false2022FY0000313616http://fasb.org/us-gaap/2022#OperatingIncomeLosshttp://fasb.org/us-gaap/2022#PropertyPlantAndEquipmentNet1212http://fasb.org/us-gaap/2022#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2022#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2022#AccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2022#AccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2022#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2022#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2022#OtherLiabilitiesNoncurrent00003136162022-01-012022-12-310000313616us-gaap:CommonStockMember2022-01-012022-12-310000313616us-gaap:SeriesBPreferredStockMember2022-01-012022-12-310000313616dhr:A17SeniorUnsecuredNotesDue2024Member2022-01-012022-12-310000313616dhr:A0.2SeniorNotesDue2026Member2022-01-012022-12-310000313616dhr:A21SeniorUnsecuredNotesDue2026Member2022-01-012022-12-310000313616dhr:A1.2SeniorNotesDue2027Member2022-01-012022-12-310000313616dhr:A0.45SeniorNotesDue2028Member2022-01-012022-12-310000313616dhr:A25SeniorUnsecuredNotesDue2030Member2022-01-012022-12-310000313616dhr:A0.75SeniorNotesDue2031Member2022-01-012022-12-310000313616dhr:A1.35SeniorNotesDue2039Member2022-01-012022-12-310000313616dhr:A1.8SeniorNotesDue2049Member2022-01-012022-12-3100003136162023-02-03xbrli:shares00003136162022-07-01iso4217:USD00003136162022-12-3100003136162021-12-31iso4217:USDxbrli:shares0000313616us-gaap:SeriesAPreferredStockMember2022-12-310000313616us-gaap:SeriesAPreferredStockMember2021-12-310000313616us-gaap:SeriesAPreferredStockMember2021-01-012021-12-31xbrli:pure0000313616us-gaap:SeriesAPreferredStockMember2022-01-012022-12-310000313616us-gaap:SeriesBPreferredStockMember2021-12-310000313616us-gaap:SeriesBPreferredStockMember2022-12-310000313616us-gaap:SeriesBPreferredStockMember2021-01-012021-12-3100003136162021-01-012021-12-3100003136162020-01-012020-12-310000313616us-gaap:PreferredStockMember2021-12-310000313616us-gaap:PreferredStockMember2020-12-310000313616us-gaap:PreferredStockMember2019-12-310000313616us-gaap:PreferredStockMember2022-01-012022-12-310000313616us-gaap:PreferredStockMember2021-01-012021-12-310000313616us-gaap:PreferredStockMember2020-01-012020-12-310000313616us-gaap:PreferredStockMember2022-12-310000313616us-gaap:CommonStockMember2021-12-310000313616us-gaap:CommonStockMember2020-12-310000313616us-gaap:CommonStockMember2019-12-310000313616us-gaap:CommonStockMember2022-01-012022-12-310000313616us-gaap:CommonStockMember2021-01-012021-12-310000313616us-gaap:CommonStockMember2020-01-012020-12-310000313616us-gaap:CommonStockMember2022-12-310000313616us-gaap:AdditionalPaidInCapitalMember2021-12-310000313616us-gaap:AdditionalPaidInCapitalMember2020-12-310000313616us-gaap:AdditionalPaidInCapitalMember2019-12-310000313616us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310000313616us-gaap:AdditionalPaidInCapitalMember2021-01-012021-12-310000313616us-gaap:AdditionalPaidInCapitalMember2020-01-012020-12-310000313616us-gaap:AdditionalPaidInCapitalMemberus-gaap:PreferredStockMember2022-01-012022-12-310000313616us-gaap:AdditionalPaidInCapitalMemberus-gaap:PreferredStockMember2021-01-012021-12-310000313616us-gaap:AdditionalPaidInCapitalMemberus-gaap:PreferredStockMember2020-01-012020-12-310000313616dhr:LiquidYieldOptionNotesMemberus-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310000313616dhr:LiquidYieldOptionNotesMemberus-gaap:AdditionalPaidInCapitalMember2021-01-012021-12-310000313616dhr:LiquidYieldOptionNotesMemberus-gaap:AdditionalPaidInCapitalMember2020-01-012020-12-310000313616us-gaap:AdditionalPaidInCapitalMember2022-12-310000313616us-gaap:RetainedEarningsMember2021-12-310000313616us-gaap:RetainedEarningsMember2020-12-310000313616us-gaap:RetainedEarningsMember2019-12-310000313616srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:RetainedEarningsMember2021-12-310000313616srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:RetainedEarningsMember2020-12-310000313616srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:RetainedEarningsMember2019-12-310000313616us-gaap:RetainedEarningsMember2022-01-012022-12-310000313616us-gaap:RetainedEarningsMember2021-01-012021-12-310000313616us-gaap:RetainedEarningsMember2020-01-012020-12-310000313616us-gaap:RetainedEarningsMember2022-12-310000313616us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310000313616us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310000313616us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-12-310000313616us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310000313616us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-12-310000313616us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310000313616us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310000313616us-gaap:NoncontrollingInterestMember2021-12-310000313616us-gaap:NoncontrollingInterestMember2020-12-310000313616us-gaap:NoncontrollingInterestMember2019-12-310000313616us-gaap:NoncontrollingInterestMember2022-01-012022-12-310000313616us-gaap:NoncontrollingInterestMember2021-01-012021-12-310000313616us-gaap:NoncontrollingInterestMember2020-01-012020-12-310000313616us-gaap:NoncontrollingInterestMember2022-12-3100003136162020-12-3100003136162019-12-31dhr:businessSegment0000313616srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccountingStandardsUpdate201613Member2020-01-012020-01-010000313616us-gaap:BuildingMember2022-01-012022-12-310000313616us-gaap:MachineryAndEquipmentMembersrt:MinimumMember2022-01-012022-12-310000313616us-gaap:MachineryAndEquipmentMembersrt:MaximumMember2022-01-012022-12-310000313616srt:MinimumMemberdhr:AssetsLeasedToCustomersMember2022-01-012022-12-310000313616srt:MaximumMemberdhr:AssetsLeasedToCustomersMember2022-01-012022-12-310000313616us-gaap:LandAndLandImprovementsMember2022-12-310000313616us-gaap:LandAndLandImprovementsMember2021-12-310000313616us-gaap:BuildingMember2022-12-310000313616us-gaap:BuildingMember2021-12-310000313616us-gaap:MachineryAndEquipmentMember2022-12-310000313616us-gaap:MachineryAndEquipmentMember2021-12-310000313616dhr:AssetsLeasedToCustomersMember2022-12-310000313616dhr:AssetsLeasedToCustomersMember2021-12-310000313616srt:MinimumMember2022-01-012022-12-310000313616srt:MaximumMember2022-01-012022-12-310000313616us-gaap:OperatingExpenseMember2022-01-012022-12-310000313616dhr:CapitalExpendituresMember2022-12-310000313616us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2022-01-012022-12-31dhr:businessdhr:segment0000313616us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2022-12-310000313616dhr:AldevronMember2021-08-302021-08-300000313616dhr:AldevronMember2021-01-012021-12-310000313616dhr:AldevronMember2021-12-310000313616us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2021-01-012021-12-310000313616us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2021-12-310000313616dhr:CytivaMember2020-03-312020-03-310000313616dhr:CytivaMember2020-03-3100003136162020-04-302020-04-3000003136162019-01-012019-12-310000313616dhr:CytivaMember2019-01-012019-12-310000313616dhr:CytivaMember2020-12-310000313616us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2020-01-012020-12-310000313616us-gaap:SeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2020-12-310000313616dhr:CytivaSeriesOfIndividuallyImmaterialBusinessAcquisitionsMember2020-01-012020-12-310000313616dhr:TotalAcquisitionsMember2022-12-310000313616dhr:TotalAcquisitionsMember2021-12-310000313616dhr:TotalAcquisitionsMember2020-12-310000313616dhr:TotalAcquisitionsMember2022-01-012022-12-310000313616dhr:TotalAcquisitionsMember2021-01-012021-12-310000313616dhr:TotalAcquisitionsMember2020-01-012020-12-310000313616dhr:CytivaMember2020-01-012020-12-310000313616dhr:AldevronMemberus-gaap:FairValueAdjustmentToInventoryMember2021-01-012021-12-310000313616dhr:CytivaMemberus-gaap:FairValueAdjustmentToInventoryMember2021-01-012021-12-310000313616dhr:CytivaMemberus-gaap:FairValueAdjustmentToInventoryMember2020-01-012020-12-310000313616dhr:EnvironmentalAppliedSolutionsMember2022-01-012022-12-310000313616us-gaap:SeriesAPreferredStockMemberus-gaap:PreferredStockMember2022-04-152022-04-150000313616srt:NorthAmericaMemberdhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2022-01-012022-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2022-01-012022-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2022-01-012022-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2022-01-012022-12-310000313616srt:NorthAmericaMember2022-01-012022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:WesternEuropeMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:WesternEuropeMemberdhr:LifeSciencesMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:WesternEuropeMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:WesternEuropeMember2022-01-012022-12-310000313616dhr:WesternEuropeMember2022-01-012022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:OtherdevelopedmarketsMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:OtherdevelopedmarketsMemberdhr:LifeSciencesMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:OtherdevelopedmarketsMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:OtherdevelopedmarketsMember2022-01-012022-12-310000313616dhr:OtherdevelopedmarketsMember2022-01-012022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:LifeSciencesMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:DiagnosticsMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:EnvironmentalAppliedSolutionsMember2022-01-012022-12-310000313616dhr:HighgrowthmarketsMember2022-01-012022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2022-01-012022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMemberdhr:LifeSciencesMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2022-01-012022-12-310000313616dhr:RevenuefromContractwithCustomerMeasurementRecurringMember2022-01-012022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMemberdhr:LifeSciencesMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2022-01-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2022-01-012022-12-310000313616dhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2022-01-012022-12-310000313616srt:NorthAmericaMemberdhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2021-01-012021-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2021-01-012021-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2021-01-012021-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2021-01-012021-12-310000313616srt:NorthAmericaMember2021-01-012021-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:WesternEuropeMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:WesternEuropeMemberdhr:LifeSciencesMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:WesternEuropeMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:WesternEuropeMember2021-01-012021-12-310000313616dhr:WesternEuropeMember2021-01-012021-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:OtherdevelopedmarketsMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:OtherdevelopedmarketsMemberdhr:LifeSciencesMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:OtherdevelopedmarketsMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:OtherdevelopedmarketsMember2021-01-012021-12-310000313616dhr:OtherdevelopedmarketsMember2021-01-012021-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:LifeSciencesMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:DiagnosticsMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:EnvironmentalAppliedSolutionsMember2021-01-012021-12-310000313616dhr:HighgrowthmarketsMember2021-01-012021-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2021-01-012021-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMemberdhr:LifeSciencesMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2021-01-012021-12-310000313616dhr:RevenuefromContractwithCustomerMeasurementRecurringMember2021-01-012021-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMemberdhr:LifeSciencesMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2021-01-012021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2021-01-012021-12-310000313616dhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2021-01-012021-12-310000313616srt:NorthAmericaMemberdhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2020-01-012020-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2020-01-012020-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2020-01-012020-12-310000313616srt:NorthAmericaMemberus-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2020-01-012020-12-310000313616srt:NorthAmericaMember2020-01-012020-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:WesternEuropeMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:WesternEuropeMemberdhr:LifeSciencesMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:WesternEuropeMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:WesternEuropeMember2020-01-012020-12-310000313616dhr:WesternEuropeMember2020-01-012020-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:OtherdevelopedmarketsMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:OtherdevelopedmarketsMemberdhr:LifeSciencesMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:OtherdevelopedmarketsMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:OtherdevelopedmarketsMember2020-01-012020-12-310000313616dhr:OtherdevelopedmarketsMember2020-01-012020-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:LifeSciencesMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:DiagnosticsMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:HighgrowthmarketsMemberdhr:EnvironmentalAppliedSolutionsMember2020-01-012020-12-310000313616dhr:HighgrowthmarketsMember2020-01-012020-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2020-01-012020-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMemberdhr:LifeSciencesMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:RevenuefromContractwithCustomerMeasurementRecurringMember2020-01-012020-12-310000313616dhr:RevenuefromContractwithCustomerMeasurementRecurringMember2020-01-012020-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMemberdhr:LifeSciencesMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2020-01-012020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMemberdhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2020-01-012020-12-310000313616dhr:RevenuefromContractwithCustomerMeasurementNonrecurringMember2020-01-012020-12-3100003136162023-01-012022-12-3100003136162024-01-012022-12-310000313616us-gaap:CorporateNonSegmentMember2022-01-012022-12-310000313616us-gaap:CorporateNonSegmentMember2021-01-012021-12-310000313616us-gaap:CorporateNonSegmentMember2020-01-012020-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2021-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2020-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2021-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2020-12-310000313616us-gaap:CorporateNonSegmentMember2022-12-310000313616us-gaap:CorporateNonSegmentMember2021-12-310000313616us-gaap:CorporateNonSegmentMember2020-12-310000313616srt:ReportableGeographicalComponentsMembercountry:US2022-01-012022-12-310000313616srt:ReportableGeographicalComponentsMembercountry:US2021-01-012021-12-310000313616srt:ReportableGeographicalComponentsMembercountry:US2020-01-012020-12-310000313616srt:ReportableGeographicalComponentsMembercountry:CN2022-01-012022-12-310000313616srt:ReportableGeographicalComponentsMembercountry:CN2021-01-012021-12-310000313616srt:ReportableGeographicalComponentsMembercountry:CN2020-01-012020-12-310000313616srt:ReportableGeographicalComponentsMembercountry:DE2022-01-012022-12-310000313616srt:ReportableGeographicalComponentsMembercountry:DE2021-01-012021-12-310000313616srt:ReportableGeographicalComponentsMembercountry:DE2020-01-012020-12-310000313616srt:ReportableGeographicalComponentsMemberus-gaap:SalesRevenueNetMemberus-gaap:GeographicConcentrationRiskMember2022-01-012022-12-310000313616srt:ReportableGeographicalComponentsMemberus-gaap:SalesRevenueNetMemberus-gaap:GeographicConcentrationRiskMember2020-01-012020-12-310000313616srt:ReportableGeographicalComponentsMemberus-gaap:SalesRevenueNetMemberus-gaap:GeographicConcentrationRiskMember2021-01-012021-12-310000313616srt:ReportableGeographicalComponentsMemberdhr:AllOtherCountriesMember2022-01-012022-12-310000313616srt:ReportableGeographicalComponentsMemberdhr:AllOtherCountriesMember2021-01-012021-12-310000313616srt:ReportableGeographicalComponentsMemberdhr:AllOtherCountriesMember2020-01-012020-12-310000313616srt:ReportableGeographicalComponentsMembercountry:US2022-12-310000313616srt:ReportableGeographicalComponentsMembercountry:US2021-12-310000313616srt:ReportableGeographicalComponentsMembercountry:US2020-12-310000313616srt:ReportableGeographicalComponentsMembercountry:SE2022-12-310000313616srt:ReportableGeographicalComponentsMembercountry:SE2021-12-310000313616srt:ReportableGeographicalComponentsMembercountry:SE2020-12-310000313616srt:ReportableGeographicalComponentsMembercountry:GB2022-12-310000313616srt:ReportableGeographicalComponentsMembercountry:GB2021-12-310000313616srt:ReportableGeographicalComponentsMembercountry:GB2020-12-310000313616srt:ReportableGeographicalComponentsMembercountry:DE2022-12-310000313616srt:ReportableGeographicalComponentsMembercountry:DE2021-12-310000313616srt:ReportableGeographicalComponentsMembercountry:DE2020-12-310000313616srt:ReportableGeographicalComponentsMemberus-gaap:PropertyPlantAndEquipmentMemberus-gaap:GeographicConcentrationRiskMember2021-01-012021-12-310000313616srt:ReportableGeographicalComponentsMemberus-gaap:PropertyPlantAndEquipmentMemberus-gaap:GeographicConcentrationRiskMember2022-01-012022-12-310000313616srt:ReportableGeographicalComponentsMemberus-gaap:PropertyPlantAndEquipmentMemberus-gaap:GeographicConcentrationRiskMember2020-01-012020-12-310000313616srt:ReportableGeographicalComponentsMemberdhr:AllOtherCountriesMember2022-12-310000313616srt:ReportableGeographicalComponentsMemberdhr:AllOtherCountriesMember2021-12-310000313616srt:ReportableGeographicalComponentsMemberdhr:AllOtherCountriesMember2020-12-310000313616dhr:AnalyticalAndPhysicalInstrumentationMember2022-01-012022-12-310000313616dhr:AnalyticalAndPhysicalInstrumentationMember2021-01-012021-12-310000313616dhr:AnalyticalAndPhysicalInstrumentationMember2020-01-012020-12-310000313616dhr:ResearchandmedicalproductsMember2022-01-012022-12-310000313616dhr:ResearchandmedicalproductsMember2021-01-012021-12-310000313616dhr:ResearchandmedicalproductsMember2020-01-012020-12-310000313616dhr:ProductIdentificationMember2022-01-012022-12-310000313616dhr:ProductIdentificationMember2021-01-012021-12-310000313616dhr:ProductIdentificationMember2020-01-012020-12-310000313616country:US2022-01-012022-12-310000313616country:US2021-01-012021-12-310000313616country:US2020-01-012020-12-310000313616us-gaap:NonUsMember2022-01-012022-12-310000313616us-gaap:NonUsMember2021-01-012021-12-310000313616us-gaap:NonUsMember2020-01-012020-12-310000313616country:US2022-12-310000313616country:US2021-12-310000313616us-gaap:NonUsMember2022-12-310000313616us-gaap:NonUsMember2021-12-310000313616dhr:GeneralBusinessAndForeignTaxCreditMember2022-12-310000313616dhr:DeferredTaxAssetOtherMember2022-12-310000313616us-gaap:InternalRevenueServiceIRSMemberus-gaap:DomesticCountryMember2022-10-012022-12-3100003136162017-01-012017-12-3100003136162017-12-222017-12-310000313616us-gaap:ForeignCountryMember2021-01-012021-04-02iso4217:DKK0000313616us-gaap:ForeignCountryMember2022-01-012022-12-310000313616us-gaap:ForeignCountryMember2021-01-012021-12-310000313616us-gaap:ForeignCountryMember2020-01-012020-12-3100003136162021-07-242021-07-240000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-01-012022-12-310000313616dhr:A2.5SeniorUnsecuredNotesDue2025Memberus-gaap:SeniorNotesMember2021-10-022021-12-31iso4217:EUR0000313616dhr:A2.5SeniorUnsecuredNotesDue2025Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A1.7SeniorUnsecuredNotesDue2022Memberus-gaap:SeniorNotesMember2020-10-032020-12-310000313616dhr:A1.7SeniorUnsecuredNotesDue2022Memberus-gaap:SeniorNotesMember2020-12-310000313616srt:MaximumMember2022-12-3100003136162022-01-012022-09-30dhr:Reporting_Unit00003136162022-10-012022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2022-10-010000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2022-10-010000313616srt:MinimumMember2022-09-300000313616srt:MaximumMember2022-09-300000313616srt:MinimumMember2022-12-310000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2022-01-012022-09-300000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2022-01-012022-09-300000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2022-01-012022-09-300000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2022-01-012022-09-300000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2022-09-300000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2022-09-300000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2022-09-300000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2022-09-3000003136162022-09-300000313616dhr:BiotechnologyMemberus-gaap:OperatingSegmentsMember2022-10-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:LifeSciencesMember2022-10-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:DiagnosticsMember2022-10-012022-12-310000313616us-gaap:OperatingSegmentsMemberdhr:EnvironmentalAppliedSolutionsMember2022-10-012022-12-310000313616us-gaap:PatentedTechnologyMember2022-12-310000313616us-gaap:PatentedTechnologyMember2021-12-310000313616us-gaap:CustomerRelationshipsMember2022-12-310000313616us-gaap:CustomerRelationshipsMember2021-12-310000313616us-gaap:TrademarksAndTradeNamesMember2022-12-310000313616us-gaap:TrademarksAndTradeNamesMember2021-12-310000313616us-gaap:FairValueInputsLevel1Member2022-12-310000313616us-gaap:FairValueInputsLevel1Member2021-12-310000313616us-gaap:FairValueInputsLevel2Member2022-12-310000313616us-gaap:FairValueInputsLevel2Member2021-12-310000313616us-gaap:FairValueInputsLevel3Member2022-12-310000313616us-gaap:FairValueInputsLevel3Member2021-12-310000313616us-gaap:PartnershipMember2022-12-310000313616us-gaap:PartnershipMember2021-12-310000313616us-gaap:CarryingReportedAmountFairValueDisclosureMember2022-12-310000313616us-gaap:EstimateOfFairValueFairValueDisclosureMember2022-12-310000313616us-gaap:CarryingReportedAmountFairValueDisclosureMember2021-12-310000313616us-gaap:EstimateOfFairValueFairValueDisclosureMember2021-12-310000313616dhr:USDollarDenominatedCommercialPaperMemberus-gaap:CommercialPaperMember2022-12-310000313616dhr:USDollarDenominatedCommercialPaperMemberus-gaap:CommercialPaperMember2021-12-310000313616dhr:EuroDenominatedCommercialPaperMemberus-gaap:CommercialPaperMember2022-12-310000313616dhr:EuroDenominatedCommercialPaperMemberus-gaap:CommercialPaperMember2021-12-310000313616dhr:FloatingRateSeniorUnsecuredNotesDue2022Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:FloatingRateSeniorUnsecuredNotesDue2022Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A2.05SeniorUnsecuredNotesDue2022Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A2.05SeniorUnsecuredNotesDue2022Memberus-gaap:SeniorNotesMember2021-12-310000313616us-gaap:BondsMemberdhr:A0.5SeniorUnsecuredBondsDue2023Member2022-12-310000313616us-gaap:BondsMemberdhr:A0.5SeniorUnsecuredBondsDue2023Member2021-12-31iso4217:CHF0000313616dhr:A17SeniorUnsecuredNotesDue2024Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A17SeniorUnsecuredNotesDue2024Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A2.2SeniorUnsecuredNotesDue2024Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A2.2SeniorUnsecuredNotesDue2024Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A335SeniorUnsecuredNotesDue2025Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A335SeniorUnsecuredNotesDue2025Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A0.2SeniorUnsecuredNotesDue2026Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A0.2SeniorUnsecuredNotesDue2026Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A21SeniorUnsecuredNotesDue2026Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A21SeniorUnsecuredNotesDue2026Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A03SeniorUnsecuredNotesDue2027Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A03SeniorUnsecuredNotesDue2027Memberus-gaap:SeniorNotesMember2021-12-31iso4217:JPY0000313616dhr:A1.2SeniorUnsecuredNotesDue2027Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A1.2SeniorUnsecuredNotesDue2027Memberus-gaap:SeniorNotesMember2022-12-310000313616us-gaap:SeniorNotesMemberdhr:A0.45SeniorUnsecuredNotesDue2028Member2021-12-310000313616us-gaap:SeniorNotesMemberdhr:A0.45SeniorUnsecuredNotesDue2028Member2022-12-310000313616us-gaap:BondsMemberdhr:A1.125SeniorUnsecuredBondsDue2028Member2021-12-310000313616us-gaap:BondsMemberdhr:A1.125SeniorUnsecuredBondsDue2028Member2022-12-310000313616dhr:A2.6SeniorUnsecuredNotesDue2029Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A2.6SeniorUnsecuredNotesDue2029Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A25SeniorUnsecuredNotesDue2030Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A25SeniorUnsecuredNotesDue2030Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A0.75SeniorUnsecuredNotesDue2031Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A0.75SeniorUnsecuredNotesDue2031Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A065SeniorUnsecuredNotesDue2032Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A065SeniorUnsecuredNotesDue2032Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A1.35SeniorUnsecuredNotesDue2039Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A1.35SeniorUnsecuredNotesDue2039Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A3.25SeniorUnsecuredNotesDue2039Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A3.25SeniorUnsecuredNotesDue2039Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A4375SeniorUnsecuredNotesDue2045Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A4375SeniorUnsecuredNotesDue2045Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A1.8SeniorUnsecuredNotesDue2049Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A1.8SeniorUnsecuredNotesDue2049Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:A3.4SeniorUnsecuredNotesDue2049Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A3.4SeniorUnsecuredNotesDue2049Memberus-gaap:SeniorNotesMember2021-12-310000313616us-gaap:SeniorNotesMemberdhr:A26SeniorUnsecuredNotesDue2050Member2021-12-310000313616us-gaap:SeniorNotesMemberdhr:A26SeniorUnsecuredNotesDue2050Member2022-12-310000313616dhr:A28SeniorUnsecuredNotesDue2051Memberus-gaap:SeniorNotesMember2022-12-310000313616dhr:A28SeniorUnsecuredNotesDue2051Memberus-gaap:SeniorNotesMember2021-12-310000313616dhr:OtherMember2022-12-310000313616dhr:OtherMember2021-12-310000313616dhr:FiveYearFacilityMemberus-gaap:LongTermDebtMemberus-gaap:RevolvingCreditFacilityMember2019-12-310000313616dhr:FiveYearFacilityMemberus-gaap:LongTermDebtMemberus-gaap:RevolvingCreditFacilityMember2019-01-012019-12-310000313616srt:MinimumMemberdhr:FiveYearFacilityMemberus-gaap:LongTermDebtMemberus-gaap:RevolvingCreditFacilityMemberus-gaap:LondonInterbankOfferedRateLIBORMember2019-01-012019-12-310000313616dhr:FiveYearFacilityMemberus-gaap:LongTermDebtMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberus-gaap:LondonInterbankOfferedRateLIBORMember2019-01-012019-12-310000313616us-gaap:FederalFundsEffectiveSwapRateMemberdhr:FiveYearFacilityMemberus-gaap:LongTermDebtMemberus-gaap:RevolvingCreditFacilityMember2019-01-012019-12-310000313616dhr:FiveYearFacilityMemberus-gaap:LongTermDebtMemberus-gaap:EurodollarMemberus-gaap:RevolvingCreditFacilityMember2019-01-012019-12-310000313616srt:MinimumMemberdhr:FiveYearFacilityMemberus-gaap:LongTermDebtMemberus-gaap:RevolvingCreditFacilityMember2019-01-012019-12-310000313616dhr:FiveYearFacilityMemberus-gaap:LongTermDebtMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMember2019-01-012019-12-310000313616srt:MinimumMemberdhr:FiveYearFacilityMemberus-gaap:LongTermDebtMemberus-gaap:RevolvingCreditFacilityMember2019-12-310000313616dhr:FiveYearFacilityMemberus-gaap:LongTermDebtMembersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMember2019-12-310000313616dhr:FiveYearFacilityMemberus-gaap:LongTermDebtMemberus-gaap:RevolvingCreditFacilityMember2022-12-310000313616dhr:EuroDenominatedCommercialPaperMemberus-gaap:CommercialPaperMember2021-12-312021-12-310000313616dhr:SwissFrancDenominatedSeniorUnsecuredBondsMemberus-gaap:BondsMember2022-01-012022-12-310000313616us-gaap:SeniorNotesMember2022-01-012022-12-310000313616dhr:A2027And2032YenNotesMemberus-gaap:SeniorNotesMember2022-01-012022-12-310000313616dhr:ZeroCouponLYONSDue2021Memberus-gaap:ConvertibleDebtMember2001-12-310000313616dhr:ZeroCouponLYONSDue2021Memberus-gaap:ConvertibleDebtMember2001-01-012001-12-310000313616dhr:ZeroCouponLYONSDue2021Memberus-gaap:ConvertibleDebtMember2021-01-012021-12-310000313616dhr:FloatingRateSeniorUnsecuredNotesDue2022Memberus-gaap:SeniorNotesMember2022-06-300000313616dhr:A2.05SeniorUnsecuredNotesDue2022Memberus-gaap:SeniorNotesMember2022-11-150000313616dhr:A0352SeniorUnsecuredNotesDue2021Memberus-gaap:SeniorNotesMember2021-01-012021-04-020000313616us-gaap:NetInvestmentHedgingMemberus-gaap:ForeignExchangeContractMember2022-12-310000313616us-gaap:NetInvestmentHedgingMemberus-gaap:ForeignExchangeContractMember2022-01-012022-12-310000313616us-gaap:NetInvestmentHedgingMemberdhr:ForeignCurrencyDenominatedDebtMember2022-01-012022-12-310000313616us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeContractMember2022-12-310000313616us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeContractMember2022-01-012022-12-310000313616us-gaap:InterestRateSwapMemberus-gaap:CashFlowHedgingMember2022-12-310000313616us-gaap:InterestRateSwapMemberus-gaap:CashFlowHedgingMember2022-01-012022-12-310000313616dhr:CashFlowAndNetInvestmentHedgesMember2022-12-310000313616dhr:CashFlowAndNetInvestmentHedgesMember2022-01-012022-12-310000313616us-gaap:NetInvestmentHedgingMemberus-gaap:ForeignExchangeContractMember2021-12-310000313616us-gaap:NetInvestmentHedgingMemberus-gaap:ForeignExchangeContractMember2021-01-012021-12-310000313616us-gaap:NetInvestmentHedgingMemberdhr:ForeignCurrencyDenominatedDebtMember2021-01-012021-12-310000313616us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeContractMember2021-12-310000313616us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeContractMember2021-01-012021-12-310000313616us-gaap:InterestRateSwapMemberus-gaap:CashFlowHedgingMember2021-12-310000313616us-gaap:InterestRateSwapMemberus-gaap:CashFlowHedgingMember2021-01-012021-12-310000313616dhr:CashFlowAndNetInvestmentHedgesMember2021-12-310000313616dhr:CashFlowAndNetInvestmentHedgesMember2021-01-012021-12-310000313616us-gaap:NetInvestmentHedgingMember2022-12-310000313616us-gaap:NetInvestmentHedgingMember2021-12-310000313616us-gaap:NetInvestmentHedgingMemberus-gaap:LongTermDebtMember2021-12-312021-12-310000313616us-gaap:NetInvestmentHedgingMemberus-gaap:LongTermDebtMember2020-12-312020-12-310000313616us-gaap:PensionPlansDefinedBenefitMembercountry:US2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMembercountry:US2020-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2020-12-310000313616us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-12-310000313616us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2020-12-310000313616us-gaap:PensionPlansDefinedBenefitMembercountry:US2022-01-012022-12-310000313616us-gaap:PensionPlansDefinedBenefitMembercountry:US2021-01-012021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2021-01-012021-12-310000313616us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-01-012022-12-310000313616us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2021-01-012021-12-310000313616us-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMember2022-12-310000313616us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMembercountry:US2020-01-012020-12-310000313616us-gaap:PensionPlansDefinedBenefitMembercountry:USsrt:ScenarioForecastMember2023-01-012023-12-310000313616us-gaap:PensionPlansDefinedBenefitMembersrt:MinimumMemberus-gaap:ForeignPlanMember2022-01-012022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembersrt:MaximumMember2022-01-012022-12-310000313616us-gaap:PensionPlansDefinedBenefitMembersrt:MinimumMemberus-gaap:ForeignPlanMember2021-01-012021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:ForeignPlanMembersrt:MaximumMember2021-01-012021-12-310000313616us-gaap:PensionPlansDefinedBenefitMembersrt:WeightedAverageMemberus-gaap:ForeignPlanMember2022-01-012022-12-310000313616us-gaap:PensionPlansDefinedBenefitMembersrt:WeightedAverageMemberus-gaap:ForeignPlanMember2021-01-012021-12-310000313616us-gaap:PensionPlansDefinedBenefitMembersrt:MinimumMembercountry:US2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMembercountry:USsrt:MaximumMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMember2021-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanEquitySecuritiesCommonStockMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanEquitySecuritiesCommonStockMemberus-gaap:PensionPlansDefinedBenefitMember2021-12-310000313616us-gaap:DefinedBenefitPlanEquitySecuritiesCommonStockMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2022-12-310000313616us-gaap:DefinedBenefitPlanEquitySecuritiesCommonStockMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2021-12-310000313616us-gaap:DefinedBenefitPlanEquitySecuritiesCommonStockMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2022-12-310000313616us-gaap:DefinedBenefitPlanEquitySecuritiesCommonStockMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2021-12-310000313616us-gaap:DefinedBenefitPlanEquitySecuritiesCommonStockMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Member2022-12-310000313616us-gaap:DefinedBenefitPlanEquitySecuritiesCommonStockMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Member2021-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberdhr:DefinedBenefitPlanEquitySecuritiesPreferredStockMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberdhr:DefinedBenefitPlanEquitySecuritiesPreferredStockMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberdhr:DefinedBenefitPlanEquitySecuritiesPreferredStockMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberdhr:DefinedBenefitPlanEquitySecuritiesPreferredStockMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberdhr:DefinedBenefitPlanEquitySecuritiesPreferredStockMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberdhr:DefinedBenefitPlanEquitySecuritiesPreferredStockMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberdhr:DefinedBenefitPlanEquitySecuritiesPreferredStockMemberus-gaap:FairValueInputsLevel12And3Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberdhr:DefinedBenefitPlanEquitySecuritiesPreferredStockMemberus-gaap:FairValueInputsLevel12And3Member2021-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:CorporateBondSecuritiesMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CorporateBondSecuritiesMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberus-gaap:CorporateBondSecuritiesMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:CorporateBondSecuritiesMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:CorporateBondSecuritiesMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Memberus-gaap:CorporateBondSecuritiesMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Memberus-gaap:CorporateBondSecuritiesMember2021-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USGovernmentDebtSecuritiesMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USGovernmentDebtSecuritiesMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:USGovernmentDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:USGovernmentDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:USGovernmentDebtSecuritiesMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberus-gaap:USGovernmentDebtSecuritiesMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:USGovernmentDebtSecuritiesMemberus-gaap:FairValueInputsLevel12And3Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:USGovernmentDebtSecuritiesMemberus-gaap:FairValueInputsLevel12And3Member2021-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMemberus-gaap:FairValueInputsLevel2Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMemberus-gaap:FairValueInputsLevel2Member2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMemberus-gaap:FairValueInputsLevel3Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMemberus-gaap:FairValueInputsLevel3Member2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMemberus-gaap:FairValueInputsLevel12And3Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:MutualFundMemberus-gaap:FairValueInputsLevel12And3Member2021-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberdhr:DefinedBenefitPlanInsuranceContractsMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMemberdhr:DefinedBenefitPlanInsuranceContractsMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberdhr:DefinedBenefitPlanInsuranceContractsMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Memberdhr:DefinedBenefitPlanInsuranceContractsMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberdhr:DefinedBenefitPlanInsuranceContractsMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Memberdhr:DefinedBenefitPlanInsuranceContractsMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Memberdhr:DefinedBenefitPlanInsuranceContractsMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Memberdhr:DefinedBenefitPlanInsuranceContractsMember2021-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMember2022-12-310000313616us-gaap:FairValueInputsLevel1Memberus-gaap:PensionPlansDefinedBenefitMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel2Member2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel3Member2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Member2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueInputsLevel12And3Member2021-12-310000313616us-gaap:DefinedBenefitPlanCommonCollectiveTrustMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2022-12-310000313616us-gaap:DefinedBenefitPlanCommonCollectiveTrustMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberdhr:DefinedBenefitPlanPartnershipsandOtherPrivateInvestmentsMember2022-12-310000313616us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FairValueMeasuredAtNetAssetValuePerShareMemberdhr:DefinedBenefitPlanPartnershipsandOtherPrivateInvestmentsMember2021-12-310000313616us-gaap:PensionPlansDefinedBenefitMember2021-12-3100003136162013-07-160000313616us-gaap:CommonStockMember2022-07-222022-07-220000313616us-gaap:PreferredStockMemberus-gaap:ConvertiblePreferredStockSubjectToMandatoryRedemptionMember2022-01-012022-12-310000313616us-gaap:PreferredStockMemberus-gaap:ConvertiblePreferredStockSubjectToMandatoryRedemptionMember2021-01-012021-12-310000313616us-gaap:PreferredStockMemberus-gaap:ConvertiblePreferredStockSubjectToMandatoryRedemptionMember2020-01-012020-12-310000313616us-gaap:ConvertiblePreferredStockSubjectToMandatoryRedemptionMemberus-gaap:CommonStockMember2022-01-012022-12-310000313616us-gaap:ConvertiblePreferredStockSubjectToMandatoryRedemptionMemberus-gaap:CommonStockMember2021-01-012021-12-310000313616us-gaap:ConvertiblePreferredStockSubjectToMandatoryRedemptionMemberus-gaap:CommonStockMember2020-01-012020-12-310000313616dhr:LiquidYieldOptionNotesMemberus-gaap:CommonStockMember2022-01-012022-12-310000313616dhr:LiquidYieldOptionNotesMemberus-gaap:CommonStockMember2021-01-012021-12-310000313616dhr:LiquidYieldOptionNotesMemberus-gaap:CommonStockMember2020-01-012020-12-310000313616us-gaap:SeriesAPreferredStockMembersrt:MinimumMember2022-04-150000313616us-gaap:CommonStockMember2020-05-012020-05-310000313616us-gaap:CommonStockMember2020-05-310000313616us-gaap:CommonStockMember2020-05-012020-05-310000313616us-gaap:SeriesBPreferredStockMember2020-05-310000313616us-gaap:SeriesBPreferredStockMember2020-05-012020-05-310000313616srt:MinimumMemberus-gaap:SeriesBPreferredStockMember2022-12-310000313616srt:MaximumMemberus-gaap:SeriesBPreferredStockMember2022-12-31dhr:day0000313616dhr:A2007OmnibusIncentivePlanMember2022-12-310000313616dhr:A2007OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-01-012022-12-310000313616dhr:AmendedAndRestated2007OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-01-012022-12-310000313616dhr:A2007OmnibusIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310000313616dhr:AmendedAndRestated2007OmnibusIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310000313616srt:MinimumMember2021-01-012021-12-310000313616srt:MaximumMember2021-01-012021-12-310000313616srt:MinimumMember2020-01-012020-12-310000313616srt:MaximumMember2020-01-012020-12-310000313616us-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310000313616us-gaap:RestrictedStockUnitsRSUMember2021-01-012021-12-310000313616us-gaap:RestrictedStockUnitsRSUMember2020-01-012020-12-310000313616us-gaap:EmployeeStockOptionMember2022-01-012022-12-310000313616us-gaap:EmployeeStockOptionMember2021-01-012021-12-310000313616us-gaap:EmployeeStockOptionMember2020-01-012020-12-310000313616us-gaap:RestrictedStockUnitsRSUMember2022-12-310000313616us-gaap:EmployeeStockOptionMember2022-12-310000313616dhr:RangeOneMember2022-01-012022-12-310000313616dhr:RangeOneMember2022-12-310000313616dhr:RangeTwoMember2022-01-012022-12-310000313616dhr:RangeTwoMember2022-12-310000313616dhr:RangeThreeMember2022-01-012022-12-310000313616dhr:RangeThreeMember2022-12-310000313616dhr:RangeFourMember2022-01-012022-12-310000313616dhr:RangeFourMember2022-12-310000313616dhr:RangeFiveMember2022-01-012022-12-310000313616dhr:RangeFiveMember2022-12-310000313616us-gaap:RestrictedStockUnitsRSUMember2019-12-310000313616us-gaap:RestrictedStockUnitsRSUMember2020-12-310000313616us-gaap:RestrictedStockUnitsRSUMember2021-12-310000313616us-gaap:AccumulatedTranslationAdjustmentMember2019-12-310000313616us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2019-12-310000313616us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2019-12-310000313616us-gaap:AccumulatedTranslationAdjustmentMember2020-01-012020-12-310000313616us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-01-012020-12-310000313616us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2020-01-012020-12-310000313616us-gaap:AccumulatedTranslationAdjustmentMember2020-12-310000313616us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2020-12-310000313616us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2020-12-310000313616us-gaap:AccumulatedTranslationAdjustmentMember2021-01-012021-12-310000313616us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2021-01-012021-12-310000313616us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2021-01-012021-12-310000313616us-gaap:AccumulatedTranslationAdjustmentMember2021-12-310000313616us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2021-12-310000313616us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2021-12-310000313616us-gaap:AccumulatedTranslationAdjustmentMember2022-01-012022-12-310000313616us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-01-012022-12-310000313616us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2022-01-012022-12-310000313616us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310000313616us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-12-310000313616us-gaap:AccumulatedGainLossCashFlowHedgeIncludingNoncontrollingInterestMember2022-12-310000313616us-gaap:AllowanceForCreditLossMember2021-12-310000313616us-gaap:AllowanceForCreditLossMember2022-01-012022-12-310000313616us-gaap:AllowanceForCreditLossMember2022-12-310000313616us-gaap:AllowanceForCreditLossMember2020-12-310000313616us-gaap:AllowanceForCreditLossMember2021-01-012021-12-310000313616us-gaap:AllowanceForCreditLossMember2019-12-310000313616us-gaap:AllowanceForCreditLossMember2020-01-012020-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________
FORM 10-K
(Mark One)
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2022
OR
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| |
| For the transition period from to |
Commission File Number: 001-08089
DANAHER CORPORATION
(Exact name of registrant as specified in its charter)
| | | | | | | | | | | |
| Delaware | | 59-1995548 |
| (State of Incorporation) | | (I.R.S. Employer Identification Number) |
| |
| 2200 Pennsylvania Avenue, N.W., Suite 800W | | 20037-1701 |
| Washington, | DC | |
| (Address of Principal Executive Offices) | | (Zip Code) |
Registrant’s telephone number, including area code: 202-828-0850
Securities Registered Pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common stock, $0.01 par value | DHR | New York Stock Exchange |
| 5.00% Mandatory Convertible Preferred Stock, Series B, without par value | DHR.PRB | New York Stock Exchange |
| 1.700% Senior Notes due 2024 | DHR 24 | New York Stock Exchange |
| | |
| 0.200% Senior Notes due 2026 | DHR/26 | New York Stock Exchange |
| 2.100% Senior Notes due 2026 | DHR 26 | New York Stock Exchange |
| 1.200% Senior Notes due 2027 | DHR/27 | New York Stock Exchange |
| 0.450% Senior Notes due 2028 | DHR/28 | New York Stock Exchange |
| 2.500% Senior Notes due 2030 | DHR 30 | New York Stock Exchange |
| 0.750% Senior Notes due 2031 | DHR/31 | New York Stock Exchange |
| 1.350% Senior Notes due 2039 | DHR/39 | New York Stock Exchange |
| 1.800% Senior Notes due 2049 | DHR/49 | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
NONE
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | |
| Large Accelerated Filer | ☒ | | | Accelerated Filer | ☐ |
| | | | |
| Non-accelerated Filer | ☐ | | | Smaller Reporting company | ☐ |
| | | | | |
| Emerging Growth Company | ☐ | | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant's executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
As of February 3, 2023, the number of shares of Registrant’s common stock outstanding was 728,576,886. The aggregate market value of common stock held by non-affiliates of the Registrant on July 1, 2022 was $167.3 billion, based upon the closing price of the Registrant’s common stock as quoted on the New York Stock Exchange on such date.
____________________________________
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates certain information by reference from the Registrant’s proxy statement for its 2023 annual meeting of shareholders to be filed pursuant to Regulation 14A within 120 days after Registrant’s fiscal year-end. With the exception of the sections of the 2023 Proxy Statement specifically incorporated herein by reference, the 2023 Proxy Statement is not deemed to be filed as part of this Form 10-K.
TABLE OF CONTENTS
| | | | | | | | | | | |
| | | PAGE |
| |
| |
| | | |
| | | |
| | | |
| Item 1. | | |
| Item 1A. | | |
| Item 1B. | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| | | |
| | | |
| | | |
| | | |
| Item 5. | | |
| Item 6. | | |
| Item 7. | | |
| Item 7A. | | |
| Item 8. | | |
| Item 9. | | |
| Item 9A. | | |
| Item 9B. | | |
| Item 9C. | | |
| | | |
| | | |
| | | |
| Item 10. | | |
| Item 11. | | |
| Item 12. | | |
| Item 13. | | |
| Item 14. | | |
| | | |
| | | |
| | | |
| Item 15. | | |
| Item 16. | | |
In this Annual Report, the terms “Danaher” or the “Company” refer to Danaher Corporation, Danaher Corporation and its consolidated subsidiaries or the consolidated subsidiaries of Danaher Corporation, as the context requires. Unless otherwise indicated, all financial data in this Annual Report refer to continuing operations only.
INFORMATION RELATING TO FORWARD-LOOKING STATEMENTS
Certain statements included or incorporated by reference in this Annual Report, in other documents we file with or furnish to the Securities and Exchange Commission (“SEC”), in our press releases, webcasts, conference calls, materials delivered to shareholders and other communications, are “forward-looking statements” within the meaning of the U.S. federal securities laws. All statements other than historical factual information are forward-looking statements, including without limitation statements regarding: projections of revenue, expenses, profit, profit margins, pricing, tax rates, tax provisions, cash flows, pension and benefit obligations and funding requirements, our liquidity position or other projected financial measures; management’s plans and strategies for future operations, including statements relating to anticipated operating performance, cost reductions, restructuring activities, new product and service developments, competitive strengths or market position, acquisitions and the integration thereof, divestitures, spin-offs, split-offs, initial public offerings, other securities offerings or other distributions, strategic opportunities, stock repurchases, dividends and executive compensation; growth, declines and other trends in markets we sell into; new or modified laws, regulations and accounting pronouncements; future regulatory approvals and the timing and conditionality thereof; outstanding claims, legal proceedings, tax audits and assessments and other contingent liabilities; future foreign currency exchange rates and fluctuations in those rates; the potential or anticipated direct or indirect impact of COVID-19 on our business, results of operations and/or financial condition; general economic and capital markets conditions; the anticipated timing of any of the foregoing; assumptions underlying any of the foregoing; and any other statements that address events or developments that Danaher intends or believes will or may occur in the future. Terminology such as “believe,” “anticipate,” “should,” “could,” “intend,” “will,” “plan,” “expect,” “estimate,” “project,” “target,” “may,” “possible,” “potential,” “forecast” and “positioned” and similar references to future periods are intended to identify forward-looking statements, although not all forward-looking statements are accompanied by such words. Forward-looking statements are based on assumptions and assessments made by our management in light of their experience and perceptions of historical trends, current conditions, expected future developments and other factors they believe to be appropriate. These forward-looking statements are subject to a number of risks and uncertainties, including but not limited to the risks and uncertainties set forth below and under “Item 1A. Risk Factors” in this Annual Report.
Forward-looking statements are not guarantees of future performance and actual results may differ materially from the results, developments and business decisions contemplated by our forward-looking statements. Accordingly, you should not place undue reliance on any such forward-looking statements. Forward-looking statements speak only as of the date of the report, document, press release, webcast, call, materials or other communication in which they are made. Except to the extent required by applicable law, we do not assume any obligation to update or revise any forward-looking statement, whether as a result of new information, future events and developments or otherwise.
Below is a summary of material risks and uncertainties we face, which are discussed more fully in “Item 1A. Risk Factors”:
Business and Strategic Risks
•The COVID-19 pandemic has adversely impacted and could in the future continue to adversely impact elements of our business and financial statements. Other conditions in the global economy, the particular markets we serve and the financial markets can also adversely affect our business and financial statements.
•We face intense competition and if we are unable to compete effectively, we may experience decreased demand and decreased market share. Even if we compete effectively, we may be required to reduce the prices we charge.
•Our growth depends on the timely development and commercialization, and customer acceptance, of new and enhanced products and services based on technological innovation. Our growth can also suffer if the markets into which we sell our products and services decline, do not grow as anticipated or experience cyclicality.
•The health care industry and related industries that we serve are undergoing significant changes in an effort to reduce (and increase the predictability of) costs, which can adversely affect our business and financial statements.
•Non-U.S. economic, political, legal, compliance, social and business factors (such as the military conflict between Russia and Ukraine) can negatively affect our business and financial statements.
•Collaborative partners and other third-parties we rely on for development, supply and/or marketing of certain products, potential products and technologies could fail to perform sufficiently.
Acquisitions, Divestitures and Investment Risks
•Any inability to consummate acquisitions at our historical rate and appropriate prices, and to make appropriate investments that support our long-term strategy, could negatively impact our business. Our acquisition of businesses, investments, joint ventures and other strategic relationships could also negatively impact our business and financial statements and our indemnification rights may not fully protect us from liabilities related thereto.
•We intend to separate our Environmental & Applied Solutions (“EAS”) segment to create a publicly-traded company in the fourth quarter of 2023 (the “EAS Separation”). The proposed transaction may not be completed on the currently contemplated timeline or at all and may not achieve the intended benefits.
•Divestitures or other dispositions (including the anticipated EAS Separation) could negatively impact our business, and contingent liabilities from EAS or from businesses that we or our predecessors have previously disposed could adversely affect our business and financial statements. For example, we could incur significant liability if the EAS Separation or any of the split-off or spin-off transactions we have previously consummated are determined to be a taxable transaction or otherwise pursuant to our indemnification obligations with respect to such transactions.
Operational Risks
•Significant disruptions in, or breaches in security of, our information technology (“IT”) systems or data; data privacy violations; other losses or disruptions to facilities, supply chains, distribution systems or IT systems due to catastrophe; and labor disputes can all adversely affect our business and financial statements.
•Defects and unanticipated use or inadequate disclosure with respect to our products or services, or allegations thereof, can adversely affect our business and financial statements.
•Our financial results are subject to fluctuations in the cost and availability of the supplies we use in, and the labor we need for, our operations. In 2022 we experienced supply chain disruptions including in some cases shortages of supply, cost inflation and shipping delays, labor availability constraints and labor cost increases.
•Climate change, legal or regulatory measures to address climate change and any inability to address stakeholder expectations with respect to climate change, may negatively affect us.
•Our success depends on our ability to recruit, retain and motivate talented employees representing diverse backgrounds, experiences and skill sets.
•Our restructuring actions can have long-term adverse effects on our business and financial statements.
Intellectual Property Risks
•Any inability to adequately protect or avoid third-party infringement of our intellectual property, and third-party claims we are infringing intellectual property rights, can adversely affect our business and financial statements.
•The U.S. government has certain rights with respect to incremental production capacity attributable to, and/or the intellectual property we have developed using, government financing. In addition, in times of national emergency the U.S. government could also control our allocation of manufacturing capacity.
Financial and Tax Risks
•Our outstanding debt has increased significantly as a result of acquisitions, and we may incur additional debt. Such indebtedness may limit our operations and use of cash flow and negatively impact our credit ratings; and failure to comply with our indebtedness-related covenants could adversely affect our business and financial statements.
•Our business and financial statements can be adversely affected by foreign currency exchange rates, changes in our tax rates (including as a result of changes in tax laws) or income tax liabilities/assessments, the outcome of tax audits, financial market risks related to our defined benefit pension plans, health care costs, recognition of impairment charges for our goodwill or other intangible assets, and fluctuations in the cost and availability of commodities.
Legal, Regulatory, Compliance and Reputational Risks
•Significant developments or changes in national laws or policies to protect or promote domestic interests and/or address foreign competition can have an adverse effect on our business and financial statements.
•Our businesses are subject to extensive regulation (including those applicable to the healthcare industry). Failure to comply with those regulations (including by our employees, agents or business partners) or significant developments or changes in U.S. laws or policies can adversely affect our business and financial statements.
•With respect to the regulated medical devices we offer, product introductions or modifications may require regulatory clearance or authorizations and we could be required to recall or cease marketing such products; off-label marketing could result in penalties; and clinical trials may have results that are unexpected or are perceived unfavorably by the market, all of which could adversely affect our business and financial statements.
•We are subject to or otherwise responsible for a variety of litigation and other legal and regulatory proceedings in the course of our business that can adversely affect our business and financial statements.
•Our operations, products and services also expose us to the risk of environmental, health and safety liabilities, costs and violations that could adversely affect our business and financial statements.
•Our By-law exclusive forum provisions could limit our stockholders’ ability to choose their preferred judicial forum for disputes.
PART I
ITEM 1. BUSINESS
General
Danaher is a global science and technology innovator committed to helping customers solve complex challenges and improving quality of life around the world. Danaher is comprised of more than 20 operating companies with leadership positions in the biotechnology, life sciences, diagnostics, environmental and applied sectors, organized under four segments (Biotechnology; Life Sciences; Diagnostics; and Environmental & Applied Solutions). United by the DANAHER BUSINESS SYSTEM (“DBS”), our businesses are also typically characterized by a high level of products and services that are sold on a recurring basis, primarily through a direct sales model and to a geographically diverse customer base. Our business’ research and development, manufacturing, sales, distribution, service and administrative facilities are located in more than 60 countries.
Danaher strives to create shareholder value primarily through three strategic priorities:
•strengthening our competitive advantage through consistent application of DBS tools;
•enhancing our portfolio in attractive science and technology markets through strategic capital allocation; and
•consistently attracting and retaining exceptional talent.
Danaher measures its progress against these strategic priorities over the long-term based primarily on financial metrics relating to revenue growth, profitability, cash flow and capital returns, as well as certain non-financial metrics. To further the strategic objectives set forth above, the Company also acquires businesses and makes investments that either complement its existing business portfolio or expand its portfolio into new and attractive markets. Given the rapid pace of technological development and the specialized expertise typical of Danaher’s served markets, acquisitions, strategic alliances and investments provide the Company access to important new technologies and domain expertise. Danaher believes there are many acquisition and investment opportunities available within its targeted markets. The extent to which we identify, consummate and effectively integrate appropriate acquisitions and consummate appropriate investments affects our overall growth and operating results. Danaher also continually assesses the strategic fit of its existing businesses and may separate or otherwise dispose businesses based on strategic and other considerations. In particular, we have announced our intention to separate Danaher’s Environmental & Applied Solutions segment to create a publicly-traded company in the fourth quarter of 2023, subject to the satisfaction of customary conditions, including obtaining final approval from the Danaher Board of Directors, satisfactory completion of financing and receipt of tax opinions, favorable rulings from the Internal Revenue Service and other regulatory approvals.
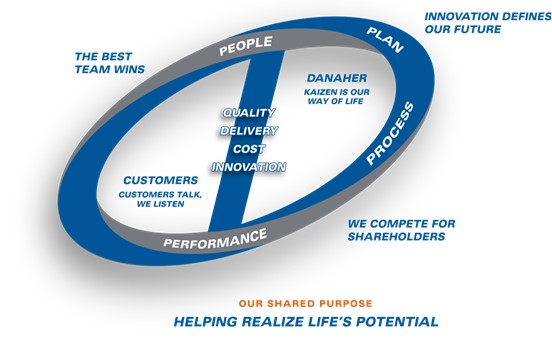
DBS is not only the set of business processes and tools our operating companies use on a daily basis, but is more broadly our culture. As reflected in our logo, DBS features five core values (the “Core Values”):
1.The Best Team Wins
2.Customers Talk, We Listen
3.Kaizen is our Way of Life
4.Innovation Defines our Future
5.We Compete for Shareholders
Underpinned by these five Core Values as well as our Shared Purpose – Helping Realize Life’s Potential, the DBS tools are organized into four pillars that are designed to apply to every aspect of our business: Growth, Lean, Leadership and the DBS Fundamentals.
The idea for Danaher originated in the early 1980s when the Company’s founders, Steven M. and Mitchell P. Rales, envisioned a business that would generate sustainable long-term value for customers, associates and shareholders. Through a series of acquisitions and divestitures, Danaher has evolved over time into the science and technology innovator it is today. While the operating companies that make up Danaher have changed, DBS continues to be the guiding philosophy for the Company.
Sales in 2022 by geographic destination (geographic destination refers to the geographic area where the final sale to the Company’s unaffiliated customer is made) as a percentage of total 2022 sales were: North America, 44% (including 42% in the United States); Western Europe, 22%; other developed markets, 5%; and high-growth markets, 29%. The Company defines North America as the United States and Canada. The Company defines high-growth markets as developing markets of the world experiencing extended periods of accelerated growth in gross domestic product and infrastructure which include Eastern Europe, the Middle East, Africa, Latin America (including Mexico) and Asia (with the exception of Japan, Australia and New Zealand). The Company defines developed markets as all markets of the world that are not high-growth markets.
BIOTECHNOLOGY
The Biotechnology segment includes the bioprocessing and discovery and medical businesses and offers a broad range of tools, consumables and services that are primarily used by customers to advance and accelerate the research, development, manufacture and delivery of biological medicines. The biotherapeutics that the Company’s solutions support range from replacement therapies such as insulin, vaccines, recombinant proteins and other biologic drugs, to novel cell, gene, mRNA and other nucleic acid therapies. Sales in 2022 for this segment by geographic destination (as a percentage of total 2022 sales) were: North America, 35%; Western Europe, 30%; other developed markets, 4%; and high-growth markets, 31%.
Danaher established the Biotechnology segment, which was previously part of the former Life Sciences segment, in 2022. The Biotechnology segment includes the Pall life sciences business, acquired in 2015, and Cytiva, acquired in 2020.
The Biotechnology segment consists of the following businesses:
Bioprocessing—The bioprocessing business is a leading provider of technologies, consumables, services and solutions that advance, accelerate and integrate the development and manufacture of therapeutics. These therapeutics include protein-based and other biological therapies as well as a new emerging class of highly-targeted therapies such as cell and gene therapies, nucleic acid-based therapies, and others requiring viral vectors and lipid nanoparticles in their manufacture. The business offers tools, solutions and services to support biomanufacturers across their workflows from the earliest stages of process development to large scale commercial and turn-key manufacturing. The bioprocessing business’ offering includes cell line and cell culture media development services; cell culture media, process liquids and buffers for manufacturing, chromatography
resins, filtration technologies, aseptic fill finish, as well as single-use hardware and consumables and services such as the design and installation of full manufacturing suites. The bioprocessing business’ offering in data connectivity and automation, advanced process training, process development services and equipment services for maintaining continuous performance, all help to ensure customers’ processes are optimized and compliant. Typical users of these products and services include pharmaceutical and biopharmaceutical companies, translational medicine institutions, biotechnology companies and contract manufacturing organizations.
Discovery and Medical—The discovery and medical business is a leading provider of solutions to accelerate biotherapeutic research and discovery through high quality sample preparation, and reliable diagnostic assays in addition to ensuring sterility and safety in medical liquids and gasses. The business provides solutions and technologies for: lab filtration, separation, and purification; lab-scale protein purification and analytical tools to support bio-molecular analysis, identification, and characterization; reagents, membranes and services for diagnostic and assay development; and healthcare filtration solutions for drug delivery and patient care that help minimize patient risk from viral infections in clinical settings. Typical users of these products include professionals in the areas of academic, translational and commercial research, medical diagnostics, clinical care and biopharmaceutical development.
Customers served by the Biotechnology segment select products based on several factors, including product quality and reliability, the product’s capacity to enhance productivity and flexibility, innovation (particularly productivity and sensitivity improvements), product performance and ergonomics, access to an advanced technical expertise, service and support network and the other factors described under “—Competition.” The businesses in Danaher’s Biotechnology segment market their products and services under several key brands including CYTIVA and PALL. Manufacturing facilities are in North America, Europe, and Asia. The business sells to customers through direct sales personnel and independent distributors.
LIFE SCIENCES
The Life Sciences segment offers a broad range of instruments and consumables that are primarily used by customers to study the basic building blocks of life, including DNA and RNA, nucleic acid, proteins, metabolites and cells, in order to understand the causes of disease, identify new therapies, and test and manufacture new drugs, vaccines and gene editing technologies. Additionally, the segment provides products and consumables used to filter and remove contaminants from a variety of liquids and gases in many end-market applications. Sales in 2022 for this segment by geographic destination (as a percentage of total 2022 sales) were: North America, 45%; Western Europe, 20%; other developed markets, 7%; and high-growth markets, 28%.
Danaher established the life sciences business in 2005 through the acquisition of Leica Microsystems and has expanded the business through numerous subsequent acquisitions, including the acquisitions of AB Sciex and Molecular Devices in 2010, Beckman Coulter in 2011, Pall in 2015, Phenomenex in 2016, IDT in 2018 and Aldevron in 2021.
The Life Sciences segment consists of the following businesses:
Flow Cytometry, Genomics, Lab Automation, Centrifugation, Particle Counting and Characterization—The business offers workflow instruments and consumables that help researchers analyze genomic, protein and cellular information. Key product areas include sample preparation equipment such as centrifugation and consumables; liquid handling automation instruments and associated consumables; flow cytometry instrumentation and associated antibodies and reagents; particle counting and characterization instrumentation; and genomic sample preparation. Researchers use these products to study biological function in the pursuit of basic research, as well as therapeutic and diagnostic development. Typical users include pharmaceutical and biotechnology companies, universities, medical schools and research institutions and in some cases industrial manufacturers.
Mass Spectrometry—The mass spectrometry business is a leading global provider of high-end mass spectrometers as well as related consumables, software and services. Mass spectrometry is a technique for identifying, analyzing and quantifying elements, chemical compounds and biological molecules, individually or in complex mixtures. The mass spectrometers utilize various combinations of quadrupole, time-of-flight and ion trap technologies. The business’ mass spectrometer systems and related products are used in numerous applications such as drug discovery and clinical development of therapeutics as well as in basic research, clinical testing, food and beverage quality testing and environmental testing. The business’ global services network provides implementation, validation, training and maintenance to support customer installations around the world. Typical users of these mass spectrometry and related products include molecular biologists, bioanalytical chemists, toxicologists and forensic scientists as well as quality assurance and quality control technicians. The business also provides high-performance bioanalytical measurement systems, including capillary electrophoresis instruments, associated reagents, software and services. Typical users of these capillary electrophoresis instruments and related products are bioanalytical chemists and quality control technicians engaged in the development and manufacture of new biotherapeutics.
Microscopy—The microscopy business is a leading global provider of professional microscopes designed to capture, manipulate and preserve images and enhance the user’s visualization and analysis of microscopic structures. The Company’s microscopy products include laser scanning (confocal) microscopes, compound microscopes and related equipment, surgical and other stereo microscopes and specimen preparation products for electron microscopy. Typical users of these products include research, medical and surgical professionals operating in research and pathology laboratories, academic settings and surgical theaters.
Industrial Filtration—The filtration, separation and purification technologies business is a leading provider of products used to remove solid, liquid and gaseous contaminants from a variety of liquids and gases in industrial settings, primarily through the sale of filtration consumables and to a lesser extent systems that incorporate filtration consumables and associated hardware. The business’ core materials and technologies can be applied in many ways to solve complex fluid separation challenges and are sold across a wide array of applications. Virtually all of the raw materials, process fluids and waste streams that are found in industry are candidates for multiple stages of filtration, separation and purification. In addition, most of the machines used in complex production processes require filtration to protect sensitive parts from degradation due to contamination. The business’ technologies enhance the quality and efficiency of manufacturing processes and prolong equipment life in applications such as microelectronics, aircraft, oil refineries, power generation turbines, petrochemical plants and food and beverage plants. Within these segments, demand is driven by end-users and original equipment manufacturers seeking to improve product performance, increase production and efficiency, reduce operating costs, extend the life of their equipment, conserve water and meet environmental regulations. The business also serves the filtration needs of the food and beverage markets, helping customers ensure the quality and safety of their products while lowering operating costs and minimizing waste.
Genomic Consumables—The genomic consumables businesses are leading providers of custom nucleic acid products for the life sciences industry, primarily through the manufacture of custom DNA and RNA oligonucleotides and gene fragments utilizing a proprietary manufacturing ecosystem. The businesses have developed proprietary technologies for genomics applications such as next generation sequencing, CRISPR genome editing, qPCR, and RNA interference. Additionally, the businesses are a leading manufacturer of high-quality plasmid DNA, RNA and proteins. These products are used in the research, development and manufacture of gene and cell therapies, DNA and RNA vaccines and gene editing technologies. Typical users of these products include professionals in the areas of academic and commercial research, agriculture, medical diagnostics, pharmaceutical development, biotechnology companies and research institutions across discovery, clinical and commercial applications.
Customers served by the Life Sciences segment select products based on a number of factors, including product quality and reliability, the product’s capacity to enhance productivity, innovation (particularly productivity and sensitivity improvements), product performance and ergonomics, access to a service and support network and the other factors described under “—Competition.” The businesses in Danaher’s Life Sciences segment market their products and services under key brands including ALDEVRON, BECKMAN COULTER, IDT, LEICA MICROSYSTEMS, MOLECULAR DEVICES, PALL, PHENOMENEX and SCIEX. Manufacturing facilities are located in North America, Europe and Asia. The business sells to customers through direct sales personnel and independent distributors.
DIAGNOSTICS
The Diagnostics segment offers clinical instruments, reagents, consumables, software and services that hospitals, physicians’ offices, reference laboratories and other critical care settings use to diagnose disease and make treatment decisions. Sales in 2022 for this segment by geographic destination (as a percentage of total 2022 sales) were: North America, 51%; Western Europe, 17%; other developed markets, 4%; and high-growth markets, 28%.
Danaher established the diagnostics business in 2004 through the acquisition of Radiometer and expanded the business through numerous subsequent acquisitions, including the acquisitions of Vision Systems in 2006, Beckman Coulter in 2011, Iris International and Aperio Technologies in 2012, HemoCue in 2013, Devicor Medical Products in 2014, the clinical microbiology business of Siemens Healthcare Diagnostics in 2015 and Cepheid in 2016. The Diagnostics segment consists of the following businesses:
Core Lab - Clinical—The core lab-clinical business is a leading manufacturer and marketer of biomedical testing instruments, systems and related consumables that are used to evaluate and analyze samples made up of body fluids and cells. The information generated is used to diagnose disease, monitor and guide treatment and therapy, assist in managing chronic disease and assess patient status in hospital, outpatient and physicians’ office settings. The business offers the following products.
•Chemistry systems use electrochemical detection and chemical reactions with patient samples to detect and quantify substances of diagnostic interest in blood, urine and other body fluids. Commonly performed tests include glucose, cholesterol, triglycerides, electrolytes, proteins and enzymes, as well as tests to detect urinary tract infections and kidney and bladder disease.
•Immunoassay systems also detect and quantify biochemicals of diagnostic interest (such as proteins and hormones) in body fluids, particularly in circumstances where more specialized diagnosis is required. Commonly performed immunoassay tests assess thyroid function, screen and monitor for cancer and cardiac risk and provide important information in fertility and reproductive testing.
•Hematology products are used for cellular analysis. The business’ hematology systems use principles of physics, optics, electronics and chemistry to separate and interrogate cells of diagnostic interest and then characterize and quantify them, allowing clinicians to study formed elements in blood (such as red and white blood cells and platelets).
•Microbiology systems are used for the identification of bacteria and antibiotic susceptibility testing (ID/AST) from human clinical samples. These systems detect and quantify bacteria related to microbial infections in urine, blood, and other body fluids, and also detect infections such as urinary tract infections, pneumonia and wound infections. The business’ technology enables direct testing of clinical isolates to ensure reliable detection of resistance to antibiotics.
•Automation systems reduce manual operation and associated cost and errors from the pre-analytical through post-analytical stages, including sample barcoding/information tracking, centrifugation, aliquoting, storage and conveyance. These systems, along with the analyzers described above, are controlled through laboratory-level software that enables laboratory managers to monitor samples, results and lab efficiency.
Typical users of the segment’s core lab products include hospitals, physician’s offices, reference laboratories and pharmaceutical clinical trial laboratories.
Molecular Diagnostics—The molecular diagnostics business is a leading provider of biomedical testing instruments, systems and related consumables that enable DNA-based testing for organisms and genetic-based diseases in both clinical and non-clinical markets. These products integrate and automate the complicated and time-intensive steps associated with DNA-based testing (including sample preparation and DNA amplification and detection) to allow the testing to be performed in both laboratory and non-laboratory environments with minimal training and infrastructure. These products also include systems which commonly test for health care-associated infections, respiratory disease, sexual health and virology.
Acute Care Diagnostics—The acute care diagnostics business is a leading worldwide provider of instruments, software and related consumables and services that are used in both laboratory and point-of-care environments to rapidly measure critical parameters, including blood gases, electrolytes, metabolites and cardiac markers, as well as for anemia and high-sensitivity glucose testing. Typical users of these products include hospital central laboratories, intensive care units, hospital operating rooms, hospital emergency rooms, physician’s office laboratories and blood banks.
Pathology Diagnostics—The pathology diagnostics business is a leader in the anatomical pathology industry, offering a comprehensive suite of instrumentation and related consumables used across the entire workflow of a pathology laboratory. The anatomical pathology diagnostics products include chemical and immuno-staining instruments, reagents, antibodies and consumables; tissue embedding, processing and slicing (microtomes) instruments and related reagents and consumables; slide cover-slipping and slide/cassette marking instruments; imaging instrumentation including slide scanners, microscopes and cameras; software solutions to store, share and analyze pathology images digitally; and minimally invasive, vacuum-assisted breast biopsy and lesion excision instruments and breast surgery localization solutions. Typical users of these products include pathologists, lab managers and researchers.
Customers in the diagnostics industry select products based on a number of factors, including product quality and reliability, the scope of tests that can be performed, the accuracy and speed of the product, the product’s ability to enhance productivity, ease of use, total cost of ownership and access to a highly qualified service and support network as well as the other factors described under “—Competition.” The businesses in Danaher’s Diagnostics segment market their products and services under key brands including BECKMAN COULTER, CEPHEID, HEMOCUE, LEICA BIOSYSTEMS, MAMMATOME and RADIOMETER. Manufacturing facilities are located in North America, Europe, Asia and Australia. The business sells to customers primarily through direct sales personnel and, to a lesser extent, through independent distributors.
ENVIRONMENTAL & APPLIED SOLUTIONS
In September 2022, the Company announced its intention to spin-off its Environmental & Applied Solutions business into a publicly traded company. The transaction is expected to be tax-free to the Company’s shareholders. The Company is targeting to complete the EAS Separation in the fourth quarter of 2023, subject to the satisfaction of certain conditions, including obtaining final approval from the Danaher Board of Directors, satisfactory completion of financing, receipt of tax opinions, receipt of favorable rulings from the Internal Revenue Service (“IRS”) and receipt of other regulatory approvals.
The Environmental & Applied Solutions segment offers products and services that help protect precious resources and keep global food and water supplies safe. Sales in 2022 for this segment by geographic destination (as a percentage of total 2022
sales) were: North America, 46%; Western Europe, 22%; other developed markets, 3%; and high-growth markets, 29%. The Company’s Environmental & Applied Solutions segment consists of the following businesses:
Water Quality—The Company’s water quality business is a leading provider of instrumentation, consumables, software, services and disinfection systems to help analyze, treat and manage the quality of ultra-pure, potable, industrial, waste, ground, source and ocean water in residential, commercial, municipal, industrial and natural resource applications. Danaher entered the water quality sector in the late 1990’s through the acquisitions of Dr. Lange and Hach Company and has enhanced the geographic coverage and capabilities of its products and services through subsequent acquisitions, including the acquisition of Trojan Technologies Inc. in 2004 and ChemTreat, Inc. in 2007. The water quality business designs, manufactures and markets:
•a wide range of analytical instruments, related consumables, software and services that detect and measure chemical, physical and microbiological parameters in ultra-pure, potable, industrial, waste, municipal, ground, source and ocean water;
•chemical treatment solutions intended to address corrosion, scaling and biological growth problems in boiler, cooling water and wastewater applications as well as associated analytical services, primarily in applied and industrial end markets; and
•ultraviolet disinfection systems, consumables and services, which disinfect billions of gallons of municipal, industrial and consumer water every day.
Typical users of these products and services include professionals in municipal drinking water and wastewater treatment plants, industrial process and discharge water facilities, wastewater treatment facilities, third-party testing laboratories and environmental operations. Customers in these industries choose suppliers based on a number of factors including the customer’s existing supplier relationships, application expertise, product performance and ease of use, the comprehensiveness of the supplier’s solutions offering, after-sales service and support and the other factors described under “—Competition.” The Company’s water quality businesses provide products under a variety of key brands, including AQUATIC INFORMATICS, CHEMTREAT, HACH, MCCROMETER, OTT HYDROMET, SEA-BIRD and TROJAN TECHNOLOGIES. Manufacturing facilities are primarily located in North America, Europe and Asia. Sales are made through the business’ direct sales personnel, e-commerce, independent representatives and independent distributors.
Product Identification—The Company’s product identification business is a leading provider of printers, instruments, software, services and consumables for various color and appearance management, packaging design and quality management, packaging converting, printing, marking, coding and traceability applications for consumer, pharmaceutical and industrial products. Danaher entered the product identification market through the acquisition of Videojet in 2002, and has expanded the product and geographic coverage through various subsequent acquisitions, including the acquisitions of EskoArtwork in 2011 and X-Rite in 2012. The product identification business designs, manufactures and markets:
•printers, consumables and solutions used to give products unique identities by printing date, lot and bar codes and other information on primary and secondary packaging, applying high-quality alphanumeric codes, logos and graphics to a wide range of surfaces at a variety of production line speeds, angles and locations on a product or package. Its vision inspection and track-and-trace solutions also help pharmaceutical and consumer goods manufacturers safeguard the authenticity of their products through supply chains.
•software for online collaboration, three-dimensional virtualization, workflow automation, quality approvals and prepress processes to manage structural design, artwork creation, color and product information for branded packaging and marketing materials. Its packaging solutions help consumer goods manufacturers improve their business processes, shorten time to market and reduce costs across internal departments and external suppliers.
•innovative color and appearance solutions through standards, software, measurement devices and related services. The business’ expertise in inspiring, virtualizing, selecting, specifying, formulating and measuring color and appearance helps users improve the quality and relevance of their products and reduce costs.
•flexographic computer-to-plate imaging equipment, solutions for print process control, press control and quality assurance systems for the packaging, labels and commercial print industries. Its automation, print process and press control solutions help packaging manufacturers reduce lead time and satisfy their customers’ demands for smaller, more frequent print jobs.
Typical users of these products include manufacturers of consumer goods, pharmaceuticals, paints, plastics and textiles, retailers, graphic design firms and packaging printers and converters. Customers in these industries choose suppliers based on a number of factors, including domain experience, speed and accuracy, ease of connection to the internet and other software
systems, equipment uptime and reliable operation without interruption, ease of maintenance, service coverage and the other factors described under “—Competition.” The product identification business’ products are primarily marketed under key brands including AVT, ESKO, LAETUS, LINX, PANTONE, VIDEOJET and X-RITE. Manufacturing and software development facilities are located in North America, Europe, Latin America and Asia. Sales are generally made through the business’ direct sales personnel, independent distributors and e-commerce.
************************************
The following discussion includes information common to all of Danaher’s segments.
Materials
The Company’s manufacturing operations employ a wide variety of raw materials, including metallic-based components, electronic components, chemistries, OEM products, plastics and other petroleum-based products. Prices of oil and gas also affect the Company’s costs for freight and utilities and also have an indirect impact on the cost of other purchased materials. While the price of, and global instability with respect to the supply of, oil and gas did not materially, adversely affect the Company’s operations in 2022, the Company is continuing to monitor the oil and gas commodity markets and will seek to mitigate price and/or availability risks as needed. The Company purchases raw materials from a large number of independent sources around the world. No single supplier is material, although for some components that require particular specifications or regulatory or other qualifications there may be a single supplier or a limited number of suppliers that can readily provide such components. The Company utilizes a number of techniques to address potential disruption in and other risks relating to its supply chain, including in certain cases the use of safety stock, alternative materials and qualification of multiple supply sources.
The supply chain disruptions that began in 2021 for a number of our businesses continued in 2022 (including in some cases shortages of supply, cost inflation and shipping delays), as well as labor availability constraints and labor cost increases. Through the application of DBS tools and processes (including the implementation of price increases), the Company largely mitigated the impact of these pressures on the Company’s profitability and as a result these pressures did not have a material, adverse effect on the business in 2022. These pressures continue to varying degrees as of the date of this Annual Report. We are continuing to work with our suppliers to understand the existing and potential future impacts of these trends on our supply chain and we continue to take actions in an effort to mitigate such impacts, including purchasing components in the open market and qualifying additional suppliers. Due to the uncertainty regarding the duration and impact of these trends in 2023, there can be no assurance that these factors will not have an adverse impact on our business and financial statements in the future. For a further discussion of risks related to the materials and components required for the Company’s operations, refer to “Item 1A. Risk Factors.”
Russia-Ukraine Conflict
In response to the ongoing conflict in Ukraine, in addition to suspending sales prohibited by sanctions, the Company has suspended the shipment of products to Russia with the exception of products for the purposes of diagnosing and treating patients and producing vaccines and therapeutics. In the first quarter of 2022, the Company recorded a pretax charge of $43 million, primarily related to the impairment of accounts receivable and inventory, as well as accruals for contractual obligations related to Russian operations. Russia has significantly reduced the export of natural gas to Europe, creating upward pressure on natural gas prices and a reduced supply of natural gas. If this trend continues, the Company’s European manufacturing facilities could face increased costs and risks of production disruptions. The Company’s European customers and suppliers could experience similar adverse impacts, which could further adversely impact the Company’s supply chain and also adversely impact the demand for its products. The Company will continue monitoring the military, social, political, regulatory and economic environment in Ukraine and Russia and its broader impacts, and will consider further actions as appropriate. For a discussion of risks related to the Company’s operations as a result of the military conflict between Russia and Ukraine, refer to “Item 1A. Risk Factors.”
Intellectual Property
The Company owns numerous patents, trademarks, copyrights, trade secrets and licenses to intellectual property owned by others. Although in aggregate the Company’s intellectual property is important to its operations, the Company does not consider any single patent, trademark, copyright, trade secret or license (or any related group of any such items) to be of material importance to any segment or to the business as a whole. From time to time the Company engages in litigation to protect its intellectual property rights. For a discussion of risks related to the Company’s intellectual property, refer to “Item 1A. Risk Factors.” All capitalized brands and product names throughout this document are trademarks owned by, or licensed to, Danaher.
Competition
Although the Company’s businesses generally operate in highly competitive markets, the Company’s competitive position cannot be determined accurately in the aggregate or by segment since none of its competitors offer all of the same product and service lines or serve all of the same markets as the Company, or any of its segments, does. Because of the range of the products and services the Company sells and the variety of markets it serves, the Company encounters a wide variety of competitors, including well-established regional competitors, competitors who are more specialized than it is in particular markets, as well as large companies or divisions of large companies with substantial sales, marketing, research and financial capabilities. The Company is facing increased competition in a number of its served markets as a result of the entry of well-resourced companies into certain markets, the entry of competitors based in low-cost manufacturing locations, the development of competitive technologies by early-stage and emerging companies and increasing consolidation in particular markets. The number of competitors varies by product and service line. Management believes that the Company has a leadership position in many of the markets it serves. Key competitive factors vary among the Company’s businesses and product and service lines, but include the specific factors noted above with respect to each particular business and typically also include price, quality and safety, performance, delivery speed, application expertise, service and support, technology and innovation, distribution network, breadth of product, service and software offerings and brand name recognition. For a discussion of risks related to competition, refer to “Item 1A. Risk Factors.”
Human Capital
As of December 31, 2022, the Company had approximately 81,000 employees (whom we refer to as “associates”), of whom approximately 32,000 were employed in the North America, 25,000 in Western Europe, 3,000 in other developed markets and 21,000 in high-growth markets. Approximately 79,000 of the Company’s total employees were full-time and 2,000 were part-time employees. Of the United States employees, approximately 400 were hourly-rated, unionized employees. Outside the United States, the Company has government-mandated collective bargaining arrangements and union contracts in certain countries, particularly in Europe where many of the Company’s employees are represented by unions and/or works councils.
Danaher is committed to attracting, developing, engaging and retaining the best people from around the world to sustain and grow our science and technology leadership. As noted above, “Consistently attracting and retaining exceptional talent” is one of our three strategic priorities and “The Best Team Wins” is one of our five Core Values, reflecting the critical role our human capital plays in supporting our strategy. Our human capital strategy spans multiple, key dimensions, including the following:
•Culture and Governance
◦Our culture is rooted in DBS and in our Shared Purpose, Helping Realize Life’s Potential. At its core, DBS reflects a commitment to use process to continuously improve every aspect of our business. Our Shared Purpose gives meaning and direction to our continuous improvement.
◦Danaher’s Board of Directors reviews the Company’s human capital strategy annually and at other times during the year in connection with significant initiatives and acquisitions, supported by the Compensation Committee’s oversight of our executive and equity compensation programs. At the management level, our Senior Vice President of Human Resources, who reports directly to our President and CEO, is responsible for the development and execution of the Company’s human capital strategy.
•Recruitment
◦As part of our commitment to the Core Value “The Best Team Wins”, we focus on identifying, attracting and recruiting diverse talent to meet our current and future business needs. We have invested in comprehensive talent acquisition capabilities across all levels of recruitment (including robust branding, labor market analytics, advanced sourcing tools, leading technology and streamlined processes). Our diversity attraction efforts are an important component of our overall talent acquisition strategy and focus on: (1) establishing and fostering partnerships with diverse organizations, and (2) effectively sourcing diverse talent.
•Engagement
◦General. Our engagement strategy focuses on developing the best workplace and best people leaders to meet our associates’ needs every day. Further, we believe that better associate engagement helps enable better retention and better business performance. We assess our engagement performance through our annual Associate Engagement Survey, which addresses engagement, direct supervisor effectiveness, behavior change and performance enablement, as well as through our voluntary turnover rate.
◦D + I. We seek to continuously improve and sustain a diverse and inclusive culture free of systemic bias and where all associates feel they belong. We believe a diverse workforce and culture of inclusion is essential to drive innovation, fuel growth and help ensure our technologies and products effectively serve a global customer base. Danaher’s Office of Diversity + Inclusion is led by our Vice President of Global Diversity + Inclusion, who is responsible for the execution of Danaher’s D+I strategy and reports to Danaher’s Senior Vice President of Human Resources. Both serve on the Danaher Diversity + Inclusion Council along with executives who lead our businesses. The D+I Council is responsible for overseeing Danaher’s D+I strategic direction; creating D+I accountability measures; and operationalizing D+I initiatives and programming across our businesses.
We have leveraged DBS with the goal of driving progress on diversity representation and inclusive culture, including by requiring all of our operating companies to implement a D+I Policy Deployment initiative in each of 2021, 2022 and 2023. Our D+I initiatives focus on broadening our candidate pools, sourcing diverse slates in the hiring process, developing people leaders’ competency in and accountability for D+I and implementing and sustaining programs (such as our Associate Resource Groups for Women, Black, Latinx, LGBTQ and Asian descent associates and friends/allies) that offer mentorship, support and engagement to help our associates succeed and thrive. As of December 31, 2022, (1) 38% of our total associates were female and females represented 32%, 34% and 39% of our executives/senior leaders, managers and individual contributors, respectively; and (2) 41% of our total U.S. associates were People of Color and People of Color represented 24%, 31% and 43% of our U.S. executives/senior leaders, managers and individual contributors, respectively.
◦In support of our D+I commitment, we conduct regular pay reviews from a race (in the United States) and gender (globally) perspective that serve to proactively identify and address potential pay differences. In 2020 we achieved base pay equity for women and for racial and ethnic minorities in the U.S. and in 2021 expanded the U.S. analysis to include both base pay and short-term incentive compensation.
•Retention
◦Compensation and Benefits. We are committed to offering competitive compensation and benefits, tailored in form and amount to geography, industry, experience and performance and designed to attract associates, motivate and reward performance, drive growth and support retention. We have a common job architecture across our businesses to provide a standardized framework for defining jobs, job families, and career levels, and set market-aligned pay structures for each career level (adjusted as appropriate for the particular job family, industry, and geography) based on a range of compensation surveys.
◦Performance Management. Performance for Growth (“P4G”), our annual performance management program, supports our high-performance culture by seeking to ensure that high-performing associates are recognized and rewarded for their contributions. P4G guides associates and their managers in setting clear personal performance goals aligned to our strategic priorities. Annual reviews under the program assess performance against these formal, annual objectives and against our Core Behaviors.
◦Talent Development and Career Mobility. Our talent development program (which is generally structured to consist of 70% on-the-job learning, 20% coaching and mentoring and 10% formal training) strives to provide every associate with appropriate development opportunities. In particular, we make available to people leaders at every level training, coaching and developmental resources to help them be effective leaders and advance their careers. We further encourage internal promotion and mobility through our Danaher Go program, which makes open positions throughout the organization visible to associates and proactively encourages our associates to seek promotional opportunities. We assess our performance in this area using metrics including internal fill rate (which tracks the percentages of open roles at particular levels filled by our own associates) as well as the percentage of eligible associates with completed talent assessments/career plans.
◦Safety and Risk Management. Associate safety is deeply embedded in our culture. Our Environment, Health and Safety (“EHS”) Policy establishes the core principles upon which our EHS management programs are built, and associates use our DBS-based “4E” toolkit to identify, assess and control hazards related to ergonomics, energetics, exposures and environment. In addition, we evaluate and manage risks relating to our human capital strategy as part of Danaher’s enterprise risk management program. Key quantitative measures that we use to assess performance in this category include total recordable incident rate (defined as the number of work-related injuries or illness cases serious enough to require treatment beyond first aid, per
100 associates) and days away, restricted or transferred (defined as the number of work-related injuries or illness cases that result in an employee working with physical restrictions, being away from work or unable to do their job or transferring to other work, per 100 associates).
The health and well-being of our associates has been a key area of focus in our response to the COVID-19 pandemic. We launched a global Employee Assistance Program in 2020 to ensure a consistent support structure for mental health and well-being across the Company and have since expanded the program to provide enhanced support with respect to childcare, eldercare and tutoring, among other areas. In the United States, we have provided benefits beyond the requirements of the Families First Act, for example by extending our leave policy to cover elder care and providing for voluntary leaves even in certain circumstances not required by the law. We have also implemented safety precautions on a facility-specific basis.
Research and Development (“R&D”)
The Company conducts R&D activities for the purpose of developing new products, enhancing the functionality, effectiveness, ease of use and reliability of its existing products and expanding the applications for which uses of its products are appropriate. The Company’s R&D efforts include internal initiatives and those that use licensed or acquired technology, and we work with a number of leading research institutions, universities and clinicians around the world to develop, evaluate and clinically test our products. The Company conducts R&D activities primarily in North America, Europe and Asia and generally on a business-by-business basis. The Company anticipates that it will continue to make significant expenditures for R&D as it seeks to provide a continuing flow of innovative products and services to maintain and improve its competitive position. For a discussion of the risks related to the need to develop and commercialize new products and product enhancements, refer to “Item 1A. Risk Factors.”
Government Contracts
Although the substantial majority of the Company’s revenue in 2022 was from customers other than governmental entities, each of Danaher’s segments has agreements relating to the sale of products to government entities. As a result, the Company is subject to various statutes and regulations that apply to companies doing business with governments. For a discussion of risks related to government contracting requirements, refer to “Item 1A. Risk Factors.” No material portion of Danaher’s business is subject to renegotiation of profits or termination of contracts at the election of a government entity.
Regulatory Matters
The Company faces extensive government regulation both within and outside the United States relating to the development, manufacture, marketing, sale and distribution of its products and services. The following sections describe certain significant regulations that the Company is subject to. These are not the only regulations that the Company’s businesses must comply with. For a description of the risks related to the regulations that the Company’s businesses are subject to, refer to “Item 1A. Risk Factors.”
Medical Device Regulations
Many of our products are classified as medical devices and are subject to restrictions under domestic and foreign laws, rules, regulations, self-regulatory codes, circulars and orders, including, but not limited to, the U.S. Food, Drug, and Cosmetic Act (the “FDCA”). The FDCA requires these products, when sold in the United States, to be safe and effective for their intended uses and to comply with the regulations administered by the U.S. Food and Drug Administration (“FDA”). The FDA regulates the design, development, research, preclinical and clinical testing, introduction, manufacture, advertising, labeling, packaging, marketing, distribution, import and export and record keeping for such products. Many medical device products are also regulated by comparable agencies in non-U.S. countries in which they are produced or sold.
Unless an exemption applies, the FDA requires that a manufacturer introducing a new medical device or a new indication for use of an existing medical device obtain either a Section 510(k) premarket notification clearance or a premarket approval (“PMA”) before introducing it into the U.S. market. The type of marketing authorization is generally linked to the classification of the device. The FDA classifies medical devices into one of three classes (Class I, II or III) based on the degree of risk the FDA determines to be associated with a device and the level of regulatory control deemed necessary to ensure the device’s safety and effectiveness.
The process of obtaining a Section 510(k) clearance generally requires the submission of performance data and clinical data, which in some cases can be extensive, to demonstrate that the device is “substantially equivalent” to a device that was on the market before 1976 or to a device that has been found by the FDA to be “substantially equivalent” to such a pre-1976 device. A predecessor device is referred to as “predicate device.” As a result, FDA clearance requirements may extend the development process for a considerable length of time.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, may require a new 510(k) clearance or PMA approval and payment of an FDA user fee. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until the required 510(k) clearance or PMA approval is obtained.
Any medical devices we manufacture and distribute are subject to pervasive and continuing regulation by the FDA and certain state and non-U.S. agencies. These include product listing and establishment registration requirements, which help facilitate inspections and other regulatory actions. As a medical device manufacturer, our manufacturing facilities are subject to inspection on a routine basis by the FDA. We are required to adhere to the Current Good Manufacturing Practices (“CGMP”) requirements, as set forth in the Quality Systems Regulation (“QSR”), which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all phases of the design and manufacturing process.
We must also comply with post-market surveillance regulations, including medical device reporting (“MDR”) requirements which require that we review and report to the FDA any incident in which our products may have caused or contributed to a death or serious injury. We must also report any incident in which our product has malfunctioned if that malfunction would likely cause or contribute to a death or serious injury if it were to recur.
Labeling and promotional activities are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission (“FTC”) (and similar regulators in other jurisdictions). Medical devices approved or cleared by the FDA may not be promoted for unapproved or uncleared uses, otherwise known as “off-label” promotion. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses.
In the European Union (“EU”), our products are subject to the medical device and in vitro medical device laws of the various member states, which for many years were based on Directives of the European Commission. However, in May 2017, the EU adopted new, formal regulations to replace such Directives; specifically, the EU Medical Device Regulation (the “EU MDR”) and In Vitro Diagnostic Regulation (the “EU IVDR”), each of which imposes stricter requirements for the marketing and sale of medical devices and in vitro devices, including in the area of clinical evaluation requirements, quality systems and post-market surveillance. The EU regulations were adopted with staggered transitional periods that have since been updated. In January 2023, the European Commission endorsed a proposal to extend the original compliance dates for both MDR and EU IVDR, subject to approval by the European Parliament and European Council. The proposal would extend the current MDR transitional period deadline of May 2024 to 2027 or 2028, based upon the risk class of the device. The EU IVDR became fully applicable in May 2022, with transition periods for certain classes of in vitro diagnostic devices extending until 2027 and the EU Commission is considering additional extensions to the EU IVDR deadlines. Regulatory requirements in the United Kingdom (“UK”) are also changing as a result of Brexit (the UK’s withdrawal from the EU), and regulatory requirements in Switzerland are changing as a result of the country’s withdrawal from its Mutual Recognition Agreement with the EU Commission. Complying with the EU MDR, EU IVDR and the evolving regulatory regimes in the UK and Switzerland requires modifications to our quality management systems, additional resources in certain functions and updates to technical files, among other changes, which has not and is not expected to have a material impact on the Company’s financial results.
Other Healthcare Laws
We are also subject to the U.S. Foreign Corrupt Practices Act and various health care related laws regulating fraud and abuse, research and development, pricing and sales and marketing practices, and the privacy and security of health information, including the U.S. federal regulations described below. Many states, foreign countries and supranational bodies have also adopted laws and regulations similar to, and in some cases more stringent than, the U.S. federal regulations discussed above and below, including the UK Bribery Act and similar anti-bribery laws.
•Many of our healthcare-related products are purchased by healthcare providers that typically bill various third-party payers, such as governmental healthcare programs (e.g., Medicare, Medicaid and comparable non-U.S. programs), private insurance plans and managed care plans, for the healthcare services provided to their patients. The ability of our customers to obtain appropriate reimbursement for products and services from third-party payers is critical because it affects which products customers purchase and the prices they are willing to pay. As a result, many of our healthcare-related products are subject to regulation regarding quality and cost by the U.S. Department of Health and Human Services (“HHS”), including the Centers for Medicare & Medicaid Services (“CMS”), as well as comparable state and non-U.S. agencies responsible for reimbursement and regulation of healthcare goods and services, including
laws and regulations related to kickbacks, false claims, self-referrals and healthcare fraud. Third-party payers are increasingly reducing reimbursements for medical products and services and, in international markets, many countries have instituted price ceilings on specific products and therapies. Price ceilings, decreases in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce usage and patient demand for the product.
•The U.S. Federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration (including any kickback or bribe), directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made in whole or in part under a federal health care program, such as Medicare or Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
•The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) prohibits knowingly and willfully (1) executing, or attempting to execute, a scheme to defraud any health care benefit program, including private payors, or (2) falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. In addition, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, also restricts the use and disclosure of patient identifiable health information, mandates the adoption of standards relating to the privacy and security of patient identifiable health information and requires the reporting of certain security breaches with respect to such information. Similar to the U.S. Federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the healthcare fraud statute implemented under HIPAA or specific intent to violate it in order to have committed a violation.
•The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal health care program, knowingly makes, uses or causes to be made or used, a false record or statement material to a false or fraudulent claim, or knowingly makes a false statement to avoid, decrease or conceal an obligation to pay money to the U.S. federal government. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. In addition, the government may assert that a claim including items and services resulting from a violation of the U.S. Federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act.
•The federal Civil Monetary Penalties Law prohibits, among other things, the offering or transferring of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of Medicare or Medicaid payable items or services.
•The Open Payments Act requires manufacturers of medical devices covered under Medicare, Medicaid or the Children’s Health Insurance Program (subject to certain exceptions) to record payments and other transfers of value to a broad range of healthcare providers and teaching hospitals and to report this data as well as ownership and investment interests held by the physicians described above and their immediate family members to HHS for subsequent public disclosure. Similar reporting requirements have also been enacted on the state level, and an increasing number of countries either have adopted or are considering similar laws requiring transparency of interactions with health care professionals.
In addition, some of the in vitro diagnostic drugs-of-abuse assays and reagents sold by the Company’s subsidiaries contain small amounts of controlled substances, and as a result some of the Company’s facilities are inspected periodically by the United States Drug Enforcement Administration to assess whether the Company properly handles, stores and disposes of controlled substances in the manufacture of those products.
Federal consumer protection and unfair competition laws broadly regulate marketplace activities and activities that potentially harm consumers. Analogous U.S. state laws and regulations, such as state anti-kickback and false claims laws, also may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by any third-party payor, including private insurers. Further, there are state laws that require medical device manufacturers to comply with the voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws and regulations that require manufacturers to file reports relating to pricing and marketing information, which requires tracking gifts and other remuneration and items of value provided to healthcare professionals and entities; state and local laws requiring the registration of sales representatives; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA.
For a discussion of risks related to regulation by the FDA and comparable agencies of other countries, and the other regulatory regimes referenced above, please refer to “Item 1A. Risk Factors.”
Healthcare Reform
In the U.S. and certain non-U.S. jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system. There is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality or expanding access. For example, in the United States, in March 2010, the U.S. Patient Protection and Affordable Care Act (as amended by the Health Care and Education Affordability Reconciliation Act) (collectively, the “PPACA”) was signed into law, which substantially changed the way healthcare is financed by both governmental and private insurers and significantly affected the healthcare industry. Since its enactment, there have been judicial, Congressional and executive challenges to certain aspects of the PPACA, and there may be additional challenges and amendments to the PPACA in the future.
Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted legislation designed, among other things, to bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for medical products. Individual states in the U.S. have also become increasingly active in implementing regulations designed to control product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures and, in some cases, mechanisms to encourage importation from other countries and bulk purchasing.
Data Privacy and Security Laws
As a global organization, we are subject to data privacy and security laws, regulations, and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal and/or sensitive data in the course of our business. For example, the European Union’s General Data Protection Regulation (“GDPR”) imposes significant restrictions on how we collect, transmit, process and retain personal data, including, among other things, in certain circumstances a requirement for almost immediate notice of data breaches to supervisory authorities with significant fines for non-compliance. In the U.S., HIPAA privacy and security rules require certain of our operations to maintain controls to protect the availability and confidentiality of patient health information, individual states regulate data breach and security requirements, and multiple governmental bodies assert authority over aspects of the protection of personal privacy. State privacy laws in California impose some of the same features as the GDPR and have prompted several other states to enact similar laws. Additionally, a bipartisan bill under consideration in Congress would, if adopted, impose broad privacy requirements at the federal level. Several other countries such as China and Russia have passed, and other countries are considering passing, privacy laws that require personal data relating to their citizens to be maintained on local servers or impose significant restrictions on data transfer. For a discussion of risks related to these laws, refer to “Item 1A. Risk Factors.”
Environmental Laws and Regulations
For a discussion of the environmental laws and regulations that the Company’s operations, products and services are subject to and other environmental contingencies, refer to Note 18 to the Consolidated Financial Statements included in this Annual Report. For a discussion of risks related to compliance with environmental and health and safety laws and risks related to past or future releases of, or exposures to, hazardous substances, refer to “Item 1A. Risk Factors.”
Antitrust Laws
The U.S. federal government, most U.S. states and many other countries have laws that prohibit certain types of conduct deemed to be anti-competitive. Violations of these laws can result in various sanctions, including criminal and civil penalties. Private plaintiffs also could bring civil lawsuits against us in the United States for alleged antitrust law violations, including claims for treble damages.
Export/Import Compliance
The Company is required to comply with various U.S. export/import control and economic sanctions laws, including:
•the International Traffic in Arms Regulations administered by the U.S. Department of State, Directorate of Defense Trade Controls, which, among other things, imposes license requirements on the export from the United States of defense articles and defense services listed on the U.S. Munitions List;
•the Export Administration Regulations administered by the U.S. Department of Commerce, Bureau of Industry and Security, which, among other things, impose licensing requirements on the export, in-country transfer and re-export of certain dual-use goods, technology and software (which are items that have both commercial and military, or proliferation applications);
•the regulations administered by the U.S. Department of Treasury, Office of Foreign Assets Control, which implement economic sanctions imposed against designated countries, governments and persons based on United States foreign policy and national security considerations; and
•the import regulatory activities of the U.S. Customs and Border Protection and other U.S. government agencies.
Other nations’ governments have implemented similar export/import control and economic sanction regulations, which may affect the Company’s operations or transactions subject to their jurisdictions.
In addition, under U.S. laws and regulations, U.S. companies and their subsidiaries and affiliates outside the U.S. are prohibited from participating or agreeing to participate in unsanctioned foreign boycotts in connection with certain business activities, including the sale, purchase, transfer, shipping or financing of goods or services within the U.S. or between the U.S. and other countries. If we, or third parties through which we sell or provide goods or services, violate anti-boycott laws and regulations, we may be subject to civil or criminal enforcement action and varying degrees of liability.
For a discussion of risks related to export/import control and economic sanctions laws, refer to “Item 1A. Risk Factors.”
International Operations
The Company’s products and services are available worldwide, and its principal markets outside the U.S. are in Europe and Asia. The Company also has operations around the world, and this geographic diversity allows the Company to draw on the skills of a worldwide workforce, provides greater stability to its operations, allows the Company to drive economies of scale, provides revenue streams that may help offset economic trends that are specific to individual economies and offers the Company an opportunity to access new markets for products. In addition, the Company believes that future growth depends in part on its ability to continue developing products and sales models that successfully target high-growth markets.
The manner in which the Company’s products and services are sold outside the U.S. differs by business and by region. Most of the Company’s sales in non-U.S. markets are made by its subsidiaries located outside the U.S., though the Company also sells directly from the U.S. into non-U.S. markets through various representatives and distributors and, in some cases, directly. In countries with low sales volumes, the Company generally sells through representatives and distributors.
Information about the effects of foreign currency fluctuations on the Company’s business is set forth in “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” (“MD&A”) included in this Annual Report. For a discussion of risks related to the Company’s non-U.S. operations and foreign currency exchange, refer to “Item 1A. Risk Factors.”
Sustainability
The Company views sustainability as a fundamental responsibility and a strategic priority. Our sustainability strategy is to help generations of our stakeholders realize life’s potential by innovating products that improve lives and our planet, building the best team, and protecting our environment. This strategy aligns with Danaher’s Shared Purpose and Core Values, as well as key UN Sustainable Development Goals (UN SDGs) under the United Nations 2030 Agenda for Sustainable Development. Our sustainability strategy is also informed by and grounded in the feedback we continually solicit from our stakeholders, including our regular sustainability prioritization assessments. Within each of the strategic elements of our sustainability program referenced above, where feasible and appropriate, we seek to quantify our performance and set goals to encourage continuous improvement.
Available Information
The Company maintains an internet website at www.danaher.com. The Company makes available free of charge on the website its annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports, filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (“the Exchange Act”), as soon as reasonably practicable after filing such material with, or furnishing such material to, the SEC. Danaher’s internet site and the information contained on or connected to that site are not incorporated by reference into this Form 10-K.
ITEM 1A. RISK FACTORS
You should carefully consider the risks and uncertainties described below, together with the information included elsewhere in this Annual Report on Form 10-K and other documents we file with the SEC. We have identified the risks and uncertainties described below as material, but they are not the only risks and uncertainties facing us. Our business is also subject to general risks and uncertainties that affect many other companies, such as market conditions, economic conditions, geopolitical events, changes in laws, regulations or accounting rules, fluctuations in interest rates, terrorism, wars or conflicts, major health concerns including pandemics, natural disasters or other disruptions of expected business conditions. Additional risks and uncertainties not currently known to us or that we currently believe are immaterial also may impair our business and financial statements, including our results of operations, liquidity and financial condition, and our stock price.
Business and Strategic Risks
The COVID-19 pandemic has adversely impacted and could in the future continue to adversely impact certain elements of our business and our financial statements.
Our global operations expose us to risks associated with public health crises, including epidemics and pandemics such as COVID-19. The global spread of COVID-19 led to unprecedented restrictions on, and disruptions in, business and personal activities, including as a result of preventive and precautionary measures that we, other businesses, our communities and governments undertook to mitigate the spread. The direct impact of COVID-19 and the preventive measures implemented as a result thereof adversely affected certain elements of our Company (including to a different degree our operations, commercial organizations, supply chains and distribution systems). Please see “Item 7 - MD&A” for a discussion of how COVID-19 impacted our results of operations in 2022. While the direct impact of COVID-19 and many of the preventive measures moderated in 2022, any resurgence of COVID-19 (or the outbreak of any other epidemic or pandemic) or the reinstatement of similar preventive measures in the future could negatively impact the economies and financial markets of the world and our businesses and financial statements.
The Company deployed its capabilities, expertise and scale to address critical health needs related to COVID-19, including developing and making available diagnostic tests for the rapid detection of COVID-19 as well as providing critical support to firms developing and producing vaccines and therapies for COVID-19. Please see “Item 7. MD&A” for a discussion of the Company products used in the fight against COVID-19. As COVID-19 and the preventive measures related thereto have moderated, demand for the Company’s COVID-19 related products has moderated as well. The duration and extent of future demand for our products supporting COVID-19 testing and for our products related to developing and producing vaccines and therapies for COVID-19 is uncertain and depends on multiple factors, including the extent to which COVID-19 persists in endemic form. Declines in demand for our COVID-19 related products that are unanticipated in timing or magnitude could adversely affect our business and financial statements.
Conditions in the global economy, the particular markets we serve and the financial markets can adversely affect our business and financial statements.
Our business is sensitive to general economic conditions. Slower economic growth in the domestic and/or international markets, inflation, actual or anticipated default on sovereign debt, volatility in the currency and credit markets, high levels of unemployment or underemployment, labor availability constraints, reduced levels of capital expenditures, changes or anticipation of potential changes in government trade, fiscal, tax and monetary policies, changes in capital requirements for financial institutions, government budget negotiation dynamics, sequestration, austerity measures and other challenges that affect economies of the world have in the past adversely affected, and may in the future adversely affect, the Company and its distributors, customers and suppliers, including having the effect of:
•reducing demand for our products and services (in this Annual Report, references to products and services also includes software), limiting the financing available to our customers and suppliers, increasing order cancellations and resulting in longer sales cycles and slower adoption of new technologies;
•increasing the difficulty in collecting accounts receivable and the risk of excess and obsolete inventories;
•increasing price competition in our served markets;
•supply interruptions, delays or cost increases, which can disrupt our ability to produce or deliver our products and/or increase our costs;
•increasing the risk of impairment of goodwill and other long-lived assets, and the risk that we may not be able to fully recover the value of other assets such as real estate and tax assets;
•increasing the risk that counterparties to our contractual arrangements will become insolvent or otherwise unable to fulfill their contractual obligations which, in addition to increasing the risks identified above, could result in preference actions against us; and
•adversely impacting market sizes and growth rates.
If growth in any key economy of the world or in any of the markets we serve slows for a significant period, if there is significant deterioration in any such economy or such markets or if economic improvements do not benefit the markets we serve, our business and financial statements can be adversely affected.
We face intense competition and if we are unable to compete effectively, we may experience decreased demand and decreased market share. Even if we compete effectively, we may be required to reduce the prices we charge.
Our businesses operate in industries that are intensely competitive and have been subject to increasing consolidation. Because of the range of the products and services we sell and the variety of markets we serve, we encounter a wide variety of competitors; refer to “Item 1. Business—Competition” for additional details. In order to compete effectively, we must retain longstanding relationships with major customers and continue to grow our business by establishing relationships with new customers, continually developing new products and services to maintain and expand our brand recognition and leadership position in various product and service categories and penetrating new markets, including high-growth markets. Our ability to compete can also be impacted by changing customer preferences and requirements (for example increased demand for products incorporating digital capabilities or more environmentally-friendly products and supplier practices) as well as changes in the way healthcare services are delivered (including the movement of some care from acute to non-acute settings and increased focus on chronic disease management). Cost containment efforts by governments and the private sector, particularly in the healthcare industry, are also resulting in increased emphasis on products that reduce costs and improve efficiency and effectiveness. In addition, significant shifts in industry market share have occurred and may in the future occur in connection with product problems, safety alerts and publications about products, reflecting the competitive significance of product quality, product efficacy and quality systems in our industry. Our failure to compete effectively and/or pricing pressures resulting from competition may adversely impact our business and financial statements, and our expansion into new markets may result in greater-than-expected risks, liabilities and expenses. In addition, the Company’s competitors and customers have from time to time introduced, and may in the future introduce, private label, generic or low-cost products that compete with the Company’s products at lower price points. New, disruptive technologies may emerge that displace the Company’s existing technologies. Competitors’ products can capture significant market share or lead to a decrease in market prices overall, resulting in an adverse effect on the Company’s business and financial statements.
Our growth depends in part on the timely development and commercialization, and customer acceptance, of new and enhanced products and services based on technological innovation.
We generally sell our products and services in industries that are characterized by rapid technological changes, frequent new product introductions and changing industry standards. If we do not develop innovative new and enhanced products and services on a timely basis, our offerings will become obsolete over time and our business and financial statements will suffer. Our success depends on several factors, including our ability to:
•correctly identify customer needs and preferences and predict future needs and preferences;
•allocate our R&D funding to products and services with higher growth prospects;
•anticipate and respond to our competitors’ development of new products and services and technological innovations;
•differentiate our offerings from our competitors’ offerings and avoid commoditization;
•innovate and develop new technologies and applications, and acquire or obtain rights to third-party technologies that may have valuable applications in our served markets;
•obtain adequate intellectual property rights with respect to key technologies before our competitors do;
•successfully commercialize new technologies in a timely manner, price them competitively and cost-effectively manufacture and deliver sufficient volumes of new products of appropriate quality on time;
•obtain necessary regulatory approvals of appropriate scope (including with respect to medical device products by demonstrating satisfactory clinical results where applicable as well as achieving third-party reimbursement); and
•stimulate customer demand for and convince customers to adopt new technologies.
If we fail to accurately predict future customer needs and preferences or fail to produce viable technologies, we may invest heavily in R&D of products and services that do not lead to significant revenue, which would adversely affect our business and financial statements. Even when we successfully innovate and develop new and enhanced products and services, we often incur substantial costs in doing so, and our profitability may suffer. In addition, promising new offerings may fail to reach the market or realize only limited commercial success because of real or perceived efficacy or safety concerns, failure to achieve positive clinical outcomes, uncertainty over third-party reimbursement or entrenched patterns of clinical practice. Competitors may also develop after-market services and parts for our products which may detract from our sales.
The health care industry and related industries that we serve have undergone, and are in the process of undergoing, significant changes in an effort to reduce (and increase the predictability of) costs, which can adversely affect our business and financial statements.
The health care industry and related industries that we serve have undergone, and are in the process of undergoing, significant changes in an effort to reduce (and increase the predictability of) costs, including the following:
•Many of our customers, and the end-users to whom our customers supply products, rely on government funding of and reimbursement for healthcare products and services and research activities. The PPACA, health care austerity measures in other countries and other potential healthcare reform changes and government austerity measures have reduced and may further reduce the amount of government funding or reimbursement available to customers or end-users of our products and services and/or the volume of medical procedures using our products and services. For example, the Protecting Access to Medicare Act of 2014 (“PAMA”) introduced a multi-year pricing program for services payable under the Clinical Laboratory Fee Schedule (“CLFS”) that is designed to bring Medicare allowable amounts in line with the amounts paid by private payors. It is still unclear whether and to what extent these new rates will affect overall pricing and reimbursement for clinical laboratory testing services, but to the extent our customers conclude that Medicare reimbursement for these services is inadequate, it can in turn adversely impact the prices at which we sell our products. Other countries, as well as some private payors, also control the price of health care products, directly or indirectly, through reimbursement, payment, pricing or coverage limitations, tying reimbursement to outcomes or (in the case of governmental entities) through compulsory licensing or limiting of intellectual property protections. Global economic uncertainty or deterioration can also adversely impact government funding and reimbursement.
•Governmental and private health care providers and payors around the world are increasingly utilizing managed care for the delivery of healthcare services, centralizing purchasing, limiting the number of vendors that may participate in purchasing programs, forming group purchasing organizations, strategic alliances and integrated health delivery networks and pursuing consolidation to improve their purchasing leverage, using competitive bid processes to procure healthcare products and services and investing in health care practices to increase their control over health care spending. Payors are also seeking to improve price predictability in an effort to mitigate exposure to future price increases.
These changes as well as other impacts from market demand, government regulations, third-party coverage and reimbursement policies and societal pressures are changing the way healthcare is delivered, reimbursed and funded and have in the past and could in the future cause participants in the healthcare industry and related industries that we serve to purchase fewer of our products and services, reduce the prices they are willing to pay for our products or services, reduce the amounts of reimbursement and funding available for our products and services from governmental agencies or third-party payors, heighten clinical data requirements, reduce the volume of medical procedures that use our products and services, affect the acceptance rate of new technologies and products and increase our compliance and other costs. In addition, we may be excluded from important market segments or unable to enter into contracts with group purchasing organizations and integrated health networks on terms acceptable to us, and even if we do enter into such contracts they may be on terms that negatively affect our current or future profitability. All of the factors described above can adversely affect our business and financial statements.
Non-U.S. economic, political, legal, compliance, social and business factors can negatively affect our business and financial statements.
In 2022 approximately 58% of our sales were derived from customers outside the U.S. In addition, many of our manufacturing operations, suppliers and employees are located outside the U.S. Since our growth strategy depends in part on our ability to further penetrate markets outside the U.S. and increase the localization of our products and services, we expect to continue to increase our sales and presence outside the U.S., particularly in the high-growth markets. Our non-U.S. business (and particularly our business in high-growth markets) is subject to risks that include:
•public health crises and epidemics, such as COVID-19;
•interruption in the transportation of materials to us and finished goods to our customers;
•differences in terms of sale, including longer payment terms than are typical in the U.S.;
•local product preferences or requirements;
•changes in a country’s or region’s political, legal, social, compliance, business or economic conditions, such as the devaluation of particular currencies;
•trade protection measures, tariffs, embargoes and import or export restrictions and requirements;
•unexpected changes in laws or regulatory requirements, including changes in tax laws;
•capital controls and limitations on ownership and on repatriation of earnings and cash;
•the potential for nationalization of enterprises;
•changes in local healthcare delivery, payment and reimbursement policies and programs;
•complex data privacy and cybersecurity requirements;
•limitations on legal rights and our ability to enforce such rights, including differing protection of intellectual property;
•difficulty in staffing and managing widespread operations;
•workforce instability and differing labor or employment regulations;
•difficulties in implementing restructuring actions on a timely or comprehensive basis;
•greater uncertainty, risk, expense and delay in commercializing products in certain foreign jurisdictions, including with respect to product and other regulatory approvals; and
•remaining uncertainties relating to the impact of the UK’s exit from the EU in 2020.
International business risks have in the past and may in the future negatively affect our business and financial statements.
In 2022 we generated approximately 13% of our sales from China. Accordingly, political, economic, legal, compliance, social and business conditions in China generally can adversely influence our business and financial statements. Additionally, China’s government continues to play a significant role in regulating industry development by imposing sector-specific policies, and it maintains control over China’s economic growth through setting monetary policy and determining treatment of particular industries or companies. Further, considerable uncertainty exists regarding the long-term effects of the expansionary monetary and fiscal policies adopted by the central banks and financial authorities of some of the world’s leading economies, including the U.S. and China. Uncertainty or adverse changes to conditions in China or the policies of China’s government or its laws and regulations can adversely affect the overall economic growth of China, or of the particular industries in which we participate, and can adversely affect our business and financial statements.
Our growth can suffer if the markets into which we sell our products and services decline, do not grow as anticipated or experience cyclicality.
Our growth depends in part on the growth of the markets which we serve, and visibility into our markets can be limited (particularly for markets into which we sell through distribution). Our quarterly sales and profits depend substantially on the volume and timing of orders received during the quarter, which are difficult to forecast. Any decline or lower than expected growth in our served markets can diminish demand for our products and services and adversely affect our business and financial statements. Certain of our businesses operate in industries that have experienced and may experience periodic, cyclical downturns. In addition, in certain of our businesses demand depends on customers’ capital spending budgets as well as government funding policies, and matters of public policy and government budget dynamics as well as product and economic cycles can affect the spending decisions of these entities. Demand for our products and services is also sensitive to changes in customer order patterns, which may be affected by announced price changes, marketing or promotional programs, new product introductions, the timing of industry trade shows and changes in distributor or customer inventory levels due to distributor or customer management thereof or other factors. Any of these factors could adversely affect our business and financial statements in any given period.
Certain of our businesses rely on relationships with collaborative partners and other third-parties for development, supply and/or marketing of certain products, potential products and technologies, and such collaborative partners or other third-parties could fail to perform sufficiently.
For certain of our businesses, success in penetrating target markets depends in part on their ability to develop and maintain collaborative relationships with other companies. Relying on collaborative relationships is risky because, among other things, our collaborative partners may (1) not devote sufficient resources to the success of our collaborations; (2) fail to obtain regulatory approvals necessary to continue the collaborations in a timely manner; (3) be acquired by other companies and terminate our collaborative partnership or become insolvent; (4) compete with us; (5) disagree with us on key details of the collaborative relationship; (6) have insufficient capital resources; (7) fail to comply with applicable laws, regulatory requirements and/or applicable contractual obligations; and (8) terminate or decline to renew existing collaborations on acceptable terms, which may require us to devote additional resources to product development and commercialization and/or cancel programs. The realization of any of these risks could adversely affect our business and financial statements.
Acquisition, Divestiture and Investment Risks
Any inability to consummate acquisitions at our historical rate and at appropriate prices, and to make appropriate investments that support our long-term strategy, could negatively impact our business.
Our ability to grow revenues, earnings and cash flow at or above our historic rates depends in part upon our ability to identify and successfully acquire and integrate businesses at appropriate prices and realize anticipated synergies, and to make appropriate investments that support our long-term strategy. We may not be able to consummate acquisitions at rates similar to the past, which could adversely impact our business. Promising acquisitions and investments are difficult to identify and complete for a number of reasons, including high valuations, competition among prospective buyers or investors, the availability of affordable funding in the capital markets and the need to satisfy applicable closing conditions and obtain applicable antitrust and other regulatory approvals on acceptable terms. In addition, competition for acquisitions and investments has resulted and may result in higher purchase prices. Changes in accounting or regulatory requirements or instability in the credit markets could also adversely impact our ability to consummate acquisitions and investments.
Our acquisition of businesses, investments, joint ventures and other strategic relationships can negatively impact our business and financial statements.
As part of our business strategy, we acquire businesses, make investments and enter into joint ventures and other strategic relationships in the ordinary course, and we also from time to time complete more significant transactions; refer to “Item 7. MD&A” for additional details. Acquisitions, investments, joint ventures and strategic relationships involve a number of financial, accounting, managerial, operational, legal, compliance and other risks and challenges, including but not limited to the following, any of which can adversely affect our business and our financial statements:
•businesses, technologies, services and products that we acquire or invest in have sometimes under-performed relative to our expectations and the price that we paid, failed to perform in accordance with our anticipated timetable or failed to achieve and/or sustain profitability;
•we from time to time incur or assume significant debt in connection with our acquisitions, investments, joint ventures or strategic relationships, which can also cause a deterioration of Danaher’s credit ratings, result in increased borrowing costs and interest expense and diminish our future access to the capital markets;
•acquisitions, investments, joint ventures or strategic relationships can cause our financial results to differ from our own or the investment community’s expectations in any given period, or over the long-term;
•pre-closing and post-closing earnings charges can adversely impact our results in any given period, and the impact may be substantially different from period-to-period;
•acquisitions, investments, joint ventures or strategic relationships can create demands on our management, operational resources and financial and internal control systems that we are unable to effectively address;
•we can experience difficulty in integrating cultures, personnel, operations and financial and other controls and systems and retaining key employees and customers, and former employees of our existing businesses or businesses we acquire sometimes compete with us;
•we are not always able to achieve cost savings or other synergies anticipated in connection with acquisitions, investments, joint ventures or strategic relationships;
•we have assumed and may assume unknown liabilities, known contingent liabilities that become realized, known liabilities that prove greater than anticipated, internal control deficiencies or exposure to regulatory sanctions resulting from the acquired company’s or investee’s activities; and the realization of any of these liabilities or deficiencies can increase our expenses, adversely affect our financial position or cause us to fail to meet our public financial reporting obligations;
•in connection with acquisitions and joint ventures, we often enter into post-closing financial arrangements such as purchase price adjustments, earn-out obligations and indemnification obligations, which can have unpredictable financial results;
•as a result of our acquisitions and investments, we have recorded significant goodwill and other assets on our balance sheet and if we are not able to realize the value of these assets, or if the value of our investments declines, we are required to incur impairment charges;
•we may have interests that diverge from those of our joint venture partners or other strategic partners or the companies we invest in, and we are not always able to direct or influence the management and operations of the joint venture, other strategic relationship or investee in the manner we believe is most appropriate, exposing us to additional risk; and
•investing in or making loans to early-stage companies often entails a high degree of risk, including uncertainty regarding the company’s ability to successfully develop new technologies and services, bring these new technologies and services to market and gain market acceptance, maintain adequate capitalization and access to cash or other forms of liquidity, and retain critical management personnel; we do not always achieve the strategic, technological, financial or commercial benefits we anticipate; we may lose our investment or fail to recoup our loan; or our investment may be illiquid for a greater-than-expected period of time.
The indemnification provisions of acquisition agreements by which we have acquired companies may not fully protect us and as a result we may face unexpected liabilities.
Certain of the acquisition agreements by which we have acquired companies require the former owners to indemnify us against certain liabilities related to the operation of the acquired company before we acquired it. In most of these agreements, however, the liability of the former owners is limited and certain former owners may be unable to meet their indemnification responsibilities. In addition, we obtain or receive the benefits of representations and warranties insurance in connection with certain acquisitions. There can be no assurance that these indemnification provisions or insurance coverages will protect us fully or at all, and as a result we may face unexpected liabilities that adversely affect our business and financial statements.
We intend to separate our Environmental & Applied Solutions segment to create a publicly-traded company in the fourth quarter of 2023. The proposed transaction may not be completed on the currently contemplated timeline or at all and may not achieve the intended benefits.
We have announced our intention to separate Danaher’s Environmental & Applied Solutions segment to create a publicly-traded company in the fourth quarter of 2023, subject to the satisfaction of customary conditions, including obtaining final approval from the Danaher Board of Directors, satisfactory completion of financing and receipt of tax opinions, favorable rulings from the Internal Revenue Service and other regulatory approvals (the “EAS Separation”). There can be no assurance that we will be able to satisfy the necessary conditions or that we will successfully complete the anticipated separation in our preferred structure, on the anticipated timeline or at all. Unanticipated developments, including possible delays in obtaining various tax rulings or regulatory approvals, uncertainty or declines in the financial markets or other adverse market conditions, changes in the Company’s cash requirements, challenges in establishing the new company’s organizational structure, infrastructure or processes, or adverse business performance could delay or prevent the proposed separation or cause the proposed separation to occur on terms or conditions that are less favorable and/or different than expected. Expenses incurred to accomplish the proposed separation may be significantly higher than what we currently anticipate. Executing the proposed separation also requires significant time and attention from management, which could distract them from other tasks in operating our business. Even if the transaction is completed, we may not realize some or all of the anticipated benefits from the separation and there can be no assurance that the separation will yield greater net benefits to Danaher and its shareholders than if such transaction had not occurred. Following the proposed separation, the combined value of the common stock of the two publicly-traded companies may not be equal to or greater than what the value of our common stock would have been had the separation not occurred.
Divestitures or other dispositions could negatively impact our business, and contingent liabilities from businesses that we or our predecessors have disposed of could adversely affect our business and financial statements.
We continually assess the strategic fit of our existing businesses and may divest, spin-off, split-off or otherwise dispose of businesses for strategic, financial or other reasons. For example, in 2015 Danaher separated and split-off to Danaher shareholders the majority of its former communications business in a Reverse Morris Trust transaction with NetScout Systems, Inc. (the “Communications Disposition”), in 2016 Danaher separated and spun-off to Danaher shareholders its former Test & Measurement segment, Industrial Technologies segment (excluding the product identification businesses) and retail/commercial petroleum business (collectively known as “Fortive Corporation”) (the “Fortive Disposition”), in 2019 Danaher consummated the separation and initial public offering (“IPO”) and subsequent split-off of its Dental segment, known as Envista Holdings Corporation (the “Envista Disposition”), and in 2022 Danaher announced the anticipated EAS Separation. Transactions such as these pose risks and challenges that could negatively impact our business and financial statements. For example, divestitures or other dispositions can dilute the Company’s earnings per share, have other adverse financial, tax and accounting impacts and distract management, and disputes can arise with the new owners of the divested/disposed business. In addition, we have retained responsibility for and/or have agreed to indemnify buyers against some known and unknown contingent liabilities related to a number of businesses we or our predecessors have sold or disposed. The resolution of these contingencies has not had a material effect on our business or financial statements but there can be no assurance that this favorable pattern will continue.
Potential indemnification liabilities pursuant to the Communications Disposition, the Fortive Disposition, the Envista Disposition or the anticipated EAS Separation could adversely affect our business and financial statements.
With respect to each of the Communications Disposition, the Fortive Disposition and the Envista Disposition, we entered into a separation agreement and related agreements to govern the separation and related transactions and the relationship between the respective companies going forward (and we expect to enter into similar agreements in connection with the EAS Separation). These agreements provide for specific indemnity and liability obligations of each party that can lead to disputes between us and the respective counterparty. If we are required to indemnify any of the other parties under the circumstances set forth in these agreements, we may be subject to substantial liabilities. In addition, with respect to the liabilities for which the other parties have agreed to indemnify us under these agreements, there can be no assurance that the indemnity rights we have against such other parties will be sufficient to protect us against the full amount of the liabilities, or that such other parties will be able to fully satisfy their respective indemnification obligations. It is also possible that a court could disregard the allocation of assets and liabilities agreed to between Danaher and such other parties and require Danaher to assume responsibility for obligations allocated to such other parties. Each of these risks could negatively affect our business and financial statements.
We could incur significant liability if any of the Fortive Disposition, the Envista Disposition or the EAS Separation is determined to be a taxable transaction.
We have received opinions from outside tax counsel to the effect that each of the Fortive Disposition and the Envista Disposition qualifies as a transaction that is described in Sections 355(a) and 368(a)(1)(D) of the Internal Revenue Code, and we anticipate obtaining a similar opinion with respect to the EAS Separation. These opinions rely on certain facts, assumptions, representations and undertakings regarding the past and future conduct of the companies’ respective businesses and other matters. If any of these facts, assumptions, representations or undertakings are incorrect or not satisfied, our stockholders and we may not be able to rely on the respective opinion of tax counsel and could be subject to significant tax liabilities. Notwithstanding the opinion of tax counsel, the IRS could determine on audit that any such transactions are taxable if it determines that any of these facts, assumptions, representations or undertakings are not correct or have been violated or if it disagrees with the conclusions in the respective opinion. If any such transaction is determined to be taxable for U.S. federal income tax purposes, our stockholders that are subject to U.S. federal income tax and we could incur significant U.S. federal income tax liabilities.
Operational Risks
Significant disruptions in, or breaches in security of, our information technology systems or data or violation of data privacy laws can adversely affect our business and financial statements.
We rely on information technology systems, some of which are provided and/or managed by third-parties, to process, transmit and store electronic information (including sensitive data such as confidential business information and personal data relating to employees, customers, other business partners and patients), and to manage or support a variety of critical business processes and activities (such as receiving and fulfilling orders, billing, collecting and making payments, shipping products, providing services and support to customers and fulfilling contractual obligations). In addition, some of our remote monitoring products and services incorporate software and information technology that house personal data and some products or software we sell to customers connect to our systems for maintenance or other purposes. These systems, products and services (including those we
acquire through business acquisitions) can be damaged, disrupted or shut down due to attacks by computer hackers, computer viruses, ransomware, human error or malfeasance (including by employees), power outages, hardware failures, telecommunication or utility failures, catastrophes or other unforeseen events, and in any such circumstances our system redundancy and other disaster recovery planning may be ineffective or inadequate. Attacks can also target hardware, software and information installed, stored or transmitted in our products after such products have been purchased and incorporated into third-party products, facilities or infrastructure. Security breaches of systems provided or enabled by us, regardless of whether the breach is attributable to a vulnerability in our products or services, or security breaches of third-party suppliers we rely on to process, store or transmit electronic information, can result in the misappropriation, destruction or unauthorized disclosure of confidential information or personal data belonging to us or to our employees, partners, customers, patients or suppliers. Like most multinational corporations, our information technology systems and data have been subject to computer viruses, malicious codes, unauthorized access and other cyber-attacks and we expect the sophistication and frequency of such attacks to continue to increase. Unauthorized tampering, adulteration or interference with our products may also adversely affect product functionality and result in loss of data, risk to patient safety and product recalls or field actions. The attacks, breaches, misappropriations and other disruptions and damage described above can interrupt our operations or the operations of our customers and partners, delay production and shipments, result in theft of our and our customers’ intellectual property and trade secrets, result in disclosure of personal data, damage customer, patient, business partner and employee relationships and our reputation and result in defective products or services, legal claims and proceedings, liability and penalties under privacy and other laws and increased costs for security and remediation, in each case resulting in an adverse effect on our business and financial statements. Our liability insurance may not be sufficient in type or amount to cover us against claims related to security breaches, cyber-attacks and other related breaches.
In addition, our information technology systems require an ongoing commitment of significant resources to maintain and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving legal and regulatory standards, evolving customer expectations, changes in the techniques used to obtain unauthorized access to data and information systems, and the information technology needs associated with our changing products and services. There can be no assurance that we will be able to successfully maintain, enhance and upgrade our systems as necessary to effectively address these requirements. Further, a greater number of our employees have been working remotely since the beginning of the COVID-19 pandemic, which exposes us to greater cybersecurity and data privacy risks.
Any inability to maintain reliable information technology systems and appropriate controls with respect to global data privacy and security requirements and prevent data breaches can result in adverse regulatory and business consequences and litigation. As a global organization, we are subject to data privacy and security laws, regulations and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal and/or sensitive data in the course of our business. Please see “Item 1. Business—Regulatory Matters” for additional information. For example, entities that are found to be in violation of HIPAA as the result of a breach of unsecured patient health information, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations. Failure to comply with the requirements of the GDPR and the applicable national data protection laws of the EU member states and other states subject to the GDPR may result in fines of up to €20 million or up to 4% of total worldwide annual turnover for the preceding financial year, whichever is higher, and other administrative penalties. Several other countries such as China and Russia have passed, and other countries have passed or are considering passing, laws that require some or all personal data relating to their citizens to be maintained on local servers or impose significant restrictions on data transfer. State privacy laws in California impose some of the same features as the GDPR and have prompted several other states to enact similar laws. Additionally, a bipartisan bill under consideration in Congress would, if adopted, impose broad privacy requirements at the U.S. federal level and provide enhanced enforcement authority to the FTC. Government investigations and enforcement actions can be costly and interrupt the regular operation of our business, and data breaches or violations of data privacy laws can result in civil and criminal, monetary and non-monetary penalties and damage to customer, patient, business partner and employee relationships and to our reputation, any of which may adversely affect our business and financial statements. In addition, compliance with the varying data privacy regulations across the U.S. and around the world has required significant expenditures and may require additional expenditures, and may require further changes in our products or business models that increase competition or reduce revenue.
Defects and unanticipated use or inadequate disclosure with respect to our products or services, or allegations thereof, can adversely affect our business and financial statements.
Manufacturing or design defects or “bugs” in, unanticipated use of, safety or quality issues (or the perception of such issues) with respect to, “off label” use of, or inadequate disclosure of risks relating to the use of products and services that we make or sell (including items that we source from third-parties) can lead to personal injury, death, property damage and/or regulatory violations that can adversely affect our business and financial statements. These events can lead to recalls or safety alerts, result in the removal of a product or service from the market and result in product liability or similar claims being brought against us. The accelerated development and production of products and services in an effort to address the COVID-19 pandemic also
increased the risk of regulatory enforcement actions, product defects or claims thereof. Recalls, removals and product liability and similar claims (regardless of their validity or ultimate outcome) can result in significant costs, as well as negative publicity and damage to our reputation that could reduce demand for our products and services. Our business can also be affected by studies of the utilization, safety and efficacy of medical device products and components that are conducted by industry participants, government agencies and others. Any of the above can result in the discontinuation of marketing of such products in one or more countries and give rise to claims for damages from persons who believe they have been injured as a result of product issues, including claims by individuals or groups seeking to represent a class.
If we suffer loss to our facilities, supply chains, distribution systems or information technology systems due to catastrophe or other events, our operations could be seriously harmed.
Our facilities, supply chains, distribution systems and information technology systems are subject to catastrophic loss due to fire, flood, cyber-attack, earthquake, hurricane, power shortage or outage, public health crisis (including epidemics and pandemics) and the reaction thereto, war, terrorism, riot, public protest or other natural or man-made disasters, such as the COVID-19 pandemic and the damage caused to our facilities by Hurricane Maria in Puerto Rico in 2017. If any of these facilities, supply chains or systems were to experience a catastrophic loss, it could disrupt our operations, delay production and shipments, result in defective products or services, diminish demand, damage customer relationships and our reputation and result in legal exposure and significant repair or replacement expenses. The third-party insurance coverage that we maintain varies from time to time in both type and amount depending on cost, availability and our decisions regarding risk retention, and may be unavailable or insufficient to protect us against such losses.
Climate change, legal or regulatory measures to address climate change and any inability on our part to address stakeholder expectations relating to climate change may negatively affect us.
Climate change resulting from increased concentrations of carbon dioxide and other greenhouse gases in the atmosphere presents risks to our operations. Physical risk resulting from acute changes (such as hurricane, tornado, wildfire or flooding) or chronic changes (such as droughts, heat waves or sea level changes) in climate patterns can adversely impact our facilities and operations and disrupt our supply chains and distribution systems. Concern over climate change can also result in new or additional legal, regulatory or quasi-regulatory requirements designed to reduce greenhouse gas emissions and/or mitigate the effects of climate change on the environment (such as taxation of, or caps on the use of, carbon-based energy). Any such new or additional requirements may increase the costs associated with, or disrupt, sourcing, manufacturing and distribution of our products, which may adversely affect our business and financial statements. In addition, any failure to adequately address stakeholder expectations with respect to environmental, social and governance (“ESG”) matters may result in the loss of business, adverse reputational impacts, diluted market valuations and challenges in attracting and retaining customers and talented employees. For example, our ability to achieve our current and future ESG goals is uncertain and remains subject to numerous risks, including evolving regulatory requirements and stakeholder expectations, our ability to recruit, develop and retain a diverse workforce, the availability of suppliers and other business partners that can meet our ESG expectations, the effects of the organic and inorganic growth of our business, cost considerations and the development and availability of cost-effective technologies or resources that support our goals.
The manufacture of many of our products is a highly exacting and complex process, and if we directly or indirectly encounter problems manufacturing products, our business and financial statements could suffer.
The manufacture of many of our products is a highly exacting and complex process, due in part to strict regulatory requirements. Problems can arise during manufacturing for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with raw materials or components, cyber-attacks, natural disasters and environmental factors, and if not discovered before the product is released to market can result in recalls and product liability exposure. Because of the time required to approve and license certain regulated manufacturing facilities and other stringent regulations of the FDA and similar agencies regarding the manufacture of certain of our products, an alternative manufacturer is not always available on a timely basis to replace such production capacity. Any of these manufacturing problems could result in adverse impacts to our business and financial statements.
Our financial results are subject to fluctuations in the cost and availability of the supplies that we use in, and the labor we need for, our operations.
Prices for and availability of the components, raw materials and other commodities we use in our business, as well as for labor, have fluctuated significantly in the past, including during 2022. Please see “Item 1. Business-Materials” for a discussion of the inputs we use in our business, supply chain and labor availability disruptions and constraints our businesses have faced and are facing, and the adverse impacts that we have incurred and may incur relating thereto. The supply chains for our businesses can be disrupted by supplier capacity constraints, fluctuations in demand, decreased availability of key raw materials or commodities, legislative or regulatory changes, bankruptcy or exiting of the business for other reasons and external events such
as natural disasters, pandemic health issues, war, terrorist actions and governmental actions (such as trade protectionism). In addition, some of our businesses purchase certain requirements from sole or limited source suppliers for reasons of quality assurance, regulatory requirements, cost effectiveness, availability or uniqueness of design. In the event of interruptions in the supply, or increases in the cost, of such supplies, we might not be able to quickly establish or qualify replacement sources of supply. Sustained interruptions in the supply of, or increase in the cost of, key components, raw materials, other commodities and labor can result in production interruptions, delays, extended lead times and inefficiencies and adversely affect our business and financial statements. In addition, due to the highly competitive nature of the industries that we serve, the cost-containment efforts of our customers and the terms of certain contracts we are party to, when supply and labor prices rise we are not always able to pass along cost increases through higher prices for our products. If we are unable to fully recover higher supply and labor costs through price increases or offset these increases through cost reductions, or if there is a time delay between the increase in costs and our ability to recover or offset these costs, our margins and profitability can decline and our business and financial statements can be adversely affected.
Our profitability could also be adversely impacted if we are unable to adjust our purchases to reflect changes in customer demand and market fluctuations, including those caused by seasonality or cyclicality. During a market upturn, suppliers from time to time extend lead times, limit supplies or increase prices. Conversely, in order to secure supplies for the production of products, we sometimes enter into noncancelable purchase commitments with vendors, which can impact our ability to adjust our inventory to reflect declining market demands. Because we cannot always immediately adapt our production capacity and related cost structures to changing market conditions, at times our manufacturing capacity exceeds or falls short of our production requirements. Any or all of these problems can result in the loss of customers or cost inefficiencies, provide an opportunity for competing products to gain market acceptance and otherwise adversely affect our business and financial statements.
Adverse changes in our relationships with, or the financial condition, performance, purchasing patterns or inventory levels of, key distributors and other channel partners can adversely affect our business and financial statements.
Certain of our businesses sell a significant amount of their products to or through key distributors and other channel partners that have valuable relationships with customers and end-users. Some of these distributors and other partners also sell our competitors’ products or compete with us directly, and if they favor competing products for any reason they may fail to market our products effectively. Adverse changes in our relationships with these distributors and other partners, reduction or discontinuation of their purchases from us or adverse developments in their financial condition, performance or purchasing patterns, can adversely affect our business and financial statements. The levels of inventory maintained by our key distributors and other channel partners, and changes in those levels, also impacts our results of operations in any given period. In addition, the consolidation of distributors and customers in certain of our served industries can adversely impact our business and financial statements.
Our success depends on our ability to recruit, retain and motivate talented employees representing diverse backgrounds, experiences and skill sets.
The market for highly skilled workers and leaders in our industries, particularly in the areas of science and technology, is extremely competitive and expectations from qualified talent in many areas of the labor market have evolved and escalated recently. In addition, in 2022 we faced labor availability constraints and labor cost inflation in certain areas of our business. If we are less successful in our recruiting efforts, if we cannot retain and motivate highly skilled workers and key leaders representing diverse backgrounds, experiences and skill sets, or if we experience labor disputes, our business and financial statements may be adversely affected.
Our restructuring actions can have long-term adverse effects on our business and financial statements.
We have implemented significant restructuring activities across our businesses to adjust our cost structure, and we may engage in similar restructuring activities in the future. These restructuring activities and our regular ongoing cost reduction activities could diminish our resources and competitiveness, and delays or failures in implementing planned restructuring activities may diminish the expected operational or financial benefits from such actions. Any of the circumstances described above could adversely impact our business and financial statements.
Intellectual Property Risks
If we are unable to adequately protect our intellectual property, or if third-parties infringe our intellectual property rights, we may suffer competitive injury or expend significant resources enforcing our rights. These risks are particularly pronounced in countries in which we do business that do not have levels of protection of intellectual property comparable to the United States.
Many of the markets we serve are technology-driven, and as a result intellectual property rights play a significant role in product development and differentiation. We own numerous patents, trademarks, copyrights, trade secrets and other intellectual property and licenses to intellectual property owned by others, which in aggregate are important to our business. The intellectual property rights that we obtain, however, are not always sufficiently broad and do not always provide us a significant competitive advantage, and patents may not be issued for pending or future patent applications owned by or licensed to us. In addition, the steps that we and our licensors have taken to maintain and protect our intellectual property do not always prevent it from being challenged, invalidated, circumvented, designed-around or becoming subject to compulsory licensing. In some circumstances, enforcement is not available to us because an infringer has a dominant intellectual property position or for other business reasons. We also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary rights. There can be no assurance that these agreements adequately protect our trade secrets and other proprietary rights and will not be breached, that we will have adequate remedies for any breach, that others will not independently develop substantially equivalent proprietary information or that third-parties will not otherwise gain access to our trade secrets or other proprietary rights. Our failure to obtain or maintain intellectual property rights that convey competitive advantage and adequately protect our intellectual property; our failure to detect or prevent circumvention or unauthorized use of such property and the cost of enforcing our intellectual property rights each can adversely impact our business and financial statements.
These risks are particularly pronounced in countries in which we do business that do not have levels of protection of corporate proprietary information, intellectual property, technology and other assets comparable to the United States. The risks we encounter in such countries include but are not limited to the following:
•Joint ventures that we participate in can include restrictions that could compromise our control over the intellectual property, technology and proprietary information of the joint venture;
•As we expand our operations globally, increasing amounts of our data, intellectual property and technology is used and stored in countries outside the United States, and regulations in certain countries require data to be stored locally. These factors increase the risk that such data, intellectual property and technology could be stolen or otherwise compromised;
•Certain of our products have been counterfeited and we may encounter additional and/or increased levels of counterfeiting in the future;
•Governmental entities may adopt regulations or other requirements that give them rights to certain of our intellectual property, technology and/or proprietary information, such as through compulsory licensing or ownership restrictions or requirements;
•In certain countries, we do not have the same ability to enforce intellectual property rights as we do in the U.S.;
•Governmental regulations relating to state secrecy or other topics limit our ability to transfer data or technology out of certain jurisdictions; and
•Risks, costs and challenges of operating in a particular jurisdiction can result in a decision to relocate or divert operations to a different jurisdiction, potentially at higher cost.
Any of these risks can adversely impact our business and financial statements. Refer to “—International economic, political, legal, compliance, social and business factors could negatively affect our financial statements” for a discussion of additional risks relating to our international operations.
Third-parties from time to time claim that we are infringing or misappropriating their intellectual property rights and we could suffer significant litigation expenses, losses or licensing expenses or be prevented from selling products or services.
From time to time, we receive notices from third parties alleging intellectual property infringement or misappropriation of third parties’ intellectual property and cannot be certain that the conduct of our business does not and will not infringe or misappropriate the intellectual property rights of others. Disputes or litigations regarding intellectual property can be costly and time-consuming to defend due to the complexity of many of our technologies and the uncertainty of intellectual property
litigation. Our intellectual property portfolio may not be useful in asserting a counterclaim, or negotiating a license, in response to a claim of infringement or misappropriation. In addition, as a result of such claims of infringement or misappropriation, we could lose our rights to critical technology, be unable to license critical technology or sell critical products and services, be required to pay substantial damages or license fees with respect to the infringed rights, be required to license technology or other intellectual property rights from others, be required to cease marketing, manufacturing or using certain products or be required to redesign, re-engineer or re-brand our products at substantial cost, any of which could adversely impact our business and financial statements. Third-party intellectual property rights may also make it more difficult or expensive for us to meet market demand for particular product or design innovations. When we are required to seek licenses under patents or other intellectual property rights of others, we are not always able to acquire these licenses on acceptable terms, if at all. Even if we successfully defend against claims of infringement or misappropriation, we may incur significant costs and diversion of management attention and resources, which could adversely affect our business and financial statements.
The U.S. government has certain rights with respect to incremental production capacity attributable to, and/or the intellectual property we have developed using government financing. In addition, in times of national emergency the U.S. government could also control our allocation of manufacturing capacity.
Certain agencies of the U.S. government, such as the Biomedical Advanced Research and Development Authority (“BARDA”) within the U.S. Department of Health and Human Services, have agreed to finance an expansion of production capacity and/or the development of technology at certain of our businesses, and our businesses may enter into similar agreements in the future. In consideration of this financing the U.S. government has certain rights, including rights with respect to the allocation of certain of the incremental production capacity associated with such expansion and/or rights in intellectual property produced with its financial assistance. If the U.S. government exercises its rights with respect to our intellectual property or allocating our production capacity, our business and financial statements could be negatively impacted.
In addition, to optimize availability of needed medical and other products in connection with any pandemic or other national emergency, we may elect or governments may require us or our customers to allocate manufacturing capacity (for example, pursuant to the U.S. Defense Production Act (“DPA”)) in a way that adversely affects our financial condition and results of operations, results in differential treatment of customers and/or adversely affects our reputation and customer relationships. For example, certain of our customers are or have been subject to DPA requirements relating to the production of COVID-19 related products and have required certain of our businesses to also comply with these requirements under our supply agreements. Under such circumstances, the levels of demand for our products can exceed our capacity to meet such demand on a timely basis or at all, which can result in negative publicity, competitive disadvantage and legal liability, and may adversely affect our business and financial statements.
Financial and Tax Risks
Our outstanding debt has increased significantly as a result of acquisitions, and we may incur additional debt in the future. Our existing and future indebtedness may limit our operations and our use of our cash flow and negatively impact our credit ratings; and any failure to comply with the covenants that apply to our indebtedness could adversely affect our business and financial statements.
As of December 31, 2022, we had approximately $19.7 billion in outstanding indebtedness. In addition, we had the ability to incur approximately $3.0 billion of additional indebtedness in direct borrowings or under our outstanding commercial paper facilities based on the amounts available under our credit facilities that were not being used to backstop outstanding commercial paper balances. Our debt level and related debt service obligations (as well as the dividend obligations pursuant to our Series B Mandatory Convertible Preferred Stock (“MCPS”)) can have negative consequences, including (1) requiring us to dedicate significant cash flow from operations to the payment of principal and interest on our debt (or dividends on our MCPS), which reduces the funds we have available for other purposes such as acquisitions and other investments; (2) reducing our flexibility in planning for or reacting to changes in our business and market conditions; and (3) exposing us to interest rate risk on any variable rate debt we may issue. If our credit ratings are downgraded or put on watch for a potential downgrade, we may not be able to sell additional debt securities or borrow money in the amounts, at the times or interest rates or upon the more favorable terms and conditions that might be available if our current credit ratings were maintained.
Our credit facilities and long-term debt obligations also impose certain restrictions on us, including certain restrictions on our ability to incur liens on our assets, and a requirement under our credit facilities to maintain a consolidated leverage ratio (the ratio of consolidated indebtedness to consolidated indebtedness plus shareholders’ equity) of 0.65 to 1.0 or less. If we breach any of these restrictions and cannot obtain a waiver from the lenders on favorable terms, subject to applicable cure periods, the outstanding indebtedness (and any other indebtedness with cross-default provisions) could be declared immediately due and payable, which would adversely affect our business and financial statements (including our liquidity). If we add new debt in the future, the risks described above would increase.
We may be required to recognize impairment charges for our goodwill and other intangible assets.
As of December 31, 2022, the net carrying value of our goodwill and other intangible assets totaled approximately $60.1 billion. Significant negative industry or economic trends, disruptions to our business, inability to effectively integrate acquired businesses, unexpected significant changes or planned changes in use of our assets, changes in the structure of our business, divestitures, market capitalization declines, or increases in associated discount rates can impair our goodwill and other intangible assets. In the past, we have recognized impairment charges relating to certain non-goodwill intangible assets, and in the future, we could recognize charges related to the impairment of goodwill or other intangible assets. Any such impairment charges adversely affect our financial statements in the periods recognized.
Foreign currency exchange rates can adversely affect our financial statements.
Sales and purchases in currencies other than the U.S. dollar expose us to fluctuations in foreign currencies relative to the U.S. dollar, which have in the past and may in the future adversely affect our financial statements. Increased strength of the U.S. dollar increases the effective price of our products sold in U.S. dollars into other countries, which can adversely affect sales or require us to lower our prices. Decreased strength of the U.S. dollar adversely affects the cost of materials, products and services we purchase overseas. Sales and expenses of our non-U.S. businesses are also translated into U.S. dollars for reporting purposes and the strengthening of the U.S. dollar generally results in unfavorable translation effects. In addition, certain of our businesses invoice customers in a currency other than the business’ functional currency, and movements in the invoiced currency relative to the functional currency can also result in unfavorable translation effects. The Company also faces exchange rate risk from its investments in subsidiaries owned and operated in foreign countries.
Changes in our tax rates or exposure to additional income tax liabilities or assessments can affect our profitability. In addition, audits by tax authorities can result in additional tax payments for prior periods.
We are subject to income taxes in the U.S. and in numerous non-U.S. jurisdictions. Due to the potential for changes to tax laws and regulations or changes to the interpretation thereof (including regulations and interpretations pertaining to the U.S. Tax Cuts and Jobs Act (“TCJA”)), the ambiguity of tax laws and regulations, the subjectivity of factual interpretations, the complexity of our intercompany arrangements, uncertainties regarding the geographic mix of earnings in any particular period, and other factors, our estimates of effective tax rate and income tax assets and liabilities can be incorrect and our financial statements could be adversely affected; please refer to “Item 7. MD&A” for a discussion of additional factors that may adversely affect our effective tax rate and decrease our profitability in any period. The impact of the factors referenced in the preceding sentence may be substantially different from period-to-period. In addition, the amount of income taxes we pay is subject to ongoing audits by U.S. federal, state and local tax authorities and by non-U.S. tax authorities, such as the audits described in MD&A and the Company’s Consolidated Financial Statements. If audits result in payments or assessments different from our reserves, our results can be adversely affected. Any further changes to the tax system in the United States or in other jurisdictions could also adversely affect our financial statements.
Changes in tax law relating to multinational corporations could adversely affect our tax position.
Legislative bodies and government agencies in the U.S. and other countries as well as the Organisation for Economic Co-operation and Development (“OECD”) have focused on issues related to the taxation of multinational corporations. One example is in the area of “base erosion and profit shifting,” for which the OECD has released several components of its comprehensive plan that have been adopted and expanded by many taxing authorities to address perceived tax abuse and inconsistencies between tax jurisdictions. As a result, the tax laws in the U.S. and other countries in which we do business could change on a prospective or retroactive basis, and any such changes could adversely affect our business and financial statements.
The military conflict between Russia and Ukraine has adversely affected and may further adversely affect our business and financial statements.
The military conflict between Russia and Ukraine has adversely affected and may further adversely affect our business and financial statements. In light of the situation in Ukraine, in addition to suspending sales prohibited by sanctions, the Company has suspended the shipment of products to Russia with the exception of products for the purposes of diagnosing and treating patients and producing vaccines and therapeutics. We incurred a pretax charge of $43 million in 2022 as a result of Russia-related asset impairments, accruals for contractual obligations and similar items and we may incur additional charges in the future. In 2021, approximately 1% of the Company’s sales were derived from customers based in Russia and a de minimis percentage of sales were derived from customers based in Ukraine, and in 2022 Russia and Ukraine sales accounted for less than 1% of the Company’s sales. The conflict in Ukraine may escalate and/or expand in scope and the broader consequences of this conflict, which have included and/or may in the future include sanctions, embargoes, regional instability, geopolitical shifts and adverse impacts on energy supplies and prices; potential retaliatory action by the Russian government against companies,
including the Company, such as nationalization of foreign businesses in Russia. Further, increased tensions between the United States and countries in which we operate cannot be predicted, nor can we predict the conflict’s future impact on the global economy and on our business and financial statements.
The Russia and Ukraine conflict also heightens many other risks disclosed in this Annual Report, any of which can adversely affect our business and financial statements. Such risks include, but are not limited to, adverse effects on macroeconomic conditions, including increased inflation, constraints on the availability of commodities, supply chain disruption and decreased business spending; disruptions to our or our business partners’ global technology infrastructure, including through cyber-attack or cyber-intrusion; adverse changes in international trade policies and relations; claims, litigation and regulatory enforcement; our ability to implement and execute our business strategy; terrorist activities; our exposure to foreign currency fluctuations; reputational risk; and constraints, volatility, or disruption in the capital markets.
Our defined benefit pension plans and health care costs are subject to financial and other market risks that could adversely affect our financial statements.
Significant changes in market interest rates, decreases in the fair value of plan assets, investment losses on plan assets and changes in discount rates can increase our defined benefit pension plan funding obligations, and upward pressure on the cost of providing health care coverage to current employees and retirees can increase our future funding obligations. Any of these risks can adversely affect our financial statements.
Legal, Regulatory, Compliance and Reputational Risks
Significant developments or changes in national laws or policies to protect or promote domestic interests and/or address foreign competition can have an adverse effect on our business and financial statements.
Significant developments or changes in national laws or policies to protect or promote domestic interests and/or address foreign competition, including laws and policies in areas such as trade, manufacturing, government purchasing, health care, intellectual property and investment/development, can adversely affect our business and financial statements. For example, certain governments have implemented policies to induce “re-shoring” of supply chains, reduce reliance on imported supplies and promote national production. The Chinese government has issued a series of policies in the past several years to promote the development and use of local medical devices. In addition, in recent years the U.S. has increased tariffs on certain imported goods and trade tensions between the U.S. and China escalated, with each country imposing significant, additional tariffs on a wide range of goods imported from the other country.
Our business and financial statements can be impaired by improper conduct by any of our employees, agents or business partners.
There can be no assurance that our internal controls and compliance systems, including our Code of Conduct, always protect us from acts committed by employees, agents or business partners of ours (or of businesses we acquire or partner with) that violate laws, including the laws governing payments to government officials, bribery, fraud, kickbacks and false claims, pricing, sales and marketing practices, conflicts of interest, competition, employment practices and workplace behavior, export and import compliance, economic and trade sanctions, money laundering and data privacy. In particular, the U.S. Foreign Corrupt Practices Act, the UK Bribery Act and similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business, and we operate in many parts of the world that have experienced governmental corruption to some degree. Any such improper actions or allegations of such acts could damage our reputation and subject us to civil or criminal investigations and related shareholder lawsuits, could lead to substantial civil and criminal, monetary and non-monetary penalties and could cause us to incur significant legal and investigatory fees. In addition, the government may seek to hold us liable for violations committed by companies in which we invest or that we acquire. We also rely on our suppliers to adhere to our Supplier Code of Conduct, and violations of such code of conduct could adversely affect our business and financial statements.
Our businesses are subject to extensive regulation; failure to comply with those regulations could adversely affect our business and financial statements.
In addition to the environmental, health, safety, health care, medical device, anticorruption, data privacy and other regulations noted elsewhere in this Annual Report, our businesses are subject to extensive regulation by U.S. and non-U.S. governmental and self-regulatory entities at the supranational, federal, state, local and other jurisdictional levels, including for example the following:
•We are required to comply with various import laws and export control and economic sanctions laws, which may affect our transactions with certain customers, business partners and other persons and dealings between our
employees and between our subsidiaries. In certain circumstances, export control and economic sanctions regulations may prohibit the export of certain products, services and technologies. In other circumstances, we may be required to obtain an export license before exporting the controlled item. Compliance with the various import laws that apply to our businesses can restrict our access to, and increase the cost of obtaining, certain products and at times can interrupt our supply of imported inventory. In addition, we sell and provide products and technology to third parties, such as agents, representatives and distributors, who may export such items to end-users. If we or any of these third parties do not comply with applicable export or import laws we may incur liability. In addition, from time to time, certain of our subsidiaries have limited business dealings in countries subject to comprehensive sanctions. These business dealings represent an insignificant amount of our consolidated revenues and income but expose us to a heightened risk of violating applicable sanctions regulations. We have established policies and procedures designed to ensure compliance with such laws and regulations but there can be no assurance that the policies and procedures have prevented and will prevent violations of these regulations, and any such violation can adversely affect our business and financial statements.
•We also have agreements to sell products and services to government entities (as well as agreements relating to government financing, as discussed above) and are subject to various statutes and regulations that apply to companies doing business with government entities (less than 5% of our 2022 sales were made to the U.S. federal government). The laws governing government contracts differ from the laws governing private contracts. For example, many government contracts contain pricing and other terms and conditions that are not applicable to private contracts. Our agreements with government entities are in some cases subject to termination, reduction or modification at the convenience of the government or in the event of changes in government requirements, reductions in federal spending and other factors, and we may underestimate our costs of performing under the contract. In certain cases, a governmental entity may require us to pay back amounts it has paid to us. Government contracts that have been awarded to us following a bid process can become the subject of a bid protest by a losing bidder, which could result in loss of the contract. We are also subject to investigation and audit for compliance with the requirements governing government contracts.
These are not the only regulations that our businesses must comply with. The regulations we are subject to have tended to become more stringent over time and can be inconsistent across jurisdictions. We, our representatives and the industries in which we operate are at times under review and/or investigation by regulatory authorities. Failure to comply (or any alleged or perceived failure to comply) with the regulations referenced above or any other regulations can result in import detentions, fines, damages, civil and administrative penalties, injunctions, consent decrees, suspensions or losses of regulatory approvals, recall or seizure of products, operating restrictions, refusal of the government to approve product export applications or allow us to enter into supply contracts, disbarment from selling to certain governmental agencies or exclusion from government funded healthcare programs, such as Medicare and Medicaid or similar programs in other countries or jurisdictions, integrity oversight and reporting obligations to resolve allegations of non-compliance, disruption of our business, limitation on our ability to manufacture, import, export and sell products and services, loss of customers, significant legal and investigatory fees, disgorgement, individual imprisonment, reputational harm, contractual damages, diminished profits, curtailment or restricting of business operations, criminal prosecution and other monetary and non-monetary penalties. Compliance with these and other regulations can also affect our returns on investment, require us to incur significant expenses or modify our business model or impair our flexibility in modifying product, marketing, pricing or other strategies for growing our business. Our products and operations are also often subject to the rules of industrial standards bodies such as the International Standards Organization, and failure to comply with these rules can result in withdrawal of certifications needed to sell our products and services and otherwise adversely impact our business and financial statements. For additional information regarding these risks, refer to “Item 1. Business—Regulatory Matters.”
We are subject to or otherwise responsible for a variety of litigation and other legal and regulatory proceedings in the course of our business that can adversely affect our business and financial statements.
We are subject to or otherwise responsible for a variety of litigation and other legal and regulatory proceedings in the course of our business (or related to the business operations of previously owned entities), including claims or counterclaims for damages arising out of the use of products or services and claims relating to intellectual property matters, employment matters, tax matters, commercial disputes, breach of contract claims, competition and sales and trading practices, environmental matters, personal injury, insurance coverage, securities matters, fiduciary duties and acquisition or divestiture-related matters, as well as regulatory subpoenas, requests for information, investigations and enforcement. We also from time to time become subject to lawsuits as a result of acquisitions or as a result of liabilities retained from, or representations, warranties or indemnities provided in connection with, businesses divested by us or our predecessors. The types of claims made in lawsuits include claims for compensatory damages, punitive and consequential damages (and in some cases, treble damages) and/or injunctive relief. The defense of these lawsuits can divert our management’s attention, we from time to time incur significant expenses in defending these lawsuits, and we can be required to pay damage awards or settlements or become subject to equitable remedies
that adversely affect our business and financial statements. Moreover, any insurance or indemnification rights that we may have may be insufficient or unavailable to protect us against such losses. Because most contingencies are resolved over long periods of time, new developments (including litigation developments, the discovery of new facts, changes in legislation and outcomes of similar cases), changes in assumptions or changes in the Company’s strategy in any given period can require us to adjust the loss contingency estimates that we have recorded in our financial statements, record estimates for liabilities or assets previously not susceptible of reasonable estimates or pay cash settlements or judgments. Any of these developments can adversely affect our business and financial statements in any particular period. There can be no assurance that our liabilities in connection with current and future litigation and other legal and regulatory proceedings will not exceed our estimates or adversely affect our financial statements and business. However, based on our experience, information and applicable law as of the date of this Annual Report, we do not believe that it is reasonably possible that any amounts we may be required to pay in connection with litigation and other legal and regulatory proceedings in excess of our reserves as of December 31, 2022 will have a material effect on our business or financial statements.
From time to time, we become aware through our internal audits and other internal control procedures, employees or other parties of possible compliance matters, such as complaints or concerns relating to accounting, internal controls, financial reporting, auditing or ethical matters or relating to compliance with laws. When we become aware of such possible compliance matters, we investigate internally and take what we believe to be appropriate corrective action. Internal investigations can lead to the assertion of claims or the commencement of legal or regulatory proceedings against us and adversely affect our business and financial statements.
Certain of our businesses are subject to extensive regulation by the FDA and by comparable agencies of other countries, as well as laws regulating fraud and abuse in the healthcare industry and the privacy and security of health information. Failure to comply with those regulations could adversely affect our business and financial statements.
Certain of our products are medical devices and other products that are subject to regulation by the FDA, by other federal and state governmental agencies, by comparable agencies of other countries and regions, by certain accrediting bodies and by regulations governing hazardous materials and drugs-of-abuse (or the manufacture and sale of products containing any such materials). The global health care regulatory environment has become increasingly stringent and unpredictable. Several countries that did not have regulatory requirements for medical devices have established such requirements in recent years, and other countries have expanded, or plan to expand, their existing regulations. For example, the proposed Verifying Accurate Leading-edge IVCT Development (VALID) Act would give FDA additional authority to regulate in vitro diagnostics, including laboratory-developed tests. Please see “Item 1. Business—Regulatory Matters” for more information. Failure to meet these requirements can adversely impact our business and financial statements in the applicable geographies.
To varying degrees, these regulators require us to comply with laws and regulations governing the development, testing, manufacturing, labeling, marketing, distribution and post-marketing surveillance of our products. We cannot guarantee that we will be able to obtain regulatory clearance (such as 510(k) clearance) or approvals for our new products or modifications to (or additional indications or uses of) existing products within our anticipated timeframe or at all, and if we do obtain such clearance or approval it may be time-consuming, costly and subject to restrictions. Our ability to obtain such regulatory clearances or approvals will depend on many factors, for example our ability to obtain the necessary clinical trial results, and the process for obtaining such clearances or approvals could change over time and may require the withdrawal of products from the market until such clearances are obtained. Even after initial regulatory clearance or approval, we are subject to periodic inspection by these regulatory authorities, and if safety issues arise we can be required to amend conditions for use of a product, such as providing additional warnings on the product’s label or narrowing its approved intended use, which could reduce the product’s market acceptance. We are also subject to various laws regulating fraud and abuse, research and development, pricing and sales and marketing practices, the privacy and security of health information as well as manufacturing and quality standards, including the federal regulations described in “Item 1. Business—Regulatory Matters.”
Government authorities may conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law. Failure to obtain required regulatory clearances or approvals before marketing our products (or before implementing modifications to or promoting additional indications or uses of our products), other violations of laws or regulations, failure to remediate inspectional observations to the satisfaction of these regulatory authorities, real or perceived efficacy or safety concerns or trends of adverse events with respect to our products (even after obtaining clearance for distribution) and unfavorable or inconsistent clinical data from existing or future clinical trials can lead to FDA Form 483 Inspectional Observations, warning letters, notices to customers, declining sales, loss of customers, loss of market share, remediation and increased compliance costs, recalls, seizures of adulterated or misbranded products, fines, expenses, injunctions, civil penalties, criminal penalties, consent decrees, administrative detentions, refusals to permit importations, partial or total shutdown of production facilities or the implementation of operating restrictions, narrowing of permitted uses for a product, refusal of the government to grant 510(k) clearance, suspension or withdrawal of approvals, pre-market notification rescissions and other adverse effects referenced under the risk factor titled “Our businesses are subject to extensive regulation;
failure to comply with those regulations could adversely affect our business and financial statements.” Further, defending against any such actions can be costly and time-consuming and may require significant personnel resources. Therefore, even if we are successful in defending against any such actions brought against us, our business may be impaired. Ensuring that our operations and business arrangements with third parties comply with applicable laws and regulations also involves substantial costs.
Our products can be subject to human clinical trials, the results of which may be unexpected, or perceived as unfavorable by the market, and could adversely affect our business and financial statements.
As a part of the regulatory process of obtaining marketing clearance for certain new products and new indications for certain existing products, we conduct and participate in clinical trials with a variety of study designs, patient populations and trial endpoints. Unexpected or inconsistent clinical data from existing or future clinical trials, or a regulator’s or market perception of these clinical data, can adversely impact our ability to obtain product approvals, our position in, and share of, the markets in which we participate and our business and financial statements.
Off-label marketing of our products could result in substantial penalties.
The FDA strictly regulates the promotional claims that may be made about approved or cleared products. In particular, any clearances we may receive only permit us to market our products for the intended uses indicated on the labeling cleared by the FDA. We may request additional label indications for our current products, and the FDA may deny those requests outright, require additional performance or clinical data to support any additional indications or impose limitations on the intended use of any cleared products as a condition of clearance. If the FDA or any other regulator determines that we have marketed our products for off-label use, we can be subject to exclusion from participation in government healthcare programs and the other adverse effects referenced under the risk factors set forth above. Any of these events could significantly harm our business and financial statements.
Certain modifications to our products may require new 510(k) clearances or other marketing authorizations and may require us to recall or cease marketing our products.
Once a medical device is permitted to be legally marketed in the United States pursuant to a 510(k) clearance or a premarket approval (“PMA”), a manufacturer may be required to notify the FDA of certain modifications to the device (similar requirements apply in other jurisdictions). Manufacturers determine in the first instance whether a change to a product requires a new 510(k) clearance or premarket submission, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances are necessary. We have made modifications to our products in the past and have determined based on our review of the applicable FDA regulations and guidance that in certain instances new 510(k) clearances or other premarket submissions were not required. We may make similar modifications or add additional features in the future that we believe do not require a new clearance or approval. If the FDA disagrees with our determinations and requires us to submit new 510(k) notifications or PMA applications, we may be required to cease marketing or to recall the modified product until we obtain clearance, and we may be subject to civil and criminal, monetary and non-monetary penalties and damage to our reputation.
Our operations, products and services expose us to the risk of environmental, health and safety liabilities, costs and violations that could adversely affect our business and financial statements.
Our operations, products and services are subject to numerous U.S. federal, state, local and non-U.S. environmental, health and safety laws and regulations concerning, among other things, the health and safety of our employees, the generation, storage, use and transportation of hazardous materials, emissions or discharges of substances into the environment, investigation and remediation of hazardous substances or materials at various sites, chemical constituents in products and end-of-life disposal and take-back programs for products sold. There can be no assurance that our environmental, health and safety compliance program (or the compliance programs of businesses we acquire) have been or will at all times be effective. Failure to comply with any of these laws can result in civil and criminal, monetary and non-monetary penalties and damage to our reputation. In addition, there can be no assurance that our costs of complying with current or future environmental protection and health and safety laws will not exceed our estimates or adversely affect our business or financial statements.
In addition, we from time to time incur costs related to remedial efforts or alleged environmental damage associated with past or current waste disposal practices or other hazardous materials handling practices. We are also from time to time party to personal injury, property damage or other claims brought by private parties alleging injury or damage due to the presence of or exposure to hazardous substances. We can also become subject to additional remedial, compliance or personal injury costs due to future events such as changes in existing laws or regulations, changes in agency direction or enforcement policies, developments in remediation technologies, changes in the conduct of our operations and changes in accounting rules. There can be no assurance that our liabilities arising from past or future releases of, or exposures to, hazardous substances will not exceed our estimates or adversely affect our reputation and financial statements or that we will not be subject to additional
claims for personal injury or remediation in the future based on our past, present or future business activities. However, based on the information we have as of the date of this Annual Report we do not believe that it is reasonably possible that any amounts we may be required to pay in connection with environmental matters in excess of our reserves as of December 31, 2022, will have a material effect on our business or financial statements.
Changes in governmental regulations can reduce demand for our products or services or increase our expenses.
We compete in markets in which we and our customers must comply with supranational, federal, state, local and other jurisdictional regulations, such as regulations governing health and safety, the environment, food and drugs and privacy. We develop, configure and market our products and services to meet customer needs created by these regulations. Any significant change in any of these regulations (or in the interpretation or application thereof) can reduce demand for, increase our costs of producing or delay the introduction of new or modified products and services, or restrict our existing activities, products and services. For example, changes in the FDA’s regulation of the drug discovery/development process can have an adverse effect on the demand for our products and services.
Exclusive forum provisions in our By-laws could limit our stockholders’ ability to choose their preferred judicial forum for disputes with us or our directors, officers or employees.
Our Amended and Restated By-laws (the “By-laws”) provide that unless the Company selects or consents to the selection of an alternative forum, the sole and exclusive forum for any complaint asserting any internal corporate claims, to the fullest extent permitted by law and subject to applicable jurisdictional requirements, will be the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have, or declines to accept, jurisdiction, another state court or a federal court located within the State of Delaware) (collectively, “Delaware Courts”). Current and former stockholders are deemed to have consented to the personal jurisdiction of the Delaware Courts in connection with any action to enforce such exclusive forum provision and to service of process in any such action. These provisions of the By-laws are not a waiver of, and do not relieve anyone of duties to comply with, federal securities laws including those specifying the exclusive jurisdiction of federal courts under the Exchange Act and concurrent jurisdiction of federal and state courts under the Securities Act of 1933, as amended. To the extent that the exclusive forum provisions of our By-laws limit a current or former stockholder’s ability to select a judicial forum other than the Delaware Courts, they might discourage the specified legal actions, might cause current or former stockholders to incur additional litigation-related expenses and might result in outcomes unfavorable to current or former stockholders. Alternatively, a court might determine that these provisions of the By-laws are inapplicable or unenforceable in any particular action, in which case we may incur additional litigation-related expenses in such action, and the action may result in outcomes unfavorable to us, which could have an adverse impact on our business and financial statements.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. PROPERTIES
As of December 31, 2022, the Company had facilities in over 60 countries, including approximately 244 significant administrative, sales, research and development, manufacturing and distribution facilities. 90 of these facilities are located in the United States in over 20 states and 154 are located outside the United States, primarily in Europe and to a lesser extent in Asia, South America, Canada and Australia. Refer to the Consolidated Financial Statements included in this Annual Report for additional information with respect to the Company’s lease commitments.
ITEM 3. LEGAL PROCEEDINGS
For information regarding legal proceedings, refer to the section titled “Legal Proceedings” in MD&A.
Consistent with SEC Regulation S-K Item 103, we have elected to disclose those environmental proceedings (if any) with a governmental entity as a party where the Company reasonably believes such proceeding would result in monetary sanctions, exclusive of interest and costs, of $1 million or more.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
INFORMATION ABOUT OUR EXECUTIVE OFFICERS
Set forth below are the names, ages, positions and experience of Danaher’s executive officers as of February 4, 2023. All of Danaher’s executive officers hold office at the pleasure of Danaher’s Board of Directors. Unless otherwise stated, the positions indicated are Danaher positions.
| | | | | | | | | | | | | | | | | | | | |
| Name | | Age | | Position | | Officer Since |
| Steven M. Rales | | 71 | | Chairman of the Board | | 1984 |
| Mitchell P. Rales | | 66 | | Chairman of the Executive Committee | | 1984 |
| Rainer M. Blair | | 58 | | President and Chief Executive Officer | | 2014 |
| Matthew R. McGrew | | 51 | | Executive Vice President and Chief Financial Officer | | 2019 |
| Jennifer L. Honeycutt | | 53 | | Executive Vice President | | 2021 |
| Joakim Weidemanis | | 53 | | Executive Vice President | | 2017 |
| Georgeann F. Couchara | | 46 | | Senior Vice President - Human Resources | | 2022 |
| Brian W. Ellis | | 56 | | Senior Vice President – General Counsel | | 2016 |
| Jose-Carlos Gutierrez-Ramos | | 60 | | Senior Vice President – Chief Science Officer | | 2020 |
| William H. King | | 55 | | Senior Vice President – Strategic Development | | 2005 |
| | | | | | |
| | | | | | |
| Daniel A. Raskas | | 56 | | Senior Vice President – Corporate Development | | 2004 |
Steven M. Rales is a co-founder of Danaher and has served on Danaher’s Board of Directors since 1983, serving as Danaher’s Chairman of the Board since 1984. He was also CEO of the Company from 1984 to 1990. Mr. Rales is a brother of Mitchell P. Rales.
Mitchell P. Rales is a co-founder of Danaher and has served on Danaher’s Board of Directors since 1983, serving as Chairman of the Executive Committee of Danaher since 1984. He was also President of the Company from 1984 to 1990. Mr. Rales is also a member of the board of directors of each of Enovis Corporation and ESAB Corporation, and is a brother of Steven M. Rales.
Rainer M. Blair has served as President and Chief Executive Officer since September 2020, after serving as Executive Vice President from January 2017 to August 2020.
Matthew R. McGrew has served as Executive Vice President and Chief Financial Officer since January 2019, after serving as Group CFO of Danaher from 2012 until December 2018.
Jennifer L. Honeycutt has served as Executive Vice President since January 2021 after serving as Vice President – Group Executive from May 2019 until December 2020 and President of Danaher’s Pall business from January 2017 until April 2019.
Joakim Weidemanis has served as Executive Vice President since December 2017.
Georgeann F. Couchara has served as Senior Vice President – Human Resources since April 2022, after serving as Vice President-Talent from January 2021 to April 2022, Vice President – Human Resources for Danaher’s Life Sciences business from July 2019 to January 2021 and Senior Vice President-Human Resources and Communications for Danaher’s Pall business from June 2017 to July 2019.
Brian W. Ellis has served as Senior Vice President – General Counsel since joining Danaher in January 2016.
Jose-Carlos Gutierrez-Ramos has served as Senior Vice President – Chief Science Officer since joining Danaher in December 2020. Prior to joining Danaher, Dr. Gutierrez-Ramos served as Vice President – Drug Discovery for AbbVie, Inc., a biopharmaceutical company, from January 2020 to December 2020; as President and CEO of Repertoire Immune Medicines, a biotechnology company, from August 2018 until January 2020; and as President and CEO of Synlogic, Inc., a biopharmaceutical company, from August 2015 until August 2018.
William H. King has served as Senior Vice President – Strategic Development since 2014.
Daniel A. Raskas has served as Senior Vice President – Corporate Development since 2010.
PART II
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is traded on the New York Stock Exchange under the symbol DHR. As of February 3, 2023, there were 2,300 holders of record of Danaher’s common stock.
Any future payments of dividends on the Company’s common stock will be determined by Danaher’s Board of Directors and will depend on business conditions, Danaher’s earnings and other factors Danaher’s Board deems relevant.
Issuer Purchases of Equity Securities
Refer to Note 19 to the Consolidated Financial Statements included in this Annual Report for a discussion of the Company’s common stock repurchase program. Neither the Company nor any “affiliated purchaser” repurchased any shares of Company common stock during 2022, 2021 or 2020, other than as described in Note 19.
Recent Issuances of Unregistered Securities
None
ITEM 6. RESERVED
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) is designed to provide material information relevant to an assessment of Danaher’s financial condition and results of operations, including an evaluation of the amounts and certainty of cash flows from operations and from outside sources. The MD&A is designed to focus specifically on material events and uncertainties known to management that are reasonably likely to cause reported financial information not to be necessarily indicative of future operating results or of future financial condition. This includes descriptions and amounts of matters that have had a material impact on reported operations, as well as matters that are reasonably likely based on management’s assessment to have a material impact on future operations. The Company’s MD&A is divided into five sections:
•Overview
•Results of Operations
•Liquidity and Capital Resources
•Critical Accounting Estimates
•New Accounting Standards
This discussion and analysis should be read together with Danaher’s audited financial statements and related Notes thereto as of December 31, 2022 and 2021 and for each of the three years in the period ended December 31, 2022 included in this Annual Report. Management's discussion and analysis of financial condition and results of operations for 2020 is included in Item 7 of the Company’s Annual Report on Form 10-K with respect to the year ended December 31, 2021 filed with the Securities and Exchange Commission, as supplemented by the discussion herein of the new Biotechnology and Life Sciences segments (which were previously reported together as the former Life Sciences segment), and should be referred to for information regarding this period.
Unless otherwise indicated, all financial results in this report refer to continuing operations.
OVERVIEW
General
Refer to “Item 1. Business—General” for a discussion of Danaher’s strategic objectives and methodologies for delivering long-term shareholder value. Danaher is a multinational business with global operations. During 2022, approximately 58% of Danaher’s sales were derived from customers outside the United States. As a diversified, global business, Danaher’s operations are affected by worldwide, regional and industry-specific economic and political factors. Danaher’s geographic and industry diversity, as well as the range of its products and services, help limit the impact of any one industry or the economy of any single country on its consolidated operating results. The Company’s individual businesses monitor key competitors and customers, including to the extent possible their sales, to gauge relative performance and the outlook for the future.
As a result of the Company’s geographic and industry diversity, the Company faces a variety of opportunities and challenges, including rapid technological development (particularly with respect to computing, automation, artificial intelligence, mobile connectivity, communications and digitization) in most of the Company’s served markets, the expansion and evolution of opportunities in high-growth markets, trends and costs associated with a global labor force, consolidation of the Company’s competitors and increasing regulation. The Company operates in a highly competitive business environment in most markets, and the Company’s long-term growth and profitability will depend in particular on its ability to expand its business in high-growth geographies and high-growth market segments, identify, consummate and integrate appropriate acquisitions and identify and consummate appropriate investments and strategic partnerships, develop innovative and differentiated new products and services with higher gross profit margins, expand and improve the effectiveness of the Company’s sales force, continue to reduce costs and improve operating efficiency and quality, and effectively address the demands of an increasingly regulated global environment. The Company is making significant investments, organically and through acquisitions and investments, to address the rapid pace of technological change in its served markets and to globalize its manufacturing, research and development and customer-facing resources (particularly in high-growth markets) in order to be responsive to the Company’s customers throughout the world and improve the efficiency of the Company’s operations.
Business Performance
Consolidated revenues for the year ended December 31, 2022 increased 7.0% as compared to 2021. Acquisitions contributed 1.5% to the increase in revenues in 2022 and the impact of currency translation decreased reported sales 4.0%. Core sales
increased 9.5% in 2022 compared to 2021 (for the definition of “core sales” refer to “—Results of Operations” below). The Company’s continued investments in sales growth initiatives and the other business-specific factors referenced below contributed to core sales growth. Geographically, both high-growth and developed markets contributed to year-over-year core sales growth during 2022. Core sales in developed markets grew at a low-teens rate in 2022 as compared to 2021 and were driven by North America and Western Europe. Core sales in high-growth markets grew at a low-single digit rate in 2022 as compared to 2021, with broad-based growth across these markets, led by growth in China. High-growth markets represented approximately 29% of the Company’s total sales in 2022.
The Company’s net earnings from continuing operations for the year ended December 31, 2022 totaled approximately $7.2 billion, compared to approximately $6.3 billion for the year ended December 31, 2021. Net earnings attributable to common stockholders for the year ended December 31, 2022 totaled approximately $7.1 billion or $9.66 per diluted common share compared to approximately $6.3 billion or $8.61 per diluted common share for the year ended December 31, 2021. The increase in net earnings in 2022 as compared to 2021 was driven by increased sales in the Company’s existing businesses and sales from acquired businesses, by a lower effective tax rate in 2022 driven by discrete tax benefits and by the impact of the non-recurring charge incurred in 2021 related to the modification and partial termination of a prior commercial arrangement and resolution of the associated litigation recorded, partially offset by investment losses recorded in 2022. Refer to “—Results of Operations” for further discussion of the year-over-year changes in net earnings and diluted net earnings per common share for the year ended December 31, 2022.
For a discussion of the impact of supply chain disruptions, labor availability constraints and increased labor costs on our businesses in 2022, please see “Item 1. Business – Materials.” For a discussion of the impact of the Russia-Ukraine conflict on our businesses in 2022, please see “Item 1. Business – Russia-Ukraine Conflict.”
The COVID-19 Pandemic
The global spread of a novel strain of coronavirus (COVID-19) has led to unprecedented restrictions on, and disruptions in, business and personal activities, including as a result of preventive and precautionary measures that we, other businesses, our communities and governments have taken and are taking to mitigate the spread of the virus and to manage its impact. The Company continues to actively monitor the COVID-19 pandemic, including the current spread of certain variants of the virus and plan for potential impacts on its business. The Company has deployed our capabilities, expertise and scale to address the critical health needs related to COVID-19, including developing and making available diagnostic tests for the rapid detection of COVID-19 as well as providing critical support to firms that are developing and producing vaccines and therapies for COVID-19. While the conditions related to the pandemic generally improved in most geographies in 2022 compared to 2021, conditions vary significantly by geography. For example, during the first half of 2022, COVID-19 considerations resulted in the re-imposition of widespread shutdowns and restrictions in China. During the fourth quarter of 2022, China relaxed many of these restrictions and began experiencing increasing COVID-19 related cases resulting in lower patient volumes for elective procedures and wellness visits as hospitals prioritized treating COVID-19 related cases. These higher COVID-19 related cases in China are anticipated to continue at least into the first quarter of 2023. The resulting impact to the Company will depend upon the prevalence of COVID-19 in the impacted regions of China and the resulting impact on economic activity, including demand and production capacity.
Demand for the Company’s products that support COVID-19 related vaccines and therapeutics (including initiatives that seek to prevent or mitigate similar, future pandemics) decreased in 2022 versus 2021. The Company expects overall demand for these products to decrease in 2023 versus 2022. Additionally, demand for the Company’s products that support COVID-19 testing continues to fluctuate significantly driven by increases or decreases in COVID-19 cases in particular geographies. While sales of COVID-19 related testing products increased in 2022 compared to 2021, the Company expects overall demand for these products to decrease in 2023 as the pandemic subsides in most geographies and evolves toward endemic status.
Due to the speed with which the COVID-19 situation has evolved, the global breadth of its spread, the range of governmental and community responses thereto and our geographic and business line diversity, its further impact on our business remains highly uncertain, but may be materially negative to certain elements of our business. The potential negative impact will depend on future developments including but not limited to:
•the degree of spread and severity of COVID-19 variants and government responses thereto;
•the timing and durability of continued recovery in the global demand for our non-COVID-19 related products and services; and
•the degree and pace of continuing declines in demand for products supporting COVID-19 testing and for products related to developing and producing vaccines and therapies for COVID-19.
For additional information on the risks of COVID-19 to the Company’s operations, refer to the “Item 1A. Risk Factors” section of this Annual Report.
Acquisitions
During 2022 the Company acquired 10 businesses for total consideration of $637 million in cash, net of cash acquired. The businesses acquired complement existing units of each of the Company’s four segments. The aggregate annual sales of the 10 businesses acquired in 2022 at the time of their acquisition, in each case based on the company’s revenues for its last completed fiscal year prior to the acquisition, were approximately $91 million.
Refer to Note 2 to the Consolidated Financial Statements for discussion regarding the Company’s acquisitions.
RESULTS OF OPERATIONS
In this report, references to the non-GAAP measures of core sales (also referred to as core revenues or sales/revenues from existing businesses) and core sales including Cytiva refer to sales from continuing operations calculated according to generally accepted accounting principles in the United States (“GAAP”) but excluding:
•sales from acquired businesses (as defined below, as applicable); and
•the impact of currency translation.
References to sales or operating profit attributable to acquisitions or acquired businesses refer to sales or operating profit, as applicable, from acquired businesses recorded prior to the first anniversary of the acquisition less any sales and operating profit, during the applicable period, attributable to divested product lines not considered discontinued operations; provided that in calculating core sales including Cytiva, Cytiva’s sales (net of the sales of the Company product lines divested in 2020 to obtain regulatory approval to acquire Cytiva, or the “divested product lines”) (“Cytiva sales”) are excluded from the definition of sales attributable to acquisitions or acquired businesses. The portion of revenue attributable to currency translation is calculated as the difference between:
•the period-to-period change in revenue (excluding sales from acquired businesses (as defined above, as applicable)); and
•the period-to-period change in revenue (excluding sales from acquired businesses (as defined above, as applicable)) after applying current period foreign exchange rates to the prior year period.
As noted above, beginning with results for the second quarter of 2020, the Company also presents core sales on a basis that includes Cytiva sales. Prior to the acquisition of Cytiva, Danaher calculated core sales solely on a basis that excluded sales from acquired businesses recorded prior to the first anniversary of the acquisition. However, given Cytiva’s significant size and historical core sales growth rate, in each case compared to Danaher’s existing businesses, management believes it is appropriate to also present core sales on a basis that includes Cytiva sales. Management believes this presentation provides useful information to investors by demonstrating beginning immediately after the acquisition Cytiva’s impact on the Company’s growth profile, rather than waiting to demonstrate such impact until 12 months after the acquisition when Cytiva would normally have been included in Danaher’s core sales calculation. Danaher calculates period-to-period core sales growth including Cytiva by adding Cytiva sales to core sales for both the baseline and current periods. Beginning in the second quarter of 2021, Cytiva sales are included in core sales, and therefore the measure “core sales including Cytiva” is no longer provided for quarterly periods beginning with the second quarter of 2021.
Core sales growth (and the related measure of core sales including Cytiva) should be considered in addition to, and not as a replacement for or superior to, sales, and may not be comparable to similarly titled measures reported by other companies. Management believes that reporting these non-GAAP financial measures provides useful information to investors by helping identify underlying growth trends in Danaher’s business and facilitating comparisons of Danaher’s revenue performance with its performance in prior and future periods and to Danaher’s peers. Management also uses these non-GAAP financial measures to measure the Company’s operating and financial performance and uses core sales growth (and previously used core sales growth including Cytiva) as one of the performance measures in the Company’s executive short-term cash incentive program. The Company excludes the effect of currency translation from these measures because currency translation is not under management’s control, is subject to volatility and can obscure underlying business trends, and excludes the effect of acquisitions (other than Cytiva sales, in the case of core sales growth including Cytiva) and divestiture-related items because the nature, size, timing and number of acquisitions and divestitures can vary dramatically from period-to-period and between the Company and its peers and can also obscure underlying business trends and make comparisons of long-term performance difficult.
Throughout this discussion, references to sales growth or decline refer to the impact of both price and unit sales and references to productivity improvements generally refer to improved cost efficiencies resulting from the ongoing application of DBS.
The Company deems acquisition-related transaction costs incurred in a given period to be significant (generally relating to the Company’s larger acquisitions) if it determines that such costs exceed the range of acquisition-related transaction costs typical for Danaher in a given period.
Sales Growth, Core Sales Growth and Core Sales Growth Including Cytiva
| | | | | | | | | | | |
| 2022 vs. 2021 | | 2021 vs. 2020 |
| Total sales growth (GAAP) | 7.0 | % | | 32.0 | % |
| Impact of: | | | |
| Acquisitions/divestitures | (1.5) | % | | (7.5) | % |
| Currency exchange rates | 4.0 | % | | (1.5) | % |
| Core sales growth (non-GAAP) | 9.5 | % | | 23.0 | % |
| | | |
| | | |
| Impact of Cytiva sales growth (net of divested product lines) | | | 2.0 | % |
| Core sales growth including Cytiva (non-GAAP) | | | 25.0 | % |
| | | |
| | | |
| | | |
2022 Sales Compared to 2021
Total sales increased 7.0% on a year-over-year basis in 2022 primarily as a result of an increase in core sales resulting from the factors discussed below by segment as well as an increase in sales from acquired businesses. The impact of changes in currency exchange rates decreased reported sales by 4.0% on a year-over-year basis in 2022 primarily due to the unfavorable impact of the strengthening of the U.S. dollar against most other major currencies in 2022.
Operating Profit Performance
Operating profit margins were 27.6% for the year ended December 31, 2022 as compared to 25.3% in 2021. The following factors impacted year-over-year operating profit margin comparisons.
2022 vs. 2021 operating profit margin comparisons were favorably impacted by:
•Third quarter 2021 impact of the modification and partial termination of a prior commercial arrangement and resolution of the associated litigation - 185 basis points
•Higher 2022 core sales and the impact of product mix, incremental year-over-year cost savings associated with continuing productivity improvement initiatives, net of incremental year-over-year costs associated with various new product development and sales, service and marketing growth investments and incremental year-over-year material, transportation and labor costs - 60 basis points
•2021 acquisition-related fair value adjustments to inventory and transaction costs deemed significant, in each case related to the acquisition of Aldevron - 20 basis points
•2021 acquisition-related fair value adjustments to inventory and deferred revenue related to the acquisition of Cytiva - 15 basis points.
2022 vs. 2021 operating profit margin comparisons were unfavorably impacted by:
•The incremental dilutive effect in 2022 of acquired businesses, net of product line dispositions which did not qualify as discontinued operations - 30 basis points
•2022 impairments of accounts receivable and inventory as well as accruals for contractual obligations in Russia - 15 basis points
•Fourth quarter 2022 costs incurred related to the anticipated separation of the Company's Environmental & Applied Solutions business - 5 basis points
Business Segments
In the fourth quarter of 2022, the Company realigned its reportable segments to reflect changes in the Company’s internal organization resulting from the rate of growth within certain of the Company’s businesses in the former Life Sciences segment. There were no changes to the Company’s Diagnostics or Environmental & Applied Solutions segments. Prior period amounts have been restated to conform to the revised segment presentation. Sales by business segment for the years ended December 31 are as follows ($ in millions):
| | | | | | | | | | | | | | | | | |
| | 2022 | | 2021 | | 2020 |
| Biotechnology | $ | 8,758 | | | $ | 8,570 | | | $ | 5,276 | |
| Life Sciences | 7,036 | | | 6,388 | | | 5,300 | |
| Diagnostics | 10,849 | | | 9,844 | | | 7,403 | |
| Environmental & Applied Solutions | 4,828 | | | 4,651 | | | 4,305 | |
| Total | $ | 31,471 | | | $ | 29,453 | | | $ | 22,284 | |
For information regarding the Company’s sales by geographical region, refer to Note 5 to the Consolidated Financial Statements.
BIOTECHNOLOGY
The Biotechnology segment includes the bioprocessing and discovery and medical businesses and offers a broad range of tools, consumables and services that are primarily used by customers to advance and accelerate the research, development, manufacture and delivery of biological medicines. The biotherapeutics that the Company’s solutions support range from replacement therapies such as insulin, vaccines, recombinant proteins and other biologic drugs, to novel cell, gene, mRNA and other nucleic acid therapies.
Biotechnology Selected Financial Data
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31 |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Sales | $ | 8,758 | | | $ | 8,570 | | | $ | 5,276 | |
| Operating profit | 3,008 | | | 3,074 | | | 1,082 | |
| Depreciation | 190 | | | 158 | | | 91 | |
| Amortization of intangible assets | 812 | | | 901 | | | 670 | |
| Operating profit as a % of sales | 34.3 | % | | 35.9 | % | | 20.5 | % |
| Depreciation as a % of sales | 2.2 | % | | 1.8 | % | | 1.7 | % |
| Amortization as a % of sales | 9.3 | % | | 10.5 | % | | 12.7 | % |
Sales Growth, Core Sales Growth and Core Sales Growth Including Cytiva
| | | | | | | | | | | |
| 2022 vs. 2021 | | 2021 vs. 2020 |
| Total sales growth (GAAP) | 2.0 | % | | 62.5 | % |
| Impact of: | | | |
| Acquisitions/divestitures | (0.5) | % | | (31.5) | % |
| Currency exchange rates | 4.5 | % | | (1.5) | % |
| Core sales growth (non-GAAP) | 6.0 | % | | 29.5 | % |
| Impact of Cytiva sales growth (net of divested product lines) | | | 7.0 | % |
| Core sales growth including Cytiva (non-GAAP) | | | 36.5 | % |
2022 Sales Compared to 2021
Price increases in the segment contributed 4.0% to sales growth on a year-over-year basis during 2022 as compared with 2021 and are reflected as a component of the change in core revenue growth.
During 2022, total Biotechnology segment sales increased 2.0% primarily as a result of increased core sales resulting from the factors discussed below, partially offset by the impact of changes in currency exchange rates due to the strengthening of the U.S. dollar in 2022 compared to 2021. Increased year-over-year core sales in the segment’s bioprocessing business were led by North America and Western Europe as the business experienced strong underlying demand for non-COVID-19 related
instruments and consumables offsetting a decline in sales of instruments and consumables used in the research, development and production of COVID-19 related treatments and vaccines and the completion of a major project in China during 2021. Core sales of the businesses’ COVID-19 related products decreased year-over-year as a result of lower underlying demand for COVID-19 related therapeutics and vaccines and as customers reduce inventory levels of these products in light of this lower demand. Core sales for the discovery and medical business increased in 2022 compared to 2021 driven by higher sales of lab filtration and protein research products. Geographically, core sales in the discovery and medical business were led by North America, Western Europe and China.
2021 Sales Compared to 2020
Price increases in the segment contributed 2.0% to sales growth on a year-over-year basis during 2021 as compared with 2020 and are reflected as a component of the change in core revenue growth.
During 2021, total Biotechnology segment sales increased 62.5% primarily as a result of increased sales from the acquisition of Cytiva on March 31, 2020 (the “Cytiva Acquisition”), increased core sales resulting from the factors discussed below and the impact of changes in currency exchange rates due to the weakening of the U.S. dollar against most major currencies in 2021 compared to 2020. In 2021, the bioprocessing business experienced significant increased year-over-year core sales, driven by demand for instruments and consumables used in the research and development and production of COVID-19 therapeutics and vaccines and increased demand for non-COVID-19 related products as well as from the completion of a major project in China. Geographically, demand for these products increased across all major geographies, led by North America, Western Europe and China. Core sales for the discovery and medical business increased in 2021 compared to 2020 driven by higher sales of protein research and lab filtration products.
Operating Profit Performance
Operating profit margins declined 160 basis points during 2022 as compared to 2021. The following factors impacted year-over-year operating profit margin comparisons.
2022 vs. 2021 operating profit margin comparisons were unfavorably impacted by:
•Incremental year-over-year costs associated with various new product development, sales, service and marketing growth investments, restructuring and continuing productivity improvement initiatives, the impact of product mix, and incremental year-over-year material, transportation and labor costs, net of the impact of higher 2022 core sales - 170 basis points
•The incremental dilutive effect in 2022 of acquired businesses - 30 basis points
•2022 impairment of accounts receivable and inventory in Russia - 15 basis points
2022 vs. 2021 operating profit margin comparisons were favorably impacted by:
•2021 acquisition-related fair value adjustments to inventory and deferred revenue, in each case related to the acquisition of Cytiva - 55 basis points
Depreciation as a percentage of sales increased in 2022 as compared with 2021 as the increase in depreciation attributable to assets related to the acquisition of Cytiva and capital expenditures exceeded the increase in sales. Amortization of intangible assets as a percentage of sales decreased in 2022 as compared with 2021 primarily due to the increase in sales.
Operating profit margins increased 1,540 basis points during 2021 as compared to 2020. The following factors favorably impacted year-over-year operating profit margin comparisons.
•2020 acquisition-related fair value adjustments to inventory and deferred revenue, transaction costs deemed significant and integration preparation costs, net of 2021 acquisition-related fair value adjustments to inventory and deferred revenue in each case related to the acquisition of Cytiva - 875 basis points
•Higher 2021 core sales, the impact of product mix and the impact of foreign currency exchange rates, net of incremental year-over-year costs associated with various new product development and sales and marketing growth investments and incremental year-over-year material and labor costs - 585 basis points
•The incremental accretive effect in 2021 of acquired businesses, net of product line dispositions which did not qualify as discontinued operations - 80 basis points
Depreciation as a percentage of sales increased in 2021 as compared with 2020 as the increase in depreciation attributable to assets related to the acquisition of Cytiva and capital expenditures exceeded the increase in sales. Amortization of intangible assets as a percentage of sales decreased in 2021 as compared with 2020 primarily due to increased sales from the acquisition of Cytiva exceeding the increase in amortization.
LIFE SCIENCES
The Life Sciences segment offers a broad range of instruments and consumables that are primarily used by customers to study the basic building blocks of life, including DNA and RNA, nucleic acid, proteins, metabolites and cells, in order to understand the causes of disease, identify new therapies, and test and manufacture new drugs, vaccines and gene editing technologies. Additionally, the segment provides products and consumables used to filter and remove contaminants from a variety of liquids and gases in many end-market applications.
Life Sciences Selected Financial Data
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31 |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Sales | $ | 7,036 | | | $ | 6,388 | | | $ | 5,300 | |
| Operating profit | 1,414 | | | 1,293 | | | 972 | |
| Depreciation | 112 | | | 100 | | | 92 | |
| Amortization of intangible assets | 419 | | | 282 | | | 200 | |
| Operating profit as a % of sales | 20.1 | % | | 20.2 | % | | 18.3 | % |
| Depreciation as a % of sales | 1.6 | % | | 1.6 | % | | 1.7 | % |
| Amortization as a % of sales | 6.0 | % | | 4.4 | % | | 3.8 | % |
Sales Growth and Core Sales Growth
| | | | | | | | | | | |
| 2022 vs. 2021 | | 2021 vs. 2020 |
| Total sales growth (GAAP) | 10.0 | % | | 20.5 | % |
| Impact of: | | | |
| Acquisitions/divestitures | (5.5) | % | | (2.0) | % |
| Currency exchange rates | 5.0 | % | | (1.5) | % |
| Core sales growth (non-GAAP) | 9.5 | % | | 17.0 | % |
| | | |
| | | |
2022 Sales Compared to 2021
Price increases in the segment contributed 5.0% to sales growth on a year-over-year basis during 2022 as compared with 2021 and are reflected as a component of the change in core revenue growth.
During 2022, total Life Sciences segment sales increased 10.0% primarily as a result of increased core sales resulting from the factors discussed below and increased sales from acquisitions, partially offset by the impact of changes in currency exchange rates due to the strengthening of the U.S. dollar in 2022 compared to 2021. Core sales for the Company’s flow cytometry, genomics, lab automation, centrifugation, particle counting and characterization business decreased in 2022 primarily as a result of declines in Western Europe due to lower demand for genomic sample preparation consumables used in COVID-19 testing. These declines were partially offset by core sales growth in all other major product lines, led geographically by North America and China. Core sales in the mass spectrometry business increased in 2022 across all major end-markets driven in part by demand from recent product launches. Geographically, demand increased across all major geographies, led by North America, Western Europe and China. In 2022, core sales for the genomic consumables businesses increased compared to 2021 due to strong demand across most major product lines, led geographically by North America. Core sales for the industrial filtration business increased in 2022 compared to 2021 due to strong demand for these products across all major end-markets, led by microelectronics, aerospace and fluid technology and asset protection. Core sales for the industrial filtration business increased across all major geographies.
2021 Sales Compared to 2020
Price increases in the segment contributed 1.5% to sales growth on a year-over-year basis during 2021 as compared with 2020 and are reflected as a component of the change in core revenue growth.
During 2021, total Life Sciences segment sales increased 20.5% primarily as a result of increased core sales resulting from the factors discussed below, increased sales from acquisitions and the impact of changes in currency exchange rates due to the
weakening of the U.S. dollar in 2021 compared to 2020. Core sales for the Company’s flow cytometry, genomics, lab automation, centrifugation, particle counting and characterization business increased in 2021 across all major geographies, led by North America and Western Europe. Core sales for the business were driven by demand earlier in the year for genomic sample preparation consumables related to COVID-19 as well as demand for flow cytometry products. Core sales in the mass spectrometry business increased in 2021 across all major end-markets driven in part by demand for new products. Geographically, demand increased across all major geographies, led by North America, Western Europe and China. In 2021, core sales for the industrial filtration business increased compared to 2020 due to strong demand for these products led by the microelectronics end-market, partially offset by weaker demand in the aerospace end-market. Geographically, core sales for the business were led by China and other high-growth markets, partially offset by North America.
The acquisition of Aldevron on August 30, 2021 has provided, and is expected to continue to provide, additional sales and earnings growth opportunities for the Company’s Life Sciences segment by expanding the business’ product line diversity, including new product and service offerings that complement the Company’s genomic medicine solutions. Since acquisition, Aldevron has seen sales growth in all major product lines compared to the prior year period.
Operating Profit Performance
Operating profit margins declined 10 basis points during 2022 as compared to 2021. The following factors impacted year-over-year operating profit margin comparisons.
2022 vs. 2021 operating profit margin comparisons were favorably impacted by:
•2021 acquisition-related fair value adjustments to inventory and transaction costs deemed significant, in each case related to the acquisition of Aldevron - 90 basis points
•Higher 2022 core sales, net of incremental year-over-year costs associated with various sales and marketing growth investments, incremental year-over-year material, transportation and labor costs and incremental restructuring and continuing productivity improvement initiatives - 50 basis points
2022 vs. 2021 operating profit margin comparisons were unfavorably impacted by:
•The incremental dilutive effect in 2022 of acquired businesses, net of product line dispositions which did not qualify as discontinued operations - 115 basis points
•2022 impairment of accounts receivable and inventory as well as accruals for contractual obligations in Russia - 35 basis points
Depreciation as a percentage of sales was consistent in 2022 as compared with 2021. Amortization of intangible assets as a percentage of sales increased in 2022 as compared with 2021 primarily as a result of the increase in intangible assets related to the acquisition of Aldevron.
Operating profit margins increased 190 basis points during 2021 as compared to 2020. The following factors impacted year-over-year operating profit margin comparisons.
2021 vs. 2020 operating profit margin comparisons were favorably impacted by:
• Higher 2021 core sales and the impact of product mix, incremental year-over-year cost savings associated with continuing productivity improvement initiatives and the impact of foreign currency exchange rates, net of incremental year-over-year costs associated with various new product development and sales and marketing growth investments and incremental year-over-year material and labor costs - 360 basis points
2021 vs. 2020 operating profit margin comparisons were unfavorably impacted by:
•2021 acquisition-related fair value adjustments to inventory and transaction costs deemed significant, in each case related to the acquisition of Aldevron - 90 basis points
•The incremental dilutive effect in 2021 of acquired businesses, net of product line dispositions which did not qualify as discontinued operations - 80 basis points
Depreciation as a percentage of sales were relatively consistent in 2021 as compared with 2020. Amortization of intangible assets as a percentage of sales increased in 2021 as compared with 2020 primarily due to the increase in intangible assets related to the acquisition of Aldevron.
DIAGNOSTICS
The Diagnostics segment offers clinical instruments, reagents, consumables, software and services that hospitals, physicians’ offices, reference laboratories and other critical care settings use to diagnose disease and make treatment decisions.
Diagnostics Selected Financial Data
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31 |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Sales | $ | 10,849 | | | $ | 9,844 | | | $ | 7,403 | |
| Operating profit | 3,436 | | | 2,313 | | | 1,538 | |
| Depreciation | 387 | | | 409 | | | 397 | |
| Amortization of intangible assets | 203 | | | 205 | | | 205 | |
| Operating profit as a % of sales | 31.7 | % | | 23.5 | % | | 20.8 | % |
| Depreciation as a % of sales | 3.6 | % | | 4.2 | % | | 5.4 | % |
| Amortization as a % of sales | 1.9 | % | | 2.1 | % | | 2.8 | % |
Sales Growth and Core Sales Growth
| | | | | | | | | | | |
| 2022 vs. 2021 | | 2021 vs. 2020 |
| Total sales growth (GAAP) | 10.0 | % | | 33.0 | % |
| Impact of: | | | |
| Acquisitions/divestitures | (0.5) | % | | (0.5) | % |
| Currency exchange rates | 4.0 | % | | (1.5) | % |
| Core sales growth (non-GAAP) | 13.5 | % | | 31.0 | % |
2022 Sales Compared to 2021
Price increases in the segment contributed 1.0% to sales growth on a year-over-year basis during 2022 as compared with 2021 and are reflected as a component of the change in core sales growth.
During 2022, total segment sales increased 10.0% primarily as a result of increased core sales resulting from the factors discussed below, particularly higher year-over-year core sales of molecular diagnostics tests for COVID-19 which contributed significantly to overall segment core sales growth, partially offset by the impact of changes in currency exchange rates. Core sales in the molecular diagnostics business increased on a year-over-year basis led by North America and Western Europe as the business experienced strong growth in sales of consumables. The increase was driven primarily by increased sales of diagnostic test solutions for COVID-19 as well as higher year-over-year demand for non-respiratory disease tests. Additional production capacity added in 2021 allowed the business to produce more diagnostic tests in 2022 and meet continued strong demand by private and government customers. Core sales in the segment’s clinical lab business grew on a year-over-year basis as increased demand in North America and Japan offset weaker demand in China where COVID-19 related restrictions reduced patient volumes. During 2022, core sales in the acute care diagnostic business increased year-over-year primarily due to increased demand for its blood gas product line. Geographically, demand was driven by Western Europe, North America and China. Core sales in the pathology business grew year-over-year across all major geographies, driven by increased demand for core histology, advanced staining and pathology imaging products.
Operating Profit Performance
Operating profit margins increased 820 basis points during 2022 as compared to 2021. The following factors impacted year-over-year operating profit margin comparisons.
2022 vs. 2021 operating profit margin comparisons were favorably impacted by:
•Third quarter 2021 impact of the modification and partial termination of a prior commercial arrangement and resolution of the associated litigation - 555 basis points
•Higher 2022 core sales and the impact of product mix, net of incremental year-over-year costs associated with material, transportation and labor, restructuring and continuing productivity improvement initiatives, sales and marketing growth initiatives and various new product development initiatives - 250 basis points
•The incremental accretive effect in 2022 of acquired businesses - 10 basis points
•First quarter 2021 impairment charge related to a trade name - 10 basis points
2022 vs. 2021 operating profit margin comparisons were unfavorably impacted by:
•2022 impairments of accounts receivable as well as accruals for contractual obligations in Russia - 5 basis points
Depreciation and amortization of intangible assets both decreased as a percentage of sales during 2022 as compared with 2021, primarily as a result of the increase in sales.
ENVIRONMENTAL & APPLIED SOLUTIONS
The Environmental & Applied Solutions segment offers products and services that help protect precious resources and keep global food and water supplies safe. The Company’s water quality business provides instrumentation, consumables, software, services and disinfection systems to help analyze, treat and manage the quality of ultra-pure, potable, industrial, waste, ground, source and ocean water in residential, commercial, municipal, industrial and natural resource applications. The Company’s product identification business provides instruments, software, services and consumables for various color and appearance management, packaging design and quality management, packaging converting, printing, marking, coding and traceability applications for consumer, pharmaceutical and industrial products.
Environmental & Applied Solutions Selected Financial Data
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31 |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Sales | $ | 4,828 | | | $ | 4,651 | | | $ | 4,305 | |
| Operating profit | 1,135 | | | 1,054 | | | 979 | |
| Depreciation | 40 | | | 44 | | | 47 | |
| Amortization of intangible assets | 50 | | | 62 | | | 63 | |
| Operating profit as a % of sales | 23.5 | % | | 22.7 | % | | 22.7 | % |
| Depreciation as a % of sales | 0.8 | % | | 0.9 | % | | 1.1 | % |
| Amortization as a % of sales | 1.0 | % | | 1.3 | % | | 1.5 | % |
Sales Growth and Core Sales Growth
| | | | | | | | | | | |
| 2022 vs. 2021 | | 2021 vs. 2020 |
| Total sales growth (GAAP) | 4.0 | % | | 8.0 | % |
| Impact of: | | | |
| Acquisitions/divestitures | 0.5 | % | | 1.5 | % |
| Currency exchange rates | 3.5 | % | | (1.5) | % |
| Core sales growth (non-GAAP) | 8.0 | % | | 8.0 | % |
2022 Sales Compared to 2021
Price increases in the segment contributed 7.5% to sales growth on a year-over-year basis during 2022 as compared with 2021 and are reflected as a component of the change in core revenue growth.
In 2022, total Environmental & Applied Solutions segment sales increased 4.0%, primarily as a result of core sales growth driven by the factors discussed below, partially offset by the impact of changes in currency exchange rates and divestitures, net of acquisitions.
On an overall basis, in 2022 core sales in the segment’s water quality businesses increased at a low-double digit rate. Year-over-year core sales in the analytical instrumentation product line increased driven by higher core sales in the municipal and industrial end-markets. Geographically, core sales growth was led by North America and Western Europe. Core sales in the chemical treatment solutions product line increased as a result of higher core sales across all major end-markets. Geographically, the increase in core sales of chemical treatment solutions was driven by North America and Latin America.
The segment’s product identification businesses’ core sales grew at a mid-single digit rate. Core sales in the marking and coding business increased led by the food and beverage end-market. Geographically, core sales growth was led by North America, Western Europe and Latin America, partially offset by the core sales decline from the suspension of shipments to Russia. Year-over-year core sales in the packaging and color solutions products and services business increased, geographically led by Western Europe.
In September 2022, the Company announced its intention to spin-off its Environmental & Applied Solutions business into a publicly traded company. The transaction is expected to be tax-free to the Company’s shareholders. The Company is targeting to complete the EAS Separation in the fourth quarter of 2023, subject to the satisfaction of certain conditions, including obtaining final approval from the Danaher Board of Directors, satisfactory completion of financing, receipt of tax opinions, receipt of favorable rulings from the Internal Revenue Service (“IRS”) and receipt of other regulatory approvals.
Operating Profit Performance
Operating profit margins increased 80 basis points during 2022 as compared to 2021. The following factors impacted year-over-year operating profit margin comparisons.
2022 vs. 2021 operating profit margin comparisons were favorably impacted by:
•Higher 2022 core sales and incrementally lower year-over-year costs associated with various new product development initiatives, net of the impact of product mix, incremental year-over-year costs associated with material, transportation and labor and restructuring and continuing productivity improvement initiatives and incremental year-over-year costs for sales, service and marketing growth investments - 85 basis points
•The incremental net accretive effect in 2022 of acquired businesses and product line dispositions which did not qualify as discontinued operations - 20 basis points
2022 vs. 2021 operating profit margin comparisons were unfavorably impacted by:
•Second quarter 2022 impairment charge related to technology and customer relationships - 20 basis points
•2022 impairments of accounts receivable and inventory in Russia - 5 basis points
Depreciation and amortization of intangible assets as a percentage of sales decreased in 2022 as compared with 2021 primarily as a result of the increase in sales.
COST OF SALES AND GROSS PROFIT
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31 |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Sales | $ | 31,471 | | | $ | 29,453 | | | $ | 22,284 | |
| Cost of sales | (12,522) | | | (11,501) | | | (9,809) | |
| Gross profit | $ | 18,949 | | | $ | 17,952 | | | $ | 12,475 | |
| Gross profit margin | 60.2 | % | | 61.0 | % | | 56.0 | % |
The year-over-year increase in cost of sales during 2022 as compared with 2021 was due primarily to the impact of higher year-over-year sales volumes, including sales volumes from recently acquired businesses and incremental year-over-year costs associated with material, transportation, labor and restructuring and continuing productivity improvement initiatives. This increase was partially offset by lower incremental year-over-year acquisition-related charges associated with fair value adjustments to inventory in connection with the 2021 acquisition of Aldevron, which increased cost of sales by $59 million in 2021.
The year-over-year decrease in gross profit margin during 2022 as compared with 2021 was driven by incremental year-over-year costs associated with material, transportation, labor and restructuring and continuing productivity improvement initiatives. In addition, the gross profit margin was negatively impacted by a 2022 inventory charge related to reduction of business activities in Russia. Gross profit margins declines were partially offset by increased year-over-year core sales and product mix as well as the impact of acquisition-related charges incurred in 2021. The 2021 acquisition-related charges of $76 million included fair value adjustments to deferred revenue related to the acquisition of Cytiva and fair value adjustments to inventory in connection with the acquisitions of Aldevron and Cytiva.
OPERATING EXPENSES
| | | | | | | | | | | | | | | | | |
| | Year Ended December 31 |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Sales | $ | 31,471 | | | $ | 29,453 | | | $ | 22,284 | |
| Selling, general and administrative (“SG&A”) expenses | (8,516) | | | (8,198) | | | (6,896) | |
| Research and development (“R&D”) expenses | (1,745) | | | (1,742) | | | (1,348) | |
| Other operating expenses | — | | | (547) | | | — | |
| SG&A as a % of sales | 27.1 | % | | 27.8 | % | | 30.9 | % |
| R&D as a % of sales | 5.5 | % | | 5.9 | % | | 6.0 | % |
| Other operating expenses as a % of sales | — | % | | 1.9 | % | | — | % |
SG&A expenses as a percentage of sales declined 70 basis points on a year-over-year basis for 2022 compared with 2021. The decline was driven by the benefit of increased leverage of the Company’s general and administrative cost base, including amortization expense, resulting from higher 2022 sales, including sales volumes from recently acquired businesses, as well as incremental year-over-year cost savings associated with continuing productivity improvement initiatives. The Company’s 2021 transaction costs for the acquisition of Aldevron also benefited the year-over-year comparison of SG&A as a percentage of sales. These decreases were partially offset by continued investments in sales and marketing growth initiatives, increased labor costs and incremental restructuring and continuing productivity improvement costs as well as higher amortization expense. Additionally, the declines were partially offset by charges related to impairments of certain accounts receivable and accrual of contractual obligations incurred in Russia and by an impairment charge related to technology and customer relationships incurred in 2022, net of the impact of an impairment charge related to a trade name in 2021.
R&D expenses (consisting principally of internal and contract engineering personnel costs) as a percentage of sales declined in 2022 as compared with 2021, primarily due to the sales growth rate exceeding the spending growth related to new product development initiatives.
Other operating expenses and other operating expenses as a percentage of sales decreased in 2022 compared with 2021 as a result of the contract settlement expense related to the modification and partial termination of a prior commercial arrangement and resolution of the associated litigation during 2021. Refer to Note 8 to the Consolidated Financial Statements.
NONOPERATING INCOME (EXPENSE)
Nonoperating income (expense) consists primarily of net unrealized and realized gains/losses resulting from changes in the fair value of the Company’s investments in equity securities and investments in partnerships, the non-service cost components of net periodic benefit costs, gains on the sale of product lines and impairments of equity method investments. Refer to Note 9 to the Consolidated Financial Statements.
LOSS ON EARLY EXTINGUISHMENT OF BORROWINGS
In the fourth quarter of 2021, the Company redeemed the €800 million aggregate principal amount of 2.5% senior unsecured notes due 2025 at a redemption price equal to the outstanding principal amount and a make-whole premium as specified in the applicable indenture, plus accrued and unpaid interest. The Company recorded a loss on early extinguishment of these borrowings related to the payment of the make-whole premiums and deferred costs in connection with the redemption of $96 million ($73 million after-tax). The Company funded the redemption using available cash balances, including proceeds from the fourth quarter 2021 issuance of the $1.0 billion aggregate principal amount of 2.8% senior unsecured notes due 2051.
In the fourth quarter of 2020, the Company redeemed the €800 million aggregate principal amount of 1.7% senior unsecured notes due 2022 at a redemption price equal to the outstanding principal amount and a make-whole premium as specified in the applicable indenture, plus accrued and unpaid interest. The Company recorded a loss on early extinguishment of these borrowings of $26 million ($20 million after-tax) related to the payment of make-whole premiums in connection with the redemption. The Company funded the redemption using available cash balances, including proceeds from the fourth quarter 2020 issuance of the $1.0 billion aggregate principal amount of 2.6% senior unsecured notes due 2050.
INTEREST COSTS
Interest expense of $211 million for 2022 was $27 million lower than in 2021, due primarily to lower average debt balances in 2022 compared to 2021 and the impact of the stronger U.S. dollar in 2022 on the interest expense for the Company’s foreign currency denominated debt (and U.S. dollar debt that has been effectively converted into foreign currency through cross-currency swap derivative contracts). Interest income of $41 million for 2022 was $30 million higher than in 2021, due primarily to higher interest rates and higher cash balances in 2022.
For a further description of the Company’s debt and cross-currency swap derivative contracts related to the debt as of December 31, 2022 refer to Notes 14 and 15 to the Consolidated Financial Statements.
INCOME TAXES
General
Income tax expense and deferred tax assets and liabilities reflect management’s assessment of future taxes expected to be paid on items reflected in the Company’s Consolidated Financial Statements. The Company records the tax effect of discrete items and items that are reported net of their tax effects in the period in which they occur.
The Company’s effective tax rate can be affected by changes in the mix of earnings in countries with different statutory tax rates (including as a result of business acquisitions and dispositions), changes in the valuation of deferred tax assets and liabilities, accruals related to contingent tax liabilities and period-to-period changes in such accruals, the results of audits and examinations of previously filed tax returns (as further discussed below), the expiration of statutes of limitations, the implementation of tax planning strategies, tax rulings, court decisions, settlements with tax authorities, changes in tax laws and regulations, and legislative policy changes that may result from the OECD’s initiative on Base Erosion and Profit Shifting. For a description of the tax treatment of earnings that are planned to be reinvested indefinitely outside the United States, refer to “—Liquidity and Capital Resources—Cash and Cash Requirements” below.
The amount of income taxes the Company pays is subject to ongoing audits by federal, state and non-U.S. tax authorities, which often result in proposed assessments. Management performs a comprehensive review of its global tax positions on a quarterly basis. Based on these reviews, which take into account the results of discussions and resolutions of matters with certain tax authorities and the other factors referenced in the prior paragraph, reserves for contingent tax liabilities are accrued or adjusted as necessary. For a discussion of risks related to these and other tax matters, refer to “Item 1A. Risk Factors”.
Year-Over-Year Changes in the Tax Provision and Effective Tax Rate
| | | | | | | | | | | | | | | | | |
| Year Ended December 31 |
| 2022 | | 2021 | | 2020 |
| Effective tax rate from continuing operations | 13.1 | % | | 16.5 | % | | 18.9 | % |
The Company’s effective tax rate for 2022 differs from the U.S. federal statutory rate of 21.0% due principally to net deferred tax benefits resulting from legal and operational actions undertaken to realign certain of its businesses, as well as excess tax benefits from stock-based compensation, the release of reserves for uncertain tax positions due to the expiration of statutes of limitation and audit settlements and changes in estimates related to prior year tax filing positions, net of changes in estimates associated with prior period uncertain tax positions.
The Company’s effective tax rate for 2021 differs from the U.S. federal statutory rate of 21.0% due principally to net discrete benefits related primarily to the release of reserves for uncertain tax positions due to the expiration of statutes of limitation and audit settlements and excess tax benefits from stock-based compensation, net of changes in estimates associated with prior period uncertain tax positions.
The Company conducts business globally and files numerous consolidated and separate income tax returns in the U.S. federal and state and non-U.S. jurisdictions. The non-U.S. countries in which the Company has a significant presence include China, Denmark, Germany, Singapore, Sweden, Switzerland and the United Kingdom. Excluding these jurisdictions, the Company believes that a change in the statutory tax rate of any individual non-U.S. country would not have a material effect on the Company’s Consolidated Financial Statements given the geographic dispersion of the Company’s taxable income.
The Company and its subsidiaries are routinely examined by various U.S. and non-U.S. taxing authorities. The IRS has completed substantially all of the examinations of the Company’s federal income tax returns through 2015 and is currently examining certain of the Company’s federal income tax returns for 2016 through 2018. In addition, the Company has subsidiaries in Belgium, Canada, China, Denmark, France, Germany, India, Italy, Japan, Korea, Switzerland, the United Kingdom and various other countries, states and provinces that are currently under audit for years ranging from 2004 through 2021.
Similar to the position it took in connection with the audit of the Company’s taxable income for the years 2012 through 2015, in the fourth quarter of 2022, the IRS proposed significant adjustments to the Company’s taxable income for the years 2016 through 2018 with respect to the deferral of tax on certain premium income related to the Company’s self-insurance programs. The settlement of this matter for the 2012 through 2015 audit was not material to the Company’s financial statements but did not preclude the IRS from proposing similar adjustments in future audit periods, as the IRS has with the 2022 assessment. For income tax purposes, the recognition of premium income has been deferred in accordance with U.S. tax laws related to insurance. The IRS is challenging the deferral of premium income for certain types of the Company’s self-insurance policies. The proposed adjustments would increase the Company’s taxable income over the 2016 through 2018 periods by approximately $2.5 billion. Due to the enactment of the TCJA in 2017 and the resulting reduction in the U.S. corporate tax rate for years after 2017, the Company remeasured its deferred tax liabilities related to the temporary differences associated with this deferred premium income from 35.0% to 21.0%. If the Company is unsuccessful in defending its position, taxes owed to the IRS may be computed under the previous 35.0% statutory tax rate and the Company may be required to remeasure the related deferred tax liabilities from 21.0% to 35.0%, which in addition to any interest due on the amounts assessed, would require a charge to future earnings. Management believes the positions the Company has taken in its U.S. tax returns are in accordance with the relevant tax laws and intends to vigorously defend these positions.
Tax authorities in Denmark have issued tax assessments related to interest accrued by certain of the Company’s subsidiaries for the years 2004 through 2015. During the first quarter of 2021, the Company received a notice from the Danish tax authorities that included a significant reduction in the interest amounts imposed in the original tax assessments. Taking into account the revised interest amounts, the assessments total approximately DKK 2.1 billion including applicable accrued interest (approximately $298 million based on the exchange rate as of December 31, 2022). The Company’s appeal of the tax assessments with the Danish National Tax Tribunal has been put on hold awaiting the final outcome of other preceding withholding tax cases that have been brought before the Danish High Court and the Danish Supreme Court. Management believes the positions the Company has taken in Denmark are in accordance with the relevant tax laws and is vigorously defending its positions. The Company intends on pursuing this matter through the Danish High Court and the Danish Supreme Court should the appeal to the Danish National Tax Tribunal be unsuccessful. While the ultimate resolution of this matter is uncertain and could take many years, taking into account the payments the Company has previously made related to these assessments in order to mitigate further interest accrual claims, the Company does not expect the resolution of this matter will have a future material adverse impact to the Company’s financial statements, including its cash flow and effective tax rate.
The Company expects its 2023 effective tax rate to be approximately 19.5% which is higher than the 2022 rate due primarily to the impact of net discrete tax benefits on the 2022 effective tax rate that are not expected to repeat in 2023 and the geographic mix of earnings anticipated for 2023. Any future legislative changes in the United States and/or potential tax reform in other jurisdictions could cause the Company’s effective tax rate to differ from this estimate. Refer to Note 7 to the Consolidated Financial Statements for additional information related to income taxes.
DISCONTINUED OPERATIONS AND ENVIRONMENTAL & APPLIED SOLUTIONS SEPARATION
Fortive Corporation Separation
On July 2, 2016, the Company completed the separation of its former Test & Measurement segment, Industrial Technologies segment (excluding the product identification businesses) and retail/commercial petroleum business by distributing to Danaher stockholders on a pro rata basis all of the issued and outstanding common stock of Fortive Corporation (“Fortive”), the entity the Company incorporated to hold such businesses. In 2021, the Company recorded an income tax benefit of $86 million related to the release of previously provided reserves associated with uncertain tax positions on certain of the Company’s tax returns which were jointly filed with Fortive entities. These reserves were released due to the expiration of statutes of limitations for those returns. This income tax benefit is included in earnings from discontinued operations, net of income taxes in the Consolidated Statements of Earnings.
Environmental & Applied Solutions Separation
In September 2022, the Company announced its intention to spin-off its Environmental & Applied Solutions business into a publicly traded company. The transaction is expected to be tax-free to the Company’s shareholders. The Company is targeting to complete the EAS Separation in the fourth quarter of 2023, subject to the satisfaction of certain conditions, including obtaining final approval from the Danaher Board of Directors, satisfactory completion of financing, receipt of tax opinions, receipt of favorable rulings from the IRS and receipt of other regulatory approvals.
Refer to Note 3 to the Consolidated Financial Statements for additional information.
COMPREHENSIVE INCOME
Comprehensive income decreased by $410 million in 2022 as compared to 2021, primarily driven by the impact of higher losses from foreign currency translation adjustments in 2022 compared to 2021 and a decrease in the income from pension and postretirement plan benefit adjustments and cash flow hedge adjustments in 2022 compared to 2021, partially offset by higher net earnings in 2022 compared to 2021. The Company recorded a foreign currency translation loss of approximately $2.1 billion for 2022 compared to a loss of approximately $1.3 billion for 2021. The Company recorded a pension and postretirement plan benefit gain of $209 million for 2022 compared to a gain of $378 million for 2021. The Company recorded gains from cash flow hedge adjustments related to the Company’s derivative contracts in 2022 of $51 million compared to gains of $247 million in 2021.
FINANCIAL INSTRUMENTS AND RISK MANAGEMENT
The Company is exposed to market risk from changes in interest rates, foreign currency exchange rates, equity prices and commodity prices as well as credit risk, each of which could impact its Consolidated Financial Statements. The Company generally addresses its exposure to these risks through its normal operating and financing activities. The Company also periodically uses derivative financial instruments to manage foreign exchange risks and interest rate risks. In addition, the Company’s broad-based business activities help to reduce the impact that volatility in any particular area or related areas may have on its financial statements as a whole.
Interest Rate Risk
The Company manages interest cost using a mixture of fixed-rate and at times variable-rate debt. A change in interest rates on fixed-rate debt impacts the fair value of the debt but not the Company’s earnings or cash flow because the interest on such debt is fixed. Generally, the fair market value of fixed-rate debt will increase as interest rates fall and decrease as interest rates rise. As of December 31, 2022, an increase of 100 basis points in interest rates would have decreased the fair value of the Company’s fixed-rate long-term debt by approximately $1.5 billion.
As of December 31, 2022, the Company had no variable-rate debt obligations, however, the interest rates of the Company’s euro-based commercial paper borrowings are fixed based on short-term market rates at the time of issuance (refer to Note 14 to the Consolidated Financial Statements for information regarding the Company’s outstanding commercial paper balances as of December 31, 2022). As a result, the Company’s primary interest rate exposure results from changes in short-term interest rates. As these shorter duration obligations mature, the Company may issue additional short-term commercial paper obligations to refinance all or part of these borrowings, to the extent commercial paper markets are available. In 2022, the average annual interest rate associated with the Company’s outstanding commercial paper borrowings was approximately 43 basis points. A hypothetical increase of this average by 100 basis points would have increased the Company’s 2022 interest expense by approximately $21 million.
Refer to “Results of Operations—Interest Costs” for discussion of the Company’s cross-currency swap derivative contracts and interest rate swap agreements.
Currency Exchange Rate Risk
The Company faces transactional exchange rate risk from transactions with customers in countries outside the United States and from intercompany transactions between affiliates. Transactional exchange rate risk arises from the purchase and sale of goods and services in currencies other than Danaher’s functional currency or the functional currency of its applicable subsidiary. The Company also faces translational exchange rate risk related to the translation of financial statements of its foreign operations into U.S. dollars, Danaher’s functional currency. Costs incurred and sales recorded by subsidiaries operating outside of the United States are translated into U.S. dollars using exchange rates effective during the respective period. As a result, the Company is exposed to movements in the exchange rates of various currencies against the U.S. dollar. In particular, the Company has more sales in European currencies than it has expenses in those currencies. Therefore, when European currencies strengthen or weaken against the U.S. dollar, operating profits are increased or decreased, respectively. The effect of a change in currency exchange rates on the Company’s net investment in non-U.S. subsidiaries is reflected in the accumulated other comprehensive income (loss) component of stockholders’ equity.
Currency exchange rates negatively impacted 2022 reported sales on a year-over-year basis primarily due to the strengthening of the U.S. dollar against most major currencies during 2022. If the currency exchange rates in effect as of December 31, 2022 were to prevail throughout 2023, the Company’s 2023 sales would be essentially flat relative to 2022 sales. Strengthening of the U.S. dollar against other major currencies compared to the exchange rates in effect as of December 31, 2022 would adversely impact the Company’s sales and results of operations on an overall basis. Any weakening of the U.S. dollar against other major currencies compared to the exchange rates in effect as of December 31, 2022 would positively impact the Company’s sales and results of operations.
The Company has generally accepted the exposure to exchange rate movements without using derivative financial instruments to manage this transactional exchange risk, although the Company has used foreign currency-denominated debt and cross-currency swaps to hedge a portion of its net investments in non-U.S. operations against adverse movements in exchange rates. Both positive and negative movements in currency exchange rates against the U.S. dollar will continue to affect the reported amount of sales and net earnings in the Company’s Consolidated Financial Statements. In addition, the Company has assets and liabilities held in foreign currencies. A 10% depreciation in major currencies relative to the U.S. dollar as of December 31, 2022 would have reduced foreign currency-denominated net assets and stockholders’ equity by approximately $1.7 billion. Refer to Note 15 to the Consolidated Financial Statements for information regarding the Company’s hedging of a portion of its net investment in non-U.S. operations.
Equity Price Risk
The Company’s investment portfolio from time to time includes publicly-traded equity securities that are sensitive to fluctuations in market price. As of December 31, 2022, the Company held $16 million of publicly-traded equity securities, excluding equity-method investments. Additionally, the Company holds non-marketable equity investments in privately held companies that may be impacted by equity price risks. These non-marketable equity investments are accounted for under the Fair Value Alternative method with changes in fair value recorded in earnings. Volatility in the equity markets or other fair value considerations could affect the value of these investments and require losses or gains to be recognized in earnings.
Commodity Price Risk
For a discussion of risks relating to commodity prices, refer to “Item 1A. Risk Factors.”
Credit Risk
The Company is exposed to potential credit losses in the event of nonperformance by counterparties to its financial instruments. Financial instruments that potentially subject the Company to credit risk consist of cash and temporary investments, receivables from customers and derivatives. The Company places cash and temporary investments with various high-quality financial institutions throughout the world and exposure is limited at any one institution. Although the Company typically does not obtain collateral or other security to secure these obligations, it does regularly monitor the third-party depository institutions that hold its cash and cash equivalents. The Company’s emphasis is primarily on safety and liquidity of principal and secondarily on maximizing yield on those funds.
In addition, concentrations of credit risk arising from receivables from customers are limited due to the diversity of the Company’s customers. The Company’s businesses perform credit evaluations of their customers’ financial conditions as deemed appropriate and also obtain collateral or other security when deemed appropriate.
The Company enters into derivative transactions infrequently and typically with high-quality financial institutions, so that exposure at any one institution is limited.
LIQUIDITY AND CAPITAL RESOURCES
Management assesses the Company’s liquidity in terms of its ability to generate cash to fund its operating, investing and financing activities. The Company continues to generate substantial cash from operating activities and believes that its operating cash flow, cash on hand and other sources of liquidity will be sufficient to allow it to continue investing in existing businesses (including capital expenditures), consummating strategic acquisitions and investments, paying interest and servicing debt, paying dividends, funding restructuring activities and managing its capital structure on a short-term and long-term basis.
The Company has relied primarily on borrowings under its commercial paper program to address liquidity requirements that exceed the capacity provided by its operating cash flows and cash on hand, while also accessing the capital markets from time to time including to secure financing for more significant acquisitions. Subject to any limitations that may result from the COVID-19 pandemic or other market disruptions (such as the disruptions in the financial and capital markets that occurred at times in 2020), the Company anticipates following the same approach in the future.
Overview of Cash Flows and Liquidity
Following is an overview of the Company’s cash flows and liquidity for the years ended December 31:
| | | | | | | | | | | | | | | | | |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Total operating cash flows provided by continuing operations | $ | 8,519 | | | $ | 8,358 | | | $ | 6,215 | |
| | | | | |
| Cash paid for acquisitions | $ | (637) | | | $ | (10,961) | | | $ | (20,971) | |
| Payments for additions to property, plant and equipment | (1,152) | | | (1,294) | | | (791) | |
| Proceeds from sales of property, plant and equipment | 9 | | | 13 | | | 2 | |
| Payments for purchases of investments | (523) | | | (934) | | | (342) | |
| Proceeds from sales of investments | 18 | | | 126 | | | 13 | |
| Proceeds from sale of product lines | — | | | 26 | | | 826 | |
| All other investing activities | 51 | | | 37 | | | 24 | |
| | | | | |
| Net cash used in investing activities for continuing operations | $ | (2,234) | | | $ | (12,987) | | | $ | (21,239) | |
| | | | | |
| Proceeds from the issuance of common stock in connection with stock-based compensation | $ | 31 | | | $ | 86 | | | $ | 153 | |
| Proceeds from the public offering of common stock, net of issuance costs | — | | | — | | | 1,729 | |
| Proceeds from the public offering of preferred stock, net of issuance costs | — | | | — | | | 1,668 | |
| | | | | |
| Payment of dividends | (818) | | | (742) | | | (615) | |
| | | | | |
| Net (repayments of) proceeds from borrowings (maturities of 90 days or less) | (723) | | | 2,265 | | | (4,637) | |
| Proceeds from borrowings (maturities longer than 90 days) | — | | | 984 | | | 8,670 | |
| Repayments of borrowings (maturities longer than 90 days) | (965) | | | (1,186) | | | (5,933) | |
| Make-whole premiums to redeem borrowings prior to maturity | — | | | (96) | | | (26) | |
| All other financing activities | (95) | | | (16) | | | (3) | |
| | | | | |
| Net cash (used in) provided by financing activities for continuing operations | $ | (2,570) | | | $ | 1,295 | | | $ | 1,006 | |
•Operating cash flows from continuing operations increased $161 million, or 2%, during 2022 as compared to 2021, due primarily to higher net earnings from continuing operations (after excluding charges for depreciation, amortization (including intangible assets and inventory step-up), stock compensation, gain on sale of product lines, unrealized investment gains/losses, loss on the extinguishment of debt and the contract settlement expense in 2021). These increases were partially offset by higher cash used in aggregate for accounts receivables, inventories, trade accounts payable and prepaid and accrued expenses, including deferred taxes, in 2022 compared to the prior year.
•Net cash used in investing activities consisted primarily of capital expenditures, cash paid for acquisitions and investments, net of proceeds from the sale of investments, and decreased primarily as a result of lower cash paid for acquisitions in 2022 compared to 2021. Refer to Notes 2 and 12 to the Consolidated Financial Statements included in this Annual Report for a discussion of the Company’s acquisitions and investments.
•As of December 31, 2022, the Company held approximately $6.0 billion of cash and cash equivalents.
Operating Activities
Cash flows from operating activities can fluctuate significantly from period-to-period as working capital needs and the timing of payments for income taxes, restructuring activities and productivity improvement initiatives, pension funding and other items impact reported cash flows.
Operating cash flows from continuing operations were approximately $8.5 billion for 2022, an increase of $161 million, or 2%, as compared to 2021. The year-over-year change in operating cash flows from 2021 to 2022 was primarily attributable to the following factors:
•2022 operating cash flows benefited from higher net earnings in 2022 as compared to 2021.
•Net earnings for 2022 reflected an increase of $160 million of depreciation, amortization, stock compensation expense, unrealized investment gains/losses, net of loss on the extinguishment of debt and contract settlement expense as
compared to 2021. Amortization expense primarily relates to the amortization of intangible assets and inventory fair value adjustments. Depreciation expense relates to both the Company’s manufacturing and operating facilities as well as instrumentation leased to customers under operating-type lease (“OTL”) arrangements. Contract settlement expense represents the pretax charge related to the modification and partial termination of a prior commercial arrangement and resolution of the associated litigation. Refer to Note 8 to the Consolidated Financial Statements for additional information on the contract settlement expense. Depreciation, amortization, stock compensation and contract settlement expense are noncash expenses that decrease earnings without a corresponding impact to operating cash flows. Cash flows from the gain on sale of product lines and loss on the extinguishment of debt are reflected in cash flows from investing activities while unrealized investment gains/losses impact net earnings without immediately impacting cash flows as the cash flow impact from investments occurs when the invested capital is returned to the Company.
•The aggregate of trade accounts receivable, inventories and trade accounts payable used $958 million in operating cash flows during 2022, compared to $564 million of operating cash flows used in 2021. The amount of cash flow generated from or used by the aggregate of trade accounts receivable, inventories and trade accounts payable depends upon how effectively the Company manages the cash conversion cycle, which effectively represents the number of days that elapse from the day it pays for the purchase of raw materials and components to the collection of cash from its customers and can be significantly impacted by the timing of collections and payments in a period.
•The aggregate of prepaid expenses and other assets, deferred income taxes and accrued expenses and other liabilities used $561 million in operating cash flows during 2022, compared to $94 million used in 2021. The timing of cash payments for taxes and the impact of deferred tax benefits and charges, various employee-related liabilities, customer funding and accrued expenses drove the majority of this change.
Investing Activities
Cash flows relating to investing activities consist primarily of cash used for acquisitions and capital expenditures, including instruments leased to customers, cash used for investments and cash proceeds from divestitures of businesses or assets.
Net cash used in investing activities was approximately $2.2 billion during 2022 compared to approximately $13.0 billion of net cash used in 2021.
Acquisitions, Divestitures and Sale of Investments
For a discussion of the Company’s 2022 and 2021 acquisitions and divestitures refer to “—Overview” and Note 2 to the Consolidated Financial Statements. In addition, in 2022 and 2021, the Company invested $523 million and $934 million, respectively, in non-marketable equity securities and partnerships.
Capital Expenditures
Capital expenditures are made primarily for increasing manufacturing capacity, replacing equipment, supporting new product development, improving information technology systems and the manufacture of instruments that are used in OTL arrangements that certain of the Company’s businesses enter into with customers. Capital expenditures totaled approximately $1.2 billion in 2022 and $1.3 billion in 2021. The year-over-year decrease in capital spending in 2022 was primarily due to higher 2021 expenditures related to diagnostic testing capacity and declines in expenditures for instruments used in OTL arrangements, partially offset by incremental capital expenditures at Aldevron and Cytiva. In 2023, the Company expects capital expenditures to be approximately $1.5 billion primarily to increase manufacturing capacity and to support other growth opportunities.
During 2021, certain agencies of the U.S. government, including the Biomedical Advanced Research and Development Authority (“BARDA”) within the U.S. Department of Health and Human Services, agreed to finance an expansion of production capacity related to chromatography, liquid cell culture media, buffers and cell culture powder media and single-use consumables at certain of the Company’s Biotechnology businesses and the development of diagnostics testing technologies and the expansion of testing production capacity at certain of the Company’s Diagnostics businesses. The Company’s businesses may enter into similar agreements in the future. In consideration of this financing, the U.S. government has certain rights, including rights with respect to the allocation of certain of the incremental production capacity associated with such expansion and/or rights in intellectual property produced with its financial assistance. The amount awarded pursuant to these grants in 2021 totaled $568 million and will be paid over periods ranging from one year to four years. In 2022, the Company received aggregate payments related to the BARDA grants and other government assistance of $137 million that offset operating expenses of $50 million and purchases of property, plant and equipment of $87 million. Property, plant and equipment purchased using funds provided by governments are recorded net of government assistance.
Financing Activities
Cash flows from financing activities consist primarily of cash flows associated with the issuance and repayments of commercial paper, issuance and repayment of long-term debt, borrowings under committed credit facilities, issuance and repurchases of common stock, issuance of preferred stock and payments of cash dividends to shareholders. Financing activities used cash of approximately $2.6 billion during 2022 compared to approximately $1.3 billion of cash provided during 2021. The year-over-year increase in cash used by financing activities was due primarily to net repayments of borrowings in 2022 compared to net proceeds from borrowings in 2021.
Total debt was approximately $19.7 billion and $22.2 billion as of December 31, 2022 and 2021, respectively, including notes payable and current portion of long-term debt was $591 million and $8 million as of December 31, 2022 and 2021, respectively. As of December 31, 2022, the Company had the ability to incur approximately $3.0 billion of additional indebtedness in direct borrowings or under the outstanding commercial paper facilities based on the amounts available under the Company’s $5.0 billion Five-Year Facility which were not being used to backstop outstanding commercial paper balances. As of December 31, 2022, the Company has classified approximately $2.0 billion of its borrowings outstanding under the euro-denominated commercial paper program as long-term debt in the Consolidated Balance Sheet as the Company has the intent and ability, as supported by availability under the Five-Year Facility, to refinance these borrowings for at least one year from the balance sheet date. As commercial paper obligations mature, the Company may issue additional short-term commercial paper obligations to refinance all or part of these borrowings, to the extent commercial paper markets are available.
Under the Company’s U.S. dollar and euro-denominated commercial paper program, the notes are typically issued at a discount from par, generally based on the ratings assigned to the Company by credit rating agencies at the time of the issuance and prevailing market rates measured by reference to the Secured Overnight Financing Rate or Euro Interbank Offer Rate, depending on the applicable currency of the borrowing.
Refer to Note 14 to the Consolidated Financial Statements for additional information regarding the Company’s financing activities and indebtedness, including the Company’s outstanding debt as of December 31, 2022, and the Company’s commercial paper program and Five-Year Facility.
Common Stock Offering and MCPS Offering
For a description of the 2020 Common Stock and MCPS Offerings, refer to Note 19 to the Consolidated Financial Statements.
Shelf Registration Statement
The Company has filed a “well-known seasoned issuer” shelf registration statement on Form S-3 with the SEC that registers an indeterminate amount of debt securities, common stock, preferred stock, warrants, depositary shares, purchase contracts and units for future issuance. The Company expects to use net proceeds realized by the Company from future securities sales off this shelf registration statement for general corporate purposes, including without limitation repayment or refinancing of debt or other corporate obligations, acquisitions, capital expenditures, share repurchases, dividends and/or working capital.
Stock Repurchase Program
Please see Note 19 to the Consolidated Financial Statements for a description of the Company’s stock repurchase program.
Dividends
The Company declared a regular quarterly dividend of $0.25 per share of Company common stock that was paid on January 27, 2023 to holders of record on December 30, 2022. In addition, the Company declared a quarterly cash dividend of $12.50 per MCPS Series B that was paid on January 15, 2023 to holders of record as of December 31, 2022. Aggregate 2022 and 2021 cash payments for dividends on Company common stock were $693 million and $578 million, respectively, and aggregate 2022 and 2021 cash payments for the dividends on the Company’s MCPS were $125 million and $164 million, respectively. The year-over-year increase in dividend payments in 2022 primarily relates to an increase in the quarterly dividend rate on common stock effective with respect to the dividend paid in the second quarter of 2022, partially offset by lower dividends paid on the MCPS Series A as a result of their conversion into common shares in April 2022.
Cash and Cash Requirements
As of December 31, 2022, the Company held approximately $6.0 billion of cash and cash equivalents that were on deposit with financial institutions or invested in highly liquid investment-grade debt instruments with a maturity of 90 days or less with an approximate weighted average annual interest rate of 2.09%. Of the cash and cash equivalents, approximately $3.4 billion was held within the United States and approximately $2.6 billion was held outside of the United States. The Company will continue to have cash requirements to support general corporate purposes, which may include working capital needs, capital expenditures, acquisitions and investments, paying interest and servicing debt, paying taxes and any related interest or penalties,
funding its restructuring activities and pension plans as required, paying dividends to shareholders, repurchasing shares of the Company’s common stock and supporting other business needs.
The Company generally intends to use available cash and internally generated funds to meet these cash requirements, but in the event that additional liquidity is required, the Company may also borrow under its commercial paper programs (if available) or borrow under the Company’s Five-Year Facility, enter into new credit facilities and either borrow directly thereunder or use such credit facilities to backstop additional borrowing capacity under its commercial paper programs (if available) and/or access the capital markets. The Company also may from time to time seek to access the capital markets to take advantage of favorable interest rate environments or other market conditions.
While repatriation of some cash held outside the United States may be restricted by local laws, most of the Company’s foreign cash could be repatriated to the United States. Following enactment of the TCJA and the associated Transition Tax, in general, repatriation of cash to the United States can be completed with no incremental U.S. tax; however, repatriation of cash could subject the Company to non-U.S. taxes on distributions. The cash that the Company’s non-U.S. subsidiaries hold for indefinite reinvestment is generally used to finance non-U.S. operations and investments, including acquisitions. The income taxes, if any, applicable to such earnings including basis differences in our non-U.S. subsidiaries are not readily determinable. As of December 31, 2022, management believes that it has sufficient sources of liquidity to satisfy its cash needs, including its cash needs in the United States.
During 2022, the Company contributed $10 million to its U.S. defined benefit pension plans and $40 million to its non-U.S. defined benefit pension plans. During 2023, the Company’s cash contribution requirements for its U.S. and its non-U.S. defined benefit pension plans are forecasted to be approximately $10 million and $35 million, respectively. The ultimate amounts to be contributed depend upon, among other things, legal requirements, underlying asset returns, the plan’s funded status, the anticipated tax deductibility of the contribution, local practices, market conditions, interest rates and other factors.
Contractual and Other Obligations
For a description of the Company’s debt and lease obligations, commitments, and litigation and contingencies, refer to Notes 10, 14, 17 and 18 to the Consolidated Financial Statements.
Legal Proceedings
Refer to Note 18 to the Consolidated Financial Statements for information regarding legal proceedings and contingencies, and for a discussion of risks related to legal proceedings and contingencies, refer to “Item 1A. Risk Factors.”
CRITICAL ACCOUNTING ESTIMATES
Management’s discussion and analysis of the Company’s financial condition and results of operations is based upon the Company’s Consolidated Financial Statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. The Company bases these estimates and judgments on historical experience, the current economic environment and on various other assumptions that are believed to be reasonable under the circumstances. Actual results may differ materially from these estimates and judgments.
The Company believes the following accounting estimates are most critical to an understanding of its financial statements. Estimates are considered to be critical if they meet both of the following criteria: (1) the estimate requires assumptions about material matters that are uncertain at the time the estimate is made, and (2) material changes in the estimate are reasonably likely from period-to-period. For a detailed discussion on the application of these and other accounting estimates, refer to Note 1 to the Consolidated Financial Statements.
Acquired Intangibles—The Company’s business acquisitions, including the Cytiva and Aldevron acquisitions, typically result in the recognition of goodwill, developed technology and other intangible assets, which affect the amount of future period amortization expense and possible impairment charges that the Company may incur. The fair values of acquired intangibles are determined using information available near the acquisition date based on estimates and assumptions that are deemed reasonable by the Company. Significant assumptions include the discount rates and certain assumptions that form the basis of the forecasted results of the acquired business including earnings before interest, taxes, depreciation and amortization (“EBITDA”), revenue, revenue growth rates, royalty rates and technology obsolescence rates. These assumptions are forward looking and could be affected by future economic and market conditions. The Company engages third-party valuation specialists who review the Company’s critical assumptions and calculations of the fair value of acquired intangible assets in connection with significant acquisitions. In connection with acquisitions during the year ended December 31, 2022, the Company recognized aggregate goodwill of $427 million and intangible assets of $218 million. Refer to Notes 1, 2 and 11 to
the Consolidated Financial Statements for a description of the Company’s policies relating to goodwill, acquired intangibles and acquisitions.
In performing its goodwill impairment testing, the Company estimates the fair value of its reporting units primarily using a market-based approach which relies on current trading multiples of forecasted EBITDA for companies operating in businesses similar to each of the Company’s reporting units to calculate an estimated fair value of each reporting unit. In evaluating the estimates derived by the market-based approach, management makes judgments about the relevance and reliability of the multiples by considering factors unique to its reporting units, including operating results, business plans, economic projections, anticipated future cash flows, and transactions and marketplace data as well as judgments about the comparability of the market proxies selected. There are inherent uncertainties related to these assumptions and management’s judgment in applying them to the analysis of goodwill impairment.
As a result of the Company’s change to its reportable segments in the fourth quarter of 2022, the Company also changed its reporting units. As of December 31, 2022, the Company had eight reporting units for goodwill impairment testing. Reporting units resulting from recent acquisitions generally present the highest risk of impairment. Management believes the impairment risk associated with these reporting units generally decreases as these businesses are integrated into the Company and better positioned for potential future earnings growth. The Company performed the annual goodwill impairments analysis on both the prior five reporting units (that existed before the change in reportable segments) as well as the Company’s eight reporting units (that resulted from the change in reportable segments). The Company’s annual goodwill impairment analysis and the analysis after the change in the Company’s reporting units in 2022 indicated that in all instances, the fair values of the Company’s reporting units exceeded their carrying values and consequently did not result in an impairment charge. The excess of the estimated fair value over carrying value (expressed as a percentage of carrying value for the respective reporting unit) for each of the Company’s eight reporting units as of the testing date ranged from approximately 145% to approximately 565%. In the test of the prior five reporting units, the excess of the estimated fair value over carrying value for each of the previous reporting units as of the testing date also ranged from approximately 145% to approximately 565%. To evaluate the sensitivity of the fair value calculations used in both goodwill impairment tests, the Company applied a hypothetical 10% decrease to the fair values of each reporting unit and compared those hypothetical values to the reporting unit carrying values in both tests. Based on this hypothetical 10% decrease, the excess of the estimated fair value over carrying value (expressed as a percentage of carrying value for the respective reporting unit) for each of the Company’s reporting units ranged from approximately 120% to approximately 500%.
The Company reviews identified intangible assets for impairment whenever events or changes in circumstances indicate that the related carrying amounts may not be recoverable. Determining whether an impairment loss occurred for finite-lived intangibles requires a comparison of the carrying amount to the sum of undiscounted cash flows expected to be generated by the asset. These analyses require management to make judgments and estimates about future revenues, expenses, market conditions and discount rates related to these assets. Indefinite-lived intangibles are subject to impairment testing at least annually or more frequently if events or changes in circumstances indicate that potential impairment exists. Determining whether an impairment loss occurred for indefinite-lived intangible assets involves calculating the fair value of the indefinite-lived intangible assets and comparing the fair value to their carrying value. If the fair value is less than the carrying value, the difference is recorded as an impairment loss. Refer to Note 11 to the Consolidated Financial Statements for a description of intangible assets impairment charges recorded during 2022.
If actual results are not consistent with management’s estimates and assumptions, goodwill and other intangible assets may be overstated and a charge would need to be taken against net earnings which would adversely affect the Company’s financial statements.
Contingent Liabilities—As discussed in “Item 3. Legal Proceedings” and Notes 8 and 18 to the Consolidated Financial Statements, the Company is, from time to time, subject to a variety of litigation and similar contingent liabilities incidental to its business (or the business operations of previously owned entities). The Company recognizes a liability for any legal contingency or contract settlement expense that is known or probable of occurrence and reasonably estimable. These assessments require judgments concerning matters such as litigation developments and outcomes, the anticipated outcome of negotiations, the number of future claims, the cost of both pending and future claims and the value of the elements in the outcome. In addition, because most contingencies are resolved over long periods of time, liabilities may change in the future due to various factors, including those discussed in Note 18 to the Consolidated Financial Statements. If the reserves established by the Company with respect to these contingent liabilities are inadequate, the Company would be required to incur an expense equal to the amount of the loss incurred in excess of the reserves, which would adversely affect the Company’s financial statements.
Income Taxes—For a description of the Company’s income tax accounting policies, refer to Notes 1 and 7 to the Consolidated Financial Statements. The Company establishes valuation allowances for its deferred tax assets if it is more likely than not that
some or all of the deferred tax asset will not be realized. This requires management to make judgments and estimates regarding: (1) the timing and amount of the reversal of taxable temporary differences, (2) expected future taxable income and (3) the impact of tax planning strategies. Future changes to tax rates would also impact the amounts of deferred tax assets and liabilities and could have an adverse impact on the Company’s financial statements.
The Company provides for unrecognized tax benefits when, based upon the technical merits, it is “more likely than not” that an uncertain tax position will not be sustained upon examination. Judgment is required in evaluating tax positions and determining income tax provisions. The Company re-evaluates the technical merits of its tax positions and may recognize an uncertain tax benefit in certain circumstances, including when: (1) a tax audit is completed; (2) applicable tax laws change, including a tax case ruling or legislative guidance; or (3) the applicable statute of limitations expires.
In addition, certain of the Company’s tax returns are currently under review by tax authorities including in Denmark and the United States (refer to “—Results of Operations—Income Taxes” and Note 7 to the Consolidated Financial Statements). Management believes the positions taken in these returns are in accordance with the relevant tax laws. However, the outcome of these audits is uncertain and could result in the Company being required to record charges for prior year tax obligations which could have a material adverse impact to the Company’s financial statements, including its effective tax rate.
An increase of 1.0% in the Company’s 2022 nominal tax rate would have resulted in an additional income tax provision for continuing operations for the year ended December 31, 2022 of $83 million.
Valuation of Investments in Equity Securities—For a description of the Company’s investments in equity securities and partnerships refer to Notes 1, 9 and 12 to the Consolidated Financial Statements. The Company invests in publicly-traded securities, non-marketable securities of early-stage companies and equity method investments, including partnerships that invest primarily in early-stage companies.
Investments in early-stage companies have significant risks, including uncertainty regarding the investee company’s ability to successfully develop new technologies and services, bring these new technologies and services to market and gain market acceptance, maintain adequate capitalization and access to cash or other forms of liquidity, and retain critical management personnel. Refer to “Item 1A. Risk Factors” for a further discussion of the risks related to investing in early-stage companies.
The Company’s investments in publicly traded securities are measured at fair value based on quotes in active markets. For investments in non-marketable equity securities where the Company does not have influence over the investee, the Company has elected the measurement alternative and records these investments at cost and adjusts the carrying value for impairments and observable price changes with a same or similar security from the same issuer adjusted to reflect the specific rights and preferences of the securities, if applicable. Valuations of non-marketable equity securities are complex and require judgment due to the absence of market prices, lack of liquidity and the risks inherent in early-stage companies. The uncertainty in the process of valuing securities for which a ready market does not exist may cause our estimated values of these securities to differ significantly from the values that would have been derived had a ready market for the securities existed, and those differences could be material.
The Company accounts for its investments in the partnerships using the equity method. Accordingly, the investments are initially recorded at cost and adjusted each period for the Company’s share of the partnership’s income or loss and distributions received. The partnerships’ investments are recorded by the partnerships on an estimated fair value basis and pose the same risks and require the same valuation judgments discussed above. As a result, changes in the value of investments in the partnership will have a direct impact on the Company’s earnings. Impairment losses are recognized to reduce the investment’s carrying value to its fair value if there is a decline in fair value below carrying value that is considered to be other-than-temporary. To determine whether there is an other-than-temporary impairment, the Company uses qualitative and quantitative valuation methods.
Realized and unrealized gains and losses for these investments in equity securities and partnerships are recorded in other income (expense), net, in the Consolidated Statements of Earnings. A 10% decrease in the carrying value of the Company’s investments in equity securities and partnerships as of December 31, 2022 would result in a loss of approximately $180 million.
NEW ACCOUNTING STANDARDS
For a discussion of the new accounting standards impacting the Company, refer to Note 1 to the Consolidated Financial Statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The information required by this item is included under “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Report of Management on Danaher Corporation’s Internal Control Over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Securities Exchange Act of 1934.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2022. In making this assessment, the Company’s management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in “Internal Control-Integrated Framework” (2013 framework). Based on this assessment, management concluded that, as of December 31, 2022, the Company’s internal control over financial reporting is effective.
The Company’s independent registered public accounting firm has issued an audit report on the effectiveness of the Company’s internal control over financial reporting. This report dated February 22, 2023 appears on page 60 of this Form 10-K.
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Danaher Corporation
Opinion on Internal Control over Financial Reporting
We have audited Danaher Corporation and subsidiaries’ internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Danaher Corporation and subsidiaries (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2022, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2022 and 2021, the related consolidated statements of earnings, comprehensive income, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2022, and the related notes and financial statement schedule listed in the Index at Item 15(a) and our report dated February 22, 2023 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Report of Management on Danaher Corporation’s Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Tysons, Virginia
February 22, 2023
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Danaher Corporation
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Danaher Corporation and subsidiaries (the Company) as of December 31, 2022 and 2021, the related consolidated statements of earnings, comprehensive income, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2022, and the related notes and financial statement schedule listed in the Index at Item 15(a) (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2022, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated February 22, 2023 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
| | | | | |
| Uncertain Tax Positions |
Description of the Matter | As discussed in Note 7 to the consolidated financial statements, the Company operates in the U.S. and multiple international tax jurisdictions and as a result files numerous tax returns in those locations. Uncertainty in a tax position may arise for multiple reasons, including because tax laws are subject to interpretation. The Company applies the applicable tax law and judgment to (1) determine whether, based on the technical merits, a tax position is more likely than not to be sustained and (2) measure the amount of tax benefit that qualifies for recognition. As of December 31, 2022, the Company’s gross unrecognized tax benefits related to uncertain tax positions were approximately $1.1 billion. Auditing the recognition and measurement of certain of the Company’s tax positions including the evaluation of whether such tax position is more likely than not to be sustained, and if applicable the measurement of the benefit, is complex and required the use of tax subject matter resources. |
How We Addressed the Matter in Our Audit | We tested controls over management’s accounting for tax positions, including assessment of the technical merits of tax positions and if applicable, the measurement of the benefit of the tax position. To evaluate whether the technical merits of certain of the Company’s income tax positions are more likely than not sustainable, our audit procedures included, among others, evaluation of applicable tax law, court cases, tax regulations and other regulatory guidance by our tax subject matter resources. For certain of the income tax positions, we also involved tax subject matter resources in corroborating our understanding of the relevant facts, examining the Company’s analysis, evaluating relevant correspondence with the tax authority and reading third-party advice obtained by management, as applicable. We also evaluated the adequacy of the Company’s disclosures included in Note 7 to the consolidated financial statements. |
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2002.
Tysons, Virginia
February 22, 2023
DANAHER CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
($ in millions, except per share amount)
| | | | | | | | | | | |
| | As of December 31 |
| | 2022 | | 2021 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and equivalents | $ | 5,995 | | | $ | 2,586 | |
Trade accounts receivable, less allowance for doubtful accounts of $126 as of December 31, 2022 and $124 as of December 31, 2021 | 4,918 | | | 4,631 | |
| Inventories | 3,110 | | | 2,767 | |
| Prepaid expenses and other current assets | 1,860 | | | 1,664 | |
| | | |
| Total current assets | 15,883 | | | 11,648 | |
| Property, plant and equipment, net | 3,956 | | | 3,790 | |
| Other long-term assets | 4,459 | | | 3,719 | |
| Goodwill | 39,752 | | | 41,184 | |
| Other intangible assets, net | 20,300 | | | 22,843 | |
| | | |
| Total assets | $ | 84,350 | | | $ | 83,184 | |
| | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | |
| Current liabilities: | | | |
| Notes payable and current portion of long-term debt | $ | 591 | | | $ | 8 | |
| Trade accounts payable | 2,296 | | | 2,569 | |
| Accrued expenses and other liabilities | 5,502 | | | 5,563 | |
| | | |
| Total current liabilities | 8,389 | | | 8,140 | |
| Other long-term liabilities | 6,785 | | | 7,699 | |
| Long-term debt | 19,086 | | | 22,168 | |
| | | |
| Stockholders’ equity: | | | |
Preferred stock, no par value, 15.0 million shares authorized; no shares and 1.65 million shares of 4.75% Mandatory Convertible Preferred Stock, Series A, issued and outstanding as of December 31, 2022 and December 31, 2021, respectively; 1.72 million shares of 5.00% Mandatory Convertible Preferred Stock, Series B, issued and outstanding as of December 31, 2022 and December 31, 2021 | 1,668 | | | 3,268 | |
Common stock - $0.01 par value, 2.0 billion shares authorized; 869.3 million issued and 728.3 million outstanding as of December 31, 2022; 855.7 million issued and 715.0 million outstanding as of December 31, 2021 | 9 | | | 9 | |
| Additional paid-in capital | 12,072 | | | 10,090 | |
| Retained earnings | 39,205 | | | 32,827 | |
| Accumulated other comprehensive income (loss) | (2,872) | | | (1,027) | |
| Total Danaher stockholders’ equity | 50,082 | | | 45,167 | |
| Noncontrolling interests | 8 | | | 10 | |
| Total stockholders’ equity | 50,090 | | | 45,177 | |
| Total liabilities and stockholders’ equity | $ | 84,350 | | | $ | 83,184 | |
See the accompanying Notes to the Consolidated Financial Statements.
DANAHER CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EARNINGS
($ and shares in millions, except per share amounts)
| | | | | | | | | | | | | | | | | | |
| | Year Ended December 31 | |
| | 2022 | | 2021 | | 2020 | |
| Sales | $ | 31,471 | | | $ | 29,453 | | | $ | 22,284 | | |
| Cost of sales | (12,522) | | | (11,501) | | | (9,809) | | |
| Gross profit | 18,949 | | | 17,952 | | | 12,475 | | |
| Operating costs: | | | | | | |
| Selling, general and administrative expenses | (8,516) | | | (8,198) | | | (6,896) | | |
| Research and development expenses | (1,745) | | | (1,742) | | | (1,348) | | |
| Other operating expenses | — | | | (547) | | | — | | |
| Operating profit | 8,688 | | | 7,465 | | | 4,231 | | |
| Nonoperating income (expense): | | | | | | |
| Other income (expense), net | (226) | | | 456 | | | 494 | | |
| | | | | | |
| Loss on early extinguishment of borrowings | — | | | (96) | | | (26) | | |
| Interest expense | (211) | | | (238) | | | (275) | | |
| Interest income | 41 | | | 11 | | | 71 | | |
| Earnings from continuing operations before income taxes | 8,292 | | | 7,598 | | | 4,495 | | |
| Income taxes | (1,083) | | | (1,251) | | | (849) | | |
| Net earnings from continuing operations | 7,209 | | | 6,347 | | | 3,646 | | |
| Earnings from discontinued operations, net of income taxes | — | | | 86 | | | — | | |
| Net earnings | 7,209 | | | 6,433 | | | 3,646 | | |
| Mandatory convertible preferred stock dividends | (106) | | | (164) | | | (136) | | |
| Net earnings attributable to common stockholders | $ | 7,103 | | | $ | 6,269 | | | $ | 3,510 | | |
| | | | | | |
| Net earnings per common share from continuing operations: | | | | | | |
| Basic | $ | 9.80 | | | $ | 8.65 | | | $ | 4.97 | | |
| Diluted | $ | 9.66 | | | $ | 8.50 | | | $ | 4.89 | | |
| Net earnings per common share from discontinued operations: | | | | | | |
| Basic | $ | — | | | $ | 0.12 | | | $ | — | | |
| Diluted | $ | — | | | $ | 0.12 | | | $ | — | | |
| Net earnings per common share: | | | | | | |
| Basic | $ | 9.80 | | | $ | 8.77 | | | $ | 4.97 | | |
| Diluted | $ | 9.66 | | | $ | 8.61 | | * | $ | 4.89 | | |
| Average common stock and common equivalent shares outstanding: | | | | | | |
| Basic | 725.1 | | | 714.6 | | | 706.2 | | |
| Diluted | 737.1 | | | 736.8 | | | 718.7 | | |
* Net earnings per common share amount does not add due to rounding.
See the accompanying Notes to the Consolidated Financial Statements.
DANAHER CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
($ in millions)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31 |
| 2022 | | 2021 | | 2020 |
| Net earnings | $ | 7,209 | | | $ | 6,433 | | | $ | 3,646 | |
| Other comprehensive income (loss), net of income taxes: | | | | | |
| Foreign currency translation adjustments | (2,105) | | | (1,284) | | | 2,919 | |
| Pension and postretirement plan benefit adjustments | 209 | | | 378 | | | (147) | |
| | | | | |
| Cash flow hedge adjustments | 51 | | | 247 | | | (72) | |
| Total other comprehensive income (loss), net of income taxes | (1,845) | | | (659) | | | 2,700 | |
| Comprehensive income | $ | 5,364 | | | $ | 5,774 | | | $ | 6,346 | |
See the accompanying Notes to the Consolidated Financial Statements.
DANAHER CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
($ in millions)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31 |
| 2022 | | 2021 | | 2020 |
| Preferred stock: | | | | | |
| Balance, beginning of period | $ | 3,268 | | | $ | 3,268 | | | $ | 1,600 | |
| Issuance of Mandatory Convertible Preferred Stock | — | | | — | | | 1,668 | |
| Conversion of Mandatory Convertible Preferred Stock to common stock | (1,600) | | | — | | | — | |
| Balance, end of period | $ | 1,668 | | | $ | 3,268 | | | $ | 3,268 | |
| Common stock: | | | | | |
| Balance, beginning of period | $ | 9 | | | $ | 9 | | | $ | 8 | |
| Common stock-based award activity | — | | | — | | | 1 | |
| | | | | |
| Balance, end of period | $ | 9 | | | $ | 9 | | | $ | 9 | |
| Additional paid-in capital: | | | | | |
| Balance, beginning of period | $ | 10,090 | | | $ | 9,698 | | | $ | 7,565 | |
| Common stock-based award activity | 396 | | | 335 | | | 351 | |
| Common stock issued in connection with Mandatory Convertible Preferred Stock conversions | 1,600 | | | — | | | — | |
| Common stock issued in connection with acquisitions | — | | | 23 | | | — | |
| Common stock issued in connection with LYONs’ conversions | — | | | 34 | | | 53 | |
| Issuance of common stock | — | | | — | | | 1,729 | |
| Acquisition of noncontrolling interests | (14) | | | — | | | — | |
| | | | | |
| Balance, end of period | $ | 12,072 | | | $ | 10,090 | | | $ | 9,698 | |
| Retained earnings: | | | | | |
| Balance, beginning of period | $ | 32,827 | | | $ | 27,159 | | | $ | 24,166 | |
| Adoption of accounting standards | — | | | — | | | (8) | |
| Net earnings | 7,209 | | | 6,433 | | | 3,646 | |
| Common stock dividends declared | (725) | | | (601) | | | (509) | |
| Mandatory Convertible Preferred Stock dividends declared | (106) | | | (164) | | | (136) | |
| | | | | |
| Balance, end of period | $ | 39,205 | | | $ | 32,827 | | | $ | 27,159 | |
| Accumulated other comprehensive income (loss): | | | | | |
| Balance, beginning of period | $ | (1,027) | | | $ | (368) | | | $ | (3,068) | |
| | | | | |
| Other comprehensive income (loss) | (1,845) | | | (659) | | | 2,700 | |
| Balance, end of period | $ | (2,872) | | | $ | (1,027) | | | $ | (368) | |
| Noncontrolling interests: | | | | | |
| Balance, beginning of period | $ | 10 | | | $ | 11 | | | $ | 11 | |
| | | | | |
| Change in noncontrolling interests | (2) | | | (1) | | | — | |
| Balance, end of period | $ | 8 | | | $ | 10 | | | $ | 11 | |
| Total stockholders’ equity, end of period | $ | 50,090 | | | $ | 45,177 | | | $ | 39,777 | |
See the accompanying Notes to the Consolidated Financial Statements.
DANAHER CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
($ in millions)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31 |
| | 2022 | | 2021 | | 2020 |
| Cash flows from operating activities: | | | | | |
| Net earnings | $ | 7,209 | | | $ | 6,433 | | | $ | 3,646 | |
| Less: earnings from discontinued operations, net of income taxes | — | | | (86) | | | — | |
| Net earnings from continuing operations | 7,209 | | | 6,347 | | | 3,646 | |
| Noncash items: | | | | | |
| Depreciation | 738 | | | 718 | | | 637 | |
| Amortization of intangible assets | 1,484 | | | 1,450 | | | 1,138 | |
| Amortization of acquisition-related inventory fair value step-up | — | | | 59 | | | 457 | |
| Stock-based compensation expense | 336 | | | 218 | | | 187 | |
| Contract settlement expense | — | | | 542 | | | — | |
| | | | | |
| Pretax loss on early extinguishment of borrowings | — | | | 96 | | | 26 | |
| Pretax gain on sale of product lines and investment (gains) losses | 271 | | | (414) | | | (455) | |
| Change in deferred income taxes | (559) | | | (229) | | | 518 | |
| Change in trade accounts receivable, net | (477) | | | (611) | | | (264) | |
| Change in inventories | (486) | | | (502) | | | (123) | |
| Change in trade accounts payable | 5 | | | 549 | | | 227 | |
| Change in prepaid expenses and other assets | (78) | | | (4) | | | 102 | |
| Change in accrued expenses and other liabilities | 76 | | | 139 | | | 119 | |
| Total operating cash provided by continuing operations | 8,519 | | | 8,358 | | | 6,215 | |
| Total operating cash used in discontinued operations | — | | | — | | | (7) | |
| Net cash provided by operating activities | 8,519 | | | 8,358 | | | 6,208 | |
| Cash flows from investing activities: | | | | | |
| Cash paid for acquisitions | (637) | | | (10,961) | | | (20,971) | |
| Payments for additions to property, plant and equipment | (1,152) | | | (1,294) | | | (791) | |
| Proceeds from sales of property, plant and equipment | 9 | | | 13 | | | 2 | |
| Payments for purchases of investments | (523) | | | (934) | | | (342) | |
| Proceeds from sales of investments | 18 | | | 126 | | | 13 | |
| Proceeds from sale of product lines | — | | | 26 | | | 826 | |
| All other investing activities | 51 | | | 37 | | | 24 | |
| | | | | |
| | | | | |
| Net cash used in investing activities for continuing operations | (2,234) | | | (12,987) | | | (21,239) | |
| Cash flows from financing activities: | | | | | |
| Proceeds from the issuance of common stock in connection with stock-based compensation | 31 | | | 86 | | | 153 | |
| Proceeds from the public offering of common stock, net of issuance costs | — | | | — | | | 1,729 | |
| Proceeds from the public offering of preferred stock, net of issuance costs | — | | | — | | | 1,668 | |
| | | | | |
| Payment of dividends | (818) | | | (742) | | | (615) | |
| | | | | |
| Net (repayments of) proceeds from borrowings (maturities of 90 days or less) | (723) | | | 2,265 | | | (4,637) | |
| Proceeds from borrowings (maturities longer than 90 days) | — | | | 984 | | | 8,670 | |
| Repayments of borrowings (maturities longer than 90 days) | (965) | | | (1,186) | | | (5,933) | |
| Make-whole premiums to redeem borrowings prior to maturity | — | | | (96) | | | (26) | |
| All other financing activities | (95) | | | (16) | | | (3) | |
| | | | | |
| | | | | |
| Net cash (used in) provided by financing activities for continuing operations | (2,570) | | | 1,295 | | | 1,006 | |
| Effect of exchange rate changes on cash and equivalents | (306) | | | (115) | | | 148 | |
| Net change in cash and equivalents | 3,409 | | | (3,449) | | | (13,877) | |
| Beginning balance of cash and equivalents | 2,586 | | | 6,035 | | | 19,912 | |
| Ending balance of cash and equivalents | $ | 5,995 | | | $ | 2,586 | | | $ | 6,035 | |
| | | | | |
| | | | | |
| | | | | |
See the accompanying Notes to the Consolidated Financial Statements.
DANAHER CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. BUSINESS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business—Danaher Corporation (“Danaher” or the “Company”) designs, manufactures and markets professional, medical, industrial and commercial products and services, which are typically characterized by strong brand names, innovative technology and major market positions. As of December 31, 2022, the Company operates in four business segments:
•The Biotechnology segment includes the bioprocessing and discovery and medical businesses and offers a broad range of tools, consumables and services that are primarily used by customers to advance and accelerate the research, development, manufacture and delivery of biological medicines. The biotherapeutics that the Company’s solutions support range from replacement therapies such as insulin, vaccines, recombinant proteins and other biologic drugs, to novel cell, gene, mRNA and other nucleic acid therapies.
•The Life Sciences segment offers a broad range of instruments and consumables that are primarily used by customers to study the basic building blocks of life, including DNA and RNA, nucleic acid, proteins, metabolites and cells, in order to understand the causes of disease, identify new therapies, and test and manufacture new drugs, vaccines and gene editing technologies. Additionally, the segment provides products and consumables used to filter and remove contaminants from a variety of liquids and gases in many end-market applications.
•The Diagnostics segment offers clinical instruments, reagents, consumables, software and services that hospitals, physicians’ offices, reference laboratories and other critical care settings use to diagnose disease and make treatment decisions.
•The Environmental & Applied Solutions segment offers products and services that help protect precious resources and keep global food and water supplies safe. The Company’s water quality business provides instrumentation, consumables, software, services and disinfection systems to help analyze, treat and manage the quality of ultra-pure, potable, industrial, waste, ground, source and ocean water in residential, commercial, municipal, industrial and natural resource applications. The Company’s product identification business provides instruments, software, services and consumables for various color and appearance management, packaging design and quality management, packaging converting, printing, marking, coding and traceability applications for consumer, pharmaceutical and industrial products.
Refer to Notes 2 and 3 for a discussion of significant acquisitions, discontinued operations and other dispositions.
Accounting Principles—The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The Consolidated Financial Statements include the accounts of the Company and its subsidiaries. All intercompany balances and transactions have been eliminated upon consolidation. The Consolidated Financial Statements also reflect the impact of noncontrolling interests. Noncontrolling interests do not have a significant impact on the Company’s consolidated results of continuing operations, therefore earnings attributable to noncontrolling interests for continuing operations are not presented separately in the Company’s Consolidated Statements of Earnings. Earnings attributable to noncontrolling interests have been reflected in selling, general and administrative expenses and were insignificant in all periods presented. Reclassifications of certain prior year amounts have been made to conform to the current year presentation.
Use of Estimates—The preparation of these financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. The Company bases these estimates on historical experience, the current economic environment and on various other assumptions that are believed to be reasonable under the circumstances. However, uncertainties associated with these estimates exist and actual results may differ materially from these estimates.
Cash and Equivalents—The Company considers all highly liquid investments with a maturity of three months or less at the date of purchase to be cash equivalents.
Accounts Receivable and Allowances for Doubtful Accounts—All trade accounts, contract and finance receivables are reported on the accompanying Consolidated Balance Sheets adjusted for any write-offs and net of allowances for doubtful accounts. The allowances for doubtful accounts represent management’s best estimate of the expected future credit losses from the Company’s trade accounts, contract and finance receivable portfolios. Determination of the allowances requires management to exercise judgment about the timing, frequency and severity of credit losses that could materially affect the provision for credit losses and, therefore, net earnings. The Company regularly performs detailed reviews of its portfolios to determine if an
impairment has occurred and evaluates the collectability of receivables based on a combination of various financial and qualitative factors that may affect customers’ ability to pay, including customers’ financial condition, collateral, debt-servicing ability, past payment experience and credit bureau information. In circumstances where the Company is aware of a specific customer’s inability to meet its financial obligations, a specific reserve is recorded against amounts due to reduce the recognized receivable to the amount reasonably expected to be collected. Additions to the allowances for doubtful accounts are charged to current period earnings, amounts determined to be uncollectible are charged directly against the allowances, while amounts recovered on previously written-off accounts increase the allowances. If the financial condition of the Company’s customers were to deteriorate, resulting in an impairment of their ability to make payments, additional reserves would be required. The Company does not believe that trade accounts receivable represents significant concentrations of credit risk because of the diversified portfolio of individual customers and geographical areas. On January 1, 2020, the Company adopted Accounting Standards Update (“ASU”) No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, using the modified retrospective transition method and recorded a net increase to the allowance for doubtful accounts of $10 million due to the cumulative impact of adoption. The Company’s allowance for doubtful accounts as of December 31, 2022 reflects the Company’s best estimate of the expected future losses for its accounts receivables; however, these estimates may change and future actual losses may differ from the Company’s estimates. The Company will continue to monitor economic conditions and will revise the estimates of the expected future losses for accounts receivable as necessary. The Company recorded $29 million, $31 million and $31 million of expense associated with doubtful accounts for the years ended December 31, 2022, 2021 and 2020, respectively.
Included in the Company’s trade accounts receivable and other long-term assets as of December 31, 2022 and 2021 are $254 million and $247 million of net aggregate financing receivables, respectively. All financing receivables are evaluated for impairment based on individual customer credit profiles.
Inventories—Inventories include the costs of material, labor and overhead. Inventories are stated at the lower of cost and net realizable value primarily using the first-in, first-out method.
The classes of inventory as of December 31 are summarized as follows ($ in millions):
| | | | | | | | | | | |
| 2022 | | 2021 |
| Finished goods | $ | 1,504 | | | $ | 1,343 | |
| Work in process | 473 | | | 473 | |
| Raw materials | 1,133 | | | 951 | |
| Total | $ | 3,110 | | | $ | 2,767 | |
Prepaid Expenses and Other Current Assets—Prepaid expenses and other current assets primarily result from advance payments to vendors for good and services and are capitalized until the related goods are received or services are performed and advanced payments to tax authorities. Included in the Company’s prepaid expenses and other current assets as of December 31, 2022 and 2021 are prepaid expenses of $802 million and $770 million, respectively, and taxes receivable for income and other taxes of $962 million and $812 million, respectively.
Property, Plant and Equipment—Property, plant and equipment are carried at cost. The provision for depreciation has been computed principally by the straight-line method based on the estimated useful lives of the depreciable assets as follows:
| | | | | | | | |
| Category | | Useful Life |
| Buildings | | 30 years |
| Leased assets and leasehold improvements | | Amortized over the lesser of the economic life of the asset or
the term of the lease |
| Machinery and equipment | | 3 – 10 years |
| Customer-leased instruments | | 5 – 7 years |
Estimated useful lives are periodically reviewed and, when appropriate, changes to estimates are made prospectively.
The classes of property, plant and equipment as of December 31 are summarized as follows ($ in millions):
| | | | | | | | | | | |
| 2022 | | 2021 |
| Land and improvements | $ | 216 | | | $ | 203 | |
| Buildings | 1,994 | | | 1,676 | |
| Machinery and equipment | 3,935 | | | 3,610 | |
| Customer-leased equipment | 1,704 | | | 1,766 | |
| Gross property, plant and equipment | 7,849 | | | 7,255 | |
| Less: accumulated depreciation | (3,893) | | | (3,465) | |
| Property, plant and equipment, net | $ | 3,956 | | | $ | 3,790 | |
Investments—Investments over which the Company has a significant influence but not a controlling interest, are accounted for using the equity method of accounting which requires the Company to record its initial investment at cost and adjust the balance each period for the Company’s share of the investee’s income or loss and dividends paid. The Company also invests in start-up companies where the Company has neither control of nor significant influence over the investee. The Company measures these non-marketable equity securities at fair value and recognizes changes in fair value in net earnings. For securities without readily available fair values, the Company has elected the measurement alternative to record these investments at cost and to adjust for impairments and observable price changes with a same or similar security from the same issuer within net earnings (the “Fair Value Alternative”). Additionally, the Company is a limited partner in partnerships that invest in start-up companies. While the partnerships record these investments at fair value, the Company’s investment in the partnerships is accounted for under the equity method of accounting. The Company made minority investments in equity method investments and non-marketable equity securities totaling $523 million, $934 million and $342 million in 2022, 2021 and 2020, respectively, including investments in partnerships of $283 million, $662 million and $172 million in 2022, 2021 and 2020, respectively. The Company recorded net realized and unrealized gains and losses related to changes in the fair value of these investments, as well as an impairment to an equity-method investment in other income (expense), net, in the Consolidated Statements of Earnings. Refer to Notes 9 and 12 for additional information about the Company’s investments.
Other Assets—Other assets principally include noncurrent financing receivables, noncurrent deferred tax assets and other investments.
Fair Value of Financial Instruments—The Company’s financial instruments consist primarily of cash and cash equivalents, trade accounts receivable, investments in equity securities, available-for-sale debt securities and cross-currency swaps, obligations under trade accounts payable and short and long-term debt. Due to their short-term nature, the carrying values for cash and cash equivalents, trade accounts receivable and trade accounts payable approximate fair value. Refer to Note 12 for the fair values of the Company’s investments in equity securities, available-for-sale debt securities and cross-currency swaps and other obligations.
Goodwill and Other Intangible Assets—Goodwill and other intangible assets result from the Company’s acquisition of existing businesses. In accordance with accounting standards related to business combinations, goodwill is not amortized; however, certain finite-lived identifiable intangible assets, primarily customer relationships and acquired technology, are amortized over their estimated useful lives. Intangible assets with indefinite lives are not amortized. In-process research and development (“IPR&D”) is initially capitalized at fair value and when the IPR&D project is complete, the asset is considered a finite-lived intangible asset and amortized over its estimated useful life. If an IPR&D project is abandoned, an impairment loss equal to the value of the intangible asset is recorded in the period of abandonment. The Company reviews identified intangible assets and goodwill for impairment whenever events or changes in circumstances indicate that the related carrying amounts may not be recoverable. The Company also tests intangible assets with indefinite lives and goodwill for impairment at least annually. Refer to Notes 2 and 11 for additional information about the Company’s goodwill and other intangible assets.
Revenue Recognition—The Company derives revenues primarily from the sale of Biotechnology, Life Sciences, Diagnostics and Environmental & Applied Solutions products and services. Revenue is recognized when control of the promised products or services is transferred to the Company’s customers, in an amount that reflects the consideration the Company expects to be entitled to in exchange for those products or services (the transaction price). A performance obligation is a promise in a contract to transfer a distinct product or service to a customer and is the unit of account under Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers. For equipment and consumables sold by the Company, control transfers to the customer at a point in time. To indicate the transfer of control, the Company must have a present right to payment, legal title must have passed to the customer, the customer must have the significant risks and rewards of ownership, and where acceptance is not a formality, the customer must have accepted the product or service. The Company’s principal terms of sale are Free On Board (“FOB”) Shipping Point, or equivalent, and, as such, the Company primarily transfers control
and records revenue for product sales upon shipment. Sales arrangements with delivery terms that are not FOB Shipping Point are not recognized upon shipment and the transfer of control for revenue recognition is evaluated based on the associated shipping terms and customer obligations. If a performance obligation to the customer with respect to a sales transaction remains to be fulfilled following shipment (typically installation or acceptance by the customer), revenue recognition for that performance obligation is deferred until such commitments have been fulfilled. Returns for products sold are estimated and recorded as a reduction of revenue at the time of sale. Customer allowances and rebates, consisting primarily of volume discounts and other short-term incentive programs, are recorded as a reduction of revenue at the time of sale because these allowances reflect a reduction in the transaction price. Product returns, customer allowances and rebates are estimated based on historical experience and known trends. For extended warranty and service, control transfers to the customer over the term of the arrangement. Revenue for extended warranty and service is recognized based upon the period of time elapsed under the arrangement. Revenue for other long-term contracts is generally recognized based upon the cost-to-cost measure of progress, provided that the Company meets the criteria associated with transferring control of the good or service over time.
Certain of the Company’s revenues relate to operating-type lease (“OTL”) arrangements. Leases are outside the scope of ASC 606 and are therefore accounted for in accordance with ASC 842, Leases. Equipment lease revenue for OTL agreements is recognized on a straight-line basis over the life of the lease, and the cost of customer-leased equipment is recorded within property, plant and equipment in the accompanying Consolidated Balance Sheets and depreciated over the equipment’s estimated useful life. Depreciation expense associated with the leased equipment under OTL arrangements is reflected in cost of sales in the accompanying Consolidated Statements of Earnings. The OTLs are generally not cancellable until after an initial term and may or may not require the customer to purchase a minimum number of consumables or tests throughout the contract term. The Company also enters into sales-type lease (“STL”) arrangements with customers which result in earlier recognition of equipment lease revenue as compared to an OTL.
For a contract with multiple performance obligations, the Company allocates the contract’s transaction price to each performance obligation on a relative standalone selling price basis using the Company’s best estimate of the standalone selling price of each distinct product or service in the contract. The primary method used to estimate standalone selling price is the price observed in standalone sales to customers. Allocation of the transaction price is determined at the contracts’ inception.
Shipping and Handling—Shipping and handling costs are included as a component of cost of sales. Revenue derived from shipping and handling costs billed to customers is included in sales.
Advertising—Advertising costs are expensed as incurred.
Research and Development—The Company conducts research and development activities for the purpose of developing new products, enhancing the functionality, effectiveness, ease of use and reliability of the Company’s existing products and expanding the applications for which uses of the Company’s products are appropriate. Research and development costs are expensed as incurred.
Income Taxes—The Company’s income tax expense represents the tax liability for the current year, the tax benefit or expense for the net change in deferred tax liabilities and assets during the year, as well as reserves for unrecognized tax benefits and return to provision adjustments. Deferred tax liabilities and assets are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted rates expected to be in effect during the year in which the differences reverse. Deferred tax assets generally represent items that can be used as a tax deduction or credit in the Company’s tax return in future years for which the tax benefit has already been reflected on the Company’s Consolidated Statements of Earnings. The Company establishes valuation allowances for its deferred tax assets if it is more likely than not that some or all of the deferred tax asset will not be realized. Deferred tax liabilities generally represent items that have already been taken as a deduction on the Company’s tax return but have not yet been recognized as an expense in the Company’s Consolidated Statements of Earnings. The effect on deferred tax assets and liabilities due to a change in tax rates is recognized in income tax expense in the period that includes the enactment date. The Company provides for unrecognized tax benefits when, based upon the technical merits, it is “more likely than not” that an uncertain tax position will not be sustained upon examination. Judgment is required in evaluating tax positions and determining income tax provisions. The Company re-evaluates the technical merits of its tax positions and may recognize an uncertain tax benefit in certain circumstances, including when: (1) a tax audit is completed; (2) applicable tax laws change, including a tax case ruling or legislative guidance; or (3) the applicable statute of limitations expires. The Company recognizes potential accrued interest and penalties associated with unrecognized tax positions in income tax expense. Refer to Note 7 for additional information.
Productivity Improvement and Restructuring—The Company periodically initiates productivity improvement and restructuring activities to appropriately position the Company’s cost base relative to prevailing economic conditions and associated customer demand as well as in connection with certain acquisitions. Costs associated with productivity improvement and restructuring actions can include one-time termination benefits and related charges in addition to facility closure, contract termination and
other related activities. The Company records the cost of the productivity improvement and restructuring activities when the associated liability is incurred.
Foreign Currency Translation—Exchange rate adjustments resulting from foreign currency transactions are recognized in net earnings, whereas effects resulting from the translation of financial statements are reflected as a component of accumulated other comprehensive income (loss) within stockholders’ equity. Assets and liabilities of subsidiaries operating outside the United States with a functional currency other than U.S. dollars are translated into U.S. dollars using year end exchange rates and income statement accounts are translated at weighted average rates. Net foreign currency transaction gains or losses were not material in any of the years presented. As discussed below, the Company uses its foreign currency-denominated debt and cross-currency swap arrangements whereby existing U.S. dollar-denominated borrowings are effectively converted to foreign currency borrowings to partially hedge its net investments in foreign operations against adverse movements in exchange rates.
Derivative Financial Instruments—The Company is neither a dealer nor a trader in derivative instruments. The Company has generally accepted the exposure to transactional exchange rate movements without using derivative instruments to manage this risk, although the Company from time to time partially hedges its net investments in foreign operations against adverse movements in exchange rates through foreign currency-denominated debt and cross-currency swaps. The Company periodically enters into foreign currency forward contracts to mitigate a portion of its foreign currency exchange risk and forward starting swaps to mitigate interest rate risk related to the Company’s debt. The Company also uses cross-currency swap derivative contracts to hedge long-term debt issuances in a foreign currency other than the functional currency of the borrower. When utilized, the derivative instruments are recorded on the Consolidated Balance Sheets as either an asset or liability measured at fair value. To the extent the derivative instrument qualifies as an effective hedge, changes in fair value are recognized in accumulated other comprehensive income (loss) in stockholders’ equity. Changes in the value of the foreign currency denominated debt and cross-currency swaps designated as hedges of the Company’s net investment in foreign operations based on spot rates are recognized in accumulated other comprehensive income (loss) in stockholders’ equity and offset changes in the value of the Company’s foreign currency denominated operations. Refer to Note 15 for additional information.
Accumulated Other Comprehensive Income (Loss)—Accumulated other comprehensive income (loss) refers to certain gains and losses that under U.S. GAAP are included in comprehensive income (loss) but are excluded from net earnings as these amounts are initially recorded as an adjustment to stockholders’ equity. Foreign currency translation adjustments are generally not adjusted for income taxes as they relate to indefinite investments in non-U.S. subsidiaries. Cash flow hedge adjustments reflect the gains or losses on the derivative contract designated as the hedging instrument. Pension and postretirement plan benefit adjustments relate to unrecognized prior service credits and actuarial losses. Refer to Notes 15, 16 and 19 for additional information.
Accounting for Stock-Based Compensation—The Company accounts for stock-based compensation by measuring the cost of employee services received in exchange for all equity awards granted, including stock options, restricted stock units (“RSUs”) and performance stock units (“PSUs”), based on the fair value of the award as of the grant date. Equity-based compensation expense is recognized net of an estimated forfeiture rate on a straight-line basis over the requisite service period of the award, except that in the case of RSUs, compensation expense is recognized using an accelerated attribution method. Refer to Note 19 for additional information on the stock-based compensation plans in which certain employees of the Company participate.
Pension and Postretirement Benefit Plans—The Company measures its pension and postretirement plans’ assets and its obligations that determine the respective plan’s funded status as of the end of the Company’s fiscal year, and recognizes an asset for a plan’s overfunded status or a liability for a plan’s underfunded status in its balance sheet. Changes in the funded status of the plans are recognized in the year in which the changes occur and reported in comprehensive income (loss). Refer to Note 16 for additional information on the Company’s pension and postretirement plans including a discussion of the actuarial assumptions, the Company’s policy for recognizing the associated gains and losses and the method used to estimate service and interest cost components.
Accounting Standards Recently Adopted—In October 2021, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2021-08, Accounting for Contract Assets and Contract Liabilities from Contracts with Customers. The ASU requires companies to apply the definition of a performance obligation under ASC 606 to recognize and measure contract assets and contract liabilities (i.e., deferred revenue) relating to contracts with customers acquired in a business combination. Prior to the adoption of this ASU, an acquirer generally recognized assets acquired and liabilities assumed in a business combination, including contract assets and contract liabilities arising from revenue contracts with customers, at fair value on the acquisition date. The ASU results in the acquirer recording acquired contract assets and liabilities on the same basis that would have been recorded by the acquiree before the acquisition under ASC 606. The ASU is effective for fiscal years beginning after December 15, 2022, with early adoption permitted. The Company early adopted the ASU effective January 1, 2021 and did not apply the standard to immaterial transactions that occurred in 2021. The impact of the adoption of the ASU was not significant.
In August 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. The ASU includes amendments to the guidance on convertible instruments and the derivative scope exception for contracts in an entity’s own equity and simplifies the accounting for convertible instruments which include beneficial conversion features or cash conversion features by removing certain separation models in Subtopic 470-20. Additionally, the ASU requires entities to use the “if-converted” method when calculating diluted earnings per common share for convertible instruments. On January 1, 2022, the Company adopted the ASU and the ASU did not have a significant impact on the Company’s financial statements.
In November 2021, the FASB issued ASU No. 2021-10 Government Assistance (Topic 832), which requires annual disclosures of transactions with a government that are accounted for by applying a grant or contribution accounting model by analogy. These required disclosures include information on the nature of transactions and related accounting policies used to account for transactions, detail on the line items on the balance sheet and income statement affected by these transactions including amounts applicable to each line, and significant terms and conditions of the transactions including commitments and contingencies. The Company prospectively adopted the ASU effective January 1, 2022 and applied the disclosure guidance to all transactions within the scope of the ASU that were reflected in the financial statements at the date of initial application and new transactions that are entered into subsequent to the date of initial application. The Company accounts for the government assistance transactions by analogy to the grant accounting model in International Accounting Standards 20 Accounting for Government Grants and Disclosure of Government Assistance.
The Company receives various forms of government assistance, primarily through grants related to the development of new products and the expansion of production capacity. During 2021, certain agencies of the U.S. government, including the Biomedical Advanced Research and Development Authority (“BARDA”) within the U.S. Department of Health and Human Services, agreed to finance an expansion of production capacity related to chromatography, liquid cell culture media, buffers and cell culture powder media and single-use consumables at certain of the Company’s Biotechnology businesses and the development of diagnostics testing technologies and the expansion of testing production capacity at certain of the Company’s Diagnostics businesses. The Company’s businesses may enter into similar agreements in the future. In consideration of this financing, the U.S. government has certain rights, including rights with respect to the allocation of certain of the incremental production capacity associated with such expansion and/or rights in intellectual property produced with its financial assistance. The amount awarded pursuant to these grants in 2021 totaled $568 million and will be paid over periods ranging from one year to four years. In 2022, the Company received aggregate payments related to the BARDA grants and other government assistance of $137 million that offset operating expenses of $50 million and purchases of property, plant and equipment of $87 million. Property, plant and equipment purchased using funds provided by governments are recorded net of government assistance.
In June 2022, the FASB issued ASU No. 2022-03, Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions. The ASU clarifies the guidance in ASC 820, Fair Value Measurement, related to the measurement of the fair value of an equity security subject to contractual sale restrictions and introduces disclosure requirements related to such equity securities. The Company early adopted the ASU effective July 1, 2022 and the impact of the adoption was not significant.
NOTE 2. ACQUISITIONS
The Company continually evaluates potential acquisitions that either strategically fit with the Company’s existing portfolio or expand the Company’s portfolio into a new and attractive business area. The Company has completed a number of acquisitions that have been accounted for as purchases and have resulted in the recognition of goodwill in the Company’s Consolidated Financial Statements. This goodwill arises because the purchase prices for these businesses exceeds the fair value of acquired identifiable net assets due to the purchase prices reflecting a number of factors including the future earnings and cash flow potential of these businesses, the multiple to earnings, cash flow and other factors at which similar businesses have been purchased by other acquirers, the competitive nature of the processes by which the Company acquired the businesses, the avoidance of the time and costs which would be required (and the associated risks that would be encountered) to enhance the Company’s existing product offerings to key target markets and enter into new and profitable businesses and the complementary strategic fit and resulting synergies these businesses bring to existing operations.
The Company makes an initial allocation of the purchase price at the date of acquisition based upon its understanding of the fair value of the acquired assets and assumed liabilities. The Company obtains the information used for the purchase price allocation during due diligence and through other sources. In the months after closing, as the Company obtains additional information about the acquired assets and liabilities, including through tangible and intangible asset appraisals, and learns more about the newly acquired business, it is able to refine the estimates of fair value and more accurately allocate the purchase price. The fair values of acquired intangibles are determined based on estimates and assumptions that are deemed reasonable by the Company. Significant assumptions include the discount rates and certain assumptions that form the basis of the forecasted results of the acquired business including earnings before interest, taxes, depreciation and amortization (“EBITDA”), revenue,
revenue growth rates, royalty rates and technology obsolescence rates. These assumptions are forward looking and could be affected by future economic and market conditions. The Company engages third-party valuation specialists who review the Company’s critical assumptions and calculations of the fair value of acquired intangible assets in connection with significant acquisitions. Only facts and circumstances that existed as of the acquisition date are considered for subsequent adjustment. The Company is continuing to evaluate certain pre-acquisition contingencies associated with certain of its 2022 acquisitions (for those acquisitions with open measurement periods) and is also in the process of obtaining valuations of certain acquisition-related assets and liabilities in connection with these acquisitions. The Company will make appropriate adjustments to the purchase price allocation prior to completion of the measurement period, as required.
The following briefly describes the Company’s acquisition activity for the three years ended December 31, 2022.
During 2022, the Company acquired 10 businesses for total consideration of $637 million in cash, net of cash acquired. The businesses acquired complement existing units of each of the Company’s four segments. The Company preliminarily recorded an aggregate of $427 million of goodwill related to these acquisitions. The aggregate annual sales of the 10 businesses acquired in 2022 at the time of their acquisition, in each case based on the company’s revenues for its last completed fiscal year prior to the acquisition, were approximately $91 million.
On August 30, 2021, the Company acquired Aldevron, L.L.C. (“Aldevron”) for a cash purchase price of approximately $9.6 billion (the “Aldevron Acquisition”). Aldevron manufactures high-quality plasmid DNA, mRNA and proteins, serving biotechnology and pharmaceutical customers across research, clinical and commercial applications, and is now part of the Company’s Life Sciences segment. Aldevron generated revenues of approximately $300 million in 2020. The acquisition of Aldevron has provided and is expected to provide additional sales and earnings opportunities for the Company by expanding product line diversity, including new product offerings supporting genomic medicine. The Company financed the Aldevron Acquisition using cash on hand and proceeds from the issuance of commercial paper. The Company recorded approximately $6.1 billion of goodwill related to the Aldevron Acquisition.
During 2021, in addition to the Aldevron Acquisition, the Company acquired 13 businesses for total consideration of approximately $1.4 billion in cash, net of cash acquired. The businesses acquired complement existing units of each of the Company’s four segments. The Company recorded an aggregate of approximately $1.1 billion of goodwill related to these acquisitions. The aggregate annual sales of the 13 other businesses acquired in 2021 at the time of their acquisition, in each case based on the company’s revenues for its last completed fiscal year prior to the acquisition, were approximately $100 million.
On March 31, 2020, the Company acquired the Biopharma business of General Electric Company’s (“GE”) Life Sciences division, now known as Cytiva, for a cash purchase price of approximately $20.7 billion (net of approximately $0.1 billion of acquired cash) and the assumption of approximately $0.4 billion of pension liabilities (the “Cytiva Acquisition”). Cytiva is a leading provider of instruments, consumables and software that support the research, discovery, process development and manufacturing workflows of biopharmaceutical drugs. Cytiva is included in the Company’s Biotechnology segment results beginning in the second quarter of 2020. The acquisition has provided and is expected to continue to provide additional sales and earnings growth opportunities for the Company’s Biotechnology segment by expanding the business’ geographic and product line diversity, including new product and service offerings that complement the Company’s current biologics workflow solutions. To fulfill a condition to obtaining certain regulatory approvals for the closing of the transaction, on April 30, 2020 the Company divested certain of its existing product lines in the Biotechnology and Life Sciences segments for a cash purchase price, net of cash transferred and transaction costs, of $826 million and recognized a pretax gain on sale of $455 million ($305 million after-tax or $0.42 per diluted common share). The divested product lines in the aggregate generated revenues of approximately $170 million in 2019. The divestiture of these product lines did not represent a strategic shift with a major effect on the Company’s operations and financial results and therefore is not reported as a discontinued operation.
The Company financed the Cytiva Acquisition with approximately $3.0 billion of proceeds from the 2019 underwritten public offerings of its Common Stock and Series A Mandatory Convertible Preferred Stock (“MCPS Series A”), approximately $10.8 billion of proceeds from the 2019 issuance of euro-denominated and U.S. dollar-denominated long-term debt, and approximately $6.9 billion from the aggregate of proceeds from commercial paper borrowings, borrowings under the Company’s Five-Year Facility (as defined below) and cash on hand. The Company recorded approximately $10.2 billion of goodwill related to the Cytiva Acquisition.
During 2020, in addition to the Cytiva Acquisition, the Company acquired four businesses for total consideration of $256 million in cash, net of cash acquired. The businesses acquired complement existing units of the Company’s Life Sciences and Environmental & Applied Solutions segments. The Company recorded an aggregate of $231 million of goodwill related to these acquisitions. The aggregate annual sales of the five businesses acquired in 2020 at the time of their acquisition, in each case based on the company’s revenues for its last completed fiscal year prior to the acquisition, were approximately $3.3 billion.
The following summarizes the estimated fair values of the assets acquired and liabilities assumed at the date of acquisition ($ in millions):
| | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 |
| Trade accounts receivable | $ | 10 | | | $ | 65 | | | $ | 487 | |
| Inventories | 9 | | | 120 | | | 934 | |
| Property, plant and equipment | 9 | | | 162 | | | 690 | |
| Goodwill | 427 | | | 7,235 | | | 10,402 | |
| Other intangible assets, primarily technology, customer relationships and trade names | 218 | | | 4,021 | | | 10,712 | |
| | | | | |
| Trade accounts payable | (4) | | | (23) | | | (250) | |
| Pension liabilities | — | | | — | | | (423) | |
| Deferred tax liabilities | (14) | | | (367) | | | (1,167) | |
| Other assets and liabilities, net | (18) | | | (177) | | | (414) | |
| Net assets acquired | 637 | | | 11,036 | | | 20,971 | |
| Less: noncash consideration | — | | | (75) | | | — | |
| Net cash consideration | $ | 637 | | | $ | 10,961 | | | $ | 20,971 | |
The following summarizes the estimated fair values of the assets acquired and liabilities assumed at the date of acquisition for the individually significant acquisition in 2021 discussed above, and all of the other 2021 acquisitions as a group ($ in millions):
| | | | | | | | | | | | | | | | | |
| Aldevron | | Other | | Total |
| Trade accounts receivable | $ | 46 | | | $ | 19 | | | $ | 65 | |
| Inventories | 93 | | | 27 | | | 120 | |
| Property, plant and equipment | 150 | | | 12 | | | 162 | |
| Goodwill | 6,149 | | | 1,086 | | | 7,235 | |
| Other intangible assets, primarily technology, customer relationships and trade names | 3,483 | | | 538 | | | 4,021 | |
| | | | | |
| Trade accounts payable | (15) | | | (8) | | | (23) | |
| | | | | |
| Deferred tax liabilities | (249) | | | (118) | | | (367) | |
| Other assets and liabilities, net | (73) | | | (104) | | | (177) | |
| | | | | |
| Net assets acquired | 9,584 | | | 1,452 | | | 11,036 | |
| Less: noncash consideration | (23) | | | (52) | | | (75) | |
| Net cash consideration | $ | 9,561 | | | $ | 1,400 | | | $ | 10,961 | |
The following summarizes the estimated fair values of the assets acquired and liabilities assumed at the date of acquisition for the individually significant acquisition in 2020 discussed above, and all of the other 2020 acquisitions as a group ($ in millions):
| | | | | | | | | | | | | | | | | |
| Cytiva | | Other | | Total |
| Trade accounts receivable | $ | 482 | | | $ | 5 | | | $ | 487 | |
| Inventories | 930 | | | 4 | | | 934 | |
| Property, plant and equipment | 689 | | | 1 | | | 690 | |
| Goodwill | 10,171 | | | 231 | | | 10,402 | |
| Other intangible assets, primarily technology, customer relationships and trade names | 10,656 | | | 56 | | | 10,712 | |
| | | | | |
| Trade accounts payable | (247) | | | (3) | | | (250) | |
| Pension liabilities | (423) | | | — | | | (423) | |
| Deferred tax liabilities | (1,157) | | | (10) | | | (1,167) | |
| Other assets and liabilities, net | (386) | | | (28) | | | (414) | |
| | | | | |
| | | | | |
| | | | | |
| Net cash consideration | $ | 20,715 | | | $ | 256 | | | $ | 20,971 | |
Transaction-related costs for the Aldevron Acquisition were $28 million for the year ended December 31, 2021. Additionally, transaction-related costs for the Cytiva Acquisition were $59 million for the year ended December 31, 2020. The Company’s earnings for 2021 also reflect the pretax impact of $30 million of non-recurring acquisition date fair value adjustments to inventory related to the Aldevron acquisition. In addition, the Company’s earnings for 2021 and 2020 reflect the pretax impact of $46 million and $509 million, respectively, of non-recurring acquisition date fair value adjustments to inventory and deferred revenue related to the Cytiva Acquisition. Transaction-related costs and acquisition-related fair value adjustments attributable to other acquisitions were not material for the years ended December 31, 2022, 2021 or 2020.
Pro Forma Financial Information (Unaudited)
The unaudited pro forma information for the periods set forth below gives effect to the 2022 and 2021 acquisitions as if they had occurred as of January 1, 2021, including the results from operations for the acquired business as well as the impact of
assumed financing of the transaction and the impact of the purchase price allocation (including the amortization of acquired
intangible assets). The pro forma information is presented for informational purposes only and is not necessarily indicative of the results of operations that actually would have been achieved had the acquisitions been consummated as of that time ($ in millions except per share amounts):
| | | | | | | | | | | |
| 2022 | | 2021 |
| Sales | $ | 31,538 | | | $ | 29,817 | |
| Net earnings from continuing operations | 7,189 | | | 6,190 | |
Diluted net earnings per common share from continuing operations (a) | 9.64 | | | 8.28 | |
(a) Diluted net earnings per common share from continuing operations is calculated by adding the interest on the Company’s Liquid Yield Option Notes (“LYONs”) to net earnings from continuing operations and deducting the MCPS dividends from net earnings from continuing operations for the anti-dilutive MCPS shares.
Acquisition-related transaction costs of $28 million for the year ended December 31, 2021 related to the Aldevron Acquisition were excluded from pro forma net earnings from continuing operations.
NOTE 3. DISCONTINUED OPERATIONS AND ENVIRONMENTAL & APPLIED SOLUTIONS SEPARATION
Fortive Corporation Separation
On July 2, 2016, the Company completed the separation of its former Test & Measurement segment, Industrial Technologies segment (excluding the product identification businesses) and retail/commercial petroleum business by distributing to Danaher stockholders on a pro rata basis all of the issued and outstanding common stock of Fortive Corporation (“Fortive”), the entity the Company incorporated to hold such businesses. For the year ended December 31, 2021, the Company recorded an income tax benefit of $86 million related to the release of previously provided reserves associated with uncertain tax positions on certain of the Company’s tax returns which were jointly filed with Fortive entities. These reserves were released due to the expiration of statutes of limitations for those returns. This income tax benefit is included in earnings from discontinued operations, net of income taxes in the accompanying Consolidated Statements of Earnings.
Environmental & Applied Solutions Separation
In September 2022, the Company announced its intention to spin-off its Environmental & Applied Solutions business into a publicly traded company (the “EAS Separation”). The Environmental & Applied Solutions business had sales for the year-ended December 31, 2022 of approximately $4.8 billion. The transaction is expected to be tax-free to the Company’s shareholders. The Company is targeting to complete the EAS Separation in the fourth quarter of 2023, subject to the satisfaction of certain conditions, including obtaining final approval from the Danaher Board of Directors, satisfactory completion of financing, receipt of tax opinions, receipt of favorable rulings from the Internal Revenue Service (“IRS”) and receipt of other regulatory approvals.
NOTE 4. NET EARNINGS PER COMMON SHARE FROM CONTINUING OPERATIONS
Basic net earnings per share from continuing operations (“EPS”) is calculated by taking net earnings from continuing operations less the MCPS dividends divided by the weighted average number of common shares outstanding for the applicable period. Diluted net EPS from continuing operations is computed by taking net earnings from continuing operations plus the interest accrued on the Company’s LYONs (prior to their redemption in January 22, 2021) less the MCPS dividends divided by the weighted average number of common shares outstanding increased by the number of additional shares that would have been outstanding had the potentially dilutive common shares been issued and reduced by the number of shares the Company could have repurchased with the proceeds from the issuance of the potentially dilutive shares. For the years ended December 31, 2022 and 2020, 1.4 million and 1.0 million options to purchase shares, respectively, were excluded from the diluted earnings
per share calculation, as the impact of their inclusion would have been anti-dilutive. For the year ended December 31, 2021, no options to purchase shares were excluded from the diluted earnings per share calculation.
Basic and diluted EPS are computed independently for each quarter and annual period, which involves the use of different weighted-average share count figures relating to quarterly and annual periods. As a result, and after factoring the effect of rounding to the nearest cent per share, the sum of prior quarter-to-date EPS figures may not equal annual EPS.
On April 15, 2022, all outstanding shares of the MCPS Series A converted into 11.0 million shares of the Company’s common stock. The impact of the MCPS Series A calculated under the if-converted method was dilutive for the years ended December 31, 2022 and December 31, 2021, and as such 3.0 million and 11.0 million shares, respectively, underlying the MCPS Series A were included in the calculation of diluted EPS and the related MCPS Series A dividends of $20 million and $78 million were excluded from the calculation of net earnings for diluted EPS for the periods. Refer to Note 19 for additional information about the MCPS Series A conversion.
The impact of the MCPS Series B calculated under the if-converted method was anti-dilutive for the years ended December 31, 2022 and 2021, and as such 8.6 million shares underlying the MCPS Series B were excluded from the diluted EPS calculation in each period and the related MCPS Series B dividends of $86 million were included in the calculation of net earnings for diluted EPS for the periods.
The impact of the MCPS Series A and Series B calculated under the if-converted method was anti-dilutive for the year ended December 31, 2020 and as such 17.1 million shares underlying the MCPS Series A and Series B were excluded from the calculation of diluted EPS and the related MCPS Series A and Series B dividends of $136 million were included in the calculation of net earnings for diluted EPS for the period.
Information related to the calculation of net earnings per common share from continuing operations for the years ended December 31 is summarized as follows ($ and shares in millions, except per share amounts):
| | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 |
| Numerator: | | | | | |
| Net earnings from continuing operations | $ | 7,209 | | | $ | 6,347 | | | $ | 3,646 | |
| MCPS dividends | (106) | | | (164) | | | (136) | |
| Net earnings from continuing operations attributable to common stockholders for Basic EPS | 7,103 | | | 6,183 | | | 3,510 | |
| Adjustment for interest on convertible debentures | — | | | — | | | 1 | |
| Adjustment for MCPS dividends for dilutive MCPS | 20 | | | 78 | | | — | |
| Net earnings from continuing operations attributable to common stockholders after assumed conversions for Diluted EPS | $ | 7,123 | | | $ | 6,261 | | | $ | 3,511 | |
| | | | | |
| Denominator: | | | | | |
| Weighted average common shares outstanding used in Basic EPS | 725.1 | | | 714.6 | | | 706.2 | |
| Incremental common shares from: | | | | | |
| Assumed exercise of dilutive options and vesting of dilutive RSUs and PSUs | 9.0 | | | 11.2 | | | 11.4 | |
| Assumed conversion of the convertible debentures | — | | | — | | | 1.1 | |
| Weighted average MCPS converted shares | 3.0 | | | 11.0 | | | — | |
| Weighted average common shares outstanding used in Diluted EPS | 737.1 | | | 736.8 | | | 718.7 | |
| | | | | |
| Basic EPS from continuing operations | $ | 9.80 | | | $ | 8.65 | | | $ | 4.97 | |
| Diluted EPS from continuing operations | $ | 9.66 | | | $ | 8.50 | | | $ | 4.89 | |
NOTE 5. REVENUE
The following table presents the Company’s revenues disaggregated by geographical region and revenue type ($ in millions). Sales taxes and other usage-based taxes collected from customers are excluded from revenues. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Biotechnology | | Life Sciences | | Diagnostics | | Environmental & Applied Solutions | | Total |
| Year ended December 31, 2022: | | | | | | | | | |
| Geographical region: | | | | | | | | | |
North America(a) | $ | 3,054 | | | $ | 3,154 | | | $ | 5,522 | | | $ | 2,238 | | | $ | 13,968 | |
| Western Europe | 2,645 | | | 1,377 | | | 1,837 | | | 1,051 | | | 6,910 | |
| Other developed markets | 358 | | | 506 | | | 481 | | | 122 | | | 1,467 | |
High-growth markets(b) | 2,701 | | | 1,999 | | | 3,009 | | | 1,417 | | | 9,126 | |
| Total | $ | 8,758 | | | $ | 7,036 | | | $ | 10,849 | | | $ | 4,828 | | | $ | 31,471 | |
| | | | | | | | | |
| Revenue type: | | | | | | | | | |
| Recurring | $ | 6,958 | | | $ | 4,220 | | | $ | 9,698 | | | $ | 2,841 | | | $ | 23,717 | |
| Nonrecurring | 1,800 | | | 2,816 | | | 1,151 | | | 1,987 | | | 7,754 | |
| Total | $ | 8,758 | | | $ | 7,036 | | | $ | 10,849 | | | $ | 4,828 | | | $ | 31,471 | |
| | | | | | | | | |
| Year ended December 31, 2021: | | | | | | | | | |
| Geographical region: | | | | | | | | | |
North America(a) | $ | 2,899 | | | $ | 2,534 | | | $ | 4,365 | | | $ | 2,031 | | | $ | 11,829 | |
| Western Europe | 2,497 | | | 1,540 | | | 1,840 | | | 1,088 | | | 6,965 | |
| Other developed markets | 368 | | | 508 | | | 481 | | | 118 | | | 1,475 | |
High-growth markets(b) | 2,806 | | | 1,806 | | | 3,158 | | | 1,414 | | | 9,184 | |
| Total | $ | 8,570 | | | $ | 6,388 | | | $ | 9,844 | | | $ | 4,651 | | | $ | 29,453 | |
| | | | | | | | | |
| Revenue type: | | | | | | | | | |
| Recurring | $ | 6,772 | | | $ | 3,756 | | | $ | 8,607 | | | $ | 2,660 | | | $ | 21,795 | |
| Nonrecurring | 1,798 | | | 2,632 | | | 1,237 | | | 1,991 | | | 7,658 | |
| Total | $ | 8,570 | | | $ | 6,388 | | | $ | 9,844 | | | $ | 4,651 | | | $ | 29,453 | |
| | | | | | | | | |
| Year ended December 31, 2020: | | | | | | | | | |
| Geographical region: | | | | | | | | | |
| North America | $ | 1,880 | | | $ | 2,039 | | | $ | 3,182 | | | $ | 1,910 | | | $ | 9,011 | |
| Western Europe | 1,683 | | | 1,256 | | | 1,375 | | | 1,009 | | | 5,323 | |
| Other developed markets | 313 | | | 441 | | | 423 | | | 122 | | | 1,299 | |
High-growth markets (a) | 1,400 | | | 1,564 | | | 2,423 | | | 1,264 | | | 6,651 | |
| Total | $ | 5,276 | | | $ | 5,300 | | | $ | 7,403 | | | $ | 4,305 | | | $ | 22,284 | |
| | | | | | | | | |
| Revenue type: | | | | | | | | | |
| Recurring | $ | 4,299 | | | $ | 3,101 | | | $ | 6,143 | | | $ | 2,435 | | | $ | 15,978 | |
| Nonrecurring | 977 | | | 2,199 | | | 1,260 | | | 1,870 | | | 6,306 | |
| Total | $ | 5,276 | | | $ | 5,300 | | | $ | 7,403 | | | $ | 4,305 | | | $ | 22,284 | |
(a) The Company defines North America as the United States and Canada.
(b) The Company defines high-growth markets as developing markets of the world experiencing extended periods of accelerated growth in gross domestic product and infrastructure which include Eastern Europe, the Middle East, Africa, Latin America (including Mexico) and Asia (with the exception of Japan, Australia and New Zealand). The Company defines developed markets as all markets of the world that are not high-growth markets.
The Company sells equipment to customers as well as consumables and services, some of which customers purchase on a recurring basis. Consumables sold for use with the equipment sold by the Company are typically critical to the use of the equipment and are typically used on a one-time or limited basis, requiring frequent replacement in the customer’s operating cycle. Examples of these consumables include reagents used in diagnostic tests, chromatography resins used for research and bioprocessing, filters used in filtration, separation and purification processes and cartridges for marking and coding equipment. Additionally, some of the Company’s consumables are used on a standalone basis, such as water treatment solutions, custom nucleic acids and genomics solutions. The Company separates its goods and services between those typically sold to a customer on a recurring basis and those typically sold to a customer on a nonrecurring basis. Recurring revenue includes revenue from consumables, services and OTLs. Nonrecurring revenue includes sales of equipment and STLs. OTLs and STLs are included in the above revenue amounts. For the years ended December 31, 2022, 2021 and 2020, lease revenue was $488 million, $483 million and $473 million, respectively.
Remaining Performance Obligations
Remaining performance obligations represent the aggregate transaction price allocated to performance obligations with an original contract term greater than one year which are fully or partially unsatisfied at the end of the period. Remaining performance obligations include noncancelable purchase orders, the non-lease portion of minimum purchase commitments under long-term consumable supply arrangements, extended warranty and service and other long-term contracts. These remaining performance obligations do not include revenue from contracts with customers with an original term of one year or less, revenue from long-term consumable supply arrangements with no minimum purchase requirements or revenue expected from purchases made in excess of the minimum purchase requirements or revenue from equipment leased to customers. While the remaining performance obligation disclosure is similar in concept to backlog, the definition of remaining performance obligations excludes leases and contracts that provide the customer with the right to cancel or terminate for convenience with no substantial penalty, even if historical experience indicates the likelihood of cancellation or termination is remote. Additionally, the Company has elected to exclude contracts with customers with an original term of one year or less from remaining performance obligations while these contracts are included within backlog.
As of December 31, 2022, the aggregate amount of the transaction price allocated to remaining performance obligations was approximately $5.0 billion. The Company expects to recognize revenue on approximately 57% of the remaining performance obligations over the next 12 months, 25% over the subsequent 12 months, and the remainder recognized thereafter.
Contract Balances
The timing of revenue recognition, billings and cash collections results in billed trade accounts receivable, unbilled receivables (“contract assets”) and deferred revenue, customer deposits and billings in excess of revenue recognized (“contract liabilities”) on the Consolidated Balance Sheets. In addition, the Company defers certain costs incurred to obtain a contract (“contract costs”). Contract assets, liabilities and costs are reported on the accompanying Consolidated Balance Sheets on a contract-by-contract basis.
Contract Assets—Most of the Company’s long-term contracts are billed as work progresses in accordance with the contract terms and conditions, either at periodic intervals or upon achievement of certain milestones. Often this results in billing occurring subsequent to revenue recognition resulting in contract assets. Contract assets are generally classified as other current assets in the Consolidated Balance Sheets. The balance of contract assets as of December 31, 2022 and 2021 was $90 million and $75 million, respectively.
Contract Liabilities—The Company often receives cash payments from customers in advance of the Company’s performance resulting in contract liabilities that are classified as either current or long-term in the Consolidated Balance Sheets based on the timing of when the Company expects to recognize revenue. As of December 31, 2022 and 2021, contract liabilities were approximately $1.9 billion and $1.8 billion, respectively, and are included within accrued expenses and other liabilities and other long-term liabilities in the accompanying Consolidated Balance Sheets. The increase in the contract liability balance during the years ended December 31, 2022 and 2021 was primarily a result of cash payments received in advance of satisfying performance obligations, partially offset by revenue recognized during the year that was included in the opening contract liability balance and the impact of foreign currency. Revenue recognized during the years ended December 31, 2022 and 2021 that was included in the opening contract liability balance was approximately $1.5 billion and $1.1 billion, respectively.
Contract Costs—The Company capitalizes certain direct incremental costs incurred to obtain a contract, typically sales-related commissions, where the amortization period for the related asset is greater than one year. These costs are amortized over the contract term or a longer period, generally the expected life of the customer relationship if renewals are expected and the renewal commission is not commensurate with the initial commission. Contract costs are classified as current or long-term other assets in the Consolidated Balance Sheets based on the timing of when the Company expects to recognize the expense and are generally amortized into earnings on a straight-line basis (which is consistent with the transfer of control for the related
goods or services). Management assesses these costs for impairment at least quarterly and as “triggering” events occur that indicate it is more likely than not that an impairment exists. The balance of contract costs as of December 31, 2022 and 2021 was not significant. Amortization expense related to these costs for the years ended December 31, 2022 and 2021 was also not significant. The costs to obtain a contract where the amortization period for the related asset is one year or less are expensed as incurred and recorded within selling, general and administrative expenses in the accompanying Consolidated Statements of Earnings.
NOTE 6. SEGMENT INFORMATION
In the fourth quarter of 2022, the Company realigned its reportable segments to reflect changes in the Company’s internal organization resulting from the rate of growth within certain of the Company’s businesses in the former Life Sciences segment. There were no changes to the Company’s Diagnostics or Environmental & Applied Solutions segments.
The Company now operates and reports its results in four separate business segments consisting of the Biotechnology, Life Sciences, Diagnostics and Environmental & Applied Solutions segments. When determining the reportable segments, the Company aggregated operating segments based on their similar economic and operating characteristics. Operating profit represents total revenues less operating expenses, excluding nonoperating income and expense, loss on early extinguishment of borrowings, interest and income taxes. Operating profit amounts in the Other segment consist of unallocated corporate costs and other costs not considered part of management’s evaluation of reportable segment operating performance. The identifiable assets by segment are those used in each segment’s operations. Intersegment amounts are not significant and are eliminated to arrive at consolidated totals. Prior period segment amounts have been restated to conform to the revised segment presentation.
Detailed segment data for the years ended December 31 is as follows ($ in millions):
| | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 |
| Sales: | | | | | |
| Biotechnology | $ | 8,758 | | | $ | 8,570 | | | $ | 5,276 | |
| Life Sciences | 7,036 | | | 6,388 | | | 5,300 | |
| Diagnostics | 10,849 | | | 9,844 | | | 7,403 | |
| Environmental & Applied Solutions | 4,828 | | | 4,651 | | | 4,305 | |
| Total | $ | 31,471 | | | $ | 29,453 | | | $ | 22,284 | |
| | | | | |
| Operating profit: | | | | | |
| Biotechnology | $ | 3,008 | | | $ | 3,074 | | | $ | 1,082 | |
| Life Sciences | 1,414 | | | 1,293 | | | 972 | |
| Diagnostics | 3,436 | | | 2,313 | | | 1,538 | |
| Environmental & Applied Solutions | 1,135 | | | 1,054 | | | 979 | |
| Other | (305) | | | (269) | | | (340) | |
| Total | $ | 8,688 | | | $ | 7,465 | | | $ | 4,231 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Depreciation and amortization of intangible assets: | | | | | |
| Biotechnology | $ | 1,002 | | | $ | 1,059 | | | $ | 761 | |
| Life Sciences | 531 | | | 382 | | | 292 | |
| Diagnostics | 590 | | | 614 | | | 602 | |
| Environmental & Applied Solutions | 90 | | | 106 | | | 110 | |
| Other | 9 | | | 7 | | | 10 | |
| Total | $ | 2,222 | | | $ | 2,168 | | | $ | 1,775 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
The following table presents additional detailed segment data for the years ended December 31 ($ in millions):
| | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Identifiable assets: | | | | | |
| Biotechnology | $ | 37,536 | | | $ | 38,118 | | | $ | 39,086 | |
| Life Sciences | 17,572 | | | 19,768 | | | 9,833 | |
| Diagnostics | 14,722 | | | 15,054 | | | 15,042 | |
| Environmental & Applied Solutions | 4,797 | | | 4,882 | | | 5,083 | |
| Other | 9,723 | | | 5,362 | | | 7,117 | |
| | | | | |
| Total | $ | 84,350 | | | $ | 83,184 | | | $ | 76,161 | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Capital expenditures, gross: | | | | | |
| Biotechnology | $ | 405 | | | $ | 385 | | | $ | 169 | |
| Life Sciences | 325 | | | 210 | | | 137 | |
| Diagnostics | 382 | | | 644 | | | 447 | |
| Environmental & Applied Solutions | 34 | | | 54 | | | 36 | |
| Other | 6 | | | 1 | | | 2 | |
| Total | $ | 1,152 | | | $ | 1,294 | | | $ | 791 | |
Operations in Geographical Areas:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31 |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Sales: | | | | | |
| United States | $ | 13,365 | | | $ | 11,283 | | | $ | 8,616 | |
| China | 4,002 | | | 3,975 | | | 2,688 | |
| Germany | 1,461 | | | 1,482 | | | 1,238 | |
All other (each country individually less than 5% of total sales) | 12,643 | | | 12,713 | | | 9,742 | |
| Total | $ | 31,471 | | | $ | 29,453 | | | $ | 22,284 | |
| | | | | |
| Property, plant and equipment, net: | | | | | |
| United States | $ | 2,007 | | | $ | 1,799 | | | $ | 1,317 | |
| Sweden | 429 | | | 513 | | | 553 | |
| United Kingdom | 254 | | | 260 | | | 214 | |
| Germany | 225 | | | 223 | | | 207 | |
All other (each country individually less than 5% of total property, plant and equipment, net) | 1,041 | | | 995 | | | 971 | |
| Total | $ | 3,956 | | | $ | 3,790 | | | $ | 3,262 | |
Sales by Major Product Group:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31 |
| ($ in millions) | 2022 | | 2021 | | 2020 |
| Analytical and physical instrumentation | $ | 2,846 | | | $ | 2,620 | | | $ | 2,443 | |
| Research and medical products | 26,642 | | | 24,802 | | | 17,979 | |
| Product identification | 1,983 | | | 2,031 | | | 1,862 | |
| Total | $ | 31,471 | | | $ | 29,453 | | | $ | 22,284 | |
NOTE 7. INCOME TAXES
Earnings from continuing operations before income taxes for the years ended December 31 were as follows ($ in millions):
| | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 |
| United States | $ | 3,054 | | | $ | 2,500 | | | $ | 1,655 | |
| Non-U.S. | 5,238 | | | 5,098 | | | 2,840 | |
| Total | $ | 8,292 | | | $ | 7,598 | | | $ | 4,495 | |
The provision for income taxes from continuing operations for the years ended December 31 were as follows ($ in millions):
| | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 |
| Current: | | | | | |
| Federal U.S. | $ | 271 | | | $ | 183 | | | $ | (321) | |
| Non-U.S. | 1,229 | | | 1,134 | | | 580 | |
| State and local | 142 | | | 163 | | | 72 | |
| Deferred: | | | | | |
| Federal U.S. | (331) | | | (156) | | | 530 | |
| Non-U.S. | (159) | | | (23) | | | (16) | |
| State and local | (69) | | | (50) | | | 4 | |
| Income tax provision | $ | 1,083 | | | $ | 1,251 | | | $ | 849 | |
Noncurrent deferred tax assets and noncurrent deferred tax liabilities are included in other assets and other long-term liabilities, respectively, in the accompanying Consolidated Balance Sheets. Deferred income tax assets and liabilities as of December 31 were as follows ($ in millions):
| | | | | | | | | | | |
| 2022 | | 2021 |
| Deferred tax assets: | | | |
| Allowance for doubtful accounts | $ | 17 | | | $ | 19 | |
| Inventories | 118 | | | 93 | |
| Pension and postretirement benefits | 17 | | | 105 | |
| Environmental and regulatory compliance | 39 | | | 38 | |
| Other accruals and prepayments | 406 | | | 299 | |
| Stock-based compensation expense | 105 | | | 76 | |
| Operating lease liabilities | 238 | | | 252 | |
| Research and development expense | 243 | | | 49 | |
| Tax credit and loss carryforwards | 479 | | | 544 | |
| Valuation allowances | (236) | | | (242) | |
| Total deferred tax asset | 1,426 | | | 1,233 | |
| Deferred tax liabilities: | | | |
| Property, plant and equipment | (92) | | | (79) | |
| Insurance, including self-insurance | (803) | | | (520) | |
| | | |
| Operating lease right-of-use assets | (219) | | | (235) | |
| Goodwill and other intangibles | (3,270) | | | (3,962) | |
| Total deferred tax liability | (4,384) | | | (4,796) | |
| Net deferred tax liability | $ | (2,958) | | | $ | (3,563) | |
The Company evaluates the future realizability of tax credits and loss carryforwards considering the anticipated future earnings of the Company’s subsidiaries as well as tax planning strategies in the associated jurisdictions. Deferred taxes associated with U.S. entities consist of net deferred tax liabilities of approximately $1.9 billion and $2.1 billion as of December 31, 2022 and 2021, respectively. Deferred taxes associated with non-U.S. entities consist of net deferred tax liabilities of approximately $1.1 billion and $1.5 billion as of December 31, 2022 and 2021, respectively. During 2022, the Company’s valuation allowance
decreased by $6 million primarily from the use of tax attributes which were previously not deemed realizable. As of December 31, 2022, the total amount of the basis difference in investments indefinitely reinvested outside the United States for which deferred taxes have not been provided is approximately $10.4 billion. The income taxes applicable to repatriating such earnings are not readily determinable. As of December 31, 2022, the Company had no plans which would subject these basis differences to income taxes in the United States or elsewhere.
The Tax Cuts and Jobs Act (“TCJA”) imposes tax on U.S. shareholders for global intangible low-taxed income (“GILTI”) earned by certain non-U.S. subsidiaries. The Company has elected the period cost method for its accounting for GILTI.
The effective income tax rate from continuing operations for the years ended December 31 varies from the U.S. statutory federal income tax rate as follows:
| | | | | | | | | | | | | | | | | |
| | Percentage of Pretax Earnings |
| | 2022 | | 2021 | | 2020 |
| Statutory federal income tax rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| Increase (decrease) in tax rate resulting from: | | | | | |
| State income taxes (net of federal income tax benefit) | 1.3 | % | | 1.1 | % | | 1.1 | % |
| Non-U.S. rate differential | (2.8) | % | | (2.0) | % | | (1.6) | % |
| Resolution and expiration of statutes of limitation of uncertain tax positions | (0.2) | % | | (3.0) | % | | (0.7) | % |
| Realignment of businesses | (4.9) | % | | — | % | | — | % |
| Research credits, uncertain tax positions and other | (0.8) | % | | 0.5 | % | | 0.7 | % |
| Excess tax benefits from stock-based compensation | (0.5) | % | | (1.1) | % | | (1.6) | % |
| | | | | |
| | | | | |
| Effective income tax rate | 13.1 | % | | 16.5 | % | | 18.9 | % |
The Company’s effective tax rate for 2022, 2021 and 2020 differs from the U.S. federal statutory rate of 21.0%, due to the Company’s earnings outside the United States that are indefinitely reinvested and taxed at rates different than the U.S. federal statutory rate as well as the impact of the following:
•The effective tax rate of 13.1% in 2022 includes net deferred tax benefits resulting from legal and operational actions undertaken to realign certain of its businesses, as well as excess tax benefits from stock-based compensation, the release of reserves for uncertain tax positions due to the expiration of statutes of limitation and audit settlements and changes in estimates related to prior year tax filing positions, net of changes in estimates associated with prior period uncertain tax positions. These items decreased the reported rate on a net basis by 6.1%.
•The effective tax rate of 16.5% in 2021 includes net tax benefits primarily related to the release of reserves for uncertain tax positions from the expiration of statutes of limitation, audit settlements and excess tax benefits from stock-based compensation, partially offset by changes in estimates associated with prior period uncertain tax positions. These items decreased the reported rate on a net basis by 3.5%.
•The effective tax rate of 18.9% in 2020 includes net tax benefits primarily related to the release of reserves for uncertain tax positions from audit settlements and expiration of statutes of limitation and excess tax benefits from stock-based compensation, partially offset by a higher tax rate associated with the gain on the divestiture of certain product lines in the Biotechnology and Life Sciences segments and changes in estimates associated with prior period uncertain tax positions. These items decreased the reported rate on a net basis by 0.7%.
The Company made income tax payments related to both continuing and discontinued operations of approximately $1.8 billion, $1.7 billion and $1.1 billion in 2022, 2021 and 2020, respectively. Current income taxes payable related to both continuing and discontinued operations has been reduced by $85 million, $118 million and $110 million in 2022, 2021 and 2020, respectively, for tax deductions attributable to stock-based compensation, of which, the excess tax benefit over the amount recorded for financial reporting purposes for both continuing and discontinued operations was $61 million, $95 million and $85 million, respectively. The excess tax benefits have been recorded as reductions to the current income tax provision and are reflected as operating cash inflows in the accompanying Consolidated Statements of Cash Flows.
Included in deferred income taxes as of December 31, 2022 are tax benefits for U.S. and non-U.S. net operating loss carryforwards totaling $346 million ($147 million of which the Company does not expect to realize and have corresponding valuation allowances). Certain of the losses can be carried forward indefinitely and others can be carried forward to various dates from 2023 through 2042. In addition, the Company had general business and non-U.S. tax credit carryforwards of $133 million ($80 million of which the Company does not expect to realize and have corresponding valuation allowances) as of
December 31, 2022, which can be carried forward to various dates from 2023 to 2032. In addition, as of December 31, 2022, the Company had $9 million of valuation allowances related to other deferred tax asset balances that are not more likely than not of being realized.
As of December 31, 2022, gross unrecognized tax benefits totaled approximately $1.1 billion (approximately $1.2 billion, net of the impact of $65 million of indirect tax benefits offset by $171 million associated with potential interest and penalties). As of December 31, 2021, gross unrecognized tax benefits totaled approximately $1.1 billion (approximately $1.2 billion, net of the impact of $58 million of indirect tax benefits offset by $163 million associated with potential interest and penalties). The Company recognized approximately $14 million of net tax expense from potential interest and penalties during 2022, $182 million of net tax benefits from the reversal of potential interest and penalties during 2021 and $41 million of net tax expense from potential interest and penalties during 2020, related to both continuing and discontinued operations associated with uncertain tax positions. To the extent unrecognized tax benefits (including interest and penalties) are recognized with respect to uncertain tax positions, approximately $1.2 billion and $1.1 billion as of December 31, 2022 and 2021, respectively, would reduce the tax expense and effective tax rate in future periods. The Company recognized interest and penalties related to unrecognized tax benefits within income taxes in the accompanying Consolidated Statements of Earnings. Unrecognized tax benefits and associated accrued interest and penalties are included in taxes, income and other accrued expenses as detailed in Note 13.
A reconciliation of the beginning and ending amount of unrecognized tax benefits, excluding amounts accrued for potential interest and penalties related to both continuing and discontinued operations, is as follows ($ in millions):
| | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 |
| Unrecognized tax benefits, beginning of year | $ | 1,095 | | | $ | 1,175 | | | $ | 1,181 | |
| Additions based on tax positions related to the current year | 44 | | | 47 | | | 47 | |
| Additions for tax positions of prior years | 49 | | | 166 | | | 24 | |
| Reductions for tax positions of prior years | (10) | | | (100) | | | (20) | |
| Acquisitions, divestitures and other | 6 | | | 53 | | | (30) | |
| Lapse of statute of limitations | (16) | | | (219) | | | (13) | |
| Settlements | (7) | | | (4) | | | (38) | |
| Effect of foreign currency translation | (22) | | | (23) | | | 24 | |
| Unrecognized tax benefits, end of year | $ | 1,139 | | | $ | 1,095 | | | $ | 1,175 | |
The Company conducts business globally and files numerous consolidated and separate income tax returns in the U.S. federal and state and non-U.S. jurisdictions. The non-U.S. countries in which the Company has a significant presence include China, Denmark, Germany, Singapore, Sweden, Switzerland and the United Kingdom. Excluding these jurisdictions, the Company believes that a change in the statutory tax rate of any individual non-U.S. country would not have a material effect on the Company’s Consolidated Financial Statements given the geographic dispersion of the Company’s taxable income.
The Company and its subsidiaries are routinely examined by various U.S. and non-U.S. taxing authorities. The IRS has completed substantially all of the examinations of the Company’s federal income tax returns through 2015 and is currently examining certain of the Company’s federal income tax returns for 2016 through 2018. In addition, the Company has subsidiaries in Belgium, Canada, China, Denmark, France, Germany, India, Italy, Japan, Korea, Switzerland, the United Kingdom and various other countries, states and provinces that are currently under audit for years ranging from 2004 through 2021.
Similar to the position it took in connection with the audit of the Company’s taxable income for the years 2012 through 2015, in the fourth quarter of 2022, the IRS proposed significant adjustments to the Company’s taxable income for the years 2016 through 2018 with respect to the deferral of tax on certain premium income related to the Company’s self-insurance programs. The settlement of this matter for the 2012 through 2015 audit was not material to the Company’s financial statements but did not preclude the IRS from proposing similar adjustments in future audit periods, as the IRS has with the 2022 assessment. For income tax purposes, the recognition of premium income has been deferred in accordance with U.S. tax laws related to insurance. The IRS is challenging the deferral of premium income for certain types of the Company’s self-insurance policies. The proposed adjustments would increase the Company’s taxable income over the 2016 through 2018 periods by approximately $2.5 billion. Due to the enactment of the TCJA in 2017 and the resulting reduction in the U.S. corporate tax rate for years after 2017, the Company remeasured its deferred tax liabilities related to the temporary differences associated with this deferred premium income from 35.0% to 21.0%. If the Company is unsuccessful in defending its position, taxes owed to the IRS may be computed under the previous 35.0% statutory tax rate and the Company may be required to remeasure the related deferred tax liabilities from 21.0% to 35.0%, which in addition to any interest due on the amounts assessed, would require a charge to
future earnings. Management believes the positions the Company has taken in its U.S. tax returns are in accordance with the relevant tax laws and intends to vigorously defend these positions.
Tax authorities in Denmark have issued tax assessments related to interest accrued by certain of the Company’s subsidiaries for the years 2004 through 2015. During the first quarter of 2021, the Company received a notice from the Danish tax authorities that included a significant reduction in the interest amounts imposed in the original tax assessments. Taking into account the revised interest amounts, the assessments total approximately DKK 2.1 billion including applicable accrued interest (approximately $298 million based on the exchange rate as of December 31, 2022). The Company’s appeal of the tax assessments with the Danish National Tax Tribunal has been put on hold awaiting the final outcome of other preceding withholding tax cases that have been brought before the Danish High Court and the Danish Supreme Court. Management believes the positions the Company has taken in Denmark are in accordance with the relevant tax laws and is vigorously defending its positions. The Company intends on pursuing this matter through the Danish High Court and the Danish Supreme Court should the appeal to the Danish National Tax Tribunal be unsuccessful. While the ultimate resolution of this matter is uncertain and could take many years, taking into account the payments the Company has previously made related to these assessments in order to mitigate further interest accrual claims, the Company does not expect the resolution of this matter will have a future material adverse impact to the Company’s financial statements, including its cash flow and effective tax rate.
Management estimates that it is reasonably possible that the amount of unrecognized tax benefits related to continuing operations may be reduced by approximately $306 million within 12 months as a result of resolution of worldwide tax matters, net of payments for tax audit settlements and/or statute of limitations expirations. Future resolution of uncertain tax positions related to discontinued operations may result in additional charges or credits to earnings from discontinued operations in the Consolidated Statements of Earnings (refer to Note 3).
The Company operates in various non-U.S. jurisdictions where income tax incentives and rulings have been granted for specific periods of time. In Switzerland, Singapore and Puerto Rico, the Company has various tax rulings and tax holiday arrangements which reduce the overall effective tax rate of the Company. The various rulings and tax holidays expire between 2022 and 2027. As of December 31, 2022, the Company had satisfied the conditions enumerated in these agreements. Included in the accompanying Consolidated Financial Statements are tax benefits of $72 million, $59 million and $43 million (or $0.10, $0.08 and $0.06 per diluted common share) for 2022, 2021 and 2020, respectively, from these rulings and tax holidays.
NOTE 8. OTHER OPERATING EXPENSES
Effective July 24, 2021, the Company’s indirect, wholly-owned subsidiary, Beckman Coulter, Inc. (“Beckman”), entered into a series of related agreements with Quidel Corporation and a subsidiary thereof (“Quidel”) to resolve litigation that Beckman initiated against Quidel and to modify and partially terminate the related prior commercial arrangement. Pursuant to the related agreements, the dispute regarding Beckman’s ability to compete in B-type Naturietic Peptide (“BNP”) test related activities has been settled, allowing Beckman to research, develop, manufacture and distribute BNP type tests. Beckman’s commitment to supply certain BNP test kits to Quidel has also been terminated. Beckman also obtained the right to distribute and sell the BNP assay currently sold by Quidel. As consideration under the agreements, Beckman will pay Quidel predominantly fixed payments of approximately $75 million per year through 2029 (subject to proration in 2021). The Company engaged a third-party valuation specialist to assist in determining the value of the elements of the transaction. The present value of the payments to Quidel was estimated to be $581 million, of which $547 million was recorded as a pretax contract settlement expense primarily due to the unfavorable nature of the prior arrangement (consisting of a cash charge of $5 million and a noncash charge of $542 million) in 2021 related to the modification and partial termination of the prior commercial arrangement and resolution of the associated litigation. The Company also capitalized $34 million in intangible assets, comprised of proprietary technology, customer relationships and the use of a trade name acquired in the settlement, which represent a noncash investing activity. Due to the extended payment terms of the arrangement, the arrangement represents a noncash financing activity of $576 million. Over the period of the arrangement, the cash payments related to servicing the obligation due to Quidel are recorded as cash outflows from financing activities and the payments related to the imputed interest on the obligation due to Quidel are recorded as cash outflows from operating activities in the Consolidated Statements of Cash Flows.
NOTE 9. NONOPERATING INCOME (EXPENSE)
The following sets forth the components of the Company’s other income (expense), net ($ in millions):
| | | | | | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 | | | | |
Other components of net periodic benefit costs | $ | 45 | | | $ | 42 | | | $ | 16 | | | | | |
| Investment gains (losses): | | | | | | | | | |
| Realized investment gains (losses) | 123 | | | 120 | | | 25 | | | | | |
| Unrealized investment gains (losses) | (394) | | | 281 | | | (7) | | | | | |
| Total investment gains (losses) | (271) | | | 401 | | | 18 | | | | | |
| Gains on sale of product lines | — | | | 13 | | | 455 | | | | | |
| Other | — | | | — | | | 5 | | | | | |
| Total other income (expense), net | $ | (226) | | | $ | 456 | | | $ | 494 | | | | | |
Other Components of Net Period Benefit Costs
The Company disaggregates the service cost component of net periodic benefit costs of noncontributory defined benefit pension plans and other postretirement employee benefit plans and presents the other components of net periodic benefit costs in other income (expense), net. These other components of net period benefit costs include the assumed rate of return on plan assets, partially offset by amortization of actuarial losses. The Company’s net periodic benefit costs for the year ended December 31, 2022 includes a settlement loss of $10 million ($9 million after-tax) as a result of the transfer of a portion of its non-U.S. pension liabilities related to one defined benefit plan to a third-party.
Investment Gains (Losses)
The Company estimates the fair value of investments in equity securities using the Fair Value Alternative and records adjustments to fair value within net earnings. Additionally, the Company is a limited partner in partnerships that invest primarily in early stage companies. While the partnerships record these investments at fair value, the Company’s investments in the partnerships are accounted for under the equity method of accounting. The investment gains (losses) include realized and unrealized gains and losses related to changes in the fair value of the Company’s investments in equity securities and the Company’s equity in earnings of the partnerships that reflect the changes in fair value of the investments of the partnerships and related management fees and operating expenses. In addition, during 2022 the Company recorded an impairment of $91 million related to an equity method investment that is reflected in unrealized investment gains (losses).
Gains on Sale of Product Lines
During 2021 the Company divested certain product lines for a cash purchase price, net of cash transferred and transaction costs, of $26 million and recognized a pretax gain on sale of $13 million ($10 million after-tax). The divested product lines generated revenues of approximately $88 million in the Environmental & Applied Solutions segment in 2020. The divestiture of these product lines did not represent a strategic shift with a major effect on the Company’s operations and financial results and therefore is not reported as a discontinued operation.
As a condition to obtaining certain regulatory approvals for the closing of the Cytiva Acquisition, the Company was required to divest certain of its existing product lines in the Biotechnology and Life Sciences segments that in the aggregate generated revenues of approximately $170 million in 2019. During 2020, the Company completed the sale of these product lines for a cash purchase price, net of cash transferred and transaction costs, of $826 million and recognized a pretax gain on sale of $455 million ($305 million after-tax) in the second quarter of 2020. The divestiture of these product lines did not represent a strategic shift with a major effect on the Company’s operations and financial results and therefore is not reported as a discontinued operation.
Loss on Early Extinguishment of Borrowings
In the fourth quarter of 2021, the Company redeemed the €800 million aggregate principal amount of 2.5% senior unsecured notes due 2025 at a redemption price equal to the outstanding principal amount and a make-whole premium as specified in the applicable indenture, plus accrued and unpaid interest. The Company recorded a loss on early extinguishment of these borrowings related to the payment of the make-whole premiums and deferred costs in connection with the redemption of $96 million ($73 million after-tax), which is reflected as a loss on early extinguishment of borrowings in the Consolidated Statements of Earnings.
In the fourth quarter of 2020, the Company redeemed the €800 million aggregate principal amount of 1.7% senior unsecured notes due 2022 at a redemption price equal to the outstanding principal amount and a make-whole premium as specified in the applicable indenture, plus accrued and unpaid interest. The Company recorded a loss of $26 million ($20 million after-tax) on early extinguishment of these borrowings, including deferred costs, related to the payment of the make-whole premiums in connection with the redemption which is reflected as a loss on early extinguishment of borrowings in the Consolidated Statements of Earnings.
NOTE 10. LEASES
The Company has operating leases for office space, warehouses, distribution centers, research and development facilities, manufacturing locations and certain equipment, primarily automobiles. Many leases include one or more options to renew, some of which include options to extend the leases for up to 30 years, and some leases include options to terminate the leases within 30 days. In certain of the Company’s lease agreements, the rental payments are adjusted periodically to reflect actual charges incurred for common area maintenance, utilities, inflation and/or changes in other indexes. The Company’s finance leases were not material as of December 31, 2022 and 2021. Right-of-use (“ROU”) assets arising from finance leases are included in property, plant and equipment, net and the liabilities are included in notes payable and current portion of long-term debt and long-term debt in the accompanying Consolidated Balance Sheets.
The Consolidated Financial Statements include the following amounts related to operating leases where the Company is the lessee ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | |
| | 2022 | | 2021 | | 2020 | | |
| Consolidated Statements of Earnings | | | | | | | | |
Fixed operating lease expense (a) | | $ | 239 | | | $ | 246 | | | $ | 216 | | | |
| Variable operating lease expense | | 73 | | | 59 | | | 46 | | | |
| Total operating lease expense | | $ | 312 | | | $ | 305 | | | $ | 262 | | | |
| | | | | | | | |
| Consolidated Statements of Cash Flows | | | | | | | | |
| Cash paid for amounts included in the measurement of operating lease liabilities | | $ | 259 | | | $ | 243 | | | $ | 221 | | | |
| ROU assets obtained in exchange for operating lease obligations | | 212 | | | 289 | | | 246 | | | |
| | | | | | | | |
| Consolidated Balance Sheets | | | | December 31, 2022 | | December 31, 2021 | | |
| Lease Assets and Liabilities | Classification | | | | | | |
| Operating lease ROU assets | Other long-term assets | | $ | 1,001 | | | $ | 1,041 | | | |
| | | | | | | | |
| Operating lease liabilities - current | Accrued expenses and other liabilities | | $ | 199 | | | $ | 207 | | | |
| Operating lease liabilities - long-term | Other long-term liabilities | | 863 | | | 889 | | | |
| Total operating lease liabilities | | | | $ | 1,062 | | | $ | 1,096 | | | |
| | | | | | | | |
| Weighted average remaining lease term | | | | 8 years | | 8 years | | |
| Weighted average discount rate | | | | 2.7 | % | | 2.7 | % | | |
(a) Includes short-term leases and sublease income, both of which were immaterial.
The following table presents the maturity of the Company’s operating lease liabilities as of December 31, 2022 ($ in millions):
| | | | | |
| 2023 | $ | 222 | |
| 2024 | 189 | |
| 2025 | 156 | |
| 2026 | 126 | |
| 2027 | 99 | |
| Thereafter | 401 | |
| Total operating lease payments | 1,193 | |
| Less: imputed interest | (131) | |
| Total operating lease liabilities | $ | 1,062 | |
As of December 31, 2022, the Company had no additional significant operating or finance leases that had not yet commenced.
NOTE 11. GOODWILL AND OTHER INTANGIBLE ASSETS
As discussed in Note 2, goodwill arises from the purchase price for acquired businesses exceeding the fair value of tangible and intangible assets acquired less assumed liabilities and noncontrolling interests. Management assesses the goodwill of each of its reporting units for impairment at least annually at the beginning of the fourth quarter and as “triggering” events occur that indicate that it is more likely than not that an impairment exists. The Company elected to bypass the optional qualitative goodwill assessment allowed by applicable accounting standards and performed a quantitative impairment test for all reporting units as this was determined to be the most effective method to assess for impairment across the reporting units.
The Company estimates the fair value of its reporting units primarily using a market approach, based on current trading multiples of EBITDA for companies operating in businesses similar to each of the Company’s reporting units, in addition to recent available market sale transactions of comparable businesses. In determining the estimated fair value of each reporting unit, the Company also applies a control premium. If the estimated fair value of the reporting unit is less than its carrying value, the Company must perform additional analysis to determine if the reporting unit’s goodwill has been impaired.
As a result of the Company’s change to its reportable segments in the fourth quarter of 2022 (refer to Note 6 for additional information), the Company also changed its reporting units for goodwill aggregation and impairment testing and the number of reporting units increased from five reporting units to eight reporting units. As of December 31, 2022, the Company had eight reporting units for goodwill impairment testing. The Company used the relative fair value method to reallocate goodwill to the associated reporting units impacted by the change in reportable segments in the fourth quarter of 2022, resulting in goodwill of approximately $21.0 billion (including the impact of 2022 acquisitions prior to the allocation date) and $8.1 billion being allocated to the Biotechnology and Life Sciences reportable segments, respectively. The Company performed the annual quantitative goodwill impairments analysis immediately prior to and following the change in reportable segments. As of the date of the 2022 annual impairment test, the carrying value of the goodwill included in each individual reporting unit ranged from $524 million to approximately $29.1 billion for the previous five reporting units and $524 million to approximately $21.0 billion for the current eight reporting units. No goodwill impairment charges were recorded for the years ended December 31, 2022, 2021 and 2020 and no “triggering” events have occurred subsequent to the performance of the 2022 annual impairment test. The factors used by management in its impairment analysis are inherently subject to uncertainty. If actual results are not consistent with management’s estimates and assumptions, goodwill and other intangible assets may be overstated and a charge would need to be taken against net earnings.
The following is a rollforward of the Company’s goodwill by segment ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Biotechnology | | Life Sciences | | Diagnostics | | Environmental & Applied Solutions | | Total |
| Balance, January 1, 2021 | $ | — | | | $ | 25,812 | | | $ | 7,082 | | | $ | 2,526 | | | $ | 35,420 | |
| Attributable to 2021 acquisitions | — | | | 7,077 | | | 110 | | | 48 | | | 7,235 | |
| Attributable to 2021 divestitures | — | | | — | | | — | | | (12) | | | (12) | |
| Adjustments due to finalization of purchase price allocations | — | | | (11) | | | — | | | — | | | (11) | |
| Foreign currency translation and other | — | | | (1,240) | | | (148) | | | (60) | | | (1,448) | |
| Balance, December 31, 2021 | — | | | 31,638 | | | 7,044 | | | 2,502 | | | 41,184 | |
Attributable to acquisitions (a) | — | | | 157 | | | — | | | 40 | | | 197 | |
| Adjustments due to finalization of purchase price allocations | — | | | 26 | | | (9) | | | 1 | | | 18 | |
| Foreign currency translation and other | — | | | (2,676) | | | (330) | | | (142) | | | (3,148) | |
| Balance, before resegmentation | — | | | 29,145 | | | 6,705 | | | 2,401 | | | 38,251 | |
Reallocation among new reporting units (a) | 21,019 | | | (21,019) | | | — | | | — | | | — | |
| Attributable to acquisitions | 176 | | | 43 | | | 13 | | | (2) | | | 230 | |
| | | | | | | | | |
| Adjustments due to finalization of purchase price allocations | — | | | (2) | | | — | | | — | | | (2) | |
| Foreign currency translation and other | 892 | | | 147 | | | 157 | | | 77 | | | 1,273 | |
| Balance, December 31, 2022 | $ | 22,087 | | | $ | 8,314 | | | $ | 6,875 | | | $ | 2,476 | | | $ | 39,752 | |
(a) A total of approximately $21.0 billion of goodwill was allocated to the Biotechnology reportable segment, of which $116 million is shown on the Attributable to acquisitions line before resegmentation as it relates to a 2022 acquisition that occurred prior to the allocation date.
Finite-lived intangible assets are amortized over their legal or estimated useful life. The following summarizes the gross carrying value and accumulated amortization for each major category of intangible assets as of December 31 ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2022 | | 2021 |
| | Gross Carrying
Amount | | Accumulated
Amortization | | Gross Carrying
Amount | | Accumulated
Amortization |
| Finite-lived intangibles: | | | | | | | |
| Patents and technology | $ | 13,508 | | | $ | (3,024) | | | $ | 14,377 | | | $ | (2,281) | |
| Customer relationships, trade names and other intangibles | 10,183 | | | (4,212) | | | 9,547 | | | (3,748) | |
| Total finite-lived intangibles | 23,691 | | | (7,236) | | | 23,924 | | | (6,029) | |
| Indefinite-lived intangibles: | | | | | | | |
| Trademarks and trade names | 3,845 | | | — | | | 4,948 | | | — | |
| Total intangibles | $ | 27,536 | | | $ | (7,236) | | | $ | 28,872 | | | $ | (6,029) | |
During 2022, the Company acquired finite-lived intangible assets, consisting primarily of developed technology, customer relationships and trade names, with a weighted average life of 12 years. During 2021, the Company acquired finite-lived intangible assets, consisting primarily of developed technology, customer relationships and trade names, with a weighted average life of 13 years primarily as a result of the Aldevron Acquisition. Refer to Note 2 for additional information on the intangible assets acquired.
The Company reviews identified intangible assets for impairment whenever events or changes in circumstances indicate that the related carrying amounts may not be recoverable. Indefinite-lived intangibles are subject to impairment testing at least annually or more frequently if events or changes in circumstances indicate that potential impairment exists. The Company identified impairment triggers during the second quarter of 2022 in the Environmental & Applied Solutions segment and the first quarter of 2021 in the Diagnostics segment which resulted in the impairment of certain long-lived assets, including technology, customer relationships and trade names. In 2022 and 2021, the Company recorded impairment charges totaling $9 million and $10 million, respectively, related to these long-lived assets in selling, general and administrative expenses in the Consolidated Statements of Earnings.
Total intangible amortization expense in 2022, 2021 and 2020 was $1,484 million, $1,450 million and $1,138 million, respectively. Based on the intangible assets recorded as of December 31, 2022, amortization expense is estimated to be approximately $1.5 billion during 2023, $1.5 billion during 2024, $1.5 billion during 2025, $1.4 billion during 2026 and $1.3 billion during 2027.
NOTE 12. FAIR VALUE MEASUREMENTS
Accounting standards define fair value based on an exit price model, establish a framework for measuring fair value where the Company’s assets and liabilities are required to be carried at fair value and provide for certain disclosures related to the valuation methods used within a valuation hierarchy as established within the accounting standards. This hierarchy prioritizes the inputs into three broad levels as follows. Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets in markets that are not active, or other observable characteristics for the asset or liability, including interest rates, yield curves and credit risks, or inputs that are derived principally from, or corroborated by, observable market data through correlation. Level 3 inputs are unobservable inputs based on the Company’s assumptions. A financial asset or liability’s classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement in its entirety.
A summary of financial assets that are measured at fair value on a recurring basis were as follows ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31 | | Quoted Prices in
Active Market
(Level 1) | | Significant Other
Observable Inputs
(Level 2) | | Significant
Unobservable
Inputs (Level 3) |
| 2022 | | 2021 | | 2022 | | 2021 | | 2022 | | 2021 | | 2022 | | 2021 |
| Assets: | | | | | | | | | | | | | | | |
| Available-for-sale debt securities | $ | 11 | | | $ | 20 | | | $ | — | | | $ | — | | | $ | 11 | | | $ | 20 | | | $ | — | | | $ | — | |
| Investment in equity securities | 315 | | | 336 | | | 16 | | | 88 | | | — | | | — | | | — | | | — | |
| Cross-currency swap derivative contracts | 653 | | | 50 | | | — | | | — | | | 653 | | | 50 | | | — | | | — | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
Available-for-sale debt securities, which are included in other long-term assets in the accompanying Consolidated Balance Sheets, are measured at fair value using quoted prices reported by investment brokers and dealers based on the underlying terms of the security and comparison to similar securities traded on an active market. As of December 31, 2022 and 2021, available-for-sale debt securities primarily included U.S. Treasury Notes and corporate debt securities.
The Company’s investments in equity securities consist of investments in publicly traded equity securities and investments in non-marketable equity securities. The publicly traded securities are classified as Level 1 in the fair value hierarchy as they are measured based on quotes in active markets. For the non-marketable equity securities, the Company estimates the fair value of the investments in equity securities based on the measurement alternative and adjusts for impairments and observable price changes with a same or similar security from the same issuer within net earnings (the “Fair Value Alternative”). The Company’s investments in these equity securities are not classified in the fair value hierarchy due to the use of these measurement methods. Additionally, the Company is a limited partner in partnerships that invest primarily in early-stage companies. While the partnerships record these investments at fair value, the Company’s investments in the partnerships are accounted for under the equity method of accounting and are not subject to fair value measurement disclosures. As of December 31, 2022 and 2021, the Company’s equity method investments included investments in partnerships with a carrying value of approximately $1.5 billion and $1.3 billion, respectively. During the years ended December 31, 2022 and 2021, the Company recorded net realized and unrealized losses of $271 million and net realized and unrealized gains of $401 million, respectively, related to changes in the fair value of the Company’s investments in equity securities and the Company’s equity in earnings of the partnerships that reflect the changes in fair value of the investments of the partnerships. Refer to Note 9 for additional information on gains and losses on the Company’s investments, including investments in the partnerships.
The cross-currency swap derivative contracts are used to partially hedge the Company’s net investments in non-U.S. operations against adverse movements in exchange rates between the U.S. dollar and the Danish kroner, Japanese yen, euro and Swiss franc. The Company also uses cross-currency swap derivative contracts to hedge the exchange rate exposure from long-term debt issuances in a foreign currency other than the functional currency of the borrower. The cross-currency swap derivative contracts are classified as Level 2 in the fair value hierarchy as they are measured using the income approach with the relevant interest rates and current foreign currency exchange rates and forward curves as inputs. Refer to Note 15 for additional information.
Fair Value of Other Financial Instruments
The carrying amounts and fair values of the Company’s other financial instruments as of December 31 were as follows ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| 2022 | | 2021 |
| Carrying
Amount | | Fair Value | | Carrying
Amount | | Fair Value |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Debt obligations: | | | | | | | |
| | | | | | | |
| Notes payable and current portion of long-term debt | $ | 591 | | | $ | 584 | | | $ | 8 | | | $ | 8 | |
| Long-term debt | 19,086 | | | 16,079 | | | 22,168 | | | 22,796 | |
As of December 31, 2022 and 2021, short and long-term borrowings were categorized as Level 1. The fair value of long-term borrowings was based on quoted market prices. The difference between the fair value and the carrying amounts of long-term borrowings is attributable to changes in market interest rates and/or the Company’s credit ratings subsequent to the incurrence of the borrowing. The fair values of borrowings with original maturities of one year or less, as well as cash and cash equivalents, trade accounts receivable, net and trade accounts payable generally approximate their carrying amounts due to the short-term maturities of these instruments.
Refer to Note 16 for information related to the fair value of the Company sponsored defined benefit pension plan assets.
NOTE 13. ACCRUED EXPENSES AND OTHER LIABILITIES
Accrued expenses and other liabilities as of December 31 were as follows ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2022 | | 2021 |
| | Current | | Noncurrent | | Current | | Noncurrent |
| Compensation and benefits | $ | 1,375 | | | $ | 255 | | | $ | 1,371 | | | $ | 269 | |
| Pension and postretirement benefits | 59 | | | 506 | | | 72 | | | 876 | |
| Taxes, income and other | 691 | | | 4,213 | | | 707 | | | 4,750 | |
| Deferred revenue and customer advance payments | 1,648 | | | 235 | | | 1,613 | | | 213 | |
| Sales and product allowances | 198 | | | 6 | | | 221 | | | 2 | |
| Operating lease liabilities | 199 | | | 863 | | | 207 | | | 889 | |
| | | | | | | |
| Contract settlement financing payable | 75 | | | 420 | | | 75 | | | 481 | |
| Other | 1,257 | | | 287 | | | 1,297 | | | 219 | |
| Total | $ | 5,502 | | | $ | 6,785 | | | $ | 5,563 | | | $ | 7,699 | |
NOTE 14. FINANCING
The components of the Company’s debt as of December 31 were as follows (amounts in millions):
| | | | | | | | | | | | | | | | |
| | Outstanding Amount | | |
| Description and Aggregate Principal Amount | | 2022 | | 2021 | | |
U.S. dollar-denominated commercial paper(f) | | $ | — | | | $ | 1,440 | | | |
Euro-denominated commercial paper (€1.9 billion and €1.2 billion, respectively)(e) | | 2,013 | | | 1,366 | | | |
| | | | | | |
| | | | | | |
| | | | | | |
Floating rate senior unsecured notes due 6/30/2022 (€250 million) (the “Floating Rate 2022 Euronotes”)(a) | | — | | | 284 | | | |
2.05% senior unsecured notes due 11/15/2022 ($700 million) (the “2022 Biopharma Notes”)(b) | | — | | | 699 | | | |
0.5% senior unsecured bonds due 12/08/2023 (CHF 540 million) (the “2023 CHF Bonds”)(c) | | 584 | | | 592 | | | |
1.7% senior unsecured notes due 3/30/2024 (€900 million) (the “2024 Euronotes”)(f) | | 962 | | | 1,021 | | | |
2.2% senior unsecured notes due 11/15/2024 ($700 million) (the “2024 Biopharma Notes”)(b) | | 698 | | | 698 | | | |
| | | | | | |
3.35% senior unsecured notes due 9/15/2025 ($500 million) (the “2025 U.S. Notes”)(f) | | 499 | | | 498 | | | |
0.2% senior unsecured notes due 3/18/2026 (€1.3 billion) (the “2026 Biopharma Euronotes”)(b) | | 1,333 | | | 1,416 | | | |
2.1% senior unsecured notes due 9/30/2026 (€800 million) (the “2026 Euronotes”)(f) | | 854 | | | 907 | | | |
0.3% senior unsecured notes due 5/11/2027 (¥30.8 billion) (the “2027 Yen Notes”)(d) | | 234 | | | 267 | | | |
1.2% senior unsecured notes due 6/30/2027 (€600 million) (the “2027 Euronotes”)(a) | | 639 | | | 680 | | | |
0.45% senior unsecured notes due 3/18/2028 (€1.3 billion) (the “2028 Biopharma Euronotes”)(b) | | 1,331 | | | 1,413 | | | |
1.125% senior unsecured bonds due 12/08/2028 (CHF 210 million) (the “2028 CHF Bonds”)(c) | | 230 | | | 233 | | | |
2.6% senior unsecured notes due 11/15/2029 ($800 million) (the “2029 Biopharma Notes”)(b) | | 796 | | | 795 | | | |
2.5% senior unsecured notes due 3/30/2030 (€800 million) (the “2030 Euronotes”)(f) | | 856 | | | 910 | | | |
0.75% senior unsecured notes due 9/18/2031 (€1.8 billion) (the “2031 Biopharma Euronotes”)(b) | | 1,863 | | | 1,980 | | | |
0.65% senior unsecured notes due 5/11/2032 (¥53.2 billion) (the “2032 Yen Notes”)(d) | | 404 | | | 461 | | | |
1.35% senior unsecured notes due 9/18/2039 (€1.3 billion) (the “2039 Biopharma Euronotes”)(b) | | 1,323 | | | 1,406 | | | |
3.25% senior unsecured notes due 11/15/2039 ($900 million) (the “2039 Biopharma Notes”)(b) | | 890 | | | 890 | | | |
4.375% senior unsecured notes due 9/15/2045 ($500 million) (the “2045 U.S. Notes”)(f) | | 499 | | | 499 | | | |
1.8% senior unsecured notes due 9/18/2049 (€750 million) (the “2049 Biopharma Euronotes”)(b) | | 794 | | | 844 | | | |
3.4% senior unsecured notes due 11/15/2049 ($900 million) (the “2049 Biopharma Notes”)(b) | | 889 | | | 889 | | | |
2.6% senior unsecured notes due 10/01/2050 ($1.0 billion) (the “2050 U.S. Notes”)(f) | | 981 | | | 980 | | | |
2.8% senior unsecured notes due 12/10/2051 ($1.0 billion) (the “2051 U.S. Notes”)(f) | | 984 | | | 983 | | | |
| Other | | 21 | | | 25 | | | |
| Total debt | | 19,677 | | | 22,176 | | | |
| Less: currently payable | | (591) | | | (8) | | | |
| Long-term debt | | $ | 19,086 | | | $ | 22,168 | | | |
(a) Issued by DH Europe Finance S.A. (“Danaher International”).
(b) Issued by DH Europe Finance II S.a.r.l. (“Danaher International II”).
(c) Issued by DH Switzerland Finance S.A. (“Danaher Switzerland”).
(d) Issued by DH Japan Finance S.A. (“Danaher Japan”).
(e) Issued by Danaher Corporation or Danaher International II.
(f) Issued by Danaher Corporation.
Debt discounts, premiums and debt issuance and other related costs totaled $118 million and $130 million as of December 31, 2022 and 2021, respectively, and have been netted against the aggregate principal amounts of the related debt in the components of debt table above.
Commercial Paper Programs and Credit Facilities
In 2019, the Company entered into a $5.0 billion unsecured revolving credit facility with a syndicate of banks that expires on August 27, 2024, subject to a one-year extension option at the request of the Company with the consent of the lenders (the “Five-Year Facility”). The Five-Year Facility also contains an expansion option permitting Danaher to request up to five increases of up to an aggregate additional $2.5 billion from lenders that elect to make such increase available, upon the satisfaction of certain conditions.
The Company expects to limit borrowings under the Five-Year Facility to amounts that would leave sufficient borrowing capacity under the facilities so that it could borrow, if needed, to repay all of the outstanding commercial paper as it matures.
On February 21, 2022, the Company and the syndicate of banks amended the Five-Year Facility to replace references to the London Interbank Offered Rate with references to the Sterling Overnight Index Average Reference Rate, the Tokyo Interbank Offer Rate or the Euro Interbank Offer Rate depending on the applicable currency of the borrowing.
Borrowings under the Five-Year Facility bear interest as follows: (1) Eurocurrency Rate Committed Loans (as defined in the Five-Year Facility) bear interest at a variable rate equal to the Sterling Overnight Index Average Reference Rate, the Tokyo Interbank Offer Rate or the Euro Interbank Offer Rate plus a margin of between 58.5 and 100 basis points, depending on Danaher’s long-term debt credit rating; (2) Base Rate Committed Loans and Swing Line Loans (each as defined in the Five-Year Facility) bear interest at a variable rate equal to the highest of (a) the Federal funds rate (as published by the Federal Reserve Bank of New York from time to time) plus 50 basis points; (b) Bank of America’s “prime rate” as publicly announced from time to time and (c) the Eurocurrency Rate (as defined in the Five-Year Facility) plus 100 basis points; and (3) Bid Loans (as defined in the Five-Year Facility) bear interest at the rate bid by the particular lender providing such loan. In addition, Danaher is required to pay a per annum facility fee of between 4.0 and 12.5 basis points (depending on Danaher’s long-term debt credit rating) based on the aggregate commitments under the Five-Year Facility, regardless of usage.
The Five-Year Facility requires the Company to maintain a consolidated leverage ratio (as defined in the facility) of 0.65 to 1.00 or less. Borrowings under the Five-Year Facility are prepayable at the Company’s option at any time in whole or in part without premium or penalty. As of December 31, 2022, no borrowings were outstanding under the Five-Year Facility and the Company was in compliance with all covenants under the facilities. The nonperformance by any member of the Five-Year Facility syndicates would reduce the maximum capacity of the Five-Year Facility by such member’s commitment amount.
The Company’s obligations under the Five-Year Facility are unsecured. The Company has unconditionally and irrevocably guaranteed the obligations of each of its subsidiaries in the event a subsidiary is named a borrower under the Five-Year Facility. The Five-Year Facility contains customary representations, warranties, conditions precedent, events of default, indemnities and affirmative and negative covenants. The Five-Year Facility is available for liquidity support for Danaher’s U.S. dollar and euro commercial paper programs, as discussed below, and for general corporate purposes.
Under the Company’s U.S. dollar and euro-denominated commercial paper programs, the Company or a subsidiary of the Company, as applicable, may issue and sell unsecured, short-term promissory notes. The notes are typically issued at a discount from par, generally based on the ratings assigned to the Company by credit rating agencies at the time of the issuance and prevailing market rates. The Five-Year Facility provides liquidity support for issuances under the Company’s commercial paper programs, and can also be used for working capital and other general corporate purposes. The availability of the Five-Year Facility as a standby liquidity facility to repay maturing commercial paper is an important factor in maintaining the existing credit ratings of the Company’s commercial paper programs. As commercial paper obligations mature, the Company may issue additional short-term commercial paper obligations to refinance all or part of these borrowings. As of December 31, 2022, borrowings outstanding under the Company’s euro-denominated commercial paper programs had a weighted average annual interest rate of 1.89% and a weighted average remaining maturity of approximately 30 days. As of December 31, 2022, the Company has classified approximately $2.0 billion of its borrowings outstanding under the euro-denominated commercial paper programs as long-term debt in the accompanying Consolidated Balance Sheet (even though such borrowings are scheduled to mature within one year of December 31, 2022) as the Company had the intent and ability, as supported by availability under the Five-Year Facility, to refinance these borrowings for at least one year from the balance sheet date.
The Company’s ability to access the commercial paper market, and the related costs of these borrowings, is affected by the strength of the Company’s credit rating and market conditions. Any downgrade in the Company’s credit rating would increase the cost of borrowings under the Company’s commercial paper program and the Five-Year Facility, and could limit or preclude the Company’s ability to issue commercial paper. If the Company’s access to the commercial paper market is adversely affected due to a credit downgrade, change in market conditions or otherwise, the Company expects it would rely on a combination of available cash, operating cash flow, the Five-Year Facility and any other available sources of financing to provide short-term funding. In such event, the cost of borrowings under the Five-Year Facility or other available sources of financing could be higher than the cost of commercial paper borrowings.
Covenants and Redemption Provisions Applicable to Notes
With respect to the 2027 and 2032 Yen Notes; the 2024, 2026, 2027 and 2030 Euronotes; the 2025, 2045, 2050 and 2051 U.S. Notes; the 2022 (prior to their repayment in the fourth quarter of 2022), 2024, 2029, 2039 and 2049 Biopharma Notes; and the 2026, 2028, 2031, 2039 and 2049 Biopharma Euronotes, at any time prior to the applicable maturity date, the Company may redeem the applicable series of notes in whole or in part, by paying the principal amount accrued and unpaid interest and, until the par call date specified in the applicable indenture or comparable governing document, the “make-whole” premium specified therein (and in the case of the Yen Notes, net of certain swap-related gains or losses as applicable). With respect to each of the 2023 and 2028 CHF Bonds, at any time after 85% or more of the applicable bonds have been redeemed or purchased and canceled, the Company may redeem some or all of the remaining bonds for their principal amount plus accrued and unpaid
interest. With respect to the 2027 and 2032 Yen Notes; Floating Rate 2022 (prior to their repayment in the second quarter of 2022), 2024, 2026, 2027 and 2030 Euronotes; the 2023 and 2028 CHF Bonds; and the 2026, 2028, 2031, 2039 and 2049 Biopharma Euronotes, the Company may redeem such notes and bonds upon the occurrence of specified, adverse changes in tax laws, or interpretations under such laws, at a redemption price equal to the principal amount of the bonds to be redeemed.
If a change of control triggering event occurs with respect to any of the 2027 and 2032 Yen Notes; the Floating Rate 2022 (prior to their repayment in the second quarter of 2022), 2024, 2026, 2027 and 2030 Euronotes; the 2025, 2045, 2050 and 2051 U.S. Notes; the 2023 and 2028 CHF Bonds; the 2022 (prior to their repayment in the fourth quarter of 2022), 2024, 2029, 2039 and 2049 Biopharma Notes; or the 2026, 2028, 2031, 2039 and 2049 Biopharma Euronotes, each holder of such notes may require the Company to repurchase some or all of such notes and bonds at a purchase price equal to 101% (100% in the case of the 2027 and 2032 Yen Notes) of the principal amount of the notes and bonds, plus accrued and unpaid interest (and in the case of the Yen Notes, certain swap-related losses as applicable). A change of control triggering event means the occurrence of both a change of control and a rating event, each as defined in the applicable indenture or comparable governing document. Except in connection with a change of control triggering event, the Company does not have any credit rating downgrade triggers that would accelerate the maturity of a material amount of outstanding debt. Each holder of the 2027 and 2032 Yen Notes may also require the Company to repurchase some or all of its notes at a purchase price equal to 100% of the principal amount of the notes, plus accrued and unpaid interest and certain swap-related losses as applicable, in certain circumstances whereby such holder comes into violation of economic sanctions laws as a result of holding such notes.
The respective indentures or comparable governing documents under which the above-described notes and bonds were issued contain customary covenants including, for example, limits on the incurrence of secured debt and sale-leaseback transactions. None of these covenants are considered restrictive to the Company’s operations and as of December 31, 2022, the Company was in compliance with all of its debt covenants.
LYONs
In 2001, the Company issued $830 million (value at maturity) in LYONs. Pursuant to the terms of the indenture that governed the Company’s LYONs, each $1,000 of principal amount at maturity could be converted into 38.1998 shares of Danaher common stock at any time on or before the maturity date of January 22, 2021.
During the year ended December 31, 2021, holders of certain of the Company’s LYONs converted such LYONs into an aggregate of approximately 912 thousand shares of the Company’s common stock, par value $0.01 per share. The Company’s deferred tax liability of $10 million associated with the book and tax basis difference in the converted LYONs was transferred to additional paid-in capital. The residual LYONS not converted into shares of the Company’s stock were redeemed at face value on January 22, 2021.
Long-Term Debt Repayments
On June 30, 2022, the Company repaid the €250 million aggregate principal amount of the Floating Rate 2022 Euronotes and on November 15, 2022 the Company repaid the €700 million aggregate principal amount of the 2022 Biopharma Euronotes upon their maturity using available cash and the proceeds from the issuance of commercial paper. The ¥30.0 billion aggregate principal amount of the 2021 Yen Notes were repaid during the first quarter of 2021 using proceeds from the issuance of commercial paper. During 2021 and 2020, the Company redeemed certain outstanding borrowings in advance of their scheduled maturities. Refer to Note 9 for details of these redemptions and the related losses on early extinguishment of borrowings incurred on such redemptions.
Guarantors of Debt
The Company has guaranteed long-term debt and commercial paper issued by certain of its wholly-owned finance subsidiaries: Danaher International, Danaher International II, Danaher Switzerland and Danaher Japan. All of the outstanding and future securities issued by each of these entities are or will be fully and unconditionally guaranteed by the Company and these guarantees rank on parity with the Company’s unsecured and unsubordinated indebtedness.
Other
The Company’s minimum principal payments for the next five years are as follows ($ in millions):
| | | | | |
| 2023 | $ | 591 | |
| 2024 | 3,661 | |
| 2025 | 489 | |
| 2026 | 2,185 | |
| 2027 | 871 | |
| Thereafter | 11,880 | |
The Company made interest payments of $347 million, $452 million and $331 million in 2022, 2021 and 2020, respectively. Interest payments decreased in 2022 due primarily to the decrease in outstanding debt in 2022 and 2021 and the year-over-year decrease in make-whole premiums on the early extinguishment of debt.
NOTE 15. HEDGING TRANSACTIONS AND DERIVATIVE FINANCIAL INSTRUMENTS
The Company uses cross-currency swap derivative contracts to partially hedge its net investments in foreign operations against adverse movements in exchange rates between the U.S. dollar and the Danish kroner, Japanese yen, euro and Swiss franc. The cross-currency swap derivative contracts are agreements to exchange fixed-rate payments in one currency for fixed-rate payments in another currency. These contracts effectively convert U.S. dollar-denominated bonds to obligations denominated in Danish kroner, Japanese yen, euro and Swiss franc, and partially offset the impact of changes in currency rates on the Company’s foreign currency denominated net investments. These contracts also reduce the interest rate from the stated interest rates on the U.S. dollar-denominated debt to the interest rates of the swaps. The changes in the spot rate of these instruments are recorded in accumulated other comprehensive income (loss) in stockholders’ equity, partially offsetting the foreign currency translation adjustment of the Company’s related net investment that is also recorded in accumulated other comprehensive income (loss). The interest income or expense from these swaps are recorded in interest expense in the accompanying Consolidated Statements of Earnings consistent with the classification of interest expense attributable to the underlying debt. These instruments mature on dates ranging from September 2025 to December 2031.
The Company also uses cross-currency swap derivative contracts to hedge U.S. dollar-denominated long-term debt issuances in a foreign subsidiary whose functional currency is the euro against adverse movements in exchange rates between the U.S. dollar and the euro. These contracts effectively convert these U.S. dollar-denominated bonds to obligations denominated in euro. The changes in the fair value of these instruments are recorded in accumulated other comprehensive income (loss), with a reclassification from accumulated other comprehensive income (loss) to net earnings to offset the remeasurement of the hedged debt that is also recorded in net earnings. The interest income or expense from these swaps are recorded in interest expense in the accompanying Consolidated Statements of Earnings consistent with the classification of interest expense attributable to the underlying debt. These instruments mature on dates ranging from November 2024 to November 2049.
The Company has also issued foreign currency denominated long-term debt as partial hedges of its net investments in foreign operations against adverse movements in exchange rates between the U.S. dollar and the euro, Japanese yen and Swiss franc. These foreign currency denominated long-term debt issuances are designated and qualify as nonderivative hedging instruments. Accordingly, the foreign currency translation of these debt instruments is recorded in accumulated other comprehensive income (loss), offsetting the foreign currency translation adjustment of the Company’s related net investment that is also recorded in accumulated other comprehensive income (loss). These instruments mature on dates ranging from January 2023 to May 2032.
The Company used interest rate swap agreements to hedge the variability in cash flows due to changes in benchmark interest rates related to a portion of the U.S. debt the Company issued to fund the Cytiva Acquisition and a portion of the 2051 Notes. These contracts effectively fixed the interest rate for a portion of the Company’s U.S. dollar-denominated debt equal to the notional amount of the swaps to the rate specified in the interest rate swap agreements and were settled in November 2019 and December 2021, respectively. The changes in the fair value of these instruments were recorded in accumulated other comprehensive income (loss) prior to the issuance of the debt and are subsequently being reclassified to interest expense over the life of the related debt.
The following table summarizes the notional values as of December 31, 2022 and 2021 and pretax impact of changes in the fair values of instruments designated as net investment hedges and cash flow hedges in accumulated other comprehensive income (“OCI”) for the year then ended ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| Original Notional Amount | | Notional Amount Outstanding | | Gain (Loss) Recognized in OCI | | Amounts Reclassified from OCI |
| Year ended December 31, 2022: | | | | | | | |
| Net investment hedges: | | | | | | | |
Cross-currency contracts | $ | 3,875 | | | $ | 3,000 | | | $ | 225 | | | $ | — | |
Foreign currency denominated debt | 5,777 | | | 5,777 | | | 248 | | | — | |
| Cash flow hedges: | | | | | | | |
Cross-currency contracts | 4,000 | | | 3,300 | | | 378 | | | (238) | |
| Interest rate swaps | 1,600 | | | — | | | — | | | 3 | |
| Total | $ | 15,252 | | | $ | 12,077 | | | $ | 851 | | | $ | (235) | |
| | | | | | | |
| Year ended December 31, 2021: | | | | | | | |
| Net investment hedges: | | | | | | | |
Cross-currency contracts | $ | 3,875 | | | $ | 3,000 | | | $ | 130 | | | $ | — | |
Foreign currency denominated debt | 3,883 | | | 3,883 | | | 333 | | | — | |
| Cash flow hedges: | | | | | | | |
Cross-currency contracts | 4,000 | | | 4,000 | | | 542 | | | (283) | |
| Interest rate swaps | 1,600 | | | — | | | (19) | | | 3 | |
| Total | $ | 13,358 | | | $ | 10,883 | | | $ | 986 | | | $ | (280) | |
Gains or losses related to the net investment hedges are classified as foreign currency translation adjustments in the schedule of changes in OCI in Note 19, as these items are attributable to the Company’s hedges of its net investment in foreign operations. Gains or losses related to the cash flow hedges are classified as cash flow hedge adjustments in the schedule of changes in OCI in Note 19. The amount reclassified from other comprehensive income (loss) for the cross-currency swap derivative contracts that are cash flow hedges of the Company’s U.S. dollar-denominated debt was equal to the remeasurement amount recorded in the period on the hedged debt.
The Company did not reclassify any other deferred gains or losses related to net investment hedges or cash flow hedges from accumulated other comprehensive income (loss) to earnings during the years ended December 31, 2022 and 2021. In addition, the Company did not have any ineffectiveness related to net investment hedges or cash flow hedges during the years ended December 31, 2022 and 2021, and, should they arise, any ineffective portions of the hedges would be reclassified from accumulated other comprehensive income (loss) into earnings during the period of change. The cash inflows and outflows associated with the Company’s derivative contracts designated as net investment hedges are classified in all other investing activities in the accompanying Consolidated Statements of Cash Flows. The cash inflows and outflows associated with the Company’s derivative contracts designated as cash flow hedges are classified in cash flows from operating activities in the accompanying Consolidated Statements of Cash Flows.
The Company’s derivative instruments, as well as its nonderivative debt instruments designated and qualifying as net investment hedges, were classified as of December 31 in the Company’s Consolidated Balance Sheets as follows ($ in millions):
| | | | | | | | | | | | | |
| 2022 | | 2021 | | |
| Derivative assets: | | | | | |
| Other long-term assets | $ | 653 | | | $ | 50 | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Nonderivative hedging instruments: | | | | | |
| Long-term debt | 5,777 | | | 3,883 | | | |
Amounts related to the Company’s derivatives expected to be reclassified from accumulated other comprehensive income (loss) to net earnings during the next 12 months, if interest rates and foreign exchange rates remain unchanged, are not significant.
NOTE 16. PENSION AND OTHER POSTRETIREMENT EMPLOYEE BENEFIT PLANS
The Company has noncontributory defined benefit pension plans which cover certain of its U.S. employees. During 2012, all remaining benefit accruals under the U.S. plans ceased. Defined benefit plans from acquisitions subsequent to 2012 are ceased as soon as practical. The Company also has noncontributory defined benefit pension plans which cover certain of its non-U.S. employees, and under certain of these plans, benefit accruals continue. In general, the Company’s policy is to fund these plans based on considerations relating to legal requirements, underlying asset returns, the plan’s funded status, the anticipated tax deductibility of the contribution, local practices, market conditions, interest rates and other factors. In addition to providing pension benefits, the Company provides certain health care and life insurance benefits for some of its retired employees in the United States. Certain employees may become eligible for these benefits as they reach normal retirement age while working for the Company.
The following sets forth the funded status of the U.S. pension, non-U.S. pension and postretirement benefit plans as of the most recent actuarial valuations using measurement dates of December 31 ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Pension Benefits | | Non-U.S. Pension Benefits | | Postretirement Benefits |
| | 2022 | | 2021 | | 2022 | | 2021 | | 2022 | | 2021 |
| Change in pension benefit obligation: | | | | | | | | | | | |
| Benefit obligation at beginning of year | $ | (2,532) | | | $ | (2,718) | | | $ | (1,944) | | | $ | (2,161) | | | $ | (135) | | | $ | (148) | |
| Service cost | — | | | — | | | (39) | | | (44) | | | — | | | — | |
| Interest cost | (54) | | | (44) | | | (23) | | | (19) | | | (3) | | | (2) | |
| Employee/retiree contributions | — | | | — | | | (7) | | | (7) | | | (1) | | | (2) | |
| Benefits and other expenses paid | 178 | | | 167 | | | 46 | | | 59 | | | 13 | | | 14 | |
| | | | | | | | | | | |
| Actuarial gain (loss) | 495 | | | 63 | | | 481 | | | 112 | | | 20 | | | 3 | |
| Amendments, settlements and curtailments | — | | | — | | | 66 | | | 18 | | | — | | | — | |
| Foreign exchange rate impact and other | — | | | — | | | 148 | | | 98 | | | — | | | — | |
| Benefit obligation at end of year | (1,913) | | | (2,532) | | | (1,272) | | | (1,944) | | | (106) | | | (135) | |
| Change in plan assets: | | | | | | | | | | | |
| Fair value of plan assets at beginning of year | 2,303 | | | 2,125 | | | 1,360 | | | 1,331 | | | — | | | — | |
| Actual return on plan assets | (278) | | | 335 | | | (322) | | | 80 | | | — | | | — | |
| Employer contributions | 10 | | | 10 | | | 40 | | | 50 | | | 12 | | | 12 | |
| Employee contributions | — | | | — | | | 7 | | | 7 | | | 1 | | | 2 | |
| Amendments and settlements | — | | | — | | | (65) | | | (10) | | | — | | | — | |
| Benefits and other expenses paid | (178) | | | (167) | | | (46) | | | (59) | | | (13) | | | (14) | |
| | | | | | | | | | | |
| Foreign exchange rate impact and other | — | | | — | | | (105) | | | (39) | | | — | | | — | |
| Fair value of plan assets at end of year | 1,857 | | | 2,303 | | | 869 | | | 1,360 | | | — | | | — | |
| Funded status | $ | (56) | | | $ | (229) | | | $ | (403) | | | $ | (584) | | | $ | (106) | | | $ | (135) | |
The largest contributor to the net actuarial gains affecting the benefit obligations in 2022 and 2021 U.S. pension, non-U.S. pension plans and the postretirement benefit plans is increases in the discount rates compared to the rates in the prior year.
Projected benefit obligation (“PBO”) and fair value of plan assets for pension plans and postretirement benefit plans with PBO’s in excess of plan assets ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Pension Benefits | | Non-U.S. Pension Benefits | | Postretirement Benefits |
| 2022 | | 2021 | | 2022 | | 2021 | | 2022 | | 2021 |
Projected benefit obligation | $ | 98 | | | $ | 2,532 | | | $ | 754 | | | $ | 1,125 | | | $ | 106 | | | $ | 135 | |
Fair value of plan assets | — | | | 2,303 | | | 260 | | | 357 | | | — | | | — | |
The year-over-year change in the amounts above reflects the changes in the benefit plans with a fair value of plan assets in excess of the projected benefit obligation.
Accumulated benefit obligation (“ABO”) and fair value of plan assets for pension plans with ABO’s in excess of plan assets ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Pension Benefits | | Non-U.S. Pension Benefits |
| 2022 | | 2021 | | 2022 | | 2021 |
Accumulated benefit obligation | $ | 98 | | | $ | 2,532 | | | $ | 694 | | | $ | 1,184 | |
Fair value of plan assets | — | | | 2,303 | | | 250 | | | 521 | |
The year-over-year change in the amounts above reflects the changes in the benefit plans with a fair value of plan assets in excess of the accumulated benefit obligation.
Weighted average assumptions used to determine benefit obligations at date of measurement:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Pension Benefits | | Non-U.S. Pension Benefits | | Postretirement Benefits |
| | 2022 | | 2021 | | 2022 | | 2021 | | 2022 | | 2021 |
| Discount rate | 5.4 | % | | 2.7 | % | | 3.9 | % | | 1.4 | % | | 5.4 | % | | 2.6 | % |
| Rate of compensation increase | N/A | | N/A | | 3.0 | % | | 2.6 | % | | N/A | | N/A |
In 2022, the medical trend rate used to determine the postretirement benefit obligation was 5.2%. The rate decreases gradually to an ultimate rate of 4.0% by 2046 and remains at that level thereafter. In 2021, the medical trend rate used to determine the postretirement benefit obligation was 5.3%, gradually decreasing to an ultimate rate of 4.0% by 2046 and remaining at that level thereafter. The trend rate is a significant factor in determining the amounts reported.
Components of net periodic pension and postretirement benefit (cost) ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Pension Benefits | | Non-U.S. Pension Benefits | | Postretirement Benefits |
| 2022 | | 2021 | | 2022 | | 2021 | | 2022 | | 2021 |
| Service cost | $ | — | | | $ | — | | | $ | (39) | | | $ | (44) | | | $ | — | | | $ | — | |
| Interest cost | (54) | | | (44) | | | (23) | | | (19) | | | (3) | | | (2) | |
| Expected return on plan assets | 130 | | | 123 | | | 37 | | | 42 | | | — | | | — | |
| Amortization of prior service (cost) credit | (1) | | | (1) | | | 2 | | | 1 | | | 2 | | | 2 | |
| Amortization of net loss | (35) | | | (46) | | | (2) | | | (11) | | | (1) | | | (2) | |
| Curtailment and settlement gains (losses) recognized | — | | | — | | | (7) | | | (1) | | | — | | | — | |
| Net periodic pension benefit (cost) | $ | 40 | | | $ | 32 | | | $ | (32) | | | $ | (32) | | | $ | (2) | | | $ | (2) | |
The components of the net periodic benefit (cost) of the noncontributory defined benefit pension plans and other postretirement employee benefit plans other than service cost are included in other income (expense), net in the Consolidated Statements of Earnings.
Weighted average assumptions used to determine net periodic pension benefit (cost) at date of measurement:
| | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Plans | | Non-U.S. Plans |
| | 2022 | | 2021 | | 2022 | | 2021 |
| Discount rate | 2.7 | % | | 2.3 | % | | 1.4 | % | | 1.1 | % |
| Expected long-term return on plan assets | 6.8 | % | | 6.8 | % | | 3.2 | % | | 3.3 | % |
| Rate of compensation increase | N/A | | N/A | | 2.6 | % | | 2.5 | % |
The discount rate reflects the market rate on December 31 of the prior year for high-quality fixed-income investments with maturities corresponding to the Company’s benefit obligations and is subject to change each year. For non-U.S. pension plans, rates appropriate for each plan are determined based on investment-grade instruments with maturities approximately equal to the average expected benefit payout under the plan. During 2021, the Company updated the mortality assumptions used to estimate the projected benefit obligation to reflect updated mortality tables.
Included in accumulated other comprehensive income (loss) as of December 31, 2022 are the following amounts that have not yet been recognized in net periodic pension cost: unrecognized prior service credit of $7 million ($5 million, after-tax) and unrecognized actuarial losses of approximately $464 million ($353 million, after-tax). The unrecognized losses and prior service cost, net, is calculated as the difference between the actuarially determined projected benefit obligation and the value of the plan assets less accrued pension costs as of December 31, 2022.
Included in accumulated other comprehensive income (loss) as of December 31, 2022 are the following amounts that have not yet been recognized in net periodic postretirement benefit cost: unrecognized prior service credits of $10 million ($8 million, after-tax) and unrecognized actuarial losses of $1 million ($1 million, after-tax). The unrecognized losses and prior service credits, net, is calculated as the difference between the actuarially determined projected benefit obligation and the value of the plan assets less accrued benefit costs as of December 31, 2022.
Selection of Expected Rate of Return on Assets
For the years ended December 31, 2022, 2021 and 2020, the Company used an expected long-term rate of return assumption of 6.75%, 6.75%, and 7.00%, respectively, for its U.S. defined benefit pension plan. The Company intends to use an expected long-term rate of return assumption of 6.75% for 2023 for such plan. This expected rate of return reflects the asset allocation of the plan, and is based primarily on broad, publicly-traded equity and fixed-income indices and forward-looking estimates of active portfolio and investment management. Long-term rate of return on asset assumptions for the non-U.S. plans were determined on a plan-by-plan basis based on the composition of assets and ranged from 0.8% to 5.3% in 2022 and 0.3% to 5.0% in 2021, with a weighted average rate of return assumption of 3.2% in 2022 and 3.3% in 2021.
Pension Plan Assets
The U.S. pension plan’s goal is to maintain between 60% and 70% of its assets in equity portfolios, which are invested in individual equity securities or funds that are expected to mirror broad market returns for equity securities or in assets with characteristics similar to equity investments, such as venture capital funds and partnerships. Asset holdings are periodically rebalanced when equity holdings are outside this range. The balance of the U.S. plan asset portfolio is invested in bond funds, real estate funds, various absolute and real return funds and private equity funds. Non-U.S. plan assets are invested in various insurance contracts, equity and debt securities as determined by the administrator of each plan. The value of the plan assets directly affects the funded status of the Company’s pension plans recorded in the Consolidated Financial Statements.
The Company has certain investments that are valued using Net Asset Value (“NAV”) as the practical expedient. In addition, certain of the investments valued using NAV as the practical expedient have limits on their redemption to monthly, quarterly, semiannually or annually and require up to 90 days prior written notice. These investments valued using NAV consist of mutual funds, venture capital funds, partnerships, real estate, and other private investments, which allow the Company to allocate investments across a broad array of types of funds and diversify the portfolio.
The fair values of the Company’s pension plan assets for both the U.S. and non-U.S. plans as of December 31, 2022 and 2021, by asset category were as follows ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Quoted Prices in Active Market (Level 1) | | Significant Other Observable Inputs (Level 2) | | Significant Unobservable Inputs (Level 3) | | Total |
| 2022 | | 2021 | | 2022 | | 2021 | | 2022 | | 2021 | | 2022 | | 2021 |
| Cash and equivalents | $ | 113 | | | $ | 85 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 113 | | | $ | 85 | |
| Equity securities: | | | | | | | | | | | | | | | |
| Common stock | 379 | | | 486 | | | — | | | — | | | — | | | — | | | 379 | | | 486 | |
| Preferred stock | — | | | 2 | | | — | | | — | | | — | | | — | | | — | | | 2 | |
| Fixed income securities: | | | | | | | | | | | | | | | |
| Corporate bonds | — | | | — | | | 129 | | | 47 | | | — | | | — | | | 129 | | | 47 | |
| Government issued | — | | | — | | | 24 | | | 46 | | | — | | | — | | | 24 | | | 46 | |
| Mutual funds | 156 | | | 223 | | | 118 | | | 248 | | | — | | | — | | | 274 | | | 471 | |
| Insurance contracts | — | | | — | | | 303 | | | 357 | | | — | | | — | | | 303 | | | 357 | |
| Total | $ | 648 | | | $ | 796 | | | $ | 574 | | | $ | 698 | | | $ | — | | | $ | — | | | 1,222 | | | 1,494 | |
Investments measured at NAV (a): | | | | | | | | | | | | | | |
| Common/collective trusts | | | | | | | | | | | | 811 | | | 1,073 | |
| Venture capital, partnerships and other private investments | | | | | | | | | | | | 693 | | | 1,096 | |
| Total assets at fair value | | | | | | | | | | | | $ | 2,726 | | | $ | 3,663 | |
(a) The fair value amounts presented in the table above are intended to permit reconciliation of the fair value hierarchy to the total plan assets.
Preferred stock and common stock traded on an active market, as well as mutual funds are valued at the quoted closing price reported on the active market on which the individual securities are traded. Preferred stock, common stock, corporate bonds, U.S. government securities and mutual funds that are not traded on an active market are valued at quoted prices reported by investment brokers and dealers based on the underlying terms of the security and comparison to similar securities traded on an active market. Insurance contracts are valued based upon the quoted prices of the underlying investments with the insurance company.
Common/collective trusts are valued based on the plan’s interest, represented by investment units, in the underlying investments held within the trust that are traded in an active market by the trustee.
Venture capital, partnerships and other private investments are valued using the NAV based on the information provided by the asset fund managers, which reflects the plan’s share of the fair value of the net assets of the investment. Depending on the nature of the assets, the underlying investments are valued using a combination of either discounted cash flows, earnings and market multiples, third-party appraisals or through reference to the quoted market prices of the underlying investments held by the venture, partnership or private entity where available. Valuation adjustments reflect changes in operating results, financial condition, or prospects of the applicable portfolio company.
The methods described above may produce a fair value estimate that may not be indicative of net realizable value or reflective of future fair values. Furthermore, while the Company believes the valuation methods are appropriate and consistent with the methods used by other market participants, the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different fair value measurement at the reporting date.
Expected Contributions
During 2023, the Company’s cash contribution requirements for its U.S. and its non-U.S. defined benefit pension plans are expected to be approximately $10 million and $35 million, respectively. During 2023, the Company’s cash contribution requirements for its other postretirement benefit plans are expected to be approximately $14 million. The ultimate amounts to be contributed depend upon, among other things, legal requirements, underlying asset returns, the plan’s funded status, the anticipated tax deductibility of the contributions, local practices, market conditions, interest rates and other factors.
The following sets forth benefit payments, which reflect expected future service, as appropriate, expected to be paid by the plans in the periods indicated ($ in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | |
| U.S. Pension Plans | | Non-U.S. Pension Plans | | | | Postretirement Benefit Plans | | All Plans |
| 2023 | $ | 186 | | | $ | 56 | | | | | $ | 14 | | | $ | 256 | |
| 2024 | 184 | | | 62 | | | | | 13 | | | 259 | |
| 2025 | 183 | | | 59 | | | | | 12 | | | 254 | |
| 2026 | 182 | | | 64 | | | | | 11 | | | 257 | |
| 2027 | 180 | | | 64 | | | | | 10 | | | 254 | |
| 2028 - 2032 | 718 | | | 352 | | | | | 42 | | | 1,112 | |
Other Matters
Substantially all employees not covered by defined benefit plans are covered by defined contribution plans, which generally provide for Company funding based on a percentage of compensation.
A limited number of the Company’s subsidiaries participate in multiemployer defined benefit and contribution plans, primarily outside of the United States, that require the Company to periodically contribute funds to the plan. The risks of participating in a multiemployer plan differ from the risks of participating in a single-employer plan in the following respects: (1) assets contributed to the multiemployer plan by one employer may be used to provide benefits to employees of other participating employers, (2) if a participating employer ceases contributing to the plan, the unfunded obligations of the plan may be required to be borne by the remaining participating employers and (3) if the Company elects to stop participating in the plan, the Company may be required to pay the plan an amount based on the unfunded status of the plan. None of the multiemployer plans in which the Company’s subsidiaries participate are considered to be quantitatively or qualitatively significant, either individually or in the aggregate. In addition, contributions made to these plans during 2022, 2021 and 2020 were not significant, either individually or in the aggregate.
The Company’s expenses for all defined benefit and defined contribution pension plans amounted to $281 million, $245 million and $224 million for the years ended December 31, 2022, 2021 and 2020, respectively.
NOTE 17. COMMITMENTS
Warranties
The Company generally accrues estimated warranty costs at the time of sale. In general, manufactured products are warranted against defects in material and workmanship when properly used for their intended purpose, installed correctly and appropriately maintained. Warranty periods depend on the nature of the product and range from the date of such sale up to twenty years. The amount of the accrued warranty liability is determined based on historical information such as past experience, product failure rates or number of units repaired, estimated cost of material and labor and in certain instances estimated property damage. As of December 31, 2022 and 2021, the Company had accrued warranty liabilities of $95 million and $97 million, respectively.
Purchase Obligations
The Company has entered into agreements to purchase goods or services that are enforceable and legally binding on the Company and that specify all significant terms, including fixed or minimum quantities to be purchased, fixed, minimum or variable price provisions and the approximate timing of the transaction. Purchase obligations exclude agreements that are cancellable at any time without penalty. As of December 31, 2022, the aggregate amount of the Company’s purchase obligations totaled approximately $2.3 billion and the majority of these obligations are expected to be settled during 2023.
NOTE 18. LITIGATION AND CONTINGENCIES
The Company is subject to or otherwise responsible for a variety of litigation and other legal and regulatory proceedings in the course of its business (or related to the business operations of previously owned entities), including claims or counterclaims for damages arising out of the use of products or services and claims relating to intellectual property matters, employment matters, tax matters, commercial disputes, breach of contract claims, competition and sales and trading practices, environmental matters, personal injury, insurance coverage, securities matters, fiduciary duties and acquisition or divestiture-related matters, as well as regulatory subpoenas, requests for information, investigations and enforcement. The Company also from time to time becomes subject to lawsuits as a result of acquisitions or as a result of liabilities retained from, or representations, warranties or indemnities provided in connection with, businesses divested by the Company or its predecessors. The types of claims made in lawsuits include claims for compensatory damages, punitive and consequential damages (and in some cases, treble damages) and/or injunctive relief.
While the Company maintains general, products, property, workers’ compensation, automobile, cargo, aviation, crime, cyber, fiduciary and directors’ and officers’ liability insurance (and has acquired rights under similar policies in connection with certain acquisitions) up to certain limits that cover certain of these claims, this insurance may be insufficient or unavailable to cover such losses. For general, products and property liability and most other insured risks, the Company purchases outside insurance coverage only for severe losses and must establish and maintain reserves with respect to amounts within the self-insured retention. In addition, while the Company believes it is entitled to indemnification from third-parties for some of these claims, these rights may also be insufficient or unavailable to cover such losses.
The Company records a liability in the Consolidated Financial Statements for loss contingencies when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss does not meet the known or probable level but is reasonably possible it is disclosed and if the loss or range of loss can be reasonably estimated, the estimated loss or range of loss is disclosed. The Company’s reserves consist of specific reserves for individual claims and additional amounts for anticipated developments of these claims as well as for incurred but not yet reported claims. The specific reserves for individual known claims are quantified with the assistance of legal counsel and outside risk professionals where appropriate. In addition, outside risk professionals assist in the determination of reserves for incurred but not yet reported claims through evaluation of the Company’s specific loss history, actual claims reported and industry trends together with statistical and other factors. Reserve estimates may be adjusted as additional information regarding a claim becomes known. Because most contingencies are resolved over long periods of time, new developments (including litigation developments, the discovery of new facts, changes in legislation and outcomes of similar cases), changes in assumptions or changes in the Company’s strategy in any given period can require the Company to adjust the loss contingency estimates that have been recorded in the financial statements, record estimates for liabilities or assets previously not susceptible of reasonable estimates or pay cash settlements or judgments. While the Company actively pursues financial recoveries from insurance providers and indemnifying parties, it does not recognize any recoveries until realized or until such time as a sustained pattern of collections is established related to historical matters of a similar nature and magnitude. If the Company’s self-insurance and litigation reserves prove inadequate, it would be required to incur an expense equal to the amount of the loss incurred in excess of the reserves, which would adversely affect the Company’s Consolidated Financial Statements.
In addition, the Company’s operations, products and services are subject to numerous U.S. federal, state, local and non-U.S. environmental, health and safety laws and regulations concerning, among other things, the health and safety of our employees, the generation, storage, use and transportation of hazardous materials, emissions or discharges of substances into the environment, investigation and remediation of hazardous substances or materials at various sites, chemical constituents in products and end-of-life disposal and take-back programs for products sold. A number of the Company’s operations involve the handling, manufacturing, use or sale of substances that are or could be classified as hazardous materials within the meaning of applicable laws. Compliance with these laws and regulations has not had and, based on current information and the applicable laws and regulations currently in effect, is not expected to have a material effect on the Company’s capital expenditures, earnings or competitive position, and the Company does not anticipate material capital expenditures for environmental control facilities.
In addition to environmental compliance costs, the Company from time to time incurs costs related to remedial efforts or alleged environmental damage associated with past or current waste disposal practices or other hazardous materials handling practices. For example, generators of hazardous substances found in disposal sites at which environmental problems are alleged to exist, as well as the current and former owners of those sites and certain other classes of persons, are subject to claims brought by state and federal regulatory agencies pursuant to statutory authority. The Company has received notification from the U.S. Environmental Protection Agency, and from state and non-U.S. environmental agencies, that conditions at certain sites where the Company and others previously disposed of hazardous wastes and/or are or were property owners require clean-up and other possible remedial action, including sites where the Company has been identified as a potentially responsible party under U.S. federal and state environmental laws. The Company has projects underway at a number of current and former facilities, in both the United States and abroad, to investigate and remediate environmental contamination resulting from past operations. Remediation activities generally relate to soil and/or groundwater contamination and may include pre-remedial activities such as fact-finding and investigation, risk assessment, feasibility study and/or design, as well as remediation actions such as contaminant removal, monitoring and/or installation, operation and maintenance of longer-term remediation systems. The Company is also from time to time party to personal injury, property damage or other claims brought by private parties alleging injury or damage due to the presence of, or exposure to, hazardous substances. The Company can also become subject to additional remedial, compliance or personal injury costs due to future events such as changes in existing laws or regulations, changes in agency direction or enforcement policies, developments in remediation technologies, changes in the conduct of the Company’s operations and changes in accounting rules.
The Company has recorded a provision for environmental investigation and remediation and environmental-related claims with respect to sites owned or formerly owned by the Company and its subsidiaries and third-party sites where the Company has been determined to be a potentially responsible party. The Company generally makes an assessment of the costs involved for its remediation efforts based on environmental studies, as well as its prior experience with similar sites. The ultimate cost of site cleanup is difficult to predict given the uncertainties of the Company’s involvement in certain sites, uncertainties regarding the extent of the required cleanup, the availability of alternative cleanup methods, variations in the interpretation of applicable laws and regulations, the possibility of insurance recoveries with respect to certain sites and the fact that imposition of joint and several liability with right of contribution is possible under the Comprehensive Environmental Response, Compensation and Liability Act of 1980 and other environmental laws and regulations. If the Company determines that potential liability for a particular site or with respect to a personal injury claim is known or considered probable and reasonably estimable, the Company accrues the total estimated loss, including investigation and remediation costs, associated with the site or claim. As of December 31, 2022, the Company had a reserve of $197 million for environmental matters which are known or considered probable and reasonably estimable (of which $164 million are noncurrent), which reflects the Company’s best estimate of the costs to be incurred with respect to such matters.
While the Company actively pursues insurance recoveries, as well as recoveries from other potentially responsible parties, it does not recognize any insurance recoveries for environmental liability claims until realized or until such time as a sustained pattern of collections is established related to historical matters of a similar nature and magnitude.
The Company’s Restated Certificate of Incorporation requires it to indemnify to the full extent authorized or permitted by law any person made, or threatened to be made a party to any action or proceeding by reason of his or her service as a director or officer of the Company, or by reason of serving at the request of the Company as a director or officer of any other entity, subject to limited exceptions. Danaher’s Amended and Restated By-laws provide for similar indemnification rights. In addition, Danaher has executed with each director and executive officer of Danaher Corporation an indemnification agreement which provides for substantially similar indemnification rights and under which Danaher has agreed to pay expenses in advance of the final disposition of any such indemnifiable proceeding. While the Company maintains insurance for this type of liability, a significant deductible applies to this coverage and any such liability could exceed the amount of the insurance coverage.
As of December 31, 2022, the Company had approximately $632 million of guarantees consisting primarily of outstanding standby letters of credit, bank guarantees and performance and bid bonds. These guarantees have been provided in connection
with certain arrangements with vendors, customers, insurance providers, financing counterparties and governmental entities to secure the Company’s obligations and/or performance requirements related to specific transactions. The Company believes that if the obligations under these instruments were triggered, it would not have a material effect on its Consolidated Financial Statements.
NOTE 19. STOCKHOLDERS' EQUITY AND STOCK-BASED COMPENSATION
Stockholders’ Equity
On July 16, 2013, the Company’s Board of Directors approved a repurchase program (the “Repurchase Program”) authorizing the repurchase of up to 20 million shares of the Company’s common stock from time to time on the open market or in privately negotiated transactions. There is no expiration date for the Repurchase Program, and the timing and amount of any shares repurchased under the program will be determined by the Company’s management based on its evaluation of market conditions and other factors. The Repurchase Program may be suspended or discontinued at any time. Any repurchased shares will be available for use in connection with the Company’s equity compensation plans (or any successor plan) and for other corporate purposes. On July 22, 2022, the Company repurchased 3,906 shares of the Company’s common stock for $1 million as part of the Repurchase Program. As of December 31, 2022, approximately 20 million shares remained available for repurchase pursuant to the Repurchase Program. The Company expects to fund any future stock repurchases using the Company’s available cash balances or proceeds from the issuance of debt.
Except as discussed above, neither the Company nor any “affiliated purchaser” repurchased any shares of Company common stock during 2022, 2021 or 2020.
The following table summarizes the Company’s share activity for the years ended December 31 (shares in millions):
| | | | | | | | | | | | | | | | | |
| 2022 | | 2021 | | 2020 |
| Preferred stock - shares issued: | | | | | |
| Balance, beginning of period | 3.4 | | | 3.4 | | | 1.7 | |
| Issuance of MCPS | — | | | — | | | 1.7 | |
| Conversion of MCPS to common stock | (1.7) | | | — | | | — | |
| Balance, end of period | 1.7 | | | 3.4 | | | 3.4 | |
| | | | | |
| Common stock - shares issued: | | | | | |
| Balance, beginning of period | 855.7 | | | 851.3 | | | 835.5 | |
| Issuance of common stock attributable to stock-based compensation | 2.6 | | | 3.4 | | | 4.5 | |
| Conversion of MCPS to common stock | 11.0 | | | — | | | — | |
| Common stock issued in connection with acquisitions | — | | | 0.1 | | | — | |
| Common stock issued in connection with LYONs’ conversions | — | | | 0.9 | | | 0.4 | |
| Other issuance of common stock | — | | | — | | | 10.9 | |
| Balance, end of period | 869.3 | | | 855.7 | | | 851.3 | |
On April 15, 2022, all outstanding shares of the Company’s 4.75% MCPS Series A converted to common shares at a rate of 6.6632 common shares per share of preferred stock into an aggregate of 11.0 million shares of the Company’s common stock, pursuant to the terms of the Certificate of Designation governing the Series A Preferred Stock. Danaher issued cash in lieu of fractional shares of common stock in the conversion. The final quarterly cash dividend of $11.875 per share was paid on April 15, 2022.
In May 2020, the Company completed the underwritten public offering of 10.9 million shares of Danaher common stock at a price to the public of $163.00 per share (the “2020 Common Stock Offering”), resulting in net proceeds of approximately $1.7 billion, after deducting expenses and the underwriters’ discount of $54 million. Simultaneously, the Company completed the underwritten public offering of 1.72 million shares of its 5.0% MCPS Series B, without par value and with a liquidation preference of $1,000 per share (the “2020 MCPS Offering”), resulting in net proceeds of approximately $1.7 billion, after deducting expenses and the underwriters’ discount of $49 million. The Company has used the net proceeds from the 2020 Common Stock Offering and the 2020 MCPS Offering for general corporate purposes.
Unless converted earlier in accordance with the terms of the applicable certificate of designations, each share of MCPS Series B will mandatorily convert on April 15, 2023 into a number of shares of the Company’s common stock between the Minimum Conversion Rate of 5.0156 shares and the Maximum Conversion Rate of 6.1441 shares (subject to further anti-dilution
adjustments). The number of shares of the Company’s common stock issuable upon conversion will be determined based on the average volume-weighted average price per share of the Company’s common stock over the 20 consecutive trading day period beginning on, and including, the 21st scheduled trading day immediately before the Mandatory Conversion Date. Subject to certain exceptions, at any time prior to the Mandatory Conversion Date, holders may elect to convert the MCPS Series B shares into common stock based on the Minimum Conversion Rate (subject to further anti-dilution adjustments). In the event of a fundamental change, the MCPS Series B shares will convert at the fundamental change rates specified in the certificate of designations, and the holders of MCPS Series B shares would be entitled to a fundamental change make-whole dividend.
Holders of MCPS Series B will be entitled to receive, when and if declared by the Company’s Board of Directors, cumulative dividends at the Annual Cumulative Dividend Rate of the Liquidation Preference per share, payable in cash or, subject to certain limitations, by delivery of shares of the Company’s common stock or any combination of cash and shares of the Company’s common stock, at the Company’s election. If declared, dividends on the MCPS Series B shares are payable quarterly on January 15, April 15, July 15 and October 15 of each year (to, and including, the Mandatory Conversion Date), to the holders of record of the MCPS Series B shares as they appear on the Company’s stock register at the close of business on the immediately preceding December 31, March 31, June 30 and September 30, respectively.
Stock-Based Compensation
Stock options, RSUs and PSUs have been issued to directors, officers and other employees under the Company’s 2007 Omnibus Incentive Plan. The 2007 Omnibus Incentive Plan provides for the grant of stock options, stock appreciation rights, RSUs, restricted stock, PSUs or any other stock-based award and cash-based awards. A total of approximately 127 million shares of Danaher common stock have been authorized for issuance under the 2007 Omnibus Incentive Plan. As of December 31, 2022, approximately 45 million shares of the Company’s common stock remain available for issuance under the 2007 Omnibus Incentive Plan.
Stock options granted prior to 2022 under the 2007 Omnibus Incentive Plan generally vest pro rata over a five-year period and terminate ten years from the grant date, though the specific terms of each grant are determined by the Compensation Committee of the Company’s Board (the “Compensation Committee”). Stock options granted subsequent to December 31, 2021 under the amended and restated 2007 Omnibus Incentive Plan generally vest pro rata over a four-year period and terminate ten years from the grant date, though specific terms of each grant are determined by the Compensation Committee. The Company’s executive officers and certain other employees have been awarded options with different vesting criteria, and options granted to outside directors are fully vested as of the grant date. Option exercise prices for options granted by the Company equal the closing price of the Company’s common stock on the New York Stock Exchange on the date of grant.
RSUs issued under the 2007 Omnibus Incentive Plan provide for the issuance of a share of the Company’s common stock at no cost to the holder. RSUs granted prior to 2022 to employees under the 2007 Omnibus Incentive Plan generally provide for pro rata time-based vesting over a five-year period, although executive officers and certain other employees have been awarded RSUs with different vesting criteria. RSUs granted subsequent to December 31, 2021 to employees under the amended and restated 2007 Omnibus Incentive Plan generally vest pro rata over a four-year period, although certain employees have been awarded RSUs with different vesting criteria. The RSUs that have been granted to directors under the 2007 Omnibus Incentive Plan vest on the earlier of the first anniversary of the grant date or the date of, and immediately prior to, the next annual meeting of the Company’s shareholders following the grant date, but the underlying shares are not issued until the earlier of the director’s death or the first day of the seventh month following the director’s retirement from the Board. Prior to vesting, RSUs granted under the 2007 Omnibus Incentive Plan do not have dividend equivalent rights, do not have voting rights and the shares underlying the RSUs are not considered issued and outstanding.
PSUs issued under the 2007 Omnibus Incentive Plan provide for the issuance of a share of the Company’s common stock at no cost to the holder, vest based on specified performance criteria, are subject to an additional holding period following vesting and are entitled to dividend equivalent rights. The PSU dividend equivalent rights are subject to the same vesting and payment restrictions as the related shares, and the shares underlying the PSUs are not considered issued and outstanding.
The equity compensation awards granted by the Company generally vest only if the employee is employed by the Company (or in the case of directors, the director continues to serve on the Company Board) on the vesting date or in other limited circumstances, including following a qualifying retirement. To cover the exercise of options and vesting of RSUs and PSUs, the Company generally issues new shares from its authorized but unissued share pool, although it may instead issue treasury shares in certain circumstances.
The Company accounts for stock-based compensation by measuring the cost of employee services received in exchange for all equity awards granted based on the fair value of the award as of the grant date. The Company recognizes the compensation expense over the requisite service period (which is generally the vesting period but may be shorter than the vesting period if the
employee becomes retirement eligible before the end of the vesting period). The fair value for RSU awards was calculated using the closing price of the Company’s common stock on the date of grant, adjusted for the fact that RSUs do not accrue dividends. The fair value of the PSU awards was calculated using a Monte Carlo pricing model. The fair value of the options granted was calculated using a Black-Scholes Merton option pricing model (“Black-Scholes”).
The following summarizes the assumptions used in the Black-Scholes model to value options granted during the years ended December 31:
| | | | | | | | | | | | | | | | | |
| | 2022 | | 2021 | | 2020 |
| Risk-free interest rate | 1.8 – 4.0% | | 0.6 – 1.5% | | 0.3 – 1.3% |
| Weighted average volatility | 30.3 | % | | 29.8 | % | | 24.3 | % |
| Dividend yield | 0.4 | % | | 0.3 | % | | 0.4 | % |
| Expected years until exercise | 5.0 – 7.5 | | 5.0 – 7.5 | | 5.0 – 8.0 |
The Black-Scholes model incorporates assumptions to value stock-based awards. The risk-free rate of interest for periods within the contractual life of the option is based on a zero-coupon U.S. government instrument whose maturity period equals or approximates the option’s expected term. Expected volatility is based on implied volatility from traded options on the Company’s stock and historical volatility of the Company’s stock. The dividend yield is calculated by dividing the Company’s annual common stock dividend, based on the most recent quarterly dividend rate, by the closing stock price on the grant date. To estimate the option exercise timing used in the valuation model (which impacts the risk-free interest rate and the expected years until exercise), in addition to considering the vesting period and contractual term of the option, the Company analyzes and considers actual historical exercise experience for previously granted options. The Company stratifies its employee population into multiple groups for option valuation and attribution purposes based upon distinctive patterns of forfeiture rates and option holding periods, as indicated by the ranges set forth in the table above for the risk-free interest rate and the expected years until exercise.
The amount of stock-based compensation expense recognized during a period is also based on the portion of the awards that are ultimately expected to vest. The Company estimates pre-vesting forfeitures at the time of grant by analyzing historical data and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. Ultimately, the total expense recognized over the vesting period will equal the fair value of awards that actually vest.
The following summarizes the components of the Company’s continuing operations stock-based compensation expense for the years ended December 31 ($ in millions):
| | | | | | | | | | | | | | | | | |
| | 2022 | | 2021 | | 2020 |
| RSUs/PSUs: | | | | | |
| Pretax compensation expense | $ | 195 | | | $ | 129 | | | $ | 114 | |
| Income tax benefit | (40) | | | (26) | | | (24) | |
| RSU/PSU expense, net of income taxes | 155 | | | 103 | | | 90 | |
| Stock options: | | | | | |
| Pretax compensation expense | 141 | | | 89 | | | 73 | |
| Income tax benefit | (28) | | | (18) | | | (15) | |
| Stock option expense, net of income taxes | 113 | | | 71 | | | 58 | |
| Total stock-based compensation: | | | | | |
| Pretax compensation expense | 336 | | | 218 | | | 187 | |
| Income tax benefit | (68) | | | (44) | | | (39) | |
| Total stock-based compensation expense, net of income taxes | $ | 268 | | | $ | 174 | | | $ | 148 | |
Stock-based compensation has been recognized as a component of selling, general and administrative expenses in the accompanying Consolidated Statements of Earnings. As of December 31, 2022, $204 million of total unrecognized compensation cost related to RSUs/PSUs is expected to be recognized over a weighted average period of approximately two years. As of December 31, 2022, $240 million of total unrecognized compensation cost related to stock options is expected to be recognized over a weighted average period of approximately two years. Future compensation amounts will be adjusted for any changes in estimated forfeitures.
The following summarizes option activity under the Company’s stock plans (in millions, except weighted exercise price and number of years):
| | | | | | | | | | | | | | | | | | | | | | | |
| Options | | Weighted Average Exercise Price | | Weighted Average Remaining Contractual Term (in years) | | Aggregate Intrinsic Value |
| Outstanding as of January 1, 2020 | 17.0 | | | $ | 82.95 | | | | | |
| Granted | 2.9 | | | 160.71 | | | | | |
| Exercised | (3.5) | | | 62.54 | | | | | |
| Cancelled/forfeited | (0.5) | | | 113.94 | | | | | |
| | | | | | | |
| Outstanding as of December 31, 2020 | 15.9 | | | 100.65 | | | | | |
| Granted | 2.8 | | | 240.75 | | | | | |
| Exercised | (2.4) | | | 79.16 | | | | | |
| Cancelled/forfeited | (0.7) | | | 144.60 | | | | | |
| Outstanding as of December 31, 2021 | 15.6 | | | 127.13 | | | | | |
| Granted | 2.3 | | | 269.10 | | | | | |
| Exercised | (1.6) | | | 89.62 | | | | | |
| Cancelled/forfeited | (0.6) | | | 198.85 | | | | | |
| Outstanding as of December 31, 2022 | 15.7 | | | 149.01 | | | 6 | | $ | 1,847 | |
Vested and expected to vest as of December 31, 2022 (a) | 15.3 | | | $ | 147.34 | | | 6 | | $ | 1,833 | |
| Vested as of December 31, 2022 | 8.2 | | | $ | 102.64 | | | 5 | | $ | 1,341 | |
(a) The “expected to vest” options are the net unvested options that remain after applying the forfeiture rate assumption to total unvested options.
The aggregate intrinsic value in the table above represents the total pretax intrinsic value (the difference between the Company’s closing stock price on the last trading day of 2022 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2022. The amount of aggregate intrinsic value will change based on the price of the Company’s common stock.
The weighted average per share grant-date fair values of options granted during 2022, 2021 and 2020 were $80.32, $64.57 and $37.42, respectively.
Options outstanding as of December 31, 2022 are summarized below (in millions, except price per share and number of years):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Outstanding | | Exercisable |
| Exercise Price | Shares | | Average Exercise Price | | Average Remaining Life (in years) | | Shares | | Average Exercise Price |
$39.6 to $66.79 | 1.8 | | | $ | 62.75 | | | 2 | | 1.8 | | | $ | 62.75 | |
$66.8 to $92.41 | 2.4 | | | 81.60 | | | 4 | | 2.4 | | | 81.60 | |
$92.42 to $141.10 | 4.4 | | | 107.41 | | | 5 | | 2.7 | | | 105.90 | |
$141.11 to $249.18 | 4.2 | | | 185.12 | | | 7 | | 1.1 | | | 173.94 | |
$249.19 to $299.68 | 2.9 | | | 272.79 | | | 9 | | 0.2 | | | 283.43 | |
The aggregate intrinsic value of options exercised during the years ended December 31, 2022, 2021 and 2020 was $288 million, $446 million and $415 million, respectively. Exercise of options during the years ended December 31, 2022, 2021 and 2020 resulted in cash receipts of $130 million, $167 million and $211 million, respectively. Upon exercise of the award by the employee, the Company derives a tax deduction measured by the excess of the market value over the grant price at the date of exercise. The Company realized a tax benefit of $48 million, $83 million and $82 million in 2022, 2021 and 2020, respectively, related to the exercise of employee stock options.
The following summarizes information on unvested RSU and PSU activity (in millions, except weighted average grant-date fair value):
| | | | | | | | | | | |
| Number of RSUs/PSUs | | Weighted Average
Grant-Date Fair Value |
| Unvested as of January 1, 2020 | 3.5 | | | $ | 94.85 | |
| Granted | 1.1 | | | 159.93 | |
| Vested | (1.0) | | | 91.08 | |
| Forfeited | (0.2) | | | 111.59 | |
| | | |
| Unvested as of December 31, 2020 | 3.4 | | | 116.03 | |
| Granted | 0.9 | | | 234.52 | |
| Vested | (1.0) | | | 101.86 | |
| Forfeited | (0.2) | | | 147.20 | |
| Unvested as of December 31, 2021 | 3.1 | | | 152.99 | |
| Granted | 1.1 | | | 268.00 | |
| Vested | (1.0) | | | 159.42 | |
| Forfeited | (0.2) | | | 202.55 | |
| Unvested as of December 31, 2022 | 3.0 | | | 189.71 | |
The Company realized a tax benefit of $37 million, $35 million and $18 million in the years ended December 31, 2022, 2021 and 2020, respectively, related to the vesting of RSUs and PSUs.
The excess tax benefit of $61 million, $95 million and $85 million related to the exercise of employee stock options and vesting of RSUs and PSUs for the years ended December 31, 2022, 2021 and 2020, respectively, has been recorded as a reduction to the current income tax provision and is reflected as an operating cash inflow in the accompanying Consolidated Statements of Cash Flows.
In connection with the exercise of certain stock options and the vesting of RSUs previously issued by the Company, a number of shares sufficient to fund statutory minimum tax withholding requirements has been withheld from the total shares issued or released to the award holder (though under the terms of the applicable plan, the shares are considered to have been issued and are not added back to the pool of shares available for grant). During the year ended December 31, 2022, 362 thousand shares with an aggregate value of $99 million were withheld to satisfy the requirement. During the year ended December 31, 2021, 346 thousand shares with an aggregate value of $81 million were withheld to satisfy the requirement. The withholding is treated as a reduction in additional paid-in capital in the accompanying Consolidated Statements of Stockholders’ Equity and a reduction in proceeds from the issuance of common stock in connection with stock-based compensation in the Consolidated Statements of Cash Flows.
Accumulated Other Comprehensive Income
The changes in accumulated other comprehensive income (loss) by component are summarized below ($ in millions).
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign Currency Translation Adjustments | | Pension and Postretirement Plan Benefit Adjustments | | | | Cash Flow Hedge Adjustments | | Accumulated Comprehensive Income (Loss) |
| Balance, January 1, 2020 | $ | (2,174) | | | $ | (781) | | | | | $ | (113) | | | $ | (3,068) | |
| Other comprehensive income (loss) before reclassifications: | | | | | | | | | |
| Increase (decrease) | 2,894 | | | (239) | | | | | (432) | | | 2,223 | |
| Income tax impact | 25 | | | 57 | | | | | — | | | 82 | |
| Other comprehensive income (loss) before reclassifications, net of income taxes | 2,919 | | | (182) | | | | | (432) | | | 2,305 | |
| Reclassification adjustments | | | | | | | | | |
| Increase (decrease) | — | | | 46 | | (a) | | | 361 | | (b) | 407 | |
| Income tax impact | — | | | (11) | | | | | (1) | | | (12) | |
| Reclassification adjustments, net of income taxes | — | | | 35 | | | | | 360 | | | 395 | |
| Net other comprehensive income (loss), net of income taxes | 2,919 | | | (147) | | | | | (72) | | | 2,700 | |
| Balance, December 31, 2020 | 745 | | | (928) | | | | | (185) | | | (368) | |
| Other comprehensive income (loss) before reclassifications: | | | | | | | | | |
| Increase (decrease) | (1,277) | | | 436 | | | | | 523 | | | (318) | |
| Income tax impact | (7) | | | (102) | | | | | 5 | | | (104) | |
| Other comprehensive income (loss) before reclassifications, net of income taxes | (1,284) | | | 334 | | | | | 528 | | | (422) | |
| Reclassification adjustments | | | | | | | | | |
| Increase (decrease) | — | | | 58 | | (a) | | | (280) | | (b) | (222) | |
| Income tax impact | — | | | (14) | | | | | (1) | | | (15) | |
| Reclassification adjustments, net of income taxes | — | | | 44 | | | | | (281) | | | (237) | |
| Net other comprehensive income (loss), net of income taxes | (1,284) | | | 378 | | | | | 247 | | | (659) | |
| Balance, December 31, 2021 | (539) | | | (550) | | | | | 62 | | | (1,027) | |
| Other comprehensive income (loss) before reclassifications: | | | | | | | | | |
| Increase (decrease) | (2,051) | | | 233 | | | | | 378 | | | (1,440) | |
| Income tax impact | (54) | | | (56) | | | | | (91) | | | (201) | |
| Other comprehensive income (loss) before reclassifications, net of income taxes | (2,105) | | | 177 | | | | | 287 | | | (1,641) | |
| Reclassification adjustments | | | | | | | | | |
| Increase (decrease) | — | | | 42 | | (a) | | | (235) | | (b) | (193) | |
| Income tax impact | — | | | (10) | | | | | (1) | | | (11) | |
| Reclassification adjustments, net of income taxes | — | | | 32 | | | | | (236) | | | (204) | |
| Net other comprehensive income (loss), net of income taxes | (2,105) | | | 209 | | | | | 51 | | | (1,845) | |
| Balance, December 31, 2022 | $ | (2,644) | | | $ | (341) | | | | | $ | 113 | | | $ | (2,872) | |
(a) This accumulated other comprehensive income (loss) component is included in the computation of net periodic pension and postretirement cost (refer to Note 16 for additional details).
(b) Reflects reclassification to earnings related to remeasurement of certain long-term debt (refer to Note 15 for additional details).
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
The Company’s management, with the participation of the Company’s President and Chief Executive Officer, and Executive Vice President and Chief Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) as of the end of the period covered by this report. Based on such evaluation, the Company’s President and Chief Executive Officer, and Executive Vice President and Chief Financial Officer, have concluded that, as of the end of such period, the Company’s disclosure controls and procedures were effective.
Management’s annual report on its internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) and the independent registered public accounting firm’s audit report on the effectiveness of Danaher’s internal control over financial reporting are included in the Company’s financial statements for the year ended December 31, 2022 included in Item 8 of this Annual Report on Form 10-K, under the headings “Report of Management on Danaher Corporation’s Internal Control Over Financial Reporting” and “Report of Independent Registered Public Accounting Firm,” respectively, and are incorporated herein by reference.
There have been no changes in the Company’s internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the Company’s most recent completed fiscal quarter that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
Disclosure Pursuant to Section 13(r) of the Exchange Act
Pursuant to Section 219 of the Iran Threat Reduction and Syria Human Rights Act of 2012, which amended the Exchange Act to add Section 13(r) thereof, an issuer is required to disclose in its annual or quarterly reports, as applicable, whether, during the relevant reporting period, it or any entity acting on its behalf knowingly engaged in certain activities, transactions or dealings related to parties subject to sanctions administered by the Office of Foreign Assets Control (“OFAC”) within the U.S. Department of the Treasury, even if those transactions are authorized by law.
On March 2, 2021, the U.S. government designated the Russian Federal Security Service (the “FSB”) as a blocked party under Executive Order 13382. On the same day, the U.S. Department of the Treasury’s Office of Foreign Assets Control issued General License No. 1B (the “OFAC General License”), which generally authorizes U.S. companies to engage in certain transactions and dealings with the FSB necessary and ordinarily incident to requesting or obtaining licenses, permits, certifications or notifications issued or registered by the FSB for the importation, distribution or use of information technology products in Russia. Section 13(r) of the Exchange Act now requires disclosure of dealings with FSB, even where the activities were conducted in compliance with applicable laws and regulations.
In the normal course of business, as permitted and authorized by the OFAC General License (but subject to the Company’s suspension of sales prohibited by sanctions and suspension of certain product shipments to Russia as a result of the conflict with Ukraine, as described above), certain of the Company’s subsidiaries may file notifications with, or apply for import licenses and permits from, the FSB as required pursuant to Russian encryption product import controls for the purpose of enabling such subsidiaries or their channel partners to import and distribute certain products in the Russian Federation. There are no gross revenues or net profits directly associated with these activities, and neither the Company nor any of its subsidiaries distribute or sell products or provide services to the FSB.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Other than the information below, the information required by this Item is incorporated by reference from the sections entitled Proposal 1–Election of Directors of Danaher, Corporate Governance and Other Information in the Proxy Statement for the Company’s 2023 annual meeting of shareholders and from the information under the caption “Information About Our
Executive Officers” in Part I hereof. No nominee for director was selected pursuant to any arrangement or understanding between the nominee and any person other than the Company pursuant to which such person is or was to be selected as a director or nominee.
Code of Ethics
Danaher has adopted a code of business conduct and ethics for directors, officers (including Danaher’s principal executive officer, principal financial officer and principal accounting officer) and employees, known as the Code of Conduct. The Code of Conduct is available in the “Sustainability” section of Danaher’s website at www.danaher.com.
Danaher intends to disclose any amendment to the Code of Conduct that relates to any element of the code of ethics definition enumerated in Item 406(b) of Regulation S-K, and any waiver from a provision of the Code of Conduct granted to any director, principal executive officer, principal financial officer, principal accounting officer, or any of its other executive officers, in the “Sustainability” section of its website, at www.danaher.com, within four business days following the date of such amendment or waiver.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item is incorporated by reference from the sections entitled Director Compensation, Compensation Discussion and Analysis, Compensation Committee Report, Compensation Tables and Information (other than the Pay Versus Performance disclosure) and Summary of Employment Agreements and Plans in the Proxy Statement for the Company’s 2023 annual meeting of shareholders (provided that the Compensation Committee Report shall not be deemed to be “filed” and the Pay-Versus-Performance disclosure shall not be deemed to be incorporated by reference herein).
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item is incorporated by reference from the sections entitled Beneficial Ownership of Danaher Common Stock by Directors, Officers and Principal Shareholders, Summary of Employment Agreements and Plans and Compensation Tables and Information in the Proxy Statement for the Company’s 2023 annual meeting of shareholders (provided that the Pay-Versus-Performance disclosure shall not be deemed to be incorporated by reference herein).
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item is incorporated by reference from the section entitled Director Independence and Related Person Transactions in the Proxy Statement for the Company’s 2023 annual meeting of shareholders.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Our independent registered public accounting firm is Ernst & Young LLP, Tysons, Virginia, PCAOB ID: 00042.
The information required by this Item is incorporated by reference from the section entitled Proposal 2–Ratification of Independent Registered Public Accounting Firm in the Proxy Statement for the Company’s 2023 annual meeting of shareholders.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
a) The following documents are filed as part of this report.
(1)Financial Statements. The financial statements are set forth under “Item 8. Financial Statements and Supplementary Data” of this Annual Report on Form 10-K.
(2)Schedules. An index of Exhibits and Schedules is on page 112 of this report. Schedules other than those listed below have been omitted from this Annual Report on Form 10-K because they are not required, are not applicable or the required information is included in the financial statements or the notes thereto. (3)Exhibits. The exhibits listed in the accompanying Exhibit Index are filed or incorporated by reference as part of this Annual Report on Form 10-K.
ITEM 16. FORM 10-K SUMMARY
Not applicable.
DANAHER CORPORATION
INDEX TO FINANCIAL STATEMENTS, SUPPLEMENTARY DATA AND FINANCIAL STATEMENT SCHEDULE
| | | | | |
| | Page Number in
Form 10-K |
| Schedule: | |
| Valuation and Qualifying Accounts | |
EXHIBIT INDEX
| | | | | | | | | | | | | | |
| Exhibit Number | | Description |
| | |
| 3.1 | | | | Incorporated by reference from Exhibit 3.1 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended June 29, 2012 |
| | | | |
| 3.2 | | | | Incorporated by reference from Exhibit 3.1 to Danaher Corporation’s Current Report on Form 8-K filed March 1, 2019 |
| | | | |
| 3.3 | | | | Incorporated by reference from Exhibit 3.1 to Danaher Corporation’s Current Report on Form 8-K filed May 12, 2020 |
| | | | |
| 3.4 | | | | Incorporated by reference from Exhibit 3.1 to Danaher Corporation’s Current Report on Form 8-K filed December 7, 2022 |
| | | | |
| 4.1 | | | | Incorporated by reference from Exhibit 1.2 to Danaher Corporation’s Current Report on Form 8-K filed on December 11, 2007 |
| | | | |
| 4.2 | | | | Incorporated by reference from Exhibit 4.1 to Danaher Corporation’s Current Report on Form 8-K filed September 15, 2015
|
| | | | |
| 4.3 | | | | Incorporated by reference from Exhibit 4.1 to Danaher Corporation’s Current Report on Form 8-K filed on July 8, 2015 |
| | | | |
| 4.4 | | | | Incorporated by reference from Exhibit 4.2 to Danaher Corporation’s Current Report on Form 8-K filed on July 8, 2015 |
| | | | |
| 4.5 | | | | Incorporated by reference from Exhibit 4.2 to Danaher Corporation’s Current Report on Form 8-K filed on June 30, 2017 |
| | | | |
| 4.6 | | | | Incorporated by reference from Exhibit 4.2 to Danaher Corporation’s Post-Effective Amendment No. 1 to Registration Statement on Form S-3 filed July 10, 2019 |
| | | | |
| 4.7 | | | | Incorporated by reference from Exhibit 4.3 to Danaher Corporation’s Current Report on Form 8-K filed on March 30, 2020 |
| | | | |
| 4.8 | | | | Incorporated by reference from Exhibit 4.4 to Danaher Corporation’s Current Report on Form 8-K filed on October 6, 2020 |
| | | | | | | | | | | | | | |
| | | | |
| 4.9 | | | | Incorporated by reference from Exhibit 4.4 to Danaher Corporation’s Current Report on Form 8-K filed on December 10, 2021 |
| | | | |
| 4.10 | | | | Incorporated by reference from Exhibit 4.5 to Danaher Corporation’s Post-Effective Amendment No. 1 to Registration Statement on Form S-3 filed July 10, 2019 |
| | | | |
| 4.11 | | | | Incorporated by reference from Exhibit 4.1 to Danaher Corporation’s Current Report on Form 8-K filed September 18, 2019 |
| | | | |
| 4.12 | | | | Incorporated by reference from Exhibit 4.2 to Danaher Corporation’s Current Report on Form 8-K filed September 18, 2019 |
| | | | |
| 4.13 | | | | Included in Exhibit 3.3 above |
| | | | |
| 4.14 | | | | |
| | | | |
| 10.1 | | | | Incorporated by reference from Exhibit 10.1 to Danaher Corporation’s Current Report on Form 8-K filed December 8, 2021 |
| | | | |
| 10.2 | | | | Incorporated by reference from Exhibit 10.2 to Danaher Corporation’s Annual Report on Form 10-K for the year ended December 31, 2008 |
| | | | |
| 10.3 | | | | Incorporated by reference from Exhibit 10.3 to Danaher Corporation’s Annual Report on Form 10-K for the year ended December 31, 2008 |
| | | | |
| 10.4 | | | | |
| | | | |
| 10.5 | | | | |
| | | | |
| 10.6 | | | | |
| | | | |
| 10.7 | | | | |
| | | | |
| 10.8 | | | | |
| | | | |
| 10.9 | | | | Incorporated by reference from Exhibit 10.8 to Danaher Corporation’s Annual Report on Form 10-K for the year ended December 31, 2018 |
| | | | |
| 10.10 | | | | Incorporated by reference from Exhibit 10.14 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended September 27, 2019 |
| | | | |
| 10.11 | | | | Incorporated by reference from Exhibit 10.9 to Danaher Corporation’s Annual Report on Form 10-K for the year ended December 31, 2018 |
| | | | |
| | | | | | | | | | | | | | |
| 10.12 | | | | Incorporated by reference from Exhibit 10.15 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended September 27, 2019 |
| | | | |
| 10.13 | | | | Incorporated by reference from Exhibit 10.12 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended September 27, 2019 |
| | | | |
| 10.14 | | | | Incorporated by reference from Exhibit 10.13 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended September 27, 2019 |
| | | | |
| 10.15 | | | | Incorporated by reference from Exhibit 10.1 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended March 29, 2013 |
| | | | |
| 10.16 | | | | Incorporated by reference from Exhibit 10.2 to Danaher Corporation’s Current Report on Form 8-K filed May 6, 2020 |
| | | | |
| 10.17 | | | | Incorporated by reference from Exhibit 10.3 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended July 3, 2020 |
| | | | |
| 10.18 | | | | Incorporated by reference from Exhibit 10.2 to Danaher Corporation’s Current Report on Form 8-K filed on November 8, 2018 |
| | | | |
| 10.19 | | | | Incorporated by reference from Exhibit 10.19 to Danaher Corporation’s Annual Report on Form 10-K for the year ended December 31, 2021 |
| | | | |
| 10.20 | | | | |
| | | | |
| 10.21 | | | | |
| | | | |
| 10.22 | | | | |
| | | | |
| 10.23 | | | | Incorporated by reference from Exhibit 10.25 to Danaher Corporation’s Annual Report on Form 10-K for the year ended December 31, 2011 |
| | | | |
| 10.24 | | | | Incorporated by reference from Exhibit 10.10 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended July 1, 2011 |
| | | | |
| 10.25 | | | | Incorporated by reference from Exhibit 10.1 to Danaher Corporation’s Quarterly Report on Form 10-Q for the quarter ended October 2, 2020 |
| | | | |
| 10.26 | | | | Incorporated by reference from Exhibit 10.35 to Danaher Corporation’s Annual Report on Form 10-K for the year ended December 31, 2008 |
| | | | |
| 10.27 | | | | Incorporated by reference from Exhibit 10.1 to Danaher Corporation’s Current Report on Form 8-K filed August 29, 2019 |
| | | | |
| | | | | | | | | | | | | | |
| 10.28 | | Amendment No. 1 to Second Amended and Restated Credit Agreement, dated as of September 20, 2019, among Danaher Corporation, Bank of America, N.A., Bank of America, N.A. London Branch and Citibank, N.A. , each in their respective roles as a Swing Line Lender, Bank of America, N.A. as Administrative Agent and the lenders referred to therein | | Incorporated by reference from Exhibit 10.8 to Danaher Corporation’s Report on Form 10-Q for the quarter ended September 27, 2019 |
| | | | |
| 10.29 | | Amendment No. 2 to Second Amended and Restated Credit Agreement, dated as of October 7, 2019, among Danaher Corporation, Bank of America, N.A., Bank of America, N.A. London Branch and Citibank, N.A. , each in their respective roles as a Swing Line Lender, Bank of America, N.A. as Administrative Agent and the lenders referred to therein | | Incorporated by reference from Exhibit 10.9 to Danaher Corporation’s Report on Form 10-Q for the quarter ended September 27, 2019 |
| | | | |
| 21.1 | | | | |
| | | | |
| 22.1 | | | | |
| | | | |
| 23.1 | | | | |
| | | | |
| 31.1 | | | | |
| | | | |
| 31.2 | | | | |
| | | | |
| 32.1 | | | | |
| | | | |
| 32.2 | | | | |
| | | | |
| 101.INS | | Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. (4) |
| | | | |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document (4) |
| | | | |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document (4) |
| | | | |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document (4) |
| | | | |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document (4) |
| | | | |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document (4) |
| | | | |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
Danaher is a party to additional long-term debt instruments under which, in each case, the total amount of debt authorized does not exceed 10% of the total assets of Danaher and its subsidiaries on a consolidated basis. Pursuant to paragraph 4(iii)(A) of Item 601(b) of Regulation S-K, Danaher agrees to furnish a copy of such instruments to the Securities and Exchange Commission upon request.
| | | | | | | | |
| | |
| * | Indicates management contract or compensatory plan, contract or arrangement. |
| (1) | In accordance with Instruction 2 to Item 601(a)(4) of Regulation S-K, FJ900, Inc. (a subsidiary of Danaher) has entered into a management agreement with Joust Capital II, LLC that is substantially identical in all material respects to the form of agreement referenced as Exhibit 10.23, except as to the referenced aircraft and the name of the counterparty. |
| (2) | In accordance with Instruction 2 to Item 601(a)(4) of Regulation S-K, Danaher Corporation or a subsidiary thereof has entered into additional interchange agreements with each of Joust Capital II, LLC and Joust Capital III, LLC that are substantially identical in all material respects to the form of agreement attached as Exhibit 10.24, except as to the referenced aircraft and, in certain cases, the name of the counterparty. |
| (3) | In accordance with Instruction 2 to Item 601(a)(4) of Regulation S-K, Danaher Corporation has entered into an aircraft time sharing agreement with Matthew R. McGrew that is substantially identical in all material respects to the form of agreement referenced as Exhibit 10.25. |
| (4) | Attached as Exhibit 101 to this report are the following documents formatted in Inline XBRL (Inline Extensible Business Reporting Language): (i) Consolidated Balance Sheets as of December 31, 2022 and 2021, (ii) Consolidated Statements of Earnings for the years ended December 31, 2022, 2021, and 2020, (iii) Consolidated Statements of Comprehensive Income for the years ended December 31, 2022, 2021, and 2020, (iv) Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2022, 2021, and 2020, (v) Consolidated Statements of Cash Flows for the years ended December 31, 2022, 2021, and 2020 and (vi) Notes to Consolidated Financial Statements. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | |
| | DANAHER CORPORATION |
| | | |
| Date: | February 22, 2023 | By: | | /s/ RAINER M. BLAIR |
| | | | Rainer M. Blair |
| | | | President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this annual report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated:
| | | | | | | | | | | |
| Name, Title and Signature | | Date | |
| | | |
| /s/ STEVEN M. RALES | | February 22, 2023 | |
| Steven M. Rales | | | |
| Chairman of the Board | | | |
| | | |
| /s/ MITCHELL P. RALES | | February 22, 2023 | |
| Mitchell P. Rales | | | |
| Chairman of the Executive Committee | | | |
| | | |
| /s/ RAINER M. BLAIR | | February 22, 2023 | |
| Rainer M. Blair | | | |
| President, Chief Executive Officer and Director | | | |
| | | |
| /s/ LINDA FILLER | | February 22, 2023 | |
| Linda Filler | | | |
| Director | | | |
| | | |
| /s/ FEROZ DEWAN | | February 22, 2023 | |
| Feroz Dewan | | | |
| Director | | | |
| | | |
| /s/ TERI LIST | | February 22, 2023 | |
| Teri List | | | |
| Director | | | |
| | | |
| /s/ WALTER G. LOHR, JR. | | February 22, 2023 | |
| Walter G. Lohr, Jr. | | | |
| Director | | | |
| | | |
| /s/ JESSICA L. MEGA, M.D., MPH | | February 22, 2023 | |
| Jessica L. Mega, M.D, MPH | | | |
| Director | | | |
| | | |
| | | |
| | | |
| | | | | | | | | | | |
| /s/ PARDIS C. SABETI, M.D., D.Phil | | February 22, 2023 | |
| Pardis C. Sabeti, M.D., D.Phil | | | |
| Director | | | |
| | | |
| /s/ A. SHANE SANDERS | | February 22, 2023 | |
A. Shane Sanders | | | |
| Director | | | |
| | | |
| /s/ JOHN T. SCHWIETERS | | February 22, 2023 | |
| John T. Schwieters | | | |
| Director | | | |
| | |
| /s/ ALAN G. SPOON | | February 22, 2023 | |
| Alan G. Spoon | | | |
| Director | | | |
| | | |
| /s/ RAYMOND C. STEVENS, Ph.D. | | February 22, 2023 | |
| Raymond C. Stevens, Ph.D. | | | |
| Director | | | |
| | | |
| /s/ ELIAS A. ZERHOUNI, M.D. | | February 22, 2023 | |
| Elias A. Zerhouni, M.D. | | | |
| Director | | | |
| | |
| /s/ MATTHEW R. MCGREW | | February 22, 2023 | |
| Matthew R. McGrew | | | |
| Executive Vice President and Chief Financial Officer | | | |
| | |
| /s/ CHRISTOPHER M. BOUDA | | February 22, 2023 | |
| Christopher M. Bouda | | | |
| Vice President and Chief Accounting Officer | | | |
DANAHER CORPORATION AND SUBSIDIARIES
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
($ in millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Classification | Balance at Beginning of Period (a) | | Charged to Costs & Expenses | | Impact of Currency | | Charged to Other Accounts (b) | | Write-Offs, Write-Downs & Deductions | | Balance at End of Period (a) |
| Year ended December 31, 2022: | | | | | | | | | | | |
| Allowances deducted from asset account | | | | | | | | | | | |
| Allowance for doubtful accounts | $ | 126 | | | 29 | | | (5) | | | — | | | (22) | | | $ | 128 | |
| Year ended December 31, 2021: | | | | | | | | | | | |
| Allowances deducted from asset account | | | | | | | | | | | |
| Allowance for doubtful accounts | $ | 134 | | | 31 | | | (5) | | | — | | | (34) | | | $ | 126 | |
| Year ended December 31, 2020: | | | | | | | | | | | |
| Allowances deducted from asset account | | | | | | | | | | | |
| Allowance for doubtful accounts | $ | 105 | | | 31 | | | 4 | | | 14 | | | (20) | | | $ | 134 | |
(a) Amounts include allowance for doubtful accounts classified as current and noncurrent.
(b) Amounts related to businesses acquired, net of amounts related to businesses disposed not included in discontinued operations, and amounts related to the adoption impact from ASU No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments.