EX-99.3

Exhibit 99.3 Corporate Presentation February 2024

Disclaimers Cautionary Statement Regarding Forward-Looking Statements
This Presentation contains certain forward-looking statements within the meaning of the federal securities laws and forward-looking information within the meaning of Canadian securities laws (collectively, forward-looking statements ).
Forward-looking statements may be identified by the use of the words such as “plan”, “forecast”, “intend”, “development”, “expect”, “anticipate”, “become”,
“believe”, “continue”, “could”, “estimate”, “expect”, “intends”, “may”, “might”, “plan”, “possible”, “project”,
“should”, “would”, “strategy”, “future”, “potential”, “opportunity”, “target”, “term”, “will”, “would”, “will be” or
similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding estimates and forecasts of financial and
performance metrics, projections of market opportunity and market share, expectations and timing related to regulatory submissions and commercial product launches. These forward-looking statements are based on various estimates and assumptions,
whether or not identified in this presentation, and on the current expectations of the management of enGene Holdings Inc. ( enGene ), are not predictions of annual performance, and are subject to risks and uncertainties. These forward-looking
statements are subject to a number of risks and uncertainties, including but not limited to, those described in the “Risk Factors” section of the Annual Report on Form 10-K filed with the United States Securities and Exchange Commission
(the “SEC”) on January 29, 2024 by enGene. You should carefully consider the risks and uncertainties described in the “Risk Factors” section of such Annual Report on Form 10-K, as well as other documents if and when filed by
enGene from time to time with the SEC and Canadian securities regulators. If any of these risks materialize or our assumptions prove incorrect, actual events and results could differ materially from those contained in the forward-looking statements.
There may be additional risks that enGene presently knows or that enGene currently believes are immaterial that could also cause actual events and results to differ. In addition, forward-looking statements reflect enGene’s expectations, plans,
or forecasts of future events and views as of the date of this presentation. enGene anticipates that subsequent events and developments will cause enGene’s assessments to change. While enGene may elect to update these forward-looking
statements at some point in the future, enGene specifically disclaim any obligation to do so, unless required by applicable law. These forward- looking statements should not be relied upon as representing enGene’s assessments as of any date
subsequent to the date of this presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements contained herein Purchasers’ Rights Securities legislation in certain of the provinces of Canada provides
purchasers with rights of rescission or damages, or both, where an offering memorandum or any amendment to it contains a misrepresentation. A “misrepresentation” is an untrue statement of a material fact or an omission to state a
material fact that is required to be stated or that is necessary to make any statement not misleading in light of the circumstances in which it was made. These remedies must be commenced by the purchaser within the time limits prescribed and are
subject to the defences contained in the applicable securities legislation. The following is a summary of the statutory rights of rescission or damages, or both, under securities legislation in certain of the provinces of Canada where such summary
is required to be disclosed under the relevant securities legislation, and as such, is subject to the express provisions of the legislation and the related regulations and rules and reference is made thereto for the complete text of such provisions.
Such provisions may contain limitations and statutory defences not described here on which the issuer and other applicable parties may rely. The rights described below are in addition to, and without derogation from, any other right or remedy
available at law to purchasers of the New Notes. Purchasers should refer to the applicable provisions of the securities legislation of their province for the particulars of these rights or consult with a legal adviser. Ontario Purchasers: Ontario
securities legislation provides that where an offering memorandum is delivered to a purchaser and contains a misrepresentation, the purchaser will, except as provided below, have a statutory right of action for damages or for rescission against the
issuer and a selling security holder on whose behalf the distribution is made, without regard to whether the purchaser relied on the misrepresentation; if the purchaser elects to exercise the right of rescission, the purchaser will have no right of
action for damages against the issuer or any selling security holder. No such action shall be commenced more than, in the case of an action for rescission, 180 days after the date of the transaction that gave rise to the cause of action, or, in the
case of any action other than an action for rescission, the earlier of: (i) 180 days after the purchaser first had knowledge of the facts giving rise to the cause of action, or (ii) three years after the date of the transaction that gave rise to the
cause of action. The Ontario legislation provides a number of limitations and defences to such actions, including: (a) the issuer or any selling security holder is not liable if it proves that the purchaser purchased the securities with knowledge of
the misrepresentation; (b) in an action for damages, the issuer shall not be liable for all or any portion of the damages that the issuer or any selling security holder proves do not represent the depreciation in value of the securities as a result
of the misrepresentation relied upon; and (c) in no case shall the amount recoverable exceed the price at which the securities were offered. These rights are not available for a purchaser that is: (a) a Canadian financial institution, meaning
either: (i) an association governed by the Cooperative Credit Associations Act (Canada) or a central cooperative credit society for which an order has been made under section 473(1) of that Act; or (ii) a bank, loan corporation, trust company, trust
corporation, insurance company, treasury branch, credit union, caisse populaire, financial services cooperative, or league that, in each case, is authorized by an enactment of Canada or a province or territory of Canada to carry on business in
Canada or a province or territory of Canada; (b) a Schedule III bank, meaning an authorized foreign bank named in Schedule III of the Bank Act (Canada); (c) the Business Development Bank of Canada incorporated under the Business Development Bank of
Canada Act (Canada); or (d) a subsidiary of any person referred to in clauses (a), (b) or (c), if the person owns all of the voting securities of the subsidiary, except the voting securities required by law to be owned by directors of that
subsidiary. Intellectual Property This Presentation contains trademarks, service marks, trade names, copyrights, and products of enGene and other companies, which are the property of their respective owners. The use or display of third
parties’ trademarks, service marks, trade names, copyrights, or products in this Presentation is not intended to, and does not, imply a relationship with enGene, or an endorsement of or sponsorship by enGene. Solely for convenience, the
trademarks, service marks, and trade names referred to in this Presentation may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that enGene will not assert, to the fullest extent permitted
under applicable law, their rights or the right of the applicable licensor in such trademarks, service marks and trade names. Industry and Market Data This Presentation relies on and refers to certain information and statistics based on estimates by
enGene’s management and/or obtained from third party sources which enGene believes to be reliable. enGene has not independently verified the accuracy or completeness of any such third party information, which involves elements of subjective
judgment and analysis that may or may not prove to be accurate. None enGene, or its affiliates or any third parties that provide information to enGene or its affiliates, such as market research firms, guarantees the accuracy, completeness,
timeliness, or availability of any information. None enGene, or its affiliates, or any third parties that provide information to enGene, and its affiliates, such as market research firms, is responsible for any errors or omissions (negligent or
otherwise), regardless of the cause, or the results obtained from the use of such content. enGene may have supplemented such information where necessary, taking into account publicly available information about other industry participants and enGene
management’s best view as to information that is not publicly available. Neither enGene nor its affiliates give any express or implied warranties with respect to the information included herein, including, but not limited to, any warranties
regarding its accuracy or of merchantability or fitness for a particular purpose or use, and they expressly disclaim any responsibility or liability for direct, indirect, incidental, exemplary, compensatory, punitive, special, or consequential
damages, costs, expenses, legal fees, or losses (including lost income or profits and opportunity costs) in connection with the use of the information herein. Lead Program (EG-70/detalimogene voraplasmid) The lead program described herein is an
investigational drug therapy that has not been subject to testing designed to demonstrate that the therapy is effective in humans or to provide a basis to predict in advance whether an adequate level of efficacy in humans will be demonstrated in
further testing. Although deemed sufficient to permit further testing, the limited, early Phase 1 testing to date is not a sufficient basis on which to predict efficacy. Although the FDA has indicated that the authorized Phase 2 portion of the
current 2 LEGEND study may potentially support BLA approval, that outcome will depend entirely on the results of Phase 2 tests, none of which are expected to be available until at least 2025.

enGene: moving genetic medicines into the mainstream
Registrational-stage EG-70 program represents estimated multi-billion
dollar opportunity, with initial entry in BCG-unresponsive NMIBC • Highly differentiated program designed for community urologists: Single-step administration, more patient-friendly, no onerous viral containment / handling requirements •
Profile supportive of expansive applications across bladder cancer: Product candidate attributes conducive to broad physician adoption • Non-viral platform supports low COGS and scalable manufacturing: Polymeric nanoparticle with
non-integrating DNA cargo; already scaled for initial US launch • Multiple anticipated value inflection points: Mid-2024 Phase 2 interim efficacy data and Q1 2026 BLA NMIBC = Non-muscle invasive bladder cancer; BCG = Bacillus Calmette-Guerin;
BLA = Biologics License Application; COGS = Cost of Goods Sold 4 Expected timelines and anticipated milestones reflect enGene management's current estimate and are subject to change.

enGene platform: localized, non-viral delivery of genetic medicines to
mucosal tissues and surfaces Mucosal tissues are underserved by Product platform tailored for streamlined, traditional gene therapy yet can be tissue-specific administration accessed via local administration Nasopharynx Carcinoma Rhinitis Mucosal
vaccines Respiratory Tract Cystic Fibrosis Asthma COPD Lung cancer Gastrointestinal Tract Colorectal cancer Inflammatory Bowel Disease Lyophilized Drug Product Reconstituted at Point of Care Short Bowel Syndrome Familial Adenomatous Polyposis No
thawing process or special and instilled into target organ Bladder (lead tissue of focus) handling required Cancer 5
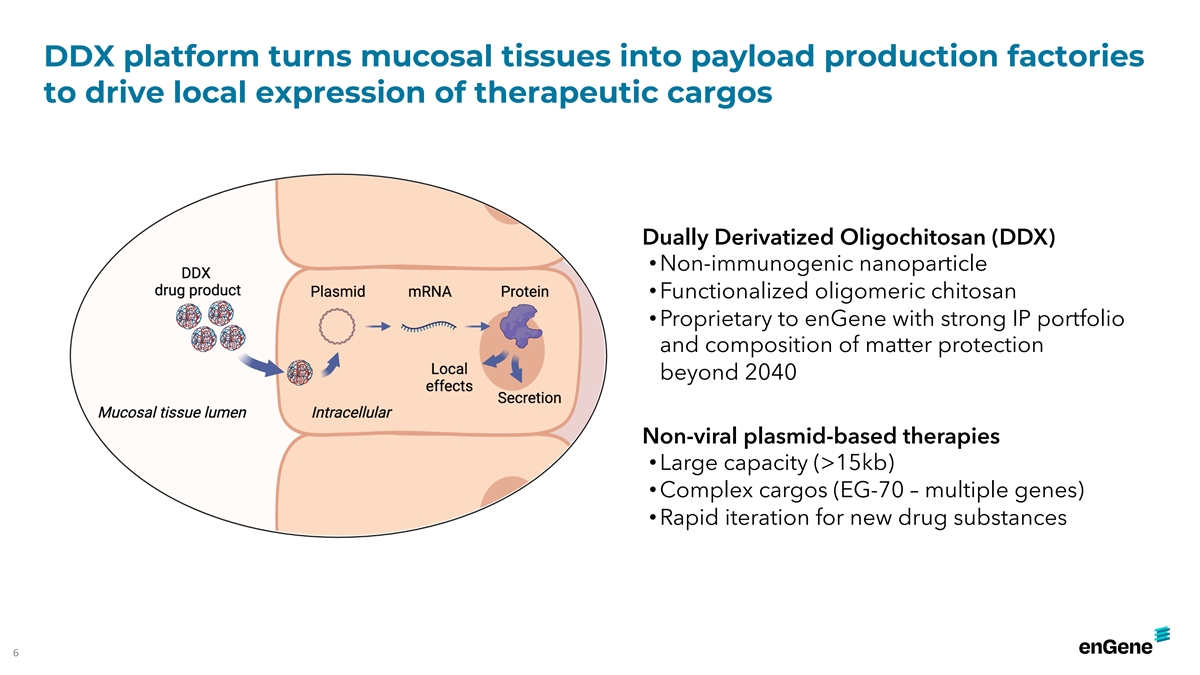
DDX platform turns mucosal tissues into payload production factories to
drive local expression of therapeutic cargos Dually Derivatized Oligochitosan (DDX) • Non-immunogenic nanoparticle • Functionalized oligomeric chitosan • Proprietary to enGene with strong IP portfolio and composition of matter
protection beyond 2040 Non-viral plasmid-based therapies • Large capacity (>15kb) • Complex cargos (EG-70 – multiple genes) • Rapid iteration for new drug substances 6
Genetic medicine designed for urologists: EG-70 combines promising
efficacy with a unique, fit-for-purpose product profile LEGEND study supports Non-viral DDX platform Product designed for ease planned Q1 2026 BLA supports favourable COGS of use in any urology clinic • 73% CR rate at any time*: All •
Drug product manufacturable • Administered analogously to Ph1 endpoints met; currently using scalable, established BCG: No pre-wash steps, no enrolling in pivotal Ph2 study techniques extended chair time, no vial thaw • Favorable safety
profile • Low COGS supports commercial • No special handling observed to date, no MTD and strategic optionality at a requirements (e.g., no ultra-cold reached platform level chain, no BSL2/USP800 facilities needed) • Encouraging
durability signals • Non-viral mechanism supportive observed in Phase 1 of repeat dosing Designed to be the first script urologists write after BCG failure • CR = Complete Response. 73% complete response rate represents the overall CR
rate at any time during the Ph1 LEGEND study in BCG-unresponsive NMIBC with Cis. MTD = Maximum tolerated dose 7
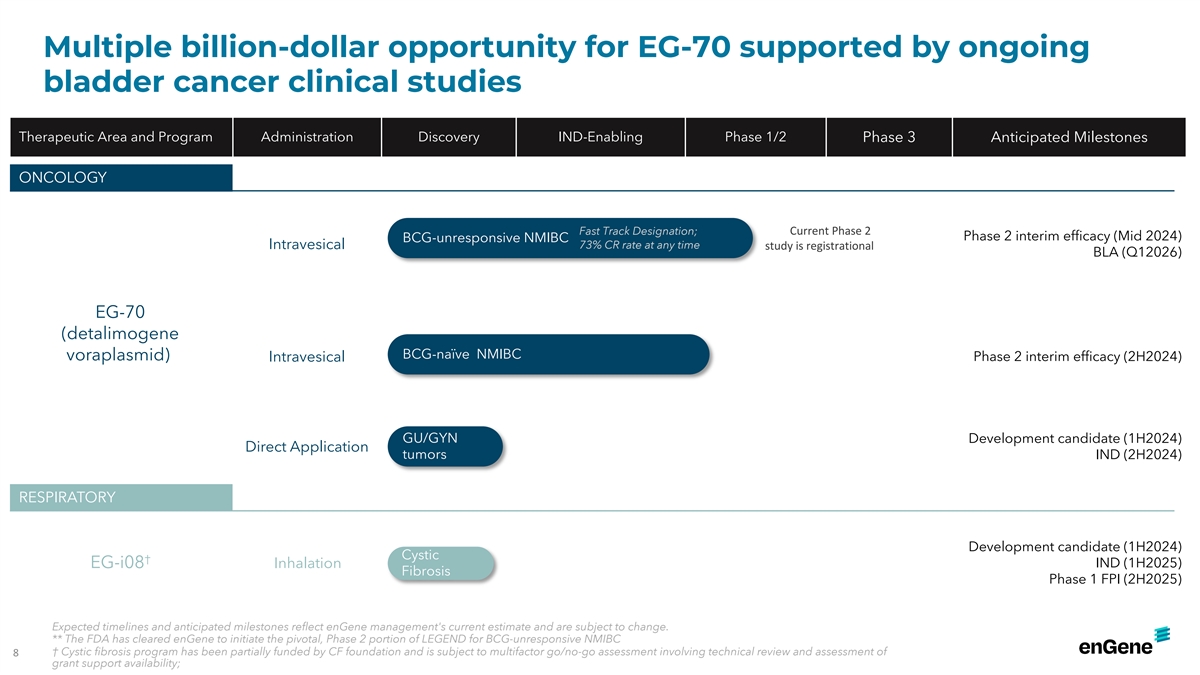
Multiple billion-dollar opportunity for EG-70 supported by ongoing
bladder cancer clinical studies Therapeutic Area and Program Administration Discovery IND-Enabling Phase 1/2 Phase 3 Anticipated Milestones ONCOLOGY Fast Track Designation; Current Phase 2 Phase 2 interim efficacy (Mid 2024) BCG-unresponsive NMIBC
Intravesical 73% CR rate at any time study is registrational BLA (Q12026) EG-70 (detalimogene BCG-naïve NMIBC voraplasmid) Intravesical Phase 2 interim efficacy (2H2024) GU/GYN Development candidate (1H2024) Direct Application tumors IND
(2H2024) RESPIRATORY Development candidate (1H2024) Cystic † IND (1H2025) EG-i08 Inhalation Fibrosis Phase 1 FPI (2H2025) Expected timelines and anticipated milestones reflect enGene management's current estimate and are subject to change. **
The FDA has cleared enGene to initiate the pivotal, Phase 2 portion of LEGEND for BCG-unresponsive NMIBC † Cystic fibrosis program has been partially funded by CF foundation and is subject to multifactor go/no-go assessment involving technical
review and assessment of 8 grant support availability;

EG-70 (detalimogene voraplasmid): addressing unmet needs in
NMIBC

Potential multi-billion dollar market opportunity in NMIBC NMIBC
remains a substantial unmet medical need 2 • Highest cost of all cancers to treat • >82K new diagnoses of bladder cancer per year in • 50% of patients fail BCG, only 1 USA, with 16K deaths approved first-line treatment •
BCG shortage has created a • Radical cystectomy is standard to public health crisis and prevent muscle invasion after BCG unmet need across the care failure continuum • Avoiding cystectomy is goal of • Potential near-term enGene
therapy in BCG-unresponsive market entry via NMIBC, per FDA guidance BCG-unresponsive NMIBC • Community urologists typically treat NMIBC, not oncologists; specialized product requirements 1 2 10 SEER database, 2023 figure; Mossanen and Gore,
Curr Opin Urol 2014
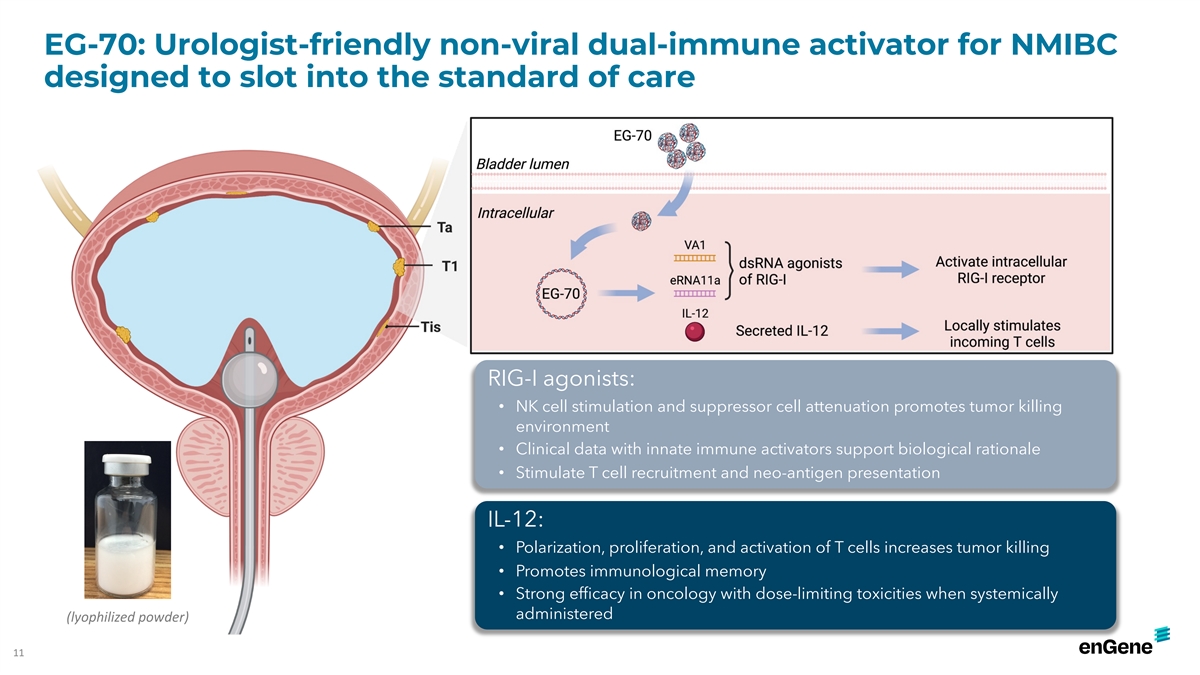
EG-70: Urologist-friendly non-viral dual-immune activator for NMIBC
designed to slot into the standard of care RIG-I agonists: • NK cell stimulation and suppressor cell attenuation promotes tumor killing environment • Clinical data with innate immune activators support biological rationale •
Stimulate T cell recruitment and neo-antigen presentation IL-12: • Polarization, proliferation, and activation of T cells increases tumor killing • Promotes immunological memory • Strong efficacy in oncology with dose-limiting
toxicities when systemically administered (lyophilized powder) 11
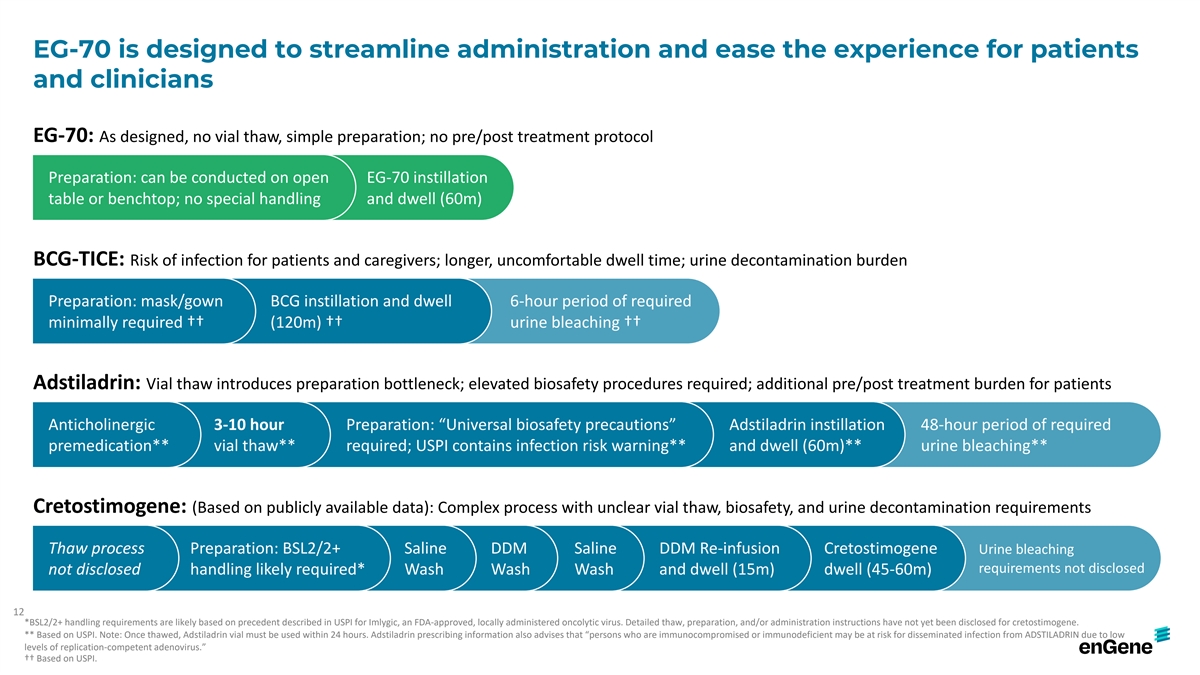
EG-70 is designed to streamline administration and ease the experience
for patients and clinicians EG-70: As designed, no vial thaw, simple preparation; no pre/post treatment protocol Preparation: can be conducted on open EG-70 instillation table or benchtop; no special handling and dwell (60m) BCG-TICE: Risk of
infection for patients and caregivers; longer, uncomfortable dwell time; urine decontamination burden Preparation: mask/gown BCG instillation and dwell 6-hour period of required minimally required †† (120m) †† urine bleaching
†† Adstiladrin: Vial thaw introduces preparation bottleneck; elevated biosafety procedures required; additional pre/post treatment burden for patients Anticholinergic 3-10 hour Preparation: “Universal biosafety precautions”
Adstiladrin instillation 48-hour period of required premedication** vial thaw** required; USPI contains infection risk warning** and dwell (60m)** urine bleaching** Cretostimogene: (Based on publicly available data): Complex process with unclear
vial thaw, biosafety, and urine decontamination requirements Thaw process Preparation: BSL2/2+ Saline DDM Saline DDM Re-infusion Cretostimogene Urine bleaching requirements not disclosed not disclosed handling likely required* Wash Wash Wash and
dwell (15m) dwell (45-60m) 12 *BSL2/2+ handling requirements are likely based on precedent described in USPI for Imlygic, an FDA-approved, locally administered oncolytic virus. Detailed thaw, preparation, and/or administration instructions have not
yet been disclosed for cretostimogene. ** Based on USPI. Note: Once thawed, Adstiladrin vial must be used within 24 hours. Adstiladrin prescribing information also advises that “persons who are immunocompromised or immunodeficient may be at
risk for disseminated infection from ADSTILADRIN due to low levels of replication-competent adenovirus.” †† Based on USPI.

EG-70 in NMIBC Patients Who Are BCG-Unresponsive and High- Risk NMIBC
Patients Who Have Been Incompletely Treated With BCG or Are BCG-Naïve NCT04752722 We would like to thank the patients, their families, and all staff at participating sites
FDA guidance: Use of a single-arm, open-label design is appropriate for
approval for BCG-unresponsive NMIBC • Clearly defined patient population and entry criteria for BCG-unresponsive NMIBC provided by guidance • Approval based on complete response rate, duration of response, and safety • Pivotal
Phase 2 design is consistent with FDA guidance 14
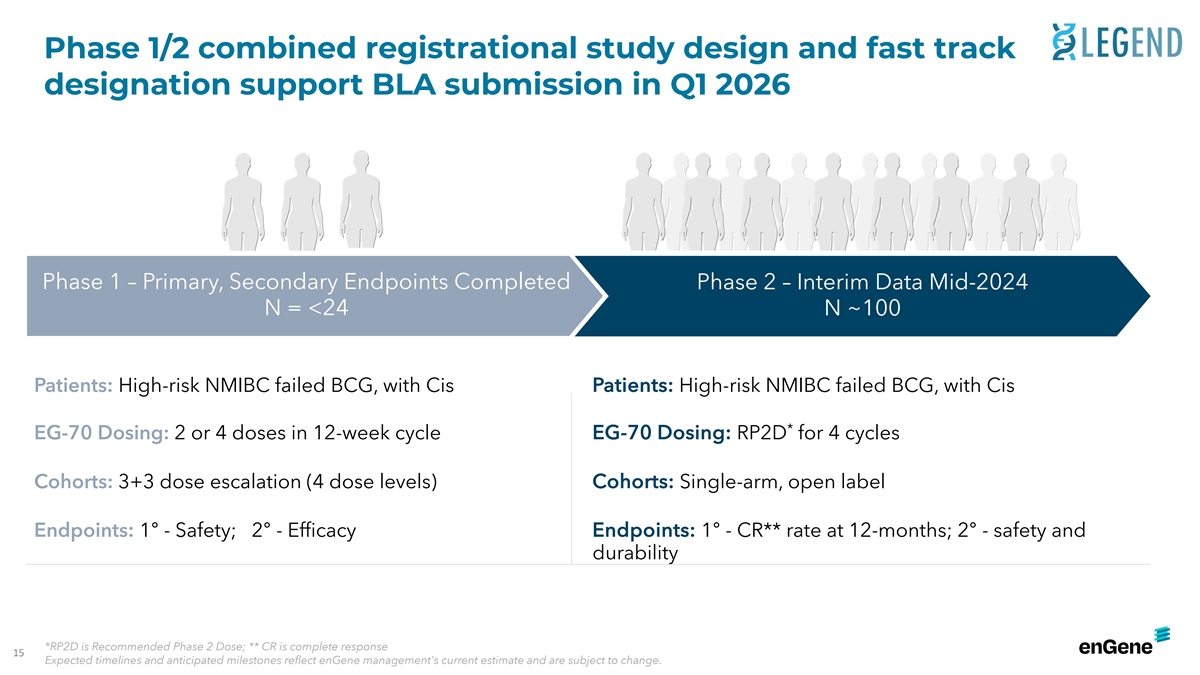
Phase 1/2 combined registrational study design and fast track
designation support BLA submission in Q1 2026 Phase 1 – Primary, Secondary Endpoints Completed Phase 2 – Interim Data Mid-2024 N = <24 N ~100 : Patients: Patients High-risk NMIBC failed BCG, with Cis High-risk NMIBC failed BCG, with
Cis * EG-70 Dosing: EG-70 Dosing: 2 or 4 doses in 12-week cycle RP2D for 4 cycles Cohorts: 3+3 dose escalation (4 dose levels) Cohorts: Single-arm, open label : Endpoints: Endpoints 1° - Safety; 2° - Efficacy 1° - CR** rate at
12-months; 2° - safety and durability *RP2D is Recommended Phase 2 Dose; ** CR is complete response 15 Expected timelines and anticipated milestones reflect enGene management's current estimate and are subject to change.
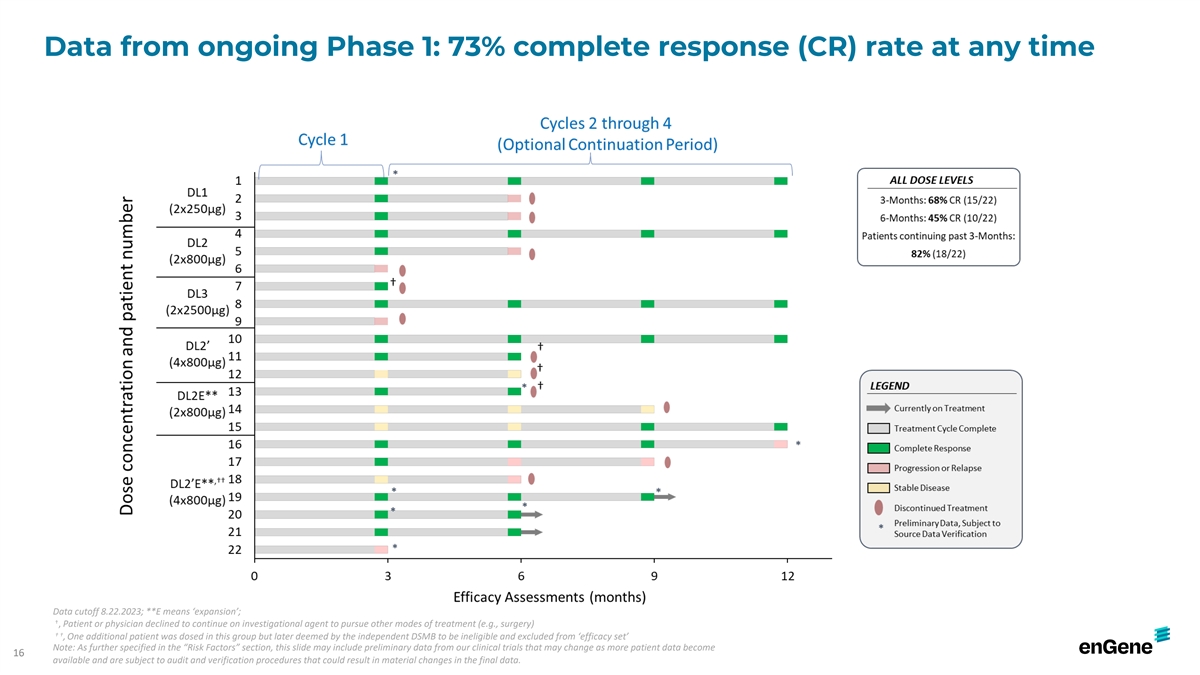
Data from ongoing Phase 1: 73% complete response (CR) rate at any time
Data cutoff 8.22.2023; **E means ‘expansion’; † , Patient or physician declined to continue on investigational agent to pursue other modes of treatment (e.g., surgery) † † , One additional patient was dosed in this
group but later deemed by the independent DSMB to be ineligible and excluded from ‘efficacy set’ Note: As further specified in the “Risk Factors” section, this slide may include preliminary data from our clinical trials that
may change as more patient data become 16 available and are subject to audit and verification procedures that could result in material changes in the final data.
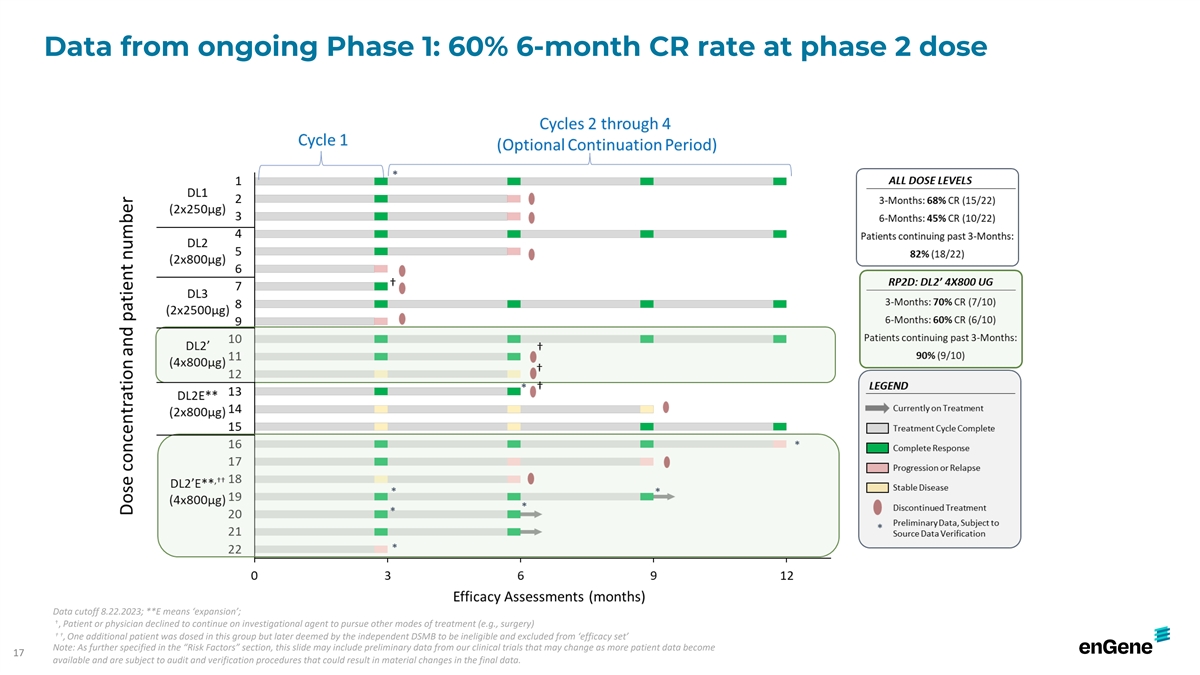
Data from ongoing Phase 1: 60% 6-month CR rate at phase 2 dose Data
cutoff 8.22.2023; **E means ‘expansion’; † , Patient or physician declined to continue on investigational agent to pursue other modes of treatment (e.g., surgery) † † , One additional patient was dosed in this group but
later deemed by the independent DSMB to be ineligible and excluded from ‘efficacy set’ Note: As further specified in the “Risk Factors” section, this slide may include preliminary data from our clinical trials that may change
as more patient data become 17 available and are subject to audit and verification procedures that could result in material changes in the final data.
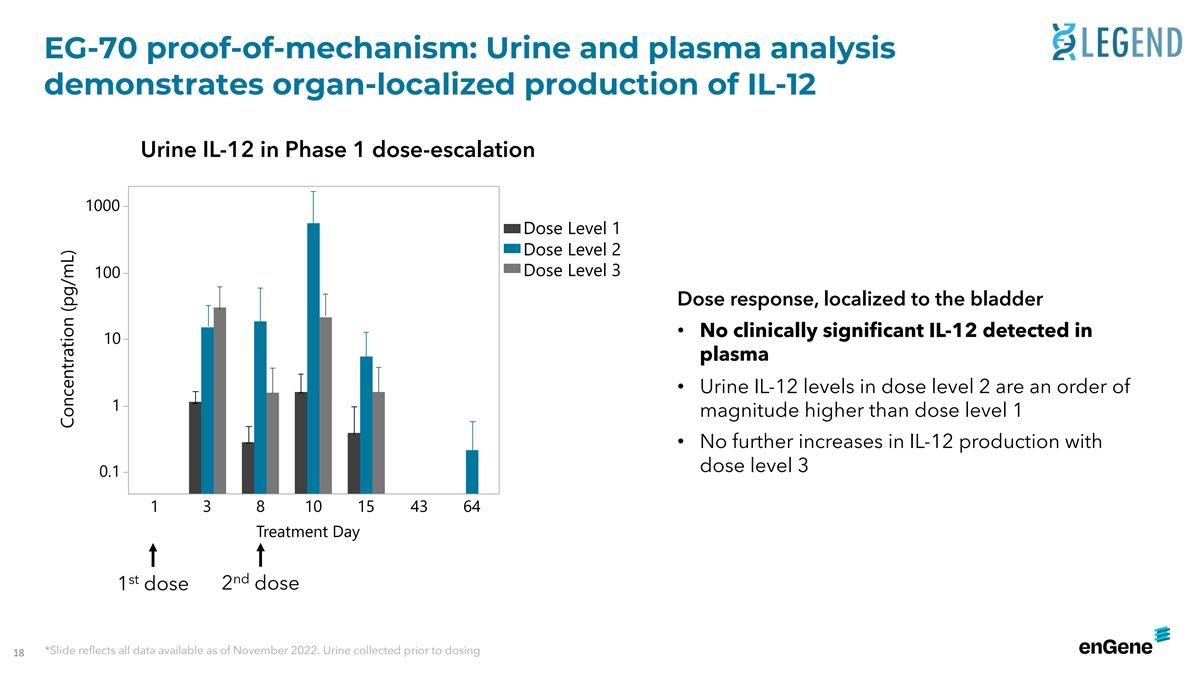
EG-70 proof-of-mechanism: Urine and plasma analysis demonstrates
organ-localized production of IL-12 Urine IL-12 in Phase 1 dose-escalation 1000 Dose Level 1 Dose Level 2 Dose Level 3 100 Dose response, localized to the bladder • No clinically significant IL-12 detected in 10 plasma • Urine IL-12
levels in dose level 2 are an order of 1 magnitude higher than dose level 1 • No further increases in IL-12 production with dose level 3 0.1 1 3 8 10 15 43 64 Treatment Day st nd 1 dose 2 dose *Slide reflects all data available as of November
2022. Urine collected prior to dosing 18
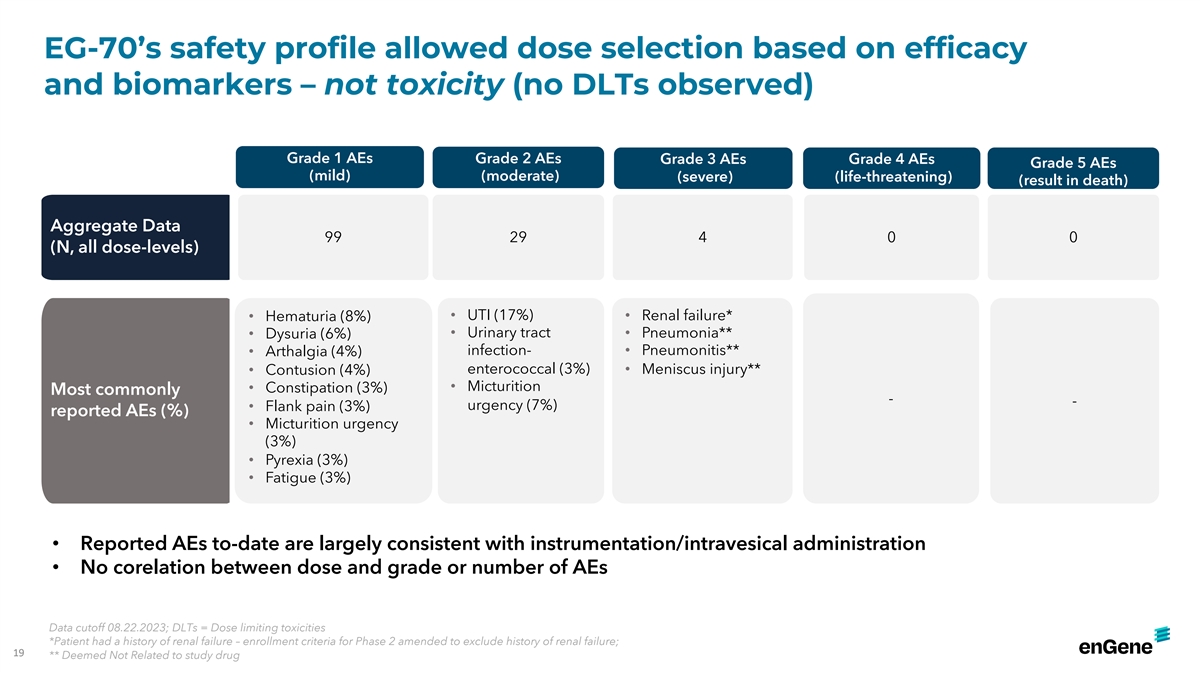
EG-70’s safety profile allowed dose selection based on efficacy
and biomarkers – not toxicity (no DLTs observed) Grade 1 AEs Grade 2 AEs Grade 3 AEs Grade 4 AEs Grade 5 AEs (mild) (moderate) (severe) (life-threatening) (result in death) Aggregate Data 99 29 4 0 0 (N, all dose-levels) • UTI (17%)
• Renal failure* • Hematuria (8%) • Urinary tract • Pneumonia** • Dysuria (6%) infection- • Pneumonitis** • Arthalgia (4%) enterococcal (3%) • Meniscus injury** • Contusion (4%) •
Micturition • Constipation (3%) Most commonly - - urgency (7%) • Flank pain (3%) reported AEs (%) • Micturition urgency (3%) • Pyrexia (3%) • Fatigue (3%) • Reported AEs to-date are largely consistent with
instrumentation/intravesical administration • No corelation between dose and grade or number of AEs Data cutoff 08.22.2023; DLTs = Dose limiting toxicities *Patient had a history of renal failure – enrollment criteria for Phase 2 amended
to exclude history of renal failure; 19 ** Deemed Not Related to study drug

Urologists play a central role in the multi-year patient journey from
diagnosis onward, with the goal of avoiding radical cystectomy Urology Referral and Diagnosis High-grade NMIBC with carcinoma in situ (CIS): Begin intravesical therapies Treatment: Surveillance: Exhaustion of intravesical options, Every 3-months
until relapse currently beginning with BCG Available Intravesical therapies have failed; tumor has recurred. ~2-years have elapsed. Surgery: Medical Oncology Referral: Radical cystectomy iv pembro or pembro combo 20
EG-70’s patient- and clinician-friendly profile potentially makes
it ideal for use in community urology clinics Designed to slot into standard of care: Requires no shift in practice for ✓ community urologists or burdensome regime for clinicians and patients Non-viral, lyophilized drug product: No heightened
exposure risks to clinicians, ✓ ◦ staff, or patient families; no BSL2 handling precautions or -80 C storage required Potential for compelling efficacy profile: 73% CR rate at any time is the highest ✓ observed in Phase 1 context
in BCG-unresponsive NMIBC patients Favorable safety profile based on available data: Does not require non-urological ✓ or specialized patient care team (e.g., medical oncology team) 21

LEGEND study will act as launchpad for an expansive EG-70 franchise
• Potent yet local immune system stimulation • Stimulation of adaptive and innate immune system via dual IL-12/RIG-I activation • Strong preclinical evidence of immunological memory Intermediate st • Favorable safety profile
based on available BCG-unresponsive 1 line Other GU and locally NMIBC NMIBC* data (IND-package and clinical data) cancers advanced (>60k pts/yr) (60k pts/yr) bladder cancer • Flexible formulation amenable to multiple routes of
administration • IP fortress through at least 2040 *Potential launch dates and indications are illustrative of EG-70’s potential; 22 *FDA has suggested including 1L patients in our study – use in 1L BCG-naïve patients would
double the potential addressable patient population

World-class leadership and investors enGene Leadership Team Selected
Investors JASON HANSON, JD ANTHONY CHEUNG, Ph.D JAMES SULLIVAN, Ph.D ALEX NICHOLS, Ph.D Chief Executive Officer Chief Technology Officer Chief Scientific Officer President and Chief Operating Chief Executive Officer, Ohana Co-founder, enGene (CEO
through VP, Pulmonary Discovery, Translate Bio Officer 2018) Founding Director, Mythic Therapeutics Executive Director, Sana Biotechnology Co-Founder and CEO, Mythic Co-inventor on all key enGene patents EVP & Chief Strategy Officer, Nuvasive
Director, R&D, Vertex Pharmaceuticals Therapeutics Former Committee Member, ASGCT Chief Operating Officer, Medicis Co-Founder, Cogen Industry Liaison Committee (2008-2014) Corporate VP, GE and GE Healthcare Associate, Flagship Pioneering RYAN
DAWS RICHARD BRYCE, MBChB, LEE GIGUERE, JD MRCGP, MFPM Chief Financial Officer Chief Legal Officer and Secretary CFO Roles: Concert Therapeutics, Chief Medical Officer CLO, Obsidian Therapeutics Obsidian Therapeutics CMO, Rain Oncology General
Counsel, Chiasma Inc. Investment Banker Roles: Cowen, Stifel, Other legal roles: Karyopharm Chief Medical & Scientific Officer, Puma Baird Biotechnology Therapeutics, Boston Scientific, Sr Director - Clinical Science, Onyx Goodwin Procter
Pharma. 23

Summary: Moving genetic medicines into the mainstream • Highly
differentiated program designed for community urologists: Single-step administration, more patient-friendly, no onerous viral containment / handling requirements • Profile supportive of expansive applications across bladder cancer: Product
candidate attributes conducive to broad physician adoption • Non-viral platform supports low COGS and scalable manufacturing: Polymeric nanoparticle with non-integrating DNA cargo; already scaled for initial US launch • Multiple
anticipated value inflection points: Mid-2024 Phase 2 interim efficacy data and Q1 2026 BLA NMIBC = Non-muscle invasive bladder cancer; BCG = Bacillus Calmette-Guerin; BLA = Biologics License Application; COGS = Cost of Goods Sold 24 Expected
timelines and anticipated milestones reflect enGene management's current estimate and are subject to change.

Corporate Presentation February 2024

Appendix
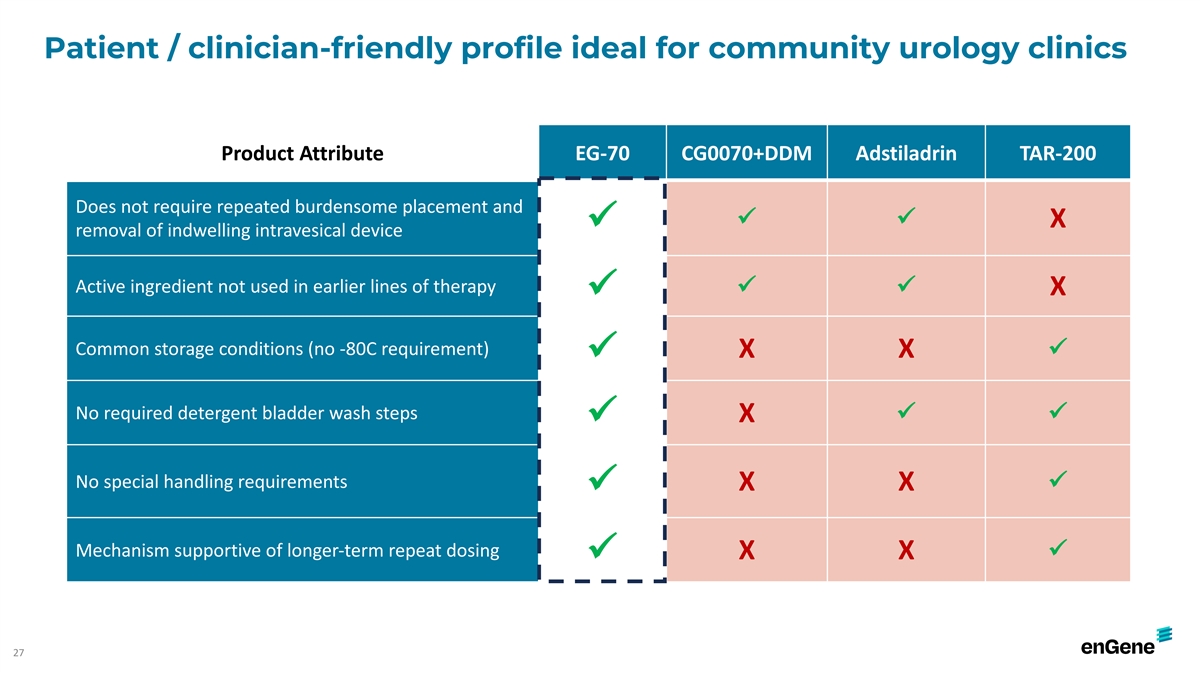
Patient / clinician-friendly profile ideal for community urology
clinics Product Attribute EG-70 CG0070+DDM Adstiladrin TAR-200 Does not require repeated burdensome placement and ü ü X ü removal of indwelling intravesical device Active ingredient not used in earlier lines of therapy ü ü X
ü Common storage conditions (no -80C requirement) X X ü ü No required detergent bladder wash steps ü ü X ü No special handling requirements ü X X ü Mechanism supportive of longer-term repeat dosing ü X X
ü 27
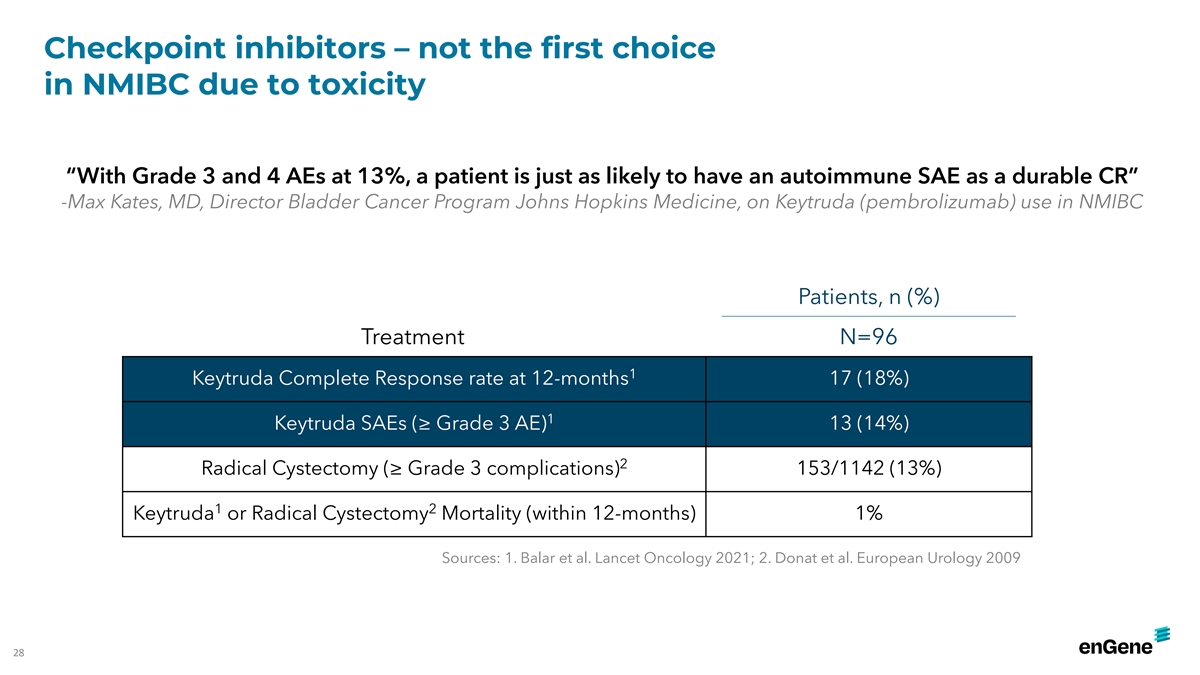
Checkpoint inhibitors – not the first choice in NMIBC due to
toxicity “With Grade 3 and 4 AEs at 13%, a patient is just as likely to have an autoimmune SAE as a durable CR” -Max Kates, MD, Director Bladder Cancer Program Johns Hopkins Medicine, on Keytruda (pembrolizumab) use in NMIBC Patients, n
(%) Treatment N=96 1 Keytruda Complete Response rate at 12-months 17 (18%) 1 Keytruda SAEs (≥ Grade 3 AE) 13 (14%) 2 Radical Cystectomy (≥ Grade 3 complications) 153/1142 (13%) 1 2 Keytruda or Radical Cystectomy Mortality (within
12-months) 1% Sources: 1. Balar et al. Lancet Oncology 2021; 2. Donat et al. European Urology 2009 28

EG-70 preclinical and CMC data
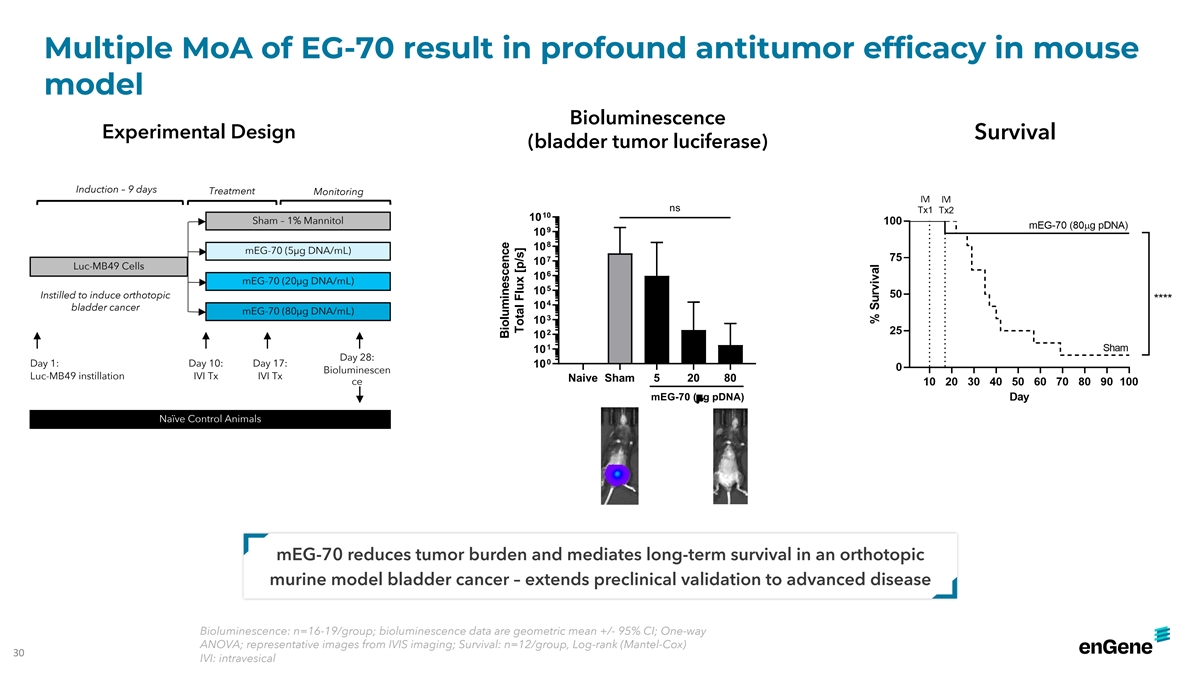
Multiple MoA of EG-70 result in profound antitumor efficacy in mouse
model Bioluminescence Experimental Design Survival (bladder tumor luciferase) Induction – 9 days Treatment Monitoring ns 10 10 Sham – 1% Mannitol 9 10 8 10 mEG-70 (5µg DNA/mL) 7 10 Luc-MB49 Cells 6 10 mEG-70 (20µg DNA/mL) 5 10
Instilled to induce orthotopic 4 10 bladder cancer mEG-70 (80µg DNA/mL) 3 10 2 10 1 10 Day 28: 0 Day 1: Day 10: Day 17: 10 Bioluminescen Luc-MB49 instillation IVI Tx IVI Tx Naive Sham 5 20 80 ce mEG-70 (µg pDNA) Naïve Control Animals
mEG-70 reduces tumor burden and mediates long-term survival in an orthotopic murine model bladder cancer – extends preclinical validation to advanced disease Bioluminescence: n=16-19/group; bioluminescence data are geometric mean +/- 95% CI;
One-way ANOVA; representative images from IVIS imaging; Survival: n=12/group, Log-rank (Mantel-Cox) 30 IVI: intravesical Bioluminescence Total Flux [p/s]
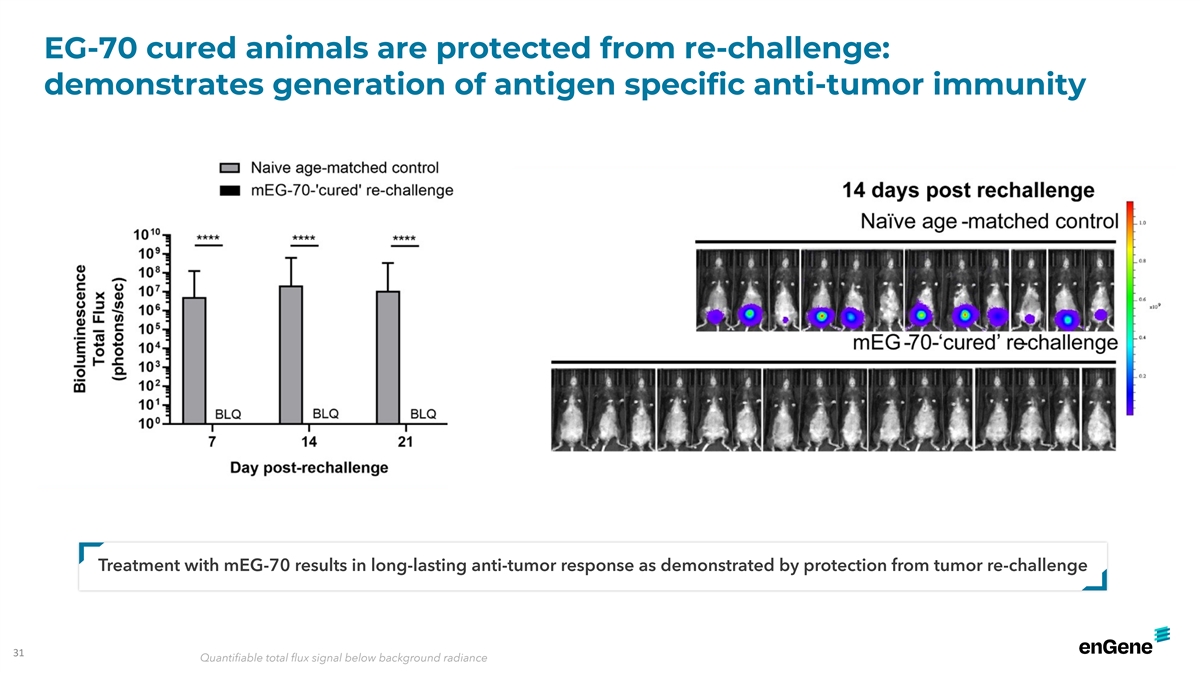
EG-70 cured animals are protected from re-challenge: demonstrates
generation of antigen specific anti-tumor immunity Treatment with mEG-70 results in long-lasting anti-tumor response as demonstrated by protection from tumor re-challenge 31 Quantifiable total flux signal below background radiance
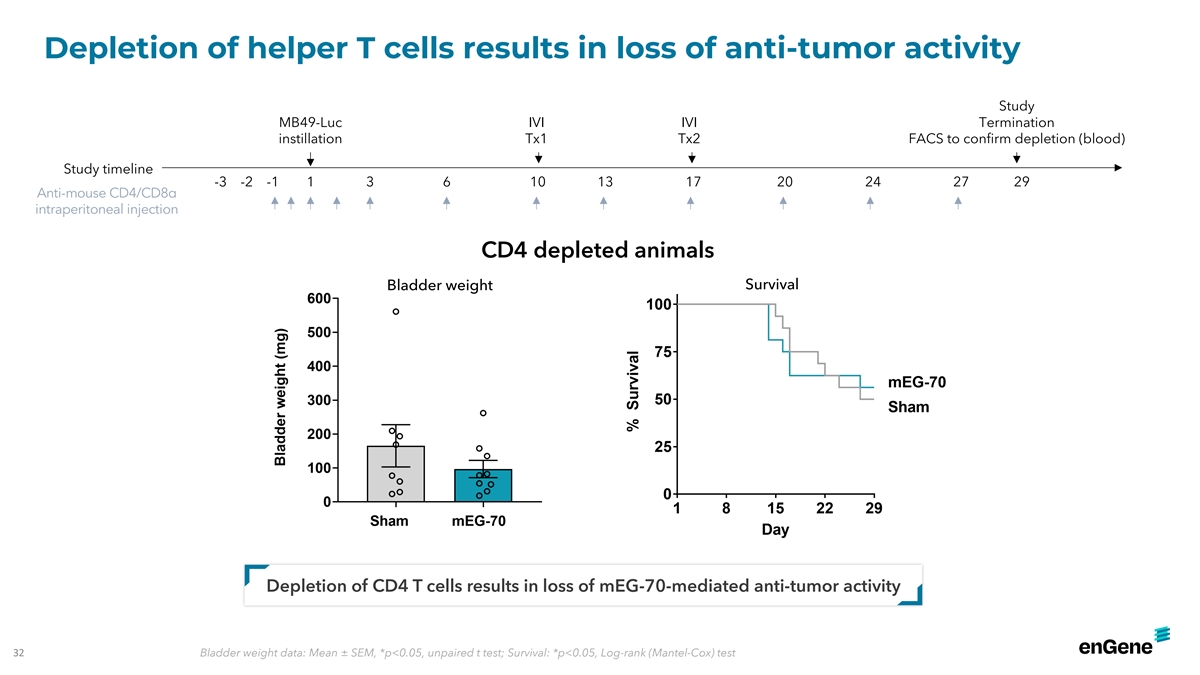
Depletion of helper T cells results in loss of anti-tumor activity
Study MB49-Luc IVI IVI Termination instillation Tx1 Tx2 FACS to confirm depletion (blood) Study timeline -3 -2 -1 1 3 6 10 13 17 20 24 27 29 Anti-mouse CD4/CD8α intraperitoneal injection CD4 depleted animals Bladder weight Survival Depletion of
CD4 T cells results in loss of mEG-70-mediated anti-tumor activity 32 Bladder weight data: Mean ± SEM, *p<0.05, unpaired t test; Survival: *p<0.05, Log-rank (Mantel-Cox) test IN IN INT T T0 0 02 2 2- - -409 429 429

EG-70 induces dose-dependent target engagement in bladders of
tumor-bearing mice Ifnb Ifna2 Ddx58 Irf7 Isg15 Cxcl10 Ifit1 Ccl5 Tnfa Ifng Strong dose-dependent induction of RIG-I and IL-12 signaling in bladders of tumor-bearing mice treated with EG-70 n=8/group; data are mean +/- SEM; Mann Whitney t-test; 33
tumor bearing mice treated with indicated doses of mEG-70; bladder harvested 24h post-administration for Taqman qPCR of bladder tissue lysates INT02-348
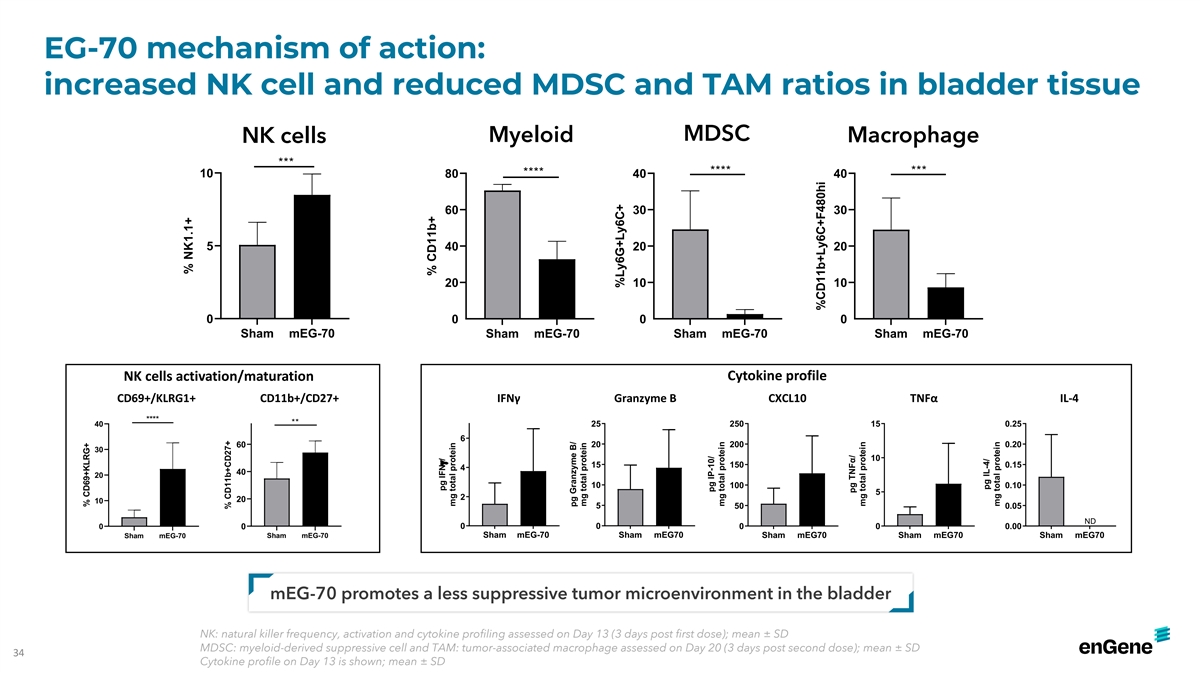
EG-70 mechanism of action: increased NK cell and reduced MDSC and TAM
ratios in bladder tissue MDSC NK cells Myeloid Macrophage *** **** *** **** 10 80 40 40 60 30 30 5 40 20 20 20 10 10 0 0 0 0 Sham mEG-70 Sham mEG-70 Sham mEG-70 Sham mEG-70 Cytokine profile NK cells activation/maturation CD69+/KLRG1+ CD11b+/CD27+
IFNγ Granzyme B CXCL10 TNFα IL-4 **** ** 40 25 250 15 0.25 6 60 20 200 0.20 30 10 15 150 0.15 4 40 20 10 100 0.10 5 2 20 10 5 50 0.05 ND 0 0 0 0 0 0 0.00 Sham mEG-70 Sham mEG70 Sham mEG-70 Sham mEG-70 Sham mEG70 Sham mEG70 Sham mEG70
mEG-70 promotes a less suppressive tumor microenvironment in the bladder NK: natural killer frequency, activation and cytokine profiling assessed on Day 13 (3 days post first dose); mean ± SD MDSC: myeloid-derived suppressive cell and TAM:
tumor-associated macrophage assessed on Day 20 (3 days post second dose); mean ± SD 34 Cytokine profile on Day 13 is shown; mean ± SD % CD69+KLRG+ % NK1.1+ % CD11b+CD27+ % CD11b+ pg IFNg/ mg total protein pg Granzyme B/ mg total protein
%Ly6G+Ly6C+ pg IP-10/ mg total protein %CD11b+Ly6C+F480hi pg TNFα/ mg total protein pg IL-4/ mg total protein

EG-70 mechanism of action: T-cell recruitment and infiltration into
tumor microenvironment CD4 CD8 CD4 CD8 **** **** 15 25 30 30 *** **** 20 10 20 20 Bladder 15 Lymph node 10 5 10 10 5 0 0 0 0 Sham mEG-70 Sham mEG-70 Sham mEG-70 Sham mEG-70 CD3 H&E CD4 CD8 Sham mEG-70 mEG-70 promotes an anti-tumor adaptive
immune cell response 35 T cells assessed on Day 23 (6 days post second dose), mean ± SD; IHC performed on bladder tissue collected on Day 22 (5 days post second dose) % CD4+ % CD8+ % CD4+ % CD8+

EG-70 induces strong target engagement of RIG-I and IL-12 signaling:
biomarkers translate across species Ifng Ifnb1 Irf7 Tnfa Mouse Non-human primate Empty EG-70 Empty EG-70 Empty EG-70 Empty EG-70 Vector Vector Vector Vector Mouse data: n=8/group; data are mean +/- SEM; Mann Whitney t-test; tumor bearing mice;
bladders collected 24h post-administration for Taqman qPCR of bladder tissue lysates NHP data: EG-70 or empty vector control; intravesival administration; tissue collected 48 h post-administration; mean ± SEM, fold change vs empty vector
control; Tbp used as internal control gene; representative of 1 animal per treatment group, with 5 pieces of bladder tissue per animal 36 INT02-348

EG-70 Preclinical Profile: profound efficacy coupled to a remarkably
clean safety profile Toxicology studies did not reveal a maximum tolerated dose (MTD) • Mice - no adverse findings attributable to mEG-70 • Monkeys - very well-tolerated with no systemic findings; • Local findings limited to
transient mild inflammation consistent with intravesical administrations • All changes showed reversibility Biodistribution - payloads are circumscribed to bladder as intended and designed • Bladder: plasmid DNA found at sustained levels
in bladder tissue and urine • Blood: Transient plasmid DNA in the NHP blood 24 to 48 hours after dosing; regressed to zero rapidly • Non-target tissues: no significant levels detected No Adverse Events Limit (NOAEL) was the highest dose
tested – maximum feasible dose • Enabled pharmacologically active dose in mice and in NHPs to be the starting dose for Phase 1 EG-70 is a potentially powerful anti-tumor agent that is well-tolerated preclinically, with no observed MTD
37

Additional lung program detail
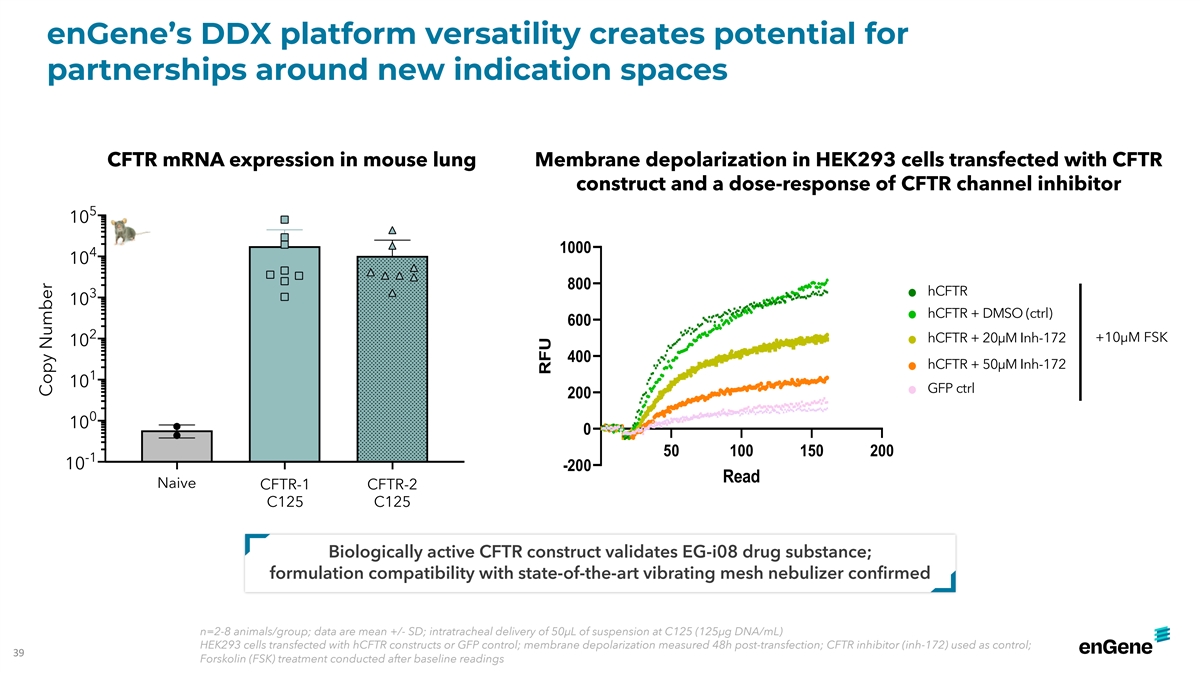
enGene’s DDX platform versatility creates potential for
partnerships around new indication spaces CFTR mRNA expression in mouse lung Membrane depolarization in HEK293 cells transfected with CFTR construct and a dose-response of CFTR channel inhibitor 5 10 1000 4 10 800 hCFTR hCFTR 3 10 hCFTR + DMSO
(ctrl) hCFTR - DMSO ctrl 600 2 hCFTR + 20µM Inh-172 +10µM FSK 10 hCFTR +50uM Inh-172 10uM FSK 400 hCFTR + 50µM Inh-172 hCFTR + 20uM Inh-172 1 10 GFP ctrl 200 GFP ctrl 0 10 0 50 100 150 200 -1 10 -200 Read Naive CFTR-1 CFTR-2 C125 C125
Biologically active CFTR construct validates EG-i08 drug substance; formulation compatibility with state-of-the-art vibrating mesh nebulizer confirmed n=2-8 animals/group; data are mean +/- SD; intratracheal delivery of 50µL of suspension at
C125 (125µg DNA/mL) HEK293 cells transfected with hCFTR constructs or GFP control; membrane depolarization measured 48h post-transfection; CFTR inhibitor (inh-172) used as control; 39 Forskolin (FSK) treatment conducted after baseline readings
Copy Number RFU
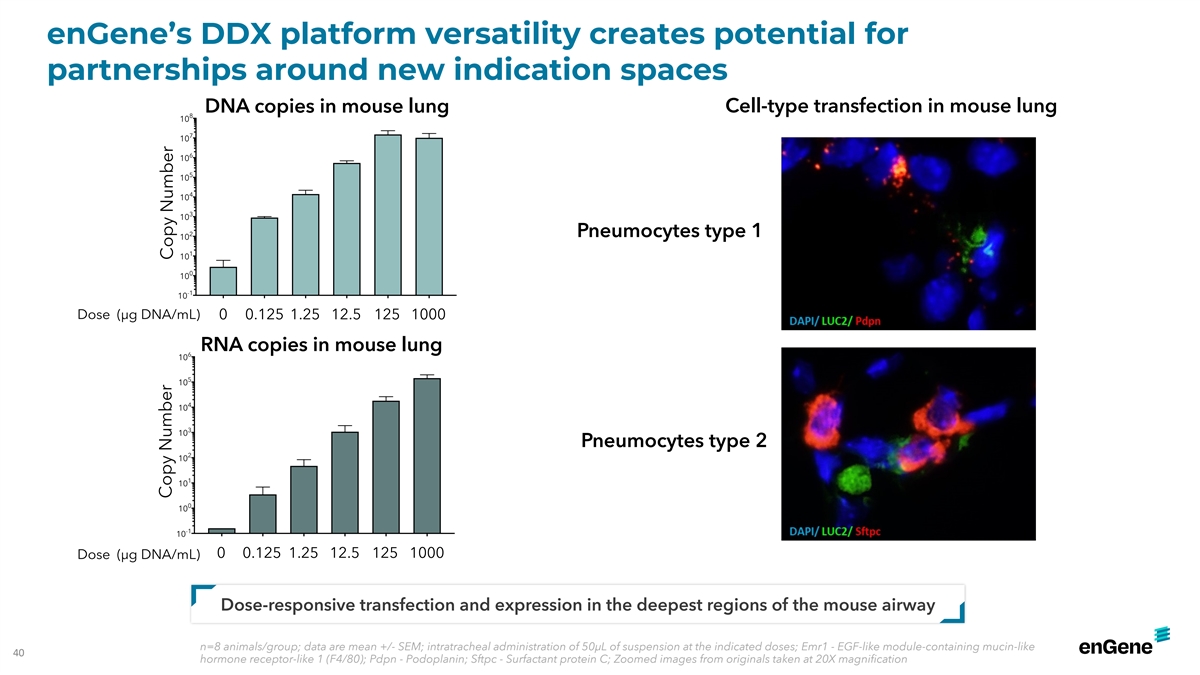
enGene’s DDX platform versatility creates potential for
partnerships around new indication spaces DNA copies in mouse lung Cell-type transfection in mouse lung 8 10 7 10 6 10 5 10 4 10 3 10 2 Pneumocytes type 1 10 1 10 0 10 -1 10 Dose (µg DNA/mL) 0 0.125 1.25 12.5 125 1000 RNA copies in mouse lung 6
10 5 10 4 10 3 10 Pneumocytes type 2 2 10 1 10 0 10 -1 10 0 0.125 1.25 12.5 125 1000 Dose (µg DNA/mL) Dose-responsive transfection and expression in the deepest regions of the mouse airway n=8 animals/group; data are mean +/- SEM; intratracheal
administration of 50µL of suspension at the indicated doses; Emr1 - EGF-like module-containing mucin-like 40 hormone receptor-like 1 (F4/80); Pdpn - Podoplanin; Sftpc - Surfactant protein C; Zoomed images from originals taken at 20X
magnification Copy Number Copy Number











































