UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934, or | ||
For the fiscal year ended December 31, 2019
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
Commission File No. 1-11083
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
(Address of Principal Executive Offices) (Zip Code)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol | Name of each exchange on which registered | ||
Securities registered pursuant to Section 12(g) of the Act:
NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes : ☑ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes: ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes : ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorted period that the registrant was required to submit such files). Yes : ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
☑ | Accelerated filer | ☐ | |
Non-accelerated filer | ☐ | Smaller reporting company | |
Emerging growth company | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes: ☐ No ☑
The aggregate market value of the registrant’s common stock held by non-affiliates was approximately $53.1 billion based on the last reported sale price of $42.98 of the registrant’s common stock on the New York Stock Exchange on June 28, 2019, the last business day of the registrant’s most recently completed second fiscal quarter. (For this computation, the registrant has excluded the market value of all shares of common stock of the registrant reported as beneficially owned by executive officers, and directors of the registrant; such exclusion shall not be deemed to constitute an admission that any such person is an affiliate of the registrant.)
The number of shares outstanding of Common Stock, $0.01 par value per share, as of January 31, 2020 was 1,396,195,349 .
Documents Incorporated by Reference
Portions of the registrant’s definitive proxy statement to be filed within 120 days of December 31, 2019 with the Securities and Exchange Commission in connection with its 2020 Annual Meeting of Stockholders are incorporated by reference into Part III of this Form 10-K.
TABLE OF CONTENTS
2
PART I
ITEM 1. BUSINESS
Our Company
Boston Scientific Corporation is a global developer, manufacturer and marketer of medical devices that are used in a broad range of interventional medical specialties. Our mission is to transform lives through innovative medical solutions that improve the health of patients around the world. As a medical technology leader for nearly 40 years, we advance science for life by providing a broad range of high performance solutions to address unmet patient needs and reduce the cost of healthcare. When used in this report, the terms "we," "us," "our" and "the Company" mean Boston Scientific Corporation and its divisions and subsidiaries.
Our history began in the late 1960s when our co-founder, John Abele, acquired an equity interest in Medi-tech, Inc., a research and development company focused on developing alternatives to surgery. In 1969, Medi-tech introduced a family of steerable catheters used in some of the world's first less-invasive procedures. In 1979, John Abele joined with Pete Nicholas to form Boston Scientific Corporation, which indirectly acquired Medi-tech. This acquisition began a period of active and focused new product development, innovation, market development and organizational growth. Since then, we have advanced the practice of less-invasive medicine by helping physicians and other medical professionals diagnose and treat a wide range of diseases and medical conditions and improve patients’ quality of life by providing alternatives to surgery and other medical procedures that are typically traumatic to the body.
Our net sales have increased substantially since our formation. Our growth has been fueled in part by strategic acquisitions designed to improve our ability to take advantage of growth opportunities in the medical device industry and to build depth of portfolio within our core businesses. These strategic acquisitions have helped us to add promising new technologies to our pipeline and to offer one of the broadest product portfolios in the world for use in less-invasive procedures in our core areas of Medical Surgical (MedSurg), Rhythm and Neuro, and Cardiovascular. We believe that the depth and breadth of our product portfolio has also enabled us to compete more effectively in the current healthcare environment that seeks to improve outcomes and lower costs. Our strategy of category leadership also enables us to compete in a changing healthcare landscape and position our products with providers and payers, while also expanding internationally and managing the complexities of the global healthcare market.
Business Strategy
We operate pursuant to five strategic imperatives: Strengthen Category Leadership, Expand into High Growth Adjacencies, Drive Global Expansion, Fund the Journey to Fuel Growth and Develop Key Capabilities. We believe that our execution of these strategic imperatives will drive innovation, accelerate profitable revenue growth and increase stockholder value while strengthening our leadership position in the medical device industry.
We expect to continue to invest in our core franchises and pursue opportunities to diversify and further expand our presence in strategic growth adjacencies and new global markets. Our approach to innovation combines internally-developed products and technologies with those we may obtain externally through strategic acquisitions, alliances and other investments. Our research and development efforts are focused largely on the development of next-generation and novel technology offerings across multiple programs and all divisions. In the past several years, we have completed numerous acquisitions in support of our growth strategy, both strengthening our core franchises and expanding into high growth adjacent markets.
Our Enterprise Risk Management program analyzes the key risks inherent to achieving our strategic and organizational imperatives. Such risk assessment helps us to anticipate and adapt to potential challenges to preserve and grow stockholder value. Our Board of Directors oversees our risk management program and focuses on monitoring, and together with management, mitigating the most significant risks facing the Company, including strategic, operational, financial, legal and compliance risks.
Products
In 2019, our products were offered for sale by seven core businesses, with 26 percent of our revenue generated by our Interventional Cardiology business, 18 percent by our Cardiac Rhythm Management business, 18 percent by our Endoscopy business, 13 percent by our Urology and Pelvic Health business, 13 percent by our Peripheral Interventions business, eight percent by our Neuromodulation business, and three percent by our Electrophysiology business, with the remaining one percent generated by our recently acquired Specialty Pharmaceuticals business. Our seven core businesses are organized into three reportable segments: MedSurg, Rhythm and Neuro, and Cardiovascular. Following our acquisition of BTG plc (BTG), which closed during the third quarter of 2019, we have included BTG’s Interventional Medicine business within our Peripheral Interventions operating segment, within the Cardiovascular reportable segment. We have presented our full year 2019 results to include BTG's Interventional
3
Medicine business within our Peripheral Interventions operating segment, following the close of the BTG acquisition. We present BTG’s Specialty Pharmaceuticals business as a standalone operating segment alongside our reportable segments. The following describes our principal product offerings by reportable segment, as well as our Specialty Pharmaceutical standalone operating segment.
MedSurg
Endoscopy
Gastroenterology and Pulmonary
Our Endoscopy business develops and manufactures devices to diagnose and treat a broad range of gastrointestinal (GI) and pulmonary conditions with innovative, less invasive technologies. Our product offerings include the following:
• | our SpyGlass™ DS II Direct Visualization System, which brings digital imaging, a wider field of view and a simpler set-up (compared to our legacy SpyGlass System), thus enabling cholangioscopy to play a greater role in the diagnosis and treatment of pancreatico-biliary diseases, |
• | our Resolution 360™ Clip, a hemostatic clipping technology designed to stop and help prevent bleeding during endoscopic procedures, |
• | our Epic™ Biliary Endoscopic Stent System, indicated for the palliation of malignant strictures, is our first laser cut self-expanding metal stent and complements our braided metal stent portfolio, |
• | our Acquire™ Endoscopic Ultrasound Fine Needle Biopsy Device, which is designed to obtain larger tissue specimens for histological assessment and is useful when diagnosing diseases such as pancreatic cancer, liver cancer and stomach lesions, |
• | our AXIOS™ Stent and Electrocautery Enhanced Delivery System, the first, and currently only, stent in the U.S. indicated for endoscopic drainage of pancreatic pseudocysts, |
• | our infection prevention portfolio, which includes a customizable Compliance EndoKit™ and single-use Orca™ Valves, designed to minimize the risk of infection transmission and improve operational efficiencies by streamlining manual cleaning or eliminating the need for cleaning and tracking, and |
• | our endoluminal surgery portfolio with ORISE™ Tissue Retractor System, designed to enable tissue retraction and countertraction during en bloc colonic tissue resection procedures and ORISE™ Gel, designed to be used for submucosal lift of polyps, adenomas, early-stage cancers or other gastrointestinal mucosal lesions prior to excision with a snare or other endoscopic device. |
In the fourth quarter of 2019, we received U.S. Food and Drug Administration (FDA) clearance for the EXALT™ Model D Single-Use Duodenoscope for use in endoscopic retrograde cholangiopancreatography (ERCP) procedures. The EXALT Model D Duodenoscope is the first and only FDA-cleared single-use (disposable) duodenoscope on the market and was granted Breakthrough Device Designation from the FDA to ensure patients and healthcare providers have timely access to this device.
Urology and Pelvic Health
Our Urology and Pelvic Health business develops and manufactures devices to treat various urological and pelvic conditions for both male and female anatomies, including kidney stones, benign prostatic hyperplasia (BPH), prostate cancer, erectile dysfunction, incontinence, pelvic floor disorders, abnormal uterine bleeding and uterine fibroids and polyps. Our product offerings include the following:
• | our comprehensive line of stone management products, including ureteral stents, catheters, baskets, guidewires, sheaths and balloons and stone laser devices, |
• | our LithoVue™ Single-Use Digital Flexible Ureteroscope, which delivers detailed high-resolution digital images for high-quality visualization and seamless navigation, |
• | our penile implants to treat erectile dysfunction and urinary control systems to treat male urinary incontinence, under our Prosthetic Urology portfolio, |
• | our GreenLight XPS™ Laser System, our MoXy™ Fiber and Rezūm™ System, purchased as part of the NxThera, Inc. (NxThera) acquisition in the second quarter of 2018, under our BPH therapies, |
• | our SpaceOAR™ Hydrogel System, purchased as part of the Augmenix, Inc. (Augmenix) acquisition in the fourth quarter of 2018, to help reduce side effects that men may experience after receiving radiotherapy to treat prostate cancer, and |
• | our range of devices for the treatment of Women's Health conditions such as stress urinary incontinence, heavy menstrual bleeding (menorrhagia) and uterine fibroids and polyps. |
4
Rhythm and Neuro
Cardiac Rhythm Management
Our Cardiac Rhythm Management (CRM) business develops and manufactures a variety of implantable devices that monitor the heart and deliver electricity to treat cardiac abnormalities. Our product offerings include the following:
• | our implantable cardioverter defibrillators (ICD) and implantable cardiac resynchronization therapy defibrillators (CRT-D) as well as the world's first, and currently only, commercially available subcutaneous implantable cardiac defibrillators (S-ICD), |
• | our pacemakers and implantable cardiac resynchronization therapy pacemakers (CRT-P), and |
• | our LATITUDE™ Remote Patient Management System, which allows for more frequent monitoring and better guided treatment decisions by enabling physicians in most geographies to monitor implantable system performance remotely. |
Our entire transvenous defibrillator portfolio leverages our EnduraLife™ Battery Technology, including our extended longevity (EL) ICD, our CRT-D’s and our MINI (smallest and thinnest) ICD.
Our most current generation of defibrillators, the RESONATE™ family of devices, is available in most major markets around the world. These devices include our proprietary HeartLogic™ Heart Failure (HF) Diagnostic, EnduraLife Battery Technology and SmartCRT™ with Multisite pacing in CRT-D. We have magnetic resonance imaging (MRI) conditional labeling across our defibrillator portfolio in most major markets around the world when used with our current generation of leads, including our current generation devices as well as our prior generation of DYNAGEN™ and INOGEN™ devices. Our implantable defibrillator portfolio is complemented by our suite of ACUITY™ X4 Quadripolar LV Leads, RELIANCE™ family of ICD Leads and INGEVITY™ Pacing Lead.
In addition to our transvenous defibrillator portfolio, we offer our EMBLEM™ MRI S-ICD System, which provides physicians the ability to treat patients who are at risk for sudden cardiac arrest without touching the heart or invading the vasculature. Our EMBLEM S-ICD devices have MRI conditional labeling and LATITUDE Remote Patient Management in most major markets.
We market our ACCOLADE™ family of pacemaker systems in nearly all major markets around the world. Approval of our ACCOLADE Pacemaker family in the U.S., Europe and Japan also included approval for use of these products in patients undergoing MRI scans. Much like our defibrillator portfolio, our pacemakers leverage our INGEVITY Pacing Leads and LATITUDE™ Remote Patient Management in nearly all major markets.
Electrophysiology
Our Electrophysiology business develops and manufactures less-invasive medical technologies used in the diagnosis and treatment of rate and rhythm disorders of the heart, including a broad portfolio of therapeutic and diagnostic catheters and a variety of equipment used in the Electrophysiology lab. Our product offerings include the following:
• | our Rhythmia™ Mapping System, a next-generation, catheter-based, 3-D cardiac mapping and navigation solution designed to help diagnose and guide treatment of a variety of arrhythmias, |
• | our Blazer™ Therapeutic Ablation Catheter line, |
• | our broad portfolio of diagnostic catheters including Blazer™ Dx-20, Dynamic Tip™ and Viking™ Catheters, |
• | our IntellaMap OrionTM Mapping Catheter, for use with our Rhythmia Mapping System to provide high-density, high-resolution maps of the heart, |
• | our intracardiac ultrasound catheters, delivery sheaths and other accessories, and |
• | our full offering of capital equipment used in Electrophysiology labs, such as recording systems, generators and pumps. |
Our cooled ablation catheter portfolio includes our U.S. and CE Mark approved Blazer™ Open-Irrigated, IntellaNav™ Open-Irrigated, and IntellaNav MiFi™ Open-Irrigated ablation catheters with a unique Total Tip Cooling™ Design. We also offer our IntellaNav XP and IntellaNav MiFi XP solid tip catheters. Our IntellaTip™ MiFi XP, IntellaNav MiFi XP and IntellaNav MiFi Open-Irrigated Catheters include MicroFidelity (MiFi) sensor technology in the catheter tip. Additionally, the European and Japan markets have access to our DIRECTSENSE™ Software which leverages our proprietary MiFi electrode catheter design to capture and present local impedance. DIRECTSENSE Software provides meaningful information on tissue to catheter tip proximity, catheter stability, and other local tissue characteristics.
5
All of our IntellaNav Catheters are designed to allow magnetic tracking when used with our Rhythmia Mapping System. We also recently received CE Mark and U.S. IDE approval for our POLARx™ Cryoablation Single-shot Pulmonary Vein Isolation Technology, purchased as part of our acquisition of Cryterion in the third quarter of 2018.
Our capital equipment offerings include our Rhythmia Mapping System, LabSystem™ PRO Recording System, Maestro™ RF Generators and the MetriQ™ Pump.
Neuromodulation
Our Neuromodulation business develops and manufactures devices to treat various neurological movement disorders and manage chronic pain. Our product offerings include the following:
• | our Precision™, Precision Spectra™, Precision Montage™, Precision Novi™ and Spectra WaveWriter™ Spinal Cord Stimulator (SCS) Systems, designed to provide improved pain relief to a wide range of patients who suffer from chronic pain, |
• | our Superion™ Indirect Decompression System, a minimally-invasive device used to improve physical function and reduce pain in patients with lumbar spinal stenosis (LSS) purchased as part of the acquisition of Vertiflex, Inc. in the second quarter of 2019, and |
• | our Vercise™, Vercise™ PC and Vercise Gevia™ Deep Brain Stimulation (DBS) Systems for the treatment of Parkinson's disease, tremor, and intractable primary and secondary dystonia, a neurological movement disorder characterized by involuntary muscle contractions. |
In January 2018, we announced FDA approval for the Spectra WaveWriter™ SCS System, the first and only system approved by the FDA to simultaneously provide paresthesia-based and sub-perception therapy. The Precision Spectra SCS System is the world's first and only SCS system with 32 contacts and 32 dedicated power sources. We believe that we continue to have a technological advantage due to our proprietary features such as Multiple Independent Current Control and our Illumina 3D™ Proprietary Programming Software, which together are intended to allow the physician to target specific areas of pain and customize stimulation of nerve fibers more precisely.
In 2018, we began commercializing our Vercise™ DBS System in the U.S. following FDA approval in late 2017. The Vercise DBS System is approved in the U.S. as an adjunctive therapy that aids in reducing some of the symptoms of moderate to advanced Parkinson’s disease. We also have regulatory approval for our Vercise DBS System in various international regions including Europe, Latin America and Asia Pacific. Our Vercise Gevia™ DBS System with the Cartesia™ Directional Lead is the first and only MRI conditional, rechargeable and directional system, using multi-directional stimulation designed for greater precision, intended to minimize side effects for patients. The Cartesia Directional Lead continues to expand our market access in Europe, Japan and various countries in Latin America. In the third quarter of 2018, we received CE mark approval in Europe for GUIDE™ XT System, the first DBS visualization system built for directionality that utilizes patient specific anatomy and stimulation field modeling. This technology provides physicians with 3-D image planning capability and when used in conjunction with the Vercise DBS Systems, enables physicians to personalize and optimize DBS treatment. In January 2019, the Vercise Gevia DBS System with the Cartesia Directional Lead was approved by the FDA and in August 2019 received ImageReady™ MRI labeling to be used in a full-body magnetic resonance imaging environment.
Cardiovascular
Interventional Cardiology
Our Interventional Cardiology business develops, manufactures and commercializes technologies for diagnosing and treating coronary artery disease and other cardiovascular disorders including structural heart conditions. Our broad, innovative product offerings have led to our leadership in the global interventional cardiology market.
6
Drug-Eluting Coronary Stent Systems
Our drug-eluting coronary stent product offerings are an important element of our global Interventional Cardiology market leadership. We believe we have enhanced the outcomes associated with the use of coronary stents, particularly the processes that lead to restenosis (the growth of neointimal tissue within an artery after angioplasty and stenting), through our scientific research and product development of drug-eluting stent systems. Our coronary stent offerings include the following:
• | our SYNERGY™ Everolimus-Eluting Platinum Chromium Coronary Stent System, featuring an ultra-thin abluminal (outer) bioabsorbable polymer coating, |
• | our Promus ELITE™ Everolimus-Eluting Stent, and |
• | our Promus PREMIER™ Everolimus-Eluting family of stents. |
Complex PCI Product Offerings
Our product offerings to perform complex percutaneous coronary interventions (PCI) include a broad line of products used to treat patients with atherosclerosis, a principal cause of coronary artery obstructive disease. These include balloon catheters, rotational atherectomy systems, guide wires, guide catheters, embolic protection devices, crossing and re-entry devices for the treatment of chronically occluded coronary vessels and diagnostic catheters used in percutaneous transluminal coronary angioplasty (PTCA) procedures.
PCI Guidance
Our PCI Guidance offerings include a family of intravascular catheter-directed ultrasound imaging catheters, complemented by our intravascular ultrasound (IVUS) imaging system and our fractional flow reserve (FFR) devices and systems for use in coronary arteries and heart chambers as well as certain peripheral vessels to assist in the diagnosis of coronary artery disease. Our PCI Guidance product offerings include the following:
• | our OptiCross™ IVUS Imaging catheter, |
• | our COMET™ FFR Pressure Guidewire, and |
• | our iLab™ Ultrasound Imaging System with Polaris Software, designed to enhance the diagnosis and treatment of blocked vessels and other heart disorders, which is compatible with our full line of imaging catheters and coronary physiology devices and continues to be our flagship console. |
The iLab Ultrasound Imaging System has been placed in cardiology labs worldwide and provides an installed base through which we expect to continue to sell associated single-use products.
Structural Heart Therapies
Structural heart therapies are one of the fastest growing areas of the medical technology market and are highly synergistic with our Interventional Cardiology and Rhythm Management businesses. Our current structural heart product offerings include the following:
• | our WATCHMAN™ Left Atrial Appendage Closure (LAAC) Technology (WATCHMAN), and WATCHMAN FLX, designed to close the left atrial appendage in patients with non-valvular atrial fibrillation who are at risk for ischemic stroke, |
• | our ACURATE TA™, ACURATE neo™, and ACURATE TF™ Aortic Valve Systems, which are based on a self-expanding architecture, |
• | our LOTUS Edge™ Aortic Valve System, which is based on mechanical-expanding architecture, and |
• | our Sentinel™ Cerebral Embolic Protection System, purchased as part of our acquisition of Claret Medical, Inc. (Claret) in the third quarter of 2018. |
WATCHMAN™ Left Atrial Appendage Closure (LAAC) Device is the first device to offer a non-pharmacologic alternative to oral anti-coagulants that has been studied in a randomized clinical trial and is marketed globally. The WATCHMAN Device has been commercially available internationally since 2009, received FDA approval in 2015 and is the leading device in percutaneous LAAC globally. In the first quarter of 2019, we received CE Mark and initiated a limited market release of the next generation WATCHMAN FLX™ LAAC Device in Europe. We believe that the WATCHMAN device will be the only LAAC technology commercially available in the U.S. throughout 2020.
7
Our Transcatheter Aortic Valve Replacement (TAVR) portfolio is comprised of our dual valve offering including the LOTUS Edge™ Valve with mechanical-expanding architecture which is well suited for intra-annular cases and was launched commercially in the U.S. and Europe in the first half of 2019 and the ACURATE neo Valve based on self-expanding architecture for supra-annular cases. In addition to our dual valve offering, our TAVR portfolio includes the Sentinel Cerebral Embolic Protection System which is used to reduce the risk of stroke in TAVR procedures.
Peripheral Interventions
Our Peripheral Interventions business develops and manufactures products to diagnose and treat peripheral vascular diseases, including a broad line of medical devices used in percutaneous transluminal angioplasty (PTA), as well as products to diagnose, treat and ease various forms of cancer. Following the completion of the acquisition of BTG during the third quarter of 2019, we began to integrate BTG's Interventional Medicine (IM) portfolio into the Peripheral Interventions division, adding complementary technologies in the areas of venous disease and interventional oncology. Our combined broad peripheral portfolio includes products to treat arterial diseases (stents, balloon catheters, wires and atherectomy) and venous diseases (thrombectomy, acoustic pulse thrombolysis, wires and stents) and for use in interventional oncology techniques to treat various cancers (peripheral embolization devices, radioactive microspheres, radiofrequency and cryotherapy ablation systems, microcatheters and drainage catheters).
Our peripheral angioplasty balloon technologies include the following:
• | our Mustang™ PTA next-generation Balloon Catheter, a 0.035" balloon with superior crossing and tracking, powerful dilatation, longer lengths and smaller sheath sizes, |
• | our Coyote™ Balloon Catheter, a highly deliverable and ultra-low profile balloon dilatation catheter designed for a wide range of peripheral angioplasty procedures, |
• | our Sterling™ Balloon Catheter, a 0.018" PTA balloon catheter designed for post-stent dilatation as well as conventional balloon angioplasty to open blocked peripheral arteries, and |
• | our Ranger™ Drug-Coated Balloon, an innovative balloon built on the Sterling balloon platform, featuring a low-dose of paclitaxel. |
Our peripheral stent technologies include the following:
• | our EPIC™ Vascular Self-Expanding Stent System, a nitinol stent designed to sustain vessel patency while providing enhanced visibility and accuracy during placement, |
• | our Innova™ Self-Expanding Stent System, a laser-cut nitinol stent built for the superficial femoral artery (SFA, a large artery in the thigh) with flexibility, strength and fracture resistance, and |
• | our Eluvia™ Drug Eluting Vascular Stent System, an innovative stent built on the Innova stent platform, designed to deliver a sustained dosage of paclitaxel during the time when restenosis is most likely to occur. |
Our venous disease technologies include the following:
• | our AngioJet™ Thrombectomy System, used in endovascular procedures to remove blood clots from blocked arteries and veins, |
• | our AngioJet Zelante DVT™ Thrombectomy Catheter to treat deep vein thrombosis (DVT) in large-diameter upper and lower limb peripheral veins, in the U.S. and Europe, |
• | our VICI VENOUS STENT™ System to treat venous obstructive disease, purchased as part of the VENITI, Inc. acquisition in the third quarter of 2018, and |
• | our EKOS™ Ultrasound Assisted Thrombolysis system used to treat deep vein thrombosis and pulmonary embolism, purchased as part of the BTG acquisition, which closed during the third quarter of 2019. |
Our interventional oncology product offerings include the following:
• | our Therasphere™ Y-90 radioactive glass microspheres used in the treatment of hepatocellular carcinoma (HCC or the most common type of liver cancer) purchased as part of the BTG acquisition, |
• | our Direxion™ Torqueable Microcatheter, and |
• | our line of interventional oncology solutions, including the Renegade™ HI-FLO™ Fathom™ Microcatheter and Guidewire System and Interlock™ - 35 Fibered IDC™ and 18 Fibered IDC™ Occlusion System for peripheral embolization. |
8
Specialty Pharmaceuticals
Following the closing of the BTG acquisition in the third quarter of 2019, Specialty Pharmaceuticals was added as an eighth operating segment. Our Specialty Pharmaceuticals business develops and manufactures acute care antidotes to treat overexposure to certain medications and toxins. These products are sold primarily in the U.S. through small, specialist sales teams and through commercial partners elsewhere, where approved or permitted, on a named patient basis. Our Specialty Pharmaceuticals product offerings include the following:
• | our CroFab®, the only FDA-approved product derived exclusively from U.S. snakes and approved to treat all North American pit viper envenomations in adult and pediatric patients, |
• | our DigiFab® Digoxin Immune Fab (Ovine), a treatment for patients with life-threatening or potentially life-threatening digoxin toxicity or overdose that is clinically proven to effectively clear digoxin from the body, and |
• | our Voraxaze®, a carboxypeptidase indicated to reduce toxic plasma methotrexate concentration (greater than one micromole per liter) in adult and pediatric patients with delayed methotrexate clearance (plasma methotrexate concentrations greater than two standard deviations of the mean methotrexate excretion curve specific for the dose of methotrexate administered) due to impaired renal function. |
Research and Development
Our investment in research and development is critical to driving our future growth. Our investment in research and development supports the following:
• | internal research and development programs, regulatory design and clinical science, as well as other programs obtained through our strategic acquisitions and alliances, and |
• | engineering efforts that incorporate customer feedback into continuous improvement efforts for currently marketed and next-generation products. |
We have directed our development efforts toward innovative technologies designed to expand current markets or enter adjacent markets. We are transforming how we conduct research and development by identifying best practices, driving efficiencies and optimizing our cost structure, which we believe will enable increased development activity and faster concept-to-market timelines.
Focused, cross-functional teams take a formal approach to new product design and development, helping us to manufacture and offer innovative products consistently and efficiently. Involving cross-functional teams early in the process is the cornerstone of our product development cycle. We believe this collaboration allows our teams to concentrate resources on the most viable and clinically relevant new products and technologies and maximize cost and time savings as we bring them to market.
In addition to internal development, we work with hundreds of leading research institutions, universities and clinicians around the world to develop, evaluate and clinically test our products. We are expanding our collaborations to include research and development teams in emerging markets; these teams will focus on both global and local market requirements at a lower cost of development. We believe that these efforts will play a significant role in our future success.
9
Marketing and Sales
In 2019, we marketed our products and solutions to approximately 37,000 hospitals, clinics, outpatient facilities and medical offices in more than 120 countries worldwide, including the U.S. The majority of our net sales are derived from countries in which we have direct sales organizations. We also have a network of distributors and dealers who offer our products in certain countries and markets. We expect to continue to leverage our infrastructure in markets where commercially appropriate and use third party distributors in those markets where it is not economical or strategic to establish or maintain a direct presence.
No single institution accounted for more than ten percent of our net sales in 2019, 2018 or 2017; however, large group purchasing organizations, hospital networks and other buying groups have become increasingly important to our business and represent a substantial portion of our net sales. We have a dedicated corporate accounts organization in the U.S. and Europe focused principally on selling to major buying groups and integrated healthcare networks. We consistently strive to understand and exceed the expectations of our customers. Each of our businesses maintains dedicated sales forces and marketing teams focused on physicians who specialize in the diagnosis and treatment of different medical conditions, as well as on key hospital service line administrators. We believe that this dual focus on disease state management and hospital administrators enables us to develop highly knowledgeable and dedicated sales representatives and to foster collaborative relationships with both physicians and key service line administrators. We believe that our strong working relationships with physicians, service line administrators and others in the medical industry enable us to gain a detailed understanding of new therapeutic and diagnostic alternatives and to respond quickly to our customers' changing needs.
International Operations
International net sales accounted for 42 percent of our net sales in 2019, 44 percent of our net sales in 2018 and 43 percent of our net sales in 2017. Maintaining and expanding our international presence is an important component of our long-term growth strategy. Through our international presence, we seek to increase net sales and market share, leverage our relationships with leading physicians and their clinical research programs, accelerate the time to bring new products to market and gain access to worldwide technological developments that we can implement across our product lines. In addition, we continue to invest in infrastructure in emerging markets to strengthen our sales and service capabilities and maximize our opportunities in these countries.
As of December 31, 2019, we had 9 principal international manufacturing facilities, in addition to our U.S. facilities, including three in Ireland, two in Costa Rica, one in Brazil, one in Malaysia, one in Puerto Rico and one in Switzerland. Approximately 52 percent of our products manufactured in 2019 were produced at these international facilities. We also maintain our primary research and development capabilities in China, Costa Rica, India, Ireland, Puerto Rico and the UK. Medical education is also a vital component of safe procedure adoption and collaboration with physicians. We continue to provide localized training programs through our 14 Institutes for Advancing Science in the Americas, Africa, Asia and Europe.
Manufacturing and Raw Materials
We are focused on continuously improving our supply chain effectiveness, strengthening our manufacturing processes and increasing operational efficiencies within our organization. We strive to improve the efficiency of our sourcing operations and to leverage the technical expertise of the broader market by partnering with strategic suppliers. In doing so, we seek to focus our internal resources on the development and commercial launch of new products and the enhancement of existing products. We continue to implement new systems designed to provide improved quality, reliability, service, greater efficiency and lower supply chain costs. We also drive continuous improvement in product quality through process controls and validations, supplier and distribution controls and then necessary training and tools for our operations team. In addition, we remain focused on examining our operations and general business activities to enhance our operational effectiveness by identifying cost-improvement opportunities.
We remain committed to maintaining appropriate investments in supply chain resiliency on an ongoing basis. Our products are designed and manufactured in technology centers around the world, either by us or third parties. We consistently monitor our inventory levels, manufacturing and distribution capabilities and maintain recovery plans to address potential disruptions that we may encounter. Supply chain resiliency also includes sterilization, which is performed and optimized through a combination of internal and third party locations and may also be subject to potential interruptions. Many components used in the manufacturing of our products are readily fabricated from commonly available raw materials or off-the-shelf items available from multiple supply sources; however, certain items are custom made to meet our specifications. We believe that in most cases, redundant capacity exists at our suppliers and that alternative sources of supply are available or could be developed within a reasonable period of time. We also have an ongoing program to identify single-source components and to develop alternative back-up supplies, and we regularly readdress the adequacy and abilities of our suppliers and sterilizers to meet our needs.
10
Quality Assurance
We are committed to providing high quality products to our customers. Our quality system starts with the initial product specification and continues through the design of the product, component specification process and the manufacturing, sale and servicing of the product. Our quality system is intended to build in quality and process control and to utilize continuous improvement concepts throughout the product life. These systems are designed to enable us to satisfy various international quality system regulations, including those of the FDA with respect to products sold in the U.S. All of our medical device manufacturing facilities and distribution centers are certified under the ISO 13485 quality system standard, established by the International Standards Organization (ISO) for medical devices, which includes requirements for an implemented quality system that applies to component quality, supplier control, product design and manufacturing operations. This certification can be obtained only after a complete audit of a company’s quality system by an independent outside auditor. Maintenance of the certification requires that these facilities undergo periodic re-examination.
Environmental Regulation and Management
We are subject to various environmental laws, directives and regulations both in the U.S. and abroad. Our operations involve the use of substances regulated under environmental laws, primarily in manufacturing and sterilization processes. We believe that strong performance across relevant environmental, health and safety metrics enhances our competitive strength while benefiting our patients, customers, stockholders and employees. We are focused on continuous improvement in these areas by reducing pollution, depletion of natural resources and our overall environmental footprint. Specifically, we are working to optimize energy and resource usage, ultimately reducing greenhouse gas emissions and waste. We are listed on the FTSE4Good Corporate Social Responsibility Index, managed by the Financial Times and the London Stock Exchange, which measures the performance of companies that meet globally recognized standards of corporate responsibility. This listing recognizes our dedication to those standards and it places us in a select group of companies with a demonstrated commitment to responsible business practices and sound environmental policies. We also recognize the need to minimize the impact of the manufacturing of our products on the environment and have committed to carbon neutrality in our manufacturing and key distribution sites by 2030.
We have obtained ISO 14001:2015 certifications at our major manufacturing plants and Tier 1 distribution centers around the world, as well as our Corporate headquarters in Marlborough, Massachusetts. ISO 14001:2015 is a globally recognized standard for Environmental Management Systems, established by the International Standards Organization, which provides a voluntary framework to identify key environmental aspects associated with our business. Using this environmental management system and the specific attributes of our certified locations in the U.S., Ireland, Costa Rica and the Netherlands, we continue to improve our environmental performance and reduce our environmental footprint.
Competition
We encounter significant competition across our product lines and in each market in which we sell our products and solutions, some from companies that may have greater financial, sales and marketing resources than we do. Our primary competitors include Abbott Laboratories and Medtronic plc, as well as a wide range of medical device companies that sell a single or limited number of competitive products or participate in only a specific market segment. In certain countries, we face competition from domestic medical device companies that may benefit from their status as local suppliers. We also face competition from non-medical device companies, which may offer alternative therapies for disease states that could also be treated using our products, or from companies offering technologies that could augment or replace procedures using our products.
We believe that our products and solutions compete primarily on their ability to deliver both clinical and economic outcomes for our customers by enabling physicians to perform diagnostic and therapeutic procedures safely and effectively often in a less-invasive manner. We also compete on ease of use, comparative effectiveness, reliability and physician familiarity. In the current environment of managed care, with economically-motivated buyers, consolidation among healthcare providers, increasing prevalence and importance of regional and national tenders, increased competition and declining reimbursement rates, we have been increasingly required to compete on the basis of price, value, reliability and efficiency. We believe the current global economic conditions and healthcare reform measures could continue to put additional competitive pressure on us, including on our average selling prices, overall procedure rates and addressable market sizes. We recognize that our continued competitive success will depend upon our ability to:
• | offer products and solutions that provide differentiated clinical and economic outcomes, |
• | create or acquire innovative, scientifically advanced technologies, |
• | apply our technology and solutions cost-effectively and with superior quality across product lines and markets, |
• | develop or acquire proprietary products and solutions, |
11
• | attract and retain skilled personnel, |
• | obtain patent or other protection for our products, |
• | obtain required regulatory and reimbursement approvals, |
• | compete in regional and national tenders for our products, |
• | continually enhance our quality systems, |
• | manufacture and market our products and solutions either directly or through third parties, and |
• | supply sufficient inventory to meet customer demand. |
Medical Device Regulatory Approvals
The medical devices that we manufacture and market are subject to regulation by numerous worldwide regulatory bodies, including the FDA and comparable international regulatory agencies. These agencies require manufacturers of medical devices to comply with applicable laws and regulations governing development, testing, manufacturing, labeling, marketing and distribution. Medical devices are also generally subject to varying levels of regulatory control based on risk level of the device.
In the U.S., authorization to distribute a new device can generally be met in one of three ways. The first process requires that a premarket notification (510(k)) be made to the FDA to demonstrate that the device is as safe and effective as, or substantially equivalent to, a legally marketed device (the “predicate” device). Applicants must submit performance data to establish substantial equivalence. In some instances, data from human clinical trials must also be submitted in support of a 510(k) premarket notification. If so, these data must be collected in a manner that conforms to the applicable Investigational Device Exemption (IDE) regulations. The FDA must issue a decision finding substantial equivalence before commercial distribution can occur. Changes to cleared devices that could not significantly affect the safety or effectiveness of the device can generally be made without additional 510(k) premarket notifications; otherwise, a new 510(k) is required.
The second process requires the submission of a premarket approval (PMA) application to the FDA to demonstrate that the device is safe and effective for its intended use. This approval process applies to most Class III devices and generally requires clinical data to support the safety and effectiveness of the device, obtained in adherence with IDE requirements. The FDA will approve the PMA application if it finds that there is a reasonable assurance that the device is safe and effective for its intended purpose and that the proposed manufacturing is in compliance with the Quality System Regulation (QSR). For novel technologies, the FDA will generally seek input from an advisory panel of medical experts and seek their views on the safety, effectiveness and benefit-risk of the device. The PMA process is generally more detailed, lengthier and more expensive than the 510(k) process.
The third process requires that an application for a Humanitarian Device Exemption (HDE) be made to the FDA for the use of a Humanitarian Use Device (HUD). A HUD is intended to benefit patients by treating or diagnosing a disease or condition that affects, or is manifested in, not more than 8,000 individuals in the U.S. per year. The application submitted to the FDA for an HDE is similar in both form and content to a PMA application, but is exempt from the effectiveness requirements of a PMA. The HUD provision of the regulation provides an incentive for the development of devices for use in the treatment or diagnosis of diseases affecting smaller patient populations.
In the European Union (EU), we will be required to comply with the new Medical Device Regulation (MDR or EU MDR) effective May 2020 which will supersede the current Medical Device Directives. Medical devices which have a valid CE Certificate to the current Directives (issued before May 2020) can continue to be sold until May 2024 or until the CE Certificate expires, whichever comes first, providing there are no significant changes to the design or intended use. The MDR was published in May 2017 with a 3-year transition period. The CE Mark required to sell medical devices in the EU is affixed following conformity assessment and either approval from the appointed independent Notified Body or through self-certification by the manufacturer. The selected pathway to CE marking is based on product risk classification. CE Marking indicates conformity to the applicable Essential Requirements of the relevant Medical Devices Directive and in the future to the General Safety and Performance Requirements for the new MDR. The MDR will change multiple aspects of the existing regulatory framework for CE marking, such as increased clinical evidence requirements and other new requirements, including Unique Device Identification (UDI) as well as many other post-market obligations. MDR also significantly modifies and increases the compliance requirements for the industry and will require significant investment over the next few years to implement.
We are also required to comply with the regulations of every other country where we commercialize products before we can launch or maintain new products on the market, such as the requirements that we obtain approval from the Japanese Ministry of Health, Labor and Welfare (MHLW), the Japanese Pharmaceutical & Medical Device Agency (PMDA) and the China Food and Drug Administration (NMPA). Many countries that previously did not have medical device regulations, or had minimal regulations, are now introducing them. For example, India is in the process of expanding its current regulations to include all medical device categories while many countries in the Middle East and Southeast Asia are introducing new regulations.
12
The FDA and other worldwide regulatory agencies and competent authorities actively monitor compliance to local laws and regulations through review and inspection of design and manufacturing practices, record-keeping, reporting of adverse events, labeling and promotional practices. The FDA can ban certain medical devices, detain or seize adulterated or misbranded medical devices, order repair, replacement or refund of these devices and require notification of health professionals and others with regard to medical devices that present unreasonable risks of substantial harm to the public health. The FDA may also enjoin and restrain a company for certain violations of the Food, Drug and Cosmetic Act and the Safe Medical Devices Act pertaining to medical devices or initiate action for criminal prosecution of such violations. Regulatory agencies and authorities in the countries where we do business can halt production in or distribution within their respective country or otherwise take action in accordance with local laws and regulations.
International sales of medical devices manufactured in the U.S. that are not approved by the FDA for use in the U.S., or that are banned or deviate from lawful performance standards, are subject to FDA export requirements. Additionally, exported devices are subject to the regulatory requirements of each country to which the device is exported. Some countries do not have medical device regulations, but in most foreign countries, medical devices are regulated. Frequently, regulatory approval may first be obtained in a foreign country prior to application in the U.S. due to differing regulatory requirements; however, other countries, such as China, for example, require approval in the country of origin first. Most countries outside of the U.S. require that product approvals be recertified on a regular basis, generally every five years. The recertification process requires that we evaluate any device changes and any new regulations or standards relevant to the device and, where needed, conduct appropriate testing to document continued compliance. Where recertification applications are required, they must be approved in order to continue selling our products in those countries.
Government Affairs
We maintain a global Government Affairs presence, headquartered in Washington, D.C., to actively monitor and advocate on myriad legislation and policies impacting us, both on a domestic and an international front. The Government Affairs office works closely with members of Congress and committee staff, the White House and Administration offices, state Governors, legislatures and regulatory agencies, embassies and global governments on issues affecting our business. Our proactive approach and depth of political and policy expertise are aimed at having our positions heard by federal, state and global decision-makers to improve patient care and to advance our business objectives by educating policymakers on our positions, key priorities and the value of our technologies. The Government Affairs office manages our political action committee and works closely with trade groups on issues affecting our industry and healthcare in general. The Government Affairs office also advocates for public policy that benefits our employees, and the patients we serve and supports the communities in which we live.
Healthcare Policies and Reimbursement
Political, economic and regulatory influences around the world continue to subject the healthcare industry to potential fundamental changes that could substantially affect our results of operations. Government and private sector initiatives related to limiting the growth of healthcare costs (including price regulation), coverage and payment policies, comparative effectiveness reviews of therapies, technology assessments and healthcare delivery structure reforms, are continuing in many countries where we do business. We believe that these changes are causing the marketplace to put increased emphasis on the delivery of treatments that can reduce costs, improve efficiencies and/or increase patient access. Although we believe our less-invasive products and technologies generate favorable clinical outcomes, value and cost efficiency, the resources necessary to demonstrate value to our customers, patients, payers and other stakeholders are significant and new therapies now take longer periods of time to gain widespread adoption.
The impact to our business of the U.S. Patient Protection and Affordable Care Act's (ACA) provisions related to coverage expansion, payment reforms and delivery system has been immaterial. The ACA and Health Care and Education Affordability Reconciliation Act were enacted into law in the U.S. in 2010. The legislation imposed on medical device manufacturers a 2.3 percent excise tax on U.S. sales of Class I, II and III medical devices beginning in January 2013. In December 2015, the Promise for Antibiotics and Therapeutics for Health Act, or PATH Act, was passed, which included legislation which temporarily suspended the 2.3 percent excise tax until December 31, 2017. In January 2018, another temporary two-year suspension of the 2.3 percent excise tax was passed, extending the suspension to December 31, 2019. On December 20, 2019, the President signed the 2020 spending bill, which included a provision to permanently repeal the excise tax.
The U.S. Federal government, as part of the ACA, and certain state governments have enacted laws aimed at increasing transparency, or "sunshine," in relationships between medical device, biologics and pharmaceutical companies and healthcare professionals (HCPs). As a result, we are required by law to report many types of payments and transfers of value provided to HCPs. Certain foreign jurisdictions have similar laws or are currently acting to implement similar laws. Failure to comply with sunshine laws and/or implement and adhere to adequate policies and practices to address changes to legal and regulatory requirements could
13
have a negative impact on our results of operations. Additional legislation at the state and federal levels may result in further changes to these laws.
As noted below, we expect certain trends to continue placing pressure on pricing and utilization in the U.S. The Tax Cuts and Jobs Acts (TCJA), enacted December 22, 2017 in the U.S., changed the tax treatment of healthcare expenses and repealed the “individual mandate” to purchase private insurance. These tax law changes have resulted in changes to insurance coverage and financing of insurance coverage in individual markets. Additional legislation may result in changes to government programs such as Medicare and Medicaid. In addition, the current U.S. Administration has enacted a number of administrative policy changes that will likely result in additional changes to insurance coverage, financing of insurance coverage and benefits offered through private insurance in both the employer-sponsored and individual markets. These changes and other similar changes being considered are likely to lead to an increase in the number of people without insurance. Other individual coverage policies will be less generous than those required under the ACA. The impact of these changes on coverage levels and patient cost-sharing could affect utilization of non-urgent, non-acute services in which our devices are used.
We expect that pricing of medical devices will remain under pressure as alternative payment reform, such as prospective payment systems for hospital care, preferential site of service payments, value-based purchasing and accountable care organizations (ACOs), continue to take shape globally. We also expect marketplace changes to place pressure on medical device pricing globally as hospitals consolidate and large group purchasing organizations, hospital networks and other groups continue to seek to aggregate purchasing power. Similarly, governments are increasing the use of tenders, placing pressure on medical device pricing. Some governments also seek to limit the growth of healthcare costs through price regulation. Implementation of cost containment initiatives and healthcare reforms in significant markets such as the U.S., Japan and Europe and other markets may limit the price of, or the level at which reimbursement is provided for, our products, which in turn may influence a hospital’s or physician's selection of products used to treat patients.
Our products are purchased principally by hospitals, physicians and other healthcare providers around the world that typically bill various third-party payers, including government programs (e.g., Medicare and Medicaid in the U.S.) and private insurance payers, for the services provided to their patients. Third-party payers and governments may approve or deny coverage for certain technologies and associated procedures based on independently determined assessment criteria. Coverage decisions by payers for these technologies and associated procedures are based on a wide range of methodologies that may reflect the assessed resource costs, clinical outcomes and economic value of the technologies and associated procedures.
Proprietary Rights and Patent Litigation
We rely on a combination of patents, trademarks, trade secrets and other forms of intellectual property to protect our proprietary rights. We generally file patent applications in the U.S. and foreign countries where patent protection for our technology is appropriate and available. As of December 31, 2019, we held more than 21,000 patents and had approximately 6,000 patent applications pending worldwide that cover various aspects of our technology. In addition, we hold exclusive and non-exclusive licenses to a variety of third-party technologies covered by patents and patent applications. In the aggregate, these intellectual property assets and licenses are of material importance to our business; however, we believe that no single patent, technology, trademark, intellectual property asset or license is material in relation to our business as a whole.
We rely on non-disclosure and non-competition agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary technology. There has been substantial litigation regarding patent and other intellectual property rights in the medical device industry, particularly in the areas in which we compete. We continue to defend ourselves against claims and legal actions alleging infringement of the patent rights of others. Additionally, we may find it necessary to initiate litigation to enforce our patent rights, to protect our trade secrets or know-how and to determine the scope and validity of the proprietary rights of others. Accordingly, we may seek to settle some or all of our pending litigation, particularly to manage risk over time. Settlement may include cross licensing of the patents that are the subject of the litigation as well as our other intellectual property and may involve monetary payments to or from third parties.
We maintain insurance policies providing limited coverage against securities claims. We are substantially self-insured with respect to product liability claims and fully self-insured with respect to intellectual property infringement claims. The absence of significant third-party insurance coverage increases our potential exposure to unanticipated claims or adverse decisions. See Note J – Commitments and Contingencies to our 2019 consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for a discussion of intellectual property, product liability and other litigation and proceedings in which we are involved.
14
Employees
As of December 31, 2019, we had approximately 36,000 employees, including approximately 14,000 in operations, 11,000 in selling, marketing and distribution, 7,000 in administration and 4,000 in clinical, regulatory and research and development. Of these employees, we employed approximately 19,000 outside the U.S., approximately 9,000 of whom are in the manufacturing operations function.
Community Outreach
We are united by a goal to make a difference in the lives of the over 30 million patients we serve annually. We seek to give our time and resources to make positive impacts upon those communities where we live, work, and serve. Guided by our core values, we seek to improve access to healthcare, to invest in educational programming for students with limited means and access to opportunities, and to support and embrace the spirit of volunteerism within our global workforce, while adhering to strong ethical standards.
In some parts of the world, access to health information, screening, care and services can be limited. Our collaborations with non-profit community organizations raise awareness of chronic disease and decrease these health disparities by improving health outcomes for underserved populations. We accomplish this through our focus on 3 P’s - Prevent, Provide and Prepare. We work to prevent chronic disease through education and awareness, provide access to healthcare through increasing the quantity and quality of healthcare workers and screenings, and preparing and empowering children at high risk of chronic disease to successfully navigate their health journey. Each year, our employees play a role in how we reach our health goals by participating in health awareness campaigns like Wear Red Day for heart health, No Shave November for men’s health, and in walks and runs for various health causes. For the last three years, Boston Scientific partnered with Project HOPE, a global health and humanitarian relief organization, to fund the United Dialogue and Action Against Non-Communicable Disorders (UDAAN) program to help reduce premature mortality in India. The program focuses on improving the health practices related to chronic disease treatment and care. Through the program, a chronic disease tool kit was developed to improve healthcare worker efficiencies and is now being scaled to other Health and Wellness centers in the area.
We are also passionate about inspiring young learners to see themselves in a Science, Technology, Engineering and Math (STEM) role in the future. Our employees work with underrepresented K-12 students around the world and share their passion for STEM by providing interactive product demos, development programs, and hands-on activities for young learners in their communities. A key effort of our STEM volunteers is to create resources that empower other employees to get involved. While each regional team focuses on what is needed most by students in their local communities, they also work together to share best practices that ensure we reach our overall goals. Resources like presentations, STEM kits, interactive displays, and an online activity database have been created to increase global collaboration and overall impact. In 2019, our employee volunteers participated in more than 160 STEM events and school programs. Through this outreach, we are helping to develop the diverse future talent that will enable Boston Scientific to create innovative health solutions for generations to come. Beyond the classroom, we empower our employees to participate in and influence the way we care for people in their local communities. We are proud of these efforts and the collective impact we have on advancing possibilities across the globe. In 2019 our employees volunteered more than 41,000 hours to make a positive impact at more than 600 global community events in 38 countries.
We also support the U.S. communities where we have significant business presences through the Boston Scientific Foundation. The mission of the foundation is simple: to help expand access to quality healthcare and educational opportunities for underserved populations. The Boston Scientific Foundation awarded approximately $1 million dollars in scholarships to children of employees and grant awards across the U.S. in 2019. The process involved more than 70 employee volunteers who evaluated proposals for the Boston Scientific Foundation Board review and approval, upon which the Boston Scientific Foundation was able to help fuel grassroots innovative solutions to improve access to quality healthcare and create new opportunities for students to learn and achieve.
Seasonality
Our net sales are influenced by many factors, including product launches, acquisitions, regulatory and reimbursement approvals, patient, physician and employee holiday schedules and other macro-economic conditions. While our consolidated net sales do not reflect any significant degree of seasonality, customer purchases of our medical devices have historically been lower in the first and third quarters of the year.
15
Available Information
Copies of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the Exchange Act), are available free of charge on our website (www.bostonscientific.com) as soon as reasonably practicable after we electronically file the material with or furnish it to the U.S. Securities and Exchange Commission (SEC). Additionally, the SEC maintains an internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Printed copies of these posted materials are also available free of charge to stockholders who request them in writing from Investor Relations, 300 Boston Scientific Way, Marlborough, MA 01752-1234. Information on our website or linked to our website is not incorporated by reference into this Annual Report.
Safe Harbor for Forward-Looking Statements
Certain statements that we may make from time to time, including statements contained in this Annual Report and information incorporated by reference into this Annual Report, constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like “anticipate,” “expect,” “project,” “believe,” “plan,” “estimate,” “intend,” “aiming” and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements.
The forward-looking statements in this Annual Report are based on certain risks and uncertainties, including the risk factors described in Item 1A under the heading “Risk Factors” and the specific risk factors discussed below and in connection with forward-looking statements throughout this Annual Report, which could cause actual results to vary materially from the expectations and projections expressed or implied by our forward-looking statements. These risks and uncertainties, in some cases, have affected and in the future could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this Annual Report. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements. Risks and uncertainties that may cause such differences include, among other things: future economic, political, competitive, reimbursement and regulatory conditions, new product introductions and the market acceptance of those products, markets for our products, expected pricing environment, expected procedural volumes, the closing and integration of acquisitions, clinical trial results, demographic trends, intellectual property rights, litigation, financial market conditions, the execution and effect of our restructuring program, the execution and effect of our business strategy, including our cost-savings and growth initiatives and future business decisions made by us and our competitors. New risks and uncertainties may arise from time to time and are difficult to predict. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Item 1A. Risk Factors contained within this Annual Report on Form 10-K filed with the SEC, which we may update in Part II, Item 1A. Risk Factors in subsequent Quarterly Reports on Form 10-Q that we will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statement to reflect any change in our expectations or in events, conditions, or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this Annual Report.
The following are some of the important risk factors that could cause our actual results to differ materially from our expectations in any forward-looking statements. For further discussion of these and other risk factors, see Item 1A. Risk Factors.
Our Businesses
• | Our ability to increase net sales, expand the market, capture market share and adapt to market volatility, |
• | The ongoing impact on our business of physician alignment to hospitals, governmental investigations and audits of hospitals and other market and economic conditions on the overall number of procedures performed, |
• | Competitive offerings and related declines in average selling prices for our products, |
• | The performance of, and physician and patient confidence in, our products and technologies or those of our competitors, |
• | The impact and outcome of ongoing and future clinical trials and market studies undertaken by us, our competitors or other third parties or perceived product performance of our or our competitors' products, |
16
• | Variations in clinical results, reliability or product performance of our and our competitors' products, |
• | Our ability to acquire or develop, launch and supply new or next-generation products and technologies worldwide and in line with our commercialization strategies in a timely and successful manner and with respect to our recent acquisitions, |
• | The effect of consolidation and competition in the markets in which we do business or plan to do business, |
• | Disruption in the manufacture or supply of certain components, materials or products, or the failure to secure in a timely manner alternative manufacturing or additional or replacement components, materials or products, |
• | Our ability to retain and attract key personnel, including those associated with recent acquisitions, |
• | The impact of natural disasters, public health crises, and other catastrophic events, |
• | The impact of enhanced requirements to obtain regulatory approval in the U.S. and around the world, including EU MDR and the associated timing and cost of product approval, and |
• | The impact of increased pressure on the availability and rate of third-party reimbursement for our products and procedures in the U.S. and around the world, including with respect to the timing and costs of creating and expanding markets for new products and technologies. |
Regulatory Compliance, Litigation and Data Protection
• | The impact of healthcare policy changes and legislative or regulatory efforts in the U.S., the EU and around the world to modify product approval or reimbursement processes, including a trend toward demonstrating clinical outcomes, comparative effectiveness and cost efficiency, as well as the impact of other healthcare reform legislation, |
• | Risks associated with our regulatory compliance and quality systems and activities in the U.S., the EU and around the world, including meeting regulatory standards applicable to manufacturing and quality processes, |
• | Our ability to minimize or avoid future field actions or FDA warning letters relating to our products and processes and the ongoing inherent risk of potential physician advisories related to our or our competitors' products, |
• | The impact of increased scrutiny of and heightened global regulatory enforcement facing the medical device industry arising from political and regulatory changes, economic pressures or otherwise, including under U.S. Anti-Kickback Statute, U.S. False Claims Act and similar laws in other jurisdictions, U.S. Foreign Corrupt Practices Act (FCPA) and similar laws in other jurisdictions, and U.S. and foreign export control, trade embargo and customs laws, |
• | Costs and risks associated with current and future asserted litigation, |
• | The effect of our litigation and risk management practices, including self-insurance and compliance activities on our loss contingencies, legal provision and cash flows, |
• | The impact of, diversion of management attention as a result of, and costs to cooperate with, litigate and/or resolve governmental investigations and our class action, product liability, contract and other legal proceedings, |
• | The possibility of failure to protect our intellectual property rights and the outcome of patent litigation, and |
• | Our ability to operate properly our information systems that support our business operations and protect our data integrity and products from a cyber-attack or other breach that has a material adverse effect on our business, reputation or results of operations. |
Innovation and Certain Growth Initiatives
• | The timing, size and nature of our strategic growth initiatives and market opportunities, including with respect to our internal research and development platforms and externally available research and development platforms and technologies and the ultimate cost and success of those initiatives and opportunities, |
17
• | Our ability to complete planned clinical trials successfully, obtain regulatory approvals and launch new and next generation products in a timely manner consistent with cost estimates, including the successful completion of projects from in-process research and development, |
• | Our ability to identify and prioritize our internal research and development project portfolio and our external investment portfolio on profitable revenue growth opportunities as well as to keep them in line with the estimated timing and costs of such projects and expected revenue levels for the resulting products and technologies, |
• | Our ability to successfully develop, manufacture and market new products and technologies in a timely manner and the ability of our competitors and other third parties to develop products or technologies that render our products or technologies noncompetitive or obsolete, |
• | Our ability to execute appropriate decisions to discontinue, write-down or reduce the funding of any of our research and development projects, including projects from in-process research and development from our acquisitions, in our growth adjacencies or otherwise, |
• | Our dependence on acquisitions, alliances or investments to introduce new products or technologies and to enter new or adjacent growth markets and our ability to fund them or to fund contingent payments with respect to those acquisitions, alliances and investments, and |
• | The potential failure to successfully integrate and realize the expected benefits from the strategic acquisitions, alliances and investments we have consummated or may consummate in the future. |
International Markets
• | Our dependency on international net sales to achieve growth, including in emerging markets, |
• | The impact of changes we may make in the future on our international structure and leadership, |
• | The timing and collectability of customer payments, |
• | Geopolitical and economic conditions (including the impact of the United Kingdom's exit from the EU, often referred to as "Brexit"), |
• | Protection of our intellectual property, |
• | Our ability to comply with established and developing U.S. and foreign legal and regulatory requirements, including FCPA and similar laws in other jurisdictions |
• | Our ability to comply with U.S. and foreign export control, trade embargo and custom laws, |
• | The impact of changes in reimbursement practices and policies, |
• | Our ability to maintain or expand our worldwide market positions in the various markets in which we compete or seek to compete, including through investments in product diversification and emerging markets such as Brazil, Russia, India and China, |
• | Our ability to execute and realize anticipated benefits from our investments in emerging markets, and |
• | The potential effect of foreign currency fluctuations and interest rate fluctuations on our net sales, expenses and resulting margins. |
Liquidity
• | Our ability to generate sufficient cash flow to fund operations, capital expenditures, global expansion initiatives, any litigation settlements and judgments, share repurchases and strategic investments and acquisitions as well as maintaining our investment grade ratings and managing our debt levels and covenant compliance, |
18
• | Our ability to access the public and private capital markets when desired and to issue debt or equity securities on terms reasonably acceptable to us, |
• | The unfavorable resolution of open tax matters, exposure to additional tax liabilities and the impact of changes in U.S. and international tax laws, |
• | The impact of examinations and assessments by domestic and international taxing authorities on our tax provision, financial condition or results of operations, |
• | The possibility of counterparty default on our derivative financial instruments, |
• | The impact of potential intangible asset impairment charges, including on our results of operations, and |
• | Our ability to collect outstanding and future receivables and/or sell receivables under our factoring programs. |
Cost Reduction and Optimization Initiatives
• | Risks associated with changes made or expected to be made to our organizational and operational structure, pursuant to our restructuring plans as well as any further restructuring or optimization plans we may undertake in the future and our ability to recognize benefits and cost reductions from such programs and |
• | Business disruption and employee distraction as we execute our global compliance program, restructuring and optimization plans and divestitures of assets or businesses and implement our other strategic and cost reduction initiatives. |
19
ITEM 1A. RISK FACTORS
In addition to the other information contained in this Annual Report and the exhibits hereto, the following risk factors should be considered carefully in evaluating our business. Our business, financial condition, cash flows or results of operations could be materially adversely affected by any of these risks. This section contains forward-looking statements. You should refer to the explanation of the qualifications and limitations on forward-looking statements set forth at the end of Item 1. Business of this Annual Report. Additional risks not presently known to us or that we currently deem immaterial may also adversely affect our business, financial condition, cash flows or results of operations.
Business and Operational Risks
We face intense competition and may not be able to keep pace with the rapid technological changes in the medical devices industry, which could have an adverse effect on our business, financial condition or results of operations.
The medical device markets in which we participate are highly competitive. We encounter significant competition across our product lines and in each market in which our products are sold from various medical device companies. Some of our competitors may have greater financial and marketing resources than we do, including as a result of consolidation among companies in our industry. Our primary competitors include Abbott Laboratories and Medtronic plc,, as well as a wide range of medical device companies that sell a single or limited number of competitive products or which participate in only a specific market segment or set of segments. We also face competition from non-medical device companies, including pharmaceutical companies, which may offer alternative therapies for disease states also amenable to treatment using our products. New direct or indirect competitors may emerge in the future, potentially including companies introducing new sales or distribution models to our industry or leveraging robotic, navigation, and/or other automation technologies into markets in which we compete.
In addition, the medical device markets in which we participate are characterized by extensive research and development and rapid technological change. Developments by other companies of products and/or services, processes or technologies may make our products or proposed products obsolete or less competitive and may negatively impact our net sales. It is necessary for us to devote continued efforts and financial resources to the development or acquisition of scientifically advanced technologies and products. In addition, we will need to apply our technologies cost-effectively across product lines and markets, obtain patent and other protection for our technologies and products, obtain required regulatory and reimbursement approvals and successfully manufacture and market our products consistent with our quality standards. If we fail to develop or acquire new products or enhance existing products, such failure could have a material adverse effect on our business, financial condition or results of operations. In addition, a delay in the timing of the launch of next-generation products and the overall performance of, and continued physician confidence in, those products may result in declines in our market share and have an adverse impact on our business, financial condition or results of operations.
We may experience declines in market size, average selling prices for our products, medical procedure volumes and our share of the markets in which we compete, which may materially adversely affect our results of operations and financial condition.
We continue to experience pressures across many of our businesses due to competitive activity, increased market power of our customers as the healthcare industry consolidates, economic pressures experienced by our customers, public perception of our products, and the impact of managed care organizations and other third-party payers. These and other factors may adversely impact market sizes, as well as our share of the markets in which we compete, the average selling prices for our products or medical procedure volumes. There can be no assurance that the size of the markets in which we compete will increase above existing levels, that we will be able to regain or gain market share or compete effectively on the basis of price or that the number of procedures in which our products are used will increase above existing levels. Decreases in market sizes or our market share and declines in average selling prices or procedural volumes could materially adversely affect our results of operations or financial condition.
Continued consolidation in the healthcare industry or additional governmental controls exerted over pricing in key markets could lead to increased demands for price concessions or limit or eliminate our ability to sell to certain of our significant market segments, which could have an adverse effect on our business, financial condition or results of operations.
Numerous initiatives and reforms by legislators, regulators and third-party payers to curb the rising cost of healthcare have catalyzed a consolidation of aggregate purchasing power within the markets in which we sell our products. Additionally, a growing number of countries have instituted or are contemplating introducing regional or national tender processes driven primarily by price. In some cases, such processes may favor local companies to multinational companies like Boston Scientific. As the healthcare industry consolidates, competition to provide products and services is expected to continue to intensify, resulting in pricing pressures, decreased average selling prices and the exclusion of certain suppliers from important market segments. We expect that market demand, government regulation, third-party coverage and reimbursement policies, government contracting requirements and
20
societal pressures will continue to change the worldwide healthcare industry, resulting in further business consolidations and alliances among our customers, which may increase competition, exert further downward pressure on the prices of our products and services and may adversely impact our business, financial condition or results of operations.
Healthcare cost containment pressures, government payment and delivery system reforms, changes in private payer policies, and marketplace consolidations could decrease the demand for our products, the prices which customers are willing to pay for those products and/or the number of procedures performed using our devices, which could have an adverse effect on our business, financial condition or results of operations.
Our products are purchased principally by hospitals, physicians and other healthcare providers around the world that typically bill various third-party payers, including government programs, authorities or agencies (e.g., Medicare and Medicaid in the U.S.) and private health plans, for the healthcare services provided to their patients. Governments and payers may also institute changes in healthcare delivery systems that may reduce funding for services or encourage greater scrutiny of healthcare costs. The ability of customers to obtain appropriate reimbursement for their products and services from private and governmental third-party payers is critical to the success of medical technology companies because it affects which products customers purchase and the prices they are willing to pay. Reimbursement and funding vary by country and can significantly impact the acceptance of new products and technologies and the use of established products and technologies. Even if we develop a promising new product, we may find limited demand for the product unless reimbursement approval is obtained from private and governmental third-party payers. Further legislative or administrative reforms to the reimbursement systems in the U.S., Japan, or other countries in a manner that significantly reduces reimbursement for procedures using our medical devices or denies coverage for those procedures, including price regulation, site of service requirements, competitive bidding and tendering, coverage and payment policies, comparative effectiveness of therapies, heightened clinical data requirements, technology assessments and managed-care arrangements, could have a material adverse effect on our business, financial condition or results of operations
We are subject to a number of market, business, financial, legal and regulatory risks and uncertainties with respect to our international operations that could have a material impact on our business, financial condition or results of operations.
International net sales accounted for 42 percent of our global net sales in 2019. An important part of our strategy is to continue pursuing growth opportunities in net sales and market share outside of the U.S. by expanding global presence, including in Emerging Markets. Our international operations are subject to a number of market, business and financial risks and uncertainties, including those related to our use of channel partners, geopolitical and economic instability, foreign currency exchange and interest rate fluctuations, competitive product offerings, local changes in healthcare financing and payment systems and healthcare delivery systems, local product preferences and requirements, including preferences for local manufacturers, workforce instability, weaker intellectual property protection in certain countries than exists in the U.S. and longer accounts receivable cycles. Such risks and uncertainties may adversely impact our ability to implement our growth strategy in these markets and, as a result, our sales growth, market share and operating profits from our international operations may be adversely affected.
Our international operations are subject to established and developing legal and regulatory requirements for medical devices in each country in which our products are marketed and sold. Most foreign countries have medical device regulations. Further, most countries outside of the U.S. require product approvals be renewed or recertified on a regular basis in order to continue to be marketed and sold there. In addition, several countries that previously did not have regulatory requirements for medical devices have established such requirements in recent years and other countries have expanded, or plan to expand, existing regulations, including requiring local clinical data in addition to global clinical data. These factors have caused or may cause us to experience more uncertainty, risk, expense and delay in commercializing products in certain foreign jurisdictions, which could affect our ability to obtain approvals for our products in those jurisdictions and adversely impact our net sales, market share and operating profits from our international operations.
Further, international markets are affected by economic pressure to contain healthcare costs, which can lead to more rigorous evidence requirements and lower reimbursement rates for either our products directly or procedures in which our products are used. Governments and payers may also institute changes in healthcare delivery systems that may reduce funding for services or encourage greater scrutiny of healthcare costs. In addition, certain international markets may also be affected by foreign government efforts to reference reimbursement rates in other countries. All of these types of changes may ultimately reduce selling prices of our products and/or reduce the number of procedures in which our products are used, which may adversely impact our net sales, market share and operating profits from our international operations.
In addition, our international operations are subject to other established and developing U.S. and foreign legal and regulatory requirements, including FCPA and/or similar laws in other countries and U.S. and foreign import and export controls and licensing requirements, trade protection and embargo measures and customs laws. Global businesses, including those in the medical device industry, are facing increasing scrutiny of, and heightened enforcement efforts with respect to, their international operations. Any
21
alleged or actual failure to comply with legal and regulatory requirements may subject us to government scrutiny, civil and/or criminal proceedings, sanctions and other liabilities, which may have a material adverse effect on our international operations, financial condition, results of operations and/or liquidity.
In a referendum on June 23, 2016, voters approved the exit of the United Kingdom (UK) from the European Union (EU). Following a formal notification by the UK to the EU that it intends to leave the EU, and after several extensions to the deadline for doing so agreed between the UK and the EU, the UK legally withdrew from the EU on January 31, 2020. Pursuant to the withdrawal agreement entered into on January 24, 2020, the UK will maintain access to the single EU market and to the global trade deals negotiated by the EU on behalf of its members and remain subject to EU for a transition period ending on December 31, 2020. Exit of the UK from the EU will have numerous consequences in all areas of our business, including, economic, regulatory and operational, and the actual impact is very difficult to assess at this time. Changes in industry regulations could have an effect on existing CE certificates being renewed and new certificates being issued which would impact the ability to trade; however, it is impossible to assess the full impact at this stage.
At this stage, the materiality to us of Brexit remains unknown and unquantifiable. However, we have implemented a Brexit Response Team and have put in place mitigation procedures to reduce any significant operational risks that have been identified to date.
Any significant changes in the political and economic, financial, competitive, legal and regulatory or reimbursement conditions where we conduct, or plan to expand, our international operations may have a material impact on our business, financial condition or results of operations.
If we are unable to manage our debt levels, maintain investment grade credit ratings at the three ratings agencies, or experience a disruption in our cash flows it could have an adverse effect on our cost of borrowing, financial condition or results of operations.
As part of our strategy to maximize stockholder value, we use financial leverage to manage our cost of capital. Our outstanding debt balance was $10.008 billion as of December 31, 2019 and $7.056 billion as of December 31, 2018. Although we currently have investment grade ratings at Moody's Investor Service, Standard & Poor's Rating Service and Fitch Ratings, our inability to maintain investment grade credit ratings could increase our cost of borrowing funds in the future and reduce our access to liquidity. Delays in our product development and new product launches could result in disruption in our cash flow or our ability to continue to effectively manage our debt levels could have an adverse effect on our cost of borrowing, financial condition or results of operations. In addition, our credit and security facilities contain covenants that require us to maintain specified financial ratios and place other limits on our business. If we are unable to satisfy these covenants, we may be required to obtain waivers from our lenders and no assurance can be made that our lenders would grant such waivers on favorable terms or at all and we could be required to repay any borrowings on demand.
We may record future intangible asset impairment charges related to one or more of our global reporting units, which could materially adversely impact our results of operations.
We test our goodwill balances during the second quarter of each year for impairment, or more frequently if indicators are present or changes in circumstances suggest that impairment may exist. We assess goodwill for impairment at the reporting unit level and, in evaluating the potential for impairment of goodwill, we make assumptions regarding estimated revenue projections, growth rates, cash flows and discount rates. In the second quarter of 2019, we performed our annual goodwill impairment test for all of our reporting units existing at the time. In conjunction with our annual test, the fair value of each reporting unit exceeded its carrying value and none of our reporting units were at risk of impairment. Refer to Critical Accounting Policies and Estimates contained in Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations of this Annual Report for a discussion of key assumptions used in our testing.
On a quarterly basis, we monitor the key drivers of fair value to detect events or other changes that would warrant an interim impairment test of our goodwill and other intangible assets. Relatively small declines in the future performance and cash flows of a reporting unit or asset group, changes in our reporting units or in the structure of our business as a result of future reorganizations, acquisitions or divestitures of assets or businesses, or small changes in other key assumptions, may result in the recognition of significant asset impairment charges, which could have a material adverse impact on our results of operations.
Failure to integrate acquired businesses into our operations successfully could adversely affect our business, financial condition and operating results.
As part of our strategy to realign our business portfolio, we have completed multiple acquisitions over the past three years and may pursue additional acquisitions in the future. Our integration of acquired businesses requires significant efforts, including
22
corporate restructuring and the coordination of information technologies, research and development, sales and marketing, operations, regulatory, supply chain, manufacturing, quality systems and finance. These efforts result in additional expenses and involve significant management time. Some of the factors that could affect the success of our acquisitions include, among others, the effectiveness of our due diligence process, our ability to execute our business plan for the acquired companies, the strength of the acquired technology, results of clinical trials, regulatory approvals and reimbursement levels of the acquired products and related procedures, the continued performance of critical transition services, our ability to adequately fund acquired in-process research and development projects and retain key employees and our ability to achieve synergies with our acquired companies, such as increasing sales of our products, achieving cost savings and effectively combining technologies to develop new products. In addition, foreign acquisitions involve unique risks, including those related to integration of operations across different geographies, cultures and languages, currency risks and risks associated with the economic, political, legal and regulatory environment in specific countries. Our failure to manage successfully and coordinate the growth of the acquired companies could have an adverse impact on our business and our future growth. In addition, we cannot be certain that the businesses we acquire will become profitable or remain so, and if our acquisitions are not successful, we may record related asset impairment charges in the future or experience other negative consequences on our results.
We may not be successful in our strategy relating to future strategic acquisitions of, investments in, or alliances with, other companies and businesses, which have been a significant source of historical growth for us, and will be key to our diversification into new markets and technologies.
Our strategic acquisitions, investments and alliances are intended to further expand our ability to offer customers effective, high quality medical devices that satisfy their interventional needs. These acquisitions, investments and alliances have been a significant source of our growth. If we are unsuccessful in our acquisitions, investments and alliances, we may be unable to grow our business. The success of our strategy relating to future acquisitions, investments or alliances will depend on a number of factors, including:
• | our ability to identify suitable opportunities for acquisition, investment or alliance, if at all, |
• | our ability to manage acquisition, investment or alliance opportunities within our capital capacity and prioritize those investments to execute on our strategy, |
• | our ability to manage our due diligence process to uncover potential issues with targets, |
• | our ability to finance any future acquisition, investment or alliance on terms acceptable to us, if at all, |
• | our ability to complete acquisitions, investments or alliances in a timely manner on terms that are satisfactory to us, if at all, |
• | our ability to successfully integrate and operate acquired businesses, |
• | our ability to successfully identify and retain key target employees, |
• | our ability to comply with applicable laws and regulations, including foreign laws and regulations, and |
• | our ability to protect intellectual property and to prevail in litigation related to newly acquired technologies. |
Any potential future acquisitions we consummate may be dilutive to our earnings and may require additional debt or equity financing, depending on their size or nature.
We may not realize the expected benefits from our restructuring and optimization initiatives, our long-term expense reduction programs may result in an increase in short-term expenses and our efforts may lead to unintended consequences.
We monitor the dynamics of the economy, the healthcare industry and the markets in which we compete and assess opportunities for improved operational effectiveness and efficiency and to better align expenses with revenues, while preserving our ability to make investments in research and development projects, capital and our people that we believe are important to our long-term success. As a result of these assessments, we have undertaken restructuring and optimization initiatives in order to enhance our growth potential and position us for long-term success. In November 2018, we announced a restructuring initiative (the 2019 Restructuring Plan) intended to support our effort to improve operating performance and meet anticipated market demands by ensuring that we are appropriately structured and resourced to deliver sustainable value to patients and customers. Key activities under the 2019 Restructuring Plan include supply chain network optimization intended to maximize our global manufacturing and distribution network capacity and building functional capabilities that support business growth. These activities were initiated in 2019, with the majority of activity expected to be complete by the end of 2021. The 2019 Restructuring Plan is expected to result in total pre-tax charges of approximately $200 million to $300 million and reduce gross annual pre-tax operating expenses by approximately $100 million to $150 million by the end of 2022 as program benefits are realized. We expect a substantial portion of the savings to be reinvested in strategic growth initiatives. These measures could yield unintended consequences, such as distraction of our management and employees, business disruption, inability to attract or retain key personnel and reduced employee productivity, which could negatively affect our business, sales, financial condition and results of operations. Moreover, our restructuring and optimization initiatives result in charges and expenses some of which impact our operating results. We cannot
23
guarantee that the activities under our restructuring plans or other optimization initiatives will result in the desired efficiencies and estimated cost savings.
Current domestic and international economic conditions could adversely affect our cash flows and results of operations.
Uncertainty about global economic conditions, including those resulting from credit and sovereign debt issues, has caused and may continue to cause disruption in the financial markets, including diminished liquidity and credit availability. These conditions may adversely affect our suppliers, leading them to experience financial difficulties or be unable to borrow money to fund their operations, which could cause disruptions in our ability to produce our products. Our customers may experience financial difficulties or be unable to borrow money to fund their operations, which may adversely impact their ability or decision to purchase our products, particularly capital equipment, or to pay for our products that they purchase on a timely basis, if at all. In addition, we have accounts receivable factoring programs in certain European and Asian countries. Continued deterioration of the global economy or increase in sovereign debt issues may impact our ability to transfer receivables to third parties in certain of those countries in the future. Third parties such as banks offering factoring programs in these countries are looking to reduce their exposure levels to government owned or supported debt. This could result in terminations of, or changes to the costs or credit limits of our existing factoring programs. Such terminations or changes could have a negative impact on our cash flow and days sales outstanding.
The strength and timing of economic recovery remains uncertain and there can be no assurance that there will not be further deterioration in the global economy. Accordingly, we cannot predict to what extent global economic conditions, including sovereign debt issues and increased focus on healthcare systems and costs in the U.S. and abroad, may continue to impact negatively our average selling prices, net sales and profit margins, procedural volumes and reimbursement rates from third party payers. In addition, conditions in the financial markets and other factors beyond our control may adversely affect our ability to borrow money in the credit markets, access the capital markets and to obtain financing for acquisitions or other general corporate and commercial purposes.
Our future growth is dependent upon the development of new products and enhancement of existing products, which requires significant research and development, clinical trials and regulatory approvals, all of which may be very expensive and time-consuming and may not result in commercially viable products.
In order to develop new products and enhance existing products, we focus our research and development programs largely on the development of next-generation and novel technology offerings across multiple programs and businesses. The development of new products and enhancement of existing products requires significant investment in research and development, clinical trials and regulatory approvals. The results of our product development efforts may be affected by a number of factors, including our ability to anticipate customer needs, innovate and develop new products, complete clinical trials, obtain regulatory approvals and reimbursement in the United States and abroad, manufacture products in a cost-effective manner, obtain appropriate intellectual property protection for our products and gain and maintain market approval of our products. There can be no assurance that any products now in development or that we may seek to develop in the future will achieve technological feasibility, obtain regulatory approval or gain market acceptance. If we are unable to develop and launch new products and enhanced products, our ability to maintain or expand our market position in the markets in which we participate may be materially adversely impacted. Further, we are continuing to investigate and have completed several acquisitions that involve opportunities to further expand our presence in and diversify into, priority growth areas by accessing new products and technologies. There can be no assurance that our investments will be successful or that we will be able to access new products and technologies on terms favorable to us, or that these products and technologies will achieve commercial feasibility, obtain regulatory approval or gain market acceptance. A delay in the development or approval of new products and technologies or our decision to reduce our investments may adversely impact the contribution of these technologies to our future growth.
Additionally, certain products or groups of products, in particular new products or enhancements of existing products, may have a disproportionate impact on our business, financial condition and results of operations. Failure to meet growth projections, poor clinical outcomes, increasing regulatory requirements, launch delays and inability to effectively scale manufacturing and achieve targeted margins with respect to any of these products or groups of products in particular may materially adversely impact on our business, financial condition and results of operations.
Natural disasters, public health crises, and other catastrophic events or other events outside of our control may affect sales of our products or disrupt our supply chain and have an adverse effect on our business, financial condition and results of operations.
If any of our facilities, or the facilities of our suppliers or customers, is affected by natural disasters, public health crises, such as pandemics and epidemics, or other events outside of our control, our business and operating results could suffer. These types of
24
events could negatively impact procedure volumes in the affected regions or depending upon the severity, globally, which could adversely impact our operating results. For example, we anticipate that the novel coronavirus may have a negative sales impact due to the potential effect on procedure volumes in China and supply chain disruption.
Interruption of our manufacturing operations could adversely affect our results of operations and financial condition.
Our products are designed and manufactured in technology centers around the world, either by us or third parties. In most cases, the manufacturing of any specific product is concentrated in one or a few locations. Factors such as a failure to follow specific internal protocols and procedures, equipment malfunction, environmental factors or damage to one or more of our facilities could adversely affect our ability to manufacture our products. In the event of an interruption in manufacturing, we may be unable to quickly move to alternate means of producing affected products or to meet customer demand. In the event of a significant interruption, for example, as a result of a failure to follow regulatory protocols and procedures, we may experience lengthy delays in resuming production of affected products due primarily to needs for regulatory approvals. As a result, we may experience loss of market share, which we may be unable to recapture and harm to our reputation, which could adversely affect our results of operations and financial condition.
Disruptions in the supply of the materials and components used in manufacturing our products or the sterilization of our products by third-party vendors could adversely affect our results of operations and financial condition.
We purchase the majority of the materials and components used in manufacturing our products from third-party vendors. Certain of these materials and components are purchased from single sources due to quality considerations, expertise, costs or constraints resulting from regulatory requirements. In certain cases, we may not be able to establish additional or replacement vendors for such materials or components in a timely or cost effective manner, largely as a result of FDA regulations that require validation of materials and components prior to their use in our products and the complex nature of our and many of our vendors' manufacturing processes. A reduction or interruption in the supply of materials and components used in manufacturing our products, an inability to timely develop and validate alternative sources if required or a significant increase in the price of such materials or components could adversely affect our results of operations and financial condition.
In addition, many of our products require sterilization prior to sale and we utilize a mix of internal resources and contract sterilizers to perform this service. To the extent we or our contract sterilizers are unable to sterilize our products, whether due to capacity, availability of materials for sterilization, regulatory or other constraints, including federal and state regulations on the use of ethylene oxide, we may be unable to transition to other internal resources, contract sterilizers, sterilizer locations or sterilization methods in a timely or cost effective manner or at all, which could have a material impact on our results of operations and financial condition.
Our share price has been volatile and may fluctuate, and accordingly, the value of an investment in our common stock may also fluctuate.
Stock markets in general and our common stock in particular have experienced significant price and trading volume volatility over recent years. The market price and trading volume of our common stock may continue to be subject to significant fluctuations due to factors described under this Item 1A. Risk Factors, as well as economic and geopolitical conditions in general and to variability in the prevailing sentiment regarding our operations or business prospects, as well as, among other things, changing investment priorities of our stockholders. Because the market price of our common stock fluctuates significantly, stockholders may not be able sell their shares at attractive prices.
If we are unable to attract or retain key personnel, it could have an adverse effect on our business, financial condition and results from operations.
In our industry, there is substantial competition for key personnel in the regions in which we operate and we may face increased competition for such employees, particularly in emerging markets as the trend toward globalization continues. Our business depends to a significant extent on the continued service of senior management and other key personnel, the development of additional management personnel and the hiring of new qualified employees. There can be no assurance that we will be successful in retaining and developing existing personnel or recruiting new personnel. The loss of one or more key employees, our ability to attract or develop additional qualified employees or any delay in hiring key personnel could have material adverse effects on our business, financial condition or results of operations.
25
Legal and Regulatory Risk Factors
Healthcare policy changes, including healthcare reform legislation, may have a material adverse effect on our business, financial condition, results of operations and cash flows.
Political, economic and policy influences are leading the healthcare industry to make substantial structural and financial changes that will continue affecting our results of operations. Government and private sector initiatives limiting the growth of healthcare costs (including price regulation), coverage and payment policies, comparative effectiveness of therapies, technology assessments and healthcare delivery structure reforms, are continuing in many countries where we do business. We believe that these changes are causing the marketplace to put increased emphasis on the delivery of more treatments that can reduce costs, improve efficiencies and/or increase patient access. Although we believe our less-invasive products and technologies generate favorable clinical outcomes, value and cost efficiency, the resources necessary and evidence necessary to demonstrate value to our customers, patients, payers and other stakeholders may be significant and it may take a longer period of time to gain widespread adoption. Moreover, there can be no assurance that our strategies will succeed for every product.
The Patient Protection and Affordable Care Act (ACA) and Health Care and Education Affordability Reconciliation Act of 2010 were enacted into law in the U.S. in March 2010. As a U.S. headquartered company with significant domestic sales, the medical device tax included in this law has materially affected us. The law imposed on medical device manufacturers a 2.3 percent excise tax on U.S. sales of Class I, II and III medical devices beginning in January 2013. In December 2015, this law was temporarily suspended until December 2019, when the law was permanently repealed as part of the 2020 fiscal spending bill. Under the current administration, there may be a permanent repeal or an alteration of other elements of the ACA, but at this time it is not definite that a change will be enacted or what new healthcare provisions may be implemented. While the implementation of the medical device tax has been repealed, other provisions of this law, including comparative effectiveness research, pilot programs to evaluate alternative payment methodologies and other changes to the payment systems, have started changing the way healthcare is delivered, reimbursed and funded. While the extent to which it has affected our business is not clear, these changes, over the long-term, may adversely affect our business and results of operations.
We cannot predict the specific healthcare programs and regulations that will be ultimately implemented by regional and national governments globally. However, any changes that lower reimbursements for either our products and/or procedures using our products reduce medical procedure volumes and/or increase cost containment pressures on us or others in the healthcare sector could adversely affect our business and results of operations.
We are subject to extensive and dynamic medical device regulation, which may impede or hinder the approval or sale of our products and, in some cases, may ultimately result in an inability to obtain approval of certain products or may result in the recall or seizure of previously approved products.
Our products, marketing, sales and development activities and manufacturing processes are subject to extensive and rigorous regulation by the FDA pursuant to the Federal Food, Drug and Cosmetic Act (FDC Act), by comparable agencies in foreign countries and by other regulatory agencies and governing bodies. Under the FDC Act, medical devices must receive FDA clearance or approval or an exemption from such clearance or approval before they can be commercially marketed in the U.S. In the European Union (EU), we will be required to comply with the new Medical Device Regulation (MDR or EU MDR) effective May 2020 which will supersede the current Medical Device Directives. Medical devices which have a valid CE Certificate to the current Directives (issued before May 2020) can continue to be sold until May 2024 or until the CE Certificate expires, whichever comes first, providing there are no significant changes to the design or intended use. The CE Mark is applied following approval from an independent notified body or declaration of conformity. The process of obtaining marketing approval or clearance from the FDA or by comparable agencies in foreign countries for new products, or with respect to enhancements or modifications to existing products, could:
• | take a significant period of time, |
• | require the expenditure of substantial resources, |
• | involve rigorous pre-clinical and clinical testing, as well as increased post-market surveillance, |
• | require changes to products and |
• | result in limitations on the indicated uses of products. |
In addition, exported devices are subject to the regulatory requirements of each country to which the device is exported. Some countries do not have medical device regulations, but in most foreign countries, medical devices are regulated. Frequently, regulatory approval may first be obtained in a foreign country prior to application in the U.S. due to differing regulatory requirements; however, other countries, such as China for example, require approval in the country of origin or legal manufacturer first. Most countries outside of the U.S. require that product approvals be renewed or recertified on a regular basis, generally every
26
four to five years. The renewal or recertification process requires that we evaluate any device changes and any new regulations or standards relevant to the device and conduct appropriate testing to document continued compliance. Where renewal or recertification applications are required, they may need to be renewed and/or approved in order to continue selling our products in those countries. There can be no assurance that we will receive the required approvals for new products or modifications to existing products on a timely basis or that any approval will not be subsequently withdrawn or conditioned upon extensive post-market study requirements.
Our global regulatory environment is becoming increasingly stringent and unpredictable, which could increase the time, cost and complexity of obtaining regulatory approvals for our products, as well as the clinical and regulatory costs of supporting those approvals. Several countries that did not have regulatory requirements for medical devices have established such requirements in recent years and other countries have expanded on existing regulations. Certain regulators are exhibiting less flexibility and are requiring local preclinical and clinical data in addition to global data. While harmonization of global regulations has been pursued, requirements continue to differ significantly among countries. We expect this global regulatory environment will continue to evolve, which could impact our ability to obtain future approvals for our products or could increase the cost and time to obtain such approvals in the future.
The FDA and other worldwide regulatory agencies actively monitor compliance with local laws and regulations through review and inspection of design and manufacturing practices, recordkeeping, reporting of adverse events, labeling and promotional practices. The FDA can ban certain medical devices, detain or seize adulterated or misbranded medical devices, order repair, replacement or refund of these devices and require notification of health professionals and others with regard to medical devices that present unreasonable risks of substantial harm to the public health. The FDA can take action against a company that promotes "off-label" uses. The FDA may also enjoin and restrain a company for certain violations of the FDC Act and other amending Acts pertaining to medical devices, or initiate action for criminal prosecution of such violations. Any adverse regulatory action, depending on its magnitude, may restrict a company from effectively marketing and selling its products, may limit a company's ability to obtain future premarket clearances or approvals and could result in a substantial modification to our business practices and operations. International sales of medical devices manufactured in the U.S. that are not approved by the FDA for use in the U.S., or that are banned or deviate from lawful performance standards, are subject to FDA export requirements.
Regulations regarding the development, manufacture and sale of medical devices are evolving and subject to future change. We cannot predict what impact, if any, those changes might have on our business. Failure to comply with regulatory requirements could have a material adverse effect on our business, financial condition and results of operations. Later discovery of previously unknown problems with a product or manufacturer could result in fines, delays or suspensions of regulatory clearances or approvals, seizures or recalls of products, physician advisories or other field actions, operating restrictions and/or criminal prosecution. We may also initiate field actions as a result of a failure to strictly comply with our internal quality policies. The failure to receive product approval clearance on a timely basis, suspensions of regulatory clearances, seizures or recalls of products, physician advisories or other field actions, or the withdrawal of product approval by the FDA or by comparable agencies in foreign countries could have a material adverse effect on our business, financial condition or results of operations.
Our products are continually subject to clinical trials and other analyses conducted by us, our competitors or other third parties, the results of which may be unexpected, or perceived as unfavorable by the market, and could have a material adverse effect on our business, financial condition or results of operations.
As a part of the regulatory process of obtaining marketing clearance for new products and new indications for existing products, we conduct and participate in numerous clinical trials with a variety of study designs, patient populations and trial endpoints. Unexpected or inconsistent clinical data from existing or future clinical trials or other analyses conducted by us, by our competitors or by third parties, including acquired businesses prior to acquisition by us, or the FDA's or the market's perception of this clinical data, may adversely impact our ability to obtain product approvals, our position in, and share of, the markets in which we participate and our business, financial condition, results of operations or future prospects.
The medical device industry and its customers continue to face scrutiny and regulation by governmental authorities and are often the subject of numerous investigations, often involving marketing and other business practices or product quality issues including device recalls or advisories. These investigations could result in the commencement of civil and criminal proceedings; imposition of substantial fines, penalties and administrative remedies, including corporate integrity agreements, stipulated judgments or exclusion; diversion of our employees' and management's attention; imposition of administrative costs and have an adverse effect on our financial condition, results of operations and liquidity; and may lead to greater governmental regulation in the future.
The medical devices we design, develop, manufacture and market are subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. These authorities continue to closely scrutinize our industry. We have received and in the future may receive, subpoenas and other requests for information from Congress and state and federal
27
governmental agencies, including, among others, the U.S. Department of Justice (DOJ), the Office of Inspector General of the Department of Health and Human Services (HHS) and the Department of Defense, as well as from foreign governments and agencies. The requests and/or subpoenas we have received relate primarily to financial arrangements with healthcare providers, regulatory compliance and sale and/or product promotional practices. We have cooperated with these subpoenas and other requests for information and expect to continue to do so in the future. We cannot predict when a matter will be resolved, the outcome of the matter or its impact on us and cooperation may involve significant costs, including document production costs. An adverse outcome in any matter could include the commencement of an investigation, civil and criminal proceedings, substantial fines, penalties and administrative remedies, including exclusion from government reimbursement programs, entry into Corporate Integrity Agreements (CIAs) with governmental agencies and amendments to any existing CIAs. In addition, resolution of any matter could involve the imposition of additional and costly compliance obligations. Cooperation with requests and investigations from external agencies result in employee resource costs and diversion of employee focus. If any requests or investigations continue over a long period of time, they could divert the attention of management from the day-to-day operations of our business and impose significant additional administrative burdens on us. These potential consequences, as well as any adverse outcome from these requests or investigations, could have a material adverse effect on our financial condition, results of operations and liquidity.
In addition, certain foreign governments, state governments (including that of Massachusetts, where we are headquartered) and the U.S. federal government have enacted legislation aimed at increasing transparency of our interactions with healthcare providers. As an example, compliance with the U.S. Physician Payment Sunshine Act requires us by law to disclose payments and other transfers of value to all U.S. physicians and U.S. teaching hospitals at the U.S. federal level made after August 1, 2013. Any failure to comply with these legal and regulatory requirements could impact our business. In addition, we have and may continue to devote substantial additional time and financial resources to further develop and implement enhanced structure, policies, systems and processes to comply with enhanced legal and regulatory requirements, which may also impact our business.
We anticipate that governmental authorities will continue to scrutinize our industry closely and that additional regulation may increase compliance and legal cost and exposure to litigation and have additional adverse effects on our operations.
Changes in tax laws, unfavorable resolution of tax contingencies, or exposure to additional income tax liabilities could have a material impact on our financial condition, results of operations and/or liquidity.
We are subject to income taxes as well as non-income based taxes and tariffs, in both the U.S. and various foreign jurisdictions. We are subject to ongoing tax audits in various jurisdictions. Tax authorities may disagree with certain positions we have taken and assess additional taxes. We regularly assess the likely outcomes of these audits to determine the appropriateness of our tax provision, and we have established contingency reserves for material, known tax exposures. However, the calculation of such tax exposures involves the application of complex tax laws and regulations in many jurisdictions, as well as interpretations as to the legality under European Union state aid rules of tax advantages granted in certain jurisdictions. Therefore, there can be no assurance that we will accurately predict the outcomes of these disputes or other tax audits or that issues raised by tax authorities will be resolved at a financial cost that does not exceed our related reserves and the actual outcomes of these disputes and other tax audits could have a material impact on our results of operations or financial condition.
Changes in tax laws and regulations, or their interpretation and application, in the jurisdictions where we are subject to tax could materially impact our effective tax rate. The U.S. enacted the Tax Cuts and Jobs Act (TCJA) on December 22, 2017 and we expect the U.S. Treasury to issue future notices and regulations under the TCJA. Certain provisions of the TCJA and the regulations issued thereunder could have a significant impact on our future results of operations as could interpretations made by the Company in the absence of regulatory guidance and judicial interpretations. The result of the upcoming U.S. presidential and congressional elections may result in additional U.S. tax law changes that could have a material impact on our future effective tax rate.
Additionally, the Organization for Economic Co-operation and Development (OECD), the European Commission (EC) and individual taxing jurisdictions where we and our affiliates do business have recently focused on issues related to the taxation of multinational corporations. The OECD has released its comprehensive plan to create an agreed set of international rules for fighting base erosion and profit shifting. In addition, the OECD, the EC and individual countries are examining changes to how taxing rights should be allocated among countries considering the digital economy. As a result, the tax laws in the U.S. and other countries in which we and our affiliates do business could change on a prospective or retroactive basis and any such changes could materially adversely affect our business.
Our operations in Puerto Rico and Costa Rica presently benefit from various tax incentives and grants. Unless these incentives and grants are extended, they will expire between 2023 and 2028. If we are unable to renew, extend, or obtain new incentive and grants, the expiration of the existing incentives and grants could have a material impact on our financial results in future periods.
Furthermore, changes in customs laws and regulations in the U.S. and various foreign jurisdictions could have a material impact on our results of operations or financial condition.
28
We may not effectively be able to protect our intellectual property or other sensitive data, which could have a material adverse effect on our business, financial condition or results of operations.
The medical device market in which we participate is largely technology driven. Physician customers have historically moved quickly to new products and new technologies. As a result, intellectual property rights, particularly patents and trade secrets, play a significant role in product development and differentiation. However, intellectual property litigation is inherently complex and unpredictable and appellate courts can overturn lower court decisions. Furthermore, as our business increasingly relies on technology systems and infrastructure, our intellectual property, other proprietary technology and other sensitive data are potentially vulnerable to loss, damage or misappropriation. Finally, our ability to protect novel business models is uncertain.
Competing parties in our industry frequently file multiple suits to leverage patent portfolios across product lines, technologies and geographies and to balance risk and exposure between the parties. In some cases, several competitors are parties in the same proceeding, or in a series of related proceedings, or litigate multiple features of a single class of devices. These forces frequently drive settlement not only of individual cases, but also of a series of pending and potentially related and unrelated cases. In addition, although monetary and injunctive relief is typically sought, remedies and restitution are generally not determined until the conclusion of the trial court proceedings and can be modified on appeal. Accordingly, the outcomes of individual cases are difficult to time, predict or quantify and are often dependent upon the outcomes of other cases in other geographies.
A number of third parties have asserted that our current and former product offerings infringe patents owned or licensed by them. We have similarly asserted that products sold by our competitors infringe patents owned or licensed by us. Adverse outcomes in one or more of the proceedings against us could limit our ability to sell certain products in certain jurisdictions, or reduce our operating margin on the sale of these products and could have a material adverse effect on our financial condition, results of operations or liquidity.
Patents and other proprietary rights are and will continue to be essential to our business and our ability to compete effectively with other companies will be dependent upon the proprietary nature of our technologies. We rely upon trade secrets, know-how, continuing technological innovations, strategic alliances and licensing opportunities to develop, maintain and strengthen our competitive position. We pursue a policy of generally obtaining patent protection in both the U.S. and abroad for patentable subject matter in our proprietary devices and attempt to review third-party patents and patent applications to the extent publicly available in order to develop an effective patent strategy, avoid infringement of third-party patents, identify licensing opportunities and monitor the patent claims of others. We currently own numerous U.S. and foreign patents and have numerous patent applications pending. We also are party to various license agreements pursuant to which patent rights have been obtained or granted in consideration for cash, cross-licensing rights or royalty payments. No assurance can be made that any pending or future patent applications will result in the issuance of patents, that any current or future patents issued to, or licensed by, us will not be challenged or circumvented by our competitors, or that our patents will not be found invalid. In addition, we may have to take legal action in the future to protect our patents, trade secrets or know-how or to assert them against claimed infringement by others. Any legal action of that type could be costly and time consuming and no assurances can be made that any lawsuit will be successful. We are generally involved as both a plaintiff and a defendant in a number of patent infringement and other intellectual property-related actions. The invalidation of key patents or proprietary rights that we own, or an unsuccessful outcome in lawsuits to protect our intellectual property, could have a material adverse effect on our business, financial condition or results of operations.
In addition, the laws of certain countries in which we market and plan on manufacturing some of our products in the near future, do not protect our intellectual property rights to the same extent as the laws of the U.S. If we are unable to protect our intellectual property in these countries, it could have a material adverse effect on our business, financial condition or results of operations.
Furthermore, our intellectual property, other proprietary technology and other sensitive data are potentially vulnerable to loss, damage or misappropriation from system malfunction, computer viruses and unauthorized access to our data or misappropriation or misuse thereof by those with permitted access and other events. While we have invested to protect our intellectual property, products and other data and continue to work diligently in this area, there can be no assurance that our precautionary measures will prevent breakdowns, breaches, cyber-attacks or other events. Such events could have a material adverse effect on our reputation, business, financial condition or results of operations.
We rely on the proper function, availability and security of information technology systems to operate our business and a cyber-attack or other breach of these systems could have a material adverse effect on our business, financial condition or results of operations.
We rely on information technology systems, including technology from third party vendors, to process, transmit and store electronic information in our day-to-day operations. Similar to other large multi-national companies, the size and complexity of our information technology systems makes them vulnerable to a cyber-attack, malicious intrusion, breakdown, destruction, loss of
29
data privacy, or other significant disruption. Our information systems require an ongoing commitment of significant resources to maintain, protect and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, the increasing need to protect patient and customer information and changing customer patterns. In addition, third parties may attempt to hack into our products to obtain data relating to patients or disrupt performance of our products or to access our proprietary information and the technology from third party vendors that we rely upon may have defects or vulnerabilities which, in turn, create vulnerabilities or disruptions in our system. Any failure by us to maintain or protect our information technology systems, products and data integrity, including from cyber-attacks, intrusions or other breaches, could result in the unauthorized access to patient data and personally identifiable information, theft of intellectual property or other misappropriation of assets, or otherwise compromise our confidential or proprietary information and disrupt our operations, or, in the worst case, could result in harm to patients. Such failure, or demonstration of vulnerability to such failure, may also result in additional regulatory scrutiny. We also grow our company through acquisitions and may face risks associated with defects and vulnerabilities in their systems as we work to integrate the acquisitions into our information technology system.
In the U.S., federal and state privacy and security laws require certain of our operations to protect the confidentiality of personal information including patient medical records and other health information, and to comply with other requirements with respect to personal data. In Europe, the Data Protection Directive requires us to manage individually identifiable information in the EU and, the new General Data Protection Regulation (GDPR) may impose fines of up to four percent of our global revenue in the event of violations after implementation of the requirements on May 25, 2018. Internationally, some countries have also passed laws that require individually identifiable data on their citizens to be maintained on local servers and that may restrict transfer or processing of that data. We believe that we meet the expectations of applicable regulations and that the ongoing costs and impacts of ensuring compliance with such rules are not material to our business. However, there is no guarantee that we will avoid enforcement actions by governmental bodies or civil actions based on this growing body of regulations. Enforcement actions could be costly and interrupt regular operations of our business. Any of these events, in turn, may cause us to lose existing customers, have difficulty preventing, detecting and controlling fraud, have disputes with customers, physicians and other healthcare professionals, be subject to legal claims and liability, have regulatory sanctions or penalties imposed, have increases in operating expenses, incur expenses or lose revenues as a result of a data privacy breach or theft of intellectual property, or suffer other adverse consequences, any of which could have a material adverse effect on our business, financial condition or results of operations.
Pending and future intellectual property litigation could be costly and disruptive to us.
We operate in an industry that is susceptible to significant intellectual property litigation and, in recent years, it has been common for companies in the medical device field to aggressively challenge the patent rights of other companies. We are currently the subject of various patent litigation proceedings and other proceedings described in more detail under Note J – Commitments and Contingencies to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report. Intellectual property litigation is expensive, complex and lengthy and its outcome is difficult to predict. Adverse outcomes in one or more of these matters could have a material adverse effect on our ability to sell certain products and on our operating margins, financial condition, results of operation or liquidity. Pending or future patent litigation may result in significant royalty or other payments or injunctions that can prevent the sale of products and may significantly divert the attention of our technical and management personnel. In the event that our right to market any of our products is successfully challenged, we may be required to obtain a license on terms which may not be favorable to us, if at all. If we fail to obtain a required license or are unable to design around a patent, our business, financial condition or results of operations could be materially adversely affected.
Pending and future product liability claims and other litigation, including private securities litigation, stockholder derivative suits and contract litigation, may adversely affect our financial condition and results of operations or liquidity.
The design, manufacturing and marketing of medical devices of the types that we produce entail an inherent risk of product liability claims. Many of the medical devices that we manufacture and sell are designed to be implanted in the human body for long periods of time or indefinitely. A number of factors could result in an unsafe condition or injury to, or death of, a patient with respect to these or other products that we manufacture or sell, including physician technique and experience in performing the surgical procedure, component failures, manufacturing flaws, design defects, off-label use or inadequate disclosure of product-related risks or product-related information. These factors could result in product liability claims, a recall of one or more of our products or a safety alert relating to one or more of our products. Product liability claims may be brought by individuals or by groups seeking to represent a class.
30
We are currently the subject of product liability litigation proceedings and other proceedings described in more detail under Note J – Commitments and Contingencies to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report. The outcome of litigation, particularly class action lawsuits, is difficult to assess or quantify. Plaintiffs in these types of lawsuits often seek recovery of very large or indeterminate amounts, including not only actual damages, but also punitive damages. The magnitude of the potential losses relating to these lawsuits may remain unknown for substantial periods of time. In addition, the cost to defend against any future litigation may be significant. Product liability claims, securities and commercial litigation and other litigation in the future, regardless of the outcome, could have a material adverse effect on our financial condition, results of operations or liquidity. Additionally, we maintain an insurance policy providing limited coverage against securities claims and we are substantially self-insured with respect to product liability claims and fully self-insured with respect to intellectual property infringement claims. The fact that we do not maintain third-party insurance coverage for all categories of losses increases our exposure to unanticipated claims and adverse decisions and these losses could have a material adverse effect on our financial condition, results of operations or liquidity.
Any failure to meet regulatory quality standards applicable to our manufacturing and quality processes could have an adverse effect on our business, financial condition and results of operations.
As a medical device manufacturer, we are required to register our establishments and list our devices with the FDA and are subject to periodic inspection by the FDA for compliance with its Quality System Regulation requirements, which require manufacturers of medical devices to adhere to certain regulations, including testing, quality control and documentation procedures. In addition, the Federal Medical Device Reporting regulations require us to provide information to the FDA whenever there is evidence that reasonably suggests that a device may have caused or contributed to a death or serious injury or, if a malfunction were to occur, could cause or contribute to a death or serious injury. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through periodic inspections by the FDA which may result in observations on Form 483 and in some cases warning letters that require corrective action. In the European Community, we are required to maintain certain International Standards Organization (ISO) certifications in order to sell our products and must undergo periodic inspections by notified bodies to obtain and maintain these certifications. Many other countries in which we do business have requirements similar to those of the U.S. or the EU and other foreign governments or agencies may subject us to periodic inspections as well. If we, or our manufacturers, fail to adhere to quality system regulations or ISO requirements, this could delay production of our products and lead to fines, difficulties in obtaining regulatory clearances, recalls, enforcement actions, including injunctive relief or consent decrees, or other consequences, which could, in turn, have a material adverse effect on our financial condition or results of operations.
31
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our world headquarters is located in Marlborough, Massachusetts, with principal regional headquarters located in Singapore and Voisins-le-Bretonneux, France. As of December 31, 2019, our principal manufacturing and technology centers were located in Minnesota, California and Indiana within the U.S., as well as internationally in Ireland, Costa Rica, Puerto Rico, Malaysia, Brazil and Switzerland. Our products are distributed worldwide from primary customer fulfillment centers in Massachusetts, the Netherlands, and Japan. As of December 31, 2019, we maintained 16 principal manufacturing facilities, including seven in the U.S., three in Ireland, two in Costa Rica, one in Puerto Rico, one in Malaysia, one in Brazil and one in Switzerland, as well as various distribution and technology centers around the world. Many of these facilities produce and manufacture products for more than one of our divisions and include research facilities. The following is a summary of our facilities as of December 31, 2019 (in approximate square feet):
Owned (1) | Leased (2) | Total | ||||||
U.S. | 4,072,000 | 1,446,000 | 5,518,000 | |||||
International | 2,258,000 | 1,624,000 | 3,882,000 | |||||
6,330,000 | 3,070,000 | 9,400,000 | ||||||
(1) | Includes our principal manufacturing facilities in Minnesota, Ireland, Puerto Rico and one facility in Costa Rica, our manufacturing facility in Malaysia, our primary customer fulfillment centers in Massachusetts, the Netherlands and Japan, and our global headquarters location in Marlborough, Massachusetts. |
(2) | Includes our principal manufacturing facilities in California, Indiana, Brazil, Switzerland and one in Costa Rica, and our regional headquarters located in Singapore and Voisins-le-Bretonneux, France. |
ITEM 3. LEGAL PROCEEDINGS
See Note J – Commitments and Contingencies to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report and incorporated herein by reference.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
32
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is traded on the New York Stock Exchange (NYSE) under the symbol “BSX.”
Holders of Record
As of January 31, 2020, there were 7,331 holders of record of our common stock.
Dividends
We did not pay a cash dividend in 2019, 2018 or 2017 and currently we do not intend to pay cash dividends. We may consider declaring and paying a cash dividend in the future; however, there can be no assurance that we will do so.
Securities Authorized for Issuance under Equity Compensation Plans
Please see Item 12. "Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters" under Part III of this Annual Report for information on where to find information required by Item 201(d) of Regulation S-K.
Purchases of Equity Securities by the Issuer and Affiliated Purchases
On January 25, 2013, our Board of Directors approved and on January 29, 2013, we announced a program authorizing the repurchase of up to $1.000 billion of our common stock. We made no share repurchases in 2019, 2018 or 2017 and as of December 31, 2019, we had approximately $535 million remaining available under the 2013 share repurchase program . Refer to Note K – Stockholders' Equity to our consolidated financial statements contained in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional information.
33
Stock Performance Graph
The graph below compares the five-year total return to stockholders on our common stock with the return of the Standard & Poor’s (S&P) 500 Stock Index and the S&P Healthcare Equipment Index. The graph assumes $100 was invested in our common stock and in each of the named indices on December 31, 2014 and that any dividends were reinvested.
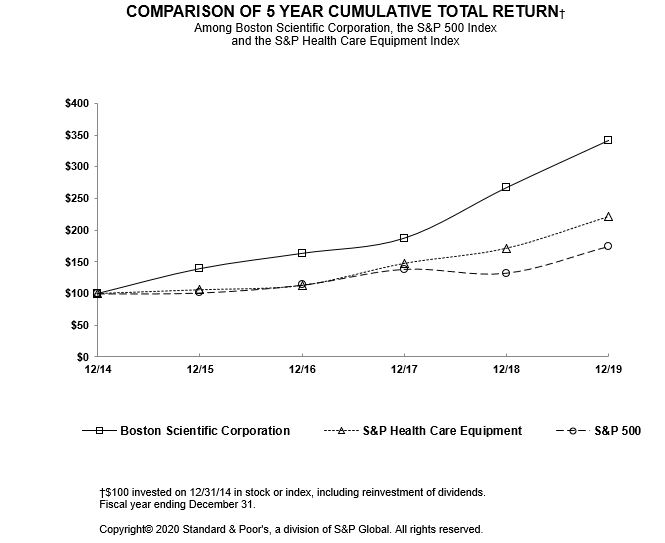
Note: The stock price performance shown on the graph above is not indicative of future price performance. This graph shall not be deemed "filed" for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, regardless of any general incorporation language in such filing.
34
ITEM 6. SELECTED FINANCIAL DATA
FIVE-YEAR SELECTED FINANCIAL DATA
(in millions, except per share data)
Operating Data
Year Ended December 31, | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||
Net sales | $ | 10,735 | $ | 9,823 | $ | 9,048 | $ | 8,386 | $ | 7,477 | |||||||||
Gross profit | 7,620 | 7,011 | 6,455 | 5,962 | 5,304 | ||||||||||||||
Total operating expenses | 6,102 | 5,504 | 5,170 | 5,515 | 5,587 | ||||||||||||||
Operating income (loss) | 1,518 | 1,506 | 1,285 | 447 | (283 | ) | |||||||||||||
Income (loss) before income taxes | 687 | 1,422 | 933 | 177 | (650 | ) | |||||||||||||
Net income (loss) | 4,700 | 1,671 | 104 | 347 | (239 | ) | |||||||||||||
Net income (loss) per common share: | |||||||||||||||||||
Basic | $ | 3.38 | $ | 1.21 | $ | 0.08 | $ | 0.26 | $ | (0.18 | ) | ||||||||
Assuming dilution | $ | 3.33 | $ | 1.19 | $ | 0.08 | $ | 0.25 | $ | (0.18 | ) | ||||||||
Balance Sheet Data
As of December 31, | 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||
Cash, cash equivalents and marketable securities | $ | 217 | $ | 146 | $ | 188 | $ | 196 | $ | 319 | |||||||||
Working capital (deficit) | (168 | ) | (1,257 | ) | (1,832 | ) | (348 | ) | 1,041 | ||||||||||
Total assets | 30,565 | 20,999 | 19,042 | 18,096 | 18,133 | ||||||||||||||
Borrowings (short-term) | 1,416 | 2,253 | 1,801 | 64 | 3 | ||||||||||||||
Borrowings (long-term) | 8,592 | 4,803 | 3,815 | 5,420 | 5,674 | ||||||||||||||
Stockholders’ equity | 13,877 | 8,726 | 7,012 | 6,733 | 6,320 | ||||||||||||||
Book value per common share † | $ | 9.95 | $ | 6.30 | $ | 5.11 | $ | 4.94 | $ | 4.69 | |||||||||
† | Book value per common share is calculated using shares outstanding as of December 31, for each year, respectively shown. |
The data above should be read in conjunction with our consolidated financial statements, including the notes thereto, included in Item 8. Financial Statements and Supplementary Data of our Annual Report on Form 10-K.
35
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis provides information management believes to be relevant to understanding the financial condition and results of operations of Boston Scientific Corporation and its subsidiaries. For a full understanding of our financial condition and results of operations, this discussion should be read in conjunction with our consolidated financial statements and accompanying notes included in Item 8. Financial Statements and Supplementary Data of this Annual Report.
For additional information on our financial condition and results of operations for 2017, refer to our Annual Report on Form 10-K for the year ended December 31, 2018.
During the third quarter of 2019, we completed the acquisition of BTG plc (BTG) which was composed of three key businesses, the largest of which was its interventional medicine (Interventional Medicine) that encompasses interventional oncology therapeutic technologies for patients with liver and kidney cancers, as well as a vascular portfolio for treatment of deep vein thrombosis, pulmonary embolism, deep venous obstruction and superficial venous disease. Following the closing of the acquisition, the Interventional Medicine business was integrated into our Peripheral Interventions division. For additional information, refer to Note B – Acquisitions and Strategic Investments.
Executive Summary
Financial Highlights and Trends
In 2019, we generated net sales of $10.735 billion, as compared to $9.823 billion in 2018. This increase of $912 million, or 9.3 percent, included operational growth of 11.1 percent and the negative impact of 180 basis points from foreign currency fluctuations. Operational net sales1 included $378 million in 2019 due to the acquisitions of NxThera, Inc. (NxThera) in the second quarter of 2018, Claret Medical, Inc. (Claret) in the third quarter of 2018, Augmenix, Inc. (Augmenix) in the fourth quarter of 2018, Vertiflex, Inc. (Vertiflex) in the second quarter of 2019, and BTG in the third quarter of 2019, each with less than a full year of prior period related net sales. Refer to the Business and Market Overview section for further discussion of our net sales by global business.
Our reported net income in 2019 was $4.700 billion, or $3.33 per diluted share. Our reported results for 2019 included certain charges and/or credits totaling $2.466 billion (after-tax), or $1.75 per diluted share. These adjustments are excluded from results reviewed by management in order to analyze the underlying trends in our business, assess our performance period over period, and make operating decisions. Excluding these items, adjusted net income1 for 2019 was $2.234 billion, or $1.58 per diluted share.
Our reported net income in 2018 was $1.671 billion, or $1.19 per diluted share. Our reported results for 2018 included certain charges and/or credits totaling $389 million (after-tax), or $0.28 per diluted share. Excluding these items, adjusted net income for 2018 was $2.060 billion, or $1.47 per diluted share.
1 Operational net sales growth rates, which exclude the impact of foreign currency fluctuations and adjusted net income and adjusted net income per share, which exclude certain items required by generally accepted accounting principles in the United States (U.S. GAAP), are not prepared in accordance with U.S. GAAP and should not be considered in isolation from, or as a replacement for, the most directly comparable GAAP measure. Refer to Additional Information for a discussion of management’s use of these non-GAAP financial measures.
36
The following is a reconciliation of our results of operations prepared in accordance with U.S. GAAP to those adjusted results considered by management. Refer to Results of Operations for a discussion of each reconciling item:
Year Ended December 31, 2019 | |||||||
(in millions, except per share data) | Net Income (Loss) | Impact per Share | |||||
GAAP net income (loss) | $ | 4,700 | $ | 3.33 | |||
Non-GAAP adjustments: | |||||||
Amortization expense | 628 | 0.44 | |||||
Intangible asset impairment charges | 102 | 0.07 | |||||
Acquisition/divestiture-related net charges (credits) | 672 | 0.48 | |||||
Restructuring and restructuring-related net charges (credits) | 68 | 0.05 | |||||
Litigation-related net charges (credits) | 72 | 0.05 | |||||
Investment impairment charges | 3 | 0.00 | |||||
EU MDR implementation charges | 5 | 0.00 | |||||
Debt extinguishment net charges (credits) | 67 | 0.05 | |||||
Deferred tax expenses (benefits) | (4,102 | ) | (2.91 | ) | |||
Discrete tax items | 18 | 0.01 | |||||
Adjusted net income | $ | 2,234 | $ | 1.58 | |||
Year Ended December 31, 2018 | |||||||
(in millions, except per share data) | Net Income (Loss) | Impact per Share | |||||
GAAP net income (loss) | $ | 1,671 | $ | 1.19 | |||
Non-GAAP adjustments: | |||||||
Amortization expense | 520 | 0.37 | |||||
Intangible asset impairment charges | 31 | 0.02 | |||||
Acquisition-related net charges (credits) | 5 | 0.00 | |||||
Restructuring and restructuring-related net charges (credits) | 77 | 0.05 | |||||
Litigation-related net charges (credits) | 79 | 0.06 | |||||
Investment impairment charges | 6 | 0.00 | |||||
Discrete tax items | (328 | ) | (0.23 | ) | |||
Adjusted net income | $ | 2,060 | $ | 1.47 | |||
Cash provided by operating activities was $1.836 billion in 2019. As of December 31, 2019, we had total debt of $10.008 billion, Cash and cash equivalents of $217 million and a working capital deficit of $168 million. Refer to Liquidity and Capital Resources for further information.
37
Business and Market Overview
The following section describes an overview of our product offerings and results of operations by business unit. For additional information on our businesses and their product offerings, see Item 1. Business of this Annual Report.
Our seven core businesses are organized into three reportable segments: MedSurg, Rhythm and Neuro, and Cardiovascular. Following our acquisition of BTG, which closed during the third quarter of 2019, we have included BTG’s Interventional Medicine business within our Peripheral Interventions operating segment, within the Cardiovascular reportable segment. We present BTG’s Specialty Pharmaceuticals business as a standalone operating segment alongside our reportable segments.
MedSurg
Endoscopy
Our Endoscopy business develops and manufactures devices to diagnose and treat a broad range of gastrointestinal (GI) and pulmonary conditions with innovative, less invasive technologies.
Our net sales of Endoscopy products of $1.894 billion represented 18 percent of our consolidated net sales in 2019. Our Endoscopy net sales increased $132 million, or 7.5 percent, in 2019, as compared to 2018. This increase included operational net sales growth of 9.2 percent and the negative impact of 170 basis points from foreign currency fluctuations, as compared to 2018. This year-over-year increase was primarily driven by growth in our pancreaticobiliary franchise with both our SpyGlass™ DS II Direct Visualization System, AXIOS™ Stent and Electrocautery Enhanced Delivery System, our core GI franchise featuring our Resolution 360™ Clip, disposable snares and Endoluminal Surgery products, and our infection prevention products.
Urology and Pelvic Health
Our Urology and Pelvic Health business develops and manufactures devices to treat various urological and pelvic conditions for both male and female anatomies.
Our net sales of Urology and Pelvic Health products of $1.413 billion represented 13 percent of our consolidated net sales in 2019. Urology and Pelvic Health net sales increased $167 million, or 13.4 percent, in 2019, as compared to 2018. This increase included operational net sales growth of 14.7 percent and the negative impact of 130 basis points from foreign currency fluctuations, as compared to 2018. This year-over-year increase was primarily attributable to growth in sales of our prostate health product family, including the SpaceOAR™ Hydrogel System purchased as part of the acquisition of Augmenix in the fourth quarter of 2018 and the Rezûm™ System purchased as part of the acquisition of NxThera in the second quarter of 2018, as well as our stone franchise, including our LithoVue™ Digital Flexible Ureteroscope.
Rhythm and Neuro
Cardiac Rhythm Management
Our Cardiac Rhythm Management (CRM) business develops and manufactures a variety of implantable devices that monitor the heart and deliver electricity to treat cardiac abnormalities.
Our net sales of CRM products of $1.939 billion represented 18 percent of our consolidated net sales in 2019. Our net sales of CRM products decreased $12 million, or 0.6 percent, in 2019, as compared to 2018. This decrease included operational net sales growth of 1.2 percent and the negative impact of 180 basis points from foreign currency fluctuations, as compared to 2018. This year-over-year increase in operational net sales was driven by share gains in our high voltage franchise. Our high voltage performance was driven by the strength of our broad high voltage portfolio including the RESONATE™ family of cardiac resynchronization therapy defibrillator (CRT-D) and implantable cardiac defibrillator's (ICD) with HeartLogic™, our EMBLEM™ magnetic resonance imaging (MRI) subcutaneous implantable cardiac defibrillator (S-ICD), and high voltage replacement device growth. This strength in our high voltage pacemaker franchise was partially offset by declines in our low voltage pacemaker franchise primarily due to U.S. pacemaker share loss.
Electrophysiology
Our Electrophysiology business develops and manufactures less-invasive medical technologies used in the diagnosis and treatment of rate and rhythm disorders of the heart.
38
Our net sales of Electrophysiology products of $329 million represented three percent of our consolidated net sales in 2019. Our Electrophysiology net sales increased $17 million, or 5.5 percent, in 2019, as compared to 2018. This increase included operational net sales growth of 7.5 percent and the negative impact of 200 basis points from foreign currency fluctuations, as compared to 2018. This year-over-year increase was primarily driven by strong growth across our global Rhythmia™ Mapping and Navigation Products, partially offset by declines in our core diagnostic and therapeutic devices due to relatively slower end markets and share loss in select product categories. Our Rhythmia Mapping System and navigation portfolio growth was driven by the continued account expansion of our global system footprint and commercialization of our IntellaNav MiFi™ Open-Irrigated catheter and DIRECTSENSE™ Software in approved markets.
Neuromodulation
Our Neuromodulation business develops and manufactures devices to treat various neurological movement disorders and manage chronic pain.
Our net sales of Neuromodulation products of $873 million represented eight percent of our consolidated net sales in 2019. Neuromodulation net sales increased $94 million, or 12.0 percent, in 2019, as compared to 2018. This increase included operational net sales growth of 13.1 percent and the negative impact of 110 basis points from foreign currency fluctuations, as compared to 2018. This year-over-year increase was primarily driven by strong performance of our deep brain stimulation (DBS) systems, sales of our Superion™ Indirect Decompression System following the acquisition of Vertiflex in the second quarter of 2019, and sales in international markets; partially offset by declines in our U.S. Spinal Cord Stimulator (SCS) Systems portfolio due to market contraction.
Cardiovascular
Interventional Cardiology
Our Interventional Cardiology business develops, manufactures and commercializes technologies for diagnosing and treating coronary artery disease and other cardiovascular disorders including structural heart conditions.
Our net sales of Interventional Cardiology products of $2.816 billion represented 26 percent of our consolidated net sales in 2019. Our Interventional Cardiology net sales increased $226 million, or 8.7 percent, in 2019, as compared to 2018. This increase included operational net sales growth of 11.0 percent and the negative impact of 230 basis points from foreign currency fluctuations, as compared to 2018. This year-over-year increase was primarily related to growth in our structural heart therapies including our Watchman™ LAAC Device, our TAVR products including our ACURATE™ Neo Valve outside the U.S. and LOTUS™ Edge Valve as well as our Sentinel™ Cerebral Embolic Protection System. Our year-over-year sales increase was also attributable to our Complex PCI and PCI Guidance product offerings, partially offset by declines in sales of drug eluting stent product offerings.
Peripheral Interventions
Our Peripheral Interventions business develops and manufactures products to diagnose and treat peripheral arterial and venous diseases, as well as products to diagnose, treat and ease various forms of cancer. In the third quarter of 2019, we completed the acquisition of BTG, and began integrating BTG's Interventional Medicine (IM) portfolio into the Peripheral Interventions division, adding complementary technologies in the areas of venous disease and interventional oncology.
Our net sales of Peripheral Interventions products of $1.392 billion represented 13 percent of our consolidated net sales in 2019, and included $144 million of sales from BTG products following the date of acquisition. Our Peripheral Interventions net sales increased $205 million, or 17.3 percent, in 2019, as compared to 2018, and $61 million or 5.1 percent excluding BTG. This increase included operational net sales growth of 19.1 percent (7.8 percent excluding BTG and the related divestiture of our drug-eluting and bland embolic microsphere portfolio) and the negative impact of 180 basis points from foreign currency fluctuations, as compared to 2018. This year-over-year increase was primarily driven by global growth of the Eluvia™ Drug Eluting Vascular Stent System, which was launched in the U.S. in the fourth quarter of 2018 and Japan in the first quarter of 2019.
39
Specialty Pharmaceuticals
Following the closing of the BTG acquisition in the third quarter of 2019, Specialty Pharmaceuticals was added as an eighth operating segment. Our Specialty Pharmaceuticals business develops and manufactures acute care antidotes to treat overexposure to certain medications and toxins. These products are sold primarily in the U.S. through small, specialist sales teams and through commercial partners elsewhere, where approved or permitted, on a named patient basis.
Our net sales of Specialty Pharmaceuticals products of $81 million following the date of acquisition represented less than one percent of our consolidated net sales in 2019.
Emerging Markets
As part of our strategic imperatives to drive global expansion, described in Item 1. Business of this Annual Report, we are seeking to grow net sales and market share by expanding our global presence, including in Emerging Markets. We define Emerging Markets as the 20 countries that we believe have strong growth potential based on their economic conditions, healthcare sectors and our global capabilities. We have increased our investment in infrastructure in these countries in order to maximize opportunities. Periodically, we assess our list of Emerging Markets; effective January 1, 2019, we updated our list of Emerging Market countries. Our current list is comprised of the following countries: Argentina, Brazil, Chile, China, Colombia, Czech Republic, India, Indonesia, Malaysia, Mexico, Philippines, Poland, Russia, Saudi Arabia, Slovakia, South Africa, South Korea, Thailand, Turkey and Vietnam. The revision had an immaterial impact on prior year Emerging Markets sales. Our Emerging Markets net sales of Medical Devices represented 12 percent of our consolidated net sales in 2019 and 11 percent in 2018. In 2019, our Emerging Markets net sales grew 14.1 percent on a reported basis including operational net sales growth of 19.5 percent and the negative impact of 540 basis points from foreign currency fluctuations, as compared to 2018. Our future net sales in Emerging Markets may be negatively impacted by geopolitical and economic instability and a number of other factors, including the impact to our net sales in China from the evolving coronavirus situation.
40
Results of Operations
Net Sales
The following table provides our net sales by business and the relative change in growth on a reported basis:
Year Ended December 31, | 2019 versus 2018 | 2018 versus 2017 | |||||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||||||
Endoscopy | $ | 1,894 | $ | 1,762 | $ | 1,619 | 7.5% | 8.8% | |||||||
Urology and Pelvic Health | 1,413 | 1,245 | 1,124 | 13.4% | 10.8% | ||||||||||
MedSurg | 3,307 | 3,007 | 2,742 | 10.0% | 9.7% | ||||||||||
Cardiac Rhythm Management | 1,939 | 1,951 | 1,895 | (0.6)% | 2.9% | ||||||||||
Electrophysiology | 329 | 311 | 278 | 5.5% | 12.1% | ||||||||||
Neuromodulation | 873 | 779 | 635 | 12.0% | 22.7% | ||||||||||
Rhythm and Neuro | 3,140 | 3,041 | 2,808 | 3.3% | 8.3% | ||||||||||
Interventional Cardiology | 2,816 | 2,590 | 2,419 | 8.7% | 7.1% | ||||||||||
Peripheral Interventions | 1,392 | 1,187 | 1,081 | 17.3% | 9.8% | ||||||||||
Cardiovascular | 4,208 | 3,777 | 3,500 | 11.4% | 7.9% | ||||||||||
Medical Devices(1) | 10,654 | 9,823 | 9,048 | 8.5% | 8.6% | ||||||||||
Specialty Pharmaceuticals | 81 | n/a | n/a | n/a | n/a | ||||||||||
Net Sales | $ | 10,735 | $ | 9,823 | $ | 9,048 | 9.3% | 8.6% | |||||||
(1) | We have three historical reportable segments comprised of Medical Surgical (MedSurg), Rhythm and Neuro, and Cardiovascular, which represent an aggregation of our operating segments that generate revenues from the sale of medical devices (Medical Devices). As part of our acquisition of BTG on August 19, 2019, we acquired an Interventional Medicine business, which is now included in our Peripheral Interventions operating segment's 2019 revenues from the date of acquisition. |
Refer to Executive Summary for further discussion of our net sales and a comparison of our 2019 and 2018 net sales.
In 2018, we generated net sales of $9.823 billion, as compared to $9.048 billion in 2017. This increase of $775 million, or 8.6 percent, included operational growth of 8.0 percent and the positive impact of 60 basis points from foreign currency fluctuations. Operational net sales included approximately $78 million in 2018 due to the acquisitions of Symetis SA (Symetis) in the second quarter of 2017, NxThera, Inc. (NxThera) in the second quarter of 2018, Claret Medical, Inc. (Claret) in the third quarter of 2018 and Augmenix, Inc. (Augmenix) in the fourth quarter of 2018, each with no prior period related net sales.
41
Gross Profit
Our gross profit was $7.620 billion in 2019 and $7.011 billion in 2018. As a percentage of net sales, our gross profit decreased to 71.0 percent in 2019, as compared to 71.4 percent in 2018. The following is a rollforward of our gross profit margins and a description of the drivers of the change from period to period:
Gross Profit Margin | |
Year Ended December 31, 2017 | 71.3% |
Manufacturing cost reductions | 0.8% |
Sales pricing and mix | (0.2)% |
Inventory step-up due to acquisition accounting | (0.1)% |
Net impact of foreign currency fluctuations | (0.8)% |
All other, including other inventory charges and other period expense | 0.4% |
Year Ended December 31, 2018 | 71.4% |
Manufacturing cost reductions | 0.8% |
Sales pricing and mix | (0.6)% |
Inventory step-up due to acquisition accounting | (0.4)% |
Net impact of foreign currency fluctuations | 0.7% |
All other, including other inventory charges and other period expense | (0.8)% |
Year Ended December 31, 2019 | 71.0% |
The primary factors contributing to the decrease in our gross profit margin for 2019 as compared to 2018 were the negative impacts of pricing declines related primarily to sales of our coronary drug-eluting stent products, as well as increased levels of scrap associated with recently launched products and excess and obsolete inventory. In addition, in connection with our recent acquisitions, we adjusted acquired inventory from manufacturing cost to fair value. The step-up in value is amortized through gross profit over an average estimated inventory turnover period. In 2019, we recorded increased cost of $46 million associated with these step-ups. This was partially offset by manufacturing cost reductions driven by our process improvement programs as well as favorable foreign currency fluctuations.
The primary factors contributing to the increase in our gross profit margin for 2018 as compared to 2017 were the positive impacts of cost reductions resulting from our process improvement programs and restructuring programs and favorable period expense, partially offset by negative impacts from foreign currency fluctuations.
EU MDR Implementation Charges
The European Union Medical Device Regulation (EU MDR) is a replacement of the existing European Medical Devices Directive (MDD) regulatory framework, and manufacturers of medical devices are required to comply with EU MDR beginning in May 2020 for new product registrations and by May 2024 for medical devices which have a valid CE Certificate to the current Directives (issued before May 2020).
We consider the adoption of EU MDR to be a significant change to a regulatory framework, and therefore, the incremental costs specific to complying with EU MDR for previously registered products are not considered to be ordinary course expenditures in connection with regulatory matters. As such, these medical device regulation charges are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. We expect to incur expenditures of approximately $150 million over the next three years associated with the implementation of EU MDR, which will be recorded primarily within Cost of products sold.
42
Operating Expenses
The following table provides a summary of certain of our operating expenses:
Year Ended December 31, | |||||||||||||||||
2019 | 2018 | 2017 | |||||||||||||||
(in millions) | $ | % of Net Sales | $ | % of Net Sales | $ | % of Net Sales | |||||||||||
Selling, general and administrative expenses | $ | 3,941 | 36.7 | % | $ | 3,569 | 36.3 | % | $ | 3,294 | 36.4 | % | |||||
Research and development expenses | 1,174 | 10.9 | % | 1,113 | 11.3 | % | 997 | 11.0 | % | ||||||||
Royalty expense | 65 | 0.6 | % | 70 | 0.7 | % | 68 | 0.8 | % | ||||||||
Selling, General and Administrative (SG&A) Expenses
In 2019, our SG&A expenses increased $371 million, or 10 percent, as compared to 2018 and were 40 basis points higher as a percentage of net sales. This increase in SG&A expenses as a percentage of net sales was primarily due to acquisition-related charges primarily associated with our acquisition and integration of BTG, partially offset by savings from ongoing cost optimization initiatives. These increased SG&A expenses were also partially offset by a $25 million net gain recorded in the first quarter primarily associated with a portion of the Edwards litigation settlement. For further details regarding the presentation of the Edwards litigation settlement see Litigation-related net charges (credits) below.
In 2018, our SG&A expenses increased $275 million, or eight percent, as compared to 2017 and were 10 basis points lower as a percentage of net sales. This decrease in SG&A expenses as a percentage of net sales was primarily due to leverage from increased sales, as well as the benefit of our targeted initiatives focused on reducing SG&A expenses such as end-to-end business process streamlining and automation, including functional expansion of global shared service and robotic process utilization.
Research and Development (R&D) Expenses
We remain committed to advancing medical technologies and investing in meaningful research and development projects across our businesses. In 2019, our R&D expenses increased $61 million, or six percent, as compared to 2018, and were 40 basis points lower as a percentage of net sales. In 2018, our R&D expenses increased $116 million, or 12 percent, as compared to 2017, and were 30 basis points higher as a percentage of sales. R&D expenses increased each year as a result of investments across our businesses in order to maintain a pipeline of new products that we believe will contribute to profitable sales growth.
Royalty Expense
In 2019, our Royalty expense decreased $5 million, or seven percent, as compared to 2018 and was 10 basis points lower as a percentage of net sales. The decrease in Royalty expense in 2019, as compared to 2018, relates primarily to contractual reductions in royalty rates associated with certain products.
In 2018, our Royalty expense increased $2 million, or three percent, as compared to 2017 and was 10 basis points lower as a percentage of net sales. The increase in Royalty expense in 2018 as compared to 2017 relates primarily to increased sales partially offset by expired royalties in certain countries.
The following table provides a summary of certain of our other operating expenses, which are excluded by management for purposes of evaluating operating performance, refer to Additional Information for a further description of certain operating expenses:
Year Ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Amortization expense | $ | 699 | $ | 599 | $ | 565 | |||||
Intangible asset impairment charges | 105 | 35 | 4 | ||||||||
Contingent consideration expense (benefit) | (35 | ) | (21 | ) | (80 | ) | |||||
Restructuring charges (credits) | 38 | 36 | 37 | ||||||||
Litigation-related net charges (credits) | 115 | 103 | 285 | ||||||||
43
Amortization Expense
In 2019, our Amortization expense increased $101 million, or 17 percent, as compared to 2018. In 2018, our Amortization expense increased $33 million, or six percent, as compared to 2017. The increases in each period were primarily due to an increase in the balance of amortizable intangible assets as a result of recent acquisitions.
Intangible Asset Impairment Charges
In 2019, our Intangible asset impairment charges were $105 million, primarily associated with technology-related amortizable intangible assets. Refer to Critical Accounting Estimates for a discussion of key assumptions used in our goodwill and intangible asset impairment testing and future events that could have a negative impact on the recoverability of our goodwill and intangible assets.
Contingent Consideration Expense (Benefit)
In 2019, 2018, and 2017, we recorded net benefits related to the change in fair value of our contingent consideration liability. Refer to Note B – Acquisitions and Strategic Investments to our consolidated financial statements contained in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional details related to our contingent consideration arrangements.
Restructuring Charges (Credits)
In June 2016, our Board of Directors approved, and we committed to a restructuring initiative (the 2016 Restructuring Plan), which was initiated in the second quarter of 2016 and substantially completed in 2019. The 2016 Restructuring Plan resulted in total pre-tax charges of $271 million and approximately $255 million in cash outlays.
In addition, in November 2018, our Board of Directors approved, and we committed to, a new global restructuring program (the 2019 Restructuring Plan). The 2019 Restructuring Plan is expected to result in total pre-tax charges of approximately $200 million to $300 million and approximately $180 million to $280 million of these charges are expected to result in cash outlays. A substantial portion of the savings are being reinvested in strategic growth initiatives.
Restructuring charges pursuant to these programs were $38 million in 2019, $36 million in 2018, and $37 million in 2017. See Note G – Restructuring-related Activities to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional details on our restructuring plans.
Litigation-related Net Charges (Credits)
In 2019, our litigation-related net charges included a net charge of $223 million in the fourth quarter of 2019, primarily related to litigation with Channel Medsystems, Inc., net charges of $25 million in the third quarter of 2019 and $15 million in the second quarter of 2019, primarily related to transvaginal surgical mesh product liability litigation, and a gain of $148 million recorded in the first quarter of 2019, which represents a portion of the total $180 million one-time settlement payment received from Edwards Lifesciences Corporation (Edwards) in January 2019. We record certain legal and product liability charges, credits and costs of defense, which we consider to be unusual or infrequent and significant as Litigation-related net charges (credits) in our consolidated financial statements. All other legal and product liability charges, credits and costs are recorded within SG&A expenses. As such, a portion of the related gain from the Edwards settlement was recorded in SG&A expenses on our consolidated statements of operations.
In 2018 and 2017, our litigation-related net charges were primarily in connection with transvaginal surgical mesh product liability cases and claims.
We continue to assess certain litigation and claims to determine the amounts, if any, that management believes will be paid as a result of such claims and litigation, and therefore, additional losses may be accrued and paid in the future, which could materially adversely impact our operating results, cash flows and/or our ability to comply with our debt covenants. Refer to Note J – Commitments and Contingencies to our consolidated financial statements contained in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional discussion of our material legal proceedings.
44
Interest Expense
The following table provides a summary of our Interest expense and average borrowing rate:
(in millions) | Year Ended December 31, | ||||||||||
2019 | 2018 | 2017 | |||||||||
Interest expense | $ | (473 | ) | $ | (241 | ) | $ | (229 | ) | ||
Weighted average borrowing rate | 4.8 | % | 3.6 | % | 3.8 | % | |||||
Interest expense increased in 2019, as compared to 2018, primarily due to the increase in our average debt balance following the February 2019 senior notes offering as well as the Euro bond offering in November 2019. A portion of the proceeds from the February 2019 senior notes offering were used to finance our acquisition of BTG. The net proceeds from our November 2019 senior notes offering were used to repay certain outstanding principal amounts of our senior notes and pay accrued and unpaid interest, premiums, and fees. In addition, Interest expense in 2019 included debt extinguishment charges following our 2019 senior notes offerings and subsequent repayment of existing senior notes and termination of the Bridge Facility.
Refer to Liquidity and Capital Resources in this Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations and Note D – Hedging Activities and Fair Value Measurements and Note E – Contractual Obligations and Commitments to our consolidated financial statements contained in Item 8. Financial Statements and Supplementary Data of this Annual Report for information regarding our debt obligations.
Other, net
The following are the components of Other, net:
Year Ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Interest income | $ | 30 | $ | 3 | $ | 5 | |||||
Net foreign currency gain (loss) | (358 | ) | 11 | (15 | ) | ||||||
Net gains (losses) on investments | (30 | ) | 155 | (92 | ) | ||||||
Other income (expense), net | (1 | ) | (14 | ) | (22 | ) | |||||
$ | (358 | ) | $ | 156 | $ | (124 | ) | ||||
Certain of our non-designated forward currency contracts were entered into for the purpose of managing our exposure to currency exchange rate risk related to the purchase price of our acquisition of BTG. In 2019, we settled all outstanding contracts, and we recognized a $323 million loss in Other, net due to changes in fair value of the contracts. These amounts are included in Acquisition/divestiture-related net charges (credits) presented in the reconciliation of our results of operations prepared in accordance with U.S. GAAP to those adjusted results considered by management. Refer to Financial Summary for the reconciliation and Additional Information for a discussion of management's use of non-GAAP financial measures.
In 2018, we recorded gains of $184 million based on the difference between the book values and the fair values of our previously-held investments immediately prior to the acquisition dates, which aggregated to $251 million. We remeasured the fair value of each previously-held investment based on the implied enterprise value and allocation of purchase price consideration according to priority of equity interests. Gains and losses recorded on previously-held investments are excluded by management for purposes of evaluating operating performance. Refer to Note B – Acquisitions and Strategic Investments to our consolidated financial statements contained in Item 8. Financial Statements and Supplementary Data of this Annual Report for information regarding our strategic investments.
45
Tax Rate
The following table provides a summary of our reported tax rate:
Year Ended December 31, | ||||||||
2019 | 2018 | 2017 | ||||||
Reported tax rate | (584.0 | )% | (17.5 | )% | 88.8 | % | ||
Impact of certain receipts/charges (1) | 594.2 | % | 30.7 | % | (75.8 | )% | ||
10.2 | % | 13.2 | % | 13.0 | % | |||
(1) | These receipts/charges are taxed at different rates than our effective tax rate. |
The change in our reported tax rate for 2019, as compared to 2018, relates primarily to the deferred tax benefit of intra-entity transfers of intellectual property rights partially offset by increased current tax expense related to the U.S. taxation of current foreign earnings.
The change in our reported tax rate for 2018, as compared to 2017, relates primarily to the impact of certain receipts and charges that are taxed at different rates than our effective tax rate. These receipts and charges included intangible asset impairment charges, acquisition-related items, restructuring and restructuring-related items, litigation-related items, as well as certain discrete tax items. Included in the discrete tax items were the effective settlement of our transfer pricing dispute with the Internal Revenue Service (IRS) for the 2001 through 2010 tax years, the conclusion of the IRS examinations of our 2011 through 2013 tax years, and the final impact of the Tax Cuts and Jobs Act (TCJA), enacted on December 22, 2017.
`
In the second quarter of 2018, a decision was entered by the U.S. Tax Court resolving all disputes for Guidant Corporation for its 2001 through 2006 tax years and our 2006 and 2007 tax years as well as the tax issues related to our 2006 transaction with Abbott Laboratories. Additionally, we resolved all issues with the IRS Office of Appeals for our 2008 through 2010 tax years. The final settlement calculation resulted in a final net tax payment of $303 million plus $307 million of estimated interest, which was remitted in the second quarter of 2018. Due to the final settlement of these disputes, we recorded a net tax benefit of $250 million in 2018 to remove a reserve related to these years.
In the fourth quarter of 2018, we received a Revenue Agent Report (RAR) from the IRS for our 2011 through 2013 tax years. We remitted $93 million to the IRS in the fourth quarter of 2018 reflecting the net balance of tax and interest due for these years after consideration of amounts owed to us by the IRS. Due to the resolution of these tax years, we recorded a net tax benefit of $90 million to remove a reserve related to these years.
See Note I – Income Taxes to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional details on our tax rate.
Liquidity and Capital Resources
Based on our current business plan, we believe our existing balance of Cash and cash equivalents, future cash generated from operations, access to capital markets and existing credit facilities will be sufficient to fund our operations, invest in our infrastructure, pay our legal-related liabilities, pay taxes due, fund possible mergers and/or acquisitions and service and repay our existing debt. Please refer to Contractual Obligations and Commitments below for additional details on our future payment obligations and commitments.
As of December 31, 2019, we had $217 million of unrestricted Cash and cash equivalents on hand, comprised of $50 million invested in money market and government funds and $165 million in interest bearing and non-interest-bearing bank accounts. We invest excess cash on hand in short-term financial instruments that earn at market interest rates while mitigating principal risk through instrument and counterparty diversification, as well as what we believe to be prudent instrument selection. We limit our direct exposure to securities in any one industry or issuer. We also have access to our $2.750 billion commercial paper program, which is backed by our 2018 revolving credit facility entered into on December 19, 2018. As of December 31, 2019, we had $711 million in commercial paper debt outstanding resulting in an additional $2.039 billion of available liquidity.
In the fourth quarter of 2019, we entered into a $700 million term loan credit agreement scheduled to mature on December 3, 2020 (2020 Term Loan). As of December 31, 2019, we had the full amount outstanding under the 2020 Term Loan, which is presented within Current debt obligations on our consolidated balance sheet. In the first quarter of 2020, we repaid $300 million of the outstanding balance of the 2020 Term Loan.
46
For the purpose of funding our acquisition of BTG, in the fourth quarter of 2018, we entered into a $1.000 billion two-year delayed draw term loan credit facility, maturing in two years from the date of the closing of our acquisition of BTG (Two-Year Delayed Draw Term Loan) and a $1.000 billion three-year delayed draw term loan credit facility, maturing in three years from the date of the closing of our acquisition of BTG (Three-Year Delayed Draw Term Loan). In 2019, we used the proceeds from the Two-Year and Three-Year Delayed Draw Term Loan facilities to refinance the Bridge Facility, as described below, and fund a portion of our acquisition of BTG. In the fourth quarter of 2019, we repaid $200 million of the Two-Year Delayed Draw Term Loan with proceeds from the sale of the Zytiga-related royalty interests obtained through the acquisition of BTG and extinguished the facility, and we repaid the remaining $800 million of the $1.000 billion with proceeds from the 2020 Term Loan and commercial paper and terminated the Two-Year Delayed Draw Term Loan. As of December 31, 2018, we had no amounts borrowed under the Two-Year Delayed Draw Term Loan or the Three-Year Delayed Draw Term Loan. As of December 31, 2019, we had $1.000 billion outstanding under the Three-Year Delayed Draw Term Loan, which is presented within Long-term debt on our condensed consolidated balance sheet.
In the fourth quarter of 2019, we completed an offering of €900 million in aggregate principal amount of 0.625% senior notes due in 2027. The Euro-denominated debt principal is a nonderivative instrument designated as a net investment hedge of our net investments in certain of our Euro functional entities. We used a portion of the net proceeds from our senior notes offering to repay certain outstanding principal amounts of our senior notes including $206 million of our $450 million 4.125% senior notes due 2023, $566 million of our $1.000 billion 4.000% senior notes due 2028 and $227 million of our $750 million 3.850% senior notes due 2025 and pay accrued and unpaid interest, premiums, fees and expenses in connection with the transaction.
In the first quarter of 2019, we completed an offering of $4.300 billion in aggregate principal amount of senior notes. We used a portion of the net proceeds from the offering to repay the $850 million plus accrued interest and premium of our 6.000% senior notes due in January 2020 (January 2020 Notes), the $600 million plus accrued interest and premium of our 2.850% senior notes due in May 2020 (May 2020 Notes) and the $1.000 billion plus accrued interest of our August 2019 Term Loan. In 2019, the remaining proceeds were used to finance a portion of our acquisition of BTG.
In the first quarter of 2019, upon the closing of our senior notes offering in aggregate principal amount of $4.300 billion described above, we terminated the Bridge Facility entered into on November 20, 2018. The termination was pursuant to the terms of the Bridge Facility, which required full termination upon the refinancing of the January 2020 Notes and May 2020 Notes discussed above. There were no amounts borrowed under the Bridge Facility as of December 31, 2018.
For additional information on our credit facilities, refer to Note E – Contractual Obligations and Commitments to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report.
47
The following provides a summary and description of our net cash inflows (outflows) and adjusted free cash flow:
Year Ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Cash provided by (used for) operating activities | $ | 1,836 | $ | 310 | $ | 1,426 | |||||
Cash provided by (used for) investing activities | (5,041 | ) | (1,921 | ) | (1,010 | ) | |||||
Cash provided by (used for) financing activities | 2,973 | 1,432 | 110 | ||||||||
Cash provided by (used for) operating activities | $ | 1,836 | $ | 310 | $ | 1,426 | |||||
Less: Purchases of property, plant and equipment | 461 | 316 | 319 | ||||||||
Add: Proceed on disposals of property, plant and equipment | 7 | 14 | — | ||||||||
Free cash flow | 1,382 | 8 | 1,107 | ||||||||
Add: Restructuring and restructuring-related payments | 66 | 89 | 72 | ||||||||
Add: Acquisitions-related payments | 266 | 205 | 95 | ||||||||
Add: EU MDR payments | 4 | — | — | ||||||||
Add: Certain discrete tax payments (refunds/credits) | (42 | ) | 977 | (239 | ) | ||||||
Add: Litigation-related settlements | 330 | 791 | 694 | ||||||||
Adjusted free cash flow2 | $ | 2,007 | $ | 2,070 | $ | 1,729 | |||||
Operating Activities
In 2019, cash provided by operating activities increased $1.526 billion, as compared to 2018. This increase was primarily due to the one-time settlement payment of $180 million that we received from Edwards Lifesciences Corporation in January 2019, comparatively fewer litigation payments in 2019 primarily associated with product liability cases or claims related to transvaginal surgical mesh products and the IRS final net tax settlement payments of $303 million plus $307 million of estimated interest that we remitted in the second quarter of 2018 and $93 million reflecting the net balance of tax and interest due in the fourth quarter of 2018.
In 2018, cash provided by operating activities decreased $1.116 billion, or 78 percent, as compared to 2017. This decrease was primarily due to the IRS tax settlement payments in 2018 described above.
Investing Activities
In 2019, cash used for investing activities primarily included Payments for acquisitions of businesses, net of cash acquired of $4.382 billion relating to our acquisitions of BTG, Vertiflex and Millipede, Inc. (Millipede), Purchases of property, plant and equipment of $461 million, Payments for investments and acquisitions of certain technologies of $149 million, partially offset by Proceeds from divestiture of certain businesses of $90 million relating to the sale of our drug-eluting and bland embolic microsphere portfolio to Varian Medical Systems, Inc. (Varian) in connection with our acquisition of BTG. Cash used for investing activities also included Payments for settlements of hedge contracts of $199 million, of which $95 million relates to the termination and settlement of our outstanding forward currency contracts designated as net investment hedges in our Euro-denominated entities and $294 million relates to the settlement of our non-designated forward currency contracts entered into for the purpose of managing our exposure to currency exchange rate risk related to the GBP-denominated purchase price of BTG. Refer to Note D – Hedging Activities and Fair Value Measurements consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for further information.
In 2018, cash used for investing activities primarily included Payments for acquisitions of businesses, net of cash acquired of $1.448 billion primarily relating to our acquisitions of Augmenix, NxThera, Cryterion Medical, Inc., Claret and nVision, Purchases of property, plant and equipment of $316 million and Payments for investments and acquisitions of certain technologies of $172 million, including our $90 million investment in Millipede in the first quarter of 2018.
2Adjusted free cash flow, which excludes certain items required by U.S. GAAP is not prepared in accordance with U.S. GAAP and should not be considered in isolation from, or as a replacement for, the most directly comparable GAAP measure. Refer to Additional Information for a discussion of management’s use of non-GAAP financial measures.
48
Financing Activities
Our cash flows provided by financing activities reflect issuances and repayments of debt, including our commercial paper program and cash used for new share settlement and stock issuances related to our equity incentive programs, as discussed in Note K – Stockholders' Equity to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report. In addition, our financing activities included Payments of contingent consideration and royalty rights established in purchase accounting of $135 million in 2019, $19 million in 2018 and $33 million in 2017. In connection with the acquisition of BTG, we acquired rights to future royalties associated with the Zytiga™ drug used to treat certain forms of prostate cancer. In the fourth quarter of 2019, we sold our rights to these royalties for $256 million in cash, included in Proceeds from royalty rights transfer.
Our liquidity plans are subject to a number of risks and uncertainties, including those described in Item 1A. Risk Factors of this Annual Report, some of which are outside our control. Macroeconomic conditions, adverse litigation outcomes and other risks and uncertainties could limit our ability to successfully execute our business plans and adversely affect our liquidity plans.
Debt
The following table presents the current and long-term portions of our total debt:
As of | |||||||
(in millions) | December 31, 2019 | December 31, 2018 | |||||
Current debt obligations | $ | 1,416 | $ | 2,253 | |||
Long-term debt | 8,592 | 4,803 | |||||
Total debt | $ | 10,008 | $ | 7,056 | |||
The following table presents the portions of our total debt that are comprised of fixed and variable rate debt instruments, which are presented on an amortized cost basis:
As of | |||||||
(in millions) | December 31, 2019 | December 31, 2018 | |||||
Fixed-rate debt instruments | $ | 7,587 | $ | 4,797 | |||
Variable rate debt instruments | 2,421 | 2,259 | |||||
Total debt | $ | 10,008 | $ | 7,056 | |||
As of and through December 31, 2019, we were in compliance with all the required covenants related to our debt obligations. For additional details related to our debt obligations, including our debt covenant requirements, refer to Note E – Contractual Obligations and Commitments to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report.
Equity
During 2019 we received $123 million in proceeds from stock issuances related to our stock option and employee stock purchase plans, as compared to $101 million in 2018. Proceeds from the exercise of employee stock options and employee stock purchases vary from period to period based upon, among other factors, fluctuations in the trading price of our common stock and in the exercise and stock purchase patterns of employees.
We did not repurchase any shares of our common stock during 2019 or 2018. As of December 31, 2019, we had remaining approximately $535 million authorized under our 2013 share repurchase program. There were approximately 248 million shares in treasury as of December 31, 2019 and December 31, 2018.
Stock-based compensation expense related to our stock ownership plans was $157 million in 2019 and $140 million in 2018. Stock-based compensation expense varies from period to period based upon, among other factors, the timing, number and fair value of awards granted during the period, forfeiture levels related to unvested awards and employee contributions to our employee stock purchase plan, as well as the retirement eligibility of stock award recipients.
49
Contractual Obligations and Commitments
The following table provides a summary of certain information concerning our obligations and commitments to make future payments and is based on conditions in existence as of December 31, 2019:
(in millions) | 2020 | 2021 | 2022 | 2023 | 2024 | Thereafter | Total | ||||||||||||||||||||
Debt obligations (1) | $ | 1,411 | $ | — | $ | 1,500 | $ | 244 | $ | 850 | $ | 6,068 | $ | 10,072 | |||||||||||||
Interest payments (2) | 343 | 326 | 308 | 280 | 252 | 2,550 | 4,059 | ||||||||||||||||||||
Lease obligations (2) | 84 | 71 | 59 | 48 | 41 | 79 | 382 | ||||||||||||||||||||
Purchase obligations (2) | 334 | 20 | 7 | 3 | 1 | 1 | 366 | ||||||||||||||||||||
Minimum royalty obligations (2) | 3 | 3 | 2 | 2 | 2 | 2 | 15 | ||||||||||||||||||||
License and software commitments (2) | 4 | 5 | 5 | 3 | 3 | — | 20 | ||||||||||||||||||||
Legal reserves | 470 | — | — | — | — | — | 470 | ||||||||||||||||||||
One-time transition tax | 40 | 40 | 40 | 75 | 100 | 125 | 420 | ||||||||||||||||||||
$ | 2,689 | $ | 465 | $ | 1,921 | $ | 655 | $ | 1,249 | $ | 8,825 | $ | 15,804 | ||||||||||||||
(1) | Debt obligations are comprised of our senior notes, term loan and commercial paper outstanding as of December 31, 2019. This does not include unamortized debt issuance discounts, deferred financing costs and gain on fair value hedges or capital lease obligations. Refer to Note E – Contractual Obligations and Commitments to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional information. In January 2020, we repaid $300 million of the outstanding balance of the 2020 Term Loan with proceeds from our commercial paper program. |
(2) | In accordance with U.S. GAAP, these obligations relate to expenses associated with future periods and are not reflected in our consolidated balance sheets. Interest payments included above are calculated based on rates and required fees applicable to our outstanding debt obligations as of December 31, 2019 described in Note E – Contractual Obligations and Commitments to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report. Interest payments above do not include interest on variable rate debt instruments. |
The amounts in the table above with respect to purchase obligations relate primarily to non-cancellable inventory commitments and capital expenditures entered in the normal course of business. Royalty obligations reported above represent minimum contractual obligations under our current royalty agreements.
The table above does not include:
• | Our long-term liability for legal matters that are probable and estimable of $227 million due to the timing of payment being uncertain. Refer to Note J – Commitments and Contingencies to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for more information, |
• | Unrecognized tax benefits, accrued interest and penalties and other related items totaling $288 million because the timing of their future cash settlement is uncertain and tax payments and interest totaling $5 million related to state obligations of recently settled IRS tax years to be remitted in 2020. Refer to Note I – Income Taxes to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for more information, and |
• | With certain of our acquisitions, we acquired IPR&D projects that require future funding to complete the projects. We estimate that the total remaining R&D cost to complete acquired IPR&D projects is between $200 million and $210 million. Net cash inflows from the projects currently in development are expected to commence in 2020 and will continue through 2037, following the respective launches of these technologies in the U.S., Europe and Japan. Certain of our acquisitions also involve the potential payment of contingent consideration, but the timing and amounts are uncertain. See Note B – Acquisitions and Strategic Investments to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for more information. |
Legal Matters
For a discussion of our material legal proceedings see Note J – Commitments and Contingencies to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report.
50
Critical Accounting Policies and Estimates
Our financial results are affected by the selection and application of accounting policies and methods. We have adopted accounting policies to prepare our consolidated financial statements in conformity with U.S. GAAP.
To prepare our consolidated financial statements in accordance with U.S. GAAP, management makes estimates and assumptions that may affect the reported amounts of our assets and liabilities, including our contingent liabilities, as of the date of our financial statements and the reported amounts of our revenues and expenses during the reporting periods. Our actual results may differ from these estimates. We consider estimates to be critical (i) if we are required to make assumptions about material matters that are uncertain at the time of estimation or (ii) if materially different estimates could have been made or it is reasonably likely that the accounting estimate will change from period to period. The following are areas considered to be critical and require management’s judgment: Revenue Recognition, Bad Debt Reserves, Inventory Provisions, Valuation of Intangible Assets and Contingent Consideration Liability, Goodwill Valuation, Legal and Product Liability Accruals and Income Taxes.
See Note A – Significant Accounting Policies to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional information related to our accounting policies and our consideration of these critical accounting areas. In addition, see Note B – Acquisitions and Strategic Investments and Note C – Goodwill and Other Intangible Assets for further discussion of the valuation of goodwill and intangible assets and contingent consideration, Note I – Income Taxes for further discussion of income tax related matters, Note J – Commitments and Contingencies for further discussion of legal and product liability matters and Note O – Revenue for further discussion of revenue recognition.
Revenue Recognition
Deferred Revenue
We record a contract liability, or deferred revenue, when we have an obligation to provide a product or service to the customer and payment is received or due in advance of our performance. When we sell a device with a future service obligation, we defer revenue on the unfulfilled performance obligation and recognize this revenue over the related service period. Many of our Cardiac Rhythm Management product offerings combine the sale of a device with our LATITUDE™ Patient Management System, which represents a future service obligation. Generally, we do not have observable evidence of the standalone selling price related to our future service obligations; therefore, we estimate the selling price using an expected cost plus a margin approach. We allocate the transaction price using the relative standalone selling price method. The use of alternative estimates could result in a different amount of revenue deferral.
Contract liabilities are classified within Other current liabilities and Other long-term liabilities on our accompanying consolidated balance sheets. Our contractual liabilities are primarily composed of deferred revenue related to the LATITUDE™ Patient Management System. Revenue is recognized over the average service period which is based on device and patient longevity.
Variable Consideration
We generally allow our customers to return defective, damaged and, in certain cases, expired products for credit. We base our estimate for sales returns upon historical trends and record the amount as a reduction to revenue when we sell the initial product. In addition, we may allow customers to return previously purchased products for next-generation product offerings. For these transactions, we defer recognition of revenue on the sale of the earlier generation product based upon an estimate of the amount of product to be returned when the next-generation products are shipped to the customer. Uncertain timing of next-generation product approvals, variability in product launch strategies, product recalls and variation in product utilization all affect our estimates related to sales returns and could cause actual returns to differ from these estimates.
We also offer sales rebates and discounts to certain customers. We treat sales rebates and discounts as a reduction of revenue and classify the corresponding liability as current. We estimate rebates for products where there is sufficient historical information available to predict the volume of expected future rebates. If we are unable to reasonably estimate the expected rebates, we record a liability for the maximum rebate percentage offered.
Post Implant Services
We provide non-contractual services to customers to ensure the safe and effective use of certain implanted devices. Since our modified retrospective adoption of FASB ASC Topic 606, Revenue from Contracts with Customers on January 1, 2018, because the revenue related to the immaterial services is recognized before they are delivered, we forward accrue the costs to provide these services at the time the devices are sold. We record these costs to Selling, general and administrative expenses. We estimate the
51
amount of time spent by our representatives performing these services and their compensation throughout the device life to determine the service cost. Changes to our business practice or the use of alternative estimates could result in a different amount of accrued cost. Refer to Note A – Significant Accounting Policies and Note O – Revenue to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for further information on our adoption of FASB ASC Topic 606 and our revenue recognition accounting policies.
Inventory Provisions
We base our provisions for excess, expired and obsolete inventory primarily on our estimates of forecasted net sales. A significant change in the timing or level of demand for our products as compared to forecasted amounts may result in recording additional provisions for excess, expired and obsolete inventory in the future. Further, the industry in which we participate is characterized by rapid product development and frequent new product introductions. Uncertain timing of next-generation product approvals, variability in product launch strategies, product recalls and variation in product utilization all affect our estimates related to excess, expired and obsolete inventory.
Valuation of Intangible Assets and Contingent Consideration Liability
We base the fair value of identifiable intangible assets acquired in a business combination, including IPR&D, on detailed valuations that use information and assumptions provided by management, which consider management’s best estimates of inputs and assumptions that a market participant would use. Further, for those arrangements that involve potential future contingent consideration, we record on the date of acquisition a liability equal to the fair value of the estimated additional consideration we may be obligated to pay in the future. We re-measure this liability each reporting period and record changes in the fair value through a separate line item within our consolidated statements of operations. Increases or decreases in the fair value of the contingent consideration liability can result from changes in discount rates, periods, timing and amount of projected revenue or timing or likelihood of achieving regulatory, revenue or commercialization-based milestones. The use of alternative valuation assumptions, including estimated revenue projections, growth rates, cash flows, discount rates, useful life or probability of achieving clinical, regulatory or revenue-based milestones could result in different purchase price allocations and recognized amortization expense and contingent consideration expense or benefit in current and future periods.
We review intangible assets subject to amortization quarterly to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment or adjustment to the remaining useful life. If we determine it is more likely than not that the asset is impaired based on our qualitative assessment of impairment indicators, we test the intangible asset for recoverability. If the carrying value of the intangible asset is determined not recoverable, we will write the carrying value down to fair value in the period identified. We calculate fair value of our intangible assets as the present value of estimated future cash flows we expect to generate from the asset using a risk-adjusted discount rate. The use of alternative assumptions, including estimated cash flows, discount rates and alternative estimated remaining useful lives could result in different calculations of impairment.
In addition, we test our indefinite-lived intangible assets at least annually for impairment and reassess their classification as indefinite-lived assets, or more frequently if indicators exist. We assess qualitative factors to determine whether the existence of events and circumstances indicate that it is more likely than not that our indefinite-lived intangible assets are impaired. If we conclude that it is more likely than not that the asset is impaired, we then determine the fair value of the intangible asset and perform the quantitative impairment test by comparing the fair value with the carrying value in accordance with FASB ASC Topic 350, Intangibles - Goodwill and Other (FASB ASC Topic 350). If the carrying value exceeds the fair value of the indefinite-lived intangible asset, we write the carrying value down to fair value. The use of alternative valuation assumptions, including estimated revenue projections, growth rates, cash flows and discount rates could result in different fair value estimates.
Goodwill Valuation
We allocate any excess purchase price over the fair value of the net tangible and identifiable intangible assets acquired in a business combination to goodwill. We test our goodwill balances during the second quarter of each year for impairment, or more frequently if indicators are present or changes in circumstances suggest that impairment may exist. We assess goodwill for impairment at the reporting unit level, which is defined as an operating segment or one level below an operating segment, referred to as a component. For our 2019, 2018 and 2017 annual impairment assessments, we identified the following reporting units: Interventional Cardiology, Peripheral Interventions, Cardiac Rhythm Management, Electrophysiology, Endoscopy, Urology and Pelvic Health and Neuromodulation. In addition, following the BTG acquisition in 2019, Specialty Pharmaceuticals was added as an additional reporting unit. We aggregated the Cardiac Rhythm Management and Electrophysiology reporting units, components of the Rhythm Management operating segment, based on the criteria prescribed in FASB ASC Topic 350.
52
Refer to Note A – Significant Accounting Policies to our consolidated financial statements contained in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional details related to our annual goodwill impairment assessments performed in 2019, 2018 and 2017.
Although we use consistent methodologies in developing the assumptions and estimates underlying the fair value calculations used in our impairment tests, these estimates are uncertain by nature and can vary from actual results. The use of alternative valuation assumptions, including estimated revenue projections, growth rates, cash flows and discount rates could result in different fair value estimates.
Future events that could have a negative impact on the levels of excess fair value over carrying value of our reporting units include, but are not limited to, the following:
• | decreases in estimated market sizes or market growth rates due to greater-than-expected declines in procedural volumes, pricing pressures, reductions in reimbursement levels, product actions and/or competitive technology developments, |
• | declines in our market share and penetration assumptions due to increased competition, an inability to develop or launch new and next-generation products and technology features in line with our commercialization strategies and market and/or regulatory conditions that may cause significant launch delays or product recalls, |
• | decreases in our forecasted profitability due to an inability to implement successfully and achieve timely and sustainable cost improvement measures consistent with our expectations, |
• | negative developments in intellectual property litigation that may impact our ability to market certain products or increase our costs to sell certain products, |
• | the level of success of ongoing and future research and development efforts, including those related to recent acquisitions and increases in the research and development costs necessary to obtain regulatory approvals and launch new products, |
• | the level of success in managing the growth of acquired companies, achieving sustained profitability consistent with our expectations, establishing government and third-party payer reimbursement, supplying the market and increases in the costs and time necessary to integrate acquired businesses into our operations successfully, |
• | changes in our reporting units or in the structure of our business as a result of future reorganizations, acquisitions or divestitures of assets or businesses and |
• | increases in our market-participant risk-adjusted weighted average cost of capital (WACC) and increases in our market-participant tax rate and/or changes in tax laws or macroeconomic conditions. |
Negative changes in one or more of these factors, among others, could result in future impairment charges.
Refer to Note C – Goodwill and Other Intangible Assets to our consolidated financial statements contained in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional details related to our annual goodwill balances.
Legal and Product Liability Accruals
In the normal course of business, we are involved in various legal and regulatory proceedings, including intellectual property, breach of contract, securities litigation and product liability suits. In some cases, the claimants seek damages, as well as other relief, which, if granted, could require significant expenditures or impact our ability to sell our products. We accrue anticipated costs of settlement, damages, losses for product liability claims and, under certain conditions, costs of defense, based on historical experience or to the extent specific losses are probable and estimable. Otherwise, we expense these costs as incurred. If the estimate of a probable loss is a range and no amount within the range is more likely, we accrue the minimum amount of the range. Litigation and product liability matters are inherently uncertain, and the outcomes of individual matters are difficult to predict and quantify. As such, significant judgment is required in determining our legal and product liability accruals. Our estimates related to our legal and product liability accruals may change as additional information becomes available to us, including information related to the nature or existence of claims against us, trial court or appellate proceedings, and mediation, arbitration or settlement proceedings.
53
Income Taxes
We establish reserves when we believe that certain positions are likely to be challenged despite our belief that our tax return positions are fully supportable. The calculation of our tax liabilities involves significant judgment based on individual facts, circumstances and information available in addition to applying complex tax regulations in various jurisdictions across our global operations. Under U.S. GAAP, in order to recognize an uncertain tax benefit, the taxpayer must determine it is more likely than not the position will be sustained, and the measurement of the benefit is calculated as the largest amount that is more than 50 percent likely to be realized upon resolution of the benefit. Although we believe that we have adequately provided for liabilities resulting from tax assessments by taxing authorities, positions taken by these tax authorities could have a material impact on our effective tax rate, results of operations, financial position and/or cash flows.
As part of the Tax Cut and Jobs Act of 2017, we are subject to a territorial tax system in which we are required to establish an accounting policy in providing for tax on Global Intangible Low Taxed Income (GILTI) earned by certain foreign subsidiaries. We have elected to treat the impact of GILTI as a period cost and will be reported as a part of continuing operations.
New Accounting Pronouncements
See Note A – Significant Accounting Policies to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional information on standards implemented since December 31, 2018 and Note Q – New Accounting Pronouncements to our consolidated financial statements included in Item 8. Financial Statements and Supplementary Data of this Annual Report for additional information on standards to be implemented.
Additional Information
Cybersecurity
We have established controls and procedures to escalate enterprise level issues, including cybersecurity matters, to the appropriate management levels within our organization and our Board of Directors, or members or committees thereof, as appropriate. Under our framework, cybersecurity issues are analyzed by subject matter experts and a crisis committee for potential financial, operational, and reputational risks, based on, among other factors, the nature of the matter and breadth of impact. Matters determined to present potential material impacts to the Company’s financial results, operations, and/or reputation are immediately reported by management to the Board of Directors, or individual members or committees thereof, as appropriate, in accordance with our escalation framework. In addition, we have established procedures to ensure that members of management responsible for overseeing the effectiveness of disclosure controls are informed in a timely manner of known cybersecurity risks and incidents that may materially impact our operations and that timely public disclosure is made, as appropriate.
Use of Non-GAAP Financial Measures
To supplement our consolidated financial statements presented on a GAAP basis, we disclose certain non-GAAP financial measures, including adjusted net income (earnings) and adjusted net income (earnings) per share that exclude certain amounts, operational net sales growth that exclude the impact of foreign currency fluctuations and adjusted free cash flow that excludes certain amounts. These non-GAAP financial measures are not in accordance with generally accepted accounting principles in the United States and should not be considered in isolation from or as a replacement for the most directly comparable GAAP financial measures. Further, other companies may calculate these non-GAAP financial measures differently than we do, which may limit the usefulness of those measures for comparative purposes.
To calculate adjusted net income (earnings) and adjusted net income (earnings) per share we exclude certain charges (credits) from GAAP net income as detailed below. Amounts are presented after-tax using our effective tax rate, unless the amount is a significant unusual or infrequently occurring item in accordance with FASB ASC section 740-270-30, "General Methodology and Use of Estimated Annual Effective Tax Rate." The GAAP financial measure most directly comparable to adjusted net income is GAAP net income (loss) and the GAAP financial measure most directly comparable to adjusted net income per share is GAAP net income (loss) per share.
To calculate operational net sales, which exclude the impact of foreign currency fluctuations, we convert actual net sales from local currency to U.S. dollars using constant foreign currency exchange rates in the current and prior periods. The GAAP financial measure most directly comparable to operational growth rate percentages is growth rate percentages using net sales on a GAAP basis.
54
Adjusted free cash flow is a non-GAAP measure that excludes from free cash flow the cash component of certain charges (credits) that are also excluded from adjusted net income as well as any cash tax benefits of such charges, as detailed below. In addition, we exclude payments or refunds that relate to resolving tax disputes related to prior periods. Free cash flow is a non-GAAP measure that excludes net purchases of property, plant and equipment from cash provided by (used for) operating activities on a GAAP basis. The GAAP measure that is most directly comparable to adjusted free cash flow and free cash flow is cash provided by (used for) operating activities on a GAAP basis.
Reconciliations of each of these non-GAAP financial measures to the corresponding GAAP financial measure are included in the relevant sections of this Annual Report.
Management uses these supplemental non-GAAP financial measures to evaluate performance period over period, to analyze the underlying trends in our business, to assess our performance relative to our competitors and to establish operational goals and forecasts that are used in allocating resources. In addition, management uses these non-GAAP financial measures to further its understanding of the performance of our operating segments. The adjustments excluded from our non-GAAP financial measures are consistent with those excluded from our operating segments’ measures of net sales and profit or loss. These adjustments are excluded from the segment measures reported to our chief operating decision maker that are used to make operating decisions and assess performance.
We believe that presenting adjusted net income, adjusted net income per share that exclude certain amounts, operational net sales growth that exclude the impact of changes in foreign currency exchange rates, and adjusted free cash flow that excludes certain amounts, in addition to the corresponding GAAP financial measures, provides investors greater transparency to the information used by management for its operational decision-making and allows investors to see our results “through the eyes” of management. We further believe that providing this information assists our investors in understanding our operating performance and the methodology used by management to evaluate and measure such performance.
The following is an explanation of each of the adjustments that management excluded as part of these non-GAAP financial measures as well as reasons for excluding each of these individual items. In each case, management has excluded the item for purposes of calculating the relevant non-GAAP financial measure to facilitate an evaluation of our current operating performance and a comparison to our past operating performance:
Adjusted Net Income, Adjusted Net Income per Share and Adjusted Free Cash Flow
• | Amortization expense - We record intangible assets at historical cost and amortize them over their estimated useful lives. Amortization expense is excluded from management's assessment of operating performance and is also excluded from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. |
• | Intangible asset impairment charges - This amount represents write-downs of certain intangible asset balances during each period. We review intangible assets subject to amortization quarterly to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment and test our indefinite-lived intangible assets at least annually for impairment. If we determine the carrying value of the amortizable intangible asset is not recoverable or we conclude that it is more likely than not that the indefinite-lived asset is impaired, we will write the carrying value down to fair value in the period identified. Impairment charges are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. |
• | Acquisition/divestiture-related net charges (credits) or payments - These adjustments may consist of (a) contingent consideration and Zytiga™ licensing arrangement fair value adjustments; (b) gains on previously held investments; (c) due diligence, deal fees and other fees and costs related to our acquisition and divestiture transactions; (d) inventory step-up amortization and accelerated compensation expense; (e) integration and exit costs; and (f) separation costs and gains primarily associated with the sale of a business or portion of a business. The contingent consideration and Zytiga licensing arrangement fair value adjustments represent accounting adjustments to state contingent consideration liabilities and Zytiga-related assets and liabilities at their estimated fair value. These adjustments can be highly variable depending on the assessed likelihood and amount of future contingent consideration and Zytiga royalty payments. In addition, we have sold our rights to retain any future royalties related to Zytiga. Refer to Note D - Hedging Activities and Fair Value Measurements for further information on the Zytiga licensing arrangement. Gains on previously held investments, due diligence, deal fees and other fees and costs, inventory step-up amortization, accelerated compensation expense, and other expenses and gains associated with prior and potential future acquisitions and divestitures can be highly variable and not representative of ongoing operations. Integration and exit costs, include contract cancellations, |
55
severance and other compensation-related charges and costs, project management fees and costs, and other direct costs associated with the integration of our acquisitions. Examples of integration and exit activities include the movement of business activities; the elimination or combination of redundant roles and business processes; the consolidation or closure of facilities and legal entities; and the transfer of product lines between manufacturing facilities. These integration and exit activities take place over a defined timeframe and have a distinct project timelines, are incremental to activities and costs that arise in the ordinary course of our business and are not considered part of our core, ongoing operations. In addition, our acquisition-related charges in 2019 included expenses for instruments entered into solely for the purpose of financing or hedging the BTG Acquisition, including net interest expense and hedging expenses. Subsequent to September 30, 2019, we did not incur and will not incur any hedging gains or losses related to the BTG Acquisition, and we are not classifying any interest expense subsequent to the BTG acquisition date as an acquisition/divestiture-related item. Acquisition/divestiture-related net charges (credits) are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance.
• | Restructuring and restructuring-related net charges (credits) or payments - These adjustments primarily represent compensation-related charges, fixed asset write-offs, contract cancellations, project management fees and other direct costs associated with our restructuring plans. These restructuring plans each consist of distinct initiatives that are fundamentally different from our ongoing, core cost reduction initiatives in terms of, among other things, the frequency with which each action is performed and the required planning, resourcing, cost and timing. Examples of such initiatives include the movement of business activities, facility consolidations and closures and the transfer of product lines between manufacturing facilities, which, due to the highly regulated nature of our industry, requires a significant investment in time and cost to create duplicate manufacturing lines, run product validations and seek regulatory approvals. Restructuring initiatives take place over a defined timeframe and have a distinct project timeline that begins subsequent to approval by our Board of Directors. In contrast to our ongoing cost reduction initiatives, restructuring initiatives typically result in duplicative cost and exit costs over this period of time, are one-time shut downs or transfers and are not considered part of our core, ongoing operations. These restructuring plans are incremental to the core activities that arise in the ordinary course of our business. Restructuring and restructuring-related net charges (credits) are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. |
• | Litigation-related net charges (credits) or payments - These adjustments include certain significant product liability and other litigation-related charges and credits. We record these charges and credits, which we consider to be unusual or infrequent and significant, within the litigation-related charges line in our consolidated statements of operations; all other legal and product liability charges, credits and costs are recorded within selling general and administrative expenses. Litigation-related net charges (credits) are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. |
• | EU MDR implementation charges - These adjustments represent incremental costs or payments specific to complying with the new European Union Medical Device Regulation (EU MDR) for previously registered products. EU MDR is a replacement of the existing European Medical Devices Directive (MDD) regulatory framework, and manufacturers of medical devices are required to comply with EU MDR beginning in May 2020 for new product registrations and by May 2024 for medical devices which have a valid CE Certificate to the current Directives (issued before May 2020). We expect to incur significant expenditures in connection with the adoption of the EU MDR requirements and we consider the adoption of EU MDR to be a significant change to a regulatory framework, and therefore, these expenditures are not considered to be ordinary course expenditures in connection with regulatory matters. As such, these medical device regulation charges are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. |
• | Debt extinguishment net charges (credits) - These amounts relate to the early extinguishment of certain outstanding principal amounts of our senior notes in November 2019. Certain debt extinguishment net charges (credits) are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. |
• | Investment impairment charges - These amounts represent write-downs relating to our investment portfolio that are considered unusual or infrequent and significant. Each reporting period, we evaluate our investments to determine if there are any events or circumstances that are likely to have a significant adverse effect on the fair value of the investment. If we identify an impairment indicator, we will estimate the fair value of the investment and compare it to its carrying |
56
value and determine if the impairment is other-than-temporary. For other-than-temporary impairments, we recognize an impairment loss equal to the difference between an investment’s carrying value and its fair value. Certain investment impairment charges are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance.
• | Deferred tax expenses (benefits) - This adjustment relates to a $4.1 billion non-cash tax benefit arising from an intra-entity asset transfer of intellectual property completed in the fourth quarter of 2019. The effects of this transfer were excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. |
• | Discrete tax items - These items represent adjustments of certain tax positions including those which a) are related to the finalization of the enactment date impact of the TCJA, or b) are related to the tax consequences of a non-GAAP adjustment item booked in a prior period. These discrete tax items are excluded from management's assessment of operating performance and from our operating segments' measures of profit and loss used for making operating decisions and assessing performance. |
Operational Net Sales Excluding the Impact of Foreign Currency Fluctuations
• | The impact of foreign currency fluctuations is highly variable and difficult to predict. Accordingly, management excludes the impact of foreign currency fluctuations for purposes of reviewing the net sales and growth rates to facilitate an evaluation of our current operating performance and a comparison to our past operating performance. |
Rule 10b5-1 Trading Plans by Executive Officers
Periodically, certain of our executive officers adopt written stock trading plans in accordance with Rule 10b5-1 under the Exchange Act and our own Stock Trading Policy. A Rule 10b5-1 Trading Plan is a written document that pre-establishes the amount, prices and dates (or formulas for determining the amounts, prices and dates) of future purchases or sales of our stock, including shares issued upon exercise of stock options or vesting of deferred stock units. These plans are entered into at a time when the person is not in possession of material non-public information about our company. We disclose details regarding individual Rule 10b5-1 Trading Plans on the Investor Relations section of our website.
57
Management’s Annual Report on Internal Control over Financial Reporting
As the management of Boston Scientific Corporation, we are responsible for establishing and maintaining adequate internal control over financial reporting. We designed our internal control process to provide reasonable assurance to management and the Board of Directors regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
We assessed the effectiveness of our internal control over financial reporting as of December 31, 2019. In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control–Integrated Framework (2013 framework). Based on our assessment, we believe that, as of December 31, 2019, our internal control over financial reporting is effective at a reasonable assurance level based on these criteria.
Ernst & Young LLP, an independent registered public accounting firm, has issued an audit report on the effectiveness of our internal control over financial reporting. This report, in which they expressed an unqualified opinion, is included below.
The BTG plc Acquisition
On August 19, 2019, we announced the closing of our acquisition of BTG plc (BTG). In accordance with the SEC Staff 's interpretive guidance for newly acquired businesses, we are permitted to omit an assessment of an acquired business's internal control over financial reporting from our assessment of internal control for up to one year from the acquisition date. As such, we have excluded BTG from our annual assessment of internal controls over financial reporting as of December 31, 2019, as the acquisition was completed on August 19, 2019. BTG represents less than 5% of total assets as of December 31, 2019 and less than 5% of revenues and net income, respectively, for the year then ended.
/s/ Michael F. Mahoney | /s/ Daniel J. Brennan | |||||
Michael F. Mahoney | Daniel J. Brennan | |||||
President and Chief Executive Officer | Executive Vice President and Chief Financial Officer | |||||
58
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Boston Scientific Corporation
Opinion on Internal Control over Financial Reporting
We have audited Boston Scientific Corporation’s internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Boston Scientific Corporation (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the 2019 consolidated financial statements of the Company and our report dated February 25, 2020 expressed an unqualified opinion thereon.
As indicated in the accompanying Management’s Annual Report on Internal Control over Financial Reporting, management’s assessment of and conclusion on the effectiveness of internal control over financial reporting did not include the internal controls of BTG plc, which is included in the 2019 consolidated financial statements of the Company and constituted less than 5% of total assets as of December 31, 2019 and less than 5% of revenues and net income, respectively, for the year then ended. Our audit of internal control over financial reporting of the Company also did not include an evaluation of the internal control over financial reporting of BTG plc.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
59
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Boston, Massachusetts
February 25, 2020
60
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We develop, manufacture and sell medical devices globally and our earnings and cash flows are exposed to market risk from changes in currency exchange rates and interest rates. We address these risks through a risk management program that includes the use of derivative financial instruments. We operate the program pursuant to documented corporate risk management policies. We do not enter derivative transactions for speculative purposes. Gains and losses on derivative financial instruments substantially offset losses and gains on underlying hedged exposures. Furthermore, we manage our exposure to counterparty risk on derivative instruments by entering into contracts with a diversified group of major financial institutions and by actively monitoring outstanding positions.
Our currency risk consists primarily of foreign currency denominated firm commitments, forecasted foreign currency denominated intercompany and third-party transactions and net investments in certain subsidiaries. We use both nonderivative (primarily European manufacturing operations) and derivative instruments to manage our earnings and cash flow exposure to changes in currency exchange rates. We had currency derivative instruments outstanding in the contract amount of $9.221 billion as of December 31, 2019 and $11.326 billion as of December 31, 2018. A ten percent appreciation in the U.S. dollar’s value relative to the hedged currencies would increase the derivative instruments’ fair value by $337 million as of December 31, 2019 as compared to $181 million as of December 31, 2018. A ten percent depreciation in the U.S. dollar’s value relative to the hedged currencies would decrease the derivative instruments’ fair value by $412 million as of December 31, 2019 as compared to $222 million as of December 31, 2018. Any increase or decrease in the fair value of our currency exchange rate sensitive derivative instruments would be substantially offset by a corresponding decrease or increase in the fair value of the hedged underlying asset, liability or forecasted transaction, resulting in minimal impact on our consolidated statements of operations.
Our interest rate risk relates primarily to U.S. dollar borrowings partially offset by U.S. dollar cash investments. We have historically used interest rate derivative instruments to manage our earnings and cash flow exposure to changes in interest rates. We had interest rate derivative instruments outstanding in the contract amount of $1.000 billion as of December 31, 2018 and none as of December 31, 2019. As of December 31, 2019, $7.661 billion of our outstanding debt obligations was at fixed interest rates, representing approximately 76 percent of our total debt. As of December 31, 2019, our outstanding debt obligations at fixed interest rates were comprised of senior notes.
See Note D – Hedging Activities and Fair Value Measurements to our consolidated financial statements contained in Item 8. Financial Statements and Supplementary Data of this Annual Report for further information regarding our derivative financial instruments.
61
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Boston Scientific Corporation
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Boston Scientific Corporation (the Company) as of December 31, 2019 and 2018, the related consolidated statements of operations, comprehensive income (loss), stockholders' equity and cash flows for each of the three years in the period ended December 31, 2019, and the related notes and financial statement schedule listed in the Index at Item 15(a) (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated February 25, 2020 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
62
Business Combinations | |
Description of the Matter | As disclosed in Note B to the consolidated financial statements, during 2019, the Company completed three acquisitions for total aggregate consideration of $4.38 billion, net of cash acquired. The most significant of these was the acquisition of all outstanding equity of BTG, plc. for consideration of approximately $3.62 billion, net of cash acquired. The transactions were accounted for as business combinations. In certain acquisitions, the Company has recognized a liability for acquisition consideration that is contingent upon achieving either research and development and commercialization milestones, or sales-based milestones. The Company determines the fair value of these arrangements, both as part of the initial purchase price allocation, and on an ongoing basis each reporting period until the arrangements are settled. As of December 31, 2019, the amount accrued for future estimated contingent consideration is $354 million. Auditing the Company’s accounting for its acquisitions was complex due to the significant estimation required by management to determine the fair value of identified intangible assets, which totaled $2.2 billion and principally consisted of developed technology and assets related to currently marketed products, and to determine the fair value of contingent consideration arrangements. A significant emphasis is placed on the appropriateness of the estimate considerations used by management to determine the fair value of acquired intangible assets due to the sensitivity of the respective fair values to the underlying assumptions. The Company used an income approach to measure the technology-related intangible assets. The significant assumptions used to estimate the value of the intangible assets included discount rates and certain assumptions that form the basis of the forecasted results, including revenue growth rates, estimates of technological obsolescence, operating profit margin and market participant synergies. The significance of the estimations used by management to determine the fair value of contingent consideration was primarily due to the sensitivity of the respective fair values to the significant underlying assumptions. The significant assumptions include estimation of the probability and timing of payment, future sales forecasts, as well as the appropriate discount rate based on the estimated timing of payments. These significant assumptions are forward looking and could be affected by future economic and market conditions. |
How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of the controls over the Company’s accounting for acquisitions. For example, we tested controls over the identification and valuation of intangible assets, including the valuation models and underlying assumptions used to develop such estimates. We also tested controls over the valuation of the contingent consideration liability, including the valuation models and underlying assumptions used to develop such estimates. For each of the Company's acquisitions, we read the purchase agreements, evaluated the significant assumptions and methods used in developing the fair value estimates, and tested the recognition of (1) the tangible assets acquired and liabilities assumed at fair value; (2) the identifiable intangible assets acquired at fair value; and (3) goodwill measured as a residual. To test the estimated fair value of the intangible assets, we performed audit procedures that included, among others, evaluating the Company's use of the income approach and testing the significant assumptions used in the model, as described above. In testing the valuation of contingent consideration, we assessed, among other things, the terms of the arrangements and the conditions that must be met for the arrangements to become payable. We evaluated the completeness and accuracy of the underlying data used in the analyses. For example, we compared the significant assumptions to current industry, market and economic trends, to the assumptions used to value similar assets in other acquisitions, to the historical results of the acquired business and to other guideline companies within the same industry. We involved our valuation professionals to assist with our evaluation of the methodology used by the Company and significant assumptions included in the fair value estimates. . |
63
Income Taxes - Intra-entity transfer of a license for intellectual property | |
Description of the Matter | As discussed in Note I - Income Taxes, the Company completed intra-entity transfers of certain intellectual property rights among various wholly-owned subsidiaries. The Company determined that some of these transactions created a step-up in the tax deductible basis in the transferred intellectual property rights in certain jurisdictions and recognized a deferred tax asset and related income tax benefit of $4.1 billion based upon the tax basis step-up to the intellectual property’s current fair value. Auditing management’s estimation of the intellectual property’s fair value was especially challenging because the estimates required significant and complex management judgments to establish assumptions about the intellectual property’s fair value, including revenue growth rates, projected profit margins, and discount rate. This also involved complex judgment to analyze, interpret and apply complex tax laws and regulations in the impacted jurisdictions. |
How We Addressed the Matter in Our Audit | We tested the effectiveness of the Company’s controls over the accounting for the intra-entity transfers, including controls over the appropriateness of the valuation approach and method selected, assumptions and data used, and application of the technical tax guidance by management. To test the estimated fair value of the intellectual property, we performed audit procedures that included, among others, evaluating the assumptions used by management related to revenue growth rates, projected profit margins, and the discount rate. We involved our valuation professionals to assist with the evaluation of the appropriateness of the valuation model used by management and the methodology used in determining the valuation of significant assumptions included in the fair value estimates. We also involved tax professionals to assess the technical merits of the Company’s tax positions related to the intra-entity transfers. To evaluate the reasonableness of management’s assumptions about revenue growth rates and projected profit margins, we compared these assumptions to historical revenue and profit margins for the business and to industry benchmarks. We also compared these assumptions to those used in the Company’s annual budget and forecasting process to determine whether they were consistent, where relevant. We recalculated the recognized deferred tax assets and assessed the adequacy of the related disclosures included in Note I - Income Taxes to the consolidated financial statements. |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 1992.
Boston, Massachusetts
February 25, 2020
64
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
BOSTON SCIENTIFIC CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
Year Ended December 31, | |||||||||||
(in millions, except per share data) | 2019 | 2018 | 2017 | ||||||||
Net sales | $ | $ | $ | ||||||||
Cost of products sold | |||||||||||
Gross profit | |||||||||||
Operating expenses: | |||||||||||
Selling, general and administrative expenses | |||||||||||
Research and development expenses | |||||||||||
Royalty expense | |||||||||||
Amortization expense | |||||||||||
Intangible asset impairment charges | |||||||||||
Contingent consideration expense (benefit) | ( | ) | ( | ) | ( | ) | |||||
Restructuring charges (credits) | |||||||||||
Litigation-related charges (credits) | |||||||||||
Operating income (loss) | |||||||||||
Other income (expense): | |||||||||||
Interest expense | ( | ) | ( | ) | ( | ) | |||||
Other, net | ( | ) | ( | ) | |||||||
Income (loss) before income taxes | |||||||||||
Income tax (benefit) expense | ( | ) | ( | ) | |||||||
Net income (loss) | $ | $ | $ | ||||||||
Net income (loss) per common share — basic | $ | $ | $ | ||||||||
Net income (loss) per common share — assuming dilution | $ | $ | $ | ||||||||
Weighted-average shares outstanding | |||||||||||
Basic | |||||||||||
Assuming dilution | |||||||||||
See notes to the consolidated financial statements.
65
BOSTON SCIENTIFIC CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
Year Ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Net income (loss) | $ | $ | $ | ||||||||
Other comprehensive income (loss), net of tax: | |||||||||||
Foreign currency translation adjustment | ( | ) | |||||||||
Net change in derivative financial instruments | ( | ) | |||||||||
Net change in available-for-sale securities | |||||||||||
Net change in defined benefit pensions and other items | ( | ) | ( | ) | |||||||
Total other comprehensive income (loss) | ( | ) | |||||||||
Total comprehensive income (loss) | $ | $ | $ | ||||||||
See notes to the consolidated financial statements.
66
BOSTON SCIENTIFIC CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
As of December 31, | |||||||
(in millions, except share and per share data) | 2019 | 2018 | |||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | $ | |||||
Trade accounts receivable, net | |||||||
Inventories | |||||||
Prepaid income taxes | |||||||
Other current assets | |||||||
Total current assets | |||||||
Property, plant and equipment, net | |||||||
Goodwill | |||||||
Other intangible assets, net | |||||||
Deferred tax assets | |||||||
Other long-term assets | |||||||
TOTAL ASSETS | $ | $ | |||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Current debt obligations | $ | $ | |||||
Accounts payable | |||||||
Accrued expenses | |||||||
Other current liabilities | |||||||
Total current liabilities | |||||||
Long-term debt | |||||||
Deferred tax liabilities | |||||||
Other long-term liabilities | |||||||
Commitments and contingencies | |||||||
Stockholders’ equity: | |||||||
Preferred stock, $0.01 par value - authorized 50,000,000 shares, none issued and outstanding | |||||||
Common stock, $0.01 par value - authorized 2,000,000,000 shares; issued 1,642,488,911 shares as of December 31, 2019 and 1,632,148,030 shares as of December 31, 2018 | |||||||
Treasury stock, at cost - 247,566,270 shares as of December 31, 2019 and December 31, 2018 | ( | ) | ( | ) | |||
Additional paid-in capital | |||||||
Accumulated deficit | ( | ) | ( | ) | |||
Accumulated other comprehensive income (loss), net of tax: | |||||||
Foreign currency translation adjustment | ( | ) | |||||
Unrealized gain (loss) on derivative financial instruments | |||||||
Unrealized costs associated with defined benefit pensions and other items | ( | ) | ( | ) | |||
Total stockholders’ equity | |||||||
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | $ | $ | |||||
See notes to the consolidated financial statements.
67
BOSTON SCIENTIFIC CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
Treasury Stock | Additional Paid-In Capital | Accumulated Deficit | Accumulated Other Comprehensive Income (Loss), Net of Tax | |||||||||||||||||||
Common Stock | ||||||||||||||||||||||
(in millions, except share data) | Shares Issued | Par Value | ||||||||||||||||||||
Balance as of December 31, 2016 | $ | $ | ( | ) | $ | $ | ( | ) | $ | |||||||||||||
Net income (loss) | ||||||||||||||||||||||
Cumulative effect adjustment for ASU 2016-09 | ||||||||||||||||||||||
Changes in other comprehensive income (loss), net of tax: | ||||||||||||||||||||||
Foreign currency translation adjustment | ||||||||||||||||||||||
Derivative financial instruments | ( | ) | ||||||||||||||||||||
Available-for-sale securities | ||||||||||||||||||||||
Defined benefit pensions and other items | ( | ) | ||||||||||||||||||||
Impact of stock-based compensation plans, net of tax | ||||||||||||||||||||||
Balance as of December 31, 2017 | $ | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | |||||||||||
Net income (loss) | ||||||||||||||||||||||
Cumulative effect adjustments for ASC Update Adoptions(1) | ( | ) | ||||||||||||||||||||
Changes in other comprehensive income (loss), net of tax: | ||||||||||||||||||||||
Foreign currency translation adjustment | ( | ) | ||||||||||||||||||||
Derivative financial instruments | ||||||||||||||||||||||
Defined benefit pensions and other items | ||||||||||||||||||||||
Impact of stock-based compensation plans, net of tax | ||||||||||||||||||||||
Balance as of December 31, 2018 | $ | $ | ( | ) | $ | $ | ( | ) | $ | |||||||||||||
Net income (loss) | ||||||||||||||||||||||
Changes in other comprehensive income (loss), net of tax: | ||||||||||||||||||||||
Foreign currency translation adjustment | ||||||||||||||||||||||
Derivative financial instruments | ||||||||||||||||||||||
Defined benefit pensions and other items | ( | ) | ||||||||||||||||||||
Impact of stock-based compensation plans, net of tax | ||||||||||||||||||||||
Balance as of December 31, 2019 | $ | $ | ( | ) | $ | $ | ( | ) | $ | |||||||||||||
(1) In 2018, we recorded cumulative effect adjustments to retained earnings to reflect the adoption of Accounting Standards Codification (ASC) Update No. 2014-09, Update No. 2016-16 and Update No. 2016-01. Please refer to Note A – Significant Accounting Policies for additional details.
See notes to the consolidated financial statements.
68
BOSTON SCIENTIFIC CORPORATION AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF CASH FLOWS | Year Ended December 31, | ||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Net income (loss) | $ | $ | $ | ||||||||
Adjustments to reconcile net income (loss) to cash provided by operating activities | |||||||||||
Gain on sale of businesses | ( | ) | |||||||||
Depreciation and amortization | |||||||||||
Deferred and prepaid income taxes | ( | ) | ( | ) | |||||||
Stock-based compensation expense | |||||||||||
Intangible asset impairment charges | |||||||||||
Net loss (gain) on investments and notes receivable | ( | ) | |||||||||
Contingent consideration expense (benefit) | ( | ) | ( | ) | ( | ) | |||||
Payment of contingent consideration in excess of amount recognized at acquisition | ( | ) | ( | ) | ( | ) | |||||
Inventory step-up amortization | |||||||||||
Exchange (gain) loss | ( | ) | |||||||||
Other, net | |||||||||||
Increase (decrease) in operating assets and liabilities, net of acquisitions: | |||||||||||
Trade accounts receivable | ( | ) | ( | ) | ( | ) | |||||
Inventories | ( | ) | ( | ) | ( | ) | |||||
Other assets | ( | ) | ( | ) | |||||||
Accounts payable and accrued expenses | ( | ) | |||||||||
Other liabilities | ( | ) | ( | ) | |||||||
Cash provided by (used for) operating activities | |||||||||||
Purchases of property, plant and equipment | ( | ) | ( | ) | ( | ) | |||||
Proceeds on disposals of property, plant and equipment | |||||||||||
Payments for acquisitions of businesses, net of cash acquired | ( | ) | ( | ) | ( | ) | |||||
Proceeds from divestiture of certain businesses | |||||||||||
Proceeds from royalty rights | |||||||||||
Payments for settlements of hedge contracts | ( | ) | |||||||||
Payments for investments and acquisitions of certain technologies | ( | ) | ( | ) | ( | ) | |||||
Cash provided by (used for) investing activities | ( | ) | ( | ) | ( | ) | |||||
Payment of contingent consideration and royalty rights previously established in purchase accounting | ( | ) | ( | ) | ( | ) | |||||
Proceeds from royalty rights transfer | |||||||||||
Proceeds from short-term borrowings, net of debt issuance costs | |||||||||||
Net increase (decrease) in commercial paper | ( | ) | |||||||||
Proceeds from borrowings on credit facilities | |||||||||||
Payments on borrowings from credit facilities | ( | ) | ( | ) | |||||||
Payments on short-term borrowings | ( | ) | |||||||||
Payments on long-term borrowings and debt extinguishment costs | ( | ) | ( | ) | ( | ) | |||||
Proceeds from long-term borrowings, net of debt issuance costs | |||||||||||
Cash used to net share settle employee equity awards | ( | ) | ( | ) | ( | ) | |||||
Proceeds from issuances of shares of common stock | |||||||||||
Cash provided by (used for) financing activities | |||||||||||
Effect of foreign exchange rates on cash | ( | ) | |||||||||
Net increase (decrease) in cash, cash equivalents, restricted cash and restricted cash equivalents | ( | ) | ( | ) | |||||||
Cash, cash equivalents, restricted cash and restricted cash equivalents at beginning of period | |||||||||||
Cash, cash equivalents, restricted cash and restricted cash equivalents at end of period | $ | $ | $ | ||||||||
Supplemental Information | |||||||||||
Cash (received) paid for income taxes, net | $ | $ | $ | ( | ) | ||||||
Cash paid for interest | |||||||||||
Fair value of contingent consideration recorded in purchase accounting | |||||||||||
As of December 31, | |||||||||||
Reconciliation to amounts within the consolidated balance sheets: | 2019 | 2018 | 2017 | ||||||||
Cash and cash equivalents | $ | $ | $ | ||||||||
Restricted cash and restricted cash equivalents included in Other current assets | |||||||||||
Restricted cash equivalents included in Other long-term assets | |||||||||||
Cash, cash equivalents, restricted cash and restricted cash equivalents at end of period | $ | $ | $ | ||||||||
See notes to the consolidated financial statements.
69
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE A – SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
Our consolidated financial statements include the accounts of Boston Scientific Corporation and our wholly-owned subsidiaries, after the elimination of intercompany transactions. When used in this report, the terms "we," "us," "our" and "the Company" mean Boston Scientific Corporation and its divisions and subsidiaries. We assess the terms of our investment interests to determine if any of our investees meet the definition of a variable interest entity (VIE). For any VIEs, we perform an analysis to determine whether our variable interests give us a controlling financial interest in a VIE. The analysis identifies the primary beneficiary of a VIE as the enterprise that has both 1) the power to direct activities of a VIE that most significantly impact the entity’s economic performance and 2) the obligation to absorb losses of the entity or the right to receive benefits from the entity. Based on our assessments under the applicable guidance, we did not have controlling financial interests in any VIEs and, therefore, did not consolidate any VIEs for 2019, 2018 and 2017.
Basis of Presentation
The accompanying consolidated financial statements and notes thereto have been prepared in accordance with accounting principles generally accepted in the United States (U.S. GAAP) and with the instructions to Form 10-K and Regulation S-X.
Amounts reported in millions within this report are computed based on the amounts in thousands. As a result, the sum of the components reported in millions may not equal the total amount reported in millions due to rounding. Certain columns and rows within tables may not add due to the use of rounded numbers. Percentages presented are calculated from the underlying numbers in dollars.
Reportable Segments
Our seven core businesses are organized into three reportable segments: MedSurg, Rhythm and Neuro, and Cardiovascular. Following our acquisition of BTG plc (BTG), which closed during the third quarter of 2019, we have included BTG’s Interventional Medicine business within our Peripheral Interventions operating segment, within the Cardiovascular reportable segment. We present BTG’s Specialty Pharmaceuticals business as a standalone operating segment alongside our reportable segments.
We evaluate events occurring after the date of our accompanying consolidated balance sheets for potential recognition or disclosure in our consolidated financial statements. We did not identify any material subsequent events requiring adjustment to our accompanying consolidated financial statements (recognized subsequent events). Those items requiring disclosure (unrecognized subsequent events) in the consolidated financial statements have been disclosed accordingly. Refer to Note E – Contractual Obligations and Commitments and Note J – Commitments and Contingencies for further details.
Accounting Estimates
To prepare our consolidated financial statements in accordance with U.S. GAAP, management makes estimates and assumptions that may affect the reported amounts of our assets and liabilities, the disclosure of contingent liabilities as of the date of our consolidated financial statements and the reported amounts of our revenues and expenses during the reporting period. Our actual results may differ from these estimates. Refer to Critical Accounting Estimates included in Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations of this Annual Report for further discussion.
Cash, Cash Equivalents, Restricted Cash and Restricted Cash Equivalents
Cash and Cash Equivalents
We record Cash and cash equivalents in our consolidated balance sheets at cost, which approximates fair value. Our policy is to invest excess cash in short-term marketable securities earning a market rate of interest without assuming undue risk of loss of principal amounts invested and we limit our direct exposure to securities in any one industry or issuer. We consider to be cash equivalents all short-term marketable securities with remaining days to maturity of 90 days or less from the purchase date that can be readily converted to cash.
70
Restricted Cash
Amounts included in restricted cash represent cash on hand required to be set aside by a contractual agreement related to receivable factoring arrangements and deferred compensation plans and are included in the Other current assets caption on our consolidated balance sheets. Generally, the restrictions related to the factoring arrangements lapse at the time we remit the customer payments collected by us as servicer of previously sold customer receivables to the purchaser. Restrictions for deferred compensation lapse when amounts are paid to the employee.
Restricted Cash Equivalents
Restricted cash equivalents primarily represent amounts paid into various qualified settlement funds related to our ongoing transvaginal surgical mesh litigation and current amounts related to our non-qualified pension plan and are included in the Other current assets caption on our consolidated balance sheets. The restrictions related to the various qualified settlement funds will lapse as we approve amounts payable to claimants, at which time we no longer have rights to a return of the amounts paid into the various qualified settlement funds. Restricted cash equivalents included in the Other long-term assets caption on our consolidated balance sheets are related to deferred compensation plans.
Concentrations of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents, derivative financial instruments and accounts and notes receivable. Our investment policy limits exposure to concentrations of credit risk and changes in market conditions. Counterparties to financial instruments expose us to credit-related losses in the event of nonperformance. We transact our financial instruments with a diversified group of major financial institutions with investment grade credit ratings and actively monitor their credit ratings and our outstanding positions to limit our credit exposure. In the normal course, our payment terms with customers, including hospitals, healthcare agencies, clinics, doctors' offices and other private and governmental institutions, are typically 30 days in the U.S. but may be longer in international markets and generally do not require collateral. We record our accounts receivable in our consolidated balance sheets at net realizable value. We perform ongoing credit evaluations of our customers and maintain allowances for potential credit losses, based on historical information and management's best estimates. We write-off amounts determined to be uncollectible against this reserve. Write-offs of uncollectible accounts receivable were immaterial in 2019, 2018 and 2017. We are not dependent on any single institution, and no single customer accounted for more than ten percent of our net sales in 2019, 2018 and 2017; however, large group purchasing organizations, hospital networks, international distributors and dealers and other buying groups have become increasingly important to our business and represent a substantial portion of our net sales.
We closely monitor outstanding receivables for potential collection risks, including those that may arise from economic conditions, in both the U.S. and international economies. Our European sales to government-owned or supported customers in Southern Europe are subject to an increased number of days outstanding prior to payment relative to other countries. Historically, receivable balances with certain publicly-owned hospitals in these countries accumulated over a period of time and are then subsequently settled as large lump sum payments, sometimes at large discounts. While we believe our allowance for doubtful accounts in these countries is adequate as of December 31, 2019 and 2018, if significant changes were to occur in the payment practices of these European governments or if government funding becomes unavailable, we may not be able to collect on receivables due to us from these customers, and our write-offs of uncollectible accounts receivable may increase.
Revenue Recognition
In May 2014, the FASB issued FASB ASC Topic 606, Revenue from Contracts with Customers (Topic 606), which was subsequently updated. We adopted the standard as of January 1, 2018, using the modified retrospective method. Under this method, we applied FASB ASC Topic 606 to contracts that were not complete as of January 1, 2018 and recognized the cumulative effect of initially applying the standard as an adjustment to the opening balance of retained earnings. Results for reporting periods beginning after January 1, 2018 are presented in accordance with FASB ASC Topic 606. Prior period amounts are not adjusted and are reported in accordance with legacy GAAP requirements in FASB ASC Topic 605, Revenue Recognition.
Due to the adoption of FASB ASC Topic 606, we recorded a net reduction to retained earnings of $177 million on January 1, 2018, primarily related to the cost of providing non-contractual post-implant support to certain customers, which we historically deemed immaterial in the context of the arrangement. Upon the adoption of FASB ASC Topic 606, when we sell a device with an implied non-contractual post-implant support obligation, we forward accrue the cost of the service within Selling, general and administrative expenses and recognize it at the point in time the associated revenue is earned. We release the accrual over the related service period. These costs were previously expensed as incurred due to such service obligation being non-contractual.
71
The impact of adopting FASB ASC Topic 606 on our consolidated balance sheets resulted in an increase in Other current liabilities of $59 million and an increase in Other long-term liabilities of $205 million as of December 31, 2018, as a result of accruing for our post-implant support obligation. We also recorded deferred tax assets primarily related to post-implant support, resulting in an increase in Other long-term assets of $12 million and a reduction in Deferred income taxes of $41 million as of December 31, 2018. The remaining impact of adopting FASB ASC Topic 606 was not material to our financial position or results of operations.
We sell our products primarily through a direct sales force. In certain international markets, we sell our products through independent distributors or dealers. We consider revenue to be earned when all of the following criteria are met:
• | We have a contract with a customer that creates enforceable rights and obligations, |
• | Promised products or services are identified, |
• | The transaction price, or the amount we expect to receive, is determinable and |
• | We have transferred control of the promised items to the customer. |
Transfer of control is evidenced upon passage of title and risk of loss to the customer unless we are required to provide additional services. We treat shipping and handling costs performed after a customer obtains control of the good as a fulfillment cost and record these costs as a selling expense when incurred. We recognize revenue from consignment arrangements based on product usage, or implant, which indicates that the sale is complete. We recognize a receivable at the point in time we have an unconditional right to payment. Payment terms are typically 30 days in the U.S. but may be longer in international markets.
Deferred Revenue
We record a contract liability, or deferred revenue, when we have an obligation to provide a product or service to the customer and payment is received or due in advance of our performance. When we sell a device with a future service obligation, we defer revenue on the unfulfilled performance obligation and recognize this revenue over the related service period. Many of our Cardiac Rhythm Management product offerings combine the sale of a device with our LATITUDE™ Patient Management System, which represents a future service obligation. Generally, we do not have observable evidence of the standalone selling price related to our future service obligations; therefore, we estimate the selling price using an expected cost plus a margin approach. We allocate the transaction price using the relative standalone selling price method. The use of alternative estimates could result in a different amount of revenue deferral.
Contract liabilities are classified within Other current liabilities and Other long-term liabilities on our accompanying consolidated balance sheets. Our contractual liabilities are primarily composed of deferred revenue related to the LATITUDE Patient Management System. Revenue is recognized over the average service period which is based on device and patient longevity. We have elected not to disclose the transaction price allocated to unsatisfied performance obligations when the original expected contract duration is one year or less. In addition, we have not identified material unfulfilled performance obligations for which revenue is not currently deferred.
Variable Consideration
We generally allow our customers to return defective, damaged and, in certain cases, expired products for credit. We base our estimate for sales returns upon historical trends and record the amount as a reduction to revenue when we sell the initial product. In addition, we may allow customers to return previously purchased products for next-generation product offerings. For these transactions, we defer recognition of revenue on the sale of the earlier generation product based upon an estimate of the amount of product to be returned when the next-generation products are shipped to the customer. Uncertain timing of next-generation product approvals, variability in product launch strategies, product recalls and variation in product utilization all affect our estimates related to sales returns and could cause actual returns to differ from these estimates.
We also offer sales rebates and discounts to certain customers. We treat sales rebates and discounts as a reduction of revenue and classify the corresponding liability as current. We estimate rebates for products where there is sufficient historical information available to predict the volume of expected future rebates. If we are unable to reasonably estimate the expected rebates, we record a liability for the maximum rebate percentage offered. We have entered certain agreements with group purchasing organizations to sell our products to participating hospitals at negotiated prices. We recognize revenue from these agreements following the same revenue recognition criteria discussed above.
72
Capitalized Contract Costs
We capitalize commission fees related to contracts with customers when the associated revenue is expected to be earned over a period that exceeds one year. Deferred commissions are primarily related to the sale of devices enabled with our LATITUDE™ Patient Management System. We have elected to expense commission costs when incurred for contracts with an expected duration of one year or less. Capitalized commission fees are amortized over the period the associated products or services are transferred. Similarly, we capitalize certain recoverable costs related to the delivery of the LATITUDE Remote Monitoring Service. These fulfillment costs are amortized over the average service period. Our total capitalized contract costs are immaterial to our consolidated financial statements.
Post-Implant Services
We provide non-contractual services to customers to ensure the safe and effective use of certain implanted devices. Following our modified retrospective adoption of FASB ASC Topic 606 on January 1, 2018, because the revenue related to the immaterial services is recognized before they are delivered, we forward accrue the costs to provide these services at the time the devices are sold. We record these costs to Selling, general and administrative expenses. We estimate the amount of time spent by our representatives performing these services and their compensation throughout the device life to determine the service cost. Changes to our business practice or the use of alternative estimates could result in a different amount of accrued cost.
Warranty Obligations
We offer warranties on certain of our product offerings. The majority of our warranty liability relates to implantable devices offered by our Cardiac Rhythm Management business, which include implantable defibrillator and pacemaker systems. Our Cardiac Rhythm Management products come with a standard limited warranty covering the replacement of these devices. We offer a full warranty for a portion of the period post-implant and a partial warranty for a period of time thereafter. We estimate the costs that we may incur under our warranty programs based on the number of units sold, historical and anticipated rates of warranty claims and cost per claim and record a liability equal to these estimated costs as cost of products sold at the time the product sale occurs. We assess the adequacy of our recorded warranty liabilities on a quarterly basis and adjust these amounts as necessary.
Inventories
We state inventories at the lower of first-in, first-out cost or net realizable value. We base our provisions for excess, expired and obsolete inventory primarily on our estimates of forecasted net sales. A significant change in the timing or level of demand for our products as compared to forecasted amounts may result in recording additional provisions for excess, expired and obsolete inventory in the future. Further, the industry in which we participate is characterized by rapid product development and frequent new product introductions. Uncertain timing of next-generation product approvals, variability in product launch strategies, product recalls and variation in product utilization all affect our estimates related to excess, expired and obsolete inventory. Approximately 32 percent of our finished goods inventory as of December 31, 2019 and approximately 40 percent as of December 31, 2018 was at customer locations pursuant to consignment arrangements or held by sales representatives.
Property, Plant and Equipment
We state property, plant, equipment and leasehold improvements at historical cost. We charge expenditures for maintenance and repairs to expense and capitalize additions and improvements that extend the life of the underlying asset. We provide for depreciation using the straight-line method at rates that approximate the estimated useful lives of the assets. We depreciate buildings over a maximum life of 40 years; building improvements over the remaining useful life of the building structure; equipment, furniture and fixtures over a three to seven year life; and leasehold improvements over the shorter of the useful life of the improvement or the term of the related lease.
Leases
In February 2016, the FASB issued ASC Update No. 2016-02, Leases (FASB ASC Topic 842, Leases). We adopted the standard as of January 1, 2019, using the modified retrospective approach and the transition method provided by ASC Update No. 2018-11, Leases (Topic 842): Targeted Improvements. Under this method, we applied the new leasing rules on the date of adoption and recognized the cumulative effect of initially applying the standard as an adjustment to our January 1, 2019 opening balance sheet, rather than at the earliest comparative period presented in the financial statements. Prior periods presented are in accordance with the previous lease guidance under FASB ASC Topic 840, Leases (FASB ASC Topic 840).
73
In addition, we applied the package of practical expedients permitted under FASB ASC Topic 842 transition guidance to our entire lease portfolio at January 1, 2019. As a result, we were not required to reassess (i) whether any expired or existing contracts are or contain leases, (ii) the classification of any expired or existing leases and (iii) the treatment of initial direct costs for any existing leases. Furthermore, we elected not to separate lease and non-lease components for the majority of our leases. Instead, for all applicable classes of underlying assets, we accounted for each separate lease component and the non-lease components associated with that lease component, as a single lease component.
As a result of adopting FASB ASC Topic 842 on January 1, 2019, we recognized right-of-use assets of $271 million and corresponding liabilities of $278 million for our existing operating lease portfolio on our consolidated balance sheet. Operating lease right-of-use assets are presented within Other long-term assets and corresponding liabilities are presented within Other current liabilities and Other long-term liabilities on our consolidated balance sheets. Finance leases are immaterial to our consolidated financial statements. Refer to Note E – Contractual Obligations and Commitments for additional information. There was no material impact to our consolidated statements of operations or consolidated statements of cash flows as a result of adopting FASB ASC Topic 842. Please refer to Note F – Leases for information regarding our lease portfolio as of December 31, 2019 as accounted for under FASB ASC Topic 842.
To meet the reporting and disclosure requirements of FASB ASC Topic 842, we implemented a new lease administration and lease accounting system in 2018 that tracks all of our material leasing arrangements. In addition, we designed and implemented new processes and internal controls during the first quarter of 2019 to ensure the completeness and accuracy of the transition adjustment and subsequent financial reporting under FASB ASC Topic 842. We have also established monitoring controls to ensure we have appropriate mechanisms in place to identify material leases in a timely manner, particularly contracts that may contain embedded lease features.
Valuation of Business Combinations
We allocate the amounts we pay for each acquisition to the assets we acquire and liabilities we assume based on their fair values at the date of acquisition, including identifiable intangible assets and in-process research and development (IPR&D), which either arise from a contractual or legal right or are separable from goodwill. We base the fair value of identifiable intangible assets acquired in a business combination, including IPR&D, on detailed valuations that use information and assumptions provided by management, which consider management’s best estimates of inputs and assumptions that a market participant would use. We allocate to goodwill any excess purchase price over the fair value of the net tangible and identifiable intangible assets acquired. Transaction costs associated with these acquisitions are expensed as incurred through Selling, general and administrative expenses.
In those circumstances where an acquisition involves a contingent consideration arrangement, we recognize a liability equal to the fair value of the contingent payments we expect to make as of the acquisition date. We re-measure this liability each reporting period and record changes in the fair value through Contingent consideration expense (benefit) on our consolidated statements of operations. Increases or decreases in the fair value of the contingent consideration liability can result from changes in discount rates, periods, timing and amount of projected revenue or timing or likelihood of achieving regulatory, revenue or commercialization-based milestones. Payment of additional consideration is generally contingent on the acquired company reaching certain performance milestones after the acquisition date, including attaining specified revenue levels, achieving product development targets and/or obtaining regulatory approvals for products in development at the date of the acquisition.
Indefinite-lived Intangibles, including IPR&D
Our indefinite-lived intangible assets, which are not subject to amortization, include acquired balloon and other technology, which is foundational to our ongoing operations within the Cardiovascular market and other markets within interventional medicine and IPR&D intangible assets acquired in a business combination. Our IPR&D represents intangible assets that are used in research and development activities but have not yet reached technological feasibility, regardless of whether they have alternative future use. The primary basis for determining the technological feasibility or completion of these projects is obtaining regulatory approval to market the underlying products in an applicable geographic region. We classify IPR&D as an indefinite-lived intangible asset until the completion or abandonment of the associated research and development efforts. Upon completion of the associated research and development efforts, we will determine the useful life of the technology and begin amortizing the assets to reflect their use over their remaining lives. Upon permanent abandonment, we write-off the remaining carrying amount of the associated IPR&D intangible asset.
74
We test our indefinite-lived intangible assets at least annually during the third quarter for impairment and reassess their classification as indefinite-lived assets. In addition, we review our indefinite-lived intangible assets for classification and impairment more frequently if impairment indicators exist. We assess qualitative factors to determine whether the existence of events and circumstances indicate that it is more likely than not that our indefinite-lived intangible assets are impaired. If we conclude that it is more likely than not that the asset is impaired, we then determine the fair value of the intangible asset and perform the quantitative impairment test by comparing the fair value with the carrying value in accordance with FASB ASC Topic 350, Intangibles - Goodwill and Other. If the carrying value exceeds the fair value of the indefinite-lived intangible asset, we write the carrying value down to the fair value.
We use the income approach to determine the fair values of our IPR&D. We base our revenue assumptions on estimates of relevant market sizes, expected market growth rates, expected trends in technology and expected levels of market share. In arriving at the value of the in-process projects, we consider, among other factors, the in-process projects’ stage of completion, the complexity of the work completed as of the acquisition date, the costs already incurred, the projected costs to complete, the contribution of other acquired assets, the expected regulatory path and introduction dates by region and the estimated useful life of the technology. See Note C – Goodwill and Other Intangible Assets for more information related to indefinite-lived intangibles, including IPR&D.
For asset purchases outside of business combinations, we expense any purchased research and development assets as of the acquisition date.
Amortization and Impairment of Intangible Assets
We record definite-lived intangible assets at historical cost and amortize them over their estimated useful lives. We use a straight-line method of amortization, unless a method that better reflects the pattern in which the economic benefits of the intangible asset are consumed or otherwise used up can be reliably determined. The approximate useful lives for amortization of our intangible assets are as follows: patents and licenses, two to 20 years; amortizable technology-related and customer relationships, five to 25 years; other intangible assets, various.
We review intangible assets subject to amortization quarterly to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment or a change in the remaining useful life. Conditions that may indicate impairment include, but are not limited to, a significant adverse change in legal factors or business climate that could affect the value of an asset, a product recall or an adverse action or assessment by a regulator. If we determine it is more likely than not that the asset is impaired based on our qualitative assessment of impairment indicators, we test the intangible asset for recoverability. For purposes of the recoverability test, we group our amortizable intangible assets with other assets and liabilities at the lowest level of identifiable cash flows if the intangible asset does not generate cash flows independent of other assets and liabilities. If the carrying value of the intangible asset or asset group exceeds the undiscounted cash flows expected to result from the use and eventual disposition of the intangible asset or asset group, we will write the carrying value down to fair value in the period impairment is identified.
We calculate fair value of our intangible assets as the present value of estimated future cash flows we expect to generate from the asset. In determining our estimated future cash flows associated with our intangible assets, we use estimates and assumptions about future revenue contributions, cost structures and remaining useful lives of the asset or asset group. See Note C – Goodwill and Other Intangible Assets for more information related to impairments of intangible assets.
For patents developed internally, we capitalize costs incurred to obtain patents, including attorney fees, registration fees, consulting fees and other expenditures directly related to securing the patent.
Goodwill Valuation
We allocate any excess purchase price over the fair value of the net tangible and identifiable intangible assets acquired in a business combination to goodwill. We test our goodwill balances during the second quarter of each year for impairment or more frequently if indicators are present or changes in circumstances suggest that impairment may exist. We assess goodwill for impairment at the reporting unit level, which is defined as an operating segment or one level below an operating segment, referred to as a component. For our 2019, 2018 and 2017 annual impairment assessment, we identified the following reporting units: Interventional Cardiology, Peripheral Interventions (including the Interventional Medicine business acquired with BTG), Cardiac Rhythm Management, Electrophysiology, Endoscopy, Urology and Pelvic Health and Neuromodulation. In addition, following the BTG acquisition in the third quarter of 2019, we added Specialty Pharmaceuticals as an additional reporting unit. We aggregated the Cardiac Rhythm Management and Electrophysiology reporting units, components of the Rhythm Management operating segment, based on the criteria prescribed in FASB ASC Topic 350.
75
In performing the goodwill impairment assessments for 2019, 2018 and 2017, we utilized both the optional qualitative assessment and the quantitative approach prescribed under FASB ASC Topic 350. The qualitative assessment was used for testing certain reporting units where fair value has historically exceeded carrying value by greater than 100 percent. All other reporting units were tested using the quantitative approach described below. The qualitative assessment requires an evaluation of whether it is more likely than not that the fair value of the reporting unit is less than its carrying amount based on an assessment of relevant events including macroeconomic factors, industry and market conditions, cost factors, overall financial performance and other entity-specific factors. After assessing the totality of events, if it is determined that it is more likely than not that the fair value of the reporting unit exceeds its carrying value, no further steps are required. If it is determined that impairment is more likely than not, then we perform the quantitative impairment test.
When allocating goodwill from business combinations to our reporting units, we assign goodwill to the reporting units that we expect to benefit from the respective business combination at the time of acquisition. In addition, for purposes of performing our goodwill impairment tests, assets and liabilities, including corporate assets, which relate to a reporting unit’s operations, and would be considered in determining its fair value, are allocated to the individual reporting units. We allocate assets and liabilities not directly related to a specific reporting unit, but from which the reporting unit benefits, based primarily on the respective revenue contribution of each reporting unit.
For our 2019, 2018 and 2017 annual impairment assessments, for those reporting units for which a quantitative test was performed, we used only the income approach, specifically the Discounted Cash Flow method, to derive the fair value of each of our reporting units in preparing our goodwill impairment assessments. We selected this method as being the most meaningful in preparing our goodwill assessments because we believe the income approach most appropriately measures our income producing assets. We have considered using the market approach and cost approach but concluded they are not appropriate in valuing our reporting units given the lack of relevant market comparisons available for application of the market approach and the inability to replicate the value of the specific technology-based assets within our reporting units for application of the cost approach. Therefore, we believe that the income approach represents the most appropriate valuation technique for which sufficient data are available to determine the fair value of our reporting units.
In applying the income approach to our accounting for goodwill, we make assumptions about the amount and timing of future expected cash flows, terminal value growth rates and appropriate discount rates. The amount and timing of future cash flows within our Discounted Cash Flow analysis is based on our most recent operational budgets, long range strategic plans and other estimates. The terminal value growth rate reflects our best estimates for stable, perpetual growth of our reporting units. We use estimates of market-participant risk-adjusted weighted average cost of capital as a basis for determining the discount rates to apply to our reporting units’ future expected cash flows.
Refer to Note C – Goodwill and Other Intangible Assets to our consolidated financial statements for additional details related to our goodwill balances.
Investments in Publicly Traded and Privately Held Entities
In January 2016, the FASB issued ASC Update No. 2016-01, Financial Instruments - Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. The purpose of Update No. 2016-01 is to improve financial reporting for financial instruments by reducing the number of items recorded to Other Comprehensive Income. We adopted Update No. 2016-01 in the first quarter of 2018, using both the modified retrospective and prospective methods. For publicly-held securities, we used the modified retrospective approach. Unrealized gains and losses previously recorded to Other comprehensive income (loss) were reclassified to retained earnings, and all future fair value changes will be recorded to Net income (loss). For privately-held securities of investee companies over which we do not have the ability to exercise significant influence, we elected the measurement alternative approach for our existing investments, which is applied prospectively upon adoption. This approach requires entities to measure their investments at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. The adoption of the standard did not have a material impact on our financial position or results of operations.
In 2017, we accounted for our publicly traded investments as available-for-sale securities based on the quoted market price at the end of the reporting period. Unrealized holding gains or losses during the period, net of tax, were recorded to Accumulated other comprehensive income (loss), net of tax. We computed realized gains and losses on sales of available-for-sale securities at fair value, adjusted for any other-than-temporary declines in fair value. We accounted for investments in privately-held entities in which we had less than a 20 percent ownership interest under the cost method of accounting if we did not have the ability to exercise significant influence over the investee in accordance with FASB ASC Topic 325, Investments - Other.
76
We account for investments in entities over which we have the ability to exercise significant influence under the equity method if we hold 50 percent or less of the voting stock and the entity is not a VIE in which we are the primary beneficiary in accordance with FASB ASC Topic 323, Investments - Equity Method and Joint Ventures. We record these investments initially at cost and adjust the carrying amount to reflect our share of the earnings or losses of the investee, including all adjustments similar to those made in preparing consolidated financial statements. Lastly, we have notes receivable from certain companies that we account for in accordance with FASB ASC Topic 320, Investments - Debt and Equity Securities. Refer to Note B – Acquisitions and Strategic Investments for additional details on our investment balances.
Each reporting period, we evaluate our investments to determine if there are any events or circumstances that are likely to have a significant adverse effect on the fair value of the investment. Examples of such impairment indicators include, but are not limited to, a significant deterioration in earnings performance, recent financing rounds at reduced valuations, a significant adverse change in the regulatory, economic or technological environment of an investee or a significant doubt about an investee’s ability to continue as a going concern. If we identify an impairment indicator, we will estimate the fair value of the investment and compare it to its carrying value. Our estimation of fair value considers financial information related to the investee available to us, including valuations based on recent third-party equity investments in the investee. If the fair value of the investment is less than its carrying value, the investment is impaired and we recognize an impairment loss equal to the difference between an investment’s carrying value and its fair value if accounted for under measurement alternative. For our equity method investments, an impairment loss is recorded if we determine the impairment is other-than-temporary. We deem an impairment to be other-than-temporary unless available evidence indicates that the valuation is more likely than not to recover up to the carrying value of the investment in a reasonable period of time, and we have both the ability and intent to hold the investment for at least the period of time needed to recover the value. For other-than-temporary impairments, we recognize an impairment loss equal to the difference between an investment’s carrying value and its fair value. Impairment losses on our investments are included in Other, net in our consolidated statements of operations.
Income Taxes
In February 2018, the FASB issued ASC Update No. 2018-02, Income Statement - Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income. The purpose of Update No. 2018-02 is to allow an entity to reclassify the income tax effects of the Tax Cut and Jobs Act of 2017 (TCJA) on items within Accumulated other comprehensive income (loss), net of tax (AOCI) to retained earnings. Update No. 2018-02 is effective for all entities for annual periods beginning after December 15, 2018, including interim periods within those annual periods. We adopted Update No. 2018-02 in the first quarter of 2019 and have not elected to reclassify the income tax effects of the TCJA from AOCI to retained earnings.
In October 2016, the FASB issued ASC Update No. 2016-16, Income Taxes (Topic 740): Intra-Entity Transfers of Assets Other Than Inventory. The purpose of Update No. 2016-16 is to allow an entity to recognize the income tax consequences of an intra-entity transfer of an asset other than inventory when the transfer occurs, as opposed to waiting until the asset is sold to a third party, or impaired. Update No. 2016-16 was effective for annual periods beginning after December 15, 2017, including interim periods within those annual periods. We adopted Update No. 2016-16 prospectively in the first quarter of 2018 and recognized a net reduction to retained earnings of $55 million for income tax consequences not previously recognized for intra-entity transfers of assets other than inventories. All future income tax consequences of intra-entity transfers of assets other than inventories will be recognized through Income tax expense (benefit).
77
Legal and Product Liability Costs
In the normal course of business, we are involved in various legal and regulatory proceedings, including intellectual property, breach of contract, securities litigation and product liability suits. In some cases, the claimants seek damages, as well as other relief, which, if granted, could require significant expenditures or impact our ability to sell our products. We are also the subject of certain governmental investigations, which could result in substantial fines, penalties and administrative remedies. We maintain an insurance policy providing limited coverage against securities claims, and we are substantially self-insured with respect to product liability claims and fully self-insured with respect to intellectual property infringement claims. We accrue anticipated costs of settlement, damages, losses for product liability claims and, under certain conditions, costs of defense, based on historical experience or to the extent specific losses are probable and estimable. Otherwise, we expense these costs as incurred. If the estimate of a probable loss is a range and no amount within the range is more likely, we accrue our best estimate of the minimum amount of the range. We analyze litigation settlements to identify each element of the arrangement. We allocate arrangement consideration to patent licenses received based on estimates of fair value and capitalize these amounts as assets if the license will provide an ongoing future benefit. We record certain legal and product liability charges, credits and costs of defense, which we consider to be unusual or infrequent and significant as Litigation-related charges (credits) in our consolidated statements of operations; all other legal and product liability charges, credits and costs are recorded within Selling, general and administrative expenses. See Note J – Commitments and Contingencies for discussion of our individual material legal proceedings.
Costs Associated with Exit Activities
We record employee termination costs in accordance with FASB ASC Topic 712, Compensation - Nonretirement and Postemployment Benefits, if we pay the benefits as part of an ongoing benefit arrangement, which includes benefits provided as part of our established severance policies or that we provide in accordance with international statutory requirements. We accrue employee termination costs associated with an ongoing benefit arrangement if the obligation is attributable to prior services rendered, the rights to the benefits have vested, the payment is probable and we can reasonably estimate the liability. We account for involuntary employee termination benefits that represent a one-time benefit in accordance with FASB ASC Topic 420, Exit or Disposal Cost Obligations. We record such costs into expense over the employee’s future service period, if any.
Other costs associated with exit activities may include contract termination costs and consulting fees, which are expensed in accordance with FASB ASC Topic 420 and are included in Restructuring charges (credits) in our consolidated statements of operations. Additionally, costs directly related to our active restructuring initiatives, including program management costs, accelerated depreciation, fixed asset write-offs and costs to transfer product lines among facilities and are included within Costs of products sold and Selling, general and administrative expenses in our consolidated statements of operations. Impairment of right of use lease assets and lease terminations directly related to our active restructuring initiatives are expensed in accordance with FASB ASC Topic 842 and included within Costs of products sold and Selling, general and administrative expenses in our consolidated statements of operations. See Note G – Restructuring-related Activities for further information and discussion of our restructuring plans.
Translation of Foreign Currency
We translate all assets and liabilities of foreign subsidiaries from the functional currency, which is generally the local currency, into U.S. dollars using the year-end exchange rate and translate revenues and expenses at the average exchange rates in effect during the year. We show the net effect of these translation adjustments in our consolidated financial statements as a component of Accumulated other comprehensive income (loss), net of tax. For any significant foreign subsidiaries located in highly inflationary economies, we would re-measure their financial statements as if the functional currency were the U.S. dollar.
Foreign currency transaction gains and losses are included in Other, net in our consolidated statements of operations, net of losses and gains from any related derivative financial instruments.
78
Financial Instruments
We recognize all derivative financial instruments in our consolidated financial statements at fair value in accordance with FASB ASC Topic 815, Derivatives and Hedging, and we present assets and liabilities associated with our derivative financial instruments on a gross basis in our financial statements. In accordance with FASB ASC Topic 815, for those derivative instruments that are designated and qualify as hedging instruments, the hedging instrument must be designated, based upon the exposure being hedged, as a fair value hedge, cash flow hedge, or a hedge of a net investment in a foreign operation. The accounting for changes in the fair value of a derivative instrument depends on whether it qualifies for, and has been designated as part of a hedging relationship, as well as on the type of hedging relationship. Our derivative instruments do not subject our earnings to material risk, as gains and losses on these derivatives generally offset gains and losses on the item being hedged, and we do not enter into derivative transactions for speculative purposes. Refer to Note D – Hedging Activities and Fair Value Measurements for more information on our hedging instruments.
Shipping and Handling Costs
Research and Development
We expense research and development costs, including new product development programs, regulatory compliance and clinical research as incurred. Refer to Indefinite-lived Intangibles, including In-Process Research and Development above for our policy regarding IPR&D acquired in connection with our business combinations and asset purchases.
Net Income (Loss) per Common Share
We base Net income (loss) per common share upon the weighted-average number of common shares and common stock equivalents outstanding during each year. Potential common stock equivalents are determined using the treasury stock method. We exclude stock options and stock awards whose effect would be anti-dilutive from the calculation.
NOTE B – ACQUISITIONS AND STRATEGIC INVESTMENTS
Our consolidated financial statements include the operating results for acquired entities from the respective dates of acquisition. With the exception of the acquisition of BTG, which was completed on August 19, 2019, we have not presented supplemental pro forma financial information for acquisitions given their results are not material to our consolidated financial statements. Transaction costs for all acquisitions in 2019, 2018 and 2017 were immaterial to our consolidated financial statements and were expensed as incurred. In 2019, we recorded approximately $125 million of purchase price adjustments, of which $95 million related to BTG.
79
2019 Acquisitions
BTG plc
On August 19, 2019, we announced the closing of our acquisition of BTG, a public company organized under the laws of England and Wales. BTG had three key portfolios, the largest of which is its interventional medicine portfolio (Interventional Medicine) that encompasses interventional oncology therapeutic technologies for patients with liver and kidney cancers, as well as a vascular portfolio for treatment of deep vein thrombosis, pulmonary embolism, deep venous obstruction and superficial venous disease. Following the closing of the acquisition, we began to integrate BTG's Interventional Medicine business into our Peripheral Interventions division.
In addition to the Interventional Medicine product lines, the BTG portfolio also included a specialty pharmaceutical business (Specialty Pharmaceuticals) comprised of acute care antidotes to treat overexposure to certain medications and toxins and a licensing portfolio (Licensing arrangements) that generates net royalties related to BTG intellectual property and product license agreements. In connection with the acquisition, we acquired rights to future royalties associated with the Zytiga™ drug used to treat certain forms of prostate cancer. In the fourth quarter of 2019, we sold our rights to these royalties for $256 million in cash, included in Proceeds from royalty rights transfer. Refer to Note D – Hedging Activities and Fair Value Measurements for additional information.
The transaction price consisted of upfront cash in the aggregate amount of £3.312 billion (or $4.023 billion based on the exchange rate at closing on August 19, 2019) for the entire issued ordinary share capital of BTG, whereby BTG stockholders received 840 pence (or $10.20 based on the exchange rate at closing) in cash for each BTG share. The transaction price included $404 million of cash and cash equivalents acquired. We implemented our acquisition of BTG by way of a court-sanctioned scheme of arrangement under Part 26 of the United Kingdom Companies Act 2006, as amended.
Purchase Price Allocation
We accounted for our acquisition of BTG as a business combination, and in accordance with FASB ASC Topic 805, Business Combinations (FASB ASC Topic 805), we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date. The preliminary purchase price was comprised of the amounts presented below, which represent the preliminary determination of the fair value of identifiable assets acquired and liabilities assumed from the acquisition. The final determination of the fair value of certain assets and liabilities will be completed within the measurement period as required by FASB ASC Topic 805. As of December 31, 2019, the valuation studies necessary to determine the fair market value of the assets acquired and liabilities assumed are preliminary, including the projection of the underlying cash flows used to determine the fair value of the identified tangible, intangible and financial assets and liabilities.
We accounted for BTG as a business combination, and in accordance with FASB ASC Topic 805, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date. The preliminary purchase price of BTG, was comprised of the following components as of December 31, 2019:
(in millions) | |||
Payment for acquisition, net of cash acquired | $ | ||
80
The following summarizes the preliminary purchase price allocation for our acquisition of BTG as of December 31, 2019:
(in millions) | |||
Goodwill | $ | ||
Trade accounts receivable, net | |||
Inventories | |||
Other current assets | |||
Other intangible assets, net | |||
Other long-term assets | |||
Accrued expenses and other current liabilities | ( | ) | |
Other long-term liabilities | ( | ) | |
Deferred tax liability | ( | ) | |
$ | |||
As a result of our acquisition of BTG, we recognized goodwill of $1.644 billion, which is attributable to the synergies expected to arise from the acquisition and revenue and cash flow projections associated with future technologies. The goodwill is not deductible for tax purposes. As of December 31, 2019, we have allocated $1.406 billion to our Peripheral Interventions reporting unit and $238 million to the Specialty Pharmaceuticals reporting unit.
We allocated a portion of the preliminary purchase price for our acquisition of BTG to specific intangible asset categories as follows:
Amount Assigned (in millions) | Amortization Period (in years) | Risk-Adjusted Discount Rates used in Purchase Price Allocation | ||||||||||
Amortizable intangible assets: | ||||||||||||
Technology-related | $ | - | % | - | ||||||||
Other intangible assets | - | |||||||||||
$ | ||||||||||||
Pro Forma Financial Information (unaudited)
BTG contributed $226 million to our Net sales and had an immaterial impact to our Net income (loss) for the period post acquisition through December 31, 2019.
The unaudited estimated pro forma results presented below include the effects of our acquisition of BTG as if it was consummated on January 1, 2018. In 2019, we incurred nonrecurring charges that we attributed to our acquisition of BTG, which are presented in our consolidated statements of operations for this period. These charges include acquisition-related costs, stock-based compensation expenses as a result of the change in control and retention bonuses and severance payments, adjusted for the related tax effects. We have reflected these nonrecurring charges as adjustments to the pro forma earnings presented below for 2019 and 2018.
Additionally, these pro forma amounts have been calculated after applying our accounting policies and adjusting the results of BTG to reflect the additional costs associated with fair value adjustments relating to inventories, property, plant, and equipment, and intangible assets as if the acquisition had occurred on January 1, 2018, with the consequential tax effects. Additionally, the pro forma amounts have been adjusted to reflect the amortization of deferred financing costs and interest expense associated with additional financing entered into as part of the acquisition. The pro forma results exclude BTG’s historical licensing revenue and related cost of sales, as these arrangements are accounted for as part of the acquisition as a financial asset and liability and are not accounted for within the scope of FASB ASC Topic 606.
81
The supplemental pro forma information presented below is for informational purposes only and should be read in conjunction with our historical financial statements. The pro forma results do not include any anticipated synergies or other expected benefits of the acquisition. Accordingly, the unaudited estimated pro forma financial information below is not necessarily indicative of what the actual results of operations of the combined companies would have been had the acquisition of BTG occurred as of January 1, 2018, nor are they indicative of future results of operations. We believe that the pro forma assumptions and adjustments are reasonable and appropriate under the circumstances and are factually supported based on information currently available.
Year Ended December 31, | |||||||
(in millions, except per share data) (Unaudited) | 2019 | 2018 | |||||
Net sales | $ | $ | |||||
Net income (loss) | $ | $ | |||||
Net income (loss) per common share — basic | $ | $ | |||||
Net income (loss) per common share — assuming dilution | $ | $ | |||||
Transaction with Varian Medical Systems, Inc.
On August 21, 2019, we completed the sale of our drug-eluting and bland embolic microsphere portfolio to Varian Medical Systems, Inc. (Varian) in connection with our acquisition of BTG. The transaction price consisted of an upfront cash payment of $90 million, a portion of which is allocated to the fair value of the services to be rendered under the Transition Services Agreement and Transition Manufacturing Agreement entered into with Varian as part of this transaction. Additionally, we transferred certain contingent consideration arrangements arising from our initial acquisition of the portfolio to Varian and agreed to indemnify Varian for any payments ultimately arising under the terms of the contingent consideration arrangement. Accordingly, as part of the disposal, we recorded a liability of $16 million to recognize the fair value of this guarantee based on our potential obligation resulting from the indemnifications. The maximum amount payable under this guarantee is $200 million in accordance with FASB ASC Topic 460, Guarantees, which is consistent with the contingent consideration arrangement executed with our initial acquisition of the portfolio in accordance with FASB ASC Topic 805.
Vertiflex, Inc.
On June 11, 2019, we announced the closing of our acquisition of Vertiflex, Inc. (Vertiflex), a privately-held company which has developed and commercialized the Superion™ Indirect Decompression System, a minimally-invasive device used to improve physical function and reduce pain in patients with lumbar spinal stenosis (LSS). The transaction price consisted of an upfront cash payment of $465 million and contingent payments that are based on a percentage of Vertiflex sales growth in the first three years following the acquisition close. We estimate the sales-based contingent payments to be in a range of zero to $100 million; however, the payments are uncapped over the three year earn-out period. Following the closing of the acquisition, we have integrated the Vertiflex business into our Neuromodulation division.
Millipede, Inc.
On January 29, 2019, we announced the closing of our acquisition of Millipede, Inc. (Millipede), a privately-held company that has developed the IRIS Transcatheter Annuloplasty Ring System for the treatment of severe mitral regurgitation. We had previously been an investor in Millipede since the first quarter of 2018 as part of an investment and acquisition option agreement, whereby we purchased a portion of the outstanding shares of Millipede, along with newly issued shares of the company, for an upfront cash payment of $90 million. In the fourth quarter of 2018, upon the successful completion of a first-in-human clinical study, we exercised our option to acquire the remaining shares of Millipede. We held an interest of approximately 20 percent immediately prior to the acquisition date. We remeasured the fair value of our previously-held investment based on the implied enterprise value and allocation of purchase price consideration according to priority of equity interests. The transaction price for the remaining stake consisted of an upfront cash payment of $325 million and up to an additional $125 million of future payments upon achievement of a commercial milestone. Following the closing of the acquisition, we have integrated the Millipede business into our Interventional Cardiology division.
82
Purchase Price Allocation
We accounted for our 2019 acquisitions of Vertiflex and Millipede as business combinations, and in accordance with FASB ASC Topic 805, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition dates. The preliminary purchase prices of our acquisitions of Vertiflex and Millipede, presented in aggregate, were comprised of the following components as of December 31, 2019:
(in millions) | |||
Payments for acquisitions, net of cash acquired | $ | ||
Fair value of contingent consideration | |||
Fair value of prior interests | |||
$ | |||
The preliminary purchase price allocations of our acquisitions of Vertiflex and Millipede, presented in aggregate, were comprised of the following components as of December 31, 2019:
(in millions) | |||
Goodwill | $ | ||
Amortizable intangible assets | |||
Indefinite-lived intangible assets | |||
Other assets acquired | |||
Liabilities assumed | ( | ) | |
Net deferred tax liabilities | ( | ) | |
$ | |||
We allocated a portion of the preliminary purchase prices of our acquisitions of Vertiflex and Millipede, presented in aggregate, to the specific intangible asset categories as follows:
Amount Assigned (in millions) | Amortization Period (in years) | Risk-Adjusted Discount Rates used in Purchase Price Allocation | |||||
Amortizable intangible assets: | |||||||
Technology-related | $ | ||||||
Other intangible assets | |||||||
Indefinite-lived intangible assets: | |||||||
In-process research and development | n/a | ||||||
$ | |||||||
2018 Acquisitions
Augmenix, Inc.
On October 16, 2018, we announced the closing of our acquisition of Augmenix, Inc. (Augmenix), a privately-held company that developed and commercialized the SpaceOAR™ Hydrogel System to help reduce common and debilitating side effects that men may experience after receiving radiotherapy to treat prostate cancer. The transaction price consisted of an upfront cash payment of $500 million and up to $100 million in payments contingent upon achieving certain revenue-based milestones. Following the closing of the acquisition, we have integrated the Augmenix business into our Urology and Pelvic Health division.
83
Claret Medical, Inc.
On August 2, 2018, we announced the closing of our acquisition of Claret Medical, Inc. (Claret), a privately-held company that has developed and commercialized the Sentinel™ Cerebral Embolic Protection System. The device is used to protect the brain during certain interventional procedures, predominately in patients undergoing transcatheter aortic valve replacement (TAVR). The transaction price consisted of an upfront cash payment of $220 million and an additional $50 million payment for achieving a reimbursement-based milestone that was achieved in the third quarter of 2018. Following the closing of the acquisition, we have integrated the Claret business into our Interventional Cardiology division.
Cryterion Medical, Inc.
On July 5, 2018, we announced the closing of our acquisition of Cryterion Medical, Inc. (Cryterion), a privately-held company developing a single-shot cryoablation platform for the treatment of atrial fibrillation. We had been an investor in Cryterion since 2016 and held an interest of approximately 35 percent immediately prior to the acquisition date. The transaction price to acquire the remaining stake consisted of an upfront cash payment of $202 million. Following the closing of the acquisition, we have integrated the Cryterion business into our Electrophysiology division.
NxThera, Inc.
On April 30, 2018, we announced the closing of our acquisition of NxThera, Inc. (NxThera), a privately-held company that developed the Rezūm™ System, a minimally invasive therapy in a growing category of treatment options for patients with benign prostatic hyperplasia (BPH). We held a minority interest immediately prior to the acquisition date. The transaction price to acquire the remaining stake consisted of an upfront cash payment of approximately $240 million and up to approximately $85 million in future potential payments contingent upon achieving commercial milestones over the four years following the date of acquisition. Following the closing of the acquisition, we have integrated the NxThera business into our Urology and Pelvic Health division.
nVision Medical Corporation
On April 16, 2018, we announced the closing of our acquisition of nVision Medical Corporation (nVision), a privately-held company focused on women’s health. nVision developed the first and only device cleared by the U.S. Food and Drug Administration (FDA) to collect cells from the fallopian tubes, offering a potential platform for earlier diagnosis of ovarian cancer. The transaction price consisted of an upfront cash payment of $150 million and up to an additional $125 million in future potential payments contingent upon achieving certain clinical and commercial milestones over the four years following the date of acquisition. Following the closing of the acquisition, we have integrated the nVision business into our Urology and Pelvic Health division.
Other Acquisitions
In addition, we completed other individually immaterial acquisitions in 2018 for total consideration of $158 million in cash at closing plus aggregate future potential contingent consideration of up to $62 million.
We recorded gains of $184 million in 2018 within Other, net on our consolidated statements of operations based on the difference between the book values and the fair values of our previously-held investments immediately prior to the acquisition dates. The aggregate fair value of our previously-held investments immediately prior to the acquisition dates was $251 million. We remeasured the fair value of each previously-held investment based on the implied enterprise value and allocation of purchase price consideration according to priority of equity interests.
Purchase Price Allocation
We accounted for these acquisitions as business combinations, and in accordance with FASB ASC Topic 805, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition dates. The components of the aggregate purchase prices are as follows for our 2018 acquisitions as of December 31, 2019:
(in millions) | |||
Payments for acquisitions, net of cash acquired | $ | ||
Fair value of contingent consideration | |||
Fair value of prior interests | |||
$ | |||
84
The following summarizes the purchase price allocations for our 2018 acquisitions as of December 31, 2019:
(in millions) | |||
Goodwill | $ | ||
Amortizable intangible assets | |||
In-process research and development | |||
Other assets acquired | |||
Liabilities assumed | ( | ) | |
Net deferred tax liabilities | ( | ) | |
$ | |||
We allocated a portion of the purchase prices to specific intangible asset categories as follows:
Amount Assigned (in millions) | Amortization Period (in years) | Risk-Adjusted Discount Rates used in Purchase Price Allocation | ||||||||||
Amortizable intangible assets | ||||||||||||
Technology-related | $ | - | % | - | ||||||||
Other intangible assets | - | % | - | |||||||||
Indefinite-lived intangible assets | ||||||||||||
In-process research and development | n/a | |||||||||||
$ | ||||||||||||
2017 Acquisitions
Apama Medical Inc.
On October 11, 2017, we announced the closing of our acquisition of Apama Medical Inc. (Apama), a privately-held company developing the Apama™ Radiofrequency single-shot Balloon Catheter System for the treatment of atrial fibrillation. The transaction price consisted of an upfront cash payment of approximately $175 million and up to approximately $125 million in future potential payments contingent upon achieving certain clinical and regulatory milestones. Following the closing of the acquisition, we have integrated the Apama business into our Electrophysiology division.
Symetis SA
On May 16, 2017, we announced the closing of our acquisition of Symetis SA (Symetis), a privately-held Swiss structural heart company focused on minimally-invasive TAVR devices, having developed the ACURATE neo™ Aortic Valve. The transaction price consisted of an upfront cash payment of approximately $430 million. Following the closing of the acquisition, we have integrated the Symetis business into our Interventional Cardiology division.
Purchase Price Allocation
We accounted for these acquisitions as business combinations and, in accordance with FASB ASC Topic 805, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition dates. The components of the aggregate purchase prices were as follows for our 2017 acquisitions:
(in millions) | |||
Payment for acquisitions, net of cash acquired | $ | ||
Fair value of contingent consideration | |||
$ | |||
85
The following summarizes the aggregate purchase price allocations for our 2017 acquisitions:
(in millions) | |||
Goodwill | $ | ||
Amortizable intangible assets | |||
Indefinite-lived intangible assets | |||
Other assets acquired | |||
Liabilities assumed | ( | ) | |
Deferred tax liabilities | ( | ) | |
$ | |||
We allocated a portion of the purchase prices to specific intangible asset categories as follows:
Amount Assigned (in millions) | Amortization Period (in years) | Risk-Adjusted Discount Rates used in Purchase Price Allocation | |||||||
Amortizable intangible assets | |||||||||
Technology-related | $ | ||||||||
Other intangible assets | - | ||||||||
Indefinite-lived intangible assets | |||||||||
In-process research and development | $ | n/a | |||||||
$ | |||||||||
Our technology-related intangible assets consist of technical processes, intellectual property and institutional understanding with respect to products and processes that we intend to leverage in future products or processes and will carry forward from one product generation to the next. We used the multi-period excess earnings method, a form of the income approach, to derive the fair value of the technology-related intangible assets and are amortizing them on a straight-line basis over their assigned estimated useful lives.
Goodwill was primarily established due to synergies expected to be gained from leveraging our existing operations as well as revenue and cash flow projections associated with future technologies and has been allocated to our reportable segments based on the relative expected benefit. Based on preliminary estimates updated for applicable regulatory changes, the goodwill recorded relating to our 2019, 2018 and 2017 acquisitions is not deductible for tax purposes.
Contingent Consideration
Changes in the fair value of our contingent consideration liability were as follows:
(in millions) | |||
Balance as of December 31, 2017 | $ | ||
Amounts recorded related to current year acquisitions | |||
Purchase price adjustments related to prior year acquisitions | ( | ) | |
Contingent consideration expense (benefit) | ( | ) | |
Contingent consideration payments | ( | ) | |
Balance as of December 31, 2018 | $ | ||
Amounts recorded related to current year acquisitions | |||
Contingent consideration arrangements transferred to Varian | ( | ) | |
Contingent consideration expense (benefit) | ( | ) | |
Contingent consideration payments | ( | ) | |
Balance as of December 31, 2019 | $ | ||
86
As of December 31, 2019, the maximum amount of future contingent consideration (undiscounted) that we could be required to pay was $697 million, which includes our estimate of maximum contingent payments of $100 million associated with the Vertiflex acquisition described above. The maximum decreased $176 million compared to the amount as of December 31, 2018 due to the contingent consideration arrangement which is now accounted for as a guarantee in connection with our transaction with Varian as discussed in the BTG section above. In addition, the aggregated maximum decreased as a result of the expiration or full payment of certain contingent consideration arrangements in 2019, partially offset by the Millipede and Vertiflex arrangements entered into in 2019.
The recurring Level 3 fair value measurements of our contingent consideration liability include the following significant unobservable inputs:
Contingent Consideration Liability | Fair Value as of December 31, 2019 | Valuation Technique | Unobservable Input | Range | Weighted Average (1) | |||
R&D, Regulatory and Commercialization-based Milestones | $ | Discounted Cash Flow | Discount Rate | % | - | |||
Probability of Payment | % | - | ||||||
Projected Year of Payment | 2020 | - | 2027 | 2021 | ||||
Revenue-based Payments | $ | Discounted Cash Flow | Discount Rate | % | - | |||
Probability of Payment | % | - | ||||||
Projected Year of Payment | 2020 | - | 2026 | 2021 | ||||
(1) | Unobservable inputs were weighted by the relative fair value of the contingent consideration liability. For projected year of payment, the amount represents the median of the inputs and is not a weighted average. |
Projected contingent payment amounts related to some of our R&D, commercialization-based and revenue-based milestones are discounted back to the current period using a discounted cash flow model. Significant increases or decreases in projected revenues, probabilities of payment, discount rates or the time until payment is made would have resulted in a significantly lower or higher fair value measurement as of December 31, 2019.
Strategic Investments
The aggregate carrying amount of our strategic investments was comprised of the following:
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Equity method investments | $ | $ | |||||
Measurement alternative investments(1) | |||||||
Publicly-held securities(2) | |||||||
Notes receivable | |||||||
$ | $ | ||||||
(1) | Measurement alternative investments are privately-held equity securities without readily determinable fair values that are measured at cost less impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. |
(2) | Publicly-held equity securities are measured at fair value with changes in fair value recognized currently in Other, net on our accompanying consolidated statements of operations. |
These investments are classified as Other long-term assets within our accompanying consolidated balance sheets, in accordance with U.S. GAAP and our accounting policies.
As of December 31, 2019, the cost of our aggregated equity method investments exceeded our share of the underlying equity in net assets by $314 million, which represents amortizable intangible assets, IPR&D, goodwill and deferred tax liabilities.
87
NOTE C – GOODWILL AND OTHER INTANGIBLE ASSETS
The gross carrying amount of goodwill and other intangible assets and the related accumulated amortization for intangible assets subject to amortization and accumulated write-offs of goodwill are as follows:
As of December 31, 2019 | As of December 31, 2018 | ||||||||||||||
(in millions) | Gross Carrying Amount | Accumulated Amortization/Write-offs | Gross Carrying Amount | Accumulated Amortization/Write-offs | |||||||||||
Amortizable intangible assets | |||||||||||||||
Technology-related | $ | $ | ( | ) | $ | $ | ( | ) | |||||||
Patents | ( | ) | ( | ) | |||||||||||
Other intangible assets | ( | ) | ( | ) | |||||||||||
$ | $ | ( | ) | $ | $ | ( | ) | ||||||||
Indefinite-lived intangible assets | |||||||||||||||
Goodwill | $ | $ | ( | ) | $ | $ | ( | ) | |||||||
In-process research and development (IPR&D) | — | — | |||||||||||||
Technology-related | |||||||||||||||
$ | $ | ( | ) | $ | $ | ( | ) | ||||||||
In the third quarter of 2019, we performed our annual impairment test of all IPR&D projects and our indefinite-lived core technology assets and determined that the assets were not impaired. In addition, we verified the classification as indefinite-lived assets continues to be appropriate.
Intangible asset impairment charges were $105 million in 2019, $35 million in 2018 and $4 million in 2017. Refer to Note A - Significant Accounting Policies for a discussion of key assumptions used in our goodwill and intangible asset impairment testing.
Effective January 1, 2018, we reclassified our Neuromodulation operating segment and associated goodwill balance from our MedSurg reportable segment to our Rhythm and Neuro reportable segment. This change did not impact our total goodwill carrying value.
Our seven core businesses are organized into three reportable segments: MedSurg, Rhythm and Neuro, and Cardiovascular. Following our acquisition of BTG, which closed during the third quarter of 2019, we have included BTG’s Interventional Medicine business within our Peripheral Interventions operating segment, within the Cardiovascular reportable segment. We present BTG’s Specialty Pharmaceuticals business as a standalone operating segment alongside our reportable segments.
88
The following represents our goodwill balance by global reportable segment and our separately reported Specialty Pharmaceuticals operating segment:
(in millions) | MedSurg | Rhythm and Neuro | Cardiovascular | Specialty Pharmaceuticals | Total | ||||||||||||||
Balance as of December 31, 2017 | $ | $ | $ | $ | $ | ||||||||||||||
Reportable segment revisions | ( | ) | — | — | — | ||||||||||||||
Foreign currency fluctuations and other changes | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||
Goodwill acquired | |||||||||||||||||||
Balance as of December 31, 2018 | $ | $ | $ | $ | $ | ||||||||||||||
Foreign currency fluctuations and other changes | ( | ) | |||||||||||||||||
Goodwill acquired | |||||||||||||||||||
Goodwill divested | — | — | ( | ) | ( | ) | |||||||||||||
Balance as of December 31, 2019 | $ | $ | $ | $ | $ | ||||||||||||||
We did not have any goodwill impairments in 2019, 2018 or 2017.
Estimated Amortization expense for each of the five succeeding fiscal years based upon our amortizable intangible asset portfolio as of December 31, 2019 is as follows (in millions):
Fiscal Year | |||
2020 | $ | ||
2021 | |||
2022 | |||
2023 | |||
2024 | |||
NOTE D – HEDGING ACTIVITIES AND FAIR VALUE MEASUREMENTS
Derivative Instruments and Hedging Activities
We address market risk from changes in foreign currency exchange rates and interest rates through risk management programs which include the use of derivative and nonderivative financial instruments. We operate these programs pursuant to documented corporate risk management policies and do not enter into derivative transactions for speculative purposes. Our derivative instruments do not subject our earnings to material risk, as the gains or losses on these derivatives generally offset losses or gains recognized on the hedged item.
We manage concentration of counterparty credit risk by limiting acceptable counterparties to major financial institutions with investment grade credit ratings, limiting the amount of credit exposure to individual counterparties and by actively monitoring counterparty credit ratings and the amount of individual credit exposure. We also employ master netting arrangements that limit the risk of counterparty non-payment on a particular settlement date to the net gain that would have otherwise been received from the counterparty. Although not completely eliminated, we do not consider the risk of counterparty default to be significant as a result of these protections. Further, none of our derivative instruments are subject to collateral or other security arrangements, nor do they contain provisions that are dependent on our credit ratings from any credit rating agency.
89
Currency Hedging Instruments
Risk Management Strategy
Our risk from changes in currency exchange rates consists primarily of monetary assets and liabilities, forecast intercompany and third-party transactions, net investments in certain subsidiaries and the purchase price of any acquisition that is denominated in a currency other than the U.S. dollar. We manage currency exchange rate risk at a consolidated level to reduce the cost of hedging by taking advantage of offsetting transactions. We employ derivative and nonderivative instruments, primarily forward currency contracts, to reduce the risk to our earnings and cash flows associated with changes in currency exchange rates.
The success of our currency risk management program depends, in part, on forecast transactions denominated primarily in Euro, Japanese yen, Chinese renminbi and British pound sterling. We may experience unanticipated currency exchange gains or losses to the extent the actual activity is different than forecast. In addition, changes in currency exchange rates related to any unhedged transactions may impact our earnings and cash flows.
Hedge Designations and Relationships
Certain of our currency derivative instruments are designated as cash flow hedges under FASB ASC Topic 815, Derivatives and Hedging (FASB ASC Topic 815), and are intended to protect the U.S. dollar value of forecasted transactions. The gain or loss on a derivative instrument designated as a cash flow hedge is recorded in the Net change in derivative financial instruments component of Other comprehensive income (loss), net of tax (OCI) on our consolidated statements of comprehensive income (loss) until the underlying third-party transaction occurs. When the underlying third-party transaction occurs, we recognize the gain or loss in earnings within the Cost of products sold caption of our consolidated statements of operations. In the event the hedging relationship is no longer effective, or if the occurrence of the hedged forecast transaction becomes no longer probable, we reclassify the gains or losses within AOCI to earnings at that time.
We also designate certain forward currency contracts as net investment hedges to hedge a portion of our net investments in certain of our entities with functional currencies denominated in the Euro, Swiss franc, Japanese yen, British pound sterling, South Korean won and Taiwan dollar. We elected to use the spot method to assess effectiveness for our derivatives that are designated as net investment hedges. Under the spot method, the change in fair value attributable to changes in the spot rate is recorded in the Foreign currency translation adjustment (CTA) component of OCI. We have elected to exclude the spot-forward difference from the assessment of hedge effectiveness and are amortizing this amount separately, as calculated at the date of designation, on a straight-line basis over the term of the currency forward contracts. Amortization of the spot-forward difference is then reclassified from AOCI to current period earnings as a component of Interest expense on our consolidated statements of operations. In November 2019, we terminated and settled all of our outstanding forward currency contracts designated as net investment hedges in our entities with Euro-denominated functional currencies and recognized a gain of $95 million presented in the CTA component of OCI on our consolidated statements of comprehensive income (loss).
We also completed an offering of €900 million (approximately $1.000 billion) in aggregate principal amount of 0.625 % senior notes due in 2027. The Euro-denominated debt principal is a nonderivative instrument designated as a net investment hedge of our net investments in certain of our Euro functional entities. As of December 31, 2019, the notional value of our outstanding net investment hedges was $1.950 billion, which includes our derivative and nonderivative instruments designated as net investment hedges.
We also use forward currency contracts that are not part of designated hedging relationships under FASB ASC Topic 815 as a part of our strategy to manage our exposure to currency exchange rate risk related to monetary assets and liabilities and related forecast transactions. These non-designated currency forward contracts have an original time to maturity consistent with the hedged currency transaction exposures, generally less than one year, and are marked-to-market with changes in fair value recorded to earnings within the Other, net caption of our consolidated statements of operations.
Certain of our non-designated forward currency contracts were entered into for the purpose of managing our exposure to currency exchange rate risk related to the GBP-denominated purchase price of BTG. In 2019, we settled all outstanding contracts, resulting in a cumulative loss on the contracts of $294 million that was recognized over time in earnings as we adjusted for changes in fair value until the final fair value was determined at maturity. As of December 31, 2018, the notional value of the contracts was $2.550 billion, and we entered into additional contracts in 2019. Upon settlement in 2019, we received £3.312 billion of cash to fund our acquisition of BTG, which translated into $4.303 billion based on hedged currency exchange rates. We recognized a $323 million loss in 2019 and a $29 million gain in 2018 within Other, net due to changes in fair value of the contracts.
90
Interest Rate Hedging Instruments
Risk Management Strategy
Our interest rate risk relates primarily to U.S. dollar borrowings partially offset by U.S. dollar cash investments. We use interest rate derivative instruments to mitigate the risk to our earnings and cash flows associated with exposure to changes in interest rates. Under these agreements we and the counterparty, at specified intervals, exchange the difference between fixed and floating interest amounts calculated by reference to an agreed-upon notional principal amount. We designate these derivative instruments either as fair value or cash flow hedges in accordance with FASB ASC Topic 815.
Hedge Designations and Relationships
We had no interest rate derivative instruments designated as cash flow hedges outstanding as of December 31, 2019 and $1.000 billion outstanding as of December 31, 2018, which were intended to manage our earnings and cash flow exposure to changes in the benchmark interest rate in connection with the forecasted issuance of fixed-rate debt. For outstanding designated cash flow hedges, we record the changes in the fair value of the derivatives within OCI until the underlying hedged transaction occurs, at which time we recognize the gain or loss within Interest expense over the same period that the hedged items affect earnings, so long as the hedge relationship remains effective. If we determine the hedging relationship is no longer effective, or if the occurrence of the hedged forecast transaction becomes no longer probable, we reclassify the amount of gains or losses from AOCI to earnings at that time.
During the fourth quarter of 2018, we entered into interest rate derivative contracts designated as cash flow hedges having a notional amount of $1.000 billion to hedge interest rate risk. In the first quarter of 2019, we terminated these instruments in connection with our senior notes issuance in the same period as discussed in Note E – Contractual Obligations and Commitments. We recognized an immaterial loss within OCI in 2019 and are reclassifying the amortization of the loss from AOCI into earnings as a component of Interest expense over the same period that the hedged item affects earnings, so long as the hedge relationship remains effective. We are also continuing to reclassify in a similar manner the amortization of the gains or losses of our other previously terminated interest rate derivative instruments that were designated as cash flow hedges. The balance of the deferred amounts on our terminated cash flow hedges within AOCI was a $34 million loss as of December 31, 2019 and a $7 million gain December 31, 2018. We recognized immaterial gains and losses in Interest expense relating to the amortization of our terminated cash flow hedges in the current and prior periods.
We had no interest rate derivative instruments designated as fair value hedges outstanding as of December 31, 2019 and December 31, 2018. Prior to 2018, we terminated interest rate derivative instruments that were designated as fair value hedges and are continuing to recognize the amortization of the gains or losses originally recorded within the Long-term debt caption on our consolidated balance sheets into earnings as a component of Interest expense over the same period that the discount or premium associated with the hedged items affects earnings. In the event that we designate outstanding interest rate derivative instruments as fair value hedges, we record the changes in the fair values of interest rate derivatives designated as fair value hedges and of the underlying hedged debt instruments in Interest expense, which generally offset. The balance of the deferred gains on our terminated fair value hedges within Long-term debt was immaterial as of December 31, 2019 and December 31, 2018. We recognized immaterial gains in Interest expense relating to the amortization of the terminated fair value hedges in the current and prior periods.
The following table presents the contractual amounts of our hedging instruments outstanding:
(in millions) | FASB ASC Topic 815 Designation | As of December 31, | ||||||||
2019 | 2018 | |||||||||
Forward currency contracts | Cash flow hedge | $ | $ | |||||||
Forward currency contracts | Net investment hedge | |||||||||
Foreign currency-denominated debt(1) | Net investment hedge | |||||||||
Forward currency contracts | Non-designated | |||||||||
Interest rate derivative contracts | Cash flow hedge | |||||||||
Total Notional Outstanding | $ | $ | ||||||||
(1) | The € |
91
The remaining time to maturity as of December 31, 2019 is within 60 months for all designated forward currency contracts and generally less than one year for all non-designated forward currency contracts. The Euro-denominated debt principal designated as a net investment hedge has a contractual maturity of December 1, 2027.
The following presents the effect of our derivative and nonderivative instruments designated as cash flow and net investment hedges under FASB ASC Topic 815 on our accompanying consolidated statements of operations. Refer to Note P – Changes in Other Comprehensive Income for the total amounts relating to derivative and nonderivative instruments presented within the consolidated statements of comprehensive income (loss).
Effect of Hedging Relationships on Accumulated Other Comprehensive Income | ||||||||||||||||||||||||
Amount Recognized in OCI on Hedges | Consolidated Statements of Operations (1) | Amount Reclassified from AOCI into Earnings | ||||||||||||||||||||||
Pre-Tax Gain (Loss) | Tax Benefit (Expense) | Gain (Loss) Net of Tax | Location of Amount Reclassified | Total Amount of Line Item Presented | Pre-Tax (Gain) Loss | Tax (Benefit) Expense | (Gain) Loss Net of Tax | |||||||||||||||||
Year Ended December 31, 2019 | ||||||||||||||||||||||||
Forward currency contracts | ||||||||||||||||||||||||
Cash flow hedges | $ | $ | ( | ) | $ | Cost of products sold | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||||
Net investment hedges (2) | ( | ) | Interest expense | ( | ) | ( | ) | |||||||||||||||||
Foreign currency-denominated debt | ||||||||||||||||||||||||
Net investment hedges | ( | ) | ( | ) | Interest expense | — | — | — | ||||||||||||||||
Interest rate derivative contracts | ||||||||||||||||||||||||
Cash flow hedges | Interest expense | ( | ) | |||||||||||||||||||||
Year Ended December 31, 2018 | ||||||||||||||||||||||||
Forward currency contracts | ||||||||||||||||||||||||
Cash flow hedges | $ | $ | ( | ) | $ | Cost of products sold | $ | $ | $ | ( | ) | $ | ||||||||||||
Net investment hedges (2) | ( | ) | Interest expense | ( | ) | ( | ) | |||||||||||||||||
Interest rate derivative contracts | ||||||||||||||||||||||||
Cash flow hedges | ( | ) | ( | ) | Interest expense | ( | ) | ( | ) | |||||||||||||||
Year Ended December 31, 2017 | ||||||||||||||||||||||||
Forward currency contracts | ||||||||||||||||||||||||
Cash flow hedges | $ | ( | ) | $ | $ | ( | ) | Cost of products sold | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||
Interest rate derivative contracts | ||||||||||||||||||||||||
Cash flow hedges | Interest expense | ( | ) | ( | ) | |||||||||||||||||||
(1) | In all periods presented in the table above, the pre-tax (gain) loss amounts reclassified from AOCI to earnings represent the effect of the hedging relationships on earnings. All other amounts included in earnings related to hedging relationships were immaterial. |
(2) | For our outstanding forward currency contracts designated as net investment hedges, the net gain or loss reclassified from AOCI to earnings as a reduction of Interest expense represents the straight-line amortization of the excluded component as calculated at the date of designation. This initial value of the excluded component has been excluded from the assessment of effectiveness in accordance with FASB ASC Topic 815. In the current period, we did not recognize any gains or losses on the components included in the assessment of hedge effectiveness in earnings. |
92
As of December 31, 2019, pre-tax net gains or losses for our derivative instruments designated, or previously designated, as cash flow and net investment hedges under FASB ASC Topic 815 that may be reclassified from AOCI to earnings within the next twelve months are presented below (in millions):
Designated Hedging Instrument | FASB ASC Topic 815 Designation | Location on Consolidated Statements of Operations | Amount of Pre-Tax Gain (Loss) that may be Reclassified to Earnings | |||||
Forward currency contracts | Cash flow hedge | Cost of products sold | $ | |||||
Forward currency contracts | Net investment hedge | Interest expense | ||||||
Interest rate derivative contracts | Cash flow hedge | Interest expense | ( | ) | ||||
Net gains and losses on currency hedge contracts not designated as hedging instruments offset by net gains and losses from currency transaction exposures are presented below:
(in millions) | Location on Consolidated Statements of Operations | Year Ended December 31, | ||||||||||||
2019 | 2018 | 2017 | ||||||||||||
Net gain (loss) on currency hedge contracts | Other, net | $ | ( | ) | $ | $ | ( | ) | ||||||
Net gain (loss) on currency transaction exposures | Other, net | ( | ) | ( | ) | |||||||||
Net currency exchange gain (loss) | $ | ( | ) | $ | $ | ( | ) | |||||||
Certain of our non-designated forward currency contracts were entered into for the purpose of managing our exposure to currency exchange rate risk related to the GBP-denominated purchase price of BTG. In 2019, we settled all outstanding contracts. We recognized a $323 million loss in 2019 and a $29 million gain in 2018 within Net gain (loss) on currency hedge contracts due to changes in fair value of the contracts.
93
Fair Value Measurements
(in millions) | Location on Consolidated Balance Sheets (1) | As of December 31, | ||||||||
2019 | 2018 | |||||||||
Derivative and Nonderivative Assets: | ||||||||||
Designated Hedging Instruments | ||||||||||
Forward currency contracts | Other current assets | $ | $ | |||||||
Forward currency contracts | Other long-term assets | |||||||||
Non-Designated Hedging Instruments | ||||||||||
Forward currency contracts | Other current assets | |||||||||
Total Derivative and Nonderivative Assets | $ | $ | ||||||||
Derivative and Nonderivative Liabilities: | ||||||||||
Designated Hedging Instruments | ||||||||||
Forward currency contracts | Other current liabilities | $ | $ | |||||||
Forward currency contracts | Other long-term liabilities | |||||||||
Foreign currency-denominated debt | Other long-term liabilities | |||||||||
Interest rate contracts | Other current liabilities | |||||||||
Non-Designated Hedging Instruments | ||||||||||
Forward currency contracts | Other current liabilities | |||||||||
Total Derivative and Nonderivative Liabilities | $ | $ | ||||||||
(1) | We classify derivative and nonderivative assets and liabilities as current when the settlement date of the contract is one year or less. |
Recurring Fair Value Measurements
On a recurring basis, we measure certain financial assets and financial liabilities at fair value based upon quoted market prices. Where quoted market prices or other observable inputs are not available, we apply valuation techniques to estimate fair value. FASB ASC Topic 820 establishes a three-level valuation hierarchy for disclosure of fair value measurements. The category of a financial asset or a financial liability within the valuation hierarchy is based upon the lowest level of input that is significant to the measurement of fair value. The three levels of the hierarchy are defined as follows:
• | Level 1 – Inputs to the valuation methodology are quoted market prices for identical assets or liabilities. |
• | Level 2 – Inputs to the valuation methodology are other observable inputs, including quoted market prices for similar assets or liabilities and market-corroborated inputs. |
• | Level 3 – Inputs to the valuation methodology are unobservable inputs based on management’s best estimate of inputs market participants would use in pricing the asset or liability at the measurement date, including assumptions about risk. |
94
Assets and liabilities measured at fair value on a recurring basis consist of the following:
As of | |||||||||||||||||||||||||||||||
December 31, 2019 | December 31, 2018 | ||||||||||||||||||||||||||||||
(in millions) | Level 1 | Level 2 | Level 3 | Total | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||||||
Assets | |||||||||||||||||||||||||||||||
Money market and government funds | $ | $ | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||
Publicly-held securities | |||||||||||||||||||||||||||||||
Hedging instruments | |||||||||||||||||||||||||||||||
Licensing arrangements | — | — | — | — | — | — | |||||||||||||||||||||||||
$ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
Liabilities | |||||||||||||||||||||||||||||||
Hedging instruments | $ | $ | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||
Contingent consideration liability | |||||||||||||||||||||||||||||||
Licensing arrangements | — | — | — | — | — | — | |||||||||||||||||||||||||
$ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
Our investments in money market and government funds are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices. These investments are classified as Cash and cash equivalents within our accompanying consolidated balance sheets, in accordance with U.S. GAAP and our accounting policies. In addition to $50 million invested in money market and government funds as of December 31, 2019, we had $165 million in interest bearing and non-interest-bearing bank accounts. In addition to $13 million invested in money market and government funds as of December 31, 2018, we had $133 million in interest bearing and non-interest bearing bank accounts.
Our recurring fair value measurements using Level 3 inputs relates to our contingent consideration liability. Refer to Note B – Acquisitions and Strategic Investments for a discussion of the changes in the fair value of our contingent consideration liability.
In addition, our recurring fair value measurements using Level 3 inputs relate to our licensing arrangements. In connection with our acquisition of BTG, we acquired intellectual property and related licensing arrangements, principally relating to Zytiga™, as a result of our acquisition of BTG that provides the contractual right to receive future royalty payments.
In the fourth quarter of 2019, we entered into a royalty purchase agreement with the Ontario Municipal Employees Retirement System (OMERS), whereby we sold to OMERS our remaining 50 percent of the future Zytiga™ royalty stream. The purchase price for these royalty interests consisted of an upfront cash payment of $256 million. Prior to our acquisition of BTG, BTG agreed to pay 50 percent of the Zytiga™ royalty stream, net of certain offsets, to the inventors associated with the intellectual property. As such, we do not expect to receive any future cash benefit from Zytiga™ royalties subsequent to our transaction with OMERS. In accordance with FASB ASC Topic 860, Transfers and Servicing, we are accounting for the transfer as a secured borrowing and continue to recognize the financial asset and financial liability in our consolidated balance sheets.
We have elected the fair value option to account for the licensing arrangements' financial asset and financial liability in accordance with FASB ASC Topic 825, Financial Instruments. As of December 31, 2019, we have recorded the fair values of the financial asset and financial liability using a discounted cash flow approach considering the probability-weighted expected future cash flows to be generated by the royalty stream. The fair value of the financial liability also considers the related contractual provisions that govern our payment obligations.
In connection with our preliminary purchase price allocation of BTG as of the acquisition date, the amount recognized for the financial asset was $567 million in aggregate, comprised of $195 million included in Other current assets and $373 million included in Other long-term assets. The amount recognized for the financial liability was $370 million, comprised of $54 million included in Accounts payable, $104 million included in Other current liabilities and $212 million included in Other long-term liabilities. The amounts above were valued using the fair value option with the exception of the Accounts payable, which was settled in the third quarter. During the fourth quarter, we also recorded the $256 million proceeds received from the sale of the Zytiga royalty stream as an increase in the fair value of the financial liability now accounted for as a secured borrowing, as discussed above. Refer to Note B – Acquisitions and Strategic Investments for further information on the preliminary purchase price allocation of BTG.
95
The recurring Level 3 fair value measurements of our licensing arrangements recognized in our consolidated balance sheets as of December 31, 2019 include the following significant unobservable inputs:
Licensing Arrangements | Fair Value as of December 31, 2019 | Valuation Technique | Unobservable Input | Range | Weighted Average (1) | |||
Financial Asset | $ | Discounted Cash Flow | Discount Rate | % | - | |||
Projected Year of Payment | 2020 | - | 2028 | 2024 | ||||
Financial Liability | $ | Discounted Cash Flow | Discount Rate | |||||
Projected Year of Payment | 2020 | - | 2027 | 2023 | ||||
(1) | Unobservable inputs relate to a single financial asset and liability. As such, unobservable inputs were not weighted by the relative fair value of the instruments. For projected year of payment, the amount represents the median of the inputs and is not a weighted average. |
Significant increases or decreases in projected cash flows of the royalty stream and the related contractual provisions that govern our payment obligations, discount rates or the time until payment is made would have resulted in a significantly lower or higher fair value measurement of the licensing arrangements' financial asset and liability as of December 31, 2019. However, increases or decreases in the financial asset would be substantially offset by increases or decreases in the financial liability, as such our earnings are not subject to material gains or losses from the licensing arrangement.
Changes in the fair value of our licensing arrangements' financial asset was as follows:
(in millions) | |||
Balance as of December 31, 2018 | $ | ||
Amounts recorded related to current year acquisition | |||
Proceeds from royalty rights | ( | ) | |
Fair value adjustment (expense) benefit | |||
Balance as of December 31, 2019 | $ | ||
Changes in the fair value of our licensing arrangements' financial liability was as follows:
(in millions) | |||
Balance as of December 31, 2018 | $ | ||
Amounts recorded related to current year acquisition | |||
Proceeds from secured borrowings relating to royalty arrangements | |||
Balance as of December 31, 2019 | $ | ||
Non-Recurring Fair Value Measurements
We hold certain assets and liabilities that are measured at fair value on a non-recurring basis in periods after initial recognition. The fair value of a measurement alternative investment is not estimated if there are no identified events or changes in circumstances that may have a significant adverse effect on the fair value of the investment. Refer to Note B – Acquisitions and Strategic Investments for a discussion of our strategic investments.
Refer to Note C – Goodwill and Other Intangible Assets for a discussion of the fair values.
The fair value of our outstanding debt obligations as of December 31, 2019 was $11.020 billion, of which $1.004 billion relates to the Euro-denominated December 2027 Notes, and as of December 31, 2018 was $7.239 billion. We determined fair value by using quoted market prices for our publicly registered senior notes, classified as Level 1 within the fair value hierarchy, and face value for commercial paper, term loans and credit facility borrowings outstanding. Refer to Note E – Contractual Obligations and Commitments for a discussion of our debt obligations.
96
NOTE E – CONTRACTUAL OBLIGATIONS AND COMMITMENTS
Borrowings and Credit Arrangements
We had total debt of $10.008 billion as of December 31, 2019 and $7.056 billion as of December 31, 2018. The debt maturity schedule for our long-term debt obligations is presented below:
Issuance Date | Maturity Date | As of December 31, | Stated Interest Rate | |||||||||||
(in millions, except interest rates) | 2019 | 2018 | ||||||||||||
January 2020 Notes | December 2009 | January 2020 | $ | $ | ||||||||||
May 2020 Notes | May 2015 | May 2020 | ||||||||||||
May 2022 Notes | May 2015 | May 2022 | ||||||||||||
August 2022 Term Loan | August 2019 | August 2022 | ||||||||||||
October 2023 Notes | August 2013 | October 2023 | ||||||||||||
March 2024 Notes | February 2019 | March 2024 | ||||||||||||
May 2025 Notes | May 2015 | May 2025 | ||||||||||||
March 2026 Notes | February 2019 | March 2026 | ||||||||||||
December 2027 Notes | November 2019 | December 2027 | ||||||||||||
March 2028 Notes | February 2018 | March 2028 | ||||||||||||
March 2029 Notes | February 2019 | March 2029 | ||||||||||||
November 2035 Notes (1) | November 2005 | November 2035 | ||||||||||||
March 2039 Notes | February 2019 | March 2039 | ||||||||||||
January 2040 Notes | December 2009 | January 2040 | ||||||||||||
March 2049 Notes | February 2019 | March 2049 | ||||||||||||
Unamortized Debt Issuance Discount and Deferred Financing Costs | 2020 - 2049 | ( | ) | ( | ) | |||||||||
Unamortized Gain on Fair Value Hedges | 2020-2023 | |||||||||||||
Finance Lease Obligation (2) | Various | |||||||||||||
Long-term debt | $ | $ | ||||||||||||
Note: The table above does not include unamortized amounts related to interest rate contracts designated as cash flow hedges.
(1) | Corporate credit rating improvements may result in a decrease in the adjusted interest rate on our November 2035 Notes to the extent that our lowest credit rating is above BBB- or Baa3. The interest rates on our November 2035 Notes will be permanently reinstated to the issuance rate if the lowest credit ratings assigned to these senior notes is either A- or A3 or higher. |
(2) | Effective January 1, 2019, we adopted FASB ASC Topic 842, which requires that we recognize finance lease obligations in our consolidated balance sheets. As of December 31, 2018, these leases were referred to as capital lease obligations in accordance with FASB ASC Topic 840. Please refer to Note A – Significant Accounting Policies for additional information. |
Revolving Credit Facility
On December 19, 2018, we entered into a $2.750 billion revolving credit facility (the 2018 Facility) with a global syndicate of commercial banks and terminated our previous $2.250 billion revolving credit facility (the 2017 Facility), which was scheduled to mature in August 2022. The 2018 Facility will mature on December 19, 2023 with one-year extension options subject to certain conditions. Eurodollar and multicurrency loans bear interest at the Eurocurrency Rate determined for the interest period plus the applicable margin, based on our corporate credit ratings (1.02 percent as of December 31, 2019). ABR loans bear interest at ABR plus the applicable margin of up to 0.40 percent, based on our corporate credit ratings. Under the credit agreement for the 2018 Facility (the 2018 Credit Agreement), we are required to pay a facility fee (0.11 percent as of December 31, 2019) based on our credit ratings and the total amount of revolving credit commitment, regardless of usage of the 2018 Facility. This facility provides backing for the commercial paper program described below. The 2018 Credit Agreement for the 2018 Facility requires that we comply with certain covenants, including financial covenants as described below. There were no amounts borrowed under our current or prior revolving credit facilities as of December 31, 2019 or December 31, 2018.
97
Term Loans
On December 5, 2019, we entered into a $700 million term loan credit agreement scheduled to mature on December 3, 2020 (2020 Term Loan). The 2020 Term Loan bears interest at an annual rate of LIBOR plus a margin of 0.65 %. In addition, we pay customary expenses. The credit agreement contains covenants, as described below, and also contains customary events of default, which may result in the acceleration of any outstanding commitments. As of December 31, 2019, we had $700 million outstanding under the 2020 Term Loan, which is presented within Current debt obligations on our consolidated balance sheet. We used the proceeds from the 2020 Term Loan to repay a portion of the Two-Year Delayed Draw Term Loan, described below. In January 2020, we repaid $300 million of the outstanding balance of the 2020 Term Loan with proceeds from our commercial paper program.
On February 25, 2019, upon the closing of our senior notes offering in aggregate principal amount of $4.300 billion described below, we terminated the $1.000 billion Term Loan Credit Agreement, entered into on August 20, 2018 and amended on December 19, 2018 (August 2019 Term Loan). The August 2019 Term Loan was scheduled to mature on August 19, 2019. As of December 31, 2018, we had $1.000 billion outstanding under our August 2019 Term Loan, which is presented within Current debt obligations on our consolidated balance sheet.
On December 19, 2018, we entered into a $2.000 billion senior unsecured delayed-draw term loan facility consisting of a $1.000 billion two-year delayed draw term loan credit facility maturing in two years from the date of the closing of our acquisition of BTG (Two-Year Delayed Draw Term Loan) and a $1.000 billion three-year delayed draw term loan credit facility maturing in three years from the date of the closing of our acquisition of BTG (Three-Year Delayed Draw Term Loan). In 2019, we used the proceeds from the Two-Year and Three-Year Delayed Draw Term Loan facilities to refinance the Bridge Facility, as described below, and fund a portion of our acquisition of BTG. On November 27, 2019, we repaid $200 million of the Two-Year Delayed Draw Term Loan with proceeds from the sale of the Zytiga-related royalty interests obtained through the acquisition of BTG and extinguished the facility. On December 5, 2019, we repaid the remaining $800 million with proceeds from the 2020 Term Loan and commercial paper and terminated the Two-Year Delayed Draw Term Loan. Borrowings of the Three-Year Delayed Draw Term Loan are available in U.S. dollars and bear interest at LIBOR or a base rate in each case plus an applicable margin based on our public debt ratings (1.13 percent as of December 31, 2019). The facility contains customary events of default, which may result in the acceleration of any outstanding commitments. As of December 31, 2018, we had no amounts borrowed under the Two-Year Delayed Draw Term Loan or the Three-Year Delayed Draw Term Loan. As of December 31, 2019, we had $1.000 billion outstanding under the Three-Year Delayed Draw Term Loan.
Debt Covenants
As of and through December 31, 2019, we were in compliance with all the required covenants related to our debt obligations.
All existing credit arrangements described above require that we maintain certain financial covenants, as follows:
Covenant Requirement as of December 31, 2019 | Actual as of December 31, 2019 | ||
Maximum leverage ratio (1) | |||
(1) Ratio of total debt to consolidated EBITDA, as defined by the credit agreements, for the preceding four consecutive fiscal quarters.
Our covenants require that we maintain a maximum leverage ratio of 3.75 times, provided, however, that for the two consecutive fiscal quarters ended immediately following the consummation of a Qualified Acquisition, as defined by each agreement, the maximum leverage ratio shall be 4.75 times, and then subject to a step-down for each succeeding fiscal quarter end to 4.50 times, 4.25 times, 4.00 times and then back to 3.75 times for each fiscal quarter end thereafter. On August 19, 2019, we announced the closing of our acquisition of BTG, a Qualified Acquisition, and our maximum leverage ratio was 4.75 times as of December 31, 2019. Refer to Note B – Acquisitions and Strategic Investments for more information.
Our covenants provide for an exclusion from the calculation of consolidated EBITDA, as defined by the agreements, through maturity, of any non-cash charges and up to $500 million in restructuring charges and restructuring-related expenses related to our current or future restructuring plans. As of December 31, 2019, we had $270 million of the restructuring charge exclusion remaining. In addition, any cash litigation payments (net of any cash litigation receipts), as defined by the agreements, are excluded from the calculation of consolidated EBITDA, as defined by the agreements, provided that the sum of any excluded net cash litigation payments does not exceed $2.624 billion in the aggregate. As of December 31, 2019, we had $1.199 billion of the litigation exclusion remaining.
Any inability to maintain compliance with these covenants could require us to seek to renegotiate the terms of our credit facility or seek waivers from compliance with these covenants, both of which could result in additional borrowing costs. Further, there
98
can be no assurance that our lenders would agree to such new terms or grant such waivers on terms acceptable to us. In this case, all credit facility commitments would terminate, and any amounts borrowed under the facility would become immediately due and payable. Furthermore, any termination of our credit facility may negatively impact the credit ratings assigned to our commercial paper program which may impact our ability to refinance any then outstanding commercial paper as it becomes due and payable.
Commercial Paper
Our commercial paper program is backed by the 2018 Facility, as discussed above. Outstanding commercial paper directly reduces borrowing capacity under the 2018 Facility.
As of December 31, | |||||||
(in millions, except maturity and yield) | 2019 | 2018 | |||||
Commercial paper outstanding | $ | $ | |||||
Maximum borrowing capacity | |||||||
Borrowing capacity available | |||||||
Weighted average maturity | |||||||
Weighted average yield | % | % | |||||
Senior Notes
We had senior notes outstanding of $7.661 billion as of December 31, 2019 and $4.800 billion as of December 31, 2018.
In November 2019, we completed an offering of €900 million (approximately $1.000 billion) in aggregate principal amount of 0.625 % senior notes due in 2027. The Euro-denominated debt principal is a nonderivative instrument designated as a net investment hedge of our net investments in certain of our Euro functional entities. Refer to Note D – Hedging Activities and Fair Value Measurements for additional information. We used a portion of the net proceeds from our November 2019 senior notes offering to repay certain outstanding principal amounts of our senior notes including $206 million of our $450 million 4.125 % senior notes due 2023, $566 million of our $1.000 billion 4.000 % senior notes due 2028 and $227 million of our $750 million 3.850 % senior notes due 2025 and pay accrued and unpaid interest, premiums, fees and expenses in connection with the transaction. In 2019, we incurred associated debt extinguishment charges of $86 million presented in Other, net on our consolidated statements of operations.
In February 2019, we completed an offering of $4.300 billion in aggregate principal amount of senior notes comprised of $850 million of 3.450 % senior notes due March 2024, $850 million of 3.750 % senior notes due March 2026, $850 million of 4.000 %
senior notes due March 2029, $750 million of 4.550 % senior notes due March 2039 and $1.000 billion of 4.700 % senior notes due March 2049. We used a portion of the net proceeds from the offering to repay the $850 million plus accrued interest and premium of our 6.000 % senior notes due in January 2020, the $600 million plus accrued interest and premium of our 2.850 % senior notes due in May 2020 and the $1.000 billion plus accrued interest of our August 2019 Term Loan. In 2019, the remaining proceeds were used to finance a portion of our acquisition of BTG.
In February 2018, we completed an offering of $1.000 billion in aggregate principal amount of 4.000 % senior notes, due March 2028. We used a portion of the net proceeds from the offering to repay the $600 million plus accrued interest of our 2.650 % senior notes due in October 2018, which were classified as short-term debt as of December 31, 2017. The remaining proceeds were used to repay a portion of our outstanding commercial paper.
Our senior notes were issued in public offerings, are redeemable prior to maturity and are not subject to sinking fund requirements. Our senior notes are unsecured, unsubordinated obligations and rank on parity with each other. These notes are effectively junior to liabilities of our subsidiaries (see Other Arrangements below).
Our $7.311 billion of senior notes issued in 2009, 2013, 2015, 2018 and 2019 contain a change-in-control provision, which provides that each holder of the senior notes may require us to repurchase all or a portion of the notes at a price equal to 101 percent of the aggregate repurchased principal, plus accrued and unpaid interest, if a rating event, as defined in the indenture, occurs as a result of a change-in-control, as defined in the indenture. Any other credit rating changes may impact our borrowing cost, but do not require us to repay any borrowings.
99
Bridge Facility
On February 25, 2019, upon the closing of our senior notes offering in aggregate principal amount of $4.300 billion described above, we terminated the Bridge Facility entered into on November 20, 2018. The termination was pursuant to the terms of the Bridge Facility, which required full termination upon the refinancing of the January 2020 Notes and May 2020 Notes discussed above. There were no amounts borrowed under the Bridge Facility as of December 31, 2018.
Other Arrangements
We have accounts receivable factoring programs in certain European countries and with commercial banks in Japan which include promissory notes discounting programs. We account for our factoring programs as sales under FASB ASC Topic 860, Transfers and Servicing. We have no retained interest in the transferred receivables, other than collection and administration, and once sold, the accounts receivable are no longer available to satisfy creditors in the event of bankruptcy. Amounts de-recognized for accounts and notes receivable, which are excluded from Trade accounts receivable, net in the accompanying consolidated balance sheets, are aggregated by contract denominated currency below (in millions):
As of December 31, 2019 | As of December 31, 2018 | ||||||||||||
Factoring Arrangements | Amount De-recognized | Weighted Average Interest Rate | Amount De-recognized | Weighted Average Interest Rate | |||||||||
Euro denominated | $ | % | $ | % | |||||||||
Yen denominated | % | % | |||||||||||
BTG Revolving Credit Facility
After closing our acquisition of BTG, we terminated BTG's revolving credit facility with a borrowing capacity of £150 million (or approximately $184 million based on the exchange rate at termination on August 27, 2019), which contained an option to increase the facility by £150 million and was scheduled to expire in November 2020. The termination was effective on August 27, 2019, and there were no amounts outstanding at the time of close of the acquisition.
Other Contractual Obligations and Commitments
We had outstanding letters of credit of $105 million as of December 31, 2019 and $111 million as of December 31, 2018, which consisted primarily of bank guarantees and collateral for workers' compensation insurance arrangements. As of December 31, 2019 and December 31, 2018, none of the beneficiaries had drawn upon the letters of credit or guarantees, accordingly, we have not recognized a related liability for our outstanding letters of credit in our consolidated balance sheets as of December 31, 2019 and December 31, 2018.
Future minimum purchase obligations as of December 31, 2019 were as follows (in millions):
Fiscal Year | Unrecorded Purchase Obligations | ||
2020 | $ | ||
2021 | |||
2022 | |||
2023 | |||
2024 | |||
Thereafter | |||
$ | |||
The amounts in the table above with respect to purchase obligations relate primarily to non-cancellable inventory commitments and capital expenditures entered in the normal course of business.
100
NOTE F – LEASES
We have operating and finance leases for real estate including corporate offices, land, warehouse space, and vehicles and certain equipment. Leases with an initial term of 12 months or less are generally not recorded on the balance sheet, unless the arrangement includes an option to purchase the underlying asset, or an option to renew the arrangement, that we are reasonably certain to exercise (short-term leases). We recognize lease expense on a straight-line basis over the lease term for short-term leases that we do not record on our balance sheet. If there is a change in our assessment of the lease term and, as a result, the remaining lease term extends more than 12 months from the end of the previously determined lease term, or we subsequently become reasonably certain that we will exercise an option to purchase the underlying asset, the lease no longer meets the definition of a short-term lease and is accounted for as either an operating or finance lease and recognized on the balance sheet. Effective January 1, 2019, we adopted FASB ASC Topic 842 as discussed in Note A – Significant Accounting Policies. Beginning in 2019, we account for the lease components and the non-lease components as a single lease component, with the exception of our warehouse leases. Our leases have remaining lease terms of less than 1 year to approximately 60 years, some of which may include options to extend the leases for up to 10 years. If we are reasonably certain we will exercise an option to extend the lease, the time period covered by the extension option is included in the lease term.
We determine whether an arrangement is or contains a lease based on the unique facts and circumstances present at the inception of the arrangement. Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of lease payments over the expected lease term. The interest rate implicit in lease contracts is typically not readily determinable. As such, we utilize the appropriate incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term at an amount equal to the lease payments in a similar economic environment. Certain adjustments to the right-of-use asset may be required for items such as initial direct costs paid or incentives received.
The following table presents supplemental balance sheet information related to our operating leases:
(in millions) | As of December 31, 2019 | ||
Assets | |||
Operating lease right-of-use assets in Other long-term assets | $ | ||
Liabilities | |||
Operating lease liabilities in Other current liabilities | |||
Operating lease liabilities in Other long-term liabilities | |||
The following table presents the weighted average remaining lease term and discount rate information related to our operating leases:
As of December 31, 2019 | |
Weighted average remaining lease term | |
Weighted average discount rate | |
Our operating lease cost under FASB ASC Topic 842 was $80 million in 2019. Rent expense under FASB ASC Topic 840 amounted to $92 million in 2018 and $88 million in 2017.
The following table presents supplemental cash flow information related to our operating leases:
Year Ended | |||
(in millions) | December 31, 2019 | ||
Cash paid for amounts included in the measurement of operating lease liabilities | |||
Operating cash flows from operating leases | $ | ||
Right-of-use assets obtained in exchange for operating lease obligations were $137 million as of December 31, 2019.
101
The following table presents the maturities of our operating lease liabilities as of December 31, 2019 (in millions):
Fiscal year | Operating Leases | ||
2020 | $ | ||
2021 | |||
2022 | |||
2023 | |||
2024 | |||
Thereafter | |||
Total future minimum operating lease payments | |||
Less: imputed interest | |||
Present value of operating lease liabilities | $ | ||
As of December 31, 2019, we have additional leases for office space and warehouse space, that have not yet commenced, of approximately $70 million. These leases will commence in 2020, with lease terms of up to 15 years.
Future minimum rental commitments as of December 31, 2018, under all noncancellable lease agreements, including capital leases, were as follows:
Fiscal year | Future Minimum Rental Commitments | ||
2019 | $ | ||
2020 | |||
2021 | |||
2022 | |||
2023 | |||
Thereafter | |||
$ | |||
NOTE G – RESTRUCTURING-RELATED ACTIVITIES
2019 Restructuring Plan
On November 15, 2018, the Board of Directors approved, and we committed to a new global restructuring program (the 2019 Restructuring Plan). The 2019 Restructuring Plan is intended to support our effort to improve operating performance and meet anticipated market demands by ensuring that we are appropriately structured and resourced to deliver sustainable value to patients and customers. Key activities under the 2019 Restructuring Plan include supply chain network optimization intended to maximize our global manufacturing and distribution network capacity and building functional capabilities that support business growth. These activities were initiated in 2019, with the majority of activity expected to be complete by the end of 2021.
The following table provides a summary of our estimates of total pre-tax charges associated with the 2019 Restructuring Plan by major type of cost:
Type of Cost | Total Estimated Amount Expected to be Incurred | ||||
Restructuring charges: | |||||
Termination benefits | $ | million | to | $ | |
Other (1) | $ | million | to | $ | |
Restructuring-related expenses: | |||||
Other (2) | $ | million | to | $ | |
$ | million | to | $ | ||
(1) | Consists primarily of consulting fees and costs associated with contractual cancellations. |
(2) | Comprised of other costs directly related to the restructuring program, including program management, accelerated depreciation, fixed asset write-offs, and costs to transfer product lines among facilities. |
102
Approximately $180 million to $280 million of these charges are expected to result in cash outlays.
2016 Restructuring Plan
On June 6, 2016, our Board of Directors approved, and we committed to a restructuring initiative (the 2016 Restructuring Plan). The 2016 Restructuring Plan was intended to develop global commercialization, technology and manufacturing capabilities in key growth markets, build on our Plant Network Optimization (PNO) strategy which is intended to simplify our manufacturing plant structure by transferring certain production lines among facilities and expand operational efficiencies in support of our operating income margin goals. Key activities under the 2016 Restructuring Plan included strengthening global infrastructure through evolving global real estate assets and workplaces, developing global commercial and technical competencies, enhancing manufacturing and distribution expertise in certain regions and continuing implementation of our PNO strategy. These activities were initiated in the second quarter of 2016 and substantially completed in 2019.
The following table provides a summary of total pre-tax charges associated with the 2016 Restructuring Plan by major type of cost:
Type of cost | Total Amount Incurred | ||
Restructuring charges: | |||
Termination benefits | $ | million | |
Other (1) | million | ||
Restructuring-related expenses: | |||
Other (2) | million | ||
$ | million | ||
(1) | Consists primarily of consulting fees and costs associated with contract cancellations. |
(2) | Comprised of other costs directly related to the 2016 Restructuring Plan, including program management, accelerated depreciation, fixed asset write-offs and costs to transfer product lines among facilities. |
Approximately $255 million of these charges are expected to result in cash outlays; the majority of which were completed as of December 31, 2019.
The following presents the restructuring and restructuring-related charges (credits) by major type and line item within our accompanying consolidated statements of operations (in millions):
Year Ended December 31, 2019 | Termination Benefits | Transfer Costs | Other | Total | |||||||||||
Restructuring charges | $ | $ | $ | $ | |||||||||||
Restructuring-related expenses: | |||||||||||||||
Cost of products sold | |||||||||||||||
Selling, general and administrative expenses | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
Year Ended December 31, 2018 | Termination Benefits | Transfer Costs | Other | Total | |||||||||||
Restructuring charges | $ | $ | $ | $ | |||||||||||
Restructuring-related expenses: | |||||||||||||||
Cost of products sold | |||||||||||||||
Selling, general and administrative expenses | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
103
Year Ended December 31, 2017 | Termination Benefits | Transfer Costs | Other | Total | |||||||||||
Restructuring charges | $ | $ | $ | $ | |||||||||||
Restructuring-related expenses: | |||||||||||||||
Cost of products sold | |||||||||||||||
Selling, general and administrative expenses | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
The following table presents cumulative restructuring and restructuring-related charges incurred as of December 31, 2019, related to our Restructuring Plans by major type:
(in millions) | 2016 Restructuring Plan | 2019 Restructuring Plan | Total | ||||||||
Termination benefits | $ | $ | $ | ||||||||
Other (1) | |||||||||||
Total restructuring charges | |||||||||||
Transfer costs | |||||||||||
Other (2) | |||||||||||
Restructuring-related charges | |||||||||||
$ | $ | $ | |||||||||
(1) | Consists primarily of consulting fees and costs associated with contract cancellations. |
(2) | Comprised of other costs directly related to our Restructuring Plans, including program management, accelerated depreciation, and fixed asset write-offs. |
Cash payments associated with our Restructuring Plans were made using cash generated from operations and are comprised of the following:
(in millions) | 2016 Restructuring Plan | 2019 Restructuring Plan | Total | ||||||||
Year Ended December 31, 2019 | |||||||||||
Termination benefits | $ | $ | $ | ||||||||
Transfer costs | |||||||||||
Other | |||||||||||
$ | $ | $ | |||||||||
NOTE H – SUPPLEMENTAL BALANCE SHEET INFORMATION
Components of selected captions in our accompanying consolidated balance sheets are as follows:
Trade accounts receivable, net
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Accounts receivable | $ | $ | |||||
Allowance for doubtful accounts | ( | ) | ( | ) | |||
$ | $ | ||||||
104
The following is a rollforward of our allowance for doubtful accounts:
Year Ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Beginning balance | $ | $ | $ | ||||||||
Net charges to expenses | |||||||||||
Utilization of allowances | ( | ) | ( | ) | ( | ) | |||||
Ending balance | $ | $ | $ | ||||||||
Inventories
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Finished goods | $ | $ | |||||
Work-in-process | |||||||
Raw materials | |||||||
$ | $ | ||||||
Other current assets
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Restricted cash and restricted cash equivalents | $ | $ | |||||
Derivative assets | |||||||
Licensing arrangements | |||||||
Taxes receivable | |||||||
Other | |||||||
$ | $ | ||||||
Property, plant and equipment, net
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Land | $ | $ | |||||
Buildings and improvements | |||||||
Equipment, furniture and fixtures | |||||||
Capital in progress | |||||||
Less: accumulated depreciation | |||||||
$ | $ | ||||||
Depreciation expense was $311 million in 2019, $296 million in 2018 and $279 million in 2017.
105
Other long-term assets
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Restricted cash equivalents | $ | $ | |||||
Operating lease right-of-use assets | |||||||
Derivative assets | |||||||
Investments | |||||||
Licensing arrangements | |||||||
Other | |||||||
$ | $ | ||||||
Accrued expenses
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Legal reserves | $ | $ | |||||
Payroll and related liabilities | |||||||
Accrued contingent consideration | |||||||
Rebates | |||||||
Other | |||||||
$ | $ | ||||||
Other current liabilities
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Deferred revenue | $ | $ | |||||
Licensing arrangements | |||||||
Taxes payable | |||||||
Other | |||||||
$ | $ | ||||||
Other long-term liabilities
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Accrued income taxes | $ | $ | |||||
Legal reserves | |||||||
Accrued contingent consideration | |||||||
Licensing arrangements | |||||||
Operating lease liabilities | |||||||
Other | |||||||
$ | $ | ||||||
106
NOTE I – INCOME TAXES
Our Income (loss) before income taxes consisted of the following:
Year Ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Domestic | $ | ( | ) | $ | $ | ( | ) | ||||
Foreign | |||||||||||
$ | $ | $ | |||||||||
The related benefit for income taxes consisted of the following:
Year Ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Current | |||||||||||
Federal | $ | $ | ( | ) | $ | ||||||
State | ( | ) | |||||||||
Foreign | |||||||||||
( | ) | ||||||||||
Deferred | |||||||||||
Federal | ( | ) | ( | ) | |||||||
State | ( | ) | |||||||||
Foreign | ( | ) | ( | ) | ( | ) | |||||
( | ) | ( | ) | ||||||||
$ | ( | ) | $ | ( | ) | $ | |||||
The reconciliation of income taxes at the federal statutory rate to the actual benefit for income taxes is as follows:
Year Ended December 31, | ||||||||
2019 | 2018 | 2017 | ||||||
(reclassified)(1) | ||||||||
U.S. federal statutory income tax rate | % | % | % | |||||
State income taxes, net of federal benefit | % | % | ( | )% | ||||
Domestic taxes on foreign earnings | % | % | % | |||||
Effect of foreign taxes | ( | )% | ( | )% | ( | )% | ||
Acquisition-related | % | % | ( | )% | ||||
Research credit | ( | )% | ( | )% | ( | )% | ||
Valuation allowance | % | ( | )% | ( | )% | |||
Compensation-related | ( | )% | ( | )% | ( | )% | ||
Non-deductible expenses | % | % | % | |||||
Uncertain tax positions | % | ( | )% | % | ||||
TCJA net impact | % | ( | )% | % | ||||
Intra-entity intangible asset transfers | ( | )% | % | % | ||||
Other, net | ( | )% | % | ( | )% | |||
( | )% | ( | )% | % | ||||
(1) | Due to the inclusion of new tax provisions in 2018 created by the TJCA, we have reclassified select items in prior years to align with the new categories established in 2018, domestic taxes on foreign earnings and uncertain tax positions. |
107
Significant components of our deferred tax assets and liabilities are as follows:
As of December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Deferred Tax Assets: | |||||||
Inventory costs and related reserves | $ | $ | |||||
Tax benefit of net operating loss and credits | |||||||
Reserves and accruals | |||||||
Restructuring-related charges | |||||||
Litigation and product liability reserves | |||||||
Investment write-down | |||||||
Compensation related | |||||||
Federal benefit of uncertain tax positions | |||||||
Intangible assets | |||||||
Other | |||||||
Less: valuation allowance | ( | ) | ( | ) | |||
Deferred Tax Liabilities: | |||||||
Property, plant and equipment | |||||||
Unrealized gains and losses on derivative financial instruments | |||||||
Intangible assets | |||||||
Inventory costs and related services | |||||||
Other | |||||||
Net Deferred Tax Assets / (Liabilities) | ( | ) | |||||
Prepaid on intercompany profit | |||||||
Net Deferred Tax Assets / (Liabilities) and Prepaid on Intercompany Profit | $ | $ | ( | ) | |||
Our deferred tax assets, deferred tax liabilities and prepaid on intercompany profit, are included in the following locations within our accompanying consolidated balance sheets (in millions):
Location on Consolidated Balance Sheets | As of December 31, | |||||||
Component | 2019 | 2018 | ||||||
Prepaid on intercompany profit | Prepaid income taxes | $ | $ | |||||
Non-current deferred tax asset | Deferred tax assets | |||||||
Deferred Tax Assets and Prepaid on Intercompany Profit | ||||||||
Non-current deferred tax liability | Deferred income taxes | |||||||
Deferred Tax Liabilities | ||||||||
Net Deferred Tax Assets (Liabilities) and Prepaid on Intercompany Profit | $ | $ | ( | ) | ||||
As of December 31, 2019, we had U.S. federal and state tax net operating loss carryforwards and tax credits, the tax effect of which was $449 million. As of December 31, 2018, we had U.S. federal and state tax net operating loss carryforwards and tax credits, the tax effect of which was $416 million. In addition, we had foreign tax net operating loss carryforwards and tax credits, the tax effect of which was $105 million as of December 31, 2019, as compared to $42 million as of December 31, 2018. These tax attributes expire periodically beginning in 2020.
During the fourth quarter of 2019, we completed intra-entity asset transfers of certain intellectual property rights among various wholly-owned subsidiaries. These transactions occurred to more closely align the global economic ownership of our intellectual property rights with our current and future business operations. These transactions did not result in a taxable gain in any jurisdiction,
108
however, some of the transactions did create a step-up in the tax-deductible basis in the transferred intellectual property rights in certain jurisdictions. As a result, we recorded deferred tax assets in the amount of $4.102 billion, which represents the book and tax basis differences measured at applicable statutory tax rates, net of a valuation allowance of $542 million.
After consideration of all positive and negative evidence, we believe that it is more likely than not that a portion of our deferred tax assets will not be realized. As a result, we established a valuation allowance of $915 million as of December 31, 2019 and $344 million as of December 31, 2018, representing an increase of $571 million. The increase in the valuation allowance as of December 31, 2019, as compared to December 31, 2018, is primarily attributable to an intra-entity transfer of certain intellectual property. The income tax impact of the unrealized gain or loss component of other comprehensive income and stockholders' equity was a charge of $13 million in 2019, a charge of $37 million in 2018 and a benefit of $63 million in 2017.
We obtain tax incentives through Free Trade Zone Regime offered in Costa Rica which allows 100.0 percent exemption from income tax in the first eight years of operations and 50.0 percent exemption in the following four years. This tax incentive resulted in income tax savings of $173 million for 2019, $146 million for 2018 and $127 million for 2017. The tax incentive for 100.0 percent exemption from income tax was renewed during 2019 and is expected to expire in 2027. The impact on per share earnings was $0.12 for 2019 and $0.10 for 2018 and $0.09 for 2017. Additionally, we benefit from tax incentives in Puerto Rico. The income tax savings from Puerto Rico were immaterial for 2019, 2018 and 2017.
As of December 31, 2019, we had $455 million of gross unrecognized tax benefits, of which a net $355 million, if recognized, would affect our effective tax rate. As of December 31, 2018, we had $427 million of gross unrecognized tax benefits, of which a net $332 million, if recognized, would affect our effective tax rate. As of December 31, 2017, we had $1.238 billion of gross unrecognized tax benefits, of which a net $1.150 billion, if recognized, would affect our effective tax rate. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
Year Ended December 31, | |||||||||||
(in millions) | 2019 | 2018 | 2017 | ||||||||
Beginning Balance | $ | $ | $ | ||||||||
Additions based on positions related to the current year | |||||||||||
Additions based on positions related to prior years | |||||||||||
Reductions for tax positions of prior years | ( | ) | ( | ) | ( | ) | |||||
Settlements with taxing authorities | ( | ) | ( | ) | ( | ) | |||||
Statute of limitation expirations | ( | ) | ( | ) | ( | ) | |||||
Ending Balance | $ | $ | $ | ||||||||
We are subject to U.S. Federal income tax as well as income tax of multiple state and foreign jurisdictions. We have concluded all U.S. federal income tax matters through 2013, with the exception of select issues in 2011 and substantially all material state and local income tax matters through 2010. We have concluded all foreign income tax matters through 2013, with the exception of issues for Italy, which have concluded through 2002.
In the second quarter of 2018, a decision was entered by the U.S. Tax Court resolving all disputes for Guidant Corporation for its 2001 through 2006 tax years and our 2006 and 2007 tax years as well as the tax issues related to our 2006 transaction with Abbott Laboratories. Additionally, we resolved all issues with the IRS Office of Appeals for our 2008 through 2010 tax years. The final settlement calculation resulted in a final net tax payment of $303 million plus $307 million of estimated interest, which was remitted in the second quarter of 2018. Due to the final settlement of these disputes, we recorded a net tax benefit of $250 million in 2018 to remove a reserve related to these years.
In the fourth quarter of 2018, we received a Revenue Agent Report (RAR) from the IRS for our 2011 through 2013 tax years. We remitted $93 million to the IRS in the fourth quarter of 2018 reflecting the net balance of tax and interest due for these years after consideration of amounts owed to us by the IRS. Due to the resolution of these tax years, we recorded a net tax benefit of $90 million to remove a reserve related to these years.
We recognize interest and penalties related to income taxes as a component of income tax expense. We had $19 million accrued for gross interest and penalties as of December 31, 2019 and $11 million as of December 31, 2018. We recognized a benefit of $643 million of gross interest and penalties in our consolidated statements of operations in 2018 primarily related to reaching settlements with the taxing authorities. We recognized net tax benefit related to interest and penalties of $1 million in 2019, as compared to a net tax benefit of $498 million in 2018 and a net tax expense of $154 million in 2017. The decrease in our net tax benefit related to interest and penalties as of December 31, 2019, as compared to December 31, 2018, is related to reaching settlements with the taxing authorities.
109
It is reasonably possible that within the next 12 months we will resolve transactional- related issues with foreign and state taxing authorities, in which case we could record a reduction in our balance of unrecognized tax benefits of up to approximately $98 million.
During 2018, we completed our analysis and recording of all tax effects related to the Tax Cuts and Jobs Act, as required under SAB 118, and recorded a net benefit of $67 million, exclusive of the one-time transition tax adjustment described below.
For the year ended December 31, 2017, we were required under the Tax Cuts and Jobs Act (TCJA) to calculate a one-time transition tax based on our total post-1986 foreign subsidiaries' earnings and profits (E&P) that we previously deferred from U.S. income taxes. As a result of settling our various tax audits, the revised amount of transition tax is approximately $856 million as of December 31, 2018 as compared to the preliminary amount recorded of approximately $1.044 billion as of December 31, 2017. We anticipate offsetting this liability against existing tax attributes reducing the required payment to approximately $499 million, which will be remitted over an eight-year period. We have begun remitting the required installment payments, with a balance remaining of $420 million as of December 31, 2019. In addition, we have provided for U.S. state income taxes of $18 million on all U.S. dollar-denominated E&P accumulated through December 31, 2017, which constitutes the preponderance of our foreign subsidiaries' accumulated E&P through December 31, 2017. We intend to indefinitely reinvest the unremitted foreign earnings of all other subsidiaries as of December 31, 2017, as well as all subsequent earnings generated by all of our foreign subsidiaries. Determining the amount of unrecognized deferred tax liability related to any remaining undistributed foreign earnings and additional outside basis difference in these entities is not practicable.
NOTE J – COMMITMENTS AND CONTINGENCIES
The medical device market in which we participate is largely technology driven. As a result, intellectual property rights, particularly patents and trade secrets, play a significant role in product development and differentiation. Over the years, there has been litigation initiated against us by others, including our competitors, claiming that our current or former product offerings infringe patents owned or licensed by them. Intellectual property litigation is inherently complex and unpredictable. In addition, competing parties frequently file multiple suits to leverage patent portfolios across product lines, technologies and geographies and to balance risk and exposure between the parties. In some cases, several competitors are parties in the same proceeding, or in a series of related proceedings, or litigate multiple features of a single class of devices. These dynamics frequently drive settlement not only for individual cases, but also for a series of pending and potentially related and unrelated cases. Although monetary and injunctive relief is typically sought, remedies and restitution are generally not determined until the conclusion of the trial court proceedings and can be modified on appeal. Accordingly, the outcomes of individual cases are difficult to time, predict or quantify and are often dependent upon the outcomes of other cases in other geographies.
During recent years, we successfully negotiated closure of several long-standing legal matters and have received favorable rulings in several other matters; however, there continues to be outstanding intellectual property litigation. Adverse outcomes in one or more of these matters could have a material adverse effect on our ability to sell certain products and on our operating margins, financial position, results of operations and/or liquidity.
In the normal course of business, product liability, securities and commercial claims are asserted against us. Similar claims may be asserted against us in the future related to events not known to management at the present time. We maintain an insurance policy providing limited coverage against securities claims and we are substantially self-insured with respect to product liability claims and fully self-insured with respect to intellectual property infringement claims. The absence of significant third-party insurance coverage increases our potential exposure to unanticipated claims or adverse decisions. Product liability claims, securities and commercial litigation and other legal proceedings in the future, regardless of their outcome, could have a material adverse effect on our financial position, results of operations and/or liquidity.
In addition, like other companies in the medical device industry, we are subject to extensive regulation by national, state and local government agencies in the U.S. and other countries in which we operate. From time to time we are the subject of qui tam actions and governmental investigations often involving regulatory, marketing and other business practices. These qui tam actions and governmental investigations could result in the commencement of civil and criminal proceedings, substantial fines, penalties and administrative remedies and have a material adverse effect on our financial position, results of operations and/or liquidity.
110
In accordance with FASB ASC Topic 450, Contingencies, we accrue anticipated costs of settlement, damages, losses for product liability claims and, under certain conditions, costs of defense, based on historical experience or to the extent specific losses are probable and estimable. Otherwise, we expense these costs as incurred. If the estimate of a probable loss is a range and no amount within the range is more likely, we accrue the minimum amount of the range.
Our accrual for legal matters that are probable and estimable was $697 million as of December 31, 2019 and $929 million as of December 31, 2018 and includes certain estimated costs of settlement, damages and defense. The decrease in our legal accrual was mainly due to settlement payments associated with product liability cases or claims related to transvaginal surgical mesh products. A portion of our legal accrual is already funded through our qualified settlement fund (QSF), which is included in our restricted cash and restricted cash equivalent balances in Other current assets of $346 million as of December 31, 2019 and $655 million as of December 31, 2018, as discussed in Note A – Significant Accounting Policies.
We recorded litigation-related net charges of $115 million in 2019, $103 million in 2018 and $285 million in 2017. These included litigation-related net charges included a net charge of $223 million in the fourth quarter of 2019, primarily related to litigation with Channel Medsystems, Inc., net charges of $25 million in the third quarter of 2019 and $15 million in the second quarter of 2019, primarily related to transvaginal surgical mesh product liability litigation, and a gain of $148 million recorded in the first quarter of 2019, which represents a portion of the total $180 million one-time settlement payment received from Edwards Lifesciences Corporation (Edwards) in January 2019. We record certain legal and product liability charges, credits and costs of defense, which we consider to be unusual or infrequent and significant as Litigation-related net charges (credits) in our consolidated financial statements. All other legal and product liability charges, credits and costs are recorded within Selling, general and administrative expenses. As such, a portion of the related gain from the Edwards settlement was recorded in Selling, general and administrative expenses on our consolidated statements of operations. We continue to assess certain litigation and claims to determine the amounts, if any, that management believes will be paid as a result of such claims and litigation and, therefore, additional losses may be accrued and paid in the future, which could materially adversely impact our operating results, cash flows and/or our ability to comply with our debt covenants.
In management's opinion, we are not currently involved in any legal proceedings other than those specifically identified below, which, individually or in the aggregate, could have a material adverse effect on our financial condition, operations and/or cash flows. Unless included in our legal accrual or otherwise indicated below, a range of loss associated with any individual material legal proceeding cannot be estimated.
Patent Litigation
On October 28, 2015, BSC filed suit against Cook Group Limited and Cook Medical LLC (collectively, “Cook”) in the District Court for the District of Delaware (1:15-cv-00980) alleging infringement of certain Company patents regarding Cook’s Instinct Endoscopic Hemoclip. The case was transferred to the District Court for the S.D. Indiana. Cook filed seven Inter Partes Review (“IPR”) requests with the U.S. Patent and Trademark Office against the four asserted patents. All IPRs have concluded with claims confirmed in each patent. Cook and Boston Scientific have both appealed the Patent Office’s IPR decisions to the Federal Circuit Court of Appeals. The district court had stayed the case pending the appeals court decision on the IPRs.
On November 29, 2016 Nevro Corp. (Nevro) filed a patent infringement action against us and one of our subsidiaries, Boston Scientific Neuromodulation Corporation, in the U.S. District Court for the Northern District of California alleging that six U.S. patents (Alataris) owned by Nevro are infringed by our spinal cord stimulation systems. On June 29, 2017, Nevro amended the complaint to add an additional patent (Fang). On July 24, 2018, summary judgment was entered in favor of the Company and on July 31, 2018, we received final judgment and dismissal of the action. On July 31, 2018, Nevro filed an appeal.
On December 9, 2016, the Company and Boston Scientific Neuromodulation Corporation filed a patent infringement action against Nevro in U.S. District Court for the District of Delaware alleging that ten U.S. patents owned by Boston Scientific Neuromodulation Corporation are infringed by Nevro’s Senza™ Spinal Cord Stimulation System.
On November 20, 2017, The Board of Regents, University of Texas System (UT) and TissueGen. Inc., served a lawsuit against us in the Western District of Texas. The complaint against us alleges patent infringement of two U.S. patents owned by UT, relating to “Drug Releasing Biodegradable Fiber Implant” and “Drug Releasing Biodegradable Fiber for Delivery of Therapeutics,” and affects the manufacture, use and sale of our Synergy™ Stent System. On March 12, 2018, the District Court for the Western District of Texas dismissed the action and transferred it to the United States District Court for the District of Delaware. On September 5, 2019, the Court of Appeals for the Federal Circuit affirmed the dismissal of the District Court for the Western District of Texas.
On April 21, 2018, the Company and Boston Scientific Neuromodulation Corporation filed a patent infringement, theft of trade secrets and tortious interference with a contract action against Nevro in U.S. District Court for the District of Delaware, and
111
amended the complaint on July 18, 2018, alleging that nine U.S. patents owned by Boston Scientific Neuromodulation Corporation are infringed by Nevro’s Senza™ I and Senza™ II Spinal Cord Stimulation Systems. On December 9, 2019, Nevro filed an answer and counterclaims, in which it alleged that our Spinal Cord Stimulation systems infringe 5 Nevro patents.
On April 30, 2019, Tissue Anchor Innovations filed a complaint for patent infringement in the United States District Court Central District of California against Fountain Valley Regional Hospital and Medical Center, Los Alamitos Medical Center and us. The complaint alleges that the Solyx™ Sling System infringes US Patent 6,506,190.
Product Liability Litigation
As of January 29, 2020, approximately 54,000 product liability cases or claims related to transvaginal surgical mesh products designed to treat stress urinary incontinence and pelvic organ prolapse have been asserted against us. As of January 29, 2020, we have entered into master settlement agreements in principle or are in the final stages of entering one with certain plaintiffs' counsel to resolve an aggregate of approximately 52,000 cases and claims. These master settlement agreements provide that the settlement and distribution of settlement funds to participating claimants are conditional upon, among other things, achieving minimum required claimant participation thresholds. Of the approximately 52,000 cases and claims, approximately 46,000 have met the conditions of the settlement and are final. All settlement agreements were entered into solely by way of compromise and without any admission or concession by us of any liability or wrongdoing. On April 16, 2019, the U.S. Food and Drug Administration (FDA) ordered that all manufacturers of surgical mesh products indicated for the transvaginal repair of pelvic organ prolapse stop selling and distributing their products in the United States immediately, stemming from the FDA’s 2016 reclassification of these devices to class III (high risk) devices, and as a result, the Company ceased global sales and distribution of surgical mesh products indicated for transvaginal pelvic organ prolapse. The pending cases are in various federal and state courts in the U.S. Generally, the plaintiffs allege personal injury associated with use of our transvaginal surgical mesh products. The plaintiffs assert design and manufacturing claims, failure to warn, breach of warranty, fraud, violations of state consumer protection laws and loss of consortium claims. Over 3,100 of the cases were specially assigned to one judge in state court in Massachusetts. On February 7, 2012, the Judicial Panel on Multi-District Litigation (MDL) established MDL-2326 in the U.S. District Court for the Southern District of West Virginia and transferred the federal court transvaginal surgical mesh cases to MDL-2326 for coordinated pretrial proceedings. During the fourth quarter of 2013, we received written discovery requests from certain state attorneys general offices regarding our transvaginal surgical mesh products. We have responded to those requests. On December 12, 2019 the Mississippi Attorney General filed suit against BSC in State Court alleging violations of the Mississippi Consumer Protection Act which the Company plans to vigorously defend. There were also fewer than 25 cases in Canada, inclusive of one certified class action and fewer than 25 claims in the United Kingdom. We have reached an agreement to settle the Canadian class action pending Court approval.
We have established a product liability accrual for known and estimated future cases and claims asserted against us as well as with respect to the actions that have resulted in verdicts against us and the costs of defense thereof associated with our transvaginal surgical mesh products. While we believe that our accrual associated with this matter is adequate, changes to this accrual may be required in the future as additional information becomes available. While we continue to engage in discussions with plaintiffs’ counsel regarding potential resolution of pending cases and claims and intend to vigorously contest the cases and claims asserted against us, that do not settle, the final resolution of the cases and claims is uncertain and could have a material impact on our results of operations, financial condition and/or liquidity. Initial trials involving our transvaginal surgical mesh products have resulted in both favorable and unfavorable judgments for us. We do not believe that the judgment in any one trial is representative of potential outcomes of all cases or claims related to our transvaginal surgical mesh products.
Governmental Investigations and Qui Tam Matters
On May 5, 2014, we were served with a subpoena from the U.S. Department of Health and Human Services, Office of the Inspector General. The subpoena seeks information relating to the launch of the Cognis™ and Teligen™ line of devices in 2008, the performance of those devices from 2007 to 2009 and the operation of the Physician Guided Learning Program. We are cooperating with this request. On May 6, 2016, a qui tam lawsuit in this matter was unsealed in the U.S. District Court for the District of Minnesota. At the same time, we learned that the U.S. government and the State of California had earlier declined to intervene in that lawsuit on April 15, 2016. The complaint was served on us on July 21, 2016. On October 7, 2016, the plaintiff/relator served an amended complaint that dropped the allegations relating to the Physician Guided Learning Program. We filed a motion to dismiss the amended complaint on December 7, 2016 and the court heard our motion to dismiss on April 5, 2017. On August 29, 2017, the Court granted the motion to dismiss, without prejudice and on September 19, 2017, the relator filed a Second Amended Complaint. We filed a motion to dismiss the Second Amended Complaint on October 10, 2017 and the Court denied that motion on December 13, 2017. On July 31, 2018, the relator filed a motion seeking leave to file a Third Amended Complaint. The Court denied the motion on October 30, 2018.
112
On February 23, 2015, a judge for the Court of Modena (Italy) ordered a trial for Boston Scientific and three of its employees, as well as numerous other defendants charged in criminal proceedings. The charges arise from allegations that the defendants made improper donations to certain healthcare providers and other employees of the Hospital of Modena in order to induce them to conduct unauthorized clinical trials, as well as related government fraud in relation to the financing of such clinical trials. A trial began on February 24, 2016. On November 10, 2017, the Court issued a ruling that convicted one Boston Scientific employee but acquitted two others and levied a fine of €245 thousand against us and imposed joint and several civil damages of €620 thousand on all defendants. We continue to deny these allegations, timely appealed the decision on May 10, 2018 and intend to continue to defend ourselves vigorously.
On December 1, 2015, the Brazilian governmental entity known as CADE (the Administrative Council of Economic Defense), served a search warrant on the offices of our Brazilian subsidiary, as well as on the Brazilian offices of several other major medical device makers who do business in Brazil, in furtherance of an investigation into alleged anti-competitive activity with respect to certain tender offers for government contracts. On June 20, 2017, CADE, through the publication of a “technical note,” announced that it was launching a formal administrative proceeding against Boston Scientific’s Brazilian subsidiary, Boston Scientific do Brasil Ltda., as well as against the Brazilian operations of Medtronic, Biotronik and St. Jude Medical, two Brazilian associations, ABIMED and AMBIMO and 29 individuals for alleged anti-competitive behavior. We deny the allegations and intend to defend ourselves vigorously.
Other Proceedings
On May 16, 2018, Arthur Rosenthal et al., filed a plenary summons against Boston Scientific Corporation and Boston Scientific Limited with the High Court of Ireland alleging that payments are due pursuant a transaction agreement regarding Labcoat Limited.
On September 6, 2019, Boston Scientific Corporation, Boston Scientific Scimed, Inc., and Fortis Advisors, LLC, as a Securityholder Representative for the former Securityholders of nVision Medical Corp. filed a declaratory judgment action against BioCardia, Inc. in the United States District Court for the Northern District of California to address threats and allegations by BioCardia challenging inventorship and ownership of various patents that Boston Scientific Corporation acquired through an April 13, 2018 merger with nVision as well as related threats and allegations by BioCardia of trade secret misappropriation and unjust enrichment. On December 11, 2019, BioCardia filed an amended answer and counterclaims.
On April 18, 2019, Blue Cross & Blue Shield of Louisiana and HMO Louisiana, Inc. filed a class action complaint against Janssen Biotech, Inc, Janssen Oncology, Inc, Janssen Research & Development, LLC and BTG International Limited in the United States District Court for the Eastern District of Virginia. The complaint alleges that the defendants violated the Sherman Act and the antitrust and consumer protections laws of several states by pursuing patent litigation relating to ZYTIGA™ in order to delay generic entry. On June 21, 2019, the case was transferred to the United States District Court for the District of New Jersey and has been consolidated with similar complaints.
On December 21, 2017, Janssen Biotech, Inc., Janssen Oncology, Inc, Janssen Research & Development, LLC, and Johnson & Johnson (collectively, Janssen) were served with a qui tam complaint filed on behalf of the United States, 28 states, and the District of Columbia. The complaint, which was filed in the United States District Court for the Northern District of California, alleges that Janssen violated the federal False Claims Act and state law when providing pricing information for ZYTIGA to the government in connection with direct government sales and government-funded drug reimbursement programs. The case has been transferred to United States District Court for the District of New Jersey. On June 20, 2019, the complaint was amended to include BTG International Limited as a defendant.
Refer to Note I – Income Taxes for information regarding our tax litigation.
Matters Concluded Since December 31, 2018
On January 15, 2019, we announced that we reached an agreement with Edwards Lifesciences Corporation (Edwards) to settle all outstanding patent disputes between us and Edwards in all venues around the world. All pending cases or appeals in courts and patent offices between the two companies will be dismissed, and the parties will not litigate patent disputes related to current portfolios of transcatheter aortic valves, certain mitral valve repair devices, and left atrial appendage closure devices. Any injunctions currently in place will be lifted. Under the terms of the agreement, Edwards made a one-time payment to us of $180 million. No further royalties will be owed by either party under the agreement. All other terms remain confidential. The previously disclosed matters that have been resolved as a result of this settlement include:
• | On October 30, 2015, a subsidiary of Boston Scientific filed suit against Edwards Lifesciences Corporation and Edwards Lifesciences Services GmbH in Düsseldorf District Court in Germany for patent infringement. We allege that Edwards’ |
113
SAPIEN 3™ Heart Valve infringes our patent related to adaptive sealing technology. On February 25, 2016, we extended the action to allege infringement of a second patent related to adaptive sealing technology. The trial began on February 7, 2017. On March 9, 2017, the court found that Edwards infringed both patents and Edwards appealed.
• | On November 9, 2015, Edwards Lifesciences, LLC filed an invalidity claim against one of our subsidiaries, Sadra Medical, Inc. (Sadra), in the High Court of Justice, Chancery Division Patents Court in the United Kingdom, alleging that a European patent owned by Sadra relating to a repositionable heart valve is invalid. On January 15, 2016, we filed our defense and counterclaim for a declaration that our European patent is valid and infringed by Edwards. On February 25, 2016, we amended our counterclaim to allege infringement of a second patent related to adaptive sealing technology. A trial was held from January 18 to January 27, 2017. On March 3, 2017, the court found one of our patents valid and infringed and some claims of the second patent invalid and the remaining claims not infringed. Both parties have filed an appeal. On March 28, 2018, the Court of Appeals affirmed the decision of the High Court. |
• | On November 23, 2015, Edwards Lifesciences PVT, Inc. filed a patent infringement action against us and one of our subsidiaries, Boston Scientific Medizintechnik GmbH, in the District Court of Düsseldorf, Germany alleging a European patent (Spenser '672) owned by Edwards is infringed by our Lotus™ Valve System. The trial began on February 7, 2017. On March 9, 2017, the court found that we did not infringe the Spenser '672 patent. Edwards filed an appeal. |
• | On November 23, 2015, Edwards Lifesciences Corporation filed a patent infringement action against us and Boston Scientific Medizintechnik GmbH in the District Court of Düsseldorf, Germany alleging an European patent (Bourang) owned by Edwards is infringed by our Lotus Valve System. The trial began on February 7, 2017. On March 28, 2017, the European Patent Office revoked the Bourang patent and on April 3, 2017, the court suspended the infringement action pending Edwards' appeal of the revocation of the patent at the European Patent Office. |
• | On April 19, 2016, a subsidiary of Boston Scientific filed suit against Edwards Lifesciences Corporation (Edwards) in the U.S. District Court for the District of Delaware for patent infringement. We allege that Edwards’ SAPIEN 3™ Valve infringes a patent related to adaptive sealing technology. On June 9, 2016, Edwards filed a counterclaim alleging that our Lotus™ Valve System infringes three patents owned by Edwards. On October 12, 2016, Edwards filed a petition for inter partes review of our patent with the U.S. Patent and Trademark Office (USPTO), Patent Trial and Appeal Board. On March 29, 2017, the USPTO granted the inter partes review request. On April 18, 2017, Edwards filed a second petition for inter partes review of our patent with the USPTO. On March 23, 2018, the USPTO found our patent invalid. The Company filed an appeal before the United States Court of Appeals for the Federal Circuit on May 24, 2018. |
• | On April 19, 2016, a subsidiary of Boston Scientific filed suit against Edwards Lifesciences Corporation in the U.S. District Court for the Central District of California for patent infringement. We allege that Edwards’ aortic valve delivery systems infringe eight of our catheter related patents. On October 13, 2016, Edwards filed a petition for inter partes review of one asserted patent with the USPTO, Patent Trial and Appeal Board. On April 21, 2017, the USPTO denied the petition. On April 19 and 20, 2017, Edwards filed multiple inter partes review petitions against the patents in suit. On September 8, 2017, the court granted a stay of the action pending an inter partes review of the patents in suit. |
• | On April 26, 2016, Edwards Lifesciences PVT, Inc. filed a patent infringement action against us and one of our subsidiaries, Boston Scientific Medizintechnik GmbH, in the District Court of Düsseldorf, Germany alleging a European patent (Spenser '550) owned by Edwards is infringed by our Lotus™ Transcatheter Heart Valve System. The trial began on February 7, 2017. On March 9, 2017, the court found that we infringed the Spenser '550 patent. The Company filed an appeal. On April 13, 2018, the ‘550 patent was revoked by the European Patent Office. |
• | On October 27, 2016, Edwards Lifesciences PVT, Inc. filed a patent infringement action against us and one of our subsidiaries, Boston Scientific, LTD, in the Federal Court of Canada alleging that three Canadian patents (Spenser) owned by Edwards are infringed by our Lotus Transcatheter Heart Valve System. |
• | On December 22, 2016, Edwards Lifesciences PVT, Inc. and Edwards Lifesciences SA (AG) filed a plenary summons against Boston Scientific Limited and Boston Scientific Group Public Company in the High Court of Ireland alleging that a European patent (Spenser) owned by Edwards is infringed by our Lotus Valve System. On April 13, 2018, the ‘550 patent was revoked by the European Patent Office. |
• | On August 1, 2018, the Company filed a patent infringement action on the merits in Dusseldorf, Germany against Edwards Lifesciences Corporation and Edwards Lifesciences GmbH (collectively Edwards) alleging that the Sapien 3™ Device and Sapien 3 Ultra Device infringed a patent owned by the Company. |
114
• | On August 3, 2018, the Company filed a preliminary injunction request in Dusseldorf, Germany against Edwards Lifesciences Corporation and Edwards Lifesciences GmbH (collectively Edwards) alleging that the Sapien 3 Ultra Device infringed a patent owned by the Company. On October 23, 2018, the court found that the Sapien 3 Ultra Device infringed the patent. Edwards had the right to appeal. |
• | On August 22, 2018, Edwards Lifesciences LLC filed a patent infringement action against Boston Scientific Corporation, in the U. S. District Court of Delaware, alleging that two U.S. patents (Schweich) owned by them are infringed by our Watchman™ Left Atrial Appendage Closure Device, Watchman Delivery System and Watchman Access System. |
On December 14, 2016, we learned that the Associacao Brasileira de Medicina de Grupo d/b/a ABRAMGE filed a complaint against us, Arthrex and Zimmer Biomet Holdings, in the U.S. District Court for the District of Delaware. This complaint, which ABRAMGE never served against us, alleges that the defendants or their agents paid kickbacks to health care providers in order to increase sales and prices and are liable under a variety of common law theories. On February 6, 2017, ABRAMGE filed and served an amended complaint on us and the other defendants. The amended complaint does not contain any material changes in the allegations against us. Subsequently, on March 2, 2017, ABRAMGE filed a motion to consolidate this lawsuit with two other similar suits that it had brought against Stryker and Abbott Laboratories, in a multidistrict litigation proceeding. On April 13, 2017, we filed a motion to dismiss the amended complaint, as well as a separate opposition to the multidistrict litigation motion and on May 31, 2017, the Joint Panel on Multi-District Litigation denied ABRAMGE’s motion for the multidistrict litigation. On September 1, 2017, ABRAMGE filed a motion for leave to file a Second Amended Complaint, while our motion to dismiss the Amended Complaint remained pending. On September 15, 2017, we filed an opposition to the motion seeking leave to amend. On November 8, 2018, the Court granted ABRAMGE’s motion for leave to file a Second Amended Complaint, while also granting us leave to renew our motion to dismiss. We filed our motion to dismiss the Second Amended Complaint on January 18, 2019. On February 28, 2019, ABRAMGE dismissed its Second Amended Complaint, concluding the lawsuit.
On or about January 12, 2016, Teresa L. Stevens filed a claim against us and three other defendants asserting, for herself and on behalf of a putative class of similarly situated women, that she was harmed by a vaginal mesh implant that she alleges contained a counterfeit or adulterated resin product that we imported from China. The complaint was filed in the U.S. District Court for the Southern District of West Virginia, before the same Court that is hearing the mesh MDL. The complaint, which alleges Racketeer Influenced and Corrupt Organizations Act (RICO) violations, fraud, misrepresentation, deceptive trade practices and unjust enrichment, seeks both equitable relief and damages under state and federal law. On January 26, 2016, the Court issued an order staying the case and directing the plaintiff to submit information to allow the FDA to issue a determination with respect to her allegations. In addition, we were in contact with the U.S. Attorney’s Office for the Southern District of West Virginia and responded voluntarily to their requests in connection with that office’s review of the allegations concerning the use of mesh resin in the complaint. We reached a settlement on this matter and this case was dismissed on May 13, 2019.
On February 27, 2017, Carolyn Turner filed a complaint against us and five other defendants asserting for herself and on behalf of a putative class of similarly situated women, that she was harmed by a vaginal mesh implant that she alleges contained a counterfeit or adulterated resin product that we imported from China. The complaint was filed in the U.S. District Court for the Middle District of Florida, Orlando Division and alleges violations of the RICO, negligence, strict liability, breach of an express or implied warranty, intentional and negligent misrepresentation, fraud and unjust enrichment. Ms. Turner served this complaint against us on April 7, 2017. As of April 27, 2017, this case was stayed, pending resolution of the transfer petition to the mesh multidistrict litigation. We reached a settlement on this matter and this case was dismissed on February 25, 2019.
On April 24, 2019, a class action complaint was filed in the U.S. District Court for the Southern District of New York against Boston Scientific Corporation, Michael F. Mahoney, our Chief Executive Officer, and Daniel J. Brennan, our Chief Financial Officer. The complaint alleges violations of federal securities laws based on false and/or misleading statements and failure to disclose facts related to the Company’s transvaginal surgical mesh products. On September 20, 2019, the case was dismissed with prejudice.
On March 10, 2017, Imran Niazi filed a patent infringement action against us in the U.S. District Court for the Western District of Wisconsin alleging that a U.S. patent owned by him is infringed by our Acuity™ Lead Delivery System. On June 30, 2017, we filed a motion to dismiss for improper venue and on November 7, 2017 the Wisconsin Court granted the motion to dismiss. On November 13, 2017 Niazi refiled the same action in the U.S. District of Minnesota. We reached a confidential settlement on this matter on February 3, 2020 pursuant to which we anticipate the court will dismiss the pending action.
On August 3, 2012, we were served with a qui tam complaint that had previously been filed under seal against Boston Scientific Neuromodulation Corporation in the U.S. District Court for the District of New Jersey on March 2, 2011. On August 8, 2012, we learned that the federal government had previously declined to intervene in this matter. The relators’ complaint, now unsealed, alleges that Boston Scientific Neuromodulation Corporation violated the federal and various states' false claims acts through
115
submission of fraudulent bills for implanted devices, under-reporting of certain adverse events and promotion of off-label uses. On September 10, 2012, the relators filed an amended complaint revising and restating certain of the claims in the original complaint. Our motion to dismiss, filed subsequently, was denied on May 31, 2013 and on June 28, 2013, we answered the amended complaint and brought certain counterclaims arising from relators’ unauthorized removal of documents from the business during their employments, which the relators moved to dismiss on July 22, 2013. The Court denied relators’ motion to dismiss the counterclaims on September 4, 2014. Following the completion of fact and expert discovery, we filed a motion for summary judgment against all claims on January 27, 2017, relators filed their own motion for summary judgment against our counterclaims that same date. On December 15, 2017, the Court denied both motions for summary judgment. The parties reached a settlement on May 2, 2019, and the Court subsequently ordered the case dismissed, except for any claim by relators to recover attorneys’ fees.
On November 1, 2017, we entered into a definitive agreement with Channel Medsystems, Inc. (Channel) pursuant to which we could have been obligated to pay $145 million in cash up-front and a maximum of $130 million in contingent payments to acquire Channel. The agreement contained a provision allowing Channel to sell the remaining equity interests of Channel to us upon achievement of a regulatory milestone and an option allowing us to acquire the remaining equity interests. We sent a notice of termination of that agreement to Channel in the second quarter of 2018. On September 12, 2018, Channel filed a complaint in Delaware Chancery Court against us for alleged breach of the agreement. Channel alleged that we breached the agreement by terminating it. We have answered the complaint, denied the claims by Channel and counterclaimed to recover part of our investment in Channel, alleging fraud in the inducement. On April 2, 2019, Channel announced its receipt of FDA approval of the Cerene™ Cryotherapy Device. Trial testimony was taken in April 2019, and the post-trial briefing and hearing have been completed. During the third quarter of 2019, Channel notified us that they were exercising their option to sell the remaining equity interests in Channel to us. We responded to the notification that we did not intend to purchase Channel since the previous agreement had been terminated. On December 18, 2019, the Chancery Court ruled that Boston Scientific was in breach of the agreement and granted Channel's request for specific performance to require the Company to complete the purchase. On January 10, 2020, we filed a Notice of Appeal of the Chancery Court’s decision to the Delaware Supreme Court. On February 4, 2020, the Company settled the dispute with Channel resulting in termination of the agreement, payment by the Company of an undisclosed sum and surrender of the Company's equity interest in Channel.
NOTE K – STOCKHOLDERS' EQUITY
Preferred Stock
We are authorized to issue 50 million shares of preferred stock in one or more series and to fix the powers, designations, preferences and relative participating, option or other rights thereof, including dividend rights, conversion rights, voting rights, redemption terms, liquidation preferences and the number of shares constituting any series, without any further vote or action by our stockholders. As of December 31, 2019 and 2018, we had no shares of preferred stock issued or outstanding.
Common Stock
We are authorized to issue 2.000 billion shares of common stock, $0.01 par value per share. Holders of common stock are entitled to one vote per share. Holders of common stock are entitled to receive dividends, if and when declared by our Board of Directors and to share ratably in our assets legally available for distribution to our stockholders in the event of liquidation. Holders of common stock have no preemptive, subscription, redemption, or conversion rights. The holders of common stock do not have cumulative voting rights. The holders of a majority of the shares of common stock can elect all of the directors and can control our management and affairs.
On January 25, 2013, our Board of Directors approved a stock repurchase program authorizing the repurchase of up to $1.000 billion of our common stock. Repurchased shares are available for reissuance under our equity incentive plans and for general corporate purposes, including acquisitions. We did not repurchase any shares of our common stock during 2019, 2018 or 2017. As of December 31, 2019, we had remaining $535 million authorized under our 2013 share repurchase program. There were approximately 248 million shares in treasury as of December 31, 2019 and December 31, 2018.
116
NOTE L – STOCK INCENTIVE AND OWNERSHIP PLANS
Employee and Director Stock Incentive Plans
In 2011, our Board of Directors and stockholders approved our 2011 Long-Term Incentive Plan (the 2011 LTIP), authorizing for issuance up to 146 million shares of our common stock. The 2011 LTIP covers officers, directors, employees and consultants and provides for the grant of restricted or unrestricted common stock, deferred stock units (DSU), options to acquire our common stock, stock appreciation rights, performance awards (market-based and performance-based DSUs) and other stock and non-stock awards. Shares reserved under our current and former stock incentive plans totaled approximately 124 million as of December 31, 2019. The Executive Compensation and Human Resources Committee (the Committee) of the Board of Directors, consisting of independent, non-employee directors may authorize the issuance of common stock and cash awards under the 2011 LTIP in recognition of the achievement of long-term performance objectives established by the Committee.
Non-qualified options issued to employees are generally granted with an exercise price equal to the market price of our stock on the grant date, vest over a four-year service period and have a ten-year contractual life. In the case of qualified options, if the recipient owns more than ten percent of the voting power of all classes of stock, the option granted will be at an exercise price of 110 percent of the fair market value of our common stock on the date of grant and will expire over a period not to exceed five years. Non-vested stock awards, including restricted stock awards and DSUs, issued to employees are generally granted with an exercise price of zero and typically vest in four or five equal annual installments. These awards represent our commitment to issue shares to recipients after the vesting period. Upon each vesting date, such awards are no longer subject to risk of forfeiture and we issue shares of our common stock to the recipient.
The following presents the impact of stock-based compensation on our consolidated statements of operations:
Year Ended December 31, | |||||||||||
(in millions, except per share data) | 2019 | 2018 | 2017 | ||||||||
Cost of products sold | $ | $ | $ | ||||||||
Selling, general and administrative expenses | |||||||||||
Research and development expenses | |||||||||||
Income tax (benefit) expense | ( | ) | ( | ) | ( | ) | |||||
$ | $ | $ | |||||||||
Net impact per common share - basic | $ | $ | $ | ||||||||
Net impact per common share - assuming dilution | $ | $ | $ | ||||||||
Stock Options
We use the Black-Scholes option-pricing model to calculate the grant-date fair value of stock options granted to employees under our stock incentive plans. We calculated the fair value for options granted using the following estimated weighted-average assumptions:
Year Ended December 31, | ||||||||||||||||||||
2019 | 2018 | 2017 | ||||||||||||||||||
Options granted (in thousands) | ||||||||||||||||||||
Weighted-average exercise price | $ | $ | $ | |||||||||||||||||
Weighted-average grant-date fair value | $ | $ | $ | |||||||||||||||||
Black-Scholes Assumptions | ||||||||||||||||||||
Expected volatility | % | % | % | |||||||||||||||||
Expected term (in years, weighted) | ||||||||||||||||||||
Risk-free interest rate | % | - | % | - | % | - | ||||||||||||||
Expected Volatility
We use our historical volatility and implied volatility as a basis to estimate expected volatility in our valuation of stock options.
117
Expected Term
We estimate the expected term of options using historical exercise and forfeiture data. We believe that this historical data provides the best estimate of the expected term of new option grants.
Risk-Free Interest Rate
We use yield rates on U.S. Treasury securities for a period approximating the expected term of the award to estimate the risk-free interest rate in our grant-date fair value assessment.
Expected Dividend Yield
We have not historically paid cash dividends to our stockholders and currently do not intend to pay cash dividends. Therefore, we have assumed an expected dividend yield of zero in our grant-date fair value assessment.
Information related to stock options under stock incentive plans are as follows:
Stock Options (in thousands) | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (in years) | Aggregate Intrinsic Value (in millions) | |||||||||
Outstanding as of December 31, 2016 | $ | |||||||||||
Granted | ||||||||||||
Exercised | ( | ) | ||||||||||
Cancelled/forfeited | ( | ) | ||||||||||
Outstanding as of December 31, 2017 | $ | |||||||||||
Granted | ||||||||||||
Exercised | ( | ) | ||||||||||
Cancelled/forfeited | ( | ) | ||||||||||
Outstanding as of December 31, 2018 | $ | |||||||||||
Granted | ||||||||||||
Exercised | ( | ) | ||||||||||
Cancelled/forfeited | ( | ) | ||||||||||
Outstanding as of December 31, 2019 | $ | $ | ||||||||||
Exercisable as of December 31, 2019 | $ | $ | ||||||||||
Expected to vest as of December 31, 2019 | ||||||||||||
Total vested and expected to vest as of December 31, 2019 | $ | $ | ||||||||||
The total intrinsic value of stock options exercised was $140 million in 2019, $90 million in 2018 and $64 million in 2017.
118
Non-Vested Stock
We value restricted stock awards and DSUs based on the closing trading value of our shares on the date of grant. Information related to non-vested stock awards is as follows:
Non-Vested Stock Award Units (in thousands) | Weighted Average Grant-Date Fair Value | |||||
Balance as of December 31, 2016 | $ | |||||
Granted | ||||||
Vested (1) | ( | ) | ||||
Forfeited | ( | ) | ||||
Balance as of December 31, 2017 | $ | |||||
Granted | ||||||
Vested (1) | ( | ) | ||||
Forfeited | ( | ) | ||||
Balance as of December 31, 2018 | $ | |||||
Granted | ||||||
Vested (1) | ( | ) | ||||
Forfeited | ( | ) | ||||
Balance as of December 31, 2019 | $ | |||||
(1) | The number of restricted stock units vested includes shares withheld on behalf of employees to satisfy statutory tax withholding requirements. |
The total vesting date fair value of stock award units that vested was approximately $193 million in 2019, $170 million in 2018 and $190 million in 2017.
Market-based DSU Awards
During 2019, 2018 and 2017, we granted market-based DSU awards to certain members of our senior management team. The number of shares ultimately issued to the recipient is based on the total stockholder return (TSR) of our common stock as compared to the TSR of the common stock of the other companies in the S&P 500 Healthcare Index over a three-year performance period. The number of DSUs ultimately granted under this program range from 0 percent to 200 percent of the target number of performance-based DSUs awarded to the participant as determined by achievement of the performance criteria of the program. In addition, award recipients must remain employed by us throughout the three-year performance period to attain the full amount of the market-based DSUs that satisfied the market performance criteria.
We determined the fair value of the market-based DSU awards to be approximately $10 million for 2019, $7 million for 2018 and $8 million for 2017. We determined these fair values based on Monte Carlo simulations as of the date of grant, utilizing the following assumptions:
2019 | 2018 | 2017 | |||||||||
Awards | Awards | Awards | |||||||||
Stock price on date of grant | $ | $ | $ | ||||||||
Measurement period (in years) | |||||||||||
Risk-free rate | % | % | % | ||||||||
We recognize the expense on these awards in our consolidated statements of operations on a straight-line basis over the three-year measurement period.
119
Free Cash Flow Performance-based DSU Awards
During 2019, 2018 and 2017, we granted free cash flow performance-based DSU awards to certain members of our senior management team. The attainment of these performance-based DSUs is based on our adjusted free cash flow (AFCF) measured against our internal annual financial plan performance for AFCF. AFCF is measured over a one-year performance period beginning January 1st of each year and ending December 31st. The number of DSUs ultimately granted under this program range from 0 percent to 150 percent of the target number of performance-based DSUs awarded to the participant as determined by achievement of the performance criteria of the program. In addition, award recipients must remain employed by us throughout a three-year service period (inclusive of the one-year performance period) to attain the full amount of the performance-based DSUs that satisfied the performance criteria.
The following table presents our assumptions used in determining the fair value of our AFCF awards currently expected to vest as of December 31, 2019:
2019 AFCF | 2018 AFCF | 2017 AFCF | |||||||||
Fair value, net of forfeitures to date (in millions) | $ | $ | $ | ||||||||
Achievement of target payout | % | % | % | ||||||||
Year-end stock price used in determining fair value | $ | $ | $ | ||||||||
We recognize the expense on these awards in our consolidated statements of operations over the vesting period which is three years after the date of grant.
Expense Attribution
We recognize compensation expense for our stock incentive plan using a straight-line method over the substantive vesting period. Most of our stock awards provide for immediate vesting upon death or disability of the participant. In addition, our stock grants to employees provide for accelerated vesting of our stock-based awards, other than performance-based and market-based awards, upon retirement, if the stock award has been held for at least one year by the recipient. In accordance with the terms of our stock grants, for employees who will become retirement eligible prior to the vest date we expense stock-based awards, other than performance-based and market-based awards, over the greater of one year or the period between grant date and retirement-eligibility. The performance-based and market-based awards discussed above do not contain provisions that would accelerate the full vesting of the awards upon retirement-eligibility.
We recognize stock-based compensation expense for the value of the portion of awards that are ultimately expected to vest. FASB ASC Topic 718, Compensation – Stock Compensation allows forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” or “expirations” and represents only the unvested portion of the surrendered stock-based award. We have applied, based on an analysis of our historical forfeitures, a weighted-average annual forfeiture rate of approximately six percent to all unvested stock-based awards as of December 31, 2019, which represents the portion that we expect will be forfeited each year over the vesting period. We re-evaluate this analysis annually or more frequently if there are significant changes in circumstances and adjust the forfeiture rate as necessary. Ultimately, we will only recognize expense for those shares that vest.
Unrecognized Compensation Cost
We expect to recognize the following future expense for awards outstanding as of December 31, 2019:
Unrecognized Compensation Cost (in millions) (1) | Weighted Average Remaining Vesting Period (in years) | ||||
Stock options | $ | ||||
Non-vested stock awards | |||||
$ | |||||
(1) | Amounts presented represent compensation cost, net of estimated forfeitures. |
120
Employee Stock Purchase Plans
Our global employee stock purchase plan provides for the granting of options to purchase up to 50 million shares of our common stock to all eligible employees. Under the global employee stock purchase plan, we grant each eligible employee, at the beginning of each six-month offering period, an option to purchase shares of our common stock equal to not more than ten percent of the employee’s eligible compensation or the statutory limit under the U.S. Internal Revenue Code. Such options may be exercised only to the extent of accumulated payroll deductions at the end of the offering period, at a purchase price equal to 85 percent of the fair market value of our common stock at the beginning or end of each offering period, whichever is less. As of December 31, 2019, there were approximately 8 million shares available for future issuance under the employee stock purchase plan.
Information related to shares issued or to be issued in connection with the employee stock purchase plan based on employee contributions and the range of purchase prices is as follows:
Year Ended December 31, | ||||||||||||||||||||
2019 | 2018 | 2017 | ||||||||||||||||||
Shares issued or to be issued (in thousands) | ||||||||||||||||||||
Range of purchase prices | $ | - | $ | $ | - | $ | $ | - | $ | |||||||||||
Expense recognized (in millions) | $ | $ | $ | |||||||||||||||||
We use the Black-Scholes option-pricing model to calculate the grant-date fair value of shares issued under the employee stock purchase plan. We recognize expense related to shares purchased through the employee stock purchase plan ratably over the offering period.
NOTE M – WEIGHTED AVERAGE SHARES OUTSTANDING
Year Ended December 31, | ||||||||
(in millions) | 2019 | 2018 | 2017 | |||||
Weighted average shares outstanding - basic | ||||||||
Net effect of common stock equivalents | ||||||||
Weighted average shares outstanding - assuming dilution | ||||||||
The impact of stock options outstanding with exercise prices greater than the average fair market value of our common stock was immaterial for all periods presented.
NOTE N – SEGMENT REPORTING
We have three reportable segments comprised of MedSurg, Rhythm and Neuro, and Cardiovascular, which represent an aggregation of our operating segments that generate revenues from the sale of medical devices. We measure and evaluate our reportable segments based on net sales of reportable segments, operating income of reportable segments, excluding intersegment profits, and operating income of reportable segments as a percentage of net sales of reportable segments. Operating income of reportable segments as a percentage of net sales of reportable segments is defined as operating income of reportable segments divided by net sales of reportable segments. We exclude from operating income of reportable segments certain corporate-related expenses and certain transactions or adjustments that our chief operating decision maker (CODM) considers to be non-operational, such as amounts related to amortization expense, intangible asset impairment charges, acquisition/divestitures-related items, restructuring and restructuring-related items, medical device regulation charges and litigation-related charges/(credits). Although we exclude these amounts from operating income of reportable segments, they are included in reported Income (loss) before income taxes on the consolidated statements of operations and are included in the reconciliation below.
Our seven core businesses are organized into three reportable segments: MedSurg, Rhythm and Neuro, and Cardiovascular. Following our acquisition of BTG, which closed during the third quarter of 2019, we have included BTG’s Interventional Medicine business within our Peripheral Interventions operating segment, within the Cardiovascular reportable segment. We present BTG’s Specialty Pharmaceuticals business as a standalone operating segment alongside our reportable segments.
121
A reconciliation of the totals reported for the reportable segments to the applicable line items in our accompanying consolidated statements of operations is as follows (in millions, except percentages):
Year Ended December 31, | |||||||||||
Net sales | 2019 | 2018 | 2017 | ||||||||
MedSurg | $ | $ | $ | ||||||||
Rhythm and Neuro | |||||||||||
Cardiovascular | |||||||||||
Total net sales of reportable segments | 9,823 | ||||||||||
All other (Specialty Pharmaceuticals) | n/a | n/a | |||||||||
Consolidated net sales | $ | $ | $ | ||||||||
Year Ended December 31, | |||||||||||
Depreciation expense | 2019 | 2018 | 2017 | ||||||||
MedSurg | $ | $ | $ | ||||||||
Rhythm and Neuro | |||||||||||
Cardiovascular | |||||||||||
Total depreciation expense of reportable segments | |||||||||||
All other (Specialty Pharmaceuticals) | n/a | n/a | |||||||||
Consolidated depreciation expense | $ | $ | $ | ||||||||
Year Ended December 31, | |||||||||||
Income (loss) before income taxes | 2019 | 2018 | 2017 | ||||||||
MedSurg | $ | $ | $ | ||||||||
Rhythm and Neuro | |||||||||||
Cardiovascular | |||||||||||
Total operating income of reportable segments | |||||||||||
All other (Specialty Pharmaceuticals) | n/a | n/a | |||||||||
Unallocated amounts: | |||||||||||
Corporate expenses, including hedging activities | ( | ) | ( | ) | ( | ) | |||||
Intangible asset impairment charges, acquisition/divestiture-related, restructuring- and restructuring-related, litigation-related net (charges) credits and EU MDR implementation costs | ( | ) | ( | ) | ( | ) | |||||
Amortization expense | ( | ) | ( | ) | ( | ) | |||||
Operating income (loss) | |||||||||||
Other expense, net | ( | ) | ( | ) | ( | ) | |||||
Income (loss) before income taxes | $ | $ | $ | ||||||||
Operating income of reportable segments as a percentage of net sales of reportable segments | Year Ended December 31, | |||||||
2019 | 2018 | 2017 | ||||||
MedSurg | % | % | % | |||||
Rhythm and Neuro | % | % | % | |||||
Cardiovascular | % | % | % | |||||
122
As of December 31, | |||||||
Total assets | 2019 | 2018 | |||||
MedSurg | $ | $ | |||||
Rhythm and Neuro | |||||||
Cardiovascular | |||||||
Total assets of reportable segments | |||||||
All other (Specialty Pharmaceuticals) | |||||||
Goodwill | |||||||
Other intangible assets, net | |||||||
All other corporate assets | |||||||
$ | $ | ||||||
As of December 31, | |||||||||||
Long-lived assets | 2019 | 2018 | 2017 | ||||||||
U.S. | $ | $ | $ | ||||||||
Ireland | |||||||||||
Other countries | |||||||||||
Property, plant and equipment, net | |||||||||||
Goodwill | |||||||||||
Other intangible assets, net | |||||||||||
Operating lease right-of-use assets in Other long-term assets | |||||||||||
$ | $ | $ | |||||||||
NOTE O – REVENUE
We generate revenue primarily from the sale of single-use medical devices and present revenue net of sales taxes in our consolidated statements of operations. The following tables disaggregate our revenue from contracts with customers by business and geographic region (in millions):
Year Ended December 31, | |||||||||||||||||||||||||||||||||||
2019 | 2018 | 2017 | |||||||||||||||||||||||||||||||||
Businesses | U.S. | OUS | Total | U.S. | OUS | Total | U.S. | OUS | Total | ||||||||||||||||||||||||||
Endoscopy | $ | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||
Urology and Pelvic Health | |||||||||||||||||||||||||||||||||||
Cardiac Rhythm Management | |||||||||||||||||||||||||||||||||||
Electrophysiology | |||||||||||||||||||||||||||||||||||
Neuromodulation | |||||||||||||||||||||||||||||||||||
Interventional Cardiology | |||||||||||||||||||||||||||||||||||
Peripheral Interventions | |||||||||||||||||||||||||||||||||||
Specialty Pharmaceuticals | n/a | n/a | n/a | n/a | n/a | n/a | |||||||||||||||||||||||||||||
Net Sales | $ | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||
123
Year Ended December 31, | |||||||||||
Geographic Regions | 2019 | 2018 | 2017 | ||||||||
U.S. | $ | $ | $ | ||||||||
EMEA (Europe, Middle East and Africa) | |||||||||||
APAC (Asia-Pacific) | |||||||||||
LACA (Latin America and Canada) | |||||||||||
Medical Devices | |||||||||||
U.S. | n/a | n/a | |||||||||
OUS | n/a | n/a | |||||||||
Specialty Pharmaceuticals | n/a | n/a | |||||||||
Net Sales | $ | $ | $ | ||||||||
Emerging Markets (1) | $ | $ | $ | ||||||||
(1) | We define Emerging Markets as the 20 countries that we believe have strong growth potential based on their economic conditions, healthcare sectors and our global capabilities. Periodically, we assess our list of Emerging Markets; effective January 1, 2019, we updated our list of Emerging Market countries. Our current list is comprised of the following countries: Argentina, Brazil, Chile, China, Colombia, Czech Republic, India, Indonesia, Malaysia, Mexico, Philippines, Poland, Russia, Saudi Arabia, Slovakia, South Africa, South Korea, Thailand, Turkey and Vietnam. We have revised prior year amounts to the current year’s presentation. |
Contract liabilities are classified within Other current liabilities and Other long-term liabilities on our accompanying consolidated balance sheets. Our deferred revenue balance was $400 million as of December 31, 2019 and $373 million as of December 31, 2018. Our contractual liabilities are primarily composed of deferred revenue related to the LATITUDE Patient Management System. Revenue is recognized over the average service period which is based on device and patient longevity. We recognized revenue of $143 million in 2019 that was included in the above December 31, 2018 contract liability balance. We have elected not to disclose the transaction price allocated to unsatisfied performance obligations when the original expected contract duration is one year or less. In addition, we have not identified material unfulfilled performance obligations for which revenue is not currently deferred. Refer to Note A – Significant Accounting Policies for additional information on our accounting policies relating to revenue recognition.
NOTE P – CHANGES IN OTHER COMPREHENSIVE INCOME
The following table provides the reclassifications out of Other comprehensive income, net of tax:
(in millions) | Foreign Currency Translation Adjustments | Net Change in Derivative Financial Instruments | Net Change in Available-for-Sale Securities | Net Change in Defined Benefit Pensions and Other Items | Total | ||||||||||||||
Balance as of December 31, 2018 | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||
Other comprehensive income (loss) before reclassifications | ( | ) | |||||||||||||||||
(Income) loss amounts reclassified from accumulated other comprehensive income | ( | ) | ( | ) | ( | ) | |||||||||||||
Total other comprehensive income (loss) | ( | ) | |||||||||||||||||
Balance as of December 31, 2019 | $ | $ | $ | $ | ( | ) | $ | ||||||||||||
124
(in millions) | Foreign Currency Translation Adjustments | Net Change in Derivative Financial Instruments | Net Change in Available-for-Sale Securities | Net Change in Defined Benefit Pensions and Other Items | Total | ||||||||||||||
Balance as of December 31, 2017 | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
Other comprehensive income (loss) before reclassifications | ( | ) | |||||||||||||||||
(Income) loss amounts reclassified from accumulated other comprehensive income | ( | ) | ( | ) | |||||||||||||||
Total other comprehensive income (loss) | ( | ) | |||||||||||||||||
Balance as of December 31, 2018 | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||
Refer to Note D – Hedging Activities and Fair Value Measurements for further detail on our net investment hedges recorded in Foreign currency translation adjustments and our cash flow hedges recorded in Net change in derivative financial instruments.
As a result of adopting ASC Update No. 2016-01 in the first quarter of 2018, we recorded a cumulative effect adjustment to retained earnings to reclassify unrealized gains and losses from our equity investments previously recorded to Accumulated other comprehensive income (loss), net of tax. These equity investments were classified as available-for-sale securities under the former accounting guidance, and we now refer to these investments as publicly-held equity securities. Refer to Note A – Significant Accounting Policies for additional information.
NOTE Q – NEW ACCOUNTING PRONOUNCEMENTS
Periodically, new accounting pronouncements are issued by the FASB or other standard setting bodies. Recently issued standards typically do not require adoption until a future effective date. Prior to their effective date, we evaluate the pronouncements to determine the potential effects of adoption on our consolidated financial statements.
Standards to be Implemented
ASC Update No. 2016-13
In June 2016, the FASB issued ASC Update No. 2016-13, Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The purpose of Update No. 2016-13 is to replace the current incurred loss impairment methodology for financial assets measured at amortized cost with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information, including forecasted information, to develop credit loss estimates. Update No. 2016-13 is effective for annual periods beginning after December 15, 2019, including interim periods within those annual periods. We expect the adoption will have an immaterial impact on our financial position and results of operations.
ASC Update No. 2018-15
In August 2018, the FASB issued ASC Update No. 2018-15, Intangibles – Goodwill and Other – Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract. The purpose of Update No. 2018-15 is to align the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software (and hosting arrangements that include an internal-use software license). Update No. 2018-15 is effective for annual periods beginning after December 15, 2019, including interim periods within those annual periods. Early adoption is permitted, including adoption in any interim period. We plan to adopt Update No. 2018-15 in the first quarter of 2020. We expect the adoption will have an immaterial impact on our financial position and results of operations.
125
ASC Update No. 2018-18
In November 2018, the FASB issued ASC Update No. 2018-18, Collaborative Arrangements (Topic 808): Clarifying The Interaction Between Topic 808 and Topic 606. The purpose of Update No. 2018-18 is to clarify the interaction between FASB ASC Topic 808 and FASB ASC Topic 606 as FASB ASC Topic 808 did not provide comprehensive recognition or measurement guidance for collaborative arrangements, and the accounting for those arrangements was often based on an analogy to other accounting literature or an accounting policy election. Update No. 2018-18 is effective for annual periods beginning after December 15, 2019. We plan to adopt Update No. 2018-18 in the first quarter of 2020. We expect the adoption will have an immaterial impact on our financial position and results of operations.
No other new accounting pronouncements, issued or effective, during the period had, or is expected to have, a material impact on our consolidated financial statements.
NOTE R - EMPLOYEE RETIREMENT PLANS
Defined Benefit Pension Plans
Domestic Retirement Plans
Following our 2006 acquisition of Guidant, we assumed the Guidant Supplemental Retirement Plan, a frozen, non-qualified defined benefit plan for certain former officers and employees of Guidant. The Guidant Supplemental Retirement Plan was partially funded through a Rabbi Trust that contains segregated company assets within restricted cash used to pay the benefit obligations related to the plan.
We also maintain an Executive Retirement Plan, a defined benefit plan covering executive officers and division presidents. Participants may retire with benefits once retirement conditions have been satisfied.
U.K. Plan
As a result of our acquisition of BTG, we assumed a benefit obligation related to a defined benefit pension plan sponsored by BTG for eligible United Kingdom (U.K.) employees (U.K. Plan). The U.K. Plan was closed to new entrants as of June 1, 2004. Prior to the acquisition close date of August 19, 2019, the Trustees of the U.K. Plan executed buy-in arrangements (Buy-in Contracts), which effectively, as structured under the Buy-in Contracts, are intended to provide payments designed to equal all future designated contractual benefit payments to covered participants. The benefit obligation of the pension plan is not transferred to the insurers, and we remain responsible for paying pension benefits. We do not anticipate any additional material contributions or payments to the U.K. Plan or the insurer.
In connection with our preliminary purchase price allocation of BTG, we recorded the assets acquired and liabilities assumed at their respective fair values as of the acquisition date. The following assumptions were used to measure the fair value of the benefit obligation and associated plan assets as of the August 19, 2019 measurement date:
Discount Rate | Expected Return on Plan Assets | Rate of Compensation Increase | |||
U.K. Plan | |||||
As of the measurement date of August 19, 2019, the funded status was as follows:
(in millions) | |||
Fair value of plan assets | $ | ||
Benefit obligation | ( | ) | |
Funded status | $ | ( | ) |
Refer to Note B – Acquisitions and Strategic Investments for additional information on our acquisition of BTG.
Information about the U.K. Plan presented below is as of the December 31, 2019 measurement date.
126
Other International Retirement Plans
In addition, we maintain retirement plans covering certain international employees.
As of December 31, 2019 | |||||||||||||||
(in millions) | Accumulated Benefit Obligation (ABO) | Projected Benefit Obligation (PBO) | Fair value of Plan Assets | Unfunded/Underfunded PBO Recognized | |||||||||||
Domestic Retirement Plans | $ | $ | $ | $ | |||||||||||
U.K. Plan | |||||||||||||||
Other International Retirement Plans | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
As of December 31, 2018 | |||||||||||||||
(in millions) | Accumulated Benefit Obligation (ABO) | Projected Benefit Obligation (PBO) | Fair value of Plan Assets | Unfunded/Underfunded PBO Recognized | |||||||||||
Domestic Retirement Plans | $ | $ | $ | $ | |||||||||||
International Retirement Plans | |||||||||||||||
$ | $ | $ | $ | ||||||||||||
A rollforward of the changes in the PBO for our retirement plans is as follows:
Year Ended December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Beginning obligations | $ | $ | |||||
Acquired and established plans(1) | |||||||
Service costs | |||||||
Interest costs | |||||||
Actuarial (gain) loss | ( | ) | |||||
Plan amendments and assumption changes | ( | ) | |||||
Benefits paid | ( | ) | ( | ) | |||
Impact of foreign currency fluctuations | ( | ) | |||||
Ending obligation | $ | $ | |||||
(1) | Plans obtained through acquisition and other increases in connection with our international operations. Refer to Note B – Acquisitions and Strategic Investments for additional information regarding the U.K. Plan we acquired with BTG on August 19, 2019. |
The critical assumptions associated with our employee retirement plans for 2019 are as follows:
Weighted Average Discount Rate | Weighted Average Expected Return on Plan | Weighted Average Rate of Compensation Increase(1) | |||
Domestic Retirement Plans | n/a | ||||
U.K. Plan | |||||
Other International Retirement Plans | |||||
(1) | Rates of compensation increase were not weighted by the relative fair value of the instruments. As such, the amount represents the median of the inputs and is not a weighted average. |
127
The critical assumptions associated with our employee retirement plans for 2018 are as follows:
Discount Rate | Expected Return on Plan Assets | Rate of Compensation Increase | ||||||||||||
Domestic Retirement Plans | % | - | n/a | |||||||||||
International Retirement Plans | % | - | % | - | % | - | ||||||||
A rollforward of the changes in the fair value of plan assets for our funded retirement plans is as follows:
Year Ended December 31, | |||||||
(in millions) | 2019 | 2018 | |||||
Beginning fair value | $ | $ | |||||
Acquired and established plans(1) | |||||||
Actual return on plan assets | ( | ) | |||||
Employer contributions | |||||||
Participant contributions | |||||||
Actuarial gain (loss) | ( | ) | |||||
Benefits paid | ( | ) | ( | ) | |||
Impact of foreign currency fluctuations | |||||||
Ending fair value | $ | $ | |||||
(1) | Plans obtained through acquisition and other increases in connection with our international operations. Refer to Note B – Acquisitions and Strategic Investments for additional information regarding the U.K. Plan we acquired with BTG on August 19, 2019. |
For our defined benefit plans excluding our U.K. Plan, we base our discount rate on the rates of return available on high-quality bonds with maturities approximating the expected period over which benefits will be paid. The rate of compensation increase is based on historical and expected rate increases. We base our rate of expected return on plan assets on historical experience, our investment guidelines and expectations for long-term rates of return. Our assets are invested in a variety of securities, primarily equity securities and government bonds. These securities are considered Level 1 and Level 2 investments.
The following table presents the fair value hierarchy of the U.K. Plan assets measured at fair value as of December 31, 2019:
As of | |||||||||||||||
December 31, 2019 | |||||||||||||||
(in millions) | Level 1 | Level 2 | Level 3 | Total | |||||||||||
Buy-in contracts | $ | $ | $ | $ | |||||||||||
Cash | |||||||||||||||
Total assets | $ | $ | $ | $ | |||||||||||
128
Changes in the fair value of the U.K. Plan Level 3 assets were as follows:
(in millions) | Buy-in Contracts | ||
Balance as of December 31, 2018 | $ | ||
Acquired plans(1) | |||
Actuarial gain (loss) | ( | ) | |
Benefits paid | ( | ) | |
Impact of foreign currency fluctuations | |||
Balance as of December 31, 2019 | $ | ||
(1) | Refer to Note B – Acquisitions and Strategic Investments for additional information regarding the U.K. Plan we acquired with BTG on August 19, 2019. |
Defined Contribution Plan
We also sponsor a voluntary 401(k) Retirement Savings Plan for eligible employees. We match 200 percent of employee elective deferrals for the first two percent of employee eligible compensation and 50 percent of employee elective deferrals greater than two percent, but not exceeding six percent, of employee eligible compensation. Total expense for our matching contributions to the plan was $98 million in 2019, $87 million in 2018 and $79 million in 2017.
129
QUARTERLY RESULTS OF OPERATIONS
(in millions, except per share data)
(unaudited)
Three Months Ended | |||||||||||||||
Mar 31, | June 30, | Sept 30, | Dec 31, | ||||||||||||
2019 | |||||||||||||||
Net sales | $ | 2,493 | $ | 2,631 | $ | 2,707 | $ | 2,905 | |||||||
Gross profit | 1,763 | 1,873 | 1,930 | 2,054 | |||||||||||
Operating income (loss) | 541 | 384 | 383 | 210 | |||||||||||
Net income (loss) | 424 | 154 | 126 | 3,996 | |||||||||||
Net income (loss) per common share - basic | $ | 0.31 | $ | 0.11 | $ | 0.09 | $ | 2.87 | |||||||
Net income (loss) per common share - assuming dilution | $ | 0.30 | $ | 0.11 | $ | 0.09 | $ | 2.83 | |||||||
2018 | |||||||||||||||
Net sales | $ | 2,379 | $ | 2,490 | $ | 2,393 | $ | 2,561 | |||||||
Gross profit | 1,707 | 1,751 | 1,720 | 1,832 | |||||||||||
Operating income (loss) | 407 | 392 | 388 | 319 | |||||||||||
Net income (loss) | 298 | 555 | 432 | 386 | |||||||||||
Net income (loss) per common share - basic | $ | 0.22 | $ | 0.40 | $ | 0.31 | $ | 0.28 | |||||||
Net income (loss) per common share - assuming dilution | $ | 0.21 | $ | 0.40 | $ | 0.31 | $ | 0.27 | |||||||
Our reported results for 2019 included EU MDR implementation charges, amortization expense, intangible asset impairment charges, acquisition/divestiture-related net charges, restructuring and restructuring-related net charges, litigation-related net charges, debt extinguishment charges, certain investment impairment charges, certain deferred tax benefits and certain discrete tax items (after tax) of: $66 million in charges in the first quarter, $396 million in charges in the second quarter, $424 million in charges in the third quarter and $3,353 million of credits in the fourth quarter. These after-tax net credits consisted primarily of: deferred tax benefits of $4,102 million arising from an intra-entity asset transfer of intellectual property, partially offset by $672 million of acquisition / divestiture-related net charges primarily related to the BTG acquisition and $628 million of amortization expense.
Our reported results for 2018 included amortization expense, intangible asset impairment charges, acquisition-related net charges, restructuring and restructuring-related net charges, litigation-related net charges, certain investment impairment charges and certain discrete tax items (after tax) of: $157 million in the first quarter, $13 million in the second quarter, $53 million in the third quarter and $166 million in the fourth quarter. These after-tax net charges consisted primarily of: $328 million credit related to certain discrete tax items and $520 million of amortization expense.
130
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (CEO) and Executive Vice President and Chief Financial Officer (CFO), evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2019 pursuant to Rule 13a-15(b) of the Securities Exchange Act of 1934, as amended. Disclosure controls and procedures are designed to ensure that material information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and ensure that such material information is accumulated and communicated to our management, including our CEO and CFO, as appropriate to allow timely decisions regarding required disclosure. Based on their evaluation, our CEO and CFO concluded that as of December 31, 2019, our disclosure controls and procedures were effective.
Management’s Annual Report on Internal Control over Financial Reporting
Management’s annual report on our internal control over financial reporting is contained in Item 7 of this Annual Report.
Report of Independent Registered Public Accounting Firm on Internal Control over Financial Reporting
The report of Ernst & Young LLP on our internal control over financial reporting is contained in Item 7 of this Annual Report.
Changes in Internal Control over Financial Reporting
During the quarter ended December 31, 2019, there were no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
131
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item is set forth in our Proxy Statement for the 2020 Annual Meeting of Stockholders to be filed with the SEC within 120 days of December 31, 2019 and is incorporated into this Annual Report by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item is set forth in our Proxy Statement for the 2020 Annual Meeting of Stockholders to be filed with the SEC within 120 days of December 31, 2019 and is incorporated into this Annual Report by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item is set forth in our Proxy Statement for the 2020 Annual Meeting of Stockholders to be filed with the SEC within 120 days of December 31, 2019 and is incorporated into this Annual Report by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
The information required by this Item is set forth in our Proxy Statement for the 2020 Annual Meeting of Stockholders to be filed with the SEC within 120 days of December 31, 2019 and is incorporated into this Annual Report by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item is set forth in our Proxy Statement for the 2020 Annual Meeting of Stockholders to be filed with the SEC within 120 days of December 31, 2019 and is incorporated into this Annual Report by reference.
132
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements.
The response to this portion of Item 15 is set forth under Item 8.
(a)(2) Financial Statement Schedules.
The response to this portion of Item 15 (Schedule II) follows the signature page to this report. All other financial statement schedules are not required under the related instructions or are inapplicable and therefore have been omitted.
(a)(3) Exhibits (* documents filed or furnished with this report, ** certain schedules and exhibits omitted pursuant to Item 601(b)(2) of Regulation S-K. We agree to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request, # compensatory plans or arrangements)
EXHIBIT NO. | TITLE | |
2.1 | ||
3.1 | ||
3.2 | ||
4.1 | Specimen Certificate for shares of the Company's Common Stock (incorporated herein by reference to Exhibit 4.1, Registration No. 33-46980). | |
4.2* | ||
4.3 | ||
4.4 | ||
4.5 | ||
4.6 | ||
4.7 | ||
133
4.8 | ||
4.9 | ||
4.10 | ||
4.11 | ||
4.12 | ||
4.13 | ||
4.14 | ||
4.15 | ||
4.16 | ||
4.17 | ||
4.18 | ||
4.19 | ||
4.20 | ||
4.21 | ||
4.22 | ||
4.23 | ||
134
4.24 | ||
10.1 | ||
10.2 | ||
10.3 | ||
10.4 | ||
10.5 | ||
10.6 | ||
10.7 | ||
10.8 | Transaction Agreement, dated as of January 8, 2006, as amended, between the Company and Abbott Laboratories (incorporated herein by reference to Exhibit 10.47, Exhibit 10.48, Exhibit 10.49 and Exhibit 10.50, Annual Report on Form 10-K for year ended December 31, 2005 and Exhibit 10.1, Current Report on Form 8-K dated April 7, 2006, File No. 1-11083). | |
10.9 | ||
10.10 | ||
10.11 | ||
10.12 | ||
135
10.13 | Form of Boston Scientific Corporation Excess Benefit Plan, as amended (incorporated herein by reference to Exhibit 10.1, Current Report on Form 8-K dated June 29, 2005 and Exhibit 10.4, Current Report on Form 8-K dated December 16, 2008, File No. 1-11083).# | |
10.14 | ||
10.15 | ||
10.16 | ||
10.17 | ||
10.18 | ||
10.19 | ||
10.20 | Boston Scientific Corporation 2000 Long-Term Incentive Plan, as amended (incorporated herein by reference to Exhibit 10.20, Annual Report on Form 10-K for the year ended December 31, 1999, Exhibit 10.18, Annual Report on Form 10-K for the year ended December 31, 2001, Exhibit 10.1, Current Report on Form 8-K dated December 22, 2004, Exhibit 10.3, Current Report on Form 8-K dated May 9, 2005, and Exhibit 10.3, Current Report on Form 8-K dated December 16, 2008, File No. 1-11083).# | |
10.21 | ||
10.22 | ||
10.23 | ||
10.24 | ||
10.25 | ||
10.26 | ||
10.27 | ||
136
10.28 | ||
10.29 | ||
10.30 | ||
10.31 | ||
10.32 | ||
10.33 | ||
10.34 | ||
10.35 | ||
10.36 | ||
10.37 | ||
10.38 | ||
10.39 | ||
10.40 | ||
10.41 | ||
137
10.42 | ||
10.43 | ||
10.44 | ||
10.45 | ||
10.46 | ||
10.47 | ||
10.48 | ||
10.49 | ||
10.50 | ||
10.51 | ||
10.52 | ||
10.53 | ||
10.54 | ||
10.55 | ||
138
10.56 | ||
10.57 | ||
10.58 | ||
10.59 | ||
10.60 | ||
10.61 | ||
10.62 | ||
10.63 | ||
10.64 | ||
10.65 | ||
10.66 | ||
10.67 | ||
10.68 | ||
10.69 | ||
139
10.70 | ||
10.71 | ||
10.72 | ||
10.73 | ||
10.74 | ||
10.75 | ||
10.76 | ||
10.77 | ||
10.78 | ||
10.79 | ||
10.80 | ||
10.81 | ||
10.82 | ||
10.83 | ||
140
10.84 | ||
10.85 | ||
10.86 | ||
10.87 | ||
10.88 | ||
10.89 | ||
10.90 | ||
10.91 | ||
10.92 | ||
10.93 | ||
10.94 | ||
10.95 | ||
10.96 | ||
141
10.97 | ||
10.98 | ||
10.99 | ||
10.100 | ||
10.101 | ||
10.102 | ||
10.103 | ||
10.104 | ||
10.105 | ||
10.106 | ||
10.107 | ||
10.108 | ||
10.109 | ||
10.110 | ||
142
10.111 | ||
10.112 | ||
10.113 | ||
10.114 | ||
10.115 | ||
10.116 | ||
10.117 | ||
10.118 | ||
10.119 | ||
10.120 | ||
10.121 | ||
21* | ||
23* | ||
31.1* | ||
31.2* | ||
32.1* | ||
143
32.2* | ||
101.SCH* | XBRL Taxonomy Extension Schema Document. | |
101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document. | |
101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document. | |
101.LAB* | XBRL Taxonomy Extension Label Linkbase Document. | |
101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document. | |
104 | Cover Page Interactive Data File (embedded within the Inline XBRL document and contained in Exhibit 101) | |
144
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: February 25, 2020 | Boston Scientific Corporation | |||
By: | /s/ Daniel J. Brennan | |||
Daniel J. Brennan | ||||
Executive Vice President and Chief Financial Officer | ||||
(duly authorized officer and principal financial officer) | ||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Dated: February 25, 2020 | By: | /s/ Daniel J. Brennan | ||
Daniel J. Brennan | ||||
Executive Vice President and Chief Financial Officer | ||||
(Principal Financial Officer) | ||||
Dated: February 25, 2020 | By: | /s/ Michael F. Mahoney | ||
Michael F. Mahoney | ||||
Director, Chairman of the Board, President and Chief Executive Officer | ||||
(Principal Executive Officer) | ||||
Dated: February 25, 2020 | By: | /s/ Jonathan R. Monson | ||
Jonathan R. Monson | ||||
Vice President, Global Controller and Chief Accounting Officer | ||||
(Principal Accounting Officer) | ||||
145
Dated: February 25, 2020 | By: | /s/ Nelda J. Connors | ||
Nelda J. Connors | ||||
Director | ||||
Dated: February 25, 2020 | By: | /s/ Charles J. Dockendorff | ||
Charles J. Dockendorff | ||||
Director | ||||
Dated: February 25, 2020 | By: | /s/ Yoshiaki Fujimori | ||
Yoshiaki Fujimori | ||||
Director | ||||
Dated: February 25, 2020 | By: | /s/ Donna A. James | ||
Donna A. James | ||||
Director | ||||
Dated: February 25, 2020 | By: | /s/ Edward J. Ludwig | ||
Edward J. Ludwig | ||||
Director | ||||
146
Dated: February 25, 2020 | By: | /s/ Stephen P. MacMillan | ||
Stephen P. MacMillan | ||||
Director | ||||
Dated: February 25, 2020 | By: | /s/ David J. Roux | ||
David J. Roux | ||||
Director | ||||
Dated: February 25, 2020 | By: | /s/ John E. Sununu | ||
John E. Sununu | ||||
Director | ||||
Dated: February 25, 2020 | By: | /s/ Ellen M. Zane | ||
Ellen M. Zane | ||||
Director | ||||
147
Schedule II
VALUATION AND QUALIFYING ACCOUNTS
(in millions)
Description | Balance at Beginning of Year | Charges to Costs and Expenses (a) | Deductions to Allowances for Uncollectible Accounts (b) | Charges to (Deductions from) Other Accounts (c) | Balance at End of Year | ||||||||||||
Year Ended December 31, 2019: | |||||||||||||||||
Allowances for uncollectible accounts (d) | $ | ( | ) | $ | |||||||||||||
Year Ended December 31, 2018: | |||||||||||||||||
Allowances for uncollectible accounts (d) | $ | ( | ) | ( | ) | $ | |||||||||||
Year Ended December 31, 2017: | |||||||||||||||||
Allowances for uncollectible accounts and sales returns and allowances | $ | ( | ) | ( | ) | $ | |||||||||||
(a) Represents allowances for uncollectible accounts established through selling, general and administrative expenses.
(b) Represents actual write-offs of uncollectible accounts.
(c) Represents net change in allowances for sales returns, recorded as contra-revenue.
(d) Following the adoption of FASB ASC Topic 606 as of January 1, 2018, the allowance for sales returns has been reclassified from Trade accounts receivable, net to Other current liabilities within the consolidated balance sheets and is not included in the ending balance for 2018 above. Prior period balances remain unchanged.
148