EXHIBIT 13
Pfizer Inc. 2019 Financial Report | |
 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
GLOSSARY OF DEFINED TERMS
Unless the context requires otherwise, references to “Pfizer,” “the Company,” “we,” “us” or “our” in this 2019 Financial Report (defined below) refer to Pfizer Inc. and its subsidiaries. We also have used several other terms in this 2019 Financial Report, most of which are explained or defined below:
2018 Financial Report | Financial Report for the fiscal year ended December 31, 2018, which was filed as Exhibit 13 to the Annual Report on Form 10-K for the fiscal year ended December 31, 2018 |
2019 Financial Report | This Financial Report for the fiscal year ended December 31, 2019, which was filed as Exhibit 13 to the Annual Report on Form 10-K for the fiscal year ended December 31, 2019 |
2019 Form 10-K | Annual Report on Form 10-K for the fiscal year ended December 31, 2019 |
ABO | Accumulated benefit obligation |
ACA (Also referred to as U.S. Healthcare Legislation) | U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act |
ACIP | Advisory Committee on Immunization Practices |
ALK | anaplastic lymphoma kinase |
Allergan | Allergan plc |
Alliance revenues | Revenues from alliance agreements under which we co-promote products discovered or developed by other companies or us |
Allogene | Allogene Therapeutics, Inc. |
Akcea | Akcea Therapeutics, Inc. |
AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
Anacor | Anacor Pharmaceuticals, Inc. |
AOCI | Accumulated Other Comprehensive Income |
Array | Array BioPharma Inc. |
Astellas | Astellas Pharma Inc., Astellas US LLC and Astellas Pharma US, Inc. |
ATM-AVI | aztreonam-avibactam |
Bain Capital | Bain Capital Private Equity and Bain Capital Life Sciences |
Bamboo | Bamboo Therapeutics, Inc. |
Biogen | Biogen Inc. |
Biopharma | Pfizer Biopharmaceuticals Group |
BMS | Bristol-Myers Squibb Company |
BRCA | BReast CAncer susceptibility gene |
CAR T | chimeric antigen receptor T cell |
CDC | U.S. Centers for Disease Control and Prevention |
Cellectis | Cellectis S.A. |
Cerevel | Cerevel Therapeutics, LLC |
CHMP | Committee for Medicinal Products for Human Use |
CIAS | cognitive impairment associated with schizophrenia |
Citibank | Citibank, N.A. |
CML | chronic myelogenous leukemia |
Developed Markets | U.S., Western Europe, Japan, Canada, South Korea, Australia, Scandinavian countries, Finland and New Zealand |
EC | European Commission |
EGFR | epidermal growth factor receptor |
EH | Essential Health |
EMA | European Medicines Agency |
Emerging Markets | Includes, but is not limited to, the following markets: Asia (excluding Japan and South Korea), Latin America, Eastern Europe, Africa, the Middle East, Central Europe and Turkey |
EPS | earnings per share |
EU | European Union |
FASB | Financial Accounting Standards Board |
FDA | U.S. Food and Drug Administration |
GAAP | Generally Accepted Accounting Principles |
GIST | gastrointestinal stromal tumors |
GPD | Global Product Development organization |
GSK | GlaxoSmithKline plc |
GS&Co. | Goldman, Sachs & Co. LLC |
HER2- | human epidermal growth factor receptor 2-negative |
hGH-CTP | human growth hormone |
HIS | Hospira Infusion Systems |
Hisun | Zhejiang Hisun Pharmaceuticals Co., Ltd. |
Hisun Pfizer | Hisun Pfizer Pharmaceuticals Company Limited |
Hospira | Hospira, Inc. |
HR+ | hormone receptor-positive |
IBT | Income before tax |
ICU Medical | ICU Medical, Inc. |
IH | Innovative Health |
2019 Financial Report | i | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Ionis | Ionis Pharmaceuticals, Inc. |
InnoPharma | InnoPharma, Inc. |
IPR&D | in-process research and development |
IRC | Internal Revenue Code |
IRS | U.S. Internal Revenue Service |
IV | intravenous |
Janssen | Janssen Biotech, Inc. |
J&J | Johnson & Johnson |
JV | joint venture |
King | King Pharmaceuticals LLC (formerly King Pharmaceuticals, Inc.) |
LDL | low density lipoprotein |
LEP | Legacy Established Products |
LIBOR | London Interbank Offered Rate |
Lilly | Eli Lilly and Company |
LOE | loss of exclusivity |
MCC | Merkel Cell Carcinoma |
MCO | Managed Care Organization |
mCRC | metastatic colorectal cancer |
Medivation | Medivation, Inc. |
Merck | Merck & Co., Inc. |
Meridian | Meridian Medical Technologies, Inc. |
Moody’s | Moody’s Investors Service |
Mylan | Mylan N.V. |
NAV | Net asset value |
NDA | new drug application |
NovaQuest | NovaQuest Co-Investment Fund II, L.P. or NovaQuest Co-Investment Fund V, L.P., as applicable |
NSCLC | non-small cell lung cancer |
NYSE | New York Stock Exchange |
OPKO | OPKO Health, Inc. |
OTC | over-the-counter |
PARP | poly ADP ribose polymerase |
PBM | Pharmacy Benefit Manager |
PBO | Projected benefit obligation |
Pharmacia | Pharmacia Corporation |
PPS | Portfolio Performance Shares |
PP&E | Property, plant & equipment |
PSAs | Performance Share Awards |
PsA | psoriatic arthritis |
PTSRUs | Performance Total Shareholder Return Units |
PTUs | Profit Units |
RA | rheumatoid arthritis |
RCC | renal cell carcinoma |
R&D | research and development |
ROU | right of use |
RPI | RPI Finance Trust |
RSUs | Restricted Stock Units |
Sandoz | Sandoz, Inc., a division of Novartis AG |
SEC | U.S. Securities and Exchange Commission |
Servier | Les Laboratoires Servier SAS |
SFJ | SFJ Pharmaceuticals |
Shire | Shire International GmbH |
SI&A | Selling, informational and administrative |
S&P | Standard and Poor’s |
SIP | Sterile Injectable Pharmaceuticals |
Tax Cuts and Jobs Act or TCJA | Legislation commonly referred to as the U.S. Tax Cuts and Jobs Act of 2017 |
Teuto | Laboratório Teuto Brasileiro S.A. |
Therachon | Therachon Holding AG |
TSR | Total Shareholder Return |
TSRUs | Total Shareholder Return Units |
UC | ulcerative colitis |
U.K. | United Kingdom |
U.S. | United States |
VBP | volume-based procurement in China |
ViiV | ViiV Healthcare Limited |
WRDM | Worldwide Research, Development and Medical |
ii | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
INTRODUCTION
See the Glossary of Defined Terms at the beginning of this 2019 Financial Report for terms used throughout this Financial Review. Our Financial Review is provided to assist readers in understanding the results of operations, financial condition and cash flows of Pfizer Inc. and its subsidiaries (the Company). It should be read in conjunction with the consolidated financial statements and Notes to Consolidated Financial Statements. The discussion in this Financial Review contains forward-looking statements that involve substantial risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors, such as those discussed in Part 1, Item 1A, “Risk Factors” of our 2019 Form 10-K and in the “Forward-Looking Information and Factors That May Affect Future Results”, “Our Operating Environment”, "The Global Economic Environment" and “Our Strategy” sections of this Financial Review.
All trademarks in this Financial Report are the property of their respective owners.
The Financial Review is organized as follows:
● | Beginning on page 2 | |||||
This section provides information about the following: Financial Highlights; Our Business; Our Business Development Initiatives; Our 2019 Performance; Our Operating Environment; The Global Economic Environment; Our Strategy; and Our Financial Guidance for 2020. | ||||||
● | Beginning on page 12 | |||||
This section discusses those accounting policies and estimates that we consider important in understanding our consolidated financial statements. For additional discussion of our accounting policies, see Notes to Consolidated Financial Statements—Note 1. Basis of Presentation and Significant Accounting Policies. | ||||||
● | Beginning on page 16 | |||||
This section includes the following sub-sections: | ||||||
○ | Beginning on page 16 | |||||
This sub-section provides a high-level summary of our revenues, including revenue deductions. | ||||||
○ | Beginning on page 18 | |||||
This sub-section provides an overview of revenues by segment and geography. | ||||||
○ | Beginning on page 21 | |||||
This sub-section provides an overview of several of our biopharmaceutical products. | ||||||
○ | Beginning on page 26 | |||||
This sub-section provides an overview of important biopharmaceutical product developments. | ||||||
○ | Beginning on page 30 | |||||
This sub-section provides a discussion about our costs and expenses. | ||||||
○ | Beginning on page 33 | |||||
This sub-section provides a discussion of items impacting our tax provisions. | ||||||
○ | Beginning on page 34 | |||||
This sub-section provides a discussion of an alternative view of performance used by management. | ||||||
● | Beginning on page 39 | |||||
This section provides a discussion of the performance of each of our operating segments. | ||||||
● | Beginning on page 48 | |||||
This section provides an analysis of our consolidated cash flows for the three years ended December 31, 2019. | ||||||
● | Beginning on page 49 | |||||
This section provides an analysis of selected measures of our liquidity and of our capital resources as of December 31, 2019 and December 31, 2018, as well as a discussion of our outstanding debt and other commitments that existed as of December 31, 2019 and December 31, 2018. Included in the discussion of outstanding debt is a discussion of the amount of financial capacity available to help fund Pfizer’s future activities. | ||||||
● | Beginning on page 54 | |||||
This section discusses accounting standards that we have recently adopted, as well as those that recently have been issued, but not yet adopted. | ||||||
● | Beginning on page 55 | |||||
This section provides a description of the risks and uncertainties that could cause actual results to differ materially from those discussed in forward-looking statements presented in this Financial Review. | ||||||
Certain amounts in our Financial Review may not add due to rounding. All percentages have been calculated using unrounded amounts.
2019 Financial Report | 1 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
OVERVIEW OF OUR PERFORMANCE, OPERATING ENVIRONMENT, STRATEGY AND OUTLOOK
Financial Highlights
The following charts provide a summary of certain financial performance (in billions, except per share data):
2019 Total Revenues––$51.8 billion | 2019 Net Cash Flow from Operations––$12.6 billion |
A decrease of 4% compared to 2018 | A decrease of 20% compared to 2018 |


2019 Reported Diluted EPS––$2.87 | 2019 Adjusted Diluted EPS (Non-GAAP)––$2.95* |
An increase of 54% compared to 2018 | An increase of 1% compared to 2018 |
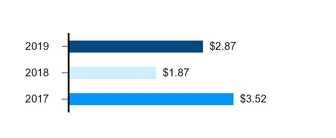

* | For additional information regarding Adjusted diluted EPS (which is a non-GAAP financial measure), including reconciliations of certain GAAP reported to non-GAAP adjusted information, see the “Non-GAAP Financial Measure (Adjusted Income)” section of this Financial Review. We have revised 2018 and 2017 Adjusted diluted EPS to conform with our 2019 presentation (see the “Non-GAAP Financial Measure (Adjusted Income)––Certain Significant Items)” section of this Financial Review). |
Our Business
We apply science and our global resources to bring therapies to people that extend and significantly improve their lives through the discovery, development, manufacture and distribution of healthcare products, including innovative medicines and vaccines. We work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. We collaborate with healthcare providers, governments and local communities to support and expand access to reliable, affordable healthcare around the world. Our revenues are derived from the sale of our products and, to a much lesser extent, from alliance agreements, under which we co-promote products discovered or developed by other companies or us (Alliance revenues).
At the beginning of our 2019 fiscal year, we began to manage our commercial operations through a new global structure consisting of three business segments––Pfizer Biopharmaceuticals Group (Biopharma), Upjohn and, through July 31, 2019, Consumer Healthcare. Biopharma and Upjohn are the only reportable segments. For additional information about this operating structure, see Notes to Consolidated Financial Statements––Note 17A. Segment, Geographic and Other Revenue Information: Segment Information. See the “Our Strategy––Organizing for Growth” section of this Financial Review below and the “Commercial Operations” section in Part I, Item 1, “Business” of our 2019 Form 10-K for additional information.
The majority of our revenues come from the manufacture and sale of biopharmaceutical products. The biopharmaceutical industry is highly competitive and highly regulated. As a result, we face a number of industry-specific factors and challenges, which can significantly impact our results. These factors include, among others: the loss or expiration of intellectual property rights and the expiration of co-promotion and licensing rights, the ability to replenish innovative biopharmaceutical products, healthcare legislation, pipeline productivity, the regulatory environment, pricing and access pressures and competition. We also face challenges as a result of the global economic environment. For additional information about these factors and challenges, see the “Our Operating Environment” and “The Global Economic Environment” sections of this Financial Review and Part I, Item 1A, “Risk Factors” of our 2019 Form 10-K.
The financial information included in our consolidated financial statements for our subsidiaries operating outside the U.S. is as of and for the year ended November 30 for each year presented. Pfizer's fiscal year-end for U.S. subsidiaries is as of and for the year ended December 31 for each year presented.
2 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
References to developed and emerging markets in this Financial Review include: | ||
Developed markets | U.S., Western Europe, Japan, Canada, South Korea, Australia, Scandinavian countries, Finland and New Zealand | |
Emerging markets (include, but are not limited to) | Asia (excluding Japan and South Korea), Latin America, Eastern Europe, Africa, the Middle East, Central Europe and Turkey | |
References to operational variances in this Financial Review pertain to period-over-period growth rates that exclude the impact of foreign exchange. The operational variances are determined by multiplying or dividing, as appropriate, our current year U.S. dollar results by the current year average foreign exchange rates and then multiplying or dividing, as appropriate, those amounts by the prior-year period average foreign exchange rates. Although exchange rate changes are part of our business, they are not within our control. Exchange rate changes, however, can mask positive or negative trends in the business; therefore, we believe presenting operational variances provides useful information to evaluate the results of our business.
On December 22, 2017, the U.S. enacted significant changes to U.S. tax law following the passage and signing of the TCJA. The TCJA is complex and significantly changes the U.S. corporate income tax system by, among other things, reducing the U.S. Federal corporate tax rate from 35% to 21%, transitioning U.S. international taxation from a worldwide tax system to a territorial tax system and imposing a repatriation tax on deemed repatriated accumulated post-1986 earnings of foreign subsidiaries. For information on the TCJA, see Notes to Consolidated Financial Statements––Note 5A. Tax Matters: Taxes on Income from Continuing Operations.
Our Business Development Initiatives
We are committed to capitalizing on growth opportunities by advancing our own pipeline and maximizing the value of our in-line products, as well as through various forms of business development, which can include alliances, licenses, joint ventures, collaborations, equity- or debt-based investments, dispositions, mergers and acquisitions. We view our business development activity as an enabler of our strategies, and we seek to generate earnings growth and enhance shareholder value by pursuing a disciplined, strategic and financial approach to evaluating business development opportunities. We continue to evaluate business development transactions that have the potential to strengthen our businesses and their capabilities, such as our acquisitions of Array, Therachon, Hospira, Medivation, Anacor and AstraZeneca’s small molecule anti-infectives business, as well as collaborations, and alliance and license agreements with other companies. We assess our businesses, assets and scientific capabilities/portfolio as part of our regular, ongoing portfolio review process and also continue to consider business development activities that will advance our businesses.
Our significant recent business development activities include:
• | License Agreement with Akcea Therapeutics, Inc.––In October 2019, we entered into a worldwide exclusive licensing agreement for AKCEA-ANGPTL3-LRx, an investigational antisense therapy being developed to treat patients with certain cardiovascular and metabolic diseases, with Akcea, a majority-owned affiliate of Ionis. The transaction closed in November 2019 and we made an upfront payment of $250 million to Akcea and Ionis, which was recorded in Research and development expenses in our fiscal fourth quarter of 2019. |
• | Formation of a New Consumer Healthcare Joint Venture––On July 31, 2019, we completed the transaction in which we and GSK combined our respective consumer healthcare businesses into a new consumer healthcare joint venture that operates globally under the GSK Consumer Healthcare name. The joint venture is a category leader in pain relief, respiratory and vitamins, minerals and supplements, and therapeutic oral health and is the largest global OTC consumer healthcare business. In accordance with our domestic and international reporting periods, our financial results, and our Consumer Healthcare segment’s operating results, for 2019 reflect seven months of Consumer Healthcare segment domestic operations and eight months of Consumer Healthcare segment international operations. Assets and liabilities associated with our Consumer Healthcare business were reclassified as held for sale in the consolidated balance sheet as of December 31, 2018. See Note 1A. Basis of Presentation and Significant Accounting Policies: Basis of Presentation. |
• | Acquisition of Array BioPharma Inc.––On July 30, 2019, we acquired Array for $48 per share in cash. The total fair value of the consideration transferred for Array was approximately $11.2 billion ($10.9 billion, net of cash acquired). Our financial statements for 2019 reflect the assets, liabilities, operating results and cash flows of Array, commencing from the acquisition date. |
• | Agreement to Combine Upjohn with Mylan N.V.––On July 29, 2019, we announced that we entered into a definitive agreement to combine Upjohn with Mylan, creating a new global pharmaceutical company, Viatris. Under the terms of the agreement, which is structured as an all-stock, Reverse Morris Trust transaction, Upjohn is expected to be spun off or split off to Pfizer’s shareholders and, immediately thereafter, combined with Mylan. Pfizer shareholders would own 57% of the combined new company, and former Mylan shareholders would own 43%. The transaction is expected to be tax free to Pfizer and Pfizer shareholders. The transaction is anticipated to close in mid-2020, subject to Mylan shareholder approval and satisfaction of other customary closing conditions, including receipt of regulatory approvals. We expect to incur costs of approximately $500 million in connection with fully separating Upjohn, inclusive of $145 million incurred in 2019. Such charges will include costs and expenses related to separation of legal entities and anticipated transaction costs. |
• | Acquisition of Therachon Holding AG––On July 1, 2019, we acquired all the remaining shares of Therachon for $340 million upfront, plus potential milestone payments of up to $470 million, contingent on the achievement of key milestones in the development and commercialization of the lead asset. The total fair value of the consideration transferred for Therachon was approximately $322 million. Our financial statements for 2019 reflect the assets, liabilities, operating results and cash flows of Therachon, commencing from the acquisition date and, in accordance with our international reporting period, reflect five months of Therachon operations and cash flows. |
• | Sale of Hospira Infusion Systems Net Assets to ICU Medical, Inc.––On February 3, 2017, we completed the sale of Pfizer’s global infusion systems net assets, HIS, to ICU Medical for up to approximately $900 million, composed of cash and contingent cash consideration, ICU Medical common stock (all of which we sold during 2018) and seller financing. The operating results of HIS are included in our consolidated statement of income through February 2, 2017 and, therefore, our financial results for 2017 reflect one month of HIS domestic operations and two months of HIS international operations. Our financial results for 2019 and 2018 do not reflect any contribution from HIS global operations. |
• | Acquisition of AstraZeneca’s Small Molecule Anti-Infectives Business––On December 22, 2016, which fell in the first fiscal quarter of 2017 for our international operations, we acquired the development and commercialization rights to AstraZeneca’s small molecule anti-infectives |
2019 Financial Report | 3 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
business, primarily outside the U.S. for approximately $1.0 billion, composed of cash and contingent consideration. Our financial statements reflect the assets, liabilities, operating results and cash flows of this business, commencing from the acquisition date and, in accordance with our international reporting period, for 2017 reflect approximately 11 months of the small molecule anti-infectives business operations and cash flows acquired from AstraZeneca.
For additional information, see Notes to Consolidated Financial Statements––Note 2. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements.
Our 2019 Performance
Revenues––2019
Revenues decreased $1.9 billion, or 4%, to $51.8 billion in 2019 from $53.6 billion in 2018, reflecting an operational decrease of $545 million, or 1%, and an unfavorable impact of foreign exchange of $1.4 billion, or 3%.
The following graph illustrates the components of the net decrease in revenues in 2019:
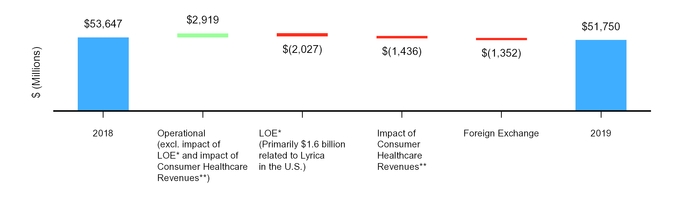
* | LOE generally pertains to period-over-period revenue impacts for products across our portfolios experiencing patent expirations or loss of regulatory exclusivity in certain developed markets. |
** | On July 31, 2019, we completed the transaction in which we and GSK combined our respective consumer healthcare businesses into a new consumer healthcare joint venture that operates globally under the GSK Consumer Healthcare name. For additional information, see Notes to Consolidated Financial Statements––Note 2. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements. |
The following provides an analysis of the changes in revenues in 2019:
(MILLIONS OF DOLLARS) | ||||
2018 Revenues | $ | 53,647 | ||
Operational growth/(decline): | ||||
Continued growth from certain key brands(a) | 2,495 | |||
Higher revenues from continued growth of anti-infective products in China, driven by increased demand for Sulperazon and new launches, the 2018 U.S. launches of immune globulin intravenous products (Panzyga and Octagam) and certain anti-infective launches in international developed and other emerging markets, all in the Hospital products business, higher revenues primarily in the U.S. for Inlyta, Biosimilars, and rare disease products driven by Vyndaqel/Vyndamax and volume-driven growth from Celebrex and Effexor, primarily in Japan and China | 1,067 | |||
Declines from Lyrica, primarily in the U.S., reflecting significantly lower volume associated with multi-source generic competition that began in July 2019, other Hospital products, Enbrel internationally, Viagra and Upjohn’s authorized generic for Viagra primarily in the U.S., Revatio and Relpax primarily in the U.S., and Norvasc and Lipitor primarily in China and Japan | (2,728 | ) | ||
Decline from Consumer Healthcare reflecting the July 31, 2019 completion of the Consumer Healthcare joint venture transaction with GSK | (1,436 | ) | ||
Other operational factors, net | 57 | |||
Operational decline, net | (545 | ) | ||
Operational revenues | 53,102 | |||
Unfavorable impact of foreign exchange | (1,352 | ) | ||
2019 Revenues | $ | 51,750 | ||
(a) | Certain key brands represent Ibrance, Eliquis, Xeljanz and Prevnar 13/Prevenar 13. See the “Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion" section of this Financial Review for product analysis information. |
For worldwide revenues and revenues by geography, for selected products, see the discussion in the “Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion” section of this Financial Review. For additional information regarding the primary indications or class of certain products, see Notes to Consolidated Financial Statements––Note 17C. Segment, Geographic and Other Revenue Information: Other Revenue Information.
4 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
See the “Analysis of the Consolidated Statements of Income––Revenues––Overview” and “––Revenues by Operating Segment and Geography” sections of this Financial Review for more information, including a discussion of key drivers of our revenue performance.
Income from Continuing Operations Before Provision/(Benefit) for Taxes on Income––2019
The following provides an analysis of the increase in Income from continuing operations before provision/benefit) for taxes on income for 2019:
(MILLIONS OF DOLLARS) | ||||
Income from continuing operations before provision/(benefit) for taxes on income for the year ended December 31, 2018 | $ | 11,885 | ||
Unfavorable change in revenues | (1,897 | ) | ||
Favorable/(Unfavorable) changes: | ||||
Gain on completion of Consumer Healthcare JV transaction | 8,086 | |||
Lower Cost of sales(a) | 1,029 | |||
Lower Selling, information and administrative expenses(b) | 105 | |||
Higher Research and development expenses(c) | (644 | ) | ||
Lower Amortization of intangible assets(d) | 283 | |||
Lower Restructuring charges and certain acquisition-related costs(e) | 296 | |||
Lower certain asset impairments(f) | 272 | |||
Higher royalty-related income(f) | 154 | |||
Favorable change in the fair value of contingent consideration(f) | 153 | |||
Non-recurrences of net realized losses on sales of investments in debt securities(f) | 141 | |||
Income from insurance recoveries related to Hurricane Maria(f) | 50 | |||
Higher charges for certain legal matters(f) | (397 | ) | ||
Higher net interest expense(f) | (365 | ) | ||
Impact of net periodic benefit costs/(credits) other than service costs(f) | (352 | ) | ||
Non-recurrence of gain on equity investment in Cerevel(f) | (343 | ) | ||
Lower income from collaborations, out-licensing arrangements and sales of compound/product rights(f) | (320 | ) | ||
Higher business and legal entity alignment costs(f) | (275 | ) | ||
Higher net losses on early retirement of debt(f) | (134 | ) | ||
Lower net gains recognized during the period on equity securities(f) | (132 | ) | ||
Non-recurrence of gain on the contribution of Pfizer’s CAR T assets(f) | (50 | ) | ||
All other items, net | 138 | |||
Income from continuing operations before provision/(benefit) for taxes on income for the year ended December 31, 2019 | $ | 17,682 | ||
(a) | See the “Costs and Expenses––Cost of Sales” section of this Financial Review. |
(b) | See the “Costs and Expenses––Selling, Informational and Administrative (SI&A) Expenses” section of this Financial Review. |
(c) | See the “Costs and Expenses––Research and Development (R&D) Expenses” section of this Financial Review. |
(d) | See the “Costs and Expenses––Amortization of Intangible Assets” section of this Financial Review. |
(e) | See the “Costs and Expenses––Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” and Notes to Consolidated Financial Statements––Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives. |
(f) | See the Notes to Consolidated Financial Statements––Note 4. Other (Income)/Deductions—Net. |
For information on our tax provision and effective tax rate see the “Provision/(Benefit) for Taxes on Income” section of this Financial Review and Notes to Consolidated Financial Statements––Note 5A. Tax Matters: Taxes on Income from Continuing Operations.
Our Operating Environment
Industry-Specific Challenges
Intellectual Property Rights and Collaboration/Licensing Rights
The loss, expiration or invalidation of intellectual property rights, patent litigation settlements with manufacturers and the expiration of co-promotion and licensing rights can have a significant adverse effect on our revenues. Certain of our current products have experienced patent-based expirations or loss of regulatory exclusivity in certain markets in the last few years, and we expect certain products to face significantly increased generic competition over the next few years. For example, Lyrica lost patent protection in the U.S. in June 2019 and multi-source generic competition began in July 2019. Also, the basic product patent for Chantix in the U.S. will expire in November 2020. We expect the impact of reduced revenues due to patent expiries will be significant in 2020, then moderating downward to a much lower level from 2021 through 2025.
For additional information, including the patent rights we consider most significant in relation to our business as a whole, together with the year in which the basic product patent expires and recent and expected losses of product exclusivity, see the “Patents and Other Intellectual Property Rights” section in Part I, Item 1, “Business” of our 2019 Form 10-K.
2019 Financial Report | 5 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
We will continue to aggressively defend our patent rights whenever we deem appropriate. For a discussion of certain recent developments with respect to patent litigation, see Notes to Consolidated Financial Statements––Note 16A1. Contingencies and Certain Commitments: Legal Proceedings––Patent Litigation.
Regulatory Environment/Pricing and Access––U.S. Healthcare Legislation
In March 2010, the ACA was enacted in the U.S. For additional information, see the “Government Regulation and Price Constraints” section in Part I, Item 1, “Business”, of our 2019 Form 10-K.
We recorded the following amounts as a result of the U.S. Healthcare Legislation: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Reduction to Revenues, related to the Medicare “coverage gap” discount provision | $ | 934 | $ | 674 | $ | 450 | ||||||
Selling, informational and administrative expenses, related to the fee payable to the federal government (which is not deductible for U.S. income tax purposes), based on our prior-calendar-year share relative to other companies of branded prescription drug sales to specified government programs. 2018 reflected a favorable true-up associated with the updated 2017 invoice received from the federal government, which reflected a lower expense than what was previously estimated for invoiced periods. | 247 | 184 | 307 | |||||||||
Regulatory Environment/Pricing and Access––Government and Other Payer Group Pressures
The pricing of medicines by pharmaceutical manufacturers and the cost of healthcare, which includes medicines, medical services and hospital services, continues to be important to payers, governments, patients, and other stakeholders. Governments, MCOs and other payor groups continue to seek increasing discounts on our products through a variety of means and could have an adverse impact on our results of operations. We believe that medicines are amongst the most powerful tools for patients in curing, treating and preventing illness and disability, and that all patients should have appropriate access to the medicines their doctors prescribe. We may consider a number of factors when determining a medicine’s price, including, for example, its impact on patients and their disease, other available treatments, the medicine’s potential to reduce other healthcare costs (such as hospital stays), and affordability. Within the U.S., in particular, we may also engage with patients, doctors and healthcare plans regarding their views. We also negotiate with insurers, including PBMs and MCOs, often providing significant discounts to them from the list price. The price that patients pay in the U.S. for the medicines their physicians prescribe is ultimately set by healthcare providers and insurers. On average, in the U.S., insurers impose a of higher out-of-pocket burden on patients for prescription medicines than for comparably-priced medical services. We will continue to work with insurance providers, governments and others to improve access to today’s innovative treatments. Certain governments, including the different EU member states, the U.K., China, Japan, Canada, South Korea and some other international markets, provide healthcare at low-to-zero direct cost to consumers at the point of care and have significant power as large single payers to regulate pharmaceutical prices or patient reimbursement levels to control costs for the government-sponsored healthcare system, particularly under recent global financing pressures. Governments may use a variety of cost-containment measures for our pharmaceutical products, including price cuts, mandatory rebates, health technology assessments, forced localization as a condition of market access, “international reference pricing” (i.e., the practice of a country linking its regulated medicine prices to those of other countries), quality consistency evaluation processes and volume-based procurement. For additional information, see the “Government Regulation and Price Constrains” section in Part I, Item 1, “Business” of our 2019 Form 10-K.
Regulatory Environment––Pipeline Productivity
The discovery and development of safe, effective new products, as well as the development of additional uses for existing products, are necessary for the continued strength of our businesses. We have encountered increasing regulatory scrutiny of drug safety and efficacy, even as we continue to gather safety and other data on our products, before and after the products have been launched. Our product lines must be replenished over time in order to offset revenue losses when products lose their market exclusivity, as well as to provide for earnings growth. We devote considerable resources to R&D activities. These activities involve a high degree of risk and cost and may take many years, and with respect to any specific R&D project, there can be no assurance that the development of any particular product candidate or new indication for an in-line product will achieve the desired clinical endpoints and safety profile, will be approved by regulators or will be successful commercially.
During the development of a product, we conduct clinical trials to provide data on the drug’s safety and efficacy to support the evaluation of its overall benefit-risk profile for a particular patient population. In addition, after a product has been approved and launched, we continue to monitor its safety as long as it is available to patients, and postmarketing trials may be conducted, including trials requested by regulators and trials that we do voluntarily to gain additional medical knowledge. For the entire life of the product, we collect safety data and report safety information to the FDA and other regulatory authorities. The FDA and regulatory authorities in other jurisdictions may evaluate potential safety concerns related to a product or a class of products and take regulatory actions in response, such as updating a product’s labeling, restricting the use of a product, communicating new safety information to the public, or, in rare cases, removing a product from the market.
Product Manufacturing
We periodically encounter difficulties or delays in manufacturing, including due to suspension of manufacturing or recall of a product, or legal or regulatory actions such as warning letters. For example, Hospira’s manufacturing facility in McPherson, Kansas is currently under the FDA inspection classification of Official Action Indicated (OAI). As a result of this classification, the FDA may refuse to grant premarket approval of applications and/or the FDA may refuse to grant export certificates related to products manufactured at our McPherson site until the site status is upgraded, which upgrade would be based on a re-inspection by the FDA. Future FDA inspections and regulatory activities will further assess the adequacy and sustainability of corrections implemented at the site. Communication with the FDA on the status of the McPherson site is
6 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
ongoing. For additional information regarding the FDA inspection of the McPherson site, see Part I, Item 1A, “Risk Factors––Product Manufacturing, Sales and Marketing Risks” of our 2019 Form 10-K.
We have been experiencing product shortages for products from the legacy Hospira portfolio, among others, largely driven by capacity constraints, technical issues, supplier quality concerns or unanticipated demand. We have made considerable progress remediating issues at legacy Hospira facilities manufacturing sterile injectables and have substantially improved supply from most of these sites. Continuing product shortage interruption at these and other manufacturing facilities could negatively impact our financial results.
Competition
Many of our prescription pharmaceutical products face competition in the form of branded or generic drugs or biosimilars that treat similar diseases or indications. For additional information, see the “Competition” section in Part I, Item 1, “Business” of our 2019 Form 10-K.
The Global Economic Environment
In addition to the industry-specific factors discussed above, we, like other businesses of our size, are exposed to the economic cycle, which impacts our biopharmaceutical operations globally.
• | Governments, corporations, and insurance companies, which provide insurance benefits to patients, have implemented increases in cost-sharing and restrictions on access to medicines, potentially causing patients to switch to generic or biosimilar products, delay treatments, skip doses or use less effective treatments. As discussed above, government financing pressures can lead to negative pricing pressure in various markets where governments take an active role in setting prices, access criteria (e.g., through public or private health technology assessments), or other means of cost control. |
• | Significant portions of our revenues, costs and expenses, as well as our substantial international net assets, are exposed to changes in foreign exchange rates. We seek to manage our foreign exchange risk in part through operational means, including managing same-currency revenues in relation to same-currency costs and same-currency assets in relation to same-currency liabilities. Depending on market conditions, foreign exchange risk also is managed through the use of derivative financial instruments and foreign currency debt. As we operate in multiple foreign currencies, including the euro, the Chinese renminbi, the Japanese yen, the Canadian dollar, the U.K. pound and approximately 100 other currencies, changes in those currencies relative to the U.S. dollar will impact our revenues and expenses. If the U.S. dollar were to weaken against another currency, assuming all other variables remained constant, our revenues would increase, having a positive impact on earnings, and our overall expenses would increase, having a negative impact on earnings. Conversely, if the U.S. dollar were to strengthen against another currency, assuming all other variables remained constant, our revenues would decrease, having a negative impact on earnings, and our overall expenses would decrease, having a positive impact on earnings. Therefore, significant changes in foreign exchange rates can impact our results and our financial guidance. |
The impact of possible currency devaluations in countries experiencing high inflation rates or significant exchange fluctuations, including Venezuela and Argentina, can impact our results and financial guidance. For additional information about our exposure to foreign currency risk, see the “Our Financial Guidance for 2020” and the “Analysis of Financial Condition, Liquidity and Capital Resources” sections of this Financial Review.
• | In June 2016, the U.K. electorate voted in a referendum to leave the EU, which is commonly referred to as “Brexit”. In March 2017, the U.K. government formally notified the European Council of its intention to leave the EU after it triggered Article 50 of the Lisbon Treaty to begin the two-year negotiation process establishing the terms of the exit and outlining the future relationship between the U.K. and the EU. Formal negotiations officially started in June 2017. Following the General Election in December 2019, the new U.K. parliament approved the negotiated withdrawal agreement and the U.K. left the EU on January 31, 2020 with status quo arrangements through a transition period scheduled to end on December 31, 2020. The transition period will be used to negotiate future trade arrangements between the U.K. and the EU. The consequences of the U.K. leaving the EU and the terms of the future trading relationship continue to be highly uncertain, which may pose certain implications to our research, commercial and general business operations in the U.K. and the EU, including the approval and supply of our products. At present, it is still unclear whether and to what extent the U.K. will remain within or aligned to the EU system of medicines regulation, after the end of the transition period. However, both the U.K. and the EU have issued detailed guidance for the industry on how medicines, medical devices and clinical trials will be separately regulated in their respective territories. Pfizer has substantially completed its preparations for Brexit, having made the changes necessary to meet relevant regulatory requirements in the EU and the U.K., through the transition period and afterwards, especially in the regulatory, research, manufacturing and supply chain areas. Between 2018 and 2021, we expect to spend up to approximately $60 million in one-time costs to make these adaptations. |
We generated approximately 2% of our worldwide revenues from the U.K. in 2019 including the foreign currency exchange impact from the weakening U.K. pound relative to the U.S. dollar to date.
• | Public health epidemics or outbreaks could adversely impact our business. In December 2019, a novel strain of coronavirus (COVID-19) emerged in Wuhan, Hubei Province, China. While initially the outbreak was largely concentrated in China and caused significant disruptions to its economy, it has now spread to several other countries and infections have been reported globally. The extent to which the coronavirus impacts our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, new information which may emerge concerning the severity of the coronavirus and the actions to contain the coronavirus or treat its impact, among others. In particular, the continued spread of the coronavirus globally could adversely impact our operations, including among others, our manufacturing and supply chain, sales and marketing and clinical trial operations and could have an adverse impact on our business and our financial results. |
Pfizer maintains a strong financial position while operating in a complex global environment. Due to our significant operating cash flows, financial assets, access to capital markets and available lines of credit and revolving credit agreements, we continue to believe that we have, and will maintain, the ability to meet our liquidity needs for the foreseeable future. Our long-term debt is rated high quality by both S&P and Moody’s. As market conditions change, we continue to monitor our liquidity position. We have taken and will continue to take a conservative approach to our financial investments. Both short-term and long-term investments consist primarily of high-quality, highly liquid, well-diversified,
2019 Financial Report | 7 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
available-for-sale debt securities. For further discussion of our financial condition and credit ratings, see the “Analysis of Financial Condition, Liquidity and Capital Resources” section of this Financial Review.
These and other industry-wide factors that may affect our businesses should be considered along with information presented in the “Forward-Looking Information and Factors That May Affect Future Results” section of this Financial Review and in Part I, Item 1A, “Risk Factors” of our 2019 Form 10-K.
Our Strategy
We believe that our medicines provide significant value for both healthcare providers and patients, not only from the improved treatment of diseases but also from a reduction in other healthcare costs, such as emergency room or hospitalization costs, as well as improvements in health, wellness and productivity. We continue to actively engage in dialogues about the value of our medicines and how we can best work with patients, physicians and payers to prevent and treat disease and improve outcomes. We continue to work within the current legal and pricing structures, as well as continue to review our pricing arrangements and contracting methods with payers, to maximize patient access and minimize any adverse impact on our revenues. We remain firmly committed to fulfilling our Company’s purpose: Breakthroughs that change patients’ lives. By doing so, we expect to create value for the patients we serve and for our colleagues and shareholders.
Organizing for Growth
We believe we have one of the strongest pipelines we have had in over a decade, and believe we are well positioned for future growth. Additional patent expiries will continue over several years, and we expect the impact of reduced revenues due to patent expiries will be significant in 2020, then moderating downward to a much lower level from 2021 through 2025. This confluence of events has provided us an opportunity to look at and refine how we organize our business to best achieve sustainable growth and to deliver our medicines and vaccines to the maximum number of people who need them.
At the beginning of our fiscal year 2019, we began to manage our commercial operations through a new global structure consisting of three businesses, each led by a single manager—Pfizer Biopharmaceuticals Group (Biopharma), Upjohn and, through July 31, 2019, Pfizer’s Consumer Healthcare business. We designed this new global structure to take advantage of new growth opportunities driven by the evolving and unique dynamics of relevant markets.
For additional information about each business, see Notes to Consolidated Financial Statements––Note 17A. Segment, Geographic and Other Revenue Information: Segment Information.
We also reorganized our R&D operations as part of our Organizing for Growth reorganization:
• | The former Worldwide Research and Development organization is renamed Worldwide Research, Development and Medical (WRDM) as we have created a new Worldwide Medical & Safety organization in WRDM that incorporates the former Chief Medical Office as well as the Worldwide Safety function; |
• | The R&D organization within our former Essential Health business has been integrated into the WRDM, GPD and Upjohn organizations, including moving biosimilars into WRDM and GPD and realigning them with the relevant therapeutic areas (e.g., Oncology and Inflammation & Immunology); |
• | The Regulatory function has been moved from the WRDM organization into the GPD organization; and |
• | Late-stage portfolio spend has been moved from our former Innovative Health business to GPD and from our former Essential Health business to GPD and Upjohn. |
We re-aligned our commercial operations in 2019 for a number of reasons, including:
• | Bringing biosimilars into our Oncology and Inflammation & Immunology therapeutic categories gives us the potential to leverage our R&D, regulatory and commercial infrastructure within the Biopharma business to more efficiently bring those assets to market; |
• | Creating a business unit (i.e., the Hospital unit within Biopharma) that is solely focused on medicines that are used in hospitals can potentially bring greater focus and attention to serving those customers and developing those relationships; |
• | Giving Upjohn more autonomy with a focus on maximizing the value of its products, particularly in emerging markets, provides it the opportunity to operate as a standalone business within Pfizer with the potential for sustainable modest growth; and |
• | We believe this new structure better positions each business to achieve its growth potential as we transition to a period post-2020 where we expect higher and more sustained revenue growth due to declining LOEs and the potential of our late-stage pipeline. |
Biopharma seeks to leverage a strong pipeline, organize around operational growth drivers, and capitalize on trends creating long-term growth opportunities, including:
• | an aging global population that is generating increased demand for innovative medicines that address patients’ unmet needs; |
• | advances in both biological science and digital technology that are enhancing the delivery of breakthrough new medicines; and |
• | the increasingly significant role of hospitals in healthcare systems. |
Urbanization and the rise of the middle class in emerging markets, particularly in Asia, provide growth opportunities for the Upjohn business. Our ability to work collaboratively within local markets and to be fast, focused and flexible is intended to position this business to seize these opportunities. Upjohn has distinct and dedicated manufacturing, marketing, regulatory and, subject to limited exceptions, enabling functions that report directly into the business providing autonomy and positioning Upjohn to operate as a true stand-alone division. We created this new structure to, among other things, position Upjohn to optimize its distinct growth potential and provide us with the flexibility to access further opportunities to enhance value.
Subsequent to the re-alignment of our commercial operations in 2019, on July 29, 2019, we announced that we entered into a definitive agreement to combine Upjohn with Mylan, creating a new global pharmaceutical company, Viatris. For additional information, see the “Our Business Development Initiatives” section above in this Financial Review. We believe the new company will transform and accelerate Upjohn’s
8 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
and Mylan’s ability to serve patients’ needs and expand their capabilities across more than 165 markets. The combination will drive a sustainable, diverse and differentiated portfolio of prescription medicines, complex generics, over-the-counter products and biosimilars supported by commercial and regulatory expertise, established infrastructure, R&D capabilities and manufacturing and supply chain excellence.
As we prepare for expected growth, we are focused on creating a simpler, more efficient organization by streamlining structures, processes and governance within each business and the functions that support them. As our innovative pipeline matures based on anticipated progression of current trials and the initiation of new pivotal trials, including new trials for medicines we may acquire or in-license, we will need to increase our R&D investments. In addition, as our pipeline potentially delivers new commercialization opportunities, we will need to increase our investments in new-market-creation activities. We have initiated an enterprise-wide digital effort to accelerate drug development, enhance experiences (patient and physician) and access and leverage technology and robotics to simplify and automate our processes.
In the fourth quarter of 2018, we took steps to simplify the organization, increase spans of control and reduce organizational layers, which impacted some managerial roles and responsibilities. We also offered enhancements to certain employee benefits for a short period of time. The expenses related to these enhancements for certain employee benefits did not have a material impact on our 2018 and 2019 results of operations.
Transforming to a More Focused Company
With the formation of the GSK Consumer Healthcare venture and the pending combination of Upjohn with Mylan, Pfizer is transforming itself into a more focused, global leader in science-based innovative medicines. As a result, we began, in the fourth quarter of 2019, to identify and undertake efforts to ensure our cost base aligns appropriately with our Biopharmaceutical revenue base, which is expected to be 20% less (based on the midpoint of the range for 2020 New Pfizer revenue guidance (see the “Our Financial Guidance for 2020” section of this Financial Review), compared to 2019 total company reported revenue) as a result of both the completed Consumer Healthcare and expected Upjohn transactions. While certain direct costs have transferred or will transfer to the Consumer Healthcare joint venture and to the Upjohn entities, there are indirect costs which are not expected to transfer. In addition, we are taking steps to restructure our organizations to appropriately support and drive the purpose of the three core functions of our focused innovative medicines business: R&D, Manufacturing and Commercial. For additional information, see the “Costs and Expenses–– Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” section of this Financial Review.
Commercial Operations
As discussed under “Organizing for Growth”, at the beginning of our 2019 fiscal year, we began to manage our commercial operations through a new global structure consisting of three business segments––Biopharma, Upjohn and, through July 31, 2019, Consumer Healthcare, each led by a single manager. Each operating segment has responsibility for its commercial activities. Upjohn, and through July 31, 2019, Consumer Healthcare are responsible for their own R&D activities while Biopharma receives its R&D services from GPD and WRDM. These services include IPR&D projects for new investigational products and additional indications for in-line products. Each business has a geographic footprint across developed and emerging markets. For additional information on our Commercial Operations, see the “Commercial Operations” section in Part I, Item 1, “Business” of our 2019 Form 10-K. For additional information about our operating structure, see Notes to Consolidated Financial Statements—Note 17A. Segment, Geographic and Other Revenue Information: Segment Information. For additional information about the 2019 performance of each of our operating segments, see the “Analysis of Operating Segment Information” section of this Financial Review.
Description of Research and Development Operations
Innovation is critical to the success of our Company, and drug discovery and development are time-consuming, expensive and unpredictable. Pfizer’s purpose is to deliver breakthroughs that change patients’ lives. R&D is at the heart of fulfilling Pfizer’s purpose as we work to translate advanced science and technologies into the therapies that matter most.
Our R&D priorities include:
• | delivering a pipeline of highly differentiated medicines and vaccines where Pfizer has a unique opportunity to bring the most important new therapies to patients in need; |
• | advancing our capabilities that can position Pfizer for long-term R&D leadership; and |
• | advancing new models for partnerships with creativity, flexibility and urgency to deliver innovation to patients as quickly as possible. |
To that end, our R&D primarily focuses on:
• | Oncology; |
• | Inflammation and Immunology; |
• | Vaccines; |
• | Internal Medicine; |
• | Rare Diseases; and |
• | Hospital. |
For additional information about R&D, see the “Research and Development” section in Part I, Item 1, “Business” of our 2019 Form 10-K. For additional information about R&D by operating segment, see the “Analysis of Operating Segment Information” section of this Financial Review.
For additional information about our pending new drug applications and supplemental filings, see the “Analysis of the Consolidated Statements of Income––Product Developments––Biopharmaceutical” section of this Financial Review.
For additional information about recent transactions and strategic investments that we believe have the potential to advance our pipeline, see the “Our Business Development Initiatives” section of this Financial Review above.
2019 Financial Report | 9 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Intellectual Property Rights
We continue to vigorously defend our patent rights against increasingly aggressive infringement, and we will continue to support efforts that strengthen worldwide recognition of patent rights while taking necessary steps to ensure appropriate patient access. In addition, we will continue to employ innovative approaches designed to prevent counterfeit pharmaceuticals from entering the supply chain and to achieve greater control over the distribution of our products, and we will continue to participate in the generics market whenever appropriate. Also, the pursuit of valid business opportunities may require us to challenge intellectual property rights held by other companies that we believe were improperly granted. Such challenges may include negotiation and litigation, which may not always be successful. For additional information about our current efforts to enforce our intellectual property rights and certain other patent proceedings, see Notes to Consolidated Financial Statements––Note 16A1. Contingencies and Certain Commitments: Legal Proceedings––Patent Litigation. For information on risks related to patent protection and intellectual property claims by third parties, see Part I, Item 1A, “Risk Factors––Risks Related to Intellectual Property” in our 2019 Form 10-K.
Capital Allocation and Expense Management
We seek to maintain a strong balance sheet and robust liquidity so that we continue to have the financial resources necessary to take advantage of prudent commercial, research and business development opportunities and to directly enhance shareholder value through share repurchases and dividends. For additional information about our financial condition, liquidity, capital resources, share repurchases (including accelerated share repurchases) and dividends, see the “Analysis of Financial Condition, Liquidity and Capital Resources” section of this Financial Review. For additional information about our recent business development activities, see the “Our Business Development Initiatives” section of this Financial Review above.
In December 2019, our Board of Directors declared a first-quarter 2020 dividend of $0.38 per share, an increase from the $0.36 per-share quarterly dividend paid during 2019. For additional information, see the “Analysis of Financial Condition, Liquidity and Capital Resources” section of this Financial Review.
We remain focused on achieving an appropriate cost structure for our company. For additional information about our various initiatives, see the “Our Strategy––Transforming to a More Focused Company” and “Costs and Expenses––Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” sections of this Financial Review and Notes to Consolidated Financial Statements––Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives.
Increasing Investment in the U.S.––After evaluating the expected positive net impact the TCJA will have on us, in early 2018, we decided to take several actions:
• | Over the five-year period from 2018 through 2022, we plan to invest approximately $5.0 billion in capital projects in the U.S., including the strengthening of our manufacturing presence in the U.S. As part of this plan: |
◦ | in July 2018, we announced that we will increase our commitment to U.S. manufacturing with a $465 million investment to build one of the most technically advanced sterile injectable pharmaceutical production facilities in the world in Portage, Michigan. This U.S. investment will strengthen our capability to produce and supply critical, life-saving injectable medicines for patients around the world, creating an estimated 450 new jobs over the next several years; and |
◦ | in August 2019, we announced an additional half billion dollar investment for the construction of a state-of-the-art gene therapy manufacturing facility in Sanford, North Carolina. This facility is anticipated to support our continuing investment in gene therapy R&D, similar to Pfizer’s Chapel Hill and Kit Creek, North Carolina R&D sites. This facility would expand our presence in North Carolina. The expanded facility is projected to add approximately 300 new jobs. |
• | We made a $500 million voluntary contribution to the U.S. Pfizer Consolidated Pension Plan in February 2018. |
• | In the first quarter of 2018, we paid a special, one-time bonus to virtually all Pfizer colleagues, excluding executives, of $119 million in the aggregate. |
Our Business Development Initiatives
As part of our strategy, we are also committed to capitalizing on growth opportunities by advancing our own pipeline and maximizing the value of our in-line products, as well as through various forms of business development, which can include alliances, licenses, joint ventures, collaborations, equity- or debt-based investments, dispositions, mergers and acquisitions. For additional information see the “Our Business Development Initiatives” section of this Financial Review above and Notes to Consolidated Financial Statements––Note 2. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements.
10 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Our Financial Guidance for 2020
2020 Financial Guidance for Total Company
2020 financial guidance for Total Company is presented below(a), (b): | |
Revenues | $48.5 to $50.5 billion |
Adjusted cost of sales as a percentage of revenues | 19.9% to 20.9% |
Adjusted selling, informational and administrative expenses | $12.0 to $13.0 billion |
Adjusted research and development expenses | $8.1 to $8.5 billion |
Adjusted other (income)/deductions | Approximately $800 million of income |
Effective tax rate on adjusted income | Approximately 15.0% |
Adjusted diluted EPS | $2.82 to $2.92 |
(a) | The 2020 financial guidance for Total Company reflects the following: |
• | Reflects a full year of revenue and expense contributions from Biopharma and Upjohn, and excludes any impact from the pending Upjohn combination with Mylan. |
• | Does not assume the completion of any business development transactions not completed as of December 31, 2019, including any one-time upfront payments associated with such transactions. |
• | Includes Pfizer’s pro rata share of the Consumer Healthcare joint venture anticipated earnings, which is recorded in Adjusted other (income)/deductions on a one-quarter lag. Therefore, 2020 financial guidance for Adjusted other (income)/deductions and Adjusted diluted EPS reflects Pfizer’s share of the joint venture’s earnings that were generated in the fourth quarter of 2019 (to be recorded by Pfizer in the first quarter of 2020) as well as Pfizer’s share of the joint venture’s projected earnings during the first three quarters of 2020. |
• | Reflects an anticipated negative revenue impact of $2.4 billion due to recent and expected generic and biosimilar competition for certain products that have recently lost or are anticipated to soon lose patent protection. |
• | Exchange rates assumed are as of mid-January 2020. Reflects the anticipated unfavorable impact of approximately $0.2 billion on revenues and approximately $0.01 on adjusted diluted EPS as a result of changes in foreign exchange rates relative to the U.S. dollar compared to foreign exchange rates from 2019. |
• | Guidance for adjusted diluted EPS assumes diluted weighted-average shares outstanding of approximately 5.65 billion shares, which assumes no share repurchases in 2020. |
(b) | For additional information regarding an understanding of Adjusted income and its components and Adjusted diluted EPS (all of which are non-GAAP financial measures), see the “Non-GAAP Financial Measure (Adjusted Income)” section of this Financial Review. |
Beginning in 2020, Upjohn began managing Pfizer’s Meridian subsidiary, the manufacturer of EpiPen and other auto-injector products, and the Mylan-Japan collaboration for generic drugs in Japan (established in 2012) (Mylan-Japan). As a result, revenues and expenses associated with Meridian and Mylan-Japan will be reported in Pfizer’s Upjohn business beginning in the first quarter of 2020. In 2019, revenues from Meridian and Mylan-Japan were recorded in Pfizer’s Biopharma business and totaled $598 million, flat operationally, compared with full-year 2018.
Pfizer, Upjohn and Mylan are in the process of negotiating the terms on which Pfizer would transfer the Meridian business and/or certain Pfizer assets that currently form part of the Mylan-Japan collaboration to Viatris following the completion of the proposed combination of Upjohn and Mylan. There can be no assurance that any agreement or transaction will result from these negotiations and if the parties are unsuccessful in their efforts to negotiate the terms of such potential transactions, the Meridian business and/or the Pfizer assets that currently form part of the Mylan-Japan collaboration will remain with Pfizer.
2020 Financial Guidance for New Pfizer
2020 financial guidance for New Pfizer is presented below(a), (b): | |
Revenues | $40.7 to $42.3 billion |
Adjusted IBT Margin(c) | Approximately 37.0% |
Adjusted Diluted EPS | $2.25 to $2.35 |
Operating Cash Flow(d) | $11.0 to $12.0 billion |
(a) | Financial guidance for New Pfizer reflects a full-year 2020 pro-forma view of the company assuming the pending Upjohn combination with Mylan was completed at the beginning of 2020. Therefore, New Pfizer reflects contributions from the Biopharma business as it is presently being managed, which excludes contributions from Pfizer’s Meridian subsidiary and the Pfizer-Mylan strategic collaboration in Japan (Mylan-Japan). Pfizer’s Meridian subsidiary and Mylan-Japan were managed by Pfizer’s Biopharma business in 2019. Financial guidance for New Pfizer also includes the full-year effect of the following items that assume the completion of the Upjohn combination with Mylan: (i) $12 billion of net proceeds from Upjohn to be retained by Pfizer, which Pfizer will use to repay its own existing indebtedness; and (ii) other transaction-related items, such as income from transition services agreements between Pfizer and Viatris. In addition, 2020 financial guidance for New Pfizer Adjusted IBT Margin and Adjusted diluted EPS reflects Pfizer’s share of the earnings generated by the Consumer Healthcare joint venture in fourth-quarter 2019 (to be recorded by Pfizer in first-quarter 2020) as well as Pfizer’s share of the Consumer Healthcare joint venture’s projected earnings during the first three quarters of 2020. |
(b) | For additional information regarding an understanding of Adjusted income and its components and Adjusted diluted EPS (all of which are non-GAAP financial measures), see the “Non-GAAP Financial Measure (Adjusted Income)” section of this Financial Review. |
(c) | Adjusted income before tax margin (Adjusted IBT margin) is defined as revenue less the sum of Adjusted cost of sales, Adjusted SI&A expenses, Adjusted R&D expenses, Adjusted amortization of intangible assets and Adjusted other (income)/deductions as a percentage of revenue. Adjusted IBT Margin is presented because management believes this performance measure supplements investors’ and other readers’ understanding and assessment of the financial performance of New Pfizer. Adjusted IBT margin is not, and should not be viewed as, a substitute for U.S. GAAP income before tax margin. |
(d) | Does not include our planned $1.25 billion voluntary contribution to the U.S. qualified pension plans in the second half of 2020. |
2020 Financial Guidance for Upjohn
2020 financial guidance for Upjohn now reflects the inclusion of revenues and expenses associated with Meridian and Mylan-Japan, which were previously recorded in Pfizer’s Biopharma business. Except for the shift of Meridian and Mylan-Japan from Biopharma to Upjohn, there are no operational changes to Upjohn’s 2020 financial guidance compared with the preliminary financial targets that were provided in July 2019.
2019 Financial Report | 11 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
2020 financial guidance for Upjohn is presented below(a): | |
Revenues | $8.0 to $8.5 billion |
Adjusted EBITDA(b) | $3.8 to $4.2 billion |
(a) | Financial guidance for Upjohn assumes a full-year 2020 contribution from the Upjohn business as it is presently being managed, which includes contributions from Pfizer’s Meridian subsidiary and Mylan-Japan. |
(b) | Adjusted Earnings Before Interest, Tax, Depreciation and Amortization (EBITDA) is defined as reported U.S. GAAP net income/(loss), and its components, adjusted for interest expense, provision/(benefit) for taxes on income/(loss) and depreciation and amortization, further adjusted to exclude purchase accounting adjustments, acquisition-related costs, discontinued operations and certain significant items (some of which may recur, such as gains on the completion of joint venture transactions, restructuring charges, legal charges or net gains and losses on equity securities, but which management does not believe are reflective of ongoing core operations). Adjusted EBITDA is presented because management believes this performance measure supplements investors’ and other readers’ understanding and assessment of the financial performance of Upjohn. Adjusted EBITDA as defined is not a measurement of financial performance under GAAP, and should not be considered as an alternative to net income/(loss) or cash flow from operations determined in accordance with GAAP. |
Pfizer does not provide guidance for GAAP Reported financial measures (other than revenues) or a reconciliation of forward-looking non-GAAP financial measures to the most directly comparable GAAP Reported financial measures on a forward-looking basis because it is unable to predict with reasonable certainty the ultimate outcome of pending litigation, unusual gains and losses, acquisition-related expenses, net gains or losses on investments in equity securities and potential future asset impairments without unreasonable effort. These items are uncertain, depend on various factors, and could have a material impact on GAAP Reported results for the guidance period.
For information about our actual costs and anticipated costs and cost savings associated with our various initiatives, see the “Costs and Expenses––Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” section of this Financial Review and Notes to Consolidated Financial Statements––Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives.
Our 2020 financial guidance is subject to a number of factors and uncertainties as described in the “Our Operating Environment”, “The Global Economic Environment”, “Our Strategy” and “Forward-Looking Information and Factors That May Affect Future Results” sections of this Financial Review; and Part I, Item 1A, “Risk Factors” of our 2019 Form 10-K.
SIGNIFICANT ACCOUNTING POLICIES AND APPLICATION OF CRITICAL ACCOUNTING ESTIMATES AND ASSUMPTIONS
For a description of our significant accounting policies, see Notes to Consolidated Financial Statements––Note 1. Basis of Presentation and Significant Accounting Policies. Of these policies, the following are considered critical to an understanding of our consolidated financial statements as they require the application of the most subjective and the most complex judgments: Acquisitions (Note 1D); Fair Value (Note 1E); Revenues (Note 1G); Asset Impairments (Note 1L); Tax Assets and Liabilities and Income Tax Contingencies (Note 1P); Pension and Postretirement Benefit Plans (Note 1Q); and Legal and Environmental Contingencies (Note 1R).
Following is a discussion about the critical accounting estimates and assumptions impacting our consolidated financial statements. See also Notes to Consolidated Financial Statements––Note 1C. Basis of Presentation and Significant Accounting Policies: Estimates and Assumptions for a discussion about the risks associated with estimates and assumptions.
Acquisitions and Fair Value
For a discussion about the application of fair value to our recent acquisitions, see Notes to Consolidated Financial Statements—Note 2A. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Acquisitions.
For a discussion about the application of fair value to our investments, see Notes to Consolidated Financial Statements—Note 7A. Financial Instruments: Fair Value Measurements.
For a discussion about the application of fair value to our benefit plan assets, see Notes to Consolidated Financial Statements––Note 11D. Pension and Postretirement Benefit Plans and Defined Contribution Plans: Plan Assets.
For a discussion about the application of fair value to our asset impairment reviews, see “Asset Impairment Reviews” below.
Revenues
Our gross product revenues are subject to a variety of deductions, which generally are estimated and recorded in the same period that the revenues are recognized. Such variable consideration represents chargebacks, rebates, sales allowances and sales returns. These deductions represent estimates of the related obligations and, as such, knowledge and judgment are required when estimating the impact of these revenue deductions on gross sales for a reporting period.
Historically, our adjustments of estimates, to reflect actual results or updated expectations, have not been material to our overall business. On a quarterly basis, our adjustments of estimates to reflect actual results generally have been less than 1% of revenues, and have resulted in either a net increase or a net decrease in revenues. Product-specific rebates, however, can have a significant impact on year-over-year individual product growth trends. If any of our ratios, factors, assessments, experiences or judgments are not indicative or accurate predictors of our future experience, our results could be materially affected. The sensitivity of our estimates can vary by program, type of customer and geographic location. However, estimates associated with U.S. Medicare, Medicaid and performance-based contract rebates are most at risk for material adjustment because of the extensive time delay between the recording of the accrual and its ultimate settlement, an interval that can generally range up to one year. Because of this time lag, in any given quarter, our adjustments to actual can incorporate revisions of several prior quarters.
12 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Asset Impairment Reviews
We review all of our long-lived assets for impairment indicators throughout the year. We perform impairment testing for indefinite-lived intangible assets and goodwill at least annually and for all other long-lived assets whenever impairment indicators are present. When necessary, we record charges for impairments of long-lived assets for the amount by which the fair value is less than the carrying value of these assets. Our impairment review processes are described in the Notes to Consolidated Financial Statements––Note 1L. Basis of Presentation and Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
Examples of events or circumstances that may be indicative of impairment include:
• | A significant adverse change in legal factors or in the business climate that could affect the value of the asset. For example, a successful challenge of our patent rights would likely result in generic competition earlier than expected. |
• | A significant adverse change in the extent or manner in which an asset is used. For example, restrictions imposed by the FDA or other regulatory authorities could affect our ability to manufacture or sell a product. |
• | A projection or forecast that indicates losses or reduced profits associated with an asset. This could result, for example, from a change in a government reimbursement program that results in an inability to sustain projected product revenues and profitability. This also could result from the introduction of a competitor’s product that results in a significant loss of market share or the inability to achieve the previously projected revenue growth, as well as the lack of acceptance of a product by patients, physicians and payers. For IPR&D projects, this could result from, among other things, a change in outlook based on clinical trial data, a delay in the projected launch date or additional expenditures to commercialize the product. |
Identifiable Intangible Assets
As a result of our identifiable intangible asset impairment review work, we recognized a number of impairments of identifiable intangible assets for the years ended December 31, 2019, 2018 and 2017. See Notes to Consolidated Financial Statements––Note 4. Other (Income)/Deductions—Net.
When we are required to determine the fair value of intangible assets other than goodwill, we use an income approach, specifically the discounted cash flow method. We start with a forecast of all the expected net cash flows associated with the asset, which includes the application of a terminal value for indefinite-lived assets, and then we apply an asset-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of competitive, legal and/or regulatory forces on the projections and the impact of technological risk associated with IPR&D assets, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
While all intangible assets other than goodwill can face events and circumstances that can lead to impairment, in general, intangible assets other than goodwill that are most at risk of impairment include IPR&D assets (approximately $5.9 billion as of December 31, 2019) and newly acquired or recently impaired indefinite-lived brand assets. IPR&D assets are high-risk assets, as R&D is an inherently risky activity. Newly acquired and recently impaired indefinite-lived assets are more vulnerable to impairment as the assets are recorded at fair value and are then subsequently measured at the lower of fair value or carrying value at the end of each reporting period. As such, immediately after acquisition or impairment, even small declines in the outlook for these assets can negatively impact our ability to recover the carrying value and can result in an impairment charge.
Goodwill
As a result of our goodwill impairment review work, we concluded that none of our goodwill was impaired as of December 31, 2019, and we do not believe the risk of impairment is significant at this time.
We first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. Qualitative factors that we consider include, for example, macroeconomic and industry conditions, overall financial performance and other relevant entity-specific events. If we conclude that it is more likely than not that the fair value of a reporting unit is less than its carrying value, we then perform a quantitative fair value test.
When we are required to determine the fair value of a reporting unit, as appropriate for the individual reporting unit, we mainly use the income approach but we may also use the market approach, or a weighted-average combination of both approaches.
• | The income approach is a forward-looking approach to estimating fair value and relies primarily on internal forecasts. Within the income approach, the method that we use is the discounted cash flow method. We start with a forecast of all the expected net cash flows associated with the reporting unit, which includes the application of a terminal value, and then we apply a reporting unit-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of technological risk and competitive, legal and/or regulatory forces on the projections, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows. |
• | The market approach is a historical approach to estimating fair value and relies primarily on external information. Within the market approach are two methods that we may use: |
◦ | Guideline public company method—this method employs market multiples derived from market prices of stocks of companies that are engaged in the same or similar lines of business and that are actively traded on a free and open market and the application of the identified multiples to the corresponding measure of our reporting unit’s financial performance. |
2019 Financial Report | 13 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
◦ | Guideline transaction method—this method relies on pricing multiples derived from transactions of significant interests in companies engaged in the same or similar lines of business and the application of the identified multiples to the corresponding measure of our reporting unit’s financial performance. |
The market approach is only appropriate when the available external information is robust and deemed to be a reliable proxy for the specific reporting unit being valued; however, these assessments may prove to be incomplete or inaccurate. Some of the more significant estimates and assumptions inherent in this approach include: the selection of appropriate guideline companies and transactions and the determination of applicable premiums and discounts based on any differences in ownership percentages, ownership rights, business ownership forms or marketability between the reporting unit and the guideline companies and transactions.
For all of our reporting units, there are a number of future events and factors that may impact future results and that could potentially have an impact on the outcome of subsequent goodwill impairment testing. For a list of these factors, see the “Forward-Looking Information and Factors That May Affect Future Results” section of this Financial Review and Part I, Item 1A, “Risk Factors” in our 2019 Form 10-K.
Benefit Plans
The majority of our employees worldwide are covered by defined benefit pension plans, defined contribution plans or both. In the U.S., we sponsor both IRC-qualified and supplemental (non-qualified) defined benefit plans and defined contribution plans, as well as other postretirement benefit plans consisting primarily of medical insurance for retirees and their eligible dependents.
The accounting for benefit plans is highly dependent on actuarial estimates, assumptions and calculations, which can result from a complex series of judgments about future events and uncertainties. The assumptions and actuarial estimates required to estimate the net employee benefit obligations for the defined benefit and postretirement plans include the discount rate; expected salary increases; certain employee-related factors, such as turnover, retirement age and mortality (life expectancy); and healthcare cost trend rates.
Effective January 1, 2018, accruals for future benefits under the Pfizer Consolidated Pension Plan (our largest U.S. defined benefit plan) and the defined benefit section of the Pfizer Group Pension Scheme (our largest pension plan in the U.K.) were frozen and resulted in elimination of future service costs for the plans. The Pfizer defined contribution savings plan provides additional annual contributions to those previously accruing benefits under the Pfizer Consolidated Pension Plan and active members of the Pfizer Group Pension Scheme started accruing benefits under the defined contribution section of that plan.
As of December 31, 2019, the noncurrent portion of our pension benefit obligations, net, increased by approximately $326 million, compared to December 31, 2018. The increase reflects, among other things, a decrease in the discount rate used in the measurement of plan obligations, partially offset by an increase in the actual returns on plan assets. As of December 31, 2019, the noncurrent portion of our postretirement benefit obligations, net decreased by approximately $247 million, compared to December 31, 2018. The decrease reflects, among other things, plan amendments related to the prescription drug coverage in our U.S. and Puerto Rico Postretirement Plans and changes to the claim cost assumptions, partially offset by the decrease in discount rate. For additional information, see Notes to Consolidated Financial Statements––Note 11C. Pension and Postretirement Benefit Plans and Defined Contribution Plans: Obligations and Funded Status.
Our assumptions reflect our historical experiences and our judgment regarding future expectations that have been deemed reasonable by management. The judgments made in determining the costs of our benefit plans can materially impact our results of operations.
The following table provides (i) at the end of each year, the expected annual rate of return on plan assets for the following year, (ii) the actual annual rate of return on plan assets achieved in each year, and (iii) the weighted-average discount rate used to measure the benefit obligations at the end of each year for our U.S. qualified pension plans and our international pension plans(a): | |||||||||
2019 | 2018 | 2017 | |||||||
U.S. Qualified Pension Plans | |||||||||
Expected annual rate of return on plan assets | 7.0 | % | 7.2 | % | 7.5 | % | |||
Actual annual rate of return on plan assets | 22.6 | (5.3 | ) | 16.2 | |||||
Discount rate used to measure the plan obligations | 3.3 | 4.4 | 3.8 | ||||||
International Pension Plans | |||||||||
Expected annual rate of return on plan assets | 3.4 | 3.9 | 4.4 | ||||||
Actual annual rate of return on plan assets | 10.7 | (0.9 | ) | 10.3 | |||||
Discount rate used to measure the plan obligations | 1.7 | 2.5 | 2.3 | ||||||
(a) | For detailed assumptions associated with our benefit plans, see Notes to Consolidated Financial Statements—Note 11B. Pension and Postretirement Benefit Plans and Defined Contribution Plans: Actuarial Assumptions. |
Expected Annual Rate of Return on Plan Assets
The assumptions for the expected annual rate of return on all of our plan assets reflect our actual historical return experience and our long-term assessment of forward-looking return expectations by asset classes, which is used to develop a weighted-average expected return based on the implementation of our targeted asset allocation in our respective plans.
The expected annual rate of return on plan assets for our U.S. plans and the majority of our international plans is applied to the fair value of plan assets at each year-end and the resulting amount is reflected in our net periodic benefit costs in the following year.
14 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
The following table illustrates the sensitivity of net periodic benefit costs to a 50 basis point decline in our assumption for the expected annual rate of return on plan assets, holding all other assumptions constant (in millions, pre-tax): | ||||
Assumption | Change | Increase in 2020 Net Periodic Benefit Costs | ||
Expected annual rate of return on plan assets | 50 basis point decline | $110 | ||
The actual return on plan assets was approximately $3.7 billion during 2019.
Discount Rate Used to Measure Plan Obligations
The weighted-average discount rate used to measure the plan obligations for our U.S. defined benefit plans is determined at least annually and evaluated and modified, as required, to reflect the prevailing market rate of a portfolio of high-quality fixed income investments, rated AA/Aa or better, that reflect the rates at which the pension benefits could be effectively settled. The discount rate used to measure the plan obligations for our international plans is determined at least annually by reference to investment grade corporate bonds, rated AA/Aa or better, including, when there is sufficient data, a yield-curve approach. These discount rate determinations are made in consideration of local requirements.
The measurement of the plan obligations at the end of the year will affect the amount of service cost, interest cost and amortization expense reflected in our net periodic benefit costs in the following year.
The following table illustrates the sensitivity of net periodic benefit costs and benefit obligations to a 10 basis point decline in our assumption for the discount rate, holding all other assumptions constant (in millions, pre-tax): | ||||||
Assumption | Change | Increase in 2020 Net Periodic Benefit Costs | 2019 Benefit Obligations | |||
Increase | Increase | |||||
Discount rate | 10 basis point decline | $4 | $461 | |||
The change in the discount rates used in measuring our plan obligations as of December 31, 2019 resulted in an increase in the measurement of our aggregate plan obligations by approximately $3.2 billion.
Income Tax Assets and Liabilities
In the fourth quarter of 2017, we recorded an estimate of certain tax effects of the TCJA, including (i) the impact on deferred tax assets and liabilities from the reduction in the U.S. Federal corporate tax rate from 35% to 21%, (ii) the impact on valuation allowances and other state income tax considerations, (iii) a repatriation tax liability on accumulated post-1986 foreign earnings for which we elected, with the filing of our 2018 U.S. Federal Consolidated Income Tax Return, payment over eight years through 2026, and (iv) deferred taxes on basis differences expected to give rise to future taxes on global intangible low-taxed income. In addition, we had provided deferred tax liabilities in the past on foreign earnings that were not indefinitely reinvested. As a result of the TCJA, in the fourth quarter of 2017, we reversed an estimate of the deferred taxes that are no longer expected to be needed due to the change to the territorial tax system.
The TCJA subjects a U.S. shareholder to current tax on global intangible low-taxed income earned by certain foreign subsidiaries. The FASB Staff Q&A, Topic 740, No. 5, Accounting for Global Intangible Low-Taxed Income, states that we are permitted to make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as global intangible low-taxed income in future years or provide for the tax expense related to such income in the year the tax is incurred. We elected to recognize deferred taxes for temporary differences expected to reverse as global intangible low-taxed income in future years. We were able to make a reasonable estimate of the deferred taxes on the temporary differences expected to reverse in the future and provided a provisional deferred tax liability as of December 31, 2017.
In 2018, we finalized our provisional accounting for the tax effects of the TCJA based on our best estimates of available information and data, and reported and disclosed the impacts within the applicable measurement period, in accordance with guidance issued by the SEC. We believe that there may be additional interpretations, clarifications and guidance from the U.S. Department of Treasury. Any change to our calculations resulting from such additional interpretations, clarifications and guidance would be reflected in the period of issuance. In addition, our obligations may vary as a result of changes in our uncertain tax positions and/or availability of attributes such as foreign tax and other credit carryforwards. The current portion of the aforementioned repatriation tax liability is reported in current Income taxes payable in our consolidated balance sheet as of December 31, 2019 (approximately $600 million due in April 2020) and the remaining liability is reported in noncurrent Other taxes payable in our consolidated balance sheet as of December 31, 2019. The first installment of $750 million was paid in April 2019.
Income tax assets and liabilities also include income tax valuation allowances and accruals for uncertain tax positions. For additional information, see Notes to Consolidated Financial Statements—Note 1C. Basis of Presentation and Significant Accounting Policies: Estimates and Assumptions; Note 1P. Basis of Presentation and Significant Accounting Policies: Tax Assets and Liabilities and Income Tax Contingencies and Note 5A. Tax Matters: Taxes on Income from Continuing Operations, as well as the “Analysis of Financial Condition, Liquidity and Capital Resources––Selected Measures of Liquidity and Capital Resources—Contractual Obligations” section of this Financial Review.
2019 Financial Report | 15 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Contingencies
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business, such as patent litigation, product liability and other product-related litigation, commercial litigation, environmental claims and proceedings, government investigations, and guarantees and indemnifications, as well as for tax matters. For additional information, see Notes to Consolidated Financial Statements—Note 1P. Basis of Presentation and Significant Accounting Policies: Tax Assets and Liabilities and Income Tax Contingencies, Note 1R. Basis of Presentation and Significant Accounting Policies: Legal and Environmental Contingencies, Note 5D. Tax Matters: Tax Contingencies and Note 16. Contingencies and Certain Commitments.
ANALYSIS OF THE CONSOLIDATED STATEMENTS OF INCOME
The following table provides the components of the consolidated statements of income: | ||||||||||||||||||
Year Ended December 31, | % Change | |||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | |||||||||||||
Revenues | $ | 51,750 | $ | 53,647 | $ | 52,546 | (4 | ) | 2 | |||||||||
Cost of sales(a) | 10,219 | 11,248 | 11,228 | (9 | ) | — | ||||||||||||
% of revenues | 19.7 | % | 21.0 | % | 21.4 | % | ||||||||||||
Selling, informational and administrative expenses(a) | 14,350 | 14,455 | 14,804 | (1 | ) | (2 | ) | |||||||||||
% of revenues | 27.7 | % | 26.9 | % | 28.2 | % | ||||||||||||
Research and development expenses(a) | 8,650 | 8,006 | 7,683 | 8 | 4 | |||||||||||||
% of revenues | 16.7 | % | 14.9 | % | 14.6 | % | ||||||||||||
Amortization of intangible assets | 4,610 | 4,893 | 4,758 | (6 | ) | 3 | ||||||||||||
% of revenues | 8.9 | % | 9.1 | % | 9.1 | % | ||||||||||||
Restructuring charges and certain acquisition-related costs | 747 | 1,044 | 351 | (28 | ) | * | ||||||||||||
% of revenues | 1.4 | % | 1.9 | % | 0.7 | % | ||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | (8,086 | ) | — | — | * | — | ||||||||||||
% of revenues | 15.6 | % | — | — | ||||||||||||||
Other (income)/deductions—net | 3,578 | 2,116 | 1,416 | 69 | 49 | |||||||||||||
Income from continuing operations before provision/(benefit) for taxes on income | 17,682 | 11,885 | 12,305 | 49 | (3 | ) | ||||||||||||
% of revenues | 34.2 | % | 22.2 | % | 23.4 | % | ||||||||||||
Provision/(benefit) for taxes on income | 1,384 | 706 | (9,049 | ) | 96 | * | ||||||||||||
Effective tax rate | 7.8 | % | 5.9 | % | (73.5 | )% | ||||||||||||
Income from continuing operations | 16,298 | 11,179 | 21,353 | 46 | (48 | ) | ||||||||||||
% of revenues | 31.5 | % | 20.8 | % | 40.6 | % | ||||||||||||
Discontinued operations—net of tax | 4 | 10 | 2 | (61 | ) | * | ||||||||||||
Net income before allocation to noncontrolling interests | 16,302 | 11,188 | 21,355 | 46 | (48 | ) | ||||||||||||
% of revenues | 31.5 | % | 20.9 | % | 40.6 | % | ||||||||||||
Less: Net income attributable to noncontrolling interests | 29 | 36 | 47 | (18 | ) | (24 | ) | |||||||||||
Net income attributable to Pfizer Inc. | $ | 16,273 | $ | 11,153 | $ | 21,308 | 46 | (48 | ) | |||||||||
% of revenues | 31.4 | % | 20.8 | % | 40.6 | % | ||||||||||||
* | Indicates calculation not meaningful or result is equal to or greater than 100%. |
(a) | Excludes amortization of intangible assets, except as disclosed in Notes to Consolidated Financial Statements––Note 10A. Identifiable Intangible Assets and Goodwill: Identifiable Intangible Assets. |
Revenues—Overview
Total revenues in 2019 compared to 2018 reflects an operational decline of $545 million, or 1%, and an unfavorable impact of foreign exchange of $1.4 billion, or 3% in 2019 compared to 2018.
Total revenues in 2018 compared to 2017 reflects an operational increase of $791 million, or 2%, and the favorable impact of foreign exchange of $310 million, or less than 1%, in 2018 compared to 2017.
See the “Revenues by Segment and Geography” and “Revenues—Selected Product Discussion” sections of this Financial Review for additional analyses.
Certain of our current products have experienced patent-based expirations or loss of regulatory exclusivity in certain markets in the last few years, and we expect certain products to face significantly increased generic competition over the next few years. For additional information, see the “Patents and Other Intellectual Property Rights” section in Part I, Item 1, “Business” of our 2019 Form 10-K.
We have significant operations outside the U.S., with revenues exceeding $500 million in eleven countries in each of 2019, 2018 and 2017.
16 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
By total revenues, the U.S., China and Japan are our three largest national markets:

Inventory Stocking
Our policy relating to the supply of pharmaceutical inventory at domestic wholesalers, and in major international markets, is to generally maintain stocking levels under one month on average and to keep monthly levels consistent from year to year based on patterns of utilization. We historically have been able to closely monitor these customer stocking levels by purchasing information from our customers directly or by obtaining other third-party information. We believe our data sources to be directionally reliable but cannot verify their accuracy. Further, as we do not control this third-party data, we cannot be assured of continuing access. Unusual buying patterns and utilization are promptly investigated.
Revenue Deductions
Our gross product revenues are subject to a variety of deductions, which generally are estimated and recorded in the same period that the revenues are recognized. Such variable consideration represents chargebacks, rebates, sales allowances and sales returns. These deductions represent estimates of related obligations and, as such, knowledge and judgment are required when estimating the impact of these revenue deductions on gross sales for a reporting period. Historically, our adjustments of estimates, to reflect actual results or updated expectations, have not been material to our overall business. On a quarterly basis, our adjustments of estimates to reflect actual results generally have been less than 1% of revenues, and have resulted in either a net increase or a net decrease in revenues. Product-specific rebates, however, can have a significant impact on year-over-year individual product growth trends.
The following table provides information about revenue deductions: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Medicare rebates(a) | $ | 1,306 | $ | 1,706 | $ | 1,316 | ||||||
Medicaid and related state program rebates(a) | 1,936 | 1,969 | 1,860 | |||||||||
Performance-based contract rebates(a), (b) | 3,767 | 3,377 | 3,245 | |||||||||
Chargebacks(c) | 5,588 | 6,461 | 6,047 | |||||||||
Sales allowances(d) | 5,678 | 5,592 | 5,165 | |||||||||
Sales returns and cash discounts | 1,315 | 1,522 | 1,493 | |||||||||
Total(e) | $ | 19,589 | $ | 20,627 | $ | 19,126 | ||||||
(a) | Rebates are product-specific and, therefore, for any given year are impacted by the mix of products sold. |
(b) | Performance-based contract rebates include contract rebates with MCOs within the U.S., including health maintenance organizations and PBMs, who receive rebates based on the achievement of contracted performance terms and claims under these contracts. Outside the U.S., performance-based contract rebates include rebates to wholesalers/distributors based on achievement of contracted performance for specific products or sales milestones. |
(c) | Chargebacks primarily represent reimbursements to U.S. wholesalers for honoring contracted prices to third parties. |
(d) | Sales allowances primarily represent price reductions that are contractual or legislatively mandated outside the U.S., discounts and distribution fees. |
(e) | For 2019, associated with the following segments: Biopharma ($12.0 billion), Upjohn ($7.2 billion) and Other ($0.4 billion). For 2018, associated with the following segments: Biopharma ($10.2 billion), Upjohn ($9.7 billion) and Other ($0.7 billion). For 2017, associated with the following segments: Biopharma ($9.2 billion), Upjohn ($9.2 billion) and Other ($0.7 billion). |
Total revenue deductions for 2019 decreased 5% compared to 2018, primarily as a result of:
• | a decrease in chargebacks primarily related to Upjohn products, including Viagra and Lyrica; and |
• | a decrease in Medicare rebates, driven by a significant decrease in Lyrica sales in the U.S. due to multi-source generic competition that began in July 2019, |
partially offset by:
• | an increase in performance-based contract rebates, primarily in the U.S. due to increased sales of certain Biopharma products, slightly offset by decreased Lyrica sales. |
For information on our accruals for Medicare rebates, Medicaid and related state program rebates, performance-based contract rebates, chargebacks, sales allowances and sales returns and cash discounts, including the balance sheet classification of these accruals, see Notes to Consolidated Financial Statements––Note 1G. Basis of Presentation and Significant Accounting Policies: Revenues and Trade Accounts Receivable.
2019 Financial Report | 17 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Revenues by Operating Segment and Geography
The following graphs show revenues by operating segment and geography:
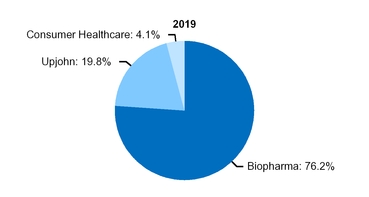
2019 Revenues by Geography | % of Total | |
U.S. | 46% | |
International | 54% | |
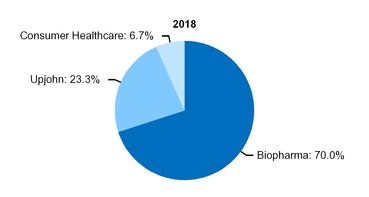
2018 Revenues by Geography | % of Total | |
U.S. | 47% | |
International | 53% | |
2017 Revenues by Geography | % of Total | |
U.S. | 50% | |
International | 50% | |
18 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
The following table provides worldwide revenues by operating segment and geography: | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Year Ended December 31, | % Change | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Worldwide | U.S. | International | Worldwide | U.S. | International | ||||||||||||||||||||||||||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 2019 | 2018 | 2017 | 2019 | 2018 | 2017 | 19/18 | 18/17 | 19/18 | 18/17 | 19/18 | 18/17 | ||||||||||||||||||||||||||||||||||||||
Operating Segments(a): | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Biopharma | $ | 39,419 | $ | 37,558 | $ | 35,530 | $ | 19,605 | $ | 18,243 | $ | 17,961 | $ | 19,814 | $ | 19,315 | $ | 17,569 | 5 | 6 | 7 | 2 | 3 | 10 | |||||||||||||||||||||||||||||
Upjohn | 10,233 | 12,484 | 13,447 | 3,259 | 5,209 | 6,150 | 6,974 | 7,275 | 7,297 | (18 | ) | (7 | ) | (37 | ) | (15 | ) | (4 | ) | — | |||||||||||||||||||||||||||||||||
Consumer Healthcare | 2,098 | 3,605 | 3,472 | 988 | 1,877 | 1,851 | 1,110 | 1,728 | 1,621 | (42 | ) | 4 | (47 | ) | 1 | (36 | ) | 7 | |||||||||||||||||||||||||||||||||||
Other(b) | — | — | 97 | — | — | 64 | — | — | 33 | — | * | — | * | — | * | ||||||||||||||||||||||||||||||||||||||
Total revenues | $ | 51,750 | $ | 53,647 | $ | 52,546 | $ | 23,852 | $ | 25,329 | $ | 26,026 | $ | 27,898 | $ | 28,318 | $ | 26,519 | (4 | ) | 2 | (6 | ) | (3 | ) | (1 | ) | 7 | |||||||||||||||||||||||||
* | Indicates the calculation is not meaningful or results are equal to or greater than 100%. |
(a) | For additional information about each operating segment, see the “Commercial Operations” section in Part I, Item 1, “Business” of our 2019 Form 10-K, the “Analysis of Operating Segment Information” section of this Financial Review and Notes to Consolidated Financial Statements––Note 17A. Segment, Geographic and Other Revenue Information: Segment Information. |
(b) | Represents HIS revenues through February 2, 2017. On February 3, 2017, we completed the sale of HIS to ICU Medical. For additional information, see Notes to Consolidated Financial Statements—Note 1A. Basis of Presentation and Significant Accounting Policies: Basis of Presentation. |
We recorded direct product and/or alliance revenues of more than $1 billion for each of: eight products in 2019, ten products in 2018 and nine products in 2017.
Direct Product And/Or Alliance Revenues of More Than $1 Billion | ||||
2019 | 2018 | 2017 | ||
Prevnar 13/Prevenar 13 | Prevnar 13/Prevenar 13 | Prevnar 13/Prevenar 13 | ||
Ibrance | Lyrica | Lyrica | ||
Eliquis* | Ibrance | Ibrance | ||
Lyrica | Eliquis* | Eliquis* | ||
Xeljanz | Enbrel | Enbrel | ||
Lipitor | Lipitor | Lipitor | ||
Enbrel | Xeljanz | Xeljanz | ||
Chantix/Champix | Chantix/Champix | Viagra | ||
Sutent | Sutent | |||
Norvasc | ||||
* Eliquis includes alliance revenues and direct sales in 2019, 2018 and 2017. | ||||
These direct product sales and/or alliance product revenues represent 49% of our revenues in 2019, 51% of our revenues in 2018 and 46% of our revenues in 2017. See the “Analysis of the Consolidated Statements of Income—Revenues—Selected Product Discussion” section of this Financial Review for additional information.
2019 Financial Report | 19 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
2019 v. 2018
The following provides an analysis of the change in worldwide revenues by geographic areas in 2019:
(MILLIONS OF DOLLARS) | Worldwide | U.S. | International | |||||||||
Operational growth/(decline): | ||||||||||||
Continued growth from certain key brands(a) | $ | 2,495 | $ | 914 | $ | 1,581 | ||||||
Higher revenue from continued growth of anti-infective products in China, driven by increased demand for Sulperazon and new launches, the 2018 U.S. launches of our immune globulin intravenous products (Panzyga and Octagam) and the launches of certain anti-infectives products (Zavicefta, Zinforo and Cresemba) in international developed and emerging markets, all in the Hospital products business | 472 | 174 | 298 | |||||||||
Higher revenues for Inlyta, primarily in the U.S. driven by increased demand resulting from the second quarter of 2019 U.S. FDA approvals for the combinations of certain immune checkpoint inhibitors plus Inlyta for the first-line treatment of patients with advanced RCC | 190 | 175 | 14 | |||||||||
Higher revenues for Biosimilars, primarily in the U.S. | 168 | 185 | (17 | ) | ||||||||
Higher revenues for rare disease products driven by the U.S. launches in May 2019 of Vyndaqel and in September 2019 of Vyndamax, for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM); and in international markets, primarily driven by continued uptake for the transthyretin amyloid polyneuropathy indication, primarily in developed Europe, as well as the March 2019 launch of the ATTR-CM indication in Japan, partially offset by lower revenues for certain rare disease products, including the hemophilia franchises (Refacto AF/Xyntha and BeneFIX), primarily due to competitive pressures, and Genotropin in developed markets, mainly due to unfavorable channel mix in the U.S. | 159 | 108 | 51 | |||||||||
Volume-driven growth from Celebrex and Effexor, primarily in Japan and China | 78 | (8 | ) | 87 | ||||||||
Lower worldwide revenues for Lyrica, primarily in the U.S., reflecting the expected significantly lower volumes associated with multi-source generic competition that began in July 2019 | (1,628 | ) | (1,582 | ) | (46 | ) | ||||||
Lower revenues for Consumer Healthcare reflecting the July 31, 2019 completion of the Consumer Healthcare joint venture transaction with GSK. As a result, 2019 revenues reflect seven months of Consumer Healthcare segment domestic operations and eight months of Consumer Healthcare segment international operations | (1,436 | ) | (889 | ) | (547 | ) | ||||||
Lower revenues from other Hospital products, primarily reflecting declines in developed markets, mostly due to the continued expected negative impact from generic competition for products that have previously lost marketing exclusivity | (447 | ) | (200 | ) | (247 | ) | ||||||
Lower revenues for Enbrel internationally, reflecting continued biosimilar competition in most developed Europe markets | (292 | ) | — | (292 | ) | |||||||
Lower revenues for Viagra and Upjohn's authorized generic for Viagra in the U.S. resulting from increased generic competition following Viagra's December 2017 patent expiration, partially offset by increased retail demand growth in China | (171 | ) | (193 | ) | 21 | |||||||
Decline in revenues for Revatio driven by lower U.S. Oral Suspension formulation sales and pricing pressures due to a recent generic entry, and for Relpax, driven by continued generic competition across developed markets | (149 | ) | (110 | ) | (39 | ) | ||||||
Decline in Norvasc and Lipitor due to pricing pressures from the implementation of the VBP in certain cities in China and lower volumes in Japan, partially offset by overall increased demand in China in the first quarter of 2019 and continued geographic expansion in China during the second half of 2019 in provinces where the VBP had not yet been implemented | (40 | ) | (4 | ) | (36 | ) | ||||||
Other operational factors, net | 57 | (47 | ) | 104 | ||||||||
Operational growth/(decline), net | (545 | ) | (1,477 | ) | 932 | |||||||
Unfavorable impact of foreign exchange | (1,352 | ) | — | (1,352 | ) | |||||||
Revenues decrease | $ | (1,897 | ) | $ | (1,477 | ) | $ | (420 | ) | |||
(a) | Certain key brands represent Ibrance, Eliquis, Xeljanz and Prevnar 13/Prevenar 13. See the “Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion" section of this Financial Review for product analysis information. |
Emerging markets revenues increased $82 million, or 1%, in 2019 to $12.7 billion, reflecting an operational increase of $877 million, or 7%. Foreign exchange had an unfavorable impact of approximately 6% on emerging markets revenues. The operational increase in emerging markets was primarily driven by Prevenar 13, Ibrance and Eliquis in our Biopharma segment and Zoloft, Viagra, Celebrex and Lipitor in our Upjohn segment.
20 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
2018 v. 2017
The following provides an analysis of the change in worldwide revenues by geographic areas in 2018:
(MILLIONS OF DOLLARS) | Worldwide | U.S. | International | |||||||||
Operational growth/(decline): | ||||||||||||
Continued growth from certain key brands(a) | $ | 2,715 | $ | 1,019 | $ | 1,696 | ||||||
Growth from Biosimilars, primarily from Inflectra in certain channels in the U.S. and developed Europe markets | 217 | 147 | 69 | |||||||||
Growth from recently launched products, including Eucrisa in the U.S., as well as Besponsa and Bavencio, primarily in the U.S. and developed Europe | 195 | 158 | 37 | |||||||||
Higher revenues for Lipitor and Norvasc primarily due to increased demand in China, partially offset by pricing pressures in China and lower volumes in Japan for Lipitor and Norvasc and the non-recurrence of favorable U.S. rebates for Lipitor that occurred in 2017 | 182 | (52 | ) | 234 | ||||||||
Growth in our Consumer Healthcare business across all markets | 107 | 26 | 81 | |||||||||
Lower revenues for Viagra in the U.S. resulting from the loss of exclusivity in December 2017 | (572 | ) | (572 | ) | — | |||||||
Decline in the Hospital products business, driven by lower revenues in developed markets, primarily due to increased competition across the portfolio and continued legacy Hospira product shortages in the U.S., partially offset by an increase in emerging markets, primarily in China | (482 | ) | (703 | ) | 221 | |||||||
Lower revenues for Enbrel, primarily in most developed Europe markets due to continued biosimilar competition | (350 | ) | — | (350 | ) | |||||||
Decline in Greenstone, Upjohn's solid oral dose generics subsidiary, due to additional generic competition in the U.S. and decline in Relpax, primarily due to loss of exclusivity in the U.S. | (318 | ) | (310 | ) | (8 | ) | ||||||
Lower revenues for the Premarin family of products and Pristiq primarily driven by generic competition in the U.S. | (241 | ) | (201 | ) | (40 | ) | ||||||
Decline in revenues for Lyrica, primarily driven by losses of exclusivity in developed Europe markets and Australia, partially offset by growth in the U.S. and growth in the orally dissolving tablet formulation in Japan | (115 | ) | 131 | (246 | ) | |||||||
Lower revenues from the hemophilia portfolio (BeneFIX and Refacto AF/Xyntha), primarily in developed Europe | (100 | ) | (13 | ) | (88 | ) | ||||||
Decline in revenues for Celebrex, primarily driven by the non-recurrence of favorable U.S. rebates that occurred in 2017 and lower volumes in the U.S. | (99 | ) | (99 | ) | — | |||||||
Impact on financial results from the sale of HIS in February 2017. 2018 does not reflect any contribution from HIS global operations, compared to approximately one month of HIS domestic operations and approximately two months of HIS international operations in the same period in 2017 | (97 | ) | (64 | ) | (33 | ) | ||||||
Other operational factors, net | (251 | ) | (166 | ) | (84 | ) | ||||||
Operational growth/(decline), net | 791 | (698 | ) | 1,489 | ||||||||
Favorable impact of foreign exchange | 310 | — | 310 | |||||||||
Revenues increase/(decrease) | $ | 1,101 | $ | (698 | ) | $ | 1,799 | |||||
(a) | Certain key brands represent Ibrance, Eliquis, Xeljanz, Prevnar/Prevenar 13, Xtandi and Chantix/Champix. See the “Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion" section of this Financial Review for product analysis information. |
Emerging markets revenues increased $1.3 billion, or 11%, in 2018 to $12.7 billion, from $11.4 billion in 2017, reflecting an operational increase of $1.5 billion, or 13%. Foreign exchange had an unfavorable impact of approximately 2% on emerging markets revenues. The operational increase in emerging markets was primarily driven by Prevenar 13, Sulperazon, Ibrance and Eliquis in our Biopharma segment and Lipitor and Norvasc in our Upjohn segment.
For additional information about operating segment revenues, see the “Analysis of Operating Segment Information” section of this Financial Review.
Revenues—Selected Product Discussion
The tables below provide worldwide revenues and revenues by geography, for selected products. References to total change pertain to period-over-period growth rates that include foreign exchange. The difference between the total change and operational change represents the impact of foreign exchange. Amounts may not add due to rounding. All percentages have been calculated using unrounded amounts. An asterisk (*) indicates the calculation is not meaningful or results are equal to or greater than 100%.
2019 Financial Report | 21 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | Prevnar 13/Prevenar 13 (Biopharma): |
Year Ended December 31, | |||||||||||||
% Change | |||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | |||||||||
U.S. | $ | 3,209 | $ | 3,360 | (4 | ) | |||||||
International | 2,638 | 2,443 | 8 | 12 | |||||||||
Worldwide revenues | $ | 5,847 | $ | 5,802 | 1 | 3 | |||||||
The decline in 2019 in the U.S. primarily reflects the continued decline in revenues for the adult indication due to a high initial capture rate of the eligible population following its successful fourth-quarter 2014 launch, which resulted in a smaller remaining “catch up” opportunity (i.e., the opportunity to reach adults aged 65 years and older who have not been previously vaccinated with Prevnar 13) as well as lower government purchases for the pediatric indication.
The operational growth in 2019 internationally was primarily driven by the pediatric indication due to higher volumes reflecting continued uptake in China, favorable impact of timing and increased volumes associated with government purchases for the pediatric indication in certain emerging markets and higher volumes resulting from increased shipments associated with Gavi, the Vaccine Alliance.
In 2014, the ACIP voted to recommend Prevnar 13 for routine use to help protect adults aged 65 years and older against pneumococcal disease, which for adults includes pneumonia caused by the 13 pneumococcal serotypes included in the vaccine. These ACIP recommendations were subsequently approved by the directors at the CDC and U.S. Department of Health and Human Services, and were published in the Morbidity and Mortality Weekly Report in September 2014 by the CDC. The CDC regularly monitors the impact of vaccination and reviews the recommendations. During the February 2019 ACIP meeting, the CDC presented a formal evaluation of evidence to the recommendation framework (grading) for ACIP’s input. On June 26, 2019, the ACIP voted to revise the pneumococcal vaccination guidelines and recommend Prevnar 13 for adults 65 and older based on the shared clinical decision making of the provider and patient, which means the decision to vaccinate should be made at the individual level between health care providers and their patients, maintaining reimbursement. The recommendation reaffirms that there remains vaccine preventable pneumococcal disease in the population of adults 65 years or older, which may be prevented through direct vaccination. The ACIP’s recommendation was approved by the directors at the CDC and U.S. Department of Health and Human Services, and published by the CDC in the Morbidity and Mortality Weekly Report in the fourth quarter of 2019. While the ACIP’s latest recommendation did not have an impact on Prevnar 13 revenues in 2019, due to timing of the Morbidity and Mortality Weekly Report publication, we expect Prevnar 13 revenues from the adult indication to continue to decline in 2020 and beyond.
• | Ibrance (Biopharma): |
Year Ended December 31, | ||||||||||||
% Change | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||
U.S. | $ | 3,250 | $ | 2,922 | 11 | |||||||
International | 1,710 | 1,196 | 43 | 53 | ||||||||
Worldwide revenues | $ | 4,961 | $ | 4,118 | 20 | 23 | ||||||
The operational growth in 2019 in international markets reflects the continued strong uptake in developed Europe and Japan as well as in certain emerging markets following launches, partially offset by pricing pressure primarily in certain developed Europe markets beginning in the fourth quarter of 2019. The growth in 2019 in the U.S. was mainly driven by cyclin-dependent kinase (CDK) class share growth and Ibrance’s continued CDK class share leadership in its approved metastatic breast cancer indications.
• | Eliquis alliance revenues and direct sales (Biopharma): Eliquis has been jointly developed and is commercialized by Pfizer and BMS. Pfizer funds between 50% and 60% of all development costs depending on the study. Profits and losses are shared equally on a global basis, except in certain countries where Pfizer commercializes Eliquis and pays BMS compensation based on a percentage of net sales. We have full commercialization rights in certain smaller markets. BMS supplies the product to us at cost plus a percentage of the net sales to end-customers in these markets. Eliquis is part of the Novel Oral Anticoagulant market; the agents in this class were developed as alternative treatment options to warfarin in appropriate patients. |
Year Ended December 31, | ||||||||||||
% Change | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||
U.S. | $ | 2,343 | $ | 1,849 | 27 | |||||||
International | 1,877 | 1,585 | 18 | 24 | ||||||||
Worldwide revenues | $ | 4,220 | $ | 3,434 | 23 | 26 | ||||||
The worldwide operational growth in 2019 was mostly driven by continued increased adoption in non-valvular atrial fibrillation, as well as oral anti-coagulant market share gains, partially offset by a higher Medicare “coverage gap” discount provision on U.S. revenues compared to the prior year.
22 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | Lyrica (Upjohn): |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | 2,012 | $ | 3,594 | (44 | ) | ||||||||
International | 1,308 | 1,375 | (5 | ) | (3 | ) | ||||||||
Worldwide revenues | $ | 3,321 | $ | 4,970 | (33 | ) | (33 | ) | ||||||
The declines in 2019 in the U.S. were due to the expected significantly lower volumes driven by multi-source generic competition which began in July 2019.
The operational decline internationally in 2019 was primarily due to generic competition in developed Europe markets and pricing pressures across international markets, partially offset by increased volumes in Japan attributable to growth in the orally dissolving tablet formulation, and increased volumes in Russia and China.
• | Xeljanz (Biopharma): |
Year Ended December 31, | ||||||||||||
% Change | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||
U.S. | $ | 1,636 | $ | 1,394 | 17 | |||||||
International | 606 | 380 | 59 | 70 | ||||||||
Worldwide revenues | $ | 2,242 | $ | 1,774 | 26 | 29 | ||||||
The growth in the U.S. in 2019 was primarily due to continued growth in the RA indication driven by access improvements, and by volume growth from the launches of the UC and PsA indications in 2018, partially offset by higher rebating from new commercial contracts.
The operational growth internationally in 2019 was mainly driven by continued uptake in the RA indication in developed Europe, emerging markets, Japan and Canada as well as from the recent launch of the UC indication in certain developed markets.
In July and December 2019, the FDA updated the U.S. prescribing information for Xeljanz to include three additional boxed warnings as well as changes to the indication and dosing for UC. In January 2020, the EC revised the summary of product characteristics (SmPC) for Xeljanz to include new warnings and recommendations for use of Xeljanz due to an increased risk of venous thromboembolism, and, due to an increased risk of infections, revised warnings in patients older than 65 years of age. These updates were based on the FDA’s and EMA’s review of 10 mg data from the ongoing post-marketing requirement RA study A3921133. We expect these updates to moderate growth. See the “Analysis of Consolidated Statements of Income—Product Development—Biopharmaceuticals” section of this Financial Review.
• | Lipitor (Upjohn): |
Year Ended December 31, | |||||||||||||
% Change | |||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | |||||||||
U.S. | $ | 104 | $ | 110 | (6 | ) | |||||||
International | 1,870 | 1,952 | (4 | ) | — | ||||||||
Worldwide revenues | $ | 1,973 | $ | 2,062 | (4 | ) | — | ||||||
The worldwide operational revenue was flat in 2019 mostly due to overall increased demand in China in the first quarter of 2019 and continued geographic expansion during the second half of 2019 in provinces where the VBP program had not yet been implemented, offset by pricing pressures in China resulting from the VBP implementation in certain cities, discontinued sales in Saudi Arabia and lower volumes in Japan.
• | Enbrel (Biopharma, outside the U.S. and Canada): |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | — | $ | — | — | |||||||||
International | 1,699 | 2,112 | (20 | ) | (14 | ) | ||||||||
Worldwide revenues | $ | 1,699 | $ | 2,112 | (20 | ) | (14 | ) | ||||||
The worldwide operational decline in 2019 was primarily due to ongoing biosimilar competition in most developed Europe markets, which is expected to continue.
2019 Financial Report | 23 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | Chantix/Champix (Biopharma): |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | 899 | $ | 838 | 7 | |||||||||
International | 208 | 247 | (16 | ) | (12 | ) | ||||||||
Worldwide revenues | $ | 1,107 | $ | 1,085 | 2 | 3 | ||||||||
The growth in the U.S. in 2019 was primarily due to stronger demand. The operational decline internationally in 2019 was mainly driven by generic entry in South Korea and Canada.
• | Norvasc (Upjohn): |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | 39 | $ | 36 | 6 | |||||||||
International | 911 | 992 | (8 | ) | (4 | ) | ||||||||
Worldwide revenues | $ | 950 | $ | 1,029 | (8 | ) | (4 | ) | ||||||
The worldwide operational decline in 2019 was primarily due to pricing pressures in China resulting from the VBP implementation in certain cities and lower volumes in Japan, partially offset by overall increased demand in China in the first quarter of 2019 and continued geographic expansion during the second half of 2019 in provinces where the VBP had not yet been implemented.
• | Sutent (Biopharma): |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | 283 | $ | 357 | (21 | ) | ||||||||
International | 653 | 692 | (6 | ) | 1 | |||||||||
Worldwide revenues | $ | 936 | $ | 1,049 | (11 | ) | (7 | ) | ||||||
The worldwide operational decline in 2019 reflects continued erosion as a result of increased competition in the U.S. and key developed markets, partially offset by growth in certain emerging markets.
• | Xtandi alliance revenues (Biopharma): Xtandi is being developed and commercialized through a collaboration with Astellas. The two companies share equally in the gross profits (losses) related to U.S. net sales of Xtandi. Subject to certain exceptions, Pfizer and Astellas also share equally all Xtandi commercialization costs attributable to the U.S. market. Pfizer and Astellas also share certain development and other collaboration expenses, and Pfizer receives tiered royalties as a percentage of international Xtandi net sales (recorded in Other (income)/deductions—net). |
Year Ended December 31, | ||||||||||||
% Change | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||
U.S. | $ | 838 | $ | 699 | 20 | |||||||
International | — | — | — | — | ||||||||
Worldwide revenues | $ | 838 | $ | 699 | 20 | 20 | ||||||
The growth in the U.S. in 2019 was primarily driven by increased demand for Xtandi in metastatic (mCRPC) and non-metastatic (nmCRPC) castration-resistant prostate cancer. Revenues continue to be unfavorably impacted by patient assistance programs (PAP) utilization.
• | The Premarin family of products (Biopharma): |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | 690 | $ | 783 | (12 | ) | ||||||||
International | 44 | 49 | (10 | ) | (6 | ) | ||||||||
Worldwide revenues | $ | 734 | $ | 832 | (12 | ) | (12 | ) | ||||||
The worldwide operational decline in 2019 was primarily driven by continued competitive pressures in the U.S., which is expected to continue.
24 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | Celebrex (Upjohn): |
Year Ended December 31, | |||||||||||||
% Change | |||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | |||||||||
U.S. | $ | 58 | $ | 65 | (11 | ) | |||||||
International | 661 | 621 | 7 | 8 | |||||||||
Worldwide revenues | $ | 719 | $ | 686 | 5 | 7 | |||||||
The worldwide operational growth in 2019 was mainly due to higher volumes in China, driven by investments in geographic expansion, and higher volumes in Japan, partially offset by pricing pressures in certain emerging markets.
• | Sulperazon (Biopharma): |
Year Ended December 31, | ||||||||||||
% Change | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||
U.S. | $ | — | $ | — | — | |||||||
International | 684 | 613 | 12 | 17 | ||||||||
Worldwide revenues | $ | 684 | $ | 613 | 12 | 17 | ||||||
The international operational growth in 2019 was mostly due to increased demand in China.
• | Inflectra/Remsima (Biopharma): |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | 300 | $ | 259 | 16 | |||||||||
International | 325 | 383 | (15 | ) | (10 | ) | ||||||||
Worldwide revenues | $ | 625 | $ | 642 | (3 | ) | — | |||||||
Worldwide operational revenues were relatively flat in 2019 due to continued volume growth in the U.S., as well as in certain developed markets in Europe and Canada, offset by pricing pressures globally as well as competitive pressures internationally.
The growth in the U.S. in 2019 was primarily driven by demand in open systems, partially offset by price erosion. While Inflectra has achieved parity access to Remicade® (infliximab) in Medicare Part B, nearly half of all commercial patients are not able to access Inflectra due to exclusionary contracting practices by J&J. In September 2017, Pfizer filed suit in the U.S. District Court for the Eastern District of Pennsylvania against J&J alleging that J&J’s exclusionary contracts and other anticompetitive practices concerning Remicade violate federal antitrust laws. In June 2019, Pfizer received a Civil Investigative Demand from the Federal Trade Commission (FTC) seeking documents and information relating to the alleged conduct and market conditions at issue in Pfizer’s lawsuit against J&J. Pfizer understands that the FTC’s investigation is focused on J&J’s alleged conduct at issue in Pfizer’s lawsuit against J&J.
• | Xalkori (Biopharma): |
Year Ended December 31, | |||||||||||||
% Change | |||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | |||||||||
U.S. | $ | 149 | $ | 158 | (5 | ) | |||||||
International | 381 | 366 | 4 | 9 | |||||||||
Worldwide revenues | $ | 530 | $ | 524 | 1 | 5 | |||||||
The worldwide operational growth in 2019 primarily resulted from growth in China, following the impact of inclusion of Xalkori in the 2019 National Reimbursement Drug Listing, partially offset by erosion due to competition in developed Europe and in the U.S.
• | Viagra (Upjohn) |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | 75 | $ | 217 | (65 | ) | ||||||||
International | 422 | 419 | 1 | 5 | ||||||||||
Worldwide revenues | $ | 497 | $ | 636 | (22 | ) | (19 | ) | ||||||
The decline in the U.S. in 2019 was driven by the loss of exclusivity in December 2017. The operational growth internationally in 2019 was mostly due to increased retail demand growth in China, partially offset by pricing pressures in China and lower volumes across certain developed and certain emerging markets.
2019 Financial Report | 25 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | Inlyta (Biopharma): |
Year Ended December 31, | ||||||||||||
% Change | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||
U.S. | $ | 295 | $ | 119 | * | |||||||
International | 182 | 178 | 2 | 8 | ||||||||
Worldwide revenues | $ | 477 | $ | 298 | 60 | 64 | ||||||
The worldwide operational growth in 2019 was primarily due to increased demand in the U.S. as a result of the FDA approvals in the second quarter of 2019 for combinations of certain immune checkpoint inhibitors plus Inlyta for the first-line treatment of patients with advanced RCC.
• | Vyndaqel/Vyndamax (Biopharma): |
Year Ended December 31, | ||||||||||||
% Change | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||
U.S. | $ | 191 | $ | — | * | |||||||
International | 282 | 148 | 91 | 96 | ||||||||
Worldwide revenues | $ | 473 | $ | 148 | * | * | ||||||
The growth in the U.S. was driven by the launches in May 2019 of Vyndaqel and in September 2019 of Vyndamax, for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM). The operational growth in international markets was primarily driven by continued uptake for the transthyretin amyloid polyneuropathy indication, primarily in developed Europe, as well as the March 2019 launch of the ATTR-CM indication in Japan.
• | Eucrisa (Biopharma): |
Year Ended December 31, | ||||||||||||||
% Change | ||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||||
U.S. | $ | 134 | $ | 147 | (9 | ) | ||||||||
International | 3 | — | * | * | ||||||||||
Worldwide revenues | $ | 138 | $ | 147 | (7 | ) | (7 | ) | ||||||
The decline in the U.S. in 2019 was primarily driven by higher rebating and unfavorable channel mix, in addition to favorable rebate adjustments in 2018, partially offset by volume growth.
• | Alliance revenues (Biopharma): |
Year Ended December 31, | ||||||||||||
% Change | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | Total | Oper. | ||||||||
U.S. | $ | 3,208 | $ | 2,576 | 25 | |||||||
International | 1,440 | 1,263 | 14 | 19 | ||||||||
Worldwide revenues | $ | 4,648 | $ | 3,838 | 21 | 23 | ||||||
The worldwide operational growth in 2019 was mainly due to increases in Eliquis and Xtandi alliance revenues included in the above discussion.
◦ | Bavencio (Biopharma) is being developed and commercialized in collaboration with Merck KGaA. Both companies jointly fund the majority of development and commercialization costs, and split equally any profits related to net sales generated from selling any products containing avelumab from this collaboration. Bavencio is currently approved in metastatic MCC in the U.S., the EU, Japan and select other markets; the second-line treatment of locally advanced or metastatic urothelial carcinoma in the U.S. and select other markets; and first-line treatment of patients with advanced RCC in combination with Inlyta in the U.S., the EU, Japan and select other markets. |
See Notes to Consolidated Financial Statements––Note 17C. Segment, Geographic and Other Revenue Information: Other Revenue Information for additional information regarding the primary indications or class of the selected products discussed above.
See the “Patents and Other Intellectual Property Rights” section in Part I, Item 1, “Business” of our 2019 Form 10-K for information regarding the expiration of various patent rights.
See Notes to Consolidated Financial Statements—Note 16. Contingencies and Certain Commitments for a discussion of recent developments concerning patent and product litigation relating to certain of the products discussed above.
PRODUCT DEVELOPMENTS—BIOPHARMACEUTICAL
We continue to invest in R&D to provide potential future sources of revenues through the development of new products, as well as through additional uses for in-line and alliance products. Notwithstanding our efforts, there are no assurances as to when, or if, we will receive regulatory approval for additional indications for existing products or any of our other products in development.
26 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
We continue to strengthen our global R&D organization and pursue strategies intended to improve innovation and overall productivity in R&D to achieve a sustainable pipeline that will deliver value in the near term and over time.
For additional information about our R&D organization, see the “Overview of Our Performance, Operating Environment, Strategy and Outlook—Our Strategy—Organizing for Growth” and “—Description of Research and Development Operations” sections of this Financial Review.
A comprehensive update of Pfizer’s development pipeline was published as of January 28, 2020 and is available at www.pfizer.com/science/drug-product-pipeline. It includes an overview of our research and a list of compounds in development with targeted indication and phase of development, as well as mechanism of action for some candidates in Phase 1 and all candidates from Phase 2 through registration.
The following series of tables provides information about significant regulatory actions by, and filings pending with, the FDA and regulatory authorities in the EU and Japan, as well as additional indications and new drug candidates in late-stage development.
RECENT FDA APPROVALS | ||
PRODUCT | INDICATION | DATE APPROVED |
Xtandi (enzalutamide) | Treatment of metastatic castration-sensitive prostate cancer, which is being developed through a collaboration with Astellas | December 2019 |
Abrilada (adalimumab-afzb)(a) | A biosimilar to Humira® (adalimumab) for the treatment of certain patients with rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, adult Crohn's disease, ulcerative colitis and plaque psoriasis | November 2019 |
Ruxience (rituximab-pvvr)(b) | A biosimilar to Rituxan® (rituximab) for the treatment of adult patients with non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and granulomatosis with polyangiitis and microscopic polyangiitis | July 2019 |
Zirabev (bevacizumab-bvzr)(c) | A biosimilar to Avastin® (bevacizumab) for the treatment of mCRC; unresectable, locally advanced, recurrent or metastatic NSCLC; recurrent glioblastoma; metastatic RCC; and persistent, recurrent or metastatic cervical cancer | June 2019 |
Bavencio (avelumab) | Bavencio (avelumab) in combination with Inlyta (axitinib) for the first-line treatment of patients with advanced RCC, which is being developed in collaboration with Merck KGaA, Germany | May 2019 |
Vyndaqel (tafamidis meglumine) | Treatment of the cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular mortality and cardiovascular-related hospitalization | May 2019 |
Vyndamax (tafamidis) | Treatment of the cardiomyopathy of wild-type or hereditary ATTR-CM in adults to reduce cardiovascular mortality and cardiovascular-related hospitalization | May 2019 |
Trazimera (trastuzumab-qyyp)(d) | A biosimilar to Herceptin® (trastuzumab) for all eligible indications of the reference product | March 2019 |
Daurismo (glasdegib) | Treatment of newly-diagnosed acute myeloid leukemia in adult patients who are 75 years or older or who have comorbidities that preclude use of intensive induction chemotherapy | November 2018 |
Lorbrena (lorlatinib) | Treatment of patients with ALK-positive metastatic NSCLC whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic disease; or whose disease has progressed on alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease | November 2018 |
(a) | Humira® is a registered trademark of AbbVie Biotechnology Ltd. Pfizer is working to make Abrilada available to U.S. patients as soon as feasible based on the terms of its agreement with AbbVie. Current plans are to launch Abrilada in 2023. |
(b) | Rituxan® is a registered trademark of Biogen MA Inc. |
(c) | Avastin® is a registered trademark of Genentech, Inc. |
(d) | Herceptin® is a registered trademark of Genentech, Inc. |
PENDING U.S. NDAs AND SUPPLEMENTAL FILINGS | ||
PRODUCT | PROPOSED INDICATION | DATE FILED* |
Braftovi (encorafenib)(a) | Braftovi (encorafenib) in combination with Erbitux® (cetuximab) for the treatment of BRAFV600E-mutant metastatic colorectal cancer after prior therapy | December 2019 |
PF-06881894(b) | A potential biosimilar to Neulasta® (pegfilgrastim) | August 2019 |
Vyndaqel (tafamidis meglumine)(c) | Treatment of transthyretin familial amyloid polyneuropathy | February 2012 |
* | The dates set forth in this column are the dates on which the FDA accepted our submissions. |
(a) | Erbitux® is a registered trademark of ImClone LLC. |
(b) | Neulasta® is a registered U.S. trademark of Amgen Inc. |
(c) | In May 2012, the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee voted that the tafamidis meglumine data provide substantial evidence of efficacy for a surrogate endpoint that is reasonably likely to predict a clinical benefit. In June 2012, the FDA issued a “complete response” letter with respect to this tafamidis NDA. The FDA has requested the completion of a second efficacy study, and also has asked for additional information on the data within the current tafamidis NDA. Pfizer has completed study B3461028, a global Phase 3 study to support the new indication of transthyretin amyloid cardiomyopathy, which includes patients with wild type and variant transthyretin. We are working with the FDA to identify next steps. |
2019 Financial Report | 27 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
REGULATORY APPROVALS AND FILINGS IN THE EU AND JAPAN | |||
PRODUCT | DESCRIPTION OF EVENT | DATE APPROVED | DATE FILED* |
Vyndaqel (tafamidis free acid) | Application approved in the EU for a once-daily 61 mg oral capsule, for the treatment of wild-type or hereditary transthyretin amyloidosis in adult patients with cardiomyopathy | February 2020 | — |
Amsparity (adalimumab)(a) | Application approved in the EU for a biosimilar to Humira® (adalimumab) for the treatment of certain patients with rheumatoid arthritis, juvenile idiopathic arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis, hidradenitis suppurativa, Crohn’s disease, ulcerative colitis, uveitis, and pediatric plaque psoriasis | February 2020 | — |
Xeljanz (tofacitinib) | Application approved in the EU for Xeljanz (tofacitinib) 11 mg prolonged release tablets in combination with methotrexate for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying antirheumatic drugs | December 2019 | — |
Bavencio (avelumab) | Application approved in Japan for Bavencio (avelumab) in combination with Inlyta (axitinib) for the first-line treatment of advanced RCC, which is being developed in collaboration with Merck KGaA, Germany | December 2019 | — |
Braftovi (encorafenib) and Mektovi (binimetinib) | Application filed in the EU for second-or-third-line treatment of BRAF-mutant mCRC in patients who have received prior systemic therapy, which is being developed in collaboration with the Pierre Fabre Group | — | November 2019 |
Bavencio (avelumab) | Application approved in the EU for Bavencio (avelumab) in combination with Inlyta (axitinib) for the first-line treatment of advanced RCC, which is being developed in collaboration with Merck KGaA, Germany | October 2019 | — |
PF-06881894(b) | Application filed in the EU for a potential biosimilar to Neulasta® (pegfilgrastim) | — | October 2019 |
Rituximab Pfizer (rituximab)(c) | Application approved in Japan for a biosimilar to Rituxan® (rituximab) for the treatment of CD20-positive, B-cell non-Hodgkin’s Lymphoma, CD20-positive, B-cell lymphoproliferative disease under immunosuppression, and Granulomatosis with polyangiitis, and microscopic polyangiitis | September 2019 | — |
Bosulif (bosutinib) | Application filed in Japan for the treatment of chronic myelogenous leukemia (CML), which is being developed in collaboration with Avillion LLP | — | July 2019 |
Xtandi (enzalutamide) | Application filed in the EU for the treatment of metastatic hormone-sensitive prostate cancer, which is being developed through a collaboration with Astellas | — | July 2019 |
Talzenna (talazoparib) | Application approved in the EU for monotherapy for the treatment of adult patients with germline breast cancer susceptibility gene (gBRCA)1/2-mutations, who have HER2- locally advanced or metastatic breast cancer | June 2019 | — |
Bevacizumab Pfizer (bevacizumab)(d) | Application approved in Japan for a biosimilar to Avastin® (bevacizumab) for the treatment of metastatic colorectal cancer | June 2019 | — |
Daurismo (glasdegib) | Application filed in the EU for treatment of newly-diagnosed acute myeloid leukemia in adult patients who are 75 years or older or who have co-morbidities that preclude use of intensive induction chemotherapy | — | May 2019 |
Lorviqua (lorlatinib) | Application approved in the EU as monotherapy, for the treatment of adult patients with ALK- positive advanced non-small cell lung cancer whose disease has progressed after: • alectinib or ceritinib as the first ALK tyrosine kinase inhibitor (TKI) therapy; or• crizotinib and at least one other ALK TKI | May 2019 | — |
Vizimpro (dacomitinib) | Application approved in the EU as monotherapy for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer with EGFR activating mutations, which is being developed in collaboration with SFJ | April 2019 | — |
Vyndaqel (tafamidis meglumine) | Application approved in Japan for treatment of transthyretin amyloid cardiomyopathy | March 2019 | — |
Zirabev(d) | Application approved in the EU for a biosimilar to Avastin® (bevacizumab) for the treatment of metastatic carcinoma of the colon or rectum, metastatic breast cancer, unresectable advanced, metastatic or recurrent NSCLC, advanced and/or metastatic renal cell cancer and persistent, recurrent, or metastatic carcinoma of the cervix | February 2019 | — |
Vizimpro (dacomitinib) | Application approved in Japan for the treatment of patients with locally advanced or metastatic non-small cell lung cancer with EGFR mutations, which is being developed in collaboration with SFJ | January 2019 | — |
PF-05280586(e) | Application filed in the EU for a potential biosimilar to MabThera® (rituximab) | — | August 2018 |
crisaborole(f) | Application filed in the EU for the treatment of mild to moderate atopic dermatitis in adults and pediatric patients from 2 years of age with ≤ 40% body surface area (BSA) affected | — | May 2018 |
* | For applications in the EU, the dates set forth in this column are the dates on which the EMA validated our submissions. |
(a) | Humira® is a registered trademark of AbbVie Biotechnology Ltd. Pfizer does not currently plan to commercialize Amsparity in the EU due to unfavorable market conditions. |
(b) | Neulasta® is a registered trademark of Amgen Inc. |
(c) | Rituxan® is a registered trademark of Biogen MA Inc. |
(d) | Avastin® is a registered trademark of Genentech, Inc. |
(e) | MabThera® is a registered trademark of Roche, Inc. In January 2020, the EMA’s CHMP adopted a positive opinion recommending the approval of PF-05280586 as a potential biosimilar to MabThera® (rituximab) for the treatment of non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, RA, granulomatosis with polyangiitis and microscopic polyangiitis, and pemphigus vulgaris. |
(f) | In January 2020, the EMA’s CHMP adopted a positive opinion recommending the approval of Staquis (crisaborole) for the treatment of mild to moderate atopic dermatitis in adults and pediatric patients from 2 years of age with ≤ 40% BSA affected. |
28 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
LATE-STAGE CLINICAL PROGRAMS FOR ADDITIONAL USES AND DOSAGE FORMS FOR IN-LINE AND IN-REGISTRATION PRODUCTS | |
PRODUCT | PROPOSED INDICATION |
Bavencio (avelumab) | A monoclonal antibody that inhibits PD-L1 for the first-line treatment of stage IIIb/IV non-small cell lung cancer, which is being developed in collaboration with Merck KGaA, Germany |
Bavencio (avelumab) | A monoclonal antibody that inhibits PD-L1 for maintenance treatment, in the first-line setting, for patients with urothelial cancer, which is being developed in collaboration with Merck KGaA, Germany |
Bavencio (avelumab) | A monoclonal antibody that inhibits PD-L1 for treatment of locally advanced squamous cell carcinoma of the head and neck, which is being developed in collaboration with Merck KGaA, Germany |
Daurismo (glasdegib) | A smoothened inhibitor, in combination with azacitidine, for the treatment of acute myeloid leukemia |
Ibrance (palbociclib) | Treatment of HER2+ advanced breast cancer, in collaboration with the Alliance Foundation Trials, LLC |
Ibrance (palbociclib) | Treatment of high-risk early breast cancer, in collaboration with the German Breast Group |
Ibrance (palbociclib) | Treatment of HR+ early breast cancer, in collaboration with the Alliance Foundation Trials, LLC, and the Austrian Breast Colorectal Cancer Study Group |
Lorbrena (lorlatinib) | Treatment of patients with metastatic non-small cell lung cancer whose tumors are ALK-positive as detected by an FDA-approved test |
Xeljanz (tofacitinib) | Treatment of ankylosing spondylitis |
Xtandi (enzalutamide) | Treatment of non-metastatic hormone-sensitive prostate cancer, which is being developed through a collaboration with Astellas |
Talzenna (talazoparib) | An oral PARP inhibitor, in combination with Xtandi (enzalutamide), for the treatment of metastatic castration-resistant prostate cancer |
In November 2019, we and our partner Merck KGaA, Germany, announced the topline results of the Phase III JAVELIN Gastric 100 study evaluating avelumab as first-line maintenance therapy following induction chemotherapy in patients with unresectable, locally advanced or metastatic HER2-negative gastric or gastroesophageal junction cancer versus continuation of chemotherapy or best supportive care. While the study showed clinical activity for avelumab in this setting, it did not meet the primary endpoints of superior overall survival compared with the standard of care in the overall intent-to-treat population. No new safety signals were observed, and the safety profile for avelumab in this trial was consistent with that observed in the overall JAVELIN clinical development program.
In February 2019, the company took steps to transition rheumatoid arthritis study patients who were on tofacitinib 10 mg twice daily to tofacitinib 5 mg twice daily in the ongoing FDA post-marketing requirement study A3921133, a study performed in patients considered to be at high risk for certain side effects. This action was taken as the result of notification from the tofacitinib Rheumatology Data Safety Monitoring Board of a safety signal regarding the tofacitinib 10 mg twice daily treatment arm in study A3921133. The 5 mg twice daily dose is the FDA approved dose in the U.S. for adult patients with moderate to severe rheumatoid arthritis. In July 2019, the FDA updated the U.S. prescribing information for Xeljanz to include two additional boxed warnings as well as changes to the indication and dosing for UC. These updates were based on the FDA’s review of data from the ongoing post-marketing requirement RA study A3921133. In January 2020, the EC revised the summary of product characteristics (SmPC) for Xeljanz to include new warnings and recommendations for use of Xeljanz due to an increased risk of venous thromboembolism and, due to an increased risk of infections, revised warnings in patients older than 65 years of age. These updates were based on the FDA’s and EMA’s review of data from the ongoing post-marketing requirement rheumatoid arthritis study A3921133.
NEW DRUG CANDIDATES IN LATE-STAGE DEVELOPMENT | |
CANDIDATE | PROPOSED INDICATION |
aztreonam-avibactam (PF-06947387) | A beta lactam/beta lactamase inhibitor for the treatment of patients with infections caused by Gram-negative bacteria, including those that produce metallo-beta-lactamases, for which there are limited or no treatment options |
fidanacogene elaparvovec (PF-06838435) | An investigational gene therapy for the treatment of hemophilia B |
PF-06482077 | A 20-Valent pneumococcal conjugate vaccine for the prevention of invasive pneumococcal disease and pneumonia caused by Streptococcus pneumoniae serotypes covered by the vaccine in adults 18 years of age and older |
PF-06651600 | A selective dual Janus kinase 3 (JAK3) and Tyrosine kinase Expressed in hepatocellular Carcinoma (TEC) family inhibitor for the treatment of patients with moderate to severe alopecia areata |
abrocitinib (PF-04965842) | A Janus kinase 1 (JAK1) inhibitor for the treatment of moderate-to-severe atopic dermatitis |
PF-06425090 | A prophylactic vaccine for prevention of primary clostridioides difficile infection (CDI) in individuals |
PF-07265803 | An oral inhibitor of p38 mitogen-activated protein kinase for the treatment of patients with symptomatic dilated cardiomyopathy due to a Lamin A/C gene mutation |
PF-06801591 | A monoclonal antibody that inhibits PD-1, in combination with Bacillus Calmette-Guerin (BCG), for the treatment of non-muscle invasive bladder cancer |
somatrogon (PF-06836922) | A long-acting hGH-CTP for the treatment of growth hormone deficiency in children, which is being developed in collaboration with OPKO |
somatrogon (PF-06836922) | A long-acting hGH-CTP for the treatment of growth hormone deficiency in adults, which is being developed in collaboration with OPKO |
tanezumab | An anti-nerve growth factor monoclonal antibody for the treatment of pain, which is being developed in collaboration with Lilly |
In August 2019, we announced that the Phase 3 Rivipansel (GMI-1070): Evaluating Safety, Efficacy and Time to Discharge (RESET) pivotal study did not meet its primary or key secondary efficacy endpoints. The objective of the trial was to evaluate the efficacy and safety of rivipansel in patients aged six and older with sickle cell disease (SCD) who were hospitalized for a vaso-occlusive crisis (VOC) and required treatment with IV opioids. We plan to share the study data in a publication or scientific meeting presentation in the near future, as we want to ensure the learnings from this trial help inform future sickle cell programs that aim to improve care for SCD disease patients experiencing a
2019 Financial Report | 29 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
VOC. In February 2020, we notified GlycoMimetics, Inc. that we were discontinuing further development of rivipansel and will transfer the program back to them.
Additional product-related programs are in various stages of discovery and development.
COSTS AND EXPENSES
The changes in expenses below reflect, among other things, a decline in expenses resulting from the July 31, 2019 completion of the Consumer Healthcare JV transaction with GSK. Our financial results, and our Consumer Healthcare segment’s operating results, for 2019 reflect seven months of Consumer Healthcare segment domestic operations and eight months of Consumer Healthcare segment international operations. For additional information, see Notes to Consolidated Financial Statements—Note 1A. Basis of Presentation and Significant Accounting Policies: Basis of Presentation.
Cost of Sales
Year Ended December 31, | % Change | ||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | ||||||||||||
Cost of sales | $ | 10,219 | $ | 11,248 | $ | 11,228 | (9 | ) | — | ||||||||
As a percentage of Revenues | 19.7 | % | 21.0 | % | 21.4 | % | |||||||||||
2019 v. 2018
Cost of sales decreased $1.0 billion, or 9% in 2019, compared to 2018, primarily due to:
• | the favorable impact of the July 31, 2019 completion of the Consumer Healthcare joint venture transaction with GSK; |
• | the favorable impact of foreign exchange of $279 million; |
• | the favorable impact of hedging activity on intercompany inventory of $261 million; and |
• | lower royalty expense for Lyrica due to the patent expiration, |
partially offset by:
• | an unfavorable change in product mix. |
The decrease in Cost of sales as a percentage of revenues in 2019, compared to 2018, was primarily due to all of the factors discussed above, as well an increase in alliance revenues, which have no associated cost of sales, partially offset by lower Lyrica revenues in developed markets, due to U.S. multi-source generic competition that began in July 2019.
2018 v. 2017
Cost of sales increased $21 million, or were relatively flat, in 2018, compared to 2017, primarily due to:
• | increased sales volumes mostly related to key products within our product portfolio; |
• | higher costs across the legacy SIP portfolio, as a result of the complexity of high quality product manufacture across the legacy Hospira plants, which was partially offset by decreases in other costs across various markets; |
• | an increase in royalty expenses based on the mix of products sold; and |
• | the unfavorable impact of hedging activity on intercompany inventory of $65 million, |
partially offset by:
• | lower volumes from the legacy SIP portfolio, in developed markets, primarily due to increased competition across the legacy SIP portfolio and continued legacy Hospira product shortages in the U.S.; |
• | the non-recurrence of $195 million in inventory losses, overhead costs, and incremental costs related to the period in 2017 during which our Puerto Rico plants were not operational due to hurricanes; |
• | the favorable impact of foreign exchange of $153 million; |
• | the non-recurrence of charges related to a product recall that occurred in 2017; and |
• | the favorable impact of the sale of HIS of $35 million. |
The decrease in Cost of sales as a percentage of revenues in 2018, compared to 2017, was mainly due to all of the factors discussed above, as well as an increase in alliance revenues, which have no associated cost of sales.
Selling, Informational and Administrative (SI&A) Expenses
Year Ended December 31, | % Change | |||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | |||||||||||||
Selling, informational and administrative expenses | $ | 14,350 | $ | 14,455 | $ | 14,804 | (1 | ) | (2 | ) | ||||||||
As a percentage of Revenues | 27.7 | % | 26.9 | % | 28.2 | % | ||||||||||||
2019 v. 2018
SI&A expenses decreased $105 million, or 1%, in 2019, compared to 2018, mostly due to:
• | the favorable impact of the July 31, 2019 completion of the Consumer Healthcare joint venture with GSK; |
30 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | reduction in field force expense as well as advertising and promotion expenses in developed markets, primarily related to Lyrica in the U.S.; and |
• | the favorable impact of foreign exchange of $291 million, |
partially offset by:
• | additional Biopharma investment in emerging markets; |
• | separation costs of $127 million associated with our planned Upjohn transaction with Mylan; |
• | additional investment in the Oncology portfolio in developed markets; |
• | increased employee deferred compensation as a result of savings plan gains; |
• | an increase due to the timing of expenses (i.e., insurance recoveries and product donations); |
• | marketing and promotional expenses associated with the U.S. launches of Vyndaqel in May 2019 and Vyndamax in September 2019; |
• | costs to separate Consumer Healthcare; |
• | increased healthcare reform expenses; and |
• | Upjohn investments in China across key brands. |
2018 v. 2017
SI&A expenses decreased $350 million, or 2%, in 2018, compared to 2017, mainly due to:
• | lower advertising, promotional and field force expenses, as well as general and administrative expenses, reflecting the benefits of cost-reduction and productivity initiatives; |
• | the non-recurrence of a $200 million charitable contribution to the Pfizer Foundation; |
• | decreased investment across several of our key products, primarily Viagra and Enbrel; and |
• | lower healthcare reform expenses as a result of a true up of the prior year amount, |
partially offset by:
• | additional investment across several of our key products, primarily, Xeljanz, Ibrance, Eucrisa and Prevnar 13/Prevenar 13; |
• | additional investments in China; and |
• | a special, one-time bonus paid to virtually all Pfizer colleagues, excluding executives, of $119 million, in the aggregate, in the first quarter of 2018. |
Research and Development (R&D) Expenses
Year Ended December 31, | % Change | |||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | |||||||||||
Research and development expenses | $ | 8,650 | $ | 8,006 | $ | 7,683 | 8 | 4 | ||||||||
As a percentage of Revenues | 16.7 | % | 14.9 | % | 14.6 | % | ||||||||||
2019 v. 2018
R&D expenses increased $644 million, or 8%, in 2019, compared to 2018, mainly due to:
• | upfront payments to Therachon and Akcea (see Notes to Consolidated Financial Statements—Note 2. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements); |
• | increased investments towards building new capabilities and driving automation; |
• | increased spending on our Inflammation & Immunology and Rare Disease portfolios due to several Phase 3 programs and |
investment in gene therapy;
• | increased spending related to assets acquired from Array; and |
• | increased medical spend for new and growing products, |
partially offset by:
• | decreased spending across the Oncology, Vaccines and Internal Medicine portfolios, as select programs have reached completion; |
• | a decrease in the value of the portfolio performance share grants reflecting changes in the price of Pfizer’s common stock, as well as management’s assessment of the probability that the specified performance criteria will be achieved; |
• | the discontinuation of the Staphylococcus aureus vaccine trial; |
• | the favorable impact of the July 31, 2019 completion of the Consumer Healthcare joint venture with GSK; and |
• | the favorable impact of foreign exchange. |
2018 v. 2017
R&D expenses increased $322 million, or 4%, in 2018, compared to 2017, mainly due to:
• | increased costs associated with our Phase 3 clinical trials related to our JAK1 inhibitor (which was initiated in December 2017) and the C. difficile vaccine program (which was initiated in March 2017) as well as increased spending for our 20 valent pneumococcal conjugate vaccine candidate; |
• | increased costs associated with the Bavencio program; and |
2019 Financial Report | 31 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | an increase in the value of the portfolio performance share grants reflecting changes in the price of Pfizer’s common stock, as well as management’s assessment of the probability that the specified performance criteria will be achieved, |
partially offset by:
• | decreased spending for biosimilars as several programs have reached completion; and |
• | the impact of our decision to end internal neuroscience discovery and early development efforts. |
For additional information on Cost of sales, SI&A and R&D expenses by operating segment, see the “Analysis of Operating Segment Information” section of this Financial Review.
Amortization of Intangible Assets
Year Ended December 31, | % Change | ||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | ||||||||||||
Amortization of intangible assets | $ | 4,610 | $ | 4,893 | $ | 4,758 | (6 | ) | 3 | ||||||||
As a percentage of Revenues | 8.9 | % | 9.1 | % | 9.1 | % | |||||||||||
Amortization of intangible assets decreased $283 million, or 6%, in 2019, compared to 2018, mainly due to the non-recurrence of amortization expense as a result of the impairment of sterile injectable products in the fourth quarter of 2018 (recorded in Other (income)/deductions––net), fully amortized assets and the contribution of our Consumer Healthcare business to the Consumer Healthcare joint venture with GSK, partially offset by an increase in amortization expense related to assets recorded as a result of the approval of Xtandi in the U.S. for the treatment of non-metastatic castration-resistant prostate cancer in July of 2018 and amortization of intangible assets as a result of our acquisition of Array.
Amortization of intangible assets increased $135 million, or 3%, in 2018, compared to 2017, primarily due to amortization expense of approximately $151 million (pre-tax) in 2018 associated with the approval of Xtandi in the U.S. for the treatment of non-metastatic castration-resistant prostate cancer. The U.S. approval resulted in the transfer of $2.7 billion from an indefinite-lived IPR&D intangible asset to a finite-lived Developed technology rights intangible asset.
For additional information, see Notes to Consolidated Financial Statements—Note 2A. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Acquisitions, —Note 2C. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Equity-Method Investment and Assets and Liabilities Held for Sale and —Note 10A. Identifiable Intangible Assets and Goodwill: Identifiable Intangible Assets.
Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
Year Ended December 31, | % Change | |||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | |||||||||||||
Restructuring charges/(credits)––acquisition-related costs(a) | $ | (192 | ) | $ | 37 | $ | 105 | * | (64 | ) | ||||||||
Restructuring charges/(credits)––cost reduction initiatives(b) | 565 | 745 | (75 | ) | (24 | ) | * | |||||||||||
Restructuring charges | 373 | 782 | 30 | (52 | ) | * | ||||||||||||
Transaction costs(c) | 63 | 1 | 4 | * | (62 | ) | ||||||||||||
Integration costs and other(c) | 311 | 260 | 317 | 20 | (18 | ) | ||||||||||||
Restructuring charges and certain acquisition-related costs | 747 | 1,044 | 351 | (28 | ) | * | ||||||||||||
Net periodic benefit costs(c) | 23 | 146 | 136 | (84 | ) | 8 | ||||||||||||
Total additional depreciation––asset restructuring | 38 | 50 | 91 | (24 | ) | (45 | ) | |||||||||||
Total implementation costs | 158 | 194 | 227 | (18 | ) | (15 | ) | |||||||||||
Costs associated with acquisitions and cost-reduction/productivity initiatives(d) | $ | 967 | $ | 1,434 | $ | 805 | (33 | ) | 78 | |||||||||
(a) | Restructuring charges/(credits)––acquisition-related costs include employee termination costs, asset impairments and other exit costs associated with business combinations. Credits for 2019 were mostly due to the reversal of certain accruals related to our acquisition of Wyeth upon the effective favorable settlement of an IRS audit for multiple tax years. See Notes Consolidated Financial Statements—Note 5D. Tax Matters: Tax Contingencies. Charges for 2018 were mainly due to asset write downs, partially offset by the reversal of previously recorded accruals for employee termination costs related to our acquisition of Hospira. Restructuring charges for 2017 mainly related to our acquisitions of Hospira and Medivation and were primarily due to asset write-downs, partially offset by the reversal of previously recorded accruals for employee termination costs. |
(b) | Restructuring charges/(credits)––cost reduction initiatives relate to employee termination costs, asset impairments and other exit costs not associated with acquisitions. For 2019, the charges were mostly related to employee termination costs. For 2018, the charges were mostly related to employee termination costs and asset write downs. The employee termination costs for 2019 and 2018 were primarily associated with our improvements to operational effectiveness as part of the realignment of our organizational structure, and for 2019, also includes employee termination costs associated with the Transforming to a More Focused Company initiative. For 2017, the credits were mostly related to the reversal of previously recorded accruals for employee termination costs, partially offset by asset write downs. |
(c) | For additional information, see Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives. |
(d) | Comprises Restructuring charges and certain acquisition-related costs as well as costs associated with our cost-reduction/productivity initiatives included in Cost of sales, Research and development expenses, Selling, informational and administrative expenses and/or Other (income)/deductions––net as appropriate. For additional information, see Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives. |
32 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
* | Indicates calculation not meaningful or result is equal to or greater than 100%. |
2017-2019 Initiatives and Organizing for Growth
During 2018, we determined that at the start of our 2019 fiscal year, we would begin operating under our new commercial structure, which reorganized our operations into three businesses––Biopharma, a science-based innovative medicines business; Upjohn, a global, primarily off-patent branded and generic established medicines business; and through July 31, 2019, a Consumer Healthcare business. To operate effectively in this structure and position ourselves for future growth, we focused on creating a simpler, more efficient operating structure within each business as well as the functions that support them. Beginning in the fourth quarter of 2018, we reviewed previously planned initiatives and new initiatives to ensure that there was alignment around our new structure and combined the 2017-2019 initiatives with our current Organizing for Growth initiatives to form one cohesive plan. For the combined programs, we achieved savings of approximately $1.6 billion and incurred approximately $2.1 billion in costs over the three-year period 2017-2019. Savings of approximately $500 million were reinvested in our R&D pipeline and in selling and marketing to support our current and recently launched products and indications.
Transforming to a More Focused Company
With the formation of the GSK Consumer Healthcare venture and the pending combination of Upjohn with Mylan, Pfizer is transforming itself into a more focused, global leader in science-based innovative medicines. As a result, we began, in the fourth quarter of 2019, to identify and undertake efforts to ensure our cost base aligns appropriately with our Biopharmaceutical revenue base, which is expected to be 20% less (based on the midpoint of the range for 2020 New Pfizer revenue guidance (see the “Our Financial Guidance for 2020” section of this Financial Review), compared to 2019 total company reported revenue) as a result of both the completed Consumer Healthcare and expected Upjohn transactions. While certain direct costs have transferred or will transfer to the Consumer Healthcare joint venture and to the Upjohn entities, there are indirect costs which are not expected to transfer. In addition, we are taking steps to restructure our organizations to appropriately support and drive the purpose of the three core functions of our focused innovative medicines business: R&D, Manufacturing and Commercial. We expect the costs associated with this multi-year effort to continue through 2022 and to total approximately $1.4 billion on a pre-tax basis and approximately 10% of this to be non-cash. Actions may include, among others, changes in location of certain activities, expanded use and co-location of centers of excellence and shared services, and increased use of digital technologies. The associated actions and the specific costs are currently in development but will include severance and benefit plan impacts, exit costs as well as associated implementation costs.
We expect net cost savings of about $1.0 billion to be achieved over the three-year period 2020-2022. Certain qualifying costs associated with this program were recorded in the fourth quarter of 2019 and are reflected as Certain Significant Items and excluded from our non-GAAP measure of Adjusted Income. See the “Non-GAAP Financial Measure (Adjusted Income)” section of this Financial Review for additional information. These savings are expected to be realized primarily in procurement and in enabling functions (as described in the Notes to Consolidated Financial Statements––Note 17A. Segment, Geographic and Other Revenue Information: Segment Information).
For additional information about this program and expected and actual total costs, see Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives.
In addition to these major initiatives, we continuously monitor our operations for cost reduction and/or productivity opportunities, especially in light of the losses of exclusivity and the expiration of collaborative arrangements for various products.
Other (Income)/Deductions––Net
Year Ended December 31, | % Change | |||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | |||||||||||
Other (income)/deductions—net | $ | 3,578 | $ | 2,116 | $ | 1,416 | 69 | 49 | ||||||||
For information about the components of Other (income)/deductions—net, see Notes to Consolidated Financial Statements—Note 4. Other (Income)/Deductions—Net.
See also the “Analysis of Operating Segment Information” section of this Financial Review.
PROVISION/(BENEFIT) FOR TAXES ON INCOME
Year Ended December 31, | % Change | |||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | |||||||||||
Provision/(benefit) for taxes on income | $ | 1,384 | $ | 706 | $ | (9,049 | ) | 96 | * | |||||||
Effective tax rate on continuing operations | 7.8 | % | 5.9 | % | (73.5 | )% | ||||||||||
* | Indicates calculation not meaningful or result is equal to or greater than 100%. |
2019 v. 2018
The higher effective tax rate in 2019 compared to 2018 was mainly the result of:
• | the tax expense of approximately $2.7 billion associated with the gain related to the completion of the Consumer Healthcare joint venture transaction with GSK; and |
• | the non-recurrence of certain tax initiatives and favorable adjustments to the provisional estimate of the TCJA, |
2019 Financial Report | 33 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
partially offset by:
• | an increase in tax benefits associated with the resolution of certain tax positions pertaining to prior years, primarily due to a benefit of $1.4 billion, representing tax and interest, resulting from the favorable settlement of a U.S. IRS audit; |
• | benefits related to certain tax initiatives associated with the implementation of our new organizational structure; |
• | the tax benefit recorded as a result of additional guidance issued by the U.S. Department of Treasury related to the enactment of the TCJA; and |
• | the favorable change in the jurisdictional mix of earnings as a result of operating fluctuations in the normal course of business. |
2018 v. 2017
The higher effective tax rate in 2018 compared to 2017 was primarily the result of:
• | the non-recurrence of a $10.7 billion tax benefit recorded in 2017 to reflect the enactment of the TCJA, |
partially offset by:
• | tax benefits related to the TCJA, including certain 2018 tax initiatives as well as favorable adjustments to the provisional estimate of the impact of the legislation, reported and disclosed within the applicable measurement period, in accordance with guidance issued by the SEC; |
• | the favorable change in the jurisdictional mix of earnings as a result of operating fluctuations in the normal course of business; as well as |
• | an increase in tax benefits associated with the resolution of certain tax positions pertaining to prior years primarily with various foreign tax authorities, and the expiration of certain statutes of limitations. |
For details about discrete elements that impacted our tax provisions, see Notes to Consolidated Financial Statements—Note 5A. Tax Matters: Taxes on Income from Continuing Operations.
Changes in Tax Laws
On December 22, 2017, the U.S. enacted significant changes to U.S. tax law following the passage and signing of the TCJA. The TCJA is complex and significantly changes the U.S. corporate income tax system by, among other things, reducing the U.S. Federal corporate tax rate from 35% to 21%, transitioning U.S. international taxation from a worldwide tax system to a territorial tax system and imposing a repatriation tax on deemed repatriated accumulated post-1986 earnings of foreign subsidiaries. In accordance with guidance issued by the SEC we recorded provisional estimates of the legislation in the fourth-quarter 2017. In 2018, we finalized our provisional accounting for the tax effects of the TCJA based on our best estimates of available information and data, and have reported and disclosed the impacts within the applicable measurement period, in accordance with guidance issued by the SEC. For additional information, see Notes to Consolidated Financial Statements—Note 5A. Tax Matters: Taxes on Income from Continuing Operations and the “Analysis of Financial Condition, Liquidity and Capital Resources––Selected Measures of Liquidity and Capital Resources—Contractual Obligations” section of this Financial Review.
On January 23, 2017, the Governor of Puerto Rico signed into law Act No. 3-2017, amending Section 2101 of the Puerto Rico Internal Revenue Code of 1994, which imposes an excise tax that was effective beginning in 2011 (Act 154). The excise tax is imposed on the purchase of products by multinational corporations and their affiliates from their Puerto Rico affiliates. As originally adopted, the excise tax was to be in effect from 2011 through 2016 and the tax rate was to decline over time from 4% in 2011 to 1% in 2016. Act No. 2-2013 extended the excise tax through 2017 and, effective July 1, 2013, increased the tax rate to 4% for all years through 2017. Act No. 3-2017 further extended the excise tax for all years through 2027 at a rate of 4%. The excise tax has been recorded in Cost of sales and Provision/(benefit) for taxes on income, as appropriate. All expected impacts in 2020 have been reflected in our financial guidance for 2020.
NON-GAAP FINANCIAL MEASURE (ADJUSTED INCOME)
General Description of Non-GAAP Financial Measure (Adjusted Income)
Adjusted income is an alternative view of performance used by management. We measure the performance of the overall Company on this basis in conjunction with other performance metrics. Because Adjusted income is an important internal measurement for Pfizer, we believe that investors’ understanding of our performance is enhanced by disclosing this performance measure. We report Adjusted income, certain components of Adjusted income, and Adjusted diluted earnings per share in order to portray the results of our major operations––the discovery, development, manufacture, marketing and sale of prescription medicines and vaccines––prior to considering certain income statement elements. We have defined Adjusted income as Net income attributable to Pfizer Inc. before the impact of purchase accounting for acquisitions, acquisition-related costs, discontinued operations and certain significant items, which are described below. Similarly, we have defined the Adjusted income components as Cost of sales, Selling, informational and administrative expenses, Research and development expenses, Amortization of intangible assets and Other (income)/deductions––net each before the impact of purchase accounting for acquisitions, acquisition-related costs and certain significant items. We have defined Adjusted diluted earnings per share as Earnings per common share attributable to Pfizer Inc.––diluted before the impact of purchase accounting for acquisitions, acquisition-related costs, discontinued operations and certain significant items. The Adjusted income measure, the Adjusted income component measures and the Adjusted diluted earnings per share measure are not, and should not be viewed as, substitutes for U.S. GAAP net income, U.S. GAAP net income components or U.S. GAAP diluted earnings per share.
The following are examples of how the Adjusted income and Adjusted diluted earnings per share measures are utilized:
• | senior management receives a monthly analysis of our operating results that is prepared on an Adjusted income and Adjusted diluted earnings per share basis; |
• | our annual budgets are prepared on an Adjusted income and Adjusted diluted earnings per share basis; and |
• | senior management’s annual compensation is derived, in part, using Adjusted income and Adjusted diluted earnings per share measures. The bonus plans for virtually all bonus-eligible, non-sales-force employees worldwide, including the Executive Leadership Team members |
34 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
and other members of senior management, are funded from a pool based on the performance measured by three financial metrics, including Adjusted diluted earnings per share, which is derived from Adjusted income. This metric accounts for 40% of the bonus pool funding. In addition, effective in 2019, Adjusted net income, which is derived from Adjusted income, is one of the measures utilized to determine payout for PSAs and is used for performance years starting in 2019, except for the 2017 PSA grant that used the previous metric, Adjusted operating income.
Adjusted income and its components and Adjusted diluted earnings per share are non-GAAP financial measures that have no standardized meaning prescribed by U.S. GAAP and, therefore, are limited in their usefulness to investors. Because of their non-standardized definitions, Adjusted income and its components (unlike U.S. GAAP net income and its components) and Adjusted diluted earnings per share (unlike U.S. GAAP diluted earnings per share) may not be comparable to the calculation of similar measures of other companies. Adjusted income and its components and Adjusted diluted earnings per share are presented solely to permit investors to more fully understand how management assesses performance.
We also recognize that, as internal measures of performance, the Adjusted income and its components and Adjusted diluted earnings per share measures have limitations, and we do not restrict our performance-management process solely to these metrics. A limitation of these measures is that they provide a view of our operations without including all events during a period, such as the effects of an acquisition or amortization of purchased intangibles, and do not provide a comparable view of our performance to other companies in the biopharmaceutical industry. We also use other specifically tailored tools designed to achieve the highest levels of performance. For example, our R&D organization has productivity targets, upon which its effectiveness is measured. In addition, total shareholder return, both on an absolute basis and relative to a publicly traded pharmaceutical index, plays a significant role in determining payouts under certain of Pfizer’s long-term incentive compensation plans.
See the accompanying reconciliations of certain GAAP reported to non-GAAP adjusted information for 2019, 2018 and 2017 below.
Purchase Accounting Adjustments
Adjusted income is calculated prior to considering certain significant purchase accounting impacts resulting from business combinations and net asset acquisitions. These impacts, primarily associated with Wyeth (acquired in 2009), Hospira (acquired in 2015), Anacor (acquired in 2016) and Medivation (acquired in 2016), can include the incremental charge to cost of sales from the sale of acquired inventory that was written up to fair value, amortization related to the increase in fair value of the acquired finite-lived intangible assets, and to a much lesser extent, depreciation related to the increase/decrease in fair value of the acquired fixed assets (primarily manufacturing facilities), amortization related to the increase in fair value of acquired debt, and the fair value changes associated with contingent consideration. Therefore, the Adjusted income measure includes the revenues earned upon the sale of the acquired products without considering the acquisition cost of those products.
Certain of the purchase accounting adjustments can occur through 20 or more years, but this presentation provides an alternative view of our performance that is used by management to internally assess business performance. We believe the elimination of amortization attributable to acquired intangible assets provides management and investors an alternative view of our business results by trying to provide a degree of parity to internally developed intangible assets for which R&D costs previously have been expensed.
However, a completely accurate comparison of internally developed intangible assets and acquired intangible assets cannot be achieved through Adjusted income. This component of Adjusted income is derived solely from the impacts of the items listed in the first paragraph of this section. We have not factored in the impacts of any other differences in experience that might have occurred if we had discovered and developed those intangible assets on our own, and this approach does not intend to be representative of the results that would have occurred in those circumstances. For example, our R&D costs in total, and in the periods presented, may have been different; our speed to commercialization and resulting sales, if any, may have been different; or our costs to manufacture may have been different. In addition, our marketing efforts may have been received differently by our customers. As such, in total, there can be no assurance that our Adjusted income amounts would have been the same as presented had we discovered and developed the acquired intangible assets.
Acquisition-Related Costs
Adjusted income is calculated prior to considering transaction, integration, restructuring charges and additional depreciation costs associated with business combinations because these costs are unique to each transaction and represent costs that were incurred to restructure and integrate two businesses as a result of the acquisition decision. For additional clarity, only transaction costs, additional depreciation and restructuring and integration activities that are associated with a business combination or a net-asset acquisition are included in acquisition-related costs. We have made no adjustments for the resulting synergies.
We believe that viewing income prior to considering these charges provides investors with a useful additional perspective because the significant costs incurred in connection with a business combination result primarily from the need to eliminate duplicate assets, activities or employees––a natural result of acquiring a fully integrated set of activities. For this reason, we believe that the costs incurred to convert disparate systems, to close duplicative facilities or to eliminate duplicate positions (for example, in the context of a business combination) can be viewed differently from those costs incurred in other, more normal, business contexts.
The integration and restructuring costs associated with a business combination may occur over several years, with the more significant impacts typically ending within three years of the transaction. Because of the need for certain external approvals for some actions, the span of time needed to achieve certain restructuring and integration activities can be lengthy. For example, due to the highly regulated nature of the pharmaceutical business, the closure of excess facilities can take several years, as all manufacturing changes are subject to extensive validation and testing and must be approved by the FDA and/or other global regulatory authorities.
2019 Financial Report | 35 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Discontinued Operations
Adjusted income is calculated prior to considering the results of operations included in discontinued operations, as well as any related gains or losses on the disposal of such operations. We believe that this presentation is meaningful to investors because, while we review our businesses and product lines for strategic fit with our operations, we do not build or run our businesses with the intent to sell them. Restatements due to discontinued operations do not impact compensation or change the Adjusted income measure for the compensation in respect of the restated periods, but are presented for consistency across all periods.
Certain Significant Items
Adjusted income is calculated prior to considering certain significant items. Certain significant items represent substantive and/or unusual items that are evaluated on an individual basis. Such evaluation considers both the quantitative and the qualitative aspects of their nature. Certain significant items may be highly variable and difficult to predict. Furthermore, in some cases it is reasonably possible that they could reoccur in future periods. For example, major non-acquisition-related cost-reduction programs stand on their own as they are specific to an event or goal with a defined term, but we may have subsequent programs based on reorganizations of the business, cost productivity or in response to loss of exclusivity or economic conditions. Legal charges to resolve litigation are also related to specific cases, which are facts and circumstances specific and, in some cases, may also be the result of litigation matters at acquired companies that were inestimable, not probable or unresolved at the date of acquisition. Unusual items may represent items that are not part of our ongoing business; items that, either as a result of their nature or size, we would not expect to occur as part of our normal business on a regular basis; items that would be non-recurring; or items that relate to products we no longer sell. While not all-inclusive, examples of items that could be included as certain significant items would be gains on the completion of joint venture transactions such as the gain on the completion of the Consumer Healthcare joint venture transaction discussed in Notes to Consolidated Financial Statements—Note 2C. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Equity-Method Investment and Assets and Liabilities Held for Sale, a major non-acquisition-related restructuring charge and associated implementation costs; amounts related to certain disposals of businesses, products or facilities that do not qualify as discontinued operations under U.S. GAAP; certain intangible asset impairments; adjustments related to the resolution of certain tax positions; the impact of adopting certain significant, event-driven tax legislation, such as the TCJA discussed in Notes to Consolidated Financial Statements—Note 5A. Tax Matters: Taxes on Income from Continuing Operations or charges related to certain legal matters, such as certain of those discussed in Notes to Consolidated Financial Statements—Note 16A. Contingencies and Certain Commitments: Legal Proceedings and in Part II, Item 1, “Legal Proceedings” in our Quarterly Reports on Form 10-Q. Normal, ongoing defense costs of the Company or settlements of and accruals for legal matters made in the normal course of our business would not be considered certain significant items.
Beginning in 2019, we exclude the gains and losses from equity securities from our measure of Adjusted income because of their inherent volatility, which we do not control and cannot predict with any level of certainty and because we do not believe that including these gains and losses assists investors in understanding our business or is reflective of our core operations and business. For example, in 2018, we contributed assets related to our allogeneic CAR T therapy to Allogene and received equity securities. We have revised Adjusted income and Adjusted diluted EPS for prior periods for consistency with our 2019 presentation.
Reconciliation of GAAP Reported to Non-GAAP Adjusted Information––Certain Line Items
2019 | ||||||||||||||||||||||||
IN MILLIONS, EXCEPT PER COMMON SHARE DATA | GAAP Reported | Purchase Accounting Adjustments(a) | Acquisition-Related Costs(a) | Discontinued Operations(a) | Certain Significant Items(a) | Non-GAAP Adjusted | ||||||||||||||||||
Revenues | $ | 51,750 | $ | — | $ | — | $ | — | $ | — | $ | 51,750 | ||||||||||||
Cost of sales | 10,219 | 19 | — | — | (208 | ) | 10,030 | |||||||||||||||||
Selling, informational and administrative expenses | 14,350 | 2 | (2 | ) | — | (309 | ) | 14,041 | ||||||||||||||||
Research and development expenses | 8,650 | 4 | — | — | (666 | ) | 7,988 | |||||||||||||||||
Amortization of intangible assets | 4,610 | (4,339 | ) | — | — | — | 271 | |||||||||||||||||
Restructuring charges and certain acquisition-related costs | 747 | — | (183 | ) | — | (565 | ) | — | ||||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | (8,086 | ) | — | — | — | 8,086 | — | |||||||||||||||||
Other (income)/deductions––net | 3,578 | (21 | ) | — | — | (3,858 | ) | (300 | ) | |||||||||||||||
Income from continuing operations before provision/(benefit) for taxes on income | 17,682 | 4,333 | 185 | — | (2,481 | ) | 19,720 | |||||||||||||||||
Provision/(benefit) for taxes on income(b) | 1,384 | 848 | 59 | — | 667 | 2,958 | ||||||||||||||||||
Income from continuing operations | 16,298 | 3,485 | 126 | — | (3,148 | ) | 16,762 | |||||||||||||||||
Discontinued operations––net of tax | 4 | — | — | (4 | ) | — | — | |||||||||||||||||
Net income attributable to noncontrolling interests | 29 | — | — | — | — | 29 | ||||||||||||||||||
Net income attributable to Pfizer Inc. | 16,273 | 3,485 | 126 | (4 | ) | (3,148 | ) | 16,733 | ||||||||||||||||
Earnings per common share attributable to Pfizer Inc.––diluted | 2.87 | 0.61 | 0.02 | — | (0.55 | ) | 2.95 | |||||||||||||||||
See end of tables for notes (a) and (b).
36 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
2018 | ||||||||||||||||||||||||
IN MILLIONS, EXCEPT PER COMMON SHARE DATA | GAAP Reported | Purchase Accounting Adjustments(a) | Acquisition-Related Costs(a) | Discontinued Operations(a) | Certain Significant Items(a) | Non-GAAP Adjusted | ||||||||||||||||||
Revenues | $ | 53,647 | $ | — | $ | — | $ | — | $ | — | $ | 53,647 | ||||||||||||
Cost of sales | 11,248 | 3 | (10 | ) | — | (110 | ) | 11,130 | ||||||||||||||||
Selling, informational and administrative expenses | 14,455 | 2 | (2 | ) | — | (222 | ) | 14,232 | ||||||||||||||||
Research and development expenses | 8,006 | 3 | — | — | (47 | ) | 7,962 | |||||||||||||||||
Amortization of intangible assets | 4,893 | (4,612 | ) | — | — | — | 281 | |||||||||||||||||
Restructuring charges and certain acquisition-related costs | 1,044 | — | (299 | ) | — | (745 | ) | — | ||||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | — | — | ||||||||||||||||||
Other (income)/deductions––net | 2,116 | (182 | ) | (7 | ) | — | (2,595 | ) | (667 | ) | ||||||||||||||
Income from continuing operations before provision/(benefit) for taxes on income | 11,885 | 4,786 | 318 | — | 3,719 | 20,709 | ||||||||||||||||||
Provision/(benefit) for taxes on income(b) | 706 | 915 | 54 | — | 1,520 | 3,196 | ||||||||||||||||||
Income from continuing operations | 11,179 | 3,871 | 264 | — | 2,199 | 17,513 | ||||||||||||||||||
Discontinued operations––net of tax | 10 | — | — | (10 | ) | — | — | |||||||||||||||||
Net income attributable to noncontrolling interests | 36 | — | — | — | — | 36 | ||||||||||||||||||
Net income attributable to Pfizer Inc. | 11,153 | 3,871 | 264 | (10 | ) | 2,199 | 17,477 | |||||||||||||||||
Earnings per common share attributable to Pfizer Inc.––diluted | 1.87 | 0.65 | 0.04 | — | 0.37 | 2.92 | ||||||||||||||||||
2017 | ||||||||||||||||||||||||
IN MILLIONS, EXCEPT PER COMMON SHARE DATA | GAAP Reported | Purchase Accounting Adjustments(a) | Acquisition-Related Costs(a) | Discontinued Operations(a) | Certain Significant Items(a) | Non-GAAP Adjusted | ||||||||||||||||||
Revenues | $ | 52,546 | $ | — | $ | — | $ | — | $ | — | $ | 52,546 | ||||||||||||
Cost of sales | 11,228 | (47 | ) | (39 | ) | — | (363 | ) | 10,778 | |||||||||||||||
Selling, informational and administrative expenses | 14,804 | (16 | ) | — | — | (299 | ) | 14,489 | ||||||||||||||||
Research and development expenses | 7,683 | 8 | — | — | (38 | ) | 7,653 | |||||||||||||||||
Amortization of intangible assets | 4,758 | (4,565 | ) | — | — | — | 193 | |||||||||||||||||
Restructuring charges and certain acquisition-related costs | 351 | — | (426 | ) | — | 75 | — | |||||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | — | — | ||||||||||||||||||
Other (income)/deductions––net | 1,416 | (138 | ) | 9 | — | (1,796 | ) | (509 | ) | |||||||||||||||
Income from continuing operations before provision/(benefit) for taxes on income | 12,305 | 4,758 | 456 | — | 2,423 | 19,941 | ||||||||||||||||||
Provision/(benefit) for taxes on income(b) | (9,049 | ) | 1,331 | 173 | — | 11,506 | 3,962 | |||||||||||||||||
Income from continuing operations | 21,353 | 3,426 | 283 | — | (9,083 | ) | 15,980 | |||||||||||||||||
Discontinued operations––net of tax | 2 | — | — | (2 | ) | — | — | |||||||||||||||||
Net income attributable to noncontrolling interests | 47 | — | — | — | — | 47 | ||||||||||||||||||
Net income attributable to Pfizer Inc. | 21,308 | 3,426 | 283 | (2 | ) | (9,083 | ) | 15,933 | ||||||||||||||||
Earnings per common share attributable to Pfizer Inc.––diluted | 3.52 | 0.57 | 0.05 | — | (1.50 | ) | 2.63 | |||||||||||||||||
(a) | For details of adjustments, see “Details of Income Statement Items Included in GAAP Reported but Excluded from Non-GAAP Adjusted Income” below. |
(b) | The effective tax rate on Non-GAAP Adjusted income was 15.0% in 2019, 15.4% in 2018 and 19.9% in 2017. The decrease in the effective tax rate on Non-GAAP Adjusted income for 2019, compared with 2018, was primarily due to a favorable change in the jurisdictional mix of earnings as a result of operating fluctuations in the normal course of business, partially offset by a decrease in tax benefits associated with the resolution of certain tax positions pertaining to prior years primarily with various foreign tax authorities. The decrease in the effective tax rate on Non-GAAP Adjusted income for 2018 compared with 2017 was primarily due to tax benefits associated with the December 2017 enactment of the TCJA, a favorable change in the jurisdictional mix of earnings as a result of operating fluctuations in the normal course of business, as well as an increase in benefits associated with the resolution of certain tax positions pertaining to prior years primarily with various foreign tax authorities, and the expiration of certain statutes of limitations. |
2019 Financial Report | 37 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Details of Income Statement Items Included in GAAP Reported but Excluded from Non-GAAP Adjusted Income
Adjusted income, as shown above, excludes the following items: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Purchase accounting adjustments | ||||||||||||
Amortization, depreciation and other(a) | $ | 4,353 | $ | 4,789 | $ | 4,711 | ||||||
Cost of sales | (19 | ) | (3 | ) | 47 | |||||||
Total purchase accounting adjustments—pre-tax | 4,333 | 4,786 | 4,758 | |||||||||
Income taxes(b) | (848 | ) | (915 | ) | (1,331 | ) | ||||||
Total purchase accounting adjustments—net of tax | 3,485 | 3,871 | 3,426 | |||||||||
Acquisition-related costs | ||||||||||||
Restructuring charges/(credits)(c) | (192 | ) | 37 | 105 | ||||||||
Transaction costs(c) | 63 | 1 | 4 | |||||||||
Integration costs and other(c) | 311 | 260 | 317 | |||||||||
Net periodic benefit costs/(credits) other than service costs(d) | — | 7 | (9 | ) | ||||||||
Additional depreciation—asset restructuring(e) | 3 | 12 | 39 | |||||||||
Total acquisition-related costs—pre-tax | 185 | 318 | 456 | |||||||||
Income taxes(f) | (59 | ) | (54 | ) | (173 | ) | ||||||
Total acquisition-related costs—net of tax | 126 | 264 | 283 | |||||||||
Discontinued operations | ||||||||||||
Total discontinued operations—net of tax, attributable to Pfizer Inc.(g) | (4 | ) | (10 | ) | (2 | ) | ||||||
Certain significant items | ||||||||||||
Restructuring charges/(credits)––cost reduction initiatives(h) | 565 | 745 | (75 | ) | ||||||||
Implementation costs and additional depreciation—asset restructuring(i) | 194 | 232 | 279 | |||||||||
Certain legal matters, net(j) | 543 | 157 | 237 | |||||||||
Certain asset impairments(j) | 2,798 | 3,101 | 379 | |||||||||
Business and legal entity alignment costs(k) | 495 | 63 | 71 | |||||||||
Net gains recognized during the period on equity securities(j) | (415 | ) | (586 | ) | (224 | ) | ||||||
(Gain) on completion of Consumer Healthcare JV transaction(l) | (8,086 | ) | — | — | ||||||||
Net losses on early retirement of debt(j) | 138 | 3 | 999 | |||||||||
Other(m) | 1,289 | 4 | 756 | |||||||||
Total certain significant items—pre-tax | (2,481 | ) | 3,719 | 2,423 | ||||||||
Income taxes(n) | (667 | ) | (1,520 | ) | (11,506 | ) | ||||||
Total certain significant items—net of tax | (3,148 | ) | 2,199 | (9,083 | ) | |||||||
Total purchase accounting adjustments, acquisition-related costs, discontinued operations and certain significant items—net of tax, attributable to Pfizer Inc. | $ | 460 | $ | 6,324 | $ | (5,376 | ) | |||||
(a) | Included primarily in Amortization of intangible assets. |
(b) | Included in Provision/(benefit) for taxes on income. Income taxes includes the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying that jurisdiction’s applicable tax rate. Income taxes recorded in 2017 do not reflect any changes associated with the enactment of the TCJA. These changes resulting from the TCJA have been reflected in the line item, Certain significant items “Income taxes”. |
(c) | Included in Restructuring charges and certain acquisition-related costs. Restructuring charges/(credits) include employee termination costs, asset impairments and other exit costs associated with business combinations. Transaction costs represent external costs for banking, legal, accounting and other similar services. Integration costs and other represent external, incremental costs directly related to integrating acquired businesses, such as expenditures for consulting and the integration of systems and processes and certain other qualifying costs. For additional information, see the “Costs and Expenses—Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” section of this Financial Review and Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives. |
(d) | Included in Other (income)/deductions—net. The credits in 2017 included a net settlement gain, partially offset by accelerated amortization of actuarial losses and prior service costs upon the settlement of the remaining obligation associated with the Hospira U.S. qualified defined benefit pension plan. See Notes to Consolidated Financial Statements—Note 11. Pension and Postretirement Benefit Plans and Defined Contribution Plans. |
(e) | In 2019, primarily included in Selling, informational and administrative expenses. In 2018 and 2017, primarily included in Cost of sales. Represents the impact of changes in estimated useful lives of assets involved in restructuring actions related to acquisitions. |
(f) | Included in Provision/(benefit) for taxes on income. Income taxes includes the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying that jurisdiction’s applicable tax rate. 2019 includes the impact of the non-taxable reversal of certain accruals related to our acquisition of Wyeth upon the effective favorable settlement of a U.S. IRS audit for multiple tax years. Income taxes recorded in 2017 do not reflect any changes associated with the December 2017 enactment of the TCJA. These changes resulting from the TCJA have been reflected in Certain significant items “Income taxes”. |
(g) | Included in Discontinued operations––net of tax. For all years presented, represents post-close adjustments. |
38 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
(h) | Amounts relate to employee termination costs, asset impairments and other exit costs not associated with acquisitions, which are included in Restructuring charges and certain acquisition-related costs (see the “Costs and Expenses—Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” section of this Financial Review and Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives). |
(i) | Amounts relate to our cost-reduction/productivity initiatives not related to acquisitions (see Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives). For 2019, included in Cost of sales ($90 million), Selling, informational and administrative expenses ($74 million) and Research and development expenses ($30 million). For 2018, included in Cost of sales ($121 million), Selling, informational and administrative expenses ($72 million) and Research and development expenses ($39 million). For 2017, included in Cost of sales ($170 million), Selling, informational and administrative expenses ($71 million) and Research and development expenses ($38 million). |
(j) | Included in Other (income)/deductions—net (see the Notes to Consolidated Financial Statements—Note 4. Other (Income)/Deductions—Net). |
(k) | In 2019, primarily included in Cost of sales ($15 million), Selling, informational and administrative expenses ($139 million) and Other (income)/deductions––net ($338 million) and represents (i) incremental costs of $350 million associated with the design, planning and implementation of our new organizational structure, effective in the beginning of 2019, and primarily includes consulting, legal, tax and advisory services and (ii) separation costs of $145 million associated with our planned Upjohn transaction with Mylan and mainly includes consulting, legal, tax and advisory services. In full-year 2018, primarily included in Other (income)/deductions––net and mainly represents incremental costs associated with the design, planning and implementation of our new organizational structure, effective in the beginning of 2019, and primarily includes consulting, legal, tax and advisory services. |
(l) | Included in (Gain) on completion of Consumer Healthcare JV transaction (see notes to Consolidated Financial Statements––Note 2C. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Equity-Method Investments and Assets and Liabilities Held for Sale). |
(m) | For 2019, included in Cost of sales ($103 million), Selling, informational and administrative expenses ($96 million), Research and development expenses ($632 million) and Other (income)/deductions––net ($457 million). For 2018, included in Cost of sales ($10 million income), Selling, informational and administrative expenses ($151 million), Research and development expenses ($8 million) and Other (income)/deductions––net ($143 million income). For 2017, included in Cost of sales ($193 million), Selling, informational and administrative expenses ($229 million) and Other (income)/deductions––net ($334 million). For 2019 includes, among other things, (i) an upfront license fee payment of $250 million to Akcea, which was recorded in Research and development expenses, (ii) charges of $112 million recorded in Other (income)/deductions––net representing our pro rata share of primarily restructuring and business combination accounting charges recorded by the Consumer Healthcare joint venture, (iii) a $337 million charge in Research and development expenses related to our acquisition of Therachon, (iv) a $99 million charge in Cost of sales related to rivipansel, primarily for inventory manufactured for expected future sale and (v) charges of $240 million, primarily in Selling, informational and administrative expenses ($87 million) and Other (income)/deductions––net ($152 million), for external incremental costs, such as transaction costs and costs to separate our Consumer Healthcare business into a separate legal entity associated with the formation of the GSK Consumer Healthcare joint venture. For 2018, includes, among other things, (i) a non-cash $343 million pre-tax gain in Other (income)/deductions––net associated with our transaction with Bain Capital to create a new biopharmaceutical company, Cerevel, to continue development of a portfolio of clinical and preclinical stage neuroscience assets primarily targeting disorders of the central nervous system, (ii) a $119 million charge, in the aggregate, in Selling, informational and administrative expenses for a special, one-time bonus paid to virtually all Pfizer colleagues, excluding executives, which was one of several actions taken by us after evaluating the expected positive net impact of the December 2017 enactment of the legislation commonly referred to as the TCJA and (iii) a non-cash $50 million pre-tax gain in Other (income)/deductions––net as a result of the contribution of our allogeneic chimeric antigen receptor T cell therapy development program assets in connection with our contribution agreement entered into with Allogene (see Notes to Consolidated Financial Statements—Note 2B. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Divestitures). For 2017, includes, among other things, (i) a charitable contribution to the Pfizer Foundation of $200 million, which is included in Selling, informational and administrative expenses; (ii) $195 million in inventory losses, overhead costs related to the period in which our Puerto Rico plants were not operational, and incremental costs, all of which resulted from hurricanes in Puerto Rico in 2017 and are included in Cost of sales; (iii) an $81 million loss related to the sale of our former 49% equity share in Hisun Pfizer, which is included in Other (income)/deductions––net; (iv) charges of $55 million in Other (income)/deductions––net representing adjustments to amounts previously recorded to write down the HIS net assets to fair value less costs to sell and (v) a net loss of $30 million related to the sale of our former 40% ownership investment in Teuto, including the extinguishment of a put option for the remaining 60% ownership interest, which is included in Other (income)/deductions––net. |
(n) | Included in Provision/(benefit) for taxes on income. Income taxes includes the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying that jurisdiction’s applicable tax rate. Also included is the effect of certain U.S. tax consequences. The amount in 2019 was favorably impacted by a benefit of $1.4 billion, representing tax and interest, resulting from the favorable settlement of a U.S. IRS audit for multiple tax years, the benefits related to certain tax initiatives associated with the implementation of our new organizational structure, as well as the tax benefit recorded as a result of additional guidance issued by the U.S. Department of Treasury related to the TCJA and unfavorably impacted by the tax expense of approximately $2.7 billion associated with the gain related to the completion of the Consumer Healthcare joint venture transaction with GSK. The amount in 2018 was favorably impacted primarily by tax benefits related to the TCJA, including certain 2018 tax initiatives as well as adjustments to the provisional estimate of the legislation, reported and disclosed within the applicable measurement period, in accordance with guidance issued by the SEC. The amount in 2017 was favorably impacted by tax benefits primarily associated with the remeasurement of deferred tax liabilities, which includes the repatriation tax on deemed repatriated accumulated post-1986 earnings of foreign subsidiaries associated with the TCJA. See Notes to Consolidated Financial Statements—Note 5A. Tax Matters: Taxes on Income from Continuing Operations. |
ANALYSIS OF OPERATING SEGMENT INFORMATION
The following tables and associated notes provide additional information about the performance of each of our two reportable operating segments for the periods presented—Biopharma and Upjohn, and our Consumer Healthcare operating segment through July 31, 2019. For additional information about each operating segment, see the “Overview of Our Performance, Operating Environment, Strategy and Outlook—Our Strategy—Organizing for Growth” section of this Financial Review, Notes to Consolidated Financial Statements—Note 17. Segment, Geographic and Other Revenue Information and the “Commercial Operations” section in Part I, Item 1, “Business” of our 2019 Form 10-K.
As described in Notes to Consolidated Financial Statements—Note 1A. Basis of Presentation and Significant Accounting Policies: Basis of Presentation, acquisitions impacted our results of operations in 2019 and 2017, the contribution of our Consumer Healthcare business to the GSK Consumer Healthcare joint venture impacted our results of operations in 2019 and divestitures impacted our results of operations in 2017.
2019 Financial Report | 39 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
The following tables provide revenue and cost information by reportable operating segment and a reconciliation of that information to our consolidated statements of income: | ||||||||||||||||||||||||
2019 | ||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | Biopharma(a) | Upjohn(a) | Other(b) | Non-GAAP Adjusted(c) | Reconciling Items(d) | GAAP Reported | ||||||||||||||||||
Revenues | $ | 39,419 | $ | 10,233 | $ | 2,098 | $ | 51,750 | $ | — | $ | 51,750 | ||||||||||||
Cost of sales | 7,579 | 1,724 | 727 | 10,030 | 189 | 10,219 | ||||||||||||||||||
% of revenue | 19.2 | % | 16.8 | % | * | 19.4 | % | * | 19.7 | % | ||||||||||||||
Selling, informational and administrative expenses | 7,000 | 1,492 | 5,549 | 14,041 | 309 | 14,350 | ||||||||||||||||||
Research and development expenses | 1,047 | 236 | 6,705 | 7,988 | 661 | 8,650 | ||||||||||||||||||
Amortization of intangible assets | 271 | 1 | — | 271 | 4,339 | 4,610 | ||||||||||||||||||
Restructuring charges and certain acquisition-related costs | — | — | — | — | 747 | 747 | ||||||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | (8,086 | ) | (8,086 | ) | ||||||||||||||||
Other (income)/deductions––net | (993 | ) | (5 | ) | 698 | (300 | ) | 3,878 | 3,578 | |||||||||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income | 24,517 | 6,785 | (11,582 | ) | 19,720 | (2,037 | ) | 17,682 | ||||||||||||||||
See end of tables for notes (a) through (d).
2018 | ||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | Biopharma(a) | Upjohn(a) | Other(b) | Non-GAAP Adjusted(c) | Reconciling Items(d) | GAAP Reported | ||||||||||||||||||
Revenues | $ | 37,558 | $ | 12,484 | $ | 3,605 | $ | 53,647 | $ | — | $ | 53,647 | ||||||||||||
Cost of sales | 7,147 | 1,964 | 2,018 | 11,130 | 118 | 11,248 | ||||||||||||||||||
% of revenue | 19.0 | % | 15.7 | % | * | 20.7 | % | * | 21.0 | % | ||||||||||||||
Selling, informational and administrative expenses | 6,678 | 1,668 | 5,886 | 14,232 | 223 | 14,455 | ||||||||||||||||||
Research and development expenses | 907 | 233 | 6,822 | 7,962 | 43 | 8,006 | ||||||||||||||||||
Amortization of intangible assets | 235 | 1 | 45 | 281 | 4,612 | 4,893 | ||||||||||||||||||
Restructuring charges and certain acquisition-related costs | — | — | — | — | 1,044 | 1,044 | ||||||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | — | — | ||||||||||||||||||
Other (income)/deductions––net | (1,148 | ) | (18 | ) | 499 | (667 | ) | 2,784 | 2,116 | |||||||||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income | 23,738 | 8,636 | (11,666 | ) | 20,709 | (8,823 | ) | 11,885 | ||||||||||||||||
2017 | ||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | Biopharma(a) | Upjohn(a) | Other(b) | Non-GAAP Adjusted(c) | Reconciling Items(d) | GAAP Reported | ||||||||||||||||||
Revenues | $ | 35,530 | $ | 13,447 | $ | 3,569 | $ | 52,546 | $ | — | $ | 52,546 | ||||||||||||
Cost of sales | 7,012 | 1,919 | 1,847 | 10,778 | 449 | 11,228 | ||||||||||||||||||
% of revenue | 19.7 | % | 14.3 | % | * | 20.5 | % | * | 21.4 | % | ||||||||||||||
Selling, informational and administrative expenses | 6,487 | 1,913 | 6,089 | 14,489 | 316 | 14,804 | ||||||||||||||||||
Research and development expenses | 847 | 275 | 6,531 | 7,653 | 31 | 7,683 | ||||||||||||||||||
Amortization of intangible assets | 149 | 1 | 44 | 193 | 4,565 | 4,758 | ||||||||||||||||||
Restructuring charges and certain acquisition-related costs | — | — | — | — | 351 | 351 | ||||||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | — | — | ||||||||||||||||||
Other (income)/deductions––net | (1,159 | ) | (8 | ) | 658 | (509 | ) | 1,925 | 1,416 | |||||||||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income | 22,194 | 9,348 | (11,600 | ) | 19,941 | (7,637 | ) | 12,305 | ||||||||||||||||
* | Indicates calculation not meaningful or result is equal to or greater than 100%. |
(a) | Amounts represent the revenues and costs managed by each of the Biopharma and Upjohn reportable operating segments for the periods presented. The expenses generally include only those costs directly attributable to the operating segment. |
40 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
(b) | Other comprises the revenues and costs included in our Adjusted income components (see footnote (c) below) that are managed outside Biopharma and Upjohn and includes the following: |
2019 | ||||||||||||||||||||
Other Business Activities | ||||||||||||||||||||
(MILLIONS OF DOLLARS) | WRDM(i) | GPD(ii) | Other(iii) | Corporate and Other Unallocated(iv) | Total | |||||||||||||||
Revenues | $ | — | $ | — | $ | 2,098 | $ | — | $ | 2,098 | ||||||||||
Cost of sales | — | 2 | 663 | 62 | 727 | |||||||||||||||
Selling, informational and administrative expenses | 146 | — | 1,218 | 4,185 | 5,549 | |||||||||||||||
Research and development expenses | 2,398 | 3,311 | 89 | 908 | 6,705 | |||||||||||||||
Amortization of intangible assets | — | — | — | — | — | |||||||||||||||
Restructuring charges and certain acquisition-related costs | — | — | — | — | — | |||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | — | |||||||||||||||
Other (income)/deductions––net | (6 | ) | — | — | 704 | 698 | ||||||||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income | (2,538 | ) | (3,313 | ) | 128 | (5,859 | ) | (11,582 | ) | |||||||||||
2018 | ||||||||||||||||||||
Other Business Activities | ||||||||||||||||||||
(MILLIONS OF DOLLARS) | WRDM(i) | GPD(ii) | Other(iii) | Corporate and Other Unallocated(iv) | Total | |||||||||||||||
Revenues | $ | — | $ | — | $ | 3,605 | $ | — | $ | 3,605 | ||||||||||
Cost of sales | — | — | 1,211 | 807 | 2,018 | |||||||||||||||
Selling, informational and administrative expenses | 159 | — | 1,753 | 3,974 | 5,886 | |||||||||||||||
Research and development expenses | 2,319 | 3,359 | 179 | 965 | 6,822 | |||||||||||||||
Amortization of intangible assets | — | — | 45 | — | 45 | |||||||||||||||
Restructuring charges and certain acquisition-related costs | — | — | — | — | — | |||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | — | |||||||||||||||
Other (income)/deductions––net | (127 | ) | (18 | ) | 7 | 637 | 499 | |||||||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income | (2,352 | ) | (3,341 | ) | 410 | (6,383 | ) | (11,666 | ) | |||||||||||
2017 | ||||||||||||||||||||
Other Business Activities | ||||||||||||||||||||
(MILLIONS OF DOLLARS) | WRDM(i) | GPD(ii) | Other(iii) | Corporate and Other Unallocated(iv) | Total | |||||||||||||||
Revenues | $ | — | $ | — | $ | 3,472 | $ | 97 | $ | 3,569 | ||||||||||
Cost of sales | — | 2 | 1,192 | 653 | 1,847 | |||||||||||||||
Selling, informational and administrative expenses | 143 | 1 | 1,741 | 4,204 | 6,089 | |||||||||||||||
Research and development expenses | 2,363 | 3,151 | 176 | 840 | 6,531 | |||||||||||||||
Amortization of intangible assets | — | — | 44 | — | 44 | |||||||||||||||
Restructuring charges and certain acquisition-related costs | — | — | — | — | — | |||||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | — | |||||||||||||||
Other (income)/deductions––net | (49 | ) | (6 | ) | 15 | 698 | 658 | |||||||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income | (2,457 | ) | (3,148 | ) | 304 | (6,299 | ) | (11,600 | ) | |||||||||||
(i) | WRDM—the R&D and Medical expenses managed by our WRDM organization, which is generally responsible for research projects for our Biopharma portfolio until proof-of-concept is achieved and then for transitioning those projects to the GPD organization for possible clinical and commercial development. R&D spending may include upfront and milestone payments for intellectual property rights. The WRDM organization also has responsibility for certain science-based and other platform-services organizations, which provide end-to-end technical expertise and other services to the various R&D projects, as well as the Worldwide Medical and Safety group, which ensures that Pfizer provides all stakeholders––including patients, healthcare providers, pharmacists, payers and health authorities––with complete and up-to-date information on the risks and benefits associated with Pfizer products so that they can make appropriate decisions on how and when to use Pfizer’s medicines. |
2019 Financial Report | 41 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
(ii) | GPD––the costs associated with our GPD organization, which is generally responsible for clinical trials from WRDM in the Biopharma portfolio, including late stage portfolio spend. GPD also provides technical support and other services to Pfizer R&D projects. GPD is responsible for facilitating all regulatory submissions and interactions with regulatory agencies. |
(iii) | Other—the operating results of our Consumer Healthcare business, through July 31, 2019, and costs associated with other commercial activities not managed as part of Biopharma or Upjohn, including all strategy, business development, portfolio management and valuation capabilities, which previously had been reported in various parts of the organization. |
(iv) | Corporate and Other Unallocated—the costs associated with platform functions (such as worldwide technology, global real estate operations, legal, finance, human resources, worldwide public affairs, compliance and worldwide procurement), patient advocacy activities and certain compensation and other corporate costs, such as interest income and expense, and gains and losses on investments, as well as overhead expenses associated with our manufacturing (which include manufacturing variances associated with production) and commercial operations that are not directly assessed to an operating segment, as business unit (segment) management does not manage these costs. |
We recognized the following amounts in Cost of sales related to forward-exchange contracts designated as cash flow hedges of a portion of our foreign exchange-denominated forecasted intercompany inventory sales:
• | a $247 million net gain in 2019; |
• | a $13 million net loss in 2018; and |
• | a $52 million net gain in 2017. |
For additional information, see Notes to Consolidated Financial Statements––Note 7F. Financial Instruments: Derivative Financial Instruments and Hedging Activities.
For information purposes only, the following tables present reconciliations of the Biopharma segment operating results and Upjohn segment operating results to Biopharma and Upjohn operating results including estimated Other costs generally associated with the Biopharma and Upjohn operating segments for 2019. While we do not manage our segments or have performance goals under such an allocated manner, we believe that some investors may find this information useful in their analyses.
The estimated Other costs generally associated with our operating segments do not purport to reflect the additional amounts that each of our operating segments would have incurred had each segment operated as a standalone company during the period presented.
For information purposes only, for 2019, we estimate that Other costs attributable to our Biopharma and Upjohn segments, as described above, for combined WRDM, GPD and other business activities costs are $6.4 billion, and combined Corporate and Other Unallocated costs are $4.8 billion, which excludes income and costs associated with our Consumer Healthcare business. The combined Corporate and Other Unallocated costs also exclude (i) net interest-related expense not attributable to an operating segment included in Corporate (approximately $1.4 billion in Other (income)/deductions––net); and (ii) net income from investments and other assets not attributable to an operating segment included in Corporate (approximately $318 million in Other (income)/deductions––net). The remaining costs have been attributed to our Biopharma and Upjohn operating segments, as follows:
2019 | ||||||||||||||||
Estimated Other Costs Associated with Biopharma(ii) | ||||||||||||||||
(MILLIONS OF DOLLARS) | Biopharma Non-GAAP Adjusted(i), (iii) | Estimated WRDM/GPD/Other Business Activities(ii) | Estimated Corporate/Other Unallocated(ii) | Biopharma with Estimated Other Costs Associated with Biopharma Non-GAAP Adjusted(ii), (iii) | ||||||||||||
Revenues | $ | 39,419 | $ | — | $ | — | $ | 39,419 | ||||||||
Cost of sales | 7,579 | 2 | 55 | 7,635 | ||||||||||||
Selling, informational and administrative expenses | 7,000 | 611 | 3,268 | 10,879 | ||||||||||||
Research and development expenses | 1,047 | 5,721 | 873 | 7,640 | ||||||||||||
Amortization of intangible assets | 271 | — | — | 271 | ||||||||||||
Restructuring charges and certain acquisition-related costs | — | — | — | — | ||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | ||||||||||||
Other (income)/deductions––net | (993 | ) | (5 | ) | (275 | ) | (1,273 | ) | ||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income | 24,517 | (6,329 | ) | (3,921 | ) | 14,267 | ||||||||||
42 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
2019 | ||||||||||||||||
Estimated Other Costs Associated with Upjohn(ii) | ||||||||||||||||
(MILLIONS OF DOLLARS) | Upjohn Non-GAAP Adjusted(i), (iii) | Estimated WRDM/GPD/Other Business Activities(ii) | Estimated Corporate/Other Unallocated(ii) | Upjohn with Estimated Other Costs Associated with Upjohn Non-GAAP Adjusted(ii), (iii) | ||||||||||||
Revenues | $ | 10,233 | $ | — | $ | — | $ | 10,233 | ||||||||
Cost of sales | 1,724 | — | (14 | ) | 1,710 | |||||||||||
Selling, informational and administrative expenses | 1,492 | 34 | 753 | 2,280 | ||||||||||||
Research and development expenses | 236 | 5 | 21 | 262 | ||||||||||||
Amortization of intangible assets | 1 | — | — | 1 | ||||||||||||
Restructuring charges and certain acquisition-related costs | — | — | — | — | ||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | — | — | — | — | ||||||||||||
Other (income)/deductions––net | (5 | ) | — | (46 | ) | (51 | ) | |||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income | 6,785 | (39 | ) | (714 | ) | 6,031 | ||||||||||
(i) | Amount represents the revenues and costs managed by each of our operating segments. The expenses generally include only those costs directly attributable to the operating segment. See note (a) above for more information. |
(ii) | Represents costs not assessed to an operating segment, as business unit (segment) management does not manage these costs. For a description of these other costs and business activities, see note (b) above. |
• | WRDM/GPD/Other Business Activities––The information provided for WRDM, GPD and Other Business Activities was substantially all derived from our estimates of the costs incurred in connection with the R&D projects associated with the Biopharma and Upjohn operating segment. |
• | Corporate/Other Unallocated––The information provided for Corporate and Other Unallocated was derived mainly using proportional allocation methods based on global, regional or country revenues or global, regional or country headcount, as well as certain cost metrics, as appropriate, such as those derived from R&D and manufacturing costs, and, to a lesser extent, specific identification and estimates. Management believes that the allocations of Corporate and Other Unallocated costs are reasonable. |
The estimated Other costs generally associated with our Biopharma and Upjohn operating segments do not purport to reflect the additional amounts that each of our operating segments would have incurred had each segment operated as a standalone company during the period presented.
(iii) | See note (c) below for an explanation of our Non-GAAP Adjusted financial measure. |
(c) | See the “Non-GAAP Financial Measure (Adjusted Income)” section of this Financial Review for a definition of these “Adjusted Income” components. |
(d) | Includes costs associated with (i) purchase accounting adjustments; (ii) acquisition-related costs; and (iii) certain significant items, which are substantive and/or unusual, and in some cases recurring, items (such as gains on the completion of joint venture transactions, restructuring charges, legal charges or net gains and losses on investment in equity securities), that are evaluated on an individual basis by management. For additional information about these reconciling items and/or our Non-GAAP adjusted measure of performance, see the “Non-GAAP Financial Measure (Adjusted Income)” section of this Financial Review. |
Biopharma Operating Segment
2019 vs. 2018
Biopharma Revenues increased $1.9 billion, or 5%, to $39.4 billion in 2019 from $37.6 billion in 2018, reflecting an operational increase of $2.9 billion, or 8%, and an unfavorable impact of foreign exchange of $1.0 billion, or 3%.
The following graph illustrates the components of the net increase in Biopharma Revenues:

* | LOE generally pertains to period-over-period revenue impacts for products across our portfolios experiencing patent expirations or loss of regulatory exclusivity in certain developed markets. |
2019 Financial Report | 43 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
The following provides an analysis of the increase in Biopharma worldwide Revenues:
(MILLIONS OF DOLLARS) | ||||
Biopharma Revenues, 2018 | $ | 37,558 | ||
Operational growth/(decline): | ||||
Continued growth from certain key brands(a) | 2,495 | |||
Higher revenue from continued growth of anti-infective products in China, driven by increased demand for Sulperazon and new launches, the 2018 U.S. launches of our immune globulin intravenous products (Panzyga and Octagam) and the launches of certain anti-infectives products (Zavicefta, Zinforo and Cresemba) in international developed and emerging markets, all in the Hospital products business | 472 | |||
Higher revenues for Inlyta, primarily in the U.S. driven by increased demand resulting from the second quarter of 2019 U.S. FDA approvals for the combinations of certain immune checkpoint inhibitors plus Inlyta for the first-line treatment of patients with advanced RCC | 190 | |||
Growth from Biosimilars, primarily in the U.S. | 168 | |||
Higher revenues for rare disease products driven by the U.S. launches in May 2019 of Vyndaqel and in September 2019 of Vyndamax, for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM); and in international markets, primarily driven by continued uptake for the transthyretin amyloid polyneuropathy indication, primarily in developed Europe, as well as the March 2019 launch of the ATTR-CM indication in Japan, partially offset by lower revenues for certain rare disease products, including the hemophilia franchises (Refacto AF/Xyntha and BeneFIX), primarily due to competitive pressures, and Genotropin in developed markets, mainly due to unfavorable channel mix in the U.S. | 159 | |||
Lower revenues from other Hospital products, primarily reflecting declines in developed markets, mostly due to the continued expected negative impact from generic competition for products that have previously lost marketing exclusivity | (447 | ) | ||
Lower revenues for Enbrel internationally, reflecting continued biosimilar competition in most developed Europe markets | (292 | ) | ||
Other operational factors, net | 150 | |||
Operational growth, net | 2,894 | |||
Unfavorable impact of foreign exchange | (1,033 | ) | ||
Biopharma Revenues increase | 1,862 | |||
Biopharma Revenues, 2019 | $ | 39,419 | ||
(a) | Certain key brands represent Ibrance, Eliquis, Xeljanz and Prevnar 13/Prevenar 13. See the “Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion" section of this Financial Review for product analysis information. |
Total Biopharma revenues from emerging markets increased $539 million, or 7%, to $8.2 billion in 2019 from $7.7 billion in 2018, reflecting a 14% operational increase. Foreign exchange had an unfavorable impact of 7% on total Biopharma revenues from emerging markets. The operational increase in emerging markets was primarily driven by Prevenar 13, Ibrance and Eliquis.
Costs and Expenses
• | Cost of sales as a percentage of Revenues was relatively flat. |
• | The increase in Cost of sales of 6% was mainly driven by an unfavorable change in product mix, an increase in royalty expenses based on the mix of products sold, and an increase in sales volumes for various products within our product portfolio, partially offset by a favorable impact of foreign exchange. |
• | The increase in Selling, informational and administrative expenses of 5% was mostly driven by additional investment in emerging markets, the Oncology portfolio in developed markets, and for marketing and promotional expenses associated with the U.S. launches of Vyndaqel in May 2019 and Vyndamax in September 2019, as well as an increase in healthcare reform expenses, partially offset by a favorable impact of foreign exchange. |
• | The increase in Research and development expenses of 15% was mainly related to the Array acquisition, as well as an increase in medical spend for new and growing products. |
• | The unfavorable change in Other (income)/deductions––net primarily reflects a $246 million decrease in income from collaborations, out-licensing arrangements and sales of compound/product rights and a $33 million decrease in dividend income from our investment in ViiV, partially offset by an increase in royalty-related income mainly due to a one-time favorable resolution in the second quarter of 2019 of a legal dispute for $82 million, as well as a favorable impact of foreign exchange. |
2018 vs. 2017
Biopharma Revenues increased $2.0 billion, or 6%, to $37.6 billion in 2018 from $35.5 billion in 2017, reflecting an operational increase of $1.9 billion, or 5%, and a favorable impact of foreign exchange of $168 million, or 1%.
44 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
The following graph illustrates the components of the net increase in Biopharma Revenues:
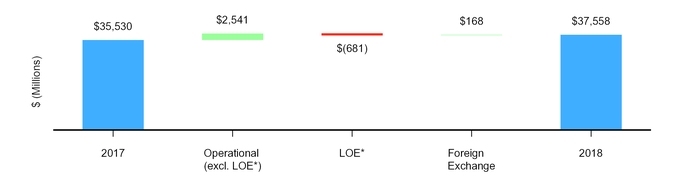
* | LOE generally pertains to period-over-period revenue impacts for products across our portfolios experiencing patent expirations or loss of regulatory exclusivity in certain developed markets. |
The following provides an analysis of the increase in Biopharma Revenues:
(MILLIONS OF DOLLARS) | ||||
Biopharma Revenues, 2017 | $ | 35,530 | ||
Operational growth/(decline): | ||||
Continued growth from certain key brands(a) | 2,715 | |||
Growth from Biosimilars, primarily from Inflectra in certain channels in the U.S. and developed Europe markets | 217 | |||
Growth from recently launched products, including Eucrisa in the U.S., as well as Besponsa and Bavencio, primarily in the U.S. and developed Europe | 195 | |||
Decline in the Hospital products business, driven by lower revenues in developed markets, primarily due to increased competition across the portfolio and continued legacy Hospira product shortages in the U.S., partially offset by an increase in emerging markets, primarily in China | (482 | ) | ||
Lower revenues for Enbrel, primarily in most developed Europe markets due to continued biosimilar competition | (350 | ) | ||
Lower revenues for the Premarin family of products and Pristiq primarily driven by generic competition in the U.S. | (241 | ) | ||
Lower revenues from the hemophilia portfolio (BeneFIX and Refacto AF/Xyntha), primarily in developed Europe | (100 | ) | ||
Other operational factors, net | (93 | ) | ||
Operational growth, net | 1,860 | |||
Favorable impact of foreign exchange | 168 | |||
Biopharma Revenues increase | 2,028 | |||
Biopharma Revenues, 2018 | $ | 37,558 | ||
(a) | Certain key brands represent Ibrance, Eliquis, Xeljanz, Prevnar 13/Prevenar 13, Xtandi and Chantix/Champix. |
Total Biopharma revenues from emerging markets increased $878 million, or 13%, to $7.7 billion in 2018 from $6.8 billion in 2017, reflecting a 16% operational increase. Foreign exchange had an unfavorable impact of 3% on total Biopharma revenues from emerging markets. The operational increase in emerging markets was primarily driven by Prevenar 13, Sulperazon, Ibrance and Eliquis.
Costs and Expenses
• | Cost of sales as a percentage of Revenues decreased 0.7 percentage points mainly driven by a favorable change in product mix, which includes an increase in alliance revenue which has no associated cost of sales, and a favorable impact of foreign exchange, partially offset by an increase in royalty expenses based on the mix of products sold. |
• | The increase in Cost of sales of 2% was mostly driven by an increase in royalty expenses based on the mix of products sold, an unfavorable change in product mix, and an increase in sales volumes for various products within our product portfolio, partially offset by a favorable impact of foreign exchange. |
• | The increase in Selling, informational and administrative expenses of 3% was mainly driven by additional investment across several of our products, primarily Xeljanz, Ibrance, Eucrisa and Prevnar 13/Prevenar 13 (pediatric indication), partially offset by lower marketing, advertising and promotion expenses, reflecting the benefits of cost-reduction and productivity initiatives. |
• | The increase in Research and development expenses of 7% was driven by an increase in medical spend, mostly in Oncology (including Ibrance, as well as investments for new global capabilities) and expenses associated with the creation of new business units, as well as support for recently launched assets (including Talzenna), partially offset by a decrease in spend on products transferred from EH to Internal Medicine and on Hospital products. |
• | Other (income)/deductions––net was relatively unchanged. |
2019 Financial Report | 45 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Upjohn Operating Segment
2019 vs. 2018
Upjohn Revenues decreased $2.3 billion, or 18%, to $10.2 billion in 2019 from $12.5 billion in 2018, reflecting an operational decrease of $2.0 billion, or 16%, and an unfavorable impact of foreign exchange of $249 million, or 2%.
The following graph illustrates the components of the decrease in Upjohn Revenues:

* | LOE generally pertains to period-over-period revenue impacts for products across our portfolios experiencing patent expirations or loss of regulatory exclusivity in certain developed markets. |
The following provides an analysis of the decrease in worldwide Upjohn Revenues:
(MILLIONS OF DOLLARS) | ||||
Upjohn Revenues, 2018 | $ | 12,484 | ||
Operational growth/(decline): | ||||
Lower worldwide revenues for Lyrica, primarily in the U.S., reflecting the expected significantly lower volumes associated with multi-source generic competition that began in July 2019 | (1,628 | ) | ||
Lower revenues for Viagra and Upjohn's authorized generic for Viagra in the U.S. resulting from increased generic competition following Viagra's December 2017 patent expiration, partially offset by increased retail demand growth in China | (171 | ) | ||
Decline in revenues for Revatio driven by lower U.S. Oral Suspension formulation sales and pricing pressures due to a recent generic entry, and for Relpax, driven by continued generic competition across developed markets | (149 | ) | ||
Decline in Norvasc and Lipitor due to pricing pressures from the implementation of the VBP in certain cities in China and lower volumes in Japan, partially offset by overall increased demand in China in the first quarter of 2019 and continued geographic expansion in China during the second half of 2019 in provinces where the VBP had not yet been implemented | (40 | ) | ||
Volume-driven growth from Celebrex and Effexor, primarily in Japan and China | 78 | |||
Other operational factors, net | (92 | ) | ||
Operational decline, net | (2,002 | ) | ||
Unfavorable impact of foreign exchange | (249 | ) | ||
Upjohn Revenues decrease | (2,251 | ) | ||
Upjohn Revenues, 2019 | $ | 10,233 | ||
Total Upjohn revenues from emerging markets decreased $128 million, or 3%, to $3.9 billion in 2019 from $4.0 billion in 2018, reflecting 1% operational growth more than offset by the unfavorable impact of foreign exchange of 5% on total Upjohn revenues from emerging markets. The operational increase in emerging markets was primarily driven by Zoloft, Viagra, Celebrex and Lipitor.
Costs and Expenses
• | Cost of sales as a percentage of Revenues increased 1.1 percentage points, driven by lower Lyrica revenues in developed markets, primarily in the U.S. due to multi-source generic competition that began in July 2019, partially offset by lower royalty expense for Lyrica due to the patent expiration. |
• | The decrease in Cost of sales of 12% was mainly driven by lower royalty expense due to the Lyrica patent expiration and multi-source generic competition that began in July 2019, as well as a favorable impact of foreign exchange. |
• | Selling, informational and administrative expenses decreased 11% driven by a reduction in field force expense as well as advertising and promotion expenses in developed markets, primarily related to Lyrica in the U.S., as well as a favorable impact of foreign exchange, partially offset by the non-recurrence of one-time general and administrative expense reversals in the second and third quarters of 2018, and investments in China across key brands. |
• | Research and development expenses and Other (income)/deductions––net were relatively unchanged. |
2018 vs. 2017
Upjohn Revenues decreased $963 million, or 7%, to $12.5 billion in 2018 from $13.4 billion in 2017, reflecting an operational decrease of $1.1 billion, or 8%, and a favorable impact from foreign exchange of $116 million, or 1%.
46 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
The following graph illustrates the components of the net decrease in Upjohn Revenues:

* | LOE generally pertains to period-over-period revenue impacts for products across our portfolios experiencing patent expirations or loss of regulatory exclusivity in certain developed markets. |
The following provides an analysis of the decrease in Upjohn Revenues:
(MILLIONS OF DOLLARS) | ||||
Upjohn Revenues, 2017 | $ | 13,447 | ||
Operational growth/(decline): | ||||
Lower revenues for Viagra in the U.S. resulting from the loss of exclusivity in December 2017 | (572 | ) | ||
Decline in Greenstone, Upjohn's solid oral dose generics subsidiary, due to additional generic competition in the U.S. and decline in Relpax, primarily due to loss of exclusivity in the U.S. | (318 | ) | ||
Decline in revenues for Lyrica, primarily driven by losses of exclusivity in developed Europe markets and Australia, partially offset by growth in the U.S. and growth in the orally dissolving tablet formulation in Japan | (115 | ) | ||
Decline in revenues for Celebrex, primarily driven by the non-recurrence of favorable U.S. rebates that occurred in 2017 and lower volumes in the U.S. | (99 | ) | ||
Higher revenues for Lipitor and Norvasc primarily due to increased demand in China, partially offset by pricing pressures in China and lower volumes in Japan for Lipitor and Norvasc and the non-recurrence of favorable U.S. rebates for Lipitor that occurred in 2017 | 182 | |||
Other operational factors, net | (158 | ) | ||
Operational decline, net | (1,079 | ) | ||
Favorable impact of foreign exchange | 116 | |||
Upjohn Revenues decrease | (963 | ) | ||
Upjohn Revenues, 2018 | $ | 12,484 | ||
Total Upjohn revenues from emerging markets increased $352 million, or 10%, to $4.0 billion in 2018 from $3.7 billion in 2017, reflecting a 9% operational increase. Foreign exchange had a favorable impact of 1%. The operational increase in emerging markets was primarily driven by Lipitor and Norvasc growth in China.
Costs and Expenses
• | Cost of sales as a percentage of Revenues increased 1.5 percentage points driven by lower revenues for Viagra in the U.S. resulting from the loss of exclusivity in December 2017, as well as increased manufacturing plant costs in Puerto Rico due to hurricane-related recovery expenses, partially offset by a favorable impact of foreign exchange. |
• | The increase in Cost of sales of 2% was mostly driven by higher sales volume of Lyrica in the U.S. and Japan, as well as key products in China, partially offset by a favorable impact of foreign exchange. |
• | Selling, informational and administrative expenses decreased 13%, primarily driven by a reduction in field force expense as well as advertising and promotion expenses in developed markets, mainly related to Viagra and Lyrica in the U.S., as well as one-time general and administrative expense reversals in the second and third quarters of 2018, partially offset by additional investments in China across key brands. |
• | Research and development expenses decreased 15% mostly due to decreased spending for post-approval commitment activities for Lyrica and Celebrex. |
• | Other (income)/deductions––net were relatively unchanged. |
2019 Financial Report | 47 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
ANALYSIS OF THE CONSOLIDATED STATEMENTS OF CASH FLOWS
Year Ended December 31, | % Change | |||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 19/18 | 18/17 | |||||||||||||
Cash provided by/(used in): | ||||||||||||||||||
Operating activities | $ | 12,588 | $ | 15,827 | $ | 16,802 | (20 | ) | (6 | ) | ||||||||
Investing activities | (3,945 | ) | 4,525 | (4,740 | ) | * | * | |||||||||||
Financing activities | (8,485 | ) | (20,441 | ) | (13,350 | ) | (58 | ) | 53 | |||||||||
Effect of exchange-rate changes on cash and cash equivalents and restricted cash and cash equivalents | (32 | ) | (116 | ) | 53 | (73 | ) | * | ||||||||||
Net increase/(decrease) in Cash and cash equivalents and restricted cash and cash equivalents | $ | 125 | $ | (205 | ) | $ | (1,235 | ) | * | (83 | ) | |||||||
* | Indicates calculation not meaningful or result is equal to or greater than 100%. |
In the consolidated statements of cash flows, the line item, Other changes in assets and liabilities, net of acquisitions and divestitures, is presented excluding the effects of changes in foreign currency exchange rates, as these changes do not reflect actual cash inflows or outflows, and excluding any other significant non-cash movements. Accordingly, the amounts shown will not necessarily agree with the changes in the assets and liabilities that are presented in our consolidated balance sheets.
Operating Activities
2019 v. 2018
Our net cash provided by operating activities was $12.6 billion in 2019, compared to $15.8 billion in 2018. The decrease in net cash provided by operating activities reflects the timing of receipts from customers and payments to vendors in the ordinary course of business, the upfront cash payment associated with our acquisition of Therachon, and the upfront cash payment associated with our licensing agreement with Akcea (see Notes to Consolidated Financial Statements––Note 2A. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Acquisitions), partially offset by an increase in net income and a decrease in benefit plan contributions.
In 2019, the change in the line item Other adjustments, net primarily reflects, among other items:
• | the non-recurrence of a non-cash gain associated with our transaction with Bain Capital to create a new biopharmaceutical company, Cerevel, to continue development of a portfolio of clinical and pre-clinical stage neuroscience assets in 2018 (see Notes to Consolidated Financial Statements––Note 2A. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Acquisitions); and |
• | the non-recurrence of a non-cash gain on the contribution of Pfizer’s allogeneic CAR T developmental program assets, in connection with our contribution agreement with Allogene in 2018 (see Notes to Consolidated Financial Statements––Note 2B. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Divestitures), |
partially offset by:
• | net gains on foreign exchange contracts hedging a portion of our forecasted intercompany inventory sales (that fixes the cost of inventory sold later to customers). |
In 2019 and 2018, the line item Other changes in assets and liabilities, net of acquisitions and divestitures, primarily reflects changes, in the normal course of business, in trade accounts receivable, inventories, other current assets, other noncurrent assets, trade accounts payable, accrued compensation, other current and noncurrent liabilities, as well as in 2019, the adjustment necessary to reflect the non-cash nature of a favorable settlement of a U.S. IRS audit for multiple tax years (see Notes to Consolidated Financial Statements––Note 5A. Tax Matters: Taxes on Income from Continuing Operations).
2018 v. 2017
Our net cash provided by operating activities was $15.8 billion in 2018, compared to $16.8 billion in 2017. The decrease in net cash provided by operating activities reflects a decrease in net cash generated from net income. The net cash generated reflects the timing of receipts from customers and payments to vendors in the ordinary course of business.
In 2018, the change in the line item Other adjustments, net primarily reflects, among other items:
• | the non-recurrence of a non-cash net loss on early retirement of debt under an exchange offer in 2017; |
• | unrealized net gains on equity securities resulting from the adoption of a new accounting standard on January 1, 2018, related to the recognition and measurement of financial assets and liabilities; |
• | a decrease in debt extinguishment costs in 2018 related to early retirement of debt under an exchange offer in 2017, which had been reclassified from operating to financing activities in 2018 and 2017 in accordance with our implementation of a new accounting standard on January 1, 2018 related to the classification of debt prepayment and extinguishment costs; |
• | a non-cash gain associated with our transaction with Bain Capital to create a new biopharmaceutical company to continue development of a portfolio of clinical and preclinical stage neuroscience assets (see Notes to Consolidated Financial Statements––Note 2B. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Divestitures); and |
48 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | a non-cash gain on the contribution of Pfizer’s allogeneic CAR T developmental program assets, in connection with our contribution agreement with Allogene (see Notes to Consolidated Financial Statements—Note 2B. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Divestitures), |
partially offset by:
• | decreases in net realized gains on sales of investments in debt and equity securities; |
• | net losses on foreign exchange contracts hedging a portion of our forecasted intercompany inventory sales (that fixes the cost of inventory later sold to customers); and |
• | a decrease in gains on the sale of property, plant and equipment. |
In 2018 and 2017, the line item Other changes in assets and liabilities, net of acquisitions and divestitures, primarily reflects changes, in the normal course of business, in trade accounts receivable, inventories, other current assets, other noncurrent assets, trade accounts payable, accrued compensation and other current and noncurrent liabilities.
Investing Activities
2019 v. 2018
Our net cash used in investing activities was $3.9 billion in 2019, compared to net cash provided by investing activities of $4.5 billion in 2018. The change in net cash used in/provided by investing activities was primarily attributable to:
• | cash used for the acquisition of Array, net of cash acquired, of $10.9 billion in 2019, |
partially offset by:
• | an increase in net proceeds generated from the sale of investments of $2.9 billion for cash needs, including financing the acquisition of Array (see Notes to Consolidated Financial Statements––Note 2A. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Acquisitions). |
2018 v. 2017
Our net cash provided by investing activities was $4.5 billion in 2018, compared to net cash used in investing activities of $4.7 billion in 2017. The change in net cash provided by/(used in) investing activities was primarily attributable to:
• | an increase in net proceeds generated from the sale of investments of $8.6 billion in 2018 for cash needs; and |
• | a decrease in cash used for acquisitions, net of cash acquired of $1.0 billion due to the acquisition of the development and commercialization rights to AstraZeneca’s small molecule anti-infectives business and substantially all of the remaining consideration for the Medivation acquisition in 2017 (see Notes to Consolidated Financial Statements—Note 2A. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Acquisitions). |
Financing Activities
2019 v. 2018
Our net cash used in financing activities was $8.5 billion in 2019, compared to $20.4 billion in 2018. The decrease in net cash used in financing activities was primarily attributable to:
• | $10.6 billion net proceeds raised from short-term borrowings in 2019, primarily in connection with the acquisition of Array, compared to net payments on short-term borrowings of $2.3 billion in 2018; and |
• | lower purchases of common stock of $3.3 billion, |
partially offset by:
• | higher repayments on long-term debt of $3.2 billion; and |
• | lower proceeds from the exercise of stock options of $864 million. |
2018 v. 2017
Our net cash used in financing activities was $20.4 billion in 2018, compared to $13.3 billion in 2017. The increase in net cash used in financing activities was primarily attributable to:
• | $2.3 billion less proceeds raised from short-term borrowings in 2018, compared to 2017; and |
• | higher purchases of common stock of $7.2 billion, |
partially offset by:
• | lower repayments on long-term debt of $2.6 billion. |
ANALYSIS OF FINANCIAL CONDITION, LIQUIDITY AND CAPITAL RESOURCES
We rely largely on operating cash flows, short-term investments, short-term commercial paper borrowings and long-term debt to provide for our liquidity requirements. We continue our efforts to improve cash inflows through working capital efficiencies. We target specific areas of focus including accounts receivable, inventories, accounts payable, and other working capital, which allows us to optimize our operating cash flows.
2019 Financial Report | 49 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Due to our significant operating cash flows as well as our financial assets, access to capital markets and available lines of credit and revolving credit agreements, we believe that we have, and will maintain, the ability to meet our liquidity needs for the foreseeable future, which include:
• | the working capital requirements of our operations, including our R&D activities; |
• | investments in our business; |
• | dividend payments and potential increases in the dividend rate; |
• | share repurchases; |
• | the cash requirements associated with our cost-reduction/productivity initiatives; |
• | paying down outstanding debt; |
• | contributions to our pension and postretirement plans; and |
• | business-development activities. |
Our long-term debt is rated high-quality by both S&P and Moody’s. See the “Credit Ratings” section below. As market conditions change, we continue to monitor our liquidity position. We have taken and will continue to take a conservative approach to our financial investments. Both short-term and long-term investments consist primarily of high-quality, highly liquid, well-diversified available-for-sale debt securities.
Selected Measures of Liquidity and Capital Resources
The following table provides certain relevant measures of our liquidity and capital resources: | ||||||||
As of December 31, | ||||||||
(MILLIONS OF DOLLARS, EXCEPT RATIOS AND PER COMMON SHARE DATA) | 2019 | 2018 | ||||||
Selected financial assets(a): | ||||||||
Cash and cash equivalents | $ | 1,305 | $ | 1,139 | ||||
Short-term investments | 8,525 | 17,694 | ||||||
Long-term investments, excluding private equity securities at cost | 2,258 | 1,823 | ||||||
12,088 | 20,656 | |||||||
Debt: | ||||||||
Short-term borrowings, including current portion of long-term debt | 16,195 | 8,831 | ||||||
Long-term debt | 35,955 | 32,909 | ||||||
52,150 | 41,740 | |||||||
Selected net financial liabilities(b) | $ | (40,062 | ) | $ | (21,084 | ) | ||
Working capital(c) | $ | (4,501 | ) | $ | 18,068 | |||
Ratio of current assets to current liabilities | 0.88:1 | 1.57:1 | ||||||
Total Pfizer Inc. shareholders’ equity per common share(d) | $ | 11.41 | $ | 11.09 | ||||
(a) | See Notes to Consolidated Financial Statements––Note 7. Financial Instruments for a description of certain assets held and for a description of credit risk related to our financial instruments held. |
(b) | The increase in selected net financial liabilities was primarily driven by a decrease in short-term investments and net increase in short-term debt, mainly as a result of cash paid for the acquisition of Array (see Notes to Consolidated Financial Statements––Note 2A. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Acquisitions). We retain a strong financial liquidity position as a result of our net cash provided by operating activities, our high-quality financial asset portfolio and access to capital markets. For additional information, see the “Credit Ratings” section of this Financial Review. |
(c) | The decrease in working capital was primarily due to: |
• | financing requirements for the acquisition of Array, share repurchase activities, dividend payments, capital expenditures and debt repayment, partially offset by operating cash flow generation, cash from employee stock option exercises and the March 2019 long-term debt issuance discussed below; |
• | the impact of the deconsolidation of the Consumer Healthcare business as a result of the completion of the joint venture transaction; and |
• | the net impact of foreign currency exchange, |
partially offset by:
• | the timing of accruals, cash receipts and payments in the ordinary course of business; and |
• | an increase in inventory for certain products, including inventory build for new product launches, supply recovery, and market demand, partially offset by a charge related to rivipansel, primarily for inventory manufactured for expected future sale (see Notes to Consolidated Financial Statements—Note 2E. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Research and Development and Collaborative Arrangements for additional information). |
(d) | Represents total Pfizer Inc. shareholders’ equity divided by the actual number of common shares outstanding (which excludes treasury stock). |
On February 11, 2020, we issued a notice for the redemption in full of all $1.065 billion principal amount of senior unsecured notes due 2047. The notes will be redeemed on March 17, 2020 at par as set forth in the indenture agreement. We do not expect this redemption to have a material impact on our consolidated financial statements.
In March 2019, we completed a public offering of $5.0 billion aggregate principal amount of senior unsecured notes with a weighted average effective interest rate of 3.57% (see Notes to Consolidated Financial Statements––Note 7D. Financial Instruments: Long-Term Debt).
For additional information about the sources and uses of our funds, see the “Analysis of the Consolidated Statements of Cash Flows” section of this Financial Review.
50 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Domestic and International Selected Financial Assets
Many of our operations are conducted outside the U.S., and significant portions of our selected financial assets are held internationally. The amount of funds held in U.S. tax jurisdictions can fluctuate due to the timing of receipts and payments in the ordinary course of business and due to other reasons, such as business-development activities. As part of our ongoing liquidity assessments, we regularly monitor the mix of domestic and international cash flows (both inflows and outflows). The changes in tax law under the TCJA, which includes transitioning U.S. international taxation from a worldwide tax system to a territorial tax system, will also allow us to more easily access our selected financial assets globally. The majority of our cash we held internationally as of year-end 2017 was repatriated in 2018.
Agreement to Combine Upjohn with Mylan
In connection with the recently-announced agreement to combine Upjohn with Mylan discussed in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Business Development Initiatives” section of this Financial Review, Upjohn will incur approximately $12 billion of debt prior to the closing of the transaction. Immediately prior to the separation, Upjohn will make a cash distribution of $12 billion to Pfizer, which will be funded by the proceeds of such debt. Following the separation, Upjohn will remain the obligor with respect to the debt.
Credit Ratings
Two major corporate debt-rating organizations, Moody’s and S&P, assign ratings to our short-term and long-term debt. A security rating is not a recommendation to buy, sell or hold securities and the rating is subject to revision or withdrawal at any time by the rating organization. Each rating should be evaluated independently of any other rating.
In June 2019, S&P placed Pfizer on “CreditWatch Negative” following the announcement of Pfizer’s intention to acquire Array. The CreditWatch placement was resolved with a one-notch downgrade of Pfizer’s debt rating to ‘AA-’ upon the consummation of the transaction. In July 2019, we announced that we entered into a definitive agreement to combine Upjohn with Mylan, which resulted in actions from both Moody’s and S&P. Moody’s placed Pfizer’s long-term rating under review for downgrade (limited to one-notch, or ‘A2’ upon close of the Mylan transaction) while S&P lowered Pfizer’s rating to ‘AA-’ (as a result of the Array transaction) and confirmed it will still remain on CreditWatch Negative (with the expectation the rating will be lowered one additional notch to ‘A+’ upon close of the Mylan transaction).
The following table provides the current ratings assigned by these rating agencies to our commercial paper and senior unsecured long-term debt: | ||||||||
NAME OF RATING AGENCY | Pfizer Commercial Paper | Pfizer Long-Term Debt | Outlook | Date of Last Rating Change | ||||
Rating | Rating | |||||||
Moody’s | P-1 | A1 | Under Review for Downgrade | October 2009 | ||||
S&P | A-1+ | AA- | CreditWatch Negative | July 2019 | ||||
Debt Capacity––Lines of Credit
We have available lines of credit and revolving credit agreements with a group of banks and other financial intermediaries. We typically maintain cash and cash equivalent balances and short-term investments which, together with our available revolving credit facilities, are in excess of our commercial paper and other short-term borrowings. As of December 31, 2019, we had access to a total of $15 billion in U.S. revolving credit facilities consisting of a $7 billion facility expiring in 2024 and an $8 billion facility expiring in September 2020, which may be used to support our commercial paper borrowings. In addition to the U.S. revolving credit facilities, our lenders have provided us an additional $537 million in lines of credit, of which $508 million expire within one year. Of these total lines of credit, $15.5 billion were unused on December 31, 2019. In connection with the recently-announced agreement to combine Upjohn with Mylan discussed in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Business Development Initiatives” section of this Financial Review, Upjohn entered into a fully underwritten, 364-day senior unsecured bridge facility for up to $12 billion. This bridge facility is expected to terminate upon the issuance of approximately $12 billion of debt securities, loans or a combination of the two by Upjohn prior to the transaction close.
LIBOR
From time to time, we issue variable rate debt based on LIBOR, or undertake interest rate swaps that contain a variable element based on LIBOR. The U.K. Financial Conduct Authority announced in July 2017 that it will no longer compel banks to submit rates that are currently used to calculate LIBOR after 2021. Various governing parties, including government agencies, are working on a benchmark transition plan for LIBOR (and other interbank offered rates globally). We are monitoring their progress, and we will likely amend contracts to accommodate any replacement rate where it is not already provided. We do not expect the transition to an alternative rate to have a material impact on our liquidity or financial resources.
Global Economic Conditions––General
The global economic environment has not had, nor do we anticipate it will have, a material impact on our liquidity or capital resources. Due to our significant operating cash flows, financial assets, access to capital markets and available lines of credit and revolving credit agreements, we continue to believe that we have, and will maintain, the ability to meet our liquidity needs for the foreseeable future. We monitor our liquidity position continuously in the face of evolving economic conditions. For additional information see the “Overview of Our Performance, Operating Environment, Strategy and Outlook––The Global Economic Environment” section in this Financial Review.
2019 Financial Report | 51 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Global Economic Conditions––Venezuela Operations and Argentina Operations
Our Venezuela and Argentina operations function in hyperinflationary economies. The impact to Pfizer is not considered material.
Contractual Obligations
Payments due under contractual obligations as of December 31, 2019, mature as follows:
Years | ||||||||||||||||||||
(MILLIONS OF DOLLARS) | Total | 2020 | 2021-2022 | 2023-2024 | Thereafter | |||||||||||||||
Long-term debt, including current portion(a) | $ | 37,417 | $ | 1,462 | $ | 4,769 | $ | 5,274 | $ | 25,912 | ||||||||||
Interest payments on long-term debt obligations(b) | 21,612 | 1,403 | 2,701 | 2,436 | 15,073 | |||||||||||||||
Other long-term liabilities(c) | 2,521 | 427 | 534 | 483 | 1,078 | |||||||||||||||
Operating leases(d) | 3,280 | 323 | 512 | 433 | 2,012 | |||||||||||||||
Purchase obligations and other(e) | 2,534 | 711 | 994 | 485 | 344 | |||||||||||||||
Other taxes payable—deemed repatriated accumulated post-1986 earnings of foreign subsidiaries(f) | 9,650 | 600 | 1,700 | 2,400 | 4,950 | |||||||||||||||
Uncertain tax positions(g) | 130 | 130 | — | — | — | |||||||||||||||
(a) | Long-term debt consists of senior unsecured notes (including fixed and floating rate, foreign currency denominated, and other notes), carried at historical proceeds, as adjusted (see Notes to Consolidated Financial Statements—Note 7. Financial Instruments). Commitments under financing leases are not significant. |
(b) | Our calculations of expected interest payments incorporate only current period assumptions for interest rates, foreign currency translation rates and hedging strategies (see Notes to Consolidated Financial Statements—Note 7. Financial Instruments), and assume that interest is accrued through the maturity date or expiration of the related instrument. |
(c) | Includes expected payments relating to our unfunded U.S. supplemental (non-qualified) pension plans, postretirement plans and deferred compensation plans. Excludes amounts relating to our U.S. qualified pension plans and international pension plans, all of which have a substantial amount of plan assets, because the required funding obligations are not expected to be material and/or because such liabilities do not necessarily reflect future cash payments, as the impact of changes in economic conditions on the fair value of the pension plan assets and/or liabilities can be significant. Also, excludes $3.9 billion of liabilities related to the fair value of derivative financial instruments, legal matters and employee terminations, among other liabilities, most of which do not represent contractual obligations. See also our liquidity discussion above in this “Analysis of Financial Condition, Liquidity and Capital Resources” section, as well as the Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives, Note 7A. Financial Instruments: Fair Value Measurements, Note 11E. Pension and Postretirement Benefit Plans and Defined Contribution Plans: Cash Flows, and Note 16. Contingencies and Certain Commitments. |
(d) | Includes future minimum rental commitments under non-cancelable operating leases. These amounts include an agreement we entered in April 2018 to lease space in an office building in New York City. We expect to take control of the property in 2021 and relocate our global headquarters to this new office building in 2022. Our future minimum rental commitment under this 20-year lease is approximately $1.7 billion. |
(e) | Includes agreements to purchase goods and services that are enforceable and legally binding and includes amounts relating to advertising, information technology services, employee benefit administration services, and potential milestone payments deemed reasonably likely to occur. Also includes obligations to make guaranteed fixed annual payments over the next 7 years in connection with the U.S. and EU approvals for Besponsa ($412 million) and an obligation to make guaranteed fixed annual payments over the next 8 years for Bosulif ($217 million), both associated with R&D arrangements. For additional information, see Notes to Consolidated Financial Statements—Note 7E. Financial Instruments: Other Noncurrent Liabilities and —Note 16C. Contingencies and Certain Commitments: Certain Commitments. |
(f) | Represents estimated cash payments related to the TCJA repatriation tax for which we elected with the filing of our 2018 U.S. Federal Consolidated Income Tax Return to pay in annual installments over eight years through 2026 (with the next installment due in April 2020). Our obligations may vary as a result of changes in our uncertain tax positions and/or availability of attributes such as foreign tax and other credit carryforwards. For additional information, see Notes to Consolidated Financial Statements—Note 5A. Tax Matters: Taxes on Income from Continuing Operations and Note 5C. Tax Matters: Deferred Taxes. |
(g) | Includes only income tax amounts currently payable. We are unable to predict the timing of tax settlements related to our noncurrent obligations for uncertain tax positions as tax audits can involve complex issues and the resolution of those issues may span multiple years, particularly if subject to negotiation or litigation. |
The above table includes amounts for potential milestone payments under collaboration, licensing or other arrangements, if the payments are deemed reasonably likely to occur. Payments under these agreements generally become due and payable only upon the achievement of certain development, regulatory and/or commercialization milestones, which may span several years and which may never occur.
In 2020, we expect to spend approximately $2.4 billion on property, plant and equipment. We rely largely on operating cash flows to fund our capital investment needs. Due to our significant operating cash flows, we believe we have the ability to meet our capital investment needs and anticipate no delays to planned capital expenditures.
Off-Balance Sheet Arrangements
In the ordinary course of business and in connection with the sale of assets and businesses and other transactions, we often indemnify our counterparties against certain liabilities that may arise in connection with a transaction or that are related to events and activities prior to or following a transaction. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we may be required to reimburse the loss. These indemnification obligations generally are subject to various restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2019, the estimated fair value of our indemnification obligations was not significant.
Certain of our co-promotion or license agreements give our licensors or partners the rights to negotiate for, or in some cases to obtain under certain financial conditions, co-promotion or other rights in specified countries with respect to certain of our products.
52 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
Share-Purchase Plans and Accelerated Share Repurchase Agreements
See Notes to Consolidated Financial Statements––Note 12. Equity for information on the shares of our common stock purchased and the cost of purchases under our publicly announced share-purchase plans, including our accelerated share repurchase agreements. At December 31, 2019, our remaining share-purchase authorization was approximately $5.3 billion, with no repurchases currently planned in 2020.
Dividends on Common Stock
We paid dividends on our common stock of $8.0 billion in 2019, $8.0 billion in 2018 and $7.7 billion in 2017. In December 2019, our Board of Directors declared a first-quarter 2020 dividend of $0.38 per share, payable on March 6, 2020, to shareholders of record at the close of business on January 31, 2020. The first-quarter 2020 cash dividend will be our 325th consecutive quarterly dividend.
Our current and projected dividends provide a return to shareholders while maintaining sufficient capital to invest in growing our businesses. Our dividends are not restricted by debt covenants. While the dividend level remains a decision of Pfizer’s Board of Directors and will continue to be evaluated in the context of future business performance, we currently believe that we can support future annual dividend increases, barring significant unforeseen events. Pfizer also expects that immediately following the closing of the proposed transaction to combine Upjohn with Mylan, the combined dividend dollar amount received by Pfizer shareholders in the event the equity distribution is structured as a spinoff, based upon the combination of continued Pfizer ownership and an expected 0.12 shares of the new company granted for each Pfizer share, will equate to Pfizer’s dividend amount in effect immediately prior to closing.
2019 Financial Report | 53 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
NEW ACCOUNTING STANDARDS
Recently Adopted Accounting Standards
See Notes to Consolidated Financial Statements—Note 1B. Basis of Presentation and Significant Accounting Policies: Adoption of New Accounting Standards in 2019.
Recently Issued Accounting Standards, Not Adopted as of December 31, 2019 | ||||
Standard/Description | Effective Date | Effect on the Financial Statements or Other Significant Matters | ||
In June 2016, the FASB issued new guidance on accounting for credit losses of financial instruments. The new guidance and related amendments replace the probable initial recognition threshold for incurred loss estimates in current GAAP with a methodology that reflects expected credit loss estimates. | January 1, 2020. | This standard includes our financial instruments, such as accounts receivable, and investments that are generally of high credit quality. Previously, when credit losses were measured under GAAP, an entity generally only considered past events and current conditions in measuring the incurred loss. The new guidance requires us to identify, analyze, document and support new methodologies for quantifying expected credit loss estimates for our financial instruments, using information such as historical experience and current economic conditions, plus the use of reasonable supportable forecast information. We do not expect this new guidance to have a material impact on our consolidated financial statements. | ||
In January 2017, the FASB issued new guidance for goodwill impairment testing. The new guidance eliminates the requirement to perform a hypothetical purchase price allocation to measure goodwill impairment. Under the new guidance the goodwill impairment test is performed by comparing the fair value of a reporting unit with its carrying amount, and recognizing an impairment charge for the amount by which the carrying amount of the reporting unit exceeds its fair value, although it cannot exceed the total amount of goodwill allocated to that reporting unit. | January 1, 2020. | We do not expect this new guidance to have a material impact on our consolidated financial statements. | ||
In August 2018, the FASB issued new guidance related to customers’ accounting for implementation costs incurred in a cloud computing arrangement that is considered a service contract. The new guidance aligns the requirements for capitalizing implementation costs in such arrangements with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. The new guidance can be adopted either prospectively or retrospectively. | January 1, 2020. | We do not expect this new guidance to have a material impact on our consolidated financial statements. | ||
In November 2018, the FASB issued new guidance clarifying the interaction between the accounting guidance for collaboration agreements and revenue from contracts with customers. | January 1, 2020. | We do not expect this new guidance to have a material impact on our consolidated financial statements. | ||
In December 2019, the FASB issued new guidance that simplifies the accounting for income taxes by eliminating certain exceptions to the guidance related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. The new guidance also simplifies aspects of the accounting for franchise taxes and enacted changes in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. | January 1, 2021. Early adoption is permitted. | We are assessing the impact of the provisions of this new guidance on our consolidated financial statements. | ||
54 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
FORWARD-LOOKING INFORMATION AND FACTORS THAT MAY AFFECT FUTURE RESULTS
This report and other written or oral statements that we make from time to time contain forward-looking statements. Such forward-looking statements involve substantial risks and uncertainties. We have tried, wherever possible, to identify such statements by using words such as “will,” “may,” “could,” “likely,” “ongoing,” “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” “assume,” “target,” “forecast,” “guidance,” “goal,” “objective,” “aim,” “seek” and other words and terms of similar meaning or by using future dates in connection with any discussion of, among other things, our anticipated operating and financial performance, business plans and prospects, expectations for our product pipeline, in-line products and product candidates, including anticipated regulatory submissions, data read-outs, study starts, approvals, revenue contribution, growth, performance, timing of exclusivity and potential benefits, strategic reviews, capital allocation objectives, plans for and prospects of our acquisitions and other business-development activities, benefits anticipated from the reorganization of our commercial operations in 2019, sales efforts, expenses, interest rates, foreign exchange rates, the outcome of contingencies, such as legal proceedings, government regulation, our ability to successfully capitalize on growth opportunities or prospects, manufacturing and product supply and plans relating to share repurchases and dividends. In particular, these include statements relating to future actions, including, among others, the expected timing, benefits, charges and/or costs in connection with our agreement to combine Upjohn with Mylan to create a new global pharmaceutical company, Viatris, set forth in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Business Development Initiatives” and “––Our Strategy” sections and the Notes to Consolidated Financial Statements—Note 1A. Basis of Presentation and Significant Accounting Policies––Basis of Presentation of this Financial Review, the expected impact of patent expiries on our business set forth in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Operating Environment––Industry-Specific Challenges––Intellectual Property Rights and Collaboration/Licensing Rights” section of this Financial Review, the benefits expected from the reorganization of our commercial operations in 2019 and our expectations regarding growth set forth in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Strategy––Organizing for Growth” section of this Financial Review, the anticipated costs related to our preparations for Brexit set forth in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––The Global Economic Environment” section of this Financial Review, our anticipated liquidity position set forth in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––The Global Economic Environment” and the “Analysis of Financial Condition, Liquidity and Capital Resources” sections of this Financial Review, the anticipated costs and savings from certain of our initiatives, including the Transforming to a More Focused Company initiative, set forth in the “Overview of Our Performance, Operating Environment, Strategy and Outlook—Transforming to a More Focused Company” and “Costs and Expenses—Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” sections of this Financial Review and in the Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives, our plans for increasing investment in the U.S. set forth in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Strategy––Capital Allocation and Expense Management––Increasing Investment in the U.S.” section of this Financial Review, the financial guidance set forth in the “Overview of Our Performance, Operating Environment, Strategy and Outlook––Our Financial Guidance for 2020” section of this Financial Review, the expected impact of ACIP’s recommendation for Prevnar 13 for adults 65 and older on Prevnar 13’s revenues set forth in the “Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion––Prevnar 13/Prevenar 13 (Biopharma)” section of this Financial Review, the expected impact of updates to the prescribing information for Xeljanz on its growth set forth in the “Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion––Xeljanz (Biopharma)” section of this Financial Review, the benefits expected from our business development transactions, the planned capital spending set forth in the “Analysis of Financial Condition, Liquidity and Capital Resources––Selected Measures of Liquidity and Capital Resources––Contractual Obligations” section of this Financial Review, the expected payments to our unfunded U.S. supplemental (non-qualified) pension plans, postretirement plans and deferred compensation plans and expected funding obligations set forth in the “Analysis of Financial Condition, Liquidity and Capital Resources––Selected Measures of Liquidity and Capital Resources––Contractual Obligations” section of this Financial Review, and the voluntary contribution we expect to make during 2020 for the U.S. qualified plans set forth in Notes to Consolidated Financial Statements––Note 11. Pension and Postretirement Benefit Plans and Defined Contribution Plans. Among the factors that could cause actual results to differ materially from past results and future plans and projected future results are the following:
• | the outcome of R&D activities, including, without limitation, the ability to meet anticipated pre-clinical or clinical endpoints, commencement and/or completion dates for our pre-clinical or clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates, as well as the possibility of unfavorable pre-clinical and clinical trial results, including the possibility of unfavorable new clinical data and further analyses of existing clinical data; |
• | the risk we may not be able to successfully address all of the comments received from regulatory authorities such as the FDA or the EMA, or obtain approval from regulators, which will depend on myriad factors, including such regulator making a determination as to whether a product’s benefits outweigh its known risks and a determination of the product’s efficacy; regulatory decisions impacting labeling, manufacturing processes, safety and/or other matters; and recommendations by technical or advisory committees, such as ACIP, that may impact the use of our vaccines; |
• | the speed with which regulatory authorizations, pricing approvals and product launches may be achieved; |
• | claims and concerns that may arise regarding the safety or efficacy of in-line products and product candidates, including claims and concerns that may arise from the outcome of post-approval clinical trials, which could result in the loss of marketing approval, changes in product labeling, and/or new or increased concerns about the side effects or efficacy of, a product that could affect its availability or commercial potential, such as the update to the U.S. and EU prescribing information for Xeljanz; |
• | the success of external business-development activities, including the ability to identify and execute on potential business development opportunities, the ability to satisfy the conditions to closing of announced transactions in the anticipated time frame or at all, the ability to realize the anticipated benefits of any such transactions, and the potential need to obtain additional equity or debt financing to pursue these opportunities, which could result in increased leverage and impact our credit ratings; |
• | competitive developments, including the impact on our competitive position of new product entrants, in-line branded products, generic products, private label products, biosimilars and product candidates that treat diseases and conditions similar to those treated by our in-line drugs and drug candidates; |
2019 Financial Report | 55 | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | the implementation by the FDA and regulatory authorities in certain countries of an abbreviated legal pathway to approve biosimilar products, which could subject our biologic products to competition from biosimilar products, with attendant competitive pressures, after the expiration of any applicable exclusivity period and patent rights; |
• | risks related to our ability to develop and commercialize biosimilars, including risks associated with “at risk” launches, defined as the marketing of a product by Pfizer before the final resolution of litigation (including any appeals) brought by a third party alleging that such marketing would infringe one or more patents owned or controlled by the third party, and access challenges for our biosimilar products where our product may not receive appropriate formulary access or remains in a disadvantaged position relative to the innovator product; |
• | the ability to meet competition from generic, branded and biosimilar products after the loss or expiration of patent protection for our products or competitor products; |
• | the ability to successfully market both new and existing products domestically and internationally; |
• | difficulties or delays in manufacturing, sales or marketing, including delays caused by natural events, such as hurricanes; supply disruptions, shortages or stock-outs at our facilities; and legal or regulatory actions, such as warning letters, suspension of manufacturing, seizure of product, injunctions, debarment, recall of a product, delays or denials of product approvals, import bans or denial of import certifications; |
• | the impact of public health epidemics or outbreaks on our operations (such as the novel strain of coronavirus impacting China and several other countries); |
• | trade buying patterns; |
• | the impact of existing and future legislation and regulatory provisions on product exclusivity; |
• | trends toward managed care and healthcare cost containment, and our ability to obtain or maintain timely or adequate pricing or favorable formulary placement for our products; |
• | the impact of any significant spending reductions or cost controls affecting Medicare, Medicaid or other publicly funded or subsidized health programs or changes in the tax treatment of employer-sponsored health insurance that may be implemented; |
• | the impact of any U.S. healthcare reform or legislation, including any replacement, repeal, modification or invalidation of some or all of the provisions of the U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act; |
• | U.S. federal or state legislation or regulatory action and/or policy efforts affecting, among other things, pharmaceutical product pricing, intellectual property, reimbursement or access, including under Medicaid, Medicare and other publicly funded or subsidized health programs; patient out-of-pocket costs for medicines, manufacturer prices and/or price increases that could result in new mandatory rebates and discounts or other pricing restrictions; general budget control actions; the importation of prescription drugs from outside the U.S. at prices that are regulated by governments of various foreign countries; revisions to reimbursement of biopharmaceuticals under government programs; restrictions on U.S. direct-to-consumer advertising; limitations on interactions with healthcare professionals; or the use of comparative effectiveness methodologies that could be implemented in a manner that focuses primarily on the cost differences and minimizes the therapeutic differences among pharmaceutical products and restricts access to innovative medicines; as well as pricing pressures for our products as a result of highly competitive insurance markets; |
• | legislation or regulatory action in markets outside the U.S., including China, affecting pharmaceutical product pricing, intellectual property, reimbursement or access, including, in particular, continued government-mandated reductions in prices and access restrictions for certain biopharmaceutical products to control costs in those markets; |
• | the exposure of our operations outside the U.S. to possible capital and exchange controls, economic conditions, expropriation and other restrictive government actions, changes in intellectual property legal protections and remedies, as well as political unrest, unstable governments and legal systems and inter-governmental disputes; |
• | contingencies related to actual or alleged environmental contamination; |
• | any significant breakdown, infiltration or interruption of our information technology systems and infrastructure; |
• | legal defense costs, insurance expenses and settlement costs; |
• | the risk of an adverse decision or settlement and the adequacy of reserves related to legal proceedings, including patent litigation, such as claims that our patents are invalid and/or do not cover the product of the generic drug manufacturer or where one or more third parties seeks damages and/or injunctive relief to compensate for alleged infringement of its patents by our commercial or other activities, product liability and other product-related litigation, including personal injury, consumer, off-label promotion, securities, antitrust and breach of contract claims, commercial, environmental, government investigations, employment and other legal proceedings, including various means for resolving asbestos litigation, as well as tax issues; |
• | the risk that our currently pending or future patent applications may not result in issued patents, or be granted on a timely basis, or any patent-term extensions that we seek may not be granted on a timely basis, if at all; |
• | our ability to protect our patents and other intellectual property, both domestically and internationally; |
• | interest rate and foreign currency exchange rate fluctuations, including the impact of possible currency devaluations in countries experiencing high inflation rates; |
• | governmental laws and regulations affecting domestic and foreign operations, including, without limitation, tax obligations and changes affecting the tax treatment by the U.S. of income earned outside the U.S. that may result from pending and possible future proposals, including further clarifications and/or interpretations of or changes to the TCJA enacted in 2017; |
• | any significant issues involving our largest wholesale distributors, which account for a substantial portion of our revenues; |
• | the possible impact of the increased presence of counterfeit medicines in the pharmaceutical supply chain on our revenues and on patient confidence in the integrity of our medicines; |
56 | 2019 Financial Report | |
Financial Review
Pfizer Inc. and Subsidiary Companies
• | uncertainties based on the formal change in relationship between the U.K. government and the EU, which could have implications on our research, commercial and general business operations in the U.K. and the EU, including the approval and supply of our products; |
• | any significant issues that may arise related to the outsourcing of certain operational and staff functions to third parties, including with regard to quality, timeliness and compliance with applicable legal or regulatory requirements and industry standards; |
• | any significant issues that may arise related to our joint ventures and other third-party business arrangements; |
• | further clarifications and/or changes in interpretations of existing laws and regulations, or changes in laws and regulations, in the U.S. and other countries, including changes in U.S. generally accepted accounting principles; |
• | uncertainties related to general economic, political, business, industry, regulatory and market conditions including, without limitation, uncertainties related to the impact on us, our customers, suppliers and lenders and counterparties to our foreign-exchange and interest-rate agreements of challenging global economic conditions and recent and possible future changes in global financial markets; the related risk that our allowance for doubtful accounts may not be adequate; and the risks related to volatility of our income due to changes in the market value of equity investments; |
• | any changes in business, political and economic conditions due to actual or threatened terrorist activity in the U.S. and other parts of the world, and related U.S. military action overseas; |
• | growth in costs and expenses; |
• | changes in our product, segment and geographic mix; |
• | the impact of purchase accounting adjustments, acquisition-related costs, discontinued operations and certain significant items; |
• | the impact of product recalls, withdrawals and other unusual items; |
• | the risk of an impairment charge related to our intangible assets, goodwill or equity-method investments; |
• | the impact of, and risks and uncertainties related to, acquisitions and divestitures, such as the acquisition of Array, our transaction with GSK which combined our respective consumer healthcare businesses into a new consumer healthcare joint venture and our agreement to combine Upjohn with Mylan to create a new global pharmaceutical company, Viatris, including, among other things, risks related to the satisfaction of the conditions to closing to any pending transaction (including the failure to obtain any necessary shareholder and regulatory approvals) in the anticipated timeframe or at all and the possibility that such transaction does not close; the ability to realize the anticipated benefits of those transactions, including the possibility that the expected cost savings and/or accretion from certain of those transactions will not be realized or will not be realized within the expected time frame; the risk that the businesses will not be integrated successfully; negative effects of the announcement or the consummation of the transaction on the market price of Pfizer’s common stock, Pfizer’s credit ratings and/or Pfizer’s operating results; disruption from the transactions making it more difficult to maintain business and operational relationships; risks related to our ability to grow revenues for certain acquired products; significant transaction costs; unknown liabilities; the risk of litigation and/or regulatory actions related to the transaction, other business effects, including the effects of industry, market, economic, political or regulatory conditions, future exchange and interest rates, changes in tax and other laws, regulations, rates and policies, future business combinations or disposals; competitive developments; and as it relates to the Consumer Healthcare JV with GSK, the possibility that a future separation of the joint venture as an independent company via a demerger of GSK’s equity interest to GSK’s shareholders and a listing of the joint venture on the U.K. equity market may not occur; and |
• | the impact of, and risks and uncertainties related to, restructurings and internal reorganizations, including the reorganization of our commercial operations in 2019, as well as any other corporate strategic initiatives, and cost-reduction and productivity initiatives, each of which requires upfront costs but may fail to yield anticipated benefits and may result in unexpected costs or organizational disruption. |
We cannot guarantee that any forward-looking statement will be realized. Achievement of anticipated results is subject to substantial risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize or should underlying assumptions prove inaccurate, actual results could vary materially from past results and those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements, and are cautioned not to put undue reliance on forward-looking statements.
We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law or by the rules and regulations of the SEC. You are advised, however, to consult any further disclosures we make on related subjects.
Certain risks, uncertainties and assumptions are discussed here and under the heading entitled “Risk Factors” in Part I, Item 1A. of our Form 10-K for the year ended December 31, 2019. We note these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider any such list to be a complete set of all potential risks or uncertainties.
The operating segment information provided in this report does not purport to represent the revenues, costs and income from continuing operations before provision for taxes on income that each of our operating segments would have recorded had each segment operated as a standalone company during the periods presented.
This report includes discussion of certain clinical studies relating to various in-line products and/or product candidates. These studies typically are part of a larger body of clinical data relating to such products or product candidates, and the discussion herein should be considered in the context of the larger body of data. In addition, clinical trial data are subject to differing interpretations, and, even when we view data as sufficient to support the safety and/or effectiveness of a product candidate or a new indication for an in-line product, regulatory authorities may not share our views and may require additional data or may deny approval altogether.
2019 Financial Report | 57 | |
Management’s Report on Internal Control Over Financial Reporting
Management’s Report
We prepared and are responsible for the financial statements that appear in our 2019 Financial Report. These financial statements are in conformity with accounting principles generally accepted in the United States of America and, therefore, include amounts based on informed judgments and estimates. We also accept responsibility for the preparation of other financial information that is included in this document.
Report on Internal Control Over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. The Company’s internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate. Management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2019. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control—Integrated Framework (2013). Based on our assessment and those criteria, management believes that the Company maintained effective internal control over financial reporting as of December 31, 2019.
The Company’s independent auditors have issued their auditors’ report on the Company’s internal control over financial reporting. That report appears in our 2019 Financial Report under the heading, Report of Independent Registered Public Accounting Firm on Internal Control Over Financial Reporting.
 | ||
Albert Bourla | ||
Chairman and Chief Executive Officer | ||
 |  | |
Frank D’Amelio | Loretta Cangialosi | |
Principal Financial Officer | Principal Accounting Officer | |
February 27, 2020 | ||
58 | 2019 Financial Report | |
Audit Committee Report
The Audit Committee reviews Pfizer’s financial reporting process on behalf of the Board of Directors. Management has the primary responsibility for the financial statements and the reporting process, including the system of internal controls.
The Committee met and held discussions with management and the independent registered public accounting firm regarding the fair and complete presentation of Pfizer’s results and the assessment of Pfizer’s internal control over financial reporting. We discussed significant accounting policies applied in Pfizer’s financial statements, as well as, when applicable, alternative accounting treatments, and critical audit matters addressed during the audit. Management represented to the Committee that the consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United States of America, and the Committee reviewed and discussed the consolidated financial statements with management and the independent registered public accounting firm. The Committee discussed with the independent registered public accounting firm matters required to be discussed under applicable Public Company Accounting Oversight Board (PCAOB) and U.S. Securities and Exchange Commission standards.
In addition, the Committee reviewed and discussed with the independent registered public accounting firm the auditor’s independence from Pfizer and its management. As part of that review, we received the written disclosures and the letter required by applicable requirements of the PCAOB regarding the independent registered public accounting firm’s communications with the Audit Committee concerning independence, and the Committee discussed the independent registered public accounting firm’s independence from Pfizer.
We also considered whether the independent registered public accounting firm’s provision of non-audit services to Pfizer is compatible with the auditor’s independence. The Committee concluded that the independent registered public accounting firm is independent from Pfizer and its management.
As part of our responsibilities for oversight of Pfizer’s Enterprise Risk Management process, we reviewed and discussed company policies with respect to risk assessment and risk management, including discussions of individual risk areas, as well as an annual summary of the overall process.
The Committee discussed with Pfizer’s Internal Audit Department and independent registered public accounting firm the overall scope of and plans for their respective audits. The Committee meets with the Chief Internal Auditor, Chief Compliance, Quality and Risk Officer and representatives of the independent registered public accounting firm, in regular and executive sessions, to discuss the results of their examinations, the evaluations of Pfizer’s internal controls, and the overall quality of Pfizer’s financial reporting and compliance programs.
In reliance on the reviews and discussions referred to above, the Committee has recommended to the Board of Directors, and the Board has approved, that the audited financial statements be included in Pfizer’s Annual Report on Form 10-K for the year ended December 31, 2019, for filing with the U.S. Securities and Exchange Commission. The Committee has selected, and the Board of Directors has ratified, the selection of Pfizer’s independent registered public accounting firm for 2020.
The Audit Committee |
Suzanne Nora Johnson, Chair |
Ronald E. Blaylock |
Joseph J. Echevarria |
James C. Smith |
February 27, 2020 |
The Audit Committee Report does not constitute soliciting material, and shall not be deemed to be filed or incorporated by reference into any Company filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Company specifically incorporates the Audit Committee Report by reference therein.
2019 Financial Report | 59 | |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Pfizer Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Pfizer Inc. and Subsidiary Companies (the Company) as of December 31, 2019 and 2018, the related consolidated statements of income, comprehensive income, equity, and cash flows for each of the years in the three-year period ended December 31, 2019, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 27, 2020 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Evaluation of certain assumptions impacting the U.S. Medicare, Medicaid, and performance-based contract rebates accrual
As discussed in Note 1G to the consolidated financial statements, the Company records estimated deductions for Medicare, Medicaid, and performance-based contract rebates (collectively, U.S. rebates) as a reduction to gross product revenues. The accrual for U.S. rebates is recorded in the same period that the corresponding revenues are recognized. The length of time between when a sale is made and when the U.S. rebate is paid by the Company can be as long as one year, which increases the need for significant management judgment and knowledge of market conditions and practices in estimating the accrual.
We identified the evaluation of the U.S. rebates accrual as a critical audit matter because evaluating the product-specific experience ratio assumption underlying the estimate of the accrual involved especially challenging auditor judgment. The product-specific experience ratio assumption relates to estimating which of the Company’s revenue transactions will ultimately be subject to a related rebate.
The primary procedures we performed to address this critical audit matter included the following. We tested certain internal controls over the Company’s U.S. rebate accrual process, including controls related to the development of the product-specific experience ratio assumptions. We recalculated the U.S. rebates accrual for a selection of products, based on a combination of Company internal data, historical actual information, and executed third-party contracts. We performed a sensitivity analysis of the Company’s accrual by recalculating the accrual using our independent assumptions. We evaluated the Company’s ability to accurately estimate the accrual for U.S. rebates by comparing historically recorded accruals to the actual amount that was ultimately paid by the Company.
Evaluation of gross unrecognized tax benefits
As of December 31, 2019 the Company has recorded gross unrecognized tax benefits, excluding associated interest, of $5.4 billion. As discussed in Notes 5D and 1P, the Company’s tax positions are subject to audit by local taxing authorities in each respective tax jurisdiction, and the resolution of such audits may span multiple years. Since tax law is complex and often subject to varied interpretations and judgments, it is uncertain whether some of the Company’s tax positions will be sustained upon audit.
We identified the evaluation of the Company’s gross unrecognized tax benefits as a critical audit matter because complex auditor judgment is required in evaluating the Company’s interpretation of tax law and its estimate of the ultimate resolution of its tax positions.
60 | 2019 Financial Report | |
Report of Independent Registered Public Accounting Firm
The primary procedures we performed to address this critical audit matter included the following. We tested certain internal controls over the Company’s liability for unrecognized tax position process, including (1) interpretation of tax law, (2) evaluation of which of the Company’s tax positions may not be sustained upon audit, and (3) estimation and recording of the gross unrecognized tax benefits. We involved tax and valuation professionals with specialized skills and knowledge. We evaluated the Company’s interpretation of tax laws, including the assessment of transfer pricing practices in accordance with applicable tax laws and regulations. We inspected settlements with applicable taxing authorities, including assessing the expiration of statutes of limitations. We tested the calculation of the liability for uncertain tax positions, including an evaluation of the Company’s assessment of the technical merits of tax positions and estimates of the amount of tax benefits expected to be sustained.
Evaluation of product and other product-related litigation
As discussed in Notes 1R and 16 to the consolidated financial statements, the Company is involved in product liability and other product-related litigation, which can include personal injury, consumer, off-label promotion, securities, antitrust and breach of contract claims, among others. Certain of these pending product and other product-related legal proceedings could result in losses that could be substantial. The accrued liability and/or disclosure for the pending product and other product-related legal proceedings requires a complex series of judgments by the Company about future events, which involves a number of uncertainties.
We identified the evaluation of the accrued liability and/or related disclosures for these legal proceedings as a critical audit matter because the nature of the estimates and assumptions, including judgments about uncertainties and future events, requires challenging auditor judgment.
The primary procedures we performed to address this critical audit matter included the following. We tested certain internal controls over the Company’s product liability and other product-related litigation processes, including (1) the evaluation of information from external and internal legal counsel, (2) forward-looking expectations, and (3) new legal proceedings, or other legal proceedings not currently reserved or disclosed. We read letters received directly from the Company’s external and internal legal counsel that described the Company’s probable or reasonably possible legal contingency to pending product and other product-related legal proceedings. We inspected the Company’s minutes from meetings of the Audit Committee, which included the status of key litigation matters. We evaluated the Company’s ability to estimate its monetary exposure to pending product and other product-related legal proceedings by comparing historically recorded liabilities to actual monetary amounts incurred upon resolution of prior legal matters. We analyzed relevant publicly available information about the Company, its competitors, and the industry.

KPMG LLP |
We have not been able to determine the specific year that KPMG and our predecessor firms began serving as the Company’s auditor, however, we are aware that KPMG and our predecessor firms have served as the Company’s auditor since at least 1942. |
New York, New York |
February 27, 2020 |
2019 Financial Report | 61 | |
Report of Independent Registered Public Accounting Firm on
Internal Control Over Financial Reporting
The Board of Directors and Shareholders of Pfizer Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited Pfizer Inc. and Subsidiary Companies’ (the Company) internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of Pfizer Inc. and Subsidiary Companies as of December 31, 2019 and 2018, and the related consolidated statements of income, comprehensive income, equity, and cash flows for each of the years in the three-year period ended December 31, 2019, and the related notes (collectively, the consolidated financial statements), and our report dated February 27, 2020 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.

KPMG LLP |
New York, New York |
February 27, 2020 |
62 | 2019 Financial Report | |
Consolidated Statements of Income
Pfizer Inc. and Subsidiary Companies
Year Ended December 31, | ||||||||||||
(MILLIONS, EXCEPT PER COMMON SHARE DATA) | 2019 | 2018 | 2017 | |||||||||
Revenues | $ | $ | $ | |||||||||
Costs and expenses: | ||||||||||||
Cost of sales(a) | ||||||||||||
Selling, informational and administrative expenses(a) | ||||||||||||
Research and development expenses(a) | ||||||||||||
Amortization of intangible assets | ||||||||||||
Restructuring charges and certain acquisition-related costs | ||||||||||||
(Gain) on completion of Consumer Healthcare JV transaction | ( | ) | ||||||||||
Other (income)/deductions––net | ||||||||||||
Income from continuing operations before provision/(benefit) for taxes on income | ||||||||||||
Provision/(benefit) for taxes on income | ( | ) | ||||||||||
Income from continuing operations | ||||||||||||
Discontinued operations: | ||||||||||||
Income from discontinued operations––net of tax | ( | ) | ||||||||||
Gain on disposal of discontinued operations––net of tax | ||||||||||||
Discontinued operations––net of tax | ||||||||||||
Net income before allocation to noncontrolling interests | ||||||||||||
Less: Net income attributable to noncontrolling interests | ||||||||||||
Net income attributable to Pfizer Inc. | $ | $ | $ | |||||||||
Earnings per common share––basic: | ||||||||||||
Income from continuing operations attributable to Pfizer Inc. common shareholders | $ | $ | $ | |||||||||
Discontinued operations––net of tax | ||||||||||||
Net income attributable to Pfizer Inc. common shareholders | $ | $ | $ | |||||||||
Earnings per common share––diluted: | ||||||||||||
Income from continuing operations attributable to Pfizer Inc. common shareholders | $ | $ | $ | |||||||||
Discontinued operations––net of tax | ||||||||||||
Net income attributable to Pfizer Inc. common shareholders | $ | $ | $ | |||||||||
Weighted-average shares––basic | ||||||||||||
Weighted-average shares––diluted | ||||||||||||
(a) | Exclusive of amortization of intangible assets, except as disclosed in Note 1L. |
Amounts may not add due to rounding.
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
2019 Financial Report | 63 | |
Consolidated Statements of Comprehensive Income
Pfizer Inc. and Subsidiary Companies
Year Ended December 31, | ||||||||||||
(MILLIONS) | 2019 | 2018 | 2017 | |||||||||
Net income before allocation to noncontrolling interests | $ | $ | $ | |||||||||
Foreign currency translation adjustments, net(a) | $ | $ | ( | ) | $ | |||||||
Reclassification adjustments(b) | ( | ) | ( | ) | ||||||||
( | ) | |||||||||||
Unrealized holding gains/(losses) on derivative financial instruments, net | ( | ) | ||||||||||
Reclassification adjustments for (gains)/losses included in net income(c) | ( | ) | ( | ) | ||||||||
( | ) | ( | ) | |||||||||
Unrealized holding gains/(losses) on available-for-sale securities, net | ( | ) | ( | ) | ||||||||
Reclassification adjustments for (gains)/losses included in net income(c) | ( | ) | ||||||||||
Reclassification adjustments for unrealized gains included in Retained earnings(d) | ( | ) | ||||||||||
( | ) | |||||||||||
Benefit plans: actuarial losses, net | ( | ) | ( | ) | ( | ) | ||||||
Reclassification adjustments related to amortization | ||||||||||||
Reclassification adjustments related to settlements, net | ||||||||||||
Other | ( | ) | ||||||||||
( | ) | ( | ) | |||||||||
Benefit plans: prior service costs and other, net | ( | ) | ( | ) | ( | ) | ||||||
Reclassification adjustments related to amortization of prior service costs and other, net | ( | ) | ( | ) | ( | ) | ||||||
Reclassification adjustments related to curtailments of prior service costs and other, net | ( | ) | ( | ) | ( | ) | ||||||
Other | ||||||||||||
( | ) | ( | ) | ( | ) | |||||||
Other comprehensive income/(loss), before tax | ( | ) | ( | ) | ||||||||
Tax provision/(benefit) on other comprehensive income/(loss)(e) | ( | ) | ||||||||||
Other comprehensive income/(loss) before allocation to noncontrolling interests | $ | ( | ) | $ | ( | ) | $ | |||||
Comprehensive income before allocation to noncontrolling interests | $ | $ | $ | |||||||||
Less: Comprehensive income attributable to noncontrolling interests | ||||||||||||
Comprehensive income attributable to Pfizer Inc. | $ | $ | $ | |||||||||
(a) | Amounts in 2019 include a gain of approximately $ |
(b) | For the year ended December 31, 2019, the foreign currency translation adjustments are primarily reclassified into (Gain) on completion of Consumer Healthcare JV transaction in the consolidated statement of income as a result of the contribution of our Consumer Healthcare business to the Consumer Healthcare joint venture with GSK. See Note 2C. For the year ended December 31, 2017, the foreign currency translation adjustments reclassified into Other (income)/deductions—net in the consolidated statement of income primarily result from the sale of our former |
Reclassified into Other (income)/deductions—net and Cost of sales in the consolidated statements of income. For additional information on amounts reclassified into Other (income)/deductions—net and Cost of sales, see Note 7F. |
(d) | For additional information, see Notes to Consolidated Financial Statements—Note 1B. Basis of Presentation and Significant Accounting Policies: Adoption of New Accounting Standards in 2018 in our 2018 Financial Report. |
(e) | For additional information. see Note 5E. |
Amounts may not add due to rounding.
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
64 | 2019 Financial Report | |
Consolidated Balance Sheets
Pfizer Inc. and Subsidiary Companies
As of December 31, | ||||||||
(MILLIONS, EXCEPT PREFERRED STOCK ISSUED AND PER COMMON SHARE DATA) | 2019 | 2018 | ||||||
Assets | ||||||||
Cash and cash equivalents | $ | $ | ||||||
Short-term investments | ||||||||
Trade accounts receivable, less allowance for doubtful accounts: 2019—$527; 2018—$541 | ||||||||
Inventories | ||||||||
Current tax assets | ||||||||
Other current assets | ||||||||
Assets held for sale | ||||||||
Total current assets | ||||||||
Equity-method investments | ||||||||
Long-term investments | ||||||||
Property, plant and equipment, less accumulated depreciation | ||||||||
Identifiable intangible assets, less accumulated amortization | ||||||||
Goodwill | ||||||||
Noncurrent deferred tax assets and other noncurrent tax assets | ||||||||
Other noncurrent assets | ||||||||
Total assets | $ | $ | ||||||
Liabilities and Equity | ||||||||
Short-term borrowings, including current portion of long-term debt: 2019—$1,462; 2018—$4,776 | $ | $ | ||||||
Trade accounts payable | ||||||||
Dividends payable | ||||||||
Income taxes payable | ||||||||
Accrued compensation and related items | ||||||||
Other current liabilities | ||||||||
Liabilities held for sale | ||||||||
Total current liabilities | ||||||||
Long-term debt | ||||||||
Pension benefit obligations, net | ||||||||
Postretirement benefit obligations, net | ||||||||
Noncurrent deferred tax liabilities | ||||||||
Other taxes payable | ||||||||
Other noncurrent liabilities | ||||||||
Total liabilities | ||||||||
Commitments and Contingencies | ||||||||
Preferred stock, no par value, at stated value; 27 shares authorized; issued: 2019—431; 2018—478 | ||||||||
Common stock, $0.05 par value; 12,000 shares authorized; issued: 2019—9,369; 2018—9,332 | ||||||||
Additional paid-in capital | ||||||||
Treasury stock, shares at cost: 2019—3,835; 2018—3,615 | ( | ) | ( | ) | ||||
Retained earnings | ||||||||
Accumulated other comprehensive loss | ( | ) | ( | ) | ||||
Total Pfizer Inc. shareholders’ equity | ||||||||
Equity attributable to noncontrolling interests | ||||||||
Total equity | ||||||||
Total liabilities and equity | $ | $ | ||||||
Amounts may not add due to rounding.
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
2019 Financial Report | 65 | |
Consolidated Statements of Equity
Pfizer Inc. and Subsidiary Companies
PFIZER INC. SHAREHOLDERS | |||||||||||||||||||||||||||||||||||||||||||||
Preferred Stock | Common Stock | Treasury Stock | |||||||||||||||||||||||||||||||||||||||||||
(MILLIONS, EXCEPT PREFERRED SHARES) | Shares | Stated Value | Shares | Par Value | Add’l Paid-In Capital | Shares | Cost | Retained Earnings | Accum. Other Comp. Loss | Share - holders’ Equity | Non-controlling Interests | Total Equity | |||||||||||||||||||||||||||||||||
Balance, January 1, 2017 | $ | $ | $ | ( | ) | $ | ( | ) | $ | $ | ( | ) | $ | $ | $ | ||||||||||||||||||||||||||||||
Net income | |||||||||||||||||||||||||||||||||||||||||||||
Other comprehensive income/(loss), net of tax | |||||||||||||||||||||||||||||||||||||||||||||
Cash dividends declared: | |||||||||||||||||||||||||||||||||||||||||||||
Common stock | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||
Preferred stock | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||
Noncontrolling interests | — | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||
Share-based payment transactions(a) | ( | ) | |||||||||||||||||||||||||||||||||||||||||||
Purchases of common stock | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||
Preferred stock conversions and redemptions | ( | ) | ( | ) | ( | ) | — | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||
Other | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
Balance, December 31, 2017 | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||
Net income | |||||||||||||||||||||||||||||||||||||||||||||
Other comprehensive income/(loss), net of tax | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||
Cash dividends declared: | |||||||||||||||||||||||||||||||||||||||||||||
Common stock | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||
Preferred stock | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||
Noncontrolling interests | — | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||
Share-based payment transactions | ( | ) | |||||||||||||||||||||||||||||||||||||||||||
Purchases of common stock | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||
Preferred stock conversions and redemptions | ( | ) | ( | ) | ( | ) | — | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||
Other(b) | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
Balance, December 31, 2018 | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||
Net income | |||||||||||||||||||||||||||||||||||||||||||||
Other comprehensive income/(loss), net of tax | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||
Cash dividends declared: | |||||||||||||||||||||||||||||||||||||||||||||
Common stock | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||
Preferred stock | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||
Noncontrolling interests | — | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||
Share-based payment transactions | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||||
Purchases of common stock | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||
Preferred stock conversions and redemptions | ( | ) | ( | ) | ( | ) | — | 1 | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||
Other(c) | ( | ) | — | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||
Balance, December 31, 2019 | $ | $ | $ | ( | ) | $ | ( | ) | $ | $ | ( | ) | $ | $ | $ | ||||||||||||||||||||||||||||||
(a) | 2017 treasury shares include the effect of the modification for a commitment to pay |
(b) | Primarily represents the cumulative effect of the adoption of new accounting standards in the first quarter of 2018 for revenues, financial assets and liabilities, income tax accounting, and the reclassification of certain tax effects from Accumulated other comprehensive income. For additional information, see Notes to Consolidated Financial Statements––Note 1B. Basis of Presentation and Significant Accounting Policies: Adoption of New Accounting Standards in 2018 in our 2018 Financial Report. |
(c) | The increase to Retained earnings in 2019 includes the cumulative effect of the adoption of a new accounting standard for leases in the first quarter of 2019. For additional information, see Note 1B. The decrease in Equity attributable to noncontrolling interests resulted from the deconsolidation of our Consumer Healthcare business in connection with the formation of the GSK Consumer Healthcare joint venture. For additional information, see Note 2C. The decrease in Additional paid in capital relates to our buyout of the remaining |
Amounts may not add due to rounding.
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
66 | 2019 Financial Report | |
Consolidated Statements of Cash Flows
Pfizer Inc. and Subsidiary Companies
Year Ended December 31, | ||||||||||||
(MILLIONS) | 2019 | 2018 | 2017 | |||||||||
Operating Activities | ||||||||||||
Net income before allocation to noncontrolling interests | $ | $ | $ | |||||||||
Adjustments to reconcile net income before allocation to noncontrolling interests to net cash provided by operating activities: | ||||||||||||
Depreciation and amortization | ||||||||||||
Asset write-offs and impairments | ||||||||||||
TCJA impact(a) | ( | ) | ( | ) | ( | ) | ||||||
Gain on completion of Consumer Healthcare JV transaction, net of cash conveyed(b) | ( | ) | ||||||||||
Deferred taxes from continuing operations(c) | ( | ) | ( | ) | ||||||||
Share-based compensation expense | ||||||||||||
Benefit plan contributions in excess of expense/income | ( | ) | ( | ) | ( | ) | ||||||
Other adjustments, net | ( | ) | ( | ) | ||||||||
Other changes in assets and liabilities, net of acquisitions and divestitures: | ||||||||||||
Trade accounts receivable | ( | ) | ( | ) | ||||||||
Inventories | ( | ) | ( | ) | ( | ) | ||||||
Other assets | ( | ) | ||||||||||
Trade accounts payable | ( | ) | ||||||||||
Other liabilities | ( | ) | ||||||||||
Other tax accounts, net | ( | ) | ( | ) | ||||||||
Net cash provided by operating activities | ||||||||||||
Investing Activities | ||||||||||||
Purchases of property, plant and equipment | ( | ) | ( | ) | ( | ) | ||||||
Purchases of short-term investments | ( | ) | ( | ) | ( | ) | ||||||
Proceeds from redemptions/sales of short-term investments | ||||||||||||
Net (purchases of)/proceeds from redemptions/sales of short-term investments with original maturities of three months or less | ( | ) | ||||||||||
Purchases of long-term investments | ( | ) | ( | ) | ( | ) | ||||||
Proceeds from redemptions/sales of long-term investments | ||||||||||||
Acquisitions of businesses, net of cash acquired | ( | ) | ( | ) | ||||||||
Acquisitions of intangible assets | ( | ) | ( | ) | ( | ) | ||||||
Other investing activities, net(b), (d) | ||||||||||||
Net cash provided by/(used in) investing activities | ( | ) | ( | ) | ||||||||
Financing Activities | ||||||||||||
Proceeds from short-term borrowings | ||||||||||||
Principal payments on short-term borrowings | ( | ) | ( | ) | ( | ) | ||||||
Net (payments on)/proceeds from short-term borrowings with original maturities of three months or less | ( | ) | ||||||||||
Proceeds from issuance of long-term debt | ||||||||||||
Principal payments on long-term debt | ( | ) | ( | ) | ( | ) | ||||||
Purchases of common stock | ( | ) | ( | ) | ( | ) | ||||||
Cash dividends paid | ( | ) | ( | ) | ( | ) | ||||||
Proceeds from exercise of stock options | ||||||||||||
Other financing activities, net | ( | ) | ( | ) | ( | ) | ||||||
Net cash used in financing activities | ( | ) | ( | ) | ( | ) | ||||||
Effect of exchange-rate changes on cash and cash equivalents and restricted cash and cash equivalents | ( | ) | ( | ) | ||||||||
Net increase/(decrease) in cash and cash equivalents and restricted cash and cash equivalents | ( | ) | ( | ) | ||||||||
Cash and cash equivalents and restricted cash and cash equivalents, at beginning of period | ||||||||||||
Cash and cash equivalents and restricted cash and cash equivalents, at end of period | $ | $ | $ | |||||||||
- Continued - | ||||||||||||
2019 Financial Report | 67 | |
Consolidated Statements of Cash Flows
Pfizer Inc. and Subsidiary Companies
Year Ended December 31, | ||||||||||||
2019 | 2018 | 2017 | ||||||||||
Supplemental Cash Flow Information | ||||||||||||
Non-cash transactions: | ||||||||||||
32% equity-method investment in GSK Consumer Healthcare JV in exchange for contributing Pfizer’s Consumer Healthcare business(b) | $ | $ | $ | |||||||||
Equity investment in Cerevel Therapeutics, Inc. in exchange for Pfizer’s portfolio of clinical and preclinical neuroscience assets(d) | ||||||||||||
Equity investment in Allogene received in exchange for Pfizer's allogeneic CAR T developmental program assets(d) | ||||||||||||
Exchange of $1.1 billion net book value 6.50% U.K. pound-denominated bonds maturing in 2038 for $1.8 billion of new 2.735% U.K. pound-denominated bonds maturing in 2043, resulting in a debt extinguishment loss of $747 million(e) | ||||||||||||
Receipt of ICU Medical common stock(d) | ||||||||||||
Promissory note from ICU Medical(d) | ||||||||||||
Cash paid (received) during the period for: | ||||||||||||
Income taxes | $ | $ | $ | |||||||||
Interest | ||||||||||||
Interest rate hedges | ( | ) | ( | ) | ( | ) | ||||||
(a) | As a result of the enactment of the TCJA in December 2017, Pfizer’s Provision/(benefit) for taxes on income (i) for the year ended December 31, 2017 was favorably impacted by approximately $ |
(b) | The $ |
(c) | Includes tax expense of approximately $ |
(d) | For additional information, see Note 2B. |
(e) | The $ |
Amounts may not add due to rounding.
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
68 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 1. Basis of Presentation and Significant Accounting Policies
A. Basis of Presentation
See the Glossary of Defined Terms at the beginning of this 2019 Financial Report for terms used throughout the consolidated financial statements and related notes in this 2019 Financial Report.
From the second quarter of our 2016 fiscal year until the end of 2018, we managed our commercial operations through two distinct business segments: Pfizer Innovative Health (IH) and Pfizer Essential Health (EH). At the beginning of our 2019 fiscal year, we began to manage our commercial operations through a new global structure consisting of three business segments––Pfizer Biopharmaceuticals Group (Biopharma), Upjohn and through July 31, 2019, Consumer Healthcare. Biopharma and Upjohn are the only reportable segments. We have revised prior-period segment information to reflect the reorganization. For additional information, see Note 17. In addition, certain amounts within Long-term investments in the December 31, 2018 consolidated balance sheet have been reclassified to Equity-method investments to conform to the current presentation. For additional information, see Note 2C.
Certain amounts in the consolidated financial statements and associated notes may not add due to rounding. All percentages have been calculated using unrounded amounts.
In the first quarter of 2019, as of January 1, 2019, we adopted four new accounting standards. See Note 1B for further information.
Our recent significant business development activities include:
• | License Agreement with Akcea Therapeutics, Inc.––In October 2019, we entered into a worldwide exclusive licensing agreement for AKCEA-ANGPTL3-LRx, an investigational antisense therapy being developed to treat patients with certain cardiovascular and metabolic diseases, with Akcea, a majority-owned affiliate of Ionis. The transaction closed in November 2019 and we made an upfront payment of $ |
• | Formation of a New Consumer Healthcare Joint Venture––On July 31, 2019, we completed the transaction in which we and GSK combined our respective consumer healthcare businesses into a new consumer healthcare joint venture that operates globally under the GSK Consumer Healthcare name. In accordance with our domestic and international reporting periods, our financial results, and our Consumer Healthcare segment’s operating results, for 2019 reflect seven months of Consumer Healthcare segment domestic operations and eight months of Consumer Healthcare segment international operations. Assets and liabilities associated with our Consumer Healthcare business were reclassified as held for sale in the consolidated balance sheet as of December 31, 2018. |
• | Acquisition of Array BioPharma Inc.––On July 30, 2019, we acquired Array for $ |
• | Agreement to Combine Upjohn with Mylan N.V.––On July 29, 2019, we announced that we entered into a definitive agreement to combine Upjohn with Mylan, creating a new global pharmaceutical company, Viatris. Under the terms of the agreement, which is structured as an all-stock, Reverse Morris Trust transaction, Upjohn is expected to be spun off or split off to Pfizer’s shareholders and, immediately thereafter, combined with Mylan. Pfizer shareholders would own |
• | Acquisition of Therachon Holding AG––On July 1, 2019, we acquired all the remaining shares of Therachon for $ |
• | Sale of Hospira Infusion Systems Net Assets to ICU Medical, Inc.––On February 3, 2017, we completed the sale of our global infusion systems net assets, HIS, to ICU Medical for up to approximately $ |
2019 Financial Report | 69 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
• | Acquisition of AstraZeneca’s Small Molecule Anti-Infectives Business––On December 22, 2016, which fell in the first fiscal quarter of 2017 for our international operations, we acquired the development and commercialization rights to AstraZeneca’s small molecule anti-infectives business, primarily outside the U.S. for approximately $ |
For additional information, see Note 2.
B. Adoption of New Accounting Standards in 2019
On January 1, 2019, we adopted four new accounting standards.
Leases––On January 1, 2019, we adopted a new accounting standard for leases and changed our lease policies accordingly. Under the new standard, the most significant change is the requirement of balance sheet recognition of ROU assets and lease liabilities by lessees for those leases classified as operating leases. We adopted the new accounting standard utilizing the modified retrospective method using a simplified transition approach, and, therefore, no adjustments were made to our prior period financial statements. We have elected the package of practical expedients for transition which are permitted in the new standard. Accordingly, we did not reassess whether (i) any expired or existing contracts are or contain leases under the new standard, (ii) classification of leases as operating leases or capital leases would be different under the new standard, or (iii) any initial direct costs would have met the definition of initial direct costs under the new standard. Additionally, we did not elect to use hindsight in determining the lease term for existing leases as of January 1, 2019. We recorded noncurrent ROU assets of $1.4 billion and current and noncurrent operating lease liabilities of $1.4 billion as of January 1, 2019. We also recorded the cumulative effect of adopting the standard as an adjustment to increase the opening balance of Retained earnings by $30 million on a pre-tax basis ($20 million after-tax), relating to previously deferred sale-leaseback gains that can be recognized under the new rules.
Adopting the standard related to leases impacted our prior period consolidated balance sheet as follows: | ||||||||||||
(MILLIONS OF DOLLARS) | As Previously Reported Balance at December 31, 2018 | Effect of Change Higher/(Lower) | Balance at January 1, 2019 | |||||||||
Other current assets | $ | $ | ( | ) | $ | |||||||
Noncurrent deferred tax assets and other noncurrent tax assets | ( | ) | ||||||||||
Other noncurrent assets | ||||||||||||
Other current liabilities | ||||||||||||
Other noncurrent liabilities | ||||||||||||
Retained earnings | ||||||||||||
Adoption of the standard related to leases did not have a material impact on our consolidated statements of income or consolidated statements of cash flows in 2019. For additional information, see Note 1T.
Amortization Period for Certain Callable Debt Securities Held at a Premium––We prospectively adopted the standard, which shortens the amortization period for certain callable debt securities held at a premium. The new guidance requires the premium to be amortized to the earliest call date. We do not have any investments with features subject to this standard and, therefore, there was no impact to our consolidated financial statements from the adoption of this new standard.
Accounting for Certain Financial Instruments with Characteristics of Liabilities and Equity and Accounting for Certain Financial Instruments with Down Round Features––We prospectively adopted the standard, which changes the accounting for warrants or convertible instruments that include a down round feature. We do not have any financial instruments with features subject to this standard and, therefore, there was no impact to our consolidated financial statements from the adoption of this new standard.
Accounting for Share-Based Payments to Nonemployees––We prospectively adopted the standard, which simplifies the accounting for share-based payments to nonemployees by aligning it with the accounting for share-based payments to employees, with certain exceptions. Under the guidance, the measurement of equity-classified nonemployee awards will be fixed at the grant date. We do not have any share-based awards issued to nonemployees and, therefore, there was no impact to our consolidated financial statements from the adoption of this new standard.
C. Estimates and Assumptions
In preparing the consolidated financial statements, we use certain estimates and assumptions that affect reported amounts and disclosures, including amounts recorded and disclosed in connection with acquisitions. These estimates and underlying assumptions can impact all elements of our financial statements. For example, in the consolidated statements of income, estimates are used when accounting for deductions from revenues (such as rebates, chargebacks, sales allowances and sales returns), determining the cost of inventory that is sold, allocating cost in the form of depreciation and amortization, and estimating restructuring charges and the impact of contingencies, as well as determining provisions for taxes on income. On the consolidated balance sheets, estimates are used in determining the valuation and recoverability of assets, such as accounts receivable, investments, inventories, deferred tax assets, fixed assets and intangible assets (including acquired IPR&D assets), and estimates are used in determining the reported amounts of liabilities, such as taxes payable, benefit obligations, accruals for contingencies, rebates, chargebacks, sales allowances and sales returns, and restructuring reserves, all of which also impact the consolidated statements of income.
70 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Our estimates are often based on complex judgments and assumptions that we believe to be reasonable, but that can be inherently uncertain and unpredictable. If our estimates and assumptions are not representative of actual outcomes, our results could be materially impacted.
As future events and their effects cannot be determined with precision, our estimates and assumptions may prove to be incomplete or inaccurate, or unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions. We are subject to risks and uncertainties that may cause actual results to differ from estimated amounts, such as changes in the healthcare environment, competition, litigation, legislation and regulations. We regularly evaluate our estimates and assumptions using historical experience and expectations about the future. We adjust our estimates and assumptions when facts and circumstances indicate the need for change.
For information on estimates and assumptions in connection with the TCJA, see Notes to Consolidated Financial Statements––Note 5A. Tax Matters: Taxes on Income from Continuing Operations.
D. Acquisitions
Our consolidated financial statements include the operations of acquired businesses after the completion of the acquisitions. We account for acquired businesses using the acquisition method of accounting, which requires, among other things, that most assets acquired and liabilities assumed be recognized at their estimated fair values as of the acquisition date and that the fair value of acquired IPR&D be recorded on the balance sheet. Transaction costs are expensed as incurred. Any excess of the consideration transferred over the assigned values of the net assets acquired is recorded as goodwill. When we acquire net assets that do not constitute a business, as defined in U.S. GAAP, no goodwill is recognized and acquired IPR&D is expensed.
Contingent consideration in a business combination is included as part of the acquisition cost and is recognized at fair value as of the acquisition date. Fair value is generally estimated by using a probability-weighted discounted cash flow approach. See Note 16D. Any liability resulting from contingent consideration is remeasured to fair value at each reporting date until the contingency is resolved. These changes in fair value are recognized in earnings in Other (income)/deductions––net.
E. Fair Value
We are often required to measure certain assets and liabilities at fair value, either upon initial recognition or for subsequent accounting or reporting. For example, we use fair value extensively in the initial recognition of net assets acquired in a business combination, when measuring certain impairment losses and when accounting for and reporting of certain financial instruments. We estimate fair value using an exit price approach, which requires, among other things, that we determine the price that would be received to sell an asset or paid to transfer a liability in an orderly market. The determination of an exit price is considered from the perspective of market participants, considering the highest and best use of non-financial assets and, for liabilities, assuming that the risk of non-performance will be the same before and after the transfer.
When estimating fair value, depending on the nature and complexity of the asset or liability, we may use one or all of the following techniques:
• | Income approach, which is based on the present value of a future stream of net cash flows. |
• | Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities. |
• | Cost approach, which is based on the cost to acquire or construct comparable assets, less an allowance for functional and/or economic obsolescence. |
Our fair value methodologies depend on the following types of inputs:
• | Quoted prices for identical assets or liabilities in active markets (Level 1 inputs). |
• | Quoted prices for similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active, or inputs other than quoted prices that are directly or indirectly observable, or inputs that are derived principally from, or corroborated by, observable market data by correlation or other means (Level 2 inputs). |
• | Unobservable inputs that reflect estimates and assumptions (Level 3 inputs). |
F. Foreign Currency Translation
For most of our international operations, local currencies have been determined to be the functional currencies. We translate functional currency assets and liabilities to their U.S. dollar equivalents at exchange rates in effect as of the balance sheet date and we translate functional currency income and expense amounts to their U.S. dollar equivalents at average exchange rates for the period. The U.S. dollar effects that arise from changing translation rates are recorded in Other comprehensive income/(loss). The effects of converting non-functional currency monetary assets and liabilities into the functional currency are recorded in Other (income)/deductions––net. For operations in highly inflationary economies, we translate monetary items at rates in effect as of the balance sheet date, with translation adjustments recorded in Other (income)/deductions––net, and we translate non-monetary items at historical rates.
2019 Financial Report | 71 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
G. Revenues and Trade Accounts Receivable
We recorded direct product sales and/or alliance revenues of more than $1 billion for each of eight products in 2019, for each of ten products in 2018 and for each of nine products in 2017. In the aggregate, these direct products sales and/or alliance product revenues represent 49 % of our revenues in 2019, 51 % of our revenues in 2018 and 46 % of our revenues in 2017. See Note 17C for additional information. The loss or expiration of intellectual property rights can have a significant adverse effect on our revenues as our contracts with customers will generally be at lower selling prices due to added competition and we generally provide for higher sales returns during the period in which individual markets begin to near the loss or expiration of intellectual property rights. Our Consumer Healthcare business, which was combined with GSK’s Consumer Healthcare business into a new consumer healthcare joint venture that operates globally under the GSK Consumer Healthcare name on July 31, 2019, included OTC brands with a focus on dietary supplements, pain management, gastrointestinal and respiratory and personal care. We sell biopharmaceutical products after patent expiration, and under patent, and, to a much lesser extent, through July 31, 2019, we sold consumer healthcare products worldwide to developed and emerging market countries.
Revenue Recognition––We record revenues from product sales when there is a transfer of control of the product from us to the customer. We determine transfer of control based on when the product is shipped or delivered and title passes to the customer.
• | Customers––Our biopharmaceutical products are sold principally to wholesalers but we also sell directly to retailers, hospitals, clinics, government agencies and pharmacies, and, in the case of our vaccine products in the U.S., we primarily sell directly to the CDC, wholesalers, individual provider offices, retail pharmacies and integrated delivery networks. Customers for our consumer healthcare business, which were part of the business that was combined with GSK’s Consumer Healthcare business into a new consumer healthcare joint venture on July 31, 2019, included retailers and, to a lesser extent, wholesalers and distributors. |
Biopharmaceutical products that ultimately are used by patients are generally covered under governmental programs, managed care programs and insurance programs, including those managed through PBMs, and are subject to sales allowances and/or rebates payable directly to those programs. Those sales allowances and rebates are generally negotiated, but government programs may have legislated amounts by type of product (e.g., patented or unpatented).
• | Our Sales Contracts––Sales on credit are typically under short-term contracts. Collections are based on market payment cycles common in various markets, with shorter cycles in the U.S. Sales are adjusted for sales allowances, chargebacks, rebates and sales returns and cash discounts. Sales returns occur due to loss of exclusivity, product recalls or a changing competitive environment. |
• | Deductions from Revenues––Our gross product revenues are subject to a variety of deductions, which generally are estimated and recorded in the same period that the revenues are recognized. Such variable consideration represents chargebacks, rebates, sales allowances and sales returns. These deductions represent estimates of the related obligations and, as such, knowledge and judgment is required when estimating the impact of these revenue deductions on gross sales for a reporting period. |
Specifically:
• | In the U.S., we sell our products to distributors and hospitals under our sales contracts. However, we also have contracts with managed care or pharmacy benefit managers and legislatively mandated contracts with the federal and state governments under which we provide rebates to them based on medicines utilized by the lives they cover. We record provisions for Medicare, Medicaid, and performance-based contract pharmaceutical rebates based upon our experience ratio of rebates paid and actual prescriptions written during prior quarters. We apply the experience ratio to the respective period’s sales to determine the rebate accrual and related expense. This experience ratio is evaluated regularly to ensure that the historical trends are as current as practicable. We estimate discounts on branded prescription drug sales to Medicare Part D participants in the Medicare “coverage gap,” also known as the “doughnut hole,” based on the historical experience of beneficiary prescriptions and consideration of the utilization that is expected to result from the discount in the coverage gap. We evaluate this estimate regularly to ensure that the historical trends and future expectations are as current as practicable. For performance-based contract rebates, we also consider current contract terms, such as changes in formulary status and rebate rates. |
• | Outside the U.S., the majority of our pharmaceutical sales allowances are contractual or legislatively mandated and our estimates are based on actual invoiced sales within each period, which reduces the risk of variations in the estimation process. In certain European countries, rebates are calculated on the government’s total unbudgeted pharmaceutical spending or on specific product sales thresholds and we apply an estimated allocation factor against our actual invoiced sales to project the expected level of reimbursement. We obtain third-party information that helps us to monitor the adequacy of these accruals. |
• | Provisions for pharmaceutical chargebacks (primarily reimbursements to U.S. wholesalers for honoring contracted prices to third parties) closely approximate actual amounts incurred, as we settle these deductions generally within two to five weeks of incurring the liability. |
• | Provisions for pharmaceutical sales returns are based on a calculation for each market that incorporates the following, as appropriate: local returns policies and practices; historical returns as a percentage of sales; an understanding of the reasons for past returns; estimated shelf life by product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could impact the estimate of future returns, such as loss of exclusivity, product recalls or a changing competitive environment. Generally, returned products are destroyed, and customers are refunded the sales price in the form of a credit. |
• | We record sales incentives as a reduction of revenues at the time the related revenues are recorded or when the incentive is offered, whichever is later. We estimate the cost of our sales incentives based on our historical experience with similar incentives programs to predict customer behavior. |
72 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides information about the balance sheet classification of these accruals: | ||||||||
As of December 31, | ||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | ||||||
Reserve against Trade accounts receivable, less allowance for doubtful accounts | $ | $ | ||||||
Other current liabilities: | ||||||||
Accrued rebates | ||||||||
Other accruals | ||||||||
Other noncurrent liabilities | ||||||||
Total accrued rebates and other accruals | $ | $ | ||||||
Amounts recorded for revenue deductions can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Note 1C.
Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from Revenues.
Trade Accounts Receivable—Trade accounts receivable are stated at their net realizable value. The allowance against gross trade accounts receivable reflects the best estimate of probable losses inherent in the receivables portfolio determined on the basis of historical experience, specific allowances for known troubled accounts and other current information. Trade accounts receivable are written off after all reasonable means to collect the full amount (including litigation, where appropriate) have been exhausted.
H. Collaborative Arrangements
Payments to and from our collaboration partners are presented in our consolidated statements of income based on the nature of the arrangement (including its contractual terms), the nature of the payments and applicable accounting guidance. Under co-promotion agreements, we record the amounts received from our collaboration partners as alliance revenues, a component of Revenues, when our collaboration partners are the principal in the transaction and we receive a share of their net sales or profits. Alliance revenues are recorded as we perform co-promotion services for the collaboration and the collaboration partners sell the products to their customers within the applicable period. The related expenses for selling and marketing these products are included in Selling, informational and administrative expenses. In collaborative arrangements where we manufacture a product for our collaboration partners, we record revenues when we transfer control of the product to our collaboration partners. In collaboration arrangements where we are the principal in the transaction, we record amounts paid to collaboration partners for their share of net sales or profits earned, and all royalty payments to collaboration partners as Cost of sales. Royalty payments received from collaboration partners are included in Other (income)/deductions—net.
Reimbursements to or from our collaboration partners for development costs are recorded net in Research and development expenses. Upfront payments and pre-approval milestone payments due from us to our collaboration partners in development stage collaborations are recorded as Research and development expenses. Milestone payments due from us to our collaboration partners after regulatory approval has been attained for a medicine are recorded in Identifiable intangible assets—Developed technology rights. Upfront and pre-approval milestone payments earned from our collaboration partners by us are recognized in Other (income)/deductions—net over the development period for the collaboration products, when our performance obligations include providing R&D services to our collaboration partners. Upfront, pre-approval and post-approval milestone payments earned by us may be recognized in Other (income)/deductions—net immediately when earned or over other periods depending upon the nature of our performance obligations in the applicable collaboration. Where the milestone event is regulatory approval for a medicine, we generally recognize milestone payments due to us in the transaction price when regulatory approval in the applicable jurisdiction has been attained. We may recognize milestone payments due to us in the transaction price earlier than the milestone event in certain circumstances when recognition of the income would not be probable of a significant reversal.
I. Cost of Sales and Inventories
We carry inventories at the lower of cost or net realizable value. The cost of finished goods, work in process and raw materials is determined using average actual cost. We regularly review our inventories for impairment and reserves are established when necessary.
J. Selling, Informational and Administrative Expenses
K. Research and Development Expenses
R&D costs are expensed as incurred. These expenses include the costs of our proprietary R&D efforts, as well as costs incurred in connection with certain licensing arrangements. Before a compound receives regulatory approval, we record upfront and milestone payments made by us to third parties under licensing arrangements as expense. Upfront payments are recorded when incurred, and milestone payments are recorded when the specific milestone has been achieved. Once a compound receives regulatory approval, we record any milestone payments in Identifiable intangible assets, less accumulated amortization and, unless the asset is determined to have an indefinite life, we amortize the payments on a straight-line basis over the remaining agreement term or the expected product life cycle, whichever is shorter.
2019 Financial Report | 73 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
L. Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets
Long-lived assets include:
• | Property, plant and equipment, less accumulated depreciation—These assets are recorded at cost and are increased by the cost of any significant improvements after purchase. Property, plant and equipment assets, other than land and construction in progress, are depreciated on a straight-line basis over the estimated useful life of the individual assets. Depreciation begins when the asset is ready for its intended use. For tax purposes, accelerated depreciation methods are used as allowed by tax laws. |
• | Identifiable intangible assets, less accumulated amortization—These acquired assets are recorded at fair value. Intangible assets with finite lives are amortized on a straight-line basis over their estimated useful lives. Intangible assets with indefinite lives that are associated with marketed products are not amortized until a useful life can be determined. |
• | Goodwill—Goodwill represents the excess of the consideration transferred for an acquired business over the assigned values of its net assets. Goodwill is not amortized. |
Amortization expense related to finite-lived acquired intangible assets that contribute to our ability to sell, manufacture, research, market and distribute products, compounds and intellectual property is included in Amortization of intangible assets as these intangible assets benefit multiple business functions. Amortization expense related to intangible assets that are associated with a single function and depreciation of property, plant and equipment are included in Cost of sales, Selling, informational and administrative expenses and/or Research and development expenses, as appropriate.
We review all of our long-lived assets for impairment indicators throughout the year. We perform impairment testing for indefinite-lived intangible assets and goodwill at least annually and for all other long-lived assets whenever impairment indicators are present. When necessary, we record charges for impairments of long-lived assets for the amount by which the fair value is less than the carrying value of these assets.
Specifically:
• | For finite-lived intangible assets, such as developed technology rights, and for other long-lived assets, such as property, plant and equipment, whenever impairment indicators are present, we calculate the undiscounted value of the projected cash flows associated with the asset, or asset group, and compare this estimated amount to the carrying amount. If the carrying amount is found to be greater, we record an impairment loss for the excess of book value over fair value. In addition, in all cases of an impairment review, we reevaluate the remaining useful lives of the assets and modify them, as appropriate. |
• | For indefinite-lived intangible assets, such as Brands and IPR&D assets, when necessary, we determine the fair value of the asset and record an impairment loss, if any, for the excess of book value over fair value. In addition, in all cases of an impairment review other than for IPR&D assets, we re-evaluate whether continuing to characterize the asset as indefinite-lived is appropriate. |
• | For goodwill, when necessary, we determine the fair value of each reporting unit and compare that value to its book value. If the carrying amount is found to be greater, we then determine the implied fair value of goodwill by subtracting the fair value of all the identifiable net assets other than goodwill from the fair value of the reporting unit and record an impairment loss, if any, for the excess of the book value of goodwill over the implied fair value. |
Impairment reviews can involve a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Note 1C.
M. Restructuring Charges and Certain Acquisition-Related Costs
We may incur restructuring charges in connection with acquisitions when we implement plans to restructure and integrate the acquired operations or in connection with our cost-reduction and productivity initiatives. Included in Restructuring charges and certain acquisition-related costs are all restructuring charges, as well as certain other costs associated with acquiring and integrating an acquired business. If the restructuring action results in a change in the estimated useful life of an asset, that incremental impact is classified in Cost of sales, Selling, informational and administrative expenses and/or Research and development expenses, as appropriate. Termination costs are generally recorded when the actions are probable and estimable. Transaction costs, such as banking, legal, accounting and other similar costs incurred in connection with a business acquisition are expensed as incurred.
N. Cash Equivalents and Statement of Cash Flows
Cash equivalents include items almost as liquid as cash, such as certificates of deposit and time deposits with maturity periods of three months or less when purchased. If items meeting this definition are part of a larger investment pool, we classify them as Short-term investments.
Cash flows associated with financial instruments designated as fair value or cash flow hedges may be included in operating, investing or financing activities, depending on the classification of the items being hedged. Cash flows associated with financial instruments designated as net investment hedges are classified according to the nature of the hedge instrument. Cash flows associated with financial instruments that do not qualify for hedge accounting treatment are classified according to their purpose and accounting nature.
O. Investments and Derivative Financial Instruments
Our investments are comprised of the following: public equity securities with readily determinable fair values, available-for-sale debt securities, held-to-maturity debt securities (when we have both the positive intent and ability to hold the investment to maturity), private equity securities
74 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
without readily determinable fair values and equity-method investments. The classification of an investment can depend on the nature of the investment, our intent and ability to hold the investment, and the degree to which we may exercise influence.
• | Public equity securities with readily determinable fair values are carried at fair value, with changes in fair value reported in Other (income)/deductions—net. |
• | Available-for-sale debt securities are carried at fair value, with changes in fair value reported in Other comprehensive income/(loss) until realized. |
• | Held-to-maturity debt securities are carried at amortized cost. |
• | Private equity securities without readily determinable fair values and where we have no significant influence are measured at cost minus any impairment and plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. |
• | For equity investments in common stock or in-substance common stock where we have significant influence over the financial and operating policies of the investee, we use the equity-method of accounting. Under the equity-method, we record our share of the investee’s income and expenses in Other (income)/deductions—net. The excess of the cost of the investment over our share of the underlying equity in the net assets of the investee as of the acquisition date is allocated to the identifiable assets and liabilities of the investee, with any remaining excess amount allocated to goodwill. Such investments are initially recorded at cost, which is the fair value of consideration paid and typically does not include contingent consideration. |
Realized gains or losses on sales of investments are determined by using the specific identification cost method.
We regularly evaluate all of our financial assets for impairment. For investments in debt and equity, when a decline in fair value, if any, is determined, an impairment charge is recorded and a new cost basis in the investment is established.
Derivative financial instruments are carried at fair value in various balance sheet categories (see Note 7A), with changes in fair value reported in Net income or, for derivative financial instruments in certain qualifying hedging relationships, in Other comprehensive income/(loss) (see Note 7F).
A single estimate of fair value and impairment reviews can involve a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Note 1C.
P. Tax Assets and Liabilities and Income Tax Contingencies
Tax Assets and Liabilities
Current tax assets primarily includes (i) tax effects associated with intercompany transfers of inventory within our combined group, which are recognized in the consolidated statements of income when the inventory is sold to a third party, as well as (ii) income tax receivables that are expected to be recovered either as refunds from taxing authorities or as a reduction to future tax obligations.
Deferred tax assets and liabilities are recognized for the expected future tax consequences of differences between the financial reporting and tax bases of assets and liabilities using enacted tax rates and laws, including the impact of the TCJA enacted in December 2017. We provide a valuation allowance when we believe that our deferred tax assets are not recoverable based on an assessment of estimated future taxable income that incorporates ongoing, prudent and feasible tax-planning strategies, that would be implemented, if necessary, to realize the deferred tax assets. All deferred tax assets and liabilities within the same tax jurisdiction are presented as a net amount in the noncurrent section of our consolidated balance sheet. Amounts recorded for valuation allowances can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Note 1C.
Other non-current tax assets primarily represent our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction. These potential benefits generally result from cooperative efforts among taxing authorities, as required by tax treaties to minimize double taxation, commonly referred to as the competent authority process. The recoverability of these assets, which we believe to be more likely than not, is dependent upon the actual payment of taxes in one tax jurisdiction and, in some cases, the successful petition for recovery in another tax jurisdiction.
Other taxes payable in our consolidated balance sheet as of December 31, 2019 includes liabilities for uncertain tax positions and the noncurrent portion of the repatriation tax liability on the deemed repatriated accumulated post-1986 foreign earnings recorded in connection with the TCJA for which we elected, with the filing of our 2018 U.S. Federal Consolidated Income Tax Return, payment over eight years through 2026. For additional information, see Note 5D for uncertain tax positions and Note 5A for the repatriation tax liability.
Income Tax Contingencies
We account for income tax contingencies using a benefit recognition model. If we consider that a tax position is more likely than not to be sustained upon audit, based solely on the technical merits of the position, we recognize the benefit. We measure the benefit by determining the amount that is greater than 50% likely of being realized upon settlement, presuming that the tax position is examined by the appropriate taxing authority that has full knowledge of all relevant information.
Under the benefit recognition model, if our initial assessment fails to result in the recognition of a tax benefit, we regularly monitor our position and subsequently recognize the tax benefit: (i) if there are changes in tax law, analogous case law or there is new information that sufficiently raise the likelihood of prevailing on the technical merits of the position to “more likely than not”; (ii) if the statute of limitations expires; or (iii) if there is a completion of an audit resulting in a favorable settlement of that tax year with the appropriate agency. We regularly re-evaluate our tax positions based on the results of audits of federal, state and local and foreign income tax filings, statute of limitations expirations, changes and clarification in tax law or receipt of new information that would either increase or decrease the technical merits of a position relative to the
2019 Financial Report | 75 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
more-likely-than-not standard. Liabilities associated with uncertain tax positions are classified as current only when we expect to pay cash within the next 12 months. Interest and penalties, if any, are recorded in Provision/(benefit) for taxes on income and are classified on our consolidated balance sheet with the related tax liability.
Q. Pension and Postretirement Benefit Plans
The majority of our employees worldwide are covered by defined benefit pension plans, defined contribution plans or both. In the U.S., we have both IRC-qualified and supplemental (non-qualified) defined benefit plans and defined contribution plans, as well as other postretirement benefit plans consisting primarily of medical insurance for retirees and their eligible dependents. We recognize the overfunded or underfunded status of each of our defined benefit plans as an asset or liability on our consolidated balance sheet. The obligations are generally measured at the actuarial present value of all benefits attributable to employee service rendered, as provided by the applicable benefit formula. Our pension and other postretirement obligations may include assumptions such as expected employee turnover and participant mortality. For our pension plans, the obligation may also include assumptions as to future compensation levels. For our other postretirement benefit plans, the obligation may include assumptions as to the expected cost of providing medical insurance benefits, as well as the extent to which those costs are shared with the employee or others (such as governmental programs). Plan assets are measured at fair value. Net periodic pension and postretirement benefit costs other than the service costs are recognized in Other (income)/deductions—net.
R. Legal and Environmental Contingencies
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business, such as patent litigation, product liability and other product-related litigation, commercial litigation, environmental claims and proceedings, government investigations and guarantees and indemnifications. We record accruals for these contingencies to the extent that we conclude that a loss is both probable and reasonably estimable. If some amount within a range of loss appears to be a better estimate than any other amount within the range, we accrue that amount. Alternatively, when no amount within a range of loss appears to be a better estimate than any other amount, we accrue the lowest amount in the range. We record anticipated recoveries under existing insurance contracts when recovery is assured.
S. Share-Based Payments
Our compensation programs can include share-based payments. Generally, grants under share-based payment programs are accounted for at fair value and these fair values are generally amortized on a straight-line basis over the vesting terms into Cost of sales, Selling, informational and administrative expenses and/or Research and development expenses, as appropriate.
T. Leases
On January 1, 2019, we adopted a new accounting standard for leases. For further information, see Note 1B.
We lease real estate, fleet, and equipment for use in our operations. Our leases generally have lease terms of 1 to 30 years, some of which include options to terminate or extend leases for up to 5 to 10 years or on a month-to-month basis. We include options that are reasonably certain to be exercised as part of the determination of lease terms. We may negotiate termination clauses in anticipation of any changes in market conditions, but generally these termination options are not exercised. Residual value guarantees are generally not included within our operating leases with the exception of some fleet leases. In addition to base rent payments, the leases may require us to pay directly for taxes and other non-lease components, such as insurance, maintenance and other operating expenses, which may be dependent on usage or vary month-to-month. Variable lease payments amounted to $328 million for the year ended December 31, 2019. We have elected the practical expedient in the new standard to not separate non-lease components from lease components in calculating the amounts of ROU assets and lease liabilities for all underlying asset classes.
We determine if an arrangement is a lease at inception of the contract in accordance with guidance detailed in the new standard and we perform the lease classification test as of the lease commencement date. ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of our leases do not provide an implicit rate, we use our estimated incremental borrowing rate based on the information available at commencement date in determining the present value of future payments.
76 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
For operating leases, the ROU assets and liabilities are presented in our consolidated balance sheet as follows: | ||||||
Balance at | ||||||
(MILLIONS OF DOLLARS) | Balance Sheet Classification | December 31, 2019 | ||||
ROU assets | Other noncurrent assets | $ | ||||
Lease liabilities (short-term) | Other current liabilities | |||||
Lease liabilities (long-term) | Other noncurrent liabilities | |||||
Our total lease costs are as follows: | ||||
Year Ended | ||||
(MILLIONS OF DOLLARS) | December 31, 2019 | |||
Operating lease cost | $ | |||
Variable lease cost | ||||
Sublease income | ( | ) | ||
Total lease cost | $ | |||
Other supplemental information includes the following: | |||||||||
Weighted-Average Remaining Contractual Lease Term (Years) as of | Weighted-Average Discount Rate as of | Year Ended | |||||||
(MILLIONS OF DOLLARS) | December 31, 2019 | December 31, 2019 | December 31, 2019 | ||||||
Operating leases | % | ||||||||
Cash paid for amounts included in the measurement of lease liabilities: | |||||||||
Operating cash flows from operating leases | $ | ||||||||
(Gains)/losses on sale and leaseback transactions, net | ( | ) | |||||||
ROU assets obtained in exchange for new operating lease liabilities | |||||||||
The table below reconciles the undiscounted cash flows for the first five years and total of the remaining years to the operating lease liabilities recorded in the consolidated balance sheet as of December 31, 2019: | ||||
(MILLIONS OF DOLLARS) | ||||
Period | Operating Lease Liabilities | |||
Next one year(a) | $ | |||
1-2 years | ||||
2-3 years | ||||
3-4 years | ||||
4-5 years | ||||
Thereafter | ||||
Total undiscounted lease payments | ||||
Less: Imputed interest | ||||
Present value of minimum lease payments | ||||
Less: Current portion | ||||
Noncurrent portion | $ | |||
(a) | Reflects lease payments due within 12 months subsequent to the balance sheet date. |
In April 2018, we entered an agreement to lease space in an office building in New York City. We expect to take control of the property in 2021 and relocate our global headquarters to this new office building in 2022. Our future minimum rental commitment under this 20 -year lease is approximately $1.7 billion.
Prior to our adoption of the new lease standard, rental expense, net of sublease income, was $301 million in 2018 and $314 million in 2017.
As of December 31, 2018, the future minimum rental commitments under non-cancelable operating leases follow: | ||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2020 | 2021 | 2022 | 2023 | After 2023 | ||||||||||||||||||
Lease commitments | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
2019 Financial Report | 77 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 2. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements
A. Acquisitions
Array BioPharma Inc.
On July 30, 2019, we acquired Array, a commercial stage biopharmaceutical company focused on the discovery, development and commercialization of targeted small molecule medicines to treat cancer and other diseases of high unmet need, for $48 per share in cash. The total fair value of the consideration transferred for Array was approximately $11.2 billion ($10.9 billion, net of cash acquired). In addition, approximately $157 million in payments to Array employees for the fair value of previously unvested stock options was recognized as post-closing compensation expense and recorded in Restructuring charges and certain acquisition-related costs in the consolidated statement of income in the third quarter of 2019 (see Note 3). We financed the majority of the transaction with debt and the balance with existing cash.
Array’s portfolio includes the approved combined use of Braftovi (encorafenib) and Mektovi (binimetinib) for the treatment of BRAFV600E- or BRAFV600K-mutant unresectable or metastatic melanoma. The combination therapy has significant potential for long-term growth via expansion into additional areas of unmet need and is currently being investigated in over 30 clinical trials across several solid tumor indications, including in BRAF-mutant mCRC, through collaborations with third parties. In December 2019, the FDA accepted and granted priority review to our supplemental new drug application for Braftovi in combination with Erbitux (cetuximab) (Braftovi Doublet) in BRAF-mutant mCRC. Pfizer has exclusive rights to commercialize Braftovi and Mektovi in the U.S. and Canada. In addition to the combination therapy for BRAF-mutant metastatic melanoma, Array brings a broad pipeline of targeted cancer medicines in different stages of R&D, as well as a portfolio of out-licensed medicines, which may generate milestones and royalties over time.
In connection with this acquisition, we provisionally recorded: (i) $7.2 billion in Identifiable intangible assets, consisting of $1.8 billion of Developed technology rights with a useful life of 16 years, $4.0 billion of IPR&D and $1.4 billion for Licensing agreements ($1.1 billion for technology in development––indefinite-lived licensing agreements and $340 million for developed technology––finite-lived licensing agreements with a useful life of 10 years), (ii) $5.4 billion of Goodwill, (iii) $1.3 billion of net deferred tax liabilities and (iv) $451 million of assumed long-term debt, which was paid in full in the third quarter of 2019. The allocation of the consideration transferred to the assets acquired and the liabilities assumed has not yet been finalized.
Therachon Holding AG
On July 1, 2019, we acquired all the remaining shares of Therachon, a privately-held clinical-stage biotechnology company focused on rare diseases, with assets in development for the treatment of achondroplasia, a genetic condition and the most common form of short-limb dwarfism, for $340 million upfront, plus potential milestone payments of up to $470 million contingent on the achievement of key milestones in the development and commercialization of the lead asset. In 2018, we acquired approximately 3 % of Therachon’s outstanding shares for $5 million. We accounted for the transaction as an asset acquisition since the lead asset represented substantially all the fair value of the gross assets acquired. The total fair value of the consideration transferred for Therachon was approximately $322 million, which consisted of $317 million of cash and our previous $5 million investment in Therachon. Therachon is a wholly-owned subsidiary of Pfizer. In connection with this asset acquisition, we recorded a charge of $337 million in Research and development expenses.
AstraZeneca’s Small Molecule Anti-Infectives Business
On December 22, 2016, which fell in the first fiscal quarter of 2017 for our international operations, we acquired the development and commercialization rights to AstraZeneca’s small molecule anti-infectives business, primarily outside the U.S., including the commercialization and development rights to the marketed products Zavicefta™ (ceftazidime-avibactam), Merrem™/Meronem™ (meropenem) and Zinforo™ (ceftaroline fosamil), and the clinical development assets ATM-AVI and CXL (ceftaroline fosamil-AVI). In 2017, under the terms of the agreement, we made payments of approximately $605 million to AstraZeneca related to the transaction. We made an additional milestone payment of $125 million in our first fiscal quarter of 2018, we made a deferred payment of $175 million to AstraZeneca in January 2019, and we made an additional milestone payment of $75 million in our third fiscal quarter of 2019. We may make payments of up to $600 million to AstraZeneca if sales of Zavicefta™ exceed certain thresholds prior to January 1, 2026, as well as tiered royalties on sales of Zavicefta™ and ATM-AVI in certain markets for a period ending on the later of 10 years from first commercial sale or the loss of patent protection or loss of regulatory exclusivity. The total royalty payments are unlimited during the royalty term and the undiscounted payments are expected to be in the range of approximately $315 million to $542 million. The total fair value of consideration transferred for AstraZeneca’s small molecule anti-infectives business was approximately $1.0 billion inclusive of cash paid of $555 million and the fair value of contingent consideration of $485 million (which is composed of the fair values of the deferred payment, the $50 million milestone payment made in the second quarter of 2017, the $125 million milestone payment made in our first fiscal quarter of 2018, the $75 million milestone payment made in the third quarter of 2019, and the future expected milestone and royalty payments). In connection with this acquisition, we recorded $894 million in Identifiable intangible assets, consisting of $728 million in Developed technology rights and $166 million in IPR&D. We also recorded $92 million in Other current assets related to the economic value of inventory which was retained by AstraZeneca for sale on our behalf, $73 million in Goodwill and $19 million of net deferred tax liabilities. The final allocation of the consideration transferred to the assets acquired and the liabilities assumed has been completed.
Medivation, Inc.
On September 28, 2016, we acquired Medivation for $81.50 per share. The total fair value of consideration transferred for Medivation was approximately $14.3 billion in cash ($13.9 billion, net of cash acquired). Of this consideration, approximately $365 million was not paid as of December 31, 2016, and was recorded in Other current liabilities. The remaining consideration was paid as of December 31, 2017. Medivation is a wholly-owned subsidiary of Pfizer. Medivation is focused on developing and commercializing small molecules for oncology. Medivation’s portfolio includes Xtandi (enzalutamide). Xtandi is FDA-approved for the treatment of non-metastatic and metastatic castration-resistant
78 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
prostate cancer as well as metastatic castration sensitive prostate cancer. Xtandi is being developed and commercialized through a collaboration with Astellas. Astellas has exclusive commercialization rights for Xtandi outside the U.S. The Medivation portfolio also includes talazoparib, which was approved by the FDA in October 2018, under the trade name Talzenna, for the treatment of adults with germline BRCA-mutated HER2-negative locally advanced or metastatic breast cancer and is currently in development for other types of cancer. In connection with this acquisition, we recorded $12.2 billion in Identifiable intangible assets, primarily consisting of $8.1 billion of Developed technology rights with an average useful life of approximately 12 years and $4.1 billion of IPR&D, and recorded $6.1 billion of Goodwill, $4.0 billion of net income tax liabilities, and $259 million of assumed contingent consideration of which $51 million has been paid through December 31, 2019. In 2017 and 2016, we recorded measurement period adjustments to the estimated fair values initially recorded in 2016, which resulted in a reduction in Identifiable intangible assets of approximately $1.0 billion with a corresponding change to Goodwill and net income tax liabilities. The measurement period adjustments were recorded to better reflect market participant assumptions about facts and circumstances existing as of the acquisition date. The 2017 results included a decrease of approximately $38 million to Amortization of intangible assets which reflected the cumulative pre-tax impact of the measurement period adjustments to Identifiable intangible assets that were amortized to the income statement since the acquisition date. The measurement period adjustments did not result from intervening events subsequent to the acquisition date. The final allocation of the consideration transferred to the assets acquired and the liabilities assumed has been completed.
B. Divestitures
Sale of Hospira Infusion Systems Net Assets to ICU Medical, Inc.
On October 6, 2016, we announced that we entered into a definitive agreement under which ICU Medical, a global device manufacturer, agreed to acquire all of our global infusion systems net assets, HIS, for approximately $1 billion in cash and ICU Medical common stock. HIS includes IV pumps, solutions, and devices. As a result of the performance of HIS relative to ICU Medical’s expectations, on January 5, 2017 we entered into a revised agreement with ICU Medical under which ICU Medical would acquire HIS for up to approximately $900 million, composed of cash and contingent cash consideration, ICU Medical common stock and seller financing.
The revised transaction closed on February 3, 2017. At closing, we received 3.2 million newly issued shares of ICU Medical common stock (as originally agreed), which we initially valued at approximately $428 million (based upon the closing price of ICU Medical common stock on the closing date less a discount for lack of marketability) and which were reported as equity securities at fair value in Long-term investments on the consolidated balance sheet as of December 31, 2017. Upon the sale of these shares in 2018, we realized a full gain of $302 million on these securities, although our income statement only reflects a gain of $47 million as the balance of the previously unrealized gain was recorded as a cumulative effect adjustment upon the adoption of a new accounting standard. We also received a promissory note in the amount of $75 million, which was repaid in full as of December 31, 2017, and net cash of approximately $200 million before customary adjustments for net working capital, which was reported in Other investing activities, net on the consolidated statement of cash flows for the year-ended December 31, 2017. In addition, we are entitled to receive a contingent amount of up to an additional $225 million in cash based on ICU Medical’s achievement of certain cumulative performance targets for the combined company through December 31, 2019. The amount of contingent payment we will receive, if any, will be determined during the first half of 2020. We recognized a pre-tax gain of $1 million in 2018 and a pre-tax loss of $55 million in 2017 in Other (income)/deductions––net, representing adjustments to amounts previously recorded in 2016 to write down the HIS net assets to fair value less costs to sell.
The sale of the HIS net assets was fully completed in all jurisdictions as of year-end 2018.
In connection with the sale transaction, we entered into certain transitional agreements designed to facilitate the orderly transition of the HIS net assets to ICU Medical. These agreements primarily related to administrative services, and were provided for a period of 24 months after the closing date. We will also manufacture and supply certain HIS products for ICU Medical and ICU Medical will manufacture and supply certain retained Pfizer products for us after closing, generally for a term of five years. These agreements are not material to Pfizer and none confers upon us the ability to influence the operating and/or financial policies of ICU Medical subsequent to the sale.
Contribution Agreement Between Pfizer and Allogene Therapeutics, Inc.
In April 2018, Pfizer and Allogene announced that the two companies entered into a contribution agreement for Pfizer’s portfolio of assets related to allogeneic CAR T therapy, an investigational immune cell therapy approach to treating cancer. Under this agreement, Allogene received from Pfizer rights to pre-clinical and clinical CAR T assets, all of which were previously licensed to Pfizer from French cell therapy company, Cellectis, beginning in 2014 and French pharmaceutical company, Servier, beginning in 2015. Allogene assumed responsibility for all potential financial obligations to both Cellectis and Servier. Pfizer continues to participate financially in the development of the CAR T portfolio through an ownership stake in Allogene. Separately, Pfizer continues to maintain its approximate 7 % ownership stake in Cellectis that was obtained in 2014 as part of the licensing agreement in which Pfizer obtained exclusive rights to pursue the development and commercialization of certain Cellectis CAR T therapies in exchange for an upfront payment of $80 million, as well as potential future development, regulatory and commercial milestone payments and royalties. In connection with the Allogene transaction, Pfizer recognized a non-cash $50 million pre-tax gain in Other (income)/deductions––net in the second quarter of 2018, representing the difference between the $127 million fair value of the equity investment received and the book value of assets transferred (including an allocation of goodwill) (see Note 4).
In October 2018, Allogene consummated an initial public offering of new shares of its common stock, which resulted in Pfizer’s preferred stock converting into common stock and a decrease in our ownership percentage from approximately 25 % to approximately 18 % as of December 31, 2018. The closing price on the day of the initial public offering was $25 per share. Beginning as of the date of the initial public offering, our investment in Allogene is being measured at fair value with changes in fair value recognized in net income (see Note 4).
2019 Financial Report | 79 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Sale of Phase 2b Ready AMPA Receptor Potentiator for CIAS to Biogen Inc.
In April 2018, we sold our Phase 2b ready AMPA receptor potentiator for CIAS to Biogen. We received $75 million upfront and have the opportunity to receive up to $515 million in total development and commercialization milestones, as well as tiered royalties in the low-to-mid-teen percentages. We recognized the $75 million upfront payment in Other (income)/deductions––net in the second quarter of 2018 (see Note 4). In the fourth quarter of 2018, we recognized an additional $10 million milestone in Other (income)/deductions––net (see Note 4). We will record the other milestones and royalties to Other (income)/deductions––net when due, or earlier if we have sufficient experience to determine such amounts are not probable of significant reversal.
Divestiture of Neuroscience Assets
In September 2018, we and Bain Capital entered into a transaction to create a new biopharmaceutical company, Cerevel, to continue development of a portfolio of clinical and preclinical stage neuroscience assets primarily targeting disorders of the central nervous system including Parkinson’s disease, epilepsy, Alzheimer’s disease, schizophrenia and addiction. These assets were part of the neuroscience discovery and early development efforts, which we announced we were ending in January 2018. In connection with this transaction, we out-licensed the portfolio to Cerevel in exchange for a 25 % ownership stake in Cerevel’s parent company, Cerevel Therapeutics, Inc., and potential future regulatory and commercial milestone payments and royalties. Bain Capital has committed to invest $350 million to develop the portfolio, with the potential for additional funding as the assets advance. In connection with the transaction, we recognized a non-cash $343 million pre-tax gain in Other (income)/deductions––net in the third quarter of 2018, representing the fair value of the equity investment received as the assets transferred had a book value of $0 (see Note 4). Our investment in Cerevel Therapeutics, Inc. is reported in Long-term investments on the consolidated balance sheets as of December 31, 2019 and December 31, 2018.
C. Equity-Method Investments and Assets and Liabilities Held for Sale
Formation of a New Consumer Healthcare Joint Venture
On July 31, 2019, we completed the transaction in which we and GSK combined our respective consumer healthcare businesses into a new consumer healthcare joint venture that operates globally under the GSK Consumer Healthcare name. In exchange for contributing our Consumer Healthcare business to the joint venture, we received a 32 % equity stake in the new company and GSK owns the remaining 68 %. Upon the closing of the transaction, we deconsolidated our Consumer Healthcare business and recognized a pre-tax gain of $8.1 billion ($5.4 billion, net of tax) in our fiscal third quarter of 2019 in (Gain) on completion of Consumer Healthcare JV transaction for the difference in the fair value of our 32 % equity stake in the new company and the carrying value of our Consumer Healthcare business. We may record additional adjustments to the gain in future periods, which we do not expect to have a material impact on our consolidated financial statements.
In valuing our investment in GSK Consumer Healthcare, we used discounted cash flow techniques. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which include the expected impact of competitive, legal or regulatory forces on the products; the long-term growth rate, which seeks to project the sustainable growth rate over the long term; the discount rate, which seeks to reflect our best estimate of the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows. As part of the joint venture transaction, we agreed to indemnify GSK with respect to certain tax matters related to periods prior to closing of the transaction as well as certain potential environmental or other legal liabilities associated with the previous operation of our Consumer Healthcare business. We recognized a liability of $45 million with respect to the tax matters indemnification. The value of the environmental and legal indemnifications was not considered to be material.
We are accounting for our interest in GSK Consumer Healthcare as an equity-method investment. The carrying value of our investment in GSK Consumer Healthcare is approximately $17.0 billion and it is reported as a private equity investment in the Equity-method investments line in our consolidated balance sheet as of December 31, 2019. Our consolidated statement of income for 2019 includes revenues and expenses associated with Pfizer’s Consumer Healthcare business through July 31, 2019. We record our share of earnings from the Consumer Healthcare joint venture on a quarterly basis on a one-quarter lag in Other (income)/deductions––net commencing from August 1, 2019. Therefore, we recorded our share of two months of the joint venture’s earnings generated in the third quarter of 2019 totaling $47 million in our operating results in the fourth quarter of 2019. As of the July 31, 2019 closing date, we estimated that the fair value of our investment in GSK Consumer Healthcare was approximately $15.7 billion and 32 % of the underlying equity in the carrying value of the net assets of GSK Consumer Healthcare was approximately $11.2 billion resulting in an initial basis difference of approximately $4.5 billion. In the fourth quarter of 2019, we preliminarily completed the allocation of the basis difference, which resulted from the excess of the initial fair value of our investment over the underlying equity in the carrying value of the net assets of the joint venture, primarily to inventory, definite-lived intangible assets, indefinite-lived intangible assets, related deferred tax liabilities and equity method goodwill within the investment account. We recorded the amortization of basis differences allocated to inventory, definite-lived intangible assets and related deferred tax liabilities in Other (income)/deductions––net commencing August 1, 2019. The amortization of these basis differences for two months of the third quarter of 2019 totaling approximately $31 million is included in our operating results in the fourth quarter of 2019. Amortization of basis differences on inventory and related deferred tax liabilities will be completely recognized by the first quarter of 2020. Basis differences on definite-lived intangible assets and related deferred tax liabilities are being amortized over approximately 17 years. The increase in the value of our investment from the closing date to December 31, 2019 is primarily due to foreign currency translation adjustments (see Note 6).
While we have received our full 32 % interest in GSK Consumer Healthcare as of the July 31, 2019 closing and transferred control of our Consumer Healthcare business to GSK Consumer Healthcare, the contribution of the business was not completed in certain non-U.S. jurisdictions due to temporary regulatory or operational constraints. In these jurisdictions, we have continued to operate the business for the net economic benefit of GSK Consumer Healthcare, and we are indemnified by GSK Consumer Healthcare against risks associated with such operations during the interim period, subject to our obligations under the definitive transaction agreements. We expect the contribution of our
80 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Consumer Healthcare business in these jurisdictions to be fully completed by the first half of 2021. As such, and as we and GSK Consumer Healthcare are contractually obligated to complete the transaction, we have treated these jurisdictions as sold for accounting purposes.
In connection with the contribution of our Consumer Healthcare business, we entered into certain transitional agreements designed to facilitate the orderly transition of the business to GSK Consumer Healthcare. These agreements primarily relate to administrative services, which are generally to be provided for a period of up to 24 months after the closing date. We will also manufacture and supply certain consumer products for GSK Consumer Healthcare and GSK Consumer Healthcare will manufacture and supply certain retained Pfizer products for us after closing, generally for a term of up to six years . These agreements are not material to Pfizer.
Assets and liabilities associated with our Consumer Healthcare business were reclassified as held for sale in the consolidated balance sheet as of December 31, 2018. The Consumer Healthcare business assets held for sale are reported in Assets held for sale and Consumer Healthcare business liabilities held for sale are reported in Liabilities held for sale in the consolidated balance sheet as of December 31, 2018. This includes the Consumer Healthcare business tax assets and liabilities related to fully dedicated consumer healthcare subsidiaries.
The amounts associated with the Consumer Healthcare business, as well as other assets classified as held for sale consisted of the following: | ||||
(MILLIONS OF DOLLARS) | December 31, 2018 | |||
Assets Held for Sale | ||||
Cash and cash equivalents | $ | |||
Trade accounts receivable, less allowance for doubtful accounts | ||||
Inventories | ||||
Other current assets | ||||
PP&E | ||||
Identifiable intangible assets, less accumulated amortization | ||||
Goodwill | ||||
Noncurrent deferred tax assets and other noncurrent tax assets | ||||
Other noncurrent assets | ||||
Total Consumer Healthcare assets held for sale | ||||
Other assets held for sale(a) | ||||
Assets held for sale | $ | |||
Liabilities Held for Sale | ||||
Trade accounts payable | $ | |||
Income taxes payable | ||||
Accrued compensation and related items | ||||
Other current liabilities | ||||
Pension benefit obligations, net | ||||
Postretirement benefit obligations, net | ||||
Noncurrent deferred tax liabilities | ||||
Other noncurrent liabilities | ||||
Total Consumer Healthcare liabilities held for sale | $ | |||
(a) | Other assets held for sale consist of PP&E. |
As a part of Pfizer, pre-tax income on a management business unit basis for the Consumer Healthcare business was $654 million through July 31, 2019, $977 million in 2018 and $863 million in 2017.
2019 Financial Report | 81 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Summarized financial information for our equity method investee, GSK Consumer Healthcare, as of and for the two months ending September 30, 2019, the most recent period available, is as follows: | ||||
(MILLIONS OF DOLLARS) | September 30, 2019 | |||
Current assets | $ | |||
Noncurrent assets | ||||
Total assets | $ | |||
Current liabilities | $ | |||
Noncurrent liabilities | ||||
Total liabilities | $ | |||
Equity attributable to shareholders | $ | |||
Equity attributable to noncontrolling interests | ||||
Total net equity | $ | |||
(MILLIONS OF DOLLARS) | For the Two Months Ending September 30, 2019 | |||
Net Sales | $ | |||
Cost of sales | ( | ) | ||
Gross profit | $ | |||
Income from continuing operations | ||||
Net income | ||||
Income attributable to shareholders | ||||
Investment in ViiV Healthcare Limited
In 2009, we and GSK created ViiV, which is focused on research, development and commercialization of human immunodeficiency virus (HIV) medicines. We own approximately 11.7 % of ViiV, and we have historically accounted for our investment in ViiV under the equity method due to the significant influence that we have over the operations of ViiV through our board representation and minority veto rights. We suspended application of the equity method to our investment in ViiV in 2016 when the carrying value of our investment was reduced to zero due to the recognition of cumulative equity method losses and dividends. Since 2016, we have recognized dividends from ViiV as income in Other (income)/deductions––net when earned, including dividends of $220 million in 2019, $253 million in 2018 and $266 million in 2017 (see Note 4).
Summarized financial information for our equity method investee, ViiV, as of December 31, 2019 and 2018 and for the years ending December 31, 2019, 2018, and 2017 is as follows: | ||||||||
As of December 31, | ||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | ||||||
Current assets | $ | $ | ||||||
Noncurrent assets | ||||||||
Total assets | ||||||||
Current liabilities | ||||||||
Noncurrent liabilities | ||||||||
Total liabilities | ||||||||
Total net equity/(deficit) attributable to shareholders | $ | ( | ) | $ | ( | ) | ||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Net Sales | $ | $ | $ | |||||||||
Cost of sales | ( | ) | ( | ) | ( | ) | ||||||
Gross profit | $ | $ | $ | |||||||||
Income from continuing operations | ||||||||||||
Net income | ||||||||||||
Income attributable to shareholders | ||||||||||||
82 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Investment in Hisun Pfizer Pharmaceuticals Company Limited
In September 2012, we and Hisun, a leading pharmaceutical company in China, formed a new company, Hisun Pfizer, to develop, manufacture, market and sell pharmaceutical products, primarily branded generic products, predominately in China. Hisun Pfizer was established with registered capital of $250 million, of which our portion was $122.5 million. As a result of the contributions from both parties, Hisun Pfizer holds a broad portfolio of branded generics covering cardiovascular disease, infectious disease, oncology, mental health and other therapeutic areas.
We accounted for our interest in Hisun Pfizer as an equity-method investment, due to the significant influence we had over the operations of Hisun Pfizer through our board representation, minority veto rights and 49 % voting interest. Our investment in Hisun Pfizer was reported in Long-term investments, and our share of Hisun Pfizer’s net income was recorded in Other (income)/deductions––net.
On November 10, 2017, we sold our 49 % equity share in Hisun Pfizer to Sapphire I (HK) Holdings Limited, an investment fund managed by Hillhouse Capital, for a total of $286 million in cash which included our carrying value of $270 million in cash plus $16 million to cover certain taxes incurred on the transaction. As a result of the sale transaction, we recognized a loss of $81 million in the fourth quarter of 2017 for the recognition in earnings of the currency translation adjustment associated with our investment. After the sale transaction, Hisun Pfizer changed its name but retained its current rights to manufacture, sell and distribute all of Hisun Pfizer’s currently marketed and pipeline products in China. We are providing technical, manufacturing and regulatory services in connection with a technology transfer process being run by Hisun Pfizer to support Hisun Pfizer’s objective that the products that we had previously licensed to Hisun Pfizer, will in the future, be manufactured locally in China. We continue to supply certain products to Hisun Pfizer for a period of time, after the sale transaction, to facilitate a smooth transition.
Investment in Laboratório Teuto Brasileiro S.A.
We entered into an agreement on June 30, 2017 to exit our investment in Teuto, a 40 %-owned generics company in Brazil, and sell our 40 % interest in Teuto to the majority shareholders. As part of the agreement, we waived our option to acquire the remaining 60 % of Teuto, and Teuto’s other shareholders have waived their option to sell their 60 % stake in the company to us. As a result, in the second quarter of 2017, we recognized a net loss of approximately $30 million in Other (income)/deductions––net (see Note 4), which included the impairment of our equity-method investment in Teuto, the reversal of a contingent liability associated with the majority shareholders’ option to sell their 60 % stake in the company to us, and the recognition in earnings of the currency translation adjustment associated with the Teuto investment. The transaction closed on August 16, 2017.
D. Licensing Arrangements
Akcea Therapeutics, Inc.
In October 2019, we entered into a worldwide exclusive licensing agreement for AKCEA-ANGPTL3-LRx, an investigational antisense therapy being developed to treat patients with certain cardiovascular and metabolic diseases, with Akcea, a majority-owned affiliate of Ionis. The transaction closed in November 2019 and we made an upfront payment of $250 million to Akcea and Ionis, which was recorded in Research and development expenses in our fiscal fourth quarter of 2019. Under the terms of the agreement, Akcea and Ionis will split equally the $250 million upfront license fee. We may be required to make development, regulatory and sales milestone payments of up to $1.3 billion and tiered, double-digit royalties on annual worldwide net sales upon marketing approval of AKCEA-ANGPTL3-LRx and these payments will also be split equally between Akcea and Ionis. Pfizer is responsible for all development and regulatory activities and costs beyond those associated with the ongoing Phase 2 study.
Shire International GmbH
In 2016, we out-licensed PF-00547659, an investigational biologic being evaluated for the treatment of moderate-to-severe inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, to Shire for an upfront payment of $90 million, up to $460 million in development and sales-based milestone payments and potential future royalty payments on commercialized products. The $90 million upfront payment was initially deferred and recognized in Other (income)/deductions––net ratably through December 2017. In the first quarter of 2018, we recognized $75 million in Other (income)/deductions––net for a milestone payment received from Shire related to their first dosing of a patient in a Phase 3 clinical trial of the compound for the treatment of ulcerative colitis, and in the third quarter of 2018, we recognized $35 million in Other (income)/deductions––net for a milestone payment received from Shire related to their first dosing of a patient in a Phase 3 clinical trial of the compound for the treatment of Crohn’s disease (see Note 4).
BionTech AG
In August 2018, a multi-year R&D arrangement went into effect between BionTech AG (BionTech), a privately held company, and Pfizer to develop mRNA-based vaccines for prevention of influenza (flu). In September 2018, we made an upfront payment of $50 million to BionTech, which was recorded in Research and development expenses, and BionTech became eligible to receive up to an additional $325 million in future development and sales based milestones and future royalty payments associated with worldwide sales. As part of the transaction, we also purchased 169,670 newly-issued ordinary shares of BionTech for $50 million in the third quarter of 2018, which are reported in Long-term investments in the consolidated balance sheets as of December 31, 2019 and December 31, 2018.
E. Research and Development and Collaborative Arrangements
Research and Development Arrangement with NovaQuest Co-Investment Fund V, L.P.
In April 2016, Pfizer entered into an agreement with NovaQuest under which NovaQuest would fund up to $200 million in development costs related to certain Phase 3 clinical trials of Pfizer’s rivipansel compound and Pfizer would use commercially reasonable efforts to develop and obtain regulatory approvals for such compound. NovaQuest’s development funding was expected to cover up to 100 % of the development
2019 Financial Report | 83 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
costs and was received over approximately 13 quarters from 2016 through the second quarter of 2019 after which Pfizer would be responsible for the remaining development costs. As there was a substantive and genuine transfer of risk to NovaQuest, the development funding was recognized by us as an obligation to perform contractual services and therefore a reduction of Research and development expenses as incurred. The funding cap was reached in 2019. The reduction to Research and Development expenses totaled $24 million for 2019, $58 million for 2018 and $72 million for 2017.
In August 2019, we announced that the Phase 3 RESET (Rivipansel Evaluating Safety, Efficacy and Time to Discharge) pivotal study did not meet its primary or key secondary efficacy endpoints. The objective of the trial was to evaluate the efficacy and safety of rivipansel in patients aged six and older with sickle cell disease who were hospitalized for a vaso-occlusive crisis and required treatment with IV opioids. As a result, in 2019, we recorded a $99 million charge in Cost of sales related to rivipansel, primarily for inventory manufactured for expected future sale, as well as $15 million of anticipated clinical development program close-out costs, which were recorded in Research and development costs in the consolidated statement of income. In January 2020, we discontinued development of rivipansel resulting in the termination of the R&D arrangement with NovaQuest. No payments have been or are expected to be received from or paid to NovaQuest as part of the termination of the arrangement.
Research and Development Arrangement with RPI Finance Trust
In January 2016, Pfizer entered into an agreement with RPI, a subsidiary of Royalty Pharma, under which RPI would fund up to $300 million in development costs related to certain Phase 3 clinical trials of Pfizer’s Ibrance (palbociclib) product primarily for adjuvant treatment of hormone receptor positive early breast cancer (the Indication). RPI’s development funding is expected to cover up to 100 % of the costs primarily for the applicable clinical trials until the first quarter of 2020 after which Pfizer will be responsible for the remaining cost of the trials. As there is a substantive and genuine transfer of risk to RPI, the development funding is recognized by us as an obligation to perform contractual services and therefore is a reduction of Research and development expenses as incurred. The reduction to Research and development expenses totaled $63 million for 2019, $99 million for 2018 and $76 million for 2017. If successful and upon approval of Ibrance in the U.S. or certain major markets in the EU for the Indication based on the applicable clinical trials, RPI will be eligible to receive a combination of approval-based fixed milestone payments of up to $250 million dependent upon results of the clinical trials and royalties on certain Ibrance sales over approximately seven years. Fixed milestone payments due upon approval will be recorded as intangible assets and amortized to Amortization of intangible assets over the estimated commercial life of the Ibrance product and sales-based royalties will be recorded as Cost of sales when incurred.
Collaborative Arrangements
In the normal course of business, we enter into collaborative arrangements with respect to in-line medicines, as well as medicines in development that require completion of research and regulatory approval. Collaborative arrangements are contractual agreements with third parties that involve a joint operating activity, typically a research and/or commercialization effort, where both we and our partner are active participants in the activity and are exposed to the significant risks and rewards of the activity. Our rights and obligations under our collaborative arrangements vary. For example, we have agreements to co-promote pharmaceutical products discovered by us or other companies, and we have agreements where we partner to co-develop and/or participate together in commercializing, marketing, promoting, manufacturing and/or distributing a drug product.
The following table provides the amounts and classification of payments (income/(expense)) between us and our collaboration partners: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Revenues—Revenues(a) | $ | $ | $ | |||||||||
Revenues—Alliance revenues(b) | ||||||||||||
Total revenues from collaborative arrangements | $ | $ | $ | |||||||||
Cost of sales(c) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
Selling, informational and administrative expenses(d) | ( | ) | ( | ) | ( | ) | ||||||
Research and development expenses(e) | ||||||||||||
Other income/(deductions)—net(f) | ||||||||||||
(a) | Represents sales to our partners of products manufactured by us. |
(b) | Substantially all relates to amounts earned from our partners under co-promotion agreements. The increases in each of the periods presented reflect increases in alliance revenues from Eliquis and Xtandi. |
(c) | Primarily relates to amounts paid to collaboration partners for their share of net sales or profits earned in collaboration arrangements where we are the principal in the transaction, and cost of sales associated with inventory purchased from our partners. |
(d) | Represents net reimbursements to our partners for selling, informational and administrative expenses incurred. |
(e) | Primarily relates to upfront payments and pre-approval milestone payments earned by our partners as well as net reimbursements. The upfront and milestone payments were as follows: $ |
(f) | Primarily relates to royalties from our collaboration partners. |
The amounts disclosed in the above table do not include transactions with third parties other than our collaboration partners, or other costs associated with the products under the collaborative arrangements.
In addition, in connection with our collaborative arrangements, we paid post-approval milestones of $80 million in 2019 and $140 million in 2017 related to our collaboration with Merck KGaA (see below). These payments were recorded in Identifiable intangible assets––Developed technology rights. We did not pay post-approval milestones to collaboration partners in 2018. We also recorded milestones earned related to (i) our collaboration with Mylan Pharmaceuticals Inc. related to the FDA’s approval and launch of Wixela Inhub®, a generic of Advair Diskus® (fluticasone propionate and salmeterol inhalation powder) of $78 million in 2019 in Other (income)/deductions––net (see Note 4) and (ii) our
84 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
collaboration with Merck (see below) of $40 million in 2018 in Other (income)/deductions––net and $150 million in 2017, substantially all of which was included in the adjustment to increase the opening balance of Retained earnings upon the adoption of a new accounting standard for revenue recognition, effective January 1, 2018.
Collaboration with Merck & Co., Inc.
Under a worldwide collaboration agreement, except for Japan, we collaborated with Merck on the clinical development of ertugliflozin and ertugliflozin-containing fixed-dose combinations with metformin and Januvia (sitagliptin) tablets, which were approved by the FDA in December 2017 and the EC in March 2018 as Steglatro, Segluromet and Steglujan. Merck exclusively promotes Steglatro and the two fixed-dose combination products and we share revenues and certain costs with Merck on a 60 %/40 % basis, with Pfizer having the 40 % share.
In the first quarter of 2017, we received a $90 million milestone payment from Merck upon the FDA’s acceptance for review of the NDAs for ertugliflozin and two fixed-dose combinations (ertugliflozin plus Januvia (sitagliptin) and ertugliflozin plus metformin), which, as of December 31, 2017, was deferred and primarily reported in Other noncurrent liabilities, and through December 31, 2017, was being recognized in Other (income)/deductions––net over a multi-year period. As of December 31, 2017, we were due a $60 million milestone payment from Merck, which we received in the first quarter of 2018, in conjunction with the approval of ertugliflozin by the FDA. As of December 31, 2017, the $60 million due from Merck was deferred and primarily reported in Other noncurrent liabilities. In the first quarter of 2018, in connection with the approval of ertugliflozin in the EU, we recognized a $40 million milestone payment from Merck in Other (income)/deductions––net (see Note 4). We are eligible for additional payments associated with the achievement of future commercial milestones. In the first quarter of 2018, in connection with the adoption of a new accounting standard for revenue recognition, as of January 1, 2018, the $60 million of deferred income and approximately $85 million of the $90 million of deferred income associated with the above-mentioned milestone payments were recorded as a cumulative effect adjustment to Retained earnings.
Collaboration with Eli Lilly & Company
In 2013, we entered into a collaboration agreement with Lilly to jointly develop and globally commercialize Pfizer’s tanezumab, which provides that Pfizer and Lilly will equally share product-development expenses as well as potential revenues and certain product-related costs. We received a $200 million upfront payment from Lilly in accordance with the collaboration agreement between Pfizer and Lilly, which was deferred and primarily reported in Other noncurrent liabilities, and through December 31, 2017, was being recognized in Other (income)/deductions––net over a multi-year period beginning in the second quarter of 2015. Pfizer and Lilly resumed the Phase 3 chronic pain program for tanezumab in July 2015. Under the collaboration agreement with Lilly, we are eligible to receive additional payments from Lilly upon the achievement of specified regulatory and commercial milestones. In the first quarter of 2018, in connection with the adoption of a new accounting standard for revenue recognition, as of January 1, 2018, approximately $107 million of deferred income associated with the above-mentioned upfront payment was recorded as a cumulative effect adjustment to Retained earnings. Approximately $9 million of the upfront payment continues to be deferred, and is reported in Other current liabilities as of December 31, 2019. This amount is being recognized in Other (income)/deductions––net over the remaining development period for the product in 2020.
Collaboration with Merck KGaA
In November 2014, we entered into a collaborative arrangement with Merck KGaA, to jointly develop and commercialize avelumab, currently approved as Bavencio for metastatic MCC in the U.S., the EU, Japan and select other markets, in combination with Inlyta for the first-line treatment of patients with advanced RCC in the U.S., the EU, Japan and select other markets, as well as for the second-line treatment of patients with locally advanced or metastatic urothelial carcinoma in the U.S. and select other markets. Avelumab is also in development as a potential treatment for multiple other types of cancer. We and Merck KGaA are exploring the therapeutic potential of this novel anti-PD-L1 antibody as a single agent as well as in various combinations with our and Merck KGaA’s broad portfolio of approved and investigational oncology therapies. Also, as part of the agreement, we gave Merck KGaA certain co-promotion rights for Xalkori in the U.S. and several other key markets. Under the terms of the agreement, in the fourth quarter of 2014, we made an upfront payment of $850 million to Merck KGaA and Merck KGaA is eligible to receive regulatory and commercial milestone payments of up to approximately $2.0 billion. During 2017, we made $140 million in milestone payments to Merck KGaA, which were recorded in Identifiable intangible assets––Developed technology rights, for approvals of avelumab received in 2017 for the MCC indication in the U.S., the EU and Japan, and for the metastatic urothelial carcinoma indication in the U.S. Both companies jointly fund the majority of development and commercialization costs, and split equally any profits related to net sales generated from selling any products containing avelumab from this collaboration. In December 2018, both companies amended the collaborative agreement such that Pfizer will be solely responsible for the development and commercialization of its anti PD-1 antibody. Under the terms of the amended agreement, we paid Merck KGaA an up-front payment and we will make a potential milestone and tiered royalty payments should the Pfizer anti PD-1 antibody achieve regulatory and commercial success. We made $80 million in milestone payments to Merck KGaA, which were recorded in Identifiable intangible assets––Developed technology rights, for the U.S. and the EU approvals received in 2019 related to the use of Bavencio in combination with Inlyta for the first-line treatment of patients with advanced RCC.
Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
We incur significant costs in connection with acquiring, integrating and restructuring businesses and in connection with our global cost-reduction/productivity initiatives. For example:
• | In connection with acquisition activity, we typically incur costs associated with executing the transactions, integrating the acquired operations (which may include expenditures for consulting and the integration of systems and processes), and restructuring the combined company (which may include charges related to employees, assets and activities that will not continue in the combined company); and |
• | In connection with our cost-reduction/productivity initiatives, we typically incur costs and charges associated with site closings and other facility rationalization actions, workforce reductions and the expansion of shared services, including the development of global systems. |
2019 Financial Report | 85 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
All of our businesses and functions may be impacted by these actions, including sales and marketing, manufacturing and R&D, as well as groups such as information technology, shared services and corporate operations.
2017-2019 Initiatives and Organizing for Growth
During 2018, we determined that at the start of our 2019 fiscal year, we would begin operating under our new commercial structure, which reorganized our operations into three businesses––Biopharma, a science-based innovative medicines business; Upjohn, a global, primarily off-patent branded and generic established medicines business; and through July 31, 2019, a Consumer Healthcare business (See Note 17). To operate effectively in this structure and position ourselves for future growth, we focused on creating a simpler, more efficient operating structure within each business as well as the functions that support them. Beginning in the fourth quarter of 2018, we reviewed previously planned initiatives and new initiatives to ensure that there was alignment around our new structure and combined the 2017-2019 initiatives with our Organizing for Growth initiatives to form one cohesive plan. Initiatives for the combined program included activities related to the optimization of our manufacturing plant network, the centralization of our corporate and platform functions, and the simplification and optimization of our operating business structure and functions that support them. From 2017 through December 31, 2019, we incurred approximately $921 million associated with manufacturing optimization, approximately $1.2 billion associated with other activities, and have substantially completed this program.
Transforming to a More Focused Company
With the formation of the GSK Consumer Healthcare venture and the pending combination of Upjohn with Mylan, Pfizer is transforming itself into a more focused, global leader in science-based innovative medicines. As a result, we began in the fourth quarter of 2019, to identify and undertake efforts to ensure our cost base aligns appropriately with our Biopharmaceutical revenue base as a result of both the completed Consumer Healthcare and expected Upjohn transactions. While certain direct costs have transferred or will transfer to the Consumer Healthcare joint venture and to the Upjohn entities, there are indirect costs which are not expected to transfer. In addition, we are taking steps to restructure our organizations to appropriately support and drive the purpose of the three core functions of our focused innovative medicines business: R&D, Manufacturing and Commercial.
We expect the costs associated with this multi-year effort to continue through 2022 and to total approximately $1.4 billion on a pre-tax basis and approximately 10 % of this to be non-cash. Actions may include, among others, changes in location of certain activities, expanded use and co-location of centers of excellence and shared services, and increased use of digital technologies. The associated actions and the specific costs are currently in development but will include severance and benefit plan impacts, exit costs as well as associated implementation costs.
Current-Period Key Activities
In 2019, we incurred costs of $967 million composed of $695 million associated with 2017-2019 Initiatives and Organizing for Growth, $288 million associated with the integration of Array, $94 million associated with the integration of Hospira, and $87 million associated with the Transforming to a More Focused Company initiative, partially offset by income of $197 million, primarily due to the reversal of certain accruals upon the effective favorable settlement of a U.S. IRS audit for multiple tax years and other acquisition-related initiatives.
The following table provides the components of costs associated with acquisitions and cost-reduction/productivity initiatives: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Restructuring charges/(credits): | ||||||||||||
Employee terminations | $ | $ | $ | ( | ) | |||||||
Asset impairments(a) | ||||||||||||
Exit costs | ||||||||||||
Restructuring charges(b) | ||||||||||||
Transaction costs(c) | ||||||||||||
Integration costs and other(d) | ||||||||||||
Restructuring charges and certain acquisition-related costs | ||||||||||||
Net periodic benefit costs recorded in Other (income)/deductions––net(e) | ||||||||||||
Additional depreciation––asset restructuring recorded in our consolidated statements of income as follows(f): | ||||||||||||
Cost of sales | ||||||||||||
Selling, informational and administrative expenses | ||||||||||||
Research and development expenses | ||||||||||||
Total additional depreciation––asset restructuring | ||||||||||||
Implementation costs recorded in our consolidated statements of income as follows(g): | ||||||||||||
Cost of sales | ||||||||||||
Selling, informational and administrative expenses | ||||||||||||
Research and development expenses | ||||||||||||
Total implementation costs | ||||||||||||
Total costs associated with acquisitions and cost-reduction/productivity initiatives | $ | $ | $ | |||||||||
(a) | The asset impairment charges for 2018 are largely associated with cost reduction initiatives not associated with acquisitions. The asset impairment charges for 2017 are largely associated with our acquisitions of Hospira and Medivation. The asset impairment charges included in restructuring charges for 2017 are primarily associated with abandoned assets. See (b) below for additional information. |
86 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
(b) | In 2019, restructuring charges mainly represent employee termination costs associated with cost-reduction and productivity initiatives, partially offset by the reversal of certain accruals related to our acquisition of Wyeth upon the effective favorable settlement of a U.S. IRS audit for multiple tax years (see Note 5B). In 2018, restructuring charges were primarily related to employee termination costs and asset write downs. The employee termination costs for 2019 and 2018 were primarily associated with our improvements to operational effectiveness as part of the realignment of our organizational structure, and for 2019, also includes employee termination costs associated with the Transforming to a More Focused Company initiative. In 2017, restructuring charges were primarily associated with our acquisitions of Hospira and Medivation, partially offset by credits associated with cost-reduction and productivity initiatives not associated with acquisitions that mostly related to the reversal of previously recorded accruals for employee termination costs resulting from revisions of our severance benefit estimates. Employee termination costs are generally recorded when the actions are probable and estimable and include accrued severance benefits, pension and postretirement benefits, many of which may be paid out during periods after termination. |
The restructuring activities in 2019 are associated with the following:
• | Biopharma ($ |
At the beginning of fiscal 2019, we revised our operating segments and are unable to directly associate 2018 and 2017 restructuring charges with the new individual segments.
The restructuring activities for 2018 are associated with the following:
• | Total reportable segments ($ |
The restructuring activities for 2017 are associated with the following:
• | Total reportable segments ($ |
(c) | Transaction costs represent external costs for banking, legal, accounting and other similar services. In 2019, transaction costs relate to our acquisition of Array. In 2017, transaction costs were directly related to our acquisitions of Hospira, Anacor and Medivation. |
Integration costs and other represent external, incremental costs directly related to integrating acquired businesses, such as expenditures for consulting and the integration of systems and processes, and certain other qualifying costs. In 2019, integration costs and other mainly related to our acquisitions of Array (including $ |
In 2018, primarily represents the net pension curtailments and settlements included in Other (income)/deductions––net upon the adoption of a new accounting standard in the first quarter of 2018. In 2017, mainly represents the net pension curtailments and settlements, partially offset by net periodic benefit credits, excluding service costs, related to our acquisition of Hospira, both of which were reclassified to Other (income)/deductions––net as a result of the retrospective adoption of a new accounting standard in the first quarter of 2018. These credits included a net settlement gain, partially offset by accelerated amortization of actuarial losses and prior service costs upon the settlement of the remaining obligation associated with the Hospira U.S. qualified defined benefit pension plan. For additional information, see Note 11. |
Additional depreciation––asset restructuring represents the impact of changes in the estimated useful lives of assets involved in restructuring actions. |
(g) | I |
The following table provides the components of and changes in our restructuring accruals: | ||||||||||||||||
(MILLIONS OF DOLLARS) | Employee Termination Costs | Asset Impairment Charges | Exit Costs | Accrual | ||||||||||||
Balance, January 1, 2018 | $ | $ | $ | $ | ||||||||||||
Provision | ||||||||||||||||
Utilization and other(a) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
Balance, December 31, 2018(b) | ||||||||||||||||
Provision(c) | ||||||||||||||||
Utilization and other(a) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
Balance, December 31, 2019(d) | $ | $ | $ | $ | ||||||||||||
(a) | Includes adjustments for foreign currency translation. |
(b) | Included in Other current liabilities ($ |
(c) | Includes the reversal of certain accruals related to our acquisition of Wyeth upon the effective favorable settlement of a U.S. IRS audit for multiple tax years. See Note 5D for additional information. |
(d) | Included in Other current liabilities ($ |
2019 Financial Report | 87 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 4. Other (Income)/Deductions—Net
The following table provides components of Other (income)/deductions––net: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Interest income(a) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
Interest expense(a) | ||||||||||||
Net interest expense | ||||||||||||
Royalty-related income(b) | ( | ) | ( | ) | ( | ) | ||||||
Net (gains)/losses on asset disposals(c) | ( | ) | ( | ) | ||||||||
Net gains recognized during the period on equity securities(d) | ( | ) | ( | ) | ( | ) | ||||||
Net realized (gains)/losses on sales of investments in debt securities(e) | ( | ) | ||||||||||
Income from collaborations, out-licensing arrangements and sales of compound/product rights(f) | ( | ) | ( | ) | ( | ) | ||||||
Net periodic benefit costs/(credits) other than service costs(g) | ( | ) | ||||||||||
Certain legal matters, net(h) | ||||||||||||
Certain asset impairments(i) | ||||||||||||
Business and legal entity alignment costs(j) | ||||||||||||
Net losses on early retirement of debt(k) | ||||||||||||
GSK Consumer Healthcare JV equity method (income)/loss(l) | ( | ) | ||||||||||
Other, net(m) | ( | ) | ( | ) | ( | ) | ||||||
Other (income)/deductions––net | $ | $ | $ | |||||||||
(a) | 2019 v. 2018––Interest income decreased primarily driven by a lower investment balance. Interest expense increased mainly as a result of an increased commercial paper balance due to the acquisition of Array, as well as the retirement of lower-coupon debt and the issuance of new debt with a higher coupon than the debt outstanding for the comparative prior year period. 2018 v. 2017––Interest income decreased primarily driven by a lower investment balance. Interest expense increased primarily as a result of higher short-term interest rates, offset, in part, by refinancing activity that occurred in the fourth quarter of 2017. Capitalized interest expense totaled $ |
(b) | Royalty-related income increased in 2019, primarily due to a one-time favorable resolution in the second quarter of 2019 of a legal dispute for $ |
(c) | In 2018, primarily included a realized gain on sale of property of $ |
(d) | The gains in 2019 include, among other things, unrealized gains of $ |
(e) | In 2018, primarily includes gross realized losses on sales of available-for-sale debt securities of $ |
(f) | Includes income from upfront and milestone payments from our collaboration partners and income from out-licensing arrangements and sales of compound/product rights. In 2019, mainly includes, among other things, $ |
(g) | I |
(h) | In 2019, mostly includes legal reserves for certain pending legal matters. In 2018, primarily includes legal reserves for certain pending legal matters, partially offset by the reversal of a legal accrual where a loss was no longer deemed probable. In 2017, primarily includes a $ |
(i) | In 2019, primarily includes intangible asset impairment charges of $ |
88 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
U.S. market only, and reflects, among other things, updated commercial forecasts; and (vi) $10 million of other IPR&D assets acquired in connection with our acquisition of Innopharma.
In 2018, primarily includes intangible asset impairment charges of $3.1 billion, mainly composed of (i) $2.6 billion related to Biopharma developed technology rights, $242 million related to Biopharma licensing agreements and $80 million related to Biopharma IPR&D, all of which relate to our acquisition of Hospira, for generic sterile injectable products associated with various indications; (ii) $117 million related to a multi-antigen vaccine IPR&D program for adults undergoing elective spinal fusion surgery; (iii) $31 million related to an Biopharma developed technology right, acquired in connection with our acquisition of Anacor, for the treatment for toenail fungus market marketed in the U.S. market only; and (iv) $17 million of other IPR&D assets acquired in connection with our acquisition of Innopharma. In 2018, the intangible asset impairment charges associated with the generic sterile injectable products reflect, among other things, updated commercial forecasts, reflecting an increased competitive environment as well as higher manufacturing costs, largely stemming from manufacturing and supply issues. The intangible asset impairment charge for the multi-antigen vaccine IPR&D program was the result of the Phase 2b trial reaching futility at a pre-planned interim analysis. The intangible asset impairment charge related to the Biopharma developed technology right reflects, among other things, updated commercial forecasts. .
In 2017, primarily includes intangible asset impairment charges of $337 million, reflecting (i) $127 million related to developed technology rights, acquired in connection with our acquisition of Hospira, for a generic sterile injectable product for the treatment of edema associated with certain conditions; (ii) $124 million related to developed technology rights, acquired in connection with our acquisition of Hospira, for a sterile injectable pain reliever; (iii) $39 million related to developed technology rights, acquired in connection with our acquisition of NextWave, for the treatment of attention deficit hyperactivity disorder; (iv) $26 million related to developed technology rights, acquired in connection with our acquisition of Hospira, for a generic injectable antibiotic product for the treatment of bacterial infections; and (v) $20 million related to other developed technology rights. The intangible asset impairment charges for 2017 are associated with Biopharma and reflect, among other things, updated commercial forecasts and an increased competitive environment. In addition, 2017 includes a loss of $43 million for an impairment of our AM-Pharma B.V. long-term investment.
(j) | In 2019 and 2018, mainly represents incremental costs associated with the design, planning and implementation of our new organizational structure, effective in the beginning of 2019, and primarily includes consulting, legal, tax and advisory services. In 2017, represents expenses for changes to our infrastructure to align our commercial operations that existed through December 31, 2018, including costs to internally separate our businesses into distinct legal entities, as well as to streamline our intercompany supply operations to better support each business. |
(k) | In 2019 and 2017, represents net losses due to the early retirement of debt, inclusive of the related termination of cross currency swaps. |
(l) | See Note 2C for additional information. |
(m) | In 2019, includes, among other things, (i) dividend income of $ |
The following table provides additional information about the intangible assets that were impaired during 2019 in Other (income)/deductions––net: | ||||||||||||||||||||
Year Ended December 31, | ||||||||||||||||||||
Fair Value(a) | 2019 | |||||||||||||||||||
(MILLIONS OF DOLLARS) | Amount | Level 1 | Level 2 | Level 3 | Impairment | |||||||||||||||
Intangible assets––Developed technology rights(b) | $ | $ | $ | $ | $ | |||||||||||||||
Intangible assets––IPR&D(b) | ||||||||||||||||||||
Total | $ | $ | $ | $ | $ | |||||||||||||||
(a) | The fair value amount is presented as of the date of impairment, as these assets are not measured at fair value on a recurring basis. See also Note 1E. |
(b) | Reflects intangible assets written down to fair value in 2019. Fair value was determined using the income approach, specifically the multi-period excess earnings method, also known as the discounted cash flow method. We started with a forecast of all the expected net cash flows associated with the asset and then applied an asset-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of competitive, legal and/or regulatory forces on the product; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows. |
Note 5. Tax Matters
A. Taxes on Income from Continuing Operations
The following table provides the components of Income from continuing operations before provision/(benefit) for taxes on income: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
United States | $ | $ | ( | ) | $ | ( | ) | |||||
International | ||||||||||||
Income from continuing operations before provision/(benefit) for taxes on income(a), (b) | $ | $ | $ | |||||||||
(a) | 2019 v. 2018––The domestic income in 2019 versus domestic loss in 2018 was mainly related to the completion of the Consumer Healthcare joint venture transaction with GSK as well as lower certain asset impairments, partially offset by reduced Lyrica revenues in the U.S., higher business and legal entity |
2019 Financial Report | 89 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
alignment costs as well as increased costs related to certain legal matters. The decrease in the international income was primarily related to higher certain asset impairments as well as the write off of assets contributed to the Consumer Healthcare joint venture with GSK.
(b) | 2018 v. 2017––The decrease in the domestic loss was primarily due to lower interest expense paid to certain foreign subsidiaries, lower net losses on the retirement of debt, higher net gains on equity securities and increased revenue related to Eliquis, partially offset by higher certain asset impairments and lower revenue for Viagra and the legacy SIP portfolio. The decrease in international income was primarily related to lower interest income received primarily from intercompany borrowings from Pfizer Inc. and higher charges related to certain cost reduction initiatives, partially offset by increased revenue related to Ibrance and Eliquis. |
The following table provides the components of Provision/(benefit) for taxes on income based on the location of the taxing authorities: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
United States | ||||||||||||
Current income taxes: | ||||||||||||
Federal | $ | ( | ) | $ | $ | |||||||
State and local | ( | ) | ||||||||||
Deferred income taxes: | ||||||||||||
Federal | ( | ) | ( | ) | ||||||||
State and local | ( | ) | ||||||||||
Total U.S. tax benefit | ( | ) | ( | ) | ( | ) | ||||||
TCJA(a) | ||||||||||||
Current income taxes | ( | ) | ( | ) | ||||||||
Deferred Income taxes | ( | ) | ( | ) | ||||||||
Total TCJA tax benefit | ( | ) | ( | ) | ( | ) | ||||||
International | ||||||||||||
Current income taxes | ||||||||||||
Deferred income taxes | ( | ) | ( | ) | ( | ) | ||||||
Total international tax provision | ||||||||||||
Provision/(benefit) for taxes on income | $ | $ | $ | ( | ) | |||||||
(a) | The 2018 current tax benefit and deferred tax expense primarily relate to the utilization of tax credit carryforwards against the repatriation tax liability associated with the enactment of the TCJA. See discussion below and Note 5C. |
In the fourth quarter of 2017, we recorded an estimate of certain tax effects of the TCJA, including (i) the impact on deferred tax assets and liabilities from the reduction in the U.S. Federal corporate tax rate from 35% to 21%, (ii) the impact on valuation allowances and other state income tax considerations, (iii) the $15.2 billion repatriation tax liability on accumulated post-1986 foreign earnings for which we elected, with the filing of our 2018 U.S. Federal Consolidated Income Tax Return, payment over eight years through 2026 and (iv) deferred taxes on basis differences expected to give rise to future taxes on global intangible low-taxed income. In addition, we had provided deferred tax liabilities in the past on foreign earnings that were not indefinitely reinvested. As a result of the TCJA, in the fourth quarter of 2017, we reversed an estimate of the deferred taxes that are no longer expected to be needed due to the change to the territorial tax system.
In 2018, we finalized our provisional accounting for the tax effects of the TCJA, based on our best estimates of available information and data, and reported and disclosed the impacts within the applicable measurement period, in accordance with guidance issued by the SEC, and recorded a favorable adjustment of approximately $100 million to Provision/(benefit) for taxes on income. We believe that there may be additional interpretations, clarifications and guidance from the U.S. Department of Treasury. Any change to our calculations resulting from such additional interpretations, clarifications and guidance would be reflected in the period of issuance. In addition, our obligations may vary as a result of changes in our uncertain tax positions and/or availability of attributes such as foreign tax and other credit carryforwards.
With respect to the aforementioned repatriation tax liability, our revised estimate is approximately $15 billion, which is reported in current Income taxes payable (approximately $600 million) and the remaining liability is reported in noncurrent Other taxes payable in our consolidated balance sheet as of December 31, 2019. The first installment of $750 million was paid in April 2019.
The TCJA subjects a U.S. shareholder to current tax on global intangible low-taxed income earned by certain foreign subsidiaries. The FASB Staff Q&A, Topic 740, No. 5, Accounting for Global Intangible Low-Taxed Income, states that we are permitted to make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as global intangible low-taxed income in future years or provide for the tax expense related to such income in the year the tax is incurred. We elected to recognize deferred taxes for temporary differences expected to reverse as global intangible low-taxed income in future years. In 2017, we provided a provisional deferred tax liability of approximately $1.0 billion based on the evaluation of certain temporary differences inside each of our foreign subsidiaries that are expected to reverse as global intangible low-taxed income. In 2018, this estimate was finalized and we provided for an additional deferred tax liability of approximately $200 million, resulting in a deferred tax liability of approximately $1.2 billion.
In 2019, the Provision/(benefit) for taxes on income was impacted by the following:
• | tax expense of approximately $ |
• | tax benefits of approximately $ |
• | tax benefits of approximately $ |
90 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
• | tax benefits of approximately $ |
• | tax benefits of approximately $ |
In 2018, the Provision/(benefit) for taxes on income was impacted by the following:
• | estimated U.S. net tax benefits of approximately $ |
◦ | approximately $ |
◦ | approximately $ |
◦ | $ |
◦ | $ |
partially offset by:
◦ | $ |
• | tax benefits of approximately $ |
• | tax benefits of approximately $ |
In 2017, the Provision/(benefit) for taxes on income was impacted by the following:
• | estimated U.S. net tax benefits of $ |
◦ | $ |
◦ | $ |
◦ | $ |
◦ | $ |
◦ | approximately $ |
• | U.S. tax expense of approximately $ |
• | tax benefits of approximately $ |
• | tax benefits of approximately $ |
• | the non-deductibility of a $ |
In all years, federal, state and international net tax liabilities assumed or established as part of a business acquisition are not included in Provision/(benefit) for taxes on income (see Note 2A).
B. Tax Rate Reconciliation
The reconciliation of the U.S. statutory income tax rate to our effective tax rate for Income from continuing operations follows: | |||||||||
Year Ended December 31, | |||||||||
2019 | 2018 | 2017 | |||||||
U.S. statutory income tax rate | % | % | % | ||||||
TCJA impact(a) | ( | ) | ( | ) | ( | ) | |||
Taxation of non-U.S. operations (b), (c) | ( | ) | ( | ) | ( | ) | |||
Tax settlements and resolution of certain tax positions(d) | ( | ) | ( | ) | ( | ) | |||
Completion of Consumer Healthcare joint venture transaction(d) | |||||||||
U.S. Healthcare Legislation(d), (e) | ( | ) | |||||||
U.S. R&D tax credit and manufacturing deduction | ( | ) | ( | ) | ( | ) | |||
Certain legal settlements and charges | ( | ) | |||||||
All other, net(f) | ( | ) | ( | ) | |||||
Effective tax rate for income from continuing operations | % | % | ( | )% | |||||
(a) | For a discussion about the enactment of the TCJA, see Note 5A. |
(b) | For taxation of non-U.S. operations, this rate impact reflects the income tax rates and relative earnings in the locations where we do business outside the U.S., together with the cost of repatriation decisions, which, for 2017, includes the repatriation tax on deemed repatriated 2017 earnings of foreign subsidiaries discussed in Note 5A, changes in uncertain tax positions not included in the reconciling item called “Tax settlements and resolution of certain tax positions,” as well as changes in valuation allowances. Specifically: (i) the jurisdictional location of earnings is a significant component of our effective tax rate each year, and the rate impact of this component is influenced by the specific location of non-U.S. earnings and the level of such earnings as compared to our total earnings; (ii) the cost of repatriation decisions, and other U.S. tax implications of our foreign operations, is a significant component of our effective tax rate each year and generally offsets some of the reduction to our effective tax rate each year resulting from the jurisdictional location of earnings; (iii) |
2019 Financial Report | 91 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
certain tax initiatives; and (iv) the impact of changes in uncertain tax positions not included in the reconciling item called “Tax settlements and resolution of certain tax positions” is a component of our effective tax rate each year that can result in either an increase or decrease to our effective tax rate. The jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs, can vary as a result of the repatriation decisions, as a result of operating fluctuations in the normal course of business and as a result of the extent and location of other income and expense items, such as restructuring charges, asset impairments and gains and losses on strategic business decisions. See also Note 5A for the components of pre-tax income and Provision/(benefit) for taxes on income, which is based on the location of the taxing authorities, and for information about settlements and other items impacting Provision/(benefit) for taxes on income.
(c) | In all periods presented, the reduction in our effective tax rate resulting from the jurisdictional location of earnings is largely due to lower tax rates in certain jurisdictions, as well as manufacturing and other incentives associated with our subsidiaries in Puerto Rico and Singapore. We benefit from a Puerto Rican incentive grant that expires in 2029. Under the grant, we are partially exempt from income, property and municipal taxes. In Singapore, we benefit from incentive tax rates effective through 2045 on income from manufacturing and other operations. |
(d) |
(e) | The favorable rate impact in 2018 is a result of the updated 2017 invoice received from the federal government, which reflected a lower expense than what was previously estimated for invoiced periods, as well as certain tax initiatives. |
(f) | All other, net in 2019 is primarily due to routine business operations. 2018 is primarily due to routine business operations and the non-recurrence of tax benefits associated with certain tax initiatives. 2017 primarily relates to tax benefits associated with certain tax initiatives in the normal course of business. |
C. Deferred Taxes
Deferred taxes arise as a result of basis differentials between financial statement accounting and tax amounts.
The components of our deferred tax assets and liabilities, shown before jurisdictional netting, follow: | ||||||||||||||||
2019 Deferred Tax* | 2018 Deferred Tax* | |||||||||||||||
(MILLIONS OF DOLLARS) | Assets | (Liabilities) | Assets | (Liabilities) | ||||||||||||
Prepaid/deferred items(a) | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||
Inventories | ( | ) | ( | ) | ||||||||||||
Intangible assets(b) | ( | ) | ( | ) | ||||||||||||
Property, plant and equipment | ( | ) | ( | ) | ||||||||||||
Employee benefits | ( | ) | ( | ) | ||||||||||||
Restructurings and other charges | — | — | ||||||||||||||
Legal and product liability reserves | — | — | ||||||||||||||
Net operating loss/tax credit carryforwards(c) | — | — | ||||||||||||||
Unremitted earnings | — | ( | ) | — | ( | ) | ||||||||||
State and local tax adjustments | — | — | ||||||||||||||
Investments(d) | ( | ) | ( | ) | ||||||||||||
All other | ( | ) | ( | ) | ||||||||||||
( | ) | ( | ) | |||||||||||||
Valuation allowances | ( | ) | — | ( | ) | — | ||||||||||
Total deferred taxes | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||
Net deferred tax liability(e) | $ | ( | ) | $ | ( | ) | ||||||||||
* | The deferred tax assets and liabilities associated with global intangible low-taxed income are included in the relevant categories above. See Note 5A. |
(a) | The increase in 2019 is primarily related to the capitalization of certain R&D-related expenses. |
The decrease in 2019 is primarily the result of amortization of intangible assets and certain impairment charges, mainly offset by deferred tax liabilities established on intangible assets from the acquisition of Array. |
(c) | The amounts in 2019 and 2018 are reduced for unrecognized tax benefits of $ |
(d) | The increase in 2019 is primarily related to the Consumer Healthcare joint venture with GSK. See Note 2C for additional information. |
(e) | In 2019, Noncurrent deferred tax assets and other noncurrent tax assets ($ |
We have carryforwards, primarily related to net operating and capital losses, general business credits and charitable contributions, which are available to reduce future U.S. federal and/or state, as well as international, income taxes payable with either an indefinite life or expiring at various times from 2020 to 2039. Certain of our U.S. net operating losses and general business credits are subject to limitations under IRC Section 382.
Valuation allowances are provided when we believe that our deferred tax assets are not recoverable based on an assessment of estimated future taxable income that incorporates ongoing, prudent and feasible tax planning strategies, that would be implemented, if necessary, to realize the deferred tax assets.
As of December 31, 2019, we have not made a U.S. tax provision on approximately $29.0 billion of unremitted earnings of our international subsidiaries. As these earnings are intended to be indefinitely reinvested overseas, the determination of a hypothetical unrecognized deferred tax liability as of December 31, 2019 is not practicable.
92 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
D. Tax Contingencies
We are subject to income tax in many jurisdictions, and a certain degree of estimation is required in recording the assets and liabilities related to income taxes. All of our tax positions are subject to audit by the local taxing authorities in each tax jurisdiction. These tax audits can involve complex issues, interpretations and judgments and the resolution of matters may span multiple years, particularly if subject to negotiation or litigation. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and variation from such estimates could materially affect our financial statements in the period of settlement or when the statutes of limitations expire, as we treat these events as discrete items in the period of resolution.
For a description of our accounting policies associated with accounting for income tax contingencies, see Note 1P. For a description of the risks associated with estimates and assumptions, see Note 1C.
Uncertain Tax Positions
As tax law is complex and often subject to varied interpretations, it is uncertain whether some of our tax positions will be sustained upon audit. As of December 31, 2019, we had approximately $4.2 billion in net unrecognized tax benefits, excluding associated interest and as of December 31, 2018, we had approximately $5.1 billion in net unrecognized tax benefits, excluding associated interest.
• | Tax assets associated with uncertain tax positions primarily represent our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction. These potential benefits generally result from cooperative efforts among taxing authorities, as required by tax treaties to minimize double taxation, commonly referred to as the competent authority process. The recoverability of these assets, which we believe to be more likely than not, is dependent upon the actual payment of taxes in one tax jurisdiction and, in some cases, the successful petition for recovery in another tax jurisdiction. As of December 31, 2019, we had approximately $ |
• | Tax liabilities associated with uncertain tax positions represent unrecognized tax benefits, which arise when the estimated benefit recorded in our financial statements differs from the amounts taken or expected to be taken in a tax return because of the uncertainties described above. These unrecognized tax benefits relate primarily to issues common among multinational corporations. Substantially all of these unrecognized tax benefits, if recognized, would impact our effective income tax rate. |
The reconciliation of the beginning and ending amounts of gross unrecognized tax benefits follows: | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Balance, beginning | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
Acquisitions(a) | ( | ) | ||||||||||
Increases based on tax positions taken during a prior period(b) | ( | ) | ( | ) | ( | ) | ||||||
Decreases based on tax positions taken during a prior period(b), (c) | ||||||||||||
Decreases based on settlements for a prior period(d) | ||||||||||||
Increases based on tax positions taken during the current period(b) | ( | ) | ( | ) | ( | ) | ||||||
Impact of foreign exchange | ( | ) | ||||||||||
Other, net(b), (e) | ||||||||||||
Balance, ending(f) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
(a) | For 2019, primarily related to the acquisition of Array. For 2017, primarily related to the acquisitions of Medivation and Anacor. See also Note 2A. |
(b) | Primarily included in Provision/(benefit) for taxes on income. |
(c) | Primarily related to effectively settling certain issues with the U.S. and foreign tax authorities. See also Note 5A. |
(d) | Primarily related to cash payments and reductions of tax attributes. |
(e) | Primarily related to decreases as a result of a lapse of applicable statutes of limitations. |
(f) | In 2019, included in Income taxes payable ($ |
• | Interest related to our unrecognized tax benefits is recorded in accordance with the laws of each jurisdiction and is recorded primarily in Provision/(benefit) for taxes on income in our consolidated statements of income. In 2019, we recorded a net decrease in interest of $ |
Status of Tax Audits and Potential Impact on Accruals for Uncertain Tax Positions
The U.S. is one of our major tax jurisdictions, and we are regularly audited by the IRS:
• | During the second quarter of 2019, Pfizer reached settlement of disputed issues at the IRS Office of Appeals, thereby settling all issues related to U.S. tax returns of Pfizer for the years 2009-2010. As a result of settling these years, in the second quarter of 2019 we recorded a benefit of approximately $ |
2019 Financial Report | 93 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
• | With respect to Pfizer, tax years 2011-2015 are currently under audit. Tax years 2016-2019 are open, but not under audit. All other tax years are closed. |
In addition to the open audit years in the U.S., we have open audit years in other major tax jurisdictions, such as Canada (2013-2019), Japan (2017-2019), Europe (2011-2019, primarily reflecting Ireland, the U.K., France, Italy, Spain and Germany), Latin America (1998-2019, primarily reflecting Brazil) and Puerto Rico (2015-2019).
Any settlements or statutes of limitations expirations could result in a significant decrease in our uncertain tax positions. We estimate that it is reasonably possible that within the next 12 months, our gross unrecognized tax benefits, exclusive of interest, could decrease by as much as $200 million, as a result of settlements with taxing authorities or the expiration of the statutes of limitations. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and variation from such estimates could materially affect our financial statements in the period of settlement or when the statutes of limitations expire, as we treat these events as discrete items in the period of resolution. Finalizing audits with the relevant taxing authorities can include formal administrative and legal proceedings, and, as a result, it is difficult to estimate the timing and range of possible changes related to our uncertain tax positions, and such changes could be significant.
E. Tax Provision/(Benefit) on Other Comprehensive Income/(Loss)
The following table provides the components of the Tax provision/(benefit) on other comprehensive income/(loss): | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Foreign currency translation adjustments, net(a) | $ | $ | $ | ( | ) | |||||||
Unrealized holding gains/(losses) on derivative financial instruments, net | ||||||||||||
Reclassification adjustments for (gains)/losses included in net income | ( | ) | ( | ) | ||||||||
Reclassification adjustments of certain tax effects from AOCI to Retained earnings(b) | ||||||||||||
( | ) | ( | ) | |||||||||
Unrealized holding gains/(losses) on available-for-sale securities, net | ( | ) | ||||||||||
Reclassification adjustments for (gains)/losses included in net income | ( | ) | ||||||||||
Reclassification adjustments for tax on unrealized gains from AOCI to Retained earnings(c) | ( | ) | ||||||||||
( | ) | |||||||||||
Benefit plans: actuarial losses, net | ( | ) | ( | ) | ( | ) | ||||||
Reclassification adjustments related to amortization | ||||||||||||
Reclassification adjustments related to settlements, net | ||||||||||||
Reclassification adjustments of certain tax effects from AOCI to Retained earnings(b) | ||||||||||||
Other | ( | ) | ( | ) | ||||||||
( | ) | |||||||||||
Benefit plans: prior service costs and other, net | ( | ) | ||||||||||
Reclassification adjustments related to amortization of prior service costs and other, net | ( | ) | ( | ) | ( | ) | ||||||
Reclassification adjustments related to curtailments of prior service costs and other, net | ( | ) | ( | ) | ( | ) | ||||||
Reclassification adjustments of certain tax effects from AOCI to Retained earnings(b) | ( | ) | ||||||||||
Other | ||||||||||||
( | ) | ( | ) | ( | ) | |||||||
Tax provision/(benefit) on other comprehensive income/(loss) | $ | $ | $ | ( | ) | |||||||
(a) | Taxes are not provided for foreign currency translation adjustments relating to investments in international subsidiaries that will be held indefinitely. |
(b) | For additional information on the adoption of a new accounting standard related to reclassification of certain tax effects from AOCI, see Notes to Consolidated Financial Statements––Note 1B. Basis of Presentation and Significant Accounting Policies: Adoption of New Accounting Standards in 2018 in our 2018 Financial Report. |
(c) | For additional information on the adoption of a new accounting standard related to financial assets and liabilities, see Notes to Consolidated Financial Statements––Note 1B. Basis of Presentation and Significant Accounting Policies: Adoption of New Accounting Standards in 2018 in our 2018 Financial Report. |
94 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 6. Accumulated Other Comprehensive Loss, Excluding Noncontrolling Interests
The following table provides the changes, net of tax, in Accumulated other comprehensive loss: | ||||||||||||||||||||||||
Net Unrealized Gain/(Losses) | Benefit Plans | |||||||||||||||||||||||
(MILLIONS OF DOLLARS) | Foreign Currency Translation Adjustments | Derivative Financial Instruments | Available-For-Sale Securities | Actuarial Gains/(Losses) | Prior Service (Costs)/ Credits and Other | Accumulated Other Comprehensive Income/(Loss) | ||||||||||||||||||
Balance, January 1, 2017 | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | $ | $ | ( | ) | ||||||||||
Other comprehensive income/(loss)(a) | ( | ) | ( | ) | ||||||||||||||||||||
Balance, December 31, 2017 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||
Other comprehensive income/(loss) due to the adoption of new accounting standards(b) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||
Other comprehensive income/(loss)(a) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||
Balance, December 31, 2018 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||
Other comprehensive income/(loss)(a) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||
Balance, December 31, 2019 | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | $ | $ | ( | ) | ||||||||||
(a) | Amounts do not include foreign currency translation adjustments attributable to noncontrolling interests of $ |
(b) | Amounts represent the cumulative effect adjustments as of January 1, 2018 from the adoption of new accounting standards related to (i) financial assets and liabilities and (ii) the reclassification of certain tax effects from AOCI. For additional information, see Notes to Consolidated Financial Statements––Note 1B. Basis of Presentation and Significant Accounting Policies: Adoption of New Accounting Standards in 2018 in our 2018 Financial Report. |
2019 Financial Report | 95 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 7. Financial Instruments
A. Fair Value Measurements
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following table presents the financial assets and liabilities measured at fair value using a market approach on a recurring basis by balance sheet categories and fair value hierarchy level as defined in Note 1E: | ||||||||||||||||||||||||
December 31, 2019 | December 31, 2018 | |||||||||||||||||||||||
(MILLIONS OF DOLLARS) | Total | Level 1 | Level 2 | Total | Level 1 | Level 2 | ||||||||||||||||||
Financial assets measured at fair value on a recurring basis: | ||||||||||||||||||||||||
Short-term investments | ||||||||||||||||||||||||
Classified as equity securities with readily determinable fair values: | ||||||||||||||||||||||||
Money market funds | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
Equity(a) | ||||||||||||||||||||||||
Classified as available-for-sale debt securities: | ||||||||||||||||||||||||
Government and agency—non-U.S. | ||||||||||||||||||||||||
Government and agency—U.S. | ||||||||||||||||||||||||
Corporate and other | ||||||||||||||||||||||||
Total short-term investments | ||||||||||||||||||||||||
Other current assets | ||||||||||||||||||||||||
Derivative assets: | ||||||||||||||||||||||||
Interest rate contracts | ||||||||||||||||||||||||
Foreign exchange contracts | ||||||||||||||||||||||||
Total other current assets | ||||||||||||||||||||||||
Long-term investments | ||||||||||||||||||||||||
Classified as equity securities with readily determinable fair values(a) | ||||||||||||||||||||||||
Classified as available-for-sale debt securities: | ||||||||||||||||||||||||
Government and agency—non-U.S. | ||||||||||||||||||||||||
Government and agency—U.S. | ||||||||||||||||||||||||
Corporate and other | ||||||||||||||||||||||||
Total long-term investments | ||||||||||||||||||||||||
Other noncurrent assets | ||||||||||||||||||||||||
Derivative assets: | ||||||||||||||||||||||||
Interest rate contracts | ||||||||||||||||||||||||
Foreign exchange contracts | ||||||||||||||||||||||||
Total derivative assets | ||||||||||||||||||||||||
Insurance contracts(b) | ||||||||||||||||||||||||
Total other noncurrent assets | ||||||||||||||||||||||||
Total assets | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
Financial liabilities measured at fair value on a recurring basis: | ||||||||||||||||||||||||
Other current liabilities | ||||||||||||||||||||||||
Derivative liabilities: | ||||||||||||||||||||||||
Interest rate contracts | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
Foreign exchange contracts | ||||||||||||||||||||||||
Total other current liabilities | ||||||||||||||||||||||||
Other noncurrent liabilities | ||||||||||||||||||||||||
Derivative liabilities: | ||||||||||||||||||||||||
Interest rate contracts | ||||||||||||||||||||||||
Foreign exchange contracts | ||||||||||||||||||||||||
Total other noncurrent liabilities | ||||||||||||||||||||||||
Total liabilities | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
(a) | As of December 31, 2019, long-term equity securities of $ |
(b) | Other noncurrent assets include life insurance policies held in restricted trusts attributable to the funding of various U.S. non-qualified employee benefit plans. The underlying invested assets in these insurance contracts are marketable securities, which are carried at fair value, with changes in fair value recognized in Other (income)/deductions––net in the consolidated statements of income (see Note 4). |
96 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Financial Assets and Liabilities Not Measured at Fair Value on a Recurring Basis
The following table presents the financial liabilities not measured at fair value on a recurring basis, including the carrying values and estimated fair values using a market approach: | ||||||||||||||||||||||||
December 31, 2019 | December 31, 2018 | |||||||||||||||||||||||
Carrying Value | Estimated Fair Value | Carrying Value | Estimated Fair Value | |||||||||||||||||||||
(MILLIONS OF DOLLARS) | Total | Level 2 | Total | Level 2 | ||||||||||||||||||||
Financial Liabilities | ||||||||||||||||||||||||
Long-term debt, excluding the current portion | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
The differences between the estimated fair values and carrying values of held-to-maturity debt securities, restricted stock and private equity securities, and short-term borrowings not measured at fair value on a recurring basis were not significant as of December 31, 2019 or December 31, 2018. The fair value measurements of our held-to-maturity debt securities and our short-term borrowings are based on Level 2 inputs. The fair value measurements of our private equity securities, which represent investments in the life sciences sector, are based on Level 3 inputs using a market approach.
In addition, as of December 31, 2019 and 2018, we had long-term receivables whose fair value is based on Level 3 inputs. As of December 31, 2019 and 2018, the differences between the estimated fair values and carrying values of these receivables were not significant.
Total Short-Term and Long-Term Investments and Equity-Method Investments
The following table represents our investments by classification type: | ||||||||
As of December 31, | ||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | ||||||
Short-term investments | ||||||||
Equity securities with readily determinable fair values(a) | $ | $ | ||||||
Available-for-sale debt securities | ||||||||
Held-to-maturity debt securities | ||||||||
Total Short-term investments | $ | $ | ||||||
Long-term investments | ||||||||
Equity securities with readily determinable fair values | $ | $ | ||||||
Available-for-sale debt securities | ||||||||
Held-to-maturity debt securities | ||||||||
Private equity securities at cost | ||||||||
Total Long-term investments | $ | $ | ||||||
Equity-method investments | ||||||||
Total long-term investments and equity-method investments | $ | $ | ||||||
Held-to-maturity cash equivalents | $ | $ | ||||||
(a) | As of December 31, 2019 and December 31, 2018, equity securities with readily determinable fair values included money market funds primarily invested in U.S. Treasury and government debt. |
Fair Value Methodology
The following inputs and valuation techniques were used to estimate the fair value of our financial assets and liabilities:
• | Available-for-sale debt securities—third-party matrix-pricing model that uses significant inputs derived from or corroborated by observable market data and credit-adjusted interest rate yield curves. |
• | Equity securities with readily determinable fair values—quoted market prices and observable net asset value prices. |
• | Derivative assets and liabilities—third-party matrix-pricing model that uses significant inputs derived from or corroborated by observable market data. Where applicable, these models discount future cash flow amounts using market-based observable inputs, including interest rate yield curves, and forward and spot prices for currencies. The credit risk impact to our derivative financial instruments was not significant. |
• | Money market funds—observable net asset value prices. |
We periodically review the methodologies, inputs and outputs of third-party pricing services for reasonableness. Our procedures can include, for example, referencing other third-party pricing models, monitoring key observable inputs (like LIBOR interest rates) and selectively performing test-comparisons of values with actual sales of financial instruments.
2019 Financial Report | 97 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
B. Investments
Debt Securities
At December 31, 2019, the investment securities portfolio consisted of debt securities that were virtually all investment-grade. Information on investments in debt securities at December 31, 2019 and December 31, 2018 is as follows, including, as of December 31, 2019, the contractual maturities, or as necessary, the estimated maturities, of the available-for-sale and held-to-maturity debt securities: | ||||||||||||||||||||||||||||||||||||||||||||
December 31, 2019 | December 31, 2018 | |||||||||||||||||||||||||||||||||||||||||||
Gross Unrealized | Maturities (in Years) | Gross Unrealized | ||||||||||||||||||||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | Amortized Cost | Gains | Losses | Fair Value | Within 1 | Over 1 to 5 | Over 5 | Amortized Cost | Gains | Losses | Fair Value | |||||||||||||||||||||||||||||||||
Available-for-sale debt securities | ||||||||||||||||||||||||||||||||||||||||||||
Government and agency––non-U.S. | $ | $ | $ | ( | ) | $ | $ | $ | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||||||||||
Government and agency––U.S. | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||
Corporate and other(a) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||
Held-to-maturity debt securities | ||||||||||||||||||||||||||||||||||||||||||||
Time deposits and other | ||||||||||||||||||||||||||||||||||||||||||||
Government and agency––non-U.S. | ||||||||||||||||||||||||||||||||||||||||||||
Total debt securities | $ | $ | $ | ( | ) | $ | $ | $ | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||||||||||
(a) | Primarily issued by a diverse group of corporations. |
Equity Securities
The following table presents the net unrealized (gains) and losses for the period that relate to equity securities, excluding equity method investments, still held at the reporting date, calculated as follows: | ||||||||
(MILLIONS OF DOLLARS) | December 31, 2019 | December 31, 2018 | ||||||
Net gains recognized during the period on equity securities(a) | $ | ( | ) | $ | ( | ) | ||
Less: Net gains recognized during the period on equity securities sold during the period | ( | ) | ( | ) | ||||
Net unrealized gains during the reporting period on equity securities still held at the reporting date | $ | ( | ) | $ | ( | ) | ||
(a) | The net gains on equity securities are reported in Other (income)/deductions––net. For additional information, see Note 4. |
C. Short-Term Borrowings
Short-term borrowings include: | ||||||||
As of December 31, | ||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | ||||||
Commercial paper | $ | $ | ||||||
Current portion of long-term debt, principal amount(a) | ||||||||
Other short-term borrowings, principal amount(b) | ||||||||
Total short-term borrowings, principal amount | ||||||||
Net fair value adjustments related to hedging and purchase accounting | ( | ) | ||||||
Net unamortized discounts, premiums and debt issuance costs | ( | ) | ( | ) | ||||
Total Short-term borrowings, including current portion of long-term debt, carried at historical proceeds, as adjusted | $ | $ | ||||||
(a) | For additional information, see Note 7D. |
(b) | Other short-term borrowings primarily include cash collateral. For additional information, see Note 7F. |
The weighted-average effective interest rate on commercial paper outstanding was approximately 1.92 % as of December 31, 2019 and 2.42 % as of December 31, 2018.
As of December 31, 2019, we had access to a total of $15 billion in U.S. revolving credit facilities consisting of a $7 billion facility expiring in 2024 and an $8 billion facility expiring in September 2020, which may be used to support our commercial paper borrowings. In addition to the U.S. revolving credit facilities, our lenders have provided us an additional $537 million in lines of credit, of which $508 million expire within one year. Of these total lines of credit, $15.5 billion were unused as of December 31, 2019.
98 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
D. Long-Term Debt
New Issuances
In the first quarter of 2019, we issued the following senior unsecured notes: | ||||||
(MILLIONS OF DOLLARS) | Principal | |||||
Interest Rate | Maturity Date | As of December 31, 2019 | ||||
2.800% notes(a) | March 11, 2022 | $ | ||||
2.950% notes(a) | March 15, 2024 | |||||
3.450% notes(a) | March 15, 2029 | |||||
3.900% notes(a) | March 15, 2039 | |||||
4.000% notes(a) | March 15, 2049 | |||||
Total long-term debt issued in the first quarter of 2019(b) | $ | |||||
(a) | Fixed rate notes may be redeemed by us at any time, in whole, or in part, at varying redemption prices plus accrued and unpaid interest. |
(b) | The weighted-average effective interest rate for the notes at issuance was |
In September 2018, we completed a public offering of $5.0 billion aggregate principal amount of senior unsecured notes with a weighted-average effective interest rate of 3.56 %.
In March 2017, we completed a public offering of $1.065 billion principal amount of senior unsecured notes due 2047 with an interest rate of 4.20 %, and also in March 2017, we completed a public offering of €4.0 billion principal amount of senior unsecured notes with a weighted-average effective interest rate of 0.23 %.
Retirements
In January 2019, we repurchased all €1.1 billion ($1.3 billion, at exchange rates on settlement) principal amount outstanding of the 5.75 % euro-denominated debt that was due June 2021 before the maturity date at a redemption value of €1.3 billion ($1.5 billion, at exchange rates on settlement). As a result, in the first quarter of 2019, we recorded a net loss of approximately $138 million, which included the related termination of cross-currency swaps, and is reported in Other (income)/deductions––net in the consolidated statements of income (see Note 4).
In December 2017, we exchanged approximately £833 million and repurchased £197 million principal amount of the outstanding 6.50 % debt before the maturity date at a redemption value of £1.7 billion, leaving £470 million principal amount of the 6.50 % debt due 2038 outstanding. Also, in December 2017, we repurchased approximately €834 million principal amount of the outstanding 5.75 % debt before the maturity date at a redemption value of €1.0 billion, leaving approximately €1.2 billion of the 5.75 % euro-denominated debt due 2021 outstanding as of December 31, 2017. As a result, we recorded a net loss of approximately $846 million and $153 million upon the exchange and early retirement of the U.K. pound-denominated debt and the early retirement of the euro-denominated debt, respectively, for a net loss on early retirement of debt of $999 million, which included the related termination of cross-currency swaps, and that were recorded in Other (income)/deductions––net in the consolidated statement of income (see Note 4).
The following table provides the components of our senior unsecured long-term debt, including the weighted-average stated interest rate for 2019 and 2018 by maturity: | ||||||||
As of December 31, | ||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | ||||||
Notes due 2020 (1.2%)(a) | $ | $ | ||||||
Notes due 2021 (0.7% and 3.4%) | ||||||||
Notes due 2022 (1.0% and 0.3%) | ||||||||
Notes due 2023 (3.7% and 3.8%) | ||||||||
Notes due 2024 (3.9% and 4.4%) | ||||||||
Notes due 2026-2029 (3.3%) | ||||||||
Notes due 2034 (6.5%) | ||||||||
Notes due 2036-2040 (5.8% and 6.0%) | ||||||||
Notes due 2043-2044 (3.5%) | ||||||||
Notes due 2046-2049 (4.1% and 4.2%) | ||||||||
Total long-term debt, principal amount | ||||||||
Net fair value adjustments related to hedging and purchase accounting | ||||||||
Net unamortized discounts, premiums and debt issuance costs | ( | ) | ( | ) | ||||
Other long-term debt | ||||||||
Total long-term debt, carried at historical proceeds, as adjusted | $ | $ | ||||||
Current portion of long-term debt, carried at historical proceeds (not included above (1.2% and 1.3%)) | $ | $ | ||||||
(a) | At December 31, 2019, the debt issuances have been reclassified to the current portion of long-term debt. |
Our long-term debt, provided in the above table, is generally redeemable by us at any time at varying redemption prices plus accrued and unpaid interest.
2019 Financial Report | 99 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
E. Other Noncurrent Liabilities
Mylotarg (gemtuzumab ozogamicin)
In April 2018, the EU approved Mylotarg for the treatment of acute myeloid leukemia. In connection with the EU approval, we incurred an obligation to make guaranteed fixed annual payments over a ten-year period aggregating $301 million related to an R&D arrangement. We recorded the estimated net present value of $240 million as a liability and an intangible asset in Developed technology rights as of the approval date. In June 2018, we entered into a transaction with the obligee to buyout the remaining liability for the fixed annual payments for a lump sum payment of $224 million. As a result of the buyout transaction, the liability was extinguished and we recognized a non-cash $17 million pre-tax gain in Other (income)/deductions––net in the second quarter of 2018 (see Note 4).
Bosulif (bosutinib)
In December 2017, the FDA approved Bosulif for the treatment of patients with newly-diagnosed chronic-phase Ph+ CML. In connection with the U.S. approval, we incurred an obligation to make guaranteed fixed annual payments over a ten-year period aggregating $416 million related to an R&D arrangement. We recorded the estimated net present value of $364 million as of the approval date as an intangible asset in Developed technology rights. In August 2018, we entered into a transaction with the obligee to buyout a portion of the remaining liability for the fixed annual payments for a lump sum payment of $71 million. As a result of the buyout transaction, the liability was reduced and we recognized a non-cash $9 million pre-tax gain in Other (income)/deductions––net in the third quarter of 2018. The present value of the remaining future payments as of December 31, 2019 is $191 million, of which $22 million is recorded in Other current liabilities and $169 million is recorded in Other noncurrent liabilities.
Besponsa (inotuzumab ozogamicin)
In August 2017, the FDA approved Besponsa and in June 2017, the EU approved Besponsa as monotherapy for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia. In connection with the U.S. approval, we incurred an obligation to make guaranteed fixed annual payments over a nine-year period aggregating $296 million related to an R&D arrangement. We recorded the estimated net present value of $248 million as of the approval date as an intangible asset in Developed technology rights. The present value of the remaining future payments as of December 31, 2019 is $242 million, of which $7 million is recorded in Other current liabilities and $235 million is recorded in Other noncurrent liabilities. In connection with the EU approval, we incurred an obligation to make guaranteed fixed annual payments over a nine-year period aggregating $148 million related to an R&D arrangement. We recorded the estimated net present value of $123 million as of the approval date as an intangible asset in Developed technology rights. The present value of the remaining future payments as of December 31, 2019 is $122 million, of which $3 million is recorded in Other current liabilities and $119 million is recorded in Other noncurrent liabilities.
As of December 31, 2019 and 2018, the differences between the estimated fair values, using a market approach in the Level 2 fair value hierarchy, and carrying values of these obligations were not significant.
F. Derivative Financial Instruments and Hedging Activities
Foreign Exchange Risk
A significant portion of our revenues, earnings and net investments in foreign affiliates is exposed to changes in foreign exchange rates. We manage our foreign exchange risk, in part, through operational means, including managing same-currency revenues in relation to same-currency costs and same-currency assets in relation to same-currency liabilities. We also manage our foreign exchange risk, depending on market conditions, through fair value, cash flow, and net investment hedging programs through the use of derivative financial instruments and foreign currency debt. These financial instruments serve to protect net income against the impact of remeasurement into another currency, or against the impact of translation into U.S. dollars of certain foreign exchange-denominated transactions.
All derivative financial instruments used to manage foreign currency risk are measured at fair value and are reported as assets or liabilities on the consolidated balance sheet. The derivative financial instruments primarily hedge or offset exposures in the euro, U.K. pound, Japanese yen, Chinese renminbi and Swedish krona. Changes in fair value are reported in earnings or in Other comprehensive income/(loss), depending on the nature and purpose of the financial instrument (hedge or offset relationship) and the effectiveness of the hedge relationships, as follows:
• | Generally, we recognize the gains and losses on foreign exchange contracts that are designated as fair value hedges in earnings upon the recognition of the change in fair value of the hedged risk. For certain foreign exchange contracts, we exclude an amount from the assessment of hedge effectiveness and recognize that excluded amount through an amortization approach. We also recognize the offsetting foreign exchange impact attributable to the hedged item in earnings. |
• | Generally, we record in Other comprehensive income/(loss) gains or losses on foreign exchange contracts that are designated as cash flow hedges and reclassify those amounts, as appropriate, into earnings in the same period or periods during which the hedged transaction affects earnings. For certain foreign exchange contracts, we exclude an amount from the assessment of hedge effectiveness and recognize that excluded amount through an amortization approach. |
• | We record in Other comprehensive income/(loss) the foreign exchange gains and losses related to foreign exchange-denominated debt and foreign exchange contracts designated as a hedge of our net investments in foreign subsidiaries and reclassify those amounts into earnings upon the sale or substantial liquidation of our net investments. For foreign exchange contracts, we exclude an amount from the assessment of hedge effectiveness and recognize that excluded amount through an amortization approach. |
• | For certain foreign exchange contracts not designated as hedging instruments, we recognize the gains and losses on foreign currency exchange contracts that are used to offset the same foreign currency assets or liabilities immediately into earnings along with the earnings impact of the items they generally offset. These contracts essentially take the opposite currency position of that reflected in the month-end balance sheet to counterbalance the effect of any currency movement. |
100 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
As a part of our cash flow hedging program, we designate foreign exchange contracts to hedge a portion of our forecasted euro, Japanese yen, Chinese renminbi, Canadian dollar, U.K. pound and Australian dollar-denominated intercompany inventory sales expected to occur no more than two years from the date of each hedge.
For 2017, any ineffectiveness was recognized immediately into earnings. There was no significant ineffectiveness for 2017.
Interest Rate Risk
Our interest-bearing investments and borrowings are subject to interest rate risk. With respect to our investments, we strive to maintain a predominantly floating-rate basis position, but our strategy may change based on prevailing market conditions. We currently borrow primarily on a long-term, fixed-rate basis. From time to time, depending on market conditions, we will change the profile of our outstanding debt by entering into derivative financial instruments like interest rate swaps. We entered into derivative financial instruments to hedge or offset the fixed interest rates on the hedged item, matching the amount and timing of the hedged item. The derivative financial instruments primarily hedge U.S. dollar fixed-rate debt.
All derivative contracts used to manage interest rate risk are measured at fair value and reported as assets or liabilities on the consolidated balance sheet. Changes in fair value are reported in earnings, as follows:
• | We recognize the gains and losses on interest rate contracts that are designated as fair value hedges in earnings upon the recognition of the change in fair value of the hedged risk. We also recognize the offsetting earnings impact of fixed-rate debt attributable to the hedged risk in earnings. |
For 2017, any ineffectiveness was recognized immediately into earnings. There was no significant ineffectiveness for 2017.
The following table provides the fair value of the derivative financial instruments and the related notional amounts presented between those derivatives that are designated as hedging instruments and those that are not designated as hedging instruments: | ||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | December 31, 2019 | December 31, 2018 | ||||||||||||||||||||||
Fair Value | Fair Value | |||||||||||||||||||||||
Notional | Asset | Liability | Notional | Asset | Liability | |||||||||||||||||||
Derivatives designated as hedging instruments: | ||||||||||||||||||||||||
Foreign exchange contracts(a) | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
Interest rate contracts | ||||||||||||||||||||||||
Derivatives not designated as hedging instruments: | ||||||||||||||||||||||||
Foreign exchange contracts | $ | $ | ||||||||||||||||||||||
Total | $ | $ | $ | $ | ||||||||||||||||||||
(a) |
2019 Financial Report | 101 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides information about the gains/(losses) incurred to hedge or offset operational foreign exchange or interest rate risk: | ||||||||||||||||||||||||
Amount of Gains/(Losses) Recognized in OID(a) | Amount of Gains/(Losses) Recognized in OCI(a), (b) | Amount of Gains/(Losses) Reclassified from OCI into OID and COS(a), (b) | ||||||||||||||||||||||
As of December 31, | ||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | ||||||||||||||||||
Derivative Financial Instruments in Cash Flow Hedge Relationships: | ||||||||||||||||||||||||
Foreign exchange contracts(c) | $ | — | $ | — | $ | $ | $ | $ | ( | ) | ||||||||||||||
Amount excluded from effectiveness testing recognized in earnings based on an amortization approach(d) | — | — | ||||||||||||||||||||||
Derivative Financial Instruments in Fair Value Hedge Relationships: | ||||||||||||||||||||||||
Interest rate contracts | ( | ) | — | — | — | — | ||||||||||||||||||
Hedged item | ( | ) | — | — | — | — | ||||||||||||||||||
Foreign exchange contracts | — | — | — | — | ||||||||||||||||||||
Hedged item | ( | ) | — | — | — | — | ||||||||||||||||||
Derivative Financial Instruments in Net Investment Hedge Relationships: | ||||||||||||||||||||||||
Foreign exchange contracts | — | — | ( | ) | ||||||||||||||||||||
The portion on foreign exchange contracts excluded from the assessment of hedge effectiveness(d) | — | — | ||||||||||||||||||||||
Non-Derivative Financial Instruments in Net Investment Hedge Relationships: | ||||||||||||||||||||||||
Foreign currency short-term borrowings(e) | — | — | ||||||||||||||||||||||
Foreign currency long-term debt(e) | — | — | ||||||||||||||||||||||
Derivative Financial Instruments Not Designated as Hedges: | ||||||||||||||||||||||||
Foreign exchange contracts | ( | ) | — | — | — | — | ||||||||||||||||||
All other net(d) | — | — | ( | ) | ( | ) | ||||||||||||||||||
$ | ( | ) | $ | $ | $ | $ | $ | |||||||||||||||||
(a) | OID = Other (income)/deductions—net, included in Other (income)/deductions—net in the consolidated statements of income. COS = Cost of Sales, included in Cost of sales in the consolidated statements of income. OCI = Other comprehensive income/(loss), included in the consolidated statements of comprehensive income. |
(b) | For derivative financial instruments in cash flow hedge relationships, the gains and losses are included in Other comprehensive income/(loss)––Unrealized holding gains/(losses) on derivative financial instruments, net. For derivative financial instruments in net investment hedge relationships and for foreign currency debt designated as hedging instruments, the gains and losses are included in Other comprehensive income/(loss)––Foreign currency translation adjustments, net. |
The amounts reclassified from OCI into COS were a net gain of $ |
(d) | These amounts were reclassified from OCI into OID. |
(e) | Short-term borrowings include foreign currency short-term borrowings with carrying values of $ |
102 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides the amounts recorded in our consolidated balance sheet related to cumulative basis adjustments for fair value hedges: | ||||||||||||||||||||||||
December 31, 2019 | December 31, 2018 | |||||||||||||||||||||||
Cumulative Amount of Fair Value Hedging Adjustment Increase/(Decrease) to Carrying Amount | Cumulative Amount of Fair Value Hedging Adjustment Increase/(Decrease) to Carrying Amount | |||||||||||||||||||||||
(MILLIONS OF DOLLARS) | Carrying Amount of Hedged Assets/Liabilities(a) | Active Hedging Relationships | Discontinued Hedging Relationships | Carrying Amount of Hedged Assets/Liabilities(a) | Active Hedging Relationships | Discontinued Hedging Relationships | ||||||||||||||||||
Long-term investments | $ | $ | $ | $ | $ | ( | ) | $ | ||||||||||||||||
Short-term borrowings, including current portion of long-term debt | ( | ) | ||||||||||||||||||||||
Long-term debt | ( | ) | ||||||||||||||||||||||
(a) | Carrying amounts exclude the cumulative amount of fair value hedging adjustments. |
Certain of our derivative financial instruments are covered by associated credit-support agreements that have credit-risk-related contingent features designed to reduce both counterparties’ exposure to risk of defaulting on amounts owed by the other party. As of December 31, 2019, the aggregate fair value of these derivative financial instruments that are in a net liability position was $449 million, for which we have posted collateral of $470 million in the normal course of business. If there had been a downgrade to below an A rating by S&P or the equivalent rating by Moody’s, we would not have been required to post any additional collateral to our counterparties.
As of December 31, 2019, we received cash collateral of $835 million from various counterparties. The collateral primarily supports the approximate fair value of our derivative contracts. With respect to the collateral received, the obligations are reported in Short-term borrowings, including current portion of long-term debt.
G. Credit Risk
On an ongoing basis, we review the creditworthiness of counterparties to our foreign exchange and interest rate agreements and do not expect to incur a significant loss from failure of any counterparties to perform under the agreements. There are no significant concentrations of credit risk related to our financial instruments with any individual counterparty. For additional information about concentrations of credit risk related to certain significant customers, see Note 17C. As of December 31, 2019, we had amounts due from a well-diversified, high quality group of banks ($1.4 billion) from around the world. For details about our investments, see Note 7B above.
In general, there is no requirement for collateral from customers. However, derivative financial instruments are executed under credit-support agreements that provide for the ability to request to receive cash collateral, depending on levels of exposure, our credit rating and the credit rating of the counterparty, see Note 7F above.
Note 8. Inventories
The following table provides the components of Inventories: | ||||||||
As of December 31, | ||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | ||||||
Finished goods | $ | $ | ||||||
Work in process | ||||||||
Raw materials and supplies | ||||||||
Inventories(a) | $ | $ | ||||||
Noncurrent inventories not included above(b) | $ | $ | ||||||
(a) | The change from December 31, 2018 reflects increases for certain products, including inventory build for new product launches, supply recovery and market demand, partially offset by a decrease due to foreign exchange and the write off of rivipansel inventory previously expected to be sold (see Note 2E). |
(b) | Included in Other noncurrent assets. There are no recoverability issues associated with these amounts. |
2019 Financial Report | 103 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 9. Property, Plant and Equipment
The following table provides the components of Property, plant and equipment: | ||||||||||
Useful Lives | As of December 31, | |||||||||
(MILLIONS OF DOLLARS) | (Years) | 2019 | 2018 | |||||||
Land | - | $ | $ | |||||||
Buildings | 33-50 | |||||||||
Machinery and equipment | 8-20 | |||||||||
Furniture, fixtures and other | 3-12 1/2 | |||||||||
Construction in progress | - | |||||||||
Less: Accumulated depreciation | ||||||||||
Property, plant and equipment(a) | $ | $ | ||||||||
(a) |
Note 10. Identifiable Intangible Assets and Goodwill
A. Identifiable Intangible Assets
Balance Sheet Information
The following table provides the components of Identifiable intangible assets: | ||||||||||||||||||||||||
December 31, 2019 | December 31, 2018 | |||||||||||||||||||||||
(MILLIONS OF DOLLARS) | Gross Carrying Amount | Accumulated Amortization | Identifiable Intangible Assets, less Accumulated Amortization | Gross Carrying Amount | Accumulated Amortization | Identifiable Intangible Assets, less Accumulated Amortization | ||||||||||||||||||
Finite-lived intangible assets | ||||||||||||||||||||||||
Developed technology rights(a) | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||||||
Brands | ( | ) | ( | ) | ||||||||||||||||||||
Licensing agreements and other(a) | ( | ) | ( | ) | ||||||||||||||||||||
( | ) | ( | ) | |||||||||||||||||||||
Indefinite-lived intangible assets | ||||||||||||||||||||||||
Brands | ||||||||||||||||||||||||
IPR&D(a) | ||||||||||||||||||||||||
Licensing agreements and other(a), (b) | ||||||||||||||||||||||||
Identifiable intangible assets(a), (c) | $ | $ | ( | ) | $ | $ | $ | ( | ) | $ | ||||||||||||||
(a) | The increase in the gross carrying amount of Identifiable intangible assets mainly reflects the impact of the acquisition of Array, including the addition of $ |
(b) | Reflects acquired licensing agreements for technology in development. |
(c) | The increase in Identifiable intangible assets, less accumulated amortization, is mostly due to the additions noted in (a) above, partially offset by amortization and intangible asset impairment charges, primarily for Eucrisa in Developed technology rights. See Note 4 for additional information on intangible asset impairments. |
Our identifiable intangible assets are associated with the following, as a percentage of total identifiable intangible assets, less accumulated amortization: | |||||||||
December 31, 2019 | |||||||||
Biopharma | Upjohn | WRDM | |||||||
Developed technology rights | % | % | |||||||
Brands, finite-lived | % | ||||||||
Brands, indefinite-lived | % | % | |||||||
IPR&D | % | % | |||||||
Licensing agreements and other, finite-lived | % | % | |||||||
Licensing agreements and other, indefinite-lived | % | ||||||||
104 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Developed technology rights represent the amortized cost associated with developed technology, which has been acquired from third parties and which can include the right to develop, use, market, sell and/or offer for sale the product, compounds and intellectual property that we have acquired with respect to products, compounds and/or processes that have been completed. We possess a well-diversified portfolio of hundreds of developed technology rights across therapeutic categories, representing the commercialized products included in our biopharmaceutical businesses. The more significant components of developed technology rights are the following (in order of significance): Xtandi, Prevnar 13/Prevenar 13 Infant, Braftovi/Mektovi, Eucrisa, Premarin, Prevnar 13/Prevenar 13 Adult, and, to a lesser extent Tygacil, Zavicefta, Pristiq, Refacto AF and Bosulif. Also included in this category are the post-approval milestone payments made under our alliance agreements for certain biopharmaceutical products.
Brands
Brands represent the amortized or unamortized cost associated with tradenames and know-how, as the products themselves do not receive patent protection. The more significant components of indefinite-lived brands are the following (in order of significance): Xanax, Medrol and Depo-Medrol. The more significant components of finite-lived brands are the following (in order of significance): Depo-Provera and Zavedos.
IPR&D
IPR&D assets represent R&D assets that have not yet received regulatory approval in a major market. The significant components of IPR&D at December 31, 2019 include IPR&D assets acquired in connection with the Array acquisition and the program for the oral PARP inhibitor for the treatment of patients with germline BRCA-mutated advanced breast cancer acquired as part of the Medivation acquisition. IPR&D assets are required to be classified as indefinite-lived assets until the successful completion or the abandonment of the associated R&D effort. Accordingly, during the development period after the date of acquisition, these assets will not be amortized until approval is obtained in a major market, typically either the U.S. or the EU, or in a series of other countries, subject to certain specified conditions and management judgment. At that time, we will determine the useful life of the asset, reclassify the asset out of IPR&D and begin amortization. If the associated R&D effort is abandoned, the related IPR&D assets will likely be written-off, and we will record an impairment charge.
For IPR&D assets, the risk of failure is significant and there can be no certainty that these assets ultimately will yield successful products. The nature of the biopharmaceutical business is high-risk and, as such, we expect that many of these IPR&D assets will become impaired and be written off at some time in the future.
Licensing Agreements
Licensing agreements for developed technology and licensing agreements for technology in development primarily relate to out-licensing arrangements acquired from third parties, including the Array acquisition. These intangible assets represent the amortized or unamortized cost associated with the license, where Pfizer has acquired the right to future royalties and/or milestones upon development or commercialization by the licensing partner. A significant component of the licensing arrangements at December 31, 2019 are for out-licensing arrangements with a number of partners for oncology technology in varying stages of development that have not yet received regulatory approval in a major market. Accordingly, during the development period after the date of acquisition, each of these assets is classified as indefinite-lived intangible assets and will not be amortized until approval is obtained in a major market. At that time we will determine the useful life of the asset, reclassify the respective licensing arrangement asset to finite-lived intangible asset and begin amortization. If the development effort is abandoned, the related licensing asset will likely be written-off, and we will record an impairment charge.
Amortization
The weighted-average life for each of our total finite-lived intangible assets and the largest component, developed technology rights, is approximately 9 years. Total amortization expense for finite-lived intangible assets was $4.7 billion in 2019, $5.0 billion in 2018 and $4.8 billion in 2017.
The following table provides the annual amortization expense expected for the years 2020 through 2024: | ||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2020 | 2021 | 2022 | 2023 | 2024 | |||||||||||||||
Amortization expense | $ | $ | $ | $ | $ | |||||||||||||||
B. Goodwill
Prior to 2019, we managed our commercial operations through two distinct business segments: Pfizer Innovative Health (IH) and Pfizer Essential Health (EH). At the beginning of our 2019 fiscal year, we reorganized our commercial operations and our businesses have been managed through three different operating segments––Biopharma, Upjohn and through July 31, 2019, Pfizer’s Consumer Healthcare business (see Note 17 for further information). Our Consumer Healthcare business was classified as held for sale as of December 31, 2018 (see Note 2C for further information). Additionally, upon closing of the transaction during the third quarter of 2019, we deconsolidated our Consumer Healthcare business and derecognized Consumer Healthcare goodwill.
As a result of the reorganization of our commercial operations, our remaining goodwill was required to be reallocated amongst the then new Biopharma and Upjohn operating segments by determining the fair value of each reporting unit under our old and new management structure and the portions being transferred. We completed this re-allocation based on relative fair value in the second quarter of 2019 and have retrospectively presented goodwill according to the new operating structure.
2019 Financial Report | 105 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides the components of and changes in the carrying amount of Goodwill: | ||||||||||||||||
(MILLIONS OF DOLLARS) | Biopharma | Upjohn | Consumer Healthcare | Total | ||||||||||||
Balance, January 1, 2018 | $ | $ | $ | $ | ||||||||||||
Other(a) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
Balance, December 31, 2018 | ||||||||||||||||
Additions(b) | ||||||||||||||||
Other(c) | ( | ) | ( | ) | ( | ) | ||||||||||
Balance, December 31, 2019 | $ | $ | $ | $ | ||||||||||||
(a) | Primarily reflects the reclassification of our Consumer Healthcare business as held for sale (see Note 2C), the impact of foreign exchange and the contribution of the allogeneic CAR T developmental program assets and operations to Allogene that constituted a business for accounting purposes (see Note 2B). |
(b) | Biopharma additions relate to our acquisition of Array (see Note 2A). |
(c) | Primarily reflects the impact of foreign exchange. |
Note 11. Pension and Postretirement Benefit Plans and Defined Contribution Plans
The majority of our employees worldwide are eligible for retirement benefits provided through defined benefit pension plans, defined contribution plans or both. In the U.S., we sponsor both IRC-qualified and supplemental (non-qualified) defined benefit plans and defined contribution plans. A qualified plan meets the requirements of certain sections of the IRC, and, generally, contributions to qualified plans are tax deductible. A qualified plan typically provides benefits to a broad group of employees with restrictions on discriminating in favor of highly compensated employees with regard to coverage, benefits and contributions. A supplemental (non-qualified) plan provides additional benefits to certain employees. In addition, we provide medical insurance benefits to certain retirees and their eligible dependents through our postretirement plans.
A. Components of Net Periodic Benefit Costs and Changes in Other Comprehensive Income/(Loss)
The following table provides the annual (credit)/cost and changes in Other comprehensive income/(loss) for our benefit plans: | ||||||||||||||||||||||||||||||||||||||||||||||||
Year Ended December 31, | ||||||||||||||||||||||||||||||||||||||||||||||||
Pension Plans | ||||||||||||||||||||||||||||||||||||||||||||||||
U.S. Qualified(a) | U.S. Supplemental (Non-Qualified) | International | Postretirement Plans | |||||||||||||||||||||||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 2019 | 2018 | 2017 | 2019 | 2018 | 2017 | 2019 | 2018 | 2017 | ||||||||||||||||||||||||||||||||||||
Service cost(b) | $ | $ | $ | $ | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||||||||||||
Interest cost | ||||||||||||||||||||||||||||||||||||||||||||||||
Expected return on plan assets | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
Amortization of: | ||||||||||||||||||||||||||||||||||||||||||||||||
Actuarial losses(b) | ||||||||||||||||||||||||||||||||||||||||||||||||
Prior service cost/(credit) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||
Curtailments | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||
Settlements | ( | ) | ||||||||||||||||||||||||||||||||||||||||||||||
Special termination benefits | ||||||||||||||||||||||||||||||||||||||||||||||||
Net periodic benefit cost/(credit) reported in income(c) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||
(Credit)/cost reported in Other comprehensive income/(loss) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||
(Credit)/cost recognized in Comprehensive income | $ | ( | ) | $ | $ | $ | $ | ( | ) | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||||||
(a) | In the second quarter of 2017, we settled the remaining obligation associated with the Hospira U.S. qualified defined benefit pension plan. We purchased a group annuity contract on behalf of the remaining plan participants with a third-party insurance provider. As a result, we were relieved of the $ |
(b) | Effective January 1, 2018, we froze |
(c) | We adopted an accounting standard on January 1, 2018 that requires the net periodic pension and postretirement benefit costs other than service costs be presented in Other (income)/deductions––net on the consolidated statements of income. For additional information, see Note 4. |
106 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides the amounts in Accumulated other comprehensive loss expected to be amortized into 2020 net periodic benefit costs: | ||||||||||||||||
Pension Plans | ||||||||||||||||
(MILLIONS OF DOLLARS) | U.S. Qualified | U.S. Supplemental (Non-Qualified) | International | Postretirement Plans | ||||||||||||
Actuarial (losses)/gains(a) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ||||||
Prior service credits and other | ||||||||||||||||
Total | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ||||||
(a) | Due to the U.S. Pfizer Consolidated Pension Plan freeze effective for January 1, 2018, the average amortization period for the U.S. qualified plans and U.S. supplemental (non-qualified) plans reflect the expected life expectancy of the plan participants, whereas prior years utilized the expected future service period of plan participants. The average amortization periods to be utilized for 2020 are |
B. Actuarial Assumptions
The following table provides the weighted-average actuarial assumptions of our benefit plans: | |||||||||
(PERCENTAGES) | 2019 | 2018 | 2017 | ||||||
Weighted-average assumptions used to determine benefit obligations | |||||||||
Discount rate: | |||||||||
U.S. qualified pension plans | % | % | % | ||||||
U.S. non-qualified pension plans | % | % | % | ||||||
International pension plans | % | % | % | ||||||
Postretirement plans | % | % | % | ||||||
Rate of compensation increase: | |||||||||
U.S. qualified pension plans(a) | % | ||||||||
U.S. non-qualified pension plans(a) | % | ||||||||
International pension plans | % | % | % | ||||||
Weighted-average assumptions used to determine net periodic benefit cost | |||||||||
Discount rate: | |||||||||
U.S. qualified pension plans | % | % | % | ||||||
U.S. non-qualified pension plans | % | % | % | ||||||
International pension plans interest cost | % | % | % | ||||||
International pension plans service cost | % | % | % | ||||||
Postretirement plans | % | % | % | ||||||
Expected return on plan assets: | |||||||||
U.S. qualified pension plans | % | % | % | ||||||
International pension plans | % | % | % | ||||||
Postretirement plans | % | % | % | ||||||
Rate of compensation increase: | |||||||||
U.S. qualified pension plans(a) | % | % | |||||||
U.S. non-qualified pension plans(a) | % | % | |||||||
International pension plans | % | % | % | ||||||
(a) | Effective January 1, 2018, we froze the defined benefit plans to future benefit accruals in the U.S. and members’ accrued benefits to that date no longer increase in line with future compensation increases. The rate of compensation increase is therefore no longer an assumption used to determine the benefit obligation and net periodic benefit cost. |
The assumptions above are used to develop the benefit obligations at fiscal year-end and to develop the net periodic benefit cost for the subsequent fiscal year. Therefore, the assumptions used to determine net periodic benefit cost for each year are established at the end of each previous fiscal year, while the assumptions used to determine benefit obligations are established at each fiscal year-end.
The net periodic benefit cost and the benefit obligations are based on actuarial assumptions that are reviewed on at least an annual basis. We revise these assumptions based on an annual evaluation of long-term trends, as well as market conditions that may have an impact on the cost of providing retirement benefits.
The weighted-average discount rate for our U.S. defined benefit plans is determined annually and evaluated and modified to reflect at year-end the prevailing market rate of a portfolio of high-quality fixed income investments, rated AA/Aa or better that reflect the rates at which the pension benefits could be effectively settled. For our international plans, the discount rates are set by benchmarking against investment grade corporate bonds rated AA/Aa or better, including, when there is sufficient data, a yield curve approach. These rate determinations are made consistent with local requirements. Overall, the yield curves used to measure the benefit obligations at year-end 2019 resulted in lower discount rates as compared to the prior year.
2019 Financial Report | 107 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides the healthcare cost trend rate assumptions for our U.S. postretirement benefit plans: | ||||||
2019 | 2018 | |||||
Healthcare cost trend rate assumed for next year (up to age 65) | % | % | ||||
Healthcare cost trend rate assumed for next year (age 65 and older) | % | % | ||||
Rate to which the cost trend rate is assumed to decline | % | % | ||||
Year that the rate reaches the ultimate trend rate | 2037 | 2037 | ||||
The following table provides the effects as of December 31, 2019 of a one-percentage-point increase or decrease in the healthcare cost trend rate assumed for postretirement benefits: | ||||||||
(MILLIONS OF DOLLARS) | Increase | Decrease | ||||||
Effect on total service and interest cost components | $ | $ | ( | ) | ||||
Effect on postretirement benefit obligation | ( | ) | ||||||
Actuarial and other assumptions for pension and postretirement plans can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. For a description of the risks associated with estimates and assumptions, see Note 1C.
C. Obligations and Funded Status
The following table provides an analysis of the changes in our benefit obligations, plan assets and funded status of our benefit plans: | ||||||||||||||||||||||||||||||||
Year Ended December 31, | ||||||||||||||||||||||||||||||||
Pension Plans | ||||||||||||||||||||||||||||||||
U.S. Qualified | U.S. Supplemental (Non-Qualified) | International | Postretirement Plans | |||||||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | ||||||||||||||||||||||||
Change in benefit obligation(a) | ||||||||||||||||||||||||||||||||
Benefit obligation, beginning | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
Service cost | ||||||||||||||||||||||||||||||||
Interest cost | ||||||||||||||||||||||||||||||||
Employee contributions | ||||||||||||||||||||||||||||||||
Plan amendments | ( | ) | ( | ) | ||||||||||||||||||||||||||||
Changes in actuarial assumptions and other | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||
Foreign exchange impact | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||
Acquisitions/divestitures/other, net | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||
Curtailments | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
Settlements | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||
Special termination benefits | ||||||||||||||||||||||||||||||||
Benefits paid | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||
Benefit obligation, ending(a) | ||||||||||||||||||||||||||||||||
Change in plan assets | ||||||||||||||||||||||||||||||||
Fair value of plan assets, beginning | ||||||||||||||||||||||||||||||||
Actual gain/(loss) on plan assets | ( | ) | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||
Company contributions | ||||||||||||||||||||||||||||||||
Employee contributions | ||||||||||||||||||||||||||||||||
Foreign exchange impact | ( | ) | ||||||||||||||||||||||||||||||
Acquisitions/divestitures, net | — | — | — | ( | ) | |||||||||||||||||||||||||||
Settlements | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||
Benefits paid | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||
Fair value of plan assets, ending | ||||||||||||||||||||||||||||||||
Funded status—Plan assets less than benefit obligation | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||
(a) | The PBO represents the present value of the benefit obligation earned through the end of the year and factors in future compensation increases. The ABO is similar to the PBO but does not factor in future compensation increases. For the U.S. qualified and supplemental (non-qualified) pension plans, the benefit obligation is the PBO, which is also equal to the ABO. Effective January 1, 2018, we froze the defined benefit plans to future benefit accruals in the U.S. and members’ accrued benefits to that date no longer increase in line with future compensation increases. The rate of compensation increase is therefore no longer an assumption used to determine the benefit obligation and net periodic benefit cost. For the international pension plans, the benefit obligation is the PBO. The ABO for our international pension plans was $ |
108 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides information as to how the funded status is recognized in our consolidated balance sheets: | ||||||||||||||||||||||||||||||||
As of December 31, | ||||||||||||||||||||||||||||||||
Pension Plans | ||||||||||||||||||||||||||||||||
U.S. Qualified | U.S. Supplemental (Non-Qualified) | International | Postretirement Plans | |||||||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | ||||||||||||||||||||||||
Noncurrent assets(a) | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
Current liabilities(b) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||
Noncurrent liabilities(c) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||
Funded status | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||
(a) | Included in Other noncurrent assets. |
(b) | Included in Accrued compensation and related items. |
(c) | As of December 31, 2019, included in Pension benefit obligations, net and Postretirement benefit obligations, net, as appropriate. In 2018, included in Pension benefit obligations, net and Postretirement benefit obligations, net, as well as in Liabilities held for sale (see Note 2C), as appropriate. |
The following table provides the pre-tax components of cumulative amounts recognized in Accumulated other comprehensive loss: | ||||||||||||||||||||||||||||||||
As of December 31, | ||||||||||||||||||||||||||||||||
Pension Plans | ||||||||||||||||||||||||||||||||
U.S. Qualified | U.S. Supplemental (Non-Qualified) | International | Postretirement Plans | |||||||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | ||||||||||||||||||||||||
Actuarial losses(a) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||
Prior service (costs)/credits | ( | ) | ( | ) | ||||||||||||||||||||||||||||
Total | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | $ | ||||||||||||
(a) | The accumulated actuarial losses primarily represent the impact of changes in discount rates and other assumptions that result in cumulative changes in our PBO, as well as the cumulative difference between the expected return and actual return on plan assets. These accumulated actuarial losses are recognized in Accumulated other comprehensive loss and are amortized into net periodic benefit costs primarily over the average remaining service period for active participants for plans that are not frozen or the average life expectancy of plan participants for frozen plans, primarily using the corridor approach. |
The following table provides information related to the funded status of selected benefit plans: | ||||||||||||||||||||||||
As of December 31, | ||||||||||||||||||||||||
Pension Plans | ||||||||||||||||||||||||
U.S. Qualified | U.S. Supplemental (Non-Qualified) | International | ||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | ||||||||||||||||||
Pension plans with an ABO in excess of plan assets: | ||||||||||||||||||||||||
Fair value of plan assets | $ | $ | $ | — | $ | — | $ | $ | ||||||||||||||||
ABO | ||||||||||||||||||||||||
Pension plans with a PBO in excess of plan assets: | ||||||||||||||||||||||||
Fair value of plan assets | — | — | ||||||||||||||||||||||
PBO | ||||||||||||||||||||||||
All of our U.S. plans and many of our international plans were underfunded as of December 31, 2019.
2019 Financial Report | 109 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
D. Plan Assets
The following table provides the components of plan assets: | ||||||||||||||||||||||||||||||||||||||||
Fair Value(a) | Fair Value(a) | |||||||||||||||||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | As of December 31, 2019 | Level 1 | Level 2 | Level 3 | Assets Measured at NAV(b) | As of December 31, 2018 | Level 1 | Level 2 | Level 3 | Assets Measured at NAV(b) | ||||||||||||||||||||||||||||||
U.S. qualified pension plans | ||||||||||||||||||||||||||||||||||||||||
Cash and cash equivalents | $ | $ | $ | $ | $ | — | $ | $ | $ | $ | $ | — | ||||||||||||||||||||||||||||
Equity securities: | ||||||||||||||||||||||||||||||||||||||||
Global equity securities | — | — | ||||||||||||||||||||||||||||||||||||||
Equity commingled funds | ||||||||||||||||||||||||||||||||||||||||
Fixed income securities: | ||||||||||||||||||||||||||||||||||||||||
Corporate debt securities | — | — | ||||||||||||||||||||||||||||||||||||||
Government and agency obligations | — | — | ||||||||||||||||||||||||||||||||||||||
Fixed income commingled funds | — | |||||||||||||||||||||||||||||||||||||||
Other investments: | ||||||||||||||||||||||||||||||||||||||||
Partnership investments(c) | — | |||||||||||||||||||||||||||||||||||||||
Insurance contracts | — | — | ||||||||||||||||||||||||||||||||||||||
Other commingled funds(d) | — | |||||||||||||||||||||||||||||||||||||||
Total | $ | $ | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||||||
International pension plans | ||||||||||||||||||||||||||||||||||||||||
Cash and cash equivalents | $ | $ | $ | $ | $ | — | $ | $ | $ | $ | $ | — | ||||||||||||||||||||||||||||
Equity securities: | ||||||||||||||||||||||||||||||||||||||||
Global equity securities | — | — | ||||||||||||||||||||||||||||||||||||||
Equity commingled funds | ||||||||||||||||||||||||||||||||||||||||
Fixed income securities: | ||||||||||||||||||||||||||||||||||||||||
Corporate debt securities | ||||||||||||||||||||||||||||||||||||||||
Government and agency obligations(e) | ||||||||||||||||||||||||||||||||||||||||
Fixed income commingled funds | ||||||||||||||||||||||||||||||||||||||||
Other investments: | ||||||||||||||||||||||||||||||||||||||||
Partnership investments(c) | ||||||||||||||||||||||||||||||||||||||||
Insurance contracts(f) | ||||||||||||||||||||||||||||||||||||||||
Other(d), (f) | ||||||||||||||||||||||||||||||||||||||||
Total | $ | $ | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||||||
U.S. postretirement plans(g) | ||||||||||||||||||||||||||||||||||||||||
Insurance contracts | $ | $ | $ | $ | — | $ | — | $ | $ | — | $ | $ | $ | — | ||||||||||||||||||||||||||
(a) | Fair values are determined based on valuation inputs categorized as Level 1, 2 or 3 (see Note 1E). |
(b) | Certain investments that are measured at NAV per share (or its equivalent) have not been classified in the fair value hierarchy. The NAV amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the amounts presented for the total pension benefits plan assets. |
(c) | Mainly includes investments in private equity, private debt, public equity limited partnerships, and, to a lesser extent, real estate and venture capital. |
(d) | Mostly includes, for U.S. plan assets, investments in hedge funds and, to a lesser extent, real estate and, for international plan assets, investments in real estate and hedge funds. |
(e) | Government and agency obligations are inclusive of repurchase agreements. |
(f) | See below for a tabular analysis of the changes in Level 3 investments valued using significant unobservable inputs. |
(g) | Reflects postretirement plan assets, which support a portion of our U.S. retiree medical plans. |
110 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides an analysis of the changes in our more significant investments valued using significant unobservable inputs: | ||||||||||||||||
Year Ended December 31, | ||||||||||||||||
International Pension Plans | ||||||||||||||||
Insurance contracts | Other | |||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2019 | 2018 | ||||||||||||
Fair value, beginning | $ | $ | $ | $ | ||||||||||||
Actual return on plan assets: | ||||||||||||||||
Assets held, ending | ||||||||||||||||
Purchases, sales, and settlements, net | ( | ) | ( | ) | ||||||||||||
Transfer into/(out of) Level 3 | ( | ) | ||||||||||||||
Exchange rate changes | ( | ) | ( | ) | ||||||||||||
Fair value, ending | $ | $ | $ | $ | ||||||||||||
A single estimate of fair value can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. For a description of our general accounting policies associated with developing fair value estimates, see Note 1E. For a description of the risks associated with estimates and assumptions, see Note 1C.
Equity securities, Fixed income securities and Other investments may each be combined into commingled funds. Most commingled funds are valued to reflect the interest in the fund based on the reported year-end NAV. Partnership and Other investments are valued based on year-end reported NAV (or its equivalent), with adjustments as appropriate for lagged reporting of up to three months.
The following methods and assumptions were used to estimate the fair value of our pension and postretirement plans’ assets:
• | Cash and cash equivalents: Level 1 investments may include cash, cash equivalents and foreign currency valued using exchange rates. Level 2 investments may include short-term investment funds which are commingled funds priced at a stable NAV by the administrator of the funds. |
• | Equity securities: Level 1 investments may include individual securities that are valued at the closing price or last trade reported on the major market on which they are traded. Level 1 and Level 2 investments may include commingled funds that have a readily determinable fair value based on quoted prices on an exchange or a published NAV derived from the quoted prices in active markets of the underlying securities. Level 3 investments may include individual securities that are unlisted, delisted, suspended, or illiquid and are typically valued using their last available price. |
• | Fixed income securities: Level 1 investments may include individual securities that are valued at the closing price or last trade reported on the major market on which they are traded. Level 2 investments may include commingled funds that have a readily determinable fair value based on observable prices of the underlying securities. Level 2 investments may include corporate bonds, government and government agency obligations and other fixed income securities valued using bid evaluation pricing models or quoted prices of securities with similar characteristics. Level 3 investments may include securities that are valued using alternative pricing sources, such as investment managers or brokers, which use proprietary pricing models that incorporate unobservable inputs. |
• | Other investments: Level 1 investments may include individual securities that are valued at the closing price or last trade reported on the major market on which they are traded. Level 2 investments may include Insurance contracts which invest in interest bearing cash, U.S. government securities and corporate debt instruments. |
Certain investments are authorized to include derivatives, such as equity or bond futures, swaps, options and currency futures or forwards for managing risks and exposures.
2019 Financial Report | 111 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides the long-term target asset allocations ranges and the percentage of the fair value of plan assets for benefit plans: | |||||||||
As of December 31, | |||||||||
Target Allocation Percentage | Percentage of Plan Assets | ||||||||
(PERCENTAGES) | 2019 | 2019 | 2018 | ||||||
U.S. qualified pension plans | |||||||||
Cash and cash equivalents | 0-10% | % | % | ||||||
Equity securities | 35-55% | % | % | ||||||
Fixed income securities | 28-53% | % | % | ||||||
Other investments | 5-20% | % | % | ||||||
Total | % | % | % | ||||||
International pension plans | |||||||||
Cash and cash equivalents | 0-10% | % | % | ||||||
Equity securities | 20-40% | % | % | ||||||
Fixed income securities | 35-60% | % | % | ||||||
Other investments | 10-35% | % | % | ||||||
Total | % | % | % | ||||||
U.S. postretirement plans | |||||||||
Cash and cash equivalents | 0-5% | ||||||||
Other investments | 95-100% | % | % | ||||||
Total | % | % | % | ||||||
Global plan assets are managed with the objective of generating returns that will enable the plans to meet their future obligations, while seeking to manage net periodic benefit costs and cash contributions over the long-term. We utilize long-term asset allocation ranges in the management of our plans’ invested assets. Our long-term return expectations are developed based on a diversified, global investment strategy that takes into account historical experience, as well as the impact of portfolio diversification, active portfolio management, and our view of current and future economic and financial market conditions. As market conditions and other factors change, we may adjust our targets accordingly and our asset allocations may vary from the target allocations.
Our long-term asset allocation ranges reflect our asset class return expectations and tolerance for investment risk within the context of the respective plans’ long-term benefit obligations. These ranges are supported by analysis that incorporates historical and expected returns by asset class, as well as volatilities and correlations across asset classes and our liability profile.
Each pension plan is overseen by a local committee or board that is responsible for the overall investment of the pension plan assets. In determining investment policies and associated target allocations, each committee or board considers a wide variety of factors. As such, the target asset allocation for each of our international pension plans is set on a standalone basis by the relevant board or committee. The target asset allocation ranges shown for the international pension plans seek to reflect the combined target allocations across all such plans, while also showing the range within which the target allocations for each plan typically falls.
The investment managers of certain separately managed accounts, commingled funds and private equity funds may be permitted to use repurchase agreements and derivative securities, including U.S. Treasury and equity futures contracts as described in each respective investment management, subscription, partnership or other governing agreement.
E. Cash Flows
It is our practice to fund amounts for our qualified pension plans that are at least sufficient to meet the minimum requirements set forth in applicable employee benefit laws and local tax laws.
112 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides the expected future cash flow information related to our benefit plans: | ||||||||||||||||
Pension Plans | ||||||||||||||||
(MILLIONS OF DOLLARS) | U.S. Qualified | U.S. Supplemental (Non-Qualified) | International | Postretirement Plans | ||||||||||||
Expected employer contributions: | ||||||||||||||||
2020(a) | $ | $ | $ | $ | ||||||||||||
Expected benefit payments: | ||||||||||||||||
2020 | $ | $ | $ | $ | ||||||||||||
2021 | ||||||||||||||||
2022 | ||||||||||||||||
2023 | ||||||||||||||||
2024 | ||||||||||||||||
2025–2029 | ||||||||||||||||
(a) | For the U.S. qualified plans, we plan to make a $ |
The above table reflects the total U.S. and international plan benefits projected to be paid from the plans or from our general assets under the current actuarial assumptions used for the calculation of the benefit obligation and, therefore, actual benefit payments may differ from projected benefit payments.
F. Defined Contribution Plans
We have defined contribution plans in the U.S. and several other countries. For the majority of the U.S. defined contribution plans, employees may contribute a portion of their salaries and bonuses to the plans, and we match, in cash, a portion of the employee contributions. Beginning on January 1, 2011, for newly hired non-union employees, rehires and transfers to the U.S. or Puerto Rico, we no longer offer a defined benefit pension plan and, instead, offer a Retirement Savings Contribution (RSC) in the defined contribution plan. The RSC is an annual non-contributory employer contribution (that is not dependent upon the participant making a contribution) determined based on each employee’s eligible compensation, age and years of service. Beginning on January 1, 2018, all non-union employees in the U.S. and Puerto Rico defined benefit plans transitioned to the RSC in the defined contribution plans. We recorded charges related to the employer contributions to global defined contribution plans of $659 million in 2019, $622 million in 2018 and $380 million in 2017.
Note 12. Equity
A. Common Stock
We purchase our common stock through privately negotiated transactions or in open market purchases as circumstances and prices warrant. Purchased shares under each of the share-purchase plans, which are authorized by our Board of Directors, are available for general corporate purposes. On October 23, 2014, we announced that the Board of Directors had authorized an $11 billion share repurchase program, which was exhausted in the first quarter of 2017. In December 2015, the Board of Directors authorized a new $11 billion share repurchase program, which was exhausted in the third quarter of 2018. In December 2017, the Board of Directors authorized an additional $10 billion share repurchase program, which was exhausted in the first quarter of 2019. In December 2018, the Board of Directors authorized a new $10 billion share repurchase program to be utilized over time and share repurchases commenced thereunder in the first quarter of 2019.
On February 2, 2017, we entered into an accelerated share repurchase agreement with Citibank to repurchase $5 billion of our common stock. Pursuant to the terms of the agreement, on February 6, 2017, we paid $5 billion to Citibank and received an initial delivery of approximately 126 million shares of our common stock from Citibank at a price of $31.73 per share, which represented, based on the closing price of our common stock on the NYSE on February 2, 2017, approximately 80 % of the notional amount of the accelerated share repurchase agreement. On May 16, 2017, the accelerated share repurchase agreement with Citibank was completed, which, per the terms of the agreement, resulted in Citibank owing us a certain number of shares of Pfizer common stock. Pursuant to the agreement’s settlement terms, we received an additional 24 million shares of our common stock from Citibank on May 19, 2017. The average price paid for all of the shares delivered under the accelerated share repurchase agreement was $33.31 per share. The common stock received is included in Treasury Stock. This agreement was entered into pursuant to our previously announced share repurchase authorization.
On March 12, 2018, we entered into an accelerated share repurchase agreement with Citibank to repurchase $4 billion of our common stock. Pursuant to the terms of the agreement, on March 14, 2018, we paid $4 billion to Citibank and received an initial delivery of approximately 87 million shares of our common stock from Citibank at a price of $36.61 per share, which represented, based on the closing price of our common stock on the NYSE on March 12, 2018, approximately 80 % of the notional amount of the accelerated share repurchase agreement. On September 5, 2018, the accelerated share repurchase agreement with Citibank was completed, which, per the terms of the agreement, resulted in Citibank owing us a certain number of shares of Pfizer common stock. Pursuant to the agreement’s settlement terms, we received an additional 21 million shares of our common stock from Citibank on September 7, 2018. The average price paid for all of the shares delivered under the accelerated share repurchase agreement was $36.86 per share. The common stock received is included in Treasury stock. This agreement was entered into pursuant to our previously announced share repurchase authorization.
On February 7, 2019, we entered into an accelerated share repurchase agreement with GS&Co. to repurchase approximately $6.8 billion of our common stock. Pursuant to the terms of the agreement, on February 12, 2019, we paid approximately $6.8 billion to GS&Co. and received an initial delivery of approximately 130 million shares of our common stock from GS&Co., which represented, based on the closing price of our common stock on the NYSE on February 7, 2019, approximately 80 % of the notional amount of the accelerated share repurchase agreement.
2019 Financial Report | 113 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
On August 1, 2019, the accelerated share repurchase agreement with GS&Co. was completed, which, per the terms of the agreement, resulted in GS&Co. owing us a certain number of shares of Pfizer common stock. Pursuant to the agreement’s settlement terms, we received an additional 33.5 million shares of our common stock from GS&Co. on August 5, 2019. The average price paid for all of the shares delivered under the accelerated share repurchase agreement was $41.42 per share. The common stock received is included in Treasury stock. This agreement was entered into pursuant to our previously announced share repurchase authorization.
Open market purchases totaled $2.1 billion in 2019 and $8.2 billion in 2018 under our publicly announced share-purchase plans.
The following table provides the number of shares of our common stock purchased and the cost of purchases under our publicly announced share purchase plans, including our accelerated share repurchase agreements: | ||||||||||||
(SHARES IN MILLIONS, DOLLARS IN BILLIONS) | 2019(a) | 2018(b) | 2017c) | |||||||||
Shares of common stock purchased | ||||||||||||
Cost of purchase | $ | $ | $ | |||||||||
(a) | Represents shares purchased pursuant to the accelerated share repurchase agreement with GS&Co. entered into on February 7, 2019, as well as other share repurchases. See above for additional information. |
(b) | Represents shares purchased pursuant to the accelerated share repurchase agreement with Citibank entered into on March 12, 2018, as well as other share repurchases. See above for additional information. |
(c) | Represents shares purchased pursuant to the accelerated share repurchase agreement with Citibank entered into on February 2, 2017. See above for additional information. |
B. Preferred Stock
The Series A convertible perpetual preferred stock (7,500 shares designated) is held by an employee stock ownership plan (Preferred ESOP) Trust and provides dividends at the rate of 6.25 %, which are accumulated and paid quarterly. The per-share stated value is $40,300 and the preferred stock ranks senior to our common stock as to dividends and liquidation rights. Each share is convertible, at the holder’s option, into 2,574.87 shares of our common stock with equal voting rights. The conversion option is indexed to our common stock and requires share settlement, and, therefore, is reported at the fair value at the date of issuance. We may redeem the preferred stock at any time or upon termination of the Preferred ESOP, at our option, in cash, in shares of common stock, or a combination of both at a price of $40,300 per share.
C. Employee Stock Ownership Plans
We have two employee stock ownership plans (collectively, the ESOPs), the Preferred ESOP and another that holds common stock of the Company (Common ESOP).
Allocated shares held by the Common ESOP, including reinvested dividends, are considered outstanding for EPS calculations and the eventual conversion of allocated preferred shares held by the Preferred ESOP are assumed in the diluted EPS calculation. As of December 31, 2019, the Preferred ESOP held preferred shares convertible into approximately 1 million shares of our common stock, and the Common ESOP held approximately 47 million shares of our common stock. As of December 31, 2019, all shares of preferred and common stock held by the ESOPs have been allocated to the Pfizer U.S. defined contribution plan participants. The compensation cost related to the Common ESOP was $20 million in 2019, $19 million in 2018 and $11 million in 2017.
Note 13. Share-Based Payments
Our compensation programs can include share-based payments. The award value is determined by reference to the fair value of share-based awards to similar employees in competitive survey data or industry peer groups used for compensation purposes; and is allocated between different long-term incentive vehicles, in the form of TSRUs, PTSRUs, PTUs, RSUs, PPSs, PSAs and stock options, as determined by the Compensation Committee.
The 2019 Stock Plan (2019 Plan) replaced and superseded the 2014 Plan. The 2019 Plan provides for 400 million shares to be authorized for grants, plus any shares remaining available for grant under the 2014 Plan as of April 25, 2019 (the carryforward shares). In addition, the 2019 Plan provides that the number of stock options, Stock Appreciation Rights (known as TSRUs and PTSRUs), RSUs, or other performance-based awards that may be granted to any one individual during any 36-month period is limited to 20 million shares, and that RSUs, PPSs and PSAs count as three shares, while TSRUs, PTSRUs and stock options count as one share, toward the maximum shares available under the 2019 plan. As of December 31, 2019, 518 million shares were available for award.
Although not required to do so, we have used authorized and unissued shares and, to a lesser extent, treasury stock to satisfy our obligations under these programs.
114 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
A. Impact on Net Income
The following table provides the components of share-based compensation expense and the associated tax benefit: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
TSRUs(a) | $ | $ | $ | |||||||||
RSUs | ||||||||||||
PPSs | ||||||||||||
PSAs | ||||||||||||
Stock options | ||||||||||||
Directors’ compensation | ||||||||||||
Share-based payment expense | ||||||||||||
Tax benefit for share-based compensation expense | ( | ) | ( | ) | ( | ) | ||||||
Share-based payment expense, net of tax | $ | $ | $ | |||||||||
(a) Includes expense for PTSRUs, described in Note 13C below, which is not significant for all years presented.
The table above excludes total expense due to the modification for share-based awards in connection with our Organizing for Growth initiative. The total expense was not significant for 2019 and 2018, the year in which the Organization for Growth Initiative began and is recorded in Restructuring charges and certain acquisition-related costs (see Note 3).
Amounts capitalized as part of inventory cost were not significant for any period presented.
B. Total Shareholder Return Units
TSRUs are awarded to senior and other key management, and to certain other employees. TSRUs entitle the holders to receive a number of shares of our common stock with a value equal to the difference between the defined settlement price and the grant price, plus the dividends accumulated during the five-year or seven-year term, if and to the extent the total value is positive. The settlement price is the average closing price of our common stock during the 20 trading days ending on the fifth or seventh anniversary of the grant, as applicable; the grant price is the closing price of our common stock on the date of the grant. The TSRUs are automatically settled on the fifth or seventh anniversary of the grant but vest on the third anniversary of the grant, after which time there is no longer a substantial risk of forfeiture.
Retiree eligible holders (at least age 55 with at least ten years of service) can elect to exercise and convert his/her TSRUs, when vested, into PTUs. The value received upon the election and conversion is calculated by taking the change in stock price (20 trading day average ending on the exercise date (Election Price) less the grant price) plus accumulated dividends from the grant date, times the number of TSRUs exercised. This value is divided by the Election Price to determine the number of PTUs. The PTUs will be entitled to earn Dividend Equivalent Units (DEUs), and the PTUs and DEUs will be settled in our common stock on the TSRUs’ original settlement date (i.e., the fifth or seventh anniversary of grant), and will be subject to all of the terms and conditions of the original grant including forfeiture provisions. We measure the value of TSRU grants as of the grant date using a Monte Carlo simulation model. The values determined through this fair value methodology generally are amortized on a straight-line basis over the vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate.
The following table provides the weighted-average assumptions used in the valuation of TSRUs: | |||||||||
Year Ended December 31, | |||||||||
2019 | 2018 | 2017 | |||||||
Expected dividend yield(a) | % | % | % | ||||||
Risk-free interest rate(b) | % | % | % | ||||||
Expected stock price volatility(c) | % | % | % | ||||||
Contractual term (years) | |||||||||
(a) | Determined using a constant dividend yield during the expected term of the TSRU. |
(b) | Determined using the interpolated yield on U.S. Treasury zero-coupon issues. |
(c) | Determined using implied volatility, after consideration of historical volatility. |
The following table summarizes all TSRU activity during 2019: | |||||||||||
TSRUs (Thousands) | Weighted-Average Grant-Date Fair Value Per TSRU | Weighted-Average Grant Price Per TSRU | |||||||||
Nonvested, December 31, 2018 | $ | $ | |||||||||
Granted | |||||||||||
Vested | ( | ) | |||||||||
Forfeited | ( | ) | |||||||||
Nonvested, December 31, 2019 | $ | $ | |||||||||
2019 Financial Report | 115 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table summarizes TSRU and PTU information as of December 31, 2019(a), (b): | ||||||||||||||||
TSRUs (Thousands) | PTUs (Thousands) | Weighted-Average Grant Price Per TSRU | Weighted-Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value (Millions) | ||||||||||||
TSRUs Outstanding | — | $ | $ | |||||||||||||
TSRUs Vested | — | |||||||||||||||
TSRUs Expected to vest(c) | — | |||||||||||||||
TSRUs exercised and converted to PTUs | — | $ | — | $ | ||||||||||||
(a) | In 2019, we settled |
(b) | In 2019, |
(c) | The number of TSRUs expected to vest takes into account an estimate of expected forfeitures. |
The following table provides data related to all TSRU activity: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS, EXCEPT PER TSRU AMOUNTS) | 2019 | 2018 | 2017 | |||||||||
Weighted-average grant-date fair value per TSRU | $ | $ | $ | |||||||||
Total compensation cost related to nonvested TSRU grants not yet recognized, pre-tax | $ | $ | $ | |||||||||
Weighted-average period over which TSRU cost is expected to be recognized (years) | ||||||||||||
C. Performance Total Shareholder Return Units
On December 29, 2017, in connection with the Board’s succession planning,1,372,213 PTSRUs were awarded to the then Chairman and Chief Executive Officer, and 343,053 PTSRUs were awarded to the Group President, Chief Business Officer (former role Group President, Pfizer Innovative Health) at a grant price of $36.22 and at a grant-date fair value of $5.83 per TSRU. In addition to having the same characteristics of TSRUs, PTSRU grants require special service and performance conditions.
We measure the value of PTSRU grants as of the grant date using a Monte Carlo simulation model. The values determined through this fair value methodology generally are amortized on a straight-line basis over the vesting term into Selling, informational and administrative expenses as appropriate.
D. Restricted Stock Units
RSUs are awarded to select employees and, when vested, entitle the holder to receive a specified number of shares of our common stock, including shares resulting from dividend equivalents paid on such RSUs. For RSUs granted during the periods presented, in virtually all instances, the units vest after three years of continuous service from the grant date.
We measure the value of RSU grants as of the grant date using the closing price of our common stock. The values determined through this fair value methodology generally are amortized on a straight-line basis over the vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate.
The following table summarizes all RSU activity during 2019: | |||||||
Shares (Thousands) | Weighted-Average Grant-Date Fair Value Per Share | ||||||
Nonvested, December 31, 2018 | $ | ||||||
Granted | |||||||
Vested | ( | ) | |||||
Reinvested dividend equivalents | |||||||
Forfeited | ( | ) | |||||
Nonvested, December 31, 2019 | $ | ||||||
The following table provides data related to all RSU activity: | ||||||||||||
(MILLIONS OF DOLLARS) | Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | ||||||||||
Total fair value of shares vested(a) | $ | $ | $ | |||||||||
Total compensation cost related to nonvested RSU awards not yet recognized, pre-tax | $ | $ | $ | |||||||||
Weighted-average period over which RSU cost is expected to be recognized (years) | ||||||||||||
(a) | 2017 includes the modification for a commitment to pay approximately |
116 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
E. Portfolio Performance Shares
PPSs are awards granted to select employees which, when vested, entitle the holder to receive, at the end of the performance period, a number of shares within a possible range of shares of our common stock, including shares resulting from dividend equivalents paid on such shares. For PPSs granted during the period presented, the awards vest after three years of continuous service from the grant date and the number of shares paid, if any, depends on the achievement of predetermined goals related to Pfizer’s long-term product portfolio during a five-year performance period from the year of the grant date. The number of shares that may be earned over the performance period ranges from 0 % to 200 % of the initial award.
We measure the value of PPS grants as of the grant date using the intrinsic value method, for which we use the closing price of our common stock. The values are amortized on a straight-line basis over the probable vesting term into Cost of sales, Selling, informational and administrative expenses and/or Research and development expenses, as appropriate, and adjusted each reporting period, as necessary, to reflect changes in the price of Pfizer’s common stock, changes in the number of shares that are probable of being earned and changes in management’s assessment of the probability that the specified performance criteria will be achieved and/or changes in management’s
assessment of the probable vesting term.
The following table summarizes all PPS activity during 2019, with the shares representing the maximum award that could be achieved: | |||||||
Shares (Thousands) | Weighted-Average Intrinsic Value Per Share | ||||||
Nonvested, December 31, 2018 | $ | ||||||
Granted | |||||||
Vested | ( | ) | |||||
Forfeited | ( | ) | |||||
Nonvested, December 31, 2019(a) | $ | ||||||
(a) | Vested and non-vested shares outstanding, but not paid as of December 31, 2019 were |
The following table provides data related to all PPS activity: | ||||||||||||
(MILLIONS OF DOLLARS) | Year Ended December 31, | |||||||||||
2019 | 2018 | 2017 | ||||||||||
Total fair value of shares vested | $ | $ | $ | |||||||||
Total compensation cost related to nonvested PPS awards not yet recognized, pre-tax | $ | $ | $ | |||||||||
Weighted-average period over which PPS cost is expected to be recognized (years) | ||||||||||||
F. Performance Share Awards
PSAs are awarded to senior and other key management. PSAs vest after three years of continuous service from the grant date. The number of shares paid, if any, including shares resulting from dividend equivalents, for awards granted in 2015 and later, depends upon the achievement of predetermined goals related to two measures: (i) adjusted operating income (for performance years through 2018) or adjusted net income (for 2019 and later years, except for the 2017 PSAs) over three one-year periods; and (ii) TSR as compared to the NYSE ARCA Pharmaceutical Index (DRG Index) over the three-year performance period. The number of shares that are earned over the performance period ranges from 0 % to 200 % of the initial award.
We measure the value of PSA grants as of the grant date using the intrinsic value method, for which we use the closing price of our common stock. The values are amortized on a straight-line basis over the probable vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate, and adjusted each reporting period, as necessary, to reflect changes in the price of Pfizer’s common stock, changes in the number of shares that are probable of being earned and changes in management’s assessment of the probability that the specified performance criteria will be achieved.
The following table summarizes all PSA activity during 2019, with the shares granted representing the maximum award that could be achieved: | |||||||
Shares (Thousands) | Weighted-Average Intrinsic Value Per Share | ||||||
Nonvested, December 31, 2018 | $ | ||||||
Granted | |||||||
Vested | ( | ) | |||||
Forfeited | ( | ) | |||||
Nonvested, December 31, 2019 | $ | ||||||
2019 Financial Report | 117 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides data related to all PSA activity: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Total fair value of shares vested(a) | $ | $ | $ | |||||||||
Total compensation cost related to nonvested PSA grants not yet recognized, pre-tax | $ | $ | $ | |||||||||
Weighted-average period over which PSA cost is expected to be recognized (years) | ||||||||||||
(a) | 2017 includes the modification for a commitment to pay |
G. Stock Options
Stock options are awarded to select employees and, when vested, entitle the holder to purchase a specified number of shares of our common stock at a price per share equal to the closing market price of our common stock on the date of grant.
Beginning in 2016, only a limited set of overseas employees received stock option grants. No stock options were awarded to senior and other key management in any period presented; however, stock options were awarded to certain other employees. In virtually all instances, stock options granted vest after three years of continuous service from the grant date and have a contractual term of 10 years. In most cases, stock options must be held for at least one year from the grant date before any vesting may occur. In the event of a sale of business or plant closing or restructuring, options held by employees are immediately vested and are exercisable for a period of 3 months following the date employment is terminated or through their remaining term, depending on various conditions.
We measure the value of stock option grants as of the grant date using the Black-Scholes-Merton option-pricing model. The values determined through this fair value methodology generally are amortized on a straight-line basis over the vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate.
The following table provides the weighted-average assumptions used in the valuation of stock options: | |||||||||
Year Ended December 31, | |||||||||
2019 | 2018 | 2017 | |||||||
Expected dividend yield(a) | % | % | % | ||||||
Risk-free interest rate(b) | % | % | % | ||||||
Expected stock price volatility(c) | % | % | % | ||||||
Expected term (years)(d) | |||||||||
(a) | Determined using a constant dividend yield during the expected term of the option. |
(b) | Determined using the interpolated yield on U.S. Treasury zero-coupon issues. |
(c) | Determined using implied volatility, after consideration of historical volatility. |
(d) | Determined using historical exercise and post-vesting termination patterns. |
The following table summarizes all stock option activity during 2019: | |||||||||||||
Shares (Thousands) | Weighted-Average Exercise Price Per Share | Weighted-Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value(a) (Millions) | ||||||||||
Outstanding, December 31, 2018 | $ | ||||||||||||
Granted | |||||||||||||
Exercised | ( | ) | |||||||||||
Forfeited | ( | ) | |||||||||||
Expired | ( | ) | |||||||||||
Outstanding, December 31, 2019 | $ | ||||||||||||
Vested and expected to vest, December 31, 2019(b) | |||||||||||||
Exercisable, December 31, 2019 | $ | $ | |||||||||||
(a) | Market price of our underlying common stock less exercise price. |
(b) | The number of options expected to vest takes into account an estimate of expected forfeitures. |
118 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table summarizes data related to all stock option activity: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS, EXCEPT PER STOCK OPTION AMOUNTS) | 2019 | 2018 | 2017 | |||||||||
Weighted-average grant-date fair value per stock option | $ | $ | $ | |||||||||
Aggregate intrinsic value on exercise | $ | $ | $ | |||||||||
Cash received upon exercise | $ | $ | $ | |||||||||
Tax benefits realized related to exercise | $ | $ | $ | |||||||||
Total compensation cost related to nonvested stock options not yet recognized, pre-tax | $ | $ | $ | |||||||||
Weighted-average period over which stock option compensation cost is expected to be recognized (years) | ||||||||||||
Note 14. Earnings Per Common Share Attributable to Pfizer Inc. Common Shareholders
The following table provides the detailed calculation of Earnings per common share (EPS): | ||||||||||||
Year Ended December 31, | ||||||||||||
(IN MILLIONS) | 2019 | 2018 | 2017 | |||||||||
EPS Numerator––Basic | ||||||||||||
Income from continuing operations | $ | $ | $ | |||||||||
Less: Net income attributable to noncontrolling interests | ||||||||||||
Income from continuing operations attributable to Pfizer Inc. | ||||||||||||
Less: Preferred stock dividends––net of tax | ||||||||||||
Income from continuing operations attributable to Pfizer Inc. common shareholders | ||||||||||||
Discontinued operations––net of tax | ||||||||||||
Less: Discontinued operations––net of tax, attributable to noncontrolling interests | ||||||||||||
Discontinued operations––net of tax, attributable to Pfizer Inc. common shareholders | ||||||||||||
Net income attributable to Pfizer Inc. common shareholders | $ | $ | $ | |||||||||
EPS Numerator––Diluted | ||||||||||||
Income from continuing operations attributable to Pfizer Inc. common shareholders and assumed conversions | $ | $ | $ | |||||||||
Discontinued operations––net of tax, attributable to Pfizer Inc. common shareholders and assumed conversions | ||||||||||||
Net income attributable to Pfizer Inc. common shareholders and assumed conversions | $ | $ | $ | |||||||||
EPS Denominator | ||||||||||||
Weighted-average number of common shares outstanding––Basic(a) | ||||||||||||
Common-share equivalents: stock options, stock issuable under employee compensation plans, convertible preferred stock and accelerated share repurchase agreements(a) | ||||||||||||
Weighted-average number of common shares outstanding––Diluted | ||||||||||||
Stock options that had exercise prices greater than the average market price of our common stock issuable under employee compensation plans(b) | ||||||||||||
Cash dividends declared per share | $ | $ | $ | |||||||||
(a) | 2017 includes the effect of the modification for a commitment to pay |
(b) | These common stock equivalents were outstanding for the periods presented, but were not included in the computation of diluted EPS for those periods because their inclusion would have had an anti-dilutive effect. |
Note 15. Insurance
Our insurance coverage reflects market conditions (including cost and availability) existing at the time it is written, and our decision to obtain insurance coverage or to self-insure varies accordingly. Depending upon the cost and availability of insurance and the nature of the risk involved, the amount of self-insurance may be significant. The cost and availability of coverage have resulted in self-insuring certain exposures, including product liability. If we incur substantial liabilities that are not covered by insurance or substantially exceed insurance coverage and that are in excess of existing accruals, there could be a material adverse effect on our cash flows or results of operations in the period in which the amounts are paid and/or accrued (see Note 16).
Note 16. Contingencies and Certain Commitments
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business, including tax and legal contingencies. For a discussion of our tax contingencies, see Note 5D. For a discussion of our legal contingencies, see below.
2019 Financial Report | 119 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
A. Legal Proceedings
Our legal contingencies include, but are not limited to, the following:
• | Patent litigation, which typically involves challenges to the coverage and/or validity of patents on various products, processes or dosage forms. We are the plaintiff in the majority of these actions. An adverse outcome in actions in which we are the plaintiff could result in loss of patent protection for a drug, a significant loss of revenues from that drug or impairment of the value of associated assets. |
• | Product liability and other product-related litigation, which can include personal injury, consumer, off-label promotion, securities, antitrust and breach of contract claims, among others, often involves highly complex issues relating to medical causation, label warnings and reliance on those warnings, scientific evidence and findings, actual, provable injury and other matters. |
• | Commercial and other matters, which can include merger-related and product-pricing claims and environmental claims and proceedings, can involve complexities that will vary from matter to matter. |
• | Government investigations, which often are related to the extensive regulation of pharmaceutical companies by national, state and local government agencies in the U.S. and in other jurisdictions. |
Certain of these contingencies could result in losses, including damages, fines and/or civil penalties, which could be substantial, and/or criminal charges.
We believe that our claims and defenses in matters in which we are a defendant are substantial, but litigation is inherently unpredictable and excessive verdicts do occur. We do not believe that any of these matters will have a material adverse effect on our financial position. However, we could incur judgments, enter into settlements or revise our expectations regarding the outcome of certain matters, and such developments could have a material adverse effect on our results of operations in the period in which the amounts are accrued and/or our cash flows in the period in which the amounts are paid.
We have accrued for losses that are both probable and reasonably estimable. Substantially all of our contingencies are subject to significant uncertainties and, therefore, determining the likelihood of a loss and/or the measurement of any loss can be complex. Consequently, we are unable to estimate the range of reasonably possible loss in excess of amounts accrued. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but the assessment process relies heavily on estimates and assumptions that may prove to be incomplete or inaccurate, and unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions.
Amounts recorded for legal and environmental contingencies can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions.
The principal pending matters to which we are a party are discussed below. In determining whether a pending matter is a principal matter, we consider both quantitative and qualitative factors in order to assess materiality, such as, among other things, the amount of damages and the nature of any other relief sought in the proceeding, if such damages and other relief are specified; our view of the merits of the claims and of the strength of our defenses; whether the action purports to be, or is, a class action and, if not certified, our view of the likelihood that a class will be certified by the court; the jurisdiction in which the proceeding is pending; whether related actions have been transferred to multidistrict litigation; any experience that we or, to our knowledge, other companies have had in similar proceedings; whether disclosure of the action would be important to a reader of our financial statements, including whether disclosure might change a reader’s judgment about our financial statements in light of all of the information that is available to the reader; the potential impact of the proceeding on our reputation; and the extent of public interest in the matter. In addition, with respect to patent matters in which we are the plaintiff, we consider, among other things, the financial significance of the product protected by the patent(s) at issue. As a result of considering qualitative factors in our determination of principal matters, there are some matters discussed below with respect to which management believes that the likelihood of possible loss in excess of amounts accrued is remote.
A1. Legal Proceedings––Patent Litigation
Like other pharmaceutical companies, we are involved in numerous suits relating to our patents, including but not limited to, those discussed below. Most of the suits involve claims by generic drug manufacturers that patents covering our products, processes or dosage forms are invalid and/or do not cover the product of the generic drug manufacturer. Also, counterclaims, as well as various independent actions, have been filed alleging that our assertions of, or attempts to enforce, patent rights with respect to certain products constitute unfair competition and/or violations of antitrust laws. In addition to the challenges to the U.S. patents on a number of our products that are discussed below, patent rights to certain of our products are being challenged in various other jurisdictions. We are also party to patent damages suits in various jurisdictions pursuant to which generic drug manufacturers, payers, governments or other parties are seeking damages from us for allegedly causing delay of generic entry. Additionally, our licensing and collaboration partners face challenges by generic drug manufacturers to patents covering products for which we have licenses or co-promotion rights. We also are often involved in other proceedings, such as inter partes review, post-grant review, re-examination or opposition proceedings, before the U.S. Patent and Trademark Office, the European Patent Office, or other foreign counterparts relating to our intellectual property or the intellectual property rights of others. Also, if one of our patents is found to be invalid by such proceedings, generic or competitive products could be introduced into the market resulting in the erosion of sales of our existing products. For example, several of the patents in our pneumococcal vaccine portfolio were challenged in inter partes review and post-grant review proceedings in the U.S. In October 2017, the Patent Trial and Appeal Board (PTAB) refused to initiate proceedings as to two patents. In June 2018, the PTAB ruled on another patent, holding that one claim was valid and that all other claims were invalid. The party challenging that patent has appealed the decision. In November 2019, the U.S. Court of Appeals for the Federal Circuit vacated the PTAB’s ruling and requested that the PTAB redecide the challenge. In March and June 2019, an additional patent was found invalid in separate proceedings by the PTAB. We have appealed. Challenges to other patents remain pending in jurisdictions outside the U.S. The invalidation of all of the patents in our pneumococcal portfolio could potentially allow a competitor pneumococcal vaccine into the marketplace. In the event that any of the patents are found valid and infringed, a competitor pneumococcal vaccine might be prohibited from entering the market or required to pay Pfizer a royalty. We are also subject to patent litigation pursuant to which one or more third parties seeks damages and/or
120 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
injunctive relief to compensate for alleged infringement of its patents by our commercial or other activities. For example, our Hospira subsidiaries are involved in patent and patent-related disputes over their attempts to bring generic pharmaceutical and biosimilar products to market. If one of our marketed products is found to infringe valid patent rights of a third party, such third party may be awarded significant damages, or we may be prevented from further sales of that product. Such damages may be enhanced as much as three-fold in the event that we or one of our subsidiaries, like Hospira, is found to have willfully infringed valid patent rights of a third party.
Actions In Which We Are The Plaintiff
EpiPen
In July 2010, King, which we acquired in 2011 and is a wholly-owned subsidiary, brought a patent-infringement action against Sandoz in the U.S. District Court for the District of New Jersey in connection with Sandoz’s abbreviated new drug application filed with the FDA seeking approval to market an epinephrine injectable product. Sandoz is challenging patents, which expire in 2025, covering the next-generation autoinjector for use with epinephrine that is sold under the EpiPen brand name.
Precedex Premix
Beginning in 2014, several generic manufacturers filed separate abbreviated new drug applications with the FDA, seeking approval to market their generic versions of our subsidiary Hospira’s premix version of Precedex prior to the expiration of one or more patents covering the product. One of those patents expired in March 2019, while others do not expire until 2032. Beginning in 2014, Hospira brought suit against these generic manufacturers, in some cases joined by Orion Corporation (co-owner of certain of our Precedex premix patents). To date, two of the actions have been settled or dismissed on terms not material to Pfizer: (i) the action filed against Ben Venue Laboratories, Inc., which was sued along with Hikma Pharmaceuticals PLC (together, succeeded by Eurohealth International Sarl) and West-Ward Pharmaceuticals Corp (collectively, the Ben Venue case); and (ii) the action filed against Baxter Healthcare Corporation. The remaining actions continue as described below.
In August 2015, Hospira filed suit against Amneal Pharmaceuticals LLC (Amneal) in the U.S. District Court for the District of Delaware asserting the validity and infringement of four patents relating to the Precedex premix formulations and their use, all of which expire in 2032. In January 2018, the District Court ruled that one of the four patents was valid and infringed, and that the other three patents were invalid. In February and March 2018, respectively, each of Amneal and Hospira appealed the District Court decision to the U.S. Court of Appeals for the Federal Circuit. In January 2019, the U.S. Court of Appeals for the Federal Circuit affirmed the District Court’s decision.
In December 2015, Fresenius Kabi USA LLC (Fresenius) notified Hospira that it had filed an abbreviated new drug application with the FDA seeking approval to market a generic version of Hospira’s premix version of Precedex and containing allegations that certain patents relating to the Precedex premix formulations and their use, all of which expire in 2032, were invalid or not infringed. In January 2016, Hospira filed suit against Fresenius in the U.S. District Court for the Northern District of Illinois, asserting the validity and infringement of those patents. In December 2018, the District Court ruled that the asserted claims of two patents were invalid. Hospira appealed the District Court’s decision as to one of the patents to the U.S. Court of Appeals for the Federal Circuit. In January 2020, the U.S. Court of Appeals for the Federal Circuit affirmed the District Court’s decision.
Separate actions in which Hospira sued Par Sterile Products LLC, Gland Pharma Limited, and Jiangsu Hengrui Medicine Co., Ltd., each in response to such generic manufacturer’s filing of a separate abbreviated new drug application with the FDA, seeking approval to market their generic versions of our subsidiary Hospira’s premix version of Precedex prior to the expiration of one or more patents covering the product, were stayed pending the outcome of the case against Fresenius described above.
Xeljanz (tofacitinib)
Beginning in 2017, we brought patent-infringement actions against several generic manufacturers that filed separate abbreviated new drug applications with the FDA, seeking approval to market their generic versions of tofacitinib tablets in one or both of 5 mg and 10 mg dosage strengths, and in both immediate and extended release forms. To date, actions against the following generic manufacturers have been settled on terms not material to Pfizer: (i) MicroLabs USA Inc. and MicroLabs Ltd.; (ii) Sun Pharmaceutical Industries Ltd.; (iii) Prinston Pharmaceutical Inc., Zhejiang Huahai Pharmaceutical Co., Ltd., Huahai US Inc. and Solco Healthcare US, LLC; and (iv) Breckenridge Pharmaceutical Inc., Pensa Pharma S.A. and Laboratorios Del Dr. Esteve, S.A. The remaining actions continue as described below.
In March 2017, we brought a patent-infringement action against Zydus Pharmaceuticals (USA) Inc. and Cadila Healthcare Ltd. (collectively, Zydus) in the U.S. District Court for the District of Delaware asserting the infringement and validity of three patents: the patent covering the active ingredient expiring in December 2025, the patent covering an enantiomer of tofacitinib expiring in 2022, and the patent covering a polymorphic form of tofacitinib expiring in 2023, which Zydus challenged in its abbreviated new drug application seeking approval to market a generic version of tofacitinib 5 mg tablets.
In December 2018, we brought a separate patent infringement action against Teva Pharmaceuticals USA, Inc. (Teva) in the U.S. District Court for the District of Delaware asserting the infringement and validity of our patent covering extended release formulations of tofacitinib that was challenged by Teva in its abbreviated new drug application seeking approval to market a generic version of tofacitinib 11 mg extended release tablets.
In March 2019, we brought a separate patent infringement action against Ajanta Pharma Ltd. and Ajanta Pharma USA Inc. (collectively, Ajanta) in the U.S. District Court for the District of Delaware asserting the infringement and validity of two patents: the patent covering the active ingredient that expires in December 2025 and the patent covering a polymorphic form of tofacitinib that expires in 2023, each of which Ajanta challenged in its abbreviated new drug application seeking approval to market a generic version of tofacitinib 5 mg tablets. In August 2019, in response to a similar challenge by Ajanta relating the tofacitinib 10 mg tablets, we brought another patent infringement action against Ajanta in the U.S. District Court for the District of Delaware.
Inlyta (axitinib)
In April 2018, Apotex Inc. notified us that it had filed an abbreviated new drug application with the FDA seeking approval to market a generic version of Inlyta. Apotex Inc. asserts the invalidity and non-infringement of the crystalline form patent for Inlyta that expires in 2030. In May
2019 Financial Report | 121 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2018, we filed suit against Apotex Inc. in the U.S. District Court for the District of Delaware, asserting the validity and infringement of the crystalline form patent for Inlyta.
In May 2019, Glenmark Pharmaceuticals Limited (Glenmark) notified us that it had filed an abbreviated new drug application with the FDA seeking approval to market a generic version of Inlyta. Glenmark asserts the invalidity and non-infringement of the crystalline form patent for Inlyta that expires in 2030. In June 2019, we filed suit against Glenmark in the U.S. District Court for the District of Delaware, asserting the validity and infringement of the crystalline form patent for Inlyta.
Kerydin (tavaborole)
In September 2018, several generic companies notified us that they had filed abbreviated new drug applications with the FDA seeking approval to market generic versions of Kerydin. The generic companies assert the invalidity and non-infringement of methods of use and formulation patents for tavaborole that expire in 2026 and 2027, including pediatric exclusivity. In October 2018, Anacor, our wholly-owned subsidiary, filed infringement lawsuits against each of the generic filers in the U.S. District Court for the District of Delaware and the U.S. District Court for the District of West Virginia.
Ibrance (palbociclib)
In March 2019, several generic companies notified us that they had filed abbreviated new drug applications with the FDA seeking approval to market generic versions of Ibrance. The generic companies assert the invalidity and non-infringement of two composition of matter patents and a method of use patent covering palbociclib, each of which expire in 2023. In April 2019, we brought patent infringement actions against each of the generic filers in various federal courts, asserting the validity and infringement of the patents challenged by the generic companies.
Chantix (varenicline)
In January 2020, we brought a patent infringement action against Viwit Pharmaceutical Co. Ltd. (Viwit) in the U.S. District Court for the District of Delaware, asserting the validity and infringement of three patents challenged by Viwit in its abbreviated new drug application seeking approval to market a generic version of varenicline, 0.5 mg and 1.0 mg tablets.
Matter Involving Our Collaboration/Licensing Partners
Eliquis
In February, March, and April 2017, twenty-five generic companies sent BMS Paragraph-IV certification letters informing BMS that they had filed abbreviated new drug applications seeking approval of generic versions of Eliquis, challenging the validity and infringement of one or more of the three patents listed in the Orange Book for Eliquis. One of the patents expired in December 2019 and the remaining patents currently are set to expire in 2026 and 2031. Eliquis has been jointly developed and is being commercialized by BMS and Pfizer. In April 2017, BMS and Pfizer filed patent-infringement actions against all generic filers in the U.S. District Court for the District of Delaware and the U.S. District Court for the District of West Virginia, asserting that each of the generic companies’ proposed products would infringe each of the patent(s) that each generic filer challenged. Some generic filers challenged only the 2031 patent, some challenged both the 2031 and 2026 patent, and one generic company challenged all three patents. We and BMS have settled with certain of the generic companies on terms not material to Pfizer, and we and BMS may settle with other generic companies in the future.
Action In Which We Are The Defendant
Inflectra (infliximab-dyyb)
In March 2015, Janssen and New York University, together, brought a patent-infringement action in the U.S. District Court for the District of Massachusetts against Hospira, Celltrion Healthcare Co. Ltd. and Celltrion Inc. alleging that infliximab-dyyb, to be marketed by Hospira in the U.S. under the brand name Inflectra, would infringe six patents relating to infliximab, its manufacture and use. Claims with respect to four of the patents were dismissed by the plaintiffs, leaving two patents at issue: the infliximab antibody patent and a patent relating to cell culture media. In January 2018, the antibody patent was declared invalid by the Court of Appeals for the Federal Circuit. In July 2018, the U.S. District Court for the District of Massachusetts granted defendants’ motion for summary judgment and ruled that the patent relating to cell culture media was not infringed. Janssen appealed the District Court’s decision to the U.S. Court of Appeals for the Federal Circuit.
A2. Legal Proceedings––Product Litigation
Like other pharmaceutical companies, we are defendants in numerous cases, including but not limited to those discussed below, related to our pharmaceutical and other products. Plaintiffs in these cases seek damages and other relief on various grounds for alleged personal injury and economic loss.
Asbestos
Between 1967 and 1982, Warner-Lambert owned American Optical Corporation (American Optical), which manufactured and sold respiratory protective devices and asbestos safety clothing. In connection with the sale of American Optical in 1982, Warner-Lambert agreed to indemnify the purchaser for certain liabilities, including certain asbestos-related and other claims. Claims against American Optical and numerous other defendants are pending in various federal and state courts seeking damages for alleged personal injury from exposure to asbestos and other allegedly hazardous materials. Warner-Lambert was acquired by Pfizer in 2000 and is a wholly owned subsidiary of Pfizer. Warner-Lambert is actively engaged in the defense of, and will continue to explore various means of resolving, these claims.
Numerous lawsuits are pending against Pfizer in various federal and state courts seeking damages for alleged personal injury from exposure to products allegedly containing asbestos and other allegedly hazardous materials sold by Pfizer and certain of its previously owned subsidiaries.
There also are a small number of lawsuits pending in various federal and state courts seeking damages for alleged exposure to asbestos in facilities owned or formerly owned by Pfizer or its subsidiaries.
122 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Effexor
Beginning in May 2011, actions, including purported class actions, were filed in various federal courts against Wyeth and, in certain of the actions, affiliates of Wyeth and certain other defendants relating to Effexor XR, which is the extended-release formulation of Effexor. The plaintiffs in each of the class actions seek to represent a class consisting of all persons in the U.S. and its territories who directly purchased, indirectly purchased or reimbursed patients for the purchase of Effexor XR or generic Effexor XR from any of the defendants from June 14, 2008 until the time the defendants’ allegedly unlawful conduct ceased. The plaintiffs in all of the actions allege delay in the launch of generic Effexor XR in the U.S. and its territories, in violation of federal antitrust laws and, in certain of the actions, the antitrust, consumer protection and various other laws of certain states, as the result of Wyeth fraudulently obtaining and improperly listing certain patents for Effexor XR in the Orange Book, enforcing certain patents for Effexor XR and entering into a litigation settlement agreement with a generic drug manufacturer with respect to Effexor XR. Each of the plaintiffs seeks treble damages (for itself in the individual actions or on behalf of the putative class in the purported class actions) for alleged price overcharges for Effexor XR or generic Effexor XR in the U.S. and its territories since June 14, 2008. All of these actions have been consolidated in the U.S. District Court for the District of New Jersey.
In October 2014, the District Court dismissed the direct purchaser plaintiffs’ claims based on the litigation settlement agreement, but declined to dismiss the other direct purchaser plaintiff claims. In January 2015, the District Court entered partial final judgments as to all settlement agreement claims, including those asserted by direct purchasers and end-payer plaintiffs, which plaintiffs appealed to the U.S. Court of Appeals for the Third Circuit. In August 2017, the U.S. Court of Appeals for the Third Circuit reversed the District Court’s decisions and remanded the claims to the District Court.
Lipitor
• | Antitrust Actions |
Beginning in November 2011, purported class actions relating to Lipitor were filed in various federal courts against, among others, Pfizer, certain affiliates of Pfizer, and, in most of the actions, Ranbaxy, Inc. (Ranbaxy) and certain affiliates of Ranbaxy. The plaintiffs in these various actions seek to represent nationwide, multi-state or statewide classes consisting of persons or entities who directly purchased, indirectly purchased or reimbursed patients for the purchase of Lipitor (or, in certain of the actions, generic Lipitor) from any of the defendants from March 2010 until the cessation of the defendants’ allegedly unlawful conduct (the Class Period). The plaintiffs allege delay in the launch of generic Lipitor, in violation of federal antitrust laws and/or state antitrust, consumer protection and various other laws, resulting from (i) the 2008 agreement pursuant to which Pfizer and Ranbaxy settled certain patent litigation involving Lipitor, and Pfizer granted Ranbaxy a license to sell a generic version of Lipitor in various markets beginning on varying dates, and (ii) in certain of the actions, the procurement and/or enforcement of certain patents for Lipitor. Each of the actions seeks, among other things, treble damages on behalf of the putative class for alleged price overcharges for Lipitor (or, in certain of the actions, generic Lipitor) during the Class Period. In addition, individual actions have been filed against Pfizer, Ranbaxy and certain of their affiliates, among others, that assert claims and seek relief for the plaintiffs that are substantially similar to the claims asserted and the relief sought in the purported class actions described above. These various actions have been consolidated for pre-trial proceedings in a Multi-District Litigation (In re Lipitor Antitrust Litigation MDL-2332) in the U.S. District Court for the District of New Jersey.
In September 2013 and 2014, the District Court dismissed with prejudice the claims by direct purchasers. In October and November 2014, the District Court dismissed with prejudice the claims of all other Multi-District Litigation plaintiffs. All plaintiffs have appealed the District Court’s orders dismissing their claims with prejudice to the U.S. Court of Appeals for the Third Circuit. In addition, the direct purchaser class plaintiffs appealed the order denying their motion to amend the judgment and for leave to amend their complaint to the U.S. Court of Appeals for the Third Circuit. In August 2017, the U.S. Court of Appeals for the Third Circuit reversed the District Court’s decisions and remanded the claims to the District Court.
Also, in January 2013, the State of West Virginia filed an action in West Virginia state court against Pfizer and Ranbaxy, among others, that asserts claims and seeks relief on behalf of the State of West Virginia and residents of that state that are substantially similar to the claims asserted and the relief sought in the purported class actions described above.
• | Personal Injury Actions |
A number of individual and multi-plaintiff lawsuits have been filed against us in various federal and state courts alleging that the plaintiffs developed type 2 diabetes purportedly as a result of the ingestion of Lipitor. Plaintiffs seek compensatory and punitive damages.
In February 2014, the federal actions were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Lipitor (Atorvastatin Calcium) Marketing, Sales Practices and Products Liability Litigation (No. II) MDL-2502) in the U.S. District Court for the District of South Carolina. Since 2016, certain cases in the Multi-District Litigation were remanded to certain state courts. In January 2017, the District Court granted our motion for summary judgment, dismissing substantially all of the remaining cases pending in the Multi-District Litigation. In January 2017, the plaintiffs appealed the District Court’s decision to the U.S. Court of Appeals for the Fourth Circuit. In June 2018, the U.S. Court of Appeals for the Fourth Circuit affirmed the District Court’s decision.
Viagra
Since April 2016, a Multi-District Litigation has been pending in the U.S. District Court for the Northern District of California (In Re: Viagra (Sildenafil Citrate) Products Liability Litigation, MDL-2691), in which plaintiffs allege that they developed melanoma and/or the exacerbation of melanoma purportedly as a result of the ingestion of Viagra. Additional cases filed against Lilly with respect to Cialis have also been consolidated in the Multi-District Litigation (In re: Viagra (Sildenafil Citrate) and Cialis (Tadalafil) Products Liability Litigation, MDL-2691). In January 2020, the District Court granted our and Lilly’s motion to exclude all of plaintiffs’ general causation opinions.
Intravenous Solutions
Beginning in November 2016, purported class actions were filed in the U.S. District Court for the Northern District of Illinois against Hospira, Hospira Worldwide, Inc. and certain other defendants relating to intravenous saline solution. Plaintiffs seek to represent a class consisting of all persons and entities in the U.S. who directly purchased intravenous saline solution sold by any of the defendants from January 1, 2013 until the time the defendants’ allegedly unlawful conduct ceases. Plaintiffs allege that the defendants’ conduct restricts output and artificially fixes, raises, maintains and/or stabilizes the prices of intravenous saline solution sold throughout the U.S. in violation of federal antitrust laws.
2019 Financial Report | 123 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Plaintiffs seek treble damages (for themselves and on behalf of the putative classes) and an injunction against defendants for alleged price overcharges for intravenous saline solution in the U.S. since January 1, 2013. All of these actions have been consolidated in the U.S. District Court for the Northern District of Illinois. In July 2018, the District Court granted defendants’ motions to dismiss the consolidated amended complaint without prejudice. Plaintiffs filed a second amended complaint in September 2018. On February 3, 2017, we completed the sale of our global infusion systems net assets, HIS, which includes intravenous saline solution, to ICU Medical. The litigation is the subject of cross-claims for indemnification by both Pfizer and ICU Medical under the purchase agreement.
Hormone Therapy Consumer Class Action
A certified consumer class action is pending against Wyeth in the U.S. District Court for the Southern District of California based on the alleged off-label marketing of its hormone therapy products. The case was originally filed in December 2003. The class consists of California consumers who purchased Wyeth’s hormone-replacement products between January 1995 and January 2003 and who do not seek personal injury damages therefrom. The class seeks compensatory and punitive damages, including a full refund of the purchase price.
EpiPen
Beginning in February 2017, purported class actions were filed in various federal courts by indirect purchasers of EpiPen against Pfizer, and/or its affiliates King and Meridian, and/or various entities affiliated with Mylan, and Mylan Chief Executive Officer, Heather Bresch. The plaintiffs in these actions seek to represent U.S. nationwide classes comprising persons or entities who paid for any portion of the end-user purchase price of an EpiPen between 2009 until the cessation of the defendants’ allegedly unlawful conduct. In August 2017, a similar lawsuit brought in the U.S. District Court for the District of New Jersey on behalf of a purported class of direct purchaser plaintiffs against Pfizer, King, Meridian and Mylan was voluntarily dismissed without prejudice. In February 2020, a similar lawsuit was filed in the U.S. District Court for the District of Kansas against Pfizer, King, Meridian and the Mylan entities on behalf of a purported U.S. nationwide class of direct purchaser plaintiffs who purchased EpiPen devices directly from the defendants (the 2020 lawsuit). Against Pfizer and/or its affiliates, plaintiffs in these actions generally allege that Pfizer’s and/or its affiliates’ settlement of patent litigation regarding EpiPen delayed market entry of generic EpiPen in violation of federal antitrust laws and various state antitrust laws. At least one lawsuit also alleges that Pfizer and/or Mylan violated the federal Racketeer Influenced and Corrupt Organizations Act. Plaintiffs also filed various federal antitrust, state consumer protection and unjust enrichment claims against, and relating to conduct attributable solely to, Mylan and/or its affiliates regarding EpiPen. Plaintiffs seek treble damages for alleged overcharges for EpiPen since 2009. In August 2017, all of these actions, except for the 2020 lawsuit, were consolidated for coordinated pre-trial proceedings in a Multi-District Litigation (In re: EpiPen (Epinephrine Injection, USP) Marketing, Sales Practices and Antitrust Litigation, MDL-2785) in the U.S. District Court for the District of Kansas with other EpiPen-related actions against Mylan and/or its affiliates to which Pfizer, King and Meridian are not parties.
Nexium 24HR and Protonix
A number of individual and multi-plaintiff lawsuits have been filed against Pfizer, certain of its subsidiaries and/or other pharmaceutical manufacturers in various federal and state courts alleging that the plaintiffs developed kidney-related injuries purportedly as a result of the ingestion of certain proton pump inhibitors. The cases against Pfizer involve Protonix and/or Nexium 24HR and seek compensatory and punitive damages and, in some cases, treble damages, restitution or disgorgement. In August 2017, the federal actions were ordered transferred for coordinated pre-trial proceedings to a Multi-District Litigation (In re: Proton-Pump Inhibitor Products Liability Litigation (No. II)) in the U.S. District Court for the District of New Jersey. On July 31, 2019, we completed the transaction in which we and GSK combined our respective consumer healthcare businesses into a new consumer healthcare joint venture that operates globally under the GSK Consumer Healthcare name. As part of the joint venture transaction, the joint venture has agreed to assume, and to indemnify Pfizer for, liabilities arising out of such litigation to the extent related to Nexium 24HR.
Docetaxel
• | Personal Injury Actions |
A number of lawsuits have been filed against Hospira and Pfizer in various federal and state courts alleging that plaintiffs who were treated with Docetaxel developed permanent hair loss. The significant majority of the cases also name other defendants, including the manufacturer of the branded product, Taxotere. Plaintiffs seek compensatory and punitive damages.
In October 2016, the federal cases were transferred for coordinated pre-trial proceedings to a Multi-District Litigation (In re Taxotere (Docetaxel) Products Liability Litigation, MDL-2740) in the U.S. District Court for the Eastern District of Louisiana.
• | Mississippi Attorney General Government Investigation |
In October 2018, the Attorney General of Mississippi filed a complaint in Mississippi state court against the manufacturer of the branded product and eight other manufacturers including Pfizer and Hospira, alleging, with respect to Pfizer and Hospira, a failure to warn about a risk of permanent hair loss in violation of the Mississippi Consumer Protection Act. The action seeks civil penalties and injunctive relief.
Array Securities Litigation
In November 2017, two purported class actions were filed in the U.S. District Court for the District of Colorado alleging that Array, which we acquired in July 2019 and is our wholly owned subsidiary, and certain of its former officers violated federal securities laws in connection with certain disclosures made, or omitted, by Array regarding the NRAS-mutant melanoma program. In March 2018, the actions were consolidated into a single proceeding.
Zantac
A number of lawsuits have been filed against Pfizer in various federal courts alleging that plaintiffs developed various types of cancer, or face an increased risk of developing cancer, purportedly as a result of the ingestion of Zantac. The significant majority of these cases also name other defendants that have historically manufactured and sold Zantac. Pfizer has not sold Zantac since 2006. Plaintiffs seek compensatory and punitive damages and, in some cases, treble damages, restitution or disgorgement.
In February 2020, these federal actions were transferred for coordinated pre-trial proceedings to a Multi-District Litigation (In re Zantac/Ranitidine NDMA Litigation, MDL-2924) in the U.S. District Court for the Southern District of Florida.
124 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
A3. Legal Proceedings––Commercial and Other Matters
Monsanto-Related Matters
In 1997, Monsanto Company (Former Monsanto) contributed certain chemical manufacturing operations and facilities to a newly formed corporation, Solutia Inc. (Solutia), and spun off the shares of Solutia. In 2000, Former Monsanto merged with Pharmacia & Upjohn Company to form Pharmacia. Pharmacia then transferred its agricultural operations to a newly created subsidiary, named Monsanto Company (New Monsanto), which it spun off in a two-stage process that was completed in 2002. Pharmacia was acquired by Pfizer in 2003 and is a wholly owned subsidiary of Pfizer.
In connection with its spin-off that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities related to Pharmacia’s former agricultural business. New Monsanto has defended and/or is defending Pharmacia in connection with various claims and litigation arising out of, or related to, the agricultural business, and has been indemnifying Pharmacia when liability has been imposed or settlement has been reached regarding such claims and litigation.
In connection with its spin-off in 1997, Solutia assumed, and agreed to indemnify Pharmacia for, liabilities related to Former Monsanto’s chemical businesses. As the result of its reorganization under Chapter 11 of the U.S. Bankruptcy Code, Solutia’s indemnification obligations relating to Former Monsanto’s chemical businesses are primarily limited to sites that Solutia has owned or operated. In addition, in connection with its spinoff that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities primarily related to Former Monsanto’s chemical businesses, including, but not limited to, any such liabilities that Solutia assumed. Solutia’s and New Monsanto’s assumption of, and agreement to indemnify Pharmacia for, these liabilities apply to pending actions and any future actions related to Former Monsanto’s chemical businesses in which Pharmacia is named as a defendant, including, without limitation, actions asserting environmental claims, including alleged exposure to polychlorinated biphenyls. Solutia and/or New Monsanto are defending Pharmacia in connection with various claims and litigation arising out of, or related to, Former Monsanto’s chemical businesses, and have been indemnifying Pharmacia when liability has been imposed or settlement has been reached regarding such claims and litigation.
Environmental Matters
In 2009, we submitted to the U.S. Environmental Protection Agency (EPA) a corrective measures study report with regard to Pharmacia’s discontinued industrial chemical facility in North Haven, Connecticut. In September 2010, our corrective measures study report was approved by the EPA, and we commenced construction of the site remedy in late 2011 under an Updated Administrative Order on Consent with the EPA. In September 2019, the EPA acknowledged that construction of the site remedy has been completed.
Also, in 2009, we submitted a revised site-wide feasibility study with regard to Wyeth Holdings Corporation’s (formerly, American Cyanamid Company) discontinued industrial chemical facility in Bound Brook, New Jersey. In July 2011, Wyeth Holdings Corporation finalized an Administrative Settlement Agreement and Order on Consent for Removal Action (the 2011 Administrative Settlement Agreement) with the EPA with regard to the Bound Brook facility. In May 2012, we completed construction of an interim remedy to address the discharge of impacted groundwater from that facility to the Raritan River. In September 2012, the EPA issued a final remediation plan for the Bound Brook facility’s main plant area, which is generally in accordance with one of the remedies evaluated in our revised site-wide feasibility study. In March 2013, Wyeth Holdings Corporation (now Wyeth Holdings LLC) entered into an Administrative Settlement Agreement and Order on Consent with the EPA to allow us to undertake detailed engineering design of the remedy for the main plant area and to perform a focused feasibility study for two adjacent lagoons. In September 2015, the U.S., on behalf of the EPA, filed a complaint and consent decree with the federal District Court for the District of New Jersey that allows Wyeth Holdings LLC to complete the design and to implement the remedy for the main plant area. In December 2015, the consent decree (which supersedes the 2011 Administrative Settlement Agreement) was entered by the District Court. In September 2018, the EPA issued a final remediation plan for the two adjacent lagoons, which is generally in accordance with one of the remedies evaluated in our focused feasibility study, and in September 2019, Wyeth Holdings LLC entered into an Administrative Settlement Agreement and Order on Consent with the EPA to allow us to undertake detailed engineering design of the remedy for the lagoons.
We have accrued for the estimated costs of the site remedies for the North Haven and Bound Brook facilities.
We are a party to a number of other proceedings brought under the Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended, and other state, local or foreign laws in which the primary relief sought is the cost of past and/or future remediation.
Contracts with Iraqi Ministry of Health
In October 2017, a number of United States service members, civilians, and their families brought a complaint in the U.S. District Court for the District of Columbia against a number of pharmaceutical and medical devices companies, including Pfizer and certain of its subsidiaries, alleging that the defendants violated the United States Anti-Terrorism Act. The complaint alleges that the defendants provided funding for terrorist organizations through their sales practices pursuant to pharmaceutical and medical device contracts with the Iraqi Ministry of Health, and seeks monetary relief. In July 2018, the U.S. Department of Justice requested documents related to this matter, which are being provided.
Allergan Complaint for Indemnity
In August 2018, Pfizer was named as a defendant in a third-party complaint for indemnity, along with King, filed by Allergan Finance LLC (Allergan) in a Multi-District Litigation (In re National Prescription Opiate Litigation MDL 2804) in the U.S. District Court for the Northern District of Ohio. The lawsuit asserted claims for indemnity related to Kadian, which was owned for a short period by King in 2008, prior to Pfizer's acquisition of King in 2010. In December 2018, the District Court dismissed the lawsuit. In February 2019, Allergan filed a similar complaint in the Supreme Court of the State of New York, asserting claims for indemnity related to Kadian.
Breach of Contract––Xalkori
Pfizer is a defendant in a breach of contract action brought by New York University (NYU) in the Supreme Court of the State of New York (Supreme Court). NYU alleges that it is entitled to royalties on Pfizer’s sales of Xalkori under the terms of a Research and License Agreement between NYU and Sugen, Inc. Sugen, Inc. was acquired by Pharmacia in August 1999, and Pharmacia was acquired by Pfizer in 2003 and is a wholly owned subsidiary of Pfizer. The action was originally filed in 2013. In December 2015, the Supreme Court dismissed the action and in May 2017, the New York State Appellate Division reversed the decision and remanded the proceedings to the Supreme Court. In January 2020, the Supreme Court denied both parties’ summary judgment motions.
2019 Financial Report | 125 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
A4. Legal Proceedings––Government Investigations
Like other pharmaceutical companies, we are subject to extensive regulation by government agencies in the U.S., other developed markets and multiple emerging markets in which we operate. Criminal charges, substantial fines and/or civil penalties, limitations on our ability to conduct business in applicable jurisdictions, corporate integrity or deferred prosecution agreements, as well as reputational harm and increased public interest in the matter could result from government investigations in the U.S. and other jurisdictions in which we do business. In addition, in a qui tam lawsuit in which the government declines to intervene, the relator may still pursue a suit for the recovery of civil damages and penalties on behalf of the government. Among the investigations by government agencies are the matters discussed below.
Phenytoin Sodium Capsules
In 2012, Pfizer sold the U.K. Marketing Authorisation for phenytoin sodium capsules to a third party, but retained the right to supply the finished product to that third party. In May 2013, the U.K. Competition & Markets Authority (CMA) informed us that it had launched an investigation into the supply of phenytoin sodium capsules in the U.K. market. In August 2015, the CMA issued a Statement of Objections alleging that Pfizer and Pfizer Limited, a U.K. subsidiary, engaged in conduct that violates U.K. and EU antitrust laws. In December 2016, the CMA imposed a £84.2 million fine on Pfizer and Pfizer Limited. Pfizer appealed the CMA decision to The Competition Appeal Tribunal in February 2017. On June 7, 2018, the Competition Appeal Tribunal overturned the CMA decision as well as the associated fine. The CMA appealed the judgment to the Court of Appeal.
Greenstone Investigations
• | U.S. Department of Justice Antitrust Division Investigation |
Since July 2017, the U.S. Department of Justice's Antitrust Division has been investigating our Greenstone generics business. We believe this is related to an ongoing broader antitrust investigation of the generic pharmaceutical industry. The government has been obtaining information from Greenstone.
• | State Attorneys General Generics Antitrust Litigation |
In April 2018, Greenstone received requests for information from the Antitrust Department of the Connecticut Office of the Attorney General. In May 2019, Attorneys General of more than 40 states plus the District of Columbia and Puerto Rico filed a complaint against a number of pharmaceutical companies, including Greenstone and Pfizer. The matter has been consolidated with a Multi-District Litigation (In re: Generic Pharmaceuticals Pricing Antitrust Litigation MDL No. 2724) in the Eastern District of Pennsylvania. As to Greenstone and Pfizer, the complaint alleges anticompetitive conduct in violation of federal and state antitrust laws and state consumer protection laws.
Subpoena relating to Manufacturing of Quillivant XR
In October 2018, we received a subpoena from the U.S. Attorney’s Office for the Southern District of New York (SDNY) seeking records relating to our relationship with another drug manufacturer and its production and manufacturing of drugs including, but not limited to, Quillivant XR. We have produced records pursuant to the subpoena.
Government Inquiries relating to Meridian Medical Technologies
In February 2019, we received a civil investigative demand from the U.S. Attorney’s Office for the SDNY. The civil investigative demand seeks records and information related to alleged quality issues involving the manufacture of auto-injectors at our Meridian site. In August 2019, we received a HIPAA subpoena from the U.S. Attorney’s Office for the Eastern District of Missouri seeking similar records and information. We are producing records in response to these requests.
U.S. Department of Justice/SEC Inquiry relating to Russian Operations
In June 2019, we received an informal request from the U.S. Department of Justice’s FCPA Unit seeking documents relating to our operations in Russia. In September 2019, we received a similar request from the SEC’s FCPA unit. We are producing records pursuant to these requests.
Contracts with Iraqi Ministry of Health
See Note 16A3. Contingencies and Certain Commitments: Legal Proceedings––Commercial and Other Matters––Contracts with Iraqi Ministry of Health above for information regarding U.S. government investigations related to contracts with the Iraqi Ministry of Health.
Docetaxel––Mississippi Attorney General Government Investigation
See Note 16A2. Contingencies and Certain Commitments: Legal Proceedings––Product Litigation––Docetaxel––Mississippi Attorney General Government Investigation above for information regarding a government investigation related to Docetaxel marketing practices.
B. Guarantees and Indemnifications
In the ordinary course of business and in connection with the sale of assets and businesses and other transactions, we often indemnify our counterparties against certain liabilities that may arise in connection with the transaction or that are related to events and activities prior to or following a transaction. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we may be required to reimburse the loss. These indemnifications are generally subject to various restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2019, the estimated fair value of these indemnification obligations was not significant.
In addition, in connection with our entry into certain agreements, our counterparties agree to indemnify us. For example, our collaboration agreement with EMD Serono, Inc. to co-promote Rebif in the U.S. expired at the end of 2015 and included certain indemnity provisions. Patent litigation brought by Biogen Idec MA Inc. against EMD Serono Inc. and Pfizer is pending in the U.S. District Court for the District of New Jersey and the United States Court of Appeals for the Federal Circuit. EMD Serono Inc. has acknowledged that it is obligated to satisfy any award of damages.
Pfizer Inc. has also guaranteed the long-term debt of certain companies that it acquired and that now are subsidiaries of Pfizer.
126 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
C. Certain Commitments
• | As of December 31, 2019, we had agreements totaling $ |
• | As of December 31, 2019, in connection with the TCJA, we have an estimated $ |
D. Contingent Consideration for Acquisitions
We may be required to make contingent consideration payments to sellers for certain prior business combinations. See Note 1D. The estimated fair value of contingent consideration as of December 31, 2019 is $711 million, of which $160 million is recorded in Other current liabilities and $551 million is recorded in Other noncurrent liabilities. The estimated fair value of contingent consideration as of December 31, 2018 is $988 million, of which $280 million is recorded in Other current liabilities and $708 million is recorded in Other noncurrent liabilities. The decrease in the contingent consideration balance from prior year is primarily due to payments made upon the achievement of certain milestones.
Note 17. Segment, Geographic and Other Revenue Information
A. Segment Information
We regularly review our segments and the approach used by management to evaluate performance and allocate resources. Prior to January 1, 2019, we managed our commercial operations through two distinct business segments: Pfizer Innovative Health (IH) and Pfizer Essential Health (EH). At the beginning of our fiscal year 2019, we reorganized our commercial operations and began to manage our commercial operations through a new global structure consisting of three distinct business segments: Pfizer Biopharmaceuticals Group (Biopharma), Upjohn and, through July 31, 2019, Pfizer’s Consumer Healthcare business (Consumer Healthcare), each led by a single manager. Each operating segment has responsibility for its commercial activities. Upjohn and through July 31, 2019, Consumer Healthcare, are responsible for their own R&D activities while Biopharma receives its R&D services from GPD and WRDM. These services include IPR&D projects for new investigational products and additional indications for in-line products. Each business has a geographic footprint across developed and emerging markets. Our chief operating decision maker uses the revenues and earnings of the operating segments, among other factors, for performance evaluation and resource allocation. Biopharma and Upjohn are the only reportable segments. We have revised prior-period information (Revenues and Earnings, as defined by management) to conform to the current management structure. As our operations were not managed under the new structure until the beginning of fiscal 2019, certain costs and expenses could not be directly attributed to one of the then new operating segments. As a result, our operating segment results for 2018 and 2017 include allocations, which management believes are reasonable. As described in Note 1A, acquisitions impacted our results of operations in 2019 and 2017, the contribution of our Consumer Healthcare business to the GSK Consumer Healthcare joint venture impacted our results of operations in 2019 and divestitures impacted our results of operations in 2017.
2019 Financial Report | 127 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Operating Segments
Some additional information about our Biopharma and Upjohn business segments follows: | |||
 | Pfizer Biopharmaceuticals Group |  | |
Biopharma is a science-based medicines business that includes six business units – Oncology, Inflammation & Immunology, Rare Disease, Hospital, Vaccines and Internal Medicine. The Hospital unit commercializes our global portfolio of sterile injectable and anti-infective medicines and includes Pfizer’s contract manufacturing operation, Pfizer CentreOne. At the beginning of our 2019 fiscal year, we also incorporated our biosimilar portfolio into the Oncology and Inflammation & Immunology business units and certain legacy established products into the Internal Medicine business unit. Each business unit is committed to delivering breakthroughs that change patients’ lives. | Upjohn is a global, primarily off-patent branded and generic medicines business, which includes a portfolio of 20 globally recognized solid oral dose brands, as well as a U.S.-based generics platform, Greenstone. | ||
Select products include: - Prevnar 13/Prevenar 13 - Ibrance - Eliquis - Xeljanz - Enbrel (outside the U.S. and Canada) - Chantix/Champix - Sutent - Xtandi - Vyndaqel/Vyndamax | Select products include: - Lyrica - Lipitor - Norvasc - Celebrex - Viagra - Certain generic medicines | ||
On July 29, 2019, we announced that we entered into a definitive agreement to combine Upjohn with Mylan, creating a new global pharmaceutical company. For additional information, see Note 1A.
On July 31, 2019, Pfizer’s Consumer Healthcare business, an over-the-counter medicines business, was combined with GSK’s consumer healthcare business to form a new consumer healthcare joint venture. See Note 1A and Note 2C for additional information.
Other Costs and Business Activities
Certain pre-tax costs are not allocated to our operating segment results, such as costs associated with the following:
• | WRDM––the R&D and Medical expenses managed by our WRDM organization, which is generally responsible for research projects for our Biopharma portfolio until proof-of-concept is achieved and then for transitioning those projects to the GPD organization for possible clinical and commercial development. R&D spending may include upfront and milestone payments for intellectual property rights. The WRDM organization also has responsibility for certain science-based and other platform-services organizations, which provide end-to-end technical expertise and other services to the various R&D projects, as well as the Worldwide Medical and Safety group, which ensures that Pfizer provides all stakeholders––including patients, healthcare providers, pharmacists, payers and health authorities––with complete and up-to-date information on the risks and benefits associated with Pfizer products so that they can make appropriate decisions on how and when to use Pfizer’s medicines. |
• | GPD––the costs associated with our GPD organization, which is generally responsible for clinical trials from WRDM in the Biopharma portfolio, including late stage portfolio spend. GPD also provides technical support and other services to Pfizer R&D projects. GPD is responsible for facilitating all regulatory submissions and interactions with regulatory agencies. |
• | Other––the operating results of our Consumer Healthcare business, through July 31, 2019, and costs associated with other commercial activities not managed as part of Biopharma or Upjohn, including all strategy, business development, portfolio management and valuation capabilities, which previously had been reported in various parts of the organization. |
• | Corporate and Other Unallocated––the costs associated with platform functions (such as worldwide technology, global real estate operations, legal, finance, human resources, worldwide public affairs, compliance, and worldwide procurement), patient advocacy activities and certain compensation and other corporate costs, such as interest income and expense, and gains and losses on investments, as well as overhead expenses associated with our manufacturing (which include manufacturing variances associated with production) and commercial operations that are not directly assessed to an operating segment, as business unit (segment) management does not manage these costs. |
• | Certain transactions and events such as (i) purchase accounting adjustments, where we incur expenses associated with the amortization of fair value adjustments to inventory, intangible assets and PP&E; (ii) acquisition-related costs, where we incur costs for executing the transaction, integrating the acquired operations and restructuring the combined company; and (iii) certain significant items, representing substantive and/or unusual, and in some cases recurring, items (such as gains on the completion of joint venture transactions, restructuring charges, legal charges or net gains and losses on investments in equity securities) that are evaluated on an individual basis by management and that, either as a result of their nature or size, would not be expected to occur as part of our normal business on a regular |
128 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
basis. Such items can include, but are not limited to, non-acquisition-related restructuring costs, as well as costs incurred for legal settlements, asset impairments and disposals of assets or businesses, including, as applicable, any associated transition activities.
Segment Assets
We manage our assets on a total company basis, not by operating segment, as many of our operating assets are shared or commingled (such as accounts receivable, as many of our customers are served by multiple operating segments). Therefore, our chief operating decision maker does not regularly review any asset information by operating segment and, accordingly, we do not report asset information by operating segment. Total assets were approximately $167 billion as of December 31, 2019 and approximately $159 billion as of December 31, 2018.
Selected Income Statement Information
The following table provides selected income statement information by reportable segment: | ||||||||||||||||||||||||||||||||||||
Revenues | Earnings(a) | Depreciation and Amortization(b) | ||||||||||||||||||||||||||||||||||
Year Ended December 31, | Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||||||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | 2019 | 2018 | 2017 | 2019 | 2018 | 2017 | |||||||||||||||||||||||||||
Reportable Segments: | ||||||||||||||||||||||||||||||||||||
Biopharma | $ | $ | $ | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||
Upjohn | ||||||||||||||||||||||||||||||||||||
Total reportable segments | ||||||||||||||||||||||||||||||||||||
Other business activities | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
Reconciling Items: | ||||||||||||||||||||||||||||||||||||
Corporate and other unallocated | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
Purchase accounting adjustments | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
Acquisition-related costs | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
Certain significant items(c) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||
$ | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||||
(a) | Income from continuing operations before provision/(benefit) for taxes on income. Biopharma’s earnings include dividend income from our investment in ViiV of $ |
(b) | Certain production facilities are shared. Depreciation is allocated based on estimates of physical production. Amounts here relate solely to the depreciation and amortization associated with continuing operations. |
(c) | Certain significant items are substantive and/or unusual, and in some cases recurring, items (as noted above) that, either as a result of their nature or size, would not be expected to occur as part of our normal business on a regular basis. |
For Earnings in 2019, certain significant items includes: (i) restructuring charges and implementation costs associated with our cost-reduction initiatives that are not associated with an acquisition of $758 million, (ii) charges for certain legal matters of $543 million, (iii) certain asset impairment charges of $2.8 billion, (iv) charges for business and legal entity alignment of $495 million, (v) net gains of $415 million recognized during the period on equity securities, (vi) a pre-tax gain of $8.1 billion associated with the completion of the GSK Consumer Healthcare joint venture transaction, (vii) net losses on early retirement of debt of $138 million and (viii) other charges of $1.3 billion, which includes, among other things: an upfront license fee payment of $250 million to Akcea, which was recorded in Research and development expenses, charges of $112 million recorded in Other (income)/deductions––net representing our pro rata share of primarily restructuring and business combination accounting charges recorded by the GSK Consumer Healthcare joint venture, a $337 million charge in Research and development expenses related to our acquisition of Therachon, a $99 million charge in Cost of sales related to rivipansel, primarily for inventory manufactured for expected future sale and charges of $240 million, primarily in Selling, informational and administrative expenses and Other (income)/deductions––net, for external incremental costs, such as transaction costs and costs to separate our Consumer Healthcare business into a separate legal entity associated with the formation of the GSK Consumer Healthcare joint venture. For additional information, see Note 1A, Note 2A, Note 2C, Note 2D, Note 3 and Note 4.
For Earnings in 2018, certain significant items includes: (i) restructuring charges and implementation costs associated with our cost-reduction initiatives that are not associated with an acquisition of $977 million, (ii) net charges for certain legal matters of $157 million, (iii) certain asset impairment charges of $3.1 billion, (iv) charges for business and legal entity alignment of $63 million, (v) net gains of $586 million recognized during the period on equity securities, (vi) net losses on early retirement of debt of $3 million and (vii) other charges of $4 million, which includes, among other things: a non-cash $343 million pre-tax gain in Other (income)/deductions––net associated with our transaction with Bain Capital to create a new biopharmaceutical company, Cerevel, to continue development of a portfolio of clinical and pre-clinical stage neuroscience assets primarily targeting disorders of the central nervous system, a $119 million charge, in the aggregate, in Selling, informational and administrative expenses for a special, one-time bonus paid to virtually all Pfizer colleagues, excluding executives, which was one of several actions taken by us after evaluating the expected positive net impact of the December 2017 enactment of the TCJA, and a non-cash $50 million pre-tax gain in Other (income)/deductions––net as a result of the contribution of our allogeneic CAR T cell therapy development program assets in connection with our contribution agreement entered into with Allogene. For additional information, see Note 2B, Note 3 and Note 4.
For Earnings in 2017, certain significant items includes: (i) restructuring charges and implementation costs associated with our cost-reduction initiatives that are not associated with an acquisition of $204 million, (ii) charges for certain legal matters of $237 million, (iii) certain asset impairment charges of $379 million, (iv) charges for business and legal entity alignment of $71 million, (v) net gains of $224 million recognized during the period on equity securities, (vi) net losses on early retirement of debt of $999 million and (vii) other charges of $756 million, which includes, among other things: a charitable contribution to the Pfizer Foundation of $200 million, which is included in Selling, informational and administrative expenses, $195 million in inventory losses, overhead costs related to the period in which our Puerto Rico plants were not operational, and incremental costs, all of which resulted from hurricanes in Puerto Rico in 2017 and are included in Cost of sales, an $81 million loss related to the sale of our former 49 % equity share in Hisun Pfizer, which is included in Other (income)/deductions––net, charges of $55 million in Other (income)/deductions––net representing adjustments to amounts previously recorded to write down the HIS net assets to fair value less costs to sell and a net loss of $30 million related to the sale of our former 40 % ownership investment in Teuto, including the extinguishment of a put option for the remaining 60 % ownership interest, which is included in Other (income)/deductions––net. For additional information, see Note 2B, Note 2C, Note 3 and Note 4.
The operating segment information does not purport to represent the revenues, costs and Income from continuing operations before provision/(benefit) for taxes on income that each of our operating segments would have recorded had each segment operated as a standalone company during the periods presented.
2019 Financial Report | 129 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
B. Geographic Information
As described in Note 1A, acquisitions impacted our results of operations in 2019 and 2017, the contribution of our Consumer Healthcare business to the GSK Consumer Healthcare joint venture impacted our results of operations in 2019 and divestitures impacted our results of operations in 2017.
The following table provides revenues by geographic area: | ||||||||||||
Year Ended December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
United States | $ | $ | $ | |||||||||
Developed Europe(a) | ||||||||||||
Developed Rest of World(b) | ||||||||||||
Emerging Markets(c) | ||||||||||||
Revenues | $ | $ | $ | |||||||||
(a) | Developed Europe region includes the following markets: Western Europe, Scandinavian countries and Finland. Revenues denominated in euros were $ |
(b) | Developed Rest of World region includes the following markets: Japan, Canada, South Korea, Australia and New Zealand. |
(c) | Emerging Markets region includes, but is not limited to, the following markets: Asia (excluding Japan and South Korea), Latin America, Eastern Europe, Africa, the Middle East, Central Europe and Turkey. |
Revenues exceeded $500 million in each of 11 countries outside the U.S. in 2019, 2018 and 2017. The U.S. is the only country to contribute more than 10 % of total revenue in 2019, 2018 and 2017. As a percentage of revenues, our two largest national markets outside the U.S. were China, which contributed 9 % of total revenue in 2019, 8 % of total revenue in 2018 and 7 % of total revenues in 2017, and Japan, which contributed 8 % of total revenue in each of 2019, 2018 and 2017.
The following table provides long-lived assets by geographic area: | ||||||||||||
As of December 31, | ||||||||||||
(MILLIONS OF DOLLARS) | 2019 | 2018 | 2017 | |||||||||
Property, plant and equipment, net | ||||||||||||
United States | $ | $ | $ | |||||||||
Developed Europe(a) | ||||||||||||
Developed Rest of World(b) | ||||||||||||
Emerging Markets(c) | ||||||||||||
Property, plant and equipment, net | $ | $ | $ | |||||||||
(a) | Developed Europe region includes the following markets: Western Europe, Scandinavian countries and Finland. |
(b) | Developed Rest of World region includes the following markets: Japan, Canada, South Korea, Australia and New Zealand. |
(c) | Emerging Markets region includes, but is not limited to, the following markets: Asia (excluding Japan and South Korea), Latin America, Eastern Europe, Africa, the Middle East, Central Europe and Turkey. |
C. Other Revenue Information
Significant Customers
We sell our biopharmaceutical products primarily to customers in the wholesale sector. In all years presented, our three largest U.S. wholesaler customers are McKesson, Inc., AmerisourceBergen Corporation and Cardinal Health, Inc. In 2019, sales to our three largest U.S. wholesaler customers represented approximately 16 %, 12 % and 10 % of total revenues, respectively, and, collectively, represented approximately 25 % of total trade accounts receivable as of December 31, 2019. In 2018, sales to our three largest U.S. wholesaler customers represented approximately 15 %, 11 % and 10 % of total revenues, respectively, and, collectively, represented approximately 34 % of total trade accounts receivable as of December 31, 2018. In 2017, sales to our three largest U.S. wholesaler customers represented approximately 16 %, 12 % and 10 % of total revenues, respectively, and, collectively, represented approximately 36 % of total trade accounts receivable as of December 31, 2017. For all years presented, these sales and related trade accounts receivable were concentrated in our biopharmaceutical businesses.
Significant Product Revenues
As described in Note 1A, acquisitions impacted our results of operations in 2019 and 2017, the contribution of our Consumer Healthcare business to the GSK Consumer Healthcare joint venture impacted our results of operations in 2019 and divestitures impacted our results of operations in 2017.
130 | 2019 Financial Report | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following table provides detailed revenue information for several of our major products:
(MILLIONS OF DOLLARS) | Year Ended December 31, | |||||||||||||
PRODUCT | PRIMARY INDICATION OR CLASS | 2019 | 2018 | 2017 | ||||||||||
TOTAL REVENUES | $ | $ | $ | |||||||||||
PFIZER BIOPHARMACEUTICALS GROUP (BIOPHARMA) | $ | $ | $ | |||||||||||
Internal Medicine(a) | $ | $ | $ | |||||||||||
Eliquis alliance revenues and direct sales | Nonvalvular Atrial fibrillation, deep vein thrombosis, pulmonary embolism | |||||||||||||
Chantix/Champix | An aid to smoking cessation treatment in adults 18 years of age or older | |||||||||||||
Premarin family | Symptoms of menopause | |||||||||||||
BMP2 | Development of bone and cartilage | |||||||||||||
Toviaz | Overactive bladder | |||||||||||||
All other Internal Medicine | Various | |||||||||||||
Oncology(b) | $ | $ | $ | |||||||||||
Ibrance | Metastatic breast cancer | |||||||||||||
Sutent | Advanced and/or metastatic RCC, adjuvant RCC, refractory GIST (after disease progression on, or intolerance to, imatinib mesylate) and advanced pancreatic neuroendocrine tumor | |||||||||||||
Xtandi alliance revenues | Non-metastatic and metastatic castration-resistant prostate cancer and non-metastatic castration-sensitive prostate cancer | |||||||||||||
Xalkori | ALK-positive and ROS1-positive advanced NSCLC | |||||||||||||
Inlyta | Advanced RCC | |||||||||||||
Bosulif | Philadelphia chromosome–positive chronic myelogenous leukemia | |||||||||||||
Retacrit(c) | Anemia | |||||||||||||
Mektovi | In combination with Braftovi for metastatic melanoma for patients who test positive for a BRAF genetic mutation | |||||||||||||
Braftovi | In combination with Mektovi for metastatic melanoma for patients who test positive for a BRAF genetic mutation | |||||||||||||
All other Oncology | Various | |||||||||||||
Hospital(d) | $ | $ | $ | |||||||||||
Sulperazon | Bacterial infections | |||||||||||||
Medrol(e) | Anti-inflammatory glucocorticoid | |||||||||||||
Vfend | Fungal infections | |||||||||||||
Zithromax(e) | Bacterial infections | |||||||||||||
EpiPen | Epinephrine injection used in treatment of life-threatening allergic reactions | |||||||||||||
Fragmin | Treatment/prevention of venous thromboembolism | |||||||||||||
Zyvox | Bacterial infections | |||||||||||||
Zosyn/Tazocin | Bacterial infections | |||||||||||||
Tygacil | Bacterial infections | |||||||||||||
Diflucan | Fungal infections | |||||||||||||
Panzyga | Primary humoral immunodeficiency | |||||||||||||
Pfizer CentreOne(f) | Various | |||||||||||||
All other Anti-infectives | Various | |||||||||||||
All other Hospital(d) | Various | |||||||||||||
Vaccines | $ | $ | $ | |||||||||||
Prevnar 13/Prevenar 13 | Pneumococcal disease | |||||||||||||
Nimenrix | Meningococcal disease | |||||||||||||
FSME/IMMUN-TicoVac | Tick-borne encephalitis disease | |||||||||||||
Trumenba | Meningococcal disease | |||||||||||||
All other Vaccines | Various | |||||||||||||
Inflammation & Immunology (I&I)(g) | $ | $ | $ | |||||||||||
Xeljanz | RA, PsA, UC | |||||||||||||
Enbrel (Outside the U.S. and Canada) | RA, juvenile idiopathic arthritis, PsA, plaque psoriasis, pediatric plaque psoriasis, ankylosing spondylitis and nonradiographic axial spondyloarthritis | |||||||||||||
Inflectra/Remsima(c), (g) | Crohn’s Disease, Pediatric Crohn’s Disease, UC, Pediatric UC, RA in combination with methotrexate, Ankylosing Spondylitis, PsA and Plaque Psoriasis | |||||||||||||
Eucrisa | Mild-to-moderate atopic dermatitis (eczema) in adults and children 2 years of age and older | |||||||||||||
All other I&I | Various | |||||||||||||
2019 Financial Report | 131 | |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
(MILLIONS OF DOLLARS) | Year Ended December 31, | |||||||||||||
PRODUCT | PRIMARY INDICATION OR CLASS | 2019 | 2018 | 2017 | ||||||||||
Rare Disease | $ | $ | $ | |||||||||||
Genotropin | Replacement of human growth hormone | |||||||||||||
BeneFIX | Hemophilia B | |||||||||||||
Vyndaqel/Vyndamax | ATTR-Cardiomyopathy and Polyneuropathy | |||||||||||||
Refacto AF/Xyntha | Hemophilia A | |||||||||||||
Somavert | Acromegaly | |||||||||||||
All other Rare Disease | Various | |||||||||||||
Upjohn(a) | $ | $ | $ | |||||||||||
Lyrica | Epilepsy, post-herepetic neuralgia and diabetic peripheral neuropathy, fibromyalgia, neuropathic pain due to spinal cord injury | |||||||||||||
Lipitor | Reduction of LDL cholesterol | |||||||||||||
Norvasc | Hypertension | |||||||||||||
Celebrex | Arthritis pain and inflammation, acute pain | |||||||||||||
Viagra | Erectile dysfunction | |||||||||||||
Effexor | Depression and certain anxiety disorders | |||||||||||||
Zoloft | Depression and certain anxiety disorders | |||||||||||||
Xalatan/Xalacom | Glaucoma and ocular hypertension | |||||||||||||
Xanax | Anxiety disorders | |||||||||||||
Revatio | Pulmonary arterial hypertension | |||||||||||||
All other Upjohn | Various | |||||||||||||
Consumer Healthcare Business(h) | $ | $ | $ | |||||||||||
Other(i) | Various | $ | $ | $ | ||||||||||
Total Alliance revenues | Various | $ | $ | $ | ||||||||||
Total Biosimilars(c) | Various | $ | $ | $ | ||||||||||
Total Sterile Injectable Pharmaceuticals(j) | $ | $ | $ | |||||||||||
(a) | We reclassified certain products from the LEP category, including Premarin family products, and certain other products from the legacy Peri-LOE category, including Pristiq, to the Internal Medicine category and reclassified Lyrica from the Internal Medicine category to the Upjohn business to conform 2018 and 2017 product revenues to the current presentation. |
(b) | We performed certain reclassifications in the All other Oncology category to conform 2018 and 2017 product revenues to the current presentation. |
(c) | Biosimilars are highly similar versions of approved and authorized biological medicines and primarily include revenues from Inflectra/Remsima and Retacrit. |
(d) | Hospital is a business unit that commercializes our global portfolio of sterile injectable and anti-infective medicines. We performed certain reclassifications, primarily from the legacy SIP category (Sulperazon, Medrol, Fragmin, Tygacil, Zosyn/Tazocin and Precedex, among other products), the LEP category (Epipen and Zithromax), and the legacy Peri-LOE category (Vfend and Zyvox) to the Hospital category to conform 2018 and 2017 product revenues to the current presentation. Hospital also includes Pfizer CentreOne(f). All other Hospital primarily includes revenues from legacy SIP products (that are not anti-infective products) and, to a much lesser extent, solid oral dose products (that are not anti-infective products). SIP anti-infective products that are not individually listed above are recorded in “All other Anti-infectives”. |
(e) | 2018 and 2017 revenues for Medrol and Zithromax may not agree to previously disclosed revenues because revenues for those products were previously split between LEP and the legacy SIP categories. All revenues for these products are currently reported in the Hospital category. |
(f) | Pfizer CentreOne includes revenues from our contract manufacturing and active pharmaceutical ingredient sales operation, including sterile injectables contract manufacturing, and revenues related to our manufacturing and supply agreements, including with Zoetis Inc. In the fourth quarter of 2017, we sold our equity share in Hisun Pfizer. As a result, effective in the first quarter of 2018, Hisun Pfizer-related revenues, previously reported in emerging markets within legacy All Other LEP and legacy All Other SIP, are reported in emerging markets within Pfizer CentreOne. |
(g) | We reclassified Inflectra/Remsima from the legacy Biosimilars category to the Inflammation & Immunology category to conform 2018 and 2017 product revenues to the current presentation. |
(h) | On July 31, 2019, Pfizer’s Consumer Healthcare business, an over-the-counter medicines business, was combined with GSK’s consumer healthcare business to form a new consumer healthcare joint venture. For additional information, see Note 1A and Note 2C. |
(i) | Represents HIS revenues through February 2, 2017, which includes Medication Management Systems products composed of infusion pumps and related software and services, as well as IV Infusion Products, including large volume IV solutions and their associated administration sets. On February 3, 2017, we completed the sale of HIS to ICU Medical. For additional information, see Note 1A and Note 2B. |
(j) | Sterile Injectable Pharmaceuticals represents the total of all branded and generic injectable products in the Hospital business, including anti-infective sterile injectable pharmaceuticals. |
132 | 2019 Financial Report | |
Selected Quarterly Financial Data (Unaudited)
Pfizer Inc. and Subsidiary Companies
Quarter | ||||||||||||||||
(MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) | First | Second | Third | Fourth | ||||||||||||
2019(a) | ||||||||||||||||
Revenues | $ | 13,118 | $ | 13,264 | $ | 12,680 | $ | 12,688 | ||||||||
Costs and expenses(b) | 8,749 | 9,239 | 9,676 | 13,743 | ||||||||||||
Restructuring charges and certain acquisition-related costs(c), (d) | 46 | (115 | ) | 365 | 452 | |||||||||||
(Gain) on completion of Consumer Healthcare JV transaction(d) | — | — | (8,087 | ) | 1 | |||||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income/(loss) | 4,323 | 4,141 | 10,727 | (1,508 | ) | |||||||||||
Provision/(benefit) for taxes on income/(loss)(e) | 433 | (915 | ) | 3,047 | (1,181 | ) | ||||||||||
Income/(loss) from continuing operations | 3,889 | 5,056 | 7,680 | (326 | ) | |||||||||||
Discontinued operations—net of tax | — | — | 4 | — | ||||||||||||
Net income/(loss) before allocation to noncontrolling interests | 3,889 | 5,056 | 7,684 | (326 | ) | |||||||||||
Less: Net income attributable to noncontrolling interests | 6 | 10 | 4 | 10 | ||||||||||||
Net income/(loss) attributable to Pfizer Inc. | $ | 3,884 | $ | 5,046 | $ | 7,680 | $ | (337 | ) | |||||||
Earnings/(loss) per common share—basic: | ||||||||||||||||
Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | $ | 0.69 | $ | 0.91 | $ | 1.38 | $ | (0.06 | ) | |||||||
Discontinued operations—net of tax | — | — | — | — | ||||||||||||
Net income/(loss) attributable to Pfizer Inc. common shareholders | $ | 0.69 | $ | 0.91 | $ | 1.38 | $ | (0.06 | ) | |||||||
Earnings/(loss) per common share—diluted: | ||||||||||||||||
Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | $ | 0.68 | $ | 0.89 | $ | 1.36 | $ | (0.06 | ) | |||||||
Discontinued operations—net of tax | — | — | — | — | ||||||||||||
Net income/(loss) attributable to Pfizer Inc. common shareholders | $ | 0.68 | $ | 0.89 | $ | 1.36 | $ | (0.06 | ) | |||||||
(a) | As described in Notes to Consolidated Financial Statements—Note 1A. Basis of Presentation and Significant Accounting Policies: Basis of Presentation, acquisitions and the contribution of our Consumer Healthcare business to the GSK Consumer Healthcare joint venture impacted our results of operations in 2019. |
(b) | The fourth quarter historically reflects higher costs in Cost of sales, Selling, informational and administrative expenses and Research and development expenses. The fourth quarter of 2019 includes $2.8 billion in certain asset impairments recorded in Other (income)/deductions—net. For additional information, see Notes to Consolidated Financial Statements—Note 4. Other (Income)/Deductions—Net. |
(c) | The second quarter of 2019 includes the reversal of certain accruals related to our acquisition of Wyeth upon the effective favorable settlement of a U.S. IRS audit from multiple tax years (see Notes to Consolidated Financial Statements—Note 5D. Tax Matters: Tax Contingencies). The third quarter of 2019 includes $217 million of integration costs and other, primarily including $157 million in payments to Array employees for the fair value of previously unvested stock options that was recognized as post-closing compensation expense. The fourth quarter of 2019 primarily includes employee termination costs, asset impairments and other exit costs associated with cost reduction initiatives. The employee termination costs are mostly associated with (i) our improvements to operational effectiveness as part of the realignment of our organizational structure effective at the beginning of 2019 and (ii) our initiatives in connection with transforming to a more focused company. For additional information, see Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives. |
(d) | See Notes to Consolidated Financial Statements—Note 2C. Acquisitions, Divestitures, Equity-Method Investments and Assets and Liabilities Held for Sale, Licensing Arrangements and Research and Development and Collaborative Arrangements: Equity-Method Investments and Assets and Liabilities Held for Sale. |
(e) | During the second quarter of 2019, Pfizer reached settlement of disputed issues at the IRS Office of Appeals, thereby settling all issues related to U.S. tax returns of Pfizer for the years 2009-2010. As a result of settling these years, in the second quarter of 2019 we recorded a benefit of approximately $1.4 billion, representing tax and interest. The third quarter of 2019 reflects tax expense of approximately $2.7 billion associated with the gain related to the completion of the Consumer Healthcare joint venture with GSK. |
Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
2019 Financial Report | 133 | |
Selected Quarterly Financial Data (Unaudited)
Pfizer Inc. and Subsidiary Companies
Quarter | ||||||||||||||||
(MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) | First | Second | Third | Fourth | ||||||||||||
2018 | ||||||||||||||||
Revenues | $ | 12,906 | $ | 13,466 | $ | 13,298 | $ | 13,976 | ||||||||
Costs and expenses(a) | 8,736 | 8,895 | 9,035 | 14,051 | ||||||||||||
Restructuring charges and certain acquisition-related costs(b) | 43 | 44 | 85 | 872 | ||||||||||||
Income/(loss) from continuing operations before provision/(benefit) for taxes on income/(loss) | 4,127 | 4,527 | 4,177 | (946 | ) | |||||||||||
Provision/(benefit) for taxes on income/(loss)(c) | 556 | 648 | 66 | (563 | ) | |||||||||||
Income/(loss) from continuing operations | 3,571 | 3,879 | 4,111 | (383 | ) | |||||||||||
Discontinued operations—net of tax | (1 | ) | — | 11 | — | |||||||||||
Net income/(loss) before allocation to noncontrolling interests | 3,570 | 3,879 | 4,122 | (383 | ) | |||||||||||
Less: Net income attributable to noncontrolling interests | 9 | 7 | 8 | 11 | ||||||||||||
Net income/(loss) attributable to Pfizer Inc. | $ | 3,561 | $ | 3,872 | $ | 4,114 | $ | (394 | ) | |||||||
Earnings/(loss) per common share—basic: | ||||||||||||||||
Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | $ | 0.60 | $ | 0.66 | $ | 0.70 | $ | (0.07 | ) | |||||||
Discontinued operations—net of tax | — | — | — | — | ||||||||||||
Net income/(loss) attributable to Pfizer Inc. common shareholders | $ | 0.60 | $ | 0.66 | $ | 0.70 | $ | (0.07 | ) | |||||||
Earnings/(loss) per common share—diluted: | ||||||||||||||||
Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | $ | 0.59 | $ | 0.65 | $ | 0.69 | $ | (0.07 | ) | |||||||
Discontinued operations—net of tax | — | — | — | — | ||||||||||||
Net income/(loss) attributable to Pfizer Inc. common shareholders | $ | 0.59 | $ | 0.65 | $ | 0.69 | $ | (0.07 | ) | |||||||
(a) | The fourth quarter historically reflects higher costs in Cost of sales, Selling, informational and administrative expenses and Research and development expenses. The fourth quarter of 2018 included $3.1 billion in certain asset impairments recorded in Other (income)/deductions––net. For additional information, see Notes to Consolidated Financial Statements—Note 4. Other (Income)/Deductions—Net. |
(b) | The fourth quarter of 2018 included restructuring charges that were primarily related to employee termination costs and asset write downs. The employee termination costs were associated with our improvements to operational effectiveness as part of the realignment of our organizational structure effective at the beginning of 2019. For additional information, see Notes to Consolidated Financial Statements—Note 3. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives. |
(c) | The third and fourth quarters of 2018 reflect the impact of the TCJA on the Provision/(benefit) for taxes on income/(loss). For additional information, see Notes to Consolidated Financial Statements—Note 5A. Tax Matters: Taxes on Income from Continuing Operations. |
Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
134 | 2019 Financial Report | |
Financial Summary
Pfizer Inc. and Subsidiary Companies
Year Ended/As of December 31,(a) | ||||||||||||||||||||
(MILLIONS, EXCEPT PER COMMON SHARE DATA) | 2019 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||
Revenues | $ | 51,750 | $ | 53,647 | $ | 52,546 | $ | 52,824 | $ | 48,851 | ||||||||||
Income from continuing operations | 16,298 | 11,179 | 21,353 | 7,229 | 6,975 | |||||||||||||||
Total assets | 167,489 | 159,422 | 171,797 | 171,615 | 167,381 | |||||||||||||||
Long-term obligations(b) | 66,739 | 63,807 | 69,714 | 80,660 | 72,985 | |||||||||||||||
Earnings per common share—basic(c) | ||||||||||||||||||||
Income from continuing operations attributable to Pfizer Inc. common shareholders | $ | 2.92 | $ | 1.90 | $ | 3.57 | $ | 1.18 | $ | 1.13 | ||||||||||
Discontinued operations—net of tax | — | — | — | — | — | |||||||||||||||
Net income attributable to Pfizer Inc. common shareholders | $ | 2.92 | $ | 1.90 | $ | 3.57 | $ | 1.18 | $ | 1.13 | ||||||||||
Earnings per common share—diluted(c) | ||||||||||||||||||||
Income from continuing operations attributable to Pfizer Inc. common shareholders | $ | 2.87 | $ | 1.86 | $ | 3.52 | $ | 1.17 | $ | 1.11 | ||||||||||
Discontinued operations—net of tax | — | — | — | — | — | |||||||||||||||
Net income attributable to Pfizer Inc. common shareholders | $ | 2.87 | $ | 1.87 | $ | 3.52 | $ | 1.17 | $ | 1.11 | ||||||||||
Cash dividends declared per common share | $ | 1.46 | $ | 1.38 | $ | 1.30 | $ | 1.22 | $ | 1.14 | ||||||||||
(a) | As described in Notes to Consolidated Financial Statements—Note 1A. Basis of Presentation and Significant Accounting Policies: Basis of Presentation, acquisitions impacted our results of operations in 2019 and 2017, the contribution of our Consumer Healthcare business to the GSK Consumer Healthcare joint venture impacted our results of operations in 2019 and divestitures impacted our results of operations in 2017. 2016 reflects the acquisition of Medivation on September 28, 2016 and the acquisition of Anacor on June 24, 2016, and 2015 reflects the acquisition of Hospira on September 3, 2015. |
(b) | Defined as Long-term debt, Pension benefit obligations, net, Postretirement benefit obligations, net, Noncurrent deferred tax liabilities, Other taxes payable and Other noncurrent liabilities. |
(c) | 2019, 2018 and 2017 reflect the impact of the TCJA on the Provision/(benefit) for taxes on income. For additional information, see Notes to Consolidated Financial Statements—Note 5A. Tax Matters: Taxes on Income from Continuing Operations. |
2019 Financial Report | 135 | |
Peer Group Performance Graph
Pfizer Inc. and Subsidiary Companies
The following graph assumes a $100 investment on December 31, 2014, and reinvestment of all dividends, in each of the Company’s Common Stock, the S&P 500 Index, and a composite peer group of the major U.S. and European-based pharmaceutical companies, which are: AbbVie Inc., Amgen, Inc., AstraZeneca plc, Bristol-Myers Squibb Company, Eli Lilly & Co., GlaxoSmithKline plc, Johnson & Johnson, Merck and Co., Inc., Novartis AG, Roche Holding AG and Sanofi SA.
Five Year Performance
2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |||||||
PFIZER | $100.0 | $107.1 | $111.9 | $129.5 | $161.7 | $150.5 | ||||||
PEER GROUP | $100.0 | $101.6 | $102.4 | $120.0 | $129.8 | $157.7 | ||||||
S&P 500 | $100.0 | $101.4 | $113.5 | $138.3 | $132.2 | $173.8 | ||||||
136 | 2019 Financial Report | |