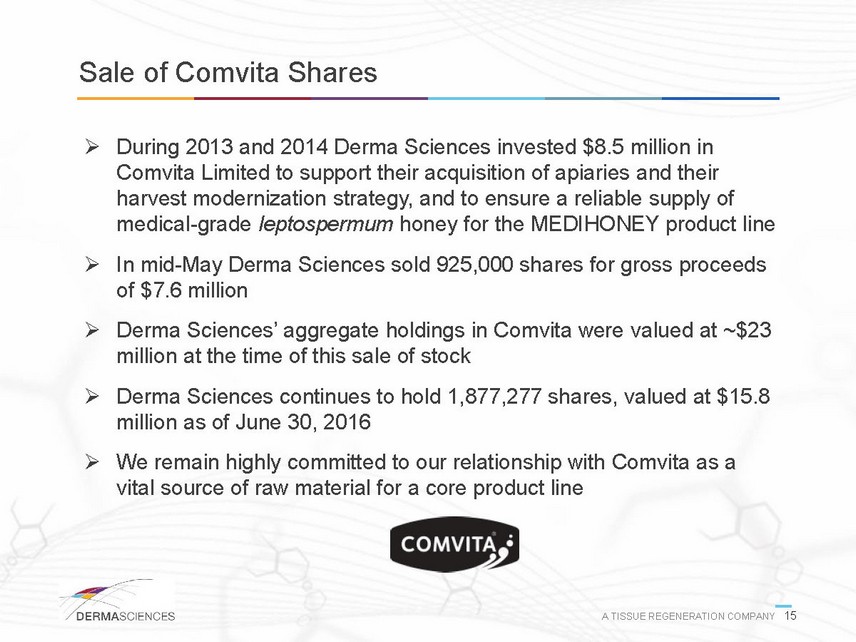
Exhibit 10.1
SEPARATION AGREEMENT
This Separation Agreement
(this “Agreement”) is made and entered into as of July 31, 2016 (the “Separation Date”),
by and between Barry Wolfenson (the “Executive”) and Derma Sciences, Inc. (the “Company”).
The Company and Executive are sometimes collectively referred to herein as the Parties and individually as a Party.
As used in this Agreement, the term “affiliate” shall mean any entity controlled by, controlling, or under common
control with, the Company.
WHEREAS, Executive
and the Company have determined to provide for the separation of Executive’s employment with the Company and its affiliates
on the terms and subject to the conditions set forth herein.
NOW, THEREFORE,
in consideration of the foregoing recitals, the mutual promises contained herein, and for other good and valuable consideration,
the receipt and adequacy of which are hereby acknowledged, the Parties hereto agree as follows:
1. Separation. As of the Separation
Date, Executive’s employment and status as an employee and officer with the Company and its affiliates (including, without
limitation, as Group President, Advanced Wound Care of the Company) will terminate and Executive will cease to be an employee
of any and all of the foregoing. In addition, as of the Separation Date, Executive shall, and by execution of this Agreement he
does, resign from any and all directorships Executive may hold with any of the Company’s affiliates.
2. Accrued Benefits. The
Company will pay and provide to Executive the following payments and benefits:
(a) Salary and PTO Pay. Within
10 calendar days after the Separation Date, or such earlier date as required by law, the Company will issue to Executive his final
paycheck, reflecting (i) his unpaid base salary through the Separation Date, and (ii) his accrued but unused PTO pay through the
Separation Date.
(b) Expense
Reimbursements. Within 30 calendar days following the Separation Date, the Company will reimburse Executive for any reasonable
unreimbursed business expenses actually and properly incurred by Executive in connection with carrying out his duties with the
Company through the Separation Date in accordance with applicable Company business expense reimbursement policies, which expenses
will be submitted by Executive to the Company with supporting receipts and/or documentation no later than 10 calendar days after
the Separation Date.
(c) Equity Awards. The Company
has granted Executive stock options and other equity based compensation that are outstanding as of the Separation Date pursuant
to the terms and conditions of the Company’s equity compensation plan and the equity award agreements between the Parties
(the “Equity Awards”). As partial consideration for Executive’s obligations under the Non-Compete provisions
set forth in Section 6(d) hereof, the following Equity Awards that remain outstanding immediately prior to the Separation Date
shall become vested and exercisable, effective as of the Separation Date, until the Expiration Date set forth on the following
table:
|
Equity Award
and
Expiration Date
|
Equity Awards to Vest as of
the Separation Date |
Exercise Price |
Time-Based
Stock Option
(Granted 2/14/14 and Expiring 2/14/24) |
5,875 option shares |
$13.39/share |
Time-Based
Stock Option
(Granted 2/12/2015 and Expiring 2/12/25) |
6,500 option shares |
$8.83/share |
Time-Based
Stock Option
(Granted 3/2/16 and Expiring 3/2/2026) |
12,000 option shares |
$3.30/share |
Any remaining unvested
Equity Awards that have not vested prior to the Separation Date and that do not vest in accordance with this Section 3(c) shall
be forfeited effective as of the Separation Date.
(d) Other
Benefits. All Company-provided benefits shall cease to accrue on the Separation Date, including, but not limited to, accrual
of PTO, sick, and other benefits. The Executive shall be eligible for COBRA coverage as set forth below.
3. Severance Benefits. In
consideration of, and subject to and conditioned upon Executive’s execution and non-revocation of the release attached as
Exhibit A to this Agreement (the “Release”) and the effectiveness of such Release as provided in Section
4 of this Agreement, and subject to Executive’s continuing compliance with his obligations in Sections 1, 6(b), 6(c) and
6(d) hereof, the Company will pay or provide to Executive the following payments and benefits, which Executive acknowledges and
agrees constitute adequate and valuable consideration, in and of themselves, for the promises contained in this Agreement:
(a) Severance. The Company
shall pay to Executive an amount equal to $314,000 (the “Severance Payment”), payable in equal installments
in accordance with the Company’s payroll practices over the one year period commencing on the first payroll date after the
Company is in receipt of the executed “Release”.
(b) Non- Compete.
The Company shall pay to Executive an additional amount equal to $78,500 as additional consideration for Executive’s obligations
under the Non-Compete provisions set forth in Section 6(d) hereof (the “Non-Compete Payment”) payable in equal installments
in accordance with the Company’s payroll practices over the one year period commencing on the first payroll date after the
Company is in receipt of the executed “Release”. Any breach or alleged breach of the agreement not to compete shall
not affect the obligation of the Company to make the Severance Payment as set forth in paragraph 3(a) above.
(b) Continued Health
Care Benefits. If Executive timely elects continued health coverage under COBRA, the Company will pay all related COBRA premiums,
and any other premiums required to maintain Executive’s health insurance coverage as in effect immediately prior to the Separation
Date, for the one year period commencing on the Separation Date and ending August 31, 2017 (the “Premium Period”),
which period shall run concurrently with the COBRA continuation period. An amount equal to the applicable premiums (or such other
amounts as may be required by law) will be included in Executive’s income for tax purposes to the extent required by applicable
law and the Company may withhold taxes from Executive’s other compensation for this purpose.
4. Release
of Claims. Executive agrees that, as a condition to Executive’s right to receive the payments and benefits set forth
in Section 3, within 21 calendar days following the Separation Date, Executive shall execute and deliver the Release to the Company.
If Executive fails to execute and deliver the Release to the Company, or if the Release is revoked by Executive or otherwise does
not become effective and irrevocable in accordance with its terms, then Executive will not be entitled to any payment or benefit
under Section 3 of this Agreement.
5. Effect on Other Arrangements.
Executive acknowledges that the payments and arrangements contained in this Agreement will constitute full and complete satisfaction
of any and all amounts properly due and owing to Executive as a result of his employment with the Company and its affiliates and
the termination thereof. Executive agrees that, as of the Separation Date, this Agreement supersedes and replaces the severance
terms under any plan, program, policy or practice or contract or agreement of the Company and its affiliates, including the terms
of the Employment Agreement between the Parties dated March 8, 2012, as amended December 20, 2012, March 27, 2013, and March 9,
2015 (the “Employment Agreement”), and that Company and its affiliates have no further obligations to Executive
under any plan, program, policy or practice or contract or agreement, with the exception of the Company’s equity compensation
plan and the equity award agreements.
6. Covenants.
(a) Claw Back Policy.
Executive acknowledges that he shall remain subject to the provisions of the Company’s Claw Back Policy (the “Claw
Back Policy”), as in effect on the Separation Date, which shall survive and continue in full force and effect notwithstanding
the termination of Executive’s employment. The Parties acknowledge that, on and after the Separation Date, the Company may
not amend or modify the Claw Back Policy in a manner that adversely affects Executive, unless the Company determines in good faith
that such amendment or modification is required in order to comply with applicable laws or exchange listing requirements.
(b) Non-Disparagement.
Executive agrees that he will not do or say anything that could reasonably be expected to disparage or impact negatively the name
or reputation in the marketplace of the Company or any of its affiliates, employees, officers, directors, stockholders, members,
principals or assigns. Nothing in this Section 6(b) shall preclude Executive from responding truthfully to any legal process or
truthfully testifying in a legal or regulatory proceeding, provided that, to the extent permitted by law, Executive promptly informs
the Company of any such obligation prior to participating in any such proceedings. The Company likewise agrees that it will not
release any information or make any statements, and its officers and directors shall not do or say anything that could reasonably
be expected to disparage or impact negatively the name or reputation in the marketplace of Executive. Nothing herein shall preclude
the Company or any of its affiliates, employees, officers, directors, stockholders, members, principals or assigns from responding
truthfully to any legal process or truthfully testifying in a legal or regulatory proceeding, provided that to the extent permitted
by law, the Company will promptly inform Executive in advance if it has reason to believe such response or testimony will directly
relate to Executive, or preclude the Company from complying with applicable disclosure requirements.
(c) Trade Secrets,
Confidential and/or Proprietary Information. Executive agrees to keep and preserve as confidential: (i) all trade secrets and/or
other proprietary and/or confidential information belonging to the Company; and (ii) all trade secrets and/or other proprietary
and/or confidential information belonging to any third party which have been confidentially disclosed to the Company, which trade
secrets and/or other proprietary and/or confidential information described in (i) and (ii) above (collectively, "Confidential
Information") may have been developed or obtained by or disclosed to Executive by reason of Executive’s employment with
the Company. Confidential Information will include, but not be limited to, all nonpublic information relating to the Company's:
(i) business, research, development and marketing plans, strategies and forecasts; (ii) products (whether existing, in development,
or being contemplated); (iii) customers' identities, usages, contract terms, pricing, and requirements; (iv) reports; (v) formulas;
(vi) specifications; (vii) designs, software and other technology; (viii) research and development programs; (ix) terms of contracts;
and (x) information obtained from or relating to any clients, customers, consultants, licensees or affiliates.
(d) Non-Compete.
During the six (6) month period following the Separation Date the “Restricted Period”), Executive will not, directly
or indirectly, whether on his own behalf or on behalf of a third party (i) engage in any part of the Business, assist others in
engaging in any part of the Business or compete with the Company in any part of the Business, in each case, in any part of the
United States of America (the “Restricted Territory”), (ii) have an interest in any Person that competes with or engages
directly or indirectly in any part of the Business anywhere in the Restricted Territory, and in any capacity whatsoever, including
as a partner, shareholder (other than as a less than two percent shareholder of a publicly traded corporation), member, employee,
principal, agent, trustee or consultant, or (iii) cause, induce or encourage any Person who is (A) an employee, client, customer,
supplier or licensor of the Company or any of its affiliates (including any existing or former client or customer of the Company
or any of its affiliates) or (B) a prospective client, customer, supplier or licensor of the Company or any of its affiliates,
in each case to terminate or modify any such relationship. Notwithstanding the above, the Company acknowledges that Executive may
without using or disclosing any Confidential Information engage in certain preliminary activities in order to assess his ability
or interest to engage in a competing business, such as contacting potential investors, inquiring about the availability of certain
advanced wound care products (except for any products for which the Company sent a letter of intent to purchase during the last
six months), and preparing a business plan (but shall not contact any key opinion leaders, customers, or employees or Company with
respect to any potential new business) without violating this agreement not to compete. Executive understands that this agreement
not to compete is an essential element of this Agreement, that the Company is paying additional consideration to Executive under
this Agreement for this agreement not to compete, and that the Company would not have entered into this Agreement without this
agreement not to compete having been included in it. Executive acknowledges that this agreement not to compete is reasonable and
appropriate in all respects and in the specific context of the Business. For the purposes of this paragraph 6(d), the “Business”
is defined as the advanced wound care business of the Company, and “Person” shall include an individual, corporation,
partnership, limited liability company or any other entity.
7. Indemnification
and Insurance. The Company will continue in force its directors and officers liability insurance coverages with respect to
any claims that may be asserted against Executive arising out of Executive’s employment with the Company. Executive shall
be eligible for indemnification on the same basis as other former officers of the Company in accordance with its bylaws and applicable
law after the Separation Date.
8. Miscellaneous.
(a) Section 409A.
The intent of the Parties is that payments and benefits under this Agreement comply with Section 409A of the Code (“Section
409A”) or are exempt therefrom and, accordingly, to the maximum extent permitted, this Agreement will be interpreted
and administered so as to be in compliance therewith. If Executive notifies the Company (with specificity as to the reason therefor)
that Executive believes that any provision of this Agreement would cause Executive to incur any additional tax or interest under
Section 409A and the Company concurs with such belief or the Company (without any obligation whatsoever to do so) independently
makes such determination, the Company will, after consulting with Executive, reform such provision in a manner that is economically
neutral to the Company to attempt to comply with Section 409A through good faith modifications to the minimum extent reasonably
appropriate to conform with Section 409A. The Parties hereby acknowledge and agree that (i) the payments and benefits due to Executive
under Section 3 above are payable or provided on account of Executive’s “separation from service” within the
meaning of Section 409A; and (ii) each installment of Severance Payment payable to Executive under Section 3(a) is intended to
be treated as a separate payment for purposes of Section 409A that is exempt from Section 409A, to the maximum extent possible,
under the “short-term deferral” exemption of Treasury Regulation Section 1.409A-1(b)(4)
and/or the “involuntary separation pay” exemption of Treasury Regulation Section 1.409A-1(b)(9)(iii). Notwithstanding
any provision of this Agreement to the contrary, if Executive is determined by the Company to be a “specified employee”
within the meaning of Section 409A, then any payment under this Agreement that is considered nonqualified deferred compensation
subject to Section 409A will be paid no earlier than (1) the date that is six months after the date of Executive’s separation
from service, or (2) the date of Executive’s death. In no event may Executive, directly or indirectly, designate the calendar
year of any payment under this Agreement.
(b) Withholding. The Company
or its affiliates, as applicable, may withhold from any amounts payable or benefits provided under this Agreement such federal,
state, local, foreign or other taxes as will be required to be withheld pursuant to any applicable law or regulation. Notwithstanding
the foregoing, Executive will be solely responsible and liable for the satisfaction of all taxes, interest and penalties that may
be imposed on Executive in connection with this Agreement (including any taxes, interest and penalties under Section 409A), and
neither the Company nor its affiliates will have any obligation to indemnify or otherwise hold Executive harmless from any or all
of such taxes, interest or penalties. To the extent the Company or any affiliate is required to withhold any federal, state, local,
foreign or other taxes in connection with the payment or exercise of an Equity Award, then the Company or an affiliate (as applicable)
shall satisfy the minimum required tax withholding obligation via a net share withholding method authorized by the applicable equity
plan.
(c) Severability. In construing
this Agreement, if any portion of this Agreement will be found to be invalid or unenforceable, the remaining terms and provisions
of this Agreement will be given effect to the maximum extent permitted without considering the void, invalid or unenforceable provision.
(d) Successors. This Agreement
is personal to Executive and without the prior written consent of the Company will not be assignable by Executive other than by
will or the laws of descent and distribution. This Agreement will inure to the benefit of and be enforceable by Executive’s
surviving spouse, heirs and legal representatives. This Agreement will inure to the benefit of and be binding upon the Company
and its affiliates, and their respective successors and assigns.
(e) Final and Entire Agreement;
Amendment. This Agreement, together with the Release, represents the final and entire agreement between the Parties with respect
to the subject matter hereof and supersedes all prior agreements, negotiations and discussions between the Parties hereto and/or
their respective counsel with respect to the subject matter hereof. Executive has not relied upon any representations, promises
or agreements of any kind except those set forth herein in signing this Agreement. Any amendment to this Agreement must be in writing,
signed by duly authorized representatives of the Parties, and stating the intent of the Parties to amend this Agreement.
(f) Governing Law; Jurisdiction.
This Agreement shall be governed by and construed in accordance with the laws of the State of New Jersey, without reference to
conflict of laws principles.
(g) Notices. All notices
and other communications hereunder will be in writing and will be given by hand delivery to the other Party or by registered or
certified mail, return receipt requested, postage prepaid, or by overnight courier, addressed as follows:
If to Executive: at Executive’s
most recent address on the records of the Company;
If to the Company: Derma Sciences,
Inc., 214 Carnegie Center, Suite 300, Princeton, NJ 08540, Attention: Chairman of the Board;
or to such other address as either Party
will have furnished to the other in writing in accordance herewith. Notice and communications will be effective on the date of
delivery if delivered by hand, on the first business day following the date of dispatch if delivered utilizing overnight courier,
or three business days after having been mailed, if sent by registered or certified mail.
(h)
Cooperation. From and after the Separation Date, the Employee agrees to be available for and to and cooperate with
the Company in any internal investigation or administrative, regulatory, or judicial proceeding. The Employee understands and agrees
that his cooperation would consist of making herself available to the Company on reasonable notice for interviews and factual investigations;
appearing at the Company’s request to give deposition and trial testimony; volunteering to the Company relevant information;
and turning over all relevant documents which are or may come into his possession. The Company understands that if the Company
asks for his cooperation under this provision, the Company will reimburse him solely for reasonable pre-approved expenses upon
his submission of proper documentation.
(i) Counterparts. This Agreement
may be executed in one or more counterparts (including by means of facsimile or other electronic transmission), each of which will
be deemed an original, but all of which taken together will constitute one original instrument.
IN WITNESS WHEREOF, the Parties hereto have each executed this
Agreement as of the date first above written.
|
Derma Sciences,
Inc.
/s/ Stephen T. Wills
By: Stephen T. Wills
Its: Chairman of the Board
|
EXECUTIVE
/s/ Barry J. Wolfenson
Barry J. Wolfenson |
EXHIBIT A
GENERAL RELEASE
This General Release
(this “Release”) is made and entered into as of this ____ day of July, 2016, by and between Derma Sciences,
Inc. (the “Company”) and Barry J. Wolfenson (“Executive”).
1. Employment
Status. Executive’s employment with the Company and its affiliates is terminated effective as of July 31, 2016 (the “Separation
Date”).
2. Payments
and Benefits. Upon the effectiveness of the terms set forth herein, the Company shall provide Executive with the benefits set
forth in Section 3 of the Separation Agreement between Executive and the Company dated as of July 31, 2016 (the “Separation
Agreement”), upon the terms, and subject to the conditions, of the Separation Agreement. Executive agrees that Executive
is not entitled to receive any additional payments as wages, vacation or bonuses except as otherwise provided under the Separation
Agreement.
3. No
Liability. This Release does not constitute an admission by the Company or its affiliates or predecessors, or their respective
officers, directors, partners, agents, or employees, or by Executive, of any unlawful acts or of any violation of federal, state
or local laws.
4. Release.
In consideration of the payments and benefits set forth in Section 2 of this Release, Executive for himself/herself, his or her
heirs, administrators, representatives, executors, successors and assigns (collectively, “Releasors”) does hereby
irrevocably and unconditionally release, acquit and forever discharge the Company, its respective affiliates and their respective
predecessors, successors and assigns (the “Company Group”) and each of its officers, directors, partners, agents,
and former and current employees, including without limitation all persons acting by, through, under or in concert with any of
them (collectively, “Releasees”), and each of them, from any and all claims, demands, actions, causes of action,
costs, expenses, attorney fees, and all liability whatsoever, whether known or unknown, fixed or contingent, which Executive has,
had, or may ever have against the Releasees relating to or arising out of Executive’s employment or separation from employment
with the Company Group, from the beginning of time and up to and including the date Executive executes this Release. This Release
includes, without limitation, (a) law or equity claims; (b) contract (express or implied) or tort claims; (c) claims for wrongful
discharge, retaliatory discharge, whistle blowing, libel, slander, defamation, unpaid compensation, wage and hour violations, intentional
infliction of emotional distress, fraud, public policy contract or tort, and implied covenant of good faith and fair dealing, whether
based in common law or any federal, state or local statute; (d) claims under or associated with any of the Company Group’s
incentive or equity compensation plans or arrangements; (e) claims arising under any federal, state, or local laws of any jurisdiction
that prohibit age, sex, race, national origin, color, disability, religion, veteran, military status, sexual orientation, or any
other form of discrimination, harassment, or retaliation (including without limitation under the Age Discrimination in Employment
Act of 1967 as amended by the Older Workers Benefit Protection Act (“ADEA”), Title VII of the Civil Rights Act
of 1964 as amended by the Civil Rights Act of 1991 (“Title VII”), the Equal Pay Act of 1963, and the Americans
with Disabilities Act of 1990 (“ADA”), the Rehabilitation Act, the Family and Medical Leave Act, the Sarbanes-Oxley
Act, the Employee Polygraph Protection Act, the Uniformed Services Employment and Reemployment Rights Act of 1994 (“USERRA”),
the Lilly Ledbetter Fair Pay Act or any other foreign, federal, state or local law or judicial decision); (f) claims arising under
the Employee Retirement Income Security Act; and (g) any other statutory or common law claims related to Executive’s employment
with the Company Group or the separation of Executive’s employment with the Company Group.
Without limiting the
foregoing paragraph, Executive represents that Executive understands that this Release specifically releases and waives any claims
of age discrimination, known or unknown, that Executive may have against the Company as of the date Executive signs this Release.
This Release specifically includes a waiver of rights and claims under the Age Discrimination in Employment Act of 1967, as amended,
and the Older Workers Benefit Protection Act. Executive acknowledges that as of the date Executive signs this Release, Executive
may have certain rights or claims under the Age Discrimination in Employment Act, 29 U.S.C. §626 and Executive voluntarily
relinquishes any such rights or claims by signing this Release.
Notwithstanding the
foregoing provisions of this Section 4, nothing herein will release the Company Group from (i) any obligation under the Separation
Agreement; and (ii) any rights or claims that relate to events or circumstances that occur after the date that Executive executes
this Release. In addition, nothing in this Release is intended to interfere with Executive’s right to file a charge with
the Equal Employment Opportunity Commission or any state or local human rights commission in connection with any claim Executive
believes he may have against the Releasees. However, by executing this Release, Executive hereby waives the right to recover any
remuneration, damages, compensation or relief of any type whatsoever from the Company, its affiliates and their respective predecessors
and successors in any proceeding that Executive may bring before the Equal Employment Opportunity Commission or any similar state
commission or in any proceeding brought by the Equal Employment Opportunity Commission or any similar state commission on Executive’s
behalf.
5. Return of
Property. Executive warrants and represents that, as of the Separation Date, Executive has surrendered to the Company all documents,
materials, and other property of the Company and/or its clients and has not photocopied or reproduced such documents. Executive
further warrants and represents that, as of the Separation Date, Executive has returned to the Company any and all Company computer
equipment and software, and any and all other equipment of the Company in Executive’s possession in good working order and
reasonable condition, including any keys; provide Executive shall be entitled to retain his laptop computer and company phone.
6. Representation
of No Pending Action and Agreement Not to Sue. Executive further agrees never to sue any Releasees or cause any Releasees to
be sued regarding any matter within the scope of the above release. If Executive violates this Release by suing any Releasees or
causing any Releasees to be sued, Executive shall continue to be bound by the release obligations of this Release and shall pay
all costs and expenses of defending against the suit incurred by the Releasees, including reasonable attorneys’ fees, unless
(1) paying such costs and expenses is prohibited by law, (2) the action is brought by Executive to enforce the terms of this Agreement,
or (3) Executive is bringing an action relating to the validity of this Agreement.
7. Acknowledgment.
Executive has read this Release, understands it, and voluntarily accepts its terms, and Executive acknowledges that he has been
advised by the Company to seek the advice of legal counsel (at Executive’s cost) before entering into this Release. Executive
acknowledges that he was given a period of at least 21 calendar days within which to consider and execute this Release, and to
the extent that he executes this Release before the expiration of the 21-day period, he does so knowingly and voluntarily and only
after consulting his attorney. Executive acknowledges and agrees that the promises made by the Company Group hereunder represent
substantial value over and above that to which Executive would otherwise be entitled.
8. Revocation.
Executive has a period of 7 calendar days following the execution of this Release during which Executive may revoke this Release
by delivering written notice to the Company pursuant to Section 8(g) of the Separation Agreement by hand or overnight courier before
5:00 p.m. on the seventh day after signing this Release. This Release will not become effective or enforceable until such revocation
period has expired. Executive understands that if he revokes this Release, it will be null and void in its entirety, and he will
not be entitled to any payments or benefits provided in this Release, including without limitation under Section 2 of this Release.
9. Counterparts.
This Release may be executed by the parties hereto in counterparts, which taken together shall be deemed one original.
|
Derma Sciences,
Inc.
By: Stephen T. Wills
Its: Chairman of the Board
|
EXECUTIVE
Barry J. Wolfenson |