00000377852020FYFALSEP3YP3YP3Yus-gaap:OtherAssetsNoncurrentus-gaap:AccruedLiabilitiesCurrentus-gaap:AccruedLiabilitiesCurrentus-gaap:OtherLiabilitiesNoncurrentus-gaap:OtherLiabilitiesNoncurrentP3YP3YP3Y00000377852020-01-012020-12-31iso4217:USD00000377852020-06-30xbrli:shares00000377852020-12-3100000377852019-01-012019-12-3100000377852018-01-012018-12-31iso4217:USDxbrli:shares00000377852019-12-310000037785fmc:FuradanProductExitMember2020-12-3100000377852018-12-3100000377852017-12-310000037785us-gaap:CommonStockMember2017-12-310000037785us-gaap:AdditionalPaidInCapitalMember2017-12-310000037785us-gaap:RetainedEarningsMember2017-12-310000037785us-gaap:AccumulatedOtherComprehensiveIncomeMember2017-12-310000037785us-gaap:TreasuryStockMember2017-12-310000037785us-gaap:NoncontrollingInterestMember2017-12-310000037785us-gaap:RetainedEarningsMember2018-01-012018-12-310000037785us-gaap:NoncontrollingInterestMember2018-01-012018-12-310000037785us-gaap:AdditionalPaidInCapitalMember2018-01-012018-12-310000037785us-gaap:TreasuryStockMember2018-01-012018-12-310000037785us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-01-012018-12-310000037785us-gaap:CommonStockMember2018-12-310000037785us-gaap:AdditionalPaidInCapitalMember2018-12-310000037785us-gaap:RetainedEarningsMember2018-12-310000037785us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-12-310000037785us-gaap:TreasuryStockMember2018-12-310000037785us-gaap:NoncontrollingInterestMember2018-12-310000037785srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:RetainedEarningsMember2018-12-310000037785srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccumulatedOtherComprehensiveIncomeMember2018-12-310000037785srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2018-12-310000037785us-gaap:RetainedEarningsMember2019-01-012019-12-310000037785us-gaap:NoncontrollingInterestMember2019-01-012019-12-310000037785us-gaap:AdditionalPaidInCapitalMember2019-01-012019-12-310000037785us-gaap:TreasuryStockMember2019-01-012019-12-310000037785us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310000037785us-gaap:CommonStockMember2019-12-310000037785us-gaap:AdditionalPaidInCapitalMember2019-12-310000037785us-gaap:RetainedEarningsMember2019-12-310000037785us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-12-310000037785us-gaap:TreasuryStockMember2019-12-310000037785us-gaap:NoncontrollingInterestMember2019-12-310000037785us-gaap:RetainedEarningsMember2020-01-012020-12-310000037785us-gaap:NoncontrollingInterestMember2020-01-012020-12-310000037785us-gaap:AdditionalPaidInCapitalMember2020-01-012020-12-310000037785us-gaap:TreasuryStockMember2020-01-012020-12-310000037785us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310000037785us-gaap:CommonStockMember2020-12-310000037785us-gaap:AdditionalPaidInCapitalMember2020-12-310000037785us-gaap:RetainedEarningsMember2020-12-310000037785us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310000037785us-gaap:TreasuryStockMember2020-12-310000037785us-gaap:NoncontrollingInterestMember2020-12-31fmc:class0000037785fmc:LiventMember2018-10-15xbrli:pure0000037785us-gaap:LandImprovementsMember2020-01-012020-12-310000037785srt:MinimumMemberus-gaap:BuildingMember2020-01-012020-12-310000037785srt:MaximumMemberus-gaap:BuildingMember2020-01-012020-12-310000037785us-gaap:MachineryAndEquipmentMembersrt:MinimumMember2020-01-012020-12-310000037785srt:MaximumMemberus-gaap:MachineryAndEquipmentMember2020-01-012020-12-310000037785us-gaap:SoftwareDevelopmentMembersrt:MinimumMember2020-01-012020-12-310000037785srt:MaximumMemberus-gaap:SoftwareDevelopmentMember2020-01-012020-12-310000037785srt:MinimumMember2020-01-012020-12-310000037785srt:MaximumMember2020-01-012020-12-310000037785fmc:PocatelloMember2019-10-012019-12-3100000377852019-01-012019-01-010000037785us-gaap:AccountingStandardsUpdate201602Member2019-01-010000037785srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:RetainedEarningsMemberus-gaap:AccountingStandardsUpdate201602Member2019-01-01fmc:product0000037785fmc:FmcAgriculturalSolutionsMember2020-01-012020-12-310000037785srt:NorthAmericaMember2020-01-012020-12-310000037785srt:NorthAmericaMember2019-01-012019-12-310000037785srt:NorthAmericaMember2018-01-012018-12-310000037785srt:LatinAmericaMember2020-01-012020-12-310000037785srt:LatinAmericaMember2019-01-012019-12-310000037785srt:LatinAmericaMember2018-01-012018-12-310000037785us-gaap:EMEAMember2020-01-012020-12-310000037785us-gaap:EMEAMember2019-01-012019-12-310000037785us-gaap:EMEAMember2018-01-012018-12-310000037785srt:AsiaPacificMember2020-01-012020-12-310000037785srt:AsiaPacificMember2019-01-012019-12-310000037785srt:AsiaPacificMember2018-01-012018-12-310000037785country:US2020-01-012020-12-310000037785country:US2019-01-012019-12-310000037785country:US2018-01-012018-12-310000037785country:BR2020-01-012020-12-310000037785country:BR2019-01-012019-12-310000037785country:BR2018-01-012018-12-310000037785fmc:InsecticidesMember2020-01-012020-12-310000037785fmc:InsecticidesMember2019-01-012019-12-310000037785fmc:InsecticidesMember2018-01-012018-12-310000037785fmc:HerbicidesMember2020-01-012020-12-310000037785fmc:HerbicidesMember2019-01-012019-12-310000037785fmc:HerbicidesMember2018-01-012018-12-310000037785fmc:FungicidesMember2020-01-012020-12-310000037785fmc:FungicidesMember2019-01-012019-12-310000037785fmc:FungicidesMember2018-01-012018-12-310000037785fmc:OtherAgriculturalSolutionsMember2020-01-012020-12-310000037785fmc:OtherAgriculturalSolutionsMember2019-01-012019-12-310000037785fmc:OtherAgriculturalSolutionsMember2018-01-012018-12-310000037785srt:MinimumMember2020-12-310000037785srt:MaximumMember2020-12-310000037785srt:MinimumMemberus-gaap:RealEstateMember2020-12-310000037785srt:MaximumMemberus-gaap:RealEstateMember2020-12-310000037785fmc:NonRealEstatePropertiesMembersrt:MinimumMember2020-12-310000037785srt:MaximumMemberfmc:NonRealEstatePropertiesMember2020-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMember2017-11-012017-11-010000037785fmc:E.I.duPontdeNemoursandCompanyMember2020-01-012020-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMember2019-01-012019-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMember2018-01-012018-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMemberfmc:LegalandProfessionalFeesMember2020-01-012020-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMemberfmc:LegalandProfessionalFeesMember2019-01-012019-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMemberfmc:LegalandProfessionalFeesMember2018-01-012018-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMemberfmc:InventoryFairValueAmortizationMember2020-01-012020-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMemberfmc:InventoryFairValueAmortizationMember2019-01-012019-12-310000037785fmc:E.I.duPontdeNemoursandCompanyMemberfmc:InventoryFairValueAmortizationMember2018-01-012018-12-310000037785fmc:DuPontCropRestructuringMember2020-01-012020-12-310000037785fmc:DuPontCropRestructuringMember2019-01-012019-12-310000037785fmc:DuPontCropRestructuringMember2018-01-012018-12-310000037785fmc:DuPontCropRestructuringMember2020-12-310000037785srt:ScenarioForecastMembersrt:MinimumMemberfmc:E.I.duPontdeNemoursandCompanyMember2021-01-012021-12-310000037785srt:ScenarioForecastMembersrt:MaximumMemberfmc:E.I.duPontdeNemoursandCompanyMember2021-01-012021-12-3100000377852020-07-012020-09-300000037785us-gaap:CustomerRelationshipsMember2020-01-012020-12-310000037785us-gaap:CustomerRelationshipsMember2020-12-310000037785us-gaap:CustomerRelationshipsMember2019-12-310000037785us-gaap:PatentsMember2020-01-012020-12-310000037785us-gaap:PatentsMember2020-12-310000037785us-gaap:PatentsMember2019-12-310000037785us-gaap:TrademarksAndTradeNamesMember2020-01-012020-12-310000037785us-gaap:TrademarksAndTradeNamesMember2020-12-310000037785us-gaap:TrademarksAndTradeNamesMember2019-12-310000037785us-gaap:DevelopedTechnologyRightsMember2020-01-012020-12-310000037785us-gaap:DevelopedTechnologyRightsMember2020-12-310000037785us-gaap:DevelopedTechnologyRightsMember2019-12-310000037785us-gaap:OtherIntangibleAssetsMember2020-01-012020-12-310000037785us-gaap:OtherIntangibleAssetsMember2020-12-310000037785us-gaap:OtherIntangibleAssetsMember2019-12-310000037785fmc:CropProtectionBrandsMember2020-12-310000037785fmc:CropProtectionBrandsMember2019-12-310000037785us-gaap:TrademarksAndTradeNamesMember2020-12-310000037785us-gaap:TrademarksAndTradeNamesMember2019-12-310000037785fmc:OtherRestructuringActivitiesMember2020-01-012020-12-310000037785fmc:FuradanProductExitMember2019-01-012019-12-310000037785fmc:OtherRestructuringActivitiesMember2019-01-012019-12-310000037785fmc:OtherRestructuringActivitiesMember2018-01-012018-12-310000037785fmc:DuPontCropRestructuringIndiaMarketOperationsMember2018-01-012018-12-310000037785fmc:ChangeToMarketAccessMemberfmc:DuPontCropRestructuringMember2018-01-012018-12-310000037785fmc:SeveranceAndOtherEmployeeBenefitsMemberfmc:DuPontCropRestructuringMember2018-01-012018-12-310000037785fmc:MigrationOfResearchAndDevelopmentActivitiesAndEmployeesMemberfmc:DuPontCropRestructuringMember2018-01-012018-12-310000037785fmc:FutureLiabilityOfRentalObligationMemberfmc:DuPontCropRestructuringMember2018-01-012018-12-310000037785fmc:DuPontCropRestructuringMemberfmc:ReductionOfCapitalLeaseLiabilityMember2018-01-012018-12-310000037785fmc:SeveranceRelocationAndOtherEmployeeRelatedChargesMemberfmc:DuPontCropRestructuringMember2018-01-012018-12-310000037785fmc:DuPontCropRestructuringMember2018-12-310000037785fmc:DuPontCropRestructuringMember2019-12-310000037785fmc:OtherRestructuringActivitiesMember2018-12-310000037785fmc:OtherRestructuringActivitiesMember2019-12-310000037785fmc:OtherRestructuringActivitiesMember2020-12-3100000377852019-10-012019-12-310000037785fmc:IsagroMember2020-10-022020-10-020000037785fmc:IsagroMember2020-01-012020-12-310000037785fmc:LiventMember2019-03-010000037785us-gaap:DiscontinuedOperationsDisposedOfBySaleMemberfmc:FMCLithiumMember2020-01-012020-12-310000037785us-gaap:DiscontinuedOperationsDisposedOfBySaleMemberfmc:FMCLithiumMember2019-01-012019-12-310000037785us-gaap:DiscontinuedOperationsDisposedOfBySaleMemberfmc:FMCLithiumMember2018-01-012018-12-310000037785fmc:Omega3Memberus-gaap:DiscontinuedOperationsDisposedOfBySaleMember2017-08-010000037785us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberfmc:FmcHealthAndNutritionMember2017-11-012017-11-010000037785us-gaap:DiscontinuedOperationsHeldforsaleMemberfmc:FmcHealthAndNutritionMember2017-11-012017-11-010000037785us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberfmc:FmcHealthAndNutritionMember2020-01-012020-12-310000037785us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberfmc:FmcHealthAndNutritionMember2019-01-012019-12-310000037785us-gaap:DiscontinuedOperationsHeldForSaleOrDisposedOfBySaleMemberfmc:FmcHealthAndNutritionMember2018-01-012018-12-310000037785fmc:DiscontinuedworkerscompensationproductliabilityandotherpostretirementbenefitsMember2020-01-012020-12-310000037785fmc:DiscontinuedworkerscompensationproductliabilityandotherpostretirementbenefitsMember2019-01-012019-12-310000037785fmc:DiscontinuedworkerscompensationproductliabilityandotherpostretirementbenefitsMember2018-01-012018-12-310000037785fmc:DiscontinuedEnvironmentalLiabilitiesMember2020-01-012020-12-310000037785fmc:DiscontinuedEnvironmentalLiabilitiesMember2019-01-012019-12-310000037785fmc:DiscontinuedEnvironmentalLiabilitiesMember2018-01-012018-12-310000037785fmc:DiscontinuedLegalExpensesMember2020-01-012020-12-310000037785fmc:DiscontinuedLegalExpensesMember2019-01-012019-12-310000037785fmc:DiscontinuedLegalExpensesMember2018-01-012018-12-310000037785fmc:FmcHealthAndNutritionMember2020-01-012020-12-310000037785fmc:FmcHealthAndNutritionMember2019-01-012019-12-310000037785fmc:FmcHealthAndNutritionMember2018-01-012018-12-310000037785fmc:FMCLithiumMember2020-01-012020-12-310000037785fmc:FMCLithiumMember2019-01-012019-12-310000037785fmc:FMCLithiumMember2018-01-012018-12-31fmc:parcelOfLand0000037785fmc:NewarkEnvironmentalSiteMember2020-01-012020-12-310000037785fmc:NewarkEnvironmentalSiteMember2019-01-012019-12-310000037785fmc:WorkersCompensationAndProductLiabilityReserveMember2020-12-310000037785fmc:WorkersCompensationAndProductLiabilityReserveMember2019-12-310000037785fmc:OtherPostretirementMedicalAndLifeInsuranceBenefitsReservesMember2020-12-310000037785fmc:OtherPostretirementMedicalAndLifeInsuranceBenefitsReservesMember2019-12-310000037785us-gaap:LegalReserveMember2020-12-310000037785us-gaap:LegalReserveMember2019-12-310000037785fmc:WorkersCompensationAndProductLiabilityReserveMember2020-01-012020-12-310000037785fmc:WorkersCompensationAndProductLiabilityReserveMember2019-01-012019-12-310000037785fmc:WorkersCompensationAndProductLiabilityReserveMember2018-01-012018-12-310000037785fmc:OtherPostretirementMedicalAndLifeInsuranceBenefitsReservesMember2020-01-012020-12-310000037785fmc:OtherPostretirementMedicalAndLifeInsuranceBenefitsReservesMember2019-01-012019-12-310000037785fmc:OtherPostretirementMedicalAndLifeInsuranceBenefitsReservesMember2018-01-012018-12-310000037785us-gaap:LegalReserveMember2020-01-012020-12-310000037785us-gaap:LegalReserveMember2019-01-012019-12-310000037785us-gaap:LegalReserveMember2018-01-012018-12-310000037785fmc:OtherAssetsIncludingLongTermReceivablesNetMember2018-12-310000037785fmc:OtherAssetsIncludingLongTermReceivablesNetMember2019-01-012019-12-310000037785fmc:OtherAssetsIncludingLongTermReceivablesNetMember2019-12-310000037785fmc:OtherAssetsIncludingLongTermReceivablesNetMember2020-01-012020-12-310000037785fmc:OtherAssetsIncludingLongTermReceivablesNetMember2020-12-310000037785us-gaap:OtherAssetsMember2018-12-310000037785us-gaap:OtherAssetsMember2019-01-012019-12-310000037785us-gaap:OtherAssetsMember2019-12-310000037785us-gaap:OtherAssetsMember2020-01-012020-12-310000037785us-gaap:OtherAssetsMember2020-12-3100000377852020-01-012020-03-310000037785fmc:PocatelloMember2020-12-310000037785fmc:PocatelloMember2019-12-310000037785fmc:PocatelloMember1998-01-011998-12-310000037785fmc:PocatelloMember2006-04-252006-04-250000037785fmc:PocatelloMember2017-09-282017-09-280000037785fmc:PocatelloMember2019-11-152019-11-150000037785us-gaap:SubsequentEventMemberfmc:PocatelloMember2021-01-012021-02-250000037785us-gaap:SubsequentEventMemberfmc:PocatelloMember2021-01-012021-12-310000037785fmc:PocatelloMember2020-01-012020-12-31fmc:operable_unit0000037785us-gaap:PendingLitigationMemberfmc:MiddleportLitigationMember2020-01-012020-12-310000037785us-gaap:PendingLitigationMemberfmc:MiddleportLitigationMember2018-10-012018-12-310000037785fmc:EnvironmentalRemediationCostsMemberus-gaap:PendingLitigationMemberfmc:MiddleportLitigationMember2018-10-012018-12-310000037785us-gaap:PendingLitigationMemberfmc:MiddleportLitigationMember2018-12-310000037785fmc:ImplementationAndCompletionOfSelectedRemedyMemberus-gaap:PendingLitigationMemberfmc:MiddleportLitigationMember2018-10-012018-12-310000037785us-gaap:PendingLitigationMemberfmc:MiddleportLitigationMemberfmc:EnvironmentalCleanUpCostsMember2018-12-310000037785fmc:MiddleportMember2020-12-310000037785fmc:MiddleportMember2019-12-310000037785fmc:MiddleportLitigationMember2020-01-012020-12-310000037785fmc:MiddleportLitigationMember2019-01-012019-12-310000037785srt:MinimumMemberus-gaap:PendingLitigationMemberfmc:MiddleportLitigationMember2020-12-310000037785srt:MaximumMemberus-gaap:PendingLitigationMemberfmc:MiddleportLitigationMember2020-12-310000037785us-gaap:PendingLitigationMemberfmc:MiddleportLitigationMember2020-12-31fmc:sitefmc:party0000037785us-gaap:StateAndLocalJurisdictionMember2020-12-310000037785us-gaap:ForeignCountryMember2020-12-310000037785us-gaap:ForeignCountryMemberus-gaap:SecretariatOfTheFederalRevenueBureauOfBrazilMember2020-12-310000037785us-gaap:ForeignCountryMemberus-gaap:LuxembourgInlandRevenueMember2020-12-310000037785us-gaap:SwissFederalTaxAdministrationFTAMemberus-gaap:ForeignCountryMember2020-12-310000037785us-gaap:MinistryOfFinanceIndiaMemberus-gaap:ForeignCountryMember2020-12-310000037785us-gaap:TaxAuthoritySpainMemberus-gaap:ForeignCountryMember2020-12-31fmc:jurisdiction0000037785fmc:ShorttermForeignDebtMember2020-12-310000037785us-gaap:CommercialPaperMember2020-12-310000037785fmc:PollutionControlAndIndustrialRevenueBondsMember2020-12-310000037785fmc:PollutionControlAndIndustrialRevenueBondsMember2019-12-310000037785srt:MinimumMemberfmc:PollutionControlAndIndustrialRevenueBondsMember2020-12-310000037785srt:MaximumMemberfmc:PollutionControlAndIndustrialRevenueBondsMember2020-12-310000037785us-gaap:SeniorNotesMember2020-12-310000037785us-gaap:SeniorNotesMember2019-12-310000037785us-gaap:SeniorNotesMembersrt:MinimumMember2020-12-310000037785srt:MaximumMemberus-gaap:SeniorNotesMember2020-12-310000037785fmc:TermLoanFacility2017Member2020-12-310000037785fmc:TermLoanFacility2017Member2019-12-310000037785us-gaap:RevolvingCreditFacilityMember2020-12-310000037785us-gaap:RevolvingCreditFacilityMember2019-12-310000037785fmc:ForeignDebtMembersrt:MinimumMember2020-12-310000037785srt:MaximumMemberfmc:ForeignDebtMember2020-12-310000037785fmc:ForeignDebtMember2020-12-310000037785fmc:ForeignDebtMember2019-12-310000037785fmc:RevolvingCreditAgreementMemberus-gaap:SubsequentEventMember2021-01-012021-06-300000037785fmc:TermLoanAgreementMemberus-gaap:SubsequentEventMember2021-01-012021-06-3000000377852020-04-220000037785us-gaap:LineOfCreditMember2020-01-012020-12-310000037785srt:ScenarioForecastMember2021-04-012021-06-300000037785srt:ScenarioForecastMember2021-01-012021-03-310000037785srt:ScenarioForecastMember2021-01-012021-12-310000037785us-gaap:PensionPlansDefinedBenefitMember2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMember2019-12-310000037785us-gaap:OtherPensionPlansDefinedBenefitMember2020-12-310000037785us-gaap:OtherPensionPlansDefinedBenefitMember2019-12-310000037785us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2020-12-310000037785us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMember2018-12-310000037785us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2018-12-310000037785us-gaap:PensionPlansDefinedBenefitMember2020-01-012020-12-310000037785us-gaap:PensionPlansDefinedBenefitMember2019-01-012019-12-310000037785us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2020-01-012020-12-310000037785us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2019-01-012019-12-310000037785country:USfmc:PensionPlanWithAssetsMember2020-12-310000037785country:USfmc:PensionPlanWithAssetsMember2019-12-310000037785country:USfmc:OtherPostretirementBenefitPlanU.S.PlanWithAssetsMember2020-12-310000037785country:USfmc:OtherPostretirementBenefitPlanU.S.PlanWithAssetsMember2019-12-310000037785fmc:PensionPlanWithoutAssetsMembercountry:US2020-12-310000037785fmc:PensionPlanWithoutAssetsMembercountry:US2019-12-310000037785fmc:OtherPostretirementBenefitPlanU.S.PlansWithoutAssetsMembercountry:US2020-12-310000037785fmc:OtherPostretirementBenefitPlanU.S.PlansWithoutAssetsMembercountry:US2019-12-310000037785us-gaap:ForeignPlanMemberfmc:PensionPlanWithAssetsMember2020-12-310000037785us-gaap:ForeignPlanMemberfmc:PensionPlanWithAssetsMember2019-12-310000037785us-gaap:ForeignPlanMemberfmc:OtherPostretirementBenefitPlanU.S.PlanWithAssetsMember2020-12-310000037785us-gaap:ForeignPlanMemberfmc:OtherPostretirementBenefitPlanU.S.PlanWithAssetsMember2019-12-310000037785fmc:OtherPostretirementBenefitPlanAllOtherPlansMember2020-12-310000037785fmc:OtherPostretirementBenefitPlanAllOtherPlansMember2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMember2018-01-012018-12-310000037785us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2018-01-012018-12-310000037785country:US2020-01-012020-12-310000037785country:US2019-01-012019-12-310000037785country:US2018-01-012018-12-310000037785country:US2018-11-012018-12-310000037785us-gaap:FixedIncomeInvestmentsMembercountry:US2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2020-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2020-12-310000037785fmc:DefinedBenefitPlanInvestmentContractsMemberus-gaap:PensionPlansDefinedBenefitMember2020-12-310000037785fmc:DefinedBenefitPlanInvestmentContractsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Member2020-12-310000037785fmc:DefinedBenefitPlanInvestmentContractsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMember2020-12-310000037785fmc:DefinedBenefitPlanInvestmentContractsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2020-12-310000037785us-gaap:USTreasuryAndGovernmentMemberus-gaap:PensionPlansDefinedBenefitMember2020-12-310000037785us-gaap:USTreasuryAndGovernmentMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Member2020-12-310000037785us-gaap:USTreasuryAndGovernmentMemberus-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMember2020-12-310000037785us-gaap:USTreasuryAndGovernmentMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMember2020-12-310000037785us-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Member2020-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMember2020-12-310000037785us-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMember2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2020-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMember2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:CorporateDebtSecuritiesMember2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Member2020-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMember2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2020-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2019-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2019-12-310000037785fmc:DefinedBenefitPlanInvestmentContractsMemberus-gaap:PensionPlansDefinedBenefitMember2019-12-310000037785fmc:DefinedBenefitPlanInvestmentContractsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Member2019-12-310000037785fmc:DefinedBenefitPlanInvestmentContractsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMember2019-12-310000037785fmc:DefinedBenefitPlanInvestmentContractsMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2019-12-310000037785us-gaap:USTreasuryAndGovernmentMemberus-gaap:PensionPlansDefinedBenefitMember2019-12-310000037785us-gaap:USTreasuryAndGovernmentMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Member2019-12-310000037785us-gaap:USTreasuryAndGovernmentMemberus-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMember2019-12-310000037785us-gaap:USTreasuryAndGovernmentMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMember2019-12-310000037785us-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Member2019-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMember2019-12-310000037785us-gaap:MutualFundMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMember2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2019-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateDebtSecuritiesMember2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:CorporateDebtSecuritiesMember2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel1Member2019-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:PensionPlansDefinedBenefitMember2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2019-12-310000037785us-gaap:PensionPlansDefinedBenefitMembercountry:USus-gaap:QualifiedPlanMember2020-01-012020-12-310000037785us-gaap:PensionPlansDefinedBenefitMembercountry:USus-gaap:QualifiedPlanMember2019-01-012019-12-310000037785us-gaap:NonqualifiedPlanMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2020-01-012020-12-310000037785us-gaap:NonqualifiedPlanMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2019-01-012019-12-310000037785us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2020-01-012020-12-310000037785us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2019-01-012019-12-310000037785us-gaap:EmployeeStockOptionMember2020-01-012020-12-310000037785fmc:RestrictedStockUnitsRsusRelatedToDirectorsMember2020-01-012020-12-310000037785fmc:RestrictedStockUnitsRsusRelatedToDirectorsMember2019-01-012019-12-310000037785us-gaap:EmployeeStockOptionMember2019-01-012019-12-310000037785us-gaap:EmployeeStockOptionMember2018-01-012018-12-310000037785us-gaap:RestrictedStockUnitsRSUMember2020-01-012020-12-310000037785us-gaap:RestrictedStockUnitsRSUMember2019-01-012019-12-310000037785us-gaap:RestrictedStockUnitsRSUMember2018-01-012018-12-310000037785us-gaap:PerformanceSharesMember2020-01-012020-12-310000037785us-gaap:PerformanceSharesMember2019-01-012019-12-310000037785us-gaap:PerformanceSharesMember2018-01-012018-12-310000037785fmc:DiscontinuedOperationsNetOfIncomeTaxesMember2020-01-012020-12-310000037785fmc:DiscontinuedOperationsNetOfIncomeTaxesMember2019-01-012019-12-310000037785fmc:DiscontinuedOperationsNetOfIncomeTaxesMember2018-01-012018-12-31utr:Rate00000377852017-01-012017-12-310000037785us-gaap:EmployeeStockOptionMember2020-12-310000037785us-gaap:RestrictedStockUnitsRSUMember2017-12-310000037785us-gaap:PerformanceSharesMember2017-12-310000037785us-gaap:RestrictedStockUnitsRSUMember2018-12-310000037785us-gaap:PerformanceSharesMember2018-12-310000037785us-gaap:RestrictedStockUnitsRSUMember2019-12-310000037785us-gaap:PerformanceSharesMember2019-12-310000037785us-gaap:RestrictedStockUnitsRSUMember2020-12-310000037785us-gaap:PerformanceSharesMember2020-12-310000037785us-gaap:AccumulatedTranslationAdjustmentMember2017-12-310000037785us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2017-12-310000037785us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2017-12-310000037785us-gaap:AccumulatedTranslationAdjustmentMember2018-01-012018-12-310000037785us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2018-01-012018-12-310000037785us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2018-01-012018-12-310000037785us-gaap:AccumulatedTranslationAdjustmentMember2018-12-310000037785us-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2018-12-310000037785us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2018-12-310000037785us-gaap:AccumulatedTranslationAdjustmentMember2019-01-012019-12-310000037785us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2019-01-012019-12-310000037785us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2019-01-012019-12-310000037785srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccumulatedTranslationAdjustmentMember2018-12-310000037785srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2018-12-310000037785srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2018-12-310000037785us-gaap:SegmentDiscontinuedOperationsMemberus-gaap:AccumulatedTranslationAdjustmentMember2019-01-012019-12-310000037785us-gaap:SegmentDiscontinuedOperationsMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2019-01-012019-12-310000037785us-gaap:SegmentDiscontinuedOperationsMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2019-01-012019-12-310000037785us-gaap:SegmentDiscontinuedOperationsMemberus-gaap:AccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310000037785us-gaap:AccumulatedTranslationAdjustmentMember2019-12-310000037785us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2019-12-310000037785us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2019-12-310000037785us-gaap:AccumulatedTranslationAdjustmentMember2020-01-012020-12-310000037785us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2020-01-012020-12-310000037785us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-01-012020-12-310000037785us-gaap:AccumulatedTranslationAdjustmentMember2020-12-310000037785us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2020-12-310000037785us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:ForeignExchangeContractMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2020-01-012020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:ForeignExchangeContractMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2019-01-012019-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:ForeignExchangeContractMemberus-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2018-01-012018-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:InterestRateSwapMember2020-01-012020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:InterestRateSwapMember2019-01-012019-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMemberus-gaap:InterestRateSwapMember2018-01-012018-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2020-01-012020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMember2019-01-012019-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember2018-01-012018-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetPriorServiceCostCreditMember2020-01-012020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetPriorServiceCostCreditMember2019-01-012019-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetPriorServiceCostCreditMember2018-01-012018-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetUnamortizedGainLossMember2020-01-012020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetUnamortizedGainLossMember2019-01-012019-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetUnamortizedGainLossMember2018-01-012018-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetTransitionAssetObligationMember2020-01-012020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetTransitionAssetObligationMember2019-01-012019-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentNetTransitionAssetObligationMember2018-01-012018-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-01-012020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2019-01-012019-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMemberus-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2018-01-012018-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310000037785us-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2018-01-012018-12-310000037785fmc:PTBinaGunaKimiaMember2020-07-3100000377852020-07-012020-07-310000037785fmc:PTBinaGunaKimiaMember2020-08-010000037785fmc:FMCLithiumMember2017-11-010000037785us-gaap:SubsequentEventMember2021-01-212021-01-210000037785fmc:RepurchaseProgramMember2020-01-012020-12-310000037785fmc:RepurchaseProgramMember2020-12-310000037785us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CashFlowHedgingMember2020-12-310000037785us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2020-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CashFlowHedgingMember2020-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CashFlowHedgingMemberus-gaap:InterestRateSwapMember2020-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CashFlowHedgingMemberus-gaap:InterestRateSwapMember2019-09-202019-09-20utr:MMBTU0000037785us-gaap:EnergyRelatedDerivativeMemberus-gaap:DesignatedAsHedgingInstrumentMember2020-12-310000037785fmc:ForeignCurrencyAndEnergyContractsMemberus-gaap:DesignatedAsHedgingInstrumentMember2020-12-310000037785us-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2020-12-310000037785us-gaap:ForeignExchangeContractMember2020-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestRateSwapMember2020-12-310000037785us-gaap:NondesignatedMemberus-gaap:InterestRateSwapMember2020-12-310000037785us-gaap:InterestRateSwapMember2020-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMember2020-12-310000037785us-gaap:NondesignatedMember2020-12-310000037785us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2019-12-310000037785us-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2019-12-310000037785us-gaap:ForeignExchangeContractMember2019-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMember2019-12-310000037785us-gaap:NondesignatedMember2019-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestRateSwapMember2019-12-310000037785us-gaap:NondesignatedMemberus-gaap:InterestRateSwapMember2019-12-310000037785us-gaap:InterestRateSwapMember2019-12-310000037785us-gaap:ForeignExchangeContractMember2017-12-310000037785us-gaap:InterestRateSwapMember2017-12-310000037785us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2018-01-012018-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestRateSwapMember2018-01-012018-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMember2018-01-012018-12-310000037785us-gaap:ForeignExchangeContractMember2018-12-310000037785us-gaap:InterestRateSwapMember2018-12-310000037785us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2019-01-012019-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestRateSwapMember2019-01-012019-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMember2019-01-012019-12-310000037785us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2020-01-012020-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:InterestRateSwapMember2020-01-012020-12-310000037785us-gaap:DesignatedAsHedgingInstrumentMember2020-01-012020-12-310000037785fmc:CostOfSalesAndServicesMemberus-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2020-01-012020-12-310000037785fmc:CostOfSalesAndServicesMemberus-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2019-01-012019-12-310000037785fmc:CostOfSalesAndServicesMemberus-gaap:ForeignExchangeContractMemberus-gaap:NondesignatedMember2018-01-012018-12-310000037785us-gaap:NondesignatedMember2020-01-012020-12-310000037785us-gaap:NondesignatedMember2019-01-012019-12-310000037785us-gaap:NondesignatedMember2018-01-012018-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMemberus-gaap:FairValueInputsLevel1Member2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMemberus-gaap:FairValueInputsLevel2Member2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMemberus-gaap:FairValueInputsLevel3Member2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Memberus-gaap:InterestRateSwapMember2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:InterestRateSwapMember2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Memberus-gaap:InterestRateSwapMember2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMember2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2020-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMemberus-gaap:FairValueInputsLevel1Member2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMemberus-gaap:FairValueInputsLevel2Member2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMemberus-gaap:FairValueInputsLevel3Member2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMember2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Member2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Member2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel1Memberus-gaap:InterestRateSwapMember2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel2Memberus-gaap:InterestRateSwapMember2019-12-310000037785us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Memberus-gaap:InterestRateSwapMember2019-12-310000037785us-gaap:FairValueMeasurementsNonrecurringMember2018-12-310000037785us-gaap:FairValueMeasurementsNonrecurringMemberus-gaap:FairValueInputsLevel1Member2018-12-310000037785us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsNonrecurringMember2018-12-310000037785us-gaap:FairValueMeasurementsNonrecurringMemberus-gaap:FairValueInputsLevel3Member2018-12-310000037785us-gaap:FairValueMeasurementsNonrecurringMember2018-01-012018-12-310000037785fmc:BrandsMember2018-01-012018-12-310000037785us-gaap:FinancialGuaranteeMember2020-12-310000037785us-gaap:GuaranteeOfIndebtednessOfOthersMember2020-12-310000037785us-gaap:GuaranteeOfIndebtednessOfOthersMember2020-01-012020-12-3100000377852020-10-282020-10-280000037785us-gaap:UnfavorableRegulatoryActionMembercountry:BR2020-12-310000037785us-gaap:UnfavorableRegulatoryActionMembercountry:BR2019-12-310000037785us-gaap:UnfavorableRegulatoryActionMembercountry:BR2020-01-012020-12-310000037785srt:NorthAmericaMember2020-12-310000037785srt:NorthAmericaMember2019-12-310000037785srt:LatinAmericaMember2020-12-310000037785srt:LatinAmericaMember2019-12-310000037785us-gaap:EMEAMember2020-12-310000037785us-gaap:EMEAMember2019-12-310000037785srt:AsiaPacificMember2020-12-310000037785srt:AsiaPacificMember2019-12-310000037785country:SG2020-12-310000037785country:SG2019-12-310000037785country:US2020-12-310000037785country:US2019-12-310000037785country:DK2020-12-310000037785country:DK2019-12-3100000377852020-04-012020-06-3000000377852020-10-012020-12-3100000377852019-01-012019-03-3100000377852019-04-012019-06-3000000377852019-07-012019-09-300000037785us-gaap:AllowanceForCreditLossMember2019-12-310000037785us-gaap:AllowanceForCreditLossMember2020-01-012020-12-310000037785us-gaap:AllowanceForCreditLossMember2020-12-310000037785us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2019-12-310000037785us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2020-01-012020-12-310000037785us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2020-12-310000037785us-gaap:AllowanceForCreditLossMember2018-12-310000037785us-gaap:AllowanceForCreditLossMember2019-01-012019-12-310000037785us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2018-12-310000037785us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2019-01-012019-12-310000037785us-gaap:AllowanceForCreditLossMember2017-12-310000037785us-gaap:AllowanceForCreditLossMember2018-01-012018-12-310000037785us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2017-12-310000037785us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2018-01-012018-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_______________________________________________________________________
FORM 10-K
_______________________________________________________________________
| | | | | |
| ☒ | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended December 31, 2020
or
| | | | | |
| ☐ | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from _______ to _______
Commission File Number 1-2376
__________________________________________________________________________
FMC CORPORATION
(Exact name of registrant as specified in its charter)
__________________________________________________________________________
| | | | | | | | | | | |
| Delaware | | 94-0479804 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
| 2929 Walnut Street | Philadelphia | Pennsylvania | 19104 |
| (Address of principal executive offices) | | | (Zip Code) |
Registrant’s telephone number, including area code: 215-299-6000
__________________________________________________________________________
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol | | Name of each exchange on which registered |
| Common Stock, par value $0.10 per share | | FMC | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | | ☒ | | Accelerated filer | | ☐ |
| | | | | | |
| Non-accelerated filer | | ☐ | | Smaller reporting company | | ☐ |
| | | | | | |
| | | | Emerging growth company | | ☐ |
| | | | | | |
| If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. | | |
| ☐ |
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes ☐ No ☒
The aggregate market value of voting stock held by non-affiliates of the registrant as of June 30, 2020, the last day of the registrant’s second fiscal quarter was $12,829,126,457. The market value of voting stock held by non-affiliates excludes the value of those shares held by executive officers and directors of the registrant.
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date:
As of December 31, 2020, there were 129,353,583 of the registrant's common shares outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
| | | | | | | | |
| DOCUMENT | | FORM 10-K REFERENCE |
| Portions of Proxy Statement for 2021 Annual Meeting of Stockholders | | Part III |
FMC Corporation
2020 Form 10-K
Table of Contents
PART I
FMC Corporation was incorporated in 1928 under Delaware law and has its principal executive offices at 2929 Walnut Street, Philadelphia, Pennsylvania 19104. Throughout this annual report on Form 10-K, except where otherwise stated or indicated by the context, "FMC", the "Company", "We," "Us," or "Our" means FMC Corporation and its consolidated subsidiaries and their predecessors. Copies of the annual, quarterly and current reports we file with the Securities and Exchange Commission ("SEC"), and any amendments to those reports, are available on our website at www.fmc.com as soon as practicable after we furnish such materials to the SEC.
ITEM 1. BUSINESS
General
We are a pure-play agricultural sciences company, providing innovative solutions to growers around the world with a robust product portfolio fueled by a market-driven discovery and development pipeline in crop protection, plant health, precision agriculture, and professional pest and turf management. This powerful combination of advanced technologies includes leading insect control products based on Rynaxypyr® and Cyazypyr® active ingredients; Authority®, Boral®, Centium®, Command® and Gamit® branded herbicides; Isoflex™ active herbicide ingredient; Talstar® and Hero® branded insecticides(1); and flutriafol-based fungicides. The FMC portfolio also includes Arc™ farm intelligence and biologicals such as Quartzo® and Presence® bionematicides. Our products are used in agriculture to enhance crop yield and quality by controlling a broad spectrum of insects, weeds and disease, as well as in non-agricultural markets for pest and turf management.
FMC Strategy
We have streamlined our portfolio over the past ten years to become a tier-one leader and the fifth largest global innovation provider in the global agricultural chemicals market. Our strong competitive position is driven by our technology and innovation, as well as our geographic balance and crop diversity, which helped FMC to take market share in 2018, 2019, and 2020.
We have industry-leading insecticides and herbicides (the majority of which are patented technologies), exceptional discovery research capabilities and a global manufacturing network. We expect to spend approximately 6.5 percent of sales on research and development annually. Our R&D pipeline includes 11 molecules and biological strains in our development pipeline (approximately 1-7 years away from commercialization) and more than 25 additional molecules and biological strains in our discovery pipeline (approximately 8-10 years from commercialization). We expect the first four product launches, including the first two significant active ingredients, out of this pipeline will occur in 2021. We own and operate a total of 25 manufacturing plants, and we have the scale to operate with strong resources and global reach to address changing market conditions. Our supply chain organization effectively managed to continue supplying our customers and growing our business, despite multiple shutdowns and other disruptions in the Chinese chemical sector in 2018 and 2019. In the fourth quarter of 2020, we experienced logistics and supply chain constraints in the U.S., mostly due to the COVID-19 pandemic. We do not expect this to be completely resolved by the first quarter of 2021 but we are focused on ensuring we can mitigate supply chain risks and continue to expand our market growth opportunities. We posted solid overall results in 2020, despite numerous challenges related to the COVID-19 pandemic. As an agricultural sciences company, we are considered an "essential" industry in the countries in which we operate; we have avoided significant plant closures and all our manufacturing facilities and distribution warehouses remain operational and fully staffed. We will continue to assess the need related to cost-saving measures as appropriate.
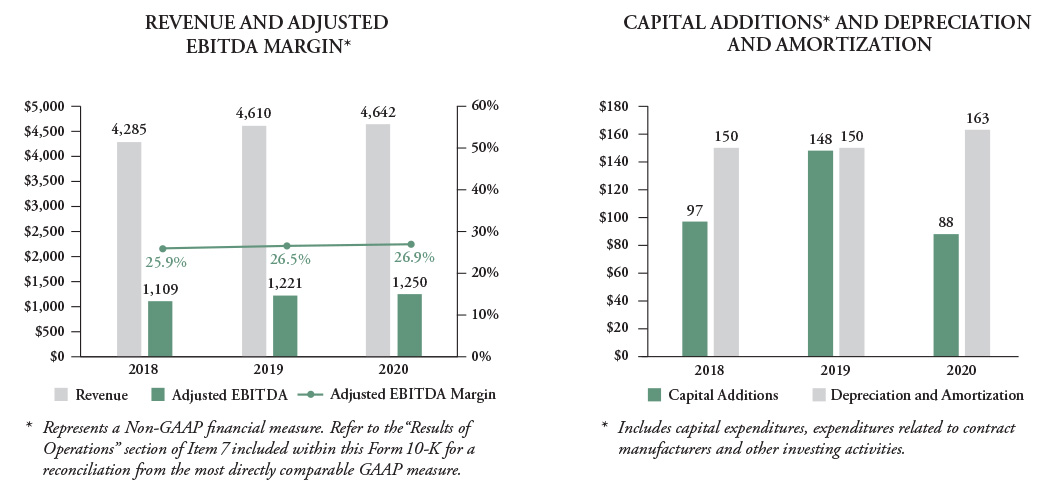
Our revenues grew approximately 1 percent, or 7 percent organically(2) excluding the impacts of foreign currency, year over year in 2020, driven by double-digit growth for our diamides, Rynaxypyr® and Cyazypyr® active ingredients. Though we saw growth in additional active ingredients, the aggregate of the rest of our portfolio (excluding diamides) amounted to a mid-single digit decline, inclusive of a 2 percent decline in product registrations and rationalizations, which mostly offset the diamide growth discussed above. Rynaxypyr® and Cyazypyr® actives now represent over $1.8 billion in combined sales, representing approximately 55 percent growth since we acquired these molecules in November 2017. Products launched in 2020 and 2019 also contributed to revenue growth. We successfully launched our new bixafen fungicide under the Lucento® fungicide brand in North America in 2019, and we are on track to accomplish the $30 million to $50 million revenue target for this new active ingredient. We also launched several new formulated products in 2020, which is key to lifecycle management of our products. Approximately $50 million of our 2020 revenue growth came from 2020 product launches.
FMC performed slightly better than the overall crop protection market in 2020, which we estimate was flat versus 2019. Growth for FMC and the market was offset by significant headwinds from foreign currency. As mentioned above, our growth rate was 1 percent, and excluding the impact of foreign currency, our organic(1) growth rate was 7 percent. FMC’s innovation, starting with our current portfolio of advanced products and continuing through our R&D discovery, development and new formulations, contributed to our performance. Our technology portfolio includes specific innovations in plant health, application technology and delivery systems, as well as advanced agronomic insights through Arc™ farm intelligence, our precision agriculture tool that leverages artificial intelligence and machine learning.
____________________
(1) Hero® insecticide is a restricted use pesticide in the U.S.
(2) Organic revenue growth is a non-GAAP term which excludes the impact of foreign currency changes. Refer to the "Results of Operations" section of our Management's Discussion and Analysis in Item 7 for our organic revenue non-GAAP reconciliation.
Acquisitions and Divestitures
In May 2020, FMC entered into a binding offer with Isagro S.p.A ("Isagro") to acquire the remaining rights for Fluindapyr active ingredient assets from Isagro. In July 2020, we entered into an asset sale and purchase agreement with Isagro. On October 2, 2020, we closed on the transaction with a purchase price of approximately $65 million. Fluindapyr has been jointly developed by FMC and Isagro under a 2012 research and development collaboration agreement. The transaction provides FMC with full global rights to the Fluindapyr active ingredient, including key U.S., European, Asian, and Latin American fungicide markets. The transaction transfers to FMC all intellectual property, know-how, registrations, product formulations and other global assets of the proprietary broad-spectrum fungicide molecule. The acquired assets have been classified as in-process research and development. See Note 9 in the consolidated financial statements included within this Form 10-K for accounting considerations. The transaction will expand our fungicide portfolio by giving us full global rights to the Fluindapyr active ingredient and is an important strategic addition to our product line.
In 2019, we completed the separation of our FMC Lithium segment, which was renamed Livent Corporation, or "Livent", following its initial public offering ("IPO") that closed on October 15, 2018. After completion of the IPO, FMC owned 123 million shares of Livent's common stock, representing approximately 84 percent of the total outstanding shares of Livent's common stock. On March 1, 2019, we completed the distribution of 123 million shares of common stock of Livent as a pro rata dividend on shares of FMC. Following the distribution, FMC has zero shares of Livent and zero exposure to lithium markets. The financial information within this filing has been recast to present the former FMC Lithium as a discontinued operation retrospectively for all relevant periods presented.
Financial Information About Our Business
(Financial Information in Millions)
The following table shows the principal products produced by our business, its raw materials and uses:
| | | | | | | | |
| Product | Raw Materials | Uses |
| Insecticides | Synthetic and biological chemical intermediates | Protection of crops, including soybean, corn, fruits and vegetables, cotton, sugarcane, rice, and cereals, from insects and for non-agricultural applications including pest control for home, garden and other specialty markets |
| Herbicides | Synthetic and biological chemical intermediates | Protection of crops, including cotton, sugarcane, rice, corn, soybeans, cereals, fruits and vegetables from weed growth and for non-agricultural applications including turf and roadsides |
| Fungicides | Synthetic and biological chemical intermediates | Protection of crops, including cereals, fruits and vegetables from fungal disease |
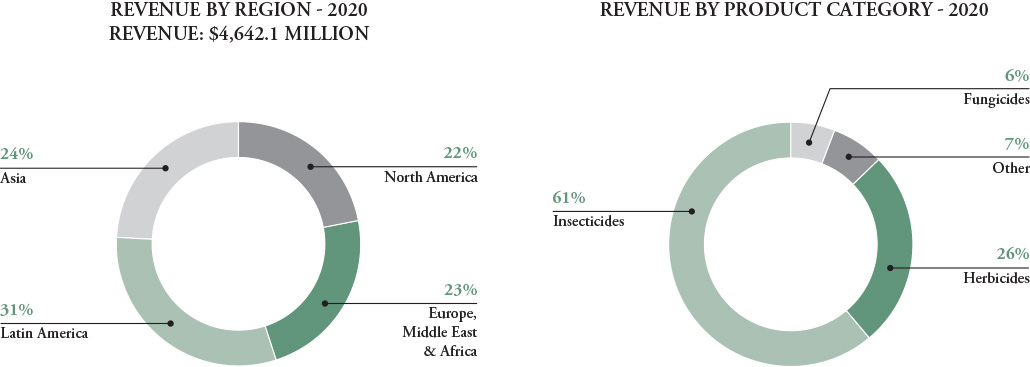
With a worldwide manufacturing and distribution infrastructure, we are better able to respond rapidly to global customer needs, offset downward economic trends in one region with positive trends in another and match local revenues to local costs to reduce the impact of currency volatility. The charts below detail our sales by major geographic region and major product category.
The following table provides our long-lived assets by major geographical region:
| | | | | | | | | | | |
| (in Millions) | December 31, |
| 2020 | | 2019 |
| Long-lived assets | | | |
| North America | $ | 1,230.2 | | | $ | 1,190.7 | |
| Latin America | 792.7 | | | 837.0 | |
| Europe, Middle East, and Africa | 1,513.9 | | | 1,448.0 | |
| Asia | 2,044.4 | | | 2,064.8 | |
| Total | $ | 5,581.2 | | | $ | 5,540.5 | |
Products and Markets
Our portfolio is comprised of three major pesticide categories: insecticides, herbicides and fungicides. The majority of our product lines consist of insecticides and herbicides, and we have a small but fast-growing portfolio of fungicides mainly used in high value crop segments. Our insecticides are used to control a wide spectrum of pests, while our herbicide portfolio primarily targets a large variety of difficult-to-control weeds. We are also investing substantially in a plant health program that includes biological crop protection products, seed treatments and micro-nutrients. Biological technologies developed by FMC’s R&D team in Denmark offer excellent sustainability profiles and serve as strong complements to our synthetic products. Our biologicals feature attributes that exceed the competition, such as high stability, long shelf life, low use rates and compatibility with other chemistries.
In the Latin American region, which includes the large agricultural market of Brazil, we sell directly to large growers through our own sales and marketing organization, and we access the market through independent distributors and co-ops. In North America, we access the market through several major national and regional distributors and have our own sales and marketing organization in Canada. We access the Europe, Middle East & Africa markets through our own sales and marketing organizations. We access key Asian markets through large distributors, in addition to either local independent distributors or our own sales and marketing organizations. Through these and other alliances, along with our own targeted marketing efforts, access to novel technologies and our innovation initiatives, we expect to maintain and enhance our access in key agricultural and non-crop markets and develop new products that will help us continue to compete effectively.
Industry Overview
The three principal categories of agricultural and non-crop chemicals are: herbicides, insecticides and fungicides, representing approximately 40 percent, 30 percent and 28 percent of global industry revenue, respectively.
The agrochemicals industry is more consolidated following several recent mergers of the leading crop protection companies, which now include FMC, ChemChina (owner of Syngenta Group, which includes the former Syngenta and Adama), Bayer AG
(acquired Monsanto in 2018), BASF AG and Corteva Agriscience (the agricultural division of former DowDuPont, spun out in June 2019). These five innovation companies currently represent approximately 75 percent of the crop protection industry’s global sales. The next group of agrochemical producers include UPL Ltd. (UPL also acquired Arysta in February 2019), Sumitomo Chemical Company Ltd., and Nufarm Ltd. FMC employs various differentiated strategies and competes with unique technologies focusing on certain crops, markets and geographies, while also being supported by a low-cost manufacturing model.
Growth
We are among the leading agrochemical producers in the world. Some of our key insecticides are predominantly based on patent-protected active ingredients and continue to grow well above market patterns. Our complementary technologies combine improved formulation capabilities and a broader innovation pipeline, resulting in new and differentiated products. We will take advantage of enhanced market access positions and an expanded portfolio to deliver near-term growth.
We will continue to grow by obtaining new and approved uses for existing product lines and acquiring, accessing, developing, marketing, distributing and/or selling complementary chemistries and related technologies in order to strengthen our product portfolio and our capabilities to effectively service our target markets and customers.
Our growth efforts focus on developing environmentally compatible and sustainable solutions that can effectively increase farmers’ yields and provide cost-effective alternatives to chemistries which may be prone to resistance. We are committed to providing unique, differentiated products to our customers by acquiring and further developing technologies as well as investing in innovation to extend product life cycles. Our external growth efforts include product acquisitions, in-licensing of chemistries and technologies and alliances that bolster our market access, complement our existing product portfolio or provide entry into adjacent spaces. We have entered into a range of development and distribution agreements with other companies that provide access to new technologies and products which we can subsequently commercialize.
In 2020, we announced the launch of our Arc™ farm intelligence platform, an exclusive precision agriculture platform that enables growers and advisors to more accurately predict pest pressure before it becomes a problem. Nearly 4 million acres across six countries were covered by our platform during its pilot rollout. It is expanding significantly and supports product recommendations for multiple FMC active ingredients, led by our diamides. We have other precision agriculture initiatives and new product launches such as Isoflex™ herbicide. We also launched FMC Ventures, our new venture capital arm targeting strategic investments in start-ups and early-stage companies that are developing and applying emerging technologies in the agricultural industry. The group will be making small, seed type investments.
Diamide Growth Strategy
Our product portfolio features two key diamide-class molecules – Rynaxypyr® (chlorantraniliprole) and Cyazypyr® (cyantraniliprole) actives – with combined annual revenues of approximately $1.8 billion in 2020. These two molecules are industry-leading in terms of performance, combining highly effective low dose rates with fast-acting, systemic, long residual control. These attributes quickly established Rynaxypyr® active as the world’s leading insect control technology and we expect it to continue on a strong growth trajectory notwithstanding the expiration of composition of matter patents covering Rynaxypyr® active in certain countries starting in late 2022. Our Cyazypyr® active, a second-generation diamide, is growing quickly as we obtain more product registrations. We expect it to continue to grow strongly notwithstanding the expiration of its active ingredient composition of matter patents starting in the mid-2020s. This expectation is based on not only our broad patent estate and the timing of key patent milestones, but also on other critical elements that will allow FMC to continue to profitably grow the diamide franchise well beyond the expiration of key patents. These other critical elements include registration and data protection, commercial strategies, brand recognition, as well as manufacturing and supply chain complexity and FMC efficiencies.
Patents and Trade Secrets. The FMC diamide insect control patent estate is made up of many different patent families which cover: Composition of matter – both active ingredients and certain intermediates; Manufacturing processes – both active ingredients and certain intermediates; Formulations; Uses; and Applications. For Rynaxypyr® and Cyazypyr® actives related patents, as of December 31, 2020, we had 33 families with granted patents filed in up to 76 countries, with a total of 897 active granted patents as well as numerous pending patent applications. See "Patents, Trademarks and Licenses" within this Item 1 for more details. FMC’s process patents cover the manufacturing processes for both active ingredients – chlorantraniliprole and cyantraniliprole – as well as key intermediates that are used to make the final products. Chlorantraniliprole is a complex molecule to produce, requiring 16 separate steps; FMC owns granted patents covering many of these 16 process steps and several of the intermediate chemicals, and we protect other aspects of the manufacturing processes by trade secret. Cyantraniliprole is similarly complex and covered by a comparable range of intellectual property. Many of these intermediate process patents run well past the expiration of the composition of matter patents, and in some cases stretch until the end of this decade. Third parties that intend to manufacture and sell generic chlorantraniliprole or cyantraniliprole and rely on FMC’s extensive product safety data will be required to demonstrate that their product has the same regulatory safety profile as FMC Rynaxypyr® and Cyazypyr® actives. To meet regulatory requirements for such difficult-to-manufacture molecules, we believe
that third parties will have to produce these active ingredients using the same processes that are patented by FMC and if so, would be infringing before patent expiration and subject to our challenge for infringement. FMC also owns formulation patents which cover the use of chlorantraniliprole or cyantraniliprole in specific formulations found in commercially important end-use products.
Regulatory Data Protection. In addition to the patent estate, various pesticide laws and regulations around the world offer added protection to the initial active ingredient registrant in the form of data protection and registration timelines that can extend after the composition or process patents have expired. These rules can effectively provide a product innovator and initial active ingredient registrant such as FMC with a further period of exclusive use of the key reference data even after the applicable AI composition of matter patents have expired. Further, in certain countries, even after the period of exclusive use has expired, a generic entrant seeking to rely on the initial registrant’s reference data may have to pay significant compensation to the initial registrant. For FMC’s diamide products, such rights apply in key markets including United States, Brazil and the European Union.
Growing the Branded FMC Diamide Franchise. FMC is executing its strategy to supply end-use pesticide products that include Rynaxypyr® and Cyazypyr® actives to a broad range of companies prior to patent expiration, and in return establishing long-term commitments from the companies to purchase the diamide active ingredients from FMC. These arrangements may also include limited patent, data and/or trademark licenses. Such partner relationships allow us to grow our business by having others develop and sell diamide-based products to meet farmers' needs not within our current portfolio, offering those farmers a better alternative to competing insecticides with product safety or efficacy profiles which are less attractive than Rynaxypyr® or Cyazypyr® actives. These agreements can require the third party to use the well-known and trusted Rynaxypyr® or Cyazypyr® brand names on the end-use products formulated with active ingredient supplied by FMC. As of December 31, 2020, we had global agreements with four major multinational companies and approximately 50 separate local-country agreements covering 14 countries. We are continuing to explore opportunities with additional companies beyond those with whom we are already engaged.
Complexity of manufacturing. Today FMC manufactures all the required intermediates in the multi-step processes, as well as the final Rynaxypyr® and Cyazypyr® actives, at our own active ingredient manufacturing plants or through key contract manufacturers who produce under long-term exclusive technology-license agreements. For a third party to replicate this complex supply chain and manufacturing network would be a major undertaking with very large capital requirements. In addition, given our manufacturing know-how, scale of our operations, and continual investment in manufacturing process improvement, we believe FMC’s manufacturing costs will be substantially lower than any other party seeking to produce these diamide products.
Collectively, these four factors -- deep patent estate, proprietary regulatory data, strong commercial approach leveraging our brand recognition, and capabilities of managing large scale manufacturing complexity – provide us the basis for our expectation that FMC will be the company of choice to supply chlorantraniliprole and cyantraniliprole products to third-party partners, and ultimately to farmers, well into the future.
Source and Availability of Raw Materials
We utilize numerous vendors to supply raw materials and intermediate chemicals to support operations. These materials are sourced on a global basis to strategically balance FMC’s vendor portfolio.
Patents, Trademarks and Licenses
As an agricultural sciences company, FMC believes in innovation and in protecting that innovation through intellectual property rights. We own and license a significant number of U.S. and foreign patents, trademarks, trade secrets and other intellectual property that are cumulatively important to our business. In addition, we seek to license our proprietary technologies through partnering arrangements that effectively allow us to capitalize from our intellectual property. The FMC intellectual property estate provides us with a significant competitive advantage which we seek to expand and renew on a continual basis. We manage our technology investment to discover and develop new active ingredients and biological products, as well as to continue to improve manufacturing processes and existing active ingredients through new formulations, mixtures or other concepts. FMC’s technology innovation processes capture those innovations and protect them through the most appropriate form of intellectual property rights. We also in-license certain active ingredients and other technologies under patents held by third parties, and have granted licenses to certain of our patents to third parties.
Our patents cover many aspects of our business, including our chemical and biological active ingredients, intermediate chemicals, manufacturing processes to produce such active ingredients or intermediates, formulations, and product uses, as well as many aspects of our research and development activities that support the FMC new product pipeline. Patents are granted by individual jurisdictions and the duration of our patents depends on their respective jurisdictions and payment of annuities.
As of December 31, 2020, the Company owned a total of approximately 220 active granted U.S. patents and 2,600 active granted foreign patents (includes Supplemental Patent Certificates); we also have approximately 1,600 patent applications pending globally.
In our current product portfolio, our diamide insect control products based on Rynaxypyr® (Chlorantraniliprole) and Cyazypyr® (Cyantraniliprole) active ingredients have a substantial patent estate which will remain in force well into the future. More details regarding our diamide granted patent estate are set forth in the tables below:
| | | | | | | | | | | |
| Numbers of active Granted Patents by type*: Chlorantraniliprole and Cyantraniliprole, as of December 31, 2020 |
| United States | | Foreign |
| Active Ingredients | 21 | | 252 |
| Intermediates and Methods of Manufacturing | 23 | | 254 |
| Formulations/Mixtures/Applications | 9 | | 338 |
| Total | 53 | | 844 |
*Patent families were only placed under one type but may cover several types.
| | | | | | | | | | | |
Remaining Life of Granted Patents: Chlorantraniliprole and Cyantraniliprole, as of December 31, 2020 |
| United States | | Foreign |
| Through December 31, 2025 | 36 | | 550 |
| 2026 - 2030 | 15 | | 266 |
| 2031 - 2036 | 2 | | 28 |
| Total | 53 | | 844 |
We also own many trademarks that are well recognized by customers or product end-users. Unlike patents, ownership rights in trademarks can be continued indefinitely so long as the trademarks are properly used and renewal fees are paid.
We actively monitor and manage our patents and trademarks to maintain our rights in these assets and we strategically take aggressive action when we believe our intellectual property rights are being infringed. While we believe that the invalidity or loss of any particular patent, trademark or license would be a remote possibility, our patent and trademark estate related to our diamide insect control products based on Rynaxypyr® and Cyazypyr® active ingredients in the aggregate are of material importance to our operations.
Seasonality
The seasonal nature of the crop protection market and the geographic spread of our business can result in significant variations in quarterly earnings among geographic locations. Our products sold in the northern hemisphere (North America, Europe and parts of Asia) serve seasonal agricultural markets from March through September, generally resulting in significant earnings in the first and second quarters, and to a lesser extent in the fourth quarter. Markets in the southern hemisphere (Latin America and parts of the Asia Pacific region, including Australia) are served from July through February, generally resulting in earnings in the third, fourth and first quarters.
Competition
We encounter substantial competition in our business. We market our products through our own sales organization and through alliance partners, independent distributors and sales representatives. The number of our principal competitors varies from market to market. In general, we compete by providing advanced technology, high product quality, reliability, quality customer and technical service, and by operating in a cost-efficient manner.
Our business competes primarily in the global chemical crop protection market for insecticides, herbicides and fungicides. Industry products include crop protection chemicals and, for certain major competitors, genetically engineered (crop biotechnology) products. Competition from generic agrochemical producers is significant as a number of key product patents have expired in the last two decades. In general, we compete as an innovator by focusing on product development, including novel formulations, proprietary mixes, and advanced delivery systems and by acquiring or licensing (mostly) proprietary chemistries or technologies that complement our product and geographic focus. We also differentiate ourselves by our global cost-competitiveness through our manufacturing strategies, establishing effective product stewardship programs and developing strategic alliances that strengthen market access in key countries and regions.
Research and Development Expense
The R&D efforts in our business focus on discovering and developing environmentally sound solutions — both new active ingredients and new product formulations — that meet the needs of farmers to maximize yields and control pests by providing new products that utilize both existing and new active ingredient chemistries. On June 24, 2019, we announced our investment of more than $50 million at our FMC Stine Research Center in Newark, Delaware, to upgrade infrastructure and complete construction on a new state-of-the-art, greenhouse and laboratory facility. Due to the pandemic, work on the greenhouse project did not progress as anticipated during 2020. We anticipate that the project will be completed by 2023.
Environmental Laws and Regulations
A discussion of environmental related factors can be found in Item 7 "Management’s Discussion and Analysis of Financial Condition and Results of Operations" and in Note 12 "Environmental Obligations" in the notes to our consolidated financial statements included in this Form 10-K.
Human Capital
Employees
We employ approximately 6,400 people with about 1,500 people in our domestic operations and 4,900 people in our foreign operations.
Approximately 3 percent of our U.S.-based and 33 percent of our foreign-based employees, respectively, are represented by collective bargaining agreements. We have successfully concluded most of our recent contract negotiations without any material work stoppages. In those rare instances where a work stoppage has occurred, there has been no material effect on consolidated sales and earnings. We cannot predict, however, the outcome of future contract negotiations. In 2021, six foreign collective-bargaining agreements will be expiring. These contracts affect approximately 15 percent of our foreign-based employees. There are no U.S. collective-bargaining agreements expiring in 2021.
Talent Engagement and Retention
At FMC, it is important that we focus our programs and initiatives on sustaining strong leaders who are committed to engaging and developing their employees, so they can lead competitively, innovate change, improve business performance, and successfully maintain a competitive advantage. FMC’s leadership development program components include in-class and self-paced learning, development planning and stretch assignments, project-based action learning and rotational learning, mentoring and coaching, and leadership and functional assessments. Our programs are designed to provide engaging, collaborative, and creative learning environments. Employees leverage their experiences in these programs to develop their leadership abilities to their highest levels, enabling them to deliver innovative solutions, strong results and continued growth. Three of our signature leadership programs are science of leadership, the art of leadership, and keys to leadership. We hold quarterly Town Hall meetings and engage with our employees continuously through regular email updates, social media, webcasts, and other channels. We ask our employees to complete surveys and participate in focus groups, we distribute certain reports to keep our employees informed, we require our employees to complete specific trainings and we are piloting a voluntary e-learning program with other development and learning opportunities. We also reach out to new talent through social media.
FMC continually strives to meet the needs of our employees, shareholders, and customers through competitive rewards, policies, and practices that support the company as an employer of choice in every market where we compete for talent. FMC compensates employees through total reward programs that are aligned with performance and competencies. Performance-based direct pay programs include competitive base pay, annual bonus opportunities, sales incentive plans, and long-term incentives. These compensation elements along with benefits, work-life flexibility, recognition awards, talent and career development, enable FMC to offer a comprehensive total reward package designed for employees throughout their career. We also enhanced our offerings during the COVID-19 pandemic to better support our employees and their families by:
•Paying our essential workers a special recognition award
•Fully covering the costs of COVID-19 testing and vaccines
•Expanding our Dependent Care offerings
•Providing more flexibility in taking out 401K loans
•Enhancing Employee Assistance Program presentations and offerings to assist employees with mental well being
•Expanding flexible work opportunities
Culture and Inclusion
We strive to be an inclusive workplace where our employees reflect the community, are valued, find purpose in their work, and grow and contribute to their fullest potential. We are broadening investments in social areas, including Diversity and Inclusion and racial and gender equity. We launched two task forces, one on Social Justice and Racial Equity, and the other focused on Gender Equity. Our goal for 2027 is to have Black/African American representation in our U.S. workforce to be 14 percent and female representation to be 50 percent of our global workforce across all regions and job levels. The company has developed new global policies and practices to attract and hire talented individuals from underrepresented minorities. For every new hire we now require diverse candidate slates and multiple dimensions of diversity represented by each interview panel. We are expanding our applicant pools and pipelines by adding a new Human Resource role for diversity talent sourcing and partnering with an external recruiting agency specializing in diverse hiring. Diverse views, backgrounds and experiences are key to our success. We launched three additional Employee Resource Groups ("ERGs") in 2019. FMC has six total ERGs with more than twenty employee resource group chapters. We scored 100 percent on the Human Rights Campaign Foundation’s 2021 Corporate Equality Index, a U.S. benchmarking survey measuring corporate policies and practices related to lesbian, gay, bisexual, transgender and queer ("LGBTQ") workplace equality. This is our second consecutive year receiving a score of 100 percent. Over the past several years, we have had significant policy changes related to parental leave and domestic partner and transgender inclusion benefits in the U.S. Due to our diversity and inclusion strategy, women in senior management positions increased from 32 percent in 2019 to 34 percent in 2020.
Safety
Safety is a core value of FMC. At FMC, people come first. We strive for an injury-free workplace, where every employee returns home the same way they arrived. We encourage a culture of open reporting, so we can learn from our mistakes and work towards continuous improvement in behaviors and processes. As a result of our firm commitment to safety, our 2020 TRIR of 0.08 is among the lowest in the industry globally and in the upper decile of peer companies in North America, placing our company among the safest organizations in the chemical industry. This milestone underscores our employees’ commitment to work every day with safety at the forefront of their thoughts and actions. We empower our employees to always put safety first. 2020 presented us with the unique challenge of the COVID-19 pandemic. FMC responded by enacting robust Business Continuity Plans ("BCPs") to ensure continued safe operation at all of our manufacturing sites. These BCPs have been so effective, FMC has not experienced an on-site transmission of the virus to date. In 2021, we continue our journey, focusing on improving management systems and tools. In addition, we continue to engage our global workforce through focused campaigns which address issues and trends identified through analysis of our environment, health and safety data – for example – our current TH!NK. SAFE. campaign addressing "Line of Fire" injuries.
Sustainability
We are committed to delivering products that maintain a safe and secure food supply and to do so in a way that protects the environment for future generations. To reflect this commitment, we reset our sustainability goals in October 2019 to challenge ourselves and ensure that we are helping to create a better world. Our new goals include achieving (i) 100 percent research and development spend on developing sustainable products by 2025, (ii) <0.1 Total Recordable Incident Rate ("TRIR") by 2025, (iii) a 25 percent reduction in Energy Intensity by 2030, (iv) a 25 percent reduction in Green House Gas ("GHG") emissions intensity by 2030, (v) a 20 percent reduction in Water-Use Intensity in High-Risk Locations by 2030, (vi) a sustained Waste Disposed Intensity through 2030 (from our 2018 base year level), and (vii) a 100 on the Community Engagement Index by 2025. In 2020, FMC made progress towards meeting its commitments on the updated goals.
FMC developed and utilizes its award-winning Sustainability Assessment Tool to determine the sustainability of new active ingredients and formulated products in the research and development pipeline and to evaluate products currently on the market. This assessment, along with other stewardship processes and tools, ensures the introduction and continued use of environmentally sustainable agricultural solutions.
At FMC we promote stewardship at each stage of the product life cycle, and stewardship priorities are built into the core of research and development, portfolio and marketing strategies for a truly proactive approach. We continue to strive for open and transparent communications about our product stewardship successes and challenges. FMC is continuing to phase out Highly Hazardous Pesticides ("HHPs") from our product portfolio. In 2020, HHPs accounted for less than 0.4 percent of our total sales.
SEC Filings
SEC filings are available free of charge on our website, www.fmc.com. Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports are posted as soon as practicable after we furnish such materials to the SEC.
REGULATION FD DISCLOSURES
The Company’s investor relations website, located at https://investors.fmc.com, should be considered as a recognized channel of distribution, and the Company may periodically post important information to the web site for investors, including information that the Company may wish to disclose publicly for purposes of complying with the federal securities laws and our disclosure obligations under the SEC's Regulation FD. We encourage investors and others interested in the Company to monitor our investor relations website for material disclosures. Our website address is included in this Form 10-K as a textual reference only and the information on the website is not incorporated by reference into this Form 10-K.
ITEM 1A. RISK FACTORS
Among the factors that could have an impact on our ability to achieve operating results and meet our other goals are:
Industry Risks:
Pricing and volumes in our markets are sensitive to a number of industry specific and global issues and events including:
•Competition and new agricultural technologies - Our business faces competition, which could affect our ability to maintain or raise prices, successfully enter certain markets or retain our market position. Competition for our business includes not only generic suppliers of the same pesticidal active ingredients but also alternative proprietary pesticide chemistries and crop protection technologies that are bred into or applied onto seeds. Increased generic presence in agricultural chemical markets has been driven by the number of significant product patents and product data protections that have expired in the last decade, and this trend is expected to continue. Also, there are changing competitive dynamics in the agrochemical industry as some of our competitors have consolidated, resulting in them having greater scale and diversity, as well as market reach. These competitive differences may not be overcome and may erode our business. Agriculture in many countries is changing and new technologies (e.g., precision pest prediction or application, data management) continue to emerge. At this time, the scope and potential impact of these technologies are largely unknown but could have the potential to disrupt our business.
•Climatic conditions - Our markets are affected by climatic conditions, which could adversely impact crop pricing and pest infestations. For example, drought may reduce the need for fungicides, which could result in fewer sales and greater unsold inventories in the market, whereas excessive rain could lead to increased plant disease or weed growth requiring growers to purchase and use more pesticides. Drought and/or increased temperatures may change insect pest pressures, requiring growers to use more, less, or different insecticides. Natural disasters can impact production at our facilities in various parts of the world. The nature of these events makes them difficult to predict.
•Geographic cyclicality - While our business is well balanced geographically, in any given calendar quarter a certain geography(ies) will predominate in light of seasonal variations in the demand for our products given the nature of the crop protection market and the geographic regions in which we operate. Unexpected market conditions in any such predominating geography(ies), such as adverse weather, pest pressures, or other risks described herein, may impact our business if occurring during a calendar quarter in which such geography(ies) is predominating.
•Changing regulatory environment and public perception - Changes in the regulatory environment, particularly in the U.S., Brazil, China, India, Argentina and the European Union, could adversely impact our ability to continue producing and/or selling certain products in our domestic and foreign markets or could increase the cost of doing so. Additionally, changes to the regulatory environment may be influenced by non-government public pressure as a result of negative perception regarding the use of our crop protection products. We are sensitive to this regulatory risk given the need to obtain and maintain pesticide registrations in every country in which we sell our products. Many countries require re-registration of pesticides to meet new and more challenging requirements; while we defend our products vigorously, these re-registration processes may result in significant additional data costs, reduced number of permitted product uses, or potential product cancellation. Compliance with changing laws and regulations may involve significant costs or capital expenditures or require changes in business practice that could result in reduced profitability. In the European Union, the regulatory risk specifically includes the chemicals regulation known as REACH (Registration, Evaluation, and Authorization of Chemicals), which requires manufacturers to verify through a special registration system that their chemicals can be marketed safely.
•Geographic presence outside of U.S. - We have a strong presence in Latin America, Europe and Asia, as well as in the U.S. Growth of our geographic footprint particularly in Europe and key Asian countries such as India means that developments outside the U.S. will generally have a more significant effect on our operations than in the past. Our operations outside the U.S. are subject to special risks and restrictions, including: fluctuations in currency values; exchange control regulations; changes in local political or economic conditions; governmental pricing directives; import and trade restrictions or tariffs; import or export licensing requirements and trade policy; restrictions on the ability to repatriate funds; and other potentially detrimental domestic and foreign governmental practices or policies affecting U.S. companies doing business abroad.
•Climate change and government regulation of greenhouse gases - The effects of climate change such as rising sea levels, drought, flooding and general volatility in seasonal temperatures could adversely affect our operations globally. Extreme weather events attributable to climate change may result in, among other things, physical damage to our property and equipment, and interruptions to our supply chain. Climate change may also impact markets in which we sell our products, where, for example, a prolonged drought may result in decreased demand for our products. The more gradual effects of persistent temperature change in geographies with significant agricultural lands may result in changes in lands suitable for agriculture or changes in the mix of crops suitable for cultivation and the pests that may be present in such geographies. For example, prolonged increase in average temperature may make northern lands suitable for growing crops not grown historically in such climes, leading farmers to shift from crops such as wheat to soybean and may result in new or different weed, plant disease or insect pressures on such crops – such changes would impact the mix of pesticide products farmers would purchase, which may be adverse for us, depending on the local market and our product mix. Additionally, changes in the governmental regulation of greenhouse gases, depending on their nature and scope, could subject our manufacturing operations to significant additional costs or limits on operations.
•Fluctuations in commodity prices - Our operating results could be significantly affected by the cost of commodities - both chemical raw material commodities and harvested crop commodities. We may not be able to raise prices or improve productivity sufficiently to offset future increases in chemical raw material commodity pricing. Accordingly, increases in such commodity prices may negatively affect our financial results. We use hedging strategies to address material commodity price risks, where hedge strategies are available on reasonable terms. However, we are unable to avoid the risk of medium- and long-term increases. Additionally, fluctuations in harvested crop commodity prices could negatively impact our customers' ability to sell their products at previously forecasted prices resulting in reduced customer liquidity. Inadequate customer liquidity could affect our customers’ abilities to pay for our products and, therefore, affect existing and future sales or our ability to collect on customer receivables.
•Supply arrangements - Certain raw materials are critical to our production processes and our purchasing strategy and supply chain design are complex. While we have made supply arrangements to meet planned operating requirements, an inability to obtain the critical raw materials or operate under contract manufacturing arrangements would adversely impact our ability to produce certain products and could lead to operational disruption and increase uncertainties around business performance. We source critical intermediates and finished products from a number of suppliers, largely outside of the U.S. and principally in China. An inability to obtain these products or execute under contract sourcing arrangements would adversely impact our ability to sell products.
Operational Risks:
•COVID-19 and global pandemic cycles - The rapid spread of the novel coronavirus (COVID-19) outbreak has caused significant disruptions in the U.S. and global economies, and economists expect the impact will continue to be significant. As an agricultural sciences company, we are considered an "essential" industry in the countries in which we operate; we have avoided significant plant closures and all our manufacturing facilities and distribution warehouses remain operational and fully staffed. While we have maintained business continuity and sustained our operations with safety as a priority, the full extent of the disruptions on either our business and operations or the global economy are on-going. In addition, the duration of the pandemic and its adverse effects are unknown and rapidly evolving. External and internal factors and events related to COVID-19 could result in employee isolation and burnout, leading to operational disruption and unexpected, regrettable attrition, which may impact the sustainability of our "high touch" agile culture. We have seen some logistics challenges and shortages of packaging materials and containers, as many industries have increased e-commerce and delivery of goods, creating extra demand on packaging materials, as well as related higher costs and pockets of demand reduction. We may continue to experience disruption caused by COVID-19 in our supply chain, logistics, and pockets of demand, as well as on farm worker labor required for planting, harvesting and packing crops (especially fruits, vegetables and other specialty crops) in the food chain going forward. This outbreak may impact access to our production sites or our ability to adequately and safely staff these sites, the ability of raw material suppliers to produce and deliver goods to us, our ability to ship our products to production, warehousing or customer sites, the ability of our sales organization to make sales or for customers (or indirect customers such as farmers) to purchase our products, or the ability to collect on customer receivables. Our supply chain and business operations could be disrupted from the temporary closure of third-party supplier and manufacturer facilities, interruptions in product supply or restrictions on the export or shipment of our products. Any disruption of our suppliers and contract manufacturers could impact our sales and operating results. The outbreak, and governmental responses to the outbreak, have caused disruption in certain food distribution systems and labor markets for planting and harvesting, which in turn have created operational and financial pressures on some farmers who are the ultimate users of the vast majority of our products. If those pressures continue and grow more widespread or severe, and if farmers materially change their planting decisions or choose not to protect their crops with our products, such pressures on farmers could impact our sales and operating results. Global health concerns, such as coronavirus, could also result in social, economic, and labor instability in the countries in which we or our customers and suppliers
operate. These uncertainties could have a material adverse effect on our business and our results of operation and financial condition. A widespread health crisis could adversely affect the global economy, resulting in an economic downturn that could impact demand for our products. Although our production operations that support agriculture have generally been viewed as "essential" and exempted from governmental lockdown orders, the future impact of the outbreak is highly uncertain and cannot be predicted and there is no assurance that the outbreak will not have a material adverse impact on the future results of the Company. The extent of the impact will depend on future developments, including the availability of vaccines and other actions taken to contain the coronavirus.
•Business disruptions - We produce products through a combination of owned facilities and contract manufacturers. We own and operate large-scale active ingredient manufacturing facilities in the U.S. (Mobile), Puerto Rico (Manati), China (Jinshan), Denmark (Ronland), and India (Panoli). Our operating results are dependent in part on the continued operation of these production facilities. Interruptions at these facilities may materially reduce the productivity of a particular manufacturing facility, or the profitability of our business as a whole. Although we take precautions to enhance the safety of our operations and minimize the risk of disruptions, our operations and those of our contract manufacturers are subject to hazards inherent in chemical manufacturing and the related storage and transportation of raw materials, products and wastes. These potential hazards include explosions, fires, severe weather and natural disasters, mechanical failure, unscheduled downtimes, supplier disruptions, labor shortages or other labor difficulties, information technology systems outages, disruption in our supply chain or manufacturing and distribution operations, transportation interruptions, chemical spills, discharges or releases of toxic or hazardous substances or gases, shipment of contaminated or off-specification product to customers, storage tank leaks, other environmental risks, or other sudden disruption in business operations beyond our control as a result of events such as acts of sabotage, terrorism or war, civil or political unrest, natural disasters, large scale power outages and public health epidemics/pandemics. Some of these hazards may cause severe damage to or destruction of property and equipment or personal injury and loss of life and may result in suspension of operations or the shutdown of affected facilities.
•Litigation and environmental risks - Current reserves relating to our ongoing litigation and environmental liabilities may ultimately prove to be inadequate.
•Hazardous materials - We manufacture and transport certain materials that are inherently hazardous due to their toxic or volatile nature. While we take precautions to handle and transport these materials in a safe manner, if they are mishandled or released into the environment, they could cause property damage or result in personal injury claims against us.
•Environmental compliance - We are subject to extensive federal, state, local, and foreign environmental and safety laws, regulations, directives, rules and ordinances concerning, among other things, emissions in the air, discharges to land and water, and the generation, handling, treatment, disposal and remediation of hazardous waste and other materials. We may face liability arising out of the normal course of business, including alleged personal injury or property damage due to exposure to chemicals or other hazardous substances at our current or former facilities or chemicals that we manufacture, handle or own. We take our environmental responsibilities very seriously, but there is a risk of environmental impact inherent in our manufacturing operations and transportation of chemicals. Any substantial liability for environmental damage could have a material adverse effect on our financial condition, results of operations and cash flows.
Technology Risks:
•Technological and new product discovery/development - Our ability to compete successfully depends in part upon our ability to maintain a superior technological capability and to continue to identify, develop and commercialize new and innovative, high value-added products for existing and future customers. Our investment in the discovery and development of new pesticidal active ingredients relies on discovery of new chemical molecules or biological strains. Such discovery processes depend on our scientists being able to find new molecules and strains, which are novel and outside of patents held by others, and such molecules/strains being efficacious against target pests, and our ability to develop those molecules and strains into new products without creating an undue risk to human health and the environment, and then meeting applicable regulatory criteria. The timeline from active ingredient discovery through full development and product launch averages 8-10 years depending on local regulatory requirements; the complexity and duration of developing new products create risks that product concepts may fail during development or, when launched, may not meet then-current market needs or competitive conditions.
Portfolio Management and Integration Risks:
•Portfolio management risks - We continuously review our portfolio which includes the evaluation of potential business acquisitions that may strategically fit our business and strategic growth initiatives. If we are unable to successfully integrate and develop our acquired businesses, we could fail to achieve anticipated synergies which would include expected cost savings and revenue growth. Failure to achieve these anticipated synergies could materially and adversely affect our financial results. In addition to strategic acquisitions we evaluate the diversity of our portfolio in light of our objectives and alignment with our growth strategy. In implementing this strategy we may not be successful in separating underperforming or non-strategic assets. The gains or losses on the divestiture of, or lost operating
income from, such assets (e.g., divesting) may affect the Company’s earnings. Moreover, we may incur asset impairment charges related to acquisitions or divestitures that reduce earnings. Significant effort will likely be required to ensure that the right mix of resources are trained, engaged and focused on achieving business objectives while adhering to our core values of safety, ethics and compliance.
•Innovation and intellectual property - Our innovation efforts are protected by patents, trade secrets and other intellectual property rights that cover many of our current products, manufacturing processes, and product uses, as well as many aspects of our research and development activities supporting our new product pipeline. Trademarks protect valuable brands associated with our products. Patents and trademarks are granted by individual jurisdictions and the duration of our patents depends on their respective jurisdictions and payment of annuities. Our future performance will depend on our ability to address active ingredient composition of matter patent expirations through effective enforcement of our patents that continue to cover key chemical intermediates and process patents, as well as portfolio life cycle management, particularly for our high value diamide insecticides (see "Diamide Growth Strategy" and "Patents, Trademarks and Licenses" in Item 1 for more details). If our innovation efforts fail to continue to make process improvements to reduce costs, such conditions could impede our competitive position. Some of our competitors may secure patents on production methods or uses of products that may limit our ability to compete cost-effectively.
•Enforcement of intellectual property rights - The composition of matter patents on our Rynaxypyr® active ingredient is nearing its expiration in several key countries. We have a broad estate of additional patents regarding the production of Rynaxypyr® active ingredient, as well as trademark and data exclusivity protection in certain countries that extend well beyond the active ingredient composition of matter patents. (See "Diamide Growth Strategy" and "Patents, Trademarks and Licenses" in Item 1). We intend to strategically and vigorously enforce our patents and other forms of intellectual property and have done so already against several third parties. Other third parties may seek to enter markets with infringing products or may find alternative production methods that avoid infringement or we may not be successful in litigating to enforce our patents due to the risks inherent in any litigation. Patents involve complex factual and legal issues and, thus, the scope, validity or enforceability of any patent claims we have or may obtain cannot be clearly predicted. Patents may be challenged in the courts, as well as in various administrative proceedings before U.S. or foreign patent offices, and may be deemed unenforceable, invalidated or circumvented. We are currently and may in the future be a party to various lawsuits or administrative proceedings involving our patents. Such challenges can result in some or all of the claims of the asserted patent being invalidated or deemed unenforceable. In such circumstances, an adverse patent enforcement decision which could lead to the entry of competing chlorantraniliprole products in relevant markets may materially and adversely impact our financial results.
•Major enterprise initiatives - In the fourth quarter of 2020 we completed the go-live on a single global instance of SAP S/4 HANA. There are execution and change management activities that may affect our ability to operationalize and monetize the investment made in the system. The post implementation period may place significant demands on certain of our internal functional groups, particularly finance and information technology, as we continue to adapt to the new system. Failure to successfully execute and realize the expected synergies from a single global instance could materially and adversely affect our expected performance.
•Potential tax implications of FMC Lithium separation - We have received an opinion from outside counsel to the effect that the spin-off of FMC Lithium as a distribution to our stockholders, completed in March 2019, qualified as a non-taxable transaction for U.S. federal income tax purposes. The opinion is based on certain assumptions and representations as to factual matters from both FMC and FMC Lithium, as well as certain covenants by those parties. The opinion cannot be relied upon if any of the assumptions, representations or covenants is incorrect, incomplete or inaccurate or is violated in any material respect. The opinion of counsel is not binding upon the IRS or the courts and there is no assurance that the IRS or a court will not take a contrary position. It is possible that the IRS or a state or local taxing authority could take the position that aforementioned transaction results in the recognition of significant taxable gain by FMC, in which case FMC may be subject to material tax liabilities.
Financial Risks:
•Foreign exchange rate risks - We are an international company operating in many countries around the world, and thus face foreign exchange rate risks in the normal course of our business. We are particularly sensitive to the Brazilian real, the euro, the Indian rupee, the Chinese yuan, the Mexican peso, the Argentine peso and the U.S. dollar. While we engage in hedging and other strategies to mitigate those risks, unexpected severe changes in foreign exchange may create risks that could materially and adversely affect our expected performance.
•Uncertain tax rates - Our future effective tax rates may be materially impacted by numerous items including: a future change in the composition of earnings from foreign and domestic tax jurisdictions, as earnings in foreign jurisdictions are typically taxed at different statutory rates than the U.S. federal statutory rate; accounting for uncertain tax positions; business combinations; expiration of statute of limitations or settlement of tax audits; changes in valuation allowance; changes in tax law; currency gains and losses; and the potential decision to repatriate certain future foreign earnings on which U.S. or foreign withholding taxes have not been previously accrued.
•Uncertain recoverability of investments in long-lived assets - We have significant investments in long-lived assets and continually review the carrying value of these assets for recoverability in light of changing market conditions and alternative product sourcing opportunities. We may recognize future impairments of long-lived assets which could adversely affect our results of operations.
•Pension and postretirement plans - Our U.S. Plan reached fully funded status during 2018. The primary investment strategy is a liability hedging approach with an objective of maintaining the funded status of the plan such that the funded status volatility is minimized and the likelihood that we will be required to make significant contributions to the plan is limited. The portfolio is comprised of 100 percent fixed income securities and cash. Nevertheless, obligations related to our pension and postretirement plans reflect certain assumptions. To the extent actual experience differs from these assumptions, our costs and funding obligations could increase or decrease significantly.
General Risk Factors:
•Market access risk - Our results may be affected by changes in distribution channels, which could impact our ability to access the market.
•Compliance with laws and regulations - The global regulatory environment is becoming increasingly complex and requires more resources to effectively manage, which may increase the potential for misunderstanding or misapplication of regulatory standards.
•Talent engagement and culture - The inability to recruit and retain key personnel, the unexpected loss of key personnel, or other external and internal factors and events could culminate in employee attrition and may adversely affect our operations. In addition, our future success depends in part on our ability to identify and develop talent to succeed senior management and other key members of the organization.
•Economic and political change - Our business has been and could continue to be adversely affected by economic and political changes in the markets where we compete including: inflation rates, recessions, trade restrictions, tariff increases or potential new tariffs, foreign ownership restrictions and economic embargoes imposed by the U.S. or any of the foreign countries in which we do business; changes in laws, taxation, and regulations and the interpretation and application of these laws, taxes, and regulations; restrictions imposed by the U.S. government or foreign governments through exchange controls or taxation policy; nationalization or expropriation of property, undeveloped property rights, and legal systems or political instability; other governmental actions; and other external factors over which we have no control. Economic and political conditions within the U.S. and foreign jurisdictions or strained relations between countries could result in fluctuations in demand, price volatility, loss of property, state sponsored cyberattacks, supply disruptions, or other disruptions. In Argentina, continued inflation and foreign exchange controls could adversely affect our business. Realignment of change in regional economic arrangements could have an operational impact on our businesses. In China, unpredictable enforcement of environmental regulations could result in unanticipated shutdowns in broad geographic areas, impacting our contract manufacturers and raw material suppliers.
•Information technology security and data privacy risks - As with all enterprise information systems, our information technology systems could be penetrated by outside parties’ intent on extracting information, corrupting information, deploying ransomware, or disrupting business processes. Remote and other work arrangements may leave the Company more vulnerable to a cyberattack. Our systems have in the past been, and likely will in the future be, subject to unauthorized access attempts. Unauthorized access could disrupt our business operations and could result in failures or interruptions in our computer systems, lockout from systems due to ransomware, or in the loss of assets and could have a material adverse effect on our business, financial condition or results of operations. In addition, breaches of our security measures or the accidental loss, inadvertent disclosure, or unapproved dissemination of proprietary information or sensitive or confidential information about the Company, our employees, our vendors, or our customers, could result in litigation, violations of various data privacy regulations in some jurisdictions, and also potentially result in a liability. While we have taken measures to assess the requirements of, and to comply with the European Union's General Data Protection Regulation and data privacy regulations in other countries, these measures may be challenged by authorities that regulate data-related compliance. We could incur significant expense in facilitating and responding to investigations and if the measures we have taken prove to be inadequate, we could face fines or penalties. This could damage our reputation, or otherwise harm our business, financial condition, or results of operations.
•Access to debt and capital markets - We rely on cash generated from operations and external financing to fund our growth and working capital needs. Limitations on access to external financing could adversely affect our operating results. Moreover, interest payments, dividends and the expansion of our business or other business opportunities may require significant amounts of capital. We believe that our cash from operations and available borrowings under our revolving credit facility will be sufficient to meet these needs in the foreseeable future. However, if we need external financing, our access to credit markets and pricing of our capital will be dependent upon maintaining sufficient credit ratings from credit rating agencies and the state of the capital markets generally. There can be no assurances that we would be able to obtain equity or debt financing on terms we deem acceptable, and it is possible that the cost of any financings could increase significantly, thereby increasing our expenses and decreasing our net income. If we are
unable to generate sufficient cash flow or raise adequate external financing, including as a result of significant disruptions in the global credit markets, we could be forced to restrict our operations and growth opportunities, which could adversely affect our operating results.
•Credit default risks - We may use our existing revolving credit facility to meet our cash needs, to the extent available. In the event of a default in this credit facility or any of our senior notes, we could be required to immediately repay all outstanding borrowings and make cash deposits as collateral for all obligations the facility supports, which we may not be able to do. Any default under any of our credit arrangements could cause a default under many of our other credit agreements and debt instruments. Without waivers from lenders party to those agreements, any such default could have a material adverse effect on our ability to continue to operate.
•Exposure to global economic conditions - Deterioration in the global economy and worldwide credit and foreign exchange markets could adversely affect our business. A worsening of global or regional economic conditions or financial markets could adversely affect both our own and our customers' ability to meet the terms of sale or our suppliers' ability to perform all their commitments to us. A slowdown in economic growth in our international markets, or a deterioration of credit or foreign exchange markets could adversely affect customers, suppliers and our overall business there. Customers in weakened economies may be unable to purchase our products, or it could become more expensive for them to purchase imported products in their local currency, or sell their commodities at prevailing international prices, and we may be unable to collect receivables from such customers.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
FMC leases executive offices in Philadelphia, Pennsylvania and operates 25 manufacturing facilities in 18 countries. Our major research and development facilities are in Newark, Delaware; Shanghai, China and Copenhagen, Denmark.
We believe our facilities are in good operating conditions. The number and location of our owned or leased production properties for continuing operations are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| North America | | Latin America | | Europe, Middle East and Africa | | Asia | | Total |
| | | | | | | | | |
| | | | | | | | | |
| Total | 5 | | 2 | | 6 | | 12 | | 25 |
ITEM 3. LEGAL PROCEEDINGS
Like hundreds of other industrial companies, we have been named as one of many defendants in asbestos-related personal injury litigation. Most of these cases allege personal injury or death resulting from exposure to asbestos in premises of FMC or to asbestos-containing components installed in machinery or equipment manufactured or sold by discontinued operations. The machinery and equipment businesses we owned or operated did not fabricate the asbestos-containing component parts at issue in the litigation, and to this day, neither the U.S. Occupational Safety and Health Administration nor the Environmental Protection Agency has banned the use of these components. Further, the asbestos-containing parts for this machinery and equipment were accessible only at the time of infrequent repair and maintenance. A few jurisdictions have permitted claims to proceed against equipment manufacturers relating to insulation installed by other companies on such machinery and equipment. We believe that, overall, the claims against FMC are without merit.
As of December 31, 2020, there were approximately 9,100 premises and product asbestos claims pending against FMC in several jurisdictions. Since the 1980s, approximately 117,000 asbestos claims against FMC have been discharged, the overwhelming majority of which have been dismissed without any payment to the claimant. Since the 1980s, settlements with claimants have totaled approximately $130 million.
We intend to continue managing these asbestos-related cases in accordance with our historical experience. We have established a reserve for this litigation within our discontinued operations and believe that any exposure of a loss in excess of the established reserve cannot be reasonably estimated. Our experience has been that the overall trends in asbestos litigation have changed over time. Over the last several years, we have seen changes in the jurisdictions where claims against FMC are being filed and changes in the mix of products named in the various claims. Because these claim trends have yet to form a predictable pattern, we are presently unable to reasonably estimate our asbestos liability with respect to claims that may be filed in the future.
Please see Note 1 "Principal Accounting Policies and Related Financial Information" - Environmental obligations, Note 12 "Environmental Obligations" and Note 20 "Guarantees, Commitments and Contingencies" in the notes to our consolidated financial statements included in this Form 10-K, the content of which are incorporated by reference to this Item 3.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
ITEM 4A. INFORMATION ABOUT OUR EXECUTIVE OFFICERS
The executive officers of FMC Corporation, the offices they currently hold, their business experience during the previous five years and their ages as of December 31, 2020, are as follows. Each executive officer has been employed by the Company for more than five years.
| | | | | | | | | | | | | | |
| Name | | Age | | Office and year of election |
| Mark A. Douglas | | 58 | | President, Chief Executive Officer, and Director (20-present), President and Chief Operating Officer (18-19), President, FMC Agricultural Solutions (12-18); President, Industrial Chemicals Group (11-12); Vice President, Global Operations and International Development (10-11); Vice President, President Asia, Dow Advanced Materials (09-10); Board Member, Quaker Houghton (13-present); Board Member CropLife International (17-present); Board Member Pennsylvania Academy of the Fine Arts (16-present) |
| Pierre R. Brondeau | | 63 | | Executive Chairman of the Board (20-Present); Chief Executive Officer and Chairman of the Board (18-20); President, Chief Executive Officer and Chairman of the Board (10-18); President and Chief Executive Officer of Dow Advanced Materials, a specialty materials company (08-09); President and Chief Operating Officer of Rohm and Haas Company, a predecessor of Dow Advanced Materials (07-08); Board Member, T.E. Connectivity Electronics (07-present); Board Member, American Chemistry Council (17-present); Board Trustee, Franklin Institute (17-present), Board Member, Livent Corporation (18-present) |
| Andrew D. Sandifer | | 51 | | Executive Vice President and Chief Financial Officer (18-present); Vice President and Treasurer (16-18); Vice President, Corporate Transformation (14-16); Board Member, Philabundance (14-present); Board Trustee, Germantown Academy (17-present) |
| Michael F. Reilly | | 57 | | Executive Vice President, General Counsel, Chief Compliance Officer and Secretary (19-present); Vice President, Associate General Counsel and Chief Compliance Officer (16-19); Associate General Counsel (13-16); Board Member, First State Montessori Academy, Inc. (18-present) |
All officers are elected to hold office for one year or until their successors are elected and qualified. No family relationships exist among any of the above-listed officers, and there are no arrangements or understandings between any of the above-listed officers and any other person pursuant to which they serve as an officer. The above-listed officers have not been involved in any legal proceedings during the past ten years of a nature for which the SEC requires disclosure that are material to an evaluation of the ability or integrity of any such officer.
PART II
ITEM 5. MARKET FOR THE REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDERS MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
FMC common stock of $0.10 par value is traded on the New York Stock Exchange (Symbol: FMC). There were 2,370 registered common stockholders as of December 31, 2020.
FMC’s annual meeting of stockholders will be held at 2:00 p.m. on Tuesday, April 27, 2021 via live webcast at www.virtualshareholdermeeting.com/FMC2021. Notice of the meeting, together with proxy materials, will be mailed approximately five weeks prior to the meeting to stockholders of record as of March 3, 2021.
Transfer Agent and Registrar of Stock:
| | | | | | | | |
| EQ Shareowner Services | | |
| 1110 Centre Pointe Curve, Suite 101 | or | P.O. Box 64874 |
| Mendota Heights, MN 55120-4100 | St. Paul, MN 55164-0854 |
| | |
| Phone: 1-800-468-9716 | | |
| (651-450-4064 local and outside the U.S.) | | |
| https://equiniti.com/us/ |
Stockholder Return Performance Presentation
The graph that follows shall not be deemed to be incorporated by reference into any filing made by FMC under the Securities Act of 1933 or the Securities Exchange Act of 1934.
The following Stockholder Performance Graph compares the five-year cumulative total return on FMC’s Common Stock with the S&P 500 Index and the S&P 500 Chemicals Index. The comparison assumes $100 was invested on December 31, 2015, in FMC’s Common Stock and in both of the indices, and the reinvestment of all dividends.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2015 | | 2016 | | 2017 | | 2018 | | 2019 | | 2020 |
| FMC Corporation | $ | 100.00 | | | $ | 146.23 | | | $ | 246.44 | | | $ | 194.27 | | | $ | 266.40 | | | $ | 311.42 | |
| S&P 500 Index | 100.00 | | | 111.76 | | 135.99 | | 130.25 | | 170.91 | | 201.81 |
| S&P 500 Chemicals Index | 100.00 | | | 109.98 | | 139.16 | | 123.23 | | 150.07 | | 176.46 |
The following table summarizes information with respect to the purchase of our common stock during the three months ended December 31, 2020:
ISSUER PURCHASES OF EQUITY SECURITIES
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Publicly Announced Program |
| Period | Total Number of Shares Purchased (1) | | Average Price Paid Per Share | | Total Number of Shares Purchased | | Total Dollar Amount Purchased | | Maximum Dollar Value of Shares that May Yet be Purchased |
| October | 187,511 | | | $ | 107.18 | | | 186,581 | | | $ | 19,999,977 | | | $ | 580,000,643 | |
| November | 224,837 | | | 113.36 | | | 210,000 | | | 23,818,775 | | | 556,181,868 | |
| December | 53,983 | | | 118.91 | | | 51,957 | | | 6,181,212 | | | 550,000,656 | |
| Total | 466,331 | | | $ | 111.52 | | | 448,538 | | | $ | 49,999,964 | | | |
___________________
(1) Includes shares purchased in open market transactions by the independent trustee of the FMC Corporation Non-Qualified Savings and Investment Plan ("NQSP").
In 2020, 0.4 million shares were repurchased under the publicly announced repurchase program. At December 31, 2020, approximately $550 million remained unused under our Board-authorized repurchase program. This repurchase program does not include a specific timetable or price targets and may be suspended or terminated at any time. Shares may be purchased through open market or privately negotiated transactions at the discretion of management based on its evaluation of market conditions and other factors. We also reacquire shares from time to time from employees in connection with the vesting, exercise and forfeiture of awards under our equity compensation plans. In addition, the independent trustee of our non-qualified deferred compensation plan reacquires shares from time to time through open-market purchases relating to investments by employees in our common stock, one of the investment options available under the Plan.
ITEM 6. SELECTED FINANCIAL DATA
SELECTED CONSOLIDATED FINANCIAL DATA
The selected consolidated financial and other data presented below for, and as of the end of, each of the years in the five-year period ended December 31, 2020, are derived from our consolidated financial statements. The selected consolidated financial data should be read in conjunction with our consolidated financial statements for the year ended December 31, 2020.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in Millions, except per share data) | 2020 | | 2019 | | 2018 | | 2017 | | 2016 |
| Income Statement Data: | | | | | | | | | |
| Revenue | $ | 4,642.1 | | | $ | 4,609.8 | | | $ | 4,285.3 | | | $ | 2,531.2 | | | $ | 2,274.8 | |
| Income from continuing operations before equity in (earnings) loss of affiliates, non-operating pension and postretirement charges (income), interest expense, net and income taxes | 902.2 | | | 821.6 | | | 740.9 | | | 158.5 | | | 197.8 | |
| Income (loss) from continuing operations before income taxes | 729.8 | | | 655.0 | | | 608.4 | | | 95.8 | | | 111.6 | |
| Income (loss) from continuing operations | $ | 578.9 | | | $ | 543.5 | | | $ | 537.6 | | | $ | (133.1) | | | $ | 73.4 | |
Discontinued operations, net of income taxes (1) | (28.3) | | | (63.3) | | | (26.1) | | | 671.5 | | | 138.3 | |
| Net income (loss) | $ | 550.6 | | | $ | 480.2 | | | $ | 511.5 | | | $ | 538.4 | | | $ | 211.7 | |
| Less: Net income (loss) attributable to noncontrolling interest | (0.9) | | | 2.8 | | | 9.4 | | | 2.6 | | | 2.6 | |
| Net income (loss) attributable to FMC stockholders | $ | 551.5 | | | $ | 477.4 | | | $ | 502.1 | | | $ | 535.8 | | | $ | 209.1 | |
| Amounts attributable to FMC stockholders: | | | | | | | | | |
| Continuing operations, net of income taxes | $ | 579.8 | | | $ | 540.7 | | | $ | 531.4 | | | $ | (135.7) | | | $ | 71.1 | |
| Discontinued operations, net of income taxes | (28.3) | | | (63.3) | | | (29.3) | | | 671.5 | | | 138.0 | |
| Net income (loss) | $ | 551.5 | | | $ | 477.4 | | | $ | 502.1 | | | $ | 535.8 | | | $ | 209.1 | |
| Basic earnings (loss) per common share attributable to FMC stockholders: | | | | | | | | | |
| Continuing operations | $ | 4.46 | | | $ | 4.12 | | | $ | 3.94 | | | $ | (1.01) | | | $ | 0.53 | |
| Discontinued operations | (0.22) | | | (0.48) | | | (0.22) | | | 5.00 | | | 1.03 | |
| Net income (loss) | $ | 4.24 | | | $ | 3.64 | | | $ | 3.72 | | | $ | 3.99 | | | $ | 1.56 | |
| Diluted earnings (loss) per common share attributable to FMC stockholders: | | | | | | | | | |
| Continuing operations | $ | 4.44 | | | $ | 4.10 | | | $ | 3.91 | | | $ | (1.01) | | | $ | 0.53 | |
| Discontinued operations | (0.22) | | | (0.48) | | | (0.22) | | | 5.00 | | | 1.03 | |
| Net income (loss) | $ | 4.22 | | | $ | 3.62 | | | $ | 3.69 | | | $ | 3.99 | | | $ | 1.56 | |
| | | | | | | | | |
| Balance Sheet Data: | | | | | | | | | |
| Total assets | $ | 10,186.4 | | | $ | 9,872.7 | | | $ | 9,974.3 | | | $ | 9,206.3 | | | $ | 6,139.3 | |
| Long-term debt | 3,023.1 | | | 3,113.9 | | | 2,531.0 | | | 3,094.2 | | | 1,801.2 | |
| | | | | | | | | |
| Other Data: | | | | | | | | | |
| Cash dividends declared per share | $ | 1.80 | | | $ | 1.64 | | | $ | 0.90 | | | $ | 0.66 | | | $ | 0.66 | |
____________________
(1) Discontinued operations, net of income taxes includes, in periods up to their respective dispositions, our discontinued FMC Lithium and FMC Health and Nutrition segments. It also includes other historical discontinued gains and losses related to adjustments to our estimates of our retained liabilities for environmental exposures, general liability, workers’ compensation, postretirement benefit obligations, legal defense, property maintenance and other costs, losses for the settlement of litigation and gains related to property sales. Amount in 2017 includes the divestiture gain associated with FMC Health and Nutrition.
FORWARD-LOOKING INFORMATION
Statement under the Safe Harbor Provisions of the Private Securities Litigation Reform Act of 1995: FMC and its representatives may from time to time make written or oral statements that are "forward-looking" and provide other than historical information, including statements contained herein, in FMC’s other filings with the SEC, and in reports or letters to FMC stockholders.
In some cases, FMC has identified forward-looking statements by such words or phrases as "will likely result," "is confident that," "expect," "expects," "should," "could," "may," "will continue to," "believe," "believes," "anticipates," "predicts," "forecasts," "estimates," "projects," "potential," "intends" or similar expressions identifying "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including the negative of those words and phrases. Such forward-looking statements are based on management’s current views and assumptions regarding future events, future business conditions and the outlook for the company based on currently available information. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from any results, levels of activity, performance or achievements expressed or implied by any forward-looking statement. Currently, one of the most significant factors is the potential adverse effect of the current COVID-19 pandemic on our financial condition, results of operations, cash flows and performance, which is substantially influenced by the potential adverse effect of the pandemic on our customers and suppliers and the global economy and financial markets. The extent to which COVID-19 impacts us will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the scope, severity and duration of the pandemic, the actions taken to contain the pandemic or mitigate its impact, and the direct and indirect economic effects of the pandemic and containment measures, among others. Additional factors include, among other things, the risk factors and other cautionary statements filed with the SEC included within this Form 10-K as well as other SEC filings and public communications. Moreover, investors are cautioned to interpret many of these factors as being heightened as a result of the ongoing and numerous adverse impacts of COVID-19. FMC cautions readers not to place undue reliance on any such forward-looking statements, which speak only as of the date made. Forward-looking statements are qualified in their entirety by the above cautionary statement. FMC undertakes no obligation, and specifically disclaims any duty, to update or revise any forward-looking statements to reflect events or circumstances arising after the date on which they were made, except as otherwise required by law.
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are an agricultural sciences company, providing innovative solutions to growers around the world with a robust product portfolio fueled by a market-driven discovery and development pipeline in crop protection, plant health, precision agriculture and professional pest and turf management. We operate in a single distinct business segment and develop, market and sell all three major classes of crop protection chemicals: insecticides, herbicides and fungicides. These products are used in agriculture to enhance crop yield and quality by controlling a broad spectrum of insects, weeds and disease, as well as in non-agricultural markets for pest control. This powerful combination of advanced technologies includes leading insect control products based on Rynaxypyr® and Cyazypyr® active ingredients; Authority®, Boral®, Centium®, Command® and Gamit® branded herbicides; Isoflex™ active herbicide ingredient; Talstar® and Hero® branded insecticides; and flutriafol-based fungicides. The FMC portfolio also includes Arc™ farm intelligence and biologicals such as Quartzo® and Presence® bionematicides.
COVID-19 Pandemic
In March 2020, the World Health Organization characterized COVID-19 as a pandemic, and the President of the United States declared the COVID-19 outbreak a national emergency. The rapid spread of the outbreak has caused significant disruptions in the U.S. and global economies.
As an agricultural sciences company, we are considered an "essential" industry in the countries in which we operate; we have avoided significant plant closures and all our manufacturing facilities and distribution warehouses remain operational and fully staffed. However, we did have a third party U.S. toller that was disrupted in the fourth quarter because of COVID-related staffing issues, which signifies one of the ongoing business risks that the pandemic creates. We do not yet know the full extent of the disruptions on either our business and operations or the global economy nor the duration of the pandemic and its adverse effects.
We have implemented new procedures to support the health and safety of our employees and we are following all U.S. Centers for Disease Control and Prevention, as well as state and regional health department guidelines. The well-being of our employees is FMC's top priority. Although most FMC office-based employees around the world have been working remotely during this period, we have implemented procedures to safely return to the workplace in regions where the pandemic is controlled and local health officials have deemed this to be safe in compliance with any government regulations. In addition, we have thousands of employees who continue operating our manufacturing sites and distribution warehouses. In all our facilities, we are using a variety of best practices to address COVID-19 risks, following the protocols and procedures recommended by leading health authorities. We are monitoring the situation in regions where the pandemic continues to escalate and in such regions will remain in a remote working environment until it is safe to return to the workplace. During 2020 we have made significant investments in our employees as a result of the COVID-19 pandemic, including through enhanced dependent care pay policies, recognition bonuses, increased flexibility of work schedules and hours of work to accommodate remote working arrangements, and investment in IT infrastructure to promote remote work. Through these efforts we have successfully avoided any COVID-19 related furloughs or workforce reductions to date.
In addition to addressing the needs of the Company and our employees, FMC has been a leader in supporting the needs of the communities in which FMC has operations and those generally in need as a result of the pandemic. Since the advent of the pandemic, we have donated in excess of 233,000 personal protective equipment supplies, including N95 masks, surgical masks, protective cover suits, goggles and similar items. We have also donated more than 1,800 containers and canisters used to transport alcohol-based disinfecting solution. Additional efforts include financial contributions to hunger-relief organizations; assisting with disinfecting schools and other public spaces in villages; and supporting various community initiatives.
In our supply chain, sourcing of raw materials and intermediates was not a significant issue, although we continued to see some logistics challenges and related higher costs. We are conscious of the potential downside risks in future periods and expect to continue to experience disruption caused by COVID-19 in our supply chain and logistics. We have also seen some pockets of reduced demand as a result of COVID-19, primarily related to disruptions of farm worker labor required for planting, harvesting and packing crops (especially fruits, vegetables and other specialty crops) which may continue going forward. As discussed in our 2020 quarterly reports, we implemented price increases and cost-saving measures across the company to offset impacts of the COVID-19 pandemic and related foreign currency headwinds. We amended our debt covenants with our banks on April 22, 2020 (see Note 11 for more details) to provide significant additional headroom above any of the COVID-19 related scenarios assessed by the company. We will continue to monitor the economic environment related to the pandemic on an ongoing basis and assess the impacts on our business.
2020 Highlights
The following are the more significant developments in our businesses during the year ended December 31, 2020:
•Revenue of $4,642.1 million in 2020 increased $32.3 million or approximately 1 percent versus last year. A more detailed review of revenues is included under the section entitled "Results of Operations". On a regional basis, sales in North America decreased 8 percent, driven primarily by timing of shipments and supply chain disruptions, including COVID related factors, sales in Latin America increased by 1 percent, sales in Europe, Middle East and Africa increased by 4 percent and sales in Asia increased 6 percent, primarily by volume growth. •Our gross margin of $2,052.0 million decreased $31.6 million or approximately 2 percent versus last year. The decrease in gross margin was primarily driven by unfavorable foreign currency impacts primarily in Latin America. Gross margin as a percent of revenue of 44 percent decreased slightly from 45 percent in the prior year period, primarily due to unfavorable foreign currency headwinds.
•Selling, general and administrative expenses decreased from $792.9 million to $729.7 million. Selling, general and administrative expenses, excluding transaction-related charges, of $676.4 million decreased $38.7 million or approximately 5 percent. These decreases were a result of cost-saving measures implemented in response to the pandemic. Transaction-related charges are presented in our Adjusted Earnings Non-GAAP financial measurement below under the section titled "Results of Operations". •Research and development expenses of $287.9 million decreased $10.2 million or 3 percent. The decrease was primarily due to cost-saving measures taken in response to the COVID-19 pandemic. We did not cancel any research and development projects, but we phased some differently to allow lower costs this year in response to the pandemic without fundamentally impacting long-term timelines. We maintain our commitment to invest resources to discover new active ingredients and formulations that support resistance management and sustainable agriculture.
•Net income (loss) attributable to FMC stockholders of $551.5 million increased $74.1 million or approximately 16 percent from $477.4 million in the prior year period. The higher results were driven by cost-saving measures of a combined $73.4 million in selling, general, and administrative and research and development expenses combined in response to the pandemic. Restructuring and other charges were $38.8 million lower versus prior year and discontinued operations expense decreased $35 million compared to the prior year. These increases to income were slightly offset by higher tax expense and higher provision for income taxes of $39.4 million and higher non-operating pension and postretirement charges of $13.1 million. Adjusted after-tax earnings from continuing operations attributable to FMC stockholders of $809.0 million increased $5.3 million or approximately 1 percent. See the disclosure of our Adjusted Earnings Non-GAAP financial measurement under the section titled "Results of Operations".
Other 2020 Highlights
In November 2020, we successfully completed the implementation of our new SAP system. We now have a single, modern system across the entire company for the first time in our history.
On October 2, 2020, we closed on the previously disclosed transaction with Isagro S.p.A ("Isagro") for a purchase price of approximately $65 million, which resulted in a charge in the fourth quarter of 2020. The Fluindapyr acquisition has been treated as an asset acquisition for accounting purposes as it does not meet the definition of a business. Therefore, any acquired in-process research and development was immediately expensed. See Note 9 in the consolidated financial statements included within this Form 10-K for further details.
In June 2020, we launched FMC Ventures, our new venture capital arm targeting strategic investments in start-ups and early-stage companies that are developing and applying emerging technologies in the agricultural industry. The group will be making small, seed type investments.
In May 2020, we announced the launch of our Arc™ farm intelligence platform, a proprietary precision agriculture platform that enables growers and advisors to more accurately predict pest pressure before it becomes a problem.
2021 Outlook
Our 2021 expectation for the overall global crop protection market growth is that it will be up low-single digits on a percentage basis in U.S. dollars. Commodity prices for many of the major crops are higher and stock-to-use ratios have improved compared to this time last year. All regions are seeing some benefit from better crop commodity prices, while the impacts from COVID on crop demand appear to be lessening.
We expect 2021 revenue will be in the range of approximately $4.9 billion to $5.1 billion, up approximately 8 percent at the midpoint versus 2020. We also expect adjusted EBITDA(1) of $1.32 billion to $1.42 billion, which represents 10 percent growth at the midpoint versus 2020 results. 2021 adjusted earnings are expected to be in the range of $6.65 to $7.35 per diluted share(1), up 13 percent at the midpoint versus 2020, excluding any impact from potential share repurchases in 2021. For cash flow outlook, refer to the liquidity and capital resources section below.
(1)Although we provide forecasts for adjusted earnings per share and adjusted EBITDA (Non-GAAP financial measures), we are not able to forecast the most directly comparable measures calculated and presented in accordance with U.S. GAAP. Certain elements of the composition of the U.S. GAAP amounts are not predictable, making it impractical for us to forecast. Such elements include, but are not limited to, restructuring, acquisition charges, and discontinued operations. As a result, no U.S. GAAP outlook is provided.
Results of Operations — 2020, 2019 and 2018
Overview
The following charts provide a reconciliation of Adjusted EBITDA, Adjusted Earnings and Organic Revenue Growth, all of which are Non-GAAP financial measures, from the most directly comparable GAAP measure. Adjusted EBITDA is provided to assist the readers of our financial statements with useful information regarding our operating results. Our operating results are presented based on how we assess operating performance and internally report financial information. For management purposes, we report operating performance based on earnings before interest, income taxes, depreciation and amortization, discontinued operations, and corporate special charges. Our Adjusted Earnings measure excludes corporate special charges, net of income taxes, discontinued operations attributable to FMC stockholders, net of income taxes, and certain Non-GAAP tax adjustments. These are excluded by us in the measure we use to evaluate business performance and determine certain performance-based compensation. Organic Revenue Growth excludes the impacts of foreign currency changes, which we believe is a meaningful metric to evaluate our revenue changes. These items are discussed in detail within the "Other Results of Operations" section that follows. In addition to providing useful information about our operating results to investors, we also believe that excluding the effect of corporate special charges, net of income taxes, and certain Non-GAAP tax adjustments from operating results and discontinued operations allows management and investors to compare more easily the financial performance of our underlying business from period to period. These measures should not be considered as substitutes for net income (loss) or other measures of performance or liquidity reported in accordance with U.S. GAAP.
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Revenue | $ | 4,642.1 | | | $ | 4,609.8 | | | $ | 4,285.3 | |
| Costs and Expenses | | | | | |
| Costs of sales and services | 2,590.1 | | | 2,526.2 | | | 2,405.5 | |
| Gross Margin | $ | 2,052.0 | | | $ | 2,083.6 | | | $ | 1,879.8 | |
| Selling, general and administrative expenses | 729.7 | | | 792.9 | | | 790.0 | |
| Research and development expenses | 287.9 | | | 298.1 | | | 287.7 | |
| Restructuring and other charges (income) | 132.2 | | | 171.0 | | | 61.2 | |
| | | | | |
| Total costs and expenses | $ | 3,739.9 | | | $ | 3,788.2 | | | $ | 3,544.4 | |
Income from continuing operations before equity in (earnings) loss of affiliates, non-operating pension and postretirement charges (income), interest income, interest expense, and provision for income taxes (1) | $ | 902.2 | | | $ | 821.6 | | | $ | 740.9 | |
| Equity in (earnings) loss of affiliates | — | | | — | | | (0.1) | |
| Non-operating pension and postretirement charges (income) | 21.2 | | | 8.1 | | | (0.5) | |
| Interest income | (0.1) | | | (1.9) | | | (1.4) | |
| Interest expense | 151.3 | | | 160.4 | | | 134.5 | |
| Income from continuing operations before income taxes | $ | 729.8 | | | $ | 655.0 | | | $ | 608.4 | |
| Provision for income taxes | 150.9 | | | 111.5 | | | 70.8 | |
| Income (loss) from continuing operations | $ | 578.9 | | | $ | 543.5 | | | $ | 537.6 | |
| Discontinued operations, net of income taxes | (28.3) | | | (63.3) | | | (26.1) | |
| Net income (loss) (GAAP) | $ | 550.6 | | | $ | 480.2 | | | $ | 511.5 | |
| Adjustments to arrive at Adjusted EBITDA: | | | | | |
| Corporate special charges (income): | | | | | |
Restructuring and other charges (income) (3) | $ | 132.2 | | | $ | 171.0 | | | $ | 61.2 | |
Non-operating pension and postretirement charges (income) (4) | 21.2 | | | 8.1 | | | (0.5) | |
Transaction-related charges (5) | 53.3 | | | 77.8 | | | 156.5 | |
| Discontinued operations, net of income taxes | 28.3 | | | 63.3 | | | 26.1 | |
| Interest expense, net | 151.2 | | | 158.5 | | | 133.1 | |
| Depreciation and amortization | 162.7 | | | 150.1 | | | 150.2 | |
| Provision (benefit) for income taxes | 150.9 | | | 111.5 | | | 70.8 | |
Adjusted EBITDA (Non-GAAP) (2) | $ | 1,250.4 | | | $ | 1,220.5 | | | $ | 1,108.9 | |
____________________
(1)Referred to as operating profit.
(2)Adjusted EBITDA is defined as operating profit excluding corporate special charges (income) and depreciation and amortization expense.
(3)See Note 9 to the consolidated financial statements included within this Form 10-K for details of restructuring and other charges (income).
(4)Our non-operating pension and postretirement charges (income) are defined as those costs (benefits) related to interest, expected return on plan assets, amortized actuarial gains and losses and the impacts of any plan curtailments or settlements. These are excluded from our operating results and are primarily related to changes in pension plan assets and liabilities which are tied to financial market performance and we consider these costs to be outside our operational performance. We continue to include the service cost and amortization of prior service cost in our operating results noted above. These elements reflect the current year operating costs to our businesses for the employment benefits provided to active employees.
(5)Charges relate to the expensing of the inventory fair value step-up resulting from the application of purchase accounting, transaction costs, costs for transitional employees, other acquired employee related costs, integration related legal and professional third-party fees. Except for the completion of certain in-flight initiatives, primarily associated with the finalization of our worldwide ERP system, we completed the integration of the DuPont Crop Protection Business as of June 30, 2020. The TSA is now terminated and the last phase of the ERP system transition went live in November 2020 with a stabilization period that will go into the first quarter of 2021. Estimated remaining costs are expected to be less than $5 million for the completion of these defined in-flight initiatives during the remaining time period. Amounts represent the following:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
DuPont Crop Protection Business Acquisition (1) | | | | | |
Legal and professional fees (2) | $ | 53.3 | | | $ | 77.8 | | | $ | 86.9 | |
Inventory fair value amortization (3) | — | | | — | | | 69.6 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Total transaction-related charges | $ | 53.3 | | | $ | 77.8 | | | $ | 156.5 | |
____________________
(1)As previously disclosed, in November 2017, we acquired certain assets relating to the crop protection business of E. I. du Pont de Nemours and Company, and the related research and development organization (the "DuPont Crop Protection Business").
(2)Represents transaction costs, costs for transitional employees, other acquired employees related costs, and transactional-related costs such as legal and professional third-party fees. These charges are recorded as a component of "Selling, general and administrative expense" on the consolidated statements of income (loss).
(3)These charges are included in "Costs of sales and services" on the consolidated statements of income (loss).
ADJUSTED EARNINGS RECONCILIATION
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Net income (loss) attributable to FMC stockholders (GAAP) | $ | 551.5 | | | $ | 477.4 | | | $ | 502.1 | |
Corporate special charges (income), pre-tax (1) | 206.7 | | | 256.9 | | | 217.2 | |
Income tax expense (benefit) on Corporate special charges (income) (2) | (23.8) | | | (49.2) | | | (52.8) | |
| Corporate special charges (income), net of income taxes | $ | 182.9 | | | $ | 207.7 | | | $ | 164.4 | |
| Adjustment for noncontrolling interest, net of tax on Corporate special charges (income) | — | | | — | | | (0.5) | |
| Discontinued operations attributable to FMC Stockholders, net of income taxes | 28.3 | | | 63.3 | | | 29.3 | |
Non-GAAP tax adjustments (3) | 46.3 | | | 55.3 | | | 17.3 | |
| Adjusted after-tax earnings from continuing operations attributable to FMC stockholders (Non-GAAP) | $ | 809.0 | | | $ | 803.7 | | | $ | 712.6 | |
____________________
(1) Represents restructuring and other charges (income), non-operating pension and postretirement charges (income) and transaction-related charges.
(2) The income tax expense (benefit) on Corporate special charges (income) is determined using the applicable rates in the taxing jurisdictions in which the Corporate special charge or income occurred and includes both current and deferred income tax expense (benefit) based on the nature of the non-GAAP performance measure.
(3) We exclude the GAAP tax provision, including discrete items, from the Non-GAAP measure of income, and instead include a Non-GAAP tax provision based upon the annual Non-GAAP effective tax rate. The GAAP tax provision includes certain discrete tax
items including, but not limited to: income tax expenses or benefits that are not related to current year ongoing business operations; tax adjustments associated with fluctuations in foreign currency remeasurement of certain foreign operations; certain changes in estimates of tax matters related to prior fiscal years; certain changes in the realizability of deferred tax assets; and changes in tax law which includes the impact of the Tax Cuts and Jobs Act ("the Act") enacted on December 22, 2017. Management believes excluding these discrete tax items assists investors and securities analysts in understanding the tax provision and the effective tax rate related to ongoing operations thereby providing investors with useful supplemental information about FMC's operational performance.
ORGANIC REVENUE GROWTH RECONCILIATION
| | | | | |
| Twelve Months Ended December 31, 2020 vs. 2019 |
| Total Revenue Change (GAAP) | 1 | % |
| Less: Foreign Currency Impact | (6%) |
| Organic Revenue Change (Non-GAAP) | 7 | % |
| |
Results of Operations
In the discussion below, all comparisons are between the periods unless otherwise noted.
Revenue
2020 vs. 2019
Revenue of $4,642.1 increased $32.3 million, or approximately 1 percent versus the prior year period. The increase was driven by higher volumes, primarily in Latin America and Asia, which accounted for an approximate 4 percent increase, as well as favorable pricing which accounted for an approximate 3 percent increase. Foreign currency headwinds had an unfavorable impact of approximately 6 percent on revenue. Excluding foreign currency impacts, revenue increased approximately 7 percent.
2019 vs. 2018
Revenue of $4,609.8 million increased $324.5 million, or approximately 8 percent versus the prior year period. The increase was driven by higher volumes, primarily in Latin America, and pricing which accounted for an approximate 8 percent and 3 percent increase, respectively, slightly offset by unfavorable foreign currency fluctuations of approximately 3 percent.
See below for a discussion of revenue by region.
| | | | | | | | | | | | | | | | | |
| Total Revenue by Region |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| North America | $ | 1,032.5 | | | $ | 1,121.1 | | | $ | 1,090.8 | |
| Latin America | 1,456.5 | | | 1,441.7 | | | 1,210.1 | |
| Europe, Middle East and Africa (EMEA) | 1,046.3 | | | 1,001.8 | | | 966.0 | |
| Asia | 1,106.8 | | | 1,045.2 | | | 1,018.4 | |
| Total | $ | 4,642.1 | | | $ | 4,609.8 | | | $ | 4,285.3 | |
2020 vs. 2019
North America: Revenue decreased approximately 8 percent in the year ended December 31, 2020. Sales were impacted due to supply chain disruptions, including COVID-related factors associated with logistics and a tolling partner in the fourth quarter. Additionally, we had channel destocking in the first half of the year. We continued market expansion of the Lucento® fungicide, which had a strong second year, and Elevest™ insect control had a good launch year.
Latin America: Revenue increased approximately 1 percent, or approximately 17 percent excluding foreign currency headwinds, for the year ended December 31, 2020 compared to the prior year period due primarily to high-single digit volume growth and solid price increases. Brazil had robust demand for our products for soybeans and sugarcane, while there was reduced acreage for cotton.
EMEA: Revenue increased approximately 4 percent versus the prior year period, or approximately 6 percent excluding foreign currency headwinds. Demand was driven by diamides on specialty crops, Battle® Delta herbicide on cereals and Spotlight® Plus herbicide on potatoes.
Asia: Revenue increased approximately 6 percent versus the prior year period, or approximately 9 percent excluding foreign currency headwinds, primarily driven by market expansion and share gains in India and the very strong market rebound in Australia. Our diamides were in high demand throughout the region in 2020, as we continue to grow on specialty crops like rice and fruit and vegetables.
2019 vs. 2018
North America: Revenue increased approximately 3 percent in the year ended December 31, 2019, primarily driven by volume growth and strength of Rynaxypyr® and Cyazypyr® actives on specialty crops, the launch of Lucento® fungicide, and strong herbicide sales in Canada.
Latin America: Revenue increased approximately 19 percent, or approximately 23 percent excluding foreign currency headwinds, for the year ended December 31, 2019 compared to the prior year period due primarily to strong demand in Brazil for insecticides on cotton, herbicides on sugarcane, and insecticides in soybean applications. Strong growth in Argentina, due to improved market access and strength of herbicides in soybean applications also contributed to the significant growth in the region.
EMEA: Revenue increased approximately 4 percent versus the prior year period, or approximately 10 percent excluding foreign currency headwinds, primarily due to the successful launch of Battle® Delta herbicides and Cyazypyr® insect control registrations across the region. Favorable weather, demand for our diamide products, and higher pricing throughout the region also contributed to the increase. These increases were partially offset by unfavorable foreign currency impacts.
Asia: Revenue increased approximately 3 percent versus the prior year period, or approximately 8 percent excluding foreign currency headwinds, primarily driven by continued strong growth in India and new products across the region. Partially offsetting the increases were adverse weather conditions in Australia and challenged rice markets in China.
In late March 2019, there was an explosion within an industrial park in China which impacted one plant operated by one of our contract manufacturing tollers. The local government had temporarily shut down the entire park to investigate the cause of the explosion. During 2020, our toller received approval for a phased re-opening that began during the fourth quarter and will continue through 2021. Our global manufacturing network provides significant supply chain flexibility. Due to the strength of our partnerships and our alternate sourcing options, we have been able to secure supply of the active ingredients normally manufactured at this location.
Gross margin
2020 vs. 2019
Gross margin of $2,052.0 million decreased $31.6 million, or approximately 2 percent versus the prior year period. The decrease was primarily due to unfavorable foreign currency impacts.
Gross margin percent of approximately 44 percent slightly decreased from 45 percent in the prior year period, primarily due to unfavorable foreign currency headwinds.
2019 vs. 2018
Gross margin of $2,083.6 million increased $203.8 million, or approximately 11 percent versus the prior year period. Gross margin, excluding transaction-related charges, increased versus the prior year period by $134.2 million. The increase was primarily due to higher revenues driven by increased volume and pricing, partially offset by higher costs, primarily raw material costs.
Gross margin percent of approximately 45 percent slightly increased from approximately 44 percent in the prior year period. The increase from higher pricing was nearly offset by higher costs, primarily raw material costs. Gross margin percent, excluding transaction-related charges, of approximately 45 percent remained relatively flat compared to the prior year period.
Selling, general, and administrative expenses
2020 vs. 2019
Selling, general and administrative expenses of $729.7 million decreased by $63.2 million, or approximately 8.0 percent versus the prior year period. Selling, general and administrative expenses, excluding transaction-related charges, decreased $38.7 million, or approximately 5 percent, versus the prior year period due to cost-saving measures implemented in response to the pandemic.
2019 vs. 2018
Selling, general and administrative expenses of $792.9 million slightly increased by $2.9 million versus the prior year period. Selling, general and administrative expenses, excluding transaction-related charges, increased $12.0 million, or approximately 2 percent, versus the prior year period.
Research and development expenses
2020 vs. 2019
Research and development expenses of $287.9 million decreased $10.2 million, or approximately 3 percent versus the prior year period due to cost-saving measures taken in response to the COVID-19 pandemic, but we did not cancel any research and development projects. We phased some projects differently to allow lower costs this year in response to the pandemic without fundamentally impacting long-term timelines.
2019 vs. 2018
Research and development expenses of $298.1 million increased $10.4 million, or approximately 4 percent versus the prior year period primarily due to investments in our global discovery and product development.
Adjusted EBITDA (Non-GAAP)
2020 vs. 2019
Adjusted EBITDA of $1,250.4 million increased $29.9 million, or approximately 2 percent versus the prior year period. The increase was due to higher volumes, higher pricing, and strong cost management which accounted for approximately 9 percent, 9 percent, and 6 percent increases respectively. These factors offset foreign currency fluctuations which had an unfavorable impact of approximately 22 percent on Adjusted EBITDA.
2019 vs. 2018
Adjusted EBITDA of $1,220.5 million increased $111.6 million, or approximately 10 percent versus the prior year period. The increase was due to the strong demand which led to higher volumes and higher pricing as discussed above which contributed approximately 18 percent and 12 percent to the increase, respectively. The price increases were primarily seen in Latin America. These factors more than offset the higher costs, primarily driven by higher raw material costs, and unfavorable foreign currency fluctuations which impacted the change in Adjusted EBITDA by approximately 15 percent and 5 percent, respectively.
Other Results of Operations
Depreciation and amortization
2020 vs. 2019
Depreciation and amortization of $162.7 million increased $12.6 million, or approximately 8 percent, as compared to 2019 of $150.1 million. The increase was mostly driven by the impacts of the amortization effects of the completion of various phases of our ERP implementation which increased amortization expense by approximately $10 million.
2019 vs. 2018
Depreciation and amortization of $150.1 million remained relatively flat as compared to 2018 of $150.2 million.
Interest expense, net
2020 vs. 2019
Interest expense, net of $151.2 million decreased by $7.3 million, or approximately 5 percent, compared to $158.5 million in 2019. The decrease was driven by lower term loan balances which decreased interest expense by approximately $17 million, lower LIBOR rates which decreased interest expense by approximately $20 million and partially offset by the impacts of our third quarter 2019 debt offering which increased interest expense by approximately $30 million.
2019 vs. 2018
Interest expense, net of $158.5 increased by $25.4 million, or approximately 19 percent compared to $133.1 million in 2018. The increase was driven by the issuance of the Senior Notes discussed further below, which increased interest expense by approximately $7 million, and higher average foreign debt balances throughout the year, which increased interest expense by approximately $17 million.
Corporate special charges (income)
Restructuring and other charges (income)
Our restructuring and other charges (income) are comprised of restructuring, assets disposals and other charges (income) as described below:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Restructuring charges | $ | 42.6 | | | $ | 62.2 | | | $ | 124.1 | |
| Other charges (income), net | 89.6 | | | 108.8 | | | (62.9) | |
Total restructuring and other charges (income) (1) | $ | 132.2 | | | $ | 171.0 | | | $ | 61.2 | |
_______________
(1) See Note 9 to the consolidated financial statements included in this Form 10-K for more information.
2020
Restructuring charges in 2020 primarily consisted of $40.2 million of charges associated with the integration of the DuPont Crop Protection Business which was completed during the second quarter of 2020 except for certain in-flight initiatives. These charges included severance, accelerated depreciation on certain fixed assets, and other costs (benefits). There were other miscellaneous restructuring charges $2.4 million.
Other charges (income), net in 2020 includes $65.6 million of charges related to our acquisition of the remaining rights for Fluindapyr active ingredient assets from Isagro. See Note 9 for further information regarding this matter. Additional charges of $24.9 million consists of charges of environmental sites.
2019
Restructuring charges in 2019 primarily consisted of $34.1 million of charges related to our decision to exit sales of all carbofuran formulations globally and $26.4 million of charges associated with the integration of the DuPont Crop Protection Business. These charges included severance, accelerated depreciation on certain fixed assets, and other costs (benefits). There were other miscellaneous restructuring charges $1.7 million.
Other charges (income), net in 2019 primarily consists of charges of environmental sites. During the fourth quarter of 2019, we recorded a charge of $72.8 million a result of an unfavorable court ruling we received in relation to the Pocatello Tribal Litigation at one of our environmental sites. See Note 12 for further information regarding this matter.
2018
Restructuring charges in 2018 were primarily associated with restructuring charges associated with the integration of the DuPont Crop Protection Business. These charges primarily consisted of approximately $59 million of charges related to the change in our market access model in India and approximately $28 million of charges due to our decision to exit the Ewing R&D center. Refer to Note 9 for more information. Other restructuring charges related to the integration of the acquired DuPont Crop Protection Business totaled approximately $22 million.
Other charges (income), net in 2018 primarily consists of income from the gain on sales of $87.2 million from the divestment of a portion of FMC's European herbicide portfolio to Nufarm Limited and certain products of our India portfolio to Crystal Crop Protection Limited. These divestitures satisfied FMC's commitment to the European Commission and the Competition Commission of India, respectively, for regulatory requirements in order to complete the DuPont Crop Protection Acquisition. Additionally, there were environmental related charges of $21.7 million for remediation activities and $2.6 million of other charges.
Non-operating pension and postretirement (charges) income
2020 vs. 2019
The charge for 2020 was $21.2 million compared to $8.1 million in 2019. The increase in non-operating pension and post retirement charges (income) is attributable to the continued approach of using the smoothed market related value of assets (MRVA) as opposed to the actual fair value of plan assets in the determination of 2020 expense. This continued approach will create some volatility in our non-operating periodic pension cost since our qualified pension plan is 100 percent fixed income securities.
2019 vs. 2018
The charge for 2019 was $8.1 million compared to income of $0.5 million in 2018. The change was due to lower expected return on plan assets of approximately $10 million resulting from the full shift to a fixed income investment portfolio for the full year of 2019 versus the shift to a primarily fixed income investment portfolio for only a portion of the year in 2018. See Note 15 for more information.
Transaction-related charges
A detailed description of the transaction related charges is included in Note 5 to the consolidated financial statements included within this Form 10-K.
Provision for income taxes
Provision for income taxes for 2020 was expense of $150.9 million resulting in an effective tax rate of 20.7 percent. Provision for income taxes for 2019 was expense of $111.5 million resulting in an effective tax rate of 17.0 percent. Provision for income taxes for 2018 was expense of $70.8 million resulting in an effective tax rate of 11.6 percent. Note 13 to the consolidated financial statements included in this Form 10-K includes more details on the drivers of the GAAP effective rate and year-over-year changes. We believe showing the reconciliation below of our GAAP to Non-GAAP effective tax rate provides investors with useful supplemental information about our tax rate on the core underlying business.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| (in Millions) | Income (Expense) | Tax Provision (Benefit) | Effective Tax Rate | | Income (Expense) | Tax Provision (Benefit) | Effective Tax Rate | | Income (Expense) | Tax Provision (Benefit) | Effective Tax Rate |
| GAAP - Continuing operations | $ | 729.8 | | $ | 150.9 | | 20.7 | % | | $ | 655.0 | | $ | 111.5 | | 17.0 | % | | $ | 608.4 | | $ | 70.8 | | 11.6 | % |
| Corporate special charges (income) | 206.7 | | 23.8 | | | | 256.9 | | 49.2 | | | | 217.2 | | 52.8 | | |
Tax adjustments (1) | | (46.3) | | | | | (55.3) | | | | | (17.3) | | |
| Non-GAAP - Continuing operations | $ | 936.5 | | $ | 128.4 | | 13.7 | % | | $ | 911.9 | | $ | 105.4 | | 11.6 | % | | $ | 825.6 | | $ | 106.3 | | 12.9 | % |
_______________
(1)Tax adjustments in 2020, 2019, and 2018 are materially attributable to the effects of certain changes in prior year tax matters and the realizability of deferred tax assets in certain jurisdictions. Tax adjustments in 2018 also include the effects of the Act, primarily related to the one-time transition tax and the decrease in the U.S. federal tax rate. See Note 13 to the consolidated financial statements included within this Form 10-K for additional discussion.
The primary drivers for the fluctuations in the effective tax rate for each period are provided in the table above. Excluding the items in the table above, the changes in the non-GAAP effective tax rate were primarily due to the impact of geographic mix of earnings among our global subsidiaries. See Note 13 to the consolidated financial statements for additional details related to the provisions for income taxes on continuing operations, as well as items that significantly impact our effective tax rate.
Discontinued operations, net of income taxes
Our discontinued operations, in periods up to its disposition, represent our discontinued FMC Lithium and FMC Health and Nutrition business results as well as adjustments to retained liabilities from other previously discontinued operations. The primary liabilities retained include environmental liabilities, other postretirement benefit liabilities, self-insurance, long-term obligations related to legal proceedings and historical restructuring activities. See Note 11 to the consolidated financial statements for additional details on our discontinued operations.
2020 vs. 2019
Discontinued operations, net of income taxes represented a loss of $28.3 million in 2020 compared to a loss of $63.3 million in 2019. The loss during both periods was primarily due to adjustments related to the retained liabilities from our previously discontinued operations. Offsetting the loss in both 2019 and 2020 were the gain on sale of two parcels of land in our discontinued site in Newark, California of $21 million and $24 million, net of taxes, respectively. Additionally, during 2019, we included the net loss from our discontinued FMC Lithium segment, primarily due to separation-related costs, up to its separation date on March 1, 2019.
2019 vs. 2018
Discontinued operations, net of income taxes represented a loss of $63.3 million in 2019 compared to a loss of $26.1 million in 2018. 2019 included the net loss from our discontinued FMC Lithium segment, primarily due to separation-related costs, up to its separation date on March 1, 2019, compared to income for the full year in 2018. Offsetting the loss was the gain on sale from the sale of the first of two parcels of land of our discontinued site in Newark, California in 2019. During 2018, we recorded a charge of approximately $106 million as a result of active negotiations for a settlement agreement primarily to address discontinued operations at our Middleport, New York plant which was the subject of an Administrative Order on Consent entered into with the EPA and NYSDEC in 1991. The charge consisted of incremental estimated costs of remediation for certain offsite operable units associated with historic site operations as we engaged in settlement discussions with NYSDEC to resolve the path forward regarding remediation. Refer to Note 12 for further details.
Net income (loss) attributable to FMC stockholders
2020 vs. 2019
Net income (loss) attributable to FMC stockholders increased to $551.5 million from $477.4 million. The higher results were driven by a slight increase in revenue as well as cost-saving measures in selling, general, and administrative and research and development expenses in response to the pandemic. Restructuring and other charges were $38.8 million lower versus prior year and discontinued operations expense decreased $35 million compared to the prior year. These increases to income were partially offset by higher tax expense and higher provision for income taxes of $39.4 million and higher non-operating pension and postretirement charges of $13.1 million.
2019 vs. 2018
Net income (loss) attributable to FMC stockholders decreased to $477.4 million from $502.1 million. The decrease was primarily due to higher costs and expenses, particularly restructuring and other charges associated with environmental remediation at our decommissioned plant near Pocatello, higher tax provisions, and higher net interest expense. This was partially offset by higher adjusted EBITDA from higher volumes and pricing.
Liquidity and Capital Resources
Cash and cash equivalents at December 31, 2020 and 2019, were $568.9 million and $339.1 million, respectively. We held more cash on the balance sheet as a result of significantly increased cash from operations year over year and held it in advance of a seasonal working capital build in the first quarter. Of the cash and cash equivalents balance at December 31, 2020, $560.5 million was held by our foreign subsidiaries. The cash held by foreign subsidiaries for permanent reinvestment is generally used to finance the subsidiaries’ operating activities and future foreign investments. We have not provided income taxes for other outside basis differences inherent in our investments in subsidiaries because the investments and related unremitted earnings are essentially permanent in duration or we have concluded that no additional tax liability will arise upon disposal or remittance. See Note 13 to the consolidated financial statements included within this Form 10-K for more information.
At December 31, 2020, we had total debt of $3,267.8 million as compared to $3,258.8 million at December 31, 2019. Total debt included $2,929.5 million and $3,031.1 million of long-term debt (excluding current portions of $93.6 million and $82.8 million) at December 31, 2020 and 2019, respectively. Early in the second quarter of 2020 we amended the Revolving Credit Facility and 2017 Term Loan Agreements to increase the maximum leverage ratio, in order to address potential liquidity constraints that might arise due to the COVID-19 pandemic. Although we had not then, and have not since, experienced any liquidity issues as a result of the economic impacts of the pandemic, we determined that it would be prudent to take this step, as the higher leverage ratio provides significant headroom above any of the COVID-19 related scenarios assessed by the company. Additionally, during the second quarter we fully repaid the $500 million revolver draw made late in the first quarter at the height of the pandemic’s impact on short-term financing markets. As of December 31, 2020, we were in compliance with all of our debt covenants. See Note 14 in the consolidated financial statements included in this Form 10-K for further details. We remain committed to solid investment grade credit metrics, and expect full-year average leverage to be in line with this commitment in 2020.
The decrease in long-term debt was primarily due to paydowns on the 2017 Term Loan Facility, which is scheduled to mature on November 1, 2022. The borrowings under the 2017 Term Loan Facility will bear interest at a floating rate, which will be a base rate or a Eurocurrency rate equal to the London interbank offered rate for the relevant interest period, plus in each case an applicable margin, as determined in accordance with the provisions of the 2017 Term Loan Facility. The decrease in long-term debt was offset by the increase in short-term debt.
Our short-term debt consists of foreign borrowings and our commercial paper program. Foreign borrowings decreased from $144.9 million at December 31, 2019 to $98.4 million at December 31, 2020 while outstanding commercial paper increased from zero at December 31, 2019 to $146.3 million at December 31, 2020. We provide parent-company guarantees to lending institutions providing credit to our foreign subsidiaries.
Our commercial paper program allows us to borrow at rates generally more favorable than those available under our credit facility. At December 31, 2020, we had $146.3 million borrowings outstanding under the commercial paper program at an average borrowing rate of 0.5 percent. Our commercial paper balances fluctuate from year to year depending on working capital needs and status on receivables collections.
Revolving Credit Facility and 2017 Term Loan Agreement Amendment
On April 22, 2020, we amended both our Revolving Credit Agreement and 2017 Term Loan Agreement which, among other things, increased the maximum leverage ratio financial covenant and added a negative covenant restricting purchases of the Company’s stock if at any time the maximum leverage ratio exceeds 3.5 through the period ending June 30, 2021. See Note 14 for further details.
Statement of Cash Flows
Cash provided (required) by operating activities was $736.8 million, $555.6 million and $362.7 million for 2020, 2019 and 2018, respectively.
The table below presents the components of net cash provided (required) by operating activities. For comparability, the prior period amounts for "Change in all other operating assets and liabilities" have been recast to reflect the current period presentation.
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year ended December 31, |
| 2020 | | 2019 | | 2018 |
| Income (loss) from continuing operations before equity in (earnings) loss of affiliates, non-operating pension expense and postretirement charges, interest expense, net and income taxes | $ | 902.2 | | | $ | 821.6 | | | $ | 740.9 | |
| Restructuring and other charges (income), transaction-related charges and depreciation and amortization | 348.2 | | | 398.9 | | | 367.9 | |
| Operating income before depreciation and amortization (Non-GAAP) | $ | 1,250.4 | | | $ | 1,220.5 | | | $ | 1,108.8 | |
Change in trade receivables, net (1) | (71.8) | | | (123.5) | | | (281.5) | |
| Change in guarantees of vendor financing | 64.8 | | | 8.6 | | | 15.4 | |
Change in advance payments from customers (2) | (145.5) | | | 34.1 | | | 80.2 | |
Change in accrued customer rebates (3) | 17.2 | | | (85.8) | | | 104.1 | |
Change in inventories (4) | (59.7) | | | 6.4 | | | (200.7) | |
Change in accounts payable (5) | 61.8 | | | 103.0 | | | 166.7 | |
Change in all other operating assets and liabilities (6) | (68.2) | | | (208.5) | | | (187.5) | |
| Operating cash flows (Non-GAAP) | $ | 1,049.0 | | | $ | 954.8 | | | $ | 805.5 | |
Restructuring and other spending (7) | (17.9) | | | (18.6) | | | (25.2) | |
Environmental spending, continuing, net of recoveries (8) | (1.9) | | | (18.3) | | | (20.3) | |
Pension and other postretirement benefit contributions (9) | (4.6) | | | (13.4) | | | (37.5) | |
Net interest payments (10) | (141.8) | | | (140.9) | | | (133.4) | |
Tax payments, net of refunds (11) | (82.1) | | | (130.9) | | | (125.3) | |
| | | | | |
| | | | | |
Transaction and integration costs (12) | (63.9) | | | (77.1) | | | (101.1) | |
| Cash provided (required) by operating activities of continuing operations | $ | 736.8 | | | $ | 555.6 | | | $ | 362.7 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
____________________
(1)The change in trade receivables in all periods include the impacts of seasonality and the receivable build intrinsic in our business. The change in cash flows related to trade receivables in 2020 was driven by timing of collections as well as higher sales. Collection timing is more pronounced in certain countries such as Brazil where there may be terms significantly longer than the rest of our business. Additionally, timing of collection is impacted as amounts for all periods include carry-over balances remaining to be collected in Latin America, where collection periods are measured in months rather than weeks. During 2020, we collected approximately $931 million of receivables in Brazil.
(2)Advance payments are typically received in the fourth quarter of each year, primarily in our North America operations as revenue associated with advance payments is recognized, generally in the first half of each year following the seasonality of that business, as shipments are made and title, ownership and risk of loss pass to the customer. The change in 2020 was primarily related to lower overall payments received and higher application of funds to accounts receivable balances year over year.
(3)These rebates are primarily associated within North America, and to a lesser extent Brazil, and in North America generally settle in the fourth quarter of each year given the end of the respective crop cycle. The changes year over year are associated with the mix in sales eligible for rebates and incentives in 2020 compared to 2019 and 2018 and timing of certain rebate payments.
(4)Changes in inventory in 2020 are a result of significant market impacts during the fourth quarter related to logistics and supply chain constraints in the U.S., reduced demand in the U.S., Brazil and Argentina, and products held by foreign customs. Changes in inventory in 2019 and 2018 are a result of inventory levels being adjusted to take into consideration the change in market conditions.
(5)The change in cash flows related to accounts payable in 2020, 2019 and 2018 is primarily due to timing of payments made to suppliers and vendors. 2019 was partially impacted during portions of 2019 from global supply chain issues, primarily in China, which required us to obtain raw materials on payment terms shorter than normal.
(6)Changes in all periods presented primarily represent timing of payments associated with all other operating assets and liabilities. Additionally, the 2020 and 2019 period includes the effects of the unfavorable contracts amortization of approximately $120 million and $116 million, respectively.
(7)See Note 9 to the consolidated financial statements included in this Form 10-K for further details.
(8)Included in our results for each of the years presented are environmental charges for environmental remediation of $24.9 million, $108.7 million and $21.7 million, respectively. The amounts in 2020 will be spent in future years. The amounts represent environmental remediation spending which were recorded against pre-existing reserves, net of recoveries. Environmental obligations for continuing operations primarily represent obligations at shut down or abandoned facilities within businesses that do not meet the criteria for presentation as discontinued operations. Additionally, during the first quarter of 2020, we entered into a confidential insurance settlement pertaining to coverage at a legacy environmental site, which settlement resulted in a cash payment to FMC in the amount of $20.0 million. Refer to Note 12 for more details.
(9)There were no voluntary contributions to our U.S. qualified defined benefit plan in 2020. Amounts in 2019 and 2018 include voluntary contributions to our U.S. qualified defined benefit plan of $7.0 million and $30.0 million, respectively.
(10)Interest payments were basically flat versus prior year.
(11)Amounts shown in the chart represent net tax payments of our continuing operations. The decrease in net tax payments in 2020 as compared to prior periods is primarily attributable to the deferral of income tax payments in various jurisdictions as a result of the COVID-19 pandemic. Tax payments in 2019 primarily represent the payments of tax attributable to the Nufarm Limited sale, transition tax, and tax payments related to the acquired DuPont Crop Protection Business. Tax payments in 2018 primarily represent the payments of tax attributable to the FMC Health and Nutrition segment disposition, transition tax and full year tax payments related to the acquired DuPont Crop Protection Business.
(12)Represents payments for legal and professional fees associated with the DuPont Crop Protection Business Acquisition in addition to costs related to integrating the DuPont Crop Protection Business. Except for the completion of certain in-flight initiatives, primarily associated with the finalization of our worldwide ERP system, we completed the integration of the DuPont Crop Protection Business as of June 30, 2020. The TSA is now terminated and the last phase of the ERP system transition went live in November 2020 with a stabilization period that will go into the first quarter of 2021. Estimated remaining cash outflows are expected to be approximately $15 million for the completion of these defined in-flight initiatives during the remaining time period. See Note 5 to the consolidated financial statements for more information.
Cash provided (required) by operating activities of discontinued operations was $(89.0) million, $(67.1) million and $5.7 million for 2020, 2019 and 2018, respectively.
Cash required by operating activities of discontinued operations is directly related to environmental, other postretirement benefit liabilities, self-insurance, long-term obligations related to legal proceedings and historical restructuring activities.
Amounts in 2019 and 2018 also include the operating activities of our discontinued FMC Lithium segment, which was separated on March 1, 2019
Cash provided (required) by investing activities of continuing operations was $(200.4) million, $(195.9) million and $(37.5) million for 2020, 2019 and 2018, respectively.
Cash required in 2020 primarily due to capital expenditures and spending related to our contract manufacturing arrangements, as well as continued spending associated with the final stages of our new SAP system implementation. 2020 also includes payments of $65.6 million to acquire the remaining rights for Fluindapyr from Isagro S.p.A ("Isagro") in an asset acquisition.
Cash required in 2019 is primarily due to capital expenditures and spending related to our contract manufacturing arrangements, as well as continued spending during that period associated with the implementation of a new SAP system.
Cash required in 2018 is primarily due to higher capital expenditure spending as well as incremental capitalizable corporate level spending associated with the implementation of a new SAP system, partially offset by the sale of product portfolios of approximately $88 million that were required to complete the DuPont Crop Protection Business Acquisition.
Cash provided (required) by investing activities of discontinued operations was $31.1 million, $9.2 million and $(93.4) million for 2020, 2019 and 2018, respectively.
Cash provided by investing activities of discontinued operations in 2020 and 2019 represents the proceeds of approximately $31 million and $26 million from the sale of our two parcels of land of our discontinued site in Newark, California. These sales resulted in a gain recognized within discontinued operations in each period of approximately $24 million and $21 million, net of taxes, respectively. In 2019, this was partially offset by capital expenditures of our discontinued FMC Lithium segment. Cash required by investing activities of discontinued operations in 2018 represents the working capital payment associated with the divestiture of FMC Health and Nutrition as well as the capital expenditures of our discontinued FMC Lithium segment.
Cash provided (required) by financing activities was $(250.3) million, $(87.0) million and $(397.3) million in 2020, 2019 and 2018, respectively.
The change in cash required by financing activities in 2020 is primarily driven by the prior year proceeds from the Senior Notes and higher dividend payments offset by a reduction in the payment of long term debt and a reduction of repurchases of common stock under our publicly announced program.
The change in cash required by financing activities in 2019 is primarily due to the proceeds from the Senior Notes offset by cash outflows including higher repurchases of common stock, repayment of long-term debt, and higher dividend payments in
2019 as compared to the prior period. 2018 included the net proceeds from the IPO of FMC Lithium which were more than offset by repayments of long-term debt, dividend payments and repurchases of common stock.
Cash provided (required) by financing activities of discontinued operations was zero , $(37.2) million and $34.0 million in 2020, 2019 and 2018, respectively.
Cash required by financing activities of discontinued operations in 2019 represents debt repayments on FMC Lithium's external debt as well as cash payments associated with its separation. Cash provided by financing activities of discontinued operations in 2018 represents the proceeds from borrowing of long-term debt of our discontinued FMC Lithium segment.
Free Cash Flow
We define free cash flow, a Non-GAAP financial measure, as all cash inflows and outflows excluding those related to financing activities (such as debt repayments, dividends, and share repurchases) and acquisition related investing activities. Free cash flow is calculated as all cash from operating activities reduced by spending for capital additions and other investing activities as well as legacy and transformation spending. Therefore, our calculation of free cash flow will almost always result in a lower amount than cash from operating activities from continuing operations, the most directly comparable U.S. GAAP measure. However, the free cash flow measure is consistent with management's assessment of operating cash flow performance and we believe it provides a useful basis for investors and securities analysts about the cash generated by routine business operations, including capital expenditures, in addition to assessing our ability to repay debt, fund acquisitions and return capital to shareholders through share repurchases and dividends.
Our use of free cash flow has limitations as an analytical tool and should not be considered in isolation or as a substitute for an analysis of our results under U.S. GAAP. First, free cash flow is not a substitute for cash provided (required) by operating activities of continuing operations, as it is not a measure of cash available for discretionary expenditures since we have non-discretionary obligations, primarily debt service, that are not deducted from the measure. Second, other companies may calculate free cash flow or similarly titled Non-GAAP financial measures differently or may use other measures to evaluate their performance, all of which could reduce the usefulness of free cash flow as a tool for comparison. Additionally, the utility of free cash flow is further limited as it does not reflect our future contractual commitments and does not represent the total increase or decrease in our cash balance for a given period. Because of these and other limitations, free cash flow should be considered along with cash provided (required) by operating activities of continuing operations and other comparable financial measures prepared and presented in accordance with U.S. GAAP.
The table below presents a reconciliation of free cash flow from the most directly comparable U.S. GAAP measure.
FREE CASH FLOW RECONCILIATION
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year ended December 31, |
| 2020 | | 2019 | | 2018 |
| Cash provided (required) by operating activities of continuing operations (GAAP) | $ | 736.8 | | | $ | 555.6 | | | $ | 362.7 | |
Transaction and integration costs (1) | 63.9 | | | 77.1 | | | 101.1 | |
Adjusted cash from operations (2) | $ | 800.7 | | | $ | 632.7 | | | $ | 463.8 | |
| | | | | |
Capital expenditures (3) | (67.2) | | | (93.9) | | | (83.0) | |
Other investing activities (3)(4) | (20.4) | | | (54.0) | | | (13.6) | |
| Capital additions and other investing activities | $ | (87.6) | | | $ | (147.9) | | | $ | (96.6) | |
| | | | | |
Cash provided (required) by operating activities of discontinued operations (5) | (89.0) | | | (67.1) | | | 5.7 | |
Cash provided (required) by investing activities of discontinued operations (5) | 31.1 | | | 9.2 | | | (93.4) | |
Transaction and integration costs (1) | (63.9) | | | (77.1) | | | (101.1) | |
Investment in Enterprise Resource Planning system (3) | (47.2) | | | (48.0) | | | (48.5) | |
Legacy and transformation (6) | $ | (169.0) | | | $ | (183.0) | | | $ | (237.3) | |
| | | | | |
| Free cash flow (Non-GAAP) | $ | 544.1 | | | $ | 301.8 | | | $ | 129.9 | |
___________________
(1) Represents payments for legal and professional fees associated with the DuPont Crop Protection Business Acquisition in addition to costs related to integrating the DuPont Crop Protection Business. See Note 5 to the consolidated financial statements for more information.
(2) Adjusted cash from operations is defined as cash provided (required) by operating activities of continuing operations excluding the effects of transaction-related cash flows, which are included within Legacy and transformation.
(3) Components of cash provided (required) by investing activities of continuing operations. Refer to the below discussion for further details.
(4) Cash spending associated with contract manufacturers was $17.4 million, $51.7 million and $13.1 million for the years ended December 31, 2020, 2019 and 2018, respectively.
(5) Refer to the above discussion for further details.
(6) Includes our legacy liabilities such as environmental remediation and other legal matters that are reported in discontinued operations as well as business integration costs associated with the DuPont Crop Protection Business Acquisition and the implementation of our new SAP system.
2021 Cash Flow Outlook
Our cash needs for 2021 include operating cash requirements (which are impacted by contributions to our pension plan, as well as environmental, asset retirement obligation, and restructuring spending), capital expenditures, and legacy and transformation spending, as well as mandatory payments of debt, dividend payments, and share repurchases. We plan to meet our liquidity needs through available cash, cash generated from operations, commercial paper issuances and borrowings under our committed revolving credit facility. At December 31, 2020 our remaining borrowing capacity under our credit facility was $1,139.6 million.
We expect 2021 free cash flow (Non-GAAP) to increase to a range of approximately $530 million to $620 million, driven by growth in adjusted cash from operations and reduced legacy and transformation spending which is forecasted to be partially offset by a significant year over year increase in capital additions. This increase in capital additions primarily relates to resuming or advancing projects that were delayed or deferred in 2020 due to the pandemic.
Although we provide a forecast for free cash flow, a Non-GAAP financial measure, we are not able to forecast the most directly comparable measure calculated and presented in accordance with U.S. GAAP, which is cash provided (required) by operating activities of continuing operations. Certain elements of the composition of the U.S. GAAP amount are not predictable, making it impractical for us to forecast. Such elements include, but are not limited to, restructuring, acquisition charges, and discontinued operations. As a result, no U.S. GAAP outlook is provided.
Cash from operating activities of continuing operations
We expect higher cash from operating activities, excluding the effects of transaction-related cash flows, primarily driven by higher forecasted Adjusted EBITDA as well as continued improvement in working capital, to be in the range of approximately $790 million to $950 million. Transaction-related cash flows are included within Legacy and transformation, which is consistent with how we evaluate our business operations from a cash flow standpoint. See below for further discussion. Cash from operating activities includes cash requirements related to our pension plans, environmental sites, restructuring and asset retirement obligations, taxes and interest on borrowings.
Pension
We do not expect to make any voluntary cash contributions to our U.S. qualified defined benefit pension plan in 2021. The plan is fully funded and our portfolio is comprised of 100 percent fixed income securities and cash. Our investment strategy is a liability hedging approach with an objective of maintaining the funded status of the plan such that the funded status volatility is minimized and the likelihood that we will be required to make significant contributions to the plan is limited.
Environmental
Projected 2021 spending includes approximately $58 million to $68 million of net environmental remediation spending for our sites accounted for within continuing operations. Environmental obligations for continuing operations primarily represent obligations at shut down or abandoned facilities within businesses that do not meet the criteria for presentation as discontinued operations. This spending includes approximately $43 million related to our environmental remediation site near Pocatello, Idaho, primarily as a result of a litigation judgment against us in the Pocatello Tribal litigation described in Note 12. Of the total 2021 projected spend at this site, $20.5 million was paid in the first quarter of 2021 and an additional $11.7 million payment for past years' permit fees plus interest associated with these payments will also be made in 2021.
Total projected 2021 environmental spending, inclusive of both sites accounted for within continuing operations and discontinued sites (discussed within Legacy and transformation below), is expected to be in the range of $115 million to $125 million.
Restructuring and asset retirement obligations
We expect to make payments of approximately $25 to $35 million in 2021, of which approximately $10 million is related to exit and disposal costs as a result of our decision to exit sales of all carbofuran formulations (including Furadan® insecticide/nematicide, as well as Curaterr® insecticide/nematicide and any other brands used with carbofuran products). See Note 9 for more information.
Capital additions and other investing activities
Projected 2021 capital expenditures and expenditures related to contract manufacturers are expected to be in the range of approximately $160 million to $200 million. The spending is mainly driven by continuing progress on projects delayed or deferred in 2020 due to the pandemic, primarily for diamide capacity expansion and new active ingredient capacity. Expenditures related to contract manufacturers are included within "other investing activities".
Legacy and transformation
Projected 2021 legacy and transformation spending are expected to be in the range of approximately $100 million to $130 million. This is primarily driven by environmental remediation spending and legacy liabilities. Except for the completion of certain in-flight initiatives, primarily associated with the finalization of our worldwide ERP system, we completed the integration of the DuPont Crop Protection Business as of June 30, 2020. As noted, the TSA is now terminated and the last phase of the ERP system transition went live in November 2020 with a stabilization period that will go into the first quarter of 2021. Cash outflows for these initiatives are expected to be approximately $15 million in 2021.
Projected 2021 spending includes approximately $53 million to $63 million of net environmental remediation spending for our discontinued sites. These projections include spending as a result of a settlement reached in the second quarter of 2019 at our Middleport, New York site. The settlement will result in spending of approximately $25 million in 2021.
Total projected 2021 environmental spending, inclusive of both sites accounted for within continuing operations (discussed within Cash from operating activities of continuing operations above) and discontinued sites, is expected to be in the range of $115 million to $125 million.
Share repurchases
During the year ended December 31, 2020, 0.4 million shares were repurchased under the publicly announced repurchase program for approximately $50 million. At December 31, 2020, approximately $550 million remained unused under our Board-authorized repurchase program. We intend to purchase between $400 million to $500 million of our common shares in 2021. This repurchase program does not include a specific timetable or price targets and may be suspended or terminated at any time. Shares may be purchased through open market or privately negotiated transactions at the discretion of management based on its evaluation of market conditions and other factors. We also reacquire shares from time to time from employees in connections with vesting, exercise and forfeiture of awards under our equity compensation plans.
Dividends
On January 21, 2021, we paid dividends aggregating $62.3 million to our shareholders of record as of December 31, 2020. This amount is included in "Accrued and other liabilities" on the consolidated balance sheet as of December 31, 2020. For the years ended December 31, 2020, 2019 and 2018, we paid $228.5 million, $210.3 million and $89.2 million in dividends, respectively. We expect to continue to make quarterly dividend payments. Future cash dividends, as always, will depend on a variety of factors, including earnings, capital requirements, financial condition, general economic conditions and other factors considered relevant by us and is subject to final determination by our Board of Directors.
Commitments
We provide guarantees to financial institutions on behalf of certain customers, principally customers in Brazil, for their seasonal borrowing. The total of these guarantees was $140.6 million at December 31, 2020. These guarantees arise during the ordinary course of business from relationships with customers and nonconsolidated affiliates. Non-performance by the guaranteed party triggers the obligation requiring us to make payments to the beneficiary of the guarantee. Based on our experience these types of guarantees have not had a material effect on our consolidated financial position or on our liquidity. Our expectation is that future payment or performance related to the non-performance of others is considered unlikely.
In connection with certain of our property and asset sales and divestitures, we have agreed to indemnify the buyer for certain liabilities, including environmental contamination and taxes that occurred prior to the date of sale. Our indemnification obligations with respect to these liabilities may be indefinite as to duration and may or may not be subject to a deductible, minimum claim amount or cap. In cases where it is not possible for us to predict the likelihood that a claim will be made or to make a reasonable estimate of the maximum potential loss or range of loss, no specific liability has been recorded. If triggered, we may be able to recover certain of the indemnity payments from third parties. In cases where it is possible, we have recorded a specific liability within our Reserve for Discontinued Operations. Refer to Note 11 for further details.
Our total significant committed contracts that we believe will affect cash over the next four years and beyond are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Contractual Commitments | Expected Cash Payments by Year |
| (in Millions) | 2021 | | 2022 | | 2023 | | 2024 | | 2025 & beyond | | Total |
Debt maturities (1) | $ | 338.3 | | | $ | 1,000.1 | | | $ | 0.2 | | | $ | 400.1 | | | $ | 1,550.0 | | | $ | 3,288.7 | |
Contractual interest (2) | 97.0 | | | 100.3 | | | 78.6 | | | 76.0 | | | 706.4 | | | 1,058.3 | |
Lease obligations (3) | 31.7 | | | 27.3 | | | 21.5 | | | 17.6 | | | 120.3 | | | 218.4 | |
| Derivative contracts | 24.5 | | | 0.8 | | | — | | | — | | | — | | | 25.3 | |
Purchase obligations (4) | 380.3 | | | 142.3 | | | 147.5 | | | 52.9 | | | 102.0 | | | 825.0 | |
Total (5) | $ | 871.8 | | | $ | 1,270.8 | | | $ | 247.8 | | | $ | 546.6 | | | $ | 2,478.7 | | | $ | 5,415.7 | |
____________________
(1) Excluding discounts.
(2) Contractual interest is the interest we are contracted to pay on our long-term debt obligations. We had $700.0 million of long-term debt subject to variable interest rates at December 31, 2020. The rate assumed for the variable interest component of the contractual interest obligation was the rate in effect at December 31, 2020. Variable rates are determined by the market and will fluctuate over time.
(3) Obligations associated with operating leases, before sub-lease rental income.
(4) Purchase obligations consist of agreements to purchase goods and services that are enforceable and legally binding and specify all significant terms, including fixed or minimum quantities to be purchased, price provisions and timing of the transaction. We have entered into a number of purchase obligations for the sourcing of materials and energy where take-or-pay arrangements apply. Since the majority of the minimum obligations under these contracts are take-or-pay commitments over the life of the contract and not a year by year take-or-pay, the obligations in the table related to these types of contacts are presented in the earliest period in which the minimum obligation could be payable under these types of contracts.
(5) As of December 31, 2020, the liability for uncertain tax positions was $83.1 million. This liability is excluded from the table above. Additionally, accrued pension and other postretirement benefits and our environmental liabilities as recorded on our consolidated balance sheets are excluded from the table above. Due to the high degree of uncertainty regarding the timing of potential future cash flows associated with these liabilities, we are unable to make a reasonably reliable estimate of the amount and periods in which these liabilities might be paid. Also excluded from the table above is the liability attributable to the transition tax on deemed repatriated foreign earnings incurred as a result of the Act of $107.8 million.
Contingencies
See Note 20 to our consolidated financial statements included in this Form 10-K.
Climate Change
As a global corporate citizen, we are concerned about the consequences of climate change and will take prudent and cost effective actions that reduce Green House Gas (GHG) emissions to the atmosphere.
FMC is committed to continuing to do its part to address climate change and its impacts. Our 2030 intensity reduction targets for energy and greenhouse gas emissions are both 25 percent from our 2018 baseline year. FMC has been reporting its GHG emissions and mitigation strategy to CDP (formerly Carbon Disclosure Project) since 2016. FMC detailed the business risks and opportunities we have due to climate change and its impacts in our CDP climate change reports. FMC received a "B" in the CDP Climate Change questionnaire in 2020. In 2021, FMC will begin conducting climate related scenario analyses in line with the Taskforce for Climate-Related Financial Disclosures recommendations to better understand our risks and opportunities with respect to climate change.
Even as we take action to control the release of GHGs, additional warming is anticipated. Long-term, higher average global temperatures could result in induced changes in natural resources, growing seasons, precipitation patterns, weather patterns, species distributions, water availability, sea levels, and biodiversity. These impacts could cause changes in supplies of raw materials used to maintain FMC’s production capacity and could lead to possible increased sourcing costs. Depending on how pervasive the climate impacts are in the different geographic locations experiencing changes in natural resources, FMC’s customers could be impacted. Demand for FMC’s products could increase if our products meet our customers’ needs to adapt to climate change impacts or decrease if our products do not meet their needs. Within our own operations, we continually assess our manufacturing sites worldwide for risks and opportunities to increase our preparedness for climate change. We are continuing to evaluate sea level rise and storm surge at our plants to understand timing of potential impacts and proactive responses that may need to be taken. To lessen FMC’s overall environmental footprint, we have taken actions to increase the energy efficiency in our manufacturing sites. We have also committed to new 2030 goals to reduce our water use intensity in
high-risk areas by 20 percent and to maintain our 2018 waste disposed intensity which otherwise would increase by 55 percent due to expected growth and shifts in production mix.
In our product portfolio, we see market opportunities for our products to address climate change and its impacts. For example, FMC's agricultural products can help customers increase yield, energy and water efficiency, and decrease greenhouse gas emissions. Our products can also help growers adapt to more unpredictable growing conditions and the effects these types of threats have on crops. FMC has committed to invest 100 percent of our innovation budget to developing sustainable products and solutions for future use.
We are improving existing products and developing new platforms and technologies that help mitigate impacts of climate change. FMC is developing products with a lighter environmental footprint in its biologicals products. These opportunities could lead to new products and services for our existing and potential customers. Beyond our products and operations, FMC recognizes that energy consumption throughout our supply chain can impact climate change and product costs. Therefore, we will actively work with our entire value chain - suppliers, contractors, and customers - to improve their energy efficiencies and to reduce their GHG emissions.
We continue to follow legislative and regulatory developments regarding climate change because the regulation of greenhouse gases, depending on their nature and scope, could subject some of our manufacturing operations to additional costs or limits on operations. In December 2015, 195 countries at the United Nations Climate Change Conference in Paris reached an agreement to reduce GHGs. It remains to be seen how and when each of these countries will implement this agreement.
FMC will actively manage climate risks and incorporate them in our decision making as indicated in our responses to the CDP Climate Change Module. The United States Climate Alliance, a coalition of 24 states (governing 55 percent of the population) and unincorporated self-governing territories in the United States have expressed their commitment to upholding the objectives of the 2015 Paris Agreement on climate change within their borders. Several of our manufacturing and R&D sites fall within this alliance territory. FMC remains deeply committed to reducing our GHG emissions and energy consumption at all our facilities around the world.
Some of our foreign operations are subject to national or local energy management or climate change regulation, such as our plant in Denmark that is subject to the EU Emissions Trading Scheme. At present, that plant’s emissions are below its designated cap.
In December 2019, the European Commission approved the European Green Deal, with the goal of making the EU carbon neutral by 2050. The Green Deal includes investment plans and a roadmap to fight against climate change. FMC is closely following updates and the discussion surrounding the Green Deal. The costs of complying with possible future requirements are difficult to estimate at this time.
Future GHG regulatory requirements may result in increased costs of energy, additional capital costs for emissions control or new equipment, and/or costs associated with cap and trade or carbon taxes. We are currently monitoring regulatory developments. The costs of complying with possible future climate change requirements are difficult to estimate at this time.
Recently Adopted and Issued Accounting Pronouncements and Regulatory Items
See Note 2 "Recently Issued and Adopted Accounting Pronouncements and Regulatory Items" to our consolidated financial statements included in this Form 10-K.
Off-Balance Sheet Arrangements
See Note 20 to our consolidated financial statements included in this Form 10-K and Part I, Item 3 - Legal Proceedings for further information regarding any off-balance sheet arrangements.
Fair Value Measurements
See Note 19 to our consolidated financial statements included in this Form 10-K for additional discussion surrounding our fair value measurements.
Critical Accounting Policies
Our consolidated financial statements are prepared in conformity with U.S. generally accepted accounting principles ("U.S. GAAP"). The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. We have described our accounting policies in Note 1 "Principal Accounting Policies and Related Financial Information" to our consolidated financial statements included in this Form 10-K. We have reviewed these accounting policies, identifying those that we believe to be critical to the preparation and understanding of our consolidated financial statements. We have reviewed these critical accounting policies with the Audit Committee of the Board of Directors. Critical accounting policies are central to our presentation of results of operations and financial condition in accordance with U.S. GAAP and require management to make estimates and judgments on certain matters. We base our estimates and judgments on historical experience, current conditions and other reasonable factors.
Revenue recognition and trade receivables
We recognize revenue when (or as) we satisfy our performance obligation which is when the customer obtains control of the good or service. Rebates due to customers are accrued as a reduction of revenue in the same period that the related sales are recorded based on the contract terms. Refer to Note 3 to our consolidated financial statements included in this Form 10-K for more information.
We record amounts billed for shipping and handling fees as revenue. Costs incurred for shipping and handling are recorded as costs of sales and services. Amounts billed for sales and use taxes, value-added taxes, and certain excise and other specific transactional taxes imposed on revenue-producing transactions are presented on a net basis and excluded from sales in the consolidated income statements. We record a liability until remitted to the respective taxing authority.
We periodically enter into prepayment arrangements with customers and receive advance payments for product to be delivered in future periods. These advance payments are recorded as deferred revenue and classified as "Advance payments from customers" on the consolidated balance sheet. Revenue associated with advance payments is recognized as shipments are made and transfer of control to the customer takes place.
Trade receivables consist of amounts owed to us from customer sales and are recorded when revenue is recognized. The allowance for trade receivables represents our best estimate of the probable losses associated with potential customer defaults. In developing our allowance for trade receivables, we use a two stage process which includes calculating a general formula to develop an allowance to appropriately address the uncertainty surrounding collection risk of our entire portfolio and specific allowances for customers where the risk of collection has been reasonably identified either due to liquidity constraints or disputes over contractual terms and conditions.
Our method of calculating the general formula consists of estimating the recoverability of trade receivables based on historical experience, current collection trends, and external business factors such as economic factors, including regional bankruptcy rates, and political factors. Our analysis of trade receivable collection risk is performed quarterly, and the allowance is adjusted accordingly.
We also hold long-term receivables that represent long-term customer receivable balances related to past-due accounts which are not expected to be collected within the current year. Our policy for the review of the allowance for these receivables is consistent with the discussion in the preceding paragraph above on trade receivables. Therefore on an ongoing basis, we continue to evaluate the credit quality of our long-term receivables utilizing aging of receivables, collection experience and write-offs, as well as existing economic conditions, to determine if an additional allowance is necessary.
Environmental obligations and related recoveries
We provide for environmental-related obligations when they are probable and amounts can be reasonably estimated. Where the available information is sufficient to estimate the amount of liability, that estimate has been used. Where the information is only sufficient to establish a range of probable liability and no point within the range is more likely than any other, the lower end of the range has been used.
Estimated obligations to remediate sites that involve oversight by the United States Environmental Protection Agency ("EPA"), or similar government agencies, are generally accrued no later than when a Record of Decision ("ROD"), or equivalent, is issued, or upon completion of a Remedial Investigation/Feasibility Study ("RI/FS"), or equivalent, that is submitted by us to the appropriate government agency or agencies. Estimates are reviewed quarterly by our environmental remediation management, as well as by financial and legal management and, if necessary, adjusted as additional information becomes available. The estimates can change substantially as additional information becomes available regarding the nature or extent of site contamination, required remediation methods, and other actions by or against governmental agencies or private parties.
Our environmental liabilities for continuing and discontinued operations are principally for costs associated with the remediation and/or study of sites at which we are alleged to have released hazardous substances into the environment. Such costs principally include, among other items, RI/FS, site remediation, costs of operation and maintenance of the remediation
plan, management costs, fees to outside law firms and consultants for work related to the environmental effort, and future monitoring costs. Estimated site liabilities are determined based upon existing remediation laws and technologies, specific site consultants’ engineering studies or by extrapolating experience with environmental issues at comparable sites.
Included in our environmental liabilities are costs for the operation, maintenance and monitoring of site remediation plans (OM&M). Such reserves are based on our best estimates for these OM&M plans. Over time we may incur OM&M costs in excess of these reserves. However, we are unable to reasonably estimate an amount in excess of our recorded reserves because we cannot reasonably estimate the period for which such OM&M plans will need to be in place or the future annual cost of such remediation, as conditions at these environmental sites change over time. Such additional OM&M costs could be significant in total but would be incurred over an extended period of years.
Included in the environmental reserve balance, other assets balance and disclosure of reasonably possible loss contingencies are amounts from third party insurance policies, which we believe are probable of recovery.
Provisions for environmental costs are reflected in income, net of probable and estimable recoveries from named Potentially Responsible Parties ("PRPs") or other third parties. In the fourth quarter of 2019, we increased our reserves for the Pocatello Tribal Matter by $72.8 million, which represents both the historical and discounted present value of future annual use permit fees as well as the associated legal costs. See Note 12 for further information. All other environmental provisions incorporate inflation and are not discounted to their present value.
In calculating and evaluating the adequacy of our environmental reserves, we have taken into account the joint and several liability imposed by Comprehensive Environmental Response, Compensation and Liability Act ("CERCLA") and the analogous state laws on all PRPs and have considered the identity and financial condition of the other PRPs at each site to the extent possible. We have also considered the identity and financial condition of other third parties from whom recovery is anticipated, as well as the status of our claims against such parties. Although we are unable to forecast the ultimate contributions of PRPs and other third parties with absolute certainty, the degree of uncertainty with respect to each party is taken into account when determining the environmental reserve by adjusting the reserve to reflect the facts and circumstances on a site-by-site basis. Our liability includes our best estimate of the costs expected to be paid before the consideration of any potential recoveries from third parties. We believe that any recorded recoveries related to PRPs are realizable in all material respects. Recoveries are recorded as either an offset in "Environmental liabilities, continuing and discontinued" or as "Other assets" in our consolidated balance sheets in accordance with U.S. accounting literature.
See Note 12 to our consolidated financial statements included in this Form 10-K for changes in estimates associated with our environmental obligations.
Impairments and valuation of long-lived and indefinite-lived assets
Our long-lived assets primarily include property, plant and equipment, goodwill and intangible assets. The assets and liabilities of acquired businesses are measured at their estimated fair values at the dates of acquisition. The excess of the purchase price over the estimated fair value of the net assets acquired, including identified intangibles, is recorded as goodwill. The determination and allocation of fair value to the assets acquired and liabilities assumed is based on various assumptions and valuation methodologies requiring considerable management judgment, including estimates based on historical information, current market data and future expectations. The principal assumptions utilized in our valuation methodologies include revenue growth rates, operating margin estimates and discount rates. Although the estimates were deemed reasonable by management based on information available at the dates of acquisition, those estimates are inherently uncertain.
We test for impairment whenever events or circumstances indicate that the net book value of our property, plant and equipment may not be recoverable from the estimated undiscounted expected future cash flows expected to result from their use and eventual disposition. In cases where the estimated undiscounted expected future cash flows are less than net book value, an impairment loss is recognized equal to the amount by which the net book value exceeds the estimated fair value of assets, which is based on discounted cash flows at the lowest level determinable. The estimated cash flows reflect our assumptions about selling prices, volumes, costs and market conditions over a reasonable period of time.
We perform an annual impairment test of goodwill and indefinite-lived intangible assets in the third quarter of each year, or more frequently whenever an event or change in circumstances occurs that would require reassessment of the recoverability of those assets. In performing our evaluation we assess qualitative factors such as overall financial performance of our reporting units, anticipated changes in industry and market structure, competitive environments, planned capacity and cost factors such as raw material prices. Based on our assessment for 2020, we determined that no goodwill and indefinite-lived intangible assets impairment charge to our continuing operations was required.
See Note 9 to our consolidated financial statements included in this Form 10-K for charges associated with long-lived asset disposal costs and the activity associated with the restructuring reserves.
Pension and other postretirement benefits
We provide qualified and nonqualified defined benefit and defined contribution pension plans, as well as postretirement health care and life insurance benefit plans to our employees and retirees. The costs (benefits) and obligations related to these benefits reflect key assumptions related to general economic conditions, including interest (discount) rates, healthcare cost trend rates, expected rates of return on plan assets and the rates of compensation increase for employees. The costs (benefits) and obligations for these benefit programs are also affected by other assumptions, such as average retirement age, mortality, employee turnover, and plan participation. To the extent our plans’ actual experience, as influenced by changing economic and financial market conditions or by changes to our own plans’ demographics, differs from these assumptions, the costs and obligations for providing these benefits, as well as the plans’ funding requirements, could increase or decrease. When actual results differ from our assumptions, the difference is typically recognized over future periods. In addition, the unrealized gains and losses related to our pension and postretirement benefit obligations may also affect periodic benefit costs (benefits) in future periods.
We use several assumptions and statistical methods to determine the asset values used to calculate both the expected rate of return on assets component of pension cost and to calculate our plans’ funding requirements. The expected rate of return on plan assets is based on a market-related value of assets that recognizes investment gains and losses over a five-year period. We use an actuarial value of assets to determine our plans’ funding requirements. The actuarial value of assets must be within a certain range, high or low, of the actual market value of assets, and is adjusted accordingly.
We select the discount rate used to calculate pension and other postretirement obligations based on a review of available yields on high-quality corporate bonds as of the measurement date. In selecting a discount rate as of December 31, 2020, we placed particular emphasis on a discount rate yield-curve provided by our actuary. This yield-curve, when populated with projected cash flows that represent the expected timing and amount of our plans' benefit payments, produced an effective discount rate of 2.49 percent for our U.S. qualified plan, 1.62 percent for our U.S. nonqualified, and 1.91 percent for our U.S. other postretirement benefit plans.
The discount rates used to determine projected benefit obligation at our December 31, 2020 and 2019 measurement dates for the U.S. qualified plan were 2.49 percent and 3.22 percent, respectively. The effect of the change in the discount rate from 3.22 percent to 2.49 percent at December 31, 2020 resulted in a $105.9 million increase to our U.S. qualified pension benefit obligations. The effect of the change in the discount rate used to determine net annual benefit cost (income) from 4.36 percent at December 31, 2019 to 3.22 percent at December 31, 2020 resulted in a $0.1 million decrease to the 2020 U.S. qualified pension expense.
The change in discount rate from 3.22 percent at December 31, 2019 to 2.49 percent at December 31, 2020 was attributable to a decrease in yields on high quality corporate bonds with cash flows matching the timing and amount of our expected future benefit payments between the 2019 and 2020 measurement dates. Using the December 31, 2020 and 2019 yield curves, our U.S. qualified plan cash flows produced a single weighted-average discount rate of approximately 2.49 percent and 3.22 percent, respectively.
In developing the assumption for the long-term rate of return on assets for our U.S. Plan, we take into consideration the technical analysis performed by our outside actuaries, including historical market returns, information on the assumption for long-term real returns by asset class, inflation assumptions, and expectations for standard deviation related to these best estimates. Our long-term rate of return for the fiscal year ended December 31, 2020, 2019 and 2018 was 3.00 percent, 4.25 percent and 5.00 percent, respectively.
For the sensitivity of our pension costs to incremental changes in assumptions see our discussion below.
Sensitivity analysis related to key pension and postretirement benefit assumptions.
A one-half percent increase in the assumed discount rate would have decreased pension and other postretirement benefit obligations by $72.6 million and $72.1 million at December 31, 2020 and 2019, respectively, and decreased pension and other postretirement benefit costs by zero, $0.6 million and $0.4 million for 2020, 2019 and 2018, respectively. A one-half percent decrease in the assumed discount rate would have increased pension and other postretirement benefit obligations by $79.3 million and $79.4 million at December 31, 2020 and 2019, respectively, and increased pension and other postretirement benefit cost by $0.1 million, $0.5 million and $0.1 million for 2020, 2019 and 2018, respectively.
A one-half percent increase in the assumed expected long-term rate of return on plan assets would have decreased pension costs by $6.2 million, $6.3 million and $6.4 million for 2020, 2019 and 2018, respectively. A one-half percent decrease in the assumed long-term rate of return on plan assets would have increased pension costs by $6.2 million, $6.3 million and $6.4 million for 2020, 2019 and 2018, respectively.
Further details on our pension and other postretirement benefit obligations and net periodic benefit costs (benefits) are found in Note 15 to our consolidated financial statements in this Form 10-K.
Income taxes
We have recorded a valuation allowance to reduce deferred tax assets in certain jurisdictions to the amount that we believe is more likely than not to be realized. In assessing the need for this allowance, we have considered a number of factors including future taxable income, the jurisdictions in which such income is earned and our ongoing tax planning strategies. In the event that we determine that we would not be able to realize all or part of our net deferred tax assets in the future, an adjustment to the deferred tax assets would be charged to income in the period such determination was made. Similarly, should we conclude that we would be able to realize certain deferred tax assets in the future in excess of the net recorded amount, an adjustment to the deferred tax assets would increase income in the period such determination was made.
Additionally, we file income tax returns in the U.S. federal jurisdiction and various state and foreign jurisdictions. Certain income tax returns for FMC entities taxable in the U.S. and significant foreign jurisdictions are open for examination and adjustment. We assess our income tax positions and record a liability for all years open to examination based upon our evaluation of the facts, circumstances and information available at the reporting date. For those tax positions where it is more likely than not that a tax benefit will be sustained, we have recorded the largest amount of tax benefit with a greater than 50 percent likelihood of being realized upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. We adjust these liabilities, if necessary, upon the completion of tax audits or changes in tax law.
On December 22, 2017, the Act was enacted in the United States. The Act reduced the U.S. federal corporate tax rate from 35 percent to 21 percent, required companies to pay a one-time transition tax on earnings of certain foreign subsidiaries that were previously tax deferred and created new taxes on certain foreign sourced earnings. At December 31, 2018, the Company had completed its accounting for the impacts of the enactment of the Act.
See Note 13 to our consolidated financial statements included in this Form 10-K for additional discussion surrounding income taxes.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our earnings, cash flows and financial position are exposed to market risks relating to fluctuations in commodity prices, interest rates and foreign currency exchange rates. Our policy is to minimize exposure to our cash flow over time caused by changes in commodity, interest and currency exchange rates. To accomplish this, we have implemented a controlled program of risk management consisting of appropriate derivative contracts entered into with major financial institutions.
The analysis below presents the sensitivity of the market value of our financial instruments to selected changes in market rates and prices. The range of changes chosen reflects our view of changes that are reasonably possible over a one-year period. Market value estimates are based on the present value of projected future cash flows considering the market rates and prices chosen.
At December 31, 2020, our net financial instrument position was a net liability of $25.3 million compared to a net liability of $8.9 million at December 31, 2019. The change in the net financial instrument position was primarily due to exchange rate fluctuations in our foreign exchange portfolio.
Since our risk management programs are generally highly effective, the potential loss in value for each risk management portfolio described below would be largely offset by changes in the value of the underlying exposure.
Foreign Currency Exchange Rate Risk
The primary currencies for which we have exchange rate exposure are the U.S. dollar versus the euro, the Chinese yuan, the Brazilian real, Mexican peso, Indian rupee and the Argentine peso. Foreign currency debt and foreign exchange forward contracts are used in countries where we do business, thereby reducing our net asset exposure. Foreign exchange forward contracts are also used to hedge firm and highly anticipated foreign currency cash flows.
To analyze the effects of changing foreign currency rates, we have performed a sensitivity analysis in which we assume an instantaneous 10 percent change in the foreign currency exchange rates from their levels at December 31, 2020 and 2019, with all other variables (including interest rates) held constant.
| | | | | | | | | | | | | | | | | |
| | | Hedged Currency vs. Functional Currency |
| (in Millions) | Net Asset / (Liability) Position on Consolidated Balance Sheets | | Net Asset / (Liability) Position with 10% Strengthening | | Net Asset / (Liability) Position with 10% Weakening |
| Net asset/(liability) position at December 31, 2020 | $ | (24.5) | | | $ | 8.4 | | | $ | (9.6) | |
| | | | | |
| Net asset/(liability) position at December 31, 2019 | (8.0) | | | 55.9 | | | (75.4) | |
Interest Rate Risk
One of the strategies that we can use to manage interest rate exposure is to enter into interest rate swap agreements. In these agreements, we agree to exchange, at specified intervals, the difference between fixed and variable interest amounts calculated on an agreed-upon notional principal amount. In the quarter ended December 31, 2020, we had outstanding interest rate swap contracts in place with an aggregate notional value of $100.0 million.
To analyze the effects of changing interest rates, we have performed a sensitivity analysis in which we assume an instantaneous one percent change in the interest rates from their levels at December 31, 2020 and 2019, with all other variables held constant.
| | | | | | | | | | | | | | | | | |
| (in Millions) | Net Asset / (Liability) Position on Consolidated Balance Sheets | | 1% Increase | | 1% Decrease |
| Net asset/(liability) position at December 31, 2020 | $ | (0.8) | | | $ | 8.8 | | | $ | (10.4) | |
| | | | | |
| Net asset/(liability) position at December 31, 2019 | (0.9) | | | — | | | (1.9) | |
Our debt portfolio at December 31, 2020 is composed of 72 percent fixed-rate debt and 28 percent variable-rate debt. The variable-rate component of our debt portfolio principally consists of borrowings under our 2017 Term Loan Facility, Credit Facility, Commercial Paper program, variable-rate industrial and pollution control revenue bonds, and amounts outstanding under foreign subsidiary credit lines. Changes in interest rates affect different portions of our variable-rate debt portfolio in different ways.
Based on the variable-rate debt in our debt portfolio at December 31, 2020, a one percentage point increase in interest rates would have increased gross interest expense by $9.3 million and a one percentage point decrease in interest rates would have decreased gross interest expense by $2.6 million for the year ended December 31, 2020.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
FMC CORPORATION
CONSOLIDATED STATEMENTS OF INCOME (LOSS)
| | | | | | | | | | | | | | | | | |
| (in Millions, Except Per Share Data) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Revenue | $ | 4,642.1 | | | $ | 4,609.8 | | | $ | 4,285.3 | |
| Costs and Expenses | | | | | |
| Costs of sales and services | 2,590.1 | | | 2,526.2 | | | 2,405.5 | |
| | | | | |
| Gross Margin | $ | 2,052.0 | | | $ | 2,083.6 | | | $ | 1,879.8 | |
| | | | | |
| Selling, general and administrative expenses | 729.7 | | | 792.9 | | | 790.0 | |
| Research and development expenses | 287.9 | | | 298.1 | | | 287.7 | |
| Restructuring and other charges (income) | 132.2 | | | 171.0 | | | 61.2 | |
| | | | | |
| Total costs and expenses | $ | 3,739.9 | | | $ | 3,788.2 | | | $ | 3,544.4 | |
| Income from continuing operations before equity in (earnings) loss of affiliates, non-operating pension and postretirement charges (income), interest expense, net and income taxes | $ | 902.2 | | | $ | 821.6 | | | $ | 740.9 | |
| Equity in (earnings) loss of affiliates | — | | | — | | | (0.1) | |
| Non-operating pension and postretirement charges (income) | 21.2 | | | 8.1 | | | (0.5) | |
| | | | | |
| Interest income | (0.1) | | | (1.9) | | | (1.4) | |
| Interest expense | 151.3 | | | 160.4 | | | 134.5 | |
| Income (loss) from continuing operations before income taxes | $ | 729.8 | | | $ | 655.0 | | | $ | 608.4 | |
| Provision (benefit) for income taxes | 150.9 | | | 111.5 | | | 70.8 | |
| Income (loss) from continuing operations | $ | 578.9 | | | $ | 543.5 | | | $ | 537.6 | |
| Discontinued operations, net of income taxes | (28.3) | | | (63.3) | | | (26.1) | |
| Net income (loss) | $ | 550.6 | | | $ | 480.2 | | | $ | 511.5 | |
| Less: Net income (loss) attributable to noncontrolling interests | (0.9) | | | 2.8 | | | 9.4 | |
| Net income (loss) attributable to FMC stockholders | $ | 551.5 | | | $ | 477.4 | | | $ | 502.1 | |
| Amounts attributable to FMC stockholders: | | | | | |
| Continuing operations, net of income taxes | $ | 579.8 | | | $ | 540.7 | | | $ | 531.4 | |
| Discontinued operations, net of income taxes | (28.3) | | | (63.3) | | | (29.3) | |
| Net income (loss) attributable to FMC stockholders | $ | 551.5 | | | $ | 477.4 | | | $ | 502.1 | |
| Basic earnings (loss) per common share attributable to FMC stockholders: | | | | | |
| Continuing operations | $ | 4.46 | | | $ | 4.12 | | | $ | 3.94 | |
| Discontinued operations | (0.22) | | | (0.48) | | | (0.22) | |
| Net income (loss) attributable to FMC stockholders | $ | 4.24 | | | $ | 3.64 | | | $ | 3.72 | |
| Diluted earnings (loss) per common share attributable to FMC stockholders: | | | | | |
| Continuing operations | $ | 4.44 | | | $ | 4.10 | | | $ | 3.91 | |
| Discontinued operations | (0.22) | | | (0.48) | | | (0.22) | |
| Net income (loss) attributable to FMC stockholders | $ | 4.22 | | | $ | 3.62 | | | $ | 3.69 | |
The accompanying notes are an integral part of these consolidated financial statements.
FMC CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Net income (loss) | $ | 550.6 | | | $ | 480.2 | | | $ | 511.5 | |
| Other comprehensive income (loss), net of tax: | | | | | |
| Foreign currency adjustments: | | | | | |
| Foreign currency translation gain (loss) arising during the period | $ | 102.0 | | | $ | (18.5) | | | $ | (100.8) | |
| | | | | |
Total foreign currency adjustments (1) | $ | 102.0 | | | $ | (18.5) | | | $ | (100.8) | |
| | | | | |
| Derivative instruments: | | | | | |
Unrealized hedging gains (losses) and other, net of tax of $1.9, $(16.7) and $2.6 | $ | (2.5) | | | $ | (69.0) | | | $ | 13.7 | |
Reclassification of deferred hedging (gains) losses and other, included in net income, net of tax of $1.7, $(3.0) and $(3.1) (3) | (4.3) | | | (8.2) | | | (7.7) | |
Total derivative instruments, net of tax of $3.6, $(19.7) and $(0.5) | $ | (6.8) | | | $ | (77.2) | | | $ | 6.0 | |
| | | | | |
| Pension and other postretirement benefits: | | | | | |
Unrealized actuarial gains (losses) and prior service (costs) credits, net of tax of $5.2, $(1.4) and $1.3 (2) | $ | 18.9 | | | $ | (6.5) | | | $ | 4.2 | |
Reclassification of net actuarial and other (gain) loss, amortization of prior service costs and settlement charges, included in net income, net of tax of $4.2, $2.6 and $4.3 (3) | 16.0 | | | 9.9 | | | 16.5 | |
Total pension and other postretirement benefits, net of tax of $9.4, $1.2 and $5.6 | $ | 34.9 | | | $ | 3.4 | | | $ | 20.7 | |
| | | | | |
| Other comprehensive income (loss), net of tax | $ | 130.1 | | | $ | (92.3) | | | $ | (74.1) | |
| Comprehensive income (loss) | $ | 680.7 | | | $ | 387.9 | | | $ | 437.4 | |
| Less: Comprehensive income (loss) attributable to the noncontrolling interest | (0.6) | | | (0.5) | | | 3.9 | |
| Comprehensive income (loss) attributable to FMC stockholders | $ | 681.3 | | | $ | 388.4 | | | $ | 433.5 | |
____________________
(1)Income taxes are not provided for other outside basis differences inherent in our investments in subsidiaries because the investments and related unremitted earnings are essentially permanent in duration or we have concluded that no additional tax liability will arise upon disposal or remittance.
(2)At December 31 of each year, we remeasure our pension and postretirement plan obligations at which time we record any actuarial gains (losses) and prior service (costs) credits to other comprehensive income. During the year ended December 31, 2018, due to the announced plan to separate FMC Lithium, we triggered a curtailment of our U.S. pension plans. As a result, we revalued our pension plans as of October 31, 2018, in addition to the normal December 31st remeasurement, which resulted in adjustments to comprehensive income. See Note 15 for more information.
(3)For more detail on the components of these reclassifications and the affected line item in the consolidated statements of income (loss) see Note 17 within these consolidated financial statements.
The accompanying notes are an integral part of these consolidated financial statements.
FMC CORPORATION
CONSOLIDATED BALANCE SHEETS
| | | | | | | | | | | |
| December 31, |
| (in Millions, Except Share and Par Value Data) | 2020 | | 2019 |
| ASSETS | | | |
| Current assets | | | |
| Cash and cash equivalents | $ | 568.9 | | | $ | 339.1 | |
Trade receivables, net of allowance of $27.9 in 2020 and $26.3 in 2019 | 2,330.3 | | | 2,231.2 | |
| Inventories | 1,095.6 | | | 1,017.0 | |
| Prepaid and other current assets | 380.8 | | | 487.5 | |
| | | |
| | | |
| Total current assets | $ | 4,375.6 | | | $ | 4,074.8 | |
| Investments | 3.1 | | | 0.7 | |
| Property, plant and equipment, net | 771.7 | | | 758.0 | |
| Goodwill | 1,468.9 | | | 1,467.5 | |
| Other intangibles, net | 2,625.2 | | | 2,629.0 | |
| Other assets including long-term receivables, net | 712.3 | | | 685.3 | |
| Deferred income taxes | 229.6 | | | 257.4 | |
| | | |
| Total assets | $ | 10,186.4 | | | $ | 9,872.7 | |
| LIABILITIES AND EQUITY | | | |
| Current liabilities | | | |
| Short-term debt and current portion of long-term debt | $ | 338.3 | | | $ | 227.7 | |
| Accounts payable, trade and other | 946.7 | | | 900.1 | |
| Advance payments from customers | 347.1 | | | 492.7 | |
| Accrued and other liabilities | 674.7 | | | 680.6 | |
| Accrued customer rebates | 295.2 | | | 280.6 | |
| Guarantees of vendor financing | 140.6 | | | 75.7 | |
| Accrued pension and other postretirement benefits, current | 4.2 | | | 4.3 | |
| Income taxes | 82.2 | | | 62.2 | |
| | | |
| Total current liabilities | $ | 2,829.0 | | | $ | 2,723.9 | |
| Long-term debt, less current portion | 2,929.5 | | | 3,031.1 | |
| Accrued pension and other postretirement benefits, long-term | 46.4 | | | 44.2 | |
| Environmental liabilities, continuing and discontinued | 443.5 | | | 470.5 | |
| Deferred income taxes | 350.0 | | | 333.2 | |
| | | |
| Other long-term liabilities | 603.8 | | | 708.4 | |
| Commitments and contingent liabilities (Note 20) | | | |
| Equity | | | |
Preferred stock, no par value, authorized 5,000,000 shares; no shares issued in 2020 or 2019 | $ | — | | | $ | — | |
Common stock, $0.10 par value, authorized 260,000,000 shares in 2020 and 2019; 185,983,792 shares issued in 2020 and 2019 | 18.6 | | | 18.6 | |
| Capital in excess of par value of common stock | 860.2 | | | 829.7 | |
| Retained earnings | 4,506.4 | | | 4,188.8 | |
| Accumulated other comprehensive income (loss) | (282.2) | | | (412.0) | |
Treasury stock, common, at cost - 2020: 56,630,209 shares, 2019: 56,859,498 shares | (2,141.2) | | | (2,092.8) | |
| Total FMC stockholders’ equity | $ | 2,961.8 | | | $ | 2,532.3 | |
| Noncontrolling interests | 22.4 | | | 29.1 | |
| Total equity | $ | 2,984.2 | | | $ | 2,561.4 | |
| Total liabilities and equity | $ | 10,186.4 | | | $ | 9,872.7 | |
The accompanying notes are an integral part of these consolidated financial statements.
FMC CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Cash provided (required) by operating activities of continuing operations: | | | | | |
| Net income (loss) | $ | 550.6 | | | $ | 480.2 | | | $ | 511.5 | |
| Discontinued operations, net of income taxes | 28.3 | | | 63.3 | | | 26.1 | |
| Income (loss) from continuing operations | $ | 578.9 | | | $ | 543.5 | | | $ | 537.6 | |
| Adjustments from income (loss) from continuing operations to cash provided (required) by operating activities of continuing operations: | | | | | |
| Depreciation and amortization | $ | 162.7 | | | $ | 150.1 | | | $ | 150.2 | |
| Equity in (earnings) loss of affiliates | — | | | — | | | (0.1) | |
| Restructuring and other charges (income) | 132.2 | | | 171.0 | | | 61.2 | |
| Deferred income taxes | 33.6 | | | 46.1 | | | (43.9) | |
| Pension and other postretirement benefits | 25.8 | | | 12.6 | | | 6.1 | |
| Share-based compensation | 18.9 | | | 25.6 | | | 22.5 | |
| | | | | |
| Changes in operating assets and liabilities, net of effect of acquisitions and divestitures: | | | | | |
| Trade receivables, net | $ | (71.8) | | | $ | (123.5) | | | $ | (281.5) | |
| Guarantees of vendor financing | 64.8 | | | 8.6 | | | 15.4 | |
| Advance payments from customers | (145.5) | | | 34.1 | | | 80.2 | |
| Accrued customer rebates | 17.2 | | | (85.8) | | | 104.1 | |
| Inventories | (59.7) | | | 6.4 | | | (200.7) | |
| Accounts payable, trade and other | 61.8 | | | 103.0 | | | 166.7 | |
| Income taxes | 36.2 | | | (25.0) | | | (94.7) | |
| Pension and other postretirement benefit contributions | (4.6) | | | (13.4) | | | (37.5) | |
| Environmental spending, continuing, net of recoveries | (1.9) | | | (18.3) | | | (20.3) | |
Restructuring and other spending (1) | (17.9) | | | (18.6) | | | (25.2) | |
| Transaction and integration costs | (63.9) | | | (77.1) | | | (101.1) | |
Change in other operating assets and liabilities, net (2) | (30.0) | | | (183.7) | | | 23.7 | |
| Cash provided (required) by operating activities of continuing operations | $ | 736.8 | | | $ | 555.6 | | | $ | 362.7 | |
| Cash provided (required) by operating activities of discontinued operations: | | | | | |
| Environmental spending, discontinued, net of recoveries | $ | (58.9) | | | $ | (51.7) | | | $ | (41.0) | |
| Operating activities of discontinued operations, net of divestiture costs | (0.2) | | | 9.0 | | | 74.5 | |
| Other discontinued spending | (29.9) | | | (24.4) | | | (27.8) | |
| Cash provided (required) by operating activities of discontinued operations | $ | (89.0) | | | $ | (67.1) | | | $ | 5.7 | |
____________________
(1)The restructuring and other spending amount includes spending of $3.6 million related to the Furadan® asset retirement obligations. For additional detail on restructuring activities, see Note 9 to our consolidated financial statements included within this Form 10-K.
(2)Changes in all periods represent timing of payments associated with all other operating assets and liabilities.
The accompanying notes are an integral part of these consolidated financial statements.
FMC CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS (CONTINUED)
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Cash provided (required) by investing activities of continuing operations: | | | | | |
| Capital expenditures | $ | (67.2) | | | $ | (93.9) | | | $ | (83.0) | |
| | | | | |
Acquisitions, net (3) | (65.6) | | | — | | | 19.6 | |
| | | | | |
| Proceeds from sale of product portfolios | — | | | — | | | 88.0 | |
| Investment in Enterprise Resource Planning system | (47.2) | | | (48.0) | | | (48.5) | |
Other investing activities (4) | (20.4) | | | (54.0) | | | (13.6) | |
| Cash provided (required) by investing activities of continuing operations | $ | (200.4) | | | $ | (195.9) | | | $ | (37.5) | |
| Cash provided (required) by investing activities of discontinued operations: | | | | | |
| Proceeds from disposal of property, plant and equipment | $ | 31.1 | | | $ | 26.2 | | | $ | — | |
| Other discontinued investing activities | — | | | (17.0) | | | (93.4) | |
| Cash provided (required) by investing activities of discontinued operations | $ | 31.1 | | | $ | 9.2 | | | $ | (93.4) | |
| Cash provided (required) by financing activities of continuing operations: | | | | | |
| | | | | |
| Increase (decrease) in short-term debt | $ | 97.0 | | | $ | (11.9) | | | $ | 79.5 | |
| Proceeds from borrowing of long-term debt | 27.1 | | | 1,500.0 | | | — | |
| Financing fees and interest rate swap settlements | (3.5) | | | (97.4) | | | (3.1) | |
| Repayments of long-term debt | (100.0) | | | (901.9) | | | (552.0) | |
| Acquisitions of noncontrolling interests | (7.4) | | | — | | | — | |
| Distributions to noncontrolling interests | (1.3) | | | — | | | — | |
Net proceeds received from initial public offering of FMC Lithium (5) | — | | | — | | | 363.6 | |
Dividends paid (6) | (228.5) | | | (210.3) | | | (89.2) | |
| Issuances of common stock, net | 24.7 | | | 50.7 | | | 10.7 | |
| | | | | |
| Repurchases of common stock under publicly announced program | (50.0) | | | (400.0) | | | (200.0) | |
| Other repurchases of common stock | (8.4) | | | (16.2) | | | (6.8) | |
| | | | | |
| Cash provided (required) by financing activities of continuing operations | $ | (250.3) | | | $ | (87.0) | | | $ | (397.3) | |
| Cash provided (required) by financing activities of discontinued operations: | | | | | |
| Proceeds from borrowing of long-term debt | $ | — | | | $ | — | | | $ | 34.0 | |
| Payment of Livent external debt | — | | | (27.0) | | | — | |
| Cash transfer to Livent due to spin | — | | | (10.2) | | | — | |
| Cash provided (required) by financing activities of discontinued operations | $ | — | | | $ | (37.2) | | | $ | 34.0 | |
| Effect of exchange rate changes on cash and cash equivalents | 1.6 | | | (0.2) | | | 4.5 | |
| Increase (decrease) in cash and cash equivalents | $ | 229.8 | | | $ | 177.4 | | | $ | (121.3) | |
| | | | | |
| Cash and cash equivalents of continuing operations, beginning of period | $ | 339.1 | | | $ | 134.4 | | | $ | 281.8 | |
Cash and cash equivalents of discontinued operations, beginning of period (7) | — | | | 27.3 | | | 1.2 | |
| Cash and cash equivalents, beginning of period | $ | 339.1 | | | $ | 161.7 | | | $ | 283.0 | |
| Less: cash and cash equivalent of discontinued operations, end of period | — | | | — | | | 27.3 | |
| Cash and cash equivalents, end of period | $ | 568.9 | | | $ | 339.1 | | | $ | 134.4 | |
____________________
(3) The acquisitions, net amount in 2020 represents payments made on October 2, 2020 to acquire the remaining rights for Fluindapyr from Isagro S.p.A ("Isagro") in an asset acquisition. For additional detail on this transaction, see Note 9 to our consolidated financial statements included within this Form 10-K.
(4) Cash spending associated with contract manufacturers was $17.4 million, $51.7 million and $13.1 million for the years ended December 31, 2020, 2019 and 2018, respectively.
The accompanying notes are an integral part of these consolidated financial statements.
(5) Pursuant to the terms of the separation and distribution agreement, we received a net distribution of approximately $364 million from the public offering of Livent representing the proceeds from the sale of its common stock and the underwriters' exercise to purchase additional shares as part of the initial public offering ("IPO"), net of underwriting discounts and commissions, financing fees and other offering related expenses.
(6) See Note 17 regarding our quarterly cash dividend.
(7) Reflected within "Current assets of discontinued operations" on the consolidated balance sheets.
Supplemental disclosure of cash flow information: Cash paid for interest, net of capitalized interest was $141.8 million, $140.9 million and $133.4 million, and income taxes paid, net of refunds was $82.1 million, $130.9 million and $135.3 million in December 31, 2020, 2019 and 2018, respectively. Net interest payments of zero and tax payments, net of refunds of $10.0 million were allocated to discontinued operations for the year ended December 31, 2018. Accrued additions to property, plant and equipment and other assets at December 31, 2020, 2019 and 2018 were $14.7 million, $18.2 million and $3.1 million, respectively.
The accompanying notes are an integral part of these consolidated financial statements.
FMC CORPORATION
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | FMC Stockholders’ Equity | | | | |
| (in Millions, Except Per Share Data) | Common Stock, $0.10 Par Value | | Capital In Excess of Par | | Retained Earnings | | Accumulated Other Comprehensive Income (Loss) | | Treasury Stock | | Non-controlling
Interest | | Total Equity |
| Balance December 31, 2017 | $ | 18.6 | | | $ | 450.7 | | | $ | 3,952.4 | | | $ | (240.3) | | | $ | (1,499.6) | | | $ | 25.3 | | | $ | 2,707.1 | |
| Net income (loss) | | | | | 502.1 | | | | | | | 9.4 | | | 511.5 | |
| Stock compensation plans | | | 26.5 | | | | | | | 7.2 | | | | | 33.7 | |
| Shares for benefit plan trust | | | | | | | | | 0.1 | | | | | 0.1 | |
| Net pension and other benefit actuarial gains (losses) and prior service cost, net of income tax | | | | | | | 20.7 | | | | | | | 20.7 | |
| Net hedging gains (losses) and other, net of income tax | | | | | | | 6.0 | | | | | | | 6.0 | |
| Foreign currency translation adjustments | | | | | | | (95.3) | | | | | (5.5) | | | (100.8) | |
Dividends ($0.90 per share) | | | | | (120.2) | | | | | | | | | (120.2) | |
| Repurchases of common stock | | | | | | | | | (206.8) | | | | | (206.8) | |
| | | | | | | | | | | | | |
Transactions with noncontrolling interests (1)(2) | | | 299.0 | | | | | | | | | 60.1 | | | 359.1 | |
| Balance December 31, 2018 | $ | 18.6 | | | $ | 776.2 | | | $ | 4,334.3 | | | $ | (308.9) | | | $ | (1,699.1) | | | $ | 89.3 | | | $ | 3,210.4 | |
| Adoption of accounting standards (Note 2) | | | | | 55.5 | | | (53.1) | | | | | | | 2.4 | |
| Net income (loss) | | | | | 477.4 | | | | | | | 2.8 | | | 480.2 | |
| Stock compensation plans | | | 53.5 | | | | | | | 21.6 | | | | | 75.1 | |
| Shares for benefit plan trust | | | | | | | | | (1.0) | | | | | (1.0) | |
| Net pension and other benefit actuarial gains (losses) and prior service cost, net of income tax | | | | | | | 3.4 | | | | | | | 3.4 | |
| Net hedging gains (losses) and other, net of income tax | | | | | | | (77.2) | | | | | | | (77.2) | |
| Foreign currency translation adjustments | | | | | | | (15.2) | | | | | (3.3) | | | (18.5) | |
Dividends ($1.64 per share) | | | | | (214.1) | | | | | | | | | (214.1) | |
| Repurchases of common stock | | | | | | | | | (414.3) | | | | | (414.3) | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Distribution of FMC Lithium (3) | | | | | (464.3) | | | 39.0 | | | | (59.7) | | | (485.0) | |
| | | | | | | | | | | | | |
| Balance December 31, 2019 | $ | 18.6 | | | $ | 829.7 | | | $ | 4,188.8 | | | $ | (412.0) | | | $ | (2,092.8) | | | $ | 29.1 | | | $ | 2,561.4 | |
| | | | | | | | | | | | | |
| Net income (loss) | | | | | 551.5 | | | | | | | (0.9) | | | 550.6 | |
| Stock compensation plans | | | 33.1 | | | | | | | 10.4 | | | | | 43.5 | |
| Shares for benefit plan trust | | | | | | | | | (0.4) | | | | | (0.4) | |
| Net pension and other benefit actuarial gains (losses) and prior service cost, net of income tax | | | | | | | 34.9 | | | | | | | 34.9 | |
| Net hedging gains (losses) and other, net of income tax | | | | | | | (6.8) | | | | | | | (6.8) | |
| Foreign currency translation adjustments | | | | | | | 101.7 | | | | | 0.3 | | | 102.0 | |
Dividends ($1.80 per share) | | | | | (233.9) | | | | | | | | | (233.9) | |
| Repurchases of common stock | | | | | | | | | (58.4) | | | | | (58.4) | |
Acquisition of noncontrolling interests (1) | | | (2.6) | | | | | | | | | (4.8) | | | (7.4) | |
| Distributions to noncontrolling interests | | | | | | | | | | | (1.3) | | | (1.3) | |
| | | | | | | | | | | | | |
| Balance December 31, 2020 | $ | 18.6 | | | $ | 860.2 | | | $ | 4,506.4 | | | $ | (282.2) | | | $ | (2,141.2) | | | $ | 22.4 | | | $ | 2,984.2 | |
____________________
(1) See Note 17 for more detail on transactions with noncontrolling interest.
(2) Primarily represents the noncontrolling interest of our FMC Lithium as a result of the IPO. Refer to Note 1 for further information.
(3) Represents the effects of the distribution of FMC Lithium. Refer to Note 1 for further information.
The accompanying notes are an integral part of these consolidated financial statements.
Note 1: Principal Accounting Policies and Related Financial Information
Nature of operations. We are an agricultural sciences company providing innovative solutions to growers around the world with a robust product portfolio fueled by a market-driven discovery and development pipeline in crop protection, plant health, and professional pest and turf management. We operate in a single distinct business segment and develop, market and sell all three major classes of crop protection chemicals: insecticides, herbicides and fungicides. These products are used in agriculture to enhance crop yield and quality by controlling a broad spectrum of insects, weeds and disease, as well as in non-agricultural markets for pest control.
In March 2017, we announced our intention to separate our FMC Lithium segment (subsequently renamed Livent Corporation, or "Livent") into a publicly traded company. The initial step of the separation, the initial public offering ("IPO") of Livent, closed on October 15, 2018. In connection with the IPO, Livent had granted the underwriters an option to purchase additional shares of common stock to cover over-allotments at the IPO price, less the underwriting discount. On November 8, 2018, the underwriters exercised in full their option to purchase additional shares. After completion of the IPO and the underwriters' exercise to purchase additional shares of common stock, FMC owned 123 million shares of Livent's common stock, representing approximately 84 percent of the total outstanding shares of Livent's common stock. On March 1, 2019, we completed the previously announced distribution of 123 million shares of common stock of Livent as a pro rata dividend on shares of FMC common stock outstanding at the close of business on the record date of February 25, 2019. We have recast all the data within this filing to present FMC Lithium as a discontinued operation retrospectively for all periods presented.
COVID-19. Given the COVID-19 pandemic, many countries, including the United States, subsequently imposed restrictions on both travel and business closures in an effort to mitigate the spread of COVID-19. As an agricultural sciences company, we are considered an "essential" industry in the countries in which we operate and have avoided significant plant closures and all our facilities are operational. While we have maintained business continuity and sustained our operations, we do not yet know the full extent of the disruptions on either our business and operations or the global economy nor the duration of the pandemic and its adverse effects.
Basis of consolidation and basis of presentation. The accompanying consolidated financial statements of FMC Corporation and its subsidiaries were prepared in accordance with accounting principles generally accepted in the United States of America ("U.S. GAAP"). Our consolidated financial statements include the accounts of FMC and all entities that we directly or indirectly control. All significant intercompany accounts and transactions are eliminated in consolidation.
Certain prior year amounts have been reclassified to conform to current year's presentation.
Estimates and assumptions. In preparing the financial statements in conformity with U.S. GAAP we are required to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results are likely to differ from those estimates, but we do not believe such differences will materially affect our financial position, results of operations or cash flows.
Cash equivalents. We consider investments in all liquid debt instruments with original maturities of 3 months or less to be cash equivalents.
Trade receivables, net of allowance. Trade receivables consist of amounts owed to us from customer sales and are recorded when revenue is recognized. The allowance for trade receivables represents our best estimate of the probable losses associated with potential customer defaults. In developing our allowance for trade receivables, we use a two-stage process which includes calculating a general formula to develop an allowance to appropriately address the uncertainty surrounding collection risk of our entire portfolio and specific allowances for customers where the risk of collection has been reasonably identified either due to liquidity constraints or disputes over contractual terms and conditions.
Our method of calculating the general formula consists of estimating the recoverability of trade receivables based on historical experience, current collection trends, and external business factors such as economic factors, including regional bankruptcy rates, and political factors. Our analysis of trade receivable collection risk is performed quarterly, and the allowance is adjusted accordingly.
We also hold long-term receivables that represent long-term customer receivable balances related to past-due accounts which are not expected to be collected within the current year. Our policy for the review of the allowance for these receivables is
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
consistent with the discussion in the preceding paragraph above on trade receivables. Therefore on an ongoing basis, we continue to evaluate the credit quality of our long-term receivables utilizing aging of receivables, collection experience and write-offs, as well as existing economic conditions, to determine if an additional allowance is necessary.
The allowance for trade receivables was $27.9 million and $26.3 million as of December 31, 2020 and 2019, respectively. The allowance for long-term receivables was $24.7 million and $61.1 million at December 31, 2020 and 2019, respectively. The provision to the allowance for receivables charged against operations was $4.7 million, $21.2 million and $71.4 million for the years ended December 31, 2020, 2019 and 2018, respectively. See Note 10 for more information. The provision in 2018 includes the effects of the stranded accounts receivables written off as part of the restructuring in India.
Investments. Investments in companies in which our ownership interest is 50 percent or less and in which we exercise significant influence over operating and financial policies are accounted for using the equity method. Under the equity method, original investments are recorded at cost and adjusted by our share of undistributed earnings and losses of these investments. Majority owned investments in which our control is restricted are also accounted for using the equity method. All other investments are carried at their fair values or at cost, as appropriate. We are party to several joint venture investments throughout the world, which individually and in the aggregate are not significant to our financial results.
Inventories. Inventories are stated at the lower of cost or net realizable value. Inventory costs include those costs directly attributable to products before sale, including all manufacturing overhead but excluding distribution costs. All domestic inventories, excluding materials and supplies, are determined on a last-in, first-out ("LIFO") basis and our remaining inventories are recorded on either a first-in, first-out ("FIFO") basis or average cost. See Note 7 for more information.
Property, plant and equipment. We record property, plant and equipment, including capitalized interest, at cost. We recognize acquired property, plant and equipment, from acquisitions at its estimated fair value. Depreciation is provided principally on the straight-line basis over the estimated useful lives of the assets (land improvements — 20 years, buildings and building equipment — 15 to 40 years, and machinery and equipment — three to 18 years). Gains and losses are reflected in income upon sale or retirement of assets. Expenditures that extend the useful lives of property, plant and equipment or increase productivity are capitalized. Ordinary repairs and maintenance are expensed as incurred through operating expense.
Capitalized interest. We capitalized interest costs of $3.5 million, $4.7 million, and $4.1 million in 2020, 2019, and 2018, respectively. These costs were primarily associated with the construction of certain long-lived assets and have been capitalized as part of the cost of those assets. We amortize capitalized interest over the assets’ estimated useful lives.
Impairments of long-lived assets. We review the recovery of the net book value of long-lived assets whenever events and circumstances indicate that the net book value of an asset may not be recoverable from the estimated undiscounted future cash flows expected to result from its use and eventual disposition. In cases where undiscounted expected future cash flows are less than the net book value, we recognize an impairment loss equal to an amount by which the net book value exceeds the fair value of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell.
Asset retirement obligations. We record asset retirement obligations ("AROs") at fair value at the time the liability is incurred if we can reasonably estimate the settlement date. The associated AROs are capitalized as part of the carrying amount of related long-lived assets. In future periods, the liability is accreted to its present value and the capitalized cost is depreciated over the useful life of the related asset. We also adjust the liability for changes resulting from the passage of time and/or revisions to the timing or the amount of the original estimate. Upon retirement of the long-lived asset, we either settle the obligation for its recorded amount or incur a gain or loss.
We have obligations at the majority of our manufacturing facilities in the event of permanent plant shutdown. For certain AROs not already accrued, we have calculated the fair value of these AROs and concluded that the present value of these obligations was inconsequential at December 31, 2020 and 2019.
The carrying amounts for the AROs for the years ended December 31, 2020 and 2019 are $30.7 million and $35.7 million, respectively. These amounts are included in "Accrued and other liabilities" and "Other long-term liabilities" on the consolidated balance sheet. During 2019, we recorded a charge to recognize the acceleration of asset retirement obligations associated with our decision to exit sales of all carbofuran formulations (including Furadan® insecticide/nematicide, Curaterr® insecticide/nematicide and any other brands used with carbofuran products) globally effective December 31, 2019. Refer to Note 9 for more information.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Restructuring and other charges. We continually perform strategic reviews and assess the return on our business. This sometimes results in a plan to restructure the operations of our business. We record an accrual for severance and other exit costs under the provisions of the relevant accounting guidance.
Additionally, as part of these restructuring plans, write-downs of long-lived assets may occur. Two types of assets are impacted: assets to be disposed of by sale and assets to be abandoned. Assets to be disposed of by sale are measured at the lower of carrying amount or estimated net proceeds from the sale. Assets to be abandoned with no remaining future service potential are written down to amounts expected to be recovered. The useful life of assets to be abandoned that have a remaining future service potential are adjusted and depreciation is recorded over the adjusted useful life.
Capitalized software. We capitalize the costs of internal use software in accordance with accounting literature which generally requires the capitalization of certain costs incurred to develop or obtain internal use software. We assess the recoverability of capitalized software costs on an ongoing basis and record write-downs to fair value as necessary. We amortize capitalized software costs over expected useful lives ranging from three to 10 years. See Note 22 for the net unamortized computer software balances.
Goodwill and intangible assets. Goodwill and other indefinite life intangible assets are not subject to amortization. Instead, they are subject to at least an annual assessment for impairment by applying a fair value-based test.
We test goodwill and indefinite life intangibles for impairment annually using the criteria prescribed by U.S. GAAP accounting guidance for goodwill and other intangible assets. Based upon our annual impairment assessments conducted in 2020, 2019 and 2018, we did not record any goodwill impairments.
Finite-lived intangible assets consist of primarily customer relationships as well as patents, brands, registration rights, industry licenses, and other intangibles and are generally being amortized over periods of approximately three to 20 years. See Note 6 for additional information on goodwill and intangible assets.
Revenue recognition. We recognize revenue when (or as) we satisfy our performance obligation which is when the customer obtains control of the good or service. Rebates due to customers are accrued as a reduction of revenue in the same period that the related sales are recorded based on the contract terms. Refer to Note 3.
We record amounts billed for shipping and handling fees as revenue. Costs incurred for shipping and handling are recorded as costs of sales and services. Amounts billed for sales and use taxes, value-added taxes, and certain excise and other specific transactional taxes imposed on revenue-producing transactions are presented on a net basis and excluded from sales in the consolidated income statements. We record a liability until remitted to the respective taxing authority.
We periodically enter into prepayment arrangements with customers and receive advance payments for product to be delivered in future periods. These advance payments are recorded as deferred revenue and classified as "Advance payments from customers" on the consolidated balance sheet. Revenue associated with advance payments is recognized as shipments are made and transfer of control to the customer takes place.
Research and development. Research and development costs are expensed as incurred. In-process research and development acquired as part of asset acquisitions, which include license and development agreements, are expensed as incurred and included as a component of "Restructuring and other charges (income)" on the consolidated statements of income (loss).
Income and other taxes. We provide current income taxes on income reported for financial statement purposes adjusted for transactions that do not enter into the computation of income taxes payable. We recognize deferred tax liabilities and assets for the expected future tax consequences of temporary differences between the carrying amounts and the tax basis of assets and liabilities. We have not provided income taxes for other outside basis differences inherent in our investments in subsidiaries because the investments and related unremitted earnings are essentially permanent in duration or we have concluded that no additional tax liability will arise upon disposal or remittance.
Foreign currency. We translate the assets and liabilities of our foreign operations at exchange rates in effect at the balance sheet date. For foreign operations for which the functional currency is not the U.S. dollar we record translation gains and losses as a component of accumulated other comprehensive income (loss) in equity. The foreign operations' income statements are translated at the monthly exchange rates for the period.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
We record remeasurement gains and losses on monetary assets and liabilities, such as accounts receivables and payables, which are not in the functional currency of the operation. These remeasurement gains and losses are recorded in income as they occur. We generally enter into foreign currency contracts to mitigate the financial risk associated with these transactions. See "Derivative financial instruments" below and Note 19.
Derivative financial instruments. We mitigate certain financial exposures, including currency risk, interest rate risk and commodity price exposures, through a controlled program of risk management that includes the use of derivative financial instruments. We enter into foreign exchange contracts, including forward and purchased option contracts, to reduce the effects of fluctuating foreign currency exchange rates.
We recognize all derivatives on the balance sheet at fair value. On the date the derivative instrument is entered into, we generally designate the derivative as either a hedge of the variability of cash flows to be received or paid related to a forecasted transaction (cash flow hedge) or a hedge of the fair value of a recognized asset or liability or of an unrecognized firm commitment (fair value hedge). We record in accumulated other comprehensive income (loss) changes in the fair value of derivatives that are designated as, and meet all the required criteria for, a cash flow hedge. We then reclassify these amounts into earnings as the underlying hedged item affects earnings. We record immediately in earnings changes in the fair value of derivatives that are not designated as cash flow hedges.
We formally document all relationships between hedging instruments and hedged items, as well as the risk management objective and strategy for undertaking various hedge transactions. This process includes relating derivatives that are designated as fair value or cash flow hedges to specific assets and liabilities on the balance sheet or to specific firm commitments or forecasted transactions. We also formally assess, both at the inception of the hedge and throughout its term, whether each derivative is highly effective in offsetting changes in fair value or cash flows of the hedged item. If we determine that a derivative is not highly effective as a hedge, or if a derivative ceases to be a highly effective hedge, we discontinue hedge accounting with respect to that derivative prospectively.
Treasury stock. We record shares of common stock repurchased at cost as treasury stock, resulting in a reduction of stockholders’ equity in the consolidated balance sheets. When the treasury shares are contributed under our employee benefit plans or issued for option exercises, we use a FIFO method for determining cost. The difference between the cost of the shares and the market price at the time of contribution to an employee benefit plan is added to or deducted from the related capital in excess of par value of common stock.
Segment information. As a result of the FMC Lithium separation on March 1, 2019, we now operate as a single business segment providing innovative solutions to growers around the world with a robust product portfolio fueled by a market-driven discovery and development pipeline in crop protection, plant health, and professional pest and turf management. The business is supported by global corporate staff functions. The determination of a single segment is consistent with the financial information regularly reviewed by the chief executive officer for purposes of evaluating performance, allocating resources, setting incentive compensation targets and both planning and forecasting future periods. Refer to Note 3 for further information on product and regional revenues.
Geographic long-lived assets include goodwill and other intangibles, net, property, plant and equipment, net and other non-current assets. Refer to Note 21.
Stock compensation plans. We recognize compensation expense in the financial statements for all share options and other equity-based arrangements. Share-based compensation cost is measured at the date of grant, based on the fair value of the award, and is recognized over the employee’s requisite service period. See Note 16 for further discussion on our share-based compensation.
Environmental obligations. We provide for environmental-related obligations when they are probable and amounts can be reasonably estimated. Where the available information is sufficient to estimate the amount of liability, that estimate has been used. Where the information is only sufficient to establish a range of probable liability and no point within the range is more likely than any other, the lower end of the range has been used.
Estimated obligations to remediate sites that involve oversight by the United States Environmental Protection Agency ("EPA"), or similar government agencies, are generally accrued no later than when a Record of Decision ("ROD"), or equivalent, is issued, or upon completion of a Remedial Investigation/Feasibility Study ("RI/FS"), or equivalent, that is submitted by us and the appropriate government agency or agencies. Estimates are reviewed quarterly and, if necessary, adjusted as additional information becomes available. The estimates can change substantially as additional information becomes available regarding
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
the nature or extent of site contamination, required remediation methods, and other actions by or against governmental agencies or private parties.
Our environmental liabilities for continuing and discontinued operations are principally for costs associated with the remediation and/or study of sites at which we are alleged to have released hazardous substances into the environment. Such costs principally include, among other items, RI/FS, site remediation, costs of operation and maintenance of the remediation plan, management costs, fees to outside law firms and consultants for work related to the environmental effort, and future monitoring costs. Estimated site liabilities are determined based upon existing remediation laws and technologies, specific site consultants’ engineering studies or by extrapolating experience with environmental issues at comparable sites.
Included in our environmental liabilities are costs for the operation, maintenance and monitoring ("M&M") of site remediation plans. Such reserves are based on our best estimates for these OM&M plans. Over time we may incur OM&M costs in excess of these reserves. However, we are unable to reasonably estimate an amount in excess of our recorded reserves because we cannot reasonably estimate the period for which such OM&M plans will need to be in place or the future annual cost of such remediation, as conditions at these environmental sites change over time. Such additional OM&M costs could be significant in total but would be incurred over an extended period of years.
Included in the environmental reserve balance, other assets balance and disclosure of reasonably possible loss contingencies are amounts from third party insurance policies which we believe are probable of recovery.
Provisions for environmental costs are reflected in income, net of probable and estimable recoveries from named Potentially Responsible Parties ("PRPs") or other third parties. In the fourth quarter of 2019, we increased our reserves for the Pocatello Tribal Matter by $72.8 million, which represents both the historical and discounted present value of future annual use permit fees as well as the associated legal costs at the time the charge was recorded. We remeasure our discounted liability balance according to current interest rates. See Note 12 for further information. All other environmental provisions incorporate inflation and are not discounted to their present value.
In calculating and evaluating the adequacy of our environmental reserves, we have taken into account the joint and several liability imposed by Comprehensive Environmental Remediation, Compensation and Liability Act ("CERCLA") and the analogous state laws on all PRPs and have considered the identity and financial condition of the other PRPs at each site to the extent possible. We have also considered the identity and financial condition of other third parties from whom recovery is anticipated, as well as the status of our claims against such parties. Although we are unable to forecast the ultimate contributions of PRPs and other third parties with absolute certainty, the degree of uncertainty with respect to each party is taken into account when determining the environmental reserve on a site-by-site basis. Our liability includes our best estimate of the costs expected to be paid before the consideration of any potential recoveries from third parties. We believe that any recorded recoveries related to PRPs are realizable in all material respects. Recoveries are recorded as either an offset in "Environmental liabilities, continuing and discontinued" or as "Other assets including long-term receivables, net" in our consolidated balance sheets in accordance with U.S. accounting literature.
Pension and other postretirement benefits. We provide qualified and nonqualified defined benefit and defined contribution pension plans, as well as postretirement health care and life insurance benefit plans to our employees and retirees. The costs (or benefits) and obligations related to these benefits reflect key assumptions related to general economic conditions, including interest (discount) rates, healthcare cost trend rates, expected rates of return on plan assets and the rates of compensation increase for employees. The costs (or benefits) and obligations for these benefit programs are also affected by other assumptions, such as average retirement age, mortality, employee turnover, and plan participation. To the extent our plans’ actual experience, as influenced by changing economic and financial market conditions or by changes to our own plans’ demographics, differs from these assumptions, the costs and obligations for providing these benefits, as well as the plans’ funding requirements, could increase or decrease. When actual results differ from our assumptions, the difference is typically recognized over future periods. In addition, the unrealized gains and losses related to our pension and postretirement benefit obligations may also affect periodic benefit costs (or benefits) in future periods. See Note 15 for additional information relating to pension and other postretirement benefits.
Note 2: Recently Issued and Adopted Accounting Pronouncements and Regulatory Items
New accounting guidance and regulatory items
In March 2020, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting. This
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
ASU provides optional guidance for a limited period of time to ease the potential burden in accounting for contracts and hedging relationships affected by reference rate reform. This applies to contracts that reference LIBOR or another rate that is expected to be discontinued as a result of rate reform and have modified terms that affect or have the potential to affect the amount and timing of contractual cash flows resulting from the discontinuance of reference rate. The new standard is currently effective and upon adoption may be applied prospectively through December 31, 2022. We are evaluating the impacts this standard will have on accounting for contracts and hedging relationships but do not believe it will have a material impact on our consolidated financial statements.
In December 2019, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. The amendments in this ASU simplify the accounting for income taxes by removing certain exceptions and simplification in several other areas. The new standard is effective for fiscal years beginning after December 15, 2020 (i.e., a January 1, 2021 effective date). We believe the adoption will not have a material impact on our consolidated financial statements.
Recently adopted accounting guidance
In August 2018, the FASB issued ASU No. 2018-14, Defined Benefit Plans - General (Subtopic 715-20): Disclosure Framework - Changes to the Disclosure Requirements for Defined Benefit Plans. The amendments in this ASU modify the disclosure requirements for employers that sponsor defined benefit pension or other postretirement plans. The new standard is effective for fiscal years ending after December 15, 2020. There was no impact to our consolidated financial statements upon adoption, however, we have updated our disclosures within to comply with the ASU.
In August 2018, the FASB issued ASU No. 2018-15, Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract. The amendments in this ASU align the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. The new standard became effective for fiscal years beginning after December 15, 2019 (i.e. a January 1, 2020 effective date). There was no material impact to our consolidated financial statements upon adoption.
In January 2017, the FASB issued ASU No. 2017-04, Intangibles - Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment. This ASU changes the subsequent measurement of goodwill impairment by eliminating Step 2 from the impairment test. Under the new guidance, an entity will measure impairment using the difference between the carrying amount and the fair value of the reporting unit. The new standard became effective for fiscal years beginning after December 15, 2019 (i.e. a January 1, 2020 effective date), with early adoption permitted for goodwill impairment tests with measurement dates after January 1, 2017. There was no material impact to our consolidated financial statements upon adoption.
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments – Credit Losses (Topic 326), Measurement of Credit Losses on Financial Instruments ("ASU 2016-13"). ASU 2016-13 replaces the incurred loss impairment methodology with a current expected credit loss ("CECL") model that immediately recognizes an estimate of credit losses that are expected to occur over the life of the financial instrument, including trade receivables. The update is intended to provide financial statement users with more decision-useful information about the expected credit losses on financial instruments and other commitments to extend credit held by a reporting entity at each reporting date. The new standard became effective January 1, 2020. As a result of the adoption, we have refined our allowance for doubtful trade receivables methodology which considers current economic conditions as well as forward-looking expectations about expected credit losses. The adoption of the new standard did not result in a material impact to our consolidated financial statements.
In February 2018, the FASB issued ASU No. 2018-02, Income Statement – Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income. This new standard permits a company to reclassify the income tax effects of the change in the U.S federal corporate income tax rate on the gross deferred tax amounts and related valuation allowances as well as other income tax effects related to the application of the Tax Cuts and Jobs Act (the "Act") within accumulated other comprehensive income ("AOCI") to retained earnings. The new standard also requires certain disclosures about stranded tax effects. The new standard is effective for fiscal years beginning after December 15, 2018 (i.e., a January 1, 2019 effective date), and interim periods within those fiscal years, with early adoption permitted. We adopted this standard prospectively as of January 1, 2019 and reclassified $53.1 million of the stranded income tax effects from accumulated other comprehensive income (loss) to retained earnings. The reclassification was related to the change in the U.S. federal corporate tax rate and the effect of the Act on our pension plans and derivative instruments. This reclassification is reflected within the consolidated statements of changes in equity for the current period.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
In February 2016, the FASB issued its new lease accounting guidance in ASU No. 2016-02, Leases (Topic 842) ("ASC 842"). Under the new guidance, lessees will be required to recognize for all leases (with the exception of short-term leases) a lease liability, which is a lessee's obligation to make lease payments arising from a lease, measured on a discounted basis and a right-of-use ("ROU") asset, which is an asset that represents the lessee's right to use, or control the use of, a specified asset for the lease term. The new standard, including related amendments, is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years (i.e., a January 1, 2019 effective date). In adopting this standard, we performed a detailed review of contracts of our business and assessed the terms under ASC 842. Additionally, we assessed potential impacts on our internal controls and processes related to both the implementation and ongoing compliance of the new guidance.
We have adopted this standard as of January 1, 2019 utilizing a modified retrospective approach and have elected the transition practical expedient package. Under this transition practical expedient package, ASC 842 was only applied to contracts that existed as of, or were entered into on or after, January 1, 2019, and a cumulative effect adjustment was made as of January 1, 2019. All comparative periods prior to January 1, 2019 will retain the financial reporting and disclosure requirements of ASC 840. The adoption of ASC 842 had a material impact on our consolidated balance sheet but did not have a material impact on the consolidated statement of income (loss), consolidated statement of comprehensive income (loss), consolidated statement of cash flows, or consolidated statement of changes in equity. As a result of adoption, we recorded additional ROU lease assets and lease liabilities of $185.3 million and $215.9 million, respectively. ROU lease assets includes a reclassification of $30.6 million of prepaid rent, accrued rent, and lease incentives previously recorded under ASC 840. Additionally, we recorded a retained earnings impact of $2.4 million as of January 1, 2019. Refer to Note 4 for further information.
The expedient package allowed us not to reassess whether existing contracts contain a lease under the new definition of a lease, the lease classification of existing leases, and initial direct cost for existing leases including whether such costs would qualify for capitalization under the standard. Additionally, we elected the practical expedient to not separate non-lease components from lease components. In addition to these practical expedients, we elected the following exemption permissible under ASC 842: the exclusion of leases with terms 12 months or less that do not have a purchase option or extension that is reasonably certain to exercise.
The adoption of ASC 842 required adjustments to record our initial ROU asset and lease liability on the balance sheet. The initial right of use asset and lease liability are presented on a discounted basis by our incremental borrowing rate at transition.
Note 3: Revenue Recognition
Disaggregation of revenue
We disaggregate revenue from contracts with customers by geographical areas and major product categories. We have three major agricultural pesticide product categories: insecticides, herbicides, and fungicides. The disaggregated revenue tables are shown below for the years ended December 31, 2020, 2019 and 2018.
The following table provides information about disaggregated revenue by major geographical region:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
North America (1) | $ | 1,032.5 | | | $ | 1,121.1 | | | $ | 1,090.8 | |
Latin America (1) | 1,456.5 | | | 1,441.7 | | | 1,210.1 | |
| Europe, Middle East & Africa | 1,046.3 | | | 1,001.8 | | | 966.0 | |
| Asia | 1,106.8 | | | 1,045.2 | | | 1,018.4 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Total Revenue | $ | 4,642.1 | | | $ | 4,609.8 | | | $ | 4,285.3 | |
____________________
(1)Countries with sales in excess of 10 percent of consolidated revenue consisted of the U.S. and Brazil. Sales for the years ended December 31, 2020, 2019, and 2018 for the U.S. totaled $941.2 million, $1,044.1 million and $991.8 million , respectively, and for Brazil totaled $1,083.4 million, $1,094.1 million and $913.7 million, respectively.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The following table provides information about disaggregated revenue by major product category:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Insecticides | $ | 2,836.8 | | | $ | 2,773.6 | | | $ | 2,476.5 | |
| Herbicides | 1,187.2 | | | 1,228.8 | | | 1,251.2 | |
| Fungicides | 275.5 | | | 271.4 | | | 268.7 | |
| Other | 342.6 | | | 336.0 | | | 288.9 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Total Revenue | $ | 4,642.1 | | | $ | 4,609.8 | | | $ | 4,285.3 | |
We earn revenue from the sale of a wide range of products to a diversified base of customers around the world. Our portfolio is comprised of three major pesticide categories: insecticides, herbicides and fungicides. These products are used in agriculture to enhance crop yield and quality by controlling a broad spectrum of insects, weeds and disease, as well as in non-agricultural markets for pest control. The majority of our product lines consist of insecticides and herbicides, with a smaller portfolio of fungicides mainly used in high value crop segments. Our insecticides are used to control a wide spectrum of pests, while our herbicide portfolio primarily targets a large variety of difficult-to-control weeds. Products in the other category include various agricultural products such as smaller classes of pesticides, growth promoters, and soil enhancements.
Sale of Goods
Revenue from product sales is recognized when (or as) we satisfy a performance obligation by transferring the promised goods to a customer, that is, when control of the good transfers to the customer. The customer is then invoiced at the agreed-upon price with payment terms generally ranging from 30 to 90 days, with some regions providing terms longer than 90 days. We do not typically give payment terms that exceed 360 days; however, in certain geographical regions such as Latin America, these extended terms may be given in limited circumstances. Additionally, a timing difference of over one year can exist between when products are delivered to the customer and when payment is received from the customer in these regions; however, the effect of these sales is not material to the financial statements as a whole. Furthermore, we have assessed the circumstances and arrangements in these regions and determined that the contracts with these customers do not contain a significant financing component.
In determining when the control of goods is transferred, we typically assess, among other things, the transfer of risk and title and the shipping terms of the contract. The transfer of title and risk typically occurs either upon shipment to the customer or upon receipt by the customer. As such, we typically recognize revenue when goods are shipped based on the relevant Incoterm for the product order, or in some regions, when delivery to the customer’s requested destination has occurred. When we perform shipping and handling activities after the transfer of control to the customer (e.g., when control transfers prior to delivery), they are considered as fulfillment activities, and accordingly, the costs are accrued for when the related revenue is recognized. For FOB shipping point terms, revenue is recognized at the time of shipment since the customer gains control at this point in time.
We record amounts billed for shipping and handling fees as revenue. Costs incurred for shipping and handling are recorded as costs of sales and services. Amounts billed for sales and use taxes, value-added taxes, and certain excise and other specific transactional taxes imposed on revenue-producing transactions are presented on a net basis and excluded from sales in the consolidated income statements. We record a liability until remitted to the respective taxing authority.
Sales Incentives and Other Variable Considerations
As a part of our customary business practice, we offer a number of sales incentives to our customers including volume discounts, retailer incentives, and prepayment options. The variable considerations given can differ by products, support levels and other eligibility criteria. For all such contracts that include any variable consideration, we estimate the amount of variable consideration that should be included in the transaction price utilizing either the expected value method or the most likely amount method depending on the nature of the variable consideration. Variable consideration is included in the transaction price if, in our judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. Although determining the transaction price for these considerations requires significant judgment, we have significant historical experience with incentives provided to customers and estimate the expected consideration considering historical patterns of incentive payouts. These estimates are reassessed each reporting period as required.
In addition to the variable considerations describe above, in certain instances, we may require our customers to meet certain volume thresholds within their contract term. We estimate what amount of variable consideration should be included in the transaction price at contract inception and continually reassess this estimation each reporting period to determine situations
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
when the minimum volume thresholds will not be met. Variable consideration is included in the transaction price if, in our judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur.
Right of Return
We extend an assurance warranty offering customers a right of refund or exchange in case the delivered product does not conform to specifications. Additionally, in certain regions and arrangements, we may offer a right of return for a specified period. Both instances are accounted for as a right of return and transaction price is adjusted for an estimate of expected returns. Replacement products are accounted for under the warranty guidance if the customer exchanges one product for another of the same kind, quality, and price. We have significant experience with historical return patterns and use this experience to include returns in the estimate of transaction price.
Contract asset and contract liability balances
We satisfy our obligations by transferring goods and services in exchange for consideration from customers. The timing of performance sometimes differs from the timing the associated consideration is received from the customer, thus resulting in the recognition of a contract asset or contract liability. We recognize a contract liability if the customer's payment of consideration is received prior to completion of our related performance obligation.
The following table presents the opening and closing balances of our receivables (net of allowances) and contract liabilities from contracts with customers:
| | | | | | | | | | | | | | | | | |
| (in Millions) | Balance as of December 31, 2019 | | Balance as of December 31, 2020 | | Increase (Decrease) |
| | | | | |
| Receivables from contracts with customers, net of allowances | $ | 2,354.3 | | | $ | 2,433.8 | | | $ | 79.5 | |
| Contract liabilities: Advance payments from customers | 492.7 | | | 347.1 | | | (145.6) | |
The amount of revenue recognized in the year ended December 31, 2020 that was included in the opening contract liability balance was $492.7 million.
The balance of receivables from contracts with customers listed in the table above include both current trade receivables and long-term receivables, net of allowance for doubtful accounts. The allowance for receivables represents our best estimate of the probable losses associated with potential customer defaults. We determine the allowance based on historical experience, current collection trends, and external business factors such as economic factors, including regional bankruptcy rates, and political factors. The change in allowance for doubtful accounts for both current trade receivables and long-term receivables is representative of the impairment of receivables as of December 31, 2020. Refer to Note 10 for further information.
We periodically enter into prepayment arrangements with customers and receive advance payments for product to be delivered in future periods. Prepayment terms are extended to customers/distributors in order to capitalize on surplus cash with growers. Growers receive bulk payments for their produce, which they leverage to buy our products from distributors through prepayment options. This in turn creates opportunity for distributors to make large prepayments to us for securing the future supply of products to be sold to growers. Prepayments are typically received in the fourth quarter of the fiscal year, primarily in North America, and are for the following marketing year indicating that the time difference between prepayment and performance of corresponding performance obligations does not exceed one year. We recognize these prepayments as a liability under "Advance Payments from customers" on the consolidated balance sheets when they are received. Revenue associated with advance payments is recognized as shipments are made and transfer of control to the customer takes place. Advance payments from customers was $492.7 million as of December 31, 2019 and $347.1 million as of December 31, 2020.
Performance obligations
At contract inception, we assess the goods and services promised in our contracts with customers and identify a performance obligation for each promise to transfer a good or service (or bundle of goods or services) that is distinct. To identify the performance obligations, we consider all the goods or services promised in the contract, whether explicitly stated or implied based on customary business practices. Based on our evaluation, we have determined that our current contracts do not contain more than one performance obligation. Revenue is recognized when (or as) the performance obligation is satisfied, which is when the customer obtains control of the good or service.
Periodically, we may enter into contracts with customers which require them to submit a forecast of non-binding purchase obligations to us. These forecasts are typically provided by the customer to us in good faith, and there are no penalties or obligations if the forecasts are not met. Accordingly, we have determined that these are optional purchases and do not represent material rights and are not considered as unsatisfied (or partially satisfied) performance obligations for the purposes of this disclosure.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
In separate and less common circumstances, we may have contracts with customers which have binding purchase requirements for just one quarter of their annual forecasts. Additionally, as noted in the Contract Liabilities section above, we periodically enter into agricultural prepayment arrangements with customers, and receive advance payments for product to be delivered in future periods within one year. We have elected not to disclose the aggregate amount of the transaction price allocated to remaining performance obligations for these two types of contracts as they have an expected duration of one year or less and the revenue is expected to be recognized within the next year.
Other arrangements
Data Licensing
We sometimes grant to third parties a license and right to rely upon pesticide regulatory data filed with government agencies. Such licenses allow a licensee to cite and rely upon our data in connection with the licensee’s application for pesticide registrations as required by law; these licenses can be granted through contract or through a mandatory statutory license, depending on circumstances. In the most common occurrence, when a license is embedded in a contract for supply of pesticide active ingredient from us to the licensee, the license grant is not considered as distinct from other promised goods or services. Accordingly, all promises are treated as a single performance obligation and revenue is recognized at a point when the control of the pesticide products is transferred to the licensee-customer. In the less frequent occurrence, when the license and right to use data is granted without a supply contract, we account for the revenue attributable to the data license as a performance obligation satisfied at a single point in time and recognize revenue on the effective date of such contract. Finally, in those circumstance of mandatory data licensing by statute, such as under U.S. pesticide law, we recognize the data compensation upon the effective date of the data compensation settlement agreement. Payment terms for these arrangements may vary by contract.
Service Arrangements
In limited cases, we engage in providing certain tolling services, such as filling and packing services using raw and packing materials supplied by the customer. However, as a result of the DuPont Crop Protection Business Acquisition, on November 1, 2017, we entered into an agreement with DuPont to provide tolling services to one another for up to five years from the acquisition date. Depending on the nature of the tolling services, we determine the appropriate method of satisfaction of the performance obligation, which may be the input or output method. Compared to other goods and services provided by us, service arrangements do not represent a significant portion of sales each year. Payment terms for service arrangements may vary by contract; however, payment is typically due within 30 days of the invoice date.
Practical Expedients and Exemptions
We have elected the following practical expedients following the adoption of ASC 606:
a.Costs of obtaining a contract: FMC incurs certain costs such as sales commissions which are incremental to obtaining the contract. We have taken the practical expedient of expensing such costs to obtain a contract, as and when they are incurred, as their expected amortization period is one year or less.
b.Significant financing component: We elected not to adjust the promised amount of consideration for the effects of a significant financing component if FMC expects, at contract inception, that the period between the transfer of a promised good or service to a customer and when the customer pays for that good or service will be one year or less.
c.Remaining performance obligations: We elected not to disclose the aggregate amount of the transaction price allocated to remaining performance obligations for its contracts that are one year or less, as the revenue is expected to be recognized within one year. Additionally, we have elected not to disclose information about variable considerations for remaining, wholly unsatisfied performance obligations for which the criteria in paragraph 606-10-32-40 have been met.
d.Shipping and handling costs: We elected to account for shipping and handling activities that occur after the customer has obtained control of a good as fulfillment activities (i.e., an expense) rather than as a promised service.
e.Measurement of transaction price: We have elected to exclude from the measurement of transaction price all taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction and collected by us from a customer.
Note 4: Leases
We lease office space, vehicles and other equipment under non-cancellable leases with initial terms typically ranging from 1 to 20 years, with some leases having terms greater than 20 years. Our lease portfolio includes agreements with renewal options, purchase options and clauses for early termination based on the terms specific to the agreement.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
At contract inception, we review the facts and circumstances of the arrangement to determine if the contract is a lease. We follow the guidance in ASC 842-10-15 and consider the following: whether the contract has an identified asset; if we have the right to obtain substantially all economic benefits from the asset; and if we have the right to direct the use of the underlying asset. When determining if a contract has an identified asset, we consider both explicit and implicit assets, and whether the supplier has the right to substitute the asset. When determining if we have the right to obtain substantially all economic benefits from the asset, we consider the primary outputs of the identified asset throughout the period of use and determine if we receive greater than 90 percent of those benefits. When determining if we have the right to direct the use of an underlying asset, we consider if we have the right to direct how and for what purpose the asset is used throughout the period of use and if we control the decision-making rights over the asset. All leased assets are classified as operating or finance under ASC 842. The lease term is determined as the non-cancellable period of the lease, together with all of the following: periods covered by an option to extend the lease which are reasonably certain to be exercised, periods covered by an option to terminate the lease if the lessee is reasonably certain not to exercise that option, and periods covered by an option to extend (or not to terminate) the lease in which exercise of the option is controlled by the lessor. At commencement, we assess whether any options included in the lease are reasonably certain to be exercised by considering all economic factors relevant including, contract-based, asset-based, market-based, and company-based factors.
To determine the present value of future minimum lease payments, we use the implicit rate when readily determinable or our incremental borrowing rate at the lease commencement date. When determining our incremental borrowing rate, we consider our centralized treasury function and our current credit profile. We then make adjustments to this rate for securitization, the length of the lease term, and leases denominated in foreign currencies. Minimum lease payments are expensed over the term of the lease on a straight-line basis. Some leases may require additional contingent or variable lease payments based on factors specific to the individual agreement. Variable lease payments for which we are typically responsible for include payment of vehicle insurance, real estate taxes, and maintenance expenses.
Most leases within our portfolio are classified as operating leases under the new standard. Operating leases are included in "Other assets including long-term receivables, net", "Accrued and other liabilities", and "Other long-term liabilities" in our consolidated balance sheet. Operating lease right-of-use ("ROU") assets are subsequently measured throughout the lease term at the carrying amount of the lease liability, plus initial direct costs, plus (minus) any prepaid (accrued) lease payments, less the unamortized balance of any lease incentives received. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
Operating leases relate to office spaces, IT equipment, transportation equipment, machinery equipment, furniture and fixtures, and plant and facilities under non-cancellable lease agreements. Leases primarily have fixed rental periods, with many of the real estate leases requiring additional payments for property taxes and occupancy-related costs. Leases for real estate typically have initial terms ranging from 1 to 20 years, with some leases having terms greater than 20 years. Leases for non-real estate (transportation, IT) typically have initial terms ranging from 1 to 10 years. We have elected not to record short-term leases on the balance sheet whose term is 12 months or less and does not include a purchase option or extension that is reasonably certain to be exercised.
We rent or sublease a small number of assets including equipment and office space to third party companies. These third-party arrangements include a small number of transition service arrangements from recent acquisitions. We also sublease a floor of our Corporate headquarters to our former subsidiary, Livent Corporation. Rental income from all subleases is not material to our business.
The ROU asset and lease liability balances as of December 31, 2020 were as follows:
| | | | | | | | | | | | | | | | | | |
| (in Millions) | | Classification | | December 31, 2020 | | December 31, 2019 |
| Assets | | | | | | |
| | | | | | |
| Operating lease ROU assets | | Other assets including long-term receivables, net | | $ | 147.3 | | | $ | 164.7 | |
| | | | | | |
| Liabilities | | | | | | |
| | | | | | |
| Operating lease current liabilities | | Accrued and other liabilities | | $ | 25.6 | | | $ | 31.5 | |
| | | | | | |
| | | | | | |
| | | | | | |
| Operating lease noncurrent liabilities | | Other long-term liabilities | | 151.1 | | | 163.2 | |
| | | | | | |
| | | | | | |
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The components of lease expense for the year ended December 31, 2020 were as follows:
| | | | | | | | | | | | | | | | | | | |
| (in Millions) | Lease Cost Classification | | 2020 | | 2019 | | |
| Lease Cost | | | | | | | |
| Operating lease cost | Costs of sales and services / Selling, general and administrative expenses | | $ | 39.5 | | | $ | 41.3 | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Variable lease cost | Costs of sales and services / Selling, general and administrative expenses | | 4.7 | | | 5.2 | | | |
| | | | | | | |
| Total lease cost | | | $ | 44.2 | | | $ | 46.5 | | | |
| | | | | | | | | | |
| | December 31, 2020 | | |
| Operating Lease Term and Discount Rate | | | | |
| Weighted-average remaining lease term (years) | | 9.6 | | |
| | | | |
| | | | |
| Weighted-average discount rate | | 4.2 | % | | |
| | | | |
| | | | |
| | | | | | | | |
| (in Millions) | Year ended December 31, 2020 | Year ended December 31, 2019 |
| Other Information | | |
| | |
| Cash paid for amounts included in the measurement of lease liabilities: | | |
| Operating cash flows from operating leases | $ | (40.8) | | $ | (42.3) | |
| | |
| | |
| Supplemental non-cash information on lease liabilities arising from obtaining right-of-use assets: | | |
| Right-of-use assets obtained in exchange for new operating lease liabilities | $ | 8.4 | | $ | 15.7 | |
| | |
The following table represents our future minimum operating lease payments as of, and subsequent to, December 31, 2020 under ASC 842:
| | | | | | | | | |
| (in Millions) | | | | | Operating Leases Total |
| Maturity of Lease Liabilities | | | | | |
| 2021 | | | | | $ | 31.7 | |
| 2022 | | | | | 27.3 | |
| 2023 | | | | | 21.5 | |
| 2024 | | | | | 17.6 | |
| 2025 | | | | | 16.8 | |
| Thereafter | | | | | 103.5 | |
| Total undiscounted lease payments | | | | | $ | 218.4 | |
| Less: Present value adjustment | | | | | (41.7) | |
| Present value of lease liabilities | | | | | $ | 176.7 | |
Rent expense for operating leases under ASC 840 (the previous U.S. GAAP lease accounting guidance) was $40 million for the year ended December 31, 2018.
Note 5: Acquisitions
DuPont Crop Protection Business
On November 1, 2017, pursuant to the terms and conditions set forth in the Transaction Agreement entered into with E. I. du Pont de Nemours and Company ("DuPont"), we completed the acquisition of certain assets relating to DuPont's Crop Protection business and research and development ("R&D") organization (the "DuPont Crop Protection Business") (collectively, the "DuPont Crop Protection Business Acquisition"). In connection with this transaction, we sold to DuPont our FMC Health and
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Nutrition segment and paid DuPont $1.2 billion in cash which was funded with the 2017 Term Loan Facility which was secured for the purposes of the Acquisition.
The DuPont Crop Protection Business has been integrated into our business and has been included within our results of operations since the date of acquisition.
As part of the DuPont Crop Protection Business Acquisition, we acquired various manufacturing contracts. The manufacturing contracts have been recognized as an asset or liability to the extent the terms of the contract are favorable or unfavorable compared with market terms of the same or similar items at the date of the acquisition.
We also entered into supply agreements with DuPont, with terms of up to five years, to supply technical insecticide products required for their retained seed treatment business at cost. The unfavorable liability is recorded within both "Accrued and other liabilities" and "Other long-term liabilities" on the consolidated balance sheets and is reduced and recognized to revenues within earnings as sales are made. The amount recognized in revenue for the years ended December 31, 2020, 2019, and 2018 was approximately $111 million, $105 million, and $92 million, respectively.
Transaction-related charges
Pursuant to U.S. GAAP, costs incurred associated with acquisition activities are expensed as incurred. Historically, these costs have primarily consisted of legal, accounting, consulting, and other professional advisory fees associated with the preparation and execution of these activities. Given the significance and complexity around the integration of the DuPont Crop Protection Business, we have incurred costs associated with integrating the DuPont Crop Protection Business, which included planning for the termination of the transitional service agreement ("TSA") as well as implementation of a new worldwide Enterprise Resource Planning ("ERP") system in connection with the termination of the TSA, the majority of which were capitalized in accordance with the relevant accounting literature.
The following table summarizes the costs incurred associated with these activities:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| | | | | |
| DuPont Crop Protection Business Acquisition | | | | | |
Legal and professional fees (1) | $ | 53.3 | | | $ | 77.8 | | | $ | 86.9 | |
Inventory fair value amortization (2) | — | | | — | | | 69.6 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Total transaction-related charges | $ | 53.3 | | | $ | 77.8 | | | $ | 156.5 | |
| Restructuring charges | | | | | |
DuPont Crop restructuring (3) | $ | 40.2 | | | $ | 26.4 | | | $ | 108.3 | |
| | | | | |
| Total restructuring charges | $ | 40.2 | | | $ | 26.4 | | | $ | 108.3 | |
____________________
(1)Represents transaction costs, costs for transitional employees, other acquired employees related costs, and transactional-related costs such as legal and professional third-party fees. These charges are recorded as a component of "Selling, general and administrative expense" on the consolidated statements of income (loss).
(2)These charges are included in "Costs of sales and services" on the consolidated statements of income (loss).
(3)See Note 9 for more information. These charges are recorded as a component of "Restructuring and other charges (income)" on the consolidated statements of income (loss).
Except for the completion of certain in-flight initiatives, primarily associated with the finalization of our worldwide ERP system, we completed the integration of the DuPont Crop Protection Business as of June 30, 2020. As noted, the TSA is now terminated and the last phase of the ERP system transition went live in November 2020 with a stabilization period that will go into the first quarter of 2021. Estimated remaining charges are expected to be less than $5 million for the completion of these defined in-flight initiatives over that time period. We will also have remaining in-flight restructuring charges as we complete the established DuPont Crop Restructuring program associated with integration which are nearing completion. Refer to Note 9 for further information.
As a result of completing the implementation of our worldwide ERP system, we will have a series of delayed restructurings under a separate initiative from those discussed above. These future restructurings are the result of consolidating activities into one system as well as into several shared service centers allowing us to improve productivity and gain efficiencies in our processes. The first wave of this new initiative is anticipated to run through 2021 and is estimated to result in pre-tax severance charges of approximately $5 million to $8 million, of which $3 million was recorded in 2020, primarily due to the fact we will
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
be performing activities in one ERP system as opposed to multiple. Severance associated with the outer years of these restructurings is not expected to be material and will be determined as we progress through the initiative.
Note 6: Goodwill and Intangible Assets
The changes in the carrying amount of goodwill for the years ended December 31, 2020 and 2019 are presented in the table below:
| | | | | | | | | |
| (in Millions) | | | | | Total |
| Balance, December 31, 2018 | | | | | $ | 1,468.1 | |
| | | | | |
| Foreign currency and other adjustments | | | | | (0.6) | |
| | | | | |
| Balance, December 31, 2019 | | | | | $ | 1,467.5 | |
| | | | | |
| | | | | |
| Foreign currency and other adjustments | | | | | 1.4 | |
| Balance, December 31, 2020 | | | | | $ | 1,468.9 | |
Our fiscal year 2020 annual goodwill and indefinite life impairment test was performed during the third quarter ended September 30, 2020. We determined no goodwill impairment existed and that the fair value was substantially in excess of the carrying value. There were no events or circumstances indicating that goodwill might be impaired as of December 31, 2020. Additionally, the estimated fair values also substantially exceeded the carrying value for each of our indefinite-lived intangible assets.
Our intangible assets, other than goodwill, consist of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2020 | | December 31, 2019 |
| (in Millions) | Weighted avg. useful life remaining at December 31, 2020 | Gross | | Accumulated Amortization | | Net | | Gross | | Accumulated Amortization | | Net |
| Intangible assets subject to amortization (finite life) | | | | | | | | | | | | |
| Customer relationships | 16 years | $ | 1,169.4 | | | $ | (249.7) | | | $ | 919.7 | | | $ | 1,139.7 | | | $ | (184.7) | | | $ | 955.0 | |
| Patents | 5 years | 1.9 | | | (1.2) | | | 0.7 | | | 1.7 | | | (0.9) | | | 0.8 | |
Brands (1) | 8 years | 18.3 | | | (8.9) | | | 9.4 | | | 16.7 | | | (6.7) | | | 10.0 | |
| Purchased and licensed technologies | 9 years | 61.1 | | | (38.1) | | | 23.0 | | | 60.2 | | | (35.2) | | | 25.0 | |
| Other intangibles | < 1 year | 3.4 | | | (2.6) | | | 0.8 | | | 1.9 | | | (1.8) | | | 0.1 | |
| | $ | 1,254.1 | | | $ | (300.5) | | | $ | 953.6 | | | $ | 1,220.2 | | | $ | (229.3) | | | $ | 990.9 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Intangible assets not subject to amortization (indefinite life) | | | | | | | | | | | | |
Crop Protection Brands (2) | | $ | 1,259.1 | | | | | $ | 1,259.1 | | | $ | 1,259.1 | | | | | $ | 1,259.1 | |
Brands (1) | | 412.5 | | | | | 412.5 | | | 379.0 | | | | | 379.0 | |
| | | | | | | | | | | | |
| | $ | 1,671.6 | | | | | $ | 1,671.6 | | | $ | 1,638.1 | | | | | $ | 1,638.1 | |
| Total intangible assets | $ | 2,925.7 | | | $ | (300.5) | | | $ | 2,625.2 | | | $ | 2,858.3 | | | $ | (229.3) | | | $ | 2,629.0 | |
____________________
(1) Represents trademarks, trade names and know-how.
(2) Represents proprietary brand portfolios, consisting of trademarks, trade names and know-how, of our crop protection brands.
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Amortization expense | $ | 61.9 | | | $ | 62.6 | | | $ | 62.2 | |
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The estimated pre-tax amortization expense for each of the five years ending December 31, 2021 to 2025 is $64.1 million, $64.1 million, $63.7 million, $62.2 million, and $61.7 million, respectively.
Note 7: Inventories
Inventories consisted of the following:
| | | | | | | | | | | |
| December 31, |
| (in Millions) | 2020 | | 2019 |
| Finished goods | $ | 434.6 | | | $ | 372.2 | |
| Work in process | 621.9 | | | 559.4 | |
| Raw materials, supplies and other | 165.7 | | | 217.3 | |
| FIFO inventory | $ | 1,222.2 | | | $ | 1,148.9 | |
| Less: Excess of FIFO cost over LIFO cost | (126.6) | | | (131.9) | |
| Net inventories | $ | 1,095.6 | | | $ | 1,017.0 | |
Approximately 33 percent and 21 percent of our inventories in 2020 and 2019, respectively, were recorded on the LIFO basis.
Note 8: Property, Plant and Equipment
Property, plant and equipment consisted of the following:
| | | | | | | | | | | |
| December 31, |
| (in Millions) | 2020 | | 2019 |
| Land and land improvements | $ | 103.1 | | | $ | 94.3 | |
| | | |
| Buildings and building equipment | 513.7 | | | 490.1 | |
| Machinery and equipment | 501.1 | | | 459.5 | |
| Construction in progress | 73.6 | | | 65.3 | |
| Total cost | $ | 1,191.5 | | | $ | 1,109.2 | |
| Accumulated depreciation | (419.8) | | | (351.2) | |
| Property, plant and equipment, net | $ | 771.7 | | | $ | 758.0 | |
____________________
Depreciation expense was $71.5 million, $69.7 million, and $73.9 million in 2020, 2019 and 2018, respectively.
Note 9: Restructuring and Other Charges (Income)
The following table shows total restructuring and other charges (income) included in the respective line items of the consolidated statements of income (loss):
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Restructuring charges | $ | 42.6 | | | $ | 62.2 | | | $ | 124.1 | |
| Other charges (income), net | 89.6 | | | 108.8 | | | (62.9) | |
| Total restructuring and other charges (income) | $ | 132.2 | | | $ | 171.0 | | | $ | 61.2 | |
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Restructuring charges
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | |
| (in Millions) | Severance and Employee Benefits | | Other Charges (Income) (1) | | Asset Disposal Charges (2) | | Total |
| DuPont Crop restructuring | $ | 9.2 | | | $ | 3.8 | | | $ | 27.2 | | | $ | 40.2 | |
| | | | | | | |
| Other items | 2.8 | | | — | | | (0.4) | | | 2.4 | |
| Year ended December 31, 2020 | $ | 12.0 | | | $ | 3.8 | | | $ | 26.8 | | | $ | 42.6 | |
| DuPont Crop restructuring | $ | 9.1 | | | $ | 5.2 | | | $ | 12.1 | | | $ | 26.4 | |
Furadan® product exit | — | | | — | | | 34.1 | | | 34.1 | |
| Other items | 1.7 | | | — | | | — | | | 1.7 | |
| Year ended December 31, 2019 | $ | 10.8 | | | $ | 5.2 | | | $ | 46.2 | | | $ | 62.2 | |
| DuPont Crop restructuring | $ | 16.3 | | | $ | 16.9 | | | $ | 75.1 | | | 108.3 | |
| Other items | 5.7 | | | 3.1 | | | 7.0 | | | 15.8 | |
| Year ended December 31, 2018 | $ | 22.0 | | | $ | 20.0 | | | $ | 82.1 | | | $ | 124.1 | |
____________________
(1)Primarily represents third-party costs associated with miscellaneous restructuring activities. Other income, if applicable, primarily represents favorable developments on previously recorded exit costs and recoveries associated with restructuring.
(2)Primarily represents asset write-offs (recoveries), and accelerated depreciation and impairment charges on long-lived assets, which were or are to be abandoned. To the extent incurred, the acceleration effect of re-estimating settlement dates and revised cost estimates associated with asset retirement obligations due to facility shutdowns, are also included within the asset disposal charges.
Furadan® Product Exit
During the fourth quarter of 2019, we decided to exit sales of all carbofuran formulations (including Furadan® insecticide/nematicide, Curaterr® insecticide/nematicide and any other brands used with carbofuran products) globally effective December 31, 2019. As a result of this decision, we accelerated the recognition of asset retirement obligations and asset write offs associated with the exit.
DuPont Crop Restructuring
On November 1, 2017, we completed the acquisition of the DuPont Crop Protection Business. See Note 5 "Acquisitions" for more details. As also discussed in Note 5, we completed the integration of the DuPont Crop Protection Business except for the completion of certain in-flight initiatives including the DuPont Crop restructuring program as of June 30, 2020. Estimated remaining restructuring charges are expected to be less than $5 million primarily associated with accelerated depreciation on certain fixed assets, severance, and other costs as we exit certain facilities.
For the years ended December 31, 2020 and 2019, we incurred restructuring charges of $40.2 million and $26.4 million, respectively, which primarily represented severance and other employee related costs as well as accelerated depreciation on fixed assets for the planned exit of certain facilities.
2018 Activities
For the year ended December 31, 2018, we incurred restructuring charges of $108 million. $63 million of these charges were related to a significant change to how we operate in the market in the combined business in India. On July 3, 2018, we announced the adoption of an innovation-focused product strategy that uses a unique market access model anchored by our key, large scale distributors rather than the vast customer base we served prior to the DuPont Crop Protection Acquisition. Additionally, we rationalized our product portfolio and decisively exited a vast majority of the low margin product range. As a result of the change to our market access, we incurred charges of approximately $59 million, which primarily included the write-off of stranded accounts receivables and inventory. We also had workforce reductions which resulted in severance and other employee benefit charges of approximately $4 million.
$27.8 million of the 2018 restructuring charges related to our decision to migrate our Ewing R&D activities and employees into the newly acquired Stine R&D facilities due to their close proximity to one another. We incurred charges of $17.4 million of accelerated depreciation charges of certain fixed assets that will no longer be used due to our exit from the facility. The cease use criteria was met as of September 30, 2018 as all employees had exited the Ewing facility and the facility became available for use. We recorded the estimated future liability associated with the rental obligation on the cease use date which resulted in a charge of $11.2 million. This charge was offset by the reduction of the capital lease liability previously recorded in "Other long-
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
term liabilities" of $6.0 million. In addition to lease termination costs, we incurred severance, relocation and other employee related charges of $5.2 million for combined charges of $27.8 million for the year for this restructuring initiative.
Roll forward of restructuring reserves
The following table shows a roll forward of restructuring reserves that will result in cash spending. These amounts exclude asset retirement obligations:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | Balance at 12/31/18 | | Change in reserves (3) | | Cash payments | | Other (4) | | Balance at 12/31/19 (5) | | Change in reserves (3) | | Cash payments (5) | | Other (4) | | Balance at 12/31/20 (6) |
DuPont Crop restructuring (1) | $ | 16.2 | | | $ | 14.3 | | | $ | (15.9) | | | $ | (0.1) | | | $ | 14.5 | | | $ | 13.0 | | | $ | (14.2) | | | $ | 0.3 | | | $ | 13.6 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Other workforce related and facility shutdowns (2) | 1.0 | | | 1.7 | | | (2.7) | | | 0.1 | | | 0.1 | | | 2.8 | | | (0.1) | | | — | | | 2.8 | |
| | | | | | | | | | | | | | | | | |
| Total | $ | 17.2 | | | $ | 16.0 | | | $ | (18.6) | | | $ | — | | | $ | 14.6 | | | $ | 15.8 | | | $ | (14.3) | | | $ | 0.3 | | | $ | 16.4 | |
____________________
(1)Primarily consists of real estate exit costs and severance associated with DuPont Crop restructuring activities.
(2)Primarily severance costs related to workforce reductions and facility shutdowns described in the Other items section of the Restructuring charges table above.
(3)Primarily severance, exited lease, contract termination and other miscellaneous exit costs. The accelerated depreciation and impairment charges noted above impacted our property, plant and equipment or intangible balances and are not included in this table.
(4)Primarily foreign currency translation adjustments.
(5)In addition to the spend above there was also $3.6 million of spending related to the Furadan® asset retirement obligation.
(6)Included in "Accrued and other liabilities" and "Other long-term liabilities" on the consolidated balance sheets.
Other charges (income), net
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Environmental charges, net | $ | 24.9 | | | $ | 108.7 | | | $ | 21.7 | |
| Product portfolio sales | — | | | 0.1 | | | (87.2) | |
| | | | | |
| Isagro Fluindapyr Acquisition | 65.6 | | | — | | | — | |
| | | | | |
| Other items, net | (0.9) | | | — | | | 2.6 | |
| Other charges (income), net | $ | 89.6 | | | $ | 108.8 | | | $ | (62.9) | |
Environmental charges, net
Environmental charges represent the net charges associated with environmental remediation at continuing operating sites. Environmental obligations for continuing operations primarily represent obligations at shut down or abandoned facilities within businesses that do not meet the criteria for presentation as discontinued operations. During the fourth quarter of 2019, we recorded a charge of $72.8 million as a result of an unfavorable court ruling we received in relation to the Pocatello Tribal Litigation at one of our environmental sites. We remeasure our discounted liability balance according to current interest rates. See Note 12 for further information regarding this matter.
Isagro Fluindapyr Acquisition
In May 2020, FMC entered into a binding offer with Isagro S.p.A ("Isagro") to acquire the remaining rights for Fluindapyr active ingredient assets from Isagro. In July 2020, we entered into an asset sale and purchase agreement with Isagro. On October 2, 2020, we closed on the transaction with a purchase price of approximately $65 million. Fluindapyr has been jointly developed by FMC and Isagro under a 2012 research and development collaboration agreement. The transaction provides FMC with full global rights to the Fluindapyr active ingredient, including key U.S., European, Asian, and Latin American fungicide markets. The transaction transfers to FMC all intellectual property, know-how, registrations, product formulations and other global assets of the proprietary broad-spectrum fungicide molecule.
The Fluindapyr acquisition does not meet the criteria within ASC 805 to qualify as a business and as a result it is treated as an asset acquisition. Based on the current development stage of the technology, the acquired assets have been classified as in-process research and development. As part of our evaluation, we consider the current development phase of the molecule being acquired. Molecules that have not received formal regulatory approval are still considered in process due to the inherent uncertainty with the approval process. As a result, these assets were immediately expensed. While this transaction resulted in an
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
immediate expense of the purchase price under the accounting rules, this acquisition expands our fungicide portfolio by giving us full global rights to the Fluindapyr active ingredient and is an important strategic addition to our product line. We recorded charges totaling $65.6 million for the year, including transaction costs.
Product portfolio sales
On February 1, 2018, we sold a portion of our European herbicide portfolio to Nufarm Limited. Additionally, on August 16, 2018, we completed the sale of certain products of our India portfolio to Crystal Crop Protection Limited. Both sales were required by regulatory authorities as part of closing conditions for the DuPont Crop Protection Business Acquisition. The gain on these sales are recorded within "Restructuring and other charges (income)" on the consolidated statements of income (loss). Proceeds from these sales are included in investing activities on the consolidated statements of cash flows.
Other items, net
Other items, net in 2020 were not material. In 2018, other items, net primarily represents a milestone payment on an agreement related to our in-process research and development. Other items, net also includes the loss associated with the divestment of a joint venture.
Note 10: Receivables
The following table displays a roll forward of the allowance for doubtful trade receivables for fiscal years 2019 and 2020:
| | | | | |
| (in Millions) | |
| Balance, December 31, 2018 | $ | 22.4 | |
| Additions — charged (credited) to expense | 3.6 | |
| Transfer from (to) allowance for credit losses (see below) | 3.4 | |
| Net recoveries, write-offs and other | (3.1) | |
| Balance, December 31, 2019 | $ | 26.3 | |
| Additions — charged (credited) to expense | 8.2 | |
| Transfer from (to) allowance for credit losses (see below) | (2.9) | |
| Net recoveries, write-offs and other | (3.7) | |
| Balance, December 31, 2020 | $ | 27.9 | |
We have non-current receivables that represent long-term customer receivable balances related to past due accounts which are not expected to be collected within the current year. The net long-term customer receivables were $103.5 million as of December 31, 2020. These long-term customer receivable balances and the corresponding allowance are included in "Other assets including long-term receivables, net" on the consolidated balance sheets.
A portion of these long-term receivables have payment contracts. We have no reason to believe payments will not be made based upon the credit quality of these customers. Additionally, we also hold significant collateral against these customers including rights to property or other assets as a form of credit guarantee. If the customer does not pay or gives indication that they will not pay, these guarantees allow us to start legal action to block the sale of the customer’s harvest. On an ongoing basis, we continue to evaluate the credit quality of our non-current receivables using aging of receivables, collection experience and write-offs, as well as evaluating existing economic conditions, to determine if an additional allowance is necessary.
The following table displays a roll forward of the allowance for credit losses related to long-term customer receivables for fiscal years 2019 and 2020:
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
| | | | | |
(in Millions) | |
| Balance, December 31, 2018 | $ | 60.5 | |
| Additions — charged (credited) to expense | 17.6 | |
| Transfer from (to) allowance for doubtful accounts (see above) | (3.4) | |
| Foreign currency adjustments | (0.5) | |
| Net recoveries, write-offs and other | (13.1) | |
| Balance, December 31, 2019 | $ | 61.1 | |
| Additions — charged (credited) to expense | (3.5) | |
| Transfer from (to) allowance for doubtful accounts (see above) | 2.9 | |
| Foreign currency adjustments | (7.6) | |
| Net recoveries, write-offs and other | (28.2) | |
| Balance, December 31, 2020 | $ | 24.7 | |
Note 11: Discontinued Operations
FMC Lithium (Livent Corporation):
On March 1, 2019, we completed the previously announced distribution of 123 million shares of common stock of Livent as a pro rata dividend on shares of FMC common stock outstanding at the close of business on the record date of February 25, 2019.
The results of our discontinued FMC Lithium operations are summarized below:
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Revenue | $ | — | | | $ | 52.1 | | | $ | 442.5 | |
| Costs of sales and services | — | | | 41.3 | | | 235.4 | |
| | | | | |
Income (loss) from discontinued operations before income taxes (1) | $ | — | | | $ | 1.1 | | | $ | 170.9 | |
| Provision (benefit) for income taxes | — | | | 6.0 | | | 25.5 | |
| Total discontinued operations of FMC Lithium, net of income taxes, before separation-related costs | $ | — | | | $ | (4.9) | | | $ | 145.4 | |
| Separation-related costs and other adjustments of discontinued operations of FMC Lithium, net of income taxes | — | | | (16.4) | | | (28.1) | |
| Discontinued operations of FMC Lithium, net of income taxes | $ | — | | | $ | (21.3) | | | $ | 117.3 | |
| Less: Discontinued operations of FMC Lithium attributable to noncontrolling interests | — | | | — | | | 3.2 | |
| Discontinued operations of FMC Lithium, net of income taxes, attributable to FMC Stockholders | $ | — | | | $ | (21.3) | | | $ | 114.1 | |
____________________
(1) For the year ended December 31, 2018, amount includes $2.5 million of restructuring and other charges (income), and $4.3 million of non-operating pension settlement charges (income).
FMC Health and Nutrition:
On August 1, 2017, we completed the sale of the Omega-3 business to Pelagia AS for $38 million.
On November 1, 2017, we completed the previously disclosed sale of our FMC Health and Nutrition business to DuPont. The sale resulted in a gain of approximately $918 million ($727 million, net of tax). In connection with the sale, we entered into a customary transitional services agreement with DuPont to provide for the orderly separation and transition of various functions and processes. These services have been provided by us to DuPont for 24 months after closing and an additional six months extension. These services included information technology services, accounting, human resource and facility services among other services, while DuPont assumed the operations of FMC Health and Nutrition.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Certain sites were to transfer at a later date due to various local timing constraints. In May 2018, the last site transferred to DuPont. The results of our discontinued FMC Health and Nutrition operations are summarized below, including the results of these delayed sites included in the year ended December 31, 2018.
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Revenue | $ | — | | | $ | — | | | $ | 3.8 | |
| Costs of sales and services | — | | | — | | | 4.0 | |
| | | | | |
Income (loss) from discontinued operations before income taxes (1) | $ | — | | | $ | — | | | $ | 2.0 | |
| Provision (benefit) for income taxes | — | | | — | | | 3.8 | |
Total discontinued operations of FMC Health and Nutrition, net of income taxes, before divestiture related costs and adjustments | $ | — | | | $ | — | | | $ | (1.8) | |
| | | | | |
| Gain on sale of FMC Health and Nutrition, net of income taxes | — | | | — | | | — | |
Adjustment to gain on sale of FMC Health and Nutrition, net of income taxes (2) | — | | | — | | | 7.8 | |
| Divestiture related costs and other adjustments of discontinued operations of FMC Health and Nutrition, net of income taxes | — | | | 0.5 | | | — | |
| Adjustment to FMC Health and Nutrition Omega-3 net assets held for sale, net of income taxes | — | | | — | | | — | |
| | | | | |
| | | | | |
| Discontinued operations of FMC Health and Nutrition, net of income taxes, attributable to FMC Stockholders | $ | — | | | $ | 0.5 | | | $ | 6.0 | |
____________________
(1)Results for the year ended December 31, 2018 include an adjustment to retained liabilities of the disposed FMC Health and Nutrition business.
(2)Amount represents the settlement of working capital adjustments subsequent to the sale.
In addition to our discontinued FMC Lithium and FMC Health and Nutrition segments, our discontinued operations in our financial statements includes adjustments to retained liabilities from previous discontinued operations. The primary liabilities retained include environmental liabilities, other postretirement benefit liabilities, self-insurance, long-term obligations related to legal proceedings and historical restructuring activities.
Our discontinued operations comprised the following:
| | | | | | | | | | | | | | | | | |
| (in Millions) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
Adjustment for workers’ compensation, product liability, and other postretirement benefits and other, net of income tax benefit (expense) of $(10.0), $(23.9) and $(5.2), respectively (1) | $ | 24.7 | | | $ | 4.3 | | | $ | (1.7) | |
Provision for environmental liabilities, net of recoveries, net of income tax benefit (expense) of $6.0, $6.3 and $32.5, respectively (2) | (24.1) | | | (23.5) | | | (121.4) | |
Provision for legal reserves and expenses, net of recoveries, net of income tax benefit (expense) of $7.6, $6.3 and $6.9, respectively | (28.9) | | | (23.3) | | | (26.3) | |
Discontinued operations of FMC Health and Nutrition, net of income tax benefit (expense) of zero, $(0.2) and $(7.1), respectively | — | | | 0.5 | | | 6.0 | |
Discontinued operations of FMC Lithium, net of income tax benefit (expense) of zero, $(12.3) and $(18.0), respectively | — | | | (21.3) | | | 117.3 | |
| Discontinued operations, net of income taxes | $ | (28.3) | | | $ | (63.3) | | | $ | (26.1) | |
____________________
(1)During the year ended December 31, 2020, we finalized the sale of the second of two parcels of land of our discontinued site in Newark, California and recorded a gain of approximately $24 million, net of tax. During the year ended December 31, 2019, we finalized the sale of the first of the two parcels of land of our discontinued site in Newark, California and recorded a gain of approximately $21 million, net of tax.
(2)See a roll forward of our environmental reserves as well as discussion on significant environmental issues that occurred during the year in Note 12.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Reserves for Discontinued Operations, other than Environmental at December 31, 2020 and 2019
| | | | | | | | | | | |
| (in Millions) | December 31, |
| 2020 | | 2019 |
| Workers’ compensation, product liability, and indemnification reserves | $ | 12.9 | | | $ | 15.7 | |
| Postretirement medical and life insurance benefits reserve, net | 5.5 | | | 5.9 | |
| Reserves for legal proceedings | 58.2 | | | 50.3 | |
Reserve for discontinued operations (1) | $ | 76.6 | | | $ | 71.9 | |
____________________
(1)Included in "Other long-term liabilities" on the consolidated balance sheets. Refer to Note 12 for discontinued environmental reserves.
The discontinued postretirement medical and life insurance benefits liability equals the accumulated postretirement benefit obligation. Associated with this liability is a net pre-tax actuarial gain and prior service credit of $4.4 million ($3.6 million after-tax) and $5.2 million ($4.2 million after-tax) at December 31, 2020 and 2019, respectively.
Net spending in 2020, 2019 and 2018 was $1.0 million, $3.8 million and $5.4 million, respectively, for workers’ compensation, product liability and other claims; $0.5 million, $0.4 million and $1.1 million, respectively, for other postretirement benefits; and $28.4 million, $20.2 million and $21.3 million, respectively, related to reserves for legal proceedings associated with discontinued operations.
Note 12: Environmental Obligations
We are subject to various federal, state, local and foreign environmental laws and regulations that govern emissions of air pollutants, discharges of water pollutants, and the manufacture, storage, handling and disposal of hazardous substances, hazardous wastes and other toxic materials and remediation of contaminated sites. We are also subject to liabilities arising under the Comprehensive Environmental Response, Compensation and Liability Act ("CERCLA") and similar state laws that impose responsibility on persons who arranged for the disposal of hazardous substances, and on current and previous owners and operators of a facility for the clean-up of hazardous substances released from the facility into the environment. We are also subject to liabilities under the Resource Conservation and Recovery Act ("RCRA") and analogous state laws that require owners and operators of facilities that have treated, stored or disposed of hazardous waste pursuant to a RCRA permit to follow certain waste management practices and to clean up releases of hazardous substances into the environment associated with past or present practices. In addition, when deemed appropriate, we enter certain sites with potential liability into voluntary remediation compliance programs, which are also subject to guidelines that require owners and operators, current and previous, to clean up releases of hazardous substances into the environment associated with past or present practices.
Environmental liabilities consist of obligations relating to waste handling and the remediation and/or study of sites at which we are alleged to have released or disposed of hazardous substances. These sites include current operations, previously operated sites, and sites associated with discontinued operations. We have provided reserves for potential environmental obligations that we consider probable and for which a reasonable estimate of the obligation can be made. Accordingly, total reserves of $574.7 million and $595.8 million, respectively, before recoveries, existed at December 31, 2020 and 2019.
The estimated reasonably possible environmental loss contingencies, net of expected recoveries, exceed amounts accrued by approximately $170 million at December 31, 2020. This reasonably possible estimate is based upon information available as of the date of the filing but the actual future losses may be higher given the uncertainties regarding the status of laws, regulations, enforcement policies, the impact of potentially responsible parties, technology and information related to individual sites.
Additionally, although potential environmental remediation expenditures in excess of the reserves and estimated loss contingencies could be significant, the impact on our future consolidated financial results is not subject to reasonable estimation due to numerous uncertainties concerning the nature and scope of possible contamination at many sites, identification of remediation alternatives under constantly changing requirements, selection of new and diverse clean-up technologies to meet compliance standards, the timing of potential expenditures and the allocation of costs among Potentially Responsible Parties ("PRPs") as well as other third parties. The liabilities arising from potential environmental obligations that have not been reserved for at this time may be material to any one quarter's or year's results of operations in the future. However, we believe any liability arising from such potential environmental obligations is not likely to have a material adverse effect on our liquidity or financial condition as it may be satisfied over many years.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The table below is a roll forward of our total environmental reserves, continuing and discontinued, from December 31, 2017 to December 31, 2020.
| | | | | |
| (in Millions) | Operating and Discontinued Sites Total |
| Total environmental reserves, net of recoveries at December 31, 2017 | $ | 411.8 | |
| 2018 | |
| Provision | 178.2 | |
| Spending, net of recoveries | (65.7) | |
| |
| Foreign currency translation adjustments | (2.8) | |
| Net Change | $ | 109.7 | |
| Total environmental reserves, net of recoveries at December 31, 2018 | $ | 521.5 | |
| |
| 2019 | |
| Provision | 138.8 | |
| Spending, net of recoveries | (73.8) | |
| |
| Foreign currency translation adjustments | (0.7) | |
| Net Change | $ | 64.3 | |
| Total environmental reserves, net of recoveries at December 31, 2019 | $ | 585.8 | |
| |
| 2020 | |
| Provision | 53.2 | |
| Spending, net of recoveries | (81.1) | |
| |
| Foreign currency translation adjustments and other adjustments | 6.5 | |
| Net Change | $ | (21.4) | |
| Total environmental reserves, net of recoveries at December 31, 2020 | $ | 564.4 | |
To ensure we are held responsible only for our equitable share of site remediation costs, we have initiated, and will continue to initiate, legal proceedings for contributions from other PRPs. At December 31, 2020 and 2019, we have recorded recoveries representing probable realization of claims against U.S. government agencies, insurance carriers and other third parties. Recoveries are recorded as either an offset to the "Environmental liabilities, continuing and discontinued" or as "Other assets including long-term receivables, net" on the consolidated balance sheets.
The table below is a roll forward of our total recorded recoveries from December 31, 2018 to December 31, 2020:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | December 31, 2018 | | Increase (Decrease) in Recoveries | | Cash Received | | Other | | December 31, 2019 | | Increase (Decrease) in Recoveries | | Cash Received (2) | | | | December 31, 2020 |
| Environmental liabilities, continuing and discontinued | $ | 7.9 | | | $ | 2.6 | | | $ | (0.5) | | | $ | — | | | $ | 10.0 | | | $ | 0.9 | | | $ | (0.6) | | | | | $ | 10.3 | |
Other assets (1) | 30.5 | | | 0.3 | | | (3.8) | | | 0.3 | | | 27.3 | | | (1.8) | | | (21.1) | | | | | 4.4 | |
| Total | $ | 38.4 | | | $ | 2.9 | | | $ | (4.3) | | | $ | 0.3 | | | $ | 37.3 | | | $ | (0.9) | | | $ | (21.7) | | | | | $ | 14.7 | |
______________
(1) The amounts are included within "Prepaid and other current assets" and "Other assets including long-term receivables, net" on the consolidated balance sheets. See Note 22 for more details.
(2) During the first quarter of 2020, we entered into a confidential insurance settlement pertaining to coverage at a legacy environmental site, which settlement resulted in a cash payment to FMC in the amount of $20.0 million.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The table below provides detail of current and long-term environmental reserves, continuing and discontinued.
| | | | | | | | | | | |
| December 31, |
| (in Millions) | 2020 | | 2019 |
Environmental reserves, current, net of recoveries (1) | $ | 120.9 | | | $ | 115.3 | |
Environmental reserves, long-term continuing and discontinued, net of recoveries (2) | 443.5 | | | 470.5 | |
| Total environmental reserves, net of recoveries | $ | 564.4 | | | $ | 585.8 | |
______________
(1)These amounts are included within "Accrued and other liabilities" on the consolidated balance sheets.
(2)These amounts are included in "Environmental liabilities, continuing and discontinued" on the consolidated balance sheets.
Our net environmental provisions relate to costs for the continued remediation of both operating sites and for certain discontinued manufacturing operations from previous years. The net provisions are comprised as follows:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
Continuing operations (1) | $ | 24.9 | | | $ | 108.7 | | | $ | 21.7 | |
Discontinued operations (2) | 30.1 | | | 29.8 | | | 153.9 | |
| Net environmental provision | $ | 55.0 | | | $ | 138.5 | | | $ | 175.6 | |
______________
(1)Recorded as a component of "Restructuring and other charges (income)" on our consolidated statements of income. See Note 9. Environmental obligations for continuing operations primarily represent obligations at shut down or abandoned facilities within businesses that do not meet the criteria for presentation as discontinued operations.
(2)Recorded as a component of "Discontinued operations, net of income taxes" on our consolidated statements of income (loss). See Note 11.
On our consolidated balance sheets, the net environmental provisions affect assets and liabilities as follows:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
Environmental reserves (1) | $ | 53.2 | | | $ | 138.8 | | | $ | 178.2 | |
Other assets (2) | 1.8 | | | (0.3) | | | (2.6) | |
| Net environmental provision | $ | 55.0 | | | $ | 138.5 | | | $ | 175.6 | |
______________
(1)See above roll forward of our total environmental reserves as presented on our consolidated balance sheets.
(2)Represents certain environmental recoveries. See Note 22 for details of "Other assets including long-term receivables, net" as presented on our consolidated balance sheets.
Significant Environmental Sites
Pocatello
From 1949 until 2001, we operated the world's largest elemental phosphorus plant in Power County, Idaho, just outside the city of Pocatello. Since the plant's closure, FMC has worked with the EPA, the State of Idaho, and the Shoshone-Bannock Tribes ("Tribes") to develop a proposed cleanup plan for the property. In September 2012, the EPA issued an Interim Record of Decision ("IROD") that is environmentally protective and that ensures the health and safety of both workers and the general public. Since the plant's closure, we have successfully decommissioned our Pocatello plant, completed closure of the RCRA ponds and formally requested that the EPA acknowledge completion of work under a June 1999 RCRA Consent Decree. Future remediation costs include completion of the IROD that addresses groundwater contamination and existing waste disposal areas on the Pocatello plant portion of the Eastern Michaud Flats Superfund Site. In June 2013, the EPA issued a Unilateral Administrative Order to us under which we will implement the IROD remedy. Our current reserves factor in the estimated costs associated with implementing the IROD. In addition to implementing the IROD, we continue to conduct work pursuant to CERCLA unilateral administrative orders to address air emissions from beneath the cap of several of the closed RCRA ponds. Actions also involve impacts of the Tribal Litigation discussed below.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The amount of the reserve for this site, which includes the Pocatello Tribal Litigation described below, was $117.8 million and $107.5 million at December 31, 2020 and 2019, respectively.
Pocatello Tribal Litigation
For a number of years, we engaged in disputes with the Tribes concerning their attempts to regulate our activities on the reservation. On March 6, 2006, a U.S. District Court Judge found that the Tribes were a third-party beneficiary of a 1998 RCRA Consent Decree and ordered us to apply for any applicable Tribal permits relating to the nearly-complete RCRA Consent Decree work. The third-party beneficiary ruling was later reversed by the Ninth Circuit Court of Appeals, but the permitting process continued in the tribal legal system. We applied for the tribal permits, but preserved objections to the Tribes' jurisdiction.
In addition, in 1998, we entered into an agreement that required us to pay the Tribes $1.5 million per year for waste generated from operating our Pocatello plant and stored on site. We paid $1.5 million per year until December 2001 when the plant closed. In our view the agreement was terminated, as the plant was no longer generating waste. The Tribes claimed that the 1998 Agreement has no end date.
On April 25, 2006, the Tribes' Land Use Policy Commission issued us a Special Use Permit for the "disposal and storage of waste" at the Pocatello plant and imposed a $1.5 million per annum permit fee.
FMC challenged this fee at various levels of the Tribal Court system and in April 2014, the Shoshone-Bannock Tribal Appellate Court issued a Statement of Decision finding in favor of the Tribes’ jurisdiction over FMC and awarding costs on appeal to the Tribes. The Tribal Appellate Court conducted further post-trial proceedings and on May 6, 2014 issued Finding and Conclusions and a Final Judgment consistent with its earlier Statement of Decision. FMC challenged the Final Judgment in the United States District Court for the District of Idaho.
On September 28, 2017, the District Court issued a decision finding that the Tribal Court has jurisdiction over FMC to require FMC to pay the $1.5 million per year fee to the Tribes. In 2017, we appealed to the United States Court of Appeals for the Ninth Circuit and oral arguments were held on May 17, 2019. On November 15, 2019, the Ninth Circuit affirmed the District Court's decision that the Tribal Court has jurisdiction over FMC to require FMC to pay the $1.5 million per year fee to the Tribes. As a result of the unfavorable court decision issued on November 15, 2019, we increased our reserves by $72.8 million, which represents both the historical and discounted present value of future annual use permit fees as well as the associated legal costs incurred through December 31, 2019. The increase in reserve was transferred from the previously estimated reasonably possible loss related to this matter. Following the Ninth Circuit's denial of our petition for rehearing en banc, we filed a motion to stay the mandate with the Ninth Circuit. On February 4, 2020, the Ninth Circuit granted our motion to stay the mandate. Because this stay was granted, payment of the judgment was not required until final disposition by the United States Supreme Court.
On March 16, 2020, FMC filed a petition in the United States Supreme Court to review the Ninth Circuit’s decision. On June 29, 2020, the Supreme Court invited the Solicitor General to file a brief in this case expressing the views of the United States with respect to the litigation. The recommendation filed by the Solicitor General on December 9, 2020 was that our petition for review by the Supreme Court should not be granted. On January 11, 2021, the Supreme Court denied our petition to review our case. In the first quarter of 2021, FMC made a $20.5 million payment to the Tribes for attorney's fees and unpaid permit fees incurred from 2002 to 2014. There was no change to our existing reserves as a result of our denied petition.
In calculating the net present value of future annual permit fees, we used a discount rate of 1.45%, which represents the appropriate risk-free rate. We believe that the application of this rate produces a result which approximates the amount that would hypothetically satisfy our liability in an arms-length transaction. The current estimate for expenditures in 2021 is $32.2 million. This includes the $20.5 million court judgement, $10.5 million of incurred past years' permit fees from 2015 through 2021, plus interest associated with these payments. Estimates for expenditures for 2022 and beyond are $1.5 million in annual fees payable each year thereafter. The expected aggregate undiscounted amount related to this matter is $104.5 million of which $82.6 million, on a discounted basis, has been recognized in environmental liabilities on the statements of financial position. The increase in our liability balance from 2019 is primarily due to the remeasurement of our discounted liability using the current U.S. Treasury bill rate.
Middleport
Our Middleport, NY facility is currently an Agricultural Solutions formulation and packaging plant that formerly manufactured arsenic-based and other products. As a result of past manufacturing operations and waste disposal practices at this facility, releases of hazardous substances have occurred at the site that have affected soil, sediment, surface water and groundwater at the facility's property and also in adjacent off-site areas. The impact of our discontinued operations was the subject of an Administrative Order on Consent ("1991 AOC") entered into with the EPA and New York State Department of Environmental Conservation ("NYSDEC", and collectively with EPA, the "Agencies") in 1991, which was replaced by a New Order on
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Consent and Administrative Settlement with the NYSDEC, effective June 6, 2019 ("2019 Order"). Like the 1991 AOC, the 2019 Order requires us to (1) define the nature and extent of contamination caused by our historical plant operations, (2) take interim corrective measures and (3) evaluate Corrective Measure Alternatives ("CMA") for discrete contaminated areas, known as "operable units" of which there are 11.
We have defined the nature and extent of the contamination in certain areas, have constructed an engineered cover, taken certain closure actions regarding RCRA regulated surface water impoundments and are collecting and treating both surface water runoff and ground water. To date, we have evaluated and proposed CMAs for six of the 11 identified operable units.
Middleport Litigation
All pending litigation with respect to the Middleport site was settled and/or dismissed in 2019. The 2019 Order supplanted the need for a separate Hazardous Waste Management Permit ("Part 373 permit"), and as a result, the administrative action challenging the Part 373 Permit was dismissed. In connection with the settlement, FMC also dismissed its claims against the EPA that were pending appeal before the United States Court of Appeals for the Second Circuit. The terms of the 2019 Order are materially consistent with our established reserve for Middleport as of December 31, 2018 as a result of the 2019 Order.
Middleport Reserves
In the fourth quarter of 2018, we increased the reserve by $106.3 million, which included our best estimate for remediation costs for OUs 2,4 and 5 in line with the drafted settlement terms between FMC and NYSDEC. Of the $106.3 million reserve increase, $60.6 million related to our best estimate for remediation costs associated with the operable unit that comprises the southern portion of the tributary ("OU 6") plus the impact of inflation. The $60.6 million increase was in addition to a previously established reserve of $29.1 million related to this operable unit.
The remaining $45.7 million reserve increase related to costs associated with the implementation and completion of NYSDEC’s selected remedy for OUs 2,4, and 5. Prior to settlement discussions, our reserve balance for OUs 2,4, and 5 of $31.1 million included the estimated liability for clean-up to reflect the costs associated with our recommended CMAs. Our total reserve for all of Middleport is $142.7 million and $159.4 million at December 31, 2020 and 2019, respectively. FMC is in various stages of evaluating the remaining operable units.
In 2020 and 2019, the Middleport settlement resulted in cash outflows of $17.9 million and $22.2 million respectively. This settlement will result in cash outflows of approximately $20 million to $30 million for 2021 due to front loading of reimbursement in installments of past costs, and thereafter an amount not to exceed an average of $10 million per year until the remediation is complete.
Other Potentially Responsible Party ("PRP") Sites
We have been named a PRP at 29 sites on the federal government’s National Priorities List ("NPL"), at which our potential liability has not yet been settled. We have received notice from the EPA or other regulatory agencies that we may be a PRP, or PRP equivalent, at other sites, including 47 sites at which we have determined that it is probable that we have an environmental liability for which we have recorded an estimate of our potential liability in the consolidated financial statements. In cooperation with appropriate government agencies, we are currently participating in, or have participated in, a Remedial Investigation/Feasibility Study ("RI/FS"), or equivalent, at most of the identified sites, with the status of each investigation varying from site to site. At certain sites, a RI/FS has only recently begun, providing limited information, if any, relating to cost estimates, timing, or the involvement of other PRPs; whereas, at other sites, the studies are complete, remedial action plans have been chosen, or a ROD has been issued.
One site where FMC is listed as a PRP is the Portland Harbor Superfund Site ("Portland Harbor"), that includes the river and sediments of a 12 mile section of the lower reach of the Willamette River in Portland, Oregon that runs through an industrialized area. Portland Harbor is listed on the NPL. FMC formerly owned and operated a manufacturing site adjacent to this section of the river and has since sold its interest in this business. Currently, FMC and approximately 70 other parties are involved in a non-judicial allocation process to determine each party’s respective share of the cleanup costs. FMC and several other parties have been sued by the Confederated Bands and Tribes of the Yakama Nation for reimbursement of cleanup costs and the costs of performing a natural damage assessment. Based on the information known to date, we are unable to develop a reasonable estimate of our potential exposure of loss at this time. We intend to defend this matter.
On January 6, 2017, EPA issued its Record of Decision ("ROD") for the Portland Harbor Superfund Site. On December 30, 2019, FMC and EPA entered into an Administrative Settlement Agreement and Order on Consent to perform a remedial design for the area at and around FMC's former operations. The cost of developing a work plan for this remedial design and performing predesign investigation work is included in our reserves. Based on the current information available in the ROD as well as the large number of responsible parties for the Superfund Site, we are unable to develop a reasonable estimate of our
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
potential exposure for Portland Harbor at this time. Because of this uncertainty, we cannot say whether the ultimate resolution of our potential obligations at Portland Harbor will have a material adverse effect on our consolidated financial position, liquidity or results of operations. However, adverse results in the outcome of the allocation could have a material adverse effect on our consolidated financial position, results of operations in any one reporting period, or liquidity.
Note 13: Income Taxes
Domestic and foreign components of income (loss) from continuing operations before income taxes are shown below:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Domestic | $ | (36.5) | | | $ | (227.4) | | | $ | (234.9) | |
| Foreign | 766.3 | | | 882.4 | | | 843.3 | |
| Total | $ | 729.8 | | | $ | 655.0 | | | $ | 608.4 | |
The provision (benefit) for income taxes attributable to income (loss) from continuing operations consisted of:
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Current: | | | | | |
| Federal | $ | 24.9 | | | $ | (12.0) | | | $ | 25.1 | |
| Foreign | 91.7 | | | 77.0 | | | 90.0 | |
| State | 0.7 | | | 0.4 | | | (0.4) | |
| Total current | $ | 117.3 | | | $ | 65.4 | | | $ | 114.7 | |
| Deferred: | | | | | |
| Federal | $ | 15.0 | | | $ | (1.2) | | | $ | (4.4) | |
| Foreign | 7.7 | | | 42.7 | | | (30.4) | |
| State | 10.9 | | | 4.6 | | | (9.1) | |
| Total deferred | $ | 33.6 | | | $ | 46.1 | | | $ | (43.9) | |
| Total | $ | 150.9 | | | $ | 111.5 | | | $ | 70.8 | |
The effective income tax rate applicable to income from continuing operations before income taxes was different from the statutory U.S. federal income tax rate due to the factors listed in the following table:
| | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | | | |
| (in Millions) | 2020 | | 2019 | | 2018 | | | | |
| U.S. Federal statutory rate | $ | 153.3 | | | $ | 137.5 | | | $ | 127.8 | | | | | |
Impacts of Tax Cuts and Jobs Act Enactment (1) | — | | | — | | | 7.8 | | | | | |
Foreign earnings subject to different tax rates (2) | (127.6) | | | (137.7) | | | (154.9) | | | | | |
| | | | | | | | | |
| State and local income taxes, less federal income tax benefit | 2.7 | | | (2.9) | | | 1.4 | | | | | |
| Research and development and miscellaneous tax credits | (6.2) | | | (3.8) | | | (3.7) | | | | | |
Tax on dividends, deemed dividends, and GILTI (3) | 46.5 | | | 46.8 | | | 45.5 | | | | | |
| Changes to unrecognized tax benefits | 5.8 | | | (5.4) | | | 2.7 | | | | | |
| Nondeductible expenses | 5.5 | | | 3.5 | | | 12.4 | | | | | |
Change in valuation allowance (4) | 52.1 | | | 49.9 | | | 7.4 | | | | | |
Exchange gains and losses (5) | (2.1) | | | (2.1) | | | 5.7 | | | | | |
| Other | 20.9 | | | 25.7 | | | 18.7 | | | | | |
| Total Tax Provision | $ | 150.9 | | | $ | 111.5 | | | $ | 70.8 | | | | | |
____________________
(1) The tax impacts of the Tax Cuts and Jobs Act ("the Act") were completed in 2018 as permitted by Staff Accounting Bulletin 118.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
(2) A significant amount of our earnings is generated by our foreign subsidiaries (e.g., Singapore, Hong Kong, and Switzerland), which tax earnings at lower statutory rates than the United States federal statutory rate. Our future effective tax rates may be materially impacted by a future change in the composition of earnings from foreign and domestic tax jurisdictions.
(3) The years ended December 31, 2020, 2019 and 2018 includes tax expense of $40.7 million, $41.6 million and $43.8 million, respectively, associated with the global intangible low-taxed income (GILTI) provisions of the Act.
(4) The year ended December 31, 2020 is primarily related to net operating losses with our Brazil operations. The year ended December 31, 2019 is primarily related to net operating losses with limited carryforward associated with our India operations.
(5) Includes the impact of transaction gains or losses on net monetary assets for which no corresponding tax expense or benefit is realized and the tax provision for statutory taxable gains or losses in foreign jurisdictions for which there is no corresponding amount in income before taxes.
Significant components of our deferred tax assets and liabilities were attributable to:
| | | | | | | | | | | |
| | December 31, |
| (in Millions) | 2020 | | 2019 |
| Reserves for discontinued operations, environmental and restructuring | $ | 161.7 | | | $ | 188.3 | |
| Accrued pension and other postretirement benefits | 1.8 | | | 2.4 | |
| | | |
| Capital loss, foreign tax and other credit carryforwards | 5.5 | | | 7.5 | |
| Net operating loss carryforwards | 311.4 | | | 227.0 | |
| Deferred expenditures capitalized for tax | 39.6 | | | 18.7 | |
| Other | 163.3 | | | 163.6 | |
| Deferred tax assets | $ | 683.3 | | | $ | 607.5 | |
| Valuation allowance, net | (335.6) | | | (303.3) | |
| Deferred tax assets, net of valuation allowance | $ | 347.7 | | | $ | 304.2 | |
| Intangibles, Property, plant and equipment, and Investments, net | 468.1 | | | 380.0 | |
| Deferred tax liabilities | $ | 468.1 | | | $ | 380.0 | |
| Net deferred tax assets (liabilities) | $ | (120.4) | | | $ | (75.8) | |
We evaluate our deferred income taxes quarterly to determine if valuation allowances are required or should be adjusted. GAAP accounting guidance requires companies to assess whether valuation allowances should be established against deferred tax assets based on all available evidence, both positive and negative, using a "more likely than not" standard. In assessing the need for a valuation allowance, appropriate consideration is given to all positive and negative evidence related to the realization of deferred tax assets. This assessment considers, among other matters, the nature and severity of current and cumulative losses, forecasts of future profitability, the duration of statutory carryforward periods, and tax planning alternatives. We operate and derive income across multiple jurisdictions. As our business experiences changes in operating results across its geographic footprint, we may encounter losses in jurisdictions that have been historically profitable, and as a result might require additional valuation allowances to be recorded. We are committed to implementing tax planning actions, when deemed appropriate, in jurisdictions that experience losses in order to realize deferred tax assets prior to their expiration.
At December 31, 2020, we had net operating loss and tax credit carryforwards as follows: U.S. state net operating loss carryforwards of $28.5 million (tax-effected) expiring in future tax years through 2040, foreign net operating loss carryforwards of $282.9 million (tax-effected) expiring in various future years, and other tax credit carryforwards of $5.5 million expiring in various future years.
At December 31, 2020, our net valuation allowance was primarily comprised of balances within continuing operations locations of Brazil of $116.8 million, Luxembourg of $30.3 million, U.S. state of $40.8 million, Switzerland of $30.2 million and India of $15.5 million and within discontinued operations in Spain of $70.1 million. The valuation allowance balances at these locations are associated mainly with net operating losses, but in some cases relate to other additional deferred tax assets in the jurisdiction.
We do not provide income taxes for other outside basis differences inherent in our investments in subsidiaries because the investments and related unremitted earnings are essentially permanent in duration or we have concluded that no additional tax liability will arise upon disposal or remittance. Determining the amount of unrecognized deferred tax liability related to any remaining undistributed foreign earnings is not practicable due to the complexity of the hypothetical calculation.
Uncertain Income Tax Positions
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
U.S. GAAP accounting guidance for uncertainty in income taxes prescribes a model for the recognition and measurement of a tax position taken or expected to be taken in a tax return, and provides guidance on derecognition, classification, interest and penalties, disclosure and transition.
We file income tax returns in the U.S. federal jurisdiction, and various states and foreign jurisdictions. The income tax returns for FMC entities taxable in the U.S. and significant foreign jurisdictions are open for examination and adjustment. As of December 31, 2020, the U. S. federal and state income tax returns are open for examination and adjustment for the years 2017 - 2020 and 2000 - 2020, respectively. Our significant foreign jurisdictions, which total 11, are open for examination and adjustment during varying periods from 2010 - 2020.
As of December 31, 2020, we had total unrecognized tax benefits of $76.2 million, of which $34.6 million would favorably impact the effective tax rate from continuing operations if recognized. As of December 31, 2019, we had total unrecognized tax benefits of $68.2 million, of which $29.4 million would favorably impact the effective tax rate if recognized. Interest and penalties related to unrecognized tax benefits are reported as a component of income tax expense. For the years ended December 31, 2020, 2019 and 2018, we recognized interest and penalties of $(1.5) million, $1.4 million, and $0.9 million, respectively, in the consolidated statements of income (loss). As of December 31, 2020 and 2019, we have accrued interest and penalties in the consolidated balance sheets of $13.9 million and $15.4 million, respectively.
Due to the potential for resolution of federal, state, or foreign examinations, and the expiration of various jurisdictional statutes of limitation, it is reasonably possible that our liability for unrecognized tax benefits will decrease within the next 12 months by a range of $33.4 million to $53.1 million.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| | | | | | | | | | | | | | | | | |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Balance at beginning of year | $ | 68.2 | | | $ | 79.1 | | | $ | 84.0 | |
| Increases related to positions taken in the current year | 1.1 | | | 4.1 | | | 11.8 | |
| | | | | |
| Increases and decreases related to positions taken in prior years | 25.7 | | | 3.4 | | | (1.8) | |
| Decreases related to lapse of statutes of limitations | (18.8) | | | (13.0) | | | (13.5) | |
| Settlements during the current year | — | | | (2.8) | | | (1.4) | |
| Decreases for tax positions on dispositions | — | | | (2.6) | | | — | |
Balance at end of year (1) | $ | 76.2 | | | $ | 68.2 | | | $ | 79.1 | |
____________________
(1) At December 31, 2020, 2019, and 2018 we recognized an offsetting non-current asset of $27.4 million, $34.0 million, and $45.3 million respectively, relating to the indirect income tax benefits associated with specific uncertain tax positions presented above.
Note 14: Debt
Debt maturing within one year:
Debt maturing within one year consists of the following:
| | | | | | | | | | | |
| December 31, |
| (in Millions) | 2020 | | 2019 |
Short-term foreign debt (1) | $ | 98.4 | | | $ | 144.9 | |
Commercial paper (2) | 146.3 | | | — | |
| Total short-term debt | $ | 244.7 | | | $ | 144.9 | |
| Current portion of long-term debt | 93.6 | | | 82.8 | |
| Short-term debt and current portion of long-term debt | $ | 338.3 | | | $ | 227.7 | |
____________________
(1) At December 31, 2020, the average effective interest rate on the borrowings was 13.2 percent.
(2) At December 31, 2020, the average effective interest rate on the borrowings was 0.5 percent.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Long-term debt:
Long-term debt consists of the following:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | December 31, 2020 | | December 31, |
Interest Rate Percentage | | Maturity Date | | 2020 | | 2019 |
Pollution control and industrial revenue bonds (less unamortized discounts of $0.1 and $0.2, respectively) | 0.30% - 6.50% | | 2021 - 2032 | | $ | 51.6 | | | $ | 51.6 | |
Senior notes (less unamortized discounts of $1.0 and $1.3, respectively) | 3.20% - 4.50% | | 2022 - 2049 | | 2,199.0 | | | 2,198.7 | |
| | | | | | | |
| 2017 Term Loan Facility | 1.4% | | 2022 | | 700.0 | | | 800.0 | |
Revolving Credit Facility (1) | 2.8% | | 2024 | | — | | | — | |
| | | | | | | |
| Foreign debt | 0% - 6.1% | | 2021 - 2024 | | 92.3 | | | 83.8 | |
| Debt issuance cost | | | | | (19.8) | | | (20.2) | |
| Total long-term debt | | | | | $ | 3,023.1 | | | $ | 3,113.9 | |
| Less: debt maturing within one year | | | | | 93.6 | | | 82.8 | |
| Total long-term debt, less current portion | | | | | $ | 2,929.5 | | | $ | 3,031.1 | |
____________________
(1)Letters of credit outstanding under the Revolving Credit Facility totaled $214.1 million and available funds under this facility were $1,139.6 million at December 31, 2020.
Revolving Credit Facility Agreement Amendment
On April 22, 2020, the Company entered into Amendment No. 1 (the "Revolving Credit Amendment") to the Third Amended and Restated Credit Agreement, dated as of May 17, 2019, among the Company, as U.S. Borrower, certain foreign subsidiaries of the Company party thereto, as Euro Borrowers, the lenders (the "Revolving Credit Lenders") and issuing banks party thereto, Citibank, N.A., as administrative agent, Citibank, N.A. and BofA Securities, Inc., as joint lead arrangers, Bank of America, N.A., as syndication agent, and certain other financial institutions party thereto as co-documentation agents (the "Revolving Credit Agreement"). Among other things, the Revolving Credit Amendment amends the maximum leverage ratio financial covenant in the Revolving Credit Agreement and adds a negative covenant restricting purchases of the Company’s stock if at any time the maximum leverage ratio exceeds 3.5 through the period ending June 30, 2021.
2017 Term Loan Agreement Amendment
On April 22, 2020, the Company entered into Amendment No. 2 (the "Term Loan Amendment") to the Term Loan Agreement, dated as of May 2, 2017, among the Company, as U.S. Borrower, certain foreign subsidiaries of the Company party thereto, as Euro Borrowers, the lenders party thereto (the "Term Loan Lenders"), Citibank, N.A., as administrative agent, Citigroup Global Markets Inc. and Merrill Lynch, Pierce, Fenner & Smith Incorporated, as joint lead arrangers, Bank of America, N.A., as syndication agent, and certain other financial institutions party thereto as co-documentation agents (as previously amended, the "Term Loan Agreement"). Among other things, the Term Loan Amendment amends the maximum leverage ratio financial covenant in the Term Loan Agreement and adds a negative covenant restricting purchases of the Company’s stock if at any time the maximum leverage ratio exceeds 3.5 through the period ending June 30, 2021.
Deferred financing fees totaling $3.5 million associated with both amendments have been deferred and is being recognized to interest expense over the life of the agreements.
Maturities of long-term debt
Maturities of long-term debt outstanding, excluding discounts, at December 31, 2020, are $93.6 million in 2021, $1,000.1 million in 2022, $0.2 million in 2023, $400.1 million in 2024, zero in 2025 and $1,550.0 million thereafter.
Covenants
Among other restrictions, the Revolving Credit Facility and 2017 Term Loan Facility contain financial covenants applicable to FMC and its consolidated subsidiaries related to leverage (measured as the ratio of debt to adjusted earnings) and interest coverage (measured as the ratio of adjusted earnings to interest expense). Our actual leverage for the four consecutive quarters ended December 31, 2020 was 2.9 which is below the maximum leverage of 4.25. As amended pursuant to the Revolving Credit Amendment and the Term Loan Amendment discussed above, the maximum leverage ratio has been increased to 4.25
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
through the period ending December 31, 2020. The maximum leverage ratio will step down to 4.0 for the quarter ending March 31, 2021 and then to 3.5 for future quarters. Our actual interest coverage for the four consecutive quarters ended December 31, 2020 was 8.3 which is above the minimum interest coverage of 3.5. We were in compliance with all covenants at December 31, 2020.
Compensating Balance Agreements
We maintain informal credit arrangements in many foreign countries. Foreign lines of credit, which include overdraft facilities, typically do not require the maintenance of compensating balances, as credit extension is not guaranteed but is subject to the availability of funds.
Note 15: Pension and Other Postretirement Benefits
The funded status of our U.S. qualified and nonqualified defined benefit pension plans, our Germany, France, and Belgium defined benefit pension plans, plus our U.S. other postretirement healthcare and life insurance benefit plans for continuing operations, together with the associated balances and net periodic benefit cost recognized in our consolidated financial statements as of December 31, are shown in the tables below.
We are required to recognize in our consolidated balance sheets the overfunded and underfunded status of our defined benefit postretirement plans. The overfunded or underfunded status is defined as the difference between the fair value of plan assets and the projected benefit obligation. We are also required to recognize as a component of other comprehensive income the actuarial gains and losses and the prior service costs and credits that arise during the period.
The following table summarizes the weighted-average assumptions used to determine the benefit obligations at December 31 for the U.S. Plans:
| | | | | | | | | | | |
| Pensions and Other Benefits |
| December 31, |
| 2020 | | 2019 |
| Discount rate qualified | 2.49 | % | | 3.22 | % |
| Discount rate nonqualified plan | 1.62 | % | | 2.74 | % |
| Discount rate other benefits | 1.91 | % | | 2.89 | % |
| Rate of compensation increase | 3.10 | % | | 3.10 | % |
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The following table summarizes the components of our defined benefit postretirement plans and reflect a measurement date of December 31:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Pensions | | Other Benefits (1) |
| | | | December 31, |
| (in Millions) | | | | 2020 | | 2019 | | 2020 | | 2019 |
| Change in projected benefit obligation | | | | | | | | | | |
| Projected benefit obligation at January 1 | | | | $ | 1,379.1 | | | $ | 1,261.3 | | | $ | 15.8 | | | $ | 18.9 | |
| Service cost | | | | 4.4 | | | 4.2 | | | — | | | — | |
| Interest cost | | | | 36.7 | | | 47.6 | | | 0.4 | | | 0.6 | |
Actuarial loss (gain) (2) | | | | 115.5 | | | 153.0 | | | 0.3 | | | (2.2) | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| Plan participants’ contributions | | | | — | | | — | | | 0.5 | | | 0.4 | |
| | | | | | | | | | |
| Settlements | | | | (1.5) | | | (3.5) | | | — | | | — | |
| | | | | | | | | | |
| | | | | | | | | | |
| Benefits paid | | | | (83.9) | | | (83.5) | | | (1.7) | | | (1.9) | |
| Projected benefit obligation at December 31 | | | | $ | 1,450.3 | | | $ | 1,379.1 | | | $ | 15.3 | | | $ | 15.8 | |
| Change in plan assets | | | | | | | | | | |
| Fair value of plan assets at January 1 | | | | $ | 1,390.6 | | | $ | 1,269.7 | | | $ | — | | | $ | — | |
| Actual return on plan assets | | | | 176.5 | | | 196.2 | | | — | | | — | |
| Foreign currency exchange rate changes | | | | — | | | (0.2) | | | — | | | — | |
| Company contributions | | | | 2.9 | | | 11.9 | | | 1.2 | | | 1.5 | |
| Plan participants’ contributions | | | | — | | | — | | | 0.5 | | | 0.4 | |
| | | | | | | | | | |
| Settlements | | | | (1.5) | | | (3.5) | | | — | | | — | |
| | | | | | | | | | |
| Benefits paid | | | | (83.9) | | | (83.5) | | | (1.7) | | | (1.9) | |
| Fair value of plan assets at December 31 | | | | $ | 1,484.6 | | | $ | 1,390.6 | | | $ | — | | | $ | — | |
| Funded Status | | | | | | | | | | |
| U.S. plans with assets | | | | $ | 69.5 | | | $ | 44.2 | | | $ | — | | | $ | — | |
| U.S. plans without assets | | | | (23.9) | | | (22.4) | | | (15.3) | | | (15.8) | |
| Non-U.S. plans with assets | | | | (3.0) | | | (1.3) | | | — | | | — | |
| All other plans | | | | (8.3) | | | (9.0) | | | — | | | — | |
| Net funded status of the plan (liability) | | | | $ | 34.3 | | | $ | 11.5 | | | $ | (15.3) | | | $ | (15.8) | |
| Amount recognized in the consolidated balance sheets: | | | | | | | | | | |
Pension asset (3) | | | | $ | 69.5 | | | $ | 44.2 | | | $ | — | | | $ | — | |
Accrued benefit liability (4) | | | | (35.2) | | | (32.7) | | | (15.3) | | | (15.8) | |
| Total | | | | $ | 34.3 | | | $ | 11.5 | | | $ | (15.3) | | | $ | (15.8) | |
____________________
(1) Refer to Note 11 for information on our discontinued postretirement benefit plans.
(2) The actuarial loss in 2020 and 2019 was primarily driven by the change in discount rate on the U.S. qualified plan. Additionally, the Society of Actuaries released an updated mortality table projection scale for measurement of retirement program obligations in both 2020 and 2019. Adoption of the most recent projection scale for each applicable year increased the U.S. defined benefit obligations by approximately $10 million and $13 million at December 31, 2020 and 2019, respectively.
(3) Recorded as "Other assets including long-term receivables, net" on the consolidated balance sheets.
(4) Recorded as "Accrued pension and other postretirement benefits, current" and "Accrued pension and other postretirement benefits, long-term" on the consolidated balance sheets.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The amounts in accumulated other comprehensive income (loss) that have not yet been recognized as components of net periodic benefit cost are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Pensions | | Other Benefits (1) |
| | December 31, |
| (in Millions) | 2020 | | 2019 | | 2020 | | 2019 |
| | | | | | | |
| | | | | | | |
| Prior service (cost) credit | $ | (0.7) | | | $ | (0.9) | | | $ | — | | | $ | — | |
| Net actuarial (loss) gain | (321.9) | | | (367.3) | | | 4.2 | | | 5.5 | |
| Accumulated other comprehensive income (loss) – pretax | $ | (322.6) | | | $ | (368.2) | | | $ | 4.2 | | | $ | 5.5 | |
Accumulated other comprehensive income (loss) – net of tax (2) | (240.7) | | | (277.2) | | | 2.7 | | | 3.7 | |
____________________
(1) Refer to Note 11 for information on our discontinued postretirement benefit plans.
(2) Accumulated other comprehensive income (loss) - net of tax as of December 31, 2019 includes the reclassification of stranded income tax effects. See Note 2 for more information.
The accumulated benefit obligation for all pension plans was $1,435.9 million and $1,364.2 million at December 31, 2020 and 2019, respectively.
| | | | | | | | | | | |
| (in Millions) | December 31 |
| Information for pension plans with projected benefit obligation in excess of plan assets | 2020 | | 2019 |
| Projected benefit obligations | $ | 42.9 | | | $ | 37.2 | |
| Accumulated benefit obligations | 43.3 | | | 37.5 | |
| Fair value of plan assets | 7.7 | | | 4.5 | |
| | | | | | | | | | | |
| (in Millions) | December 31 |
| Information for pension plans with accumulated benefit obligation in excess of plan assets | 2020 | | 2019 |
| Projected benefit obligations | $ | 42.9 | | | $ | 37.2 | |
| Accumulated benefit obligations | 43.3 | | | 37.5 | |
| Fair value of plan assets | 7.7 | | | 4.5 | |
Other changes in plan assets and benefit obligations for continuing operations recognized in other comprehensive loss (income) are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| | Pensions | | Other Benefits (1) |
| | Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2020 | | 2019 |
| Current year net actuarial loss (gain) | $ | (23.5) | | | $ | 11.0 | | | $ | 0.4 | | | $ | (2.3) | |
| | | | | | | |
| Amortization of net actuarial (loss) gain | (21.3) | | | (12.9) | | | 0.9 | | | 1.0 | |
| Amortization of prior service (cost) credit | (0.2) | | | (0.2) | | | — | | | (0.1) | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Settlement loss | (0.6) | | | (1.4) | | | — | | | — | |
| | | | | | | |
| Total recognized in other comprehensive (income) loss, before taxes | $ | (45.6) | | | $ | (3.5) | | | $ | 1.3 | | | $ | (1.4) | |
| Total recognized in other comprehensive (income) loss, after taxes | (36.5) | | | (3.0) | | | 1.0 | | | (1.1) | |
____________________
(1) Refer to Note 11 for information on our discontinued postretirement benefit plans.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The following table summarizes the weighted-average assumptions used for and the components of net annual benefit cost (income):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | Pensions | | Other Benefits (1) |
| (in Millions, except for percentages) | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 |
| Discount rate | 3.22 | % | | 4.36 | % | | 3.68 | % | | 2.89 | % | | 4.08 | % | | 3.41 | % |
| Expected return on plan assets | 3.00 | % | | 4.25 | % | | 5.00 | % | | — | | | — | | | — | |
| Rate of compensation increase | 3.10 | % | | 3.10 | % | | 3.10 | % | | — | | | — | | | — | |
| Components of net annual benefit cost: | | | | | | | | | | | |
| Service cost | $ | 4.4 | | $ | 4.2 | | $ | 6.3 | | $ | — | | $ | — | | $ | — |
| Interest cost | 36.7 | | 47.6 | | 44.5 | | 0.4 | | 0.6 | | 0.7 |
| Expected return on plan assets | (37.1) | | (53.4) | | (63.0) | | — | | — | | — |
| | | | | | | | | | | |
| Amortization of prior service cost | 0.2 | | 0.2 | | 0.4 | | — | | 0.1 | | (0.1) |
| Amortization of net actuarial and other (gain) loss | 21.4 | | 12.9 | | 16.0 | | (0.9) | | (1.0) | | (0.5) |
| | | | | | | | | | | |
| | | | | | | | | | | |
| Recognized (gain) loss due to settlement | 0.7 | | 1.4 | | 1.8 | | — | | — | | — |
| Net annual benefit cost (income) | $ | 26.3 | | $ | 12.9 | | $ | 6.0 | | $ | (0.5) | | $ | (0.3) | | $ | 0.1 |
___________________
(1) Refer to Note 11 for information on our discontinued postretirement benefit plans.
For the year ended December 31, 2018 we recognized a $4.3 million loss due to curtailment and special termination benefits associated with the planned separation of FMC Lithium which was recorded within "Discontinued operations, net of income taxes" within the consolidated statements of income (loss).
Our U.S. qualified defined benefit pension plan ("U.S. Plan") holds the majority of our pension plan assets. The expected long-term rate of return on these plan assets was 3.00 percent for the year ended December 31, 2020, 4.25 percent for the year ended December 31, 2019, and 5.0 percent for the year ended December 31, 2018 (except for the period between the November 1, 2018 remeasurement and December 31, 2018 during which it was 4.5 percent). The expected long-term rate of return on these plan assets decreased by 1.25 percent in 2020 compared to 2019 primarily due to falling yields on corporate bonds. In developing the assumption for the long-term rate of return on assets for our U.S. Plan, we take into consideration the technical analysis performed by our outside actuaries, including historical market returns, information on the assumption for long-term real returns by asset class, inflation assumptions and expectations for standard deviation related to these best estimates. Given an actively managed investment portfolio, the expected annual rates of return by asset class for our portfolio, assuming an estimated inflation rate of approximately 2.1 percent, is in line with our assumption for the rate of return on assets. The target asset allocation at December 31, 2020 by asset category is 100 percent fixed income investments.
Our U.S. Plan reached fully funded status during 2018. The primary investment strategy is a liability hedging approach with an objective of maintaining the funded status of the plan such that the funded status volatility is minimized and the likelihood that we will be required to make significant contributions to the plan is limited. The portfolio is comprised of 100 percent fixed income securities and cash. Investment performance and related risks are measured and monitored on an ongoing basis through monthly liability measurements, periodic asset liability studies, and quarterly investment portfolio reviews. The increase in our non-operating pension and post retirement charges (income) is attributable to the continued approach of using the smoothed market related value of assets (MRVA) as opposed to the actual fair value of plan assets in the determination of 2020 expense. This continued approach will create some volatility in our non-operating periodic pension cost since our qualified pension plan is 100 percent fixed income securities.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The following tables present our fair value hierarchy for our major categories of pension plan assets by asset class. See Note 19 for the definition of fair value and the descriptions of Level 1, 2 and 3 in the fair value hierarchy.
| | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | December 31, 2020 | | Quoted Prices in Active Markets for Identical Assets (Level 1) | | Significant Other Observable Inputs (Level 2) | | Significant Unobservable Inputs (Level 3) |
| Cash and short-term investments | $ | 24.7 | | | $ | 24.6 | | | $ | 0.1 | | | $ | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Fixed income investments: | | | | | | | |
| Investment contracts | 151.4 | | | — | | | 151.4 | | | — | |
| U.S. Government Securities | 307.0 | | | 297.9 | | | 9.1 | | | — | |
| Mutual funds | 70.5 | | | 70.5 | | | — | | | — | |
| Corporate debt instruments | 931.0 | | | — | | | 931.0 | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Total assets | $ | 1,484.6 | | | $ | 393.0 | | | $ | 1,091.6 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | December 31, 2019 | | Quoted Prices
in Active
Markets for
Identical
Assets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) |
| Cash and short-term investments | $ | 19.8 | | | $ | 19.8 | | | $ | — | | | $ | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Fixed income investments: | | | | | | | |
| Investment contracts | 150.1 | | | — | | | 150.1 | | | — | |
| U.S. Government Securities | 331.0 | | | 294.3 | | | 36.7 | | | — | |
| Mutual funds | 65.2 | | | 65.2 | | | — | | | — | |
| Corporate debt instruments | 824.5 | | | — | | | 824.5 | | | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Total assets | $ | 1,390.6 | | | $ | 379.3 | | | $ | 1,011.3 | | | $ | — | |
We made the following contributions to our pension and other postretirement benefit plans:
| | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 |
| U.S. qualified pension plan | $ | — | | | $ | 7.0 | |
| U.S. nonqualified pension plan | 2.9 | | | 4.9 | |
| Non-U.S. plans | 0.5 | | | — | |
| Other postretirement benefits | 1.2 | | | 1.5 | |
| Total | $ | 4.6 | | | $ | 13.4 | |
The following table reflects the estimated future benefit payments for our pension and other postretirement benefit plans. These estimates take into consideration expected future service, as appropriate:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Estimated Net Future Benefit Payments |
| (in Millions) | 2021 | | 2022 | | 2023 | | 2024 | | 2025 | | 2026 - 2030 |
| Pension Benefits | $ | 90.6 | | | $ | 87.9 | | | $ | 86.1 | | | $ | 86.6 | | | $ | 84.7 | | | $ | 404.6 | |
| Other Benefits | 1.7 | | | 1.6 | | | 1.5 | | | 1.4 | | | 1.3 | | | 5.0 | |
FMC Corporation Savings and Investment Plan. The FMC Corporation Savings and Investment Plan is a qualified salary-reduction plan under Section 401(k) of the Internal Revenue Code in which substantially all of our U.S. employees may participate by contributing a portion of their compensation. For eligible employees participating in the Plan, except for those employees covered by certain collective bargaining agreements, the Company makes matching contributions of 80 percent of the portion of those contributions up to 5 percent of the employee’s compensation. Eligible employees participating in the Plan that do not participate in the U.S. qualified pension plan are entitled to receive an employer contribution of 5 percent of the
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
employee’s eligible compensation. Charges against income for all contributions were $16.6 million in 2020, $15.3 million in 2019, and $15.0 million in 2018.
Note 16: Share-based Compensation
Stock Compensation Plans
We have a share-based compensation plan, which has been approved by the stockholders, for certain employees, officers and directors. This plan is described below.
FMC Corporation Incentive Compensation and Stock Plan
The FMC Corporation Incentive Compensation and Stock Plan (the "Plan") provides for the grant of a variety of cash and equity awards to officers, directors, employees and consultants, including stock options, restricted stock, performance units (including restricted stock units), stock appreciation rights, and multi-year management incentive awards payable partly in cash and partly in common stock. The Compensation and Organization Committee of the Board of Directors (the "Committee"), subject to the provisions of the Plan, approves financial targets, award grants, and the times and conditions for payment of awards to employees. The total number of shares of common stock authorized for issuance under the Plan is 30.2 million of which approximately 3.2 million shares of common stock are available for future grants of share based awards under the Plan as of December 31, 2020. The FMC Corporation Non-Employee Directors’ Compensation Policy, administered by the Nominating and Corporate Governance Committee of the Board of Directors, sets forth the compensation to be paid to the directors, including stock options, stock appreciation rights, restricted stock, restricted stock units, performance-based restricted stock units, and cash awards to be made to directors under the Plan.
Stock options granted under the Plan may be incentive or nonqualified stock options. The exercise price for stock options may not be less than the fair market value of the stock at the date of grant. Awards granted under the Plan vest or become exercisable or payable at the time designated by the Committee, which has generally been three years from the date of grant. Incentive and nonqualified options granted under the Plan expire no later than 10 years from the grant date.
Under the Plan, awards of restricted stock and restricted stock units may be made to selected employees. The awards vest over periods designated by the Committee, which has generally been three years, with vesting conditional upon continued employment. Compensation cost is recognized over the vesting periods based on the market value of the stock on the date of the award. Restricted stock units granted to directors under the Plan vest immediately if granted as part of, or in lieu of, the annual retainer; other restricted stock units granted to directors vest at the Annual Meeting of Shareholders in the calendar year following the May 1 annual grant date (but are subject to forfeiture on a pro rata basis if the director does not serve the full year except under certain circumstances).
At December 31, 2020 and 2019, there were restricted stock units representing an aggregate of 267,988 shares and 276,145 shares of common stock, respectively, credited to the directors’ accounts.
Stock Compensation
We recognized the following stock compensation expense:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
Stock option expense, net of taxes of $1.1, $1.5 and $1.3 (1) | $ | 4.0 | | | $ | 5.7 | | | $ | 4.9 | |
Restricted stock expense, net of taxes of $2.0, $2.2 and $2.3 (2) | 7.4 | | | 8.2 | | | 8.4 | |
Performance based expense, net of taxes of $0.9, $1.7 and $1.2 | 3.5 | | | 6.3 | | | 4.4 | |
| | | | | |
Total stock compensation expense, net of taxes of $4.0, $5.4 and $4.8 (3) | $ | 14.9 | | | $ | 20.2 | | | $ | 17.7 | |
____________________
(1) We applied an estimated forfeiture rate of 4.0% per stock option grant in the calculation of the expense.
(2) We applied an estimated forfeiture rate of 2.0% of outstanding grants in the calculation of the expense.
(3) This expense is classified as "Selling, general and administrative expenses" in our consolidated statements of income (loss). Total stock compensation expense, net of tax, not included in the above table of $2.2 million, $0.1 million, and $4.0 million for the years ended December 31, 2020, 2019 and 2018, respectively, is included in "Discontinued operations, net of income taxes" in the consolidated statements of income (loss).
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
We received $24.7 million, $50.7 million and $10.7 million in cash related to stock option exercises for the years ended December 31, 2020, 2019 and 2018, respectively. The shares used for the exercise of stock options occurring during the years ended December 31, 2020, 2019 and 2018 came from treasury shares.
Impacts of Livent Distribution
On March 1, 2019, we completed the previously announced distribution of 123 million shares of common stock of Livent as a pro rata dividend on shares of FMC common stock outstanding at the close of business on the record date of February 25, 2019. All outstanding and nonvested equity awards relating to FMC’s stock immediately prior to the effective date were generally converted into FMC and Livent units pursuant to the employee matters agreement.
Stock Options
The grant-date fair values of the stock options we granted in the years ended December 31, 2020, 2019 and 2018 were estimated using the Black-Scholes option valuation model, the key assumptions for which are listed in the table below. The dividend yield assumption reflects anticipated dividends on our common stock. The expected volatility assumption is based on the actual historical experience of our common stock. The expected life represents the period of time that options granted are expected to be outstanding. The risk-free interest rate is based on U.S. Treasury securities with terms equal to the expected timing of stock option exercises as of the grant date. Employee stock options generally vest after a three year period and expire ten years from the date of grant.
Black Scholes valuation assumptions for stock option grants:
| | | | | | | | | | | | | | | | | |
| 2020 | | 2019 | | 2018 |
| Expected dividend yield | 1.91% | | 1.83% | | 0.77% |
| Expected volatility | 26.60% | | 26.07% | | 26.85% |
| Expected life (in years) | 6.5 | | 6.5 | | 6.5 |
| Risk-free interest rate | 1.19% | | 2.53% | | 2.79% |
The weighted-average grant-date fair value of options granted during the years ended December 31, 2020, 2019 and 2018 was $20.28, $18.66 and $25.70 per share, respectively.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The following summary shows stock option activity for employees under the Plan for the three years ended December 31, 2020:
| | | | | | | | | | | | | | | | | | | | | | | |
| (Shares in Thousands) | Number of Options Granted But Not Exercised | | Weighted-Average Remaining Contractual Life | | Weighted-Average Exercise Price Per Share | | Aggregate Intrinsic Value (in Millions) |
December 31, 2017 (920 shares exercisable and 1,452 shares expected to vest or be exercised) | 2,435 | | | 6.3 years | | $ | 48.37 | | | $ | 112.7 | |
| Granted | 250 | | | | | 85.19 | | | |
| Exercised | (260) | | | | | 41.80 | | | 11.7 | |
| Forfeited | (61) | | | | | 52.51 | | | |
December 31, 2018 (1,044 shares exercisable and 1,287 shares expected to vest or be exercised) | 2,364 | | | 6.0 years | | $ | 52.87 | | | $ | 52.5 | |
| Granted | 380 | | | | | 75.76 | | | |
Conversion impact from Livent spin (1) | 210 | | | | | 53.09 | | | |
| Exercised | (1,414) | | | | | 39.17 | | | 67.2 | |
| Forfeited | (36) | | | | | 67.82 | | | |
December 31, 2019 (628 shares exercisable and 835 shares expected to vest or be exercised) | 1,504 | | | 6.5 years | | $ | 58.06 | | | $ | 62.8 | |
| Granted | 302 | | | | | 92.24 | | | |
| | | | | | | |
| Exercised | (549) | | | | | 48.02 | | | 31.3 | |
| Forfeited | (22) | | | | | 81.84 | | | |
December 31, 2020 (388 shares exercisable and 818 shares expected to vest or be exercised) | 1,235 | | | 7.0 years | | $ | 70.44 | | | $ | 54.9 | |
____________________
(1)Awards converted as a result of March 1, 2019 Livent separation.
The number of stock options indicated in the above table as being exercisable as of December 31, 2020, had an intrinsic value of $26.2 million, a weighted-average remaining contractual term of 4.2 years, and a weighted-average exercise price of $47.56.
As of December 31, 2020, we had total remaining unrecognized compensation cost related to unvested stock options of $4.5 million which will be amortized over the weighted-average remaining requisite service period of approximately 1.78 years.
Restricted and Performance Based Equity Awards
The grant-date fair value of restricted stock awards and stock units under the Plan is based on the market price per share of our common stock on the date of grant. The related compensation cost is amortized to expense on a straight-line basis over the vesting period during which the employees perform related services, which is typically three years except for those eligible for retirement prior to the stated vesting period as well as non-employee directors.
Starting in 2015, we began granting performance based restricted stock awards. The performance based share awards represent a number of shares of common stock to be awarded upon settlement based on the achievement of a total shareholder return ("TSR") relative to peer companies over a three year period. These awards generally vest upon the completion of a three year period from the date of grant; however, starting with the 2016 grants, certain performance criteria is measured on an annual basis. Starting with the 2019 grants, vesting was based on a TSR relative to peer companies and a cumulative operating cash flow metric. The fair value of the equity classified performance-based share awards is determined based on the number of shares of common stock expected to be awarded and a Monte Carlo valuation model.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The following table shows our employee restricted award activity for the three years ended December 31, 2020:
| | | | | | | | | | | | | | | | | | | | | | | |
| Restricted Equity | | Performance Based Equity |
| (Number of Awards in Thousands) | Number of awards | | Weighted-Average Grant Date Fair Value Per Share | | Number of
awards | | Weighted-Average Grant Date Fair Value Per Share |
| Nonvested at December 31, 2017 | 489 | | | $ | 47.63 | | | 260 | | | $ | 53.36 | |
| Granted | 137 | | | 84.94 | | | 133 | | | 88.65 | |
| Vested | (154) | | | 55.14 | | | (58) | | | 81.15 | |
| Forfeited | (13) | | | 65.39 | | | — | | | — | |
| Nonvested at December 31, 2018 | 459 | | | $ | 55.75 | | | 335 | | | $ | 56.42 | |
| Granted | 108 | | | 76.22 | | | 106 | | | 83.89 | |
Conversion impact from Livent spin (1) | (29) | | | 67.46 | | | (12) | | | 84.58 | |
| Vested | (223) | | | 37.54 | | | (222) | | | 42.18 | |
| Forfeited | (13) | | | 69.69 | | | (1) | | | 78.92 | |
| Nonvested at December 31, 2019 | 302 | | | $ | 67.89 | | | 206 | | | $ | 72.06 | |
| Granted | 92 | | | 91.83 | | | 111 | | | 108.74 | |
| | | | | | | |
| Vested | (84) | | | 50.14 | | | (115) | | | 58.37 | |
| Forfeited | (12) | | | 77.42 | | | — | | | — | |
| Nonvested at December 31, 2020 | 298 | | | $ | 79.91 | | | 202 | | | $ | 88.48 | |
____________________
(1)Awards transferred to Livent employees as a result of March 1, 2019 Livent separation.
As of December 31, 2020, we had total remaining unrecognized compensation cost related to unvested restricted awards of $11.5 million which will be amortized over the weighted-average remaining requisite service period of approximately 1.83 years.
Note 17: Equity
The following is a summary of our capital stock activity over the past three years:
| | | | | | | | | | | |
| Common Stock Shares | | Treasury Stock Shares |
| December 31, 2017 | 185,983,792 | | | 51,653,236 | |
| Stock options and awards | — | | | (390,553) | |
| Repurchases of common stock, net | — | | | 2,439,495 | |
| December 31, 2018 | 185,983,792 | | | 53,702,178 | |
| Stock options and awards | — | | | (1,563,307) | |
| Repurchases of common stock, net | — | | | 4,720,627 | |
| December 31, 2019 | 185,983,792 | | | 56,859,498 | |
| Stock options and awards | — | | | (677,827) | |
| Repurchases of common stock, net | — | | | 448,538 | |
| December 31, 2020 | 185,983,792 | | | 56,630,209 | |
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Accumulated other comprehensive income (loss)
Summarized below is the roll forward of accumulated other comprehensive income (loss), net of tax.
| | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | Foreign currency adjustments | | Derivative Instruments (1) | | Pension and other postretirement benefits (2) | | Total |
| Accumulated other comprehensive income (loss), net of tax at December 31, 2017 | $ | (6.2) | | | $ | 5.2 | | | $ | (239.3) | | | $ | (240.3) | |
| 2018 Activity | | | | | | | |
| Other comprehensive income (loss) before reclassifications | $ | (95.3) | | | $ | 13.7 | | | $ | 4.2 | | | $ | (77.4) | |
| Amounts reclassified from accumulated other comprehensive income (loss) | — | | | (7.7) | | | 16.5 | | | 8.8 | |
| | | | | | | |
| Accumulated other comprehensive income (loss), net of tax at December 31, 2018 | $ | (101.5) | | | $ | 11.2 | | | $ | (218.6) | | | $ | (308.9) | |
| 2019 Activity | | | | | | | |
| Other comprehensive income (loss) before reclassifications | $ | (15.2) | | | $ | (69.0) | | | $ | (6.5) | | | $ | (90.7) | |
| Amounts reclassified from accumulated other comprehensive income (loss) | — | | | (8.2) | | | 9.9 | | | 1.7 | |
| Net current period other comprehensive income (loss) | $ | (15.2) | | | $ | (77.2) | | | $ | 3.4 | | | $ | (89.0) | |
| Adoption of accounting standard (Note 2) | — | | | 1.0 | | | (54.1) | | | $ | (53.1) | |
Distribution of FMC Lithium (3) | 39.0 | | | — | | | — | | | 39.0 | |
| | | | | | | |
| Accumulated other comprehensive income (loss), net of tax at December 31, 2019 | $ | (77.7) | | | $ | (65.0) | | | $ | (269.3) | | | $ | (412.0) | |
| 2020 Activity | | | | | | | |
| Other comprehensive income (loss) before reclassifications | $ | 101.7 | | | $ | (2.5) | | | $ | 18.9 | | | $ | 118.1 | |
| Amounts reclassified from accumulated other comprehensive income (loss) | — | | | (4.3) | | | 16.0 | | | 11.7 | |
| | | | | | | |
| Accumulated other comprehensive income (loss), net of tax at December 31, 2020 | $ | 24.0 | | | $ | (71.8) | | | $ | (234.4) | | | $ | (282.2) | |
____________________
(1)See Note 19 for more information.
(2)See Note 15 for more information.
(3)Represents the effects of the distribution of FMC Lithium.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Reclassifications of accumulated other comprehensive income (loss)
The table below provides details about the reclassifications from accumulated other comprehensive income (loss) and the affected line items in the consolidated statements of income (loss) for each of the periods presented.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Details about Accumulated Other Comprehensive Income (Loss) Components | | Amounts Reclassified from Accumulated Other Comprehensive Income (Loss) (1) | | Affected Line Item in the Consolidated Statements of Income (Loss) |
| | Year Ended December 31, | | |
| (in Millions) | | 2020 | | 2019 | | 2018 | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Derivative instruments: | | | | | | | | |
| Foreign currency contracts | | $ | 24.6 | | | $ | 10.0 | | | $ | 18.9 | | | Costs of sales and services |
| | | | | | | | |
| Foreign currency contracts | | (19.3) | | | 1.9 | | | (8.0) | | | Selling, general and administrative expenses |
| Interest rate contracts | | (2.7) | | | (0.7) | | | (0.4) | | | Interest expense |
| Total before tax | | $ | 2.6 | | | $ | 11.2 | | | $ | 10.5 | | | |
| | 1.7 | | | (3.0) | | | (2.8) | | | Provision for income taxes |
| Amount included in net income | | $ | 4.3 | | | $ | 8.2 | | | $ | 7.7 | | | |
| | | | | | | | |
Pension and other postretirement benefits (2): | | | | | | | | |
| Amortization of prior service costs | | $ | (0.3) | | | $ | (0.3) | | | $ | (0.3) | | | Selling, general and administrative expenses |
| Amortization of unrecognized net actuarial and other gains (losses) | | (19.2) | | | (10.8) | | | (14.4) | | | Non-operating pension and postretirement charges (income) |
| Recognized loss due to settlement/curtailment | | (0.7) | | | (1.4) | | | (6.1) | | | Non-operating pension and postretirement charges (income); Discontinued operations, net of income taxes |
| Total before tax | | $ | (20.2) | | | $ | (12.5) | | | $ | (20.8) | | | |
| | 4.2 | | | 2.6 | | | 4.3 | | | Provision for income taxes; Discontinued operations, net of income taxes |
| Amount included in net income | | $ | (16.0) | | | $ | (9.9) | | | $ | (16.5) | | | |
| | | | | | | | |
| Total reclassifications for the period | | $ | (11.7) | | | $ | (1.7) | | | $ | (8.8) | | | Amount included in net income |
____________________
(1)Amounts in parentheses indicate charges to the consolidated statements of income (loss).
(2)Pension and other postretirement benefits amounts include the impact from both continuing and discontinued operations. For detail on the continuing operations components of pension and other postretirement benefits, see Note 15.
Transactions with Noncontrolling Interest
In July 2020, we purchased the remaining 49 percent ownership interest in our Indonesia joint venture, PT Bina Guna Kimia ("BGK"), for $7.4 million which increased our ownership from 51 percent to 100 percent.
As a result of the IPO and underwriters' exercise to purchase additional shares of common stock in the fourth quarter of 2018, our controlling interest in FMC Lithium was approximately 84 percent. On March 1, 2019, we completed the previously announced distribution of the remaining shares of common stock of Livent. See Note 1 for further information.
Dividends and Share Repurchases
On January 21, 2021, we paid dividends totaling $62.3 million to our shareholders of record as of December 31, 2020. This amount is included in "Accrued and other liabilities" on the consolidated balance sheets as of December 31, 2020. For the years ended December 31, 2020, 2019 and 2018, we paid $228.5 million, $210.3 million and $89.2 million in dividends, respectively.
In 2020, 0.4 million shares were repurchased under the publicly announced repurchase program. At December 31, 2020, approximately $550 million remained unused under our Board-authorized repurchase program. This repurchase program does not include a specific timetable or price targets and may be suspended or terminated at any time. Shares may be purchased through open market or privately negotiated transactions at the discretion of management based on its evaluation of market conditions and other factors. We also reacquire shares from time to time from employees in connection with the vesting, exercise and forfeiture of awards under our equity compensation plans.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Note 18: Earnings Per Share
Earnings per common share ("EPS") is computed by dividing net income by the weighted average number of common shares outstanding during the period on a basic and diluted basis.
Our potentially dilutive securities include potential common shares related to our stock options, restricted stock and restricted stock units. Diluted earnings per share ("Diluted EPS") considers the impact of potentially dilutive securities except in periods in which there is a loss because the inclusion of the potential common shares would have an antidilutive effect. Diluted EPS excludes the impact of potential common shares related to our stock options in periods in which the option exercise price is greater than the average market price of our common stock for the period. For the years ended December 31, 2020, 2019 and 2018 there were 0.2 million, 0.3 million and 0.2 million potential common shares excluded from Diluted EPS, respectively.
Our non-vested restricted stock awards contain rights to receive non-forfeitable dividends, and thus, are participating securities requiring the two-class method of computing EPS. The two-class method determines EPS by dividing the sum of distributed earnings to common stockholders and undistributed earnings allocated to common stockholders by the weighted average number of shares of common stock outstanding for the period. In calculating the two-class method, undistributed earnings are allocated to both common shares and participating securities based on the weighted average shares outstanding during the period.
Earnings applicable to common stock and common stock shares used in the calculation of basic and diluted earnings per share are as follows:
| | | | | | | | | | | | | | | | | |
| (in Millions, Except Share and Per Share Data) | Year Ended December 31, |
| 2020 | | 2019 | | 2018 |
| Earnings (loss) attributable to FMC stockholders: | | | | | |
| Continuing operations, net of income taxes | $ | 579.8 | | | $ | 540.7 | | | $ | 531.4 | |
| Discontinued operations, net of income taxes | (28.3) | | | (63.3) | | | (29.3) | |
| Net income (loss) attributable to FMC stockholders | $ | 551.5 | | | $ | 477.4 | | | $ | 502.1 | |
| Less: Distributed and undistributed earnings allocable to restricted award holders | (1.4) | | | (1.5) | | | (2.4) | |
| Net income (loss) allocable to common stockholders | $ | 550.1 | | | $ | 475.9 | | | $ | 499.7 | |
| | | | | |
| Basic earnings (loss) per common share attributable to FMC stockholders: | | | | | |
| Continuing operations | $ | 4.46 | | | $ | 4.12 | | | $ | 3.94 | |
| Discontinued operations | (0.22) | | | (0.48) | | | (0.22) | |
| Net income (loss) | $ | 4.24 | | | $ | 3.64 | | | $ | 3.72 | |
| | | | | |
| Diluted earnings (loss) per common share attributable to FMC stockholders: | | | | | |
| Continuing operations | $ | 4.44 | | | $ | 4.10 | | | $ | 3.91 | |
| Discontinued operations | (0.22) | | | (0.48) | | | (0.22) | |
| Net income (loss) | $ | 4.22 | | | $ | 3.62 | | | $ | 3.69 | |
| | | | | |
| Shares (in thousands): | | | | | |
| Weighted average number of shares of common stock outstanding - Basic | 129,701 | | | 130,761 | | | 134,406 | |
| Weighted average additional shares assuming conversion of potential common shares | 883 | | | 1,241 | | | 1,473 | |
| Shares – diluted basis | 130,584 | | | 132,002 | | | 135,879 | |
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Note 19: Financial Instruments, Risk Management and Fair Value Measurements
Our financial instruments include cash and cash equivalents, trade receivables, other current assets, certain receivables classified as other long-term assets, accounts payable, and amounts included in investments and accruals meeting the definition of financial instruments. The carrying value of these financial instruments approximates their fair value. Our other financial instruments include the following:
| | | | | | | | |
| Financial Instrument | | Valuation Method |
| | |
| Foreign exchange forward contracts | | Estimated amounts that would be received or paid to terminate the contracts at the reporting date based on current market prices for applicable currencies. |
| | |
| Commodity forward and option contracts | | Estimated amounts that would be received or paid to terminate the contracts at the reporting date based on quoted market prices for applicable commodities. |
| | |
| Debt | | Our estimates and information obtained from independent third parties using market data, such as bid/ask spreads for the last business day of the reporting period. |
The estimated fair value of the financial instruments in the above table have been determined using standard pricing models which take into account the present value of expected future cash flows discounted to the balance sheet date. These standard pricing models utilize inputs derived from, or corroborated by, observable market data such as interest rate yield curves and currency and commodity spot and forward rates. In addition, we test a subset of our valuations against valuations received from the transaction's counterparty to validate the accuracy of our standard pricing models. Accordingly, the estimates presented may not be indicative of the amounts that we would realize in a market exchange at settlement date and do not represent potential gains or losses on these agreements. The estimated fair values of foreign exchange forward contracts, commodity forward and option contracts, and interest rate contracts are included in the tables within this Note. The estimated fair value of debt is $3,640.0 million and $3,393.8 million and the carrying amount is $3,267.8 million and $3,258.8 million as of December 31, 2020 and 2019, respectively.
Use of Derivative Financial Instruments to Manage Risk
We mitigate certain financial exposures, including currency risk, commodity purchase exposures and interest rate risk through a program of risk management that includes the use of derivative financial instruments. We enter into foreign exchange contracts, including forward and purchased option contracts, to reduce the effects of fluctuating foreign currency exchange rates.
We formally document all relationships between hedging instruments and hedged items, as well as the risk management objective and strategy for undertaking various hedge transactions. This process includes relating derivatives that are designated as fair value or cash flow hedges to specific assets and liabilities on the balance sheet or to specific firm commitments or forecasted transactions. We also assess both at the inception of the hedge and on an ongoing basis, whether each derivative is highly effective in offsetting changes in fair values or cash flows of the hedged item. If we determine that a derivative is not highly effective as a hedge, or if a derivative ceases to be a highly effective hedge, we discontinue hedge accounting with respect to that derivative prospectively.
Foreign Currency Exchange Risk Management
We conduct business in many foreign countries, exposing earnings, cash flows, and our financial position to foreign currency risks. The majority of these risks arise as a result of foreign currency transactions. Our policy is to minimize exposure to adverse changes in currency exchange rates. This is accomplished through a controlled program of risk management that includes the use of foreign currency debt and forward foreign exchange contracts. We also use forward foreign exchange contracts to hedge firm and highly anticipated foreign currency cash flows, with an objective of balancing currency risk to provide adequate protection from significant fluctuations in the currency markets.
The primary currencies for which we have exchange rate exposure are the U.S. dollar versus the Brazilian Real, the Euro, the Chinese yuan, the Mexican peso, Indian rupee and the Argentine peso.
Commodity Price Risk
We are exposed to risks in energy costs due to fluctuations in energy prices, particularly natural gas. We attempt to mitigate our exposure to increasing energy costs by hedging the cost of future deliveries of natural gas.
Interest Rate Risk
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
We use various strategies to manage our interest rate exposure, including entering into interest rate swap agreements to achieve a targeted mix of fixed and variable-rate debt. In the agreements we exchange, at specified intervals, the difference between fixed and variable-interest amounts calculated on an agreed-upon notional principal amount.
Concentration of Credit Risk
Our counterparties to derivative contracts are primarily major financial institutions. We limit the dollar amount of contracts entered into with any one financial institution and monitor counterparties’ credit ratings. We also enter into master netting agreements with each financial institution, where possible, which helps mitigate the credit risk associated with our financial instruments. While we may be exposed to credit losses due to the nonperformance of counterparties, we consider this risk remote.
Financial Guarantees and Letter-of-Credit Commitments
We enter into various financial instruments with off-balance sheet risk as part of the normal course of business. These off-balance sheet instruments include financial guarantees and contractual commitments to extend financial guarantees under letters of credit and other assistance to customers. See Notes 1 and 20 for more information. Decisions to extend financial guarantees to customers, and the amount of collateral required under these guarantees, is based on our evaluation of creditworthiness on a case-by-case basis.
Accounting for Derivative Instruments and Hedging Activities
Cash Flow Hedges
We recognize all derivatives on the balance sheet at fair value. On the date we enter into the derivative instrument, we generally designate the derivative as a hedge of the variability of cash flows to be received or paid related to a forecasted transaction (cash flow hedge). We record in AOCI changes in the fair value of derivatives that are designated as, and meet all the required criteria for, a cash flow hedge. We then reclassify these amounts into earnings as the underlying hedged item affects earnings. In contrast we immediately record in earnings changes in the fair value of derivatives that are not designated as cash flow hedges.
As of December 31, 2020, we had open foreign currency forward contracts in AOCI in a net after-tax loss position of $18.3 million designated as cash flow hedges of underlying forecasted sales and purchases. Current open contracts hedge forecasted transactions until December 31, 2021. At December 31, 2020, we had open forward contracts with various expiration dates to buy, sell or exchange foreign currencies with a U.S. dollar equivalent of approximately $1,881 million.
As of December 31, 2020, we had open interest rate contracts in AOCI in a net after-tax loss position of $0.7 million designated as cash flow hedges of the anticipated fixed rate coupon of debt forecasted to be issued within a designated window. At December 31, 2020 we had interest rate swap contracts outstanding with a total aggregate notional value of approximately $100 million.
In conjunction with the issuance of the Senior Notes, on September 20, 2019 we settled on various interest rate swap agreements which were entered into to hedge the variability in treasury rates. This settlement resulted in a loss of $83.1 million which was recorded in other comprehensive income and will be amortized over the various terms of the Senior Notes. Refer to Note 14 for further details on the Senior Notes.
As of December 31, 2020, we had no open commodity contracts in AOCI designated as cash flow hedges of underlying forecasted purchases. At December 31, 2020, we had no mmBTUs (millions of British Thermal Units) in aggregate notional volume of outstanding natural gas commodity forward contracts.
Approximately $18.3 million of net after-tax losses, representing open foreign currency exchange contracts will be realized in earnings during the twelve months ending December 31, 2021 if spot rates in the future are consistent with forward rates as of December 31, 2020. The actual effect on earnings will be dependent on the actual spot rates when the forecasted transactions occur. We recognize derivative gains and losses in the "Costs of sales and services" line in the consolidated statements of income (loss).
Derivatives Not Designated As Hedging Instruments
We hold certain forward contracts that have not been designated as cash flow hedging instruments for accounting purposes. Contracts used to hedge the exposure to foreign currency fluctuations associated with certain monetary assets and liabilities are not designated as cash flow hedging instruments, and changes in the fair value of these items are recorded in earnings.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
We had open forward contracts not designated as cash flow hedging instruments for accounting purposes with various expiration dates to buy, sell or exchange foreign currencies with a U.S. dollar equivalent of approximately $2,026 million at December 31, 2020.
Fair Value of Derivative Instruments
The following tables provide the gross fair value and net balance sheet presentation of our derivative instruments as of December 31, 2020 and 2019:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 |
| Gross Amount of Derivatives | | | | | | |
| (in Millions) | Designated as Cash Flow Hedges | | Not Designated as Hedging Instruments | | Total Gross Amounts | | Gross Amounts Offset in the Consolidated Balance Sheet (3) | | Net Amounts |
| Derivatives | | | | | | | | | |
| Foreign exchange contracts | $ | 19.4 | | | $ | 1.9 | | | $ | 21.3 | | | $ | (21.1) | | | $ | 0.2 | |
| Interest rate contracts | 0.1 | | | — | | | 0.1 | | | — | | | 0.1 | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
Total derivative assets (1) | $ | 19.5 | | | $ | 1.9 | | | $ | 21.4 | | | $ | (21.1) | | | $ | 0.3 | |
| | | | | | | | | |
| Foreign exchange contracts | $ | (42.7) | | | $ | (3.1) | | | $ | (45.8) | | | $ | 21.1 | | | $ | (24.7) | |
| | | | | | | | | |
| | | | | | | | | |
| Interest rate contracts | (0.9) | | | — | | | (0.9) | | | — | | | (0.9) | |
Total derivative liabilities (2) | $ | (43.6) | | | $ | (3.1) | | | $ | (46.7) | | | $ | 21.1 | | | $ | (25.6) | |
| | | | | | | | | |
| Net derivative assets (liabilities) | $ | (24.1) | | | $ | (1.2) | | | $ | (25.3) | | | $ | — | | | $ | (25.3) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2019 |
| Gross Amount of Derivatives | | |
| (in Millions) | Designated as Cash Flow Hedges | | Not Designated as Hedging Instruments | | Total Gross Amounts | | Gross Amounts Offset in the Consolidated Balance Sheet (3) | | Net Amounts |
| Derivatives | | | | | | | | | |
| Foreign exchange contracts | $ | 8.0 | | | $ | 0.3 | | | $ | 8.3 | | | $ | (8.1) | | | $ | 0.2 | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
Total derivative assets (1) | $ | 8.0 | | | $ | 0.3 | | | $ | 8.3 | | | $ | (8.1) | | | $ | 0.2 | |
| | | | | | | | | |
| Foreign exchange contracts | $ | (12.1) | | | $ | (4.2) | | | $ | (16.3) | | | $ | 8.1 | | | $ | (8.2) | |
| Interest rate contracts | (0.9) | | | — | | | (0.9) | | | — | | | (0.9) | |
| | | | | | | | | |
| | | | | | | | | |
Total derivative liabilities (2) | $ | (13.0) | | | $ | (4.2) | | | $ | (17.2) | | | $ | 8.1 | | | $ | (9.1) | |
| | | | | | | | | |
| Net derivative assets (liabilities) | $ | (5.0) | | | $ | (3.9) | | | $ | (8.9) | | | $ | — | | | $ | (8.9) | |
____________________
(1) Net balance is included in "Prepaid and other current assets" in the consolidated balance sheets.
(2) Net balance is included in "Accrued and other liabilities" in the consolidated balance sheets.
(3) Represents net derivatives positions subject to master netting arrangements.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The following tables summarize the gains or losses related to our cash flow hedges and derivatives not designated as hedging instruments:
Derivatives in Cash Flow Hedging Relationships
| | | | | | | | | | | | |
| Contracts | |
| (in Millions) | Foreign exchange | | Interest rate | Total |
| Accumulated other comprehensive income (loss), net of tax at December 31, 2017 | $ | 4.4 | | | $ | 0.8 | | $ | 5.2 | |
| 2018 Activity | | | | |
| Unrealized hedging gains (losses) and other, net of tax | $ | 14.2 | | | $ | (0.5) | | $ | 13.7 | |
Reclassification of deferred hedging (gains) losses, net of tax (1) | (8.2) | | | 0.5 | | (7.7) | |
| Total derivative instrument impact on comprehensive income, net of tax | $ | 6.0 | | | $ | — | | $ | 6.0 | |
| | | | |
| Accumulated other comprehensive income (loss), net of tax at December 31, 2018 | $ | 10.4 | | | $ | 0.8 | | $ | 11.2 | |
| 2019 Activity | | | | |
| Unrealized hedging gains (losses) and other, net of tax | $ | (3.1) | | | $ | (65.9) | | $ | (69.0) | |
Reclassification of deferred hedging (gains) losses, net of tax (1) | (8.7) | | | 0.5 | | (8.2) | |
| Total derivative instrument impact on comprehensive income, net of tax | $ | (11.8) | | | $ | (65.4) | | $ | (77.2) | |
| | | | |
| Accumulated other comprehensive income (loss), net of tax at December 31, 2019 | $ | (1.4) | | | $ | (64.6) | | $ | (66.0) | |
| 2020 Activity | | | | |
| Unrealized hedging gains (losses) and other, net of tax | $ | (3.8) | | | $ | 1.3 | | $ | (2.5) | |
Reclassification of deferred hedging (gains) losses, net of tax (1) | (6.4) | | | 2.1 | | (4.3) | |
| Total derivative instrument impact on comprehensive income, net of tax | $ | (10.2) | | | $ | 3.4 | | $ | (6.8) | |
| | | | |
| Accumulated other comprehensive income (loss), net of tax at December 31, 2020 | $ | (11.6) | | | $ | (61.2) | | $ | (72.8) | |
____________________
(1)Amounts are included in "Costs of sales and services", "Selling, general and administrative expenses", and "Interest expense" on the consolidated statements of income (loss).
Derivatives Not Designated as Hedging Instruments
| | | | | | | | | | | | | | | | | |
| Amount of Pre-tax Gain (Loss) Recognized in Income on Derivatives (1) |
| Year Ended December 31, |
| (in Millions) | 2020 | | 2019 | | 2018 |
| Foreign exchange contracts | $ | (62.9) | | | $ | (26.7) | | | $ | (10.9) | |
| | | | | |
| Total | $ | (62.9) | | | $ | (26.7) | | | $ | (10.9) | |
____________________
(1) Amounts in the columns represent the gain or loss on the derivative instrument offset by the gain or loss on the hedged item. These amounts are included in "Costs of sales and services" on the consolidated statements of income (loss).
Fair Value Measurements
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Market participants are defined as buyers or sellers in the principle or most advantageous market for the asset or liability that are independent of the reporting entity, knowledgeable and able and willing to transact for the asset or liability.
Fair Value Hierarchy
We have categorized our assets and liabilities that are recorded at fair value, based on the priority of the inputs to the valuation technique, into a three-level fair value hierarchy. The fair value hierarchy gives the highest priority to quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). If the inputs used to measure the assets and liabilities fall within different levels of the hierarchy, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Recurring Fair Value Measurements
The following tables present our fair value hierarchy for those assets and liabilities measured at fair value on a recurring basis in our consolidated balance sheets:
| | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | December 31, 2020 | | Quoted Prices in Active Markets for Identical Assets (Level 1) | | Significant Other Observable Inputs (Level 2) | | Significant Unobservable Inputs (Level 3) |
| Assets | | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Derivatives – Foreign exchange (1) | $ | 0.2 | | | $ | — | | | $ | 0.2 | | | $ | — | |
Derivatives - Interest Rate (1) | 0.1 | | | — | | | 0.1 | | | — | |
Other (2) | 24.1 | | | 24.1 | | | — | | | — | |
| Total Assets | $ | 24.4 | | | $ | 24.1 | | | $ | 0.3 | | | $ | — | |
| | | | | | | |
| Liabilities | | | | | | | |
| | | | | | | |
| | | | | | | |
Derivatives – Foreign exchange (1) | $ | 24.7 | | | $ | — | | | $ | 24.7 | | | $ | — | |
Derivatives - Interest Rate (1) | 0.9 | | | — | | | 0.9 | | | — | |
Other (3) | 35.2 | | | 35.2 | | | — | | | — | |
| Total Liabilities | $ | 60.8 | | | $ | 35.2 | | | $ | 25.6 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | December 31, 2019 | | Quoted Prices in Active Markets for Identical Assets (Level 1) | | Significant Other Observable Inputs (Level 2) | | Significant Unobservable Inputs (Level 3) |
| Assets | | | | | | | |
| | | | | | | |
| | | | | | | |
Derivatives – Foreign exchange (1) | $ | 0.2 | | | $ | — | | | $ | 0.2 | | | $ | — | |
Other (2) | 20.2 | | | 20.2 | | | — | | | — | |
| Total Assets | $ | 20.4 | | | $ | 20.2 | | | $ | 0.2 | | | $ | — | |
| | | | | | | |
| Liabilities | | | | | | | |
| | | | | | | |
| | | | | | | |
Derivatives – Foreign exchange (1) | $ | 8.2 | | | $ | — | | | $ | 8.2 | | | $ | — | |
Derivatives - Interest Rate (1) | 0.9 | | | — | | | 0.9 | | | — | |
Other (3) | 32.8 | | | 29.7 | | | 3.1 | | | — | |
| Total Liabilities | $ | 41.9 | | | $ | 29.7 | | | $ | 12.2 | | | $ | — | |
____________________
(1)See the Fair Value of Derivative Instruments table within this Note for classifications on our consolidated balance sheets.
(2)Consists of a deferred compensation arrangement, through which we hold various investment securities, recognized on our balance sheet. Both the asset and liability are recorded at fair value. Asset amounts included in "Other assets including long-term receivables, net" in the consolidated balance sheets.
(3)Primarily consists of a deferred compensation arrangement recognized on our balance sheet. Both the asset and liability are recorded at fair value. Liability amounts included in "Other long-term liabilities" in the consolidated balance sheets.
Nonrecurring Fair Value Measurements
The following table presents our fair value hierarchy for those assets and liabilities measured at fair value on a non-recurring basis in our consolidated balance sheets during the year ended December 31, 2018. There were no non-recurring fair value measurements in the consolidated balance sheets during the years ended December 31, 2020 and 2019.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions) | December 31, 2018 | | Quoted Prices in Active Markets for Identical Assets (Level 1) | | Significant Other Observable Inputs (Level 2) | | Significant Unobservable Inputs (Level 3) | | Total Gains (Losses) (Year Ended December 31, 2018) |
| Assets | | | | | | | | | |
| | | | | | | | | |
Impairment of intangibles (1) | $ | 3.1 | | | $ | — | | | $ | — | | | $ | 3.1 | | | $ | (1.8) | |
| Total Assets | $ | 3.1 | | | $ | — | | | $ | — | | | $ | 3.1 | | | $ | (1.8) | |
____________________
(1) We recorded an impairment charge to write down the carrying value of the generic brand portfolio of approximately $2 million to its fair value.
Note 20: Guarantees, Commitments and Contingencies
We continue to monitor the conditions that are subject to guarantees and indemnifications to identify whether a liability must be recognized in our financial statements.
The following table provides the estimated undiscounted amount of potential future payments for each major group of guarantees at December 31, 2020. These guarantees arise during the ordinary course of business from relationships with customers and nonconsolidated affiliates. Non-performance by the guaranteed party triggers the obligation requiring us to make payments to the beneficiary of the guarantee. Based on our experience these types of guarantees have not had a material effect on our consolidated financial position or on our liquidity. Our expectation is that future payment or performance related to the non-performance of others is considered unlikely.
| | | | | |
| (in Millions) | |
| Guarantees: | |
Guarantees of vendor financing - short term (1) | $ | 140.6 | |
| |
Other debt guarantees (2) | — | |
| Total | $ | 140.6 | |
____________________
(1)Represents guarantees to financial institutions on behalf of certain customers for their seasonal borrowing. The short-term amount is recorded as "Guarantees of vendor financing" on the consolidated balance sheets.
(2)These guarantees represent support provided to third-party banks for credit extended to various customers and nonconsolidated affiliates. The liability for the guarantees is recorded at an amount that approximates fair value (i.e. representing the stand-ready obligation) based on our historical collection experience and a current assessment of credit exposure. In the past, the fair value of these guarantees has been immaterial and the majority of these guarantees have had an expiration date of less than one year.
Excluded from the chart above are parent-company guarantees we provide to lending institutions that extend credit to our foreign subsidiaries. Since these guarantees are provided for consolidated subsidiaries, the consolidated financial position is not affected by the issuance of these guarantees. Also excluded from the chart, in connection with our property and asset sales and divestitures, we have agreed to indemnify the buyer for certain liabilities, including environmental contamination and taxes that occurred prior to the date of sale or provided guarantees to third parties relating to certain contracts assumed by the buyer. Our indemnification or guarantee obligations with respect to certain liabilities may be indefinite as to duration and may or may not be subject to a deductible, minimum claim amount or cap. As such, it is not possible for us to predict the likelihood that a claim will be made or to make a reasonable estimate of the maximum potential loss or range of loss. If triggered, we may be able to recover some of the indemnity payments from third parties. Therefore, we have not recorded any specific liabilities for these guarantees. For certain obligations related to our divestitures for which we can make a reasonable estimate of the maximum potential loss or range of loss and is probable, a liability in those instances has been recorded.
Commitments
Purchase Obligations
Our minimum commitments under our take-or-pay purchase obligations associated with the sourcing of materials and energy total approximately $825 million. Since the majority of our minimum obligations under these contracts are over the life of the contract on a year-by-year basis, we are unable to determine the periods in which these obligations could be payable under these contracts. However, we intend to fulfill the obligations associated with these contracts through our purchases associated with the normal course of business.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Contingencies
Livent Corporation class action. On May 13, 2019, purported stockholders of our former subsidiary Livent Corporation ("Livent") filed a putative class action complaint in the Pennsylvania Court of Common Pleas, Philadelphia County, in connection with Livent’s October 2018 initial public offering (the "Livent IPO"). The complaint in this case, Plymouth County Retirement Association v. Livent Corp., et al., named as defendants Livent, certain of its current and former executives and directors, FMC Corporation, and underwriters involved in the Livent IPO ("Defendants"). The complaint alleges generally that the offering documents for the Livent IPO failed to adequately disclose certain information related to Livent’s business and prospects. The complaint alleges violations of Sections 11, 12(a)(2), and 15 of the Securities Act of 1933 and seeks unspecified damages and other relief on behalf of all persons and entities who purchased or otherwise acquired Livent common stock pursuant and/or traceable to the Livent IPO offering documents. On July 2, 2019, Defendants moved to stay the Plymouth County action, in favor of two similar putative class actions relating to the Livent IPO, in which FMC had not been named as a Defendant, which are pending in the United States District Court of the Eastern District of Pennsylvania. On July 18, 2019, a separate state action was filed against the same Defendants in the Pennsylvania Court of Common Pleas, Philadelphia County, Bizzaria v. Livent Corp., et al. On July 26, 2019, Plymouth County filed an amended complaint in its state court case. On September 23, 2019, the actions were consolidated under the caption In re Livent Corporation Securities Litigation, No. 190501229. On October 11, 2019, Defendants filed preliminary objections seeking to dismiss the case in its entirety. On October 22, 2019, the Court denied Defendants’ motion to stay the case, but granted a separate motion of the Defendants to stay all discovery. On June 29, 2020 the court overruled the preliminary objections filed by the Defendants and on July 29, 2020, Defendants filed a motion seeking permission to appeal the state court's order.
Separately, on October 18, 2019, purported stockholders of Livent amended a putative class action complaint filed in the U.S. District Court for the Eastern District of Pennsylvania, to add FMC Corporation as a defendant. The operative complaint in that case, Bisser Nikolov v. Livent Corp., et al. makes similar substantive allegations as the state court case, including alleged violations of Sections 11, 12(a)(2), and 15 of the Securities Act of 1933 and seeks unspecified damages and other relief on behalf of all persons and entities who purchased or otherwise acquired Livent common stock pursuant and/or traceable to the Livent IPO offering documents. Pursuant to a stipulated scheduling order, Defendants filed a motion to dismiss the Nikolov case on November 18, 2019. Plaintiffs filed their opposition to the motion to dismiss on December 30, 2019. On July 2, 2020, the federal court granted the Defendants' motion to dismiss and dismissed the federal complaint in its entirety. On July 31, 2020, Plaintiffs filed a notice of appeal.
On October 28, 2020, Defendants entered into a stipulation of settlement with the state court plaintiffs in which Livent, on behalf of the Defendants, will pay $7.4 million to resolve all claims related to the IPO. On October 29, 2020, the state court plaintiffs filed a motion seeking preliminary approval of the settlement. The court entered a preliminary approval order and the final hearing is scheduled for April 15, 2021. If approved, the settlement would resolve all pending litigation relating to the IPO, including the claims in both the state and federal actions. All deadlines in the state and federal actions are currently stayed in light of the settlement. There is no financial impact to FMC as a result of the settlement. Livent has agreed to defend and indemnify FMC with regard to these cases. FMC is cooperating with Livent and other Defendants to defend the litigation.
Competition / antitrust litigation related to the discontinued FMC Peroxygens segment. We are subject to actions brought by private plaintiffs relating to alleged violations of European and Canadian competition and antitrust laws, as further described below.
European competition action. Multiple European purchasers of hydrogen peroxide who claim to have been harmed as a result of alleged violations of European competition law by hydrogen peroxide producers assigned their legal claims to a single entity formed by a law firm. The single entity then filed a lawsuit in Germany in March 2009 against European producers, including our wholly-owned Spanish subsidiary, FMC Foret SA ("Foret"). Foret executed a Settlement Agreement on December 14, 2020 wherein it agreed to pay a confidential settlement amount to the plaintiffs on December 17, 2020. The case was withdrawn on December 18, 2020.
Asbestos claims. Like hundreds of other industrial companies, we have been named as one of many defendants in asbestos-related personal injury litigation. Most of these cases allege personal injury or death resulting from exposure to asbestos in premises of FMC or to asbestos-containing components installed in machinery or equipment manufactured or sold by discontinued operations.
We intend to continue managing these asbestos-related cases in accordance with our historical experience. We have established a reserve for this litigation within our discontinued operations and believe that any exposure of a loss in excess of the established reserve cannot be reasonably estimated. Our experience has been that the overall trends in asbestos litigation have changed over time. Over the last several years, we have seen changes in the jurisdictions where claims against FMC are being filed and changes in the mix of products named in the various claims. Because these claim trends have yet to form a predictable
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
pattern, we are presently unable to reasonably estimate our asbestos liability with respect to claims that may be filed in the future.
Other contingent liabilities. In addition to the matters disclosed above, we have certain other contingent liabilities arising from litigation, claims, products we have sold, guarantees or warranties we have made, contracts we have entered into, indemnities we have provided, and other commitments or obligations incident to the ordinary course of business. In Brazil, we are subject to claims from various governmental agencies regarding alleged additional indirect (non-income) taxes or duties as well as product liability matters and labor cases related to our operations. These disputes take many years to resolve as the matters move through administrative or judicial courts. We have provided reserves for such Brazilian matters that we consider probable and for which a reasonable estimate of the obligation can be made in the amount of $4.1 million and $4.9 million as of December 31, 2020 and 2019, respectively. The aggregate estimated reasonably possible loss contingencies related to such Brazilian matters exceed amounts accrued by approximately $80 million at December 31, 2020. This reasonably possible estimate is based upon information available as of the date of the filing and the actual future losses may be higher given the uncertainties regarding the ultimate decision by administrative or judicial authorities in Brazil. Regarding other contingencies arising from operations, some of these contingencies are known - for example pending product liability litigation or claims - but are so preliminary that the merits cannot be determined, or if more advanced, are not deemed material based on current knowledge. Some contingencies are unknown - for example, claims with respect to which we have no notice or claims which may arise in the future, resulting from products we have sold, guarantees or warranties we have made, or indemnities we have provided. Therefore, we are unable to develop a reasonable estimate of our potential exposure of loss for these contingencies, either individually or in the aggregate, at this time. Based on information currently available and established reserves, we have no reason to believe that the ultimate resolution of our known contingencies, including the matters described in this Note, will have a material adverse effect on our consolidated financial position, liquidity or results of operations. However, there can be no assurance that the outcome of these contingencies will be favorable, and adverse results in certain of these contingencies could have a material adverse effect on our consolidated financial position, results of operations in any one reporting period, or liquidity.
See Note 12 for the Pocatello Tribal litigation, Middleport litigation, and Portland Harbor site for legal proceedings associated with our environmental contingencies.
Note 21: Segment Information
As discussed in Note 1, we operate as a single business segment providing innovative solutions to growers around the world with a robust product portfolio fueled by a market-driven discovery and development pipeline in crop protection, plant health, and professional pest and turf management.
For revenue by major geographical region, refer to Note 3. The following table provides our long-lived assets by major geographical region:
| | | | | | | | | | | |
| (in Millions) | December 31, |
| 2020 | | 2019 |
Long-lived assets (1) | | | |
North America (2) | $ | 1,230.2 | | | $ | 1,190.7 | |
| Latin America | 792.7 | | | 837.0 | |
Europe, Middle East, and Africa (2) | 1,513.9 | | | 1,448.0 | |
Asia (2) | 2,044.4 | | | 2,064.8 | |
| Total | $ | 5,581.2 | | | $ | 5,540.5 | |
| | | |
____________________
(1)Geographic long-lived assets exclude long-term deferred income taxes and assets of discontinued operations on the consolidated balance sheets.
(2)The countries with long-lived assets in excess of 10 percent of consolidated long-lived assets at December 31, 2020 and 2019 are Singapore, which totaled $1,582.5 million and $1,547.0 million, the U.S., which totaled $1,221.3 million and $1,177.7 million and Denmark, which totaled $1,104.6 million and $1,045.3 million, respectively.
Note 22: Supplemental Information
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
The following tables present details of prepaid and other current assets, other assets including long-term receivables, net, accrued and other liabilities and other long-term liabilities as presented on the consolidated balance sheets:
| | | | | | | | | | | |
| (in Millions) | December 31, |
| 2020 | | 2019 |
| Prepaid and other current assets | | | |
| Prepaid insurance | $ | 11.1 | | | $ | 8.2 | |
| | | |
| | | |
| Tax related items including value added tax receivables | 197.7 | | | 229.2 | |
Refund asset (1) | 28.4 | | | 37.7 | |
| Environmental obligation recoveries (Note 12) | 0.8 | | | 12.3 | |
| Derivative assets (Note 19) | 0.3 | | | 0.2 | |
| | | |
| Acquisition related items | 3.0 | | | 3.0 | |
| | | |
| Other prepaid and current assets | 139.5 | | | 196.9 | |
| Total | $ | 380.8 | | | $ | 487.5 | |
| | | | | | | | | | | |
| (in Millions) | December 31, |
| 2020 | | 2019 |
| Other assets including long-term receivables, net | | | |
| Non-current receivables (Note 10) | $ | 103.5 | | | $ | 123.1 | |
| Advance to contract manufacturers | 122.2 | | | 116.3 | |
| Capitalized software, net | 158.0 | | | 117.0 | |
| Environmental obligation recoveries (Note 12) | 3.6 | | | 15.0 | |
| | | |
| Income taxes indirect benefits | 37.9 | | | 32.7 | |
| Operating lease ROU asset (Note 4) | 147.3 | | | 164.7 | |
| Deferred compensation arrangements (Note 19) | 24.1 | | | 20.2 | |
| Pension and other postretirement benefits (Note 15) | 69.5 | | | 44.2 | |
| Other long-term assets | 46.2 | | | 52.1 | |
| Total | $ | 712.3 | | | $ | 685.3 | |
____________________
(1) In accordance with revenue standard requirements, a sales return liability is recognized for the consideration paid by a customer to which FMC does not expect to be entitled, together with a corresponding refund asset to recover the product from the customer.
| | | | | | | | | | | |
| (in Millions) | December 31, |
| 2020 | | 2019 |
| Accrued and other liabilities | | | |
| Restructuring reserves (Note 9) | $ | 11.9 | | | $ | 8.1 | |
| Dividend payable (Note 17) | 62.3 | | | 57.0 | |
| Accrued payroll | 87.0 | | | 101.2 | |
| | | |
| Environmental reserves, current, net of recoveries (Note 12) | 120.9 | | | 115.3 | |
| Derivative liabilities (Note 19) | 24.8 | | | 8.9 | |
Furadan® product exit asset retirement obligations | 10.0 | | | 33.0 | |
Unfavorable contracts (1) | 105.8 | | | 109.2 | |
| Operating lease current liabilities (Note 4) | 25.6 | | | 31.5 | |
Other accrued and other liabilities (2) | 226.4 | | | 216.4 | |
| Total | $ | 674.7 | | | $ | 680.6 | |
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
| | | | | | | | | | | |
| (in Millions) | December 31, |
| 2020 | | 2019 |
| Other long-term liabilities | | | |
| Restructuring reserves (Note 9) | $ | 4.5 | | | $ | 6.5 | |
| Asset retirement obligations, long-term (Note 1) | 20.7 | | | 2.7 | |
Transition tax related to Tax Cuts and Jobs Act (3) | 107.8 | | | 123.6 | |
| Contingencies related to uncertain tax positions (Note 13) | 83.1 | | | 71.4 | |
| Deferred compensation arrangements (Note 19) | 35.2 | | | 29.7 | |
| Derivative liabilities (Note 19) | 0.8 | | | 0.2 | |
| Self-insurance reserves (primarily workers' compensation) | 1.9 | | | 1.8 | |
| Lease obligations (Note 4) | 151.1 | | | 163.2 | |
| Reserve for discontinued operations (Note 11) | 76.6 | | | 71.9 | |
| | | |
Unfavorable contracts (1) | 89.4 | | | 206.0 | |
| Other long-term liabilities | 32.7 | | | 31.4 | |
| Total | $ | 603.8 | | | $ | 708.4 | |
____________________
(1) Primarily represents the technical insecticide product supply agreements with DuPont for use in their retained seed treatment business. Refer to Note 5 for more details.
(2) Other accrued and other liabilities includes the gross up of the estimated sales returns as part of our adoption of ASC 606. The impact of the adoption impacted accrued and other liabilities by $28.4 million and $37.7 million, respectively.
(3) Represents noncurrent portion of overall transition tax to be paid over the next five years.
FMC CORPORATION
Notes to Consolidated Financial Statements — (Continued)
Note 23: Quarterly Financial Information (Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in Millions, Except Share and Per Share Data) | 2020 | | 2019 |
| 1Q | | 2Q | | 3Q | | 4Q | | 1Q | | 2Q | | 3Q | | 4Q |
| Revenue | $ | 1,250.0 | | | $ | 1,155.3 | | | $ | 1,084.6 | | | $ | 1,152.2 | | | $ | 1,192.1 | | | $ | 1,206.1 | | | $ | 1,014.3 | | | $ | 1,197.3 | |
| Gross margin | 561.5 | | | 522.7 | | | 466.4 | | | 501.4 | | | 544.7 | | | 550.5 | | | 432.4 | | | 556.0 | |
| Income (loss) from continuing operations before equity in (earnings) loss of affiliates, non-operating pension and postretirement charges (income), interest expense, net and income taxes | 291.4 | | | 267.9 | | | 196.0 | | | 146.9 | | | 281.8 | | | 267.8 | | | 159.9 | | | 112.1 | |
| Income (loss) from continuing operations | 213.7 | | | 195.8 | | | 130.5 | | | 38.9 | | | 207.6 | | | 194.4 | | | 110.8 | | | 30.7 | |
| Discontinued operations, net of income taxes | (7.5) | | | (10.8) | | | (18.4) | | | 8.4 | | | 9.6 | | | (18.1) | | | (21.3) | | | (33.5) | |
| Net income (loss) | $ | 206.2 | | | $ | 185.0 | | | $ | 112.1 | | | $ | 47.3 | | | $ | 217.2 | | | $ | 176.3 | | | $ | 89.5 | | | $ | (2.8) | |
| Less: Net income (loss) attributable to noncontrolling interests | — | | | 0.6 | | | 0.7 | | | (2.2) | | | 1.5 | | | 1.8 | | | (0.9) | | | 0.4 | |
| Net income (loss) attributable to FMC stockholders | $ | 206.2 | | | $ | 184.4 | | | $ | 111.4 | | | $ | 49.5 | | | $ | 215.7 | | | $ | 174.5 | | | $ | 90.4 | | | $ | (3.2) | |
| Amounts attributable to FMC stockholders: | | | | | | | | | | | | | | | |
| Continuing operations, net of income taxes | $ | 213.7 | | | $ | 195.2 | | | $ | 129.8 | | | $ | 41.1 | | | $ | 206.1 | | | $ | 192.6 | | | $ | 111.7 | | | $ | 30.3 | |
| Discontinued operations, net of income taxes | (7.5) | | | (10.8) | | | (18.4) | | | 8.4 | | | 9.6 | | | (18.1) | | | (21.3) | | | (33.5) | |
| Net income (loss) | $ | 206.2 | | | $ | 184.4 | | | $ | 111.4 | | | $ | 49.5 | | | $ | 215.7 | | | $ | 174.5 | | | $ | 90.4 | | | $ | (3.2) | |
Basic earnings (loss) per common share attributable to FMC stockholders (1): | | | | | | | | | | | | | | | |
| Continuing operations | $ | 1.65 | | | $ | 1.50 | | | $ | 1.00 | | | $ | 0.32 | | | $ | 1.56 | | | $ | 1.46 | | | $ | 0.85 | | | $ | 0.23 | |
| Discontinued operations | (0.06) | | | (0.08) | | | (0.14) | | | 0.06 | | | 0.07 | | | (0.14) | | | (0.16) | | | (0.25) | |
| Basic net income (loss) per common share | $ | 1.59 | | | $ | 1.42 | | | $ | 0.86 | | | $ | 0.38 | | | $ | 1.63 | | | $ | 1.32 | | | $ | 0.69 | | | $ | (0.02) | |
Diluted earnings (loss) per common share attributable to FMC stockholders (1): | | | | | | | | | | | | | | | |
| Continuing operations | $ | 1.64 | | | $ | 1.49 | | | $ | 0.99 | | | $ | 0.32 | | | $ | 1.55 | | | $ | 1.46 | | | $ | 0.85 | | | $ | 0.23 | |
| Discontinued operations | (0.06) | | | (0.08) | | | (0.14) | | | 0.06 | | | 0.07 | | | (0.14) | | | (0.16) | | | (0.25) | |
| Diluted net income (loss) per common share | $ | 1.58 | | | $ | 1.41 | | | $ | 0.85 | | | $ | 0.38 | | | $ | 1.62 | | | $ | 1.32 | | | $ | 0.69 | | | $ | (0.02) | |
| Weighted average shares outstanding: | | | | | | | | | | | | | | | |
| Basic | 129.5 | | | 129.7 | | | 129.9 | | | 129.8 | | | 131.9 | | | 131.1 | | | 130.4 | | | 129.7 | |
| Diluted | 130.5 | | | 130.6 | | | 130.8 | | | 130.7 | | | 133.2 | | | 132.3 | | | 131.6 | | | 130.9 | |
____________________
(1)The sum of quarterly earnings per common share may differ from the full-year amount.
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
FMC Corporation:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of FMC Corporation and subsidiaries (the Company) as of December 31, 2020 and 2019, the related consolidated statements of income (loss), comprehensive income (loss), changes in equity, and cash flows for each of the years in the three‑year period ended December 31, 2020, and the related notes and schedule II – valuation and qualifying accounts and reserves (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the years in the three‑year period ended December 31, 2020, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 25, 2021 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Change in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, the Company has changed its method of accounting for leases as of January 1, 2019 due to the adoption of Accounting Standards Update (ASU) No. 2016-02, Leases (Topic 842) and the related amendments.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Evaluation of the allowance for trade receivables and long-term receivables associated with customers located in Brazil
As discussed in Notes 1 and 10 to the consolidated financial statements, the Company develops an analysis of trade receivables and long-term receivables to determine its best estimate of the probable losses associated with potential customer defaults. The most significant portion of the allowance for trade receivables and long-term receivables is related to customers located in Brazil.
We identified the evaluation of the allowance for trade receivables and long-term receivables associated with customers located in Brazil as a critical auditing matter. Specifically, the length of standard credit terms offered and customer liquidity may be significantly influenced by economic conditions and unfavorable weather conditions impacting crop quality. This increased the need for subjective judgment and knowledge in assessing customer liquidity constraints to estimate probable losses.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls over the Company’s collectability determination process, including controls over the identification of at-risk trade receivables and long-term receivables balances and related estimate of probable losses associated with such balances. We inspected underlying documentation for collateral arrangements, legal disputes, and historical trends and analysis performed by the Company for historical collection results. The Company’s assumptions underlying the collectability of trade receivables and long-term receivables were tested by evaluating:
•The Company’s rationale for and appropriateness of changes in assumptions from those used in the prior year related to its expected collection period for specific customers;
•Local Brazil economic and weather conditions that might impact the assumptions;
•Adjustments to the prior period reserve and assessing if those adjustments provided information that was contradictory to the current year’s assumptions; and
•Deterioration of trade receivables and long-term receivables balances subsequent to year-end, to identify the presence of trends not considered by the Company when it developed its assumptions.
Evaluation of unrecognized tax benefits
As discussed in Note 13, the Company has $76.2 million of unrecognized tax benefits as of December 31, 2020. The Company recognizes the largest amount of tax benefit that it believes is more than 50 percent likely to be sustained. A significant amount of the Company’s earnings are generated by certain foreign subsidiaries whose earnings are taxed at lower rates than the United States federal statutory rate.
We identified the evaluation of the Company’s unrecognized tax benefits related to the earnings of certain foreign subsidiaries as a critical audit matter. Complex auditor judgment was required in evaluating the Company’s interpretation of tax law, the transfer pricing structure, and its analysis of the recognition of its tax benefits.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls over the unrecognized tax benefits process, including controls related to the transfer pricing structure which affects the determination of earnings of certain foreign subsidiaries. We also involved tax and transfer pricing professionals with specialized skills and knowledge, who assisted in:
•Examining the Company’s tax positions, including the methodology for evaluating unrecognized tax benefits;
•Assessing transfer pricing studies with applicable laws and regulations;
•Evaluating the Company’s interpretation of tax laws and income tax consequences of intercompany transactions;
•Considering applicable settlements with taxing authorities; and
•Evaluating the Company’s determination of unrecognized tax benefits.
/s/ KPMG LLP
We have served as the Company's auditor since 1928.
Philadelphia, Pennsylvania
February 25, 2021
MANAGEMENT’S ANNUAL REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Exchange Act Rule 13a-15(f). FMC’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Internal control over financial reporting includes those written policies and procedures that:
•pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of FMC;
•provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles;
•provide reasonable assurance that receipts and expenditures of FMC are being made only in accordance with authorization of management and directors of FMC; and
•provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements.
Internal control over financial reporting includes the controls themselves, monitoring and internal auditing practices and actions taken to correct deficiencies as identified.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
We assessed the effectiveness of our internal control over financial reporting as of December 31, 2020. We based this assessment on criteria for effective internal control over financial reporting described in "Internal Control—Integrated Framework (COSO 2013)" issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included an evaluation of the design of our internal control over financial reporting and testing of the operational effectiveness of our internal control over financial reporting. We reviewed the results of our assessment with the Audit Committee of our Board of Directors.
Based on this assessment, we determined that, as of December 31, 2020, FMC has effective internal control over financial reporting.
KPMG LLP, our independent registered public accounting firm, has issued an attestation report on the effectiveness of internal control over financial reporting as of December 31, 2020, which appears on the following page.
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
FMC Corporation:
Opinion on Internal Control Over Financial Reporting
We have audited FMC Corporation and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2020 and 2019, the related consolidated statements of income (loss), comprehensive income (loss), changes in equity, and cash flows for each of the years in the three-year period ended December 31, 2020, and the related notes and schedule II – valuation and qualifying accounts and reserves (collectively, the consolidated financial statements), and our report dated February 25, 2021 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Philadelphia, Pennsylvania
February 25, 2021
FMC CORPORATION
SCHEDULE II - VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Provision (Benefit) | | | | |
| (in Millions) | Balance, Beginning of Year | | Charged to Costs and Expenses | | Charged to Other Comprehensive Income | | Net recoveries, write-offs and other (1) | | Balance, End of Year |
| December 31, 2020 | | | | | | | | | |
Reserve for doubtful accounts (2) | $ | 87.4 | | | 4.7 | | | — | | | (39.5) | | | $ | 52.6 | |
| Deferred tax valuation allowance | 303.3 | | | 34.0 | | | (1.7) | | | — | | | 335.6 | |
| December 31, 2019 | | | | | | | | | |
Reserve for doubtful accounts (2) | $ | 82.9 | | | 21.2 | | | — | | | (16.7) | | | $ | 87.4 | |
| Deferred tax valuation allowance | 261.4 | | | 42.2 | | | (0.3) | | | — | | | 303.3 | |
| December 31, 2018 | | | | | | | | | |
Reserve for doubtful accounts (2)(3) | $ | 85.7 | | | 71.4 | | | — | | | (74.2) | | | $ | 82.9 | |
| Deferred tax valuation allowance | 272.0 | | | (8.8) | | | (1.8) | | | — | | | 261.4 | |
____________________
(1)Write-offs are net of recoveries.
(2)Includes short-term and long-term portion.
(3)Includes the charge and write-off of approximately $42 million associated with the stranded accounts receivables written off as part of the restructuring in India. The charge was recorded as a component of "Restructuring and other charges (income)" on the consolidated statements of income (loss).
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
(a) Evaluation of disclosure controls and procedures. Based on management’s evaluation (with the participation of the Company’s Chief Executive Officer and Chief Financial Officer), the Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of the period covered by this report, the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934) are effective to provide reasonable assurance that information required to be disclosed by the Company in reports filed or submitted under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and is accumulated and communicated to management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s annual report on internal control over financial reporting. Refer to Management’s Annual Report on Internal Control Over Financial Reporting which is included in Item 8 of Part II of this Annual Report on Form 10-K and is incorporated by reference to this Item 9A.
Audit report of the independent registered public accounting firm. Refer to Report of Independent Registered Public Accounting Firm which is included in Item 8 of Part II of this Annual Report on Form 10-K and is incorporated by reference to this Item 9A.
(b) Change in Internal Controls. During the fourth quarter of 2020, we completed the final phase of our ERP implementation by migrating the remaining legacy entities to the new system. As a result, we have implemented updates and changes to our current processes and related control activities and have evaluated the operating effectiveness of related key controls.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information concerning directors, appearing under the caption "III. Board of Directors" in our Proxy Statement to be filed with the SEC in connection with the Annual Meeting of Stockholders scheduled to be held on April 27, 2021 (the "Proxy Statement"), information concerning executive officers, appearing under the caption "Item 4A. Information about our Executive Officers" in Part I of this Annual Report on Form 10-K, information concerning the Audit Committee, appearing under the caption "IV. Information About the Board of Directors and Corporate Governance - Committees and Independence of Directors - Audit Committee" in the Proxy Statement, and information concerning the Code of Ethics, appearing under the caption "IV. Information About the Board of Directors and Corporate Governance - Corporate Governance - Code of Ethics and Business Conduct Policy" in the Proxy Statement, is incorporated herein by reference in response to this Item 10.
ITEM 11. EXECUTIVE COMPENSATION
The information contained in the Proxy Statement in the section titled "VI. Executive Compensation" with respect to executive compensation, in the section titled "IV. Information About the Board of Directors and Corporate Governance—Director Compensation" and "—Corporate Governance—Compensation and Organization Committee Interlocks and Insider Participation" is incorporated herein by reference in response to this Item 11.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information contained in the section titled "V. Security Ownership of FMC Corporation" in the Proxy Statement, with respect to security ownership of certain beneficial owners and management, is incorporated herein by reference in response to this Item 12.
Equity Compensation Plan Information
The table below sets forth information with respect to compensation plans under which equity securities of FMC are authorized for issuance as of December 31, 2020. All of the equity compensation plans pursuant to which we are currently granting equity awards have been approved by stockholders.
| | | | | | | | | | | | | | | | | | | | |
| (Shares in thousands) | | Number of Securities to be issued upon exercise of outstanding options and restricted stock awards (A) (2) | | Weighted-average exercise price of outstanding options awards (B) (1) | | Number of Securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (A)) (C) |
| Equity Compensation Plans approved by stockholders | | 2,003 | | | $ | 70.44 | | | 3,200 | |
____________________
(1)Taking into account all outstanding awards included in this table, the weighted-average exercise price of such stock options is $70.44 and the weighted-average term-to-expiration is 7.0 years.
(2)Includes 1,235 thousand stock options and 500 thousand restricted stock awards granted to employees and 268 thousand restricted stock units held by directors.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information contained in the Proxy Statement concerning our independent directors and related party transactions under the caption "IV. Information About the Board of Directors and Corporate Governance—Committees and Independence of Directors," and the information contained in the Proxy Statement concerning our related party transactions policy, appearing under the caption "IV. Information About the Board of Directors and Corporate Governance—Corporate Governance—Related Party Transactions Policy," is incorporated herein by reference in response to this Item 13.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information contained in the Proxy Statement in the section titled "II. The Proposals to be Voted On—Ratification of Appointment of Independent Registered Public Accounting Firm" is incorporated herein by reference in response to this Item 14.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)Documents filed with this Report
1. Consolidated financial statements of FMC Corporation and its subsidiaries are incorporated under Item 8 of this Form 10-K.
2. The following supplementary financial information is filed in this Form 10-K:
| | | | | |
| | Page |
| Financial Statements Schedule II – Valuation and qualifying accounts and reserves for the years ended December 31, 2020, 2019, and 2018 | |
The schedules not included herein are omitted because they are not applicable or the required information is presented in the financial statements or related notes.
3. Exhibits – The following exhibits are filed as a part of, or incorporated by reference into, this Form 10-K:
(a)Exhibits
| | | | | |
| Exhibit No. | Exhibit Description |
| (2) | | Plan of acquisition, reorganization, arrangement, liquidation or succession |
| |
| *2.1a | |
| |
| *2.1b | |
| |
| (3) | | Articles of Incorporation and By-Laws |
| |
| *3.1 | |
| |
| *3.2 | |
| |
| (4) | | Instruments defining the rights of security holders, including indentures. FMC Corporation undertakes to furnish to the SEC upon request, a copy of any instrument defining the rights of holders of long-term debt of FMC Corporation and its consolidated subsidiaries and for any of its unconsolidated subsidiaries for which financial statements are required to be filed. |
| |
| *4.1 | |
| |
| *4.2 | |
| |
| *4.3 | |
| |
| *4.4 | |
| |
| *4.5 | |
| |
| *4.6 | |
| |
| (10) | | Material contracts |
| |
| *10.1a | |
| | | | | |
| |
| *10.1b | Amendment No. 1, dated September 28, 2018, to the Term Loan Agreement, dated as of May 2, 2017, among FMC Corporation, certain subsidiaries of FMC Corporation from time to time party thereto as borrowers, Citibank, N.A., as Administrative Agent, each lender from time to time party thereto and the other parties thereto. (Exhibit 10.2 to the Current Report on Form 8-K filed on October 3, 2018) |
| |
| *10.1c | |
| |
| *10.1d | Amendment No. 1, dated as of April 22, 2020, to the Third Amended and Restated Credit Agreement, dated as of May 17, 2019, among FMC Corporation, certain subsidiaries of FMC Corporation party thereto, the lenders and issuing banks party thereto, and Citibank, N.A., as Administrative Agent for such lenders. (Exhibit 10.1 to the Current Report on Form 8-K filed on April 22, 2020) |
| |
| *10.1e | Amendment No. 2, dated as of April 22, 2020, to the Term Loan Agreement, dated as of May 2, 2017, among FMC Corporation, certain subsidiaries of FMC Corporation party thereto, the lenders party thereto, and Citibank, N.A., as Administrative Agent for such lenders. (Exhibit 10.2 to the Current Report on Form 8-K filed on April 22, 2020) |
| |
| †10.2 | |
| |
| †*10.2.a | |
| |
| †*10.2.b | |
| |
| †*10.3 | |
| |
| †*10.4 | |
| |
| †*10.5 | |
| |
| †*10.5a | |
| |
| †*10.5b | |
| |
| †*10.6 | |
| |
| †* 10.6a | |
| |
| †* 10.6b | |
| |
| †*10.6c | |
| |
| †*10.6d | |
| |
| †*10.6e | |
| |
| †*10.6f | |
| |
| | | | | |
| †*10.6g | |
| |
| †*10.7 | |
| |
| †*10.7a | |
| |
| †*10.7b | |
| |
| †*10.7c | |
| |
| *10.7d | |
| |
| †*10.7e | |
| |
| †*10.7f | |
| |
| †*10.8 | |
| |
| †*10.9 | |
| |
| †10.10 | |
| |
| *10.11 | |
| |
| *10.12 | |
| |
| *10.13 | |
| |
| *10.14 | |
| |
| *10.15 | |
| |
| †10.16 | |
| |
| *10.17 | |
| |
| †10.18 | |
| |
| †10.19 | |
| |
| †*10.20 | |
| |
| 21 | | |
| |
| 23.1 | | |
| |
| 31.1 | | |
| |
| 31.2 | | |
| |
| | | | | |
| 32.1 | | |
| |
| 32.2 | | |
| |
| 101 | | Interactive Data File |
* Incorporated by reference
† Management contract or compensatory plan or arrangement
ITEM 16. FORM 10-K SUMMARY
Optional disclosure, not included in this Report.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
FMC CORPORATION
(Registrant)
| | | | | |
| By: | /S/ ANDREW D. SANDIFER |
| Andrew D. Sandifer Executive Vice President and Chief Financial Officer |
Date: February 25, 2021
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
| | | | | | | | |
| Signature | Title | Date |
/S/ ANDREW D. SANDIFER Andrew D. Sandifer | Executive Vice President and Chief Financial Officer | February 25, 2021 |
| | |
/S/ NICHOLAS L. PFEIFFER Nicholas L. Pfeiffer | Vice President, Chief Accounting Officer, and Corporate Controller | February 25, 2021 |
| | |
/S/ PIERRE R. BRONDEAU Pierre R. Brondeau | Executive Chairman | February 25, 2021 |
| | |
/S/ MARK A. DOUGLAS Mark A. Douglas | President, Chief Executive Officer, and Director | February 25, 2021 |
| | |
/S/ EDUARDO E. CORDEIRO Eduardo E. Cordeiro | Director | February 25, 2021 |
| | |
/S/ CAROL ANTHONY ("JOHN") DAVIDSON Carol Anthony ("John") Davidson | Director | February 25, 2021 |
| | |
/S/ C. SCOTT GREER C. Scott Greer | Director | February 25, 2021 |
| | |
/S/ K'LYNNE JOHNSON K'Lynne Johnson | Director | February 25, 2021 |
| | |
/S/ DIRK A. KEMPTHORNE Dirk A. Kempthorne | Director | February 25, 2021 |
| | |
/S/ PAUL J. NORRIS Paul J. Norris | Director | February 25, 2021 |
| | |
/S/ MARGARETH ØVRUM Margareth Øvrum | Director | February 25, 2021 |
| | |
/S/ ROBERT C. PALLASH Robert C. Pallash | Director | February 25, 2021 |
| | |
/S/ VINCENT R. VOLPE, JR. Vincent R. Volpe, Jr. | Director | February 25, 2021 |