000074211212/312020FYFALSE207,010,502P5DP5DP5DP5D00007421122020-01-012020-12-31iso4217:USD0000742112us-gaap:CommonStockMember2020-06-300000742112us-gaap:CommonClassBMember2020-06-30xbrli:shares0000742112us-gaap:CommonStockMember2021-03-010000742112us-gaap:CommonClassBMember2021-03-0100007421122020-06-3000007421122019-01-012019-12-3100007421122018-01-012018-12-31iso4217:USDxbrli:shares00007421122020-12-3100007421122019-12-310000742112us-gaap:CommonStockMember2020-12-310000742112us-gaap:CommonStockMember2019-12-310000742112us-gaap:CommonClassBMember2020-12-310000742112us-gaap:CommonClassBMember2019-12-3100007421122018-12-3100007421122017-12-310000742112us-gaap:CommonStockMember2017-12-310000742112us-gaap:CommonClassBMember2017-12-310000742112us-gaap:AdditionalPaidInCapitalMember2017-12-310000742112us-gaap:RetainedEarningsMember2017-12-310000742112us-gaap:AccumulatedOtherComprehensiveIncomeMember2017-12-310000742112us-gaap:TreasuryStockMember2017-12-310000742112us-gaap:CommonStockMember2018-01-012018-12-310000742112us-gaap:AdditionalPaidInCapitalMember2018-01-012018-12-310000742112us-gaap:TreasuryStockMember2018-01-012018-12-310000742112us-gaap:RetainedEarningsMember2018-01-012018-12-310000742112us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-01-012018-12-310000742112us-gaap:CommonStockMember2018-12-310000742112us-gaap:CommonClassBMember2018-12-310000742112us-gaap:AdditionalPaidInCapitalMember2018-12-310000742112us-gaap:RetainedEarningsMember2018-12-310000742112us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-12-310000742112us-gaap:TreasuryStockMember2018-12-310000742112us-gaap:CommonStockMember2019-01-012019-12-310000742112us-gaap:AdditionalPaidInCapitalMember2019-01-012019-12-310000742112us-gaap:TreasuryStockMember2019-01-012019-12-310000742112us-gaap:RetainedEarningsMember2019-01-012019-12-310000742112us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310000742112us-gaap:CommonStockMember2019-12-310000742112us-gaap:AdditionalPaidInCapitalMember2019-12-310000742112us-gaap:RetainedEarningsMember2019-12-310000742112us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-12-310000742112us-gaap:TreasuryStockMember2019-12-310000742112us-gaap:CommonStockMember2020-01-012020-12-310000742112us-gaap:AdditionalPaidInCapitalMember2020-01-012020-12-310000742112us-gaap:TreasuryStockMember2020-01-012020-12-310000742112us-gaap:RetainedEarningsMember2020-01-012020-12-310000742112us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310000742112us-gaap:CommonStockMember2020-12-310000742112us-gaap:AdditionalPaidInCapitalMember2020-12-310000742112us-gaap:RetainedEarningsMember2020-12-310000742112us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310000742112us-gaap:TreasuryStockMember2020-12-310000742112us-gaap:FurnitureAndFixturesMembersrt:MinimumMember2020-01-012020-12-310000742112us-gaap:FurnitureAndFixturesMembersrt:MaximumMember2020-01-012020-12-310000742112us-gaap:BuildingAndBuildingImprovementsMembersrt:MinimumMember2020-01-012020-12-310000742112srt:MaximumMemberus-gaap:BuildingAndBuildingImprovementsMember2020-01-012020-12-310000742112ivc:InstitutionalProductsGroupMemberus-gaap:TrademarksMember2020-01-012020-12-310000742112ivc:InstitutionalProductsGroupMemberus-gaap:TrademarksMember2019-01-012019-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2016-03-31xbrli:pure0000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2020-12-310000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2017-06-300000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2020-12-3100007421122020-01-012020-03-0700007421122020-03-070000742112us-gaap:RiskLevelLowMember2020-01-012020-12-310000742112us-gaap:RiskLevelMediumMember2020-01-012020-12-310000742112us-gaap:RiskLevelHighMember2020-01-012020-12-31ivc:payment0000742112ivc:USAMember2020-12-310000742112ivc:USAMember2020-01-012020-12-310000742112ivc:AsiaPacMember2020-12-310000742112ivc:AsiaPacMember2020-01-012020-12-310000742112ivc:IVCCanadaMember2020-12-310000742112ivc:IVCCanadaMember2020-01-012020-12-310000742112ivc:USAMember2019-12-310000742112ivc:USAMember2019-01-012019-12-310000742112ivc:IVCCanadaMember2019-12-310000742112ivc:IVCCanadaMember2019-01-012019-12-310000742112us-gaap:MachineryAndEquipmentMember2020-12-310000742112us-gaap:MachineryAndEquipmentMember2019-12-310000742112us-gaap:LandBuildingsAndImprovementsMember2020-12-310000742112us-gaap:LandBuildingsAndImprovementsMember2019-12-310000742112us-gaap:FurnitureAndFixturesMember2020-12-310000742112us-gaap:FurnitureAndFixturesMember2019-12-310000742112us-gaap:LeaseholdImprovementsMember2020-12-310000742112us-gaap:LeaseholdImprovementsMember2019-12-310000742112ivc:CapitalizedSoftwareMember2020-12-310000742112ivc:CapitalizedSoftwareMember2019-12-3100007421122018-09-3000007421122020-04-012020-06-300000742112ivc:InstitutionalProductsGroupMember2018-12-310000742112ivc:EuropeEMEAMember2018-12-310000742112ivc:InstitutionalProductsGroupMember2019-01-012019-12-310000742112ivc:EuropeEMEAMember2019-01-012019-12-310000742112ivc:InstitutionalProductsGroupMember2019-12-310000742112ivc:EuropeEMEAMember2019-12-310000742112ivc:InstitutionalProductsGroupMember2020-01-012020-12-310000742112ivc:EuropeEMEAMember2020-01-012020-12-310000742112ivc:InstitutionalProductsGroupMember2020-12-310000742112ivc:EuropeEMEAMember2020-12-310000742112us-gaap:CustomerListsMember2020-12-310000742112us-gaap:CustomerListsMember2019-12-310000742112us-gaap:TrademarksMember2020-12-310000742112us-gaap:TrademarksMember2019-12-310000742112us-gaap:DevelopedTechnologyRightsMember2020-12-310000742112us-gaap:DevelopedTechnologyRightsMember2019-12-310000742112us-gaap:PatentsMember2020-12-310000742112us-gaap:PatentsMember2019-12-310000742112us-gaap:LicensingAgreementsMember2020-12-310000742112us-gaap:LicensingAgreementsMember2019-12-310000742112us-gaap:OtherIntangibleAssetsMember2020-12-310000742112us-gaap:OtherIntangibleAssetsMember2019-12-310000742112srt:MinimumMember2020-01-012020-12-310000742112srt:MaximumMember2020-01-012020-12-310000742112srt:WeightedAverageMember2020-01-012020-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2019-12-310000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2019-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00November2024Member2020-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00November2024Member2019-12-310000742112ivc:SeriesIIConvertibleSeniorNotesAt500November2024Memberus-gaap:ConvertibleSubordinatedDebtMember2020-12-310000742112ivc:SeriesIIConvertibleSeniorNotesAt500November2024Memberus-gaap:ConvertibleSubordinatedDebtMember2019-12-310000742112ivc:OtherNotesandCapitalLeaseObligationsMember2020-12-310000742112ivc:OtherNotesandCapitalLeaseObligationsMember2019-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorSubordinatedDebenturesat5.00February2021Domain2020-01-012020-12-310000742112us-gaap:LetterOfCreditMember2020-12-310000742112us-gaap:LetterOfCreditMember2019-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMemberus-gaap:RevolvingCreditFacilityMember2020-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMemberus-gaap:RevolvingCreditFacilityMembercurrency:EUR2020-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMemberus-gaap:RevolvingCreditFacilityMembercurrency:GBP2020-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMemberus-gaap:LetterOfCreditMember2020-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMemberus-gaap:RevolvingCreditFacilityMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:LondonInterbankOfferedRateLIBORMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMemberus-gaap:RevolvingCreditFacilityMembersrt:MinimumMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:LondonInterbankOfferedRateLIBORMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMemberus-gaap:RevolvingCreditFacilityMemberus-gaap:BaseRateMembersrt:MinimumMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberus-gaap:BaseRateMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMemberus-gaap:RevolvingCreditFacilityMembersrt:MinimumMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberivc:RevolvingCreditandSecurityAgreementNewCreditAgreementMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:RevolvingCreditFacilityMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomain2020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:LetterOfCreditMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomain2020-12-310000742112us-gaap:LineOfCreditMemberivc:SwingLineLoansDomainivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomain2020-12-310000742112us-gaap:LineOfCreditMemberivc:AmountAvailabletoInvacareLimitedandInvacarePoirierSASDomainivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomain2020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:RevolvingCreditFacilityMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomain2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:LondonInterbankOfferedRateLIBORMemberus-gaap:RevolvingCreditFacilityMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomainsrt:MinimumMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:LondonInterbankOfferedRateLIBORMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomain2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:RevolvingCreditFacilityMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomainus-gaap:BaseRateMembersrt:MinimumMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomainus-gaap:BaseRateMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMemberus-gaap:RevolvingCreditFacilityMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomainsrt:MinimumMember2020-01-012020-12-310000742112us-gaap:LineOfCreditMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberivc:RevolvingCreditandSecurityAgreementEuropeCreditAgreementMemberDomain2020-01-012020-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2019-09-300000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2020-09-3000007421122020-07-012020-09-300000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2020-01-012020-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2019-01-012019-12-3100007421122016-01-012016-03-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2016-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2016-01-012016-03-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2019-10-012019-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2020-04-012020-06-300000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2020-04-012020-06-300000742112ivc:SeriesIIConvertibleSeniorNotesAt500November2024Memberus-gaap:ConvertibleSubordinatedDebtMember2020-04-012020-06-300000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2020-01-012020-12-310000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2017-12-310000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2019-01-012019-12-310000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2017-04-012017-06-300000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00November2024Member2020-01-012020-12-310000742112ivc:ConvertibleSeniorNotesat5.00November2024Domainus-gaap:ConvertibleSubordinatedDebtMember2020-01-012020-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00November2024Member2019-10-012019-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00November2024Member2019-01-012019-12-310000742112ivc:SeriesIIConvertibleSeniorNotesAt500November2024Memberus-gaap:ConvertibleSubordinatedDebtMember2020-01-012020-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:SeriesIIConvertibleSeniorNotesAt500November2024Domain2020-01-012020-12-310000742112ivc:SeriesIIConvertibleSeniorNotesAt500November2024Memberus-gaap:ConvertibleSubordinatedDebtMember2020-06-300000742112ivc:CARESActLoanMember2020-05-150000742112ivc:CARESActLoanMember2020-12-3100007421122015-04-230000742112srt:MinimumMember2020-12-310000742112srt:MaximumMember2020-12-3100007421122015-01-012015-12-3100007421122015-04-222015-04-2300007421122020-07-010000742112us-gaap:SupplementalEmployeeRetirementPlanDefinedBenefitMember2020-01-012020-12-310000742112us-gaap:SupplementalEmployeeRetirementPlanDefinedBenefitMember2020-12-310000742112us-gaap:SupplementalEmployeeRetirementPlanDefinedBenefitMember2019-12-31utr:Y0000742112us-gaap:SupplementalEmployeeRetirementPlanDefinedBenefitMember2019-01-012019-12-310000742112us-gaap:SupplementalEmployeeRetirementPlanDefinedBenefitMember2018-01-012018-12-310000742112us-gaap:DomesticPlanMember2020-01-012020-12-310000742112us-gaap:DomesticPlanMember2019-01-012019-12-310000742112us-gaap:DomesticPlanMember2018-01-012018-12-310000742112us-gaap:ForeignPlanMemberus-gaap:ForeignPlanMember2020-01-012020-12-310000742112us-gaap:ForeignPlanMemberus-gaap:ForeignPlanMember2019-01-012019-12-310000742112us-gaap:ForeignPlanMemberus-gaap:ForeignPlanMember2018-01-012018-12-310000742112us-gaap:ProductMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112us-gaap:ServiceMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112us-gaap:ProductMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112us-gaap:ServiceMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112ivc:NorthAmericaNAMember2020-01-012020-12-310000742112us-gaap:ProductMemberivc:AllOtherMember2020-01-012020-12-310000742112us-gaap:ServiceMemberivc:AllOtherMember2020-01-012020-12-310000742112ivc:AllOtherMember2020-01-012020-12-310000742112us-gaap:ProductMember2020-01-012020-12-310000742112us-gaap:ServiceMember2020-01-012020-12-310000742112us-gaap:ProductMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112us-gaap:ServiceMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112us-gaap:ProductMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112us-gaap:ServiceMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112ivc:NorthAmericaNAMember2019-01-012019-12-310000742112us-gaap:ProductMemberivc:AllOtherMember2019-01-012019-12-310000742112us-gaap:ServiceMemberivc:AllOtherMember2019-01-012019-12-310000742112ivc:AllOtherMember2019-01-012019-12-310000742112us-gaap:ProductMember2019-01-012019-12-310000742112us-gaap:ServiceMember2019-01-012019-12-310000742112ivc:GeneralTermsandConditionsMember2020-01-012020-12-310000742112ivc:LargeNationalCustomersMember2020-01-012020-12-310000742112ivc:GovernmentTendersMember2020-01-012020-12-310000742112ivc:OtherCustomersMember2020-01-012020-12-31ivc:votes0000742112us-gaap:CommonClassBMember2020-01-012020-12-310000742112us-gaap:CommonStockMember2020-01-012020-12-310000742112ivc:A2018PlanMember2020-12-310000742112ivc:A2018PlanMember2020-01-012020-12-310000742112ivc:A2018PlanMember2020-07-012020-09-300000742112ivc:A2013PlanMember2020-12-310000742112us-gaap:EmployeeStockOptionMember2020-01-012020-12-310000742112us-gaap:EmployeeStockOptionMember2019-01-012019-12-310000742112us-gaap:EmployeeStockOptionMember2018-01-012018-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:EmployeeStockOptionMember2020-01-012020-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:EmployeeStockOptionMember2019-01-012019-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:EmployeeStockOptionMember2018-01-012018-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberivc:RestrictedStockandRestrictedStockUnitsRSUsMember2020-01-012020-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberivc:RestrictedStockandRestrictedStockUnitsRSUsMember2019-01-012019-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberivc:RestrictedStockandRestrictedStockUnitsRSUsMember2018-01-012018-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:PerformanceSharesMember2020-01-012020-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:PerformanceSharesMember2019-01-012019-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:PerformanceSharesMember2018-01-012018-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMember2020-01-012020-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMember2019-01-012019-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMember2018-01-012018-12-310000742112us-gaap:EmployeeStockOptionMember2020-12-310000742112us-gaap:EmployeeStockOptionMember2019-12-310000742112us-gaap:EmployeeStockOptionMember2018-12-310000742112ivc:RestrictedStockandRestrictedStockUnitsRSUsMember2020-12-310000742112ivc:RestrictedStockandRestrictedStockUnitsRSUsMember2019-12-310000742112ivc:RestrictedStockandRestrictedStockUnitsRSUsMember2018-12-310000742112us-gaap:PerformanceSharesMember2020-12-310000742112us-gaap:PerformanceSharesMember2019-12-310000742112us-gaap:PerformanceSharesMember2018-12-310000742112ivc:RangeOfExercisePriceOneMembersrt:MinimumMember2019-01-012019-12-310000742112ivc:RangeOfExercisePriceOneMembersrt:MinimumMember2018-01-012018-12-310000742112ivc:RangeOfExercisePriceThreeMembersrt:MaximumMember2020-01-012020-12-310000742112ivc:RangeOfExercisePriceThreeMembersrt:MaximumMember2019-01-012019-12-310000742112ivc:RangeOfExercisePriceThreeMembersrt:MaximumMember2018-01-012018-12-310000742112ivc:A2013PlanMember2019-12-310000742112ivc:A2013PlanMember2018-12-310000742112us-gaap:RestrictedStockMember2020-01-012020-12-310000742112us-gaap:PerformanceSharesMember2020-01-012020-12-310000742112ivc:RangeOfExercisePriceOneMembersrt:MinimumMember2020-01-012020-12-310000742112ivc:RangeOfExercisePriceOneMembersrt:MaximumMember2020-01-012020-12-310000742112ivc:RangeOfExercisePriceOneMemberus-gaap:EmployeeStockOptionMember2020-12-310000742112ivc:RangeOfExercisePriceOneMemberus-gaap:EmployeeStockOptionMember2020-01-012020-12-310000742112ivc:RangeOfExercisePriceTwoMembersrt:MinimumMember2020-01-012020-12-310000742112srt:MaximumMemberivc:RangeOfExercisePriceTwoMember2020-01-012020-12-310000742112us-gaap:EmployeeStockOptionMemberivc:RangeOfExercisePriceTwoMember2020-12-310000742112us-gaap:EmployeeStockOptionMemberivc:RangeOfExercisePriceTwoMember2020-01-012020-12-310000742112ivc:RangeOfExercisePriceThreeMembersrt:MinimumMember2020-01-012020-12-310000742112ivc:RangeOfExercisePriceThreeMemberus-gaap:EmployeeStockOptionMember2020-12-310000742112ivc:RangeOfExercisePriceThreeMemberus-gaap:EmployeeStockOptionMember2020-01-012020-12-310000742112ivc:RestrictedStockandRestrictedStockUnitsRSUsMember2017-12-310000742112ivc:RestrictedStockandRestrictedStockUnitsRSUsMember2020-01-012020-12-310000742112ivc:RestrictedStockandRestrictedStockUnitsRSUsMember2019-01-012019-12-310000742112ivc:RestrictedStockandRestrictedStockUnitsRSUsMember2018-01-012018-12-310000742112us-gaap:PerformanceSharesMember2017-12-310000742112us-gaap:PerformanceSharesMember2019-01-012019-12-310000742112us-gaap:PerformanceSharesMember2018-01-012018-12-310000742112us-gaap:PerformanceSharesMembersrt:WeightedAverageMember2020-01-012020-12-310000742112us-gaap:PerformanceSharesMembersrt:MinimumMember2019-12-310000742112srt:MaximumMemberus-gaap:PerformanceSharesMember2019-12-310000742112us-gaap:AccumulatedTranslationAdjustmentMember2019-12-310000742112ivc:AccumulatedLongTermNotesAdjustmentMember2019-12-310000742112us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2019-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2019-12-310000742112us-gaap:AccumulatedTranslationAdjustmentMember2020-01-012020-12-310000742112ivc:AccumulatedLongTermNotesAdjustmentMember2020-01-012020-12-310000742112us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-01-012020-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2020-01-012020-12-310000742112us-gaap:AccumulatedTranslationAdjustmentMember2020-12-310000742112ivc:AccumulatedLongTermNotesAdjustmentMember2020-12-310000742112us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2020-12-310000742112us-gaap:AccumulatedTranslationAdjustmentMember2018-12-310000742112ivc:AccumulatedLongTermNotesAdjustmentMember2018-12-310000742112us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2018-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2018-12-310000742112us-gaap:AccumulatedTranslationAdjustmentMember2019-01-012019-12-310000742112ivc:AccumulatedLongTermNotesAdjustmentMember2019-01-012019-12-310000742112us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2019-01-012019-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2019-01-012019-12-310000742112us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310000742112us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMemberus-gaap:ForeignExchangeForwardMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310000742112us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310000742112us-gaap:CommonStockMember2017-12-310000742112us-gaap:CommonStockMember2018-01-012018-12-310000742112us-gaap:CommonClassBMember2018-01-012018-12-310000742112us-gaap:TreasuryStockMember2018-01-012018-12-310000742112us-gaap:CommonStockMember2018-12-310000742112us-gaap:CommonStockMember2019-01-012019-12-310000742112us-gaap:CommonClassBMember2019-01-012019-12-310000742112us-gaap:TreasuryStockMember2019-01-012019-12-310000742112us-gaap:TreasuryStockMember2020-01-012020-12-310000742112ivc:NorthAmericaNAMember2018-01-012018-12-310000742112ivc:EuropeEMEAMember2018-01-012018-12-310000742112ivc:AllOtherMember2018-01-012018-12-310000742112us-gaap:EmployeeSeveranceMemberivc:NorthAmericaNAMember2018-01-012018-12-310000742112us-gaap:ContractTerminationMemberivc:NorthAmericaNAMember2018-01-012018-12-310000742112us-gaap:EmployeeSeveranceMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112us-gaap:ContractTerminationMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112us-gaap:EmployeeSeveranceMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112us-gaap:ContractTerminationMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112us-gaap:EmployeeSeveranceMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112us-gaap:ContractTerminationMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112us-gaap:EmployeeSeveranceMemberivc:NorthAmericaNAMember2017-12-310000742112us-gaap:ContractTerminationMemberivc:NorthAmericaNAMember2017-12-310000742112ivc:NorthAmericaNAMember2017-12-310000742112us-gaap:EmployeeSeveranceMemberivc:EuropeEMEAMember2017-12-310000742112us-gaap:ContractTerminationMemberivc:EuropeEMEAMember2017-12-310000742112ivc:EuropeEMEAMember2017-12-310000742112ivc:AllOtherMemberus-gaap:EmployeeSeveranceMember2017-12-310000742112ivc:AllOtherMemberus-gaap:ContractTerminationMember2017-12-310000742112ivc:AllOtherMember2017-12-310000742112us-gaap:EmployeeSeveranceMember2017-12-310000742112us-gaap:ContractTerminationMember2017-12-310000742112us-gaap:EmployeeSeveranceMemberivc:EuropeEMEAMember2018-01-012018-12-310000742112us-gaap:ContractTerminationMemberivc:EuropeEMEAMember2018-01-012018-12-310000742112ivc:AllOtherMemberus-gaap:EmployeeSeveranceMember2018-01-012018-12-310000742112ivc:AllOtherMemberus-gaap:ContractTerminationMember2018-01-012018-12-310000742112us-gaap:EmployeeSeveranceMember2018-01-012018-12-310000742112us-gaap:ContractTerminationMember2018-01-012018-12-310000742112us-gaap:EmployeeSeveranceMemberivc:NorthAmericaNAMember2018-12-310000742112us-gaap:ContractTerminationMemberivc:NorthAmericaNAMember2018-12-310000742112ivc:NorthAmericaNAMember2018-12-310000742112us-gaap:EmployeeSeveranceMemberivc:EuropeEMEAMember2018-12-310000742112us-gaap:ContractTerminationMemberivc:EuropeEMEAMember2018-12-310000742112ivc:AllOtherMemberus-gaap:EmployeeSeveranceMember2018-12-310000742112ivc:AllOtherMemberus-gaap:ContractTerminationMember2018-12-310000742112ivc:AllOtherMember2018-12-310000742112us-gaap:EmployeeSeveranceMember2018-12-310000742112us-gaap:ContractTerminationMember2018-12-310000742112ivc:AllOtherMemberus-gaap:EmployeeSeveranceMember2019-01-012019-12-310000742112ivc:AllOtherMemberus-gaap:ContractTerminationMember2019-01-012019-12-310000742112us-gaap:EmployeeSeveranceMember2019-01-012019-12-310000742112us-gaap:ContractTerminationMember2019-01-012019-12-310000742112us-gaap:EmployeeSeveranceMemberivc:NorthAmericaNAMember2019-12-310000742112us-gaap:ContractTerminationMemberivc:NorthAmericaNAMember2019-12-310000742112ivc:NorthAmericaNAMember2019-12-310000742112us-gaap:EmployeeSeveranceMemberivc:EuropeEMEAMember2019-12-310000742112us-gaap:ContractTerminationMemberivc:EuropeEMEAMember2019-12-310000742112ivc:AllOtherMemberus-gaap:EmployeeSeveranceMember2019-12-310000742112ivc:AllOtherMemberus-gaap:ContractTerminationMember2019-12-310000742112ivc:AllOtherMember2019-12-310000742112us-gaap:EmployeeSeveranceMember2019-12-310000742112us-gaap:ContractTerminationMember2019-12-310000742112us-gaap:EmployeeSeveranceMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112us-gaap:ContractTerminationMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112ivc:AllOtherMemberus-gaap:EmployeeSeveranceMember2020-01-012020-12-310000742112ivc:AllOtherMemberus-gaap:ContractTerminationMember2020-01-012020-12-310000742112us-gaap:EmployeeSeveranceMember2020-01-012020-12-310000742112us-gaap:ContractTerminationMember2020-01-012020-12-310000742112us-gaap:EmployeeSeveranceMemberivc:NorthAmericaNAMember2020-12-310000742112us-gaap:ContractTerminationMemberivc:NorthAmericaNAMember2020-12-310000742112ivc:NorthAmericaNAMember2020-12-310000742112us-gaap:EmployeeSeveranceMemberivc:EuropeEMEAMember2020-12-310000742112us-gaap:ContractTerminationMemberivc:EuropeEMEAMember2020-12-310000742112ivc:AllOtherMemberus-gaap:EmployeeSeveranceMember2020-12-310000742112ivc:AllOtherMemberus-gaap:ContractTerminationMember2020-12-310000742112ivc:AllOtherMember2020-12-310000742112us-gaap:EmployeeSeveranceMember2020-12-310000742112us-gaap:ContractTerminationMember2020-12-310000742112us-gaap:DomesticCountryMember2020-12-310000742112ivc:TaxYear2034to2037Memberus-gaap:DomesticCountryMember2020-12-310000742112us-gaap:DomesticCountryMemberivc:TaxYear2020to2022Member2020-12-310000742112ivc:TaxYear2023to2027Memberus-gaap:DomesticCountryMember2020-12-310000742112ivc:TaxYear2031to2037Memberus-gaap:DomesticCountryMember2020-12-310000742112ivc:TaxYear2020to2023Memberus-gaap:StateAndLocalJurisdictionMember2020-12-310000742112ivc:Taxyear2024to2033Memberus-gaap:StateAndLocalJurisdictionMember2020-12-310000742112us-gaap:StateAndLocalJurisdictionMemberivc:TaxYear2033andThereafterMember2020-12-310000742112us-gaap:StateAndLocalJurisdictionMemberivc:UnlimitedCarryoverMember2020-12-310000742112us-gaap:ForeignCountryMember2020-12-310000742112us-gaap:ForeignCountryMemberivc:TaxYear2026Member2020-12-310000742112us-gaap:EmployeeStockOptionMember2020-01-012020-12-310000742112us-gaap:EmployeeStockOptionMember2019-01-012019-12-310000742112us-gaap:EmployeeStockOptionMember2018-01-012018-12-310000742112us-gaap:EmployeeStockOptionMember2020-12-310000742112us-gaap:EmployeeStockOptionMember2019-12-310000742112us-gaap:EmployeeStockOptionMember2018-12-310000742112us-gaap:PaymentGuaranteeMember2020-12-310000742112ivc:OtherLongTermObligationsMemberus-gaap:PaymentGuaranteeMember2020-12-31ivc:Customer0000742112us-gaap:CustomerConcentrationRiskMemberus-gaap:SalesRevenueNetMember2020-01-012020-12-310000742112us-gaap:CustomerConcentrationRiskMemberus-gaap:SalesRevenueNetMember2020-12-310000742112us-gaap:ForeignExchangeForwardMember2020-01-012020-12-310000742112us-gaap:ForeignExchangeForwardMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDAUDMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDAUDMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDAUDMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDAUDMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDCADMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDCADMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDCADMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDCADMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDCHFMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDCHFMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDCHFMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDCHFMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDEURMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDEURMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDEURMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDEURMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDGBPMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDGBPMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDGBPMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDGBPMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDNZDMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDNZDMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDNZDMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDNZDMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDSEKMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDSEKMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDSEKMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDSEKMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDMXPMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDMXPMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDMXPMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDMXPMember2019-01-012019-12-310000742112ivc:ForeignExchangeForwardEURCADMemberus-gaap:DesignatedAsHedgingInstrumentMember2020-12-310000742112ivc:ForeignExchangeForwardEURCADMemberus-gaap:DesignatedAsHedgingInstrumentMember2020-01-012020-12-310000742112ivc:ForeignExchangeForwardEURCADMemberus-gaap:DesignatedAsHedgingInstrumentMember2019-12-310000742112ivc:ForeignExchangeForwardEURCADMemberus-gaap:DesignatedAsHedgingInstrumentMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURCHFMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURCHFMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURCHFMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURCHFMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURGBPMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURGBPMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURGBPMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURGBPMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURNOKMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURNOKMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURNOKMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURNOKMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURSEKMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURSEKMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURSEKMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURSEKMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardAUDNZDMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardAUDNZDMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardAUDNZDMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardAUDNZDMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardDKKSEKMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardDKKSEKMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardDKKSEKMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardDKKSEKMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardNOKSEKMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardNOKSEKMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardNOKSEKMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardNOKSEKMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardAUDTHBMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardAUDTHBMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardAUDTHBMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardAUDTHBMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardNZDTHBMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardNZDTHBMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardNZDTHBMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardNZDTHBMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDTHBMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDTHBMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDTHBMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardUSDTHBMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURTHBMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURTHBMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURTHBMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardEURTHBMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardGBPTHBMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardGBPTHBMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardGBPTHBMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberivc:ForeignExchangeForwardGBPTHBMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMember2020-01-012020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMember2019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMember2019-01-012019-12-310000742112us-gaap:NondesignatedMemberivc:ForeignExchangeForwardAUDUSDMember2020-12-310000742112us-gaap:NondesignatedMemberivc:ForeignExchangeForwardAUDUSDMember2020-01-012020-12-310000742112us-gaap:NondesignatedMemberivc:ForeignExchangeForwardAUDUSDMember2019-12-310000742112us-gaap:NondesignatedMemberivc:ForeignExchangeForwardAUDUSDMember2019-01-012019-12-310000742112ivc:ForeignExchangeForwardCADUSDMemberus-gaap:NondesignatedMember2020-12-310000742112ivc:ForeignExchangeForwardCADUSDMemberus-gaap:NondesignatedMember2020-01-012020-12-310000742112ivc:ForeignExchangeForwardCADUSDMemberus-gaap:NondesignatedMember2019-12-310000742112ivc:ForeignExchangeForwardCADUSDMemberus-gaap:NondesignatedMember2019-01-012019-12-310000742112ivc:ForeignExchangeForwardEURUSDMemberus-gaap:NondesignatedMember2020-12-310000742112ivc:ForeignExchangeForwardEURUSDMemberus-gaap:NondesignatedMember2020-01-012020-12-310000742112ivc:ForeignExchangeForwardEURUSDMemberus-gaap:NondesignatedMember2019-12-310000742112ivc:ForeignExchangeForwardEURUSDMemberus-gaap:NondesignatedMember2019-01-012019-12-310000742112ivc:ForeignExchangeForwardDKKUSDMemberus-gaap:NondesignatedMember2020-12-310000742112ivc:ForeignExchangeForwardDKKUSDMemberus-gaap:NondesignatedMember2020-01-012020-12-310000742112ivc:ForeignExchangeForwardDKKUSDMemberus-gaap:NondesignatedMember2019-12-310000742112ivc:ForeignExchangeForwardDKKUSDMemberus-gaap:NondesignatedMember2019-01-012019-12-310000742112ivc:ForeignExchangeForwardGBPUSDMemberus-gaap:NondesignatedMember2020-12-310000742112ivc:ForeignExchangeForwardGBPUSDMemberus-gaap:NondesignatedMember2020-01-012020-12-310000742112ivc:ForeignExchangeForwardGBPUSDMemberus-gaap:NondesignatedMember2019-12-310000742112ivc:ForeignExchangeForwardGBPUSDMemberus-gaap:NondesignatedMember2019-01-012019-12-310000742112us-gaap:NondesignatedMemberivc:ForeignExchangeForwardNZDUSDMember2020-12-310000742112us-gaap:NondesignatedMemberivc:ForeignExchangeForwardNZDUSDMember2020-01-012020-12-310000742112us-gaap:NondesignatedMemberivc:ForeignExchangeForwardNZDUSDMember2019-12-310000742112us-gaap:NondesignatedMemberivc:ForeignExchangeForwardNZDUSDMember2019-01-012019-12-310000742112ivc:ForeignExchangeForwardNZDAUDMemberus-gaap:NondesignatedMember2020-12-310000742112ivc:ForeignExchangeForwardNZDAUDMemberus-gaap:NondesignatedMember2020-01-012020-12-310000742112ivc:ForeignExchangeForwardNZDAUDMemberus-gaap:NondesignatedMember2019-12-310000742112ivc:ForeignExchangeForwardNZDAUDMemberus-gaap:NondesignatedMember2019-01-012019-12-310000742112ivc:ForeignExchangeForwardNOKUSDMemberus-gaap:NondesignatedMember2020-12-310000742112ivc:ForeignExchangeForwardNOKUSDMemberus-gaap:NondesignatedMember2020-01-012020-12-310000742112ivc:ForeignExchangeForwardNOKUSDMemberus-gaap:NondesignatedMember2019-12-310000742112ivc:ForeignExchangeForwardNOKUSDMemberus-gaap:NondesignatedMember2019-01-012019-12-310000742112us-gaap:NondesignatedMember2020-12-310000742112us-gaap:NondesignatedMember2020-01-012020-12-310000742112us-gaap:NondesignatedMember2019-12-310000742112us-gaap:NondesignatedMember2019-01-012019-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentAssetsMemberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:AccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentAssetsMemberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:AccruedLiabilitiesMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:OtherCurrentAssetsMemberus-gaap:ForeignExchangeForwardMemberus-gaap:NondesignatedMember2020-12-310000742112us-gaap:AccruedLiabilitiesMemberus-gaap:ForeignExchangeForwardMemberus-gaap:NondesignatedMember2020-12-310000742112us-gaap:OtherCurrentAssetsMemberus-gaap:ForeignExchangeForwardMemberus-gaap:NondesignatedMember2019-12-310000742112us-gaap:AccruedLiabilitiesMemberus-gaap:ForeignExchangeForwardMemberus-gaap:NondesignatedMember2019-12-310000742112us-gaap:OtherCurrentAssetsMember2020-12-310000742112us-gaap:AccruedLiabilitiesMember2020-12-310000742112us-gaap:OtherCurrentAssetsMember2019-12-310000742112us-gaap:AccruedLiabilitiesMember2019-12-310000742112us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeForwardMember2020-01-012020-12-310000742112us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeForwardMember2019-01-012019-12-310000742112us-gaap:ForeignExchangeForwardMemberus-gaap:NondesignatedMember2020-01-012020-12-310000742112us-gaap:ForeignExchangeForwardMemberus-gaap:NondesignatedMember2019-01-012019-12-310000742112us-gaap:CashFlowHedgingMemberus-gaap:SalesMember2020-01-012020-12-310000742112us-gaap:CashFlowHedgingMemberus-gaap:CostOfSalesMember2020-01-012020-12-310000742112us-gaap:CashFlowHedgingMember2020-01-012020-12-310000742112us-gaap:CashFlowHedgingMemberus-gaap:SalesMember2019-01-012019-12-310000742112us-gaap:CashFlowHedgingMemberus-gaap:CostOfSalesMember2019-01-012019-12-310000742112us-gaap:CashFlowHedgingMember2019-01-012019-12-310000742112us-gaap:CashFlowHedgingMemberus-gaap:SalesMember2018-01-012018-12-310000742112us-gaap:CashFlowHedgingMemberus-gaap:CostOfSalesMember2018-01-012018-12-310000742112us-gaap:CashFlowHedgingMember2018-01-012018-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:ForeignExchangeForwardMember2020-01-012020-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:ForeignExchangeForwardMember2019-01-012019-12-310000742112us-gaap:SellingGeneralAndAdministrativeExpensesMemberus-gaap:ForeignExchangeForwardMember2018-01-012018-12-310000742112us-gaap:ConvertibleSubordinatedDebtMemberivc:ConvertibleSeniorNotesat5.00February2021Member2016-01-012016-12-310000742112ivc:ConvertibleSeniorNotesat4.50February2022Domainus-gaap:ConvertibleSubordinatedDebtMember2017-01-012017-12-310000742112us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberivc:ConvertibleDebt2021ConversionFeatureMember2020-12-310000742112ivc:ConvertibleDebt2022ConversionFeatureDomainus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-12-310000742112ivc:ConvertibleDebtBondHedgeMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-12-310000742112us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Memberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Memberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:CarryingReportedAmountFairValueDisclosureMember2020-12-310000742112us-gaap:EstimateOfFairValueFairValueDisclosureMember2020-12-310000742112us-gaap:CarryingReportedAmountFairValueDisclosureMember2019-12-310000742112us-gaap:EstimateOfFairValueFairValueDisclosureMember2019-12-310000742112us-gaap:OtherCurrentAssetsMemberus-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:OtherCurrentAssetsMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:OtherCurrentAssetsMemberus-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:OtherCurrentAssetsMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:AccountsPayableAndAccruedLiabilitiesMemberus-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:AccountsPayableAndAccruedLiabilitiesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:ForeignExchangeForwardMember2020-12-310000742112us-gaap:AccountsPayableAndAccruedLiabilitiesMemberus-gaap:CarryingReportedAmountFairValueDisclosureMemberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:AccountsPayableAndAccruedLiabilitiesMemberus-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:ForeignExchangeForwardMember2019-12-310000742112us-gaap:ForeignExchangeForwardMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMember2018-01-012018-12-310000742112us-gaap:IntersegmentEliminationMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112us-gaap:IntersegmentEliminationMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112us-gaap:IntersegmentEliminationMemberivc:EuropeEMEAMember2018-01-012018-12-310000742112us-gaap:IntersegmentEliminationMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112us-gaap:IntersegmentEliminationMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112us-gaap:IntersegmentEliminationMemberivc:NorthAmericaNAMember2018-01-012018-12-310000742112ivc:AllOtherMemberus-gaap:IntersegmentEliminationMember2020-01-012020-12-310000742112ivc:AllOtherMemberus-gaap:IntersegmentEliminationMember2019-01-012019-12-310000742112ivc:AllOtherMemberus-gaap:IntersegmentEliminationMember2018-01-012018-12-310000742112us-gaap:IntersegmentEliminationMember2020-01-012020-12-310000742112us-gaap:IntersegmentEliminationMember2019-01-012019-12-310000742112us-gaap:IntersegmentEliminationMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:LifestyleProductsMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:LifestyleProductsMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:LifestyleProductsMemberivc:EuropeEMEAMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:MobilityAndSeatingMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:MobilityAndSeatingMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:MobilityAndSeatingMemberivc:EuropeEMEAMember2018-01-012018-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2018-01-012018-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2020-01-012020-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2019-01-012019-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:EuropeEMEAMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:LifestyleProductsMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:LifestyleProductsMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:LifestyleProductsMemberivc:NorthAmericaNAMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:MobilityAndSeatingMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:MobilityAndSeatingMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:MobilityAndSeatingMemberivc:NorthAmericaNAMember2018-01-012018-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2018-01-012018-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2020-01-012020-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2019-01-012019-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:NorthAmericaNAMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMemberivc:MobilityAndSeatingMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMemberivc:MobilityAndSeatingMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMemberivc:MobilityAndSeatingMember2018-01-012018-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMemberivc:LifestyleProductsMember2020-01-012020-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMemberivc:LifestyleProductsMember2019-01-012019-12-310000742112us-gaap:OperatingSegmentsMemberivc:AllOtherMemberivc:LifestyleProductsMember2018-01-012018-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:AllOtherMember2020-01-012020-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:AllOtherMember2019-01-012019-12-310000742112ivc:RespiratoryTherapyMemberus-gaap:OperatingSegmentsMemberivc:AllOtherMember2018-01-012018-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:AllOtherMember2020-01-012020-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:AllOtherMember2019-01-012019-12-310000742112ivc:OtherProductsAndServicesMemberus-gaap:OperatingSegmentsMemberivc:AllOtherMember2018-01-012018-12-3100007421122020-01-012020-03-3100007421122020-10-012020-12-3100007421122019-01-012019-03-3100007421122019-04-012019-06-3000007421122019-07-012019-09-3000007421122019-10-012019-12-310000742112us-gaap:AllowanceForCreditLossMember2019-12-310000742112us-gaap:AllowanceForCreditLossMember2020-01-012020-12-310000742112us-gaap:AllowanceForCreditLossMember2020-12-310000742112us-gaap:InventoryValuationReserveMember2019-12-310000742112us-gaap:InventoryValuationReserveMember2020-01-012020-12-310000742112us-gaap:InventoryValuationReserveMember2020-12-310000742112us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2019-12-310000742112us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2020-01-012020-12-310000742112us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2020-12-310000742112us-gaap:WarrantyReservesMember2019-12-310000742112us-gaap:WarrantyReservesMember2020-01-012020-12-310000742112us-gaap:WarrantyReservesMember2020-12-310000742112ivc:ProductLiabilityReservesMember2019-12-310000742112ivc:ProductLiabilityReservesMember2020-01-012020-12-310000742112ivc:ProductLiabilityReservesMember2020-12-310000742112us-gaap:AllowanceForCreditLossMember2018-12-310000742112us-gaap:AllowanceForCreditLossMember2019-01-012019-12-310000742112us-gaap:InventoryValuationReserveMember2018-12-310000742112us-gaap:InventoryValuationReserveMember2019-01-012019-12-310000742112us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2018-12-310000742112us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2019-01-012019-12-310000742112us-gaap:WarrantyReservesMember2018-12-310000742112us-gaap:WarrantyReservesMember2019-01-012019-12-310000742112ivc:ProductLiabilityReservesMember2018-12-310000742112ivc:ProductLiabilityReservesMember2019-01-012019-12-310000742112us-gaap:AllowanceForCreditLossMember2017-12-310000742112us-gaap:AllowanceForCreditLossMember2018-01-012018-12-310000742112us-gaap:InventoryValuationReserveMember2017-12-310000742112us-gaap:InventoryValuationReserveMember2018-01-012018-12-310000742112us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2017-12-310000742112us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2018-01-012018-12-310000742112us-gaap:WarrantyReservesMember2017-12-310000742112us-gaap:WarrantyReservesMember2018-01-012018-12-310000742112ivc:ProductLiabilityReservesMember2017-12-310000742112ivc:ProductLiabilityReservesMember2018-01-012018-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
—————————————————————
FORM 10-K
—————————————————————
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2020
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number 1-15103
—————————————————————
INVACARE CORPORATION
(Exact name of Registrant as specified in its charter)
| | | | | |
| Ohio | 95-2680965 |
(State or other Jurisdiction of
Incorporation or Organization) | (I.R.S. Employer
Identification Number) |
One Invacare Way, Elyria, Ohio 44035
(Address of principal executive offices) (Zip Code)
Registrant's telephone number, including area code: (440) 329-6000
—————————————————————
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol | Name of exchange on which registered |
| Common Shares, without par value | IVC | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
—————————————————————
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined by Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the Registrant (1) has filed all reports to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports) and (2) has been subject to the filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such short period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | |
| Large Accelerated filer | ☐ | Accelerated filer | ☒ | Emerging growth company | ☐ |
| Non-accelerated filer | ☐ | Smaller reporting company | ☐ | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report Yes ☒ No ☐
Indicate by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Act). Yes ☐ No ☒
As of June 30, 2020, the aggregate market value of the 32,491,367 Common Shares of the Registrant held by non-affiliates was $206,970,008 and the aggregate market value of the 6,357 Class B Common Shares of the Registrant held by non-affiliates was $40,494. While the Class B Common Shares are not listed for public trading on any exchange or market system, shares of that class are convertible into Common Shares at any time on a share-for-share basis. The market values indicated were calculated based upon the last sale price of the Common Shares as reported by The New York Stock Exchange on June 30, 2020, which was $6.37. For purposes of this information, the 1,921,755 Common Shares and 0 Class B Common Shares which were held by Executive Officers and Directors of the Registrant were deemed to be the Common Shares and Class B Common Shares held by affiliates.
As of March 1, 2021, there were 34,420,502 Common Shares and 3,667 Class B Common Shares outstanding.
Documents Incorporated By Reference
Portions of the Registrant's definitive Proxy Statement to be filed in connection with its 2021 Annual Meeting of Shareholders are incorporated by reference into Part III (Items 10, 11, 12, 13 and 14) of this report.
Except as otherwise stated, the information contained in this Annual Report on Form 10-K is as of December 31, 2020.
INVACARE CORPORATION
2020 ANNUAL REPORT ON FORM 10-K CONTENTS
| | | | | | | | |
| | |
| Item | | Page |
| PART I: |
| 1 | | |
| 1A. | | |
| 1B. | | |
| 2 | | |
| 3 | | |
| 4 | | |
| | |
|
| PART II: |
| 5 | | |
| 6 | | |
| 7 | | |
| 7A. | | |
| 8 | | |
| 9 | | |
| 9A. | | |
| 9B. | | |
|
| PART III: |
| 10 | | |
| 11 | | |
| 12 | | |
| 13 | | |
| 14 | | |
|
| PART IV: |
| 15 | | |
| 16 | | |
| |
Item 1. Business.
GENERAL
Invacare Corporation (“Invacare,” the “company,” including its subsidiaries, unless otherwise noted) is a leading manufacturer and distributor in its markets for medical equipment used in non-acute care settings. At its core, the company designs, manufactures and distributes medical devices that help people to move, breathe, rest and perform essential hygiene. The company provides clinically complex medical device solutions for congenital (e.g., cerebral palsy, muscular dystrophy, spina bifida), acquired (e.g., stroke, spinal cord injury, traumatic brain injury, post-acute recovery, pressure ulcers) and degenerative (e.g., ALS, multiple sclerosis, chronic obstructive pulmonary disease (COPD), age related, bariatric) conditions. The company's products are an important component of care for people facing a wide range of medical challenges, from those who are active and heading to work or school each day and may need additional mobility or respiratory support, to those who are cared for in residential care settings, at home and in rehabilitation centers. The company sells its products principally to home medical equipment providers through retail and e-commerce channels, residential care operators, dealers and government health services in North America, Europe and Asia Pacific. Invacare's products are sold through its worldwide distribution network by its sales force, independent manufacturers' representatives, and distributors.
Invacare is committed to providing medical products that deliver the best clinical value; promote recovery, independence and active lifestyles; and support long-term conditions and palliative care. The company's global tagline - Yes, You Can.® is indicative of the "can do" attitude of many of the people who use the company's products and their care providers. In everything it does, the company strives to leave its stakeholders with its brand promise - Making Life's Experiences Possible®.
The company is a corporation organized under the laws of the State of Ohio in 1971. When the company was first established as a stand-alone enterprise in December 1979, it had $19.5 million in net sales and a limited product line of basic wheelchairs and patient aids. Since then, the company has made approximately fifty acquisitions and, after some recent divestitures to harmonize its portfolio, Invacare's net sales in 2020 were approximately $900 million. Based upon the company's distribution channels, breadth of product lines and net sales, Invacare is a leading company in many of the following medical product categories: custom power wheelchairs; custom manual wheelchairs; electromotive technology to augment wheelchairs and recreational products; recreational adaptive sports products; non-acute bed systems; patient
transfer and bathing equipment; and supplementary respiratory therapy devices.
THE NON-ACUTE DURABLE MEDICAL EQUIPMENT INDUSTRY
The non-acute durable medical equipment market includes a broad range of equipment and services that enable the care and lifestyle needs of individuals with a broad range of conditions. With expected long-term pressure to control healthcare spending per capita, the company believes the market for equipment and services that support higher acuity care in lower acuity settings will continue to grow. Healthcare payors and providers continue to seek to optimize therapies which result in improved outcomes, reduced cost protocols, and ultimately, earlier discharge, including recovery and treatment in non-acute settings. Care in these settings may reduce exposure to concomitant issues and be preferred by patients.
As healthcare costs continue to increase, the interests of patients and healthcare providers are converging to focus on the most cost-effective delivery of the best care. As healthcare payors become more judicious in their spending, companies that provide better care or demonstrate better clinical outcomes will have an advantage. With its diverse product portfolio, clinical solutions, global scale and focus on the non-acute care setting, the company believes it is well positioned to serve this growing market.
Macro trends are impacting the world's aging population. While institutional care will likely remain an important part of healthcare systems in the wealthiest economies, the company believes care settings other than traditional hospitals will increasingly provide higher acuity care. With a broad product offering, diversified channels of trade, and infrastructure capable of serving many of the largest healthcare economies, the company believes it is well positioned to benefit from these global demographic trends and changes to the provision of healthcare.
North America Market
The population of the United States is growing and aging. As a result, there is a greater prevalence of disability among major U.S. population groups and an increasing need for assistance and care. The U.S. Census Bureau has projected the U.S. population will continue to grow to an estimated 400 million by 2050. Along the way, the bolus of Baby Boomers is expected to continue to raise the average age of the U.S. population. By 2030, the government estimates that more than 20% of the U.S. population will
consist of individuals over the age of 65, a 50% increase compared to the population in 2010.
In the United States, healthcare provision is supported by reimbursement from the federal Centers for Medicare and Medicaid Services (“CMS”), the Department of Veterans Affairs, state agencies, private payors and healthcare recipients themselves. In total, CMS estimates U.S. national healthcare expenditures will grow by more than 5% annually between 2019 and 2028. At this rate, healthcare spending would exceed GDP growth by 1%, which will sustain pressure to deploy care in ways that deliver the best outcomes for lower cost.
The Canadian health care system is a publicly funded model that provides coverage to all citizens. Provinces and territories are primarily responsible for the administration and delivery of Canada's health care services, and all health insurance plans are expected to meet the national guidelines established by the Canada Health Act. The objective of the Canada Health Act is to provide consumer-centered support and funding to residents with long-term physical disabilities and to provide access to personalized assistive devices that meet the basic needs of each patient. Each provincial and territorial health insurance plan differs with respect to reimbursement policies and product specification standards, allowing healthcare services to be adjusted based on regional needs. Invacare sells across Canada, taking into consideration the regional differences among the various provinces and territories.
Europe, Middle East and Africa Markets
While the healthcare equipment market in each country in Europe has distinct characteristics, many of the factors driving demand and affecting reimbursement are consistent with those in North America: population aging; more patients with chronic illnesses; an increasing preference to deliver healthcare outside hospitals; and a focus on the use of technology to increase productivity and reduce ancillary costs. Each European country has variations in product specifications and service requirements, regulations, distribution needs and reimbursement policies. These differences, as well as differences in the competitive landscape, require the company to tailor its approach based on the local market and the reimbursement requirements into which the products are being sold. The company's core strategy is to address these distinct markets with global product platforms that are localized with country-specific adjustments as necessary. This is especially the case for power wheelchairs, manual wheelchairs, and respiratory products. Customers in all European markets typically make product selections based upon quality, features, alignment with local reimbursement requirements, ability to reduce total cost of care, and customer service.
The company serves various markets in Eastern Europe, Russia, Middle East and Africa. It approaches these markets with the global portfolio of products developed and manufactured elsewhere. Sales in these markets are made somewhat opportunistically to balance changes in demand and specific product requirements. Often, sales in the Middle East and Africa represent episodic tenders as well as in some cases consistent sustained trade over the years. Most of the company's sales in these markets result from business conducted in Western Europe, as well as through dedicated local distributors.
Asia Pacific Market
The company's Asia Pacific market comprises revenue from products sold in Australia, New Zealand, China, Japan, Korea, India and Southeast Asia. Invacare's Asia Pacific businesses sell through multiple channels. Mobility and seating products are sold directly in New Zealand and through a network of dealers in all other countries, with almost all sales funded to reimbursed levels directly by governmental payors. Homecare products are sold via a dealer network that sells products to the consumer market. Long-term care products are sold via a dealer network and directly to care facilities. The company operates a rental business in New Zealand supporting the three largest hospital districts on New Zealand's North Island. Sales to other parts of Asia are sold via distributors and agents based in China, Japan, Korea, India and Southeast Asia. The company has a distribution and assembly center in Thailand.
Reimbursement
In most markets, the company does not make significant sales directly to end-users. In some markets, such as the United States, the United Kingdom and certain Scandinavian countries, the company sells directly to a government payor. In other markets, the company's customers purchase products to have available for use by, or re-sale to, end-users. These customers then work with end-users to determine what equipment may be needed to address the end-user's particular medical needs. Products are then provided to the end-user, and the company's customer may seek reimbursement on behalf of the consumer or sell the products, as appropriate. Product mix, pricing and payment terms vary by market. The company believes its market position and technical expertise will allow it to respond to ongoing changes in demand and reimbursement.
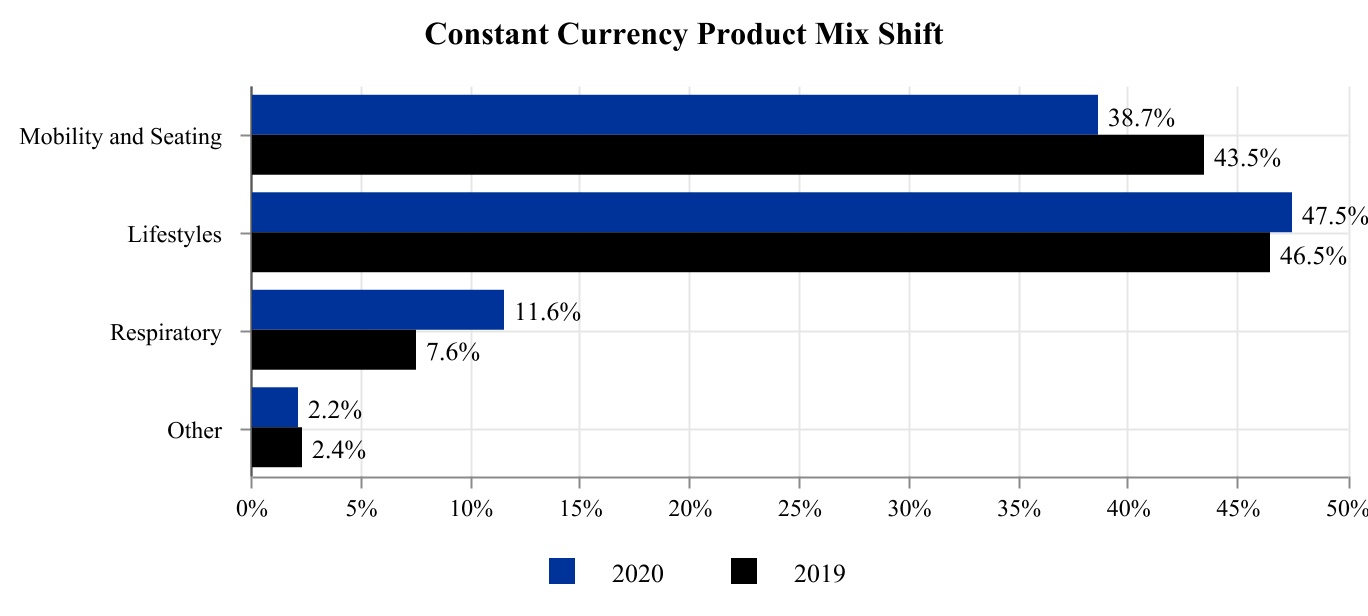
PRODUCT CATEGORIES
The company designs, manufactures, markets and distributes products in three key product categories: Mobility and Seating, Lifestyle and Respiratory.
Mobility and Seating
•Power Wheelchairs. This product category includes complex power wheelchairs for individuals who require powered mobility. The company's power wheelchair product offerings include products that can be highly customized to meet an individual end-user's needs, as well as products that are inherently versatile and designed to meet a broad range of requirements. Center-wheel drive power wheelchair lines include the Invacare® TDX® (Total Driving eXperience) product line and the ROVI® X3 and A3 power base product line, offered through the company's Motion Concepts subsidiary and also sold in Asia Pacific. The TDX line of power wheelchairs offers a combination of power, stability and maneuverability, including the Invacare® SureStep® suspension with Stability Lock and available G-Trac™ Technology. Seating systems offer elevate, power tilt and recline features. The ROVI A3 also offers the Multi-Positioning-Standing-MAXX System (MPS), an innovative, highly adjustable system that provides consumers the medical benefits of adjusting to a standing position throughout the day, adding additional independence, function and accessibility.
The company also offers rear-wheel drive power wheelchair technology through the Invacare® Storm Series®. The company launched a new generation of front and rear wheel drive power wheelchairs in early 2020 under the AVIVA® brand name. AVIVA replaces some of the legacy products, with compelling enhancements, and provides new market share opportunities. The market feedback after some months from the launch has been extremely positive. Several of the company's subsidiaries specialize in the development and implementation of complementary technology designed to enhance the utility of wheelchairs to meet unique and complex physiological needs. For example, Adaptive Switch Labs has developed alternative electronic control systems and human/machine input devices that enable wheelchair and environmental control via alternative interfaces to joysticks, such as sip/puff, eye-gaze, or head position inputs. Motion Concepts designs and produces custom powered seating and power positioning systems. The company continues to be a leader in this market with unique intellectual property in wheelchair suspension, and alternative controls.
•Custom Manual Wheelchairs. This product category includes products for independent everyday use, outdoor recreation, and casual and competitive sports, such as basketball, racing and tennis. These products are marketed under the Invacare® and Invacare® Top End® brand names. The company
markets a premiere line of lightweight, aesthetically-stylish custom manual wheelchairs under the Küschall® brand name. This Küschall family of products was renewed during 2020, adding to the traditional features, the new hydroforming technology. This technology forms material specifically to the areas of the highest strength needs using less total material. As a consequence, the rigidity and the driving performance of the chair improve significantly while the weight is reduced. These custom manual wheelchairs feature precision components and outstanding driving performance. The company provides a wide range of mobility solutions for everyday activities for active and passive users. The company's competitive advantages include a wide range of features and functionality and the ability to build purposeful custom wheelchairs, along with components which feature cross compatibility across the portfolio and wheelchairs that collapse to fit into very small spaces for ease of transportability.
•Seating and Positioning Products. At the core of care for seated end-users is the need for proper seating and positioning. Invacare designs, manufactures and markets some of the industry's best custom seating and positioning systems, custom molded and modular seat cushions, back supports and accessories to enable care givers to optimize the posture of their patients in mobility products. The Invacare® Seating and Positioning series provides seating solutions for less complex end-user needs. The Invacare® Matrx® Series offers versatile modular seating components with unique proprietary designs and materials designed to optimize pressure management and to help ensure long-term proper posture. The company's PinDot® series provides custom molded seat modules that can accommodate the most unique anatomic needs, and that can be adapted to fit with a wide range of mobility products. The company's ability to rapidly produce highly-customized products is highly specialized in the market, and is valued by therapists who need timely solutions for their patient's most complex clinical needs.
•Power Add-Ons The company sells innovative power add-on devices that enable manual wheelchair users to have optional electric power to augment manual propulsion and enable caretakers to more easily maneuver manual wheelchairs. This product category includes six main product lines: stairclimbers (scalamobil® and scalacombi); push and brake aids (viamobil® and viamobil eco), push-rim activated power assists (e-motion®, twion®, smoov®), Joystick controlled add-on kits (e-fix® and Esprit); Handbikes (e-pilot®) and E-Bike drive train components (neodrives®). Add-on drives can be retrofitted to almost any manual wheelchair. The
products are characterized by their light weight design and compactness, which makes them an ideal travel companion. Highly efficient hub motors along with the latest Lithium-Ion battery technology are used to enhance freedom of movement for users and their caregivers.
Lifestyle Products
•Pressure Relieving Sleep Surfaces. This product category includes a complete line of therapeutic pressure relieving overlays and mattress systems. The Invacare® Softform and microAIR® brand names feature a broad range of pressure relieving foam mattresses and powered mattresses with alternating pressure, low-air-loss, or rotational design features, which redistribute weight and assist with moisture management. These mattresses are designed to provide comfort, support and relief to those patients who are immobile or have limited mobility; who may have fragile skin or be susceptible to skin breakdown; and who spend long periods in bed.
•Safe Patient Handling. This product category includes products needed to assist caregivers in transferring individuals from surface to surface (e.g., bed to chair). Designed for use in the home or in institutional settings, these products include ceiling and floor lifts, sit-to-stand devices and a comprehensive line of slings. The company has very strong development in these areas with numerous launches in the last year: Birdie™, Evo, ISA™, and new Optislings.
•Beds. This product category includes wide variety of Invacare® branded semi-electric and fully-electric bed systems designed for both residential and institutional care for a range of patient sizes. The company's offering includes bed accessories, such as bedside rails, overbed tables and trapeze bars. The company's bed systems introduced the split-spring bed design, which is easier for home medical equipment providers to deliver, assemble and clean than other bed designs. Invacare's bed systems also feature patented universal bed-ends, where the headboard and footboard may be used interchangeably. This enables customers to more efficiently deploy their inventory.
•Manual Wheelchairs. This product category includes a complete line of manual wheelchairs. The company's manual wheelchairs are sold for use in the home and in institutional care settings. Consumers include people who are chronically or temporarily-disabled, require basic mobility with little or no frame modification, and may propel themselves or be moved by a caregiver. The company's manual wheelchairs are marketed under the Invacare® brand
name. Examples include the 9000, Tracer® wheelchair product lines, as well as the Action family of products in Europe.
•Personal Care. This product category includes a full line of personal care products, including ambulatory aids such as rollators, walkers, and wheeled walkers. The company also distributes bathing safety aids, such as tub transfer benches and shower chairs, as well as patient care products, such as commodes and other toileting aids. In markets where payors value durable long-lasting devices, especially those markets outside of the U.S., personal care products continue to be an important part of the company's lifestyle products business. In certain other markets, and in the U.S. in particular, this product area is focused on residential care.
Respiratory Therapy Products
The company designs and manufactures products that concentrate oxygen for consumers who need supplemental oxygen for breathing. Invacare® oxygen products are designed to meet a wide variety of patient needs, including stationary systems for use while at home and portable systems for mobile use. Historically, oxygen therapy required the delivery of large tanks of liquid oxygen or the routine delivery of tanks of compressed oxygen to patients. Industry trends continue to displace modes of oxygen therapy that involve delivery, which is costlier to provide and less convenient for patients who need to coordinate the exchange of oxygen containers. Published industry data suggests a large portion of the costs associated with home oxygen therapy are directly associated with delivery-related activities required to meet the ambulatory oxygen therapy needs of patients. Invacare's newer modalities of oxygen supply replace these costlier and constraining delivery-based forms of care.
•Stationary Oxygen Concentrators. Invacare oxygen concentrators are manufactured under the Platinum® and Perfecto2™ brand names and are available in five-, nine-, and ten-liter models. All Invacare stationary oxygen concentrators are designed to provide patients with durable equipment that reliably concentrates oxygen at home or in a healthcare setting. Stationary oxygen concentrators are typically used by people needing home or nocturnal oxygen, or by patients who have advanced-stage lung diseases and whose lifestyles keep them largely at home.
•Portable Oxygen Concentrators. One of two primary modalities for non-delivery supplementary ambulatory oxygen is the battery-powered portable category. Invacare's Platinum® Mobile Oxygen Concentrator has among the most competitive features in the five-liter equivalent category, including the industry's first wireless informatics
platform in the five-pound category. The informatics platform includes a user centric app which allows remote flow control of the portable concentrator from up to 25ft and a provider facing portal for remote fleet monitoring to help reduce unplanned dispatches and total operating costs.
•Oxygen Refilling Devices. The Invacare® HomeFill® Oxygen System is an alternative source of ambulatory oxygen that allows patients to fill their own convenient small portable oxygen cylinders from a stationary oxygen concentrator at home. This enables users to access high-flow stationary oxygen while at home and provides an easy-to-use form of mobile oxygen while away. As a result, medical equipment providers can significantly reduce time-consuming and costly service calls associated with cylinder and/or liquid oxygen deliveries, limit recurring high maintenance expenses and total cost of ownership while at the same time enhancing the lifestyle of the patient.
Other Products and Services
Other products and services include various services, including repair services, equipment rentals and external contracting. In certain regions of Europe and Asia Pacific, refurbishing of products is increasing as governments look for ways to lower costs while still providing needed equipment. A range of distributed products including a heart rate monitor, thermometer and nebulizer were launched for the Asia Pacific market. Portable ramps are sold throughout Asia and Europe, largely to public transport providers.
GEOGRAPHIC SEGMENTS
Europe
The company's Europe segment operates as an integrated unit across the European, Middle Eastern and African markets with sales and operations throughout Europe. The Europe segment is coordinated with other global business units for new product development, supply chain resources and additional corporate resources. This segment primarily includes mobility and seating; lifestyle; and respiratory therapy product lines. Products are sold through home healthcare providers and government provider agencies. In total, the Europe segment comprised 55.0%, 57.4% and 57.4% of net sales in 2020, 2019 and 2018, respectively.
North America
The company's North America segment comprises sales and operations throughout the United States, Mexico and Canada. This segment primarily includes mobility and
seating, lifestyle and respiratory therapy product lines. Products are sold through rehabilitation providers, home healthcare providers, and government provider agencies, such as the U.S. Department of Veterans Affairs. The North America segment represented 40.9%, 37.5% and 37.5% of net sales in 2020, 2019 and 2018, respectively.
All Other
All Other combines sales and services operations supporting customers principally in Australia and New Zealand and increasingly in other regions in the Asia Pacific market. All Other included the Dynamic Controls business, until it was divested in March 2020. All Other represented 4.1%, 5.1% and 5.1% of net sales in 2020, 2019 and 2018, respectively.
Refer to Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
For financial information regarding reportable segments, including revenues from external customers, products, segment profitability, assets and other information by segments, refer to Business Segments in the Notes to the Consolidated Financial Statements of this Annual Report on Form 10-K.
WARRANTY
Generally, the company's products are covered by warranties against defects in material and workmanship for product-specific warranty periods starting from the date of sale to the customer. Certain components, principally wheelchair and bed frames, carry a lifetime warranty.
COMPETITION
The durable medical equipment markets are highly competitive, and Invacare products face significant competition from other well-established manufacturers and distributors in the industry. Each country into which the company sells and markets its products has a set of unique conditions that impact competition, including healthcare coverage, forms and levels of reimbursement, presence of payor and provider structures and various competitors. Many factors may play a role in the selection of products and success of the company including specific features, aesthetics, quality, availability, service levels and price. Various competitors, from time to time, have instituted price-cutting programs in an effort to gain market share, and they may do so again in the future. In addition, reimbursement pressures may continue to persist in major markets, such as the U.S. These pressures have and may again significantly alter market dynamics. Increasingly, customers have access to manufacturers in low cost locations and are able to source certain products directly in
lieu of purchasing from Invacare or its traditional competitors, particularly for less complex products where price is the primary selection criterion.
The company believes that successfully increasing its market share is dependent on its ability to provide value to its customers based on clinical benefits, quality, performance, and durability of the company's products and services. In addition, the company's cost reduction achievements are expected to improve the market competitiveness of its products. Customers also value the technical and clinical expertise of the company's sales force, the effectiveness of the company's distribution system, the strength of its dealer and distributor network, the availability of prompt and reliable service for its products, and the ease of doing business with the company. The company's focus on quality is paramount. By embracing quality in all aspects of the company's activities, the company believes that its products will be better aligned with customer needs and, brought to market more quickly, resulting in a better customer experience and economic return.
SALES, MARKETING AND DISTRIBUTION
Europe
The company's European operations primarily conduct manufacturing, marketing and distribution functions in Western Europe and coordinate export sales activities through local distributors for markets in Eastern Europe, Russia, Middle East and Africa. The company utilizes an employee sales force and independent distributors. In markets where the company has its own sales force, product sales are made to medical equipment dealers and directly to government agencies. Marketing functions are staffed by central and regional teams to optimize coverage and content. The company operates distribution centers in various locations to optimize cost and delivery performance.
Prior to the pandemic, company representatives attended more than 50 trade shows annually across the European and Middle eastern geographies. With the pandemic, most of these trade shows have been cancelled and the company has changed to webinars and digital events. The company builds brand awareness through a strong presence in social media (LinkedIn, Facebook, Twitter, YouTube, Instagram) and has a dedicated blog that has achieved more than one million readers in 2020. In some European countries, the company sponsors key events and several individual wheelchair athletes and teams. In addition, every year the company conducts numerous marketing campaigns targeted to dealers, therapists, and end users to promote current and new products.
North America
In the United States, Invacare products are marketed primarily to clinical specialists in rehabilitation centers, long-term care facilities, hospice facilities, government agencies and residential care settings. The company markets to these medical professionals, who refer their patients to rehabilitation, HME, and government providers to obtain specific types of the company's medical equipment. The company sells its products to these providers.
In 2020, the North America sales force was primarily organized into three groups of specialized sales professionals focused on complex rehabilitation, post-acute care and respiratory products. Each team is focused on clinically complex products and solutions to support customer needs.
The company contributes extensively to editorial coverage in trade publications concerning the products the company manufactures. Company representatives attend numerous online trade shows and conferences on a national and regional basis in which Invacare products are displayed to providers, health care professionals, managed care professionals and consumers. The company also drives brand awareness through its website, as well as online communities of people who may use its products.
The company raises consumer awareness of its products through a strong presence in social and digital media as well as its sponsorship of a variety of wheelchair sporting events and its support of various philanthropic causes benefiting consumers of the company's products. The company's sponsorship of several individual wheelchair athletes and teams continued in 2020, including top-ranked male and female racers and handcyclists and wheelchair basketball teams. In addition, the company supports disabled veterans with its continuous sponsorship of the National Veterans Wheelchair Games, the largest annual wheelchair sporting event in the world, although the event did not take place in 2020 as a result of the pandemic. These sporting events bring a competitive and recreational sports experience to military veterans who, due to spinal cord injury, neurological conditions or amputation, use various assistive technology devices for their mobility needs.
The company's products are distributed through a network of facilities and directly from some manufacturing sites to optimize cost, inventory and delivery performance.
All Other
All Other comprises revenue primarily from Australia and New Zealand and to a lesser extent from other regions of the Asia Pacific market. The New Zealand revenues
include rental of durable medical equipment. It uses an employee sales force and service representatives to support this revenue. Products are distributed throughout Asia Pacific from a regional distribution center in Thailand, with complex rehabilitation product assembled in Thailand and sourced from global sources via a network of distribution nodes designed to optimize cost, inventory and delivery performance.
Sales and marketing efforts in Asia Pacific region are managed within the region and leveraged from other regions of the company. Sponsorship efforts are focused at the grass roots level and around programs designed to introduce people with disabilities to sports as a pathway to inclusion. While efforts were suspended in 2020 due to the pandemic, historically Invacare Australia sponsored the Summer Down Under Series, which culminated in the Oz Day 10K classic wheelchair race on Australia Day; Invacare New Zealand sponsored the Halberg Junior Disability Games and worked with local organizations to improve access for people with disabilities. Invacare supports a number of sporting organizations in the region, primarily focused on those that introduce people to sports.
PRODUCT LIABILITY COSTS
The company is self-insured in North America for product liability exposures through its captive insurance company, Invatection Insurance Company, which currently has a policy year that runs from September 1 to August 31 and insures annual policy losses up to $10,000,000 per occurrence and $13,000,000 in the aggregate. The company has additional layers of external insurance coverage, related to all lines of insurance, insuring up to $75,000,000 in aggregate losses per policy year arising from individual claims anywhere in the world that exceed the captive insurance company policy limits or the limits of the company's per-country foreign liability limits, as applicable. There can be no assurance that Invacare's current insurance levels will continue to be adequate or available at affordable rates.
Product liability reserves are recorded for individual claims based upon historical experience, industry expertise and other indicators. Additional reserves, in excess of the specific individual case reserves, are provided for incurred unreported claims based upon actuarial valuations at the time such valuations are conducted. Historical claims experience and other assumptions are taken into consideration by the company in estimating the ultimate reserves. For example, the actuarial analysis assumes that historical loss experience is an indicator of future experience, that the distribution of exposures by geographic area and nature of operations for ongoing operations is expected to be very similar to historical operations with no dramatic changes and that the government indices used to trend losses and exposures are
appropriate. Estimated amounts used in the calculation of reserves are adjusted on a regular basis and can be impacted by actual loss awards and claim settlements. While actuarial analysis is used to help determine adequate reserves, the company is responsible for determining and recording adequate reserves in accordance with accepted loss reserving standards and practices and applicable accounting principles.
PRODUCT DEVELOPMENT AND ENGINEERING
The company's strategy includes developing a cadence of meaningful new products in key markets and product areas. The intent is to have close collaboration in the development phase of new products with input from potential end users. As the result of work among the company's development groups in North America, Europe and Asia, Invacare launched a series of new innovations in 2020, including the following:
•AVIVA FX is a Front Wheel Drive (FWD) wheelchair that was designed for maximum comfort and control for both indoor and outdoor rides. It is ideal for getting around in indoor spaces negotiating tight corners with a small turn radius. The design also allows users to get up close to tables and into Wheelchair Accessible Vehicles. It has powerful climbing ability even at slow speeds. AVIVA FX features Invacare 4Sure™ suspension system designed to isolate the seat from vibrations and shock while keeping the seat angle virtually flat when traversing obstacles. All four wheels consistently keep contact with the ground providing optimum weight distribution for stability and traction control.
•AVIVA RX is a Rear Wheel Drive (RWD) wheelchair for indoor and outdoor use. Bringing together the key elements that define performance, these new rear wheel drive power chairs combine an innovative method of managing center of gravity for optimal weight distribution with superior C.T.C. (Control, Traction, Comfort) Suspension. It re-defines rear wheel performance, delivering unbeatable control, exceptional traction and extreme ride comfort. Designed to combat the challenges of everyday modern life whether at home, work or out and about, ready to face the world the AVIVA RX is a mobility product designed to enhance everyday life.
•AVIVA FX MPS Maxx - Multi-Position Power Standing System is an innovative, highly adjustable system incorporating power standing and a full range of power positioning features in a package that offers a unique combination of independence, functionality, and accessibility. ROVI A3 MPS Maxx - Modular Power Standing System is an innovative, highly adjustable system that combines a power standing function with a full range of power positioning
options, all in a modular design that provides consumers with a unique combination of independence, functionality, and accessibility.
•The Motion Concepts 407 seating system utilizes a simple design to optimize the stability of the base of the wheelchair, while providing up to 40º of tilt. This allows the end user to achieve a unique position where the head is below the feet, and the feet are above the heart, to assist with respiratory and circulatory conditions.
•The Motion Concepts UpFront thoughtfully combines a fully functioning power positioning system with 5.5” of forward shift, allowing the end user to access previously difficult to reach surfaces and objects.
•The Motion Concepts HD Series is a seating system which meets the individual needs of larger end users. The HD Series is designed to allow for exceptional driving performance, elegant design and maximum comfort and can accommodate higher weight capacities.
•For bariatric users a new product has been developed with additional functionalities for ease of transport and transfer. The Action AMPLA received seven international awards for innovative product solutions and will improve the quality of life for customers.
•In passive wheelchairs the new Clematis Pro has been launched and includes several improvements for customers. The user’s perception under tilting improved due to a geometry specially designed with human factors in consideration.
•A new bed and mattress system has been developed based on the NordBed, which was introduced in 2019. The NordBed Kid and the new mattresses are especially developed for children to fulfil their special needs with an aesthetically pleasing design.
•In Lifestyle for hygiene products, introduction of new shower chairs built from environmentally friendly material, PICO green. A high content of wood ratio in the material composites improves the antibacterial capabilities as well.
•The Lifestyle walking aids portfolio has been enhanced with a clean design and lightweight new rollator. The Gloss™ is cross foldable and the most lightweight rollator in its segment.
•For all Patient Lifters a new sling line has been launched under the Optislings brand, with improved material mix, improved shape and clarity regarding sizes.
MANUFACTURING AND SUPPLIERS
The company's objective is to efficiently deploy resources in its supply network to achieve the best quality, service performance and lowest total cost. The company seeks to achieve this result through a combination of inputs from Invacare facilities, contract manufacturers and key suppliers.
The company continues to emphasize quality excellence and efficiency across its manufacturing and distribution operations. The company is expanding its culture of deploying current Good Manufacturing Practices (“cGMP”) and Lean Manufacturing principles to eliminate waste throughout the network and will continue to pursue improvements in its manufacturing processes. At its core, the company's operations produce and distribute both custom-configured products for use in specialized clinical situations and standard products.
The company procures raw materials, components and finished goods from a global network of internal and external sources. The company utilizes regional sourcing offices to identify, develop and manage its external supply base. Where appropriate, Invacare utilizes suppliers across multiple regions to ensure flexibility, continuity and responsiveness. The company's network of engineering design centers, product management groups and sources of supply are used to optimize cost and satisfy customer demand.
The company continually reviews its operations network capacity, workforce skills and technologies along with its distribution network to optimize design, manufacture, sourcing and delivery performance, inventory and cost.
Europe
The company's manufacturing and assembly facilities in Europe are operated as centers of excellence, i.e., factories, with specific capabilities. The company manufactures power wheelchair products, wheelchair power add-ons and hygiene products in one single facility in Albstadt, Germany. Manual wheelchair production is based in Fondettes, France. The company manufactures beds in Portugal and Sweden for various markets. Invacare manufactures therapeutic support surfaces as well as seating and positioning products in the U.K. The Europe segment uses these internal sources and some external sources of finished goods and components to create the portfolio of products it distributes. Products manufactured or assembled in Europe are sold to external European customers as well as to other internal customers.
North America
The company operates several vertically integrated centers of excellence, i.e., factories, in North America, each with specific capabilities: custom powered wheelchairs, seating products and respiratory therapy products (Elyria, OH); manual and passive manual wheelchairs and patient aids (Reynosa, MX); beds, institutional case goods and respiratory therapy products (Sanford, FL); manual recreational and sport wheelchair products (Pinellas Park, FL), passive manual and pediatric wheelchairs (Simi Valley, CA); and seating and positioning systems (Toronto, Canada). Products designed and produced in North American operations are sold in North America and are shipped as finished goods and as subcomponents to internal and external customers globally.
Asia Pacific
Invacare Asia Pacific assembles components used primarily in rehabilitation products that serve Asia Pacific markets at a facility based in Thailand. The company operates a centralized distribution node in Thailand, with additional nodes in Australia and New Zealand, to supply customer needs while optimizing cost, inventory and service levels.
BUSINESS OPTIMIZATION ACTIONS
The company is executing a multi-year strategy to return the company to profitability by focusing its resources on products and solutions that provide greater healthcare value in clinically complex rehabilitation and post-acute care. The company's business optimization actions and growth plan balances innovative organic growth, product portfolio changes across all regions, and cost improvements in supply chain and administrative functions. Key elements of the business optimization and growth plans are:
•Globally, continue to drive all business segments and product lines based on their potential to achieve a leading market position and to support profitability goals;
•In Europe, leverage centralized innovation and supply chain capabilities while reducing the cost and complexity of a legacy infrastructure;
•In North America, adjust the portfolio to consistently grow profitability amid cost increases by adding new products, reducing costs and continuing to improve customers' experience;
•In Asia Pacific, remain focused on sustainable growth and expansion in the southeast Asia region; and,
•Take actions globally to reduce working capital and improve free cash flow.
As it navigates the uncertain business environment resulting from the pandemic, the company continues to allocate more resources to the business units experiencing increased demand and expects to continue taking actions to mitigate the potential negative financial and operational impacts on other parts of the company's business that have declined. In the medium-term, the company still expects to execute on its business improvement actions, such as the global IT modernization initiative which is expected to ultimately result in optimization of the operating structure.
The company believes that continued generation of earnings driven by operational performance, cash balances on hand, and expected free cash flow will support the company's on-going business improvement plans and enable it to address future debt maturities.
GOVERNMENT REGULATION
The company is governed by regulations that affect the manufacture, distribution, marketing and sale of its products and regulate healthcare reimbursement that may affect its customers and the company directly. These policies differ among and within every country in which the company operates. Changes in regulations, guidelines, procedural precedents, enforcement and healthcare policy take place frequently and can impact the size, growth potential and profitability of products sold in each market.
In many markets, healthcare costs have been consistently increasing in excess of the rate of inflation and as a percentage of GDP. Efforts to control payor's budgets have impacted reimbursement levels for healthcare programs. Private insurance companies often mimic changes in government programs. Reimbursement guidelines in the home healthcare industry have a substantial impact on the nature and type of equipment consumers can obtain and thus, affect the product mix, pricing and payment patterns of the company's customers who are typically the medical equipment providers to end-users.
The company has continued its efforts to influence public policies that impact home-based and long-term non-acute healthcare. The company has been actively educating federal and state legislators about the needs of the patient communities it serves and has worked with policy authors to ensure the industry's healthcare consumer needs are represented. The company believes its efforts have given the company a competitive advantage. Customers and end-users recognize the company's advocacy efforts, and the company has the benefit of remaining apprised of emerging policy direction.
FDA
The United States Food and Drug Administration (“FDA”) regulates the manufacture, distribution and marketing of medical devices. Under such regulation, medical devices are classified as Class I, Class II or Class III devices, depending on the level of risk posed to patients, with Class III designating the highest-risk devices. The company's principal products are designated as Class I or Class II. In general, Class I devices must comply with general controls, including, but not limited to, requirements related to establishment registration and device listing, labeling, medical device reporting, and the Quality System Regulation (QSR). In addition to general controls, certain Class II devices must comply with design controls, premarket notification and clearance, and applicable special controls. Domestic and foreign manufacturers of medical devices sold in the U.S. are subject to routine inspections by FDA. In addition, some foreign governments have adopted regulations relating to the design, manufacture and marketing of health care products, and imposing similar controls as the FDA regulations.
Other Medical Device Regulators
Outside the U.S., it is customary for foreign governments to have a ministry of health or similar government body that regulates and enforces regulations relating to the design, manufacture, distribution and marketing of medical devices. In some cases, there are common standards for design and testing. In some cases, there are country-specific requirements. These regulations are not always harmonized with those from other jurisdictions and in some cases, the consequence in costs, time to enter a market or support a product may be significant.
The company is currently conducting a program to bring its products designed, manufactured and sold into the European Union market, into conformance with the EU MDR (Medical Device Regulations). The company is compliant for all class I devices. Class II devices will be completed on or before the required date of May 27, 2024 date.
2012 Consent Decree, Taylor Street and Corporate Facilities
In December 2012, the company became subject to a consent decree of injunction filed by FDA with respect to the company's Corporate Headquarters and its Taylor Street facility's operations in Elyria, Ohio. The consent decree initially limited the company's (i) manufacture and distribution of power and manual wheelchairs, wheelchair components and wheelchair sub-assemblies at or from its Taylor Street manufacturing facility, except in verified
cases of medical necessity, (ii) design activities related to wheelchairs and power beds that take place at the impacted Elyria facilities and (iii) replacement, service and repair of products already in use and supplied from the Taylor Street manufacturing facility. Under the terms of the consent decree, in order to resume full operations, the company had to successfully complete independent, third-party expert certification audits at the impacted Elyria facilities, comprised of three distinct certification reports separately submitted to, and accepted by, the FDA; submit its own report to the FDA; and successfully complete a reinspection by the FDA of the company's Corporate and Taylor Street facilities.
On July 24, 2017, following its June 2017 reinspection of the Corporate and Taylor Street facilities, the FDA notified the company that it was in substantial compliance with the Federal Food, Drug and Cosmetic Act (FDA Act), FDA regulations and the terms of the consent decree and that the company was permitted to resume full operations at those facilities including the resumption of unrestricted sales of products made in those facilities.
The consent decree will continue in effect for at least five years from July 24, 2017, during which time the company's Corporate and Taylor Street facilities must complete two semi-annual audits in the first year following the lifting of the injunction and then an annual audit in each of the next four years performed by an independent company-retained audit firm. The expert audit firm will determine whether the facilities remain in continuous compliance with the FDA Act, regulations and the terms of the consent decree and issue post audit reports contemporaneously to the FDA and Invacare. The FDA has the authority to inspect these facilities and any other FDA registered facility, at any time.
In 2018, the company completed the two semi-annual independent expert audits and, in 2019 and 2020, the company completed the first two annual independent expert audit of the Corporate and Taylor Street facilities, as required under the consent decree, and in each case the facilities were found to remain in compliance with the FDA Act, the FDA regulations and the consent decree. The audit reports have been submitted to FDA. Following the 2020 independent audit, the FDA requested the company's corrective actions to address three of four issued minor observations. These corrective actions were provided to the FDA and there were no further requests concerning the audit or its results.
Under the consent decree, the FDA has the authority to order the company to take a wide variety of actions if the FDA finds that the company is not in compliance with the consent decree, FDA Act or FDA regulations, including requiring the company to cease all operations relating to Taylor Street products. The FDA also can order
the company to undertake a partial cessation of operations or a recall, issue a safety alert, public health advisory, or press release, or to take any other corrective action the FDA deems necessary with respect to Taylor Street products.
The FDA also has authority under the consent decree to assess liquidated damages of $15,000 per violation per day for any violations of the consent decree, FDA regulations or the FDA Act. The FDA also may assess liquidated damages for shipments of adulterated or misbranded devices in the amount of twice the sale price of any such adulterated or misbranded device. The liquidated damages, if assessed, are limited to a total of $7,000,000 for each calendar year. The authority to assess liquidated damages is in addition to any other remedies otherwise available to the FDA, including civil money penalties.
For additional information regarding the consent decree, refer to the following sections of this Annual Report on Form 10-K: Item 1. Business - Government Regulation; Item 1A. Risk Factors.
Other FDA Matters
As required, the company's facilities which produce products for sale in the U.S. are registered with the FDA. Those facilities are subject to inspections by the FDA at any time. Recent inspections of company facilities by or on behalf of the FDA are summarized in the following paragraphs.
From January 13, 2020 through January 31, 2020, FDA conducted a routine inspection under the Consent Decree of Invacare Headquarters and Taylor Street Operations. At the close of the inspection, three FDA Form 483 observations were issued. Invacare has responded to the FDA to address the 483 observations and the inspection was closed as No Action Indicated (NAI) meaning no objectionable conditions or practices were found during the inspection.
The company expects that substantially all of its facilities will be inspected by the FDA or other regulatory agencies from time to time. The frequency, duration, scope, findings and consequences of these inspections cannot be predicted.
From time to time, the company may undertake voluntary recalls or field corrective actions of the company's products to correct potential product safety issues that may arise, in furtherance of the company's high standards of quality, safety and effectiveness.
In December 2020, the company’s operations in Sanford, Florida and Elyria, Ohio underwent a successful MDSAP (Medical Device Single Audit Program)
surveillance audit. No findings were issued as a result of this audit.
Other Quality Accomplishments
In 2020, the company's main facilities in Europe, Asia and North America were again certified as meeting ISO 13485-2016 requirements, a stringent international standard for quality management systems, demonstrating its continued commitment to quality excellence.
National Competitive Bidding
In the United States, CMS is a significant payor and governs healthcare reimbursement for Medicare services. On January 1, 2011, CMS began its National Competitive Bidding ("NCB") program in nine metropolitan statistical areas (MSA) across the country ("Round 1") to reduce healthcare spending, pursuant to a 2003 federal law. On July 1, 2013, CMS expanded the program to an additional 91 MSAs ("Round 2"). These bid programs have resulted in new, lower Medicare payment rates in these 100 areas. In January 2016, CMS began the deployment of NCB rates to the remainder of the Medicare population that had not yet been impacted by the program. These were primarily less densely populated, rural areas. In 2016, CMS divided the United States into eight regions and applied the average reimbursement reduction per NCB product category in each region from Round 1 and Round 2 to the rural providers in those eight regions.
In November 2018, CMS announced that it was suspending the NCB program for approximately two years, from January 1, 2019 through approximately December 31, 2020, and in the interim will implement changes to the NCB program. CMS' November 2018 rule also modified payment rates for oxygen, based on Medicare's “budget neutrality” mandate. For the oxygen devices the company sells, the total Medicare payment rate remained substantially similar to 2018 payment rates.
On March 7, 2019, the CMS announced plans to consolidate the competitive bidding areas (CBAs) included in the Round 2 Recompete and Round 1 2017 Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) Competitive Bidding Program (CBP) into a single round of competition named Round 2021.
On October 27, 2020, CMS revealed that the Agency would only move forward with off-the-shelf (OTS) back braces and OTS knee braces for Round 2021 of the CBP effective January 1, 2021 and extending through December 31, 2023. CMS removed the remaining 13 product categories for Round 2021 and delayed any changes for three years.
The company's exposure to effects of NCB rate reductions and any similar reductions from private payors
or state agencies can increase the company's credit risk associated with customers whose revenue, based on reimbursement, may be significantly reduced. As reimbursement rates are reduced, the company's customers may experience pressure on profitability and liquidity. The company therefore remains focused on being judicious in its extension of credit to its customers and vigilant about collections efforts.
In addition, the consequence of reduced reimbursement has and may continue to compel customers to consider alternative sources of supply, which may be available at lower purchase prices, thereby reducing sales or the price at which customers will transact for certain products.
Although reductions in CMS payments are disruptive to the homecare industry, the company believes it can grow and thrive in this environment. The company expects to continue pursuing productivity initiatives intended to lower the costs to serve customers, in an effort to profitably meet lower customer price targets. The company also produces certain solutions, which can provide lower total cost of business for its customers. As an example, the company's respiratory therapy products can help offset reimbursement reductions by eliminating the need for routine home exchange services of pre-filled oxygen cylinders with end-users. Delivery costs can be a substantial element of cost for its customers. The company's HomeFill oxygen system, Platinum Mobile oxygen concentrator, as well as the company's oxygen concentrators, can provide effective convenient therapy for consumers and cost-effective equipment solutions for providers by eliminating customer's costs associated with home cylinder exchange. Similarly, the informatics capabilities the company launched for power wheelchairs and respiratory devices in 2017 enable customers to more cost effectively provide service and support their end-user customers. The company intends to continue developing solutions that help providers improve profitability and reduce the overall cost of care for payors.
BACKLOG
The company generally manufactures its products to meet near-term demands by shipping from stock or by building to order based on the specialized nature of certain products. As a result, the company does not ordinarily have a substantial backlog of orders for any particular product. However, at the end of 2020, the order backlogs in Europe and North America are at higher levels than normal as a result of strong demand for respiratory products driven by COVID-19 and supply chain constraints due to pandemic-related disruptions in supply chain, transportation and logistics which are affecting access to components and finished products.
HUMAN CAPITAL
As of December 31, 2020, the company had approximately 3,400 employees. The company believes that its employees are integral to its success and strive to create a rewarding culture through commitment to its core values of Integrity, Innovation, Leadership, Excellence and Accountability. The company's compensation programs are designed to attract, retain and motivate employees to be part of the company's success. The company provides wages that are competitive locally and consistent globally to reward employees for performance. The company's long-term incentive program is equity based to align leadership with the interests of shareholders.
Invacare is committed to its Environmental, Social and Governance program and embraces diversity, equity and inclusion. The company believes that an innovative workforce needs to be diverse, with skills and perspectives drawn from a broad spectrum of backgrounds and experiences.
Global and U.S. demographics include:
| | | | | | | | | |
| December 31, 2020 global gender demographics | |
| Female | Male | |
| Manager Level and Above | 26% | 74% | |
| Individual Contributors | 42% | 58% | |
| Manufacturing and Warehouse Associates | 31% | 69% | |
| Total Invacare | 35% | 65% | |
| | | |
| | | | | | | | | | | | | | | |
| December 31, 2020 U.S. race and ethnicity demographics | |
| Total U.S. | M&W 1 | IC 2 | Mgr and Above 3 | |
| Black / African American | 8% | 11% | 6% | 5% | |
| Asian | 3% | 2% | 1% | 5% | |
| Hispanic / Latino | 24% | 40% | 11% | 6% | |
| White | 64% | 46% | 80% | 82% | |
| Multiracial, Native American and Pacific Islander | 1% | 1% | 2% | 2% | |
1 Manufacturing and Warehousing | |
2 Individual Contributors (below manager who do not supervise others) | |
3 Manager and Above | |
| | | | | |
The company focuses on training employees and conducting self-audits to create a safe work environment. As an essential business during the COVID-19 pandemic, the company instituted policies across the organization to
help protect employees who continue to work onsite, while at the same time requiring employees to work remotely whenever possible.
FOREIGN OPERATIONS AND EXPORT SALES
The company also markets its products for export to other foreign countries. In 2020, the company's products were sold in over 100 countries. For information relating to net sales, operating income and identifiable assets of the company's foreign operations, refer to Business Segments in the Notes to the Consolidated Financial Statements.
AVAILABLE INFORMATION
The company files Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and any amendments thereto, as well as proxy statements and other documents with the Securities and Exchange Commission (SEC). The SEC maintains a website, http://www.sec.gov, which contains all reports, proxy and information statements and other information filed by the company with the SEC.
Additionally, Invacare's filings with the SEC are available on or through the company's website, www.invacare.com, as soon as reasonably practicable after they are filed electronically with, or furnished to, the SEC. Copies of the company's filings also can be requested, free of charge, by writing to: Shareholder Relations Department, Invacare Corporation, One Invacare Way, Elyria, OH 44035. The contents of the company's website are not part of this Annual Report on Form 10-K.
FORWARD-LOOKING INFORMATION
This Form 10-K contains forward-looking statements within the meaning of the “Safe Harbor” provisions of the Private Securities Litigation Reform Act of 1995. Terms such as “will,” “should,” “could,” “plan,” “intend,” “expect,” “continue,” “believe” and “anticipate,” as well as similar comments, denote forward-looking statements that are subject to inherent uncertainties that are difficult to predict. Actual results and events may differ significantly from those expressed or anticipated as a result of risks and uncertainties, which include, but are not limited to, the following: the duration and scope of the COVID-19 pandemic, the resumption of access to healthcare, including clinics and elective care, and loosening of public health restrictions, or any reimposed restrictions on access to health care or tightening of public health restrictions which could impact the demand for the company’s products; global shortages in, or increasing costs for, transportation and logistics services and capacity; the inability of the company to obtain needed products, components or raw materials from its suppliers; actions that governments, businesses and individuals take in response to the pandemic, including mandatory business closures and restrictions on onsite commercial interactions; the impact of the pandemic and actions taken in response to the pandemic on global and regional economies and economic activity; the pace of recovery when the COVID-19 pandemic subsides; general economic uncertainty in key global markets and a worsening of global economic conditions or low levels of economic growth; the effects of steps the company takes to reduce operating costs; the inability of the company to sustain profitable sales growth, achieve anticipated improvements in segment operating performance, convert high inventory or accounts receivable levels to cash or reduce its costs to maintain competitive prices for its products; lack of market acceptance of the company's new product innovations; circumstances or developments that may make the company unable to implement or realize the anticipated benefits, or that may increase the costs, of its current and planned business initiatives, in particular the key elements of its growth plan such as its new product introductions, additional investments in sales force and demonstration equipment, supply chain actions and global information technology outsourcing and ERP implementation activities, finance and accounting systems outsourcing and other outsourcing activities; possible adverse effects on the company's liquidity, including the company's ability to address future debt maturities; adverse changes in government and other third-party payor reimbursement levels and practices both in the U.S. and in other countries; consolidation of health care providers; increasing pricing pressures in the markets for the company's products; risks of failures in, or disruptions to, legacy IT systems; risks of cybersecurity attack, data breach or data loss and/or delays in or inability to recover or restore data and IT systems; adverse effects of the company's consent decree of injunction with the U.S. Food
and Drug Administration (FDA), including but not limited to, compliance costs, inability to rebuild negatively impacted customer relationships, unabsorbed capacity utilization, including fixed costs and overhead; any circumstances or developments that might adversely impact the third-party expert auditor's required audits of the company's quality systems at the facilities impacted by the consent decree, including any possible failure to comply with the consent decree or FDA regulations; regulatory proceedings or the company's failure to comply with regulatory requirements or receive regulatory clearance or approval for the company's products or operations in the United States or abroad; adverse effects of regulatory or governmental inspections of company facilities at any time and governmental enforcement actions; including the investigation of pricing practices at one of the company's former rentals businesses; product liability or warranty claims; product recalls, including more extensive warranty or recall experience than expected; possible adverse effects of being leveraged, including interest rate or event of default risks; exchange rate fluctuations, particularly in light of the relative importance of the company's foreign operations to its overall financial performance and including the existing and potential impacts from Brexit; legal actions, including adverse judgments or settlements of litigation or claims in excess of available insurance limits; tax rate fluctuations; additional tax expense or additional tax exposures, which could affect the company's future profitability and cash flow; uncollectible accounts receivable; risks inherent in managing and operating businesses in many different foreign jurisdictions; decreased availability or increased costs of materials which could increase the company's costs of producing or acquiring the company's products, including the adverse impacts of tariffs and increases in commodity costs or freight costs; heightened vulnerability to a hostile takeover attempt or other shareholder activism; provisions of Ohio law or in the company's debt agreements, charter documents or other agreements that may prevent or delay a change in control, as well as the risks described elsewhere in this Annual Report on Form 10-K and from time to time in the company's reports as filed with the Securities and Exchange Commission. Except to the extent required by law, the company does not undertake and specifically declines any obligation to review or update any forward-looking statements or to publicly announce the results of any revisions to any of such statements to reflect future events or developments or otherwise.
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
Item 1A. Risk Factors.
The company's business, financial condition, results of operations and prospects are subject to various risks and uncertainties. One should carefully consider the risks and uncertainties described below, together with all the other information in this Annual Report on Form 10-K and in the company's other filings with the SEC, before making any investment decision with respect to the company's securities. The risks and uncertainties described below may not be the only ones the company faces. Additional risks and uncertainties not presently known by the company or that the company currently deems immaterial may also affect the company's business. If any of these known or unknown risks or uncertainties occur, develop or worsen, the company's business, financial condition, results of operations and prospects could change substantially.
Summary of Risk Factors
As noted above, the company is subject to a number of risks that if realized could materially harm its business, financial condition, results of operations and prospects. Some of the more significant risks and uncertainties the company faces include those summarized below. The summary below is not exhaustive and is qualified by reference to the full set of risk factors set forth in this “Item 1A. Risk Factors” section.
Risks Related to the COVID-19 Pandemic
•The COVID-19 pandemic has disrupted, and may continue to disrupt, the company’s operations and could have a material adverse effect on the company’s business, financial condition and liquidity, including the ability for end users to gain access to the company's products.
•The COVID-19 pandemic has contributed to a global shipping and logistics crisis, including shortages in the availability of, and increasing costs for, container shipping and has disrupted, and may continue to disrupt, the company’s ability to obtain critical products, components and raw materials which could have a material, adverse effect on the company’s business, financial condition and liquidity.
Commercial and Operational Risks
•If the company's business improvement efforts are ineffective, the company's strategic goals, business plans, financial performance or liquidity could be negatively impacted.
•If the company is unable to attract and retain critical IT Governance, Project Management and Contract Management competencies, it may limit the effectiveness of the company's cost improvement and business efficiency initiatives and adversely affect the company's profitability and growth.
•Changes in government and other third-party payor reimbursement levels and practices have negatively impacted and could continue to negatively impact the company's revenues and profitability.
•If the company's products are not included within an adequate number of customer formularies, or if pricing policies otherwise favor competitors' products, the company's market share and gross margin could be negatively affected.
•The industry in which the company operations is highly competitive and the consolidation of health care customers and the company's competitors could result in a loss of customers or in additional competitive pricing pressures.
•The company's business strategy relies on certain assumptions concerning demographic trends that impact the market for its products. If these assumptions prove to be incorrect, demand for the company's products may be lower than expected.
Risks Related to Financial Results and Liquidity
•The terms of the company's debt facilities and financing arrangements may limit the company's flexibility in operating its business.
•The company's leverage and debt obligations could adversely affect its financial condition, limit its ability to raise additional capital to fund its operations, impact the way it operates its business and prevent it from fulfilling its debt service obligations.
•The company may not be able to repay or refinance its convertible notes, and the issuance of common shares upon conversion of the convertible notes could cause dilution to the company's existing shareholders.
•The company's convertible notes have certain fundamental change and conditional conversion features which, if triggered, may adversely affect the company's financial condition.
| | | | | | | | |
| Part I | | |
| Item 1A. Risk Factors | | |
| | |
Risks Related to Information Technology and Reliance on Third Parties
•Any major disruption or failure of the company’s information technology systems, or its failure to successfully implement new technology effectively, could adversely affect the company.
•Cyber security threats and more sophisticated and targeted computer crime pose a risk the company's systems, networks, products and services, and a risk to the company's compliance with data privacy laws.
•As the company outsources functions, it becomes more dependent on the entities performing those functions. Disruptions or delays at the company’s third-party service providers could adversely impact its operations.
Regulatory and Development Risks
•The company remains subject to a consent decree of injunction with the U.S. Food and Drug Administration.
•Any failure by the company to comply with medical device regulatory requirements or receive regulatory clearance or approval for the company's products or operations in the United States or abroad could adversely affect the company.
•If the company fails to comply with applicable health care laws or regulations, the company could suffer severe civil or criminal sanctions or may be required to make significant changes to the company's operations.
•Legislative developments in all regions in which the company operates may adversely affect the company.
Intellectual Property Risks
•The company's operating results and financial condition could be adversely affected if the company becomes involved in litigation regarding its patents or other intellectual property rights.
•If the company is unable to protect its intellectual property rights or resolve successfully claims of infringement brought against it, the company's
product sales and business could be affected adversely.
Manufacturing and Supply Risks
•Decreased availability or increased costs of materials could increase the company's costs of producing its products.
•The company's ability to manage an effective supply chain is a key success factor.
Other Regulatory and Litigation Risks
•The company is subject to certain risks inherent in managing and operating businesses in many different foreign jurisdictions.
•The impact of "Brexit" may adversely affect the company.
•The company's products may be subject to product liability claims or recalls, which could be costly, harm the company's reputation and adversely affect its business.
Other Risk Factors – Other Financial Risks, Risks Related to Employees and the Company's Common Shares
•The company has long-term finance leases on significant facilities which can affect the company's liquidity and cash flow.
•The company's revenues and profits are subject to exchange rate and interest rate fluctuations which can affect the company's profitability and cash flow.
•Additional tax expense or additional tax exposures could affect the company's future profitability and cash flow.
•The company's reported results may be adversely affected by increases in reserves for uncollectible accounts receivable.
•The inability to attract and retain, or loss of the services of, the company's key management and personnel could adversely affect its ability to operate the company's business.
•Volatility in the market price of the company's common shares could adversely affect its shareholders, its ability to finance operations or attract and retain leadership.
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
Risks Related to the COVID-19 Pandemic
The COVID-19 pandemic has disrupted, and may continue to disrupt, the company’s operations and could have a material adverse effect on the company’s business, financial condition and liquidity, including the ability for end users to access to the company's products.
The present COVID-19 pandemic has disrupted the company’s operations and affected the company’s business, and may continue to do so, due to many factors, including imposition by government authorities of mandatory closures, work-from-home orders and social distancing protocols, and voluntary facility closures or other restrictions that could materially adversely affect the company’s ability to adequately staff and maintain its operations. Specifically, the company experienced at least one mandatory temporary facility closure, and may experience in the future other temporary facility closures, in response to government mandates in certain jurisdictions in which the company operates or in response to positive diagnoses for COVID-19 in certain facilities for the safety of the company’s associates.
The COVID-19 pandemic has also disrupted, and may continue to disrupt, the company’s operations and supply chain and may materially adversely impact its ability to secure raw materials, components and supplies for its facilities and to provide personal protective equipment for its employees. There may also be long-term negative effects on the economic well-being of company’s customers and in the economies of affected countries, which may limit their access or expenditures on health care. The pandemic has caused, and may in the future cause, temporary closure of, or restricted access to, health care provider facilities where the company's products are demonstrated, trialed, fitted and sold. Limitations on non-acute care due to the pandemic have delayed or reduced, and if reimposed may in the future delay or reduce, the ability for end users to gain access to the company's products.
Government actions in response to the COVID-19
pandemic continue to change and evolve. As a result, the countries in which the company’s products are manufactured and distributed remain under varying stages of restrictions. Certain jurisdictions have had to, or may in the future have to, re-establish restrictions due to a resurgence in COVID-19 cases or the development of new strains of COVID-19. Additionally, although access to elective healthcare by patients and access to healthcare for many of the company’s customers has been at least partially restored or increased, such access may again be limited or closed if a resurgence of COVID-19 cases occurs, new strains of COVID-19 develop, or if efforts to combat COVID-19, including vaccine development or
distribution, are ineffective or prolonged. Even as government restrictions have been lifted and economies gradually reopened, the shape of the economic recovery remains uncertain and may continue to negatively impact the company's results of operations, cash flows and financial position in subsequent quarters. Given this current level of economic and operational uncertainty over the impacts of COVID-19, the ultimate financial impact cannot be reasonably estimated at this time.
The COVID-19 pandemic or a similar widespread outbreak of disease in the future could have a material adverse effect on the company’s ability to operate, results of operations, financial condition and liquidity. In addition, public health restrictions and preventive measures the company may voluntarily put in place, could have a material adverse effect on the company's business for an indefinite period of time, such as the potential shut down of certain locations, decreased employee availability and border closures, among others. The company’s suppliers and customers may also face these and other challenges, which could lead to continued disruption in the company’s supply chain, as well as decreased customer demand for the company’s products. These issues may also materially affect the company’s future access to its sources of liquidity, particularly its cash flows from operations, financial condition and capitalization. Although these disruptions may continue to occur, the long-term economic impact and near-term financial impacts of such issues, may not be reasonably estimated due to the uncertainty of future developments.
The COVID-19 pandemic has contributed to a global shipping and logistics crisis, including shortages in the availability of, and increasing costs for, container shipping and has disrupted, and may continue to disrupt, the company’s ability to obtain critical products, components and raw materials which could have a material, adverse effect on the company’s business, financial condition and liquidity.
The global impacts surrounding the COVID-19 pandemic, including operational and manufacturing disruptions, logistical constraints and travel restrictions, have contributed to substantial and ongoing delays, disruptions and shortages in the availability of shipping and logistics services. In particular, shortages of the availability of, and soaring cost increases for, container shipping have disrupted the company’s ability to obtain, and increased the cost of obtaining, critical products, components and raw materials for its business. These factors have negatively impacted the company's ability to operate and to the extent that these negative impacts continue, they could have a material adverse effect on the company’s business, financial condition and liquidity.
| | | | | | | | |
| Part I | | |
| Item 1A. Risk Factors | | |
| | |
Commercial and Operational Risks
If the company's business improvement efforts are ineffective, the company's strategic goals, business plans, financial performance or liquidity could be negatively impacted.
The company is implementing a multi-year business improvement strategy intended to substantially improve its business and re-orient its resources to a more clinically complex mix of products and solutions. To date, this strategy has included actions to re-orient the company's global commercial team, restart the company's innovation pipeline, shift its product mix, develop and expand its talent, and strengthen its balance sheet. The company also has taken steps to realign infrastructure and processes that are intended to drive efficiency and reduce costs.
The company may not be successful in achieving the full long-term growth and profitability, operating efficiencies and cost reductions, or other benefits expected from these business improvement efforts in a timely manner or at all. The company also may experience business disruptions associated with these activities. Further, the full benefits of the strategy, if realized, may be realized later than expected, the costs of implementing the strategy may be greater than anticipated, and the company may lack adequate cash or capital or may not be able to attract and retain the necessary talent, to complete the improvement actions. In addition, the negative impacts of the COVID-19 pandemic may result in increased costs associated with, and delayed execution of, business improvement initiatives. If these business improvement measures are not successful, the company may undertake additional actions, which could result in future expenses. If the company's business improvement efforts prove ineffective, the company's ability to achieve its strategic goals and business plans, and the company's financial performance, including its ability to repay or refinance its indebtedness, may be materially adversely affected. Under these circumstances, the company may require additional financing, which may be difficult or expensive to obtain, and the company can make no assurances that it would be available on terms acceptable to the company, if at all.
If the company is unable to attract and retain critical IT Governance, Project Management and Contract Management competencies it may limit the effectiveness of the company's cost improvement and business efficiency initiatives and adversely affect the company's profitability and growth.
The company is implementing a multi-year business improvement strategy which involves projects focused on streamlining the company’s supply chain and operations infrastructure, upgrading and modernizing its information
technology capabilities and implementation of new ERP systems in conjunction with its third-party outsourcing service provider. In addition, the company has outsourced certain key functions to third-party service providers and may continue to do so in the future. The success of these activities is dependent on the company’s ability to maintain an adequate IT governance management structure and adequate capabilities in project management and contract management functions. Despite its efforts to build and maintain these capabilities, the company could have inadequate skills, personnel, management skills, or processes necessary to successfully implement the programs and projects necessary to successfully improve the business and achieve the intended operating efficiencies and cost reductions from such programs and projects, which in turn may adversely affect the company's profitability and growth.
Changes in government and other third-party payor reimbursement levels and practices have negatively impacted and could continue to negatively impact the company's revenues and profitability.
The company's products are sold primarily through a network of medical equipment and home health care providers, extended care facilities and other providers such as various government-provider agencies throughout the world. Many of these providers (the company's customers) are reimbursed for the products and services provided to their customers and patients by third-party payors, such as government programs, including Medicare and Medicaid, private insurance plans and managed care programs. Most of these programs set maximum reimbursement levels for some of the products sold by the company in the United States and abroad. If third-party payors deny coverage, make the reimbursement process or documentation requirements more uncertain or reduce their levels of reimbursement, or if the company is unable to reduce its costs of production to keep pace with decreases in reimbursement levels, the company may be unable to sell the affected product(s) through its distribution channels on a profitable basis.
Reduced government reimbursement levels and changes in reimbursement policies have in the past added, and could continue to add, significant pressure to the company's revenues and profitability. For example, the National Competitive Bidding, or “NCB”, program introduced by CMS beginning in January 2011 has had the effect of substantially reducing reimbursement and payment rates for medical equipment and supplies by Medicare. The reduced reimbursement and payment rates have, in some cases, prompted customers to consider lower-priced alternatives to the company's products and compelled the company to reduce prices on certain products, which has negatively impacted the company's
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
revenues and profitability. In October 2020, CMS announced the delay to changes to NCB for three years. The potential impact of any modifications may be uncertain and may further negatively impact the company's revenues and profitability. Refer to “Item 1. Business -Government Regulation-National Competitive Bidding.”
Similar trends and concerns are occurring in state Medicaid programs. These recent changes to reimbursement policies, and any additional unfavorable reimbursement policies or budgetary cuts that may be adopted in the future, could adversely affect the demand for the company's products by customers who depend on reimbursement from the government-funded programs. The percentage of the company's overall sales that are dependent on Medicare or other insurance programs may increase as the portion of the U.S. population over age 65 continues to grow, making the company more vulnerable to reimbursement level reductions by these organizations. Reduced government reimbursement levels also could result in reduced private payor reimbursement levels because some third-party payors index their reimbursement schedules to Medicare fee schedules. Reductions in reimbursement levels also may affect the profitability of the company's customers and ultimately force some customers without strong financial resources to become unable to pay their bills as they come due or go out of business. The reimbursement reductions may prove to be so dramatic that some of the company's customers may not be able to adapt quickly enough to survive. The company is one of the industry's significant creditors and an increase in bankruptcies or financial weakness in the company's customer base could have an adverse effect on the company's financial results.
Outside the U.S., reimbursement systems vary significantly by country. Many foreign markets have government-managed health care systems that govern reimbursement for home health care products. The ability of hospitals and other providers supported by such systems to purchase the company's products is dependent, in part, upon public budgetary constraints. Various countries have tightened reimbursement rates and other countries may follow. If adequate levels of reimbursement from third-party payors outside of the U.S. are not obtained, international sales of the company's products may decline, which could adversely affect the company's net sales.
The impact of all the above is uncertain and could have a material adverse effect on the company's business, financial condition, liquidity and results of operations.
If the company's products are not included within an adequate number of customer formularies, or if pricing policies otherwise favor competitors' products, the
company's market share and gross margin could be negatively affected.
Many of the medical equipment and home health care providers to whom the company sells its products negotiate the price of products and develop formularies which establish pricing and reimbursement levels. Many of these providers also compensate their sales personnel based on the formulary position of the products they sell. Exclusion of a product from a formulary, unfavorable positioning of a product within a formulary, or lower compensation levels for customer sales personnel associated with the products can lead to such product's sharply reduced usage in the provider's patient population. If the company's products are not included, or favorably positioned, within an adequate number of formularies, or if the pricing policies or sales compensation programs of providers otherwise favor other products, the company's sales revenues, market share and gross margin could be negatively affected, which could have a material adverse effect on the company's results of operations and financial condition.
The industry in which the company operates is highly competitive and the consolidation of health care provider customers and the company's competitors could result in a loss of customers or in additional competitive pricing pressures.
The home medical equipment market is highly competitive and the company's products face significant competition from other well established manufacturers. Numerous initiatives and reforms instituted by legislators, regulators and third-party payors to reduce home medical equipment costs have caused pricing pressures which have resulted in a consolidation trend in the home medical equipment industry as well as among the company's customers, including home health care providers. In the past, some of the company's competitors, which may include distributors, have been lowering the purchase prices of their products in an effort to attract customers. This in turn has resulted in greater pricing pressures, including pressure to offer customers more competitive pricing terms, exclusion of products from or unfavorable position on provider formularies and the exclusion of certain suppliers from important market segments as group purchasing organizations, independent delivery networks and large single accounts continue to consolidate purchasing decisions for some of the company's customers. Further consolidation could result in a loss of customers, increased collectability risks, or increased competitive pricing pressures. In addition, as reimbursement pressures persist, some customers may directly source their own products to secure a low-cost advantage.
The company's business strategy relies on certain assumptions concerning demographic trends that impact
| | | | | | | | |
| Part I | | |
| Item 1A. Risk Factors | | |
| | |
the market for its products. If these assumptions prove to be incorrect, demand for the company's products may be lower than expected.
The company's ability to achieve its business objectives is subject to a variety of factors, including the relative increase in the aging of the general population. The company believes that these trends will increase the need for its products. The projected demand for the company's products could materially differ from actual demand if the company's assumptions regarding these trends and acceptance of its products by health care professionals and patients prove to be incorrect or do not materialize. If the company's assumptions regarding these factors prove to be incorrect, the company may not be able to successfully implement the company's business strategy, which could adversely affect the company's results of operations. In addition, the perceived benefits of these trends may be offset by competitive or business factors, such as the introduction of new products by the company's competitors or the emergence of other countervailing trends, including lower reimbursement and pricing.
Risks Related to Financial Results and Liquidity
The terms of the company's debt facilities and financing arrangements may limit the company's flexibility in operating its business.
The company's credit agreement provides the company and certain of the company's U.S., Canadian, U.K. and French subsidiaries with the ability to borrow under senior secured revolving credit, letter of credit and swing line loan facilities. The aggregate borrowing availability under the credit facilities is determined based on borrowing base formulas set forth in the credit agreement. The credit facilities are secured by substantially all the company's domestic and Canadian assets, other than real estate, and by substantially all the personal property assets of the company's U.K. subsidiaries and all of the receivables of the company's French subsidiaries. On January 15, 2021 the company entered into the Eighth Amendment to the credit agreement which, among other things, expanded to include the company's Netherlands subsidiary as a guarantor under the European revolving credit facility. The credit agreement contains customary default provisions, with certain grace periods and exceptions, that include, among other things, failure to pay amounts due, breach of covenants, representations or warranties, bankruptcy, the occurrence of a material adverse effect, exclusion from any medical reimbursement program, and an interruption of any material manufacturing facilities for more than ten consecutive days.
The restrictive terms of the company's credit agreement may limit the company's ability to conduct and expand its business and pursue its business strategies. The company's ability to comply with the provisions of its credit agreements can be affected by events beyond its control, including changes in general economic and business conditions, or by government enforcement actions, such as, for example, adverse impacts from the FDA consent decree of injunction. If the company is unable to comply with the provisions in the credit agreement, it could result in a default which could trigger acceleration of, or the right to accelerate, the related debt. Because of cross-default provisions in its agreements and instruments governing certain of the company's indebtedness, a default under the credit agreement could result in a default under, and the acceleration of, certain other company indebtedness. In addition, the company's lenders would be entitled to proceed against the collateral securing the indebtedness.
The company's ability to meet its liquidity needs will depend on many factors, including the operating performance of the business, as well as the company's continued compliance with the covenants under its credit agreement. Notwithstanding the company's expectations, if the company's operating results decline, the company may be unable to comply with the financial covenants, and its lenders could demand repayment of the amounts outstanding under the company's credit facility.
The company also has an agreement with De Lage Landen, Inc. (“DLL”), a third-party financing company, to provide financing to the company's U.S. customers. Either party could terminate this agreement with 180 days' notice or 90 days' notice by DLL upon the occurrence of certain events. Should this agreement be terminated, the company's borrowing needs under the credit agreement could increase.
The company's leverage and debt obligations could adversely affect its financial condition, limit its ability to raise additional capital to fund its operations, impact the way it operates its business and prevent it from fulfilling its debt service obligations.
The company has significant outstanding indebtedness. As of December 31, 2020, the company had outstanding $1,250,000 aggregate principal amount of 5.00% Convertible Senior Notes that mature in February 2021 (the “2021 Notes”), $81,500,000 aggregate principal amount of 4.50% Convertible Senior Notes that mature in June 2022 (the “2022 Notes”), $72,909,000 aggregate principal amount of 5.00% Series I Convertible Senior Notes that mature in November 2024 (the “Series I 2024 Notes”) and $75,688,000 aggregate principal and accretion amount of 5.00% Series II Convertible Senior Notes that mature in November 2024 (the "Series II 2024 Notes").
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
The company has an Amended and Restated Credit Agreement providing for asset-based lending senior secured revolving credit facilities which mature in January 2024 with outstanding indebtedness at December 31, 2020 of $31,502,000. The company also has $10,000,000 of outstanding indebtedness under a CARES Act loan which may be forgivable, partially or in full, if certain conditions are met, principally based on whether the borrowings were disbursed for permissible purposes and on average levels of employment over a designated period of time. However, no assurance can be given that the company will be granted forgiveness of the CARES Act loan in whole or in part.
The company's indebtedness could have important negative consequences, including:
•reduced availability of cash for the company's operations and other business activities after satisfying interest payments and other requirements under the terms of its debt instruments;
•less flexibility to plan for or react to competitive challenges, and suffer a competitive disadvantage relative to competitors that do not have as much indebtedness;
•difficulty in obtaining additional financing in the future;
•inability to comply with covenants in, and potential for default under, the company's debt instruments; and
•challenges to repaying or refinancing any of the company's debt.
The company’s ability to satisfy its debt obligations will depend principally upon its future operating performance. As a result, prevailing economic conditions and financial, business, legal and regulatory and other factors, many of which are beyond the company’s control, may affect its ability to make payments on its debt. If it does not generate sufficient cash flow to satisfy its debt obligations, the company may have to undertake alternative financing plans, such as refinancing or restructuring its debt, selling assets, seeking additional capital or reducing or delaying capital investments. The company’s ability to restructure or refinance its debt will depend on the capital markets and the company’s financial condition at the time. Restructuring or refinancing indebtedness could require the company to issue additional debt, pay additional fees and interest, issue potentially dilutive additional equity, further encumber certain of the company’s assets, agree to covenants that could restrict its future operations and pay related transaction fees and expenses. Any such measures would require agreements
with counterparties, including potentially the company’s existing creditors, and may not be successful on attractive terms or otherwise. Whether or not successful, any such measures may have a negative impact on the company’s financial condition and results of operations, including on the market price of the company’s common stock and debt securities.
Refer to Item 7. “Management's Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources.”
The company may not be able to repay or refinance its convertible notes, and the issuance of common shares upon conversion of the convertible notes could cause dilution to the company's existing shareholders.
As of December 31, 2020, the company had outstanding $81,500,000, $72,909,000 and $75,688,000 aggregate principal amount (with accretion in the case of the Series II 2024 Notes) of its 2022 Notes, Series I 2024 Notes and its Series II 2024 Notes, respectively. Prior to the close of business on the business day immediately preceding December 1, 2021 (with respect to the 2022 Notes) and prior to the close of business on the business day preceding May 15, 2024 (with respect to the Series I 2024 Notes and Series II 2024 Notes), the notes will be convertible only upon satisfaction of certain conditions. Holders may convert their 2022 Notes at their option at any time after December 1, 2021 until the close of business on the second scheduled trading day immediately preceding June 1, 2022 and holders may convert their Series I 2024 and Series II 2024 Notes at their option at any time after May 15, 2024 until the close of business on the second scheduled trading day immediately preceding November 15, 2024.
Any use of cash upon conversion or maturity of the notes could adversely affect the company’s liquidity, and the company may not have enough available cash or be able to obtain financing at the time it is required to pay cash in settlement of notes being converted or maturing. Furthermore, the company may seek to refinance the 2022 Notes, the Series I 2024 and/or Series II 2024 Notes prior to maturity, and there is no assurance that the company will be able to do so on attractive terms or at all.
The company may settle conversions of the notes by paying or delivering, as the case may be, cash, common shares, or a combination of cash and common shares, at the company's election. If any such conversions occur and the company has authority, and so elects, to settle some or all of the converted notes in common shares, the number of shares issued could be significant and such an issuance could cause dilution to the interests of the existing shareholders.
| | | | | | | | |
| Part I | | |
| Item 1A. Risk Factors | | |
| | |
The company's convertible notes have certain fundamental change and conditional conversion features which, if triggered, may adversely affect the company's financial condition.
If a fundamental change occurs under the company's 2022 Notes, Series I 2024 or its Series II 2024 Notes, the holders of the notes may require the company to purchase for cash any or all of the notes. However, there can be no assurance that the company will have sufficient funds at the time of the fundamental change to purchase all of the notes delivered for purchase, and it may not be able to arrange necessary financing on acceptable terms, if at all. Likewise, if one of the conversion contingencies of the notes is triggered, holders of notes will be entitled to convert the notes at any time during specified periods. If the company desires to settle any portion of any converted notes through the payment of cash, there can be no assurance that it will have sufficient funds to purchase all of the notes delivered for purchase, and the company may not be able to arrange necessary financing on acceptable terms, if at all. If the company elects to settle any converted notes through the issuance of common shares, it would have a dilutive effect on shareholders’ interests.
If a fundamental change occurs under the company's 2022 Notes, the company may be obligated to settle the outstanding common shares warrants issued to the respective counterparties in connection with the issuance of the 2022 Notes. If exercised, the warrants may obligate the company to issue a substantial number of common shares to the counterparty, which would have a dilutive effect on shareholders’ interests. If the warrant is terminated upon a fundamental change, the company may be obligated to make a substantial cash termination payment to the counterparty, and there can be no assurance that the company will have sufficient funds to do so.
In addition, whether following a fundamental change or otherwise, the counterparties to the company’s convertible note hedge and warrant transactions or their respective affiliates may modify their initial hedge positions by entering into or unwinding various derivatives contracts with respect to the company’s common shares and/or purchasing or selling common shares or other securities of the company in secondary market transactions prior to the maturity of the notes. This activity could cause or avoid a significant change in the market price of the company’s common shares.
Risks Related to Information Technology and Reliance on Third Parties
Any major disruption or failure of the company’s information technology systems, or its failure to
successfully implement new technology effectively, could adversely affect the company.
The company relies on various information technology systems to manage its operations. The company recently outsourced substantially all of its information technology services to Birlasoft Solutions, Inc. Over the next several years, the company expects Birlasoft to implement modifications and upgrades to the company's systems, including making changes to legacy systems, replacing legacy systems with successor systems with new functionality and acquiring new systems with new functionality. For example, over the next several years, the company plans to implement with the assistance of Birlasoft a new enterprise resource planning ("ERP") system across the company. These activities subject the company to inherent risks associated with replacing and upgrading these systems, including impairment of its ability to fulfill customer orders, potential disruption of its internal control structure, additional administration and operating expenses, reliance on Birlasoft providing sufficiently skilled personnel to implement and operate the new systems, demands on management time, and other risks and costs of delays or difficulties in transitioning to new or upgraded systems or of integrating new or upgraded systems into the company’s current systems. The company’s system implementations may not result in productivity improvements at a level that outweighs the risks and burdens of implementation, or at all. In addition, the difficulties with implementing new or upgraded technology systems may cause disruptions in its business operations and have an adverse effect on its business and operations, if not anticipated and appropriately mitigated.
Cybersecurity threats and more sophisticated and targeted computer crime pose a risk to the company's systems, networks, products and services, and a risk to the company's compliance with data privacy laws.
Global IT security threats and more sophisticated and targeted computer crime pose a risk to the security of the company's systems and networks as well as the confidentiality, protection, availability and integrity of the company's data and any personal data on such networks or systems, including regulatory risks under the EU General Data Protection Regulation (GDPR), the California Consumer Privacy Act (CCPA) and the U.S. Health Insurance Portability and Accountability Act (HIPAA), among other risks. In addition, data security breaches can also occur as a result of a failure by the company or its employees to follow policies, procedures or training, or by acts, omissions or breaches by persons with whom the company has commercial relationships that result in the unauthorized release of personal or confidential information.
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
Through its sales channels, the company may collect and store personal or confidential information that customers provide to purchase products or services, enroll in promotional programs and register on the company's website, among other reasons. The company may also acquire and retain information about customers, product end users, suppliers and employees in the normal course of
business. The company also creates and maintains proprietary information that is critical to its business, such
as its product designs and manufacturing processes. In addition to the company’s own databases, it uses third-party service providers to store, process and transmit confidential or personal information on its behalf. Although the company contractually requires these service providers to implement and use reasonable security measures and to comply with laws relating to privacy and data protection, the company cannot control third parties and cannot guarantee that a data security breach will not occur in the future either at their location or within their systems. Some of the company’s information technology systems have aged and are no longer supported or maintained by the original system vendors. Despite the company's efforts to secure its systems and networks, and any personal or sensitive information stored thereon, the company could experience a significant data security breach. Computer hackers may attempt to penetrate the company's or its vendors' information systems and, if successful, misappropriate confidential customer, supplier, employee or other business or personal information, including company intellectual property. Third parties could also gain control of company systems and use them for criminal purposes. Depending on their nature and scope, such threats could result in the loss of existing customers, difficulty in attracting new customers, exposure to claims from customers, governmental or data privacy or data protection authorities, financial institutions, payment card associations, employees and other persons, imposition of regulatory sanctions or penalties, incurring of additional expenses or lost revenues, or other adverse consequences, any of which could have a material adverse effect on the company's business and results of operations.
As the company outsources functions, it becomes more dependent on the entities performing those functions. Disruptions or delays at the company’s third-party service providers could adversely impact its operations.
As part of its actions to improve business efficiency, the company has sought opportunities to provide essential business services in a more cost-effective manner. In some cases, this results in the outsourcing of functions or parts of functions that can be performed more effectively by external service providers. For example, the company recently outsourced a significant portion of its information technology functions to Birlasoft Solutions Inc. While the
company believes it conducts appropriate diligence before entering into agreements with any outsourcing entity, the failure of one or more of such entities to meet the company’s performance standards and expectations, including with respect to service levels, data security, compliance with data protection and privacy laws, providing services on a timely basis or providing services at the prices the company expects, may have an adverse effect on the company’s results of operations or financial condition. In addition, the company could face increased costs or disruption associated with finding replacement vendors or hiring new employees in order to return these services in-house. The company may outsource other functions in the future, which would increase its reliance on third parties.
Regulatory and Development Risks
The company remains subject to a consent decree of injunction with the U.S. Food and Drug Administration.
In December 2012, the company became subject to a consent decree of injunction filed by the FDA with respect to the company's Corporate facility and its Taylor Street manufacturing facility in Elyria, Ohio. The consent decree initially limited the company's (i) manufacture and distribution of power and manual wheelchairs, wheelchair components and wheelchair sub-assemblies at or from its Taylor Street manufacturing facility, except in verified cases of medical necessity, (ii) design activities related to wheelchairs and power beds that take place at the impacted Elyria facilities and (iii) replacement, service and repair of products already in use from the Taylor Street manufacturing facility. Under the terms of the consent decree, in order to resume full operations, the company had to successfully complete independent, third-party expert certification audits at the impacted Elyria facilities, comprising three distinct certification reports separately submitted to, and accepted by, the FDA; submit its own report to the FDA; and successfully complete a reinspection by the FDA of the company's Corporate and Taylor Street facilities.
On July 24, 2017, following its reinspection, the FDA notified the company that it was in substantial compliance with the QSR and the Federal Food, Cosmetic & Drug Act (The FDA Act), the FDA regulations and the terms of the consent decree that the company was permitted to resume full operations at those facilities including the resumption of unrestricted sales of products made in those facilities.
The consent decree will continue in effect for a minimum of five years from July 24, 2017, during which time the company's Corporate and Taylor Street facilities must complete two semi-annual audits in the first year and
| | | | | | | | |
| Part I | | |
| Item 1A. Risk Factors | | |
| | |
then four annual audits in the next four years performed by a company retained expert firm. The expert audit firm will determine whether the facilities remain in continuous compliance with the FDA Act, regulations and the terms of the consent decree. Thus far, the two semi-annual audits and the first two annual audits have been completed successfully. The FDA has the authority to inspect these facilities and any other FDA registered facility, at any time. The FDA also has the authority to order the company to take a wide variety of remedial actions if the FDA finds that the company is not in compliance with the consent decree or FDA regulations. The FDA also has authority under the consent decree to assess liquidated damages for any violations of the consent decree, FDA regulations or the FDA Act. Any such failure by the company to comply with the consent decree, the FDA Act or FDA regulations, or any need to complete significant remediation as a result of any such audits or inspections, or actions taken by the FDA as a result of any such failure to comply, could have a material adverse effect on the company's business, financial condition, liquidity or results of operations.
The limitations previously imposed by the FDA consent decree negatively affected net sales in the North America segment and, to a certain extent, the Asia Pacific region beginning in 2012. The limitations led to delays in new product introductions. Further, uncertainty regarding how long the limitations would be in effect limited the company's ability to renegotiate and bid on certain customer contracts and otherwise led to a decline in customer orders.
Although the company has been permitted to resume full operations at the Corporate and Taylor Street facilities, the negative effect of the consent decree on customer orders and net sales in the North America segment and Asia Pacific region has been considerable, and it is uncertain as to whether, or how quickly, the company will be able to rebuild net sales to more typical historical levels, irrespective of market conditions. Accordingly, when compared to the company's 2010 results, the previous limitations in the consent decree had, and likely may continue to have, a material adverse effect on the company's business, financial condition and results of operations. Refer to Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
Any failure by the company to comply with medical device regulatory requirements or receive regulatory clearance or approval for the company's products or operations in the United States or abroad could adversely affect the company's business.
The company's medical devices are subject to extensive regulation in the United States by the FDA, and by similar governmental authorities in the foreign countries
where the company does business. The FDA regulates virtually all aspects of a medical device's development, testing, manufacturing, labeling, promotion, distribution and marketing. In addition, the company is required to file reports with the FDA if the company's products may have caused, or contributed to, a death or serious injury, or if they malfunction and would be likely to cause, or contribute to, a death or serious injury if the malfunction were to recur. In general, unless an exemption applies, the company's mobility and respiratory therapy products must receive a pre-market clearance from the FDA before they can be marketed in the United States. The FDA also regulates the export of medical devices to foreign countries. The company cannot be assured that any of the company's devices, to the extent required, will be cleared by the FDA through the pre-market clearance process or that the FDA will provide export certificates that are necessary to export certain of the company's products for sale in certain foreign countries. If the company is unable to obtain export certificates for its products, it will limit the company's ability to support foreign markets with such products, which may have an adverse impact on the company's business and results of operations.
Additionally, the company is required to obtain pre-market clearances to market modifications to the company's existing products or market its existing products for new indications. The FDA requires device manufacturers themselves to make and document a determination as to whether a modification requires a new clearance; however, the FDA can review and disagree with a manufacturer's decision. The company may not be successful in receiving clearances in the future or the FDA may not agree with the company's decisions not to seek clearances for any particular device modification. The FDA may require a clearance for any past or future modification or a new indication for the company's existing products. Such submissions may require the submission of additional data and may be time consuming and costly, and ultimately, may not be cleared by the FDA.
If the FDA requires the company to obtain pre-market clearances for any modification to a previously cleared device, the company may be required to cease manufacturing and marketing the modified device or to recall the modified device until the company obtains FDA clearance, and the company may be subject to significant regulatory fines or penalties. In addition, the FDA may not clear these submissions in a timely manner, if at all. The FDA also may change its policies, adopt additional regulations or revise existing regulations, each of which could prevent or delay pre-market clearance of the company's devices, or could impact the company's ability to market a device that was previously cleared. Any of the foregoing could adversely affect the company's business.
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
Any failure by the company to comply with the regulatory requirements of the FDA and other applicable U.S. regulatory requirements may subject the company to administrative or judicially imposed sanctions. These sanctions include warning letters, civil penalties, criminal penalties, injunctions, consent decrees, product seizure or detention, product recalls and total or partial suspension of production, any of which could materially adversely affect the company's business, financial condition, liquidity and results of operations.
As part of its regulatory function, the FDA routinely inspects the facilities of medical device companies and has continued to actively inspect the company's facilities, other than through the processes established under the consent decree. The company expects that the FDA will from time to time, inspect substantially all the company's domestic and foreign FDA-registered operational facilities and may do so repeatedly. The results of regulatory claims, proceedings or investigations are difficult to predict. An unfavorable resolution or outcome of any matter that may arise out of any FDA inspection of the company's facilities, could materially and adversely affect the company's business, financial condition, liquidity and results of operations.
In many of the foreign countries in which the company manufactures or markets its products, the company is subject to extensive medical device regulations that are similar to those of the FDA, including those in Europe. The regulation of the company's products in Europe falls primarily within the United Kingdom and the European Economic Area, which consists of the European Union member states, as well as Iceland, Liechtenstein and Norway. Only medical devices that comply with certain conformity requirements of the Medical Device Directive ("MDD") are allowed to be marketed within the European Economic Area and the United Kingdom. The company's products are required to comply with the European Medical Device Regulation ("MDR"), for class I products by May 2021. Class II products will be required to comply with the MDR by no later than the expiration of their respective current MDD certifications, which will begin to expire in 2024. Products that fail to be certified with the MDR may not be marketed or sold in the European Union. As a result of Brexit, beginning on January 1, 2021, the company's products sold in Great Britain are required to be registered with the Medical and Healthcare Products Regulatory Agency ("MHRA"). Products in conformity with the MDD may continue to be marked with their CE marking in Great Britain until June 2023, after which time products must be certified by a United Kingdom recognized Notified Body. In addition, the national health or social security organizations of certain foreign countries, including those outside Europe, require the company's products to be qualified before they can be marketed in
those countries. Failure to receive, or delays in the receipt of, relevant foreign qualifications in the European Economic Area, the United Kingdom or other foreign countries could have a material adverse effect on the company's business.
If the company fails to comply with applicable health care laws or regulations, the company could suffer severe civil or criminal sanctions or may be required to make significant changes to the company's operations.
The company sells its products principally to medical equipment and home health care providers who resell or rent those products to consumers. Many of those providers (the company's customers) are reimbursed by third-party payors, including Medicare and Medicaid, for the company products sold to their customers and patients. The U.S. federal government and the governments in the states and other countries in which the company operates regulate many aspects of the company's business and the business of the company's customers. As a part of the health care industry, the company and its customers are subject to extensive government regulation, including numerous laws directed at preventing fraud and abuse and laws regulating reimbursement under various government programs. The marketing, invoicing, documenting and other practices of health care suppliers and manufacturers are all subject to government scrutiny. Government agencies periodically open investigations and obtain information from health care suppliers and manufacturers pursuant to the legal process. Violations of law or regulations can result in severe administrative, civil and criminal penalties and sanctions, including disqualification from Medicare and other reimbursement programs, which could have a material adverse effect on the company's business. While the company has established numerous policies and procedures to address compliance with these laws and regulations, there can be no assurance that the company's efforts will be effective to prevent a material adverse effect on the company's business from noncompliance issues.
Health care is an area of rapid regulatory change. Changes in the law and new interpretations of existing laws may affect permissible activities, the costs associated with doing business, and reimbursement amounts paid by federal, state and other third-party payors, all of which may affect the company and its customers. The company cannot predict the future of federal, state and local regulation or legislation, including Medicare and Medicaid statutes and regulations, or possible changes in health care policies in any country in which the company conducts business. Future legislation and regulatory changes could have a material adverse effect on the company's business.
| | | | | | | | |
| Part I | | |
| Item 1A. Risk Factors | | |
| | |
Legislative developments in all regions in which the company operates may adversely affect the company.
Future healthcare legislation, including any significant reform of existing healthcare laws, whether in the U.S. or in our other global markets, along with any programs implemented by such laws, whether at a federal or state level, may reduce reimbursements for the company's products, may impact the demand for the company's products and may impact the prices at which the company sells its products. Such changes could have a material adverse effect on the company's business, results of operations and/or financial condition.
Intellectual Property Risks
The company's operating results and financial condition could be adversely affected if the company becomes involved in litigation regarding its patents or other intellectual property rights.
Litigation involving patents and other intellectual property rights is common in the company's industry, and other companies within the company's industry have used intellectual property litigation in an attempt to gain a competitive advantage. The company in the past has been, and in the future may become, a party to lawsuits involving patents or other intellectual property. If the company were to receive an adverse judgment in any such proceeding, a court or a similar foreign governing body could invalidate or render unenforceable the company's owned or licensed patents, require the company to pay significant damages, seek licenses and/or pay ongoing royalties to third parties, require the company to redesign its products, or prevent the company from manufacturing, using or selling its products, any of which could have an adverse effect on the company's results of operations and financial condition. The company in the past has brought, and may in the future also bring, actions against third parties for infringement of the company's intellectual property rights. The company may not succeed in these actions. The defense and prosecution of intellectual property suits, proceedings before the U.S. Patent and Trademark Office or its foreign equivalents and related legal and administrative proceedings are both costly and time consuming. Protracted litigation to defend or prosecute the company's intellectual property rights could seriously detract from the time the company's management would otherwise devote to running its business. Intellectual property litigation relating to the company's products could cause its customers or potential customers to defer or limit their purchase or use of the affected products until resolution of the litigation.
If the company is unable to protect its intellectual property rights or resolve successfully claims of
infringement brought against it, the company's product sales and business could be affected adversely.
The company's business depends in part on its ability to establish, protect, safeguard and enforce its intellectual property and contractual rights and to defend against any claims of infringement, both of which involve complex legal, factual and marketplace uncertainties. The company relies on a combination of patent, trade secret, copyright and trademark law and security measures to protect its intellectual property, but effective intellectual property protection may not be available in all places that the company sells its products or services, particularly in certain foreign jurisdictions, and patents provide protection for finite time periods. In addition, the company uses nondisclosure, confidentiality agreements and invention assignment agreements with many of its employees, and nondisclosure and confidentiality agreements with certain third parties, in an effort to help protect its proprietary technology and know-how. If these agreements are breached or the company's intellectual property is otherwise infringed, misappropriated or violated, the company may have to rely on litigation to enforce its intellectual property rights. If any of these measures are unsuccessful in protecting the company's intellectual property, the company's business may be affected adversely.
In addition, the company may face claims of infringement, misappropriation or other violation of third parties' intellectual property that could interfere with its ability to use technology or other intellectual property rights that are material to the company's business operations. In the event that a claim of infringement, misappropriation or other violation against the company is successful, the company may be required to pay royalties or license fees to continue to use technology or other intellectual property rights that the company was using, or the company may be unable to obtain necessary licenses from third parties at a reasonable cost or within a reasonable time. If the company is unable to obtain licenses on reasonable terms, it may be forced to cease selling or using the products that incorporate the challenged intellectual property, or to redesign or, in the case of trademark claims, rename its products to avoid infringing the intellectual property rights of third parties, which may not be possible, or if possible, may be time-consuming. Any litigation of this type, whether successful or unsuccessful, could result in substantial costs to the company and adversely affect the company's business and financial condition.
The company also holds patent and other intellectual property licenses from third parties for some of its products and on technologies that are necessary in the design and manufacture of some of the company's products. The loss
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
of these licenses could prevent the company from, or could cause additional disruption or expense in, manufacturing, marketing and selling these products, which could harm the company's business.
Manufacturing and Supply Risks
Decreased availability or increased costs of materials could increase the company's costs of producing its products.
The company purchases raw materials, fabricated components, some finished goods and services from a variety of suppliers. Raw materials such as plastics, steel and aluminum, purchased electronics and other components are considered key raw materials. Where appropriate, the company employs contracts with its suppliers, both domestic and international. From time to time, however, the prices, availability, or quality of these materials fluctuate due to global market demands, import duties and tariffs, delays or interruptions in production or delivery, including shipping and logistics disruptions exacerbated by the COVID-19 pandemic or economic conditions, which could impair the company's ability to procure necessary materials or increase the cost of these materials. Inflationary and other increases in costs of these materials have occurred in the past and may recur from time to time. In addition, freight costs associated with shipping and receiving product and sales are impacted by fluctuations in the cost of oil and gas. In addition, freight and transportation costs have been negatively impacted by the pandemic. A reduction in the supply or increase in the cost or change in quality of those materials or transportation costs, could impact the company's ability to manufacture its products and could increase the cost of production, which could negatively impact the company's revenues and profitability. For example, the tariffs on steel and aluminum on a wide range of products and components imported from China imposed by the U.S. as well as material cost increases imposed by domestic suppliers influenced by the tariffs, have had, and may continue to have, a significant adverse effect on the company's cost of product. The company is attempting to mitigate the adverse impacts of these tariffs, through identifying long-term alternative supply chain opportunities and other actions, including tariff exemptions which expired on January 1, 2021. The company's actions to date have greatly reduced the impact of tariffs. However, if the company is unsuccessful in mitigating the impact of tariffs in the future, its revenues, profitability and results of operations may continue to be adversely affected.
The company's ability to manage an effective supply chain is a key success factor.
The company needs to manage its supply chain efficiently from sourcing to manufacturing and distribution. Successful supply chain management is based on building strong supplier relationships, built on conforming, quality products delivered on-time and at a fair price and operating efficiency. Cost reduction efforts depend on the company's execution of global and regional product platforms that create leverage in sourcing. If the company's supply chain management or cost reduction optimization efforts are ineffective, or if the supply chain is adversely effected by disruption due to shortages, trade barriers or other factors, such as the coronavirus pandemic, the company's revenues and profitability can be negatively impacted.
Other Regulatory and Litigation Risks
The company is subject to certain risks inherent in managing and operating businesses in many different foreign jurisdictions.
The company has significant international operations, including operations in Australia, Canada, New Zealand, Mexico, Asia (primarily Thailand) and Europe. There are risks inherent in operating and selling products internationally, including:
•different regulatory environments and reimbursement systems;
•difficulties in enforcing agreements and collecting receivables through certain foreign legal systems;
•foreign customers who may have longer payment cycles than customers in the United States;
•fluctuations in foreign currency exchange rates;
•tax rates in certain foreign countries that may exceed those in the United States and foreign earnings that may be subject to withholding requirements;
•the imposition of tariffs, exchange controls or other trade restrictions including transfer pricing restrictions when products produced in one country are sold to an affiliated entity in another country;
•potential adverse changes in trade agreements between the United States and foreign countries, including the United States-Mexico-Canada Agreement (USMCA);
•potential adverse changes in economic and political conditions in countries where the company operates or where end-users of the
| | | | | | | | |
| Part I | | |
| Item 1A. Risk Factors | | |
| | |
company's products reside, or in their diplomatic relations with the United States;
•government control of capital transactions, including the borrowing of funds for operations or the expatriation of cash;
•potential adverse tax consequences, including those that may result from new United States tax laws, rules, regulations or policies;
•security concerns and potential business interruption risks associated with political and/or social unrest, or public health crisis, in foreign countries where the company's facilities or assets are located;
•difficulties associated with managing a large organization spread throughout various countries;
•difficulties in enforcing intellectual property rights and weaker intellectual property rights protection in some countries;
•required compliance with a variety of foreign laws and regulations; and
•differing consumer product preferences.
The factors described above also could disrupt the company's product manufacturing and assembling operations or its key suppliers located outside of the United States or increase the cost to the company of conducting those operations or using those suppliers. For example, the company relies on its manufacturing operation in Mexico and suppliers in China and other countries to produce its products or components, and the global COVID-19 pandemic resulted in interruptions in production and supply of components and product on a global basis. Disruptions in, or increased costs related to, the company's foreign operations, particularly in Mexico, may impact the company's revenues and profitability.
The impact of "Brexit" may adversely affect the company.
The United Kingdom (“UK”) exited the European Union (the “EU”) on January 31, 2020. On January 1, 2021 the UK entered into a Free Trade Agreement ("FTA") with the EU. Customs and value added tax accounting requirements have changed and led to increased pressures on supply chain. The UK effectively remains in the EU's customs union and single market and continues to follow EU rules; however, it is no longer part of the EU political institutions. The company markets its main products in the UK, has contracts with the UK government and manufactures mattresses, seating and upholstery products in the UK. Due to Brexit, the company will likely need to re-register products with the MHRA and make labeling and
other changes for products delivered from the UK into the EU. Brexit will pose supply chain risks as the company will need to make various changes, including changes to transportation documentation. Brexit may increase the company's foreign exchange risk should the exchange rates between the British Pound and other currencies such as the U.S. Dollar and Euro materially change. The company expects to take actions to mitigate such risks associated with Brexit but there is no assurance that its efforts will be entirely successful. If the company's mitigation efforts are not sufficient, the company's financial results could be adversely affected.
The company's products may be subject to product liability claims or recalls, which could be costly, harm the company's reputation and adversely affect its business.
The manufacture and sale of medical devices and related products exposes the company to a significant risk of product liability claims. From time to time, the company has been, and currently is, subject to a number of product liability claims alleging that the use of the company's products has resulted in serious injury or even death.
Even if the company is successful in defending against any liability claims, these claims could nevertheless distract the company's management, result in substantial costs, harm the company's reputation, adversely affect the sales of all the company's products and otherwise harm the company's business. If there is a significant increase in the number of product liability claims, the company's business could be adversely affected.
The company is self-insured in North America for product liability exposures through its captive insurance company, Invatection Insurance Company, which currently has a policy year that runs from September 1 to August 31 and insures annual policy losses up to $10,000,000 per occurrence and $13,000,000 in the aggregate. The company also has additional layers of external insurance coverage, related to all lines of insurance coverage, insuring up to $75,000,000 in aggregate losses per policy year arising from individual claims anywhere in the world that exceed the captive insurance company policy limits or the limits of the company's per country foreign liability limits, as applicable. There can be no assurance that Invacare's current insurance levels will continue to be adequate or available at affordable rates.
Product liability reserves are recorded for individual claims based upon historical experience, industry expertise and indications from the third-party actuary. Additional reserves, in excess of the specific individual case reserves, are provided for incurred but not reported claims based upon actuarial valuations at the time such valuations are conducted. Historical claims experience and other
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
assumptions are taken into consideration to estimate the ultimate reserves. For example, the actuarial analysis assumes that historical loss experience is an indicator of future experience, that the distribution of exposures by geographic area and nature of operations for ongoing operations is expected to be very similar to historical operations with no dramatic changes and that the government indices used to trend losses and exposures are appropriate. Estimates made are adjusted on a regular basis and can be impacted by actual loss awards and settlements on claims. While actuarial analysis is used to help determine adequate reserves, the company is responsible for the determination and recording of adequate reserves in accordance with accepted loss reserving standards and practices. If the company's reserves are not adequate to cover actual claims experience, the company's financial results could be adversely affected.
In addition, as a result of a product liability claim or if the company's products are alleged to be defective, the company may have to recall some of its products, may have to incur significant costs or may suffer harm to its business reputation.
The company is subject to ongoing medical device reporting regulations that require the company to report to the FDA or similar governmental authorities in other countries if the company's products cause, or contribute to, death or serious injury, or if they malfunction and would be likely to cause, or contribute to, death or serious injury if the malfunction were to recur. If a deficiency, defect in design or manufacturing or defect in labeling is discovered, the company may voluntarily elect to recall or correct the company's products. In addition, the FDA and similar regulatory authorities in other countries could force the company to do a field correction or recall the company's products in the event of material deficiencies or defects in design or manufacturing. A government mandated or voluntary recall or field correction by the company could occur for various reasons, such as component failures, manufacturing errors or design defects, including defects in labeling. Any recall or field correction could divert managerial and financial resources and could harm the company's reputation with its customers, product users and the health care professionals that use, prescribe and recommend the company's products. The company could have product recalls or field actions that result in significant costs to the company in the future, and these actions could have a material adverse effect on the company's business.
Other Risk Factors - Other Financial Risks, Risks Related to Employees and the Company's Common Shares
The company has long-term finance leases on significant facilities which can affect the company's liquidity and cash flow.
Under the terms of the real estate leases for the company’s facilities in Elyria and North Ridgeville, Ohio, and Sanford, Florida, defaults by the company under any one of such leases, would trigger a cross default under all related leases with the owner/landlord. The company also has a finance lease for its Albstadt, Germany facility. Should a default by the company occur, there could be a material adverse effect on the company's business, operations, financial condition or liquidity.
The company's revenues and profits are subject to exchange rate and interest rate fluctuations which can affect the company's profitability and cash flow.
Currency exchange rates are subject to fluctuation due to, among other things, changes in local, regional or global economic conditions, the imposition of currency exchange restrictions and unexpected changes in regulatory or taxation environments. The predominant currency used by the company's subsidiaries outside the U.S. to transact business is the functional currency used for each subsidiary. Through the company's international operations, the company is exposed to foreign currency fluctuations, and changes in exchange rates can have a significant impact on net sales and elements of cost. The company conducts a significant number of transactions in currencies other than the U.S. dollar. In addition, because certain of the company's costs and revenues are denominated in other currencies, such as those from its European operations, the company's results of operations are exposed to foreign exchange rate fluctuations as the financial results of those operations are translated from local currency into U.S. dollars upon consolidation. For example, in prior years, the devaluation of the Euro had a negative impact on the translation of company's European segment net income into U.S. dollars, and the foreign currency impact of the Brexit referendum in the U.K. had a negative impact on acquisition of dollar and Euro denominated goods in the U.K. If other countries also exit the European Union, similar negative impacts may result.
The company uses foreign exchange forward contracts primarily to help reduce its exposure to transactional exchange rate risk. Despite the company's efforts to mitigate these risks, however, the company's revenues and profitability may be materially adversely affected by exchange rate fluctuations. The company does not have any similar arrangements that mitigate the company's exposure to foreign exchange translation risk, and does not believe that any meaningful arrangement to do so is available to the company.
| | | | | | | | |
| Part I | | |
| Item 1A. Risk Factors | | |
| | |
The company also is exposed to market risk through various financial instruments, including fixed rate and floating rate debt instruments. The company does at times use interest rate swap contracts to mitigate its exposure to interest rate fluctuations, but those efforts may not adequately protect the company from significant interest rate risks. Interest on some of the company's debt is based on the London Interbank Offered Rate (LIBOR), which is currently historically low. Increases in LIBOR could have a significant impact on the company's reported interest expense, to the extent that the company has outstanding borrowings subject to LIBOR-based interest rates.
Additional tax expense or additional tax exposures could affect the company's future profitability and cash flow.
The company is subject to income taxes in the United States and various non-U.S. jurisdictions. The domestic and international tax liabilities are dependent upon the allocation of income among these different jurisdictions. The company's tax expense includes estimates of additional tax which may be incurred for tax exposures and reflects various other estimates and assumptions. In addition, the assumptions include assessments of future earnings of the company that could impact the valuation of its deferred tax assets. The company's future results of operations could be adversely affected by changes in the company's effective tax rate which could result from changes in the mix of earnings in countries with differing statutory tax rates, changes in the overall profitability of the company, changes in tax legislation and rates, changes in generally accepted accounting principles, changes in the valuation of deferred tax assets and liabilities, the results of audits and examinations of previously filed tax returns and continuing assessments of its tax exposures. Corporate tax reform and tax law changes continue to be analyzed in many jurisdictions, including the potential impacts of new United States tax laws, rules, regulations or policies, and any legislation or regulations which may result from those policies.
The Tax Cuts and Jobs Act (“Tax Act”) was enacted on December 22, 2017. The Tax Act significantly revamped U.S. taxation of corporations, including a reduction of the federal income tax rate from 35% to 21%, a limitation on interest deductibility, and a new tax regime for foreign earnings. The limitation on interest deductibility, the new U.S. taxes on accumulated and future foreign earnings, other adverse changes resulting from the Tax Act, or a change in the mix of domestic and foreign earnings, might offset the benefit from the reduced tax rate, and the company's future effective tax rates and/or cash taxes may increase, even significantly, or not decrease much, compared to recent or historical trends. Many of the
provisions of the Tax Act are highly complex and may be subject to further interpretive guidance from the IRS or others. Some of the provisions of the Tax Act may be changed by a future Congress or challenged by the World Trade Organization (“WTO”) or be subject to trade or tax retaliation by other countries. Although the company cannot predict the nature or outcome of such future interpretive guidance, or actions by a future Congress, WTO or other countries, they could adversely impact the company's financial condition, results of operations and cash flows.
The company's reported results may be adversely affected by increases in reserves for uncollectible accounts receivable.
The company has a large balance of accounts receivable and has established a reserve for the portion of such accounts receivable that the company estimates will not be collected because of the company's customers' non-payment. The specific reserve is based on historical trends and a general reserve is recorded to capture macroeconomic trends.
The inability to attract and retain, or loss of the services of, the company's key management and personnel could adversely affect its ability to operate the company's business.
The company's future success will depend, in part, upon the continued service of key managerial, engineering, marketing, sales and technical and operational personnel. In addition, the company's future success will depend on its ability to continue to attract and retain other highly qualified personnel, including personnel experienced in sales, marketing and manufacturing of medical equipment and in quality systems and regulatory affairs. If the company is not successful in retaining its current personnel or in hiring or retaining qualified personnel in the future, the company's business may be adversely affected. The company's future success depends, to a significant extent, on the abilities and efforts of its executive officers and other members of its management team, such as the company's Chairman, President and Chief Executive Officer and its Senior Vice President and Chief Financial Officer, as well as other members of its management team. The company had significant turnover in personnel in recent years and cannot be certain it can adequately recruit, hire and retain replacement personnel or that its executive officers and other key employees will continue in their respective capacities for any period of time, and these employees may be difficult to replace. If the company loses the services of any of its management team, the company's business may be adversely affected.
| | | | | | | | |
| | Part I |
| | Item 1A. Risk Factors |
| | |
Volatility in the market price of the company's common shares could adversely affect its shareholders, its ability to finance operations and retain leadership.
The market price of the company's common shares may be influenced by lower trading volume and concentrated ownership relative to many other publicly-held companies. Because several of the company's shareholders own significant amounts of the company's outstanding common shares, the common shares are relatively less liquid and therefore more susceptible to price fluctuations than many other companies' shares. If any one or more of these shareholders were to sell all or a portion of their holdings of company common shares at once or within short periods of time, or there was an expectation that such a sale was imminent, then the market price of the company's common shares could be negatively affected.
| | | | | | | | |
| Part I | | |
| Item 1B. Unresolved Staff Comments | | |
| | |
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
The company owns or leases its manufacturing facilities, warehouses and offices and believes that these facilities are well maintained, adequately insured and suitable for their present and intended uses. Information concerning certain leased facilities of the company as of December 31, 2020 is set forth in Leases and Commitments in the Notes to the Consolidated Financial Statements of the company included in this report. The company's corporate headquarters is in Elyria, Ohio and a summary of the company's materially important properties by segment is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Owned | | Leased |
| Number | | Square Feet | | Number | | Square Feet |
| Manufacturing Facilities | | | | | | | |
| Europe | 2 | | 302,380 | | | 5 | | 587,280 | |
| North America | 1 | | 152,256 | | | 10 | | 481,656 | |
| | | | | | | |
| 3 | | 454,636 | | | 15 | | 1,068,936 | |
| | | | | | | |
| Warehouse and Office Facilities | | | | | | | |
| Europe | 2 | | 33,444 | | | 38 | | 459,737 | |
| North America | — | | — | | | 9 | | 321,892 | |
| All Other (Asia Pacific) | — | | — | | | 4 | | 77,254 | |
| 2 | | 33,444 | | | 51 | | 858,883 | |
| | | | | | | |
| | | | | | | | |
| Part I | | |
| Item 3. Legal Proceedings | | |
| | |
Item 3. Legal Proceedings.
In the ordinary course of its business, the company is a defendant in a number of lawsuits, primarily product liability actions in which various plaintiffs seek damages for injuries allegedly caused by defective products. All the product liability lawsuits that the company faces in the United States have been referred to the company's captive insurance company and/or excess insurance carriers while all non-U.S. lawsuits have been referred to the company's commercial insurance carriers. All such lawsuits are generally contested vigorously. The coverage territory of the company's insurance is worldwide with the exception of those countries with respect to which, at the time the product is sold for use or at the time a claim is made, the U.S. government has suspended or prohibited diplomatic or trade relations. Management does not believe that the outcome of any of these actions will have a material adverse effect upon the company's business or financial condition.
In December 2012, the company became subject to a consent decree of injunction filed by the FDA in the U.S. District Court for the Northern District of Ohio with respect to the company's Corporate facility and its Taylor Street manufacturing facility in Elyria, Ohio. On July 24, 2017, following its reinspection of the Corporate and Taylor Street facilities, FDA notified the company that it was in substantial compliance with the FDA Act, FDA regulations and the terms of the consent decree and that the company was permitted to resume full operations at those facilities, including the resumption of unrestricted sales of products made in those facilities.
The consent decree will continue in effect for at least five years from July 24, 2017, during which time the company's Corporate and Taylor Street facilities must complete to two semi-annual audits in the first year and then four annual audits in the next four years performed by a company-retained expert firm. The expert audit firm will determine whether the facilities remain in continuous compliance with the FDA Act, regulations and the terms of the consent decree.
The FDA has the authority to inspect the Corporate and Taylor Street facilities, and any other FDA registered facility, at any time. The FDA also has the authority to order the company to take a wide variety of actions if the FDA finds that the company is not in compliance with the consent decree, FDA Act or FDA regulations, including requiring the company to cease all operations relating to Taylor Street products. The FDA also can order the company to undertake a partial cessation of operations or a recall, issue a safety alert, public health advisory, or press release, or to take any other corrective action the FDA deems necessary with respect to Taylor Street products.
The FDA also has authority under the consent decree to assess liquidated damages of $15,000 per violation per day for any violations of the consent decree, FDA Act or FDA regulations. The FDA also may assess liquidated damages for shipments of adulterated or misbranded devices in the amount of twice the sale price of any such adulterated or misbranded device. The liquidated damages, if assessed, are limited to a total of $7,000,000 for each calendar year. The authority to assess liquidated damages is in addition to any other remedies otherwise available to the FDA, including civil money penalties.
Additional information regarding the consent decree is included in Item 1. Business - Government Regulation; Item 1A. Risk Factors.
Item 4. Mine Safety Disclosures.
None.
| | | | | | | | |
| | Part I |
| | Executive Officers of the Registrant |
| | |
Executive Officers of the Registrant*
The following table sets forth the names of the executive officers of the company, each of whom serves at the pleasure of the Board of Directors, as well as certain other information.
| | | | | | | | |
| Name | Age | Position |
| Matthew E. Monaghan | 53 | Chairman, President and Chief Executive Officer |
| Kathleen P. Leneghan | 57 | Senior Vice President and Chief Financial Officer |
| Anthony C. LaPlaca | 62 | Senior Vice President, General Counsel, Chief Administrative Officer and Secretary |
| Ralf A. Ledda | 53 | Senior Vice President and General Manager, Europe, Middle East & Africa |
| Joost Beltman | 52 | Senior Vice President and General Manager, North America |
| Angela Goodwin | 57 | Chief Information Technology Officer |
* The description of executive officers is included pursuant to Instruction 3 to Section (b) of Item 401 of Regulation S-K.
Matthew E. Monaghan serves as the company’s President and Chief Executive Officer since April 2015 and was appointed Chairman of the Board in May 2015. Prior to joining Invacare, Mr. Monaghan served as a business unit leader at Zimmer Holdings (now Zimmer Biomet NYSE: ZBH), a major orthopedic implant company, serving first as Vice President and General Manager of the company’s Global Hips business (December 2009 to January 2014) and later as Senior Vice President of Hips and Reconstructive Research (January 2014 until joining Invacare). While at Zimmer, Mr. Monaghan was responsible for the Hip Division’s new product development, engineering, marketing, clinical studies, quality, regulatory affairs and results of the shared sales and supply chain functions. Later, those responsibilities also included directing global research for various areas of material, process and product innovation. Prior to joining Zimmer in 2009, Mr. Monaghan spent eight years as an operating executive for two leading private equity firms, Texas Pacific Group (TPG) and Cerberus Capital Management, where he led acquisitions and operational improvements of portfolio companies, which included the carve-out from Baxter Healthcare of a global medical business, making significant improvements at a U.S. personal insurance business and as COO of a consumer durable goods business spun off from Newell-Rubbermaid. For the first 13 years of his career, Mr. Monaghan held various engineering, financial and management positions at General Electric (NYSE:GE). Since November 2016, Mr. Monaghan has served as a director of Syneos Health (NASDAQ:SYNH), formerly known as INC Research (NASDAQ: INCR), a contract research and contract commercial organization serving the needs of biopharmaceutical clients.
Kathleen P. Leneghan serves as the Senior Vice President and Chief Financial Officer of the company since February 2018, after having served as interim Chief Financial Officer since November 2017. She served as Vice President and Corporate Controller of the company since 2003. Ms. Leneghan has been employed by the company since 1990, serving in various financial roles of increasing responsibility in North America and Europe.
Prior to joining the company, Ms. Leneghan was an audit manager with Ernst & Young LLP.
Anthony C. LaPlaca serves as Senior Vice President, General Counsel Chief Administrative Officer and Secretary of the company and oversees legal affairs, corporate governance and global human resources. Prior to joining the company in October 2008, Mr. LaPlaca served as Vice President and General Counsel of Bendix Commercial Vehicle Systems LLC, Elyria, Ohio, a member of the Knorr-Bremse group, a supplier of commercial vehicle safety systems, since 2002. Prior to that, he served as Vice President and General Counsel of Honeywell Transportation & Power Systems and General Counsel to Honeywell Commercial Vehicle Systems LLC. Before joining Honeywell's predecessor, AlliedSignal Inc. in 1997, Mr. LaPlaca practiced law at a Cleveland-based national law firm for 13 years, the last 3 years of which as a partner in the firm.
Ralf A. Ledda serves as the company's Senior Vice President and General Manager, Europe, Middle East & Africa since November 2016. Previously he served for 21 years as Managing Director of Alber GmbH, Albstadt, Germany, Invacare’s subsidiary that specializes in innovative electromotive technology and power add-on devices used with medical and recreational products. Prior to joining Alber, he worked at Siemens AG.
Joost Beltman serves as the company's Senior Vice President and General Manager, North America, since August 2020. Previously he served as Vice President of Sales and Marketing North America since 2018. Prior to that, Mr. Beltman was the Managing Director for the BENELUX region and Italy of the company. Prior to joining the company in 2008, Mr. Beltman held several management positions of increasing responsibility with providers in the telecommunications industry.
Angela Goodwin serves as Chief Information Technology Officer of the company since March 2019. Prior to joining Invacare, Ms. Goodwin served as CIO for Fresenius Kabi, a global healthcare company specializing
| | | | | | | | |
| Part I | | |
| Executive Officers of the Registrant | | |
| | |
in pharmaceutical and technological innovations for critically and chronically ill patients from 2012 to 2018. Prior to that, she was CIO of Fenwal, Inc., a global blood technology company supporting transfusion and cell therapies from 2009 to 2012. Ms. Goodwin has over 30 years of global IT leadership experience.
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Invacare's Common Shares, without par value, trade on the New York Stock Exchange (NYSE) under the symbol “IVC.” Ownership of the company's Class B Common Shares (which are not listed on the NYSE or any other established trading market) cannot be transferred, except, in general, to family members without first being converted into Common Shares. Class B Common Shares may be converted into Common Shares at any time on a share-for-share basis. The number of record holders of the company Common Shares and Class B Common Shares at March 1, 2021 was 1,840 and 15, respectively.
SHAREHOLDER RETURN PERFORMANCE GRAPH
The following graph compares the yearly cumulative total return on Invacare's Common Shares against the yearly cumulative total return of the companies listed on the Standard & Poor's 500 Stock Index, the Russell 2000 Stock Index and the S&P Healthcare Equipment & Supplies Index. The S&P Healthcare Equipment & Supplies Index is a capitalization-weighted average index comprised of health care companies in the S&P 500 Index.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 12/15 | | 12/16 | | 12/17 | | 12/18 | | 12/19 | | 12/20 |
| Invacare Corporation | $ | 100.00 | | | $ | 75.35 | | | $ | 97.56 | | | $ | 24.97 | | | $ | 52.84 | | | $ | 52.60 | |
| S&P 500 | 100.00 | | | 111.96 | | | 136.40 | | | 130.42 | | | 171.49 | | | 203.04 | |
| Russell 2000 | 100.00 | | | 121.31 | | | 139.08 | | | 123.76 | | | 155.35 | | | 186.36 | |
| S&P Healthcare Equipment & Supplies | 100.00 | | | 107.56 | | | 141.29 | | | 162.86 | | | 210.89 | | | 256.72 | |
Copyright© 2021 Standard & Poor's, a division of S&P Global. All rights reserved.
Copyright© 2021 Russell Investment Group. All rights reserved.
The graph assumes $100 invested on December 31, 2015 in the Common Shares of Invacare Corporation, S&P 500 Index, Russell 2000 Index and the S&P Healthcare Equipment & Supplies Index, including reinvestment of dividends, through December 31, 2020.
The following table presents information with respect to repurchases of Common Shares made by the company during the three months ended December 31, 2020.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Period | | | Total Number
of Shares Purchased (1) | | Average Price
Paid Per Share | | Total Number of Shares
Purchased as Part of
Publicly Announced
Plans or Programs | | Maximum Number
of Shares That May Yet
Be Purchased Under
the Plans or Programs (2) |
| 10/1/2020 | - | 10/31/20 | — | | $ __ | | — | | 2,453,978 |
| 11/1/2020 | - | 11/30/20 | 452 | | 8.21 | | — | | 2,453,978 |
| 12/1/2020 | - | 12/31/20 | — | | — | | — | | 2,453,978 |
| Total | | | 452 | | $8.21 | | — | | 2,453,978 |
________________________
(1)All 452 shares repurchased between October 1, 2020 and December 31, 2020 were surrendered to the company by employees for minimum tax withholding purposes in conjunction with the vesting of restricted shares awarded to the employees or exercise of non-qualified options under the company's equity compensation plans.
(2)In 2001, the Board of Directors authorized the company to purchase up to 2,000,000 Common Shares, excluding any shares acquired from employees or directors as a result of the exercise of options or vesting of restricted shares or restricted stock units pursuant to the company's performance plans. The Board of Directors reaffirmed its authorization of this repurchase program on November 5, 2010, and on August 17, 2011 authorized an additional 2,046,500 shares for repurchase under the plan. To date, the company has purchased 1,592,522 shares under this program, with authorization remaining to purchase 2,453,978 shares. The company purchased no shares pursuant to this Board authorized program during 2020.
Under the terms of the company's senior credit facilities, repurchases of shares by the company generally are not permitted except in certain limited circumstances in connection with the vesting or exercise of employee equity compensation awards. Refer to Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources, regarding covenants of the company's senior credit facilities with respect to share purchases.
The equity compensation plan information required under Item 201(d) of Regulation S-K is incorporated by reference to the information under the caption "Equity Compensation Plan Information" in the company's definitive Proxy Statement on Schedule 14A for the 2021 Annual Meeting of Shareholders.
Item 6. Selected Financial Data.
The selected consolidated financial data set forth below with respect to the company's consolidated statements of comprehensive income (loss), cash flows and shareholders' equity for the fiscal years ended December 31, 2020, 2019 and 2018, and the consolidated balance sheets as of December 31, 2020 and 2019 are derived from the Consolidated Financial Statements included elsewhere in this Form 10-K. The consolidated statements of comprehensive income (loss), cash flows and shareholders' equity data for the fiscal years ended December 31, 2017 and 2016 and consolidated balance sheet data for the fiscal years ended December 31, 2018, 2017 and 2016 are derived from the company's previously filed Consolidated Financial Statements.
The data set forth in the following table should be read in conjunction with Item 7—“Management's Discussion and Analysis of Financial Condition and Results of Operations” and the company's Consolidated Financial Statements and Notes thereto included elsewhere in this Form 10-K.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2020 * | | 2019 ** | | 2018 *** | | 2017**** | | 2016 ***** |
| | (In thousands, except per share and ratio data) |
| Earnings (Loss) | | | | | | | | | |
| Net sales | $ | 850,689 | | | $ | 927,964 | | | $ | 972,347 | | | $ | 966,497 | | | $ | 1,047,474 | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Net Loss | (28,280) | | | (53,327) | | | (43,922) | | | (76,541) | | | (42,856) | |
| | | | | | | | | |
| Net Loss per Share—Basic: | | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Net Loss per Share—Basic | (0.83) | | | (1.59) | | | (1.33) | | | (2.34) | | | (1.32) | |
| | | | | | | | | |
| Net Loss per Share—Assuming Dilution: | | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Net Loss per Share—Assuming Dilution | (0.83) | | | (1.59) | | | (1.33) | | | (2.34) | | | (1.32) | |
| | | | | | | | | |
| Dividends per Common Share | 0.0125 | | | 0.05 | | | 0.05 | | | 0.05 | | | 0.05 | |
| Dividends per Class B Common Share | — | | | — | | | 0.02273 | | | 0.04545 | | | 0.04545 | |
| | | | | | | | | |
| Balance Sheet | | | | | | | | | |
| Current Assets | $ | 374,466 | | | $ | 355,877 | | | $ | 397,410 | | | $ | 456,914 | | | $ | 409,072 | |
| Total Assets | 945,981 | | | 852,126 | | | 885,855 | | | 1,066,033 | | | 903,743 | |
| Current Liabilities | 230,386 | | | 218,657 | | | 198,208 | | | 218,064 | | | 220,861 | |
| Working Capital | 144,080 | | | 137,220 | | | 199,202 | | | 238,850 | | | 188,211 | |
| Long-Term Debt (including leases) | 311,275 | | | 258,004 | | | 253,535 | | | 241,405 | | | 146,088 | |
| Other Long-Term Obligations | 70,474 | | | 66,949 | | | 74,965 | | | 183,270 | | | 114,407 | |
| Shareholders' Equity | 333,846 | | | 308,516 | | | 359,147 | | | 423,294 | | | 422,387 | |
| | | | | | | | | |
| Other Data | | | | | | | | | |
| Research and Development Expenditures | $ | 12,275 | | | $ | 15,836 | | | $ | 17,377 | | | $ | 17,796 | | | $ | 17,123 | |
| Capital Expenditures | 22,304 | | | 10,874 | | | 9,823 | | | 14,569 | | | 10,151 | |
| Depreciation and Amortization | 14,317 | | | 15,563 | | | 15,556 | | | 14,631 | | | 14,635 | |
| | | | | | | | | |
| Key Ratios | | | | | | | | | |
| Return on Sales % | (3.3) | | | (5.7) | | | (4.5) | | | (7.9) | | | (4.1) | |
| Return on Average Assets % | (3.1) | | | (6.1) | | | (4.5) | | | (7.8) | | | (4.9) | |
| Return on Beginning Shareholders' Equity % | (9.2) | | | (14.8) | | | (10.4) | | | (18.1) | | | (9.3) | |
| Current Ratio | 1.6:1 | | 1.6:1 | | 2:1 | | 2.1:1 | | 1.9:1 |
| Debt-to-Equity Ratio | 0.98:1 | | 0.87:1 | | 0.71:1 | | 0.58:1 | | 0.38:1 |
________________________
* Reflects gain on sale of Dynamic Controls of $9,790,000 ($10,778,000 after-tax or $0.31 per share assuming dilution), charges related to restructuring of $7,358,000 ($5,639,000 after-tax expense or $0.16 per share assuming dilution) and loss on debt extinguishment including debt finance charges and fees of $7,360,000 ($7,360,000 after-tax expense or $0.21 per share assuming dilution).
** Reflects charges related to restructuring of $11,829,000 ($9,003,000 after-tax expense or $0.27 per share assuming dilution), loss on debt extinguishment including debt finance charges and fees of $6,165,000 ($6,165,000 after-tax expense or $0.18 per share assuming dilution), net gains on convertible debt derivatives of $1,197,000 ($1,197,000 after-tax income or $0.04 per share assuming dilution) and an intangible asset impairment of $587,000 ($435,000 after-tax expense or $0.01 per share assuming dilution).
*** Reflects charges related to restructuring of $3,481,000 ($3,249,000 after-tax expense or $0.10 per share assuming dilution), net loss on convertible debt derivatives of $11,994,000 ($11,994,000 after-tax income or $0.36 per share assuming dilution), an intangible asset impairment of $583,000 ($431,000 after-tax expense or $0.01 per share assuming dilution) and a non-cash tax benefit of $2,023,000 ($0.06 per share assuming dilution) related to U.S. tax reform legislation.
**** Reflects charges related to restructuring of $12,274,000 ($11,872,000 after-tax expense or $0.36 per share assuming dilution), net loss on convertible debt derivatives of $3,657,000 ($3,657,000 after-tax income or $0.11 per share assuming dilution), an intangible asset impairment of $320,000 ($237,000 after-tax expense or $0.01 per share assuming dilution) and a non-cash tax benefit of $1,580,000 ($0.05 per share assuming dilution) related to the revaluation of net deferred tax liabilities as a result of the new U.S. tax reform legislation.
***** Reflects gain on sale of Garden City Medical, Inc. of $7,386,000 ($7,386,000 after-tax income or $0.23 per share assuming dilution), charges related to restructuring of $2,447,000 ($2,447,000 after-tax expense or $0.08 per share assuming dilution), incremental warranty expense of $2,856,000 ($2,856,000 after-tax expense or $0.09 per share assuming dilution related to three product recalls) and net gain on convertible debt derivatives of $1,268,000 ($1,268,000 after-tax income or $0.04 per share assuming dilution).
| | | | | | | | |
| | Part II |
| | Management Discussion & Analysis - Overview |
| | |
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
OVERVIEW
Management's discussion and analysis should be read in conjunction with the consolidated financial statements and accompanying notes that appear elsewhere in this Annual Report on Form 10-K.
Invacare is a multi-national company with integrated capabilities to design, manufacture and distribute durable medical devices. The company makes products that help people move, breathe, rest and perform essential hygiene, and with those products the company supports people with congenital, acquired and degenerative conditions. The company's products and solutions are important parts of care for people with a range of challenges, from those who are active and involved in work or school each day and may need additional mobility or respiratory support, to those who are cared for in residential care settings, at home and in rehabilitation centers. The company operates in facilities in North America, Europe and Asia Pacific, which are the result of dozens of acquisitions made over the company's forty one year history. Some of these acquisitions have been combined into integrated operating units, while others have remained relatively independent.
COVID-19 Impact
The company continues to actively monitor the impact of the coronavirus (COVID-19), which negatively impacted the company’s business in 2020 with regard to reduced net sales on a global basis year-over-year primarily related to mobility and seating products. In addition, the company experienced a decrease in 2020 operating income in the Europe segment as a result of a significant decline in net sales related to the pandemic. The extent to which the company’s operations will be impacted by the pandemic will depend largely on future developments, which remain highly uncertain and difficult to accurately predict, including, among other things, new information which may emerge concerning the severity of the pandemic and actions by government authorities to contain COVID-19 or treat its impact, such as reimposed public health restrictions or restrictions on access to healthcare facilities.
In 2020, as an “essential” business making medical devices, the company had continued to operate in all but one of its facilities which had temporarily suspended operations, having taken the recommended public health measures to ensure worker and workplace safety. The company continues to experience high demand globally for its respiratory products. These products are being deployed in the fight against the COVID-19
pandemic in expanded medical facilities to relieve the strain on hospital systems by providing more medical beds and access to purified oxygen needed in respiratory care. The company continues to work to increase its capacity to produce these critical products and resolve global supply chain challenges that are compounded by the effects of the pandemic. As a result, there are practical limits to the extent the company can increase output. In addition, the company has continued to experience cost increases from pandemic-related supply chain disruptions.
The initial stages of the pandemic appropriately focused the provision of healthcare to urgent non-elective care, reducing access to clinicians and healthcare facilities on which other parts of the company’s business rely to engage with customers for product trials and fittings. As a result, and combined with various stay-at-home orders, the company experienced a global decline in quotes for mobility and seating products, and a resultant decline in orders starting in the second quarter of 2020. The company realized sequential sales growth on a consolidated basis in the third and fourth quarters of 2020, primarily driven by Europe, as access to customers improved from the low point of the second quarter of 2020. While the company believes the decline in demand for mobility and seating product is temporary in nature, the rebound of the business will depend on the continued restoration of access to healthcare and loosening of public health restrictions, and will be impacted by several items including government actions and policies related to the pandemic, and the magnitude of the pandemic.
The company continues to optimize its balance sheet for the current environment. In the third quarter of 2020, the company repurchased $24,466,000 aggregate principal amount of its 2021 Notes. Earlier in the year, the company entered into separate privately negotiated agreements with certain holders to exchange an aggregate of $73,875,000 of its 2021 and 2022 Notes into new 5% Series II Convertible Senior Exchange Notes (the "Series II 2024 Notes") of the company. This transaction enhanced the company's financial flexibility in reducing short-term debt by extending a significant portion of near-term convertible debt to November 2024. These actions, as well as other actions completed in the first half of 2020, for example completing the divestiture of Dynamic Controls and obtaining an unsecured loan under the Coronavirus Aid, Relief, and Economic Security ("CARES") Act, borrowing availability under the ABL revolver, and the anticipated generation of Adjusted EBITDA and free cash flow, should provide the company sufficient liquidity to manage the business and meet its obligations.
Strategy
The company historically had a strategy to be a leading provider of durable medical equipment to health
| | | | | | | | |
| Part II | | |
| Management Discussion & Analysis - Overview | | |
| | |
care providers in global markets by providing the broadest portfolio available. This strategy has not kept pace with certain reimbursement changes, competitive dynamics and company-specific challenges. Since 2015, the company has made a major shift in its strategy. The company has since been aligning its resources to produce products and solutions that assist customers and end-users with their most clinically complex needs. By focusing the company's efforts to provide the best possible assistance and outcomes to the people and caregivers who use its products, the company aims to improve its financial condition for sustainable profit and growth. As a result, the company is undertaking a substantial multi-year business optimization plan.
Business Optimization Efforts
The company continues to execute a multi-year strategy to return the company to profitability by focusing its resources on products and solutions that provide greater healthcare value in clinically complex rehabilitation and post-acute care. The company's business optimization actions and growth plan balance innovative organic growth, product portfolio changes across all regions, and cost improvements in supply chain and administrative functions. Key elements of the business optimization and growth plans are:
•Globally, continue to drive all business segments and product lines based on their potential to achieve a leading market position and to support profitability goals;
•In Europe, leverage centralized innovation and supply chain capabilities while reducing the cost and complexity of a legacy infrastructure;
•In North America, adjust the portfolio to consistently grow profitability amid cost increases by adding new products, reducing costs and continuing to improve customers' experience;
•In Asia Pacific, remain focused on sustainable growth and expansion in the southeast Asia region; and,
•Take actions globally to reduce working capital and improve free cash flow.
As it navigates the uncertain business environment resulting from the pandemic, the company continues to allocate more resources to the business units experiencing increased demand and expects to continue taking actions to mitigate the potential negative financial and operational impacts on other parts of the company's business that have declined. In the medium-term, the company still expects to execute on its business optimization strategy, such as the
global IT modernization initiative which is expected to ultimately result in optimization of the operating structure.
In 2020, the company made significant progress with improved financial performance and strengthened its overall business profile by taking actions such as:
•Significantly reduced expenses given the reduced net sales as a result of the pandemic;
•Enhanced its product portfolio including the introduction of power wheelchairs with standing capabilities, power add-ons for manual wheelchairs, beds, lifts and slings, all of which are expected to drive incremental sales and expand margins;
•Completed the outsourcing of the IT infrastructure and commenced the roll out of modernized IT systems in Canada and New Zealand;
•Completed the consolidation of three German facilities;
•Divested Dynamic Controls and entered into a long-term supply agreement; and,
•Strengthened the balance sheet by retiring $24,466,000 of 2021 convertible notes and executed a convertible note exchange that effectively extended the maturity of $73,875,000 of convertible notes to 2024.
The company made great progress in 2020 with more work to be done in 2021. The company intends to continue to make significant investments in its business improvement initiatives, may reduce sales in certain areas, refocus resources away from less accretive activities, and look at its global infrastructure for opportunities to drive efficiency with a focus on improving profitability and free cash flow generation. As part of the company’s efforts to streamline its operations and focus its resources on core product lines that provide the greatest value and financial returns, the company continuously evaluates opportunities and activities, including potential divestitures, which it considers from time to time, particularly if they involve businesses or assets outside of the company’s primary areas of focus.
Outlook
Given the continued progress of the company's performance, increased visibility into the path of recovery for its customers, expected improved access to healthcare, and successful vaccine execution, improvements to global pandemic-related supply chain disruptions, and pent-up
| | | | | | | | |
| Table of Contents | | Part II |
| | MD&A - Accounting Estimates and Pronouncements |
| | |
demand from the prior year, the company expects improved operating results for the full year 2021.
The company participates in durable healthcare markets and serves a persistent need for its products. The company will continue to improve operating efficiency, together with the lifting of public health restrictions, yielding improved financial performance. As a result, the company expects to grow revenue, profitability, and improve its cash flow performance in the year. As approximately 95% of the company's revenues are generated in the Northern Hemisphere, the company expects continued impact from the pandemic on the business throughout the winter months, with growth thereafter as the pandemic subsides, amplified by the adoption of new products and pent-up demand from the prior year.
While the company expects full year revenue growth over 2020, revenues in the first quarter will decline in the mid-single digits and related profitability will decline compared to the first quarter of 2020, excluding the divestiture of Dynamic Controls, due to the current challenges related to the pandemic including supply chain disruptions. These challenges will also hamper the company's ability to reduce backlog from the end of 2020. In addition, margins will be negatively impacted due to unfavorable supply chain costs, manufacturing variances and product mix. Further, the first quarter of 2020 was not impacted by the pandemic.
The company's earnings performance is expected to benefit from: (1) new product introductions with improved commercialization plans and additional investments in the sales force and demonstration equipment, which are expected to result in profitable incremental sales, as well as higher sales and margins on existing products; and (2) margin expansion expected as a result of efficiencies related to the plant consolidations in Germany; supply chain actions to expand gross profits, offset by higher freight and logistics costs, and the impact of U.S. tariff exclusions which expired on January 1, 2021. The company expects SG&A expense to be higher than 2020 levels but lower than 2019 levels as it adds back sales and marketing related spending to support sales growth and activity-based spending. Stock compensation expense is expected to be closer to 2019 levels. In addition, while the new IT system implementation is a key project for the company in North America during 2021, benefits related to improved customer experience and efficiencies have not been considered in the guidance as a result of the anticipated timing to roll out the new system in North America.
Cash flow is expected to fund one-time payments of approximately $5 million for severance costs related to the German plant consolidation, and approximately $10
million to fund the payment of the majority of VAT and other taxes deferred from 2020 implemented by many jurisdictions as result of the pandemic. The company has historically generated negative cash flow performance during the first half of the year. This pattern is expected to continue due to the timing of annual one-time payments such as customer rebates and employee bonuses earned during the prior year, and higher working capital usage from seasonal inventory increases. Further, the first quarter of 2021 is expected to continue to be negatively impacted by limitations to access to healthcare impacted by the pandemic. The absence of these payments and seasonally stronger sales in the second half of the year typically result in more favorable cash flow performance in the second half of the year. The company expects improvement in segment operating performance, reduced working capital and slightly lower capital expenditures. The company anticipates spending $20 million on capital expenditures in 2021.
The company participates in growing markets and believes its long-term economic potential remains strong. The company believes that the continued generation of earnings driven by operational performance, cash balances on hand, and expected free cash flow will support the company's on-going business improvement plans and enable it to address future debt maturities.
Favorable Long-term Demand
Ultimately, demand for the company's products and services is based on the need to provide care for people with certain conditions. The company's medical devices provide solutions for end-users and caregivers. Therefore, the demand for the company's medical equipment is largely driven by population growth and the incidence of certain conditions where treatment may be supplemented by the company's devices. The company also provides solutions to help equipment providers and residential care operators deliver cost-effective and high-quality care. The company believes that its commercial team, customer relationships, products and solutions, supply chain infrastructure, and strong research and development pipeline will create favorable business potential.
| | | | | | | | |
| Part II | | |
| Results of Operations | | |
| | |
RESULTS OF OPERATIONS
NET SALES
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in thousands USD) | | 2020 | | 2019 | | % Change
Fav/(Unfav) | | Foreign Exchange % Impact | | Divestiture % Impact | | Constant Currency % Change
Fav/(Unfav) |
| Europe | | 468,041 | | | 533,048 | | | (12.2) | | | 0.6 | | | — | | | (12.8) | |
| North America | | 348,307 | | | 348,201 | | | — | | | (0.1) | | | — | | | 0.1 | |
| All Other (Asia Pacific) | | 34,341 | | | 46,715 | | | (26.5) | | | (2.6) | | | (29.2) | | | 5.3 | |
| Consolidated | | 850,689 | | | 927,964 | | | (8.3) | | | 0.4 | | | (1.5) | | | (7.2) | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
The table above provides net sales change as reported and as adjusted to exclude the impact of foreign exchange translation and divestitures (constant currency net sales). “Constant currency net sales" is a non-Generally Accepted Accounting Principles ("GAAP") financial measure, which is defined as net sales excluding the impact of foreign currency translation and divestitures. The current year's functional currency net sales are translated using the prior year's foreign exchange rates. These amounts are then compared to the prior year's sales to calculate the constant currency net sales change. For the divestiture impact, the company adjusted the net sales of the Dynamic Controls business which was divested as of March 7, 2020. Management believes that this financial measure provides meaningful information for evaluating the core operating performance of the company.
Consolidated net sales for 2020 decreased 8.3% for the year, to $850,689,000 from $927,964,000 in 2019. Foreign currency translation increased net sales by 0.4 percentage points with the divestiture decreasing net sales by 1.5 percentage points. Constant currency net sales decreased 7.2% compared to 2019 as mobility and seating declined by $60,432,000, or 15.6% and lifestyle products declined by $28,520,000 or 6.6%. Both of these product categories were negatively impacted by the pandemic as a result of restrictions which limited access to certain product selections. These were partially offset by increases for respiratory products of $26,168,000 or 36.1%.
Europe - European net sales decreased 12.2% in 2020 compared to 2019 to $468,041,000 from $533,048,000 as foreign currency translation increased net sales by 0.6 percentage points. Constant currency net sales decreased 12.8% compared to 2019 driven by a 18.3% decrease in sales of mobility and seating products and 8.8% decrease in sales of lifestyle products. Net sales were significantly impacted by the pandemic and by public health restrictions in certain countries limiting access to
healthcare professionals and institutions needed for certain product selections. The countries which the company as a significant portion of the operations are France, Germany, UK and Nordic countries. Changes in exchange rates have had, and may continue to have, a significant impact on sales in this segment.
North America - North America net sales were flat in 2020 versus the prior year to $348,307,000 from $348,201,000. Foreign currency translation decreased net sales by 0.1 percentage points. Constant currency net sales increased by respiratory products of $20,688,000 or 40.1%, which offset declines in mobility and seating and lifestyle products. Demand for mobility and seating and non-bed lifestyle products were impacted by the pandemic, with public restrictions limiting access to healthcare professionals and institutions to provide certain products.
All Other - Net sales, which relate entirely to the Asia Pacific region, decreased 26.5% in 2020 from the prior year to $34,341,000 from $46,715,000. Foreign currency translation decreased net sales by 2.6 percentage points and the Dynamic Controls divestiture decreased net sales by 29.2 percentage points. Constant currency net sales increased 5.3% compared to 2019 primarily from net sales increases in lifestyle products of 21.7%, primarily bed and bed related products. Changes in exchange rates have had, and may continue to have, a significant impact on sales in the Asia Pacific region.
The sales mix shift in 2020 reflects the significant impacts of the pandemic limiting the access to healthcare professionals and institutions needed for certain product selections, specifically mobility and seating and non-bed lifestyle products as well as higher demand for pandemic related respiratory and bed and mattress products.
2019 Versus 2018
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in thousands USD) | | 2019 | | 2018 | | Reported % Change | | Foreign Exchange % Impact | | Constant Currency % Change |
| Europe | | 533,048 | | | 558,518 | | | (4.6) | | | (5.8) | | | 1.2 | |
| North America | | 348,201 | | | 364,590 | | | (4.5) | | | 0.2 | | | (4.3) | |
| All Other (Asia Pacific) | | 46,715 | | | 49,239 | | | (5.1) | | | (5.5) | | | 0.4 | |
| Consolidated | | 927,964 | | | 972,347 | | | (4.6) | | | (3.7) | | | (0.9) | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
Consolidated net sales for 2019 decreased 4.6% for the year, to $927,964,000 from $972,347,000 in 2018. Foreign currency translation decreased net sales by 3.7 percentage points. Constant currency net sales decreased 0.9% compared to 2018 as a decline in respiratory products of $19,249,000 or 20.7%, was only partially offset by increases in mobility and seating products of $10,211,000.
Europe - European net sales decreased 4.6% in 2019 compared to 2018 to $533,048,000 from $558,518,000 as foreign currency translation decreased net sales by 5.8 percentage points. Constant currency net sales increased 1.2% compared to 2018 driven by a 4.4% increase in sales of mobility and seating products. Changes in exchange rates have had, and may continue to have, a significant impact on sales in the segment.
North America - North America net sales decreased 4.5% in 2019 versus the prior year to $348,201,000 from $364,590,000 as foreign currency translation decreased net sales by 0.2 percentage points. Constant currency net sales decreased as net sales growth in mobility and seating and lifestyle products was completely offset by a $15,925,000 decrease in respiratory net sales.
All Other - Net sales, which relate entirely to the Asia pacific region decreased 5.1% in 2019 from the prior year to $46,715,000 from $49,239,000. Foreign currency translation decreased net sales by 5.5 percentage points. Constant currency net sales increased 0.4% compared to 2018 as net sales increases in lifestyle products were largely offset by declines in sales of mobility and seating products. The second half of 2019 sales growth was impacted by payor process changes which were temporarily affected by funding availability in New Zealand. Changes in exchange rates have had, and may continue to have, a significant impact on sales in the segment.
| | | | | | | | |
| | Part II |
| | MD&A - Gross Profit |
| | |
GROSS PROFIT
2020 Versus 2019
Consolidated gross profit as a percentage of net sales increased by 60 basis points to 28.8% in 2020 as compared to 28.2% in 2019. Gross profit as a percentage of net sales improved significantly for North America and All Other, while Europe margins declined significantly impacted by the pandemic. Gross profit dollars decreased due to lower net sales in 2020, primarily in Europe.
Europe - Gross profit as a percentage of net sales decreased 110 basis points in 2020 compared to the prior year and gross margin dollars decreased by $22,951,000. The decrease in margin dollars was principally due to lower sales as result of the pandemic and unfavorable manufacturing variances on lower volume.
North America - Gross profit as a percentage of net sales increased by 260 basis points in 2020 compared to the prior year while gross margin dollars increased by $11,133,000. The increase in gross profit dollars was primarily due to favorable material costs, improved product mix and lower freight costs offset by unfavorable operational variances.
All Other - Gross profit as a percentage of net sales, increased 610 basis points in 2020 compared to the prior year and gross profit dollars decreased $4,997,000. All other primarily relates to the company's Asia Pacific businesses. The decrease in gross profit dollars was primarily driven by reduced sales from the divestiture of the Dynamic Controls business as of Mach 7, 2020.
Research and Development
The company continued to invest strategically in research and development activities in 2020. The company dedicated funds to applied research activities to ensure that new and enhanced design concepts are available to its businesses. Research and development expenditures, which are included in costs of products sold, decreased to $12,275,000 in 2020 from $15,836,000 in 2019. The decline in expense in 2020 was primarily related to the divestiture of Dynamic Controls. The expenditures, as a percentage of net sales, were 1.4% and 1.7% in 2020 and 2019, respectively.
| | | | | | | | |
| Part II | | |
| MD&A - Gross Profit | | |
| | |
2019 Versus 2018
Consolidated gross profit as a percentage of net sales was 28.2% in 2019 as compared to 27.5% in 2018. Gross profit as a percentage of net sales for 2018 decreased by 70 basis points as compared to 2018. The gross margin improvement reflects the effective mitigation of the previously estimated approximately $5,000,000 annual negative impact of tariffs, to an actual negative impact of less than $2,000,000, as well as lower material and freight costs. Gross profit as a percentage of net sales improved significantly for North America while Europe margins were flat and All Other declined. Gross profit dollars decreased due to lower net sales and unfavorable foreign currency translation which negatively impacted consolidated gross profit by $10,742,000 in 2019.
Europe - Gross profit as a percentage of net sales was unchanged in 2019 compared to the prior year and gross margin dollars decreased by $7,913,000. The decrease in margin dollars was principally due to unfavorable foreign currency and unfavorable sales mix.
North America - Gross profit as a percentage of net sales increased by 200 basis points in 2019 from the prior year while gross margin dollars increased by $3,811,000. The increase in gross profit dollars was primarily due to favorable material costs, improved product mix and also lower freight and warranty costs. The favorable material costs were partially due to mitigating the negative impact of tariffs, which were reduced to a combined negative impact of less than $2,000,000.
All Other - Gross profit, which primarily relates to the company's Asia Pacific businesses increased 60 basis points in 2019 from the prior year and gross margin dollars decreased $1,507,000. The decrease was primarily attributable to reduced sales volumes.
Research and Development
The company continued to invest strategically in research and development activities in 2019. The company dedicated funds to applied research activities to ensure that new and enhanced design concepts are available to its businesses. Research and development expenditures, which are included in costs of products sold, decreased to $15,836,000 in 2019 from $17,377,000 in 2018. The expenditures, as a percentage of net sales, were 1.7% and 1.8% in 2019 and 2018, respectively.
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
2020 Versus 2019
| | | | | | | | | | | | | | | | | | | | |
| ($ in thousands USD) | 2020 | 2019 | Reported Change | Foreign Exchange Impact | Divestiture Impact | Constant Currency Change |
| SG&A Expenses - $ | 236,357 | | 260,061 | | (23,704) | | 1,756 | | (3,484) | | (21,976) | |
| SG&A Expenses - % change | | | (9.1) | | 0.8 | | (1.3) | | (8.6) | |
| % to net sales | 27.8 | | 28.0 | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
The table above provides selling, general and administrative (SG&A) expense change as reported and as adjusted to exclude the impact of foreign exchange translation (constant currency SG&A). “Constant currency SG&A" is a non-GAAP financial measure, which is defined as SG&A expenses excluding the impact of foreign currency translation and divestitures. The current year's functional currency SG&A expenses are translated using the prior year's foreign exchange rates. These amounts are then compared to the prior year's SG&A expenses to calculate the constant currency SG&A expense change. Management believes that this financial measure provides meaningful information for evaluating the core operating performance of the company.
The divestiture impact is related to the SG&A expenses related to the Dynamic Controls business divested on March 7, 2020.
Consolidated SG&A expenses as a percentage of net sales were 27.8% in 2020 and 28.0% in 2019. The overall dollar decrease was $23,704,000, or 9.1%, with foreign currency translation increasing expense by $1,756,000. Excluding the impact of foreign currency translation and the divestiture of Dynamic Controls, SG&A expenses decreased $21,976,000, or 8.6%, primarily driven by reduced employee-related costs.
Europe - European SG&A expenses decreased by 7.9%, or $9,459,000, in 2020 compared to 2019. Foreign currency translation increased expense by approximately $1,775,000 or 1.5%. Excluding the foreign currency translation impact, SG&A expenses decreased by $11,234,000, or 9.4%, primarily driven by reduced employee-related costs.
North America - SG&A expenses for North America decreased 6.1%, or $5,909,000, in 2020 compared to 2019 with foreign currency translation decreasing expense by $132,000 or 0.2%. Excluding the foreign currency translation, SG&A expense decreased $5,777,000, or 5.9%, driven primarily driven by reduced employee-related costs.
All Other - SG&A expenses decreased $8,336,000 in 2020 compared to 2019. Foreign currency translation increased expense by $113,000. All Other includes SG&A related to the Asia Pacific businesses and non-allocated corporate costs.
SG&A expenses related to non-allocated corporate costs for 2020 decreased 7.4%, or $2,137,000, compared to 2019. The decrease was primarily driven by reduced employee-related costs, primarily stock compensation. Stock compensation was lower in 2020 due to lowered projected vesting assumptions on multi-year cycle performance awards impacted by the pandemic and was partially offset by modification of performance awards in the fourth quarter of 2020.
Related to the Asia Pacific businesses, 2020 SG&A decreased 44.0%, or $6,199,000, compared to 2019 with foreign currency translation increasing SG&A expenses $113,000, or 7.4%. The divestiture of Dynamic Controls decreased expense by $3,484,000 or 24.7%. Constant currency SG&A expenses decreased $2,828,000, or 26.7%, primarily driven by reduced employee-related costs.
2019 Versus 2018
| | | | | | | | | | | | | | | | | |
| ($ in thousands USD) | 2019 | 2018 | Reported Change | Foreign Exchange Impact | Constant Currency Change |
| SG&A Expenses - $ | 260,061 | | 281,906 | | (21,845) | | (7,228) | | (14,617) | |
| SG&A Expenses - % change | | | (7.7) | | (2.5) | | (5.2) | |
| % to net sales | 28.0 | | 29.0 | | | | |
| | | | | |
| | | | | |
| | | | | |
Consolidated SG&A expenses as a percentage of net sales were 28.0% in 2019 and 29.0% in 2018. The overall dollar decrease was $21,845,000, or 7.7%, with foreign currency translation decreasing expense by $7,228,000. Excluding the impact of foreign currency translation, SG&A expenses decreased $14,617,000, or 5.2%, primarily driven by reduced employment and product liability costs partially offset by higher bonus and stock compensation expense.
Europe - European SG&A expenses decreased by 8.7%, or $11,413,000, in 2019 compared to 2018. Foreign currency translation decreased expense by approximately $6,135,000 or 4.7%. Excluding the foreign currency translation impact, SG&A expenses decreased by $5,278,000, or 4.0%, primarily attributable to lower employment costs.
North America - SG&A expenses for North America decreased 17.8%, or $21,104,000, in 2019 compared to 2018 with foreign currency translation decreasing expense by $302,000 or 0.2%. Excluding the foreign currency translation, SG&A expense decreased $20,802,000, or 17.6%, driven primarily by decreased employment, consulting and product liability costs.
All Other - SG&A expenses increased $10,672,000 in 2019 compared to 2018. Foreign currency translation decreased expense by $791,000. All Other includes SG&A related to the Asia Pacific businesses and non-allocated corporate costs. SG&A expenses related to non-allocated corporate costs for 2019 increased 61.3%, or $11,036,000, compared to 2018. The increase was driven primarily by increased employment costs, including stock compensation and bonus expense. Related to the Asia Pacific businesses, 2019 SG&A decreased 2.5%, or $364,000, compared to 2018 with foreign currency translation decreasing SG&A expenses $791,000, or 5.5%. Constant currency SG&A expenses increased $427,000, or 3.0%, primarily due to higher sales and marketing and employment costs.
| | | | | | | | |
| | Part II |
| | MD&A - Operating Income |
| | |
OPERATING INCOME (LOSS)
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | 2020 vs. 2019 | 2019 vs. 2018 |
| ($ in thousands USD) | 2020 | 2019 | 2018 | $ Change | % Change | $ Change | % Change |
| Europe | 22,682 | | 36,174 | | 32,673 | | (13,492) | | (37.3) | | 3,501 | | 10.7 | |
| North America | 9,449 | | (7,592) | | (32,506) | | 17,041 | | 224.5 | | 24,914 | | 76.6 | |
| | | | | | | |
| | | | | | | |
| All Other | (23,236) | | (26,576) | | (14,397) | | 3,340 | | 12.6 | | (12,179) | | (84.6) | |
| Gain on sale of business | 9,790 | | — | | — | | 9,790 | | — | | — | | — | |
| Charges related to restructuring | (7,358) | | (11,829) | | (3,481) | | 4,471 | | 37.8 | | (8,348) | | (239.8) | |
| Impairment of an intangible asset | — | | (587) | | (583) | | 587 | | (100.0) | | (4) | | 0.7 | |
| Consolidated Operating Income (Loss) | 11,327 | | (10,410) | | (18,294) | | 21,737 | | 208.8 | | 7,884 | | 43.1 | |
| | | | | | | |
2020 Versus 2019
Consolidated operating income increased by $21,737,000 to $11,327,000 in 2020 from an operating loss of $10,410,000 in 2019 primarily due to a $23,704,000 decrease in SG&A expenses, principally attributable to lower employment costs, gain on sale of Dynamic Controls of $9,790,000 and decrease in restructuring costs of $4,471,000, which more than offset lower gross profit on lower sales, primarily impacted by the pandemic.
Europe - Operating income decreased by $13,492,000 in 2020 compared to 2019 primarily related to lower gross profit on lower sales and unfavorable manufacturing variances, partially offset by reduced SG&A expenses, primarily driven by reduced employee-related costs. This segment was significantly impacted by the pandemic.
North America - Operating income increased by $17,041,000 in 2020 compared to 2019 driven primarily by lower SG&A expenses, due to decreased employee-related costs, as well as improved gross margin driven by product mix and cost reductions.
All Other - Operating loss improved in 2020 compared to 2019 driven by decreased SG&A expense driven by reduced employee-related costs, primarily stock compensation expense, and improved profitability in the Asia Pacific business.
Charge Related to Restructuring Activities
The company's restructuring charges were originally necessitated primarily by continued declines in Medicare and Medicaid reimbursement by the U.S. government, as well as similar healthcare reimbursement pressures abroad, which negatively affect the company's customers (e.g. home health care providers) and continued pricing pressures faced by the company due to the outsourcing by competitors to lower cost locations. Restructuring decisions were also the result of reduced profitability in
each of the segments. In addition, as a result of the company's business improvement strategy, additional restructuring actions have continued into 2020. The company expects reduced salary and benefit costs principally impacting Selling, General and Administrative expenses and Costs of Products Sold.
Charges for the year ended December 31, 2020 totaled $7,358,000 which were related to Europe ($5,934,000), North America ($1,306,000) and All Other ($118,000). The European charges incurred related to severance ($5,588,000) and lease termination costs ($346,000) primarily related to the German facility consolidation. In North America, all charges incurred related to severance. All Other charges were related to severance. Payments for the year ended December 31, 2020 were $8,132,000 and the cash payments were funded with company's cash on hand. The majority of the 2020 accrued balances are expected to be paid out within twelve months.
Charges for the year ended December 31, 2019 totaled $11,829,000 which were related to Europe ($9,579,000), North America ($1,617,000) and All Other ($633,000). The European charges were incurred related to severance ($9,356,000) and lease termination costs ($223,000). In North America, costs were incurred related to severance ($1,573,000) and lease termination costs ($44,000). All Other charges were related to severance. Payments for the year ended December 31, 2019 were $6,484,000 and the cash payments were funded with company's cash on hand.
To date, the company's liquidity has been sufficient to absorb these charges; however, the company's disclosure below in Liquidity and Capital Resources and in "Item 1A. Risk Factors" highlights risks that could negatively impact the company's liquidity. Refer also to "Charges Related to Restructuring Activities" in the Notes to the Consolidated Financial Statements included in this Annual Report on Form 10-K.
| | | | | | | | |
| Part II | | |
| MD&A - Operating Income | | |
| | |
Impairment of Intangible Asset
In accordance with ASC 350, Intangibles - Goodwill and Other, the company assesses intangible assets for impairment. As a result of the company's 2020 and 2019 intangible asset impairment assessments, the company recognized no impairment on intangible assets for 2020 as compared to $587,000 ($435,000 after-tax) impairment in 2019 related to a trademark with an indefinite life in the Industrial Products Group reporting unit, which is part of the North America segment. The fair value of trademarks were calculated using a relief from royalty payment methodology which requires applying an estimated market royalty rate to forecasted net sales and discounting the resulting cash flows to determine fair value.
2019 Versus 2018
Consolidated operating loss decreased by $7,884,000 to a loss of $10,410,000 in 2019 from a loss of $18,294,000 in 2018 primarily due to a $21,845,000 decrease in SG&A expenses, principally attributable to lower employment costs, partially offset by an increase in restructuring costs of $8,348,000.
Europe - Operating income increased $3,501,000 in 2019 compared to 2018 primarily related to improved gross profit and reduced SG&A expenses, driven by lower employment costs, partially offset by unfavorable foreign exchange of $3,200,000.
North America - Operating loss decreased by $24,914,000 in 2019 compared to 2018 primarily by lower SG&A expenses, due to decreased employment and consulting costs, as well as improved gross margin driven by cost reductions.
All Other - Operating loss increased in 2019 compared to 2018 driven by increased SG&A expenses related to stock compensation and bonuses as well as a decline in operating income for the Asia Pacific business driven by lower net sales.
Charge Related to Restructuring Activities
Charges for the year ended December 31, 2019 totaled $11,829,000 which were related to Europe ($9,579,000), North America ($1,617,000) and All Other ($633,000). The European charges were incurred related to severance ($9,356,000) and lease termination costs ($223,000). In North America, costs were incurred related to severance ($1,573,000) and lease termination costs ($44,000). All Other charges were related to severance. Payments for the year ended December 31, 2019 were $6,484,000 and the cash payments were funded with company's cash on hand.
Charges for the year ended December 31, 2018 totaled $3,481,000 which were related to Europe ($1,773,000), North America ($1,359,000) and All Other ($349,000). The European and All Other charges were incurred related to severance costs. In North America, costs were incurred related to severance ($1,471,000) and lease termination reversals were recognized ($112,000). Payments for the year ended December 31, 2018 were $5,804,000 and the cash payments were funded with company's cash on hand. The 2018 charges have been paid out.
Impairment of Intangible Asset
In accordance with ASC 350, Intangibles - Goodwill and Other, the company assesses intangible assets for impairment. As a result of the company's 2019 and 2018 intangible asset assessments, the company recognized an intangible impairment charge in the Institutional Products Group reporting unit, which is part of the North America segment, of $587,000 ($435,000 after-tax) in 2019 as compared to impairment of $583,000 ($431,000 after-tax) in 2018 related to a trademark with an indefinite life. The fair value of trademarks were calculated using a relief from royalty payment methodology which requires applying an estimated market royalty rate to forecasted net sales and discounting the resulting cash flows to determine fair value.
| | | | | | | | |
| | Part II |
| | MD&A - Other Items |
| | |
OTHER ITEMS
2020 Versus 2019
Net Gain on Convertible Debt Derivatives
| | | | | | | | | |
| ($ in thousands USD) | Change in Fair Value - Gain | |
| 2020 | 2019 | |
| Convertible Note Hedge Assets | — | | 9,600 | | |
| Convertible Debt Conversion Liabilities | — | | (8,403) | | |
| Net gain on convertible debt derivatives | — | | 1,197 | | |
| | | |
The company recognized a net gain of $0 in 2020 compared to a net gain of $1,197,000 in 2019 related to the fair value of convertible debt derivatives. As a result of the company's receipt of shareholder approval authorizing the company to elect to settle future conversions of its convertible notes in common shares, the second quarter of 2019 was the last quarter for which the company could recognize gain (or loss) on the fair value of its note hedge assets and convertible debt conversion liabilities. Refer to "Long-Term Debt" in the notes to the Consolidated Financial Statements included elsewhere in this report for more detail.
Loss on debt extinguishment including debt finance changes and fees
| | | | | | | | | | |
| | | | |
| ($ in thousands USD) | 2020 | 2019 | | |
| Loss on debt extinguishment including debt finance fees | 7,360 | | 6,165 | | | |
| | | | |
During the second quarter of 2020, the company entered into separate, privately negotiated agreements with certain holders of its 2021 Notes and certain holders of its 2022 Notes to exchange $35,375,000 in aggregate principal amount of 2021 Notes and $38,500,000 in aggregate principal amount of 2022 Notes, for aggregate consideration of $73,875,000 in aggregate principal amount of new Series II 2024 Notes of the company and $5,593,000 in cash. During the third quarter of 2020, the company repurchased and retired $24,466,000 its 2021 Notes. These transactions resulted in a combined loss on debt extinguishment including debt and finance fees of $7,360,000.
In the third quarter of 2019, the company repurchased $16,000,000 in principal amount of the 2021 Notes and in the fourth quarter of 2019, the company entered into separate privately negotiated agreements with certain holders of its 2021 Notes to exchange $72,909,000 in aggregate principal amount of 2021 Notes, for aggregate consideration of $72,909,000 in aggregate principal
amount of new Series I 2024 Notes of the company and approximately $6,928,000 in cash. These transactions resulted in a combined loss on debt extinguishment including debt and finance fees of $6,165,000.
Interest
| | | | | | | | | | | | | | |
| ($ in thousands USD) | 2020 | 2019 | $ Change | % Change |
| Interest Expense | 28,499 | | 29,076 | | (577) | | (2.0) | |
| Interest Income | (93) | | (429) | | 336 | | 78.3 | |
Interest expense declined as a result of lower debt levels and interest income declined as a result of lower interest-bearing cash balances.
Income Taxes
The company had an effective tax rate charge of 15.7% and 21.1% on losses before taxes in 2020 and 2019, respectively, compared to an expected benefit at the U.S. statutory rate of 21.0% on the pre-tax losses for each period, respectively. The company's effective tax rate in 2020 and 2019 was unfavorable compared to the U.S. federal statutory rate expected benefit, principally due to the negative impact of the company's inability to record tax benefits related to the significant losses in countries which had tax valuation allowances. The gain on the divestiture of Dynamic Controls was not taxable locally. In addition, the company had accrued withholding taxes on earnings of its Chinese subsidiary based on the expectation of not permanently reinvesting those earnings. The sale of this entity, without such distribution resulted in the reversal of this accrual in an amount of $988,000 in 2020. The 2020 and 2019 effective tax rate was increased by certain taxes outside the United States, excluding countries with tax valuation allowances, that were at an effective rate higher than the U.S. statutory rate.
| | | | | | | | |
| Part II | | Table of Contents |
| MD&A - Other Items | | |
| | |
2019 Versus 2018
Net Gain on Convertible Debt Derivatives
| | | | | | | | | |
| ($ in thousands USD) | Change in Fair Value - Gain (Loss) | |
| 2019 | 2018 | |
| Convertible Note Hedge Assets | 9,600 | | (90,505) | | |
| Convertible Debt Conversion Liabilities | (8,403) | | 102,499 | | |
| Net gain on convertible debt derivatives | 1,197 | | 11,994 | | |
| | | |
The company recognized a net gain of 1,997,000 in 2019 compared to a net gain of $11,994,000 in 2018 related to the fair value of convertible debt derivatives. As a result of the company's receipt of shareholder approval authorizing the company to elect to settle future conversions of its convertible notes in common shares, the second quarter of 2019 was the last quarter for which the company could recognize gain (or loss) on the fair value of its note hedge assets and convertible debt conversion liabilities. Refer to "Long-Term Debt" in the notes to the Consolidated Financial Statements included elsewhere in this report for more detail.
Loss on debt extinguishment including debt finance changes and fees
| | | | | | | | | | |
| | | | |
| ($ in thousands USD) | 2019 | 2018 | | |
| Loss on debt extinguishment including debt finance fees | 6,165 | | — | | | |
| | | | |
In the third quarter of 2019, the company repurchased $16,000,000 in principal amount of the 2021 Notes and in the fourth quarter of 2019, the company entered into separate privately negotiated agreements with certain holders of its 2021 Notes to exchange $72,909,000 in aggregate principal amount of 2021 Notes, for aggregate consideration of $72,909,000 in aggregate principal amount of new Series I 2024 Notes of the company and approximately $6,928,000 in cash. These transactions resulted in a combined loss on debt extinguishment including debt and finance fees of $6,165,000.
Interest
| | | | | | | | | | | | | | |
| ($ in thousands USD) | 2019 | 2018 | $ Change | % Change |
| Interest Expense | 29,076 | | 28,336 | | 740 | | 2.6 | |
| Interest Income | (429) | | (534) | | 105 | | 19.7 | |
Interest expense did not materially change in 2019 compared to 2018.
Income Taxes
The company had an effective tax rate charge of 21.1% and 28.8% on losses before taxes in 2019 and 2018, respectively, compared to an expected benefit at the U.S. statutory rate of 21.0% on the pre-tax losses for each period, respectively. The company's effective tax rate in 2019 and 2018 was unfavorable compared to the U.S. federal statutory rate expected benefit, principally due to the negative impact of the company's inability to record tax benefits related to the significant losses in countries which had tax valuation allowances. The 2019 and 2018 effective tax rates were increased by certain taxes outside the United States, excluding countries with tax valuation allowances, that were at an effective rate higher than the U.S. statutory rate. The 2018 effective rate was also benefited by 5.9% as a result of the effect of indefinite intangibles and a related 2018 indefinite loss carryforward created in 2018 due to U.S. tax reform.
| | | | | | | | |
| | Part II |
| | MD&A - Liquidity and Capital Resources |
| | |
LIQUIDITY AND CAPITAL RESOURCES
The company continues to maintain an adequate liquidity position through its cash balances and unused bank lines of credit (refer to Long-Term Debt in the Notes to the Consolidated Financial Statements included in this report) as described below.
Key balances on the company's balance sheet and related metrics:
| | | | | | | | | | | | | | |
| ($ in thousands USD) | December 31, 2020 | December 31, 2019 | $ Change | % Change |
| Cash and cash equivalents | 105,298 | | 80,063 | | 25,235 | | 31.5 | |
Working capital (1) | 144,080 | | 137,220 | | 6,860 | | 5.0 | |
Total debt (2) | 339,928 | | 283,256 | | 56,672 | | 20.0 | |
Long-term debt (2) | 330,903 | | 280,684 | | 50,219 | | 17.9 | |
| Total shareholders' equity | 333,846 | | 308,516 | | 25,330 | | 8.2 | |
Credit agreement borrowing availability (3) | 36,509 | | 34,516 | | 1,993 | | 5.8 | |
(1) Current assets less current liabilities.
(2) Long-term debt and Total debt include finance leases but exclude debt issuance costs recognized as a deduction from the carrying amount of debt liability and debt discounts classified as debt or equity and operating leases.
(3) Reflects the combined availability of the company's North American and European asset-based revolving credit facilities before borrowings. The change in borrowing availability is due to changes in the calculated borrowing base. At December 31, 2020 the company had drawn $20,000,000 on the North American credit facility and $11,502,000 on the European credit facility. Outstanding borrowings and availability are calculated on a month lag related to the European credit facility. The company had no borrowings on its credit facilities as of December 31, 2019.
The company's cash and cash equivalents were $105,298,000 and $80,063,000 at December 31, 2020 and December 31, 2019, respectively. The increase in cash balances at December 31, 2020 compared to December 31, 2019 is attributable to borrowing $31,502,000 on the North America and Europe credit facilities under the company's credit agreement which is an asset-based-lending senior secured revolving credit facility; the proceeds of $14,563,000 from the sale of Dynamic Controls in March 2020; and an unsecured loan of $10,000,000 pursuant to sections 1102 and 1106 of the Coronavirus Aid, Relief, and Economic Security ("CARES") Act in May 2020. This increase in cash was primarily offset by cash used for continued investment in the company's business improvement strategy and debt related payments.
Refer to "Long-Term Debt" in the Notes to the Consolidated Financial statements included in this report for a summary of the material terms of the Company's long-term indebtedness.
Debt repayments, acquisitions, divestitures, the timing of vendor payments, the timing of customer rebate payments, the granting of extended payment terms to significant national accounts and other activity can have a significant impact on the company's cash flow and borrowings outstanding such that the cash reported at the end of a given period may be materially different than cash levels during a given period. While the company has cash balances in various jurisdictions around the world, there
are no material restrictions regarding the use of such cash for dividends within the company, loans or other purposes.
The company's total debt outstanding, inclusive of the debt discount related to the debentures included in equity as well as the debt discount and fees associated with the company's convertible senior notes due 2021 ("2021 Notes"), 2022 ("2022 Notes") and 2024 ("Series I 2024 Notes" and "Series II 2024 Notes") and finance leases, increased by $56,672,000 to $339,928,000 at December 31, 2020 from $283,256,000 as of December 31, 2019. The increase is primarily driven by borrowing on the company's credit agreement of $31,502,000, new finance leases primarily in the third quarter of 2020, and $10,000,000 of loan proceeds obtained pursuant to the CARES Act. The CARES Act funds may be forgiven partially or in full, if certain conditions are met. While the company believes it has utilized the funds for forgivable eligible expenditures under the program, there can be no assurance that all, or any portion, of the loan will be forgiven.
The debt discount and fees associated with the 2021, 2022, Series I 2024 Notes and Series II 2024 Notes reduced the company's reported debt balance by $28,333,000 and $34,740,000 as of December 31, 2020 and December 31, 2019, respectively. At December 31, 2020 the company had drawn $20,000,000 on the North American credit facility and $11,502,000 on the European credit facility. At December 31, 2019, the company had
| | | | | | | | |
| Part II | | |
| MD&A - Liquidity and Capital Resources | | |
| | |
zero borrowings outstanding under its revolving credit facility.
The company may from time to time seek to retire or purchase its convertible notes, in open market purchases, privately negotiated transactions or otherwise. Such purchases, if any, will depend on prevailing market conditions, the company's liquidity requirements, contractual restrictions and other factors. The amount involved in any such transactions, individually or in the aggregate, may be material.
The company has an asset-based lending Amended and Restated Revolving Credit and Security Agreement (the “Credit Agreement”), which provides for a revolving line of credit, letter of credit and swing line facility for the company's U.S. and Canadian borrowers in an aggregate principal amount of up to $60,000,000 (the "U.S. and Canadian Credit Facility") and a similar facility for European borrowers in an aggregate principal amount of up to $30,000,000 (the "European Credit Facility") each of which is subject to variable rates and availability based on a borrowing base formula.
As determined pursuant to the borrowing base formula for the U.S. and Canadian borrowers, the company's borrowing base including the period ending December 31, 2020 under the U.S. and Canadian Credit Facility of the Credit Agreement was approximately $43,874,000, with aggregate borrowing availability of approximately $24,508,000, taking into account the $4,250,000 minimum availability reserve, then-outstanding applicable letters of credit and other reserves of $7,616,000, and the $7,500,000 dominion trigger amount noted below. As determined pursuant to the borrowing base formula for the European borrowers, the company's borrowing base including the period ending December 31, 2020 under the European Credit Facility of the Credit Agreement was approximately $18,376,000, with aggregate borrowing availability of approximately $12,001,000, considering the $3,000,000 minimum availability reserve and the $3,375,000 dominion trigger amount noted below. As of December 31, 2020, the combined aggregate borrowing availability under the U.S. and Canadian Credit Facility and the European Credit Facility of the Credit Agreement was $36,509,000.
As a result of entering into the Credit Agreement, the company incurred fees which were capitalized and are being amortized as interest expense through January 16, 2024 of which $619,000 are yet to be amortized as of December 31, 2020.
As of December 31, 2020, the company was in compliance with all covenant requirements under the Credit Agreement. The Credit Agreement contains customary representations, warranties and covenants
including dominion triggers requiring the company to maintain borrowing capacity of not less than $7,500,000 on any given business day or for five consecutive days related to the U.S. and Canadian borrowers, and $3,750,000 on any given business day or $3,375,000 for five consecutive days related to European borrowers, in order to avoid triggering full control by an agent for the lenders of the company's cash receipts for application to the company's obligations under the agreement.
If the company is unable to comply with the provisions in the Credit Agreement, it could result in a default, which could trigger acceleration of, or the right to accelerate, the related debt. Because of cross-default provisions in its agreements and instruments governing certain of the company's indebtedness, a default under the Credit Agreement could result in a default under, and the acceleration of, certain other company indebtedness. In addition, the company's lenders would be entitled to proceed against the collateral securing the indebtedness.
Based on the company's current expectations, the company believes that its cash balances and available borrowing capacity under its Credit Agreement should be sufficient to meet working capital needs, capital requirements, and commitments for at least the next twelve months. Notwithstanding the company's expectations, if the company's operating results decrease as the result of pressures on the business due to, for example, prolonged, or worsening of, negative impacts of the COVID-19 pandemic, currency fluctuations or regulatory issues or the company's failure to execute its business plans or if the company's business improvement actions take longer than expected to materialize, the company may require additional financing, or may be unable to comply with its obligations under the credit facilities, and its lenders could demand repayment of any amounts outstanding under the company's credit facilities. If additional financing is required, there can be no assurance that it will be available on terms satisfactory to the company, if at all. Refer to "Item 1A. Risk Factors" for a further discussion of risks applicable to the company's liquidity, capital resources and financial condition.
In the first quarter of 2016, the company issued $150,000,000 aggregate principal amount of 2021 Notes in a private offering which bear interest at a rate of 5.00% per year payable semi-annually and will mature in February 2021, unless repurchased or converted in accordance with their terms prior to such date. Prior to August 15, 2020, the notes were be convertible only upon satisfaction of certain conditions and during certain periods, and thereafter, at any time until the close of business on the second scheduled trading day immediately preceding the maturity date. The net proceeds from the offering of the 2021 Notes were $144,034,000, after deducting fees and offering expenses payable by the company. Approximately $5,000,000 of the
| | | | | | | | |
| | Part II |
| | MD&A - Liquidity and Capital Resources |
| | |
net proceeds from the offering was used to repurchase the company's common shares, and $15,600,000 of the net proceeds was used to pay the net cost of the convertible note hedge and warrant transactions. The company incurred fees which were capitalized and are being amortized as interest expense through February 2021 (net of loss on extinguishment of debt from 2020 transactions as discussed herein) of which $1,000 have yet to be amortized as of December 31, 2020. At December 31, 2020, $1,250,000 in principal amount of 2021 Notes remained outstanding, all of which was subsequently repaid and retired upon its maturity on February 16, 2021. .
In the second quarter of 2017, the company issued $120,000,000 aggregate principal amount of the 2022 Notes in a private offering which bear interest at a rate of 4.50% per year payable semi-annually and will mature in June 2022, unless repurchased or converted in accordance with their terms prior to such date. Prior to December 1, 2021, the 2022 Notes will be convertible only upon satisfaction of certain conditions and during certain periods, and thereafter, at any time until the close of business on the second scheduled trading day immediately preceding the maturity date. The net proceeds from the offering of the 2022 Notes were approximately $115,289,000, after deducting fees and offering expenses of $4,711,000. These debt issuance costs were capitalized and are being amortized as interest expense through June 2022 (net of loss on extinguishment of debt from 2020 transactions as discussed herein) of which $859,000 have yet to be amortized as of December 31, 2020. A portion of the net proceeds from the offering were used to pay the cost of the convertible note hedge transactions (after such cost is partially offset by the proceeds to the company from the warrant transactions), which was a net cost of $10,680,000. At December 31, 2020, $81,500,000 in principal amount of 2022 Notes remained outstanding.
In the fourth quarter of 2019, the company entered into separate privately negotiated agreements with certain holders of its 2021 Notes to exchange $72,909,000 in aggregate principal amount of 2021 Notes, for aggregate consideration of $72,909,000 in aggregate principal amount of new Series I 2024 Notes of the company and approximately $6,928,000 in cash. The Series I 2024 Notes bear interest at a fixed rate of 5.00% per year payable semi-annually and will mature in November 2024, unless earlier repurchased, redeemed or converted. Prior to May 2024, the Series I 2024 Notes will be convertible only upon satisfaction of certain conditions and during certain periods, and thereafter, at any time until the close of business on the second scheduled trading day immediately preceding the maturity date. Prior to maturity, the Series I 2024 Notes will be redeemable by the company upon satisfaction of certain conditions and during certain periods. The fees paid totaled $1,394,000. These debt issuance costs were capitalized and are being amortized as
interest expense through November 2024 of which $1,037,000 have yet to be amortized as of December 31, 2020.
In the second quarter of 2020, the company entered into separate privately negotiated agreements with certain holders of its 2021 Notes and 2022 Notes to exchange $35,375,000 in aggregate principal amount of the company's 2021 Notes and $38,500,000 in aggregate principal amount of the company's 2022 Notes, for $73,875,000 in aggregate principal amount of new Series II 2024 Notes and approximately $5,593,000 in cash. The new Series II 2024 Notes bear interest at a fixed rate of 5% per year payable semi-annually and will mature in November 2024, unless earlier repurchased, redeemed or converted. Prior to May 2024, the Series II 2024 Notes will be convertible only upon satisfaction of certain conditions and during certain periods, and thereafter, at any time until the close of business on the second scheduled trading day immediately preceding the maturity date. Prior to maturity, the Series II 2024 Notes will be redeemable by the company upon satisfaction of certain conditions and during certain periods. The principal amount of the Series II 2024 Notes also will accrete at a rate of approximately 4.7% compounding on a semi-annual basis. The accreted portion of the principal is payable in cash upon maturity but does not bear interest and is not convertible into the company's common shares. The amount accreted as of December 31, 2020 was $1,813,000. The issuance costs were capitalized and are being amortized as interest expense through November 2024 of which $1,308,000 have yet to be amortized as of December 31, 2020. The fees paid in 2020 totaled $1,505,000. A loss on debt extinguishment of $6,599,000 was recorded as part of the exchange transaction, which included the write-off of fees related to portions of the 2021 Notes and 2022 Notes exchanged.
In the third quarter of 2020, the company repurchased $24,466,000 aggregate principal amount of its 2021 Notes, resulting in a $761,000 loss on debt extinguishment.
The company has used, and intends to continue to use, the remaining net proceeds from the 2021 and 2022 Notes offerings for working capital and general corporate purposes, which may include funding portions of the company's ongoing turnaround and addressing potential risks and contingencies. The net proceeds have allowed the company to invest in new products, people, marketing initiatives and working capital to transform the business and pursue growth.
The company also has an agreement with De Lage Landen, Inc. (“DLL”), a third-party financing company, to provide lease financing to the company's U.S. customers. Either party could terminate this agreement with 180 days' notice or 90 days' notice by DLL upon the occurrence of
| | | | | | | | |
| Part II | | |
| MD&A - Liquidity and Capital Resources | | |
| | |
certain events. Should this agreement be terminated, the company's borrowing needs under its credit facilities could increase.
Should interest rates increase, the company expects that it would be able to absorb modest rate increases without any material impact on its liquidity or capital resources. For 2020 and 2019, the weighted average interest rate on all borrowings, excluding finance leases, was 4.6% and 4.78%, respectively.
Refer to "Long-Term Debt" and "Leases and Commitments" in the Notes to the Consolidated Financial Statements for more details regarding the company's convertible notes and credit facilities and lease liabilities, respectively.
CAPITAL EXPENDITURES
There were no individually material capital expenditure commitments outstanding as of December 31, 2020. The company estimates that capital investments for 2021 will be approximately $20,000,000 compared to actual capital expenditures of $22,304,000 in 2020. The continued investment at this level relates primarily to the new ERP system. The company believes that its balances of cash and cash equivalents and existing borrowing facilities will be sufficient to meet its operating cash requirements and fund required capital expenditures (refer to "Liquidity and Capital Resources"). The Credit Agreement limits the company's annual capital expenditures to $35,000,000.
DIVIDEND POLICY
On May 21, 2020, the Board of Directors decided to suspend the quarterly dividend on the company's common shares in light of the impacts of COVID-19 on the business. The Board of Directors suspended the company's regular quarterly dividend on the Class B Common Shares starting in Q3 2018. Less than 4,000 Class B Common Shares remain outstanding and suspending the regular Class B dividend allows the company to save on the administrative costs and compliance expenses associated with that dividend. Holders of Class B Common Shares are entitled to convert their shares into Common Shares at any time on a share-for-share basis and would be eligible for any Common Share dividends declared following any such conversion.
CASH FLOWS
Cash flows provided by operating activities were $21,917,000 in 2020, compared to $2,743,000 in the previous year. The 2020 operating cash flows benefited from improved operating income and reduced inventory and accounts receivable. In 2019, operating cash flows benefited from reduced operating loss and reduced inventory.
Cash flows used by investing activities were $9,547,000 in 2020, compared to $11,614,000 in 2019. The decrease in cash flows used for investing was primarily driven by gross proceeds of $14,563,000 from the sale of Dynamic Controls in the first quarter of 2020. This was partially offset by higher purchases of property, plant and equipment compared to 2019 driven in part by an ERP implementation.
Cash flows provided by financing activities in 2020 were $6,259,000 compared to cash flow used of $27,941,000 in 2019.
Cash flows provided in 2020 was driven primarily by borrowing of $31,502,000 on the North America and Europe credit facility under the company's credit agreement which provides for an asset-based-lending senior secured revolving credit facility as well as an unsecured CARES Act loan of $10,000,000. This was offset by cash paid of $24,466,000 to retire the majority of the company's outstanding 2021 Notes in the third quarter of 2020; and $7,098,000 of cash paid in connection with the exchange 2021 Notes and 2022 Notes for Series II 2024 Notes executed during the second quarter of 2020 for debt fees and payments to note holders.
Cash flows used in 2019 reflects $14,708,000 in cash to repurchase $16,000,000 in principal amount of the 2021 Notes, cash payments of $6,928,000 to debt holders that exchanged 2021 Notes for Series I 2024 Notes and debt fee payments of $1,278,000.
Free cash flow is a non-GAAP financial measure and is reconciled to the corresponding GAAP measure as follows:
| | | | | | | | | | | |
| ($ in thousands USD) | Twelve Months Ended
December 31, |
| | 2020 | | 2019 |
| Net cash provided by operating activities | $ | 21,917 | | | $ | 2,743 | |
| | | |
| Plus: Sales of property and equipment | 396 | | | 73 | |
| | | |
| Less: Purchases of property and equipment | (22,304) | | | (10,874) | |
| Free Cash Flow | $ | 9 | | | $ | (8,058) | |
| | | |
Free cash flow was positive $9,000 in 2020 compared to use of $8,058,000 in 2019. The change in free cash flow was driven by profit improvement and reduced inventory. Free cash flow is a non-GAAP financial measure composed of net cash used by operating activities less purchases of property and equipment plus proceeds from sales of property and equipment. Management believes that this financial measure provides meaningful information for evaluating the overall financial
performance of the company and its ability to repay debt or make future investments (including acquisitions, etc.).
The company historically generates negative free cash flow during the first half of the year. This pattern is expected to continue due to the timing of annual one-time payments such as customer rebates and employee bonuses earned during the prior year, and higher working capital usage from seasonal inventory increases. In addition, for 2021, the company will fund severance payments related to the German facility closure in the first quarter of 2021 and repayment of deferred VAT and other taxes from 2020, as a result of government programs introduced as a result of the pandemic. The absence of these payments and seasonally stronger sales in the second half of the year typically result in more favorable free cash flow in the second half of the year.
The company's approximate cash conversion days at December 31, 2020 and December 31, 2019 are as follows:
Days in receivables are equal to current quarter net current receivables divided by trailing four quarters of net sales multiplied by 365 days. Days in inventory and accounts payable are equal to current quarter net inventory and accounts payable, respectively, divided by trailing four quarters of cost of sales multiplied by 365 days. Total cash conversion days are equal to days in receivables plus days in inventory less days in accounts payable.
The company provides a summary of days of cash conversion for the components of working capital, so investors may see the rate at which cash is disbursed, collected and how quickly inventory is converted and sold.
| | | | | | | | |
| | Part II |
| | MD&A - Accounting Estimates and Pronouncements |
| | |
ACCOUNTING ESTIMATES AND PRONOUNCEMENTS
CRITICAL ACCOUNTING POLICIES
The Consolidated Financial Statements included in the report include accounts of the company and all majority-owned subsidiaries. The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions in certain circumstances that affect amounts reported in the accompanying Consolidated Financial Statements and related footnotes. In preparing the financial statements, management has made its best estimates and judgments of certain amounts included in the financial statements, giving due consideration to materiality. However, application of these accounting policies involves the exercise of judgment and use of assumptions as to future uncertainties and, as a result, actual results could differ from these estimates. The following critical accounting policies, among others, affect the more significant judgments and estimates used in preparation of the company's consolidated financial statements.
Revenue Recognition
The company recognizes revenues when control of the product or service is transferred to unaffiliated customers. Revenues from Contracts with Customers, ASC 606, provides guidance on the application of generally accepted accounting principles to revenue recognition issues. The company has concluded that its revenue recognition policy is appropriate and in accordance with GAAP under ASC 606.
All of the company's product-related contracts, and a portion related to services, have a single performance obligation, which is the promise to transfer an individual good or service, with revenue recognized at a point in time. Certain service-related contracts contain multiple performance obligations that require the company to allocate the transaction price to each performance obligation. For such contracts, the company allocates revenue to each performance obligation based on its relative standalone selling price at inception of the contract. The company determined the standalone selling price based on the expected cost-plus margin methodology. Revenue related to the service contracts with multiple performance obligations is recognized over time. To the extent performance obligations are satisfied over time, the company defers revenue recognition until the performance obligations are satisfied.
The determination of when and how much revenue to recognize can require the use of significant judgment. Revenue is recognized when obligations under the terms of
a contract with the customer are satisfied; generally, this occurs with the transfer of control of the company's products and services to the customer.
Revenue is measured as the amount of consideration expected to be received in exchange for transferring the product or providing services. The amount of consideration received and recognized as revenue by the company can vary as a result of variable consideration terms included in the contracts such as customer rebates, cash discounts and return policies. Customer rebates and cash discounts are estimated based on the most likely amount principle and these estimates are based on historical experience and anticipated performance. Customers have the right to return product within the company's normal terms policy, and as such, the company estimates the expected returns based on an analysis of historical experience. The company adjusts its estimate of revenue at the earlier of when the most likely amount of consideration the company expects to receive changes or when the consideration becomes fixed. The company generally does not expect that there will be significant changes to its estimates of variable consideration (refer to Receivables in the Notes to the Consolidated Financial Statements include elsewhere in this report).
Depending on the terms of the contract, the company may defer recognizing a portion of the revenue at the end of a given period as the result of title transfer terms that are based upon delivery and or acceptance which align with transfer of control of the company's products to its customers.
Sales are made only to customers with whom the company believes collection is reasonably assured based upon a credit analysis, which may include obtaining a credit application, a signed security agreement, personal guarantee and/or a cross corporate guarantee depending on the credit history of the customer. Credit lines are established for new customers after an evaluation of their credit report and/or other relevant financial information. Existing credit lines are regularly reviewed and adjusted with consideration given to any outstanding past due amounts.
The company records distributed product sales gross as a principal since the company takes title to the products and has the risks of loss for collections, delivery and returns. The company's payment terms are for relatively short periods and thus do not contain any element of financing. Additionally, no contract costs are incurred that would require capitalization and amortization.
| | | | | | | | |
| Part II | | |
| MD&A - Accounting Estimates and Pronouncements | | |
| | |
Sales, value added, and other taxes the company collects concurrent with revenue producing activities are excluded from revenue. Incidental items that are immaterial in the context of the contract are recognized as expense. Shipping and handling costs are included in cost of products sold.
The majority of the company's warranties are considered assurance-type warranties and continue to be recognized as expense when the products are sold (refer to Current Liabilities in the Notes to the Consolidated Financial Statements include elsewhere in this report). These warranties cover against defects in material and workmanship for various periods depending on the product from the date of sale to the customer. Certain components carry a lifetime warranty. In addition, the company has sold extended warranties that, while immaterial, require the company to defer the revenue associated with those warranties until earned. The company has established procedures to appropriately defer such revenue. A provision for estimated warranty cost is recorded at the time of sale based upon actual experience. The company continuously assesses the adequacy of its product warranty accruals and makes adjustments as needed. Historical analysis is primarily used to determine the company's warranty reserves. Claims history is reviewed and provisions are adjusted as needed. However, the company does consider other events, such as a product recall, which could require additional warranty reserve provisions. Refer to Accrued Expenses in the Notes to the Consolidated Financial Statements for a reconciliation of the changes in the warranty accrual.
Allowance for Uncollectible Accounts Receivable
The estimated allowance for uncollectible amounts is based primarily on management's evaluation of the financial condition of the customer. In addition, as a result of the third-party financing arrangement, management monitors the collection status of these contracts in accordance with the company's limited recourse obligations and provides amounts necessary for estimated losses in the allowance for doubtful accounts and establishing reserves for specific customers as needed and general reserve for macroeconomic considerations.
The company continues to closely monitor the credit-worthiness of its customers and adhere to tight credit policies. The Centers for Medicare and Medicaid Services publishes Medicare contract prices under its NCB program which includes 100% of the Medicare population. The company believes that the NCB program contract pricing could have a significant impact on the collectability of accounts receivable for those customers which have a portion of their revenues tied to Medicare reimbursement. In addition, there is a risk that these precedent-setting price reductions could influence other
non-CMS payors' reimbursement rates for the same product categories. As a result, this is an additional risk factor which the company considers when assessing the collectability of accounts receivable.
The company has an agreement with DLL, a third-party financing company, to provide lease financing to Invacare's U.S. customers. The DLL agreement provides for direct leasing between DLL and the Invacare customer. The company retains a recourse obligation for events of default under the contracts. The company monitors the collections status of these contracts and has provided amounts for estimated losses in its allowances for doubtful accounts.
Goodwill, Intangible and Other Long-Lived Assets
Property, equipment, intangibles and certain other long-lived assets are amortized over their useful lives. Useful lives are based on management's estimates of the period that the assets will generate revenue. Under Intangibles-Goodwill and Other, ASC 350, goodwill and intangible assets deemed to have indefinite lives are subject to annual impairment tests. The company's measurement date for its annual goodwill impairment test is October 1 and the analysis is completed in the fourth quarter. Furthermore, goodwill and other long-lived assets are assessed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Most of the company's goodwill and intangible assets relate to the company's Europe and Institutional Products Group reporting units which were profitable in 2020.
To assess goodwill for impairment in accordance with ASC 350, the company first estimates the fair value of each reporting unit and compares the calculated fair value to the carrying value of each reporting unit. A reporting unit is defined as an operating segment or one level below. The company has determined that its reporting units are the same as its operating segments with the exception of North America which has two reporting units. The company completes its annual impairment tests in the fourth quarter of each year. To estimate the fair values of the reporting units, the company utilizes a discounted cash flow (DCF) method in which the company forecasts income statement and balance sheet amounts based on assumptions regarding future sales growth, operating income, inventory turns, days' sales outstanding, etc. to forecast future cash flows. The cash flows are discounted using a weighted average cost of capital (WACC) where the cost of debt is based on quoted rates for 20-year debt of companies of similar credit risk and the cost of equity is based upon the 20-year treasury rate for the risk-free rate, a market risk premium, the industry average beta and a small cap stock adjustment. The assumptions used are based on a market participant's point of view and yielded a WACC of 11.27%
| | | | | | | | |
| | Part II |
| | MD&A - Accounting Estimates and Pronouncements |
| | |
in 2020 for the company's annual impairment analysis for the reporting units with goodwill compared to 11.88% in 2019 and 12.41% in 2018. The financial forecast assumptions and WACC used have a significant impact upon the discounted cash flow methodology utilized in the company's annual impairment testing as lower projections of operating income or a higher WACC decrease the fair value estimates.
The company also utilizes an Enterprise Value (EV) to Earnings Before Interest, Taxes, Depreciation and Amortization (EBITDA) method to compute the fair value of its reporting units which considers potential acquirers and their EV to EBITDA multiples adjusted by an estimated premium. While more weight is given to the discounted cash flow method, the EV to EBITDA method does provide corroborative evidence of the reasonableness of the discounted cash flow method results.
While there was no impairment in 2020 related to goodwill for the Europe or Institutional Products Group reporting units, a future potential impairment is possible for any of the company's reporting units should actual results differ materially from forecasted results used in the valuation analysis or if business changes impact the company's assessment of reporting units. Furthermore, the company's annual valuation of goodwill can differ materially if the financial projections or market inputs used to determine the WACC change significantly. For instance, higher interest rates or greater stock price volatility would increase the WACC and thus increase the chance of impairment. In consideration of this potential, the company assessed the results if the WACC used were 100 basis points higher for the 2020 impairment analysis and determined that there still would not be any indicator of potential impairment for the Europe or Institutional Products Group reporting units.
The company also considers the potential for impairment of other intangible assets and other long-lived assets annually or whenever events or circumstances indicate impairment. In 2020, the company performed an assessment for potential impairments and recognized no intangible asset impairment charge or other long-lived asset impairment charge. In 2019, the company performed an assessment for potential impairments and recognized an intangible asset impairment charge for the Institutional Products Group reporting unit, which is part of the North America operating segment, of $587,000 ($435,000 after-tax) compared to $583,000 ($431,000 after-tax) in 2018 related to a trademark with an indefinite life. The fair value of the trademark was calculated using a relief from royalty payment methodology which requires applying an estimated market royalty rate to forecasted net sales and discounting the resulting cash flows to determine fair value.
The company's intangible assets consist of intangible assets with defined lives as well as intangible assets with indefinite lives. Defined-lived intangible assets consist principally of customer lists and developed technology. The company's indefinite lived intangible assets consist entirely of trademarks.
The company evaluates the carrying value of definite-lived assets whenever events or circumstances indicate possible impairment. Definite-lived assets are determined to be impaired if the future undiscounted cash flows expected to be generated by the asset or asset group are less than the carrying value of the asset or asset group. Actual impairment amounts for definite-lived assets are then calculated using a discounted cash flow calculation. The company assesses indefinite-lived assets for impairment annually in the fourth quarter of each year and whenever events or circumstances indicate possible impairment. Any impairment amounts for indefinite-lived assets are calculated as the difference between the future discounted cash flows expected to be generated by the asset less than the carrying value for the asset.
Product Liability
The company is essentially self-insured in North America for product liability exposures through its captive insurance company, Invatection Insurance Company, which currently has a policy year that runs from September 1 to August 31 and insures annual policy losses up to $10,000,000 per occurrence and $13,000,000 in the aggregate. The company also has additional layers of external insurance coverage, related to all lines of insurance coverage, insuring up to $75,000,000 in aggregate losses per policy year arising from individual claims anywhere in the world that exceed the captive insurance company policy limits or the limits of the company's per country foreign liability limits, as applicable. There can be no assurance that Invacare's current insurance levels will continue to be adequate or available at affordable rates.
Product liability reserves are recorded for individual claims based upon historical experience, industry expertise and other indicators. Additional reserves, in excess of the specific individual case reserves, are provided for incurred but not reported claims based upon actuarial valuations at the time such valuations are conducted. Historical claims experience and other assumptions are taken into consideration by the company in estimating the ultimate reserves. For example, the actuarial analysis assumes that historical loss experience is an indicator of future experience, that the distribution of exposures by geographic area and nature of operations for ongoing operations is expected to be very similar to historical operations with no dramatic changes and that the government indices used to trend losses and exposures are
| | | | | | | | |
| Part II | | |
| MD&A - Accounting Estimates and Pronouncements | | |
| | |
appropriate. Estimates made are adjusted on a regular basis and can be impacted by actual loss awards and settlements on claims. While actuarial analysis is used to help determine adequate reserves, the company is responsible for the determination and recording of adequate reserves in accordance with accepted loss reserving standards and practices.
Warranty
Generally, the company's products are covered by assurance-type warranties against defects in material and workmanship for various periods depending on the product from the date of sale to the customer. Certain components carry a lifetime warranty. In addition, the company has sold extended warranties that, while immaterial, require the company to defer the revenue associated with those warranties until earned. A provision for estimated warranty cost is recorded at the time of sale based upon actual experience. The company continuously assesses the adequacy of its product warranty accrual and makes adjustments as needed. Historical analysis is primarily used to determine the company's warranty reserves. Claims history is reviewed and provisions are adjusted as needed. However, the company does consider other events, such as a product recall, which could warrant additional warranty reserve provision. Refer to Accrued Expenses in the Notes to the Consolidated Financial Statements for a reconciliation of the changes in the warranty accrual.
Accounting for Stock Compensation
The company accounts for stock compensation under the provisions of Compensation—Stock Compensation, ASC 718. The company has not made any modifications to the terms of any previously granted awards (other than the modification in the fourth quarter of 2020 (refer to Equity Compensation in the Notes to the Consolidated Financial Statements) and no changes have been made regarding the valuation methodologies or assumptions used to determine the fair value of awards granted and the company continues to use a Black-Scholes valuation model to value options granted. As of December 31, 2020, there was $14,749,000 of total unrecognized compensation cost from stock compensation arrangements, which is related to non-vested options and shares, and includes $7,489,000 related to restricted stock awards and $7,260,000 related to performance awards.
Most of the options awarded have been granted at exercise prices equal to the market value of the underlying stock on the date of grant. Restricted stock awards granted without cost to the recipients are expensed on a straight-line basis over the vesting periods. Performance awards granted are expensed based on estimated achievement of the performance objectives over the relevant performance award periods.
Income Taxes
As part of the process of preparing its financial statements, the company is required to estimate income taxes in various jurisdictions. The process requires estimating the company's current tax liability, including assessing uncertainties related to tax return filing positions, as well as estimating temporary differences due to the different treatment of items for tax and accounting policies. The temporary differences are reported as deferred tax assets and or liabilities. The company also must estimate whether it will more likely than not realize its deferred tax assets and whether a valuation allowance should be established. The company's deferred tax assets are offset by a valuation allowance in the U.S., Australia, Switzerland and New Zealand. In the event that actual results differ from its estimates, the company's provision for income taxes could be materially impacted. The company does not believe that there is a substantial likelihood that materially different amounts would be reported related to its critical accounting policies.
On December 22, 2017 the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the “Tax Act”). The Tax Act makes broad and complex changes the U.S. tax code, including, but not limited to, (1) reducing the U.S. federal corporate tax rate from 35 percent to 21 percent; (2) requiring companies to pay a one-time transition tax on certain unrepatriated earnings, if any, of foreign subsidiaries; (3) generally eliminating U.S. federal income taxes on dividends from foreign subsidiaries; (4) requiring a current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations; (5) eliminating the corporate alternative minimum tax (AMT) and changing how existing AMT credits can be realized; (6) creating a base erosion anti-abuse tax (BEAT), a new minimum tax, (7) creating a new limitation on deductible interest expense; and (8) changing rules related to uses and limitations of net operating loss carryforwards created in tax years beginning after December 31, 2017.
The SEC staff issued SAB 118, which provides guidance on accounting for the tax effects of the Tax Act. SAB 118 provides a measurement period that should not extend beyond one year form the Tax Act enactment date for companies to complete the accounting under ASC 740. In accordance with SAB 118, a company must reflect the income tax effects of those aspects of the Tax Act for which the accounting under ASC 740 is complete. To the extent that a company's accounting for certain income tax effects of the Tax Act is incomplete but it is able to determine a reasonable estimate, it must record a provisional estimate in the financial statements. If a company cannot determine a provisional estimate to be included in the financial statements, it should continue to
| | | | | | | | |
| | Part II |
| | MD&A - Accounting Estimates and Pronouncements |
| | |
apply ASC 740 on the basis of the provisions of the tax laws that were in effect immediately before the enactment of the Tax Act.
Accounting for Convertible Debt and Related Derivatives
In 2016 and 2017, the company issued $150,000,000 and $120,000,000 aggregate principal amount of the 2021 and 2022 Notes, respectively. In 2019, the company repurchased $16,000,000 in aggregate principal amount of 2021 Notes for cash and entered into separate privately negotiated agreements with certain holders of its 2021 Notes to exchange $72,909,000 in aggregate principal amount of 2021 Notes for new 5.00% Convertible Senior Exchange Notes Series I due 2024 of the company and $6,928,000 in cash.
In 2020, the company repurchased $24,466,000 in aggregate principal amount of 2021 Notes for cash and entered into separate privately negotiated agreements with certain holders of its 2021 Notes to exchange $35,375,000 in aggregate principal amount of the company's 2021 Notes and certain holders of its 2022 Notes to its 2022 Notes to exchange $38,500,000 in aggregate principal amount of the company's 2022 Notes for new 5.00% Convertible Senior Exchange Notes Series II due 2024 of the company and $5,593,000 in cash. In connection with the original offering of the 2021 Notes and 2022 Notes, the company entered into privately negotiated convertible note hedge transactions with certain counterparties. These transactions cover, subject to customary anti-dilution adjustments, the number of the company's common shares that will initially underlie the Notes, and are expected generally to reduce the potential equity dilution, and/or offset any cash payments in excess of the principal amount due, as the case may be, upon conversion of the Notes.
The company entered into separate, privately negotiated warrant transactions with the option counterparties at a higher strike price relating to the same number of the company's common shares, subject to customary anti-dilution adjustments, pursuant to which the company sold warrants to the option counterparties. The warrants could have a dilutive effect on the company's outstanding common shares and the company's earnings per share to the extent that the price of the company's common shares exceeds the strike price of those warrants. The initial strike price of the warrants is $22.4175 and $21.4375 per share on the 2021 and 2022 Notes, respectively, and is subject to certain adjustments under the terms of the warrant transactions.
As a result of the repurchase of 2021 Notes in third quarter of 2019 and the exchange of 2021 Notes for new notes in the fourth quarter of 2019, a partial unwind of the note hedge options and warrants entered into with the issuance of the 2021 Notes also occurred during the fourth
quarter of 2019. Note hedge options outstanding were reduced from the original number of 300,000 to 138,182 and warrants were reduced from the initial number of 9,007,380 to 3,860,624. The partial unwind of the note hedge options and warrants resulted in no net impact to cash or paid in capital.
As a result of the repurchase of 2021 Notes in the third quarter of 2020 and the exchange of 2021 Notes for new notes in the second quarter of 2020, a partial unwind of the note hedge options and warrants entered into with the issuance of the 2021 Notes also occurred. Note hedge options outstanding were reduced from the original number of 300,000 to 62,341 and warrants were reduced from the initial number of 9,007,380 to 3,141,943. The partial unwind of the note hedge options and warrants resulted in no net impact to cash or paid in capital.
The convertible debt conversion liabilities and the convertible note hedges were accounted for as derivatives and accounted for at fair value quarterly until no longer accounted for separately as a result of obtaining shareholder approval in May 2019 to settle the Notes with common shares. The warrants are included as equity. The fair value of the convertible debt conversion liabilities and the convertible note hedges were estimated using a lattice model incorporating the terms and conditions of the notes and considering, for example, changes in the prices of the company's common shares, company stock price volatility, risk-free rates and changes in market rates. The valuations were, among other things, subject to changes in both the company's credit worthiness and the counter-parties to the instruments as well as change in general market conditions.
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS
For the company's disclosure regarding recently issued accounting pronouncements, refer to Accounting Policies - Recent Accounting Pronouncements in the Notes to the Consolidated Financial Statements.
| | | | | | | | |
| Part II | | |
| MD&A - Contractual Obligations | | |
| | |
CONTRACTUAL OBLIGATIONS
The company's contractual obligations as of December 31, 2020 are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Payments due by period |
| | Total | | Less than
1 year | | 1-3 years | | 3-5 years | | More than
5 years |
| Purchase obligations (primarily computer systems contracts) (1) | $ | 207,540 | | | $ | 29,620 | | | $ | 59,240 | | | $ | 53,850 | | | $ | 64,830 | |
| Finance lease obligations | 111,257 | | | 7,416 | | | 12,859 | | | 12,635 | | | 78,347 | |
| Convertible senior notes Series II at 5.00%, due in November 2024 | 104,811 | | | 3,694 | | | 7,388 | | | 93,729 | | | — | |
| Convertible senior notes at 4.50%, due in June 2022 | 89,752 | | | 3,668 | | | 86,084 | | | — | | | — | |
| Convertible senior notes Series I at 5.00%, due in November 2024 | 87,035 | | | 3,645 | | | 7,291 | | | 76,099 | | | — | |
| U.S. and Canadian Credit Facility | 21,811 | | | 530 | | | 1,060 | | | 20,221 | | | — | |
| Operating lease obligations | 18,315 | | | 7,182 | | | 6,256 | | | 2,502 | | | 2,375 | |
| Product liability | 14,757 | | | 2,453 | | | 5,878 | | | 2,782 | | | 3,644 | |
| | | | | | | | | |
| European Credit Facility | 12,327 | | | 346 | | | 464 | | | 11,517 | | | — | |
| CARES Act Loan | 10,169 | | | 4,210 | | | 5,959 | | | — | | | — | |
Future lease obligations (2)
| 8,591 | | | 683 | | | 1,366 | | | 1,366 | | | 5,176 | |
| Supplemental Executive Retirement Plan | 5,759 | | | 391 | | | 782 | | | 782 | | | 3,804 | |
| Convertible senior notes at 5.00%, due in February 2021 | 1,258 | | | 1,258 | | | — | | | — | | | — | |
| Other, principally deferred compensation | 5,318 | | | — | | | — | | | — | | | 5,318 | |
| Total | $ | 698,700 | | | $ | 65,096 | | | $ | 194,627 | | | $ | 275,483 | | | $ | 163,494 | |
________________________
(1) In October 2019, the company entered into an agreement to outsource substantially all of the company’s information technology business service activities, including, among other things, support, rationalization and upgrading of the company’s legacy information technology systems and implementation of a global enterprise resource planning system and eCommerce platform.
(2) The company entered into a lease agreement in Germany that commenced in Q1 2021.
The table does not include any payments related to liabilities recorded for uncertain tax positions as the company cannot make a reasonably reliable estimate as to the timing of payments. Refer to Income Taxes in the Notes to the Consolidated Financial Statements included in this report.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
The company is at times exposed to market risk through various financial instruments, including fixed rate and floating rate debt instruments. Based on December 31, 2020 debt levels, a 1% change in interest rates would have no impact on annual interest expense as the company did not have any variable rate debt outstanding. Additionally, the company operates internationally and, as a result, is exposed to foreign currency fluctuations. Specifically, the exposure results from intercompany loans, intercompany sales or payments and third-party sales or payments. In an attempt to reduce this exposure, foreign currency forward contracts are utilized to hedge intercompany purchases and sales as well as third-party purchases and sales. The company does not believe that any potential loss related to these financial instruments would have a material adverse effect on the company's financial condition or results of operations.
The company is party to the Credit Agreement which was originally entered into on January 16, 2015 and matures in January 2024, as extended by an amendment to the Credit Agreement which became effective on May 29, 2020. Accordingly, while the company is exposed to increases in interest rates, its exposure to the volatility of the current market environment is currently limited until the Credit Agreement expires. The Credit Agreement contains customary default provisions, with certain grace periods and exceptions, which provide that events of default that include, among other things, failure to pay amounts due, breach of covenants, representations or warranties, bankruptcy, the occurrence of a material adverse effect, exclusion from any medical reimbursement program, and an interruption of any material manufacturing facilities for more than ten consecutive days. Should the company fail to comply with these requirements, the company would potentially have to attempt to obtain alternative financing and thus likely be required to pay much higher interest rates.
As of December 31, 2020, the company had $20,000,000 on the North American credit facility and $11,502,000 on the European credit facility under its Credit Agreement, which provides for a senior secured revolving credit facility for U.S. and Canadian borrowers of up to $60,000,000 at variable rates, subject to availability based on a borrowing base formula, and in addition provides for a revolving credit, letters of credit and swing line loan facility for European borrowers allowing borrowing up to an aggregate principal amount of $30,000,000 at variable rates, also subject to availability based on a borrowing base formula. As of December 31, 2020, the company had $1,250,000, $81,500,000, $72,909,000 and $75,688,000 in principal (including
accretion, only applicable for the Series II 2024 Notes) amount outstanding of its fixed rate 2021 Notes, 2022 Notes, Series I 2024 Notes and Series II 2024 Notes, respectively.
Item 8. Financial Statements and Supplementary Data.
Reference is made to the Report of Independent Registered Public Accounting Firm, Consolidated Balance Sheets, Consolidated Statements of Comprehensive Income (Loss), Consolidated Statements of Cash Flows, Consolidated Statement of Shareholders' Equity, Notes to Consolidated Financial Statements and Financial Statement Schedule of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
(a) Evaluation of Disclosure Controls and Procedures
As of December 31, 2020, an evaluation was performed, under the supervision and with the participation of the company's management, including the Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of the company's disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)). Based on that evaluation, the company's management, including the Chief Executive Officer and Chief Financial Officer, concluded that the company's disclosure controls and procedures were effective as of December 31, 2020, in ensuring that information required to be disclosed by the company in the reports it files and submits under the Exchange Act is (1) recorded, processed, summarized and reported, within the time periods specified in the Commission's rules and forms and (2) accumulated and communicated to the company's management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow for timely decisions regarding required disclosure.
(b) Management's Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining a system of adequate internal control over financial reporting that provides reasonable assurance that assets are safeguarded and that transactions are authorized, recorded and reported properly. The system includes self-monitoring mechanisms; regular testing by the company's internal auditors; a Code of Conduct; written policies and
procedures; and a careful selection and training of employees. Actions are taken to correct deficiencies as they are identified. An effective internal control system, no matter how well designed, has inherent limitations—including the possibility of the circumvention or overriding of controls—and therefore can provide only reasonable assurance that errors and fraud that can be material to the financial statements are prevented or would be detected on a timely basis. Further, because of changes in conditions, internal control system effectiveness may vary over time.
Management's assessment of the effectiveness of the company's internal control over financial reporting is based on the Internal Control—Integrated Framework published by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework).
In management's opinion, internal control over financial reporting is effective as of December 31, 2020.
(c) Attestation Report of the Independent Registered Public Accounting Firm
The company's independent registered public accounting firm, Ernst & Young LLP, audited the company's internal control over financial reporting and, based on that audit, issued its report regarding the company's internal control over financial reporting, which is included in this Annual Report on Form 10-K.
(d) Changes in Internal Control Over Financial Reporting
There have been no changes in the company’s internal control over financial reporting during the company’s last fiscal quarter that have materially affected, or are reasonably likely to materially affect, the company’s internal control over financial reporting.
Item 9B. Other Information.
None.
Item 10. Directors, Executive Officers and Corporate Governance.
Information required by Item 10 as to the executive officers of the company is included in Part I of this Annual Report on Form 10-K. The other information required by Item 10 as to the directors of the company, the Audit Committee, the Audit Committee financial experts, the procedures by which security holders may recommend nominees to the Board of Directors, compliance with Section 16(a) of the Exchange Act, code of ethics and corporate governance is incorporated herein by reference to the information set forth under the captions “Election of Directors,” “Corporate Governance,” and “Delinquent Section 16(a) Reports” in the company's definitive Proxy Statement on Schedule 14A for the 2021 Annual Meeting of Shareholders.
Item 11. Executive Compensation.
The information required by Item 11 is incorporated by reference to the information set forth under the captions “Corporate Governance”, “Executive Compensation” and “CEO Pay Ratio” in the company's definitive Proxy Statement on Schedule 14A for the 2021 Annual Meeting of Shareholders.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters.
The information required by Item 12 is incorporated by reference to the information set forth under the caption “Security Ownership of Certain Beneficial Holders and Management” in the company's definitive Proxy Statement on Schedule 14A for the 2021 Annual Meeting of Shareholders.
Information regarding the securities authorized for issuance under the company's equity compensation plans is incorporated by reference to the information set forth under the captions “Equity Compensation Plan Information” in the company's definitive Proxy Statement on Schedule 14A for the 2021 Annual Meeting of Shareholders.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by Item 13 is incorporated by reference to the information set forth under the caption “Certain Relationships and Related Transactions” in the company's definitive Proxy Statement on Schedule 14A for the 2021 Annual Meeting of Shareholders.
Item 14. Principal Accountant Fees and Services.
The information required by Item 14 is incorporated by reference to the information set forth under the caption “Independent Registered Public Accounting Firm Fees and Services” in the company's definitive Proxy Statement on Schedule 14A for the 2021 Annual Meeting of Shareholders.
Item 15. Exhibits and Financial Statement Schedules.
(a)(1) Financial Statements.
The following financial statements of the company are included in Part II, Item 8:
Consolidated Statements of Comprehensive Income (Loss)—years ended December 31, 2020, 2019 and 2018
Consolidated Balance Sheets—December 31, 2020 and 2019
Consolidated Statements of Cash Flows—years ended December 31, 2020, 2019 and 2018
Consolidated Statement of Shareholders' Equity—years ended December 31, 2020, 2019 and 2018
Notes to Consolidated Financial Statements
(a)(2) Financial Statement Schedules.
The following financial statement schedule of the company is included in Part II, Item 8:
Schedule II—Valuation and Qualifying Accounts
All other schedules have been omitted because they are not applicable or not required, or because the required information is included in the Consolidated Financial Statements or notes thereto.
(a)(3) Exhibits.
Refer to Exhibit Index of this Annual Report on Form 10-K.
Item 16. Form 10-K Summary.
None.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized as of March 3, 2021.
| | | | | | | | |
| INVACARE CORPORATION |
| | |
| By: | /s/ MATTHEW E. MONAGHAN |
| | Matthew E. Monaghan |
| | Chairman of the Board of Directors, President and Chief Executive Officer |
INVACARE CORPORATION
Report on Form 10-K for the fiscal year ended December 31, 2020.
| | | | | | | | | | | |
Official
Exhibit No. | Description | | Sequential
Page No. |
| Securities Purchase Agreement among Allied Motion Christchurch Limited, Invacare Holdings New Zealand and Invacare Corporation, dated March 6, 2020. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the Securities and Exchange Commission upon request any omitted schedule or exhibit to the agreement.) | | (A) |
| Second Amended and Restated Articles of Incorporation | | (B) |
| Second Amended and Restated Code of Regulations, as amended | | (C) |
| Amendment No. 1 to the Second Amended and Restated Articles of Incorporation | | (D) |
| Indenture, dated as of February 23, 2016, by and between Invacare Corporation and Wells Fargo Bank, National Association (including the form of the 5.00% Convertible Senior Notes due 2021). | | (E) |
| Indenture, dated as of June 14, 2017, by and between Invacare Corporation and Wells Fargo Bank, National Association (including the form of the 4.50% Convertible Senior Notes due 2022). | | (F) |
| Indenture, dated as of November 19, 2019, by and between Invacare Corporation and Wells Fargo Bank, N.A., as Trustee (including the form of the 5.00% Convertible Senior Exchange Notes due 2024). | | (G) |
| Indenture, dated as of June 4, 2020, by and between Invacare Corporation and Wells
Fargo Bank, N.A., as Trustee (including the form of the 5.00% Series II Convertible
Senior Exchange Notes due 2024). | | (H) |
| Description of Securities Registered Under the Exchange Act. | | (I) |
| Invacare Retirement Savings Plan, effective January 1, 2001, as amended | | (J)* |
| Invacare Corporation 401(K) Plus Benefit Equalization Plan, effective January 1, 2003, as amended and restated | | (J)* |
| Invacare Corporation Deferred Compensation Plus Plan, effective January 1, 2005, as amended August 19, 2009 and on November 23, 2010 | | (K)* |
| Amendment No. 3 to Invacare Corporation Deferred Compensation Plus Plan, effective January 1, 2005 | | (L)* |
| Invacare Corporation Death Benefit Only Plan, effective January 1, 2005, as amended | | (J)* |
| Supplemental Executive Retirement Plan, as amended and restated effective February 1, 2000 | | (M)* |
| Cash Balance Supplemental Executive Retirement Plan, as amended and restated, effective December 31, 2008 | | (N)* |
| Amendment No. 1 to the Cash Balance Supplemental Executive Retirement Plan, effective August 19, 2009 | | (O)* |
| Form of Participation Agreement, for current participants in the Cash Balance Supplemental Executive Retirement Plan, as of December 31, 2008, entered into by and between the company and certain participants and a schedule of all such agreements with participants | | (P)* |
| Invacare Corporation Amended and Restated 2003 Performance Plan | | (O)* |
| Form of Director Stock Option Award under Invacare Corporation 2003 Performance Plan | | (J)* |
| Form of Director Deferred Option Award under Invacare Corporation 2003 Performance Plan | | (K)* |
| Form of Restricted Stock Award under Invacare Corporation 2003 Performance Plan | | (L) |
| Form of Stock Option Award under Invacare Corporation 2003 Performance Plan | | (J)* |
| Form of Executive Stock Option Award under Invacare Corporation 2003 Performance Plan | | (J)* |
| Form of Switzerland Stock Option Award under Invacare Corporation 2003 Performance Plan | | (J)* |
| | | | | | | | | | | |
Official
Exhibit No. | Description | | Sequential
Page No. |
| Form of Switzerland Executive Stock Option Award under Invacare Corporation 2003 Performance Plan | | (J)* |
| Invacare Corporation 2013 Equity Compensation Plan | | (Q)* |
| Amendment No. 1 to the Invacare Corporation 2013 Equity Compensation Plan | | (R)* |
| Form of Executive Stock Option Award under the Invacare Corporation 2013 Equity Compensation Plan | | (S)* |
| Form of Stock Option Award under the Invacare Corporation 2013 Equity Compensation Plan | | (S)* |
| Form of Executive Stock Option Award for Swiss Employees under the Invacare Corporation 2013 Equity Compensation Plan | | (S)* |
| Form of Stock Option Award for Swiss Employees under the Invacare Corporation 2013 Equity Compensation Plan | | (S)* |
| Form of Director Restricted Stock Award under the Invacare Corporation 2013 Equity Compensation Plan | | (S)* |
| Form of Restricted Stock Award under the Invacare Corporation 2013 Equity Compensation Plan | | (S)* |
| Form of Performance Share Award Agreement under the Invacare Corporation 2013 Equity Compensation Plan | | (T)* |
| Form of Restricted Stock Award Agreement for Employees under the Invacare Corporation 2013 Equity Compensation Plan | | (U)* |
| Form of Director Restricted Stock Unit under the Invacare Corporation 2013 Equity Compensation Plan | | (V)* |
| Invacare Corporation Executive Incentive Bonus Plan, as amended and restated | | (R)* |
| Employment Agreement, dated as of March 27, 2020, by and between the company and Matthew E. Monaghan. | | (W)* |
| Letter Agreement, dated as of February 20, 2018, by and between Invacare Corporation and Kathleen P. Leneghan. | | (X)* |
| Letter agreement, dated as of July 31, 2008, by and between the company and Anthony C. LaPlaca. | | (P)* |
| Employment Agreement, dated as of October 21, 2016, by and between the company and Ralf Ledda. | | (V)* |
| Letter Agreement, dated as of August 1, 2020, by and between the company and
Joost Beltman. | | (V)* |
| Letter Agreement, dated as of February 10, 2019, by and between the company and
Angela Goodwin. | | (V)* |
| Change of Control Agreement, dated as of December 31, 2008, by and between the company and Anthony C. LaPlaca | | (Y)* |
| Form of Change of Control Agreement entered into by and between the company and certain of its executive officers and schedule of all such agreements with certain executive officers | | (Z)* |
| Technical Information & Non-Competition Agreement, dated April 1, 2015, entered into by and between the company and Matthew E. Monaghan | | (P)* |
| Technical Information & Non-Competition Agreement, dated April 6, 2008, entered into by and between the company and Robert K. Gudbranson | | (P)* |
| Technical Information & Non-Competition Agreement entered into by and between the company and certain of its executive officers and schedule of all such agreements with executive officers | | (Z)* |
| Indemnity Agreement, dated April 1, 2015, entered into by and between the company and Matthew E. Monaghan. | | (P)* |
| Form of Indemnity Agreement entered into by and between the company and its directors and certain of its executive officers and schedule of all such agreements with directors and executive officers | | (Z)* |
| | | | | | | | | | | |
Official
Exhibit No. | Description | | Sequential
Page No. |
| Form of Rule 10b5-1 Sales Plan entered into between the company and certain of its executive officers and other employees and a schedule of all such agreements with executive officers and other employees | | (K) |
| Director Compensation Schedule | | * |
| 2012 Non-employee Directors Deferred Compensation Plan, effective January 1, 2012, Amended and Restated as of November 17, 2016 | | (V) |
| Retirement Agreement and Release, dated as of November 14, 2014, by and between Invacare Corporation and A. Malachi Mixon, III. | | (AA)* |
| Purchase and Sale Agreement, dated as of February 24, 2015, by and between the company and Industrial Realty Group, LLC. | | (BB) |
| Form of Lease Agreement by and among the company and the affiliates of
Industrial Realty Group, LLC named therein. | | (BB) |
| Amended and Restated Revolving Credit and Security Agreement, dated as of September 30, 2015, by and among the company, the other Borrowers party thereto, the Guarantors party thereto, the Lenders party thereto, PNC Bank, National Association, as administrative agent, JP Morgan Chase Bank, N.A. and J.P. Morgan Europe Limited, as European agent. | | (CC) |
| First Amendment to Amended and Restated Revolving Credit and Security Agreement, dated as of February 16, 2016, by and among the company, the other borrowers party thereto, the guarantors party thereto, the lenders party thereto, PNC Bank, National Association, as administrative agent, and J.P. Morgan Europe Limited, as European agent. | | (DD) |
| Second Amendment to Amended and Restated Revolving Credit and Security Agreement, dated as of May 3, 2016 by and among the company, the other borrowers party thereto, the guarantors party thereto, the lenders party thereto, PNC Bank, National Association, as administrative agent, and J.P. Morgan Europe Limited, as European agent. | | (V) |
| Third Amendment to Amended and Restated Revolving Credit and Security Agreement, dated as of September 30, 2016, by and among the company, the other borrowers party thereto, the guarantors party thereto, the lenders party thereto, PNC Bank, National Association, as administrative agent, and J.P. Morgan Europe Limited, as European agent. | | (V) |
| Fourth Amendment to Amended and Restated Revolving Credit and Security Agreement, dated as of November 30, 2016, by and among the company, the other borrowers party thereto, the guarantors party thereto, the lenders party thereto, PNC Bank, National Association, as agent for the lenders, and J.P. Morgan Europe Limited, as European agent for the lenders. | | (EE) |
| Waiver and Fifth Amendment to Amended and Restated Revolving Credit and Security Agreement, dated as of November 30, 2016, by and among the company, the other borrowers party thereto, the guarantors party thereto, the lenders party thereto, PNC Bank, National Association, as agent for the lenders, and J.P. Morgan Europe Limited, as European agent for the lenders. | | (FF) |
| Sixth Amendment to Amended and Restated Revolving Credit and Security Agreement, dated as of November 13, 2019, by and among the company, the other borrowers party thereto, the guarantors party thereto, the lenders party thereto, PNC Bank, National Association, as agent for the lenders, and J.P. Morgan Europe Limited, as European agent for the lenders. | | (GG) |
| Seventh Amendment to Amended and Restated Revolving Credit and Security Agreement, dated as of May 29, 2020, by and among the company, the other borrowers party thereto, the guarantors party thereto, the lenders party thereto, PNC Bank, National Association, as agent for the lenders, and J.P. Morgan Europe Limited, as European agent for the lenders. | | (HH) |
| Eighth Amendment to Amended and Restated Revolving Credit and Security Agreement, dated as of January 15, 2021, by and among the company, the other borrowers party thereto, the guarantors party thereto, the lenders party thereto, PNC Bank, National Association, as agent for the lenders, and J.P. Morgan Europe Limited, as European agent for the lenders. | | (II) |
| Call Option Transaction Confirmation entered into between JPMorgan Chase Bank, National Association, London Branch and Invacare Corporation as of February 17, 2016 | | (E) |
| Call Option Transaction Confirmation entered into between Wells Fargo Bank, National Association and Invacare Corporation as of February 17, 2016 | | (E) |
| | | | | | | | | | | |
Official
Exhibit No. | Description | | Sequential
Page No. |
| Warrants Confirmation between Invacare Corporation to JPMorgan Chase Bank, National Association, London Branch as of February 17, 2016 | | (E) |
| Warrants Confirmation between Invacare Corporation to Wells Fargo Bank, National Association as of February 17, 2016 | | (E) |
| Additional Call Option Transaction Confirmation, dated March 4, 2016, between JPMorgan Chase Bank, National Association, London Branch and Invacare Corporation. | | (JJ) |
| Additional Call Option Transaction Confirmation, dated March 4, 2016, between Wells Fargo Bank, National Association and Invacare Corporation. | | (JJ) |
| Additional Warrants Confirmation, dated March 4, 2016, between JPMorgan Chase Bank, National Association, London Branch and Invacare Corporation. | | (JJ) |
| Additional Warrants Confirmation, dated March 4, 2016, between Wells Fargo Bank, National Association and Invacare Corporation. | | (JJ) |
| Partial Unwind Agreement, dated as of November 26, 2019, between Invacare Corporation and JPMorgan Chase Bank, National Association, London Branch. | | (I) |
| Partial Unwind Agreement, dated as of November 22, 2019, between Invacare Corporation and Wells Fargo Bank, National Association. | | (I) |
| Partial Unwind Agreement, dated as of June 4, 2020, between Invacare Corporation and
Wells Fargo Bank, National Association. | | |
| Partial Unwind Agreement, dated as of September 11, 2020, between Invacare Corporation and Wells Fargo Bank, National Association. | | |
| Promissory Note dated May 13, 2020, between Invacare Corporation and Key Bank
National Association. | | (KK) |
| Form of Performance-Based Stock Option Award under Invacare Corporation 2013 Equity Compensation Plan. | | (LL)* |
| Base Call Option Transaction Confirmation, dated June 8, 2017, between Goldman Sachs & Co. LLC and Invacare Corporation. | | (F) |
| Base Warrants Confirmation, dated June 8, 2017, between Goldman Sachs & Co. LLC and Invacare Corporation. | | (F) |
| Additional Call Option Transaction Confirmation, dated June 9, 2017, between Goldman Sachs & Co. LLC and Invacare Corporation. | | (F) |
| Additional Warrants Confirmation, dated June 9, 2017, between Goldman Sachs & Co. LLC and Invacare Corporation. | | (F) |
| Invacare Corporation 2018 Equity Compensation Plan | | (MM) |
| Amendment No. 1 to Invacare Corporation 2018 Equity Compensation Plan | | (D)* |
| Amendment No. 2 to Invacare Corporation 2018 Equity Compensation Plan | | (NN)* |
| Form of Restricted Stock Award under Invacare Corporation 2018 Equity Compensation Plan | | (OO)* |
| Form of Restricted Stock Unit Award under Invacare Corporation 2018 Equity Compensation Plan | | (OO)* |
| Form of Director Restricted Stock Unit Award under Invacare Corporation 2018 Equity Compensation Plan | | (OO)* |
| Form of Performance Award under Invacare Corporation 2018 Equity Compensation Plan | | (OO)* |
| Form of Performance Unit Award under Invacare Corporation 2018 Equity Compensation Plan | | (OO)* |
| Letter agreement, dated as of May 9, 2018, by and between the company and Darcie L. Karol | | (OO)* |
| Omnibus Amendment | | (Z)* |
| Master Information Technology Services Agreement by and between Invacare Corporation and Birlasoft Solutions, Inc. effective October 1, 2019. | | (PP) |
| Form of Letter Agreement regarding salary deferrals and reductions entered into by and
between the company and certain of its executive officers and schedule of all such
agreements with certain executive officers | | * |
| | | | | | | | | | | |
Official
Exhibit No. | Description | | Sequential
Page No. |
| Subsidiaries of the company | | |
| Consent of Independent Registered Public Accounting Firm | | |
| Certification of the Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | |
| Certification of the Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | |
| Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | |
| Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | |
| Consent Decree of Permanent Injunction, as filed with the U.S. District Court for the Northern District of Ohio on December 20, 2012. | | (QQ) |
| 101.INS** | Inline XBRL instance document | | |
| 101.SCH** | Inline XBRL taxonomy extension schema | | |
| 101.CAL** | Inline XBRL taxonomy extension calculation linkbase | | |
| 101.DEF** | Inline XBRL taxonomy extension definition linkbase | | |
| 101.LAB** | Inline XBRL taxonomy extension label linkbase | | |
| 101.PRE** | Inline XBRL taxonomy extension presentation linkbase | | |
| 104 | Cover Page Interactive Data File - The cover page from the company's Annual Report on Form 10-K for the year ended December 31, 2020, formatted in Inline XBRL (included in Exhibit 101). | | |
________________________
* Management contract, compensatory plan or arrangement
** Filed herewith
(A)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated March 9, 2020, which Exhibit is incorporated herein by reference.
(B)Reference is made to Exhibit 3(a) of the company report on Form 10-K for the fiscal year ended December 31, 2008, which Exhibit is incorporated herein by reference.
(C)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated February 13, 2014, which Exhibit is incorporated herein by reference.
(D)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated May 16, 2019, which Exhibit is incorporated herein by reference.
(E)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated February 23, 2016, which Exhibit is incorporated herein by reference.
(F)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated June 14, 2017, which Exhibit is incorporated herein by reference.
(G)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated November 13, 2019, which Exhibit is incorporated herein by reference.
(H)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated June 4, 2020, which Exhibit is incorporated herein by reference.
(I)Reference is made to the appropriate Exhibit of the company report on Form 10-K, for the fiscal year ended December 31, 2019, which Exhibit is incorporated herein by reference.
(J)Reference is made to the appropriate Exhibit of the company report on Form 10-K for the fiscal year ended December 31, 2007, which Exhibit is incorporated herein by reference.
(K)Reference is made to the appropriate Exhibit of the company report on Form 10-K for the fiscal year ended December 31, 2010, which Exhibit is incorporated herein by reference.
(L)Reference is made to the appropriate Exhibit of the company report on Form 10-K for the fiscal year ended December 31, 2011, which Exhibit is incorporated herein by reference.
(M)Reference is made to the appropriate Exhibit of the company report on Form 10-K for the fiscal year ended December 31, 2004, which Exhibit is incorporated herein by reference.
(N)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated December 31, 2008, which Exhibit is incorporated herein by reference.
(O)Reference is made to Exhibit 10.1 of the company report on Form 8-K, dated May 21, 2009, which Exhibit is incorporated herein by reference.
(P)Reference is made to the appropriate Exhibit of the company report on Form 10-K for the fiscal year ended December 31, 2015, which Exhibit is incorporated herein by reference.
(Q)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated May 16, 2013, which Exhibit is incorporated herein by reference.
(R)Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated May 14, 2015, which Exhibit is incorporated herein by reference.
(S)Reference is made to the appropriate Exhibit of the company report on Form 10-Q, for the fiscal quarter ended September 30, 2013, which Exhibit is incorporated herein by reference.
(T)Reference is made to Exhibit 10.1 of the company report on Form 8-K, dated March 7, 2014, which Exhibit is incorporated herein by reference.
(U)Reference is made to Exhibit 10.2 of the company report on Form 8-K, dated March 7, 2014, which Exhibit is incorporated herein by reference.
(V)Reference is made to the appropriate Exhibit of the company report on Form 10-K for the fiscal year ended December 31, 2016, which Exhibit is incorporated herein by reference.
(W)Reference is made to Exhibit 99.1 of the company report on Form 8-K, dated March 27, 2020, which Exhibit is incorporated herein by reference.
(X)Reference is made to Exhibit 10.1 of the company report on Form 8-K, dated February 22, 2018, which Exhibit is incorporated herein by reference.
(Y)Reference is made to the appropriate Exhibit of the company report on Form 10-K for the fiscal year ended December 31, 2017, which Exhibit is incorporated herein by reference.
(Z)Reference is made to the appropriate Exhibit of the company report on Form 10-K for the fiscal year ended December 31, 2018, which Exhibit is incorporated herein by reference.
(AA)Reference is made to Exhibit 10.1 of the company report on Form 8-K, dated November 14, 2014, which Exhibit is incorporated herein by reference.
(BB) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated April 23, 2015, which Exhibit is incorporated herein by reference.
(CC) Reference is made to Exhibit 10.1 of the company report on Form 8-K, dated September 30, 2015, which Exhibit is incorporated herein by reference.
(DD) Reference is made to Exhibit 10.1 of the company report on Form 8-K, dated February 16, 2016, which Exhibit is incorporated herein by reference.
(EE) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated November 30, 2016, which Exhibit is incorporated herein by reference.
(FF) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated June 7, 2017, which Exhibit is incorporated herein by reference.
(GG) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated November 14, 2019, which Exhibit is incorporated herein by reference.
(HH) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated June 1, 2020, which Exhibit is incorporated herein by reference.
(II) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated January 21, 2021, which Exhibit is incorporated herein by reference.
(JJ) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated March 7, 2016, which Exhibit is incorporated herein by reference.
(KK) Reference is made to the appropriate Exhibit of the company report on Form 10-Q, for the fiscal quarter ended June 30, 2020, which Exhibit is incorporated herein by reference.
(LL) Reference is made to the appropriate Exhibit of the company report on Form 10-Q, for the fiscal quarter ended March 31, 2017, which Exhibit is incorporated herein by reference.
(MM) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated May 18, 2018, which Exhibit is incorporated herein by reference.
(NN) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated May 21, 2020, which Exhibit is incorporated herein by reference.
(OO) Reference is made to the appropriate Exhibit of the company report on Form 10-Q, for the fiscal quarter ended June 30, 2018, which Exhibit is incorporated herein by reference.
(PP) Reference is made to the appropriate Exhibit of the company report on Form 10-Q, for the fiscal quarter ended September 30, 2019, which Exhibit is incorporated herein by reference.
(QQ) Reference is made to the appropriate Exhibit of the company report on Form 8-K, dated December 20, 2012, which Exhibit is incorporated herein by reference.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated as of March 3, 2021.
| | | | | | | | |
| | |
| Signature | | Title |
| |
| /s/ MATTHEW E. MONAGHAN | | Chairman of the Board of Directors, President and Chief Executive Officer (Principal Executive Officer) |
| Matthew E. Monaghan | |
| | |
| /s/ KATHLEEN P. LENEGHAN | | Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) |
| Kathleen P. Leneghan | |
| | |
| /s/ SUSAN H. ALEXANDER | | Director |
| Susan H. Alexander | |
| | |
| /s/ JULIE A. BECK | | Director |
| Julie A. Beck | |
| | |
| /s/ PETRA DANIELSOHN-WEIL, PhD | | Director |
| Petra Danielsohn-Weil, PhD | | |
| | |
| /s/ DIANA S. FERGUSON | | Director |
| Diana S. Ferguson | | |
| | |
| /s/ MARC M. GIBELEY | | Director |
| Marc M. Gibeley | |
| | |
| /s/ C. MARTIN HARRIS, M.D. | | Director |
| C. Martin Harris, M.D. | |
| | |
| /s/ CLIFFORD D. NASTAS | | Director |
| Clifford D. Nastas | |
| |
| /s/ BAIJU R. SHAH | | Director |
| Baiju R. Shah | | |
| | | | | | | | |
| | Reports of Independent Registered Public Accounting Firm |
| | |
| | |
To the Shareholders and Board of Directors of Invacare Corporation
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Invacare Corporation and subsidiaries (the Company) as of December 31, 2020 and 2019, the related consolidated statements of comprehensive income (loss), shareholders' equity and cash flows for each of the three years in the period ended December 31, 2020, and the related notes and financial statement schedule listed in the Index at Item 15(a), (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2020, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated March 3, 2021 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the account or disclosures to which it relates.
| | | | | | | | |
| Reports of Independent Registered Public Accounting Firm | | |
| | |
| | |
| | | | | |
| Valuation of Goodwill |
| Description of the matter | At December 31, 2020, the carrying amount of the Company’s goodwill was $402 million. As discussed in the Accounting Policies and Long-Term Assets - Goodwill notes to the consolidated financial statements, goodwill is assessed for impairment at the reporting unit level at least annually or whenever events or changes in circumstances indicate its carrying value may not be recoverable. |
| |
| Auditing management’s goodwill impairment assessment was complex and judgmental due to the significant estimation required to determine the fair values of the reporting units. In particular, the fair value estimates were sensitive to significant assumptions, such as projected future cash flows of the reporting units and the weighted average cost of capital used in the valuation process to discount future cash flows, which are affected by expectations about future market or economic conditions and the planned business and operating strategies. |
| |
| How we addressed the matter in our audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company’s goodwill impairment assessment process, including controls over management’s review of the significant data and assumptions used in the calculation of the fair values of the reporting units. |
| |
| To test the estimated fair values of the reporting units, our audit procedures included, among others, assessing the valuation methodologies, testing the significant assumptions used to develop the projected financial information, and testing the underlying data used by the Company in its analysis. We compared the projected financial information developed by management to current industry and economic trends as well as to the historical performance of each reporting unit and evaluated the expected impacts of the Company’s operating strategies and initiatives on the significant assumptions. We also performed analyses to evaluate the sensitivity of the fair values of the reporting units resulting from changes in the significant assumptions. In addition, we tested management’s reconciliation of the fair values of the reporting units to the market capitalization of the Company and assessed its reasonableness. In addition, we involved our internal valuation specialists to assist in our evaluation of the methodologies applied and assumptions used by management. |
/s/ Ernst & Young LLP
We have served as the Company's auditor since 1984.
Cleveland, Ohio
March 3, 2021
| | | | | | | | |
| | Reports of Independent Registered Public Accounting Firm |
| | |
| | |
To the Shareholders and Board of Directors of Invacare Corporation
Opinion on Internal Control over Financial Reporting
We have audited Invacare Corporation and subsidiaries’ internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control— Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Invacare Corporation and subsidiaries (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2020 based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2020 and 2019, the related consolidated statements of comprehensive income (loss), shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2020, and the related notes and financial statement schedule listed in the Index at Item 15(a) and our report dated March 3, 2021 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying “Management’s Annual Report on Internal Control over Financial Reporting”. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Cleveland, Ohio
March 3, 2021
| | | | | | | | |
| Financial Statements | | |
| Consolidated Statements of Comprehensive Income (Loss) | | |
| | |
INVACARE CORPORATION AND SUBSIDIARIES
Consolidated Statements of Comprehensive Income (Loss)
| | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| | (In thousands, except per share data) |
| Net sales | $ | 850,689 | | | $ | 927,964 | | | $ | 972,347 | |
| Cost of products sold | 605,437 | | | 665,897 | | | 704,671 | |
| Gross Profit | 245,252 | | | 262,067 | | | 267,676 | |
| Selling, general and administrative expenses | 236,357 | | | 260,061 | | | 281,906 | |
| Gain on sale of business | (9,790) | | | — | | | — | |
| Charges related to restructuring activities | 7,358 | | | 11,829 | | | 3,481 | |
| Impairment of an intangible asset | — | | | 587 | | | 583 | |
| Operating Income (Loss) | 11,327 | | | (10,410) | | | (18,294) | |
| Net gain on convertible debt derivatives | — | | | (1,197) | | | (11,994) | |
| Loss on debt extinguishment including debt finance charges and fees | 7,360 | | | 6,165 | | | — | |
| Interest expense | 28,499 | | | 29,076 | | | 28,336 | |
| Interest income | (93) | | | (429) | | | (534) | |
| Loss Before Income Taxes | (24,439) | | | (44,025) | | | (34,102) | |
| Income tax provision | 3,841 | | | 9,302 | | | 9,820 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Net Loss | $ | (28,280) | | | $ | (53,327) | | | $ | (43,922) | |
| | | | | |
| | | | | |
| | | | | |
| Net Loss per Share—Basic | $ | (0.83) | | | $ | (1.59) | | | $ | (1.33) | |
| Weighted Average Shares Outstanding—Basic | 34,266 | | | 33,594 | | | 33,124 | |
| | | | | |
| | | | | |
| | | | | |
| Net Loss per Share—Assuming Dilution | $ | (0.83) | | | $ | (1.59) | | | $ | (1.33) | |
| Weighted Average Shares Outstanding—Assuming Dilution | 34,375 | | | 33,642 | | | 33,543 | |
| | | | | |
| Net Loss | $ | (28,280) | | | $ | (53,327) | | | $ | (43,922) | |
| Other comprehensive income (loss): | | | | | |
| Foreign currency translation adjustments | 43,405 | | | (8,499) | | | (30,858) | |
| Defined benefit plans: | | | | | |
| Amortization of prior service costs and unrecognized losses | (375) | | | (596) | | | 4,949 | |
| | | | | |
| Deferred tax adjustment resulting from defined benefit plan activity | 55 | | | 48 | | | (51) | |
| Valuation reserve associated with defined benefit plan activity | (55) | | | (48) | | | 51 | |
| Current period gain (loss) on cash flow hedges | (825) | | | (571) | | | 1,894 | |
| Deferred tax benefit (loss) related to gain (loss) on cash flow hedges | 103 | | | 1 | | | (62) | |
| Other Comprehensive Income (Loss) | 42,308 | | | (9,665) | | | (24,077) | |
| Comprehensive Income (Loss) | $ | 14,028 | | | $ | (62,992) | | | $ | (67,999) | |
See notes to consolidated financial statements.
| | | | | | | | |
| | Financial Statements |
| | Balance Sheets |
| | |
INVACARE CORPORATION AND SUBSIDIARIES
Consolidated Balance Sheets
| | | | | | | | | | | |
| December 31,
2020 | | December 31,
2019 |
| Assets | (In thousands) |
| | | |
| Current Assets | | | |
| Cash and cash equivalents | $ | 105,298 | | | $ | 80,063 | |
| Trade receivables, net | 108,588 | | | 116,669 | |
| Installment receivables, net | 379 | | | 736 | |
| Inventories, net | 115,484 | | | 120,500 | |
| | | |
| Other current assets | 44,717 | | | 37,909 | |
| | | |
| Total Current Assets | 374,466 | | | 355,877 | |
| Other Assets | 5,925 | | | 4,216 | |
| Intangibles | 27,763 | | | 26,447 | |
| Property and Equipment, net | 56,243 | | | 46,607 | |
| Finance Lease Assets, net | 64,031 | | | 26,900 | |
| Operating Lease Assets, net | 15,092 | | | 18,676 | |
| Goodwill | 402,461 | | | 373,403 | |
| Total Assets | $ | 945,981 | | | $ | 852,126 | |
| Liabilities and Shareholders' Equity | | | |
| Current Liabilities | | | |
| Accounts payable | $ | 85,424 | | | $ | 88,003 | |
| Accrued expenses | 126,273 | | | 120,947 | |
| Current taxes payable | 3,359 | | | 345 | |
| Current portion of long-term debt | 5,612 | | | 58 | |
| Current portion of finance lease obligations | 3,405 | | | 2,514 | |
| Current portion of operating lease obligations | 6,313 | | | 6,790 | |
| | | |
| Total Current Liabilities | 230,386 | | | 218,657 | |
| Long-Term Debt | 239,441 | | | 219,464 | |
| Long-Term Obligations - Finance Leases | 63,137 | | | 26,480 | |
| Long-Term Obligations - Operating Leases | 8,697 | | | 12,060 | |
| Other Long-Term Obligations | 70,474 | | | 66,949 | |
| Shareholders' Equity | | | |
Preferred Shares (Authorized 300 shares; none outstanding) | — | | | — | |
Common Shares (Authorized 150,000 shares; 38,613 and 37,609 issued and outstanding at December 31, 2020 and December 31, 2019, respectively)—no par | 9,816 | | | 9,588 | |
Class B Common Shares (Authorized 12,000 shares; 4 and 6 issued and outstanding at December 31, 2020 and December 31, 2019, respectively)—no par | 2 | | | 2 | |
| Additional paid-in-capital | 326,088 | | | 312,650 | |
| Retained earnings | 58,538 | | | 87,475 | |
| Accumulated other comprehensive income | 45,436 | | | 3,128 | |
Treasury Shares (4,184 and 3,953 shares in 2020 and 2019, respectively) | (106,034) | | | (104,327) | |
| Total Shareholders' Equity | 333,846 | | | 308,516 | |
| Total Liabilities and Shareholders' Equity | $ | 945,981 | | | $ | 852,126 | |
See notes to consolidated financial statements.
| | | | | | | | |
| Financial Statements | | |
| Consolidated Statements of Cash Flows | | |
| | |
INVACARE CORPORATION AND SUBSIDIARIES
Consolidated Statements of Cash Flows
| | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| Operating Activities | (In thousands) |
| Net loss | $ | (28,280) | | | $ | (53,327) | | | $ | (43,922) | |
| Adjustments to reconcile net earnings to net cash used by operating activities: | | | | | |
| Gain on sale of business | (9,790) | | | — | | | — | |
| | | | | |
| Depreciation and amortization | 14,317 | | | 15,563 | | | 15,556 | |
| Amortization operating lease right of use assets | 6,951 | | | 8,927 | | | — | |
| Provision for losses on trade and installment receivables | 427 | | | 955 | | | 2,029 | |
| Benefit for deferred income taxes | (2,192) | | | (830) | | | (2,800) | |
| Provision (benefit) for other deferred liabilities | 971 | | | 1,144 | | | (121) | |
| Provision for equity compensation | 8,645 | | | 11,110 | | | 5,283 | |
| Loss (gain) on disposals of property and equipment | (1,046) | | | 182 | | | 928 | |
| Loss on debt extinguishment including debt finance charges and associated fees | 7,360 | | | 6,165 | | | — | |
| Impairment of an intangible asset | — | | | 587 | | | 583 | |
| | | | | |
| Amortization of convertible debt discount and accretion of convertible debt | 11,487 | | | 12,325 | | | 11,608 | |
| Amortization of debt fees | 1,690 | | | 2,384 | | | 2,489 | |
| Net gain on convertible debt derivatives | — | | | (1,197) | | | (11,994) | |
| Changes in operating assets and liabilities: | | | | | |
| Trade receivables | 7,692 | | | 1,474 | | | (666) | |
| Installment sales contracts, net | (481) | | | 434 | | | (603) | |
| Inventories, net | 8,955 | | | 6,466 | | | (11,497) | |
| Other current assets | (5,313) | | | (7,314) | | | (873) | |
| Accounts payable | (2,359) | | | (3,603) | | | 4,505 | |
| Accrued expenses | 1,713 | | | 2,276 | | | (17,158) | |
| Other long-term liabilities | 1,170 | | | (978) | | | 230 | |
| Net Cash Provided (Used) by Operating Activities | 21,917 | | | 2,743 | | | (46,423) | |
| Investing Activities | | | | | |
| Purchases of property and equipment | (22,304) | | | (10,874) | | | (9,823) | |
| Proceeds from sale of property and equipment | 396 | | | 73 | | | 40 | |
| Advance payment from sale of property | — | | | — | | | 3,524 | |
| Proceeds from sale of business | 14,563 | | | — | | | — | |
| | | | | |
| Change in other long-term assets | (27) | | | (781) | | | (116) | |
| Other | (2,175) | | | (32) | | | 12 | |
| Net Cash Used by Investing Activities | (9,547) | | | (11,614) | | | (6,363) | |
| Financing Activities | | | | | |
| Proceeds from revolving lines of credit and long-term borrowings | 86,081 | | | — | | | — | |
| Repurchases of convertible debt, payments on revolving lines of credit and finance leases | (70,603) | | | (17,196) | | | (1,493) | |
| Proceeds from exercise of stock options | — | | | — | | | 2,626 | |
| Payment of financing costs | (1,505) | | | (1,278) | | | — | |
| Payment of dividends | (414) | | | (1,645) | | | (1,630) | |
| | | | | |
| Payments to debt holders | (5,593) | | | (6,928) | | | — | |
| Purchases of treasury shares | (1,707) | | | (894) | | | (2,427) | |
| Net Cash Provided (Used) by Financing Activities | 6,259 | | | (27,941) | | | (2,924) | |
| Effect of exchange rate changes on cash | 6,606 | | | (32) | | | (3,911) | |
| Increase (decrease) in cash and cash equivalents | 25,235 | | | (36,844) | | | (59,621) | |
| Cash and cash equivalents at beginning of year | 80,063 | | | 116,907 | | | 176,528 | |
| Cash and cash equivalents at end of year | $ | 105,298 | | | $ | 80,063 | | | $ | 116,907 | |
See notes to consolidated financial statements.
| | | | | | | | |
| | Financial Statements |
| | Consolidated Statement of Shareholders' Equity |
| | |
INVACARE CORPORATION AND SUBSIDIARIES
Consolidated Statement of Shareholders' Equity
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands) | Common
Shares | | Class B
Shares | | Additional
Paid-in-
Capital | | Retained
Earnings | | Accumulated Other
Comprehensive
Earnings | | Treasury
Shares | | Total | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| January 1, 2018 Balance | $ | 9,304 | | | $ | 2 | | | $ | 290,125 | | | $ | 187,999 | | | $ | 36,870 | | | $ | (101,006) | | | $ | 423,294 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of share options | 46 | | | — | | | 2,580 | | | — | | | — | | | (919) | | | 1,707 | | | | | | | | | |
| Performance awards | — | | | — | | | 777 | | | — | | | — | | | — | | | 777 | | | | | | | | | |
| Non-qualified share options | — | | | — | | | 201 | | | — | | | — | | | — | | | 201 | | | | | | | | | |
| Restricted share awards | 69 | | | — | | | 4,236 | | | — | | | — | | | (1,508) | | | 2,797 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | (43,922) | | | — | | | — | | | (43,922) | | | | | | | | | |
| Foreign currency translation adjustments | — | | | — | | | — | | | — | | | (30,858) | | | — | | | (30,858) | | | | | | | | | |
| Unrealized gain on cash flow hedges | — | | | — | | | — | | | — | | | 1,832 | | | — | | | 1,832 | | | | | | | | | |
| Defined benefit plans: Amortization of prior service costs and unrecognized losses and credits | — | | | — | | | — | | | — | | | 4,949 | | | — | | | 4,949 | | | | | | | | | |
| Total comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | (67,999) | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Dividends | — | | | — | | | — | | | (1,630) | | | — | | | — | | | (1,630) | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| December 31, 2018 Balance | 9,419 | | | 2 | | | 297,919 | | | 142,447 | | | 12,793 | | | (103,433) | | | 359,147 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Performance awards | 29 | | | — | | | 4,370 | | | — | | | — | | | (348) | | | 4,051 | | | | | | | | | |
| Non-qualified share options | — | | | — | | | 1,939 | | | — | | | — | | | — | | | 1,939 | | | | | | | | | |
| Restricted share awards | 140 | | | — | | | 4,632 | | | — | | | — | | | (546) | | | 4,226 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | (53,327) | | | — | | | — | | | (53,327) | | | | | | | | | |
| Foreign currency translation adjustments | — | | | — | | | — | | | — | | | (8,499) | | | — | | | (8,499) | | | | | | | | | |
| Unrealized loss on cash flow hedges | — | | | — | | | — | | | — | | | (570) | | | — | | | (570) | | | | | | | | | |
| Defined benefit plans: Amortization of prior service costs and unrecognized losses and credits | — | | | — | | | — | | | — | | | (596) | | | — | | | (596) | | | | | | | | | |
| Total comprehensive loss | — | | | — | | | — | | | — | | | — | | | — | | | (62,992) | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Convertible debt derivative adjustments | | | | | (220) | | | | | | | | | (220) | | | | | | | | | |
| Exchange of convertible notes | | | | | 4,010 | | | | | | | | | 4,010 | | | | | | | | | |
| Dividends | — | | | — | | | — | | | (1,645) | | | — | | | — | | | (1,645) | | | | | | | | | |
| December 31, 2019 Balance | 9,588 | | | 2 | | | 312,650 | | | 87,475 | | | 3,128 | | | (104,327) | | | 308,516 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Performance awards | 91 | | | — | | | 3,222 | | | — | | | — | | | (1,123) | | | 2,190 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Restricted share awards | 137 | | | — | | | 5,195 | | | — | | | — | | | (584) | | | 4,748 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | — | | | — | | | — | | | (28,280) | | | — | | | — | | | (28,280) | | | | | | | | | |
| Foreign currency translation adjustments | — | | | — | | | — | | | — | | | 43,405 | | | — | | | 43,405 | | | | | | | | | |
| Unrealized loss on cash flow hedges | — | | | — | | | — | | | — | | | (722) | | | — | | | (722) | | | | | | | | | |
| Defined benefit plans: Amortization of prior service costs and unrecognized losses and credits | — | | | — | | | — | | | — | | | (375) | | | — | | | (375) | | | | | | | | | |
| Total comprehensive income | — | | | — | | | — | | | — | | | — | | | — | | | 14,028 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Exchange of convertible notes | | | | | 5,021 | | | | | | | | | 5,021 | | | | | | | | | |
| Dividends | — | | | — | | | — | | | (414) | | | — | | | — | | | (414) | | | | | | | | | |
| Adoption of credit loss standard | | | | | | | (243) | | | | | | | (243) | | | | | | | | | |
| December 31, 2020 Balance | 9,816 | | | 2 | | | 326,088 | | | 58,538 | | | 45,436 | | | (106,034) | | | 333,846 | | | | | | | | | |
See notes to consolidated financial statements.
| | | | | | | | |
| Notes to Financial Statements | | |
| Accounting Policies | | |
| | |
Accounting Policies
Nature of Operations: Invacare Corporation is a leading manufacturer and distributor of medical equipment used in the home based upon the company's distribution channels, breadth of product line and net sales. The company designs, manufactures and distributes an extensive line of health care products for the non-acute care environment, including the home health care, retail and continuing care markets.
Principles of Consolidation: The consolidated financial statements include the accounts of the company and its wholly owned subsidiaries and include all adjustments, which were of a normal recurring nature, necessary to present fairly the financial position of the company as of December 31, 2020 and the results of its operations and changes in its cash flow for the years ended December 31, 2020, 2019 and 2018, respectively. Certain foreign subsidiaries, represented by the European segment, are consolidated using a November 30 fiscal year end to meet filing deadlines. No material subsequent events have occurred related to the European segment, which would require disclosure or adjustment to the company's financial statements. All significant intercompany transactions are eliminated.
Use of Estimates: The consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States, which require management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results may differ from these estimates.
Cash and Cash Equivalents: The company's policy is to treat investments that are readily convertible to cash and with maturities so near that there is little risk of changes in value due to changes in interest rates as cash and cash equivalents. Cash and cash equivalents are carried at cost, which approximates fair value.
Accounts Receivable: The company records accounts receivable when control of the product or service transfers to its unaffiliated customers, risk of loss is passed and title is transferred. The estimated allowance for uncollectible amounts is based primarily on management's evaluation of the financial condition of specific customers. The company records accounts receivable reserves for amounts that may become uncollectible in the future. The company writes off accounts receivable when it becomes apparent, based upon customer circumstances, that such amounts will not be collected and legal remedies are exhausted.
Reserves for customer bonus and cash discounts are recorded as a reduction in revenue and netted against gross
accounts receivable. Customer rebates in excess of a given customer's accounts receivable balance are classified in Accrued Expenses. Customer rebates and cash discounts are estimated based on the most likely amount principal as well as historical experience and anticipated performance. In addition, customers have the right to return product within the company's normal terms policy, and as such the company estimates the expected returns based on an analysis of historical experience and adjusts revenue accordingly.
Inventories: Inventories are stated at the lower of cost or net realizable value with cost determined by the first-in, first-out method. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. Finished goods and work in process inventories include material, labor and manufacturing overhead costs. Inventories have been reduced by an allowance for excess and obsolete inventories. The estimated allowance is based on management's review of inventories on hand compared to estimated future usage and sales.
Property and Equipment: Property and equipment are stated based on cost. The company principally uses the straight-line method of depreciation for financial reporting purposes based on annual rates sufficient to amortize the cost of the assets over their estimated useful lives. Machinery and equipment, internal use software as well as furniture and fixtures are generally depreciated using lives of 3 to 10 years, while buildings and improvements are depreciated using lives of 5 to 40 years. Accelerated methods of depreciation are used for federal income tax purposes. Expenditures for maintenance and repairs are charged to expense as incurred. Amortization of assets under finance leases is included in depreciation expense.
Long-lived assets are assessed for impairment whenever events or changes in circumstances indicate the carrying amount may not be recoverable. An asset would be considered impaired when the future net undiscounted cash flows generated by the asset or asset group are less than its carrying value. An impairment loss would be recognized based on the amount by which the carrying value of the asset exceeds its fair value.
Goodwill and Other Intangibles: In accordance with Intangibles—Goodwill and Other, ASC 350, goodwill and indefinite lived intangibles are subject impairment. The company completes its annual impairment assessment in the fourth quarter of each year or whenever events or changes in circumstances indicate the carrying value could be below a reporting unit's fair value. For purposes of the
| | | | | | | | |
| | Notes to Financial Statements |
| | Accounting Policies |
| | |
goodwill impairment assessment, the fair value of each reporting unit is estimated using an income approach by forecasting cash flows and discounting those cash flows using an appropriate weighted average cost of capital (WACC) as well as considering market and cost approaches as appropriate. The fair values are then compared to the carrying value of the net assets of each reporting unit. Intangible assets are also assessed for impairment by estimating forecasted cash flows and discounting those cash flows as needed to calculate impairment amounts. During 2020, 2019 and 2018 the company recognized an intangible asset impairment charge of $0, $587,000 and $583,000 respectively, related to an indefinite-lived trademark recorded in the Institutional Products Group reporting unit which is part of the North America segment.
Accrued Warranty Cost: Generally, the company's products are covered by assurance-type warranties against defects in material and workmanship for various periods depending on the product from the date of sale to the customer. Certain components carry a lifetime warranty. In addition, the company has sold extended warranties that, while immaterial, require the company to defer the revenue associated with those warranties until earned. A provision for estimated warranty cost is recorded at the time of sale based upon actual experience. The company continuously assesses the adequacy of its product warranty accrual and makes adjustments as needed. Historical analysis is primarily used to determine the company's warranty reserves. Claims history is reviewed and provisions are adjusted as needed. However, the company does consider other events, such as a product recall, which could necessitate additional warranty reserve provisions. Refer to Accrued Expenses in the Notes to the Consolidated Financial Statements for a reconciliation of the changes in the warranty accrual.
Product Liability Cost: The company is self-insured in North America for product liability exposures through its captive insurance company, Invatection Insurance Company, which currently has a policy year that runs from September 1 to August 31 and insures annual policy losses up to $10,000,000 per occurrence and $13,000,000 in the aggregate. The company also has additional layers of external insurance coverage, related to all lines of insurance coverage, insuring up to $75,000,000 in aggregate losses per policy year arising from individual claims anywhere in the world that exceed the captive insurance company policy limits or the limits of the company's per country foreign liability limits, as applicable. There can be no assurance that Invacare's current insurance levels will continue to be adequate or available at affordable rates.
Product liability reserves are recorded for individual claims based upon historical experience, industry expertise
and other indicators. Additional reserves, in excess of the specific individual case reserves, are provided for incurred but not reported claims based upon actuarial valuations at the time such valuations are conducted. Historical claims experience and other assumptions are taken into consideration by the company in estimating the ultimate reserves. For example, the actuarial analysis assumes that historical loss experience is an indicator of future experience, that the distribution of exposures by geographic area and nature of operations for ongoing operations is expected to be very similar to historical operations with no dramatic changes and that the government indices used to trend losses and exposures are appropriate. Estimates made are adjusted on a regular basis and can be impacted by actual loss awards and settlements on claims. While actuarial analysis is used to help determine adequate reserves, the company is responsible for the determination and recording of adequate reserves in accordance with accepted loss reserving standards and practices.
Revenue Recognition: The company recognizes revenues when control of the product or service is transferred to unaffiliated customers. Revenues from Contracts with Customers, ASC 606, provides guidance on the application of generally accepted accounting principles to revenue recognition issues. The company has concluded that its revenue recognition policy is appropriate and in accordance with GAAP under ASC 606.
All of the company's product-related contracts, and a portion related to services, have a single performance obligation, which is the promise to transfer an individual good or service, with revenue recognized at a point in time. Certain service-related contracts contain multiple performance obligations that require the company to allocate the transaction price to each performance obligation. For such contracts, the company allocates revenue to each performance obligation based on its relative standalone selling price at inception of the contract. The company determined the standalone selling price based on the expected cost-plus margin methodology. Revenue related to the service contracts with multiple performance obligations is recognized over time. To the extent performance obligations are satisfied over time, the company defers revenue recognition until the performance obligations are satisfied.
The determination of when and how much revenue to recognize can require the use of significant judgment. Revenue is recognized when obligations under the terms of a contract with the customer are satisfied; generally, this occurs with the transfer of control of the company's products and services to the customer.
Revenue is measured as the amount of consideration expected to be received in exchange for transferring the
| | | | | | | | |
| Notes to Financial Statements | | |
| Accounting Policies | | |
| | |
product or providing services. The amount of consideration received and recognized as revenue by the company can vary as a result of variable consideration terms included in the contracts such as customer rebates, cash discounts and return policies. Customer rebates and cash discounts are estimated based on the most likely amount principle and these estimates are based on historical experience and anticipated performance. Customers have the right to return product within the company's normal terms policy, and as such, the company estimates the expected returns based on an analysis of historical experience. The company adjusts its estimate of revenue at the earlier of when the most likely amount of consideration the company expects to receive changes or when the consideration becomes fixed. The company generally does not expect that there will be significant changes to its estimates of variable consideration (refer to Receivables in the Notes to the Consolidated Financial Statements include elsewhere in this report).
Depending on the terms of the contract, the company may defer recognizing a portion of the revenue at the end of a given period as the result of title transfer terms that are based upon delivery and or acceptance which align with transfer of control of the company's products to its customers.
Sales are made only to customers with whom the company believes collection is reasonably assured based upon a credit analysis, which may include obtaining a credit application, a signed security agreement, personal guarantee and/or a cross corporate guarantee depending on the credit history of the customer. Credit lines are established for new customers after an evaluation of their credit report and/or other relevant financial information. Existing credit lines are regularly reviewed and adjusted with consideration given to any outstanding past due amounts.
The company records distributed product sales gross as a principal since the company takes title to the products and has the risks of loss for collections, delivery and returns. The company's payment terms are for relatively short periods and thus do not contain any element of financing. Additionally, no contract costs are incurred that would require capitalization and amortization.
Sales, value added, and other taxes the company collects concurrent with revenue producing activities are excluded from revenue. Incidental items that are immaterial in the context of the contract are recognized as expense. Shipping and handling costs are included in cost of products sold.
The majority of the company's warranties are considered assurance-type warranties and continue to be recognized as expense when the products are sold (refer to
Current Liabilities in the Notes to the Consolidated Financial Statements include elsewhere in this report). These warranties cover against defects in material and workmanship for various periods depending on the product from the date of sale to the customer. Certain components carry a lifetime warranty. In addition, the company has sold extended warranties that, while immaterial, require the company to defer the revenue associated with those warranties until earned. A provision for estimated warranty cost is recorded at the time of sale based upon actual experience. The company continuously assesses the adequacy of its product warranty accruals and makes adjustments as needed. Historical analysis is primarily used to determine the company's warranty reserves. Claims history is reviewed and provisions are adjusted as needed. However, the company does consider other events, such as a product recall, which could require additional warranty reserve provisions. Refer to Accrued Expenses in the Notes to the Consolidated Financial Statements for a reconciliation of the changes in the warranty accrual. In addition, the company has sold extended warranties that, while immaterial, require the company to defer the revenue associated with those warranties until earned. The company has established procedures to appropriately defer such revenue.
Research and Development: Research and development costs are expensed as incurred and included in cost of products sold. The company's annual expenditures for product development and engineering were approximately $12,275,000, $15,836,000 and $17,377,000 for 2020, 2019 and 2018, respectively.
Advertising: Advertising costs are expensed as incurred and included in selling, general and administrative expenses. Advertising expenses amounted to $5,107,000, $7,871,000 and $10,109,000 for 2020, 2019 and 2018, respectively, the majority of which is incurred for advertising in the United States and Europe.
Income Taxes: The company uses the liability method in measuring the provision for income taxes and recognizing deferred tax assets and liabilities on the balance sheet. The liability method requires that deferred income taxes reflect the tax consequences of currently enacted rates for differences between the tax and financial reporting bases of assets and liabilities.
Value Added Taxes: The company operates internationally and is required to comply with value added tax (VAT) or goods and service tax (GST) regulations, particularly in Europe and Asia Pacific. VAT and GST are taxes on consumption in which the company pays tax on its purchases of goods and services and charges customers on the sale of product. The difference between billings to customers and payments on purchases is then remitted or received from the government as filings are due. The
| | | | | | | | |
| | Notes to Financial Statements |
| | Accounting Policies |
| | |
company records tax assets and liabilities related to these taxes and the balances in these accounts can vary significantly from period to period based on the timing of the underlying transactions.
Derivative Instruments: Derivatives and Hedging, ASC 815, requires companies to recognize all derivative instruments in the consolidated balance sheet as either assets or liabilities at fair value. The accounting for changes in fair value of a derivative is dependent upon whether or not the derivative has been designated and qualifies for hedge accounting treatment and the type of hedging relationship. For derivatives designated and qualifying as hedging instruments, the company must designate the hedging instrument, based upon the exposure being hedged, as a fair value hedge, cash flow hedge, or a hedge of a net investment in a foreign operation.
A majority of the company's derivative instruments are designated and qualify as cash flow hedges. Accordingly, the effective portion of the gain or loss on the derivative instrument is reported as a component of other comprehensive income and reclassified into earnings in the same period or periods during which the hedged transaction affects earnings. The remaining gain or loss on the derivative instrument in excess of the cumulative change in the fair value of the hedged item, if any, is recognized in current earnings during the period of change.
In 2016, the company issued $150,000,000 aggregate principal amount of 5.00% convertible senior notes due in 2021 and, in the second quarter of 2017, issued $120,000,000 aggregate principal amount of 4.50% convertible senior notes due 2022 (the “2021 Notes and 2022 Notes”). In connection with the offering of the 2021 Notes and 2022 Notes, the company entered into privately negotiated convertible note hedge transactions with certain financial institutions (the “option counterparties”). The convertible debt conversion liabilities and the convertible note hedges were accounted for as derivatives that were fair valued quarterly until the company obtained shareholder approval on May 16, 2019 to settle its convertible debt using cash or shares, which resulted in no longer accounting for the conversion liabilities and note hedges as derivatives. The fair value of the convertible debt conversion liabilities and the convertible note hedge assets were estimated using a lattice model incorporating the terms and conditions of the 2021 Notes and 2022 Notes and considering, for example, changes in the prices of the company's common stock, company stock price volatility, risk-free rates and changes in market rates. The valuations were, among other things, subject to changes in both the company's credit worthiness and the counter-parties to the instruments as well as change in general market conditions. The change in the fair value of the convertible note hedges and convertible debt conversion liabilities were recognized in net income (loss) for the respective period.
Foreign Currency Translation: The functional currency of the company's subsidiaries outside the United States is the applicable local currency. The assets and liabilities of the company's foreign subsidiaries are translated into U.S. dollars at year-end exchange rates. Revenues and expenses are translated at monthly average exchange rates. Gains and losses resulting from translation of balance sheet items are included in accumulated other comprehensive earnings.
Net Earnings Per Share: Basic earnings per share are computed based on the weighted-average number of Common Shares and Class B Common Shares outstanding during the year. Diluted earnings per share are computed based on the weighted-average number of Common Shares and Class B Common Shares outstanding plus the effects of dilutive stock options and awards outstanding during the year. For periods in which there was a net loss, loss per share assuming dilution utilized weighted average shares-basic.
Defined Benefit Plans: The company's benefit plans are accounted for in accordance with Compensation-Retirement Benefits, ASC 715 which requires plan sponsors to recognize the funded status of their defined benefit postretirement benefit plans in the consolidated balance sheet, measure the fair value of plan assets and benefit obligations as of the balance sheet date and to recognize changes in that funded status in the year in which the changes occur through comprehensive income.
Recent Accounting Pronouncements (Already Adopted):
In June 2016, the FASB issued ASU 2016-13, "Measurement of Credit Losses on Financial Statements." ASU 2016-13 requires a new credit loss standard for most financial assets and certain other instruments. For example, entities are required to use an "expected loss" model that will generally require earlier recognition of allowances for losses for trade receivables. The standard also requires additional disclosures, including disclosures regarding how an entity tracks credit quality. The company adopted ASU 2016-13, effective on January 1, 2020, which resulted in an increase for credit losses of $243,000 with the offsetting impact recorded to retained earnings.
In January 2017, the FASB issued ASU 2017-04, "Intangibles - Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment." The guidance in ASU 2017-04 eliminates the requirement to determine the fair value of individual assets and liabilities of a reporting unit to measure goodwill impairment. Under the amendments in the new ASU, goodwill impairment testing will be performed by comparing the fair value of the reporting unit with its carrying amount and recognizing
| | | | | | | | |
| Notes to Financial Statements | | |
| Accounting Policies | | |
| | |
an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value. The company adopted ASU 2017-04 as of January 1, 2020 with no impact to the company's financial statements upon adoption.
In December 2019, the FASB issued ASU 2019-12, "Income Taxes (Topic 740) - Simplifying the Accounting for Income Taxes," which simplifies the accounting for income taxes by removing certain exceptions to the general principles in Topic 740. ASU 2019-12 removes the following exceptions: 1) exception to the incremental approach for intraperiod tax allocation when there is a loss from continuing operations and income or a gain from other items (for example, discontinued operations or other comprehensive income), 2) exception to the requirement to recognize a deferred tax liability for equity method investments when a foreign subsidiary becomes an equity method investment, 3) exception to the ability not to recognize a deferred tax liability for a foreign subsidiary when a foreign subsidiary becomes a subsidiary and 4) the exception to the general methodology for calculating income taxes in an interim period when a year-to-date loss exceeds the anticipated loss for the year. The ASU also simplifies other areas of Topic 740 by clarifying and amending existing guidance. The amendments in the ASU will be applied using different approaches depending on what the specific amendments relate to. The company early adopted ASU 2019-12 on a prospective basis as of January 1, 2020 with no impact to the company's financial statements upon adoption.
Recent Accounting Pronouncements (Not Yet Adopted):
In August 2020, the FASB issued ASU 2020-06 "Debt with Conversion and Other Options" (Subtopic 470-20) and Derivatives and Hedging Contracts in Entity's Own Equity (Subtopic 815-40)", which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. ASU 2020-06 removes from U.S. GAAP the separation models for (1) convertible debt with a cash conversion feature (CCF) and (2) convertible instrument with a beneficial conversion feature (BCF). As a result, after adopting the ASU’s guidance, entities will not separately present in equity an embedded conversion feature in such debt. Instead, they will account for a convertible debt instrument wholly as debt, and for convertible preferred stock wholly as preferred stock (i.e., as a single unit of account), unless (1) a convertible instrument contains features that require bifurcation as a derivative under ASC 815 or (2) a convertible debt instrument was issued at a substantial premium. The guidance may be early adopted for fiscal years beginning after December 15, 2020, and interim periods within those
fiscal years. The company is currently reviewing the impact of the adoption of ASU 2020-06 but does not expect the adoption to have a material impact on the company's financial statements.
In March 2020, the FASB issued ASU 2020-04 "Reference Rate Reform (Topic 848): Facilitation of Effects of Reference Rate Reform on Financial Reporting," which is intended to provide temporary optional expedients an exceptions to the U.S. GAAP guidance on contract modifications and hedge accounting to ease the financial reporting burden related to the expected market transition from the London Interbank Offered Rate (LIBOR) and other interbank offered rates to alternative reference rates if certain criteria are met. The guidance may be adopted in any period prior to the guidance expiration on December 31, 2022. The company is currently reviewing the impact of the adoption of 2020-04 but does not expect the adoption to have a material impact on the company's financial statements.
| | | | | | | | |
| | Notes to Financial Statements |
| | Divested Businesses |
| | |
Divested Businesses
On March 7, 2020, the company completed the sale (the “Transaction”) of its subsidiary, Dynamic Controls, a New Zealand incorporated unlimited company (“Dynamic Controls”), to Allied Motion Christchurch Limited, a New Zealand limited company (the “Purchaser”), pursuant to a Securities Purchase Agreement among the company, Invacare Holdings New Zealand, a New Zealand incorporated unlimited company, and the Purchaser, dated March 6, 2020 (the “Purchase Agreement”). Dynamic Controls was a producer of electronic control systems for powered medical mobility devices, including systems incorporating the LiNX™ technology platform. Dynamic Controls was a component of the All Other Segment.
Dynamic Controls was a supplier of power mobility products and respiratory components to the company as well as supplying power mobility products to external customers. Sales in 2020 through the date of disposition were $5,331,000, including intercompany sales of $2,532,000, compared to sales for the full year of 2019 of $30,261,000, including intercompany sales of $13,087,000. Income before income taxes was approximately $445,000 in 2020, through the date of disposition, compared to $853,000 in 2019, inclusive of intercompany profits on sales to the company.
The transaction was the result of considering options for the products sold by Dynamic Controls which resulted in selling the business to a third-party which can provide access to further technological innovations to further differentiate the company’s power mobility products.
The gross proceeds from the Transaction were $14,563,000, net of taxes and expenses. The company realized a pre-tax gain of $9,790,000 and recorded expenses related to the sale of the business totaling $2,150,000, of which $1,796,000 have been paid as of December 31, 2020.
The Purchase Agreement contains customary indemnification obligations of each party with respect to breaches of their respective representations, warranties and covenants, and certain other specified matters, which are subject to certain exceptions, terms and limitations described further in the Purchase Agreement.
At the closing of the Transaction, the parties entered into a supply agreement pursuant to which Dynamic Controls will supply certain electronic components as required by the company for the five-year period following the Transaction, including ongoing supply and support of the LiNX™ electronic control system with informatics technology, continued contract manufacturing of certain electronic components for the company’s respiratory
products and continued infrastructure and applications support for the informatics solution for the company’s respiratory products. The estimated continued inflows and outflows following the disposal with the Purchaser are not expected to be material to the company.
The assets and liabilities of Dynamic Controls as of March 7, 2020 and December 31, 2019 consisted of the following (in thousands):
| | | | | | | | | | | |
| March 7, 2020 | | December 31, 2019 |
| Trade receivables, net | $ | 4,129 | | | $ | 1,804 | |
| Inventories, net | 3,082 | | | 3,008 | |
| Other assets | 855 | | | 933 | |
| Property and equipment, net | 600 | | | 707 | |
| Operating lease assets, net | 2,127 | | | 1,870 | |
| Total assets | $ | 10,793 | | | $ | 8,322 | |
| | | |
| Accounts payable | $ | 4,692 | | | $ | 4,501 | |
| Accrued expenses | 2,473 | | | 2,108 | |
| Current taxes payable | 41 | | | 92 | |
| Current portion of operating lease obligations | 366 | | | 393 | |
| Long-term obligations | 1,019 | | | 1,754 | |
| Total liabilities | $ | 8,591 | | | $ | 8,848 | |
Trade receivables as of March 7, 2020 includes receivables previously classified as intercompany related to product sold by Dynamic Controls to other Invacare entities.
Operations Held for Sale
Prior to 2020, the company recorded expense related to the sale of operations held for sale of $2,892,000 of which $2,377,000 has been paid as of December 31, 2020.
Discontinued Operations
From 2012 through 2014, the company sold three businesses which were classified as discontinued operations. Prior to 2020, the company had recorded cumulative expenses related to the sale of discontinued operations totaling $8,801,000, of which $8,405,000 were paid as of December 31, 2020.
| | | | | | | | |
| Notes to Financial Statements | | |
| Current Assets | | |
| | |
Current Assets
Receivables
Receivables as of December 31, 2020 and 2019 consist of the following (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| Accounts receivable, gross | $ | 131,055 | | | $ | 141,732 | |
| Customer rebate reserve | (10,730) | | | (13,922) | |
| Allowance for doubtful accounts | (4,031) | | | (4,804) | |
| Cash discount reserves | (7,320) | | | (5,326) | |
| Other, principally returns and allowances reserves | (386) | | | (1,011) | |
| | | |
| Accounts receivable, net | $ | 108,588 | | | $ | 116,669 | |
Reserves for customer rebates and cash discounts are recorded as a reduction in revenue and netted against gross accounts receivable. Customer rebates in excess of a given customer's accounts receivable balance are classified in Accrued Expenses. Customer rebates and cash discounts are estimated based on the most likely amount principle as well as historical experience and anticipated performance. In addition, customers have the right to return product within the company’s normal terms policy, and as such, the company estimates the expected returns based on an analysis of historical experience and adjusts revenue accordingly.
Accounts receivable are reduced by an allowance for amounts that may become uncollectible in the future. Substantially all the company’s receivables are due from health care, medical equipment providers and long-term care facilities predominantly located throughout the United States, Australia, Canada, New Zealand and Europe. A significant portion of products sold to providers, both foreign and domestic, are ultimately funded through government reimbursement programs such as Medicare and Medicaid in the U.S. As a consequence, changes in these programs can have an adverse impact on dealer liquidity and profitability.
The company adopted ASU 2016-13, "Measurement of Credit Losses on Financial Statements" on January 1, 2020. Accordingly, the company is now applying an "expected loss" model that will generally require earlier recognition of allowances for losses for trade receivables. In addition, the company expects more variability in its allowance for doubtful accounts as it previously provided for bad debts based on a specific reserve methodology while the new expected loss methodology requires companies to provide for estimated losses beginning at the time of sale. The adoption of the new standard resulted in an increase in credit losses and adjustment to retained
earnings of $243,000 which is reflected in the Consolidated Statement of Shareholders' Equity.
The company's approach is to separate its receivables into good-standing and collection receivables. Good-standing receivables are assigned to risk pools of high, medium and low. The risk pools are driven by the specifics associated with the geography of origination. Expected loss percentages are calculated and assigned to each risk pool, driven primarily by historical experience. The historical loss percentages are calculated for each risk pool and then judgmentally revised to consider current risk factors as well as consideration of the impact of forecasted events, as applicable. The expected loss percentages are then applied to receivables balances each period to determine the allowance for doubtful accounts.
In North America, excluding Canada, good-standing receivables are assigned to the low risk pool and assigned an expected loss percentage of 1.0% as these receivables are deemed to share the same risk profile and collections efforts are the same. Installment receivables in North America are characterized as collection receivables and thus reserves based on specific analysis of each customer. In Canada, good-standing receivables and installment receivables are deemed low risk and assigned a loss percentage of 0.2%.
In Europe, expected losses are determined by each location in each region. Most locations have a majority of their receivables assigned to the low risk pool, which has an average expected loss percentage of 0.4%. About half of the locations have a portion of their receivables assigned as medium risk with an average expected loss percentage of 1.5%. Only a few locations have any receivables characterized as high risk and the average credit loss percentage for those locations is 2.5%. Collection risk is generally low as payment terms in certain key markets, such as Germany, are immediate and in many locations the ultimate customer is the government.
In the Asia Pacific region, receivables are characterized as low risk, which have an average expected loss percentage of 0.3%. Historical losses are low in this region where the use of credit insurance is often customary.
| | | | | | | | |
| | Notes to Financial Statements |
| | Current Assets |
| | |
| | |
The movement in the trade receivables allowance for
doubtful accounts was as follows (in thousands):
| | | | | |
| 2020 |
Balance as of beginning of period | $ | 4,804 | |
| Current period provision | 361 | |
| Direct write-offs charged against the allowance | (1,134) | |
| Balance as of end of period | $ | 4,031 | |
The direct write-offs charged against the allowance includes the immaterial impact of the adoption of ASU 2016-13. The company did not make any material changes to the assignment of receivables to the different risk pools or to the expected loss reserves in the quarter. The company is monitoring the impacts of the COVID-19 pandemic and the possibility for an impact on collections, but to date this has not materially impacted 2020.
For collections receivables, the estimated allowance for uncollectible amounts is based primarily on management’s evaluation of the financial condition of each customer. In addition, as a result of the company's financing arrangement with DLL, a third-party financing company which the company has worked with since 2000, management monitors the collection status of these contracts in accordance with the company’s limited recourse obligations and provides amounts necessary for estimated losses in the allowance for doubtful accounts and establishes reserves for specific customers as needed.
The company writes off uncollectible trade accounts receivable after such receivables are moved to collection status and legal remedies are exhausted. Refer to Concentration of Credit Risk in the Notes to the Consolidated Financial Statements for a description of the financing arrangement. Long-term installment receivables are included in “Other Assets” on the consolidated balance sheet.
Upon adoption of ASU 2016-13, the company recorded a new contingent liability in the amount of $306,000 related to the contingent aspect of the company's guarantee associated with its arrangement with DLL. The contingent liability is recorded applying the same expected loss model used for the trade and installment receivables recorded on the company's books. Specifically, historical loss history is used to determine the expected loss percentage, which is then adjusted judgmentally to consider other factors, as needed.
The company’s U.S. customers electing to finance their purchases can do so using DLL. In addition, the company, prior to 2020, provided financing directly for its Canadian customers for which DLL is not an option, as DLL typically provides financing to Canadian customers
only on a limited basis. The installment receivables recorded on the books of the company represent a single portfolio segment of finance receivables to the independent provider channel and long-term care customers. The portfolio segment is comprised of two classes of receivables distinguished by geography and credit quality. The U.S. installment receivables are the first class and represent installment receivables repurchased from DLL because the customers were in default. Default with DLL is defined as a customer being delinquent by three payments. The Canadian installment receivables represent the second class of installment receivables which were originally financed by the company because third party financing was not available to the HME providers. The Canadian installment receivables were typically financed for twelve months and historically have had a very low risk of default.
The estimated allowance for uncollectible amounts and evaluation for both classes of installment receivables is based on the company’s quarterly review of the financial condition of each individual customer with the allowance for doubtful accounts adjusted accordingly. Installments are individually and not collectively reviewed. The company assesses the bad debt reserve levels based upon the status of the customer’s adherence to a legally negotiated payment schedule and the company’s ability to enforce judgments, liens, etc.
For purposes of granting or extending credit, the company utilizes a scoring model to generate a composite score that considers each customer’s consumer credit score and/or D&B credit rating, payment history, security collateral and time in business. Additional analysis is performed for most customers desiring credit greater than $250,000, which generally includes a detailed review of the customer’s financial statements as well as consideration of other factors such as exposure to changing reimbursement laws.
Interest income is recognized on installment receivables based on the terms of the installment agreements. Installment accounts are monitored and if a customer defaults on payments and is moved to collection, interest income is no longer recognized. Subsequent payments received once an account is put on non-accrual status are generally first applied to the principal balance and then to the interest. Accruing of interest on collection accounts would only be restarted if the account became current again.
All installment accounts are accounted for using the same methodology regardless of the duration of the installment agreements. When an account is placed in collection status, the company goes through a legal process for pursuing collection of outstanding amounts, the length of which typically approximates eighteen months. Any
| | | | | | | | |
| Notes to Financial Statements | | |
| Current Assets | | |
| | |
write-offs are made after the legal process has been completed.
Installment receivables as of December 31, 2020 and 2019 consist of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2020 | | 2019 |
| | Current | | Long-
Term | | Total | | Current | | Long-
Term | | Total |
| Installment receivables | $ | 704 | | | $ | 1,105 | | | $ | 1,809 | | | $ | 1,192 | | | $ | 1,257 | | | $ | 2,449 | |
| | | | | | | | | | | |
| Less: Unearned interest | — | | | — | | | — | | | (22) | | | — | | | (22) | |
| 704 | | | 1,105 | | | 1,809 | | | 1,170 | | | 1,257 | | | 2,427 | |
| Allowance for doubtful accounts | (325) | | | (162) | | | (487) | | | (434) | | | (1,080) | | | (1,514) | |
| Installment receivables, net | $ | 379 | | | $ | 943 | | | $ | 1,322 | | | $ | 736 | | | $ | 177 | | | $ | 913 | |
Installment receivables purchased from DLL during the twelve months ended December 31, 2020 increased the gross installment receivables balance by $346,000 during the year compared to $89,000 in 2019. No sales of installment receivables were made by the company during the year. There was no impact on the allowance for
doubtful accounts for installment receivables as a result of the adoption of ASU 2016-13 as the installment receivables in the U.S. are specifically reserved for by account and no adjustment was needed to the allowance for doubtful accounts for Canada or Asia Pacific installment receivables.
The movement in the installment receivables allowance for doubtful accounts was as follows (in thousands):
| | | | | | | | | | | |
| | 2020 | | 2019 |
| Balance as of beginning of period | $ | 1,514 | | | $ | 1,542 | |
| Current period provision | 66 | | | 479 | |
| Direct write-offs charged against the allowance | (1,093) | | | (507) | |
| Balance as of end of period | $ | 487 | | | $ | 1,514 | |
Installment receivables by class as of December 31, 2020 consist of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| | Total
Installment
Receivables | | Unpaid
Principal
Balance | | Related
Allowance for
Doubtful
Accounts | | Interest
Income
Recognized |
| U.S. | | | | | | | |
| Impaired installment receivables with a related allowance recorded | $ | 615 | | | $ | 615 | | | $ | 487 | | | $ | — | |
| Asia Pacific | | | | | | | |
| Non-impaired installment receivables with no related allowance recorded | 1,194 | | | 1,194 | | | — | | | — | |
| Canada | | | | | | | |
| Non-impaired installment receivables with no related allowance recorded | — | | | — | | | — | | | 29 | |
| Total | | | | | | | |
| Non-impaired installment receivables with no related allowance recorded | 1,194 | | | 1,194 | | | — | | | 29 | |
| Impaired installment receivables with a related allowance recorded | 615 | | | 615 | | | 487 | | | — | |
| Total installment receivables | $ | 1,809 | | | $ | 1,809 | | | $ | 487 | | | $ | 29 | |
| | | | | | | | |
| | Notes to Financial Statements |
| | Current Assets |
| | |
| | |
Installment receivables by class as of December 31, 2019 consist of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| | Total
Installment
Receivables | | Unpaid
Principal
Balance | | Related
Allowance for
Doubtful
Accounts | | Interest
Income
Recognized |
| U.S. | | | | | | | |
| Impaired installment receivables with a related allowance recorded | $ | 1,762 | | | $ | 1,762 | | | $ | 1,497 | | | $ | — | |
| Canada | | | | | | | |
| Non-impaired installment receivables with no related allowance recorded | 670 | | | 648 | | | — | | | 92 | |
| Impaired installment receivables with a related allowance recorded | 17 | | | 17 | | | 17 | | | — | |
| Total Canadian installment receivables | 687 | | | 665 | | | 17 | | | 92 | |
| Total | | | | | | | |
| Non-impaired installment receivables with no related allowance recorded | 670 | | | 648 | | | — | | | 92 | |
| Impaired installment receivables with a related allowance recorded | 1,779 | | | 1,779 | | | 1,514 | | | — | |
| Total installment receivables | $ | 2,449 | | | $ | 2,427 | | | $ | 1,514 | | | $ | 92 | |
Installment receivables with a related allowance recorded as noted in the table above represent those installment receivables on a non-accrual basis. As of December 31, 2020, the company had no U.S. installment receivables past due of 90 days or more for which the company is still accruing interest. Individually, all U.S.
installment receivables are assigned a specific allowance for doubtful accounts based on management's review when the company does not expect to receive both the contractual principal and interest payments as specified in the loan agreement.
The aging of the company's installment receivables was as follows as of December 31, 2020 and 2019 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 | | December 31, 2019 |
| Total | | U.S. | | Asia Pacific | | Total | | U.S. | | Canada |
| Current | $ | 1,194 | | | $ | — | | | $ | 1,194 | | | $ | 659 | | | $ | — | | | $ | 659 | |
| 0-30 days past due | — | | | — | | | — | | | 2 | | | — | | | 2 | |
| 31-60 days past due | — | | | — | | | — | | | 4 | | | — | | | 4 | |
| 61-90 days past due | — | | | — | | | — | | | — | | | — | | | — | |
| 90+ days past due | 615 | | | 615 | | | — | | | 1,784 | | | 1,762 | | | 22 | |
| $ | 1,809 | | | $ | 615 | | | $ | 1,194 | | | $ | 2,449 | | | $ | 1,762 | | | $ | 687 | |
| | | | | | | | |
| Notes to Financial Statements | | |
| Current Assets | | |
| | |
Inventories
Inventories as of December 31, 2020 and 2019 consist of the following (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| Finished goods | $ | 55,264 | | | $ | 54,064 | |
| Raw materials | 51,174 | | | 54,638 | |
| Work in process | 9,046 | | | 11,798 | |
| Inventories, net | $ | 115,484 | | | $ | 120,500 | |
Other current assets as of December 31, 2020 and 2019 consist of the following (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| Tax receivables principally value added taxes | $ | 22,500 | | | $ | 16,049 | |
| | | |
| Prepaid insurance | 3,963 | | | 2,918 | |
| Receivable due from information technology provider | 2,995 | | | 6,262 | |
| Prepaid inventory and freight | 2,700 | | | 684 | |
| Recoverable income taxes | 2,182 | | | 297 | |
| Derivatives (foreign currency forward contracts) | 1,321 | | | 838 | |
| Service contracts | 633 | | | 2,013 | |
| Prepaid debt fees | 208 | | | 207 | |
| Prepaid social charges | 43 | | | 1,216 | |
| Prepaid and other current assets | 8,172 | | | 7,425 | |
| Other Current Assets | $ | 44,717 | | | $ | 37,909 | |
In the fourth quarter of 2019, the company entered into an agreement to outsource substantially all of the company’s information technology ("IT") business service activities, including, among other things, support, rationalization and upgrading of the company’s legacy information technology systems and implementation of a global enterprise resource planning system. The agreement provides for reimbursement by the IT provider of IT expenses incurred by the company which are shown as Receivable due from IT provider above. The amount of pass-through charges will diminish as IT expenses are recorded directly by the IT provider. In addition, a corresponding current payable is due to the IT provider. Refer to "Accrued Expenses" in the notes to the Consolidated Financial Statements included elsewhere in this report.
| | | | | | | | |
| | Notes to Financial Statements |
| | Long-Term Assets |
| | |
Long-Term Assets
Other long-term assets as of December 31, 2020 and 2019 consist of the following (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| | | |
| | | |
| Cash surrender value of life insurance policies | 2,327 | | | 2,124 | |
| Deferred income taxes | 2,048 | | | 928 | |
| Installment receivables | 943 | | | 177 | |
| Deferred financing fees | 411 | | | 602 | |
| Investments | 85 | | | 85 | |
| Other | 111 | | | 300 | |
| Other Long-Term Assets | $ | 5,925 | | | $ | 4,216 | |
Property and Equipment
Property and equipment as of December 31, 2020 and 2019 consist of the following (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| Machinery and equipment | $ | 294,045 | | | $ | 296,078 | |
| Land, buildings and improvements | 28,509 | | | 33,054 | |
| Furniture and fixtures | 10,001 | | | 9,898 | |
| Leasehold improvements | 8,194 | | | 9,023 | |
| Capitalized software | 17,527 | | | 3,509 | |
| Property and Equipment, gross | 358,276 | | | 351,562 | |
| Accumulated depreciation | (302,033) | | | (304,955) | |
| Property and Equipment, net | $ | 56,243 | | | $ | 46,607 | |
Machinery and equipment includes demonstration units placed in provider locations which are depreciated to their estimated recoverable values over their estimated useful lives.
In the fourth quarter of 2019, the company initiated the first stage of an Enterprise Resource Planning ("ERP") software implementation. As a result of the initiation of the ERP project, the company capitalized certain costs in accordance with ASC 350 as shown in capitalized software above.
In the third quarter of 2018, the company agreed to sell its Isny, Germany location with a net book value at the signing of the agreement of approximately $2,900,000, which was included in Land, buildings and improvements in the table above as of December 31, 2019. In accordance with the agreement, control transferred to the buyer in April 2020. The company recorded a gain on the transaction of $971,000 in 2020.
| | | | | | | | |
| Notes to Financial Statements | | |
| Long-Term Assets | | |
| | |
The carrying amount of goodwill by reporting unit is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Institutional
Products Group | | Europe | | Consolidated |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Balance at December 31, 2018 | $ | 27,377 | | | $ | 353,896 | | | $ | 381,273 | |
| Foreign currency translation adjustments | 785 | | | (8,655) | | | (7,870) | |
| Balance at December 31, 2019 | 28,162 | | | 345,241 | | | 373,403 | |
| Foreign currency translation adjustments | 323 | | | 28,735 | | | 29,058 | |
| Balance at December 31, 2020 | $ | 28,485 | | | $ | 373,976 | | | $ | 402,461 | |
In accordance with Intangibles—Goodwill and Other, ASC 350, goodwill is assessed for impairment. The company first estimates the fair value of each reporting unit and compares the calculated fair value to the carrying value of each reporting unit. A reporting unit is defined as an operating segment or one level below. The company has determined that its reporting units are North America / HME, Europe, Institutional Products Group and Asia Pacific. North America/HME and Asia Pacific Reporting units have no goodwill.
In consideration of the negative impact of the pandemic on the global economy, the company performed an interim test of goodwill for impairment in the first quarter of 2020 and no impairment was recorded. For the second and third quarters of 2020, the company concluded that the facts and circumstances compared to the first quarter of 2020 review had not changed materially.
The company completes its annual impairment assessment in the fourth quarter of each year or whenever events or changes in circumstances indicate the carrying value could be below a reporting unit's fair value. The fair values of the company's reporting units were calculated using inputs that are not observable in the market and included management's own estimates regarding the assumptions that market participants would use and thus these inputs are deemed Level III inputs in regard to the fair value hierarchy. To calculate the fair values of the reporting units, the company utilizes a discounted cash flow method model in which the company forecasts income statement and balance sheet amounts based on assumptions regarding projected sales growth, operating income, inventory turns, days' sales outstanding, etc. to forecast future cash flows. The projected operating income used has a significant impact upon the discounted cash flow methodology utilized in the company's annual impairment assessment as lower projected operating income would result in lower fair value estimates. The cash flows are discounted using a weighted average cost of capital discount rate where the cost of debt is based on quoted rates for 20-year debt of potential acquirer companies of similar credit risk and the cost of equity is based upon the 20-year treasury rate for the risk-free rate, a
market risk premium, the industry average beta and a small cap stock adjustment. The assumptions used are based on a market participant's point of view and yielded a discount rate of 11.27% in 2020 for the company's annual impairment analysis for the reporting units with goodwill compared to 11.88% in 2019 and 12.41% in 2018. The WACC used has a significant impact upon the discounted cash flow methodology utilized in the company's annual impairment assessment as a higher WACC would decrease the fair value estimates.
The company also utilizes an Enterprise Value (EV) to Earnings Before Interest, Taxes, Depreciation and Amortization (EBITDA) Method to compute the fair value of its reporting units which considers potential acquirers and their EV to EBITDA multiples adjusted by an estimated premium. While more weight is given to the discounted cash flow method, the EV to EBITDA Method does provide corroborative evidence of the reasonableness of the discounted cash flow method results.
While there was no impairment in 2020 related to goodwill for the Europe or Institutional Products Group reporting units, a future potential impairment is possible for these reporting units should actual results differ materially from forecasted results used in the valuation analysis. Furthermore, the company's annual valuation of goodwill can differ materially if financial projections or the market inputs used to determine the WACC change significantly. For instance, higher interest rates or greater stock price volatility would increase the WACC and thus increase the chance of impairment. In consideration of this potential, the company assessed the results if the discount rate used were 100 basis points higher for the 2020 impairment analysis and determined that there still would not be an indicator of potential impairment for the Europe or Institutional Products Group reporting units. In addition, business changes impacting the company's assessment of reporting units could also have a material impact on impairment assessment results.
As part of the company's assessment of goodwill for impairment, the company also considers the potential for impairment of any intangible assets other long-lived assets.
| | | | | | | | |
| | Notes to Financial Statements |
| | Long-Term Assets |
| | |
Refer to Other Long-Term Assets, Property and Equipment and Intangibles in the Notes to the Consolidated Financial Statements for a description of any other asset impairments.
The change in goodwill from December 31, 2019 to December 31, 2020 was due to foreign currency translation.
| | | | | | | | |
| Notes to Financial Statements | | |
| Long-Term Assets | | |
| | |
The company's intangibles consist of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | December 31, 2019 |
| | Historical
Cost | | Accumulated
Amortization | | Historical
Cost | | Accumulated
Amortization |
| Customer lists | $ | 54,502 | | | $ | 54,502 | | | $ | 51,108 | | | $ | 51,108 | |
| Trademarks | 25,112 | | | — | | | 23,479 | | | — | |
| Developed technology | $ | 7,924 | | | $ | 7,204 | | | $ | 7,483 | | | $ | 6,642 | |
| Patents | 5,556 | | | 5,556 | | | 5,521 | | | 5,521 | |
| License agreements | 2,899 | | | 979 | | | 2,884 | | | 770 | |
| Other | 1,162 | | | 1,151 | | | 1,163 | | | 1,150 | |
| Intangibles | $ | 97,155 | | | $ | 69,392 | | | $ | 91,638 | | | $ | 65,191 | |
All of the company's intangible assets have been assigned definite lives and continue to be amortized over their useful lives, except for trademarks shown above, which have indefinite lives.
The changes in intangible asset balances reflected on the balance sheet from December 31, 2019 to December 31, 2020 were the result of foreign currency translation on historical cost and accumulated amortization.
The company evaluates the carrying value of definite-lived assets annually in the fourth quarter and whenever events or circumstances indicate possible impairment. In consideration of the negative impact of the pandemic on the global economy, the company performed an assessment of intangible assets for impairment in the first quarter of 2020 and concluded there was no impairment to be recorded. For the second and third quarter of 2020, the company concluded that the facts and circumstances compared to the first quarter of 2020 had not changed materially. For the fourth quarter of 2020, the company concluded there was no impairment to be recorded. Definite-lived assets are determined to be impaired if the future undiscounted cash flows expected to be generated by the asset are less than the carrying value. Actual impairment amounts for definite-lived assets are then calculated using a discounted cash flow calculation.
Any impairment for indefinite-lived intangible assets is calculated as the difference between the future discounted cash flows expected to be generated by the asset less than the carrying value for the asset. The company evaluated indefinite-lived intangible assets in the fourth quarter of 2020 and concluded there was no impairment to be recorded.
During 2019 and 2018, the company recognized an intangible asset impairment charge in the Institutional Products Group reporting unit, which is part of the North America segment, of $587,000 ($435,000 after-tax) and $583,000 ($431,000 after-tax) respectively, related to a
trademark with an indefinite life. The fair value of the trademark was calculated using a relief from royalty payment methodology which requires applying an estimated market royalty rate to forecasted net sales and discounting the resulting cash flows to determine fair value. The fair values of the company's intangible assets were calculated using inputs that are not observable in the market and included management's own estimates regarding the assumptions that market participants would use and thus these inputs are deemed Level III inputs in regard to the fair value hierarchy.
Amortization expense related to intangible assets was $377,000, $1,827,000 and $2,218,000 for 2020, 2019 and 2018, respectively. Amortization expense for 2019 and 2018 includes impairments. Estimated amortization expense for each of the next five years is expected to be $402,000 for 2021, $402,000 in 2022, $401,000 in 2023, $369,000 in 2024 and $213,000 in 2025. Amortized intangible assets are being amortized on a straight-line basis over remaining lives of 4 to 9 years with a weighted average remaining life of approximately 7.6 years.
| | | | | | | | |
Table of Contents | | Notes to Financial Statements |
| | Current Liabilities |
| | |
Current Liabilities
Accrued Expenses
Accrued expenses as of December 31, 2020 and 2019 consisted of accruals for the following (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| Salaries and wages | $ | 34,029 | | | $ | 29,725 | |
| Taxes other than income taxes, primarily value added taxes | 32,710 | | | 22,194 | |
| Warranty | 10,991 | | | 11,626 | |
| Rebates | 8,644 | | | 10,743 | |
| Professional | 7,375 | | | 6,869 | |
| Severance | 6,249 | | | 7,023 | |
| IT service contracts | 3,799 | | | 6,125 | |
| Deferred revenue | 3,516 | | | 3,173 | |
| Freight | 3,190 | | | 3,744 | |
| Product liability, current portion | 2,453 | | | 2,736 | |
| Interest | 2,076 | | | 3,608 | |
| Derivatives (foreign currency forward exchange contracts) | 1,432 | | | 905 | |
| Insurance | 878 | | | 699 | |
| Rent | 585 | | | 415 | |
| Supplemental Executive Retirement Program liability Plan (SERP) | 391 | | | 391 | |
| Advance payment on sale of land & buildings | — | | | 3,471 | |
| IT licenses | — | | | 2,114 | |
| Other items, principally trade accruals | 7,955 | | | 5,386 | |
| Accrued Expenses | $ | 126,273 | | | $ | 120,947 | |
Generally, the company's products are covered by warranties against defects in material and workmanship for various periods depending on the product from the date of sales to the customer. Certain components carry a lifetime warranty. A provision for estimated warranty cost is recorded at the time of sale based upon actual experience. In addition, the company has sold extended warranties that, while immaterial, require the company to defer the revenue associated with those warranties until earned. The company has established procedures to appropriate defer such revenue. The company continuously assesses the adequacy of its product warranty accrual and makes adjustments as needed. Historical analysis is primarily used to determine the company's warranty reserves. Claims history is reviewed and provisions are adjusted as needed. However, the company does consider other events, such as a product field action and recalls, which could require additional warranty reserve provision.
The increase in liability for taxes other than income taxes, primarily value added taxes, is attributable to measures introduced by the U.S. and other jurisdictions to allow the deferment of payment of 2020 liabilities to 2021.
This benefit for the company is approximately $10,600,000 in 2020 and will be paid starting in 1Q21.
Accrued rebates relate to several volume incentive programs the company offers its customers. The company accounts for these rebates as a reduction of revenue when the products are sold. Rebates are netted against gross accounts receivables. If rebates are in excess of such receivables, they are then classified as accrued expenses.
In the fourth quarter of 2019, the company entered into an agreement with an IT provider to outsource substantially all of the company’s information technology business service activities, including, among other things, support, rationalization and upgrading of the company’s legacy information technology systems and implementation of a global ERP. Accrued expenses related to IT outsourcing are reflected in IT service contracts. Separately, the company entered into licenses for a new ERP system which are shown as IT licenses.
| | | | | | | | |
| Notes to Financial Statements | | Table of Contents |
| Current Liabilities | | |
| | |
The following is a reconciliation of the changes in accrued warranty costs for the reporting period (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| Balance as of January 1 | $ | 11,626 | | | $ | 16,353 | |
| Warranties provided during the period | 6,144 | | | 5,504 | |
| Settlements made during the period | (8,043) | | | (10,882) | |
| Changes in liability for pre-existing warranties during the period, including expirations | 1,264 | | | 651 | |
| Balance as of December 31 | $ | 10,991 | | | $ | 11,626 | |
Warranty reserves are subject to adjustment in future periods as new developments change the company's estimate of the total cost. In 2020, warranty expense includes a provision of $768,000 for a product recall which was related to a component on a respiratory product, recorded in the North America segment.
| | | | | | | | |
| | Notes to Financial Statements |
| | Long-Term Debt |
| | |
Long-Term Debt
Debt as of December 31, 2020 and 2019 consisted of the following (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| | | |
| Convertible senior notes at 5.00%, due in February 2021 | $ | 1,242 | | | $ | 56,628 | |
| Convertible senior notes at 4.50%, due in June 2022 | 73,869 | | | 101,815 | |
| Convertible senior notes Series I at 5.00%, due in November 2024 | 62,984 | | | 60,817 | |
| Convertible senior notes Series II at 5.00%, due in November 2024 | 64,919 | | | — | |
| Other obligations | 42,039 | | | 262 | |
| 245,053 | | | 219,522 | |
| Less current maturities of long-term debt | (5,612) | | | (58) | |
| Long-Term Debt | $ | 239,441 | | | $ | 219,464 | |
On September 30, 2015, the company entered into an Amended and Restated Revolving Credit and Security Agreement, which was subsequently amended (the "Credit Agreement") and which matures on January 16, 2024. The Credit Agreement was entered into by and among the company, certain of the company’s direct and indirect U.S. and Canadian subsidiaries and certain of the company’s European subsidiaries (together with the company, the “Borrowers”), certain other of the company’s direct and indirect U.S., Canadian and European subsidiaries (the “Guarantors”), and PNC Bank, National Association (“PNC”), JPMorgan Chase Bank, N.A., J.P. Morgan Europe Limited, KeyBank National Association, and Citizens Bank, National Association (the “Lenders”). PNC is the administrative agent (the “Administrative Agent”) and J.P. Morgan Europe Limited is the European agent (the “European Agent”) under the Credit Agreement. In connection with entering into the company's Credit Agreement, the company incurred fees which were capitalized and are being amortized as interest expense. As of December 31, 2020, debt fees yet to be amortized through January 2024 totaled $619,000.
The company had outstanding letters of credit of $7,752,000 and $8,011,000 as of December 31, 2020 and 2019, respectively. Outstanding letters of credit and other reserves impacting borrowing capacity were $7,616,000 and $8,827,000 as of December 31, 2020 and December 31, 2019, respectively. The company had outstanding borrowings of $20,000,000 under its U.S. and Canadian Credit Facility as of December 31, 2020. The company had outstanding borrowings of $7,636,000 (€6,400,000) under its French Credit Facility and $3,866,000 (£2,900,000) under its UK Credit Facility as of December 31, 2020, together referred to as the European Credit Facility. The company had no borrowings under the Credit Facilities as of December 31, 2019. For 2020 and 2019, the weighted average interest rate on all borrowings, excluding finance leases, was 4.6% and 4.78%, respectively.
U.S. and Canadian Borrowers Credit Facility
For the company's U.S. and Canadian Borrowers, the Credit Agreement provides for an asset-based-lending senior secured revolving credit facility which is secured by substantially all the company's U.S. and Canadian assets, other than real estate. The Credit Agreement provides the company and the other Borrowers with a credit facility in an aggregate principal amount of $60,000,000, subject to availability based on a borrowing base formula, under a senior secured revolving credit, letter of credit and swing line loan facility (the “U.S. and Canadian Credit Facility”). Up to $20,000,000 of the U.S. and Canadian Credit Facility will be available for issuance of letters of credit. The aggregate principal amount of the U.S. and Canadian Credit Facility may be increased by up to $25,000,000 to the extent requested by the company and agreed to by any Lender or new financial institution approved by the Administrative Agent.
The aggregate borrowing availability under the U.S. and Canadian Credit Facility is determined based on a borrowing base formula. The aggregate usage under the U.S. and Canadian Credit Facility may not exceed an amount equal to the sum of (a) 85% of eligible U.S. accounts receivable plus (b) the lesser of (i) 70% of eligible U.S. inventory and eligible foreign in-transit inventory and (ii) 85% of the net orderly liquidation value of eligible U.S. inventory and eligible foreign in-transit inventory (not to exceed $4,000,000), plus (c) the lesser of (i) 85% of the net orderly liquidation value of U.S. eligible machinery and equipment and (ii) $0 as of December 31, 2020 (subject to reduction as provided in the Credit Agreement), plus (d) 85% of eligible Canadian accounts receivable, plus (e) the lesser of (i) 70% of eligible Canadian inventory and (ii) 85% of the net orderly liquidation value of eligible Canadian inventory, less (f) swing loans outstanding under the U.S. and Canadian Credit Facility, less (g) letters of credit issued and undrawn under the U.S. and Canadian Credit Facility, less (h) a
| | | | | | | | |
| Notes to Financial Statements | | |
| Long-Term Debt | | |
| | |
$4,250,000 minimum availability reserve, less (i) other reserves required by the Administrative Agent, and in each case subject to the definitions and limitations in the Credit Agreement. As of December 31, 2020, the company was in compliance with all covenant requirements. As of December 31, 2020, the company had gross borrowing base of $43,874,000 and net borrowing availability of $24,508,000 under the U.S. and Canadian Credit Facility under the Credit Agreement, considering the minimum availability reserve, then-outstanding letters of credit, other reserves and the $7,500,000 dominion trigger amount described below. Borrowings under the U.S. and Canadian Credit Facility are secured by substantially all of the company's U.S. and Canadian assets, other than real estate.
Interest will accrue on outstanding indebtedness under the Credit Agreement at the LIBOR rate, plus a margin ranging from 2.25% to 2.75%, or at the alternate base rate, plus a margin ranging from 1.25% to 1.75%, as selected by the company. Borrowings under the U.S. and Canadian Credit Facility are subject to commitment fees of 0.25% or 0.375% per year, depending on utilization.
The Credit Agreement contains customary representations, warranties and covenants. Exceptions to the operating covenants in the Credit Agreement provide the company with flexibility to, among other things, enter into or undertake certain sale and leaseback transactions, dispositions of assets, additional credit facilities, sales of receivables, additional indebtedness and intercompany indebtedness, all subject to limitations set forth in the Credit Agreement, as amended. The Credit Agreement also contains a covenant requiring the company to maintain minimum availability under the U.S. and Canadian Credit Facility of not less than the greater of (i) 11.25% of the maximum amount that may be drawn under the U.S. and Canadian Credit Facility for five (5) consecutive business days, or (ii) $6,000,000 on any business day. The company also is subject to dominion triggers under the U.S. and Canadian Credit Facility requiring the company to maintain borrowing capacity of not less than $7,500,000 on any business day or any five consecutive days in order to avoid triggering full control by an agent for the Lenders of the company's cash receipts for application to the company's obligations under the agreement.
The Credit Agreement contains customary default provisions, with certain grace periods and exceptions, which provide for events of default that include, among other things, failure to pay amounts due, breach of covenants, representations or warranties, bankruptcy, the occurrence of a material adverse effect, exclusion from any medical reimbursement program, and an interruption of any material manufacturing facilities for more than 10 consecutive days. The proceeds of the U.S. and Canadian Credit Facility will be used to finance the working capital and other business needs of the company. There was
$20,000,000 outstanding under the U.S. and Canadian Credit Facility at December 31, 2020.
European Credit Facility
The Credit Agreement also provides for a revolving credit, letter of credit and swing line loan facility which gives the company and the European Borrowers the ability to borrow up to an aggregate principal amount of $30,000,000, with a $5,000,000 sublimit for letters of credit and a $2,000,000 sublimit for swing line loans (the “European Credit Facility”). Up to $15,000,000 of the European Credit Facility will be available to each of Invacare Limited (the “UK Borrower”) and Invacare Poirier SAS (the “French Borrower” and, together with the UK Borrower, the “European Borrowers”). The European Credit Facility matures in January 2024, together with the U.S. and Canadian Credit Facility.
The aggregate borrowing availability for each European Borrower under the European Credit Facility is determined based on a borrowing base formula. The aggregate borrowings of each of the European Borrowers under the European Credit Facility may not exceed an amount equal to (a) 85% of the European Borrower's eligible accounts receivable, less (b) the European Borrower's borrowings and swing line loans outstanding under the European Credit Facility, less (c) the European Borrower's letters of credit issued and undrawn under the European Credit Facility, less (d) a $3,000,000 minimum availability reserve, less (e) other reserves required by the European Agent, and in each case subject to the definitions and limitations in the Credit Agreement. As of December 31, 2020, the gross borrowing base to the European Borrowers under the European Credit Facility was $18,376,000 and net borrowing availability was $12,001,000, considering the $3,000,000 minimum availability reserve and a $3,375,000 dominion trigger amount described below.
The aggregate principal amount of the European Credit Facility may be increased by up to $10,000,000 to the extent requested by the company and agreed to by any Lender or Lenders that wish to increase their lending participation or, if not agreed to by any Lender, a new financial institution that agrees to join the European Credit Facility and that is approved by the Administrative Agent and the European Agent.
Interest will accrue on outstanding indebtedness under the European Credit Facility at the LIBOR rate, plus a margin ranging from 2.50% to 3.00%, or for swing line loans, at the overnight LIBOR rate, plus a margin ranging from 2.50% to 3.00%, as selected by the company. The margin will be adjusted quarterly based on utilization. Borrowings under the European Credit Facility are subject
| | | | | | | | |
| | Notes to Financial Statements |
| | Long-Term Debt |
| | |
to commitment fees of 0.25% or 0.375% per year, depending on utilization.
The European Credit Facility is secured by substantially all the personal property assets of the UK Borrower and its in-country subsidiaries, and all the receivables of the French Borrower and its in-country subsidiaries. The UK and French facilities (which comprise the European Credit Facility) are cross collateralized, and the US personal property assets previously pledged under the U.S. and Canadian Credit Facility also serve as collateral for the European Credit Facility.
The European Credit Facility is subject to customary representations, warranties and covenants generally consistent with those applicable to the U.S. and Canadian Credit Facility. Exceptions to the operating covenants in the Credit Agreement provide the company with flexibility to, among other things, enter into or undertake certain sale/leaseback transactions, dispositions of assets, additional credit facilities, sales of receivables, additional indebtedness and intercompany indebtedness, all subject to limitations set forth in the Credit Agreement. The Credit Agreement also contains a covenant requiring the European Borrowers to maintain undrawn availability under the European Credit Facility of not less than the greater of (i) 11.25% of the maximum amount that may be drawn under the European Credit Facility for five (5) consecutive business days, or (ii) $3,000,000 on any business day. The European Borrowers also are subject to cash dominion triggers under the European Credit Facility requiring the European Borrower to maintain borrowing capacity of not less than $3,750,000 on any business day or $3,375,000 for five consecutive business days in order to avoid triggering full control by an agent for the Lenders of the European Borrower's cash receipts for application to its obligations under the European Credit Facility.
The European Credit Facility is subject to customary default provisions, with certain grace periods and exceptions, consistent with those applicable to the U.S. and Canadian Credit Facility, which provide that events of default include, among other things, failure to pay amounts due, breach of covenants, representations or warranties, cross-default, bankruptcy, the occurrence of a material adverse effect, exclusion from any medical reimbursement program, and an interruption in the operations of any material manufacturing facility for more than 10 consecutive days. The proceeds of the European Credit Facility will be used to finance the working capital and other business needs of the company. As of December 31, 2020, the company had borrowings of $7,636,000 (€6,400,000) under its French Credit Facility and $3,866,000 (£2,900,000) under its UK Credit Facility as of December 31, 2020, together referred to as the European Credit Facility.
On January 15, 2021, the company entered into an Eighth Amendment to Amended and Restated Revolving Credit and Security Agreement (the “Credit Agreement Amendment”), which amends the Amended and Restated Revolving Credit and Security Agreement, dated as of September 30, 2015 and amended as of February 16, 2016, May 3, 2016, September 30, 2016, November 30, 2016, June 7, 2017, November 13, 2019 and May 29, 2020 (as so amended, the “Credit Agreement”).
The Credit Agreement Amendment provides for, among other things, the addition of the company's Netherlands subsidiary as a guarantor under the European revolving credit facility, amendments to the restrictive covenants in the Credit Agreement to (1) increase the maximum amount of permitted miscellaneous indebtedness to $30,000,000 from $10,000,000 and (2) permit up to $9,000,000 of financing based on certain European public and government receivables, and terms that, upon the occurrence of certain events related to a transition from the use of LIBOR, permit the agent for the lenders to amend the Credit Agreement to replace the LIBOR rate and/or the Euro rate with a benchmark replacement rate.
Convertible senior notes due 2021
In the first quarter of 2016, the company issued $150,000,000 aggregate principal amount of 5.00% Convertible Senior Notes due 2021 (the “2021 Notes”) in a private offering to qualified institutional buyers pursuant to Rule 144A under the Securities Act. The 2021 Notes bear interest at a rate of 5.00% per year payable semi-annually in arrears on February 15 and August 15 of each year, beginning August 15, 2016. The 2021 Notes will mature on February 15, 2021, unless repurchased or converted in accordance with their terms prior to such date. Prior to August 15, 2020, the 2021 Notes will be convertible only upon satisfaction of certain conditions and during certain periods, and thereafter, at any time until the close of business on the second scheduled trading day immediately preceding the maturity date. Prior to May 16, 2019, the 2021 Notes were convertible, subject to certain conditions, into cash only. On May 16, 2019, the company obtained shareholder approval under applicable New York Stock Exchange rules such that conversion of the 2021 Notes may be settled in cash, the company’s common shares or a combination of cash and the company’s common shares, at the company’s election. At December 31, 2020, $1,250,000 aggregate principal amount of the 2021 Notes remained outstanding, following the repurchase of $16,000,000 aggregate principal amount of 2021 Notes for cash in the third quarter of 2019, the exchange transactions completed in the fourth quarter of 2019 and the second quarter of 2020, as further discussed below, and the repurchases of $24,466,000 aggregate principal amount in the third quarter of 2020. The $24,466,000 repurchases in
| | | | | | | | |
| Notes to Financial Statements | | |
| Long-Term Debt | | |
| | |
the third quarter of 2020, resulted in a $761,000 loss on debt extinguishment.
Holders of the 2021 Notes could convert their 2021 Notes at their option at any time prior to the close of business on the business day immediately preceding August 15, 2020 only under the following circumstances: (1) during any fiscal quarter commencing after March 31, 2016 (and only during such fiscal quarter), if the last reported sale price of the company’s common shares for at least 20 trading days (whether or not consecutive) during the period of 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding fiscal quarter is greater than or equal to 130% of the applicable conversion price for the 2021 Notes on each applicable trading day; (2) during the five business day period after any 10 consecutive trading day period (the “measurement period”) in which the “trading price” (as defined in the Indenture) per one thousand U.S. dollar principal amount of 2021 Notes for each trading day of such measurement period was less than 98% of the product of the last reported sale price of the company’s common shares and the applicable conversion rate for the 2021 Notes on each such trading day; or (3) upon the occurrence of specified corporate events described in the Indenture. On or after August 15, 2020 until the close of business on the second scheduled trading day immediately preceding the maturity of the 2021 Notes, holders could convert their 2021 Notes, at the option of the holder, regardless of the foregoing circumstances.
Holders of the 2021 Notes will have the right to require the company to repurchase all or some of their 2021 Notes at 100% of their principal, plus any accrued and unpaid interest, upon the occurrence of certain fundamental changes. The initial conversion rate is 60.0492 common shares per $1,000 principal amount of 2021 Notes (equivalent to an initial conversion price of approximately $16.65 per common share). Until the company received shareholder approval on May 16, 2019 authorizing it to elect to settle future conversions of the 2021 Notes in common shares, the company separately accounted for the conversion features as a derivative. The derivative was capitalized on the balance sheet as a long-term liability with adjustment to reflect fair value each quarter until the change to the conversion features as a result of the shareholder approval received on May 16, 2019 resulted in the termination of the derivative. The fair value of the convertible debt conversion liability at issuance was $34,480,000. The company recognized a loss of $2,210,000 in 2019 related to the convertible debt conversion liability.
In connection with the offering of the 2021 Notes, the company entered into privately negotiated convertible note hedge transactions with two financial institutions (the “option counterparties”). These transactions cover, subject
to customary anti-dilution adjustments, the number of the company's common shares that will initially underlie the 2021 Notes, and are expected generally to reduce the potential equity dilution, and/or offset any cash payments in excess of the principal amount due, as the case may be, upon conversion of the 2021 Notes. The company evaluated the note hedges under the applicable accounting literature, including Derivatives and Hedging, ASC 815, and determined that the note hedges should be accounted for as derivatives. These derivatives were capitalized on the balance sheet as long-term assets and adjusted to reflect fair value each quarter. The fair value of the convertible note hedge assets at issuance was $27,975,000. The company recognized a gain of $2,852,000 in 2019 related to the convertible note hedge asset.
The company entered into separate, privately negotiated warrant transactions with the option counterparties at a higher strike price relating to the same number of the company's common shares, subject to customary anti-dilution adjustments, pursuant to which the company sold warrants to the option counterparties. The warrants could have a dilutive effect on the company's outstanding common shares and the company's earnings per share to the extent that the price of the company's common shares exceeds the strike price of those warrants. The initial strike price of the warrants is $22.4175 per share and is subject to certain adjustments under the terms of the warrant transactions. The company evaluated the warrants under the applicable accounting literature, including Derivatives and Hedging, ASC 815, and determined that the warrants met the definition of a derivative, are indexed to the company's own stock and should be classified in shareholder's equity. The amount paid for the warrants and capitalized in shareholder's equity was $12,376,000.
The net proceeds from the offering of the 2021 Notes were approximately $144,034,000, after deducting fees and offering expenses of $5,966,000, which were paid in 2016. These debt issuance costs were capitalized and are being amortized as interest expense through February 2021. Debt issuance costs are presented on the balance sheet as a direct deduction from the carrying amount of the related debt liability. Approximately $5,000,000 of the net proceeds from the offering were used to repurchase the company's common shares from purchasers of 2021 Notes in the offering in privately negotiated transactions. A portion of the net proceeds from the offering were used to pay the cost of the convertible note hedge transactions (after such cost is partially offset by the proceeds to the company from the warrant transactions), with a net cost of $15,600,000.
During the third quarter of 2019, the company used an aggregate of $14,708,000 in cash to repurchase a total amount of $16,000,000 in principal amount of 2021 Notes. After recognizing expenses on unamortized fees and
| | | | | | | | |
| | Notes to Financial Statements |
| | Long-Term Debt |
| | |
discounts associated with the repurchased 2021 Notes, the repurchases resulted in a net reduction of debt of $14,367,000 and a net loss on the repurchases of $280,000.
During the fourth quarter of 2019, the company entered into separate privately negotiated agreements with certain holders of its 2021 Notes to exchange $72,909,000 in aggregate principal amount of 2021 Notes (the “Exchange Transactions”) for aggregate consideration of $72,909,000 in aggregate principal amount of new 5.00% Convertible Senior Exchange Notes due 2024 (the “Series I 2024 Notes”) of the company and $6,928,000 in cash. Refer to "Convertible senior notes Series I due 2024" below for more information. As a result of the exchange transaction in the fourth quarter of 2019 and the repurchase of $16,000,000 in principal amount of 2021 Notes in the third quarter of 2019, a partial unwind of the note hedge options and warrants entered into with the issuance of the 2021 Notes also occurred during the fourth quarter of 2019. Note hedge options outstanding were reduced from the original number of 300,000 to 138,182 and warrants were reduced from the initial number of 9,007,380 to 3,860,624. The partial unwind of the note hedge options and warrants resulted in no net impact to cash or paid in capital.
During the second quarter of 2020, the company entered into separate, privately negotiated agreements with certain holders of its 2021 Notes and certain holders of its 2022 Notes to exchange $35,375,000 in aggregate principal amount of 2021 Notes and $38,500,000 in aggregate principal amount of 2022 notes, for aggregate consideration of $73,875,000 in aggregate principal amount of new 5.00% Series II Convertible Senior Exchange Notes due 2024 (the “Series II 2024 Notes”) of the company and $5,593,000 in cash.
During the third quarter of 2020, the company repurchased $24,466,000 aggregate principal amount of 2021 Notes, resulting in a $761,000 loss on debt extinguishment. As a result of the repurchase of 2021 Notes in the third quarter of 2020 and the exchange of 2021 Notes for new notes in the second quarter of 2020, a partial unwind of the note hedge options and warrants entered into with the issuance of the 2021 Notes also occurred. Note hedge options outstanding were reduced from the original number of 300,000 to 62,341 and warrants were reduced from the initial number of 9,007,380 to 3,141,943. The partial unwind of the note hedge options and warrants resulted in no net impact to cash or paid in capital.
The liability components of the 2021 Notes consist of the following (in thousands):
| | | | | | | | | | | | | |
| December 31, 2020 | | December 31, 2019 | | |
| Principal amount of liability component | $ | 1,250 | | | $ | 61,091 | | | |
| Unamortized discount | (7) | | | (3,916) | | | |
| Debt fees | (1) | | | (547) | | | |
| Net carrying amount of liability component | $ | 1,242 | | | $ | 56,628 | | | |
The unamortized discount of $7,000 is to be amortized through February 2021. The effective interest rate on the liability component was 11.1%. Non-cash interest expense of $1,782,000 and $6,672,000 was recognized in 2020 and 2019, respectively, in comparison to actual interest expense accrued of $1,632,000 and $6,803,000 in 2020 and 2019, respectively, based on the stated coupon rate of 5.0%. The 2021 Notes were not convertible as of December 31, 2020 nor was the applicable conversion threshold met.
Convertible senior notes due 2022
In the second quarter of 2017, the company issued $120,000,000 aggregate principal amount of 4.50% Convertible Senior Notes due 2022 (the “2022 Notes”) in a private offering to qualified institutional buyers pursuant to Rule 144A under the Securities Act. The 2022 Notes bear interest at a rate of 4.50% per year payable semi-annually in arrears on June 1 and December 1 of each year, beginning December 1, 2017. The 2022 Notes will mature on June 1, 2022, unless repurchased or converted in accordance with their terms prior to such date. Prior to December 1, 2021, the 2022 Notes will be convertible only upon satisfaction of certain conditions and during certain periods, and thereafter, at any time until the close of business on the second scheduled trading day immediately preceding the maturity date. Prior to May 16, 2019, the 2022 Notes were convertible, subject to certain conditions, into cash only. On May 16, 2019, the company obtained shareholder approval under applicable New York Stock Exchange rules such that conversion of the 2022 Notes may be settled in cash, the company’s common shares or a combination of cash and the company’s common shares, at the company’s election. At December 31, 2020, $81,500,000 aggregate principal amount of the 2022 Notes remained outstanding, following the exchange transactions completed in the second quarter of 2020, as further discussed below.
Holders of the 2022 Notes may convert their 2022 Notes at their option at any time prior to the close of business on the business day immediately preceding December 1, 2021 only under the following circumstances: (1) during any fiscal quarter commencing after September 30, 2017 (and only during such fiscal quarter), if the last reported sale price of the company’s common
| | | | | | | | |
| Notes to Financial Statements | | |
| Long-Term Debt | | |
| | |
shares for at least 20 trading days (whether or not consecutive) during the period of 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding fiscal quarter is greater than or equal to 130% of the applicable conversion price for the 2022 Notes on each applicable trading day; (2) during the five business day period after any 10 consecutive trading day period (the “measurement period”) in which the “trading price” (as defined in the Indenture) per one thousand U.S. dollar principal amount of 2022 Notes for each trading day of such measurement period was less than 98% of the product of the last reported sale price of the company’s Common Shares and the applicable conversion rate for the 2022 Notes on each such trading day; or (3) upon the occurrence of specified corporate events described in the Indenture. On or after December 1, 2021 until the close of business on the second scheduled trading day immediately preceding the maturity of the 2022 Notes, holders may convert their 2022 Notes, at the option of the holder, regardless of the foregoing circumstances.
Holders of the 2022 Notes will have the right to require the company to repurchase all or some of their 2022 Notes at 100% of their principal, plus any accrued and unpaid interest, upon the occurrence of certain fundamental changes. The initial conversion rate is 61.6095 common shares per $1,000 principal amount of 2022 Notes (equivalent to an initial conversion price of approximately $16.23 per common share). Until the company received shareholder approval on May 16, 2019 authorizing it to elect to settle future conversions of the 2022 Notes in common shares, the company separately accounted for the conversion features as a derivative. The derivative was capitalized on the balance sheet as a long-term liability with adjustment to reflect fair value each quarter until the change to the conversion features as a result of the shareholder approval received on May 16, 2019 resulted in the termination of the derivative. The fair value of the convertible debt conversion liability at issuance was $28,859,000. The company recognized a loss of $6,193,000 in 2019 related to the convertible debt conversion liability.
In connection with the offering of the 2022 Notes, the company entered into privately negotiated convertible note hedge transactions with one financial institution (the “option counterparty”). These transactions cover, subject to customary anti-dilution adjustments, the number of the company's common shares that will initially underlie the 2022 Notes, and are expected generally to reduce the potential equity dilution, and/or offset any cash payments in excess of the principal amount due, as the case may be, upon conversion of the 2022 Notes. The company evaluated the note hedges under the applicable accounting literature, including Derivatives and Hedging, ASC 815, and determined that the note hedges should be accounted for as derivatives. These derivatives were capitalized on
the balance sheet as long-term assets and will be adjusted to reflect fair value each quarter. The fair value of the convertible note hedge assets at issuance was $24,780,000. The company recognized a gain of $6,748,000 in 2019 related to the convertible note hedge asset.
The company entered into separate, privately negotiated warrant transactions with the option counterparty at a higher strike price relating to the same number of the company's common shares, subject to customary anti-dilution adjustments, pursuant to which the company sold warrants to the option counterparties. The warrants could have a dilutive effect on the company's outstanding common shares and the company's earnings per share to the extent that the price of the company's common shares exceeds the strike price of those warrants. The initial strike price of the warrants is $21.4375 per share and is subject to certain adjustments under the terms of the warrant transactions. The company evaluated the warrants under the applicable accounting literature, including Derivatives and Hedging, ASC 815, and determined that the warrants meet the definition of a derivative, are indexed to the company's own shares and should be classified in shareholder's equity. The amount paid for the warrants and capitalized in shareholder's equity was $14,100,000.
The net proceeds from the offering of the 2022 Notes were approximately $115,289,000, after deducting fees and offering expenses of $4,711,000, which were paid in 2017. These debt issuance costs were capitalized and are being amortized as interest expense through June 2022. Debt issuance costs are presented on the balance sheet as a direct deduction from the carrying amount of the related debt liability. A portion of the net proceeds from the offering were used to pay the cost of the convertible note hedge transactions (after such cost is partially offset by the proceeds to the company from the warrant transactions), which net cost was $10,680,000.
During the second quarter of 2020, the company entered into separate, privately negotiated agreements with certain holders of its 2021 Notes and certain holders of its 2022 Notes to exchange $35,375,000 in aggregate principal amount of 2021 Notes and $38,500,000 in aggregate principal amount of 2022 Notes, for aggregate consideration of $73,875,000 in aggregate principal amount of new 5.00% Series II Convertible Senior Exchange Notes due 2024 (the “Series II 2024 Notes”) of the company and $5,593,000 in cash.
| | | | | | | | |
| | Notes to Financial Statements |
| | Long-Term Debt |
| | |
The liability components of the 2022 Notes consist of the following (in thousands):
| | | | | | | | | | | |
| December 31, 2020 | | December 31, 2019 |
| Principal amount of liability component | $ | 81,500 | | | $ | 120,000 | |
| Unamortized discount | (6,772) | | | (16,027) | |
| Debt fees | (859) | | | (2,158) | |
| Net carrying amount of liability component | $ | 73,869 | | | $ | 101,815 | |
The unamortized discount of $6,772,000 is to be amortized through June 2022. The effective interest rate on the liability component was 10.9%. Non-cash interest expense of $4,894,000 and $5,448,000 was recognized in 2020 and 2019, respectively, in comparison to actual interest expense accrued of $4,404,000 and $5,400,000 for the same periods, based on the stated coupon rate of 4.5%. The 2022 Notes were not convertible as of December 31, 2020 nor was the applicable conversion threshold met.
Convertible senior notes Series I due 2024
During the fourth quarter of 2019, the company entered into separate privately negotiated agreements with certain holders of its 2021 Notes to exchange $72,909,000 in aggregate principal amount of 2021 Notes for aggregate consideration of $72,909,000 in aggregate principal amount of new 5.00% Convertible Senior Exchange Notes due 2024 (the “Series I 2024 Notes”) of the company and $6,928,000 in cash.
The notes bear interest at a rate of 5.00% per year payable semi-annually in arrears on May 15 and November 15 of each year, beginning May 15, 2020. The notes will mature on November 15, 2024, unless repurchased, redeemed or converted in accordance with their terms prior to such date. Prior to May 15, 2024, the Series I 2024 Notes will be convertible only upon satisfaction of certain conditions and during certain periods, and thereafter, at any time until the close of business on the second scheduled trading day immediately preceding the maturity date. The Series I 2024 Notes may be settled in cash, the company’s common shares or a combination of cash and the company’s common shares, at the company’s election.
Prior to the maturity of the Series I 2024 Notes, the company may, at its election, redeem for cash all or part of the Series I 2024 Notes if the last reported sale price of the company’s common shares equals or exceeds 130% of the conversion price then in effect for at least 20 trading days (whether or not consecutive) during any 30 consecutive trading day period (including the last trading day of such period) ending on, and including, the trading day immediately preceding the date on which the company provides notice of redemption. The redemption price will
be equal to 100% of the principal amount of the Series I 2024 Notes to be redeemed, plus accrued and unpaid interest to, but excluding, the redemption date (subject to certain limited exceptions). No sinking fund is provided for the Series I 2024 Notes, which means the company is not required to redeem or retire the Series I 2024 Notes periodically.
Holders of the Series I 2024 Notes may convert their Series I 2024 Notes at their option at any time prior to the close of business on the business day immediately preceding May 15, 2024 only under the following circumstances: (1) during any calendar quarter commencing after the calendar quarter ending December 31, 2019 (and only during such calendar quarter), if the last reported sale price of the company’s common shares for at least 20 trading days (whether or not consecutive) during the period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter is greater than or equal to 130% of the conversion price for the Series I 2024 Notes on each applicable trading day; (2) during the five business day period after any 10 consecutive trading day period (the “measurement period”) in which the “trading price” (as defined in the Indenture) per one thousand U.S. dollar principal amount of Series I 2024 Notes for each trading day of such measurement period was less than 98% of the product of the last reported sale price of the company’s common shares and the applicable conversion rate for the 2024 notes on each such trading day; (3) upon the occurrence of specified corporate events described in the Indenture; or (4) if the company calls the Series I 2024 Notes for redemption pursuant to the terms of the Indenture. Holders of the Series I 2024 Notes will have the right to require the company to repurchase all or some of their Series I 2024 Notes at 100% of their principal, plus any accrued and unpaid interest, upon the occurrence of certain fundamental changes. The initial conversion rate is 67.6819 common shares per $1,000 principal amount of Series I 2024 Notes (equivalent to an initial conversion price of approximately $14.78 per common share). On or after May 15, 2024 until the close of business on the second scheduled trading day immediately preceding the maturity of the Series I 2024 Notes, holders may convert their Series I 2024 Notes, at the option of the holder, regardless of the foregoing circumstances.
A loss of $5,885,000 was recorded a part of the exchange transaction, which included the write-off of fees related to the portion of the 2021 Note exchanged. Debt issuance costs of $1,394,000 were capitalized and are being amortized as interest expense through November 15, 2024. Debt issuance costs are presented on the balance sheet as a direct deduction from the carrying amount of the related debt liability.
| | | | | | | | |
| Notes to Financial Statements | | |
| Long-Term Debt | | |
| | |
The liability components of the Series I 2024 Notes consist of the following (in thousands):
| | | | | | | | | | | | | | |
| December 31, 2020 | | | | December 31, 2019 | |
| Principal amount of liability component | $ | 72,909 | | | | | $ | 72,909 | | |
| Unamortized discount | (8,888) | | | | | (10,733) | | |
| Debt fees | (1,037) | | | | | (1,359) | | |
| Net carrying amount of liability component | $ | 62,984 | | | | | $ | 60,817 | | |
The unamortized discount of $8,888,000 is to be amortized through November 15, 2024. The effective interest rate on the liability component was 8.77%. Non-cash interest expense of $1,845,000 and $205,000 was recognized in 2020 and 2019, respectively, in comparison to actual interest expense accrued of $3,646,000 and $456,000 in 2020 and 2019, respectively, based on the stated coupon rate of 5.0%. The Series I 2024 Notes were not convertible as of December 31, 2020 nor was the applicable conversion threshold met.
Convertible senior notes Series II due 2024
During the second quarter of 2020, the company entered into separate, privately negotiated agreements with certain holders of its 2021 Notes and certain holders of its 2022 Notes to exchange $35,375,000 in aggregate principal amount of 2021 Notes and $38,500,000 in aggregate principal amount of 2022 Notes, for aggregate consideration of $73,875,000 in aggregate principal amount of new 5.00% Series II Convertible Senior Exchange Notes due 2024 (the “Series II 2024 Notes”) of the company and $5,593,000 in cash.
The Series II 2024 Notes bear interest at a rate of 5.00% per year, payable semi-annually in arrears on May 15 and November 15 of each year, beginning November 15, 2020. The Series II 2024 Notes will mature on November 15, 2024, unless repurchased, redeemed or converted in accordance with their terms prior to such date. Prior to May 15, 2024, the Series II 2024 Notes will be convertible only upon satisfaction of certain conditions and during certain periods, and thereafter, at any time until the close of business on the second scheduled trading day immediately preceding the maturity date. The Series II 2024 Notes may be settled in cash, the company’s common shares or a combination of cash and the company’s common shares, at the company’s election.
Prior to the maturity of the Series II 2024 Notes, the company may, at its election, redeem for cash all or part of the Series II 2024 Notes, if the last reported sale price of the company’s common shares equals or exceeds 130% of the conversion price then in effect for at least 20 trading days (whether or not consecutive) during any 30
consecutive trading day period (including the last trading day of such period) ending on, and including, the trading day immediately preceding the date on which the company provides notice of redemption. The redemption price will be equal to 100% of the accreted principal amount of the Series II 2024 Notes to be redeemed, plus any accrued and unpaid interest, if any, on the original principal amount of the New Notes redeemed to, but excluding, the redemption date (subject to certain limited exceptions). No sinking fund is provided for the Series II 2024 Notes, which means the company is not required to redeem or retire the Series 2024 II Notes periodically.
Holders of the Series II 2024 Notes may convert their Series II 2024 Notes at their option at any time prior to the close of business on the business day immediately preceding May 15, 2024 only under the following circumstances: (1) during any calendar quarter commencing after the calendar quarter ending June 30, 2020 (and only during such calendar quarter), if the last reported sale price of the company’s common shares for at least 20 trading days (whether or not consecutive) during the period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter is greater than 130% of the conversion price for the Series II 2024 Notes on each applicable trading day; (2) during the five business day period after any 10 consecutive trading day period (the “measurement period”) in which the “trading price” (as defined in the Indenture) per one thousand U.S. dollar principal amount of Series II 2024 Notes for each trading day of such measurement period was less than 98% of the product of the last reported sale price of the company’s common shares and the applicable conversion rate for the Series II 2024 Notes on each such trading day; (3) upon the occurrence of specified corporate events described in the Indenture; or (4) if the company calls the Series II 2024 Notes for redemption pursuant to the terms of the Indenture. Holders of the Series II 2024 Notes will have the right to require the company to repurchase all or some of their Series II 2024 Notes at 100% of the accreted principal amount, plus any accrued and unpaid interest, upon the occurrence of certain fundamental changes. The initial conversion rate is 67.6819 common shares per $1,000 principal amount of Series II 2024 Notes (equivalent to an initial conversion price of approximately $14.78 per common share). On or after May 15, 2024 until the close of business on the second scheduled trading day immediately preceding the maturity of the Series II 2024 Notes, holders may convert their Series II 2024 Notes, at the option of the holder, regardless of the foregoing circumstances.
The principal amount of the Series II 2024 Notes also will accrete at a rate of approximately 4.7% per year commencing June 4, 2020, compounding on a semi-annual basis. The accreted portion of the principal is payable in cash upon maturity but does not bear interest and is not
| | | | | | | | |
| | Notes to Financial Statements |
| | Long-Term Debt |
| | |
convertible into the company’s common shares. The amount accreted as of December 31, 2020 was $1,813,000.
A loss of $6,599,000 was recorded a part of the exchange transaction, which included the write-off of fees related to portions of the 2021 Notes and 2022 Notes exchanged. Debt issuance costs of $1,505,000 were capitalized and are being amortized as interest expense through November 2024. Debt issuance costs are presented on the balance sheet as a direct deduction from the carrying amount of the related debt liability.
The liability components of the Series II 2024 Notes consist of the following (in thousands):
| | | | | | | | | |
| December 31, 2020 | | | | |
| Principal amount of liability component - including accretion | $ | 75,688 | | | | | |
| Unamortized discount | (9,461) | | | | | |
| Debt fees | (1,308) | | | | | |
| Net carrying amount of liability component | $ | 64,919 | | | | | |
| | | | | |
The unamortized discount of $9,461,000 is to be amortized through November 15, 2024. The effective interest rate on the liability component was 8.99%. Non-cash interest expense, including accretion, of $2,966,000 was recognized in 2020 in comparison to actual interest expense accrued of $2,123,000 in 2020 based on the stated coupon rate of 5.0%. The Series II 2024 Notes were not convertible as of December 31, 2020 nor was the applicable conversion threshold met.
CARES Act Loan
On May 15, 2020 the company entered into an unsecured loan agreement in the aggregate amount of $10,000,000 pursuant to sections 1102 and 1106 of the Coronavirus Aid, Relief and Economic Security, "CARES," Act which was evidenced by a promissory note, dated May 13, 2020, and bears interest at a fixed rate of 1.00%. Originally, monthly payments were to commence in December 2020 however, payments have been subsequently deferred to commence in March 2021 under the program. This loan may be forgivable, partially or in full, if certain conditions are met, principally based on having been disbursed for permissible purposes and based on average levels of employment over a designated period of time. No assurance can be given that the company will be granted forgiveness of the loan in whole or in part. The company has recorded $4,062,000 of the amount borrowed in current maturities of long term debt.
The aggregate minimum maturities of long-term debt (excluding finance leases) for each of the next five years are as follows: $5,620,000 in 2021, $87,667,000 in 2022, $0 in 2023, $180,099,000 in 2024, and $0 in 2025. Interest
paid on all borrowings was $16,909,000, $15,042,000 and $14,526,000 in 2020, 2019 and 2018, respectively.
| | | | | | | | |
| Notes to Financial Statements | | |
| Other Long-Term Obligations | | |
| | |
Other Long-Term Obligations
Other long-term obligations as of December 31, 2020 and 2019 consist of the following (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| Deferred income taxes | $ | 23,234 | | | $ | 23,376 | |
| Product liability | 12,304 | | | 13,414 | |
| Pension | 9,088 | | | 7,006 | |
| Deferred gain on sale leaseback | 5,502 | | | 5,819 | |
| Supplemental Executive Retirement Plan liability | 5,368 | | | 5,433 | |
| Deferred compensation | 5,318 | | | 5,354 | |
| Uncertain tax obligation including interest | 3,114 | | | 2,612 | |
| Other | 6,546 | | | 3,935 | |
| Other long-term obligations | $ | 70,474 | | | $ | 66,949 | |
On April 23, 2015, the company entered into a real estate sales leaseback transaction which resulted in the recording of an initial deferred gain of $7,414,000, the majority of which is included in Other Long-Term Obligations and will be recognized over the 20-year life of the leases. The gain realized was $305,000 and $295,000 for December 31, 2020 and 2019, respectively.
| | | | | | | | |
| | Notes to Financial Statements |
| | Leases and Commitments |
| | |
Leases and Commitments
The company reviews new contracts to determine if the contracts include a lease. To the extent a lease agreement includes an extension option that is reasonably certain to be exercised, the company has recognized those amounts as part of the right-of-use assets and lease liabilities. The company combines lease and certain non-lease components, such as common area maintenance, in the calculation of the lease assets and related liabilities. As most lease agreements do not provide an implicit rate, the company uses an incremental borrowing rate (IBR) based on information available at commencement date in determining the present value of lease payments and to help classify the lease as operating or financing. The company calculates its IBR based on the secured rates of the company's recent debt issuances, the credit rating of the company, changes in currencies, lease repayment timing as well as other publicly available data.
The company leases a portion of its facilities, transportation equipment, data processing equipment and certain other equipment. These leases have terms from 1 to 20 years and provide for renewal options. Generally, the company is required to pay taxes and normal expenses associated with operating the facilities and equipment. As of December 31, 2020, the company is committed under non-cancelable leases, which have initial or remaining terms in excess of one year and expire on various dates through 2040.
On April 23, 2015, the company sold and leased back, under four separate lease agreements, four properties located in Ohio and one property in Florida for net proceeds of $23,000,000, which were used to reduce debt under the U.S. and Canadian Credit Facility. The initial total annual rent for the properties was $2,275,000 and can increase annually over the 20-year term of the leases based on the applicable geographical consumer price index (CPI). Each of the four lease agreements contains three 10-year renewals with the rent for each option term based on the greater of the then-current fair market rent for each property or the then- current rate and increasing annually by the applicable CPI. Under the terms of the lease agreements, the company is responsible for all taxes, insurance and utilities. The company is required to adequately maintain each of the properties and any leasehold improvements will be amortized over the lesser of the lives of the improvements or the remaining lease lives, consistent with any other company leases.
In connection with the transaction, the requirements for sale lease-back accounting were met. Accordingly, the company recorded the sale of the properties, removed the related property and equipment from the company's balance sheet, recognized an initial deferred gain of $7,414,000 and an immediate loss of $257,000 related to
one property and recorded new lease liabilities. Specifically, the company recorded four finance leases totaling $32,339,000 and one operating lease related to leased land, which was not a material component of the transaction. The gains on the sales of the properties were required to be deferred and recognized over the life of the leases as the property sold is being leased back. The deferred gain is classified under Other Long-Term Obligations on the consolidated balance sheet. The gains realized were $305,000 and $295,000 in 2020 and 2019, respectively.
In December 2018, the company entered into a 20-year lease agreement in Germany. The lease commenced in July 2020. The lease increased the company's finance lease obligation by $36,916,000.
Lease expenses for the year ended December 31, 2020 and December 31, 2019, respectively, were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| | 2020 | | 2019 | |
| Operating leases | | $ | 8,138 | | | $ | 10,550 | | |
| Variable and short-term leases | | 3,968 | | | 2,848 | | |
| Total operating leases | | $ | 12,106 | | | $ | 13,398 | | |
| | | | | |
| Finance lease interest cost | | $ | 2,544 | | | $ | 1,316 | | |
| Finance lease depreciation | | 3,479 | | | 2,658 | | |
| Total finance leases | | $ | 6,023 | | | $ | 3,974 | | |
| | | | | |
| | | | | | | | |
| Notes to Financial Statements | | |
| Leases and Commitments | | |
| | |
Future minimum operating and finance lease commitments, as of December 31, 2020, are as follows (in thousands):
| | | | | | | | | | | |
| Finance
Leases | | Operating Leases |
| 2021 | $ | 7,416 | | | $ | 7,182 | |
| 2022 | 6,466 | | | 4,282 | |
| 2023 | 6,393 | | | 1,974 | |
| 2024 | 6,335 | | | 1,389 | |
| 2025 | 6,300 | | | 1,113 | |
| Thereafter | 78,347 | | | 2,375 | |
| Total future minimum lease payments | 111,257 | | | 18,315 | |
| Amounts representing interest | (44,715) | | | (3,305) | |
| Present value of minimum lease payments | 66,542 | | | 15,010 | |
| Less: current maturities of lease obligations | (3,405) | | | (6,313) | |
| Long-term lease obligations | $ | 63,137 | | | $ | 8,697 | |
Supplemental cash flow amounts for the year ended December 31, 2020 were as follows (in thousands):
| | | | | | | | |
| Cash Activity: Cash paid in measurement of amounts for lease liabilities | | December 31, 2020 |
| Operating leases | | $ | 12,527 | |
| Finance leases | | 5,316 | |
| Total | | $ | 17,843 | |
| | |
| Non-Cash Activity: Right-of-use assets obtained in exchange for lease obligations | | December 31, 2020 |
| Operating leases | | $ | 6,155 | |
| Finance leases | | 40,078 | |
| Total | | $ | 46,233 | |
| | |
Weighted-average remaining lease terms and discount rates for finance and operating leases are as follows as of December 31, 2020:
| | | | | |
| December 31, 2020 |
| Weighted-average remaining lease term - finance leases | 17.0 years |
| Weighted-average remaining lease term - operating leases | 4.6 years |
| Weighted-average discount rate - finance leases | 6.41% |
| Weighted-average discount rate - operating leases | 7.82% |
| | | | | | | | |
| | Notes to Financial Statements |
| | Retirement and Benefit Plans |
| | |
Retirement and Benefit Plans
Substantially all full-time salaried and hourly domestic employees are included in the Invacare Retirement Savings Plan sponsored by the company. The company makes matching cash contributions up to 66.7% of employees' contributions up to 3% of compensation. The company also makes quarterly contributions to this Plan equal to a percentage of qualified wages. This quarterly contribution, of 1% of qualified wages, was only made in the second quarter of 2020. The company may make discretionary contributions to the domestic plans based on an annual resolution of the Board of Directors. Contribution expense for the Invacare Retirement Savings Plan in 2020, 2019 and 2018 was $1,214,000, $1,765,000 and $1,786,000, respectively.
The company sponsors a Deferred Compensation Plus Plan covering certain employees, which provides for elective deferrals and the company retirement deferrals so that the total retirement deferrals equal amounts that would have contributed to the company's principal retirement plans if it were not for limitations imposed by income tax regulations.
The company sponsors a non-qualified defined benefit Supplemental Executive Retirement Plan (SERP) for certain key executives. Effective December 31, 2008, the SERP was amended, in part to comply with IRS Section 409A. As a result of the amendment, the plan became a defined benefit cash balance plan for the non-retired participants and thus, payments by the company since December 31, 2008 have been based upon a cash balance formula with interest credited at a rate determined annually by the Compensation and Management Development Committee of the Board of Directors. In 2020, 2019 and 2018, respectively, interest was credited at 0% for active participants in the SERP. The plan continues to be unfunded with individual hypothetical accounts maintained for each participant.
The SERP projected benefit obligation related to this unfunded plan was $5,759,000 and $5,824,000 at December 31, 2020 and December 31, 2019, respectively, and the accumulated benefit obligation was $5,759,000 and $5,824,000 at December 31, 2020 and December 31, 2019, respectively. The projected benefit obligations for the SERP as well as the Death Benefit Only Plan discussed below were calculated using an assumed future salary increase of 3.25% at December 31, 2020 and 2019, respectively. The assumed discount rate, relevant for three participants unaffected by the plan conversion was 2.52% and 3.22% for 2020 and 2019, respectively, based upon the discount rate on high-quality fixed-income investments without adjustment. The retirement age was 67 for 2020 and 2019, respectively. The mortality assumptions used for
2020 and 2019 were based upon the Pri.A-2012 White Collar Fully Generational Mortality Table using Scale MP-2020 and the RP-2014 White Collar Fully Generational Mortality Table using Scale MP-2018, respectively.
Expense for the SERP in 2020, 2019 and 2018 was $326,000, $574,000 and $5,000 , respectively. The expense was composed of interest expense of $213,000 in 2020, interest expense of $392,000 in 2019 and interest income of $193,000 in 2018, with the remaining non-interest expense related to service costs, prior service costs and other gains/losses. Benefit payments in 2020, 2019 and 2018 were $391,000, $391,000 and $391,000, respectively.
The company also sponsors a Death Benefit Only Plan (DBO) for certain key executives that provides a benefit equal to three times the participant's final target earnings should the participant's death occur while an employee and a benefit equal to one time the participant's final earnings upon the participant's death after normal retirement or if a participant dies after his or her employment with the company is terminated following a change in control of the company. Expense for the plan in 2020 and 2019 was $640,000 and $561,000, respectively and income for the plan in 2018 of $151,000 . The 2020 and 2019 amounts contained service and accrual adjustment expense of $569,000 and $488,000, respectively, compared to income of $253,000 in 2018, with the remaining activity in each year related to interest costs. There were no benefit payments in 2020, 2019 or 2018. In conjunction with the company's DBO, the company has invested in life insurance policies related to certain employees to help satisfy the DBO obligations.
In Europe, the company maintains a defined benefit plan in Switzerland. The statutory pension plan is maintained with a private insurance company and, in accordance with Swiss law, the plan functions as defined contribution plan whereby employee and employer contributions are defined as a percentage of individual salary depending on the age of the employee and a guaranteed interest rate, which is annually defined by the Swiss Pension Fund. Under U.S. GAAP, the plan is treated as defined benefit plan. Expense for the European plan was $1,678,000, $34,000 and $1,079,000 in 2020, 2019 and 2018, respectively.
| | | | | | | | |
| Notes to Financial Statements | | |
| Revenue | | |
| | |
Revenue
The company has two revenue streams: products and services. Services include repair, refurbishment, preventive maintenance and rental of products. Services for the North America (N.A.) segment include maintenance and repair of products. Services for the Europe segment include repair, refurbishment and preventive maintenance services. Services in All Other, are in the Asia Pacific region, and include rental and repair of products.
The following tables disaggregate the company's revenues by major source and by reportable segment for the year ended December 31, 2020 and December 31, 2019 (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | 2020 |
| | Products | | Service | | Total |
| Europe | | $ | 455,638 | | | $ | 12,403 | | | $ | 468,041 | |
| N.A. | | 347,476 | | | 831 | | | 348,307 | |
| | | | | | |
| All Other | | 29,755 | | | 4,586 | | | 34,341 | |
| Total | | $ | 832,869 | | | $ | 17,820 | | | $ | 850,689 | |
| % Split | | 98% | | 2% | | 100% |
| | | | | | | | | | | | | | | | | | | | |
| | 2019 |
| | Products | | Service | | Total |
| Europe | | $ | 519,160 | | | $ | 13,888 | | | $ | 533,048 | |
| N.A. | | 346,642 | | | 1,559 | | | 348,201 | |
| | | | | | |
| All Other | | 41,852 | | | 4,863 | | | 46,715 | |
| Total | | $ | 907,654 | | | $ | 20,310 | | | $ | 927,964 | |
| % Split | | 98% | | 2% | | 100% |
The company's revenues are principally related to the sale of products, approximately 98%, with the remaining 2% related to services including repair, refurbishment, preventive maintenance and rental of products. While the company has a significant amount of contract types, the sales split by contract type is estimated as follows: general terms and conditions (30%), large national customers (30%), governments, principally pursuant to tender contracts (21%) and other customers including buying groups and independent customers (19%).
All product revenues and substantially all service revenues are recognized at a point in time. The remaining service revenue, recognized over time, are reflected in the Europe segment and include multiple performance obligations. For such contracts, the company allocates revenue to each performance obligation based on its relative standalone selling price. The company generally determines the standalone selling price based on the expected cost-plus margin methodology.
Revenue is recognized when obligations under the terms of a contract with the customer are satisfied; generally, this occurs with the transfer of control of the company's products and services. Revenue is measured as the amount of consideration expected to be received in exchange for transferring products or providing services. The amount of consideration received and revenue recognized by the company can vary as a result of variable consideration terms included in the contracts related to customer rebates, cash discounts and return policies. Customer rebates and cash discounts are estimated based on the most likely amount principle and these estimates are based on historical experience and anticipated performance. In addition, customers have the right to return products within the company's normal terms policy, and as such the company estimates the expected returns based on an analysis of historical experience. The company adjusts its estimate of revenue at the earlier of when the most likely amount of consideration it expects to receive changes or when the consideration becomes fixed. The company generally does not expect that there will be significant changes to its estimates of variable consideration (refer to “Receivables” and "Accrued Expenses" in the Notes to the Consolidated Financial Statements include elsewhere in this report for more detail).
Depending on the terms of the contract, the company may defer the recognition of a portion of the revenue at the end of a reporting period to align with transfer of control of the company's products to the customer. In addition, to the extent performance obligations are satisfied over time, the company defers revenue recognition until the performance obligations are satisfied. As of December 31, 2020 and December 31, 2019, the company had deferred revenue of $3,516,000 and $3,173,000, respectively, related to outstanding performance obligations.
| | | | | | | | |
| | Notes to Financial Statements |
| | Equity Compensation |
| | |
Equity Compensation
The company's Common Shares have a $0.25 stated value. The Common Shares and the Class B Common Shares generally have identical rights, terms and conditions and vote together as a single class on most issues, except that the Class B Common Shares have ten votes per share and, in general, can only be transferred to family members or for estate planning purposes. Holders of Class B Common Shares are entitled to convert their shares into Common Shares at any time on a share-for-share basis. When Class B Common Shares are transferred out of a familial relationship, they automatically convert to Common Shares. The Board of Directors suspended further dividends on the Class B Common Shares.
As of December 31, 2020, 3,667 Class B Common Shares remained outstanding. Prior conversions of Class B Common Shares have substantially diminished the significance of the company's dual class voting structure. As of December 31, 2020, the holders of the Common Shares represent approximately 99.9% of the company's total outstanding voting power.
Equity Compensation Plan
On May 17, 2018, the shareholders of the company approved the Invacare Corporation 2018 Equity Compensation Plan (the “2018 Plan”), which was adopted on March 27, 2018 by the company's Board of Directors (the “Board”). The company's Board adopted the 2018 Plan in order to authorize additional Common Shares for grant as equity compensation, and to reflect changes to Section 162(m) of the Internal Revenue Code (the “Code”) resulting from the U.S. Tax Cuts and Jobs Act of 2017.
Following shareholder approval of the 2018 Plan, all of the Common Shares then-remaining available for issuance under the Invacare Corporation 2013 Equity Compensation Plan (the “2013 Plan”) and all of the Common Shares that were forfeited or remained unpurchased or undistributed upon termination or expiration of awards under the 2013 Plan and under the Invacare Corporation 2003 Performance Plan (the “2003 Plan”), become available for issuance under the 2018 Plan. Awards granted previously under the 2013 Plan and 2003 Plan will remain in effect under their original terms.
The 2018 Plan uses a fungible share-counting method, under which each Common Share underlying an award of stock options or stock appreciation rights ("SAR") will count against the number of total shares available under the 2018 Plan as one share; and each Common Share underlying any award other than a stock option or a SAR will count against the number of total shares available under the 2018 Plan as two shares. Shares underlying
awards made under the 2003 Plan or 2013 Plan that are forfeited or remain unpurchased or undistributed upon termination or expiration of the awards will become available under the 2018 Plan for use in future awards. Any Common Shares that are added back to the 2018 Plan as the result of forfeiture, termination or expiration of an award granted under the 2018 Plan or the 2013 Plan will be added back in the same manner such shares were originally counted against the total number of shares available under the 2018 Plan or 2013 Plan, as applicable. Each Common Share that is added back to the 2018 Plan due to a forfeiture, termination or expiration of an award granted under the 2003 Plan will be added back as one Common Share.
The Compensation and Management Development Committee of the Board (the “Compensation Committee”), in its discretion, may grant an award under the 2018 Plan to any director or employee of the company or an affiliate. As of December 31, 2020, 3,540,534 Common Shares were available for future issuance under the 2018 Plan in connection with the following types of awards with respect to the company's Common Shares: incentive stock options, nonqualified stock options, SARs, restricted stock, restricted stock units, unrestricted stock and performance shares. The Compensation Committee also may grant performance units that are payable in cash. The Compensation Committee has the authority to determine which participants will receive awards, the amount of the awards and the other terms and conditions of the awards. The Common Shares authorized for issuance under the 2018 Plan includes an additional 1,400,000 Common Shares that were approved by shareholders at the company’s 2020 annual meeting on May 21, 2020.
In the second quarter of 2020 the company transferred 1,004,079 shares into the 2018 Plan from the 2003 and 2013 Plans in total. At December 31, 2020, an aggregate of 336,666 Common Shares underlie awards which forfeited or expired unexercised under the 2003 and 2013 Plans and thus are available to be transferred under the 2018 Plan.
The 2018 Plan provides that shares granted come from the company's authorized but unissued Common Shares or treasury shares. In addition, the company's stock-based compensation plans allow employee participants to exchange shares for minimum withholding taxes, which results in the company acquiring treasury shares. Under these provisions, the company acquired approximately 231,000 treasury shares for $1,707,000 in 2020, 112,000 shares for $894,000 in 2019 and 140,000 shares for $2,427,000 in 2018.
| | | | | | | | |
| Notes to Financial Statements | | |
| Equity Compensation | | |
| | |
The amounts of equity-based compensation expense recognized as part of SG&A expenses in All Other in business segment reporting were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Non-qualified and performance stock options | $ | — | | | $ | 1,939 | | | $ | 201 | |
| Restricted stock / units | 5,332 | | | 4,772 | | | 4,305 | |
| Performance shares / units | 3,313 | | | 4,399 | | | 777 | |
| Total stock-based compensation expense | $ | 8,645 | | | $ | 11,110 | | | $ | 5,283 | |
As of December 31, 2020, unrecognized compensation expense related to equity-based compensation arrangements granted under the company's 2018 Plan and previous plans, which is related to non-vested options and shares, was as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Non-qualified and performance stock options | $ | — | | | $ | — | | | $ | 1,939 | |
| Restricted stock and restricted stock units | 7,489 | | | 8,453 | | | 7,469 | |
| Performance shares and performance share units | 7,260 | | | 8,269 | | | 7,441 | |
| Total unrecognized stock-based compensation expense | $ | 14,749 | | | $ | 16,722 | | | $ | 16,849 | |
Total unrecognized compensation cost will be adjusted for future changes in actual and estimated forfeitures and for updated vesting assumptions for the performance share awards (refer to "Stock Options" and "Performance Shares and Performance Share Units" below). No tax benefits for stock compensation were realized during 2020, 2019 and 2018 due to a valuation allowance against deferred tax assets. In accordance with ASC 718, any tax benefits resulting from tax deductions in excess of the compensation expense recognized is classified as a component of financing cash flows.
Stock Options
Generally, non-qualified stock option awards have a term of 10 years and were granted with an exercise price per share equal to the fair market value of the company's Common Shares on the date of grant. Stock option awards granted in 2017 were performance-based awards which became exercisable based upon achievement of the performance goals established by the Compensation Committee as achieved over a 3-year period ending in 2019 which were subject to the Compensation Committee's exercise of negative discretion to reduce the number of options vested based on the progress towards other initiatives.
The following table summarizes information about stock option activity for the three years ended 2020, 2019 and 2018:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2020 | | Weighted
Average
Exercise
Price | | 2019 | | Weighted
Average
Exercise
Price | | 2018 | | Weighted
Average
Exercise
Price |
| Options outstanding at January 1 | 1,441,202 | | | $ | 18.26 | | | 1,885,262 | | | $ | 18.78 | | | 2,631,469 | | | $ | 19.44 | |
| | | | | | | | | | | |
| Exercised | — | | | — | | | — | | | — | | | (184,549) | | | 14.28 | |
| Forfeited | (359,398) | | | 24.84 | | (444,060) | | | 20.49 | | | (561,658) | | | 23.34 | |
| Options outstanding at December 31 | 1,081,804 | | | $ | 16.07 | | | 1,441,202 | | | $ | 18.26 | | | 1,885,262 | | | $ | 18.78 | |
| Options exercise price range at December 31 | $ | 12.15 | | | | | $ 12.15 | | | | $ 12.15 | | |
| to | | | | to | | | | to | | |
| $ | 33.36 | | | | | $ | 33.36 | | | | | $ | 33.36 | | | |
| Options exercisable at December 31 | 1,081,804 | | | | | 910,267 | | | | | 1,354,202 | | | |
| Shares available for grant at December 31* | 3,540,534 | | | | | 3,851,945 | | | | | 3,994,255 | | | |
________________________
* Shares available for grant under the 2018 Plan as of December 31, 2020 reduced by net restricted stock and restricted stock unit and performance share and performance share unit award activity of 668,992 shares and 1,727,470 shares, respectively.
| | | | | | | | |
| | Notes to Financial Statements |
| | Equity Compensation |
| | |
The following table summarizes information about stock options outstanding at December 31, 2020:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Options Outstanding | | Options Exercisable |
| Exercise Prices | Number
Outstanding
At 12/31/20 | | Weighted Average
Remaining
Contractual Life (Years) | | Weighted Average
Exercise Price | | Number
Exercisable
At 12/31/20 | | Weighted Average
Exercise Price |
$12.15 – $20.00 | 777,159 | | | 4.9 | | $ | 12.73 | | | 777,159 | | | $ | 12.73 | |
$20.01 – $30.00 | 300,149 | | | 0.7 | | 24.45 | | | 300,149 | | | 24.45 | |
| | | | | | | | | |
$30.01 – $33.36 | 4,496 | | | 0.4 | | 33.36 | | | 4,496 | | | 33.36 | |
| Total | 1,081,804 | | | 3.7 | | $ | 16.07 | | | 1,081,804 | | | $ | 16.07 | |
The 2018 Plan provides for a one-year minimum vesting period for stock options and, generally, options must be exercised within ten years from the date granted. No stock options were issued in 2020, 2019 or 2018 and those issued in 2017 were performance-based and vested after the conclusion of the three-year performance period ended December 31, 2019 based on achievement of performance goals established by the Compensation Committee and subject to the Compensation Committee's exercise of negative discretion to reduce the number of options vested based on the progress towards other initiatives. All other outstanding stock options were issued in 2014 or prior years and were not performance-based.
For the stock options issued in 2014 and prior, 25% of such options vested one year following the issuance and provided a 4 years vesting period whereby options vest equally in 25% installments in each year. Options granted with graded vesting were accounted for as single options. The fair value of options granted is estimated on the date of grant using the Black-Scholes option-pricing model. The calculated fair value of the 2017 performance option awards was $5.38 based on the following assumptions:
| | | | | | | | | |
| | | | | |
| Expected dividend yield | 0.4 | % | | | | |
| Expected stock price volatility | 39.1 | % | | | | |
| Risk-free interest rate | 2.31 | % | | | | |
| Expected life in years | 7.8 | | | | |
| Forfeiture percentage | 5.0 | % | | | | |
Expected dividend yields was based on historical dividends. Expected stock price volatility percentage was calculated at each date of grant based on historical stock prices for a period of time commensurate with the expected life of the option. The assumed expected life and forfeiture percentage were based on the company's historical analysis of option history.
The weighted-average fair value of options granted in 2017 was $5.38. The weighted-average remaining contractual life of options outstanding at December 31, 2020, 2019 and 2018 was 3.7, 3.7 and 3.8 years, respectively. The weighted-average contractual life of options exercisable at December 31, 2020 was 3.7 years. The total intrinsic value of stock awards exercised in 2020, 2019 and 2018 was $0, $0 and $755,000, respectively. As of December 31, 2020 and 2019, the intrinsic value of all options outstanding and of all options exercisable was $0 and $0, respectively.
The exercise of stock awards in 2020, 2019 and 2018 resulted in cash received by the company totaling $0, $0 and $2,626,000 for each period, respectively with no tax benefits for any period. The total fair value of awards vested during 2020, 2019 and 2018 was $0, $2,844,000 and $1,000, respectively.
| | | | | | | | |
| Notes to Financial Statements | | |
| Equity Compensation | | |
| | |
Restricted Stock and Restricted Stock Units
The following table summarizes information about restricted shares and restricted share units (primarily for non-U.S. recipients):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2020 | Weighted Average Fair Value | | 2019 | Weighted Average Fair Value | | 2018 | Weighted Average Fair Value |
| Stock / Units unvested at January 1 | 965,085 | | $ | 11.32 | | | 637,663 | | $ | 15.04 | | | 776,520 | | $ | 13.75 | |
| Granted | 764,012 | | 7.11 | | | 828,484 | | 9.86 | | | 377,299 | | 17.48 | |
| Vested | (475,113) | | 11.39 | | | (309,150) | | 14.26 | | | (386,275) | | 15.05 | |
| Forfeited | (108,526) | | 9.90 | | | (191,912) | | 12.60 | | | (129,881) | | 14.43 | |
| Stock / Units unvested at December 31 | 1,145,458 | | $ | 8.62 | | | 965,085 | | $ | 11.32 | | | 637,663 | | $ | 15.04 | |
| | | | | | | | |
The restricted stock awards generally vest ratably over the 3 years after the award date. Unearned restricted stock compensation, determined as the market value of the shares at the date of grant, is being amortized on a straight-line basis over the vesting period.
Performance Shares and Performance Share Units
The following table summarizes information about performance shares and performance share units (primarily for non-U.S. recipients):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2020 | | Weighted Average Fair Value | | 2019 | | Weighted Average Fair Value | | 2018 | | Weighted Average Fair Value |
| Shares / Units unvested at January 1 | 753,272 | | | $ | 11.82 | | | 448,294 | | | $ | 14.37 | | | 457,879 | | | $ | 12.33 | |
| Granted | 523,329 | | | 7.82 | | | 576,737 | | | 9.93 | | | 205,164 | | | 17.48 | |
| Vested | (183,840) | | | 17.48 | | | (255,259) | | | 12.02 | | | (155,766) | | | 12.82 | |
| Forfeited | (65,976) | | | 9.48 | | | (16,500) | | | 11.99 | | | (58,983) | | | 13.43 | |
| Shares / Units unvested at December 31 | 1,026,785 | | | $ | 8.55 | | | 753,272 | | | $ | 11.82 | | | 448,294 | | | $ | 14.37 | |
| | | | | | | | | | | |
During 2020, 2019 and 2018, the performance shares and performance share units (for non-U.S. recipients) were granted as performance awards with a 3-year performance period with payouts based on achievement of certain performance goals. The awards are classified as equity awards as they will be settled in common shares upon vesting. The number of shares earned will be determined at the end of the three-year performance period based on achievement of performance criteria for January 1, 2018 through December 31, 2020, January 1, 2019 through December 31, 2021 and January 1, 2020 through December 31 2022, respectively established by the Compensation Committee at the time of grant. Recipients will be entitled to receive a number of Common Shares equal to the number of performance shares that vest based upon the levels of achievement which may range between 0% and 150% of the target number of shares with the target being 100% of the initial grant.
The fair value of the performance awards is based on the stock price on the date of grant discounted for the
estimated value of dividends foregone as the awards are not eligible for dividends except to the extent vested. The grant fair value is further updated each reporting period while variable accounting applies. The company assesses the probability that the performance targets will be met with expense recognized whenever it is probable that at least the minimum performance criteria will be achieved. Depending upon the company's assessment of the probability of achievement of the goals, the company may not recognize any expense associated with performance awards in a given period, may reverse prior expense recorded or record additional expense to recognize the cumulative estimated achievement level of proportionate term of the award. Performance award compensation expense is generally expected to be recognized over three years. Performance award expense was recognized at 75% of target for the 2016 awards, which vested on December 31, 2018, at 122.5% to 146.45% for the 2017 awards, which vested on December 31, 2019 and 114% of target for the 2018 awards, which vested on December 31, 2020 subject to the approval of the Compensation Committee.
| | | | | | | | |
| | Notes to Financial Statements |
| | Equity Compensation |
| | |
The company continues to recognize expense related to the awards granted in 2019 and 2020 as it is considered probable that the performance goals for those awards will be met.
In the fourth quarter of 2020 the Compensation Committee considered the adverse impacts of the pandemic and approved the modification of the performance shares and performance share unit awards for the 2019-2021 performance period (the "modification"). Due to the adverse impacts of the pandemic, the previous performance targets which were established prior to the pandemic were deemed to be no longer reasonable or achievable, and accordingly, the vesting of the performance awards were no longer probable. The modification aligned updated performance targets such that vesting of at least a portion of the awards became probable. The modification of the performance awards for the 2019-2021 performance period impacted seven grantees. Incremental stock compensation expense resulting from the modification was $605,000 in the fourth quarter of 2020.
| | | | | | | | |
| Notes to Financial Statements | | |
| Accumulated Other Comprehensive Income | | |
| | |
Accumulated Other Comprehensive Income (Loss) by Component
Changes in accumulated other comprehensive income ("OCI") during the year ended December 31, 2020 were as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Foreign Currency | | Long-Term Notes | | Defined Benefit Plans | | Derivatives | | Total |
| December 31, 2019 | | $ | 8,898 | | | $ | (2,491) | | | $ | (3,299) | | | $ | 20 | | | $ | 3,128 | |
| OCI before reclassifications | | 41,431 | | | 1,974 | | | (1,015) | | | (2,129) | | | 40,261 | |
| Amount reclassified from accumulated OCI | | — | | | — | | | 640 | | | 1,407 | | | 2,047 | |
| Net current-period OCI | | 41,431 | | | 1,974 | | | (375) | | | (722) | | | 42,308 | |
| December 31, 2020 | | $ | 50,329 | | | $ | (517) | | | $ | (3,674) | | | $ | (702) | | | $ | 45,436 | |
Changes in OCI during the year ended December 31, 2019 were as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Foreign Currency | | Long-Term Notes | | Defined Benefit Plans | | Derivatives | | Total |
| December 31, 2018 | | $ | 12,244 | | | $ | 2,662 | | | $ | (2,703) | | | $ | 590 | | | $ | 12,793 | |
| | | | | | | | | | |
| OCI before reclassifications | | (3,346) | | | (5,153) | | | (1,157) | | | 1,958 | | | (7,698) | |
| Amount reclassified from accumulated OCI | | — | | | — | | | 561 | | | (2,528) | | | (1,967) | |
| Net current-period OCI | | (3,346) | | | (5,153) | | | (596) | | | (570) | | | (9,665) | |
| | | | | | | | | | |
| December 31, 2019 | | $ | 8,898 | | | $ | (2,491) | | | $ | (3,299) | | | $ | 20 | | | $ | 3,128 | |
Reclassifications out of accumulated OCI for the year ended December 31, 2020 and December 31, 2019 were as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | Amount reclassified from OCI | | Affected line item in the Statement of Comprehensive (Income) Loss |
| | 2020 | | 2019 | | |
| Defined Benefit Plans: | | | | | | |
| Service and interest costs | | $ | 640 | | | $ | 561 | | | Selling, General and Administrative |
| Tax | | — | | | — | | | Income Taxes |
| Total after tax | | $ | 640 | | | $ | 561 | | | |
| | | | | | |
| Derivatives: | | | | | | |
| Foreign currency forward contracts hedging sales | | $ | (1,359) | | | $ | (52) | | | Net Sales |
| Foreign currency forward contracts hedging purchases | | 2,826 | | | (2,673) | | | Cost of Products Sold |
| | | | | | |
| Total loss (income) before tax | | 1,467 | | | (2,725) | | | |
| Tax | | (60) | | | 197 | | | Income Taxes |
| Total after tax | | $ | 1,407 | | | $ | (2,528) | | | |
| | | | | | | | |
| | Notes to Financial Statements |
| | Capital Stock |
| | |
Capital Stock
Capital stock activity for 2020, 2019 and 2018 consisted of the following (in thousands of shares):
| | | | | | | | | | | | | | | | | |
| Common Stock
Shares | | Class B
Shares | | Treasury
Shares |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| January 1, 2018 Balance | 36,532 | | | 6 | | | (3,701) | |
| | | | | |
| Exercise of stock options | 185 | | | — | | | (50) | |
| Restricted stock awards | 293 | | | — | | | (90) | |
| | | | | |
| December 31, 2018 Balance | 37,010 | | | 6 | | | (3,841) | |
| | | | | |
| | | | | |
| Restricted and performance stock awards | 599 | | | — | | | (112) | |
| | | | | |
| December 31, 2019 Balance | 37,609 | | | 6 | | | (3,953) | |
| | | | | |
| Conversion of Class B to Common | 2 | | | (2) | | | — | |
| Restricted and performance stock awards | 1,002 | | | — | | | (231) | |
| December 31, 2020 Balance | 38,613 | | | 4 | | | (4,184) | |
Stock awards for 108,526, 191,912 and 129,881 shares were forfeited in 2020, 2019 and 2018, respectively.
In 2020, dividends of $0.0125 per Common Share were declared and paid as the Board of Directors suspended the quarterly dividend in light of the impact of COVID-19 on the business effective May 2020.
In 2019 and 2018, dividends of $0.05 per Common Share were declared and paid.
In 2018, dividends of $0.023 and $0.034 were declared and paid, respectively, per Class B Common Share. The Board of Directors suspended further dividends on the Class B Common Shares in 2018.
| | | | | | | | |
| Notes to Financial Statements | | |
| Charges Related to Restructuring | | |
| | |
Charges Related to Restructuring Activities
The company's restructuring charges were originally necessitated primarily by continued declines in Medicare and Medicaid reimbursement by the U.S. government, as well as similar healthcare reimbursement pressures abroad, which negatively affect the company's customers (e.g. home health care providers) and continued pricing pressures faced by the company due to the outsourcing by competitors to lower cost locations. Restructuring decisions were also the result of reduced profitability in each of the segments. Restructuring actions have continued into 2020.
Charges for the year ended December 31, 2018 totaled $3,481,000 which were related to North America ($1,359,000), Europe ($1,773,000) and All Other ($349,000). In North America, costs were incurred related to severance ($1,471,000) and lease termination reversals were recognized ($112,000). The European and All Other charges were incurred related to severance costs. Payments for the year ended December 31, 2018 were $5,804,000 and the cash payments were funded with company's cash on hand. The 2018 charges have been paid out.
Charges for the year ended December 31, 2019 totaled $11,829,000 which were related to North America ($1,617,000), Europe ($9,579,000) and All Other ($633,000). In North America, costs were incurred related
to severance ($1,573,000) and lease termination costs ($44,000). The European charges were incurred related to severance ($9,356,000) and lease termination costs ($223,000) primarily related to the closure of a German Manufacturing facility. All Other charges were related to severance. Most of the 2019 charges have been paid out.
Charges for the year ended December 31, 2020 totaled $7,358,000 which were related to North America ($1,306,000), Europe ($5,934,000) and All Other ($118,000). The North America and All Other costs were for severance costs. The European charges were incurred related to severance ($5,588,000) and contract terminations of ($346,000) related to the closure of a German manufacturing facility. The 2020 charges are expected to be paid out within twelve months.
There have been no material changes in accrued balances related to the charges, either as a result of revisions to the plans or changes in estimates. In addition, the savings anticipated as a result of the company's restructuring plans have been or are expected to be achieved, primarily resulting in reduced salary and benefit costs principally impacting Selling, General and Administrative expenses, and to a lesser extent, Costs of Products Sold. To date, the company's liquidity has been sufficient to absorb these charges.
A progression by reporting segment of the accruals recorded as a result of the restructuring is as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Severance | | | | Contract Terminations | | | | Total |
| January 1, 2018 Balance | | | | | | | | | |
| North America | $ | 2,439 | | | | | $ | 167 | | | | | $ | 2,606 | |
| Europe | 249 | | | | | 134 | | | | | 383 | |
| All Other | 1,016 | | | | | — | | | | | 1,016 | |
| | | | | | | | | |
| Total | 3,704 | | | | | 301 | | | | | 4,005 | |
| Charges | | | | | | | | | |
| North America | 1,471 | | | | | (112) | | | | | 1,359 | |
| Europe | 1,773 | | | | | — | | | | | 1,773 | |
| All Other | 349 | | | | | — | | | | | 349 | |
| Total | 3,593 | | | | | (112) | | | | | 3,481 | |
| Payments | | | | | | | | | |
| North America | (3,254) | | | | | (30) | | | | | (3,284) | |
| Europe | (1,841) | | | | | (134) | | | | | (1,975) | |
| All Other | (545) | | | | | — | | | | | (545) | |
| | | | | | | | | |
| Total | (5,640) | | | | | (164) | | | | | (5,804) | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | |
| | Notes to Financial Statements |
| | Charges Related to Restructuring |
| | |
| | | | | | | | | | | | | | | | | | | | | |
| Severance | | | | Contract Terminations | | | | Total |
| December 31, 2018 Balance | | | | | | | | | |
| North America | 656 | | | | | 25 | | | | | 681 | |
| Europe | 181 | | | | | — | | | | | 181 | |
| All Other | 820 | | | | | — | | | | | 820 | |
| | | | | | | | | |
| Total | 1,657 | | | | | 25 | | | | | 1,682 | |
| Charges | | | | | | | | | |
| North America | 1,573 | | | | | 44 | | | | | 1,617 | |
| Europe | 9,356 | | | | | 223 | | | | | 9,579 | |
| All Other | 633 | | | | | — | | | | | 633 | |
| Total | 11,562 | | | | | 267 | | | | | 11,829 | |
| Payments | | | | | | | | | |
| North America | (2,018) | | | | | (69) | | | | | (2,087) | |
| Europe | (3,131) | | | | | (219) | | | | | (3,350) | |
| All Other | (1,047) | | | | | — | | | | | (1,047) | |
| Total | (6,196) | | | | | (288) | | | | | (6,484) | |
| December 31, 2019 Balance | | | | | | | | | |
| North America | 211 | | | | | — | | | | | 211 | |
| Europe | 6,406 | | | | | 4 | | | | | 6,410 | |
| All Other | 406 | | | | | — | | | | | 406 | |
| Total | 7,023 | | | | | 4 | | | | | 7,027 | |
| Charges | | | | | | | | | |
| North America | 1,306 | | | | | 0 | | | | 1,306 |
| Europe | 5,588 | | | | | 346 | | | | 5,934 |
| All Other | 118 | | | | | 0 | | | | 118 |
| Total | 7,012 | | | | 346 | 346 | | | 7,358 |
| Payments | | | | | | | | | |
| North America | (1,338) | | | | | — | | | | | (1,338) | |
| Europe | (6,090) | | | | | (346) | | | | | (6,436) | |
| All Other | (358) | | | | | — | | | | | (358) | |
| Total | (7,786) | | | | | (346) | | | | | (8,132) | |
| December 31, 2020 Balance | | | | | | | | | |
| North America | 179 | | | | | — | | | | | 179 | |
| Europe | 5,904 | | | | | 4 | | | | | 5,908 | |
| All Other | 166 | | | | | — | | | | | 166 | |
| Total | $ | 6,249 | | | | | $ | 4 | | | | | $ | 6,253 | |
| | | | | | | | | |
| | | | | | | | |
| Notes to Financial Statements | | |
| Income Taxes | | |
| | |
Income Taxes
Earnings (loss) before income taxes consist of the following (in thousands):
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Domestic | $ | (42,213) | | | $ | (66,135) | | | $ | (72,703) | |
| Foreign | 17,774 | | | 22,110 | | | 38,601 | |
| $ | (24,439) | | | $ | (44,025) | | | $ | (34,102) | |
The company has provided for income taxes (benefits) as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Current: | | | | | |
| Federal | $ | 45 | | | $ | 152 | | | $ | (202) | |
| State | (180) | | | (90) | | | 147 | |
| Foreign | 6,168 | | | 10,070 | | | 12,675 | |
| 6,033 | | | 10,132 | | | 12,620 | |
| Deferred: | | | | | |
| Federal | (26) | | | (148) | | | (2,073) | |
| State | — | | | — | | | — | |
| Foreign | (2,166) | | | (682) | | | (727) | |
| (2,192) | | | (830) | | | (2,800) | |
| Income Taxes | $ | 3,841 | | | $ | 9,302 | | | $ | 9,820 | |
Included in the 2018 Federal deferred taxes is a benefit of $680,000 related to an intra-period allocation. A charge in an equal amount is in other comprehensive income. In addition, included in deferred federal taxes is a benefit of $148,000 in 2019 which resulted from the effect of indefinite intangibles and a related 2018 indefinite loss carryforward created, due to the U.S. tax reform legislation, resulting in a deferred tax benefit.
The company has historically considered the undistributed earnings of the company's foreign subsidiaries to be indefinitely reinvested, and, accordingly, no taxes have been provided on such earnings (other than earnings from the company's Chinese subsidiary which was sold in March 2020 as part of the sale of the Dynamic business). The company reversed withholding taxes in the amount of $988,000 which were previously provided as a result of the company position that the earnings from the Chinese subsidiary were not permanently reinvested. The sale of the business occurred without dividends paid from
this subsidiary. The company continues to evaluate its plans for reinvestment or repatriation of unremitted foreign. As a result of U.S. tax reform legislation, distributions of profits from non-U.S. subsidiaries are not expected to cause a significant incremental U.S. tax impact in the future. However, these distributions may be subject to non-U.S. withholding taxes if profits are distributed from certain jurisdictions. Undistributed profits of non-U.S. subsidiaries of approximately $24,600,000 are considered indefinitely reinvested. Determination of the amount of unrecognized deferred tax liability related to indefinitely reinvested profits is not practicable.
The company regularly reviews its cash positions and its determination of permanent reinvestment of foreign earnings. If the company determines all or a portion of such foreign earnings are no longer indefinitely reinvested, the company may be subject to additional foreign withholding taxes and U.S. state income taxes.
| | | | | | | | |
| | Notes to Financial Statements |
| | Income Taxes |
| | |
A reconciliation to the effective income tax rate from the federal statutory rate is as follows:
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Statutory federal income tax rate (benefit) | (21.0) | % | | (21.0) | % | | (21.0) | % |
| State and local income taxes, net of federal income tax benefit | (0.6) | | | (0.2) | | | 0.3 | |
Non-taxable disposition of subsidiaries | (11.2) | | | — | | | — | |
| | | | | |
| Expiring foreign tax credits | 16.5 | | | 40.2 | | | 4.7 | |
| Foreign taxes at other than the federal statutory rate (including tax holidays) | 8.8 | | | 5.1 | | | 12.9 | |
| Federal and foreign valuation allowances | (4.3) | | | (20.4) | | | 35.6 | |
| | | | | |
| Withholding taxes | 0.1 | | | 0.1 | | | 0.2 | |
| Unremitted earnings | (4.0) | | | 0.1 | | | — | |
| | | | | |
| | | | | |
| | | | | |
| Debt repurchase | 3.2 | | | 1.7 | | | — | |
| | | | | |
| Foreign branch activity | 19.3 | | | 12.4 | | | 0.1 | |
| Uncertain tax positions | 2.9 | | | 1.4 | | | (1.9) | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Intraperiod allocations to OCI | — | | | — | | | (2.0) | |
| | | | | |
| Other, net | 6.0 | | | 1.7 | | | (0.1) | |
| Effective federal income tax rate | 15.7 | % | | 21.1 | % | | 28.8 | % |
At December 31, 2020, total deferred tax assets were $179,985,000, total deferred tax liabilities were $37,873,000 and the tax valuation allowances total was $163,298,000 for a net deferred income tax liability of $21,186,000 compared to total deferred tax assets of $178,632,000, total deferred tax liabilities of $38,290,000 and a tax valuation allowances total of $162,790,000 for a net deferred income tax liability of $22,448,000 at December 31, 2019. The company recorded a valuation allowance for its U.S. and certain foreign country net deferred tax assets where it is or is projected to be in a three-year cumulative loss.
| | | | | | | | |
| Notes to Financial Statements | | |
| Income Taxes | | |
| | |
Significant components of long-term deferred income tax assets and liabilities at December 31, 2020 and 2019 are as follows (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| Bad debt | $ | 417 | | | $ | 841 | |
| Warranty | 1,280 | | | 1,391 | |
| Other accrued expenses and reserves | 1,709 | | | 1,515 | |
| Inventory | 3,797 | | | 2,993 | |
| Goodwill and intangibles | (24,291) | | | (22,686) | |
| Convertible debt | 2,623 | | | (1,530) | |
| Fixed assets | (13,582) | | | (13,421) | |
| Compensation and benefits | 6,349 | | | 5,965 | |
| Loss and credit carryforwards | 118,290 | | | 121,602 | |
| Product liability | 1,797 | | | 3,113 | |
| State and local taxes | 32,835 | | | 31,499 | |
| Valuation allowances | (163,298) | | | (162,790) | |
| Lease liability | 9,258 | | | 9,713 | |
| Other, net | 1,630 | | | (653) | |
| | | |
| | | |
| Net Deferred Income Taxes | $ | (21,186) | | | $ | (22,448) | |
The company made net payments for income taxes of $4,377,000, $12,463,000, and $15,820,000 during the years ended December 31, 2020, 2019 and 2018, respectively.
The company has a federal domestic net operating loss carryforward of $365,509,000 of which $276,958,000 expires between 2034 and 2037 and the remaining are non-expiring; domestic interest carryforward of $74,164,000 which is non-expiring and federal tax credit carryforwards of $11,816,000 of which $885,000 expire between 2021 and 2022 and $9,070,000 expire between 2023 and 2027, $1,861,000 expire between 2031 and 2037.
At December 31, 2020, the company also had $651,804,000 of domestic state and local tax loss carryforwards, of which $148,967,000 expire between 2021 and 2024, $270,823,000 expire between 2025 and 2034 and $205,272,000 expire after 2034 and $26,742,000 have an unlimited carryforward.
At December 31, 2020, the company had foreign tax loss carryforwards of approximately $59,075,000 of which $25,365,000 expire by 2027 and the remaining are non-expiring all of which are offset by valuation allowances except for $2,583,000.
As of December 31, 2020 and 2019, the company had a liability for uncertain tax positions, excluding interest and penalties of $2,604,000 and $2,082,000, respectively. The total liabilities associated with unrecognized tax benefits that, if recognized, would impact the effective tax rates were $2,604,000 and $2,082,000 at December 31, 2020 and 2019, respectively.
A reconciliation of the beginning and ending balance of unrecognized tax benefits is as follows (in thousands):
| | | | | | | | | | | |
| 2020 | | 2019 |
| Balance at beginning of year | $ | 2,872 | | | $ | 2,355 | |
| Additions to: | | | |
| Positions taken during the current year | 782 | | | 641 | |
| Positions taken during a prior year | 3 | | | 52 | |
| Exchange rate impact | 52 | | | 14 | |
| Deductions due to: | | | |
| | | |
| Positions taken during a prior year | (167) | | | — | |
| | | |
| Lapse of statute of limitations | (280) | | | (190) | |
| Balance at end of year | $ | 3,262 | | | $ | 2,872 | |
The company recognizes interest and penalties associated with uncertain tax positions in income tax expense. During 2020, 2019 and 2018 the expense (benefit) for interest and penalties was $(20,000), $13,000 and $(322,000), respectively. The company had approximately $510,000 and $530,000 of accrued interest and penalties as of December 31, 2020 and 2019, respectively.
The company and its subsidiaries file income tax returns in the U.S. and certain foreign jurisdictions. The company is subject to U.S. federal income tax examinations for calendar years 2017 to 2020 with limited exceptions, and is subject to various U.S. state income tax examinations for 2016 to 2020. With regards to foreign income tax jurisdictions, the company is generally subject to examinations for the periods 2014 to 2020.
| | | | | | | | |
| | Notes to Financial Statements |
| | Net Loss per Common Share |
| | |
Net Loss Per Common Share
The following table sets forth the computation of basic and diluted net earnings (loss) per common share for the periods indicated.
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| | (In thousands, except per share data) |
| Basic | | | | | |
| Average common shares outstanding | 34,266 | | | 33,594 | | | 33,124 | |
| | | | | |
| | | | | |
| | | | | |
| Net loss | $ | (28,280) | | | $ | (53,327) | | | $ | (43,922) | |
| | | | | |
| | | | | |
| | | | | |
| Net loss per common share | $ | (0.83) | | | $ | (1.59) | | | $ | (1.33) | |
| Diluted | | | | | |
| Average common shares outstanding | 34,266 | | | 33,594 | | | 33,124 | |
| | | | | |
| Stock options and awards | 109 | | | 48 | | | 419 | |
| Average common shares assuming dilution | 34,375 | | | 33,642 | | | 33,543 | |
| | | | | |
| | | | | |
| | | | | |
| Net loss | $ | (28,280) | | | $ | (53,327) | | | $ | (43,922) | |
| | | | | |
| | | | | |
| | | | | |
| Net loss per common share * | $ | (0.83) | | | $ | (1.59) | | | $ | (1.33) | |
* Net earnings (loss) per share assuming dilution calculated utilizing weighted average shares outstanding - basic for the periods in which there was a net loss.
At December 31, 2020, 2019 and 2018, incremental shares associated with equity compensation plans of 2,275,832, 3,626,828 and 741,380, respectively, were excluded from the average common shares assuming dilution, as they were anti-dilutive.
At December 31, 2020, the majority of the anti-dilutive shares were granted at an exercise price of $12.15, which was higher than the average fair market value price of $7.42 for 2020. In 2019, the majority of the anti-dilutive shares were granted at an exercise price of $25.24, which was higher than the average fair market value price of $6.93 for 2019. In 2018, the majority of the anti-dilutive shares were granted at an exercise price of $25.24, which was higher than the average fair market value price of $15.27 for 2018.
For 2020, 2019 and 2018 the diluted net loss per share calculation, all the shares associated with stock options were anti-dilutive because of the company's loss.
For 2020, 2019 and 2018, no shares were included in the common shares assuming dilution related to the company's issued warrants as the average market price of the company stock for these periods did not exceed the strike price of the warrants.
| | | | | | | | |
| Notes to Financial Statements | | |
| Concentration of Credit Risk | | |
| | |
Concentration of Credit Risk
The company manufactures and distributes durable medical equipment to the home health care, retail and extended care markets. The company performs credit evaluations of its customers' financial condition. The company utilizes De Lage Landen, Inc. (“DLL”), a third-party financing company, to provide lease financing to Invacare's U.S. customers. The DLL agreement provides for direct leasing between DLL and the Invacare customer. The company retains a recourse obligation of $1,312,000 at December 31, 2020 to DLL for events of default under the contracts, which total $8,923,000 at December 31, 2020. Guarantees, ASC 460, requires the company to record a guarantee liability as it relates to the limited recourse obligation. As such, the company has recorded a liability of $28,000 for this guarantee obligation within accrued expenses. The company's recourse is reevaluated by DLL biannually, considers activity between the biannual dates and excludes any receivables purchased by the company from DLL. The company monitors the collections status of these contracts and has provided amounts for estimated losses in its allowances for doubtful accounts in accordance with Receivables, ASC 310-10-05-4. Credit losses are provided for in the financial statements.
Substantially all the company's receivables are due from health care, medical equipment providers and long-term care facilities located throughout the United States, Australia, Canada, New Zealand and Europe or also direct from governmental entities in certain countries. A significant portion of products sold to dealers, both foreign and domestic, is ultimately funded through government reimbursement programs such as Medicare and Medicaid. The company has also seen a significant shift in reimbursement to customers from managed care entities. As a consequence, changes in these programs can have an adverse impact on dealer liquidity and profitability. In addition, reimbursement guidelines in the home health care industry have a substantial impact on the nature and type of equipment an end user can obtain as well as the timing of reimbursement and, thus, affect the product mix, pricing and payment patterns of the company's customers.
The company's top 10 customers accounted for approximately 18.2% of 2020 net sales. The loss of business of one or more of these customers may have a significant impact on the company, although no single customer accounted for more than 5.9% of the company's 2020 net sales. Providers who are part of a buying group generally make individual purchasing decisions and are invoiced directly by the company.
| | | | | | | | |
| | Notes to Financial Statements |
| | Derivatives |
| | |
Derivatives
ASC 815 requires companies to recognize all derivative instruments in the consolidated balance sheet as either assets or liabilities at fair value. The accounting for changes in fair value of a derivative is dependent upon whether or not the derivative has been designated and qualifies for hedge accounting treatment and the type of hedging relationship. For derivatives designated and qualifying as hedging instruments, the company must designate the hedging instrument, based upon the exposure being hedged, as a fair value hedge, cash flow hedge, or a hedge of a net investment in a foreign operation.
Cash Flow Hedging Strategy
The company uses derivative instruments in an attempt to manage its exposure to transactional foreign currency exchange risk. Foreign forward exchange contracts are used to manage the price risk associated with forecasted sales denominated in foreign currencies and the price risk associated with forecasted purchases of inventory over the next twelve months.
The company recognizes its derivative instruments as assets or liabilities in the consolidated balance sheet measured at fair value. A majority of the company's derivative instruments are designated and qualify as cash flow hedges. Accordingly, the effective portion of the gain or loss on the derivative instrument is reported as a component of other comprehensive income and reclassified into earnings in the same period or periods during which the hedged transaction affects earnings. The remaining gain or loss on the derivative instrument in excess of the cumulative change in the fair value of the hedged item, if any, is recognized in current earnings during the period of change.
To protect against increases/decreases in forecasted foreign currency cash flows resulting from inventory purchases/sales over the next year, the company utilizes foreign currency forward contracts to hedge portions of its forecasted purchases/sales denominated in foreign currencies. The gains and losses are included in cost of products sold and selling, general and administrative expenses on the consolidated statement of comprehensive income (loss). If it is later determined that a hedged forecasted transaction is unlikely to occur, any prospective gains or losses on the forward contracts would be recognized in earnings. The company does not expect any material amount of hedge ineffectiveness related to forward contract cash flow hedges during the next twelve months.
The company has historically not recognized any material amount of ineffectiveness related to forward contract cash flow hedges because the company generally limits its hedges to between 50% and 90% of total forecasted transactions for a given entity's exposure to currency rate changes and the transactions hedged are recurring in nature. Furthermore, most of the hedged transactions are related to intercompany sales and purchases for which settlement occurs on a specific day each month. Forward contracts with a total notional amount in USD of $210,029,000 and $148,874,000 matured during the twelve months ended December 31, 2020 and 2019, respectively.
| | | | | | | | |
| Notes to Financial Statements | | |
| Derivatives | | |
| | |
Outstanding foreign currency forward exchange contracts qualifying and designated for hedge accounting treatment were as follows (in thousands USD):
| | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | December 31, 2019 |
| | Notional
Amount | | Unrealized
Net Gain
(Loss) | | Notional
Amount | | Unrealized
Net Gain
(Loss) |
| USD / AUD | $ | — | | | $ | — | | | $ | 3,840 | | | $ | (106) | |
| USD / CAD | — | | | — | | | 3,888 | | | 32 | |
| USD / CHF | 1,675 | | | (11) | | | — | | | — | |
| | | | | | | |
| USD / EUR | 56,187 | | | (636) | | | 110,905 | | | 122 | |
| USD / GBP | 2,467 | | | (19) | | | 3,972 | | | (8) | |
| USD / NZD | — | | | — | | | 2,760 | | | (166) | |
| USD / SEK | 2,658 | | | (41) | | | 5,062 | | | (38) | |
| USD / MXN | 2,230 | | | 334 | | | 6,763 | | | 346 | |
| | | | | | | |
| EUR / CAD | — | | | — | | | 4,151 | | | 24 | |
| EUR / CHF | 5,037 | | | 10 | | | 9,821 | | | 10 | |
| | | | | | | |
| EUR / GBP | 19,060 | | | 44 | | | 29,824 | | | (216) | |
| EUR / NOK | 4,167 | | | (64) | | | 5,797 | | | 15 | |
| EUR / SEK | 10,162 | | | (73) | | | 9,493 | | | (46) | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| AUD / NZD | 781 | | | (13) | | | — | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| DKK / SEK | 3,329 | | | 9 | | | 5,936 | | | 24 | |
| | | | | | | |
| NOK / SEK | 3,431 | | | (50) | | | 5,151 | | | 18 | |
| AUD / THB | 4,963 | | | (221) | | | — | | | — | |
| NZD / THB | 1,755 | | | (55) | | | — | | | — | |
| USD / THB | 4,152 | | | (56) | | | — | | | — | |
| EUR / THB | 1,332 | | | 18 | | | — | | | — | |
| GBP / THB | 842 | | | 10 | | | — | | | — | |
| $ | 124,228 | | | $ | (814) | | | $ | 207,363 | | | $ | 11 | |
Derivatives Not Qualifying or Designated for Hedge Accounting Treatment
The company utilizes foreign currency forward contracts that are not designated as hedges in accordance with ASC 815. These contracts are entered into to eliminate the risk associated with the settlement of short-term intercompany trading receivables and payables between Invacare Corporation and its foreign subsidiaries. The currency forward contracts are entered into at the same time as the intercompany receivables or payables are created so that upon settlement, the gain/loss on the settlement is offset by the gain/loss on the foreign currency forward contract. No material net gain or loss was realized by the company in 2020 or 2019 related to these contracts and the associated short-term intercompany trading receivables and payables.
| | | | | | | | |
| | Notes to Financial Statements |
| | Derivatives |
| | |
Foreign currency forward exchange contracts not qualifying or designated for hedge accounting treatment, as well as ineffective hedges, entered into in 2020 and 2019, respectively, and outstanding were as follows (in thousands USD):
| | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | December 31, 2019 |
| | Notional
Amount | | Gain
(Loss) | | Notional
Amount | | Gain
(Loss) |
| AUD / USD | $ | 6,046 | | | $ | (159) | | | $ | 10,000 | | | $ | (94) | |
| CAD / USD | 8,320 | | | 88 | | | 8,000 | | | $ | (50) | |
| | | | | | | |
| EUR / USD | — | | | — | | | 10,000 | | | 104 | |
| | | | | | | |
| DKK / USD | 8,690 | | | 207 | | | — | | | — | |
| GBP / USD | 16,062 | | | 338 | | | 7,000 | | | 40 | |
| NZD / USD | — | | | — | | | 4,500 | | | (101) | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| NZD / AUD | 6,579 | | | (35) | | | 7,900 | | | 23 | |
| NOK / USD | 9,053 | | | 264 | | | — | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| $ | 54,750 | | | $ | 703 | | | $ | 47,400 | | | $ | (78) | |
The fair values of the company's derivative instruments were as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | December 31, 2019 |
| | Assets | | Liabilities | | Assets | | Liabilities |
| Derivatives designated as hedging instruments under ASC 815 | | | | | | | |
| Foreign currency forward exchange contracts | $ | 424 | | | $ | 1,238 | | | $ | 668 | | | $ | 657 | |
| | | | | | | |
| Derivatives not designated as hedging instruments under ASC 815 | | | | | | | |
| Foreign currency forward exchange contracts | 897 | | | 194 | | | 170 | | | 248 | |
| Total derivatives | $ | 1,321 | | | $ | 1,432 | | | $ | 838 | | | $ | 905 | |
The fair values of the company's foreign currency forward exchange contract assets and liabilities are included in Other Current Assets and Accrued Expenses, respectively in the Consolidated Balance Sheets.
The effect of derivative instruments on Accumulated Other Comprehensive Income (OCI) and the Statement of Comprehensive Income (Loss) was as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Derivatives (foreign currency forward exchange contracts) in ASC 815 cash flow hedge relationships | Amount of Gain
(Loss) Recognized in Accumulated OCI on Derivatives
(Effective Portion) | | Amount of Gain (Loss)
Reclassified from
Accumulated OCI into
Income (Effective
Portion) | | Amount of Gain (Loss)
Recognized in Income
on Derivatives (Ineffective Portion
and Amount Excluded from
Effectiveness Testing) |
| | | | | |
| Year ended December 31, 2020 | $ | (2,129) | | | $ | (1,407) | | | $ | — | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Year ended December 31, 2019 | $ | 1,958 | | | $ | 2,528 | | | $ | — | |
| | | | | |
| | | | | |
| | | | | |
| Derivatives (foreign currency forward exchange contracts) not designated as hedging instruments under ASC 815 | Amount of Gain (Loss)
Recognized in Income on
Derivatives | | | | |
| | | | | |
| Year ended December 31, 2020 | $ | 703 | | | | | |
| Year ended December 31, 2019 | $ | (78) | | | | | |
| | | | | | | | |
| Notes to Financial Statements | | |
| Derivatives | | |
| | |
The gains or losses recognized as the result of the settlement of cash flow hedge foreign currency forward contracts are recognized in net sales for hedges of inventory sales and in cost of product sold for hedges of inventory purchases. In 2020, net sales were increased by $1,359,000 and cost of product sold was increased by $2,826,000 for a net pre-tax realized loss of $1,467,000. In 2019, net sales were increased by $52,000 and cost of products sold was decreased by $2,673,000 for a net pre-tax realized gain of $2,725,000. In 2018, net sales were decreased by $1,352,000 and cost of products sold was decreased by $1,591,000 for a net realized pre-tax gain of $239,000.
A gain of $703,000 in 2020, a loss of $78,000 in 2019 and a gain of $150,000 in 2018 were recognized in selling, general and administrative (SG&A) expenses related to forward contracts not designated as hedging instruments. The forward contracts were entered into to offset gains/losses that were also recorded in SG&A expenses on intercompany trade receivables or payables. The gains/losses on the non-designated hedging instruments were substantially offset by gains/losses on intercompany trade payables.
The company's derivative agreements provide the counterparties with a right of set off in the event of a default. The right of set off would enable the counterparty to offset any net payment due by the counterparty to the company under the applicable agreement by any amount due by the company to the counterparty under any other agreement. For example, the terms of the agreement would permit a counterparty to a derivative contract that is also a lender under the company's Credit Agreement to reduce any derivative settlement amounts owed to the company under the derivative contract by any amounts owed to the counterparty by the company under the Credit Agreement. In addition, the agreements contain cross-default provisions that could trigger a default by the company under the agreement in the event of a default by the
company under another agreement with the same counterparty. The company does not present any derivatives on a net basis in its financial statements, other than the conversion and bond hedge derivatives which are presented net on the Consolidated Statement of Comprehensive Income (Loss), and all derivative balances presented are subject to provisions that are similar to master netting agreements.
During the first quarter of 2016, the company entered into privately negotiated convertible 2021 note hedges and 2021 warrants in connection with its sale of $150,000,000 in aggregate principal amount of the company's 5.00% Convertible Senior Notes due 2021. The 2021 warrants, which increased paid in capital by $12,376,000, are clearly and closely related to the convertible 2021 notes and thus classified as equity. The 2021 note hedge asset and 2021 convertible debt conversion liability were recorded, based on initial fair values, as an asset of $27,975,000 and a liability of $34,480,000, respectively, with the offset to the income statement.
During the second quarter of 2017, the company entered into privately negotiated convertible 2022 note hedges and warrants in connection with its sale of $120,000,000 in aggregate principal amount of the company's 4.50% Convertible Senior Notes due 2022. The 2022 warrants, which increased paid in capital by $14,100,000, are clearly and closely related to the convertible 2022 notes and thus classified as equity. The 2022 note hedge assets and 2022 convertible debt conversion liability were recorded, based on initial fair values, as an asset of $24,780,000 and a liability of $28,859,000, respectively, with the offset to the income statement.
| | | | | | | | |
| | Notes to Financial Statements |
| | Derivatives |
| | |
The fair values of the outstanding convertible note derivatives as of December 31, 2020 and their effect on the Statement of Comprehensive Income (Loss) were as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| | | | Gain (Loss) |
| | Fair Value | | Twelve Months Ended |
| December 31, 2020 | | December 31, 2020 | | December 31, 2019 |
| Convertible 2021 debt conversion long-term liability | $ | — | | | $ | — | | | $ | (2,210) | |
| Convertible 2022 debt conversion long-term liability | — | | | — | | | (6,193) | |
| Convertible 2021 note hedge long-term asset | — | | | — | | | 2,852 | |
| Convertible 2022 note hedge long-term asset | — | | | — | | | 6,748 | |
| Net fair value and net gains (losses) on convertible debt derivatives | $ | — | | | $ | — | | | $ | 1,197 | |
The 2021 and 2022 convertible debt conversion liability amounts and the 2021 and 2022 note hedge asset amounts are included in Other Long-Term Obligations and Other Long-Term Assets, respectively, in the company's Consolidated Balance Sheets. The year-to-date changes in the fair values of the convertible debt conversion liabilities and note hedge derivatives were significantly impacted by the change in the company's stock price.
On May 16, 2019, the company received shareholder approval authorizing it to elect to settle future conversions of convertible notes in common shares. As a result of the shareholder approval, the note hedge assets and conversion liabilities may no longer be bifurcated and accounted for as separate derivatives and thus were eliminated together with a corresponding offset to additional paid-in-capital.
| | | | | | | | |
| Notes to Financial Statements | | |
| Fair Values | | |
| | |
Fair Values
Pursuant to ASC 820, the inputs used to derive the fair value of assets and liabilities are analyzed and assigned a level I, II or III priority, with level I being the highest and level III being the lowest in the hierarchy. Level I inputs are quoted prices in active markets for identical assets or liabilities.
Level II inputs are quoted prices for similar assets or liabilities in active markets: quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations in which all significant inputs are observable in active markets. Level III inputs are based on valuations derived from valuation techniques in which one or more significant inputs are unobservable.
The following table provides a summary of the company's assets and liabilities that are measured on a recurring basis (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | | Basis for Fair Value Measurements at Reporting Date |
| | Quoted Prices in Active Markets
for Identical
Assets / (Liabilities) | | Significant
Other
Observable
Inputs | | Significant
Other
Unobservable
Inputs |
| | Level I | | Level II | | Level III |
| December 31, 2020 | | | | | | | |
| Forward exchange contracts—net | | | — | | | $ | (111) | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| December 31, 2019 | | | | | | | |
| Forward exchange contracts—net | | | — | | | $ | (67) | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
The carrying and fair values of the company's financial instruments at December 31, 2020 and 2019 are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2020 | | 2019 |
| | Carrying
Value | | Fair Value | | Carrying
Value | | Fair Value |
| Cash and cash equivalents | $ | 105,298 | | | $ | 105,298 | | | $ | 80,063 | | | $ | 80,063 | |
| Other investments | 85 | | | 85 | | | 85 | | | 85 | |
| Installment receivables, net of reserves | 1,322 | | | 1,322 | | | 913 | | | 913 | |
| Total debt (including current maturities of long-term debt) * | (245,053) | | | (237,948) | | | (219,522) | | | (207,193) | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Forward contracts in Other Current Assets | 1,321 | | | 1,321 | | | 838 | | | 838 | |
| Forward contracts in Accrued Expenses | (1,432) | | | (1,432) | | | (905) | | | (905) | |
________
* The company's total debt is shown net of discount and fees associated with the convertible senior notes due 2021, 2022 and 2024 on the company's consolidated balance sheet. Accordingly, the fair values of the convertible senior notes due 2021, 2022 and 2024 are included in the long-term debt presented in this table are also shown net of the discount and fees. Total debt amounts exclude operating and finance lease obligations.
The company, in estimating its fair value disclosures for financial instruments, used the following methods and assumptions:
Cash, cash equivalents: The carrying value reported in the balance sheet for cash, cash equivalents equals its fair value.
Other investments: The company has an investment in a limited partnership, which is accounted for using the cost method, adjusted for any estimated declines in value. The investment was acquired in a private placement and
there is no quoted market price or stated rate of return. The company does not have the ability to easily sell the investment. The company completes an evaluation of the residual value related to such investments in the fourth quarter of each year. No impairment was recognized in 2020, 2019 or 2018.
Installment receivables: The carrying value reported in the balance sheet for installment receivables approximates its fair value. The interest rates associated with these receivables have not varied significantly since inception. Management believes that after consideration of
| | | | | | | | |
| | Notes to Financial Statements |
| | Fair Values |
| | |
the credit risk, the net book value of the installment receivables approximates market value.
Total debt: Fair value for the company's convertible debt is based on quoted market-based estimates as of the end of the period, while the revolving credit facility fair value is based upon an estimate of the market for similar borrowing arrangements. The fair values are deemed to be categorized as Level 2 in the fair value hierarchy.
Forward Contracts: The company operates internationally, and as a result, is exposed to foreign currency fluctuations. Specifically, the exposure includes intercompany loans and third-party sales or payments. In an attempt to reduce this exposure, foreign currency forward contracts are utilized and accounted for as hedging instruments. The forward contracts are used to hedge the following currencies: AUD, CAD, CHF, DKK, EUR, GBP, MXN, NOK, NZD, SEK, THB and USD. The company does not use derivative financial instruments for speculative purposes. Fair values for the company's foreign exchange forward contracts are based on quoted market prices for contracts with similar maturities. The company's forward contracts are included in Other Current Assets or Accrued Expenses in the consolidated balance sheets.
The gains and losses that result from the majority of the forward contracts are deferred and recognized when the offsetting gains and losses for the identified transactions are recognized. The company recognized a net pre-tax loss of $1,467,000 in 2020 compared to net pre-tax gains of $2,725,000 and $239,000 in 2019 and 2018, respectively, related to ASC 815 designated derivatives. Gains or losses recognized as the result of the settlement of forward contracts are recognized in cost of products sold for hedges of inventory transactions, sales for hedges of forecasted sales or selling, general and administrative expenses for other hedged transactions.
| | | | | | | | |
| Notes to Financial Statements | | |
| Business Segments | | |
| | |
Business Segments
The company operates in two primary business segments: North America and Europe with each selling the company's primary product categories, which include: lifestyle, mobility and seating and respiratory therapy products. Sales in Asia Pacific are reported in All Other and include products similar to those sold in North America and Europe. The accounting policies of each segment are the same as those described in the summary of significant accounting policies for the company's consolidated financial statements. Intersegment sales and transfers are based on the costs to manufacture plus a reasonable profit element.
Segment performance is measured and resources are allocated based on a number of factors, with the primary profit or loss measure being segment operating income (loss). Segment operating income (loss) represents net sales less cost of products sold less selling general and
administrative expenses. Segment operating income (loss) excludes unallocated corporate general and administrative expenses not allocated to the segments and intersegment sales and profit eliminations, which are included in All Other. In addition, segment operating income (loss) further excludes charges related to restructuring activities, asset impairment and gain on sale of business (as applicable).
This performance measure, segment operating income (loss), is used by the Chief Operating Decision Maker (CODM) for purposes of making decisions about allocating resources to a segment and assessing its performance. In addition, this metric is reviewed by the company's Board of Directors regarding segment performance and is a key metric in the performance management assessment of the company's employees.
The information by segment is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Revenues from external customers | | | | | |
| Europe | $ | 468,041 | | | $ | 533,048 | | | $ | 558,518 | |
| North America | 348,307 | | | 348,201 | | | 364,590 | |
| All Other (Asia Pacific) | 34,341 | | | 46,715 | | | 49,239 | |
| Consolidated | $ | 850,689 | | | $ | 927,964 | | | $ | 972,347 | |
| Intersegment revenues | | | | | |
| Europe | $ | 17,384 | | | $ | 14,185 | | | $ | 15,784 | |
| North America | 80,748 | | | 80,727 | | | 90,944 | |
| | | | | |
| All Other (Asia Pacific) | 2,528 | | | 13,033 | | | 17,737 | |
| Consolidated | $ | 100,660 | | | $ | 107,945 | | | $ | 124,465 | |
| | | | | |
| Restructuring charges before income taxes | | | | | |
| Europe | $ | 5,934 | | | $ | 9,579 | | | $ | 1,773 | |
| North America | 1,306 | | | 1,617 | | | 1,359 | |
| All Other | 118 | | | 633 | | | 349 | |
| Consolidated | $ | 7,358 | | | $ | 11,829 | | | $ | 3,481 | |
| | | | | |
| | | | | |
| Depreciation and amortization | | | | | |
| Europe | $ | 7,615 | | | $ | 7,851 | | | $ | 8,125 | |
| North America | 6,013 | | | 6,429 | | | 6,228 | |
| | | | | |
| | | | | |
| All Other (1) | 689 | | | 1,283 | | | 1,203 | |
| | | | | |
| Consolidated | $ | 14,317 | | | $ | 15,563 | | | $ | 15,556 | |
| | | | | |
| | | | | |
| | | | | |
| Net interest expense | | | | | |
| Europe | $ | 1,884 | | | $ | 368 | | | $ | 225 | |
| North America | 26,510 | | | 28,070 | | | 27,355 | |
| | | | | |
| All Other | 12 | | | 209 | | | 222 | |
| Consolidated | $ | 28,406 | | | $ | 28,647 | | | $ | 27,802 | |
| Operating income (loss) | | | | | |
| Europe | $ | 22,682 | | | $ | 36,174 | | | $ | 32,673 | |
| | | | | | | | |
| | Notes to Financial Statements |
| | Business Segments |
| | |
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| North America | 9,449 | | | (7,592) | | | (32,506) | |
| | | | | |
| | | | | |
| All Other (1) | (23,236) | | | (26,576) | | | (14,397) | |
| Charges related to restructuring activities | (7,358) | | | (11,829) | | | (3,481) | |
| Gain on sale of business | 9,790 | | | — | | | — | |
| Asset write-off | — | | | (587) | | | (583) | |
| Consolidated operating income (loss) | 11,327 | | | (10,410) | | | (18,294) | |
| Net gain on convertible derivatives | — | | | 1,197 | | | 11,994 | |
| Loss on debt extinguishment including debt finance charges and fees | (7,360) | | | (6,165) | | | — | |
| Net interest expense | (28,406) | | | (28,647) | | | (27,802) | |
| Loss before income taxes | $ | (24,439) | | | $ | (44,025) | | | $ | (34,102) | |
| Assets | | | | | |
| Europe | $ | 705,314 | | | $ | 602,471 | | | $ | 611,230 | |
| North America | 207,347 | | | 212,733 | | | 242,341 | |
| | | | | |
| | | | | |
| All Other | 33,320 | | | 36,922 | | | 32,284 | |
| | | | | |
| Consolidated | $ | 945,981 | | | $ | 852,126 | | | $ | 885,855 | |
| Long-lived assets | | | | | |
| Europe | $ | 472,599 | | | $ | 408,847 | | | $ | 407,021 | |
| North America | 92,195 | | | 79,369 | | | 77,009 | |
| | | | | |
| | | | | |
| All Other | 6,721 | | | 8,033 | | | 4,415 | |
| | | | | |
| Consolidated | $ | 571,515 | | | $ | 496,249 | | | $ | 488,445 | |
| Expenditures for assets | | | | | |
| Europe | $ | 5,221 | | | $ | 6,041 | | | $ | 5,348 | |
| North America (2) | 16,473 | | | 3,679 | | | 3,648 | |
| | | | | |
| | | | | |
| All Other | 610 | | | 1,154 | | | 827 | |
| | | | | |
| Consolidated | $ | 22,304 | | | $ | 10,874 | | | $ | 9,823 | |
________________________
(1) Consists of unallocated corporate SG&A costs and intercompany profits, which do not meet the quantitative criteria for determining reportable segments.
(2) The 2020 expenditure for assets primarily driven by the company's ERP project.
| | | | | | | | |
| Notes to Financial Statements | | |
| Business Segments | | |
| | |
Net sales by product line, are as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Europe | | | | | |
| Lifestyle | $ | 222,668 | | | $ | 245,987 | | | $ | 263,340 | |
| Mobility and Seating | 200,687 | | | 249,144 | | | 252,997 | |
| Respiratory Therapy | 24,786 | | | 19,258 | | | 23,736 | |
| Other (1) | 19,900 | | | 18,659 | | | 18,445 | |
| $ | 468,041 | | | $ | 533,048 | | | $ | 558,518 | |
| North America | | | | | |
| Lifestyle | $ | 165,267 | | | $ | 173,039 | | | $ | 172,622 | |
| Mobility and Seating | 109,923 | | | 121,955 | | | 122,013 | |
| Respiratory Therapy | 72,285 | | | 51,649 | | | 67,797 | |
| Other (1) | 832 | | | 1,558 | | | 2,158 | |
| $ | 348,307 | | | $ | 348,201 | | | $ | 364,590 | |
| | | | | |
| | | | | |
| | | | | |
| All Other (Asia Pacific) | | | | | |
| Mobility and Seating | $ | 14,150 | | | $ | 28,448 | | | $ | 31,286 | |
| Lifestyle | 13,503 | | | 10,831 | | | 10,829 | |
| | | | | |
| Respiratory Therapy | 1,383 | | | 1,283 | | | 1,330 | |
| Other (1) | 5,305 | | | 6,153 | | | 5,794 | |
| $ | 34,341 | | | $ | 46,715 | | | $ | 49,239 | |
| | | | | |
| Total Consolidated | $ | 850,689 | | | $ | 927,964 | | | $ | 972,347 | |
________________________
(1)Includes various services, including repair services, equipment rentals and external contracting.
| | | | | | | | |
| | Notes to Financial Statements |
| | Contingencies |
| | |
Contingencies
General
In the ordinary course of its business, the company is a defendant in a number of lawsuits, primarily product liability actions in which various plaintiffs seek damages for injuries allegedly caused by defective products. All the product liability lawsuits that the company faces in the United States have been referred to the company's captive insurance company and/or excess insurance carriers while all non-U.S. lawsuits have been referred to the company's commercial insurance carriers. All such lawsuits are generally contested vigorously. The coverage territory of the company's insurance is worldwide with the exception of those countries with respect to which, at the time the product is sold for use or at the time a claim is made, the U.S. government has suspended or prohibited diplomatic or trade relations. The amount recorded for identified contingent liabilities is based on estimates. Amounts recorded are reviewed periodically and adjusted to reflect additional technical and legal information that becomes available. Actual costs to be incurred in future periods may vary from the estimates, given the inherent uncertainties in evaluating certain exposures.
As a medical device manufacturer, the company is subject to extensive government regulation, including numerous laws directed at preventing fraud and abuse and laws regulating reimbursement under various government programs. The marketing, invoicing, documenting, developing, testing, manufacturing, labeling, promoting, distributing and other practices of health care suppliers and medical device manufacturers are all subject to government scrutiny. Most of the company's facilities are subject to inspection at any time by the FDA or similar medical device regulatory agencies in other jurisdictions. Violations of law or regulations can result in administrative, civil and criminal penalties and sanctions, which could have a material adverse effect on the company's business.
Medical Device Regulatory Matters
The FDA in the United States and comparable medical device regulatory authorities in other jurisdictions regulate virtually all aspects of the marketing, invoicing, documenting, development, testing, manufacturing, labeling, promotion, distribution and other practices regarding medical devices. The company and its products are subject to the laws and regulations of the FDA and other regulatory bodies in the various jurisdictions where the company's products are manufactured or sold. The company's failure to comply with the regulatory requirements of the FDA and other applicable medical device regulatory requirements can subject the company to administrative or judicially imposed sanctions or
enforcement actions. These sanctions include injunctions, consent decrees, warning letters, civil penalties, criminal penalties, product seizure or detention, product recalls and total or partial suspension of production.
In December 2012, the company became subject to a consent decree of injunction filed by the FDA with respect to the company's Corporate facility and its Taylor Street manufacturing facility in Elyria, Ohio. The consent decree initially limited the company's (i) manufacture and distribution of power and manual wheelchairs, wheelchair components and wheelchair sub-assemblies at or from its Taylor Street manufacturing facility, except in verified cases of medical necessity, (ii) design activities related to wheelchairs and power beds that take place at the impacted Elyria facilities and (iii) replacement, service and repair of products already in use from the Taylor Street manufacturing facility. Under the terms of the consent decree, in order to resume full operations, the company had to successfully complete independent, third-party expert certification audits at the impacted Elyria facilities, comprising three distinct certification reports separately submitted to, and subject to acceptance by, the FDA; submit its own report to the FDA; and successfully complete a reinspection by the FDA of the company's Corporate and Taylor Street facilities.
On July 24, 2017, following its June 2017 reinspection of the Corporate and Taylor Street facilities, the FDA notified the company that it is in substantial compliance with the FDA Act, FDA regulations and the terms of the consent decree and, that the company was permitted to resume full operations at those facilities including the resumption of unrestricted sales of products made in those facilities.
The consent decree will continue in effect for at least five years from July 24, 2017, during which time the company's Corporate and Taylor Street facilities must complete two semi-annual audits in the first year and then four annual audits in the next four years performed by a company-retained expert firm. The expert audit firm will determine whether the facilities remain in continuous compliance with the FDA Act, FDA regulations and the terms of the consent decree. The FDA has the authority to inspect these facilities and any other FDA registered facility, at any time.
The FDA has continued to actively inspect the company's facilities, other than through the processes established under the consent decree. The company expects that the FDA will, from time to time, inspect substantially all the company's domestic and foreign FDA-registered facilities.
| | | | | | | | |
| Notes to Financial Statements | | |
| Contingencies | | |
| | |
The results of regulatory claims, proceedings, investigations, or litigation are difficult to predict. An unfavorable resolution or outcome of any FDA warning letters or inspectional observations, or other FDA enforcement related to company facilities, could materially and adversely affect the company's business, financial condition, and results of operations.
The limitations previously imposed by the FDA consent decree negatively affected net sales in the North America segment and, to a certain extent, the Asia Pacific region beginning in 2012. The limitations led to delays in new product introductions. Further, uncertainty regarding how long the limitations would be in effect limited the company's ability to renegotiate and bid on certain customer contracts and otherwise led to a decline in customer orders.
Although the company has been permitted to resume full operations at the Corporate and Taylor Street facilities, the negative effect of the consent decree on customer orders and net sales in the North America segment and Asia Pacific region has been considerable, and it is uncertain as to whether, or how quickly, the company will be able to rebuild net sales to more typical historical levels, irrespective of market conditions. Accordingly, when compared to the company's 2010 results, the previous limitations in the consent decree had, and likely may continue to have, a material adverse effect on the company's business, financial condition and results of operations.
Warranty Matters
The company's warranty reserves are subject to adjustment in future periods based on historical analysis of warranty claims and as new developments occur that may change the company's estimates related to specific product recalls. Refer to Current Liabilities in the Notes to the Consolidated Financial Statements for the total provision amounts and a reconciliation of the changes in the warranty accrual.
Any of the above contingencies could have an adverse impact on the company's financial condition or results of operations.
| | | | | | | | |
| | Notes to Financial Statements |
| | Interim Financial Information |
| | |
Interim Financial Information
| | | | | | | | | | | | | | | | | | | | | | | |
| (In thousands, except per share data - unaudited) | QUARTER ENDED |
| 2020 | March 31, | | June 30, | | September 30, | | December 31, |
| | | | | | | |
| Net sales | $ | 218,440 | | | $ | 196,300 | | | $ | 211,906 | | | $ | 224,043 | |
| Gross profit | 62,988 | | | 56,650 | | | 60,040 | | | 65,574 | |
| Income (Loss) before income taxes | 2,832 | | | (15,869) | | | (5,226) | | | (6,176) | |
| | | | | | | |
| | | | | | | |
| Net income (loss) | 732 | | | (16,619) | | | (7,276) | | | (5,117) | |
| | | | | | | |
| | | | | | | |
| Net income (loss) per share—basic | 0.02 | | | (0.48) | | | (0.21) | | | (0.15) | |
| | | | | | | |
| | | | | | | |
| Net income (loss) per share—assuming dilution * | 0.02 | | | (0.48) | | | (0.21) | | | (0.15) | |
| | | | | | | |
| 2019 | March 31, | | June 30, | | September 30, | | December 31, |
| | | | | | | |
| Net sales | $ | 223,419 | | | $ | 235,858 | | | $ | 235,774 | | | $ | 232,913 | |
| Gross profit | 61,455 | | | 65,066 | | | 67,585 | | | 67,961 | |
| Loss from before income taxes | (11,936) | | | (10,642) | | | (4,741) | | | (16,706) | |
| | | | | | | |
| | | | | | | |
| Net loss | (13,886) | | | (12,717) | | | (8,041) | | | (18,683) | |
| | | | | | | |
| | | | | | | |
| Net loss per share—basic | (0.42) | | | (0.38) | | | (0.24) | | | (0.56) | |
| | | | | | | |
| | | | | | | |
| Net loss per share—assuming dilution * | (0.42) | | | (0.38) | | | (0.24) | | | (0.56) | |
* Net earnings (loss) per share assuming dilution calculated utilizing weighted average shares outstanding - basic in periods in which there is a net loss.
The description of significant items affecting each quarter presented are detailed below.
Net income and earnings per share for the quarter ended March 31, 2020 reflects restructuring charges of $1,392,000 ($1,181,000 after tax or $0.03 per share assuming dilution) and net gain on sale of business of $9,590,000 ($10,578,000 after tax or $0.31 per share assuming dilution).
Loss and loss per share for the quarter ended June 30, 2020 reflects restructuring charges of $1,685,000 ($1,304,000 after tax or $0.04 per share assuming dilution) and loss on debt extinguishment including debt finance charges and fees of $6,599,000 ($6,599,000 after tax or $0.19 per share assuming dilution).
Loss and loss per share for the quarter ended September 30, 2020 reflects restructuring charges of $1,580,000 ($1,092,000 after tax or $0.03 per share assuming dilution) and loss on debt extinguishment including debt finance charges and fees of $761,000 ($761,000 after tax or $0.02 per share assuming dilution).
Loss and loss per share for the quarter ended December 31, 2020 reflects restructuring charges of $2,701,000 pre-tax ($2,062,000 after tax or $0.06 per share assuming dilution).
Loss and loss per share for the quarter ended March 31, 2019 reflects restructuring charges of $692,000 ($642,000 after tax or $0.02 per share assuming dilution) and net loss on convertible debt derivatives of $273,000 ($273,000 after tax or $0.01 per share assuming dilution).
Loss and loss per share for the quarter ended June 30, 2019 reflects restructuring charges of $1,321,000 ($1,200,000 after tax or $0.04 per share assuming dilution) and net gain on convertible debt derivatives of $1,470,000 ($1,470,000 after tax or $0.04 per share assuming dilution).
Loss and loss per share for the quarter ended September 30, 2019 reflects restructuring charges of $1,628,000 ($1,229,000 after tax or $0.04 per share assuming dilution).
Loss and loss per share for the quarter ended December 31, 2019 reflects restructuring charges of $8,188,000 pre-tax ($5,932,000 after tax or $0.18 per share assuming dilution), loss on debt extinguishment including debt finance charges and fees of $5,885,000 pre-tax ($5,885,000 after tax or $0.17 per share assuming dilution) and an intangible asset impairment of $587,000 ($435,000 after-tax expense or $0.01 per share assuming dilution).
| | | | | | | | |
| Schedule II - Valuation and Qualifying Accounts | | |
| | |
| | |
SCHEDULE II - VALUATION AND QUALIFYING ACCOUNTS
| | | | | | | | | | | | | | | | | | | | | | | |
| COL A. | | COL B. | | COL C. | | COL D. |
| | Balance
At
Beginning
of Period | | Charged
To Cost
And
Expenses | | Additions
(Deductions)
Described Below | | Balance
At End
of Period |
| | | | (In thousands) | | |
| Year Ended December 31, 2020 | | | | | | | |
| Deducted from asset accounts— | | | | | | | |
| Allowance for doubtful accounts | $ | 6,318 | | | $ | 427 | | | $ | (2,227) | | (A) | $ | 4,518 | |
| Inventory obsolescence reserve | 18,178 | | | 3,304 | | | (817) | | (B) | 20,665 | |
| Tax valuation allowances | 162,790 | | | (701) | | | 1,209 | | (C) | 163,298 | |
| Accrued warranty cost | 11,626 | | | 7,408 | | | (8,043) | | (B) | 10,991 | |
| Accrued product liability | 16,150 | | | 1,139 | | | (2,532) | | (D) | 14,757 | |
| Year Ended December 31, 2019 | | | | | | | |
| Deducted from asset accounts— | | | | | | | |
| Allowance for doubtful accounts | $ | 6,810 | | | $ | 955 | | | $ | (1,447) | | (A) | $ | 6,318 | |
| Inventory obsolescence reserve | 18,342 | | | 3,542 | | | (3,706) | | (B) | 18,178 | |
| Tax valuation allowances | 174,659 | | | (8,413) | | | (3,456) | | (C) | 162,790 | |
| Accrued warranty cost | 16,353 | | | 6,155 | | | (10,882) | | (B) | 11,626 | |
| Accrued product liability | 16,593 | | | 2,527 | | | (2,970) | | (D) | 16,150 | |
| Year Ended December 31, 2018 | | | | | | | |
| Deducted from asset accounts— | | | | | | | |
| Allowance for doubtful accounts | $ | 7,757 | | | $ | 2,029 | | | $ | (2,976) | | (A) | $ | 6,810 | |
| Inventory obsolescence reserve | 19,003 | | | 3,673 | | | (4,334) | | (B) | 18,342 | |
| Tax valuation allowances | 167,203 | | | 13,517 | | | (6,061) | | (C) | 174,659 | |
| Accrued warranty cost | 22,468 | | | 7,616 | | | (13,731) | | (B) | 16,353 | |
| Accrued product liability | 16,480 | | | 5,586 | | | (5,473) | | (D) | 16,593 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
________________________
Note (A)—Uncollectible accounts written off, net of recoveries and net of foreign currency translation adjustment.
Note (B)—Amounts written off or payments incurred, net of foreign currency translation adjustment.
Note (C)—Other activity not affecting federal or foreign tax expense, net of foreign currency translation adjustment.
Note (D)—Losses paid and loss adjustments, net of foreign currency translation adjustment.