|
Exhibit 15.1
|

Table of Contents

| > Our Strategy and Performance |
| > Business Review |
| > Corporate Governance |
| > Development Pipeline |
| > Shareholder Information |
| > Corporate Information |
astrazeneca.com/annualreport2010
2 |
Overview | |||
| 2 | AstraZeneca at a glance | |||
| 4 | Our year in brief | |||
| 6 | Chairman’s Statement | |||
| 8 | Chief Executive Officer’s Review | |||
| 10 | Our Strategy and Performance | |||
| 10 | Our marketplace | |||
| 14 | Our strategy | |||
| 18 | Performance in 2010 | |||
| 20 | Life-cycle of a medicine | |||
| 24 | Business Review | |||
| 24 | Delivering our strategy | |||
| 26 | Research and Development | |||
| 30 | Intellectual Property | |||
| 32 | Sales and Marketing | |||
| 34 | Supply and Manufacturing | |||
| 36 | People | |||
| 40 | Responsible Business | |||
50 |
Therapy Area Review | |
70 |
Geographical Review | |
75 |
Other Businesses | |
78 |
Financial Review | |
94 |
Corporate Governance | |
94 |
Risk | |
106 |
Board of Directors and Senior Executive Team |
|
109 |
Corporate Governance Report | |
119 |
Directors’ Remuneration Report | |
135 |
Financial Statements | |
135 |
Financial Statements | |
205 |
Additional Information | |
206 |
Development Pipeline | |
211 |
Shareholder Information | |
216 |
Corporate Information | |
217 |
Glossary | |
220 |
Index |
| AstraZeneca Annual Report and Form 20-F Information 2010 | Contents 1 |
Table of Contents
AstraZeneca at a glance |
||||||
| Who we are 6 Focus on six areas of healthcare 61,000 61,000 employees worldwide 10 10 medicines with sales of over $1 billion in 2010 100 Active in over 100 countries AstraZeneca is a focused, integrated, innovation-driven, global, prescription-based biopharmaceutical business Our mission is to make a meaningful difference to patient health through great medicines We are committed to acting responsibly and to the sustainable development of our business Our mission requires us to do things in the right way – to behave in accordance with our values and to act with integrity We believe that our approach delivers lasting value for patients, society and our shareholders |
What we do We discover, develop and commercialise prescription medicines for six important areas of healthcare: Cardiovascular, Gastrointestinal, Infection, Neuroscience, Oncology and Respiratory & Inflammation We have a broad range of medicines that includes established treatments for many serious illnesses, such as our antibiotic, Merrem/Meronem and Losec/Prilosec for acid-related diseases We use our scientific and commercial skills to develop a pipeline of innovative new medicines to meet medical need We had 10 medicines with sales of more than $1 billion each in 2010 Cardiovascular Crestor for managing cholesterol levels Seloken/Toprol-XL for hypertension, heart failure and angina Atacand for hypertension and heart failure Gastrointestinal Nexium for acid reflux Infection Synagis for RSV, a respiratory infection in infants Neuroscience Seroquel IR for schizophrenia and bipolar disorder Seroquel XR for schizophrenia, bipolar disorder and major depressive disorder Oncology Arimidex for breast cancer Zoladex for prostate and breast cancer Respiratory & Inflammation Symbicort for asthma and chronic obstructive pulmonary disease |
|||||
| 2 AstraZeneca at a glance | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
How we work Our activities touch many people’s lives and we are committed to working in a spirit of collaboration to achieve our goal of better health for patients For patients and doctors, we provide medicines for some of the world’s most serious illnesses For the people who pay for healthcare, we work to make sure that our medicines offer value for money For our employees, we provide a culture in which they can feel appreciated, energised and rewarded for their contribution For our shareholders, we aim to deliver value through our continued focus on innovation and running our business efficiently For the wider community, we want to be valued for the contribution our medicines make to society and trusted for the way in which we do business We recognise the value of collaborative work and so continually seek to develop new ways of working with others who complement our existing skills, enhance our internal innovation or bring extra value to what we do |
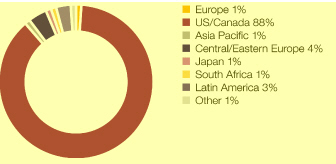
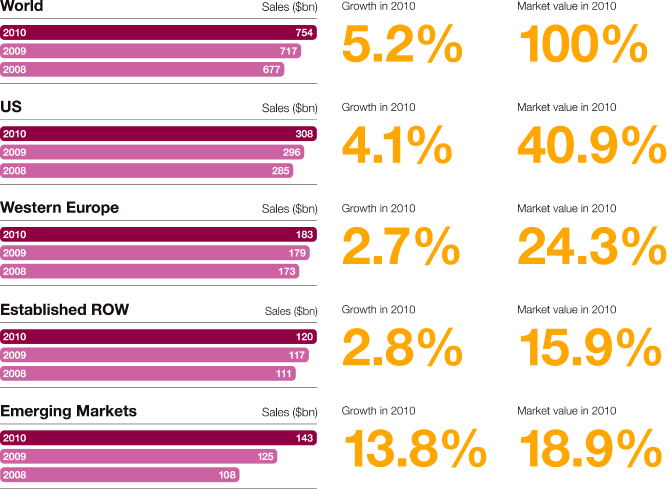
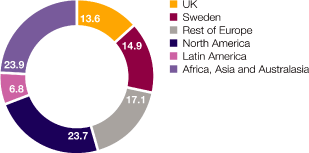
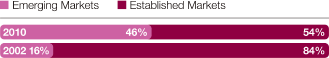
Where we work We have a global reach but local knowledge, being active in over 100 countries, with a growing presence in emerging markets such as China, Mexico, Brazil and Russia In 2010, we had sales of $13,727 million in the US, $9,168 million in Western Europe, $5,176 million in Established ROW and $5,198 million in Emerging Markets Combining our disease area expertise with country-specific knowledge helps us to market and sell medicines that best meet local needs Of our 61,000 employees worldwide, 45.6% are in Europe, 30.5% in the Americas and 23.9% in Asia, Africa and Australasia Around 15,700 people work in our R&D organisation and we have 14 principal R&D centres in eight countries, including Sweden, the US and the UK We have 9,300 employees at 23 Supply and Manufacturing sites in 16 countries $4.2bn Core investment of $4.2 billion in our R&D organisation in 2010 80 Over 80 major externalisation transactions completed over the past three years 46% 46% of sales and marketing workforce based in Emerging Markets compared with 16% in 2002 23 23 Supply and Manufacturing sites |
 |
|||||
| AstraZeneca Annual Report and Form 20-F Information 2010 | AstraZeneca at a glance 3 |
Table of Contents
operational information for 2010
| 4 Our year in brief | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | Single R&D organisation in place, including new leadership team, global organisation structure and governance framework | |
| > | Vimovo approved in the US and the EU; Brilique approved in the EU with Complete Response Letter received for Brilinta in the US; Kombiglyze™ XR (Onglyza™/metformin combination) approved in the US; decisions made in December to discontinue development of motavizumab and Certriad | |
| > | Completed a deal with Rigel for the Phase III development of fostamatinib (for rheumatoid arthritis), and TC-5214, our neuroscience collaboration with Targacept, also entered Phase III development | |
| > | Agreement with HealthCore, which maintains the largest commercially insured population data environment in the US, enables ‘real world’ studies of health outcomes | |
| > | Portfolio of more than 100 generic products being licensed across 30 Emerging Markets for marketing under our brand | |
| > | Crestor substance patent upheld in the US courts | |
| > | Ranked in the top 8% in the sector in the Dow Jones Sustainability World and European Indexes | |
| > | Reviewed and revised Responsible Business Plan to align it with strategic business priorities | |
| > | Additional ways of reporting sales and marketing performance introduced to support increased transparency | |
| > | Improvement in senior leader communications with employees but slight decline in employee engagement |
| AstraZeneca Annual Report and Form 20-F Information 2010 | Our year in brief 5 |
Table of Contents
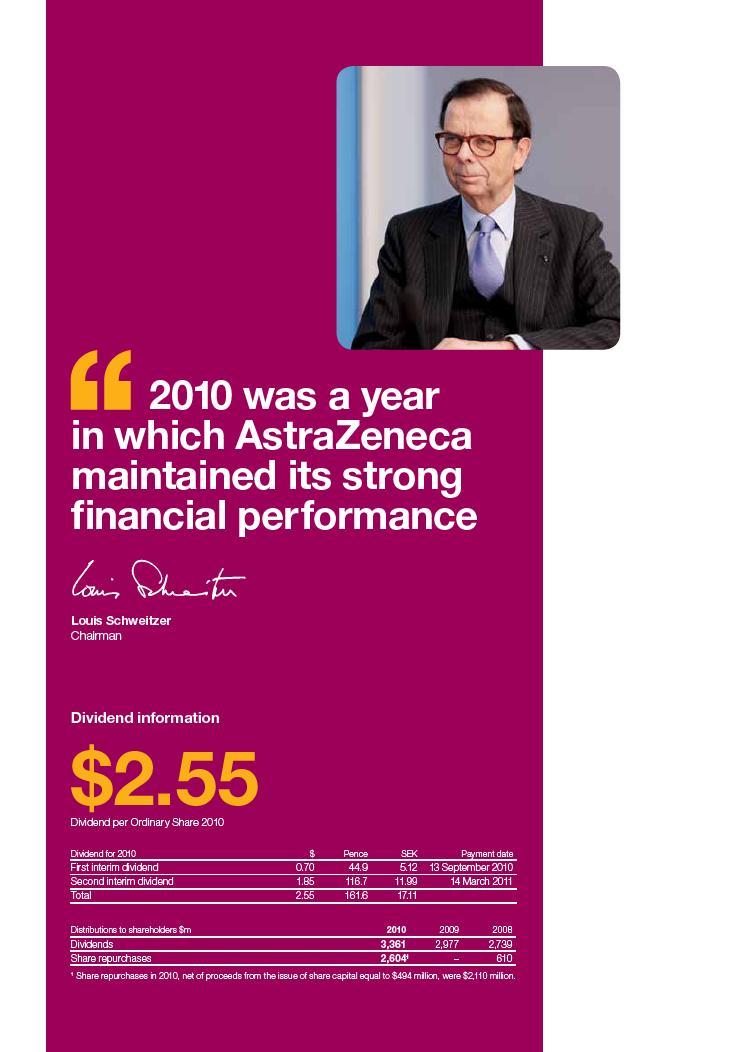
Table of Contents
| AstraZeneca Annual Report and Form 20-F Information 2010 | Chairman’s Statement 7 |
Table of Contents

Table of Contents
| AstraZeneca Annual Report and Form 20-F Information 2010 | Chief Executive Officer’s Review 9 |
Table of Contents
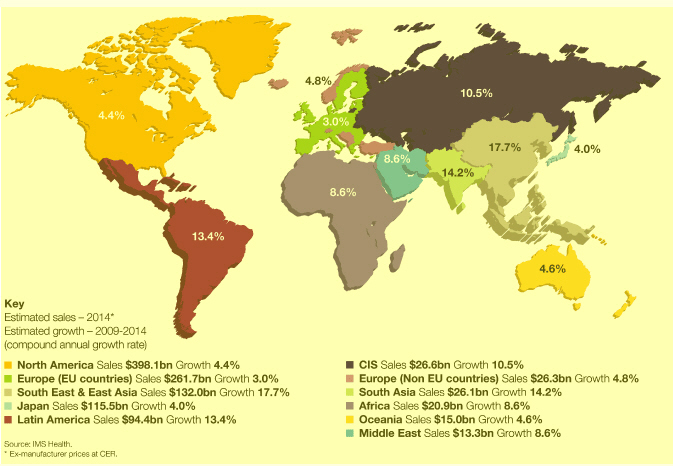
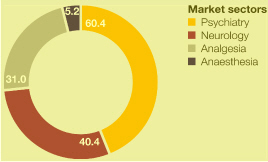
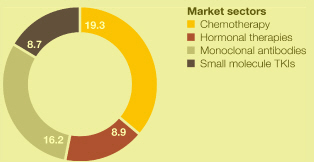
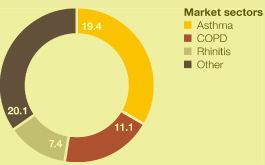
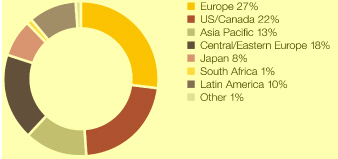
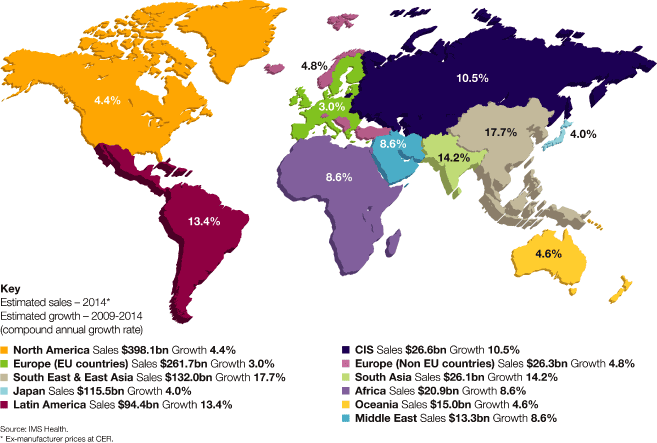
Market definitions table on page 217. Source: IMS Health.
| 10 Our Strategy and Performance Our marketplace | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| AstraZeneca Annual Report and Form 20-F Information 2010 | Our Strategy and Performance Our marketplace 11 |
Table of Contents
| 12 Our Strategy and Performance Our marketplace | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

Table of Contents
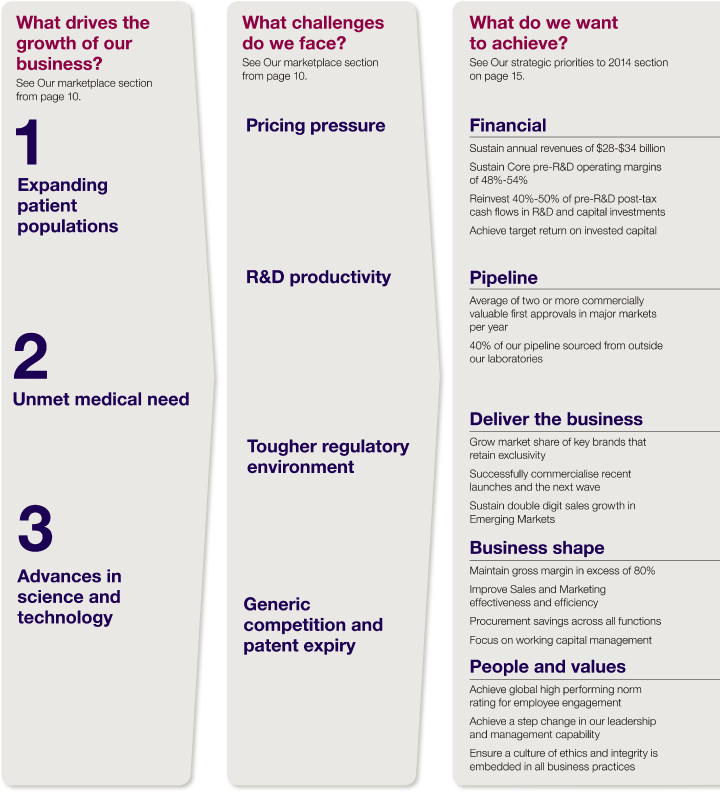
| > | focused in that we will continue to be selective about those areas of the industry in which we choose to compete, targeting those product categories where medical innovation or brand equity will continue to enable us to make acceptable levels of return on our investments | |
| > | integrated in that we believe the best way to capture value within this industry is to span the full value chain of discovery, development and commercialisation, while remaining open to working with partners and outsourcing to capture operational efficiencies | |
| > | innovation-driven in that we believe our technology base will continue to deliver innovative products that will benefit patients and for which payers will pay | |
| > | global in that we believe we have the ability to meet healthcare needs in both established and emerging markets efficiently and effectively. |

| 14 Our Strategy and Performance Our strategy | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | ‘payer partnering’ and personalised healthcare | |
| > | further improving leadership and management capability | |
| > | acquiring and retaining talent, for example in support of our growth plans in Emerging Markets | |
| > | increasing the diversity of our talent pool, so that it better reflects our future business shape. |
| AstraZeneca Annual Report and Form 20-F Information 2010 | Our Strategy and Performance Our strategy 15 |
Table of Contents
| 16 Our Strategy and Performance Our strategy | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Our Strategy and Performance Our strategy 17 |
Table of Contents
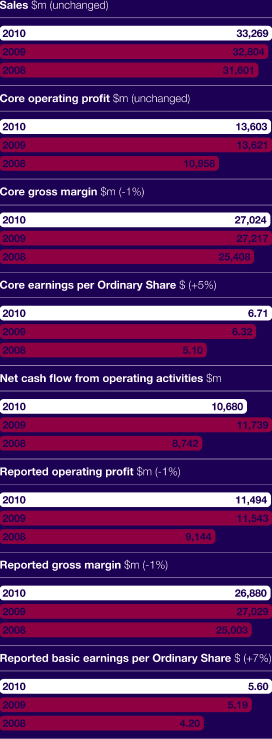
| > | Global revenue was unchanged at CER at $33.3 billion. This was ahead of target primarily as a result of strong operational performance, delayed generic entry and less generic erosion across the US and Europe than assumed in our targets. | |
| > | Core pre-R&D operating margin was 53.5%, near the top end of the medium-term planning assumption range of 48% to 54%. | |
| > | Core EPS increased by 5% at CER to $6.71. This was ahead of target as a result of the above revenue performance and strong operational execution. |
| > | Reinvestment rate was just below the medium-term planning assumption range of 40% to 50% due to the better than expected pre-R&D post-tax cash flows. AstraZeneca continues to expect this range to hold over the planning period although anticipates variances within any particular year. | |
| > | Net cash inflow before financing activities of $8,340 million reflects the strong business performance. |
| > | Vimovo approved in the US and the EU; Brilique approved in the EU with Complete Response Letter received for Brilinta in the US; Kombiglyze™ XR (Onglyza™/metformin combination) approved in the US; additional indications approved for Crestor in the US and the EU and for Seroquel XR in the EU; decisions made in December to discontinue development of motavizumab and Certriad. |
| > | Dapagliflozin and vandetanib NDAs submitted in the US and the EU; Zinforo and Axanum MAAs submitted in the EU; Recentin trials data did not support regulatory submissions; no submissions planned for zibotentan. | |
| > | Completed deal with Rigel for the development of fostamatinib. | |
| > | Phase III trials started for fostamatinib and TC-5214. |
| > | Strong double-digit sales growth for Crestor, Symbicort and Seroquel XR. Annual Crestor and Seroquel sales exceeded $5 billion each. | |
| > | Launches under way and planned for Brilinta/Brilique, Vimovo and Kombiglyze™ XR. |
| > | Achieved target of double-digit growth in Emerging Markets with revenues over $5.1 billion, a 16% increase over 2009. |
| > | Core gross margin of 81.2% slightly ahead of 80% target. | |
| > | Achieved planned improvement in Core SG&A costs with 2% reduction. | |
| > | Achieved Core R&D efficiency savings with spend of $4.2 billion. |
| > | Achieved $541 million of procurement savings against a target of $500 million. |
| > | Employee engagement score as measured by our global employee survey (FOCUS) reduced from 84% in 2009 to 83% in 2010. | |
| > | Improvement of 4% in senior leader communications measure in 2010 over 2009 as measured by FOCUS. |
| > | Maintained position in the DJSI World Index (the top 10% of the largest 2,500 companies) as sustainability leader, as well as listing on the DJSI STOXX European Index. | |
| > | 11 confirmed breaches of external sales and marketing regulations or codes globally (2009: 24). |
| 18 Our Strategy and Performance Performance in 2010 | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

Table of Contents
| Our Strategy and Performance |

Table of Contents

Table of Contents

Table of Contents

Table of Contents
| 24 Delivering our strategy | AstraZeneca Annual Report and Form 20-F Information 2010 | |
Table of Contents
1
|
An R&D function with
world-class productivity |
|
| focused on delivering a range of innovative, differentiated and commercially attractive medicines through collaboration, and underpinned by patent and intellectual property rights. See page 26 | ||
2
|
A sales and marketing activity undertaken in the right way
|
|
| and focused on our customers and their patients’ needs. See page 32 | ||
3
|
A reliable supply and manufacturing operation
|
|
| that ensures our customers and patients receive their medicines when they want and need them. See page 34 | ||
4
|
A diverse and talented workforce
|
|
| with the right skills, in the right place at the right time. See page 36 | ||
5
|
A commitment to responsible development of our business
|
|
| which delivers value for our shareholders and for our other stakeholders.
See page 40 |
||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy 25 | |
Table of Contents
innovative and valued
medicines

President, Global R&D
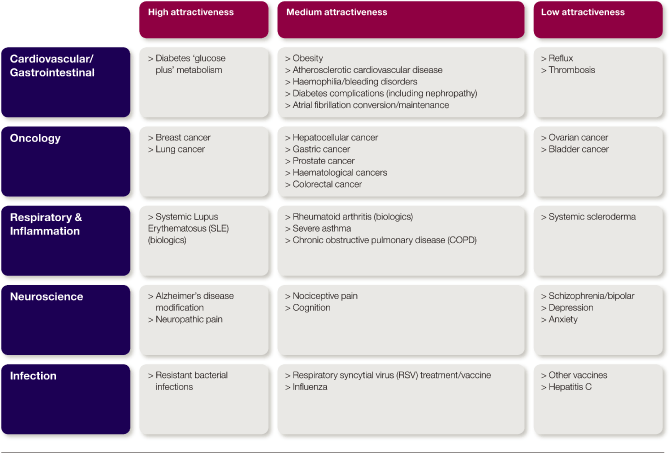
| > | prioritising our resources in those areas where we believe we can be most successful through a focused disease area strategy | |
| > | ensuring shareholders’ funds are invested wisely, using an effective and flexible R&D operating model | |
| > | building the capabilities we need to ensure delivery of our strategy | |
| > | ensuring we access the best new opportunities, regardless of their origin. |
| 26 Delivering our strategy Research and Development | AstraZeneca Annual Report and Form 20-F Information 2010 | |
Table of Contents
| 1 | Includes seven life-cycle management projects re-introduced from BRIC-MT and Japan. |
| > | Small molecule iMeds |
| > | Oncology | ||
| > | Infection | ||
| > | Respiratory & Inflammation |
| > | Aligned small molecule and biologics iMeds |
| > | Cardiovascular and Gastrointestinal | ||
| > | Neuroscience |
| > | Biologics iMeds |
| > | Oncology | ||
| > | Infection | ||
| > | Respiratory & Inflammation |
| > | A ninth, New Opportunities iMed, will focus on identifying opportunities in disease areas outside, or complementary to, our current research areas. This will be done by seeking to acquire commercially viable late stage assets as well as by generating additional value from existing internal assets through alternative uses of such assets in disease areas of high unmet medical need. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Research and Development 27 | |
Table of Contents
| 28 Delivering our strategy Research and Development | AstraZeneca Annual Report and Form 20-F Information 2010 | |
Table of Contents


| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Research and Development 29 | |
Table of Contents
of our inventions

General Counsel
| 30 Delivering our strategy Research and Development | AstraZeneca Annual Report and Form 20-F Information 2010 | |
Table of Contents
| US revenue ($m) | ||||||||||||||||||||||||
| Key marketed products*# | US Patent expiry | 2010 | 2009 | 2008 | ||||||||||||||||||||
Nexium |
20151 | 2,695 | 2,835 | 3,101 | ||||||||||||||||||||
Crestor |
2016 | 2,640 | 2,100 | 1,678 | ||||||||||||||||||||
Toprol-XL/Seloken |
Expired | 689 | 964 | 295 | ||||||||||||||||||||
Atacand |
2012 | 216 | 263 | 262 | ||||||||||||||||||||
Symbicort |
2014 (combination), 2023 (formulation), 2026 (pMDI device) | 721 | 488 | 255 | ||||||||||||||||||||
Pulmicort/Pulmicort Respules |
20192 (Respules), 2018 (Turbuhaler formulation), 2019 (Turbuhaler device) |
305 | 804 | 982 | ||||||||||||||||||||
Arimidex |
Expired | 494 | 878 | 754 | ||||||||||||||||||||
Zoladex |
Expired | 46 | 54 | 72 | ||||||||||||||||||||
Seroquel IR |
2012 | 3,107 | 3,074 | 2,895 | ||||||||||||||||||||
Seroquel XR |
2017 (formulation) | 640 | 342 | 120 | ||||||||||||||||||||
Synagis |
2015 (composition), 2023 (formulation) | 646 | 782 | 923 | ||||||||||||||||||||
Prilosec/Losec |
Expired | 47 | 64 | 171 | ||||||||||||||||||||
Merrem/Meronem |
Expired | 127 | 177 | 207 | ||||||||||||||||||||
Casodex |
Expired | 16 | 148 | 292 | ||||||||||||||||||||
| Revenue ($m)3 | ||||||||||||||||||||||||
Key marketed products*# |
EU Patent expiry | Canadian Patent expiry | Japanese Patent expiry | 2010 | 2009 | 2008 | ||||||||||||||||||
Nexium |
2014 | 2014 | 2014 | 1,422 | 1,395 | 1,387 | ||||||||||||||||||
Crestor |
2017 | 2012 | 2017 | 2,201 | 1,782 | 1,410 | ||||||||||||||||||
Toprol-XL/Seloken |
Expired | Expired | Expired | 169 | 181 | 220 | ||||||||||||||||||
Atacand |
2010 to 2014 depending | 2011 | 2014 | 837 | 808 | 836 | ||||||||||||||||||
| on country | ||||||||||||||||||||||||
Symbicort |
2018 (formulation) | 2012 (combination) | 2017 (combination) | 1,621 | 1,459 | 1,420 | ||||||||||||||||||
| 2019 (Turbuhaler device) | 2018 (formulation) | 2018 (formulation) | ||||||||||||||||||||||
| 2019 (Turbuhaler device) | 2019 (Turbuhaler device) | |||||||||||||||||||||||
Pulmicort/ |
2018 (Respules) | 2018 (Respules) | 2018 (Respules) | 353 | 347 | 364 | ||||||||||||||||||
Pulmicort Respules |
2018 (Turbuhaler formulation) | 2018 (Turbuhaler | 2018 (Turbuhaler formulation) | |||||||||||||||||||||
| 2022 (pMDI device) | formulation) | 2022 (pMDI device) | ||||||||||||||||||||||
Arimidex |
2011 | 2012 | 2012 | 843 | 875 | 930 | ||||||||||||||||||
Zoladex |
Expired | Expired | Expired | 718 | 744 | 775 | ||||||||||||||||||
Seroquel IR |
2012 | Expired | 2012 | 705 | 792 | 1,009 | ||||||||||||||||||
Seroquel XR |
2017 (formulation) | 2017 (formulation) | N/A | 401 | 301 | 90 | ||||||||||||||||||
Synagis |
2015 (composition) | 2015 (composition) | 2015 (composition) | 392 | 300 | 307 | ||||||||||||||||||
Prilosec/Losec |
Expired | Expired | Expired | 660 | 641 | 619 | ||||||||||||||||||
Merrem/Meronem |
Expired | Expired | Expired | 377 | 409 | 387 | ||||||||||||||||||
Casodex |
Expired | Expired | Expired | 457 | 588 | 838 | ||||||||||||||||||
| * | Patents are or may be challenged by third parties and generics may be launched ‘at risk’. See the Principal risks and uncertainties section from page 96. Many of our products are subject to challenges by third parties. Details of material challenges by third parties can be found in Note 25 to the Financial Statements from page 178. | |
| # | Additional patents relating to the stated products may have terms extending beyond the quoted dates. | |
| 1 | Licence agreements with Teva and Ranbaxy Pharmaceuticals Inc. allow each to launch a generic version in the US from May 2014, subject to regulatory approval. | |
| 2 | A licence agreement with Teva permits their ongoing US sale of a generic version from December 2009. | |
| 3 | Aggregate revenue for the EU, Canada and Japan. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Intellectual Property 31 | |
Table of Contents
positions and investing
in new regions

Executive Vice-President, Global Commercial Operations
| 32 Delivering our strategy Sales and Marketing | AstraZeneca Annual Report and Form 20-F Information 2010 | |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Sales and Marketing 33 | |
Table of Contents
the reliable provision of high
quality medicines

Executive Vice-President, Global Operations and
Information Services
| 34 Delivering our strategy Supply and Manufacturing | AstraZeneca Annual Report and Form 20-F Information 2010 | |
Table of Contents
 |
The WHO estimates that between 1% and 30% (rising to 50% on the internet) of medicines sold worldwide are counterfeit. Every year, thousands of patients are seriously harmed or killed as a
result of taking these products rather than the real thing. Counterfeiting is particularly prevalent in the developing world and with medicines bought online.
To combat the problem we have a comprehensive product security strategy which includes: > partnering with others to strengthen enforcement and raise awareness
> securing products through pack features and enhanced integrity of the supply chain
>
combating illegal operations through proactive investigation of suspicious activity and reported incidents.
In 2010, a life-threatening counterfeiting operation was thwarted following an investigation in Colombia. Twenty four members of a criminal gang were arrested in May on charges relating to making and selling a counterfeit of our antibiotic, Meronem. Suspicions first came to light in 2007 when we received reports from employees about suspect Meronem bearing the same batch number. A painstaking investigation was carried out, including the use of undercover techniques to gather evidence. By early 2010, we were able to hand over enough evidence for the Colombian police to conduct a series of raids. |
|||
|
“What is being done to make sure my medicines are genuine? |
||||
 |
Because health connects us all For more information on our work to prevent and detect counterfeiting, go to our website, astrazeneca.com/responsibility. |
|||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Supply and Manufacturing 35 | |
Table of Contents
talented workforce
(%)

around the key priorities we believe to be
critical to delivering our business objectives.
These include developing a high performance
culture, strengthening our talent pipeline and
building capabilities”
Executive Vice-President, Human Resources
and Corporate Affairs
| 36 Delivering our strategy People | AstraZeneca Annual Report and Form 20-F Information 2010 | |
Table of Contents


| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy People 37 | |
Table of Contents

| 38 Delivering our strategy People |
Table of Contents

Table of Contents
value responsibly

|
 |
|
Michele Hooper
|
Dame Nancy Rothwell | |
Senior independent
|
Non-Executive Director with | |
Non-Executive Director and
|
responsibility for overseeing | |
Chairman of the Audit Committee
|
Responsible Business |
| 40 Delivering our strategy Responsible Business | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | R&D ethics – underpinning our drive for innovation with sound ethical practice worldwide | |
| > | Sales and marketing practices – driving consistently high ethical standards to promote our medicines responsibly worldwide | |
| > | Human rights – making sure that we continue to develop and drive a consistent approach across all our activities | |
| > | Access to healthcare – exploring ways of increasing access to healthcare for underserved patient populations | |
| > | Suppliers – working only with organisations who embrace ethical standards that are consistent with our own. |
| > | Patient safety | |
| > | Environment | |
| > | Employee safety, health and wellbeing | |
| > | Community investment. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Responsible Business 41 |
Table of Contents
by geographical area in 2010
by geographical area in 2010
| 42 Delivering our strategy Responsible Business | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
and regulations ruled by external bodies

| 1 | Includes self-reporting activity globally which resulted in a breach being ruled. |
Commercial employees 2010
| Number of employees | ||||||||
| Action taken | 20101 | 20092 | ||||||
Removed from role |
117 | 99 | ||||||
Formal warning |
740 | 687 | ||||||
Guidance and coaching |
768 | 416 | ||||||
Total |
1,625 | 1,202 | ||||||
| 1 | 2010 data reflects improved data capture mechanisms that will be used going forward to report breaches by Commercial employees year-on-year. | |
| 2 | 2009 data shows breaches of Code of Conduct by all employees and is included for comparative purposes only. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Responsible Business 43 |
Table of Contents
| 44 Delivering our strategy Responsible Business | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents


| Figures are calculated in line with the Greenhouse Gas (GhG) protocol guidance (ghgprotocol.org). | ||
| 1 | Data excludes MedImmune. | |


| 1 | Data excludes MedImmune. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Responsible Business 45 |
Table of Contents


| 46 Delivering our strategy Responsible Business | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Delivering our strategy Responsible Business 47 |
Table of Contents

Table of Contents

Table of Contents
areas do we
focus on?
develop and
commercialise
medicines for six
areas of healthcare:
| 2010 | 2009 | 2008 | ||||||||||||||||||||||||||
| Reported | CER | Reported | CER | |||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | growth | Sales | ||||||||||||||||||||||
| $m | % | % | $m | % | % | $m | ||||||||||||||||||||||
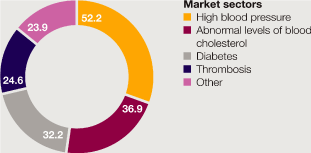
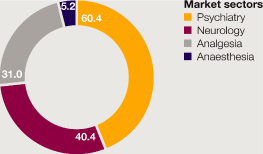
Cardiovascular |
9,403 | 12 | 11 | 8,376 | 20 | 25 | 6,963 | |||||||||||||||||||||
Gastrointestinal |
6,088 | 1 | – | 6,011 | (5 | ) | (2 | ) | 6,344 | |||||||||||||||||||
Infection and Other |
2,176 | (17 | ) | (18 | ) | 2,631 | 7 | 10 | 2,451 | |||||||||||||||||||
Neuroscience |
6,704 | 7 | 7 | 6,237 | 7 | 10 | 5,837 | |||||||||||||||||||||
Oncology |
4,045 | (10 | ) | (12 | ) | 4,518 | (9 | ) | (7 | ) | 4,954 | |||||||||||||||||
Respiratory & Inflammation |
4,099 | (1 | ) | (1 | ) | 4,132 | – | 6 | 4,128 | |||||||||||||||||||
Other businesses |
754 | (16 | ) | (15 | ) | 899 | (3 | ) | 1 | 924 | ||||||||||||||||||
Total |
33,269 | 1 | – | 32,804 | 4 | 7 | 31,601 | |||||||||||||||||||||
| 50 Therapy Area Review | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Phase I | Phase II | Phase III/ | Line | |||||||||||||||
| Registration | extensions | |||||||||||||||||
Cardiovascular
|
> AZD6714 ▲ | > AZD1656 ▲ | > Brilinta/Brilique ¨ | > KombiglyzeTM XR ¨ / | ||||||||||||||
| > AZD8329 ▲ | > Dapagliflozin# » | OnglyzaTM/metformin | ||||||||||||||||
| > AZD7687 ▲ | IR FDC#* | |||||||||||||||||
| > AZD5658 v | > Dapagliflozin/ | |||||||||||||||||
| > AZD4017 ▲ | metformin FDC# ▲ | |||||||||||||||||
| > OnglyzaTM SAVOR# v | ||||||||||||||||||
| > Brilinta | ||||||||||||||||||
| PEGASUS-TIMI v | ||||||||||||||||||
>
Crestor (elevated CRP) ¨ |
||||||||||||||||||
| > Axanum » | ||||||||||||||||||
Gastrointestinal
|
> Nexium ▲ | |||||||||||||||||
(peptic ulcer bleeding) |
||||||||||||||||||
| > Nexium v | ||||||||||||||||||
(GERD) |
||||||||||||||||||
Infection
|
> MEDI-534 ▲ | > AZD9773# ▲ | > MEDI-3250 + | > FluMist/Fluenz ▲ | ||||||||||||||
| > MEDI-550 ▲ | > CAZ104# ▲ | > Zinforo # » | ||||||||||||||||
| > MEDI-559 ▲ | > Motavizumab# l | (ceftaroline) |
||||||||||||||||
| > AZD5847 ▲ | > CXL104# + | |||||||||||||||||
| > AZD9742 ▲ | (CEF104) |
|||||||||||||||||
Neuroscience
|
> AZD3241 ▲ | > AZD3480# ▲ | > TC-5214# v | > Vimovo # ¨ | > Seroquel XR ¨ | |||||||||||||
| > AZD3043# ▲ | > AZD6765 ▲ | > TC-5619# ▲ | > TC-5214# + | > Diprivan # v | ||||||||||||||
| > MEDI-578 v | > AZD2066 ▲ | > AZD1446# ▲ | > EMLA# v | |||||||||||||||
| > AZD5213 v | (chronic neuropathic pain)
|
> AZD2423 + | ||||||||||||||||
| > AZD2066 (MDD) v | ||||||||||||||||||
| > NKTR-118# ▲ | ||||||||||||||||||
Oncology
|
> AZD2461 v | > MEDI-551 v | > Selumetinib ▲ | > Recentin ▲ | > AZD8931 + | > Vandetanib » | > Iressa ▲ | |||||||||||
| > AZD3514 v | > AZD8055 ▲ | (AZD6244)
|
> Selumetinib# ▲ | > MEDI-575# + | (Zactima)
|
> Faslodex ¨ | ||||||||||||
| > AZD7762 ▲ | > MEDI-573# ▲ | (ARRY-142886)/
|
(AZD6244)
|
> Zibotentan ▲ | ||||||||||||||
| > AZD8330# ▲ | > AZD1480 ▲ | MK2206#
|
(ARRY-142886) |
|||||||||||||||
(ARRY-424704)
|
> AZD4547 ▲ | > MEDI-3617 v | > Olaparib ▲ | |||||||||||||||
| > CAT-8015 ▲ | > AZD2014 ▲ | > AZD5363 v | > AZD1152 ▲ | |||||||||||||||
| > MEDI-565 v | ||||||||||||||||||
Respiratory & Inflammation
|
> AZD9819 v | > AZD1981 ▲ | > AZD8848 ▲ | > Fostamatinib# v + | > Oxis v | |||||||||||||
| > MEDI-546# ▲ | > MEDI-528# ▲ | > CAM-3001# + | > Symbicort v | |||||||||||||||
| > MEDI-551 v | > CAT-354 ▲ | > AZD2423 v | (COPD) |
|||||||||||||||
| > MEDI-570# v | > AZD3199 ▲ | > AZD8683 + | > Symbicort v | |||||||||||||||
| > MEDI-557 ▲ | > MEDI-563# ▲ | > AZD5423 + | (SMART) |
|||||||||||||||
| > MEDI-545# ▲ | > AZD5069 + |
v Addition
|
» New filing | # Partnered product | ||
▲ No change
|
¨ Launched | * KombiglyzeTM XR in the US; OnglyzaTM/metformin IR FDC in the EU | ||
+ Progression
|
l Reclassified |
| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review 51 |

Table of Contents
>
|
Crestor sales up 24% to $5.7 billion. | |
>
|
New indications were approved for Crestor in the US and the EU based on data from the landmark JUPITER clinical trial. | |
>
|
In June, the US District Court for the District of Delaware, decided in AstraZeneca’s favour the consolidated ANDA infringement case involving eight ANDA filers seeking approvals for generic Crestor. The defendants have appealed the Court’s judgment and decision of our patent’s infringement, validity and enforceability to the US Court of Appeals for the Federal Circuit. | |
>
|
In September, we received a Paragraph IV Certification notice-letter from Watson Laboratories, Inc. (Watson), informing us of its filing of a 505(b)(2) NDA for rosuvastatin zinc tablets and challenging the substance and formulation patents protecting Crestor. We commenced a patent infringement action against Watson in October in the US District Court for the District of Delaware. | |
> |
Torrent do Brasil launched its generic versions of Crestor in October. AstraZeneca was granted an injunction ordering Torrent do Brasil to discontinue the sale and marketing of these generic products and recall products already on the market. This injunction has subsequently been suspended and the matter is now awaiting the decision of the Court of Appeal which is expected in the first quarter of 2011. | |
>
|
Atacand sales up 3% to $1.5 billion. | |
>
|
Toprol-XL US sales down 29% as a result of increased generic competition. |
>
|
In December, the European Commission granted a marketing authorisation for Brilique (ticagrelor tablets) for the prevention of atherothrombotic events in adult patients with acute coronary syndromes. The decision is applicable to the 27 member states and the three European Economic Area countries of the EU. In the same month, the FDA issued a Complete Response Letter for the Brilinta (ticagrelor) NDA. AstraZeneca announced that it had replied to the Complete Response Letter on 21 January 2011. | |
>
|
In December, AstraZeneca notified Abbott that it would discontinue development of Certriad, a fixed dose combination of the active ingredient in Crestor (rosuvastatin calcium) and Abbott’s Trilipix™ (fenofibric acid), which was being co-developed with Abbott for the treatment of mixed dyslipidemia. | |
>
|
In May, AstraZeneca received a Complete Response Letter from the FDA for the NDA for Axanum, a single capsule of low-dose acetylsalicylic acid (ASA) and esomeprazole. In June, AstraZeneca filed an MAA for Axanum in several countries in the EU for prevention of cardio- and cerebro-vascular events in patients requiring continuous low-dose ASA treatment who are at risk of developing ASA associated gastric and/or duodenal ulcers. | |
>
|
In November, AstraZeneca and BMS received FDA approval for Kombiglyze™ XR, a fixed-dose combination of Onglyza™ plus metformin hydrochloride extended-release tablets. | |
>
|
In December, AstraZeneca and BMS filed regulatory submissions in the US and the EU seeking approval for dapagliflozin, a first-in-class sodium-glucose cotransporter-2 inhibitor, as a once-daily oral therapy for the treatment of adult patients with Type 2 diabetes. | |
| Our marketed products | ||
>
|
Crestor1 (rosuvastatin calcium) is a statin used for the treatment of dyslipidaemia and hypercholesterolemia. In some markets it is also indicated to slow the progression of atherosclerosis and to reduce the risk of first cardiovascular (CV) events. | |
>
|
Atacand2 (candesartan cilexetil) is an angiotensin II antagonist used for the 1st line treatment of hypertension and symptomatic heart failure. | |
>
|
Seloken/Toprol-XL (metoprolol succinate) is a beta-blocker once-daily tablet used for 24-hour control of hypertension and for use in heart failure and angina. | |
>
|
Tenormin (atenolol) is a cardioselective beta-blocker used for hypertension, angina pectoris and other CV disorders. | |
>
|
Zestril3 (lisinopril dihydrate) is an angiotensin-converting enzyme inhibitor used for the treatment of a wide range of CV diseases, including hypertension. | |
>
|
Plendil (felodipine) is a calcium antagonist used for the treatment of hypertension and angina. | |
>
|
Onglyza™4 (saxagliptin) is a dipeptidyl peptidase IV inhibitor used for the treatment of Type 2 diabetes. | |
| 1 | Licensed from Shionogi & Co. Ltd. | |
| 2 | Licensed from Takeda Chemicals Industries Ltd. | |
| 3 | Licensed from Merck. | |
| 4 | Co-developed and co-commercialised with BMS. |
| 52 Therapy Area Review Cardiovascular | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| World | US | Western Europe | Established ROW | Emerging Markets | Prior year | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reported | CER | Reported | Reported | CER | Reported | CER | Reported | CER | World | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | Sales | growth | growth | Sales | growth | growth | Sales | growth | growth | sales | ||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | $m | % | % | $m | % | $m | % | % | $m | % | % | $m | % | % | $m | |||||||||||||||||||||||||||||||||||||||||||||
Crestor |
5,691 | 26 | 24 | 2,640 | 26 | 1,111 | 15 | 20 | 1,332 | 37 | 25 | 608 | 31 | 26 | 4,502 | |||||||||||||||||||||||||||||||||||||||||||||
Atacand |
1,483 | 3 | 3 | 216 | (18 | ) | 736 | — | 4 | 224 | 21 | 8 | 307 | 21 | 17 | 1,436 | ||||||||||||||||||||||||||||||||||||||||||||
Seloken/
Toprol-XL |
1,210 | (16 | ) | (17 | ) | 689 | (29 | ) | 91 | (11 | ) | (9 | ) | 39 | (7 | ) | (14 | ) | 391 | 17 | 13 | 1,443 | ||||||||||||||||||||||||||||||||||||||
Tenormin |
276 | (7 | ) | (9 | ) | 13 | (13 | ) | 61 | (13 | ) | (9 | ) | 127 | (5 | ) | (10 | ) | 75 | (4 | ) | (8 | ) | 296 | ||||||||||||||||||||||||||||||||||||
Plendil |
255 | 6 | 4 | 15 | 7 | 27 | (34 | ) | (32 | ) | 14 | 8 | — | 199 | 15 | 13 | 241 | |||||||||||||||||||||||||||||||||||||||||||
Zestril |
157 | (15 | ) | (14 | ) | 10 | (44 | ) | 81 | (23 | ) | (19 | ) | 17 | (11 | ) | (21 | ) | 49 | 17 | 14 | 184 | ||||||||||||||||||||||||||||||||||||||
Onglyza™ |
69 | n/m | n/m | 54 | n/m | 10 | n/m | n/m | 2 | n/m | n/m | 3 | n/m | n/m | 11 | |||||||||||||||||||||||||||||||||||||||||||||
Others |
262 | — | (1 | ) | 15 | (25 | ) | 113 | (14 | ) | (11 | ) | 26 | (7 | ) | (14 | ) | 108 | 29 | 25 | 263 | |||||||||||||||||||||||||||||||||||||||
Total |
9,403 | 12 | 11 | 3,652 | 7 | 2,230 | 4 | 8 | 1,781 | 28 | 16 | 1,740 | 22 | 18 | 8,376 | |||||||||||||||||||||||||||||||||||||||||||||
| 2009 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Crestor |
4,502 | 25 | 29 | 2,100 | 25 | 968 | 16 | 24 | 970 | 40 | 44 | 464 | 18 | 32 | 3,597 | |||||||||||||||||||||||||||||||||||||||||||||
Atacand |
1,436 | (2 | ) | 5 | 263 | — | 734 | (4 | ) | 2 | 185 | (2 | ) | 8 | 254 | (1 | ) | 13 | 1,471 | |||||||||||||||||||||||||||||||||||||||||
Seloken/
Toprol-XL |
1,443 | 79 | 84 | 964 | 227 | 102 | (25 | ) | (18 | ) | 42 | (13 | ) | (8 | ) | 335 | 2 | 11 | 807 | |||||||||||||||||||||||||||||||||||||||||
Tenormin |
296 | (5 | ) | (5 | ) | 15 | (17 | ) | 70 | (11 | ) | (6 | ) | 133 | 5 | (6 | ) | 78 | (12 | ) | — | 313 | ||||||||||||||||||||||||||||||||||||||
Plendil |
241 | (10 | ) | (7 | ) | 14 | (44 | ) | 41 | (29 | ) | (24 | ) | 13 | (35 | ) | (30 | ) | 173 | 5 | 7 | 268 | ||||||||||||||||||||||||||||||||||||||
Zestril |
184 | (22 | ) | (17 | ) | 18 | (10 | ) | 105 | (27 | ) | (22 | ) | 19 | (21 | ) | (21 | ) | 42 | (14 | ) | (4 | ) | 236 | ||||||||||||||||||||||||||||||||||||
Onglyza™ |
11 | n/m | n/m | 11 | n/m | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
Others |
263 | (3 | ) | 3 | 20 | n/m | 131 | (13 | ) | (6 | ) | 28 | (7 | ) | (10 | ) | 84 | (7 | ) | 2 | 271 | |||||||||||||||||||||||||||||||||||||||
Total |
8,376 | 20 | 25 | 3,405 | 48 | 2,151 | (1 | ) | 6 | 1,390 | 23 | 26 | 1,430 | 4 | 15 | 6,963 | ||||||||||||||||||||||||||||||||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Cardiovascular 53 |
Table of Contents
| 1 | The collaboration for saxagliptin excludes Japan. |
| 54 Therapy Area Review Cardiovascular | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Cardiovascular 55 |
Table of Contents

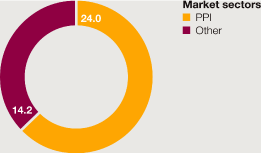
>
|
Sales of Nexium $5 billion, unchanged from the previous year. | |
>
|
Losec/Prilosec sales up 1% to $986 million. | |
>
|
In February 2010, AstraZeneca submitted Nexium for approval in Japan, the only major market yet to launch, and in October, we entered into an agreement with Daiichi Sankyo for the co-promotion and supply of Nexium in Japan. | |
>
|
AstraZeneca received a Complete Response Letter from the FDA in May for the sNDA for Nexium for the risk reduction of low-dose aspirin-associated peptic ulcers. | |
>
|
In October, AstraZeneca filed requests for preliminary injunctions to restrain six companies from marketing and selling generic forms of Nexium in Germany. The court rejected the requests in December. The decision has not yet been published. AstraZeneca has four weeks from the date of publication of the decision to determine whether or not it will appeal the decision. |
>
|
In January 2010, AstraZeneca entered into an agreement with Teva Pharmaceutical Industries Ltd. and affiliates (Teva) to settle patent litigation regarding Teva’s ANDA submission for a generic version of Nexium delayed-release capsules. Under the agreement, AstraZeneca has granted Teva a licence for its ANDA product to enter the US market, subject to regulatory approval, on 27 May 2014. | |
>
|
In June, the Federal Court of Canada dismissed AstraZeneca’s request to prohibit the Canadian Minister of Health from issuing a Notice of Compliance for the regulatory applications for generic esomeprazole magnesium submitted by Apotex Inc. (Apotex). In October, AstraZeneca commenced a patent infringement action against Apotex alleging infringement of five Canadian patents related to Nexium. | |
>
|
In January 2011, AstraZeneca entered into a settlement agreement with Dr. Reddy’s Laboratories, Ltd. and Dr. Reddy’s Laboratories, Inc. (together, DRL) regarding DRL’s ANDA submission for a generic version of Nexium delayed-release capsules. Under the agreement, AstraZeneca has granted DRL a licence for its ANDA product to enter the US market, subject to regulatory approval, on 27 May 2014. | |
>
|
Thirteen third parties have opposed the grant of a European patent covering Nexium, which is due to remain in force until 2014. The patent includes claims to Nexium of very high optical purity and has been asserted by AstraZeneca in litigation against generic companies in Europe. While the European Patent Office has not yet set a date for the oppositions to be heard, a hearing could occur in the first half of 2011. |
>
|
Nexium (esomeprazole) is the first proton pump inhibitor (PPI) used for the treatment of acid-related diseases to offer clinical improvements over other PPIs and other treatments. | |
>
|
Losec/Prilosec (omeprazole) is used for the short-term and long-term treatment of acid-related diseases. | |
>
|
Entocort (budesonide) is a locally acting corticosteroid used for the treatment of inflammatory bowel disease. |
| 56 Therapy Area Review Gastrointestinal | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| World | US | Western Europe | Established ROW | Emerging Markets | Prior year | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reported | CER | Reported | Reported | CER | Reported | CER | Reported | CER | World | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | Sales | growth | growth | Sales | growth | growth | Sales | growth | growth | sales | ||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | $m | % | % | $m | % | $m | % | % | $m | % | % | $m | % | % | $m | |||||||||||||||||||||||||||||||||||||||||||||
Nexium |
4,969 | – | – | 2,695 | (5 | ) | 1,202 | (2 | ) | 2 | 453 | 17 | 4 | 619 | 21 | 18 | 4,959 | |||||||||||||||||||||||||||||||||||||||||||
Losec/Prilosec |
986 | 4 | 1 | 47 | (28 | ) | 253 | (3 | ) | (2 | ) | 437 | 6 | (1 | ) | 249 | 19 | 16 | 946 | |||||||||||||||||||||||||||||||||||||||||
Others |
133 | 25 | 26 | 76 | 49 | 45 | – | 2 | 6 | – | (17 | ) | 6 | 50 | 75 | 106 | ||||||||||||||||||||||||||||||||||||||||||||
Total |
6,088 | 1 | – | 2,818 | (4 | ) | 1,500 | (2 | ) | 1 | 896 | 12 | 1 | 874 | 20 | 17 | 6,011 | |||||||||||||||||||||||||||||||||||||||||||
2009 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nexium |
4,959 | (5 | ) | (1 | ) | 2,835 | (9 | ) | 1,225 | (1 | ) | 7 | 386 | – | 10 | 513 | 7 | 15 | 5,200 | |||||||||||||||||||||||||||||||||||||||||
Losec/Prilosec |
946 | (10 | ) | (10 | ) | 64 | (63 | ) | 261 | (12 | ) | (3 | ) | 411 | 6 | (1 | ) | 210 | 3 | 6 | 1,055 | |||||||||||||||||||||||||||||||||||||||
Others |
106 | 19 | 24 | 51 | 55 | 45 | (2 | ) | 4 | 6 | – | – | 4 | – | 25 | 89 | ||||||||||||||||||||||||||||||||||||||||||||
Total |
6,011 | (5 | ) | (2 | ) | 2,950 | (11 | ) | 1,531 | (3 | ) | 5 | 803 | 3 | 4 | 727 | 6 | 13 | 6,344 | |||||||||||||||||||||||||||||||||||||||||
GI sales grew by 1% to $6,088 million in 2010 from $6,011 million in 2009.

| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Gastrointestinal 57 |
Table of Contents

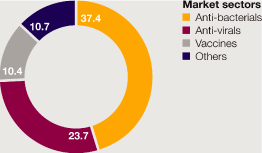
>
|
Synagis sales of $1 billion; in the US $646 million, down 17%. | |
>
|
Merrem/Meronem sales of $817 million, down 7%. | |
>
|
FluMist sales of $174 million, up 20%. | |
>
|
In December, the biological license application submitted to the FDA relating to motavizumab was withdrawn and AstraZeneca recorded a financial impairment charge of $445 million. | |
>
|
Zinforo (ceftaroline) was submitted for marketing approval in the EU in December for the treatment of complicated skin and soft tissue infections as well as for community acquired pneumonia. | |
>
|
Initiation of Phase IIb study with AZD9773 (formerly known as CytoFab™). |
>
|
Synagis (palivizumab) is a humanised MAb used for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in paediatric patients at high risk of acquiring RSV disease. | |
>
|
Merrem/Meronem1 (meropenem) is a carbapenem anti-bacterial used for the treatment of serious infections in hospitalised patients. | |
>
|
FluMist/Fluenz (influenza vaccine live, intranasal) is an intranasal live, attenuated, trivalent influenza vaccine. | |
>
|
Cubicin™2 (daptomycin) is a cyclic lipopeptide anti-bacterial used for the treatment of serious infections in hospitalised patients. |
| 1 | Licensed from Dainippon Sumitomo. | |
| 2 | Licensed from Cubist Pharmaceuticals, Inc. |
| 58 Therapy Area Review Infection | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| World | US | Western Europe | Established ROW | Emerging Markets | Prior year | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reported | CER | Reported | Reported | CER | Reported | CER | Reported | CER | World | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | Sales | growth | growth | Sales | growth | growth | Sales | growth | growth | sales | ||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | $m | % | % | $m | % | $m | % | % | $m | % | % | $m | % | % | $m | |||||||||||||||||||||||||||||||||||||||||||||
Synagis |
1,038 | (4 | ) | (4 | ) | 646 | (17 | ) | 392 | 31 | 31 | – | – | – | – | – | – | 1,082 | ||||||||||||||||||||||||||||||||||||||||||
Merrem |
817 | (6 | ) | (7 | ) | 127 | (28 | ) | 328 | (9 | ) | (7 | ) | 57 | 10 | (4 | ) | 305 | 8 | 4 | 872 | |||||||||||||||||||||||||||||||||||||||
FluMist |
174 | 20 | 20 | 173 | 19 | – | – | – | – | – | – | 1 | – | – | 145 | |||||||||||||||||||||||||||||||||||||||||||||
Non Seasonal |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Flu |
39 | (90 | ) | (90 | ) | 39 | (90 | ) | – | – | – | – | – | – | – | – | – | 389 | ||||||||||||||||||||||||||||||||||||||||||
Others |
108 | (24 | ) | (25 | ) | 68 | (16 | ) | – | (100 | ) | (93 | ) | 20 | (5 | ) | (43 | ) | 20 | 54 | 92 | 143 | ||||||||||||||||||||||||||||||||||||||
Total |
2,176 | (17 | ) | (18 | ) | 1,053 | (33 | ) | 720 | 4 | 6 | 77 | 5 | (15 | ) | 326 | 11 | 8 | 2,631 | |||||||||||||||||||||||||||||||||||||||||
2009 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Synagis |
1,082 | (12 | ) | (12 | ) | 782 | (15 | ) | 300 | (2 | ) | (2 | ) | – | – | – | – | – | – | 1,230 | ||||||||||||||||||||||||||||||||||||||||
Merrem |
872 | (3 | ) | 5 | 177 | (14 | ) | 361 | 5 | 13 | 52 | 8 | 19 | 282 | (5 | ) | 6 | 897 | ||||||||||||||||||||||||||||||||||||||||||
FluMist |
145 | 39 | 39 | 145 | 39 | – | – | – | – | – | – | – | – | – | 104 | |||||||||||||||||||||||||||||||||||||||||||||
Non Seasonal |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Flu |
389 | n/m | n/m | 389 | n/m | – | – | – | – | – | – | – | – | – | – | |||||||||||||||||||||||||||||||||||||||||||||
Others |
143 | (35 | ) | (31 | ) | 82 | (29 | ) | 27 | (61 | ) | (55 | ) | 19 | 19 | 31 | 15 | (25 | ) | (15 | ) | 220 | ||||||||||||||||||||||||||||||||||||||
Total |
2,631 | 7 | 10 | 1,575 | 17 | 688 | (5 | ) | – | 71 | 11 | 22 | 297 | (6 | ) | 5 | 2,451 | |||||||||||||||||||||||||||||||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Infection 59 |
Table of Contents
MEDI-559 and MEDI-534.
Infection sales were down 17% to $2,176 million from $2,631 million in 2009.
| 60 Therapy Area Review Infection | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | Total Seroquel sales up 9% to $5.3 billion. | |
| > | In August, the European Commission approved Seroquel XR as an add-on treatment of major depressive episodes in patients with major depressive disorder (MDD) who have had sub-optimal response to anti-depressant monotherapy. | |
| > | Seroquel XR submissions for generalised anxiety disorder (GAD) were withdrawn in the US in July and from the European Mutual Recognition Procedure in October. | |
| > | The first patients were enrolled in the MDD Adjunct Phase III clinical development programme for TC-5214, a neuronal nicotinic receptor modulator, being developed with Targacept, in June. | |
| > | In April 2010, the FDA approved Vimovo (naproxen/esomeprazole magnesium) for arthritis patients at risk of developing NSAID-associated gastric ulcers. In October, EU approval was received for Vimovo for the symptomatic treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis in patients who are at risk of developing NSAID-associated gastric and/or duodenal ulcers and where treatment with lower doses of naproxen or of other NSAIDs is not considered sufficient. | |
| > | As previously disclosed, in 2010, AstraZeneca reached a civil settlement with the US Attorney’s Office (Department of Justice) and the state attorneys general National Medicaid Fraud Control Unit (NMFCU) to resolve an investigation relating to the marketing of Seroquel, pursuant to which AstraZeneca paid to the United States Federal Government a fine of $302 million plus accrued interest and to participating states a proportional share of up to $218 million |
| plus accrued interest. In September, AstraZeneca entered into individual settlement agreements with 41 states and Washington, D.C. for an aggregate amount of approximately $210 million. | ||
| > | In 2010, AstraZeneca reached agreements in principle on monetary terms with attorneys representing 24,591 Seroquel product liability claimants. AstraZeneca has made provisions in the year totalling $592 million in respect of the ongoing Seroquel product liability litigation and state attorney general investigations into sales and marketing practices in the aggregate. For further details relating to Seroquel product liability claims and state attorney general investigations into Seroquel sales and marketing practices, see Note 25 to the Financial Statements from page 178. | |
| > | In January 2011, the US District Court for the District of New Jersey scheduled a trial date of 3 October 2011 in the consolidated seven ANDA patent litigations relating to Seroquel XR. The District Court also entered a stipulation and consent order concerning US Patent No. 4,879,288 (the ’288 patent), one of the two patents-in-suit, staying litigation between AstraZeneca and Handa Pharmaceuticals, LLC (Handa) concerning the ’288 patent, until and including 26 March 2012, the date AstraZeneca’s paediatric exclusivity relating to its ’288 patent expires. After expiration of the stay, AstraZeneca’s infringement claims against Handa relating to the ’288 patent, and Handa’s related counterclaims, will be dismissed as moot. Under the stipulation, Handa agrees not to engage in the commercial sale of its generic extended release quetiapine fumarate products until after 26 March 2012. |
| > | Seroquel IR (quetiapine fumarate) is an atypical anti-psychotic drug generally approved for the treatment of schizophrenia and bipolar disorder (mania, depression and maintenance). | |
| Seroquel XR (an extended release formulation of quetiapine fumarate) is generally approved for the treatment of schizophrenia, bipolar disorder, MDD and in some territories for GAD. Approved use for Seroquel IR and Seroquel XR varies based on territory. | ||
| > | Vimovo (naproxen/esomeprazole magnesium) is a fixed-dose combination of enteric-coated naproxen (an NSAID) with the gastroprotection of immediate release esomeprazole (a proton pump inhibitor) approved for the relief of signs and symptoms of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis, and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID-associated gastric ulcers. | |
| > | Zomig (zolmitriptan) is used for the treatment of migraines with or without aura. | |
| > | Diprivan (propofol) is an intravenous general anaesthetic used in the induction and maintenance of anaesthesia, light sedation for diagnostic procedures and for intensive care sedation. | |
| > | Naropin (ropivacaine) is used as a long-acting local anaesthetic, replacing the previous standard treatment of bupivacaine. | |
| > | Xylocaine (lidocaine) is a widely used short-acting local anaesthetic. | |
| > | EMLA (lidocaine + prilocaine) is used as a local anaesthetic for topical application. |
| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Neuroscience 61 |
Table of Contents
| World | US | Western Europe | Established ROW | Emerging Markets | Prior year | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reported | CER | Reported | Reported | CER | Reported | CER | Reported | CER | World | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | Sales | growth | growth | Sales | growth | growth | Sales | growth | growth | sales | ||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | $m | % | % | $m | % | $m | % | % | $m | % | % | $m | % | % | $m | |||||||||||||||||||||||||||||||||||||||||||||
Seroquel IR |
4,148 | (1 | ) | (1 | ) | 3,107 | 1 | 560 | (14 | ) | (11 | ) | 223 | 10 | 1 | 258 | 7 | – | 4,171 | |||||||||||||||||||||||||||||||||||||||||
Seroquel XR |
1,154 | 66 | 67 | 640 | 87 | 359 | 30 | 36 | 61 | 85 | 67 | 94 | 114 | 109 | 695 | |||||||||||||||||||||||||||||||||||||||||||||
Local |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anaesthetics |
605 | 1 | (1 | ) | 29 | (28 | ) | 265 | (4 | ) | (1 | ) | 186 | 9 | (1 | ) | 125 | 13 | 8 | 599 | ||||||||||||||||||||||||||||||||||||||||
Zomig |
428 | (1 | ) | (2 | ) | 176 | (3 | ) | 172 | (4 | ) | (2 | ) | 69 | 17 | 8 | 11 | (15 | ) | (23 | ) | 434 | ||||||||||||||||||||||||||||||||||||||
Diprivan |
322 | 11 | 8 | 45 | – | 50 | (19 | ) | (16 | ) | 76 | 29 | 20 | 151 | 22 | 17 | 290 | |||||||||||||||||||||||||||||||||||||||||||
Others |
47 | (2 | ) | (4 | ) | 6 | (25 | ) | 27 | (7 | ) | (7 | ) | 3 | – | – | 11 | 38 | 25 | 48 | ||||||||||||||||||||||||||||||||||||||||
Total |
6,704 | 7 | 7 | 4,003 | 8 | 1,433 | (3 | ) | – | 618 | 17 | 7 | 650 | 20 | 14 | 6,237 | ||||||||||||||||||||||||||||||||||||||||||||
2009 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Seroquel |
4,866 | 9 | 12 | 3,416 | 13 | 928 | 9 | 17 | 236 | (24 | ) | (24 | ) | 286 | 6 | 18 | 4,452 | |||||||||||||||||||||||||||||||||||||||||||
Local |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anaesthetics |
599 | (1 | ) | 4 | 40 | 18 | 277 | (4 | ) | 3 | 171 | 5 | 4 | 111 | (7 | ) | 5 | 605 | ||||||||||||||||||||||||||||||||||||||||||
Zomig |
434 | (3 | ) | – | 182 | (3 | ) | 180 | (5 | ) | 3 | 59 | 2 | 2 | 13 | (7 | ) | 7 | 448 | |||||||||||||||||||||||||||||||||||||||||
Diprivan |
290 | 4 | 6 | 45 | 15 | 62 | (19 | ) | (14 | ) | 59 | – | (7 | ) | 124 | 20 | 26 | 278 | ||||||||||||||||||||||||||||||||||||||||||
Others |
48 | (11 | ) | (4 | ) | 8 | (11 | ) | 29 | (12 | ) | (3 | ) | 3 | (25 | ) | (25 | ) | 8 | – | 13 | 54 | ||||||||||||||||||||||||||||||||||||||
Total |
6,237 | 7 | 10 | 3,691 | 12 | 1,476 | 2 | 10 | 528 | (11 | ) | (12 | ) | 542 | 5 | 16 | 5,837 | |||||||||||||||||||||||||||||||||||||||||||
| 62 Therapy Area Review Neuroscience | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 1 | Decision Resources 2008. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Neuroscience 63 |
Table of Contents

| > | Arimidex sales down 22% to $1.5 billion, impacted by patent expiry in the US in June. However, market exclusivity has been extended in many EU markets from August 2010 to February 2011. | |
| > | Zoladex sales $1.1 billion, unchanged from the previous year. | |
| > | Casodex sales $579 million, down 34%, as a result of generic competition in the US, Western Europe and Japan. | |
| > | Iressa sales $393 million, up 28%, having been launched in the EU as the first approved personalised medicine for the treatment of adults with locally advanced or metastatic non-small cell lung cancer (NSCLC) with activating mutations of the epidermal growth factor receptor-tyrosine kinase (EGFR-TK). In January 2011, AstraZeneca informed the FDA that it will be withdrawing the accelerated approval NDA for Iressa. | |
| > | Vandetanib has been submitted for regulatory approval for the treatment of unresectable, locally advanced medullary thyroid cancer in the US and the EU. In January 2011, the FDA extended the time to complete its review of the vandetanib NDA by three months to 7 April 2011. | |
| > | Recentin (cediranib) did not meet its primary endpoints in two pivotal studies examining cediranib in 1st line metastatic colorectal cancer (mCRC) and a third pivotal study in recurrent glioblastoma (rGBM) and therefore no regulatory submissions will be filed in 1st line mCRC or rGBM. However, studies continue in NSCLC. | |
| > | Zibotentan (ZD4054) did not demonstrate a significant improvement in overall survival in a Phase III study in patients with metastatic castration resistant prostate cancer (CRPC). Therefore, no regulatory submissions for zibotentan are planned at this time. However, clinical studies continue in other CRPC settings. | |
| > | Olaparib (AZD2281) is in ongoing Phase II studies for the treatment of certain types of breast and ovarian cancer and a decision in relation to Phase III studies has been delayed until later in 2011. |
| > | Arimidex (anastrozole) is an aromatase inhibitor used for the treatment of early breast cancer. | |
| > | Zoladex (goserelin acetate implant), in one- and three-month depots, is a luteinising hormone-releasing hormone agonist used for the treatment of prostate cancer, breast cancer and certain benign gynaecological disorders. | |
| > | Casodex (bicalutamide) is an anti-androgen therapy used for the treatment of prostate cancer. | |
| > | Iressa (gefitinib) is used as an EGFR-TK inhibitor that acts to block signals for cancer cell growth and survival in NSCLC. | |
| > | Faslodex (fulvestrant) is an injectable oestrogen receptor antagonist used for the treatment of hormone receptor-positive metastatic breast cancer for post menopausal women whose disease has spread following treatment with an antioestrogen medicine. | |
| > | Nolvadex (tamoxifen citrate) remains a widely used breast cancer treatment outside the US. |
| 64 Therapy Area Review Oncology | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| World | US | Western Europe | Established ROW | Emerging Markets | Prior year | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reported | CER | Reported | Reported | CER | Reported | CER | Reported | CER | World | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | Sales | growth | growth | Sales | growth | growth | Sales | growth | growth | sales | ||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | $m | % | % | $m | % | $m | % | % | $m | % | % | $m | % | % | $m | |||||||||||||||||||||||||||||||||||||||||||||
Arimidex |
1,512 | (21 | ) | (22 | ) | 494 | (44 | ) | 580 | (7 | ) | (4 | ) | 287 | 10 | 2 | 151 | (3 | ) | (6 | ) | 1,921 | ||||||||||||||||||||||||||||||||||||||
Zoladex |
1,115 | 3 | – | 46 | (15 | ) | 276 | (19 | ) | (17 | ) | 451 | 8 | – | 342 | 24 | 23 | 1,086 | ||||||||||||||||||||||||||||||||||||||||||
Casodex |
579 | (31 | ) | (34 | ) | 16 | (89 | ) | 113 | (39 | ) | (37 | ) | 347 | (14 | ) | (18 | ) | 103 | (6 | ) | (8 | ) | 844 | ||||||||||||||||||||||||||||||||||||
Iressa |
393 | 32 | 28 | 4 | (20 | ) | 49 | 600 | 643 | 182 | 15 | 9 | 158 | 24 | 20 | 297 | ||||||||||||||||||||||||||||||||||||||||||||
Others |
446 | 21 | 21 | 161 | 27 | 135 | 14 | 19 | 61 | 9 | 4 | 89 | 29 | 25 | 370 | |||||||||||||||||||||||||||||||||||||||||||||
Total |
4,045 | (10 | ) | (12 | ) | 721 | (41 | ) | 1,153 | (10 | ) | (7 | ) | 1,328 | 3 | (4 | ) | 843 | 15 | 12 | 4,518 | |||||||||||||||||||||||||||||||||||||||
2009 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Arimidex |
1,921 | 3 | 7 | 878 | 16 | 626 | (9 | ) | – | 261 | 5 | – | 156 | (8 | ) | 3 | 1,857 | |||||||||||||||||||||||||||||||||||||||||||
Zoladex |
1,086 | (5 | ) | – | 54 | (25 | ) | 341 | (10 | ) | 1 | 416 | 6 | – | 275 | (6 | ) | 6 | 1,138 | |||||||||||||||||||||||||||||||||||||||||
Casodex |
844 | (33 | ) | (34 | ) | 148 | (49 | ) | 185 | (60 | ) | (56 | ) | 402 | 6 | (5 | ) | 109 | (12 | ) | (1 | ) | 1,258 | |||||||||||||||||||||||||||||||||||||
Iressa |
297 | 12 | 8 | 5 | (29 | ) | 7 | 250 | 250 | 158 | 22 | 9 | 127 | 1 | 4 | 265 | ||||||||||||||||||||||||||||||||||||||||||||
Others |
370 | (15 | ) | (13 | ) | 127 | (37 | ) | 118 | 2 | 9 | 56 | 4 | (7 | ) | 69 | 6 | 20 | 436 | |||||||||||||||||||||||||||||||||||||||||
Total |
4,518 | (9 | ) | (7 | ) | 1,212 | (9 | ) | 1,277 | (22 | ) | (15 | ) | 1,293 | 7 | (1 | ) | 736 | (5 | ) | 5 | 4,954 | ||||||||||||||||||||||||||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Oncology 65 |
Table of Contents
| 66 Therapy Area Review Oncology | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents


| > | Total Symbicort sales $2.7 billion, up 20%. | |
| > | Total Pulmicort sales $872 million, down 34%. | |
| > | In November, the US Court of Appeals for the Federal Circuit affirmed the US District Court, District of New Jersey’s issuance of a preliminary injunction barring Apotex, Inc. and Apotex Corp. (Apotex) from launching a generic version of Pulmicort Respules. Apotex has petitioned the appellate court for a rehearing of its appeal, en banc. | |
| > | Fostamatinib (previously known as R788) was in-licensed from Rigel in February 2010 and in September, the first patient was enrolled in a Phase III clinical development programme for rheumatoid arthritis. |
| > | Symbicort pMDI (budesonide/formoterol in a pressurised metered-dose inhaler) is used for the treatment of asthma and chronic obstructive pulmonary disease (COPD) in the US. | |
| > | Symbicort Turbuhaler (budesonide/formoterol in a dry powder inhaler) is a combination of an inhaled corticosteroid and a fast onset, long-acting bronchodilator used for the treatment of asthma and COPD. It is also approved for maintenance and reliever therapy (SMART) in persistent asthma. | |
| > | Pulmicort (budesonide) is a corticosteroid anti-inflammatory inhalation drug that is used to help prevent the symptoms of and improve the control of asthma. | |
| > | Pulmicort Respules (budesonide inhalation suspension) is a first nebulised corticosteroid used for the treatment of asthma in both children and adults. Approved use for Pulmicort Respules varies based on territory. | |
| > | Rhinocort (budesonide) is a nasal steroid used as a treatment for allergic rhinitis (hay fever), perennial rhinitis and nasal polyps. | |
| > | Oxis (formoterol) is a fast onset, long-acting beta-agonist used for the treatment of asthma and COPD. | |
| > | Accolate (zafirlukast) is an oral leukotriene receptor antagonist used for the treatment of asthma. |
| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Respiratory & Inflammation 67 |
Table of Contents
| World | US | Western Europe | Established ROW | Emerging Markets | Prior year | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reported | CER | Reported | Reported | CER | Reported | CER | Reported | CER | World | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | Sales | growth | growth | Sales | growth | growth | Sales | growth | growth | sales | ||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | $m | % | % | $m | % | $m | % | % | $m | % | % | $m | % | % | $m | |||||||||||||||||||||||||||||||||||||||||||||
Symbicort |
2,746 | 20 | 20 | 721 | 48 | 1,367 | 2 | 5 | 286 | 75 | 59 | 372 | 25 | 23 | 2,294 | |||||||||||||||||||||||||||||||||||||||||||||
Pulmicort |
872 | (33 | ) | (34 | ) | 305 | (62 | ) | 215 | (6 | ) | (4 | ) | 114 | 13 | 5 | 238 | 35 | 32 | 1,310 | ||||||||||||||||||||||||||||||||||||||||
Rhinocort |
227 | (14 | ) | (16 | ) | 93 | (28 | ) | 39 | (13 | ) | (11 | ) | 16 | 14 | – | 79 | 4 | – | 264 | ||||||||||||||||||||||||||||||||||||||||
Others |
254 | (4 | ) | (5 | ) | 41 | (15 | ) | 118 | (4 | ) | (3 | ) | 22 | (4 | ) | (13 | ) | 73 | 4 | 1 | 264 | ||||||||||||||||||||||||||||||||||||||
Total |
4,099 | (1 | ) | (1 | ) | 1,160 | (21 | ) | 1,739 | – | 3 | 438 | 46 | 33 | 762 | 23 | 20 | 4,132 | ||||||||||||||||||||||||||||||||||||||||||
2009 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Symbicort |
2,294 | 14 | 23 | 488 | 91 | 1,345 | 2 | 11 | 163 | 3 | 13 | 298 | 8 | 21 | 2,004 | |||||||||||||||||||||||||||||||||||||||||||||
Pulmicort |
1,310 | (12 | ) | (10 | ) | 804 | (18 | ) | 229 | (8 | ) | (1 | ) | 101 | 9 | 4 | 176 | 2 | 12 | 1,495 | ||||||||||||||||||||||||||||||||||||||||
Rhinocort |
264 | (18 | ) | (15 | ) | 129 | (29 | ) | 45 | (6 | ) | 2 | 14 | (7 | ) | – | 76 | (1 | ) | 6 | 322 | |||||||||||||||||||||||||||||||||||||||
Others |
264 | (14 | ) | (7 | ) | 48 | (9 | ) | 123 | (16 | ) | (7 | ) | 23 | (12 | ) | (8 | ) | 70 | (15 | ) | (4 | ) | 307 | ||||||||||||||||||||||||||||||||||||
Total |
4,132 | – | 6 | 1,469 | – | 1,742 | (1 | ) | 8 | 301 | 3 | 8 | 620 | 2 | 14 | 4,128 | ||||||||||||||||||||||||||||||||||||||||||||
| 68 Therapy Area Review Respiratory & Inflammation | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 1 | Decision Resources 2010. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Therapy Area Review Respiratory & Inflammation 69 |
Table of Contents
business
performing
around the world?
in markets outside
the US in 2010
broadly offset the
loss of revenue
in the US
| 70 Geographical Review | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | In the US, combined sales of our key growth brands of Crestor, Onglyza™, Seroquel, Symbicort and Vimovo were up 19% to $7,167 million (2009: $6,014 million). Despite this strong performance, overall sales decreased by 7% to $13,727 million as a result of increased generic competition for Arimidex, Casodex, Pulmicort Respules and Toprol-XL and its authorised generic and the absence of the H1N1 pandemic influenza (swine flu) vaccine revenue. | |
| > | Western Europe reported a strong performance in the context of increased competition and governmental controls over healthcare expenditure. Crestor outperformed the statin market in Western Europe with double-digit growth by volume and Seroquel grew three times as fast as the atypical anti-psychotic market segment in Western Europe by value. | |
| > | Established ROW sales were up 7%, driven by the strong performance for Crestor as well as the successful launch for Symbicort Turbuhaler in Japan. | |
| > | Emerging Markets delivered strong double-digit sales growth of 16% to $5,198 million, with sales growth in China of 28%, Russia of 26% and Brazil of 17%. | |
| > | AstraZeneca is the third largest pharmaceutical company in the US, with a 6% share of US prescription pharmaceutical sales and the seventh largest prescription-based pharmaceutical company in Western Europe, with a 4.8% market share of prescription sales by value. |
| 2010 | 2009 | 2008 | ||||||||||||||||||||||||||
| Reported | CER | Reported | CER | |||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | growth | Sales | ||||||||||||||||||||||
| $m | % | % | $m | % | % | $m | ||||||||||||||||||||||
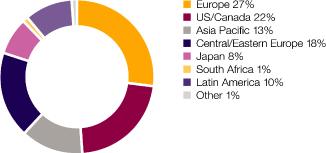
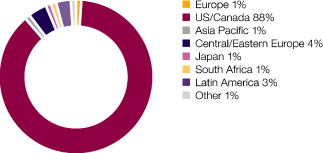
US |
13,727 | (7 | ) | (7 | ) | 14,777 | 9 | 9 | 13,510 | |||||||||||||||||||
Western Europe |
9,168 | (1 | ) | 2 | 9,252 | (5 | ) | 3 | 9,743 | |||||||||||||||||||
Canada |
1,510 | 26 | 14 | 1,203 | (6 | ) | 3 | 1,275 | ||||||||||||||||||||
Japan |
2,617 | 11 | 4 | 2,367 | 20 | 7 | 1,957 | |||||||||||||||||||||
Other Established ROW |
1,049 | 23 | 6 | 853 | 1 | 12 | 843 | |||||||||||||||||||||
Established ROW |
5,176 | 17 | 7 | 4,423 | 8 | 7 | 4,075 | |||||||||||||||||||||
Emerging Europe |
1,165 | 7 | 6 | 1,091 | (10 | ) | 7 | 1,215 | ||||||||||||||||||||
China |
1,047 | 29 | 28 | 811 | 29 | 27 | 627 | |||||||||||||||||||||
Emerging Asia Pacific |
890 | 14 | 7 | 780 | (3 | ) | 6 | 802 | ||||||||||||||||||||
Other Emerging ROW |
2,096 | 26 | 20 | 1,670 | 3 | 13 | 1,629 | |||||||||||||||||||||
Emerging Markets |
5,198 | 19 | 16 | 4,352 | 2 | 12 | 4,273 | |||||||||||||||||||||
Total |
33,269 | 1 | – | 32,804 | 4 | 7 | 31,601 | |||||||||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Geographical Review 71 |
Table of Contents
| 72 Geographical Review | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Geographical Review 73 |
Table of Contents
| 74 Geographical Review | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| World | US | Western Europe | Established ROW | Emerging Markets | Prior year | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reported | CER | Reported | Reported | CER | Reported | CER | Reported | CER | World | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sales | growth | growth | Sales | growth | Sales | growth | growth | Sales | growth | growth | Sales | growth | growth | sales | ||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | $m | % | % | $m | % | $m | % | % | $m | % | % | $m | % | % | $m | |||||||||||||||||||||||||||||||||||||||||||||
Aptium |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Oncology |
219 | (44 | ) | (44 | ) | 219 | (44 | ) | – | – | – | – | – | – | – | – | – | 393 | ||||||||||||||||||||||||||||||||||||||||||
Astra Tech |
535 | 6 | 7 | 101 | 22 | 393 | 2 | 4 | 38 | 6 | (3 | ) | 3 | 200 | 100 | 506 | ||||||||||||||||||||||||||||||||||||||||||||
Total |
754 | (16 | ) | (15 | ) | 320 | (33 | ) | 393 | 2 | 4 | 38 | 6 | (3 | ) | 3 | 200 | 100 | 899 | |||||||||||||||||||||||||||||||||||||||||
2009 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Aptium |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Oncology |
393 | (1 | ) | (1 | ) | 393 | (1 | ) | – | – | – | – | – | – | – | – | – | 395 | ||||||||||||||||||||||||||||||||||||||||||
Astra Tech |
506 | (4 | ) | 2 | 83 | 4 | 386 | (6 | ) | 2 | 36 | 10 | 20 | 1 | – | – | 529 | |||||||||||||||||||||||||||||||||||||||||||
Total |
899 | (3 | ) | 1 | 476 | – | 386 | (6 | ) | 2 | 36 | 10 | 20 | 1 | – | – | 924 | |||||||||||||||||||||||||||||||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Other Businesses 75 |
Table of Contents

Table of Contents

Table of Contents
business perform
financially in 2010?
in 2010 underlines
the resilience
and strength of
AstraZeneca’s
business
| 78 Financial Review | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| Contents | ||||
| 79 | Introduction |
|||
| 80 | ||||
| 81 | ||||
| 82 | ||||
| 84 | ||||
| 85 | ||||
| 86 | ||||
| 87 | ||||
| 87 | ||||
| 89 | ||||
| 89 | ||||
| 90 | ||||
| 90 | ||||
| 93 | ||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Review Introduction 79 |
Table of Contents
| > | Reported performance. Reported performance takes into account all the factors (including those which we cannot influence, principally currency exchange rates) that have affected the results of our business as reflected in our Group Financial Statements prepared in accordance with IFRS as adopted by the EU and as issued by the IASB. | |
| > | Core financial measures. These are non-GAAP measures because, unlike Reported performance, they cannot be derived directly from the information in the Group’s Financial Statements. These measures are adjusted to exclude certain significant items, such as charges and provisions related to our global restructuring programmes, amortisation and impairment of the significant intangibles relating to the acquisition of MedImmune in 2007, the amortisation and impairment of the significant intangibles relating to our current and future exit arrangements with Merck in the US and other specified items. See the 2010 Reconciliation of Reported results to Core results table on page 82 for a reconciliation of Reported to Core performance. | |
| > | Constant exchange rate (CER) growth rates. These are also non-GAAP measures. These measures remove the effects of currency movements (by retranslating the current year’s performance at previous year’s exchange rates and adjusting for other exchange effects, including hedging). A reconciliation of the Reported results adjusted for the impact of currency movements is provided in the 2010 Reported operating profit table on page 82. | |
| > | Core pre-R&D operating margin. This is a non-GAAP measure of our Core financial performance. A reconciliation of Core pre-R&D operating margin to our operating profit is provided on pages 82 and 88. | |
| > | Gross margin and operating profit margin percentages. These measures set out the progression of key performance margins and demonstrate the overall quality of the business. | |
| > | Prescription volumes and trends for key products. These measures can represent the real business growth and the progress of individual products better and more immediately than invoiced sales. | |
| > | Net funds/debt. This represents our cash and cash equivalents, current investments and derivative financial instruments less interest-bearing loans and borrowings. |
| 80 Financial Review Measuring Performance | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | The adverse impact on pharmaceutical prices as a result of the macroeconomic and regulatory environment. For instance, although there is no direct governmental control on prices in the US, action from federal and individual state programmes and health insurance bodies is leading to downward pressures on realised prices. In other parts of the world, there are a variety of price and volume control mechanisms and retrospective rebates based on sales levels that are imposed by governments. In 2010, we saw the introduction of the US healthcare reform legislation and government imposed price reductions in Western Europe (as detailed in the Pricing pressure section from page 11). | |
| > | The risk of generic competition following loss of patent protection or patent expiry or an ‘at risk’ launch by a competitor, with the potential adverse effects on sales volumes and prices. For example in 2010, our performance was affected by generic competition in the US for Arimidex, Pulmicort Respules and Toprol-XL. Further details of the impact of patent expiry on our revenue streams are included in the Patent expiries section on page 31. | |
| > | The timings of new product launches, which can be influenced by national regulators, and the risk that such new products do not succeed as anticipated, together with the rate of sales growth and costs following new product launches. | |
| > | Currency fluctuations. Our functional and reporting currency is the US dollar, but we have substantial exposures to other currencies, in particular the euro, Japanese yen, pound sterling and Swedish krona. | |
| > | Macro factors such as greater demand from an ageing population and increasing requirements of servicing Emerging Markets. |
| > | Reported revenue of $33,269 million was unchanged (Reported: up 1%). | |
| > | Strong revenue growth in markets outside the US broadly offset the loss of more than $1.6 billion of revenue in the US from generic competition on several products and the absence of H1N1 pandemic influenza vaccine revenue. | |
| > | Strong double-digit sales growth at CER for Crestor, Symbicort and Seroquel XR. Crestor and Seroquel franchise sales now exceed $5 billion each for the full year. | |
| > | Revenue in Emerging Markets grew to over $5.1 billion, a 16% increase (Reported: 19%). Sales in China increased to over $1.0 billion. | |
| > | Core operating profit for the full year was unchanged on both a Reported and a CER basis at $13,603 million. Operating profit decreased by 1% (Reported: unchanged). | |
| > | Excluded from Core results were specific legal provisions of $612 million (which impacted Reported results in the year) mainly in respect of the ongoing Seroquel product liability litigation and state attorney general investigations into sales and marketing practices, and a gain of $791 million arising from changes made to benefits under certain of the Group’s post-retirement plans, chiefly the Group’s UK pension plan. | |
| > | Basic EPS of $5.60 represented an increase of 7% (Reported: 8%). Core EPS for the full year increased by 5% to $6.71 (Reported: 6%). | |
| > | Net cash inflow from operating activities was $10,680 million (2009: $11,739 million). | |
| > | Dividends paid increased to $3,361 million (2009: $2,977 million). | |
| > | Net funds at 31 December were $3,653 million, an improvement of $3,118 million on $535 million in the previous year. | |
| > | Total restructuring costs associated with the global programme to reshape the cost base of the business were $1,202 million in 2010 (2009: $659 million). This brings the total restructuring costs charged to date to $3,708 million. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Review 2010 Business background and results overview 81 |
Table of Contents
| 2010 | 2009 | Percentage of sales | 2010 compared with 2009 | |||||||||||||||||||||||||||||
| Growth | ||||||||||||||||||||||||||||||||
| due to | ||||||||||||||||||||||||||||||||
| CER | exchange | Reported | Reported | CER | Reported | |||||||||||||||||||||||||||
| Reported | growth | effects | Reported | 2010 | 2009 | growth | growth | |||||||||||||||||||||||||
| $m | $m | $m | $m | % | % | % | % | |||||||||||||||||||||||||
Revenue |
33,269 | 164 | 301 | 32,804 | – | 1 | ||||||||||||||||||||||||||
Cost of sales |
(6,389 | ) | (497 | ) | (117 | ) | (5,775 | ) | (19.2 | ) | (17.6 | ) | 9 | 11 | ||||||||||||||||||
Gross profit |
26,880 | (333 | ) | 184 | 27,029 | 80.8 | 82.4 | (1 | ) | (1 | ) | |||||||||||||||||||||
Distribution costs |
(335 | ) | (31 | ) | (6 | ) | (298 | ) | (1.0 | ) | (0.9 | ) | 10 | 12 | ||||||||||||||||||
Research and development |
(5,318 | ) | (871 | ) | (38 | ) | (4,409 | ) | (16.0 | ) | (13.5 | ) | 20 | 21 | ||||||||||||||||||
Selling, general and administrative costs |
(10,445 | ) | 955 | (68 | ) | (11,332 | ) | (31.4 | ) | (34.5 | ) | (8 | ) | (8 | ) | |||||||||||||||||
Other operating income and expense |
712 | 159 | – | 553 | 2.1 | 1.7 | 29 | 29 | ||||||||||||||||||||||||
Operating profit |
11,494 | (121 | ) | 72 | 11,543 | 34.5 | 35.2 | (1 | ) | – | ||||||||||||||||||||||
Net finance expense |
(517 | ) | (736 | ) | ||||||||||||||||||||||||||||
Profit before tax |
10,977 | 10,807 | ||||||||||||||||||||||||||||||
Taxation |
(2,896 | ) | (3,263 | ) | ||||||||||||||||||||||||||||
Profit for the period |
8,081 | 7,544 | ||||||||||||||||||||||||||||||
Basic earnings per share ($) |
5.60 | 5.19 | ||||||||||||||||||||||||||||||
2010 Core operating results |
||||||||||||||||||||||||||||||||
| 2010 | 2009 | 2010 compared with 2009 | ||||||||||||||||||||||||||||||
| Growth | ||||||||||||||||||||||||||||||||
| due to | Total | |||||||||||||||||||||||||||||||
| CER | exchange | CER | Core | |||||||||||||||||||||||||||||
| Core | growth | effects | Core | growth | growth | |||||||||||||||||||||||||||
| $m | $m | $m | $m | % | % | |||||||||||||||||||||||||||
Gross margin |
27,024 | (386 | ) | 193 | 27,217 | (1 | ) | (1 | ) | |||||||||||||||||||||||
Distribution costs |
(335 | ) | (30 | ) | (7 | ) | (298 | ) | 10 | 12 | ||||||||||||||||||||||
Research and development |
(4,219 | ) | 176 | (61 | ) | (4,334 | ) | (4 | ) | (3 | ) | |||||||||||||||||||||
Selling, general and administrative costs |
(9,777 | ) | 190 | (77 | ) | (9,890 | ) | (2 | ) | (1 | ) | |||||||||||||||||||||
Other operating income and expense |
910 | (16 | ) | – | 926 | (2 | ) | (2 | ) | |||||||||||||||||||||||
Operating profit |
13,603 | (66 | ) | 48 | 13,621 | – | – | |||||||||||||||||||||||||
Net finance expense |
(517 | ) | (736 | ) | ||||||||||||||||||||||||||||
Profit before tax |
13,086 | 12,885 | ||||||||||||||||||||||||||||||
Taxation |
(3,416 | ) | (3,703 | ) | ||||||||||||||||||||||||||||
Profit for the period |
9,670 | 9,182 | ||||||||||||||||||||||||||||||
Basic earnings per share ($) |
6.71 | 6.32 | ||||||||||||||||||||||||||||||
| Merck & MedImmune | ||||||||||||||||||||||||||||||||
| Post- | ||||||||||||||||||||||||||||||||
| retirement | ||||||||||||||||||||||||||||||||
| 2010 Restructuring | Intangible | Legal | plan | |||||||||||||||||||||||||||||
| Reported | costs | Amortisation | impairments | provisions | amendments | 2010 Core | ||||||||||||||||||||||||||
| $m | $m | $m | $m | $m | $m | $m | ||||||||||||||||||||||||||
Gross margin |
26,880 | 144 | – | – | – | – | 27,024 | |||||||||||||||||||||||||
Distribution costs |
(335 | ) | – | – | – | – | – | (335 | ) | |||||||||||||||||||||||
Research and development |
(5,318 | ) | 654 | – | 445 | – | – | (4,219 | ) | |||||||||||||||||||||||
Selling, general and administrative costs |
(10,445 | ) | 404 | 443 | – | 612 | (791 | ) | (9,777 | ) | ||||||||||||||||||||||
Other operating income and expense |
712 | – | 75 | 123 | – | – | 910 | |||||||||||||||||||||||||
Operating profit |
11,494 | 1,202 | 518 | 568 | 612 | (791 | ) | 13,603 | ||||||||||||||||||||||||
Add back: Research and development |
5,318 | (654 | ) | – | (445 | ) | – | – | 4,219 | |||||||||||||||||||||||
Pre-R&D operating margin |
16,812 | 548 | 518 | 123 | 612 | (791 | ) | 17,822 | ||||||||||||||||||||||||
Net finance expense |
(517 | ) | – | – | – | – | – | (517 | ) | |||||||||||||||||||||||
Profit before tax |
10,977 | 1,202 | 518 | 568 | 612 | (791 | ) | 13,086 | ||||||||||||||||||||||||
Taxation |
(2,896 | ) | (317 | ) | (100 | ) | (150 | ) | (162 | ) | 209 | (3,416 | ) | |||||||||||||||||||
Profit for the period |
8,081 | 885 | 418 | 418 | 450 | (582 | ) | 9,670 | ||||||||||||||||||||||||
Basic earnings per share ($) |
5.60 | 0.62 | 0.29 | 0.29 | 0.31 | (0.40 | ) | 6.71 | ||||||||||||||||||||||||
| 82 Financial Review Results of operations – summary analysis of year to 31 December 2010 | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | impairment charges of $568 million, arising from impairments in respect of motavizumab ($445 million) and our HPV cervical cancer vaccine income stream ($123 million), both capitalised as part of the MedImmune acquisition. Total impairment charges relating to intangible fixed assets were $833 million in the year. |
| > | $612 million of legal provision charges, of which $592 million is in respect of the ongoing Seroquel product liability litigation and state attorney general investigations into sales and marketing practices in aggregate. In line with prior years these have been excluded from our Core performance and full details of these matters are included in Note 25 to the Financial Statements from page 178. | |
| > | restructuring costs totalling $1,202 million, incurred as the Group continues its previously announced efficiency programmes. | |
| > | amortisation totalling $518 million relating to assets capitalised as part of the MedImmune acquisition and the Merck exit arrangements. | |
| > | a credit of $791 million chiefly attributable to a curtailment gain related to changes made to benefits under the Group’s UK pension arrangements. In 2010, we amended our UK defined benefit fund. Pensionable pay was frozen at its 30 June 2010 level but the defined benefit fund remains open to existing members. Members of the pension fund were given the option of remaining in the fund or leaving the fund. Those that chose to leave the fund were offered funding which they could contribute to a new Group Self Invested Personal Pension Plan. This change to the UK defined benefit scheme represented an accounting curtailment of certain pension obligations and, in accordance with IAS 19 ‘Employee Benefits’, these obligations were revalued by the scheme actuaries immediately prior to the curtailment and the assumptions updated at that date. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Review Results of operations – summary analysis of year to 31 December 2010 83 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Net funds/(debt) brought forward at 1 January |
535 | (7,174 | ) | (9,112 | ) | |||||||
Earnings before interest, tax, depreciation, amortisation and impairment |
14,235 | 13,630 | 11,764 | |||||||||
Movement in working capital and provisions |
82 | 1,329 | (210 | ) | ||||||||
Tax paid |
(2,533 | ) | (2,381 | ) | (2,209 | ) | ||||||
Interest paid |
(641 | ) | (639 | ) | (690 | ) | ||||||
Other non-cash movements |
(463 | ) | (200 | ) | 87 | |||||||
Net cash available from operating activities |
10,680 | 11,739 | 8,742 | |||||||||
Purchase of intangibles (net) |
(1,180 | ) | (355 | ) | (2,944 | ) | ||||||
Other capital expenditure (net) |
(708 | ) | (824 | ) | (1,057 | ) | ||||||
Acquisitions |
(348 | ) | – | – | ||||||||
Investments |
(2,236 | ) | (1,179 | ) | (4,001 | ) | ||||||
Dividends |
(3,361 | ) | (2,977 | ) | (2,739 | ) | ||||||
Net share (repurchases)/issues |
(2,110 | ) | 135 | (451 | ) | |||||||
Distributions |
(5,471 | ) | (2,842 | ) | (3,190 | ) | ||||||
Other movements |
145 | (9 | ) | 387 | ||||||||
Net funds/(debt) carried forward at 31 December |
3,653 | 535 | (7,174 | ) | ||||||||
Comprised of: |
||||||||||||
Cash, short-term investments and derivatives (net) |
12,875 | 11,598 | 4,674 | |||||||||
Loans and borrowings |
(9,222 | ) | (11,063 | ) | (11,848 | ) | ||||||
| Less than | Over | |||||||||||||||||||
| 1 year | 1-3 years | 3-5 years | 5 years | Total | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Bank loans and other borrowings |
646 | 2,691 | 2,532 | 10,095 | 15,964 | |||||||||||||||
Operating leases |
161 | 137 | 105 | 103 | 506 | |||||||||||||||
Contracted capital expenditure |
259 | – | – | – | 259 | |||||||||||||||
Total |
1,066 | 2,828 | 2,637 | 10,198 | 16,729 | |||||||||||||||
| 84 Financial Review Cash flow and liquidity – 2010 | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | Movement | 2009 | Movement | 2008 | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Property, plant and equipment |
6,957 | (350 | ) | 7,307 | 264 | 7,043 | ||||||||||||||
Goodwill and intangible assets |
22,029 | (86 | ) | 22,115 | (82 | ) | 22,197 | |||||||||||||
Inventories |
1,682 | (68 | ) | 1,750 | 114 | 1,636 | ||||||||||||||
Trade and other receivables |
7,847 | 138 | 7,709 | 448 | 7,261 | |||||||||||||||
Trade and other payables |
(9,034 | ) | (103 | ) | (8,931 | ) | (1,604 | ) | (7,327 | ) | ||||||||||
Provisions |
(1,938 | ) | (252 | ) | (1,686 | ) | (544 | ) | (1,142 | ) | ||||||||||
Net income tax payable |
(3,855 | ) | (1,002 | ) | (2,853 | ) | (885 | ) | (1,968 | ) | ||||||||||
Net deferred tax liabilities |
(1,670 | ) | 285 | (1,955 | ) | (65 | ) | (1,890 | ) | |||||||||||
Retirement benefit obligations |
(2,472 | ) | 882 | (3,354 | ) | (622 | ) | (2,732 | ) | |||||||||||
Non-current other investments |
211 | 27 | 184 | 28 | 156 | |||||||||||||||
Net funds/(debt) |
3,653 | 3,118 | 535 | 7,709 | (7,174 | ) | ||||||||||||||
Net assets |
23,410 | 2,589 | 20,821 | 4,761 | 16,060 | |||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Review Financial position – 2010 85 |
Table of Contents
| > | In January 2007, AstraZeneca signed an exclusive co-development and co-promotion agreement with BMS for the development and commercialisation of saxagliptin, a dipeptidyl peptidase IV inhibitor (DPP-IV) for the treatment of Type 2 diabetes, and dapagliflozin, a selective sodium-glucose co-transporter 2 (SGLT2) inhibitor. The agreement is global (with the exception of Japan) for saxagliptin. Under each agreement the two companies jointly develop the clinical and marketing strategy and share development and commercialisation expenses on a global basis. To date, AstraZeneca has made upfront and milestone payments totalling $300 million for saxagliptin and $50 million for dapagliflozin and may make future milestone payments of $350 million on dapagliflozin contingent on achievement of regulatory milestones and launch in key markets. | |
| Following launch, profits and losses globally are shared equally and an additional $300 million of sales-related payments for each product may be triggered based on worldwide sales success. The Group made milestone payments to BMS of $50 million in 2010, $150 million in 2009 and $50 million in 2008. | ||
| > | In December 2009, AstraZeneca and Targacept entered into an in-licence agreement for AstraZeneca to obtain exclusive global development and commercialisation rights to Targacept’s investigational product for major depressive disorder (MDD), TC-5214. TC-5214, which recently completed a Phase IIb clinical trial, is a nicotinic channel blocker that is thought to treat depression by modulating the activity of various neuronal nicotinic receptor (NNR) subtypes. Under the deal, AstraZeneca made an upfront payment of $200 million and may make milestone payments to a maximum of $540 million up to launch. In addition, Targacept will be entitled to receive royalties on worldwide product sales and additional milestone payments linked to worldwide product sales. |
| $ | Pence | SEK | Payment date | ||||||||||||||
First interim dividend |
0.70 | 44.9 | 5.12 | 13.09.10 | |||||||||||||
Second interim dividend |
1.85 | 116.7 | 11.99 | 14.03.11 | |||||||||||||
Total |
2.55 | 161.6 | 17.11 | ||||||||||||||
| Shares | Dividend per | Dividend | Shareholder | |||||||||||||||||
| repurchased | Cost | share | cost | distributions | ||||||||||||||||
| (million) | $m | $ | $m | $m | ||||||||||||||||
2000 |
9.4 | 352 | 0.70 | 1,236 | 1,588 | |||||||||||||||
2001 |
23.5 | 1,080 | 0.70 | 1,225 | 2,305 | |||||||||||||||
2002 |
28.3 | 1,190 | 0.70 | 1,206 | 2,396 | |||||||||||||||
2003 |
27.2 | 1,154 | 0.795 | 1,350 | 2,504 | |||||||||||||||
2004 |
50.1 | 2,212 | 0.94 | 1,555 | 3,767 | |||||||||||||||
2005 |
67.7 | 3,001 | 1.30 | 2,068 | 5,069 | |||||||||||||||
2006 |
72.2 | 4,147 | 1.72 | 2,649 | 6,796 | |||||||||||||||
2007 |
79.9 | 4,170 | 1.87 | 2,740 | 6,910 | |||||||||||||||
2008 |
13.6 | 610 | 2.05 | 2,971 | 3,581 | |||||||||||||||
2009 |
– | – | 2.30 | 3,339 | 3,339 | |||||||||||||||
2010 |
53.7 | 2,604 | 2.55 | 3,617 | 1 | 6,221 | ||||||||||||||
Total |
425.6 | 20,520 | 15.625 | 23,956 | 44,476 | |||||||||||||||
| 1 Total dividend cost estimated based upon number of shares in issue at 31 December 2010. | ||
| 86 Financial Review Capitalisation and shareholder return | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2009 | 2008 | Percentage of sales | 2009 compared with 2008 | |||||||||||||||||||||||||||||
| Growth | ||||||||||||||||||||||||||||||||
| due to | ||||||||||||||||||||||||||||||||
| CER | exchange | Reported | Reported | CER | Reported | |||||||||||||||||||||||||||
| Reported | growth | effects | Reported | 2009 | 2008 | growth | growth | |||||||||||||||||||||||||
| $m | $m | $m | $m | % | % | % | % | |||||||||||||||||||||||||
Revenue |
32,804 | 2,317 | (1,114 | ) | 31,601 | 7 | 4 | |||||||||||||||||||||||||
Cost of sales |
(5,775 | ) | 540 | 283 | (6,598 | ) | (17.6 | ) | (20.9 | ) | (8 | ) | (12 | ) | ||||||||||||||||||
Gross profit |
27,029 | 2,857 | (831 | ) | 25,003 | 82.4 | 79.1 | 11 | 8 | |||||||||||||||||||||||
Distribution costs |
(298 | ) | (37 | ) | 30 | (291 | ) | (0.9 | ) | (0.9 | ) | 13 | 3 | |||||||||||||||||||
Research and development |
(4,409 | ) | 298 | 472 | (5,179 | ) | (13.5 | ) | (16.4 | ) | (6 | ) | (15 | ) | ||||||||||||||||||
Selling, general and administrative costs |
(11,332 | ) | (945 | ) | 526 | (10,913 | ) | (34.5 | ) | (34.6 | ) | 9 | 4 | |||||||||||||||||||
Other operating income and expense |
553 | 33 | (4 | ) | 524 | 1.7 | 1.7 | 6 | 6 | |||||||||||||||||||||||
Operating profit |
11,543 | 2,206 | 193 | 9,144 | 35.2 | 28.9 | 24 | 26 | ||||||||||||||||||||||||
Net finance expense |
(736 | ) | (463 | ) | ||||||||||||||||||||||||||||
Profit before tax |
10,807 | 8,681 | ||||||||||||||||||||||||||||||
Taxation |
(3,263 | ) | (2,551 | ) | ||||||||||||||||||||||||||||
Profit for the period |
7,544 | 6,130 | ||||||||||||||||||||||||||||||
Basic earnings per share ($) |
5.19 | 4.20 | ||||||||||||||||||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Review Future prospects 87 |
Table of Contents
| 2009 | 2008 | 2009 compared with 2008 | ||||||||||||||||||||||
| Growth | ||||||||||||||||||||||||
| due to | Total | |||||||||||||||||||||||
| CER | exchange | CER | Core | |||||||||||||||||||||
| Core | growth | effects | Core | growth | growth | |||||||||||||||||||
| $m | $m | $m | $m | % | % | |||||||||||||||||||
Gross margin |
27,217 | 2,660 | (851 | ) | 25,408 | 10 | 7 | |||||||||||||||||
Distribution costs |
(298 | ) | (37 | ) | 30 | (291 | ) | 13 | 3 | |||||||||||||||
Research and development |
(4,334 | ) | 150 | 469 | (4,953 | ) | (3 | ) | (13 | ) | ||||||||||||||
Selling, general and administrative costs |
(9,890 | ) | (452 | ) | 502 | (9,940 | ) | 5 | (1 | ) | ||||||||||||||
Other operating income and expense |
926 | 194 | (2 | ) | 734 | 26 | 26 | |||||||||||||||||
Operating profit |
13,621 | 2,515 | 148 | 10,958 | 23 | 24 | ||||||||||||||||||
Net finance expense |
(736 | ) | (463 | ) | ||||||||||||||||||||
Profit before tax |
12,885 | 10,495 | ||||||||||||||||||||||
Taxation |
(3,703 | ) | (3,056 | ) | ||||||||||||||||||||
Profit for the period |
9,182 | 7,439 | ||||||||||||||||||||||
Basic earnings per share ($) |
6.32 | 5.10 | ||||||||||||||||||||||
| Merck & MedImmune | ||||||||||||||||||||||||
| 2009 | Restructuring | Intangible | Legal | |||||||||||||||||||||
| Reported | costs | Amortisation | impairments | provisions | 2009 Core | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
Gross margin |
27,029 | 188 | – | – | – | 27,217 | ||||||||||||||||||
Distribution costs |
(298 | ) | – | – | – | – | (298 | ) | ||||||||||||||||
Research and development |
(4,409 | ) | 68 | – | 7 | – | (4,334 | ) | ||||||||||||||||
Selling, general and administrative costs |
(11,332 | ) | 403 | 403 | – | 636 | (9,890 | ) | ||||||||||||||||
Other operating income and expense |
553 | – | 108 | 265 | – | 926 | ||||||||||||||||||
Operating profit |
11,543 | 659 | 511 | 272 | 636 | 13,621 | ||||||||||||||||||
| Add back: Research and development | 4,409 | (68 | ) | – | (7 | ) | – | 4,334 | ||||||||||||||||
| Pre-R&D operating margin | 15,952 | 591 | 511 | 265 | 636 | 17,955 | ||||||||||||||||||
Net finance expense |
(736 | ) | – | – | – | – | (736 | ) | ||||||||||||||||
Profit before tax |
10,807 | 659 | 511 | 272 | 636 | 12,885 | ||||||||||||||||||
Taxation |
(3,263 | ) | (199 | ) | (125 | ) | (82 | ) | (34 | ) | (3,703 | ) | ||||||||||||
Profit for the period |
7,544 | 460 | 386 | 190 | 602 | 9,182 | ||||||||||||||||||
Basic earnings per share ($) |
5.19 | 0.32 | 0.27 | 0.13 | 0.41 | 6.32 | ||||||||||||||||||
| 88 Financial Review Results of operations – summary analysis of year to 31 December 2009 | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Review Cash flow and liquidity – 2009 89 |
Table of Contents
| > | Revenue recognition | |
| > | Research and development | |
| > | Impairment testing of goodwill and intangible assets | |
| > | Litigation | |
| > | Post-retirement benefits | |
| > | Taxation | |
| > | Segmental reporting. |
| > | Chargebacks, where we enter into arrangements under which certain parties, typically hospitals, the Department of Veterans Affairs, Public Health Service Covered Entities and the Department of Defense, are able to buy products from wholesalers at the lower prices we have contracted with them. The chargeback is the difference between the price we invoice to the wholesaler and the contracted price charged by the wholesaler. Chargebacks are paid directly to the wholesalers. | |
| > | Regulatory, including Medicaid and other federal and state programmes, where we pay rebates based on the specific terms of agreements with the US Department of Health and Human Services and with individual states, which include product usage and information on best prices and average market prices benchmarks. | |
| > | Contractual, under which entities such as third party managed-care organisations, long-term care facilities and group purchasing organisations are entitled to rebates depending on specified performance provisions, which vary from contract to contract. |
| 90 Financial Review Financial risk management | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Gross sales |
22,909 | 22,646 | 20,029 | |||||||||
Chargebacks |
(2,075 | ) | (1,841 | ) | (1,726 | ) | ||||||
Regulatory – US government and state programmes |
(1,949 | ) | (1,357 | ) | (1,005 | ) | ||||||
Contractual – Managed-care and group purchasing organisation rebates |
(4,755 | ) | (4,752 | ) | (3,658 | ) | ||||||
Cash and other discounts |
(437 | ) | (428 | ) | (390 | ) | ||||||
Customer returns |
(21 | ) | (193 | ) | (48 | ) | ||||||
Other |
(265 | ) | (196 | ) | (167 | ) | ||||||
Net sales |
13,407 | 13,879 | 13,035 | |||||||||
| Brought | Carried | |||||||||||||||||||
| forward at | Adjustment in | forward at | ||||||||||||||||||
| 1 January | Provision for | respect of | Returns and | 31 December | ||||||||||||||||
| 2010 | current year | prior years | payments | 2010 | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Chargebacks |
396 | 2,107 | (32 | ) | (1,948 | ) | 523 | |||||||||||||
Regulatory – US government and state programmes |
775 | 1,984 | (35 | ) | (1,602 | ) | 1,122 | |||||||||||||
Contractual – Managed-care and group purchasing organisation rebates |
1,447 | 4,826 | (71 | ) | (5,008 | ) | 1,194 | |||||||||||||
Cash and other discounts |
41 | 438 | (1 | ) | (437 | ) | 41 | |||||||||||||
Customer returns |
177 | 22 | (1 | ) | (65 | ) | 133 | |||||||||||||
Other |
59 | 269 | (4 | ) | (260 | ) | 64 | |||||||||||||
Total |
2,895 | 9,646 | (144 | ) | (9,320 | ) | 3,077 | |||||||||||||
| Brought | Carried | |||||||||||||||||||
| forward at | Adjustment in | forward at | ||||||||||||||||||
| 1 January | Provision for | respect of | Returns and | 31 December | ||||||||||||||||
| 2009 | current year | prior years | payments | 2009 | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Chargebacks |
359 | 1,947 | (106 | ) | (1,804 | ) | 396 | |||||||||||||
Regulatory – US government and state programmes |
520 | 1,373 | (16 | ) | (1,102 | ) | 775 | |||||||||||||
Contractual – Managed-care and group purchasing organisation rebates |
1,084 | 4,732 | 20 | (4,389 | ) | 1,447 | ||||||||||||||
Cash and other discounts |
39 | 428 | – | (426 | ) | 41 | ||||||||||||||
Customer returns |
77 | 194 | (1 | ) | (93 | ) | 177 | |||||||||||||
Other |
57 | 198 | (2 | ) | (194 | ) | 59 | |||||||||||||
Total |
2,136 | 8,872 | (105 | ) | (8,008 | ) | 2,895 | |||||||||||||
| Brought | Carried | |||||||||||||||||||
| forward at | Adjustment in | forward at | ||||||||||||||||||
| 1 January | Provision for | respect of | Returns and | 31 December | ||||||||||||||||
| 2008 | current year | prior years | payments | 2008 | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Chargebacks |
186 | 1,745 | (19 | ) | (1,553 | ) | 359 | |||||||||||||
Regulatory – US government and state programmes |
428 | 997 | 8 | (913 | ) | 520 | ||||||||||||||
Contractual – Managed-care and group purchasing organisation rebates |
900 | 3,622 | 36 | (3,474 | ) | 1,084 | ||||||||||||||
Cash and other discounts |
38 | 390 | – | (389 | ) | 39 | ||||||||||||||
Customer returns |
85 | 48 | – | (56 | ) | 77 | ||||||||||||||
Other |
53 | 167 | – | (163 | ) | 57 | ||||||||||||||
Total |
1,690 | 6,969 | 25 | (6,548 | ) | 2,136 | ||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Review Critical accounting policies and estimates 91 |
Table of Contents
| 92 Financial Review Critical accounting policies and estimates | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Review Sarbanes-Oxley Act section 404 93 |
Table of Contents
approach to risk
management?
decision making as
to which risks we
take and how we
manage them
| 94 Risk | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Risk 95 |
Table of Contents
| > | Group Internal Audit (GIA) – independent assurance reports on the Group’s risk management and control framework. | |
| > | Global Compliance – compliance programme reports on key compliance risks, updates on key compliance initiatives, performance against the Global Compliance scorecard, compliance incidents and investigations including calls made by employees to the AZethics and MedImmune helplines. | |
| > | Financial Control and Compliance Group – reports on Sarbanes-Oxley Act compliance and the financial control framework. | |
| > | Management – the Group-level risk summary from the annual business planning process and QBRs and reports on the performance management and monitoring processes. |
| > | Failure to obtain the required regulatory or marketing approvals for the product candidate or the facilities in which it is manufactured. | |
| > | Unfavourable data from key studies. | |
| > | Adverse reactions to the product candidate or indications of other safety concerns. | |
| > | Failure of R&D to develop new and differentiated product candidates. | |
| > | Failure to demonstrate adequately cost-effective benefits to regulators. | |
| > | The emergence of competing products. |
| 96 Risk | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
for new products

| AstraZeneca Annual Report and Form 20-F Information 2010 | Risk 97 |
Table of Contents
| > | Inability to manufacture sufficient quantities of the product candidate for development or commercialisation activities in a timely and cost-efficient manner. | |
| > | Excessive costs of, or difficulty in, manufacturing. | |
| > | Erosion of patent term and other intellectual property rights, and infringement of those rights and the intellectual property rights owned by third parties. | |
| > | Failure to show value or a differentiated profile for our products. |
| > | more volatile economic conditions | |
| > | competition from companies that are already present in the market | |
| > | the need to identify correctly and to leverage appropriate opportunities for sales and marketing | |
| > | poor protection of intellectual property | |
| > | inadequate protection against crime (including counterfeiting, corruption and fraud) | |
| > | the need to impose developed market compliance standards in emerging markets | |
| > | inadvertent breaches of local and international law/regulation | |
| > | not being able to recruit sufficient personnel with appropriate skills and experience | |
| > | identification of the most appropriate and effective sales channels and route to market interventions by national governments or regulators to restrict access to a market and/or to introduce adverse price controls. |
| 98 Risk | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Risk 99 |
Table of Contents
| 100 Risk | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Risk 101 |
Table of Contents
| 102 Risk | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Risk 103 |
Table of Contents

Table of Contents
structured and
managed?
structure in which
the Board reserves
and delegates
its powers
| AstraZeneca Annual Report and Form 20-F Information 2010 | Corporate Governance 105 |
Table of Contents

| 106 Board of Directors and Senior Executive Team | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents


| AstraZeneca Annual Report and Form 20-F Information 2010 | Board of Directors and Senior Executive Team 107 |
Table of Contents

| 108 Board of Directors and Senior Executive Team | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents


| AstraZeneca Annual Report and Form 20-F Information 2010 | Corporate Governance Report 109 |
Table of Contents
The Nomination and Governance Committee and, where appropriate, the full Board regularly review the composition of the Board and the status of succession to both senior executive management and Board-level positions. Directors have regular contact with, and access to, succession candidates for senior executive management positions.
| > | Bo Angelin and John Buchanan, both Non-Executive Directors, retired from the Board on 29 April 2010. | |
| > | Bruce Burlington was appointed as a Non-Executive Director and member of the Science Committee with effect from 1 August 2010. | |
| > | Shriti Vadera was appointed as a Non-Executive Director and a member of the Audit Committee with effect from 1 January 2011. |
| 110 Corporate Governance Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Name | Board | Audit | Remuneration | Nomination and Governance | ||||||||||||
Bo Angelin1 |
2 | (2) | – | – | – | |||||||||||
David Brennan |
6 | (6) | – | – | – | |||||||||||
John Buchanan2 |
2 | (2) | 2 (2) | 1 (3) | – | |||||||||||
Bruce Burlington3 |
3 | (3) | – | – | – | |||||||||||
Jean-Philippe Courtois |
5 | (6) | 3 (4) | – | – | |||||||||||
Jane Henney |
5 | (6) | 4 (4) | – | 4 (6) | |||||||||||
Michele Hooper |
6 | (6) | 4 (4) | – | 6 (6) | |||||||||||
Simon Lowth |
6 | (6) | – | – | – | |||||||||||
Rudy Markham4 |
6 | (6) | 3 (4) | 4 (4) | – | |||||||||||
Nancy Rothwell |
5 | (6) | – | 6 (7) | – | |||||||||||
Louis Schweitzer |
6 | (6) | – | 7 (7) | 6 (6) | |||||||||||
John Varley |
5 | (6) | – | 7 (7) | 5 (6) | |||||||||||
Marcus Wallenberg5 |
4 | (6) | – | – | – | |||||||||||
| 1 | Bo Angelin retired from the Board on 29 April 2010. | |
| 2 | John Buchanan retired from the Board on 29 April 2010. | |
| 3 | Bruce Burlington was appointed as a Director with effect from 1 August 2010. | |
| 4 | Rudy Markham was appointed as a member of the Remuneration Committee with effect from 29 April 2010. | |
| 5 | Marcus Wallenberg was appointed as a member of the Science Committee with effect from 28 April 2010. |
The Non-Executive Directors have various responsibilities concerning the integrity of financial information, internal controls and risk management.

| AstraZeneca Annual Report and Form 20-F Information 2010 | Corporate Governance Report 111 |
Table of Contents
| Nomination and | ||||||||||||||||||||
| Name | Audit | Remuneration | Governance | Science | Independent1 | |||||||||||||||
Bo Angelin2 |
ü | ü | ||||||||||||||||||
David Brennan |
n/a | |||||||||||||||||||
John Buchanan3 |
Chair3 | ü | ü | |||||||||||||||||
Bruce Burlington |
ü | ü | ||||||||||||||||||
Jean-Philippe Courtois |
ü | ü | ||||||||||||||||||
Jane Henney |
ü | ü | ü | ü | ||||||||||||||||
Michele Hooper4 |
Chair3 | ü | ü | |||||||||||||||||
Simon Lowth |
n/a | |||||||||||||||||||
Rudy Markham |
ü | ü | 5 | ü | ||||||||||||||||
Nancy Rothwell |
ü | Chair | ü | |||||||||||||||||
Louis Schweitzer |
ü | Chair | n/a | 6 | ||||||||||||||||
John Varley |
Chair | ü | ü | |||||||||||||||||
Marcus Wallenberg |
ü | 7 | û | |||||||||||||||||
| 1 | As determined by the Board for UK Corporate Governance Codes purposes. | |
| 2 | Bo Angelin retired from the Board on 29 April 2010. | |
| 3 | John Buchanan retired from the Board on 29 April 2010. Michele Hooper was appointed Chairman of the Audit Committee with effect from 29 April 2010. | |
| 4 | Michele Hooper is the Senior independent Non-Executive Director. | |
| 5 | Rudy Markham was appointed as a member of the Remuneration Committee with effect from 29 April 2010. | |
| 6 | Louis Schweitzer was considered independent by the Board upon his appointment as Chairman; in accordance with the UK Corporate Governance Codes, the test of independence is not appropriate in relation to the Chairman after his appointment. | |
| 7 | Marcus Wallenberg was appointed as a member of the Science Committee with effect from 28 April 2010. |
| 112 Corporate Governance Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | Matters relating to the audit plans of the external auditor and GIA as well as oversight of the work of the Global Compliance function. | |
| > | Our overall framework for internal control over financial reporting and for other internal controls and processes. | |
| > | Our overall framework for risk management, particularly financial risks. | |
| > | Our accounting policies and practices. | |
| > | Our annual and quarterly financial reporting. | |
| > | Compliance with the Corporate Integrity Agreement. |
| > | Our financial disclosures were reviewed and various accounting matters considered. | |
| > | Reports were received from the external auditor concerning its audit of the Financial Statements of the Group and from management, GIA, Global Compliance and the external auditor on the effectiveness of our system of internal controls and, in particular, our internal control over financial reporting. This included review and discussion of the results of the ‘continuous assurance’ and annual ‘letter of assurance’ processes. The Committee also reviewed quarterly activity reports of audit work carried out by GIA and the status of follow-up actions with management as well as reports from the Global Compliance function. | |
| > | The systems and processes that management has developed pertaining to risk identification, classification and mitigation. | |
| > | Continuing work to comply with the applicable provisions of the Sarbanes-Oxley Act. In particular, the Committee regularly reviewed the status of compliance with the programme of internal controls over financial reporting implemented pursuant to section 404 of the Sarbanes-Oxley Act. Further information about this is set out in the Sarbanes-Oxley Act section 404 section on page 93. | |
| > | Data about calls made by employees via the AZethics telephone lines and other routes regarding potential breaches of the Code of Conduct together with the results of inquiries into these matters. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Corporate Governance Report 113 |
Table of Contents
| > | Quarterly reports were received from the compliance officer responsible for monitoring the US business’s compliance with the Corporate Integrity Agreement. | |
| > | Accounting issues relevant to litigation and taxation matters. | |
| > | Reports from the Group Treasury function and, in particular, the Group’s liquidity and cash position and the appropriateness of its cash management policies in the context of the current economic situation. | |
| > | Other reports concerning the GIA, Global Compliance and Finance functions, including the internal audit plan and progress and plans of the Global Compliance Officer. | |
| > | The amount of audit and non-audit fees of the external auditor throughout 2010. The Committee was satisfied throughout the year that the objectivity and independence of the external auditor were not in any way impaired by the nature of the non-audit work undertaken by the external auditor during the year, the level of non-audit fees charged for such work or any other facts or circumstances. Further information about the audit and non-audit fees for 2010 is disclosed in Note 27 to the Financial Statements on page 196. | |
| > | A review and assessment of the Committee’s performance which concluded that such performance was satisfactory. |
| 114 Corporate Governance Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | The approaches we adopt in respect of our chosen Therapy Areas | |
| > | The scientific technology and R&D capabilities deployed | |
| > | The decision making processes for R&D projects and programmes | |
| > | The quality of our scientists. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Corporate Governance Report 115 |
Table of Contents
The CEO has established and chairs the SET. The SET normally meets once a month to consider and decide major business issues, or as otherwise required by business needs. Typically, it also reviews, in advance of submission to the Board, those matters that are to be submitted to the Board for review and decision.
| > | Reviewing the R&D portfolio – by conducting an objective and transparent review of R&D performance, product launch profile and alignment with corporate strategy. The review is also an important step in reconfirming the R&D three-year budget. | |
| > | Approving the business plans of the Innovative Medicines Units and the Global Medicines Development demand forecast – by confirming the allocation of resources across early- and late-stage elements of R&D as well as assessing licensing and acquisition opportunities. | |
| > | Approving late-stage (internal and external) investment decisions. | |
| > | Monitoring environmental events that could have a major transformational or disruptive impact on our business. |
| > | The processes for ensuring that key business risks are effectively managed. | |
| > | The financial and operational controls that help to ensure that the Group’s assets are properly safeguarded from losses, including fraud. | |
| > | The controls that help to ensure the reliability and integrity of management information systems. | |
| > | The processes for ensuring compliance with policies and procedures, external legislation and regulation. |
| 116 Corporate Governance Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
The disclosures that fulfil the requirements of a corporate governance statement under the Disclosure and Transparency Rules can be found in this section and in other parts of this Annual Report as listed below, each of which is incorporated into this section by reference:
| > | Significant holders of the Company’s shares (contained in the Shareholder Information section from page 211). | |
| > | Articles (contained in the Corporate Information section on page 216). | |
| > | Amendments to the Company’s Articles (contained in the Corporate Information section on page 216). |
In accordance with the Companies Act 2006, we disclose below our subsidiary companies that have representative or scientific branches/offices outside the UK:
| > | AstraZeneca UK Limited: Albania, Algeria, Angola, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Bulgaria, Chile, Costa Rica, Croatia, Cuba, Georgia, Ghana (scientific office), Ireland, Jordan, Kazakhstan, Kenya (scientific office), Macedonia, Romania, Russia, Serbia and Montenegro, Slovenia and Ukraine | |
| > | AstraZeneca AB: Egypt (scientific office), Latvia, Saudi Arabia (scientific office) and Slovakia | |
| > | AstraZeneca Export and Trading AB: Estonia, Lithuania, Romania and the United Arab Emirates | |
| > | AstraZeneca Singapore Pte Limited: Cambodia and Vietnam. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Corporate Governance Report 117 |
Table of Contents
| > | make donations to political parties | |
| > | make donations to political organisations other than political parties | |
| > | incur political expenditure, up to an aggregate limit of $250,000. |
| > | Our Strategy and Performance | |
| > | Business Review | |
| > | Corporate Governance | |
| > | Development Pipeline | |
| > | Shareholder Information | |
| > | Corporate Information |
27 January 2011
| 118 Corporate Governance Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents


| AstraZeneca Annual Report and Form 20-F Information 2010 | Directors’ Remuneration Report 119 |
Table of Contents
| > | The eligibility, structure, award/grant levels, performance metrics and targets, costs and final vesting levels under LTI plans for Directors, other SET members and the Company Secretary. | |
| > | Annual bonus payments for Executive Directors, other SET members and senior executives below SET level. | |
| > | The pension entitlements of Executive Directors and other SET members. | |
| > | The Chairman of the Board’s remuneration (which is approved by the other members of the Committee and the Senior independent Non-Executive Director). | |
| > | Any single payment or award over $1 million. | |
| > | Shareholding guidelines for Executive Directors and other SET members. | |
| > | The contractual terms and conditions of, and any potential or actual payments arising on termination to, Executive Directors, other SET members and the Company Secretary so as to ensure that they are fair to the individual and the Company, that failure is not rewarded and that the duty to mitigate loss is fully recognised. |
| > | Completion of the strategic review of the remuneration and incentive framework for Executive Directors and other SET members. This included significant consultation with major shareholders and institutional investor organisations. | |
| > | A review of the terms of senior executives’ remuneration packages on appointment, promotion and termination, including the remuneration packages on the appointment of a number of senior leaders in R&D. | |
| > | The assessment of Group and individual performance against performance targets to determine the level of executive bonuses for 2009 and to set executive bonus performance targets for 2010. | |
| > | The approval of the rules of the new AstraZeneca Investment Plan (AZIP) prior to the AZIP being proposed to shareholders for approval at the 2010 AGM, and the approval of the rules of the new AstraZeneca Global Restricted Stock Plan (GRSP), which did not require shareholder approval. | |
| > | The approval of the introduction of a new cash flow target for the AstraZeneca Performance Share Plan (PSP), to be used in conjunction with the existing TSR performance condition. | |
| > | The approval of awards made under the Group’s main LTI plans: the PSP; the AZIP; and the GRSP to SET members and other participants. |
| 120 Directors’ Remuneration Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | The approval of restricted share awards to a limited number of senior executives under the AstraZeneca Restricted Share Plan (RSP). | |
| > | A review of the use of claw-back provisions by the Company. | |
| > | A review of the Company’s governance arrangements for global compensation matters. | |
| > | A benchmarking review of the Committee’s activities and policies against institutional investor guidelines. | |
| > | A review of the levels of share ownership of Executive Directors and other SET members. | |
| > | A review of the pension entitlements of Executive Directors and other SET members. | |
| > | A review of the impact on compensation policies and practices of the current economic environment, including ensuring the appropriate degree of risk adjustment to aggregate and individual compensation decisions. | |
| > | A review of the voluntary code of conduct operated under the aegis of the Remuneration Consultants Group in relation to executive remuneration consulting in the UK. | |
| > | In conjunction with the Senior independent Non-Executive Director and not in the presence of the Chairman of the Board, a review of the basic fee paid to the Chairman of the Board for his services. | |
| > | The preparation, review and approval (in January 2011) of this Report. |
| > | All aspects of executive remuneration should be designed to help AstraZeneca create sustainable growth in shareholder value by the successful implementation of strategy and be developed in the context of shareholder views on best practice. | |
| > | Reward structures and performance measures should support a strong performance culture enabling delivery of the business strategy, where all employees have a clear understanding of the Group’s objectives, how their work will impact those objectives and how they will benefit from delivering high levels of performance. | |
| > | Base pay and total compensation positioning against the market should be appropriate to attract and develop high-calibre talent and SET remuneration should continue to be referenced to competitive levels of remuneration. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Directors’ Remuneration Report 121 |
Table of Contents
| Component of remuneration | Role within the remuneration framework | Summary of policy | Applies to | |||
Base salary (fixed)
|
Base fixed remuneration. | Based on conditions in the relevant market and recognising the value of an individual’s sustained personal performance and contribution to the business, taking account of the market rate for an individual’s skills and experience. Benchmarked against external comparators. | All employees | |||
Pension arrangements (fixed) |
Provision of retirement benefits. | Benchmarked against the relevant local employment market. | All employees | |||
Benefits (fixed)
|
Provision of standard non-cash employment benefits, such as healthcare, insurances and, for certain employees, facilitated car purchase arrangements. | Cost-effective and compatible with relevant welfare arrangements and local market norms. | All employees | |||
Short-term bonus (variable) |
An annual cash incentive opportunity determined by reference to Group, functional and individual performance, measured over a single financial year of the Company and taking into account external expectations of performance. | Differs by market, but the Group performance measures ensure that all eligible employees receive an element of reward based on the Group’s overall financial performance. | All eligible employees | |||
| The functional goals are agreed by the Committee at the start of the year and are derived from the business scorecard, the key elements of which are set out in the Business objectives and key performance indicators section from page 16, and are monitored as part of the quarterly business review (QBR) process. | ||||||
| Individual goals are based on annual objectives, which are linked to functional goals. | ||||||
Deferred bonus plan (variable) |
Aligns SET members’ interests with those of shareholders. | SET members must defer a proportion of their short-term bonus (one-third of pre-tax bonus for Executive Directors and one-sixth for other SET members) into Ordinary Shares or ADSs for a three-year period. | SET members | |||
LTI plans (variable)
|
Long-term equity incentive awards to provide individual executives and employees with total compensation opportunities that are competitive against local market practice, for the achievement of operational excellence, strong financial performance and actions that are closely aligned with the interests of shareholders. The primary LTI plans in which SET members participate are the PSP and the AZIP. | AstraZeneca Performance Share Plan. | SET members and other senior executives | |||
| AstraZeneca Investment Plan. | SET members | |||||
| Share Option Plan (final awards made in 2009). | Senior management and SET members | |||||
| Global Restricted Stock Plan. | Eligible employees globally | |||||
Other share plans
|
‘All employee’ share participation arrangements, including some that are tax-approved, for example in the UK. | Examples include the Share Incentive Plan and Savings-Related Share Option Plan (UK)1. | Eligible employees | |||
Shareholding guidelines
|
Aligning SET members’ interests with those of shareholders. | The CEO is expected to hold shares equivalent to 200% of base salary and the CFO and other SET members are expected to hold 125% of base salary in shares. | SET members | |||
| Overall approach | When assessing the overall value of a SET member’s remuneration the Committee considers, both separately and in aggregate, each component of the SET member’s total remuneration. | |||||
| 1 | Further information on these plans is provided in Note 24 to the Financial Statements from page 173. |
| 122 Directors’ Remuneration Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
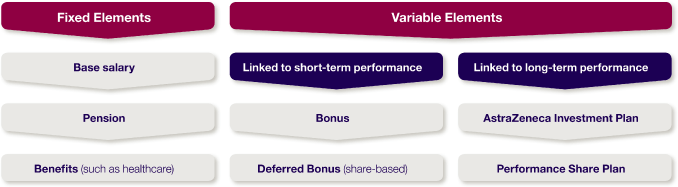
Based on AstraZeneca’s remuneration policy, the Components of remuneration – expected value charts below illustrate the potential weighting given to fixed and variable performance-related elements of the remuneration package at Executive Director level. Variable performance-related elements of the package are shown on an ‘expected value’ basis, and in the event that performance conditions are not met, such elements would not deliver any value. The ‘expected value’ approach considers the range of possible outcomes and the probability attached to each, in order to provide a value that represents the average that would be delivered if the arrangements were operated over many years. The ‘expected value’ for bonus payment is taken to be the target payout level.
| Annual salary | Annual salary | |||||||||||
| in 2010 | in 2011 | Increase | ||||||||||
| Executive Director | £ | £ | % | |||||||||
David Brennan |
972,900 | 997,223 | 2.5 | |||||||||
Simon Lowth |
620,000 | 635,500 | 2.5 | |||||||||
Components of remuneration – expected value |
||
Chief Executive Officer (%)
|
Chief Financial Officer (%) | |
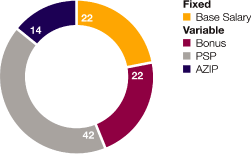
|
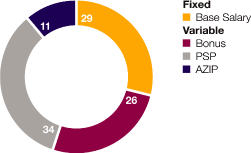 |
|

| AstraZeneca Annual Report and Form 20-F Information 2010 | Directors’ Remuneration Report 123 |
Table of Contents
| 1 | The 401(k) savings plan is a qualified plan to which eligible employees may make salary-deferral contributions on a post-tax and/or pre-tax basis. Employers may also make matching or non-elective contributions to the plan. There is a supplementary non-qualified plan in place for all eligible employees whose earnings exceed specific limits under the US Tax Code. |
| > | 60% by reference to EPS, cash flow targets and the objectives in each of the strategic priority areas identified by the Board for the business, the key elements of which are set out in the Business objectives and key performance indicators section from page 16 and which are monitored as part of the quarterly business review (QBR) process against targets set by the Committee at the beginning of the year and, which among other things, take into account external expectations of performance; | |
| > | 40% by reference to individual measures and initiatives which link to the business objectives relevant to the individual’s functional accountability (or, in the case of the CEO, the average of these individual outcomes). |
| Bonus range for 2011 | ||||
| Executive Director | % | |||
David Brennan |
0-180 | |||
Simon Lowth |
0-150 | |||
| 124 Directors’ Remuneration Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | Pipeline | |
| > | Deliver the business | |
| > | Business shape | |
| > | People and values. |
| > | In relation to the pipeline and life-cycle management, Vimovo was approved in the US and the EU; Brilique was approved in the EU; Kombiglyze™ XR (Onglyza™/metformin combination) was approved in the US; additional indications were approved for other products; decisions were made in December to discontinue development of motavizumab and Certriad. | |
| > | Although we suffered some pipeline disappointments, the new R&D leadership team has made significant progress in 2010 with the creation of a single R&D organisation. | |
| > | The Commercial organisation has continued to drive strong sales performance in the face of an increasingly challenging environment. | |
| > | Our Operations function has continued to deliver on efficiency. | |
| > | The business broadly maintained a good level of employee engagement despite a period of significant organisational change. | |
| > | The business response to the Corporate Integrity Agreement has been rigorous with significant work undertaken to continue to promote a culture of responsibility and accountability across the organisation. |
| Short-term bonus (delivered as a combination | ||||||||
| of cash and shares, as shown in the Directors' | ||||||||
| emoluments in 2010 section from page 129)1 | ||||||||
| Executive Director | £ | % of salary | ||||||
David Brennan |
1,583,025 | 162.71 | ||||||
Simon Lowth |
918,245 | 148.10 | ||||||
| 1 | Bonuses for Executive Directors are not pensionable. |
| > | 50% of the award is based on relative TSR against a selected peer group of global pharmaceutical companies, of which: |
| – | 25% of the maximum award vests for performance at the median of the peer group; | ||
| – | 75% of the maximum award vests for upper quartile performance; and |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Directors’ Remuneration Report 125 |
Table of Contents
| – | 100% of the maximum award may vest at the Committee’s discretion if the Company’s TSR performance is substantially better than that of the upper quartile of the comparator group. For PSP Share Awards to vest at this level the Company would need to have sustained a level of performance significantly in excess of upper quartile over a period of years and the Committee would need to be satisfied that this was warranted. |
| > | 50% of the award vests subject to the achievement of the free cash flow target, which operates as a cumulative cash flow target over a three-year performance period. |
| TSR ranking of the Company | Vesting % | |||
Below median |
0 | |||
Median |
25 | |||
Between median and upper quartile |
Pro rata | |||
Upper quartile |
75 | |||
Significantly above upper quartile |
Up to 100 | |||
| Adjusted cumulative cash flow | Vesting % | |||
Less than $16 billion |
0 | |||
$16 billion |
25 | |||
Between $16 billion and $23 billion |
Pro rata | |||
$23 billion and above |
100 | |||
| > | PSP Share Awards in 2007: the TSR performance for Schering-Plough Corporation and Wyeth Inc. was adjusted from a date a week before the announcement of the relevant corporate action to the end of the relevant performance period so as to track the TSR of the acquiring companies (Merck in the case of Schering-Plough and Pfizer Inc. in the case of Wyeth Inc.). | |
| > | PSP Share Awards from 2008 onwards: Schering-Plough Corporation and Wyeth Inc. were removed from the peer group thus reducing the size of the peer group to 10 companies (excluding AstraZeneca). For these awards, AstraZeneca’s TSR will be compared with the TSR for the 10 companies remaining in the peer group in respect of the relevant performance period. |
| 126 Directors’ Remuneration Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Directors’ Remuneration Report 127 |
Table of Contents
| 128 Directors’ Remuneration Report | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Date of service | Unexpired term at | Notice | ||||||||||
| Executive Director1 | contract | 31 December 2010 | period | |||||||||
David Brennan |
1 January 2006 | 12 months | 12 months | |||||||||
Simon Lowth |
5 November 2007 | 12 months | 12 months | |||||||||
1 Neither of the Executive Directors has any provision in their service contracts
giving them a right to liquidated damages or an automatic entitlement to a bonus for the
duration of their notice period. |
||
| Non-Executive Director1, 2 | Effective date of appointment | |||
Bruce Burlington |
1 August 2010 | |||
Jean-Philippe Courtois |
18 February 2008 | |||
Jane Henney |
24 September 2001 | |||
Michele Hooper |
1 July 2003 | |||
Rudy Markham |
12 September 2008 | |||
Nancy Rothwell |
27 April 2006 | |||
Louis Schweitzer |
11 March 2004 | |||
John Varley |
26 July 2006 | |||
Marcus Wallenberg |
6 April 1999 | |||
1 None of the letters of appointment applicable to Non-Executive Directors confers
upon them any right to compensation payable on early termination of their appointment. |
||
2 Pursuant to the Articles, the continued appointment of each Non-Executive Director is
subject to their election or re-election at each AGM. |
||
| £ | ||||
Basic Fee |
75,000 | |||
Senior independent Non-Executive Director |
30,000 | |||
Membership of the Audit Committee |
20,000 | |||
Membership of the Remuneration Committee |
15,000 | |||
Chairman of the Audit Committee or the Remuneration Committee1 |
20,000 | |||
Membership of the Science Committee |
10,000 | |||
Chairman of the Science Committee1 |
7,000 | |||
1 This fee is in addition to the fee for membership of the relevant Committee. |
||
| Other | ||||||||||||||||||||||||||||||||
| payments | ||||||||||||||||||||||||||||||||
| Salary | Bonus | Bonus | Taxable | and | Total | Total | Total | |||||||||||||||||||||||||
| and fees | cash | Shares1 | benefits | allowances | 2010 | 2009 | 2008 | |||||||||||||||||||||||||
| Name | £000 | £000 | £000 | £000 | £000 | £000 | £000 | £000 | ||||||||||||||||||||||||
Louis Schweitzer |
456 | – | – | – | – | 456 | 325 | 303 | ||||||||||||||||||||||||
David Brennan |
973 | 2 | 1,055 | 528 | 24 | 464 | 3 | 3,044 | 3,186 | 2,506 | ||||||||||||||||||||||
Simon Lowth |
620 | 612 | 306 | 8 | 96 | 4 | 1,642 | 1,426 | 1,304 | |||||||||||||||||||||||
Bruce Burlington5 |
33 | – | – | – | – | 33 | – | – | ||||||||||||||||||||||||
Jean-Philippe Courtois |
80 | – | – | – | – | 80 | 75 | 58 | ||||||||||||||||||||||||
Jane Henney |
90 | – | – | – | – | 90 | 85 | 76 | ||||||||||||||||||||||||
Michele Hooper |
120 | – | – | – | – | 120 | 100 | 90 | ||||||||||||||||||||||||
Rudy Markham |
90 | – | – | – | – | 90 | 75 | 23 | ||||||||||||||||||||||||
Nancy Rothwell |
96 | – | – | – | – | 96 | 92 | 80 | ||||||||||||||||||||||||
John Varley |
99 | – | – | – | – | 99 | 95 | 83 | ||||||||||||||||||||||||
Marcus Wallenberg |
71 | – | – | – | – | 71 | 60 | 53 | ||||||||||||||||||||||||
Former Directors |
||||||||||||||||||||||||||||||||
Bo Angelin6 |
23 | – | – | – | – | 23 | 70 | 63 | ||||||||||||||||||||||||
John Buchanan6 |
36 | – | – | – | – | 36 | 110 | 96 | ||||||||||||||||||||||||
Others |
– | – | – | – | – | – | 479 | 1,181 | ||||||||||||||||||||||||
Total |
2,787 | 1,667 | 834 | 32 | 560 | 5,880 | 6,178 | 5,916 | ||||||||||||||||||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Directors’ Remuneration Report 129 |
Table of Contents
| Other | ||||||||||||||||||||||||||||||||
| payments | ||||||||||||||||||||||||||||||||
| Salary | Bonus | Bonus | Taxable | and | Total | Total | Total | |||||||||||||||||||||||||
| and fees | cash | Shares1 | benefits | allowances | 2010 | 2009 | 2008 | |||||||||||||||||||||||||
| Name | $000 | $000 | $000 | $000 | $000 | $000 | $000 | $000 | ||||||||||||||||||||||||
Louis Schweitzer |
705 | – | – | – | – | 705 | 504 | 567 | ||||||||||||||||||||||||
David Brennan |
1,504 | 2 | 1,631 | 816 | 37 | 717 | 3 | 4,705 | 4,937 | 4,692 | ||||||||||||||||||||||
Simon Lowth |
958 | 946 | 473 | 12 | 148 | 4 | 2,537 | 2,209 | 2,442 | |||||||||||||||||||||||
Bruce Burlington5 |
51 | – | – | – | – | 51 | – | – | ||||||||||||||||||||||||
Jean-Philippe Courtois |
124 | – | – | – | – | 124 | 116 | 109 | ||||||||||||||||||||||||
Jane Henney |
139 | – | – | – | – | 139 | 132 | 142 | ||||||||||||||||||||||||
Michele Hooper |
185 | – | – | – | – | 185 | 155 | 169 | ||||||||||||||||||||||||
Rudy Markham |
139 | – | – | – | – | 139 | 116 | 43 | ||||||||||||||||||||||||
Nancy Rothwell |
148 | – | – | – | – | 148 | 143 | 150 | ||||||||||||||||||||||||
John Varley |
153 | – | – | – | – | 153 | 147 | 155 | ||||||||||||||||||||||||
Marcus Wallenberg |
110 | – | – | – | – | 110 | 93 | 99 | ||||||||||||||||||||||||
Former Directors |
||||||||||||||||||||||||||||||||
Bo Angelin6 |
36 | – | – | – | – | 36 | 108 | 118 | ||||||||||||||||||||||||
John Buchanan6 |
56 | – | – | – | – | 56 | 170 | 180 | ||||||||||||||||||||||||
Others |
– | – | – | – | – | – | 743 | 2,211 | ||||||||||||||||||||||||
Total |
4,308 | 2,577 | 1,289 | 49 | 865 | 9,088 | 9,573 | 11,077 | ||||||||||||||||||||||||
1 These figures represent that portion of the 2010 bonuses required to be deferred
into Shares to be held for a three-year period, as explained in the Bonus share deferral
requirements section on page 125. |
||
2 This figure includes a sum of $356,000/£230,000 in respect of member contributions to
the AstraZeneca Executive Deferred Compensation Plan and 401(k) plan which were paid into the
plan by means of a salary sacrifice (see the Defined contribution arrangements section on page
131 for further details). |
||
3 Relates to relocation allowances, a car allowance and cash payments in respect of
dividends accrued on vesting of LTI share plan awards. |
||
4 Relates to remaining cash following selection of benefits within AstraZeneca’s UK
flexible benefits programme and a cash payment in respect of dividends accrued on vesting of
an LTI share plan award. |
||
5 Part-year only as appointed as a Director with effect from 1 August 2010. |
||
6 Part-year only as ceased to be a Director on 29 April 2010. |
||
| GBP/USD | ||||
2010 |
0.647 | |||
2009 |
0.645 | |||
2008 |
0.534 | |||
| David Brennan | ||||||||
| £000 | $000 | |||||||
Defined benefit arrangements |
||||||||
1. Accrued pension at 1 January 2010 |
918 | 1,419 | ||||||
2. Increase in accrued pension during year as a result of inflation |
– | – | ||||||
3. Adjustment to accrued pension as a result of salary increase relative to inflation |
69 | 107 | ||||||
4. Increase in accrued pension as a result of additional service |
19 | 29 | ||||||
5. Accrued pension at 31 December 2010 |
1,006 | 1,555 | ||||||
6. Employee contributions during 2010 |
– | – | ||||||
7. Transfer value of accrued pension at 31 December 2009 |
12,890 | 19,922 | ||||||
8. Transfer value of accrued pension at 31 December 20101 |
14,711 | 22,738 | ||||||
9. Change in transfer value during the period less employee contributions |
1,822 | 2,816 | ||||||
10. Age at 31 December 2010 |
574/12 | 574/12 | ||||||
11. Pensionable service (years) at 31 December 2010 |
35 | 35 | ||||||
1 For David Brennan, transfer values are calculated to be consistent with the value
of the lump sum distribution equivalent to his deferred accrued pension annuity. The value
shown at 31 December 2010 has been adjusted to reflect a change in the lump sum factor at 31
December 2009. |
||
130 Directors’ Remuneration Report
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
TSR over a five-year period
|
TSR–AstraZeneca compared with peer group | |
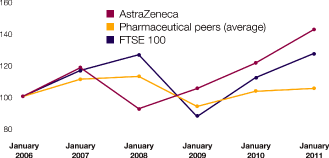 |
1 January 2008 to 31 December 2010 (%) | |
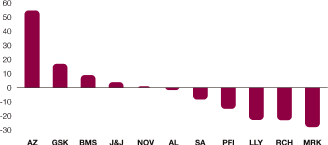 |
||
TSR –AstraZeneca compared with peer group
|
TSR –AstraZeneca compared with peer group | |
1 January 2009 to 31 December 2010 (%)
|
1 January 2010 to 31 December 2010 (%) | |
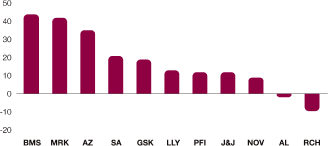
|
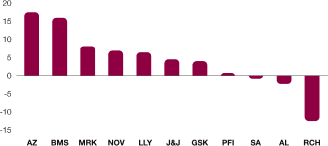 |
|

| AstraZeneca Annual Report and Form 20-F Information 2010 | Directors’ Remuneration Report 131 |
Table of Contents
| Beneficial interest in Ordinary | Beneficial interest in Ordinary | |||||||||||
| Shares at 1 January 2010 or | Change to beneficial | Shares at 31 December 2010 | ||||||||||
| Name | (if later) appointment date | interest | or (if earlier) resignation date | |||||||||
Louis Schweitzer |
5,356 | 11,259 | 16,615 | |||||||||
David Brennan |
128,375 | 58,607 | 186,982 | |||||||||
Simon Lowth |
850 | 8,496 | 9,346 | |||||||||
Bo Angelin1 |
787 | – | 787 | |||||||||
John Buchanan1 |
2,500 | – | 2,500 | |||||||||
Bruce Burlington2 |
553 | – | 553 | |||||||||
Jean-Philippe Courtois |
2,635 | – | 2,635 | |||||||||
Jane Henney |
787 | 527 | 1,314 | |||||||||
Michele Hooper |
1,700 | 700 | 2,400 | |||||||||
Rudy Markham |
1,420 | 520 | 1,940 | |||||||||
Nancy Rothwell |
787 | 527 | 1,314 | |||||||||
John Varley |
500 | 794 | 1,294 | |||||||||
Marcus Wallenberg3 |
67,264 | (3,618 | ) | 63,646 | ||||||||
| 1 | Part-year only as ceased to be a Director on 29 April 2010. | |
| 2 | Part-year only as appointed as a Director on 1 August 2010. | |
| 3 | Ceased to have an interest in 3,618 shares held by a family member who ceased to be a Connected Person during 2010. |
| ADSs held at | Net ADSs acquired | ADSs held | ||||||||||
| Unitised stock plan | 1 January 2010 | during 2010 | at 31 December 2010 | |||||||||
AstraZeneca Executive Deferral Plan |
35,906 | 1,835 | 37,741 | |||||||||
AstraZeneca Savings and Security Plan |
8,103 | 420 | 8,523 | |||||||||
132 Directors’ Remuneration Report
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Price on | ||||||||||||||||||||||||
| Number of | Award price | vesting | ||||||||||||||||||||||
| shares | (pence) | date (pence) | Grant date1 | Vesting date1 | Performance period1 | |||||||||||||||||||
David Brennan |
||||||||||||||||||||||||
2007 Share Award |
107,051 | 2744 | 30.03.07 | 30.03.10 | 01.01.07 – 31.12.09 | |||||||||||||||||||
2008 Share Award |
161,546 | 1882 | 28.03.08 | 28.03.11 | 01.01.08 – 31.12.10 | |||||||||||||||||||
2009 Share Award |
133,347 | 2280 | 27.03.09 | 27.03.12 | 01.01.09 – 31.12.11 | |||||||||||||||||||
Total at 1 January 2010 |
401,944 | |||||||||||||||||||||||
Partial vesting of 2007 Share Award2 |
(83,499) | 3,5 | 2980 | |||||||||||||||||||||
Partial lapse of 2007 Share Award |
(23,552 | ) | ||||||||||||||||||||||
2010 Share Award |
127,520 | 2861 | 07.05.10 | 07.05.13 | 01.01.10 – 31.12.12 | |||||||||||||||||||
Total at 31 December 2010 |
422,413 | |||||||||||||||||||||||
Simon Lowth |
||||||||||||||||||||||||
2007 Share Award |
15,554 | 2210 | 16.11.07 | 16.11.10 | 01.01.07 – 31.12.09 | |||||||||||||||||||
2008 Share Award |
58,448 | 1882 | 28.03.08 | 28.03.11 | 01.01.08 – 31.12.10 | |||||||||||||||||||
2009 Share Award |
54,276 | 2280 | 27.03.09 | 27.03.12 | 01.01.09 – 31.12.11 | |||||||||||||||||||
Total at 1 January 2010 |
128,278 | |||||||||||||||||||||||
Partial vesting of 2007 Share Award2 |
(12,132) | 4,5 | 3016 | |||||||||||||||||||||
Partial lapse of 2007 Share Award |
(3,422 | ) | ||||||||||||||||||||||
2010 Share Award |
52,009 | 2861 | 07.05.10 | 07.05.13 | 01.01.10 – 31.12.12 | |||||||||||||||||||
Total at 31 December 2010 |
164,733 | |||||||||||||||||||||||
| 1 | UK date convention applies. | |
| 2 | Share Awards granted in 2007 vested in 2010 at 78% based on the outcome of the performance conditions and targets (which are set out in the AstraZeneca Performance Share Plan section from page 125). | |
| 3 | Following certain mandatory tax deductions, David Brennan became beneficially interested in a net number of 49,264 Ordinary Shares. | |
| 4 | Following certain mandatory tax deductions, Simon Lowth became beneficially interested in a net number of 5,944 Ordinary Shares. | |
| 5 | Cash payments equivalent to dividends accruing over the vesting period are made at the date of vesting and are included in ‘Other payments and allowances’ in the Directors’ remuneration tables from page 129. |
| Number of | Award price | |||||||||||||||||||
| shares | (pence) | Grant date1 | Vesting date1 | Performance period1 | ||||||||||||||||
David Brennan |
||||||||||||||||||||
2010 Share Award |
21,253 | 2861 | 07.05.10 | 01.01.18 | 01.01.10 – 31.12.13 | |||||||||||||||
Total at 31 December 2010 |
21,253 | |||||||||||||||||||
Simon Lowth |
||||||||||||||||||||
2010 Share Award |
8,668 | 2861 | 07.05.10 | 01.01.18 | 01.01.10 – 31.12.13 | |||||||||||||||
Total at 31 December 2010 |
8,668 | |||||||||||||||||||
| 1 | UK date convention applies. |
| Price on | ||||||||||||||||||||
| Number of | Award price | vesting | ||||||||||||||||||
| shares | (pence) | date (pence) | Grant date1 | Vesting date1 | ||||||||||||||||
David Brennan |
||||||||||||||||||||
2007 Award |
12,014 | 2911 | 23.02.07 | 23.02.10 | ||||||||||||||||
2008 Award |
16,810 | 1999 | 25.02.08 | 25.02.11 | ||||||||||||||||
2009 Award |
17,992 | 2400 | 25.02.09 | 25.02.12 | ||||||||||||||||
Total at 1 January 2010 |
46,816 | |||||||||||||||||||
Vesting of 2007 Award |
(12,014) | 2,3 | 2810 | |||||||||||||||||
2010 Award |
20,718 | 2817.5 | 25.02.10 | 25.02.13 | ||||||||||||||||
Total at 31 December 2010 |
55,520 | |||||||||||||||||||
Simon Lowth |
||||||||||||||||||||
2008 Award |
1,340 | 1999 | 25.02.08 | 25.02.11 | ||||||||||||||||
2009 Award |
9,775 | 2400 | 25.02.09 | 25.02.12 | ||||||||||||||||
Total at 1 January 2010 |
11,115 | |||||||||||||||||||
2010 Award |
9,760 | 2817.5 | 25.02.10 | 25.02.13 | ||||||||||||||||
Total at 31 December 2010 |
20,875 | |||||||||||||||||||
| 1 | UK date convention applies. | |
| 2 | Following certain mandatory tax deductions, David Brennan became beneficially interested in a net number of 7,088 Ordinary Shares. | |
| 3 | Cash payments equivalent to dividends accruing over the vesting period are made at the date of vesting and are included in ‘Other payments and allowances’ in the Directors’ remuneration tables from page 129. |

AstraZeneca Annual Report and Form 20-F Information 2010
|
Directors’ Remuneration Report 133 |
Table of Contents
| Number of | Exercise | |||||||||||||||||||||
| Ordinary | price per | Market price | ||||||||||||||||||||
| Shares under | Ordinary | on date | First day | Last day | ||||||||||||||||||
| option1 | Share2 | of exercise | exercisable3,4 | exercisable3,4 | ||||||||||||||||||
David Brennan |
At 1 January 2010 – options over Ordinary Shares | 592,975 | 2375p | 24.03.09 | 26.03.19 | |||||||||||||||||
| – market price above option price (Ordinary Shares) | 505,244 | 2271p | 19.05.09 | 26.03.19 | ||||||||||||||||||
| – market price at or below option price (Ordinary Shares) | 87,731 | 2975p | 24.03.09 | 23.03.16 | ||||||||||||||||||
| At 31 December 2010 – options over Ordinary Shares | 592,975 | 2375p | 24.03.09 | 26.03.19 | ||||||||||||||||||
| – market price above option price (Ordinary Shares) | 505,244 | 2271p | 19.05.09 | 26.03.19 | ||||||||||||||||||
| – market price at or below option price (Ordinary Shares) | 87,731 | 2975p | 24.03.09 | 23.03.16 | ||||||||||||||||||
| At 1 January 2010 – options over ADSs | 355,246 | $45.22 | 16.03.03 | 23.03.15 | ||||||||||||||||||
| – market price above option price (ADSs) | 210,255 | $42.91 | 26.03.07 | 23.03.15 | ||||||||||||||||||
| – market price at or below option price (ADSs) | 144,991 | $48.58 | 29.03.04 | 27.03.12 | ||||||||||||||||||
| Exercised 2 March 2010 | (32,727 | )5 | $44.00 | $44.35 | 16.03.03 | 26.03.19 | ||||||||||||||||
| At 31 December 2010 – options over ADSs | 322,519 | $45.35 | 29.03.04 | 23.03.15 | ||||||||||||||||||
| – market price above option price (ADSs) | 110,987 | $40.35 | 24.03.08 | 23.03.15 | ||||||||||||||||||
| – market price at or below option price (ADSs) | 211,532 | $47.97 | 29.03.04 | 25.03.14 | ||||||||||||||||||
Simon Lowth |
At 1 January 2010 | 153,934 | 2090p | 16.11.10 | 26.03.19 | |||||||||||||||||
| – market price above option price | 153,934 | 2090p | 16.11.10 | 26.03.19 | ||||||||||||||||||
| – market price at or below option price | – | n/a | n/a | n/a | ||||||||||||||||||
| Exercised 17 November 2010 | (18,665 | ) | 2210p | 3048p | 16.11.10 | 15.11.17 | ||||||||||||||||
| At 31 December 2010 | 135,269 | 2074p | 28.03.11 | 26.03.19 | ||||||||||||||||||
| – market price above option price | 135,269 | 2074p | 28.03.11 | 26.03.19 | ||||||||||||||||||
| – market price at or below option price | – | n/a | n/a | n/a | ||||||||||||||||||
| 1 | Vesting is subject to satisfying the relevant performance conditions set out in each of the relevant share option plans. Further information on the performance conditions applicable to the SOP is set out in the AstraZeneca Share Option Plan section on page 127. As a Save As You Earn scheme, the AstraZeneca Savings-Related Share Option Plan is not subject to any performance conditions. Awards granted under the Zeneca Plan are no longer subject to any performance conditions. | |
| 2 | Exercise prices are weighted averages. | |
| 3 | First and last exercise dates of groups of options, within which period there may be shorter exercise periods. | |
| 4 | UK date convention applies. | |
| 5 | Option granted under the Zeneca Plan. |
| Gains made by Directors | Gains made by the | |||||||
| on the exercise of share options | highest paid Director | |||||||
| Year | $ | $ | ||||||
2010 |
260,182.40 | 11,454.45 | ||||||
2009 |
– | – | ||||||
2008 |
1,764.96 | – | ||||||
|
During 2010, the market price of Ordinary Shares or ADSs was as follows:
| ||||||||
| Ordinary Share/ADS market price | Range of the Ordinary Share/ | |||||||
| Stock Exchange | at 31 December 2010 | ADS market price during 2010 | ||||||
London |
2922p | 2732p to 3385p | ||||||
Stockholm |
309.3 SEK | 309.3 SEK to 382.2 SEK | ||||||
New York |
$46.19 | $40.91 to $53.50 | ||||||
27 January 2011
134 Directors’ Remuneration Report
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
financial statements
structured?
| 158 | 14 | |||||
| 158 | 15 | |||||
| 161 | 16 | |||||
| 161 | 17 | |||||
| 162 | 18 | |||||
| 166 | 19 | |||||
| 167 | 20 | |||||
| 167 | 21 | |||||
| 167 | 22 | |||||
| 168 | 23 | |||||
| 173 | 24 | |||||
| 178 | 25 | |||||
| 196 | 26 | |||||
| 196 | 27 | |||||
| 197 | Principal Subsidiaries | |||||
| 198 | Independent Auditor’s Report to the Members of AstraZeneca PLC |
|||||
| 199 | Company Balance Sheet at 31 December | |||||
| 200 | Company Accounting Policies | |||||
| 201 | Notes to the Company Financial Statements | |||||
| 201 | 1 | |||||
| 201 | 2 | |||||
| 201 | 3 | |||||
| 202 | 4 | |||||
| 202 | 5 | |||||
| 202 | 6 | |||||
| 203 | 7 | |||||
| 203 | 8 | |||||
| 204 | Group Financial Record | |||||
| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 135 |
Table of Contents
| > | Select suitable accounting policies and then apply them consistently. | |
| > | Make judgements and estimates that are reasonable and prudent. | |
| > | For the Group Financial Statements, state whether they have been prepared in accordance with IFRSs as adopted by the EU. | |
| > | For the Parent Company Financial Statements, state whether applicable UK Accounting Standards have been followed, subject to any material departures disclosed and explained in the Parent Company Financial Statements. | |
| > | Prepare the financial statements on the going concern basis unless it is inappropriate to presume that the Group and the Parent Company will continue in business. |
| > | The Financial Statements, prepared in accordance with the applicable set of accounting standards, give a true and fair view of the assets, liabilities, financial position and profit or loss of the Company and the undertakings included in the consolidation taken as a whole. | |
| > | The Directors’ Report includes a fair review of the development and performance of the business and the position of the issuer and the undertakings included in the consolidation taken as a whole, together with a description of the principal risks and uncertainties that they face. |
Director
Internal Control over Financial Reporting
| 136 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | Give a true and fair view of the state of the Group’s affairs as at 31 December 2010 and of its profit for the year then ended. | |
| > | Have been properly prepared in accordance with IFRSs as adopted by the EU. | |
| > | Have been prepared in accordance with the requirements of the Companies Act 2006 and Article 4 of the IAS Regulation. |
Companies Act 2006
| > | Certain disclosures of Directors’ Remuneration specified by law are not made. | |
| > | We have not received all the information and explanations we require for our audit. |
| > | The Directors’ Statement, set out on page 142, in relation to going concern. | |
| > | The part of the corporate governance statement relating to the Company’s compliance with the nine provisions of the June 2008 Combined Code specified for our review. | |
| > | Certain elements of the report to shareholders by the Board on directors’ remuneration. |
Senior Statutory Auditor
Statutory Auditor Chartered Accountants 15
Canada Square, London, E14 5GL

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 137 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||||||
| Notes | $m | $m | $m | |||||||||||||
Revenue |
1 | 33,269 | 32,804 | 31,601 | ||||||||||||
Cost of sales |
(6,389 | ) | (5,775 | ) | (6,598 | ) | ||||||||||
Gross profit |
26,880 | 27,029 | 25,003 | |||||||||||||
Distribution costs |
(335 | ) | (298 | ) | (291 | ) | ||||||||||
Research and development |
2 | (5,318 | ) | (4,409 | ) | (5,179 | ) | |||||||||
Selling, general and administrative costs |
2 | (10,445 | ) | (11,332 | ) | (10,913 | ) | |||||||||
Other operating income and expense |
2 | 712 | 553 | 524 | ||||||||||||
Operating profit |
2 | 11,494 | 11,543 | 9,144 | ||||||||||||
Finance income |
3 | 516 | 462 | 854 | ||||||||||||
Finance expense |
3 | (1,033 | ) | (1,198 | ) | (1,317 | ) | |||||||||
Profit before tax |
10,977 | 10,807 | 8,681 | |||||||||||||
Taxation |
4 | (2,896 | ) | (3,263 | ) | (2,551 | ) | |||||||||
Profit for the period |
8,081 | 7,544 | 6,130 | |||||||||||||
Other comprehensive income: |
||||||||||||||||
Foreign exchange arising on consolidation |
26 | 388 | (1,336 | ) | ||||||||||||
Foreign exchange differences on borrowings forming net investment hedges |
101 | (68 | ) | 291 | ||||||||||||
Amortisation of loss on cash flow hedge |
1 | 1 | 1 | |||||||||||||
Net available for sale gains taken to equity |
4 | 2 | 2 | |||||||||||||
Actuarial loss for the period |
18 | (46 | ) | (569 | ) | (1,232 | ) | |||||||||
Income tax relating to components of other comprehensive income |
4 | (61 | ) | 192 | 368 | |||||||||||
Other comprehensive income for the period, net of tax |
25 | (54 | ) | (1,906 | ) | |||||||||||
Total comprehensive income for the period |
8,106 | 7,490 | 4,224 | |||||||||||||
Profit attributable to: |
||||||||||||||||
Owners of the Parent |
8,053 | 7,521 | 6,101 | |||||||||||||
Non-controlling interests |
28 | 23 | 29 | |||||||||||||
Total comprehensive income attributable to: |
||||||||||||||||
Owners of the Parent |
8,058 | 7,467 | 4,176 | |||||||||||||
Non-controlling interests |
48 | 23 | 48 | |||||||||||||
Basic earnings per $0.25 Ordinary Share |
5 | $5.60 | $5.19 | $4.20 | ||||||||||||
Diluted earnings per $0.25 Ordinary Share |
5 | $5.57 | $5.19 | $4.20 | ||||||||||||
Weighted average number of Ordinary Shares in issue (millions) |
5 | 1,438 | 1,448 | 1,453 | ||||||||||||
Diluted weighted average number of Ordinary Shares in issue (millions) |
5 | 1,446 | 1,450 | 1,453 | ||||||||||||
Dividends declared and paid in the period |
21 | 3,494 | 3,026 | 2,767 | ||||||||||||
138 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||||||
| Notes | $m | $m | $m | |||||||||||||
Assets |
||||||||||||||||
Non-current assets |
||||||||||||||||
Property, plant and equipment |
7 | 6,957 | 7,307 | 7,043 | ||||||||||||
Goodwill |
8 | 9,871 | 9,889 | 9,874 | ||||||||||||
Intangible assets |
9 | 12,158 | 12,226 | 12,323 | ||||||||||||
Derivative financial instruments |
15 | 324 | 262 | 449 | ||||||||||||
Other investments |
10 | 211 | 184 | 156 | ||||||||||||
Deferred tax assets |
4 | 1,475 | 1,292 | 1,236 | ||||||||||||
| 30,996 | 31,160 | 31,081 | ||||||||||||||
Current assets |
||||||||||||||||
Inventories |
11 | 1,682 | 1,750 | 1,636 | ||||||||||||
Trade and other receivables |
12 | 7,847 | 7,709 | 7,261 | ||||||||||||
Other investments |
10 | 1,482 | 1,484 | 105 | ||||||||||||
Derivative financial instruments |
15 | 9 | 24 | – | ||||||||||||
Income tax receivable |
3,043 | 2,875 | 2,581 | |||||||||||||
Cash and cash equivalents |
13 | 11,068 | 9,918 | 4,286 | ||||||||||||
| 25,131 | 23,760 | 15,869 | ||||||||||||||
Total assets |
56,127 | 54,920 | 46,950 | |||||||||||||
Liabilities |
||||||||||||||||
Current liabilities |
||||||||||||||||
Interest-bearing loans and borrowings |
14 | (125 | ) | (1,926 | ) | (993 | ) | |||||||||
Trade and other payables |
16 | (8,661 | ) | (8,687 | ) | (7,178 | ) | |||||||||
Derivative financial instruments |
15 | (8 | ) | (90 | ) | (95 | ) | |||||||||
Provisions |
17 | (1,095 | ) | (1,209 | ) | (600 | ) | |||||||||
Income tax payable |
(6,898 | ) | (5,728 | ) | (4,549 | ) | ||||||||||
| (16,787 | ) | (17,640 | ) | (13,415 | ) | |||||||||||
Non-current liabilities |
||||||||||||||||
Interest-bearing loans and borrowings |
14 | (9,097 | ) | (9,137 | ) | (10,855 | ) | |||||||||
Derivative financial instruments |
15 | – | – | (71 | ) | |||||||||||
Deferred tax liabilities |
4 | (3,145 | ) | (3,247 | ) | (3,126 | ) | |||||||||
Retirement benefit obligations |
18 | (2,472 | ) | (3,354 | ) | (2,732 | ) | |||||||||
Provisions |
17 | (843 | ) | (477 | ) | (542 | ) | |||||||||
Other payables |
16 | (373 | ) | (244 | ) | (149 | ) | |||||||||
| (15,930 | ) | (16,459 | ) | (17,475 | ) | |||||||||||
Total liabilities |
(32,717 | ) | (34,099 | ) | (30,890 | ) | ||||||||||
Net assets |
23,410 | 20,821 | 16,060 | |||||||||||||
Equity |
||||||||||||||||
Capital and reserves attributable to equity holders of the Company |
||||||||||||||||
Share capital |
20 | 352 | 363 | 362 | ||||||||||||
Share premium account |
2,672 | 2,180 | 2,046 | |||||||||||||
Capital redemption reserve |
107 | 94 | 94 | |||||||||||||
Merger reserve |
433 | 433 | 433 | |||||||||||||
Other reserves |
19 | 1,377 | 1,392 | 1,405 | ||||||||||||
Retained earnings |
19 | 18,272 | 16,198 | 11,572 | ||||||||||||
| 23,213 | 20,660 | 15,912 | ||||||||||||||
Non-controlling interests |
197 | 161 | 148 | |||||||||||||
Total equity |
23,410 | 20,821 | 16,060 | |||||||||||||
David R Brennan
|
Simon Lowth | |
Director
|
Director |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 139 |
Table of Contents
| Share | Capital | Non- | ||||||||||||||||||||||||||||||||||
| Share | premium | redemption | Merger | Other | Retained | controlling | Total | |||||||||||||||||||||||||||||
| capital | account | reserve | reserve | reserves | earnings | Total | interests | equity | ||||||||||||||||||||||||||||
| $m | $m | $m | $m | $m | $m | $m | $m | $m | ||||||||||||||||||||||||||||
At 1 January 2008 |
364 | 1,888 | 91 | 433 | 1,378 | 10,624 | 14,778 | 137 | 14,915 | |||||||||||||||||||||||||||
Profit for the period |
– | – | – | – | – | 6,101 | 6,101 | 29 | 6,130 | |||||||||||||||||||||||||||
Other comprehensive income |
– | – | – | – | – | (1,925 | ) | (1,925 | ) | 19 | (1,906 | ) | ||||||||||||||||||||||||
Transfer to other reserves1 |
– | – | – | – | 27 | (27 | ) | – | – | – | ||||||||||||||||||||||||||
Transactions with owners |
||||||||||||||||||||||||||||||||||||
Dividends |
– | – | – | – | – | (2,767 | ) | (2,767 | ) | – | (2,767 | ) | ||||||||||||||||||||||||
Issue of Ordinary Shares |
1 | 158 | – | – | – | – | 159 | – | 159 | |||||||||||||||||||||||||||
Repurchase of Ordinary Shares |
(3 | ) | – | 3 | – | – | (610 | ) | (610 | ) | – | (610 | ) | |||||||||||||||||||||||
Share-based payments |
– | – | – | – | – | 176 | 176 | – | 176 | |||||||||||||||||||||||||||
Transfer from non-controlling interests to payables |
– | – | – | – | – | – | – | (11 | ) | (11 | ) | |||||||||||||||||||||||||
Dividend paid by subsidiary to non-controlling interests |
– | – | – | – | – | – | – | (26 | ) | (26 | ) | |||||||||||||||||||||||||
Net movement |
(2 | ) | 158 | 3 | – | 27 | 948 | 1,134 | 11 | 1,145 | ||||||||||||||||||||||||||
At 31 December 2008 |
362 | 2,046 | 94 | 433 | 1,405 | 11,572 | 15,912 | 148 | 16,060 | |||||||||||||||||||||||||||
Profit for the period |
– | – | – | – | – | 7,521 | 7,521 | 23 | 7,544 | |||||||||||||||||||||||||||
Other comprehensive income |
– | – | – | – | – | (54 | ) | (54 | ) | – | (54 | ) | ||||||||||||||||||||||||
Transfer to other reserves1 |
– | – | – | – | (13 | ) | 13 | – | – | – | ||||||||||||||||||||||||||
Transactions with owners |
||||||||||||||||||||||||||||||||||||
Dividends |
– | – | – | – | – | (3,026 | ) | (3,026 | ) | – | (3,026 | ) | ||||||||||||||||||||||||
Issue of Ordinary Shares |
1 | 134 | – | – | – | – | 135 | – | 135 | |||||||||||||||||||||||||||
Share-based payments |
– | – | – | – | – | 172 | 172 | – | 172 | |||||||||||||||||||||||||||
Transfer from non-controlling interests to payables |
– | – | – | – | – | – | – | (9 | ) | (9 | ) | |||||||||||||||||||||||||
Dividend paid by subsidiary to non-controlling interests |
– | – | – | – | – | – | – | (1 | ) | (1 | ) | |||||||||||||||||||||||||
Net movement |
1 | 134 | – | – | (13 | ) | 4,626 | 4,748 | 13 | 4,761 | ||||||||||||||||||||||||||
At 31 December 2009 |
363 | 2,180 | 94 | 433 | 1,392 | 16,198 | 20,660 | 161 | 20,821 | |||||||||||||||||||||||||||
Profit for the period |
– | – | – | – | – | 8,053 | 8,053 | 28 | 8,081 | |||||||||||||||||||||||||||
Other comprehensive income |
– | – | – | – | – | 5 | 5 | 20 | 25 | |||||||||||||||||||||||||||
Transfer to other reserves1 |
– | – | – | – | (15 | ) | 15 | – | – | – | ||||||||||||||||||||||||||
Transactions with owners |
||||||||||||||||||||||||||||||||||||
Dividends |
– | – | – | – | – | (3,494 | ) | (3,494 | ) | – | (3,494 | ) | ||||||||||||||||||||||||
Issue of Ordinary Shares |
2 | 492 | – | – | – | – | 494 | – | 494 | |||||||||||||||||||||||||||
Repurchase of Ordinary Shares |
(13 | ) | – | 13 | – | – | (2,604 | ) | (2,604 | ) | – | (2,604 | ) | |||||||||||||||||||||||
Share-based payments |
– | – | – | – | – | 99 | 99 | – | 99 | |||||||||||||||||||||||||||
Transfer from non-controlling interests to payables |
– | – | – | – | – | – | – | (11 | ) | (11 | ) | |||||||||||||||||||||||||
Dividend paid by subsidiary to non-controlling interests |
– | – | – | – | – | – | – | (1 | ) | (1 | ) | |||||||||||||||||||||||||
Net movement |
(11 | ) | 492 | 13 | – | (15 | ) | 2,074 | 2,553 | 36 | 2,589 | |||||||||||||||||||||||||
At 31 December 2010 |
352 | 2,672 | 107 | 433 | 1,377 | 18,272 | 23,213 | 197 | 23,410 | |||||||||||||||||||||||||||
| 1 | Amounts charged or credited to other reserves relate to exchange adjustments arising on goodwill. |
140 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||||||
| Notes | $m | $m | $m | |||||||||||||
Cash flows from operating activities |
||||||||||||||||
Profit before tax |
10,977 | 10,807 | 8,681 | |||||||||||||
Finance income and expense |
3 | 517 | 736 | 463 | ||||||||||||
Depreciation, amortisation and impairment |
2,741 | 2,087 | 2,620 | |||||||||||||
Decrease/(increase) in trade and other receivables |
10 | (256 | ) | (1,032 | ) | |||||||||||
Decrease in inventories |
88 | 6 | 185 | |||||||||||||
(Decrease)/increase in trade and other payables and provisions |
(16 | ) | 1,579 | 637 | ||||||||||||
Other non-cash movements |
(463 | ) | (200 | ) | 87 | |||||||||||
Cash generated from operations |
13,854 | 14,759 | 11,641 | |||||||||||||
Interest paid |
(641 | ) | (639 | ) | (690 | ) | ||||||||||
Tax paid |
(2,533 | ) | (2,381 | ) | (2,209 | ) | ||||||||||
Net cash inflow from operating activities |
10,680 | 11,739 | 8,742 | |||||||||||||
Cash flows from investing activities |
||||||||||||||||
Acquisitions of business operations |
22 | (348 | ) | – | – | |||||||||||
Movement in short-term investments and fixed deposits |
(239 | ) | (1,371 | ) | 1 | |||||||||||
Purchase of property, plant and equipment |
(791 | ) | (962 | ) | (1,095 | ) | ||||||||||
Disposal of property, plant and equipment |
83 | 138 | 38 | |||||||||||||
Purchase of intangible assets |
(1,390 | ) | (624 | ) | (2,944 | ) | ||||||||||
Disposal of intangible assets |
210 | 269 | – | |||||||||||||
Purchase of non-current asset investments |
(34 | ) | (31 | ) | (40 | ) | ||||||||||
Disposal of non-current asset investments |
5 | 3 | 32 | |||||||||||||
Interest received |
174 | 113 | 149 | |||||||||||||
Payments made by subsidiaries to non-controlling interests |
(10 | ) | (11 | ) | (37 | ) | ||||||||||
Net cash outflow from investing activities |
(2,340 | ) | (2,476 | ) | (3,896 | ) | ||||||||||
Net cash inflow before financing activities |
8,340 | 9,263 | 4,846 | |||||||||||||
Cash flows from financing activities |
||||||||||||||||
Proceeds from issue of share capital |
494 | 135 | 159 | |||||||||||||
Repurchase of shares |
(2,604 | ) | – | (610 | ) | |||||||||||
Issue of loans |
– | – | 787 | |||||||||||||
Repayment of loans |
(1,741 | ) | (650 | ) | – | |||||||||||
Dividends paid |
(3,361 | ) | (2,977 | ) | (2,739 | ) | ||||||||||
Movement in short-term borrowings |
(8 | ) | (137 | ) | (3,959 | ) | ||||||||||
Net cash outflow from financing activities |
(7,220 | ) | (3,629 | ) | (6,362 | ) | ||||||||||
Net increase/(decrease) in cash and cash equivalents in the period |
1,120 | 5,634 | (1,516 | ) | ||||||||||||
Cash and cash equivalents at the beginning of the period |
9,828 | 4,123 | 5,727 | |||||||||||||
Exchange rate effects |
33 | 71 | (88 | ) | ||||||||||||
Cash and cash equivalents at the end of the period |
13 | 10,981 | 9,828 | 4,123 | ||||||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 141 |
Table of Contents
| > | Contingent consideration is measured at fair value, with subsequent changes to the fair value being recognised in profit. | |
| > | Transaction costs, other than share and debt issue costs, are expensed as incurred. | |
| > | Any pre-existing interest in the acquiree is measured at fair value with the gain or loss recognised in profit. | |
| > | Any non-controlling (minority) interest is measured at either fair value, or at its proportionate interest in the identifiable assets and liabilities of the acquiree, on a transaction-by-transaction basis. |
statements on a going concern basis
| 142 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 143 |
Table of Contents
| > | Cash and cash equivalents | |
| > | Fixed deposits | |
| > | Other investments | |
| > | Bank and other borrowings | |
| > | Derivatives. |
| 144 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 145 |
Table of Contents
| > | Business combinations – IFRS 3 ‘Business Combinations’ has been applied from 1 January 2003, the date of transition, rather than being applied fully retrospectively. As a result, the combination of Astra and Zeneca is still accounted for as a merger, rather than through purchase accounting. If purchase accounting had been adopted, Zeneca would have been deemed to have acquired Astra. | |
| > | Cumulative exchange differences – the Group chose to set the cumulative exchange difference reserve at 1 January 2003 to zero. |
issued but not yet adopted
| > | Amendments to IFRIC 13 ‘Customer Loyalty Programmes’ – Fair value of award credit | |
| > | Amendments to IFRIC 14 ‘Prepayments of a Minimum Funding Requirement’ | |
| > | IFRIC 19 ‘Extinguishing Financial Liabilities with Equity Instruments’. |
| 146 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Gastrointestinal: |
||||||||||||
Nexium |
4,969 | 4,959 | 5,200 | |||||||||
Losec/Prilosec |
986 | 946 | 1,055 | |||||||||
Others |
133 | 106 | 89 | |||||||||
Total Gastrointestinal |
6,088 | 6,011 | 6,344 | |||||||||
Cardiovascular: |
||||||||||||
Crestor |
5,691 | 4,502 | 3,597 | |||||||||
Atacand |
1,483 | 1,436 | 1,471 | |||||||||
Seloken/Toprol-XL |
1,210 | 1,443 | 807 | |||||||||
Plendil |
255 | 241 | 268 | |||||||||
Zestril |
157 | 184 | 236 | |||||||||
Onglyza™ |
69 | 11 | – | |||||||||
Others |
538 | 559 | 584 | |||||||||
Total Cardiovascular |
9,403 | 8,376 | 6,963 | |||||||||
Respiratory & Inflammation: |
||||||||||||
Symbicort |
2,746 | 2,294 | 2,004 | |||||||||
Pulmicort |
872 | 1,310 | 1,495 | |||||||||
Rhinocort |
227 | 264 | 322 | |||||||||
Oxis |
63 | 63 | 71 | |||||||||
Others |
191 | 201 | 236 | |||||||||
Total Respiratory & Inflammation |
4,099 | 4,132 | 4,128 | |||||||||
Oncology: |
||||||||||||
Arimidex |
1,512 | 1,921 | 1,857 | |||||||||
Zoladex |
1,115 | 1,086 | 1,138 | |||||||||
Casodex |
579 | 844 | 1,258 | |||||||||
Iressa |
393 | 297 | 265 | |||||||||
Faslodex |
345 | 262 | 249 | |||||||||
Nolvadex |
89 | 88 | 85 | |||||||||
Abraxane™ |
– | – | 64 | |||||||||
Ethyol |
8 | 15 | 28 | |||||||||
Others |
4 | 5 | 10 | |||||||||
Total Oncology |
4,045 | 4,518 | 4,954 | |||||||||
Neuroscience: |
||||||||||||
Seroquel |
5,302 | 4,866 | 4,452 | |||||||||
Local anaesthetics |
605 | 599 | 605 | |||||||||
Zomig |
428 | 434 | 448 | |||||||||
Diprivan |
322 | 290 | 278 | |||||||||
Others |
47 | 48 | 54 | |||||||||
Total Neuroscience |
6,704 | 6,237 | 5,837 | |||||||||
Infection and Other: |
||||||||||||
Synagis |
1,038 | 1,082 | 1,230 | |||||||||
Merrem |
817 | 872 | 897 | |||||||||
FluMist |
174 | 145 | 104 | |||||||||
Non Seasonal Flu |
39 | 389 | – | |||||||||
Other Products |
108 | 143 | 220 | |||||||||
Total Infection and Other |
2,176 | 2,631 | 2,451 | |||||||||
Astra Tech |
535 | 506 | 529 | |||||||||
Aptium Oncology |
219 | 393 | 395 | |||||||||
Total |
33,269 | 32,804 | 31,601 | |||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 147 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Royalties |
||||||||||||
Income |
522 | 255 | 288 | |||||||||
Amortisation |
(59 | ) | (79 | ) | (84 | ) | ||||||
Impairment |
(123 | ) | (150 | ) | (91 | ) | ||||||
Net gain on disposal of property, plant and equipment |
66 | 8 | 6 | |||||||||
Gains on disposal of product rights |
– | 170 | – | |||||||||
Net (loss)/gain on disposal of other intangible assets |
(1 | ) | 1 | (17 | ) | |||||||
Gains on divestments of non-core products |
– | 216 | 118 | |||||||||
Impairment of intangible assets relating to future licensing and contractual income |
– | (115 | ) | – | ||||||||
Other income |
307 | 265 | 304 | |||||||||
Other expense |
– | (18 | ) | – | ||||||||
Other operating income and expense |
712 | 553 | 524 | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Cost of sales |
144 | 188 | 405 | |||||||||
Research and development |
654 | 68 | 166 | |||||||||
Selling, general and administrative costs |
404 | 403 | 310 | |||||||||
Total charge |
1,202 | 659 | 881 | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Severance costs |
505 | 262 | 499 | |||||||||
Accelerated depreciation and impairment |
299 | 148 | 219 | |||||||||
Other |
398 | 249 | 163 | |||||||||
Total charge |
1,202 | 659 | 881 | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Finance income |
||||||||||||
Returns on fixed deposits and equity securities |
9 | 20 | 15 | |||||||||
Returns on short-term deposits |
33 | 22 | 127 | |||||||||
Expected return on post-employment defined benefit plan assets |
451 | 388 | 584 | |||||||||
Fair value gains on debt, interest rate swaps and investments |
23 | 1 | 128 | |||||||||
Net exchange gains |
– | 31 | – | |||||||||
Total |
516 | 462 | 854 | |||||||||
148 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Finance expense |
||||||||||||
Interest on debt and commercial paper |
(450 | ) | (542 | ) | (664 | ) | ||||||
Interest on overdrafts and other financing costs |
(29 | ) | (18 | ) | (50 | ) | ||||||
Interest on post-employment defined benefit plan liabilities |
(543 | ) | (493 | ) | (589 | ) | ||||||
Fair value charges on debt, interest rate swaps and investments |
– | (145 | ) | (2 | ) | |||||||
Net exchange losses |
(11 | ) | – | (12 | ) | |||||||
Total |
(1,033 | ) | (1,198 | ) | (1,317 | ) | ||||||
Net finance expense |
(517 | ) | (736 | ) | (463 | ) | ||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Current tax expense |
||||||||||||
Current year |
3,065 | 2,854 | 2,946 | |||||||||
Adjustment for prior years |
370 | 251 | 130 | |||||||||
| 3,435 | 3,105 | 3,076 | ||||||||||
Deferred tax expense |
||||||||||||
Origination and reversal of temporary differences |
(369 | ) | 98 | (486 | ) | |||||||
Adjustment to prior years |
(170 | ) | 60 | (39 | ) | |||||||
| (539 | ) | 158 | (525 | ) | ||||||||
Taxation recognised in the profit for the period |
2,896 | 3,263 | 2,551 | |||||||||
Taxation relating to components of other comprehensive income is as follows: |
||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Current and deferred tax |
||||||||||||
Foreign exchange arising on consolidation |
(29 | ) | 16 | 20 | ||||||||
Actuarial loss for the period |
(18 | ) | 158 | 340 | ||||||||
Share-based payments |
9 | 17 | 9 | |||||||||
Deferred tax impact of reduction in UK tax rate |
(23 | ) | – | – | ||||||||
Other |
– | 1 | (1 | ) | ||||||||
Taxation relating to components of other comprehensive income |
(61 | ) | 192 | 368 | ||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 149 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Profit before tax |
10,977 | 10,807 | 8,681 | |||||||||
Notional taxation charge at UK corporation tax rate of 28% (28% for 2009, 28.5% for 2008) |
3,074 | 3,026 | 2,474 | |||||||||
Differences in effective overseas tax rates |
(333 | ) | (212 | ) | (8 | ) | ||||||
Deferred tax credit relating to reduction in Swedish, UK and other tax rates1,2 |
(21 | ) | – | (70 | ) | |||||||
Unrecognised deferred tax asset |
– | 2 | (7 | ) | ||||||||
Items not deductible for tax purposes |
12 | 156 | 119 | |||||||||
Items not chargeable for tax purposes |
(36 | ) | (20 | ) | (48 | ) | ||||||
Adjustments in respect of prior periods |
200 | 311 | 91 | |||||||||
Total tax charge for the year |
2,896 | 3,263 | 2,551 | |||||||||
| 1 | The 2010 item relates to the reduction in the UK statutory corporation tax rate from 28% to 27% effective from 1 April 2011. | |
| 2 | The 2008 item relates to the reduction in the Swedish statutory corporation tax rate from 28% to 26.3% effective from 1 January 2009. |
| Pension | Inter- | Losses and | ||||||||||||||||||||||||||||||||||||||||||
| Property, | and post- | company | Deferred | tax credits | ||||||||||||||||||||||||||||||||||||||||
| plant and | Intangible | retirement | inventory | Untaxed | Accrued | Share | capital | carried | ||||||||||||||||||||||||||||||||||||
| equipment | assets | benefits | transfers | reserves1 | expenses | schemes | gains | forward | Other | Total | ||||||||||||||||||||||||||||||||||
| $m | $m | $m | $m | $m | $m | $m | $m | $m | $m | $m | ||||||||||||||||||||||||||||||||||
Deferred tax assets at 1 January 2008 |
66 | 59 | 531 | 907 | – | 611 | 62 | – | 330 | 71 | 2,637 | |||||||||||||||||||||||||||||||||
Deferred tax liabilities at 1 January 2008 |
(693 | ) | (3,653 | ) | (3 | ) | (21 | ) | (1,171 | ) | (13 | ) | – | (88 | ) | – | (70 | ) | (5,712 | ) | ||||||||||||||||||||||||
Net deferred tax balance at 1 January 2008 |
(627 | ) | (3,594 | ) | 528 | 886 | (1,171 | ) | 598 | 62 | (88 | ) | 330 | 1 | (3,075 | ) | ||||||||||||||||||||||||||||
Taxation expense |
122 | 375 | 24 | 55 | (119 | ) | 37 | 43 | – | 12 | (24 | ) | 525 | |||||||||||||||||||||||||||||||
Other comprehensive income |
– | – | 340 | – | – | – | 9 | – | – | (1 | ) | 348 | ||||||||||||||||||||||||||||||||
Exchange |
168 | 130 | (113 | ) | (35 | ) | 199 | (37 | ) | (14 | ) | 24 | (7 | ) | (3 | ) | 312 | |||||||||||||||||||||||||||
Net deferred tax balance at 31 December 2008 |
(337 | ) | (3,089 | ) | 779 | 906 | (1,091 | ) | 598 | 100 | (64 | ) | 335 | (27 | ) | (1,890 | ) | |||||||||||||||||||||||||||
Deferred tax assets at 31 December 2008 |
136 | 42 | 786 | 935 | – | 598 | 100 | – | 335 | 45 | 2,977 | |||||||||||||||||||||||||||||||||
Deferred tax liabilities at 31 December 2008 |
(473 | ) | (3,131 | ) | (7 | ) | (29 | ) | (1,091 | ) | – | – | (64 | ) | – | (72 | ) | (4,867 | ) | |||||||||||||||||||||||||
Net deferred tax balance at 31 December 2008 |
(337 | ) | (3,089 | ) | 779 | 906 | (1,091 | ) | 598 | 100 | (64 | ) | 335 | (27 | ) | (1,890 | ) | |||||||||||||||||||||||||||
Taxation expense |
175 | 232 | (61 | ) | 17 | (303 | ) | (146 | ) | 5 | – | (100 | ) | 23 | (158 | ) | ||||||||||||||||||||||||||||
Other comprehensive income |
– | – | 140 | – | – | – | 17 | – | – | – | 157 | |||||||||||||||||||||||||||||||||
Exchange |
(46 | ) | (36 | ) | 54 | 29 | (80 | ) | 18 | 7 | (7 | ) | (4 | ) | 1 | (64 | ) | |||||||||||||||||||||||||||
Net deferred tax balance at 31 December 2009 |
(208 | ) | (2,893 | ) | 912 | 952 | (1,474 | ) | 470 | 129 | (71 | ) | 231 | (3 | ) | (1,955 | ) | |||||||||||||||||||||||||||
Deferred tax assets at 31 December 2009 |
266 | 47 | 918 | 968 | – | 553 | 129 | – | 231 | 34 | 3,146 | |||||||||||||||||||||||||||||||||
Deferred tax liabilities at 31 December 2009 |
(474 | ) | (2,940 | ) | (6 | ) | (16 | ) | (1,474 | ) | (83 | ) | – | (71 | ) | – | (37 | ) | (5,101 | ) | ||||||||||||||||||||||||
Net deferred tax balance at 31 December 2009 |
(208 | ) | (2,893 | ) | 912 | 952 | (1,474 | ) | 470 | 129 | (71 | ) | 231 | (3 | ) | (1,955 | ) | |||||||||||||||||||||||||||
Taxation expense |
131 | 465 | (178 | ) | 3 | 24 | 66 | (5 | ) | 2 | 50 | (19 | ) | 539 | ||||||||||||||||||||||||||||||
Other comprehensive income |
– | – | (46 | ) | – | – | – | 4 | – | – | 1 | (41 | ) | |||||||||||||||||||||||||||||||
Acquisition of subsidiary undertaking2 |
– | (143 | ) | – | – | – | – | – | – | – | 2 | (141 | ) | |||||||||||||||||||||||||||||||
Exchange |
(6 | ) | 5 | (9 | ) | 15 | (81 | ) | 12 | (1 | ) | 3 | (10 | ) | – | (72 | ) | |||||||||||||||||||||||||||
Net deferred tax balance at 31 December 2010 |
(83 | ) | (2,566 | ) | 679 | 970 | (1,531 | ) | 548 | 127 | (66 | ) | 271 | (19 | ) | (1,670 | ) | |||||||||||||||||||||||||||
Deferred tax assets at 31 December 2010 |
357 | 54 | 686 | 988 | – | 558 | 127 | – | 271 | 25 | 3,066 | |||||||||||||||||||||||||||||||||
Deferred tax liabilities at 31 December 2010 |
(440 | ) | (2,620 | ) | (7 | ) | (18 | ) | (1,531 | ) | (10 | ) | – | (66 | ) | – | (44 | ) | (4,736 | ) | ||||||||||||||||||||||||
Net deferred tax balance at 31 December 2010 |
(83 | ) | (2,566 | ) | 679 | 970 | (1,531 | ) | 548 | 127 | (66 | ) | 271 | (19 | ) | (1,670 | ) | |||||||||||||||||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Analysed in the statement of financial position, after offset of balances within countries, as: | $m | $m | $m | |||||||||
Deferred tax assets |
1,475 | 1,292 | 1,236 | |||||||||
Deferred tax liabilities |
(3,145 | ) | (3,247 | ) | (3,126 | ) | ||||||
Net deferred tax balance |
(1,670 | ) | (1,955 | ) | (1,890 | ) | ||||||
| 1 | Untaxed reserves relate to taxable profits where the tax liability is deferred to later periods. | |
| 2 | The deferred tax liability of $143m relates to the acquisition of Novexel S.A. |
150 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
Profit for the financial year attributable to equity holders ($m) |
8,053 | 7,521 | 6,101 | |||||||||
Basic earnings per Ordinary Share |
$5.60 | $5.19 | $4.20 | |||||||||
Diluted earnings per Ordinary Share |
$5.57 | $5.19 | $4.20 | |||||||||
Weighted average number of Ordinary Shares in issue for basic earnings (millions) |
1,438 | 1,448 | 1,453 | |||||||||
Dilutive impact of share options outstanding (millions) |
8 | 2 | – | |||||||||
Diluted weighted average number of Ordinary Shares in issue (millions) |
1,446 | 1,450 | 1,453 | |||||||||
| Revenue | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
UK |
||||||||||||
External |
1,952 | 1,809 | 1,910 | |||||||||
Intra-Group |
9,957 | 9,056 | 8,460 | |||||||||
| 11,909 | 10,865 | 10,370 | ||||||||||
Continental Europe |
||||||||||||
Belgium |
331 | 353 | 380 | |||||||||
France |
1,929 | 1,880 | 1,945 | |||||||||
Germany |
1,151 | 1,197 | 1,225 | |||||||||
Italy |
1,000 | 1,012 | 1,145 | |||||||||
Spain |
762 | 742 | 832 | |||||||||
Sweden |
1,157 | 1,070 | 1,135 | |||||||||
Others |
2,440 | 2,622 | 2,696 | |||||||||
Intra-Group |
5,144 | 4,944 | 3,895 | |||||||||
| 13,914 | 13,820 | 13,253 | ||||||||||
The Americas |
||||||||||||
Canada |
1,492 | 1,188 | 1,269 | |||||||||
US |
14,010 | 14,994 | 13,657 | |||||||||
Others |
1,387 | 1,113 | 1,155 | |||||||||
Intra-Group |
2,341 | 1,962 | 1,169 | |||||||||
| 19,230 | 19,257 | 17,250 | ||||||||||
Asia, Africa & Australasia |
||||||||||||
Australia |
981 | 790 | 763 | |||||||||
Japan |
2,458 | 2,214 | 1,861 | |||||||||
China |
1,047 | 811 | 627 | |||||||||
Others |
1,172 | 1,009 | 1,001 | |||||||||
Intra-Group |
67 | 80 | 78 | |||||||||
| 5,725 | 4,904 | 4,330 | ||||||||||
Continuing operations |
50,778 | 48,846 | 45,203 | |||||||||
Intra-Group eliminations |
(17,509 | ) | (16,042 | ) | (13,602 | ) | ||||||
| 33,269 | 32,804 | 31,601 | ||||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 151 |
Table of Contents
| Operating profit | Profit before tax | |||||||||||||||||||||||
| 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Profit from | $m | $m | $m | $m | $m | $m | ||||||||||||||||||
UK |
3,258 | 3,124 | 2,907 | 3,098 | 2,813 | 2,612 | ||||||||||||||||||
Continental Europe |
4,591 | 4,809 | 3,136 | 4,581 | 4,821 | 3,233 | ||||||||||||||||||
The Americas |
3,278 | 3,265 | 2,705 | 2,932 | 2,832 | 2,440 | ||||||||||||||||||
Asia, Africa & Australasia |
367 | 345 | 396 | 366 | 341 | 396 | ||||||||||||||||||
Continuing operations |
11,494 | 11,543 | 9,144 | 10,977 | 10,807 | 8,681 | ||||||||||||||||||
| Non-current assets1 | Total assets | |||||||||||||||||||||||
| 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
UK |
3,397 | 3,810 | 3,524 | 17,171 | 17,092 | 9,870 | ||||||||||||||||||
Continental Europe |
4,470 | 3,966 | 3,674 | 7,596 | 6,706 | 6,275 | ||||||||||||||||||
The Americas |
20,808 | 21,354 | 21,762 | 28,175 | 28,397 | 28,290 | ||||||||||||||||||
Asia, Africa & Australasia |
522 | 476 | 436 | 3,185 | 2,725 | 2,515 | ||||||||||||||||||
Continuing operations |
29,197 | 29,606 | 29,396 | 56,127 | 54,920 | 46,950 | ||||||||||||||||||
| Assets acquired2 | Net operating assets3 | |||||||||||||||||||||||
| 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
UK |
314 | 537 | 440 | 3,273 | 4,473 | 4,234 | ||||||||||||||||||
Continental Europe |
1,053 | 643 | 295 | 4,827 | 4,094 | 3,683 | ||||||||||||||||||
The Americas |
1,125 | 711 | 3,252 | 18,795 | 19,186 | 21,033 | ||||||||||||||||||
Asia, Africa & Australasia |
107 | 79 | 67 | 2,021 | 1,707 | 1,732 | ||||||||||||||||||
Continuing operations |
2,599 | 1,970 | 4,054 | 28,916 | 29,460 | 30,682 | ||||||||||||||||||
| 1 | ‘Non-current assets’ exclude deferred tax assets and derivative financial instruments. | |
| 2 | Included in ‘assets acquired’ are those assets that are expected to be used during more than one period (property, plant and equipment, goodwill and intangible assets). | |
| 3 | ‘Net operating assets’ exclude short-term investments, cash, short-term borrowings, loans, retirement benefit obligations and non-operating receivables and payables. |
| Property, plant and equipment | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
UK |
1,628 | 1,901 | 1,750 | |||||||||
Sweden |
1,647 | 1,700 | 1,722 | |||||||||
US |
2,381 | 2,386 | 2,200 | |||||||||
Rest of the world |
1,301 | 1,320 | 1,371 | |||||||||
Continuing operations |
6,957 | 7,307 | 7,043 | |||||||||
Geographic markets |
||||||||||||
The table below shows revenue in each geographic market in which customers are located. |
||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
UK |
1,033 | 1,057 | 994 | |||||||||
Continental Europe |
9,315 | 9,286 | 9,937 | |||||||||
The Americas |
16,629 | 17,096 | 15,945 | |||||||||
Asia, Africa & Australasia |
6,292 | 5,365 | 4,725 | |||||||||
Continuing operations |
33,269 | 32,804 | 31,601 | |||||||||
152 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Total property, | ||||||||||||||||
| Land and | Plant and | Assets in course | plant and | |||||||||||||
| buildings | equipment | of construction | equipment | |||||||||||||
| $m | $m | $m | $m | |||||||||||||
Cost |
||||||||||||||||
At 1 January 2008 |
5,819 | 10,174 | 842 | 16,835 | ||||||||||||
Capital expenditure |
49 | 239 | 825 | 1,113 | ||||||||||||
Transfer of assets into use |
275 | 404 | (679 | ) | – | |||||||||||
Disposals and other movements |
(123 | ) | (558 | ) | (25 | ) | (706 | ) | ||||||||
Exchange adjustments |
(803 | ) | (1,725 | ) | (100 | ) | (2,628 | ) | ||||||||
At 31 December 2008 |
5,217 | 8,534 | 863 | 14,614 | ||||||||||||
Capital expenditure |
8 | 209 | 750 | 967 | ||||||||||||
Transfer of assets into use |
218 | 388 | (606 | ) | – | |||||||||||
Disposals and other movements |
(400 | ) | (937 | ) | (20 | ) | (1,357 | ) | ||||||||
Exchange adjustments |
293 | 609 | 42 | 944 | ||||||||||||
At 31 December 2009 |
5,336 | 8,803 | 1,029 | 15,168 | ||||||||||||
Capital expenditure |
13 | 225 | 570 | 808 | ||||||||||||
Transfer of assets into use |
342 | 668 | (1,010 | ) | – | |||||||||||
Disposals and other movements |
(40 | ) | (449 | ) | (4 | ) | (493 | ) | ||||||||
Exchange adjustments |
48 | 46 | 6 | 100 | ||||||||||||
At 31 December 2010 |
5,699 | 9,293 | 591 | 15,583 | ||||||||||||
Depreciation |
||||||||||||||||
At 1 January 2008 |
2,015 | 6,521 | 1 | 8,537 | ||||||||||||
Charge for year |
247 | 812 | – | 1,059 | ||||||||||||
Impairment |
91 | 32 | – | 123 | ||||||||||||
Disposals and other movements |
(120 | ) | (529 | ) | (2 | ) | (651 | ) | ||||||||
Exchange adjustments |
(303 | ) | (1,192 | ) | (2 | ) | (1,497 | ) | ||||||||
At 31 December 2008 |
1,930 | 5,644 | (3 | ) | 7,571 | |||||||||||
Charge for year |
219 | 674 | – | 893 | ||||||||||||
Impairment |
44 | 6 | – | 50 | ||||||||||||
Disposals and other movements |
(343 | ) | (859 | ) | (4 | ) | (1,206 | ) | ||||||||
Exchange adjustments |
117 | 434 | 2 | 553 | ||||||||||||
At 31 December 2009 |
1,967 | 5,899 | (5 | ) | 7,861 | |||||||||||
Charge for year |
302 | 774 | – | 1,076 | ||||||||||||
Impairment |
2 | 20 | – | 22 | ||||||||||||
Disposals and other movements |
(29 | ) | (396 | ) | 5 | (420 | ) | |||||||||
Exchange adjustments |
32 | 55 | – | 87 | ||||||||||||
At 31 December 2010 |
2,274 | 6,352 | – | 8,626 | ||||||||||||
Net book value |
||||||||||||||||
At 31 December 2008 |
3,287 | 2,890 | 866 | 7,043 | ||||||||||||
At 31 December 2009 |
3,369 | 2,904 | 1,034 | 7,307 | ||||||||||||
At 31 December 2010 |
3,425 | 2,941 | 591 | 6,957 | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
The net book value of land and buildings comprised: |
||||||||||||
Freeholds |
3,425 | 3,369 | 3,287 | |||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 153 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Cost |
||||||||||||
At 1 January |
10,228 | 10,211 | 10,225 | |||||||||
Additions through business combinations |
– | – | – | |||||||||
Exchange adjustments |
(22 | ) | 17 | (14 | ) | |||||||
At 31 December |
10,206 | 10,228 | 10,211 | |||||||||
Amortisation and impairment losses |
||||||||||||
At 1 January |
339 | 337 | 341 | |||||||||
Exchange adjustments |
(4 | ) | 2 | (4 | ) | |||||||
At 31 December |
335 | 339 | 337 | |||||||||
Net book value at 31 December |
9,871 | 9,889 | 9,874 | |||||||||
154 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Product, | Software | |||||||||||||||
| marketing and | Other | development | ||||||||||||||
| distribution rights | intangibles | costs | Total | |||||||||||||
| $m | $m | $m | $m | |||||||||||||
Cost |
||||||||||||||||
At 1 January 2008 |
11,549 | 2,385 | 976 | 14,910 | ||||||||||||
Additions – separately acquired |
2,743 | 20 | 178 | 2,941 | ||||||||||||
Disposals |
– | (33 | ) | (30 | ) | (63 | ) | |||||||||
Exchange and other adjustments |
(770 | ) | (197 | ) | (133 | ) | (1,100 | ) | ||||||||
At 31 December 2008 |
13,522 | 2,175 | 991 | 16,688 | ||||||||||||
Additions – separately acquired |
764 | 46 | 193 | 1,003 | ||||||||||||
Disposals |
(200 | ) | (1 | ) | – | (201 | ) | |||||||||
Exchange and other adjustments |
267 | 84 | 28 | 379 | ||||||||||||
At 31 December 2009 |
14,353 | 2,304 | 1,212 | 17,869 | ||||||||||||
Additions through business combinations |
548 | – | – | 548 | ||||||||||||
Additions – separately acquired |
1,017 | 20 | 206 | 1,243 | ||||||||||||
Disposals |
(239 | ) | (2 | ) | – | (241 | ) | |||||||||
Exchange and other adjustments |
125 | 13 | (19 | ) | 119 | |||||||||||
At 31 December 2010 |
15,804 | 2,335 | 1,399 | 19,538 | ||||||||||||
Amortisation and impairment losses |
||||||||||||||||
At 1 January 2008 |
2,373 | 528 | 542 | 3,443 | ||||||||||||
Amortisation for year |
529 | 182 | 96 | 807 | ||||||||||||
Impairment |
516 | 91 | 24 | 631 | ||||||||||||
Disposals |
– | (9 | ) | (10 | ) | (19 | ) | |||||||||
Exchange and other adjustments |
(357 | ) | (104 | ) | (36 | ) | (497 | ) | ||||||||
At 31 December 2008 |
3,061 | 688 | 616 | 4,365 | ||||||||||||
Amortisation for year |
481 | 162 | 86 | 729 | ||||||||||||
Impairment |
93 | 273 | 49 | 415 | ||||||||||||
Disposals |
(67 | ) | – | – | (67 | ) | ||||||||||
Exchange and other adjustments |
159 | 25 | 17 | 201 | ||||||||||||
At 31 December 2009 |
3,727 | 1,148 | 768 | 5,643 | ||||||||||||
Amortisation for year |
573 | 121 | 116 | 810 | ||||||||||||
Impairment |
699 | 131 | 3 | 833 | ||||||||||||
Disposals |
– | (1 | ) | – | (1 | ) | ||||||||||
Exchange and other adjustments |
89 | 26 | (20 | ) | 95 | |||||||||||
At 31 December 2010 |
5,088 | 1,425 | 867 | 7,380 | ||||||||||||
Net book value |
||||||||||||||||
At 31 December 2008 |
10,461 | 1,487 | 375 | 12,323 | ||||||||||||
At 31 December 2009 |
10,626 | 1,156 | 444 | 12,226 | ||||||||||||
At 31 December 2010 |
10,716 | 910 | 532 | 12,158 | ||||||||||||
|
Other intangibles consist mainly of licensing and rights to contractual income streams. |
||||||||||||||||
|
Amortisation charges are recognised in profit as follows: |
||||||||||||||||
| Product, | Software | |||||||||||||||
| marketing and | Other | development | ||||||||||||||
| distribution rights | intangibles | costs | Total | |||||||||||||
| $m | $m | $m | $m | |||||||||||||
Year ended 31 December 2008 |
||||||||||||||||
Cost of sales |
39 | – | – | 39 | ||||||||||||
Research and development |
10 | – | – | 10 | ||||||||||||
Selling, general and administrative costs |
480 | 35 | 96 | 611 | ||||||||||||
Other operating income and expense |
– | 147 | – | 147 | ||||||||||||
| 529 | 182 | 96 | 807 | |||||||||||||
Year ended 31 December 2009 |
||||||||||||||||
Cost of sales |
48 | – | – | 48 | ||||||||||||
Selling, general and administrative costs |
433 | 27 | 86 | 546 | ||||||||||||
Other operating income and expense |
– | 135 | – | 135 | ||||||||||||
| 481 | 162 | 86 | 729 | |||||||||||||
Year ended 31 December 2010 |
||||||||||||||||
Cost of sales |
60 | – | – | 60 | ||||||||||||
Selling, general and administrative costs |
513 | 22 | 116 | 651 | ||||||||||||
Other operating income and expense |
– | 99 | – | 99 | ||||||||||||
| 573 | 121 | 116 | 810 | |||||||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 155 |
Table of Contents
| Product, | Software | |||||||||||||||
| marketing and | Other | development | ||||||||||||||
| distribution rights | intangibles | costs | Total | |||||||||||||
| $m | $m | $m | $m | |||||||||||||
Year ended 31 December 2008 |
||||||||||||||||
Cost of sales |
115 | – | – | 115 | ||||||||||||
Research and development |
144 | – | – | 144 | ||||||||||||
Selling, general and administrative costs |
257 | – | 24 | 281 | ||||||||||||
Other operating income and expense |
– | 91 | – | 91 | ||||||||||||
| 516 | 91 | 24 | 631 | |||||||||||||
Year ended 31 December 2009 |
||||||||||||||||
Research and development |
93 | 7 | – | 100 | ||||||||||||
Selling, general and administrative costs |
– | 1 | 49 | 50 | ||||||||||||
Other operating income and expense |
– | 265 | – | 265 | ||||||||||||
| 93 | 273 | 49 | 415 | |||||||||||||
Year ended 31 December 2010 |
||||||||||||||||
Cost of sales |
128 | – | – | 128 | ||||||||||||
Research and development |
571 | – | – | 571 | ||||||||||||
Selling, general and administrative costs |
– | 3 | 3 | 6 | ||||||||||||
Other operating income and expense |
– | 128 | – | 128 | ||||||||||||
| 699 | 131 | 3 | 833 | |||||||||||||
| Carrying value | Remaining amortisation | |||||||||||
| Description | $m | period | ||||||||||
Intangible assets arising from joint venture with Merck1 |
Product, marketing and distribution rights | 186 | 3 and 7 years | |||||||||
Advance payment1 |
Product, marketing and distribution rights | 490 | 8 years | |||||||||
Partial retirement1 |
Product, marketing and distribution rights | 735 | 4-17 years | |||||||||
First Option1 |
Product, marketing and distribution rights | 1,651 | 1-13 years | |||||||||
Non-refundable deposit1 |
Product, marketing and distribution rights | 474 | Not amortised | |||||||||
Intangible assets arising from the acquisition of CAT2 |
Product, marketing and distribution rights | 363 | 5 and 10 years | |||||||||
Intangible assets arising from the acquisition of KuDOS2 |
Product, marketing and distribution rights | 285 | Not amortised | |||||||||
RSV franchise assets arising from the acquisition of MedImmune |
Product, marketing and distribution rights | 4,174 | 15 years | |||||||||
Intangible assets arising from the acquisition of MedImmune |
Licensing and contractual income | 522 | 1-9 years | |||||||||
Intangible assets arising from the acquisition of MedImmune |
Product, marketing and distribution rights | 603 | 21 years | |||||||||
Intangible assets arising from the collaboration with BMS3 |
Product, marketing and distribution rights | 419 | 12-13 years | |||||||||
Intangible assets arising from the acquisition of Novexel2 |
Product, marketing and distribution rights | 302 | Not amortised | |||||||||
Intangible assets arising from the collaboration with Pozen4 |
Product, marketing and distribution rights | 213 | 13 years | |||||||||
| 1 | These assets are associated with the restructuring of the joint venture with Merck & Co., Inc. Further information can be found in Note 25. | |
| 2 | Assets in development are not amortised but are tested annually for impairment. | |
| 3 | These assets arise from the collaboration agreement with BMS for Onglyza™ and dapagliflozin. | |
| 4 | These assets arise from the collaboration agreement with Pozen for Vimovo. |
156 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Non-current investments |
||||||||||||
Equity securities available for sale |
211 | 184 | 156 | |||||||||
| 211 | 184 | 156 | ||||||||||
Current investments |
||||||||||||
Equity securities and bonds available for sale |
355 | – | – | |||||||||
Equity securities held for trading |
20 | 18 | 51 | |||||||||
Fixed deposits |
1,107 | 1,466 | 54 | |||||||||
| 1,482 | 1,484 | 105 | ||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Raw materials and consumables |
539 | 445 | 409 | |||||||||
Inventories in process |
665 | 726 | 631 | |||||||||
Finished goods and goods for resale |
478 | 579 | 596 | |||||||||
| 1,682 | 1,750 | 1,636 | ||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Amounts due within one year |
||||||||||||
Trade receivables |
6,328 | 5,863 | 5,657 | |||||||||
Less: Amounts provided for doubtful debts (Note 23) |
(81 | ) | (81 | ) | (99 | ) | ||||||
| 6,247 | 5,782 | 5,558 | ||||||||||
Other receivables |
607 | 1,170 | 978 | |||||||||
Prepayments and accrued income |
733 | 580 | 552 | |||||||||
| 7,587 | 7,532 | 7,088 | ||||||||||
Amounts due after more than one year |
||||||||||||
Other receivables |
64 | 27 | 44 | |||||||||
Prepayments and accrued income |
196 | 150 | 129 | |||||||||
| 260 | 177 | 173 | ||||||||||
Trade and other receivables |
7,847 | 7,709 | 7,261 | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Cash at bank and in hand |
1,750 | 1,077 | 1,039 | |||||||||
Short-term deposits |
9,318 | 8,841 | 3,247 | |||||||||
Cash and cash equivalents |
11,068 | 9,918 | 4,286 | |||||||||
Unsecured bank overdrafts |
(87 | ) | (90 | ) | (163 | ) | ||||||
Cash and cash equivalents in the cash flow statement |
10,981 | 9,828 | 4,123 | |||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 157 |
Table of Contents
| Repayment | 2010 | 2009 | 2008 | |||||||||||||||||
| dates | $m | $m | $m | |||||||||||||||||
Current liabilities |
||||||||||||||||||||
Bank overdrafts |
On demand | 87 | 90 | 163 | ||||||||||||||||
Floating rate note |
US dollars | 2009 | – | – | 650 | |||||||||||||||
4.625% Non-callable bond |
Euros | 2010 | – | 1,073 | – | |||||||||||||||
5.625% Non-callable bond |
Euros | 2010 | – | 717 | – | |||||||||||||||
Other loans |
Within one year | 38 | 46 | 180 | ||||||||||||||||
| 125 | 1,926 | 993 | ||||||||||||||||||
Non-current liabilities |
||||||||||||||||||||
4.625% Non-callable bond |
Euros | 2010 | – | – | 1,053 | |||||||||||||||
5.625% Non-callable bond |
Euros | 2010 | – | – | 702 | |||||||||||||||
5.4% Callable bond |
US dollars | 2012 | 1,800 | 1,805 | 1,823 | |||||||||||||||
5.4% Callable bond |
US dollars | 2014 | 837 | 821 | 789 | |||||||||||||||
5.125% Non-callable bond |
Euros | 2015 | 993 | 1,072 | 1,051 | |||||||||||||||
5.9% Callable bond |
US dollars | 2017 | 1,855 | 1,818 | 1,896 | |||||||||||||||
7% Guaranteed debentures |
US dollars | 2023 | 359 | 346 | 324 | |||||||||||||||
5.75% Non-callable bond |
Pounds sterling | 2031 | 535 | 558 | 501 | |||||||||||||||
6.45% Callable bond |
US dollars | 2037 | 2,718 | 2,717 | 2,716 | |||||||||||||||
| 9,097 | 9,137 | 10,855 | ||||||||||||||||||
| Non-current | Current | Current | Non-current | |||||||||||||||||
| assets | assets | liabilities | liabilities | Total | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Designated in a fair value hedge |
164 | – | – | – | 164 | |||||||||||||||
Related to instruments designated at fair value through profit or loss |
160 | – | – | – | 160 | |||||||||||||||
Other derivatives |
– | 9 | (8 | ) | – | 1 | ||||||||||||||
31 December 2010 |
324 | 9 | (8 | ) | – | 325 | ||||||||||||||
| Non-current | Current | Current | Non-current | |||||||||||||||||
| assets | assets | liabilities | liabilities | Total | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Designated in a fair value hedge |
135 | – | – | – | 135 | |||||||||||||||
Related to instruments designated at fair value through profit or loss |
127 | – | – | – | 127 | |||||||||||||||
Other derivatives |
– | 24 | (90 | ) | – | (66 | ) | |||||||||||||
31 December 2009 |
262 | 24 | (90 | ) | – | 196 | ||||||||||||||
| Non-current | Current | Current | Non-current | |||||||||||||||||
| assets | assets | liabilities | liabilities | Total | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Designated in a fair value hedge |
229 | – | – | – | 229 | |||||||||||||||
Related to instruments designated at fair value through profit or loss |
220 | – | – | – | 220 | |||||||||||||||
Other derivatives |
– | – | (95 | ) | (71 | ) | (166 | ) | ||||||||||||
31 December 2008 |
449 | – | (95 | ) | (71 | ) | 283 | |||||||||||||
158 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Instruments | Instruments | Other financial | Total | |||||||||||||||||||||||||||||
| in a hedge | designated | instruments | Available | Held for | Amortised | carrying | Fair | |||||||||||||||||||||||||
| relationship | 1 | at fair value | 2 | at fair value | 3 | for sale | trading | cost | value | value | ||||||||||||||||||||||
| $m | $m | $m | $m | $m | $m | $m | $m | |||||||||||||||||||||||||
2010 |
||||||||||||||||||||||||||||||||
Cash and cash equivalents |
— | — | — | — | — | 11,068 | 11,068 | 11,068 | ||||||||||||||||||||||||
Overdrafts |
— | — | — | — | — | (87 | ) | (87 | ) | (87 | ) | |||||||||||||||||||||
Loans due within one year |
— | — | — | — | — | (38 | ) | (38 | ) | (38 | ) | |||||||||||||||||||||
Loans due after more than one year |
(1,659 | ) | (1,196 | ) | — | — | — | (6,242 | ) | (9,097 | ) | (10,022 | ) | |||||||||||||||||||
Derivative financial instruments |
164 | 160 | 1 | — | — | — | 325 | 325 | ||||||||||||||||||||||||
Other investments |
— | — | — | 566 | 20 | 1,107 | 1,693 | 1,693 | ||||||||||||||||||||||||
Other financial assets |
— | — | 25 | — | — | 6,893 | 6,918 | 6,918 | ||||||||||||||||||||||||
Other financial liabilities |
— | — | (50 | ) | — | — | (8,963 | ) | (9,013 | ) | (9,013 | ) | ||||||||||||||||||||
2009 |
||||||||||||||||||||||||||||||||
Cash and cash equivalents |
— | — | — | — | — | 9,918 | 9,918 | 9,918 | ||||||||||||||||||||||||
Overdrafts |
— | — | — | — | — | (90 | ) | (90 | ) | (90 | ) | |||||||||||||||||||||
Loans due within one year |
— | — | — | — | — | (1,836 | ) | (1,836 | ) | (1,867 | ) | |||||||||||||||||||||
Loans due after more than one year |
(1,629 | ) | (1,167 | ) | — | — | — | (6,341 | ) | (9,137 | ) | (9,832 | ) | |||||||||||||||||||
Derivative financial instruments |
135 | 127 | (66 | ) | — | — | — | 196 | 196 | |||||||||||||||||||||||
Other investments |
— | — | — | 184 | 18 | 1,466 | 1,668 | 1,668 | ||||||||||||||||||||||||
Other financial assets |
— | — | — | — | — | 6,979 | 6,979 | 6,979 | ||||||||||||||||||||||||
Other financial liabilities |
— | — | — | — | — | (8,872 | ) | (8,872 | ) | (8,872 | ) | |||||||||||||||||||||
2008 |
||||||||||||||||||||||||||||||||
Cash and cash equivalents |
— | — | — | — | — | 4,286 | 4,286 | 4,286 | ||||||||||||||||||||||||
Overdrafts |
— | — | — | — | — | (163 | ) | (163 | ) | (163 | ) | |||||||||||||||||||||
Loans due within one year |
— | — | — | — | — | (830 | ) | (830 | ) | (830 | ) | |||||||||||||||||||||
Loans due after more than one year |
(1,727 | ) | (1,113 | ) | — | — | — | (8,015 | ) | (10,855 | ) | (11,238 | ) | |||||||||||||||||||
Derivative financial instruments |
229 | 220 | (166 | ) | — | — | — | 283 | 283 | |||||||||||||||||||||||
Other investments |
— | — | — | 156 | 50 | 54 | 260 | 260 | ||||||||||||||||||||||||
Other financial assets |
— | — | — | — | — | 6,580 | 6,580 | 6,580 | ||||||||||||||||||||||||
Other financial liabilities |
— | — | — | — | — | (7,239 | ) | (7,239 | ) | (7,239 | ) | |||||||||||||||||||||
| 1 | Includes borrowings and derivatives designated as hedged items in fair value hedge relationships with respect to interest rate risk. | |
| 2 | Includes borrowings designated at fair value through profit or loss, and related derivatives. | |
| 3 | Includes derivatives not designated in hedge relationships or related to financial instruments designated at fair value through profit or loss, and contingent consideration arising on business combinations (Note 22). |
| > | Cash and overdrafts — held on the consolidated statement of financial position at amortised costs. Fair value approximates to carrying value. | |
| > | Loans due within one year and after more than one year — the fair value of fixed-rate publicly traded debt is based on year-end quoted market prices; the fair value of floating rate debt is nominal value, as mark to market differences would be minimal given the frequency of resets. The carrying value of loans designated at fair value through profit or loss is the fair value. For loans designated in a fair value hedge relationship, carrying value is initially measured at fair value and remeasured for fair value changes in respect of the hedged risk at each reporting date. All other loans are held at amortised cost. | |
| > | Derivative financial instruments — consists of interest rate swaps (included in designated as fair value through profit or loss if related to debt designated at fair value, or instruments in a hedge relationship as a fair value hedge or other derivatives) and forward foreign exchange contracts (included in other derivatives). All derivatives are held at fair value. |
| – | Interest rate swaps — the fair value is estimated using appropriate zero coupon curve valuation techniques to discount future contractual cash flows based on rates current at year end. | ||
| – | Forward foreign exchange contracts — the majority of contracts for existing transactions had maturities of six months or less from year end. The fair value of forward foreign exchange contracts is estimated by discounting the future contractual cash flows using appropriate yield curves based on market forward foreign exchange rates at the year end. |
| > | Other investments — held on the consolidated statement of financial position at fair value. These include equity securities held on the consolidated statement of financial position as other investments (Note 10). The fair value of listed investments is based on year end quoted market prices. Unlisted investments are held at cost which approximates to fair value. | |
| > | Other financial assets and other financial liabilities — with the exception of contingent consideration which is held at fair value (see Note 22), other financial assets and liabilities are held on the consolidated statement of financial position at amortised costs with carrying value being a reasonable approximation of fair value. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 159 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
Derivatives |
0.7% to 4.0% | 2.0% to 4.6% | 3.8% to 4.6% | |||||||||
Loans and borrowings |
0.7% to 4.0% | 2.0% to 4.6% | 3.8% to 4.6% | |||||||||
| > | Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities. | |
| > | Level 2: inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (ie as prices) or indirectly (ie derived from prices). | |
| > | Level 3: inputs for the asset or liability that are not based on observable market data (unobservable inputs). |
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| $m | $m | $m | $m | |||||||||||||
31 December 2010 |
||||||||||||||||
Equity securities and bonds available for sale |
399 | — | 167 | 566 | ||||||||||||
Equity securities held for trading |
20 | — | — | 20 | ||||||||||||
Derivative assets |
— | 333 | — | 333 | ||||||||||||
Other financial assets |
— | — | 25 | 25 | ||||||||||||
Assets |
419 | 333 | 192 | 944 | ||||||||||||
Borrowing designated at fair value through profit or loss |
(1,196 | ) | — | — | (1,196 | ) | ||||||||||
Derivative liabilities |
— | (8 | ) | — | (8 | ) | ||||||||||
Other financial liabilities |
— | — | (50 | ) | (50 | ) | ||||||||||
Liabilities |
(1,196 | ) | (8 | ) | (50 | ) | (1,254 | ) | ||||||||
31 December 2009 |
||||||||||||||||
Equity securities available for sale |
41 | — | 143 | 184 | ||||||||||||
Equity securities held for trading |
18 | — | — | 18 | ||||||||||||
Derivative assets |
— | 286 | — | 286 | ||||||||||||
Assets |
59 | 286 | 143 | 488 | ||||||||||||
Borrowing designated at fair value through profit or loss |
(1,167 | ) | — | — | (1,167 | ) | ||||||||||
Derivative liabilities |
— | (90 | ) | — | (90 | ) | ||||||||||
Liabilities |
(1,167 | ) | (90 | ) | — | (1,257 | ) | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Included in operating profit |
||||||||||||
Gains/(losses) on forward foreign exchange contracts |
29 | 114 | (399 | ) | ||||||||
(Losses)/gains on receivables and payables |
(80 | ) | (141 | ) | 391 | |||||||
Losses on available for sale current investments |
(2 | ) | (18 | ) | (25 | ) | ||||||
| (53 | ) | (45 | ) | (33 | ) | |||||||
Included in finance income and expense |
||||||||||||
Interest and fair value adjustments in respect of debt
designated at fair value through profit or loss, net of derivatives |
(5 | ) | (169 | ) | 87 | |||||||
Interest and changes in carrying values of debt designated
as hedged items, net of derivatives |
(18 | ) | (35 | ) | (64 | ) | ||||||
Interest and fair value changes on fixed and short-term
deposits and equity securities |
61 | 43 | 140 | |||||||||
Interest on debt, overdrafts and commercial paper held at amortised cost |
(452 | ) | (501 | ) | (609 | ) | ||||||
Exchange (losses)/gains on financial assets and liabilities |
(11 | ) | 31 | (12 | ) | |||||||
| (425 | ) | (631 | ) | (458 | ) | |||||||
Included in other comprehensive income |
||||||||||||
Foreign exchange differences on borrowings forming net investment hedges |
101 | (68 | ) | 291 | ||||||||
Amortisation of loss on cash flow hedge through profit |
1 | 1 | 1 | |||||||||
Available for sale (losses)/gains taken to equity |
4 | 2 | 2 | |||||||||
| 106 | (65 | ) | 294 | |||||||||
| 160 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Current liabilities |
||||||||||||
Trade payables |
2,257 | 2,316 | 1,940 | |||||||||
Value added and payroll taxes and social security |
323 | 342 | 371 | |||||||||
Rebates and chargebacks |
2,839 | 2,618 | 1,963 | |||||||||
Other payables |
945 | 1,038 | 1,026 | |||||||||
Accruals |
2,297 | 2,373 | 1,878 | |||||||||
| 8,661 | 8,687 | 7,178 | ||||||||||
Non-current liabilities |
||||||||||||
Other payables |
373 | 244 | 149 | |||||||||
| Employee | Other | |||||||||||||||||||||||
| Severance | Environmental | benefits | Legal | provisions | Total | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
At 1 January 2008 |
643 | 111 | 100 | 25 | 141 | 1,020 | ||||||||||||||||||
Charge/(credit) for year |
469 | 37 | (23 | ) | — | 164 | 647 | |||||||||||||||||
Cash paid |
(405 | ) | (39 | ) | (1 | ) | — | (12 | ) | (457 | ) | |||||||||||||
Exchange and other movements |
(88 | ) | 21 | 8 | — | (9 | ) | (68 | ) | |||||||||||||||
At 31 December 2008 |
619 | 130 | 84 | 25 | 284 | 1,142 | ||||||||||||||||||
Charge for year |
309 | 6 | 12 | 636 | 101 | 1,064 | ||||||||||||||||||
Cash paid |
(341 | ) | (23 | ) | — | (13 | ) | (34 | ) | (411 | ) | |||||||||||||
Reversals |
(89 | ) | — | — | — | (28 | ) | (117 | ) | |||||||||||||||
Exchange and other movements |
13 | (1 | ) | (1 | ) | — | (3 | ) | 8 | |||||||||||||||
At 31 December 2009 |
511 | 112 | 95 | 648 | 320 | 1,686 | ||||||||||||||||||
Charge for year |
497 | 48 | 11 | 617 | 188 | 1,361 | ||||||||||||||||||
Cash paid |
(335 | ) | (43 | ) | — | (709 | ) | (22 | ) | (1,109 | ) | |||||||||||||
Reversals |
(26 | ) | — | — | (1 | ) | (22 | ) | (49 | ) | ||||||||||||||
Exchange and other movements |
12 | 2 | 21 | 7 | 7 | 49 | ||||||||||||||||||
At 31 December 2010 |
659 | 119 | 127 | 562 | 471 | 1,938 | ||||||||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Due within one year |
1,095 | 1,209 | 600 | |||||||||
Due after more than one year |
843 | 477 | 542 | |||||||||
| 1,938 | 1,686 | 1,142 | ||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 161 |
Table of Contents
| > | The Group has a fundamental belief in funding the benefits it promises to employees. | |
| > | The Group considers its pension arrangements in the context of its broader capital structure. In general it does not believe in committing excessive capital for funding while it has better uses of capital within the business nor does it wish to generate surpluses. | |
| > | The pension funds are not part of the Group’s core business. The Group believes in taking some rewarded risks with the investments underlying the funding, subject to a medium to long-term plan to reduce those risks if opportunities arise. | |
| > | The Group recognises that deciding to hold certain investments may cause volatility in the funding position. The Group would not wish to amend its contribution level for relatively small deviations from its preferred funding level, because it is expected that there will be short-term volatility, but it is prepared to react appropriately to more significant deviations. | |
| > | In the event that local regulations require an additional level of financing, the Group would consider the use of alternative methods of providing this that do not require immediate cash funding but help mitigate exposure of the pension arrangement to the credit risk of the Group. |
| 162 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | The US defined benefits programme was actuarially revalued at 31 December 2010, when plan obligations were $1,757m and plan assets were $1,525m. This includes obligations in respect of the non-qualified plan which is largely unfunded. | |
| > | The Swedish defined benefits programme was actuarially revalued at 31 December 2010, when plan obligations were estimated to amount to $1,338m and plan assets were $809m. | |
| > | The German defined benefits programme was actuarially revalued at 31 December 2010, when plan obligations amounted to $241m and plan assets were $25m. |
| 2010 | 2009 | |||||||||||||||
| UK | Rest of Group | UK | Rest of Group | |||||||||||||
Inflation assumption |
3.6 | % | 2.3 | % | 3.5 | % | 2.3 | % | ||||||||
Rate of increase in salaries |
-1 | 3.4 | % | 4.5 | % | 3.4 | % | |||||||||
Rate of increase in pensions in payment |
3.5 | % | 0.9 | % | 3.5 | % | 0.9 | % | ||||||||
Discount rate |
5.5 | % | 4.9 | % | 5.5 | % | 5.0 | % | ||||||||
Long-term rate of return expected at 31 December |
||||||||||||||||
Equities |
8.0 | % | 7.9 | % | 8.0 | % | 8.1 | % | ||||||||
Bonds |
5.1 | % | 5.0 | % | 5.5 | % | 5.2 | % | ||||||||
Others |
6.1 | % | 4.7 | % | 6.5 | % | 4.8 | % | ||||||||
Rate of increase in medical costs (initial rate) |
10.0 | % | 10.0 | % | 10.0 | % | 10.0 | % | ||||||||
| 1 | Pensionable pay frozen at 30 June 2010 levels following UK fund changes. |
| Life expectancy assumption for a male member retiring at age 65 | ||||||||||||||||
| Country | 2010 | 2030 | 2009 | 2029 | ||||||||||||
UK |
22.7 | 24.6 | 23.8 | 25.8 | ||||||||||||
US |
19.8 | 21.3 | 19.6 | 21.1 | ||||||||||||
Sweden |
20.4 | 22.4 | 20.4 | 22.4 | ||||||||||||
Germany |
17.9 | 20.7 | 17.7 | 20.5 | ||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 163 |
Table of Contents
| 2010 | 2009 | |||||||||||||||||||||||
| UK | Rest of Group | Total | UK | Rest of Group | Total | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
Scheme assets |
||||||||||||||||||||||||
Equities |
2,437 | 1,153 | 3,590 | 2,309 | 1,241 | 3,550 | ||||||||||||||||||
Bonds |
2,660 | 1,124 | 3,784 | 2,279 | 903 | 3,182 | ||||||||||||||||||
Others |
52 | 341 | 393 | 265 | 258 | 523 | ||||||||||||||||||
Total fair value of scheme assets |
5,149 | 2,618 | 7,767 | 4,853 | 2,402 | 7,255 | ||||||||||||||||||
Present value of scheme obligations |
(6,554 | ) | (3,691 | ) | (10,245 | ) | (7,055 | ) | (3,591 | ) | (10,646 | ) | ||||||||||||
Past service cost not yet recognised |
— | 6 | 6 | — | 37 | 37 | ||||||||||||||||||
Deficit in the scheme as recognised
in the statement of financial position |
(1,405 | ) | (1,067 | ) | (2,472 | ) | (2,202 | ) | (1,152 | ) | (3,354 | ) | ||||||||||||
| 2010 | 2009 | |||||||||||||||||||||||
| UK | Rest of Group | Total | UK | Rest of Group | Total | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
At beginning of year |
4,853 | 2,402 | 7,255 | 3,835 | 2,013 | 5,848 | ||||||||||||||||||
Expected return on scheme assets |
305 | 146 | 451 | 261 | 127 | 388 | ||||||||||||||||||
Expenses |
(7 | ) | — | (7 | ) | (6 | ) | — | (6 | ) | ||||||||||||||
Actuarial gains/(losses) |
244 | (4 | ) | 240 | 293 | 180 | 473 | |||||||||||||||||
Exchange |
(204 | ) | (4 | ) | (208 | ) | 430 | 17 | 447 | |||||||||||||||
Employer contributions |
224 | 245 | 469 | 304 | 262 | 566 | ||||||||||||||||||
Participant contributions |
28 | 3 | 31 | 31 | 3 | 34 | ||||||||||||||||||
Benefits paid |
(294 | ) | (170 | ) | (464 | ) | (295 | ) | (200 | ) | (495 | ) | ||||||||||||
Scheme assets fair value at end of year |
5,149 | 2,618 | 7,767 | 4,853 | 2,402 | 7,255 | ||||||||||||||||||
| 2010 | 2009 | |||||||||||||||||||||||
| UK | Rest of Group | Total | UK | Rest of Group | Total | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
Present value of obligation in scheme at beginning of year |
(7,055 | ) | (3,591 | ) | (10,646 | ) | (5,029 | ) | (3,591 | ) | (8,620 | ) | ||||||||||||
Current service cost |
(97 | ) | (114 | ) | (211 | ) | (96 | ) | (126 | ) | (222 | ) | ||||||||||||
Past service (cost)/credit |
(39 | ) | 106 | 67 | (53 | ) | (21 | ) | (74 | ) | ||||||||||||||
Participant contributions |
(28 | ) | (3 | ) | (31 | ) | (31 | ) | (3 | ) | (34 | ) | ||||||||||||
Benefits paid |
294 | 170 | 464 | 295 | 200 | 495 | ||||||||||||||||||
Other finance expense |
(371 | ) | (172 | ) | (543 | ) | (330 | ) | (163 | ) | (493 | ) | ||||||||||||
Expenses |
7 | — | 7 | 6 | — | 6 | ||||||||||||||||||
Actuarial (loss)/gain |
(221 | ) | (65 | ) | (286 | ) | (1,218 | ) | 176 | (1,042 | ) | |||||||||||||
Settlements and curtailments |
693 | 6 | 699 | — | — | — | ||||||||||||||||||
Exchange |
263 | (28 | ) | 235 | (599 | ) | (63 | ) | (662 | ) | ||||||||||||||
Present value of obligations in scheme at end of year |
(6,554 | ) | (3,691 | ) | (10,245 | ) | (7,055 | ) | (3,591 | ) | (10,646 | ) | ||||||||||||
| 2010 | 2009 | |||||||||||||||
| UK | Rest of Group | UK | Rest of Group | |||||||||||||
| $m | $m | $m | $m | |||||||||||||
Funded |
(6,526 | ) | (3,232 | ) | (7,026 | ) | (3,159 | ) | ||||||||
Unfunded |
(28 | ) | (459 | ) | (29 | ) | (432 | ) | ||||||||
Total |
(6,554 | ) | (3,691 | ) | (7,055 | ) | (3,591 | ) | ||||||||
| 164 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | |||||||||||||||||||||||
| UK | Rest of Group | Total | UK | Rest of Group | Total | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
Operating profit |
||||||||||||||||||||||||
Current service cost |
(97 | ) | (114 | ) | (211 | ) | (96 | ) | (126 | ) | (222 | ) | ||||||||||||
Past service (cost)/credit |
(39 | ) | 75 | 36 | (53 | ) | (24 | ) | (77 | ) | ||||||||||||||
Settlements and curtailments |
693 | 6 | 699 | — | — | — | ||||||||||||||||||
Total charge to operating profit |
557 | (33 | ) | 524 | (149 | ) | (150 | ) | (299 | ) | ||||||||||||||
Finance expense |
||||||||||||||||||||||||
Expected return on post-retirement scheme assets |
305 | 146 | 451 | 261 | 127 | 388 | ||||||||||||||||||
Interest on post-retirement scheme obligations |
(371 | ) | (172 | ) | (543 | ) | (330 | ) | (163 | ) | (493 | ) | ||||||||||||
Net return |
(66 | ) | (26 | ) | (92 | ) | (69 | ) | (36 | ) | (105 | ) | ||||||||||||
Charge before taxation |
491 | (59 | ) | 432 | (218 | ) | (186 | ) | (404 | ) | ||||||||||||||
Other comprehensive income |
||||||||||||||||||||||||
Difference between the actual return and the expected
return on the post-retirement scheme assets |
244 | (4 | ) | 240 | 293 | 180 | 473 | |||||||||||||||||
Experience (losses)/gains arising on the post-retirement
scheme obligations |
(81 | ) | 5 | (76 | ) | 105 | (67 | ) | 38 | |||||||||||||||
Changes in assumptions underlying the present value
of the post-retirement scheme obligations |
(140 | ) | (70 | ) | (210 | ) | (1,323 | ) | 243 | (1,080 | ) | |||||||||||||
Actuarial gains/(losses) recognised |
23 | (69 | ) | (46 | ) | (925 | ) | 356 | (569 | ) | ||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
UK |
||||||||||||||||||||
Present value of obligations ($m) |
(6,554 | ) | (7,055 | ) | (5,029 | ) | (7,644 | ) | (7,352 | ) | ||||||||||
Fair value of scheme assets ($m) |
5,149 | 4,853 | 3,835 | 6,310 | 6,078 | |||||||||||||||
Deficit in the scheme ($m) |
(1,405 | ) | (2,202 | ) | (1,194 | ) | (1,334 | ) | (1,274 | ) | ||||||||||
Experience adjustments on: |
||||||||||||||||||||
Scheme assets |
||||||||||||||||||||
Amount ($m) |
244 | 293 | (1,185 | ) | (185 | ) | (259 | ) | ||||||||||||
Percentage of scheme assets |
4.7% | 6.0% | 30.9% | 2.9% | 4.3% | |||||||||||||||
Scheme obligations |
||||||||||||||||||||
Amount ($m) |
(221 | ) | (1,218 | ) | 972 | 114 | 71 | |||||||||||||
Percentage of scheme obligations |
3.4% | 17.3% | 19.3% | 1.5% | 1.0% | |||||||||||||||
Rest of Group |
||||||||||||||||||||
Present value of obligations ($m) |
(3,691 | ) | (3,591 | ) | (3,591 | ) | (3,348 | ) | (3,109 | ) | ||||||||||
Fair value of scheme assets ($m) |
2,618 | 2,402 | 2,013 | 2,644 | 2,493 | |||||||||||||||
Deficit in the scheme ($m) |
(1,073 | ) | (1,189 | ) | (1,578 | ) | (704 | ) | (616 | ) | ||||||||||
Experience adjustments on: |
||||||||||||||||||||
Scheme assets |
||||||||||||||||||||
Amount ($m) |
(4 | ) | 180 | (700 | ) | (24 | ) | 55 | ||||||||||||
Percentage of scheme assets |
0.2% | 7.5% | 34.8% | 0.9% | 2.2% | |||||||||||||||
Scheme obligations |
||||||||||||||||||||
Amount ($m) |
(65 | ) | 176 | (319 | ) | (18 | ) | 25 | ||||||||||||
Percentage of scheme obligations |
1.8% | 4.9% | 8.9% | 0.5% | 0.8% | |||||||||||||||
Total |
||||||||||||||||||||
Present value of obligations ($m) |
(10,245 | ) | (10,646 | ) | (8,620 | ) | (10,992 | ) | (10,461 | ) | ||||||||||
Fair value of scheme assets ($m) |
7,767 | 7,255 | 5,848 | 8,954 | 8,571 | |||||||||||||||
Deficit in the scheme ($m) |
(2,478 | ) | (3,391 | ) | (2,772 | ) | (2,038 | ) | (1,890 | ) | ||||||||||
Experience adjustments on: |
||||||||||||||||||||
Scheme assets |
||||||||||||||||||||
Amount ($m) |
240 | 473 | (1,885 | ) | (209 | ) | (204 | ) | ||||||||||||
Percentage of scheme assets |
3.1% | 6.5% | 32.2% | 2.3% | 2.4% | |||||||||||||||
Scheme obligations |
||||||||||||||||||||
Amount ($m) |
(286 | ) | (1,042 | ) | 653 | 96 | 96 | |||||||||||||
Percentage of scheme obligations |
2.8% | 9.8% | 7.6% | 0.9% | 0.9% | |||||||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 165 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
At 1 January |
(1,800 | ) | (1,371 | ) | (479 | ) | ||||||
Actuarial losses |
(46 | ) | (569 | ) | (1,232 | ) | ||||||
Deferred tax |
(19 | ) | 140 | 340 | ||||||||
At 31 December |
(1,865 | ) | (1,800 | ) | (1,371 | ) | ||||||
| 2010 | 2009 | |||||||||||||||
| +1% | -1% | +1% | -1% | |||||||||||||
UK ($m) |
937 | (1,093 | ) | 973 | (1,129 | ) | ||||||||||
US ($m) |
203 | (232 | ) | 225 | (256 | ) | ||||||||||
Sweden ($m) |
222 | (293 | ) | 192 | (229 | ) | ||||||||||
Germany ($m) |
37 | (44 | ) | 35 | (42 | ) | ||||||||||
Total ($m) |
1,399 | (1,662 | ) | 1,425 | (1,656 | ) | ||||||||||
| Effect of change in medical cost assumption increase/(decrease) | ||||||||||||||||
| 2010 | 2009 | |||||||||||||||
| +1% | -1% | +1% | -1% | |||||||||||||
Current service and interest cost of net periodic post-employment medical costs ($m) |
4 | (3 | ) | 4 | (3 | ) | ||||||||||
Accumulated post-employment benefit obligation for medical costs ($m) |
10 | (11 | ) | 32 | (28 | ) | ||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Cumulative translation differences included within retained earnings |
||||||||||||
Balance at beginning of year |
1,656 | 1,323 | 2,414 | |||||||||
Foreign exchange arising on consolidation |
26 | 388 | (1,355 | ) | ||||||||
Exchange adjustments on goodwill (recorded against other reserves) |
15 | 13 | (27 | ) | ||||||||
Foreign exchange differences on borrowings forming net investment hedges |
101 | (68 | ) | 291 | ||||||||
Net exchange movement in retained earnings |
142 | 333 | (1,091 | ) | ||||||||
Balance at end of year |
1,798 | 1,656 | 1,323 | |||||||||
| 166 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Allotted, called-up and fully paid | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Issued Ordinary Shares ($0.25 each) |
352 | 363 | 362 | |||||||||
Redeemable Preference Shares (£1 each — £50,000) |
— | — | — | |||||||||
| 352 | 363 | 362 | ||||||||||
| No. of shares (million) | ||||||||
| 2010 | 2009 | |||||||
At 1 January |
1,451 | 1,447 | ||||||
Issues of shares |
12 | 4 | ||||||
Repurchase of shares |
(54 | ) | — | |||||
At 31 December |
1,409 | 1,451 | ||||||
| 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Per share | Per share | Per share | $m | $m | $m | |||||||||||||||||||
Final |
$1.710 | $1.500 | $1.350 | 2,484 | 2,171 | 1,967 | ||||||||||||||||||
Interim |
$0.700 | $0.590 | $0.550 | 1,010 | 855 | 800 | ||||||||||||||||||
| $2.410 | $2.090 | $1.900 | 3,494 | 3,026 | 2,767 | |||||||||||||||||||
| Fair value | ||||||||||||
| Book value | adjustment | Fair value | ||||||||||
| $m | $m | $m | ||||||||||
Non-current assets |
1 | 548 | 549 | |||||||||
Current assets |
89 | — | 89 | |||||||||
Current liabilities |
(18 | ) | — | (18 | ) | |||||||
Non-current liabilities |
(85 | ) | (58 | ) | (143 | ) | ||||||
Total assets acquired |
(13 | ) | 490 | 477 | ||||||||
Goodwill |
— | |||||||||||
Fair value of total consideration |
477 | |||||||||||
Less: fair value of contingent consideration |
(50 | ) | ||||||||||
Total upfront consideration |
427 | |||||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 167 |
Table of Contents
| $m | ||||
Total upfront consideration |
427 | |||
Cash and cash equivalents included in undertaking acquired |
(79 | ) | ||
Net cash consideration |
348 | |||
| > | managing funding and liquidity risk | |
| > | optimising shareholder return | |
| > | maintaining a strong investment-grade credit rating. |
| 168 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Bank | Total | Total | ||||||||||||||||||||||||||||||
| overdrafts | Trade | non-derivative | derivative | |||||||||||||||||||||||||||||
| and other | and other | financial | Interest | Currency | financial | |||||||||||||||||||||||||||
| loans | Bonds | payables | instruments | rate swaps | swaps | instruments | Total | |||||||||||||||||||||||||
| $m | $m | $m | $m | $m | $m | $m | $m | |||||||||||||||||||||||||
Within one year |
128 | 518 | 8,640 | 9,286 | (120 | ) | – | (120 | ) | 9,166 | ||||||||||||||||||||||
In one to two years |
– | 2,268 | 373 | 2,641 | (121 | ) | – | (121 | ) | 2,520 | ||||||||||||||||||||||
In two to three years |
– | 423 | – | 423 | (87 | ) | – | (87 | ) | 336 | ||||||||||||||||||||||
In three to four years |
– | 1,153 | – | 1,153 | (69 | ) | – | (69 | ) | 1,084 | ||||||||||||||||||||||
In four to five years |
– | 1,379 | – | 1,379 | (50 | ) | – | (50 | ) | 1,329 | ||||||||||||||||||||||
In more than five years |
– | 10,095 | – | 10,095 | (192 | ) | – | (192 | ) | 9,903 | ||||||||||||||||||||||
| 128 | 15,836 | 9,013 | 24,977 | (639 | ) | – | (639 | ) | 24,338 | |||||||||||||||||||||||
Effect of interest |
(3 | ) | (7,012 | ) | – | (7,015 | ) | 639 | – | 639 | (6,376 | ) | ||||||||||||||||||||
Effect of discounting, fair values and issue costs |
– | 273 | – | 273 | (324 | ) | – | (324 | ) | (51 | ) | |||||||||||||||||||||
31 December 2010 |
125 | 9,097 | 9,013 | 18,235 | (324 | ) | – | (324 | ) | 17,911 | ||||||||||||||||||||||
| Bank | Total | Total | ||||||||||||||||||||||||||||||
| overdrafts | Trade | non-derivative | derivative | |||||||||||||||||||||||||||||
| and other | and other | financial | Interest | Currency | financial | |||||||||||||||||||||||||||
| loans | Bonds | payables | instruments | rate swaps | swaps | instruments | Total | |||||||||||||||||||||||||
| $m | $m | $m | $m | $m | $m | $m | $m | |||||||||||||||||||||||||
Within one year |
139 | 2,373 | 8,687 | 11,199 | (117 | ) | 89 | (28 | ) | 11,171 | ||||||||||||||||||||||
In one to two years |
– | 523 | 185 | 708 | (117 | ) | – | (117 | ) | 591 | ||||||||||||||||||||||
In two to three years |
– | 2,246 | – | 2,246 | (116 | ) | – | (116 | ) | 2,130 | ||||||||||||||||||||||
In three to four years |
– | 429 | – | 429 | (86 | ) | – | (86 | ) | 343 | ||||||||||||||||||||||
In four to five years |
– | 405 | – | 405 | (64 | ) | – | (64 | ) | 341 | ||||||||||||||||||||||
In more than five years |
– | 12,209 | – | 12,209 | (239 | ) | – | (239 | ) | 11,970 | ||||||||||||||||||||||
| 139 | 18,185 | 8,872 | 27,196 | (739 | ) | 89 | (650 | ) | 26,546 | |||||||||||||||||||||||
Effect of interest |
(3 | ) | (7,467 | ) | – | (7,470 | ) | 739 | – | 739 | (6,731 | ) | ||||||||||||||||||||
Effect of discounting, fair values and issue costs |
– | 209 | – | 209 | (262 | ) | 1 | (261 | ) | (52 | ) | |||||||||||||||||||||
31 December 2009 |
136 | 10,927 | 8,872 | 19,935 | (262 | ) | 90 | (172 | ) | 19,763 | ||||||||||||||||||||||
| Bank | Total | Total | ||||||||||||||||||||||||||||||
| overdrafts | Trade | non-derivative | derivative | |||||||||||||||||||||||||||||
| and other | and other | financial | Interest | Currency | financial | |||||||||||||||||||||||||||
| loans | Bonds | payables | instruments | rate swaps | swaps | instruments | Total | |||||||||||||||||||||||||
| $m | $m | $m | $m | $m | $m | $m | $m | |||||||||||||||||||||||||
Within one year |
345 | 1,271 | 7,178 | 8,794 | (60 | ) | – | (60 | ) | 8,734 | ||||||||||||||||||||||
In one to two years |
– | 2,335 | 61 | 2,396 | (60 | ) | 66 | 6 | 2,402 | |||||||||||||||||||||||
In two to three years |
– | 465 | – | 465 | (59 | ) | – | (59 | ) | 406 | ||||||||||||||||||||||
In three to four years |
– | 2,241 | – | 2,241 | (59 | ) | – | (59 | ) | 2,182 | ||||||||||||||||||||||
In four to five years |
– | 424 | – | 424 | (46 | ) | – | (46 | ) | 378 | ||||||||||||||||||||||
In more than five years |
– | 12,478 | – | 12,478 | (163 | ) | – | (163 | ) | 12,315 | ||||||||||||||||||||||
| 345 | 19,214 | 7,239 | 26,798 | (447 | ) | 66 | (381 | ) | 26,417 | |||||||||||||||||||||||
Effect of interest |
(2 | ) | (7,956 | ) | – | (7,958 | ) | 447 | – | 447 | (7,511 | ) | ||||||||||||||||||||
Effect of discounting, fair values and issue costs |
– | 247 | – | 247 | (449 | ) | 5 | (444 | ) | (197 | ) | |||||||||||||||||||||
31 December 2008 |
343 | 11,505 | 7,239 | 19,087 | (449 | ) | 71 | (378 | ) | 18,709 | ||||||||||||||||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 169 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||||||||||||||||||||||||||
| Total | Fixed rate | Floating rate | Total | Fixed rate | Floating rate | Total | Fixed rate | Floating rate | ||||||||||||||||||||||||||||
| $m | $m | $m | $m | $m | $m | $m | $m | $m | ||||||||||||||||||||||||||||
Financial liabilities |
||||||||||||||||||||||||||||||||||||
Interest-bearing loans and borrowings |
||||||||||||||||||||||||||||||||||||
Current |
125 | – | 125 | 1,926 | 1,790 | 136 | 993 | – | 993 | |||||||||||||||||||||||||||
Non-current |
9,097 | 6,242 | 2,855 | 9,137 | 6,340 | 2,797 | 10,855 | 8,015 | 2,840 | |||||||||||||||||||||||||||
| 9,222 | 6,242 | 2,980 | 11,063 | 8,130 | 2,933 | 11,848 | 8,015 | 3,833 | ||||||||||||||||||||||||||||
Financial assets |
||||||||||||||||||||||||||||||||||||
Fixed deposits |
1,107 | – | 1,107 | 1,466 | – | 1,466 | 54 | – | 54 | |||||||||||||||||||||||||||
Cash and cash equivalents |
11,068 | – | 11,068 | 9,918 | – | 9,918 | 4,286 | – | 4,286 | |||||||||||||||||||||||||||
| 12,175 | – | 12,175 | 11,384 | – | 11,384 | 4,340 | – | 4,340 | ||||||||||||||||||||||||||||
170 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| GBP | 1 | SEK | EUR | AUD | JPY | CAD | ||||||||||||||||||
| 2010 | $m | $m | $m | $m | $m | $m | ||||||||||||||||||
Gross exposure |
732 | (806 | ) | 478 | 117 | 133 | 33 | |||||||||||||||||
Forward exchange contracts |
(38 | ) | 806 | (478 | ) | (117 | ) | (133 | ) | (33 | ) | |||||||||||||
Net exposure |
694 | – | – | – | – | – | ||||||||||||||||||
2009 |
||||||||||||||||||||||||
Gross exposure |
(124 | ) | (811 | ) | 556 | 75 | 197 | 43 | ||||||||||||||||
Forward exchange contracts |
124 | 811 | (556 | ) | (75 | ) | (197 | ) | (43 | ) | ||||||||||||||
Net exposure |
– | – | – | – | – | – | ||||||||||||||||||
2008 |
||||||||||||||||||||||||
Gross exposure |
(676 | ) | (444 | ) | 505 | 57 | 166 | 49 | ||||||||||||||||
Forward exchange contracts |
690 | 445 | (512 | ) | (52 | ) | (166 | ) | (24 | ) | ||||||||||||||
Net exposure |
14 | 1 | (7 | ) | 5 | – | 25 | |||||||||||||||||
| 1 | The sterling hedge position was updated in early January 2011. |
| Interest rates | Exchange rates | |||||||||||||||
| +1% | -1% | +10% | -10% | |||||||||||||
Increase/(decrease) in fair value of financial instruments |
595 | (684 | ) | 36 | (36 | ) | ||||||||||
Impact on profit: gain/(loss) |
– | – | (133 | ) | 133 | |||||||||||
Impact on equity: gain/(loss) |
– | – | 169 | (169 | ) | |||||||||||
| Interest rates | Exchange rates | |||||||||||||||
| +1% | -1% | +10% | -10% | |||||||||||||
Increase/(decrease) in fair value of financial instruments |
602 | (709 | ) | 137 | (137 | ) | ||||||||||
Impact on profit: gain/(loss) |
– | – | (134 | ) | 134 | |||||||||||
Impact on equity: gain/(loss) |
– | – | 271 | (271 | ) | |||||||||||
| Interest rates | Exchange rates | |||||||||||||||
| +1% | -1% | +10% | -10% | |||||||||||||
Increase/(decrease) in fair value of financial instruments |
587 | (706 | ) | 217 | (217 | ) | ||||||||||
Impact on profit: gain/(loss) |
– | – | (57 | ) | 57 | |||||||||||
Impact on equity: gain/(loss) |
– | – | 274 | (274 | ) | |||||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 171 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
US |
2,168 | 2,229 | 2,032 | |||||||||
UK |
530 | 482 | 459 | |||||||||
Sweden |
285 | 245 | 226 | |||||||||
Euro zone countries |
878 | 762 | 833 | |||||||||
Other European countries |
290 | 295 | 257 | |||||||||
Japan |
1,091 | 950 | 955 | |||||||||
Other countries |
1,005 | 819 | 796 | |||||||||
| 6,247 | 5,782 | 5,558 | ||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Not past due |
5,953 | 5,542 | 5,262 | |||||||||
Overdue but renegotiated |
– | – | 3 | |||||||||
Past due 0-90 days |
104 | 65 | 106 | |||||||||
Past due 90-180 days |
67 | 75 | 60 | |||||||||
Past due > 180 days |
123 | 100 | 127 | |||||||||
| 6,247 | 5,782 | 5,558 | ||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $ m | ||||||||||
Movements in provisions for trade receivables |
||||||||||||
Balance at beginning of year |
81 | 99 | 89 | |||||||||
Income statement (credit)/charge |
(1 | ) | (20 | ) | 23 | |||||||
Amounts utilised, exchange and other movements |
1 | 2 | (13 | ) | ||||||||
Balance at end of year |
81 | 81 | 99 | |||||||||
172 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
Employees |
||||||||||||
UK |
10,100 | 10,600 | 11,000 | |||||||||
Continental Europe |
20,100 | 21,200 | 23,100 | |||||||||
The Americas |
18,300 | 19,800 | 20,900 | |||||||||
Asia, Africa & Australasia |
13,200 | 12,300 | 11,100 | |||||||||
Continuing operations |
61,700 | 63,900 | 66,100 | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Salaries |
4,837 | 4,713 | 5,080 | |||||||||
Social security costs |
693 | 644 | 743 | |||||||||
Pension costs |
501 | 1 | 516 | 497 | ||||||||
Other employment costs |
408 | 560 | 596 | |||||||||
| 6,439 | 6,433 | 6,916 | ||||||||||
| 1 | Pension costs excludes gains of $791m arising from changes made to benefits under certain of the Group’s post-retirement benefit plans. |

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 173 |
Table of Contents
| 174 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Shares | WAFV | 1 | WAFV | 1 | ||||||||
| ’000 | pence | $ | ||||||||||
Shares awarded in March 2008 |
1,338 | 941 | 18.88 | |||||||||
Shares awarded in August 2008 |
14 | 1326 | 24.46 | |||||||||
Shares awarded in March 2009 |
1,190 | 1140 | 16.70 | |||||||||
Shares awarded in August 2009 |
8 | 1424 | 23.18 | |||||||||
Shares awarded in March 2010 |
2,002 | 1495 | 22.38 | |||||||||
Shares awarded in May 2010 |
436 | 1431 | 21.48 | |||||||||
Shares awarded in August 2010 |
139 | 1614 | 24.95 | |||||||||
Shares awarded in November 2010 |
4 | N/A | 25.11 | |||||||||
| Shares | WAFV | 1 | ||||||
| ’000 | $ | |||||||
Shares awarded in March 2008 |
2,094 | 18.88 | ||||||
Shares awarded in August 2008 |
20 | 24.46 | ||||||
Shares awarded in March 2009 |
2,288 | 16.70 | ||||||
Shares awarded in August 2009 |
6 | 23.18 | ||||||
| Shares | WAFV | 1 | WAFV | 1 | ||||||||
| ’000 | pence | $ | ||||||||||
Shares awarded in May 2010 |
76 | 2575 | 38.66 | |||||||||
Shares awarded in August 2010 |
15 | 2904 | N/A | |||||||||
| Shares | WAFV | 1 | WAFV | 1 | ||||||||
| ’000 | pence | $ | ||||||||||
Shares awarded in March 2010 |
2,672 | 2989 | 44.75 | |||||||||
Shares awarded in August 2010 |
8 | 3227 | 49.89 | |||||||||
| Units | WAFV | 1 | ||||||
| ’000 | $ | |||||||
Units awarded in March 2008 |
1,313 | 37.76 | ||||||
Units awarded in March 2009 |
1,283 | 33.39 | ||||||
| Units | WAFV | 1 | ||||||
| ’000 | $ | |||||||
Units awarded in March 2008 |
130 | 37.76 | ||||||
Units awarded in March 2009 |
177 | 33.39 | ||||||
| Shares | WAFV | 1 | WAFV | 1 | ||||||||
| ’000 | pence | $ | ||||||||||
Shares awarded in March 2008 |
51 | 1882 | N/A | |||||||||
Shares awarded in May 2008 |
35 | N/A | 44.20 | |||||||||
Shares awarded in August 2009 |
9 | N/A | 46.36 | |||||||||
Shares awarded in September 2009 |
22 | N/A | 44.61 | |||||||||
Shares awarded in February 2010 |
159 | 2954 | 47.70 | |||||||||
Shares awarded in May 2010 |
25 | 2861 | 42.96 | |||||||||
Shares awarded in August 2010 |
108 | 3227 | 49.89 | |||||||||
Shares awarded in November 2010 |
27 | N/A | 50.21 | |||||||||
Shares awarded in December 2010 |
20 | N/A | 48.30 | |||||||||
| 1 | Weighted average fair value. |

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 175 |
Table of Contents
| a) | 90% of the arithmetical average of the middle-market quotations for an Ordinary Share on the London Stock Exchange on three consecutive dealing days shortly before the date on which invitations to apply for options are issued (provided that no such day may fall before the Company last announced its results for any period) or such other dealing day or days falling within the six-week period for the issue of invitations, as the Directors may decide; and |
| b) | the nominal value of an Ordinary Share (unless the option is expressed to relate only to existing Ordinary Shares). |
| 176 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| AstraZeneca Share Option Plan | SAYE Schemes | 1994 Scheme | ||||||||||||||||||||||
| Options | WAEP | 1 | Options | WAEP | 1 | Options | WAEP | 1 | ||||||||||||||||
| ’000 | pence | ’000 | pence | ’000 | pence | |||||||||||||||||||
At 1 January 2008 |
||||||||||||||||||||||||
Options outstanding |
42,560 | 2451 | 2,720 | 2226 | 1,490 | 2364 | ||||||||||||||||||
Movements during 2008 |
||||||||||||||||||||||||
Options granted |
14,858 | 1887 | 483 | 2398 | – | – | ||||||||||||||||||
Options exercised |
(2,577 | ) | 2204 | (675 | ) | 2062 | (99 | ) | 2620 | |||||||||||||||
Options forfeited |
(2,273 | ) | 2622 | (388 | ) | 2291 | (106 | ) | 2594 | |||||||||||||||
Weighted average fair value of options granted during the year |
404 | 499 | – | |||||||||||||||||||||
At 31 December 2008 |
||||||||||||||||||||||||
Options outstanding |
52,568 | 2978 | 2,140 | 2304 | 1,285 | 2934 | ||||||||||||||||||
Movements during 2009 |
||||||||||||||||||||||||
Options granted |
15,246 | 2281 | 351 | 2563 | – | – | ||||||||||||||||||
Options exercised |
(2,275 | ) | 2213 | (286 | ) | 2258 | (317 | ) | 2670 | |||||||||||||||
Options forfeited |
(3,141 | ) | 2604 | (169 | ) | 2340 | (51 | ) | 2688 | |||||||||||||||
Weighted average fair value of options granted during the year |
423 | 425 | – | |||||||||||||||||||||
At 31 December 2009 |
||||||||||||||||||||||||
Options outstanding |
62,398 | 2601 | 2,036 | 2349 | 917 | 2734 | ||||||||||||||||||
Movements during 2010 |
||||||||||||||||||||||||
Options granted |
– | – | 276 | 2907 | – | – | ||||||||||||||||||
Options exercised |
(10,144 | ) | 2538 | (455 | ) | 2216 | (765 | ) | 2714 | |||||||||||||||
Options forfeited |
(3,189 | ) | 2470 | (183 | ) | 2559 | (152 | ) | 2714 | |||||||||||||||
Weighted average fair value of options granted during the year |
– | 267 | – | |||||||||||||||||||||
At 31 December 2010 |
||||||||||||||||||||||||
Options outstanding |
49,065 | 2439 | 1,674 | 2455 | – | – | ||||||||||||||||||
| 1882 to | 2164 to | |||||||||||||||||||||||
Range of exercise prices |
3487 | 3001 | – | |||||||||||||||||||||
Weighted average remaining contractual life |
2,185 days | 1,062 days | – | |||||||||||||||||||||
Options exercisable |
24,292 | 2799 | 65 | 2204 | – | – | ||||||||||||||||||
| 1 | Weighted average exercise price. |
| 2010 | 2009 | 2008 | ||||||||||
Average share price (pence) |
3058 | 2651 | 2295 | |||||||||
Weighted average exercise price (pence) |
||||||||||||
AstraZeneca Share Option Plan |
– | 2281 | 1887 | |||||||||
SAYE Schemes |
2907 | 2563 | 2398 | |||||||||
Expected volatility (%) |
20.0 | 25.0 | 25.0 | |||||||||
Dividend yield (%) |
5.5 | 4.0 | 3.4 | |||||||||
Risk-free interest rate (%) |
2.5 | 3.7 | 4.3 | |||||||||
Expected lives: AstraZeneca Share Option Plan (years) |
6.0 | 6.0 | 6.0 | |||||||||
Expected lives: SAYE Schemes (years) |
4.2 | 4.2 | 4.0 | |||||||||

AstraZeneca Annual Report and Form 20-F Information 2010
|
Financial Statements 177 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Commitments |
||||||||||||
Contracts placed for future capital expenditure on property, plant and equipment and |
||||||||||||
software development costs not provided for in these accounts |
259 | 368 | 178 | |||||||||
| Years 5 | ||||||||||||||||||||
| Total | Under 1 year | Years 1 and 2 | Years 3 and 4 | and greater | ||||||||||||||||
| $m | $m | $m | $m | $m | ||||||||||||||||
Future potential research and development milestone payments |
3,106 | 115 | 711 | 646 | 1,634 | |||||||||||||||
Future potential revenue milestone payments |
3,234 | – | 2 | 70 | 3,162 | |||||||||||||||
| > | A payment to Merck in the event of a business combination between Astra and a third party in order for Merck to relinquish certain claims to that third party’s products. | |
| > | Annual contingent payments. | |
| > | Termination arrangements which cause Merck to relinquish its interests in AstraZeneca’s products and activities in stages, some of which are mandatory and others optional. |
| > | the Advance Payment | |
| > | the Partial Retirement | |
| > | the True-Up | |
| > | the Loan Note Receivable | |
| > | the First Option | |
| > | Second Option. |
178 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | Currently, from the substantial freedom over products acquired or discovered post-merger. | |
| > | On occurrence of each stage of such arrangements, from enhanced contributions from, and substantial freedom over, those products that have already been launched (for example, Pulmicort, Symbicort, Rhinocort and Atacand), and those that are in development. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 179 |
Table of Contents
| 180 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 181 |
Table of Contents
| 182 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
and 7,030,152 (the ‘152 patent)

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 183 |
Table of Contents
| 184 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 185 |
Table of Contents
| 186 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 187 |
Table of Contents
| 188 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 189 |
Table of Contents
| 190 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 191 |
Table of Contents
AstraZeneca LP, et al
| 192 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 193 |
Table of Contents
marketing practices
| 194 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| > | The tax accrual at 31 December 2008 and 2009 included amounts in relation to a long running transfer pricing dispute between AstraZeneca and HM Revenue & Customs (HMRC) covering all periods from 1996 onwards. In February 2010, AstraZeneca announced that the company had entered into an agreement with HMRC in the UK to settle this dispute. As a consequence of the settlement, AstraZeneca and HMRC have withdrawn the joint referral of this issue to the UK Tax Court. The agreement will result in AstraZeneca paying £505m to HMRC to resolve all claims made by HMRC in relation to this issue for the 15-year period from 1996 to the end of 2010. The £505m settlement is payable in two instalments of which the first instalment of £350m ($562m) was paid in February 2010. A second final instalment of £155m is due to be paid in March 2011 and is included in ordinary tax payable at 31 December 2010. | |
| > | AstraZeneca has applied for an advance pricing agreement in relation to intra-group transactions between the UK and the US which is being progressed through competent authority proceedings under the relevant double tax treaty. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 195 |
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Operating leases |
212 | 198 | 206 | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Obligations under leases comprise: |
||||||||||||
No later than one year |
161 | 132 | 101 | |||||||||
Rentals due after more than one year: |
||||||||||||
Later than five years |
103 | 131 | 145 | |||||||||
Later than one year and not later than five years |
242 | 208 | 212 | |||||||||
| 345 | 339 | 357 | ||||||||||
| 506 | 471 | 458 | ||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $m | $m | $m | ||||||||||
Fees payable to KPMG Audit Plc and its associates: |
||||||||||||
Group audit fee |
2.3 | 2.4 | 3.2 | |||||||||
Fees payable to KPMG Audit Plc and its associates for other services: |
||||||||||||
The audit of subsidiaries pursuant to legislation |
6.5 | 6.6 | 7.1 | |||||||||
Other services pursuant to legislation |
3.3 | 2.9 | 3.3 | |||||||||
Taxation |
1.1 | 1.0 | 0.9 | |||||||||
All other services |
0.1 | 0.7 | 1.7 | |||||||||
Fees payable to KPMG Audit Plc in respect of the Group’s pension schemes: |
||||||||||||
The audit of subsidiaries’ pension schemes |
0.6 | 0.5 | 0.6 | |||||||||
| 13.9 | 14.1 | 16.8 | ||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| $000 | $000 | $000 | ||||||||||
Short-term employee benefits |
21,925 | 20,784 | 21,973 | |||||||||
Post-employment benefits |
1,793 | 2,080 | 2,290 | |||||||||
Termination benefits |
– | 3,639 | – | |||||||||
Share-based payments |
11,563 | 12,547 | 13,210 | |||||||||
| 35,281 | 39,050 | 37,473 | ||||||||||
196 Financial Statements
|
AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Percentage of voting | ||||||||||||
| At 31 December 2010 | Country | share capital held | Principal activity | |||||||||
UK |
||||||||||||
AstraZeneca UK Limited |
England | 100 | Research and development, manufacturing, marketing | |||||||||
AstraZeneca Treasury Limited |
England | 100 | Treasury | |||||||||
Continental Europe |
||||||||||||
NV AstraZeneca SA |
Belgium | 100 | Marketing | |||||||||
AstraZeneca Dunkerque Production SCS |
France | 95 | Manufacturing | |||||||||
AstraZeneca SAS |
France | 100 | Research, manufacturing, marketing | |||||||||
Novexel SA |
France | 100 | Research | |||||||||
AstraZeneca GmbH |
Germany | 100 | Development, manufacturing, marketing | |||||||||
AstraZeneca Holding GmbH |
Germany | 100 | Manufacturing, marketing | |||||||||
AstraZeneca SpA |
Italy | 100 | Marketing | |||||||||
AstraZeneca Farmaceutica Spain SA |
Spain | 100 | Marketing | |||||||||
AstraZeneca AB |
Sweden | 100 | Research and development, manufacturing, marketing | |||||||||
AstraZeneca BV |
The Netherlands | 100 | Marketing | |||||||||
The Americas |
||||||||||||
AstraZeneca Canada Inc. |
Canada | 100 | Research, marketing | |||||||||
AZ Reinsurance Limited |
Cayman Islands | 100 | Insurance and reinsurance underwriting | |||||||||
IPR Pharmaceuticals Inc. |
Puerto Rico | 100 | Development, manufacturing, marketing | |||||||||
AstraZeneca LP |
US | 99 | Research and development, manufacturing, marketing | |||||||||
AstraZeneca Pharmaceuticals LP |
US | 100 | Research and development, manufacturing, marketing | |||||||||
Zeneca Holdings Inc. |
US | 100 | Manufacturing, marketing | |||||||||
MedImmune, LLC |
US | 100 | Research and development, manufacturing, marketing | |||||||||
Asia, Africa & Australasia |
||||||||||||
AstraZeneca Pty Limited |
Australia | 100 | Development, manufacturing, marketing | |||||||||
AstraZeneca Pharmaceuticals Co., Limited |
China | 100 | Research and development, manufacturing, marketing | |||||||||
AstraZeneca KK |
Japan | 80 | Manufacturing, marketing | |||||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 197 |
Table of Contents
| > | Give a true and fair view of the state of the Company’s affairs as at 31 December 2010. | |
| > | Have been properly prepared in accordance with UK Generally Accepted Accounting Practice. | |
| > | Have been prepared in accordance with the requirements of the Companies Act 2006. |
| > | The part of the Directors’ Remuneration Report to be audited has been properly prepared in accordance with the Companies Act 2006. | |
| > | The information given in the Directors’ Report for the financial year for which the financial statements are prepared is consistent with the Parent Company Financial Statements. |
| > | Adequate accounting records have not been kept by the Parent Company, or returns adequate for our audit have not been received from branches not visited by us. | |
| > | The Parent Company Financial Statements and the part of the Directors’ Remuneration Report to be audited are not in agreement with the accounting records and returns. | |
| > | Certain disclosures of Directors’ Remuneration specified by law are not made. | |
| > | We have not received all the information and explanations we require for our audit. |
| 198 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2010 | 2009 | |||||||||||
| At 31 December | Notes | $m | $m | |||||||||
Fixed assets |
||||||||||||
Fixed asset investments |
1 | 25,232 | 25,230 | |||||||||
Current assets |
||||||||||||
Debtors – other |
1 | 1 | ||||||||||
Debtors – amounts owed by Group undertakings |
3,558 | 8,966 | ||||||||||
| 3,559 | 8,967 | |||||||||||
Creditors: Amounts falling due within one year |
||||||||||||
Non-trade creditors |
2 | (194 | ) | (252 | ) | |||||||
Interest-bearing loans and borrowings |
3 | – | (1,790 | ) | ||||||||
| (194 | ) | (2,042 | ) | |||||||||
Net current assets |
3,365 | 6,925 | ||||||||||
Total assets less current liabilities |
28,597 | 32,155 | ||||||||||
Creditors: Amounts falling due after more than one year |
||||||||||||
Amounts owed to Group undertakings |
3 | (283 | ) | (283 | ) | |||||||
Interest-bearing loans and borrowings |
3 | (8,486 | ) | (8,582 | ) | |||||||
| (8,769 | ) | (8,865 | ) | |||||||||
Net assets |
19,828 | 23,290 | ||||||||||
Capital and reserves |
||||||||||||
Called-up share capital |
6 | 352 | 363 | |||||||||
Share premium account |
4 | 2,672 | 2,180 | |||||||||
Capital redemption reserve |
4 | 107 | 94 | |||||||||
Other reserves |
4 | 3,020 | 2,922 | |||||||||
Profit and loss account |
4 | 13,677 | 17,731 | |||||||||
Shareholders’ funds |
5 | 19,828 | 23,290 | |||||||||
David R Brennan
|
Simon Lowth |
|
Director
|
Director |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 199 |
Table of Contents
| 200 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Investments in subsidiaries | ||||||||||||
| Shares | Loans | Total | ||||||||||
| $m | $m | $m | ||||||||||
Cost and net book value at 1 January 2010 |
16,367 | 8,863 | 25,230 | |||||||||
Capital contribution |
98 | – | 98 | |||||||||
Exchange |
– | (100 | ) | (100 | ) | |||||||
Amortisation |
– | 4 | 4 | |||||||||
Cost and net book value at 31 December 2010 |
16,465 | 8,767 | 25,232 | |||||||||
| 2010 | 2009 | |||||||
| $m | $m | |||||||
Amounts due within one year |
||||||||
Short-term borrowings (unsecured) |
12 | 12 | ||||||
Other creditors |
169 | 226 | ||||||
Amounts owed to Group undertakings |
13 | 14 | ||||||
| 194 | 252 | |||||||
| Repayment | 2010 | 2009 | ||||||||||
| dates | $m | $m | ||||||||||
Amounts due within one year |
||||||||||||
Interest-bearing loans and borrowings (unsecured) |
||||||||||||
Euros |
||||||||||||
4.625% Non-callable bond |
2010 | – | 1,073 | |||||||||
5.625% Non-callable bond |
2010 | – | 717 | |||||||||
| – | 1,790 | |||||||||||
Amounts due after more than one year |
||||||||||||
Amounts owed to subsidiaries (unsecured) |
||||||||||||
US dollars |
||||||||||||
7.2% Loan |
2023 | 283 | 283 | |||||||||
Interest-bearing loans and borrowings (unsecured) |
||||||||||||
US dollars |
||||||||||||
5.4% Callable bond |
2012 | 1,747 | 1,744 | |||||||||
5.4% Callable bond |
2014 | 749 | 748 | |||||||||
5.9% Callable bond |
2017 | 1,744 | 1,743 | |||||||||
6.45% Callable bond |
2037 | 2,718 | 2,717 | |||||||||
Euros |
||||||||||||
5.125% Non-callable bond |
2015 | 993 | 1,072 | |||||||||
Pounds sterling |
||||||||||||
5.75% Non-callable bond |
2031 | 535 | 558 | |||||||||
| 8,486 | 8,582 | |||||||||||
| 2010 | 2009 | |||||||
| $m | $m | |||||||
Loans or instalments thereof are repayable: |
||||||||
After five years from balance sheet date |
5,280 | 6,373 | ||||||
From two to five years |
1,742 | 2,492 | ||||||
From one to two years |
1,747 | – | ||||||
Within one year |
– | 1,790 | ||||||
Total unsecured |
8,769 | 10,655 | ||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 201 |
Table of Contents
| Share | Capital | Profit | ||||||||||||||||||||||
| premium | redemption | Other | and loss | 2010 | 2009 | |||||||||||||||||||
| account | reserve | reserves | account | Total | Total | |||||||||||||||||||
| $m | $m | $m | $m | $m | $m | |||||||||||||||||||
At beginning of year |
2,180 | 94 | 2,922 | 17,731 | 22,927 | 22,981 | ||||||||||||||||||
Profit for the year |
– | – | – | 2,043 | 2,043 | 2,658 | ||||||||||||||||||
Dividends |
– | – | – | (3,494 | ) | (3,494 | ) | (3,026 | ) | |||||||||||||||
Amortisation of loss on cash flow hedge |
– | – | – | 1 | 1 | 1 | ||||||||||||||||||
Share-based payment |
– | – | 98 | – | 98 | 179 | ||||||||||||||||||
Share repurchases |
– | 13 | – | (2,604 | ) | (2,591 | ) | – | ||||||||||||||||
Share premium |
492 | – | – | – | 492 | 134 | ||||||||||||||||||
At end of year |
2,672 | 107 | 3,020 | 13,677 | 19,476 | 22,927 | ||||||||||||||||||
Distributable reserves at end of year |
– | – | 1,841 | 13,677 | 15,518 | 19,572 | ||||||||||||||||||
| 2010 | 2009 | |||||||
| $m | $m | |||||||
At beginning of year |
23,290 | 23,343 | ||||||
Net profit for the financial year |
2,043 | 2,658 | ||||||
Dividends |
(3,494 | ) | (3,026 | ) | ||||
Amortisation of loss on cash flow hedge |
1 | 1 | ||||||
Share-based payment |
98 | 179 | ||||||
Issue of AstraZeneca PLC Ordinary Shares |
494 | 135 | ||||||
Repurchase of AstraZeneca PLC Ordinary Shares |
(2,604 | ) | – | |||||
Net decrease in shareholders’ funds |
(3,462 | ) | (53 | ) | ||||
Shareholders’ funds at end of year |
19,828 | 23,290 | ||||||
| Allotted, called-up and fully paid | ||||||||
| 2010 | 2009 | |||||||
| $m | $m | |||||||
Issued Ordinary Shares ($0.25 each) |
352 | 363 | ||||||
Redeemable Preference Shares (£1 each – £50,000) |
– | – | ||||||
| 352 | 363 | |||||||
| No. of shares (million) | $m | |||||||
At 1 January 2010 |
1,451 | 363 | ||||||
Issues of shares |
12 | 2 | ||||||
Repurchase of shares |
(54 | ) | (13 | ) | ||||
At 31 December 2010 |
1,409 | 352 | ||||||
| 202 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Financial Statements 203 |
Table of Contents
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||
| For the year ended 31 December | $m | $m | $m | $m | $m | |||||||||||||||
Revenue and profits |
||||||||||||||||||||
Revenue |
26,475 | 29,559 | 31,601 | 32,804 | 33,269 | |||||||||||||||
Cost of sales |
(5,559 | ) | (6,419 | ) | (6,598 | ) | (5,775 | ) | (6,389 | ) | ||||||||||
Distribution costs |
(226 | ) | (248 | ) | (291 | ) | (298 | ) | (335 | ) | ||||||||||
Research and development |
(3,902 | ) | (5,162 | ) | (5,179 | ) | (4,409 | ) | (5,318 | ) | ||||||||||
Selling, general and administrative costs |
(9,096 | ) | (10,364 | ) | (10,913 | ) | (11,332 | ) | (10,445 | ) | ||||||||||
Other operating income and expense |
524 | 728 | 524 | 553 | 712 | |||||||||||||||
Operating profit |
8,216 | 8,094 | 9,144 | 11,543 | 11,494 | |||||||||||||||
Finance income |
888 | 959 | 854 | 462 | 516 | |||||||||||||||
Finance expense |
(561 | ) | (1,070 | ) | (1,317 | ) | (1,198 | ) | (1,033 | ) | ||||||||||
Profit before tax |
8,543 | 7,983 | 8,681 | 10,807 | 10,977 | |||||||||||||||
Taxation |
(2,480 | ) | (2,356 | ) | (2,551 | ) | (3,263 | ) | (2,896 | ) | ||||||||||
Profit for the period |
6,063 | 5,627 | 6,130 | 7,544 | 8,081 | |||||||||||||||
Other comprehensive income for the period, net of tax |
931 | 342 | (1,906 | ) | (54 | ) | 25 | |||||||||||||
Total comprehensive income for the period |
6,994 | 5,969 | 4,224 | 7,490 | 8,106 | |||||||||||||||
Profit attributable to: |
||||||||||||||||||||
Equity holders of the Company |
6,043 | 5,595 | 6,101 | 7,521 | 8,053 | |||||||||||||||
Non-controlling interests |
20 | 32 | 29 | 23 | 28 | |||||||||||||||
Earnings per share |
||||||||||||||||||||
Earnings per $0.25 Ordinary Share (basic) |
$3.86 | $3.74 | $4.20 | $5.19 | $5.60 | |||||||||||||||
Earnings per $0.25 Ordinary Share (diluted) |
$3.85 | $3.73 | $4.20 | $5.19 | $5.57 | |||||||||||||||
Dividends |
$1.410 | $1.750 | $1.900 | $2.090 | $2.410 | |||||||||||||||
Return on revenues |
||||||||||||||||||||
Operating profit as a percentage of revenues |
31.0% | 27.4% | 28.9% | 35.2% | 34.5% | |||||||||||||||
Ratio of earnings to fixed charges |
92.7 | 15.6 | 13.5 | 19.9 | 24.0 | |||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||
| At 31 December | $m | $m | $m | $m | $m | |||||||||||||||
Statement of Financial Position |
||||||||||||||||||||
Property, plant and equipment, goodwill and intangible assets |
11,657 | 29,649 | 29,240 | 29,422 | 28,986 | |||||||||||||||
Other investments |
146 | 299 | 605 | 446 | 535 | |||||||||||||||
Deferred tax assets |
1,220 | 1,044 | 1,236 | 1,292 | 1,475 | |||||||||||||||
Current assets |
16,909 | 16,996 | 15,869 | 23,760 | 25,131 | |||||||||||||||
Total assets |
29,932 | 47,988 | 46,950 | 54,920 | 56,127 | |||||||||||||||
Current liabilities |
(9,447 | ) | (15,218 | ) | (13,415 | ) | (17,640 | ) | (16,787 | ) | ||||||||||
Non-current liabilities |
(5,069 | ) | (17,855 | ) | (17,475 | ) | (16,459 | ) | (15,930 | ) | ||||||||||
Net assets |
15,416 | 14,915 | 16,060 | 20,821 | 23,410 | |||||||||||||||
Share capital |
383 | 364 | 362 | 363 | 352 | |||||||||||||||
Reserves attributable to equity holders |
14,921 | 14,414 | 15,550 | 20,297 | 22,861 | |||||||||||||||
Non-controlling interests |
112 | 137 | 148 | 161 | 197 | |||||||||||||||
Total equity and reserves |
15,416 | 14,915 | 16,060 | 20,821 | 23,410 | |||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||
| For the year ended 31 December | $m | $m | $m | $m | $m | |||||||||||||||
Cash flows |
||||||||||||||||||||
Net cash inflow/(outflow) from: |
||||||||||||||||||||
Operating activities |
7,693 | 7,510 | 8,742 | 11,739 | 10,680 | |||||||||||||||
Investing activities |
(272 | ) | (14,887 | ) | (3,896 | ) | (2,476 | ) | (2,340 | ) | ||||||||||
Financing activities |
(5,366 | ) | 6,051 | (6,362 | ) | (3,629 | ) | (7,220 | ) | |||||||||||
| 2,055 | (1,326 | ) | (1,516 | ) | 5,634 | 1,120 | ||||||||||||||
| 204 Financial Statements | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
find out more?
contains more
information about
our business and
about being an
AstraZeneca
shareholder
| AstraZeneca Annual Report and Form 20-F Information 2010 | Additional Information 205 |
Table of Contents
| Estimated Filing | ||||||||||||||||||||||
| Compound | Mechanism | Area Under Investigation | Phase | US | EU | Japan | Emerging | |||||||||||||||
Cardiovascular |
||||||||||||||||||||||
Kombiglyze™ XR/ Onglyza™/metformin IR FDC#*
|
DPP-4 inhibitor + metformin FDC | diabetes | III | Launched | Filed | Filed | ||||||||||||||||
Dapagliflozin/metformin FDC#
|
SGLT2 inhibitor + metformin FDC | diabetes | III | H1 2012 | H1 2012 | |||||||||||||||||
Onglyza™ SAVOR#
|
DPP-4 inhibitor | outcomes study | III | 2016 | ||||||||||||||||||
Brilinta PEGASUS-TIMI
|
ADP receptor antagonist | outcomes study | III | 2014 | 2014 | 2014 | 2014 | |||||||||||||||
Crestor
|
statin | outcomes in subjects with elevated CRP |
III | Launched | Launched | TBC | Filed | |||||||||||||||
Axanum
|
proton pump inhibitor + low dose aspirin FDC | low dose aspirin associated peptic ulcer |
III | Filed | *** | Filed | 2014 | Filed | ||||||||||||||
Gastrointestinal |
||||||||||||||||||||||
Nexium
|
proton pump inhibitor | peptic ulcer bleeding | III | Filed | Launched | |||||||||||||||||
Nexium
|
proton pump inhibitor | GERD | III | Launched | Launched | Filed | Launched | |||||||||||||||
Neuroscience |
||||||||||||||||||||||
Seroquel XR
|
D2/5HT2 antagonist | major depressive disorder | III | Launched | ** | Launched | ** | Launched | ||||||||||||||
Diprivan#
|
sedative and anaesthetic | conscious sedation | III | Launched | H2 2012 | Launched | ||||||||||||||||
EMLA#
|
local anaesthetic | topical anaesthesia | III | Launched | Filed | Launched | ||||||||||||||||
Oncology |
||||||||||||||||||||||
Iressa
|
EGFR tyrosine kinase inhibitor | 1st line EGFR mut+ NSCLC | III | Launched | Filed | Launched | ||||||||||||||||
Faslodex
|
oestrogen receptor antagonist | high dose (500mg) 2nd line advanced breast cancer | III | Launched | Launched | Filed | Filed | |||||||||||||||
Infection |
||||||||||||||||||||||
FluMist/Fluenz
|
live, attenuated, intranasal influenza virus vaccine |
influenza | III | Launched | Filed | |||||||||||||||||
Respiratory & Inflammation |
||||||||||||||||||||||
Oxis
|
long-acting ß2 agonist | COPD | III | Launched | Q3 2011 | |||||||||||||||||
Symbicort
|
inhaled steroid/ long-acting ß2 agonist |
COPD | III | Launched | Launched | Q4 2011 | Launched | |||||||||||||||
Symbicort
|
inhaled steroid/ long-acting ß2 agonist |
SMART | III | Launched | Q3 2011 | Launched | ||||||||||||||||
| # | Partnered product | |
| * | Kombiglyze™ XR in the US; Onglyza™/metformin IR FDC in the EU | |
| ** | Adjunct only, monotherapy withdrawn | |
| *** | Complete Response Letter received |
| 206 Development Pipeline | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Estimated Filing | ||||||||||||||||||||||
| Compound | Mechanism | Area Under Investigation | Phase | US | EU | Japan | Emerging | |||||||||||||||
Cardiovascular |
||||||||||||||||||||||
Brilinta/Brilique
|
ADP receptor antagonist | arterial thrombosis | III | Filed | * | Launched | 2013 | Approved | ||||||||||||||
Dapagliflozin#
|
SGLT2 inhibitor | diabetes | III | Filed | Filed | 2013 | Q2 2011 | |||||||||||||||
Neuroscience |
||||||||||||||||||||||
Vimovo#
|
naproxen + esomeprazole | signs and symptoms of OA, RA and AS |
III | Launched | Launched | Filed | ||||||||||||||||
TC-5214#
|
neuronal nicotinic receptor modulator |
major depressive disorder (adjunct) |
III | H2 2012 | 2015 | |||||||||||||||||
Oncology |
||||||||||||||||||||||
Vandetanib (Zactima)
|
VEGFR/EGFR tyrosine kinase inhibitor with RET kinase activity |
medullary thyroid cancer |
III | Filed | Filed | Q3 2011 | Q3 2011 | |||||||||||||||
Zibotentan
|
endothelin A receptor antagonist | castrate resistant prostate cancer |
III | H1 2012 | H1 2012 | H1 2012 | ||||||||||||||||
Infection |
||||||||||||||||||||||
MEDI-3250
|
live, attenuated, intranasal influenza virus vaccine (quadrivalent) |
seasonal influenza | III | H1 2011 | TBC | |||||||||||||||||
Zinforo# (ceftaroline)
|
extended spectrum cephalosporin with affinity to penicillin-binding proteins |
pneumonia/skin infections |
III | Filed | Q3 2011 | |||||||||||||||||
Respiratory & Inflammation |
||||||||||||||||||||||
Fostamatinib#
|
spleen tyrosine kinase (SYK) inhibitor | rheumatoid arthritis | III | 2013 | 2013 | 2013 | ||||||||||||||||
| # | Partnered product | |
| * | Complete Response Letter received |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Development Pipeline 207 |
Table of Contents
| Estimated Filing | ||||||||||||||||||||||
| Compound | Mechanism | Area Under Investigation | Phase | US | EU | Japan | Emerging | |||||||||||||||
Cardiovascular |
||||||||||||||||||||||
AZD1656
|
GK activator | diabetes | II | |||||||||||||||||||
AZD6714
|
GK activator | diabetes | I | |||||||||||||||||||
AZD8329
|
11BHSD inhibitor | diabetes/obesity | I | |||||||||||||||||||
AZD7687
|
diacylglycerol acyl transferase –1 inhibitor | diabetes/obesity | I | |||||||||||||||||||
AZD5658
|
GK activator | diabetes/obesity | I | |||||||||||||||||||
AZD4017
|
11BHSD inhibitor | glaucoma | I | |||||||||||||||||||
Neuroscience |
||||||||||||||||||||||
AZD3480#
|
alpha4/beta2 neuronal nicotinic receptor agonist | ADHD | II | |||||||||||||||||||
AZD6765
|
NMDA receptor antagonist | major depressive disorder | II | 2016 | 2016 | |||||||||||||||||
AZD2066
|
metabotropic glutamate receptor 5 antagonist | chronic neuropathic pain | II | |||||||||||||||||||
AZD2066
|
metabotropic glutamate receptor 5 antagonist | major depressive disorder | II | |||||||||||||||||||
NKTR-118#
|
oral peripherally-acting opioid antagonist | opioid-induced constipation | II | 2013 | 2013 | |||||||||||||||||
TC-5214#
|
neuronal nicotinic receptor modulator | major depressive disorder (monotherapy) | II | |||||||||||||||||||
TC-5619#
|
alpha7 neuronal nicotinic receptor agonist | cognitive disorders in schizophrenia | II | |||||||||||||||||||
AZD1446#
|
alpha4/beta2 neuronal nicotinic receptor agonist | Alzheimer’s disease/ADHD | II | |||||||||||||||||||
AZD2423
|
chemokine antagonist | chronic neuropathic pain | II | |||||||||||||||||||
AZD3241
|
myeloperoxidase (MPO) inhibitor | Parkinson’s disease | I | |||||||||||||||||||
AZD3043#
|
GABA-A receptor modulator | short acting sedative/anaesthetic | I | |||||||||||||||||||
MEDI-578
|
anti-NGF MAb | OA pain | I | |||||||||||||||||||
AZD5213
|
H3AN | Alzheimer’s disease/ADHD | I | |||||||||||||||||||
Oncology |
||||||||||||||||||||||
Recentin
|
VEGFR tyrosine kinase inhibitor | NSCLC | II | 2016 | 2016 | |||||||||||||||||
Selumetinib# (AZD6244) (ARRY-142886) |
MEK inhibitor | solid tumours | II | 2015 | 2015 | |||||||||||||||||
Olaparib
|
PARP inhibitor | serous ovarian cancer | II | 2015 | 2015 | 2016 | 2016 | |||||||||||||||
AZD1152
|
aurora kinase inhibitor | haematological malignancies | II | |||||||||||||||||||
AZD8931
|
erbB kinase inhibitor | breast cancer chemo combi/solid tumours | II | 2015 | 2015 | |||||||||||||||||
MEDI-575#
|
anti PDGFR-alpha MAb | solid tumours | II | |||||||||||||||||||
AZD2461
|
PARP inhibitor | solid tumours | I | |||||||||||||||||||
AZD3514
|
androgen receptor downregulator | prostate cancer | I | |||||||||||||||||||
AZD7762
|
CHK1 kinase inhibitor | solid tumours | I | |||||||||||||||||||
AZD8330# (ARRY-424704)
|
MEK inhibitor | solid tumours | I | |||||||||||||||||||
CAT-8015
|
anti-CD22 recombinant immunotoxin | haematological malignancies | I | |||||||||||||||||||
MEDI-551
|
anti-CD19 MAb | haematological malignancies | I | |||||||||||||||||||
AZD8055
|
TOR kinase inhibitor | range of tumours | I | |||||||||||||||||||
MEDI-573#
|
anti-IGF MAb | solid tumours | I | |||||||||||||||||||
AZD1480
|
JAK2 inhibitor | myeloproliferative diseases/solid tumours | I | |||||||||||||||||||
AZD4547
|
FGFR tyrosine kinase inhibitor | solid tumours | I | |||||||||||||||||||
AZD2014
|
TOR kinase inhibitor | solid tumours | I | |||||||||||||||||||
Selumetinib (AZD6244) (ARRY-142886)/MK2206# |
MEK/AKT inhibitor | solid tumours | I | |||||||||||||||||||
MEDI-3617
|
anti-ANG-2 MAb | solid tumours | I | |||||||||||||||||||
AZD5363
|
AKT inhibitor | solid tumours | I | |||||||||||||||||||
MEDI-565
|
anti-CEA BiTE | solid tumours | I | |||||||||||||||||||
| # | Partnered product |
| 208 Development Pipeline | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Estimated Filing | ||||||||||||||||||||||
| Compound | Mechanism | Area Under Investigation | Phase | US | EU | Japan | Emerging | |||||||||||||||
Infection |
||||||||||||||||||||||
AZD9773#
|
anti-TNF-alpha polyclonal antibody | severe sepsis | II | 2015 | 2015 | 2015 | 2015 | |||||||||||||||
CAZ104#
|
beta lactamase inhibitor/cephalosporin | serious infections | II | 2013 | 2014 | |||||||||||||||||
Motavizumab#
|
humanized MAb binding to RSV F protein | early and late treatment of RSV in paeds >1 yr | II | |||||||||||||||||||
CXL104# (CEF104)
|
beta lactamase inhibitor/cephalosporin | MRSA | II | 2015 | ||||||||||||||||||
MEDI-534
|
RSV/PIV-3 vaccine | RSV/PIV prophylaxis | I | |||||||||||||||||||
MEDI-550
|
pandemic influenza virus vaccine | pandemic influenza prophylaxis | I | |||||||||||||||||||
MEDI-559
|
RSV vaccine | RSV prophylaxis | I | |||||||||||||||||||
AZD5847
|
oxazolidinone anti-bacterial inhibitor | tuberculosis | I | |||||||||||||||||||
AZD9742
|
BTGT4 IV | MRSA | I | |||||||||||||||||||
Respiratory & Inflammation |
||||||||||||||||||||||
AZD1981
|
CRTh2 receptor antagonist | asthma/COPD | II | |||||||||||||||||||
MEDI-528#
|
anti-IL-9 MAb | asthma | II | |||||||||||||||||||
CAT-354
|
anti-IL-13 MAb | asthma | II | |||||||||||||||||||
AZD3199
|
iLABA | asthma/COPD | II | |||||||||||||||||||
MEDI-563#
|
anti-IL-5R MAb | asthma | II | |||||||||||||||||||
MEDI-545#
|
anti-IFN-alpha MAb | SLE, myositis | II | |||||||||||||||||||
AZD8848
|
Toll-like receptor 7 agonist | asthma | II | |||||||||||||||||||
CAM-3001#
|
anti-GM-CSFR MAb | rheumatoid arthritis | II | |||||||||||||||||||
AZD2423
|
CCR2b antagonist | COPD | II | |||||||||||||||||||
AZD8683
|
muscarinic antagonist | COPD | II | |||||||||||||||||||
AZD5423
|
inhaled SEGRA | COPD | II | |||||||||||||||||||
AZD5069
|
CXCR2 | COPD | II | |||||||||||||||||||
AZD9819
|
neutrophil elastase inhibitor | COPD | I | |||||||||||||||||||
MEDI-546#
|
anti-IFN-alphaR MAb | scleroderma | I | |||||||||||||||||||
MEDI-551
|
anti-CD19 MAb | scleroderma | I | |||||||||||||||||||
MEDI-570#
|
anti-ICOS MAb | SLE | I | |||||||||||||||||||
MEDI-557
|
RSV MAb – extended half-life | COPD | I | |||||||||||||||||||
| # | Partnered product |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Development Pipeline 209 |
Table of Contents
| Compound | Area Under Investigation | |
Cardiovascular |
||
AZD6370
|
diabetes | |
Certriad
|
dyslipidaemia | |
AZD4017
|
diabetes/obesity | |
AZD0837
|
thrombosis | |
AZD6482
|
thrombosis | |
Gastrointestinal |
||
Lesogaberan (AZD3355)
|
GERD | |
AZD1386
|
GERD | |
AZD2066
|
GERD | |
AZD2516
|
GERD | |
Infection |
||
MEDI-560
|
PIV prophylaxis | |
Motavizumab
|
RSV prevention | |
AZD7295
|
Hepatitis C | |
Neuroscience |
||
AZD6280
|
anxiety | |
AZD8418
|
schizophrenia | |
AZD8529
|
schizophrenia | |
AZD7268
|
depression/anxiety | |
AZD2327
|
depression/anxiety | |
AZD2516
|
chronic neuropathic pain | |
Seroquel XR
|
generalised anxiety disorder US | |
| Compound | Area Under Investigation | |
Oncology |
||
MEDI-547
|
solid tumours | |
AZD4769
|
solid tumours | |
Faslodex
|
1st line advanced breast cancer | |
Olaparib
|
gBRCA breast | |
Recentin
|
CRC | |
Recentin
|
recurrent glioblastoma | |
Respiratory & Inflammation |
||
AZD9668
|
COPD | |
AZD6553
|
COPD | |
AZD2551
|
COPD | |
AZD5122
|
COPD | |
AZD5985
|
asthma/COPD | |
AZD8075
|
asthma/COPD | |
AZD8566
|
COPD | |
AZD1236
|
COPD | |
AZD9164
|
COPD | |
| 210 Development Pipeline | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||
Ordinary Shares in issue – millions |
||||||||||||||||||||
At year end |
1,532 | 1,457 | 1,447 | 1,451 | 1,409 | |||||||||||||||
Weighted average for year |
1,564 | 1,495 | 1,453 | 1,448 | 1,438 | |||||||||||||||
Stock market price – per Ordinary Share |
||||||||||||||||||||
Highest (pence) |
3529 | 2984 | 2888 | 2947 | 3385 | |||||||||||||||
Lowest (pence) |
2574 | 2093 | 1748 | 2147 | 2732 | |||||||||||||||
At year end (pence) |
2744 | 2164 | 2807 | 2910.5 | 2922 | |||||||||||||||
| By size of account | 2006 | 2007 | 2008 | 2009 | 2010 | |||||||||||||||
| No. of shares | % | % | % | % | % | |||||||||||||||
1 – 250 |
0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |||||||||||||||
251 – 500 |
0.7 | 0.7 | 0.7 | 0.7 | 0.6 | |||||||||||||||
501 – 1,000 |
0.9 | 0.9 | 0.9 | 0.8 | 0.8 | |||||||||||||||
1,001 – 5,000 |
1.3 | 1.3 | 1.2 | 1.1 | 1.1 | |||||||||||||||
5,001 – 10,000 |
0.2 | 0.2 | 0.2 | 0.2 | 0.2 | |||||||||||||||
10,001 – 50,000 |
1.0 | 1.0 | 1.0 | 1.1 | 1.0 | |||||||||||||||
50,001 – 1,000,000 |
12.3 | 12.9 | 13.6 | 13.0 | 12.8 | |||||||||||||||
Over 1,000,0001 |
83.1 | 82.5 | 81.9 | 82.6 | 83.0 | |||||||||||||||
| 1 | Includes VPC and ADR holdings. |
| > | For shares listed on the LSE the reported high and low middle market closing quotations are derived from the Daily Official List. | |
| > | For shares listed on the SSE the high and low closing sales prices are as stated in the Official List. | |
| > | For ADSs listed on the NYSE the reported high and low sales prices are as reported by Dow Jones (ADR quotations). |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Shareholder Information 211 |
Table of Contents
| Ordinary LSE | ADS | Ordinary SSE1 | ||||||||||||||||||||||||||
| High (pence) | Low (pence) | High (US$) | Low (US$) | High (SEK) | Low (SEK) | |||||||||||||||||||||||
| 2009 | – Quarter 1 |
2947.0 | 2147.0 | 41.60 | 30.24 | 331.0 | 261.5 | |||||||||||||||||||||
– Quarter 2 |
2728.0 | 2276.0 | 45.01 | 33.40 | 351.0 | 279.5 | ||||||||||||||||||||||
– Quarter 3 |
2878.0 | 2644.0 | 47.54 | 43.01 | 356.0 | 305.0 | ||||||||||||||||||||||
– Quarter 4 |
2930.0 | 2690.5 | 47.00 | 43.64 | 339.5 | 308.0 | ||||||||||||||||||||||
| 2010 | – Quarter 1 |
3102.5 | 2732.0 | 50.40 | 43.05 | 363.8 | 310.1 | |||||||||||||||||||||
– Quarter 2 |
3169.0 | 2772.0 | 48.74 | 40.91 | 368.0 | 314.0 | ||||||||||||||||||||||
– July |
3289.0 | 3051.5 | 51.51 | 47.05 | 371.9 | 353.4 | ||||||||||||||||||||||
– August |
3348.0 | 3216.5 | 53.41 | 49.43 | 382.2 | 365.1 | ||||||||||||||||||||||
– September |
3385.0 | 3233.5 | 52.69 | 50.49 | 376.1 | 345.0 | ||||||||||||||||||||||
– October |
3359.0 | 3129.5 | 53.50 | 50.43 | 354.7 | 336.7 | ||||||||||||||||||||||
– November |
3144.0 | 2995.5 | 50.34 | 46.93 | 336.9 | 325.7 | ||||||||||||||||||||||
– December |
3153.0 | 2922.0 | 49.28 | 45.80 | 336.5 | 309.3 | ||||||||||||||||||||||
| 1 | Principally held in bearer form. |
| Percentage of | ||||||||||||
| Number of | Date of disclosure to | issued share | ||||||||||
| Shareholder | shares | Company1 | capital | |||||||||
BlackRock, Inc. |
100,885,181 | 8 December 2009 | 7.18 | |||||||||
Invesco Limited |
72,776,277 | 6 October 2009 | 5.18 | |||||||||
Axa SA |
56,991,117 | 3 February 2009 | 4.06 | |||||||||
Investor AB |
51,587,810 | 3 February 2009 | 3.67 | |||||||||
Legal & General Investment Management Limited |
57,675,232 | 5 August 2010 | 4.10 | |||||||||
| 1 | Since the date of disclosure to the Company, the interest of any person listed above in Ordinary Shares may have increased or decreased. No requirement to notify the Company of any increase or decrease would have arisen unless the holding moved up or down through a whole number percentage level. The percentage level may increase (on the cancellation of shares following a repurchase of shares under the Company’s share repurchase programme) or decrease (on the issue of new shares under any of the Company’s share plans). |
| Percentage of issued share capital | ||||||||||||||||
| Shareholder | 27 Jan 2011 | 28 Jan 2010 | 29 Jan 2009 | 31 Jan 2008 | ||||||||||||
BlackRock, Inc. |
7.18 | 6.94 | – | – | ||||||||||||
Invesco Limited |
5.18 | 5.01 | – | – | ||||||||||||
Axa SA |
4.06 | 3.92 | 4.90 | 4.87 | ||||||||||||
Investor AB |
3.67 | 3.55 | 4.38 | 4.36 | ||||||||||||
Legal & General Investment Management Limited |
4.10 | 4.64 | 4.09 | 4.06 | ||||||||||||
Capital Research and Management Company |
– | – | 4.92 | 4.89 | ||||||||||||
Wellington Management Co., LLP |
– | – | 4.18 | 4.16 | ||||||||||||
Barclays PLC |
– | – | 4.26 | 4.24 | ||||||||||||
> In the US |
771 | |||
> Total |
120,325 |
> In the US |
2,211 | |||
> Total |
2,237 |
| 212 Shareholder Information | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| Title of class | Amount owned | Percentage of class | ||||||
Ordinary Shares |
390,106 | 0.03 | ||||||
| Number of shares | Subscription price (pence) | Normal expiry date | ||||||
50,058,484 |
1882 – 3487 | 2011 – 2019 | ||||||
| Number of shares | Subscription price (pence) | Normal expiry date | ||||||
1,928,024 |
1882 – 3487 | 2011 – 2019 | ||||||
| (c) | Included in paragraph (b) are options granted to individually named Directors. Details of these option holdings at 31 December 2010 are shown in the Share option plans table on page 134. |
First interim:
|
Announced in July and paid in September. | |
Second interim:
|
Announced in January and paid in March. |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Shareholder Information 213 |
Table of Contents
| 214 Shareholder Information | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| SEK/US$ | US$/GBP | |||||||
Average rates (profit and loss account, cash flow) |
||||||||
1995 |
7.1100 | 1.5796 | ||||||
1996 |
6.7000 | 1.5525 | ||||||
1997 |
7.6225 | 1.6386 | ||||||
1998 |
7.9384 | 1.6603 | ||||||
1999 |
8.2189 | 1.6247 | ||||||
End of year spot rates (balance sheet) |
||||||||
1995 |
6.6500 | 1.5500 | ||||||
1996 |
6.8400 | 1.6900 | ||||||
1997 |
7.8500 | 1.6600 | ||||||
1998 |
8.0400 | 1.6600 | ||||||
1999 |
8.5130 | 1.6185 | ||||||
| SEK/US$ | US$/GBP | |||||||
Average rates (income statement, cash flow) |
||||||||
2008 |
6.5130 | 1.8728 | ||||||
2009 |
7.6552 | 1.5496 | ||||||
2010 |
6.9256 | 1.5852 | ||||||
End of year spot rates (balance sheet) |
||||||||
2008 |
7.7740 | 1.4437 | ||||||
2009 |
7.1636 | 1.6072 | ||||||
2010 |
6.7511 | 1.5422 | ||||||

| AstraZeneca Annual Report and Form 20-F Information 2010 | Shareholder Information 215 |
Table of Contents
The Company’s objects were originally set out in its Memorandum of Association. By operation of law, on 1 October 2009, these objects were deemed to be provisions of the Articles. However, by a special resolution of the shareholders at the Company’s AGM held on 29 April 2010, those deemed objects were deleted from the Articles. The Company’s objects are now unrestricted.
| > | The Redeemable Preference Shares carry no rights to receive dividends. | |
| > | The holders of Redeemable Preference Shares have no rights to receive notices of, attend or vote at general meetings except in certain limited circumstances. They have one vote for every 50,000 Redeemable Preference Shares held. | |
| > | On a distribution of assets of the Company, on a winding-up or other return of capital (subject to certain exceptions), the holders of Redeemable Preference Shares have priority over the holders of Ordinary Shares to receive the capital paid up on those shares. | |
| > | Subject to the provisions of the Companies Act 2006, the Company has the right to redeem the Redeemable Preference Shares at any time on giving not less than seven days’ written notice. |
| 216 Corporate Information | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
| United States of America | Other Established Markets | Emerging Markets | ||||||||
US
|
Western Europe | Japan | Emerging Europe | China | Other Emerging ROW | |||||
| Austria | Albania* | Egypt | ||||||||
| Belgium | Canada | Belarus* | Emerging Asia Pacific | Gulf States | ||||||
| Denmark | Bosnia-Herzegovina* | Bangladesh* | Israel* | |||||||
| Finland | Other Established ROW | Bulgaria* | Cambodia* | Latin America | ||||||
| France | Australia | Croatia* | Hong Kong* | Lebanon | ||||||
| Germany | New Zealand* | Czech Republic | India | Maghreb | ||||||
| Greece | Estonia* | Indonesia* | Saudi Arabia | |||||||
| Holland | Georgia* | Laos* | South Africa | |||||||
| Iceland* | Hungary* | Malaysia* | ||||||||
| Ireland | Kazakhstan* | Philippines | ||||||||
| Italy | Latvia* | Singapore | ||||||||
| Luxembourg* | Lithuania* | South Korea | ||||||||
| Norway | Macedonia* | Sri Lanka* | ||||||||
| Portugal | Poland | Taiwan | ||||||||
| Spain | Romania* | Thailand | ||||||||
| Sweden | Russia | Vietnam* | ||||||||
| Switzerland | Serbia/Montenegro* | |||||||||
| UK | Slovakia | |||||||||
| Slovenia* | ||||||||||
| Turkey | ||||||||||
| Ukraine* | ||||||||||
Terms used in this Annual Report and Form 20-F Information
|
US equivalent or brief description | |
Accruals
|
Accrued expenses | |
Allotted
|
Issued | |
Called-up share capital
|
Issued share capital | |
Creditors
|
Liabilities/payables | |
Debtors
|
Receivables and prepaid expenses | |
Earnings
|
Net income | |
Employee share schemes
|
Employee stock benefit plans | |
Fixed asset investments
|
Non-current investments | |
Freehold
|
Ownership with absolute rights in perpetuity | |
Interest payable
|
Interest expense | |
Interest receivable
|
Interest income | |
Loans
|
Long-term debt | |
Prepayments
|
Prepaid expenses | |
Profit
|
Income | |
Profit and loss account
|
Income statement/consolidated statement of income | |
Reserves
|
Retained earnings | |
Share premium account
|
Premiums paid in excess of par value of Ordinary Shares | |
Short-term investments
|
Redeemable securities and short-term deposits | |

| AstraZeneca Annual Report and Form 20-F Information 2010 | Glossary 217 |
Table of Contents
| 218 Glossary | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents

| AstraZeneca Annual Report and Form 20-F Information 2010 | Glossary 219 |
Table of Contents
| 2010 performance summary | 18 | |
| Accounting policies | 90, 142, 200 | |
| Acquisitions | 167 | |
| Annual general meeting | 118, 216 | |
| Aptium Oncology, Inc. | 75 | |
| Articles of association | 214, 216 | |
| Astra Tech AB | 75 | |
| AstraZeneca PLC financial statements | 198 | |
| AstraZeneca PLC balance sheet | 199 | |
| Audit Committee | 111, 112, 113 | |
| Biologics | 11, 12, 42, 66, 99, 101 | |
| Board | 106 | |
| Branches | 117 | |
| Business background and results overview | 81 | |
| Capital and reserves | 166 | |
| Capitalisation | 86 | |
| Cardiovascular | 2, 27, 50, 52, 147, 206 | |
| Cash and cash equivalents | 144, 157 | |
| Chairman’s statement | 6 | |
| Chief Executive Officer’s review | 8 | |
| Combined Code | 109 | |
| Commitments and contingent liabilities | 178 | |
| Company history | 211, 216 | |
| Competition | 12, 99 | |
| Compliance and Group Internal Audit | 116 | |
| Consolidated statement of cash flows | 141 | |
| Consolidated statement of changes in equity | 140 | |
| Consolidated statement of comprehensive income | 138 | |
| Consolidated statement of financial position | 139 | |
| Corporate Responsibility | See Responsible Business | |
| Directors’ interest in shares | 132 | |
| Directors’ responsibility statement | 136 | |
| Dividends | 6, 86, 117, 167, 213, 214 | |
| Earnings per ordinary share | 4, 151 | |
| Emerging markets | 10, 32, 33, 38, 71, 73, 98, 217 | |
| Employee costs and share options for employees | 173 | |
| Environmental sustainability | 45 | |
| Established markets | 10, 32, 38, 71, 73, 217 | |
| Ethics (including stem cell research | 30, 33, 41, 42, 43 | |
| and animal research) | ||
| Executive Directors’ and Senior Executive Team’s | 123 | |
| remuneration and terms of employment | ||
| Finance income and expense | 148 | |
| Financial highlights | inside front cover | |
| Financial instruments | 146, 158 | |
| Financial position 2009 | 89 | |
| Financial position 2010 | 85 | |
| Financial risk management | 90, 168 | |
| Form 20-F | 115 | |
| Gastrointestinal | 2, 27, 50, 56, 147, 206 | |
| Glossary | 217 | |
| Goodwill | 85, 89, 92, 143, 154 | |
| Group financial record | 204 | |
| Group financial statements | 135 | |
| Growth drivers | 10 | |
| Independent auditor’s report | 137, 198 | |
| Infection | 2, 27, 50, 58, 147, 206 | |
| Inflammation | see Respiratory & Inflammation | |
| Intangible assets | 85, 89, 92, 143, 155, 178 | |
| Intellectual property | 14, 30, 97, 98 | |
| Interest-bearing loans and borrowings | 145, 158, 170, 201 | |
| Inventories | 89, 144, 157 | |
| Key performance indicators | 16, 41, 43 | |
| Leases | 144, 196 | |
| Litigation | 92, 98, 101, 102, 145, 178, 200, 203 | |
| Managing risk | 95 | |
| Market definitions | 217 | |
| Medicines | 5, 20, 50 | |
| Neuroscience | 2, 27, 50, 61, 147, 206 | |
| Nomination and Governance Committee | 111, 112, 115 | |
| Oncology | 2, 27, 50, 64, 147, 206 | |
| Operating profit | 4, 16, 82, 148 | |
| Operational overview | 4 | |
| Other investments | 144, 157, 159 | |
| Our marketplace | 10 | |
| Outsourcing | 14, 15, 40, 44, 101 | |
| Patents | see Intellectual property | |
| Patient safety | 44 | |
| People | 15, 16, 18, 36, 46, 173 | |
| Pipeline | 15, 16, 18, 27, 51, 96, 206 | |
| Political donations | 118 | |
| Portfolio Investment Board (PIB) | 116 | |
| Post-retirement benefits | 93, 103, 162 | |
| Pricing | 11, 16, 33 | |
| Principal risks and uncertainties | 96 | |
| Product revenue information | 147 | |
| Property, plant and equipment | 85, 89, 144, 153 | |
| Provisions for liabilities and charges | 85, 89, 161 | |
| Regulatory requirements | 12 | |
| Related party transactions | 213 | |
| Relations with shareholders | 112 | |
| Remuneration Committee | 111, 112, 115, 119, 120 | |
| Research and development | 9, 12, 26, 92, 143, 148 | |
| Respiratory & Inflammation | 2, 27, 50, 67, 206 | |
| Responsible Business | 9, 15, 18, 40 | |
| Rest of World | 4, 72, 217 | |
| Restructuring | 4, 38, 79, 148 | |
| Results of operations 2009 | 87 | |
| Results of operations 2010 | 82 | |
| Safety, health and wellbeing | 46 | |
| Sales and marketing | 16, 32 | |
| Sales by therapy area | 50 | |
| Science Committee | 111, 112, 115 | |
| Segment information | 151 | |
| Senior Executive Team (SET) | 108, 116 | |
| Share capital | 117, 167, 202, 211 | |
| Share repurchase | 4, 6, 7, 86, 87, 117, 167, 202 | |
| Statutory and other information | 196 | |
| Strategy | 10 | |
| Subsidiaries | 117, 197 | |
| Supply and manufacturing | 34 | |
| Taxation | 85, 89, 90, 93, 103, 143, 149, 195 | |
| Taxation information for shareholders | 214 | |
| Trade and other payables | 85, 89, 144, 161 | |
| Trade and other receivables | 85, 89, 144, 157 | |
| Trade marks | 98, inside back cover | |
| Transactions with directors | 131 | |
| UK corporate governance | 109 | |
| UK Corporate Governance Code | 109 | |
| US | 10, 72, 217 | |
| US corporate governance | 115 | |
| Working with others | 3 | |
| Surviving coronary heart disease | 13 | |
| Taking medicines | 19 | |
| Managing asthma | 23 | |
| Tackling counterfeit drugs | 35 | |
| Searching for new antibiotics | 39 | |
| Improving healthcare in Uganda | 49 | |
| Improving healthcare in China | 77 | |
| Personalising cancer treatments | 104 | |
| 220 Index | AstraZeneca Annual Report and Form 20-F Information 2010 |
Table of Contents
Trade marks of the AstraZeneca group of companies appear throughout this Annual Report in italics. AstraZeneca, the AstraZeneca logotype and the AstraZeneca symbol are all trade marks of the AstraZeneca group of companies. Trade marks of companies other than AstraZeneca appear with a ™ sign and include: Abraxane™, a trade mark of Abraxis BioScience, LLC.; Advair Diskus™, a trade mark of Glaxo Group Limited; Celebrex™, a trade mark of G. D. Searle LLC; Cubicin™, a trade mark of Cubist Pharmaceuticals, Inc.; CytoFab™, a trade mark of Protherics Inc.; Ethyol™, a trade mark of Scherico Ltd in certain countries and AstraZeneca in others; Iscover™, a trade mark of Sanofi-Aventis; Kombiglyze™, a trade mark of Bristol-Myers Squibb Company; Lipitor™, a trade mark of Pfizer Ireland Pharmaceuticals; Onglyza™, a trade mark of Bristol-Myers Squibb Company; Plavix™, a trade mark of Sanofi-Aventis; Teflaro™, a trade mark of Forest Laboratories, Inc.; and Trilipix™, a trade mark of Fournier Industrie et Santé.

|
This product is printed on Heaven 42 paper. This paper is made from virgin wood fibre from
well-managed forests independently certified according to the rules of the Forest Stewardship
Council (FSC). The mill uses pulps that are totally chlorine free (TCF), and some pulp is bleached
using an elemental chlorine free (ECF) process. The inks in printing this Annual Report are all
vegetable-based. Printed at St Ives Westerham Press Ltd, ISO9001, ISO14001, FSC certified and CarbonNeutral® |
Table of Contents

headquarters
AstraZeneca PLC
2 Kingdom Street
London W2 6BD
UK
Tel: +44 (0)20 7604 8000
Fax: +44 (0)20 7604 8151
E-mail: ir@astrazeneca.com
AstraZeneca AB
SE-151 85 Södertälje
Sweden
Tel: +46 (0)8 553 260 00
Fax: +46 (0)8 553 290 00
Investor relations
AstraZeneca Pharmaceuticals LP
1800 Concord Pike
PO Box 15437
Wilmington
DE 19850-5437
US
Tel: +1 (302) 886 3000
Fax: +1 (302) 886 2972
This Annual Report is also available on our
website, astrazeneca.com/annualreport2010.
Equiniti Limited
Aspect House
Spencer Road
Lancing
West Sussex BN99 6DA
UK
Tel (freephone in the UK):
0800 389 1580
Tel (outside the UK):
+44 (0)121 415 7033
Depository
Euroclear Sweden AB
PO Box 7822
SE-103 97 Stockholm
Sweden
Tel: +46 (0)8 402 9000
JPMorgan Chase & Co
PO Box 64504
St Paul
MN 55164-0504
US
Tel (toll free in the US):
800 990 1135
Tel (outside the US):
+1 (651) 453 2128
E-mail: jpmorgan.adr@wellsfargo.com