VERTEX PHARMACEUTICALS INC / MA000087532012/312024Q2falseP1M478365xbrli:sharesiso4217:USDiso4217:USDxbrli:sharesvrtx:segmentvrtx:targetxbrli:pure00008753202024-01-012024-06-3000008753202024-07-3100008753202024-04-012024-06-3000008753202023-04-012023-06-3000008753202023-01-012023-06-3000008753202024-06-3000008753202023-12-310000875320us-gaap:CommonStockMember2023-03-310000875320us-gaap:AdditionalPaidInCapitalMember2023-03-310000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310000875320us-gaap:RetainedEarningsMember2023-03-3100008753202023-03-310000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300000875320us-gaap:RetainedEarningsMember2023-04-012023-06-300000875320us-gaap:CommonStockMember2023-04-012023-06-300000875320us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-300000875320us-gaap:CommonStockMember2023-06-300000875320us-gaap:AdditionalPaidInCapitalMember2023-06-300000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300000875320us-gaap:RetainedEarningsMember2023-06-3000008753202023-06-300000875320us-gaap:CommonStockMember2024-03-310000875320us-gaap:AdditionalPaidInCapitalMember2024-03-310000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-03-310000875320us-gaap:RetainedEarningsMember2024-03-3100008753202024-03-310000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-04-012024-06-300000875320us-gaap:RetainedEarningsMember2024-04-012024-06-300000875320us-gaap:CommonStockMember2024-04-012024-06-300000875320us-gaap:AdditionalPaidInCapitalMember2024-04-012024-06-300000875320us-gaap:CommonStockMember2024-06-300000875320us-gaap:AdditionalPaidInCapitalMember2024-06-300000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-06-300000875320us-gaap:RetainedEarningsMember2024-06-300000875320us-gaap:CommonStockMember2022-12-310000875320us-gaap:AdditionalPaidInCapitalMember2022-12-310000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310000875320us-gaap:RetainedEarningsMember2022-12-3100008753202022-12-310000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-06-300000875320us-gaap:RetainedEarningsMember2023-01-012023-06-300000875320us-gaap:CommonStockMember2023-01-012023-06-300000875320us-gaap:AdditionalPaidInCapitalMember2023-01-012023-06-300000875320us-gaap:CommonStockMember2023-12-310000875320us-gaap:AdditionalPaidInCapitalMember2023-12-310000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310000875320us-gaap:RetainedEarningsMember2023-12-310000875320us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-06-300000875320us-gaap:RetainedEarningsMember2024-01-012024-06-300000875320us-gaap:CommonStockMember2024-01-012024-06-300000875320us-gaap:AdditionalPaidInCapitalMember2024-01-012024-06-300000875320vrtx:TRIKAFTAKAFTRIOMember2024-04-012024-06-300000875320vrtx:TRIKAFTAKAFTRIOMember2023-04-012023-06-300000875320vrtx:TRIKAFTAKAFTRIOMember2024-01-012024-06-300000875320vrtx:TRIKAFTAKAFTRIOMember2023-01-012023-06-300000875320vrtx:OtherCysticFibrosisProductsMember2024-04-012024-06-300000875320vrtx:OtherCysticFibrosisProductsMember2023-04-012023-06-300000875320vrtx:OtherCysticFibrosisProductsMember2024-01-012024-06-300000875320vrtx:OtherCysticFibrosisProductsMember2023-01-012023-06-300000875320country:US2024-04-012024-06-300000875320country:US2023-04-012023-06-300000875320country:US2024-01-012024-06-300000875320country:US2023-01-012023-06-300000875320srt:EuropeMember2024-04-012024-06-300000875320srt:EuropeMember2023-04-012023-06-300000875320srt:EuropeMember2024-01-012024-06-300000875320srt:EuropeMember2023-01-012023-06-300000875320vrtx:OtherNonU.S.Member2024-04-012024-06-300000875320vrtx:OtherNonU.S.Member2023-04-012023-06-300000875320vrtx:OtherNonU.S.Member2024-01-012024-06-300000875320vrtx:OtherNonU.S.Member2023-01-012023-06-300000875320us-gaap:NonUsMember2024-04-012024-06-300000875320us-gaap:NonUsMember2023-04-012023-06-300000875320us-gaap:NonUsMember2024-01-012024-06-300000875320us-gaap:NonUsMember2023-01-012023-06-300000875320vrtx:AlphineImmuneSciencesIncMember2024-05-202024-05-200000875320vrtx:AlphineImmuneSciencesIncMember2024-01-012024-06-300000875320vrtx:AlphineImmuneSciencesIncMember2024-04-012024-06-300000875320vrtx:UnvestedEquityAwardsMembervrtx:AlphineImmuneSciencesIncMember2024-05-202024-05-200000875320vrtx:UnvestedEquityAwardsMembervrtx:AlphineImmuneSciencesIncMember2024-01-012024-06-300000875320vrtx:UnvestedEquityAwardsMembervrtx:AlphineImmuneSciencesIncMember2024-04-012024-06-300000875320us-gaap:CommonStockMembervrtx:AlphineImmuneSciencesIncMember2024-05-012024-05-310000875320vrtx:VestedAndUnvestedEquityAwardsMembervrtx:AlphineImmuneSciencesIncMember2024-05-012024-05-310000875320vrtx:AlphineImmuneSciencesIncMember2024-05-012024-05-310000875320vrtx:CRISPRTherapeuticsAGMember2019-01-012019-12-310000875320vrtx:CRISPRJDCAMember2024-01-012024-01-310000875320vrtx:CRISPRMember2024-06-300000875320vrtx:CRISPRJDCAMember2024-04-012024-06-300000875320vrtx:CRISPRJDCAMember2024-01-012024-06-300000875320vrtx:CRISPRJDCAMember2021-12-310000875320vrtx:CRISPRJDCAMembervrtx:CRISPRMember2021-12-310000875320vrtx:CRISPRJDCAMember2023-04-012023-06-300000875320vrtx:CRISPRJDCAMember2023-01-012023-06-300000875320vrtx:CRISPRT1DMember2023-03-012023-03-310000875320vrtx:CRISPRT1DMember2023-04-012023-06-300000875320vrtx:CRISPRT1DMember2023-01-012023-03-310000875320vrtx:EntradaTherapeuticsMember2023-02-012023-02-280000875320vrtx:EntradaTherapeuticsMember2024-01-012024-03-310000875320us-gaap:RestrictedStockUnitsRSUMember2024-04-012024-06-300000875320us-gaap:RestrictedStockUnitsRSUMember2023-04-012023-06-300000875320us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-06-300000875320us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-06-300000875320us-gaap:EmployeeStockOptionMember2024-04-012024-06-300000875320us-gaap:EmployeeStockOptionMember2023-04-012023-06-300000875320us-gaap:EmployeeStockOptionMember2024-01-012024-06-300000875320us-gaap:EmployeeStockOptionMember2023-01-012023-06-300000875320us-gaap:RestrictedStockUnitsRSUMember2024-04-012024-06-300000875320us-gaap:RestrictedStockUnitsRSUMember2023-04-012023-06-300000875320us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-06-300000875320us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-06-300000875320us-gaap:EmployeeStockOptionMember2024-04-012024-06-300000875320us-gaap:EmployeeStockOptionMember2023-04-012023-06-300000875320us-gaap:EmployeeStockOptionMember2024-01-012024-06-300000875320us-gaap:EmployeeStockOptionMember2023-01-012023-06-300000875320us-gaap:FairValueMeasurementsRecurringMember2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMember2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:EquitySecuritiesMember2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:EquitySecuritiesMemberus-gaap:FairValueInputsLevel1Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EquitySecuritiesMember2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:EquitySecuritiesMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:EquitySecuritiesMember2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:EquitySecuritiesMemberus-gaap:FairValueInputsLevel1Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:EquitySecuritiesMember2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:EquitySecuritiesMemberus-gaap:FairValueInputsLevel3Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel1Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel2Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMember2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel1Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel2Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueInputsLevel3Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2023-12-310000875320us-gaap:AssetBackedSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-06-300000875320us-gaap:AssetBackedSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-06-300000875320us-gaap:AssetBackedSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-06-300000875320us-gaap:AssetBackedSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:AssetBackedSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310000875320us-gaap:AssetBackedSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2023-12-310000875320us-gaap:AssetBackedSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310000875320us-gaap:AssetBackedSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMember2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMemberus-gaap:FairValueInputsLevel1Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CertificatesOfDepositMember2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMember2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMemberus-gaap:FairValueInputsLevel1Member2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CertificatesOfDepositMember2023-12-310000875320us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CertificatesOfDepositMemberus-gaap:FairValueInputsLevel3Member2023-12-310000875320us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2024-06-300000875320us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-06-300000875320us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-06-300000875320us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310000875320us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2023-12-310000875320us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310000875320us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-12-310000875320us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2024-06-300000875320us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2024-06-300000875320us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2024-06-300000875320us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310000875320us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2023-12-310000875320us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2023-12-310000875320us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2023-12-310000875320us-gaap:EquitySecuritiesMember2024-06-300000875320us-gaap:FairValueInputsLevel3Member2019-12-310000875320us-gaap:MeasurementInputDiscountRateMembersrt:MinimumMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320srt:MaximumMemberus-gaap:MeasurementInputDiscountRateMemberus-gaap:FairValueInputsLevel3Member2024-06-300000875320us-gaap:USTreasurySecuritiesMember2024-06-300000875320us-gaap:USTreasurySecuritiesMember2023-12-310000875320us-gaap:USGovernmentAgenciesDebtSecuritiesMember2024-06-300000875320us-gaap:USGovernmentAgenciesDebtSecuritiesMember2023-12-310000875320us-gaap:AssetBackedSecuritiesMember2024-06-300000875320us-gaap:AssetBackedSecuritiesMember2023-12-310000875320us-gaap:CertificatesOfDepositMember2024-06-300000875320us-gaap:CertificatesOfDepositMember2023-12-310000875320us-gaap:CorporateDebtSecuritiesMember2024-06-300000875320us-gaap:CorporateDebtSecuritiesMember2023-12-310000875320us-gaap:CommercialPaperNotIncludedWithCashAndCashEquivalentsMember2024-06-300000875320us-gaap:CommercialPaperNotIncludedWithCashAndCashEquivalentsMember2023-12-310000875320vrtx:MarketableSecuritiesAndOtherNoncurrentAssetsMember2024-06-300000875320vrtx:MarketableSecuritiesAndOtherNoncurrentAssetsMember2023-12-3100008753202023-01-012023-12-310000875320us-gaap:CashAndCashEquivalentsMember2024-06-300000875320us-gaap:CashAndCashEquivalentsMember2023-12-310000875320vrtx:MarketableSecuritiesMember2024-06-300000875320vrtx:MarketableSecuritiesMember2023-12-310000875320vrtx:MarketableSecuritiesNoncurrentMember2024-06-300000875320vrtx:MarketableSecuritiesNoncurrentMember2023-12-310000875320vrtx:PubliclyTradedCompaniesSaleMember2023-01-012023-06-300000875320vrtx:PubliclyTradedCompaniesSaleMember2023-06-300000875320vrtx:PubliclyTradedCompaniesSaleMember2024-01-012024-06-300000875320us-gaap:OtherAssetsMember2024-06-300000875320us-gaap:AccumulatedTranslationAdjustmentMember2023-12-310000875320us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-12-310000875320us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2023-12-310000875320us-gaap:AccumulatedTranslationAdjustmentMember2024-01-012024-06-300000875320us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-01-012024-06-300000875320us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2024-01-012024-06-300000875320us-gaap:AccumulatedTranslationAdjustmentMember2024-06-300000875320us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-06-300000875320us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2024-06-300000875320us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310000875320us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-12-310000875320us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2022-12-310000875320us-gaap:AccumulatedTranslationAdjustmentMember2023-01-012023-06-300000875320us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-01-012023-06-300000875320us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2023-01-012023-06-300000875320us-gaap:AccumulatedTranslationAdjustmentMember2023-06-300000875320us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-06-300000875320us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2023-06-300000875320us-gaap:CashFlowHedgingMembersrt:MinimumMemberus-gaap:ForeignExchangeForwardMember2024-01-012024-06-300000875320us-gaap:CashFlowHedgingMembersrt:MaximumMemberus-gaap:ForeignExchangeForwardMember2024-01-012024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMembercurrency:EURus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMembercurrency:EURus-gaap:ForeignExchangeForwardMember2023-12-310000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMembercurrency:GBPus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMembercurrency:GBPus-gaap:ForeignExchangeForwardMember2023-12-310000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeForwardMembercurrency:CAD2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeForwardMembercurrency:CAD2023-12-310000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMembercurrency:AUDus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMembercurrency:AUDus-gaap:ForeignExchangeForwardMember2023-12-310000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMembercurrency:CHFus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMembercurrency:CHFus-gaap:ForeignExchangeForwardMember2023-12-310000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeForwardMember2023-12-310000875320us-gaap:NondesignatedMemberus-gaap:ForeignExchangeForwardMember2024-01-012024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:NondesignatedMemberus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:AccumulatedTranslationAdjustmentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2024-04-012024-06-300000875320us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:AccumulatedTranslationAdjustmentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300000875320us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:AccumulatedTranslationAdjustmentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2024-01-012024-06-300000875320us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:AccumulatedTranslationAdjustmentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2023-01-012023-06-300000875320us-gaap:NondesignatedMemberus-gaap:AccumulatedTranslationAdjustmentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2024-04-012024-06-300000875320us-gaap:NondesignatedMemberus-gaap:AccumulatedTranslationAdjustmentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300000875320us-gaap:NondesignatedMemberus-gaap:AccumulatedTranslationAdjustmentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2024-01-012024-06-300000875320us-gaap:NondesignatedMemberus-gaap:AccumulatedTranslationAdjustmentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2023-01-012023-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentLiabilitiesMemberus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentAssetsMemberus-gaap:ForeignExchangeForwardMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeForwardMemberus-gaap:OtherNoncurrentLiabilitiesMember2024-06-300000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeForwardMember2023-12-310000875320us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentLiabilitiesMemberus-gaap:ForeignExchangeForwardMember2023-12-310000875320us-gaap:RestrictedStockMember2024-04-012024-06-300000875320us-gaap:RestrictedStockMember2023-04-012023-06-300000875320us-gaap:RestrictedStockMember2024-01-012024-06-300000875320us-gaap:RestrictedStockMember2023-01-012023-06-300000875320us-gaap:EmployeeStockMember2024-04-012024-06-300000875320us-gaap:EmployeeStockMember2023-04-012023-06-300000875320us-gaap:EmployeeStockMember2024-01-012024-06-300000875320us-gaap:EmployeeStockMember2023-01-012023-06-300000875320us-gaap:CostOfSalesMember2024-04-012024-06-300000875320us-gaap:CostOfSalesMember2023-04-012023-06-300000875320us-gaap:CostOfSalesMember2024-01-012024-06-300000875320us-gaap:CostOfSalesMember2023-01-012023-06-300000875320us-gaap:ResearchAndDevelopmentExpenseMember2024-04-012024-06-300000875320us-gaap:ResearchAndDevelopmentExpenseMember2023-04-012023-06-300000875320us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-06-300000875320us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-06-300000875320us-gaap:SellingGeneralAndAdministrativeExpensesMember2024-04-012024-06-300000875320us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-04-012023-06-300000875320us-gaap:SellingGeneralAndAdministrativeExpensesMember2024-01-012024-06-300000875320us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-01-012023-06-3000008753202023-02-280000875320us-gaap:RevolvingCreditFacilityMember2022-07-310000875320us-gaap:LetterOfCreditMember2022-07-310000875320us-gaap:RevolvingCreditFacilityMembersrt:MinimumMemberus-gaap:BaseRateMember2022-07-012022-07-310000875320srt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberus-gaap:BaseRateMember2022-07-012022-07-310000875320us-gaap:RevolvingCreditFacilityMemberus-gaap:SecuredOvernightFinancingRateSofrMembersrt:MinimumMember2022-07-012022-07-310000875320srt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberus-gaap:SecuredOvernightFinancingRateSofrMember2022-07-012022-07-310000875320us-gaap:RevolvingCreditFacilityMember2022-07-012022-07-310000875320vrtx:JeffreyLeidenMember2024-04-012024-06-300000875320vrtx:JeffreyLeidenMember2024-06-300000875320vrtx:SangeetaBhatiaMember2024-04-012024-06-300000875320vrtx:SangeetaBhatiaMember2024-06-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
________________________________________________________
FORM 10-Q
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE QUARTERLY PERIOD ENDED JUNE 30, 2024
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE TRANSITION PERIOD FROM TO
Commission file number 000-19319
____________________________________________
Vertex Pharmaceuticals Incorporated
(Exact name of registrant as specified in its charter)
Massachusetts
(State or other jurisdiction of incorporation or organization)
50 Northern Avenue, Boston, Massachusetts
(Address of principal executive offices)
04-3039129
(I.R.S. Employer Identification No.)
02210
(Zip Code)
Registrant’s telephone number, including area code (617) 341-6100
____________________________________________
| | | | | | | | | | | | | | |
Securities registered pursuant to Section 12(b) of the Act: |
Title of each class | | Trading Symbol | | Name of each exchange on which registered |
Common Stock, $0.01 Par Value Per Share | | VRTX | | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Smaller reporting company ☐ Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date.
| | | | | | | | |
Common Stock, par value $0.01 per share | 258,102,203 | Outstanding at July 31, 2024 |
VERTEX PHARMACEUTICALS INCORPORATED
FORM 10-Q
FOR THE QUARTER ENDED JUNE 30, 2024
TABLE OF CONTENTS
“Vertex,” “we,” “us,” and “our” as used in this Quarterly Report on Form 10-Q refer to Vertex Pharmaceuticals Incorporated, a Massachusetts corporation, and its subsidiaries.
“Vertex®,” “KALYDECO®,” “ORKAMBI®,” “SYMDEKO®,” “SYMKEVI®,” “TRIKAFTA®,” “KAFTRIO®,” and CASGEVY™” are registered trademarks of Vertex. Other brands, names and trademarks contained in this Quarterly Report on Form 10-Q are the property of their respective owners.
We use the brand name for our products when we refer to the product that has been approved and with respect to the indications on the approved label. Otherwise, including in discussions of our development programs, we refer to our compounds and therapies by their scientific (or generic) name or VX developmental designation.
Part I. Financial Information
Item 1. Financial Statements
VERTEX PHARMACEUTICALS INCORPORATED
Condensed Consolidated Statements of Income
(in millions, except per share amounts)(unaudited)
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
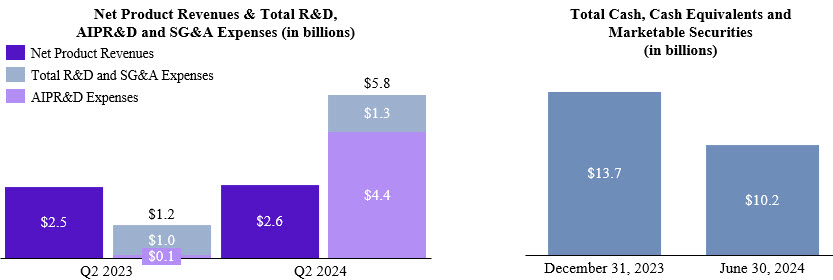
| Product revenues, net | $ | 2,645.6 | | | $ | 2,493.2 | | | $ | 5,336.2 | | | $ | 4,868.0 | |
| Costs and expenses: | | | | | | | |
| Cost of sales | 371.9 | | | 308.6 | | | 714.5 | | | 575.5 | |
| Research and development expenses | 966.6 | | | 785.7 | | | 1,755.7 | | | 1,528.3 | |
| Acquired in-process research and development expenses | 4,449.1 | | | 110.5 | | | 4,525.9 | | | 457.6 | |
| Selling, general and administrative expenses | 372.2 | | | 262.6 | | | 714.9 | | | 503.7 | |
| Change in fair value of contingent consideration | 0.5 | | | (0.6) | | | 0.4 | | | (2.5) | |
| Total costs and expenses | 6,160.3 | | | 1,466.8 | | | 7,711.4 | | | 3,062.6 | |
| (Loss) income from operations | (3,514.7) | | | 1,026.4 | | | (2,375.2) | | | 1,805.4 | |
| Interest income | 156.5 | | | 144.7 | | | 337.7 | | | 267.3 | |
| Interest expense | (9.9) | | | (11.2) | | | (20.3) | | | (22.6) | |
| Other (expense) income, net | (23.1) | | | 1.6 | | | (54.3) | | | 2.9 | |
| (Loss) income before provision for income taxes | (3,391.2) | | | 1,161.5 | | | (2,112.1) | | | 2,053.0 | |
| Provision for income taxes | 202.4 | | | 245.8 | | | 381.9 | | | 437.5 | |
| Net (loss) income | $ | (3,593.6) | | | $ | 915.7 | | | $ | (2,494.0) | | | $ | 1,615.5 | |
| | | | | | | |
| Net (loss) income per common share: | | | | | | | |
| Basic | $ | (13.92) | | | $ | 3.55 | | | $ | (9.66) | | | $ | 6.27 | |
| Diluted | $ | (13.92) | | | $ | 3.52 | | | $ | (9.66) | | | $ | 6.21 | |
| Shares used in per share calculations: | | | | | | | |
| Basic | 258.1 | | | 257.7 | | | 258.1 | | | 257.6 | |
| Diluted | 258.1 | | | 260.4 | | | 258.1 | | | 260.3 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
VERTEX PHARMACEUTICALS INCORPORATED
Condensed Consolidated Statements of Comprehensive Income
(in millions)(unaudited)
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| Net (loss) income | $ | (3,593.6) | | | $ | 915.7 | | | $ | (2,494.0) | | | $ | 1,615.5 | |
Other comprehensive income (loss): | | | | | | | |
Unrealized holding losses on available-for-sale debt securities, net of tax of $1.5, $4.3, $6.9 and $3.5, respectively | (5.4) | | | (15.5) | | | (25.1) | | | (12.6) | |
Unrealized gains (losses) on foreign currency forward contracts, net of tax of $(3.2), $4.2, $(15.5) and $11.6, respectively | 11.8 | | | (15.3) | | | 56.3 | | | (42.1) | |
| Foreign currency translation adjustment | (1.2) | | | 4.1 | | | 5.6 | | | 14.1 | |
Total other comprehensive income (loss) | 5.2 | | | (26.7) | | | 36.8 | | | (40.6) | |
Comprehensive (loss) income | $ | (3,588.4) | | | $ | 889.0 | | | $ | (2,457.2) | | | $ | 1,574.9 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
VERTEX PHARMACEUTICALS INCORPORATED
Condensed Consolidated Balance Sheets
(in millions, except share data)(unaudited)
| | | | | | | | | | | |
| June 30, 2024 | | December 31, 2023 |
Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 4,580.1 | | | $ | 10,369.1 | |
| Marketable securities | 1,215.4 | | | 849.2 | |
| Accounts receivable, net | 1,656.1 | | | 1,563.4 | |
| Inventories | 914.6 | | | 738.8 | |
Prepaid expenses and other current assets | 575.4 | | | 623.7 | |
Total current assets | 8,941.6 | | | 14,144.2 | |
| Property and equipment, net | 1,200.9 | | | 1,159.3 | |
| Goodwill | 1,088.0 | | | 1,088.0 | |
Other intangible assets, net | 837.5 | | | 839.9 | |
Deferred tax assets | 2,185.6 | | | 1,812.1 | |
Operating lease assets | 569.8 | | | 293.6 | |
| Long-term marketable securities | 4,393.1 | | | 2,497.8 | |
Other assets | 915.6 | | | 895.3 | |
Total assets | $ | 20,132.1 | | | $ | 22,730.2 | |
Liabilities and Shareholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 327.9 | | | $ | 364.9 | |
| Accrued expenses | 2,940.0 | | | 2,655.3 | |
Other current liabilities | 279.3 | | | 527.2 | |
Total current liabilities | 3,547.2 | | | 3,547.4 | |
| Long-term finance lease liabilities | 346.6 | | | 376.1 | |
| Long-term operating lease liabilities | 586.8 | | | 348.6 | |
Other long-term liabilities | 876.8 | | | 877.7 | |
Total liabilities | 5,357.4 | | | 5,149.8 | |
| Commitments and contingencies | — | | | — | |
| Shareholders’ equity: | | | |
Preferred stock, $0.01 par value; 1,000,000 shares authorized; none issued and outstanding | — | | | — | |
Common stock, $0.01 par value; 500,000,000 shares authorized, 258,015,301 and 257,695,221 shares issued and outstanding, respectively | 2.6 | | | 2.6 | |
| Additional paid-in capital | 7,101.2 | | | 7,449.7 | |
| Accumulated other comprehensive income (loss) | 22.5 | | | (14.3) | |
Retained earnings | 7,648.4 | | | 10,142.4 | |
Total shareholders’ equity | 14,774.7 | | | 17,580.4 | |
Total liabilities and shareholders’ equity | $ | 20,132.1 | | | $ | 22,730.2 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
VERTEX PHARMACEUTICALS INCORPORATED
Condensed Consolidated Statements of Shareholders’ Equity
(in millions)(unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended |
| Common Stock | | Additional Paid-in Capital | | Accumulated Other Comprehensive Income (Loss) | | Retained Earnings | | Total Shareholders’ Equity |
| Shares | | Amount | | | | |
| Balance at March 31, 2023 | 257.5 | | | $ | 2.6 | | | $ | 7,220.2 | | | $ | (13.1) | | | $ | 7,222.6 | | | $ | 14,432.3 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | (26.7) | | | — | | | (26.7) | |
| Net income | — | | | — | | | — | | | — | | | 915.7 | | | 915.7 | |
| Repurchases of common stock | (0.0) | | | (0.0) | | | (25.5) | | | — | | | — | | | (25.5) | |
| Common stock withheld for employee tax obligations | (0.0) | | | (0.0) | | | (3.1) | | | — | | | — | | | (3.1) | |
| Issuance of common stock under benefit plans | 0.3 | | | 0.0 | | | 57.6 | | | — | | | — | | | 57.6 | |
Stock-based compensation expense | — | | | — | | | 119.9 | | | — | | | — | | | 119.9 | |
| Balance at June 30, 2023 | 257.8 | | | $ | 2.6 | | | $ | 7,369.1 | | | $ | (39.8) | | | $ | 8,138.3 | | | $ | 15,470.2 | |
| | | | | | | | | | | |
| Balance at March 31, 2024 | 258.3 | | | $ | 2.6 | | | $ | 7,284.7 | | | $ | 17.3 | | | $ | 11,242.0 | | | $ | 18,546.6 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 5.2 | | | — | | | 5.2 | |
| Net loss | — | | | — | | | — | | | — | | | (3,593.6) | | | (3,593.6) | |
| Repurchases of common stock | (0.8) | | | 0.0 | | | (315.8) | | | — | | | — | | | (315.8) | |
| Common stock withheld for employee tax obligations | (0.1) | | | 0.0 | | | (80.5) | | | — | | | — | | | (80.5) | |
| Issuance of common stock under benefit plans | 0.6 | | | 0.0 | | | 55.8 | | | — | | | — | | | 55.8 | |
Stock-based compensation expense | — | | | — | | | 157.0 | | | — | | | — | | | 157.0 | |
| Balance at June 30, 2024 | 258.0 | | | $ | 2.6 | | | $ | 7,101.2 | | | $ | 22.5 | | | $ | 7,648.4 | | | $ | 14,774.7 | |
| | | | | | | | | | | |
| Six Months Ended |
| Common Stock | | Additional Paid-in Capital | | Accumulated Other Comprehensive Income (Loss) | | Retained Earnings | | Total Shareholders’ Equity |
| Shares | | Amount | | | | |
| Balance at December 31, 2022 | 257.0 | | | $ | 2.6 | | | $ | 7,386.5 | | | $ | 0.8 | | | $ | 6,522.8 | | | $ | 13,912.7 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | (40.6) | | | — | | | (40.6) | |
Net income | — | | | — | | | — | | | — | | | 1,615.5 | | | 1,615.5 | |
| Repurchases of common stock | (0.5) | | | (0.0) | | | (161.1) | | | — | | | — | | | (161.1) | |
| Common stock withheld for employee tax obligations | (0.6) | | | (0.0) | | | (169.7) | | | — | | | — | | | (169.7) | |
| Issuance of common stock under benefit plans | 1.9 | | | 0.0 | | | 70.7 | | | — | | | — | | | 70.7 | |
Stock-based compensation expense | — | | | — | | | 242.7 | | | — | | | — | | | 242.7 | |
| Balance at June 30, 2023 | 257.8 | | | $ | 2.6 | | | $ | 7,369.1 | | | $ | (39.8) | | | $ | 8,138.3 | | | $ | 15,470.2 | |
| | | | | | | | | | | |
| Balance at December 31, 2023 | 257.7 | | | $ | 2.6 | | | $ | 7,449.7 | | | $ | (14.3) | | | $ | 10,142.4 | | | $ | 17,580.4 | |
| Other comprehensive income, net of tax | — | | | — | | | — | | | 36.8 | | | — | | | 36.8 | |
Net loss | — | | | — | | | — | | | — | | | (2,494.0) | | | (2,494.0) | |
| Repurchases of common stock | (1.1) | | | (0.0) | | | (456.2) | | | — | | | — | | | (456.2) | |
| Common stock withheld for employee tax obligations | (0.7) | | | (0.0) | | | (314.0) | | | — | | | — | | | (314.0) | |
| Issuance of common stock under benefit plans | 2.1 | | | 0.0 | | | 71.7 | | | — | | | — | | | 71.7 | |
Stock-based compensation expense | — | | | — | | | 350.0 | | | — | | | — | | | 350.0 | |
| Balance at June 30, 2024 | 258.0 | | | $ | 2.6 | | | $ | 7,101.2 | | | $ | 22.5 | | | $ | 7,648.4 | | | $ | 14,774.7 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
VERTEX PHARMACEUTICALS INCORPORATED
Condensed Consolidated Statements of Cash Flows
(in millions)(unaudited)
| | | | | | | | | | | |
| Six Months Ended June 30, |
| 2024 | | 2023 |
| Cash flows from operating activities: | | | |
| Net (loss) income | $ | (2,494.0) | | | $ | 1,615.5 | |
| Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: | | | |
| Stock-based compensation expense | 346.1 | | | 241.7 | |
| Depreciation and amortization expense | 107.5 | | | 80.2 | |
| Deferred income taxes | (277.1) | | | (290.0) | |
| Losses (gains) on equity securities | 39.7 | | | (6.0) | |
| Increase (decrease) in fair value of contingent consideration | 0.4 | | | (2.5) | |
| Other non-cash items, net | (57.5) | | | 11.1 | |
| Changes in operating assets and liabilities: | | | |
| Accounts receivable, net | (116.5) | | | (93.4) | |
| Inventories | (187.3) | | | (155.4) | |
| Prepaid expenses and other assets | (56.7) | | | 26.6 | |
| Accounts payable | (25.0) | | | 71.3 | |
| Accrued expenses | 362.4 | | | 417.4 | |
| Other liabilities | (89.0) | | | 117.8 | |
| Net cash (used in) provided by operating activities | (2,447.0) | | | 2,034.3 | |
| Cash flows from investing activities: | | | |
| Purchases of available-for-sale debt securities | (3,895.1) | | | (2,390.8) | |
| Sales and maturities of available-for-sale debt securities | 1,893.1 | | | 289.8 | |
| Acquisition of available-for-sale debt securities from Alpine Immune Sciences, Inc. | (258.0) | | | — | |
| Purchases of property and equipment | (137.4) | | | (101.7) | |
| Net payments related to finite-lived intangible assets | (187.7) | | | — | |
| Sale of equity securities | — | | | 95.1 | |
| Other investing activities | (15.0) | | | (29.9) | |
| Net cash used in investing activities | (2,600.1) | | | (2,137.5) | |
| Cash flows from financing activities: | | | |
| Issuances of common stock under benefit plans | 71.9 | | | 72.8 | |
| Repurchases of common stock | (451.5) | | | (161.1) | |
| Payments in connection with common stock withheld for employee tax obligations | (314.0) | | | (169.7) | |
| Payments on finance leases | (26.9) | | | (21.6) | |
| Other financing activities | 4.4 | | | 2.2 | |
| Net cash used in financing activities | (716.1) | | | (277.4) | |
| Effect of changes in exchange rates on cash | (18.1) | | | 22.0 | |
| Net decrease in cash, cash equivalents and restricted cash | (5,781.3) | | | (358.6) | |
| Cash, cash equivalents and restricted cash—beginning of period | 10,372.3 | | | 10,512.0 | |
| Cash, cash equivalents and restricted cash—end of period | $ | 4,591.0 | | | $ | 10,153.4 | |
| | | |
| Supplemental disclosure of cash flow information: | | | |
| Cash paid for income taxes | $ | 531.8 | | | $ | 618.7 | |
| Cash paid for interest | $ | 19.7 | | | $ | 22.0 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
A.Basis of Presentation and Accounting Policies
Basis of Presentation
The accompanying condensed consolidated financial statements are unaudited and have been prepared by Vertex Pharmaceuticals Incorporated (“Vertex,” “we,” “us” or “our”) in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
The condensed consolidated financial statements reflect the operations of Vertex and our wholly-owned subsidiaries. All material intercompany balances and transactions have been eliminated. We operate in one segment, pharmaceuticals.
Certain information and footnote disclosures normally included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023 (the “2023 Annual Report on Form 10-K”) have been condensed or omitted. These interim financial statements, in the opinion of management, reflect all normal recurring adjustments necessary for a fair presentation of the financial position and results of income for the interim periods ended June 30, 2024 and 2023.
The results of operations for the interim periods are not necessarily indicative of the results of operations to be expected for the full fiscal year. These interim financial statements should be read in conjunction with the audited financial statements for the year ended December 31, 2023, which are contained in our 2023 Annual Report on Form 10-K.
Use of Estimates
The preparation of condensed consolidated financial statements in accordance with U.S. GAAP requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of our condensed consolidated financial statements, and the amounts of revenues and expenses during the reported periods. We base our estimates on historical experience and various other assumptions, including in certain circumstances future projections that we believe to be reasonable under the circumstances. Actual results could differ from those estimates. Changes in estimates are reflected in reported results in the period in which they become known.
Recently Adopted Accounting Standards
As noted in Note A, “Nature of Business and Accounting Policies,” in our 2023 Annual Report on Form 10-K, we did not adopt any accounting standards that had a significant impact on our consolidated financial statements in the three years ended December 31, 2023.
Recently Issued Accounting Standards
Segment Reporting
In 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures (“ASU 2023-07”), which requires public entities to disclose significant segment expenses and other segment items. ASU 2023-07 also requires public entities to provide in interim periods all disclosures about a reportable segment’s profit or loss and assets that are currently required annually. ASU 2023-07 becomes effective for the annual period starting on January 1, 2024, and for the interim periods starting on January 1, 2025. We are in the process of analyzing the impact that the adoption of ASU 2023-07 will have on our segment disclosures.
Income Tax Disclosures
In 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures (“ASU 2023-09”), which requires public entities to disclose in their rate reconciliation table additional categories of information about federal, state and foreign income taxes and to provide more details about the reconciling items in some categories if items meet a quantitative threshold. ASU 2023-09 becomes effective for the annual period starting on January 1, 2025. We are in the process of analyzing the impact that the adoption of ASU 2023-09 will have on our income tax disclosures.
Summary of Significant Accounting Policies
Our significant accounting policies are described in Note A, “Nature of Business and Accounting Policies,” in our 2023 Annual Report on Form 10-K.
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
B.Revenue Recognition
Disaggregation of Revenue
Revenues by Product
“Product revenues, net” consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| (in millions) |
| TRIKAFTA/KAFTRIO | $ | 2,449.2 | | | $ | 2,240.4 | | | $ | 4,932.8 | | | $ | 4,337.1 | |
| Other CF products | 196.4 | | | 252.8 | | | 403.4 | | | 530.9 | |
| Total product revenues, net | $ | 2,645.6 | | | $ | 2,493.2 | | | $ | 5,336.2 | | | $ | 4,868.0 | |
Product Revenues by Geographic Location
“Product revenues, net” by geographic region, based on the location of the customer, consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| (in millions) |
| United States | $ | 1,614.3 | | | $ | 1,507.8 | | | $ | 3,134.2 | | | $ | 2,911.6 | |
| Outside of the United States | | | | | | | |
| Europe | 806.8 | | | 800.0 | | | 1,774.2 | | | 1,607.2 | |
Other | 224.5 | | | 185.4 | | | 427.8 | | | 349.2 | |
| Total product revenues outside of the United States | 1,031.3 | | | 985.4 | | | 2,202.0 | | | 1,956.4 | |
Total product revenues, net | $ | 2,645.6 | | | $ | 2,493.2 | | | $ | 5,336.2 | | | $ | 4,868.0 | |
Contract Liabilities
We had contract liabilities of $128.3 million and $170.3 million as of June 30, 2024 and December 31, 2023, respectively, related to annual contracts with government-owned and supported customers in international markets that limit the amount of annual reimbursement we can receive for our cystic fibrosis (“CF”) products. Upon exceeding the annual reimbursement amount provided by the customer’s contract with us, our CF products are provided free of charge, which is a material right. These contracts include upfront payments and fees. If we estimate that we will exceed the annual reimbursement amount under a contract, we defer a portion of the consideration received for shipments made up to the annual reimbursement limit as a portion of “Other current liabilities.” Once the reimbursement limit has been reached, we recognize the deferred amount as revenue when we ship the free products. Our CF product revenue contracts include performance obligations that are one year or less.
Our contract liabilities at the end of each fiscal year relate to contracts with CF annual reimbursement limits in international markets in which the annual period associated with the contract is not the same as our fiscal year. In these markets, we recognize revenues related to performance obligations satisfied in previous years; however, these revenues do not relate to any performance obligations that were satisfied more than 12 months prior to the beginning of the current year.
C.Collaboration, License and Other Arrangements
We have entered into numerous business development agreements with third parties to collaborate on research, development and commercialization programs, license technologies, or acquire assets. Our “Acquired in-process research and development expenses” (“AIPR&D”) included $4.4 billion and $4.5 billion in the three and six months ended June 30, 2024, respectively, primarily due to our acquisition of Alpine Immune Sciences, Inc. (“Alpine”) as discussed below. Our AIPR&D
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
included $110.5 million and $457.6 million in the three and six months ended June 30, 2023, respectively, related to upfront, contingent milestone, or other payments pursuant to our business development transactions.
Our collaboration, licensing and asset acquisition agreements that had a significant impact on our financial statements for the three and six months ended June 30, 2024 and 2023 or were new or materially revised during the three and six months ended June 30, 2024, are described below. Additional agreements were described in Note B, “Collaboration, License and Other Arrangements,” of our 2023 Annual Report on Form 10-K.
Asset Acquisition
Alpine Immune Sciences, Inc. - povetacicept
On May 20, 2024, we acquired all of the issued and outstanding shares of common stock of Alpine, a publicly traded biotechnology company focused on discovering and developing innovative, protein-based immunotherapies for approximately $5.0 billion in cash. We funded the Alpine acquisition with our cash and cash equivalents.
Alpine’s lead molecule, povetacicept, is a highly potent and effective dual antagonist of B cell activating factor (“BAFF”) and a proliferation inducing ligand (“APRIL”). Through Phase 2 development, povetacicept has shown potential best-in-class efficacy in IgA nephropathy (“IgAN”), a serious, progressive, autoimmune disease of the kidney that can lead to end-stage-renal disease. Due to its mechanism of action as a dual BAFF/APRIL antagonist, povetacicept also holds the potential to benefit patients with other serious autoimmune diseases of the kidney, such as membranous nephropathy and lupus nephritis. We accounted for the Alpine transaction as an asset acquisition because povetacicept represents substantially all of the fair value of the gross assets that we acquired. As a result, $4.4 billion of fair value attributed to povetacicept was expensed to AIPR&D in the three and six months ended June 30, 2024.
We paid total cash of $5.0 billion at the acquisition date, which included $4.8 billion to acquire Alpine and $197.6 million for cash-settled unvested Alpine equity awards. The $197.6 million represents post-acquisition expense, which was recorded as $165.0 million of “Research and development expenses” and $32.6 million of “Selling, general and administrative expense” in the three and six months ended June 30, 2024.
The total cash paid to acquire Alpine, allocation of consideration to the assets acquired and liabilities assumed and AIPR&D was as follows:
| | | | | | | | |
| | (in millions) |
| Cash consideration to acquire Alpine’s outstanding common stock | | $ | 4,536.9 | |
| Cash consideration for Alpine’s vested and unvested equity awards | | 420.6 | |
| Total cash consideration paid to Alpine | | 4,957.5 | |
| Less: Expense related to unvested equity awards | | (197.6) | |
| Transaction costs | | 40.7 | |
| Total consideration allocated | | $ | 4,800.6 | |
| | |
| Cash and cash equivalents | | $ | 31.9 | |
| Current marketable securities | | 209.5 | |
| Long-term marketable securities | | 48.5 | |
| Deferred tax asset | | 105.5 | |
| Total other assets | | 19.5 | |
| Total liabilities | | (37.5) | |
| Total identifiable assets acquired, net | | 377.4 | |
| Acquired in-process research and development expense | | 4,423.2 | |
| Total consideration allocated | | $ | 4,800.6 | |
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
In-license Agreements
CRISPR Therapeutics AG
CRISPR-Cas9 Gene-editing Therapies Agreements
In 2015, we entered into a strategic collaboration, option, and license agreement (the “CRISPR Agreement”) with CRISPR Therapeutics AG and its affiliates (“CRISPR”) to collaborate on the discovery and development of potential new treatments aimed at the underlying genetic causes of human diseases using CRISPR-Cas9 gene-editing technology. We had the exclusive right to license certain targets. In 2019, we elected to exclusively license three targets, including CF, pursuant to the CRISPR Agreement. For each of the three targets that we elected to license, CRISPR has the potential to receive up to an additional $410.0 million in development, regulatory and commercial milestones as well as royalties on resulting net product sales.
In 2017, we entered into a joint development and commercialization agreement with CRISPR (the “CRISPR JDCA”), which we amended and restated in 2021, pursuant to the terms of the CRISPR Agreement. Under the CRISPR JDCA, we and CRISPR were co-developing and preparing to co-commercialize CASGEVY for the treatment of hemoglobinopathies, including treatments for severe sickle cell disease (“SCD”) and transfusion-dependent beta thalassemia.
Pursuant to the CRISPR JDCA, we lead global development, manufacturing and commercialization of CASGEVY, with support from CRISPR. We also conduct all research, development, manufacturing, and commercialization activities relating to other product candidates and products under the CRISPR JDCA throughout the world subject to CRISPR’s reserved right to conduct certain activities.
CASGEVY was approved by the U.S. Food and Drug Administration in December 2023 for the treatment of SCD. In connection with this approval, we made a $200.0 million milestone payment to CRISPR in January 2024, which we accrued to “Other current liabilities” and recorded within “Other intangible assets, net” on our consolidated balance sheet as of December 31, 2023. Subsequent to receiving marketing approval for CASGEVY, we continue to lead the research and development activities under the CRISPR JDCA, subject to CRISPR’s reserved right to conduct certain activities. We are reimbursed by CRISPR for its 40% share of these research and development activities, subject to certain adjustments, and we record this reimbursement from CRISPR as a credit within “Research and development expenses.” We also share with CRISPR 40% of the net commercial profits or losses incurred with respect to CASGEVY, subject to certain adjustments, which is recorded to “Cost of sales.” The net commercial profits or losses equal the sum of the product revenues, cost of sales and selling, general and administrative expenses that we have recognized related to the CRISPR JDCA.
In the three and six months ended June 30, 2024, we recognized net reimbursements from CRISPR pursuant to the CRISPR JDCA as credits to “Cost of sales” of $15.9 million and $31.7 million, respectively, related to CRISPR’s share of the CRISPR JDCA’s net commercial loss, and to “Research and development expenses” of $11.6 million and $23.3 million, respectively, related to CRISPR’s share of the CRISPR JDCA’s research and development activities.
Prior to receiving marketing approvals for CASGEVY in various markets beginning in December 2023, we accounted for the CRISPR JDCA as a cost-sharing arrangement, with costs incurred related to CASGEVY allocated 60% to us and 40% to CRISPR, subject to certain adjustments. In the three and six months ended June 30, 2023, we recognized net reimbursements from CRISPR as credits to “Research and development expenses” of $17.9 million and $35.8 million, respectively, and to “Selling, general and administrative expenses” of $8.3 million and $14.2 million, respectively, related to CRISPR’s share of the CRISPR JDCA’s operating expenses.
CRISPR-Cas9 Gene-editing Hypoimmune Cell Therapies Agreement
In March 2023, we entered into a non-exclusive license agreement (the “CRISPR T1D Agreement”) for the use of CRISPR’s CRISPR-Cas9 gene-editing technology to accelerate the development of our hypoimmune cell therapies for type 1 diabetes (“T1D”). Pursuant to the CRISPR T1D Agreement, we made a $100.0 million upfront payment to CRISPR, and we determined that substantially all the fair value of our upfront payment was attributable to in-process research and development, for which there is no alternative future use, and that no substantive processes were acquired that would constitute a business. In the second quarter of 2023, we achieved a research milestone that resulted in a $70.0 million payment to CRISPR. We recorded the upfront payment and the research milestone to AIPR&D in the first and second quarters of 2023, respectively, resulting in $70.0 million and $170.0 million of AIPR&D in the three and six month ended June 30, 2023, respectively. CRISPR is eligible to receive up to an additional $160.0 million in research, development, regulatory and commercial milestones for any products that may result from the agreement, as well as royalties on resulting net product sales.
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
Entrada Therapeutics, Inc.
In February 2023, we closed a strategic collaboration and license agreement (the “Entrada Agreement”) with Entrada Therapeutics, Inc. (“Entrada”) focused on discovering and developing intracellular therapeutics for myotonic dystrophy type 1 (“DM1”). Upon closing, we made an upfront payment of $225.1 million to Entrada, and purchased $24.9 million of Entrada’s common stock in connection with the Entrada Agreement. We determined that substantially all the fair value of our upfront payment was attributable to in-process research and development, for which there is no alternative future use, and that no substantive processes were acquired that would constitute a business. We recorded the upfront payment to AIPR&D in the first quarter of 2023. We recorded the investment in Entrada’s common stock at fair value on our condensed consolidated balance sheet within “Marketable securities.” In the first quarter of 2024, Entrada earned a $75.0 million milestone, which we recorded to AIPR&D in the three months ended March 31, 2024 and paid in the second quarter of 2024. We had accrued the milestone to “Other current liabilities” as of March 31, 2024. Entrada is eligible to receive up to an additional $335.0 million in development, regulatory and commercial milestones for any products that may result from the Entrada Agreement, as well as royalties on resulting net product sales.
Cystic Fibrosis Foundation
In 2004, we entered into a collaboration agreement with the Cystic Fibrosis Foundation, as successor in interest to the Cystic Fibrosis Foundation Therapeutics, Inc., to support research and development activities. Pursuant to the collaboration agreement, as amended, we have agreed to pay tiered royalties ranging from single digits to sub-teens on covered compounds first synthesized and/or tested during a research term on or before February 28, 2014, including ivacaftor, lumacaftor and tezacaftor and royalties ranging from low-single digits to mid-single digits on potential net sales of certain compounds first synthesized and/or tested between March 1, 2014 and August 31, 2016, including elexacaftor. We do not have any royalty obligations on compounds first synthesized and tested on or after September 1, 2016. For combination products, such as ORKAMBI, SYMDEKO/SYMKEVI and TRIKAFTA/KAFTRIO, sales are allocated equally to each of the active pharmaceutical ingredients in the combination product. We record expenses related to these royalty obligations to “Cost of sales.”
D.Earnings Per Share
The following table sets forth the computation of basic and diluted net (loss) income per common share for the periods ended:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| (in millions, except per share amounts) |
| Net (loss) income | $ | (3,593.6) | | | $ | 915.7 | | | $ | (2,494.0) | | | $ | 1,615.5 | |
| | | | | | | |
| Basic weighted-average common shares outstanding | 258.1 | | | 257.7 | | | 258.1 | | | 257.6 | |
| Effect of potentially dilutive securities: | | | | | | | |
Restricted stock units (including performance-based restricted stock units (“PSUs”)) | — | | | 1.4 | | | — | | | 1.5 | |
| Stock options | — | | | 1.3 | | | — | | | 1.2 | |
Employee stock purchase program | — | | | 0.0 | | | — | | | 0.0 | |
| Diluted weighted-average common shares outstanding | 258.1 | | | 260.4 | | | 258.1 | | | 260.3 | |
| | | | | | | |
| Basic net (loss) income per common share | $ | (13.92) | | | $ | 3.55 | | | $ | (9.66) | | | $ | 6.27 | |
| Diluted net (loss) income per common share | $ | (13.92) | | | $ | 3.52 | | | $ | (9.66) | | | $ | 6.21 | |
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
We did not include the securities in the following table in the computation of the diluted net (loss) income per common share because the effect would have been anti-dilutive during each period:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| (in millions) |
| Unvested restricted stock units (including PSUs) | 3.2 | | | — | | | 1.6 | | | 0.3 | |
| Stock options | 1.7 | | | 0.0 | | | 0.8 | | | 0.0 | |
E.Fair Value Measurements
The following fair value hierarchy is used to classify assets and liabilities based on observable inputs and unobservable inputs used to determine the fair value of our financial assets and liabilities:
| | | | | |
Level 1: | Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. |
Level 2: | Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active. |
Level 3: | Unobservable inputs based on our assessment of the assumptions that market participants would use in pricing the asset or liability. |
The following tables set forth our financial assets and liabilities subject to fair value measurements by level within the fair value hierarchy:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2024 | | As of December 31, 2023 |
| Total | | Level 1 | | Level 2 | | Level 3 | | Total | | Level 1 | | Level 2 | | Level 3 |
| | | | | | | | | | | | | | | |
| (in millions) |
Financial instruments carried at fair value (asset positions): |
| Cash equivalents | $ | 1,836.1 | | | $ | 1,152.5 | | | $ | 683.6 | | | $ | — | | | $ | 7,033.9 | | | $ | 5,397.3 | | | $ | 1,636.6 | | | $ | — | |
Marketable securities: | | | | | | | | | | | | | | | |
| Corporate equity securities | 30.6 | | | 30.6 | | | — | | | — | | | 46.0 | | | 46.0 | | | — | | | — | |
| U.S. Treasury securities | 1,389.5 | | | 1,389.5 | | | — | | | — | | | 546.5 | | | 546.5 | | | — | | | — | |
U.S. government agency securities | 367.6 | | | — | | | 367.6 | | | — | | | 425.2 | | | — | | | 425.2 | | | — | |
| Asset-backed securities | 891.9 | | | — | | | 891.9 | | | — | | | 306.0 | | | — | | | 306.0 | | | — | |
| Certificates of deposit | 20.2 | | | — | | | 20.2 | | | — | | | 33.7 | | | — | | | 33.7 | | | — | |
| Corporate debt securities | 2,776.9 | | | — | | | 2,776.9 | | | — | | | 1,802.8 | | | — | | | 1,802.8 | | | — | |
| Commercial paper | 131.8 | | | — | | | 131.8 | | | — | | | 186.8 | | | — | | | 186.8 | | | — | |
| Prepaid expenses and other current assets: | | | | | | | | | | | | | | | |
| Foreign currency forward contracts | 36.0 | | | — | | | 36.0 | | | — | | | 1.8 | | | — | | | 1.8 | | | — | |
| Other assets: | | | | | | | | | | | | | | | |
| Foreign currency forward contracts | 4.8 | | | — | | | 4.8 | | | — | | | — | | | — | | | — | | | — | |
Total financial assets | $ | 7,485.4 | | | $ | 2,572.6 | | | $ | 4,912.8 | | | $ | — | | | $ | 10,382.7 | | | $ | 5,989.8 | | | $ | 4,392.9 | | | $ | — | |
| | | | | | | | | | | | | | | |
Financial instruments carried at fair value (liability positions): |
| Other current liabilities: | | | | | | | | | | | | | | | |
Foreign currency forward contracts | $ | (0.9) | | | $ | — | | | $ | (0.9) | | | $ | — | | | $ | (33.7) | | | $ | — | | | $ | (33.7) | | | $ | — | |
| | | | | | | | | | | | | | | |
| Other long-term liabilities: | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| Contingent consideration | (77.8) | | | — | | | — | | | (77.8) | | | (77.4) | | | — | | | — | | | (77.4) | |
Total financial liabilities | $ | (78.7) | | | $ | — | | | $ | (0.9) | | | $ | (77.8) | | | $ | (111.1) | | | $ | — | | | $ | (33.7) | | | $ | (77.4) | |
Please refer to Note F, “Marketable Securities and Equity Investments,” for the carrying amount and related unrealized gains (losses) by type of investment. Our cash equivalents primarily include money market funds and time deposits.
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
Fair Value of Corporate Equity Securities
We classify our investments in publicly traded corporate equity securities as “Marketable securities” on our condensed consolidated balance sheets. Generally, our investments in the common stock of publicly traded companies are valued based on Level 1 inputs because they have readily determinable fair values. However, certain of our investments in publicly traded companies have been or continue to be valued based on Level 2 inputs due to transfer restrictions associated with these investments.
As of June 30, 2024, one of our investments in publicly traded corporate equity securities was subject to a contractual sales restriction expiring partially in the third quarter of 2024 and partially in the first quarter of 2025 with a total fair value of $23.1 million. We purchased this investment directly from the publicly traded company in the first quarter of 2023, and do not anticipate any circumstances that would cause this restriction to lapse prior to the periods listed above.
Please refer to Note F, “Marketable Securities and Equity Investments,” for further information on these investments.
Fair Value of Contingent Consideration
In 2019, we acquired Exonics Therapeutics, Inc. (“Exonics”), a privately-held company focused on creating transformative gene-editing therapies to repair mutations that cause Duchenne muscular dystrophy (“DMD”) and other severe neuromuscular diseases, including DM1. Our Level 3 contingent consideration liabilities are related to $678.3 million of development and regulatory milestones potentially payable to former Exonics equity holders. We base our estimates of the probability of achieving the milestones relevant to the fair value of contingent payments on industry data attributable to gene therapies and our knowledge of the progress and viability of the programs. The discount rates used in the valuation model for contingent payments, which were between 5.1% and 5.6% as of June 30, 2024, represent a measure of credit risk and market risk associated with settling the liabilities. Significant judgment is used in determining the appropriateness of these assumptions at each reporting period.
The following table represents a rollforward of the fair value of our contingent consideration liabilities:
| | | | | | | | | | | |
| Six Months Ended June 30, 2024 | | | | | | |
| (in millions) | | | | | | |
| Balance at December 31, 2023 | $ | 77.4 | | | | | | | |
Increase in fair value of contingent payments | 0.4 | | | | | | | |
| Balance at June 30, 2024 | $ | 77.8 | | | | | | | |
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
F.Marketable Securities and Equity Investments
A summary of our cash equivalents and marketable debt and equity securities, which are recorded at fair value, is shown below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2024 | | As of December 31, 2023 |
| Amortized Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value | | Amortized Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value |
| | | | | | | | | | | | | | | |
| (in millions) |
Cash equivalents | $ | 1,836.1 | | | $ | — | | | $ | — | | | $ | 1,836.1 | | | $ | 7,033.9 | | | $ | — | | | $ | — | | | $ | 7,033.9 | |
| Marketable securities: | | | | | | | | | | | | | | | |
| U.S. Treasury securities | 1,394.8 | | | 0.5 | | | (5.8) | | | 1,389.5 | | | 544.5 | | | 3.0 | | | (1.0) | | | 546.5 | |
U.S. government agency securities | 369.0 | | | 0.1 | | | (1.5) | | | 367.6 | | | 424.8 | | | 0.9 | | | (0.5) | | | 425.2 | |
| Asset-backed securities | 893.3 | | | 0.7 | | | (2.1) | | | 891.9 | | | 304.9 | | | 1.4 | | | (0.3) | | | 306.0 | |
| Certificates of deposit | 20.2 | | | 0.0 | | | (0.0) | | | 20.2 | | | 33.7 | | | 0.0 | | | (0.0) | | | 33.7 | |
Corporate debt securities | 2,788.4 | | | 2.4 | | | (13.9) | | | 2,776.9 | | | 1,794.0 | | | 10.5 | | | (1.7) | | | 1,802.8 | |
Commercial paper | 131.9 | | | — | | | (0.1) | | | 131.8 | | | 186.8 | | | 0.1 | | | (0.1) | | | 186.8 | |
Total marketable available-for-sale debt securities | 5,597.6 | | | 3.7 | | | (23.4) | | | 5,577.9 | | | 3,288.7 | | | 15.9 | | | (3.6) | | | 3,301.0 | |
Corporate equity securities | 72.1 | | | — | | | (41.5) | | | 30.6 | | | 72.1 | | | — | | | (26.1) | | | 46.0 | |
Total marketable securities | 5,669.7 | | | 3.7 | | | (64.9) | | | 5,608.5 | | | 3,360.8 | | | 15.9 | | | (29.7) | | | 3,347.0 | |
| Total cash equivalents and marketable securities | $ | 7,505.8 | | | $ | 3.7 | | | $ | (64.9) | | | $ | 7,444.6 | | | $ | 10,394.7 | | | $ | 15.9 | | | $ | (29.7) | | | $ | 10,380.9 | |
Amounts in the table above at fair value were classified on our condensed consolidated balance sheets as follows:
| | | | | | | | | | | |
| As of June 30, 2024 | | As of December 31, 2023 |
| | | |
| (in millions) |
| Cash and cash equivalents | $ | 1,836.1 | | | $ | 7,033.9 | |
Marketable securities | 1,215.4 | | | 849.2 | |
| Long-term marketable securities | 4,393.1 | | | 2,497.8 | |
Total | $ | 7,444.6 | | | $ | 10,380.9 | |
Marketable available-for-sale debt securities by contractual maturity were as follows:
| | | | | | | | | | | |
| As of June 30, 2024 | | As of December 31, 2023 |
| | | |
| (in millions) |
| Matures within one year | $ | 1,184.8 | | | $ | 803.2 | |
Matures after one year through five years | 4,334.3 | | | 2,495.6 | |
| Matures after five years | 58.8 | | | 2.2 | |
Total | $ | 5,577.9 | | | $ | 3,301.0 | |
We did not record any allowances for credit losses to adjust the fair value of our marketable available-for-sale debt securities or gross realized gains or losses in the three and six months ended June 30, 2024 and 2023. Additionally, we did not record any realized gains or losses that were material to our condensed consolidated statements of income during the three and six months ended June 30, 2024 and 2023. As of June 30, 2024, we held marketable available-for-sale debt securities with a total fair value of $4.6 billion that were in unrealized loss positions totaling $23.4 million. Included in this amount were marketable available-for sale debt securities with a total fair value of $382.4 million and total unrealized loss of $3.5 million that had been in unrealized loss positions for greater than twelve months. We intend to hold these investments until maturity and do not expect to incur realized losses on these investments when they mature.
We record changes in the fair value of our investments in corporate equity securities to “Other (expense) income, net” in our condensed consolidated statements of income. During the three and six months ended June 30, 2024 and 2023, our net
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
unrealized (losses) gains on corporate equity securities with readily determinable fair values held at the conclusion of each period were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| (in millions) |
Net unrealized (losses) gains | $ | (12.7) | | | $ | 9.2 | | | $ | (15.4) | | | $ | (0.9) | |
During the six months ended June 30, 2023, we received proceeds of $95.1 million related to the sale of the common stock of a publicly traded company, which had a total original cost basis of $57.3 million. There were no sales of the common stock of publicly traded companies during the six months ended June 30, 2024.
As of June 30, 2024, the carrying value of our equity investments without readily determinable fair values, which are recorded in “Other assets” on our condensed consolidated balance sheets, was $85.3 million. During the six months ended June 30, 2024, we reduced the carrying value of one of our equity investments without a readily determinable fair value by $24.3 million based on an observable change in price.
G.Accumulated Other Comprehensive Income (Loss)
The following table summarizes the changes in accumulated other comprehensive income (loss) by component:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | Unrealized Holding Gains (Losses), Net of Tax | | |
| Foreign Currency Translation Adjustment | | On Available-For-Sale Debt Securities | | On Foreign Currency Forward Contracts | | Total |
| | | | | | | |
| (in millions) |
| Balance at December 31, 2023 | $ | 1.1 | | | $ | 9.6 | | | $ | (25.0) | | | $ | (14.3) | |
| Other comprehensive income (loss) before reclassifications | 5.6 | | | (29.2) | | | 67.5 | | | 43.9 | |
| Amounts reclassified from accumulated other comprehensive income (loss) | — | | | 4.1 | | | (11.2) | | | (7.1) | |
| Net current period other comprehensive income (loss) | 5.6 | | | (25.1) | | | 56.3 | | | 36.8 | |
| Balance at June 30, 2024 | $ | 6.7 | | | $ | (15.5) | | | $ | 31.3 | | | $ | 22.5 | |
| | | | | | | |
| Balance at December 31, 2022 | $ | (25.0) | | | $ | (0.1) | | | $ | 25.9 | | | $ | 0.8 | |
| Other comprehensive income (loss) before reclassifications | 14.1 | | | (12.6) | | | (18.4) | | | (16.9) | |
| Amounts reclassified from accumulated other comprehensive income (loss) | — | | | — | | | (23.7) | | | (23.7) | |
| Net current period other comprehensive income (loss) | 14.1 | | | (12.6) | | | (42.1) | | | (40.6) | |
| Balance at June 30, 2023 | $ | (10.9) | | | $ | (12.7) | | | $ | (16.2) | | | $ | (39.8) | |
H.Hedging
Foreign currency forward contracts - Designated as hedging instruments
We maintain a hedging program intended to mitigate the effect of changes in foreign exchange rates for a portion of our forecasted product revenues denominated in certain foreign currencies. The program includes foreign currency forward contracts that are designated as cash flow hedges under U.S. GAAP having contractual durations from one to eighteen
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
months. We recognize realized gains and losses for the effective portion of such contracts in “Product revenues, net” in our condensed consolidated statements of income in the same period that we recognize the product revenues that were impacted by the hedged foreign exchange rate changes.
We formally document the relationship between foreign currency forward contracts (hedging instruments) and forecasted product revenues (hedged items), as well as our risk management objective and strategy for undertaking various hedging activities, which includes matching all foreign currency forward contracts that are designated as cash flow hedges to forecasted transactions. We also formally assess, both at the hedge’s inception and on an ongoing basis, whether the foreign currency forward contracts are highly effective in offsetting changes in cash flows of hedged items on a prospective and retrospective basis. If we were to determine that a (i) foreign currency forward contract is not highly effective as a cash flow hedge, (ii) foreign currency forward contract has ceased to be a highly effective hedge or (iii) forecasted transaction is no longer probable of occurring, we would discontinue hedge accounting treatment prospectively. We measure effectiveness based on the change in fair value of the forward contracts and the fair value of the hypothetical foreign currency forward contracts with terms that match the critical terms of the risk being hedged. As of June 30, 2024, all hedges were determined to be highly effective.
We consider the impact of our counterparties’ credit risk on the fair value of the foreign currency forward contracts. As of June 30, 2024 and December 31, 2023, credit risk did not change the fair value of our foreign currency forward contracts.
The following table summarizes the notional amount in U.S. dollars of our outstanding foreign currency forward contracts designated as cash flow hedges under U.S. GAAP:
| | | | | | | | | | | |
| As of June 30, 2024 | | As of December 31, 2023 |
| | | |
| Foreign Currency | (in millions) |
| Euro | $ | 2,045.4 | | | $ | 1,720.6 | |
British pound sterling | 128.0 | | | 225.0 | |
Canadian dollar | 127.5 | | | 229.5 | |
Australian dollar | 83.1 | | | 153.3 | |
| Swiss Franc | 34.7 | | | 63.9 | |
Total foreign currency forward contracts | $ | 2,418.7 | | | $ | 2,392.3 | |
Foreign currency forward contracts - Not designated as hedging instruments
We also enter into foreign currency forward contracts with contractual maturities of less than one month, which are designed to mitigate the effect of changes in foreign exchange rates on monetary assets and liabilities, including intercompany balances. These contracts are not designated as hedging instruments under U.S. GAAP. We recognize realized gains and losses for such contracts in “Other (expense) income, net” in our condensed consolidated statements of income each period. As of June 30, 2024, we did not have any outstanding foreign currency forward contracts where hedge accounting under U.S. GAAP was not applied.
During the three and six months ended June 30, 2024 and 2023, we recognized the following related to foreign currency forward contracts in our condensed consolidated statements of income:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| (in millions) |
| Designated as hedging instruments - Reclassified from AOCI | | | | | | |
| Product revenues, net | $ | 10.9 | | | $ | 8.2 | | | $ | 14.3 | | | $ | 30.2 | |
| Not designated as hedging instruments | | | | | | | |
| Other (expense) income, net | $ | (13.4) | | | $ | 0.6 | | | $ | (15.8) | | | $ | 4.2 | |
| | | | | | | |
| Total reported in the Condensed Consolidated Statements of Income | | | | | | |
| Product revenues, net | $ | 2,645.6 | | | $ | 2,493.2 | | | $ | 5,336.2 | | | $ | 4,868.0 | |
| Other (expense) income, net | $ | (23.1) | | | $ | 1.6 | | | $ | (54.3) | | | $ | 2.9 | |
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
The following table summarizes the fair value of our outstanding foreign currency forward contracts designated as cash flow hedges under U.S. GAAP included on our condensed consolidated balance sheets:
| | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2024 |
| Assets | | Liabilities |
| Classification | | Fair Value | | Classification | | Fair Value |
| | | | | | |
| (in millions) |
| Prepaid expenses and other current assets | | $ | 36.0 | | | Other current liabilities | | $ | (0.9) | |
| Other assets | | 4.8 | | | Other long-term liabilities | | — | |
Total assets | | $ | 40.8 | | | Total liabilities | | $ | (0.9) | |
| | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2023 |
| Assets | | Liabilities |
| Classification | | Fair Value | | Classification | | Fair Value |
| | | | | | |
| (in millions) |
| Prepaid expenses and other current assets | | $ | 1.8 | | | Other current liabilities | | $ | (33.7) | |
| | | | | | |
| | | | | | |
As of June 30, 2024, we expect the amounts that are related to foreign exchange forward contracts designated as cash flow hedges under U.S. GAAP recorded in “Prepaid expenses and other current assets” and “Other current liabilities” to be reclassified to earnings within twelve months.
We present the fair value of our foreign currency forward contracts on a gross basis within our condensed consolidated balance sheets. The following table summarizes the potential effect of offsetting derivatives by type of financial instrument designated as cash flow hedges under U.S. GAAP on our condensed consolidated balance sheets:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of June 30, 2024 |
| Gross Amounts Recognized | | Gross Amounts Offset | | Gross Amounts Presented | | Gross Amounts Not Offset | | Legal Offset |
| | | | | | | | | |
| Foreign currency forward contracts | (in millions) |
| Total assets | $ | 40.8 | | | $ | — | | | $ | 40.8 | | | $ | (0.9) | | | $ | 39.9 | |
| Total liabilities | (0.9) | | | — | | | (0.9) | | | 0.9 | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2023 |
| Gross Amounts Recognized | | Gross Amounts Offset | | Gross Amounts Presented | | Gross Amounts Not Offset | | Legal Offset |
| | | | | | | | | |
| Foreign currency forward contracts | (in millions) |
| Total assets | $ | 1.8 | | | $ | — | | | $ | 1.8 | | | $ | (1.8) | | | $ | — | |
| Total liabilities | (33.7) | | | — | | | (33.7) | | | 1.8 | | | (31.9) | |
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
I.Inventories
Inventories consisted of the following:
| | | | | | | | | | | |
| As of June 30, 2024 | | As of December 31, 2023 |
| | | |
| (in millions) |
| Raw materials | $ | 112.1 | | | $ | 78.7 | |
| Work-in-process | 657.6 | | | 525.1 | |
Finished goods | 144.9 | | | 135.0 | |
Total | $ | 914.6 | | | $ | 738.8 | |
During the first quarter of 2024, following positive results we announced related to our two Phase 3 trials for suzetrigine (formerly VX-548) for acute pain and vanzacaftor/tezacaftor/deutivacaftor for CF, we began capitalizing inventories produced in preparation for our planned product launches. As of June 30, 2024, we continued to conclude that capitalization of these inventories was appropriate. We made these determinations based on our evaluation, among other factors, the safety and efficacy results, and expected likelihood of regulatory approval and commercial success. Prior to the first quarter of 2024, we expensed inventoriable and related costs associated with these product candidates as “Research and development expenses.” As of June 30, 2024, these inventories were not material to our condensed consolidated financial statements.
J.Stock-based Compensation Expense and Share Repurchase Programs
Stock-based compensation expense
During the three and six months ended June 30, 2024 and 2023, we recognized the following stock-based compensation expense:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| (in millions) |
| Stock-based compensation expense by type of award: | | | | | | | |
| Restricted stock units (including PSUs) | $ | 150.7 | | | $ | 113.6 | | | $ | 337.9 | | | $ | 229.5 | |
| ESPP share issuances | 4.5 | | | 3.9 | | | 10.3 | | | 9.4 | |
| Stock options | 1.8 | | | 2.4 | | | 1.8 | | | 3.8 | |
Stock-based compensation expense related to inventories | (2.8) | | | (0.6) | | | (3.9) | | | (1.0) | |
Total stock-based compensation expense included in “Total costs and expenses” | $ | 154.2 | | | $ | 119.3 | | | $ | 346.1 | | | $ | 241.7 | |
| | | | | | | |
| Stock-based compensation expense by line item: | | | | | | | |
| Cost of sales | $ | 1.8 | | | $ | 1.8 | | | $ | 3.6 | | | $ | 3.7 | |
| Research and development expenses | 97.1 | | | 74.5 | | | 216.5 | | | 150.8 | |
| Selling, general and administrative expenses | 55.3 | | | 43.0 | | | 126.0 | | | 87.2 | |
Total stock-based compensation expense included in costs and expenses | 154.2 | | | 119.3 | | | 346.1 | | | 241.7 | |
| Income tax effect | (80.7) | | | (31.3) | | | (159.7) | | | (71.9) | |
Total stock-based compensation expense, net of tax | $ | 73.5 | | | $ | 88.0 | | | $ | 186.4 | | | $ | 169.8 | |
Share repurchase program
In February 2023, our Board of Directors approved a share repurchase program, pursuant to which we are authorized to repurchase up to $3.0 billion of our common stock. The program does not have an expiration date and can be discontinued at any time. During the six months ended June 30, 2024 and 2023, we repurchased 1.1 million and 0.5 million shares of our
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
common stock under the program, respectively, for aggregate repurchases of $456.2 million and $161.1 million, respectively. As of June 30, 2024, we had $2.1 billion remaining authorization under this program.
K.Income Taxes
We are subject to U.S. federal, state, and foreign income taxes. During the three and six months ended June 30, 2024 and 2023, we recorded the following provisions for income taxes and effective tax rates as compared to our income (loss) before provision for income taxes. | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | Six Months Ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| | | | | | | |
| (in millions, except percentages) |
| (Loss) income before provision for income taxes | $ | (3,391.2) | | | $ | 1,161.5 | | | $ | (2,112.1) | | | $ | 2,053.0 | |
| Provision for income taxes | $ | 202.4 | | | $ | 245.8 | | | $ | 381.9 | | | $ | 437.5 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Effective tax rate | (6.0) | % | | 21.2 | % | | (18.1) | % | | 21.3 | % |
Our effective tax rate for the three and six months ended June 30, 2024 was materially different than the U.S. statutory rate primarily due to the $4.4 billion of non-deductible AIPR&D resulting from our acquisition of Alpine, which drove our pre-tax loss in each of these periods.
Our effective tax rate for the three and six months ended June 30, 2023 was similar to the U.S. statutory rate.
We have reviewed the tax positions taken, or to be taken, in our tax returns for all tax years currently open to examination by a taxing authority. As of June 30, 2024 and December 31, 2023, we had $309.3 million and $288.7 million, respectively, of net unrecognized tax benefits, which would affect our tax rate if recognized.
We file U.S. federal income tax returns and income tax returns in various state, local and foreign jurisdictions. We have various income tax audits ongoing at any time throughout the world. Except for jurisdictions where we have net operating losses or tax credit carryforwards, we are no longer subject to any tax assessment from tax authorities for years prior to 2015 in jurisdictions that have a material impact on our consolidated financial statements. In 2023, we came to settlement with the United Kingdom’s HM Revenue & Customs (“HMRC”) with respect to our tax positions for 2015 through 2020 and subsequently received Closure Notices for those periods during the three months ended March 31, 2024. Due to the nature of the adjustments, we are asserting our rights under the U.S./U.K. Income Tax Convention pursuant to the mutual agreement procedures for the relief of double taxation for these matters.
In December 2022, European Union member states reached an agreement to implement the minimum tax component (“Pillar Two”) of the Organization for Economic Co-operation and Development’s (the “OECD’s”), global international tax reform initiative with effective dates of January 1, 2024 and 2025. In July 2023, the OECD published Administrative Guidance proposing certain safe harbors that effectively extend certain effective dates to January 1, 2027. The assessment of our potential 2024 exposure for the global per-country minimum tax of 15%, based on our forecasted 2024 results, is immaterial to our condensed consolidated financial statements as the effective tax rates in most of the jurisdictions in which we operate are above 15%.
L.Commitments and Contingencies
2022 Credit Facility
In July 2022, Vertex and certain of its subsidiaries entered into a $500.0 million unsecured revolving facility (the “Credit Agreement”) with Bank of America, N.A., as administrative agent and the lenders referred to therein (the “Lenders”), which matures on July 1, 2027. The Credit Agreement was not drawn upon at closing and we have not drawn upon it to date. Amounts drawn pursuant to the Credit Agreement, if any, will be used for general corporate purposes. Subject to satisfaction of certain conditions, we may request that the borrowing capacity for the Credit Agreement be increased by an additional $500.0 million. Additionally, the Credit Agreement provides a sublimit of $100.0 million for letters of credit.
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
Any amounts borrowed under the Credit Agreement will bear interest, at our option, at either a base rate or a Secured Overnight Financing Rate (“SOFR”), in each case plus an applicable margin. Under the Credit Agreement, the applicable margins on base rate loans range from 0.000% to 0.500% and the applicable margins on SOFR loans range from 1.000% to 1.500%, in each case based on our consolidated leverage ratio (the ratio of our total consolidated funded indebtedness to our consolidated EBITDA for the most recently completed four fiscal quarter period).
Any amounts borrowed pursuant to the Credit Agreement are guaranteed by certain of our existing and future domestic subsidiaries, subject to certain exceptions.
The Credit Agreement contains customary representations and warranties and affirmative and negative covenants, including a financial covenant to maintain subject to certain limited exceptions, a consolidated leverage ratio of 3.50 to 1.00, subject to an increase to 4.00 to 1.00 following a material acquisition. As of June 30, 2024, we were in compliance with the covenants described above. The Credit Agreement also contains customary events of default. In the case of a continuing event of default, the administrative agent would be entitled to exercise various remedies, including the acceleration of amounts due under outstanding loans.
Direct costs related to the Credit Agreement are recorded over its term and were not material to our financial statements.
Guaranties and Indemnifications
As permitted under Massachusetts law, our Articles of Organization and By-laws provide that we will indemnify certain of our officers and directors for certain claims asserted against them in connection with their service as an officer or director. The maximum potential amount of future payments that we could be required to make under these indemnification provisions is unlimited. However, we have purchased directors’ and officers’ liability insurance policies that could reduce our monetary exposure and enable us to recover a portion of any future amounts paid. No indemnification claims currently are outstanding, and we believe the estimated fair value of these indemnification arrangements is minimal.
We customarily agree in the ordinary course of our business to indemnification provisions in agreements with clinical trial investigators and sites in our product development programs, sponsored research agreements with academic and not-for-profit institutions, various comparable agreements involving parties performing services for us, and our real estate leases. We also customarily agree to certain indemnification provisions in our drug discovery, development and commercialization collaboration agreements. With respect to our clinical trials and sponsored research agreements, these indemnification provisions typically apply to any claim asserted against the investigator or the investigator’s institution relating to personal injury or property damage, violations of law or certain breaches of our contractual obligations arising out of the research or clinical testing of our compounds or product candidates. With respect to lease agreements, the indemnification provisions typically apply to claims asserted against the landlord relating to personal injury or property damage caused by us, to violations of law by us or to certain breaches of our contractual obligations. The indemnification provisions appearing in our collaboration agreements are similar to those for the other agreements discussed above, but in addition provide some limited indemnification for our collaborator in the event of third-party claims alleging infringement of intellectual property rights. In each of the cases above, the indemnification obligation generally survives the termination of the agreement for some extended period, although we believe the obligation typically has the most relevance during the contract term and for a short period of time thereafter. The maximum potential amount of future payments that we could be required to make under these provisions is generally unlimited. We have purchased insurance policies covering personal injury, property damage and general liability that reduce our exposure for indemnification and would enable us in many cases to recover all or a portion of any future amounts paid. We have never paid any material amounts to defend lawsuits or settle claims related to these indemnification provisions. Accordingly, we believe the estimated fair value of these indemnification arrangements is minimal.
Other Contingencies
We have certain contingent liabilities that arise in the ordinary course of our business activities. We accrue for such contingent liabilities when it is probable that future expenditures will be made and such expenditures can be reasonably estimated. Other than our contingent consideration liabilities discussed in Note E, “Fair Value Measurements,” there were no material contingent liabilities accrued as of June 30, 2024 or December 31, 2023.
VERTEX PHARMACEUTICALS INCORPORATED
Notes to Condensed Consolidated Financial Statements (unaudited)
M.Additional Cash Flow Information
The cash, cash equivalents and restricted cash at the beginning and ending of each period presented in our condensed consolidated statements of cash flows consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| Six Months Ended June 30, |
| 2024 | | 2023 |
| Beginning of period | | End of period | | Beginning of period | | End of period |
| | | | | | | |
| (in millions) |
| Cash and cash equivalents | $ | 10,369.1 | | | $ | 4,580.1 | | | $ | 10,504.0 | | | $ | 10,151.1 | |
Prepaid expenses and other current assets | 3.2 | | | 10.9 | | | 8.0 | | | 2.3 | |
| | | | | | | |
| Cash, cash equivalents and restricted cash per condensed consolidated statement of cash flows | $ | 10,372.3 | | | $ | 4,591.0 | | | $ | 10,512.0 | | | $ | 10,153.4 | |
Consistent with our policy for asset acquisitions, we have presented the cost to acquire the AIPR&D associated with Alpine, including attributable direct costs, as an operating cash flow within "Cash flows used in operating activities" for the six months ended June 30, 2024.
We obtained $295.0 million right-of-use operating lease assets in exchange for a similar amount of operating lease obligations in the six months ended June 30, 2024, which represent non-cash operating activities.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
OVERVIEW
We are a global biotechnology company that invests in scientific innovation to create transformative medicines for people with serious diseases, with a focus on specialty markets. We have four approved medicines that treat the underlying cause of cystic fibrosis (“CF”), a life-threatening genetic disease, and one approved therapy that treats severe sickle cell disease (“SCD”) and transfusion dependent beta thalassemia (“TDT”), life-shortening inherited blood disorders. Our pipeline includes clinical-stage programs in CF, sickle cell disease, beta thalassemia, acute and neuropathic pain, APOL1-mediated kidney disease, IgA nephropathy (as well as other autoimmune renal diseases and cytopenias), type 1 diabetes, myotonic dystrophy type 1, and autosomal dominant polycystic kidney disease.
Our four approved CF medicines, led by TRIKAFTA/KAFTRIO (elexacaftor/tezacaftor/ivacaftor and ivacaftor), are being used to treat nearly three quarters of the approximately 92,000 people with CF in North America, Europe, and Australia. We are evaluating our CF medicines in additional patient populations, including younger children, with the goal of having small molecule treatments for all people who have at least one mutation in their cystic fibrosis transmembrane conductance regulator (“CFTR”) gene that is responsive to our CFTR modulators. We also are pursuing messenger ribonucleic acid (“mRNA”) and genetic therapies for people with CF who do not make full-length CFTR protein and, as a result, cannot benefit from our current CF medicines.
In late 2023 and early 2024, CASGEVY (exagamglogene autotemcel or “exa-cel”), an ex-vivo, non-viral CRISPR/Cas9 gene-edited cell therapy, was approved in the U.S., the European Union (the “E.U.”), the United Kingdom (“U.K.”), the Kingdom of Saudi Arabia (“Saudi Arabia”), and the Kingdom of Bahrain (“Bahrain”) for the treatment of people 12 years of age and older with SCD or TDT. We estimate approximately 35,000 people with severe SCD or TDT could be eligible for CASGEVY in the U.S. and Europe, with additional people in Saudi Arabia and Bahrain. In addition, we are preparing for near-term launches of potential new products in CF and acute pain.
Financial Highlights
| | | | | |
| Revenues | In the second quarter of 2024, our net CF product revenues increased to $2.6 billion as compared to $2.5 billion in the second quarter of 2023. The increase was primarily due to the performance of TRIKAFTA in the U.S., following the launch of TRIKAFTA in children with CF 2 to 5 years of age and the continued strong uptake of TRIKAFTA/KAFTRIO in ex-U.S. markets and label extensions in younger age groups. |
| Expenses | Our total research and development (“R&D”) and selling, general and administrative (“SG&A”) expenses increased to $1.3 billion in the second quarter of 2024 as compared to $1.0 billion in the second quarter of 2023. The largest driver of this increase was compensation expense associated with cash-settled unvested equity awards resulting from our acquisition of Alpine Immune Sciences, Inc. (“Alpine”) in May 2024. Acquired in-process research and development expenses (“AIPR&D”) included $4.4 billion resulting from our acquisition of Alpine. Cost of sales was 14% in the second quarter of 2024 as compared to 12% in the second quarter of 2023, primarily due to cost of sales associated with CASGEVY. |
| Cash | Our total cash, cash equivalents and marketable securities decreased to $10.2 billion as of June 30, 2024 as compared to $13.7 billion as of December 31, 2023 primarily due to cash paid to acquire Alpine partially offset by cash flows provided by other operating activities. |
Note: Charts above may not add due to rounding.
Business Updates
Marketed Products
Cystic Fibrosis
We expect to grow our CF business with (i) label expansions, including into younger patient groups and rare mutations, (ii) continued uptake in younger patient groups, and (iii) growth in the number of people living with CF. Recent progress in activities supporting continued uptake and label expansions is included below:
•We entered into an extended long-term reimbursement agreement with NHS England providing access to KAFTRIO, SYMKEVI and ORKAMBI, and continued access to KALYDECO, for existing and future eligible CF patients in England. We have entered into similar reimbursement agreements in Wales, Northern Ireland and Scotland. These reimbursement agreements include access to any future license extensions of these medicines.
•The European Commission approved KALYDECO for the treatment of infants with CF from 1 month to less than 4 months of age with specific mutations in the CFTR gene.
•Health Canada approved TRIKAFTA for the treatment of people with CF with 152 rare responsive mutations in the CFTR gene.
•We submitted regulatory applications to the U.S. Food and Drug Administration (“FDA”) and the European Medicines Agency (the “EMA”) for TRIKAFTA/KAFTRIO for the treatment of people with CF and rare responsive mutations.
Sickle Cell Disease and Beta Thalassemia
•CASGEVY is approved in the U.S., the E.U., the U.K., Saudi Arabia, and Bahrain for people 12 years of age and older with SCD or TDT.
•We completed regulatory submissions for CASGEVY for SCD and TDT in Switzerland and Canada, and our regulatory submission in Canada has been granted Priority Review.
•We have activated more than 35 authorized treatment centers globally, and patients across all regions have initiated cell collection.
•The French National Authority for Health (“HAS”) approved our request for the implementation of an early access program for the use of CASGEVY in indicated patients with SCD. HAS previously approved the implementation of an early access program for CASGEVY in indicated patients with TDT in the first quarter of 2024.
Potential Near-Term Launch Opportunities
We are preparing for the following near-term launches of potential new products:
Vanzacaftor/tezacaftor/deutivacaftor in CF
•The FDA accepted the new drug application (“NDA”) for the once-daily vanzacaftor triple in people with CF 6 years of age and older and granted Priority Review with a PDUFA target action date of January 2, 2025.
•We received validation of our vanzacaftor triple marketing authorization application (“MAA”) submissions from the EMA in the E.U. and the Medicines and Healthcare products Regulatory Agency (“MHRA”) in the U.K. We have also completed regulatory submissions for the vanzacaftor triple in Canada, Australia, New Zealand, and Switzerland.
Suzetrigine in Acute Pain
•The FDA accepted the NDA submission for suzetrigine for the treatment of moderate-to-severe acute pain and granted Priority Review with a PDUFA target action date of January 30, 2025. Suzetrigine has been granted Fast Track and Breakthrough Therapy designations by the FDA for the treatment of moderate-to-severe acute pain.
Pipeline
We continue to advance a diversified pipeline of potentially transformative medicines for serious diseases utilizing a range of modalities. Recent and anticipated progress in activities supporting these efforts is included below:
Cystic Fibrosis
•We have initiated a new cohort in the Phase 3 clinical trial evaluating the vanzacaftor triple in children with CF 2 to 5 years of age who have at least one F508del mutation or a mutation responsive to triple combination CFTR modulators.
•In collaboration with Moderna, Inc. (“Moderna”), we are developing VX-522, a nebulized mRNA therapy for the treatment of people with CF who do not produce full-length CFTR protein. The multiple ascending dose portion of the Phase 1/2 clinical trial of VX-522 in people with CF is ongoing. We expect to complete this clinical trial and share data in the first half of 2025.
•We continue to advance new oral small molecule combination therapies through preclinical and clinical development. The most advanced next-wave CFTR modulators have completed, or are in the process of completing, Phase 1 clinical trials.
Sickle Cell Disease and Transfusion-Dependent Beta Thalassemia
•We have completed enrollment in two global Phase 3 clinical trials evaluating CASGEVY in children 5 to 11 years of age with SCD or TDT, and the trials are ongoing.
•We continue to work on preclinical assets for myeloablative conditioning agents that would have milder side-effects and could be used in connection with CASGEVY, which could broaden the eligible patient population.
Acute Pain
•We are enrolling and dosing patients in a Phase 1 clinical trial evaluating an intravenous formulation of VX-993, a next-generation selective NaV1.8 pain signal inhibitor.
•We expect to initiate a Phase 2 clinical trial evaluating an oral formulation of VX-993 for the treatment of moderate-to-severe acute pain following bunionectomy surgery in the third quarter of 2024.
•We are advancing additional NaV1.8 inhibitors and NaV1.7 pain signal inhibitors, which could be used alone or in combination, for the treatment of acute pain.
Peripheral Neuropathic Pain
•We expect to initiate the Phase 3 pivotal program evaluating suzetrigine in people with diabetic peripheral neuropathy, a common form of chronic peripheral neuropathic pain, in the third quarter of 2024. The FDA has granted suzetrigine Breakthrough Therapy designation in diabetic peripheral neuropathy.
•We have completed enrollment in the Phase 2 clinical trial evaluating suzetrigine in people with lumbosacral radiculopathy, a second type of peripheral neuropathic pain. We expect to share results from this clinical trial in late 2024.
•We expect to initiate a Phase 2 clinical trial evaluating the oral formulation of VX-993 for the treatment of diabetic peripheral neuropathy in the third quarter of 2024.
•We are advancing additional NaV1.8 inhibitors and NaV1.7 pain signal inhibitors, which could be used alone or in combination, for the treatment of peripheral neuropathic pain.
APOL1-Mediated Kidney Disease
•Inaxaplin is our small molecule for the treatment of APOL1-mediated kidney disease (“AMKD”), including APOL1-mediated focal segmental glomerulosclerosis (“FSGS”). We continue to enroll and dose people with AMKD in the Phase 3 portion of the global Phase 2/3 pivotal clinical trial.
•The clinical trial is designed to have a pre-planned interim analysis at Week 48 evaluating estimated glomerular filtration rate (“eGFR”) slope, a measure of kidney function, supported by a percentage change from baseline in proteinuria, in the inaxaplin arm versus placebo. If positive, we expect that the interim analysis may serve as the basis to seek accelerated approval in the U.S.
IgA Nephropathy and Other B Cell-Mediated Diseases
•We are developing povetacicept, a dual inhibitor of the BAFF and APRIL pathways, as a potentially best-in-class approach to treat IgA nephropathy (“IgAN”), a serious progressive, autoimmune kidney disease that can lead to end-stage renal disease. We have completed end-of-phase 2 regulatory interactions and we expect to initiate the Phase 3 clinical trial evaluating povetacicept in IgAN (the “RAINIER” trial) in the third quarter of 2024.
•RAINIER is a global pivotal trial evaluating povetacicept in approximately 480 people with IgAN. The trial is designed to have a pre-planned interim analysis evaluating urine protein creatinine ratio (“UPCR”) after a certain number of patients reach 36 weeks of treatment. If positive, the interim analysis may serve as the basis for seeking accelerated approval in the U.S. Final analysis will occur at two years of treatment, with a primary endpoint of total eGFR slope through Week 104.
•The RUBY3 (autoimmune kidney diseases) and RUBY4 (autoimmune cytopenias) Phase 2 basket trials are ongoing. We expect data readouts from certain cohorts later in 2024 and into 2025.
Type 1 Diabetes
•VX-880 is an allogeneic stem cell-derived, fully differentiated, insulin-producing islet cell replacement therapy, using standard immunosuppression to protect the implanted cells. We are evaluating VX-880 as a potential treatment for type 1 diabetes (“T1D”) in a sequential, three-part Phase 1/2 clinical trial. Based on positive data announced in June 2024, we have expanded the Phase 1/2 clinical trial in people with T1D to include a total of 37 patients. We have completed enrollment and dosing in the original Phase 1/2 17-patient clinical trial.
•Our second Phase 1/2 program in T1D evaluates VX-264, which encapsulates the same VX-880 islet cells in a novel device designed to eliminate the need for immunosuppression. This trial is a sequential, multi-part study to evaluate the safety, tolerability and efficacy of VX-264. We have completed Part A of the clinical trial and we are enrolling and dosing people with TID in Part B of the clinical trial, where patients receive the full-target dose with a stagger period between patients. In Part C, we expect that patients will receive the full target dose with no stagger.
•Our hypoimmune islet cell program uses CRISPR/Cas9 technology to gene-edit the same allogeneic stem cell-derived, fully differentiated islet cells used in the VX-880 and VX-264 programs. The goal is to protect the cells from the immune system to explore another possible path to eliminate the need for immunosuppressive therapy. This program continues to progress through the research stage.
Myotonic Dystrophy Type 1
•Our lead approach for myotonic dystrophy type 1 (“DM1”), VX-670, was in-licensed from Entrada Therapeutics, Inc. (“Entrada”). VX-670 is an oligonucleotide connected to a cyclic peptide to promote effective delivery into cells, which holds the potential to address the underlying cause of DM1.
•We are enrolling and dosing people with DM1 in the global Phase 1/2 clinical trial for VX-670 and expect to complete the single ascending dose portion of the clinical trial by the end of 2024. Following the completion of the single ascending dose portion, we expect to move into the multiple ascending dose portion of the trial, which will evaluate the safety and efficacy of VX-670.
Autosomal Dominant Polycystic Kidney
•We are enrolling and dosing in a Phase 1 clinical trial in healthy volunteers evaluating VX-407, our first-in-class small molecule corrector that targets the underlying cause of autosomal dominant polycystic kidney disease (“ADPKD”) in people with a subset of PKD1 variants.
Alpha-1 Antitrypsin Deficiency
•We are working to address the underlying genetic cause of alpha-1 antitrypsin (“AAT”) deficiency by developing novel small molecule correctors of Z-AAT protein folding, with a goal of increasing the secretion of functional AAT into the blood and addressing both the lung and the liver aspects of AAT deficiency (“AATD”).
•Based on Phase 1 biomarker analyses, we have determined that VX-634 and VX-668, two investigational small molecule AAT correctors, would not deliver transformative efficacy for people with AATD. We have discontinued development of both molecules.
•Consistent with our portfolio approach to research and development, we are using our learnings from VX-634, VX-668 and prior molecules to continue to optimize the small molecule corrector and other approaches in the preclinical research phase.
Investment in External Innovation
•We completed the previously announced acquisition of Alpine for approximately $5.0 billion in cash, including Alpine’s lead asset, povetacicept.
Our Business Environment
In the first half of 2024, our net product revenues came from the sale of our medicines for the treatment of CF. Our CF strategy involves continuing to develop and obtain approval and reimbursement for treatment regimens that will provide benefits to all people with CF and increasing the number of people with CF eligible and able to receive our medicines, including through label expansions, expanded reimbursement, and the development of new medicines. We are advancing our pipeline of product candidates for the treatment of serious diseases outside of CF, including CASGEVY, which has received marketing approvals in the U.S., the E.U., the U.K., Saudi Arabia, and Bahrain for the treatment of SCD and TDT.
Our strategy is to combine transformative advances in the understanding of causal human biology and the science of therapeutics to discover and develop innovative medicines. This approach includes advancing multiple compounds or therapies from each program, spanning multiple modalities, into early clinical trials to obtain patient data that can inform selection of the most promising therapies for later-stage development, as well as to inform discovery and development efforts. We aim to rapidly follow our first-in-class therapies that achieve proof-of-concept with potential best-in-class candidates to provide durable clinical and commercial success.
In pursuit of new product candidates and therapies in specialty markets, we invest in research and development. We believe that pursuing research in diverse areas allows us to balance the risks inherent in product development and may provide product candidates that will form our pipeline in future years. To supplement our internal research programs, we acquire technologies and programs and collaborate with biopharmaceutical and technology companies, leading academic research institutions, government laboratories, foundations and other organizations, as needed, to advance research in our areas of therapeutic interest and to access technologies needed to execute on our strategy.
Discovery and development of a new pharmaceutical or biological product is a difficult and lengthy process that requires significant financial resources along with extensive technical and regulatory expertise. Across the industry, most potential drug or biological products never progress into development, and most products that do advance into development never receive marketing approval. Our investments in product candidates are subject to considerable risks. We closely monitor the results of our discovery, research, clinical trials and nonclinical studies and frequently evaluate our product development programs in light of new data and scientific, business and commercial insights, with the objective of balancing risk and potential. This process can result in rapid changes in focus and priorities as new information becomes available and as we gain additional understanding of our ongoing programs and potential new programs, as well as those of our competitors.
Our business also requires ensuring appropriate manufacturing and reimbursement of our products. As we advance our product candidates through clinical development toward commercialization and market and sell our approved products, we build and maintain our supply chain and quality assurance resources. We rely on a global network of third parties, including some in China, and our internal capabilities to manufacture and distribute our products for commercial sale and post-approval clinical trials and to manufacture and distribute our product candidates for clinical trials. In addition to establishing supply chains for each new approved product, we adapt our supply chain for existing products to include additional formulations or to increase scale of production for existing products as needed. Our foreign third-party manufacturers and suppliers may be subject to U.S. legislation, including the BIOSECURE Act, sanctions, trade restrictions and other foreign regulatory requirements which could increase costs or reduce the supply of material available to us, or delay the procurement or supply of such material. The processes for cell and genetic therapies can be more complex than those required for small molecule drugs and require additional investments in different systems, equipment, facilities and expertise. We are focused on ensuring the stability of the supply chains for our current products, as well as for our pipeline programs.
Sales of our products depend, to a large degree, on the extent to which our products are reimbursed by third-party payors, such as government health programs, commercial insurance and managed health care organizations. Reimbursement for our products, including our potential pipeline therapies, cannot be assured and may take significant periods of time to obtain. We dedicate substantial management and other resources to obtain and maintain appropriate levels of reimbursement for our products from third-party payors, including governmental organizations in the U.S. and ex-U.S. markets.
In the U.S., we have worked successfully with third-party payors to promptly obtain appropriate levels of reimbursement for our CF medicines. In addition, we are working with U.S. government and commercial payors with respect to CASGEVY. We anticipate broad access with government and commercial payors for CASGEVY in the U.S., and we have recently entered into multiple agreements with government and commercial health insurance providers to provide access to CASGEVY. We plan to continue to engage in discussions with numerous commercial insurers and managed health care organizations, along with government health programs that are typically managed by authorities in the individual states, to ensure that payors recognize the significant benefits that all of our therapies provide and provide patients with appropriate levels of access to our medicines and therapies now and in the future. We cannot, however, predict how recent changes in the law, including through the Inflation Reduction Act of 2022 and passage of state laws (e.g., transparency laws and prescription drug affordability boards), will affect our ability to negotiate successfully with third-party payors and distribute our products. Similarly, in ex-U.S. markets, we seek government reimbursement for our medicines on a country-by-country or region-by-region basis, as required. This is necessary for each new medicine, as well as for label expansions for our current medicines. We are working with ex-U.S. payors with respect to CASGEVY, and we are pursuing long-term reimbursement agreements. We have secured reimbursed access for people with SCD or TDT in Saudi Arabia and Bahrain, and for people with SCD or TDT in France through an expanded access program. We expect to continue to focus significant resources to expand and maintain reimbursement for our CF medicines, CASGEVY and, ultimately, pipeline therapies, in U.S. and ex-U.S. markets.
Strategic Transactions
Acquisitions
As part of our business strategy, we seek to acquire technologies, products, product candidates and other businesses that are aligned with our corporate and research and development strategies and complement and advance our ongoing research and development efforts. We have engaged in a number of acquisitions of biotechnology companies over the last several years and expect to continue to identify and evaluate opportunities to acquire additional biotechnology companies. The accounting for these acquisitions can vary significantly based on whether we conclude the transactions represent business combinations or asset acquisitions. In May 2024, we acquired Alpine as described above. Alpine’s lead molecule, povetacicept, has shown potential best-in-class efficacy in IgAN through Phase 2 development. We accounted for the Alpine transaction as an asset acquisition because povetacicept represents substantially all of the fair value of the gross assets that we acquired. As a result, $4.4 billion of the fair value attributed to povetacicept was expensed as AIPR&D in the second quarter and first half of 2024. In 2022, we acquired ViaCyte, Inc. (“ViaCyte”), which had intellectual property, tools, technologies and assets with the potential to accelerate development of our T1D programs. We accounted for our acquisition of ViaCyte as a business combination.
Collaboration and In-Licensing Arrangements
We enter into arrangements with third parties, including collaboration and licensing arrangements, for the development, manufacture and commercialization of products, product candidates and other technologies that have the potential to complement our ongoing research and development efforts. Over the last several years, we entered into collaboration agreements with a number of companies, including CRISPR Therapeutics AG (“CRISPR”), Entrada, and Moderna. In July 2024, we entered into a multi-target license and option agreement with Orum Therapeutics for the use of their degrader-
antibody conjugate technology for the discovery of novel targeted conditioning agents for gene-edited therapies, such as CASGEVY, which included an upfront payment of approximately $15.0 million.
Generally, when we in-license a technology or product candidate, we make upfront payments to the collaborator, assume the costs of the program and/or agree to make contingent payments, which could consist of milestone, royalty and option payments. Most of these collaboration payments are expensed as AIPR&D, including a $75.0 million milestone due to Entrada in the first quarter of 2024, and our upfront payment of $225.1 million to Entrada and $170.0 million in total upfront and milestone payments to CRISPR related to T1D in the first half of 2023. These payments were expensed to AIPR&D because they were primarily attributable to acquired in-process research and development for which there was no alternative future use. However, depending on many factors, including the structure of the collaboration, the stage of development of the acquired technology, the significance of the in-licensed product candidate to the collaborator’s operations and the other activities in which our collaborators are engaged, the accounting for these transactions can vary significantly. We expect to continue to identify and evaluate collaboration and licensing opportunities that may be similar to or different from the collaborations and licenses that we have engaged in previously.
Acquired In-Process Research and Development Expenses
In the first half of 2024 and 2023, our AIPR&D included $4.5 billion and $457.6 million, respectively, related to upfront, contingent milestone, or other payments pursuant to our business development transactions, including the asset acquisitions, collaborations, and licenses of third-party technologies described above. Please refer to Note C, “Collaboration, License and Other Arrangements,” for further information regarding our asset acquisitions, collaborations and in-license agreements.
RESULTS OF OPERATIONS
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages and per share amounts) |
Product revenues, net | $ | 2,645.6 | | | $ | 2,493.2 | | | 6% | | $ | 5,336.2 | | | $ | 4,868.0 | | | 10% |
| Acquired in-process research and development expenses | 4,449.1 | | | 110.5 | | | ** | | 4,525.9 | | | 457.6 | | | ** |
Other operating costs and expenses | 1,711.2 | | | 1,356.3 | | | 26% | | 3,185.5 | | | 2,605.0 | | | 22% |
(Loss) income from operations | (3,514.7) | | | 1,026.4 | | | ** | | (2,375.2) | | | 1,805.4 | | | ** |
| Other non-operating income, net | 123.5 | | | 135.1 | | | (9)% | | 263.1 | | | 247.6 | | | 6% |
Provision for income taxes | 202.4 | | | 245.8 | | | (18)% | | 381.9 | | | 437.5 | | | (13)% |
Net (loss) income | $ | (3,593.6) | | | $ | 915.7 | | | ** | | $ | (2,494.0) | | | $ | 1,615.5 | | | ** |
| | | | | | | | | | | |
Net (loss) income per diluted common share | $ | (13.92) | | | $ | 3.52 | | | | | $ | (9.66) | | | $ | 6.21 | | | |
Diluted shares used in per share calculations | 258.1 | | | 260.4 | | | | | 258.1 | | | 260.3 | | | |
| | | | | | | ** Not meaningful |
Product Revenues, net
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages) |
| TRIKAFTA/KAFTRIO | $ | 2,449.2 | | | $ | 2,240.4 | | | 9% | | $ | 4,932.8 | | | $ | 4,337.1 | | | 14% |
| Other CF products | 196.4 | | | 252.8 | | | (22)% | | 403.4 | | | 530.9 | | | (24)% |
| Product revenues, net | $ | 2,645.6 | | | $ | 2,493.2 | | | 6% | | $ | 5,336.2 | | | $ | 4,868.0 | | | 10% |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | |
In the second quarter and first half of 2024, our net product revenues increased by $152.4 million and $468.2 million, or 6% and 10%, as compared to the second quarter and first half of 2023, respectively. The increase was primarily due to the performance of TRIKAFTA in the U.S., following the launch of TRIKAFTA in children with CF 2 to 5 years of age, and continued strong uptake of TRIKAFTA/KAFTRIO in ex-U.S. markets and label extensions in younger age groups. Decreases
in revenues for our CF products other than TRIKAFTA/KAFTRIO were primarily the result of patients switching from these medicines to TRIKAFTA/KAFTRIO.
Our net product revenues from the U.S. and from ex-U.S. markets were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages) |
| United States | $ | 1,614.3 | | | $ | 1,507.8 | | | 7% | | $ | 3,134.2 | | | $ | 2,911.6 | | | 8% |
| ex-U.S. | 1,031.3 | | | 985.4 | | | 5% | | 2,202.0 | | | 1,956.4 | | | 13% |
| Product revenues, net | $ | 2,645.6 | | | $ | 2,493.2 | | | 6% | | $ | 5,336.2 | | | $ | 4,868.0 | | | 10% |
Operating Costs and Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages) |
| Cost of sales | $ | 371.9 | | | $ | 308.6 | | | 21% | | $ | 714.5 | | | $ | 575.5 | | | 24% |
| Research and development expenses | 966.6 | | | 785.7 | | | 23% | | 1,755.7 | | | 1,528.3 | | | 15% |
| Acquired in-process research and development expenses | 4,449.1 | | | 110.5 | | | ** | | 4,525.9 | | | 457.6 | | | ** |
| Selling, general and administrative expenses | 372.2 | | | 262.6 | | | 42% | | 714.9 | | | 503.7 | | | 42% |
| Change in fair value of contingent consideration | 0.5 | | | (0.6) | | | ** | | 0.4 | | | (2.5) | | | ** |
| Total costs and expenses | $ | 6,160.3 | | | $ | 1,466.8 | | | 320% | | $ | 7,711.4 | | | $ | 3,062.6 | | | 152% |
| | | | | | | | | | | |
| | | | | | | ** Not meaningful |
Cost of Sales
Our cost of sales primarily consists of third-party royalties payable on net sales of our CF products as well as the cost of producing inventories. Pursuant to our agreement with the Cystic Fibrosis Foundation, our tiered third-party royalties on sales of TRIKAFTA/KAFTRIO, SYMDEKO/SYMKEVI, KALYDECO, and ORKAMBI, calculated as a percentage of net sales, range from the single digits to the sub-teens, with lower royalties on sales of TRIKAFTA/KAFTRIO than for our other products. Over the last several years, our cost of sales has been increasing due to increased net product revenues. Our cost of sales as a percentage of our net product revenues increased to 14% and 13% in the second quarter and first half of 2024, respectively, as compared to 12% in the second quarter and first half of 2023, primarily due to cost of sales associated with CASGEVY following its regulatory approval in the fourth quarter of 2023.
Research and Development Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages) |
| Research expenses | $ | 207.3 | | | $ | 170.7 | | | 21% | | $ | 403.4 | | | $ | 337.5 | | | 20% |
Development expenses | 759.3 | | | 615.0 | | | 23% | | 1,352.3 | | | 1,190.8 | | | 14% |
Total research and development expenses | $ | 966.6 | | | $ | 785.7 | | | 23% | | $ | 1,755.7 | | | $ | 1,528.3 | | | 15% |
Our research and development expenses include internal and external costs incurred for research and development of our products and product candidates. We do not assign our internal costs, such as salary and benefits, stock-based compensation expense, laboratory supplies and other direct expenses and infrastructure costs, to individual products or product candidates, because the employees within our research and development groups typically are deployed across multiple research and development programs. We assign external costs of services provided to us by clinical research organizations and other
outsourced research by individual program. Our internal costs are greater than our external costs. All research and development costs for our products and product candidates are expensed as incurred.
Since January 2022, we have incurred approximately $7.5 billion in total research and development expenses associated with product discovery and development. The successful development of our product candidates is highly uncertain and subject to a number of risks. In addition, the duration of clinical trials may vary substantially according to the type, complexity and novelty of the product candidate and the disease indication being targeted. The FDA and comparable agencies in foreign countries impose substantial requirements on the introduction of therapeutic pharmaceutical products, typically requiring lengthy and detailed laboratory and clinical testing procedures, sampling activities and other costly and time-consuming procedures. Data obtained from nonclinical and clinical activities at any step in the testing process may be adverse and lead to discontinuation or redirection of development activities. Data obtained from these activities also are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The duration and cost of discovery, nonclinical studies and clinical trials may vary significantly over the life of a project and are difficult to predict. Therefore, accurate and meaningful estimates of the ultimate costs to bring our product candidates to market are not available.
Any estimates regarding development and regulatory timelines for our product candidates are highly subjective and subject to change. Until we have data from Phase 3 clinical trials, we cannot make a meaningful estimate regarding when, or if, a clinical development program will generate revenues and cash flows.
Research Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages) |
| Research Expenses: | | | | | | | | | | | |
| Salary and benefits | $ | 61.9 | | | $ | 45.7 | | | 35% | | $ | 114.9 | | | $ | 91.2 | | | 26% |
| Stock-based compensation expense | 28.6 | | | 18.5 | | | 55% | | 58.5 | | | 38.7 | | | 51% |
| Outsourced services and other direct expenses | 67.0 | | | 60.6 | | | 11% | | 131.4 | | | 114.2 | | | 15% |
Infrastructure costs | 49.8 | | | 45.9 | | | 8% | | 98.6 | | | 93.4 | | | 6% |
Total research expenses | $ | 207.3 | | | $ | 170.7 | | | 21% | | $ | 403.4 | | | $ | 337.5 | | | 20% |
| | | | | | | | | | | |
| | | | | | | |
Our research expenses have been increasing over the last several years as we have invested in our pipeline and expanded our cell and genetic therapy capabilities, resulting in increased headcount, and outside services and other direct expenses. We expect to continue to invest in our research programs with a focus on creating transformative medicines for serious diseases.
Development Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages) |
| Development Expenses: | | | | | | | | | | | |
| Salary and benefits | $ | 167.2 | | | $ | 142.3 | | | 17% | | $ | 337.3 | | | $ | 286.5 | | | 18% |
| Stock-based compensation expense | 68.5 | | | 56.0 | | | 22% | | 158.0 | | | 112.1 | | | 41% |
| Compensation expense for cash-settled unvested Alpine equity awards | 151.9 | | | — | | | ** | | 151.9 | | | — | | | ** |
| Outsourced services and other direct expenses | 267.2 | | | 329.1 | | | (19)% | | 503.3 | | | 624.4 | | | (19)% |
Infrastructure costs | 104.5 | | | 87.6 | | | 19% | | 201.8 | | | 167.8 | | | 20% |
Total development expenses | $ | 759.3 | | | $ | 615.0 | | | 23% | | $ | 1,352.3 | | | $ | 1,190.8 | | | 14% |
| | | | | | | | | | | |
| | | | | | | ** Not meaningful |
Our development expenses increased by $144.3 million and $161.5 million, or 23% and 14%, in the second quarter and first half of 2024 as compared to the second quarter and first half of 2023, respectively, primarily due to compensation expense associated with cash-settled unvested Alpine equity awards as well as increased salaries and benefits, stock-based
compensation expense and infrastructure costs to support our clinical trials. These increases were partially offset by decreased outsourced services and other direct expenses.
We are investing significantly in internal headcount and in infrastructure to support our advancing pipeline. Additional headcount over the last several years has resulted in increased stock-based compensation expense. Our stock-based compensation expense has historically fluctuated and is expected to continue to fluctuate from one period to another based on the probability of achieving milestones associated with our performance-based awards. Our outsourced services and other direct expenses decreased as compared to the three and six months ended June 30, 2023 primarily due to lower clinical trial costs resulting from advancements in both our suzetrigine program in acute pain and the vanzacaftor triple for CF as well as the commercialization of CASGEVY. These decreased clinical trial expenses were partially offset by increased costs to support our T1D program.
Acquired In-process Research and Development Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages) |
| Acquired in-process research and development expenses | $ | 4,449.1 | | | $ | 110.5 | | | ** | | $ | 4,525.9 | | | $ | 457.6 | | | ** |
| | | | | | | ** Not meaningful |
AIPR&D in the three and six months ended June 30, 2024 was primarily related to $4.4 billion of AIPR&D resulting from our acquisition of Alpine, which was accounted for as an asset acquisition. AIPR&D in the first half of 2024 also included the $75.0 million milestone to Entrada from the first quarter of 2024. AIPR&D in the second quarter of 2023 was primarily related to a $70.0 million T1D research milestone to CRISPR and various other milestones. AIPR&D in the first half of 2023 also included our upfront payments of $225.1 million to Entrada and $100.0 million to CRISPR related to T1D from the first quarter of 2023. Our AIPR&D has historically fluctuated, and is expected to continue to fluctuate, from one period to another due to upfront, contingent milestone, and other payments pursuant to our existing and future business development transactions, including collaborations, licenses of third-party technologies, and asset acquisitions.
Selling, General and Administrative Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended June 30, | | | | Six Months Ended June 30, | | |
| 2024 | | 2023 | | Change | | 2024 | | 2023 | | Change |
| | | | | | | | | | | |
| (in millions, except percentages) |
| Selling, general and administrative expenses | $ | 372.2 | | | $ | 262.6 | | | 42% | | $ | 714.9 | | | $ | 503.7 | | | 42% |
Selling, general and administrative expenses increased by 42% in each of the second quarter and first half of 2024 as compared to the second quarter and first half of 2023, primarily due to increased investments to support the launches of our therapies globally and prepare for near-term launches of multiple potential new products. Our selling, general and administrative expenses also increased as compared to the second quarter and first half of 2023 as a result of compensation expense associated with cash-settled unvested Alpine equity awards.
Contingent Consideration
The fair value of our contingent consideration increased by $0.5 million and $0.4 million in the second quarter and first half of 2024, respectively, and decreased by $0.6 million and $2.5 million in the second quarter and first half of 2023, respectively.
Other Non-Operating Income (Expense), Net
Interest Income
Interest income increased to $156.5 million and $337.7 million in the second quarter and first half of 2024, respectively, as compared to $144.7 million and $267.3 million in the second quarter and first half of 2023, respectively, primarily due to the mix of our cash equivalents and available-for-sale debt securities. Our future interest income is dependent on the amount of, and prevailing market interest rates on, our outstanding cash equivalents and available-for-sale debt securities.
Interest Expense
Interest expense was $9.9 million and $11.2 million in the second quarter of 2024 and 2023, respectively, and $20.3 million and $22.6 million in the first half of 2024 and 2023, respectively. The majority of our interest expense in these periods was related to imputed interest expense associated with our leased corporate headquarters in Boston.
Other Income (Expense), Net
Other income (expense), net was expense of $23.1 million and income of $1.6 million in the second quarter of 2024 and 2023, respectively, and expense of $54.3 million and income of $2.9 million in the first half of 2024 and 2023, respectively. These amounts related primarily to net unrealized gains or losses resulting from changes in the fair value of our strategic equity investments, which consist of investments in our collaborators that may be public or private companies. To the extent that we continue to hold strategic equity investments in publicly traded companies, we expect that due to the volatility of the stock price of biotechnology companies, our other income (expense), net will fluctuate in future periods based on increases or decreases in the fair value of our strategic equity investments. As of June 30, 2024, the fair value of our investments in publicly traded companies was $30.6 million.
Income Taxes
Our effective tax rate fluctuates from period to period due to the global nature of our operations. The factors that most significantly impact our effective tax rate include changes in tax laws, variability in the allocation of our taxable earnings among multiple jurisdictions, the amount and characterization of our research and development expenses, the levels of certain deductions and credits, adjustments to the value of our uncertain tax positions, acquisitions and third-party collaboration and licensing transactions.
We recorded provisions for income taxes of $202.4 million and $245.8 million in the second quarter of 2024 and 2023, respectively, and $381.9 million and $437.5 million in the first half of 2024 and 2023, respectively. Our effective tax rate of (18.1)% in the first half of 2024 was materially different than the U.S. statutory rate primarily due to the $4.4 billion of AIPR&D resulting from our acquisition of Alpine, which drove our pre-tax loss in each of these periods. Our effective tax rate of 21.3% in the first half of 2023 was similar to the U.S. statutory rate.
LIQUIDITY AND CAPITAL RESOURCES
The following table summarizes the components of our financial condition as of June 30, 2024 and December 31, 2023:
| | | | | | | | | | | | | | | | | |
| As of June 30, 2024 | | As of December 31, 2023 | | Change |
| | | | | |
| (in millions, except percentages) |
| Cash, cash equivalents and marketable securities: | | | | | |
| Cash and cash equivalents | $ | 4,580.1 | | | $ | 10,369.1 | | | |
| Marketable securities | 1,215.4 | | | 849.2 | | | |
| Long-term marketable securities | 4,393.1 | | | 2,497.8 | | | |
| Total cash, cash equivalents and marketable securities | $ | 10,188.6 | | | $ | 13,716.1 | | | (26)% |
| | | | | |
| Working Capital: | | | | | |
| Total current assets | $ | 8,941.6 | | | $ | 14,144.2 | | | (37)% |
| Total current liabilities | (3,547.2) | | | (3,547.4) | | | —% |
| Total working capital | $ | 5,394.4 | | | $ | 10,596.8 | | | (49)% |
Working Capital
As of June 30, 2024, total working capital was $5.4 billion, which represented a decrease of $5.2 billion from $10.6 billion as of December 31, 2023 primarily due to the cash consideration paid to acquire Alpine.
Cash Flows
| | | | | | | | | | | |
| Six Months Ended June 30, |
| 2024 | | 2023 |
| | | |
| (in millions) |
| Net cash provided by (used in): | | | |
| Operating activities | $ | (2,447.0) | | | $ | 2,034.3 | |
| Investing activities | $ | (2,600.1) | | | $ | (2,137.5) | |
| Financing activities | $ | (716.1) | | | $ | (277.4) | |
Operating Activities
Cash used in operating activities was $2.4 billion in the first half of 2024 primarily due to our acquisition of Alpine partially offset by cash flows provided by other operating activities. Cash provided by operating activities was $2.0 billion in the first half of 2023 primarily due to income from operations of $1.8 billion driven by our net product revenues.
Investing Activities
Cash used in investing activities were $2.6 billion and $2.1 billion in the first half of 2024 and 2023, respectively. The largest portion of our investing activities in each of these periods were net purchases of available-for-sale debt securities.
Financing Activities
Cash used in financing activities were $716.1 million and $277.4 million in the first half of 2024 and 2023, respectively. Our financing activities in each of these periods were primarily related to repurchases of our common stock pursuant to our share repurchase program and payments related to our employee stock benefit plans.
Sources and Uses of Liquidity
We intend to rely on our existing cash, cash equivalents and current marketable securities together with cash flows from product sales as our primary source of liquidity. We expect that cash flows from our product sales together with our cash, cash equivalents and current marketable securities will be sufficient to fund our operations for at least the next twelve months. The adequacy of our available funds to meet our future operating and capital requirements will depend on many factors, including our future product sales, and the potential introduction of one or more of our other product candidates to the market, our business development activities, and the number, breadth, cost and prospects of our research and development programs.
Credit Facilities & Financing Strategy
We may borrow up to a total of $500.0 million pursuant to a revolving credit facility that we entered into in July 2022 and could repay and reborrow amounts under this revolving credit agreement without penalty. Subject to certain conditions, we could request that the borrowing capacity be increased by an additional $500.0 million, for a total of $1.0 billion. Negative covenants in our credit agreement could prohibit or limit our ability to access this source of liquidity. As of June 30, 2024, the facility was undrawn, and we were in compliance with these covenants.
We may also raise additional capital by borrowing under credit agreements, through public offerings or private placements of our securities or securing new collaborative agreements or other methods of financing. We will continue to manage our capital structure and will consider all financing opportunities, whenever they may occur, that could strengthen our long-term liquidity profile. There can be no assurance that any such financing opportunities will be available on acceptable terms, if at all.
Future Capital Requirements
We have significant future capital requirements, including:
•Expected operating expenses to conduct research and development activities, manufacture and commercialize our existing and future products, and to operate our organization.
•Cash that we pay for income taxes.
•Royalties we pay related to sales of our CF products.
•Facility, operating and finance lease obligations.
•Firm purchase obligations related to our supply and manufacturing processes.
In addition, other potential significant future capital requirements may include:
•We have entered into certain business development-related and strategic agreements with third parties that include the funding of certain research, development, manufacturing and commercialization efforts. Certain of our transactions, including collaborations, licensing arrangements, and asset acquisitions, include the potential for future milestone and royalty payments by us upon the achievement of pre-established developmental and regulatory targets and/or commercial targets. Other transactions include the potential for future lease-related expenses and other costs. Our obligation to fund these research and development and commercialization efforts and to pay these potential milestones, expenses and royalties is contingent upon continued involvement in the programs and/or the lack of any adverse events that could cause their discontinuance. We may enter into additional business development transactions and strategic agreements, including acquisitions, collaborations, licensing arrangements and equity investments, which require additional capital.
•To the extent we borrow amounts under our existing credit agreement, we would be required to repay any outstanding principal amounts in 2027.
•As of June 30, 2024, we had $2.1 billion remaining authorization available under our Share Repurchase Program that our Board of Directors approved in February 2023. We expect to fund repurchases of our common stock through a combination of cash on hand and cash generated by operations. This program does not have an expiration date and can be discontinued at any time.
There have not been any material changes to our future capital requirements disclosed in our Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the Securities and Exchange Commission, or SEC, on February 15, 2024.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and results of operations are based upon our condensed consolidated financial statements prepared in accordance with generally accepted accounting principles in the U.S. The preparation of these financial statements requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amounts of revenues and expenses during the reported periods. These items are monitored and analyzed by management for changes in facts and circumstances, and material changes in these estimates could occur in the future. Changes in estimates are reflected in reported results for the period in which the change occurs. We base our estimates on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from our estimates if past experience or other assumptions do not turn out to be substantially accurate. During the six months ended June 30, 2024, there were no material changes to our critical accounting policies as reported in our Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the SEC on February 15, 2024.
RECENT ACCOUNTING PRONOUNCEMENTS
For a discussion of recent accounting pronouncements, please refer to Note A, “Basis of Presentation and Accounting Policies.”
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Information required by this item is incorporated by reference from the discussion in Part II, Item 7A, “Quantitative and Qualitative Disclosures About Market Risk,” of our Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the SEC on February 15, 2024.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management (under the supervision and with the participation of our chief executive officer and chief financial officer), after evaluating the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this Quarterly Report on Form 10-Q, has concluded that, based on such evaluation, as of June 30, 2024 our disclosure controls and procedures were effective and designed to provide reasonable assurance that the information required to be disclosed is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. In designing and evaluating our disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Changes in Internal Controls Over Financial Reporting
No change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended) occurred during the three months ended June 30, 2024 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II. Other Information
Item 1. Legal Proceedings
We are not currently subject to any material legal proceedings.
Item 1A. Risk Factors
The information presented below supplements the risk factors set forth in Part I, Item 1A. “Risk Factors” of our Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the SEC on February 15, 2024.
We may be unable to successfully integrate Alpine’s business which could adversely affect our business and financial condition.
Our inability to successfully integrate Alpine could have a material adverse effect on our business. Our realization of the value from the acquisition of Alpine relies on successful integration, continued operations of the Alpine business and continued successful development of the pipeline products and candidates that we acquired. We may not be able to make Alpine’s business profitable, retain key employees or realize anticipated cost savings or synergies, if any, from this acquisition, which could adversely affect our business and financial condition.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q and, in particular, our Management’s Discussion and Analysis of Financial Condition and Results of Operations set forth in Part I, Item 2, contain a number of forward-looking statements. Forward-looking statements are not purely historical and may be accompanied by words such as “anticipates,” “may,” “forecasts,” “expects,” “intends,” “plans,” “potentially,” “believes,” “seeks,” “estimates,” and other words and terms of similar meaning. Such statements may relate to:
•our expectations regarding the amount of, timing of, and trends with respect to our financial performance, including revenues, costs and expenses, and other gains and losses;
•our expectations regarding our clinical trials and pipeline programs, including expectations for patient enrollment, development timelines, the expected timing of data from our ongoing and planned clinical trials, regulatory authority filings and other submissions for our therapies, communications with regulatory authorities and anticipated regulatory approvals;
•our ability to maintain and obtain adequate reimbursement for our products, our ability to launch, commercialize and market our products or any of our other therapies for which we obtain regulatory approval and our ability to obtain label expansions for existing therapies;
•our expectations regarding our ability to continue to grow our CF business by increasing the number of people with CF eligible and able to receive our medicines, providing improved treatment options for people who are already eligible for one of our medicines, and pursuing genetic therapies for people with CF who cannot currently benefit from our medicines;
•the data that will be generated by ongoing and planned clinical trials and the ability to use that data to advance compounds, continue development or support regulatory filings;
•our beliefs regarding the support provided by clinical trials and preclinical and nonclinical studies of our therapies for further investigation, clinical trials or potential use as a treatment;
•our plans to continue investing in our research and development programs, including anticipated timelines for our programs, and our strategy to develop our pipeline programs, alone or with third party-collaborators;
•our beliefs regarding the approximate patient populations for the disease areas on which we focus;
•plans for and prospects of our business development activities, including the potential benefits and therapeutic scope of our collaborations, our ability to integrate and continue operations of acquired businesses, and our ability to successfully capitalize on these opportunities;
•the establishment, development and maintenance of collaborative relationships, including potential milestone payments or other obligations;
•potential business development activities, including the identification of potential collaborative partners or acquisition targets;
•our ability to expand and protect our intellectual property portfolio and otherwise maintain exclusive rights to products;
•potential fluctuations in foreign currency exchange rates and the effectiveness of our foreign currency management program;
•our expectations regarding cash generated by operations, our cash balance and expected generation and interest income;
•our expectations regarding our provision for or benefit from income taxes and the utilization of our deferred tax assets;
•our ability to use our research programs to identify and develop new product candidates to address serious diseases and significant unmet medical needs;
•our plans to build and maintain our global supply chains and manufacturing infrastructure and capabilities, including for cell and gene therapies; and
•our liquidity and our expectations regarding the possibility of raising additional capital.
Forward-looking statements are subject to certain risks, uncertainties, or other factors that are difficult to predict and could cause actual events or results to differ materially from those indicated in any such statements. These risks, uncertainties, and other factors include, but are not limited to, those described in our “Risk Factors” in Item 1A of our Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the SEC on February 15, 2024, and those described from time to time in our future reports filed with the Securities and Exchange Commission.
Any such forward-looking statements are made on the basis of our views and assumptions as of the date of the filing and are not estimates of future performance. Except as required by law, we undertake no obligation to publicly update any forward-looking statements. The reader is cautioned not to place undue reliance on any such statements.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
Issuer Repurchases of Equity Securities
In February 2023, our Board of Directors approved a share repurchase program (our “Share Repurchase Program”), pursuant to which we are authorized to repurchase up to $3.0 billion of our common stock. Our Share Repurchase Program does not have an expiration date and can be discontinued at any time. The table set forth below shows repurchases of securities by us during the three months ended June 30, 2024 under our Share Repurchase Program.
| | | | | | | | | | | | | | | | | | | | | | | |
| Period | Total Number
of Shares Purchased | | Average Price
Paid per Share | | Total Number of Shares
Purchased as Part of
Publicly Announced
Plans or Programs (1) | | Approximate Dollar Value
of Shares that May Yet be
Purchased Under the
Plans or Programs (1) |
April 1, 2024 to April 30, 2024 | 306,000 | | | $ | 400.75 | | | 306,000 | | | $ | 2,309,344,013 | |
May 1, 2024 to May 31, 2024 | 240,000 | | | $ | 428.13 | | | 240,000 | | | $ | 2,206,593,837 | |
June 1, 2024 to June 30, 2024 | 190,000 | | | $ | 475.84 | | | 190,000 | | | $ | 2,116,184,531 | |
| Total | 736,000 | | | $ | 429.06 | | | 736,000 | | | $ | 2,116,184,531 | |
(1) Under our Share Repurchase Program, we are authorized to purchase shares from time to time through open market or privately negotiated transactions. Such purchases may be pursuant to Rule 10b5-1 plans or other means as determined by our management and in accordance with the requirements of the Securities and Exchange Commission.
Item 5. Other Information
Rule 10b5-1 Trading Plans
Our policy governing transactions in our securities by our directors, officers, and employees permits our officers, directors and employees to enter into trading plans complying with Rule 10b5-1 under the Securities Exchange Act of 1934, as amended. The following table describes the written plans for the sale of our securities adopted by our executive officers and directors during the second quarter of 2024, each of which is intended to satisfy the affirmative defense conditions of Rule 10b5-1 (each, a “Trading Plan”).
| | | | | | | | | | | | | | | | | | | | |
| Name and Title | | Date of Adoption of Trading Plan | | Scheduled Expiration Date of Trading Plan (1) | | Maximum Shares Subject to Trading Plan |
Jeffrey Leiden Executive Chairman | | 5/10/2024 | | 8/31/2025 | | 11,355 |
Sangeeta Bhatia Director | | 5/09/2024 | | 5/09/2025 | | 2,339 |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| (1) A Trading Plan may expire on an earlier date if all contemplated transactions are completed before such Trading Plan’s expiration date, upon termination by broker or the holder of the Trading Plan, or as otherwise provided in the Trading Plan. |
Item 6. Exhibits
| | | | | |
Exhibit Number | Exhibit Description |
| 10.1 | |
| 31.1 | |
| 31.2 | |
| 32.1 | |
| 101.INS | XBRL Instance - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| 101.SCH | XBRL Taxonomy Extension Schema |
| 101.CAL | XBRL Taxonomy Extension Calculation |
| 101.LAB | XBRL Taxonomy Extension Labels |
| 101.PRE | XBRL Taxonomy Extension Presentation |
| 101.DEF | XBRL Taxonomy Extension Definition |
| 104 | Cover Page Interactive Data File––the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| |
| |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | | | | |
| Vertex Pharmaceuticals Incorporated |
| | |
| August 2, 2024 | By: | /s/ Charles F. Wagner, Jr. |
| | Charles F. Wagner, Jr. |
| | Executive Vice President, Chief Financial Officer
(principal financial officer and
duly authorized officer) |