Use these links to rapidly review the document
ImmunoGen, Inc. Form 10-K TABLE OF CONTENTS
Item 8. Financial Statements and Supplementary Data
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the fiscal year ended June 30, 2013 |
||
OR |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
||
Commission file number 0-17999
ImmunoGen, Inc.
| Massachusetts (State or other jurisdiction of incorporation or organization) |
04-2726691 (I.R.S. Employer Identification No.) |
|
830 Winter Street, Waltham, MA 02451 (Address of principal executive offices, including zip code) |
||
(781) 895-0600 (Registrant's telephone number, including area code) |
||
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered | |
|---|---|---|
| Common Stock, $.01 par value | NASDAQ Global Select Market |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ý Yes o No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. o Yes ý No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ý Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ý Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ý | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). o Yes ý No
Aggregate market value, based upon the closing sale price of the shares as reported by the NASDAQ Global Select Market, of voting stock held by non-affiliates at December 31, 2012: $1,070,664,433 (excludes shares held by executive officers and directors). Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of management or policies of the registrant, or that such person is controlled by or under common control with the registrant. Common Stock outstanding at August 20, 2013: 85,109,108 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive Proxy Statement to be delivered to shareholders in connection with the Annual Meeting of Shareholders to be held on November 12, 2013 are incorporated by reference into Part III.
ImmunoGen, Inc.
Form 10-K
TABLE OF CONTENTS
2
In this Annual Report on Form 10-K, ImmunoGen, Inc. (ImmunoGen, Inc., together with its subsidiaries, is referred to in this document as "we", "us", "ImmunoGen", or the "Company"), incorporates by reference certain information from parts of other documents filed with the Securities and Exchange Commission. The Securities and Exchange Commission allows us to disclose important information by referring to it in that manner. Please refer to all such information when reading this Annual Report on Form 10-K. All information is as of June 30, 2013 unless otherwise indicated. For a description of the risk factors affecting or applicable to our business, see "Risk Factors," below.
Overview
We are a biotechnology company that develops targeted anticancer therapeutics. All of our wholly owned clinical and preclinical product candidates are antibody-drug conjugates, or ADCs, which use a monoclonal antibody to deliver a small molecule to targeted cells.
We developed our Targeted Antibody Payload, or TAP, ADC technology to enable the creation of highly effective, well-tolerated anticancer products. A TAP compound consists of an antibody that binds specifically to an antigen found on the targeted cancer cells with one of our potent cancer-cell killing agents attached using one of our engineered linkers. The antibody component enables a TAP compound to target cancer cells expressing its antigen, and the highly potent cell-killing agent serves to kill these cells. Our linkers are engineered to keep the cell-killing agent securely attached to the antibody while traveling through the bloodstream, and then control its release and activation once inside a cancer cell. Depending on the target, the antibody component of the TAP compound may serve only as a targeting vehicle or it may also have anticancer activity.
We develop our own product candidates using our TAP technology with antibodies from our research programs. We now have four wholly owned, clinical-stage anticancer compounds—IMGN901, IMGN853, IMGN289, and IMGN529. We also license to other companies limited rights to use our technology with their antibodies to create products. The most advanced compound with our TAP technology is Kadcyla® (ado-trastuzumab emtansine), which was launched in the U.S.and Switzerland earlier this year by Genentech, a member of the Roche Group. Kadcyla comprises Roche's trastuzumab antibody, which is the active ingredient of their Herceptin® product, with our DM1 cell-killing agent attached using our thioether engineered linker. Six other TAP compounds and one unconjugated, or "naked," antibody product candidate are in clinical testing through our partnerships. Our partnership agreements entitle us to earn royalties on product sales, if any, and milestone payments with agreed upon achievements. Our current partners are: Amgen Inc., Bayer HealthCare (a subgroup of Bayer AG), Biotest AG, Eli Lilly and Company, or Lilly, Novartis Institutes for BioMedical Research, Inc., or Novartis, the Roche Group and Sanofi. We began receiving royalties on Kadcyla sales in the fourth quarter of our 2013 fiscal year.
We were organized as a Massachusetts corporation in 1981. Our principal offices are located at 830 Winter Street, Waltham, Massachusetts (MA) 02451, and our telephone number is (781) 895-0600. We maintain a website at www.immunogen.com, where certain information about us is available. Please note that information contained on the website is not a part of this document. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and any amendments to those reports are available free of charge through the "Investor Information" section of our website as soon as reasonably practicable after those materials have been electronically filed with, or furnished to, the Securities and Exchange Commission. We have adopted a Code of Corporate Conduct that applies to all our directors, officers and employees and a Senior Officer and Financial Personnel Code of Ethics that applies to our senior officers and financial personnel. Our Code of Corporate Conduct and Senior Officer and Financial Personnel Code of Ethics are available free of charge through the "Investor Information" section of our website.
3
Pipeline: Kadcyla and Earlier-Stage Product Candidates
Listed in the tables below are the compounds in the clinic through our own programs and our collaborations with other companies. Additional earlier-stage compounds are in development by us and our partners. The results in early clinical trials may not be predictive of results obtained in subsequent clinical trials and there can be no assurance that all of our or our collaborators' product candidates will advance or will demonstrate the level of safety and efficacy necessary to obtain regulatory approval.
Compounds Wholly Owned by ImmunoGen
Compound
|
Lead Indication(s) | Target | Most Advanced Status | |||||
|---|---|---|---|---|---|---|---|---|
IMGN901 |
Small-cell lung cancer | CD56 | Phase II | |||||
IMGN853 |
Ovarian cancer, non-small cell lung cancer | Folate receptor a | Phase I | |||||
IMGN289 |
Head and neck cancer, non-small cell lung cancer | EGFR | Active IND* | |||||
IMGN529 |
Non-Hodgkin lymphoma | CD37 | Phase I | |||||
Collaborative Partner Compounds**
Compound
|
Lead Indication(s) | Target | Partner | Most Advanced Status |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
Kadcyla |
Previously treated HER2-positive metastatic breast cancer | HER2 | Roche | Marketed | ||||||
SAR3419 |
DLBCL, B-ALL*** | CD19 | Sanofi | Phase II | ||||||
SAR566658 |
Solid tumors | CA6 | Sanofi | Phase I | ||||||
SAR650984 |
Multiple myeloma | CD38 | Sanofi | Phase I | ||||||
AMG 172 |
Kidney cancer | CD70 | Amgen | Phase I | ||||||
AMG 595 |
Glioblastoma | EGFRvIII | Amgen | Phase I | ||||||
BAY 94-9343 |
Mesothelioma, ovarian cancer | Mesothelin | Bayer | Phase I | ||||||
BT-062 |
Multiple myeloma | CD138 | Biotest | Phase I | ||||||
- *
- IND: Investigational New Drug Application
- **
- All are ADCs, except for SAR650984, which is a "naked" antibody therapeutic
- ***
- DLBCL: diffuse large B-cell lymphoma; B-ALL: B-cell acute lymphoblastic leukemia
Kadcyla (previously referred to as T-DM1)
Kadcyla is an ADC that consists of trastuzumab, which is the active component of Roche's antibody therapeutic, Herceptin (trastuzumab), with our DM1 cell-killing agent attached using our thioether engineered linker. Kadcyla is in global development by Roche, under a license from us.
Kadcyla was granted marketing approval in February 2013 by the U.S. Food and Drug Administration, or FDA. It is approved for the treatment of HER2-positive metastatic breast cancer in patients who previously received Herceptin and a taxane. In the EMILIA Phase III trial, Kadcyla was found to significantly improve both overall survival and progression-free survival compared to standard of care. These were the co-primary endpoints of the trial. Kadcyla was also associated with fewer Grade 3 or greater adverse events, which are more severe side effects.
Roche also has applied for marketing approval of Kadcyla in the European Union and in Japan, with decisions there expected in late 2013 and in 2014, respectively.
4
Roche is developing Kadcyla for a number of additional indications, including for patients with
- •
- Metastatic HER2-positive breast cancer that has not previously been treated—Roche is assessing Kadcyla for
this use in the Phase III trial, MARIANNE. Roche intends to use MARIANNE results, if favorable, to apply in 2015 for marketing approval of Kadcyla for this indication, both used alone and used
with Perjeta® (pertuzumab). Roche expects the data from this trial in 2014.
- •
- Early stage HER2-positive breast cancer—Roche plans to evaluate Kadcyla in several early stage HER2-positive
breast cancer settings. In early 2013, patient enrollment commenced in its KATHERINE Phase III trial, which evaluates Kadcyla for the treatment of patients with residual invasive disease
following pre-operative therapy. Roche has said it plans to initiate registration trials evaluating Kadcyla for use in the adjuvant and neoadjuvant settings.
- •
- Advanced HER2-positive gastric cancer—Roche is evaluating Kadcyla for the treatment of patients with this cancer in its GATSBY trial. Roche intends to use the results from GATSBY, if favorable, to apply in 2015 for marketing approval for this use.
IMGN901
Our most advanced wholly owned product candidate is IMGN901, or lorvotuzumab mertansine. We created IMGN901 for the treatment of CD56-positive cancers, which include small cell lung cancer, or SCLC, Merkel cell carcinoma, multiple myeloma, ovarian cancers, carcinoid tumors, and other cancers of neuroendocrine origin. IMGN901 consists of a CD56-targeting antibody with our DM1 cell-killing agent attached using one of our hindered disulfide linkers.
In early clinical testing, IMGN901 has been found to be generally well tolerated and to demonstrate evidence of activity when used as a single agent to treat CD56-positive solid tumors and multiple myeloma. IMGN901 also has been found to be generally well tolerated and to demonstrate evidence of activity when used in combination with Revlimid® (lenalidomide) and dexamethasone for treating CD56-positive multiple myeloma.
In preclinical models of SCLC, use of IMGN901 in combination with etoposide plus carboplatin, or E/C, was found to achieve markedly greater anticancer activity than either IMGN901 or E/C alone. E/C is a standard of care for the treatment of newly diagnosed SCLC. Based on the encouraging activity seen both preclinically and in early clinical testing, we are assessing IMGN901 for the treatment of patients with newly diagnosed extensive disease SCLC in our Phase II NORTH trial. Patients in the NORTH trial receive treatment with IMGN901 plus E/C or with E/C alone. We expect to make certain development-related decisions based on the results from this trial.
IMGN853
We created our IMGN853 product candidate to treat cancers that highly express folate receptor a, or FRa. FRa-positive cancers include many cases of ovarian cancer, endometrial cancer and adenocarcinoma non-small cell lung cancer, or NSCLC. IMGN853 consists of a FRa-targeting antibody with our potent DM4 cell-killing agent attached using one of our linkers engineered to counteract one drug resistance pathway that cancer cells can develop.
IMGN853 is currently in Phase I clinical testing. Once the maximum tolerated dose, or MTD, has been established in the dose-finding phase of the trial, we plan to evaluate IMGN853 used at that dose in the expansion phase of the trial. There, IMGN853 will be evaluated specifically in patients with either FRa-positive ovarian cancer, endometrial cancer or adenocarcinoma NSCLC. The first IMGN853 clinical data were reported at a medical conference in June 2013. The compound was found to be generally well tolerated and to demonstrate evidence of activity.
5
IMGN289
Our IMGN289 product candidate is a potential new treatment for cancers that highly express EGFR. EGFR-positive cancers include squamous cell carcinoma of the head and neck, or SCCHN, and types of NSCLC. IMGN289 consists of our EGFR-binding antibody with our DM1 cell-killing agent attached using our thioether linker. In preclinical testing, the antibody component of IMGN289 was found to have meaningful anticancer activity against EGFR-positive cancer cells sensitive to EGFR inhibition. The full product candidate, which includes DM1, demonstrates superior activity against these cancers, and also against EFGR-positive cancers not sensitive to EGFR inhibitors. The DM1 enables IMGN289 to kill EGFR-positive cancer cells by a second method that is independent of the sensitivity of these cells to EGFR inhibitors.
We submitted an IND for IMGN289 to the FDA in late June 2013; it became active in late July 2013. We are preparing to begin Phase I testing of the compound.
IMGN529
Our IMGN529 TAP compound targets CD37, which is expressed on B-cell malignancies such as non-Hodgkin lymphoma, or NHL, and on chronic lymphocytic leukemia. ImmunoGen scientists have found the expression profile of CD37 on NHL subtypes to be similar to that of CD20, the target of Rituxan®.
IMGN529 comprises an antibody that, in preclinical testing, has demonstrated meaningful anticancer activity, our DM1 cell-killing agent, and our thioether engineered linker. We believe IMGN529 is a highly differentiated product candidate for B-cell malignancies because it combines the anticancer activity of its antibody component with the actions of one of our potent cell-killing agents. IMGN529 is in Phase I clinical testing for the treatment of NHL.
Compounds in Development by Our Partners
In addition to Kadcyla, seven other compounds are in clinical testing through our collaborations with other companies. Additionally, several of our collaborative partners have TAP compounds in earlier stages of development.
Sanofi
Each of the three clinical-stage Sanofi compounds—SAR3419, SAR566658 and SAR650984—was created as part of a broader research collaboration between ImmunoGen and Sanofi. The antibodies in all three compounds were developed by ImmunoGen scientists. The two TAP compounds, SAR3419 and SAR566658, contain our DM4 cell-killing agent attached with one of our hindered disulfide linkers. Sanofi has additional compounds created under that agreement in earlier stages of development.
SAR3419 targets CD19 and is a potential treatment for CD19-expressing B-cell malignancies. Sanofi is evaluating SAR3419 in Phase II clinical testing for both a type of NHL called diffuse large B-cell lymphoma, or DLBCL, and in B-cell acute lymphoblastic leukemia, or B-ALL. In Phase I clinical testing, SAR3419 showed encouraging efficacy and tolerability in the treatment of NHL previously treated with approved anticancer agents.
SAR566658 is a TAP compound in development as a potential treatment for CA6-expressing solid tumors, including ovarian cancers. Sanofi is evaluating SAR566658 in Phase I clinical testing.
SAR650984 is a CD38-targeting therapeutic, or "naked", antibody created by ImmunoGen for the treatment of hematological malignancies. It is in Phase I clinical testing for the treatment of blood cancers including multiple myeloma.
6
Amgen
Pursuant to the terms of a separate right-to-test agreement entered into in 2000, Amgen took licenses in 2009 for rights to use our TAP technology to develop therapeutics targeting CD70 and EGFRvIII and has since advanced product candidates AMG 172 and AMG 595 into clinical testing. In late 2012 and in early 2013, Amgen took licenses under the same right-to-test agreement for rights to use our TAP technology to develop therapeutics to two additional targets, which are undisclosed. Amgen has no remaining rights under the right-to-test agreement to take licenses to additional targets.
AMG 172 is a potential treatment for CD70-expressing cancers, and is in Phase I clinical testing for the treatment of patients with clear cell renal cell carcinoma. AMG 595 is a potential treatment for EGFRvIII-expressing cancers. It is in Phase I clinical testing for the treatment of patients with glioblastoma.
Bayer
BAY 94-9343 was created by Bayer under a single-target license agreement that granted Bayer rights to use our technology to develop TAP compounds to the target, mesothelin, and is currently in Phase I clinical testing.
The first clinical data for BAY 94-9343 were reported at a scientific conference in early 2013. These data were from the dose-escalation part of its Phase I trial and showed that the compound was generally well tolerated and demonstrated evidence of activity among patients with mesothelin-expressing solid tumors treated at higher dose levels. The compound is now being evaluated in the expansion phase of the trial specifically in patients with mesothelioma and in patients with ovarian cancer. It has been granted orphan drug status for the treatment of mesothelioma.
Biotest
BT-062 was created by Biotest under a single-target license agreement that granted Biotest rights to use our TAP technology with antibodies that target CD138, an antigen found on multiple myeloma and certain solid tumors. We have opt-in rights with respect to BT-062 in the U.S.
Encouraging Phase I clinical data have been reported with BT-062 used as a single agent to treat patients with multiple myeloma. Biotest is assessing BT-062 used as part of a combination regimen for this cancer. In preclinical testing, BT-062 demonstrated activity against several types of aggressive solid tumors. It is expected to advance into clinical testing for the treatment of one or more types of solid tumors.
Lilly and Novartis
Our most recent partnerships are with Lilly and Novartis. Compounds are in preclinical development under these agreements.
Incidence of Relevant Cancers
Cancer remains a leading cause of death worldwide, and is the second leading cause of death in the U.S. The American Cancer Society, or ACS, estimates that in 2013 approximately 1.7 million new cases of cancer will be diagnosed in the U.S. and that approximately 580,000 people will die from the disease. The total number of people living with cancer significantly exceeds the number of patients diagnosed with cancer in a given year as patients can live with cancer for a year or longer. Additionally, the potential market for anticancer drugs exceeds the number of patients treated as many types of cancer typically are treated with multiple compounds at the same time and because patients often receive a number of drug regimens sequentially.
7
Below is information about incidence of cancers we are seeking to treat with our wholly owned compounds. For the approved product, Kadcyla, information about the incidence of HER2-positive breast cancer is available from Roche, the marketer of the product.
IMGN901—The lead indication for IMGN901is for the treatment of extensive disease SCLC. Based on our own studies and scientific literature, we believe that CD56 is expressed on almost all cases of SCLC. Based on ACS estimates and other sources, we believe that at least 22,800 new cases of SCLC will be diagnosed in the U.S. in 2013. SCLC tends to spread broadly through the body quite early in the course of the disease, and—according to the ACS—approximately two-thirds of patients with SCLC have extensive disease at the time of diagnosis.
IMGN853—Our IMGN853 compound is a potential treatment for many cases of epithelial ovarian cancer, endometrial cancer and adenocarcinoma NSCLC. Based on published sources, we believe approximately 22,000 new cases of ovarian cancer will be diagnosed in the US in 2013 and epithelial ovarian cancer accounts for approximately 85% to 90% of these ovarian cancer cases. We believe that approximately 49,600 cases of endometrial cancers will be diagnosed in the US in 2013. Additionally, based on published sources, we believe that approximately 194,000 new cases of NSCLC will be diagnosed in the US in 2013, and that approximately 40% of these cases are the adenocarcinoma subtype.
IMGN289—Our IMGN289 compound is a potential treatment for many cases of head and neck cancer and types of NSCLC. The ACS estimates that approximately 53,600 new cases of head and neck cancers will be diagnosed in 2013. Research conducted at ImmunoGen found that over 90% of these types of cancer strongly express EGFR. Based on ACS estimates, we believe approximately 194,000 new cases of NSCLC will be diagnosed in the U.S. in 2013. This figure comprises three main subtypes—adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. These subtypes account for approximately 40%, 25-30%, and 10-15% of NSCLC diagnoses, respectively. Research with tumor samples conducted at ImmunoGen found that approximately 20% of adenocarcinoma cases and about half of squamous and of large cell carcinoma cases strongly express EGFR.
IMGN529—We are assessing our IMGN529 compound for the treatment of NHL. Based on ACS estimates, we believe approximately 69,700 new cases of NHL will be diagnosed in the U.S. in 2013.
Out-licenses and Collaborations
We selectively out-license restricted access to our TAP technology to other companies to provide us with cash to fund our own product programs and to expand the utilization of our technology. These agreements typically provide the licensee with rights to use our TAP technology with antibodies to a defined target to develop products. The licensee is generally responsible for the development, clinical testing, manufacturing, registration and commercialization of any resulting product candidate. As part of these agreements, we are generally entitled to receive upfront fees, potential milestone payments, royalties on the sales of any resulting products and research and development funding based on activities performed at our collaborative partner's request. We are also compensated for preclinical and clinical materials supplied to our partners.
We only receive royalty payments from our out-licenses after a product candidate developed under the license has been approved for marketing and commercialized. Additionally, the largest milestone payments under our existing collaborations usually are on later-stage events, such as commencement of pivotal clinical trials and product approval. Achievement of product approval requires, at a minimum, favorable completion of preclinical development and evaluation, assessment of early-stage clinical trials, advancement into pivotal Phase II and/or Phase III clinical testing, completion of this later-stage clinical testing with favorable results, and completion of regulatory submissions and a positive regulatory decision. Currently, we have a license with Roche relating to Kadcyla that provides us with royalty revenue and may provide us with significant milestone payments in the foreseeable future.
8
Below is a table setting forth our active agreements, the number of targets licensed and current status of the product candidates being developed thereunder:
Partner
|
Agreement Type | Effective Date(s) | Development Status(1) | |||
|---|---|---|---|---|---|---|
| Roche(2) | Multiple single-targets | 2000 | US Marketing Approval | |||
| Amgen(3) | Multiple single-targets | 2000 | Phase I | |||
| Sanofi | Multiple single-targets | 2003 | Phase II | |||
| Sanofi(4) | Right-to-test | 2006 | Research/Preclinical | |||
| Biotest | Single-target | 2006 | Phase I | |||
| Bayer HealthCare | Single-target | 2008 | Phase I | |||
| Novartis(4) | Right-to-test | 2010 | Research/Preclinical | |||
| Lilly(4) | Right-to-test | 2011 | Research/Preclinical |
- (1)
- For
agreements involving multiple targets, development status denotes the most advanced program under the collaboration.
- (2)
- Roche
has five single-target licenses. Pursuant to the license covering the target HER2, which was entered into in 2000, a product candidate,
Kadcyla, has received marketing approval in the US and Switzerland and Roche has submitted a marketing application for the compound in the EU and Japan. The remaining four licenses were entered into
between 2005 and 2008, and the development status of product candidates under each of those licenses is research/preclinical.
- (3)
- Amgen
has three exclusive, single-target licenses and one non-exclusive, single-target license.
- (4)
- Sanofi, Novartis and Lilly each have the right to take a defined number of exclusive, single-target options that provide the right to take single-target licenses, on pre-negotiated terms, to specified targets during the respective option periods. As of June 30, 2013, Novartis has taken one license to two related targets, one on an exclusive basis and one on a non-exclusive basis. In August 2013, Lilly took an exclusive license to a single target.
Roche
In May 2000, we granted Roche, through its Genentech unit, an exclusive development and commercialization license to our maytansinoid TAP technology for use with antibodies or other proteins that target HER2, such as trastuzumab. In February 2013, the US FDA granted marketing approval to the anti-HER2 TAP compound, Kadcyla. We received a $2 million upfront payment from Roche upon execution of the agreement. We are also entitled to receive up to a total of $44 million in milestone payments, plus tiered royalties on the commercial sales of Kadcyla or any other resulting products as described below.
The royalty term is determined on a country-by-country basis, and is initially 10 years from the date of first commercial sale of Kadcyla in the country. If, on such 10th anniversary, Kadcyla is covered by a valid claim under any patents controlled by us (excluding patents jointly owned by us and Genentech), then royalties remain payable on sales of Kadcyla in that country for an additional 2 years and no more.
The following two territories are used in our agreement with Genentech to determine the Kadcyla sales levels for the calculation of the applicable tiered royalty levels: (1) the U.S. and (2) the rest of the world. Royalties on sales of Kadcyla are determined based on annual calendar year net sales in each
9
territory in accordance with a tiered structure calculated separately in each of the two territories as follows:
- •
- 3% of net sales up to $250 million;
- •
- 3.5% of net sales above $250 million and up to $400 million;
- •
- 4% of net sales above $400 million and up to $700 million; and
- •
- 5% of net sales above $700 million.
The sales in the country count towards the annual sales in that territory for purposes of calculation of sales tiers.
Royalties will be reduced to a flat 2% of net sales in any country at any time during the royalty term in which Kadcyla is not covered by a valid claim under any patents controlled by us (excluding patents jointly owned by us and Genentech or solely owned by Genentech) in such country.
The license agreement also provides for certain adjustments to the royalties payable to us if:
- •
- Genentech makes certain third party license payments in order to exploit the TAP technology components of Kadcyla,
although such adjustments would in no event reduce the royalties payable for any country below the greater of 50% of the royalties otherwise payable with respect to sales of Kadcyla in such country,
or 2% of net sales in such country; or
- •
- a third party obtains regulatory approval in a country to market and sell a product containing a conjugate of an anti-HER2 antibody with a maytansinoid, in which case royalties will be reduced to a flat 1% of net sales of Kadcyla in such country during the royalty term as long as such competing product has not been withdrawn from the market in such country.
As of the date of this annual report on Form 10-K, we are unaware of any facts or circumstances that would give rise to the adjustments described in either of the above two circumstances.
Roche may terminate this agreement for convenience at any time upon 90 days' prior written notice to us. The agreement may also be terminated by either party for a material breach by the other, subject to notice and cure provisions. Unless earlier terminated, the agreement will continue in effect until the expiration of Roche's royalty obligations.
The US marketing approval of Kadcyla in February 2013 triggered a $10.5 million milestone payment to us. Through June 30, 2013, we have received and recognized a total of $24.0 million in milestone payments under this agreement. The next potential milestone we will be entitled to receive will be either a $5 million regulatory milestone for marketing approval of Kadcyla in Europe or a $5 million regulatory milestone for marketing approval of Kadcyla in Japan, depending on which occurs first.
Roche, through its Genentech unit, also has licenses for the exclusive right to use our maytansinoid TAP technology with antibodies to four undisclosed targets, which were granted under the terms of a separate May 2000 right-to-test agreement with Genentech. For each of these licenses we received a $1 million license fee and are entitled to receive up to a total of $38 million in milestone payments and also royalties on the sales of any resulting products. We have not received any milestone payments from these agreements through June 30, 2013. Roche is responsible for the development, manufacturing, and marketing of any products resulting from these licenses. Roche no longer has the right to take additional licenses under the right-to-test agreement.
Amgen
In September 2000, we entered into a ten-year right-to-test agreement with Abgenix, Inc. which was later acquired by Amgen. The agreement provided Amgen with the right to (a) test our maytansinoid TAP technology with Amgen's antibodies under a right-to-test, or research, license,
10
(b) take options, with certain restrictions, to individual targets selected by Amgen on either an exclusive or non-exclusive basis for specified option periods and (c) upon exercise of those options, take exclusive or non-exclusive licenses to use our maytansinoid TAP technology to develop and commercialize products directed to the specified targets on previously agreed-upon terms. Amgen no longer has the right to take additional options under the right-to-test agreement and there are no unexercised options outstanding.
Under the right-to-test agreement, in September 2009, November 2009 and December 2012, Amgen took three exclusive development and commercialization licenses, for which we received an exercise fee of $1 million for each license taken. In May 2013, Amgen took one non-exclusive development and commercialization license, for which we received an exercise fee of $500,000. We are entitled to receive up to a total of $34 million in milestone payments for each exclusive license and up to a total of $17 million in milestone payments for the non-exclusive license, plus in each case, royalties on the commercial sales of any resulting products.
In November 2011, the IND applications to the FDA for two compounds developed under two of the exclusive development and commercialization licenses became active, which triggered two $1 million milestone payments to us. The next potential milestone we will be entitled to receive under either of these two development and commercialization licenses will be a development milestone for the first dosing of a patient in a Phase II clinical trial, which will result in a $3 million payment being due. The next potential milestone we will be entitled to receive under the December 2012 development and commercialization license will be a development milestone for IND approval which will result in a $1 million payment being due to us. The next potential milestone we will be entitled to receive under the May 2013 development and commercialization license will be a development milestone for IND approval which will result in a $500,000 payment being due to us.
Amgen may terminate each development and commercialization license for convenience upon prior notice to us. Each license may also be terminated by either party for a material breach by the other, subject to notice and cure provisions. Unless earlier terminated, each license will continue in effect until the expiration of Amgen's royalty obligations, which are determined on a product-by-product and country-by-country basis. For each product and country, Amgen's royalty obligations commence with the first commercial sale of that product in that country, and extend until the later of either the expiration of the last-to-expire ImmunoGen patent covering that product in that country or the expiration for that country of the minimum royalty period specified in each development and commercialization license.
Sanofi
Collaboration Agreement
In July 2003, we entered into a broad collaboration agreement with Sanofi (formerly Aventis) to discover, develop and commercialize antibody-based products. The collaboration agreement provides Sanofi with worldwide development and commercialization rights to new antibody-based products directed to targets that are included in the collaboration, including the exclusive right to use our maytansinoid TAP technology in the creation of products directed to these targets. The product candidates (targets) currently in development under the collaboration include SAR3419 (CD19), SAR650984 (CD38), SAR566658 (DS6, also known as CA6) and two earlier-stage compounds that have yet to be disclosed. For each of the targets included in the collaboration at this time, we are entitled to receive up to a total of $21.5 million in milestone payments, plus royalties on the commercial sales of any resulting products.
The agreement may be terminated by either party for a material breach by the other, subject to notice and cure provisions. Unless earlier terminated, the agreement will continue in effect until the expiration of Sanofi's royalty obligations, which are determined on a product-by-product and country-by-country basis. For each product and country, Sanofi's royalty obligations commence upon
11
first commercial sale of that product in that country, and extend until the later of either the expiration of the last-to-expire ImmunoGen patent covering that product in that country or the expiration for that country of the minimum royalty period specified in the agreement.
The collaboration agreement also provides us an option to certain co-promotion rights in the U.S. on a product-by-product basis. The terms of the collaboration agreement allow Sanofi to terminate our co-promotion rights if there is a change in control of our company.
Through June 30, 2013, we have received and recognized a total of $16.5 million in milestone payments related to compounds covered under this agreement now and in the past, including a total of $8 million in milestone payments related to two product candidates previously in the collaboration that have been returned to us along with the rights to the respective targets.
The next potential milestone we will be entitled to receive with respect to SAR3419 will be for initiation of a Phase III clinical trial, which will result in a $3 million payment being due. The next potential milestone we will be entitled to receive with respect to each of SAR566658 and for SAR650984 will be a development milestone for initiation of a Phase IIb clinical trial (as defined in the agreement), which will result in each case in a $3 million payment being due. The next potential milestone we will be entitled to receive for each of the unidentified targets will be a development milestone for commencement of a Phase I clinical trial, which will result in a $1 million payment being due.
Right-to-Test Agreement
In December 2006, we entered into a separate right-to-test agreement with Sanofi. The agreement provides Sanofi with the right to (a) test our maytansinoid TAP technology with Sanofi's antibodies to targets that were not included in the collaboration agreement described above under a right-to-test, or research, license, (b) take exclusive options, with certain restrictions, to individual targets selected by Sanofi for specified time periods and (c) upon exercise of those options, take exclusive licenses to use our maytansinoid TAP technology to develop and commercialize products directed to the specified targets on terms agreed upon at the inception of the right-to-test agreement. The right-to-test agreement had a three-year original term from the activation date that was renewed by Sanofi in August 2011for its final three-year term by payment of a $2 million extension fee. No additional extensions are included in this agreement.
For each development and commercialization license taken, we are entitled to receive an exercise fee of $2 million and up to a total of $30 million in milestone payments, plus royalties on the commercial sales of any resulting products.
Each development and commercialization license may be terminated by either party for a material breach by the other, subject to notice and cure provisions. Unless earlier terminated, each license will continue in effect until the expiration of Sanofi's royalty obligations, which are determined on a product-by-product and country-by-country basis. For each product and country, Sanofi's royalty obligations commence with the first commercial sale of that product in that country, and extend until the later of either the expiration of the last-to-expire ImmunoGen patent covering that product in that country or the expiration for that country of the minimum royalty period specified in each development and commercialization license. No development and commercialization license has yet been taken under the right-to-test agreement.
Biotest
In July 2006, we granted Biotest an exclusive development and commercialization license to our maytansinoid TAP technology for use with antibodies that target CD138. The product candidate BT-062 is in development under this agreement. We received a $1 million upfront payment from Biotest upon
12
execution of the agreement. We are also entitled to receive up to a total of $35.5 million in milestone payments, plus royalties on the commercial sales of any resulting products.
The agreement also provides us with the right to elect, at specific stages during the clinical evaluation of any compound created under the agreement, to participate in the U.S. development and commercialization of that compound in lieu of receiving the milestone payments not yet earned and royalties on sales in the U.S. We can exercise this right during an exercise period specified in the agreement by notice and payment to Biotest of an agreed upon opt-in fee of $15 million. Upon exercise of this right, we would share equally with Biotest the associated costs of product development and commercialization in the U.S. along with the profit, if any, from product sales in the U.S.
Biotest may terminate the agreement for convenience at any time prior to our election to participate in the U.S. development and commercialization of a compound created under this agreement upon prior notice to us. The agreement may also be terminated by either party for a material breach by the other, subject to notice and cure provisions. Unless earlier terminated, the agreement will continue in effect until the expiration of Biotest's royalty obligations, which are determined on a product-by-product and country-by-country basis. For each product and country, Biotest's royalty obligations commence upon first commercial sale of that product in that country, and extend until the later of either the expiration of the last-to-expire ImmunoGen patent covering that product in that country or the expiration for that country of the minimum royalty period specified in the agreement.
Through June 30, 2013, we have received and recognized a total of $500,000 in milestone payments under this agreement. The next potential milestone we will be entitled to receive will be a development milestone for commencement of a Phase IIb clinical trial (as defined in the agreement) which will result in a $2 million payment being due.
Bayer HealthCare
In October 2008, we granted Bayer HealthCare an exclusive development and commercialization license to our maytansinoid TAP technology for use with antibodies or other proteins that target mesothelin. The product candidate BAY 94-9343 is currently in development under this agreement. We received a $4 million upfront payment upon execution of the agreement. We are also entitled to receive, for each product developed and marketed by Bayer HealthCare under this agreement, up to a total of $170.5 million in milestone payments, plus royalties on the commercial sales of any resulting products.
Bayer HealthCare may terminate the agreement for convenience at any time upon prior written notice to us. The agreement may also be terminated by either party for a material breach by the other, subject to notice and cure provisions. We may also terminate the agreement upon the occurrence of specified events. Unless earlier terminated, the agreement will continue in effect until the expiration of Bayer HealthCare's royalty obligations, which are determined on a product-by-product and country-by-country basis. For each product and country, Bayer HealthCare's royalty obligations commence upon first commercial sale of that product in that country, and extend until the later of either the expiration of the last-to-expire ImmunoGen patent covering that product in that country or the expiration for that country of the minimum royalty period specified in the agreement.
Through June 30, 2013, we have received and recognized a total of $3 million in milestone payments under this agreement. The next potential milestone we will be entitled to receive will be a development milestone for commencement of a non-pivotal Phase II clinical trial, which will result in a $4 million payment being due.
13
Novartis
In October 2010, we entered into a right-to-test agreement with Novartis. The agreement provides Novartis with a right to (a) test our TAP technology with Novartis' antibodies directed to individual targets selected by Novartis under a right-to-test, or research, license, (b) take exclusive options, with certain restrictions, to individual targets selected by Novartis for specified option periods, and (c) upon exercise of those options take exclusive licenses to use our TAP technology to develop and commercialize products for a specified number of individual targets on terms agreed upon at the inception of the right-to-test agreement. The initial term of the right-to-test agreement is three years, which may be extended by Novartis for up to two additional one-year periods by the payment of additional consideration. Novartis must exercise its options for the development and commercialization licenses by the end of the term of the right-to-test agreement, after which any then outstanding options will lapse.
We received a $45 million upfront payment in connection with the execution of the right-to-test agreement, and we are also entitled to receive additional payments under the agreement for research and development activities performed on behalf of Novartis during the term of the agreement. For each development and commercialization license taken, we are entitled to receive an exercise fee of $1 million and up to a total of $199.5 million in milestone payments, plus royalties on the commercial sales of any resulting products.
Effective March 29, 2013, we and Novartis amended the right-to-test agreement so that Novartis can take a license to develop and commercialize products directed at two pre-defined and related undisclosed targets, one target licensed on an exclusive basis and the other target initially licensed on a non-exclusive basis. The target licensed on a non-exclusive basis may be converted to an exclusive target by notice and payment to us of an agreed upon fee of at least $5 million, depending on specific circumstances. We received a $3.5 million fee in connection with the execution of the amendment to the agreement. We may be required to credit this fee against future milestone payments if Novartis discontinues the development of a specified product under certain circumstances.
In connection with the amendment, on March 29, 2013, Novartis took the license referenced above under the right-to-test agreement, as amended, enabling it to develop and commercialize products directed at the two targets. We received a $1 million upfront fee with the execution of this license. Additionally, the execution of this license provides us the opportunity to receive milestone payments totaling $199.5 million or $238 million, depending on the composition of any resulting products. The first potential milestone we will be entitled to receive will be a $5.0 million development milestone for commencement of a Phase I clinical trial.
Novartis may terminate any development and commercialization license for convenience upon prior notice to us. Each license may also be terminated by either party for a material breach by the other, subject to notice and cure provisions. Unless earlier terminated, each development and commercialization license will continue in effect until the expiration of Novartis' royalty obligations, which are determined on a product-by-product and country-by-country basis. For each product and country, Novartis' royalty obligations commence upon first commercial sale of that product in that country, and extend until the later of either the expiration of the last-to-expire ImmunoGen patent covering that product in that country or the expiration for that country of the minimum royalty period specified in each license.
Lilly
In December 2011, we entered into a three-year right-to-test agreement with Lilly. The agreement provides Lilly with the right to (a) take exclusive options, with certain restrictions, to individual targets selected by Lilly for specified option periods, (b) test our maytansinoid TAP technology with Lilly's antibodies directed to the optioned targets under a right-to-test, or research, license, and (c) upon exercise of those options take exclusive licenses to use our maytansinoid TAP technology to develop
14
and commercialize products for a specified number of individual targets on terms agreed upon at the inception of the right-to-test agreement. Lilly must exercise its options for the development and commercialization licenses by the end of the term of the right-to-test agreement, after which any then outstanding options will lapse. Under the terms of the agreement, Lilly took an exclusive development and commercialization license to a single target in August 2013.
We received a $20 million upfront payment in connection with the execution of the agreement, and we are also entitled to receive additional payments under the agreement for research and development activities performed under the agreement on behalf of Lilly during the term of the research license. For the first development and commercialization license taken, which occurred in August 2013, we are entitled to receive up to a total of $200.5 million in milestone payments, plus tiered royalties in the mid-single to low-double digits on the commercial sales of any resulting products. For each subsequent development and commercialization license taken, we are entitled to receive an exercise fee of $2 million and up to a total of $199 million in milestone payments, plus royalties on the commercial sales of any resulting products.
Lilly may terminate any development and commercialization license for convenience upon prior notice to us. Each license may also be terminated by either party for a material breach by the other, subject to notice and cure provisions. We may also terminate the agreement upon the occurrence of specified events. Unless earlier terminated, each development and commercialization license will continue in effect until the expiration of Lilly's royalty obligations, which are determined on a product-by-product and country-by-country basis. For each product and country, Lilly's royalty obligations commence upon first commercial sale of that product in that country, and extend until the later of either the expiration of the last-to-expire ImmunoGen patent covering that product in that country or the expiration for that country of the minimum royalty period specified in each license.
In-Licenses
From time to time we may in-license certain rights to targets or technologies for use in conjunction with our internal efforts to develop TAP compounds and related technologies. These licenses include rights to certain antibodies. In exchange, we may be obligated to pay upfront fees, potential milestone payments and royalties on any product sales.
Patents, Trademarks and Trade Secrets
Our intellectual property strategy centers on obtaining patent protection for our proprietary technologies and product candidates. As of June 30, 2013, our patent portfolio had a total of 459 issued patents worldwide and 477 pending patent applications worldwide that we own or license from third parties. We seek to protect our TAP technology and our product candidates through a multi-pronged approach. In this regard, we have patents and patent applications covering antibodies and other cell-binding agents, linkers, maytansinoid and other cell-killing agents, and complete antibody-drug conjugates, or immunoconjugates, comprising these components and methods of making and using each of the above. Typically, multiple issued patents and pending patent applications cover various aspects of each product candidate.
We consider our maytansinoid technology to be a key component of our overall corporate strategy. We currently own 42 issued U.S. patents covering various embodiments of our maytansinoid technology including claims directed to certain maytansinoids, antibody-maytansinoid conjugates and other cell-binding agents used with maytansinoids, and methods of making and using the same. In all cases, we have received or are applying for comparable patents in other jurisdictions including Europe and Japan. We have issued patents that cover numerous aspects of the manufacture of both our DM1 and DM4 cell-killing agents. These issued patents remain in force until various times between 2020 and 2026. We also have several composition of matter patents covering various aspects of our DM4
15
cell-killing agent and antibody-maytansinoid conjugates incorporating DM4 that are expected to remain in force until 2024-2025.
Our intellectual property strategy also includes pursuing patents directed to linkers, antibodies, conjugation methods, immunoconjugate formulations and the use of specific antibodies and immunoconjugates to treat certain diseases. In this regard, we have issued patents and pending patent applications related to many of our linker technologies. These issued patents, expiring in 2021-2029, and any patents which may issue from the patent applications, cover antibody-maytansinoid conjugates using these linkers. We also have issued U.S. patents and pending patent applications covering methods of assembling immunoconjugates from their constituent antibody, linker and cell-killing agent moieties. These issued patents will expire in 2021-2027, while any patents that may issue from pending patent applications also covering various aspects of these technologies will, if issued, expire between 2021 and 2034. We also have issued patents and pending patent applications related to monoclonal antibodies that may be a component of a TAP compound or may be developed as a therapeutic, or "naked," antibody anticancer compound. Among these patents is an issued U.S. patent claiming a method of humanizing murine antibodies to avoid their detection by the human immune system. We have received patents in other jurisdictions, including Europe and Japan, that correspond to our antibody humanization U.S. patent. These patents will expire between 2013 and 2014.
We expect our continued work in each of these areas will lead to other patent applications. In all such cases, we will either be the assignee or owner of such patents or have an exclusive license to the technology covered by the patents. For example, we also own issued patents covering proprietary derivatives of non-maytansinoid cell-killing molecules. However, we do not currently consider these additional patent families to be material to our business.
The rates at which we are entitled to receive royalties based on sales of Kadcyla in any particular country depend in part on whether the manufacture, use or sale of Kadcyla is covered by ImmunoGen patent rights in that country. In this regard, we own patents in the U.S. and Europe covering the composition of matter of Kadcyla that expire at the earliest in 2023 and 2024, respectively, and may be eligible for extension of those terms under applicable patent laws in those jurisdictions. We also own patents in the U.S. and Europe that cover various elements of the manufacture of Kadcyla, with expiration dates extending to at least 2027 and 2026, respectively. Notwithstanding these patent terms, the period during which we are entitled to receive royalties based on sales of Kadcyla in any country does not extend beyond the 12th anniversary of the date of the first commercial sale of Kadcyla in such country.
We have in-licensed intellectual property relating to our IMGN901 product candidate from Dana-Farber Cancer Institute. We do not believe that the terms of this license are material to our business or prospects.
We cannot provide assurance that the patent applications will issue as patents or that any patents, if issued, will provide us with adequate protection against competitors with respect to the covered products, technologies or processes. Defining the scope and term of patent protection involves complex legal and factual analyses and, at any given time, the result of such analyses may be uncertain. In addition, other parties may challenge our patents in litigation or administrative proceedings resulting in a partial or complete loss of certain patent rights owned or controlled by ImmunoGen, Inc. Furthermore, as a patent does not confer any specific freedom to operate, other parties may have patents that may block or otherwise hinder the development and commercialization of our technology.
In addition, many of the processes and much of the know-how that are important to us depend upon the skills, knowledge and experience of our key scientific and technical personnel, which skills, knowledge and experience are not patentable. To protect our rights in these areas, we require that all employees, consultants, advisors and collaborators enter into confidentiality agreements with us. Further, we require that all employees enter into assignment of invention agreements as a condition of
16
employment. We cannot provide assurance, however, that these agreements will provide adequate or any meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure of such trade secrets, know-how or proprietary information. Further, in the absence of patent protection, we may be exposed to competitors who independently develop substantially equivalent technology or otherwise gain access to our trade secrets, know-how or other proprietary information.
Competition
We focus on highly competitive areas of product development. Our competitors include major pharmaceutical companies and other biotechnology firms. For example, Pfizer, Seattle Genetics, Rocheand Bristol-Myers Squibb have programs to attach a proprietary cell-killing small molecule to an antibody for targeted delivery to cancer cells. Pharmaceutical and biotechnology companies, as well as other institutions, also compete with us for promising targets for antibody-based therapeutics and in recruiting highly qualified scientific personnel. Many competitors and potential competitors have substantially greater scientific, research and product development capabilities, as well as greater financial, marketing and human resources than we do. In addition, many specialized biotechnology firms have formed collaborations with large, established companies to support the research, development and commercialization of products that may be competitive with ours.
In particular, competitive factors within the antibody and cancer therapeutic market include:
- •
- the safety and efficacy of products;
- •
- the timing of regulatory approval and commercial introduction;
- •
- special regulatory designation of products, such as Orphan Drug designation; and
- •
- the effectiveness of marketing, sales, and reimbursement efforts.
Our competitive position depends on our ability to develop effective proprietary products, implement clinical development programs, production plans and marketing plans, including collaborations with other companies with greater marketing resources than ours, and to obtain patent protection and secure sufficient capital resources.
Continuing development of conventional and targeted chemotherapeutics by large pharmaceutical companies and biotechnology companies may result in new compounds that may compete with our product candidates. Antibodies developed by certain of these companies have been approved for use as cancer therapeutics. In the future, new antibodies or other targeted therapies may compete with our product candidates. Other companies have created or have programs to create potent cell-killing agents for attachment to antibodies. These companies may compete with us for technology out-license arrangements.
Regulatory Matters
Government Regulation and Product Approval
Government authorities in the U.S., at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing and export and import of products such as those we are developing. A new drug must be approved by the FDA through the new drug application, or NDA, process and a new biologic must be approved by the FDA through the biologics license application, or BLA, process before it may be legally marketed in the U.S.
17
U.S. Drug Development Process
In the U.S., the FDA regulates drugs under the federal Food, Drug, and Cosmetic Act, or FDCA, and in the case of biologics, also under the Public Health Service Act, or PHSA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA's refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement, or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us. The process required by the FDA before a drug or biologic may be marketed in the U.S. generally involves the following:
- •
- completion of preclinical laboratory tests, animal studies and formulation studies according to current Good Laboratory
Practices (cGLP) or other applicable regulations;
- •
- submission to the FDA of an IND which must become effective before human clinical trials may begin;
- •
- performance of adequate and well-controlled human clinical trials according to Good Clinical Practices (cGCP) to establish
the safety and efficacy of the proposed drug for its intended use;
- •
- submission to the FDA of an NDA or BLA;
- •
- satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to
assess compliance with current Good Manufacturing Practice (cGMP) to assure that the facilities, methods and controls are adequate to preserve the drug's identity, strength, quality and purity; and
- •
- FDA review and approval of the NDA or BLA.
Once a pharmaceutical candidate is identified for development it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The sponsor will also include a protocol detailing, among other things, the objectives of the first phase of the clinical trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated, if the first phase lends itself to an efficacy evaluation. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during studies due to safety concerns or non-compliance.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with cGCP regulations. They must be conducted under protocols detailing the objectives of the trial, dosing procedures, subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND, and progress reports detailing the results of the clinical trials must be submitted at least annually. In addition, timely safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. An institutional review board, or IRB, at each institution participating in the clinical trial must review and approve each protocol before a clinical trial commences at that institution and must also approve the information regarding the trial and the consent form that must be provided to each trial
18
subject or his or her legal representative, monitor the study until completed and otherwise comply with IRB regulations.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
- •
- Phase I: The product candidate is initially introduced into healthy human
subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, such as cancer, especially
when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients.
- •
- Phase II: This phase involves studies in a limited patient population to
identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
- •
- Phase III: Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product candidate and provide, if appropriate, an adequate basis for product labeling.
The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB's requirements or if the drug has been associated with unexpected serious harm to patients. Phase I, Phase II, and Phase III testing may not be completed successfully within any specified period, if at all.
During the development of a new drug, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase II, and before an NDA or BLA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and FDA to reach agreement on the next phase of development. Sponsors typically use the End of Phase II meeting to discuss their Phase II clinical results and present their plans for the pivotal Phase III clinical trial that they believe will support approval of the new drug. If this type of discussion occurs, a sponsor may be able to request a Special Protocol Assessment, or SPA, the purpose of which is to reach agreement with the FDA on the design of the Phase III clinical trial protocol design and analysis that will form the primary basis of an efficacy claim.
According to FDA guidance for industry on the SPA process, a sponsor that meets the prerequisites may make a specific request for a special protocol assessment and provide information regarding the design and size of the proposed clinical trial. The FDA is required to evaluate the protocol within 45 days of the request to assess whether the proposed trial is adequate, and that evaluation may result in discussions and a request for additional information. A SPA request must be made before the proposed trial begins, and all open issues must be resolved before the trial begins. If a written agreement is reached, it will be documented and made part of the record. The agreement will be binding on the FDA and may not be changed by the sponsor or the FDA after the trial begins except with the written agreement of the sponsor and the FDA or if the FDA determines that a substantial scientific issue essential to determining the safety or efficacy of the drug was identified after the testing began. If the sponsor makes any unilateral changes to the approved protocol, the agreement will be invalidated.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP
19
requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling, and other relevant information are submitted to the FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of user fees; a waiver of such fees may be obtained under certain limited circumstances. The FDA reviews all NDAs and BLAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an NDA or BLA for filing. In this event, the NDA or BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. FDA may refer the NDA or BLA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The approval process is lengthy and often difficult, and the FDA may refuse to approve an NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. Even if such data and information is submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. The FDA may issue a complete response letter, which may require additional clinical or other data or impose other conditions that must be met in order to secure final approval of the NDA or BLA, or an approved letter following satisfactory completion of all aspects of the review process. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product's identity, strength, quality and purity. The FDA reviews a BLA to determine, among other things whether the product is safe, pure and potent and the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product's continued safety, purity and potency. Before approving an NDA or BLA, the FDA will inspect the facility or facilities where the product is manufactured.
NDAs or BLAs receive either standard or priority review. A drug representing a significant improvement in treatment, prevention or diagnosis of disease may receive priority review. Priority review for an NDA for a new molecular entity and original BLAs will be 6 months from the date that the NDA or BLA is filed. In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. Priority review and accelerated approval do not change the standards for approval, but may expedite the approval process.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the
20
commercial value of the product. In addition, the FDA may require us to conduct Phase IV testing which involves clinical trials designed to further assess a drug's safety and effectiveness after NDA or BLA approval, and may require testing and surveillance programs to monitor the safety of approved products which have been commercialized.
The recently enacted Food and Drug Administration Safety and Innovation Act, or FDASIA, made permanent the Pediatric Research Equity Act, or PREA, which requires a sponsor to conduct pediatric studies for most drugs and biologicals, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs, BLAs and supplements thereto, must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric studies for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug or biologic is ready for approval for use in adults before pediatric studies are complete or that additional safety or effectiveness data needs to be collected before the pediatric studies begin. After April 2013, the FDA must send a non-compliance letter to any sponsor that fails to submit the required assessment, keep a deferral current or fails to submit a request for approval of a pediatric formulation.
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of our drugs, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product's approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA or BLA, plus the time between the submission date of an NDA or BLA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension, and the extension must be applied for prior to expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond their current expiration date, depending on the expected length of clinical trials and other factors involved in the filing of the relevant NDA.
Market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant, are deemed by FDA to be essential to the approval of the application, for example, for new indications, dosages, or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical
21
investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Pediatric exclusivity is another type of marketing exclusivity available in the U.S. The FDASIA made permanent the Best Pharmaceuticals for Children Act, or BPCA, which provides for an additional six months of marketing exclusivity if a sponsor conducts clinical trials in children in response to a written request from the FDA, or a Written Request. If the Written Request does not include studies in neonates, the FDA is required to include its rationale for not requesting those studies. The FDA may request studies on approved or unapproved indications in separate Written Requests. The issuance of a Written Request does not require the sponsor to undertake the described studies. To date, we have not received any Written Requests.
Biologics Price Competition and Innovation Act of 2009
On March 23, 2010, President Obama signed into law the Patient Protection and Affordable Care Act which included the Biologics Price Competition and Innovation Act of 2009, or BPCIA. The BPCIA amended the PHSA to create an abbreviated approval pathway for two types of "generic" biologics—biosimilars and interchangeable biologic products, and provides for a twelve-year data exclusivity period for the first approved biological product, or reference product, against which a biosimilar or interchangeable application is evaluated; however if pediatric studies are performed and accepted by the FDA, the twelve-year data exclusivity period will be extended for an additional six months A biosimilar product is defined as one that is highly similar to a reference product notwithstanding minor differences in clinically inactive components and for which there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity and potency of the product. An interchangeable product is a biosimilar product that may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.
The biosimilar applicant must demonstrate that the product is biosimilar based on data from (1) analytical studies showing that the biosimilar product is highly similar to the reference product; (2) animal studies (including toxicity); and (3) one or more clinical studies to demonstrate safety, purity and potency in one or more appropriate conditions of use for which the reference product is approved. In addition, the applicant must show that the biosimilar and reference products have the same mechanism of action for the conditions of use on the label, route of administration, dosage and strength, and the production facility must meet standards designed to assure product safety, purity and potency.
An application for a biosimilar product may not be submitted until four years after the date on which the reference product was first approved. The first approved interchangeable biologic product will be granted an exclusivity period of up to one year after it is first commercially marketed, but the exclusivity period may be shortened under certain circumstances.
Between February 2012 and March 2013, the FDA issued several draft guidance documents on biosimilar product development. The draft guidance documents are: "Scientific Considerations in Demonstrating Biosimilarity to a Reference Product," "Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product," "Biosimilars: Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009," and "Formal Meetings Between the FDA and Biosimilar Biological Product Sponsors or Applicants." The guidance documents provide FDA's current thinking on approaches to demonstrating that a proposed biological product is biosimilar to a reference product. The FDA received public comments on the draft
22
documents and intends to issue final guidance documents in the future. Nevertheless, the absence of a final guidance document does not prevent a sponsor from seeking licensure of a biosimilar under the BPCIA.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the U.S., or more than 200,000 individuals in the U.S. and for which there is no reasonable expectation that the cost of developing and making available in the U.S. a drug for this type of disease or condition will be recovered from sales in the U.S. for that drug. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use will be disclosed publicly by the FDA; the posting will also indicate whether a drug is no longer designated as an orphan drug. More than one product candidate may receive an orphan drug designation for the same indication. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to seven years of orphan product exclusivity, except in very limited circumstances. The FDA issued a final rule, effective August 12, 2013, intended to clarify several regulatory provisions, among which was a clarification of some of those limited circumstances. One of the provisions makes clear that the FDA will not recognize orphan drug exclusive approval if a sponsor fails to demonstrate upon approval that the drug is clinically superior to a previously approved drug, regardless of whether or not the approved drug was designated an orphan drug or had orphan drug exclusivity. Thus orphan drug exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug as defined by the FDA and we are not able to show the clinical superiority of our drug or if our product candidate is determined to be contained within the competitor's product for the same indication or disease.
The FDA granted Orphan Drug designation to our lorvotuzumab mertansine compound when used for the treatment of Merkel cell carcinoma (MCC), small-cell lung cancer (SCLC) and multiple myeloma (MM). Orphan drug designation provides ImmunoGen with seven years of market exclusivity that begins once lorvotuzumab mertansine receives FDA marketing approval for the use for which the orphan drug status was granted. Also, through a separate process, lorvotuzumab mertansine has been granted orphan medicinal product designation for the treatment of MCC, SCLC and MM in the European Union. Orphan medicinal product designation provides ImmunoGen with ten years of market exclusivity that begins once lorvotuzumab mertansine receives European approval for the use for which it was granted. We may pursue these designations for other indications for lorvotuzumab mertansine, and for other product candidates intended for qualifying patient populations.
Expedited Review and Approval
The FDA has various programs, including Fast Track, priority review, and accelerated approval, which are intended to expedite or simplify the process for reviewing drugs, and/or provide for approval on the basis of surrogate endpoints. Even if a drug qualifies for one or more of these programs, the FDA may later decide that the drug no longer meets the conditions for qualification or that the time period for FDA review or approval will not be shortened. Generally, drugs that may be eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs, and those that offer meaningful benefits over existing treatments. For example, Fast Track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious diseases and fill an unmet medical need. The request may be made at the time of IND submission and generally no later than the pre-BLA or pre-NDA meeting. The FDA will respond
23
within 60 calendar days of receipt of the request. Priority review, which is requested at the time of BLA or NDA submission, is designed to give drugs that offer major advances in treatment or provide a treatment where no adequate therapy exists an initial review within six months as compared to a standard review time of ten months. Although Fast Track and priority review do not affect the standards for approval, the FDA will attempt to facilitate early and frequent meetings with a sponsor of a Fast Track designated drug and expedite review of the application for a drug designated for priority review. Accelerated approval provides an earlier approval of drugs to treat serious diseases, and that fill an unmet medical need based on a surrogate endpoint, which is a laboratory measurement or physical sign used as an indirect or substitute measurement representing a clinically meaningful outcome. Discussions with the FDA about the feasibility of an accelerated approval typically begin early in the development of the drug in order to identify, among other things, an appropriate endpoint. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform post-marketing clinical trials to confirm the appropriateness of the surrogate marker trial.
In FDASIA, Congress encouraged the FDA to utilize innovative and flexible approaches to the assessment of products under accelerated approval. The law required the FDA to issue related draft guidance within a year after the law's enactment and also promulgate confirming regulatory changes. The FDA published a draft guidance in June 2013, entitled "Expedited Programs for Serious Conditions—Drugs and Biologics." One of the expedited programs added by FDASIA is that for Breakthrough Therapy. A Breakthrough Therapy designation is designed to expedite the development and review of drugs that are intended to treat a serious condition where preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s). A sponsor may request Breakthrough Therapy designation at the time that the IND is submitted, or no later than at the end-of-Phase II meeting. The FDA will respond to a Breakthrough Therapy designation request within sixty days of receipt of the request. A drug that receives Breakthrough Therapy designation is eligible for all fast track designation features, intensive guidance on an efficient drug development program, beginning as early as Phase I and commitment from the FDA involving senior managers. FDA has already granted this designation to about 20 new drugs.
Post-Approval Requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws and regulations. We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products. Future inspections by the FDA and other regulatory agencies may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct.
Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the drug, providing the FDA with updated safety and efficacy information, drug sampling and distribution requirements, complying with certain electronic records and signature requirements, and complying with FDA promotion and advertising requirements. FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed
24
on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label.
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. It is impossible to predict whether further legislative changes will be enacted, or FDA regulations, guidance or interpretations changed or what the impact of such changes, if any, may be.
Foreign Regulation
In addition to regulations in the U.S., we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries or economic areas, such as the European Union, before we may commence clinical trials or market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Under European Union regulatory systems, a company may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for medicinal products produced by biotechnology or those medicinal products containing new active substances for specific indications such as the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, viral diseases and designated orphan medicines, and optional for other medicines which are highly innovative. Under the centralized procedure, a marketing application is submitted to the European Medicines Agency where it will be evaluated by the Committee for Medicinal Products for Human Use and a favorable opinion typically results in the grant by the European Commission of a single marketing authorization that is valid for all European Union member states within 67 days of receipt of the opinion. The initial marketing authorization is valid for five years, but once renewed is usually valid for an unlimited period. The decentralized procedure provides for approval by one or more "concerned" member states based on an assessment of an application performed by one member state, known as the "reference" member state. Under the decentralized approval procedure, an applicant submits an application, or dossier, and related materials to the reference member state and concerned member states. The reference member state prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. Within 90 days of receiving the reference member state's assessment report, each concerned member state must decide whether to approve the assessment report and related materials. If a member state does not recognize the marketing authorization, the disputed points are eventually referred to the European Commission, whose decision is binding on all member states.
As in the U.S., we may apply for designation of a product as an orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including up to 10 years of market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product.
Reimbursement
Sales of pharmaceutical products depend in significant part on the availability of third-party reimbursement. Third-party payors include government healthcare programs such as Medicare, managed care providers, private health insurers and other organizations. We anticipate third-party payors will provide reimbursement for our products. However, these third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition,
25
significant uncertainty exists as to the reimbursement status of newly approved healthcare products. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. Our product candidates may not be considered cost-effective. It is time consuming and expensive for us to seek reimbursement from third-party payors. Reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis.
Medicare is a federal healthcare program administered by the federal government that covers individuals age 65 and over as well as some individuals with certain disabilities. Drugs may be covered under one or more sections of Medicare depending on the nature of the drug and the conditions associated with and site of administration. For example, under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities which provide coverage of outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Part A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level.
Medicare Part B covers most injectable drugs given in an in-patient setting and some drugs administered by a licensed medical provider in hospital outpatient departments and doctors' offices. Medicare Part B is administered by Medicare Administrative Contractors, which generally have the responsibility of making coverage decisions. Subject to certain payment adjustments and limits, Medicare generally pays for a Part B covered drug based on a percentage of manufacturer-reported average sales price which is regularly updated We believe that most of our drugs, when approved, will be subject to the Medicare Part B rules.
The American Recovery and Reinvestment Act of 2009 provides funding for the federal government to compare the effectiveness of different treatments for the same illness. A plan for the research will be developed by the Department of Health and Human Services, the Agency for Healthcare Research and Quality and the National Institutes for Health, and periodic reports on the status of the research and related expenditures will be made to Congress. Although the results of the comparative effectiveness studies are not intended to mandate coverage policies for public or private payors, it is not clear what effect, if any, the research will have on the sales of our product candidates, if any such product or the condition that it is intended to treat is the subject of a study. It is also possible that comparative effectiveness research demonstrating benefits in a competitor's product could adversely affect the sales of our product candidates. If third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
We expect that there will continue to be a number of federal and state proposals to implement governmental pricing controls and limit the growth of healthcare costs, including the cost of prescription drugs. For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, ACA) enacted in March 2010, is expected to have a significant impact on the health care industry. ACA is expected to expand coverage for the uninsured while at the same time containing overall healthcare costs. With regard to pharmaceutical products, among other things, ACA is expected to expand and increase industry rebates for drugs covered under Medicaid programs and make changes to the coverage requirements under the Medicare Part D program. We cannot predict the impact of ACA on pharmaceutical companies as many of the ACA reforms require the promulgation of detailed regulations implementing the statutory provisions which has not yet occurred. In addition, although the United States Supreme Court upheld the constitutionality of most of the ACA, some states have stated their intentions to not implement certain sections of ACA and some members of Congress are still
26
working to repeal ACA. These challenges add to the uncertainty of the changes enacted as part of ACA.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the U.S. and generally tend to by significantly lower.
Research and Development Spending
During each of the three years ended June 30, 2013, 2012 and 2011, we spent approximately $87.1 million, $69.2 million and $63.5 million, respectively, on research and development activities.
Raw Materials and Manufacturing
We procure certain raw material components of finished conjugate, including antibodies, DM1, DM4, and linker, for ourselves and on behalf of our collaborators. In order to meet our commitments to our collaborators as well as our own needs, we are required to enter into agreements with third parties to produce these components well in advance of our production needs. Our principal suppliers for these components include Boehringer Ingelheim, Cytovance Biologics LLC, SAFC, Inc. and Società Italiana Corticosteroidi S.r.l.
In addition, we operate a conjugate manufacturing facility. A portion of the cost of operating this facility, including the cost of manufacturing personnel, is incurred to conjugate material on behalf of our collaborators for which we receive payments based on the number of batches of preclinical and clinical materials produced on their behalf. Over the past few years, we have expanded and upgraded the capabilities of our manufacturing facility.
Employees
As of June 30, 2013, we had 280 full-time employees, of whom 239 were engaged in research and development activities. Of the 239 research and development employees, 113 research and development employees hold post-graduate degrees, of which 51 hold Ph.D. degrees and five hold M.D. degrees. We consider our relations with our employees to be good. None of our employees is covered by a collective bargaining agreement.
We have entered into confidentiality agreements with all of our employees, members of our board of directors and consultants. Further, we have entered into assignment of invention agreements with all of our employees.
Third-Party Trademarks
Herceptin, Kadcyla and Perjeta are registered trademarks of Genentech. Revlimid is a registered trademark of Celgene Corporation. Rituxan is a registered trademark of Biogen Idec Inc.
27
THE RISKS AND UNCERTAINTIES DESCRIBED BELOW ARE THOSE THAT WE CURRENTLY BELIEVE MAY MATERIALLY AFFECT OUR COMPANY. ADDITIONAL RISKS AND UNCERTAINTIES THAT WE ARE UNAWARE OF OR THAT WE CURRENTLY DEEM IMMATERIAL ALSO MAY BECOME IMPORTANT FACTORS THAT AFFECT OUR COMPANY.
We have a history of operating losses and expect to incur significant additional operating losses.
We have generated operating losses since our inception. As of June 30, 2013, we had an accumulated deficit of $576.8 million. For the years ended June 30, 2013, 2012, and 2011, we generated losses of $72.8 million, $73.3 million and $58.3 million, respectively. We may never be profitable. We expect to incur substantial additional operating expenses over the next several years as our research, development, preclinical testing, clinical trials and collaborator support activities continue. We intend to continue to invest significantly in our product candidates. Further, we expect to invest significant resources supporting our existing collaborators as they work to develop, test and commercialize TAP compounds. We or our collaborators may encounter technological or regulatory difficulties as part of this development and commercialization process that we cannot overcome or remedy. We may also incur substantial marketing and other costs in the future if we decide to establish marketing and sales capabilities to commercialize our product candidates. Only one of our collaborators' product candidates has generated commercial revenue and our only revenues to date have been primarily from upfront and milestone payments, research and development support and clinical materials reimbursement from our collaborative partners. We do not expect to generate revenues from the commercial sale of our internal product candidates in the near future, and we may never generate revenues from the commercial sale of internal products. Even if we do successfully develop products that can be marketed and sold commercially, we will need to generate significant revenues from those products to achieve and maintain profitability. Even if we do become profitable, we may not be able to sustain or increase profitability on a quarterly or annual basis.
If we are unable to obtain additional funding when needed, we may have to delay or scale back some of our programs or grant rights to third parties to develop and market our product candidates.
We will continue to expend substantial resources developing new and existing product candidates, including costs associated with research and development, acquiring new technologies, conducting preclinical studies and clinical trials, obtaining regulatory approvals and manufacturing products as well as providing certain support to our collaborators in the development of their products. We believe that our current working capital and expected future payments from our existing collaboration arrangements will be sufficient to meet our current and projected operating and capital requirements through fiscal 2015. However, we cannot provide assurance that such collaborative agreement funding will, in fact, be received. Should such future collaborator payments not be earned and paid as currently anticipated, we expect we could seek additional funding from other sources. We may need additional financing sooner due to a number of other factors as well, including:
- •
- if either we incur higher than expected costs or we or any of our collaborators experience slower than expected progress
in developing product candidates and obtaining regulatory approvals;
- •
- acquisition of technologies and other business opportunities that require financial commitments.
Additional funding may not be available to us on favorable terms, or at all. We may raise additional funds through public or private financings, collaborative arrangements or other arrangements. Debt financing, if available, may involve covenants that could restrict our business activities. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, scale back or eliminate expenditures for some of our development programs or grant rights to develop and market product candidates that we would otherwise prefer to internally
28
develop and market. If we are required to grant such rights, the ultimate value of these product candidates to us may be reduced.
If our TAP technology does not produce safe, effective and commercially viable products, our business will be severely harmed.
Our TAP technology yields novel product candidates for the treatment of cancer. To date, only one TAP product candidate has obtained marketing approval. Our TAP product candidates and/or our collaborators' TAP product candidates may not prove to be safe, effective or commercially viable treatments for cancer and our TAP technology may not result in any future meaningful benefits to us or for our current or potential collaborative partners. Furthermore, we are aware of only two other compounds that are a conjugate of an antibody and a cytotoxic small molecule that have obtained marketing approval by the FDA and are based on technology similar to our TAP technology. One of these products was later taken off the market by its owner due to toxicity concerns. If our TAP technology fails to generate product candidates that are safe, effective and commercially viable treatments for cancer, or fail to obtain FDA approval, our business will be severely harmed.
Clinical trials for our and our collaborative partners' product candidates will be lengthy and expensive and their outcome is uncertain.
Before obtaining regulatory approval for the commercial sale of any product candidates, we and our collaborative partners must demonstrate through clinical testing that our product candidates are safe and effective for use in humans. Conducting clinical trials is a time-consuming, expensive and uncertain process and typically requires years to complete. In our industry, the results from preclinical studies and early clinical trials often are not predictive of results obtained in later-stage clinical trials. Some compounds that have shown promising results in preclinical studies or early clinical trials subsequently fail to establish sufficient safety and efficacy data necessary to obtain regulatory approval. At any time during the clinical trials, we, our collaborative partners, or the FDA might delay or halt any clinical trials of our product candidates for various reasons, including:
- •
- occurrence of unacceptable toxicities or side effects;
- •
- ineffectiveness of the product candidate;
- •
- insufficient drug supply;
- •
- negative or inconclusive results from the clinical trials, or results that necessitate additional studies or clinical
trials;
- •
- delays in obtaining or maintaining required approvals from institutions, review boards or other reviewing entities at
clinical sites;
- •
- delays in patient enrollment;
- •
- insufficient funding or a reprioritization of financial or other resources; or
- •
- other reasons that are internal to the businesses of our collaborative partners, which they may not share with us.
Any failure or substantial delay in successfully completing clinical trials and obtaining regulatory approval for our product candidates or our collaborative partners' product candidates could severely harm our business.
29
We and our collaborative partners are subject to extensive government regulations and we and our collaborative partners may not be able to obtain necessary regulatory approvals.
We and our collaborative partners may not receive the regulatory approvals necessary to commercialize our product candidates, which would cause our business to be severely harmed. Pharmaceutical product candidates, including those in development by us and our collaborative partners, are subject to extensive and rigorous government regulation. The FDA regulates, among other things, the development, testing, manufacture, safety, record-keeping, labeling, storage, approval, advertising, promotion, sale and distribution of pharmaceutical products. If our potential products or our collaborators' potential products are marketed abroad, they will also be subject to extensive regulation by foreign governments. The regulatory review and approval process, which includes preclinical studies and clinical trials of each product candidate, is lengthy, complex, expensive and uncertain. Securing FDA approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA for each indication to establish the product candidate's safety and efficacy. Data obtained from preclinical studies and clinical trials are susceptible to varying interpretation, which may delay, limit or prevent regulatory approval. The approval process may take many years to complete and may involve ongoing requirements for post-marketing studies. Any FDA or other regulatory approvals of our or our collaborative partners' product candidates, once obtained, may be withdrawn. The effect of government regulation may be to:
- •
- delay marketing of potential products for a considerable period of time;
- •
- limit the indicated uses for which potential products may be marketed;
- •
- impose costly requirements on our activities; and
- •
- place us at a competitive disadvantage to other pharmaceutical and biotechnology companies.
We may encounter delays or rejections in the regulatory approval process because of additional government regulation from future legislation or administrative action or changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. Failure to comply with FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our product candidates or us. Outside the U.S., our ability to market a product is contingent upon receiving clearances from the appropriate regulatory authorities. The foreign regulatory approval process includes similar risks to those associated with the FDA approval process. In addition, we are, or may become, subject to various federal, state and local laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices, the experimental use of animals and the use and disposal of hazardous substances, including radioactive compounds and infectious disease agents, used in connection with our research work. If we fail to comply with the laws and regulations pertaining to our business, we may be subject to sanctions, including the temporary or permanent suspension of operations, product recalls, marketing restrictions and civil and criminal penalties.
Our and our collaborative partners' product candidates will remain subject to ongoing regulatory review even if they receive marketing approval. If we or our collaborative partners fail to comply with continuing regulations, these approvals could be lost and the sale of our or our collaborative partners' products could be suspended.
Even if we or our collaborative partners receive regulatory approval to market a particular product candidate, the approval could be conditioned on us or our collaborative partners conducting costly post-approval studies or could limit the indicated uses included in product labeling. Moreover, the product may later cause adverse effects that limit or prevent its widespread use, force us or our collaborative partners to withdraw it from the market or impede or delay our or our collaborative
30
partners' ability to obtain regulatory approvals in additional countries. In addition, the manufacturer of the product and its facilities will continue to be subject to FDA review and periodic inspections to ensure adherence to applicable regulations. After receiving marketing approval, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion and record-keeping related to the product remain subject to extensive regulatory requirements. We or our collaborative partners may be slow to adapt, or we or our collaborative partners may never adapt, to changes in existing regulatory requirements or adoption of new regulatory requirements.
If we or our collaborative partners fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, or if previously unknown problems with our or our partners' products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including:
- •
- restrictions on the products, manufacturers or manufacturing processes;
- •
- warning letters;
- •
- civil or criminal penalties;
- •
- fines;
- •
- injunctions;
- •
- product seizures or detentions;
- •
- import bans;
- •
- voluntary or mandatory product recalls and publicity requirements;
- •
- suspension or withdrawal of regulatory approvals;
- •
- total or partial suspension of production; and
- •
- refusal to approve pending applications for marketing approval of new drugs or supplements to approved applications.
Any one of these could have a material adverse effect on our business or financial condition.
If our collaborative partners fail to perform their obligations under our agreements with them, or determine not to continue with clinical trials for particular product candidates, our business could be severely impacted.
Our strategy for the development and commercialization of our product candidates depends, in large part, upon the formation and maintenance of collaborative arrangements. Collaborations provide an opportunity for us to:
- •
- generate cash flow and revenue;
- •
- fund some of the costs associated with our internal research and development, preclinical testing, clinical trials and
manufacturing;
- •
- seek and obtain regulatory approvals faster than we could on our own;
- •
- successfully commercialize existing and future product candidates; and
- •
- secure access to targets which, due to intellectual property restrictions, would otherwise be unavailable to our technology.
If we fail to secure or maintain successful collaborative arrangements, the development and marketing of compounds that use our technology may be delayed, scaled back or otherwise may not occur. In addition, we may be unable to negotiate other collaborative arrangements or, if necessary,
31
modify our existing arrangements on acceptable terms. We cannot control the amount and timing of resources our collaborative partners may devote to our product candidates. Our collaborative partners may separately pursue competing product candidates, therapeutic approaches or technologies to develop treatments for the diseases targeted by us or our collaborative efforts, or may decide, for reasons not known to us, to discontinue development of product candidates under our agreements with them. Any of our collaborative partners may slow or discontinue the development of a product candidate covered by a collaborative arrangement for reasons that can include, but are not limited to:
- •
- a change in the collaborative partner's strategic focus as a result of merger, management changes, adverse business
events, or other causes;
- •
- a change in the priority of the product candidate relative to other programs in the collaborator's pipeline;
- •
- a reassessment of the patent situation related to the compound or its target;
- •
- a change in the anticipated competition for the product candidate;
- •
- preclinical studies and clinical trial results; and
- •
- a reduction in the financial resources the collaborator can or is willing to apply to the development of new compounds.
Even if our collaborative partners continue their collaborative arrangements with us, they may nevertheless determine not to actively pursue the development or commercialization of any resulting products. Also, our collaborative partners may fail to perform their obligations under the collaborative agreements or may be slow in performing their obligations. Our collaborative partners can terminate our collaborative agreements under certain conditions. The decision to advance a product that is covered by a collaborative agreement through clinical trials and ultimately to commercialization is in the discretion of our collaborative partners. If any collaborative partner were to terminate or breach our agreements, fail to complete its obligations to us in a timely manner, or decide to discontinue its development of a product candidate, our anticipated revenue from the agreement and from the development and commercialization of the products would be severely limited. If we are not able to establish additional collaborations or any or all of our existing collaborations are terminated and we are not able to enter into alternative collaborations on acceptable terms, or at all, our continued development, manufacture and commercialization of our product candidates could be delayed or scaled back as we may not have the funds or capability to continue these activities. If our collaborators fail to successfully develop and commercialize TAP compounds, our business prospects would be severely harmed.
We depend on a small number of collaborators for a substantial portion of our revenue. The loss of, or a material reduction in activity by, any one of these collaborators could result in a substantial decline in our revenue.
We have and will continue to have collaborations with a limited number of companies. As a result, our financial performance depends on the efforts and overall success of these companies. Also, the failure of any one of our collaborative partners to perform its obligations under its agreement with us, including making any royalty, milestone or other payments to us, could have an adverse effect on our financial condition. Further, any material reduction by any one of our collaborative partners in its level of commitment of resources, funding, personnel, and interest in continued development under its agreement with us could have an adverse effect on our financial condition. Also, if consolidation trends in the healthcare industry continue, the number of our potential collaborators could decrease, which could have an adverse impact on our development efforts. If a present or future collaborator of ours were to be involved in a business combination, the collaborator's continued pursuit and emphasis on our product development program could be delayed, diminished or terminated.
32
Our royalty revenues will likely fluctuate and may become more difficult to forecast in future periods.
On February 22, 2013, the FDA granted marketing approval to Kadcyla. Kadcyla was developed by Roche, through its Genentech unit, under a license we granted in May 2000, pursuant to which we are entitled to receive milestone payments plus royalties on commercial sales of Kadcyla. Roche and its affiliates have also applied for marketing approval of Kadcyla in Europe and Japan. As a result of the start of commercialization of Kadcyla in the U.S. and the possible marketing approvals elsewhere, we expect an increasing proportion of our revenue and operating results to derive from royalties based on the commercial sales of Kadcyla. These royalty revenues may fluctuate considerably because they depend upon, among other things, the rate of growth of sales of Kadcyla as well as the mix of U.S.-based sales and ex-U.S.-based sales and our valid patent claims. Kadcyla is currently the only product with respect to which we are entitled to receive royalties that has received marketing approval.
The Roche agreement provides for separate tiered royalty structures with respect to sales in two territories: 1) the U.S. and 2) the rest of the world. The royalty rate Roche must pay on sales in each of these two territories increases on incremental sales in a given calendar year in the applicable territory above certain net sales thresholds. As a result of the tiered royalty structure, Roche's average royalty rate should increase over the course of a calendar year as more Kadcyla is sold in that year. However, we recognize royalty revenues in the quarter in which they are received, which are based on Kadcyla sales in the preceding quarter. Accordingly, we anticipate that the average royalty rate for payments we receive from Roche will generally increase between the second quarter of one calendar year (our fourth fiscal quarter) and the first calendar quarter of the next (our third quarter of the next fiscal year).
We depend on our collaborative partners for the determination of royalty payments. We may not be able to detect errors and payment calculations may call for retroactive adjustments.
The royalty payments we receive are determined by our collaborative partners based on their reported net sales. Each collaborative partner's calculation of the royalty payments is subject to and dependent upon the adequacy and accuracy of its sales and accounting functions, and errors may occur from time to time in the calculations made by a collaborative partner. Our agreement with Genentech provides us the right to audit the calculations and sales data for the associated royalty payments related to sales of Kadcyla; however, such audits may occur many months following our recognition of the royalty revenue, may require us to adjust our royalty revenues in later periods and generally require expense on our part.
If our collaborative partners' requirements for clinical materials to be manufactured by us are significantly lower than we have estimated, our financial results and condition could be adversely affected.
We procure certain components of finished conjugate, including DM1, DM4, and linker, on behalf of several of our collaborators. In order to meet our commitments to our collaborative partners, we are required to enter into agreements with third parties to produce these components well in advance of our production of clinical materials on behalf of our collaborative partners. If our collaborative partners do not require as much clinical material as we have contracted to produce and we are unable to use these materials for our own products, we may not be able to recover our investment in these components and we may suffer losses. Collaborators have discontinued development of product candidates in the past and in the periods subsequent to these discontinuations, we had significantly reduced demand for DM1 and DM4 which adversely impacted our financial results.
In addition, we operate a conjugate manufacturing facility. A portion of the cost of operating this facility, including the cost of manufacturing personnel, is reimbursed by our collaborators based on the number of batches of preclinical and clinical materials produced on their behalf. If we produce fewer
33
batches of clinical materials for our collaborators, a smaller amount of the cost of operating the conjugate manufacturing facility will be charged to our collaborative partners and our financial condition could be adversely affected.
If our product requirements for clinical trials are significantly higher than we estimated, the inability to procure additional antibody or fill/finish services in a timely manner could impair our ability to initiate or advance our clinical trials.
We rely on third-party suppliers to manufacture antibodies used in our own proprietary compounds. Due to the specific nature of the antibody and availability of production capacity, there is significant lead time required by these suppliers to provide us with the needed materials. If our antibody requirements for clinical materials to be manufactured are significantly higher than we estimated, we may not be able to readily procure additional antibody which would impair our ability to advance our clinical trials currently in process or initiate additional trials. We also rely on third parties to convert the bulk drug substance we manufacture into filled and finished vials of drug product for clinical use. Unanticipated difficulties or delays in the fill/finish process could impair our ability to advance our clinical trials currently in process or initiate additional trials. There can be no assurance that we will not have supply problems that could delay or stop our clinical trials or otherwise could have a material adverse effect on our business.
We currently rely on one third-party manufacturer with commercial production experience to produce our cell-killing agents, DM1 and DM4.
We rely on a third-party supplier to manufacture one of the materials used to make TAP compounds. Our cell-killing agents DM1 and DM4, collectively DMx, are manufactured from a precursor, ansamitocin P3. We currently use a single supplier, Societá Italiana Corticosteroidi S.r.l.,that converts ansamitocin P3 to DMx. Any delay or interruption in our supply of DMx could lead to a delay or interruption in our manufacturing operations, preclinical studies and clinical trials of our product candidates and our collaborators' product candidates, which could negatively affect our business.
We may be delayed or unable to establish the manufacturing capabilities necessary to develop and commercialize our and our collaborative partners' potential products.
Currently, we have one conjugate manufacturing facility that we use to manufacture conjugated compounds for us and most of our collaborative partners for preclinical studies and early-stage clinical testing. Two of our partners have contracted for separate, large-scale manufacturing capacity to make materials to support potential future commercialization of their TAP compounds. We do not currently have the manufacturing capacity needed to make our product candidates for commercial sale. In addition, our manufacturing capacity may be insufficient to complete all clinical trials contemplated by us and our collaborative partners over time. We intend to rely in part on third-party contract manufacturers to produce sufficiently large quantities of drug materials that are and will be needed for later-stage clinical trials and commercialization of our potential products. We are currently in the process of developing relationships with third-party manufacturers that we believe will be necessary to continue the development of our product candidates. Third-party manufacturers may not be able to meet our needs with respect to timing, quantity or quality of materials. If we are unable to contract for a sufficient supply of needed materials on acceptable terms, or if we should encounter delays or difficulties in our relationships with manufacturers, our clinical trials may be delayed, thereby delaying the submission of product candidates for regulatory approval and the market introduction and subsequent commercialization of our potential products. Any such delays may lower our revenues and potential profitability.
34
We have one conjugate manufacturing facility and any prolonged and significant disruption at that facility could impair our ability to manufacture our and our collaborative partners' product candidates for clinical testing.
Currently, in certain cases, we are contractually obligated to manufacture Phase I and non-pivotal Phase II clinical products for companies licensing our TAP technology. We manufacture this material, as well as material for our own product candidates, in our conjugate manufacturing facility. We have only one such manufacturing facility in which we can manufacture clinical products. Our current manufacturing facility contains highly specialized equipment and utilizes complicated production processes developed over a number of years that would be difficult, time-consuming and costly to duplicate. Any prolonged disruption in the operations of our manufacturing facility would have a significant negative impact on our ability to manufacture products for clinical testing on our own and would cause us to seek additional third-party manufacturing contracts, thereby increasing our development costs. Even though we carry business interruption insurance policies, we may suffer losses as a result of business interruptions that exceed the coverage available or any losses which may be excluded under our insurance policies. Certain events, such as natural disasters, fire, political disturbances, sabotage or business accidents, which could impact our current or future facilities, could have a significant negative impact on our operations by disrupting our product development efforts until such time as we are able to repair our facility or put in place third-party contract manufacturers to assume this manufacturing role.
Unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives applicable to our product candidates could limit our potential product revenue.
Antibody-based anticancer products are often much more costly to produce than traditional chemotherapeutics and tend to have significantly higher prices. Factors that help justify the price include the high mortality associated with many types of cancer and the need for more and better treatment options.
Regulations governing drug pricing and reimbursement vary widely from country to country. Some countries require approval of the sales price of a drug before it can be marketed. Some countries restrict the physicians that can authorize the use of more expensive medications. Some countries establish treatment guidelines to help limit the use of more expensive therapeutics and the pool of patients that receive them. In some countries, including the U.S., third-party payers frequently seek discounts from list prices and are increasingly challenging the prices charged for medical products. Because our product candidates are in the development stage, we do not know the level of reimbursement, if any, we will receive for any products that we are able to successfully develop. If the reimbursement for any of our product candidates is inadequate in light of our development and other costs, our ability to achieve profitability would be affected.
We believe that the efforts of governments and third-party payors to contain or reduce the cost of healthcare will continue to affect the business and financial condition of pharmaceutical and biopharmaceutical companies. A number of legislative and regulatory proposals to change the healthcare system in the U.S. and other major healthcare markets have been proposed and adopted in recent years. For example, the U.S. Congress enacted a limited prescription drug benefit for Medicare recipients as part of the Medicare Prescription Drug, Improvement and Modernization Act of 2003. While the program established by this statute may increase demand for any products that we are able to successfully develop, if we participate in this program, our prices will be negotiated with drug procurement organizations for Medicare beneficiaries and are likely to be lower than prices we might otherwise obtain. Non-Medicare third-party drug procurement organizations may also base the price they are willing to pay on the rate paid by drug procurement organizations for Medicare beneficiaries. The PPACA will also require discounts under the Medicare drug benefit program and increased rebates on drugs covered by Medicaid. In addition, the PPACA imposes an annual fee, which will increase
35
annually, on sales by branded pharmaceutical manufacturers. The financial impact of these discounts, increased rebates and fees and the other provisions of the PPACA on our business is unclear and there can be no assurance that our business will not be materially adversely affected by the PPACA. In addition, ongoing initiatives in the U.S. have increased and will continue to increase pressure on drug pricing. The announcement or adoption of any such initiative could have an adverse effect on potential revenues from any product candidate that we may successfully develop.
We may be unable to establish sales and marketing capabilities necessary to successfully commercialize our potential products.
We currently have no direct sales or marketing capabilities. We may rely on third parties to market and sell most of our primary product candidates or we may outlicense these products prior to the time when these capabilities are needed. If we decide to market our potential products through a direct sales force, we would need either to hire a sales force with expertise in pharmaceutical sales or to contract with a third party to provide a sales force which meets our needs. We may be unable to establish marketing, sales and distribution capabilities necessary to commercialize and gain market acceptance for our potential products and be competitive. In addition, co-promotion or other marketing arrangements with third parties to commercialize potential products could significantly limit the revenues we derive from these potential products, and these third parties may fail to commercialize our compounds successfully.
If our product candidates or those of our collaborative partners do not gain market acceptance, our business will suffer.
Even if clinical trials demonstrate the safety and efficacy of our and our collaborative partners' product candidates and the necessary regulatory approvals are obtained, our and our collaborative partners' product candidates may not gain market acceptance among physicians, patients, healthcare payors and other members of the medical community. The degree of market acceptance of any product candidates that we or our collaborative partners develop will depend on a number of factors, including:
- •
- their degree of clinical efficacy and safety;
- •
- their advantage over alternative treatment methods;
- •
- our/the marketer's and our collaborative partners' ability to gain acceptable reimbursement and the reimbursement policies
of government and third- party payors; and
- •
- the quality of the distribution capabilities for product candidates, both ours and our collaborative partners.
Physicians may not prescribe any of our future products until such time as clinical data or other factors demonstrate the safety and efficacy of those products as compared to conventional drug and other treatments. Even if the clinical safety and efficacy of therapies using our products is established, physicians may elect not to recommend the therapies for any number of other reasons, including whether the mode of administration of our products is effective for certain conditions, and whether the physicians are already using competing products that satisfy their treatment objectives. Physicians, patients, third-party payors and the medical community may not accept and use any product candidates that we, or our collaborative partners, develop. If our products do not achieve significant market acceptance and use, we will not be able to recover the significant investment we have made in developing such products and our business will be severely harmed.
We may be unable to compete successfully.
The markets in which we compete are well established and intensely competitive. We may be unable to compete successfully against our current and future competitors. Our failure to compete
36
successfully may result in lower volume sold, pricing reductions, reduced gross margins and failure to achieve market acceptance for our potential products. Our competitors include research institutions, pharmaceutical companies and biotechnology companies, such as Pfizer, Seattle Genetics, Roche and Bristol-Myers Squibb. Many of these organizations have substantially more experience and more capital, research and development, regulatory, manufacturing, human and other resources than we do. As a result, they may:
- •
- develop products that are safer or more effective than our product candidates;
- •
- obtain FDA and other regulatory approvals or reach the market with their products more rapidly than we can, reducing the
potential sales of our product candidates;
- •
- devote greater resources to market or sell their products;
- •
- adapt more quickly to new technologies and scientific advances;
- •
- initiate or withstand substantial price competition more successfully than we can;
- •
- have greater success in recruiting skilled scientific workers from the limited pool of available talent;
- •
- more effectively negotiate third-party licensing and collaboration arrangements; and
- •
- take advantage of acquisition or other opportunities more readily than we can.
A number of pharmaceutical and biotechnology companies are currently developing products targeting the same types of cancer that we target, and some of our competitors' products have entered clinical trials or already are commercially available.
Our product candidates, if approved and commercialized, will also compete against well-established, existing, therapeutic products that are currently reimbursed by government healthcare programs, private health insurers and health maintenance organizations. In addition, if our product candidates are approved and commercialized, we may face competition from biosimilars. The route to market for biosimilars was established with the passage of the PPACA in March 2010. The PPACA establishes a pathway for the FDA approval of follow-on biologics and provides twelve years data exclusivity for reference products and an additional six months exclusivity period if pediatric studies are conducted. In Europe, the European Medicines Agency has issued guidelines for approving products through an abbreviated pathway, and biosimilars have been approved in Europe. If a biosimilar version of one of our potential products were approved in the U.S. or Europe, it could have a negative effect on sales and gross profits of the potential product and our financial condition.
We face and will continue to face intense competition from other companies for collaborative arrangements with pharmaceutical and biotechnology companies, for relationships with academic and research institutions and for licenses to proprietary technology. In addition, we anticipate that we will face increased competition in the future as new companies enter our markets and as scientific developments surrounding antibody-based therapeutics for cancer continue to accelerate. While we will seek to expand our technological capabilities to remain competitive, research and development by others may render our technology or product candidates obsolete or noncompetitive or result in treatments or cures superior to any therapy developed by us.
If we are unable to protect our intellectual property rights adequately, the value of our technology and our product candidates could be diminished.
Our success depends in part on obtaining, maintaining and enforcing our patents and other proprietary rights and our ability to avoid infringing the proprietary rights of others. Patent law relating to the scope of claims in the biotechnology field in which we operate is still evolving, is surrounded by a great deal of uncertainty and involves complex legal, scientific and factual questions. To date, no
37
consistent policy has emerged regarding the breadth of claims allowed in biotechnology patents. Accordingly, our pending patent applications may not result in issued patents or in patent claims as broad as in the original applications. Although we own numerous patents, the issuance of a patent is not conclusive as to its validity or enforceability. Through litigation, a third party may challenge the validity or enforceability of a patent after its issuance.
Patents and applications owned or licensed by us may become the subject of interference, opposition, nullity, or other proceedings in a court or patent office in the U.S. or in a foreign jurisdiction to determine validity, enforceability or priority of invention, which could result in substantial cost to us. An adverse decision in such a proceeding may result in our loss of rights under a patent or patent application. It is unclear how much protection, if any, will be given to our patents if we attempt to enforce them or if they are challenged in court or in other proceedings. A competitor may successfully invalidate our patents or a challenge could result in limitations of the patents' coverage. In addition, the cost of litigation or interference proceedings to uphold the validity of patents can be substantial. If we are unsuccessful in these proceedings, third parties may be able to use our patented technology without paying us licensing fees or royalties. Moreover, competitors may infringe our patents or successfully avoid them through design innovation. To prevent infringement or unauthorized use, we may need to file infringement claims, which are expensive and time-consuming. In an infringement proceeding, a court may decide that a patent of ours is not valid. Even if the validity of our patents were upheld, a court may refuse to stop the other party from using the technology at issue on the ground that its activities are not covered by our patents.
The Leahy-Smith America Invents Act was signed into law on September 16, 2011, and became fully effective in March 2013. In general, the legislation attempts to address issues surrounding the enforceability of patents and the increase in patent litigation by, among other things, moving to a first inventor-to-file system, establishing new procedures for challenging patents and establishing different methods for invalidating patents. While we cannot predict what form any new patent reform regulations ultimately may take, final governmental rule-making and case law interpreting the new statute could introduce new substantive rules, procedures and case law bases for challenging patents, and certain reforms that make it easier for competitors to challenge our patents could have a material adverse effect on our business and prospects.
Policing unauthorized use of our intellectual property is difficult, and we may not be able to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the U.S.
In addition to our patent rights, we also rely on unpatented technology, trade secrets, know-how and confidential information. Third parties may independently develop substantially equivalent information and techniques or otherwise gain access to or disclose our technology. We may not be able to effectively protect our rights in unpatented technology, trade secrets, know-how and confidential information. We require each of our employees, consultants and corporate partners to execute a confidentiality agreement at the commencement of an employment, consulting or collaborative relationship with us. Further, we require that all employees enter into assignment of invention agreements as a condition of employment. However, these agreements may not provide effective protection of our information or, in the event of unauthorized use or disclosure, they may not provide adequate remedies.
Any inability to license proprietary technologies or processes from third parties which we use in connection with the development and manufacture of our product candidates may impair our business.
Other companies, universities and research institutions have or may obtain patents that could limit our ability to use, manufacture, market or sell our product candidates or impair our competitive position. As a result, we would have to obtain licenses from other parties before we could continue
38
using, manufacturing, marketing or selling our potential products. Any necessary licenses may not be available on commercially acceptable terms, if at all. If we do not obtain required licenses, we may not be able to market our potential products at all or we may encounter significant delays in product development while we redesign products or methods that are found to infringe on the patents held by others.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights held by third parties and we may be unable to protect our rights to, or to commercialize, our product candidates.
Patent litigation is very common in the biotechnology and pharmaceutical industries. Third parties may assert patent or other intellectual property infringement claims against us with respect to our technologies, products or other matters. From time to time, we have received correspondence from third parties alleging that we infringe their intellectual property rights. Any claims that might be brought against us alleging infringement of patents may cause us to incur significant expenses and, if successfully asserted against us, may cause us to pay substantial damages and limit our ability to use the intellectual property subject to these claims. Even if we were to prevail, any litigation would be costly and time-consuming and could divert the attention of our management and key personnel from our business operations. Furthermore, as a result of a patent infringement suit, we may be forced to stop or delay developing, manufacturing or selling potential products that incorporate the challenged intellectual property unless we enter into royalty or license agreements. There may be third-party patents, patent applications and other intellectual property relevant to our potential products that may block or compete with our products or processes. In addition, we sometimes undertake research and development with respect to potential products even when we are aware of third-party patents that may be relevant to our potential products, on the basis that such patents may be challenged or licensed by us. If our subsequent challenge to such patents were not to prevail, we may not be able to commercialize our potential products after having already incurred significant expenditures unless we are able to license the intellectual property on commercially reasonable terms. We may not be able to obtain royalty or license agreements on terms acceptable to us, if at all. Even if we were able to obtain licenses to such technology, some licenses may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. Ultimately, we may be unable to commercialize some of our potential products or may have to cease some of our business operations, which could severely harm our business.
We use hazardous materials in our business, and any claims relating to improper handling, storage or disposal of these materials could harm our business.
Our research and development and manufacturing activities involve the controlled use of hazardous materials, chemicals, biological materials and radioactive compounds. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and certain waste products. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by applicable laws and regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of such an accident, we could be held liable for any resulting damages, and any liability could exceed our resources. We may be required to incur significant costs to comply with these laws in the future. Failure to comply with these laws could result in fines and the revocation of permits, which could prevent us from conducting our business.
We face product liability risks and may not be able to obtain adequate insurance.
While we secure waivers from all participants in our clinical trials, the use of our product candidates during testing or after approval entails an inherent risk of adverse effects, which could
39
expose us to product liability claims. Regardless of their merit or eventual outcome, product liability claims may result in:
- •
- decreased demand for our product;
- •
- injury to our reputation and significant negative media attention;
- •
- withdrawal of clinical trial volunteers;
- •
- costs of litigation;
- •
- distraction of management; and
- •
- substantial monetary awards to plaintiffs.
We may not have sufficient resources to satisfy any liability resulting from these claims. We currently have product liability insurance for products which are in clinical testing, however, our coverage may not be adequate in scope to protect us in the event of a successful product liability claim. Further, we may not be able to maintain our current insurance or obtain general product liability insurance on reasonable terms and at an acceptable cost if we or our collaborative partners begin commercial production of our proposed product candidates. This insurance, even if we can obtain and maintain it, may not be sufficient to provide us with adequate coverage against potential liabilities.
We depend on our key personnel and we must continue to attract and retain key employees and consultants.
We depend on our key scientific and management personnel. Our ability to pursue the development of our current and future product candidates depends largely on retaining the services of our existing personnel and hiring additional qualified scientific personnel to perform research and development. We will also need to hire personnel with expertise in clinical testing, government regulation, manufacturing, marketing and finance. Attracting and retaining qualified personnel will be critical to our success. We may not be able to attract and retain personnel on acceptable terms given the competition for such personnel among biotechnology, pharmaceutical and healthcare companies, universities and non-profit research institutions. Failure to retain our existing key management and scientific personnel or to attract additional highly qualified personnel could delay the development of our product candidates and harm our business.
Our stock price can fluctuate significantly and results announced by us and our collaborators can cause our stock price to decline.
Our stock price can fluctuate significantly due to business developments announced by us and by our collaborators, as a result of market trends and daily trading volume. The business developments that could impact our stock price include disclosures related to clinical findings with compounds that make use of our TAP technology, new collaborations and clinical advancement or discontinuation of product candidates that make use of our TAP technology. Our stock price can also fluctuate significantly with the level of overall investment interest in small-cap biotechnology stocks.
Our operating results have fluctuated in the past and are likely to continue to do so in the future. Our revenue is unpredictable and may fluctuate due to the timing of non-recurring licensing fees, decisions of our collaborative partners with respect to our agreements with them, reimbursement for manufacturing services, the achievement of milestones and our receipt of the related milestone payments under new and existing licensing and collaboration agreements. Revenue historically recognized under our prior collaboration agreements may not be an indicator of revenue from any future collaborations. In addition, our expenses are unpredictable and may fluctuate from quarter to quarter due to the timing of expenses, which may include obligations to manufacture or supply product or payments owed by us under licensing or collaboration agreements. It is possible that our quarterly
40
and/or annual operating results will not meet the expectations of securities analysts or investors, causing the market price of our common stock to decline. We believe that quarter-to-quarter and year-to-year comparisons of our operating results are not good indicators of our future performance and should not be relied upon to predict the future performance of our stock price.
The potential sale of additional shares of our common stock may cause our stock price to decline.
Pursuant to shelf registration statements filed with the Securities and Exchange Commission, in July 2012, we sold 6,250,000 shares of our common stock at $16.00 per share in a public offering resulting in gross proceeds of $100 million; in fiscal 2011, we sold 7,800,000 shares of our common stock at $12.00 per share in a public offering resulting in gross proceeds of $93.6 million; in fiscal 2010, we sold 10,350,000 shares of our common stock at $8.00 per share in a public offering resulting in gross proceeds of $82.8 million; and in fiscal 2009, we sold 5,750,000 shares of our common stock at $7.00 per share in a public offering resulting in gross proceeds of $40.3 million. The potential sale of additional shares of our common stock may be dilutive to our shares outstanding and may cause our stock price to decrease.
We do not intend to pay cash dividends on our common stock.
We have not paid cash dividends since our inception and do not intend to pay cash dividends in the foreseeable future. Therefore, shareholders will have to rely on appreciation in our stock price, if any, in order to achieve a gain on an investment.
41
A WARNING ABOUT FORWARD-LOOKING STATEMENTS
This report includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to analyses and other information which are based on forecasts of future results and estimates of amounts that are not yet determinable. These statements also relate to our future prospects, developments and business strategies.
These forward-looking statements are identified by their use of terms and phrases, such as "anticipate," "believe," "could," "estimate," "expect," "intend," "may," "plan," "predict," "project," "will" and other similar terms and phrases, including references to assumptions. These statements are contained in the "Business," "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections, as well as other sections of this Annual Report on Form 10-K.
These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those contemplated by our forward-looking statements. These known and unknown risks, uncertainties and other factors are described in detail in the "Risk Factors" section and in other sections of this Annual Report on Form 10-K. We disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Item 1B. Unresolved Staff Comments
None.
We lease approximately 89,000 square feet of laboratory and office space in a building located at 830 Winter Street, Waltham, MA. The initial term of the 830 Winter Street lease expires on March 31, 2020, with an option for us to extend the lease for two additional five-year terms. In December 2009, we entered into a sublease, as sublessor, to rent 14,100 square feet of our original office and laboratory space at 830 Winter Street, Waltham, MA through January 2015. Due to space requirements, in April 2012, we entered into a sublease agreement for the rental of 7,310 square feet of additional laboratory and office space at 830 Winter Street, Waltham, MA for an initial term of three years with a conditional option to extend through October 2017. We also lease approximately 43,850 square feet of space at 333 Providence Highway, Norwood, MA, which serves as our conjugate manufacturing facility and office space. The 333 Providence Highway lease expires on June 30, 2018, with an option for us to extend the lease for an additional five-year term. Due to space requirements, in April 2013, we entered into a lease agreement for the rental of 7,507 square feet of office space at 100 River Ridge Drive, Norwood, MA. The initial term of the lease is for five years and two months commencing in July 2013 with an option for us to extend the lease for an additional five-year term.
From time to time we may be a party to various legal proceedings arising in the ordinary course of our business. We are not currently subject to any material legal proceedings.
Item 3.1. Executive Officers of the Registrant
ImmunoGen's executive officers are appointed by the Board of Directors at the first meeting of the Board following the annual meeting of shareholders or at other Board meetings as appropriate, and hold office until the first Board meeting following the next annual meeting of shareholders and until a successor is chosen, subject to prior death, resignation or removal. Information regarding our executive officers is presented below.
42
Daniel M. Junius, age 61, joined ImmunoGen in 2005, and has served as our President and Chief Executive Officer since 2009. Prior to that he served as our President and Chief Operating Officer and Acting Chief Financial Officer from July 2008 to December 2008, as our Executive Vice President and Chief Financial Officer from 2006 to July 2008, and as our Senior Vice President and Chief Financial Officer from 2005 to 2006. Mr. Junius has also served as a director of ImmunoGen since 2008. Mr. Junius holds a Masters of Management from Northwestern University's Kellogg School of Management.
John M. Lambert, Ph.D., age 62, joined ImmunoGen in 1987, and has served as our Executive Vice President and Chief Scientific Officer since July 2008. Prior to that he served in various capacities of increasing responsibility in the areas of research and development. Dr. Lambert holds a Ph.D. in Biochemistry from University of Cambridge in England, and completed his postdoctoral work at the University of California at Davis and at Glasgow University in Scotland.
Charles Q. Morris, MB, ChB, MRCP (UK), age 48, joined ImmunoGen in November 2012, and has served as our Executive Vice President and Chief Development Officer since that date. Prior to joining ImmunoGen, he served as Executive Vice President and Chief Medical Officer of Allos Therapeutics, Inc., a biotechnology company, from 2010 until its acquisition in 2012. Prior to that he served as Vice President, Worldwide Clinical Research, at Cephalon, Inc., a biotechnology company, from 2008 to 2010, and as Vice President, Clinical Research, Oncology, at Cephalon from 2007 to 2008. Dr. Morris holds two Bachelor of Medicine degrees from Sheffield University Medical School and is a member of the Royal College of Physicians of London.
James J. O'Leary, MD, age 49, joined ImmunoGen in 2008, and has served as our Vice President and Chief Medical Officer since that date. Prior to joining ImmunoGen, Dr. O'Leary served as Senior Medical Director Clinical Oncology of Bayer Corporation, a pharmaceutical company, from 2006 to 2008. Dr. O'Leary has a Doctor of Medicine degree from the State University of New York—Health Science Center at Brooklyn.
Gregory D. Perry, age 53, joined ImmunoGen in 2009, and has served as our Executive Vice President and Chief Financial Officer since April 2011. Prior to that, he served as our Senior Vice President and Chief Financial Officer from 2009 to April 2011. Prior to joining ImmunoGen, he served as Chief Financial Officer of Elixir Pharmaceuticals, Inc., a pharmaceutical company, from 2007 to 2008. Mr. Perry is also a director of Advanced Cell Technology, Inc. Mr. Perry has notified us of his intention to resign from ImmunoGen, effective September 13, 2013.
Craig Barrows, age 58, joined ImmunoGen in 2007, and has served as our Vice President, General Counsel and Secretary since that date.
Peter J. Williams, age 59, joined ImmunoGen in August 2009, and has served as our Vice President, Business Development since that date. Prior to joining ImmunoGen, he served as a Senior Director of Business Development at Alnylam Pharmaceuticals, Inc., a biopharmaceutical company, from 2006 to August 2009.
Theresa G. Wingrove, Ph.D., age 55, joined ImmunoGen in January 2011, and has served as our Vice President, Regulatory Affairs since that date. Prior to joining ImmunoGen, she served as Vice President, Regulatory and Clinical Affairs, at Histogenics, Inc., a medical device company, from 2006 to January 2011. Dr. Wingrove holds a Ph.D. in biochemical toxicology from the University of Rochester School of Medicine and Dentistry, and completed her postdoctoral work at the University of Rochester Medical Center.
Item 4. Mine Safety Disclosures
None.
43
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Price of Our Common Stock and Related Stockholder Matters
Our common stock is quoted on the NASDAQ Global Select Market under the symbol "IMGN." The table below sets forth the high and low closing price per share of our common stock as reported by NASDAQ:
| |
Fiscal Year 2013 | Fiscal Year 2012 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
High | Low | High | Low | |||||||||
First Quarter |
$ | 18.10 | $ | 12.51 | $ | 15.55 | $ | 9.42 | |||||
Second Quarter |
$ | 15.77 | $ | 10.85 | $ | 14.44 | $ | 10.09 | |||||
Third Quarter |
$ | 16.54 | $ | 12.92 | $ | 14.61 | $ | 11.38 | |||||
Fourth Quarter |
$ | 18.83 | $ | 13.91 | $ | 16.74 | $ | 12.22 | |||||
As of August 20, 2013, the closing price per share of our common stock was $16.16, as reported by NASDAQ, and we had approximately 658 holders of record of our common stock.
We have not paid any cash dividends on our common stock since our inception and do not intend to pay any cash dividends in the foreseeable future.
Recent Sales of Unregistered Securities; Uses of Proceeds from Registered Securities; Issuer Repurchases of Equity Securities
None.
Item 6. Selected Financial Data
The following table (in thousands, except per share data) sets forth our consolidated financial data for each of our five fiscal years through our fiscal year ended June 30, 2013. The information set forth below should be read in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the consolidated financial statements and related notes included elsewhere in this Annual Report on Form 10-K.
| |
Year Ended June 30, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | 2011 | 2010 | 2009 | |||||||||||
Consolidated Statement of Operations Data: |
||||||||||||||||
Total revenues |
$ | 35,535 | $ | 16,357 | $ | 19,305 | $ | 13,943 | $ | 27,988 | ||||||
Total operating expenses |
108,544 | 89,614 | 79,493 | 65,178 | 59,804 | |||||||||||
Other income (expense), net |
198 | (62 | ) | 1,914 | 58 | (221 | ) | |||||||||
(Benefit) provision for income taxes |
— | — | — | (265 | ) | (100 | ) | |||||||||
Net loss |
$ | (72,811 | ) | $ | (73,319 | ) | $ | (58,274 | ) | $ | (50,912 | ) | $ | (31,937 | ) | |
Basic and diluted net loss per common share |
$ | (0.87 | ) | $ | (0.95 | ) | $ | (0.85 | ) | $ | (0.87 | ) | $ | (0.63 | ) | |
Basic and diluted weighted average common shares outstanding |
84,063 | 76,814 | 68,919 | 58,845 | 51,068 | |||||||||||
Consolidated Balance Sheet Data: |
||||||||||||||||
Cash, cash equivalents and marketable securities |
$ | 194,960 | $ | 160,938 | $ | 191,206 | $ | 110,298 | $ | 71,125 | ||||||
Total assets |
213,596 | 180,308 | 217,641 | 137,208 | 100,704 | |||||||||||
Shareholders' equity |
121,847 | 83,890 | 139,969 | 102,048 | 66,857 | |||||||||||
44
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
Overview
Since our inception, we have been principally engaged in the development of novel, antibody-drug conjugates (ADC's) for the treatment of cancer using our expertise in cancer biology, monoclonal antibodies, highly potent cytotoxic, or cell-killing, agents, and the design of linkers that enable these agents to remain stably attached to the antibodies while in the blood stream and released in their fully active form after delivery to a cancer cell. An anticancer compound made using our Targeted Antibody Payload, or TAP, technology consists of a monoclonal antibody that binds specifically to an antigen target found on cancer cells with multiple copies of one of our proprietary cell-killing agents attached to the antibody using one of our engineered linkers. Its antibody component enables a TAP compound to bind specifically to cancer cells that express its target antigen, the highly potent cytotoxic agent serves to kill the cancer cell, and the engineered linker controls the release and activation of the cytotoxic agent inside the cancer cell. With some TAP compounds, the antibody component also has anticancer activity of its own. Our TAP technology is designed to enable the creation of highly effective, well-tolerated anticancer product candidates. All of the TAP compounds currently in clinical testing contain either DM1 or DM4 as the cytotoxic agent. Both DM1 and DM4, collectively DMx, are our proprietary derivatives of a cytotoxic agent called maytansine. We also use our expertise in antibodies and cancer biology to develop "naked," or non-conjugated, antibody anticancer product candidates.
We have used our proprietary TAP technology in conjunction with our in-house antibody expertise to develop our own anticancer product candidates. We have also entered into agreements that enable companies to use our TAP technology to develop and commercialize product candidates to specified targets. Under the terms of our agreements, we are generally entitled to upfront fees, milestone payments, and royalties on any commercial product sales. In addition, under certain agreements we are compensated for research and development activities performed at our collaborative partner's request at negotiated prices which are generally consistent with what other third parties would charge. We are compensated to manufacture preclinical and clinical materials and deliver cytotoxic agent at negotiated prices which are generally consistent with what other third parties would charge. Currently, our partners include Amgen, Bayer HealthCare, Biotest, Lilly, Novartis, Roche and Sanofi. We expect that substantially all of our revenue for the foreseeable future will result from payments under our collaborative arrangements. Details for some of our major and recent collaborative agreements can be found in this Form 10-K under Item 1. Business.
To date, we have not generated revenues from commercial sales of internal products and we expect to incur significant operating losses for the foreseeable future. As of June 30, 2013, we had approximately $195 million in cash and cash equivalents compared to $160.9 million as of June 30, 2012.
We anticipate that future cash expenditures will be partially offset by collaboration-derived proceeds, including milestone payments, royalties and upfront fees. Accordingly, period-to-period operational results may fluctuate dramatically based upon the timing of receipt of the proceeds. We believe that our established collaborative agreements, while subject to specified milestone achievements, will provide funding to assist us in meeting obligations under our collaborative agreements while also assisting in providing funding for the development of internal product candidates and technologies. However, we can give no assurances that such collaborative agreement funding will, in fact, be realized in the time frames we expect, or at all. Should we or our partners not meet some or all of the terms and conditions of our various collaboration agreements, we may be required to secure alternative financing arrangements, find additional partners and/or defer or limit some or all of our research, development and/or clinical projects. However, we cannot provide assurance that any such opportunities
45
presented by additional partners or alternative financing arrangements will be entirely available to us, if at all.
Critical Accounting Policies
We prepare our consolidated financial statements in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to our collaborative agreements, inventory and stock-based compensation. We base our estimates on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from these estimates.
We believe the following critical accounting policies reflect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We enter into licensing and development agreements with collaborative partners for the development of monoclonal antibody-based anticancer therapeutics. The terms of these agreements contain multiple deliverables which may include (i) licenses, or options to obtain licenses, to our TAP technology, (ii) rights to future technological improvements, (iii) research activities to be performed on behalf of the collaborative partner, (iv) delivery of cytotoxic agents and (v) the manufacture of preclinical or clinical materials for the collaborative partner. Payments to us under these agreements may include upfront fees, option fees, exercise fees, payments for research activities, payments for the manufacture of preclinical or clinical materials, payments based upon the achievement of certain milestones and royalties on product sales. We follow the provisions of the Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 605-25, "Revenue Recognition—Multiple-Element Arrangements," and ASC Topic 605-28, "Revenue Recognition—Milestone Method," in accounting for these agreements. In order to account for these agreements, we must identify the deliverables included within the agreement and evaluate which deliverables represent separate units of accounting based on if certain criteria are met, including whether the delivered element has stand-alone value to the collaborator. The consideration received is allocated among the separate units of accounting, and the applicable revenue recognition criteria are applied to each of the separate units.
At June 30, 2013, we had the following two types of agreements with the parties identified below:
- •
- Development and commercialization licenses to use our TAP technology and/or certain other intellectual property to develop compounds to a specified target antigen (referred to as development and commercialization licenses, as distinguished from our right-to-test agreements described elsewhere):
Amgen (three exclusive single-target licenses; one non-exclusive single-target license)
Bayer HealthCare (one exclusive single-target license)
Biotest (one exclusive single-target license)
Novartis (one license to two related targets, one target on an exclusive basis and the second target on a non-exclusive basis)
Roche, through its Genentech unit (five exclusive single-target licenses)
Sanofi (exclusive license to multiple individual targets)
46
- •
- Option/research agreement for a defined period of time to secure development and commercialization licenses to use our TAP technology to develop anticancer compounds to specified targets on established terms (referred to herein as right-to-test agreements):
Sanofi
Novartis
Lilly
There are no performance, cancellation, termination or refund provisions in any of the arrangements that contain material financial consequences to us.
Development and Commercialization Licenses
The deliverables under a development and commercialization license agreement generally include the exclusive license to our TAP technology with respect to a specified antigen target, and may also include deliverables related to rights to future technological improvements, research activities to be performed on behalf of the collaborative partner and the manufacture of preclinical or clinical materials for the collaborative partner.
Generally, development and commercialization licenses contain non-refundable terms for payments and, depending on the terms of the agreement, provide that we will (i) at the collaborator's request, provide research services at negotiated prices which are generally consistent with what other third parties would charge, (ii) at the collaborator's request, manufacture and provide to it preclinical and clinical materials or deliver cytotoxic agents at negotiated prices which are generally consistent with what other third parties would charge, (iii) earn payments upon the achievement of certain milestones and (iv) earn royalty payments, generally until the later of the last applicable patent expiration or 10 to 12 years after product launch. In the case of Kadcyla, however, the minimum royalty term is 10 years and the maximum royalty term is 12 years on a country-by-country basis. Royalty rates may vary over the royalty term depending on our intellectual property rights. We may provide technical assistance and share any technology improvements with our collaborators during the term of the collaboration agreements. We do not directly control when or whether any collaborator will request research or manufacturing services, achieve milestones or become liable for royalty payments. As a result, we cannot predict when or if we will recognize revenues in connection with any of the foregoing.
In determining the units of accounting, management evaluates whether the license has stand-alone value from the undelivered elements to the collaborative partner based on the consideration of the relevant facts and circumstances for each arrangement. Factors considered in this determination include the research capabilities of the partner and the availability of TAP technology research expertise in the general marketplace. If we conclude that the license has stand alone value and therefore will be accounted for as a separate unit of accounting, we then determine the estimated selling prices of the license and all other units of accounting based on market conditions, similar arrangements entered into by third parties, and entity-specific factors such as the terms of our previous collaborative agreements, recent preclinical and clinical testing results of therapeutic products that use our TAP technology, our pricing practices and pricing objectives, the likelihood that technological improvements will be made, the likelihood that technological improvements made will be used by our collaborators and the nature of the research services to be performed on behalf of our collaborators and market rates for similar services.
Upfront payments on development and commercialization licenses are deferred if facts and circumstances dictate that the license does not have stand-alone value. Prior to the adoption of Accounting Standards Update (ASU) No. 2009-13, "Revenue Arrangements with Multiple Deliverables" on July 1, 2010, we determined that our licenses lacked stand-alone value and were combined with other elements of the arrangement and any amounts associated with the license were
47
deferred and amortized over a certain period, which we refer to as our period of substantial involvement. The determination of the length of the period over which to defer revenue is subject to judgment and estimation and can have an impact on the amount of revenue recognized in a given period. Historically our involvement with the development of a collaborator's product candidate has been significant at the early stages of development, and lessens as it progresses into clinical trials. Also, as a drug candidate gets closer to commencing pivotal testing our collaborators have sought an alternative site to manufacture their products, as our facility does not produce pivotal or commercial drug product. Accordingly, we generally estimate this period of substantial involvement to begin at the inception of the collaboration agreement and conclude at the end of non-pivotal Phase II testing. We believe this period of substantial involvement is, depending on the nature of the license, on average six and one-half years. Quarterly, we reassess our periods of substantial involvement over which we amortize our upfront license fees and make adjustments as appropriate. In the event a collaborator elects to discontinue development of a specific product candidate under a development and commercialization license, but retains its right to use our technology to develop an alternative product candidate to the same target or a target substitute, we would cease amortization of any remaining portion of the upfront fee until there is substantial preclinical activity on another product candidate and its remaining period of substantial involvement can be estimated. In the event that a development and commercialization license were to be terminated, we would recognize as revenue any portion of the upfront fee that had not previously been recorded as revenue, but was classified as deferred revenue, at the date of such termination.
Subsequent to the adoption of ASU No. 2009-13, we determined that our research licenses lack stand-alone value and are considered for aggregation with the other elements of the arrangement and accounted for as one unit of accounting.
Upfront payments on development and commercialization licenses may be recognized upon delivery of the license if facts and circumstances dictate that the license has stand-alone value from the undelivered elements, which generally include rights to future technological improvements, research services, delivery of cytotoxic agents and the manufacture of preclinical and clinical materials.
We recognize revenue related to research services that represent separate units of accounting as they are performed, as long as there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collection of the related receivable is probable. We recognize revenue related to the rights to future technological improvements over the estimated term of the applicable license.
We may also provide cytotoxic agents to our collaborators or produce preclinical and clinical materials for them at negotiated prices which are generally consistent with what other third parties would charge. We recognize revenue on cytotoxic agents and on preclinical and clinical materials when the materials have passed all quality testing required for collaborator acceptance and title and risk of loss have transferred to the collaborator. Arrangement consideration allocated to the manufacture of preclinical and clinical materials in a multiple-deliverable arrangement is below our full cost, and our full cost is not expected to ever be below our contract selling prices for our existing collaborations. During the fiscal years ended June 30, 2013, 2012 and 2011, the difference between our full cost to manufacture preclinical and clinical materials on behalf of our collaborators as compared to total amounts received from collaborators for the manufacture of preclinical and clinical materials was $755,000, $85,000, and $1.3 million, respectively. The majority of our costs to produce these preclinical and clinical materials are fixed and then allocated to each batch based on the number of batches produced during the period. Therefore, our costs to produce these materials are significantly impacted by the number of batches produced during the period. The volume of preclinical and clinical materials we produce is directly related to the number of clinical trials we and our collaborators are preparing for or currently have underway, the speed of enrollment in those trials, the dosage schedule of each clinical trial and the time period such trials last. Accordingly, the volume of preclinical and clinical
48
materials produced, and therefore our per batch costs to manufacture these preclinical and clinical materials, may vary significantly from period to period.
We may also produce research material for potential collaborators under material transfer agreements. Additionally, we perform research activities, including developing antibody specific conjugation processes, on behalf of our collaborators and potential collaborators during the early evaluation and preclinical testing stages of drug development. We record amounts received for research materials produced or services performed as a component of research and development support revenue. We also develop conjugation processes for materials for later stage testing and commercialization for certain collaborators. We are compensated at negotiated rates and may receive milestone payments for developing these processes which are recorded as a component of research and development support revenue.
Our development and commercialization license agreements have milestone payments which for reporting purposes are aggregated into three categories: (i) development milestones, (ii) regulatory milestones, and (iii) sales milestones. Development milestones are typically payable when a product candidate initiates or advances into different clinical trial phases. Regulatory milestones are typically payable upon submission for marketing approval with the FDA or other countries' regulatory authorities or on receipt of actual marketing approvals for the compound or for additional indications. Sales milestones are typically payable when annual sales reach certain levels.
At the inception of each agreement that includes milestone payments, we evaluate whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity's performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity's performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We evaluate factors such as the scientific, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required to achieve the respective milestone and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment.
Non-refundable development and regulatory milestones that are expected to be achieved as a result of our efforts during the period of substantial involvement are considered substantive and are recognized as revenue upon the achievement of the milestone, assuming all other revenue recognition criteria are met. Milestones that are not considered substantive because we do not contribute effort to the achievement of such milestones are generally achieved after the period of substantial involvement and are recognized as revenue upon achievement of the milestone, as there are no undelivered elements remaining and no continuing performance obligations, assuming all other revenue recognition criteria are met.
Under our development and commercialization license agreements, we receive royalty payments based upon its licensees' net sales of covered products. Generally, under these agreements we are to receive royalty reports and payments from its licensees approximately one quarter in arrears, that is, generally in the second month of the quarter after the licensee has sold the royalty bearing product or products. We recognize royalty revenues when it can reliably estimate such amounts and collectability is reasonably assured. As such, we generally recognizes royalty revenues in the quarter reported to us by our licensees, or one quarter following the quarter in which sales by our licensees occurred.
Right-to-Test Agreements
Our right-to-test agreements provide collaborators the right to (a) test our TAP technology for a defined period of time through a research, or right-to-test, license, (b) take options, for a defined
49
period of time, to specified targets and (c) upon exercise of those options, secure or "take" licenses to develop and commercialize products for the specified targets on established terms. Under these agreements, fees may be due to us (i) at the inception of the arrangement (referred to as "upfront" fees or payments), (ii) upon taking an option with respect to a specific target (referred to as option fees or payments earned, if any, when the option is "taken"), (iii) upon the exercise of a previously taken option to acquire a development and commercialization license(s) (referred to as exercise fees or payments earned, if any, when the development and commercialization license is "taken"), or (iv) some combination of all of these fees.
The accounting for right-to-test agreements is dependent on the nature of the option granted to the collaborative partner. Options are considered substantive if, at the inception of a right-to-test agreement, we are at risk as to whether the collaborative partner will choose to exercise the options to secure development and commercialization licenses. Factors that are considered in evaluating whether options are substantive include the overall objective of the arrangement, the benefit the collaborator might obtain from the agreement without exercising the options, the cost to exercise the options relative to the total upfront consideration, and the additional financial commitments or economic penalties imposed on the collaborator as a result of exercising the options.
For right-to-test agreements where the options to secure development and commercialization licenses to our TAP technology are considered substantive, we do not consider the development and commercialization licenses to be a deliverable at the inception of the agreement. For those right-to-test agreements entered into prior to the adoption of ASU No. 2009-13 where the options to secure a development and commercialization license are considered substantive, we have deferred the upfront payments received and recognize this revenue over the period during which the collaborator could elect to take options for development and commercialization licenses. These periods are specific to each collaboration agreement. If a collaborator takes an option to acquire a development and commercialization license under these agreements, any substantive option fee is deferred and recognized over the life of the option, generally 12 to 18 months. If a collaborator exercises an option and takes a development and commercialization license to a specific target, we attribute the exercise fee to the development and commercialization license. Upon exercise of an option to acquire a development and commercialization license, we would also attribute any remaining deferred option fee to the development and commercialization license and apply the multiple-element revenue recognition criteria to the development and commercialization license and any other deliverables to determine the appropriate revenue recognition, which will be consistent with our accounting policy for upfront payments on single-target licenses. In the event a right-to-test agreement were to be terminated, we would recognize as revenue any portion of the upfront fee that had not previously been recorded as revenue, but was classified as deferred revenue, at the date of such termination. None of our right-to-test agreements entered into subsequent to the adoption of ASU No. 2009-13 has been determined to contain substantive options.
For right-to-test agreements where the options to secure development and commercialization licenses to our TAP technology are not considered substantive, we consider the development and commercialization license to be a deliverable at the inception of the agreement and apply the multiple-element revenue recognition criteria to determine the appropriate revenue recognition. None of our right-to-test agreements entered into prior to the adoption of ASU No. 2009-13 has been determined to contain non-substantive options.
We do not directly control when or if any collaborator will exercise its options for development and commercialization licenses. As a result, we cannot predict when or if we will recognize revenues in connection with any of the foregoing.
50
Inventory
We review our estimates of the net realizable value of our inventory at each reporting period. Our estimate of the net realizable value of our inventory is subject to judgment and estimation. The actual net realizable value of our inventory could vary significantly from our estimates. We consider quantities of raw materials in excess of twelve-month projected usage that are not supported by firm, fixed collaborator orders and projections at the time of the assessment to be excess. During fiscal years 2013, 2012 and 2011, we obtained additional quantities of DMx from our supplier which amounted to more material than would be required by our collaborators over the next twelve months and as a result, we recorded $798,000, $748,000 and $1.7 million, respectively, of charges to research and development expense related to raw material inventory identified as excess. We also recorded $38,000 to write down certain raw material inventory to its net realizable value, which is also included in research and development expense for the year ended June 30, 2012. Our collaborators' estimates of their clinical material requirements are based upon expectations of their clinical trials, including the timing, size, dosing schedule and the maximum tolerated dose likely to be reached for the compound being evaluated. Our collaborators' actual requirements for clinical materials may vary significantly from their projections. Significant differences between our collaborators' actual manufacturing orders and their projections could result in our actual twelve-month usage of raw materials varying significantly from our estimated usage at an earlier reporting period. Such differences and/or reductions in collaborators' projections could indicate that we have excess raw material inventory and we would then evaluate the need to record write-downs, which would be included as charges to research and development expense.
Stock-based Compensation
As of June 30, 2013, we are authorized to grant future awards under one share-based compensation plan, which is the ImmunoGen, Inc. 2006 Employee, Director and Consultant Equity Incentive Plan. The stock-based awards are accounted for under ASC Topic 718, "Compensation—Stock Compensation," pursuant to which the estimated grant date fair value of awards is charged to the statement of operations over the requisite service period, which is the vesting period. Such amounts have been reduced by our estimate of forfeitures for unvested awards.
The fair value of each stock option is estimated on the date of grant using the Black-Scholes option-pricing model. Expected volatility is based exclusively on historical volatility data of our stock. The expected term of stock options granted is based exclusively on historical data and represents the period of time that stock options granted are expected to be outstanding. The expected term is calculated for and applied to one group of stock options as we do not expect substantially different exercise or post-vesting termination behavior amongst our employee population. The risk-free rate of the stock options is based on the U.S. Treasury rate in effect at the time of grant for the expected term of the stock options. Estimated forfeitures are based on historical data as well as current trends. Stock compensation cost incurred during the years ended June 30, 2013, 2012 and 2011 was $12.4 million, $9.9 million and $5.5 million, respectively.
Future stock-based compensation may significantly differ based on changes in the fair value of our common stock and our estimates of expected volatility and the other relevant assumptions.
Results of Operations
Revenues
Our total revenues for the year ended June 30, 2013 were $35.5 million compared with $16.4 million and $19.3 million for the years ended June 30, 2012 and 2011, respectively. The $19.1 million increase in revenues in fiscal year 2013 from fiscal year 2012 is attributable to all revenue categories, as discussed below. The $2.9 million decrease in revenues in fiscal year 2012 from fiscal year 2011 is attributable to lower revenues from research and development support and clinical materials revenue, partially offset by higher revenues from license and milestone fees.
51
Revenue from license and milestone fees for the year ended June 30, 2013 increased approximately $15 million to $24.2 million from $9.2 million in the year ended June 30, 2012. Revenue from license and milestone fees for the year ended June 30, 2011 was $6.4 million. Included in license and milestone fees for the year ended June 30, 2013 was a $10.5 million regulatory milestone achieved under our collaboration agreement with Roche, a $500,000 development milestone achieved under our collaboration agreement with Sanofi and $11.1 million of license revenue earned upon the execution of a development and commercialization license by Novartis. Included in license and milestone fees for the year ended June 30, 2012 was a $3 million milestone payment related to the initiation of Phase II clinical testing of SAR3419 achieved under our collaboration agreement with Sanofi and two $1 million milestone payments related to regulatory milestones achieved under our license agreements with Amgen. Also during the year ended June 30, 2012, Biogen Idec terminated its exclusive license to our TAP technology to develop and commercialize therapeutic compounds to the target Cripto and as a result, we recognized the remaining $270,000 of the $1 million upfront fee received from Biogen Idec upon execution of the license which had been previously deferred. Also, during fiscal 2012, we made a change in the estimate of our period of substantial involvement as it relates to our exclusive license with Bayer HealthCare which resulted in an increase to license and milestone fees of $1.2 million for the fiscal year ending June 30, 2012 compared to amounts that would have been recognized pursuant to our previous estimate. Included in license and milestone fees for the year ended June 30, 2011 were a $1.0 million milestone payment related to the initiation of Phase I clinical testing of SAR566658 by Sanofi and a $2.0 million milestone payment related to the IND filing of BAY 94-9343 by Bayer HealthCare. The amount of license and milestone fees we earn is directly related to the number of our collaborators, the collaborators' advancement of the product candidates, and the overall success in the clinical trials of the product candidates. As such, the amount of license and milestone fees may vary widely from quarter to quarter and year to year. Total revenue recognized from license and milestone fees from each of our collaborative partners in the years ended June 30, 2013, 2012 and 2011 is included in the following table (in thousands):
| |
Year Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
License and Milestone Fees
|
2013 | 2012 | 2011 | |||||||
Collaborative Partner: |
||||||||||
Amgen |
$ | 883 | $ | 3,118 | $ | 1,123 | ||||
Bayer HealthCare |
521 | 1,839 | 2,615 | |||||||
Biogen Idec |
— | 270 | 28 | |||||||
Biotest |
25 | 120 | 130 | |||||||
Novartis |
11,131 | — | — | |||||||
Roche |
10,500 | — | — | |||||||
Sanofi |
1,167 | 3,795 | 2,435 | |||||||
Other |
— | 19 | 62 | |||||||
Total |
$ | 24,227 | $ | 9,161 | $ | 6,393 | ||||
Deferred revenue of $64.9 million at June 30, 2013 represents payments received from our collaborators pursuant to our license agreements, including a $20 million upfront payment received from Lilly during fiscal 2012 and $38.4 million remaining of a $45 million upfront payment received from Novartis during fiscal 2011, which we have yet to earn pursuant to our revenue recognition policy.
Research and development support revenue was $7.9 million for the year ended June 30, 2013, $4.5 million for the year ended June 30, 2012, and $7.3 million for the year ended June 30, 2011. These amounts primarily represent research funding earned based on actual resources utilized under our agreements with our collaborators as shown in the table below. Also included in research and development support revenue are fees for developing antibody-specific conjugation processes on behalf of our collaborators and potential collaborators during the early evaluation and preclinical testing
52
stages of drug development. The amount of research and development support revenue we earn is directly related to the number of our collaborators and potential collaborators, the stage of development of our collaborators' product candidates and the resources our collaborators allocate to the development effort. As such, the amount of development fees may vary widely from quarter to quarter and year to year. Total revenue recognized from research and development support from each of our collaborative partners in the years ended June 30, 2013, 2012 and 2011 is included in the following table (in thousands):
| |
Year Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Research and Development Support
|
2013 | 2012 | 2011 | |||||||
Collaborative Partner: |
||||||||||
Amgen |
$ | 417 | $ | 1,011 | $ | 3,971 | ||||
Bayer HealthCare |
30 | 27 | 452 | |||||||
Biotest |
921 | 627 | 896 | |||||||
Lilly |
806 | 250 | — | |||||||
Novartis |
5,605 | 2,588 | 1,338 | |||||||
Sanofi |
22 | 14 | 144 | |||||||
Other |
72 | — | 455 | |||||||
Total |
$ | 7,873 | $ | 4,517 | $ | 7,256 | ||||
Clinical materials revenue increased by approximately $164,000 to $2.8 million in the year ended June 30, 2013 compared to $2.7 million in the year ended June 30, 2012. We earned clinical materials revenue of $5.7 million during the year ended June 30, 2011. During the years ended June 30, 2013, 2012 and 2011, we shipped clinical materials in support of a number of our collaborators' clinical trials, as well as preclinical materials in support of certain collaborators' development efforts and DMx shipments to certain collaborators in support of development and manufacturing efforts. The increase in clinical materials revenue in fiscal year 2013 as compared to fiscal year 2012 is primarily due to greater DMx shipments to certain collaborators in support of development and manufacturing efforts. The decrease in clinical materials revenue in fiscal year 2012 as compared to fiscal year 2011 is primarily related to less clinical material shipped in support of one of our collaborator's trials due to larger scale material requirements being provided by another vendor, as well as less preclinical materials shipped during the year. We are compensated at negotiated prices which are generally consistent with what other third-parties would charge. The amount of clinical materials revenue we earn, and the related cost of clinical materials charged to research and development expense, is directly related to the number of clinical trials our collaborators who use us to manufacture clinical materials are preparing or have underway, the speed of enrollment in those trials, the dosage schedule of each clinical trial and the time period, if any, during which patients in the trial receive clinical benefit from the clinical materials, and the demand our collaborators have for clinical-grade material for process development and analytical purposes. As such, the amount of clinical materials revenue and the related cost of clinical materials charged to research and development expense may vary significantly from quarter to quarter and year to year.
In February 2013, the US FDA granted marketing approval to Kadcyla, a product resulting from one of our development and commercialization licenses with Roche, through its Genentech unit. We receive royalty reports and payments related to sales of Kadcyla from Roche one quarter in arrears. In accordance with our revenue recognition policy, $592,000 of royalties on net sales of Kadcyla for the period ended March 31, 2013 was recorded in our fourth quarter of fiscal 2013. No royalty revenue was recorded in fiscal years 2012 and 2013. We expect royalty revenue to increase in future periods as the underlying net sales of Kadcyla increase.
53
Research and Development Expenses
Our net research and development expenses relate to (i) research to evaluate new targets and to develop and evaluate new antibodies, linkers and cytotoxic agents, (ii) preclinical testing of our own and, in certain instances, our collaborators' product candidates, and the cost of our own clinical trials, (iii) development related to clinical and commercial manufacturing processes and (iv) manufacturing operations which also includes raw materials. Our research and development efforts have been primarily focused in the following areas:
- •
- evaluation of potential antigen targets;
- •
- evaluation of internally developed and/or in-licensed product candidates and technologies;
- •
- development and evaluation of additional cytotoxic agents and linkers;
- •
- activities related to the process, preclinical and clinical development of our internal product candidates;
- •
- process improvements to our TAP technology;
- •
- process improvements related to the production of DM1, DM4 and strain development of their precursor, ansamitocin P3;
- •
- operation and maintenance of our conjugate manufacturing facility, including production of our own and our collaborators'
clinical materials;
- •
- production costs for the supply of antibody for our internal product candidates, including fill/finish services;
- •
- production costs for the supply of DMx for our and our partners' preclinical and clinical activities;
- •
- non-pivotal and pivotal development activities with contract manufacturers for the antibody component of our internal
product candidates, linkers, and DM1, DM4 and their precursor, ansamitocin P3; and
- •
- activities pursuant to our development and license agreements with various collaborators.
Research and development expense for the year ended June 30, 2013 increased $17.9 million to $87.1 million from $69.2 million for the year ended June 30, 2012. Research and development expense was $63.5 million for the year ended June 30, 2011. Research and development salaries and related expenses increased by $6.2 million to $39.3 million in the year ended June 30, 2013 compared to the year ended June 30, 2012 and increased by $5.4 million in the year ended June 30, 2012 compared to the year ended June 30, 2011. The average number of our research personnel increased to 226 for the year ended June 30, 2013 compared to 207 for the year ended June 30, 2012. We had an average of 192 for the year ended June 30, 2011. Included in salaries and related expenses for the year ended June 30, 2013 is $7.3 million of stock compensation costs compared to $5.3 million and $3.3 million of stock compensation costs for fiscal years 2012 and 2011, respectively. The higher stock compensation costs in fiscal years 2013 and 2012 are driven by higher stock prices and increases in the number of annual options granted due to increases in personnel. Clinical trial costs increased $3.2 million to $8.9 million during fiscal year 2013 compared to fiscal year 2012 and increased $845,000 in fiscal year 2012 compared to fiscal year 2011 due primarily to new trials initiated, including a Phase II trial for IMGN901 in small-cell lung cancer, increased site management costs driven from expanded sites and higher patient enrollment. Additionally, antibody development and supply expense increased $5.9 million to $10.8 million during fiscal year 2013 compared to fiscal year 2012 and increased $1.2 million in fiscal year 2012 compared to fiscal year 2011 due to the advancement of our internal programs and timing of supply requirements.
54
We are unable to accurately estimate which potential product candidates, if any, will eventually move into our internal preclinical research program. We are unable to reliably estimate the costs to develop these products as a result of the uncertainties related to discovery research efforts as well as preclinical and clinical testing. Our decision to move a product candidate into the clinical development phase is predicated upon the results of preclinical tests. We cannot accurately predict which, if any, of the discovery stage product candidates will advance from preclinical testing and move into our internal clinical development program. The clinical trial and regulatory approval processes for our product candidates that have advanced or that we intend to advance to clinical testing are lengthy, expensive and uncertain in both timing and outcome. As a result, the pace and timing of the clinical development of our product candidates is highly uncertain and may not ever result in approved products. Completion dates and development costs will vary significantly for each product candidate and are difficult to predict. A variety of factors, many of which are outside our control, could cause or contribute to the prevention or delay of the successful completion of our clinical trials, or delay or prevent our obtaining necessary regulatory approvals. The costs to take a product through clinical trials are dependent upon, among other factors, the clinical indications, the timing, size and design of each clinical trial, the number of patients enrolled in each trial, and the speed at which patients are enrolled and treated. Product candidates may be found to be ineffective or to cause unacceptable side effects during clinical trials, may take longer to progress through clinical trials than anticipated, may fail to receive necessary regulatory approvals or may prove impractical to manufacture in commercial quantities at reasonable cost or with acceptable quality.
The lengthy process of securing FDA approvals for new drugs requires the expenditure of substantial resources. Any failure by us to obtain, or any delay in obtaining, regulatory approvals, would materially adversely affect our product development efforts and our business overall. Accordingly, we cannot currently estimate, with any degree of certainty, the amount of time or money that we will be required to expend in the future on our product candidates prior to their regulatory approval, if such approval is ever granted. As a result of these uncertainties surrounding the timing and outcome of our clinical trials, we are currently unable to estimate when, if ever, our product candidates that have advanced into clinical testing will generate revenues and cash flows.
We do not track our research and development costs by project. Since we use our research and development resources across multiple research and development projects, we manage our research and development expenses within each of the categories listed in the following table and described in more detail below (in thousands):
| |
Year Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Research and Development Expense
|
2013 | 2012 | 2011 | |||||||
Research |
$ | 17,506 | $ | 16,827 | $ | 15,208 | ||||
Preclinical and Clinical Testing |
27,839 | 21,143 | 16,884 | |||||||
Process and Product Development |
7,777 | 7,203 | 7,238 | |||||||
Manufacturing Operations |
33,951 | 24,019 | 24,123 | |||||||
Total Research and Development Expense |
$ | 87,073 | $ | 69,192 | $ | 63,453 | ||||
Research—Research includes expenses associated with activities to evaluate new targets and to develop and evaluate new antibodies, linkers and cytotoxic agents for our products and in support of our collaborators. Such expenses primarily include personnel, fees to in-license certain technology, facilities and lab supplies. Research expenses increased $679,000 to $17.5 million in fiscal year 2013 from fiscal year 2012 and $1.6 million to $16.8 million in fiscal year 2012 from fiscal year 2011. The increase in fiscal years 2013 and 2012 was principally due to an increase in salaries and related expenses.
55
Preclinical and Clinical Testing—Preclinical and clinical testing includes expenses related to preclinical testing of our own and, in certain instances, our collaborators' product candidates, regulatory activities, and the cost of our own clinical trials. Such expenses include personnel, patient enrollment at our clinical testing sites, consultant fees, contract services, and facility expenses. Preclinical and clinical testing expenses increased $6.7 million to $27.8 million in fiscal year 2013 from fiscal year 2012 and $4.2 million to $21.1 million in fiscal year 2012 from fiscal year 2011. The increase in fiscal year 2013 was primarily the result of an increase in clinical trial costs due primarily to site expansion and higher patient enrollment for the IMGN901 007 Phase II study for small-cell lung cancer and increased costs incurred for the IMGN853 Phase I trial for ovarian cancer which was initiated during the second half of fiscal 2012 and began enrolling patients in fiscal 2013, as well as an increase in salaries and related expenses. The increase in fiscal year 2012 was principally due to increases in salaries and related expenses and increases in clinical trial costs. The increase in clinical trial costs for fiscal 2012 was primarily the result of advancing two new wholly owned product candidates, IMGN529 and IMGN853, into clinical testing during the year. The increase was partially offset by lower costs incurred related to our IMGN901 and IMGN388 clinical programs due to completion of earlier-stage IMGN901 clinical trials and our returning the rights to IMGN388 to its originator.
Process and Product Development—Process and product development expenses include costs for development of clinical and commercial manufacturing processes for our own and collaborator compounds. Such expenses include the costs of personnel, contract services and facility expenses. Total development expenses increased $574,000 to $7.8 million in fiscal year 2013 from fiscal year 2012 and expenses decreased $35,000 to $7.2 million in fiscal year 2012 from fiscal year 2011. The increase in fiscal year 2013 was primarily the result of an increase in salaries and related expenses. The decrease in fiscal year 2012 was primarily due to a decrease in contract service expense due to transferring responsibility for certain outsourced costs to manufacturing operations, partially offset by an increase in salaries and related expenses.
Manufacturing Operations—Manufacturing operations expense includes costs to manufacture preclinical and clinical materials for our own and our collaborators' product candidates, quality control and quality assurance activities and costs to support the operation and maintenance of our conjugate manufacturing facility. Such expenses include personnel, raw materials for our and our collaborators' preclinical studies and clinical trials, non-pivotal and pivotal development costs with contract manufacturing organizations, manufacturing supplies, and facilities expense. Manufacturing operations expense increased $10 million to $34 million in fiscal year 2013 from fiscal year 2012 and decreased $104,000 to $24.0 million in fiscal year 2012 from fiscal year 2011. The increase in fiscal year 2013 was primarily the result of (i) an increase in antibody development and supply expense driven by our IMGN901, IMGN853, IMGN529 and IMGN289 programs; (ii) a decrease in costs capitalized into inventory due to a lower number of manufactured batches of conjugated materials on behalf of our collaborators; and (iii) an increase in salaries and related expenses. The decrease in fiscal year 2012 was primarily the result of (i) a decrease in cost of clinical materials revenue due to decreased orders of such clinical materials from our partners and lower amounts of DMx written off as excess; (ii) a decrease in raw materials and disposables used in production due to timing and mix of manufacturing requirements; and (iii) a decrease in quality-related consultant fees due to internal resources being added to perform this work. Partially offsetting these decreases, (i) costs capitalized into inventory due to a lower number of manufactured batches of conjugated materials on behalf of our collaborators decreased; (ii) antibody development and supply expense increased, driven primarily by IMGN853 and IMGN289; and (iii) contract service expense increased due to increased fill/finish costs for IMGN901 and IMGN853, greater linker development costs and increased release and stability testing of our internal antibodies.
Antibody development and supply expense in anticipation of potential future clinical trials, as well as our ongoing trials, was $10.8 million in fiscal year 2013, $4.9 million in fiscal year 2012, and
56
$3.7 million in fiscal year 2011. The process of antibody production is lengthy due in part to the lead time to establish a satisfactory production process at a vendor. Accordingly, costs incurred related to antibody production and development have fluctuated from period to period and we expect these cost fluctuations to continue in the future.
We expect that future research and development expenses will increase due to our continuing advancement of our internal product candidates through clinical trials, as well as expected increases in salaries and related expenses.
General and Administrative Expenses
General and administrative expenses for the year ended June 30, 2013 increased $1.1 million to $21.5 million from $20.4 million for the year ended June 30, 2012. General and administrative expenses for the year ended June 30, 2011 were $16.0 million. The increase in fiscal year 2013 was primarily due to an increase in salaries and related expenses, particularly stock compensation costs. The increase in fiscal year 2012 was primarily due to an increase in salaries and related expenses, particularly stock compensation costs, an increase in patent expenses and an increase in professional service fees, including increased accounting, legal and public reporting fees. We expect general and administrative expenses to increase in fiscal 2014 compared to fiscal 2013 due primarily to increases in salaries and related expenses, particularly stock compensation expense, and patent expenses.
Investment Income, net
Investment income for the years ended June 30, 2013, 2012 and 2011 was $126,000, $66,000 and $218,000, respectively.
Other Income (Expense), net
Other income (expense), net for the years ended June 30, 2013, 2012 and 2011 was $72,000, $(128,000) and $1.7 million, respectively. Net realized gains on investments were $341,000 for the year ended June 30, 2011. There were no gains or losses recognized during the years ended June 30, 2013 and 2012. During the years ended June 30, 2013, 2012 and 2011, we recorded net gains (losses) on foreign currency forward contracts of $197,000, $(173,000) and $189,000, respectively. We incurred $(153,000), $17,000, and $(57,000) in foreign currency exchange (losses) and gains related to obligations with non-U.S. dollar-based suppliers during the years ended June 30, 2013, 2012 and 2011, respectively. In addition, during fiscal year 2011, we recognized $1.2 million of federal grant funding awarded under the Patient Protection and Affordable Care Act of 2010 to develop new anticancer therapies.
Liquidity and Capital Resources
| |
As of June 30, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | |||||
| |
(In thousands) |
||||||
Cash and cash equivalents |
$ | 194,960 | $ | 160,938 | |||
Working capital |
181,511 | 150,016 | |||||
Shareholders' equity |
121,847 | 83,890 | |||||
| |
Year Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | 2011 | |||||||
| |
(In thousands) |
|||||||||
Cash used for operating activities |
$ | (60,299 | ) | $ | (34,288 | ) | $ | (7,989 | ) | |
Cash used for investing activities |
(3,696 | ) | (2,968 | ) | (660 | ) | ||||
Cash provided by financing activities |
98,017 | 6,988 | 90,699 | |||||||
57
Cash Flows
We require cash to fund our operating expenses, including the advancement of our own clinical programs, and to make capital expenditures. Historically, we have funded our cash requirements primarily through equity financings in public markets and payments from our collaborators, including license fees, milestones and research funding. As of June 30, 2013, we had approximately $195.0 million in cash and cash equivalents. Net cash used for operations was $60.3 million, $34.3 million and $8.0 million during the years ended June 30, 2013, 2012 and 2011, respectively. The principal use of cash in operating activities for all periods presented was to fund our net loss. Cash used in operations in fiscal 2012 benefited from the $20 million upfront payment received from Lilly in January 2012 with the execution of a right-to-test agreement between the companies and cash used in operations in fiscal 2011 benefited from the $45 million upfront payment received from Novartis in October 2010 with the execution of a right-to-test agreement between the companies.
Net cash used for investing activities was $3.7 million, $3.0 million and $660,000 for the years ended June 30, 2013, 2012 and 2011, respectively, and substantially represents cash outflows from capital expenditures, partially offset in fiscal 2011 by cash inflows from the sales and maturities of marketable securities. Capital expenditures were $3.8 million, $2.9 million and $2.0 million for the fiscal years ended June 30, 2013, 2012 and 2011, respectively. Capital expenditures for the years ended June 30, 2013, 2012 and 2011 consisted primarily of leasehold improvements to the laboratory and office space at our corporate headquarters and manufacturing facility, laboratory equipment and computer software applications.
Net cash provided by financing activities was $98.0 million, $7.0 million and $90.7 million for the years ended June 30, 2013, 2012 and 2011, respectively, which includes the proceeds from the exercise of 666,000, 1.4 million and 550,000 stock options, respectively. Also, pursuant to public offerings, in fiscal 2013, we issued and sold 6,250,000 shares of our common stock resulting in net proceeds of $94.0 million, and in fiscal 2011, we issued and sold 7,800,000 shares of our common stock resulting in net proceeds of $88.0 million.
We anticipate that our current capital resources and expected future collaborator payments under existing collaborations will enable us to meet our operational expenses and capital expenditures through fiscal year 2015. However, we cannot provide assurance that such collaborative agreement funding will, in fact, be received. Should we or our partners not meet some or all of the terms and conditions of our various collaboration agreements, we may be required to pursue additional strategic partners, secure alternative financing arrangements, and/or defer or limit some or all of our research, development and/or clinical projects.
Contractual Obligations
Below is a table that presents our contractual obligations and commercial commitments as of June 30, 2013 (in thousands):
| |
Payments Due by Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Total | Less than One Year |
1-3 Years |
4-5 Years |
More than 5 Years |
|||||||||||
Waltham lease obligations(1) |
$ | 37,976 | $ | 5,594 | $ | 11,154 | $ | 11,228 | $ | 10,000 | ||||||
Other operating lease obligations |
5,473 | 1,042 | 2,175 | 2,227 | 29 | |||||||||||
Total |
$ | 43,449 | $ | 6,636 | $ | 13,329 | $ | 13,455 | $ | 10,029 | ||||||
- (1)
- Lease agreements were signed in July 2007 and April 2012. In December 2009, we entered into a sublease for 14,100 square feet of our office and laboratory space at 830 Winter Street, Waltham, MA through January 2015. We will receive approximately $1.1 million in minimum rental payments over the remaining term of the sublease, which is not included in the table above.
58
In addition to the above table, we are contractually obligated to make future success-based regulatory milestone payments in conjunction with certain collaborative agreements. These payments are contingent upon the occurrence of certain future events and, given the nature of these events, it is unclear when, if ever, we may be required to pay such amounts. Therefore, the timing of any future payment is not reasonably estimable. As a result, these contingent payments have not been included in the table above or recorded in our consolidated financial statements.
During fiscal 2013, our development and commercialization license with Janssen Biotech was terminated and, accordingly, we are no longer obligated to make $41.0 million of potential future success-based milestone and third-party payments under such agreement. As of June 30, 2013 the maximum amount that may be payable in the future under our current collaborative agreements is approximately $2.0 million, $1.4 million of which is reimbursable by a third party under a separate agreement.
Recent Accounting Pronouncements
In July 2013, the FASB issued guidance to address the diversity in practice related to the financial statement presentation of unrecognized tax benefits as either a reduction of a deferred tax asset or a liability when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists. This guidance is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2013. The adoption of this guidance is not expected to have a material impact on our consolidated financial statements.
Off-Balance Sheet Arrangements
None.
Item 7A. Quantitative and Qualitative Disclosure About Market Risk
We maintain an investment portfolio in accordance with our investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Although our investments are subject to credit risk, our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure from any single issue, issuer or type of investment. Our investments are also subject to interest rate risk and will decrease in value if market interest rates increase. However, due to the conservative nature of our investments and relatively short duration, interest rate risk is mitigated. We do not own derivative financial instruments in our investment portfolio. Accordingly, we do not believe there is any material market risk exposure with respect to derivative or other financial instruments that would require disclosure under this item.
Our foreign currency hedging program uses forward contracts and a Euro-denominated bank account to manage the foreign currency exposures that exist as part of our ongoing business operations. The contracts are denominated in Euros and have maturities of less than one year. Our foreign currency risk management strategy is principally designed to mitigate the future potential financial impact of changes in the value of transactions, anticipated transactions and balances denominated in foreign currency, resulting from changes in foreign currency exchange rates.
Our market risks associated with changes in foreign currency exchange rates include a short duration foreign currency forward contract and a Euro-denominated bank account. The contract provides that we receive certain foreign currencies and pay U.S. dollars at specified exchange rates at a specified future date. Although we are exposed to credit and market risk in the event of future nonperformance by a counterparty, management has no reason to believe that such an event will occur.
59
Item 8. Financial Statements and Supplementary Data
IMMUNOGEN, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
60
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of ImmunoGen, Inc.
We have audited the accompanying consolidated balance sheets of ImmunoGen, Inc. as of June 30, 2013 and 2012, and the related consolidated statements of operations and comprehensive loss, shareholders' equity, and cash flows for each of the three years in the period ended June 30, 2013. Our audits also included the financial statement schedule listed in the Index at Item 15. These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of ImmunoGen, Inc. at June 30, 2013 and 2012, and the consolidated results of its operations and its cash flows for each of the three years in the period ended June 30, 2013, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), ImmunoGen, Inc.'s internal control over financial reporting as of June 30, 2013, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated August 29, 2013 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG LLP |
Boston,
Massachusetts
August 29, 2013
61
IMMUNOGEN, INC.
CONSOLIDATED BALANCE SHEETS
In thousands, except per share amounts
| |
June 30, 2013 |
June 30, 2012 |
|||||
|---|---|---|---|---|---|---|---|
ASSETS |
|||||||
Cash and cash equivalents |
$ | 194,960 | $ | 160,938 | |||
Accounts receivable |
— | 129 | |||||
Unbilled revenue |
2,121 | 1,196 | |||||
Inventory |
703 | 1,288 | |||||
Restricted cash |
319 | 319 | |||||
Prepaid and other current assets |
2,581 | 2,400 | |||||
Total current assets |
200,684 | 166,270 | |||||
Property and equipment, net of accumulated depreciation |
10,783 | 11,633 | |||||
Long-term restricted cash |
1,912 | 2,231 | |||||
Other assets |
217 | 174 | |||||
Total assets |
$ | 213,596 | $ | 180,308 | |||
LIABILITIES AND SHAREHOLDERS' EQUITY |
|||||||
Accounts payable |
$ | 4,498 | $ | 3,395 | |||
Accrued compensation |
6,153 | 4,942 | |||||
Other accrued liabilities |
6,049 | 4,589 | |||||
Current portion of deferred lease incentive |
979 | 979 | |||||
Current portion of deferred revenue |
1,494 | 2,349 | |||||
Total current liabilities |
19,173 | 16,254 | |||||
Deferred lease incentive, net of current portion |
5,626 | 6,605 | |||||
Deferred revenue, net of current portion |
63,384 | 69,761 | |||||
Other long-term liabilities |
3,566 | 3,798 | |||||
Total liabilities |
91,749 | 96,418 | |||||
Commitments and contingencies (Note H) |
|||||||
Shareholders' equity: |
|||||||
Preferred stock, $.01 par value; authorized 5,000 shares; no shares issued and outstanding |
— | — | |||||
Common stock, $.01 par value; authorized 150,000 shares; issued and outstanding 84,725 and 77,759 shares as of June 30, 2013 and 2012, respectively |
847 | 778 | |||||
Additional paid-in capital |
697,767 | 587,068 | |||||
Accumulated deficit |
(576,767 | ) | (503,956 | ) | |||
Total shareholders' equity |
121,847 | 83,890 | |||||
Total liabilities and shareholders' equity |
$ | 213,596 | $ | 180,308 | |||
The accompanying notes are an integral part of the consolidated financial statements.
62
IMMUNOGEN, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
In thousands, except per share amounts
| |
Year Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | 2011 | |||||||
Revenues: |
||||||||||
License and milestone fees |
$ | 24,227 | $ | 9,161 | $ | 6,393 | ||||
Research and development support |
7,873 | 4,517 | 7,256 | |||||||
Clinical materials revenue |
2,843 | 2,679 | 5,656 | |||||||
Royalty revenue |
592 | — | — | |||||||
Total revenues |
35,535 | 16,357 | 19,305 | |||||||
Operating Expenses: |
||||||||||
Research and development |
87,073 | 69,192 | 63,453 | |||||||
General and administrative |
21,471 | 20,422 | 16,040 | |||||||
Total operating expenses |
108,544 | 89,614 | 79,493 | |||||||
Loss from operations |
(73,009 | ) | (73,257 | ) | (60,188 | ) | ||||
Investment income, net |
126 | 66 | 218 | |||||||
Other income (expense), net |
72 | (128 | ) | 1,696 | ||||||
Net loss |
$ | (72,811 | ) | $ | (73,319 | ) | $ | (58,274 | ) | |
Basic and diluted net loss per common share |
$ | (0.87 | ) | $ | (0.95 | ) | $ | (0.85 | ) | |
Basic and diluted weighted average common shares outstanding |
84,063 | 76,814 | 68,919 | |||||||
Other Comprehensive Loss |
— | — | (282 | ) | ||||||
Total Comprehensive Loss |
$ | (72,811 | ) | $ | (73,319 | ) | $ | (58,556 | ) | |
The accompanying notes are an integral part of the consolidated financial statements.
63
IMMUNOGEN, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY
In thousands
| |
Common Stock | |
|
Accumulated Other Comprehensive Income (Loss) |
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid-In Capital |
Accumulated Deficit |
Total Shareholders' Equity |
||||||||||||||||
| |
Shares | Amount | |||||||||||||||||
Balance at June 30, 2010 |
67,931 | $ | 679 | $ | 473,450 | $ | (372,363 | ) | $ | 282 | $ | 102,048 | |||||||
Unrealized gains on marketable securities |
— | — | — | — | (282 | ) | (282 | ) | |||||||||||
Net loss |
— | — | — | (58,274 | ) | — | (58,274 | ) | |||||||||||
Stock options exercised |
550 | 6 | 2,713 | — | — | 2,719 | |||||||||||||
Stock-based compensation expense |
— | — | 5,452 | — | — | 5,452 | |||||||||||||
Issuance of common stock in a public offering, net of issuance costs |
7,800 | 78 | 87,902 | — | — | 87,980 | |||||||||||||
Directors' deferred share unit compensation |
— | — | 326 | — | — | 326 | |||||||||||||
Balance at June 30, 2011 |
76,281 | $ | 763 | $ | 569,843 | $ | (430,637 | ) | $ | — | $ | 139,969 | |||||||
Net loss |
— | — | — | (73,319 | ) | — | (73,319 | ) | |||||||||||
Stock options exercised |
1,432 | 14 | 6,974 | — | — | 6,988 | |||||||||||||
Stock-based compensation expense |
— | — | 9,938 | — | — | 9,938 | |||||||||||||
Directors' deferred share units converted |
46 | 1 | (1 | ) | — | — | — | ||||||||||||
Directors' deferred share unit compensation |
— | — | 314 | — | — | 314 | |||||||||||||
Balance at June 30, 2012 |
77,759 | $ | 778 | $ | 587,068 | $ | (503,956 | ) | $ | — | $ | 83,890 | |||||||
Net loss |
— | — | — | (72,811 | ) | — | (72,811 | ) | |||||||||||
Stock options exercised |
666 | 6 | 4,020 | — | — | 4,026 | |||||||||||||
Restricted stock award |
50 | — | — | — | — | — | |||||||||||||
Stock-based compensation expense |
— | — | 12,400 | — | — | 12,400 | |||||||||||||
Issuance of common stock in a public offering, net of issuance costs |
6,250 | 63 | 93,928 | — | — | 93,991 | |||||||||||||
Directors' deferred share unit compensation |
— | — | 351 | — | — | 351 | |||||||||||||
Balance at June 30, 2013 |
84,725 | $ | 847 | $ | 697,767 | $ | (576,767 | ) | $ | — | $ | 121,847 | |||||||
The accompanying notes are an integral part of the consolidated financial statements.
64
IMMUNOGEN, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
In thousands
| |
Year Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | 2011 | |||||||
Cash flows from operating activities: |
||||||||||
Net loss |
$ | (72,811 | ) | $ | (73,319 | ) | $ | (58,274 | ) | |
Adjustments to reconcile net loss to net cash used for operating activities: |
||||||||||
Depreciation and amortization |
4,641 | 4,633 | 4,937 | |||||||
(Gain) Loss on sale/disposal of fixed assets |
(21 | ) | 51 | 9 | ||||||
Gain on sale of marketable securities |
— | — | (341 | ) | ||||||
(Gain) Loss on forward contracts |
(197 | ) | 173 | (189 | ) | |||||
Stock and deferred share unit compensation |
12,751 | 10,252 | 5,778 | |||||||
Deferred rent |
(109 | ) | (109 | ) | (4 | ) | ||||
Change in operating assets and liabilities: |
||||||||||
Accounts receivable |
129 | 4,539 | (2,873 | ) | ||||||
Unbilled revenue |
(925 | ) | 292 | 107 | ||||||
Inventory |
585 | (808 | ) | 762 | ||||||
Prepaid and other current assets |
(181 | ) | 253 | (1,038 | ) | |||||
Restricted cash |
319 | 1,018 | 574 | |||||||
Other assets |
(43 | ) | (16 | ) | 38 | |||||
Accounts payable |
1,103 | 182 | 149 | |||||||
Accrued compensation |
1,211 | 219 | 522 | |||||||
Other accrued liabilities |
481 | 133 | (375 | ) | ||||||
Deferred revenue |
(7,232 | ) | 18,219 | 42,229 | ||||||
Net cash used for operating activities |
(60,299 | ) | (34,288 | ) | (7,989 | ) | ||||
Cash flows from investing activities: |
||||||||||
Proceeds from maturities or sales of marketable securities |
— | — | 1,201 | |||||||
Purchases of property and equipment, net |
(3,770 | ) | (2,908 | ) | (2,029 | ) | ||||
Proceeds (payments) from settlement of forward contracts |
74 | (60 | ) | 168 | ||||||
Net cash used for investing activities |
(3,696 | ) | (2,968 | ) | (660 | ) | ||||
Cash flows from financing activities: |
||||||||||
Proceeds from stock options exercised |
4,026 | 6,988 | 2,719 | |||||||
Proceeds from common stock issuance, net |
93,991 | — | 87,980 | |||||||
Net cash provided by financing activities |
98,017 | 6,988 | 90,699 | |||||||
Net change in cash and cash equivalents |
34,022 | (30,268 | ) | 82,050 | ||||||
Cash and cash equivalents, beginning of period |
160,938 | 191,206 | 109,156 | |||||||
Cash and cash equivalents, end of period |
$ | 194,960 | $ | 160,938 | $ | 191,206 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
65
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
AS OF JUNE 30, 2013
A. Nature of Business and Plan of Operations
ImmunoGen, Inc. (the Company) was incorporated in Massachusetts in 1981 and is focused on the development of antibody-based anticancer therapeutics. The Company has incurred operating losses and negative cash flows from operations since inception, incurred a net loss of approximately $72.8 million during the fiscal year ended June 30, 2013, and has an accumulated deficit of approximately $576.8 million as of June 30, 2013. The Company has primarily funded these losses through payments received from its collaborations and equity financings. To date, the Company has no product revenue and management expects operating losses to continue for the foreseeable future.
At June 30, 2013, the Company had $195.0 million of cash and cash equivalents on hand. The Company may raise additional funds through equity or debt financings or generate revenues from collaborative partners through a combination of upfront license payments, milestone payments, royalty payments, research funding, and clinical material reimbursement. There can be no assurance that the Company will be able to obtain additional debt or equity financing or generate revenues from collaborative partners on terms acceptable to the Company or at all. The failure of the Company to obtain sufficient funds on acceptable terms when needed could have a material adverse effect on the Company's business, results of operations and financial condition and require the Company to defer or limit some or all of its research, development and/or clinical projects.
The Company is subject to risks common to companies in the biotechnology industry including, but not limited to, the development by its competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, manufacturing and marketing limitations, collaboration arrangements, third-party reimbursements and compliance with governmental regulations.
B. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries, ImmunoGen Securities Corp., and ImmunoGen Europe Limited. All intercompany transactions and balances have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (U.S.) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Subsequent Events
The Company has evaluated all events or transactions that occurred after June 30, 2013 up through the date the Company issued these financial statements. In August 2013, as part of its right-to-test agreement, Eli Lilly and Company took an exclusive development and commercialization license to a single target. The Company did not have any other material recognizable or unrecognizable subsequent events.
66
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
Revenue Recognition
The Company enters into licensing and development agreements with collaborative partners for the development of monoclonal antibody-based anticancer therapeutics. The terms of these agreements contain multiple deliverables which may include (i) licenses, or options to obtain licenses, to the Company's Targeted Antibody Payload, or TAP, technology, (ii) rights to future technological improvements, (iii) research activities to be performed on behalf of the collaborative partner, (iv) delivery of cytotoxic agents and (v) the manufacture of preclinical or clinical materials for the collaborative partner. Payments to the Company under these agreements may include upfront fees, option fees, exercise fees, payments for research activities, payments for the manufacture of preclinical or clinical materials, payments based upon the achievement of certain milestones and royalties on product sales. The Company follows the provisions of the Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 605-25, "Revenue Recognition—Multiple-Element Arrangements," and ASC Topic 605-28, "Revenue Recognition—Milestone Method," in accounting for these agreements. In order to account for these agreements, the Company must identify the deliverables included within the agreement and evaluate which deliverables represent separate units of accounting based on if certain criteria are met, including whether the delivered element has stand-alone value to the collaborator. The consideration received is allocated among the separate units of accounting, and the applicable revenue recognition criteria are applied to each of the separate units.
At June 30, 2013, the Company had the following two types of agreements with the parties identified below:
- •
- Development and commercialization licenses to use the Company's TAP technology and/or certain other intellectual property to develop compounds to a specified target antigen (referred to as development and commercialization licenses, as distinguished from the Company's right-to-test agreements described elsewhere):
- •
- Option/research agreement for a defined period of time to secure development and commercialization licenses to use the Company's TAP technology to develop anticancer compounds to specified targets on established terms (referred to herein as right-to-test agreements):
Amgen (three exclusive single-target licenses; one non-exclusive single-target license)
Bayer HealthCare (one exclusive single-target license)
Biotest (one exclusive single-target license)
Novartis (one license to two related targets, one target on an exclusive basis and the second target on a non-exclusive basis)
Roche, through its Genentech unit (five exclusive single-target licenses)
Sanofi (exclusive license to multiple individual targets)
Sanofi
Novartis
Lilly
67
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
There are no performance, cancellation, termination or refund provisions in any of the arrangements that contain material financial consequences to the Company.
Development and Commercialization Licenses
The deliverables under a development and commercialization license agreement generally include the license to the Company's TAP technology with respect to a specified antigen target, and may also include deliverables related to rights to future technological improvements, research activities to be performed on behalf of the collaborative partner and the manufacture of preclinical or clinical materials for the collaborative partner.
Generally, development and commercialization licenses contain non-refundable terms for payments and, depending on the terms of the agreement, provide that the Company will (i) at the collaborator's request, provide research services at negotiated prices which are generally consistent with what other third parties would charge, (ii) at the collaborator's request, manufacture and provide to it preclinical and clinical materials or deliver cytotoxic agents at negotiated prices which are generally consistent with what other third parties would charge, (iii) earn payments upon the achievement of certain milestones and (iv) earn royalty payments, generally until the later of the last applicable patent expiration or 10 to 12 years after product launch. In the case of Kadcyla, however, the minimum royalty term is 10 years and the maximum royalty term is 12 years on a country-by-country basis. Royalty rates may vary over the royalty term depending on the Company's intellectual property rights. The Company may provide technical assistance and share any technology improvements with its collaborators during the term of the collaboration agreements. The Company does not directly control when or whether any collaborator will request research or manufacturing services, achieve milestones or become liable for royalty payments. As a result, the Company cannot predict when or if it will recognize revenues in connection with any of the foregoing.
In determining the units of accounting, management evaluates whether the license has stand-alone value from the undelivered elements to the collaborative partner based on the consideration of the relevant facts and circumstances for each arrangement. Factors considered in this determination include the research capabilities of the partner and the availability of TAP technology research expertise in the general marketplace. If the Company concludes that the license has stand alone value and therefore will be accounted for as a separate unit of accounting, the Company then determines the estimated selling prices of the license and all other units of accounting based on market conditions, similar arrangements entered into by third parties, and entity-specific factors such as the terms of the Company's previous collaborative agreements, recent preclinical and clinical testing results of therapeutic products that use the Company's TAP technology, the Company's pricing practices and pricing objectives, the likelihood that technological improvements will be made, the likelihood that technological improvements made will be used by the Company's collaborators and the nature of the research services to be performed on behalf of its collaborators and market rates for similar services.
Upfront payments on development and commercialization licenses are deferred if facts and circumstances dictate that the license does not have stand-alone value. Prior to the adoption of Accounting Standards Update (ASU) No. 2009-13, "Revenue Arrangements with Multiple Deliverables" on July 1, 2010, the Company determined that its licenses lacked stand-alone value and were combined with other elements of the arrangement and any amounts associated with the license
68
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
were deferred and amortized over a certain period, which the Company refers to as the Company's period of substantial involvement. The determination of the length of the period over which to defer revenue is subject to judgment and estimation and can have an impact on the amount of revenue recognized in a given period. Historically the Company's involvement with the development of a collaborator's product candidate has been significant at the early stages of development, and lessens as it progresses into clinical trials. Also, as a drug candidate gets closer to commencing pivotal testing the Company's collaborators have sought an alternative site to manufacture their products, as the Company's facility does not produce pivotal or commercial drug product. Accordingly, the Company generally estimates this period of substantial involvement to begin at the inception of the collaboration agreement and conclude at the end of non-pivotal Phase II testing. The Company believes this period of substantial involvement is, depending on the nature of the license, on average six and one-half years. Quarterly, the Company reassesses its periods of substantial involvement over which the Company amortizes its upfront license fees and makes adjustments as appropriate. In the event a collaborator elects to discontinue development of a specific product candidate under a development and commercialization license, but retains its right to use the Company's technology to develop an alternative product candidate to the same target or a target substitute, the Company would cease amortization of any remaining portion of the upfront fee until there is substantial preclinical activity on another product candidate and its remaining period of substantial involvement can be estimated. In the event that a development and commercialization license were to be terminated, the Company would recognize as revenue any portion of the upfront fee that had not previously been recorded as revenue, but was classified as deferred revenue, at the date of such termination.
Subsequent to the adoption of ASU No. 2009-13, the Company determined that its research licenses lack stand-alone value and are considered for aggregation with the other elements of the arrangement and accounted for as one unit of accounting.
Upfront payments on development and commercialization licenses may be recognized upon delivery of the license if facts and circumstances dictate that the license has stand-alone value from the undelivered elements, which generally include rights to future technological improvements, research services, delivery of cytotoxic agents and the manufacture of preclinical and clinical materials.
The Company recognizes revenue related to research services that represent separate units of accounting as they are performed, as long as there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collection of the related receivable is probable. The Company recognizes revenue related to the rights to future technological improvements over the estimated term of the applicable license.
The Company may also provide cytotoxic agents to its collaborators or produce preclinical and clinical materials at negotiated prices which are generally consistent with what other third parties would charge. The Company recognizes revenue on cytotoxic agents and on preclinical and clinical materials when the materials have passed all quality testing required for collaborator acceptance and title and risk of loss have transferred to the collaborator. Arrangement consideration allocated to the manufacture of preclinical and clinical materials in a multiple-deliverable arrangement is below the Company's full cost, and the Company's full cost is not expected to ever be below its contract selling prices for its existing collaborations. During the fiscal years ended June 30, 2013, 2012 and 2011, the difference between the Company's full cost to manufacture preclinical and clinical materials on behalf
69
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
of its collaborators as compared to total amounts received from collaborators for the manufacture of preclinical and clinical materials was $755,000, $85,000 and $1.3 million, respectively. The majority of the Company's costs to produce these preclinical and clinical materials are fixed and then allocated to each batch based on the number of batches produced during the period. Therefore, the Company's costs to produce these materials are significantly impacted by the number of batches produced during the period. The volume of preclinical and clinical materials the Company produces is directly related to the number of clinical trials the Company and its collaborators are preparing for or currently have underway, the speed of enrollment in those trials, the dosage schedule of each clinical trial and the time period such trials last. Accordingly, the volume of preclinical and clinical materials produced, and therefore the Company's per batch costs to manufacture these preclinical and clinical materials, may vary significantly from period to period.
The Company may also produce research material for potential collaborators under material transfer agreements. Additionally, the Company performs research activities, including developing antibody specific conjugation processes, on behalf of its collaborators and potential collaborators during the early evaluation and preclinical testing stages of drug development. The Company records amounts received for research materials produced or services performed as a component of research and development support revenue. The Company also develops conjugation processes for materials for later stage testing and commercialization for certain collaborators. The Company is compensated at negotiated rates and may receive milestone payments for developing these processes which are recorded as a component of research and development support revenue.
The Company's development and commercialization license agreements have milestone payments which for reporting purposes are aggregated into three categories: (i) development milestones, (ii) regulatory milestones, and (iii) sales milestones. Development milestones are typically payable when a product candidate initiates or advances into different clinical trial phases. Regulatory milestones are typically payable upon submission for marketing approval with the U.S. Food and Drug Administration, or FDA, or other countries' regulatory authorities or on receipt of actual marketing approvals for the compound or for additional indications. Sales milestones are typically payable when annual sales reach certain levels.
At the inception of each agreement that includes milestone payments, the Company evaluates whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity's performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity's performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. The Company evaluates factors such as the scientific, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required to achieve the respective milestone and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment.
Non-refundable development and regulatory milestones that are expected to be achieved as a result of the Company's efforts during the period of substantial involvement are considered substantive and are recognized as revenue upon the achievement of the milestone, assuming all other revenue
70
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
recognition criteria are met. Milestones that are not considered substantive because we do not contribute effort to the achievement of such milestones are generally achieved after the period of substantial involvement and are recognized as revenue upon achievement of the milestone, as there are no undelivered elements remaining and no continuing performance obligations, assuming all other revenue recognition criteria are met.
Under the Company's development and commercialization license agreements, the Company receives royalty payments based upon its licensees' net sales of covered products. Generally, under these agreements the Company is to receive royalty reports and payments from its licensees approximately one quarter in arrears, that is, generally in the second month of the quarter after the licensee has sold the royalty bearing product or products. The Company recognizes royalty revenues when it can reliably estimate such amounts and collectability is reasonably assured. As such, the Company generally recognizes royalty revenues in the quarter reported to the Company by its licensees, or one quarter following the quarter in which sales by the Company's licensees occurred.
Right-to-Test Agreements
The Company's right-to-test agreements provide collaborators the right to (a) test the Company's TAP technology for a defined period of time through a research, or right-to-test, license, (b) take options, for a defined period of time, to specified targets and (c) upon exercise of those options, secure or "take" licenses to develop and commercialize products for the specified targets on established terms. Under these agreements, fees may be due to the Company (i) at the inception of the arrangement (referred to as "upfront" fees or payments), (ii) upon taking an option with respect to a specific target (referred to as option fees or payments earned, if any, when the option is "taken"), (iii) upon the exercise of a previously taken option to acquire a development and commercialization license(s) (referred to as exercise fees or payments earned, if any, when the development and commercialization license is "taken"), or (iv) some combination of all of these fees.
The accounting for right-to-test agreements is dependent on the nature of the options granted to the collaborative partner. Options are considered substantive if, at the inception of a right-to-test agreement, the Company is at risk as to whether the collaborative partner will choose to exercise the options to secure development and commercialization licenses. Factors that are considered in evaluating whether options are substantive include the overall objective of the arrangement, the benefit the collaborator might obtain from the agreement without exercising the options, the cost to exercise the options relative to the total upfront consideration, and the additional financial commitments or economic penalties imposed on the collaborator as a result of exercising the options.
For right-to-test agreements where the options to secure development and commercialization licenses to the Company's TAP technology are considered substantive, the Company does not consider the development and commercialization licenses to be a deliverable at the inception of the agreement. For those right-to-test agreements entered into prior to the adoption of ASU No. 2009-13 where the options to secure development and commercialization licenses are considered substantive, the Company has deferred the upfront payments received and recognizes this revenue over the period during which the collaborator could elect to take options for development and commercialization licenses. These periods are specific to each collaboration agreement. If a collaborator takes an option to acquire a development and commercialization license under these agreements, any substantive option fee is
71
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
deferred and recognized over the life of the option, generally 12 to 18 months. If a collaborator exercises an option and takes a development and commercialization license to a specific target, the Company attributes the exercise fee to the development and commercialization license. Upon exercise of an option to acquire a development and commercialization license, the Company would also attribute any remaining deferred option fee to the development and commercialization license and apply the multiple-element revenue recognition criteria to the development and commercialization license and any other deliverables to determine the appropriate revenue recognition, which will be consistent with the Company's accounting policy for upfront payments on single-target licenses. In the event a right-to-test agreement were to be terminated, the Company would recognize as revenue any portion of the upfront fee that had not previously been recorded as revenue, but was classified as deferred revenue, at the date of such termination. None of the Company's right-to-test agreements entered into subsequent to the adoption of ASU No. 2009-13 has been determined to contain substantive options.
For right-to-test agreements where the options to secure development and commercialization licenses to the Company's TAP technology are not considered substantive, the Company considers the development and commercialization licenses to be a deliverable at the inception of the agreement and applies the multiple-element revenue recognition criteria to determine the appropriate revenue recognition. None of the Company's right-to-test agreements entered into prior to the adoption of ASU No. 2009-13 has been determined to contain non-substantive options.
The Company does not directly control when or if any collaborator will exercise its options for development and commercialization licenses. As a result, the Company cannot predict when or if it will recognize revenues in connection with any of the foregoing.
Inventory
Inventory costs relate to clinical trial materials being manufactured for sale to the Company's collaborators. Inventory is stated at the lower of cost or market as determined on a first-in, first-out (FIFO) basis.
Inventory at June 30, 2013 and 2012 is summarized below (in thousands):
| |
June 30, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | |||||
Raw materials |
$ | 75 | $ | 129 | |||
Work in process |
628 | 1,159 | |||||
Total |
$ | 703 | $ | 1,288 | |||
Raw materials inventory consists entirely of DM1 and DM4, proprietary cell-killing agents the Company developed as part of its TAP technology. All raw materials inventory is currently procured from a single supplier.
Work in process inventory consists of conjugate manufactured for sale to the Company's collaborators to be used in preclinical and clinical studies. All conjugate is made to order at the request
72
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
of the collaborators and subject to the terms and conditions of respective supply agreements. As such, no reserve for work in process inventory is required.
Raw materials inventory cost is stated net of write-downs of $810,000 and $1.3 million as of June 30, 2013 and June 30, 2012, respectively. The write-downs represent the cost of raw materials that the Company considers to be in excess of a twelve-month supply based on firm, fixed orders and projections from its collaborators as of the respective balance sheet date.
Due to yield fluctuations, the actual amount of raw materials that will be produced in future periods under third-party supply agreements is highly uncertain. As such, the amount of raw materials produced could be more than is required to support the development of the Company's collaborators' product candidates. Such excess supply, as determined under the Company's inventory reserve policy, is charged to research and development expense.
The Company produces preclinical and clinical materials for its collaborators either in anticipation of or in support of preclinical studies and clinical trials, or for process development and analytical purposes. Under the terms of supply agreements with its collaborators, the Company generally receives rolling six-month firm, fixed orders for conjugate that the Company is required to manufacture, and rolling twelve-month manufacturing projections for the quantity of conjugate the collaborator expects to need in any given twelve-month period. The amount of clinical material produced is directly related to the number of collaborator anticipated or on-going clinical trials for which the Company is producing clinical material, the speed of enrollment in those trials, the dosage schedule of each clinical trial and the time period, if any, during which patients in the trial receive clinical benefit from the clinical materials. Because these elements are difficult to estimate over the course of a trial, substantial differences between collaborators' actual manufacturing orders and their projections could result in the Company's usage of raw materials varying significantly from estimated usage at an earlier reporting period. To the extent that a collaborator has provided the Company with a firm, fixed order, the collaborator is required by contract to reimburse the Company the full negotiated price of the conjugate, even if the collaborator subsequently cancels the manufacturing run.
The Company capitalizes raw material as inventory upon receipt and accounts for the raw material inventory as follows:
- a)
- to
the extent that the Company has up to twelve months of firm, fixed orders and/or projections from its collaborators, the Company capitalizes the value of
raw materials that will be used in the production of conjugate subject to these firm, fixed orders and/or projections;
- b)
- the
Company considers more than a twelve month supply of raw materials that is not supported by firm, fixed orders and/or projections from its collaborators
to be excess and establishes a reserve to reduce to zero the value of any such excess raw material inventory with a corresponding charge to research and development expense; and
- c)
- the Company also considers any other external factors and information of which it becomes aware and assesses the impact of such factors or information on the net realizable value of the raw material inventory at each reporting period.
73
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
During fiscal years 2013, 2012 and 2011, the Company obtained additional amounts of DMx from its supplier which yielded more material than would be required by the Company's collaborators over the next twelve months and as a result, the Company recorded $798,000, $748,000 and $1.7 million respectively, of charges to research and development expense related to raw material inventory identified as excess. The Company also recorded $38,000 as research and development expense to write down certain raw material inventory to its net realizable value in fiscal year 2012. No similar charges were recorded during fiscal years 2013 and 2011. Increases in the Company's on-hand supply of raw materials, or a reduction to the Company's collaborators' projections, could result in significant changes in the Company's estimate of the net realizable value of such raw material inventory. Reductions in collaborators' projections could indicate that the Company has excess raw material inventory and the Company would then evaluate the need to record write-downs as charges to research and development expense.
Unbilled Revenue
The majority of the Company's unbilled revenue at June 30, 2013 and 2012 represents research funding earned based on actual resources utilized under the Company's various collaborator agreements.
Restricted Cash
Restricted cash at June 30, 2013 and 2012 are cash balances securing irrevocable letters of credit required for security deposits for the Company's leased facilities.
Other Accrued Liabilities
Other accrued liabilities consisted of the following at June 30, 2013 and 2012 (in thousands):
| |
June 30, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | |||||
Accrued contract payments |
$ | 2,406 | $ | 1,773 | |||
Accrued clinical trial costs |
1,849 | 865 | |||||
Accrued professional services |
678 | 677 | |||||
Accrued employee benefits |
411 | 351 | |||||
Accrued public reporting charges |
179 | 208 | |||||
Other current accrued liabilities |
526 | 715 | |||||
Total |
$ | 6,049 | $ | 4,589 | |||
Research and Development Expenses
The Company's research and development expenses are charged to expense as incurred and relate to (i) research to evaluate new targets and to develop and evaluate new antibodies, linkers and cytotoxic agents, (ii) preclinical testing of its own and, in certain instances, its collaborators' product candidates, and the cost of its own clinical trials, (iii) development related to clinical and commercial manufacturing processes and (iv) manufacturing operations which also include raw materials. Payments
74
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
made by the Company in advance for research and development services not yet provided and/or materials not yet delivered and accepted are recorded as prepaid expenses and are included in the accompanying Consolidated Balance Sheets as prepaid and other current assets.
Income Taxes
The Company uses the liability method to account for income taxes. Deferred tax assets and liabilities are determined based on differences between the financial reporting and income tax basis of assets and liabilities, as well as net operating loss carry forwards and tax credits and are measured using the enacted tax rates and laws that will be in effect when the differences reverse. A valuation allowance against net deferred tax assets is recorded if, based on the available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Financial Instruments and Concentration of Credit Risk
Cash and cash equivalents are primarily maintained with three financial institutions in the U.S. Deposits with banks may exceed the amount of insurance provided on such deposits. Generally, these deposits may be redeemed upon demand and, therefore, bear minimal risk. The Company's cash equivalents consist of money market funds with underlying investments primarily being U.S. Government- issued securities and high quality, short-term commercial paper. Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash, cash equivalents and marketable securities. The Company held no marketable securities as of June 30, 2013. The Company's investment policy, approved by the Board of Directors, limits the amount it may invest in any one type of investment, thereby reducing credit risk concentrations.
Derivative instruments include a portfolio of short duration foreign currency forward contracts intended to mitigate the risk of exchange fluctuations for existing or anticipated receivable and payable balances denominated in foreign currency. Derivatives are recorded at fair value and classified as other current assets or liabilities. The fair value of these instruments represents the present value of estimated future cash flows under the contracts, which are a function of underlying interest rates, currency rates, related volatility, counterparty creditworthiness and duration of the contracts. Changes in these factors or a combination thereof may affect the fair value of these instruments.
The Company does not designate foreign currency forward contracts as hedges for accounting purposes, and changes in the fair value of these instruments are recognized in earnings during the period of change. Because the Company enters into forward contracts only as an economic hedge, any gain or loss on the underlying foreign-denominated existing or anticipated receivable or payable balance would be offset by the loss or gain on the forward contract. Net gains (losses) on forward contracts for the years ended June 30, 2013, 2012 and 2011 were $197,000, $(173,000) and $189,000, respectively, and are included in the accompanying Consolidated Statement of Operations as other income (expense), net. As of June 30, 2013, the Company had an outstanding forward contract with a notional amount equivalent to approximately $57,000 (€41,000), maturing on October 7, 2013. As of June 30, 2012, the Company had outstanding forward contracts with notional amounts equivalent to approximately $3.3 million (€2.5 million). The Company does not anticipate using derivative instruments for any purpose other than hedging exchange rate exposure.
75
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
Cash Equivalents
All highly liquid financial instruments with maturities of three months or less when purchased are considered cash equivalents. As of June 30, 2013 and 2012, cash equivalents consisted of money market funds with underlying investments primarily being U.S. Government-issued securities and high quality, short-term commercial paper.
Fair Value of Financial Instruments
ASC Topic 820 defines fair value, establishes a framework for measuring fair value in accordance with accounting principles generally accepted in the U.S., and expands disclosures about fair value measurements. Certain provisions of ASC Topic 820 related to other non-financial assets and liabilities were adopted by the Company on July 1, 2009 and did not have a material impact on its financial position or results of operations upon adoption; however, this standard may impact the Company in subsequent periods and require additional disclosures.
Fair value is defined under ASC Topic 820 as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes a fair value hierarchy to measure fair value which is based on three levels of inputs, of which the first two are considered observable and the last unobservable, as follows:
- •
- Level 1—Quoted prices in active markets for identical assets or liabilities.
- •
- Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as
quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially
the full term of the assets or liabilities.
- •
- Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
As of June 30, 2013, the Company held certain assets that are required to be measured at fair value on a recurring basis. The following table represents the fair value hierarchy for the Company's financial assets measured at fair value on a recurring basis as of June 30, 2013 (in thousands):
| |
Fair Value Measurements at June 30, 2013 Using | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
|||||||||
| |
Total | (Level 1) | (Level 2) | (Level 3) | |||||||||
Cash, cash equivalents and restricted cash |
$ | 197,191 | $ | 197,191 | $ | — | $ | — | |||||
|
$ | 197,191 | $ | 197,191 | $ | — | $ | — | |||||
76
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
As of June 30, 2012, the Company held certain assets that are required to be measured at fair value on a recurring basis. The following table represents the fair value hierarchy for the Company's financial assets measured at fair value on a recurring basis as of June 30, 2012 (in thousands):
| |
Fair Value Measurements at June 30, 2012 Using | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
|||||||||
| |
Total | (Level 1) | (Level 2) | (Level 3) | |||||||||
Cash, cash equivalents and restricted cash |
$ | 163,488 | $ | 163,488 | $ | — | $ | — | |||||
|
$ | 163,488 | $ | 163,488 | $ | — | $ | — | |||||
The fair value of the Company's cash equivalents is based primarily on quoted prices from active markets.
The carrying amounts reflected in the consolidated balance sheets for accounts receivable, unbilled revenue, prepaid and other current assets, accounts payable, accrued compensation, and other accrued liabilities approximate fair value due to their short-term nature.
Property and Equipment
Property and equipment are stated at cost. The Company provides for depreciation based upon expected useful lives using the straight-line method over the following estimated useful lives:
Machinery and equipment |
5 years | |
Computer hardware and software |
3 years | |
Furniture and fixtures |
5 years | |
Leasehold improvements |
Shorter of remaining lease term or 7 years |
Maintenance and repairs are charged to expense as incurred. Upon retirement or sale, the cost of disposed assets and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is included in the statement of operations. The Company recorded $21,000, $(51,000) and $(9,000) of gains (losses) on the sale/disposal of certain furniture and equipment during the years ended June 30, 2013, 2012, and 2011, respectively.
Impairment of Long-Lived Assets
In accordance with ASC Topic 360, "Property, Plant, and Equipment," the Company continually evaluates whether events or circumstances have occurred that indicate that the estimated remaining useful life of its long-lived assets may warrant revision or that the carrying value of these assets may be impaired. The Company evaluates the realizability of its long-lived assets based on cash flow expectations for the related asset. Any write-downs are treated as permanent reductions in the carrying amount of the assets. Based on this evaluation, the Company believes that, as of each of the balance sheet dates presented, none of the Company's long-lived assets were impaired.
77
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
Computation of Net Loss Per Common Share
Basic and diluted net loss per common share is calculated based upon the weighted average number of common shares outstanding during the period. The Company's common stock equivalents, as calculated in accordance with the treasury-stock method, are shown in the following table (in thousands):
| |
June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | 2011 | |||||||
Options outstanding to purchase common stock and unvested restricted stock |
7,703 | 6,442 | 6,491 | |||||||
Common stock equivalents under treasury stock method |
2,149 | 2,194 | 1,901 | |||||||
The Company's common stock equivalents have not been included in the net loss per share calculation because their effect is anti-dilutive due to the Company's net loss position.
Stock-based Compensation
As of June 30, 2013, the Company is authorized to grant future awards under one employee share-based compensation plan, which is the ImmunoGen, Inc. 2006 Employee, Director and Consultant Equity Incentive Plan, or the 2006 Plan. At the annual meeting of shareholders on November 13, 2012, an amendment to the 2006 Plan was approved and an additional 3,500,000 shares were authorized for issuance under this plan. As amended, the 2006 Plan provides for the issuance of Stock Grants, the grant of Options and the grant of Stock-Based Awards for up to 12,000,000 shares of the Company's common stock, as well as any shares of common stock that are represented by awards granted under the previous stock option plan, the ImmunoGen, Inc. Restated Stock Option Plan, or the Former Plan, that are forfeited, expire or are cancelled without delivery of shares of common stock; provided, however, that no more than 5,900,000 shares shall be added to the 2006 Plan from the Former Plan, pursuant to this provision. Option awards are granted with an exercise price equal to the market price of the Company's stock at the date of grant. Options vest at various periods of up to four years and may be exercised within ten years of the date of grant.
The stock-based awards are accounted for under ASC Topic 718, "Compensation—Stock Compensation." Pursuant to Topic 718, the estimated grant date fair value of awards is charged to the statement of operations over the requisite service period, which is the vesting period. Such amounts have been reduced by an estimate of forfeitures of all unvested awards. The fair value of each stock option is estimated on the date of grant using the Black-Scholes option-pricing model with the weighted average assumptions noted in the following table. As the Company has not paid dividends since inception, nor does it expect to pay any dividends for the foreseeable future, the expected dividend yield assumption is zero. Expected volatility is based exclusively on historical volatility data of the Company's stock. The expected term of stock options granted is based exclusively on historical data and represents the period of time that stock options granted are expected to be outstanding. The expected term is calculated for and applied to one group of stock options as the Company does not expect substantially different exercise or post-vesting termination behavior amongst its employee
78
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
population. The risk-free rate of the stock options is based on the U.S. Treasury rate in effect at the time of grant for the expected term of the stock options.
| |
Year Ended June 30, | |||||
|---|---|---|---|---|---|---|
| |
2013 | 2012 | 2011 | |||
Dividend |
None | None | None | |||
Volatility |
60.44% | 59.70% | 58.81% | |||
Risk-free interest rate |
0.87% | 2.16% | 2.43% | |||
Expected life (years) |
6.3 | 7.1 | 7.2 | |||
Using the Black-Scholes option-pricing model, the weighted average grant date fair values of options granted during fiscal 2013, 2012 and 2011 were $8.60, $9.00, and $5.51 per share, respectively.
A summary of option activity under the 2006 Plan as of June 30, 2013, and changes during the twelve month period then ended is presented below (in thousands, except weighted-average data):
| |
Number of Stock Options |
Weighted- Average Exercise Price |
Weighted- Average Remaining Life in Yrs |
Aggregate Intrinsic Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Outstanding at June 30, 2012 |
6,442 | $ | 8.98 | ||||||||||
Granted |
2,047 | $ | 15.22 | ||||||||||
Exercised |
(666 | ) | $ | 6.05 | |||||||||
Forfeited/Canceled |
(170 | ) | $ | 13.87 | |||||||||
Outstanding at June 30, 2013 |
7,653 | $ | 10.79 | 6.89 | $ | 44,351 | |||||||
Outstanding at June 30, 2013—vested or unvested and expected to vest |
7,466 | $ | 10.70 | 6.84 | $ | 43,991 | |||||||
Exercisable at June 30, 2013 |
4,202 | $ | 7.97 | 5.53 | $ | 36,220 | |||||||
In November 2012, the Company granted an officer of the Company 50,000 shares of restricted stock upon hire. Pursuant to the agreement, the shares vest ratably in quarterly installments over the subsequent four years. The fair value of the restricted stock was determined by the closing price on the date of grant. A summary of restricted stock activity under the 2006 Plan as of June 30, 2013, and changes during the twelve month period then ended is presented below (in thousands, except weighted-average data):
| |
Number of Restricted Stock |
Weighted- Average Exercise Price |
|||||
|---|---|---|---|---|---|---|---|
Unvested at June 30, 2012 |
— | $ | — | ||||
Granted |
50,000 | 11.93 | |||||
Unvested at June 30, 2013 |
50,000 | $ | 11.93 | ||||
79
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
Stock compensation expense related to stock options and restricted stock awards granted under the 2006 Plan was $12.4 million, $9.9 million and $5.5 million during the fiscal years ended June 30, 2013, 2012, and 2011, respectively. As of June 30, 2013, the estimated fair value of unvested employee awards was approximately $17.4 million, net of estimated forfeitures. The weighted-average remaining vesting period for these awards is approximately two years.
A summary of option activity for options vested during the fiscal years ended June 30, 2013, 2012 and 2011 is presented below (in thousands):
| |
Year Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | 2011 | |||||||
Total fair value of options vested |
$ | 9,670 | $ | 5,647 | $ | 3,427 | ||||
Total intrinsic value of options exercised |
6,737 | 12,476 | 3,467 | |||||||
Cash received for exercise of stock options |
4,026 | 6,988 | 2,719 | |||||||
Comprehensive Loss
The Company presents comprehensive loss in accordance with ASC Topic 220, Comprehensive Income. Comprehensive loss is comprised of the Company's net loss for the years ended June 30, 2013 and 2012 and the Company's net loss and unrealized gains on available-for-sale marketable securities for the year ended June 30, 2011.
Segment Information
During the three fiscal years ended June 30, 2013, the Company continued to operate in one reportable business segment under the management approach of ASC Topic 280, Segment Reporting, which is the business of discovery of monoclonal antibody-based anticancer therapeutics.
The percentages of revenues recognized from significant customers of the Company in the years ended June 30, 2013, 2012 and 2011 are included in the following table:
| |
Year Ended June 30, | |||||
|---|---|---|---|---|---|---|
Collaborative Partner:
|
2013 | 2012 | 2011 | |||
Amgen |
6% | 30% | 41% | |||
Bayer HealthCare |
4% | 15% | 17% | |||
Biotest |
5% | 14% | 9% | |||
Novartis |
49% | 16% | 7% | |||
Roche |
30% | 0% | 0% | |||
Sanofi |
3% | 23% | 23% | |||
There were no other customers of the Company with significant revenues in the years ended June 30, 2013, 2012 and 2011.
80
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
B. Summary of Significant Accounting Policies (Continued)
Recent Accounting Pronouncements
In July 2013, the FASB issued guidance to address the diversity in practice related to the financial statement presentation of unrecognized tax benefits as either a reduction of a deferred tax asset or a liability when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists. This guidance is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2013. The adoption of this guidance is not expected to have a material impact on the Company's consolidated financial statements.
C. Agreements
Significant Collaborative Agreements
Roche
In May 2000, the Company granted Roche, through its Genentech unit, an exclusive license to the Company's maytansinoid TAP technology for use with antibodies or other proteins that target HER2, such as trastuzumab. Under the terms of this agreement, Roche has exclusive worldwide rights to develop and commercialize maytansinoid TAP compounds targeting HER2. In February 2013, the US FDA granted marketing approval to the anti-HER2 TAP compound Kadcyla. Roche is responsible for the manufacturing, product development and marketing of any products resulting from the agreement. The Company is compensated for any preclinical and clinical materials that the Company manufactures under the agreement. The Company received a $2 million non-refundable upfront payment from Roche upon execution of the agreement. The Company is also entitled to receive up to a total of $44 million in milestone payments, plus royalties on the commercial sales of Kadcyla or any other resulting products. Total milestones are categorized as follows: development milestones—$13.5 million; and regulatory milestones—$30.5 million. Through June 30, 2013, the Company has received and recognized $13.5 million and $10.5 million in development and regulatory milestone payments, respectively, related to Kadcyla. The US marketing approval of Kadcyla in February 2013 triggered a $10.5 million regulatory milestone payment to the Company. Based on an evaluation of the effort contributed to the achievement of this milestone, the Company determined this milestone was not substantive. In consideration that there were no undelivered elements remaining, no continuing performance obligations and all other revenue recognition criteria had been met, the Company recognized the $10.5 million non-refundable payment as revenue upon achievement of the milestone, which is included in license and milestone fees for the fiscal year ended June 30, 2013. The next potential milestone the Company will be entitled to receive will be either a $5 million regulatory milestone for marketing approval of Kadcyla in Europe or a $5 million regulatory milestone for marketing approval of Kadcyla in Japan depending on which occurs first. Based on an evaluation of the effort contributed to the achievement of these milestones, the Company has determined these milestones are not substantive. The Company receives royalty reports and payments related to sales of Kadcyla from Roche one quarter in arrears. In accordance with the Company's revenue recognition policy, $592,000 of royalties on net sales of Kadcyla for the period ended March 31, 2013 were recorded in the Company's fourth quarter of fiscal 2013 and are included in royalty revenues for the fiscal year ended June 30, 2013.
Roche, through its Genentech unit, also has licenses for the exclusive right to use the Company's maytansinoid TAP technology with antibodies to four undisclosed targets, which were granted under the
81
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
terms of a separate May 2000 right-to-test agreement with Genentech. For each of these licenses the Company received a $1 million license fee and is entitled to receive up to a total of $38 million in milestone payments and also royalties on the sales of any resulting products. The total milestones are categorized as follows: development milestones—$8 million; regulatory milestones—$20 million; and sales milestones—$10 million. The Company has not received any milestone payments from these agreements through June 30, 2013. Roche is responsible for the development, manufacturing, and marketing of any products resulting from these licenses. The next potential milestone the Company will be entitled to receive under any of these agreements will be a development milestone for filing of an IND application which will result in a $1 million payment being due. At the time of execution of each of these development and commercialization licenses, there was significant uncertainty as to whether this milestone would be achieved. In consideration of this, as well as the Company's past involvement in the research and manufacturing these products, this milestone was deemed substantive. Roche no longer has the right to take additional licenses under the right-to-test agreement. The Company received non-refundable technology access fees totaling $5 million for the eight-year term of the right-to-test agreement. The upfront fees were deferred and recognized ratably over the period during which Genentech could elect to obtain product licenses.
Amgen
In September 2000, the Company entered into a ten-year right-to-test agreement with Abgenix, Inc., which was later acquired by Amgen. The agreement provided Amgen with the right to (a) test the Company's maytansinoid TAP technology with Amgen's antibodies under a right-to-test, or research, license, (b) take options, with certain restrictions, to individual targets selected by Amgen on either an exclusive and non-exclusive basis for specified option periods and (c) upon exercise of those options, take exclusive or non-exclusive licenses to use the Company's maytansinoid TAP technology to develop and commercialize products for the specified targets on previously agreed-upon terms. The Company received a $5 million technology access fee in September 2000. For each exclusive development and commercialization license taken, the Company is entitled to receive an exercise fee of $1 million and up to a total of $34 million in milestone payments, plus royalties on the commercial sales of any resulting products. The total milestones per exclusive development and commercialization license are categorized as follows: development milestones—$9 million; regulatory milestones—$20 million; and sales milestones—$5 million. For each non-exclusive development and commercialization license taken, the Company is entitled to receive an exercise fee of $500,000 and up to a total of $17 million in milestone payments, plus royalties on the commercial sales of any resulting products. The total milestones per non-exclusive development and commercialization license are categorized as follows: development milestones—$4.5 million; regulatory milestones—$10 million; and sales milestones—$2.5 million. Amgen is responsible for the manufacturing, product development and marketing of any products resulting from the agreement. Amgen no longer has the right to take additional options under the agreement and there are no unexercised options outstanding.
Under the right-to-test agreement, in September 2009, November 2009 and December 2012, Amgen took three exclusive development and commercialization licenses, for which the Company received an exercise fee of $1 million for each license taken. In May 2013, Amgen took one non-exclusive development and commercialization license, for which the Company received an exercise
82
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
fee of $500,000. The Company has deferred each exercise fee and is recognizing these amounts as revenue ratably over the respective estimated periods of its substantial involvement.
In November 2011, the IND applications to the FDA for two compounds developed under two of the exclusive development and commercialization licenses became effective, which triggered two $1 million milestone payments to the Company. These payments are included in license and milestone fees for the year ended June 30, 2012. At the time of execution of each of these exclusive development and commercialization licenses, there was significant uncertainty as to whether these received and recognized milestones would be achieved. In consideration of this, as well as the Company's past involvement in the research and manufacturing of these product candidates, these milestones were deemed substantive. The next potential milestone the Company will be entitled to receive under either of these development and commercialization licenses will be a development milestone for the first dosing of a patient in a Phase II clinical trial, which will result in a $3 million payment being due. The next potential milestones the Company will be entitled to receive under the December 2012 and May 2013 development and commercialization licenses will be a development milestone for IND approval which will result in a $1 million payment and a $500,000 milestone payment, respectively, being due to the Company. At the time of execution of each of these development and commercialization licenses, there was significant uncertainty as to whether these milestones would be achieved. In consideration of this, as well as the Company's past involvement in the research and manufacturing of these product candidates, these milestones were deemed substantive.
Sanofi
In July 2003, the Company entered into a broad collaboration agreement with Sanofi (formerly Aventis) to discover, develop and commercialize antibody-based products. The collaboration agreement provides Sanofi with worldwide development and commercialization rights to new antibody-based products directed to targets that are included in the collaboration, including the exclusive right to use the Company's maytansinoid TAP technology in the creation of products developed to these targets. The product candidates (targets) as of June 30, 2013 in the collaboration include SAR3419 (CD19), SAR650984 (CD38), SAR566658 (DS6, also known as CA6) and two earlier-stage compounds that have yet to be disclosed.
For each of the targets included in the collaboration at this time, the Company is entitled to receive up to a total of $21.5 million in milestone payments, plus royalties on the commercial sales of any resulting products. The total milestones are categorized as follows: development milestones—$7.5 million; and regulatory milestones—$14 million. Through June 30, 2013, the Company has received and recognized an aggregate of $16.5 million in milestone payments for compounds covered under this agreement now or in the past, including a $500,000 development milestone related to an undisclosed target which is included in license and milestone fee revenue for the year ended June 30, 2013, a $3 million milestone payment related to the initiation of a Phase IIb clinical trial (as defined in the agreement) for SAR3419, which is included in license and milestone fee revenue for the year ended June 30, 2012, as well as a $1 million milestone payment earned in September 2010 related to the initiation of Phase I clinical testing of SAR566658 which is included in license and milestone fee revenue for the year ended June 30, 2011. At the time of execution of this agreement, there was significant uncertainty as to whether these received and recognized milestones would be achieved. In
83
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
consideration of this, as well as the Company's past involvement in the research and manufacturing of these product candidates, these milestones were deemed substantive. The next potential milestone the Company will be entitled to receive with respect to both SAR566658 and SAR650984 will be a development milestone for initiation of a Phase IIb clinical trial (as defined in the agreement), which will result in each case in a $3 million payment being due. The next potential milestone the Company will be entitled to receive with respect to SAR3419 will be for initiation of a Phase III clinical trial, which will result in a $3 million payment being due. The next potential milestone the Company will be entitled to receive for each of the unidentified targets will be a development milestone for commencement of a Phase I clinical trial, which will result in a $1 million payment being due. At the time of execution of this agreement, there was significant uncertainty as to whether these milestones would be achieved. In consideration of this, as well as the Company's past involvement in the research and manufacturing of these product candidates, these milestones were deemed substantive.
In December 2006, the Company entered into a separate right-to-test agreement with Sanofi. The agreement provides Sanofi with the right to (a) test the Company's maytansinoid TAP technology with Sanofi's antibodies to targets that were not included in the collaboration agreement described above under a right-to-test, or research, license, (b) take exclusive options, with certain restrictions, to specified targets for specified option periods and (c) upon exercise of those options, take exclusive licenses to use the Company's maytansinoid TAP technology to develop and commercialize products directed to the specified targets on terms agreed upon at the inception of the right-to-test agreement. For each development and commercialization license taken, the Company is entitled to receive an exercise fee of $2 million and up to a total of $30 million in milestone payments, plus royalties on the commercial sales of any resulting products. The total milestones are categorized as follows: development milestones—$10 million; and regulatory milestones—$20 million. No development and commercialization license has yet been taken under this agreement. Execution of the first license will entitle the Company to receive an exercise fee in the amount of $2 million. Sanofi is responsible for the manufacturing, product development and marketing of any products resulting from the agreement.
The Company received an aggregate of $4 million under the right-to-test agreement, of which $500,000 was received in December 2006 upon execution of the agreement, and $3.5 million of which was received in August 2008 upon Sanofi's activation of its rights under the agreement. The right-to-test agreement had a three-year original term from the activation date and was renewed by Sanofi in August 2011for its final three-year term by payment of a $2 million fee. The Company has deferred the $2 million extension fee and is recognizing this amount as revenue over the period during which Sanofi can take an option for a development and commercialization license.
Biotest
In July 2006, the Company granted Biotest an exclusive development and commercialization license to our maytansinoid TAP technology for use with antibodies that target CD138. The product candidate BT-062 is in development under this agreement. Biotest is responsible for the manufacturing, product development and marketing of any products resulting from the agreement. The Company received a $1 million upfront payment upon execution of the agreement and could receive up to $35.5 million in milestone payments, as well as royalties on the commercial sales of any resulting products. The total milestones are categorized as follows: development milestones—$4.5 million; and
84
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
regulatory milestones—$31 million. The Company receives payments for manufacturing any preclinical and clinical materials made at the request of Biotest. In September 2008, Biotest began Phase I evaluation of BT062 which triggered a $500,000 milestone payment to the Company. At the time of execution of this agreement, there was significant uncertainty as to whether this received and recognized milestone would be achieved. In consideration of this, as well as the Company's past involvement in the research and manufacturing of this product candidate, this milestone was deemed substantive. The next potential milestone the Company will be entitled to receive will be a development milestone for commencement of a Phase IIb clinical trial (as defined in the agreement) which will result in a $2 million payment being due. At the time of execution of this agreement, there was significant uncertainty as to whether this milestone would be achieved. In consideration of this, as well as the Company's past involvement in the research and manufacturing of this product, this milestone was deemed substantive.
The agreement also provides the Company with the right to elect at specific stages during the clinical evaluation of any compound created under this agreement, to participate in the U.S. development and commercialization of that compound in lieu of receiving the milestone payments not yet earned and royalties on sales in the U.S. The Company can exercise this right during an exercise period specified in the agreement by notice and payment to Biotest of an agreed upon opt-in fee of $15 million. Upon exercise of this right, the Company would share equally with Biotest the associated costs of product development and commercialization in the U.S. along with the profit, if any, from product sales in the U.S.
Bayer HealthCare
In October 2008, the Company granted Bayer HealthCare an exclusive development and commercialization license to the Company's maytansinoid TAP technology for use with antibodies or other proteins that target mesothelin. Bayer HealthCare is responsible for the research, development, manufacturing and marketing of any products resulting from the license. The Company received a $4 million upfront payment upon execution of the agreement, and—for each compound developed and marketed by Bayer HealthCare under this collaboration—the Company is entitled to receive a total of $170.5 million in milestone payments, plus royalties on the commercial sales of any resulting products. The total milestones are categorized as follows: development milestones—$16 million; regulatory milestones—$44.5 million; and sales milestones—$110 million. Through June 30, 2013, the Company has received and recognized an aggregate of $3 million in milestone payments under this agreement. At the time of execution of this agreement, there was significant uncertainty as to whether these received and recognized milestones would be achieved. In consideration of this, as well as the Company's past involvement in the research and supply of cytotoxic agent for this product candidate, these milestones were deemed substantive. The next potential milestone the Company will be entitled to receive will be a development milestone for commencement of a non-pivotal Phase II clinical trial, which will result in a $4 million payment being due. At the time of execution of this agreement, there was significant uncertainty as to whether this milestone would be achieved. In consideration of this, as well as the Company's past involvement in the research and supply of cytotoxic agent for this product candidate, this milestone was deemed substantive.
85
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
The Company had previously deferred the $4 million upfront payment received and was recognizing this amount as revenue ratably over the estimated period of substantial involvement. The Company had previously estimated this development period would conclude at the end of non-pivotal Phase II testing. During the first quarter of fiscal 2012, Bayer HealthCare initiated Phase I clinical testing of its product candidate. In reaching this stage of clinical testing, Bayer HealthCare developed its own processes for manufacturing required clinical material and produced clinical material in its own manufacturing facility. Considering that Bayer HealthCare was able to accomplish this without significant reliance on the Company, and considering that the Company's expected future involvement would be primarily supplying Bayer HealthCare with small quantities of cytotoxic agents for a limited period of time, the Company believed its period of substantial involvement would end prior to the completion of non-pivotal Phase II testing. As a result of this determination, beginning in September 2011, the Company recognized the balance of the upfront payment as revenue ratably through September 2012. This change in estimate resulted in an increase to license and milestone fees of approximately $1.2 million for the fiscal year ending June 30, 2012 compared to amounts that would have been recognized pursuant to the Company's previous estimate.
Novartis
In October 2010, the Company entered into a three-year right-to-test agreement with Novartis Institutes for BioMedical Research, Inc. (Novartis). The agreement provides Novartis with the right to (a) test the Company's TAP technology with individual antibodies selected by Novartis under a right-to-test, or research, license, (b) take exclusive options, with certain restrictions, to individual targets selected by Novartis for specified option periods and (c) upon exercise of those options, take exclusive licenses to use the Company's TAP technology to develop and commercialize products for a specified number of individual targets on terms agreed upon at the inception of the right-to-test agreement. The initial three-year term of the right-to-test agreement may be extended by Novartis for up to two additional one-year periods by payment of additional consideration. The terms of the right-to-test agreement require Novartis to exercise its options for the development and commercialization licenses by the end of the term of the research license. The Company received a $45 million upfront payment in connection with the execution of the right-to-test agreement, and for each development and commercialization license for a specific target, the Company is entitled to receive an exercise fee of $1 million and up to a total of $199.5 million in milestone payments, plus royalties on the commercial sales of any resulting products. The total milestones are categorized as follows: development milestones—$22.5 million; regulatory milestones—$77 million; and sales milestones—$100 million. The Company also is entitled to receive payments for research and development activities performed on behalf of Novartis. Novartis is responsible for the manufacturing, product development and marketing of any products resulting from this agreement.
Effective March 29, 2013, the Company and Novartis amended the right-to-test agreement so that Novartis can take a license to develop and commercialize products directed at two pre-defined and related undisclosed targets, one target licensed on an exclusive basis and the other target initially licensed on a non-exclusive basis. The target licensed on a non-exclusive basis may be converted to an exclusive target by notice and payment to us of an agreed upon fee of at least $5 million, depending on specific circumstances. The Company was entitled to a $3.5 million fee in connection with the execution of the amendment to the agreement. The Company may be required to credit this fee against future
86
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
milestone payments if Novartis discontinues the development of a specified product under certain circumstances.
In connection with the amendment, on March 29, 2013, Novartis took the license referenced above under the right-to-test agreement, as amended, enabling it to develop and commercialize products directed at the two targets. The Company was entitled to a $1 million upfront fee with the execution of this license. Additionally, the execution of this license provides the Company the opportunity to receive milestone payments totaling $199.5 million (development milestones—$22.5 million; regulatory milestones—$77 million; and sales milestones—$100 million) or $238 million (development milestones—$22.5 million; regulatory milestones—$115.5 million; and sales milestones—$100 million), depending on the composition of any resulting products. The first potential milestone the Company will be entitled to receive will be a $5.0 million development milestone for commencement of a Phase I clinical trial. At the time of execution of this agreement, there was significant uncertainty as to whether this milestone would be achieved. In consideration of this, as well as the Company's past involvement in the research and manufacturing of this product candidate, this milestone was deemed substantive. Additionally, the Company is entitled to receive royalties on product sales, if any. Novartis also has the right to convert the noted non-exclusive license to an exclusive license, in which case the Company would be entitled to receive a conversion fee and, depending on the composition of resultant products, an upward adjustment on milestone payments.
In accordance with ACS 605-25 (as amended by ASU No. 2009-13), the Company identified all of the deliverables at the inception of the right-to-test agreement and subsequently when amended. The significant deliverables were determined to be the right-to-test, or research, license, the development and commercialization licenses, rights to future technological improvements, and the research services. The options to obtain development and commercialization licenses in the right-to-test agreement were determined not to be substantive and, as a result, the exclusive development and commercialization licenses were considered deliverables at the inception of the right-to-test agreement. Factors that were considered in determining the options were not substantive included (i) the overall objective of the agreement was for Novartis to obtain development and commercialization licenses, (ii) the size of the exercise fee of $1 million for each development and commercialization license obtained is not significant relative to the $45 million upfront payment that was due at the inception of the right-to-test agreement, (iii) the limited economic benefit that Novartis could obtain from the right-to-test agreement unless it exercised its options to obtain development and commercialization licenses, and (iv) the lack of economic penalties as a result of exercising the options.
The Company has determined that the research license together with the development and commercialization licenses represent one unit of accounting as the research license does not have stand-alone value from the development and commercialization licenses due to the lack of transferability of the research license and the limited economic benefit Novartis would derive if they did not obtain any development and commercialization licenses. The Company has also determined that this unit of accounting does have stand-alone value from the rights to future technological improvements and the research services. The rights to future technological improvements and the research services are considered separate units of accounting as each of these was determined to have stand-alone value. The rights to future technological improvements have stand-alone value as Novartis
87
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
would be able to use those items for their intended purpose without the undelivered elements. The research services have stand-alone value as similar services are sold separately by other vendors.
The estimated selling prices for the development and commercialization licenses are the Company's best estimate of selling price and were determined based on market conditions, similar arrangements entered into by third parties, including pricing terms offered by our competitors for single-target development and commercialization licenses that utilize antibody-drug conjugate technology, and entity-specific factors such as the pricing terms of the Company's previous single-target development and commercialization licenses, recent preclinical and clinical testing results of therapeutic products that use the Company's TAP technology, and the Company's pricing practices and pricing objectives. The estimated selling price of the right to technological improvements is the Company's best estimate of selling price and was determined by estimating the probability that technological improvements will be made and the probability that such technological improvements made will be used by Novartis. In estimating these probabilities, we considered factors such as the technology that is the subject of the development and commercialization licenses, our history of making technological improvements, and when such improvements, if any, were likely to occur relative to the stage of development of any product candidates pursuant to the development and commercialization licenses. The Company's estimate of probability considered the likely period of time that any improvements would be utilized, which was estimated to be ten years following delivery of a commercialization and development license. The value of any technological improvements made available after this ten year period was considered to be de minimis due to the significant additional costs that would be incurred to incorporate such technology into any existing product candidates. The estimate of probability was multiplied by the estimated selling price of the development and commercialization licenses and the resulting cash flow was discounted at a rate of 16%, representing the Company's estimate of its cost of capital. The estimated selling price of the research services was based on third-party evidence given the nature of the research services to be performed for Novartis and market rates for similar services.
The total arrangement consideration of $55.2 million (which comprises the $45 million upfront payment, the amendment fee of $3.5 million, the exercise fee for each license, and the expected fees for the research services to be provided under the remainder of the arrangement) was allocated to the deliverables based on the relative selling price method as follows: $50.4 million to the development and commercialization licenses; $4.1 million to the rights to future technological improvements; and $710,000 to the research services. Upon execution of the development and commercialization license taken by Novartis in March 2013, the Company recorded $11.1 million of the $50.4 million of the arrangement consideration outlined above, which is included in license and milestone fee revenue for the fiscal year ended June 30, 2013. With this first development and commercialization license taken, the amount of the total arrangement consideration allocated to future technological improvements will commence to be recognized as revenue ratably over the period the Company is obligated to make available any technological improvements, which is equivalent to the estimated term of the agreement. The Company estimates the term of a development and commercialization license to be approximately 25 years, which reflects management's estimate of the time necessary to develop and commercialize products pursuant to the license plus the estimated royalty term. The Company reassesses the estimated term at the end of each reporting period. The Company will recognize as license revenue an equal amount of the total remaining $39.3 million of arrangement consideration allocated to the development and commercialization licenses as each individual license is delivered to Novartis upon Novartis'
88
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
exercise of its remaining options to such licenses. The Company does not control when Novartis will exercise its options for development and commercialization licenses. As a result, the Company cannot predict when it will recognize the related license revenue except that it will be within the term of the research license. The Company will recognize research services revenue as the related services are delivered.
Lilly
In December 2011, the Company entered into a three-year right-to-test agreement with Eli Lilly and Company (Lilly). The agreement provides Lilly with the right to (a) take exclusive options, with certain restrictions, to individual targets selected by Lilly for specified option periods, (b) test the Company's maytansinoid TAP technology with Lilly's antibodies directed to the optioned targets under a right-to-test, or research, license, and (c) upon exercise of those options, take exclusive licenses to use the Company's maytansinoid TAP technology to develop and commercialize products for a specified number of individual targets on terms agreed upon at the inception of the right-to-test agreement. The terms of the right-to-test agreement require Lilly to exercise its options for the development and commercialization licenses by the end of the term of the research license. In August 2013, Lilly took its first exclusive license to a single target.
The Company received a $20 million upfront payment in connection with the execution of the right-to-test agreement, and for the first development and commercialization license taken, which occurred in August 2013, the Company is entitled to receive up to a total of $200.5 million in milestone payments, plus royalties on the commercial sales of any resulting products. For each subsequent development and commercialization license taken, the Company is entitled to receive an exercise fee in the amount of $2 million and up to a total of $199 million in milestone payments, plus royalties on the commercial sales of any resulting products. The total milestones are categorized as follows: development milestones—$30.5 million for the first development and commercialization license and $29 million for each subsequent license; regulatory milestones—$70 million; and sales milestones—$100 million. The next payment the Company could receive would either be a $5 million development milestone payment with the initiation of a Phase I clinical trial under the first development and commercialization license taken, or a $2 million exercise fee for the execution of a second license. At the time of execution of this agreement, there was significant uncertainty as to whether the milestone related to initiation of a Phase I clinical trial under the first development and commercialization license would be achieved. In consideration of this, as well as the Company's expected involvement in the research and manufacturing of these product candidates, this milestone was deemed substantive. The Company also is entitled to receive payments for delivery of cytotoxic agents to Lilly and research and development activities performed on behalf of Lilly. Lilly is responsible for the manufacturing, product development and marketing of any products resulting from this collaboration.
In accordance with ASC 605-25 (as amended by ASU No. 2009-13), the Company identified all of the deliverables at the inception of the right-to-test agreement. The significant deliverables were determined to be the right-to-test, or research, license, the exclusive development and commercialization licenses, rights to future technological improvements, delivery of cytotoxic agents and the research services. The options to obtain development and commercialization licenses in the right-to-test agreement were determined not to be substantive and, as a result, the exclusive
89
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
development and commercialization licenses were considered deliverables at the inception of the right-to-test agreement. Factors that were considered in determining the options were not substantive included (i) the overall objective of the agreement was for Lilly to obtain development and commercialization licenses, (ii) the size of the exercise fees of $2 million for each development and commercialization license taken beyond the first license is not significant relative to the $20 million upfront payment that was due at the inception of the right-to-test agreement, (iii) the limited economic benefit that Lilly could obtain from the right-to-test agreement unless it exercised its options to obtain development and commercialization licenses, and (iv) the lack of economic penalties as a result of exercising the options.
The Company has determined that the research license together with the development and commercialization licenses represent one unit of accounting as the research license does not have stand-alone value from the development and commercialization licenses due to the lack of transferability of the research license and the limited economic benefit Lilly would derive if they did not obtain any development and commercialization licenses. The Company has also determined that this unit of accounting has stand-alone value from the rights to future technological improvements, the delivery of cytotoxic agents and the research services. The rights to future technological improvements, delivery of cytotoxic agents and the research services are considered separate units of accounting as each of these was determined to have stand-alone value. The rights to future technological improvements have stand-alone value as Lilly would be able to use those items for their intended purpose without the undelivered elements. The research services and cytotoxic agents have stand-alone value as similar services and products are sold separately by other vendors.
The estimated selling prices for the development and commercialization licenses are the Company's best estimate of selling price and were determined based on market conditions, similar arrangements entered into by third parties, including pricing terms offered by our competitors for single-target development and commercialization licenses that utilize antibody-drug conjugate technology, and entity-specific factors such as the pricing terms of the Company's previous single-target development and commercialization licenses, recent preclinical and clinical testing results of therapeutic products that use the Company's TAP technology, and the Company's pricing practices and pricing objectives. The estimated selling price of the rights to technological improvements is the Company's best estimate of selling price and was determined by estimating the probability that technological improvements will be made, and the probability that technological improvements made will be used by Lilly. In estimating these probabilities, we considered factors such as the technology that is the subject of the development and commercialization licenses, our history of making technological improvements, and when such improvements, if any, were likely to occur relative to the stage of development of any product candidates pursuant to the development and commercialization licenses. over the company's estimate of probability considered the likely period of time that any improvements would be utilized, which was estimated to be ten years following delivery of a commercialization and development license. The value of any technological improvements made available after this ten year period was considered to be de minimis due to the significant additional costs that would be incurred to incorporate such technology into any existing product candidates. The estimate of probability was multiplied by the estimated selling price of the development and commercialization licenses and the resulting cash flow was discounted at a rate of 16%, representing the Company's estimate of its cost of capital. The estimated selling price of the cytotoxic agent was based on third-party evidence given market rates for
90
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
the manufacture of such cytotoxic agents. The estimated selling price of the research services was based on third-party evidence given,the nature of the research services to be performed for Lilly and market rates for similar services.
The total arrangement consideration of $28.2 million (which comprises the $20 million upfront payment, the exercise fee, if any, for each license, the expected fees for the research services to be provided and the cytotoxic agent to be delivered under the arrangement) was allocated to the deliverables based on the relative selling price method as follows: $23.5 million to the development and commercialization licenses; $0.6 million to the rights to future technological improvements, $0.8 million to the sale of cytotoxic agent; and $3.3 million to the research services. The Company will recognize as license revenue an equal amount of the total arrangement consideration allocated to the development and commercialization licenses as each individual license is delivered to Lilly upon Lilly's exercise of its options to such licenses. At the time the first license is taken, the amount of the total arrangement consideration allocated to future technological improvements will commence to be recognized as revenue ratably over the period the Company is obligated to make available any technological improvements, which is the equivalent to the estimated term of the license. The Company estimates the term of a development and commercialization license to be approximately 25 years, which reflects management's estimate of the time necessary to develop and commercialize therapeutic products pursuant to the license plus the estimated royalty term. The Company will be required to reassess the estimated term at each subsequent reporting period. The Company does not control when Lilly will exercise its options for development and commercialization licenses. As a result, the Company cannot predict when it will recognize the related license revenue except that it will be within the term of the research license. The Company will recognize research services revenue and revenue from the delivery of cytotoxic agents as the related services and cytotoxic agents are delivered.
No license revenue has been recognized related to this agreement through June 30, 2013 as the options to take development and commercialization licenses were not considered to be substantive and no development and commercialization licenses had been delivered at this time. Accordingly, the entire $20 million upfront payment is included in long-term deferred revenue at June 30, 2013.
Other Collaborative Agreements
In December 2004, the Company entered into a development and license agreement with a predecessor to Janssen Biotech (formerly known as Centocor), a wholly owned subsidiary of Johnson & Johnson. Under the terms of this agreement, Janssen was granted exclusive worldwide rights to develop and commercialize anticancer therapeutics that consist of the Company's maytansinoid cell-killing agent attached to an av integrin-targeting antibody that was developed by Janssen. Under the terms of the agreement, the Company received an upfront payment of $1 million upon execution of the agreement.
In December 2007, the Company licensed from Janssen the exclusive, worldwide right to develop and commercialize a TAP compound, IMGN388, that consists of an av integrin-targeting antibody developed by them and one of the Company's maytansinoid cell-killing agents. This license reallocated the parties' respective responsibilities and financial obligations from the license referenced above. In November 2011, the Company announced its decision to discontinue development of IMGN388. During the first quarter of fiscal 2013, the 2007 license agreement was terminated with rights to the product candidate reverting back to Janssen. The remaining $241,000 of the $1 million upfront fee received
91
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
C. Agreements (Continued)
from Janssen upon execution of the 2004 license agreement is included in long-term deferred revenue at June 30, 2013.
Effective July 2011, Biogen Idec terminated its exclusive license to the Company's TAP technology to develop and commercialize therapeutic compounds to the target Cripto. This license was granted pursuant to the Development and License Agreement between the Company and Biogen Idec dated October 1, 2004. As a result of the termination, during the first quarter of fiscal 2012, the Company recognized the remaining $270,000 of the $1 million upfront fee received from Biogen Idec upon execution of the license which had been previously deferred.
D. Marketable Securities
As of June 30, 2013 and 2012, $195.0 million and $160.9 million, respectively, in cash and money market funds consisting principally of U.S. Government-issued securities and high quality, short-term commercial paper were classified as cash and cash equivalents.
During fiscal year 2011, the Company sold the remaining marketable securities held in its investment portfolio at June 30, 2010, resulting in realized gains of $347,000 and realized losses of $(6,000). In 2013 and 2012, the Company had no realized losses or gains.
E. Property and Equipment
Property and equipment consisted of the following at June 30, 2013 and 2012 (in thousands):
| |
June 30, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | |||||
Leasehold improvements |
$ | 26,777 | $ | 25,661 | |||
Machinery and equipment |
14,741 | 13,808 | |||||
Computer hardware and software |
4,894 | 4,168 | |||||
Furniture and fixtures |
1,540 | 1,315 | |||||
Assets under construction |
814 | 660 | |||||
|
$ | 48,766 | $ | 45,612 | |||
Less accumulated depreciation |
(37,983 | ) | (33,979 | ) | |||
Property and equipment, net |
$ | 10,783 | $ | 11,633 | |||
Depreciation expense was approximately $4.6 million, $4.6 million and $4.9 million for the years ended June 30, 2013, 2012 and 2011, respectively.
92
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
F. Income Taxes
The difference between the Company's expected tax benefit, as computed by applying the U.S. federal corporate tax rate of 34% to loss before the benefit for income taxes, and actual tax is reconciled in the following chart (in thousands):
| |
Year Ended June 30, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | 2011 | |||||||
Loss before income tax expense |
$ | (72,811 | ) | $ | (73,319 | ) | $ | (58,274 | ) | |
Expected tax benefit at 34% |
$ | (24,756 | ) | $ | (24,928 | ) | $ | (19,813 | ) | |
Permanent differences |
1,540 | 1,469 | — | |||||||
State tax benefit net of federal benefit |
(3,921 | ) | (4,204 | ) | (1,815 | ) | ||||
Increase in valuation allowance, net |
25,192 | 26,574 | 16,410 | |||||||
Expired loss and credit carryforwards |
1,945 | 1,089 | 5,610 | |||||||
Other |
— | — | (392 | ) | ||||||
Benefit for income taxes |
$ | — | $ | — | $ | — | ||||
At June 30, 2013, the Company has net operating loss carryforwards of approximately $329.5 million available to reduce federal taxable income, if any, that expire in 2014 through 2033 and $187.4 million available to reduce state taxable income, if any, that expire in fiscal 2014 through fiscal 2033. Included in the federal and state carryforwards is $18.5 million and $15.8 million, respectively, related to deductions from the exercise of stock options and the related tax benefit will result in an increase in additional paid-in capital if and when realized through a reduction of taxes paid in cash. The Company also has federal and state research tax credits of approximately $14.8 million available to offset federal and state income taxes, which expire beginning in fiscal 2014. Due to the degree of uncertainty related to the ultimate use of the loss carryforwards and tax credits, the Company has established a valuation allowance to fully reserve these tax benefits.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax
93
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
F. Income Taxes (Continued)
purposes. Significant components of the Company's deferred tax assets as of June 30, 2013 and 2012 are as follows (in thousands):
| |
June 30, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2012 | |||||
Net operating loss carryforwards |
$ | 121,937 | $ | 98,601 | |||
Research and development tax credit carryforwards |
12,806 | 10,393 | |||||
Property and other intangible assets |
2,077 | 1,486 | |||||
Deferred revenue |
25,484 | 28,325 | |||||
Stock-based compensation |
5,759 | 3,302 | |||||
Deferred lease incentive |
4,771 | 5,100 | |||||
Other liabilities |
508 | 512 | |||||
Total deferred tax assets |
$ | 173,342 | $ | 147,719 | |||
Valuation allowance |
(173,342 | ) | (147,719 | ) | |||
Net deferred tax assets |
$ | — | $ | — | |||
The valuation allowance increased by $25.6 million during 2013 due primarily to the additional net loss recognized during the year, partially offset by the expiration of net operating loss carryforwards.
Utilization of the NOL and R&D credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that have occurred previously or that could occur in the future as provided by Section 382 of the Internal Revenue Code of 1986, as well as similar state and foreign provisions. These ownership changes may limit the amount of NOL and R&D credit carry forwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. Since the Company's formation, it has raised capital through the issuance of capital stock on several occasions (both pre and post initial public offering) which, combined with the purchasing shareholders' subsequent disposition of those shares, may have resulted in a change of control, as defined by Section 382, or could result in a change of control in the future upon subsequent disposition. The Company has not currently completed a study to assess whether a change of control has occurred or whether there have been multiple changes of control since its formation due to the significant complexity, costs associated with such study and the possibility that there could be additional changes in control in the future. If the Company has experienced a change of control at any time since its formation, utilization of its NOL or R&D credit carry forwards would be subject to an annual limitation under Section 382 which is determined by first multiplying the value of the Company's stock at the time of the ownership change by the applicable long-term tax-exempt rate, and then could be subject to additional adjustments, as required. Any limitation may result in expiration of a portion of the NOL or R&D credit carry forwards before utilization. Further, until a study is completed and any limitation known, no amounts are being presented as an uncertain tax position. The Company does not expect to have any taxable income for at least the next several years.
Interest and penalties related to the settlement of uncertain tax positions, if any, will be reflected in income tax expense. The Company did not recognize any interest and penalties associated with
94
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
F. Income Taxes (Continued)
unrecognized tax benefits in the accompanying consolidated financial statements. The Company does not expect any material changes to the unrecognized benefits within 12 months of the reporting date. Due to existence of the valuation allowance, future changes in the Company's unrecognized tax benefits will not impact our effective tax rate. The Company's loss carryforwards are subject to adjustment by state and federal taxing authorities, commencing when those losses are utilized to reduce taxable income.
Included in other (expense) income, net for the fiscal year ended June 30, 2011 is $1.2 million of federal grant funding the Company was awarded under the Patient Protection and Affordable Care Act of 2010 to develop new anticancer therapies.
G. Capital Stock
Sale of Common Stock
On May 19, 2011, the Company filed a Registration Statement on Form S-3 with the Securities and Exchange Commission. Pursuant to the shelf registration statement, in May 2011 and June 2011, the Company issued and sold a total of 7,800,000 shares of its common stock at $12.00 per share through a public offering resulting in gross proceeds of $93.6 million. Pursuant to the shelf registration statement filed in May 2011, in July 2012, the Company issued and sold a total of 6,250,000 shares of its common stock at $16.00 per share through a public offering resulting in gross proceeds of $100 million.
Common Stock Reserved
At June 30, 2013, the Company has reserved 12.57 million shares of authorized common stock for the future issuance of shares under the 2006 Plan and the 2004 Director Plan. See "Stock-Based Compensation" in Note B for a description of the 2006 Plan and the Former Plan and Note G below for a description of the 2004 Director Plan.
Stock Options
As of June 30, 2013, the 2006 Plan was the only employee share-based compensation plan of the Company. During the year ended June 30, 2013, holders of options issued under the 2006 Plan and the Former Plan exercised their rights to acquire an aggregate of 666,000 shares of common stock at prices ranging from $2.91 to $15.20 per share. The total proceeds to the Company from these option exercises were approximately $4.0 million.
95
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
G. Capital Stock (Continued)
The Company granted options with an exercise price equal to the fair market value of the common stock on the date of such grant. The following options and their respective weighted- average exercise prices per share were exercisable at June 30, 2013, 2012 and 2011:
| |
Exercisable (in thousands) |
Weighted- Average Exercise Price |
|||||
|---|---|---|---|---|---|---|---|
June 30, 2013 |
4,202 | $ | 7.97 | ||||
June 30, 2012 |
3,416 | $ | 6.34 | ||||
June 30, 2011 |
3,834 | $ | 5.25 | ||||
2001 Non-Employee Director Stock Plan
In November 2001, the Company's shareholders approved the establishment of the 2001 Non-Employee Director Stock Plan, or the 2001 Director Plan, and 50,000 shares of common stock to be reserved for grant thereunder. The 2001 Director Plan provided for the granting of awards to Non-Employee Directors and, at the election of Non-Employee Directors, to have all or a portion of their awards in the form of cash, stock, or stock units. All stock or stock units are immediately vested. The number of stock or stock units issued was determined by the market value of the Company's common stock on the last date of the Company's fiscal quarter for which the services are rendered. The 2001 Director Plan was administered by the Board of Directors which was authorized to interpret the provisions of the 2001 Director Plan, determine which Non-Employee Directors would be granted awards, and determine the number of shares of stock for which a stock right will be granted. The 2001 Director Plan was replaced in 2004 by the 2004 Non-Employee Director Compensation and Deferred Share Unit Plan.
During the years ended June 30, 2013, 2012 and 2011, the Company recorded approximately $(1,000), $29,000, and $44,000 in (expense reduction) compensation expense, respectively, related to approximately 6,000, 6,000, and 15,000 stock units outstanding, respectively, under the 2001 Director Plan. The value of the stock units is adjusted to market value at each reporting period. No stock units have been issued under the 2001 Plan subsequent to June 30, 2004. Pursuant to the 2001 Plan, in November 2011, the Company paid a retiring director approximately $115,000 to settle outstanding stock units.
2004 Non-Employee Director Compensation and Deferred Share Unit Plan
In June 2004, the Board of Directors approved the establishment of the 2004 Non-Employee Director Compensation and Deferred Share Unit Plan, or the 2004 Director Plan. The 2004 Director Plan provided for the compensation of Non-Employee Directors, awarding their annual retainers in the form of deferred share units, and, at their discretion, to have all or a portion of their other compensation such as meeting fees in the form of cash or deferred share units. The deferred share units for annual retainers vested one-twelfth monthly over the next year after the award; other deferred share units vested immediately upon issuance. The number of deferred share units issued was determined by the market value of the Company's common stock on the last date of the Company's fiscal year prior to the fiscal year for which services were rendered. The deferred share units were to be paid out in cash to each non-employee director based upon the market value of the Company's
96
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
G. Capital Stock (Continued)
common stock on the date of such director's retirement from the Board of Directors of the Company. The 2004 Director Plan was administered by the Board of Directors.
The 2004 Director Plan was amended on September 5, 2006. Under the terms of the amended 2004 Director Plan, the redemption amount of deferred share units will be paid in shares of common stock of the Company under the 2006 Plan in lieu of cash. As a result of the change in payout structure, the value of the vested awards was transferred to additional paid-in capital as of the modification date and the total value of the awards, as calculated on the modification date, was expensed over the remainder of the vesting period. Accordingly, the value of the share units is fixed and will no longer be adjusted to market value at each reporting period. In addition, the amended 2004 Director Plan changed the vesting for annual retainers to take place quarterly over the three years after the award and the number of deferred share units awarded for all compensation is now based on the market value of the Company's common stock on the date of the award.
Compensation Policy for Non-Employee Directors
On September 16, 2009, the Board adopted a new Compensation Policy for Non-Employee Directors, which superseded the 2004 Plan and made certain changes to the compensation of its non-employee directors. The policy was amended on November 11, 2009 to provide that, whenever the Board has a non-employee Chairman in lieu of a Lead Director, the cash payment for the non-employee Chairman of the Board shall be the same as the cash compensation that would otherwise have been payable to the Lead Director. Effective November 12, 2009, non-employee directors became entitled to receive annual meeting fees and committee fees under the new policy. The new policy made changes to the equity portion of the non-employee director compensation, but left the cash portion unchanged. Effective November 11, 2009, non-employee directors became entitled to receive deferred stock units under the new policy as follows:
- •
- New non-employee directors will be initially awarded a number of deferred stock units having an aggregate market value of
$65,000, based on the closing price of our common stock on the date of their initial election to the Board. These awards will vest quarterly over three years from the date of grant, contingent upon
the individual remaining a director of ImmunoGen as of each vesting date.
- •
- On the first anniversary of a non-employee director's initial election to the Board, such non-employee director will be
awarded a number of deferred stock units having an aggregate market value of $30,000, based on the closing price of our common stock on such date of grant and pro-rated based on the number of whole
months remaining between the first day of the month in which such grant date occurs and the first October 31 following the grant date. These awards will generally vest quarterly over
approximately the period from the grant date to the first November 1 following the grant date, contingent upon the individual remaining a director of ImmunoGen as of each vesting date.
- •
- Thereafter, non-employee directors in general will be annually awarded a number of deferred stock units having an aggregate market value of $30,000, based on the closing price of our common stock on the date of our annual meeting of shareholders. These awards will vest
97
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
G. Capital Stock (Continued)
quarterly over approximately one year from the date of grant, contingent upon the individual remaining a director of ImmunoGen as of each vesting date.
As with the 2004 Plan, vested deferred stock units are redeemed on the date a director ceases to be a member of the Board, at which time such director's deferred stock units will be settled in shares of our common stock issued under our 2006 Plan at a rate of one share for each vested deferred stock unit then held. Any deferred stock units that remain unvested at that time will be forfeited. The new policy provides that all unvested deferred stock units will automatically vest immediately prior to the occurrence of a change of control, as defined in the 2006 Plan. Pursuant to the Compensation Policy for Non-Employee Directors, in November 2011, the Company issued two retiring directors an aggregate 46,298 shares of common stock of the Company to settle outstanding deferred share units.
In connection with the adoption of the new compensation policy, the Board also amended the 2004 Plan as follows:
- •
- All unvested deferred stock awards (other than any unvested initial awards) were vested in full on September 16,
2009 unless the date such deferred stock units were credited to the non-employee director was less than one year prior to September 16, 2009, in which case such unvested deferred stock units
will vest on the first anniversary of the date such deferred stock units were credited to the non-employee director.
- •
- All unvested deferred stock awards will automatically vest immediately prior to the occurrence of a change of control.
On September 22, 2010, the Board revised the Compensation Policy for Non-Employee Directors to provide that, in addition to the compensation they received previously, they would also become entitled to receive stock option awards having a grant date fair value of $30,000, determined using the Black-Scholes option pricing model measured on the date of grant, which would be the date of the annual meeting of shareholders. These options will vest quarterly over approximately one year from the date of grant. Any new directors will receive a pro-rated award, depending on their date of election to the Board. The directors received a total of 41,805, 33,187 and 49,688 options in fiscal years 2013, 2012 and 2011, respectively, and the related compensation expense is included in the amounts discussed in the "Stock-based Compensation" section of footnote B above.
Pursuant to the Compensation Policy for Non-Employee Directors and the 2004 Director Plan, as amended, the Company recorded approximately:
- •
- $351,000 in compensation expense during the year ended June 30, 2013 related to the issuance of 26,000 deferred
share units and 251,000 deferred share units previously issued under the 2004 Director Plan;
- •
- $314,000 in compensation expense during the year ended June 30, 2012 related to the issuance of 33,000 deferred
share units and 264,000 deferred share units previously issued under the 2004 Director Plan; and
- •
- $326,000 in compensation expense during the year ended June 30, 2011 related to the issuance of 39,000 deferred share units and 225,000 deferred share units previously issued under the 2004 Director Plan.
98
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
H. Commitments and Contingencies
Leases
Effective July 27, 2007, the Company entered into a lease agreement with Intercontinental Fund III for the rental of approximately 89,000 square feet of laboratory and office space at 830 Winter Street, Waltham, MA. The Company uses this space for its corporate headquarters and other operations. The initial term of the lease is for twelve years with an option for the Company to extend the lease for two additional terms of five years. The Company is required to pay certain operating expenses for the leased premises subject to escalation charges for certain expense increases over a base amount. The Company entered into a sublease in December 2009 for 14,100 square feet of this space in Waltham through January 2015, with the sublessee having a conditional option to extend the term for an additional two years.
Effective April 2012, the Company entered into a sublease agreement for the rental of 7,310 square feet of laboratory and office space at 830 Winter Street, Waltham, MA from Histogenics Corporation. The initial term of the sublease is for three years with a conditional option for the Company to extend the lease through October 2017. The Company is required to pay certain operating expenses for the leased premises subject to escalation charges for certain expense increases over a base amount.
As part of the 2007 lease agreement, the Company received a construction allowance of up to approximately $13.3 million to build out laboratory and office space to the Company's specifications. After completion, the Company had recorded $12.0 million of leasehold improvements under the construction allowance. The Company received $10.8 million from the landlord and paid out the same amount towards these leasehold improvements. The remaining balance of the improvements was paid directly by the landlord. The lease term began on October 1, 2007, when the Company obtained physical control of the space in order to begin construction.
The Company also leases manufacturing and office space at 333 Providence Highway, Norwood, MA under an agreement through 2018 with an option to extend the lease for an additional term of five years. The Company is required to pay certain operating expenses for the leased premises subject to escalation charges for certain expense increases over a base amount.
Effective April 2013, the Company entered into a lease agreement with River Ridge Limited Partnership for the rental of 7,507 square feet of additional office space at 100 River Ridge Drive, Norwood, MA. The initial term of the lease is for five years and two months commencing in July 2013 with an option for the Company to extend the lease for an additional term of five years. The Company is required to pay certain operating expenses for the leased premises subject to escalation charges for certain expense increases over a base amount.
Facilities rent expense, net of sublease income, was approximately $4.8 million, $4.8 million and $4.6 million during fiscal years 2013, 2012 and 2011, respectively.
99
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
H. Commitments and Contingencies (Continued)
As of June 30, 2013, the minimum rental commitments, including real estate taxes and other expenses, for the next five fiscal years and thereafter under the non-cancelable operating lease agreements discussed above are as follows (in thousands):
2013 |
$ | 6,636 | ||
2014 |
6,780 | |||
2015 |
6,549 | |||
2016 |
6,624 | |||
2017 |
6,831 | |||
Thereafter |
10,029 | |||
Total minimum lease payments |
$ | 43,449 | ||
Total minimum rental income from subleases |
(1,088 | ) | ||
Total minimum lease payments, net |
$ | 42,361 | ||
Collaborations
The Company is contractually obligated to make potential future success-based regulatory milestone payments in conjunction with certain collaborative agreements. These payments are contingent upon the occurrence of certain future events and, given the nature of these events, it is unclear when, if ever, the Company may be required to pay such amounts. Further, the timing of any future payment is not reasonably estimable. During the first quarter of fiscal 2013, the Company's license agreement with Janssen Biotech was terminated and, accordingly, the Company is no longer obligated to make $41.0 million of potential future success-based milestone and third-party payments under such agreement. As of June 30, 2013, the maximum amount that may be payable in the future under the Company's current collaborative agreements is $2.0 million, $1.4 million of which is reimbursable by a third party under a separate agreement.
Litigation
The Company is not party to any material litigation.
I. Employee Benefit Plans
The Company has a deferred compensation plan under Section 401(k) of the Internal Revenue Code (the 401(k) Plan). Under the 401(k) Plan, eligible employees are permitted to contribute, subject to certain limitations, up to 100% of their gross salary and the Company's matching contribution is 50% of the first 6% of the eligible employees' contributions. In fiscal years 2013, 2012 and 2011, the Company's contributions to the 401(k) Plan totaled approximately $593,000, $548,000, and $467,000, respectively.
100
IMMUNOGEN, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
AS OF JUNE 30, 2013
J. Quarterly Financial Information (Unaudited)
| |
Fiscal Year 2013 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
First Quarter Ended September 30, 2012 |
Second Quarter Ended December 31, 2012 |
Third Quarter Ended March 31, 2013 |
Fourth Quarter Ended June 30, 2013 |
|||||||||
| |
(In thousands, except per share data) |
||||||||||||
Revenues: |
|||||||||||||
License and milestone fees |
$ | 933 | $ | 429 | $ | 22,010 | $ | 855 | |||||
Research and development support |
1,377 | 2,036 | 2,257 | 2,203 | |||||||||
Clinical materials revenue |
1,781 | 147 | 734 | 181 | |||||||||
Royalty revenue |
— | — | — | 592 | |||||||||
Total revenues |
4,091 | 2,612 | 25,001 | 3,831 | |||||||||
Expenses: |
|||||||||||||
Research and development |
23,700 | 21,656 | 21,318 | 20,399 | |||||||||
General and administrative |
5,639 | 5,464 | 4,995 | 5,373 | |||||||||
Total expenses |
29,339 | 27,120 | 26,313 | 25,772 | |||||||||
Loss from operations |
(25,248 | ) | (24,508 | ) | (1,312 | ) | (21,941 | ) | |||||
Other income (expense), net |
56 | 115 | (39 | ) | 66 | ||||||||
Net loss |
$ | (25,192 | ) | $ | (24,393 | ) | $ | (1,351 | ) | $ | (21,875 | ) | |
Basic and diluted net loss per common share |
$ | (0.30 | ) | $ | (0.29 | ) | $ | (0.02 | ) | $ | (0.26 | ) | |
| |
Fiscal Year 2012 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
First Quarter Ended September 30, 2011 |
Second Quarter Ended December 31, 2011 |
Third Quarter Ended March 31, 2012 |
Fourth Quarter Ended June 30, 2012 |
|||||||||
| |
(In thousands, except per share data) |
||||||||||||
Revenues: |
|||||||||||||
License and milestone fees |
$ | 1,187 | $ | 6,025 | $ | 999 | $ | 950 | |||||
Research and development support |
1,068 | 945 | 1,320 | 1,184 | |||||||||
Clinical materials reimbursement |
281 | 647 | 933 | 818 | |||||||||
Total revenues |
2,536 | 7,617 | 3,252 | 2,952 | |||||||||
Expenses: |
|||||||||||||
Research and development |
17,161 | 15,559 | 16,933 | 19,539 | |||||||||
General and administrative |
4,841 | 4,834 | 5,021 | 5,726 | |||||||||
Total expenses |
22,002 | 20,393 | 21,954 | 25,265 | |||||||||
Loss from operations |
(19,466 | ) | (12,776 | ) | (18,702 | ) | (22,313 | ) | |||||
Other income, net |
(17 | ) | 23 | 33 | (101 | ) | |||||||
Net loss |
$ | (19,483 | ) | $ | (12,753 | ) | $ | (18,669 | ) | $ | (22,414 | ) | |
Basic and diluted net loss per common share |
$ | (0.26 | ) | $ | (0.17 | ) | $ | (0.24 | ) | $ | (0.29 | ) | |
101
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
1. Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this Annual Report on Form 10-K. Based on such evaluation, our principal executive officer and principal financial officer have concluded that, as of the end of such period, our disclosure controls and procedures were adequate and effective.
2. Internal Control Over Financial Reporting
- (a)
- Management's Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the U.S. and includes those policies and procedures that:
- •
- pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and
dispositions of our assets;
- •
- provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in
accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
- •
- provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of June 30, 2013. In making this assessment, management used the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO.
Based on this assessment, management has concluded that, as of June 30, 2013 our internal control over financial reporting is effective.
Ernst & Young LLP, our independent registered public accounting firm, has issued a report on the effectiveness of our internal control over financial reporting as of June 30, 2013. This report appears immediately below.
102
- (b)
- Attestation Report of the Independent Registered Public Accounting Firm
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of ImmunoGen, Inc.
We have audited ImmunoGen, Inc.'s internal control over financial reporting as of June 30, 2013, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). ImmunoGen, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, ImmunoGen, Inc. maintained, in all material respects, effective internal control over financial reporting as of June 30, 2013 based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of ImmunoGen, Inc. as of June 30, 2013 and 2012, and the related consolidated statements of operations and comprehensive loss, shareholders' equity, and cash flows for each of the three years in the period ended June 30, 2013 and our report dated August 29, 2013 expressed an unqualified opinion thereon.
| /s/ ERNST & YOUNG LLP |
Boston,
Massachusetts
August 29, 2013
103
- (c)
- Changes in Internal Control Over Financial Reporting
There have not been any changes in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the quarter ended June 30, 2013 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
3. Limitations on the Effectiveness of Controls
Our management, including our principal executive officer and principal financial officer, does not expect that our disclosure controls and procedures or its internal control over financial reporting will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within an organization have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake.
Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving our stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
None.
104
The information called for by Part III of Form 10-K (Item 10—Directors, Executive Officers and Corporate Governance of the Registrant, Item 11—Executive Compensation, Item 12—Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters, Item 13—Certain Relationships and Related Transactions, and Director Independence, and Item 14—Principal Accounting Fees and Services) is incorporated by reference from our proxy statement related to our 2013 annual meeting of shareholders, which will be filed with the Securities and Exchange Commission not later than October 28, 2013 (120 days after the end of the fiscal year covered by this Annual Report on Form 10-K), except that information required by Item 10 concerning our executive officers appears in Part I, Item 3.1 of this Annual Report on Form 10-K.
105
Item 15. Exhibits, Financial Statement Schedules
- (a)
- Financial Statements:
(1) See "Index to Consolidated Financial Statements" at Item 8 of this Annual Report on Form 10-K. Schedules not included herein are omitted because they are not applicable or the required information appears in the accompanying Consolidated Financial Statements or Notes thereto.
(2) The following schedule is filed as part of this Annual Report on Form 10-K:
Schedule II—Valuation and Qualifying Accounts for the years ended June 30, 2013, 2012 and 2011.
(3) See Exhibit Index following the signature page to this Annual Report on Form 10-K.
106
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| IMMUNOGEN, INC. | ||||
By: |
/s/ DANIEL M. JUNIUS Daniel M. Junius President and Chief Executive Officer (Principal Executive Officer) |
|||
Dated: August 29, 2013
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
/s/ DANIEL M. JUNIUS |
President, Chief Executive Officer and Director |
August 29, 2013 | ||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
107
| |
|
|
Incorporated by Reference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Exhibit Description | Filed with this Form 10-K |
Form | Filing Date with SEC |
Exhibit Number |
|||||||||||
| 3.1 | Restated Articles of Organization, as amended | 10-Q | April 30, 2010 | 3.1 | ||||||||||||
| 3.1(a) | Articles of Amendment | 10-Q | January 30, 2013 | 3.1 | ||||||||||||
| 3.2 | Amended and Restated By-Laws | 8-K | April 6, 2007 | 3.1 | ||||||||||||
| 4.1 | Article 4 of Restated Articles of Organization, as amended (see Exhibit 3.1) | |||||||||||||||
| 4.2 | Form of Common Stock certificate | S-1 | November 15, 1989 (File No. 33-31219) |
4.2 | ||||||||||||
| 10.1 | Leases dated as of December 1, 1986 and June 21, 1988 by and between James H. Mitchell, Trustee of New Providence Realty Trust, lessor, and Charles River Biotechnical Services, Inc. ("Lessee"), together with Assignment of Leases dated June 29, 1989 between Lessee and the Registrant | S-1 | September 22, 1989 (File No. 33-31219) |
10.10 | ||||||||||||
| 10.1(a) | First Amendment to Lease dated May 9, 1991 by and between James H. Mitchell, Trustee of New Providence Realty Trust, lessor, and the Registrant | S-1 | November 6, 1991 (File No. 33-43725) |
10.10a | ||||||||||||
| 10.1(b) | Confirmatory Second Amendment to Lease dated September 17, 1997 by and between James H. Mitchell, Trustee of New Providence Realty Trust, lessor, and the Registrant | 10-K | September 26, 1997 | 10.10 | ||||||||||||
| 10.1(c) | Third Amendment and Partial Termination of Lease dated as of August 8, 2000 by and between James H. Mitchell, Trustee of New Providence Realty Trust, lessor, and the Registrant | 10-K | September 2, 2008 | 10.1(c | ) | |||||||||||
| 10.1(d) | Fourth Amendment to Lease dated as of October 3, 2000 by and between James H. Mitchell, Trustee of New Providence Realty Trust, lessor, and the Registrant | 10-K | September 2, 2008 | 10.1(d | ) | |||||||||||
| 10.1(e) | Fifth Amendment to Lease dated as of June 7, 2001 by and between James H. Mitchell, Trustee of New Providence Realty Trust, lessor, and the Registrant | 10-K | September 2, 2008 | 10.1(e | ) | |||||||||||
| 10.1(f) | Sixth Amendment to Lease dated as of April 30, 2002 by and between Bobson 333 L.L.C., lessor, and the Registrant | 10-K | September 2, 2008 | 10.1(f | ) | |||||||||||
| 10.1(g) | Seventh Amendment to Lease dated as of October 20, 2005 by and between Bobson 333 L.L.C., lessor, and the Registrant | 10-K | September 2, 2008 | 10.1(g | ) | |||||||||||
| 10.1(h) | Eighth Amendment to Lease dated as of February 21, 2007 by and between Bobson 333 L.L.C., lessor, and the Registrant | 10-K | September 2, 2008 | 10.1(h | ) | |||||||||||
108
| |
|
|
Incorporated by Reference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Exhibit Description | Filed with this Form 10-K |
Form | Filing Date with SEC |
Exhibit Number |
|||||||||||
| 10.1(i) | Ninth Amendment to Lease dated as of November 17, 2010 by and between Bobson 333 LLC and the Registrant | 8-K | November 18, 2010 | 10.1 | ||||||||||||
| 10.2 | Lease Agreement, dated as of July 27, 2007, by and between Intercontinental Fund III 830 Winter Street LLC, landlord, and the Registrant | 10-Q | November 7, 2007 | 10.2 | ||||||||||||
| 10.3* | License Agreement dated effective May 2, 2000 by and between the Registrant and Genentech, Inc. | 10-Q | October 31, 2011 | 10.1 | ||||||||||||
| 10.3(a)* | Amendment to License Agreement for Anti-HER2 Antibodies, dated as of May 3, 2006, between the Registrant and Genentech, Inc. | 10-K | August 28, 2006 | 10.32 | ||||||||||||
| 10.3(b)* | Amendment to License Agreements made effective as of March 11, 2009, between the Registrant and Genentech, Inc. | 10-Q | May 7, 2009 | 10.1 | ||||||||||||
| 10.3(c) | Third Amendment to License Agreement for Anti-HER2 Antibodies, made effective as of December 18, 2012, between the Registrant and Genentech, Inc. | 10-Q | January 30, 2013 | 10.11 | ||||||||||||
| 10.4* | Collaboration and License Agreement dated as of July 30, 2003 by and between the Registrant and sanofi-aventis U.S. LLC (as successor-in-interest to Aventis Pharmaceuticals Inc.) | 10-Q | November 14, 2003 | 10.1 | ||||||||||||
| 10.4(a)* | Amendment No. 1, dated as of August 31, 2006, to the Collaboration and License Agreement between the Registrant and sanofi-aventis U.S. LLC | 10-Q | November 3, 2006 | 10.1 | ||||||||||||
| 10.4(b)* | Amendment No. 2, dated as of October 11, 2007, to the Collaboration and License Agreement between the Registrant and sanofi-aventis U.S. LLC | 10-Q | February 7, 2008 | 10.4 | ||||||||||||
| 10.4(c)* | Amendment No. 3, dated as of August 31, 2008, to the Collaboration and License Agreement between the Registrant and sanofi-aventis U.S. LLC | 10-Q | February 6, 2009 | 10.7 | ||||||||||||
| 10.5* | Option and License Agreement dated as of December 21, 2006 by and between the Registrant and sanofi-aventis U.S. LLC | 10-Q | February 8, 2007 | 10.2 | ||||||||||||
| 10.6* | Collaborative Development and License Agreement dated as of July 7, 2006 by and between the Registrant and Biotest AG | 10-Q | November 3, 2006 | 10.2 | ||||||||||||
| 10.6(a)* | Amendment No. 1, dated August 23, 2006, to Collaborative Development and License Agreement by and between the Registrant and Biotest AG | 10-Q | November 3, 2006 | 10.3 | ||||||||||||
| 10.7* | Development and License Agreement dated as of October 20, 2008 by and between the Registrant and Bayer HealthCare AG | 10-Q/A | October 10, 2012 | 10.1 | ||||||||||||
109
| |
|
|
Incorporated by Reference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Exhibit Description | Filed with this Form 10-K |
Form | Filing Date with SEC |
Exhibit Number |
|||||||||||
| 10.8* | Multi-Target Agreement dated as of October 8, 2010 by and between the Registrant and Novartis Institutes for BioMedical Research, Inc. | 10-Q/A | October 10, 2012 | 10.2 | ||||||||||||
| 10.8(a)* | First Amendment, effective as of March 29, 2013, to Multi-Target Agreement by and between the Registrant and Novartis Institutes for BioMedical Research, Inc. | 10-Q | May 6, 2013 | 10.1 | ||||||||||||
| 10.9* | Clinical Supply Agreement effective as of December 12, 2010 by and between the Registrant and Societá Italiana Corticosteroidi S.r.l. (Sicor) | 10-Q | February 8, 2011 | 10.1 | ||||||||||||
| 10.10* | Multi-Target Agreement dated as of December 19, 2011 by and between the Registrant and Eli Lilly and Company | 10-Q/A | October 10, 2012 | 10.3 | ||||||||||||
| 10.11† | Restated Stock Option Plan | 8-K | February 7, 2006 | 10.1 | ||||||||||||
| 10.11(a)† | Form of Incentive Stock Option Agreement | 8-K | February 7, 2006 | 10.2 | ||||||||||||
| 10.11(b)† | Form of Non-Qualified Stock Option Agreement | 8-K | February 7, 2006 | 10.3 | ||||||||||||
| 10.12† | 2006 Employee, Director and Consultant Equity Incentive Plan, as amended and restated through November 13, 2012 | 8-K | November 16, 2012 | 10.1 | ||||||||||||
| 10.12(a)† | Form of Incentive Stock Option Agreement for Executives | S-8 | November 15, 2006 | 99.4 | ||||||||||||
| 10.12(b)† | Form of Non-Qualified Stock Option Agreement for Executives | S-8 | November 15, 2006 | 99.5 | ||||||||||||
| 10.12(c)† | Form of Non-Qualified Stock Option Agreement for Directors | 10-Q | October 29, 2010 | 10.1 | ||||||||||||
| 10.12(d)† | Form of Director Deferred Stock Unit Agreement | 10-Q | October 29, 2010 | 10.1 | ||||||||||||
| 10.12(e)† | Form of Incentive Stock Option Agreement for all employees (including executives) | 10-K | August 29, 2012 | 10.14(g | ) | |||||||||||
| 10.12(f)† | Form of Non-Qualified Stock Option Agreement for all employees (including executives) | 10-K | August 29, 2012 | 10.14(h | ) | |||||||||||
| 10.12(g)† | Form of Non-Qualified Stock Option Agreement for Directors | 10-K | August 29, 2012 | 10.14(i | ) | |||||||||||
| 10.12(h)† | Form of Restricted Stock Agreement for all employees (including executives) | S-8 | November 21, 2012 | 99.1 | ||||||||||||
| 10.13† | 2001 Non-Employee Director Stock Plan | S-8 | December 18, 2001 | 99 | ||||||||||||
| 10.14† | 2004 Non-Employee Director Compensation and Deferred Stock Unit Plan, as amended on September16, 2009 | 10-Q | November 4, 2009 | 10.1 | ||||||||||||
| 10.15† | Form of Proprietary Information, Inventions and Competition Agreement between the Registrant and each of its executive officers | 10-Q | February 8, 2007 | 10.15 | ||||||||||||
110
| |
|
|
Incorporated by Reference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Exhibit Description | Filed with this Form 10-K |
Form | Filing Date with SEC |
Exhibit Number |
|||||||||||
| 10.16† | Change in Control Severance Agreement dated as of November 30, 2012 between the Registrant and Craig Barrows | 10-Q | January 30, 2013 | 10.1 | ||||||||||||
| 10.17† | Change in Control Severance Agreement dated as of November 30, 2012 between the Registrant and Daniel M. Junius | 10-Q | January 30, 2013 | 10.2 | ||||||||||||
| 10.18† | Change in Control Severance Agreement dated as of November 30, 2012 between the Registrant and John M. Lambert | 10-Q | January 30, 2013 | 10.3 | ||||||||||||
| 10.19† | Change in Control Severance Agreement dated as of November 30, 2012 between the Registrant and Charles Q. Morris | 10-Q | January 30, 2013 | 10.4 | ||||||||||||
| 10.20† | Change in Control Severance Agreement dated as of November 30, 2012 between the Registrant and James J. O'Leary | 10-Q | January 30, 2013 | 10.5 | ||||||||||||
| 10.21† | Change in Control Severance Agreement dated as of November 30, 2012 between the Registrant and Gregory D. Perry | 10-Q | January 30, 2013 | 10.6 | ||||||||||||
| 10.22† | Change in Control Severance Agreement dated as of November 30, 2012 between the Registrant and Peter Williams | 10-Q | January 30, 2013 | 10.7 | ||||||||||||
| 10.23† | Change in Control Severance Agreement dated as of November 30, 2012 between the Registrant and Theresa G. Wingrove | 10-Q | January 30, 2013 | 10.8 | ||||||||||||
| 10.24† | Compensation Policy for Non-Employee Directors, as amended through September 22, 2010 | 10-Q | October 29, 2010 | 10.1 | ||||||||||||
| 10.25† | Summary of Annual Executive Bonus Program | 10-Q | November 7, 2007 | 10.1 | ||||||||||||
| 10.26† | Employment Agreement dated as of July 27, 2011 between the Registrant and Gregory D. Perry | 10-K | August 29, 2011 | 10.26 | ||||||||||||
| 10.27† | Employment offer letter between the Registrant and Charles Q. Morris | 10-Q | January 30, 2013 | 10.9 | ||||||||||||
| 10.28† | Employment Agreement dated as of November 26, 2012 between the Registrant and Charles Q. Morris | 10-Q | January 30, 2013 | 10.10 | ||||||||||||
| 21 | Subsidiaries of the Registrant | 10-K | August 30, 2007 | 21 | ||||||||||||
| 23 | Consent of Ernst & Young LLP | X | ||||||||||||||
| 31.1 | Certification of the Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||||||
| 31.2 | Certification of the Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||||||
111
| |
|
|
Incorporated by Reference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit Number |
Exhibit Description | Filed with this Form 10-K |
Form | Filing Date with SEC |
Exhibit Number |
|||||||||||
| 32 | Certifications of Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||||||
| 101.INS | XBRL Instance Document | |||||||||||||||
| 101.SCH | XBRL Taxonomy Extension Schema | |||||||||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase | |||||||||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase | |||||||||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase | |||||||||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase | |||||||||||||||
- *
- Portions
of this Exhibit were omitted, as indicated by [***], and have been filed separately with the Secretary of the Commission
pursuant to the Registrant's application requesting confidential treatment.
- †
- Exhibit is a management contract or compensatory plan, contract or arrangement required to be filed as an exhibit to the annual report on Form 10-K.
112
IMMUNOGEN, INC.
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
(In thousands)
COLUMN A—DESCRIPTION
|
COLUMN B | COLUMN C— ADDITIONS |
COLUMN D | COLUMN E | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Inventory Valuation Allowance
|
Balance at Beginning of Period |
Charged to Costs and Expenses |
Use of Zero Value Inventory |
Balance at End of Period |
|||||||||
Year End June 30, 2013 |
$ | 1,291 | $ | 798 | $ | (1,279 | ) | $ | 810 | ||||
Year End June 30, 2012 |
$ | 1,993 | $ | 786 | $ | (1,488 | ) | $ | 1,291 | ||||
Year End June 30, 2011 |
$ | 939 | $ | 1,664 | $ | (610 | ) | $ | 1,993 | ||||
113
IMMUNOGEN, INC.
Stock Price Performance Graph
The graph and table below compare the annual percentage change in our cumulative total shareholder return on our common stock for the period from June 30, 2008 through June 30, 2013 (as measured by dividing (i) the sum of (A) the cumulative amount of dividends for the measurement period, assuming dividend reinvestment, and (B) the difference between our share price at the end and the beginning of the measurement period; by (ii) the share price at the beginning of the measurement period) with the total cumulative return of the NASDAQ Stock Market Index (U.S.) and the NASDAQ Pharmaceutical Stocks Total Return Index during such period. We have not paid any dividends on our common stock, and no dividends are included in the representation of our performance. The stock price performance on the graph below is not necessarily indicative of future price performance. This graph is not "soliciting material," is not deemed filed with the Commission and is not to be incorporated by reference in any of our filings under the Securities Act of 1933, or the Securities Exchange Act of 1934, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. Information used on the graph for the NASDAQ Pharmaceutical Stocks Total Return Index and the NASDAQ Stock Market Index (U.S.) was prepared by the Center for Research in Security Prices, a source believed to be reliable, but we are not responsible for any errors or omissions in such information.
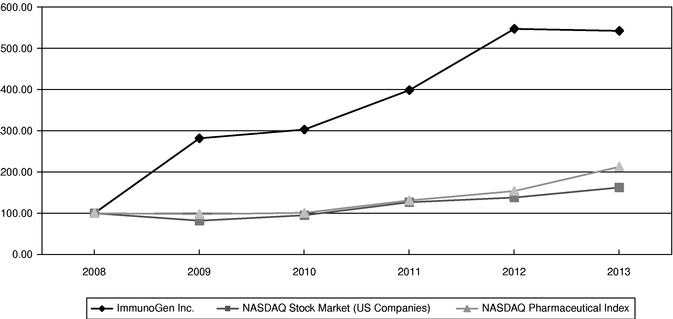
| |
2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
IMMUNOGEN, INC. |
$ | 100.00 | $ | 281.68 | $ | 302.91 | $ | 398.43 | $ | 547.14 | $ | 542.24 | |||||||
NASDAQ STOCK MARKET INDEX (U.S.) |
$ | 100.00 | $ | 81.85 | $ | 95.01 | $ | 126.63 | $ | 137.96 | $ | 162.34 | |||||||
NASDAQ PHARMACEUTICAL STOCKS TOTAL RETURN INDEX * |
$ | 100.00 | $ | 97.73 | $ | 100.83 | $ | 130.95 | $ | 153.72 | $ | 212.53 | |||||||
- *
- This index represents a group of peer issuers compiled by the Center for Research in Security Prices.
The above graph and table assume $100 invested on June 30, 2008 with all dividends reinvested, in each of our common stock, the NASDAQ Stock Market Index (U.S.) and the NASDAQ Pharmaceutical Stocks Total Return Index. Upon written request by any shareholder, we will promptly provide a list of the companies comprising the NASDAQ Pharmaceutical Stocks Total Return Index.
114