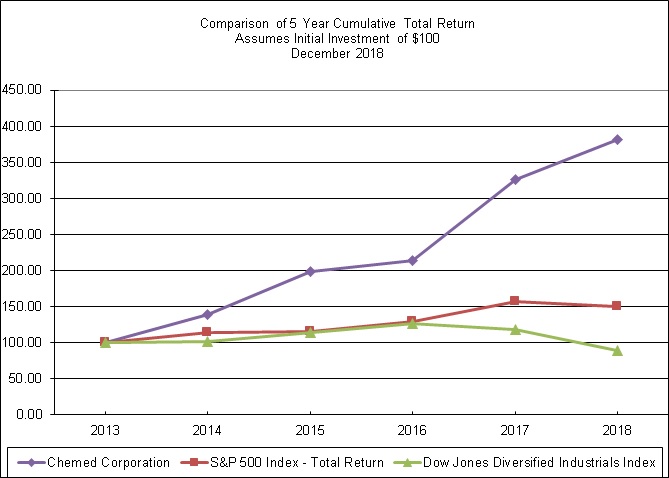
EXHIBIT 13
Financial Review
| Contents |
|
| |
|
|
| |
Report of Independent Registered Public Accounting Firm
|
61
|
| |
Consolidated Statements of Income
|
63
|
| |
Consolidated Balance Sheets
|
64
|
| |
Consolidated Statements of Cash Flows
|
65
|
| |
Consolidated Statements of Changes in Stockholders’ Equity
|
66
|
| |
Notes to Consolidated Financial Statements
|
67
|
| |
Unaudited Summary of Quarterly Results
|
93
|
| |
Selected Financial Data
|
95
|
| |
Unaudited Consolidating Statements of Income
|
96
|
|
Management’s Discussion and Analysis of Financial Conditions and Results
of Operations |
99 |
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, as
that term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that,
in reasonable detail, accurately and fairly reflect the transactions and disposition of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in
accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorization of management and directors of the company; and (iii) provide reasonable assurance
regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements.
Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management, including the President and Chief Executive Officer, Executive Vice President and Chief
Financial Officer and Vice President and Controller, has conducted an evaluation of the effectiveness of its internal control over financial reporting as of December 31, 2018, based on the framework established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on this evaluation, management
concluded that internal control over financial reporting was effective as of December 31, 2018, based on criteria in Internal Control—Integrated
Framework issued by COSO.
PricewaterhouseCoopers LLP, our independent registered public accounting firm, has audited the effectiveness of the
Company’s internal control over financial reporting as of December 31, 2018, as stated in their report which appears on pages 61 and 62.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Chemed Corporation
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Chemed Corporation and its subsidiaries (the “Company”) as of December 31,
2018 and 2017, and the related consolidated statements of income, changes in stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2018, including the related notes (collectively referred to as the
“consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of
the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2018 in conformity with accounting principles generally accepted in the United States of
America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Change in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for revenues from
contracts with customers in 2018.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over
financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Report on Internal Control over Financial Reporting . Our responsibility is to
express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board
(United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain
reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the
consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated
financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal
control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal
control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of
financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that
(i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to
permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the
company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of
any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/PricewaterhouseCoopersLLP
Cincinnati, Ohio
February 27, 2019
We have served as the Company’s auditor since 1971.
|
CONSOLIDATED STATEMENTS OF INCOME
|
|
| |
|
|
|
|
|
|
|
|
|
|
Chemed Corporation and Subsidiary Companies
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
2018
|
|
|
2017
|
|
|
2016
|
|
| |
|
|
|
|
|
|
|
|
|
|
Service revenues and sales (Note 2)
|
|
$
|
1,782,648
|
|
|
$
|
1,666,724
|
|
|
$
|
1,576,881
|
|
|
Cost of services provided and goods sold (excluding depreciation)
|
|
|
1,228,644
|
|
|
|
1,150,532
|
|
|
|
1,115,431
|
|
|
Selling, general and administrative expenses
|
|
|
270,209
|
|
|
|
276,652
|
|
|
|
243,572
|
|
|
Depreciation
|
|
|
38,464
|
|
|
|
35,488
|
|
|
|
34,279
|
|
|
Amortization
|
|
|
399
|
|
|
|
137
|
|
|
|
359
|
|
|
Other operating expenses (Note 20)
|
|
|
1,300
|
|
|
|
90,880
|
|
|
|
4,491
|
|
|
Total costs and expenses
|
|
|
1,539,016
|
|
|
|
1,553,689
|
|
|
|
1,398,132
|
|
|
Income from operations
|
|
|
243,632
|
|
|
|
113,035
|
|
|
|
178,749
|
|
|
Interest expense
|
|
|
(4,990
|
)
|
|
|
(4,272
|
)
|
|
|
(3,715
|
)
|
|
Other income--net (Note 10)
|
|
|
958
|
|
|
|
8,154
|
|
|
|
2,020
|
|
|
Income before income taxes
|
|
|
239,600
|
|
|
|
116,917
|
|
|
|
177,054
|
|
|
Income taxes (Note 11)
|
|
|
(34,056
|
)
|
|
|
(18,740
|
)
|
|
|
(68,311
|
)
|
|
Net Income
|
|
$
|
205,544
|
|
|
$
|
98,177
|
|
|
$
|
108,743
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings Per Share (Note 15)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Income
|
|
$
|
12.80
|
|
|
$
|
6.11
|
|
|
$
|
6.64
|
|
|
Average number of shares outstanding
|
|
|
16,059
|
|
|
|
16,057
|
|
|
|
16,383
|
|
|
Diluted Earnings Per Share (Note 15)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Income
|
|
$
|
12.23
|
|
|
$
|
5.86
|
|
|
$
|
6.48
|
|
|
Average number of shares outstanding
|
|
|
16,803
|
|
|
|
16,742
|
|
|
|
16,789
|
|
The Notes to Consolidated Financial Statements are integral parts of these statements.
|
CONSOLIDATED BALANCE SHEETS
|
|
| |
|
|
|
|
Chemed Corporation and Subsidiary Companies
|
|
|
|
|
|
|
|
(in thousands, except shares and per share data)
|
|
|
|
|
|
|
|
December 31,
|
|
2018
|
|
|
2017
|
|
|
Assets
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
Cash and cash equivalents (Note 9)
|
|
$
|
4,831
|
|
|
$
|
11,121
|
|
|
Accounts receivable less allowances of $15,175 for 2017
|
|
|
119,504
|
|
|
|
113,651
|
|
|
Inventories
|
|
|
5,705
|
|
|
|
5,334
|
|
|
Prepaid income taxes
|
|
|
10,646
|
|
|
|
29,848
|
|
|
Prepaid expenses
|
|
|
19,154
|
|
|
|
16,092
|
|
|
Total current assets
|
|
|
159,840
|
|
|
|
176,046
|
|
|
Investments of deferred compensation plans held in trust (Notes 14 and 16)
|
|
|
65,624
|
|
|
|
62,067
|
|
|
Properties and equipment, at cost, less accumulated depreciation (Note 12)
|
|
|
162,033
|
|
|
|
143,034
|
|
|
Identifiable intangible assets less accumulated amortization of $33,283 (2017 - $32,887) (Note 6)
|
|
|
68,253
|
|
|
|
54,865
|
|
|
Goodwill
|
|
|
510,570
|
|
|
|
476,887
|
|
|
Other assets
|
|
|
9,209
|
|
|
|
7,127
|
|
|
Total Assets
|
|
$
|
975,529
|
|
|
$
|
920,026
|
|
| |
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
50,150
|
|
|
$
|
48,372
|
|
|
Current portion of long-term debt (Note 3)
|
|
|
-
|
|
|
|
10,000
|
|
|
Accrued insurance
|
|
|
46,095
|
|
|
|
46,968
|
|
|
Accrued compensation
|
|
|
63,329
|
|
|
|
62,933
|
|
|
Accrued legal
|
|
|
1,857
|
|
|
|
1,786
|
|
|
Other current liabilities
|
|
|
30,239
|
|
|
|
23,463
|
|
|
Total current liabilities
|
|
|
191,670
|
|
|
|
193,522
|
|
|
Deferred income taxes (Note 11)
|
|
|
21,598
|
|
|
|
16,640
|
|
|
Long-term debt (Note 3)
|
|
|
89,200
|
|
|
|
91,200
|
|
|
Deferred compensation liabilities (Note 14)
|
|
|
64,616
|
|
|
|
61,800
|
|
|
Other liabilities
|
|
|
17,111
|
|
|
|
16,510
|
|
|
Total Liabilities
|
|
|
384,195
|
|
|
|
379,672
|
|
|
Commitments and contingencies (Notes 13 and 17)
|
|
|
|
|
|
|
|
|
|
Stockholders' Equity
|
|
|
|
|
|
|
|
|
|
Capital stock - authorized 80,000,000 shares $1 par; issued 35,311,418 shares
|
|
|
|
|
|
|
|
|
|
(2017 - 34,732,192 shares)
|
|
|
35,311
|
|
|
|
34,732
|
|
|
Paid-in capital
|
|
|
774,358
|
|
|
|
695,797
|
|
|
Retained earnings
|
|
|
1,225,617
|
|
|
|
1,038,955
|
|
|
Treasury stock - 19,438,358 shares (2017 - 18,694,047 shares), at cost
|
|
|
(1,446,296
|
)
|
|
|
(1,231,332
|
)
|
|
Deferred compensation payable in Company stock (Note 14)
|
|
|
2,344
|
|
|
|
2,202
|
|
|
Total Stockholders' Equity
|
|
|
591,334
|
|
|
|
540,354
|
|
|
Total Liabilities and Stockholders' Equity
|
|
$
|
975,529
|
|
|
$
|
920,026
|
|
The Notes to Consolidated Financial Statements are integral parts of these statements.
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
Chemed Corporation and Subsidiary Companies
|
|
|
|
|
|
|
|
|
|
|
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31,
|
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Cash Flows from Operating Activities
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
205,544
|
|
|
$
|
98,177
|
|
|
$
|
108,743
|
|
|
Adjustments to reconcile net income to net cash provided by operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
38,863
|
|
|
|
35,625
|
|
|
|
34,638
|
|
|
Stock option expense
|
|
|
12,611
|
|
|
|
10,485
|
|
|
|
8,330
|
|
|
Noncash portion of long-term incentive compensation
|
|
|
5,405
|
|
|
|
3,774
|
|
|
|
1,301
|
|
|
Provision/(benefit) for deferred income taxes (Note 11)
|
|
|
5,187
|
|
|
|
2,407
|
|
|
|
(6,707
|
)
|
|
Noncash directors' compensation
|
|
|
766
|
|
|
|
766
|
|
|
|
541
|
|
|
Amortization of restricted stock awards
|
|
|
446
|
|
|
|
1,231
|
|
|
|
1,855
|
|
|
Amortization of debt issuance costs
|
|
|
441
|
|
|
|
516
|
|
|
|
519
|
|
|
Provision for uncollectible accounts receivable
|
|
|
-
|
|
|
|
17,306
|
|
|
|
16,319
|
|
|
Loss on sale of transportation equipment (Note 20)
|
|
|
-
|
|
|
|
5,266
|
|
|
|
-
|
|
|
Noncash early retirement expense (Note 20)
|
|
|
-
|
|
|
|
-
|
|
|
|
1,747
|
|
|
Changes in operating assets and liabilities, excluding amounts acquired in business combinations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Decrease/(increase) in accounts receivable
|
|
|
(5,570
|
)
|
|
|
1,072
|
|
|
|
(42,142
|
)
|
|
Decrease/(increase) in inventories
|
|
|
(351
|
)
|
|
|
421
|
|
|
|
559
|
|
|
Increase in prepaid expenses
|
|
|
(2,665
|
)
|
|
|
(2,987
|
)
|
|
|
(253
|
)
|
|
Increase in accounts payable and other current liabilities
|
|
|
8,935
|
|
|
|
12,890
|
|
|
|
891
|
|
|
(Decrease)/increase in income taxes
|
|
|
18,898
|
|
|
|
(26,104
|
)
|
|
|
13,886
|
|
|
Increase in other assets
|
|
|
(5,544
|
)
|
|
|
(8,330
|
)
|
|
|
(5,224
|
)
|
|
Increase in other liabilities
|
|
|
3,451
|
|
|
|
8,561
|
|
|
|
7,105
|
|
|
Excess tax benefit on stock-based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
(7,195
|
)
|
|
Other sources
|
|
|
721
|
|
|
|
1,419
|
|
|
|
480
|
|
|
Net cash provided by operating activities
|
|
|
287,138
|
|
|
|
162,495
|
|
|
|
135,393
|
|
|
Cash Flows from Investing Activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital expenditures
|
|
|
(52,872
|
)
|
|
|
(64,300
|
)
|
|
|
(39,772
|
)
|
|
Business combinations, net of cash acquired (Note 7)
|
|
|
(53,177
|
)
|
|
|
(4,725
|
)
|
|
|
-
|
|
|
Other sources/(uses)
|
|
|
824
|
|
|
|
1,417
|
|
|
|
(90
|
)
|
|
Net cash used by investing activities
|
|
|
(105,225
|
)
|
|
|
(67,608
|
)
|
|
|
(39,862
|
)
|
|
Cash Flows from Financing Activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from revolving line of credit
|
|
|
469,550
|
|
|
|
212,350
|
|
|
|
127,050
|
|
|
Payments on revolving line of credit
|
|
|
(406,550
|
)
|
|
|
(211,150
|
)
|
|
|
(102,050
|
)
|
|
Purchases of treasury stock
|
|
|
(158,884
|
)
|
|
|
(94,640
|
)
|
|
|
(102,313
|
)
|
|
Payments on other long-term debt
|
|
|
(75,000
|
)
|
|
|
(8,750
|
)
|
|
|
(7,500
|
)
|
|
Proceeds from exercise of stock options (Note 4)
|
|
|
32,412
|
|
|
|
27,092
|
|
|
|
8,421
|
|
|
Capital stock surrendered to pay taxes on stock-based compensation
|
|
|
(27,548
|
)
|
|
|
(14,223
|
)
|
|
|
(8,772
|
)
|
|
Dividends paid
|
|
|
(18,662
|
)
|
|
|
(17,371
|
)
|
|
|
(16,439
|
)
|
|
Change in cash overdraft payable
|
|
|
(1,531
|
)
|
|
|
6,700
|
|
|
|
(736
|
)
|
|
Debt issuance costs
|
|
|
(1,052
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Excess tax benefit on stock-based compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
7,195
|
|
|
Other sources/(uses)
|
|
|
(938
|
)
|
|
|
916
|
|
|
|
196
|
|
|
Net cash used by financing activities
|
|
|
(188,203
|
)
|
|
|
(99,076
|
)
|
|
|
(94,948
|
)
|
|
Increase/(decrease) in cash and cash equivalents
|
|
|
(6,290
|
)
|
|
|
(4,189
|
)
|
|
|
583
|
|
|
Cash and cash equivalents at beginning of year
|
|
|
11,121
|
|
|
|
15,310
|
|
|
|
14,727
|
|
|
Cash and cash equivalents at end of year
|
|
$
|
4,831
|
|
|
$
|
11,121
|
|
|
$
|
15,310
|
|
The Notes to Consolidated Financial Statements are integral parts of these statements.
|
CONSOLIDATED STATEMENTS OF CHANGES
|
|
|
IN STOCKHOLDERS' EQUITY
|
|
|
Chemed Corporation and Subsidiary Companies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Compensation
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
Treasury
|
|
|
Payable in
|
|
|
|
|
| |
|
Capital
|
|
|
Paid-in
|
|
|
Retained
|
|
|
Stock-
|
|
|
Company
|
|
|
|
|
| |
|
Stock
|
|
|
Capital
|
|
|
Earnings
|
|
|
at Cost
|
|
|
Stock
|
|
|
Total
|
|
|
Balance at December 31, 2015
|
|
$
|
33,985
|
|
|
$
|
603,006
|
|
|
$
|
865,845
|
|
|
$
|
(991,978
|
)
|
|
$
|
2,395
|
|
|
$
|
513,253
|
|
|
Net income
|
|
|
-
|
|
|
|
-
|
|
|
|
108,743
|
|
|
|
-
|
|
|
|
-
|
|
|
|
108,743
|
|
|
Dividends paid ($1.00 per share)
|
|
|
-
|
|
|
|
-
|
|
|
|
(16,439
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(16,439
|
)
|
|
Stock awards and exercise of stock options (Note 4)
|
|
|
285
|
|
|
|
36,453
|
|
|
|
-
|
|
|
|
(16,127
|
)
|
|
|
-
|
|
|
|
20,611
|
|
|
Purchases of treasury stock (Note 19)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(102,313
|
)
|
|
|
-
|
|
|
|
(102,313
|
)
|
|
Other
|
|
|
-
|
|
|
|
244
|
|
|
|
-
|
|
|
|
(118
|
)
|
|
|
118
|
|
|
|
244
|
|
|
Balance at December 31, 2016
|
|
|
34,270
|
|
|
|
639,703
|
|
|
|
958,149
|
|
|
|
(1,110,536
|
)
|
|
|
2,513
|
|
|
|
524,099
|
|
|
Net income
|
|
|
-
|
|
|
|
-
|
|
|
|
98,177
|
|
|
|
-
|
|
|
|
-
|
|
|
|
98,177
|
|
|
Dividends paid ($1.08 per share)
|
|
|
-
|
|
|
|
-
|
|
|
|
(17,371
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(17,371
|
)
|
|
Stock awards and exercise of stock options (Note 4)
|
|
|
462
|
|
|
|
55,264
|
|
|
|
-
|
|
|
|
(26,467
|
)
|
|
|
-
|
|
|
|
29,259
|
|
|
Purchases of treasury stock (Note 19)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(94,640
|
)
|
|
|
-
|
|
|
|
(94,640
|
)
|
|
Other
|
|
|
-
|
|
|
|
830
|
|
|
|
-
|
|
|
|
311
|
|
|
|
(311
|
)
|
|
|
830
|
|
|
Balance at December 31, 2017
|
|
|
34,732
|
|
|
|
695,797
|
|
|
|
1,038,955
|
|
|
|
(1,231,332
|
)
|
|
|
2,202
|
|
|
|
540,354
|
|
|
Net income
|
|
|
-
|
|
|
|
-
|
|
|
|
205,544
|
|
|
|
-
|
|
|
|
-
|
|
|
|
205,544
|
|
|
Dividends paid ($1.16 per share)
|
|
|
-
|
|
|
|
-
|
|
|
|
(18,662
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(18,662
|
)
|
|
Stock awards and exercise of stock options (Note 4)
|
|
|
579
|
|
|
|
79,452
|
|
|
|
-
|
|
|
|
(55,939
|
)
|
|
|
-
|
|
|
|
24,092
|
|
|
Purchases of treasury stock (Note 19)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(158,884
|
)
|
|
|
-
|
|
|
|
(158,884
|
)
|
|
Other
|
|
|
-
|
|
|
|
(891
|
)
|
|
|
(220
|
)
|
|
|
(141
|
)
|
|
|
142
|
|
|
|
(1,110
|
)
|
|
Balance at December 31, 2018
|
|
$
|
35,311
|
|
|
$
|
774,358
|
|
|
$
|
1,225,617
|
|
|
$
|
(1,446,296
|
)
|
|
$
|
2,344
|
|
|
$
|
591,334
|
|
The Notes to Consolidated Financial Statements are integral parts of these statements.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Summary of Significant Accounting Policies
NATURE OF OPERATIONS
We operate through our two wholly-owned subsidiaries: VITAS Healthcare Corporation (“VITAS”) and
Roto-Rooter Group, Inc. (“Roto-Rooter”). VITAS focuses on hospice care that helps make terminally ill patients' final days as comfortable as possible. Through its team of doctors, nurses, home health aides, social workers, clergy and volunteers,
VITAS provides direct medical services to patients, as well as spiritual and emotional counseling to both patients and their families. Roto-Rooter provides plumbing, drain cleaning and water restoration services to both residential and commercial
customers. Through its network of company-owned branches, independent contractors and franchisees, Roto-Rooter offers plumbing, drain cleaning service and water restoration to approximately 90% of the U.S. population.
PRINCIPLES OF ACCOUNTING
The consolidated financial statements have been prepared on a going-concern basis. Management has
adopted the evaluation requirements of Accounting Stanadards Update “ASU No. 2014-15 – Presentation of Financial Statements – Going Concern”.
The consolidated financial statements include the accounts of Chemed Corporation and its wholly owned
subsidiaries. All intercompany transactions have been eliminated. We have analyzed the provisions of the Financial Accounting Standards Board (“FASB”) authoritative guidance on the consolidation of variable interest entities relative to our
contractual relationships with Roto-Rooter’s independent contractors and franchisees. The guidance requires the primary beneficiary of a Variable Interest Entity (“VIE”) to consolidate the accounts of the VIE. We have concluded that neither the
independent contractors nor the franchisees are VIEs.
Certain reclassifications have been made to prior year financial statements to conform to current
presentation.
CASH EQUIVALENTS
Cash equivalents comprise short-term, highly liquid investments, including money market funds that
have original maturities of three months or less.
CONCENTRATION OF RISK
As of December 31, 2018 and 2017, approximately 68% and 59%, respectively, of VITAS’ total accounts
receivable balance were due from Medicare and 26% and 32%, respectively, of VITAS’ total accounts receivable balance were due from various state Medicaid or managed Medicaid programs. Combined accounts receivable from Medicare, Medicaid, and
managed Medicaid represent approximately 75% of the consolidated net accounts receivable in the accompanying consolidated balance sheets as of December 31, 2018.
As further described in Note 18, we had agreements with a vendor to provide specified pharmacy
services for VITAS and its hospice patients. In 2018 and 2017, respectively, purchases made from this vendor represent 99% and 85%, respectively, of all pharmacy services used by VITAS.
INVENTORIES
Substantially all of the inventories are either general merchandise or finished goods. Inventories
are stated at the lower of cost or net realizable value. For determining the value of inventories, cost methods that reasonably approximate the first-in, first-out (“FIFO”) method are used.
DEPRECIATION AND PROPERTIES AND EQUIPMENT
Depreciation of properties and equipment is computed using the straight-line method over the
estimated useful lives of the assets. Leasehold improvements are amortized over the lesser of the remaining lease terms (excluding option terms) or their useful lives. Expenditures for maintenance, repairs, renewals and betterments that do not
materially prolong the useful lives of the assets are expensed as incurred. The cost of property retired or sold and the related accumulated depreciation are removed from the accounts, and the resulting gain or loss is reflected currently in
other operating expense or other income, net.
Expenditures for major software purchases and software developed for internal use are capitalized and
depreciated using the straight-line method over the estimated useful lives of the assets. For software developed for internal use, external direct costs for materials and services and certain internal payroll and related fringe benefit costs are
capitalized in accordance with the FASB’s authoritative guidance on accounting for the costs of computer software developed or obtained for internal use.
The weighted average lives of our property and equipment at December 31, 2018, were:
|
Buildings and building improvements
|
32.8
|
yrs.
|
|
Transportation equipment
|
10.5
|
|
|
Machinery and equipment
|
5.1
|
|
|
Computer software
|
4.6
|
|
|
Furniture and fixtures
|
4.8
|
|
GOODWILL AND INTANGIBLE ASSETS
The table below shows a rollforward of Goodwill (in thousands):
| |
|
|
|
|
Roto-
|
|
|
|
|
| |
|
Vitas
|
|
|
Rooter
|
|
|
Total
|
|
|
Balance at December 31, 2016
|
|
$
|
328,301
|
|
|
$
|
144,065
|
|
|
$
|
472,366
|
|
|
Business combinations
|
|
|
-
|
|
|
|
4,396
|
|
|
|
4,396
|
|
|
Foreign currency adjustments
|
|
|
-
|
|
|
|
125
|
|
|
|
125
|
|
|
Balance at December 31, 2017
|
|
$
|
328,301
|
|
|
$
|
148,586
|
|
|
$
|
476,887
|
|
|
Business combinations
|
|
|
5,030
|
|
|
|
28,780
|
|
|
|
33,810
|
|
|
Foreign currency adjustments
|
|
|
-
|
|
|
|
(127
|
)
|
|
|
(127
|
)
|
|
Balance at December 31, 2018
|
|
$
|
333,331
|
|
|
$
|
177,239
|
|
|
$
|
510,570
|
|
Identifiable, definite-lived intangible assets arise from purchase business combinations and are
amortized using either an accelerated method or the straight-line method over the estimated useful lives of the assets. The selection of an amortization method is based on which method best reflects the economic pattern of usage of the asset.
Reacquired franchise rights are amortized over the remaining term of the franchise agreement at the time of acquisition. The weighted average lives of our identifiable, definite-lived intangible assets at December 31, 2018, were:
|
Covenants not to compete
|
6.5
|
yrs.
|
|
Reacquired franchise rights
|
7.9
|
|
|
Referral networks
|
10.0
|
|
|
Customer lists
|
12.0
|
|
The date of our annual goodwill and indefinite-lived intangible asset impairment analysis is October
1. The VITAS trade name is considered to have an indefinite life. We also capitalize the direct costs of obtaining licenses to operate either hospice programs or plumbing operations subject to a minimum capitalization threshold. These costs
are amortized over the life of the license using the straight-line method. Certificates of Need (“CON”), which are required in certain states for hospice operations, are generally granted without expiration and thus, we believe them to be
indefinite-lived assets subject to impairment testing.
We consider that Roto-Rooter Corp. (“RRC”), Roto-Rooter Services Co. (“RRSC”) and VITAS are
appropriate reporting units for testing goodwill impairment. We consider RRC and RRSC separate reporting units but one operating segment. This is appropriate as they each have their own set of general ledger accounts that can be analyzed at
“one level below an operating segment” per the definition of a reporting unit in FASB guidance.
We completed our qualitative analysis for impairment of goodwill and our indefinite-lived intangible
assets as of October 1, 2018. Based on our assessment, we do not believe that it is more likely than not that our reporting units or indefinite-lived assets fair values are less than their carrying values.
LONG-LIVED ASSETS
If we believe a triggering event may have occurred that indicates a possible impairment of our
long-lived assets, we perform an estimate and valuation of the future benefits of our long-lived assets (other than goodwill, the VITAS trade name and capitalized CON costs) based on key financial indicators. If the projected undiscounted cash
flows of a major business unit indicate that properties and equipment or identifiable, definite-lived intangible assets have been impaired, a write-down to fair value is made.
OTHER ASSETS
Debt issuance costs are included in other assets. Issuance costs related to revolving credit
agreements are amortized using the straight-line method, over the life of the agreement. All other issuance costs are amortized using the effective interest method over the life of the debt. There are no amounts included in other assets that
individually exceed 5% of total assets.
SALES TAX
The Roto-Rooter segment collects sales tax from customers when required by state and federal laws.
We record the amount of sales tax collected net in the accompanying consolidated statements of income.
OPERATING EXPENSES
Cost of services provided and goods sold (excluding depreciation) includes salaries, wages and
benefits of service providers and field personnel, material costs, medical supplies and equipment, pharmaceuticals, insurance costs, service vehicle costs and other expenses directly related to providing service revenues or generating sales.
Selling, general and administrative expenses include salaries, wages, stock-based compensation expense and benefits of selling, marketing and administrative employees, advertising expenses, communications and branch telephone expenses, office
rent and operating costs, legal, banking and professional fees and other administrative costs. The cost associated with VITAS sales personnel is included in cost of services provided and goods sold (excluding depreciation).
ADVERTISING
We expense the production costs of advertising the first time the advertising takes place. We pay
for and expense the cost of internet advertising and placement on a “per click” basis. Similarly, the majority of our telephone directory listings are paid for and expensed on a “cost per call” basis. For those directories that are not on this
billing basis, the cost of the directory is expensed when the directories are placed in circulation. Advertising expense for the year ended December 31, 2018, was $47.0 million (2017 – $40.9 million; 2016 - $37.2 million).
COMPUTATION OF EARNINGS PER SHARE
In March 2016, the FASB issued Accounting Standards Update “ASU No. 2016-09 - Compensation – Stock
Compensation” which is part of the FASB’s Simplification Initiative. The object of this initiative is to identify, evaluate, and improve specific areas of financial reporting. The areas of simplification in this initiative involve several
aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. The guidance was effective for
fiscal years beginning after December 15, 2016. We adopted the applicable provisions of ASU 2016-09 on a prospective basis. The impact of this ASU on our financial statements for the year ended December 31, 2018 was to decrease our income tax
expense by $22.9 million as the result of excess tax benefits on stock based compensation being recorded on the statements of income. This, combined with the required change in diluted share count, resulted in an increase to basic and diluted
earnings per share of $1.42 and $1.27, respectively. The impact of this ASU on our financial statements for the year ended December 31, 2017 was to decrease our income tax expense by $18.9 million as the result of excess tax benefits on stock
based compensation being recorded on the statements of income. This, combined with the required change in diluted share count, resulted in an increase to basic and diluted earnings per share of $1.18 and $1.08, respectively.
OTHER CURRENT LIABILITIES
There are no amounts included in other current liabilities that individually exceed 5% of total
current liabilities.
OTHER LIABILITIES (NON-CURRENT)
There are no amounts included in other liabilities that individually exceed 5% of total liabilities.
STOCK-BASED COMPENSATION PLANS
Stock-based compensation cost is measured at the grant date, based on the fair value of the award and
recognized as expense over the employee’s requisite service period on a straight-line basis.
INSURANCE ACCRUALS
For our Roto-Rooter segment and Corporate Office, we initially self-insure for all casualty insurance
claims (workers’ compensation, auto liability and general liability). As a result, we closely monitor and frequently evaluate our historical claims experience to estimate the appropriate level of accrual for self-insured claims. Our third-party
administrator (“TPA”) processes and reviews claims on a monthly basis. Currently, our exposure on any single claim is capped by stop-loss coverage at $750,000. In developing our estimates, we accumulate historical claims data for the previous
10 years to calculate loss development factors (“LDF”) by insurance coverage type. LDFs are applied to known claims to estimate the ultimate potential liability for known and unknown claims for each open policy year. LDFs are updated annually.
Because this methodology relies heavily on historical claims data, the key risk is whether the historical claims are an accurate predictor of future claims exposure. The risk also exists that certain claims have been incurred and not reported on
a timely basis. To mitigate these risks, in conjunction with our TPA, we closely monitor claims to ensure timely accumulation of data and compare claims trends with the industry experience of our TPA.
For the VITAS segment, we initially self-insure for workers’ compensation claims. Currently, VITAS’
exposure on any single claim is capped by stop-loss coverage at $1,000,000. For VITAS’ self-insurance accruals for workers’ compensation, the valuation methods used are similar to those used internally for our other business units. We are also
insured for other risks with respect to professional liability with a deductible of $750,000.
Our casualty insurance liabilities are recorded gross before any estimated recovery for amounts
exceeding our stop loss limits. Estimated recoveries from insurance carriers are recorded as accounts receivable. Claims experience adjustments to our casualty and workers’ compensation accrual for the years ended December 31, 2018, 2017 and
2016, were net pretax debits/(credits) of ($3,437,000), ($1,800,000), and $1,147,000 respectively.
TAXES ON INCOME
On December 22, 2017, the President of the United States signed into law H.R.1, “An Act to Provide
for Reconciliation Pursuant to Titles II and V of the Concurrent Resolution on the Budget for Fiscal Year 2018” (previously known as “The Tax Cuts and Jobs Act”) or (the “Act”). The Act amends the Internal Revenue Code to reduce tax rates and
modify policies, credits, and deductions for individuals and businesses. For businesses, U.S. generally accepted accounting principles requires resulting tax effects for the Act, to be recorded in the reporting period of enactment.
The SEC issued SAB 118, which provides guidance on accounting for the Act’s impact. Under SAB 118, an
entity would use something similar to the measurement period in a business combination, not to exceed one year. For matters that have not been completed, the Company would recognize provisional amounts to the extent that they are reasonably
estimable, adjust them over time as more information becomes available, and disclose this information in its financial statements.
Our accounting for all elements of the Tax Act is complete. The Company did not record any material
changes to the provisional amounts previously recorded, net benefit recorded in 2017 of $8.3 million. The Company also determined new rules, such as the Global Intangible Low-Taxed Income (GILTI) and Base Erosion and Anti-Abuse Tax (BEAT), have
no material impact to the financial statements.
Historically, the Company has not provided for deferred taxes on undistributed earnings because such
earnings are considered to be indefinitely reinvested outside of the U.S. The Company continues this assertion that foreign earnings are permanently reinvested under the Act.
The Act provides for 100 percent bonus depreciation on personal tangible property expenditures
starting September 27, 2017 through 2022. The bonus depreciation percentage is phased down from 100 percent beginning in 2023 through 2026. The Company expects to take full benefit of these bonus depreciation rules.
The IRS and other tax authorities are still issuing guidance on the Act, through various regulations
some of which are still proposed and not final. The Company will implement any changes related to finalized regulations and other guidance in the period issued.
Deferred taxes are provided on an asset and liability method whereby deferred tax assets are
recognized for deductible temporary differences and operating loss carryforwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amount of assets and
liabilities and their tax basis. Deferred tax assets are reduced by a valuation allowance when, in our opinion, it is more likely than not that some portion or all of the deferred tax assets will not be realized due to insufficient taxable income
within the carryback or carryforward period available under the tax laws. Deferred tax assets and liabilities are adjusted for the effects of changes in law and rates on the date of enactment.
We are subject to income taxes in Canada, U.S. federal and most state jurisdictions. Judgement is
required to determine our provision for income taxes. Our financial statements reflect expected future tax consequences of such uncertain positions assuming the taxing authorities’ full knowledge of the position and all relevant facts.
CONTINGENCIES
As discussed in Note 17, we are subject to various lawsuits and claims in the normal course of our
business. In addition, we periodically receive communications from governmental and regulatory agencies concerning compliance with Medicare and Medicaid billing requirements at our VITAS subsidiary. We establish reserves for specific, uninsured
liabilities in connection with regulatory and legal action that we deem to be probable and reasonably estimable. We record legal fees associated with legal and
regulatory actions as the costs are incurred. We disclose material loss contingencies that are probable but not reasonably estimable and those that are at least reasonably possible.
ESTIMATES
The preparation of consolidated financial statements in conformity with accounting principles
generally accepted in the United States requires us to make estimates and assumptions that affect amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates. Disclosures of
after-tax expenses and adjustments are based on estimates of the effective income tax rates for the applicable segments.
2. Revenue Recognition
In May 2014, the FASB issued Accounting Standards Update “ASU No. 2014-09 – Revenue from Contracts
with Customers.” The standard and subsequent amendments are theoretically intended to develop a common revenue standard for removing inconsistencies and weaknesses, improve comparability, provide for more useful information to users through
improved disclosure requirements and simplify the preparation of financial statements. The standard is also referred to as Accounting Standards Codification No. 606 (“ASC 606”). We adopted ASC 606 effective January 1, 2018. The required
disclosures of ASC 606 and impact of adoption are discussed below for each of our operating subsidiaries.
VITAS
Service revenue for VITAS is reported at the amount that reflects the ultimate consideration we
expect to receive in exchange for providing patient care. These amounts are due from third-party payors, primarily commercial health insurers and government programs (Medicare and Medicaid), and includes variable consideration for revenue
adjustments due to settlements of audits and reviews, as well as certain hospice-specific revenue capitations. Amounts are generally billed monthly or subsequent to patient discharge. Subsequent changes in the transaction price initially
recognized are not significant.
Hospice services are provided on a daily basis and the type of service provided is determined based
on a physician’s determination of each patient’s specific needs on that given day. Reimbursement rates for hospice services are on a per diem
basis regardless of the type of service provided or the payor. Reimbursement rates from government programs are established by the appropriate governmental agency and are standard across all hospice providers. Reimbursement rates from health
insurers are negotiated with each payor and generally structured to closely mirror the Medicare reimbursement model. The types of hospice services provided and associated reimbursement model for each are as follows:
Routine Home Care
occurs when a patient receives hospice care in their home, including a nursing home setting. The routine home care rate is paid for each day that a patient is in a hospice program and is not receiving one of the other categories of hospice
care. For Medicare patients, the routine home care rate reflects a two-tiered rate, with a higher rate for the first 60 days of a hospice patient’s care and a lower rate for days 61 and after. In addition, there is a Service Intensity Add-on
payment which covers direct home care visits conducted by a registered nurse or social worker in the last seven days of a hospice patient’s life, reimbursed up to four hours per day in fifteen minute increments at the continuous home care rate.
General Inpatient Care
occurs when a patient requires services in a controlled setting for a short period of time for pain control or symptom management which cannot be managed in other settings. General inpatient care services must be provided in a Medicare or
Medicaid certified hospital or long-term care facility or at a freestanding inpatient hospice facility with the required registered nurse staffing.
Continuous Home Care
is provided to patients while at home, including a nursing home setting, during periods of crisis when intensive monitoring and care, primarily nursing care, is required in order to achieve palliation or management of acute medical symptoms.
Continuous home care requires a minimum of 8 hours of care within a 24-hour day, which begins at midnight. The care must be predominantly nursing care provided by either a registered nurse or licensed nurse practitioner. While the published
Medicare continuous home care rates are daily rates, Medicare pays for continuous home care in fifteen minute increments. This fifteen minute rate is calculated by dividing the daily rate by 96.
Respite Care
permits a hospice patient to receive services on an inpatient basis for a short period of time in order to provide relief for the patient’s family or other caregivers from the demands of caring for the patient. A hospice can receive payment for
respite care for a given patient for up to five consecutive days at a time, after which respite care is reimbursed at the routine home care rate.
Each level of care represents a separate promise under the contract of care and is provided
independently for each patient contingent upon the patient’s specific medical needs as determined by a physician. However, the clinical criteria used to determine a patient’s level of care is consistent across all patients, given that, each
patient is subject to the same payor rules and regulations. As a result, we have concluded that each level of care is capable of being distinct and is distinct in the context of the contract. Furthermore, we have determined that each level of
care represents a stand ready service provided as a series of either days or hours of patient care. We believe that the performance obligations for each level of care meet criteria to be satisfied over time. VITAS recognizes revenue based on
the service output. VITAS believes this to be the most faithful depiction of the transfer of control of services as the patient simultaneously receives and consumes the benefits provided by our performance. Revenue is recognized on a daily or
hourly basis for each patient in accordance with the reimbursement model for each type of service. VITAS’ performance obligations relate to contracts with an expected duration of less than one year. Therefore, VITAS has elected to apply the
optional exception provided in ASC 606 and is not required to disclose the aggregate amount of the transaction price allocated to performance obligations that are unsatisfied or partially unsatisfied at the end of the reporting period. The
unsatisfied or partially satisfied performance obligations referred to above relate to bereavement services provided to patients’ families for at least 12 months after discharge.
Care is provided to patients regardless of their ability to pay. Patients who meet our criteria for
charity care are provided care without charge. There is no revenue or associated accounts receivable in the accompanying consolidated financial statements related to charity care. The cost of providing charity care during the years ended
December 31, 2018, 2017 and 2016, was $8.2 million, $7.7 million and $7.0 million, respectively and is included in cost of services provided and goods sold. The cost of charity care is calculated by taking the ratio of charity care days to total
days of care and multiplying by total cost of care.
Generally, patients who are covered by third-party payors are responsible for related deductibles and
coinsurance which vary in amount. VITAS also provides service to patients without a reimbursement source and may offer those patients discounts from standard charges. VITAS estimates the transaction price for patients with deductibles and
coinsurance, along with those uninsured patients, based on historical experience and current conditions. The estimate of any contractual adjustments, discounts or implicit price concessions reduces the amount of revenue initially recognized.
Subsequent changes to the estimate of the transaction price are recorded as adjustments to patient service revenue in the period of change. Subsequent changes that are determined to be the result of an adverse change in the patients’ ability to
pay (i.e. change in credit risk) are recorded as bad debt expense. VITAS has no material adjustments related to subsequent changes in the estimate of the transaction price or subsequent changes as the result of an adverse change in the patient’s
ability to pay for any period reported.
Laws and regulations concerning government programs, including Medicare and Medicaid, are complex and
subject to varying interpretation. Compliance with such laws and regulations may be subject to future government review and interpretation. Additionally, the contracts we have with commercial health insurance payors provide for retroactive
audit and review of claims. Settlement with third party payors for retroactive adjustments due to audits, reviews or investigations are considered variable consideration and are included in the determination of the estimated transaction price
for providing patient care. The variable consideration is estimated based on the terms of the payment agreement, existing correspondence from the payor and our historical settlement activity. These estimates are adjusted in future periods, as
new information becomes available.
We are subject to certain limitations on Medicare payments for services which are considered variable
consideration, as follows:
Inpatient Cap. If
the number of inpatient care days any hospice program provides to Medicare beneficiaries exceeds 20% of the total days of hospice care such program provided to all Medicare patients for an annual period beginning September 28, the days in excess
of the 20% figure may be reimbursed only at the routine homecare rate. None of VITAS’ hospice programs exceeded the payment limits on inpatient services during the years ended December 31, 2018, 2017 and 2016.
Medicare Cap. We
are also subject to a Medicare annual per-beneficiary cap (“Medicare cap”). Compliance with the Medicare cap is measured in one of two ways based on a provider election. The “streamlined” method compares total Medicare payments received under a
Medicare provider number with respect to services provided to all Medicare hospice care beneficiaries in the program or programs covered by that Medicare provider number with the product of the per-beneficiary cap amount and the number of
Medicare beneficiaries electing hospice care for the first time from that hospice program or programs from September 28 through September 27 of the following year. At December 31, 2018, all our programs except one are using the “streamlined”
method.
The “proportional” method compares the total Medicare payments received under a Medicare provider number with respect to
services provided to all Medicare hospice care beneficiaries in the program or programs covered by the Medicare provider number between September 28 and September 27 of the following year with the product of the per beneficiary cap amount and a
pro-rated number of Medicare beneficiaries receiving hospice services from that program during the same period. The pro-rated number of Medicare beneficiaries is calculated based on the ratio of days the beneficiary received hospice services
during the measurement period to the total number of days the beneficiary received hospice services.
We actively monitor each of our hospice programs, by provider number, as to their specific admission, discharge rate and
median length of stay data in an attempt to determine whether revenues are likely to exceed the annual per-beneficiary Medicare cap. Should we determine that revenues for a program are likely to exceed the Medicare cap based on projected trends,
we attempt to institute corrective actions, which include changes to the patient mix and increased patient admissions. However, should we project our corrective action will not prevent that program from exceeding its Medicare cap, we estimate
revenue recognized during the government fiscal year that will require repayment to the Federal government under the Medicare cap and record an adjustment to revenue of an amount equal to a ratable portion of our best estimate for the year.
In 2013, the U.S.
government implemented automatic budget reductions of 2.0% for all government payees, including hospice benefits paid under the Medicare program. In 2015, CMS determined that the Medicare cap should be calculated “as if” sequestration did not
occur. As a result of this decision, VITAS has received notification from our third-party intermediary that an additional $3.6 million is owed for Medicare cap
in three programs arising during the 2013 through 2018 measurement periods. The amounts are automatically deducted from our semi-monthly periodic interim payments (“PIP”). We do not believe that CMS is authorized under the sequestration
authority or the statutory methodology for establishing the Medicare cap to the amounts they have withheld and intend to withhold under their current “as if” methodology. We have appealed CMS’s methodology change.
During the years ended December 31, 2018, we recorded $4.1 million in Medicare cap revenue
reduction related to two programs’ 2018 measurement period liability and 2 programs’ projected 2019 measurement period liability.
During the year ended December 31, 2017, we recorded $ 2.4 million in Medicare cap revenue
reduction related to two programs’ projected 2018 measurement period liability and $247,000 for two programs’ cap liability for prior periods.
During the year ended December 31, 2016, we recorded $228,000 in Medicare cap revenue reduction
related to one programs’ projected 2015 measurement period liability.
For VITAS’ patients in the nursing home setting in which Medicaid pays the nursing home room and
board, VITAS serves as a pass-through between Medicaid and the nursing home. We are responsible for paying the nursing home for that patient’s room and board. Medicaid reimburses us for 95% of the amount we have paid. This results in a 5% net
expense for VITAS related to nursing home room and board. This transaction creates a performance obligation in that VITAS is facilitating room and board being delivered to our patient. As a result, the 5% net expense is recognized as a
contra-revenue account under ASC 606 in the accompanying financial statements.
The composition of patient care service revenue by payor and level of care for the year ended
December 31, 2018 is as follows (in thousands):
| |
|
Medicare
|
|
|
Medicaid
|
|
|
Commercial
|
|
|
Total
|
|
|
Routine home care
|
|
$
|
939,951
|
|
|
$
|
47,609
|
|
|
$
|
22,958
|
|
|
$
|
1,010,518
|
|
|
Continuous care
|
|
|
110,596
|
|
|
|
6,126
|
|
|
|
5,776
|
|
|
|
122,498
|
|
|
Inpatient care
|
|
|
69,354
|
|
|
|
8,156
|
|
|
|
5,167
|
|
|
|
82,677
|
|
| |
|
$
|
1,119,901
|
|
|
$
|
61,891
|
|
|
$
|
33,901
|
|
|
$
|
1,215,693
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All other revenue - self-pay, respite care, etc.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,831
|
|
|
Subtotal
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,223,524
|
|
|
Medicare cap adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4,123
|
)
|
|
Implicit price concessions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(11,785
|
)
|
|
Room and board, net
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(10,054
|
)
|
|
Net revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,197,562
|
|
Roto-Rooter
Roto-Rooter provides plumbing, drain cleaning, water restoration and other related services to
both residential and commercial customers primarily in the United States. Services are provided through a network of company-owned branches, independent contractors and franchisees. Service revenue for Roto-Rooter is reported at the amount that
reflects the ultimate consideration we expect to receive in exchange for providing services.
Roto-Rooter owns and operates branches focusing mainly on large population centers in the United
States. Roto-Rooter’s primary lines of business in company-owned branches consist of plumbing, sewer and drain cleaning, excavation and water restoration. For purposes of ASC 606 analysis, plumbing, sewer and drain cleaning, and excavation have
been combined into one portfolio and are referred to as “short-term core services”. Water restoration is analyzed as a separate portfolio. The following describes the key characteristics of these portfolios:
Short-term Core
Services are plumbing, drain and sewer cleaning and excavation services. These services are provided to both commercial and residential customers. The duration of services provided in this category range from a few hours to a few
days. There are no significant warranty costs or on-going obligations to the customer once a service has been completed. For residential customers, payment is received at the time of job completion before the Roto-Rooter technician leaves the
residence. Commercial customers may be granted credit subject to internally designated authority limits and credit check guidelines. If credit is granted, payment terms are 30 days or less.
Each job in this category is a distinct service with a distinct performance obligation to the customer. Revenue is
recognized at the completion of each job. Variable consideration consists of pre-invoice discounts and post-invoice discounts. Pre-invoice discounts are given in the form of coupons or price concessions. Post-invoice discounts consist of credit
memos generally granted to resolve customer service issues. Variable consideration is estimated based on historical activity and recorded at the time service is completed.
Water Restoration
Services involve the remediation of water and humidity after a flood. These services are provided to both commercial and residential customers. The duration of services provided in this category generally ranges from 3 to 5 days.
There are no significant warranties or on-going obligations to the customer once service has been completed. The majority of these services are paid by the customer’s insurance company. Variable consideration relates primarily to allowances
taken by insurance companies upon payment. Variable consideration is estimated based on historical activity and recorded at the time service is completed.
For both short-term core services and water restoration services, Roto-Rooter satisfies its
performance obligation at a point in time. The services provided generally involve fixing plumbing, drainage or flood-related issues at the customer’s property. At the time service is complete, the customer acknowledges its obligation to pay
for service and its satisfaction with the service performed. This provides evidence that the customer has accepted the service and Roto-Rooter is now entitled to payment. As such, Roto-Rooter recognizes revenue for these services upon
completion of the job and receipt of customer acknowledgement. Roto-Rooter’s performance obligations for short-term core services and water restoration services relate to contracts with an expected duration of less than a year. Therefore,
Roto-Rooter has elected to apply the optional exception provided in ASC 606 and is not required to disclose the aggregate amount of the transaction price allocated to performance obligations that are unsatisfied or partially unsatisfied at the
end of the reporting period. Roto-Rooter does not have significant unsatisfied or partially unsatisfied performance obligations at the time of initial revenue recognition for short-term core or water restoration services.
Roto-Rooter owns the rights to certain territories and contracts with an independent third-party
to operate the territory under Roto-Rooter’s registered trademarks. The contract is for a specified term but cancellable by either party without penalty with 90 days advance notice. Under the terms of these arrangements, Roto-Rooter provides
certain back office support and advertising along with a limited license to use Roto-Rooter’s registered trademarks. The independent contractor is responsible for all day-to-day management of the business including staffing decisions and pricing
of services provided. All performance obligations of Roto-Rooter cease at the termination of the arrangement.
Independent contractors pay Roto-Rooter a standard fee calculated as a percentage of their weekly
labor sales. The primary value for the independent contractors under these arrangements is the right to use Roto-Rooter’s registered trademarks. Roto-Rooter recognizes revenue from independent contractors over-time (weekly) as the independent
contractor’s labor sales are completed. Payment from independent contractors is also received on a weekly basis. The use of Roto-Rooter’s registered trademarks and advertising provides immediate value to the independent contractor as a result
of Roto-Rooter’s nationally recognized brand. Therefore, over-time recognition provides the most faithful depiction of the transfer of services as the customer simultaneously receives and consumes the benefits provided. There is no significant
variable consideration related to these arrangements.
Roto-Rooter has licensed the rights to operate under Roto-Rooter’s registered trademarks in other
territories to franchisees. The contract is for a 10 year term but cancellable by Roto-Rooter for cause with 60 day advance notice without penalty. The franchisee may cancel the contract for any reason with 60 days advance notice without
penalty. Under the terms of the contract, Roto-Rooter provides national advertising and consultation on various aspects of operating a Roto-Rooter business along with the right to use Roto-Rooter’s registered trademarks. The franchisee is
responsible for all day- to-day management of the business including staffing decisions, pricing of services provided and local advertising spend and placement. All performance obligations of Roto-Rooter cease at the termination of the
arrangement.
Franchisees pay Roto-Rooter a standard monthly fee based on the population within the franchise
territory. The standard fee is revised on a yearly basis based on changes in the Consumer Price Index for All Urban Consumers. The primary value for the franchisees under this arrangement is the right to use Roto-Rooter’s registered
trademarks. Roto-Rooter recognizes revenue from franchisees over-time (monthly). Payment from franchisees is also received on a monthly basis. The use of Roto-Rooter’s registered trademarks and advertising provides immediate value to the
franchisees as a result of Roto-Rooter’s nationally recognized brand. Therefore, over-time recognition provides the most faithful depiction of the transfer of services as the customer simultaneously receives and consumes the benefits provided.
There is no significant variable consideration related to these arrangements.
The composition of disaggregated revenue for the year ended December 31, 2018 is as follows (in
thousands):
|
Short-term core service jobs
|
|
$
|
421,790
|
|
|
Water restoration
|
|
|
101,784
|
|
|
Contractor revenue
|
|
|
50,093
|
|
|
Franchise fees
|
|
|
6,382
|
|
|
All other
|
|
|
11,958
|
|
|
Subtotal
|
|
$
|
592,007
|
|
|
Implicit price concessions and credit memos
|
|
|
(6,921
|
)
|
|
Net revenue
|
|
$
|
585,086
|
|
Initial Adoption of ASC 606
The Company utilized the modified retrospective method of adoption for all contracts. Except for
the changes discussed below, the Company has consistently applied the accounting policies to all periods presented in the consolidated financial statements. Sales tax collected from customers at Roto-Rooter is excluded from revenue under ASC 606
and prior revenue standards.
For VITAS, expenses related to payor audits and reviews, as well as variable consideration
estimated for patient deductibles and coinsurance, have been historically estimated as revenue was recognized and classified as bad debt expense, included in the consolidated statements of income as selling, general and administrative expense.
Upon adoption of ASC 606, these expenses are classified as contra-revenue. There is no change in the timing of recognition related to the variable consideration. The amount of these expenses during the year ended December 31, 2018 was $11.8
million.
Also for VITAS, the 5% net expense related to Medicaid room and board has been historically
recorded on a net basis in cost of services provided in the consolidated income statements. Upon adoption of ASC 606, due to the change in the residual value method required by ASC 606, the expense will be classified as a contra-revenue. The
amount of the change in the classification for these expenses during the year ended December 31, 2018 was $10.1 million. There has been no change in the evaluation of Medicaid room and board related to net versus gross presentation.
Related to Roto-Rooter, expenses related to post-invoice variable consideration in our short-term
core portfolio, and adjustments made subsequent to initial estimates related to allowances taken by insurance companies for water restoration, have been classified as a contra-revenue account in the statements of income. These amounts were
previously classified as bad debt expense in SG&A. The amount of the change in classification for these expenses during the year ended December 31, 2018 was $6.9 million. The initial estimate related to allowances taken by insurance
companies for water restoration services has historically been classified as contra-revenue and did not change as a result of the transition.
There was no material impact on the consolidated balance sheets related to the initial adoption.
There is no impact to consolidated net income as a result of the initial adoption. As a result of the change in classification in the statements of income, amounts previously included in the provision for uncollectible accounts in the statements
of cash flow have been included in the decrease/(increase) in accounts receivable line item in 2018. The total impact of the change from prior revenue guidance (ASC 605) to guidance adopted on January 1, 2018 related to classification in the
statements of income is as follows (in thousands):
| |
|
Impact for the year
ended December 31, 2018 |
|
| |
|
ASC 605
|
|
|
Adjustment
|
|
|
ASC 606
|
|
|
Service revenue and sales
|
|
$
|
1,811,408
|
|
|
$
|
(28,760
|
)
|
|
$
|
1,782,648
|
|
|
Cost of services provided and goods sold
|
|
|
1,238,698
|
|
|
|
(10,054
|
)
|
|
|
1,228,644
|
|
|
Selling, general and administrative expenses
|
|
|
288,915
|
|
|
|
(18,706
|
)
|
|
|
270,209
|
|
3. Long-Term Debt and Lines of Credit
On June 20, 2018, we replaced our existing credit agreement with the Fourth Amended and Restated
Credit Agreement (“2018 Credit Agreement”). Terms of the 2018 Credit Agreement consist of a five-year, $450 million revolving credit facility and a $150 million expansion feature, which may consist of term loans or additional revolving
commitments. The 2018 Credit Agreement has a floating interest rate that is generally LIBOR plus a tiered additional rate which varies based on our current leverage ratio. For December 31, 2018 and 2017, respectively, the interest rate is
LIBOR plus 100 basis points.
The debt outstanding at December 31, 2018 and 2017 consists of the following (in thousands):
| |
|
December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
Revolver
|
|
$
|
89,200
|
|
|
$
|
26,200
|
|
|
Term loan
|
|
|
-
|
|
|
|
75,000
|
|
|
Total
|
|
|
89,200
|
|
|
|
101,200
|
|
|
Current portion of term loan
|
|
|
-
|
|
|
|
(10,000
|
)
|
|
Long-term debt
|
|
$
|
89,200
|
|
|
$
|
91,200
|
|
Capitalized interest was not material for any of the periods shown. Summarized below are the total
amounts of interest paid during the years ended December 31 (in thousands):
|
2018
|
|
$
|
4,178
|
|
|
2017
|
|
|
3,626
|
|
|
2016
|
|
|
3,047
|
|
The 2018 Credit Agreement contains the following quarterly financial covenants:
|
Description
|
|
Requirement
|
|
Chemed
|
| |
|
|
|
|
| |
|
|
|
|
|
Leverage Ratio (Consolidated Indebtedness/Consolidated Adj. EBITDA)
|
|
< 3.50 to 1.00
|
|
0.41 to 1.00
|
| |
|
|
|
|
|
Fixed Charge Coverage Ratio (Consolidated Free Cash Flow/Consolidated
|
|
|
|
|
|
Fixed Charges)
|
|
> 1.50 to 1.00
|
|
7.61 to 1.00
|
We are in compliance with all debt covenants as of December 31, 2018. We have issued $36.4 million in
standby letters of credit as of December 31, 2018 for insurance purposes. Issued letters of credit reduce our available credit under the 2018 Credit Agreement. As of December 31, 2018, we have approximately $324.4 million of unused lines of
credit available and eligible to be drawn down under our revolving credit facility.
4. Stock-Based Compensation Plans
We have three stock incentive plans under which a total of 5.1 million shares were able to be issued
to key employees and directors through a grant of stock options, stock awards and/or performance stock units (“PSUs”). The Compensation/Incentive Committee (“CIC”) of the Board of Directors administers these plans.
We grant
stock options, stock awards and PSUs to our officers, other key employees and directors to better align their long-term interests with those of our shareholders. We grant stock options at an exercise price equal to the market price of our stock
on the date of grant. Options vest ratably annually over a three-year period. Those granted in 2018, 2017, 2016 and 2015 have a contractual life of 5 years; those granted prior to 2014 have a contractual life of 10 years. Restricted stock
awards granted in 2015 vest ratably annually over a three year period. Unrestricted stock awards generally are granted to our non-employee directors annually at the time of our annual meeting. PSUs are contingent upon achievement of multi-year
earnings per share (“EPS”) targets or total shareholder return (“TSR”) targets. Upon achievement of targets, PSUs are converted to unrestricted shares of stock.
We recognize the cost of stock options, stock awards and PSUs on a straight-line basis over the service life of the
award, generally the vesting period. We include the cost of all stock-based compensation in selling, general and administrative expense.
In May 2018, the CIC granted 2,295 unrestricted shares of stock to the Company’s outside directors.
PERFORMANCE AWARDS
In February 2016, 2017 and 2018, the CIC granted PSUs contingent upon the achievement of certain TSR
targets as compared to the TSR of a group of peer companies for the three-year measurement period, at which date the awards may vest. We utilize a Monte Carlo simulation approach in a risk-neutral framework with inputs including historical
volatility and the risk-free rate of interest to value these TSR awards. We amortize the total estimated cost over the service period of the award.
In February 2016, 2017 and 2018, the CIC granted PSUs contingent on the achievement of certain EPS
targets over the three-year measurement period. At the end of each reporting period, we estimate the number of shares of stock we believe will ultimately vest and record that expense over the service period of the award.
Comparative data for the PSUs include:
| |
|
2018 Awards
|
|
|
2017 Awards
|
|
|
2016 Awards
|
|
|
TSR Awards
|
|
|
|
|
|
|
|
|
|
|
Shares of stock granted
|
|
|
7,523
|
|
|
|
7,304
|
|
|
|
9,541
|
|
|
Per-share fair value
|
|
$
|
341.20
|
|
|
$
|
226.95
|
|
|
$
|
150.74
|
|
|
Volatility
|
|
|
22.9
|
%
|
|
|
21.8
|
%
|
|
|
26.7
|
%
|
|
Risk-free interest rate
|
|
|
2.34
|
%
|
|
|
1.44
|
%
|
|
|
0.89
|
%
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
EPS Awards
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares of stock granted
|
|
|
7,523
|
|
|
|
7,304
|
|
|
|
9,541
|
|
|
Per-share fair value
|
|
$
|
256.29
|
|
|
$
|
172.60
|
|
|
$
|
126.37
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Assumptions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service period (years)
|
|
|
2.9
|
|
|
|
2.9
|
|
|
|
2.9
|
|
|
Three-year measurement period ends December 31,
|
|
|
2020
|
|
|
|
2019
|
|
|
|
2018
|
|
The following table summarizes total stock option, stock award and PSU activity during 2018:
| |
|
Stock Options
|
|
|
Stock Awards
|
|
|
Performance Units (PSUs)
|
|
| |
|
|
|
|
Weighted Average
|
|
|
Aggregate
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
| |
|
|
|
|
|
|
|
Remaining
|
|
|
Intrinsic
|
|
|
|
|
|
Average
|
|
|
Number of
|
|
|
Average
|
|
| |
|
Number of
|
|
|
Exercise
|
|
|
Contractual
|
|
|
Value
|
|
|
Number of
|
|
|
Grant-Date
|
|
|
Target
|
|
|
Grant-Date
|
|
| |
|
Options
|
|
|
Price
|
|
|
Life (Years)
|
|
|
(thousands)
|
|
|
Awards
|
|
|
Price
|
|
|
Units
|
|
|
Price
|
|
|
Outstanding at January 1, 2018
|
|
|
1,698,458
|
|
|
$
|
141.62
|
|
|
|
|
|
|
|
|
|
9,706
|
|
|
$
|
121.75
|
|
|
|
53,732
|
|
|
$
|
151.09
|
|
|
Granted
|
|
|
246,350
|
|
|
|
306.70
|
|
|
|
|
|
|
|
|
|
2,295
|
|
|
|
333.75
|
|
|
|
32,255
|
|
|
|
209.49
|
|
|
Exercised/Vested
|
|
|
(539,104
|
)
|
|
|
112.79
|
|
|
|
|
|
|
|
|
|
(12,001
|
)
|
|
|
162.29
|
|
|
|
(37,827
|
)
|
|
|
129.48
|
|
|
Canceled/ Forfeited
|
|
|
(10,670
|
)
|
|
|
153.31
|
|
|
|
|
|
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Outstanding at December 31, 2018
|
|
|
1,395,034
|
|
|
$
|
181.82
|
|
|
|
3.6
|
|
|
$
|
144,271
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
48,160
|
|
|
$
|
207.17
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and expected to vest
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at December 31, 2018
|
|
|
1,395,034
|
|
|
$
|
181.82
|
|
|
|
3.6
|
|
|
$
|
144,271
|
|
|
|
-
|
|
|
$
|
-
|
|
|
|
83,250
|
|
|
|
202.10
|
|
|
Exercisable at December 31, 2018
|
|
|
770,385
|
|
|
|
137.48
|
|
|
|
3.3
|
|
|
|
110,291
|
|
|
n.a.
|
|
|
n.a.
|
|
|
n.a.
|
|
|
n.a.
|
|
|
* Amount includes 32,134 share units which vested and were converted to shares of stock and distributed in the first quarter of 2019.
|
|
We estimate the fair value of stock options using the Black-Scholes valuation model. We determine
expected term, volatility, and dividend yield and forfeiture rate based on our historical experience. We believe that historical experience is the best indicator of these factors.
Comparative data for stock options, stock awards and PSUs include (in thousands, except per-share
amounts):
| |
|
Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Total compensation expense of stock-based compensation
|
|
|
|
|
|
|
|
|
|
|
plans charged against income
|
|
$
|
19,229
|
|
|
$
|
16,256
|
|
|
$
|
13,773
|
|
|
Total income tax benefit recognized in income for stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
based compensation expense charged against income
|
|
|
4,788
|
|
|
|
5,690
|
|
|
|
5,062
|
|
|
Total intrinsic value of stock options exercised
|
|
|
102,144
|
|
|
|
50,192
|
|
|
|
17,635
|
|
|
Total intrinsic value of stock awards vested during the period
|
|
|
4,003
|
|
|
|
6,983
|
|
|
|
7,429
|
|
|
Per-share weighted average grant-date fair value of
|
|
|
|
|
|
|
|
|
|
|
|
|
|
stock awards granted
|
|
|
333.75
|
|
|
|
203.52
|
|
|
|
126.53
|
|
The assumptions we used to value stock option grants are as follows:
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Stock price on date of issuance
|
|
$
|
306.70
|
|
|
$
|
231.91
|
|
|
$
|
135.85
|
|
|
Grant date fair value per share
|
|
$
|
67.16
|
|
|
$
|
46.27
|
|
|
$
|
22.74
|
|
|
Number of options granted
|
|
|
246,350
|
|
|
|
330,550
|
|
|
|
505,775
|
|
|
Expected term (years)
|
|
|
4.0
|
|
|
|
4.0
|
|
|
|
4.0
|
|
|
Risk free rate of return
|
|
|
2.99
|
%
|
|
|
1.86
|
%
|
|
|
1.09
|
%
|
|
Volatility
|
|
|
22.42
|
%
|
|
|
22.80
|
%
|
|
|
21.10
|
%
|
|
Dividend yield
|
|
|
0.4
|
%
|
|
|
0.5
|
%
|
|
|
0.8
|
%
|
|
Forfeiture rate
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
Other data for stock options, stock awards and PSUs for 2018 include (dollar amounts in thousands):
| |
|
Stock
|
|
|
Stock
|
|
|
|
|
| |
|
Options
|
|
|
Awards
|
|
|
PSUs
|
|
| |
|
|
|
|
|
|
|
|
|
|
Total unrecognized compensation at the end of the year
|
|
$
|
27,556
|
|
|
$
|
-
|
|
|
$
|
5,925
|
|
|
Weighted average period over which unrecognized compensation to be recognized (years)
|
|
|
2.3
|
|
|
|
-
|
|
|
|
1.8
|
|
|
Actual income tax benefit realized
|
|
$
|
24,075
|
|
|
$
|
944
|
|
|
$
|
2,086
|
|
|
Aggregate intrinsic value vested and expected to vest
|
|
$
|
144,271
|
|
|
$
|
-
|
|
|
$
|
23,363
|
|
EMPLOYEE STOCK PURCHASE PLAN (“ESPP”)
The ESPP allows eligible participants to purchase shares of stock through payroll deductions at current
market value. We pay administrative and broker fees associated with the ESPP. Shares of stock purchased for the ESPP are purchased on the open market and credited directly to participants’ accounts. In accordance with the FASB’s guidance, the
ESPP is non-compensatory.
5. Segments and Nature of the Business
Our segments include the VITAS segment and the Roto-Rooter segment. Relative contributions of each
segment to service revenues and sales were 67% and 33%, respectively, in 2018 and 69% and 31%, respectively, in 2017 and 2016. The vast majority of our service revenues and sales from continuing operations are generated from business within the
United States.
The reportable segments have been defined along service lines, which is consistent with the way the
businesses are managed. In determining reportable segments, the RRSC and RRC operating units of the Roto-Rooter segment have been aggregated on the basis of possessing similar operating and economic characteristics. The characteristics of these
operating segments and the basis for aggregation are reviewed annually.
We report corporate administrative expenses and unallocated investing and financing income and
expense not directly related to either segment as “Corporate”. Corporate administrative expense includes the stewardship, accounting and reporting, legal, tax and other costs of operating a publicly held corporation. Corporate investing and
financing income and expenses include the costs and income associated with corporate debt and investment arrangements.
Segment data are set forth below (in thousands):
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Revenues by Type of Service
|
|
|
|
|
|
|
|
|
|
|
VITAS
|
|
|
|
|
|
|
|
|
|
|
Routine homecare
|
|
$
|
1,010,518
|
|
|
$
|
935,913
|
|
|
$
|
887,940
|
|
|
Continuous care
|
|
|
122,498
|
|
|
|
124,557
|
|
|
|
138,025
|
|
|
General inpatient
|
|
|
82,677
|
|
|
|
90,472
|
|
|
|
97,580
|
|
|
Other
|
|
|
7,831
|
|
|
|
-
|
|
|
|
-
|
|
|
Subtotal revenue
|
|
|
1,223,524
|
|
|
|
1,150,942
|
|
|
|
1,123,545
|
|
|
Room and board, net
|
|
$
|
(10,054
|
)
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Implicit price concessions
|
|
|
(11,785
|
)
|
|
|
-
|
|
|
|
.
|
|
|
Medicare cap adjustment
|
|
|
(4,123
|
)
|
|
|
(2,682
|
)
|
|
|
(228
|
)
|
|
Total segment
|
|
|
1,197,562
|
|
|
|
1,148,260
|
|
|
|
1,123,317
|
|
|
Roto-Rooter
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term core service jobs
|
|
|
421,790
|
|
|
|
373,579
|
|
|
|
345,638
|
|
|
Water restoration
|
|
|
101,784
|
|
|
|
82,272
|
|
|
|
50,229
|
|
|
Contractor revenue
|
|
|
50,093
|
|
|
|
43,770
|
|
|
|
40,097
|
|
|
Franchise fees
|
|
|
6,382
|
|
|
|
6,130
|
|
|
|
5,090
|
|
|
Other
|
|
|
11,958
|
|
|
|
12,713
|
|
|
|
12,510
|
|
|
Implicit price concessions and credit memos
|
|
|
(6,921
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Total segment
|
|
|
585,086
|
|
|
|
518,464
|
|
|
|
453,564
|
|
|
Total service revenues and sales
|
|
$
|
1,782,648
|
|
|
$
|
1,666,724
|
|
|
$
|
1,576,881
|
|
|
After-tax Segment Earnings/(Loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
138,846
|
|
|
$
|
57,645
|
|
|
$
|
84,961
|
|
|
Roto-Rooter
|
|
|
98,711
|
|
|
|
73,299
|
|
|
|
52,893
|
|
|
Total
|
|
|
237,557
|
|
|
|
130,944
|
|
|
|
137,854
|
|
|
Corporate
|
|
|
(32,013
|
)
|
|
|
(32,767
|
)
|
|
|
(29,111
|
)
|
|
Net income
|
|
$
|
205,544
|
|
|
$
|
98,177
|
|
|
$
|
108,743
|
|
|
Interest Income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
13,412
|
|
|
$
|
12,044
|
|
|
$
|
8,294
|
|
|
Roto-Rooter
|
|
|
7,000
|
|
|
|
5,635
|
|
|
|
3,653
|
|
|
Total
|
|
|
20,412
|
|
|
|
17,679
|
|
|
|
11,947
|
|
|
Intercompany eliminations
|
|
|
(19,741
|
)
|
|
|
(17,252
|
)
|
|
|
(11,564
|
)
|
|
Total interest income
|
|
$
|
671
|
|
|
$
|
427
|
|
|
$
|
383
|
|
|
Interest Expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
175
|
|
|
$
|
188
|
|
|
$
|
211
|
|
|
Roto-Rooter
|
|
|
319
|
|
|
|
323
|
|
|
|
332
|
|
|
Total
|
|
|
494
|
|
|
|
511
|
|
|
|
543
|
|
|
Corporate
|
|
|
4,496
|
|
|
|
3,761
|
|
|
|
3,172
|
|
|
Total interest expense
|
|
$
|
4,990
|
|
|
$
|
4,272
|
|
|
$
|
3,715
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Income Tax Provision
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
40,847
|
|
|
$
|
16,436
|
|
|
$
|
51,910
|
|
|
Roto-Rooter
|
|
|
28,850
|
|
|
|
32,782
|
|
|
|
32,719
|
|
|
Total
|
|
|
69,697
|
|
|
|
49,218
|
|
|
|
84,629
|
|
|
Corporate
|
|
|
(35,641
|
)
|
|
|
(30,478
|
)
|
|
|
(16,318
|
)
|
|
Total income tax provision
|
|
$
|
34,056
|
|
|
$
|
18,740
|
|
|
$
|
68,311
|
|
|
Identifiable Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
553,949
|
|
|
$
|
545,304
|
|
|
$
|
542,142
|
|
|
Roto-Rooter
|
|
|
351,030
|
|
|
|
294,663
|
|
|
|
261,641
|
|
|
Total
|
|
|
904,979
|
|
|
|
839,967
|
|
|
|
803,783
|
|
|
Corporate
|
|
|
70,550
|
|
|
|
80,059
|
|
|
|
76,276
|
|
|
Total identifiable assets
|
|
$
|
975,529
|
|
|
$
|
920,026
|
|
|
$
|
880,059
|
|
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Additions to Long-Lived Assets
|
|
|
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
36,969
|
|
|
$
|
23,469
|
|
|
$
|
22,000
|
|
|
Roto-Rooter
|
|
|
68,786
|
|
|
|
45,386
|
|
|
|
17,709
|
|
|
Total
|
|
|
105,755
|
|
|
|
68,855
|
|
|
|
39,709
|
|
|
Corporate
|
|
|
128
|
|
|
|
483
|
|
|
|
63
|
|
|
Total additions to long-lived assets
|
|
$
|
105,883
|
|
|
$
|
69,338
|
|
|
$
|
39,772
|
|
|
Depreciation and Amortization
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
19,700
|
|
|
$
|
18,630
|
|
|
$
|
19,090
|
|
|
Roto-Rooter
|
|
|
19,016
|
|
|
|
16,790
|
|
|
|
15,002
|
|
|
Total
|
|
|
38,716
|
|
|
|
35,420
|
|
|
|
34,092
|
|
|
Corporate
|
|
|
147
|
|
|
|
205
|
|
|
|
546
|
|
|
Total depreciation and amortization
|
|
$
|
38,863
|
|
|
$
|
35,625
|
|
|
$
|
34,638
|
|
6. Intangible Assets
Amortization of definite-lived intangible assets for the years ended December 31, 2018, 2017, 2016,
was $399,000, $137,000 and $359,000 respectively. The following is a schedule by year of projected amortization expense for definite-lived intangible assets (in thousands):
|
2019
|
|
$
|
1,628
|
|
|
2020
|
|
|
1,624
|
|
|
2021
|
|
|
1,620
|
|
|
2022
|
|
|
1,611
|
|
|
2023
|
|
|
1,590
|
|
|
Thereafter
|
|
|
8,880
|
|
The balance in identifiable intangible assets comprises the following (in thousands):
| |
|
Gross
|
|
|
Accumulated
|
|
|
Net Book
|
|
| |
|
Asset
|
|
|
Amortization
|
|
|
Value
|
|
|
December 31, 2018
|
|
|
|
|
|
|
|
|
|
|
Referral networks
|
|
$
|
21,850
|
|
|
$
|
(21,152
|
)
|
|
$
|
698
|
|
|
Covenants not to compete
|
|
|
9,796
|
|
|
|
(9,367
|
)
|
|
|
429
|
|
|
Customer lists
|
|
|
2,025
|
|
|
|
(1,235
|
)
|
|
|
790
|
|
|
Reacquired franchise rights
|
|
|
12,447
|
|
|
|
(1,529
|
)
|
|
|
10,918
|
|
|
Subtotal - definite-lived intangibles
|
|
|
46,118
|
|
|
|
(33,283
|
)
|
|
|
12,835
|
|
|
VITAS trade name
|
|
|
51,300
|
|
|
|
-
|
|
|
|
51,300
|
|
|
Roto-Rooter trade name
|
|
|
150
|
|
|
|
-
|
|
|
|
150
|
|
|
Operating licenses
|
|
|
3,968
|
|
|
|
-
|
|
|
|
3,968
|
|
|
Total
|
|
$
|
101,536
|
|
|
$
|
(33,283
|
)
|
|
$
|
68,253
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Referral networks
|
|
$
|
21,140
|
|
|
$
|
(21,140
|
)
|
|
$
|
-
|
|
|
Covenants not to compete
|
|
|
9,519
|
|
|
|
(9,291
|
)
|
|
|
228
|
|
|
Customer lists
|
|
|
1,217
|
|
|
|
(1,217
|
)
|
|
|
-
|
|
|
Reacquired franchise rights
|
|
|
1,298
|
|
|
|
(1,239
|
)
|
|
|
59
|
|
|
Subtotal - definite-lived intangibles
|
|
|
33,174
|
|
|
|
(32,887
|
)
|
|
|
287
|
|
|
VITAS trade name
|
|
|
51,300
|
|
|
|
-
|
|
|
|
51,300
|
|
|
Roto-Rooter trade name
|
|
|
150
|
|
|
|
-
|
|
|
|
150
|
|
|
Operating licenses
|
|
|
3,128
|
|
|
|
-
|
|
|
|
3,128
|
|
|
Total
|
|
$
|
87,752
|
|
|
$
|
(32,887
|
)
|
|
$
|
54,865
|
|
7. Business Combinations
During 2018, we completed four business combinations of former franchisees within the Roto-Rooter
segment for $42.2 million in cash to increase our market penetration. The Vitas segment completed one business combination in Florida for $11.0 million to increase our market penetration. The purchase price of these acquisition was allocated as
follows (in thousands):
|
Reacquired franchise rights
|
|
$
|
11,161
|
|
|
All other identifiable intangible assets
|
|
|
2,500
|
|
|
Goodwill
|
|
|
33,828
|
|
|
Other assets and liabilities - net
|
|
|
5,688
|
|
| |
|
$
|
53,177
|
|
There was no gain or loss as a result of the settlement of the preexisting relationship with the
former franchisees at Roto-Rooter. Goodwill, substantially all of which is deductible for tax purposes, was determined based on the residual difference between the fair value of consideration transferred and the value assigned to all tangible
and intangible assets. Among factors that contributed to the recognition of goodwill for both Roto-Rooter and VITAS were expected synergies associated with combining operations of the acquiree into our existing operating platforms.
During 2017, we completed two business combinations of former franchisees within the Roto-Rooter
segment for $4.7 million in cash to increase our market penetration. The purchase price of these acquisition was allocated as follows (in thousands):
|
Identifiable intangible assets
|
|
$
|
98
|
|
|
Goodwill
|
|
|
4,396
|
|
|
Other assets and liabilities - net
|
|
|
231
|
|
| |
|
$
|
4,725
|
|
We did not complete any business combinations during 2016.
The unaudited pro forma results of operations, assuming purchase business combinations completed in
2018 were completed on January 1, 2017 are presented below (in thousands, except per share data):
| |
For the Years Ended
|
|
| |
December 31,
|
|
| |
2018
|
|
2017
|
|
|
Service revenues and sales
|
|
$
|
1,811,532
|
|
|
$
|
1,705,747
|
|
|
Net income
|
|
$
|
209,891
|
|
|
$
|
103,920
|
|
|
Earnings per share
|
|
$
|
13.07
|
|
|
$
|
6.47
|
|
|
Diluted earnings per share
|
|
$
|
12.49
|
|
|
$
|
6.21
|
|
The pro-forma revenue and net income amounts associated with the acquisitions are based on unaudited
historical results of the acquiree. No material pro-forma adjustments were made to the acquiree’s historical results.
8. Discontinued Operations
At December 31, 2018 and 2017, the accrual for our estimated liability for potential environmental
cleanup and related costs arising from the 1991 sale of DuBois amounted to $1.7 million. Of the 2018 balance, $826,000 is included in other current liabilities and $901,000 is included in other liabilities (long-term). The estimated amounts
and timing of payments of these liabilities follows (in thousands):
|
2019
|
|
$
|
826
|
|
|
2020
|
|
|
300
|
|
|
Thereafter
|
|
|
601
|
|
| |
|
$
|
1,727
|
|
We are contingently liable for additional DuBois-related environmental cleanup and related costs up
to a maximum of $14.9 million. On the basis of a continuing evaluation of the potential liability, we believe it is not probable this additional liability will be paid. Accordingly, no provision for this contingent liability has been recorded.
The potential liability is not insured, and the recorded liability does not assume the recovery of insurance proceeds. Also, the environmental liability has not been discounted because it is not possible to reliably project the timing of
payments. We believe that any adjustments to our recorded liability will not materially adversely affect our financial position, results of operations or cash flows.
9. Cash Overdrafts and Cash Equivalents
Included in accounts payable are cash overdrafts of $13.8 million and $15.3 million as of December
31, 2018 and 2017, respectively.
From time to time throughout the year, we invest excess cash in money market funds directly with
major commercial banks. We closely monitor the creditworthiness of the institutions with which we invest our overnight funds. The amount invested was less than $100,000 for each balance sheet date presented.
10. Other Income -- Net
Other income -- net from continuing operations comprises the following (in thousands):
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
| |
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
$
|
671
|
|
|
$
|
427
|
|
|
$
|
383
|
|
|
Market value gains related to deferred
|
|
|
|
|
|
|
|
|
|
|
|
|
|
compensation trusts
|
|
|
287
|
|
|
|
8,430
|
|
|
|
2,061
|
|
|
Other--net
|
|
|
-
|
|
|
|
(703
|
)
|
|
|
(424
|
)
|
|
Total other income
|
|
$
|
958
|
|
|
$
|
8,154
|
|
|
$
|
2,020
|
|
The market value gain relates to gains on the assets in the deferred compensation trust. There is an
offsetting expense in selling, general and administrative expense to reflect the corresponding increase in the liability.
11. Income Taxes
The provision for income taxes comprises the following (in thousands):
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Current
|
|
|
|
|
|
|
|
|
|
|
U.S. federal
|
|
$
|
23,934
|
|
|
$
|
11,724
|
|
|
$
|
64,698
|
|
|
U.S. state and local
|
|
|
4,484
|
|
|
|
4,144
|
|
|
|
9,927
|
|
|
Foreign
|
|
|
452
|
|
|
|
465
|
|
|
|
393
|
|
|
Deferred
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. federal, state and local
|
|
|
5,185
|
|
|
|
2,402
|
|
|
|
(6,712
|
)
|
|
Foreign
|
|
|
1
|
|
|
|
5
|
|
|
|
5
|
|
|
Total
|
|
$
|
34,056
|
|
|
$
|
18,740
|
|
|
$
|
68,311
|
|
A summary of the temporary differences that give rise to deferred tax assets/ (liabilities)
follows (in thousands):
| |
|
December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
Accrued liabilities
|
|
$
|
30,702
|
|
|
$
|
30,419
|
|
|
Stock compensation expense
|
|
|
5,894
|
|
|
|
6,282
|
|
|
State net operating loss carryforwards
|
|
|
2,422
|
|
|
|
2,243
|
|
|
Implicit price concessions
|
|
|
1,171
|
|
|
|
291
|
|
|
Other
|
|
|
626
|
|
|
|
565
|
|
|
Deferred income tax assets
|
|
|
40,815
|
|
|
|
39,800
|
|
|
Amortization of intangible assets
|
|
|
(38,346
|
)
|
|
|
(36,882
|
)
|
|
Accelerated tax depreciation
|
|
|
(19,685
|
)
|
|
|
(14,057
|
)
|
|
Market valuation of investments
|
|
|
(1,068
|
)
|
|
|
(2,277
|
)
|
|
State income taxes
|
|
|
(1,261
|
)
|
|
|
(1,722
|
)
|
|
Currents assets
|
|
|
(1,861
|
)
|
|
|
(1,255
|
)
|
|
Other
|
|
|
(192
|
)
|
|
|
(247
|
)
|
|
Deferred income tax liabilities
|
|
|
(62,413
|
)
|
|
|
(56,440
|
)
|
|
Net deferred income tax liabilities
|
|
$
|
(21,598
|
)
|
|
$
|
(16,640
|
)
|
At December 31, 2018 and 2017, state net operating loss carryforwards were $39.3 million and $36.5
million, respectively. These net operating losses will expire, in varying amounts, between 2024 and 2038. Based on our history of operating earnings, we have determined that our operating income will, more likely than not, be sufficient to
ensure realization of our deferred income tax assets.
A reconciliation of the beginning and ending of year amount of our unrecognized tax benefit is as
follows (in thousands):
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Balance at January 1,
|
|
$
|
1,123
|
|
|
$
|
1,069
|
|
|
$
|
1,052
|
|
|
Unrecognized tax benefits due to positions taken in current year
|
|
|
453
|
|
|
|
268
|
|
|
|
218
|
|
|
Decrease due to expiration of statute of limitations
|
|
|
(228
|
)
|
|
|
(214
|
)
|
|
|
(201
|
)
|
|
Balance at December 31,
|
|
$
|
1,348
|
|
|
$
|
1,123
|
|
|
$
|
1,069
|
|
We file tax returns in the U.S. federal jurisdiction and various states. The years ended December
31, 2015 and forward remain open for review for federal income tax purposes. The earliest open year relating to any of our major state jurisdictions is the fiscal year ended December 31, 2013. During the next twelve months, we do not anticipate
a material net change in unrecognized tax benefits.
We classify interest related to our accrual for uncertain tax positions in separate interest
accounts. As of December 31, 2018 and 2017, we have approximately $136,000 and $134,000, respectively, accrued in interest payable related to uncertain tax positions. These accruals are included in other current liabilities in the accompanying
consolidated balance sheet. Net interest expense related to uncertain tax positions included in interest expense in the accompanying consolidated statement of income is not material.
The difference between the actual income tax provision for continuing operations and the income tax
provision calculated at the statutory U.S. federal tax rate is explained as follows (in thousands):
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
| |
|
|
|
|
|
|
|
|
|
|
Income tax provision calculated using the statutory rate of 21%
|
|
$
|
50,316
|
|
|
$
|
40,921
|
|
|
$
|
61,969
|
|
|
Stock compensation tax benefits
|
|
|
(22,862
|
)
|
|
|
(18,932
|
)
|
|
|
-
|
|
|
State and local income taxes, less federal income tax effect
|
|
|
7,150
|
|
|
|
4,600
|
|
|
|
6,044
|
|
|
Nondeductible expenses
|
|
|
2,280
|
|
|
|
1,041
|
|
|
|
881
|
|
|
Enactment of the tax reform act
|
|
|
-
|
|
|
|
(8,305
|
)
|
|
|
-
|
|
|
Other--net
|
|
|
(2,828
|
)
|
|
|
(585
|
)
|
|
|
(583
|
)
|
|
Income tax provision
|
|
$
|
34,056
|
|
|
$
|
18,740
|
|
|
$
|
68,311
|
|
|
Effective tax rate
|
|
|
14.2
|
%
|
|
|
16.0
|
%
|
|
|
38.6
|
%
|
Summarized below are the total amounts of income taxes paid during the years ended December 31 (in
thousands):
|
2018
|
|
$
|
9,749
|
|
|
2017
|
|
|
42,311
|
|
|
2016
|
|
|
60,905
|
|
Provision has not been made for additional taxes on $35.1 million of undistributed earnings of our
domestic subsidiaries. Should we elect to sell our interest in these businesses rather than to affect a tax-free liquidation, additional taxes amounting to approximately $8.4 million would be incurred based on current income tax rates.
12. Properties and Equipment
A summary of properties and equipment follows (in thousands):
| |
|
December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
Land
|
|
$
|
7,964
|
|
|
$
|
7,108
|
|
|
Buildings and building improvements
|
|
|
96,361
|
|
|
|
85,570
|
|
|
Transportation equipment
|
|
|
51,559
|
|
|
|
47,243
|
|
|
Machinery and equipment
|
|
|
111,183
|
|
|
|
99,234
|
|
|
Computer software
|
|
|
49,928
|
|
|
|
47,840
|
|
|
Furniture and fixtures
|
|
|
72,898
|
|
|
|
74,191
|
|
|
Projects under development
|
|
|
20,510
|
|
|
|
11,882
|
|
|
Total properties and equipment
|
|
|
410,403
|
|
|
|
373,068
|
|
|
Less accumulated depreciation
|
|
|
(248,370
|
)
|
|
|
(230,034
|
)
|
|
Net properties and equipment
|
|
$
|
162,033
|
|
|
$
|
143,034
|
|
The net book value of computer software at December 31, 2018 and 2017, was $6.6 million and $7.3
million, respectively. Depreciation expense for computer software was $5.4 million, $4.4 million and $4.0 million for the years ended December 31, 2018, 2017 and 2016, respectively.
13. Lease Arrangements
We have operating leases that cover our corporate office headquarters, various warehouse and office
facilities, office equipment and transportation equipment. The remaining terms of these leases range from monthly to nine years, and in most cases we expect that these leases will be renewed or replaced by other leases in the normal course of
business. We have no significant capital leases as of December 31, 2018 or 2017.
The following is a summary of future minimum rental payments and sublease rentals to be received
under operating leases that have initial or remaining noncancelable terms in excess of one year at December 31, 2018 (in thousands):
|
2019
|
|
$
|
26,791
|
|
|
2020
|
|
|
24,152
|
|
|
2021
|
|
|
19,669
|
|
|
2022
|
|
|
13,851
|
|
|
2023
|
|
|
8,179
|
|
|
Thereafter
|
|
|
10,974
|
|
|
Total minimum rental payments
|
|
$
|
103,616
|
|
Total rental expense incurred under operating leases for continuing operations follows (in
thousands):
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
| |
|
|
|
|
|
|
|
|
|
|
Total rental expense
|
|
$
|
41,685
|
|
|
$
|
41,210
|
|
|
$
|
40,034
|
|
14. Retirement Plans
Retirement obligations under various plans cover substantially all full-time employees who meet age
and/or service eligibility requirements. All plans providing retirement benefits to our employees are defined contribution plans. Expenses for our retirement and profit-sharing plans, excess benefit plans and other similar plans are as follows
(in thousands):
| For the
Years Ended December 31, |
|
|
2018
|
|
|
2017
|
|
|
2016
|
|
| |
|
|
|
|
|
|
|
|
$ 16,502
|
|
|
$
|
22,025
|
|
|
$
|
14,467
|
|
These expenses include the impact of market gains and losses on assets held in deferred compensation plans.
We have excess benefit plans for key employees whose participation in the qualified plans is limited
by U.S. Employee Retirement Income Security Act requirements. Benefits are determined based on theoretical participation in the qualified plans. Benefits are only invested in mutual funds, and participants are not permitted to diversify
accumulated benefits in shares of our capital stock. Trust assets invested in shares of our stock are included in treasury stock, and the corresponding liability is included in a separate component of stockholders’ equity. At December 31, 2018,
these trusts held 80,584 shares at historical average cost or $2.3 million of our stock (2017 – 83,125 shares or $2.2 million).
15. Earnings Per Share
The computation of earnings per share follows (in thousands, except per share data):
| |
|
Net Income
|
|
|
For the Years Ended December 31,
|
|
Net Income
|
|
|
Shares
|
|
|
Earnings per Share
|
|
|
2018
|
|
|
|
|
|
|
|
|
|
|
Earnings
|
|
$
|
205,544
|
|
|
|
16,059
|
|
|
$
|
12.80
|
|
|
Dilutive stock options
|
|
|
-
|
|
|
|
650
|
|
|
|
|
|
|
Nonvested stock awards
|
|
|
-
|
|
|
|
94
|
|
|
|
|
|
|
Diluted earnings
|
|
$
|
205,544
|
|
|
|
16,803
|
|
|
$
|
12.23
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
2017
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings
|
|
$
|
98,177
|
|
|
|
16,057
|
|
|
$
|
6.11
|
|
|
Dilutive stock options
|
|
|
-
|
|
|
|
596
|
|
|
|
|
|
|
Nonvested stock awards
|
|
|
-
|
|
|
|
89
|
|
|
|
|
|
|
Diluted earnings
|
|
$
|
98,177
|
|
|
|
16,742
|
|
|
$
|
5.86
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
2016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings
|
|
$
|
108,743
|
|
|
|
16,383
|
|
|
$
|
6.64
|
|
|
Dilutive stock options
|
|
|
-
|
|
|
|
296
|
|
|
|
|
|
|
Nonvested stock awards
|
|
|
-
|
|
|
|
110
|
|
|
|
|
|
|
Diluted earnings
|
|
$
|
108,743
|
|
|
|
16,789
|
|
|
$
|
6.48
|
|
During 2018, 246,000 stock options were excluded from the computation of diluted earnings per share
as their exercise prices were greater than the average market price during most of the year. During 2017, 328,000 stock options were also excluded. During 2016, 923,000 stock options were also excluded.
16. Financial Instruments
FASB’s authoritative guidance on fair value measurements defines a hierarchy which prioritizes the
inputs in fair value measurements. Level 1 measurements are measurements using quoted prices in active markets for identical assets or liabilities. Level 2 measurements use significant other observable inputs. Level 3 measurements are
measurements using significant unobservable inputs which require a company to develop its own assumptions. In recording the fair value of assets and liabilities, companies must use the most reliable measurement available.
The following shows the carrying value, fair value and the hierarchy for our financial instruments as
of December 31, 2018 (in thousands):
| |
|
|
|
|
Fair Value Measure
|
|
| |
|
Carrying Value
|
|
|
Quoted Prices in Active Markets for Identical Assets (Level 1)
|
|
|
Significant Other Observable Inputs
(Level 2)
|
|
|
Significant
Unobservable
Inputs (Level 3)
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Investments of deferred compensation plans held in trust
|
|
$
|
65,624
|
|
|
$
|
65,624
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Long-term debt and current portion of long-term debt
|
|
|
89,200
|
|
|
|
-
|
|
|
|
89,200
|
|
|
|
-
|
|
The following shows the carrying value, fair value and the hierarchy for our financial instruments as
of December 31, 2017 (in thousands):
| |
|
|
|
|
Fair Value Measure
|
|
| |
|
Carrying Value
|
|
|
Quoted Prices in Active Markets for Identical Assets (Level 1)
|
|
|
Significant Other Observable Inputs
(Level 2)
|
|
|
Significant
Unobservable
Inputs (Level 3)
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Investments of deferred compensation plans held in trust
|
|
$
|
62,067
|
|
|
$
|
62,067
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Long-term debt and current portion of long-term debt
|
|
|
101,200
|
|
|
|
-
|
|
|
|
101,200
|
|
|
|
-
|
|
For cash and cash equivalents, accounts receivable and accounts payable, the carrying amount is a
reasonable estimate of fair value because of the liquidity and short-term nature of these instruments. As further described in Footnote 3, our outstanding long-term debt and current portion of long-term debt have floating interest rates that are
reset at short-term intervals, generally 30 or 60 days. The interest rate we pay also includes an additional amount based on our current leverage ratio. As such, we believe our borrowings reflect significant nonperformance risks, mainly credit
risk. Based on these factors, we believe the fair value of our long-term debt and current portion of long-term debt approximate the carrying value.
17. Legal and Regulatory Matters
The VITAS segment of the Company’s business operates in a heavily-regulated industry. As a result,
the Company is subjected to inquiries and investigations by various government agencies, as well as to lawsuits, including qui tam actions.
The following sections describe the various ongoing material lawsuits and investigations of which the Company is currently aware. It is not possible at this time for us to estimate either the timing or outcome of any of those matters, or whether
any potential loss, or range of potential losses, is probable or reasonably estimable.
Regulatory Matters and Litigation
The Company and certain current and former directors and officers are defendants in a case captioned
In re Chemed Corp. Shareholder Derivative Litigation, No. 13 Civ. 1854 (LPS) (CJB) (D. Del.), which was consolidated on February 2, 2015.
On February 2, 2015, the Court appointed KBC Asset Management NV the sole lead plaintiff and its
counsel, the sole lead and liaison counsel. On March 3, 2015, Lead Plaintiff KBC designated its Complaint as the operative complaint in the consolidated proceedings and defendants renewed a previously filed motion to dismiss those claims and
allegations. The consolidated Complaint named fourteen individual defendants, together with the Company as nominal defendant. The Complaint alleges a claim for breach of fiduciary duty against the individual defendants for allegedly permitting
the Company to submit false claims to the U.S. government. The Complaint seeks (a) a declaration that the individual defendants breached their fiduciary duties to the Company; (b) an order requiring those defendants to pay compensatory damages,
restitution and exemplary damages, in unspecified amounts, to the Company; (c) an order directing the Company to implement new policies and procedures; and (d) costs and disbursements incurred in bringing the action, including attorneys’ fees.
On May 12, 2016, the Court issued a Memorandum Order granting Chemed’s motion to dismiss, and dismissing Lead Plaintiff KBC’s Complaint without prejudice to KBC’s opportunity to file within 30 days of the date of the Court’s Order (i.e., by June
13, 2016) an amended Complaint addressing the deficiencies in its duty of loyalty claim. Lead Plaintiff KBC did not file an amended Complaint within the time specified by the Court.
However, on June 13, 2016, counsel for Chemed shareholder Michael Kvint filed a letter with the Court
requesting a two-week extension to file a motion to substitute Mr. Kvint as lead plaintiff, in place of Lead Plaintiff KBC and to file an amended Complaint. Alternatively, counsel for Mr. Kvint requested that any dismissal of the action be with
prejudice to KBC only. On June 14, 2016, Chemed filed a reply letter with the Court, reserving its rights to oppose any motion filed by Mr. Kvint and, if warranted, to oppose any other actions taken by Mr. Kvint to proceed with the action
(including by filing an untimely amended Complaint). On June 21, 2016, the Court entered an Oral Order providing Mr. Kvint until June 30, 2016 to file a Motion to Substitute and Motion for Leave to File an Amended Complaint. On that date, Mr.
Kvint filed, under seal, a Motion to Substitute Plaintiff and File Amended Complaint, and attached a Proposed Amended Complaint. Mr. Kvint’s motion was fully briefed by the parties. On April 25, 2017, Magistrate Judge Burke issued a Report and
Recommendation recommending that the Court permit Mr. Kvint to intervene as Lead Plaintiff and grant leave to amend the complaint to replead the duty of loyalty claim only. On May 16, 2017, Chief Judge Stark signed an Order adopting that Report
and Recommendation. Plaintiff Kvint filed a Corrected Amended Complaint on May 30, 2017. On September 13, 2017, the Court entered an order dismissing with prejudice the claims against defendants Timothy S. O’Toole and Joel F. Gemunder and
permitting Defendants to file a Motion to Dismiss the Corrected Amended Complaint. The matter has been fully briefed and argued. On February 26, 2019, Magistrate
Judge Burke issued a Report and Recommendations recommending that Defendants' motion to dismiss the amended complaint be granted. Any objections to that Report are due March 12, 2019, and any responses thereto are due March 26, 2019. The Court will then decide whether to accept the Report and Recommendations. As the
Company has previously disclosed, the legal fees and costs associated with defending against this lawsuit are presently being paid by insurance. For additional procedural history of this litigation, please refer to our prior quarterly and annual
filings.
On October 30, 2017, the Company entered into a settlement agreement (the “Settlement Agreement”) to
resolve civil litigation under the False Claims Act brought by the United States Department of Justice (“DOJ”) on behalf of the OIG and various relators concerning VITAS, filed in the U.S. District Court of the Western District of Missouri (the
“2013 Action”). The Company denied any violation of law and agreed to settlement without admission of wrongdoing.
In connection with the settlement VITAS and certain of its subsidiaries entered into a corporate
integrity agreement (“CIA”) on October 30, 2017. The CIA formalizes various aspects of VITAS’ already existing Compliance Program and contains requirements designed to document compliance with federal healthcare program requirements. It has a
term of five years during which it imposes monitoring, reporting, certification, oversight, screening and training obligations, certain of which had previously been implemented by VITAS. It also requires VITAS to engage an Independent Review
Organization to perform audit and review functions and to prepare reports regarding compliance with federal healthcare programs. In the event of breach of the CIA, VITAS could become liable for payment of stipulated penalties or could be
excluded from participation in federal healthcare programs.
Under the Settlement Agreement, the United States agreed to release the Company, VITAS, and its
hospice operation subsidiaries from any civil or administrative monetary liability relating to any patients’ disputed terminal medical prognosis of six months or less; a lack of medical necessity for billed Continuous Home Care, General Inpatient
Care, or Respite Care levels of hospice care; or that the claims for those levels of hospice care were not eligible for payment for any other reason. The OIG agreed, conditioned on the Company’s full payment and in consideration of VITAS’
obligations under the CIA, to release its permissive exclusion rights and refrain from instituting any administrative action seeking to exclude the Company, VITAS, and its affiliates from participating in Medicare, Medicaid, or other federal
healthcare programs in this regard.
Under the Settlement Agreement, the Company paid $75 million plus interest, plus certain attorney
fees and expenses of qui tam relators. The Company made these payments during the fourth quarter of 2017. The Company previously recorded a $90 million loss reserve ($55.8 million after-tax) related to the Settlement Agreement, and associated
costs in the second quarter of 2017. During the fourth quarter of 2017, approximately $5.5 million ($3.4 million after-tax) recorded as part of the $90 million was reversed as relator attorney' fees were less than originally estimated
The costs incurred related to U.S. v. VITAS and related regulatory matters, exclusive of the
settlement were $5.2 million and $5.3 million for 2017, and 2016 respectively.
Jordan Seper (“Seper”), a Registered Nurse at VITAS’ Inland Empire program from May 12, 2014 to March
21, 2015, filed a lawsuit in San Francisco Superior Court on September 26, 2016. She alleged VITAS Healthcare Corp of CA (“VITAS CA”) (1) failed to provide minimum wage for all hours worked; (2) failed to provide overtime for all hours worked;
(3) failed to provide a second meal period; (4) failed to provide rest breaks; (5) failed to indemnify for necessary expenditures; (6) failed to timely pay wages due at time of separation; and (7) engaged in unfair business practices. Seper
seeks a state-wide class action of current and former non-exempt employees employed with VITAS in California within the four years preceding the filing of the lawsuit. She seeks court determination that this action may be maintained as a class
action for the entire California class and subclasses, designation as class representative, declaratory relief, injunctive relief, damages (including wages for regular or overtime hours allegedly worked but not paid, premium payments for missed
meal or rest periods, and unreimbursed expenses), all applicable penalties associated with each claim, pre and post-judgment interest, and attorneys’ fees and costs. Seper served VITAS CA with the lawsuit, Jordan A. Seper on behalf of herself and others similarly situated v. VITAS Healthcare Corporation of California, a Delaware corporation; VITAS Healthcare Corp of CA, a business
entity unknown; and DOES 1 to 100, inclusive; Los Angeles Superior Court Case Number BC 642857 on October 13, 2016 (“Jordan Seper case”).
On November 14, 2016, the Parties filed a Stipulation to transfer the venue of the lawsuit from San
Francisco to Los Angeles. The Los Angeles Superior Court Complex Division accepted transfer of the case on December 6, 2016 and stayed the case. On December 16, 2016, VITAS CA filed its Answer and served written discovery on Seper.
Jiwann Chhina (“Chhina”), hired by VITAS as a Home Health Aide on February 5, 2002, is currently a
Licensed Vocational Nurse for VITAS’ San Diego program. On September 27, 2016, Chhina filed a lawsuit in San Diego Superior Court, alleging (1) failure to pay minimum wage for all hours worked; (2) failure to provide overtime for all hours
worked; (3) failure to pay wages for all hours at the regular rate; (4) failure to provide meal periods; (5) failure to provide rest breaks; (6) failure to provide complete and accurate wage statements; (7) failure to pay for all reimbursement
expenses; (8) unfair business practices; and (9) violation of the California Private Attorneys General Act. Chhina seeks to pursue these claims in the form of a state-wide class action of current and former non-exempt employees employed with
VITAS in California within the four years preceding the filing of the lawsuit. He seeks court determination that this action may be maintained as a class action for the entire California class and subclasses, designation as class representative,
declaratory relief, injunctive relief, damages (including wages for regular or overtime hours allegedly worked but not paid, premium payments for missed meal or rest period, and unreimbursed expenses), all applicable penalties associated with
each claim, pre-judgment interest, and attorneys’ fees and costs. Chhina served VITAS CA with the lawsuit, Jiwan Chhina v. VITAS Health Services of
California, Inc., a California corporation; VITAS Healthcare Corporation of California, a Delaware corporation; VITAS Healthcare Corporation of California, a Delaware corporation dba VITAS Healthcare Inc.; and DOES 1 to 100, inclusive;
San Diego Superior Court Case Number 37-2015-00033978-CU-OE-CTL on November 3, 2016 (“Jiwann Chhina case”). On December 1, 2016, VITAS CA filed its Answer and served written discovery on Chhina.
On May 19, 2017, Chere Phillips (a Home Health Aide in Sacramento) and Lady Moore (a former Social
Worker in Sacramento) filed a lawsuit against VITAS CA in Sacramento County Superior Court, alleging claims for (1) failure to pay all wages due; (2) failure to authorize and permit rest periods; (3) failure to provide off-duty meal periods; (4)
failure to furnish accurate wage statements; (5) unreimbursed business expenses; (6) waiting time penalties; (7) violations of unfair competition law; and (8) violation of the Private Attorneys General Act. The case is captioned: Chere Phillips and Lady Moore v. VITAS Healthcare Corporation of California, Sacramento County Superior Court, Case No. 34-2017-0021-2755.
Plaintiffs sought to pursue these claims in the form of a state-wide class action of current and former non-exempt employees employed with VITAS CA in California within the four years preceding the filing of the lawsuit. Plaintiffs served VITAS
with the lawsuit on June 5, 2017. VITAS CA timely answered the Complaint generally denying the Plaintiffs’ allegations. The Court has stayed all class discovery in this case pending resolution of mediation in the Jordan Seper and Jiwann Chhina
cases.
There are currently three other lawsuits against VITAS pending in the superior courts of other
California counties that contain claims and class periods that substantially overlap with Phillips’ and Moore’s claims: the Jordan Seper and Jiwann Chhina cases, and Williams v. VITAS Healthcare Corporation of California, filed on May 22, 2017 in Alameda County Superior Court, RG 17853886.
Jazzina Williams’ (a Home Health Aide in Sacramento) lawsuit alleges claims for (1) failure to pay
all wages due; (2) failure to authorize and permit rest periods; (3) failure to provide off-duty meal periods; (4) failure to furnish accurate wage statements; (5) unreimbursed business expenses; (6) waiting time penalties; and (7) violations of
the Private Attorneys General Act (“PAGA”). Williams seeks to pursue these claims both individually and as a representative action under the PAGA on behalf of current and former California non-exempt employees. Plaintiff served VITAS with the
lawsuit on May 31, 2017. VITAS CA timely answered the Complaint generally denying Plaintiff’s allegations. Williams is pursing discovery of her individual claim and has agreed to a stay of class discovery pending possible resolution through
ongoing mediation in the Jordan Seper and Jiwann Chhina cases. Defendant filed and served each of Plaintiffs Williams, Phillips, and Moore with a Notice of Related Cases on July 19, 2017.
The Jordan
Seper and Jiwann Chhina cases have been consolidated in Los Angeles County Superior court; Chhina was dismissed as a separate action and joined with Seper through
the filing of an amended complaint in Seper in which Chhina is also identified as a named plaintiff, on August 28, 2018.
Alfred Lax (“Lax”), a current employee of Roto-Rooter Services Company (“RRSC”), was hired in the
RRSC’s Menlo Park branch in 2007. On November 30, 2018, Lax filed a class action lawsuit in Santa Clara County Superior Court alleging (1) failure to provide or compensate for required rest breaks; (2) failure to properly pay for all hours
worked; (3) failure to provide accurate wage statements; (4) failure to reimburse for work-related expenses; and (5) unfair business practices. Lax has stated these claims as a representative of a class defined as all service technicians
employed by RRSC in California during the four years preceding the filing of the complaint. He seeks a determination that the action may proceed and be maintained as a class action and for compensatory and statutory damages (premium payments for
missed rest periods, uncompensated rest periods, wages for time allegedly not paid such as travel time, repair time, and vehicle maintenance time, and unreimbursed expenses), penalties and restitutions, pre- and post-judgement interest and
attorneys’ fees and costs. The lawsuit, Alfred Lax, on behalf of himself and all others similarly situated v. Roto-Rooter Services Company, and
Does 1 through 50 inclusive; Santa Clara County Superior Court Case Number 18CV338652, was received by RRSC on December 11, 2018 and RRSC timely filed its answer denying the claims.
The Company is not able to reasonably estimate the probability of loss or range of loss for any of
these lawsuits at this time.
The Company intends to defend vigorously against the allegations in each of the above lawsuits.
Regardless of the outcome of any of the preceding matters, dealing with the various regulatory agencies and opposing parties can adversely affect us through defense costs, potential payments, diversion of management time, and related publicity.
Although the Company intends to defend them vigorously, there can be no assurance that those suits will not have a material adverse effect on the Company.
18. Concentration of Risk
During the year VITAS had pharmacy services agreements (“Agreements”) with one service provider to
provide specified pharmacy services for VITAS and its hospice patients. VITAS made purchases from this provider of $31.4 million, $32.7 million and $35.2 million for the years ended December 31, 2018, 2017 and 2016, respectively. For the years
ended December 31, 2018, 2017 and 2016, respectively, purchases from this vendor represent approximately 99%, 85% and 90%, respectively of all pharmacy services used by VITAS. VITAS’ accounts payable for pharmacy services was $2.6 million at
December 31, 2018. At December 31, 2017, VITAS’ accounts payable for pharmacy services was $2.0 million.
19. Capital Stock Transactions
We repurchased the following capital stock:
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Total cost of repurchased shares (in thousands):
|
|
$
|
158,884
|
|
|
$
|
94,640
|
|
|
$
|
102,313
|
|
|
Shares repurchased
|
|
|
561,146
|
|
|
|
500,000
|
|
|
|
780,134
|
|
|
Weighted average price per share
|
|
$
|
283.14
|
|
|
$
|
189.28
|
|
|
$
|
131.15
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
In March 2018, the Board of
Directors authorized an additional $150.0 million for stock repurchase under the February 2011 repurchase program. We currently have $46.6 million of authorization remaining under this share purchase plan.
20. Other Operating Expenses
| |
|
December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Litigation settlement
|
|
$
|
796
|
|
|
$
|
84,476
|
|
|
$
|
-
|
|
|
Loss on disposal of property and equipment
|
|
|
504
|
|
|
|
5,266
|
|
|
|
-
|
|
|
Program closure expenses
|
|
|
-
|
|
|
|
1,138
|
|
|
|
-
|
|
|
Retirement expenses
|
|
|
-
|
|
|
|
-
|
|
|
|
4,491
|
|
|
Total other operating expenses
|
|
$
|
1,300
|
|
|
$
|
90,880
|
|
|
$
|
4,491
|
|
During 2017, the Company recorded $84.5 million related to the Settlement Agreement and a related qui
tam case. See footnote 17 for further discussion. The company recorded $5.3 million related to the loss on the sale of transportation equipment. Also during 2017, the Company recorded $1.1 million related to the closure of three Alabama
programs at VITAS.
During 2016, the Company recorded early retirement related costs and accelerated stock-based
compensation expense of approximately $4.5 million related to the early retirement of VITAS’ former Chief Executive Officer. The costs were calculated in accordance with the terms of this employment agreement.
21. Recent Accounting Statements
In February 2016, the FASB issued Accounting Standards Update “ASU No. 2016-02 – Leases” which
introduces a lessee model that brings most leases on to the balance sheets and updates lessor accounting to align with changes in the lessee model and the revenue recognition standard. The guidance is effective for fiscal years beginning after
December 15, 2018. We plan to elect the new transition method approved by the FASB on July 30, 2018, which allows companies to apply the provisions of the new leasing standard as of January 1, 2019, without adjusting comparative periods
presented by recognizing a cumulative-effect adjustment to the opening balance of retained earnings. We do not expect the cumulative-effect adjustment to be material.
We have contracted with a software vendor for the technology to support compliance with the
ASU. We currently have all real estate and equipment leases identified and populated into the software. We are in the process of completing testing of the data in the software for completeness and accuracy. We have implemented controls over the
implementation and are also in the process of developing and testing controls once the new standard takes effect.
While we continue to evaluate the effect of the standard on our consolidated financial statements,
the adoption of the ASU will result in the recognition of a right of use asset and related liability in the range of approximately $105 million to $125 million. This estimate is developed based partially on our anticipated utilization of the
allowable practical expedients, as follows:
|
•
|
We will utilize the “package” of practical expedients related to the ASU’s adoption. As such, we will not re-assess our
historical conclusions related to capital versus operating lease classification, the existence of embedded leases in service contracts and indirect initial costs for existing leases.
|
|
•
|
We will combine the lease and non-lease components of our building/space leases for purposes of calculating our right of use
asset and related lease liability. We do not have material non-lease components in our existing equipment leases.
|
|
•
|
We will not capitalize leases with less than a 12-month expiration without a purchase option that is reasonably certain to be
exercised.
|
While we intend to utilize the practical expedients as outlined above, we continue to analyze their
impact and the final conclusions may change upon adoption. Any change to the practical expedients could cause a significant change to the estimated right-of-use asset and associated lease liability. The remaining practical expedients are either
not applicable or are not anticipated to have a significant impact. We do not anticipate a material impact to overall net income or cash flows as a result of the adoption of the ASU.
In January 2017, the FASB issued Accounting Standards Update “ASU No. 2017-4 – Intangibles – Goodwill
and Other”. To simplify the subsequent measurement of goodwill, the FASB eliminated Step 2 from the goodwill impairment test. The guidance in the ASU is effective for the Company in fiscal years beginning after December 15, 2019. Early
adoption is permitted. We anticipate adoption of this standard will have no impact on our consolidated financial statements.
In June 2018, the FASB issued Accounting Standards Update “ASU No. 2018-07 – Compensation – Stock
Compensation”. The ASU expands the scope of current guidance to include all share-based payment arrangements related to the acquisition of goods and services from both non-employees and employees. The guidance in the ASU is effective for the
Company in all fiscal years beginning after December 15, 2018. Adoption of this standard will have no material impact on our consolidated financial statements.
In August 2018, the FASB issued Accounting Standards Update “ASU No. 2018-15” to provide guidance on
implementation costs incurred in a cloud computing arrangement that is a service contract. This ASU aligns the accounting for such costs with the guidance on capitalizing costs associated with developing or obtaining internal use software. The
ASU is effective for fiscal years beginning after December 15, 2019 and interim periods thereafter. Early adoption is permitted. We are currently evaluating the impact of this standard on our consolidated financial statements.
|
UNAUDITED SUMMARIES OF QUARTERLY RESULTS
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chemed Corporation and Subsidiary Companies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share and footnote data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
First
|
|
|
Second
|
|
|
Third
|
|
|
Fourth
|
|
|
Total
|
|
|
For the Year Ended December 31, 2018
|
|
Quarter
|
|
|
Quarter
|
|
|
Quarter
|
|
|
Quarter
|
|
|
Year
|
|
|
Total service revenues and sales
|
|
$
|
439,176
|
|
|
$
|
441,813
|
|
|
$
|
444,151
|
|
|
$
|
457,508
|
|
|
$
|
1,782,648
|
|
|
Gross profit (excluding depreciation)
|
|
$
|
134,640
|
|
|
$
|
136,072
|
|
|
$
|
138,839
|
|
|
$
|
144,453
|
|
|
$
|
554,004
|
|
|
Income/(loss) from operations
|
|
$
|
56,397
|
|
|
$
|
58,141
|
|
|
$
|
61,713
|
|
|
$
|
67,381
|
|
|
$
|
243,632
|
|
|
Interest expense
|
|
|
(1,207
|
)
|
|
|
(1,524
|
)
|
|
|
(1,082
|
)
|
|
|
(1,177
|
)
|
|
|
(4,990
|
)
|
|
Other income--net
|
|
|
1,018
|
|
|
|
1,038
|
|
|
|
2,300
|
|
|
|
(3,398
|
)
|
|
|
958
|
|
|
Income before income taxes
|
|
|
56,208
|
|
|
|
57,655
|
|
|
|
62,931
|
|
|
|
62,806
|
|
|
|
239,600
|
|
|
Income taxes
|
|
|
(11,212
|
)
|
|
|
(2,684
|
)
|
|
|
(11,682
|
)
|
|
|
(8,478
|
)
|
|
|
(34,056
|
)
|
|
Net income/(loss) (a)
|
|
$
|
44,996
|
|
|
$
|
54,971
|
|
|
$
|
51,249
|
|
|
$
|
54,328
|
|
|
$
|
205,544
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings/(Loss) Per Share (a)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income/(loss)
|
|
$
|
2.79
|
|
|
$
|
3.43
|
|
|
$
|
3.19
|
|
|
$
|
3.39
|
|
|
$
|
12.80
|
|
|
Average number of shares outstanding
|
|
|
16,100
|
|
|
|
16,035
|
|
|
|
16,074
|
|
|
|
16,026
|
|
|
|
16,059
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted Earnings/(Loss) Per Share (a)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income/(loss)
|
|
$
|
2.66
|
|
|
$
|
3.27
|
|
|
$
|
3.06
|
|
|
$
|
3.26
|
|
|
$
|
12.23
|
|
|
Average number of shares outstanding
|
|
|
16,887
|
|
|
|
16,811
|
|
|
|
16,772
|
|
|
|
16,670
|
|
|
|
16,803
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) The following amounts are included in income during the respective quarter (in thousands):
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
First
|
|
|
Second
|
|
|
Third
|
|
|
Fourth
|
|
|
Total
|
|
| |
|
Quarter
|
|
|
Quarter
|
|
|
Quarter
|
|
|
Quarter
|
|
|
Year
|
|
|
Pretax (cost)/benefit:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
(3,653
|
)
|
|
$
|
(3,652
|
)
|
|
$
|
(2,055
|
)
|
|
$
|
(3,251
|
)
|
|
$
|
(12,611
|
)
|
|
Long-term incentive compensation
|
|
|
(1,920
|
)
|
|
|
(1,222
|
)
|
|
|
(1,234
|
)
|
|
|
(2,242
|
)
|
|
|
(6,618
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(352
|
)
|
|
|
(185
|
)
|
|
|
(503
|
)
|
|
|
(456
|
)
|
|
|
(1,496
|
)
|
|
Expenses related to litigation settlements
|
|
|
-
|
|
|
|
204
|
|
|
|
-
|
|
|
|
(1,000
|
)
|
|
|
(796
|
)
|
|
Acquisition expenses
|
|
|
-
|
|
|
|
-
|
|
|
|
(354
|
)
|
|
|
(403
|
)
|
|
|
(757
|
)
|
|
Total
|
|
$
|
(5,925
|
)
|
|
$
|
(4,855
|
)
|
|
$
|
(4,146
|
)
|
|
$
|
(7,352
|
)
|
|
$
|
(22,278
|
)
|
|
After-tax (cost)/benefit:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
(2,891
|
)
|
|
$
|
(2,900
|
)
|
|
$
|
(1,674
|
)
|
|
$
|
(2,653
|
)
|
|
$
|
(10,118
|
)
|
|
Long-term incentive compensation
|
|
|
(1,499
|
)
|
|
|
(1,003
|
)
|
|
|
(1,013
|
)
|
|
|
(1,792
|
)
|
|
|
(5,307
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(263
|
)
|
|
|
(138
|
)
|
|
|
(376
|
)
|
|
|
(337
|
)
|
|
|
(1,114
|
)
|
|
Expenses related to litigation settlements
|
|
|
-
|
|
|
|
152
|
|
|
|
-
|
|
|
|
(746
|
)
|
|
|
(594
|
)
|
|
Acquisition expenses
|
|
|
-
|
|
|
|
-
|
|
|
|
(262
|
)
|
|
|
(297
|
)
|
|
|
(559
|
)
|
|
Excess tax benefits on stock compensation
|
|
|
3,798
|
|
|
|
11,702
|
|
|
|
3,118
|
|
|
|
4,244
|
|
|
|
22,862
|
|
|
Total
|
|
$
|
(855
|
)
|
|
$
|
7,813
|
|
|
$
|
(207
|
)
|
|
$
|
(1,581
|
)
|
|
$
|
5,170
|
|
|
UNAUDITED SUMMARIES OF QUARTERLY RESULTS
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Chemed Corporation and Subsidiary Companies
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share and footnote data)
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
First
|
|
Second
|
|
Third
|
|
Fourth
|
|
Total
|
|
|
For the Year Ended December 31, 2017
|
Quarter
|
|
Quarter
|
|
Quarter
|
|
Quarter
|
|
Year
|
|
|
Total service revenues and sales
|
|
$
|
405,864
|
|
|
$
|
415,059
|
|
|
$
|
417,444
|
|
|
$
|
428,357
|
|
|
$
|
1,666,724
|
|
|
Gross profit (excluding depreciation)
|
|
$
|
120,724
|
|
|
$
|
129,207
|
|
|
$
|
129,397
|
|
|
$
|
136,864
|
|
|
$
|
516,192
|
|
|
Income from operations
|
|
$
|
41,454
|
|
|
$
|
(38,948
|
)
|
|
$
|
53,997
|
|
|
$
|
56,532
|
|
|
$
|
113,035
|
|
|
Interest expense
|
|
|
(995
|
)
|
|
|
(1,121
|
)
|
|
|
(1,048
|
)
|
|
|
(1,108
|
)
|
|
|
(4,272
|
)
|
|
Other income/(expense)--net
|
|
|
2,463
|
|
|
|
1,653
|
|
|
|
1,323
|
|
|
|
2,715
|
|
|
|
8,154
|
|
|
Income before income taxes
|
|
|
42,922
|
|
|
|
(38,416
|
)
|
|
|
54,272
|
|
|
|
58,139
|
|
|
|
116,917
|
|
|
Income taxes
|
|
|
(13,078
|
)
|
|
|
16,760
|
|
|
|
(18,835
|
)
|
|
|
(3,587
|
)
|
|
|
(18,740
|
)
|
|
Net income (a)
|
|
$
|
29,844
|
|
|
$
|
(21,656
|
)
|
|
$
|
35,437
|
|
|
$
|
54,552
|
|
|
$
|
98,177
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings Per Share (a)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
1.84
|
|
|
$
|
(1.35
|
)
|
|
$
|
2.22
|
|
|
$
|
3.40
|
|
|
$
|
6.11
|
|
|
Average number of shares outstanding
|
|
|
16,219
|
|
|
|
16,010
|
|
|
|
15,976
|
|
|
|
16,026
|
|
|
|
16,057
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted Earnings Per Share (a)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
1.78
|
|
|
$
|
(1.35
|
)
|
|
$
|
2.13
|
|
|
$
|
3.25
|
|
|
$
|
5.86
|
|
|
Average number of shares outstanding
|
|
|
16,801
|
|
|
|
16,010
|
|
|
|
16,676
|
|
|
|
16,776
|
|
|
|
16,742
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) The following amounts are included in income during the respective quarter (in thousands):
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
First
|
|
|
Second |
|
|
Third
|
|
|
Fourth
|
|
|
Total
|
|
| |
|
Quarter |
|
|
Quarter |
|
|
Quarter |
|
|
Quarter |
|
|
Year
|
|
|
Pretax (cost)/benefit:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
(3,001
|
)
|
|
$
|
(3,054
|
)
|
|
$
|
(1,683
|
)
|
|
$
|
(2,747
|
)
|
|
$
|
(10,485
|
)
|
|
Long-term incentive compensation
|
|
|
(961
|
)
|
|
|
(956
|
)
|
|
|
(1,104
|
)
|
|
|
(1,973
|
)
|
|
|
(4,994
|
)
|
|
Loss on sale of transportation equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(5,266
|
)
|
|
|
(5,266
|
)
|
|
Expenses related to litigation settlements
|
|
|
-
|
|
|
|
(90,213
|
)
|
|
|
-
|
|
|
|
5,524
|
|
|
|
(84,689
|
)
|
|
Program closure expenses
|
|
|
(873
|
)
|
|
|
(636
|
)
|
|
|
371
|
|
|
|
-
|
|
|
|
(1,138
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
-
|
|
|
|
(105
|
)
|
|
|
-
|
|
|
|
(342
|
)
|
|
|
(447
|
)
|
|
Expenses related to the Office
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
of Inspector General investigation
|
|
|
(2,150
|
)
|
|
|
(2,093
|
)
|
|
|
(935
|
)
|
|
|
(16
|
)
|
|
|
(5,194
|
)
|
|
Total
|
|
$
|
(6,985
|
)
|
|
$
|
(97,057
|
)
|
|
$
|
(3,351
|
)
|
|
$
|
(4,820
|
)
|
|
$
|
(112,213
|
)
|
|
After-tax (cost)/benefit:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
(1,897
|
)
|
|
$
|
(1,931
|
)
|
|
$
|
(1,064
|
)
|
|
$
|
(2,000
|
)
|
|
$
|
(6,892
|
)
|
|
Long-term incentive compensation
|
|
|
(608
|
)
|
|
|
(604
|
)
|
|
|
(699
|
)
|
|
|
(1,332
|
)
|
|
|
(3,243
|
)
|
|
Loss on sale of transportation equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,314
|
)
|
|
|
(3,314
|
)
|
|
Expenses related to litigation settlements
|
|
|
-
|
|
|
|
(55,929
|
)
|
|
|
-
|
|
|
|
3,425
|
|
|
|
(52,504
|
)
|
|
Program closure expenses
|
|
|
(513
|
)
|
|
|
(385
|
)
|
|
|
223
|
|
|
|
-
|
|
|
|
(675
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
-
|
|
|
|
(65
|
)
|
|
|
-
|
|
|
|
(211
|
)
|
|
|
(276
|
)
|
|
Expenses related to the Office
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
of Inspector General investigation
|
|
|
(1,328
|
)
|
|
|
(1,292
|
)
|
|
|
(578
|
)
|
|
|
(9
|
)
|
|
|
(3,207
|
)
|
|
Impact of tax reform
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
8,302
|
|
|
|
8,302
|
|
|
Excess tax benefits on stock compensation
|
|
|
3,695
|
|
|
|
2,643
|
|
|
|
1,783
|
|
|
|
10,811
|
|
|
|
18,932
|
|
|
Total
|
|
$
|
(651
|
)
|
|
$
|
(57,563
|
)
|
|
$
|
(335
|
)
|
|
$
|
15,672
|
|
|
$
|
(42,877
|
)
|
|
SELECTED FINANCIAL DATA
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chemed Corporation and Subsidiary Companies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share and footnote data, ratios, percentages and personnel)
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
2015
|
|
|
2014
|
|
|
Summary of Operations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Continuing operations (a)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service revenues and sales
|
|
$
|
1,782,648
|
|
|
$
|
1,666,724
|
|
|
$
|
1,576,881
|
|
|
$
|
1,543,388
|
|
|
$
|
1,456,282
|
|
|
Gross profit (excluding depreciation)
|
|
|
554,004
|
|
|
|
516,192
|
|
|
|
461,450
|
|
|
|
455,778
|
|
|
|
421,609
|
|
|
Depreciation
|
|
|
38,464
|
|
|
|
35,488
|
|
|
|
34,279
|
|
|
|
32,369
|
|
|
|
29,881
|
|
|
Amortization
|
|
|
399
|
|
|
|
137
|
|
|
|
359
|
|
|
|
1,130
|
|
|
|
720
|
|
|
Income from operations
|
|
|
243,632
|
|
|
|
113,035
|
|
|
|
178,749
|
|
|
|
184,458
|
|
|
|
168,419
|
|
|
Net income
|
|
|
205,544
|
|
|
|
98,177
|
|
|
|
108,743
|
|
|
|
110,274
|
|
|
|
99,317
|
|
|
Earnings per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
12.80
|
|
|
$
|
6.11
|
|
|
$
|
6.64
|
|
|
$
|
6.54
|
|
|
$
|
5.79
|
|
|
Average number of shares outstanding
|
|
|
16,059
|
|
|
|
16,057
|
|
|
|
16,383
|
|
|
|
16,870
|
|
|
|
17,165
|
|
|
Diluted earnings per share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
12.23
|
|
|
$
|
5.86
|
|
|
$
|
6.48
|
|
|
$
|
6.33
|
|
|
$
|
5.57
|
|
|
Average number of shares outstanding
|
|
|
16,803
|
|
|
|
16,742
|
|
|
|
16,789
|
|
|
|
17,422
|
|
|
|
17,840
|
|
|
Cash dividends per share
|
|
$
|
1.16
|
|
|
$
|
1.08
|
|
|
$
|
1.00
|
|
|
$
|
0.92
|
|
|
$
|
0.84
|
|
|
Financial Position--Year-End
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
4,831
|
|
|
$
|
11,121
|
|
|
$
|
15,310
|
|
|
$
|
14,727
|
|
|
$
|
14,132
|
|
|
Working capital/(deficit)
|
|
|
(31,830
|
)
|
|
|
(17,476
|
)
|
|
|
(1,932
|
)
|
|
|
(20,528
|
)
|
|
|
(990
|
)
|
|
Current ratio
|
|
|
0.83
|
|
|
|
0.91
|
|
|
|
0.99
|
|
|
|
0.88
|
|
|
|
0.99
|
|
|
Properties and equipment, at cost less
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
accumulated depreciation
|
|
$
|
162,033
|
|
|
$
|
143,034
|
|
|
$
|
121,302
|
|
|
$
|
117,370
|
|
|
$
|
105,336
|
|
|
Total assets
|
|
|
975,529
|
|
|
|
920,026
|
|
|
|
880,059
|
|
|
|
852,325
|
|
|
|
859,932
|
|
|
Long-term debt
|
|
|
89,200
|
|
|
|
91,200
|
|
|
|
100,000
|
|
|
|
83,750
|
|
|
|
141,250
|
|
|
Stockholders' equity
|
|
|
591,334
|
|
|
|
540,354
|
|
|
|
524,099
|
|
|
|
513,253
|
|
|
|
451,356
|
|
|
Other Statistics
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capital expenditures
|
|
$
|
52,872
|
|
|
$
|
64,300
|
|
|
$
|
39,772
|
|
|
$
|
44,135
|
|
|
$
|
43,571
|
|
|
Number of employees
|
|
|
15,707
|
|
|
|
14,813
|
|
|
|
14,613
|
|
|
|
14,406
|
|
|
|
14,190
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) The following amounts are included in income from continuing operations during the respective year (in thousands):
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
2018
|
|
|
|
2017
|
|
|
|
2016
|
|
|
|
2015
|
|
|
|
2014
|
|
|
After-tax benefit/(cost):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Excess tax benefits on stock compensation
|
|
$
|
22,862
|
|
|
$
|
18,932
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
Stock option expense
|
|
|
(10,118
|
)
|
|
|
(6,892
|
)
|
|
|
(5,266
|
)
|
|
|
(3,439
|
)
|
|
|
(3,022
|
)
|
|
Long-term incentive compensation
|
|
|
(5,307
|
)
|
|
|
(3,243
|
)
|
|
|
(1,221
|
)
|
|
|
(4,752
|
)
|
|
|
(1,625
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(1,114
|
)
|
|
|
(276
|
)
|
|
|
(141
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Litigation settlements
|
|
|
(594
|
)
|
|
|
(52,504
|
)
|
|
|
(28
|
)
|
|
|
(3
|
)
|
|
|
(74
|
)
|
|
Acquisition expense
|
|
|
(559
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(104
|
)
|
|
|
(15
|
)
|
|
Program closure expenses
|
|
|
-
|
|
|
|
(675
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Impact of tax reform
|
|
|
-
|
|
|
|
8,302
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Loss on sale of transportation equipment
|
|
|
-
|
|
|
|
(3,314
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Expenses incurred in connection with the Office of Inspector
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General investigation
|
|
|
-
|
|
|
|
(3,207
|
)
|
|
|
(3,248
|
)
|
|
|
(3,072
|
)
|
|
|
(1,328
|
)
|
|
Early retirement expenses
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,840
|
)
|
|
|
-
|
|
|
|
-
|
|
|
Noncash impact of change in accounting for convertible debt
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,143
|
)
|
|
Expenses of securities litigation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(23
|
)
|
|
|
(207
|
)
|
|
Total
|
|
$
|
5,170
|
|
|
$
|
(42,877
|
)
|
|
$
|
(12,744
|
)
|
|
$
|
(11,393
|
)
|
|
$
|
(8,414
|
)
|
|
CHEMED CORPORATION AND SUBSIDIARY COMPANIES
|
|
|
UNAUDITED CONSOLIDATING STATEMENTS OF INCOME
|
|
|
FOR THE YEAR ENDED DECEMBER 31, 2018
|
|
|
(in thousands)(unaudited)
|
|
| |
|
|
|
|
Roto-
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
2018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service revenues and sales
|
|
$
|
1,197,562
|
|
|
$
|
585,086
|
|
|
$
|
-
|
|
|
$
|
1,782,648
|
|
|
Cost of services provided and goods sold
|
|
|
929,306
|
|
|
|
299,338
|
|
|
|
-
|
|
|
|
1,228,644
|
|
|
Selling, general and administrative expenses
|
|
|
80,969
|
|
|
|
145,683
|
|
|
|
43,557
|
|
|
|
270,209
|
|
|
Depreciation
|
|
|
19,688
|
|
|
|
18,629
|
|
|
|
147
|
|
|
|
38,464
|
|
|
Amortization
|
|
|
12
|
|
|
|
387
|
|
|
|
-
|
|
|
|
399
|
|
|
Other operating expenses
|
|
|
1,130
|
|
|
|
170
|
|
|
|
-
|
|
|
|
1,300
|
|
|
Total costs and expenses
|
|
|
1,031,105
|
|
|
|
464,207
|
|
|
|
43,704
|
|
|
|
1,539,016
|
|
|
Income/(loss) from operations
|
|
|
166,457
|
|
|
|
120,879
|
|
|
|
(43,704
|
)
|
|
|
243,632
|
|
|
Interest expense
|
|
|
(175
|
)
|
|
|
(319
|
)
|
|
|
(4,496
|
)
|
|
|
(4,990
|
)
|
|
Intercompany interest income/(expense)
|
|
|
12,832
|
|
|
|
6,908
|
|
|
|
(19,740
|
)
|
|
|
-
|
|
|
Other income/(expense)—net
|
|
|
579
|
|
|
|
93
|
|
|
|
286
|
|
|
|
958
|
|
|
Income/(loss) before income taxes
|
|
|
179,693
|
|
|
|
127,561
|
|
|
|
(67,654
|
)
|
|
|
239,600
|
|
|
Income taxes
|
|
|
(40,847
|
)
|
|
|
(28,850
|
)
|
|
|
35,641
|
|
|
|
(34,056
|
)
|
|
Net income/(loss)
|
|
$
|
138,846
|
|
|
$
|
98,711
|
|
|
$
|
(32,013
|
)
|
|
$
|
205,544
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) The following amounts are included in income from continuing operations (in thousands):
|
|
| |
|
|
|
|
|
Roto-
|
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
Pretax benefit/(cost):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
(12,611
|
)
|
|
$
|
(12,611
|
)
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
(6,618
|
)
|
|
|
(6,618
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(1,496
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,496
|
)
|
|
Litigation settlements
|
|
|
(796
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(796
|
)
|
|
Acquisition expenses
|
|
|
(209
|
)
|
|
|
(548
|
)
|
|
|
-
|
|
|
|
(757
|
)
|
|
Total
|
|
$
|
(2,501
|
)
|
|
$
|
(548
|
)
|
|
$
|
(19,229
|
)
|
|
$
|
(22,278
|
)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Roto-
|
|
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
After-tax benefit/(cost):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Excess tax benefits on stock compensation
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
22,862
|
|
|
$
|
22,862
|
|
|
Stock option expense
|
|
|
-
|
|
|
|
-
|
|
|
|
(10,118
|
)
|
|
|
(10,118
|
)
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
(5,307
|
)
|
|
|
(5,307
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(1,114
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,114
|
)
|
|
Litigation settlements
|
|
|
(594
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(594
|
)
|
|
Acquisition expenses
|
|
|
(156
|
)
|
|
|
(403
|
)
|
|
|
-
|
|
|
|
(559
|
)
|
|
Total
|
|
$
|
(1,864
|
)
|
|
$
|
(403
|
)
|
|
$
|
7,437
|
|
|
$
|
5,170
|
|
|
CHEMED CORPORATION AND SUBSIDIARY COMPANIES
|
|
|
UNAUDITED CONSOLIDATING STATEMENTS OF INCOME
|
|
|
FOR THE YEAR ENDED DECEMBER 31, 2017
|
|
|
(in thousands)(unaudited)
|
|
| |
|
|
|
|
Roto-
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
2017
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service revenues and sales
|
|
$
|
1,148,260
|
|
|
$
|
518,464
|
|
|
$
|
-
|
|
|
$
|
1,666,724
|
|
|
Cost of services provided and goods sold
|
|
|
886,062
|
|
|
|
264,470
|
|
|
|
-
|
|
|
|
1,150,532
|
|
|
Selling, general and administrative expenses
|
|
|
95,215
|
|
|
|
136,248
|
|
|
|
45,189
|
|
|
|
276,652
|
|
|
Depreciation
|
|
|
18,616
|
|
|
|
16,667
|
|
|
|
205
|
|
|
|
35,488
|
|
|
Amortization
|
|
|
14
|
|
|
|
123
|
|
|
|
-
|
|
|
|
137
|
|
|
Other operating expenses
|
|
|
85,614
|
|
|
|
-
|
|
|
|
5,266
|
|
|
|
90,880
|
|
|
Total costs and expenses
|
|
|
1,085,521
|
|
|
|
417,508
|
|
|
|
50,660
|
|
|
|
1,553,689
|
|
|
Income/(loss) from operations
|
|
|
62,739
|
|
|
|
100,956
|
|
|
|
(50,660
|
)
|
|
|
113,035
|
|
|
Interest expense
|
|
|
(188
|
)
|
|
|
(323
|
)
|
|
|
(3,761
|
)
|
|
|
(4,272
|
)
|
|
Intercompany interest income/(expense)
|
|
|
11,656
|
|
|
|
5,596
|
|
|
|
(17,252
|
)
|
|
|
-
|
|
|
Other income/(expense)—net
|
|
|
(126
|
)
|
|
|
(148
|
)
|
|
|
8,428
|
|
|
|
8,154
|
|
|
Income/(loss) before income taxes
|
|
|
74,081
|
|
|
|
106,081
|
|
|
|
(63,245
|
)
|
|
|
116,917
|
|
|
Income taxes
|
|
|
(16,436
|
)
|
|
|
(32,782
|
)
|
|
|
30,478
|
|
|
|
(18,740
|
)
|
|
Net income/(loss)
|
|
$
|
57,645
|
|
|
$
|
73,299
|
|
|
$
|
(32,767
|
)
|
|
$
|
98,177
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) The following amounts are included in income from continuing operations (in thousands):
|
|
| |
|
|
|
|
|
Roto-
|
|
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
Pretax benefit/(cost):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
(10,485
|
)
|
|
$
|
(10,485
|
)
|
|
Loss on sale of transportation equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
(5,266
|
)
|
|
|
(5,266
|
)
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,994
|
)
|
|
|
(4,994
|
)
|
|
Program closure expenses
|
|
|
(1,138
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,138
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(447
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(447
|
)
|
|
Expenses related to litigation settlements
|
|
|
(84,476
|
)
|
|
|
(213
|
)
|
|
|
-
|
|
|
|
(84,689
|
)
|
|
Expenses incurred in connection with the Office of Inspector
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General investigation
|
|
|
(5,194
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(5,194
|
)
|
|
Total
|
|
$
|
(91,255
|
)
|
|
$
|
(213
|
)
|
|
$
|
(20,745
|
)
|
|
$
|
(112,213
|
)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Roto-
|
|
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
After-tax benefit/(cost):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
(6,892
|
)
|
|
$
|
(6,892
|
)
|
|
Loss on sale of transportation equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,314
|
)
|
|
|
(3,314
|
)
|
|
Excess tax benefits on stock compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
18,932
|
|
|
|
18,932
|
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,243
|
)
|
|
|
(3,243
|
)
|
|
Impact of tax reform
|
|
|
11,057
|
|
|
|
7,761
|
|
|
|
(10,516
|
)
|
|
|
8,302
|
|
|
Program closure expenses
|
|
|
(675
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(675
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(276
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(276
|
)
|
|
Expenses related to litigation settlements
|
|
|
(52,375
|
)
|
|
|
(129
|
)
|
|
|
-
|
|
|
|
(52,504
|
)
|
|
Expenses incurred in connection with the Office of Inspector
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General investigation
|
|
|
(3,207
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,207
|
)
|
|
Total
|
|
$
|
(45,476
|
)
|
|
$
|
7,632
|
|
|
$
|
(5,033
|
)
|
|
$
|
(42,877
|
)
|
|
CHEMED CORPORATION AND SUBSIDIARY COMPANIES
|
|
|
UNAUDITED CONSOLIDATING STATEMENTS OF INCOME
|
|
|
FOR THE YEAR ENDED DECEMBER 31, 2016
|
|
|
(in thousands)(unaudited)
|
|
| |
|
|
|
|
Roto-
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
2016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Service revenues and sales
|
|
$
|
1,123,317
|
|
|
$
|
453,564
|
|
|
$
|
-
|
|
|
$
|
1,576,881
|
|
|
Cost of services provided and goods sold
|
|
|
878,092
|
|
|
|
237,339
|
|
|
|
-
|
|
|
|
1,115,431
|
|
|
Selling, general and administrative expenses
|
|
|
92,550
|
|
|
|
118,812
|
|
|
|
32,210
|
|
|
|
243,572
|
|
|
Depreciation
|
|
|
19,035
|
|
|
|
14,698
|
|
|
|
546
|
|
|
|
34,279
|
|
|
Amortization
|
|
|
55
|
|
|
|
304
|
|
|
|
-
|
|
|
|
359
|
|
|
Other operating expenses
|
|
|
4,491
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4,491
|
|
|
Total costs and expenses
|
|
|
994,223
|
|
|
|
371,153
|
|
|
|
32,756
|
|
|
|
1,398,132
|
|
|
Income/(loss) from operations
|
|
|
129,094
|
|
|
|
82,411
|
|
|
|
(32,756
|
)
|
|
|
178,749
|
|
|
Interest expense
|
|
|
(211
|
)
|
|
|
(332
|
)
|
|
|
(3,172
|
)
|
|
|
(3,715
|
)
|
|
Intercompany interest income/(expense)
|
|
|
7,969
|
|
|
|
3,595
|
|
|
|
(11,564
|
)
|
|
|
-
|
|
|
Other income/(expense)—net
|
|
|
19
|
|
|
|
(62
|
)
|
|
|
2,063
|
|
|
|
2,020
|
|
|
Income/(loss) before income taxes
|
|
|
136,871
|
|
|
|
85,612
|
|
|
|
(45,429
|
)
|
|
|
177,054
|
|
|
Income taxes
|
|
|
(51,910
|
)
|
|
|
(32,719
|
)
|
|
|
16,318
|
|
|
|
(68,311
|
)
|
|
Net income/(loss)
|
|
$
|
84,961
|
|
|
$
|
52,893
|
|
|
$
|
(29,111
|
)
|
|
$
|
108,743
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) The following amounts are included in income from continuing operations (in thousands):
|
|
| |
|
|
|
|
|
Roto-
|
|
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
Pretax benefit/(cost):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
(8,330
|
)
|
|
$
|
(8,330
|
)
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,930
|
)
|
|
|
(1,930
|
)
|
|
Early retirement expenses
|
|
|
(4,491
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,491
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(228
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(228
|
)
|
|
Expenses related to litigation settlements
|
|
|
-
|
|
|
|
(45
|
)
|
|
|
-
|
|
|
|
(45
|
)
|
|
Expenses incurred in connection with the Office of Inspector
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General investigation
|
|
|
(5,260
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(5,260
|
)
|
|
Total
|
|
$
|
(9,979
|
)
|
|
$
|
(45
|
)
|
|
$
|
(10,260
|
)
|
|
$
|
(20,284
|
)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Roto-
|
|
|
|
|
|
|
Chemed
|
|
| |
|
VITAS
|
|
|
Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
|
After-tax benefit/(cost):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
(5,266
|
)
|
|
$
|
(5,266
|
)
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
(1,221
|
)
|
|
|
(1,221
|
)
|
|
Early retirement expenses
|
|
|
(2,840
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(2,840
|
)
|
|
Medicare cap sequestration adjustment
|
|
|
(141
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(141
|
)
|
|
Expenses related to litigation settlements
|
|
|
-
|
|
|
|
(28
|
)
|
|
|
-
|
|
|
|
(28
|
)
|
|
Expenses incurred in connection with the Office of Inspector
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General investigation
|
|
|
(3,248
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,248
|
)
|
|
Total
|
|
$
|
(6,229
|
)
|
|
$
|
(28
|
)
|
|
$
|
(6,487
|
)
|
|
$
|
(12,744
|
)
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
EXECUTIVE SUMMARY
We operate through our two wholly owned subsidiaries: VITAS Healthcare Corporation (“VITAS”) and Roto-Rooter Group, Inc.
(“Roto-Rooter”). VITAS focuses on hospice care that helps make terminally ill patients' final days as comfortable as possible. Through its team of doctors, nurses, home health aides, social workers, clergy and volunteers, VITAS provides direct
medical services to patients, as well as spiritual and emotional counseling to both patients and their families. Roto-Rooter is focused on providing plumbing, drain cleaning, water restoration and other related services to both residential and
commercial customers. Through its network of company-owned branches, independent contractors and franchisees, Roto-Rooter offers plumbing and drain cleaning service to approximately 90% of the U.S. population.
The following is a summary of the key operating
results for the years ended December 31, 2018, 2017 and 2016 (in thousands except percentages and per share amounts):
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Consolidated service revenues and sales
|
|
$
|
1,782,648
|
|
|
$
|
1,666,724
|
|
|
$
|
1,576,881
|
|
|
Consolidated net income
|
|
$
|
205,544
|
|
|
$
|
98,177
|
|
|
$
|
108,743
|
|
|
Diluted EPS
|
|
$
|
12.23
|
|
|
$
|
5.86
|
|
|
$
|
6.48
|
|
|
Adjusted net income
|
|
$
|
200,374
|
|
|
$
|
141,054
|
|
|
$
|
121,487
|
|
|
Adjusted diluted EPS
|
|
$
|
11.93
|
|
|
$
|
8.43
|
|
|
$
|
7.24
|
|
|
Adjusted EBITDA
|
|
$
|
305,506
|
|
|
$
|
268,459
|
|
|
$
|
236,979
|
|
|
Adjusted EBITDA as a % of revenue
|
|
|
17.1
|
%
|
|
|
16.1
|
%
|
|
|
15.0
|
%
|
Adjusted net income, adjusted diluted EPS, earnings before interest, taxes and depreciation and amortization (“EBITDA”)
and Adjusted EBITDA are not measures derived in accordance with GAAP. We use Adjusted EPS as a measure of earnings for certain long-term incentive awards. We use adjusted EBITDA to determine compliance with certain debt covenants. We provide
non-GAAP measures to help readers evaluate our operating results, compare our operating performance with that of similar companies that have different capital structures. Our non-GAAP measures should not be considered in isolation or as a
substitute for comparable measures presented in accordance with GAAP. Reconciliations of our non-GAAP measures are presented in tables following the Critical Accounting Policies section.
2018 versus 2017
The increase in consolidated service revenues and sales from 2017 to 2018 was a result of a 12.8% increase at Roto-Rooter
and a 4.3% increase at VITAS. The increase in service revenues at Roto-Rooter was driven by an increase in all major service lines offset by a $6.9 million decrease related to the adoption of the new revenue recognition standard. The increase in
service revenues at VITAS is comprised primarily of a 1.1% geographically weighted average Medicare reimbursement rate increase, a 7.2% increase in average daily census, offset by $4.1 million in Medicare cap revenue reduction (compared to $2.7
million for 2017), acuity mix shift and a $21.8 million decrease related to the adoption of the new revenue recognition standard.
2017 versus 2016
The increase in consolidated service revenues and sales from 2016 to 2017 was a result of a 14.3% increase at Roto-Rooter
and a 2.2% increase at VITAS. Of Roto-Rooter’s total revenue increase, $32.0 million is related to water restoration and $22.2 million is related to plumbing. The increase in service revenues at VITAS was a result of Medicare reimbursement rates
increasing approximately 1.3%, a 3.2% increase in days of care offset by acuity mix shift which negatively impacted revenue when compared to the prior year. Adjusted EBITDA as a percent of revenue increased 110 basis points when compared to the
prior year. Net income for 2017 includes $52.4 million of after-tax expense ($84.5 million pre-tax) for the Settlement Agreement and Spottiswood Agreement. See Commitments and Contingencies section for details.
Impact of Current Market Conditions
On December 22, 2017, the President of the United States signed into law H.R.1, “An Act to Provide for Reconciliation
Pursuant to Titles II and V of the Concurrent Resolution on the Budget for Fiscal Year 2018” (previously known as “The Tax Cuts and Jobs Act”) or (the “Act”). The Act amends the Internal Revenue Code to reduce tax rates and modify policies,
credits, and deductions for individuals and businesses. For businesses, U.S. generally accepted accounting principles requires resulting tax effects for the Act, to be recorded in the reporting period of enactment.
The SEC issued SAB 118, which provides guidance on accounting for the Act’s impact. Under SAB 118, an entity would use
something similar to the measurement period in a business combination, not to exceed one year. For matters that have not been completed, the Company would recognize provisional amounts to the extent that they are reasonably estimable, adjust them
over time as more information becomes available, and disclose this information in its financial statements.
Our accounting for all elements of the Tax Act is complete. The Tax Act reduced our statutory Federal tax rate from 35%
in 2017 to 21% in 2018. The Company did not record any material changes to the provisional amounts previously recorded. The Company also determined new rules, such as the Global Intangible Low-Taxed Income (GILTI) and Base Erosion and Anti-Abuse
Tax (BEAT), have no material impact to the financial statements.
Historically, the Company has not provided for deferred taxes on undistributed earnings because such earnings are
considered to be indefinitely reinvested outside of the U.S. The Company continues this assertion that foreign earnings are permanently reinvested under the Act.
The Act provides for 100 percent bonus depreciation on personal tangible property expenditures starting September 27, 2017
through 2022. The bonus depreciation percentage is phased down from 100 percent beginning in 2023 through 2026. The Company expects to take full benefit of these bonus depreciation rules.
The IRS and other tax authorities are still issuing guidance on the Act, through various regulations some of which are
still proposed and not final. The Company will implement any changes related to finalized regulations and other guidance in the period issued.
On January 1, 2016, CMS implemented a refinement to the Medicare hospice reimbursement per diem. This refinement
eliminated the single-tier per diem for routine home care (RHC) and replaced it with a two-tiered rate, with a higher rate for the first 60 days of a hospice patient’s care, and a lower rate for days 61 and after. In addition, CMS provided for a
Service Intensity Add-on (SIA) payment which provides for reimbursement of care provided by a registered nurse or social worker for RHC patients within seven days prior to death. The reimbursement for continuous care, inpatient care and respite
care are not impacted by this rebasing.
Full-year 2019 revenue growth for VITAS, prior to Medicare Cap, is estimated to be in the range of 5.5% to 6.0%.
Admissions are estimated to expand approximately 3.0% to 4.0% and Average Daily Census in 2019 are estimated to expand approximately 4.0% to 5.0% and full-year Adjusted EBITDA margin, prior to Medicare Cap, is estimated to be 15.9%. We are
currently estimating $10.0 million for Medicare Cap billing limitations in the 2019 calendar year.
Roto-Rooter is forecasted to achieve full-year 2019 revenue growth of 9.0% to 10.0%. This revenue estimate is based upon
increased job pricing of approximately 2.0% and continued growth in core plumbing and drain cleaning services as well as continued but slowing revenue growth from water restoration services. Adjusted EBITDA margin for 2019 is estimated at 23.7%.
Based upon the above, full-year 2019 adjusted earnings per diluted share, excluding non-cash expense for stock options, tax
benefits from stock options, costs related to litigation, and other discrete items, is estimated to be in the range of $12.65 to $12.85. This 2019 guidance assumes an effective corporate tax rate of 25.2%. This compares to Chemed’s 2018 reported
adjusted earnings per diluted share of $11.93.
LIQUIDITY AND CAPITAL RESOURCES
Significant factors affecting our cash flows during 2018 and financial position at December 31, 2018, include the
following:
|
•
|
Our operations generated cash of $287.1 million.
|
|
•
|
We repurchased $158.9 million of our stock.
|
|
•
|
We spent $53.2 million on business combinations.
|
|
•
|
We spent $52.9 million on capital expenditures.
|
|
•
|
We paid $18.7 million in dividends.
|
|
•
|
On a net basis, we repaid $12.0 million of long-term debt.
|
The ratio of total debt to total capital was 13.1% at December 31, 2018, compared with 15.7% at December 31, 2017. Our
current ratio was 0.83 and 0.91 at December 31, 2018 and 2017, respectively. The decrease in the current ratio is primarily a result of the decrease in prepaid income taxes.
On June 20, 2018, we replaced our existing credit agreement with the Fourth Amended and Restated Credit Agreement (“2018
Credit Agreement”). Terms of the 2018 Credit Agreement consist of a five-year, $450 million revolving credit facility and a $150 million expansion feature, which may consist of term loans or additional revolving commitments. The interest rate at
inception of the agreement is LIBOR plus 100 basis points. The 2018 Credit Agreement has a floating interest rate that is generally LIBOR plus a tiered additional rate which varies based on our current leverage ratio. For December 31, 2018 and
2017, respectively, the interest rate is LIBOR plus 100 basis points. We are in compliance with all financial debt covenants as of December 31, 2018, as follows:
|
Description
|
|
Requirement
|
|
Chemed
|
| |
|
|
|
|
| |
|
|
|
|
|
Leverage Ratio (Consolidated Indebtedness/Consolidated Adj. EBITDA)
|
|
< 3.50 to 1.00
|
|
0.41 to 1.00
|
| |
|
|
|
|
|
Fixed Charge Coverage Ratio (Consolidated Free Cash Flow/Consolidated
|
|
|
|
|
|
Fixed Charges
|
|
> 1.50 to 1.00
|
|
7.61 to 1.00
|
We forecast to be in compliance with all debt covenants through fiscal 2019.
We have issued $36.4 million in standby letters of credit as of December 31, 2018, mainly for insurance purposes. Issued
letters of credit reduce our available credit under the revolving credit agreement. As of December 31, 2018, we have approximately $324.4 million of unused lines of credit available and eligible to be drawn down under our revolving credit
facility. We believe our cash flow from operating activities and our unused eligible lines of credit are sufficient to fund our obligations and operate our business in the near and long term. We continually evaluate cash utilization alternatives,
including share repurchase, debt repurchase, acquisitions, and increased dividends to determine the most beneficial use of available capital resources.
CASH FLOW
Our cash flows for 2018, 2017 and 2016 are summarized as follows (in millions):
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Net cash provided by operating activities
|
|
$
|
287.1
|
|
|
$
|
162.5
|
|
|
$
|
135.4
|
|
|
Capital expenditures
|
|
|
(52.9
|
)
|
|
|
(64.3
|
)
|
|
|
(39.8
|
)
|
|
Operating cash after capital expenditures
|
|
|
234.2
|
|
|
|
98.2
|
|
|
|
95.6
|
|
|
Purchase of treasury stock in the open market
|
|
|
(158.9
|
)
|
|
|
(94.6
|
)
|
|
|
(102.3
|
)
|
|
Business combinations
|
|
|
(53.2
|
)
|
|
|
(4.7
|
)
|
|
|
-
|
|
|
Proceeds from exercise of stock options
|
|
|
32.4
|
|
|
|
27.1
|
|
|
|
8.4
|
|
|
Capital stock surrendered to pay taxes on
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on stock-based compensation
|
|
|
(27.5
|
)
|
|
|
(14.2
|
)
|
|
|
(8.8
|
)
|
|
Dividends paid
|
|
|
(18.7
|
)
|
|
|
(17.4
|
)
|
|
|
(16.4
|
)
|
|
Net increase/(decrease) in long-term debt
|
|
|
(12.0
|
)
|
|
|
(7.6
|
)
|
|
|
17.5
|
|
|
Increase/(decrease) in cash overdraft payable
|
|
|
(1.5
|
)
|
|
|
6.7
|
|
|
|
(0.7
|
)
|
|
Other--net
|
|
|
(1.1
|
)
|
|
|
2.3
|
|
|
|
7.3
|
|
|
Increase/(decrease) in cash and cash equivalents
|
|
$
|
(6.3
|
)
|
|
$
|
(4.2
|
)
|
|
$
|
0.6
|
|
2018 versus 2017
The change in net cash provided by operating activities is mainly the result of a $107.4 million increase in net income.
Significant changes in our accounts receivable balances are driven mainly by the timing of payments received from the Federal government at our VITAS subsidiary. We typically receive a payment in excess of $35.0 million from the Federal government
from hospice services every other Friday. The timing of year end will have a significant impact on the accounts receivable at VITAS. These changes generally normalize over a two year period, as cash flow variations in one year are offset in the
following year.
In 2018, we repurchased 561,146 shares of Chemed capital stock at a weighted average price of $283.14 per share. In 2017,
we repurchased approximately 500,000 shares of Chemed stock at a weighted average price of $189.28 per share. Based on our current operations and our current sources of capital, we believe we have the ability to continue our current share
repurchase program into the foreseeable future.
We made two significant acquisitions in 2018. We acquired five Roto-Rooter franchises in northern California and assets
of a non-profit hospice in Florida to increase market penetration.
The change in overdrafts payable is also a function of the timing of cash payments made and cash receipts near year end.
2017 versus 2016
The change in net cash provided by operating activities is mainly the result of a $43.2 million increase in cash flows
related to accounts receivable and a $12.0 million increase in accounts payable and other current liabilities offset by a $40.0 million decrease in cash flows related to income tax payments and a $10.6 million decrease in net income. The decrease
in net income is the result mainly of a $84.5 million charge for the Settlement and Spottiswood Agreements offset by income increase from normal operations. Significant changes in our accounts receivable balances are driven mainly by the timing of
payments received from the Federal government at our VITAS subsidiary. We typically receive a payment in excess of $35.0 million from the Federal government from hospice services every other Friday. The timing of year end will have a significant
impact on the accounts receivable at VITAS. These changes generally normalize over a two year period, as cash flow variations in one year are offset in the following year.
In 2017, we repurchased 500,000 shares of Chemed capital stock at a weighted average price of $189.28 per share. In 2016,
we repurchased approximately 780,134 shares of Chemed stock at a weighted average price of $131.15 per share. Based on our current operations and our current sources of capital, we believe we have the ability to continue our current share
repurchase program into the foreseeable future.
The change in overdrafts payable is also a function of the timing of cash payments made and cash receipts near year end.
COMMITMENTS AND CONTINGENCIES
We are subject to various lawsuits and claims in the normal course of our business. In addition, we periodically receives
communications from governmental and regulatory agencies concerning compliance with Medicare and Medicaid billing requirements at our VITAS subsidiary. We establish reserves for specific, uninsured liabilities in connection with regulatory and
legal action that we deem to be probable and estimable. We disclose the existence of regulatory and legal actions when we believe it is reasonably possible that a loss could occur in connection with the specific action. In most instances, we are
unable to make a reasonable estimate of any reasonably possible liability due to the uncertainty of the outcome and stage of litigation. We record legal fees associated
with legal and regulatory actions as the costs are incurred.
In connection with the sale of DuBois Chemicals, Inc. ("DuBois") in 1991, we provided allowances and accruals relating to
several long-term costs, including income tax matters, lease commitments and environmental costs. Additionally, we retained liability for casualty insurance claims for Service America and Patient Care that were incurred prior to the respective
disposal dates, 2005 and 2002. In the aggregate, we believe these allowances and accruals are adequate as of December 31, 2018. Based on reviews of our environmental-related liabilities under the DuBois sale agreement, we have
estimated our remaining liability to be $1.7 million. As of December 31, 2018, we are contingently liable for additional cleanup and related costs up to a maximum of $14.9 million. We do not believe it is probable that we will be required to make
any payment towards this contingent liability. Thus, no provision has been recorded in accordance with the applicable accounting guidance.
The VITAS segment of the Company’s business operates in a heavily-regulated industry. As a result, the Company is
subjected to inquiries and investigations by various government agencies, as well as to lawsuits, including qui tam actions. The following
sections describe the various ongoing material lawsuits and investigations of which the Company is currently aware. It is not possible at this time for us to estimate either the timing or outcome of any of those matters, or whether any potential
loss, or range of potential losses, is probable or reasonably estimable.
The Company and certain current and former directors and officers are defendants in a case captioned In re Chemed Corp. Shareholder Derivative Litigation, No. 13 Civ. 1854 (LPS) (CJB) (D. Del.), which was consolidated on February 2, 2015.
On February 2, 2015, the Court appointed KBC Asset Management NV the sole lead plaintiff and its counsel, the sole lead
and liaison counsel. On March 3, 2015, Lead Plaintiff KBC designated its Complaint as the operative complaint in the consolidated proceedings and defendants renewed a previously filed motion to dismiss those claims and allegations. The
consolidated Complaint named fourteen individual defendants, together with the Company as nominal defendant. The Complaint alleges a claim for breach of fiduciary duty against the individual defendants for allegedly permitting the Company to
submit false claims to the U.S. government. The Complaint seeks (a) a declaration that the individual defendants breached their fiduciary duties to the Company; (b) an order requiring those defendants to pay compensatory damages, restitution and
exemplary damages, in unspecified amounts, to the Company; (c) an order directing the Company to implement new policies and procedures; and (d) costs and disbursements incurred in bringing the action, including attorneys’ fees. On May 12, 2016,
the Court issued a Memorandum Order granting Chemed’s motion to dismiss, and dismissing Lead Plaintiff KBC’s Complaint without prejudice to KBC’s opportunity to file within 30 days of the date of the Court’s Order (i.e., by June 13, 2016) an
amended Complaint addressing the deficiencies in its duty of loyalty claim. Lead Plaintiff KBC did not file an amended Complaint within the time specified by the Court.
However, on June 13, 2016, counsel for Chemed shareholder Michael Kvint filed a letter with the Court requesting a
two-week extension to file a motion to substitute Mr. Kvint as lead plaintiff, in place of Lead Plaintiff KBC and to file an amended Complaint. Alternatively, counsel for Mr. Kvint requested that any dismissal of the action be with prejudice to
KBC only. On June 14, 2016, Chemed filed a reply letter with the Court, reserving its rights to oppose any motion filed by Mr. Kvint and, if warranted, to oppose any other actions taken by Mr. Kvint to proceed with the action (including by filing
an untimely amended Complaint). On June 21, 2016, the Court entered an Oral Order providing Mr. Kvint until June 30, 2016 to file a Motion to Substitute and Motion for Leave to File an Amended Complaint. On that date, Mr. Kvint filed, under seal,
a Motion to Substitute Plaintiff and File Amended Complaint, and attached a Proposed Amended Complaint. Mr. Kvint’s motion was fully briefed by the parties. On April 25, 2017, Magistrate Judge Burke issued a Report and Recommendation recommending
that the Court permit Mr. Kvint to intervene as Lead Plaintiff and grant leave to amend the complaint to replead the duty of loyalty claim only. On May 16, 2017, Chief Judge Stark signed an Order adopting that Report and Recommendation. Plaintiff
Kvint filed a Corrected Amended Complaint on May 30, 2017. On September 13, 2017, the Court entered an order dismissing with prejudice the claims against defendants Timothy S. O’Toole and Joel F. Gemunder and permitting Defendants to file a Motion
to Dismiss the Corrected Amended Complaint. The matter has been fully briefed and argued. On February 26, 2019, Magistrate Judge Burke issued a Report and
Recommendations recommending that Defendants' motion to dismiss the amended complaint be granted. Any objections to that Report are due March 12, 2019, and any responses thereto are due March 26, 2019. The Court will then decide whether to accept the Report and Recommendations. As the Company has previously disclosed, the legal fees and costs associated with defending against this lawsuit
are presently being paid by insurance. For additional procedural history of this litigation, please refer to our prior quarterly and annual filings.
On October 30, 2017, the Company entered into a settlement agreement (the “Settlement Agreement”) to resolve civil
litigation under the False Claims Act brought by the United States Department of Justice (“DOJ”) on behalf of the OIG and various relators concerning VITAS, filed in the U.S. District Court of the Western District of Missouri (the “2013 Action”).
The Company denied any violation of law and agreed to settlement without admission of wrongdoing.
In connection with the settlement VITAS and certain of its subsidiaries entered into a corporate integrity agreement
(“CIA”) on October 30, 2017. The CIA formalizes various aspects of VITAS’ already existing Compliance Program and contains requirements designed to document compliance with federal healthcare program requirements. It has a term of five years
during which it imposes monitoring, reporting, certification, oversight, screening and training obligations, certain of which had previously been implemented by VITAS. It also requires VITAS to engage an Independent Review Organization to perform
audit and review functions and to prepare reports regarding compliance with federal healthcare programs. In the event of breach of the CIA, VITAS could become liable for payment of stipulated penalties or could be excluded from participation in
federal healthcare programs.
Under the Settlement Agreement, the United States agreed to release the Company, VITAS, and its hospice operation
subsidiaries from any civil or administrative monetary liability relating to any patients’ disputed terminal medical prognosis of six months or less; a lack of medical necessity for billed Continuous Home Care, General Inpatient Care, or Respite
Care levels of hospice care; or that the claims for those levels of hospice care were not eligible for payment for any other reason. The OIG agreed, conditioned on the Company’s full payment and in consideration of VITAS’ obligations under the
CIA, to release its permissive exclusion rights and refrain from instituting any administrative action seeking to exclude the Company, VITAS, and its affiliates from participating in Medicare, Medicaid, or other federal healthcare programs in this
regard.
Under the Settlement Agreement, the Company paid $75 million plus interest, plus certain attorney fees and expenses of qui
tam relators. The Company made these payments during the fourth quarter of 2017. The Company previously recorded a $90 million loss reserve ($55.8 million after-tax) related to the Settlement Agreement, and associated costs in the second quarter
of 2017. During the fourth quarter of 2017, approximately $5.5 million ($3.4 million after-tax) recorded as part of the $90 million was reversed as relator attorney' fees were less than originally estimated.
The costs incurred related to U.S. v. VITAS and related regulatory matters, exclusive of the settlement were $5.2 million
and $5.3 million for 2017, and 2016 respectively.
Jordan Seper (“Seper”), a Registered Nurse at VITAS’ Inland Empire program from May 12, 2014 to March 21, 2015, filed a
lawsuit in San Francisco Superior Court on September 26, 2016. She alleged VITAS Healthcare Corp of CA (“VITAS CA”) (1) failed to provide minimum wage for all hours worked; (2) failed to provide overtime for all hours worked; (3) failed to provide
a second meal period; (4) failed to provide rest breaks; (5) failed to indemnify for necessary expenditures; (6) failed to timely pay wages due at time of separation; and (7) engaged in unfair business practices. Seper seeks a state-wide class
action of current and former non-exempt employees employed with VITAS in California within the four years preceding the filing of the lawsuit. She seeks court determination that this action may be maintained as a class action for the entire
California class and subclasses, designation as class representative, declaratory relief, injunctive relief, damages (including wages for regular or overtime hours allegedly worked but not paid, premium payments for missed meal or rest periods, and
unreimbursed expenses), all applicable penalties associated with each claim, pre and post-judgment interest, and attorneys’ fees and costs. Seper served VITAS CA with the lawsuit, Jordan A. Seper on behalf of herself and others similarly situated v. VITAS Healthcare Corporation of California, a Delaware corporation; VITAS Healthcare Corp of CA, a business entity unknown; and DOES 1 to
100, inclusive; Los Angeles Superior Court Case Number BC 642857 on October 13, 2016 (“Jordan Seper case”).
On November 14, 2016, the Parties filed a Stipulation to transfer the venue of the lawsuit from San Francisco to Los
Angeles. The Los Angeles Superior Court Complex Division accepted transfer of the case on December 6, 2016 and stayed the case. On December 16, 2016, VITAS CA filed its Answer and served written discovery on Seper.
Jiwann Chhina (“Chhina”), hired by VITAS as a Home Health Aide on February 5, 2002, is currently a Licensed Vocational
Nurse for VITAS’ San Diego program. On September 27, 2016, Chhina filed a lawsuit in San Diego Superior Court, alleging (1) failure to pay minimum wage for all hours worked; (2) failure to provide overtime for all hours worked; (3) failure to pay
wages for all hours at the regular rate; (4) failure to provide meal periods; (5) failure to provide rest breaks; (6) failure to provide complete and accurate wage statements; (7) failure to pay for all reimbursement expenses; (8) unfair business
practices; and (9) violation of the California Private Attorneys General Act. Chhina seeks to pursue these claims in the form of a state-wide class action of current and former non-exempt employees employed with VITAS in California within the four
years preceding the filing of the lawsuit. He seeks court determination that this action may be maintained as a class action for the entire California class and subclasses, designation as class representative, declaratory relief, injunctive
relief, damages (including wages for regular or overtime hours allegedly worked but not paid, premium payments for missed meal or rest period, and unreimbursed expenses), all applicable penalties associated with each claim, pre-judgment interest,
and attorneys’ fees and costs. Chhina served VITAS CA with the lawsuit, Jiwan Chhina v. VITAS Health Services of California, Inc., a California
corporation; VITAS Healthcare Corporation of California, a Delaware corporation; VITAS Healthcare Corporation of California, a Delaware corporation dba VITAS Healthcare Inc.; and DOES 1 to 100, inclusive; San Diego Superior Court Case
Number 37-2015-00033978-CU-OE-CTL on November 3, 2016 (“Jiwann Chhina case”). On December 1, 2016, VITAS CA filed its Answer and served written discovery on Chhina.
On May 19, 2017, Chere Phillips (a Home Health Aide in Sacramento) and Lady Moore (a former Social Worker in Sacramento)
filed a lawsuit against VITAS CA in Sacramento County Superior Court, alleging claims for (1) failure to pay all wages due; (2) failure to authorize and permit rest periods; (3) failure to provide off-duty meal periods; (4) failure to furnish
accurate wage statements; (5) unreimbursed business expenses; (6) waiting time penalties; (7) violations of unfair competition law; and (8) violation of the Private Attorneys General Act. The case is captioned: Chere Phillips and Lady Moore v. VITAS Healthcare Corporation of California, Sacramento County Superior Court, Case No. 34-2017-0021-2755. Plaintiffs sought to pursue
these claims in the form of a state-wide class action of current and former non-exempt employees employed with VITAS CA in California within the four years preceding the filing of the lawsuit. Plaintiffs served VITAS with the lawsuit on June 5,
2017. VITAS CA timely answered the Complaint generally denying the Plaintiffs’ allegations. The Court has stayed all class discovery in this case pending resolution of mediation in the Jordan Seper and Jiwann Chhina cases.
There are currently three other lawsuits against VITAS pending in the superior courts of other California counties that
contain claims and class periods that substantially overlap with Phillips’ and Moore’s claims: the Jordan Seper and Jiwann Chhina cases, and Williams
v. VITAS Healthcare Corporation of California, filed on May 22, 2017 in Alameda County Superior Court, RG 17853886.
Jazzina Williams’ (a Home Health Aide in Sacramento) lawsuit alleges claims for (1) failure to pay all wages due; (2)
failure to authorize and permit rest periods; (3) failure to provide off-duty meal periods; (4) failure to furnish accurate wage statements; (5) unreimbursed business expenses; (6) waiting time penalties; and (7) violations of the Private Attorneys
General Act (“PAGA”). Williams seeks to pursue these claims both individually and as a representative action under the PAGA on behalf of current and former California non-exempt employees. Plaintiff served VITAS with the lawsuit on May 31, 2017.
VITAS CA timely answered the Complaint generally denying Plaintiff’s allegations. Williams is pursing discovery of her individual claim and has agreed to a stay of class discovery pending possible resolution through ongoing mediation in the Jordan
Seper and Jiwann Chhina cases. Defendant filed and served each of Plaintiffs Williams, Phillips, and Moore with a Notice of Related Cases on July 19, 2017.
The Jordan Seper
and Jiwann Chhina cases have been consolidated in Los Angeles County Superior court; Chhina was dismissed as a separate action and joined with Seper through the filing of an
amended complaint in Seper in which Chhina
is also identified as a named plaintiff, on August 28, 2018.
Alfred Lax (“Lax”), a current employee of Roto-Rooter Services Company (“RRSC”), was hired in the RRSC’s Menlo Park branch
in 2007. On November 30, 2018, Lax filed a class action lawsuit in Santa Clara County Superior Court alleging (1) failure to provide or compensate for required rest breaks; (2) failure to properly pay for all hours worked; (3) failure to provide
accurate wage statements; (4) failure to reimburse for work-related expenses; and (5) unfair business practices. Lax has stated these claims as a representative of a class defined as all service technicians employed by RRSC in California during
the four years preceding the filing of the complaint. He seeks a determination that the action may proceed and be maintained as a class action and for compensatory and statutory damages (premium payments for missed rest periods, uncompensated rest
periods, wages for time allegedly not paid such as travel time, repair time, and vehicle maintenance time, and unreimbursed expenses), penalties and restitutions, pre- and post-judgement interest and attorneys’ fees and costs. The lawsuit, Alfred Lax, on behalf of himself and all others similarly situated v. Roto-Rooter Services Company, and Does 1 through 50 inclusive; Santa Clara
County Superior Court Case Number 18CV338652, was received by RRSC on December 11, 2018 and RRSC timely filed its answer denying the claims.
The Company is not able to reasonably estimate the probability of loss or range of loss for any of these lawsuits at this
time.
The Company intends to defend vigorously against the allegations in each of the above lawsuits. Regardless of the outcome
of any of the preceding matters, dealing with the various regulatory agencies and opposing parties can adversely affect us through defense costs, potential payments, diversion of management time, and related publicity. Although the Company intends
to defend them vigorously, there can be no assurance that those suits will not have a material adverse effect on the Company.
CONTRACTUAL OBLIGATIONS
The table below summarizes our debt and contractual obligations as of December 31, 2018 (in thousands):
| |
|
|
|
|
Less than
|
|
|
|
|
|
|
|
|
After
|
|
| |
|
Total
|
|
|
1 year
|
|
|
1-3 Years
|
|
|
4 -5 Years
|
|
|
5 Years
|
|
|
Long-term debt obligations (a)
|
|
$
|
89,200
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
$
|
89,200
|
|
|
$
|
-
|
|
|
Interest on long-term debt
|
|
|
13,929
|
|
|
|
3,095
|
|
|
|
6,190
|
|
|
|
4,644
|
|
|
|
-
|
|
|
Operating lease obligations
|
|
|
103,616
|
|
|
|
26,791
|
|
|
|
43,821
|
|
|
|
22,030
|
|
|
|
10,974
|
|
|
Purchase obligations (b)
|
|
|
50,150
|
|
|
|
50,150
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Other long-term obligations (c)
|
|
|
77,704
|
|
|
|
-
|
|
|
|
9,816
|
|
|
|
3,272
|
|
|
|
64,616
|
|
|
Total contractual cash obligations
|
|
$
|
334,599
|
|
|
$
|
80,036
|
|
|
$
|
59,827
|
|
|
$
|
119,146
|
|
|
$
|
75,590
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) Represents the face value of the obligation.
|
|
|
(b) Purchase obligations consist of accounts payable at December 31, 2018.
|
|
|
(c) Other long-term obligations comprise largely excess benefit obligations.
|
|
RESULTS OF OPERATIONS
2018 Versus 2017 – Consolidated Results
Set forth below are the year-to-year changes in the components of the statement of operations relating to income for 2018
versus 2017 (in thousands, except percentages):
| |
|
Favorable/(Unfavorable)
|
|
| |
|
Amount
|
|
|
Percent
|
|
|
Service revenues and sales
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
49,302
|
|
|
|
4
|
|
|
Roto-Rooter
|
|
|
66,622
|
|
|
|
13
|
|
|
Total
|
|
|
115,924
|
|
|
|
7
|
|
|
Cost of services provided and goods sold
|
|
|
(78,112
|
)
|
|
|
(7
|
)
|
|
Selling, general and administrative expenses
|
|
|
6,443
|
|
|
|
2
|
|
|
Depreciation
|
|
|
(2,976
|
)
|
|
|
(8
|
)
|
|
Amortization
|
|
|
(262
|
)
|
|
|
(191
|
)
|
|
Other operating expenses
|
|
|
89,580
|
|
|
|
99
|
|
|
Income from operations
|
|
|
130,597
|
|
|
|
116
|
|
|
Interest expense
|
|
|
(718
|
)
|
|
|
(17
|
)
|
|
Other income - net
|
|
|
(7,196
|
)
|
|
|
(88
|
)
|
|
Income before income taxes
|
|
|
122,683
|
|
|
|
105
|
|
|
Income taxes
|
|
|
(15,316
|
)
|
|
|
(82
|
)
|
|
Net income
|
|
$
|
107,367
|
|
|
|
109
|
|
The VITAS segment revenue increase is the result of the following (dollars in thousands):
| |
|
2018
|
|
|
2017
|
|
|
Routine homecare
|
|
$
|
1,010,518
|
|
|
$
|
935,913
|
|
|
Continuous care
|
|
|
122,498
|
|
|
|
124,557
|
|
|
Inpatient care
|
|
|
82,677
|
|
|
|
90,472
|
|
|
Other
|
|
|
7,831
|
|
|
|
-
|
|
|
Medicare cap adjustment
|
|
|
(4,123
|
)
|
|
|
(2,682
|
)
|
|
Implicit price concessions
|
|
|
(11,785
|
)
|
|
|
-
|
|
|
Room and board, net
|
|
|
(10,054
|
)
|
|
|
-
|
|
|
Net revenue
|
|
$
|
1,197,562
|
|
|
$
|
1,148,260
|
|
Days of care changed as follows:
| |
|
Days of Care
|
|
Increase/(Decrease)
|
| |
|
2018
|
|
|
2017
|
|
Percent
|
| |
|
|
|
|
|
|
|
|
Routine homecare
|
|
6,192,858
|
|
|
5,743,414
|
|
8
|
|
Continuous care
|
|
169,828
|
|
|
171,395
|
|
(1)
|
|
General inpatient
|
|
113,453
|
|
|
125,971
|
|
(10)
|
|
Total days of care
|
|
6,476,139
|
|
|
6,040,780
|
|
7
|
The remaining increase in VITAS’ revenues for 2018 versus 2017 was primarily comprised of a geographically weighted
average Medicare reimbursement rate increase of approximately 1.1%, offset by $4.1 million in Medicare cap liability compared to $2.7 million contra-revenue in the same period of 2017 and $21.8 million change in classification of implicit price
concessions and room and board, net related to the adoption of the new revenue recognition standard.
The Roto-Rooter segment revenue increase is the result of the following (dollars in thousands):
| |
|
2018
|
|
|
2017
|
|
| |
|
|
|
|
|
|
|
Short-term core service jobs
|
|
$
|
421,790
|
|
|
$
|
373,579
|
|
|
Water restoration
|
|
|
101,784
|
|
|
|
82,272
|
|
|
Contractor revenue
|
|
|
50,093
|
|
|
|
43,770
|
|
|
Franchise Fees
|
|
|
6,382
|
|
|
|
6,130
|
|
|
All other
|
|
|
11,958
|
|
|
|
12,713
|
|
|
Implicit price concessions and credit memos
|
|
|
(6,921
|
)
|
|
|
-
|
|
| |
|
$
|
585,086
|
|
|
$
|
518,464
|
|
Short-term core service revenue increased 12.9% as a result of an 8.8% increase in price and service mix shift as well as
a 4.1% increase in the number of jobs performed. Water restoration revenue increased 23.3% as a result of a 16.3% increase in the number of jobs performed and a 7.0% increase in price and service mix shift. Contractor operations increased 14.4%
due mainly to their continued expansion into water restoration. Revenue was negatively impacted by the change in the classification of implicit price concessions and credit memos from selling, general and administrative expenses of $6.9 million
due to the adoption of the new revenue recognition standard.
The consolidated gross margin excluding depreciation was 31.1% in 2018 versus 31.0% in 2017. On a segment basis, VITAS’
gross margin excluding depreciation was 22.4% in 2018 and 22.8% in 2017. Roto-Rooter’s gross margin excluding depreciation was 48.8% in 2018 and 49.0% in 2017.
Selling, general and administrative expenses (“SG&A”) for 2018 and 2017 comprise (in thousands):
| |
|
2018
|
|
|
2017
|
|
|
SG&A expenses before long-term incentive
|
|
|
|
|
|
|
|
compensation, OIG expenses and the impact
|
|
|
|
|
|
|
|
of market gains of deferred compensation plans
|
|
$
|
263,304
|
|
|
$
|
258,034
|
|
|
Long-term incentive compensation
|
|
|
6,618
|
|
|
|
4,994
|
|
|
Impact of market value gains on liabilities
|
|
|
|
|
|
|
|
|
|
held in deferred compensation trusts
|
|
|
287
|
|
|
|
8,430
|
|
|
Expenses related to OIG investigation
|
|
|
-
|
|
|
|
5,194
|
|
|
Total SG&A expenses
|
|
$
|
270,209
|
|
|
$
|
276,652
|
|
SG&A expenses before long-term incentive compensation, expenses related to OIG investigation and the impact of market
value adjustments related to deferred compensation trusts for 2018 were up 2.0% when compared to 2017. This increase was mainly a result of the increase in variable selling expenses caused by increased revenue and increased advertising expense at
Roto-Rooter offset by $18.7 million of implicit price concessions being classified in revenue versus selling, general and administrative expenses due to the new revenue recognition standard.
Other operating expense for 2018 and 2017 comprise (in thousands):
| |
|
2018
|
|
|
2017
|
|
| |
|
|
|
|
|
|
|
Litigation settlements
|
|
$
|
796
|
|
|
$
|
84,476
|
|
|
Loss on disposal of property and equipment
|
|
|
504
|
|
|
|
5,266
|
|
|
Program closure expenses
|
|
|
-
|
|
|
|
1,138
|
|
|
Total other operating expenses
|
|
$
|
1,300
|
|
|
$
|
90,880
|
|
During 2017, the Company recorded $84.5 million related to the Settlement Agreement and a related qui tam case. See
footnote 17 for further discussion. The Company recorded $5.3 million related to the loss on the sale of transportation equipment. Also during 2017, the Company recorded $1.1 million related to the closure of three Alabama programs at VITAS.
Other income-net for 2018 and 2017 comprise (in thousands):
| |
|
2018
|
|
|
2017
|
|
| |
|
|
|
|
|
|
|
Interest income
|
|
$
|
671
|
|
|
$
|
427
|
|
|
Market value gains on assets held in deferred
|
|
|
|
|
|
|
|
|
|
compensation trusts
|
|
|
287
|
|
|
|
8,430
|
|
|
Other
|
|
|
-
|
|
|
|
(703
|
)
|
|
Total other income
|
|
$
|
958
|
|
|
$
|
8,154
|
|
Our effective tax rate reconciliation is as follows:
| |
|
2018
|
|
|
2017
|
|
| |
|
|
|
|
|
|
|
Income tax provision calculated using the statutory rate
|
|
$
|
50,316
|
|
|
$
|
40,921
|
|
|
Stock compensation tax benefits
|
|
|
(22,862
|
)
|
|
|
(18,932
|
)
|
|
State and local income taxes, less federal income tax effect
|
|
|
7,150
|
|
|
|
4,600
|
|
|
Nondeductible expenses
|
|
|
2,280
|
|
|
|
1,041
|
|
|
Enactment of the tax reform act
|
|
|
-
|
|
|
|
(8,305
|
)
|
|
Other--net
|
|
|
(2,828
|
)
|
|
|
(585
|
)
|
|
Income tax provision
|
|
$
|
34,056
|
|
|
$
|
18,740
|
|
|
Effective tax rate
|
|
|
14.2
|
%
|
|
|
16.0
|
%
|
Net income for both periods include the following aftertax adjustments that increased/ (reduced) aftertax earnings (in
thousands):
| |
|
2018
|
|
|
2017
|
|
|
VITAS
|
|
|
|
|
|
|
|
Medicare cap sequestration adjustment
|
|
$
|
(1,114
|
)
|
|
$
|
(276
|
)
|
|
Expenses related to litigation settlements
|
|
|
(594
|
)
|
|
|
(52,375
|
)
|
|
Acquisition expenses
|
|
|
(156
|
)
|
|
|
-
|
|
|
Impact of tax reform
|
|
|
-
|
|
|
|
11,057
|
|
|
Costs associated with the OIG investigation
|
|
|
-
|
|
|
|
(3,207
|
)
|
|
Program closure expenses
|
|
|
-
|
|
|
|
(675
|
)
|
|
Roto-Rooter
|
|
|
|
|
|
|
|
|
|
Acquisition expenses
|
|
|
(403
|
)
|
|
|
-
|
|
|
Impact of tax reform
|
|
|
-
|
|
|
|
7,761
|
|
|
Expenses related to litigation settlements
|
|
|
-
|
|
|
|
(129
|
)
|
|
Corporate
|
|
|
|
|
|
|
|
|
|
Excess tax benefits on stock compensation
|
|
|
22,862
|
|
|
|
18,932
|
|
|
Stock option expense
|
|
|
(10,118
|
)
|
|
|
(6,892
|
)
|
|
Long-term incentive compensation
|
|
|
(5,307
|
)
|
|
|
(3,243
|
)
|
|
Impact of tax reform
|
|
|
-
|
|
|
|
(10,516
|
)
|
|
Loss on sale of transportation equipment
|
|
|
-
|
|
|
|
(3,314
|
)
|
|
Total
|
|
$
|
5,170
|
|
|
$
|
(42,877
|
)
|
2018 Versus 2017 – Segment Results
Net income/(loss) for 2018 versus 2017 (in thousand):
| |
|
2018
|
|
|
2017
|
|
|
VITAS
|
|
$
|
138,846
|
|
|
$
|
57,645
|
|
|
Roto-Rooter
|
|
|
98,711
|
|
|
|
73,299
|
|
|
Corporate
|
|
|
(32,013
|
)
|
|
|
(32,767
|
)
|
| |
|
$
|
205,544
|
|
|
$
|
98,177
|
|
VITAS’ after-tax earnings were increased as the result the result of higher revenues and a lower effective tax rate.
Additionally, VITAS settled a lawsuit in 2017 for $55.8 million (after-tax) which did not recur in 2018.
Roto-Rooter’s net income was positively impacted in 2018 compared to 2017 primarily by an increase in revenue as well as a
reduced effective tax rate.
After-tax Corporate expenses for 2018 decreased by 2.3% when compared to 2017 by a $3.9 million increase in tax benefit
related to the adoption of ASU No. 2016-09 offset by increased stock compensation expense, and a lower effective tax rate (results in a lower tax benefit).
2017 Versus 2016 – Consolidated Results
Set forth below are the year-to-year changes in the components of the statement of operations relating to income for 2017
versus 2016 (in thousands, except percentages):
| |
|
Favorable/(Unfavorable)
|
|
| |
|
Amount
|
|
|
Percent
|
|
|
Service revenues and sales
|
|
|
|
|
|
|
|
VITAS
|
|
$
|
24,943
|
|
|
|
2
|
|
|
Roto-Rooter
|
|
|
64,900
|
|
|
|
14
|
|
|
Total
|
|
|
89,843
|
|
|
|
6
|
|
|
Cost of services provided and goods sold
|
|
|
(35,101
|
)
|
|
|
(3
|
)
|
|
Selling, general and administrative expenses
|
|
|
(33,080
|
)
|
|
|
(14
|
)
|
|
Depreciation
|
|
|
(1,209
|
)
|
|
|
(4
|
)
|
|
Amortization
|
|
|
222
|
|
|
|
62
|
|
|
Other operating expenses
|
|
|
(86,389
|
)
|
|
|
(1,924
|
)
|
|
Income from operations
|
|
|
(65,714
|
)
|
|
|
(37
|
)
|
|
Interest expense
|
|
|
(557
|
)
|
|
|
(15
|
)
|
|
Other income - net
|
|
|
6,134
|
|
|
|
304
|
|
|
Income before income taxes
|
|
|
(60,137
|
)
|
|
|
(34
|
)
|
|
Income taxes
|
|
|
49,571
|
|
|
|
73
|
|
|
Net income
|
|
$
|
(10,566
|
)
|
|
|
(10
|
)
|
The VITAS segment revenue increase is the result of the following (dollars in thousands):
| |
|
2017
|
|
|
2016
|
|
|
Routine homecare
|
|
$
|
935,913
|
|
|
$
|
887,940
|
|
|
Continuous care
|
|
|
124,557
|
|
|
|
138,025
|
|
|
Inpatient care
|
|
|
90,472
|
|
|
|
97,580
|
|
|
Medicare cap adjustment
|
|
|
(2,682
|
)
|
|
|
(228
|
)
|
|
Net revenue
|
|
$
|
1,148,260
|
|
|
$
|
1,123,317
|
|
Net Medicare reimbursement rates increased 1.3% in 2017 as compared to 2016.
Days of care change as follows:
| |
Days of Care
|
|
Increase/(Decrease)
|
| |
|
2017
|
|
|
2016
|
|
Percent
|
| |
|
|
|
|
|
|
|
|
Routine homecare
|
|
5,743,414
|
|
|
5,518,002
|
|
4
|
|
Continuous care
|
|
171,395
|
|
|
188,657
|
|
(9)
|
|
General inpatient
|
|
125,971
|
|
|
146,516
|
|
(14)
|
|
Total days of care
|
|
6,040,780
|
|
|
5,853,175
|
|
3
|
The Roto-Rooter segment revenue increase is the result of the following (dollars in thousands):
| |
|
2017
|
|
|
2016
|
|
| |
|
|
|
|
|
|
|
Short-term core service jobs
|
|
$
|
373,579
|
|
|
$
|
345,638
|
|
|
Water restoration
|
|
|
82,272
|
|
|
|
50,229
|
|
|
Contractor revenue
|
|
|
43,770
|
|
|
|
40,097
|
|
|
Franchise Fees
|
|
|
6,130
|
|
|
|
5,090
|
|
|
All other
|
|
|
12,713
|
|
|
|
12,510
|
|
| |
|
$
|
518,464
|
|
|
$
|
453,564
|
|
Short-term core service jobs increased 9.0% due to price and service mix shift offset by a 0.9% decrease in job count.
Water restoration increased 63.8% as a result of continued expansion of this service offering. There was an increase in water restoration jobs of 30.8% between years.
The consolidated gross margin excluding depreciation was 31.0% in 2017 versus 29.3% in 2016. On a segment basis, VITAS’
gross margin excluding depreciation was 22.8% in 2017 and 21.8% in 2016. The increase in VITAS’ gross margin is the result of mix shift to higher margin care, labor and ancillary cost management. Roto-Rooter’s gross margin excluding depreciation
was 49.0% in 2017 and 47.7% in 2016. The increase in Roto-Rooter gross margin is the result mainly of higher revenues, particularly in water restoration, with relatively low increases in branch level fixed costs.
Selling, general and administrative expenses (“SG&A”) for 2017 and 2016 comprise (in thousands):
| |
|
2017
|
|
|
2016
|
|
|
SG&A expenses before long-term incentive
|
|
|
|
|
|
|
|
compensation, OIG expenses and the impact
|
|
|
|
|
|
|
|
of market gains of deferred compensation plans
|
|
$
|
258,034
|
|
|
$
|
234,321
|
|
|
Long-term incentive compensation
|
|
|
4,994
|
|
|
|
1,930
|
|
|
Expenses related to OIG investigation
|
|
|
5,194
|
|
|
|
5,260
|
|
|
Impact of market value gains on liabilities
|
|
|
|
|
|
|
|
|
|
held in deferred compensation trusts
|
|
|
8,430
|
|
|
|
2,061
|
|
|
Total SG&A expenses
|
|
$
|
276,652
|
|
|
$
|
243,572
|
|
SG&A expenses before long-term incentive compensation, OIG expenses and the impact of market gains of deferred
compensation plans increased $23.7 million (10.1%) from 2016 to 2017. This increase was mainly a result of the increase in variable expenses caused by increased revenue, increased advertising expense at Roto-Rooter and normal inflationary
increases in 2017.
Other operating expense was $90.9 million in 2017. This was due to an $84.5 million litigation settlement, $5.3 million
related to the loss on the sale of transportation equipment and $1.1 million related to the closure of the programs in one state at Vitas. This is compared to a $4.5 million payment of early retirement expenses during 2016.
Other income-net for 2017 and 2016 comprise (in thousands):
| |
|
2017
|
|
|
2016
|
|
|
Market value gains on assets held in deferred
|
|
|
|
|
|
|
|
compensation trusts
|
|
$
|
8,430
|
|
|
$
|
2,061
|
|
|
Loss on disposal of property and equipment
|
|
|
(707
|
)
|
|
|
(424
|
)
|
|
Interest income
|
|
|
427
|
|
|
|
383
|
|
|
Other
|
|
|
4
|
|
|
|
-
|
|
|
Total other income
|
|
$
|
8,154
|
|
|
$
|
2,020
|
|
Our effective tax rate reconciliation is as follows:
| |
|
2017
|
|
|
2016
|
|
| |
|
|
|
|
|
|
|
Income tax provision calculated using the statutory rate of 35%
|
|
$
|
40,921
|
|
|
$
|
61,969
|
|
|
State and local income taxes, less federal income tax effect
|
|
|
4,600
|
|
|
|
6,044
|
|
|
Nondeductible expenses
|
|
|
1,041
|
|
|
|
881
|
|
|
Stock compensation tax benefits
|
|
|
(18,932
|
)
|
|
|
-
|
|
|
Enactment of the tax reform act
|
|
|
(8,305
|
)
|
|
|
-
|
|
|
Other--net
|
|
|
(585
|
)
|
|
|
(583
|
)
|
|
Income tax provision
|
|
$
|
18,740
|
|
|
$
|
68,311
|
|
|
Effective tax rate
|
|
|
16.0
|
%
|
|
|
38.6
|
%
|
Net income for both periods include the following aftertax adjustments that increased/ (reduced) aftertax earnings (in
thousands):
| |
|
2017
|
|
|
2016
|
|
|
VITAS
|
|
|
|
|
|
|
|
Expenses related to litigation settlements
|
|
$
|
(52,375
|
)
|
|
$
|
-
|
|
|
Impact of tax reform
|
|
|
11,057
|
|
|
|
(3,248
|
)
|
|
Costs associated with the OIG investigation
|
|
|
(3,207
|
)
|
|
|
-
|
|
|
Program closure expenses
|
|
|
(675
|
)
|
|
|
-
|
|
|
Medicare cap sequestration adjustment
|
|
|
(276
|
)
|
|
|
(141
|
)
|
|
Early retirement expenses
|
|
|
-
|
|
|
|
(2,840
|
)
|
|
Roto-Rooter
|
|
|
|
|
|
|
|
|
|
Impact of tax reform
|
|
|
7,761
|
|
|
|
-
|
|
|
Expenses related to litigation settlements
|
|
|
(129
|
)
|
|
|
(28
|
)
|
|
Corporate
|
|
|
|
|
|
|
|
|
|
Excess tax benefits on stock compensation
|
|
|
18,932
|
|
|
|
-
|
|
|
Impact of tax reform
|
|
|
(10,516
|
)
|
|
|
-
|
|
|
Stock option expense
|
|
|
(6,892
|
)
|
|
|
(5,266
|
)
|
|
Loss on sale of transportation equipment
|
|
|
(3,314
|
)
|
|
|
-
|
|
|
Long-term incentive compensation
|
|
|
(3,243
|
)
|
|
|
(1,221
|
)
|
|
Total
|
|
$
|
(42,877
|
)
|
|
$
|
(12,744
|
)
|
2017 Versus 2016 – Segment Results
Net income/(loss) for 2017 versus 2016 (in thousands):
| |
|
2017
|
|
|
2016
|
|
|
VITAS
|
|
$
|
57,645
|
|
|
$
|
84,961
|
|
|
Roto-Rooter
|
|
|
73,299
|
|
|
|
52,893
|
|
|
Corporate
|
|
|
(32,767
|
)
|
|
|
(29,111
|
)
|
| |
|
$
|
98,177
|
|
|
$
|
108,743
|
|
VITAS’ after-tax earnings were negatively impacted in 2017 compared to 2016 by a $52.4 million after-tax litigation
settlement as well as $675,000 after-tax related to the closure of programs in one state offset by a $11.1 million dollar decrease in tax provision related to tax reform. After-tax earnings as a percent of revenue in 2017 were 5.0% as compared
to 7.6% in 2016.
Roto-Rooter’s after-tax earnings were positively impacted in 2017 compared to 2016 by a $32.0 million revenue increase in
Roto-Rooter’s water restoration line of business and a $22.2 million revenue increase in Roto-Rooter’s plumbing line of business as well as a $7.8 million dollar decrease in tax provision related to tax reform. After-tax earnings as a percent of
revenue at Roto-Rooter in 2017 were 14.1% as compared to 11.7% in 2016.
After-tax Corporate expenses for 2017 increased 12.6% when compared to 2016 due to increased long term incentive
compensation expense, the loss on the sale of transportation equipment and increase cash bonus expense in 2017 as well as a $10.5 million decrease in tax benefit related to tax reform offset by $18.9 million increase due to excess tax benefits on
stock compensation which are recorded to the tax provision starting in 2017 in accordance with ASU 2016-09.
CRITICAL ACCOUNTING POLICIES
Revenue Recognition
In May 2014, the FASB issued Accounting Standards Update “ASU No. 2014-09 – Revenue from Contracts with Customers.” The
standard and subsequent amendments are theoretically intended to develop a common revenue standard for removing inconsistencies and weaknesses, improve comparability, provide for more useful information to users through improved disclosure
requirements and simplify the preparation of financial statements. The standard is also referred to as Accounting Standards Codification No. 606 (“ASC606”). We adopted ASC 606 effective January 1, 2018. The required disclosures of ASC 606 and
impact of adoption are discussed below for each of our operating subsidiaries.
VITAS
Service revenue for VITAS is reported at the amount that reflects the ultimate consideration we expect to receive in
exchange for providing patient care. These amounts are due from third-party payors, primarily commercial health insurers and government programs (Medicare and Medicaid), and includes variable consideration for revenue adjustments due to
settlements of audits and reviews, as well as certain hospice-specific revenue capitations. Amounts are generally billed monthly or subsequent to patient discharge. Subsequent changes in the transaction price initially recognized are not
significant.
Hospice services are provided on a daily basis and the type of service provided is determined based on a physician’s
determination of each patient’s specific needs on that given day. Reimbursement rates for hospice services are on a per diem basis regardless
of the type of service provided or the payor. Reimbursement rates from government programs are established by the appropriate governmental agency and are standard across all hospice providers. Reimbursement rates from health insurers are
negotiated with each payor and generally structured to closely mirror the Medicare reimbursement model. The types of hospice services provided and associated reimbursement model for each are as follows:
Routine Home Care
occurs when a patient receives hospice care in their home, including a nursing home setting. The routine home care rate is paid for each day that a patient is in a hospice program and is not receiving one of the other categories of hospice care.
For Medicare patients, the routine home care rate reflects a two-tiered rate, with a higher rate for the first 60 days of a hospice patient’s care and a lower rate for days 61 and after. In addition, there is a Service Intensity Add-on payment
which covers direct home care visits conducted by a registered nurse or social worker in the last seven days of a hospice patient’s life, reimbursed up to four hours per day in fifteen minute increments at the continuous home care rate.
General Inpatient Care occurs
when a patient requires services in a controlled setting for a short period of time for pain control or symptom management which cannot be managed in other settings. General inpatient care services must be provided in a Medicare or Medicaid
certified hospital or long-term care facility or at a freestanding inpatient hospice facility with the required registered nurse staffing.
Continuous Home Care
is provided to patients while at home, including a nursing home setting, during periods of crisis when intensive monitoring and care, primarily nursing care, is required in order to achieve palliation or management of acute medical symptoms.
Continuous home care requires a minimum of 8 hours of care within a 24-hour day, which begins at midnight. The care must be predominantly nursing care provided by either a registered nurse or licensed nurse practitioner. While the published
Medicare continuous home care rates are daily rates, Medicare pays for continuous home care in fifteen minute increments. This fifteen minute rate is calculated by dividing the daily rate by 96.
Respite Care
permits a hospice patient to receive services on an inpatient basis for a short period of time in order to provide relief for the patient’s family or other caregivers from the demands of caring for the patient. A hospice can receive payment for
respite care for a given patient for up to five consecutive days at a time, after which respite care is reimbursed at the routine home care rate.
Each level of care represents a separate promise under the contract of care and is provided independently for each patient
contingent upon the patient’s specific medical needs as determined by a physician. However, the clinical criteria used to determine a patient’s level of care is consistent across all patients, given that, each patient is subject to the same payor
rules and regulations. As a result, we have concluded that each level of care is capable of being distinct and is distinct in the context of the contract. Furthermore, we have determined that each level of care represents a stand ready service
provided as a series of either days or hours of patient care. We believe that the performance obligations for each level of care meet criteria to be satisfied over time. VITAS recognizes revenue based on the service output. VITAS believes this
to be the most faithful depiction of the transfer of control of services as the patient simultaneously receives and consumes the benefits provided by our performance. Revenue is recognized on a daily or hourly basis for each patient in accordance
with the reimbursement model for each type of service. VITAS’ performance obligations relate to contracts with an expected duration of less than one year. Therefore, VITAS has elected to apply the optional exception provided in ASC 606 and is not
required to disclose the aggregate amount of the transaction price allocated to performance obligations that are unsatisfied or partially unsatisfied at the end of the reporting period. The unsatisfied or partially satisfied performance
obligations referred to above relate to bereavement services provided to patients’ families for at least 12 months after discharge.
Care is provided to patients regardless of their ability to pay. Patients who meet our criteria for charity care are
provided care without charge. There is no revenue or associated accounts receivable in the accompanying consolidated financial statements related to charity care. The cost of providing charity care during the years ended December 31, 2018, 2017
and 2016, was $8.2 million, $7.7 million and $7.0 million, respectively and is included in cost of services provided and goods sold. The cost of charity care is calculated by taking the ratio of charity care days to total days of care and
multiplying by total cost of care.
Generally, patients who are covered by third-party payors are responsible for related deductibles and coinsurance which vary
in amount. VITAS also provides service to patients without a reimbursement source and may offer those patients discounts from standard charges. VITAS estimates the transaction price for patients with deductibles and coinsurance, along with those
uninsured patients, based on historical experience and current conditions. The estimate of any contractual adjustments, discounts or implicit price concessions reduces the amount of revenue initially recognized. Subsequent changes to the estimate
of the transaction price are recorded as adjustments to patient service revenue in the period of change. Subsequent changes that are determined to be the result of an adverse change in the patients’ ability to pay (i.e. change in credit risk) are
recorded as bad debt expense. VITAS has no material adjustments related to subsequent changes in the estimate of the transaction price or subsequent changes as the result of an adverse change in the patient’s ability to pay for any period
reported.
Laws and regulations concerning government programs, including Medicare and Medicaid, are complex and subject to varying
interpretation. Compliance with such laws and regulations may be subject to future government review and interpretation. Additionally, the contracts we have with commercial health insurance payors provide for retroactive audit and review of
claims. Settlement with third party payors for retroactive adjustments due to audits, reviews or investigations are considered variable consideration and are included in the determination of the estimated transaction price for providing patient
care. The variable consideration is estimated based on the terms of the payment agreement, existing correspondence from the payor and our historical settlement activity. These estimates are adjusted in future periods, as new information becomes
available.
We are subject to certain limitations on Medicare payments for services which are considered variable consideration, as
follows:
Inpatient Cap. If
the number of inpatient care days any hospice program provides to Medicare beneficiaries exceeds 20% of the total days of hospice care such program provided to all Medicare patients for an annual period beginning September 28, the days in excess of
the 20% figure may be reimbursed only at the routine homecare rate. None of VITAS’ hospice programs exceeded the payment limits on inpatient services during the years ended December 31, 2018, 2017, and 2016.
Medicare Cap. We
are also subject to a Medicare annual per-beneficiary cap (“Medicare cap”). Compliance with the Medicare cap is measured in one of two ways based on a provider election. The “streamlined” method compares total Medicare payments received under a
Medicare provider number with respect to services provided to all Medicare hospice care beneficiaries in the program or programs covered by that Medicare provider number with the product of the per-beneficiary cap amount and the number of Medicare
beneficiaries electing hospice care for the first time from that hospice program or programs from September 28 through September 27 of the following year. At December 31, 2018 all our programs except one are using the “streamlined” method.
The “proportional” method compares the total Medicare payments received under a Medicare provider number with respect to
services provided to all Medicare hospice care beneficiaries in the program or programs covered by the Medicare provider number between September 28 and September 27 of the following year with the product of the per beneficiary cap amount and a
pro-rated number of Medicare beneficiaries receiving hospice services from that program during the same period. The pro-rated number of Medicare beneficiaries is calculated based on the ratio of days the beneficiary received hospice services during
the measurement period to the total number of days the beneficiary received hospice services.
We actively monitor each of our hospice programs, by provider number, as to their specific admission, discharge rate and
median length of stay data in an attempt to determine whether revenues are likely to exceed the annual per-beneficiary Medicare cap. Should we determine that revenues for a program are likely to exceed the Medicare cap based on projected trends, we
attempt to institute corrective actions, which include changes to the patient mix and increased patient admissions. However, should we project our corrective action will not prevent that program from exceeding its Medicare cap, we estimate revenue
recognized during the government fiscal year that will require repayment to the Federal government under the Medicare cap and record an adjustment to revenue of an amount equal to a ratable portion of our best estimate for the year.
In 2013, the U.S.
government implemented automatic budget reductions of 2.0% for all government payees, including hospice benefits paid under the Medicare program. In 2015, CMS determined that the Medicare cap should be calculated “as if” sequestration did not
occur. As a result of this decision, VITAS has received notification from our third-party intermediary that an additional $3.6 million is owed for Medicare cap in
three programs arising during the 2013 through 2018 measurement periods. The amounts are automatically deducted from our semi-monthly PIP payments. We do not believe that CMS is authorized under the sequestration authority or the statutory
methodology for establishing the Medicare cap to the amounts they have withheld and intend to withhold under their current “as if” methodology. We have appealed CMS’s methodology change.
During the year ended December 31, 2018, we recorded $4.1 million in Medicare cap revenue reduction
related to two programs’ 2018 measurement period liability and 2 programs’ projected 2019 measurement period liability.
During the year ended December 31, 2017, we recorded $2.4 million in Medicare cap revenue reduction
related to two programs’ projected 2018 measurement period liability and $247,000 for two programs’ cap liability for prior periods.
During the year ended December 31, 2016, we recorded $228,000 in Medicare cap revenue reduction
related to one programs’ projected 2015 measurement period liability.
For VITAS’ patients in the nursing home setting in which Medicaid pays the nursing home room and
board, VITAS serves as a pass-through between Medicaid and the nursing home. We are responsible for paying the nursing home for that patient’s room and board. Medicaid reimburses us for 95% of the amount we have paid. This results in a 5% net
expense for VITAS related to nursing home room and board. This transaction creates a performance obligation in that VITAS is facilitating room and board being delivered to our patient. As a result, the 5% net expense is recognized as a
contra-revenue account under ASC 606 in the accompanying financial statements.
The composition of patient care service revenue by payor and level of care for the year ended December 31, 2018 is as
follows (in thousands):
| |
|
Medicare
|
|
|
Medicaid
|
|
|
Commercial
|
|
|
Total
|
|
|
Routine home care
|
|
$
|
939,951
|
|
|
$
|
47,609
|
|
|
$
|
22,958
|
|
|
$
|
1,010,518
|
|
|
Continuous care
|
|
|
110,596
|
|
|
|
6,126
|
|
|
|
5,776
|
|
|
|
122,498
|
|
|
Inpatient care
|
|
|
69,354
|
|
|
|
8,156
|
|
|
|
5,167
|
|
|
|
82,677
|
|
| |
|
$
|
1,119,901
|
|
|
$
|
61,891
|
|
|
$
|
33,901
|
|
|
$
|
1,215,693
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All other revenue - self-pay, respite care, etc.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,831
|
|
|
Subtotal
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,223,524
|
|
|
Medicare cap adjustment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4,123
|
)
|
|
Implicit price concessions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(11,785
|
)
|
|
Room and board, net
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(10,054
|
)
|
|
Net revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,197,562
|
|
Roto-Rooter
Roto-Rooter provides plumbing, drain cleaning, water restoration and other related services to both residential and
commercial customers primarily in the United States. Services are provided through a network of company-owned branches, independent contractors and franchisees. Service revenue for Roto-Rooter is reported at the amount that reflects the ultimate
consideration we expect to receive in exchange for providing services.
Roto-Rooter owns and operates branches focusing mainly on large population centers in the United States. Roto-Rooter’s
primary lines of business in company-owned branches consist of plumbing, sewer and drain cleaning, excavation and water restoration. For purposes of ASC 606 analysis, plumbing, sewer and drain cleaning, and excavation have been combined into one
portfolio and are referred to as “short-term core services”. Water restoration is analyzed as a separate portfolio. The following describes the key characteristics of these portfolios:
Short-term Core Services
are plumbing, drain and sewer cleaning and excavation services. These services are provided to both commercial and residential customers. The duration of services provided in this category range from a few hours to a few days. There are no
significant warranty costs or on-going obligations to the customer once a service has been completed. For residential customers, payment is received at the time of job completion before the Roto-Rooter technician leaves the residence. Commercial
customers may be granted credit subject to internally designated authority limits and credit check guidelines. If credit is granted, payment terms are 30 days or less.
Each job in this category is a distinct service with a distinct performance obligation to the customer. Revenue is
recognized at the completion of each job. Variable consideration consists of pre-invoice discounts and post-invoice discounts. Pre-invoice discounts are given in the form of coupons or price concessions. Post-invoice discounts consist of credit
memos generally granted to resolve customer service issues. Variable consideration is estimated based on historical activity and recorded at the time service is completed.
Water Restoration
Services involve the remediation of water and humidity after a flood. These services are provided to both commercial and residential customers. The duration of services provided in this category generally ranges from 3 to 5 days.
There are no significant warranties or on-going obligations to the customer once service has been completed. The majority of these services are paid by the customer’s insurance company. Variable consideration relates primarily to allowances taken
by insurance companies upon payment. Variable consideration is estimated based on historical activity and recorded at the time service is completed.
For both short-term core services and water restoration services, Roto-Rooter satisfies its performance obligation at a
point in time. The services provided generally involve fixing plumbing, drainage or flood-related issues at the customer’s property. At the time service is complete, the customer acknowledges its obligation to pay for service and its satisfaction
with the service performed. This provides evidence that the customer has accepted the service and Roto-Rooter is now entitled to payment. As such, Roto-Rooter recognizes revenue for these services upon completion of the job and receipt of
customer acknowledgement. Roto-Rooter’s performance obligations for short-term core services and water restoration services relate to contracts with an expected duration of less than a year. Therefore, Roto-Rooter has elected to apply the
optional exception provided in ASC 606 and is not required to disclose the aggregate amount of the transaction price allocated to performance obligations that are unsatisfied or partially unsatisfied at the end of the reporting period. Roto-Rooter
does not have significant unsatisfied or partially unsatisfied performance obligations at the time of initial revenue recognition for short-term core or water restoration services.
Roto-Rooter owns the rights to certain territories and contracts with an independent third-party to operate the territory
under Roto-Rooter’s registered trademarks. The contract is for a specified term but cancellable by either party without penalty with 90 days advance notice. Under the terms of these arrangements, Roto-Rooter provides certain back office support
and advertising along with a limited license to use Roto-Rooter’s registered trademarks. The independent contractor is responsible for all day-to-day management of the business including staffing decisions and pricing of services provided. All
performance obligations of Roto-Rooter cease at the termination of the arrangement.
Independent contractors pay Roto-Rooter a standard fee calculated as a percentage of their weekly labor sales. The
primary value for the independent contractors under these arrangements is the right to use Roto-Rooter’s registered trademarks. Roto-Rooter recognizes revenue from independent contractors over-time (weekly) as the independent contractor’s labor
sales are completed. Payment from independent contractors is also received on a weekly basis. The use of Roto-Rooter’s registered trademarks and advertising provides immediate value to the independent contractor as a result of Roto-Rooter’s
nationally recognized brand. Therefore, over-time recognition provides the most faithful depiction of the transfer of services as the customer simultaneously receives and consumes the benefits provided. There is no significant variable
consideration related to these arrangements.
Roto-Rooter has licensed the rights to operate under Roto-Rooter’s registered trademarks in other territories to
franchisees. The contract is for a 10 year term but cancellable by Roto-Rooter for cause with 60 day advance notice without penalty. The franchisee may cancel the contract for any reason with 60 days advance notice without penalty. Under the
terms of the contract, Roto-Rooter provides national advertising and consultation on various aspects of operating a Roto-Rooter business along with the right to use Roto-Rooter’s registered trademarks. The franchisee is responsible for all day-
to-day management of the business including staffing decisions, pricing of services provided and local advertising spend and placement. All performance obligations of Roto-Rooter cease at the termination of the arrangement.
Franchisees pay Roto-Rooter a standard monthly fee based on the population within the franchise territory. The standard
fee is revised on a yearly basis based on changes in the Consumer Price Index for All Urban Consumers. The primary value for the franchisees under this arrangement is the right to use Roto-Rooter’s registered trademarks. Roto-Rooter recognizes
revenue from franchisees over-time (monthly). Payment from franchisees is also received on a monthly basis. The use of Roto-Rooter’s registered trademarks and advertising provides immediate value to the franchisees as a result of Roto-Rooter’s
nationally recognized brand. Therefore, over-time recognition provides the most faithful depiction of the transfer of services as the customer simultaneously receives and consumes the benefits provided. There is no significant variable
consideration related to these arrangements.
The composition of disaggregated revenue for the year ended 31, 2018 is as follows (in thousands):
|
Short-term core service jobs
|
|
$
|
421,790
|
|
|
Water restoration
|
|
|
101,784
|
|
|
Contractor revenue
|
|
|
50,093
|
|
|
Franchise fees
|
|
|
6,382
|
|
|
All other
|
|
|
11,958
|
|
|
Subtotal
|
|
$
|
592,007
|
|
|
Implicit price concessions and credit memos
|
|
|
(6,921
|
)
|
|
Net revenue
|
|
$
|
585,086
|
|
Initial Adoption of ASC 606
The Company utilized the modified retrospective method of adoption for all contracts. Except for the changes discussed
below, the Company has consistently applied the accounting policies to all periods presented in the consolidated financial statements. Sales tax collected from customers at Roto-Rooter is excluded from revenue under ASC 606 and prior revenue
standards.
For VITAS, expenses related to payor audits and reviews, as well as variable consideration estimated for patient
deductibles and coinsurance, have been historically estimated as revenue was recognized and classified as bad debt expense, included in the consolidated statements of income as selling, general and administrative expense. Upon adoption of ASC 606,
these expenses are classified as contra-revenue. There is no change in the timing of recognition related to the variable consideration. The amount of these expenses during the year ended December 31, 2018 was $11.8 million.
Also for VITAS, the 5% net expense related to Medicaid room and board has been historically recorded on a net basis in
cost of services provided in the consolidated income statements. Upon adoption of ASC 606, due to the change in the residual value method required by ASC 606, the expense will be classified as a contra-revenue. The amount of the change in the
classification for these expenses during the year ended December 31, 2018 was $10.1 million. There has been no change in the evaluation of Medicaid room and board related to net versus gross presentation.
Related to Roto-Rooter, expenses related to post-invoice variable consideration in our short-term core portfolio, and
adjustments made subsequent to initial estimates related to allowances taken by insurance companies for water restoration, have been classified as a contra-revenue account in the statements of income. These amounts were previously classified as
bad debt expense in SG&A. The amount of the change in classification for these expenses during the year ended December 31, 2018 was $6.9 million. The initial estimate related to allowances taken by insurance companies for water restoration
services have historically been classified as contra-revenue and did not change as a result of the transition.
There was no material impact on the consolidated balance sheets related to the initial adoption. There is no impact to
consolidated net income as a result of the initial adoption. As a result of the change in classification in the statements of income, amounts previously included in the provision for uncollectible accounts in the statements of cash flow have been
included in the decrease/(increase) in accounts receivable line item in 2018. The total impact of the change from prior revenue guidance (ASC 605) to guidance adopted on January 1, 2018 related to classification in the statements of income is as
follows (in thousands):
|
|
Impact for the year ended
December 31, 2018 |
|
| |
|
ASC 605
|
|
|
Adjustment
|
|
|
ASC 606
|
|
|
Service revenue and sales
|
|
$
|
1,811,408
|
|
|
$
|
(28,760
|
)
|
|
$
|
1,782,648
|
|
|
Cost of services provided and goods sold
|
|
|
1,238,698
|
|
|
|
(10,054
|
)
|
|
|
1,228,644
|
|
|
Selling, general and administrative expenses
|
|
|
288,915
|
|
|
|
(18,706
|
)
|
|
|
270,209
|
|
Insurance Accruals
For the Roto-Rooter segment and Chemed’s Corporate Office, we initially self-insure for all casualty insurance claims
(workers’ compensation, auto liability and general liability). As a result, we closely monitor and frequently evaluate our historical claims experience to estimate the appropriate level of accrual for self-insured claims. Our third-party
administrator (“TPA”) processes and reviews claims on a monthly basis. Currently, our exposure on any single claim is capped at $750,000. In developing our estimates, we accumulate historical claims data for the previous 10 years to calculate
loss development factors (“LDF”) by insurance coverage type. LDFs are applied to known claims to estimate the ultimate potential liability for known and unknown claims for each open policy year. LDFs are updated annually. Because this
methodology relies heavily on historical claims data, the key risk is whether the historical claims are an accurate predictor of future claims exposure. The risk also exists that certain claims have been incurred and not reported on a timely
basis. To mitigate these risks, in conjunction with our TPA, we closely monitor claims to ensure timely accumulation of data and compare claims trends with the industry experience of our TPA.
For the VITAS segment, we initially self-insure for workers’ compensation claims. Currently, VITAS’ exposure on any
single claim is capped at $1,000,000. For VITAS’ self-insurance accruals for workers’ compensation, the valuation methods used are similar to those used internally for our other business units. We are also insured for other risks with respect to
professional liability with a deductible of $750,000.
Our casualty insurance liabilities are recorded gross before any estimated recovery for amounts exceeding our stop loss limits. Estimated recoveries from insurance carriers are recorded as accounts
receivable. Claims experience adjustments to our casualty and workers’ compensation accrual for the years ended December 31, 2018, 2017 and 2016, were net pretax debits/(credits) of ($3,437,000), ($1,800,000), and $1,147,000 respectively.
As an indication of the sensitivity of the accrued liability to reported claims, our analysis indicates that a 1%
across-the-board increase or decrease in the amount of projected losses would increase or decrease the accrued insurance liability at December 31, 2018 by $3.3 million or 7.0%. While the amount recorded represents our best estimate of the casualty
and workers’ compensation insurance liability, we have calculated, based on historical claims experience, the actual loss could reasonably be expected to increase or decrease by approximately $3.0 million as of December 31, 2018.
Income Taxes
Deferred taxes are provided on an asset and liability method whereby deferred tax assets are recognized for deductible
temporary differences and operating loss carry-forwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amount of assets and liabilities and their tax
basis. Deferred tax assets are reduced by a valuation allowance when, in our opinion, it is more likely than not that some portion or all of the deferred tax assets will not be realized due to insufficient taxable income within the carryback or
carryforward period available under the tax laws. Deferred tax assets and liabilities are adjusted for the effects of changes in laws and rates on the date of enactment.
In November 2015, the FASB issued ASU No. 2015-17 which simplifies the balance sheet classification required for deferred
tax balances. It allows for a company’s deferred tax assets and liabilities to be netted into a noncurrent account, either asset or liability, by jurisdiction. The ASU is required to be adopted for annual periods beginning after December 15, 2016
and the interim periods within that annual period. Early adoption is permitted. Companies have the choice to adopt prospectively or retrospectively. In order to simplify our balance sheet classification required for deferred tax balances, we
adopted the ASU for our annual balance sheet as of December 31, 2015 on a prospective basis.
We are subject to income taxes in the federal and most state jurisdictions. We are periodically audited by various taxing
authorities. Significant judgment is required to determine our provision for income taxes. We adopted FASB’s authoritative guidance on accounting for uncertainty in income taxes, which prescribes a comprehensive model for how to recognize,
measure, present and disclose in financial statements uncertain tax positions taken or expected to be taken on a tax return. Upon adoption of this guidance, the financial statements reflect expected future tax consequences of such uncertain
positions assuming the taxing authorities’ full knowledge of the position and all relevant facts.
Goodwill and Intangible Assets
Identifiable, definite-lived intangible assets arise from purchase business combinations and are amortized using either an
accelerated method or the straight-line method over the estimated useful lives of the assets. The selection of an amortization method is based on which method best reflects the economic pattern of usage of the asset.
The date of our annual goodwill and indefinite-lived intangible asset impairment analysis is October
1. The VITAS trade name is considered to have an indefinite life. We also capitalize the direct costs of obtaining licenses to operate either hospice programs or plumbing operations subject to a minimum capitalization threshold. These costs are
amortized over the life of the license using the straight line method. Certificates of Need (CON), which are required in certain states for hospice operations, are generally granted without expiration and thus, we believe them to be
indefinite-lived assets subject to impairment testing.
We consider that RRC, RRSC and VITAS are appropriate reporting units for testing goodwill
impairment. We consider RRC and RRSC as separate reporting units but one operating segment. This is appropriate as they each have their own set of general ledger accounts that can be analyzed at “one level below an operating segment” per the
definition of a reporting unit in FASB guidance.
We completed our qualitative analysis for impairment of goodwill and our indefinite-lived intangible
assets as of October 1, 2018. We assessed such qualitative factors as macroeconomic conditions, industry and market conditions, cost factors, financial performance and the legislative and regulatory environment. Based on our assessment, we do not
believe that it is more likely than not that our reporting units’ or indefinite-lived assets fair values are less than their carrying values.
In January 2017, the FASB issued Accounting Standards Update “ASU No. 2017-4 – Intangibles – Goodwill and Other”. To
simplify the subsequent measurement of goodwill, the FASB eliminated Step 2 from the goodwill impairment test. The guidance in the ASU is effective for the Company in fiscal years beginning after December 15, 2019. Early adoption is permitted.
We anticipate adoption of this standard will have no impact on our consolidated financial statements.
Stock-based Compensation Plans
Stock-based compensation cost is measured at the grant date, based on the fair value of the award and recognized as
expense over the employee’s requisite service period on a straight-line basis. We estimate the fair value of stock options using the Black-Scholes valuation model. We estimate the fair value and derived service periods of market based awards using
a Monte Carlo simulation approach in a risk neutral framework. We determine expected term, volatility, dividend yield and forfeiture rate based on our historical experience. We believe that historical experience is the best indicator of these
factors.
Contingencies
We are subject to various lawsuits and claims in the normal course of our business. In addition, we periodically receive
communications from governmental and regulatory agencies concerning compliance with Medicare and Medicaid billing requirements at our VITAS subsidiary. We establish reserves for specific, uninsured liabilities in connection with regulatory and
legal action that we deem to be probable and estimable. We record legal fees associated with legal and regulatory actions as the costs are incurred. We disclose
material loss contingencies that probable but not reasonably estimable and those that are at least reasonably possible.
Unaudited Consolidating
Summaries and Reconciliations of Adjusted EBITDA
|
|
| Chemed Corporation and Subsidiary
Companies |
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
Chemed
|
|
|
2018
|
|
VITAS
|
|
|
Roto-Rooter
|
|
|
Corporate
|
|
|
Consolidated
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income/(loss)
|
|
$
|
138,846
|
|
|
$
|
98,711
|
|
|
$
|
(32,013
|
)
|
|
$
|
205,544
|
|
|
Add/(deduct):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
175
|
|
|
|
319
|
|
|
|
4,496
|
|
|
|
4,990
|
|
|
Income taxes
|
|
|
40,847
|
|
|
|
28,850
|
|
|
|
(35,641
|
)
|
|
|
34,056
|
|
|
Depreciation
|
|
|
19,688
|
|
|
|
18,629
|
|
|
|
147
|
|
|
|
38,464
|
|
|
Amortization
|
|
|
12
|
|
|
|
387
|
|
|
|
-
|
|
|
|
399
|
|
|
EBITDA
|
|
|
199,568
|
|
|
|
146,896
|
|
|
|
(63,011
|
)
|
|
|
283,453
|
|
|
Add/(deduct):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intercompany interest/(expense)
|
|
|
(12,832
|
)
|
|
|
(6,908
|
)
|
|
|
19,740
|
|
|
|
-
|
|
|
Medicare cap sequestration adjustment
|
|
|
1,496
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,496
|
|
|
Litigation settlements
|
|
|
796
|
|
|
|
-
|
|
|
|
-
|
|
|
|
796
|
|
|
Interest income
|
|
|
(580
|
)
|
|
|
(92
|
)
|
|
|
1
|
|
|
|
(671
|
)
|
|
Acquisition expense
|
|
|
209
|
|
|
|
548
|
|
|
|
-
|
|
|
|
757
|
|
|
Stock option expense
|
|
|
-
|
|
|
|
-
|
|
|
|
12,611
|
|
|
|
12,611
|
|
|
Stock award expense
|
|
|
107
|
|
|
|
100
|
|
|
|
239
|
|
|
|
446
|
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
6,618
|
|
|
|
6,618
|
|
|
Adjusted EBITDA
|
|
$
|
188,764
|
|
|
$
|
140,544
|
|
|
$
|
(23,802
|
)
|
|
$
|
305,506
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chemed |
|
2017
|
|
|
VITAS
|
|
|
|
Roto-Rooter
|
|
|
|
Corporate
|
|
|
|
Consolidated
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income/(loss)
|
|
$
|
57,645
|
|
|
$
|
73,299
|
|
|
$
|
(32,767
|
)
|
|
$
|
98,177
|
|
|
Add/(deduct):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
188
|
|
|
|
323
|
|
|
|
3,761
|
|
|
|
4,272
|
|
|
Income taxes
|
|
|
16,436
|
|
|
|
32,782
|
|
|
|
(30,478
|
)
|
|
|
18,740
|
|
|
Depreciation
|
|
|
18,616
|
|
|
|
16,667
|
|
|
|
205
|
|
|
|
35,488
|
|
|
Amortization
|
|
|
14
|
|
|
|
123
|
|
|
|
-
|
|
|
|
137
|
|
|
EBITDA
|
|
|
92,899
|
|
|
|
123,194
|
|
|
|
(59,279
|
)
|
|
|
156,814
|
|
|
Add/(deduct):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intercompany interest/(expense)
|
|
|
(11,656
|
)
|
|
|
(5,596
|
)
|
|
|
17,252
|
|
|
|
-
|
|
|
Interest income
|
|
|
(388
|
)
|
|
|
(39
|
)
|
|
|
-
|
|
|
|
(427
|
)
|
|
Loss on sale of transportation equipment
|
|
|
-
|
|
|
|
-
|
|
|
|
5,266
|
|
|
|
5,266
|
|
|
Expenses related to OIG investigation
|
|
|
5,194
|
|
|
|
-
|
|
|
|
-
|
|
|
|
5,194
|
|
|
Program closure expenses
|
|
|
1,138
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,138
|
|
|
Medicare cap sequestration adjustment
|
|
|
447
|
|
|
|
-
|
|
|
|
-
|
|
|
|
447
|
|
|
Litigation settlements
|
|
|
84,476
|
|
|
|
213
|
|
|
|
-
|
|
|
|
84,689
|
|
|
Advertising cost adjustment
|
|
|
-
|
|
|
|
(1,371
|
)
|
|
|
-
|
|
|
|
(1,371
|
)
|
|
Stock option expense
|
|
|
-
|
|
|
|
-
|
|
|
|
10,485
|
|
|
|
10,485
|
|
|
Stock award expense
|
|
|
291
|
|
|
|
269
|
|
|
|
670
|
|
|
|
1,230
|
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
4,994
|
|
|
|
4,994
|
|
|
Adjusted EBITDA
|
|
$
|
172,401
|
|
|
$
|
116,670
|
|
|
$
|
(20,612
|
)
|
|
$
|
268,459
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chemed |
|
|
2016
|
|
|
VITAS |
|
|
|
Roto-Rooter |
|
|
|
Corporate |
|
|
|
Consolidated |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income/(loss)
|
|
$
|
84,961
|
|
|
$
|
52,893
|
|
|
$
|
(29,111
|
)
|
|
$
|
108,743
|
|
|
Add/(deduct):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense
|
|
|
211
|
|
|
|
332
|
|
|
|
3,172
|
|
|
|
3,715
|
|
|
Income taxes
|
|
|
51,910
|
|
|
|
32,719
|
|
|
|
(16,318
|
)
|
|
|
68,311
|
|
|
Depreciation
|
|
|
19,035
|
|
|
|
14,698
|
|
|
|
546
|
|
|
|
34,279
|
|
|
Amortization
|
|
|
55
|
|
|
|
304
|
|
|
|
-
|
|
|
|
359
|
|
|
EBITDA
|
|
|
156,172
|
|
|
|
100,946
|
|
|
|
(41,711
|
)
|
|
|
215,407
|
|
|
Add/(deduct):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intercompany interest/(expense)
|
|
|
(7,969
|
)
|
|
|
(3,595
|
)
|
|
|
11,564
|
|
|
|
-
|
|
|
Interest income
|
|
|
(325
|
)
|
|
|
(58
|
)
|
|
|
-
|
|
|
|
(383
|
)
|
|
Expenses related to OIG investigation
|
|
|
5,260
|
|
|
|
-
|
|
|
|
-
|
|
|
|
5,260
|
|
|
Retirement expenses
|
|
|
4,491
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4,491
|
|
|
Medicare cap sequestration adjustment
|
|
|
228
|
|
|
|
-
|
|
|
|
-
|
|
|
|
228
|
|
|
Expenses related to litigation settlements
|
|
|
1,149
|
|
|
|
45
|
|
|
|
-
|
|
|
|
1,194
|
|
|
Advertising cost adjustment
|
|
|
-
|
|
|
|
(1,333
|
)
|
|
|
-
|
|
|
|
(1,333
|
)
|
|
Stock option expense
|
|
|
-
|
|
|
|
-
|
|
|
|
8,330
|
|
|
|
8,330
|
|
|
Stock award expense
|
|
|
387
|
|
|
|
307
|
|
|
|
1,161
|
|
|
|
1,855
|
|
|
Long-term incentive compensation
|
|
|
-
|
|
|
|
-
|
|
|
|
1,930
|
|
|
|
1,930
|
|
|
Expenses related to securities litigation
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
Adjusted EBITDA
|
|
$
|
159,393
|
|
|
$
|
96,312
|
|
|
$
|
(18,726
|
)
|
|
$
|
236,979
|
|
|
CHEMED CORPORATION AND SUBSIDIARY COMPANIES
|
|
|
RECONCILIATION OF ADJUSTED NET INCOME
|
|
|
(in thousands, except per share data)(unaudited)
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
For the Years Ended December 31,
|
|
| |
|
2018
|
|
|
2017
|
|
|
2016
|
|
|
Net income as reported
|
|
$
|
205,544
|
|
|
$
|
98,177
|
|
|
$
|
108,743
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Add/(deduct) pre-tax cost of:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock option expense
|
|
|
12,611
|
|
|
|
10,485
|
|
|
|
8,330
|
|
|
Long-term incentive compensation
|
|
|
6,618
|
|
|
|
4,994
|
|
|
|
1,930
|
|
|
Medicare cap sequestration adjustment
|
|
|
1,496
|
|
|
|
447
|
|
|
|
228
|
|
|
Litigation settlements
|
|
|
796
|
|
|
|
84,476
|
|
|
|
-
|
|
|
Acquisition expenses
|
|
|
757
|
|
|
|
-
|
|
|
|
-
|
|
|
Loss on sale of transportation equipment
|
|
|
-
|
|
|
|
5,266
|
|
|
|
-
|
|
|
Expenses related to OIG investigation
|
|
|
-
|
|
|
|
5,194
|
|
|
|
5,260
|
|
|
Program closure expenses
|
|
|
-
|
|
|
|
1,138
|
|
|
|
-
|
|
|
Early retirement expenses
|
|
|
-
|
|
|
|
-
|
|
|
|
4,491
|
|
|
Net expenses related to litigation settlements
|
|
|
-
|
|
|
|
213
|
|
|
|
45
|
|
|
Add/(deduct) tax impacts:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax impact of the above pre-tax adjustments (1)
|
|
|
(4,586
|
)
|
|
|
(42,102
|
)
|
|
|
(7,540
|
)
|
|
Impact of tax reform
|
|
|
-
|
|
|
|
(8,302
|
)
|
|
|
-
|
|
|
Excess tax benefits on stock compensation
|
|
|
(22,862
|
)
|
|
|
(18,932
|
)
|
|
|
-
|
|
|
Adjusted net income
|
|
$
|
200,374
|
|
|
$
|
141,054
|
|
|
$
|
121,487
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted Earnings Per Share As Reported
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
12.23
|
|
|
$
|
5.86
|
|
|
$
|
6.48
|
|
|
Average number of shares outstanding
|
|
|
16,803
|
|
|
|
16,742
|
|
|
|
16,789
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjusted Diluted Earnings Per Share
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
11.93
|
|
|
$
|
8.43
|
|
|
$
|
7.24
|
|
|
Average number of shares outstanding
|
|
|
16,803
|
|
|
|
16,742
|
|
|
|
16,789
|
|
(1) The tax impact of pre-tax adjustments was calculated using the effective tax rate of the operating unit for which each adjustment is associated.
The "Footnotes to Financial Statements" are integral parts of this financial information.
|
CHEMED CORPORATION AND SUBSIDIARY COMPANIES
|
|
|
OPERATING STATISTICS FOR VITAS SEGMENT
|
|
|
(unaudited)
|
|
| |
|
|
|
|
|
|
| |
|
Three Months Ended December 31,
|
|
|
Year Ended December 31,
|
|
|
OPERATING STATISTICS
|
|
2018
|
|
|
2017
|
|
|
2018
|
|
|
2017
|
|
|
Net revenue ($000)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Homecare
|
|
$
|
261,972
|
|
|
$
|
242,554
|
|
|
$
|
1,010,518
|
|
|
$
|
935,913
|
|
|
Inpatient
|
|
|
20,874
|
|
|
|
22,033
|
|
|
|
82,677
|
|
|
|
90,472
|
|
|
Continuous care
|
|
|
30,834
|
|
|
|
30,131
|
|
|
|
122,498
|
|
|
|
124,557
|
|
|
Other
|
|
|
1,986
|
|
|
|
-
|
|
|
|
7,831
|
|
|
|
-
|
|
|
Subtotal
|
|
$
|
315,666
|
|
|
$
|
294,718
|
|
|
$
|
1,223,524
|
|
|
$
|
1,150,942
|
|
|
Room and board, net
|
|
|
(2,191
|
)
|
|
|
-
|
|
|
|
(10,054
|
)
|
|
|
-
|
|
|
Contractual allowances
|
|
|
(3,036
|
)
|
|
|
-
|
|
|
|
(11,785
|
)
|
|
|
-
|
|
|
Medicare cap allowance
|
|
|
(3,454
|
)
|
|
|
(2,435
|
)
|
|
|
(4,123
|
)
|
|
|
(2,682
|
)
|
|
Total
|
|
$
|
306,985
|
|
|
$
|
292,283
|
|
|
$
|
1,197,562
|
|
|
$
|
1,148,260
|
|
|
Net revenue as a percent of total before Medicare cap allowance
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Homecare
|
|
|
83.0
|
%
|
|
|
82.3
|
%
|
|
|
82.6
|
%
|
|
|
81.2
|
%
|
|
Inpatient
|
|
|
6.6
|
|
|
|
7.5
|
|
|
|
6.8
|
|
|
|
7.9
|
|
|
Continuous care
|
|
|
9.8
|
|
|
|
10.2
|
|
|
|
10.0
|
|
|
|
10.9
|
|
|
Other
|
|
|
0.6
|
|
|
|
-
|
|
|
|
0.6
|
|
|
|
-
|
|
|
Subtotal
|
|
|
100.0
|
|
|
|
100.0
|
|
|
|
100.0
|
|
|
|
100.0
|
|
|
Room and board, net
|
|
|
(0.7
|
)
|
|
|
-
|
|
|
|
(0.8
|
)
|
|
|
-
|
|
|
Contractual allowances
|
|
|
(1.0
|
)
|
|
|
-
|
|
|
|
(1.1
|
)
|
|
|
-
|
|
|
Medicare cap allowance
|
|
|
(1.1
|
)
|
|
|
(0.8
|
)
|
|
|
(0.2
|
)
|
|
|
(0.2
|
)
|
|
Total
|
|
|
97.2
|
%
|
|
|
99.2
|
%
|
|
|
97.9
|
%
|
|
|
99.8
|
%
|
|
Average daily census (days)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Homecare
|
|
|
14,062
|
|
|
|
12,861
|
|
|
|
13,652
|
|
|
|
12,549
|
|
|
Nursing home
|
|
|
3,297
|
|
|
|
3,265
|
|
|
|
3,298
|
|
|
|
3,177
|
|
|
Routine homecare
|
|
|
17,359
|
|
|
|
16,126
|
|
|
|
16,950
|
|
|
|
15,726
|
|
|
Inpatient
|
|
|
326
|
|
|
|
342
|
|
|
|
327
|
|
|
|
354
|
|
|
Continuous care
|
|
|
464
|
|
|
|
452
|
|
|
|
465
|
|
|
|
470
|
|
|
Total
|
|
|
18,149
|
|
|
|
16,920
|
|
|
|
17,742
|
|
|
|
16,550
|
|
|
Total Admissions
|
|
|
16,579
|
|
|
|
16,575
|
|
|
|
68,119
|
|
|
|
66,449
|
|
|
Total Discharges
|
|
|
16,623
|
|
|
|
16,553
|
|
|
|
66,868
|
|
|
|
65,637
|
|
|
Average length of stay (days)
|
|
|
92.6
|
|
|
|
91.4
|
|
|
|
89.9
|
|
|
|
88.8
|
|
|
Median length of stay (days)
|
|
|
17.0
|
|
|
|
16.0
|
|
|
|
17.0
|
|
|
|
16.0
|
|
|
ADC by major diagnosis
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cerebro
|
|
|
35.8
|
%
|
|
|
36.1
|
%
|
|
|
36.3
|
%
|
|
|
35.5
|
%
|
|
Neurological
|
|
|
18.6
|
|
|
|
18.5
|
|
|
|
19.0
|
|
|
|
19.2
|
|
|
Cardio
|
|
|
16.3
|
|
|
|
16.4
|
|
|
|
16.4
|
|
|
|
16.5
|
|
|
Cancer
|
|
|
13.7
|
|
|
|
14.1
|
|
|
|
13.7
|
|
|
|
14.6
|
|
|
Respiratory
|
|
|
8.0
|
|
|
|
8.0
|
|
|
|
8.2
|
|
|
|
7.9
|
|
|
Other
|
|
|
7.6
|
|
|
|
6.9
|
|
|
|
6.4
|
|
|
|
6.3
|
|
|
Total
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
Admissions by major diagnosis
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cerebro
|
|
|
20.9
|
%
|
|
|
22.3
|
%
|
|
|
21.8
|
%
|
|
|
22.0
|
%
|
|
Neurological
|
|
|
11.5
|
|
|
|
10.7
|
|
|
|
11.4
|
|
|
|
10.6
|
|
|
Cancer
|
|
|
31.1
|
|
|
|
30.0
|
|
|
|
30.2
|
|
|
|
30.6
|
|
|
Cardio
|
|
|
14.6
|
|
|
|
14.9
|
|
|
|
15.4
|
|
|
|
15.0
|
|
|
Respiratory
|
|
|
10.1
|
|
|
|
10.7
|
|
|
|
10.9
|
|
|
|
10.8
|
|
|
Other
|
|
|
11.8
|
|
|
|
11.4
|
|
|
|
10.3
|
|
|
|
11.0
|
|
|
Total
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
Direct patient care margins
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Routine homecare
|
|
|
53.9
|
%
|
|
|
53.9
|
%
|
|
|
53.0
|
%
|
|
|
52.6
|
%
|
|
Inpatient
|
|
|
3.9
|
|
|
|
8.5
|
|
|
|
4.7
|
|
|
|
5.4
|
|
|
Continuous care
|
|
|
18.4
|
|
|
|
16.8
|
|
|
|
17.7
|
|
|
|
16.9
|
|
|
Homecare margin drivers (dollars per patient day)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Labor costs
|
|
$
|
56.82
|
|
|
$
|
55.65
|
|
|
$
|
57.59
|
|
|
$
|
56.80
|
|
|
Combined drug, home medical equipment and medical supplies cost
|
|
$
|
13.58
|
|
|
$
|
14.30
|
|
|
$
|
14.06
|
|
|
$
|
14.65
|
|
|
Inpatient margin drivers (dollars per patient day) - Labor costs
|
|
$
|
379.17
|
|
|
$
|
355.96
|
|
|
$
|
376.53
|
|
|
$
|
366.41
|
|
|
Continuous care margin drivers (dollars per patient day) - Labor costs
|
|
$
|
571.18
|
|
|
$
|
583.45
|
|
|
$
|
575.36
|
|
|
$
|
584.49
|
|
|
Bad debt expense as a percent of revenues
|
|
|
1.0
|
%
|
|
|
1.1
|
%
|
|
|
1.0
|
%
|
|
|
1.1
|
%
|
|
Accounts receivable --
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Days of revenue outstanding- excluding unapplied Medicare payments
|
|
|
35.0
|
|
|
|
33.7
|
|
|
N.A.
|
|
|
N.A.
|
|
|
Days of revenue outstanding- including unapplied Medicare payments
|
|
|
24.6
|
|
|
|
25.0
|
|
|
N.A.
|
|
|
N.A.
|
|
SAFE HARBOR STATEMENT UNDER THE PRIVATE
SECURITIES LITIGATION REFORM ACT OF 1995 REGARDING FORWARD-LOOKING INFORMATION
In addition to historical information, this report contains forward-looking statements and performance trends that are
based upon assumptions subject to certain known and unknown risks, uncertainties, contingencies and other factors. Such forward-looking statements and trends include, but are not limited to, the impact of laws and regulations on our operations, our
estimate of future effective income tax rates and the recoverability of deferred tax assets. Variances in any or all of the risks, uncertainties, contingencies, and other factors from our assumptions could cause actual results to differ materially
from these forward-looking statements and trends. Our ability to deal with the unknown outcomes of these events, many of which are beyond our control, may affect the reliability of our projections and other financial matters.