
|
• |
effective coordination and integration of our research facilities and teams, particularly those located in our product development facility in Minnesota and our Irish operations; |
|
• |
successful hiring and training of personnel; |
|
• |
effective management of a business geographically located both in the U.S. and Ireland; |
|
• |
commercialization of our products, including through strategic partnerships with our medical device customers, third-party distributors, or via other distribution channels; |
|
• |
commitment from our medical device customers to market our products effectively or to devote resources necessary to provide effective sales; |
|
• |
sufficient liquidity to support substantial investments in R&D required to make our strategy successful; and |
|
• |
increased marketing, field clinical support specialists, and sales-related activities. |
There is no assurance that we will be able to successfully implement our whole-product strategy and related strategic initiatives in accordance with our expectations, which could impact our ability to realize an acceptable return on the investments we are making in connection with this strategy, and may result in an adverse impact on our business and financial results.
Increases in operating expenses related to the development and commercialization of new technologies and products may adversely affect our operating results and may not be effective.
Our future success depends, in part, upon our continued development, validation and commercial support of new products and technologies. In fiscal 2019 and fiscal 2018, our R&D expenses increased 29.1% and 28.8%, respectively, over the prior year levels, primarily due to the development of our DCB platform and costs associated with the ongoing TRANSCEND clinical trial for our SurVeil DCB. In fiscal 2020, R&D costs decreased 5.1% from fiscal 2019, representing 53% of our total revenue, primarily due to the progression of the TRANSCEND clinical trial from active enrollment in fiscal 2019 to patient follow-up in fiscal 2020. In fiscal 2021, we expect to continue the clinical evaluation of the SurVeil DCB and will conduct additional development and clinical activities for the below-the-knee, AV fistula and other whole-product solutions products, which will result in significant R&D expenses. Our agreement with Abbott provides that we are responsible for conducting all necessary clinical trials and other activities required to achieve U.S. regulatory clearance for the SurVeil DCB, which will continue to involve significant costs.
Our selling, general and administrative expenses (“SG&A”) increased 18.5% in fiscal 2020 from fiscal 2019 and represented 29.9% of our fiscal 2020 revenue. We anticipate that SG&A expenses will continue to increase in fiscal 2021, as we continue to invest in our capacity to support our DCB and other whole-product solutions products. Increases in our operating expenses may materially impact our operating results, including our profitability, in fiscal 2021 and beyond. In addition to the operating expenses associated with product development and commercialization activities, such activities are subject to risks of failure that are inherent in the development and commercialization of new medical technologies or products. There can be no assurance that we will be successful in developing new technologies or products, or that any such technologies or products will be commercialized. Even if we are successful in developing and commercializing new technologies or products, there can be no assurance that gross profits from their sales will exceed our operating expenses related to their development and commercialization.
Failure to identify acquisition opportunities, to accurate financially model the impact of acquisitions, or to integrate acquired businesses or technologies into our operations successfully may limit our growth and adversely impact operating results, cash flows and liquidity.
An important part of our growth may involve the acquisition of complementary businesses or technologies. Our identification of suitable acquisition candidates involves risks inherent in assessing the technology, value, strengths, weaknesses, overall risks and profitability, if any, of acquisition candidates. We may not be able to identify suitable acquisition candidates, or we may be unable to execute acquisitions due to competition from buyers with more resources. If we do not make suitable investments and acquisitions, we may find it more difficult to realize our growth objectives.
Our ability to realize the anticipated benefits of a potential acquisition depends, in part, on the accuracy of our financial model of the anticipate timing and magnitude of cash flows, expenses and revenues related to the acquired business. If the expectations reflected in our financial models for acquisitions are not realized, our operating results, cash flows and liquidity may be materially adversely affected.
22
The process of integrating acquired businesses into our operations poses numerous risks, including:
|
• |
an inability to effectively or efficiently integrate acquired operations, personnel, technology, information systems, and internal control systems and products; |
|
• |
diversion of management’s attention, including the need to manage several remote locations with a limited management team; |
|
• |
difficulties and uncertainties in transitioning the customers or other business relationships from the acquired entity to us; and |
|
• |
the loss of key employees of acquired companies. |
In addition, future acquisitions may be dilutive to our shareholders’ ownership and/or cause large one-time expenses or create goodwill or other intangible assets that could result in future significant asset impairment charges. In addition, if we acquire entities that have not yet commercialized products, but rather are developing technologies for future commercialization, our earnings per share may fluctuate as we expend significant funds for continued R&D efforts necessary to commercialize such acquired technology. We cannot guarantee that we will be able to successfully complete any acquisitions or that we will realize any anticipated benefits from acquisitions that we complete.
Our failure to expand our management systems and controls to support anticipated growth or integrate acquisitions could seriously harm our operating results and business.
Our operations are expanding, and we expect this trend to continue as we execute our business strategy. Executing our business strategy has placed significant demands on management and our administrative, development, operational, information technology, manufacturing, financial and personnel resources. Accordingly, our future operating results will depend on the ability of our officers and other key employees to continue to implement and improve our operational, development, customer support and financial control systems, and effectively expand, train and manage our employee base. Otherwise, we may not be able to manage our growth successfully.
Goodwill or other assets on our balance sheet may become impaired, which could have a material adverse effect on our operating results.
We have a significant amount of goodwill and intangible assets on our balance sheet in connection with our acquisitions. As of September 30, 2020, we had $40.5 million of goodwill and indefinite-lived intangible assets on our consolidated balance sheet related to our Medical Device and IVD segments, of which $31.8 million related to our Medical Device reporting unit. As required by the accounting guidance for non-amortizing intangible assets, we evaluate at least annually the potential impairment of the goodwill and trademarks. Testing for impairment of non-amortizing intangible assets involves the determination of the fair value of our reporting units. The estimation of fair values involves a high degree of judgment and subjectivity in the assumptions used. We also evaluate other assets on our balance sheet, including strategic investments and intangible assets, whenever events or changes in circumstances indicate that their carrying value may not be recoverable. Our estimate of the fair value of the assets may be based on fair value appraisals or discounted cash flow models using various inputs. During fiscal 2020, we recorded a charge of $0.5 million for the impairment of a strategic investment to reduce the carrying value of the investment to zero. Future impairment charges could materially adversely affect our results of operations.
We recognize revenue in accordance with complex accounting standards, and changes in circumstances or interpretations may lead to accounting adjustments and failure to implement these standards might impact the effectiveness of our internal control over financial reporting or impact the reliability of our financial reporting.
Our revenue recognition policies involve application of complex accounting standards, including the determination of when control is transferred to the customer and the allocation of the transaction price to multiple performance obligations. Our compliance with such accounting standards often involves management’s judgment regarding whether the criteria set forth in the standards have been met such that we can recognize as revenue the amounts that we expect to receive as payment for our products or services. We base our judgments on assumptions that we believe to be reasonable under the circumstances. However, these judgments, or the assumptions underlying them, may change over time. In particular, disruptions related to the COVID-19 pandemic in the performance of medical procedures have made it increasingly challenging to make estimates of sales volumes for medical device products that incorporate our licensed technologies, which estimates we use to determine royalties revenue. In addition, the SEC or the Financial Accounting Standards Board (“FASB”) may issue new positions or revised guidance on the treatment of complex accounting matters. Changes in circumstances or third-party guidance could cause our judgments to change with respect to our interpretations of these complex standards, and transactions recorded, including revenue recognized, for one or more prior reporting periods, could be adversely affected.
23
Our credit facility contains covenants that restrict our business and financing activities, and the property that secures our obligations under the credit facility may be subject to foreclosure.
On September 14, 2020, we entered into a $25 million secured revolving credit facility with Bridgewater Bank pursuant to a Loan and Security Agreement (the “Loan Agreement”). The Loan Agreement contains a number of restrictions and covenants, which, among other things, limit our ability to incur additional debt, make certain investments, create or permit certain liens, create or permit restrictions on the ability of subsidiaries to pay dividends or make other distributions, make acquisitions, or consolidate or merge with another entity. The Loan Agreement also requires us to maintain compliance with covenants regarding a minimum level of liquidity; a minimum current ratio; a minimum level of EBITDA, calculated quarterly on the preceding four quarters; and a minimum level of tangible net worth. These provisions impose significant operating and financial restrictions on us and may limit our ability to compete effectively, take advantage of new business opportunities, or take other actions that may be in our best interests.
Our obligations under the Loan Agreement are secured by substantially all of our assets, other than intellectual property, real estate and foreign assets, including equity in foreign subsidiaries. Our ability to obtain additional or other financing or to dispose of certain assets also could be negatively impacted based on the assets we have pledged as collateral in connection with the Loan Agreement. Our ability to borrow under the Loan Agreement is subject to a borrowing base that equals 80% of the margin value of securities collateral that we have pledged to the lender.
Our ability to comply with the provisions under the Loan Agreement may be affected by events beyond our control, and our inability to comply with any of these provisions could result in a default under the Loan Agreement. If such a default occurs, the lender may elect to declare all borrowings outstanding, together with accrued interest and other fees, to be immediately due and payable, and it would have the right to terminate any commitments it has to provide further funds. If we are unable to repay outstanding borrowings when due, the lender under the Loan Agreement also has the right to proceed against the collateral granted to them to secure the indebtedness under that facility. The occurrence of any of these events could have a material adverse effect on our business, financial condition, results of operations and liquidity.
Our business includes foreign operations which exposes us to certain risks related to fluctuations in U.S. dollar and foreign currency exchange rates.
The Company reports its consolidated financial statements in U.S. dollars. In a period where the U.S. dollar is strengthening or weakening relative to the Euro, our revenue and expenses denominated in the Euro are translated into U.S. dollars at a lower or higher value than they would be in an otherwise constant currency exchange rate environment. As our foreign operations expand, the effects may become material to our consolidated financial statements.
Changes in product mix and increased manufacturing costs could cause our product gross margin percentage to fluctuate or decline in the future.
Changes in our product mix and increases in manufacturing costs could cause our gross profit percentage to fluctuate or decline in the future. These factors, together with the scale-up of our manufacturing operations, particularly in Ireland, adversely affected our gross margin percentage for the last fiscal year and these factors will likely continue to affect our gross profit percentage in fiscal 2021 and beyond.
RISKS RELATING TO OUR OPERATIONS AND RELIANCE ON THIRD PARTIES
We rely on third parties to market, distribute and sell most products incorporating our coating and device technologies, as well as our whole-product solutions.
A principal element of our business strategy is to enter into licensing arrangements with medical device and other companies that manufacture products incorporating our technologies. For fiscal years 2020, 2019 and 2018, we derived 43%, 48%, and 44%, respectively, of our revenue from royalties and license fees derived from such licensing arrangements. The revenue that we derive from such arrangements depends upon our ability, or our licensees’ ability, to successfully develop, obtain regulatory approval for, manufacture (if applicable), market, and sell products incorporating our technologies. Many of these factors are outside of our control. Our failure, or the failure of our licensees, to meet these requirements could have a material adverse effect on our business, financial condition and results of operations.
24
Additionally, a licensee could modify their product in such a way that it no longer incorporates our technology. Moreover, under our standard license agreements, licensees can terminate the license for any reason upon 90 days’ prior written notice. Existing and potential licensees have no obligation to deal exclusively with us and may pursue parallel development or licensing of competing technologies on their own or with third parties. A decision by a licensee to terminate its relationship with us could have a material adverse effect on our business, financial condition and results of operations.
In fiscal 2018, we entered into an agreement with Abbott whereby Abbott will have exclusive worldwide commercialization rights for the SurVeil DCB. Upon receipt of regulatory approval for the SurVeil DCB, Abbott has the right to purchase commercial units from us and we will realize revenue from product sales to Abbott at an agreed-upon transfer price, as well as a share of net profits resulting from third-party product sales by Abbott. Upon receipt of regulatory approval, we will rely on Abbott to effectively market and sell the SurVeil DCB. If Abbott is unable or unwilling to effectively market and sell the SurVeil DCB, it could have a have material adverse effect on our business, financial condition and results of operations.
A portion of our IVD business relies on distribution agreements and relationships with various third parties, and any adverse change in those relationships could result in a loss of revenue and harm that business.
We sell many of our IVD products outside of the U.S. through distributors. Some of our distributors also sell our competitors’ products. If they favor our competitors’ products for any reason, they may fail to market our products as effectively or to devote resources necessary to provide significant sales, which would cause our results to suffer. Additionally, we serve as the exclusive distributor in the U.S., Canada and Puerto Rico for DIARECT GmbH for its recombinant and native antigens. The success of these arrangements with these third parties depends, in part, on the continued adherence to the terms of our agreements with them. Any disruption in these arrangements will adversely affect our financial condition and results of operations.
We rely on our customers to accurately report and make payments under our agreements with them.
We rely on our customers to determine whether the products that they sell are royalty-bearing and, if so, to report and pay the amount of royalties owed to us under our agreements with them. The majority of our license agreements with our customers give us the right to audit their records to verify the accuracy of their reports to us. However, these audits can be expensive, time-consuming and possibly detrimental to our ongoing business relationships with our customers. Inaccuracies in customer royalty reports have resulted in, and could result in, additional overpayments or underpayments of royalties, which could have a material adverse effect on our business, financial condition and results of operations.
We currently have limited or no redundancy in our manufacturing facilities for certain products, and we may lose revenue and be unable to maintain our customer relationships if we lose our production capacity.
We manufacture all of our medical device coating reagents (and provide coating manufacturing services for certain customers) and our IVD products at one of our Eden Prairie, Minnesota facilities. We also manufacture balloon catheter products at our facility in Ballinasloe, Ireland and catheter-based medical devices in limited quantities in one of our facilities in Eden Prairie, Minnesota. If we receive the necessary FDA regulatory approvals, we plan to manufacture the majority of our whole-product solutions devices in our Ireland facility, including our SurVeil DCB. We plan to maintain secondary, redundant manufacturing capacity for our SurVeil DCB in our manufacturing facility in Eden Prairie, Minnesota. If our existing production facilities become incapable of manufacturing products for any reason, we may be unable to meet production requirements, we may lose revenue and we may not be able to maintain our relationships with our customers, including certain of our licensees. In addition, because most of our customers use our coating reagents to manufacture their own products that generate royalty revenue for us, failure by us to supply these reagents could result in decreased royalty revenue, as well as decreased revenue from our surface modification coating technologies product sales. Without our existing production facilities, we would have no other means of manufacturing products until we were able to restore the manufacturing capability at these facilities or develop one or more alternative manufacturing facilities. Although we carry business interruption insurance to cover lost revenue and profits in an amount we consider adequate, this insurance does not cover all possible situations. In addition, our business interruption insurance would not compensate us for the loss of opportunity and potential adverse impact on relations with our existing customers resulting from our inability to produce products for them.
25
We may face product liability claims related to participation in clinical trials or the use or misuse of our products.
The development and sale of medical devices and component products involves an inherent risk of product liability claims. For medical device products that incorporate our coating technology, most of the licenses provide us with indemnification against such claims. However, there can be no guarantee that product liability claims will not be filed against us for such products, or for medical device products that we manufacture as part of our whole-product solutions strategy, that parties indemnifying us will have the financial ability to honor their indemnification obligations or that such manufacturers will not seek indemnification or other relief from us for any such claims. Any product liability claims, with or without merit, could result in costly litigation, reduced sales, significant liabilities and diversion of our management’s time, attention and resources. We have obtained a level of liability insurance coverage that we believe is appropriate to our activities, however, we cannot be sure that our product liability insurance coverage is adequate or that it will continue to be available to us on acceptable terms, if at all. Furthermore, we do not expect to be able to obtain insurance covering our costs and losses as a result of any recall of products or devices incorporating our technologies because of alleged defects, whether such recall is instituted by us, by a customer, or is required by a regulatory agency. A product liability claim, recall or other claim with respect to uninsured liabilities, or for amounts in excess of insured liabilities, could have a material adverse effect on our business, financial condition and results of operations.
Our revenue will be harmed if we cannot purchase sufficient components that we use in our manufacture of reagents.
We currently purchase some of the components we use to manufacture reagents from sole suppliers. If any of our sole suppliers becomes unwilling to supply components to us, experiences an interruption in its production, or is otherwise unable to provide us with sufficient material to manufacture our reagents, we will experience production interruptions. If we lose our sole supplier of any particular reagent component or are otherwise unable to procure all components required for our reagent manufacturing for an extended period of time, we may lose the ability to manufacture the reagents our customers require to commercialize products incorporating our technology. This could result in lost royalties and product sales, which would harm our financial results. Adding suppliers to our approved vendor list may require significant time and resources. We routinely attempt to maintain multiple suppliers of each of our significant materials, so we have alternative suppliers, if necessary. However, if the number of suppliers of a material is reduced, or if we are otherwise unable to obtain our material requirements on a timely basis and on favorable terms, our operations may be harmed.
We depend upon key personnel and may not be able to attract qualified personnel in the future.
Our success depends upon our ability to retain and attract highly qualified management and technical personnel. We face intense competition for such qualified personnel. We do not maintain key person insurance, and we generally do not enter into employment agreements, except with certain executive officers. Although we have non-compete agreements with most employees, there can be no assurance that such agreements will be enforceable. The loss of the services of one or more key employees or the failure to attract and retain additional qualified personnel could have a material adverse effect on our business, financial condition and results of operations.
Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer.
We collect and store sensitive data, including intellectual property, our proprietary business information and that of our customers, suppliers and business partners, and personally identifiable information of our customers and employees, on our networks. The secure maintenance of this information is critical to our operations and business strategy, and our customers expect that we will securely maintain their information. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers resulting from employee error, malfeasance or other disruptions. Any information technology breach could compromise our networks and the information stored on them could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under personal privacy laws and regulatory penalties, disrupt our operations and the services that we provide to our customers, damage our reputation and cause a loss of confidence in our products and services, any of which could adversely affect our business and competitive position.
26
RISKS RELATING TO OUR INTELLECTUAL PROPERTY
We may not be able to obtain, maintain or protect proprietary rights necessary for the commercialization of our technologies.
Our success depends, in large part, on our ability to obtain and maintain patents and trade secrets. We have been granted U.S. and foreign patents and have U.S. and foreign patent applications pending related to our proprietary technologies. There can be no assurance that any pending patent application will be approved, that we will develop additional proprietary technologies that are patentable, that any patents issued will provide us with competitive advantages or will not be challenged or invalidated by third parties, that the patents of others will not prevent the commercialization of products incorporating our technologies, or that others will not independently develop similar technologies or design around our patents. Furthermore, because we generate a significant amount of our revenue through licensing arrangements, the loss or expiration of patent protection for our licensed technologies will result in a reduction of the revenue derived from these arrangements which may have a material adverse effect on our business, cash flow, results of operations, financial position and prospects.
We may become involved in expensive and unpredictable patent litigation or other intellectual property proceedings which could result in liability for damages, or impair our development and commercialization efforts.
Our commercial success also will depend, in part, on our ability to avoid infringing patent or other intellectual property rights of third parties. There has been substantial litigation regarding patent and other intellectual property rights in the medical device and pharmaceutical industries, and intellectual property litigation may be used against us as a means of gaining a competitive advantage. Intellectual property litigation is complex, time consuming and expensive, and the outcome of such litigation is difficult to predict. If we were found to be infringing any third-party patent or other intellectual property right, we could be required to pay significant damages, alter our products or processes, obtain licenses from others, which we may not be able to do on commercially reasonable terms, if at all, or cease commercialization of our products and processes. Any of these outcomes could have a material adverse effect on our business, financial condition and results of operations.
Patent litigation or certain other administrative proceedings may also be necessary to enforce our patents or to determine the scope and validity of third-party proprietary rights. These activities could result in substantial cost to us, even if the eventual outcome is favorable to us. An adverse outcome from any such litigation or interference proceeding could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using our technology. Any action to defend or prosecute intellectual property would be costly and result in significant diversion of the efforts of our management and technical personnel, regardless of outcome, and could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to keep our trade secrets confidential, our technology and proprietary information may be used by others to compete against us.
We rely significantly upon proprietary technology, information, processes and know-how that are not subject to patent protection. We seek to protect this information through trade secret or confidentiality agreements with our employees, consultants, potential licensees, or other parties as well as through other security measures. There can be no assurance that these agreements or any security measure will provide meaningful protection for our un-patented proprietary information. In addition, our trade secrets may otherwise become known or be independently developed by competitors. If we determine that our proprietary rights have been misappropriated, we may seek to enforce our rights which would draw upon our financial resources and divert the time and efforts of our management, and could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to convince our customers to adopt our advanced generation of hydrophilic coating technologies, our royalty revenue may decrease.
In our Medical Device segment, we have licensed our PhotoLink hydrophilic technology to a number of our customers for use in a variety of medical device surface applications. We have several U.S. and international issued patents and pending international patent applications protecting various aspects of these technologies, including compositions, methods of manufacture and methods of coating devices. The expiration dates for these patents and the anticipated expiration dates of the patent applications range from fiscal 2022 to 2035. These patents and patent applications represent distinct families, with each family generally covering a successive generation of the technology, including improvements that enhance coating performance, manufacturability, or other important features desired by our customers.
27
Approximately 14% of our total revenue in fiscal 2020 was generated from our fourth-generation PhotoLink technology, which is protected by a family of patents that expired in the first quarter of fiscal 2020 in all countries where patent coverage existed for the technology, except in Japan, where the relevant patent will expire in the first quarter of fiscal 2021. Of the license agreements using our early generation technologies, most will continue to generate royalty revenue at a reduced royalty rate beyond patent expiration.
We continue to encourage our customers to adopt our advanced technology in their products. While we are actively working to support our customers’ adoption of our advanced generations of our hydrophilic coating technology, there can be no assurance that they will do so, or that those customers that have adopted, or will adopt, our hydrophilic coating technology will sell products utilizing our technology which will generate earned royalty revenue for us.
If we or any of our licensees breach any of the agreements under which we have in-licensed intellectual property from others, we could be deprived of important intellectual property rights and future revenue.
We are a party to various agreements through which we have in-licensed or otherwise acquired rights to certain technologies that are important to our business. In exchange for the rights granted to us under these agreements, we have agreed to meet certain research, development, commercialization, sublicensing, royalty, indemnification, insurance or other obligations. If we or one of our licensees fails to comply with these obligations set forth in the relevant agreement through which we have acquired rights, we may be unable to effectively use, license, or otherwise exploit the relevant intellectual property rights and may be deprived of current or future revenue that is associated with such intellectual property.
RISKS RELATING TO CLINICAL AND REGULATORY MATTERS
The development of new products and enhancement of existing products requires significant research and development and regulatory approvals, which may require clinical trials, all of which may be very expensive and time-consuming and may not result in commercially viable products.
The development of new products and enhancement of existing products requires significant investment in research and development and regulatory approvals. Regulators may require successful clinical trials prior to granting approvals for new or enhanced products.
There can be no assurance that any products now in development, or that we may seek to develop in the future, will achieve technological feasibility, obtain regulatory approval or gain market acceptance. If we are unable to obtain regulatory approval for new products or enhanced products, our ability to successfully compete in the markets in which we participate may be materially adversely impacted. A delay in the development or approval of new products and technologies may also adversely impact the timing of when these products contribute to our future revenue and earnings growth.
Delays in clinical studies are common and have many causes, and any significant delay in clinical studies being conducted by us could result in delays in obtaining regulatory approvals and jeopardize the ability to proceed to commercialization of our products.
In the third quarter of fiscal 2020, we commenced a first-in-human clinical study of our Sundance DCB. There are risks involved in this and other clinical studies, including that they may fail to enroll a sufficient number of patients for a variety of reasons or be completed on schedule, if at all. Clinical trials for any of our products could be delayed or terminated for a variety of reasons, including, but not limited to:
|
|
• |
delays in reaching agreement with applicable regulatory authorities on a clinical study design; |
|
|
• |
issuance of publications or communications relating to the safety of certain medical devices, including recent studies and communications regarding the evaluation of risks associated with paclitaxel-coated products including the FDA communication mentioned above which resulted in a temporary pause in enrollment in our TRANSCEND trial in fiscal 2019; |
|
|
• |
suspension or termination of a clinical study by us, the FDA or foreign regulatory authorities due to adverse events or safety concerns relating to our product; and |
|
|
• |
delays in recruiting suitable patients willing to participate in a trial, or delays in having patients complete participation or return for post-treatment follow-up. |
If the initiation or completion of any of the ongoing or planned clinical studies for our products is delayed for any of the above or other reasons, the regulatory approval process would be delayed and the ability to commercialize and commence sales of our products could be materially harmed. Additionally, clinical study delays may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates. Any of these events could have a material adverse effect on our business, financial condition and results of operations.
28
We cannot be sure that clinical trials of our products will be successful, or that their results will be adequate to obtain or maintain regulatory approvals.
Enrollment in the TRANSCEND pivotal clinical study for our SurVeil DCB began in the first quarter of fiscal 2018 and was completed in the fourth quarter of fiscal 2019. Patient 12-month follow-up visits in the TRANSCEND pivotal clinical study have successfully concluded, and we have achieved a sufficient level of follow-up visits to evaluate the primary endpoints. However, we have not yet received the results of the TRANSCEND clinical trial. We cannot be sure that the endpoints of the TRANSCEND clinical trial, or any other clinical trials that we may commence, will be met. Moreover, we cannot be sure that if successful, the TRANSCEND clinical trial, or any other clinical trials that we may commence, will support regulatory approvals of our products. We may expend significant financial and human capital resources on clinical trials. If they fail to achieve their endpoints, or support regulatory approvals, it could have a material adverse effect on our business, financial condition and results of operations.
Healthcare policy changes may have a material adverse effect on us.
Healthcare costs have risen significantly during the past decade. There have been and continue to be proposals by legislators, regulators and third-party payers to reduce healthcare expenditures. Certain proposals, if implemented, would impose limitations on the prices our customers will be able to charge for our products, or the amounts of reimbursement available for their products from governmental agencies or third-party payers, or otherwise negatively impact pricing and reimbursement. Because a significant portion of our revenue is currently derived from royalties on products which constitute a percentage of our customer’s product’s selling price, these limitations could have an adverse effect on our revenue.
Healthcare reform continues to be a prominent political topic. We cannot predict what healthcare programs and regulations may ultimately be implemented at the federal or state level or the effect of any future legislation or regulation in the U.S. or internationally may have on our business.
Whole-product solutions medical devices and other products incorporating our technologies are subject to increasing scrutiny and regulations, including extensive approval/clearance processes and manufacturing requirements. Any adverse regulatory and/or enforcement action (for us or our licensees) may materially affect our financial condition and business operations.
Our products and our business activities are subject to a complex regime of regulations both in the U.S. and internationally. Additionally, certain state governments and the federal government have enacted legislation aimed at increasing transparency of industry interactions with healthcare providers. Any failure to comply with these legal and regulatory requirements could impact our business. In addition, we will continue to devote substantial human capital and financial resources to further developing and implementing policies, systems and processes to comply with enhanced legal and regulatory requirements, which may impact our business and results of operations. We anticipate that governmental authorities will continue to scrutinize our industry closely, and that additional regulation may increase compliance and legal costs, exposure to litigation, and other adverse effects to our operations.
To varying degrees, the FDA and comparable agencies outside the U.S. require us to comply with laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of our products. Our compliance with these laws and regulations takes significant human capital and financial resources; involves stringent testing and surveillance; involves attention to any needed product improvements (such as modifications, repairs, or replacements); and may include significant limitations of the uses of our products.
Changes in existing regulations or adoption of new governmental regulations or policies could prevent or delay regulatory approval of products incorporating our technologies or subject us to additional regulation. Failure or delay by us or our licensees in obtaining FDA, E.U., and other necessary regulatory approval or clearance, or the loss of previously obtained approvals, could have a material adverse effect on our business, financial condition and results of operations.
29
Our facilities and procedures are subject to periodic inspections by the FDA to determine compliance with the FDA’s requirements. The results of these inspections can include inspectional observations on FDA’s Form-483, warning letters, or other forms of enforcement. The FDA has significantly increased its oversight of companies subject to its regulations, including medical device companies. If the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical devices are ineffective or pose an unreasonable health risk, the FDA could ban such medical devices; detain or seize adulterated or misbranded medical devices; order a recall, repair, replacement or refund of such devices; refuse to grant pending pre-market approval applications or require certificates of non-U.S governments for exports; and/or require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health. The FDA may also assess civil or criminal penalties against us, our officers or employees and impose operating restrictions on a company-wide basis, or enjoin and/or restrain certain conduct resulting in violations of applicable law. The FDA may also recommend prosecution to the U.S. Department of Justice. Any adverse regulatory action, depending on its magnitude, may restrict us from effectively marketing and selling our products and limit our ability to obtain future pre-market clearances or approvals, and could result in a substantial modification to our business practices and operations.
We may face liability if we mishandle or improperly dispose of the hazardous materials used in some of our research, development and manufacturing processes.
Our research, development and manufacturing activities sometimes involve the controlled use of various hazardous materials. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. While we currently maintain insurance in amounts that we believe are appropriate, we could be held liable for any damages that might result from any such event. Any such liability could exceed our insurance and could have a material adverse effect on our business, financial condition and results of operations.
Additionally, certain of our activities are regulated by federal and state agencies in addition to the FDA. For example, activities in connection with disposal of certain chemical waste are subject to regulation by the U.S. Environmental Protection Agency. We could be held liable in the event of improper disposal of such materials, even if done by third parties. Some of our reagent chemicals must be registered with the agency, with basic information filed related to toxicity during the manufacturing process as well as the toxicity of the final product. Failure to comply with existing or future regulatory requirements could have a material adverse effect on our business, financial condition and results of operations.
RISKS RELATING TO OUR SECURITIES
Our stock price has been volatile and may continue to be volatile.
The trading price of our common stock has been, and may continue to be, highly volatile, in large part attributable to developments and circumstances related to factors identified in “Forward-looking Statements” and “Risk Factors.” Our common stock price may rise or fall sharply at any time because of this volatility, as a result of sales executed by significant holders of our stock, and also because of short positions taken by investors from time to time in our stock. For instance, the market prices for securities of medical technology, drug-delivery and biotechnology companies historically have been highly volatile, and the market has experienced significant price and volume fluctuations that may be unrelated to the operating performance of particular companies.
30
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
ITEM 2. PROPERTIES.
Our principal operations are located in Eden Prairie, a suburb of Minneapolis, Minnesota, where we own a building that has approximately 64,000 square feet of space utilized by our Corporate, Medical Device and IVD operating segments. We also own a 45,000 square foot building in Ballinasloe, Ireland dedicated to our Medical Device operating segment. We lease a warehouse through December 2025 and a 50,000 square foot facility through April 2028, which is primarily used for Medical Device segment operations, R&D and redundant manufacturing capacity. Both of the leased properties are located near our principal operations in Eden Prairie, Minnesota. We also own an undeveloped parcel of land adjacent to our principal facility, which we may use to accommodate our growth needs.
ITEM 3. LEGAL PROCEEDINGS.
See the discussion of “Litigation” in Note 11 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
ITEM 4. MINE SAFETY DISCLOSURES.
Not Applicable.
31
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Our stock is traded on the NASDAQ Global Select Market under the symbol “SRDX.”
Our transfer agent is:
Broadridge Corporate Issuer Solutions, Inc.
P.O. Box 1342
Brentwood, NY 11717
1-877-830-4936
According to the records of our transfer agent, as of November 30, 2020, there were 206 holders of record of our common stock.
We have never declared or paid any dividends on our common stock. We currently intend to retain future earnings for the operation and expansion of our business and to repurchase shares of our common stock under the repurchase authorization described below, if appropriate, and therefore we do not anticipate declaring or paying cash dividends in the foreseeable future. The declaration and payment by Surmodics of future dividends, if any, on our common stock will be at the sole discretion of the Board of Directors and will depend on Surmodics’ continued earnings, financial condition, capital requirements and other factors that the Board of Directors deems relevant. In addition, the Loan and Security Agreement that governs our revolving credit facility contains certain restrictions on our ability to pay dividends.
On November 6, 2015, the Company’s Board of Directors authorized it to repurchase up to an additional $20.0 million (“fiscal 2016 authorization”) of the Company’s outstanding common stock in open-market purchases, privately negotiated transactions, block trades, accelerated share repurchase (“ASR”) transactions, tender offers or by any combination of such methods. The share repurchase program does not have a fixed expiration date.
On November 5, 2014, the Company’s Board of Directors authorized it to repurchase up to $30.0 million (“fiscal 2015 authorization”) of the Company’s outstanding common stock in open-market purchases, privately negotiated transactions, block trades, ASR transactions, tender offers or by any combination of such methods. An aggregate of $20.0 million of the fiscal 2015 authorization was utilized in fiscal 2015, with an additional $4.7 million utilized in fiscal 2017. The share repurchase program does not have a fixed expiration date.
The Company has an aggregate of $25.3 million available for future common stock purchases under the current authorizations.
32
Stock Performance Chart
The following chart compares the cumulative total shareholder return on the Company’s Common Stock with the cumulative total return on the NASDAQ US Benchmark Total Return (our broad equity market index) and the NASDAQ Medical Supplies Index (our published industry index). The comparisons assume $100 was invested on September 30, 2015 and assume reinvestment of dividends.
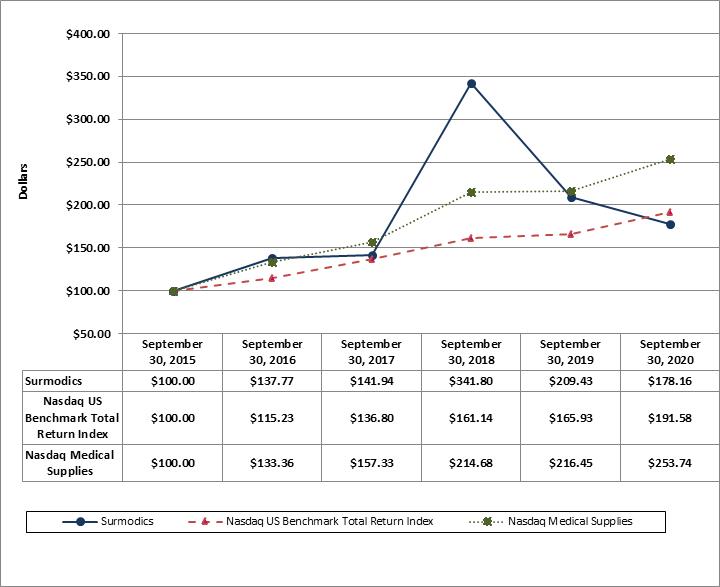
33
ITEM 6. SELECTED FINANCIAL DATA.
The following financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and accompanying notes in “Financial Statements and Supplementary Data” contained in Items 7 and 8, respectively, of Part II of this Annual Report on Form 10-K.
|
|
|
Fiscal Year |
|
|||||||||||||||||
|
(In thousands, except per share data) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|
2016 |
|
|||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
|
$ |
94,864 |
|
|
$ |
100,077 |
|
|
$ |
81,336 |
|
|
$ |
73,112 |
|
|
$ |
71,366 |
|
|
Operating (loss) income |
|
|
(1,251 |
) |
|
|
6,469 |
|
|
|
(8,799 |
) |
|
|
7,103 |
|
|
|
16,859 |
|
|
Net income (loss) |
|
|
1,123 |
|
|
|
7,592 |
|
|
|
(4,457 |
) |
|
|
3,926 |
|
|
|
9,985 |
|
|
Diluted income (loss) per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
0.08 |
|
|
$ |
0.55 |
|
|
$ |
(0.34 |
) |
|
$ |
0.29 |
|
|
$ |
0.76 |
|
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, short-term and long-term investments |
|
$ |
61,098 |
|
|
$ |
55,292 |
|
|
$ |
65,020 |
|
|
$ |
48,336 |
|
|
$ |
46,941 |
|
|
Total assets |
|
|
168,763 |
|
|
|
159,865 |
|
|
|
164,135 |
|
|
|
136,593 |
|
|
|
132,894 |
|
|
Retained earnings |
|
|
111,828 |
|
|
|
110,705 |
|
|
|
97,615 |
|
|
|
102,072 |
|
|
|
98,146 |
|
|
Total stockholders’ equity |
|
|
131,055 |
|
|
|
122,516 |
|
|
|
108,610 |
|
|
|
111,557 |
|
|
|
106,833 |
|
|
Statement of Cash Flows Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities |
|
$ |
14,010 |
|
|
$ |
8,038 |
|
|
$ |
34,052 |
|
|
$ |
14,053 |
|
|
$ |
25,166 |
|
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following discussion and analysis provide information management believes is useful in understanding the operating results, cash flows and financial condition of Surmodics. The following discussion should be read together with “Selected Financial Data” and our audited consolidated financial statements and related notes appearing elsewhere in this report. Any discussion and analysis regarding our future financial condition and results of operations are forward-looking statements that involve risks, uncertainties and assumptions, as more fully identified in “Forward-looking Statements” and “Risk Factors.” Our actual future financial condition and results of operations may differ materially from those anticipated in the forward-looking statements.
Overview
Surmodics, Inc. and subsidiaries (referred to as “Surmodics,” the “Company,” “we,” “us,” “our” and other like terms) is a leading provider of surface modification technologies for intravascular medical devices and chemical components for in vitro diagnostic (“IVD”) immunoassay tests and microarrays. Surmodics is pursuing development and commercialization of highly differentiated medical devices that are designed to address unmet clinical needs and engineered to the most demanding requirements. This key growth strategy leverages the combination of the Company’s expertise in proprietary surface technologies, along with enhanced device design, development, and manufacturing capabilities. The Company mission remains to improve the detection and treatment of disease.
Product Development
Our business model for our whole-product solutions strategy within our Medical Device segment is to design, develop and manufacture highly differentiated products that incorporate our proprietary catheter, balloon, thrombectomy and/or surface modification coating technologies to improve patient outcomes and reduce procedure costs, while maintaining patient safety. We are focused on developing devices that meet the needs of a spectrum of care settings ranging from hospitals, to ambulatory surgery centers, to office-based interventional labs in order to provide improved care and address unmet needs in the treatment of peripheral artery disease (“PAD”) and other vascular diseases.
34
Below is a brief summary of our pipeline of medical device products under development and recently commercialized, grouped by product platform.
Drug-coated balloons
|
• |
SurVeilTM DCB – paclitaxel-coated DCB to treat PAD in the superficial femoral artery. In fiscal 2018, we entered into an agreement (the “Abbott Agreement”) with Abbott Vascular, Inc. (“Abbott”) that provides Abbott with exclusive worldwide commercialization rights to the SurVeil DCB product. Patient 12-month follow-up visits in the TRANSCEND pivotal clinical trial have successfully concluded, and we have achieved a sufficient level of follow-up visits to evaluate the primary endpoints. In fiscal 2021, we expect to submit the final clinical report to the FDA for premarket approval (“PMA”). |
In the third quarter of fiscal 2020, we received Conformité Européenne Mark (“CE Mark”) approval, which is a prerequisite for commercialization of the SurVeil DCB in the European Union (E.U.). The timeline for commercialization of the SurVeil DCB in the E.U. is to be determined at the discretion of Abbott, subject to the terms of the Abbott Agreement.
|
• |
AvessTM DCB – paclitaxel-coated DCB for the treatment of arteriovenous (“AV”) fistulae commonly associated with hemodialysis. In fiscal 2019, we commenced and completed enrollment in a first in-human, 12-patient clinical study of our Avess DCB. In fiscal 2020, initial study results were received and demonstrated promising early safety data and performance insights, with greater than 90% of treated patients free from revascularization at six months. We plan to further evaluate initial study results in conjunction with relevant TRANSCEND study data when it is available. |
|
• |
SundanceTM DCB – sirolimus-coated DCB for the treatment of below-the-knee PAD. We commenced the SWING first-in-human, 35-patient clinical study of our Sundance DCB in the third quarter of fiscal 2020. We expect to complete enrollment in the SWING clinical study in the second half of fiscal 2021. |
Radial access
|
• |
Sublime™ radial access platform – access and therapeutic devices designed to provide radial (wrist) access to the peripheral vasculature. In fiscal 2019, we received FDA 510(k) clearance for our Sublime guide sheath, which enables the performance of lower extremity interventions from the radial artery. In the third quarter of fiscal 2020, we received FDA 510(k) clearance for the Sublime radial-access 0.014” percutaneous transluminal angioplasty (“PTA”) balloon catheter for treatment of lesions in arteries below the knee. An important precursor to commercialization of our radial access platform is establishment of product performance experience through physician evaluations in real-world case settings. These evaluations are designed to assess the human use factors and performance of these devices. We are targeting the first half of fiscal 2021 to initiate product evaluation activities for our Sublime guide sheath and 0.014” PTA balloon catheter products. |
In the fourth quarter of fiscal 2020, we froze the design of our Sublime 0.018” PTA balloon catheter, and we expect to pursue FDA 510(k) clearance for this product in fiscal 2021.
Thrombectomy
|
• |
Pounce™ thrombectomy platform – mechanical thrombectomy technology designed to remove thrombus or emboli (clots) from the vasculature. The Pounce technology platform has the potential to extend to other vascular indications beyond arterial with further development. |
In the fourth quarter of fiscal 2020, we received FDA 510(k) clearance on our first thrombectomy device, the Pounce Thrombus Retrieval System, intended for the non-surgical removal of thrombi and emboli (clots) from the peripheral arterial vasculature. We expect to initiate product evaluation activities for our Pounce Thrombus Retrieval System in the second half of fiscal 2021 to assess human-use factors and product performance prior to commercialization.
Specialty catheters
|
• |
Telemark™ – coronary/peripheral support microcatheter. In fiscal 2019, we executed an agreement with Medtronic plc (“Medtronic”) to distribute our Telemark microcatheter in the U.S. and Europe for coronary applications. Shipment of initial U.S. orders of our Telemark microcatheter commenced early in fiscal 2020. In the third quarter of fiscal 2020, we obtained CE Mark for our Telemark microcatheter and shipped initial European orders. |
|
• |
0.014” and 0.018” low-profile PTA balloon dilation catheters – specialty PTA balloon catheters for difficult-to-treat lesions. In fiscal 2019, we executed an agreement with Cook Medical for worldwide distribution, excluding Japan, of these products. Shipment of initial orders and the U.S. commercial launch commenced in the third quarter of fiscal 2020. |
35
For more information regarding our product development and commercialization strategy, see Part I, Item 1 of this Annual Report on Form 10-K.
Coating Technology Patents
We generate royalties revenue from licensing our proprietary surface coating technology to customers. Medical Device royalties revenue was 30%, 35% and 38% of our total revenue for fiscal 2020, 2019 and 2018, respectively. The most significant source of royalties revenue was derived from our hydrophilic coating technology. The latest generation of our hydrophilic coating technology, our SereneTM hydrophilic coating, is protected by a family of patents that begin to expire in 2033. Royalties revenue associated with our Serene hydrophilic coating technology increased approximately 27% in fiscal 2020, compared to the prior year, driven by customer product launches and resulting market share increases associated with customer device applications that incorporate this next-generation coating technology.
The family of patents that protected our fourth-generation PhotoLink hydrophilic coating technology expired in the first quarter of fiscal 2020 in all countries where patent coverage existed for the technology, except in Japan, where the relevant patent will expire in the first quarter of fiscal 2021. Medical Device royalties revenue associated with our fourth-generation hydrophilic coating technology was approximately 14%, 21% and 21% of our total revenue for fiscal 2020, 2019 and 2018, respectively. Of the license agreements using our fourth-generation and early-generation Photolink technologies, most continue to generate royalties revenue for know-how and other proprietary rights, at a reduced royalty rate, beyond patent expiration. The amount of the decline in royalties and license fee revenue in fiscal 2020, compared to the prior year, related specifically to the expiration of fourth-generation hydrophilic coating patents was approximately $5.5 million. In fiscal 2021, we expect a decline of approximately $3.0 million royalties and license fee revenue, compared to the prior year, specific to the tail-end impact of these fourth-generation patent expirations. We expect this decline to be partly offset by continued growth in our next-generation Serene hydrophilic coating royalties portfolio.
COVID-19 Pandemic Update
The COVID-19 pandemic has negatively impacted the global economy, disrupted global supply chains and healthcare delivery, and created significant volatility in financial markets. In response to the pandemic and business disruptions, first and foremost, we have prioritized the health and safety of our employees, customers, suppliers and others with whom we partner in our business activities. We have implemented business policies intended to protect our employees from the spread of COVID-19. Those policies include employees working from home when possible, but the majority of our employees have continued to work from our facilities, where we have adopted health screening, implemented socially distancing and personal protective equipment requirements, enhanced cleaning and sanitation procedures, and modified workspaces to reduce the potential for disease transmission. We also have eliminated non-essential in-person contact with customers, suppliers and other third parties.
On March 18, 2020, the Centers for Medicare & Medicaid Services (“CMS”) released guidance for U.S. healthcare providers to limit non-emergent elective medical procedures other than high acuity treatments in order to conserve personal protective equipment and limit exposure to COVID-19. On April 16, 2020, the White House issued “Guidelines for Opening Up America Again” (the “White House Guidelines”) that described a phased resumption of economic activities with gating conditions for a region or state to move from one phase to another. On June 9, 2020, CMS issued recommendations for regions and states in Phase II of the White House Guidelines that non-emergent, non-COVID-19 care should be offered to patients, as clinically appropriate, in localities or facilities that have the resources to provide such care, as well as the ability to quickly respond to a surge in COVID-19 cases, if necessary.
Since the White House Guidelines and related CMS recommendations were issued, rates of COVID-19 have vacillated by region and state, in many cases surging in the fall of 2020. Accordingly, consistent with the CMS recommendations, the degree to which elective medical procedures have been offered varies by region, state, and even between healthcare systems within a state. Where elective procedures have been offered, and even for emergency procedures, some people appear to have avoided healthcare facilities, presumably out of concern for contracting COVID-19. In addition to limiting medical procedures, hospitals and other healthcare providers vary in the degree to which they are permitting access to their facilities during the pandemic.
Many of our customers use our licensed technology and purchased materials to manufacture products used in elective procedures. In addition, our customers and business partners need access to healthcare providers and facilities to effectively market, distribute and sell products incorporating our coating and device technologies, as well as our whole-product solutions. Likewise, we and our business partners need access to healthcare providers and facilities to conduct clinical trials and other activities required to achieve regulatory clearing for our products under development. We are carefully monitoring rapidly evolving changes in healthcare delivery systems and may adjust our operating and product development plans accordingly.
36
Given the unprecedented and dynamic nature of the COVID-19 pandemic, we cannot reasonably estimate the impacts it may have on our financial condition, results of operations or cash flows in the future. However, we expect that differences in the rates of delivery and utilization of elective procedures in response to CMS recommendations and the pandemic will have an adverse impact, which may be material, on our future revenues, profitability and cash flows. The extent and duration of that impact will depend upon the extent of procedure postponements and the duration of the pandemic.
Results of Operations
Fiscal Years Ended September 30, 2020, 2019 and 2018
Revenue. Fiscal 2020 revenue was $94.9 million, a ($5.2) million or (5%) decrease from fiscal 2019 revenue. Fiscal 2019 revenue was $100.1 million, a $18.7 million or 23% increase from fiscal 2018 revenue of $81.3 million. The following is a summary of revenue by reportable segment.
|
|
|
Fiscal Year |
|
|
Increase/(Decrease) |
|
|
Increase/(Decrease) |
|
|||||||||||||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|
2020 vs. 2019 |
|
|
2019 vs. 2018 |
|
|||||||||||||
|
Revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical Device |
|
$ |
71,401 |
|
|
$ |
78,353 |
|
|
$ |
60,513 |
|
|
$ |
(6,952 |
) |
|
|
(9 |
)% |
|
$ |
17,840 |
|
|
|
29 |
% |
|
In Vitro Diagnostics |
|
|
23,463 |
|
|
|
21,724 |
|
|
|
20,823 |
|
|
|
1,739 |
|
|
|
8 |
% |
|
|
901 |
|
|
|
4 |
% |
|
Total Revenue |
|
$ |
94,864 |
|
|
$ |
100,077 |
|
|
$ |
81,336 |
|
|
$ |
(5,213 |
) |
|
|
(5 |
)% |
|
$ |
18,741 |
|
|
|
23 |
% |
Medical Device. Revenue in our Medical Device segment was $71.4 million in fiscal 2020, a (9%) decline from $78.4 million in fiscal 2019. The decrease in fiscal 2020 revenue was primarily driven by the expiration of our fourth-generation hydrophilic coating patents and the impact of COVID-19. Product revenue increased by $2.8 million in fiscal 2020, compared to the prior year, largely driven by recently commercialized medical device products, partly offset by softness in orders in the second half of fiscal 2020 as our customers managed inventory in response to reductions in procedures due to COVID-19. Royalties and license fee revenue decreased (16%), or ($7.8) million, compared to fiscal 2019. Royalties revenue decreased to $28.6 million in fiscal 2020, compared to $34.8 million in fiscal 2019. Royalties revenue in fiscal 2020 declined by approximately $5.5 million due to the previously communicated expiration of our fourth-generation hydrophilic patents. In addition, royalties revenue in fiscal 2020 was impacted by the reduction in procedures as a result of COVID-19, as well as by $1.0 million in revenue recognized in fiscal 2019 associated with the extension of an existing hydrophilic coating technology license. These decreases were partly offset by growth in royalties revenue from our next-generation Serene hydrophilic coating technology driven by customer product launches and resulting market share increases associated with the customer device applications that incorporate this next-generation coating technology. License fee revenue under our SurVeil DCB license and development agreement with Abbott (“the Abbott Agreement”) decreased to $12.0 million in fiscal 2020, compared to $13.5 million in the prior year, driven primarily by relatively higher spending in fiscal 2019 to support the TRANSCEND clinical trial during the active trial enrollment phase. Abbott Agreement license fee revenue is recognized as costs are incurred on a proportional basis to total expected costs. In fiscal 2020, Abbott Agreement license fee revenue included $7.0 million in revenue recognized on a $10.8 million milestone payment received during the period. In fiscal 2019, Abbott Agreement license fee revenue included $5.1 million in revenue recognized on a $10.0 million milestone payment received during the period. Research, development and other revenue decreased by $1.9 million in fiscal 2020, compared to the prior year, due to the timing of new product development projects with several of our contract R&D customers, as well as by a decline in coating services revenue in the second half of fiscal 2020 as a result of COVID-19.
Revenue in our Medical Device segment was $78.4 million in fiscal 2019, a 29% increase from $60.5 million in fiscal 2018. The increase in fiscal 2019 revenue was a result of growth in all our revenue categories. Product revenue increased by $1.4 million, largely driven by increased balloon catheter sales volume, as well as growth in demand for our chemical reagents. Royalties and license fee revenue increased by $13.0 million in fiscal 2019, compared to the prior year. Driving the increase in royalties and license fee revenue from the prior year was $13.5 million in license fee revenue from our SurVeil DCB license and development agreement with Abbott, an increase of $9.1 million from fiscal 2018. Revenue from the Abbott agreement was primarily driven by $5.1 million of revenue recognized on a $10.0 million milestone payment received during fiscal 2019, as well as by higher spending in fiscal 2019 to support the TRANSCEND clinical trial during the active trial enrollment phase. Growth in royalties totaled $4.2 million as a result of increased customer sales of products utilizing our hydrophilic coatings technologies, as well as $1.0 million associated with the extension of an existing hydrophilic coating technology license in fiscal 2019. Increased activity in our customer research and development programs, particularly our coating services and technology feasibility services offerings, resulted in growth of $3.4 million in our research and development and other revenue.
In Vitro Diagnostics. Revenue in our IVD segment was $23.5 million in fiscal 2020, an 8% increase from $21.7 million in fiscal 2019. Revenue growth in fiscal 2020 was driven by continued demand for our microarray DNA slide products, partly offset by a decline in demand for our antigen and stabilization products in the second half of fiscal 2020 as certain customers managed inventory levels in response to the impacts of COVID-19.
37
IVD revenue was $21.7 million in fiscal 2019, a 4% increase from $20.8 million in fiscal 2018. Revenue growth in fiscal 2019 was driven by sales volume increases in our microarray DNA slides, stabilization and BioFX products, partly offset by a decline in sales of distributed antigen products.
Major costs and expenses as a percentage of total revenue were as follows:
|
|
|
Fiscal Year |
|
|||||||||||||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||||||||||||||
|
(In thousands) |
|
Amount |
|
|
% Total Revenue |
|
|
Amount |
|
|
% Total Revenue |
|
|
Amount |
|
|
% Total Revenue |
|
||||||
|
Product costs |
|
$ |
15,317 |
|
|
|
16 |
% |
|
$ |
13,639 |
|
|
|
14 |
% |
|
$ |
13,997 |
|
|
|
17 |
% |
|
Research and development |
|
|
50,188 |
|
|
|
53 |
% |
|
|
52,885 |
|
|
|
53 |
% |
|
|
40,973 |
|
|
|
50 |
% |
|
Selling, general and administrative |
|
|
28,392 |
|
|
|
30 |
% |
|
|
23,950 |
|
|
|
24 |
% |
|
|
24,111 |
|
|
|
30 |
% |
|
Acquired in-process research and development |
|
|
— |
|
|
|
— |
|
|
|
890 |
|
|
|
1 |
% |
|
|
7,888 |
|
|
|
8 |
% |
|
Acquired intangible asset amortization |
|
|
2,218 |
|
|
|
2 |
% |
|
|
2,405 |
|
|
|
2 |
% |
|
|
2,491 |
|
|
|
3 |
% |
|
Contingent consideration (gain) expense |
|
|
— |
|
|
|
— |
|
|
|
(161 |
) |
|
|
— |
|
|
|
675 |
|
|
|
1 |
% |
Product costs. Product gross margins (defined as product sales less related product costs, as a percentage of product sales) were 65%, 66% and 63% in fiscal 2020, 2019 and 2018, respectively. As we grow our Medical Device business, product gross margins may continue to be impacted by the shift in revenue mix to towards medical device sales at relatively lower margins, particularly during the scale-up phase after initial commercialization.
Research and development expense. Research and development (“R&D”) expense decreased by $2.7 million in fiscal 2020, compared to fiscal 2019, and was 53% of revenue in both fiscal 2020 and 2019. Clinical trial spending decreased in fiscal 2020, principally for the TRANSCEND clinical trial for our SurVeil DCB with the progression from active enrollment in fiscal 2019 to patient follow up in fiscal 2020, as well as for the fiscal 2019 clinical study for our Avess DCB. These decreases were partly offset by fiscal 2020 expenses related to our SWING first-in-human clinical study for the Sundance DCB, manufacturing readiness activities for our Sublime radial access platform, and continued investments in human capital within our R&D team.
R&D expense increased $11.9 million to 53% of revenue in fiscal 2019, compared to 50% of revenue in fiscal 2019, primarily due to expense related to the TRANSCEND clinical trial for our SurVeil DCB, as well as pre-commercial manufacturing and inventory-related costs for our SurVeil DCB, as we established manufacturing capabilities for this product. Additionally, we continued to increase investment into development of our radial access and thrombectomy device platforms, as well as development and clinical study activities related to our Sundance and Avess DCBs. Internal R&D costs include employee costs, supplies, materials, facilities and overhead related to the design, development, testing and pursuit of regulatory approval for our products, including clinical costs.
Selling, general and administrative expense. Selling, general and administrative (“SG&A”) expense increased by $4.4 million to 30% of revenue in fiscal 2020, compared to 24% of revenue in fiscal 2019. The increase in SG&A expense in fiscal 2020 was primarily driven by personnel and other investments to support product development and strategic initiatives. Also contributing to the increase in SG&A expense in fiscal 2020 was a $0.6 million reduction to expense in fiscal 2019 resulting from a claim that was settled for less than the amount we had reserved. In fiscal 2019, SG&A expense decreased by $0.2 million to 24% of revenue, compared to 30% of revenue in fiscal 2018. In fiscal 2019, increases in compensation-related SG&A costs were more than offset by a $0.6 million benefit from a customer claim which was settled in fiscal 2019 for less than the amount reserved in fiscal 2018.
Acquired in-process R&D. We acquired certain intellectual property assets in fiscal 2019 that resulted in a charge to acquired in-process R&D expense totaling $0.9 million in fiscal 2019. In fiscal 2018, we acquired an innovative thrombectomy technology platform from Embolitech, LLC. As a result, we recognized acquired in-process R&D expense totaling $7.9 million in fiscal 2018, representing the present value of upfront and probable future payments expected to be made under the agreement.
Acquisition related intangible asset amortization. We have previously acquired certain intangible assets through business combinations, which are amortized over periods ranging from six to 14 years. Amortization expense on acquired intangible assets was generally consistent for fiscal 2020, 2019 and 2018.
Contingent consideration (gain) expense. We recorded ($0.2) million contingent consideration gain in fiscal 2019 and $0.7 million contingent consideration expense in fiscal 2018 from changes in the estimated fair value of our contingent consideration obligations stemming from fiscal 2016 business acquisitions. (Gain) expense in each fiscal year related to changes in the probability and timing of achieving certain revenue and operational milestones, as well as expense for the passage of time (i.e. accretion). In the first quarter of fiscal 2020, we completed the final contingent consideration payment of $3.2 million to the sellers of NorMedix, Inc. (“NorMedix”).
38
Other (expense) income. Major classifications of other (expense) income were as follows:
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Investment income, net |
|
$ |
656 |
|
|
$ |
1,097 |
|
|
$ |
851 |
|
|
Interest expense |
|
|
(133 |
) |
|
|
(152 |
) |
|
|
— |
|
|
Foreign exchange (loss) gain |
|
|
(248 |
) |
|
|
134 |
|
|
|
239 |
|
|
(Loss) gain on strategic investments and other |
|
|
(478 |
) |
|
|
10 |
|
|
|
177 |
|
|
Other (expense) income |
|
$ |
(203 |
) |
|
$ |
1,089 |
|
|
$ |
1,267 |
|
Investment income in fiscal 2020 declined relative to the prior year commensurate with a decline in interest rates. Investment income increased in fiscal 2019 relative to the prior year as a result of an increase in average investment principal stemming from the $35 million of total payments received related to the Abbott agreement in fiscal 2019 and 2018. Fiscal 2020 and 2019 interest expense included accreted expense on liabilities related to our acquisitions of certain in-process R&D technology assets in fiscal 2019 and 2018. Foreign currency (losses) gains result primarily from the impact of U.S. to Euro exchange rate fluctuations on certain intercompany obligations, as well as on Euro-denominated contingent consideration liabilities outstanding in fiscal 2019 and 2018 related to the Creagh Medical acquisition. Foreign exchange (losses) gains reflect (strengthening) weakening of the Euro relative to the U.S. dollar in each respective period. In fiscal 2020, we recognized a $0.5 million impairment loss on our investment in ViaCyte, Inc. to reduce the carrying value to zero.
Income tax benefit. We recorded an income tax benefit of $2.6 million, less than $0.1 million and $3.1 million in fiscal 2020, 2019 and 2018, respectively. In March 2020, the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”) was enacted and included significant business tax provisions. In particular, the CARES Act modified the rules associated with net operating losses (“NOLs”). In fiscal 2020, we recorded a discrete tax benefit of $1.7 million as a result of our ability under the CARES Act to carry back NOLs incurred to periods when the statutory tax rate was 35% versus our current tax rate of 21%.
In December 2017, the Tax Cuts and Jobs Act (“TCJA”) tax legislation was enacted, which reduced the U.S. Federal statutory tax rate from 35% to 21%, among other changes. In fiscal 2018, we recorded discrete tax expense of $1.6 million stemming from the revaluation of our net deferred tax assets based on the change in the enacted tax rate under the TCJA. U.S. tax law requires that taxpayers with a fiscal year beginning before and ending after the effective date of a rate change calculate a blended tax rate for the year based on the pro rata number of days in the year before and after such effective date. Accordingly, for fiscal 2018, our statutory income tax rate was 24.5% in the U.S.
The following is a reconciliation of our statutory U.S. federal tax rates and our effective tax rates:
|
|
|
Fiscal Year |
|
|||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Statutory U.S. federal income tax rate |
|
|
21.0 |
% |
|
|
21.0 |
% |
|
|
24.5 |
% |
|
State income taxes, net of federal benefit |
|
|
37.9 |
|
|
|
(6.0 |
) |
|
|
9.6 |
|
|
Federal and foreign research and development tax credits |
|
|
108.1 |
|
|
|
(32.6 |
) |
|
|
22.7 |
|
|
Foreign and state rate differential |
|
|
(14.5 |
) |
|
|
2.1 |
|
|
|
(4.9 |
) |
|
Valuation allowance change |
|
|
(56.7 |
) |
|
|
8.9 |
|
|
|
(12.7 |
) |
|
Stock based compensation (1) |
|
|
5.6 |
|
|
|
(2.2 |
) |
|
|
27.4 |
|
|
Contingent consideration gain and related foreign currency revaluation |
|
|
— |
|
|
|
(0.8 |
) |
|
|
(2.2 |
) |
|
U.S. federal & state rate change |
|
|
(1.2 |
) |
|
|
0.6 |
|
|
|
(21.0 |
) |
|
Tax reserve change |
|
|
(41.9 |
) |
|
|
10.2 |
|
|
|
(2.1 |
) |
|
Foreign-derived income deduction |
|
|
6.1 |
|
|
|
(2.0 |
) |
|
|
— |
|
|
Impact of CARES Act |
|
|
116.9 |
|
|
|
— |
|
|
|
— |
|
|
Other |
|
|
(4.1 |
) |
|
|
0.4 |
|
|
|
(0.5 |
) |
|
Effective tax rate |
|
|
177.2 |
% |
|
|
(0.4 |
)% |
|
|
40.8 |
% |
(1)Includes non-deductible stock-based compensation.
The difference between the respective U.S. federal statutory tax rates and our annual effective tax rates reflects the impact of differences between amounts recorded in our consolidated financial statements and our tax returns.
39
Our effective tax rate in fiscal 2020 differed from the U.S. federal statutory rate due primarily to the $1.7 million discrete tax benefit recorded as a result of the CARES Act. In addition, our effective tax rate in fiscal 2020 was impacted by the U.S. federal R&D tax credit, the impact of which was partly offset by related tax reserve changes as well as operating losses in Ireland, where the 12.5% statutory rate tax benefits are offset by a full valuation allowance.
Our effective tax rate in fiscal 2019 differed from the U.S. federal statutory rate due primarily to our U.S. federal R&D tax credit, the impact of which was partly offset by related tax reserve changes, as well as operating losses in Ireland, where the 12.5% statutory rate tax benefits are offset by a full valuation allowance.
Our effective tax rate in fiscal 2018 differed from the U.S. federal statutory rate due primarily to the impact of U.S. tax rate decreases on our deferred tax assets, research and development tax credits, and excess tax benefits associated with stock-based compensation. Additionally, as in prior years, operating losses in Ireland, where the 12.5% statutory rate tax benefits are offset by a full valuation allowance, non-deductible amortization expense, and contingent consideration expense impacted the effective tax rate in fiscal 2018.
During fiscal 2020, 2019 and 2018, we recognized net excess tax benefits from share options exercised, expired, forfeited or vested totaling $0.4 million, $0.5 million and $2.0 million, respectively.
Segment Operating Results
Operating results for each of our reportable segments were as follows:
|
|
|
Fiscal Year |
|
|
Increase/(Decrease) |
|
|
Increase/(Decrease) |
|
|||||||||||||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|
2020 vs. 2019 |
|
|
2019 vs. 2018 |
|
|||||||||||||
|
Operating (loss) income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical Device |
|
$ |
(3,246 |
) |
|
$ |
4,794 |
|
|
$ |
(8,478 |
) |
|
$ |
(8,040 |
) |
|
|
(168 |
)% |
|
$ |
13,272 |
|
|
|
(157 |
)% |
|
In Vitro Diagnostics |
|
|
11,771 |
|
|
|
10,620 |
|
|
|
8,619 |
|
|
|
1,151 |
|
|
|
11 |
% |
|
|
2,001 |
|
|
|
23 |
% |
|
Total segment operating (loss) income |
|
|
8,525 |
|
|
|
15,414 |
|
|
|
141 |
|
|
|
(6,889 |
) |
|
|
(45 |
)% |
|
|
15,273 |
|
|
|
10832 |
% |
|
Corporate |
|
|
(9,776 |
) |
|
|
(8,945 |
) |
|
|
(8,940 |
) |
|
|
(831 |
) |
|
|
9 |
% |
|
|
(5 |
) |
|
|
— |
|
|
Total operating (loss) income |
|
$ |
(1,251 |
) |
|
$ |
6,469 |
|
|
$ |
(8,799 |
) |
|
$ |
(7,720 |
) |
|
|
(119 |
)% |
|
$ |
15,268 |
|
|
|
(174 |
)% |
Medical Device. Our Medical Device business reported an operating loss of ($3.2) million in fiscal 2020, compared to operating income of $4.8 million in fiscal 2019, primarily driven by a $7.8 million decline in royalties and license fee revenue related to the expiration of our fourth-generation hydrophilic coating patents, the impact of COVID-19 on procedure volume, and a decline in Abbott Agreement license fee revenue. Operating expenses, excluding product costs, increased $0.8 million in fiscal 2020, compared to the prior year. Fiscal 2020 increases in SG&A to support our whole-product solutions strategy were offset by a decline in R&D from higher clinical study costs in fiscal 2019. SG&A increased in fiscal 2020 as we invested in the talent and capabilities necessary to support product development decisions and accelerate the development and management of our existing product platforms, including physician feedback and product evaluations. Operating performance was positively impacted by an increase in product gross profit of $1.3 million in fiscal 2020, compared to fiscal 2019, as a result of leverage on higher product revenue. Product gross margin declined to 61.3% for fiscal 2020, compared to 63.2% in fiscal 2019, due primarily to the unfavorable impact of fiscal 2020 product mix. In fiscal 2020, certain legacy medical device customers reduced order volume as a result of COVID-19, and revenue volume increased from initial orders of newly developed specialty catheter products. Fiscal 2020 gross margins were also a factor of our Medical Device business sustaining the necessary manufacturing infrastructure and capacity for the future commercialization of our SurVeil DCB.
Our Medical Device business reported operating income of $4.8 million in fiscal 2019, compared to an operating loss of ($8.5) million in fiscal 2018, primarily due to $17.8 million in higher revenue, partly offset by $11.9 million in higher R&D expenses. Additionally, in fiscal 2019 we recorded $0.9 million in expense for acquisitions of early-stage medical device technology, compared to $7.9 million in fiscal 2018. R&D expense increased as we completed enrollment in the TRANSCEND clinical trial, initiated and completed enrollment in a first-in-human trial of our Avess DCB, and incurred pre-clinical expenses in our Sundance DCB program to prepare for an expected first-in-human trial in fiscal 2020. Additionally, we continued to invest significantly into development of our medical device pipeline as well as continued investment in sales and marketing infrastructure, including additional headcount, to support our whole-product solutions strategy. Operating income in fiscal 2019, compared to fiscal 2018, was positively impacted by contingent consideration activity of ($0.8) million, as well as by ($1.7) million related to the settlement of a customer claim. Product gross margins increased to 63.2% for fiscal 2019, compared to 61.4% in fiscal 2018, due primarily to favorability in fiscal 2019 product mix.
In Vitro Diagnostics. Operating income in our IVD business increased by 11% or $1.2 million in fiscal 2020, compared to fiscal 2019, as a result of revenue growth. Product gross profit increased by $1.1 million in fiscal 2020, compared to the prior year. Product gross margin increased to 69.4% in fiscal 2020 from 68.6% in fiscal 2019 driven by both leverage on revenue growth and a shift in revenue mix towards products with relatively higher gross margins.
40
Operating income in our IVD business increased by 23% or $2.0 million in fiscal 2019, compared to fiscal 2018, resulting from revenue increases and product gross margin improvement, as well as reduced SG&A and allocated corporate costs. Product gross margin increased to 68.6% in fiscal 2019 from 64.6% in 2018 due to increased revenue, favorable product mix and manufacturing leverage.
Corporate. The Corporate category includes expenses for administrative corporate functions, such as executive, corporate accounting, legal, human resources and Board of Directors related fees and expenses, which have not been fully allocated to the Medical Device and IVD segments. Corporate also includes expenses, such as litigation, which are not specific to a segment and thus not allocated to our reportable segments. The unallocated Corporate expense operating loss was $9.8 million in fiscal 2020 and $8.9 million in both fiscal 2019 and 2018. The $0.8 million, or 9% increase in Corporate expense from fiscal 2020 to 2019 was primarily driven by compensation expenses. Corporate expense was flat in fiscal 2019 compared to fiscal 2018.
Liquidity and Capital Resources
As of September 30, 2020, working capital totaled $67.7 million, an increase of $6.5 million from September 30, 2019. We define working capital as current assets minus current liabilities. Cash and cash equivalents and available-for-sale investments totaled $61.1 million as of September 30, 2020, an increase of $5.8 million from $55.3 million as of September 30, 2019. This change was primarily driven by the $10.8 million SurVeil DCB milestone payment received from Abbott and a relative increase in cash provided by operations in fiscal 2020, compared to fiscal 2019, partially offset by the following in fiscal 2020: $3.2 million contingent consideration payment related to the NorMedix acquisition, $3.7 million in capital expenditures, $2.5 million cash payments for taxes related to net share settlement of equity awards, and the $1.0 million payment to Embolitech, LLC for the acquisition of in-process R&D.
During fiscal 2020, the Company took proactive steps to secure our future ability to access capital to support liquidity and continued growth. In the fourth quarter of fiscal 2020, the Company entered into a secured revolving credit facility pursuant to a Loan and Security Agreement (the ‘‘Loan Agreement’’). The Loan Agreement provides for availability of up to $25 million under a secured revolving line of credit. The outstanding balance on the revolving credit facility was zero as of September 30, 2020. In the third quarter of fiscal 2020, the Company filed a universal shelf registration statement with the SEC as a matter of standard corporate governance to provide the flexibility to access public capital markets in order to respond to future business needs and opportunities. The shelf registration statement became effective on May 29, 2020 and allows the Company to offer potentially up to $200 million in debt securities, common stock, preferred stock, warrants, and other securities or any such combination of such securities in amounts, at prices, and on terms announced if and when the securities are ever offered.
The Company’s investment policy excludes ownership of collateralized mortgage obligations, mortgage-backed derivatives and other derivative securities without prior written approval of the Board of Directors. Our investments primarily consist of money market, corporate bond and commercial paper securities and are reported at fair value as available-for-sale investments totaling $30.3 million as of September 30, 2020. Our investment policy requires that no more than 5% of investments be held in any one credit or issue, excluding U.S. government and government agency obligations. The primary investment objective of the portfolio is to provide for the safety of principal and appropriate liquidity, while generating an above-benchmark (Barclays Short Treasury 1-3 Month Index) total rate of return on a pre-tax basis. Management plans to continue to direct its investment advisors to manage the Company’s securities investments primarily for the safety of principal and the enhancement of liquidity for the time being as it continues to assess the impact of the COVID-19 pandemic on the Company, its business and its cash flows.
We believe that our existing cash and cash equivalents and investments, which totaled $61.1 million as of September 30, 2020, together with cash flow from operations, will provide liquidity sufficient to meet our cash needs and fund our operations and planned capital expenditures for the next twelve months. There can be no assurance, however, that our business will continue to generate cash flows at historic levels. Uncertainty related to the COVID-19 pandemic may cause us to seek additional funding to meet our operating needs. We cannot be certain that additional funding will be available on acceptable terms, if at all. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or otherwise curtail our operations.
41
Cash Flow Operating Results. The following is a summary of cash flow results:
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Cash provided by (used in): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
14,010 |
|
|
$ |
8,038 |
|
|
$ |
34,052 |
|
|
Investing activities |
|
|
(9,066 |
) |
|
|
9,754 |
|
|
|
(23,500 |
) |
|
Financing activities |
|
|
(4,648 |
) |
|
|
(11,029 |
) |
|
|
(3,393 |
) |
|
Effect of exchange rates on changes in cash and cash equivalents |
|
|
128 |
|
|
|
(70 |
) |
|
|
(25 |
) |
|
Net change in cash and cash equivalents |
|
$ |
424 |
|
|
$ |
6,693 |
|
|
$ |
7,134 |
|
Operating Activities. Cash provided by operating activities totaled $14.0 million, $8.0 million and $34.1 million in fiscal 2020, 2019 and 2018, respectively. During fiscal 2020, 2019 and 2018, we reported net income (loss) of $1.1 million, $7.6 million and ($4.5) million, respectively. Net changes in operating assets and liabilities increased (reduced) cash flows from operating activities by $1.1 million, ($9.9) million and $21.2 million in fiscal 2020, 2019 and 2018, respectively. Significant changes in operating assets and liabilities affecting cash flows during these years included:
|
|
• |
Cash provided by (used in) accounts receivable and contract asset was $3.5 million, ($1.6) million and ($1.8) million in fiscal 2020, 2019 and 2018, respectively. In fiscal 2020, the cash provided was primarily due to a reduction in fiscal 2020 contract asset related to reduced royalty payments receivable from customers subsequent to the expiration of our fourth-generation hydrophilic coatings patents and as a result of the impact of COVID-19. Fiscal 2019 and 2018 cash used was primarily driven by fluctuations in product sales volume. |
|
|
• |
Cash provided by (used in) accrued liabilities was $1.8 million, ($2.2) million and $5.1 million in fiscal 2020, 2019 and 2018, respectively. In fiscal 2020, the cash provided by accrued liabilities was primarily due to a $2.0 million increase in accrued compensation related to incentive compensation and accrued vacation in fiscal 2020. In fiscal 2019, the cash used in accrued liabilities was primarily due to a $1.1 million reduction in accrued compensation due to lower incentive compensation in fiscal 2019, as well as a $1.0 million reduction from a customer claim settlement in fiscal 2019. In fiscal 2018, the cash provided by accrued liabilities was driven primarily by a $2.4 million increase in accrued clinical study expense, a $1.0 million accrued customer claim related to an estimated overpayment of coating royalties, and a $1.7 million increase in accrued compensation related to incentive compensation. |
|
|
• |
Cash (used in) provided by deferred revenue was ($1.2) million, ($3.5) million and $20.7 million in fiscal 2020, 2019 and 2018, respectively. This was driven by the timing of the receipt of SurVeil DCB upfront and milestone payments from Abbott which totaled $10.8 million, $10.0 million and $25.0 million in fiscal 2020, 2019 and 2018, respectively, offset by related license fee revenue recognition of $12.0 million, $13.5 million and $4.4 million in fiscal 2020, 2019 and 2018, respectively. |
For fiscal 2020, income taxes also impacted cash provided by operating activities. Primarily as a result of the NOL carryback provisions of the CARES Act, income tax receivable increased to $2.4 million as of September 30, 2020, compared to $0.6 million as of September 30, 2019, and deferred income taxes increased to $7.3 million, compared to $6.2 million as of September 30, 2019.
Additionally, the portion of acquisition-related contingent consideration payments classified as reduction of cash flows from operations was $0.6 million and $2.0 million in fiscal 2020 and 2019, respectively, as it related to accretion expense, which increased these obligations from the acquisition date through settlement.
Investing Activities. Cash (used in) provided by investing activities totaled ($9.1) million, $9.8 million and ($23.5) million in fiscal 2020, 2019 and 2018, respectively. We invested $3.7 million, $6.0 million and $9.1 million in property and equipment in fiscal 2020, 2019 and 2018, respectively. Capital expenditures in each fiscal year were primarily related to investments in property and equipment to facilitate our whole-products strategy, including the buildout of our R&D facility in Eden Prairie, Minnesota. In fiscal 2018, these investments also included expansion of R&D and manufacturing clean rooms and assembly space as well as an analytical lab in our Irish facility. Net purchases and maturities of available-for-sale investments were a (use) source of cash totaling ($5.4) million, $16.5 million and ($9.6) million in fiscal 2020, 2019 and 2018, respectively. In fiscal 2019 and 2018, we invested $0.8 million and $5.0 million, respectively, in in-process R&D assets to expand our product development pipeline.
42
Financing Activities. Cash used in financing activities totaled $4.6 million, $11.0 million and $3.4 million in fiscal 2020, 2019 and 2018, respectively. In fiscal 2020, we paid contingent consideration of $3.2 million related to the NorMedix acquisition, with $0.6 million and $2.6 million classified as cash used in operating and financing activities, respectively. In fiscal 2019, we paid contingent consideration of $11.0 million related to the Creagh Medical acquisition, with $2.0 million and $9.1 million classified as cash used in operating and financing activities, respectively. In fiscal 2020, 2019 and 2018, we paid $2.5 million, $2.7 million and $4.5 million, respectively, to purchase common stock to pay employee taxes resulting from the exercise of stock options and vesting of other stock awards. In fiscal 2020, we paid $1.0 million to Embolitech, LLC related to our fiscal 2018 acquisition of in-process R&D. We also generated $1.6 million, $0.7 million and $2.1 million in fiscal 2020, 2019 and 2018, respectively, from the sale of common stock pursuant to our stock-based compensation arrangements.
Share Purchase Activity
Our Board of Directors has authorized the repurchase of up to an additional $25.3 million of the Company’s outstanding common stock in open-market purchases, privately negotiated transactions, block trades, accelerated share repurchase transactions, tender offers or by any combination of such methods. The authorization has no fixed expiration date.
Customer Concentrations
Revenue from customers that equaled or exceeded 10% of total revenue was as follows:
|
|
|
Fiscal Year |
|
|||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Abbott |
|
|
19 |
% |
|
|
19 |
% |
|
|
11 |
% |
|
Medtronic |
|
|
14 |
% |
|
|
14 |
% |
|
|
16 |
% |
Our licensed technologies provide royalties and license fee revenue. We have agreements with a diverse base of customers, and certain customers have multiple products using our technology. Abbott and Medtronic plc (“Medtronic”) are our largest customers. Abbott has several separately licensed products, including the SurVeil DCB license, which generate royalties and license fee revenue for Surmodics. Revenue from the SurVeil DCB license represented 13%, 13% and 5% of total revenue for fiscal 2020, 2019 and 2018, respectively. Medtronic has several separately licensed products that generate royalty revenue for Surmodics, none of which represented more than 5% of our total revenue for fiscal 2020.
Our licensing agreements with many of our customers, including most of our significant customers, cover many licensed products that each separately generates royalties revenue. This structure reduces the potential risk to our operations that may result from reduced sales (or the termination of a license) of a single product for any specific customer.
Off-balance Sheet Arrangements and Contractual Obligations
As of September 30, 2020, we did not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures, or capital resources that is material to investors.
Presented below is a summary of contractual obligations as of September 30, 2020 and payments due under these arrangements by period. For additional information regarding the below obligations, see Notes 2 and 11 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
|
(In thousands) |
|
Total |
|
|
Less than 1 Year |
|
|
1-3 Years |
|
|
4-5 Years |
|
|
More than 5 Years |
|
|||||
|
Operating leases (1) |
|
$ |
4,633 |
|
|
$ |
586 |
|
|
$ |
1,237 |
|
|
$ |
1,289 |
|
|
$ |
1,521 |
|
|
Asset acquisition obligations (2) |
|
|
3,650 |
|
|
|
1,150 |
|
|
|
1,500 |
|
|
|
1,000 |
|
|
- |
|
|
|
Minimum annual royalty obligation (3) |
|
|
1,698 |
|
|
|
234 |
|
|
|
468 |
|
|
|
468 |
|
|
|
528 |
|
|
Clinical trial CRO obligations (4) |
|
|
9,767 |
|
|
|
3,033 |
|
|
|
4,100 |
|
|
|
2,634 |
|
|
- |
|
|
|
Total |
|
$ |
19,748 |
|
|
$ |
5,003 |
|
|
$ |
7,305 |
|
|
$ |
5,391 |
|
|
$ |
2,049 |
|
(1)The Company leases U.S. facilities for research, office, manufacturing and warehousing.
(2)Asset acquisition obligations are payments to be made in connection with the in-process R&D technology asset acquisitions completed in fiscal 2019 and 2018, excluding amounts that are contingent upon unmet regulatory or commercial milestones.
43
(3)Minimum annual royalty obligation relates to payments associated with an in-bound license agreement whereby we pay an annual minimum royalty of approximately $0.2 million (at the Euro to U.S. dollar exchange rate of as of September 30, 2020) to gain access to polymer technology which is utilized in a drug-delivery customer license. The agreement includes an early termination clause. However, the future obligations above are presented through May 2027, the remaining term of the agreement, as it is not currently more likely than not that the agreement will be terminated early.
(4) Clinical Research Organization (“CRO”) obligations represent contractual periodic payments for services performed and milestone payments to third-party CROs for services related to our ongoing clinical trials. The timing of payments and recognition of expenses under these contracts is dependent on enrollment in our ongoing clinical trials and may be different from the amounts presented, which are estimated based on projected enrollment rates.
As of September 30, 2020, our gross liability, including interest and penalties, for uncertain tax positions was $3.1 million. We are not able to reasonably estimate the amount by which the liability will increase or decrease over an extended period of time or whether a cash settlement of the liability will be required. Therefore, this amount has been excluded from the schedule of contractual obligations above.
New Accounting Pronouncements
Information regarding new accounting pronouncements is included in Note 2 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
Critical Accounting Policies Estimates
The discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. (“GAAP”). The preparation of these consolidated financial statements is based in part on the application of significant accounting policies, many of which require management to make estimates and assumptions; see Notes 1 and 2 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K. Actual results may differ from these estimates and such differences could materially impact our results of operations. Critical accounting policies are those policies that require the application of management’s most challenging subjective or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Critical accounting policies involve judgments and uncertainties that are sufficiently likely to result in materially different results under different assumptions and conditions. We believe the following are critical areas in the application of our accounting policies that currently affect our financial condition and results of operations.
Revenue Recognition
We license technology to medical device manufacturers (third parties) and collect royalties based on the greater of the contractual percentage of a customer’s sales of products incorporating our licensed technologies or minimum contractual royalties. Beginning in fiscal 2019, in connection with the adoption of ASC Topic 606, the financial information included in this Annual Report on Form 10-K includes sales-based royalties revenue recognized as our license customers sell products containing our technologies, which is generally reported to us a quarter after those sales occur. This requires us to estimate the revenue earned on these arrangements and record it prior to our customers reporting the underlying sales to us. Sales-based royalties are estimated using the most-likely amount method based on historical sales information, adjusted for known changes, such as product launches and patent expirations. We also consider macroeconomic factors affecting the medical device market. These inputs require significant management judgement and are updated quarterly. Minimum royalty fees are recognized through the non-cancellable period, which is generally 90 days, but can be up to one year. Revenue related to contingent milestones is recognized upon the achievement of the milestone, provided collectability is assured. Customer advances are accounted for as a liability (deferred revenue) until all criteria for revenue recognition have been met.
We license technology to third parties and, at times, these arrangements include multiple performance obligations that require us to determine the appropriate unit(s) of account and allocate the transfer price to each of the unit(s) of account identified. The performance obligations may include license(s) to Surmodics’ technology, research, development and clinical activities, and/or product sales. We did not generate revenue from any arrangements with multiple performance obligations in fiscal 2020 or 2019.
44
Revenue associated with our license and development agreement with Abbott is recognized as the clinical and regulatory activities are performed on a proportional performance basis based on actual costs incurred relative to the expected total cost of the underlying activities, most notably the completion of the TRANSCEND clinical trial. A significant component of the cost of this trial is the cost of our outsourced clinical trial CRO consultants, which are estimated based on executed statements of work, project budgets, and patient enrollment and follow-up timing, among other things. Costs related to the clinical and regulatory activities are expensed in the period incurred. A significant change to the Company’s estimate of the costs to complete the TRANSCEND clinical trial could have a material effect on the Company’s results of operations. The total expected cost of the trial is a significant management estimate and is reviewed and assessed each reporting period. The current portion of deferred revenue on the consolidated balance sheet represents the amount of deferred revenue that is expected to be recognized over the next year, based on estimated costs to be incurred. The estimate of future revenue from the Abbott agreement will continue to be monitored and adjusted based on estimates in effect each period-end. For further disclosures related to revenue recognition, see Notes 2 and 4 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
Goodwill and Other Indefinite-lived Intangible Assets
We record all assets and liabilities acquired in purchase acquisitions at fair value, including goodwill and other intangible assets. The initial recognition of goodwill and other intangible assets requires management to make subjective judgments concerning estimates of how the acquired assets will perform in the future using valuation methods including discounted cash flow analysis.
On an ongoing basis, goodwill and certain indefinite-lived intangible assets are not amortized but are subject, at a minimum, to annual tests for impairment at the reporting unit level. A reporting unit is an operating segment, or component thereof, for which discrete financial information is available and reviewed by management on a regular basis. Management has determined that our reporting units consist of our Medical Device and IVD segments.
Goodwill in our reporting units is evaluated for impairment in two ways. First, an assessment of qualitative factors is performed to determine whether the existence of events or circumstances leads to a determination that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If, after assessing the totality of events or circumstances, the Company determines it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, then performing an impairment test, as described below, becomes unnecessary. If events or circumstances occur that would indicate that the carrying amount may be impaired, or if the Company otherwise determines it necessary, the quantitative impairment test would be performed. These tests require management to make significant judgments and estimates, most of which are based on each reporting unit’s projected future cash flows. Our estimates associated with the annual test of goodwill and indefinite-lived intangible assets are considered critical due to the amount of these assets recorded on our consolidated balance sheets and the judgment required in determining fair value, including projected future cash flows and, in the case of a quantitative test and impairment measurement, applicable discount rates.
We perform our annual assessment of goodwill for impairment as of July 1st of each fiscal year. In fiscal 2020, the annual assessment date of goodwill for impairment was changed to July 1st from the August 31st date used in fiscal 2019 and prior years. Based on the results of these assessments, no goodwill impairment charges were recorded during fiscal 2020, 2019 or 2018. During fiscal 2019, we recorded impairment charges on our indefinite-lived intangible assets of $0.3 million as a result of decreases in future revenue estimates associated with these assets. No impairment charges were recorded in fiscal 2020 and 2018 related to indefinite-lived intangible assets.
Income Taxes
Significant judgment is required in evaluating our tax positions, and in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our deferred tax assets. As of September 30, 2020 and 2019, deferred tax assets totaled $7.3 million and $6.2 million, respectively, net of valuation allowances of $6.2 million and $5.3 million, respectively. The valuation allowances are principally driven by the following factors:
|
|
• |
Financial statement other-than-temporary losses on strategic investments that were unrealized for tax purposes as we did not foresee future offsetting taxable capital gains. Therefore, as of September 30, 2020 and 2019, a valuation allowance has been recorded for all other-than-temporary impairment losses, as realized tax capital losses from sales of the underlying strategic assets have not occurred. |
|
|
• |
Deferred tax assets related to state R&D tax credit carryforwards have been offset by valuation allowances to the extent they are not expected to be utilized in future years. |
|
|
• |
Deferred tax assets related to NOLs of Creagh Medical, including those incurred prior to the acquisition in fiscal 2016, have been offset by a valuation allowance as it is not more likely than not that the tax assets will be realized in future periods, due to Creagh Medical’s history of taxable losses. |
45
We applied the accounting guidance associated with uncertain tax positions, which defines standards for recognizing the benefits of tax return positions in the consolidated financial statements as “more-likely-than-not” to be sustained by the taxing authorities based solely on the technical merits of the position. If the recognition threshold is met, the tax benefit is measured and recognized as the largest amount of tax benefit that, in our judgment, has a greater than 50% likelihood of being realized. We regularly monitor our uncertain tax positions and adjust the related liabilities to reflect completion of tax audits, expiration of an applicable statute of limitations, changes in tax laws or interpretations, and changes in our business that result in uncertainties that previously did not meet the recognition criteria. For further disclosures related to income taxes, see Note 9 to the consolidated financial statements in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Our investment policy requires investments with high credit quality issuers and limits the amount of credit exposure to any one issuer. Our investments consist principally of interest-bearing corporate debt securities with varying maturity dates, which are less than one year. Because of the credit criteria of our investment policies, the primary market risk associated with these investments is interest rate risk. We do not use derivative financial instruments to manage interest rate risk or to speculate on future changes in interest rates. As of September 30, 2020, we held $30.3 million in available-for-sale debt securities, all with maturity dates of less than one year; therefore, interest rate fluctuations would have an insignificant impact on our results of operations or cash flows. Our policy also allows the Company to hold a substantial portion of funds in cash and cash equivalents, which are defined as financial instruments with original maturities of three months or less and may include money market instruments, certificates of deposit, repurchase agreements and commercial paper instruments.
Management believes that a reasonable change in raw material prices would not have a material impact on future earnings or cash flows because the Company’s inventory exposure is not material.
We are exposed to increasing Euro currency risk with respect to our manufacturing operations in Ireland. In a period where the U.S. dollar is strengthening or weakening relative to the Euro, our revenue and expenses denominated in Euro currency are translated into U.S. dollars at a lower or higher value than they would be in an otherwise constant currency exchange rate environment. All sales transactions are denominated in U.S. dollars or Euros. We generate royalties revenue from the sale of customer products in foreign jurisdictions. Royalties generated in foreign jurisdictions by customers are converted and paid in U.S. dollars per contractual terms. Substantially all of our purchasing transactions are denominated in U.S. Dollars or Euros. To date, we have not entered into any foreign currency forward exchange contracts or other derivative financial instruments to hedge the effects of adverse fluctuations in foreign currency exchange rates.
46
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
TABLE OF CONTENTS
|
|
Page (s) |
|
48 to 49 |
|
|
50 |
|
|
51 |
|
|
52 |
|
|
53 |
|
|
54 to 55 |
|
|
56 to 80 |
47
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Surmodics, Inc.
Eden Prairie, Minnesota
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Surmodics, Inc. and subsidiaries (the "Company") as of September 30, 2020 and 2019, and the related consolidated statements of operations, comprehensive income (loss), stockholders' equity, and cash flows for each of the three years in the period ended September 30, 2020, and the related notes and the financial statement schedule listed in the Index at Item 15 (collectively referred to as the "financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of September 30, 2020 and 2019, and the results of its operations and its cash flows for each of the three years in the period ended September 30, 2020, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of September 30, 2020, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated December 2, 2020, expressed an unqualified opinion on the Company's internal control over financial reporting.
Change in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, effective October 1, 2018, the Company adopted Accounting Standard Update ASU 2014-09, Revenue from Contracts with Customers (ASC Topic 606), as amended, using the modified retrospective approach.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ DELOITTE & TOUCHE LLP
Minneapolis, Minnesota
December 2, 2020
We have served as the Company's auditor since 2002.
48
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Surmodics, Inc.
Eden Prairie, Minnesota
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of Surmodics, Inc. and subsidiaries (the “Company”) as of September 30, 2020, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of September 30, 2020, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended September 30, 2020, of the Company and our report dated December 2, 2020, expressed an unqualified opinion on those financial statements and included an explanatory paragraph regarding the Company's adoption of the new accounting standard.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ DELOITTE & TOUCHE LLP
Minneapolis, Minnesota
December 2, 2020
49
Surmodics, Inc. and Subsidiaries
Consolidated Balance Sheets
As of September 30
|
(In thousands, except per share data) |
|
2020 |
|
|
2019 |
|
||
|
ASSETS |
|
|
|
|
|
|
|
|
|
Current Assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
|
|
|
$ |
|
|
|
Available-for-sale securities |
|
|
|
|
|
|
|
|
|
Accounts receivable, net of allowances of $ September 30, 2020 and 2019, respectively |
|
|
|
|
|
|
|
|
|
Contract assets — royalties and license fees |
|
|
|
|
|
|
|
|
|
Inventories, net |
|
|
|
|
|
|
|
|
|
Income tax receivable |
|
|
|
|
|
|
|
|
|
Prepaids and other |
|
|
|
|
|
|
|
|
|
Total Current Assets |
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
|
|
|
|
|
|
|
Deferred income taxes |
|
|
|
|
|
|
|
|
|
Intangible assets, net |
|
|
|
|
|
|
|
|
|
Goodwill |
|
|
|
|
|
|
|
|
|
Other assets |
|
|
|
|
|
|
|
|
|
Total Assets |
|
$ |
|
|
|
$ |
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
|
|
|
$ |
|
|
|
Accrued liabilities: |
|
|
|
|
|
|
|
|
|
Compensation |
|
|
|
|
|
|
|
|
|
Accrued other |
|
|
|
|
|
|
|
|
|
Deferred revenue |
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
|
— |
|
|
|
|
|
|
Total Current Liabilities |
|
|
|
|
|
|
|
|
|
Deferred revenue, less current portion |
|
|
|
|
|
|
|
|
|
Other long-term liabilities |
|
|
|
|
|
|
|
|
|
Total Liabilities |
|
|
|
|
|
|
|
|
|
Commitments and Contingencies (Note 11) |
|
|
|
|
|
|
|
|
|
Stockholders’ Equity: |
|
|
|
|
|
|
|
|
|
Series A preferred stock — $ issued and outstanding |
|
|
|
|
|
|
|
|
|
Common stock — $ respectively |
|
|
|
|
|
|
|
|
|
Additional paid-in capital |
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income |
|
|
|
|
|
|
|
|
|
Retained earnings |
|
|
|
|
|
|
|
|
|
Total Stockholders’ Equity |
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Equity |
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
50
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Operations
For the Fiscal Year Ended September 30
|
(In thousands, except per share data) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product sales |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Royalties and license fees |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research, development and other |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product costs |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquired in-process research and development |
|
|
— |
|
|
|
|
|
|
|
|
|
|
Acquired intangible asset amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration (gain) expense |
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
Total operating costs and expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating (loss) income |
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|
Other (expense) income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Investment income, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
Foreign exchange (loss) gain |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
(Loss) gain on strategic investments and other |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Other (expense) income |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
(Loss) income before income taxes |
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|
Income tax benefit |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income (loss) per share: |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
Diluted net income (loss) per share: |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares outstanding: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted |
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
51
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Comprehensive Income (Loss)
For the Fiscal Year Ended September 30
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Net income (loss) |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net changes related to available-for-sale securities, net of tax |
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|
Foreign currency translation adjustments |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Other comprehensive income (loss) |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Comprehensive income (loss) |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
52
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Equity
For the Fiscal Year Ended September 30
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Other |
|
|
|
|
|
|
Total |
|
|||
|
|
|
Common Stock |
|
|
Paid-In |
|
|
Comprehensive |
|
|
Retained |
|
|
Stockholders’ |
|
|||||||||
|
(In thousands) |
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income |
|
|
Earnings |
|
|
Equity |
|
||||||
|
Balance at September 30, 2017 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Other comprehensive loss, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
Issuance of common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Common stock options exercised, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Purchase of common stock to pay employee taxes |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Balance at September 30, 2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net impact from adoption of ASC Topic 606 (Note 2) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Other comprehensive loss, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
|
Issuance of common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Common stock options exercised, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Purchase of common stock to pay employee taxes |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Balance at September 30, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
Other comprehensive income, net of tax |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
Issuance of common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Common stock options exercised, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Purchase of common stock to pay employee taxes |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
Balance at September 30, 2020 |
|
|
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
53
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
For the Fiscal Year Ended September 30
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Operating Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
Adjustments to reconcile net income (loss) to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
Payment of contingent consideration obligations in excess of acquisition-date value |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
Contingent consideration (gain) expense |
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
Acquired in-process research and development |
|
|
— |
|
|
|
|
|
|
|
|
|
|
Deferred taxes (1) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Losses (gains) on strategic investments |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Provision for bad debts |
|
|
|
|
|
|
|
|
|
|
|
|
|
Noncash lease expense |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Impairment losses on intangible assets |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
Unrealized foreign exchange gain |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Other |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Change in operating assets and liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable and contract asset (1) |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Inventories |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Prepaids and other |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Accounts payable |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
Accrued liabilities |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Income taxes (1) |
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|
Deferred revenue (1) |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
Net cash provided by operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Cash proceeds from sale of property and equipment |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
Purchases of available-for-sale securities |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Sales and maturities of available-for-sale securities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition of in-process research and development (Note 11) |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Cash received from strategic investments |
|
|
— |
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by investing activities |
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|
Financing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments for taxes related to net share settlement of equity awards |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Payment of deferred financing costs |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
Payment of contingent consideration obligations |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Payments for acquisition of in-process research and development (Note 11) |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
Net cash used in financing activities |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Effect of exchange rate changes on cash |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
Net change in cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Beginning of year |
|
|
|
|
|
|
|
|
|
|
|
|
|
End of year |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
For the fiscal year ended September 30, 2018, amounts presented are net of impact from adoption of ASC Topic 606. |
The accompanying notes are an integral part of these consolidated financial statements.
54
Surmodics, Inc. and Subsidiaries
Consolidated Statements of Cash Flows (Continued)
For the Fiscal Year Ended September 30
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Supplemental Information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for income taxes |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Noncash financing and investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition of property and equipment and intangible assets, net of refundable credits in other current assets and liabilities |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Right-of-use assets and property and equipment obtained in exchange for new operating lease liabilities |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
Acquisition of in-process research and development in other long-term liabilities |
|
|
— |
|
|
|
|
|
|
|
|
|
|
Accrual of employee taxes on common stock exercises |
|
|
— |
|
|
|
|
|
|
|
|
|
|
Acquisition of property and equipment in long-term deferred rent |
|
|
— |
|
|
|
— |
|
|
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
55
Surmodics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1. Description
Surmodics, Inc. and subsidiaries (“Surmodics,” the “Company,” “we,” “us,” “our” and other like terms) is a leading provider of surface modification technologies for intravascular medical devices and chemical components for in vitro diagnostic (“IVD”) immunoassay tests and microarrays. Surmodics is pursuing development and commercialization of highly differentiated medical devices that are designed to address unmet clinical needs and engineered to the most demanding requirements. This key growth strategy leverages the combination of the Company’s expertise in proprietary surface technologies, along with enhanced device design, development, and manufacturing capabilities. The Company mission remains to improve the detection and treatment of disease. Surmodics is headquartered in Eden Prairie, Minnesota.
Basis of Presentation
The consolidated financial statements include all accounts and wholly-owned subsidiaries and have been prepared in accordance with accounting principles generally accepted in the U.S. (“GAAP”). All intercompany transactions have been eliminated. The Company operates on a fiscal year ending on September 30.
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Ultimate results could differ from those estimates.
Certain reclassifications have been made to the prior year's consolidated financial statements to conform to the current year presentation.
Risk and Uncertainties
The COVID-19 pandemic is having, and will likely continue to have, an adverse effect on our business, results of operations, financial condition, and cash flows, and its future impacts remain highly uncertain and unpredictable. The Company has considered the disruptions caused by COVID-19, including lower than forecasted sales and customer demand and macroeconomic factors, that may impact its estimates. The Company has assessed the potential impact of the pandemic on certain accounting matters including, but not limited to, estimated sales-based royalties revenue; allowance for doubtful accounts; inventory reserves; and the valuation of goodwill, intangible assets, other long-lived assets and investments, as of September 30, 2020 and through the date of this Annual Report on Form 10-K. As of the date of issuance of these consolidated financial statements, the extent to which the COVID-19 pandemic may materially impact the Company's financial condition, liquidity or results of operations is uncertain. For further information, refer to “Risk Factors” in Part II, Item 1A of this Annual Report on Form 10-K.
2. Summary of Significant Accounting Policies and Select Balance Sheet Information
Cash and Cash Equivalents
Cash and cash equivalents consist of financial instruments with maturities of three months or less at the Company’s acquisition date of the security and are stated at cost which approximates fair value and may include money market instruments, certificates of deposit, repurchase agreements and commercial paper instruments.
Accounts Receivable, Net
We grant credit to customers in the normal course of business and maintain an allowance for doubtful accounts which reflects our estimate of uncollectible accounts as of the balance sheet date. We consider various factors in establishing, monitoring, and adjusting the allowance for credit losses including the aging of the accounts and aging trends, the historical level of charge-offs, and specific exposures related to particular customers.
56
Investments
Investments consisted of commercial paper and corporate bond securities and are classified as available-for-sale as of September 30, 2020 and 2019. Available-for-sale debt securities are reported at fair value with unrealized gains and losses, net of tax, excluded from the consolidated statements of operations and reported in the consolidated statements of comprehensive income (loss) and as a separate component of stockholders’ equity in the consolidated balance sheets, except for other-than-temporary impairments, which are reported as a charge to current earnings as they occur. A loss would be recognized when there is an other-than-temporary impairment in the fair value of any individual security classified as available-for-sale, with the associated net unrealized loss reclassified out of accumulated other comprehensive income with a corresponding adjustment to other (expense) income. This adjustment would result in a new cost basis for the investment.
The amortized cost, unrealized holding gains and losses, and fair value of available-for-sale securities were as follows:
|
|
|
September 30, 2020 |
|
|||||||||||||
|
(In thousands) |
|
Amortized Cost |
|
|
Unrealized Gains |
|
|
Unrealized Losses |
|
|
Fair Value |
|
||||
|
Commercial paper and corporate bonds |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
Total |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
|
|
September 30, 2019 |
|
|||||||||||||
|
(In thousands) |
|
Amortized Cost |
|
|
Unrealized Gains |
|
|
Unrealized Losses |
|
|
Fair Value |
|
||||
|
Commercial paper and corporate bonds |
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
Total |
|
$ |
|
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
There were
Inventories
Inventories are principally stated at the lower of cost or market using the specific identification method and include direct labor, materials and overhead, with cost of product sales determined on a first-in, first-out basis.
|
|
|
September 30, |
|
|||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
||
|
Raw materials |
|
$ |
|
|
|
$ |
|
|
|
Work-in process |
|
|
|
|
|
|
|
|
|
Finished products |
|
|
|
|
|
|
|
|
|
Total |
|
$ |
|
|
|
$ |
|
|
Property and Equipment
Property and equipment are stated at cost, less any impairment, and are depreciated using the straight-line method over the estimated useful lives of the assets. The Company recorded depreciation expense of $
The September 30, 2020 and 2019 balances in construction-in-progress include the cost of equipment and building improvements not yet placed in service in the Company’s Ballinasloe, Ireland and Eden Prairie, Minnesota facilities. As assets are placed in service, construction-in-progress is transferred to the specific property and equipment categories and depreciated over the estimated useful lives of the assets. Leasehold improvements are amortized over the shorter of the term of the lease or the estimated useful life of the asset. Expenditures for maintenance and repairs and minor renewals and betterments that do not extend or improve the life of the respective assets are expensed as incurred.
57
Property and equipment consisted of the following components:
|
|
|
Useful Life |
|
September 30, |
|
|||||
|
(Dollars in thousands) |
|
(Years) |
|
2020 |
|
|
2019 |
|
||
|
Land |
|
N/A |
|
$ |
|
|
|
$ |
|
|
|
Laboratory fixtures and equipment |
|
|
|
|
|
|
|
|
|
|
|
Buildings and improvements |
|
|
|
|
|
|
|
|
|
|
|
Leasehold improvements |
|
|
|
|
|
|
|
|
|
|
|
Office furniture and equipment |
|
|
|
|
|
|
|
|
|
|
|
Construction-in-progress |
|
|
|
|
|
|
|
|
|
|
|
Less: Accumulated depreciation |
|
|
|
|
( |
) |
|
|
( |
) |
|
Property and equipment, net |
|
|
|
$ |
|
|
|
$ |
|
|
Other Assets
Other assets consisted of the following:
|
|
|
September 30, |
|
|||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
||
|
ViaCyte, Inc. |
|
$ |
— |
|
|
$ |
|
|
|
Operating lease right-of-use assets |
|
|
|
|
|
|
— |
|
|
Other noncurrent assets |
|
|
|
|
|
|
|
|
|
Other assets, net |
|
$ |
|
|
|
$ |
|
|
The Company has invested a total of $
The total carrying value of cost method investments is reviewed quarterly for changes in circumstances or the occurrence of events that suggest the Company’s investment may not be recoverable. The carrying value of cost method investments is not adjusted if there are no identified events or changes in circumstances that may have a material adverse effect on the fair value of the investment. In fiscal 2020, the Company recorded a $
Operating lease right-of-use assets as of September 30, 2020 are recorded in accordance with ASC Topic 842, which we adopted as of October 1, 2019. Other noncurrent assets include a receivable related to refundable Irish research and development tax credits and prepaid expenses related to our ongoing clinical trials.
58
Intangible Assets
Intangible assets consisted of the following:
|
|
|
September 30, 2020 |
|
|||||||||||||
|
(Dollars in thousands) |
|
Weighted Average Original Life (Years) |
|
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Net |
|
||||
|
Definite-lived intangible assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer lists and relationships |
|
|
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
Developed technology |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Non-compete |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
— |
|
|
Patents and other |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Total definite-lived intangible assets |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Unamortized intangible assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trademarks and trade names |
|
|
|
|
|
|
580 |
|
|
|
— |
|
|
|
|
|
|
Total intangible assets |
|
|
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
|
|
September 30, 2019 |
|
|||||||||||||
|
(Dollars in thousands) |
|
Weighted Average Original Life (Years) |
|
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Net |
|
||||
|
Definite-lived intangible assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer lists and relationships |
|
|
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
|
Developed technology |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Non-compete |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Patents and other |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Total definite-lived intangible assets |
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Unamortized intangible assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trademarks and trade names |
|
|
|
|
|
|
580 |
|
|
|
— |
|
|
|
|
|
|
Total intangible assets |
|
|
|
|
|
$ |
|
|
|
$ |
( |
) |
|
$ |
|
|
The Company recorded amortization expense of $
Based on the intangible assets in service as of September 30, 2020, estimated amortization expense for each of the next five fiscal years is as follows:
|
(In thousands) |
|
|
|
|
|
2021 |
|
$ |
|
|
|
2022 |
|
|
|
|
|
2023 |
|
|
|
|
|
2024 |
|
|
|
|
|
2025 |
|
|
|
|
Future amortization amounts presented above are estimates. Actual future amortization expense may be different as a result of future acquisitions, impairments, changes in amortization periods, foreign currency exchange rates or other factors.
The Company defines in-process research and development (“IPR&D”) as the value of technology acquired for which the related projects have substance and are incomplete. IPR&D acquired in a business combination is recognized at fair value and is capitalized as an indefinite-lived intangible asset until completion or abandonment of the IPR&D project. Upon completion of the development project (generally when regulatory approval to market the product is obtained), an impairment assessment is performed prior to amortizing the asset over its estimated useful life. In cases where the IPR&D projects are abandoned, the related IPR&D assets are written off. The Company assesses indefinite-lived assets for impairment annually in the fourth quarter and whenever an event occurs or circumstances change that would indicate that the carrying amount may be impaired. Similar to the goodwill impairment assessment, the indefinite-lived assets impairment assessment requires the Company to make several estimates about fair value, most of which are based on projected future cash flows.
59
The Company performs its annual assessment of indefinite-lived intangible assets for impairment as of July 1st of each fiscal year. In fiscal 2020, the annual assessment date for impairment was changed to July 1st from the August 31st date used in fiscal 2019 and prior years. After completing the fiscal 2019 impairment analysis, the fair value of certain IPR&D and trade name assets were deemed to be less than their carrying value, due to decreases in estimated future revenue associated with the assets. Accordingly, in fiscal 2019, impairment losses on indefinite-lived intangible assets totaling $
Goodwill
Goodwill represents the excess of the purchase price of an acquired business over the fair value assigned to the assets purchased and liabilities assumed. Goodwill is not amortized but is subject, at a minimum, to annual tests for impairment in accordance with accounting guidance for goodwill. The carrying amount of goodwill is evaluated annually, and between annual evaluations if events occur or circumstances change indicating that it is more likely than not that the fair value of a reporting unit is less than its carrying amount.
The Company’s reporting units are the In Vitro Diagnostics and Medical Device operating segments. Inherent in the determination of fair value of the reporting units are certain estimates and judgments, including the interpretation of current economic indicators and market valuations, as well as the Company’s strategic plans with regard to its operations.
The Company performs its annual assessment of goodwill for impairment as of July 1st of each fiscal year. In fiscal 2020, the annual assessment date of goodwill for impairment was changed to July 1st from the August 31st date used in fiscal 2019 and prior years. The impairment assessment is reliant on forecasted cash flows, as well as the selected discount rate when a quantitative assessment is necessary, which are inherently subjective and require significant management estimates. Differences in the reporting units’ actual future operating results compared to these forecasted estimates could materially affect the estimation of the fair value of the reporting units.
Goodwill was not impaired in either reporting unit based on the outcome of the fiscal 2020 annual impairment test which utilized a quantitative assessment.
Goodwill in the Medical Device reporting unit represents the gross value from the acquisitions of Creagh Medical, Ltd. (“Creagh Medical”) and NorMedix, Inc. (“NorMedix”), which were completed in fiscal 2016. Goodwill in the In Vitro Diagnostics reporting unit represents the gross value from the acquisition of BioFX Laboratories, Inc. in 2007.
Changes in the carrying amount of goodwill by reportable segment were as follows:
|
(In thousands) |
|
In Vitro Diagnostics |
|
|
Medical Device |
|
|
Total |
|
|||
|
Balance as of September 30, 2018 |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Foreign currency translation adjustment |
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
|
Balance as of September 30, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
|
|
— |
|
|
|
|
|
|
|
|
|
|
Balance as of September 30, 2020 |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Valuation of Long-lived Assets
The Company periodically evaluates whether events and circumstances have occurred that may affect the estimated useful life or the recoverability of the remaining balance of long-lived assets, such as property and equipment, right-of-use assets, and definite-lived intangible assets. If such events or circumstances were to indicate that the carrying amount of these assets may not be recoverable, the Company would estimate the future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected future cash flows (undiscounted and without interest charges) were less than the carrying amount of the assets, the Company would recognize an impairment charge to reduce such assets to their fair value. In fiscal 2020, 2019 and 2018, there were
60
Accrued Other Liabilities
Accrued other liabilities consisted of the following:
|
|
|
September 30, |
|
|||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
||
|
Accrued professional fees |
|
$ |
|
|
|
$ |
|
|
|
Accrued clinical study expense |
|
|
|
|
|
|
|
|
|
Accrued purchases |
|
|
|
|
|
|
|
|
|
Acquisition of in-process research and development and intangible assets |
|
|
|
|
|
|
|
|
|
Due to customers |
|
|
|
|
|
|
|
|
|
Construction-in-progress |
|
|
|
|
|
|
— |
|
|
Operating lease liability, current portion |
|
|
|
|
|
|
— |
|
|
Deferred rent, current portion |
|
|
— |
|
|
|
|
|
|
Other |
|
|
|
|
|
|
|
|
|
Total accrued other liabilities |
|
$ |
|
|
|
$ |
|
|
Revenue Recognition
Adoption of ASC Topic 606 in Fiscal 2019
Effective in fiscal 2019 (October 1, 2018), the Company adopted Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (“ASC Topic 606”) using the modified retrospective adoption method. The adoption of ASC Topic 606 resulted in an acceleration of minimum license fees and sales-based royalties revenue earned under the Company’s hydrophilic coating technology license agreements by approximately one quarter. Prior to the adoption of ASC Topic 606, sales-based royalties were recognized in the period the Company’s customers reported the underlying sales, which is generally one quarter after the sales occur. Additionally, minimum royalties were recognized in the period they were contractually owed to the Company. Upon adoption of ASC Topic 606, sales-based royalties are recognized in the period the underlying customer sale occurs, while the minimum royalties are recognized at each renewal of the license contract, which generally occurs on the last day of the quarter for minimum royalties contractually due in the following quarter.
The adoption of ASC Topic 606 in fiscal 2019 resulted in cumulative-effect adjustments to opening retained earnings, contract assets, deferred tax assets and income tax receivable as summarized below:
|
(In thousands) |
|
September 30, 2018, As Reported |
|
|
Adjustments for Adoption of Topic 606 |
|
|
October 1, 2018 Opening Balance |
|
|||
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Contract assets - royalties and license fees |
|
$ |
— |
|
|
$ |
|
|
|
$ |
|
|
|
Deferred income taxes |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Income tax receivable |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred revenue, current portion |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Deferred revenue, less current portion |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
Retained earnings |
|
|
|
|
|
|
|
|
|
|
|
|
The impact of adoption of ASC Topic 606 to the Company’s consolidated statements of operations for fiscal 2019 was an increase in royalties and license fee revenue of $
Revenue is recognized when control of the promised goods or services is transferred to our customers in an amount that reflects the consideration we expect to be entitled to receive in exchange for those goods or services. The Company primarily sells or licenses its products, technologies and services to other medical device and diagnostics companies. Taxes collected from customers and remitted to governmental authorities that are imposed on, and concurrent with, a specific revenue producing transaction are excluded from revenue. For contracts that have an original duration of one year or less, the Company uses the practical expedient applicable to such contracts and does not adjust the transaction price for the time value of money.
61
Performance Obligations
We derive our revenue from three primary sources:
|
• |
Product revenues from the sale of chemical components within our IVD segment, including stabilization products, substrates, surface coatings, and antigens to the diagnostic and biomedical research markets; the sale of reagent chemicals to licensees within our Medical Device segment; and the sale of medical devices and related products (such as balloons and catheters) to original equipment manufacturer (“OEM”) suppliers and distributors within our Medical Device segment; |
|
• |
Royalties and license fees from licensing our proprietary surface modification coating and medical device technologies to our medical device manufacturers within our Medical Device segment; and |
|
• |
Research and development fees generated from contract coating services within our Medical Device segment and new product development and testing within our Medical Device and IVD segments. |
The Company recognizes revenue when control is transferred to the customer. The transfer of control varies by revenue classification and is described below. If a contract contains more than one distinct performance obligation, the transaction price is allocated to each performance obligation based on relative standalone selling price.
Product Sales. Revenue from product sales is recognized at the point in time control of the products is transferred, generally upon shipment based upon the standard contract terms. Shipping and handling activities are considered to be fulfillment activities rather than promised services and are not, therefore, considered to be separate performance obligations. The Company’s sales terms provide no right of return outside of a standard warranty policy, and returns are generally not significant. Payment terms for product sales are generally set at 30-
Royalties. Royalties revenue consists of sales-based and recurring minimum royalties earned under licenses of our surface modification coating technologies. Performance obligations under these licenses, which consist of the right to use the Company’s proprietary technology, are satisfied at a point in time corresponding with delivery of the underlying technology rights to the customer, which is generally upon transfer of the licensed technology to the customer. Sales-based royalties revenue represents variable consideration under the license agreements and is recognized in the period a customer sells products incorporating the Company’s licensed technologies. The Company estimates sales-based royalties revenue earned but unpaid at each reporting period using the expected value method based on historical sales information, adjusted for known changes such as product launches and patent expirations. The Company also considers macroeconomic factors affecting the medical device market. The Company's license arrangements also often provide for recurring fees (minimum royalties), which the Company recognizes at the later of the satisfaction of the underlying performance obligation or upon renewal of the contract, which generally occurs on a quarterly basis. Sales-based and minimum royalties are generally due within
License Fees. For distinct license performance obligations, upfront license fees are recognized when the Company satisfies the underlying performance obligation. This generally occurs upon transfer of the right to use the Company’s licensed technology to the customer, with the exception of the license of the Company’s SurVeil™ drug-coated balloon (the “SurVeil DCB”) disclosed below. Certain license arrangements include contingent milestone payments, which are due following achievement by our customers of specified sales or regulatory milestones. Contingent milestone payment terms vary by contract. The Company has generally fulfilled its performance obligation prior to achievement of these milestones. However, because of the uncertainty of the milestone achievement, and/or the dependence on sales of our customers, variable consideration for contingent milestones is fully constrained and excluded from the contract price until the milestone is achieved by our customer, to the extent collectability is reasonably certain.
62
The Company has a collaborative arrangement contract with Abbott Vascular, Inc. (“Abbott”) disclosed in Note 4 (the “Abbott Agreement”). Under the Abbott Agreement, the Company has received payments totaling $
Revenue from the upfront fee and contingent clinical and regulatory milestone payments, once the underlying contingencies are achieved, is recognized within royalties and license fees in the consolidated statements of operations as the clinical and regulatory activities are performed on a proportional performance basis. Performance is measured based on actual costs incurred relative to the expected total cost of the underlying activities, most notably the completion of the TRANSCEND clinical trial. A significant component of the cost of this trial is the cost of the Company’s outsourced clinical trial clinical research organization (“CRO”) consultants, which is estimated based on executed statements of work, project budgets, and patient enrollment timing, among other factors. A significant change to the Company’s estimate of the costs to complete the TRANSCEND clinical trial could have a material effect on the Company’s results of operations. Significant judgment is used to estimate total revenue and cost at completion for this contract.
To account for the Abbott Agreement, the Company applied the guidance in ASC Topic 808 (Collaborative Arrangements) as the parties are active participants and are exposed to significant risks and rewards dependent on commercial success of the collaborative activity. See Note 4 “Collaborative Arrangements” for further disclosures related to the Abbott Agreement.
Research and Development. The Company performs research and development (“R&D”) activities as a service to customers, which are typically charged to customers on a time-and-materials basis. Generally, revenue for R&D services is recorded over time as the services are provided to the customer in the amount to which the Company has the right to invoice. These services are generally charged to the customer as they are provided. Payment terms for R&D services are generally set at 30-
Contract Assets, Deferred Revenue and Remaining Performance Obligations
Contract assets are generally short in duration given the nature of products produced and services provided by the Company. Contract assets consist of sales-based and minimum royalties revenue earned for which unconditional right to payment does not exist as of the balance sheet date. These assets are comprised of estimated sales-based royalties earned, but not yet reported by the Company’s customers, minimum royalties on non-cancellable contracts, and contingent milestones earned, but not yet billable based on the terms of the contract. The decrease in contract assets from September 30, 2019 to September 30, 2020 resulted primarily from changes in sales-based and minimum royalties earned, but not collected at each balance sheet date, notably due to the expiration of our fourth-generation hydrophilic coating patents in fiscal 2020 and the impact of COVID-19.
The Company records a contract liability, or deferred revenue, when there is an obligation to provide a product or service to the customer, and payment is received or due in advance of performance, or when payment is received for a period outside the contract term. The Company’s deferred revenue as of September 30, 2020 and 2019 is primarily related to the upfront and milestone payments received pursuant to the Abbott Agreement (Note 4).
63
Remaining performance obligations include deferred revenue and amounts the Company expects to receive for goods and services that have not yet been delivered or provided under existing, noncancellable contracts. For contracts that have an original duration of one year or less, the Company has elected the practical expedient applicable to such contracts and does not disclose the transaction price for remaining performance obligations at the end of each reporting period or the expecting timing of recognition of related revenue. As of September 30, 2020, the estimated revenue expected to be recognized in future periods totaled approximately $
Concentrations
The Company has licenses and supply agreements with a diverse base of customers, and certain customers have multiple products using the Company’s technology. Abbott and its affiliates and Medtronic plc (“Medtronic”) are our largest customers, comprising
The Company’s licensing agreements with many of its customers, including most of its significant customers, cover many licensed products that each separately generates royalties revenue. This structure reduces the potential risk to the Company’s operations that may result from reduced sales (or the termination of a license) of a single product for any specific customer.
The Company believes that the credit risk related to marketable securities is limited due to the adherence to an investment policy and that credit risk related to accounts receivable is limited due to a large customer base.
Stock-based Compensation
We measure the cost of employee services received in exchange for the award of equity instruments based on the fair value of the award at the date of grant. Share-based payments are expensed based on their grant-date fair values on a straight-line basis over the requisite service period of the total award, less estimated forfeitures based on historical experience. Shares awarded under the Company’s stock-based compensation plans, with the exception of restricted stock awards, are not considered issued or outstanding common stock of the Company until they vest and the shares are released. New awards and forfeitures of unvested restricted stock result in an increase (decrease), respectively, in common stock issued and outstanding.
Income Taxes
We record a tax benefit (provision) for the anticipated tax consequences of the reported results of operations. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. A valuation allowance is provided when it is more likely than not that some portion, or all, of a deferred tax asset will not be realized. The ultimate realization of deferred tax assets depends on the generation of future taxable income during the period in which related temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in this assessment. Deferred tax assets and liabilities are measured using the enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in earnings in the period that includes the enactment date of such change.
64
Research and Development
R&D expenses include costs associated with the design, development, testing, enhancement and regulatory approval of the Company’s products. R&D expenses include employee compensation (including stock-based compensation), internal and external costs associated with our regulatory compliance and quality assurance functions, the costs of product used in development and clinical trials, consulting expenses, and facilities overhead. The Company also incurs significant R&D expenses to operate clinical trials. R&D costs are expensed as incurred.
Certain R&D costs are related to customer contracts, and the related revenue is recognized as described in “Revenue Recognition” above. Costs associated with customer-related R&D include specific project direct labor and materials expenses, as well as an allocation of overhead costs based on direct labor costs.
Clinical Trial Costs. The Company sponsors clinical trials intended to obtain the necessary clinical data required to obtain approval from various regulatory agencies to market medical devices developed by the Company. Costs associated with clinical trials include trial design and management expenses, clinical site reimbursements and third-party fees, among other costs. The Company’s clinical trials are administered by third-party CROs. These CROs generally bill monthly for certain services performed, as well as upon achievement of certain milestones. The Company monitors patient enrollment, the progress of clinical studies, and related activities through internal reviews of data reported to the Company by the CROs and correspondence with the CROs. We periodically evaluates our estimates to determine if adjustments are necessary or appropriate based on information received. These estimates often require significant judgement on the part of the Company’s management.
Government Funding. The Company is eligible to receive reimbursement for certain qualifying R&D expenditures under a grant from the Industrial Development Agency of Ireland (“IDA”). Reimbursements are recognized as a reduction of R&D expense when there is reasonable assurance that the funding will be received and conditions associated with the funding are met. The Company recorded reimbursements from IDA grants of $
Net Income (Loss) Per Share Data
Basic net income (loss) per common share is calculated by dividing net income (loss) by the weighted average number of common shares outstanding during the period. Diluted income per common share is computed by dividing net income by the weighted average number of common and common equivalent shares outstanding during the period. The Company’s potentially dilutive common shares are those that result from dilutive common stock options and non-vested stock relating to restricted stock awards and restricted stock units. However, these items have been excluded from the calculation of diluted net loss per share for fiscal 2018, as their effect was antidilutive as a result of the net loss incurred for that period. Therefore, diluted weighted average number of shares outstanding and diluted net loss per share were the same as basic weighted average number of shares outstanding and net loss per share for fiscal 2018.
The following table sets forth the denominator for the computation of basic and diluted net income (loss) per share:
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Net income (loss) available to common shareholders |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
|
Basic weighted average shares outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
|
Dilutive effect of outstanding stock options, non-vested restricted stock, and non-vested restricted stock units |
|
|
|
|
|
|
|
|
|
|
— |
|
|
Diluted weighted average shares outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
The calculation of weighted average diluted shares outstanding excludes outstanding common stock options associated with the right to purchase less than
65
Leases
Effective in fiscal 2020 (October 1, 2019), the Company adopted ASU No. 2016-02, Leases using the optional transition method. Refer to “New Accounting Pronouncements” within this Note 2 for further information on the impact of adoption.
Operating lease right-of-use assets and lease liabilities were as follows:
|
|
|
|
|
|
|
(In thousands) |
|
September 30, 2020 |
|
|
|
Right-of-use assets: |
|
|
|
|
|
Other assets |
|
$ |
|
|
|
|
|
|
|
|
|
Operating lease liabilities: |
|
|
|
|
|
Other accrued liabilities |
|
$ |
|
|
|
Other long-term liabilities |
|
|
|
|
|
Total operating lease liabilities |
|
$ |
|
|
As of September 30, 2020, operating lease maturities for each of the next five fiscal years were as follows:
|
(In thousands) |
|
|
|
|
|
2021 |
|
$ |
|
|
|
2022 |
|
|
|
|
|
2023 |
|
|
|
|
|
2024 |
|
|
|
|
|
2025 |
|
|
|
|
|
Thereafter |
|
|
|
|
|
Total expected operating lease payments |
|
|
|
|
|
Less: Imputed interest |
|
|
( |
) |
|
Total operating lease liabilities |
|
$ |
|
|
Operating lease cost was $
66
Currency Translation
The Company’s reporting currency is the U.S. dollar. Assets and liabilities of non-U.S. dollar functional currency subsidiaries are translated into U.S. dollars at the period-end exchange rates, and revenue and expenses are translated at the average quarterly exchange rates during the period. The net effect of these translation adjustments on the consolidated financial statements is recorded as a foreign currency translation adjustment, a component of accumulated other comprehensive income on the consolidated balance sheets. Realized foreign currency transaction gains and losses are included in other (expense) income in the consolidated statements of operations.
New Accounting Pronouncements
Accounting Standards Implemented
In February 2016, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-02, Leases (“ASC Topic 842” or the “new lease accounting standard”). The standard maintains two classifications of leases: finance leases, which replace capital leases, and operating leases. Lessees recognize a right-of-use asset and a lease liability on the consolidated balance sheets for those leases previously classified as operating leases under the previous guidance. The liability is equal to the present value of lease payments, while the asset is based on the liability, subject to adjustment, such as for direct costs.
Effective in fiscal 2020 (October 1, 2019), the Company adopted the new lease accounting standard using the optional transition method which allowed us to continue to apply the guidance under the lease standard in effect at the time in the comparative periods presented. In addition, the Company elected the package of practical expedients, including opting not to reassess whether any existing contracts contain a lease, historical lease classification as operating or finance leases, or initial direct costs. The Company has also elected the practical expedient to not separate the lease and non-lease components for all classes of underlying assets. The Company elected the short-term lease recognition exemption for all leases that qualified and has accordingly excluded short-term leases from the recognition of right-of-use assets and lease liabilities.
As a result of adoption of ASC Topic 842, we recorded operating lease right-of-use assets and corresponding operating lease liabilities of approximately $
Accounting Standards to be Adopted
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments – Credit Losses, Measurement of Credit Losses on Financial Statements. This ASU updates the guidance for measuring and recording credit losses on financial assets measured at amortized cost by replacing the incurred loss model with an expected loss model. Accordingly, these financial assets will be presented at the net amount expected to be collected. The amendment also requires that credit losses related to available-for-sale debt securities be recorded as an allowance through net income rather than reducing the carrying amount under the current, other-than-temporary-impairment model. The accounting standard will be adopted by the Company in the first quarter of fiscal 2021 (October 1, 2020), using a modified retrospective approach. We have evaluated the impact of this standard on the Company’s results of operations, cash flows and financial position, including accounting policies, processes and systems. We do not expect the impact to be material upon adoption.
In December 2019, the FASB issued ASU No. 2019-12, Simplifying the Accounting for Income Taxes, which eliminates certain exceptions related to the approach for intraperiod tax allocation and to the methodology for calculating taxes during the quarters, as well as clarifies the accounting for enacted changes in tax laws. The accounting standard will be adopted by the Company in the first quarter of fiscal 2021 (October 1, 2020) using a prospective approach. We have evaluated the impact of this standard on the Company’s results of operations, cash flows and financial position, and we do not expect the impact to be material upon adoption.
No other new accounting pronouncement issued or effective has had, or is expected to have, a material impact on the Company’s consolidated financial statements.
67
3. Revenue
The following table presents the Company’s revenues disaggregated by product classification and by reportable segment:
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Medical Device |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product sales |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Royalties |
|
|
|
|
|
|
|
|
|
|
|
|
|
License fees |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research, development and other |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Revenue — Medical Device |
|
|
|
|
|
|
|
|
|
|
|
|
|
In Vitro Diagnostics |
|
|
|
|
|
|
|
|
|
|
|
|
|
Product sales |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Revenue — In Vitro Diagnostics |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Revenue |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
4. Collaborative Arrangement
On February 26, 2018, the Company entered into an agreement with Abbott whereby Abbott has exclusive worldwide commercialization rights for Surmodics' SurVeil DCB to treat the superficial femoral artery (the “Abbott Agreement”), which is currently being evaluated in a U.S. pivotal clinical trial. Separately, Abbott also received the option to negotiate an agreement for Surmodics' below-the-knee SundanceTM DCB product, which is currently in development. Surmodics is responsible for conducting all necessary clinical trials and other activities required to achieve U.S. regulatory clearance for the SurVeil DCB, including completion of the ongoing TRANSCEND pivotal clinical trial. Abbott and Surmodics participate on a joint development committee charged with providing guidance on the Company’s clinical and regulatory activities with regard to the SurVeil DCB product. Upon receipt of regulatory approval for our SurVeil DCB, Abbott will have the right to purchase commercial units from the Company and Surmodics will realize revenue from product sales to Abbott at an agreed-upon transfer price, as well as a share of net profits resulting from third-party product sales by Abbott.
Under the Abbott Agreement, the Company has received payments totaling $
Revenue recognized from the Abbott Agreement totaled $
5. Fair Value Measurements
The accounting guidance on fair value measurements defines fair value, establishes a framework for measuring fair value under GAAP, and expands disclosures about fair value measurements. The guidance is applicable for all financial assets and financial liabilities and for all nonfinancial assets and nonfinancial liabilities recognized or disclosed at fair value in the consolidated financial statements on a recurring basis (at least annually). Fair value is defined as the exchange price that would be received from selling an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and also considers assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions and risk of nonperformance.
68
Fair Value Hierarchy
Assets and liabilities carried at fair value are classified and disclosed in one of the following three categories:
Level 1 — Quoted (unadjusted) prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the asset or liability.
Level 3 — Unobservable inputs to the valuation methodology that are supported by little or no market activity and that are significant to the measurement of the fair value of the assets or liabilities. Level 3 assets and liabilities include those whose fair value measurements are determined using pricing models, discounted cash flow methodologies or similar valuation techniques, as well as significant management judgment or estimation. In valuing Level 3 assets and liabilities, the Company is required to maximize the use of quoted market prices and minimize the use of unobservable inputs.
In valuing assets and liabilities, the Company is required to maximize the use of quoted market prices and minimize the use of unobservable inputs. In instances where the inputs used to measure fair value fall into different levels of the fair value hierarchy, the fair value measurement has been determined based on the lowest level input that is significant to the fair value measurement in its entirety. The Company’s assessment of the significance of a particular item to the fair value measurement in its entirety requires judgment, including the consideration of inputs specific to the asset or liability.
The Company did not have any Level 1 assets as of September 30, 2020 or 2019.
The Company’s Level 2 assets as of September 30, 2020 and 2019 consisted of cash equivalents (money market funds and commercial paper instruments) and available-for-sale debt securities (commercial paper instruments and corporate bonds). Cash equivalents are carried at historical cost which is a reasonable estimate of fair value because of the relatively short time between origination of the instrument and its expected realization. Available-for-sale securities are valued based on quoted vendor prices in active markets underlying the securities. Fair market values for cash equivalents and available-for-sale debt securities are based on quoted vendor prices and broker pricing where all significant inputs are observable. To ensure the accuracy of quoted vendor prices and broker pricing, the Company performs regular reviews of investment returns to industry benchmarks and sample tests of individual securities to validate quoted vendor prices with other available market data.
The Company’s Level 3 liabilities consisted of contingent consideration liabilities arising from business and asset acquisitions as of September 30, 2019. The fair value of contingent consideration liabilities was determined based on discounted cash flow analyses that included revenue estimates, probability of strategic milestone achievement and a discount rate, which are considered significant unobservable inputs as of the acquisition dates and as of September 30, 2019.
The Company did not significantly change its valuation techniques from prior periods. There were
Assets and Liabilities Measured at Fair Value on a Recurring Basis
Assets and liabilities measured at fair value on a recurring basis by level of the fair value hierarchy were as follows:
|
|
|
September 30, 2020 |
|
|||||||||||||
|
(In thousands) |
|
Quoted Prices in Active Markets for Identical Instruments (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
|
Total Fair Value |
|
||||
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents |
|
$ |
— |
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
Available-for-sale securities |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
$ |
|
|
|
Total assets |
|
$ |
— |
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
69
|
|
|
September 30, 2019 |
|
|||||||||||||
|
(In thousands) |
|
Quoted Prices in Active Markets for Identical Instruments (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
|
Total Fair Value |
|
||||
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents |
|
$ |
— |
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
Available-for-sale securities |
|
|
— |
|
|
|
|
|
|
|
— |
|
|
$ |
|
|
|
Total assets |
|
$ |
— |
|
|
$ |
|
|
|
$ |
— |
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
$ |
|
|
|
Total liabilities |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes in the contingent consideration liabilities measured at fair value using Level 3 inputs were as follows:
|
(In thousands) |
|
|
|
|
|
Contingent consideration liability at September 30, 2018 |
|
$ |
|
|
|
Additions |
|
|
— |
|
|
Fair value adjustments |
|
|
( |
) |
|
Settlements |
|
|
( |
) |
|
Interest accretion |
|
|
|
|
|
Foreign currency translation |
|
|
( |
) |
|
Contingent consideration liability at September 30, 2019 |
|
|
|
|
|
Additions |
|
|
— |
|
|
Fair value adjustments |
|
|
— |
|
|
Settlements |
|
|
( |
) |
|
Interest accretion |
|
|
— |
|
|
Foreign currency translation |
|
|
— |
|
|
Contingent consideration liability at September 30, 2020 |
|
$ |
— |
|
As of September 30, 2019, contingent consideration liabilities consisted of $3.2 million related to the fiscal 2016 acquisition of NorMedix which was paid in fiscal 2020. As of September 30, 2018, contingent consideration liabilities totaled $
The contingency period for the NorMedix acquisition ended September 30, 2019. Based on the milestones achieved during the contingency period, the Company paid the NorMedix shareholders $
The contingency period for the Creagh Medical contingent consideration obligation ended September 30, 2018. Based on the milestones achieved during the contingency period, the Company paid Creagh Medical shareholders $
The €
70
Assets and Liabilities Measured at Fair Value on a Non-recurring Basis
We measure certain assets at fair value on a nonrecurring basis, primarily goodwill, intangible assets, and long-lived assets, as well as cost method equity investments recorded within other assets on the consolidated balance sheets. These assets were initially measured and recognized at amounts equal to the fair value determined as of the date of acquisition or purchase and subject to changes in value only for foreign currency translation and impairment. See Note 2 for additional information on impairment assessments and related Level 3 inputs for goodwill, indefinite-lived intangible assets, long-lived assets, and cost method equity investments.
Assets and Liabilities Not Measured at Fair Value
Certain financial instruments are not measured at fair value but are recorded at carrying amounts approximating fair value based on their short-term nature. The carrying value of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximated fair value as of September 30, 2020 and 2019.
6. Debt
On September 14, 2020, the Company entered into a secured revolving credit facility pursuant to a Loan and Security Agreement (the "Loan Agreement") with Bridgewater Bank (the “Bank”). The Loan Agreement provides for availability under a secured revolving line of credit of up to $
The Loan Agreement contains affirmative and negative covenants customary for a transaction of this type which, among other things, require the Company to meet certain financial tests, including (i) minimum liquidity, (ii) minimum current ratio, (iii) minimum adjusted EBITDA, and (iv) minimum tangible net worth. The Loan Agreement also contains covenants which, among other things, limit the Company's ability to incur additional debt, make certain investments, create or permit certain liens, create or permit restrictions on the ability of subsidiaries to pay dividends or make other distributions, consolidate or merge and engage in other activities customarily restricted in such agreements, in each case subject to exceptions permitted by the Loan Agreement. The Loan Agreement also contains customary events of default, the occurrence of which would permit the Bank to terminate its commitment and accelerate the Loan.
7. Stockholders’ Equity
Repurchase of Common Stock
Shares are repurchased from time to time to support the Company’s stock-based compensation programs and to return capital to stockholders. The Company accounts for repurchases of common stock using the par value method.
On November 6, 2015 and November 5, 2014, the Company’s Board of Directors authorized the repurchase of up to $
71
8. Stock-based Compensation Plans
The Company has stock-based compensation plans under which it grants stock options, restricted stock awards, restricted stock units and deferred stock units.
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Product costs |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Research and development |
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stock-based compensation expense |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
As of September 30, 2020, approximately $
Under the 2019 Equity Incentive Plan (“2019 Plan”), the Company is authorized to issue
Stock Option Awards
The Company grants non-qualified stock options at fair market value on the grant date to certain key employees and members of the Board. The Company uses the Black-Scholes option pricing model to determine the fair value of stock options as of the date of each grant.
|
|
|
Fiscal Year |
|
|||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Stock option fair value assumptions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Risk-free interest rates |
|
|
|
% |
|
|
|
% |
|
|
|
% |
|
Expected life (years) |
|
|
|
|
|
|
|
|
|
|||
|
Expected volatility |
|
|
|
% |
|
|
|
% |
|
|
|
% |
|
Dividend yield |
|
|
|
% |
|
|
— |
% |
|
|
— |
% |
|
Weighted average grant date fair value of stock options granted |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
The risk-free interest rate assumption is based on the U.S. Treasury’s rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the awards. The expected life of options granted is determined based on the Company’s experience. Expected volatility is based on the Company’s stock price movement over a period approximating the expected term. Based on management’s judgment, dividend yields are expected to be zero for the expected life of the options.
With respect to members of the Board, non-qualified stock options generally become exercisable on a monthly pro-rata basis within the period following the date of grant. With respect to employees, non-qualified stock options generally become exercisable at a
As of September 30, 2020, the aggregate intrinsic value of the option shares outstanding and option shares exercisable was $
72
Stock option activity was as follows:
|
(In thousands, except per share data) |
|
Number of Shares |
|
|
Weighted Average Exercise Price |
|
||
|
Options outstanding at September 30, 2017 |
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Exercised |
|
|
( |
) |
|
|
|
|
|
Forfeited and expired |
|
|
( |
) |
|
|
|
|
|
Options outstanding at September 30, 2018 |
|
|
|
|
|
|
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Exercised |
|
|
( |
) |
|
|
|
|
|
Forfeited and expired |
|
|
( |
) |
|
|
|
|
|
Options outstanding at September 30, 2019 |
|
|
|
|
|
|
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Exercised |
|
|
( |
) |
|
|
|
|
|
Forfeited and expired |
|
|
( |
) |
|
|
|
|
|
Options outstanding at September 30, 2020 |
|
|
|
|
|
|
|
|
|
Options vested and exercisable at September 30, 2020 |
|
|
|
|
|
$ |
|
|
Restricted Stock Awards
The Company has entered into restricted stock agreements with certain key employees, covering the issuance of common stock (“Restricted Stock”). Restricted Stock generally vests at a
Restricted Stock activity was as follows:
|
(In thousands, except per share data) |
|
Number of Shares |
|
|
Weighted Average Grant Price |
|
||
|
Unvested restricted stock awards at September 30, 2017 |
|
|
|
|
|
$ |
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Vested |
|
|
( |
) |
|
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
|
Unvested restricted stock awards at September 30, 2018 |
|
|
|
|
|
|
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Vested |
|
|
( |
) |
|
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
|
Unvested restricted stock awards at September 30, 2019 |
|
|
|
|
|
|
|
|
|
Granted |
|
|
|
|
|
|
|
|
|
Vested |
|
|
( |
) |
|
|
|
|
|
Forfeited |
|
|
( |
) |
|
|
|
|
|
Unvested restricted stock awards at September 30, 2020 |
|
|
|
|
|
$ |
|
|
73
Restricted Stock Units and Deferred Stock Units
The Company has entered into restricted stock unit agreements with certain key employees in foreign jurisdictions and members of the Board, covering the issuance of common stock (“RSUs”). With respect to employees, RSUs generally vest at a
Directors may elect to receive their annual fees for services to the Board in deferred stock units (“DSUs”). DSUs are fully vested and expensed upon grant at the market value of the shares on the grant date. DSUs are settled in shares and issued to the Director upon termination of service as a Board member. As of September 30, 2020 and 2019, outstanding, fully vested DSUs totaled approximately
Performance Share Awards
In fiscal 2017 and prior years, the Company entered into performance share agreements with certain key employees covering the issuance of common stock (“Performance Shares”). The Organization and Compensation Committee of the Board of Directors (the “Committee”) established cumulative revenue and cumulative earnings before interest, income taxes, depreciation and amortization (“EBITDA”) for the applicable performance period as the performance objectives for the fiscal 2017 and 2016 awards. The fiscal 2017 and 2016 awards also included performance objectives related to achievement of the Company’s strategic initiatives. The fair value of the Performance Shares, at target, was $
1999 Employee Stock Purchase Plan
Under the amended 1999 Employee Stock Purchase Plan (“ESPP”), the Company is authorized to issue up to
74
9. Income Taxes
Income taxes in the accompanying consolidated statements of operations were as follows:
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Current (benefit) provision: |
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Federal |
|
$ |
( |
) |
|
$ |
|
|
|
$ |
( |
) |
|
U.S. State |
|
|
|
|
|
|
|
|
|
|
|
|
|
International |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current (benefit) provision |
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|
Deferred (benefit) provision: |
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. Federal |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
U.S. State |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
International |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total deferred benefit (1) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Total income tax benefit |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
(1) |
Both the current and deferred benefit include the impact of the adoption of ASC Topic 606 in fiscal 2019, which reduced deferred income taxes and income taxes receivable by $ |
The following is a reconciliation of the difference between amounts calculated at the statutory U.S. federal income tax rate of
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Amount at statutory U.S. federal income tax rate |
|
$ |
( |
) |
|
$ |
|
|
|
$ |
( |
) |
|
Change because of the following items: |
|
|
|
|
|
|
|
|
|
|
|
|
|
State income taxes, net of federal benefit |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
U.S. Federal and foreign research and development credits |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Foreign and state rate differential |
|
|
|
|
|
|
|
|
|
|
|
|
|
Valuation allowance change |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation (1) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Contingent consideration (gain) expense and related foreign currency revaluation |
|
|
— |
|
|
|
( |
) |
|
|
|
|
|
U.S. Federal and state rate change |
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax reserve change |
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign-derived income deduction |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
Impact of CARES Act |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
Other |
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax benefit |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
Includes non-deductible stock-based compensation. |
In March 2020, the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”) was enacted and included significant business tax provisions. In particular, the CARES Act modified the rules associated with net operating losses (“NOLs”) and made technical corrections to tax depreciation methods for qualified improvement property. Under the temporary provisions of CARES Act, NOL carryforwards and carrybacks may offset 100% of taxable income for taxable years beginning before 2021. In addition, NOLs arising in 2018, 2019 and 2020 taxable years may be carried back to each of the preceding five years to generate a refund. In fiscal 2020, the income tax benefit includes a discrete tax benefit of $
75
In December 2017, the Tax Cuts and Jobs Act (“TCJA”) tax legislation was signed into law, which reduced the U.S. federal statutory tax rate from
Excess tax benefits related to stock-based compensation expense are recorded within income tax benefit in the consolidated statements of operations and totaled $
The components of deferred income taxes consisted of the following and result from differences in the recognition of transactions for income tax and financial reporting purposes:
|
|
|
September 30, |
|
|||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
||
|
Depreciable assets |
|
$ |
( |
) |
|
$ |
( |
) |
|
Deferred revenue |
|
|
|
|
|
|
|
|
|
Accruals and reserves |
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
|
|
|
|
|
|
|
Impaired strategic investments |
|
|
|
|
|
|
|
|
|
NOL carryforwards |
|
|
|
|
|
|
|
|
|
U.S. Federal and State R&D credits |
|
|
|
|
|
|
|
|
|
Other |
|
|
|
|
|
|
|
|
|
Valuation allowance |
|
|
( |
) |
|
|
( |
) |
|
Total deferred tax assets |
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
As of September 30, 2020 and 2019, deferred tax asset valuation allowances totaled $
Unrecognized tax benefits are the differences between a tax position taken, or expected to be taken in a tax return, and the benefit recognized for accounting purposes pursuant to accounting guidance. The following is a reconciliation of the changes in unrecognized tax benefits, excluding interest and penalties:
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Unrecognized tax benefits, beginning balance |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Increases in tax positions for prior years |
|
|
|
|
|
|
|
|
|
|
|
|
|
Decreases in tax positions for prior years |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
Increases in tax positions for current year |
|
|
|
|
|
|
|
|
|
|
|
|
|
Settlements with taxing authorities |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
Lapse of the statute of limitations |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Unrecognized tax benefits, ending balance |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The total amount of unrecognized tax benefits excluding interest and penalties that, if recognized, would affect the effective tax rate was $
76
The Company files income tax returns, including returns for its subsidiaries, in the U.S. federal jurisdiction and in various state jurisdictions, as well as several non-U.S. jurisdictions. Uncertain tax positions are related to tax years that remain subject to examination. U.S. federal income tax returns for years prior to fiscal 2017 are no longer subject to examination by federal tax authorities. For tax returns for state and local jurisdictions, the Company is no longer subject to examination for tax years generally before fiscal 2009. For tax returns for non-U.S. jurisdictions, the Company is no longer subject to income tax examination for years prior to 2014. Additionally, the Company has been indemnified of liability for any taxes relating to Creagh Medical and NorMedix for periods prior to the respective acquisition dates, pursuant to the terms of the related share purchase agreements. As of September 30, 2020 and 2019, there were
10. Defined Contribution Plans
The Company has a 401(k) retirement and savings plan for the benefit of qualifying U.S. employees, and a defined contribution Personal Retirement Savings Account plan for the benefit of qualifying Ireland employees. For U.S. employees, the Company matches
11. Commitments and Contingencies
Litigation. From time to time, the Company may become involved in various legal actions involving its operations, products and technologies, including intellectual property and employment disputes. The outcomes of these legal actions are not within the Company’s complete control and may not be known for prolonged periods of time. In some actions, the claimants seek damages as well as other relief, including injunctions barring the sale of products that are the subject of the lawsuit, which if granted, could require significant expenditures or result in lost revenue. The Company records a liability in the consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate, the minimum amount of the range is accrued. If a loss is possible but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded.
On January 17, 2018, the Company entered into a settlement agreement fully resolving the previously disclosed litigation involving Merit Medical Systems, Inc. (“Merit”) and NorMedix.
In April 2018, a customer notified the Company that it believed it had overpaid hydrophilic coating royalties to the Company from January 2009 through December 2017. During fiscal 2018, the Company recorded $
InnoCore Technologies BV. In
Clinical Trials. The Company has engaged CRO consultants to assist with the administration of its ongoing clinical trials. The Company has executed separate contracts with two CROs for services rendered in connection with the TRANSCEND pivotal clinical trial for the SurVeil DCB, including pass-through expenses paid by the CROs, of up to $
Asset Acquisitions. In fiscal 2019, the Company acquired certain intellectual property assets to support ongoing development of the Company’s medical device pipeline and paid the sellers $
77
In fiscal 2018, the Company acquired certain intellectual property assets of Embolitech, LLC (the “Embolitech Transaction”). As part of the Embolitech Transaction, the Company paid the sellers $
As of September 30, 2020, $
12. Reportable Segment Information
Operating segments are components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker, who is the Company’s Chief Executive Officer, in deciding how to allocate resources and in assessing performance. We operate
|
• |
Medical Device: Surface modification coating technologies to improve access, deliverability, and predictable deployment of medical devices; drug-delivery coating technologies to provide site-specific drug-delivery from the surface of a medical device, with end markets that include coronary, peripheral, neuro-vascular and structural heart, among others; and the design, development and manufacture of interventional medical devices, primarily balloons and catheters, including drug-coated balloons, for peripheral arterial disease treatment and other applications; and |
|
• |
In Vitro Diagnostics: Design, development and manufacture of component products and technologies for diagnostic immunoassay as well as molecular test and biomedical research applications, with products that include protein stabilization reagents, substrates, surface coatings and antigens. |
Segment revenue, operating (loss) income, and depreciation and amortization were as follows:
|
|
|
Fiscal Year |
|
|||||||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical Device |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
In Vitro Diagnostics |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating (loss) income: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical Device |
|
$ |
( |
) |
|
$ |
|
|
|
$ |
( |
) |
|
In Vitro Diagnostics |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total segment operating (loss) income |
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
Total operating (loss) income |
|
$ |
( |
) |
|
$ |
|
|
|
$ |
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Medical Device |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
In Vitro Diagnostics |
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total depreciation and amortization |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
The Corporate category includes expenses that are not fully allocated to Medical Device and In Vitro Diagnostics segments. These Corporate costs are related to administrative corporate functions, such as executive management, corporate accounting, legal, human resources and Board of Directors. Corporate may also include expenses, such as litigation, which are not specific to a segment and thus not allocated to the operating segments.
78
Asset information by segment is not presented because the Company does not provide its chief operating decision maker assets by segment, as the data is not readily available.
Revenue from customers that equaled or exceeded 10% of total revenue was as follows:
|
|
|
Fiscal Year |
|
|||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Abbott |
|
|
|
% |
|
|
|
% |
|
|
|
% |
|
Medtronic |
|
|
|
% |
|
|
|
% |
|
|
|
% |
The revenue from the customers listed is derived from two primary sources: licensing and product sales (primarily in the Medical Device segment).
Revenue by geographic region was as follows:
|
|
|
Fiscal Year |
|
|||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2018 |
|
|||
|
Domestic |
|
|
|
% |
|
|
|
% |
|
|
|
% |
|
Foreign |
|
|
|
% |
|
|
|
% |
|
|
|
% |
Long-lived assets by country, including property and equipment and intangible assets net of accumulated depreciation and amortization, respectively, were as follows:
|
|
|
September 30, |
|
|||||
|
(In thousands) |
|
2020 |
|
|
2019 |
|
||
|
U.S. |
|
$ |
|
|
|
$ |
|
|
|
Ireland |
|
|
|
|
|
|
|
|
13. Quarterly Financial Data (Unaudited)
The following is a summary of the unaudited quarterly results:
|
(In thousands, except per share data) |
|
First Quarter |
|
|
Second Quarter |
|
|
Third Quarter |
|
|
Fourth Quarter |
|
||||
|
Fiscal Year 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Operating (loss) income |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
( |
) |
|
Net income (loss) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
Basic net income (loss) per share (1): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
Diluted net income (loss) per share (1): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
Fiscal Year 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenue |
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Operating income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income per share (1): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted net income per share (1): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
The sum of the quarterly net income (loss) per share amounts may not equal the annual income per share total because of changes in the weighted average number of shares outstanding that occurred during the year. |
In the second quarter of fiscal 2020, the Company recorded an impairment charge of $
In the second quarter of fiscal 2020, the Company recorded a $
79
In the third quarter of fiscal 2020, the Company achieved a regulatory milestone related to its agreement with Abbott, which resulted in the receipt of a $
In the third quarter of fiscal 2019, the Company recorded royalties revenue from the extension of a customer license agreement totaling $
In the fourth quarter of fiscal 2019, the Company acquired certain technology assets resulting in a $
In the fourth quarter of fiscal 2019, the Company achieved a clinical milestone related to its agreement with Abbott, which resulted in the receipt of a $
80
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
|
1. |
Disclosure Controls and Procedures |
The Company maintains disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) that are designed to ensure that information required to be disclosed in our reports filed under the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and no evaluation can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected.
The Company’s management, under the supervision and with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, referred to collectively herein as the Certifying Officers, carried out an evaluation of the effectiveness of the design and operation of the Company’s disclosure controls and procedures as of September 30, 2020, the end of the period covered by this Annual Report on Form 10-K. Based on that evaluation, the Certifying Officers concluded that the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) were effective as of September 30, 2020, as designed and implemented to ensure that information required to be disclosed by the Company in reports that it files under the Exchange Act is recorded, processed, summarized and reported within the time period specified in the Securities Exchange Commission rules and forms, and to ensure that information required to be disclosed by the Company in the reports the Company files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its Certifying Officers, as appropriate, to allow timely decisions regarding required disclosures.
|
2. |
Internal Control over Financial Reporting |
a. Management’s Annual Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rules 13a-15(f) and 15d-15(f). The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our consolidated financial statements.
Management evaluated the design and operating effectiveness of the Company’s internal control over financial reporting based on the criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the evaluation, management concluded that internal control over financial reporting was effective as of September 30, 2020.
The Company’s independent registered public accounting firm, Deloitte & Touche LLP, who audited the consolidated financial statements included in this Annual Report on Form 10-K, has issued an attestation report on the effectiveness of the Company’s internal control over financial reporting as of September 30, 2020. This report states that internal control over financial reporting was effective and appears in “Financial Statements and Supplementary Data” in Part II, Item 8 of this Annual Report on Form 10-K.
b. Changes in Internal Controls Over Financial Reporting. There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the quarter ended September 30, 2020 that materially affected, or are reasonable likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
81
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The information required by Item 10 relating to directors, our audit committee, the nature of changes, if any, to procedures by which our shareholders may recommend nominees for directors, our code of ethics and compliance with Section 16(a) of the Exchange Act will appear in the Company’s Proxy Statement for its 2021 Annual Meeting of Shareholders and is incorporated herein by reference. The information required by Item 10 relating to executive officers appears in Part I, Item 1 of this Annual Report on Form 10-K.
ITEM 11. EXECUTIVE COMPENSATION.
The information required by Item 11 will appear in the Company’s Proxy Statement for its 2021 Annual Meeting of Shareholders and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The information required by Item 12 will appear in the Company’s Proxy Statement for its 2021 Annual Meeting of Shareholders and is incorporated herein by reference.
Equity Compensation Plan Information
The following table provides information related to the Company’s equity compensation plans in effect as of September 30, 2020:
|
Plan Category |
|
(a) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
|
|
|
(b) Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights |
|
|
|
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) |
|
|
|||
|
Equity compensation plans approved by shareholders |
|
|
1,036,910 |
|
(1) |
|
$ |
31.87 |
|
(1) |
|
|
1,002,040 |
|
|
|
Equity compensation plans not approved by shareholders |
|
|
— |
|
|
|
N/A |
|
|
|
|
— |
|
|
|
|
Total |
|
|
1,036,910 |
|
|
|
$ |
31.87 |
|
|
|
|
1,002,040 |
|
|
|
|
(1) |
Excludes shares that may be issued under the Company’s amended and restated 1999 Employee Stock Purchase Plan. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
The information required by Item 13 will appear in the Company’s Proxy Statement for its 2021 Annual Meeting of Shareholders and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
The information required by Item 14 will appear in the Company’s Proxy Statement for its 2021 Annual Meeting of Shareholders and is incorporated herein by reference.
82
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a) 1. Financial Statement Schedules
Schedule II —Valuation and Qualifying Accounts for fiscal years ended September 30, 2020, 2019 and 2018. All other schedules are omitted because they are inapplicable, not required, or the information is in the consolidated financial statements or related notes.
Surmodics, Inc.
Schedule II – Valuation and Qualifying Accounts
|
(In thousands) |
|
Balance at Beginning of Fiscal Year |
|
|
Additions: Charges to Income |
|
|
Deductions: Other Changes (Debit) Credit |
|
|
|
Balance at End of Fiscal Year |
|
||||
|
Allowance for doubtful accounts: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal year ended September 30, 2018 |
|
$ |
|
|
|
$ |
|
|
|
$ |
( |
) |
(a) |
|
$ |
|
|
|
Fiscal year ended September 30, 2019 |
|
|
|
|
|
|
|
|
|
|
( |
) |
(a) |
|
|
|
|
|
Fiscal year ended September 30, 2020 |
|
|
|
|
|
|
|
|
|
|
( |
) |
(a) |
|
|
|
|
|
|
(a) |
Primarily consists of uncollectible accounts written off, less recoveries. |
2. Exhibits
83
84
|
Exhibit |
|
Description |
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
10.32*† |
|
|
|
21† |
|
|
|
23† |
|
|
|
|
Power of Attorney (included on signature page of this Form 10-K). |
|
|
31.1† |
|
|
|
31.2† |
|
|
|
32.1† |
|
|
|
32.2† |
|
|
|
101.INS† |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File as its XBRL tags are embedded within the inline XBRL document. |
|
101.SCH† |
|
Inline XBRL Taxonomy Extension Schema. |
|
101.CAL† |
|
Inline XBRL Taxonomy Extension Calculation Linkbase. |
|
101.DEF† |
|
Inline XBRL Taxonomy Extension Definition Linkbase. |
|
101.LAB† |
|
Inline XBRL Taxonomy Extension Label Linkbase. |
|
101.PRE† |
|
Inline XBRL Taxonomy Extension Presentation Linkbase. |
|
104† |
|
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101). |
|
* |
Management contract or compensatory plan or arrangement |
|
† |
Filed herewith |
|
** |
Portions of this document, which have been separately filed with the Securities and Exchange Commission, have been omitted pursuant to a request for confidential treatment. |
85
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
SURMODICS, INC. |
||
|
|
|
|
|
By: |
|
/s/ Gary R. Maharaj |
|
|
|
Gary R. Maharaj |
|
|
|
President and Chief Executive Officer |
Dated: December 2, 2020
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant, in the capacities, and on the dates indicated.
(Power of Attorney)
Each person whose signature appears below authorizes GARY R. MAHARAJ or TIMOTHY J. ARENS, and constitutes and appoints said persons as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any or all amendments to this Annual Report on Form 10-K and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, authorizing said persons and granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all said attorneys-in-fact and agents, or his substitute or substitutes, may lawfully do or cause to be done by virtue thereof.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Gary R. Maharaj Gary R. Maharaj |
|
President and Chief Executive Officer (principal executive officer) and Director |
|
December 2, 2020 |
|
|
|
|
|
|
|
/s/ Timothy J. Arens Timothy J. Arens |
|
Vice President of Finance and Chief Financial Officer (principal financial officer) |
|
December 2, 2020 |
|
|
|
|
|
|
|
/s/ John D. Manders |
|
Corporate Controller |
|
December 2, 2020 |
|
John D. Manders /s/ Susan E. Knight Susan E. Knight |
|
(principal accounting officer) Chairman of the Board of Directors |
|
December 2, 2020 |
|
|
|
|
|
|
|
/s/ José H. Bedoya José H. Bedoya |
|
Director |
|
December 2, 2020 |
|
|
|
|
|
|
|
/s/ David R. Dantzker, M.D. David R. Dantzker, M.D. |
|
Director |
|
December 2, 2020 |
|
|
|
|
|
|
|
/s/ Ronald B. Kalich Ronald B. Kalich |
|
Director |
|
December 2, 2020 |
|
|
|
|
|
|
|
/s/ Shawn T McCormick Shawn T McCormick |
|
Director |
|
December 2, 2020 |
|
/s/ Lisa Wipperman Heine |
|
Director |
|
December 2, 2020 |
|
Lisa Wipperman Heine |
|
|
|
|
86