x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Virginia | 13-3260245 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
6601 West Broad Street, Richmond, Virginia | 23230 |
(Address of principal executive offices) | (Zip Code) |
Title of each class | Name of each exchange on which registered |
Common Stock, $0.33 1/3 par value | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. þ Yes ¨ No |
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ¨ Yes þ No |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days þ Yes ¨ No |
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files) þ Yes ¨ No |
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K þ |
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. |
Large accelerated filer þ Accelerated filer ¨ |
Non-accelerated filer ¨ (Do not check if smaller reporting company) Smaller operating company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). ¨Yes þ No |
Class | Outstanding at February 15, 2013 |
Common Stock, $0.33 1/3 par value | 2,009,855,261 shares |
Portions of the registrant's definitive proxy statement for use in connection with its annual meeting of shareholders to be held on May 16, 2013, to be filed with the Securities and Exchange Commission on or about April 4, 2013 are incorporated by reference into Part III hereof. |
TABLE OF CONTENTS | ||
Page | ||
PART I | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
PART II | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
PART III | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
PART IV | ||
Item 15. | ||
Report of Independent Registered Public Accounting Firm on Financial Statement Schedule | S-1 | |
Valuation and Qualifying Accounts | S-2 | |
2012 | 2011 | 2010 | ||||
Smokeable products | 83.7 | % | 90.5 | % | 84.6 | % |
Smokeless products | 12.5 | 13.6 | 12.1 | |||
Wine | 1.4 | 1.4 | 0.9 | |||
Financial services | 2.4 | (5.5 | ) | 2.4 | ||
100.0 | % | 100.0 | % | 100.0 | % | |
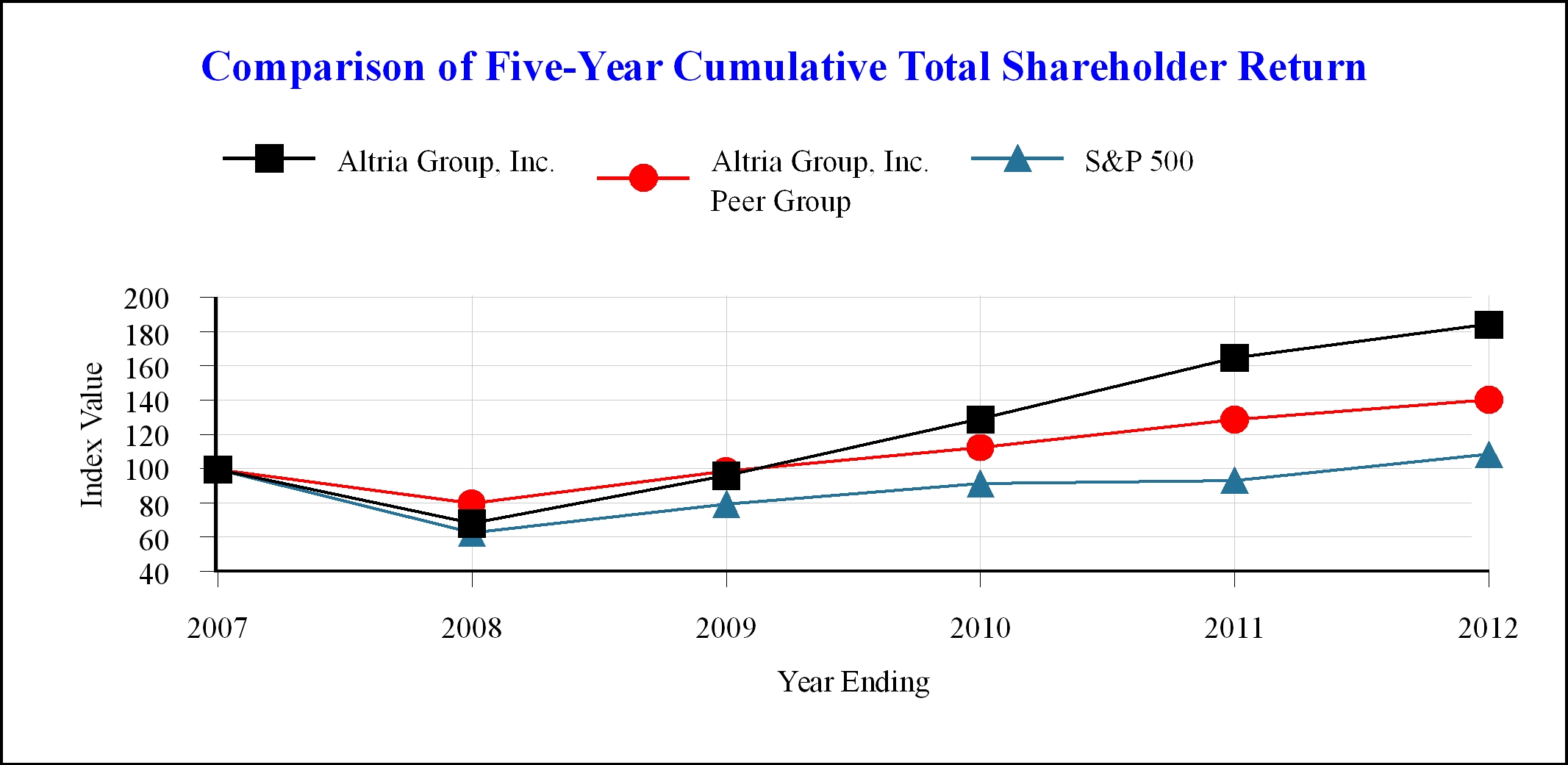
Date | Altria Group, Inc. | Altria Group, Inc. Peer Group | S&P 500 | |||||||||
December 2007 | $ | 100.00 | $ | 100.00 | $ | 100.00 | ||||||
December 2008 | $ | 68.69 | $ | 80.27 | $ | 63.00 | ||||||
December 2009 | $ | 96.38 | $ | 98.98 | $ | 79.67 | ||||||
December 2010 | $ | 129.07 | $ | 112.44 | $ | 91.67 | ||||||
December 2011 | $ | 164.77 | $ | 128.86 | $ | 93.60 | ||||||
December 2012 | $ | 184.17 | $ | 140.34 | $ | 108.58 | ||||||
Period | Total Number of Shares Purchased (1) | Average Price Paid Per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (2) | Approximate Dollar Value of Shares that May Yet be Purchased Under the Plans or Programs (3) | ||||||||||
October 1- October 31, 2012 | 481,227 | $ | 31.93 | 31,840,000 | $ | 534,813,024 | ||||||||
November 1- November 30, 2012 | 8,730,000 | $ | 32.13 | 40,570,000 | $ | 254,316,339 | ||||||||
December 1- December 31, 2012 | 6,052,480 | $ | 32.61 | 46,620,000 | $ | 57,021,354 | ||||||||
For the Quarter Ended December 31, 2012 | 15,263,707 | $ | 32.31 | |||||||||||
(1) | The total number of shares purchased include (a) shares purchased under the October 2011 share repurchase program (which totaled 480,000 shares in October, 8,730,000 shares in November and 6,050,000 shares in December) and (b) shares withheld by Altria Group, Inc. in an amount equal to statutory withholding for employees who vested in restricted and deferred stock and used shares to pay all or a portion of the related taxes, and forfeitures of restricted stock for which consideration was paid in connection with termination of employment of certain employees (which totaled 1,227 shares in October and 2,480 shares in December). |
(2) | Aggregate number of shares purchased under the October 2011 share repurchase program as of the end of the period presented. |
(3) | Reflects the expansion of the October 2011 share repurchase program from $1.0 billion to $1.5 billion, which was authorized by Altria Group, Inc.'s Board of Directors in October 2012. |
2012 | 2011 | 2010 | 2009 | 2008 | |||||||||||||||
Summary of Operations: | |||||||||||||||||||
Net revenues | $ | 24,618 | $ | 23,800 | $ | 24,363 | $ | 23,556 | $ | 19,356 | |||||||||
Cost of sales | 7,937 | 7,680 | 7,704 | 7,990 | 8,270 | ||||||||||||||
Excise taxes on products | 7,118 | 7,181 | 7,471 | 6,732 | 3,399 | ||||||||||||||
Operating income | 7,253 | 6,068 | 6,228 | 5,462 | 4,882 | ||||||||||||||
Interest and other debt expense, net | 1,126 | 1,216 | 1,133 | 1,185 | 167 | ||||||||||||||
Earnings from equity investment in SABMiller | 1,224 | 730 | 628 | 600 | 467 | ||||||||||||||
Earnings from continuing operations before income taxes | 6,477 | 5,582 | 5,723 | 4,877 | 4,789 | ||||||||||||||
Pre-tax profit margin from continuing operations | 26.3 | % | 23.5 | % | 23.5 | % | 20.7 | % | 24.7 | % | |||||||||
Provision for income taxes | 2,294 | 2,189 | 1,816 | 1,669 | 1,699 | ||||||||||||||
Earnings from continuing operations | 4,183 | 3,393 | 3,907 | 3,208 | 3,090 | ||||||||||||||
Earnings from discontinued operations, net of income taxes | — | — | — | — | 1,901 | ||||||||||||||
Net earnings | 4,183 | 3,393 | 3,907 | 3,208 | 4,991 | ||||||||||||||
Net earnings attributable to Altria Group, Inc. | 4,180 | 3,390 | 3,905 | 3,206 | 4,930 | ||||||||||||||
Basic EPS — continuing operations | 2.06 | 1.64 | 1.87 | 1.55 | 1.49 | ||||||||||||||
— discontinued operations | — | — | — | — | 0.88 | ||||||||||||||
— net earnings attributable to Altria Group, Inc. | 2.06 | 1.64 | 1.87 | 1.55 | 2.37 | ||||||||||||||
Diluted EPS — continuing operations | 2.06 | 1.64 | 1.87 | 1.54 | 1.48 | ||||||||||||||
— discontinued operations | — | — | — | — | 0.88 | ||||||||||||||
— net earnings attributable to Altria Group, Inc. | 2.06 | 1.64 | 1.87 | 1.54 | 2.36 | ||||||||||||||
Dividends declared per share | 1.70 | 1.58 | 1.46 | 1.32 | 1.68 | ||||||||||||||
Weighted average shares (millions) — Basic | 2,024 | 2,064 | 2,077 | 2,066 | 2,075 | ||||||||||||||
Weighted average shares (millions) — Diluted | 2,024 | 2,064 | 2,079 | 2,071 | 2,084 | ||||||||||||||
Capital expenditures | 124 | 105 | 168 | 273 | 241 | ||||||||||||||
Depreciation | 205 | 233 | 256 | 271 | 208 | ||||||||||||||
Property, plant and equipment, net (consumer products) | 2,102 | 2,216 | 2,380 | 2,684 | 2,199 | ||||||||||||||
Inventories (consumer products) | 1,746 | 1,779 | 1,803 | 1,810 | 1,069 | ||||||||||||||
Total assets | 35,329 | 36,751 | 37,402 | 36,677 | 27,215 | ||||||||||||||
Total long-term debt | 12,419 | 13,089 | 12,194 | 11,185 | 7,339 | ||||||||||||||
Total debt — consumer products | 13,878 | 13,689 | 12,194 | 11,960 | 6,974 | ||||||||||||||
— financial services | — | — | — | — | 500 | ||||||||||||||
Total stockholders’ equity | 3,170 | 3,683 | 5,195 | 4,072 | 2,828 | ||||||||||||||
Common dividends declared as a % of Basic EPS | 82.5 | % | 96.3 | % | 78.1 | % | 85.2 | % | 70.9 | % | |||||||||
Common dividends declared as a % of Diluted EPS | 82.5 | % | 96.3 | % | 78.1 | % | 85.7 | % | 71.2 | % | |||||||||
Book value per common share outstanding | 1.58 | 1.80 | 2.49 | 1.96 | 1.37 | ||||||||||||||
Market price per common share — high/low | 36.29-28.00 | 30.40-23.20 | 26.22-19.14 | 20.47-14.50 | 79.59-14.34 | ||||||||||||||
Closing price per common share at year end | 31.44 | 29.65 | 24.62 | 19.63 | 15.06 | ||||||||||||||
Price/earnings ratio at year end — Basic | 15 | 18 | 13 | 13 | 6 | ||||||||||||||
Price/earnings ratio at year end — Diluted | 15 | 18 | 13 | 13 | 6 | ||||||||||||||
Number of common shares outstanding at year end (millions) | 2,010 | 2,044 | 2,089 | 2,076 | 2,061 | ||||||||||||||
Approximate number of employees | 9,100 | 9,900 | 10,000 | 10,000 | 10,400 | ||||||||||||||
(in millions, except per share data) | Net Earnings | Diluted EPS | |||||
For the year ended December 31, 2011 | $ | 3,390 | $ | 1.64 | |||
2011 Asset impairment, exit, | |||||||
implementation and integration costs | 142 | 0.07 | |||||
2011 SABMiller special items | 54 | 0.03 | |||||
2011 PMCC leveraged lease charge | 627 | 0.30 | |||||
2011 Tobacco and health judgments | 102 | 0.05 | |||||
2011 UST acquisition-related costs | 5 | — | |||||
2011 Tax items (*) | (77 | ) | (0.04 | ) | |||
Subtotal 2011 special items | 853 | 0.41 | |||||
2012 Asset impairment, exit and implementation costs | (35 | ) | (0.01 | ) | |||
2012 SABMiller special items | 161 | 0.08 | |||||
2012 PMCC leveraged lease benefit | 68 | 0.03 | |||||
2012 Tobacco and health judgments | (4 | ) | — | ||||
2012 Loss on early extinguishment of debt | (559 | ) | (0.28 | ) | |||
2012 Tax items (*) | 66 | 0.03 | |||||
Subtotal 2012 special items | (303 | ) | (0.15 | ) | |||
Fewer shares outstanding | — | 0.04 | |||||
Change in tax rate | (140 | ) | (0.07 | ) | |||
Operations | 380 | 0.19 | |||||
For the year ended December 31, 2012 | $ | 4,180 | $ | 2.06 | |||
▪ | Fewer Shares Outstanding: Fewer shares outstanding during 2012 compared with 2011 were due primarily to shares repurchased by Altria Group, Inc. under its share repurchase programs. |
▪ | Change in Tax Rate: The change in tax rate includes a reduction in certain consolidated tax benefits resulting from the 2012 debt tender offer. |
▪ | Operations: The increase of $380 million in operations shown in the table above was due primarily to the following: |
2013 | 2012 | ||||||
Loss on early extinguishment of debt | $ | — | $ | 0.28 | |||
Asset impairment, exit | |||||||
and implementation costs | — | 0.01 | |||||
SABMiller special items | 0.01 | (0.08 | ) | ||||
PMCC leveraged lease benefit | — | (0.03 | ) | ||||
Tax items* | — | (0.03 | ) | ||||
$ | 0.01 | $ | 0.15 | ||||
(in millions) | Goodwill | Indefinite-Lived Intangible Assets | |||||
Cigarettes | $ | — | $ | 2 | |||
Smokeless products | 5,023 | 8,801 | |||||
Cigars | 77 | 2,640 | |||||
Wine | 74 | 258 | |||||
Total | $ | 5,174 | $ | 11,701 | |||
(in millions) | Carrying Value | Excess Fair Value Over Carrying Value | ||||
Certain smokeless products trademarks, primarily Red Seal and Husky | $ | 921 | 8 | % | ||
Cigars trademarks, primarily Black & Mild | $ | 2,640 | 10 | % | ||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Net Revenues: | |||||||||||
Smokeable products | $ | 22,216 | $ | 21,970 | $ | 22,191 | |||||
Smokeless products | 1,691 | 1,627 | 1,552 | ||||||||
Wine | 561 | 516 | 459 | ||||||||
Financial services | 150 | (313 | ) | 161 | |||||||
Net revenues | $ | 24,618 | $ | 23,800 | $ | 24,363 | |||||
Excise Taxes on Products: | |||||||||||
Smokeable products | $ | 6,984 | $ | 7,053 | $ | 7,348 | |||||
Smokeless products | 113 | 108 | 105 | ||||||||
Wine | 21 | 20 | 18 | ||||||||
Excise taxes on products | $ | 7,118 | $ | 7,181 | $ | 7,471 | |||||
Operating Income: | |||||||||||
Operating companies income (loss): | |||||||||||
Smokeable products | $ | 6,239 | $ | 5,737 | $ | 5,618 | |||||
Smokeless products | 931 | 859 | 803 | ||||||||
Wine | 104 | 91 | 61 | ||||||||
Financial services | 176 | (349 | ) | 157 | |||||||
Amortization of intangibles | (20 | ) | (20 | ) | (20 | ) | |||||
General corporate expenses | (228 | ) | (256 | ) | (216 | ) | |||||
Changes to Mondelēz and | |||||||||||
PMI tax-related receivables | 52 | 14 | (169 | ) | |||||||
Corporate asset impairment | |||||||||||
and exit costs | (1 | ) | (8 | ) | (6 | ) | |||||
Operating income | $ | 7,253 | $ | 6,068 | $ | 6,228 | |||||
For the Year Ended December 31, 2012 | |||||||||||
(in millions) | Asset Impairment and Exit Costs | Implementation (Gain) Costs | Total | ||||||||
Smokeable | |||||||||||
products | $ | 38 | $ | (10 | ) | $ | 28 | ||||
Smokeless | |||||||||||
products | 22 | 6 | 28 | ||||||||
General | |||||||||||
corporate | 1 | (1 | ) | — | |||||||
Total | $ | 61 | $ | (5 | ) | $ | 56 | ||||
For the Year Ended December 31, 2011 | |||||||||||||||
(in millions) | Asset Impairment and Exit Costs | Implementation Costs | Integration Costs | Total | |||||||||||
Smokeable | |||||||||||||||
products | $ | 182 | $ | 1 | $ | — | $ | 183 | |||||||
Smokeless | |||||||||||||||
products | 32 | — | 3 | 35 | |||||||||||
General | |||||||||||||||
corporate | 8 | — | — | 8 | |||||||||||
Total | $ | 222 | $ | 1 | $ | 3 | $ | 226 | |||||||
For the Year Ended December 31, 2010 | |||||||||||||||
(in millions) | Asset Impairment and Exit Costs | Implementation Costs | Integration Costs | Total | |||||||||||
Smokeable | |||||||||||||||
products | $ | 24 | $ | 75 | $ | 2 | $ | 101 | |||||||
Smokeless | |||||||||||||||
products | 6 | — | 16 | 22 | |||||||||||
Wine | — | — | 2 | 2 | |||||||||||
General | |||||||||||||||
corporate | 6 | — | — | 6 | |||||||||||
Total | $ | 36 | $ | 75 | $ | 20 | $ | 131 | |||||||
For the Year Ended December 31, 2012 | For the Year Ended December 31, 2011 | |||||||||||||||||||||||
(in millions) | Net Revenues | Benefit for Income Taxes | Total | Net Revenues | (Benefit) Provision for Income Taxes | Total | ||||||||||||||||||
Reduction to cumulative lease earnings | $ | 7 | $ | (2 | ) | $ | 5 | $ | 490 | $ | (175 | ) | $ | 315 | ||||||||||
Interest on tax underpayments | — | (73 | ) | (73 | ) | — | 312 | 312 | ||||||||||||||||
Total | $ | 7 | $ | (75 | ) | $ | (68 | ) | $ | 490 | $ | 137 | $ | 627 | ||||||||||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Smokeable products | $ | 4 | $ | 98 | $ | 11 | |||||
Smokeless products | — | — | 5 | ||||||||
Interest and other debt | |||||||||||
expense, net | 1 | 64 | 5 | ||||||||
Total | $ | 5 | $ | 162 | $ | 21 | |||||
▪ | FSPTCA and FDA Regulation; |
▪ | Excise Taxes; |
▪ | International Treaty on Tobacco Control; |
▪ | State Settlement Agreements; |
▪ | Other Federal, State and Local Regulation and Activity; |
▪ | Illicit Trade; |
▪ | Tobacco Price, Availability and Quality; and |
▪ | Timing of Sales. |
▪ | impose restrictions at retail; |
▪ | result in increased illicit trade activities; or |
▪ | otherwise significantly increase the cost of doing business. |
▪ | TPSAC |
▪ | The Role of the TPSAC: As required by the FSPTCA, the FDA has established a tobacco product scientific advisory committee (the "TPSAC"), which consists of both voting and non-voting members, to provide advice, reports, information and recommendations to the FDA on scientific and health issues relating to tobacco products. For example, the TPSAC advises the FDA about modified risk products (products marketed with reduced risk claims), good manufacturing practices, the effects of the alteration of nicotine yields from tobacco products and nicotine dependence thresholds. The TPSAC previously made reports and recommendations to the FDA on menthol cigarettes, including the impact of the use of menthol in cigarettes on the public health, and the nature and impact of dissolvable tobacco products on the public health. The FDA may seek advice from the TPSAC about other safety, dependence or health issues relating to tobacco products, including tobacco product standards and applications to market new tobacco products. |
▪ | TPSAC Membership: Beginning in March 2010, PM USA and USSTC raised with the FDA their concerns that four of the voting members of the TPSAC have financial and other conflicts (including services as paid experts for plaintiffs in tobacco litigation) that could hamper the full and fair consideration of issues by the TPSAC and requested that their appointments be withdrawn. The FDA declined PM USA's and USSTC's requests, stating that the FDA had satisfied itself, after inquiry, that the TPSAC members did not have disqualifying conflicts of interest. The FDA stated further that it would continue to screen, in accordance with relevant statutory and regulatory provisions and FDA guidance, all TPSAC members for potential conflicts of interest for matters that the TPSAC would be considering. The FDA also engaged two individuals to serve as consultants to a TPSAC subcommittee who also served as paid experts for plaintiffs in tobacco litigation. PM USA and USSTC raised similar concerns related to the engagement of these individuals and the FDA similarly declined to terminate |
▪ | FDA Regulatory Actions |
▪ | Graphic Warnings: In June 2011, as required by the FSPTCA, the FDA issued its final rule to modify the required warnings that appear on cigarette packages and in cigarette advertisements. The FSPTCA requires the warnings to |
▪ | New Product Marketing Authorization Processes: In January 2011, the FDA issued guidance concerning reports that manufacturers must submit for certain FDA-regulated tobacco products that the manufacturer modified or introduced for the first time into the market after February 15, 2007. These reports must be reviewed by the agency to determine if such tobacco products are "substantially equivalent" to products commercially available as of February 15, 2007. In general, in order to continue marketing these products sold before March 22, 2011, manufacturers of FDA-regulated tobacco products were required to send to the FDA a report demonstrating substantial equivalence by March 22, 2011. PM USA and USSTC submitted timely reports. PM USA and USSTC can continue marketing these products unless the FDA makes a determination that a specific product is not substantially equivalent. If the FDA ultimately makes such a determination, it could require the removal of such products or subject them to the New Product Application Process and, if any such applications are denied, prevent the continued distribution and sale of such products. PM USA and USSTC believe all of their current products meet the statute's requirements, but cannot predict when or how the FDA will respond to their reports. |
▪ | Federal, State and Local Laws |
▪ | State and Local Laws Addressing Certain Characterizing Flavors: In a growing number of states and localities, legislation has been enacted or proposed that prohibits or would prohibit the sale of certain tobacco products with certain characterizing flavors. The legislation varies in terms of the type of tobacco products subject to prohibition, the conditions under which the sale of such products is or would be prohibited, and exceptions to the prohibitions. For example, a number of proposals would prohibit characterizing flavors in smokeless tobacco products, with no exception for mint- or wintergreen-flavored products. |
▪ | State and Local Laws Imposing Certain Speech Requirements and Restrictions: In several jurisdictions, legislation or regulations have been enacted or proposed that would require the disclosure of health information separate from or in addition to federally-mandated health warnings or that would restrict commercial speech in certain respects. For example, in July 2012, the United States Court of Appeals for the Second Circuit determined that an effort by New York City to require retailers selling tobacco products to display a sign, issued by the New York City Board of Health, depicting graphic images of the potential health consequences of smoking and urging smokers to quit was preempted by federal law. This litigation has concluded. |
▪ | Federal Tobacco Quota Buy-Out: In October 2004, FETRA was signed into law. PM USA, Middleton and USSTC are subject to the requirements of FETRA. FETRA eliminated the federal tobacco quota and price support program through an industry-funded buy-out of tobacco growers and quota holders. The cost of the buy-out is approximately $9.5 billion and is being paid over 10 years ending in 2014 by manufacturers and importers of each kind of tobacco product. The cost is being allocated based on the relative market shares of manufacturers and importers of each kind of tobacco product. |
▪ | Health Effects of Tobacco Consumption and Exposure to Environmental Tobacco Smoke ("ETS"): It is the policy of Altria Group, Inc. and its tobacco subsidiaries to defer to the judgment of public health authorities as to the content of warnings in advertisements and on product packaging regarding the health effects of tobacco consumption, addiction and exposure to ETS. Altria Group, Inc. and its tobacco subsidiaries believe that the public should be guided by the messages of the United States Surgeon General and public health authorities worldwide in making decisions concerning the use of tobacco products. |
For the Years Ended December 31, | |||||||||||||||||||||||
Net Revenues | Operating Companies Income | ||||||||||||||||||||||
(in millions) | 2012 | 2011 | 2010 | 2012 | 2011 | 2010 | |||||||||||||||||
Smokeable products | $ | 22,216 | $ | 21,970 | $ | 22,191 | $ | 6,239 | $ | 5,737 | $ | 5,618 | |||||||||||
Smokeless products | 1,691 | 1,627 | 1,552 | 931 | 859 | 803 | |||||||||||||||||
Total tobacco space | $ | 23,907 | $ | 23,597 | $ | 23,743 | $ | 7,170 | $ | 6,596 | $ | 6,421 | |||||||||||
Shipment Volume | ||||||||
For the Years Ended December 31, | ||||||||
(sticks in millions) | 2012 | 2011 | 2010 | |||||
Cigarettes: | ||||||||
Marlboro | 116,377 | 117,201 | 121,893 | |||||
Other premium | 8,629 | 9,381 | 10,315 | |||||
Discount | 9,868 | 8,556 | 8,630 | |||||
Total cigarettes | 134,874 | 135,138 | 140,838 | |||||
Cigars: | ||||||||
Black & Mild | 1,219 | 1,226 | 1,222 | |||||
Other | 18 | 20 | 24 | |||||
Total cigars | 1,237 | 1,246 | 1,246 | |||||
Total smokeable products | 136,111 | 136,384 | 142,084 | |||||
Retail Share | ||||||||
For the Years Ended December 31, | ||||||||
2012 | 2011 | 2010 | ||||||
Cigarettes: | ||||||||
Marlboro | 42.6 | % | 42.0 | % | 42.6 | % | ||
Other premium | 3.4 | 3.7 | 3.9 | |||||
Discount | 3.8 | 3.3 | 3.3 | |||||
Total cigarettes | 49.8 | % | 49.0 | % | 49.8 | % | ||
Cigars: | ||||||||
Black & Mild | 30.0 | % | 29.5 | % | 29.0 | % | ||
Other | 0.2 | 0.2 | 0.4 | |||||
Total cigars | 30.2 | % | 29.7 | % | 29.4 | % | ||
Shipment Volume For the Years Ended December 31, | ||||||||
(cans and packs in millions) | 2012 | 2011 | 2010 | |||||
Copenhagen | 392.5 | 354.2 | 327.5 | |||||
Skoal | 288.4 | 286.8 | 274.4 | |||||
Copenhagen and Skoal | 680.9 | 641.0 | 601.9 | |||||
Other | 82.4 | 93.6 | 122.5 | |||||
Total smokeless products | 763.3 | 734.6 | 724.4 | |||||
Retail Share For the Years Ended December 31, | ||||||||
2012 | 2011 | 2010 | ||||||
Copenhagen | 28.4 | % | 26.2 | % | 24.7 | % | ||
Skoal | 22.2 | 22.8 | 23.3 | |||||
Copenhagen and Skoal | 50.6 | 49.0 | 48.0 | |||||
Other | 4.8 | 6.1 | 7.2 | |||||
Total smokeless products | 55.4 | % | 55.1 | % | 55.2 | % | ||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Net revenues | $ | 561 | $ | 516 | $ | 459 | |||||
Operating companies income | $ | 104 | $ | 91 | $ | 61 | |||||
Shipment Volume For the Years Ended December 31, | ||||||||
(cases in thousands) | 2012 | 2011 | 2010 | |||||
Chateau Ste. Michelle | 2,780 | 2,522 | 2,338 | |||||
Columbia Crest | 1,716 | 2,055 | 2,054 | |||||
Other | 3,093 | 2,744 | 2,289 | |||||
Total wine | 7,589 | 7,321 | 6,681 | |||||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Net revenues | $ | 150 | $ | (313 | ) | $ | 161 | ||||
Operating companies income | $ | 176 | $ | (349 | ) | $ | 157 | ||||
▪ | higher dividends paid during 2012; |
▪ | higher debt issuances during 2012; and |
▪ | lower share repurchases during 2012. |
Short-term Debt | Long-term Debt | Outlook | |||
Moody’s | P-2 | Baa1 | Stable | ||
Standard & Poor’s | A-2 | BBB | Stable | ||
Fitch | F2 | BBB+ | Stable | ||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Average daily short-term borrowings | $ | 8 | $ | 68 | $ | 186 | |||||
Peak short-term borrowings outstanding | $ | 190 | $ | 865 | $ | 1,419 | |||||
Weighted-average interest rate on short-term borrowings | 0.42 | % | 0.40 | % | 0.39 | % | |||||
Payments Due | |||||||||||||||||||
(in millions) | Total | 2013 | 2014 - 2015 | 2016 - 2017 | 2018 and Thereafter | ||||||||||||||
Long-term debt (1) | $ | 13,926 | $ | 1,459 | $ | 1,525 | $ | — | $ | 10,942 | |||||||||
Interest on borrowings (2) | 12,689 | 1,006 | 1,704 | 1,601 | 8,378 | ||||||||||||||
Operating leases (3) | 318 | 55 | 91 | 58 | 114 | ||||||||||||||
Purchase obligations: (4) | |||||||||||||||||||
Inventory and production costs | 1,940 | 773 | 618 | 283 | 266 | ||||||||||||||
Other | 836 | 482 | 269 | 74 | 11 | ||||||||||||||
2,776 | 1,255 | 887 | 357 | 277 | |||||||||||||||
Other long-term liabilities (5) | 3,158 | 510 | 370 | 470 | 1,808 | ||||||||||||||
$ | 32,867 | $ | 4,285 | $ | 4,577 | $ | 2,486 | $ | 21,519 | ||||||||||
at December 31, | 2012 | 2011 | |||||
Assets | |||||||
Consumer products | |||||||
Cash and cash equivalents | $ | 2,900 | $ | 3,270 | |||
Receivables | 193 | 268 | |||||
Inventories: | |||||||
Leaf tobacco | 876 | 934 | |||||
Other raw materials | 173 | 170 | |||||
Work in process | 349 | 316 | |||||
Finished product | 348 | 359 | |||||
1,746 | 1,779 | ||||||
Deferred income taxes | 1,216 | 1,207 | |||||
Other current assets | 260 | 396 | |||||
Total current assets | 6,315 | 6,920 | |||||
Property, plant and equipment, at cost: | |||||||
Land and land improvements | 292 | 290 | |||||
Buildings and building equipment | 1,276 | 1,271 | |||||
Machinery and equipment | 3,068 | 3,097 | |||||
Construction in progress | 114 | 70 | |||||
4,750 | 4,728 | ||||||
Less accumulated depreciation | 2,648 | 2,512 | |||||
2,102 | 2,216 | ||||||
Goodwill | 5,174 | 5,174 | |||||
Other intangible assets, net | 12,078 | 12,098 | |||||
Investment in SABMiller | 6,637 | 5,509 | |||||
Other assets | 425 | 1,257 | |||||
Total consumer products assets | 32,731 | 33,174 | |||||
Financial services | |||||||
Finance assets, net | 2,581 | 3,559 | |||||
Other assets | 17 | 18 | |||||
Total financial services assets | 2,598 | 3,577 | |||||
Total Assets | $ | 35,329 | $ | 36,751 | |||
at December 31, | 2012 | 2011 | |||||
Liabilities | |||||||
Consumer products | |||||||
Current portion of long-term debt | $ | 1,459 | $ | 600 | |||
Accounts payable | 451 | 503 | |||||
Accrued liabilities: | |||||||
Marketing | 568 | 430 | |||||
Employment costs | 184 | 225 | |||||
Settlement charges | 3,616 | 3,513 | |||||
Other | 1,085 | 1,320 | |||||
Dividends payable | 888 | 841 | |||||
Total current liabilities | 8,251 | 7,432 | |||||
Long-term debt | 12,419 | 13,089 | |||||
Deferred income taxes | 4,953 | 4,751 | |||||
Accrued pension costs | 1,735 | 1,662 | |||||
Accrued postretirement health care costs | 2,504 | 2,359 | |||||
Other liabilities | 556 | 602 | |||||
Total consumer products liabilities | 30,418 | 29,895 | |||||
Financial services | |||||||
Deferred income taxes | 1,699 | 2,811 | |||||
Other liabilities | 8 | 330 | |||||
Total financial services liabilities | 1,707 | 3,141 | |||||
Total liabilities | 32,125 | 33,036 | |||||
Contingencies (Note 18) | |||||||
Redeemable noncontrolling interest | 34 | 32 | |||||
Stockholders' Equity | |||||||
Common stock, par value $0.33 1/3 per share (2,805,961,317 shares issued) | 935 | 935 | |||||
Additional paid-in capital | 5,688 | 5,674 | |||||
Earnings reinvested in the business | 24,316 | 23,583 | |||||
Accumulated other comprehensive losses | (2,040 | ) | (1,887 | ) | |||
Cost of repurchased stock (796,221,021 shares in 2012 and 761,542,032 shares in 2011) | (25,731 | ) | (24,625 | ) | |||
Total stockholders' equity attributable to Altria Group, Inc. | 3,168 | 3,680 | |||||
Noncontrolling interests | 2 | 3 | |||||
Total stockholders' equity | 3,170 | 3,683 | |||||
Total Liabilities and Stockholders' Equity | $ | 35,329 | $ | 36,751 | |||
for the years ended December 31, | 2012 | 2011 | 2010 | ||||||||
Net revenues | $ | 24,618 | $ | 23,800 | $ | 24,363 | |||||
Cost of sales | 7,937 | 7,680 | 7,704 | ||||||||
Excise taxes on products | 7,118 | 7,181 | 7,471 | ||||||||
Gross profit | 9,563 | 8,939 | 9,188 | ||||||||
Marketing, administration and research costs | 2,281 | 2,643 | 2,735 | ||||||||
Changes to Mondelēz and PMI tax-related receivables | (52 | ) | (14 | ) | 169 | ||||||
Asset impairment and exit costs | 61 | 222 | 36 | ||||||||
Amortization of intangibles | 20 | 20 | 20 | ||||||||
Operating income | 7,253 | 6,068 | 6,228 | ||||||||
Interest and other debt expense, net | 1,126 | 1,216 | 1,133 | ||||||||
Loss on early extinguishment of debt | 874 | — | — | ||||||||
Earnings from equity investment in SABMiller | (1,224 | ) | (730 | ) | (628 | ) | |||||
Earnings before income taxes | 6,477 | 5,582 | 5,723 | ||||||||
Provision for income taxes | 2,294 | 2,189 | 1,816 | ||||||||
Net earnings | 4,183 | 3,393 | 3,907 | ||||||||
Net earnings attributable to noncontrolling interests | (3 | ) | (3 | ) | (2 | ) | |||||
Net earnings attributable to Altria Group, Inc. | $ | 4,180 | $ | 3,390 | $ | 3,905 | |||||
Per share data: | |||||||||||
Basic earnings per share attributable to Altria Group, Inc. | $ | 2.06 | $ | 1.64 | $ | 1.87 | |||||
Diluted earnings per share attributable to Altria Group, Inc. | $ | 2.06 | $ | 1.64 | $ | 1.87 | |||||
for the years ended December 31, | 2012 | 2011 | 2010 | |||||||||
Net earnings | $ | 4,183 | $ | 3,393 | $ | 3,907 | ||||||
Other comprehensive (losses) earnings, net of deferred income taxes: | ||||||||||||
Currency translation adjustments | — | (2 | ) | 1 | ||||||||
Benefit plans: | ||||||||||||
Actuarial losses and prior service cost/credit before reclassifications to net earnings | (500 | ) | (385 | ) | (64 | ) | ||||||
Amounts reclassified to net earnings | 148 | 134 | 99 | |||||||||
(352 | ) | (251 | ) | 35 | ||||||||
SABMiller: | ||||||||||||
Ownership share of SABMiller's other comprehensive earnings (losses) before reclassifications to net earnings | 197 | (162 | ) | 32 | ||||||||
Amounts reclassified to net earnings | 2 | 12 | 9 | |||||||||
199 | (150 | ) | 41 | |||||||||
Other comprehensive (losses) earnings, net of deferred income taxes | (153 | ) | (403 | ) | 77 | |||||||
Comprehensive earnings | 4,030 | 2,990 | 3,984 | |||||||||
Comprehensive earnings attributable to noncontrolling interests | (3 | ) | (3 | ) | (2 | ) | ||||||
Comprehensive earnings attributable to Altria Group, Inc. | $ | 4,027 | $ | 2,987 | $ | 3,982 | ||||||
for the years ended December 31, | 2012 | 2011 | 2010 | ||||||||||
Cash Provided by (Used in) Operating Activities | |||||||||||||
Net earnings (loss) | — Consumer products | $ | 4,006 | $ | 3,905 | $ | 3,819 | ||||||
— Financial services | 177 | (512 | ) | 88 | |||||||||
Net earnings | 4,183 | 3,393 | 3,907 | ||||||||||
Adjustments to reconcile net earnings to operating cash flows: | |||||||||||||
Consumer products | |||||||||||||
Depreciation and amortization | 225 | 253 | 276 | ||||||||||
Deferred income tax provision | 406 | 382 | 408 | ||||||||||
Earnings from equity investment in SABMiller | (1,224 | ) | (730 | ) | (628 | ) | |||||||
Dividends from SABMiller | 402 | 357 | 303 | ||||||||||
Asset impairment and exit costs, net of cash paid | (73 | ) | 179 | (188 | ) | ||||||||
IRS payment related to LILO and SILO transactions | (456 | ) | — | (945 | ) | ||||||||
Loss on early extinguishment of debt | 874 | — | — | ||||||||||
Cash effects of changes: | |||||||||||||
Receivables, net | 202 | (19 | ) | 15 | |||||||||
Inventories | 33 | 24 | 7 | ||||||||||
Accounts payable | 5 | (60 | ) | 48 | |||||||||
Income taxes | (449 | ) | (151 | ) | (53 | ) | |||||||
Accrued liabilities and other current assets | (14 | ) | 21 | (221 | ) | ||||||||
Accrued settlement charges | 103 | (22 | ) | (100 | ) | ||||||||
Pension plan contributions | (557 | ) | (240 | ) | (30 | ) | |||||||
Pension provisions and postretirement, net | 192 | 243 | 185 | ||||||||||
Other | 126 | 47 | 96 | ||||||||||
Financial services | |||||||||||||
Deferred income tax benefit | (1,335 | ) | (825 | ) | (284 | ) | |||||||
PMCC leveraged lease charges | 7 | 490 | — | ||||||||||
Net (decrease) increase to allowance for losses | (10 | ) | 25 | — | |||||||||
Other liabilities - income taxes | 1,332 | 298 | (5 | ) | |||||||||
Other | (69 | ) | (52 | ) | (24 | ) | |||||||
Net cash provided by operating activities | 3,903 | 3,613 | 2,767 | ||||||||||
for the years ended December 31, | 2012 | 2011 | 2010 | ||||||||||
Cash Provided by (Used in) Investing Activities | |||||||||||||
Consumer products | |||||||||||||
Capital expenditures | $ | (124 | ) | $ | (105 | ) | $ | (168 | ) | ||||
Other | (5 | ) | 2 | 115 | |||||||||
Financial services | |||||||||||||
Proceeds from finance assets | 1,049 | 490 | 312 | ||||||||||
Net cash provided by investing activities | 920 | 387 | 259 | ||||||||||
Cash Provided by (Used in) Financing Activities | |||||||||||||
Consumer products | |||||||||||||
Long-term debt issued | 2,787 | 1,494 | 1,007 | ||||||||||
Long-term debt repaid | (2,600 | ) | — | (775 | ) | ||||||||
Repurchases of common stock | (1,082 | ) | (1,327 | ) | — | ||||||||
Dividends paid on common stock | (3,400 | ) | (3,222 | ) | (2,958 | ) | |||||||
Issuances of common stock | — | 29 | 104 | ||||||||||
Financing fees and debt issuance costs | (22 | ) | (24 | ) | (6 | ) | |||||||
Tender premiums and fees related to early extinguishment of debt | (864 | ) | — | — | |||||||||
Other | (12 | ) | 6 | 45 | |||||||||
Net cash used in financing activities | (5,193 | ) | (3,044 | ) | (2,583 | ) | |||||||
Cash and cash equivalents: | |||||||||||||
(Decrease) Increase | (370 | ) | 956 | 443 | |||||||||
Balance at beginning of year | 3,270 | 2,314 | 1,871 | ||||||||||
Balance at end of year | $ | 2,900 | $ | 3,270 | $ | 2,314 | |||||||
Cash paid: Interest | $ | 1,219 | $ | 1,154 | $ | 1,084 | |||||||
Income taxes | $ | 3,338 | $ | 2,865 | $ | 1,884 | |||||||
Attributable to Altria Group, Inc. | |||||||||||||||||||||||||||
Common Stock | Additional Paid-in Capital | Earnings Reinvested in the Business | Accumulated Other Comprehensive Losses | Cost of Repurchased Stock | Non- controlling Interests | Total Stockholders’ Equity | |||||||||||||||||||||
Balances, December 31, 2009 | $ | 935 | $ | 5,997 | $ | 22,599 | $ | (1,561 | ) | $ | (23,901 | ) | $ | 3 | $ | 4,072 | |||||||||||
Net earnings (a) | — | — | 3,905 | — | — | 1 | 3,906 | ||||||||||||||||||||
Other comprehensive earnings, net | |||||||||||||||||||||||||||
of deferred income taxes | — | — | — | 77 | — | — | 77 | ||||||||||||||||||||
Exercise of stock options and other | |||||||||||||||||||||||||||
stock award activity | — | (246 | ) | — | — | 432 | — | 186 | |||||||||||||||||||
Cash dividends declared ($1.46 per share) | — | — | (3,045 | ) | — | — | — | (3,045 | ) | ||||||||||||||||||
Other | — | — | — | — | — | (1 | ) | (1 | ) | ||||||||||||||||||
Balances, December 31, 2010 | 935 | 5,751 | 23,459 | (1,484 | ) | (23,469 | ) | 3 | 5,195 | ||||||||||||||||||
Net earnings (a) | — | — | 3,390 | — | — | 1 | 3,391 | ||||||||||||||||||||
Other comprehensive losses, net | |||||||||||||||||||||||||||
of deferred income tax benefit | — | — | — | (403 | ) | — | — | (403 | ) | ||||||||||||||||||
Exercise of stock options and other | |||||||||||||||||||||||||||
stock award activity | — | (77 | ) | — | — | 171 | — | 94 | |||||||||||||||||||
Cash dividends declared ($1.58 per share) | — | — | (3,266 | ) | — | — | — | (3,266 | ) | ||||||||||||||||||
Repurchases of common stock | — | — | — | — | (1,327 | ) | — | (1,327 | ) | ||||||||||||||||||
Other | — | — | — | — | — | (1 | ) | (1 | ) | ||||||||||||||||||
Balances, December 31, 2011 | 935 | 5,674 | 23,583 | (1,887 | ) | (24,625 | ) | 3 | 3,683 | ||||||||||||||||||
Net earnings (a) | — | — | 4,180 | — | — | — | 4,180 | ||||||||||||||||||||
Other comprehensive losses, net | |||||||||||||||||||||||||||
of deferred income tax benefit | — | — | — | (153 | ) | — | — | (153 | ) | ||||||||||||||||||
Exercise of stock options and other | |||||||||||||||||||||||||||
stock award activity | — | 14 | — | — | 10 | — | 24 | ||||||||||||||||||||
Cash dividends declared ($1.70 per share) | — | — | (3,447 | ) | — | — | — | (3,447 | ) | ||||||||||||||||||
Repurchases of common stock | — | — | — | — | (1,116 | ) | — | (1,116 | ) | ||||||||||||||||||
Other | — | — | — | — | — | (1 | ) | (1 | ) | ||||||||||||||||||
Balances, December 31, 2012 | $ | 935 | $ | 5,688 | $ | 24,316 | $ | (2,040 | ) | $ | (25,731 | ) | $ | 2 | $ | 3,170 | |||||||||||
▪ | Dividends and Share Repurchases: During the third quarter of 2012, Altria Group, Inc.'s Board of Directors approved a 7.3% increase in the quarterly dividend rate to $0.44 per common share versus the previous rate of $0.41 per common share. The current annualized dividend rate is $1.76 per Altria Group, Inc. common share. Future dividend payments remain subject to the discretion of Altria Group, Inc.'s Board of Directors. |
Level 1 | Unadjusted quoted prices in active markets for identical assets or liabilities. |
Level 2 | Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
Level 3 | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
Goodwill | Other Intangible Assets, net | ||||||||||||||
(in millions) | December 31, 2012 | December 31, 2011 | December 31, 2012 | December 31, 2011 | |||||||||||
Smokeable products | $ | 77 | $ | 77 | $ | 2,971 | $ | 2,988 | |||||||
Smokeless products | 5,023 | 5,023 | 8,839 | 8,841 | |||||||||||
Wine | 74 | 74 | 268 | 269 | |||||||||||
Total | $ | 5,174 | $ | 5,174 | $ | 12,078 | $ | 12,098 | |||||||
December 31, 2012 | December 31, 2011 | ||||||||||||||
(in millions) | Gross Carrying Amount | Accumulated Amortization | Gross Carrying Amount | Accumulated Amortization | |||||||||||
Indefinite-lived intangible assets | $ | 11,701 | $ | — | $ | 11,701 | $ | — | |||||||
Definite-lived intangible assets | 464 | 87 | 464 | 67 | |||||||||||
Total other intangible assets | $ | 12,165 | $ | 87 | $ | 12,165 | $ | 67 | |||||||
For the Year Ended December 31, 2012 | |||||||||||
(in millions) | Asset Impairment and Exit Costs | Implementation (Gain) Costs | Total | ||||||||
Smokeable products | $ | 38 | $ | (10 | ) | $ | 28 | ||||
Smokeless products | 22 | 6 | 28 | ||||||||
General corporate | 1 | (1 | ) | — | |||||||
Total | $ | 61 | $ | (5 | ) | $ | 56 | ||||
For the Year Ended December 31, 2011 | |||||||||||||||
(in millions) | Asset Impairment and Exit Costs | Implementation Costs | Integration Costs | Total | |||||||||||
Smokeable products | $ | 182 | $ | 1 | $ | — | $ | 183 | |||||||
Smokeless products | 32 | — | 3 | 35 | |||||||||||
General corporate | 8 | — | — | 8 | |||||||||||
Total | $ | 222 | $ | 1 | $ | 3 | $ | 226 | |||||||
For the Year Ended December 31, 2010 | |||||||||||||||
(in millions) | Asset Impairment and Exit Costs | Implementation Costs | Integration Costs | Total | |||||||||||
Smokeable products | $ | 24 | $ | 75 | $ | 2 | $ | 101 | |||||||
Smokeless products | 6 | — | 16 | 22 | |||||||||||
Wine | — | — | 2 | 2 | |||||||||||
General corporate | 6 | — | — | 6 | |||||||||||
Total | $ | 36 | $ | 75 | $ | 20 | $ | 131 | |||||||
(in millions) | Severance | Other | Total | ||||||||
Severance liability balance, December 31, 2010 | $ | 26 | $ | — | $ | 26 | |||||
Charges, net | 154 | 68 | 222 | ||||||||
Cash spent | (24 | ) | (20 | ) | (44 | ) | |||||
Other | — | (48 | ) | (48 | ) | ||||||
Severance liability balance, December 31, 2011 | 156 | — | 156 | ||||||||
Charges, net | (7 | ) | 68 | 61 | |||||||
Cash spent | (112 | ) | (22 | ) | (134 | ) | |||||
Other | — | (46 | ) | (46 | ) | ||||||
Severance liability balance, December 31, 2012 | $ | 37 | $ | — | $ | 37 | |||||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Equity earnings | $ | 1,181 | $ | 703 | $ | 578 | |||||
Gains resulting from issuances | |||||||||||
of common stock | |||||||||||
by SABMiller | 43 | 27 | 50 | ||||||||
$ | 1,224 | $ | 730 | $ | 628 | ||||||
At December 31, | |||||||
(in millions) | 2012 | 2011 | |||||
Current assets | $ | 5,742 | $ | 5,967 | |||
Long-term assets | $ | 51,733 | $ | 46,438 | |||
Current liabilities | $ | 8,944 | $ | 7,591 | |||
Long-term liabilities | $ | 22,000 | $ | 22,521 | |||
Non-controlling interests | $ | 1,105 | $ | 1,013 | |||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Net revenues | $ | 23,449 | $ | 20,780 | $ | 18,981 | |||||
Operating profit | $ | 5,243 | $ | 3,603 | $ | 2,821 | |||||
Net earnings | $ | 4,362 | $ | 2,596 | $ | 2,133 | |||||
(in millions) | For the Year Ended December 31, 2012 | For the Year Ended December 31, 2011 | ||||||||||||||||||||||
Net Revenues | Benefit for Income Taxes | Total | Net Revenues | (Benefit) Provision for Income Taxes | Total | |||||||||||||||||||
Reduction to cumulative lease earnings | $ | 7 | $ | (2 | ) | $ | 5 | $ | 490 | $ | (175 | ) | $ | 315 | ||||||||||
Interest on tax underpayments | — | (73 | ) | (73 | ) | — | 312 | 312 | ||||||||||||||||
Total | $ | 7 | $ | (75 | ) | $ | (68 | ) | $ | 490 | $ | 137 | $ | 627 | ||||||||||
Leveraged Leases | Direct Finance Leases | Total | |||||||||||||||||||||
(in millions) | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | |||||||||||||||||
Rents receivable, net | $ | 2,378 | $ | 3,926 | $ | 116 | $ | 162 | $ | 2,494 | $ | 4,088 | |||||||||||
Unguaranteed residual values | 1,068 | 1,306 | 87 | 86 | 1,155 | 1,392 | |||||||||||||||||
Unearned income | (968 | ) | (1,692 | ) | (1 | ) | (2 | ) | (969 | ) | (1,694 | ) | |||||||||||
Investments in finance leases | 2,478 | 3,540 | 202 | 246 | 2,680 | 3,786 | |||||||||||||||||
Deferred income taxes | (1,654 | ) | (2,793 | ) | (89 | ) | (107 | ) | (1,743 | ) | (2,900 | ) | |||||||||||
Net investments in finance leases | $ | 824 | $ | 747 | $ | 113 | $ | 139 | $ | 937 | $ | 886 | |||||||||||
(in millions) | Leveraged Leases | Direct Finance Leases | Total | ||||||||
2013 | $ | 92 | $ | 45 | $ | 137 | |||||
2014 | 136 | 45 | 181 | ||||||||
2015 | 275 | — | 275 | ||||||||
2016 | 99 | — | 99 | ||||||||
2017 | 151 | — | 151 | ||||||||
Thereafter | 1,625 | 26 | 1,651 | ||||||||
Total | $ | 2,378 | $ | 116 | $ | 2,494 | |||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Balance at beginning of year | $ | 227 | $ | 202 | $ | 266 | |||||
(Decrease) increase to allowance | (10 | ) | 25 | — | |||||||
Amounts written-off | (118 | ) | — | (64 | ) | ||||||
Balance at end of year | $ | 99 | $ | 227 | $ | 202 | |||||
(in millions) | 2012 | 2011 | |||||
Credit Rating by Standard & Poor’s/Moody’s: | |||||||
“AAA/Aaa” to “A-/A3” | $ | 961 | $ | 1,570 | |||
“BBB+/Baa1” to “BBB-/Baa3” | 938 | 1,080 | |||||
“BB+/Ba1” and Lower | 781 | 1,136 | |||||
Total | $ | 2,680 | $ | 3,786 | |||
(in millions) | 2012 | 2011 | |||||
Notes, 2.85% to 10.20%, interest payable semi-annually (average coupon interest rate 7.2%), due through 2042 | $ | 13,836 | $ | 13,647 | |||
Debenture, 7.75% due 2027, interest payable semi-annually | 42 | 42 | |||||
13,878 | 13,689 | ||||||
Less current portion of long-term debt | 1,459 | 600 | |||||
$ | 12,419 | $ | 13,089 | ||||
(in millions) | Altria Group, Inc. | UST | Total Long-Term Debt | ||||||||
2013 | $ | 1,459 | $ | — | $ | 1,459 | |||||
2014 | 525 | — | 525 | ||||||||
2015 | 1,000 | — | 1,000 | ||||||||
2018 | 1,949 | 300 | 2,249 | ||||||||
2019 | 1,351 | — | 1,351 | ||||||||
Thereafter | 7,342 | — | 7,342 | ||||||||
▪ | Tender Offer for Altria Group, Inc. Senior Notes: During the third quarter of 2012, Altria Group, Inc. completed a tender offer to purchase for cash $2.0 billion aggregate principal amount of certain of its senior unsecured notes. Altria Group, Inc. repurchased $1,151 million aggregate principal amount of its 9.70% notes due 2018, and $849 million aggregate principal amount of its 9.25% notes due 2019. As a result of the tender offer, during the third quarter of 2012, Altria Group, Inc. recorded a pre-tax loss on early extinguishment of debt of $874 million, which included debt tender premiums and fees of $864 million and the write-off of related unamortized debt discounts and debt issuance costs of $10 million. |
Shares Issued | Shares Repurchased | Shares Outstanding | ||||||
Balances, December 31, 2009 | 2,805,961,317 | (729,932,673 | ) | 2,076,028,644 | ||||
Exercise of stock options and issuance of other stock-based awards | — | 12,711,022 | 12,711,022 | |||||
Balances, December 31, 2010 | 2,805,961,317 | (717,221,651 | ) | 2,088,739,666 | ||||
Exercise of stock options and issuance of other stock-based awards | — | 5,004,502 | 5,004,502 | |||||
Repurchases of common stock | — | (49,324,883 | ) | (49,324,883 | ) | |||
Balances, December 31, 2011 | 2,805,961,317 | (761,542,032 | ) | 2,044,419,285 | ||||
Exercise of stock options and issuance of other stock-based awards | — | 181,011 | 181,011 | |||||
Repurchases of common stock | — | (34,860,000 | ) | (34,860,000 | ) | |||
Balances, December 31, 2012 | 2,805,961,317 | (796,221,021 | ) | 2,009,740,296 | ||||
Number of Shares | Weighted-Average Grant Date Fair Value Per Share | |||||
Balance at December 31, 2011 | 8,410,416 | $ | 20.17 | |||
Granted | 1,841,740 | 28.77 | ||||
Vested | (2,747,426 | ) | 16.97 | |||
Forfeited | (922,747 | ) | 22.73 | |||
Balance at December 31, 2012 | 6,581,983 | 23.55 | ||||
Shares Subject to Options | Weighted- Average Exercise Price | |||||
Balance at December 31, 2011 | 4,590 | $ | 12.48 | |||
Options exercised | (4,590 | ) | 12.48 | |||
Balance at December 31, 2012 | — | — | ||||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Net earnings attributable to Altria Group, Inc. | $ | 4,180 | $ | 3,390 | $ | 3,905 | |||||
Less: Distributed and undistributed earnings attributable to unvested restricted and deferred shares | (13 | ) | (13 | ) | (15 | ) | |||||
Earnings for basic and diluted EPS | $ | 4,167 | $ | 3,377 | $ | 3,890 | |||||
Weighted-average shares for basic EPS | 2,024 | 2,064 | 2,077 | ||||||||
Add: Incremental shares from stock options | — | — | 2 | ||||||||
Weighted-average shares for diluted EPS | 2,024 | 2,064 | 2,079 | ||||||||
(in millions) | Currency Translation Adjustments | Benefit Plans | SABMiller | Accumulated Other Comprehensive Losses | |||||||||||
Balances, December 31, 2009 | $ | 3 | $ | (1,846 | ) | $ | 282 | $ | (1,561 | ) | |||||
Period change, before deferred income taxes | 1 | 58 | 63 | 122 | |||||||||||
Deferred income taxes | — | (23 | ) | (22 | ) | (45 | ) | ||||||||
Balances, December 31, 2010 | 4 | (1,811 | ) | 323 | (1,484 | ) | |||||||||
Period change, before deferred income taxes | (2 | ) | (415 | ) | (231 | ) | (648 | ) | |||||||
Deferred income taxes | — | 164 | 81 | 245 | |||||||||||
Balances, December 31, 2011 | 2 | (2,062 | ) | 173 | (1,887 | ) | |||||||||
Period change, before deferred income taxes | — | (574 | ) | 306 | (268 | ) | |||||||||
Deferred income taxes | — | 222 | (107 | ) | 115 | ||||||||||
Balances, December 31, 2012 | $ | 2 | $ | (2,414 | ) | $ | 372 | $ | (2,040 | ) | |||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Earnings before income taxes: | |||||||||||
United States | $ | 6,461 | $ | 5,568 | $ | 5,709 | |||||
Outside United States | 16 | 14 | 14 | ||||||||
Total | $ | 6,477 | $ | 5,582 | $ | 5,723 | |||||
Provision for income taxes: | |||||||||||
Current: | |||||||||||
Federal | $ | 2,870 | $ | 2,353 | $ | 1,430 | |||||
State and local | 348 | 275 | 258 | ||||||||
Outside United States | 5 | 4 | 4 | ||||||||
3,223 | 2,632 | 1,692 | |||||||||
Deferred: | |||||||||||
Federal | (920 | ) | (458 | ) | 120 | ||||||
State and local | (9 | ) | 15 | 4 | |||||||
(929 | ) | (443 | ) | 124 | |||||||
Total provision for income taxes | $ | 2,294 | $ | 2,189 | $ | 1,816 | |||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Balance at beginning of year | $ | 381 | $ | 399 | $ | 601 | |||||
Additions based on tax positions | |||||||||||
related to the current year | 15 | 22 | 21 | ||||||||
Additions for tax positions of | |||||||||||
prior years | 170 | 71 | 30 | ||||||||
Reductions for tax positions due to | |||||||||||
lapse of statutes of limitations | (16 | ) | (39 | ) | (58 | ) | |||||
Reductions for tax positions of | |||||||||||
prior years | (102 | ) | (67 | ) | (164 | ) | |||||
Settlements | (186 | ) | (5 | ) | (31 | ) | |||||
Balance at end of year | $ | 262 | $ | 381 | $ | 399 | |||||
(in millions) | 2012 | 2011 | |||||
Unrecognized tax benefits — Altria Group, Inc. | $ | 156 | $ | 191 | |||
Unrecognized tax benefits — Mondelēz | 9 | 112 | |||||
Unrecognized tax benefits — PMI | 97 | 78 | |||||
Unrecognized tax benefits | 262 | 381 | |||||
Accrued interest and penalties | 66 | 618 | |||||
Tax credits and other indirect benefits | (20 | ) | (211 | ) | |||
Liability for tax contingencies | $ | 308 | $ | 788 | |||
2012 | 2011 | 2010 | ||||||
U.S. federal statutory rate | 35.0 | % | 35.0 | % | 35.0 | % | ||
Increase (decrease) resulting from: | ||||||||
State and local income taxes, net | ||||||||
of federal tax benefit | 3.5 | 3.8 | 3.7 | |||||
Uncertain tax positions | (0.7 | ) | 5.5 | (2.3 | ) | |||
SABMiller dividend benefit | (0.1 | ) | (2.0 | ) | (2.3 | ) | ||
Domestic manufacturing deduction | (2.0 | ) | (2.4 | ) | (2.4 | ) | ||
Other | (0.3 | ) | (0.7 | ) | — | |||
Effective tax rate | 35.4 | % | 39.2 | % | 31.7 | % | ||
(in millions) | 2012 | 2011 | |||||
Deferred income tax assets: | |||||||
Accrued postretirement and | |||||||
postemployment benefits | $ | 1,101 | $ | 1,087 | |||
Settlement charges | 1,419 | 1,382 | |||||
Accrued pension costs | 549 | 458 | |||||
Net operating losses and tax credit | |||||||
carryforwards | 208 | 96 | |||||
Total deferred income tax assets | 3,277 | 3,023 | |||||
Deferred income tax liabilities: | |||||||
Property, plant and equipment | (475 | ) | (511 | ) | |||
Intangible assets | (3,787 | ) | (3,721 | ) | |||
Investment in SABMiller | (2,198 | ) | (1,803 | ) | |||
Other | (166 | ) | (251 | ) | |||
Total deferred income tax liabilities | (6,626 | ) | (6,286 | ) | |||
Valuation allowances | (184 | ) | (82 | ) | |||
Net deferred income tax liabilities | $ | (3,533 | ) | $ | (3,345 | ) | |
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Net revenues: | |||||||||||
Smokeable products | $ | 22,216 | $ | 21,970 | $ | 22,191 | |||||
Smokeless products | 1,691 | 1,627 | 1,552 | ||||||||
Wine | 561 | 516 | 459 | ||||||||
Financial services | 150 | (313 | ) | 161 | |||||||
Net revenues | $ | 24,618 | $ | 23,800 | $ | 24,363 | |||||
Earnings before income taxes: | |||||||||||
Operating companies income (loss): | |||||||||||
Smokeable products | $ | 6,239 | $ | 5,737 | $ | 5,618 | |||||
Smokeless products | 931 | 859 | 803 | ||||||||
Wine | 104 | 91 | 61 | ||||||||
Financial services | 176 | (349 | ) | 157 | |||||||
Amortization of intangibles | (20 | ) | (20 | ) | (20 | ) | |||||
General corporate expenses | (228 | ) | (256 | ) | (216 | ) | |||||
Changes to Mondelēz and | |||||||||||
PMI tax-related receivables | 52 | 14 | (169 | ) | |||||||
Corporate asset impairment | |||||||||||
and exit costs | (1 | ) | (8 | ) | (6 | ) | |||||
Operating income | 7,253 | 6,068 | 6,228 | ||||||||
Interest and other debt | |||||||||||
expense, net | (1,126 | ) | (1,216 | ) | (1,133 | ) | |||||
Loss on early | |||||||||||
extinguishment of debt | (874 | ) | — | — | |||||||
Earnings from equity | |||||||||||
investment in SABMiller | 1,224 | 730 | 628 | ||||||||
Earnings before income taxes | $ | 6,477 | $ | 5,582 | $ | 5,723 | |||||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Smokeable products | $ | 4 | $ | 98 | $ | 11 | |||||
Smokeless products | — | — | 5 | ||||||||
Total | $ | 4 | $ | 98 | $ | 16 | |||||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Depreciation expense: | |||||||||||
Smokeable products | $ | 125 | $ | 145 | $ | 167 | |||||
Smokeless products | 26 | 31 | 32 | ||||||||
Wine | 27 | 25 | 23 | ||||||||
Corporate | 27 | 32 | 34 | ||||||||
Total depreciation expense | $ | 205 | $ | 233 | $ | 256 | |||||
Capital expenditures: | |||||||||||
Smokeable products | $ | 48 | $ | 46 | $ | 70 | |||||
Smokeless products | 36 | 24 | 19 | ||||||||
Wine | 30 | 25 | 22 | ||||||||
Corporate | 10 | 10 | 57 | ||||||||
Total capital expenditures | $ | 124 | $ | 105 | $ | 168 | |||||
(in millions) | 2012 | 2011 | |||||
Projected benefit obligation at beginning of year | $ | 6,965 | $ | 6,439 | |||
Service cost | 79 | 74 | |||||
Interest cost | 344 | 351 | |||||
Benefits paid | (420 | ) | (371 | ) | |||
Actuarial losses | 956 | 460 | |||||
Termination and curtailment | — | 17 | |||||
Other | — | (5 | ) | ||||
Projected benefit obligation at end of year | 7,924 | 6,965 | |||||
Fair value of plan assets at beginning of year | 5,275 | 5,218 | |||||
Actual return on plan assets | 755 | 188 | |||||
Employer contributions | 557 | 240 | |||||
Benefits paid | (420 | ) | (371 | ) | |||
Fair value of plan assets at end of year | 6,167 | 5,275 | |||||
Net pension liability recognized at December 31 | $ | (1,757 | ) | $ | (1,690 | ) | |
(in millions) | 2012 | 2011 | |||||
Other accrued liabilities | $ | (22 | ) | $ | (28 | ) | |
Accrued pension costs | (1,735 | ) | (1,662 | ) | |||
$ | (1,757 | ) | $ | (1,690 | ) | ||
2012 | 2011 | ||||
Discount rate | 4.0 | % | 5.0 | % | |
Rate of compensation increase | 4.0 | 4.0 | |||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Service cost | $ | 79 | $ | 74 | $ | 80 | |||||
Interest cost | 344 | 351 | 356 | ||||||||
Expected return on plan assets | (442 | ) | (422 | ) | (421 | ) | |||||
Amortization: | |||||||||||
Net loss | 224 | 171 | 126 | ||||||||
Prior service cost | 10 | 14 | 13 | ||||||||
Termination, settlement and curtailment | 21 | 41 | — | ||||||||
Net periodic pension cost | $ | 236 | $ | 229 | $ | 154 | |||||
(in millions) | 2012 | 2011 | |||||
Benefit obligation | $ | — | $ | 39 | |||
Other comprehensive earnings/losses: | |||||||
Net losses | 21 | — | |||||
Prior service cost | — | 2 | |||||
$ | 21 | $ | 41 | ||||
2012 | 2011 | 2010 | ||||||
Discount rate | 5.0 | % | 5.5 | % | 5.9 | % | ||
Expected rate of return on plan assets | 8.0 | 8.0 | 8.0 | |||||
Rate of compensation increase | 4.0 | 4.0 | 4.5 | |||||
(in millions) | Level 1 | Level 2 | Level 3 | Total | |||||||||||
Common/collective trusts: | |||||||||||||||
U.S. large cap | $ | — | $ | 1,566 | $ | — | $ | 1,566 | |||||||
U.S. small cap | — | 499 | — | 499 | |||||||||||
International developed markets | — | 179 | — | 179 | |||||||||||
Long duration fixed income | — | 494 | — | 494 | |||||||||||
U.S. and foreign government securities or their agencies: | |||||||||||||||
U.S. government and agencies | — | 625 | — | 625 | |||||||||||
U.S. municipal bonds | — | 71 | — | 71 | |||||||||||
Foreign government and agencies | — | 311 | — | 311 | |||||||||||
Corporate debt instruments: | |||||||||||||||
Above investment grade | — | 714 | — | 714 | |||||||||||
Below investment grade and no rating | — | 391 | — | 391 | |||||||||||
Common stock: | |||||||||||||||
International equities | 759 | — | — | 759 | |||||||||||
U.S. equities | 300 | — | — | 300 | |||||||||||
Registered investment companies | 128 | 50 | — | 178 | |||||||||||
U.S. and foreign cash and cash equivalents | 16 | 4 | — | 20 | |||||||||||
Asset backed securities | — | 35 | — | 35 | |||||||||||
Other, net | 9 | 2 | 14 | 25 | |||||||||||
Total investments at fair value, net | $ | 1,212 | $ | 4,941 | $ | 14 | $ | 6,167 | |||||||
(in millions) | Level 1 | Level 2 | Level 3 | Total | |||||||||||
Common/collective trusts: | |||||||||||||||
U.S. large cap | $ | — | $ | 1,482 | $ | — | $ | 1,482 | |||||||
U.S. small cap | — | 441 | — | 441 | |||||||||||
International developed markets | — | 152 | — | 152 | |||||||||||
International emerging markets | — | 100 | — | 100 | |||||||||||
Long duration fixed income | — | 585 | — | 585 | |||||||||||
U.S. and foreign government securities or their agencies: | |||||||||||||||
U.S. government and agencies | — | 510 | — | 510 | |||||||||||
U.S. municipal bonds | — | 44 | — | 44 | |||||||||||
Foreign government and agencies | — | 204 | — | 204 | |||||||||||
Corporate debt instruments: | |||||||||||||||
Above investment grade | — | 618 | — | 618 | |||||||||||
Below investment grade and no rating | — | 255 | — | 255 | |||||||||||
Common stock: | |||||||||||||||
International equities | 550 | — | — | 550 | |||||||||||
U.S. equities | 21 | — | — | 21 | |||||||||||
Registered investment companies | 124 | 63 | — | 187 | |||||||||||
U.S. and foreign cash and cash equivalents | 42 | 4 | — | 46 | |||||||||||
Asset backed securities | — | 49 | — | 49 | |||||||||||
Other, net | 16 | 2 | 13 | 31 | |||||||||||
Total investments at fair value, net | $ | 753 | $ | 4,509 | $ | 13 | $ | 5,275 | |||||||
▪ | Common/Collective Trusts: Common/collective trusts consist of pools of investments used by institutional investors to obtain exposure to equity and fixed income markets by investing in equity index funds that are intended to mirror indices such as Standard & Poor's 500 Index, Russell Small Cap Completeness Index, State Street Global Advisor's Fundamental Index, MSCI EAFE Index and an actively managed long duration fixed income fund. They are valued on the basis of the relative interest of each participating investor in the fair value of the underlying assets of each of the respective common/collective trusts. The underlying assets are valued based on the net asset value ("NAV") as provided by the investment account manager and are classified in level 2 of the fair value hierarchy. These common/collective trusts have defined redemption terms that vary from a two-day prior notice to semi-monthly openings for redemption. There were no other restrictions on redemption at December 31, 2012 and 2011. |
▪ | U.S. and Foreign Government Securities: U.S. and foreign government securities consist of investments in Treasury Nominal Bonds and Inflation Protected Securities, investment grade municipal securities and unrated or non-investment grade municipal securities. Government securities, that are traded in a non-active over-the-counter market, are valued at a price that is based on a broker quote, and are classified in level 2 of the fair value hierarchy. |
▪ | Corporate Debt Instruments: Corporate debt instruments are valued at a price that is based on a compilation of primarily observable market information or a broker quote in a non-active over-the-counter market, and are classified in level 2 of the fair value hierarchy. |
▪ | Common Stock: Common stocks are valued based on the price of the security as listed on an open active exchange on last trade date, and are classified in level 1 of the fair value hierarchy. |
▪ | Registered Investment Companies: Investments in mutual funds sponsored by a registered investment company are valued based on exchange listed prices and are classified in level 1 of the fair value hierarchy. Registered investment company funds which are designed specifically to meet Altria Group, Inc.'s pension plans investment strategies but are not traded on an active market are valued based on the NAV of the underlying securities as provided by the investment account manager on the last business day of the period and are classified in level 2 of the fair value hierarchy. The registered investment company funds measured at NAV have daily liquidity and were not subject to any redemption restrictions at December 31, 2012 and 2011. |
▪ | U.S. and Foreign Cash & Cash Equivalents: Cash and cash equivalents are valued at cost that approximates fair value, and are classified in level 1 of the fair value hierarchy. Cash collateral for forward contracts on U.S. Treasury notes, which approximates fair value, is classified in level 2 of the fair value hierarchy. |
▪ | Asset Backed Securities: Asset backed securities are fixed income securities such as mortgage backed securities and auto loans that are collateralized by pools of underlying assets that are unable to be sold individually. They are valued at a price which is based on a compilation of primarily observable market information or a broker quote in a non-active over-the-counter market, and are classified in level 2 of the fair value hierarchy. |
(in millions) | |||
2013 | $ | 400 | |
2014 | 412 | ||
2015 | 414 | ||
2016 | 420 | ||
2017 | 427 | ||
2018-2022 | 2,227 | ||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Service cost | $ | 18 | $ | 34 | $ | 29 | |||||
Interest cost | 115 | 139 | 135 | ||||||||
Amortization: | |||||||||||
Net loss | 40 | 39 | 32 | ||||||||
Prior service credit | (45 | ) | (21 | ) | (21 | ) | |||||
Termination and curtailment | (26 | ) | (4 | ) | — | ||||||
Net postretirement health care costs | $ | 102 | $ | 187 | $ | 175 | |||||
(in millions) | 2012 | 2011 | |||||
Accrued postretirement health care costs | $ | — | $ | 11 | |||
Other comprehensive earnings/losses: | |||||||
Prior service credit | (26 | ) | (15 | ) | |||
$ | (26 | ) | $ | (4 | ) | ||
2012 | 2011 | 2010 | ||||||
Discount rate | 4.9 | % | 5.5 | % | 5.8 | % | ||
Health care cost trend rate | 8.0 | 8.0 | 7.5 | |||||
(in millions) | 2012 | 2011 | |||||
Accrued postretirement health care costs at beginning of year | $ | 2,505 | $ | 2,548 | |||
Service cost | 18 | 34 | |||||
Interest cost | 115 | 139 | |||||
Benefits paid | (135 | ) | (136 | ) | |||
Plan amendments | — | (282 | ) | ||||
Actuarial losses | 160 | 191 | |||||
Termination and curtailment | — | 11 | |||||
Accrued postretirement health care costs at end of year | $ | 2,663 | $ | 2,505 | |||
2012 | 2011 | ||||
Discount rate | 3.9 | % | 4.9 | % | |
Health care cost trend rate assumed for next year | 7.5 | 8.0 | |||
Ultimate trend rate | 5.0 | 5.0 | |||
Year that the rate reaches the ultimate trend rate | 2018 | 2018 | |||
One-Percentage-Point Increase | One-Percentage-Point Decrease | ||||
Effect on total of service and interest cost | 7.1 | % | (6.0 | )% | |
Effect on postretirement benefit obligation | 6.8 | (5.8 | ) | ||
(in millions) | |||
2013 | $ | 159 | |
2014 | 168 | ||
2015 | 174 | ||
2016 | 177 | ||
2017 | 177 | ||
2018-2022 | 825 | ||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Service cost | $ | 1 | $ | 1 | $ | 1 | |||||
Interest cost | 1 | 2 | 1 | ||||||||
Amortization of net loss | 17 | 16 | 12 | ||||||||
Other | (7 | ) | 121 | 5 | |||||||
Net postemployment costs | $ | 12 | $ | 140 | $ | 19 | |||||
(in millions) | 2012 | 2011 | |||||
Accrued postemployment costs at beginning of year | $ | 270 | $ | 151 | |||
Service cost | 1 | 1 | |||||
Interest cost | 1 | 2 | |||||
Benefits paid | (143 | ) | (48 | ) | |||
Actuarial losses and assumption changes | 27 | 43 | |||||
Other | (7 | ) | 121 | ||||
Accrued postemployment costs at end of year | $ | 149 | $ | 270 | |||
(in millions) | Pensions | Post- retirement | Post- employment | Total | |||||||||||
Net losses | $ | (3,186 | ) | $ | (917 | ) | $ | (169 | ) | $ | (4,272 | ) | |||
Prior service (cost) credit | (36 | ) | 354 | — | 318 | ||||||||||
Deferred income taxes | 1,254 | 221 | 65 | 1,540 | |||||||||||
Amounts recorded in accumulated other comprehensive losses | $ | (1,968 | ) | $ | (342 | ) | $ | (104 | ) | $ | (2,414 | ) | |||
(in millions) | Pensions | Post- retirement | Post- employment | Total | |||||||||||
Net losses | $ | (2,788 | ) | $ | (796 | ) | $ | (175 | ) | $ | (3,759 | ) | |||
Prior service (cost) credit | (46 | ) | 425 | — | 379 | ||||||||||
Deferred income taxes | 1,104 | 146 | 68 | 1,318 | |||||||||||
Amounts recorded in accumulated other comprehensive losses | $ | (1,730 | ) | $ | (225 | ) | $ | (107 | ) | $ | (2,062 | ) | |||
(in millions) | Pensions | Post- retirement | Post- employment | Total | |||||||||||
Amounts transferred to earnings as components of net periodic benefit cost: | |||||||||||||||
Amortization: | |||||||||||||||
Net losses | $ | 224 | $ | 40 | $ | 17 | $ | 281 | |||||||
Prior service cost/credit | 10 | (45 | ) | — | (35 | ) | |||||||||
Other expense (income): | |||||||||||||||
Net losses | 21 | — | — | 21 | |||||||||||
Prior service cost/credit | — | (26 | ) | — | (26 | ) | |||||||||
Deferred income taxes | (99 | ) | 12 | (6 | ) | (93 | ) | ||||||||
156 | (19 | ) | 11 | 148 | |||||||||||
Other movements during the year: | |||||||||||||||
Net losses | (643 | ) | (161 | ) | (11 | ) | (815 | ) | |||||||
Deferred income taxes | 249 | 63 | 3 | 315 | |||||||||||
(394 | ) | (98 | ) | (8 | ) | (500 | ) | ||||||||
Total movements in other comprehensive earnings/losses | $ | (238 | ) | $ | (117 | ) | $ | 3 | $ | (352 | ) | ||||
(in millions) | Pensions | Post- retirement | Post- employment | Total | |||||||||||
Amounts transferred to earnings as components of net periodic benefit cost: | |||||||||||||||
Amortization: | |||||||||||||||
Net losses | $ | 171 | $ | 39 | $ | 16 | $ | 226 | |||||||
Prior service cost/credit | 14 | (21 | ) | — | (7 | ) | |||||||||
Deferred income taxes | (72 | ) | (7 | ) | (6 | ) | (85 | ) | |||||||
113 | 11 | 10 | 134 | ||||||||||||
Other movements during the year: | |||||||||||||||
Net losses | (672 | ) | (188 | ) | (40 | ) | (900 | ) | |||||||
Prior service cost/credit | 2 | 264 | — | 266 | |||||||||||
Deferred income taxes | 262 | (27 | ) | 14 | 249 | ||||||||||
(408 | ) | 49 | (26 | ) | (385 | ) | |||||||||
Total movements in other comprehensive earnings/losses | $ | (295 | ) | $ | 60 | $ | (16 | ) | $ | (251 | ) | ||||
(in millions) | Pensions | Post- retirement | Post- employment | Total | |||||||||||
Amounts transferred to earnings as components of net periodic benefit cost: | |||||||||||||||
Amortization: | |||||||||||||||
Net losses | $ | 126 | $ | 32 | $ | 12 | $ | 170 | |||||||
Prior service cost/credit | 13 | (21 | ) | — | (8 | ) | |||||||||
Deferred income taxes | (55 | ) | (4 | ) | (4 | ) | (63 | ) | |||||||
84 | 7 | 8 | 99 | ||||||||||||
Other movements during the year: | |||||||||||||||
Net losses | (41 | ) | (95 | ) | (10 | ) | (146 | ) | |||||||
Prior service cost/credit | (16 | ) | 58 | — | 42 | ||||||||||
Deferred income taxes | 21 | 15 | 4 | 40 | |||||||||||
(36 | ) | (22 | ) | (6 | ) | (64 | ) | ||||||||
Total movements in other comprehensive earnings/losses | $ | 48 | $ | (15 | ) | $ | 2 | $ | 35 | ||||||
For the Years Ended December 31, | |||||||||||
(in millions) | 2012 | 2011 | 2010 | ||||||||
Research and development expense | $ | 136 | $ | 128 | $ | 144 | |||||
Advertising expense | $ | 6 | $ | 5 | $ | 5 | |||||
Interest and other debt expense, net: | |||||||||||
Interest expense | $ | 1,128 | $ | 1,220 | $ | 1,136 | |||||
Interest income | (2 | ) | (4 | ) | (3 | ) | |||||
$ | 1,126 | $ | 1,216 | $ | 1,133 | ||||||
Rent expense | $ | 49 | $ | 63 | $ | 58 | |||||
(in millions) | Rental Commitments | Sublease Income | |||||
2013 | $ | 55 | $ | 3 | |||
2014 | 50 | 3 | |||||
2015 | 41 | 5 | |||||
2016 | 32 | 5 | |||||
2017 | 26 | 4 | |||||
Thereafter | 114 | 28 | |||||
$ | 318 | $ | 48 | ||||
Type of Case | Number of Cases Pending as of December 31, 2012 | Number of Cases Pending as of December 31, 2011 | Number of Cases Pending as of December 31, 2010 |
Individual Smoking and Health Cases (1) | 77 | 82 | 92 |
Smoking and Health Class Actions and Aggregated Claims Litigation (2) | 7 | 7 | 11 |
Health Care Cost Recovery Actions (3) | 1 | 1 | 4 |
"Lights/Ultra Lights" Class Actions | 14 | 17 | 27 |
Tobacco Price Cases | 1 | 1 | 1 |
(3) | See Health Care Cost Recovery Litigation - Federal Government's Lawsuit below. |
▪ | D. Boeken: In August 2011, a California jury returned a verdict in favor of plaintiff, awarding $12.8 million in compensatory damages against PM USA. PM USA's motions for judgment notwithstanding the verdict and for a new trial were denied in October 2011. PM USA appealed and posted a bond in the amount of $12.8 million in November 2011. |
▪ | Bullock: This litigation has concluded. In the fourth quarter of 2011, PM USA recorded a pre-tax provision of $14 million related to damages and costs and $3 million related to interest and in March 2012, paid an amount of approximately $19.1 million in satisfaction of the judgment and associated costs and interest. |
▪ | Schwarz: In March 2002, an Oregon jury awarded against PM USA $168,500 in compensatory damages and $150 million in punitive damages. In May 2002, the trial court reduced the punitive damages award to $100 million. In May 2006, the Oregon Court of Appeals affirmed the compensatory damages verdict, reversed the award of punitive damages and remanded the case to the trial court for a second trial to determine the amount of punitive damages, if any. In June 2006, plaintiff petitioned the Oregon Supreme Court to review the portion of the court of appeals' decision reversing and remanding the case for a new trial on punitive damages. In June 2010, the Oregon Supreme Court affirmed the court of appeals' decision and remanded the case to the trial court for a new trial limited to the question of punitive damages. In December 2010, the Oregon Supreme Court reaffirmed its earlier ruling and awarded PM USA approximately $500,000 in costs. In March 2011, PM USA filed a claim against the plaintiff for its costs and disbursements on appeal, plus interest. Trial on the amount of punitive damages began in January 2012. In February 2012, the jury awarded plaintiff $25 million in punitive damages. In March 2012, PM USA filed motions to set aside the verdict, for a new trial or, in the alternative, for a remittitur. The trial court denied these motions in May 2012. In September 2012, PM USA filed a notice of appeal from the trial court's judgment with the Oregon Court of Appeals. |
▪ | Williams: This litigation has concluded. In the fourth quarter of 2011, PM USA recorded a provision of approximately $48 million related to damages and costs and $54 million related to interest and in January 2012 paid an amount of approximately $102 million in satisfaction of the judgment and associated costs and interest. |
Date | Plaintiff | Verdict | Post-Trial Developments |
December 2012 | Buchanan | On December 7, 2012, a Leon County jury returned a verdict in favor of the plaintiff and against PM USA and Liggett Group LLC ("Liggett Group"). The jury awarded $5.5 million in compensatory damages and allocated 37% of the fault to each of the defendants (an amount of approximately $2 million). | On December 17, 2012, the defendants filed several post-trial motions, including motions for a new trial and to set aside the verdict. Argument on these motions was heard on January 16, 2013. |
October 2012 | Lock | A Pinellas County jury returned a verdict in favor of plaintiff and against PM USA and R.J. Reynolds. The jury awarded $1.15 million in compensatory damages and allocated 9% of the fault to each of the defendants (an amount of $103,500). | On November 5, 2012, the defendants filed several post-trial motions, including motions for a new trial, to set aside the verdict and to reduce the damages award by the amount of economic damages paid by third parties. On January 23, 2013, the trial court orally denied all post-trial motions. Judgment has yet to be entered. |
Date | Plaintiff | Verdict | Post-Trial Developments |
August 2012 | Hancock | A Broward County jury returned a verdict in the amount of zero damages and allocated 5% of the fault to each of the defendants (PM USA and R.J. Reynolds). The trial court granted an additur of $110,000, which is subject to the jury's comparative fault finding. | In August 2012, the defendants moved to set aside the verdict and to enter judgment in accordance with their motion for directed verdict. The defendants also moved to reduce damages, which motion the court granted. The trial court granted defendants' motion to set off the damages award by the amount of economic damages paid by third parties, which will reduce further any final award. On October 16, 2012, the trial court entered final judgment. PM USA's portion of the damages was approximately $700. Both sides have filed notices of appeal to the Florida Fourth District Court of Appeal. |
May 2012 | Calloway | A Broward County jury returned a verdict in favor of plaintiff and against PM USA, R.J. Reynolds, Lorillard Tobacco Company ("Lorillard") and Liggett Group. The jury awarded approximately $21 million in compensatory damages and allocated 25% of the fault against PM USA but the trial court ruled that it will not apply the comparative fault allocations because the jury found against each defendant on the intentional tort claims. The jury also awarded approximately $17 million in punitive damages against PM USA, approximately $17 million in punitive damages against R.J. Reynolds, approximately $13 million in punitive damages against Lorillard and approximately $8 million in punitive damages against Liggett Group. | In May and June, 2012, the defendants filed motions to set aside the verdict and for a new trial. In August 2012, the trial court denied the remaining post-trial motions and entered final judgment, reducing the total compensatory damages award to $16.1 million but leaving undisturbed the separate punitive damages awards. In September 2012, PM USA posted a bond in an amount of $1.5 million and the defendants filed a notice of appeal to the Florida Fourth District Court of Appeal. |
January 2012 | Hallgren | A Highland County jury returned a verdict in favor of plaintiff and against PM USA and R.J. Reynolds. The jury awarded approximately $2 million in compensatory damages and allocated 25% of the fault to PM USA (an amount of approximately $500,000). The jury also awarded $750,000 in punitive damages against each of the defendants. | The trial court entered final judgment in March 2012. In April 2012, PM USA posted a bond in an amount of approximately $1.25 million. In May 2012, the defendants filed a notice of appeal to the Florida Second District Court of Appeal. |
July 2011 | Weingart | A Palm Beach County jury returned a verdict in the amount of zero damages and allocated 3% of the fault to each of the defendants (PM USA, R.J. Reynolds and Lorillard). | In September 2011, the trial court granted plaintiff's motion for additur or a new trial, concluding that an additur of $150,000 is required for plaintiff's pain and suffering. The trial court entered final judgment and, since PM USA was allocated 3% of the fault, its portion of the damages was $4,500. In October 2011, PM USA filed its notice of appeal to the Florida Fourth District Court of Appeal and, in November 2011, posted bonds in an aggregate amount of $48,000. |
April 2011 | Allen | A Duval County jury returned a verdict in favor of plaintiffs and against PM USA and R.J. Reynolds. The jury awarded a total of $6 million in compensatory damages and allocated 15% of the fault to PM USA (an amount of $900,000). The jury also awarded $17 million in punitive damages against each of the defendants. | In May 2011, the trial court entered final judgment. In October 2011, the trial court granted the defendants' motion for remittitur, reducing the punitive damages award against PM USA to $2.7 million, and denied defendants' remaining post-trial motions. PM USA filed a notice of appeal to the Florida First District Court of Appeal and posted a bond in the amount of $1.25 million in November 2011. Oral argument was heard on January 16, 2013. |
Date | Plaintiff | Verdict | Post-Trial Developments |
April 2011 | Tullo | A Palm Beach County jury returned a verdict in favor of plaintiff and against PM USA, Lorillard and Liggett Group. The jury awarded a total of $4.5 million in compensatory damages and allocated 45% of the fault to PM USA (an amount of $2,025,000). | In April 2011, the trial court entered final judgment. In July 2011, PM USA filed its notice of appeal to the Florida Fourth District Court of Appeal and posted a $2 million bond. |
February 2011 | Huish | An Alachua County jury returned a verdict in favor of plaintiff and against PM USA. The jury awarded $750,000 in compensatory damages and allocated 25% of the fault to PM USA (an amount of $187,500). The jury also awarded $1.5 million in punitive damages against PM USA. | In March 2012, the Florida First District Court of Appeal affirmed per curiam the trial court's decision without issuing an opinion. In the second quarter of 2012, PM USA recorded a provision on its condensed consolidated balance sheet of approximately $2.5 million. In July 2012, PM USA paid an amount of $2.5 million in satisfaction of the judgment and associated costs. This litigation has concluded. |
February 2011 | Hatziyannakis | A Broward County jury returned a verdict in favor of plaintiff and against PM USA. The jury awarded approximately $270,000 in compensatory damages and allocated 32% of the fault to PM USA (an amount of approximately $86,000). | In April 2011, the trial court denied PM USA's post-trial motions for a new trial and to set aside the verdict. In June 2011, PM USA filed its notice of appeal to the Florida Fourth District Court of Appeal and posted an $86,000 appeal bond. On January 16, 2013, the Fourth District affirmed per curiam the trial court's decision without issuing an opinion. |
August 2010 | Piendle | A Palm Beach County jury returned a verdict in favor of plaintiff and against PM USA and R.J. Reynolds. The jury awarded $4 million in compensatory damages and allocated 27.5% of the fault to PM USA (an amount of approximately $1.1 million). The jury also awarded $90,000 in punitive damages against PM USA. | In June 2012, the Florida Fourth District Court of Appeal affirmed per curiam the trial court's decision without issuing an opinion. In the third quarter of 2012, PM USA recorded a provision on its condensed consolidated balance sheet of approximately $2.7 million for the judgment plus interest and associated costs and paid such amount on November 27, 2012. This litigation has concluded. |
July 2010 | Kayton (formerly Tate) | A Broward County jury returned a verdict in favor of the plaintiff and against PM USA. The jury awarded $8 million in compensatory damages and allocated 64% of the fault to PM USA (an amount of approximately $5.1 million). The jury also awarded approximately $16.2 million in punitive damages against PM USA. | In August 2010, the trial court entered final judgment, and PM USA filed its notice of appeal and posted a $5 million appeal bond. On November 28, 2012, the Florida Fourth District Court of Appeal reversed the punitive damages award and remanded the case for a new trial on plaintiff's conspiracy claim. Upon retrial, if the jury finds in plaintiff's favor on that claim, the original $16.2 million punitive damages award will be reinstated. PM USA filed a motion for rehearing, which was denied on January 18, 2013. On January 29, 2013, plaintiffs filed a notice to invoke discretionary jurisdiction with the Florida Supreme Court. |
April 2010 | Putney | A Broward County jury returned a verdict in favor of the plaintiff and against PM USA, R.J. Reynolds and Liggett Group. The jury awarded approximately $15.1 million in compensatory damages and allocated 15% of the fault to PM USA (an amount of approximately $2.3 million). The jury also awarded $2.5 million in punitive damages against PM USA. | In August 2010, the trial court entered final judgment. PM USA filed its notice of appeal to the Florida Fourth District Court of Appeal and posted a $1.6 million appeal bond. Argument on the merits of the appeal occurred in September 2012. |
Date | Plaintiff | Verdict | Post-Trial Developments |
March 2010 | R. Cohen | A Broward County jury returned a verdict in favor of the plaintiff and against PM USA and R.J. Reynolds. The jury awarded $10 million in compensatory damages and allocated 33 1/3% of the fault to PM USA (an amount of approximately $3.3 million). The jury also awarded a total of $20 million in punitive damages, assessing separate $10 million awards against each defendant. | In July 2010, the trial court entered final judgment and, in August 2010, PM USA filed its notice of appeal. In October 2010, PM USA posted a $2.5 million appeal bond. In September 2012, the Florida Fourth District Court of Appeal affirmed the compensatory damages award but reversed and remanded the punitive damages verdict. The Fourth District returned the case to the trial court for a new jury trial on the plaintiff's fraudulent concealment claim. If the jury finds in plaintiff's favor on that claim, the $10 million punitive damages award against each defendant will be reinstated. On October 8, 2012, both plaintiff and defendants filed petitions for rehearing, which the Fourth District denied on December 31, 2012. On January 14, 2013, the defendants filed a notice to invoke discretionary jurisdiction with the Florida Supreme Court. Plaintiffs filed a similar notice on January 18, 2013. |
March 2010 | Douglas | A Hillsborough County jury returned a verdict in favor of the plaintiff and against PM USA, R.J. Reynolds and Liggett Group. The jury awarded $5 million in compensatory damages. Punitive damages were dismissed prior to trial. The jury allocated 18% of the fault to PM USA, resulting in an award of $900,000. | In June 2010, PM USA filed its notice of appeal and posted a $900,000 appeal bond. In March 2012, the Florida Second District Court of Appeal issued a decision affirming the judgment and upholding the use of the Engle jury findings but certified to the Florida Supreme Court the question of whether granting res judicata effect to the Engle jury findings violates defendants' federal due process rights. In April 2012, the defendants filed a notice to invoke discretionary jurisdiction with the Florida Supreme Court. In May 2012, the Florida Supreme Court accepted jurisdiction of the case. Argument occurred in September 2012. |
November 2009 | Naugle | A Broward County jury returned a verdict in favor of the plaintiff and against PM USA. The jury awarded approximately $56.6 million in compensatory damages and $244 million in punitive damages. The jury allocated 90% of the fault to PM USA. | In March 2010, the trial court entered final judgment reflecting a reduced award of approximately $13 million in compensatory damages and $26 million in punitive damages. In April 2010, PM USA filed its notice of appeal and posted a $5 million appeal bond. In August 2010, upon the motion of PM USA, the trial court entered an amended final judgment of approximately $12.3 million in compensatory damages and approximately $24.5 million in punitive damages to correct a clerical error. In June 2012, the Fourth District Court of Appeal affirmed the amended final judgment. In July 2012, PM USA filed a motion for rehearing. On December 12, 2012, the Fourth District withdrew its prior decision, reversed the verdict as to compensatory and punitive damages and returned the case to the trial court for a new trial on the question of damages. On December 26, 2012, the plaintiff filed a motion for rehearing en banc or for certification to the Florida Supreme Court, which was denied on January 25, 2013. |
Date | Plaintiff | Verdict | Post-Trial Developments |
August 2009 | F. Campbell | An Escambia County jury returned a verdict in favor of the plaintiff and against R.J. Reynolds, PM USA and Liggett Group. The jury awarded $7.8 million in compensatory damages. In September 2009, the trial court entered final judgment and awarded the plaintiff $156,000 in damages against PM USA due to the jury allocating only 2% of the fault to PM USA. | In March 2011, the Florida First District Court of Appeal affirmed per curiam the trial court's decision without issuing an opinion. In May 2012, PM USA paid an amount of approximately $262,000 in satisfaction of the judgment and associated costs and interest. This litigation has concluded. |
August 2009 | Barbanell | A Broward County jury returned a verdict in favor of the plaintiff, awarding $5.3 million in compensatory damages. The judge had previously dismissed the punitive damages claim. In September 2009, the trial court entered final judgment and awarded the plaintiff $1.95 million in actual damages. The judgment reduced the jury's $5.3 million award of compensatory damages due to the jury allocating 36.5% of the fault to PM USA. | A notice of appeal was filed by PM USA in September 2009, and PM USA posted a $1.95 million appeal bond. In February 2012, the Florida Fourth District Court of Appeal reversed the judgment, holding that the statute of limitations barred the plaintiff's claims. On October 17, 2012, on motion for rehearing, the Fourth District withdrew its prior decision and affirmed the trial court's judgment. On November 16, 2012, PM USA filed a notice to invoke the jurisdiction of the Florida Supreme Court. On December 5, 2012, the Florida Supreme Court granted a partial stay pending its disposition of the J. Brown case against R.J. Reynolds. |
February 2009 | Hess | A Broward County jury found in favor of plaintiffs and against PM USA. The jury awarded $3 million in compensatory damages and $5 million in punitive damages. In June 2009, the trial court entered final judgment and awarded plaintiffs $1.26 million in actual damages and $5 million in punitive damages. The judgment reduced the jury's $3 million award of compensatory damages due to the jury allocating 42% of the fault to PM USA. | PM USA noticed an appeal to the Fourth District Court of Appeal in July 2009. In May 2012, the Fourth District reversed and vacated the punitive damages award and affirmed the judgment in all other respects, upholding the compensatory damages award of $1.26 million. In June 2012, both parties filed rehearing motions with the Fourth District, which were denied in September 2012. On October 15, 2012, PM USA and plaintiffs filed notices to invoke the Florida Supreme Court's discretionary jurisdiction. |
Year for which NPM Adjustment calculated | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 |
Year in which deduction for NPM Adjustment may be taken | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
PM USA's Approximate Share of Disputed NPM Adjustment (in millions) | $337 | $388 | $181 | $154 | $185 | $252 | $206 | $208 | $137 |
▪ | defendants falsely denied, distorted and minimized the significant adverse health consequences of smoking; |
▪ | defendants hid from the public that cigarette smoking and nicotine are addictive; |
▪ | defendants falsely denied that they control the level of nicotine delivered to create and sustain addiction; |
▪ | defendants falsely marketed and promoted "low tar/light" cigarettes as less harmful than full-flavor cigarettes; |
▪ | defendants falsely denied that they intentionally marketed to youth; |
▪ | defendants publicly and falsely denied that ETS is hazardous to non-smokers; and |
▪ | defendants suppressed scientific research. |
▪ | its application to defendants' subsidiaries; |
▪ | the prohibition on the use of express or implied health messages or health descriptors, but only to the extent of extraterritorial application; |
▪ | its point-of-sale display provisions; and |
▪ | its application to Brown & Williamson Holdings. |
▪ | Aspinall: In August 2004, the Massachusetts Supreme Judicial Court affirmed the class certification order. In August 2006, the trial court denied PM USA's motion for summary judgment and granted plaintiffs' motion for summary judgment on the defenses of federal preemption and a state law exemption to Massachusetts' consumer protection statute. On motion of the parties, the trial court subsequently reported its decision to deny summary judgment to the appeals court for review and stayed further proceedings pending completion of the appellate review. In December 2008, subsequent to the United States Supreme Court's decision in Good, the Massachusetts Supreme Judicial Court issued an order requesting that the parties advise the court within 30 days whether the Good decision is dispositive of federal preemption issues pending on appeal. In January 2009, PM USA notified the Massachusetts Supreme Judicial |
▪ | Brown: In May 2009, the California Supreme Court reversed the trial court decision decertifying the class and remanded the case to the trial court. At this time, the sole remaining theory of liability in this action is whether the marketing of "Lights" cigarettes was deceptive to consumers. In September 2012, at the plaintiffs' request, the trial court dismissed all defendants except PM USA from the lawsuit. Trial is currently scheduled for April 19, 2013. |
▪ | Larsen: In August 2005, a Missouri Court of Appeals affirmed the class certification order. In December 2009, the trial court denied plaintiffs' motion for reconsideration of the period during which potential class members can qualify to become part of the class. The class period remains 1995 through 2003. In June 2010, PM USA's motion for partial summary judgment regarding plaintiffs' request for punitive damages was denied. In April 2010, plaintiffs moved for partial summary judgment as to an element of liability in the case, claiming collateral estoppel from the findings in the case brought by the Department of Justice (see Federal Government's Lawsuit described above). The plaintiffs' motion was denied in December 2010. In June 2011, PM USA filed various summary judgment motions challenging the plaintiffs' claims. In August 2011, the trial court granted PM USA's motion for partial summary judgment, ruling that plaintiffs could not present a damages claim based on allegations that Marlboro Lights are more dangerous than Marlboro Reds. The trial court denied PM USA's remaining summary judgment motions. Trial in the case began in September 2011 and, in October 2011 the court declared a mistrial after the jury failed to reach a verdict. The court has continued the new trial through January 2014, with an exact date to be determined. |
(in millions) | Altria Group, Inc. | PM USA | |||||
For the years ended: | |||||||
December 31, 2011 | $ | 3,666 | $ | 213 | |||
December 31, 2010 | $ | 3,438 | $ | 179 | |||
For the three months ended: | |||||||
March 31, 2012 | $ | 923 | $ | 59 | |||
March 31, 2011 | $ | 890 | $ | 26 | |||
at December 31, 2012 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Assets | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Cash and cash equivalents | $ | 2,862 | $ | — | $ | 38 | $ | — | $ | 2,900 | |||||||||
Receivables | 101 | 7 | 85 | — | 193 | ||||||||||||||
Inventories: | |||||||||||||||||||
Leaf tobacco | — | 512 | 364 | — | 876 | ||||||||||||||
Other raw materials | — | 127 | 46 | — | 173 | ||||||||||||||
Work in process | — | 3 | 346 | — | 349 | ||||||||||||||
Finished product | — | 117 | 231 | — | 348 | ||||||||||||||
— | 759 | 987 | — | 1,746 | |||||||||||||||
Due from Altria Group, Inc. and subsidiaries | 834 | 3,424 | 1,157 | (5,415 | ) | — | |||||||||||||
Deferred income taxes | — | 1,246 | 16 | (46 | ) | 1,216 | |||||||||||||
Other current assets | — | 193 | 175 | (108 | ) | 260 | |||||||||||||
Total current assets | 3,797 | 5,629 | 2,458 | (5,569 | ) | 6,315 | |||||||||||||
Property, plant and equipment, at cost | 2 | 3,253 | 1,495 | — | 4,750 | ||||||||||||||
Less accumulated depreciation | 2 | 2,073 | 573 | — | 2,648 | ||||||||||||||
— | 1,180 | 922 | — | 2,102 | |||||||||||||||
Goodwill | — | — | 5,174 | — | 5,174 | ||||||||||||||
Other intangible assets, net | — | 2 | 12,076 | — | 12,078 | ||||||||||||||
Investment in SABMiller | 6,637 | — | — | — | 6,637 | ||||||||||||||
Investment in consolidated subsidiaries | 9,521 | 3,018 | — | (12,539 | ) | — | |||||||||||||
Due from Altria Group, Inc. and subsidiaries | 4,500 | — | — | (4,500 | ) | — | |||||||||||||
Other assets | 136 | 530 | 124 | (365 | ) | 425 | |||||||||||||
Total consumer products assets | 24,591 | 10,359 | 20,754 | (22,973 | ) | 32,731 | |||||||||||||
Financial services | |||||||||||||||||||
Finance assets, net | — | — | 2,581 | — | 2,581 | ||||||||||||||
Due from Altria Group, Inc. and subsidiaries | — | — | 14 | (14 | ) | — | |||||||||||||
Other assets | — | — | 17 | — | 17 | ||||||||||||||
Total financial services assets | — | — | 2,612 | (14 | ) | 2,598 | |||||||||||||
Total Assets | $ | 24,591 | $ | 10,359 | $ | 23,366 | $ | (22,987 | ) | $ | 35,329 | ||||||||
at December 31, 2012 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Liabilities | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Current portion of long-term debt | $ | 1,459 | $ | — | $ | — | $ | — | $ | 1,459 | |||||||||
Accounts payable | 4 | 155 | 292 | — | 451 | ||||||||||||||
Accrued liabilities: | |||||||||||||||||||
Marketing | — | 526 | 42 | — | 568 | ||||||||||||||
Employment costs | 27 | 10 | 147 | — | 184 | ||||||||||||||
Settlement charges | — | 3,610 | 6 | — | 3,616 | ||||||||||||||
Other | 469 | 506 | 264 | (154 | ) | 1,085 | |||||||||||||
Dividends payable | 888 | — | — | — | 888 | ||||||||||||||
Due to Altria Group, Inc. and subsidiaries | 3,965 | 409 | 1,055 | (5,429 | ) | — | |||||||||||||
Total current liabilities | 6,812 | 5,216 | 1,806 | (5,583 | ) | 8,251 | |||||||||||||
Long-term debt | 12,120 | — | 299 | — | 12,419 | ||||||||||||||
Deferred income taxes | 2,034 | — | 3,284 | (365 | ) | 4,953 | |||||||||||||
Accrued pension costs | 235 | — | 1,500 | — | 1,735 | ||||||||||||||
Accrued postretirement health care costs | — | 1,759 | 745 | — | 2,504 | ||||||||||||||
Due to Altria Group, Inc. and subsidiaries | — | — | 4,500 | (4,500 | ) | — | |||||||||||||
Other liabilities | 222 | 178 | 156 | — | 556 | ||||||||||||||
Total consumer products liabilities | 21,423 | 7,153 | 12,290 | (10,448 | ) | 30,418 | |||||||||||||
Financial services | |||||||||||||||||||
Deferred income taxes | — | — | 1,699 | — | 1,699 | ||||||||||||||
Other liabilities | — | — | 8 | — | 8 | ||||||||||||||
Total financial services liabilities | — | — | 1,707 | — | 1,707 | ||||||||||||||
Total liabilities | 21,423 | 7,153 | 13,997 | (10,448 | ) | 32,125 | |||||||||||||
Contingencies | |||||||||||||||||||
Redeemable noncontrolling interest | — | — | 34 | — | 34 | ||||||||||||||
Stockholders' Equity | |||||||||||||||||||
Common stock | 935 | — | 9 | (9 | ) | 935 | |||||||||||||
Additional paid-in capital | 5,688 | 3,321 | 10,272 | (13,593 | ) | 5,688 | |||||||||||||
Earnings reinvested in the business | 24,316 | 314 | 943 | (1,257 | ) | 24,316 | |||||||||||||
Accumulated other comprehensive losses | (2,040 | ) | (429 | ) | (1,891 | ) | 2,320 | (2,040 | ) | ||||||||||
Cost of repurchased stock | (25,731 | ) | — | — | — | (25,731 | ) | ||||||||||||
Total stockholders' equity attributable to Altria Group, Inc. | 3,168 | 3,206 | 9,333 | (12,539 | ) | 3,168 | |||||||||||||
Noncontrolling interests | — | — | 2 | — | 2 | ||||||||||||||
Total stockholders' equity | 3,168 | 3,206 | 9,335 | (12,539 | ) | 3,170 | |||||||||||||
Total Liabilities and Stockholders' Equity | $ | 24,591 | $ | 10,359 | $ | 23,366 | $ | (22,987 | ) | $ | 35,329 | ||||||||
at December 31, 2011 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Assets | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Cash and cash equivalents | $ | 3,245 | $ | — | $ | 25 | $ | — | $ | 3,270 | |||||||||
Receivables | 174 | 16 | 78 | — | 268 | ||||||||||||||
Inventories: | |||||||||||||||||||
Leaf tobacco | — | 565 | 369 | — | 934 | ||||||||||||||
Other raw materials | — | 128 | 42 | — | 170 | ||||||||||||||
Work in process | — | 4 | 312 | — | 316 | ||||||||||||||
Finished product | — | 126 | 233 | — | 359 | ||||||||||||||
— | 823 | 956 | — | 1,779 | |||||||||||||||
Due from Altria Group, Inc. and subsidiaries | 403 | 3,007 | 1,765 | (5,175 | ) | — | |||||||||||||
Deferred income taxes | 9 | 1,157 | 41 | — | 1,207 | ||||||||||||||
Other current assets | 6 | 224 | 242 | (76 | ) | 396 | |||||||||||||
Total current assets | 3,837 | 5,227 | 3,107 | (5,251 | ) | 6,920 | |||||||||||||
Property, plant and equipment, at cost | 2 | 3,280 | 1,446 | — | 4,728 | ||||||||||||||
Less accumulated depreciation | 2 | 2,005 | 505 | — | 2,512 | ||||||||||||||
— | 1,275 | 941 | — | 2,216 | |||||||||||||||
Goodwill | — | — | 5,174 | — | 5,174 | ||||||||||||||
Other intangible assets, net | — | 2 | 12,096 | — | 12,098 | ||||||||||||||
Investment in SABMiller | 5,509 | — | — | — | 5,509 | ||||||||||||||
Investment in consolidated subsidiaries | 7,009 | 3,035 | — | (10,044 | ) | — | |||||||||||||
Due from Altria Group, Inc. and subsidiaries | 6,500 | — | — | (6,500 | ) | — | |||||||||||||
Other assets | 941 | 586 | 111 | (381 | ) | 1,257 | |||||||||||||
Total consumer products assets | 23,796 | 10,125 | 21,429 | (22,176 | ) | 33,174 | |||||||||||||
Financial services | |||||||||||||||||||
Finance assets, net | — | — | 3,559 | — | 3,559 | ||||||||||||||
Due from Altria Group, Inc. and subsidiaries | — | — | 292 | (292 | ) | — | |||||||||||||
Other assets | — | — | 18 | — | 18 | ||||||||||||||
Total financial services assets | — | — | 3,869 | (292 | ) | 3,577 | |||||||||||||
Total Assets | $ | 23,796 | $ | 10,125 | $ | 25,298 | $ | (22,468 | ) | $ | 36,751 | ||||||||
at December 31, 2011 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Liabilities | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Current portion of long-term debt | $ | — | $ | — | $ | 600 | $ | — | $ | 600 | |||||||||
Accounts payable | 69 | 159 | 275 | — | 503 | ||||||||||||||
Accrued liabilities: | |||||||||||||||||||
Marketing | — | 390 | 40 | — | 430 | ||||||||||||||
Employment costs | 29 | 12 | 184 | — | 225 | ||||||||||||||
Settlement charges | — | 3,508 | 5 | — | 3,513 | ||||||||||||||
Other | 384 | 623 | 389 | (76 | ) | 1,320 | |||||||||||||
Dividends payable | 841 | — | — | — | 841 | ||||||||||||||
Due to Altria Group, Inc. and subsidiaries | 3,792 | 474 | 1,201 | (5,467 | ) | — | |||||||||||||
Total current liabilities | 5,115 | 5,166 | 2,694 | (5,543 | ) | 7,432 | |||||||||||||
Long-term debt | 12,790 | — | 299 | — | 13,089 | ||||||||||||||
Deferred income taxes | 1,787 | — | 3,345 | (381 | ) | 4,751 | |||||||||||||
Accrued pension costs | 236 | — | 1,426 | — | 1,662 | ||||||||||||||
Accrued postretirement health care costs | — | 1,562 | 797 | — | 2,359 | ||||||||||||||
Due to Altria Group, Inc. and subsidiaries | — | — | 6,500 | (6,500 | ) | — | |||||||||||||
Other liabilities | 188 | 216 | 198 | — | 602 | ||||||||||||||
Total consumer products liabilities | 20,116 | 6,944 | 15,259 | (12,424 | ) | 29,895 | |||||||||||||
Financial services | |||||||||||||||||||
Deferred income taxes | — | — | 2,811 | — | 2,811 | ||||||||||||||
Other liabilities | — | — | 330 | — | 330 | ||||||||||||||
Total financial services liabilities | — | — | 3,141 | — | 3,141 | ||||||||||||||
Total liabilities | 20,116 | 6,944 | 18,400 | (12,424 | ) | 33,036 | |||||||||||||
Contingencies | |||||||||||||||||||
Redeemable noncontrolling interest | — | — | 32 | — | 32 | ||||||||||||||
Stockholders' Equity | |||||||||||||||||||
Common stock | 935 | — | 9 | (9 | ) | 935 | |||||||||||||
Additional paid-in capital | 5,674 | 3,283 | 8,238 | (11,521 | ) | 5,674 | |||||||||||||
Earnings reinvested in the business | 23,583 | 210 | 265 | (475 | ) | 23,583 | |||||||||||||
Accumulated other comprehensive losses | (1,887 | ) | (312 | ) | (1,649 | ) | 1,961 | (1,887 | ) | ||||||||||
Cost of repurchased stock | (24,625 | ) | — | — | — | (24,625 | ) | ||||||||||||
Total stockholders' equity attributable to Altria Group, Inc. | 3,680 | 3,181 | 6,863 | (10,044 | ) | 3,680 | |||||||||||||
Noncontrolling interests | — | — | 3 | — | 3 | ||||||||||||||
Total stockholders' equity | 3,680 | 3,181 | 6,866 | (10,044 | ) | 3,683 | |||||||||||||
Total Liabilities and Stockholders' Equity | $ | 23,796 | $ | 10,125 | $ | 25,298 | $ | (22,468 | ) | $ | 36,751 | ||||||||
for the year ended December 31, 2012 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Net revenues | $ | — | $ | 21,531 | $ | 3,110 | $ | (23 | ) | $ | 24,618 | ||||||||
Cost of sales | — | 7,067 | 893 | (23 | ) | 7,937 | |||||||||||||
Excise taxes on products | — | 6,831 | 287 | — | 7,118 | ||||||||||||||
Gross profit | — | 7,633 | 1,930 | — | 9,563 | ||||||||||||||
Marketing, administration and research costs | 210 | 1,867 | 204 | — | 2,281 | ||||||||||||||
Changes to Mondelēz & PMI tax-related receivables | (52 | ) | — | — | — | (52 | ) | ||||||||||||
Asset impairment and exit costs | 1 | 59 | 1 | — | 61 | ||||||||||||||
Amortization of intangibles | — | — | 20 | — | 20 | ||||||||||||||
Operating (expense) income | (159 | ) | 5,707 | 1,705 | — | 7,253 | |||||||||||||
Interest and other debt expense (income), net | 705 | (3 | ) | 424 | — | 1,126 | |||||||||||||
Loss on early extinguishment of debt | 874 | — | — | — | 874 | ||||||||||||||
Earnings from equity investment in SABMiller | (1,224 | ) | — | — | — | (1,224 | ) | ||||||||||||
(Loss) earnings before income taxes and equity earnings of subsidiaries | (514 | ) | 5,710 | 1,281 | — | 6,477 | |||||||||||||
(Benefit) provision for income taxes | (196 | ) | 2,100 | 390 | — | 2,294 | |||||||||||||
Equity earnings of subsidiaries | 4,498 | 218 | — | (4,716 | ) | — | |||||||||||||
Net earnings | 4,180 | 3,828 | 891 | (4,716 | ) | 4,183 | |||||||||||||
Net earnings attributable to noncontrolling interests | — | — | (3 | ) | — | (3 | ) | ||||||||||||
Net earnings attributable to Altria Group, Inc. | $ | 4,180 | $ | 3,828 | $ | 888 | $ | (4,716 | ) | $ | 4,180 | ||||||||
Net earnings | $ | 4,180 | $ | 3,828 | $ | 891 | $ | (4,716 | ) | $ | 4,183 | ||||||||
Other comprehensive losses, net of deferred income tax benefit | (153 | ) | (117 | ) | (242 | ) | 359 | (153 | ) | ||||||||||
Comprehensive earnings | 4,027 | 3,711 | 649 | (4,357 | ) | 4,030 | |||||||||||||
Comprehensive earnings attributable to noncontrolling interests | — | — | (3 | ) | — | (3 | ) | ||||||||||||
Comprehensive earnings attributable to Altria Group, Inc. | $ | 4,027 | $ | 3,711 | $ | 646 | $ | (4,357 | ) | $ | 4,027 | ||||||||
for the year ended December 31, 2011 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Net revenues | $ | — | $ | 21,330 | $ | 2,496 | $ | (26 | ) | $ | 23,800 | ||||||||
Cost of sales | — | 6,883 | 823 | (26 | ) | 7,680 | |||||||||||||
Excise taxes on products | — | 6,846 | 335 | — | 7,181 | ||||||||||||||
Gross profit | — | 7,601 | 1,338 | — | 8,939 | ||||||||||||||
Marketing, administration and research costs | 186 | 2,164 | 293 | — | 2,643 | ||||||||||||||
Changes to Mondelēz and PMI tax-related receivables | (14 | ) | — | — | — | (14 | ) | ||||||||||||
Asset impairment and exit costs | 8 | 200 | 14 | — | 222 | ||||||||||||||
Amortization of intangibles | — | — | 20 | — | 20 | ||||||||||||||
Operating (expense) income | (180 | ) | 5,237 | 1,011 | — | 6,068 | |||||||||||||
Interest and other debt expense, net | 698 | 61 | 457 | — | 1,216 | ||||||||||||||
Earnings from equity investment in SABMiller | (730 | ) | — | — | — | (730 | ) | ||||||||||||
(Loss) earnings before income taxes and equity earnings of subsidiaries | (148 | ) | 5,176 | 554 | — | 5,582 | |||||||||||||
(Benefit) provision for income taxes | (199 | ) | 1,930 | 458 | — | 2,189 | |||||||||||||
Equity earnings of subsidiaries | 3,339 | 153 | — | (3,492 | ) | — | |||||||||||||
Net earnings | 3,390 | 3,399 | 96 | (3,492 | ) | 3,393 | |||||||||||||
Net earnings attributable to noncontrolling interests | — | — | (3 | ) | — | (3 | ) | ||||||||||||
Net earnings attributable to Altria Group, Inc. | $ | 3,390 | $ | 3,399 | $ | 93 | $ | (3,492 | ) | $ | 3,390 | ||||||||
Net earnings | $ | 3,390 | $ | 3,399 | $ | 96 | $ | (3,492 | ) | $ | 3,393 | ||||||||
Other comprehensive losses, net of deferred income tax benefit | (403 | ) | (36 | ) | (209 | ) | 245 | (403 | ) | ||||||||||
Comprehensive earnings (losses) | 2,987 | 3,363 | (113 | ) | (3,247 | ) | 2,990 | ||||||||||||
Comprehensive earnings attributable to noncontrolling interests | — | — | (3 | ) | — | (3 | ) | ||||||||||||
Comprehensive earnings attributable to Altria Group, Inc. | $ | 2,987 | $ | 3,363 | $ | (116 | ) | $ | (3,247 | ) | $ | 2,987 | |||||||
for the year ended December 31, 2010 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Net revenues | $ | — | $ | 21,580 | $ | 2,809 | $ | (26 | ) | $ | 24,363 | ||||||||
Cost of sales | — | 6,990 | 740 | (26 | ) | 7,704 | |||||||||||||
Excise taxes on products | — | 7,136 | 335 | — | 7,471 | ||||||||||||||
Gross profit | — | 7,454 | 1,734 | — | 9,188 | ||||||||||||||
Marketing, administration and research costs | 147 | 2,280 | 308 | — | 2,735 | ||||||||||||||
Changes to Mondelēz and PMI tax-related receivables | 169 | — | — | — | 169 | ||||||||||||||
Asset impairment and exit costs | — | 24 | 12 | — | 36 | ||||||||||||||
Amortization of intangibles | — | — | 20 | — | 20 | ||||||||||||||
Operating (expense) income | (316 | ) | 5,150 | 1,394 | — | 6,228 | |||||||||||||
Interest and other debt expense, net | 549 | 2 | 582 | — | 1,133 | ||||||||||||||
Earnings from equity investment in SABMiller | (628 | ) | — | — | — | (628 | ) | ||||||||||||
(Loss) earnings before income taxes and equity earnings of subsidiaries | (237 | ) | 5,148 | 812 | — | 5,723 | |||||||||||||
(Benefit) provision for income taxes | (329 | ) | 1,864 | 281 | — | 1,816 | |||||||||||||
Equity earnings of subsidiaries | 3,813 | 143 | — | (3,956 | ) | — | |||||||||||||
Net earnings | 3,905 | 3,427 | 531 | (3,956 | ) | 3,907 | |||||||||||||
Net earnings attributable to noncontrolling interests | — | — | (2 | ) | — | (2 | ) | ||||||||||||
Net earnings attributable to Altria Group, Inc. | $ | 3,905 | $ | 3,427 | $ | 529 | $ | (3,956 | ) | $ | 3,905 | ||||||||
Net earnings | $ | 3,905 | $ | 3,427 | $ | 531 | $ | (3,956 | ) | $ | 3,907 | ||||||||
Other comprehensive earnings, net of deferred income taxes | 77 | 15 | 25 | (40 | ) | 77 | |||||||||||||
Comprehensive earnings | 3,982 | 3,442 | 556 | (3,996 | ) | 3,984 | |||||||||||||
Comprehensive earnings attributable to noncontrolling interests | — | — | (2 | ) | — | (2 | ) | ||||||||||||
Comprehensive earnings attributable to Altria Group, Inc. | $ | 3,982 | $ | 3,442 | $ | 554 | $ | (3,996 | ) | $ | 3,982 | ||||||||
for the year ended December 31, 2012 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Cash Provided by Operating Activities | |||||||||||||||||||
Net cash provided by operating activities | $ | 3,059 | $ | 4,206 | $ | 565 | $ | (3,927 | ) | $ | 3,903 | ||||||||
Cash Provided by (Used in) Investing Activities | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Capital expenditures | — | (35 | ) | (89 | ) | — | (124 | ) | |||||||||||
Other | — | — | (5 | ) | — | (5 | ) | ||||||||||||
Financial services | |||||||||||||||||||
Proceeds from finance assets | — | — | 1,049 | — | 1,049 | ||||||||||||||
Net cash (used in) provided by investing activities | — | (35 | ) | 955 | — | 920 | |||||||||||||
Cash Provided by (Used in) Financing Activities | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Long-term debt issued | 2,787 | — | — | — | 2,787 | ||||||||||||||
Long-term debt repaid | (2,000 | ) | — | (600 | ) | — | (2,600 | ) | |||||||||||
Repurchases of common stock | (1,082 | ) | — | — | — | (1,082 | ) | ||||||||||||
Dividends paid on common stock | (3,400 | ) | — | — | — | (3,400 | ) | ||||||||||||
Changes in amounts due to/from Altria Group, Inc. and subsidiaries | 1,128 | (475 | ) | (653 | ) | — | — | ||||||||||||
Financing fees and debt issuance costs | (22 | ) | — | — | — | (22 | ) | ||||||||||||
Tender premiums and fees related to early extinguishment of debt | (864 | ) | — | — | — | (864 | ) | ||||||||||||
Cash dividends paid to parent | — | (3,690 | ) | (237 | ) | 3,927 | — | ||||||||||||
Other | 11 | (6 | ) | (17 | ) | — | (12 | ) | |||||||||||
Net cash used in financing activities | (3,442 | ) | (4,171 | ) | (1,507 | ) | 3,927 | (5,193 | ) | ||||||||||
Cash and cash equivalents: | |||||||||||||||||||
(Decrease) increase | (383 | ) | — | 13 | — | (370 | ) | ||||||||||||
Balance at beginning of year | 3,245 | — | 25 | — | 3,270 | ||||||||||||||
Balance at end of year | $ | 2,862 | $ | — | $ | 38 | $ | — | $ | 2,900 | |||||||||
for the year ended December 31, 2011 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Cash Provided by Operating Activities | |||||||||||||||||||
Net cash provided by operating activities | $ | 3,515 | $ | 3,775 | $ | 202 | $ | (3,879 | ) | $ | 3,613 | ||||||||
Cash Provided by (Used in) Investing Activities | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Capital expenditures | — | (26 | ) | (79 | ) | — | (105 | ) | |||||||||||
Other | — | 1 | 1 | — | 2 | ||||||||||||||
Financial services | |||||||||||||||||||
Proceeds from finance assets | — | — | 490 | — | 490 | ||||||||||||||
Net cash (used in) provided by investing activities | — | (25 | ) | 412 | — | 387 | |||||||||||||
Cash Provided by (Used in) Financing Activities | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Long-term debt issued | 1,494 | — | — | — | 1,494 | ||||||||||||||
Repurchases of stock | (1,327 | ) | — | — | — | (1,327 | ) | ||||||||||||
Dividends paid on common stock | (3,222 | ) | — | — | — | (3,222 | ) | ||||||||||||
Issuances of common stock | 29 | — | — | — | 29 | ||||||||||||||
Changes in amounts due to/from Altria Group, Inc. and subsidiaries | 441 | (28 | ) | (413 | ) | — | — | ||||||||||||
Financing fees and debt issuance costs | (24 | ) | — | — | — | (24 | ) | ||||||||||||
Cash dividends paid to parent | — | (3,666 | ) | (213 | ) | 3,879 | — | ||||||||||||
Other | 41 | (56 | ) | 21 | — | 6 | |||||||||||||
Net cash used in financing activities | (2,568 | ) | (3,750 | ) | (605 | ) | 3,879 | (3,044 | ) | ||||||||||
Cash and cash equivalents: | |||||||||||||||||||
Increase | 947 | — | 9 | — | 956 | ||||||||||||||
Balance at beginning of year | 2,298 | — | 16 | — | 2,314 | ||||||||||||||
Balance at end of year | $ | 3,245 | $ | — | $ | 25 | $ | — | $ | 3,270 | |||||||||
for the year ended December 31, 2010 | Altria Group, Inc. | PM USA | Non- Guarantor Subsidiaries | Total Consolidating Adjustments | Consolidated | ||||||||||||||
Cash Provided by Operating Activities | |||||||||||||||||||
Net cash provided by operating activities | $ | 2,726 | $ | 3,172 | $ | 486 | $ | (3,617 | ) | $ | 2,767 | ||||||||
Cash Provided by (Used in) Investing Activities | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Capital expenditures | — | (54 | ) | (114 | ) | — | (168 | ) | |||||||||||
Other | — | 3 | 112 | — | 115 | ||||||||||||||
Financial services | |||||||||||||||||||
Proceeds from finance assets | — | — | 312 | — | 312 | ||||||||||||||
Net cash (used in) provided by investing activities | — | (51 | ) | 310 | — | 259 | |||||||||||||
Cash Provided by (Used in) Financing Activities | |||||||||||||||||||
Consumer products | |||||||||||||||||||
Long-term debt issued | 1,007 | — | — | — | 1,007 | ||||||||||||||
Long-term debt repaid | (775 | ) | — | — | — | (775 | ) | ||||||||||||
Dividends paid on common stock | (2,958 | ) | — | — | — | (2,958 | ) | ||||||||||||
Issuances of common stock | 104 | — | — | — | 104 | ||||||||||||||
Changes in amounts due to/from Altria Group, Inc. and subsidiaries | 279 | 325 | (604 | ) | — | — | |||||||||||||
Financing fees and debt insurance costs | (6 | ) | — | — | — | (6 | ) | ||||||||||||
Cash dividends paid to parent | — | (3,438 | ) | (179 | ) | 3,617 | — | ||||||||||||
Other | 59 | (8 | ) | (6 | ) | — | 45 | ||||||||||||
Net cash used in financing activities | (2,290 | ) | (3,121 | ) | (789 | ) | 3,617 | (2,583 | ) | ||||||||||
Cash and cash equivalents: | |||||||||||||||||||
Increase | 436 | — | 7 | — | 443 | ||||||||||||||
Balance at beginning of year | 1,862 | — | 9 | — | 1,871 | ||||||||||||||
Balance at end of year | $ | 2,298 | $ | — | $ | 16 | $ | — | $ | 2,314 | |||||||||
2012 Quarters | |||||||||||||||
(in millions, except per share data) | 1st (a) | 2nd | 3rd | 4th | |||||||||||
Net revenues | $ | 5,647 | $ | 6,487 | $ | 6,242 | $ | 6,242 | |||||||
Gross profit | $ | 2,202 | $ | 2,494 | $ | 2,484 | $ | 2,383 | |||||||
Net earnings | $ | 1,195 | $ | 1,226 | $ | 657 | $ | 1,105 | |||||||
Net earnings attributable to noncontrolling interests | — | (1 | ) | — | (2 | ) | |||||||||
Net earnings attributable to Altria Group, Inc. | $ | 1,195 | $ | 1,225 | $ | 657 | $ | 1,103 | |||||||
Per share data: | |||||||||||||||
Basic EPS attributable to Altria Group, Inc. | $ | 0.59 | $ | 0.60 | $ | 0.32 | $ | 0.55 | |||||||
Diluted EPS attributable to Altria Group, Inc. | $ | 0.59 | $ | 0.60 | $ | 0.32 | $ | 0.55 | |||||||
Dividends declared | $ | 0.41 | $ | 0.41 | $ | 0.44 | $ | 0.44 | |||||||
Market price — high | $ | 31.00 | $ | 34.60 | $ | 36.29 | $ | 34.25 | |||||||
— low | $ | 28.00 | $ | 30.74 | $ | 32.72 | $ | 30.01 | |||||||
2011 Quarters | |||||||||||||||
(in millions, except per share data) | 1st | 2nd | 3rd | 4th | |||||||||||
Net revenues | $ | 5,643 | $ | 5,920 | $ | 6,108 | $ | 6,129 | |||||||
Gross profit | $ | 2,148 | $ | 1,972 | $ | 2,445 | $ | 2,374 | |||||||
Net earnings | $ | 938 | $ | 444 | $ | 1,174 | $ | 837 | |||||||
Net earnings attributable to noncontrolling interests | (1 | ) | — | (1 | ) | (1 | ) | ||||||||
Net earnings attributable to Altria Group, Inc. | $ | 937 | $ | 444 | $ | 1,173 | $ | 836 | |||||||
Per share data: | |||||||||||||||
Basic EPS attributable to Altria Group, Inc. | $ | 0.45 | $ | 0.21 | $ | 0.57 | $ | 0.41 | |||||||
Diluted EPS attributable to Altria Group, Inc. | $ | 0.45 | $ | 0.21 | $ | 0.57 | $ | 0.41 | |||||||
Dividends declared | $ | 0.38 | $ | 0.38 | $ | 0.41 | $ | 0.41 | |||||||
Market price — high | $ | 26.27 | $ | 28.13 | $ | 27.41 | $ | 30.40 | |||||||
— low | $ | 23.34 | $ | 25.81 | $ | 23.20 | $ | 25.94 | |||||||
2012 Quarters | |||||||||||||||
(in millions) | 1st | 2nd | 3rd | 4th | |||||||||||
Asset impairment, exit and implementation costs | $ | 4 | $ | 25 | $ | 11 | $ | 16 | |||||||
Tobacco and health judgments, including accrued interest | — | 1 | 3 | 1 | |||||||||||
PMCC decrease to allowance for losses and recoveries | — | (11 | ) | (33 | ) | — | |||||||||
Reduction to cumulative lease earnings related to the Closing Agreement | — | 7 | — | — | |||||||||||
SABMiller special items (a) | (309 | ) | 26 | 19 | 16 | ||||||||||
Loss on early extinguishment of debt | — | — | 874 | — | |||||||||||
$ | (305 | ) | $ | 48 | $ | 874 | $ | 33 | |||||||
2011 Quarters | |||||||||||||||
(in millions) | 1st | 2nd | 3rd | 4th | |||||||||||
Asset impairment, exit, implementation and integration costs | $ | 2 | $ | 3 | $ | 1 | $ | 220 | |||||||
Tobacco and health judgments, including accrued interest | — | 41 | — | 121 | |||||||||||
UST acquisition-related costs | 4 | — | 1 | 1 | |||||||||||
PMCC (decrease) increase to allowance for losses | — | — | (35 | ) | 60 | ||||||||||
Reduction to cumulative lease earnings related to the 2011 PMCC Leveraged Lease Charge | — | 490 | — | — | |||||||||||
SABMiller special items | (32 | ) | 57 | 11 | 46 | ||||||||||
$ | (26 | ) | $ | 591 | $ | (22 | ) | $ | 448 | ||||||
(a) | During the second quarter of 2012, Altria Group, Inc. determined that it had not recorded in its financial statements for the three months ended March 31, 2012, its share of non-cash gains from its equity investment in SABMiller, relating to SABMiller's strategic alliance transactions with Anadolu Efes and Castel that were closed during the first quarter of 2012. Because Altria Group, Inc. did not record these gains, it understated by $342 million, $222 million and $0.11 earnings before income taxes, net earnings and diluted earnings per share attributable to Altria Group, Inc., respectively, for the three months ended March 31, 2012. Altria Group, Inc. revised its first quarter of 2012 financial statements and reflected this revision in the financial statements as of and for the six months ended June 30, 2012. Financial results for the first quarter of 2012 reported above reflect this revision. |
Name | Office | Age |
Martin J. Barrington | Chairman of the Board and Chief Executive Officer | 59 |
David R. Beran | President and Chief Operating Officer | 58 |
Ivan S. Feldman | Vice President and Controller | 46 |
Michael B. French | Senior Vice President and Chief Marketing and Innovation Officer, Altria Client Services Inc. | 58 |
William F. Gifford, Jr. | President and Chief Executive Officer, Philip Morris USA Inc. | 42 |
Louanna O. Heuhsen | Vice President, Corporate Governance and Associate General Counsel | 62 |
Craig A. Johnson | President and Chief Executive Officer, Altria Group Distribution Company | 60 |
Denise F. Keane | Executive Vice President and General Counsel | 60 |
Salvatore Mancuso | Vice President and Treasurer, Finance and Strategy | 47 |
John R. Nelson | Executive Vice President and Chief Technology Officer | 60 |
Brian W. Quigley | President and Chief Executive Officer, U.S. Smokeless Tobacco Company LLC | 39 |
W. Hildebrandt Surgner, Jr. | Corporate Secretary and Senior Assistant General Counsel | 47 |
Howard A. Willard III | Executive Vice President and Chief Financial Officer | 49 |
Charles N. Whitaker | Senior Vice President, Human Resources & Compliance and Chief Compliance Officer | 46 |
Number of Shares to be Issued upon Exercise of Outstanding Options and Vesting of Deferred Stock (a) | Weighted Average Exercise Price of Outstanding Options (b) | Number of Shares Remaining Available for Future Issuance Under Equity Compensation Plans (c) | |
Equity compensation plans approved by shareholders (1) | 54,903 (2) | $— | 47,167,008 (3) |
(1) | The following plans have been approved by Altria Group, Inc. shareholders and have shares referenced in column (a) or column (c): the 2005 Performance Incentive Plan, the 2010 Performance Incentive Plan and the Stock Compensation Plan for Non-Employee Directors. |
(2) | Represents 54,903 shares of deferred stock. |
(3) | Includes 46,574,327 shares available under the 2010 Performance Incentive Plan and 592,681 shares available under the Stock Compensation Plan for Non-Employee Directors, and excludes shares reflected in column (a). |
Page | |
Consolidated Balance Sheets at December 31, 2012 and 2011 | |
Consolidated Statements of Earnings for the years ended December 31, 2012, 2011 and 2010 | |
Consolidated Statements of Comprehensive Earnings for the years ended December 31, 2012, 2011 and 2010 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2012, 2011 and 2010 | |
Consolidated Statements of Stockholders' Equity for the years ended December 31, 2012, 2011 and 2010 | |
Notes to Consolidated Financial Statements | |
Report of Independent Registered Public Accounting Firm | |
Report of Management on Internal Control Over Financial Reporting | |
Report of Independent Registered Public Accounting Firm on Financial Statement Schedule | S-1 |
Financial Statement Schedule - Valuation and Qualifying Accounts | S-2 |
2.1 | Distribution Agreement by and between Altria Group, Inc. and Kraft Foods Inc. (now known as Mondelēz International, Inc.), dated as of January 31, 2007. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 31, 2007 (File No. 1-08940). | ||
2.2 | Distribution Agreement by and between Altria Group, Inc. and Philip Morris International Inc., dated as of January 30, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 30, 2008 (File No. 1-08940). | ||
2.3 | Agreement and Plan of Merger by and among UST Inc., Altria Group, Inc., and Armchair Merger Sub, Inc., dated as of September 7, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on September 8, 2008 (File No. 1-08940). | ||
2.4 | Amendment No. 1 to the Agreement and Plan of Merger, dated as of September 7, 2008, by and among UST Inc., Altria Group, Inc., and Armchair Merger Sub, Inc., dated as of October 2, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on October 3, 2008 (File No. 1-08940). | ||
3.1 | Articles of Amendment to the Restated Articles of Incorporation of Altria Group, Inc. and Restated Articles of Incorporation of Altria Group, Inc. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2002 (File No. 1-08940). | ||
3.2 | Amended and Restated By-laws of Altria Group, Inc., effective February 26, 2013. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on February 26, 2013 (File No. 1-08940). | ||
4.1 | Indenture between Altria Group, Inc. and The Bank of New York (as successor in interest to JPMorgan Chase Bank, formerly known as The Chase Manhattan Bank), as Trustee, dated as of December 2, 1996. Incorporated by reference to Altria Group, Inc.'s Registration Statement on Form S-3/A filed on January 29, 1998 (No. 333-35143). | ||
4.2 | First Supplemental Indenture to Indenture, dated as of December 2, 1996, between Altria Group, Inc. and The Bank of New York (as successor in interest to JPMorgan Chase Bank, formerly known as The Chase Manhattan Bank), as Trustee, dated as of February 13, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on February 15, 2008 (File No. 1-08940). | ||
4.3 | Indenture among Altria Group, Inc., as Issuer, Philip Morris USA Inc., as Guarantor, and Deutsche Bank Trust Company Americas, as Trustee, dated as of November 4, 2008. Incorporated by reference to Altria Group, Inc.'s Registration Statement on Form S-3 filed on November 4, 2008 (No. 333-155009). | ||
4.4 | 5-Year Revolving Credit Agreement among Altria Group, Inc. and the Initial Lenders named therein and JPMorgan Chase Bank, N.A. and Citibank, N.A., as Administrative Agents, Barclays Capital, Credit Suisse Securities (USA) LLC, Deutsche Bank Securities Inc., Goldman Sachs Bank USA, The Bank of Nova Scotia and The Royal Bank of Scotland plc, as Syndication Agents and Sovereign Bank, HSBC Bank USA, National Association, Morgan Stanley Senior Funding, Inc., Wells Fargo Bank, National Association and U.S. Bank National Association, as Documentation Agents, dated as of June 30, 2011. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on June 30, 2011 (File No. 1-08940). | ||
4.5 | The Registrant agrees to furnish copies of any instruments defining the rights of holders of long-term debt of the Registrant and its consolidated subsidiaries that does not exceed 10 percent of the total assets of the Registrant and its consolidated subsidiaries to the Commission upon request. | ||
10.1 | Comprehensive Settlement Agreement and Release related to settlement of Mississippi health care cost recovery action, dated as of October 17, 1997. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 1997 (File No. 1-08940). | ||
10.2 | Settlement Agreement related to settlement of Florida health care cost recovery action, dated August 25, 1997. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on September 3, 1997 (File No. 1-08940). | ||
10.3 | Comprehensive Settlement Agreement and Release related to settlement of Texas health care cost recovery action, dated as of January 16, 1998. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 28, 1998 (File No. 1-08940). | ||
10.4 | Settlement Agreement and Stipulation for Entry of Judgment regarding the claims of the State of Minnesota, dated as of May 8, 1998. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended March 31, 1998 (File No. 1-08940). | ||
10.5 | Settlement Agreement and Release regarding the claims of Blue Cross and Blue Shield of Minnesota, dated as of May 8, 1998. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended March 31, 1998 (File No. 1-08940). | ||
10.6 | Stipulation of Amendment to Settlement Agreement and For Entry of Agreed Order regarding the settlement of the Mississippi health care cost recovery action, dated as of July 2, 1998. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended June 30, 1998 (File No. 1-08940). | ||
10.7 | Stipulation of Amendment to Settlement Agreement and For Entry of Consent Decree regarding the settlement of the Texas health care cost recovery action, dated as of July 24, 1998. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended June 30, 1998 (File No. 1-08940). | ||
10.8 | Stipulation of Amendment to Settlement Agreement and For Entry of Consent Decree regarding the settlement of the Florida health care cost recovery action, dated as of September 11, 1998. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended September 30, 1998 (File No. 1-08940). | ||
10.9 | Master Settlement Agreement relating to state health care cost recovery and other claims, dated as of November 23, 1998. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on November 25, 1998, as amended by Form 8-K/A filed on December 24, 1998 (File No. 1-08940). | ||
10.10 | Stipulation and Agreed Order Regarding Stay of Execution Pending Review and Related Matters, dated as of May 7, 2001. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on May 8, 2001 (File No. 1-08940). | ||
10.11 | Term Sheet effective December 17, 2012, between Philip Morris USA Inc., the other participating manufacturers, and various states and territories for settlement of the 2003-2012 Non-Participating Manufacturer Adjustment with those states. Incorporated by reference to Altria Group, Inc.'s Current Report on From 8-K filed on December 18, 2012 (File No. 1-08940). | ||
10.12 | Stock Purchase Agreement by and among Altria Group, Inc., Bradford Holdings, Inc. and John Middleton, Inc., dated as of October 31, 2007. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended September 30, 2007 (File No. 1-08940). | ||
10.13 | Employee Matters Agreement by and between Altria Group, Inc. and Kraft Foods Inc. (now known as Mondelēz International, Inc.), dated as of March 30, 2007. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on March 30, 2007 (File No. 1-08940). | ||
10.14 | Tax Sharing Agreement by and between Altria Group, Inc. and Kraft Foods Inc. (now known as Mondelēz International, Inc.), dated as of March 30, 2007. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on March 30, 2007 (File No. 1-08940). | ||
10.15 | Transition Services Agreement by and between Altria Corporate Services, Inc. and Kraft Foods Inc. (now known as Mondelēz International, Inc.), dated as of March 30, 2007. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on March 30, 2007 (File No. 1-08940). | ||
10.16 | Intellectual Property Agreement by and between Philip Morris International Inc. and Philip Morris USA Inc., dated as of January 1, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on March 28, 2008 (File No. 1-08940). | ||
10.17 | Employee Matters Agreement by and between Altria Group, Inc. and Philip Morris International Inc., dated as of March 28, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on March 28, 2008 (File No. 1-08940). | ||
10.18 | Tax Sharing Agreement by and between Altria Group, Inc. and Philip Morris International Inc., dated as of March 28, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on March 28, 2008 (File No. 1-08940). | ||
10.19 | Transition Services Agreement by and between Altria Corporate Services, Inc. and Philip Morris International Inc., dated as of March 28, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on March 28, 2008 (File No. 1-08940). | ||
10.20 | Guarantee made by Philip Morris USA Inc., in favor of the lenders party to the 5-Year Revolving Credit Agreement, dated as of June 30, 2011, among Altria Group, Inc., the lenders named therein, and JPMorgan Chase Bank, N.A. and Citibank, N.A., as Administrative Agents, dated as of June 30, 2011. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on June 30, 2011 (File No. 1-08940). | ||
10.21 | Financial Counseling Program. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2009 (File No. 1-08940).* | ||
10.22 | Benefit Equalization Plan, effective September 2, 1974, as amended.* | ||
10.23 | Form of Employee Grantor Trust Enrollment Agreement. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 1995 (File No. 1-08940).* | ||
10.24 | Form of Supplemental Employee Grantor Trust Enrollment Agreement. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2005 (File No. 1-08940).* | ||
10.25 | Automobile Policy. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 1997 (File No. 1-08940).* | ||
10.26 | Supplemental Management Employees' Retirement Plan of Altria Group, Inc., effective as of October 1, 1987, as amended and in effect as of January 1, 2012. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended March 31, 2012 (File No. 1-08940).* | ||
10.27 | Unit Plan for Incumbent Non-Employee Directors, effective January 1, 1996, as amended effective October 1, 2012.* | ||
10.28 | Grantor Trust Agreement by and between Altria Client Services Inc. and Wells Fargo Bank, National Association, dated February 23, 2011. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2010 (File No. 1-08940).* | ||
10.29 | Long-Term Disability Benefit Equalization Plan, effective as of January 1, 1989, as amended. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended June 30, 2009 (File No. 1-08940).* | ||
10.30 | Survivor Income Benefit Equalization Plan, effective as of January 1, 1985, as amended and in effect as of January 1, 2010. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended June 30, 2011 (File No. 1-08940).* | ||
10.31 | 2000 Stock Compensation Plan for Non-Employee Directors, as amended and restated as of March 1, 2003. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2002 (File No. 1-08940).* | ||
10.32 | 2005 Performance Incentive Plan, effective on May 1, 2005. Incorporated by reference to Altria Group, Inc.'s definitive proxy statement filed on March 14, 2005 (File No. 1-08940).* | ||
10.33 | Deferred Fee Plan for Non-Employee Directors, as amended and restated effective October 1, 2012.* | ||
10.34 | Stock Compensation Plan for Non-Employee Directors, as amended and restated effective October 1, 2012.* | ||
10.35 | 2010 Performance Incentive Plan, effective on May 20, 2010. Incorporated by reference to Altria Group, Inc.'s definitive proxy statement filed on April 9, 2010 (File No. 1-08940).* | ||
10.36 | Kraft Foods Inc. (now known as Mondelēz International, Inc.) Supplemental Benefits Plan I (including First Amendment adding Supplement A), as amended and restated effective as of January 1, 1996. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2006 (File No. 1-08940).* | ||
10.37 | Agreement among Altria Group, Inc., Philip Morris USA Inc. and Michael E. Szymanczyk, dated as of May 15, 2002. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended June 30, 2002 (File No. 1-08940).* | ||
10.38 | Form of Indemnity Agreement. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on October 30, 2006 (File No. 1-08940). | ||
10.39 | Form of Restricted Stock Agreement, dated as of April 23, 2008. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on April 29, 2008 (File No. 1-08940).* | ||
10.40 | Form of Restricted Stock Agreement, dated as of January 27, 2009. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 29, 2009 (File No. 1-08940).* | ||
10.41 | Form of Restricted Stock Agreement, dated as of December 31, 2009. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2009 (File No. 1-08940).* | ||
10.42 | Form of Restricted Stock Agreement, dated as of January 26, 2010. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 28, 2010 (File No. 1-08940).* | ||
10.43 | Form of Restricted Stock Agreement, dated as of January 25, 2011. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 27, 2011(File No. 1-08940).* | ||
10.44 | Form of Deferred Stock Agreement, dated as of January 25, 2011. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 27, 2011 (File No. 1-08940).* | ||
10.45 | Form of Restricted Stock Agreement, dated as of January 25, 2012. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 27, 2012 (File No. 1-08940).* | ||
10.46 | Amendment to Restricted Stock Agreement, dated January 26, 2010, and Restricted Stock Agreement, dated April 23, 2008, each between Altria Group, Inc. and Michael E. Szymanczyk. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 27, 2012 (File No. 1-08940).* | ||
10.47 | Form of Restricted Stock Agreement, dated as of May 16, 2012. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on May 17, 2012 (File No. 1-08940).* | ||
10.48 | Form of Executive Confidentiality and Non-Competition Agreement. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 27, 2011 (File No. 1-08940).* | ||
10.49 | Time Sharing Agreement between Altria Client Services Inc. and Michael E. Szymanczyk, dated January 28, 2009. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 29, 2009 (File No. 1-08940).* | ||
10.50 | First Amendment to the Time Sharing Agreement between Altria Client Services Inc. and Michael E. Szymanczyk, dated November 12, 2009. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2009 (File No. 1-08940).* | ||
10.51 | Second Amendment to the Time Sharing Agreement between Altria Client Services Inc. and Michael E. Szymanczyk, effective October 14, 2010. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2010 (File No. 1-08940).* | ||
10.52 | Time Sharing Termination Letter to Michael E. Szymanczyk, dated May 17, 2012. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on May 17, 2012 (File No. 1-08940).* | ||
10.53 | Time Sharing Agreement between Altria Client Services Inc. and Martin J. Barrington, dated as of July 25, 2012. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended June 30, 2012 (File No. 1-08940).* | ||
10.54 | Time Sharing Agreement between Altria Client Services Inc. and David R. Beran, dated as of July 25, 2012. Incorporated by reference to Altria Group, Inc.'s Quarterly Report on Form 10-Q for the period ended June 30, 2012 (File No. 1-08940).* | ||
10.55 | Consulting Agreement between Altria Group, Inc. and Michael E. Szymanczyk, dated January 26, 2012. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 27, 2012 (File No. 1-08940).* | ||
10.56 | Agreement and General Release between Altria Group, Inc. and Michael E. Szymanczyk, dated January 26, 2012. Incorporated by reference to Altria Group, Inc.'s Current Report on Form 8-K filed on January 27, 2012 (File No. 1-08940).* | ||
12 | Statements regarding computation of ratios of earnings to fixed charges. | ||
21 | Significant subsidiaries of Altria Group, Inc. | ||
23 | Consent of independent registered public accounting firm. | ||
24 | Powers of attorney. | ||
31.1 | Certification of Chief Executive Officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934, as amended, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | ||
31.2 | Certification of Chief Financial Officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934, as amended, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | ||
32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | ||
32.2 | Certification of Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | ||
99.1 | Certain Litigation Matters. | ||
99.2 | Trial Schedule for Certain Cases. | ||
99.3 | Definitions of Terms Related to Financial Covenants Included in Altria Group, Inc.'s 5-Year Revolving Credit Agreement, dated as of June 30, 2011. Incorporated by reference to Altria Group, Inc.'s Annual Report on Form 10-K for the year ended December 31, 2011 (File No. 1-08940). | ||
101.INS | XBRL Instance Document. | ||
101.SCH | XBRL Taxonomy Extension Schema. | ||
101.CAL | XBRL Taxonomy Extension Calculation Linkbase. | ||
101.DEF | XBRL Taxonomy Extension Definition Linkbase. | ||
101.LAB | XBRL Taxonomy Extension Label Linkbase. | ||
101.PRE | XBRL Taxonomy Extension Presentation Linkbase. | ||
ALTRIA GROUP, INC. | |
By: /s/ MARTIN J. BARRINGTON | |
(Martin J. Barrington Chairman of the Board and Chief Executive Officer) | |
Signature | Title | Date | |||
/s/ MARTIN J. BARRINGTON (Martin J. Barrington) | Director, Chairman of the Board and Chief Executive Officer | February 27, 2013 | |||
/s/ HOWARD A. WILLARD III (Howard A. Willard III) | Executive Vice President and Chief Financial Officer | February 27, 2013 | |||
/s/ IVAN S. FELDMAN (Ivan S. Feldman) | Vice President and Controller | February 27, 2013 | |||
*ELIZABETH E. BAILEY, GERALD L. BALILES, JOHN T. CASTEEN III, DINYAR S. DEVITRE, THOMAS F. FARRELL II, THOMAS W. JONES, DEBRA J. KELLY-ENNIS W. LEO KIELY III, KATHRYN B. MCQUADE, GEORGE MUÑOZ, NABIL Y. SAKKAB | Directors | ||||
*By: | /s/ MARTIN J. BARRINGTON (MARTIN J. BARRINGTON ATTORNEY-IN-FACT) | February 27, 2013 | |||
Col. A | Col. B | Col. C | Col. D | Col. E | ||||||||||||||||
Additions | ||||||||||||||||||||
Description | Balance at Beginning of Period | Charged to Costs and Expenses | Charged to Other Accounts | Deductions | Balance at End of Period | |||||||||||||||
(a) | ||||||||||||||||||||
2012: | ||||||||||||||||||||
CONSUMER PRODUCTS: | ||||||||||||||||||||
Allowance for discounts | $ | — | $ | 619 | $ | — | $ | 619 | $ | — | ||||||||||
Allowance for returned goods | 54 | 114 | — | 126 | 42 | |||||||||||||||
$ | 54 | $ | 733 | $ | — | $ | 745 | $ | 42 | |||||||||||
FINANCIAL SERVICES: | ||||||||||||||||||||
Allowance for losses | $ | 227 | $ | (10 | ) | $ | — | $ | 118 | $ | 99 | |||||||||
2011: | ||||||||||||||||||||
CONSUMER PRODUCTS: | ||||||||||||||||||||
Allowance for discounts | $ | — | $ | 602 | $ | — | $ | 602 | $ | — | ||||||||||
Allowance for returned goods | 46 | 102 | — | 94 | 54 | |||||||||||||||
$ | 46 | $ | 704 | $ | — | $ | 696 | $ | 54 | |||||||||||
FINANCIAL SERVICES: | ||||||||||||||||||||
Allowance for losses | $ | 202 | $ | 25 | $ | — | $ | — | $ | 227 | ||||||||||
2010: | ||||||||||||||||||||
CONSUMER PRODUCTS: | ||||||||||||||||||||
Allowance for discounts | $ | — | $ | 606 | $ | — | $ | 606 | $ | — | ||||||||||
Allowance for doubtful accounts | 3 | — | — | 3 | — | |||||||||||||||
Allowance for returned goods | 47 | 86 | — | 87 | 46 | |||||||||||||||
$ | 50 | $ | 692 | $ | — | $ | 696 | $ | 46 | |||||||||||
FINANCIAL SERVICES: | ||||||||||||||||||||
Allowance for losses | $ | 266 | $ | — | $ | — | $ | 64 | $ | 202 | ||||||||||
Notes: | ||||||||||||||||||||
(a) Represents charges for which allowances were created | ||||||||||||||||||||