00018607422023FYFALSEBausch & Lomb CorpP30DP1YP1YP6Mhttp://fasb.org/us-gaap/2023#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2023#OtherAssetsNoncurrenthttp://fasb.org/us-gaap/2023#AccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2023#AccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2023#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2023#OtherLiabilitiesNoncurrentP3YP4YP10Y33.3333.3333.3333.3333.3333.3300018607422023-01-012023-12-3100018607422023-06-30iso4217:USD00018607422024-02-16xbrli:shares00018607422023-12-3100018607422022-12-310001860742us-gaap:ProductMember2023-01-012023-12-310001860742us-gaap:ProductMember2022-01-012022-12-310001860742us-gaap:ProductMember2021-01-012021-12-310001860742us-gaap:ProductAndServiceOtherMember2023-01-012023-12-310001860742us-gaap:ProductAndServiceOtherMember2022-01-012022-12-310001860742us-gaap:ProductAndServiceOtherMember2021-01-012021-12-3100018607422022-01-012022-12-3100018607422021-01-012021-12-31iso4217:USDxbrli:shares0001860742us-gaap:CommonStockMember2020-12-310001860742blco:NetParentInvestmentMember2020-12-310001860742us-gaap:AdditionalPaidInCapitalMember2020-12-310001860742us-gaap:RetainedEarningsMember2020-12-310001860742us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310001860742us-gaap:ParentMember2020-12-310001860742us-gaap:NoncontrollingInterestMember2020-12-3100018607422020-12-310001860742blco:NetParentInvestmentMember2021-01-012021-12-310001860742us-gaap:ParentMember2021-01-012021-12-310001860742us-gaap:NoncontrollingInterestMember2021-01-012021-12-310001860742us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-12-310001860742us-gaap:CommonStockMember2021-12-310001860742blco:NetParentInvestmentMember2021-12-310001860742us-gaap:AdditionalPaidInCapitalMember2021-12-310001860742us-gaap:RetainedEarningsMember2021-12-310001860742us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310001860742us-gaap:ParentMember2021-12-310001860742us-gaap:NoncontrollingInterestMember2021-12-3100018607422021-12-310001860742us-gaap:CommonStockMember2022-01-012022-12-310001860742blco:NetParentInvestmentMember2022-01-012022-12-310001860742us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310001860742us-gaap:ParentMember2022-01-012022-12-310001860742us-gaap:NoncontrollingInterestMember2022-01-012022-12-310001860742us-gaap:RetainedEarningsMember2022-01-012022-12-310001860742us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310001860742us-gaap:CommonStockMember2022-12-310001860742blco:NetParentInvestmentMember2022-12-310001860742us-gaap:AdditionalPaidInCapitalMember2022-12-310001860742us-gaap:RetainedEarningsMember2022-12-310001860742us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001860742us-gaap:ParentMember2022-12-310001860742us-gaap:NoncontrollingInterestMember2022-12-310001860742us-gaap:CommonStockMember2023-01-012023-12-310001860742us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310001860742us-gaap:ParentMember2023-01-012023-12-310001860742us-gaap:NoncontrollingInterestMember2023-01-012023-12-310001860742us-gaap:RetainedEarningsMember2023-01-012023-12-310001860742us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310001860742us-gaap:CommonStockMember2023-12-310001860742blco:NetParentInvestmentMember2023-12-310001860742us-gaap:AdditionalPaidInCapitalMember2023-12-310001860742us-gaap:RetainedEarningsMember2023-12-310001860742us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001860742us-gaap:ParentMember2023-12-310001860742us-gaap:NoncontrollingInterestMember2023-12-31blco:segment0001860742blco:BHCMemberus-gaap:SubsequentEventMemberblco:BauschLombMember2024-02-162024-02-16xbrli:pure0001860742blco:BHCMemberus-gaap:SubsequentEventMemberblco:BauschLombMember2024-02-160001860742blco:RevisionOfPriorPeriodErrorCorrectionOverstatementAmountMember2022-01-012022-12-310001860742blco:RevisionOfPriorPeriodErrorCorrectionUnderstatementAmountMember2021-12-310001860742srt:RevisionOfPriorPeriodErrorCorrectionAdjustmentMember2022-01-012022-12-310001860742blco:AlgeriaArgentinaBrazilBelarusGreeceIranRussiaSerbiaSouthAfricaTurkeyUkraineAndVenezuelaMember2023-12-310001860742blco:AlgeriaArgentinaBrazilBelarusGreeceIranRussiaSerbiaSouthAfricaTurkeyUkraineAndVenezuelaMember2022-12-310001860742blco:AlgeriaArgentinaBrazilBelarusGreeceIranRussiaSerbiaSouthAfricaTurkeyUkraineAndVenezuelaMember2023-01-012023-12-310001860742srt:MinimumMemberus-gaap:LandImprovementsMember2023-12-310001860742srt:MaximumMemberus-gaap:LandImprovementsMember2023-12-310001860742srt:MaximumMemberus-gaap:BuildingAndBuildingImprovementsMember2023-12-310001860742us-gaap:MachineryAndEquipmentMembersrt:MaximumMember2023-12-310001860742us-gaap:OtherMachineryAndEquipmentMembersrt:MinimumMember2023-12-310001860742us-gaap:OtherMachineryAndEquipmentMembersrt:MaximumMember2023-12-310001860742us-gaap:LeaseholdsAndLeaseholdImprovementsMembersrt:MaximumMember2023-12-310001860742blco:ProductBrandMembersrt:MinimumMember2023-12-310001860742blco:ProductBrandMembersrt:MaximumMember2023-12-310001860742us-gaap:TradeNamesMembersrt:MinimumMember2023-12-310001860742us-gaap:TradeNamesMembersrt:MaximumMember2023-12-310001860742us-gaap:ContractualRightsMembersrt:MinimumMember2023-12-310001860742us-gaap:ContractualRightsMembersrt:MaximumMember2023-12-310001860742blco:OutLicensedTechnologyMember2023-12-310001860742blco:ReserveForDiscountsAndAllowancesMember2021-12-310001860742blco:ReserveForCustomerReturnsMember2021-12-310001860742blco:ReserveForRebatesMember2021-12-310001860742blco:ReserveForChargebacksMember2021-12-310001860742blco:ReserveForDistributionFeesMember2021-12-310001860742blco:ReserveForDiscountsAndAllowancesMember2022-01-012022-12-310001860742blco:ReserveForCustomerReturnsMember2022-01-012022-12-310001860742blco:ReserveForRebatesMember2022-01-012022-12-310001860742blco:ReserveForChargebacksMember2022-01-012022-12-310001860742blco:ReserveForDistributionFeesMember2022-01-012022-12-310001860742blco:ReserveForDiscountsAndAllowancesMember2022-12-310001860742blco:ReserveForCustomerReturnsMember2022-12-310001860742blco:ReserveForRebatesMember2022-12-310001860742blco:ReserveForChargebacksMember2022-12-310001860742blco:ReserveForDistributionFeesMember2022-12-310001860742blco:ReserveForDiscountsAndAllowancesMember2023-01-012023-12-310001860742blco:ReserveForCustomerReturnsMember2023-01-012023-12-310001860742blco:ReserveForRebatesMember2023-01-012023-12-310001860742blco:ReserveForChargebacksMember2023-01-012023-12-310001860742blco:ReserveForDistributionFeesMember2023-01-012023-12-310001860742blco:ReserveForDiscountsAndAllowancesMember2023-12-310001860742blco:ReserveForCustomerReturnsMember2023-12-310001860742blco:ReserveForRebatesMember2023-12-310001860742blco:ReserveForChargebacksMember2023-12-310001860742blco:ReserveForDistributionFeesMember2023-12-310001860742blco:ReserveForRebatesAdvertisingCreditsPortionMember2023-12-310001860742blco:ReserveForRebatesAdvertisingCreditsPortionMember2022-12-310001860742srt:MinimumMember2023-01-012023-12-310001860742srt:MaximumMember2023-01-012023-12-310001860742srt:MinimumMember2023-12-310001860742srt:MaximumMember2023-12-310001860742blco:A2022OmnibusIncentivePlanMember2022-05-050001860742blco:A2022OmnibusIncentivePlanMember2023-04-242023-04-240001860742blco:A2022OmnibusIncentivePlanMember2023-04-240001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-05-052022-05-050001860742srt:AffiliatedEntityMember2023-12-310001860742srt:AffiliatedEntityMember2022-12-310001860742blco:PromissoryNoteMembersrt:AffiliatedEntityMember2022-01-010001860742blco:PromissoryNoteMembersrt:AffiliatedEntityMember2022-05-102022-05-100001860742srt:AffiliatedEntityMember2022-05-102022-05-100001860742blco:PromissoryNoteMembersrt:AffiliatedEntityMember2022-01-012022-12-310001860742srt:MinimumMembersrt:AffiliatedEntityMember2022-05-102022-05-100001860742srt:MaximumMembersrt:AffiliatedEntityMember2022-05-102022-05-100001860742srt:AffiliatedEntityMember2023-01-012023-12-310001860742srt:AffiliatedEntityMember2022-01-012022-12-310001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMember2023-09-292023-09-290001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMember2023-09-290001860742blco:CashPayableUponTheAchievementOfSpecifiedCommercializationAndSalesMilestonesForCertainPipelineProductsMemberblco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMember2023-09-290001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMemberblco:CashPayableUponTheAchievementOfSpecifiedSalesMilestonesMember2023-09-290001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMember2023-09-292023-12-310001860742blco:ProductBrandMemberblco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMember2023-09-292023-09-290001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMemberus-gaap:InProcessResearchAndDevelopmentMember2023-09-292023-09-290001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMember2023-01-012023-12-310001860742blco:SalesBasedMilestonePaymentsMemberblco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMember2023-09-290001860742blco:SalesBasedMilestonePaymentsMemberblco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMemberus-gaap:MeasurementInputDiscountRateMember2023-09-29blco:rate0001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMember2022-01-012022-12-310001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMemberblco:AcquisitionRelatedTransactionCostsMember2023-01-012023-12-310001860742blco:XIIDRAAndCertainOtherOphthalmologyAssetsAcquisitionMemberblco:AcquisitionRelatedFinanceCostsMember2023-01-012023-12-310001860742blco:JohnsonJohnsonVisionBlinkProductLineAcquisitionMember2023-07-062023-07-060001860742us-gaap:TradeNamesMemberblco:JohnsonJohnsonVisionBlinkProductLineAcquisitionMember2023-07-062023-07-060001860742blco:ProductBrandMemberblco:JohnsonJohnsonVisionBlinkProductLineAcquisitionMember2023-07-062023-07-060001860742blco:OutLicensedTechnologyMemberblco:JohnsonJohnsonVisionBlinkProductLineAcquisitionMember2023-07-062023-07-060001860742blco:AcuFocusIncAcquisitionMember2023-01-172023-01-170001860742blco:AcuFocusIncAcquisitionMember2023-01-012023-01-310001860742blco:AcuFocusIncAcquisitionMember2023-01-170001860742blco:AcuFocusIncAcquisitionMember2023-12-310001860742blco:AcuFocusIncAcquisitionMember2023-01-172023-09-300001860742blco:ParagonBioTeckIncAssetAcquisitionAndTotalTitaniumIncBusinessCombinationMember2022-01-012022-12-310001860742blco:SanoculisLtdMemberus-gaap:CollaborativeArrangementTransactionWithPartyToCollaborativeArrangementMember2022-07-282022-07-280001860742blco:ParagonBioTeckIncAssetAcquisitionAndTotalTitaniumIncBusinessCombinationMember2023-12-310001860742us-gaap:FairValueMeasurementsRecurringMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001860742us-gaap:FairValueMeasurementsRecurringMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2022-12-310001860742us-gaap:NondesignatedMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:NondesignatedMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:NondesignatedMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:NondesignatedMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2023-12-310001860742us-gaap:NondesignatedMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:NondesignatedMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:NondesignatedMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2022-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:NondesignatedMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2022-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2023-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2023-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2023-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2023-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-09-300001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:OtherNoncurrentLiabilitiesMemberus-gaap:NetInvestmentHedgingMember2023-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:OtherNoncurrentLiabilitiesMemberus-gaap:NetInvestmentHedgingMember2022-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2023-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2023-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2023-01-012023-12-310001860742us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-01-012022-12-310001860742us-gaap:InterestExpenseMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2023-01-012023-12-310001860742us-gaap:InterestExpenseMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:CurrencySwapMemberus-gaap:NetInvestmentHedgingMember2022-01-012022-12-310001860742us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2023-12-310001860742us-gaap:NondesignatedMemberblco:AccruedLiabilitiesCurrentMemberus-gaap:ForeignExchangeContractMember2023-12-310001860742us-gaap:NondesignatedMemberblco:AccruedLiabilitiesCurrentMemberus-gaap:ForeignExchangeContractMember2022-12-310001860742us-gaap:NondesignatedMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeContractMember2023-12-310001860742us-gaap:NondesignatedMemberus-gaap:PrepaidExpensesAndOtherCurrentAssetsMemberus-gaap:ForeignExchangeContractMember2022-12-310001860742us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2022-12-310001860742us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2023-01-012023-12-310001860742us-gaap:NondesignatedMemberus-gaap:ForeignExchangeContractMember2022-01-012022-12-310001860742srt:MinimumMemberus-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MeasurementInputDiscountRateMember2023-12-310001860742us-gaap:FairValueInputsLevel3Membersrt:MaximumMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MeasurementInputDiscountRateMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberblco:MeasurementInputWeightedAverageDiscountRateMember2023-12-310001860742blco:AccretionForTimeValueOfMoneyMember2023-01-012023-12-310001860742blco:AccretionForTimeValueOfMoneyMember2022-01-012022-12-310001860742blco:FairValueAdjustmentsMember2023-01-012023-12-310001860742blco:FairValueAdjustmentsMember2022-01-012022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsNonrecurringMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsNonrecurringMember2022-12-310001860742us-gaap:LandMember2023-12-310001860742us-gaap:LandMember2022-12-310001860742us-gaap:BuildingAndBuildingImprovementsMember2023-12-310001860742us-gaap:BuildingAndBuildingImprovementsMember2022-12-310001860742us-gaap:MachineryAndEquipmentMember2023-12-310001860742us-gaap:MachineryAndEquipmentMember2022-12-310001860742blco:OtherEquipmentAndLeaseholdImprovementMember2023-12-310001860742blco:OtherEquipmentAndLeaseholdImprovementMember2022-12-310001860742us-gaap:ConstructionInProgressMember2023-12-310001860742us-gaap:ConstructionInProgressMember2022-12-310001860742blco:ProductBrandMember2023-12-310001860742blco:ProductBrandMember2022-12-310001860742us-gaap:TradeNamesMember2023-12-310001860742us-gaap:TradeNamesMember2022-12-310001860742us-gaap:ContractualRightsMember2023-12-310001860742us-gaap:ContractualRightsMember2022-12-310001860742blco:OutLicensedTechnologyMember2022-12-310001860742blco:AcquiredInProcessResearchAndDevelopmentMember2023-12-310001860742blco:AcquiredInProcessResearchAndDevelopmentMember2022-12-310001860742us-gaap:TrademarksMember2023-12-310001860742us-gaap:TrademarksMember2022-12-310001860742blco:DiscontinuedProductLinesMember2023-01-012023-12-310001860742blco:DiscontinuedProductLinesMember2022-01-012022-12-310001860742blco:DiscontinuedProductLinesMember2021-01-012021-12-310001860742blco:BauschLombMember2020-12-310001860742blco:VisionCareMember2020-12-310001860742blco:PharmaceuticalsMember2020-12-310001860742blco:SurgicalMember2020-12-310001860742blco:BauschLombMember2021-01-012021-12-310001860742blco:VisionCareMember2021-01-012021-12-310001860742blco:PharmaceuticalsMember2021-01-012021-12-310001860742blco:SurgicalMember2021-01-012021-12-310001860742blco:BauschLombMember2021-12-310001860742blco:VisionCareMember2021-12-310001860742blco:PharmaceuticalsMember2021-12-310001860742blco:SurgicalMember2021-12-310001860742blco:BauschLombMember2022-01-012022-12-310001860742blco:VisionCareMember2022-01-012022-12-310001860742blco:PharmaceuticalsMember2022-01-012022-12-310001860742blco:SurgicalMember2022-01-012022-12-310001860742blco:BauschLombMember2022-12-310001860742blco:VisionCareMember2022-12-310001860742blco:PharmaceuticalsMember2022-12-310001860742blco:SurgicalMember2022-12-310001860742blco:BauschLombMember2023-01-012023-12-310001860742blco:VisionCareMember2023-01-012023-12-310001860742blco:PharmaceuticalsMember2023-01-012023-12-310001860742blco:SurgicalMember2023-01-012023-12-310001860742blco:BauschLombMember2023-12-310001860742blco:VisionCareMember2023-12-310001860742blco:PharmaceuticalsMember2023-12-310001860742blco:SurgicalMember2023-12-3100018607422021-04-012021-06-300001860742blco:VisionCarePharmaceuticalsAndSurgicalMembersrt:MinimumMember2021-06-300001860742blco:VisionCarePharmaceuticalsAndSurgicalMembersrt:MaximumMember2021-06-300001860742blco:VisionCarePharmaceuticalsAndSurgicalMember2021-06-300001860742blco:VisionCarePharmaceuticalsAndSurgicalMember2021-04-012021-06-300001860742blco:VisionCarePharmaceuticalsAndSurgicalMembersrt:MinimumMember2022-10-010001860742blco:VisionCarePharmaceuticalsAndSurgicalMembersrt:MaximumMember2022-10-010001860742blco:VisionCarePharmaceuticalsAndSurgicalMember2022-10-010001860742blco:VisionCarePharmaceuticalsAndSurgicalMember2022-10-012022-10-010001860742blco:VisionCarePharmaceuticalsAndSurgicalMembersrt:MinimumMember2023-10-010001860742blco:VisionCarePharmaceuticalsAndSurgicalMembersrt:MaximumMember2023-10-010001860742blco:VisionCarePharmaceuticalsAndSurgicalMember2023-10-010001860742blco:VisionCarePharmaceuticalsAndSurgicalMember2023-10-012023-10-010001860742blco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMember2023-12-310001860742blco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMember2022-12-310001860742blco:TermLoanDueMay2027Member2023-12-310001860742blco:TermLoanDueMay2027Member2022-12-310001860742blco:TermLoanDueSeptember2028Member2023-12-310001860742blco:TermLoanDueSeptember2028Member2022-12-310001860742blco:SeniorSecured8375NotesDueOctober2028Memberus-gaap:SecuredDebtMember2023-12-310001860742blco:SeniorSecured8375NotesDueOctober2028Memberus-gaap:SecuredDebtMember2022-12-310001860742blco:TermLoanDueMay2027Member2022-05-100001860742blco:TermLoanDueMay2027Member2022-05-102022-05-100001860742blco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMember2022-05-102022-05-100001860742blco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMember2022-05-100001860742blco:TermLoanDueSeptember2028Member2023-09-290001860742blco:TermLoanDueSeptember2028Member2023-09-292023-09-290001860742us-gaap:LetterOfCreditMemberblco:RevolvingCreditFacilityDueMay2027Member2023-12-310001860742blco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMemberblco:SOFRCDOREURIBORAndSONIARatesMember2022-05-102022-05-100001860742blco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMemberblco:USDollarBaseRateAndCanadianDollarPrimeRateMember2022-05-102022-05-100001860742blco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMemberus-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMember2022-05-102022-05-100001860742srt:MinimumMemberblco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMemberblco:USDollarBaseRateAndCanadianDollarPrimeRateMember2022-05-102022-05-100001860742blco:RevolvingCreditFacilityDueMay2027Membersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberblco:USDollarBaseRateAndCanadianDollarPrimeRateMember2022-05-102022-05-100001860742srt:MinimumMemberblco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMemberblco:SOFRCDOREURIBORAndSONIARatesMember2022-05-102022-05-100001860742blco:RevolvingCreditFacilityDueMay2027Membersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMemberblco:SOFRCDOREURIBORAndSONIARatesMember2022-05-102022-05-100001860742blco:TermLoanDueMay2027AndTermLoanDueSeptember2028Membersrt:MinimumMemberblco:USDollarBaseRateAndCanadianDollarPrimeRateMember2022-05-102022-05-100001860742blco:TermLoanDueMay2027AndTermLoanDueSeptember2028Membersrt:MaximumMemberblco:USDollarBaseRateAndCanadianDollarPrimeRateMember2022-05-102022-05-100001860742blco:TermLoanDueMay2027AndTermLoanDueSeptember2028Membersrt:MinimumMemberblco:SOFRCDOREURIBORAndSONIARatesMember2022-05-102022-05-100001860742blco:TermLoanDueMay2027AndTermLoanDueSeptember2028Membersrt:MaximumMemberblco:SOFRCDOREURIBORAndSONIARatesMember2022-05-102022-05-100001860742srt:MinimumMemberblco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMember2023-12-310001860742blco:RevolvingCreditFacilityDueMay2027Membersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMember2023-12-310001860742srt:MinimumMemberblco:RevolvingCreditFacilityDueMay2027Memberus-gaap:RevolvingCreditFacilityMember2022-05-102022-05-100001860742blco:RevolvingCreditFacilityDueMay2027Membersrt:MaximumMemberus-gaap:RevolvingCreditFacilityMember2022-05-102022-05-100001860742us-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMemberblco:TermLoanDueMay2027Member2022-05-102022-05-100001860742us-gaap:BaseRateMemberblco:TermLoanDueMay2027Member2022-05-102022-05-100001860742srt:MinimumMemberus-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMemberblco:TermLoanDueMay2027Member2022-05-102022-05-100001860742us-gaap:BaseRateMembersrt:MinimumMemberblco:TermLoanDueMay2027Member2022-05-102022-05-100001860742us-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMemberblco:TermLoanDueSeptember2028Member2023-09-292023-09-290001860742us-gaap:BaseRateMemberblco:TermLoanDueSeptember2028Member2023-09-292023-09-290001860742srt:MinimumMemberus-gaap:SecuredOvernightFinancingRateSofrOvernightIndexSwapRateMemberblco:TermLoanDueSeptember2028Member2023-09-292023-09-290001860742us-gaap:BaseRateMembersrt:MinimumMemberblco:TermLoanDueSeptember2028Member2023-09-292023-09-290001860742blco:TermLoanDueMay2027AndTermLoanDueSeptember2028Memberus-gaap:RevolvingCreditFacilityMember2022-05-102022-05-100001860742blco:TermLoanDueMay2027Member2023-12-312023-12-310001860742blco:TermLoanDueSeptember2028Member2023-12-312023-12-310001860742blco:SeniorSecured8375NotesDueOctober2028Memberus-gaap:SecuredDebtMember2023-09-290001860742blco:SeniorSecured8375NotesDueOctober2028Memberus-gaap:SecuredDebtMember2023-09-292023-09-290001860742blco:SeniorSecured8375NotesDueOctober2028Membersrt:MaximumMemberus-gaap:SecuredDebtMember2023-09-292023-09-290001860742us-gaap:RevolvingCreditFacilityMember2022-05-100001860742us-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742country:IEus-gaap:PensionPlansDefinedBenefitMember2023-12-31blco:defined_benefit_plan0001860742country:IEus-gaap:PensionPlansDefinedBenefitMember2023-01-012023-12-310001860742us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMember2023-01-012023-12-310001860742us-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:PensionPlansDefinedBenefitMembercountry:US2023-01-012023-12-310001860742us-gaap:PensionPlansDefinedBenefitMembercountry:US2022-01-012022-12-310001860742us-gaap:PensionPlansDefinedBenefitMembercountry:US2021-01-012021-12-310001860742us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2023-01-012023-12-310001860742us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2022-01-012022-12-310001860742us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2021-01-012021-12-310001860742us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMembercountry:US2023-01-012023-12-310001860742us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMembercountry:US2022-01-012022-12-310001860742us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMembercountry:US2021-01-012021-12-310001860742us-gaap:PensionPlansDefinedBenefitMembercountry:US2021-12-310001860742us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2021-12-310001860742us-gaap:OtherPostretirementBenefitPlansDefinedBenefitMembercountry:US2021-12-310001860742country:US2023-12-310001860742country:US2022-12-310001860742us-gaap:ForeignPlanMember2023-12-310001860742us-gaap:ForeignPlanMember2022-12-310001860742srt:ScenarioForecastMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2024-01-012024-12-310001860742srt:ScenarioForecastMembercountry:IEus-gaap:PensionPlansDefinedBenefitMember2024-01-012024-12-310001860742us-gaap:CashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:CashAndCashEquivalentsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:DefinedBenefitPlanEquitySecuritiesMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:DefinedBenefitPlanEquitySecuritiesMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FixedIncomeSecuritiesMembercountry:US2023-12-310001860742us-gaap:PensionPlansDefinedBenefitMemberus-gaap:FixedIncomeSecuritiesMembercountry:US2022-12-310001860742us-gaap:CashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:CashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:DefinedBenefitPlanEquitySecuritiesMemberus-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:DefinedBenefitPlanEquitySecuritiesMemberus-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FixedIncomeSecuritiesMember2023-12-310001860742us-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:FixedIncomeSecuritiesMember2022-12-310001860742us-gaap:OtherDebtSecuritiesMemberus-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:OtherDebtSecuritiesMemberus-gaap:ForeignPlanMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:USblco:U.S.BroadMarketMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:USblco:U.S.BroadMarketMember2023-12-310001860742us-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:USblco:U.S.BroadMarketMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:USblco:U.S.BroadMarketMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:USblco:U.S.BroadMarketMember2022-12-310001860742us-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:USblco:U.S.BroadMarketMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel1Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel2Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742blco:NonU.S.DevelopedMarketsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel1Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel2Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742blco:NonU.S.DevelopedMarketsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742blco:NonU.S.OtherAssetsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel2Memberblco:NonU.S.OtherAssetsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742blco:NonU.S.OtherAssetsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742blco:NonU.S.OtherAssetsMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel2Memberblco:NonU.S.OtherAssetsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742blco:NonU.S.OtherAssetsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:DefinedBenefitPlanCashAndCashEquivalentsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberblco:EmergingMarketsMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742blco:NonU.S.DevelopedMarketsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel3Memberblco:NonU.S.DevelopedMarketsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742blco:NonU.S.DevelopedMarketsMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742blco:InvestmentGradeMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742blco:InvestmentGradeMemberus-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742blco:InvestmentGradeMemberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USTreasuryAndGovernmentMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USTreasuryAndGovernmentMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USTreasuryAndGovernmentMember2023-12-310001860742us-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USTreasuryAndGovernmentMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USTreasuryAndGovernmentMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USTreasuryAndGovernmentMember2022-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USTreasuryAndGovernmentMember2022-12-310001860742us-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberus-gaap:USTreasuryAndGovernmentMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberblco:OtherAssetsFixedIncomeSecuritiesMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberblco:OtherAssetsFixedIncomeSecuritiesMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberblco:OtherAssetsFixedIncomeSecuritiesMember2023-12-310001860742us-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberblco:OtherAssetsFixedIncomeSecuritiesMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberblco:OtherAssetsFixedIncomeSecuritiesMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberblco:OtherAssetsFixedIncomeSecuritiesMember2022-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberblco:OtherAssetsFixedIncomeSecuritiesMember2022-12-310001860742us-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMemberblco:OtherAssetsFixedIncomeSecuritiesMember2022-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2023-12-310001860742us-gaap:FairValueInputsLevel1Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel2Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:FairValueInputsLevel3Memberus-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742us-gaap:ForeignPlanMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:PensionPlansDefinedBenefitMember2022-12-310001860742country:IEus-gaap:PensionPlansDefinedBenefitMember2022-01-012022-12-310001860742blco:BauschLombAndBauschHealthCompaniesMember2023-01-012023-12-310001860742blco:BauschLombAndBauschHealthCompaniesMember2022-01-012022-12-310001860742blco:BauschLombAndBauschHealthCompaniesMember2021-01-012021-12-310001860742blco:IPOFoundersGrantsMembersrt:ExecutiveOfficerMember2022-05-052022-05-050001860742blco:IPOFoundersGrantsMembersrt:ExecutiveOfficerMemberus-gaap:EmployeeStockOptionMember2022-05-050001860742blco:IPOFoundersGrantsMembersrt:ExecutiveOfficerMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-050001860742blco:ShareBasedPaymentArrangementOptionAndRestrictedStockUnitsRSUsMemberblco:NonExecutiveEligibleRecipientsMember2022-05-052022-05-050001860742blco:IPOFoundersGrantsMemberblco:ShareBasedPaymentArrangementOptionAndRestrictedStockUnitsRSUsMemberblco:NonExecutiveEligibleRecipientsMember2022-05-052022-05-050001860742blco:IPOFoundersGrantsMemberblco:NonExecutiveEligibleRecipientsMemberus-gaap:EmployeeStockOptionMember2022-05-052022-05-050001860742blco:IPOFoundersGrantsMemberus-gaap:ShareBasedCompensationAwardTrancheOneMemberblco:NonExecutiveEligibleRecipientsMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-052022-05-050001860742us-gaap:ShareBasedCompensationAwardTrancheTwoMemberblco:IPOFoundersGrantsMemberblco:NonExecutiveEligibleRecipientsMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-052022-05-050001860742blco:IPOFoundersGrantsMemberblco:ShareBasedPaymentArrangementOptionAndRestrictedStockUnitsRSUsMemberblco:ExecutiveOfficerExcludingTheChiefExecutiveOfficerMember2022-07-012022-09-300001860742blco:IPOFoundersGrantsMemberblco:ShareBasedPaymentArrangementOptionAndRestrictedStockUnitsRSUsMemberblco:ExecutiveOfficerExcludingTheChiefExecutiveOfficerMember2022-05-052022-05-050001860742blco:IPOFoundersGrantsMemberus-gaap:EmployeeStockOptionMemberblco:ExecutiveOfficerExcludingTheChiefExecutiveOfficerMember2022-07-012022-09-300001860742blco:ExecutiveOfficerAndCertainOtherEmployeesExcludingTheChiefExecutiveOfficerMemberblco:A2022OmnibusIncentivePlanRetentionProgramMemberus-gaap:RestrictedStockUnitsRSUMember2022-07-012022-09-300001860742blco:A2022OmnibusIncentivePlanMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMemberblco:PerformanceRestrictedShareUnitsMember2023-02-232023-02-230001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMember2023-02-232023-02-230001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMember2023-02-232023-02-230001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:ShareBasedCompensationAwardTrancheOneMemberus-gaap:RestrictedStockUnitsRSUMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMember2023-02-232023-02-230001860742us-gaap:ShareBasedCompensationAwardTrancheTwoMemberblco:A2022OmnibusIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMember2023-02-232023-02-230001860742blco:A2022OmnibusIncentivePlanMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMemberblco:PerformanceRestrictedShareUnitsMember2023-03-062023-03-060001860742blco:A2022OmnibusIncentivePlanMember2023-12-310001860742us-gaap:EmployeeStockOptionMember2023-01-012023-12-310001860742us-gaap:EmployeeStockOptionMember2022-01-012022-12-310001860742us-gaap:EmployeeStockOptionMember2021-01-012021-12-310001860742blco:PerformanceRestrictedShareUnitsAndRestrictedStockUnitsMember2023-01-012023-12-310001860742blco:PerformanceRestrictedShareUnitsAndRestrictedStockUnitsMember2022-01-012022-12-310001860742blco:PerformanceRestrictedShareUnitsAndRestrictedStockUnitsMember2021-01-012021-12-310001860742us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-12-310001860742us-gaap:ResearchAndDevelopmentExpenseMember2022-01-012022-12-310001860742us-gaap:ResearchAndDevelopmentExpenseMember2021-01-012021-12-310001860742us-gaap:SellingGeneralAndAdministrativeExpensesMember2023-01-012023-12-310001860742us-gaap:SellingGeneralAndAdministrativeExpensesMember2022-01-012022-12-310001860742us-gaap:SellingGeneralAndAdministrativeExpensesMember2021-01-012021-12-310001860742srt:AffiliatedEntityMember2021-01-012021-12-310001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:ShareBasedCompensationAwardTrancheThreeMemberus-gaap:EmployeeStockOptionMember2022-05-052022-05-050001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:ShareBasedCompensationAwardTrancheOneMemberus-gaap:EmployeeStockOptionMember2022-05-052022-05-050001860742us-gaap:ShareBasedCompensationAwardTrancheTwoMemberblco:A2022OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-05-052022-05-050001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-12-310001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMember2023-01-012023-12-310001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMember2023-12-310001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMember2022-01-012022-12-310001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:ShareBasedCompensationAwardTrancheOneMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-052022-05-050001860742us-gaap:ShareBasedCompensationAwardTrancheTwoMemberblco:A2022OmnibusIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-052022-05-050001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:ShareBasedCompensationAwardTrancheThreeMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-052022-05-050001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-052022-05-050001860742blco:IPOFoundersGrantsMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-05blco:installment0001860742blco:IPOFoundersGrantsMemberus-gaap:ShareBasedCompensationAwardTrancheOneMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-052022-05-050001860742us-gaap:ShareBasedCompensationAwardTrancheTwoMemberblco:IPOFoundersGrantsMemberus-gaap:RestrictedStockUnitsRSUMember2022-05-052022-05-050001860742us-gaap:RestrictedStockUnitsRSUMember2022-12-310001860742us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310001860742us-gaap:RestrictedStockUnitsRSUMember2023-12-310001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310001860742blco:A2022OmnibusIncentivePlanMemberblco:PerformanceBasedRestrictedStockUnitsMember2023-12-310001860742blco:TotalShareholderReturnPerformanceBasedRestrictedStockUnitsMember2021-01-012021-12-310001860742blco:TotalShareholderReturnPerformanceBasedRestrictedStockUnitsMember2022-01-012022-12-310001860742blco:TotalShareholderReturnPerformanceBasedRestrictedStockUnitsMember2023-01-012023-12-310001860742blco:PerformanceBasedRestrictedStockUnitsMember2022-12-310001860742blco:PerformanceBasedRestrictedStockUnitsMember2023-01-012023-12-310001860742blco:PerformanceBasedRestrictedStockUnitsMember2023-12-310001860742blco:A2022OmnibusIncentivePlanMemberblco:PerformanceBasedRestrictedStockUnitsMember2023-01-012023-12-310001860742blco:A2022OmnibusIncentivePlanMemberblco:TotalShareholderReturnPerformanceBasedRestrictedStockUnitsMember2023-01-012023-12-310001860742blco:A2022OmnibusIncentivePlanMemberblco:OrganicRevenueGrowthPerformanceBasedRestrictedStockUnitsMember2023-01-012023-12-310001860742blco:A2022OmnibusIncentivePlanMemberblco:CertainEmployeesMemberblco:PerformanceBasedRestrictedStockUnitsMembersrt:AffiliatedEntityMember2023-12-310001860742blco:ExecutiveOfficerAndCertainOtherEmployeesExcludingTheChiefExecutiveOfficerMemberblco:A2022OmnibusIncentivePlanRetentionProgramMemberus-gaap:ShareBasedCompensationAwardTrancheOneMemberus-gaap:RestrictedStockUnitsRSUMember2022-07-012022-09-300001860742us-gaap:ShareBasedCompensationAwardTrancheTwoMemberblco:ExecutiveOfficerAndCertainOtherEmployeesExcludingTheChiefExecutiveOfficerMemberblco:A2022OmnibusIncentivePlanRetentionProgramMemberus-gaap:RestrictedStockUnitsRSUMember2022-07-012022-09-300001860742blco:ExecutiveOfficerAndCertainOtherEmployeesExcludingTheChiefExecutiveOfficerMemberblco:A2022OmnibusIncentivePlanRetentionProgramMemberus-gaap:ShareBasedCompensationAwardTrancheThreeMemberus-gaap:RestrictedStockUnitsRSUMember2022-07-012022-09-300001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:ShareBasedCompensationAwardTrancheOneMemberus-gaap:EmployeeStockOptionMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMember2023-03-062023-03-060001860742us-gaap:ShareBasedCompensationAwardTrancheTwoMemberblco:A2022OmnibusIncentivePlanMemberus-gaap:EmployeeStockOptionMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMember2023-03-062023-03-060001860742blco:A2022OmnibusIncentivePlanMemberus-gaap:ShareBasedCompensationAwardTrancheThreeMemberus-gaap:EmployeeStockOptionMemberblco:ChiefExecutiveOfficerAndBoardOfDirectorsChairmanMember2023-03-062023-03-060001860742us-gaap:AccumulatedTranslationAdjustmentMember2023-12-310001860742us-gaap:AccumulatedTranslationAdjustmentMember2022-12-310001860742us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-12-310001860742us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-12-310001860742blco:SeparationCostsMemberus-gaap:OtherOperatingIncomeExpenseMember2023-01-012023-12-310001860742blco:SeparationCostsMemberus-gaap:OtherOperatingIncomeExpenseMember2022-01-012022-12-310001860742blco:SeparationCostsMemberus-gaap:OtherOperatingIncomeExpenseMember2021-01-012021-12-310001860742us-gaap:SellingGeneralAndAdministrativeExpensesMemberblco:SeparationRelatedCostsMember2023-01-012023-12-310001860742us-gaap:SellingGeneralAndAdministrativeExpensesMemberblco:SeparationRelatedCostsMember2022-01-012022-12-310001860742us-gaap:SellingGeneralAndAdministrativeExpensesMemberblco:SeparationRelatedCostsMember2021-01-012021-12-310001860742us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2022-12-310001860742us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2021-12-310001860742us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2020-12-310001860742us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2023-01-012023-12-310001860742us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2022-01-012022-12-310001860742us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2021-01-012021-12-310001860742us-gaap:ValuationAllowanceOfDeferredTaxAssetsMember2023-12-310001860742blco:ForeignCountryStateAndLocalMember2023-01-012023-12-310001860742us-gaap:DomesticCountryMember2023-12-310001860742us-gaap:RevenueCommissionersIrelandMemberus-gaap:ForeignCountryMember2023-12-31blco:subsidiary0001860742us-gaap:DomesticCountryMembersrt:MinimumMember2023-01-012023-12-310001860742us-gaap:DomesticCountryMembersrt:MaximumMember2023-01-012023-12-310001860742country:CAsrt:MinimumMember2023-01-012023-12-310001860742country:CAsrt:MaximumMember2023-01-012023-12-310001860742country:DEsrt:MinimumMember2023-01-012023-12-310001860742country:DEsrt:MaximumMember2023-01-012023-12-310001860742srt:MinimumMembercountry:FR2023-01-012023-12-310001860742srt:MaximumMembercountry:FR2023-01-012023-12-310001860742srt:MinimumMembercountry:IE2023-01-012023-12-310001860742srt:MaximumMembercountry:IE2023-01-012023-12-310001860742srt:MinimumMembercountry:CN2023-01-012023-12-310001860742srt:MaximumMembercountry:CN2023-01-012023-12-3100018607422022-04-280001860742blco:EmployeeStockOptionsRestrictedStockUnitsAndPerformanceBasedRestrictedStockUnitsMember2023-01-012023-12-310001860742blco:EmployeeStockOptionsTimeBasedRestrictedStockUnitsandPerformanceBasedRestrictedStockUnitsMember2023-01-012023-12-310001860742blco:EmployeeStockOptionsAndRestrictedStockUnitsRSUsMemberblco:IPOFoundersGrantsMember2023-01-012023-12-310001860742blco:PerformanceRestrictedShareUnitsMember2023-01-012023-12-310001860742blco:EmployeeStockOptionsTimeBasedRestrictedStockUnitsandPerformanceBasedRestrictedStockUnitsMember2022-01-012022-12-310001860742blco:EmployeeStockOptionsAndRestrictedStockUnitsRSUsMemberblco:IPOFoundersGrantsMember2022-01-012022-12-310001860742us-gaap:PendingLitigationMemberblco:BHCMemberblco:ShowerToShowerProductLiabilityLitigationMember2023-12-31blco:case0001860742us-gaap:PendingLitigationMemberblco:BHCMemberblco:ShowerToShowerProductLiabilityLitigationAtlanticCountyNewJerseyMultiCountyStipulationsOfDismissalSubmittedMember2023-12-310001860742us-gaap:PendingLitigationMemberblco:BHCMemberblco:ShowerToShowerProductLiabilityLitigationAllegingCausedOvarianCancerMesotheliomaOrBreastCancerMember2023-12-310001860742blco:BHCMemberblco:ShowerToShowerProductLiabilityLitigationMembercountry:CA2023-12-310001860742blco:BHCMemberstpr:CA-BCblco:ShowerToShowerProductLiabilityLitigationMember2023-12-310001860742blco:BHCMemberblco:ShowerToShowerProductLiabilityLitigationMemberstpr:CA-QC2023-12-310001860742blco:CaliforniaProposition65RelatedMatterLitigationMemberblco:BauschHealthCompaniesMember2023-02-172023-02-170001860742srt:MinimumMemberblco:DoctorsAllergyFormulaLLCLitigationMemberblco:BauschHealthAmericasMember2018-04-012018-04-300001860742us-gaap:PendingLitigationMemberblco:PreserVisionAREDSPatentLitigationMember2021-03-012021-03-31blco:defendantblco:proceeding0001860742blco:PreserVisionAREDSPatentLitigationMemberus-gaap:SettledLitigationMember2023-01-012023-12-310001860742blco:PreserVisionAREDSPatentLitigationMemberblco:DefaultJudgementMember2023-01-012023-12-310001860742us-gaap:PendingLitigationMemberus-gaap:SubsequentEventMemberblco:PreserVisionAREDSPatentLitigationMember2024-02-212024-02-210001860742blco:LumifyParagraphIVProceedingsSlaybackANDALitigationMember2021-09-102021-09-100001860742blco:NovaliqGmbHMember2023-12-310001860742us-gaap:OperatingSegmentsMemberblco:VisionCareMember2023-01-012023-12-310001860742us-gaap:OperatingSegmentsMemberblco:VisionCareMember2022-01-012022-12-310001860742us-gaap:OperatingSegmentsMemberblco:VisionCareMember2021-01-012021-12-310001860742blco:PharmaceuticalsMemberus-gaap:OperatingSegmentsMember2023-01-012023-12-310001860742blco:PharmaceuticalsMemberus-gaap:OperatingSegmentsMember2022-01-012022-12-310001860742blco:PharmaceuticalsMemberus-gaap:OperatingSegmentsMember2021-01-012021-12-310001860742blco:SurgicalMemberus-gaap:OperatingSegmentsMember2023-01-012023-12-310001860742blco:SurgicalMemberus-gaap:OperatingSegmentsMember2022-01-012022-12-310001860742blco:SurgicalMemberus-gaap:OperatingSegmentsMember2021-01-012021-12-310001860742us-gaap:OperatingSegmentsMember2023-01-012023-12-310001860742us-gaap:OperatingSegmentsMember2022-01-012022-12-310001860742us-gaap:OperatingSegmentsMember2021-01-012021-12-310001860742us-gaap:CorporateNonSegmentMember2023-01-012023-12-310001860742us-gaap:CorporateNonSegmentMember2022-01-012022-12-310001860742us-gaap:CorporateNonSegmentMember2021-01-012021-12-310001860742blco:PharmaceuticalProductsMemberblco:VisionCareMember2023-01-012023-12-310001860742blco:PharmaceuticalProductsMemberblco:VisionCareMember2022-01-012022-12-310001860742blco:PharmaceuticalProductsMemberblco:VisionCareMember2021-01-012021-12-310001860742blco:PharmaceuticalsMemberblco:PharmaceuticalProductsMember2023-01-012023-12-310001860742blco:PharmaceuticalsMemberblco:PharmaceuticalProductsMember2022-01-012022-12-310001860742blco:PharmaceuticalsMemberblco:PharmaceuticalProductsMember2021-01-012021-12-310001860742blco:PharmaceuticalProductsMemberblco:SurgicalMember2023-01-012023-12-310001860742blco:PharmaceuticalProductsMemberblco:SurgicalMember2022-01-012022-12-310001860742blco:PharmaceuticalProductsMemberblco:SurgicalMember2021-01-012021-12-310001860742blco:PharmaceuticalProductsMember2023-01-012023-12-310001860742blco:PharmaceuticalProductsMember2022-01-012022-12-310001860742blco:PharmaceuticalProductsMember2021-01-012021-12-310001860742blco:DeviceProductsMemberblco:VisionCareMember2023-01-012023-12-310001860742blco:DeviceProductsMemberblco:VisionCareMember2022-01-012022-12-310001860742blco:DeviceProductsMemberblco:VisionCareMember2021-01-012021-12-310001860742blco:PharmaceuticalsMemberblco:DeviceProductsMember2023-01-012023-12-310001860742blco:PharmaceuticalsMemberblco:DeviceProductsMember2022-01-012022-12-310001860742blco:PharmaceuticalsMemberblco:DeviceProductsMember2021-01-012021-12-310001860742blco:SurgicalMemberblco:DeviceProductsMember2023-01-012023-12-310001860742blco:SurgicalMemberblco:DeviceProductsMember2022-01-012022-12-310001860742blco:SurgicalMemberblco:DeviceProductsMember2021-01-012021-12-310001860742blco:DeviceProductsMember2023-01-012023-12-310001860742blco:DeviceProductsMember2022-01-012022-12-310001860742blco:DeviceProductsMember2021-01-012021-12-310001860742blco:OvertheCounterProductsMemberblco:VisionCareMember2023-01-012023-12-310001860742blco:OvertheCounterProductsMemberblco:VisionCareMember2022-01-012022-12-310001860742blco:OvertheCounterProductsMemberblco:VisionCareMember2021-01-012021-12-310001860742blco:PharmaceuticalsMemberblco:OvertheCounterProductsMember2023-01-012023-12-310001860742blco:PharmaceuticalsMemberblco:OvertheCounterProductsMember2022-01-012022-12-310001860742blco:PharmaceuticalsMemberblco:OvertheCounterProductsMember2021-01-012021-12-310001860742blco:SurgicalMemberblco:OvertheCounterProductsMember2023-01-012023-12-310001860742blco:SurgicalMemberblco:OvertheCounterProductsMember2022-01-012022-12-310001860742blco:SurgicalMemberblco:OvertheCounterProductsMember2021-01-012021-12-310001860742blco:OvertheCounterProductsMember2023-01-012023-12-310001860742blco:OvertheCounterProductsMember2022-01-012022-12-310001860742blco:OvertheCounterProductsMember2021-01-012021-12-310001860742blco:BrandedandOtherGenericProductsMemberblco:VisionCareMember2023-01-012023-12-310001860742blco:BrandedandOtherGenericProductsMemberblco:VisionCareMember2022-01-012022-12-310001860742blco:BrandedandOtherGenericProductsMemberblco:VisionCareMember2021-01-012021-12-310001860742blco:PharmaceuticalsMemberblco:BrandedandOtherGenericProductsMember2023-01-012023-12-310001860742blco:PharmaceuticalsMemberblco:BrandedandOtherGenericProductsMember2022-01-012022-12-310001860742blco:PharmaceuticalsMemberblco:BrandedandOtherGenericProductsMember2021-01-012021-12-310001860742blco:SurgicalMemberblco:BrandedandOtherGenericProductsMember2023-01-012023-12-310001860742blco:SurgicalMemberblco:BrandedandOtherGenericProductsMember2022-01-012022-12-310001860742blco:SurgicalMemberblco:BrandedandOtherGenericProductsMember2021-01-012021-12-310001860742blco:BrandedandOtherGenericProductsMember2023-01-012023-12-310001860742blco:BrandedandOtherGenericProductsMember2022-01-012022-12-310001860742blco:BrandedandOtherGenericProductsMember2021-01-012021-12-310001860742blco:OtherRevenuesMemberblco:VisionCareMember2023-01-012023-12-310001860742blco:OtherRevenuesMemberblco:VisionCareMember2022-01-012022-12-310001860742blco:OtherRevenuesMemberblco:VisionCareMember2021-01-012021-12-310001860742blco:PharmaceuticalsMemberblco:OtherRevenuesMember2023-01-012023-12-310001860742blco:PharmaceuticalsMemberblco:OtherRevenuesMember2022-01-012022-12-310001860742blco:PharmaceuticalsMemberblco:OtherRevenuesMember2021-01-012021-12-310001860742blco:OtherRevenuesMemberblco:SurgicalMember2023-01-012023-12-310001860742blco:OtherRevenuesMemberblco:SurgicalMember2022-01-012022-12-310001860742blco:OtherRevenuesMemberblco:SurgicalMember2021-01-012021-12-310001860742blco:OtherRevenuesMember2023-01-012023-12-310001860742blco:OtherRevenuesMember2022-01-012022-12-310001860742blco:OtherRevenuesMember2021-01-012021-12-31blco:product0001860742blco:CustomerTopTenProductsMemberus-gaap:ProductConcentrationRiskMemberblco:RevenuesNetMember2023-01-012023-12-310001860742blco:CustomerTopTenProductsMemberus-gaap:ProductConcentrationRiskMemberblco:RevenuesNetMember2022-01-012022-12-310001860742blco:CustomerTopTenProductsMemberus-gaap:ProductConcentrationRiskMemberblco:RevenuesNetMember2021-01-012021-12-310001860742blco:UnitedStatesandPuertoRicoMember2023-01-012023-12-310001860742blco:UnitedStatesandPuertoRicoMember2022-01-012022-12-310001860742blco:UnitedStatesandPuertoRicoMember2021-01-012021-12-310001860742country:CN2023-01-012023-12-310001860742country:CN2022-01-012022-12-310001860742country:CN2021-01-012021-12-310001860742country:FR2023-01-012023-12-310001860742country:FR2022-01-012022-12-310001860742country:FR2021-01-012021-12-310001860742country:JP2023-01-012023-12-310001860742country:JP2022-01-012022-12-310001860742country:JP2021-01-012021-12-310001860742country:DE2023-01-012023-12-310001860742country:DE2022-01-012022-12-310001860742country:DE2021-01-012021-12-310001860742country:GB2023-01-012023-12-310001860742country:GB2022-01-012022-12-310001860742country:GB2021-01-012021-12-310001860742country:CA2023-01-012023-12-310001860742country:CA2022-01-012022-12-310001860742country:CA2021-01-012021-12-310001860742country:RU2023-01-012023-12-310001860742country:RU2022-01-012022-12-310001860742country:RU2021-01-012021-12-310001860742country:ES2023-01-012023-12-310001860742country:ES2022-01-012022-12-310001860742country:ES2021-01-012021-12-310001860742country:IT2023-01-012023-12-310001860742country:IT2022-01-012022-12-310001860742country:IT2021-01-012021-12-310001860742country:MX2023-01-012023-12-310001860742country:MX2022-01-012022-12-310001860742country:MX2021-01-012021-12-310001860742country:PL2023-01-012023-12-310001860742country:PL2022-01-012022-12-310001860742country:PL2021-01-012021-12-310001860742country:KR2023-01-012023-12-310001860742country:KR2022-01-012022-12-310001860742country:KR2021-01-012021-12-310001860742blco:OtherCountriesMember2023-01-012023-12-310001860742blco:OtherCountriesMember2022-01-012022-12-310001860742blco:OtherCountriesMember2021-01-012021-12-310001860742blco:UnitedStatesandPuertoRicoMember2023-12-310001860742blco:UnitedStatesandPuertoRicoMember2022-12-310001860742country:IE2023-12-310001860742country:IE2022-12-310001860742country:DE2023-12-310001860742country:DE2022-12-310001860742country:CA2023-12-310001860742country:CA2022-12-310001860742country:FR2023-12-310001860742country:FR2022-12-310001860742country:CN2023-12-310001860742country:CN2022-12-310001860742country:IT2023-12-310001860742country:IT2022-12-310001860742country:ES2023-12-310001860742country:ES2022-12-310001860742blco:OtherCountriesMember2023-12-310001860742blco:OtherCountriesMember2022-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________________
FORM 10-K
| | | | | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the Fiscal Year Ended | December 31, 2023 |
| OR |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the transition period from ____________ to ____________ |
| | |
Commission File Number: 001-41380
Bausch + Lomb Corporation
(Exact Name of Registrant as Specified in its Charter)
| | | | | | | | | | | | | | | | | | | | | | | |
| Canada | 98-1613662 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
520 Applewood Crescent, Vaughan, Ontario, Canada L4K 4B4
(Address of Principal Executive Offices) (Zip Code)
Registrant's telephone number, including area code (905) 695-7700
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Shares, No Par Value | BLCO | New York Stock Exchange | Toronto Stock Exchange |
Securities registered pursuant to section 12(g) of the Act:
None
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or Section 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ý No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Large accelerated filer | x | Accelerated filer | ☐ | Non-accelerated filer
(Do not check if a smaller
reporting company) | ☐ | Smaller reporting company | ☐ | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ý
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the common shares held by non-affiliates of the registrant as of the last business day of the registrant’s most recently completed second fiscal quarter was $802,313,182 based on the last reported sale price on the New York Stock Exchange on June 30, 2023.
The number of outstanding shares of the registrant’s common shares as of February 16, 2024 was 350,992,243.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates certain information by reference from the registrant’s proxy statement for the 2024 Annual Meeting of Shareholders. Such proxy statement will be filed no later than 120 days after the close of the registrant’s fiscal year ended December 31, 2023.
TABLE OF CONTENTS
GENERAL INFORMATION
| | | | | | | | | | | | | | |
| | | | | Page |
| PART I |
| Item 1. | | Business | | |
| Item 1A. | | Risk Factors | | |
| Item 1B. | | Unresolved Staff Comments | | |
| Item 1C. | | Cybersecurity | | |
| Item 2. | | Properties | | |
| Item 3. | | Legal Proceedings | | |
| Item 4. | | Mine Safety Disclosures | | |
| PART II |
| Item 5. | | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | | |
| Item 6. | | Reserved | | |
| Item 7. | | Management’s Discussion and Analysis of Financial Condition and Results of Operations | | |
| Item 7A. | | Quantitative and Qualitative Disclosures About Market Risk | | |
| Item 8. | | Financial Statements and Supplementary Data | | |
| Item 9. | | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | | |
| Item 9A. | | Controls and Procedures | | |
| Item 9B. | | Other Information | | |
| Item 9C. | | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | | |
| PART III |
| Item 10. | | Directors, Executive Officers and Corporate Governance | | |
| Item 11. | | Executive Compensation | | |
| Item 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | | |
| Item 13. | | Certain Relationships and Related Transactions, and Director Independence | | |
| Item 14. | | Principal Accounting Fees and Services | | |
| PART IV |
| Item 15. | | Exhibits and Financial Statement Schedules | | |
| Item 16. | | Form 10-K Summary | | |
| SIGNATURES | | |
| | | | |
Basis of Presentation
General
Except where the context otherwise requires, all references in this Annual Report on Form 10-K ("Form 10-K") to the “Company”, “Bausch + Lomb”, “we”, “us”, “our” or similar words or phrases are to Bausch + Lomb Corporation and its subsidiaries, taken together. In this Form 10-K, references to “$” are to United States (“U.S.”) dollars, references to “€” are to euros, references to “£” or “GBP” are to British pounds and references to “CAD” are to Canadian dollars. Unless otherwise indicated, the statistical and financial data contained in this Form 10-K are presented as of December 31, 2023.
Trademarks
The following words are some of the trademarks in our Company’s trademark portfolio and are the subject of either registration, or application for registration, in one or more of the U.S., Canada or certain other jurisdictions: ACUSTREAM®, AERGEL®, AKREOS®, ALAWAY®, ALREX®, AMVISC®, AQUALOX®, ARTELAC®, B & L®, B + L®, BAUSCH & LOMB®, BAUSCH + LOMB®, BAUSCH + LOMB INFUSE®, BAUSCH + LOMB ULTRA®, BESIVANCE®, BLINK®, BLINK CONTACTS®, BLINK GELTEARS®, BLINK-N-CLEAN®, BIOTRUE®, BOSTON®, CLEARVISC®, COMFORTMOIST®, CRYSTALENS®, ENVISTA®, ENVISTA ASPIRETM, ENVISTA BEYONDTM, ENVISTA ENVYTM, EYEFILL®, IC-8®, IC-8 APTHERATM, INFUSE®, LOTEMAX®, LUMIFY®, LUMIFY EYE ILLUMINATIONSTM, LUXLIFE®, LUXSMARTTM, MIEBO®, MILLENNIUM®, MINIMS®, MIOCLEAR®, MOISTURESEAL®, OCUVITE®, OPTICALIGN®, PRESERVISION®, PROLENSA®, PUREVISION®, RENU®, RENU MULTIPLUS®, SHOWER TO SHOWER®, SOFLENS®, SOOTHE®, STABLEVISCTM, STELLARIS®, STELLARIS ELITE®, STORZ®, SYNERGETICS®, TENEOTM, TRULIGN®, VICTUS®, VYZULTA®, XIIDRA®, YELLOXTM, ZYLET® and ZYOPTIXTM.
In addition to the trademarks previously noted, we have filed trademark applications and/or obtained trademark registrations for many of our other trademarks in the U.S., Canada and in other jurisdictions and have implemented, on an ongoing basis, a trademark protection program for new trademarks.
XIPERE® and SCS MICROINJECTOR® are trademarks of Clearside Biomedical, Inc. and are used by us under license. VISUDYNE® is a trademark of Cheplapharm Arzneimittel GmbH and is used by us under license.
Forward-Looking Statements
Caution regarding forward-looking information and statements and “Safe-Harbor” statements under the U.S. Private Securities Litigation Reform Act of 1995 and applicable Canadian securities laws:
To the extent any statements made in this Form 10-K contain information that is not historical, these statements are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and may be forward-looking information within the meaning defined under applicable Canadian securities laws (collectively, “forward-looking statements”).
These forward-looking statements relate to, among other things: our business strategy, business plans, business prospects and forecasts and changes thereto; product pipeline, prospective products and product approvals, expected launches of new products, product development and results of current and anticipated products; our recently consummated acquisition of XIIDRA® and certain other ophthalmology assets (the “XIIDRA Acquisition”); anticipated revenues for our products; expected R&D and marketing spend; our expected primary cash and working capital requirements for 2024 and beyond; our plans for continued improvement in operational efficiency and the anticipated impact of such plans; our expectations regarding the implementation of a system upgrade to our Lynchburg distribution facility; our liquidity and our ability to satisfy our debt maturities as they become due; our ability to comply with the covenants contained in our credit agreement, as recently amended, (the “Amended Credit Agreement”) and in the indenture governing our October 2028 Secured Notes (as defined below); any proposed pricing actions; exposure to foreign currency exchange rate changes and interest rate changes; the outcome of contingencies, such as litigation, subpoenas, investigations, reviews, audits and regulatory proceedings; the anticipated impact of the adoption of new accounting standards; general market conditions and economic uncertainty; our expectations regarding our financial performance, including our future financial and operating performance, revenues, expenses, gross margins and income taxes; our impairment assessments, including the assumptions used therein and the results thereof; the anticipated effect of current market conditions and recessionary pressures in one or more of our markets; the anticipated effect of macroeconomic factors, including inflation; the anticipated impact from the ongoing conflicts between Russia and Ukraine and in the Middle East involving Israel and Hamas; and the anticipated separation from Bausch Health Companies Inc. (“BHC”), including the structure and expected timetable for completing such separation transaction.
Forward-looking statements can generally be identified by the use of words such as “believe,” “anticipate,” “expect,” “intend,” “estimate,” “plan,” “schedule,” “continue,” “future,” “will,” “may,” “can,” “might,” “could,” “would,” “should,” “target,” “potential,” “opportunity,” “designed,” “create,” “predict,” “project,” “timeline,” “forecast,” “outlook,” “guidance,” “seek,” “strive,” “suggest,” “prospective,” “strategy,” “indicative,” “intend,” “ongoing,” “decrease” or “increase” and positive and negative variations thereof or other similar expressions. In addition, any statements that refer to expectations, intentions, projections or other characterizations of future events or circumstances are forward-looking statements. These forward-looking statements may not be appropriate for other purposes. Although we have previously indicated certain of these statements set out herein, all of the statements in this Form 10-K that contain forward-looking statements are qualified by these cautionary statements. These statements are based upon the current expectations and beliefs of management. Although we believe that the expectations reflected in such forward-looking statements are reasonable, such statements involve risks and uncertainties, and undue reliance should not be placed on such statements. Certain material factors or assumptions are applied in making such forward-looking statements, including, but not limited to, factors and assumptions regarding the items previously outlined, those factors, risks and uncertainties outlined below and the assumption that none of these factors, risks and uncertainties will cause actual results or events to differ materially from those described in such forward-looking statements. Actual results may differ materially from those expressed or implied in such statements. Important factors, risks and uncertainties that could cause actual results to differ materially from these expectations include, among other things, the following:
•adverse economic conditions and other macroeconomic factors, including inflation, slower growth or a potential recession, which could adversely impact our revenues, expenses and resulting margins;
•the effect of current market conditions and recessionary pressures in one or more of our markets;
•the challenges the Company faces following its initial public offering (the “B+L IPO”), including the challenges and difficulties associated with managing an independent, complex business, the transitional services being provided by and to BHC, and any potential, actual or perceived conflict of interest of some of our directors and officers because of their equity ownership in BHC and/or because they also serve as directors of BHC;
•our status as a controlled company, and the possibility that BHC’s interest may conflict with our interests and the interests of our other shareholders and other stakeholders;
•the risks and uncertainties associated with the proposed plan to separate or spinoff Bausch + Lomb from BHC, which include, but are not limited to, the expected benefits and costs of the spinoff transaction, the expected timing of completion of the spinoff transaction and its terms (including the expectation that the spinoff transaction will be completed following the achievement of targeted debt leverage ratios, subject to receipt of applicable shareholder and other necessary approvals and other factors), the ability to complete the spinoff transaction considering the various conditions to the completion of the spinoff transaction (some of which are outside the Company’s and BHC's control, including conditions related to regulatory matters and receipt of applicable shareholder approvals), the impact of any potential sales of our common shares by BHC, that market or other conditions are no longer favorable to completing the transaction, that applicable shareholder, stock exchange, regulatory or other approval is not obtained on the terms or timelines anticipated or at all, the impact on the spinoff transaction (and the timing thereof) of the filing by Norwich Pharmaceuticals Inc. ("Norwich") of its Abbreviated New Drug Application ("ANDA") for Xifaxan® (rifaxamin) 550 mg tablets and BHC's related lawsuit filed against Norwich in connection therewith (including BHC’s ability to successfully appeal the decision of the U.S. District Court for the District of Delaware in such lawsuit), business disruption during the pendency of, or following, the spinoff transaction, diversion of management time on spinoff transaction-related issues, retention of existing management team members, the reaction of customers and other parties to the spinoff transaction, the structure of the spinoff transaction and related distribution, the qualification of the spinoff transaction as a tax-free transaction for Canadian and/or U.S. federal income tax purposes (including whether or not an advance ruling from the Canada Revenue Agency and/or the Internal Revenue Service will be sought or obtained), the ability of the Company and BHC to satisfy the conditions required to maintain the tax-free status of the spinoff transaction (some of which are beyond their control), other potential tax or other liabilities that may arise as a result of the spinoff transaction, the potential dis-synergy costs resulting from the spinoff transaction, the impact of the spinoff transaction on relationships with customers, suppliers, employees and other business counterparties, general economic conditions, conditions in the markets the Company is engaged in, behavior of customers, suppliers and competitors, technological developments, as well as legal and regulatory rules affecting the Company’s business. In particular, the Company can offer no assurance that any spinoff transaction will occur at all, or that any such transaction will occur on the timelines or in the manner anticipated by the Company and BHC;
•ongoing litigation and potential additional litigation, claims, challenges and/or regulatory investigations challenging or otherwise relating to the B+L IPO and the proposed separation from BHC and the costs, expenses, use of resources, diversion of management time and efforts, liability and damages that may result therefrom;
•pricing decisions that we have implemented or may in the future elect to implement at the direction of our pricing committees or otherwise;
•legislative or policy efforts, including those that may be introduced and passed by the U.S. Congress, designed to reduce patient out-of-pocket costs for medicines and other products, which could result in new mandatory rebates and discounts or other pricing restrictions, controls or regulations (including mandatory price reductions);
•ongoing oversight and review of our products and facilities by regulatory and governmental agencies, including periodic audits by the U.S. Food and Drug Administration (the “FDA”) and equivalent agencies outside of the United States and the results thereof;
•actions by the FDA or other regulatory authorities with respect to our products or facilities;
•compliance with the legal and regulatory requirements of our marketed products;
•our ability to comply with the financial and other covenants contained in our Amended Credit Agreement, the indenture governing our October 2028 Secured Notes and other current or future debt agreements, including the limitations, restrictions and prohibitions such covenants may impose on the way we conduct our business, including prohibitions on incurring additional debt if certain financial covenants are not met, our ability to draw under the revolving credit facility under our Amended Credit Agreement (the “Revolving Credit Facility”) and restrictions on our ability to make certain investments and other restricted payments;
•any downgrade or additional downgrade by rating agencies in our or BHC's credit ratings, which may impact, among other things, our ability to raise debt and the cost of capital for additional debt issuances;
•changes in the assumptions used in connection with our impairment analyses or assessments, which would lead to a change in such impairment analyses and assessments and which could result in an impairment in the goodwill associated with any of our reporting units or impairment charges related to certain of our products or other intangible assets;
•the risks and uncertainties relating to the XIIDRA Acquisition, including our ability to effectively and efficiently integrate the acquired XIIDRA® product, pipeline products, transferred sales force and other assets into our existing business, risks that such integration efforts will potentially divert the efforts and attention of management and other employees away from our ongoing business operations, the effect of the transaction on our ability to maintain relationships with customers, suppliers, and other business partners, risks relating to our increased levels of debt as a result of debt incurred to finance such acquisition and risks that we may not realize the expected benefits of the acquisition on a timely basis or at all;
•the possibility that the unaudited pro forma financial information included in this Form 10-K may not necessarily be indicative of what the consolidated results of operations would have been had the XIIDRA Acquisition been completed on January 1, 2022 and may differ materially from our actual results of operations;
•the uncertainties associated with the acquisition and launch of new products, assets and businesses (including the recently-acquired XIIDRA® product and Blink® product line and our recently launched MIEBO® product), including, but not limited to, our ability to provide the time, resources, expertise and funds required for the commercial launch of new products, the acceptance and demand for new products, the failure to obtain required regulatory approvals, clearances or authorizations, and the impact of competitive products and pricing, which could lead to material impairment charges;
•our ability or inability to extend the profitable life of our products, including through line extensions and other life-cycle programs;
•our ability to manage the transition to our new Chairman and Chief Executive Officer and other new executive officers and key employees, the success of such individuals in assuming their respective roles and the ability of such individuals to implement and achieve the strategies and goals of the Company as they develop;
•our ability to retain, motivate and recruit executives and other key employees;
•our ability to implement effective succession planning for our executives and other key employees;
•factors impacting our ability to achieve anticipated revenues for our products, including changes in anticipated marketing spend on such products and launch of competing products;
•factors impacting our ability to achieve anticipated market acceptance for our products, including the pricing of such products, effectiveness of promotional efforts, reputation of our products and launch of competing products;
•our ability to compete against companies that are larger and have greater financial, technical and human resources than we do, as well as other competitive factors, such as technological advances achieved, patents obtained and new products introduced by our competitors;
•the extent to which our products are reimbursed by government authorities, pharmacy benefit managers (“PBMs”) and other third-party payors; the impact our distribution, pricing and other practices may have on the decisions of such government authorities, PBMs and other third-party payors to reimburse our products; and the impact of obtaining or maintaining such reimbursement on the price and sales of our products;
•the inclusion of our products on formularies or our ability to achieve favorable formulary status, as well as the impact on the price and sales of our products in connection therewith;
•the consolidation of wholesalers, retail drug chains and other customer groups and the impact of such industry consolidation on our business;
•our ability to maintain strong relationships with physicians and other health care professionals;
•our eligibility for benefits under tax treaties and the continued availability of low effective tax rates for the business profits of certain of our subsidiaries;
•the implementation of the Organisation for Economic Co-operation and Development inclusive framework on Base Erosion and Profit Shifting, including the global minimum corporate tax rate, by the countries in which we operate;
•the actions of our third-party partners or service providers of research, development, manufacturing, marketing, distribution or other services, including their compliance with applicable laws and contracts, which actions may be beyond our control or influence, and the impact of such actions on us;
•the risks associated with the international scope of our operations, including our presence in emerging markets and the challenges we face when entering and operating in new and different geographic markets (including the challenges created by new and different regulatory regimes in such countries and the need to comply with applicable anti-bribery and economic sanctions, laws and regulations);
•adverse global economic conditions and credit markets and foreign currency exchange uncertainty and volatility in certain of the countries in which we do business;
•trade conflicts, including the trade conflict between the United States and China;
•risks associated with the ongoing conflict between Russia and Ukraine and the export controls, sanctions and other restrictive actions that have been or may be imposed by the United States, Canada, the EU and other countries against governmental and other entities and individuals in or associated with Russia, Belarus and parts of Ukraine, including its potential escalation and the potential impact on sales, earnings, market conditions and the ability of the Company to manage resources and historical investment in Russia;
•risks associated with the ongoing conflict in the Middle East involving Israel and Hamas, including its potential escalation and the potential impact on our operations, sale of products and revenues in this region;
•our ability to obtain, maintain and license sufficient intellectual property rights over our products and enforce and defend against challenges to such intellectual property;
•the introduction of generic, biosimilar or other competitors of our branded products and other products, including the introduction of products that compete against our products that do not have patent or data exclusivity rights;
•the expense, timing and outcome of pending or future legal and governmental proceedings, arbitrations, investigations, subpoenas, tax and other regulatory audits, examinations, reviews and regulatory proceedings against us or relating to us and settlements thereof;
•our ability to obtain components, raw materials or finished products supplied by third parties (some of which may be single-sourced) and other manufacturing and related supply difficulties, interruptions and delays;
•the disruption of delivery of our products and the routine flow of manufactured goods;
•potential work stoppages, slowdowns or other labor problems at our facilities and the resulting impact on our manufacturing, distribution and other operations;
•economic factors over which we have no control, including inflationary pressures as a result of historically high domestic and global inflation and otherwise, interest rates, foreign currency rates, and the potential effect of such factors on revenues, expenses and resulting margins;
•interest rate risks associated with our floating rate debt borrowings;
•our ability to effectively distribute our products and the effectiveness and success of our distribution arrangements;
•our ability to effectively promote our own products and those of our co-promotion partners;
•our ability to secure and maintain third-party research, development, manufacturing, licensing, marketing or distribution arrangements;
•the risk that our products could cause, or be alleged to cause, personal injury and adverse effects, leading to potential lawsuits, product liability claims and damages and/or recalls or withdrawals of products from the market;
•the mandatory or voluntary recall or withdrawal of our products from the market and the costs associated therewith;
•the availability of, and our ability to obtain and maintain, adequate insurance coverage and/or our ability to cover or insure against the total amount of the claims and liabilities we face, whether through third-party insurance or self-insurance;
•our indemnity agreements, which may result in an obligation to indemnify or reimburse the relevant counterparty, which amounts may be material;
•the difficulty in predicting the expense, timing and outcome within our legal and regulatory environment, including with respect to approvals by the FDA, Health Canada, the European Medicines Agency (“EMA”) and similar agencies in other jurisdictions, legal and regulatory proceedings and settlements thereof, the protection afforded by our patents and other intellectual and proprietary property, successful generic challenges to our products and infringement or alleged infringement of the intellectual property of others;
•the results of continuing safety and efficacy studies by industry and government agencies;
•the success of preclinical and clinical trials for our drug development pipeline or delays in clinical trials that adversely impact the timely commercialization of our pipeline products, as well as other factors impacting the commercial success of our products, which could lead to material impairment charges;
•uncertainties around the successful improvement and modification of our existing products and development of new products, which may require significant expenditures and efforts;
•the results of management reviews of our research and development portfolio (including following the receipt of clinical results or feedback from the FDA or other regulatory authorities), which could result in terminations of specific projects which, in turn, could lead to material impairment charges;
•the seasonality of sales of certain of our products;
•declines in the pricing and sales volume of certain of our products that are distributed or marketed by third parties, over which we have no or limited control;
•compliance by us or our third-party partners and service providers (over whom we may have limited influence), or the failure by us or these third parties to comply, with health care “fraud and abuse” laws and other extensive regulation of our marketing, promotional and business practices (including with respect to pricing), worldwide anti-bribery laws (including the U.S. Foreign Corrupt Practices Act and the Canadian Corruption of Foreign Public Officials Act), worldwide economic sanctions and/or export laws, worldwide environmental laws and regulation and privacy and security regulations;
•the impacts of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (the “Health Care Reform Act”) and any potential amendment thereof and other legislative and regulatory health care reforms in the countries in which we operate, including with respect to recent government inquiries on pricing;
•the impact of any changes in or reforms to the legislation, laws, rules, regulation and guidance that apply to us and our businesses and products or the enactment of any new or proposed legislation, laws, rules, regulations or guidance that will impact or apply to us or our businesses or products;
•the impact of changes in federal laws and policy that may be undertaken under the Biden administration;
•illegal distribution or sale of counterfeit versions of our products;
•interruptions, breakdowns or breaches in our information technology systems; and
•risks in Item 1A. “Risk Factors” in this Form 10-K.
Additional information about these factors and about the material factors or assumptions underlying such forward-looking statements may be found elsewhere in this Form 10-K, including under Item 1A. "Risk Factors", and in the Company's other filings with the U.S. Securities and Exchange Commission (the “SEC”) and the Canadian Securities Administrators (the “CSA”). When relying on our forward-looking statements to make decisions with respect to the Company, investors and others should carefully consider the foregoing factors and other uncertainties and potential events. These forward-looking statements speak only as of the date made. We undertake no obligation to update or revise any of these forward-looking statements to reflect events or circumstances after the date of this Form 10-K or to reflect actual outcomes, except as required by law. We caution that, as it is not possible to predict or identify all relevant factors that may impact forward-looking statements, the foregoing list of important factors that may affect future results is not exhaustive and should not be considered a complete statement of all potential risks and uncertainties.
PART I
Item 1. Business
Bausch + Lomb Corporation (and its subsidiaries) (“Bausch + Lomb,” “we,” “us,” “our” or the “Company”) is a leading global eye health company dedicated to protecting and enhancing the gift of sight for millions of people around the world – from the moment of birth through every phase of life. Our mission is simple, yet powerful: helping you see better, to live better. We develop, manufacture and market a range of products, primarily in the areas of eye health, which are marketed directly or indirectly in approximately 100 countries. As a fully integrated eye health business, Bausch + Lomb has an established line of contact lenses, intraocular lenses ("IOLs") and other medical devices, surgical systems and devices, vitamin and mineral supplements, lens care products, prescription eye-medications and other consumer products that positions us to compete in all areas of the eye health market.
Our comprehensive portfolio of approximately 400 products is built to serve our customers across the full spectrum of their eye health needs throughout their lives. Our iconic brand is built on the deep trust and loyalty of our customers established over our 170-year history. We have a significant global research, development, manufacturing and commercial footprint of approximately 13,300 employees and a presence in approximately 100 countries, extending our reach to billions of potential customers across the globe. We have long been associated with many of the most significant advances in eye health, and we believe we are well positioned to continue leading the advancement of eye health in the future.
Initial Public Offering and Separation of the Bausch + Lomb Eye Health Business
On August 6, 2020, our parent company, BHC, announced its plan to separate our eye health business into an independent publicly traded entity, separate from the remainder of BHC (the “Separation”). This resulted in the initial public offering of Bausch + Lomb (the “B+L IPO”), and our common shares began trading on the New York Stock Exchange and the Toronto Stock Exchange, in each case under the ticker symbol “BLCO”, on May 6, 2022. Prior to the completion of the B+L IPO, we were an indirect wholly-owned subsidiary of BHC. See Note 2, “SIGNIFICANT ACCOUNTING POLICIES” to our audited Consolidated Financial Statements for additional information.
As of February 16, 2024, BHC directly or indirectly holds 310,449,643 Bausch + Lomb common shares, which represents approximately 88.4% of the issued and outstanding common shares of Bausch + Lomb. The completion of the full Separation of Bausch + Lomb, which includes the transfer of all or a portion of BHC’s remaining direct or indirect equity interest in Bausch + Lomb to its shareholders (the “Distribution”), is subject to the achievement of targeted debt leverage ratios and the receipt of applicable shareholder and other necessary approvals and other factors and is subject to various risk factors relating to the Separation. See Item 1A. “Risk Factors” of this Form 10-K for additional information on the risks associated with the Separation. Bausch + Lomb understands that BHC continues to believe that completing the Separation makes strategic sense and that BHC continues to evaluate all relevant factors and considerations related to completing the Separation, including the effect of the lawsuit filed against Norwich Pharmaceuticals Inc.
We believe the Separation presents Bausch + Lomb with a unique opportunity, provides us operating flexibility and puts us in a strong position to unlock additional value in our eye health business as a separate and dissimilar business from the remainder of BHC’s product portfolios and businesses. As a separate entity, Bausch + Lomb’s management believes that it is positioned to focus on its core businesses to drive additional growth, more effectively allocate capital and better manage our capital needs. Although management believes these transactions will unlock value for our shareholders, there can be no assurance that the Separation will be consummated, or, even if consummated, that the Separation will be successful in doing so.
See Item 1A. ''Risk Factors — Risks Relating to the Separation” included in this Form 10-K for additional information.
Business Strategy
Our strategy is to enhance our position as a leading global eye health company dedicated to helping people see better to live better, through the delivery of high quality, innovative products. To achieve this goal, we plan to generate sustainable and profitable growth by employing the following strategies:
•Leverage our expertise as an eye health-focused company to strengthen our market position - Our comprehensive product offering—spanning over-the counter (“OTC”) products, nutritional supplements, eye health products, ophthalmic pharmaceuticals, contact lenses, lens care products and ophthalmic surgical devices and instruments— allows us to build strong brand loyalty and engage with patients and consumers throughout the entire continuum of their eye health needs over time. We intend to leverage the synergistic nature of our products, our brand equity and our relationships with physicians, patients, consumers and retailers to grow our business globally.
•Increase adoption of our products by growing our addressable market - To increase adoption of our products, we intend to continue our focus on patient, consumer and eye care professional education. In addition, we believe that
we can grow our market opportunity by expanding into emerging therapeutic areas, new geographies and researching and securing other indications for our products. We intend to leverage our global regulatory and commercial capabilities to accelerate product approvals and launches across current and future markets.
•Continuous investment in our product portfolio - We continuously search for new product opportunities through internal development and through strategic licensing and acquisition opportunities, that, if successful, will allow us to leverage our commercial footprint and supplement our existing product portfolio and address specific unmet needs in the market. We plan to develop and, where applicable, commercialize our global pipeline of over 60 projects, many of which are global projects being developed in and for multiple countries. These global and individual projects are in various stages of pre-clinical and clinical development, including new contact lenses for myopia, next-generation cataract equipment, premium IOLs, investigational treatments for dry eye, novel formulations for eye vitamins and preservative free formulations of eye drops, among others, that are designed to grow our portfolio and accelerate future growth.
We believe there is significant opportunity in each of our businesses and we believe our existing portfolio, commercial footprint and pipeline of product development projects position us to build value for our investors.
Segment Information
Our portfolio of products fall into three operating and reportable segments: (i) Vision Care, (ii) Pharmaceuticals and (iii) Surgical. Segment revenues for the years 2023, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2023 | | 2022 | | 2021 |
| (in millions) | | Amount | | Pct. | | Amount | | Pct. | | Amount | | Pct. |
| Vision Care | | $ | 2,543 | | | 61 | % | | $ | 2,369 | | | 63 | % | | $ | 2,327 | | | 62 | % |
| Pharmaceuticals | | 836 | | | 20 | % | | 681 | | | 18 | % | | 720 | | | 19 | % |
| Surgical | | 767 | | | 19 | % | | 718 | | | 19 | % | | 718 | | | 19 | % |
| Total revenues | | $ | 4,146 | | | 100 | % | | $ | 3,768 | | | 100 | % | | $ | 3,765 | | | 100 | % |
Effective in the first quarter of 2023, certain products historically included in the reported results of the Pharmaceuticals segment are now included in the reported results of the Vision Care segment and certain products included in the reported results of the Vision Care segment are now included in the reported results of the Pharmaceuticals segment. The net impact of these product movements were not material to the periods presented. Prior period presentations of segment revenues have been conformed to the current segment reporting structure. Comparative segment information for 2023, 2022 and 2021 is further presented in Note 22, “SEGMENT INFORMATION” to our audited Consolidated Financial Statements.
Vision Care
Our Vision Care segment consists of our consumer eye care and contact lens businesses. For the year ended December 31, 2023, our revenue from the Vision Care segment breaks down as follows: 65% from our consumer eye care business and 35% from our contact lens business.
Our consumer eye care business consists of contact lens care products, OTC eye drops that address various conditions, including eye allergies, conjunctivitis, dry eye and redness relief, and eye vitamin and mineral supplements. Our principal consumer eye care products include:
•PreserVision® AREDS 2 is a patented eye vitamin and mineral supplement that contains the exact nutrient formula recommended by the National Eye Institute for people with moderate to advanced age-related macular degeneration ("AMD") following the landmark AREDS 2 clinical study.
•Ocuvite® is a family of nutritional supplements that contain antioxidant vitamins and minerals and other nutrients beneficial for eye health, including lutein and zeaxanthin (antioxidant carotenoids), nutrients that support macular health by helping filter harmful blue light.
•Biotrue® multi-purpose solution helps prevent certain tear proteins from denaturing and fights germs for healthy contact lens wear. Biotrue® multi-purpose solution contains hyaluronic acid (sodium hyaluronate) a lubricant naturally found in eyes and is pH balanced to match healthy tears.
•Bausch + Lomb Renu® Advanced Formula multi-purpose solution is a novel soft and silicone hydrogel contact lens solution that makes use of three disinfectants and two moisture agents.
•Boston® solution is a specialty cleansing solution design for gas permeable contact lenses.
•Artelac® is an eye moisturizer eye drop which enables quick wetting of dry eyes. Artelac® contains hyaluronic acid (sodium hyaluronate), a natural lubricant which instantly refreshes and hydrates the eyes. Artelac® is particularly suitable for alleviating mild symptoms of dry eyes and can also be used to moisten hard contact lenses while being worn.
•Lumify® (brimonidine tartrate ophthalmic solution, 0.025%) is an OTC redness reliever eye drop that significantly reduces redness to help eyes look whiter and brighter, revealing eyes’ natural beauty. To date, we have launched and acquired the right to launch Lumify® in various countries. We also have several innovative new line extension formulations that were recently launched or are under development, including Lumify Eye IlluminationsTM, three clinically proven products for the sensitive eye area which launched in the U.S. in September 2023, Lumify® Preservative Free, for which the New Drug Application (“NDA”) was submitted to the U.S. Food and Drug Administration (the “FDA”) in May 2023, and Lumify® Eye Allergy, for which we expect to submit an NDA during 2024.
•Blink® OTC product line consists of a variety of eye drops and contact lens rewetting drops designed to provide immediate and long-lasting symptom relief. In July 2023, we acquired the Blink® OTC product line of eye and contact lens drops from Johnson & Johnson Vision, which consists of Blink® Tears Lubricating Eye Drops, Blink® Tears Preservative Free Lubricating Eye Drops, Blink GelTears® Lubricating Eye Drops, Blink® Triple Care Lubricating Eye Drops, Blink Contacts® Lubricating Eye Drops and Blink-N-Clean® Lens Drops (collectively, the “Blink® Product Line”).
Our contact lens business includes sales of traditional, planned replacement disposable and daily disposable soft contact lenses; multifocal, toric and multifocal toric soft contact lenses (commonly known as specialty contact lenses); and rigid gas permeable (RGP) materials. Our principal contact lens products include:
•SiHy Daily, a silicone hydrogel daily disposable contact lens designed to provide outstanding comfort and clear vision throughout the day. To date SiHy Daily has been launched in approximately 50 countries, under the brand names INFUSE®, Bausch + Lomb ULTRA® ONE DAY and AQUALOX® ONE DAY. We launched our first silicone hydrogel daily disposable multifocal contact lens in May 2023, and plan to launch a toric lens in 2024.
•Bausch + Lomb ULTRA®, a silicone hydrogel frequent replacement contact lens for patients with myopia or hyperopia that uses our proprietary MoistureSeal® technology, which allows the contact lens to retain 95% of moisture after 16 hours of wear, limiting lens dryness and resulting symptoms.
•Bausch + Lomb ULTRA® for Astigmatism, a monthly planned replacement contact lens for astigmatic patients developed using our proprietary MoistureSeal® technology. Bausch + Lomb ULTRA® for Astigmatism lenses integrate an OpticAlign® design engineered for lens stability and to promote a successful wearing experience for the astigmatic patient.
•Bausch + Lomb ULTRA® for Presbyopia, a monthly planned replacement contact lens for presbyopic patients developed using the Company’s proprietary MoistureSeal® technology. Bausch + Lomb ULTRA® for Presbyopia lenses integrate our 3-Zone Progressive™ multifocal design with seamless transitions between near, far and intermediate distances for clear, comfortable vision across all distances.
•Bausch + Lomb ULTRA® multifocal for astigmatism, a monthly planned replacement multifocal toric lens combining our 3-Zone ProgressiveTM multifocal design with the stability of its OpticAlign® toric design to address the lifestyle and vision needs of patients with both astigmatism and presbyopia.
•Biotrue® ONEday daily disposable contact lenses for patients with myopia or hyperopia, which are made of a unique material inspired by the natural biology of the eye and feature Surface Active TechnologyTM, a patented dehydration barrier. The lens contains 78% water, more moisture than any other soft contact lens and the same water content as the cornea, and maintains nearly 100% of its moisture for up to 16 hours.
•Biotrue® ONEday for Astigmatism, a daily disposable contact lens for astigmatic patients developed using the Company’s proprietary Surface Active TechnologyTM. Biotrue® ONEday for Astigmatism includes evolved peri-ballast geometry designed to work with natural blink patterns to deliver stability, clear vision and comfort for the astigmatic patient.
•Biotrue® ONEday for Presbyopia daily disposable contact lens for presbyopic patients developed using the Company’s proprietary Surface Active Technology. Biotrue® ONEday for Presbyopia integrates the Company’s 3-Zone Progressive™ design with seamless transitions between near, far and intermediate distances for clear, comfortable vision across all distances.
•PureVision®, a silicone hydrogel frequent replacement contact lens using AerGel® technology lens material to allow natural levels of oxygen to reach the eye as well as resist protein buildup. The lens also incorporates an aspheric optical design that reduces spherical aberration.
•SofLens® Daily Disposable Contact Lenses, which use ComfortMoist® Technology (a combination of thin lens design and moisture-rich packaging solution) and High Definition Optics™ which is an aspheric design that reduces spherical aberration over a range of powers, especially in low light.
Pharmaceuticals
Our Pharmaceuticals segment consists of a broad line of proprietary and generic pharmaceutical products for post-operative treatments and treatments for a number of eye conditions, such as glaucoma, eye inflammation, ocular hypertension, dry eyes and retinal diseases. Effective June 30, 2023, the Company renamed its former Ophthalmic Pharmaceuticals segment to the Pharmaceuticals segment. Aside from the change in name, there were no other changes made to this segment at that time. Our principal Pharmaceuticals products include:
•XIIDRA® (lifitegrast ophthalmic solution) 5%, a non-steroid eye drop specifically approved to treat the signs and symptoms of dry eye disease focusing on inflammation associated with dry eye. We completed our acquisition of XIIDRA® during the third quarter of 2023, in conjunction with which, the Company also acquired libvatrep (also known as SAF312), an investigational compound being studied for the treatment of chronic ocular surface pain, AcuStream® technology, an investigational device that may have the potential to facilitate precise dosing and accurate delivery of certain topical ophthalmic medications to the eye, and OJL332, a non-competitive antagonist (inhibitor) of TRPV1 that is still in the pre-clinical stage.
•MIEBO® (perfluorohexyloctane ophthalmic solution) is the first and only FDA-approved eye drop that directly targets the leading cause of dry eye: tear evaporation and is used to treat the signs and symptoms of dry eye disease. We launched MIEBO® in the U.S. during the third quarter of 2023.
•XIPERE® (triamcinolone acetonide suprachoroidal injectable suspension) is a proprietary suspension of the corticosteroid triamcinolone acetonide formulated for suprachoroidal administration via Clearside’s proprietary SCS Microinjector®. We launched XIPERE® during the first quarter of 2022, and believe that it is the first and only therapy currently available in the U.S. for suprachoroidal use for the treatment of macular edema associated with uveitis.
•Vyzulta® (latanoprostene bunod ophthalmic solution, 0.024%) is an intraocular pressure lowering single-agent eye drop with dual activity dosed once daily for patients with open angle glaucoma or ocular hypertension.
•Lotemax® SM (loteprednol etabonate ophthalmic gel 0.38%), a new gel drop formulation of loteprednol etabonate, which was designed with novel SubMicron (SM) technology for efficient penetration to key ocular tissues at a low preservative (BAK) level (3.5-10) and a pH close to human tears, indicated for the treatment of postoperative inflammation and pain following ocular surgery.
•Besivance® (besifloxacin ophthalmic suspension, 0.6%) is the first and only chloro-fluoroquinolone indicated for the treatment of bacterial conjunctivitis. It is a new generation potent quinolone antibiotic specifically designed for the ophthalmic use and has no systemic formulation.
•Visudyne® (verteporfin for injection) therapy is a photoenhancer indicated for the treatment of patients with predominantly classic subfoveal choroidal neovascularization due to age-related macular degeneration, pathologic myopia or presumed ocular histoplasmosis.
•Minims® portfolio including ocular anaesthetics, corticosteroids, mydriatics, cycloplegics, artificial tears, irrigating solutions and diagnostic stain products.
•Prolensa® (bromfenac ophthalmic solution) 0.07% is a nonsteroidal anti-inflammatory drug (NSAID) indicated to treat inflammation and reduce eye pain in patients after cataract surgery. In international markets, we market YelloxTM (bromfenac ophthalmic solution, 0.9%) which is indicated for the treatment of postoperative ocular inflammation following cataract extraction.
•Lotemax® Suspension (loteprednol etabonate ophthalmic suspension, 0.5%) is a topical corticosteroid indicated for the treatment of steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe and for the treatment of post-operative inflammation following ocular surgery.
•Alrex® (loteprednol etabonate ophthalmic suspension, 0.2%) is indicated for the temporary relief of the signs and symptoms of seasonal allergic conjunctivitis.
•Zylet® (loteprednol etabonate 0.5% and tobramycin 0.3% ophthalmic suspension) indicated for the steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
Surgical
Our Surgical segment consists of medical device equipment, consumables and technologies for the treatment of cataracts, corneal, vitreous and retinal eye conditions, which includes IOLs and delivery systems, phacoemulsification equipment and other surgical instruments and devices necessary for cataract surgery. For the year ended December 31, 2023, our revenue from Surgical products was comprised as follows: 25% from equipment, 24% from implantables and 51% from consumables.
Our principal Surgical products include:
•Vitreoretinal Surgery
◦Stellaris Elite® vision enhancement system, is a combined system with cataract and vitreoretinal capability featuring the Bi-Blade vitrectomy handpiece.
◦Synergetics® instruments include reusable and single use devices and are marketed for use in vitreoretinal surgery.
•Cataract Surgery and Laser Systems
◦The Stellaris Elite® vision enhancement system configured for cataract procedures is our latest generation phacoemulsification cataract platform, Stellaris Elite® is the first phacoemulsification platform on the market to offer Adaptive FluidicsTM, which combines aspiration control with predictive infusion management to create a responsive and controlled surgical environment for efficient cataract lens removal. Our Stellaris Elite® vision enhancement system was launched in the United States in 2017 and internationally in 2018.
◦VICTUS® femtosecond laser for cataract and corneal refractive surgery, which delivers multi- mode versatility for cataract and corneal procedures on a single platform. This single laser platform enables surgeons to perform capsulotomies, fragmentation, arcuate incisions, corneal incisions, and LASIK flaps.
◦TeneoTM Excimer Laser system for corneal refractive surgery.
•Intraocular Lenses
◦A portfolio of ophthalmic surgical IOLs, including implantable IOLs such as Akreos®, enVista®, Crystalens® and Trulign®. We are expanding our portfolio of premium IOLs built on the enVista® platform with enVista AspireTM (Monofocal Plus), enVista EnvyTM Trifocal and enVista BeyondTM (extended depth of focus (“EDOF”)) optical designs with two options: non-Toric and Toric for astigmatism patients. enVista AspireTM monofocal and toric IOLs with Intermediate Optimized optics launched in the U.S. during October 2023 and we anticipate launching the Trifocal and EDOF optical designs for presbyopia in the U.S. in 2024 and 2026, respectively.
•Surgical Instruments
◦Storz® Ophthalmic instruments are our suite of surgical instruments which include precision microsurgical instruments, diamond knives and single-use surgical instruments, as well as instruments customized for individual surgeons under the Storz® Ophthalmic Instrument brand.
Research and Development
We are focused on bringing innovative products to market to serve doctors, patients and consumers in the pursuit of helping people see better to live better all over the world. Our product development approach starts with the identification of key patient and customer needs with feedback from our deep relationships with physicians and optometrists, and involves all of the functional experts responsible for creating a solution from origination through commercial launch. This approach harnesses the cross-functional expertise of our R&D, quality, clinical, medical and regulatory affairs, supply chain and commercial representatives at every phase of product development.
Our R&D organization focuses on the development of products through clinical trials. Currently, we have over 60 R&D projects in our global pipeline, which are being developed in and for multiple countries. As of December 31, 2023, approximately 850 dedicated R&D personnel globally in 12 R&D facilities were involved in our R&D efforts.
In addition, we continuously search for new ways to augment our in-house research efforts with externally-sourced innovations that allow us to gain access to unique products and investigational treatments, that, if successful, will allow us to
leverage our commercial footprint and supplement our existing product portfolio and address specific unmet needs in the market.
For additional information and for details of key projects in our pipeline, see Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Overview — Positioning for Growth” of this Form 10-K.
Trademarks, Patents, Exclusivity and Proprietary Know-How
The development of new and innovative products, as well as protecting the underlying intellectual property of our product portfolio, is important to our success in all areas of our business. We rely on a combination of contractual provisions, confidentiality policies and procedures and patent, trademark, copyright and trade secrecy laws to protect the proprietary aspects of our technology and business. These legal measures afford limited protection and may not prevent our competitors from gaining access to our intellectual property and proprietary information. Our policy is to vigorously protect, enforce and defend our rights to our intellectual property and proprietary rights, as appropriate. Our commercial success will also depend in part on not infringing, misappropriating or otherwise violating the intellectual or proprietary rights of third parties. Some of our products either: (i) have no meaningful exclusivity protection via patent or marketing or data exclusivity rights or (ii) are protected by patents or regulatory exclusivity periods that will be expiring in the near future. See Item 1A. “Risk Factors” of this Form 10-K for additional information on the risks associated with our intellectual property and proprietary rights.
Trademarks
We believe that trademark protection is an important part of establishing product and brand recognition. We own or license a number of registered trademarks and trademark applications in the U.S., Canada and in various other countries throughout the world. U.S. federal registrations for trademarks remain in force for 10 years and may be renewed every 10 years after issuance, provided the mark is still being used in commerce. Trademark registrations in Canada issued on or before June 17, 2019 remain in force for 15 years and may thereafter be renewed for 10-year terms. Trademark registrations in Canada issued after June 17, 2019 remain in force for 10 years and may be renewed every 10 years after issuance. Other countries generally have similar but varying terms and renewal policies with respect to trademarks registered in those countries.
Data and Patent Exclusivity
For certain of our products, we rely on a combination of regulatory and patent rights to protect the value of our investment in the development of these products.
As of February 16, 2024, we own or exclusively license approximately 2,349 granted patents throughout the world, approximately 462 of which are U.S. patents. Of our issued patents, approximately 84% will expire within the next 10 years and the remaining approximately 16% will expire thereafter. Within the next three years, the following number of U.S. patents held by us is set to expire: approximately 30 patents in 2024, approximately 29 patents in 2025 and approximately 23 patents in 2026. The expiration of these patents is not expected to have a material adverse effect on our business. We currently own or exclusively license approximately 126 pending U.S. patent applications.
A patent is the grant of a property right which allows its holder to exclude others from, among other things, selling the subject invention in, or importing such invention into, the jurisdiction that granted the patent. In the U.S., Canada and the European Union (“EU”), generally patents expire 20 years from the date of application. We have obtained, acquired or in-licensed a number of patents and patent applications covering key aspects of certain of our principal products. In the aggregate, our patents are of material importance to our business taken as a whole.
In the U.S., the Hatch-Waxman Act provides non-patent regulatory exclusivity for five years from the date of the first FDA approval of a new drug compound in a New Drug Application (“NDA”). The FDA, with one exception, is prohibited during those five years from accepting for filing a generic, or an Abbreviated New Drug Application (“ANDA”), that references the NDA. In reference to the foregoing exception, if a patent is indexed in the FDA Orange Book for the new drug compound, a generic may file an ANDA four years from the NDA approval date if it also files a Paragraph IV Certification with the FDA challenging the patent. Protection under the Hatch-Waxman Act will not prevent the filing or approval of another NDA. However, the NDA applicant would be required to conduct its own pre-clinical trials and adequate and well-controlled clinical trials to independently demonstrate safety and effectiveness.
A similar data exclusivity scheme exists in the EU, whereby only the pioneer drug company can use data obtained at the pioneer’s expense for up to eight years from the date of the first approval of a drug by the European Medicines Agency (“EMA”) and no generic drug can be marketed for ten years from the approval of the innovator product. Under both the U.S. and the EU data exclusivity programs, products without patent protection can be marketed by others so long as they repeat the clinical trials necessary to show safety and efficacy.
In the United States, the Biologics Price Competition and Innovation Act ("BPCIA") allows companies to seek FDA approval to manufacture and sell biosimilar or interchangeable versions of brand name biological products. Due to the size and complexity of biological products, as compared to small molecule drugs, a biosimilar must be “highly similar” to the reference product with “no clinically meaningful differences” in safety, purity and potency between the two. The BPCIA provides reference product sponsors with 12 years (with potential for six additional months of pediatric exclusivity) of market exclusivity, but unlike the Hatch-Waxman Act which covers small molecules, it does not require reference product sponsors to list patents in an Orange Book equivalent and does not include an automatic 30-month stay of FDA approval upon the timely filing of a lawsuit. The BPCIA, however, does provide pre-litigation procedures for the parties to follow, including identification of relevant patents and each party’s basis for infringement and invalidity. A biosimilar patent application cannot be filed until four years after the reference product is first licensed and a biosimilar cannot be launched, at the earliest (assumes no patent litigation or an adverse decision on all patents), until the expiration of the twelve years of marketing exclusivity from the approval of the reference product.
Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a disease or condition that affects populations of fewer than 200,000 individuals in the U.S. or a disease whose incidence rates number more than 200,000 where the sponsor establishes that it does not realistically anticipate that its product sales will be sufficient to recover its costs. The sponsor that obtains the first marketing approval for a designated orphan drug for a given rare disease is eligible to receive marketing exclusivity for use of that drug for the orphan indication for a period of seven years.
In Canada, the Patented Medicines (Notice of Compliance) Regulations (“PM(NOC) Regulations”) create a regime analogous to the U.S. Hatch-Waxman Act, and link the regulatory approval process for generic and biosimilar drugs to the adjudication of innovator patent rights. To be eligible for protection under the PM(NOC) Regulations, patents must first be listed on the Patent Register in connection with an innovator’s drug submission to Health Canada. A generic or biosimilar manufacturer must then provide notice to the innovator of its plans to market a drug that it compared to the innovator’s patented drug in the Health Canada approval process. Within 45 days of receiving such a notice of allegation, an innovator drug company may commence patent infringement proceedings against the generic or biosimilar manufacturer. The commencement of an action by the innovator under the PM(NOC) Regulations may stay Health Canada’s regulatory approval of the generic or biosimilar drug for a period of 24 months.
Canada also employs a data exclusivity regime for innovative drugs that provides an eight-year period of data protection from the date of market approval by Health Canada. An additional six months of data exclusivity is provided for drugs studied in clinical trials relating to use in pediatric populations. Drug submissions seeking approval based on a comparison to an innovative drug cannot be filed during the first six years of the data exclusivity period. Generic or biosimilar drug submissions remain on hold until expiry of the innovator’s data protection term, unless the innovative product is a patented drug subject to further protection under the PM(NOC) Regulations. Canada has no distinct drug submission process for biosimilar or orphan drug products.
Proprietary Know-How
We also rely upon unpatented proprietary know-how, trade secrets and technological innovation in the development and manufacture of many of our principal products. However, the foregoing rights, technologies and information are difficult to protect. We seek to protect our proprietary rights through a variety of methods, including confidentiality and non-disclosure agreements and proprietary information agreements with vendors, employees, consultants and others who may have access to proprietary information.
These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. These agreements may be breached, and we may not have adequate remedies for any breach. There can be no assurance that these agreements will be self-executing or otherwise provide meaningful protection for our trade secrets or other intellectual property or proprietary information. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Government Regulations
Government authorities in the United States, at the federal, state and local level, in Canada, in the EU and in other countries extensively regulate, among other things, the research, development, testing, approval, clearance, manufacturing, labeling, post-approval monitoring and reporting, packaging, advertising and promotion, storage, distribution, marketing and export and import of pharmaceutical products and medical devices. As such, our products and product candidates are subject to extensive regulation both before and after approval. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with these regulations could result in, among other things, warning letters, civil penalties, delays in approving or refusal to approve a product candidate, product recall, product seizure, interruption of production, operating restrictions, suspension or withdrawal of product approval, injunctions or criminal prosecution.
Prior to human use, FDA approval (drugs (in the form of an NDA or ANDA for generic equivalents), biologics (in the form of a Biologics License Application (BLA)) and some medical devices) or premarket approval or marketing clearance (other devices) must be obtained in the United States, approval by Health Canada must be obtained in Canada, EMA approval (drugs) or a CE Marking (devices) and or registration under the MDR 2017/475 must be obtained for countries that are part of the EU and approval must be obtained from comparable agencies in other countries prior to manufacturing or marketing new pharmaceutical products or medical devices. Generally, preclinical studies and clinical trials of the products must first be conducted and the results submitted to the applicable regulatory agency (such as the FDA) for approval.
In addition, with respect to medical devices, in April 2017, the European Commission adopted the Medical Device Regulation (“MDR”), which replaced the Medical Device Directive (“MDD”). Pursuant to the terms of the new regulations, in order to continue to market medical device products in the EU, such products must achieve compliance with these new regulations and be re-registered in the EU within a specified transition period, which, for a portion of products, ended as early as May 26, 2021. While EU law is applicable in Northern Ireland, the UK Medical Devices Regulations 2002/618 (“UK MDR 2002”) also need to be complied with in Great Britain. Medical device manufacturers who have CE marked devices will be able to continue to place them on the market in the whole of the United Kingdom (the “UK”) if they have an EU MDR CE certificate, until June 30, 2030, without a change in labeling. Legacy medical devices with an EU MDD CE certificate or an EU Declaration of Conformity may continue to be placed on the UK market as long as they meet the transitional provisions of the EU MDR for Northern Ireland and the UK MDR 2002 for Great Britain. For class III and class IIb implantable devices (subject to some exclusions), these transitional provisions will end on December 31, 2027 in both Great Britain and Northern Ireland and, for all other classes in scope, these transitional provisions will end on June 30, 2028 in Great Britain and December 31, 2028 in Northern Ireland. After that, devices destined for Great Britain will be required to follow the future UK regulatory regime, which is expected to come into force in 2025. Northern Ireland will, however, continue to accept CE marked devices. There are some additional requirements for manufacturers who are based outside the UK, such as the requirement to appoint a UK Responsible Person (“UKRP”) to take on certain regulatory responsibilities. To enable devices to be placed on the market in the UK after January 1, 2021 (even for CE marked devices), a UK manufacturer, a UKRP for an overseas manufacturer or an EU Authorised Representative based in Northern Ireland (for the purposes of the Northern Ireland market) must register with the Medicines and Healthcare products Regulatory Agency (“MHRA”). The registering entity will then register each of the devices for which they are responsible for placing on the market in the UK, whether in Great Britain or Northern Ireland, as required by the UM MDR 2002. Until May 25, 2021, our products bearing a CE mark could be exported from the EEA to Switzerland. However, as of May 26, 2021, the Mutual Recognition Agreement between the European Economic Area (“EEA”) and Switzerland has not been updated to include the requirements of EU MDR. Accordingly, legal manufacturers in Switzerland are required to appoint a European Union authorized representative, and manufacturers outside of Switzerland are required to appoint a Swiss authorized representative in compliance with the Swiss Medical Device Ordinance. As a consequence, beginning in June 2021 through May 2022, we have been required to appoint an authorized representative in Switzerland in order to export our CE- marked medical devices to Switzerland. Additionally, the name and address of the Swiss authorized representative must be placed on the packaging.
Regulation by other federal agencies, such as the Drug Enforcement Administration (“DEA”), and state and local authorities in the United States, and by comparable agencies in certain foreign countries, is also required. In the United States, the Federal Trade Commission (the “FTC”), the FDA and state and local authorities regulate the advertising of medical devices, prescription drugs, OTC drugs and cosmetics. The U.S. Federal Food, Drug and Cosmetic Act, as amended and the regulations promulgated thereunder, and other federal and state statutes and regulations, govern, among other things, the testing, manufacture, safety, effectiveness, labeling, storage, record keeping, approval, sale, distribution, advertising and promotion of our products.
Manufacturers of pharmaceutical products and medical devices are required to comply with manufacturing regulations, including current good manufacturing practices and quality system management requirements, enforced by the FDA and Health Canada, in the United States and Canada respectively, and similar regulations enforced by regulatory agencies in other countries and we face periodic audits of our facilities and plants and those of our contract manufacturers by the FDA and
such other regulatory agencies. In addition, we are subject to price control restrictions on our pharmaceutical products in many countries in which we operate.
We are also subject to extensive U.S. federal and state health care marketing and fraud and abuse regulations, such as the federal False Claims Act, federal and provincial marketing regulations in Canada and similar regulations in foreign countries in which we may conduct our business. The federal False Claims Act imposes civil and criminal liability on individuals or entities who submit (or cause the submission of) false or fraudulent claims for payment to the government. The U.S. federal Anti-Kickback Statute prohibits persons or entities from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal or state health care program such as the Medicare and Medicaid programs. Some state anti-kickback laws also prohibit such conduct where commercial insurance, rather than federal or state, programs are involved. Due to recent legislative changes, violations of the U.S. federal Anti-Kickback Statute also carry potential federal False Claims Act liability. In addition, in the United States and Canada, companies may not promote drugs or medical devices for “off-label” uses—that is, uses that are not described in the product’s labeling and that differ from those that were approved or cleared by the FDA or Health Canada, respectively—and “off-label promotion” in the United States has also formed the predicate for False Claims Act liability resulting in significant financial settlements. These and other laws and regulations, rules and policies may significantly impact the manner in which we are permitted to market our products. If our operations are found to be in violation of any of these laws, regulations, rules or policies or any other law or governmental regulation, or if interpretations of the foregoing change, we may be subject to civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations, including consent orders or corporate integrity agreements.
In addition, the U.S. Department of Health and Human Services Office of Inspector General recommends, and increasingly states require pharmaceutical companies to have comprehensive compliance programs. Moreover, the Physician Payment Sunshine Act enacted in 2010 imposes reporting and disclosure requirements on device and drug manufacturers for any “transfer of value” made or distributed to prescribers and other health care providers. Failure to submit this required information may result in significant civil monetary penalties.
We are also subject to the U.S. Foreign Corrupt Practices Act (“FCPA”), the Canadian Corruption of Foreign Public Officials Act and similar worldwide anti-bribery laws, which generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. Violations of these laws could result in criminal or civil penalties or remedial measures.
We are also subject to various state, federal and international laws and regulations governing the collection, transmission, dissemination, use, privacy, confidentiality, security, retention, availability, integrity and other processing of health-related and other sensitive and personal information, including, but not limited to, the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HIPAA”). HIPAA mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common health care transactions (e.g., health care claims information and plan eligibility, referral certification and authorization, claims status, plan enrollment, coordination of benefits and related information), as well as standards relating to the privacy and security of individually identifiable health information. These standards require the adoption of administrative, physical and technical safeguards to protect such information. Many states in which we operate have laws that protect the privacy and security of sensitive and personal information, including health-related information. Certain state laws may be more stringent or broader in scope, or offer greater individual rights, with respect to sensitive and personal information than federal, international or other state laws, and such laws may differ from each other, which may complicate compliance efforts. For example, the California Consumer Privacy Act, as amended by the California Privacy Rights Act (“CPRA,” and collectively, the “CCPA”), imposes stringent data privacy and security requirements and obligations with respect to the personal information of California residents, including, among other things, disclosures to California consumers and providing such consumers data protection and privacy rights, including the ability to opt out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal data that may increase the likelihood of, and risks associated with, data breach litigation. The CPRA significantly modifies the CCPA, including by expanding consumers’ rights with respect to certain sensitive personal information. The CPRA also creates a new state agency vested with authority to implement and enforce the CCPA and the CPRA. In addition, multiple states have enacted or are expected to enact similar laws. The effects on our business of the CCPA, CPRA and other similar state laws are potentially significant, and may require us to modify our data processing practices and policies and to incur substantial costs and expenses in an effort to comply. State laws are changing rapidly and there is discussion in Congress of a new federal data protection and privacy law to which we may be subject.
Additionally, some statutory requirements, both in the United States and abroad, include obligations for companies to notify individuals of security breaches involving particular personal information, which could result from breaches experienced by us or our service providers. For example, laws in all 50 U.S. states require businesses to provide notice to
customers whose personal data has been disclosed as a result of a data breach. The laws are not consistent, and compliance in the event of a widespread data breach is difficult and may be costly. Moreover, states have been frequently amending existing laws, requiring attention to changing regulatory requirements.
Internationally, laws and regulations in many jurisdictions apply broadly to the collection, transmission, dissemination, use, privacy, confidentiality, security, retention, availability, integrity and other processing of health-related and other sensitive and personal information. For example, in the EEA, the collection and use of personal data, including clinical trial data, is governed by the provisions of the General Data Protection Regulation (“GDPR”). The GDPR became effective on May 25, 2018, repealing its predecessor directive and increasing responsibility and liability of companies in relation to the processing of personal data of EU data subjects. The GDPR, together with national legislation, regulations and guidelines of the EU member states and the United Kingdom governing the processing of personal data, impose strict obligations and restrictions on the ability to collect, analyze, store, transfer and otherwise process personal data, including health data from clinical trials and adverse event reporting. In particular, the GDPR includes obligations and restrictions concerning the consent and rights of the individuals to whom the personal data relates, the transfer of personal data out of the EEA, security breach notifications and the security and confidentiality of personal data. Guidance on implementation and compliance practices is often updated or otherwise revised. The GDPR authorizes fines for certain violations of up to 4% of global annual revenue or €20 million, whichever is greater. European data protection authorities may interpret the GDPR and national laws differently and impose additional requirements, which contributes to the complexity of processing personal data in or from the EEA or United Kingdom. For example, the EU-U.S. Data Privacy Framework (“EU-U.S. DPF”), the UK Extension to the EU-U.S. Data Privacy Framework (UK Extension to the EU-U.S. DPF), and the Swiss-U.S. Data Privacy Framework (“Swiss-U.S. DPF”) were developed to facilitate transatlantic commerce by providing U.S. organizations with reliable mechanisms for personal data transfers to the United States from the European Union / European Economic Area, the United Kingdom and Switzerland that are consistent with EU, UK, and Swiss law. On July 10, 2023, the European Commission’s adequacy decision for the EU-U.S. Data Privacy Framework (“DPF”) was entered into force and made effective the EU-U.S. DPF Principles, including the Supplemental Principles and Annex I of the Principles. The adequacy decision enables the transfer of EU personal data to participating organizations consistent with EU law. Organizations participating in the EU-U.S. DPF may receive personal data from the European Union / European Economic Area in reliance on the EU-U.S. DPF effective July 10, 2023.
Further, following the United Kingdom’s withdrawal from the EU and the EEA, and the expiry of the transition period, companies have to comply with both the GDPR and the GDPR as incorporated into the United Kingdom national law, the Data Protection Act of 2018, the latter regime having the ability to separately fine up to the greater of £17.5 million, or 4% of global turnover. The relationship between the United Kingdom and the EU in relation to certain aspects of data protection law remains unclear, for example around how data can lawfully be transferred between each jurisdiction, which exposes us to further compliance risk. Beginning in 2021, the United Kingdom is a “third country” under the GDPR. We may incur liabilities, expenses, costs and other operational losses under the GDPR and privacy laws of the applicable EU and EEA Member States and the United Kingdom in connection with any measures we take to comply with them.
In addition, in China, the Personal Information Protection Law (the “PIPL”) came into force in November 2021. The PIPL is the first national-level law comprehensively regulating issues in relation to personal information protection. The PIPL provides for very specific administrative requirements and security controls when transferring personal data outside the Peoples Republic of China. These transfer requirements came into effect on March 1, 2023.
We are also subject to Canada’s federal Personal Information Protection and Electronic Documents Act (“PIPEDA”) and substantially similar equivalents at the provincial level with respect to the collection, use and disclosure of personal information in Canada. Such federal and provincial legislation impose data privacy and security obligations on our processing of personal information of Canadian residents. The federal, Quebec and Alberta legislation include mandatory data breach notification requirements. Canada’s Anti-Spam Legislation (“CASL”) also applies to the extent that we send commercial electronic messages from Canada or to electronic addresses in Canada. CASL contains prescriptive consent, form, content and unsubscribe mechanism requirements. Penalties for non-compliance with CASL are up to CAD $10 million per violation. These laws and regulations may be interpreted and applied differently over time and from jurisdiction to jurisdiction, and it is possible they will be interpreted and applied in ways that will materially and adversely affect our business. The regulatory framework for data privacy, data security and data transfers worldwide is rapidly evolving and is likely to remain uncertain for the foreseeable future. Complying with all of these laws and regulations involves costs to our business, and failure to comply with these laws and regulations can result in the imposition of significant civil and criminal penalties, as well as litigation.
Successful commercialization of our products may depend, in part, on the availability of governmental and third-party payor reimbursement for the cost of our products. Third-party payors may include government health administration authorities, private health insurers and other organizations. In the United States, the EU and other significant or potentially significant markets for our products and product candidates, government authorities and third-party payors are increasingly
attempting to limit or regulate the price of medical products and services, which has resulted in lower average realized prices. In the United States, these pressures can arise from rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and health care reform, pharmaceutical reimbursement policies and pricing in general. In particular, sales of our products may be subject to discounts from list price and rebate obligations, as well as formulary coverage decisions impacting or limiting the types of patients for whom coverage will be provided. Various U.S. health care and other laws regulate our interactions with government agencies, private insurance companies and other third-party payors regarding coverage and reimbursement for our products. Failure to comply with these laws could subject us to civil, criminal and administrative sanctions. In countries outside the United States, the success of our products may depend, at least in part, on obtaining and maintaining government reimbursement because, in many countries, patients are unlikely to use prescription drugs that are not reimbursed by their governments. In addition, negotiating prices with certain governmental authorities for newly developed products can delay commercialization. In Canada and many international markets, governments control the prices of prescription pharmaceuticals, including through the implementation of reference pricing, price cuts, rebates, revenue-related taxes, tenders and profit control, and they expect prices of prescription pharmaceuticals to decline over the life of the product or as volumes increase.
See Item 1A. “Risk Factors” of this Form 10-K for additional information on the risks associated with these regulations and related matters.
Environmental and Other Regulation
We are subject to a broad range of federal, state, provincial and local environmental laws and regulations concerning the environment, safety matters, regulation of chemicals and product safety in the countries where we manufacture and sell our products or otherwise operate our business. These requirements include, among other matters, regulation of the handling, manufacture, transportation, storage, use and disposal of materials, including the discharge of pollutants, hazardous substances and waste into the environment. Compliance with environmental, health and safety laws and regulations could require us to incur significant operating or capital expenditures or result in significant restrictions on our operations. If we fail to comply with these environmental, health and safety laws and regulations, including failing to obtain any necessary permits, we could incur substantial civil or criminal fines or penalties or enforcement actions, including regulatory or judicial orders enjoining or curtailing our operations or requiring us to conduct or fund remedial or corrective measures, install pollution control equipment, reformulate or cease the marketing of our products or perform other actions. In the normal course of our business, such substances and waste may be released into the environment, which could cause environmental or property damage or personal injuries, and which could subject us to remediation obligations regarding contaminated soil and groundwater, potential liability for damage claims or to social or reputational harm and other similar adverse impacts. Under certain of these laws and regulations, we may be subject to joint and several liability for environmental investigations and cleanups, including at properties that we currently or previously owned or operated, or at sites at which waste we generated was disposed, even if the contamination was not caused by us or was legal at the time it occurred. We are also subject to extensive and evolving regulations regarding the manufacturing, processing, distribution, importing, exporting, and labeling of our products and their raw materials.
In light of the rapid and ongoing global regulations and expectations relating to environmental, social and governance (“ESG”) matters, we are developing an integrated ESG program.
We believe we are in compliance in all material respects with applicable environmental and occupational health and safety laws and regulations. We are not aware of any pending environmental or occupational health and safety litigation or significant liabilities that are likely to have a material adverse effect on our financial position. We cannot assure, however, that environmental liabilities relating to us or facilities owned, leased or operated by us will not develop in the future, and we cannot predict whether any such liabilities, if they were to develop, would require significant expenditures on our part. In addition, we are unable to predict what environmental or and occupational health and safety legislation or regulations may be proposed, adopted or enacted in the future. See Item 1A. “Risk Factors” of this Form 10-K for additional information.
Sales and Marketing
We sell our portfolio of products and services through direct sales forces and independent distributors depending on specific market and product needs. Our global business sells and distributes products in approximately 100 countries. Our footprint is bolstered by a global commercial team of approximately 4,250 employees.
In the United States, we have approximately 1,100 employees on our commercial team dedicated to our efforts to sell and market contact lens, lens care, consumer eye health, surgical and prescription pharmaceutical products, which are sold through wholesalers, retailers and eye care professional practices.
Our international commercial footprint is represented through approximately 3,150 employees on our commercial team as well as a network of distribution partners.
Our sales effort allows us to deliver the full suite of Bausch + Lomb products to key clinician decision makers, recognize cross-selling opportunities for key products from other product categories and impact consumer purchasing decisions.
•Our sales representatives within the global consumer products and global vision care business categories are focused on promoting and selling our products to large and mid-sized retailers, pharmacies and eye care professionals as well as optimizing and expanding our shelf presence at retailers.
•Our sales representatives within the ophthalmic pharmaceuticals business category are focused on promoting and marketing our products to wholesalers, large retailers, eye care professionals, independent pharmacies and hospitals.
•Our sales representatives within the global surgical business category are focused on selling products and equipment to eye care professionals, physicians, including ophthalmic surgeons, hospitals and ambulatory surgery centers.
We reinforce our sales efforts and continue to drive demand and awareness of our brands and the clinical benefits of our products through multiple initiatives to both eye care professionals and consumers. These initiatives include the sponsorship of various industry congresses and symposia throughout the world. We also conduct training programs to provide eye care professionals with the latest information concerning clinical experience with our products. We provide and sponsor eye health education and programs for consumers. We continually seek input from eye care professionals through medical and scientific advisory boards to help us refresh and update all of these initiatives as well as to create new opportunities to provide our customers with the necessary resources to use our products safely and effectively.
No individual customers accounted for 10% or more of our total revenue for 2023, 2022 and 2021.
Competition
Our competitors include specialty and other large pharmaceutical companies, medical device companies, biotechnology companies, OTC companies and generic manufacturers, in the United States, Canada, Europe, Asia, Latin America, the Middle East, Africa and in other countries in which we market our products. The market for Bausch + Lomb products is very competitive, both across product categories and geographies. In addition to larger diversified pharmaceutical and medical device companies, we face competition in the eye health market from mid-size and smaller, regional and entrepreneurial companies with fewer products in niche areas or regions. We sell a broad range of products, and competitive factors vary by product line and geographic area in which the products are sold. The principal methods of competition for our products include quality, efficacy, market acceptance, price and marketing and promotional efforts.
Our sole focus on eye health with one of the most comprehensive portfolios in the industry enables us to reach a broad set of customers through coordinated delivery of solutions across the pharmaceutical, vision and surgical product lines. See Item 1A. “Risk Factors” of this Form 10-K for additional information on our competition risks.
Manufacturing and Supply
We manufacture the significant majority of our products at 25 manufacturing facilities in 10 countries worldwide, including the United States, Ireland, China, Germany, France and Italy, with the remainder of our production assigned to high quality third-party manufacturers. Our manufacturing facilities are generally organized based on product categories and tend to be specifically focused on manufacturing either pharmaceuticals, contact lenses, solutions or surgical devices due to the unique differences in regulatory requirements and technical skills required for the different product categories. Our manufacturing sites are clustered by business unit reporting and technology mapping. This organizational construct provides tight managerial control while permitting a strong focus on a limited set of technologies per business unit. We believe that our manufacturing facilities and relationships will support our potential capacity needs for the foreseeable future.
In the normal course of business, our products, devices and facilities are the subject of ongoing oversight and review by regulatory and governmental agencies, including general, for cause and pre-approval inspections by the relevant competent authorities where we have business operations. Through the date of this filing, all of our global operations and facilities have the relevant operational good manufacturing practices certificates and all Company products and operating sites are in good compliance standing with all relevant notified bodies and global health authorities. Further, all sites under FDA jurisdiction are rated as No Action Indicated (where there was no Form 483 observation).
We use a diverse and broad range of raw materials in manufacturing our products. We purchase the materials and components for each of our product categories from a wide variety of suppliers. In order to manage any single-sourced suppliers we maintain sufficient inventory consistent with good practice and production lead-times. We believe that the loss of any one supplier would not adversely affect our business to a significant extent.
Some of our products are provided by suppliers under a private label distribution agreement. Under these agreements, the supplier generally retains the intellectual property and exclusive manufacturing rights. The supplier private labels the
products under the Bausch + Lomb brand for sale in certain fields of use and geographic territories. These agreements may be subject to minimum purchase or sales obligations. Our private label distribution agreements do not, individually or in the aggregate, represent a material portion of our business and we are not substantially dependent on them.
We also subcontract the manufacturing of certain of our products, including products manufactured under the rights acquired from other pharmaceutical companies. Products representing approximately 26% of our product sales for 2023 are produced in total, or in part, by third-party manufacturers under manufacturing arrangements.
In some cases, the principal raw materials, including active pharmaceutical ingredients, used by us (or our third-party manufacturers) for our various products are purchased in the open market or are otherwise available from several sources. However, some of the active pharmaceutical ingredients and other raw materials used in our products and some of the finished products themselves are currently only available from a single source; or others may in the future become available from only one source. For example, with respect to some of our largest or most significant products, the supply of the finished product for each of our Lumify®, Vyzulta®, SofLens®, MIEBO®, XIIDRA® and PureVision® products are only available from a single source, the supply of the active pharmaceutical ingredients or other components for our Vyzulta®, MIEBO® and PreserVision® products are also only available from a single source and certain of our Biotrue®, Softlens® and Ultra® contact lens products are also only available from a single source. Any disruption in the supply of any such single-sourced active pharmaceutical ingredient, other raw material or finished product or an increase in the cost of such materials or products could adversely impact our ability to manufacture or sell such products, the ability of our third-party manufacturers to supply us with such products, or our profitability. We attempt to manage the risks associated with reliance on single sources of active pharmaceutical ingredient, other raw materials or finished products by carrying additional inventories or, where possible, developing second sources of supply. See Item 1A. “Risk Factors” for additional information on the risks associated with our manufacturing arrangements.
Our global supply team continues to work diligently to manage the inflationary and supply-chain challenges presented by ongoing macroeconomic conditions. See Item 7. "Management's Discussion and Analysis — Inflation and Supply Chain" for further information.
Human Capital Resources
As of December 31, 2023, we had approximately 13,300 employees, which included approximately 7,200 in production, 4,250 in sales and marketing, 1,000 in general and administrative positions and 850 in R&D. These employees are located around the world, with approximately 5,100 in the United States, 3,300 in Europe excluding Ireland, 2,300 in Asia-Pacific countries, 1,600 in Ireland, 400 in Latin America, 400 in Russia and Commonwealth of Independent State countries, 100 in Canada and 100 in the Middle East and Africa.
Collective bargaining exists for some employees in several countries. Bausch + Lomb considers relations with employees to be good and have not experienced any work stoppages, slowdowns or other serious labor problems that have materially impeded business operations. During 2023, Bausch + Lomb did not experience any business disruption as a result of normal course employee turnover.
In 2023, we implemented action plans based on the feedback received from more than 9,500 colleagues across the world in the 2022 employee survey, our first such survey as a standalone company. These action plans include key areas of focus, including talent management and operating efficiency. While we continue to expand learning and development opportunities for employees to support their professional development, in the area of operating efficiency, we launched a rapid response process during 2023, providing employees with the opportunity to identify ways to be more efficient and effective. From these submissions, we took immediate action to simplify how we work and increase productivity.
Health, Safety and Wellness
Our employees' health, safety and wellness are of utmost importance to us. On an ongoing basis, we measure how well we are fostering the health and safety of our employees through our Days Away Rate (“DAR”), which captures globally the number of days that our employees are away from work due to illness or injury. In 2023, we achieved an annual DAR of 6, which met our annual not to exceed goal of 6 and is far below other similar industry standard DAR of 22.
In recognizing that physical, emotional and financial well-being are significant contributors to employees’ success at work and home, we support employees in all aspects of their everyday life by centering programs and activities around these three pillars of well-being. Across each pillar, a range of resources are offered to help employees be healthy and feel successful in both their professional and personal lives, including employee assistance programs that offer resources and support on various topics, including relationship issues, stress management, fitness and nutrition and grief and loss. In the 2022 employee survey, 75% of employees viewed our health care and wellness benefit programs as meeting their needs, which is significantly higher than the external norm against which we compare.
Diversity, Equity and Inclusion
We are dedicated to fostering an inclusive work environment where everyone feels welcomed, supported and valued for their talents and contributions. The Bausch + Lomb Diversity, Equity & Inclusion strategy centers on connecting employees to the Company, each other and our communities to cultivate a sense of trust, respect and belonging for all.
We strive to advance candid conversations among employees regarding such key topics as inclusion, racism and gender equality. Through our diversity and inclusion training and education efforts, all employees have been provided with educational tools and resources to understand how to talk about these topics at work and how to become more aware of unconscious biases they may have. During 2023, new career workshops were introduced on various topics including writing development plans, personal branding, and networking, and various playlists were launched on our e-learning platform to provide employees with targeted educational opportunities in key areas.
We continue to utilize our Employee Resource Groups to provide opportunities for professional growth, development and informal networking, including the Women’s Inclusive Network, the LGBTQ+ Network, the Military Network, the Black and African Heritage Network and the Asian Heritage Network. Our employees around the world participated in activities hosted by these networks throughout the year, including an International Women’s Day event, a virtual concert sharing the history of Black artistry in honor of Juneteenth, a session fostering LGBTQ+ allyship, and a Veteran’s Day event.
Talent Development and Total Rewards
We are committed to the development of employees and believe that our success coincides with employees’ achievements of personal and professional goals. Through our Employee Development Framework, the Company endeavors to support employees’ interests to grow to their full potential, achieve career goals and contribute to the success of the Company. Employees are empowered to explore roles that are of interest and gain insights into their strengths and development needs. A variety of development programs are provided to support employees at every stage of their career and incorporate individual development plans that aim to help employees reach their career goals. The Company also has a robust, global succession planning process that allows us to define talent needs based on business strategy, identify talent and drive development and growth, strengthen the pipeline for critical leadership positions, and optimize talent deployment across the business. In 2023, we expanded our nomination programs for individuals who have been identified through succession planning, to include a Business Leadership Excellence program for experienced people managers that focuses on developing leaders, executing on strategy, and driving engagement and wellbeing.
The Company's total rewards philosophy is designed to attract, retain, motivate and engage employees, providing comprehensive and market competitive compensation and benefit programs across our geographies. The compensation program includes base pay, short-term incentives and long-term incentives. This program provides the opportunity for employees to earn more when objectives are delivered – both as a total company and individually. The Company also provides competitive benefit programs based on local practice in the countries where employees work. These programs include medical coverage, retirement benefits, paid time off and life and other insurances.
Corporate Social Responsibility
The Bausch Foundation was established to improve the lives of patients globally by providing access to safe, effective medicines and by financially supporting health care education and causes. It subsidizes initiatives aimed at disease prevention, improving patient outcomes and lives, education and community support related to our core businesses, and supports relief efforts and those who need help in the communities in which we live and work.
Our unique recycling programs make it possible to properly recycle used contact lens, eye care and lens care items, which can be used to help create a variety of post-consumer products. These materials are not typically processed in standard recycling facilities and can end up in landfills or waterways and contribute to plastic pollution. In 2023, our ONE by ONE and Biotrue Eye Care Recycling programs were recognized as Sustainability Award winners by Business Intelligence Group in the “Sustainability Service of the Year” category. The ONE by ONE Recycling program has collected more than 76 million used contact lenses, blister packs and top foils since the program's launch in November 2016.
Seasonality of Business
Historically, revenues from our business tend to be weighted toward the second half of the year. Sales in the first quarter tend to be lower as patient co-pays and deductibles reset at the beginning of each year. Sales in the fourth quarter tend to be higher based on consumer and customer purchasing patterns associated with health care reimbursement programs. However, there are no assurances that these historical trends will continue in the future.
Geographic Areas
A significant portion of our revenues is generated from operations or otherwise earned outside the U.S. and Canada. All of our foreign operations are subject to risks inherent in conducting business abroad, including price and currency exchange controls, fluctuations in the relative values of currencies, political and economic instability and restrictive governmental actions including possible nationalization or expropriation. Changes in the relative values of currencies may materially affect our results of operations. For a discussion of these risks, see Item 1A. “Risk Factors” of this Form 10-K.
See Note 22, “SEGMENT INFORMATION” to our audited Consolidated Financial Statements for revenues and long-lived assets by geographic area.
Available Information
Our Internet address is www.bausch.com. We post links on our website to the following filings as soon as reasonably practicable after they are electronically filed or furnished to the SEC: annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and any amendment to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended. All such filings are available through our website free of charge. The information on our Internet website is not incorporated by reference into this Form 10-K or our other securities filings and is not a part of such filings. The SEC also maintains an Internet website at www.sec.gov that contains reports, proxy and information statements and other information regarding issuers, including us, that file electronically with the SEC.
We are also required to file reports and other information with the securities commissions in all provinces and territories in Canada. You are invited to read and copy any reports, statements or other information, other than confidential filings, that we file with the CSA. These filings are also electronically available from the Canadian System for Electronic Document Analysis and Retrieval + (“SEDAR+”) at www.sedarplus.ca, the Canadian equivalent of the SEC’s electronic document gathering and retrieval system.
Item 1A. Risk Factors
Our business, financial condition, cash flows and results of operations are subject to various risks and uncertainties. You should carefully consider the risks and uncertainties described below, together with all of the other information in this Form 10-K, including those risks set forth under the heading entitled “Forward-Looking Statements” and in other documents that we file with the SEC and the CSA, before making any investment decision with respect to our common shares or debt securities. If any of the risks or uncertainties actually occur or develop, our business, financial condition, cash flows, results of operations and/or future growth prospects could change, and such change could be materially adverse. Under these circumstances, the market value of our common shares and/or debt securities could decline, and you could lose all or part of your investment in our common shares and/or debt securities.
Summary of Risk Factors
The following is a summary of the risk factors our business faces. The list below is not exhaustive, and investors should read this “Risk Factors” section in full. Some of the risks we face include:
•Current market and economic conditions in one or more of our markets could impact our ability to grow our business;
•Inflation could materially adversely affect out business and operations;
•We may not realize the anticipated benefits from the Separation, and the Separation could harm our business;
•The Separation is subject to certain uncertainties. Furthermore, the Distribution (as defined below) may not occur;
•The Separation is subject to challenge and could be subject to further challenges in the future, any of which could delay or prevent the consummation of such transactions or cause them to occur on worse terms than we currently expect;
•We have limited history of operating as an independent company, and our historical financial information prior to the B+L IPO is not necessarily representative of the results that we would have achieved as an independent or standalone company and may not be a reliable indicator of our future results;
•Until the completion of the Separation, BHC will control the direction of our business, and the concentrated ownership of our common shares will prevent you and other shareholders from influencing significant decisions;
•Some of our directors and officers may have actual or potential conflicts of interest because of their equity ownership in BHC, and some of our directors may have actual or potential conflicts of interest because they also serve as directors of BHC;
•Potential tax liabilities that may arise as a result of the Separation or related transactions;
•Certain requirements of the public company “butterfly reorganization” rules in Section 55 of the Income Tax Act (Canada) (the “Tax Act”) depend on events that may not be within our control;
•We potentially could have received better terms from unaffiliated third parties than the terms we received in our agreements with BHC;
•The potential indemnification obligations to BHC and the ability of BHC to satisfy its corresponding indemnification obligations to us;
•As long as BHC owns a majority of our common shares, we may rely on certain exemptions from the corporate governance requirements of the New York Stock Exchange (“NYSE”) available to “controlled companies” and of the Toronto Stock Exchange (“TSX”) available to “majority controlled” companies;
•The impact of the actual or perceived future sales of our common shares (including via the Distribution) on our common share price;
•The services that BHC provides to us may not be sufficient to meet our needs;
•The transfer of certain outstanding assets, liabilities and contracts relating to the Separation and any delays thereof;
•Our ability to successfully develop our pipeline of products, which is highly uncertain and requires significant expenditures and time, including risks relating to obtaining necessary government approvals;
•Failure to comply with post-approval legal and regulatory requirements for our marketed products;
•Interruptions to our manufacturing operations and those of our third-party manufacturers, including as a result of failure to comply with applicable regulations;
•Certain of our products or components thereof are available from a single source or a limited number of sources;
•Issues relating to inventory levels or fluctuations in buying patterns by our large distributors and retail customers and supply chain disruptions;
•Failure to yield new products that achieve commercial success;
•Changes in market acceptance of our products due to inadequate reimbursement for such products or otherwise.
•The impact of competition and new medical and technological developments in our markets;
•The loss of the services of, or our inability to recruit, retain, motivate, our executives and other key employees;
•Pricing decisions, including as a result of price changes and/or new programs to enhance patient access to our products;
•Failure to maintain our relationships with health care providers who recommend our products to their patients;
•Our inability to control certain aspects of our third party distribution arrangements:
•The impact on our revenues of our policies and programs relating to returns, allowances, chargebacks and marketing;
•Risks associated with wholesaler concentration;
•Acquisition and integration risks;
•Potential obligations under our indemnity agreements and arrangements;
•Environmental, social and governance (ESG) matters and our ability to monitor and respond appropriately;
•Our indebtedness could adversely affect our business and our ability to meet our obligations;
•International operations risks associated with conducting a significant portion of our business outside the United States, including with respect to foreign currency risk and the ongoing Ukraine-Russia and Israel-Hamas conflicts;
•The loss of patent protection or exclusivity rights and, even where we retain patent protection or exclusivity rights, competition from similar products in the markets in which we participate;
•The inability to obtain, maintain, license, enforce, defend or otherwise protect our intellectual property rights;
•Breakdown, interruption or breach of our information technology systems;
•Competition for our pharmaceutical, OTC products or medical devices;
•The potential increase of our effective tax rates, including as a result of changes in applicable tax laws;
•The impact of ongoing and potential legal and governmental proceedings, including with respect to intellectual property;
•Compliance by our third party partners and service providers of their contractual, legal and regulatory obligations;
•Product liability matters, including potential product recalls or voluntary market withdrawals;
•Compliance with various laws and regulations, including with respect to marketing, promotional and business practices and fraud and abuse, anti-bribery, environmental and privacy and security matters; and
•Enactment of new regulations or changes in existing regulations related to the health care system.
Risks Relating to Economic and Market Conditions
Current market and economic conditions in one or more of our markets could impact our ability to grow our business.
Over the last few years in the U.S. and globally, market and economic conditions have been challenging, particularly in light of the COVID-19 pandemic and, more recently, as a result of uncertainty concerning government shutdowns, debt ceilings and government funding. Any negative impact on economic conditions and international markets, continued volatility or deterioration in the debt and equity capital markets, inflation, deflation or other adverse economic conditions may adversely affect our business, liquidity, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline. Ongoing uncertain economic and financial market conditions may also adversely affect the financial condition of our customers, suppliers and other business partners. When our customers’ financial conditions are adversely affected, it could materially and adversely affect our sales and financial results, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline. Our global business may be negatively affected by local economic conditions, including inflation, increasing labor costs, potential recession and currency exchange rate fluctuations, which could adversely affect our cost to manufacture and provide our products and services and revenues generated through sales of such products and services. There is no guarantee that we will be able to fully absorb any such additional costs or revenue declines in the prices for our products and services. We also continue to monitor the impacts on our businesses of the COVID-19 virus and variant and subvariant strains thereof in order to timely address new issues if and when they arise. To the extent the COVID-19 pandemic persists, with surges in infection and associated government responses, it could have a significant adverse effect on our business, financial condition, cash flows, results of operations and could cause the market value of our common shares and/or debt securities to decline and may exacerbate other risk factors disclosed elsewhere in this “Risk Factors” section.
Inflation and other macroeconomic factors could materially adversely affect our business and operations.
Our operating results could be materially impacted by changes in the overall global macroeconomic environment and other economic factors that impact our cost structure and revenue results. Changes in economic conditions, including supply chain constraints, logistics challenges, labor shortages and steps taken by governments and central banks, particularly in response to the COVID-19 pandemic, as well as other stimulus and spending programs, have, in the past, led to (and could, in the future lead to) higher inflation, resulting in an increase in costs and changes in fiscal and monetary policy, including increased interest rates. In a higher inflationary environment, we may be unable to raise the prices of our products and services sufficiently to keep up with the rate of inflation. Moreover, negative macroeconomic conditions could adversely impact our ability to obtain financing in the future on terms acceptable to us, or at all. In addition, geopolitical instability (such as the ongoing conflict between Russia and Ukraine and the ongoing conflict in the Middle East involving Israel and Hamas) and related sanctions could continue to have significant ramifications on global financial markets, including volatility in the U.S. and global financial markets. These inflationary pressures and other negative macroeconomic conditions could impact our revenues and resulting margins and could have an adverse impact on results of operations and could cause the market value of our common shares and/or debt securities to decline.
Risks Relating to the Separation
We may not realize the anticipated benefits from the Separation, and the Separation could harm our business.
From 2013 until the completion of the B+L IPO, we operated as a business within BHC. Since completion of the B+L IPO, we have operated as an independent company from BHC, although BHC controls a majority of the voting power of our outstanding common shares and therefore generally is able to determine the outcome of all corporate actions that require shareholder approval. The completion of the full Separation of the Company from BHC remains subject to the achievement of targeted debt leverage ratios and the receipt of applicable shareholder and other necessary approvals and the various risk factors set forth herein.
We may not be able to achieve the full strategic and financial benefits expected to result from the Separation, or such benefits may be delayed or not occur at all. The Separation is expected to enhance strategic and management focus, provide a distinct investment identity and allow us to efficiently allocate resources and deploy capital. We may not achieve these and other anticipated benefits for a variety of reasons, including, among others:
•the Separation has required significant amounts of management’s time and effort and may continue to require management’s further time and effort, which may divert management’s attention from operating and growing our business;
•as a result of the Separation, we may be more susceptible to economic downturns and other adverse events than if we were still a part of BHC;
•following the B+L IPO, we commenced operating as an independent company and, as a result, our business is less diversified than BHC’s business prior to the completion of the B+L IPO;
•our business has and may continue to experience a loss of scale and purchasing power and access to certain financial, managerial and professional resources from which we have benefited at lower cost in the past;
•the other actions required to complete the Separation could disrupt our operations; and
•the development of our operations and infrastructure in connection with the Separation, and any future expansion of such operations and infrastructure, may not be entirely successful, and may strain our operations and increase our operating expenses.
If we fail to achieve some or all of the benefits expected to result from the Separation, or if such benefits are delayed, our business could be harmed and could cause the market value of our common shares and/or debt securities to decline.
The Separation is subject to certain uncertainties. Furthermore, the Distribution (as defined below) may not occur.
Unanticipated developments, including disruptions to business and commerce induced by changes in global market, financial and economic conditions (such as the COVID-19 pandemic and international conflicts), possible delays in obtaining any necessary shareholder, stock exchange, regulatory or other approval or the failure to obtain any such approvals, possible delays in obtaining any required tax opinions or rulings or the failure to obtain any such tax opinions or rulings, that a portion of BHC’s ownership of Bausch + Lomb is pledged as collateral securing BHC’s 9.00% senior secured notes, negotiating challenges, the uncertainty of the financial markets, changes in the law, and other challenges could delay or prevent the completion of the Separation, result in changes to the anticipated structure of the Separation, or cause the Separation to occur on terms or conditions that are different or less favorable than expected. Any changes to the Separation or delay in completing the Separation could cause us not to realize some or all of the expected benefits or realize them on a different timeline than expected.
In particular, as part of the Separation, BHC has indicated that it intends to transfer all or a portion of its remaining direct or indirect equity interest in us to its shareholders (the “Distribution”). BHC informed us in the past that it intended to conduct the Distribution by way of a statutory plan of arrangement under applicable corporate law (the “Distribution Arrangement”) to be implemented in accordance with the terms and subject to the conditions set out in the plan of arrangement (the “Distribution Plan of Arrangement”) appended to the Arrangement Agreement entered into between Bausch + Lomb and BHC (the “Distribution Arrangement Agreement”). Subject to the terms of the Distribution Arrangement Agreement, BHC may instead also effect the Distribution through one or more distributions effected as a dividend or a tax-free reduction of capital to all BHC shareholders, one or more distributions in exchange for BHC shares or other securities, or any combination thereof. Prior to the completion of any such Distribution, BHC may also sell a portion of its remaining direct or indirect equity interest in us through an offering to third parties. In August 2023, BHC disclosed that it continues to evaluate all relevant factors and circumstances around the Distribution, and that while its initial intent was to effect the Distribution by way of the Distribution Plan of Arrangement, BHC has since determined it may be optimal to effect the Distribution through a tax-free reduction of capital rather than pursuant to the terms of the Distribution Plan of Arrangement; however, BHC has indicated that it continues to evaluate the structure of any Distribution and other related details.
However, BHC has no obligation to complete the Distribution on the terms that have been previously disclosed or at all, and it will have the ability to unilaterally terminate the Distribution Arrangement Agreement in its sole discretion at any time before the Distribution Arrangement is implemented, and Bausch + Lomb may terminate the Distribution Arrangement Agreement in accordance with its terms as of the outside date of December 31, 2024 (unless the parties otherwise agree). Whether BHC proceeds with the Distribution pursuant to the Distribution Arrangement or otherwise, in whole or in part, is subject to a number of conditions precedent, many of which are outside our control. These conditions precedent are expected to include, but are not limited to the following: achievement of targeted debt leverage ratios by BHC, receipt of any necessary regulatory or other approvals, existence of satisfactory market conditions, and in the case of a tax-free transaction, an opinion of counsel (and, at the election of BHC, a tax ruling from the IRS as to certain issues related to the Distribution (the “U.S. Tax Ruling”)) and a tax ruling requested from the Canada Revenue Agency (the “CRA”) confirming the tax-free treatment of the transaction to BHC, the Company and their respective shareholders (the “Tax Ruling”). Completion of any plan of arrangement under applicable corporate law (including the Distribution Plan of Arrangement) would also be subject to approvals, including by receipt of applicable shareholder approvals and receipt of and compliance with the interim and final orders from the Supreme Court of British Columbia (the “Interim Order” and the “Final Order,” respectively). If BHC proceeds with the Distribution pursuant to the Distribution Arrangement, at the hearing for the Final Order, the Supreme Court of British Columbia would consider whether to approve the Distribution based on the applicable legal requirements and the evidence and submissions before the Court as to, among other things, whether the Distribution Plan of Arrangement is fair and reasonable. There can be no certainty, nor can we provide any assurance, that all conditions precedent to the Distribution, whether under the Distribution Arrangement Agreement, through a reduction of capital or otherwise, will be satisfied or waived, or, if satisfied or waived, when they will be satisfied or waived. If certain approvals and consents are not received prior to the anticipated effective date of the Distribution, we and BHC may decide to proceed nonetheless, or we and BHC may either delay or amend the implementation of all or part of the Distribution, including possibly delaying the
completion of the Distribution in order to allow sufficient time to complete such matters or effecting the Distribution other than by way of a plan of arrangement under applicable corporate law (such as through a reduction of capital). Any such changes in timing or manner of effecting the Distribution could result in other conditions needing to be satisfied or waived. If the Distribution is delayed, restructured or not completed, the market price of our common shares may be materially adversely affected. Furthermore, if the Distribution does not occur, or if BHC does not otherwise dispose of its ownership of our equity interests, the risks relating to BHC’s control of us and the potential business conflicts of interest between BHC and us will continue to be relevant to our securityholders. The liquidity of our common shares and/or debt securities in the market may be constrained for as long as BHC continues to hold a significant position in our common shares and/or debt securities. A lack of liquidity in our common shares and/or debt securities could depress the price of our common shares.
It is possible that future factors may arise that make it inadvisable to proceed with, or advisable to delay, all or part of the Distribution, which may include an amendment to the Distribution Plan of Arrangement to modify, add or remove certain steps in the Distribution Arrangement, or to amend the terms of the Distribution Arrangement Agreement. BHC will have the right, in its sole discretion to amend the Distribution Plan of Arrangement and to make any necessary conforming changes to the Distribution Arrangement Agreement so long as it has determined, acting reasonably, that such amendment(s) are not materially adverse to us or to our shareholders from a financial perspective. The Distribution Arrangement Agreement may also be terminated in certain circumstances, including by BHC in its sole discretion at any time before the Distribution Arrangement is implemented. BHC will have the right to abandon or change the structure of the Distribution if BHC determines to do so in its sole discretion.
Additionally, if the Distribution, does not occur on the timelines or in the manner currently anticipated or at all, it may have a negative effect on the price of our common shares and/or debt securities or value of our common shares and/or debt securities.
The Separation is subject to challenge and could be subject to further challenges in the future, any of which could delay or prevent the consummation of such transactions or cause them to occur on worse terms than we currently expect.
The Separation is subject to challenge, which could delay or prevent the consummation of such transactions or cause them to occur on worse terms than we currently expect. For example, in March 2022, we and BHC were named in a declaratory judgment action in the Superior Court of New Jersey, Somerset County, Chancery Division (which was removed to the U.S. District Court for the District of New Jersey, but subsequently remanded back to the Superior Court of New Jersey), brought by certain individual investors in BHC’s common shares and debt securities who are also maintaining individual securities fraud claims against BHC and certain of its current or former officers and directors. This action seeks a declaratory judgment that the transfer of assets from BHC to us would constitute a voidable transfer under New Jersey’s Uniform Voidable Transactions Act and that we would become liable for damages awarded against BHC in the individual opt-out actions. In addition, we could, in the future, face additional legal proceedings and investigations and inquiries by governmental agencies relating to these or similar matters. For more information regarding legal proceedings, see Note 20, “LEGAL PROCEEDINGS” to our audited Consolidated Financial Statements.
We are unable to predict the outcome of any such proceedings, investigations and inquiries, but we may incur significant costs and diversion of management attention as a result of these matters, regardless of the outcome. Some or all of these proceedings, investigations and inquiries may lead to damages, settlement payments, fines, penalties, consent orders or other administrative sanctions against us, even if they relate solely to alleged actions or misstatements of BHC. Furthermore, publicity surrounding these proceedings, investigations and inquiries or any enforcement action as a result thereof, even if ultimately resolved favorably for us could result in additional investigations and legal proceedings. As a result, these proceedings, investigations and inquiries could have a material adverse effect on our reputation, business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We have limited history of operating as an independent company, and our historical financial information prior to the B+L IPO is not necessarily representative of the results that we would have achieved as an independent or standalone company and may not be a reliable indicator of our future results.
Our historical financial information, for periods prior to the B+L IPO, is not necessarily indicative of our future results of operations, financial condition or cash flows, nor does it reflect what our results of operations, financial condition or cash flows would have been as an independent public company during the periods presented. In particular, such historical financial information included in this Form 10-K is not necessarily indicative of our future results of operations, financial condition or cash flows primarily because of the following factors, among others:
•Prior to the B+L IPO, our business had been operated by BHC as part of its broader corporate organization, rather than as an independent company; BHC or one of its affiliates provided support for various corporate functions for us, such as information technology, compensation and benefits, human resources, engineering, finance and internal audit.
•Our historical financial results prior to the B+L IPO reflect the direct, indirect and allocated costs for such services historically provided by BHC. Following the B+L IPO, BHC continues to provide some of these services to us on a transitional basis, pursuant to a transition services agreement that we entered into with BHC in connection with the Separation. Our historical financial information does not reflect our obligations under the various transitional and other agreements we entered into with BHC in connection with the Separation. At the end of this transition period, we will need to perform these functions ourselves or hire third parties to perform these functions on our behalf, and these costs may differ significantly from the comparable expenses we have incurred in the past.
•Prior to the B+L IPO, our working capital requirements and capital expenditures historically were satisfied as part of BHC’s corporate-wide cash management and centralized funding programs, and our cost of debt and other capital may significantly differ from the historical amounts reflected in our historical financial statements.
•Prior to the B+L IPO, our business was integrated with that of BHC and we benefited from BHC’s size and scale in costs, employees and vendor and customer relationships. Thus, costs we incur as an independent company may significantly exceed comparable costs we incurred as part of BHC.
•As a standalone public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, applicable Canadian securities laws and the regulations of the NYSE and the TSX. Such requirements have increased our legal, accounting and financial compliance costs, make some activities more difficult, time-consuming and costly and could be burdensome on our personnel, systems and resources. We have devoted and expect to continue to devote significant resources to address these public company-associated requirements, including compliance programs and investor relations, as well as our financial reporting obligations. Complying with these rules and regulations has and will substantially increase our legal and financial compliance costs and make some activities more time-consuming and costly.
•In addition, as a public company, our management is required to conduct an annual evaluation of our internal controls over financial reporting and include a report of management on our internal controls in our annual reports on Form 10-K, commencing with this Form 10-K. In addition, commencing with this Form 10-K, we are required to have our independent registered public accounting firm attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002. If we are unable to conclude that we have effective internal controls over financial reporting, or if our registered public accounting firm is unable to provide us with an attestation and an unqualified report as to the effectiveness of our internal controls over financial reporting, investors could lose confidence in the reliability of our financial statements, which could result in a decrease in the value of our common shares and/or debt securities. Moreover, failure to accurately report our financial performance on a timely basis could also jeopardize our continued listing on the NYSE, the TSX or any other exchange on which our common shares may be listed. Delisting of our common shares on any exchange would reduce the liquidity of the market for our common shares, which would reduce the price of and increase the volatility of the market price of our common shares.
Until the completion of the Separation, BHC will control the direction of our business, and the concentrated ownership of our common shares will prevent you and other shareholders from influencing significant decisions.
As of February 16, 2024, BHC beneficially owns approximately 88.4% of our issued and outstanding common shares. As long as BHC controls a majority of the voting power of our issued and outstanding common shares with respect to a particular matter, it will generally be able to determine the outcome of all corporate actions requiring shareholder approval (as further described below) and will be able to block a takeover bid made for the shares of the Company as Canadian securities laws require that a minimum of 50% of the issued and outstanding shares be tendered to the bid in order for the bid to succeed. In addition, as controlling shareholder, BHC will have significant influence over our plans and strategies, including strategies relating to marketing and growth. Even if BHC were to control less than a majority of the voting power of our outstanding common shares, it may be able to influence the outcome of such corporate actions so long as it owns a significant portion of our common shares. If BHC does not complete the Distribution or otherwise dispose of its ownership of our equity interests, it could remain our controlling shareholder for an extended period of time or indefinitely. In such a case, the concentration of BHC’s holdings may delay or prevent any acquisition or delay or discourage takeover attempts that shareholders may consider to be favorable, or make it more difficult or impossible for a third-party to acquire control of the Company or effect a change in the Board of Directors and management, any of which may cause the market price of our common shares and/or debt securities to decline. Any delay or prevention of a change of control transaction could deter potential acquirors or prevent the completion of a transaction in which the Company’s shareholders could receive a premium over the then current market price for their common shares.
As long as BHC controls the majority of the voting power of our outstanding common shares, except where Canadian law requires that a matter be determined by a majority of the votes cast by minority shareholders and excludes BHC from the
minority for that purpose, BHC will generally be able to control, whether directly or indirectly through its ability to remove and elect directors, and subject to applicable law, substantially all matters affecting us, including:
•any determination with respect to our business direction and policies, including the election and removal of directors and the appointment and removal of officers;
•any determinations with respect to mergers, amalgamations, business combinations or dispositions of assets;
•our financing and dividend policy, and the payment of dividends on our common shares, if any;
•compensation and benefit programs and other human resources policy decisions;
•changes to any other agreements that may adversely affect us; and
•determinations with respect to our tax returns and other tax matters.
In addition, pursuant to the Master Separation Agreement entered into by us and BHC in connection with the B+L IPO (the “MSA”), until BHC ceases to hold 50% of the total voting power of our outstanding share capital entitled to vote in the election of our directors, we will not be permitted, without BHC’s prior written consent, (or, in certain circumstances, the approval of the BHC Board of Directors), to take certain significant actions. As a result, our ability to take such actions may be delayed or prevented. We will not be able to terminate or amend the MSA, except in accordance with its terms.
BHC’s interests may not be the same as, or may conflict with, our interests or the interests of our other shareholders Because BHC’s interests may differ from ours or from those of our other shareholders, actions that BHC takes with respect to us, as our controlling shareholder and pursuant to its rights under the MSA, may not be favorable to us or our other securityholders.
In addition, BHC will have the ability, should it choose to do so, to sell some or all of our common shares that it owns in a privately negotiated transaction, which, if sufficient in size, could result in a change of control of our company. If BHC privately sells its significant equity interests in our company, we may become subject to the control of a presently unknown third party. Such third party may have interests that conflict with those of other securityholders, and may attempt to cause us to revise or change our plans and strategies. A new owner may also have different plans with respect to the Separation, including not effecting such Separation.
Some of our directors and officers may have actual or potential conflicts of interest because of their equity ownership in BHC, and some of our directors may have actual or potential conflicts of interest because they also serve as directors of BHC.
Because of their current or former positions with BHC, some of our directors and executive officers may own common shares of BHC or have options to acquire shares of BHC, and the individual holdings may be significant for some of these individuals compared to their total assets. In addition, certain of our directors also serve as directors of BHC. While our Board of Directors has determined that Thomas W. Ross, Sr., Nathalie Bernier, Andrew C. von Eschenbach, Sarah B. Kavanagh, John A. Paulson, Russel C. Robertson, Richard U. De Schutter, Brett Icahn and Gary Hu are “independent directors” within the meaning of applicable regulatory and stock exchange requirements in the United States and within the meaning of Canadian securities regulations, certain of them have served and, in some cases, continue to serve, as directors of BHC.
A director who has a material interest in a matter before our Board of Directors or any committee on which he or she serves is required to disclose such interest as soon as the director becomes aware of it in accordance with applicable law. In situations where a director has a material interest in a matter to be considered by our Board of Directors or any committee on which he or she serves, such director may be required to excuse himself or herself from the meeting while discussions and voting with respect to the matter are taking place. Although all transactions with related parties will be approved by independent members of our Board of Directors that may meet in the absence of senior executive officers or non-independent directors, the ownership of BHC equity or service to BHC may create the appearance of conflicts of interest when the BHC-affiliated directors and officers are faced with decisions that could have different implications for BHC or us. For example, potential conflicts of interest could arise in connection with the resolution of any dispute that may arise between BHC and us regarding the terms of the agreements governing the Separation and the relationship thereafter between the companies. Potential conflicts of interest could also arise if we enter into commercial arrangements with BHC in the future. As a result of these actual or apparent conflicts, we may be precluded from pursuing certain growth initiatives.
While the Board of Directors believes that, given its size and structure, such actual or potential conflicts of interest can be managed adequately, including that the independent members of our Board of Directors may meet in the absence of senior executive officers or non-independent directors in respect of the relevant matter, the actual or perceived conflicts of interest that may arise could cause reputational or other harm.
To preserve the tax-free treatment of certain transactions related to the Distribution, we may not be able to engage in certain transactions. We could incur significant tax liabilities, or be liable to BHC, if certain transactions occur which result in these transactions or the Distribution being subject to tax.
To preserve the tax-free treatment of certain transactions related to the Distribution, certain agreements we entered into with BHC in connection with the Separation (including the Distribution Arrangement Agreement) contain certain tax-related covenants. We previously expected that the Distribution would be effected pursuant to the public company “butterfly reorganization” rules in Section 55 of the Tax Act (although BHC has recently announced it is considering other alternative structures, including a tax-free reduction of capital) and so these covenants include agreements that, among other things and subject to certain limited exceptions: (a) we and BHC will: (i) not, on or before the effective date of the Distribution Arrangement, take or perform or fail to take or perform any act, including entering into any transaction or permitting any act or transaction within our respective control to be taken or performed or to occur, that, in each case, could reasonably be considered to interfere or be inconsistent with the Tax Ruling; (ii) not take or perform or fail to take or perform any act, including entering into any transaction or permitting any act or transaction within our respective control to be taken or performed or to occur, in each case, that would cause BHC to cease to be a “specified corporation” within the meaning of the Tax Act on or prior to the effective date of the Distribution Arrangement, except as specifically contemplated by the Distribution Arrangement Agreement and in the Tax Ruling; and (iii) fulfill all representations and undertakings provided by us (or by any of our subsidiaries), or on our behalf (or on behalf of any of our subsidiaries) with our knowledge and consent, in the Tax Ruling; and (b) we and BHC will not, for a period of three years after the effective date of the Distribution Arrangement, take or perform or fail to take or perform any act, including entering into any transaction or permitting any act or transaction within our respective control to be taken or performed or to occur, that, in each case, could reasonably be expected to cause the Distribution Arrangement and/or any transaction contemplated by the Distribution Arrangement and/or the Distribution Arrangement Agreement to be taxed in a manner inconsistent with that provided for in the Tax Ruling. Although BHC recently announced that it may effect the Distribution through a tax-free reduction of capital, we remain subject to these tax covenants, which may restrict us from taking certain actions that we might otherwise choose to take or from pursuing certain strategic transactions or engaging in other transactions, some of which could be material. The nature, extent and effect of these restrictions will depend on the manner in which the Distribution is effected.
If the Distribution were to be effected pursuant to the public company “butterfly reorganization” rules in Section 55 of the Tax Act as BHC initially anticipated, the Company and BHC would recognize a taxable gain on the completion of the Distribution if (a) within three years of completing the Distribution, we engage in a subsequent spin-off or split-up transaction under Section 55 of the Tax Act or BHC engages in a split-up (but not spin-off) transaction under Section 55 of the Tax Act, (b) a “specified shareholder” as defined for purposes of the “butterfly reorganization” rules in Section 55 of the Tax Act disposes of our shares or shares of BHC, or property that derives 10% or more of its value from such shares and an unrelated person or a partnership acquires such property or property substituted therefor as part of the “series of transactions” which includes the Distribution; (c) there is an acquisition of control of the Company or BHC that is part of the “series of transactions” that includes the Distribution; or (d) certain persons acquire shares in our capital (other than in specified permitted transactions) in contemplation of, and as part of the “series of transactions” that includes, the Distribution. If any of the above events were to occur and to cause the Distribution to be taxable to BHC and/or to the Company, then BHC or the Company, as applicable, and, in some cases, both BHC and the Company, would be liable for a substantial amount of tax. In addition, if such an event were due to an act of BHC (or one of its subsidiaries or controlled affiliates, other than the Company or its subsidiaries) or the Company (or one of its subsidiaries or controlled affiliates), or an omission by BHC or the Company to act, then BHC (in the case of an action taken by it or one of its subsidiaries or controlled affiliates (other than the Company and its subsidiaries)) or the Company (in the case of any action taken by it or one of its subsidiaries or controlled affiliates), as applicable, would generally be required to indemnify the other party for tax under the Distribution Arrangement Agreement. A breach by BHC or the Company of the other tax-related covenants in any of the Separation related agreements (including these tax covenants) may also require BHC or the Company, as applicable, to indemnify the other against any loss suffered or incurred from or in connection with such breach.
The applicability of these restrictions and the extent and nature of any indemnity obligations will depend on the manner in which the Distribution is ultimately effected, including whether or not the Distribution is effected pursuant to the public company “butterfly reorganization” rules of the Tax Act, which may be outside of our control. If the Distribution is effected through a tax free reduction of capital, certain of these restrictions may no longer apply to us or to BHC. See also “—The Separation is subject to certain uncertainties. Furthermore, the Distribution may not occur.”
In addition, in order to preserve the tax-free treatment of the Distribution, if effected, for U.S. federal income tax purposes, we will be restricted from taking certain actions, including, during the two-year period after the Distribution, discontinuing the active conduct of our trade or business, merging or amalgamating with any other person (other than in connection with the Distribution), redeeming or otherwise acquiring our shares (other than pursuant to certain open-market repurchases of less than 20% of our common shares, in the aggregate), soliciting, participating or supporting any acquisition of our shares by any person or business combination having a similar effect, or otherwise taking any action that could
reasonably be expected to adversely affect the tax-free treatment of the Distribution for U.S. federal income tax purposes. Notwithstanding the foregoing, we may be permitted to take certain of these actions if we receive a tax ruling or opinion of counsel, acceptable to BHC, to the effect that the action will not adversely affect the tax-free treatment of the Distribution for U.S. federal income tax purposes. Regardless of whether we are so permitted to take such action, we will be required to indemnify BHC for any tax-related losses that result from the taking of any such action. Due to these restrictions and indemnification obligations, we may be limited in our ability to pursue strategic transactions or other transactions that may be in our best interests, and our potential indemnity obligation to BHC could discourage, delay or prevent a merger or other business combination with us.
Certain requirements of the public company “butterfly reorganization” rules in Section 55 of the Tax Act depend on events that may not be within our control.
If the Distribution is to be achieved in accordance with the Distribution Plan of Arrangement, we would expect the Tax Ruling to require, among other things, that the Distribution complies with all of the requirements of the public company “butterfly reorganization” rules in Section 55 of the Tax Act. Although the Distribution would be expected to be structured to comply with these rules, and although BHC and the Company have each agreed to provide certain tax-related covenants in the Distribution Arrangement Agreement, certain events could occur that may not be within the control of the Company and/or BHC, including certain actions taken by one or more of the shareholders of the Company and/or BHC, none of whom are, to the Company’s knowledge, bound by any similar covenants (other than BHC pursuant to its tax-related covenants).
These events include circumstances where: (i) a “specified shareholder” as defined for purposes of the “butterfly reorganization” rules in Section 55 of the Tax Act disposes of our shares or shares of BHC, or property that derives 10% or more of its value from such shares and an unrelated person or a partnership acquires such property or property substituted therefor as part of the “series of transactions” which includes the Distribution; (ii) there is an acquisition of control of the Company or BHC that is part of the “series of transactions” that includes the Distribution; or (iii) certain persons acquire shares in our capital (other than in specified permitted transactions) in contemplation of, and as part of the “series of transactions” that includes, the Distribution.
If the Distribution is effected through a “butterfly reorganization,” and if the requirements of the public company “butterfly reorganization” rules in Section 55 of the Tax Act are not met, then this could cause the Distribution to be taxable to BHC and/or to the Company, with the result that BHC or the Company, as applicable, and, in some cases, both BHC and the Company, would be liable for a substantial amount of tax for which indemnification from the other party may not be available. If incurred, tax liabilities could have a material effect on our financial position.
We potentially could have received better terms from unaffiliated third parties than the terms we received in our agreements with BHC.
The agreements we entered into with BHC in connection with the Separation (including the Distribution Arrangement Agreement) were negotiated while we were still part of BHC’s business. Accordingly, during the period in which the terms of those agreements were negotiated, we did not have an independent Board of Directors or a management team independent of BHC. The terms of the agreements negotiated in the context of the Separation relate to, among other things, the allocation of assets, intellectual property, liabilities, rights and other obligations between BHC and us. Arm’s-length negotiations between us and an unaffiliated third party in another form of transaction, such as a seller in a sale of a business, may have resulted in more favorable terms to us.
We have agreed to indemnify BHC for certain liabilities, and BHC has agreed to indemnify us for certain liabilities. However, there can be no assurance that BHC’s indemnity will be sufficient to insure us against the full amount of such liabilities, or that BHC’s ability to satisfy its indemnification obligation will not be impaired in the future.
Pursuant to the various Separation-related agreements with BHC, BHC has agreed to indemnify us for certain liabilities. However, there can be no assurance that the indemnity from BHC will be sufficient to protect us against the full amount of such liabilities, or that BHC will be able to fully satisfy its indemnification obligations in the future. Even if we ultimately succeed in recovering from BHC any amounts for which we are held liable, we may be temporarily required to bear these losses. Each of these risks could negatively affect our business, financial condition, results of operations and cash flows.
Furthermore, any indemnification claim against the Company, by BHC, including for a breach of the tax-related covenants, could be substantial, may not be able to be satisfied and may have a material adverse effect on us. Each of these risks could also negatively affect our business, financial condition, results of operations and cash flows.
As long as BHC owns a majority of our common shares, we may rely on certain exemptions from the corporate governance requirements of the NYSE available to “controlled companies” and of the TSX available to “majority controlled” companies.
We are currently a “controlled company” within the meaning of the corporate governance requirements of the NYSE because BHC beneficially owns more than 50% of our outstanding common shares. Until such time as we are no longer a “controlled company,” we are exempt from certain corporate governance requirements, including requirements that a majority of the Board of Directors consist of independent directors and having a compensation committee and a nominating and corporate governance committee that is composed entirely of independent directors. We may take advantage of these exemptions from time to time. Upon completion of the Distribution, we will no longer qualify as a controlled company and will be required to fully implement NYSE corporate governance requirements within one year of the Distribution. While BHC controls a majority of the voting power of our outstanding common shares, we may not have a majority of independent directors or our Talent and Compensation Committee may not consist entirely of independent directors. Prior to such time, shareholders may not have certain of the protections afforded to shareholders of companies that are required to comply with all of the corporate governance requirements of the NYSE.
In Canada, National Policy 58-201 (“NP 58-201”) provides guidance on corporate governance practices, which reflect best practices established by the Canadian securities regulatory authorities but are not intended to be prescriptive. NP 58-201 provides, among other things, that (i) the board of directors of a reporting issuer should have a majority of independent directors; (ii) the chair of the board of directors should be an independent director; (iii) the board of directors should appoint a nominating committee composed entirely of independent directors; and (iv) the board of directors should appoint a compensation committee composed entirely of independent directors. National Instrument 58-101 requires a company to disclose the extent to which it complies with the best practices set forth in NP 58-201. To the extent that we take advantage of the “controlled company” exemption of the NYSE, and as a result do not comply with NP 58-201, we will be required to explain why we do not comply with Canadian director independence standards.
The Distribution or future sales by BHC or others of our common shares, or the perception that the Distribution or such sales may occur, could depress our common share price.
Future sales of our common shares in the public market will be subject to the volume and other restrictions of Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”), for so long as BHC is deemed to be our affiliate, unless such sales of shares are registered with the SEC or qualify for another applicable exemption from registration. Similarly, any sale of any of our common shares by BHC will constitute a “control distribution” under Canadian securities laws (generally a sale by a person or a group of persons holding more than 20% of our outstanding voting securities) and will be subject to restrictions under Canadian securities laws, unless the sale is qualified under a prospectus filed with Canadian securities regulatory authorities, is made pursuant to a prospectus exemption, or if prior notice of the sale is filed with the Canadian securities regulatory authorities at least seven days before any sale and there has been compliance with certain other requirements and restrictions regarding the manner of sale, payment of commissions, reporting and availability of current public information about us and compliance with applicable Canadian securities laws. We have granted certain registration rights to BHC. We are unable to predict with certainty whether or when BHC will sell a substantial number of our common shares to the extent it retains shares following the Distribution or in the event the Distribution does not occur. The Distribution or sale by BHC of a substantial number of our common shares, or a perception that the Distribution or such sales could occur, could significantly reduce the market price of our common shares.
The services that BHC provides to us may not be sufficient to meet our needs, which may result in increased costs and otherwise adversely affect our business.
Pursuant to the Transition Services Agreement entered into with BHC in connection with the B+L IPO, BHC agreed to provide us with corporate and shared services for a transitional period, including information technology services, technical and engineering support, application support for operations, legal, payroll, finance, tax and accounting, general administrative services and other support services and other services in exchange for the fees specified in the Transition Services Agreement between us and BHC. Certain of these transitional services are still being provided to us by BHC. If we no longer receive these services from BHC due to the termination of the Transition Services Agreement or otherwise, we may not be able to perform these services ourselves and/or find appropriate third party arrangements at a reasonable cost (and any such costs may be higher than those charged by BHC). In addition, we have received informal support from BHC, which may not be addressed in the agreements we have entered into with BHC, and the level of this informal support may diminish as we become a more independent company. Any failure or significant downtime in our own administrative systems or in BHC’s administrative systems during the remainder of the transitional period could result in unexpected costs, impact our results and/or prevent us from paying our suppliers or employees and performing other administrative services on a timely basis.
Certain contracts used in our business will need to be replaced, or assigned from BHC or its affiliates to us in connection with the Separation, which may require the consent of the counterparty to such an assignment, and failure to obtain such replacement contracts or consents could increase our expenses or otherwise adversely affect our results of operations. In addition, the transfer of certain other assets and liabilities from BHC to us contemplated by the Separation are not yet complete.
The Separation requires us to replace shared contracts and, with respect to certain contracts that are to be assigned from BHC or its affiliates to us or our affiliates, to obtain consents and assignments from third parties. It is possible that, in connection with the replacement or consent process, some parties may seek more favorable contractual terms from us. While many of these replacement contracts and consents have already been obtained in connection with the Separation and the B+L IPO, certain replacement contracts and consents remain outstanding. BHC has agreed to use commercially reasonable efforts to ensure that we receive the economic benefits of the contract in question. Nonetheless, we may be unable to obtain some of the benefits, assets and contractual commitments that are intended to be allocated to us as part of the Separation. If we are unable to obtain such replacement contracts or consents, the loss of these contracts could increase our expenses or otherwise materially adversely affect our business, results of operations and financial condition.
Development and Regulatory Risks
The successful development of our pipeline products is highly uncertain and requires significant expenditures and time. In addition, obtaining necessary government approvals is time-consuming and not assured. The failure to commercialize certain of our pipeline products could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We currently have a number of pipeline products in development. We and our development partners, as applicable, conduct extensive preclinical studies and clinical trials to demonstrate the safety and efficacy in humans of our pipeline products in order to obtain regulatory approval for the sale of our pipeline products. Preclinical studies and clinical trials are expensive, complex, can take many years and have uncertain outcomes. None of, or only a small number of, our research and development programs may actually result in the commercialization of a product. We will not be able to commercialize our pipeline products if preclinical studies do not produce successful results or if clinical trials do not demonstrate safety and efficacy in humans.
Furthermore, success in preclinical studies or early-stage clinical trials does not ensure that later stage clinical trials will be successful nor does it ensure that regulatory approval for the product candidate will be obtained. In addition, the process for the completion of pre-clinical and clinical trials is lengthy and may be subject to a number of delays for various reasons, which would delay the commercialization of any successful product. If our development projects are not successful or are significantly delayed, we may not recover our substantial investments in the pipeline product and our failure to bring these pipeline products to market on a timely basis, or at all, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
In addition, the FDA and Health Canada approval must be obtained in the U.S. and Canada, respectively, EMA approval (drugs) and CE Marking (devices) and/or registration under the European Commission’s Medical Device Regulation (“MDR”) 2017/745 must be obtained in countries in the EU and similar approvals must be obtained from comparable agencies in other countries, prior to marketing or manufacturing new pharmaceutical and medical device products for use by humans. Obtaining such regulatory approvals for new products and devices and manufacturing processes can take a number of years and involves the expenditure of substantial resources. We may face additional challenges with respect to EMA approval and CE Marking in the EU as a result of additional requirements for approval in the EU that may be more burdensome than those required by the FDA and Health Canada. Even if such products appear promising in development stages, regulatory approval may not be achieved and no assurance can be given that we will obtain approval in those countries where we wish to commercialize such products. Nor can any assurance be given that if such approval is secured, the approved labeling will not have significant labeling limitations, including limitations on the indications for which we can market a product, or require onerous risk management programs. Furthermore, from time to time, changes to the applicable legislation, regulations or policies may be introduced that change these review and approval processes for our products, which changes may make it more difficult and costly to obtain or maintain regulatory approvals.
Our marketed products will be subject to ongoing regulatory review.
Following initial regulatory approval of any products, we or our partners may develop or acquire, we will be subject to continuing regulatory review by various government authorities in those countries where our products are marketed or intended to be marketed, including the review of adverse drug events and clinical results that are reported after product candidates become commercially available. This also includes the need to monitor our medical device products both before and after receipt of the applicable market authorizations, including with respect to managing adverse device cases for reportable events, which may, for example, result in the need to file field safety notifications to competent health authorities. In addition, we are subject to ongoing audits and investigations of our facilities and products by the FDA, as well as other regulatory agencies in and outside the United States.
If we fail to comply with the regulatory requirements in those countries where our products are sold, we could lose our marketing approvals or be subject to fines or other sanctions. Also, as a condition to granting marketing approval of a product, the applicable regulatory agencies may require a company to conduct additional clinical trials or remediate Current Good Manufacturing Practice (“cGMP”) issues, the results of which could result in the subsequent loss of marketing approval, changes in product labeling or new or increased concerns about side effects or efficacy of a product. Also our compliance requirements extend to other current good practices with which we must comply and adhere to with respect to the development and commercialization of our products and medical devices, including not only cGMP, but also Current Good Laboratory Practices (“cGLP”), Current Good Clinical Practices (“cGLP”) and Current Good Distribution Practices (“cGDP”).
In April 2017, the European Union adopted MDR, which repeals and replaces the Medical Device Directive (“MDD”) and active implantable medical devices Directive (“AIMDD”) 90/385/EEC. The MDR, for most parts, became applicable on May 26, 2021. Under the MDR, several transitional measures apply to medical devices that are certified under the MDD or AIMDD prior to May 26, 2021 or, for class I device, for which a declaration of conformity was drawn up prior to May 26, 2021, allowing these devices to be placed on the market after May 26, 2021 under certain conditions for a transitional period. However, if we make any significant changes in the design or intended purpose of our devices, they will no longer benefit from such transitional periods. Generally, the MDR imposes stricter requirements on manufacturers, importers and distributors of medical devices. Moreover, the requirements to provide clinical data for medical devices has become stricter and as a result we may need to conduct new time consuming and costly clinical investigations with our existing medical devices to meet the new requirements, including to obtain CE certificates under the MDR. We may, or may not, be able to provide this data in time to obtain MDR certifications in a timely fashion when our existing certificates expire. These new regulations impact all of our existing and pipeline medical device products being sold in the EEA for which we are legal manufacturer, importer and/or distributor, including contact lens, lens care, eye health, aesthetic and surgical areas, as well as certain of our products outside the EEA, which rely on the EEA registration to support registration in those other countries. These products, in the aggregate, account for a meaningful portion of our net revenue in this region. While we are working to ensure compliance with these new regulations for all impacted products, we may not be able to achieve compliance for all products within the applicable transition period. If we fail to achieve compliance, we will not be able to market and sell the non-compliant products in the EEA, nor will we be able to rely on the non-compliant registration for such products in regions outside of the EEA, which could have a material adverse effect on our business, financial condition, cash flows and results of operations in the EEA and, possibly, on a consolidated basis, and could cause the market value of our common shares and/or debt securities to decline.
While EU law is applicable in Northern Ireland, the UK Medical Devices Regulations 2002/618 (“UK MDR 2002”) also need to be complied with in Great Britain. Medical device manufacturers who have CE marked devices will be able to continue to place them on the market in the whole of the UK if they have an EU MDR CE certificate, until June 30, 2030, without a change in labeling. Legacy medical devices with an EU MDD CE certificate or an EU Declaration of Conformity may continue to be placed on the UK market as long as they meet the transitional provisions of the EU MDR for Northern Ireland and the UK MDR 2002 for Great Britain. For class III and class IIb implantable devices (subject to some exclusions), these transitional provisions will end on December 31, 2027 in both Great Britain and Northern Ireland and, for all other classes in scope, these transitional provisions will end on June 30, 2028 in Great Britain and December 31, 2028 in Northern Ireland. After that, devices destined for Great Britain will be required to follow the future UK regulatory regime, which is expected to come into force in 2025. Northern Ireland will, however, continue to accept CE marked devices. There are some additional requirements for manufacturers who are based outside the UK such as the requirement to appoint a UK Responsible Person (“UKRP”) to take on certain regulatory responsibilities. To enable devices to be placed on the market in the UK after January 1, 2021 (even for CE marked devices), a UK manufacturer, a UKRP for an overseas manufacturer or an EU Authorised Representative based in Northern Ireland (for the purposes of the Northern Ireland market) must register with the Medicines and Healthcare products Regulatory Agency (“MHRA”). The registering entity will then register each of the devices for which they are responsible for placing on the market in the UK, whether in Great Britain or Northern Ireland, as required by the UM MDR 2002. This may create added expense and challenges as explained below.
Until May 25, 2021, our products bearing a CE mark could be exported from the EEA to Switzerland. However, as of May 26, 2021, the Mutual Recognition Agreement between the EEA and Switzerland has not been updated to include the requirements of EU MDR. Accordingly, legal manufacturers in Switzerland are required to appoint a European Union authorized representative, and manufacturers outside of Switzerland are required to appoint a Swiss authorized representative in compliance with the Swiss Medical Device Ordinance. As a consequence, beginning in June 2021 through May 2022, we have been required to appoint an authorized representative in Switzerland in order to export our CE-marked medical devices to Switzerland. Additionally, the name and address of the Swiss authorized representative must be placed on the packaging. This has created added expenses and challenges.
In addition, incidents of adverse drug reactions, unintended side effects or misuse relating to our products could result in additional regulatory controls or restrictions, or even lead to the regulatory authority requiring us to recall or withdraw the
product from the market. Further, if faced with these incidents of adverse drug reactions, unintended side effects or misuse relating to our products, we may elect to voluntarily implement a recall or market withdrawal of our product. A recall or market withdrawal, whether voluntary or required by a regulatory authority, may involve significant costs to us, potential disruptions in the supply of our products to our customers and reputational harm to our products and business, all of which could harm our ability to market our products and could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Complying with existing government regulation of dietary supplements, including our eye vitamins and mineral supplements, in the U.S., Canada and elsewhere could increase our costs significantly and adversely affect our financial results.
The manufacturing, formulation, packaging, labeling and advertising of the Company’s dietary supplement products are also subject to regulation by certain federal, state and foreign agencies, including the FDA, the Federal Trade Commission (the “FTC”), and the Consumer Product Safety Commission, in the U.S., and by Health Canada in Canada. The FDA has authority in the U.S. over the adulteration or misbranding of dietary supplements. There are requirements relating to ingredient safety, new dietary ingredient notifications, labeling, claims notifications, and adverse event reporting among other requirements. While we believe our products comply with those requirements, the FDA may challenge positions we have taken with respect to the formulation or labeling of a dietary supplement product or the claims we make with respect to such products. We are also subject to risks relating to evolving regulations of dietary supplement products, including our eye vitamins and mineral supplements, as the FDA and other applicable agencies have in the past and may in the future consider additional or more stringent regulations of dietary supplements and other products. Such developments could require reformulation of certain of our products to meet new standards, additional record-keeping obligations, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, adverse event reporting or similar obligations, amended or different promotional claims and materials, or could result in recalls or the discontinuance of certain of our products that are not able to be reformulated. Any such developments could increase our costs significantly. In addition, the FDA also has comprehensive regulations for cGMP for those who manufacture, package or hold dietary supplement products. These regulations focus on practices that ensure the identity, purity, quality, strength and composition of dietary supplements manufacture. We or our contract manufacturers may not be able to comply with such regulations without incurring additional expenses, which could be significant.
Manufacturing and Supply Risks
If we or our third-party manufacturers are unable to manufacture our products or the manufacturing process is interrupted due to failure to comply with regulations or for other reasons, the interruption of the manufacture of our products could adversely affect our business. Other manufacturing and supply difficulties or delays may also have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Our manufacturing facilities and those of our contract manufacturers must be inspected and found to be in full compliance with cGMP, quality system management requirements or similar standards before approval for marketing. Compliance with cGMP regulations requires the dedication of substantial resources and requires significant expenditures. In addition, while we attempt to build in certain contractual obligations on our third party manufacturers, we may not be able to ensure that such third-parties comply with these obligations. Our failure or that of our contract manufacturers to comply with cGMP regulations, quality system management requirements or similar regulations outside of the United States, or compliance with environmental laws or regulations, could result in enforcement action by the FDA or its foreign counterparts, or other regulatory bodies, including, but not limited to, warning letters, fines, injunctions, civil or criminal penalties, recall or seizure of products, total or partial suspension of production or importation, suspension or withdrawal of regulatory approval for approved or in-market products, refusal of the government to renew marketing applications or approve pending applications or supplements, refusal of certificates for export to foreign jurisdictions, suspension of ongoing clinical trials, imposition of new manufacturing requirements, closure of facilities and criminal prosecution. These enforcement actions could lead to a delay or suspension in production, which could have a material adverse effect on our competitive position, business, financial condition, results of operations and cash flows and could cause the market value of our common shares and/or debt securities to decline.
In addition, our manufacturing and other processes use complicated and sophisticated equipment, which sometimes requires a significant amount of time to obtain and install. Manufacturing complexity, testing requirements and safety and security processes combine to increase the overall difficulty of manufacturing these products and resolving manufacturing problems that we may encounter. Although we endeavor to properly maintain our equipment (and require our contract manufacturers to properly maintain their equipment), including through on-site quality control and experienced manufacturing supervision, periodic upgrades and have key spare parts on hand, our business could suffer if certain manufacturing or other equipment, or all or a portion of our or their facilities, were to become inoperable for a period of time. We could experience substantial production delays or inventory shortages in the event of any such occurrence until we or
they repair such equipment or facility or we or they build or locate replacement equipment or a replacement facility, as applicable, and seek to obtain necessary regulatory approvals for such replacement. Any interruption in our manufacture of products could adversely affect the sales of our current products or introduction of new products and could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
The supply of our products to our customers (or, in some cases, supply from our contract manufacturers to us) is subject to and dependent upon the availability and cost of transportation services. Disruption of our or our contract manufacturer's manufacturing operations or such transportation services (including as a result of weather conditions or other natural disasters) could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline. In addition, any prolonged disruption in the operations of our existing manufacturing or distribution facilities, whether due to technical, labor or other difficulties, weather conditions, equipment malfunction, contamination, failure to follow specific protocols and procedures, destruction of or damage to any facility or other reasons, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We have been experiencing certain supply chain challenges which have caused disruptions in availability and delays in shipping, which has led to challenges in meeting end market demand, primarily within our contact lens and surgical businesses. These supply-chain challenges have impacted our revenues and resulting margins, despite our effort to manage these impacts through strategic pricing actions and other initiatives. We are unable to predict the duration and extent of these challenges and they could have an adverse impact on results of operations and could cause the market value of our common shares and/or debt securities to decline.
For some of our finished products and raw materials, we obtain supply from one or a limited number of sources. If we are unable to obtain components or raw materials, or products supplied by third parties, our ability to manufacture and deliver our products to the market would be impeded, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Some components and raw materials used in our manufactured products and some finished products sold by us, are currently available only from one or a limited number of domestic or foreign suppliers. For example, with respect to some of our largest or most significant products, the supply of the finished product for each of our Lumify®, Vyzulta®, SofLens®, MIEBO®, XIIDRA® and PureVision® products are only available from a single source, the supply of the active pharmaceutical ingredients (“API”) or other components for our Vyzulta®, MIEBO® and PreserVision® products are also only available from a single source and certain of our Biotrue®, Softlens® and Ultra® contact lens products are also only available from a single source. In the event an existing supplier fails to supply product on a timely basis and/or in the requested amount, supplies product that fails to meet regulatory requirements, becomes unavailable through business interruption or financial insolvency or loses its regulatory status as an approved source or we are unable to renew current supply agreements when such agreements expire and we do not have a second supplier, we may be unable to obtain the required components, raw materials or products on a timely basis or at commercially reasonable prices. We attempt to mitigate these risks by maintaining safety stock of these products, but such safety stock may not be sufficient. In addition, in some cases, only a single source of active pharmaceutical ingredient is identified in filings with regulatory agencies, including the FDA, and cannot be changed without prior regulatory approval, which would involve time and expense to us. A prolonged interruption in the supply of a single-sourced raw material, including the API, or single-sourced finished product could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline. In addition, these third-party manufacturers may have the ability to increase the supply price payable by us for the manufacture and supply of our products, in some cases without our consent.
As a result, our dependence upon others to manufacture and supply our products may adversely affect our profit margins and our ability to obtain approval for and produce our products on a timely and competitive basis, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Changes in inventory levels or fluctuations in buying patterns by our large distributor and retail customers may adversely affect our sales and earnings and add to sales variability from quarter to quarter.
We balance the need to maintain inventory levels that are sufficient to ensure competitive lead times against the risk of inventory obsolescence because of changing customer requirements, fluctuating commodity prices, changes to our products, product transfers or the life-cycle of our products. In order to successfully manage our inventories, we must estimate demand from our customers and produce products that substantially correspond to that demand. If we fail to adequately forecast demand for any new or existing product, or fail to determine the appropriate product mix for production purposes, we may
face production capacity issues in manufacturing sufficient quantities of a given product. In addition, failures in our information technology systems or human error could also lead to inadequate forecasting of our overall demand or product mix.
We have a significant number of unique products and we anticipate that number will continue to grow over time. As a result, the demand forecasting precision required for us to avoid production capacity issues will also increase, which could increase the risk of product unavailability and lost sales. Additionally, an increasing number of unique products could increase global inventory requirements, negatively impacting our working capital performance and leading to write-offs due to obsolescence and expired products.
Due to the lead times necessary to obtain and install new equipment and ramp up production of product lines, if we fail to adequately forecast the need for additional manufacturing capacity, whether for new or existing products, we may be unable to scale production in a timely manner to meet demand for our products. In addition, the technically complex manufacturing processes required to manufacture many of our products increase the risk of production failures and can increase the cost of producing our goods. As a result, because the production process for many of our products is complex and sensitive, the cost of production and the chance of production failures and lengthy supply interruptions is increased, which can have a substantial impact on our inventory levels.
Finally, a significant portion of our products are sold to major health care distributors and major retail chains in Canada, the United States and abroad. Consequently, our sales and quarterly growth comparisons, as well as our estimates for required inventory levels, may be affected by fluctuations in the buying patterns of major distributors, retail chains and other trade buyers. These fluctuations may result from seasonality, pricing, large retailers’ and distributors’ buying decisions or other factors. If we overestimate demand and produce too much of a particular product, we face a risk of inventory obsolescence, leaving us with inventory that we cannot sell profitably or at all. In addition, we may have to write down such inventory if we are unable to sell it for its recorded value. Conversely, if we underestimate demand and produce insufficient quantities of a product, we could be forced to produce that product at a higher price and forego profitability in order to meet customer demand. For example, if a competitor initiates a recall and there is an unexpected increase in the demand for our products, we may not be able to meet such increased demand. Insufficient inventory levels may lead to shortages that result in loss of sales opportunities altogether as potential end-customers turn to competitors’ products that are readily available. If any of these situations occur frequently or in large volumes or if we are unable to effectively manage our inventory and that of our distribution partners, this could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Commercialization Risks
Our approved products may not achieve or maintain expected levels of market acceptance.
Even if we are able to obtain and maintain regulatory approvals for our pharmaceutical and medical device products, generic or branded, the success of these products is dependent upon achieving and maintaining market acceptance. Launching and commercializing products is time consuming, expensive and unpredictable. The commercial launch of a product takes significant time, resources, personnel and expertise, which we may not have in sufficient levels to achieve success, and is subject to various market conditions, some of which may be beyond our control. There can be no assurance that we will be able to, either by ourselves or in collaboration with our partners or through our licensees or distributors, successfully launch and commercialize new products or gain market acceptance for such products. New product candidates that appear promising in development may fail to reach the market or may have only limited or no commercial success. While we have been successful in launching some of our products, we may not achieve the same level of success with respect to all of our new products, and we may face additional challenges associated with operating as an independent company following the completion of the Separation. Our inability to successfully launch our new products may negatively impact the commercial success of such products, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline. Our inability to successfully launch our new products could also lead to material impairment charges.
Levels of market acceptance for our new products could be impacted by several factors, some of which are not within our control, including but not limited to the following:
•safety, efficacy, convenience and cost-effectiveness of our products compared to the products of our competitors;
•scope of approved uses and marketing approval;
•availability of patent or regulatory exclusivity;
•timing of market approvals and market entry;
•ongoing regulatory obligations following approval, such as the requirement to conduct Risk Evaluation and Mitigation Strategy (“REMS”) programs;
•any restrictions or “black box” warnings required on the labeling of such products;
•availability of alternative products from our competitors;
•acceptance of the price of our products;
•effectiveness of our sales forces and promotional efforts;
•the level of reimbursement of our products;
•acceptance of our products on government and private formularies;
•ability to market our products effectively at the retail level or in the appropriate setting of care; and
•the reputation of our products.
Further, the market perception and reputation of our products and their safety and efficacy are important to our business and the continued acceptance of our products. Any negative publicity about our products, such as the discovery of safety issues with our products, adverse events involving our products, or even public rumors about such events, could have a material adverse effect on our business, financial condition, cash flows or results of operation or could cause the market value of our common shares and/or debt securities to decline. In addition, the discovery of significant problems with a product similar to one of our products that implicate (or are perceived to implicate) an entire class of products or the withdrawal or recall of such similar products could have a material adverse effect on sales of our products. Accordingly, new data about our products, or products similar to our products, could cause us reputational harm and could negatively impact demand for our products due to real or perceived side effects or uncertainty regarding safety or efficacy and, in some cases, could result in product withdrawal.
If our products fail to gain, or lose, market acceptance, our revenues would be adversely impacted and we may be required to record material impairment charges, all of which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
For certain of our products, we depend on reimbursement from governmental and other third-party payors and a reduction in reimbursement could reduce our product sales and revenue. In addition, failure to be included in formularies developed by managed care organizations and coverage by other organizations may negatively impact the utilization of our products, which could harm our market share and could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Sales of certain of our products are dependent, in part, on the availability and extent of reimbursement from government health administration authorities, private health insurers, pharmacy benefit managers and other organizations of the costs of our products and the continued reimbursement and coverage of our products in such programs. Changes in government regulations or private third-party payors’ reimbursement policies may reduce reimbursement for our products. In addition, such third-party payors may otherwise make the decision to reduce reimbursement of some or all our products or fail to cover some or all our products in such programs or assert that reimbursements were not in accordance with applicable requirements. For example, these decisions may be based on the price of our products or our current or former pricing practices and decisions. Any reduction or elimination of such reimbursement or coverage could result in a negative impact on the utilization of our products and, as a result, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Managed care organizations and other third-party payors try to negotiate the pricing of medical services and products to control their costs. Managed care organizations and pharmacy benefit managers typically develop formularies to reduce their cost for medications. Formularies can be based on the prices and therapeutic benefits of the available products. Due to their lower costs, generic products are often favored. The breadth of the products covered by formularies varies considerably from one managed care organization to another, and many formularies include alternative and competitive products for treatment of particular medical conditions. Failure to be included in such formularies or to achieve favorable formulary status may negatively impact the utilization and market share of our products. If our products are not included within an adequate number of formularies or adequate reimbursement levels are not provided, or if those policies increasingly favor generic products, this could have a material adverse effect on our business, financial condition, cash flows and results of operations or result in additional pricing pressure on our products and could cause the market value of our common shares and/or debt securities to decline.
Catastrophic events may disrupt our business.
We have operations and facilities which manufacture, sell and/or distribute our products in many parts of the world. Natural events (such as a hurricane or major earthquake), terrorist attack, pandemics, epidemics, outbreaks of an infectious disease or similar events or other catastrophic events, including adverse weather events associated with global climate change which have increased in severity and frequency in recent years, could cause delays in developing, manufacturing or selling our products. Such events that occur in major markets where we sell our products could reduce the demand for our products in those areas and, as a result, impact our sales into those markets. In either case, any such disruption could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Employment-related Risks
The loss of the services of, or our inability to recruit, retain, motivate, our executives and other key employees could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We must retain and motivate our executives and other key employees and recruit other executives and employees in order to strengthen our management team and workforce. Our ability to retain or recruit executive and other key employees may be hindered or delayed by, among other things, competition from other employers who may be able to offer more attractive compensation packages. We have a limited history of operating as an independent company and do not have the same resources we had as a part of BHC and, as a result, we may experience additional challenges retaining and motivating our key personnel. A failure by us to retain, motivate and recruit executives and other key employees or the unanticipated loss of the services of any of these executives or key employees for any reason, whether temporary or permanent, could create disruptions in our business, could cause concerns and instability for management and employees, current and potential customers, credit rating agencies and other third parties with whom we do business and our securityholders and could cause concern regarding our ability to execute our business strategy or to manage operations in the manner previously conducted and, as a result, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
In addition, we may experience challenges in building and retaining our workforce in certain markets, where pressure from inflation and competition have exacerbated turnover and retention trends continuing from the COVID-19 pandemic. Labor shortages and competition for qualified personnel could cause disruptions in our business operations.
Furthermore, as a result of any failure to retain, or loss of, any executives or key employees, we may experience increased costs in order to identify and recruit a suitable replacement in a timely manner (and, even if we are able to hire a qualified successor, the search process and transition period may be difficult to manage and result in additional periods of uncertainty), which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline. In addition, once identified and recruited, the transition of new executives and key employees may be difficult to manage and we cannot guarantee that new executives and employees will efficiently transition into their roles or ultimately be successful in their roles. Finally, as a result of changes in our executives and key employees, there may be changes in the way we conduct our business, as well as changes to our business strategy. We cannot predict what these changes may involve or the timing of any such changes and how they will impact our product sales, revenue, business, financial condition, cash flows or results of operation, but any such changes could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We have recently appointed a new chairman and chief executive officer, as well as a number of other new executive officers and other key employees, and our inability to successfully manage the transition to our new executive officers and other key employees or other operational disruptions resulting from such transitions could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Effective March 6, 2023, we appointed Brent Saunders to become our new Chairman of the Board of Directors and Chief Executive Officer. Subsequent to that appointment, we also appointed or hired a number of other new executive officers and key employees, including a new Chief Legal Officer, a new Chief Human Resources Officer, a new Chief Supply Chain and Operations Officer and a new President of our Global Pharmaceuticals and International Consumer businesses. The transition of these new executive officers and other new key employees may be difficult to manage and we cannot guarantee that all such individuals will efficiently transition into their respective roles or ultimately be successful in such roles. As a result of any such inadequate transitions, we may experience operational disruptions and there may be additional uncertainty, instability and concerns for management, employees, current and potential customers, other third parties with whom we do business, credit rating agencies and our shareholders regarding our ability to continue to execute our business
strategy and manage operations in the manner previously conducted, and we may also experience difficulties in executing our business strategies and goals. Furthermore, our new chairman and chief executive officer and other executive officers have implemented and may continue to implement changes to our business strategies, which could create further disruption and uncertainty among management, employees, current and potential customers, other third parties with whom we do business and shareholders. Any of these factors relating to the appointment and transition of these new executive officers and other key employees could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Risks Relating to Our Business and our Business Strategy
We are, and may in the future be, subject to certain limitations or restrictions on pricing increases for certain of our products. These pricing limitations or restrictions could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
For certain of our products, we are, and may in the future be, subject to certain restrictions that limit our ability to increase or make changes to the pricing of those products. These restrictions or limitations are or may be imposed contractually (such as through our contracts with group purchasing organizations or others), through legislation (such as the new Inflation Reduction Act, which, among other things, requires manufacturers to pay rebates to Medicare if prices increase faster than inflation for products used by Medicare beneficiaries) or through decisions or commitments we decide to make ourselves (such as through the pricing committees we have established or may establish for certain of our businesses).
At this time, we cannot predict what pricing changes we will make (or be required to make) nor can we predict what other changes in our business practices we may implement with respect to pricing. We also cannot predict the impact such pricing decisions or changes will or would have on our business. However, any such changes could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
For example, any pricing changes and programs could affect the average realized prices for our products and may have a significant impact on our revenue trends. In addition, limiting or eliminating price increases on certain of our products will result in fewer or lower price appreciation credits from certain of our wholesalers. Price appreciation credits are generated when we increase a product’s wholesaler acquisition cost (“WAC”) under our contracts with certain wholesalers. Under such contracts, we are entitled to credits from such wholesalers for the impact of that WAC increase on inventory currently on hand at the wholesalers. In wholesaler contracts, such credits, which can be significant, are offset against the total distribution service fees we pay on all of our products to each wholesaler. As a result, to the extent we decide to cease or limit price increases, we will have fewer or lower price appreciation credits to use to offset against our distribution fees owing to these wholesalers. In addition, under certain of our agreements with our wholesaler customers, we have price protection or price depreciation provisions, pursuant to which we have agreed to adjust the value of any on-hand or in-transit inventory with such customers in the event we reduce the price of any of our products. As a result, to the extent we reduce the WAC price for any of our products, we may owe a payment to such customers (or such customers may earn a credit to be offset against any amounts owing to us) equal to the amount of such inventory multiplied by the difference between the price at which they acquired the product inventory and the new reduced price.
If we fail to maintain our relationships with, and provide appropriate training in our products to, health care providers, including physicians, eyecare professionals, hospitals, large drug store chains, wholesale distributors, pharmacies, government entities and group purchasing organizations, customers may not buy certain of our products and our sales and profitability may decline.
We market our products to physicians, hospitals, pharmacies and wholesalers through our own sales force and sell through wholesalers. In some markets, we additionally sell directly to physicians, hospitals and large drug store chains and we sell through distributors in countries where we do not have our own sales staff. We have developed and strive to maintain strong relationships with members of each of these groups who assist in product research and development and advise us on how to satisfy the full range of consumer needs. We rely on these groups to educate their patients and other members of their organizations regarding our products. Consumers in the pharmaceutical industry, particularly the contact lens and lens care customers in the eye health industry, have a tendency not to switch products regularly and are repeat consumers. Our ability to maintain strong relationships is essential to our future performance.
The success of certain of our products, particularly our vision care and consumer health care products, is impacted by a physician’s initial recommendation of such products and a consumer’s initial choice to use such products. As a result, the failure of certain of our products, particularly in our vision care business, to retain the support of pharmaceutical professionals, hospitals or group purchasing organizations and to retain the support of the end-users and the distributors and retailers to whom we sell such products, could have a material adverse effect on our sales and profitability.
We have entered into distribution agreements with other companies to distribute certain of our products at supply prices based on net sales. Declines in the pricing and/or volume, over which we have no or limited control, of such products, and therefore the amounts paid to us, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Certain of our products are the subject of third-party distribution or sublicense agreements, pursuant to which we may manufacture and sell products to other companies, which distribute such products in return for a royalty or a supply price, in both cases which are often based on net sales. Our ability to control pricing and volume of these products may be limited and, in some cases, these companies make all distribution and pricing decisions independently of us. If the pricing or volume of such products declines, our revenues would be adversely impacted which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Our policies regarding returns, allowances and chargebacks, and marketing programs adopted by wholesalers, may reduce our revenues in future fiscal periods.
We provide certain rebates, allowances, chargebacks and other credits to our customers with respect to certain of our products. For example, we make payments or give credits to certain wholesalers for the difference between the invoice price paid to us by our wholesaler customer for a particular product and the negotiated price that such wholesaler sells such products to its hospitals, group purchasing organizations, pharmacies or other retail customers. We also give certain of our customers credits on our products that such customers hold in inventory after we have decreased the WAC prices of such products, such credit being for the difference between the old and new price. In addition, we also implement and maintain returns policies, pursuant to which our customers may return product to us in certain circumstances in return for a credit. Although we establish reserves based on our prior experience, wholesaler data, then-current on-hand inventory, our best estimates of the impact that these policies may have in subsequent periods and certain other considerations, we cannot ensure that our reserves are adequate or that actual product returns, rebates, allowances and chargebacks will not exceed our estimates, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We may experience declines in sales volumes or prices of certain of our products as the result of the concentration of sales to wholesalers and the continuing trend towards consolidation of such wholesalers and other customer groups and this could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
For certain of our products, a significant portion of our sales are to a relatively small number of customers. If our relationship with one or more of such customers is disrupted or changes adversely or if one or more of such customers experience financial difficulty or other material adverse changes in their businesses, it could materially and adversely affect our sales and financial results, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
In addition, wholesalers and retail drug chains have undergone, and are continuing to undergo, significant consolidation. This consolidation may result in these groups gaining additional purchasing leverage and consequently increasing the product pricing pressures facing our business. The result of these developments could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We have recently completed a number of acquisition and in-licensing transactions and may, in the future, seek to identify and acquire certain other assets, products and businesses. We may experience difficulties in integrating any acquired assets, products and businesses and we may fail to realize the anticipated benefits of any such acquisitions.
We have recently completed a number of acquisitions and in-licensing transactions, including our recent acquisition of XIIDRA® (lifitegrast ophthalmic solution) and certain other ophthalmology assets from Novartis Pharma AG and Novartis Finance Corporation (the “XIIDRA Acquisition”). We may in the future seek to identify and acquire complementary businesses, products, technologies or other assets to augment our pipeline. Such transactions may be complex, time consuming and expensive. There can be no guarantee that we will be able to successfully consummate acquisitions or other arrangements, which could result in significant diversion of management and other employee time, as well as substantial out-of-pocket costs. If such transactions are not completed for any reason, we may incur significant costs and the market price of our common shares and/or debt securities may decline.
In addition, even if an acquisition is consummated, the integration of the acquired business, product or other assets into our Company may be complex and time-consuming, and we may not achieve the anticipated benefits, cost-savings or growth opportunities we expect. Potential difficulties that may be encountered in the integration process include the
following: integrating personnel (such as the XIIDRA salesforce brought on as part of the XIIDRA Acquisition), operations and systems, while maintaining focus on selling and promoting existing and newly-acquired products; coordinating geographically dispersed organizations; distracting management and employees from operations; retaining existing customers and attracting new customers; maintaining the business relationships the acquired company has established, including with health care providers; and managing inefficiencies associated with integrating the operations of the Company and the acquired business, product or other assets. In addition, delays encountered in the integration process could result in a failure to realize the anticipated benefits on the anticipated timeline, or at all.
Finally, these acquisitions and other arrangements, even if successfully integrated, may fail to further our business strategy as anticipated or to achieve anticipated benefits and success, expose us to increased competition or challenges with respect to our products or geographic markets, and expose us to additional liabilities associated with an acquired business, product, technology or other asset or arrangement. Any one of these challenges or risks could impair our ability to realize any benefit from our acquisition or arrangement after we have expended resources on them.
With respect to the XIIDRA Acquisition, in addition to the integration challenges we face, the anticipated benefits we expect from this acquisition are subject to numerous assumptions, including assumptions derived from our diligence efforts concerning the status of and prospects for the XIIDRA® business and the pipeline assets. We cannot provide any assurances with respect to the accuracy of our assumptions, including our assumptions with respect to future revenues of the XIIDRA® products or assumptions regarding our ability to successfully develop and obtain regulatory approval for the acquired pipeline assets. There are a variety of risks and uncertainties, some of which are outside of our control, which could cause actual results to differ materially from these anticipated benefits. As a result, there can be no assurance that we will realize the anticipated benefits from the XIIDRA Acquisition in the anticipated timelines, or at all.
In addition, as described above, we may expend significant expenses in connection with the consummation of these transactions and the integration of the acquired business with our business. These expenses may include, but are not limited to, fees paid to legal, financial and accounting advisors, filing fees and fees associated with any financing required in connection with the funding for such transactions. Many of these expenses must be paid regardless of whether the transaction is consummated. Additional unanticipated costs may be incurred in the integration of the acquired business with our business. In addition, as was the case with the XIIDRA Acquisition, we may also incur additional indebtedness to finance the transaction, which indebtedness may be material and may limit our operating or financial flexibility relative to our then current position.
We have various indemnity agreements and indemnity arrangements in place, which may result in an obligation to indemnify or reimburse the relevant counterparty, which amounts may be material.
We have entered into customary indemnification agreements with our directors and certain of our officers. We have also obtained directors’ and officers’ liability insurance to mitigate the cost of any potential future lawsuits or actions. The maximum amount of any potential future payment cannot be reasonably estimated but could have a material adverse effect on the Company.
In the normal course of business, we have entered or may enter into agreements that include indemnities in favor of third parties, such as purchase and sale agreements, license agreements, engagement letters with advisors and consultants and various product and service agreements. These indemnification arrangements may require us to compensate counterparties for losses incurred by the counterparties as a result of breaches in representations, covenants and warranties provided by us or as a result of litigation or other third-party claims or statutory sanctions that may be suffered by the counterparties as a consequence of the relevant transaction. In some instances, the terms of these indemnities are not explicitly defined. We, whenever possible, try to limit this potential liability within the particular agreement or contract (such as through maximum claim amounts, specified claim periods and other conditions and limits), but due to the unpredictability of future events the maximum amount of any potential reimbursement cannot be reasonably estimated, but could have a material adverse effect on the Company.
Our ability to effectively monitor and respond to the rapid and ongoing developments and expectations relating to environmental, social and governance (“ESG”) matters, including related social expectations and concerns, may impose unexpected costs or result in reputational or other harm that could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
There are rapid and ongoing developments and changing expectations relating to ESG matters and factors such as the impact of our operations on climate change, water and waste management, our practices relating to sustainability and product stewardship, product safety, access to health care and affordable drugs, management of business ethics and human capital development, which may result in increased regulatory, social or other scrutiny on us. If we are unable to adequately recognize and respond to such developments and governmental, societal, investor and consumer expectations relating to such
ESG matters, we may miss corporate opportunities, become subject to additional scrutiny, incur unexpected costs or experience damage to our reputation or our various brands. If any of these events were to occur, there may be a material adverse effect on our business, financial condition, cash flows and results of operations and the market value of our common shares and/or debt securities may decline.
Debt-Related Risks
Our indebtedness could adversely affect our business and our ability to meet our obligations.
As of December 31, 2023, we had $3,236 million and $1,400 million in outstanding aggregate principal amount of issued variable rate and fixed rate debt, respectively. Our variable rate debt exposes us to interest rate risk. When interest rates increase, our debt service obligations on the variable rate indebtedness increase even though the amount borrowed remains the same.
Our credit facilities and our indenture governing the October 2028 Secured Notes contain customary affirmative and negative covenants and specified events of default. These affirmative and negative covenants include, among other things, and subject to certain qualifications and exceptions, covenants that restrict our ability and the ability of our subsidiaries to: incur or guarantee additional indebtedness; create or permit liens on assets; pay dividends on capital stock or redeem, repurchase or retire capital stock or subordinated indebtedness; make certain investments and other restricted payments; engage in mergers, acquisitions, consolidations and amalgamations; transfer and sell certain assets; and engage in transactions with affiliates. The Revolving Credit Facility also contains financial covenants that: (1) prior to the IG Trigger (as defined herein), require us to, if, as of the last day of any fiscal quarter (commencing with the fiscal quarter ending December 31, 2022), loans under the Revolving Credit Facility and swingline loans are outstanding in an aggregate amount greater than 40% of the total commitments in respect of the Revolving Credit Facility at such time, maintain a maximum first lien net leverage ratio of not greater than 4.50:1.00 and (2) after the IG Trigger, require us to, as of the last day of each fiscal quarter ending after the IG Trigger, (a) maintain a total leverage ratio of not greater than 4.00:1.00 (provided that such ratio will increase to 4.50:1.00 in connection with certain acquisitions for the four fiscal quarter period commencing with the quarter in which such acquisition is consummated) and (b) maintain an interest coverage ratio of not less than 3.00:1.00. The financial covenant in effect prior to the IG Trigger may be waived or amended without the consent of the term loan facility lenders and contains a customary term loan facility standstill and customary cure rights. The indenture governing the October 2028 Secured Notes also contains negative covenants and events of default that are similar to those contained in the Credit Facilities (as defined herein).
Such financial or other covenants limit our operational flexibility in a number of other ways, including:
•causing us to be less able to take advantage of business opportunities, such as making certain investments and other restricted payments and engaging in mergers, acquisitions consolidations and amalgamations, and to react to changes in market or industry conditions;
•increasing our vulnerability to adverse economic, industry, or competitive developments;
•affecting our ability to pay or refinance debts as they become due during adverse economic, financial market, and industry conditions;
•requiring us to use a portion of cash flow for debt service, reducing funds available for other purposes;
•decreasing our profitability and/or cash flow;
•causing us to be disadvantaged compared to competitors with less leverage; and
•limiting our ability to borrow additional funds in the future to fund working capital, capital expenditures, and other general corporate purposes.
Further, our credit facilities have Secured Overnight Financing Rate (“SOFR”)-based interest rates. SOFR is a relatively new reference rate, has a very limited history and is based on short-term repurchase agreements, backed by Treasury securities. Changes in SOFR can be volatile and difficult to predict. As a result, the amount of interest we may pay on our credit facilities is difficult to predict.
Risks Relating to the International Scope of our Business
Our business, financial condition, cash flows and results of operations are subject to risks arising from the international scope of our operations.
We conduct a significant portion of our business outside the United States and Canada and may, in the future, expand our operations into new countries, including emerging markets. We sell our products in many countries around the world. All of our foreign operations are subject to risks inherent in conducting business abroad, including, among other things:
•difficulties in coordinating and managing foreign operations, including ensuring that foreign operations comply with foreign laws as well as Canadian and U.S. laws applicable to Canadian companies with U.S. and foreign operations, such as export and sanctions laws and the FCPA, the Canadian Corruption of Foreign Public Officials Act and other applicable worldwide anti-bribery laws;
•price and currency exchange controls;
•restrictions on the repatriation of funds;
•scarcity of hard currency, including the U.S. dollar, which may require a transfer or loan of funds to the operations in such countries, which they may not be able to repay on a timely basis;
•political and economic instability;
•ongoing uncertainties as a result of instability or changes in geopolitical conditions, including military or political conflicts, such as those caused by the ongoing conflicts between Russia and Ukraine or Israel and Hamas (the potential escalation or geographic expansion of which could heighten other risks identified elsewhere in this “Risk Factors” section);
•compliance with multiple regulatory regimes;
•compliance with economic sanctions laws and other laws that apply to our activities in the countries where we operate;
•less established legal and regulatory regimes in certain jurisdictions, including as relates to enforcement of anti-bribery and anti-corruption laws and the reliability of the judicial systems;
•differing degrees of protection for intellectual property;
•unexpected changes in foreign regulatory requirements, including quality standards and other certification requirements;
•new export license requirements;
•adverse changes in tariff and trade protection measures;
•differing labor regulations;
•potentially negative consequences from changes in or interpretations of tax laws;
•restrictive governmental actions;
•possible nationalization or expropriation;
•credit market uncertainty;
•restrictions on business activities and other challenges associated with pandemics, including the lingering COVID-19 pandemic, epidemics, outbreaks of an infectious disease or similar events;
•differing local practices, customs and cultures, some of which may not align or comply with our Company practices and policies or U.S. laws and regulations;
•difficulties with licensees, contract counterparties, or other commercial partners; and
•differing local product preferences and product requirements.
As a result of changes to U.S. policy, there may be changes to existing trade agreements and greater restrictions on trade generally. In addition, support for protectionism and rising anti-globalization sentiment in the United States and other countries may slow global growth. In particular, a protracted and wide-ranging trade conflict between the United States and China could adversely affect global economic growth. Concerns also remain around the social, political and economic impacts of the changing political landscape in Europe and elsewhere. In addition, there are growing concerns over an economic slowdown in emerging markets in light of capital outflows in favor of developed markets and expected interest rate increases. Broader geopolitical tensions remain high among the United States, Russia, China and across the Middle East.
Given the international scope of our operations, any of the above factors, including sanctions, export controls, tariffs, trade wars and other governmental actions, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Similarly, adverse economic conditions impacting our customers in these countries or uncertainty about global economic conditions could cause purchases of our products to decline, which would adversely affect our revenues and operating results. Moreover, our projected revenues and operating results are based on assumptions concerning certain levels of customer spending. Any failure to attain our projected revenues and operating results as a result of adverse economic or market conditions could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Due to the large portion of our business conducted in currency other than U.S. dollars, we have significant foreign currency risk.
We face foreign currency exposure on the translation into U.S. dollars of the financial results of our operations in numerous jurisdictions, including Europe, Canada, Latin America and Asia. Where possible, we manage foreign currency risk by managing same currency revenue in relation to same currency expenses. We may also use derivative financial instruments from time to time to mitigate our foreign currency risk and not for trading or speculative purposes. We face foreign currency exposure in those countries where we have revenue denominated in the local foreign currency and expenses denominated in other currencies. Both favorable and unfavorable foreign currency impacts to our foreign currency-denominated operating expenses are mitigated to a certain extent by the natural, opposite impact on our foreign currency-denominated revenue. In addition, the repurchase of our U.S. dollar denominated debt may result in foreign exchange gains or losses for Canadian income tax purposes. One half of any foreign exchange gains or losses will be included in our Canadian taxable income. Any foreign exchange gain will result in a corresponding reduction in our available Canadian tax attributes. The strengthening of the U.S. dollar in 2022 has adversely impacted our results of operations. The dollar has also strengthened to date in 2023 and may continue to adversely impact our results of operations.
As a result of the current conflict between Russia and Ukraine, the current and any future responses by the global community to such conflict and any counter responses by the Russian government or other entities or individuals, and the potential expansion of the conflict to other countries, we have experienced and may continue to experience an adverse impact on our business and operations in this region, as well as on our business and operations generally, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
On February 24, 2022, Russia launched a military invasion of Ukraine. The ongoing military conflict between Ukraine and Russia has provoked strong reactions from the United States, the UK, the EU, Canada and various other countries around the world, including the imposition of export controls and broad financial and economic sanctions against Russia, Belarus and specific areas of Ukraine. In retaliation against new international sanctions and as part of measures to stabilize and support the volatile Russian financial and currency markets, the Russian authorities also imposed significant currency control measures aimed at restricting the outflow of foreign currency and capital from Russia, imposed various restrictions on transacting with non-Russian parties, banned exports of various products and imposed other economic and financial restrictions. Additional sanctions or other measures may be imposed by the global community, and counteractive measures may be taken by the Russian government, other entities in Russia or governments or other entities outside of Russia.
In 2023, we derived approximately 3% of our revenues from sales of our products in Russia, Ukraine and Belarus. As of the date of this Form 10-K, the conflict between Ukraine and Russia has continued to impact our business in the region, and we are continuously monitoring developments to assess any potential future impact that may arise. Given the nature of our products, we do not believe that the current sanctions and other measures imposed by the United States and other countries preclude us from conducting business in the region. However, we anticipate that the ongoing conflict in this region and the sanctions and other actions by the global community in response may continue to hinder our ability to conduct business with customers and vendors in this region. For example, we have experienced and may in the future experience disruption and delays in the supply of our products to our customers in Russia, Belarus and Ukraine. We have experienced and may in the future also experience decreased demand for our products in these countries as a result of the conflict and invasion. In addition, we may experience difficulties in collecting receivables from such customers. If we are hampered in our ability to conduct business with new or existing customers and vendors in this region, our business, and operations, including our revenues, profitability and cash flows, could be adversely impacted. Furthermore, if the sanctions and other retaliatory measures imposed by the global community change, we may be required to cease or suspend our operations in the region or, should the conflict worsen, we may voluntarily elect to do so. We cannot provide assurance that current sanctions or potential future changes in these sanctions or other measures will not have a material impact on our operations in Russia, Belarus and Ukraine. The disruption to, or suspension of, our business and operations in Russia, Belarus and Ukraine would adversely impact our business, financial condition, cash flows and results of operations in this region which may, in turn, materially adversely impact our overall business, financial condition, cash flows and results of operations, which impact
could be material, and could cause the market value of our common shares and/or debt securities to decline. Finally, we are also subject to risks if exchange controls were to be imposed that would limit the repatriation of profits from our operations in Russia. While we do not rely on profits or dividends from our Russian operations to fund our debt repayment or other business activities generally, as our operations from Russia primarily involve the sale of products purchased from our affiliates located outside of Russia, any exchange controls that would limit the purchase of or payment for products or goods from outside of Russia may have an adverse impact on our operations in Russia or the way we conduct business in Russia.
The ongoing military conflict and sanctions on the Russian and global economies have resulted in significant volatility in financial markets and depreciation of the Russian ruble and the Ukrainian hryvnia against the U.S. dollar, as well as in an increase in energy and commodity prices globally. As the conflict continues, certain economic and security consequences have been experienced and may continue or worsen, including, but not limited to, supply shortages of different kinds, further increases in prices of commodities, including piped gas, oil and agricultural goods, reduced consumer purchasing power, significant disruptions in logistics infrastructure, telecommunications services and risks relating to the unavailability of information technology systems and infrastructure. The resulting impacts to the global economy, financial markets, inflation, interest rates and unemployment, among others, could adversely impact economic and financial conditions. Other potential consequences include, but are not limited to, growth in the number of popular uprisings in the region, increased political discontent, especially in the regions most affected by the conflict or economic sanctions, increase in cyberterrorism activities and attacks, displacement of persons to regions close to the areas of conflict and an increase in the number of refugees fleeing across Europe, among other unforeseen social and humanitarian effects.
In addition, as a result of the ongoing conflict between Russia and Ukraine, we may experience other risks, difficulties and challenges in the way we conduct our business and operations generally. For example, there may be an increased risk of cybersecurity attacks due to the current conflict between Russia and Ukraine, including cybersecurity attacks perpetrated by Russia or others at its direction in response to economic sanctions and other actions taken against Russia as a result of its invasion of Ukraine. Any increase in such attacks on us or our third- party providers or other systems could adversely affect our network systems or other operations. In order to address the risks associated with cybersecurity attacks from the region (including state-sponsored cybersecurity attacks), we have taken action to consolidate network traffic from Russia and Belarus through a single point, which is designed to allow us to more closely inspect that traffic. In addition, if required, this consolidation provides a single point to quickly and efficiently disconnect the region from our corporate network. At this time, to the best of our knowledge, we do not believe we have experienced any cyberattacks that are related to the conflict between Russia and Ukraine. Although we have taken steps to enhance our protections against such attacks, we may not be able to address these cybersecurity threats proactively or implement adequate preventative measures and there can be no assurance that we will promptly detect and address any such disruption or security breach, if at all. In addition, as a result of the risk of collectability of receivables from our customers in Russia, Belarus and Ukraine, we may be required to adjust our accounting practices relating to revenue recognition in this region, with the result that we may not be able to recognize revenue from these customers until collected. We may also suffer reputational harm as a result of our continued operations in Russia, which may adversely impact our sales and other businesses in other countries. Finally, while we are not currently conducting clinical trials in Russia, Belarus or Ukraine, certain planned trials in Russia and any future trials in this region will need to be postponed and/or relocated; however, we do not anticipate that the impact of this postponement or relocation will have a material impact to any of our development programs or pipeline products.
A further protracted conflict between Ukraine and Russia, any escalation of that conflict, and the financial and economic sanctions and import and/or export controls imposed on Russia by the United States, the UK, the EU, Canada and others, and the above-mentioned adverse effect on our operations (both in this region and generally) and on the wider global economy and market conditions could, in turn, have a material adverse impact on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Risks Relating to Intellectual Property and Exclusivity
The expiration or loss of patent protection or regulatory exclusivity rights for our key products could adversely impact our business. In addition, we have faced competition in the past and expect to face additional competition in the future, including with respect to our products that have patent protection or exclusivity rights. Competitors (including generic and potential biosimilar competitors) of our products could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
The development of new and innovative products, as well as protecting the underlying intellectual property of our product portfolio, is important to our success in all areas of our business. Some of our products either: (i) have no meaningful exclusivity protection via patent or marketing or data exclusivity rights or (ii) are protected by patents or regulatory exclusivity periods that will be expiring in the near future. The expiration or loss of patent protection or regulatory exclusivity rights for our key products could adversely impact our business. In addition, even for our products that have patent protection
or exclusivity rights, we face competition from similar products in the markets in which we participate. As a result, we face significant competition with respect to a substantial majority of our products.
Without patent protection or regulatory exclusivity, competitors (including generics and biosimilars) face fewer barriers in introducing competing products. Upon the expiration or loss of patent protection or regulatory exclusivity for our products or otherwise upon the introduction of generic, biosimilar or other competitors (which may be sold at significantly lower prices than our products), we could lose a significant portion of sales and market share of that product in a very short period and, as a result, our revenues could be lower. In addition, the introduction of generic and biosimilar competitors may have a significant downward pressure on the pricing of our branded products which compete with such generics and biosimilars. Where we have the rights, we may elect to launch an authorized generic of such product (either ourselves or through a third party) prior to, upon or following generic entry, which may mitigate the anticipated decrease in product sales; however, even with the launch of an authorized generic, the decline in product sales of such product would still be expected to be significant and the effect on our future revenues could be material. The introduction of competing products (including generic products and biosimilars) could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We may fail to obtain, maintain, license, enforce or defend the intellectual property rights required to conduct our business, or third parties may allege that we are infringing, misappropriating or otherwise violating their intellectual property rights, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We strive to acquire, maintain and defend patent, trademark and other intellectual property protections over our products and the processes used to manufacture these products. However, we may not be successful in obtaining such protections, or the patent, trademark and intellectual property rights we do obtain may not be sufficient in breadth and scope to fully protect our products or prevent competing products, or such patent, trademark and intellectual property rights may be susceptible to third-party challenges, which could result in the loss of such intellectual property rights or the narrowing of scope of protection afforded by such rights. Our intellectual property rights may also be circumvented by third parties and we may not be able to enforce our intellectual property rights against such third parties. The failure to obtain, maintain, enforce or defend such intellectual property rights, for any reason, could allow third parties to develop, manufacture and sell products that compete with our products or may impact our ability to develop, manufacture and market our own products, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Further, the pharmaceutical and medical device industries historically have generated substantial litigation concerning the manufacture, use and sale of products and we expect this litigation activity to continue. As a result, we expect that patents related to our products will be routinely challenged, and the validity or enforceability of our patents may not be upheld. In order to protect or enforce patent rights, we may initiate litigation against third parties. Our patents may also be challenged in administrative proceedings in the United States Patent and Trademark Office and patent offices outside of the United States. If we are not successful in defending an attack on our patents and maintaining exclusive rights to market one or more of our products still under patent protection, we could lose a significant portion of sales in a very short period. Even in cases where we prevail in an infringement claim, legal remedies available for harm caused to us may not be sufficient to make us whole. We may also become subject to infringement claims by third parties and may have to defend against charges that we infringed, misappropriated or otherwise violated patents or the intellectual property or proprietary rights of third parties. Third parties may also request a preliminary or permanent injunction from a court of law to prevent us from marketing a product. Even if we believe third-party intellectual property claims are without merit, there is no assurance that a court would find in our favor on questions of infringement, validity, enforceability or priority. If we are found to infringe, misappropriate or otherwise violate the intellectual property rights of others, we could lose our right to develop, manufacture or sell products, including our generic products, or could be required to pay monetary damages or royalties to license proprietary rights from third parties, which could be substantial and include treble damages if we are found to willfully infringe intellectual property rights or others. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Any of the foregoing events could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
For certain of our products and manufacturing processes, we rely on trade secrets and other proprietary information, which we seek to protect, in part, through information technology systems discussed in more detail in the following section, and, in part, by confidentiality and nondisclosure agreements with our employees, consultants, advisors and partners. Trade secrets and proprietary information are difficult to protect. We also attempt to enter into agreements whereby such employees, consultants, advisors and partners assign to us the rights in any intellectual property they develop in the course of their engagement with us. These agreements may be breached, and we may not have adequate remedies for any breach. There can be no assurance that these agreements will be self-executing or otherwise provide meaningful protection for our trade secrets or other intellectual property or proprietary information. These agreements may not effectively prevent disclosure or
misappropriation of such information and disputes may still arise with respect to the ownership of intellectual property. In addition, third parties may independently develop the same or similar proprietary information. Further, we have employed and expect to employ individuals who were previously employed at universities or other companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, advisors and partners do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or such persons have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information of their former employers or other third parties, or to claims that we have improperly used or obtained such trade secrets. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights and face increased competition to our business. The unauthorized access to or disclosure of our proprietary information or the loss of such intellectual property rights may impact our ability to develop, manufacture and market our own products or may assist competitors in the development, manufacture and sale of competing products, which could have a material adverse effect on our revenues, financial condition, cash flows or results of operations and could cause the market value of our common shares and/or debt securities to decline.
For a number of our commercialized products and pipeline products, including MIEBO®, XIPERE®, LUMIFY® and VYZULTA®, we rely on licenses to patents and other technologies, know-how and proprietary rights held by third parties. Any loss, expiration, termination or suspension of our rights to such licensed intellectual property could result in our inability to continue to develop, manufacture and market our products or product candidates and, as a result, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline. If these licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, third parties, including our competitors, could have the freedom to seek regulatory approval of, and to market, products identical to ours. Under some license agreements, we may not control the preparation, filing, prosecution or maintenance of the licensed intellectual property, or may not have the first right to enforce the intellectual property. In those cases, we may not be able to adequately influence patent prosecution or enforcement, or prevent inadvertent lapses of coverage due to failure to pay maintenance fees and we cannot be certain that these patents and patent applications will be prepared, filed, prosecuted, maintained, enforced and defended in a manner consistent with the best interests of our business and that does not compromise the patent rights. In the future, we may also need to obtain such licenses from third parties to develop, manufacture, market or continue to develop, manufacture or market our products. If we are unable to timely obtain these licenses on commercially reasonable terms, our ability to develop, manufacture and market our products may be inhibited or prevented, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Intellectual property litigation could cause us to spend substantial resources, distract our personnel from their normal responsibilities and cause the value of our common shares and/or debt securities to decline.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the value of our common shares and/or debt securities. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace, including compromising our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties or enter into development collaborations that would help us commercialize our product candidates, if approved. Any of the foregoing events would harm our business, financial condition, results of operations and prospects and could cause the market value of our common shares and/or debt securities to decline.
Risks Relating to Information Technology
We have become increasingly dependent on information technology systems and infrastructure and any breakdown, interruption, breach or other compromise of our information technology systems or those of our third party service providers could subject us to liability or interrupt the operation of our business, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We are increasingly dependent upon our information technology systems and infrastructure, as well as those of third parties with whom we interact, and internal and public internet sites, data hosting and processing facilities, cloud-based services and hardware, social media sites and mobile technology, in connection with the conduct of our business.
We must constantly update our information technology systems and infrastructure and undertake investments in new information technology systems and infrastructure. However, we cannot provide assurance that the information technology systems and infrastructure on which we depend, including those of third parties, will continue to meet our current and future business needs or adequately safeguard our operations. Furthermore, modification, upgrade or replacement of such systems and infrastructure may be costly or out of our control.
Any failure to so modify, upgrade or replace such systems and infrastructure, any disruptions that occur during the process of such modification, upgrade or replacement and/or any breakdown, interruption or corruption of our information technology systems and infrastructure could create system disruptions, shutdowns, delays in generating or the corruption or loss of data and information or other disruptions that could result in negative financial, operational, business or reputational consequences for us.
The size and complexity of the information technology systems and infrastructure on which we rely makes such systems and infrastructure potentially vulnerable to internal or external inadvertent or intentional security breaches, including as a result of private or state-sponsored cybercrimes, terrorism, war, malware, ransomware, human error, system malfunction, telecommunication and electrical failures, natural disaster, misplaced or lost data, socially engineered breaches or other similar events.
In addition, during the normal course of our business operations, including through the use of information technology systems and infrastructure, we are involved in the collection, processing, transmission, use and retention of sensitive, confidential, non-public or personal data including personal health data and information in Canada, the United States and abroad.
Cyber-attacks are increasing in frequency, sophistication and intensity and are made by groups and individuals with a wide range of motives and expertise. Cyber-attacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, worms, social engineering, improper modification of information, fraudulent “phishing” e-mails and other means to affect service reliability or threaten data confidentiality, integrity or availability. Techniques used in these attacks are often highly sophisticated, change frequently and may be difficult to detect for periods of time.
We have established: (i) physical, electronic and organizational measures to safeguard and secure our systems to prevent a compromise and (ii) policies and procedures designed to provide for the timely investigation of cybersecurity incidents and the timely disclosure of cybersecurity incidents consistent with our legal and contractual obligations. We also rely on commercially available systems, software, tools and monitoring to provide security for the processing, transmission and storage of digital information.
While we attempt to take appropriate security and cybersecurity measures to protect our information technology systems and infrastructure (including any trade secrets, confidential or other sensitive information) and to prevent and detect breakdowns, unauthorized breaches and cyber-attacks, we cannot guarantee that these measures will be successful and that breakdowns and breaches of, or attacks on, our systems and data, or those of third parties upon which we rely, will be prevented. Such breakdowns and breaches of, or attacks on, our systems and infrastructure, or the public perception that we or any third party upon which we rely have suffered a cybersecurity incident or breakdown, may cause business interruption and could have a material adverse effect on our business, financial condition, cash flows and results of operations, damage our reputation with customers, employees and third parties with whom we do business and cause the market value of our common shares and/or debt securities to decline, and we may suffer financial damage or other loss, including fines or criminal penalties or may be subject to litigation, including potentially class action law suits because of lost or misappropriated information.
While we maintain insurance against some of these risks, this insurance may not be sufficient to cover the financial, legal, business or reputational losses that may result from a breakdown, breach, cyber-attack or other compromise of or interruption to our information technology systems and infrastructure or confidential and other sensitive information.
In addition, we provide confidential and other sensitive information to third parties when necessary to pursue our business objectives. While we obtain assurances that these third parties will protect this information and, where appropriate, monitor the protections employed by these third parties, there is a risk that the confidentiality of information held by third parties, including trade secrets and sensitive personal information, may be compromised. If personal information of our customers or employees is misappropriated, our reputation with our customers and employees may be injured, resulting in loss of business and/or morale. Any such incidents could require us to incur costs to remediate possible injury to our customers and employees, to further improve our protective measures or to pay fines or take other action with respect to litigation, judicial or regulatory actions arising out of such incidents which may be significant. Any of the foregoing could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Competitive Risks
We operate in an extremely competitive industry. If competitors develop or acquire more effective or less costly pharmaceutical, OTC products or medical devices for our target indications, it could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Our vision care business operates within an extremely competitive environment. In contact lenses, we face intense competition from competitors’ products and may face increasing competition as other new products enter the market, for example, with increased product entries from contact lens manufacturers in Asia. New market entrants and existing competitors are also challenging distribution models with innovation in non-traditional, disruptive models such as direct-to-consumer, Internet and other e-commerce sales opportunities, which could adversely impact the traditional eye care professional (“ECP”) channel in which we have a significant presence. The market for contact lenses is intensely competitive and is characterized by declining sales volumes for older and reusable product lines and growing demand for daily lenses and advanced materials lenses. As the market for contact lenses shifts toward daily lenses, we expect our sales in daily lenses to, at least in part, cannibalize sales of our reusable contact lenses and contact lens care offerings. Furthermore, our ocular health product category is also highly competitive.
Many of our competitors spend significantly more on research and development related activities than we do. Others may succeed in developing or acquiring products and technologies that are more effective, more advanced or less costly than those currently marketed or proposed for development by us. In addition, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products and may also establish exclusive collaborative or licensing relationships with our competitors. These competitors and the introduction of competing products (that may be more effective or less costly than our products) could make our products less competitive or obsolete, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We cannot predict the timing or impact of the introduction of competitive products, including new market entries, “generic” versions of our approved products, or private label products that treat the same conditions as those of our products. In addition, the introduction of alternatives in medical devices and medical prescriptions could also alter the eye health markets and impede our sales growth. Our ability to respond to these competitive pressures will depend on our ability to decrease our costs and maintain gross margins and operating results and to introduce new products successfully and on a timely basis, and to achieve manufacturing efficiencies and sufficient manufacturing capacity and capabilities for such products.
Tax- and Accounting-related Risks
Our effective tax rates may increase.
We have operations in various countries that have differing tax laws and rates. Our tax reporting is supported by current domestic tax laws in the countries in which we operate and the application of tax treaties between the various countries in which we operate. Our income tax reporting is subject to audit by domestic and foreign authorities. Our effective tax rate may change from year to year based on changes in the mix of activities and income earned among the different jurisdictions in which we operate; changes in tax laws in these jurisdictions; changes in the tax treaties between various countries in which we operate; changes in our eligibility for benefits under those tax treaties; and changes in the estimated values of deferred tax assets and liabilities. Tax laws, regulations and administrative practices in various jurisdictions may be subject to significant change, with or without notice, due to economic, political and other conditions, and significant judgment is required in evaluating and estimating our provision and accruals for these taxes. Such changes could result in a substantial increase in the effective tax rate on all or a portion of our income.
In August 2022, the Inflation Reduction Act (the “IRA”) was signed into law, which includes implementation of a new corporate alternative minimum tax (the “CAMT”), among other provisions. The CAMT imposes a minimum tax on the adjusted financial statement income (“AFSI”) for “applicable corporations” with average annual AFSI over a three-year period in excess of $1 billion. A corporation that is a member of a foreign-parented multinational group, as defined, must include the AFSI (with certain modifications) of all members of the group in applying the $1 billion test, but would only be subject to the CAMT if the three-year average AFSI of its US members, US trades or business of foreign group members that are not subsidiaries of US members, and foreign subsidiaries of US members exceeds $100 million. We are currently considered a member of BHC’s foreign-parented multinational group and our “applicable corporations” would be combined with that of BHC’s “applicable corporations” to determine the applicability of the CAMT to the US members of our group. Although we currently do not believe that the CAMT will have a significant impact on our tax results, there are a number of uncertainties and ambiguities as to the interpretation and application of the CAMT, and it is possible that any future guidance with respect to the interpretation and application of the CAMT could result in the CAMT having a material effect on our liability for corporate taxes and our consolidated effective tax rate.
On October 8, 2021, the Organisation for Economic Co-operation and Development (“OECD”)/G20 inclusive framework on Base Erosion and Profit Shifting (the “Inclusive Framework”) published a statement updating and finalizing the key components of a two-pillar plan on global tax reform originally agreed on July 1, 2021, and a timetable for implementation by 2023. The timetable for implementation has since been extended to 2024 or, with respect to certain components of the plan, to 2025. The Inclusive Framework plan has now been agreed to by 145 OECD members, including several countries which did not agree to the initial plan. Under pillar one, a portion of the residual profits of multinational businesses with global turnover above €20 billion and a profit margin above 10% will be allocated to market countries where such allocated profits would be taxed. Under pillar two, the Inclusive Framework has agreed on a global minimum corporate tax rate of 15% for companies with revenue above €750 million, calculated on a country-by-country basis. On October 30, 2021, the G20 formally endorsed the new global minimum corporate tax rate rules. The Inclusive Framework agreement must now be implemented by the OECD Members who have agreed to the plan, effective in 2024. Many members of the Inclusive Framework have either introduced or announced their intention to introduce certain components of the global minimum tax in line with the model rules for fiscal year beginning on or after December 31, 2023. For example, on December 15, 2022, the European Union member states unanimously adopted the directive to implement pillar two rules. According to the directive, the member states were expected to enact pillar two rules into domestic law in 2023, with certain elements becoming effective for fiscal years beginning on or after December 31, 2023. On August 4, 2023, Canada released draft legislation to enact certain components of the pillar two proposals into Canadian law as the Global Minimum Tax Act (“GMTA”). The GMTA is generally aligned with the model rules proposed by the OECD and is expected to become effective for fiscal years beginning on or after December 31, 2023. The United States did not announce plans to enact the tax measures under the two-pillar plan. On February 1, 2023, the U.S. Financial Accounting Standards Board indicated that they believe the minimum tax imposed under pillar two is an alternative minimum tax, and, accordingly, deferred tax assets and liabilities associated with the minimum tax would not be recognized or adjusted for the estimated future effects of the minimum tax but would be recognized in the period incurred. The OECD has published model rules and other guidance with respect to pillar two, which are generally consistent with the agreement reached by the Inclusive Framework in October 2021. On February 1, 2023, the Inclusive Framework released a package of technical and administrative guidance on the implementation of pillar two, including the scope of companies that will be subject to the Global Anti-Base Erosion Rules, transition rules, and guidance on domestic minimum taxes that countries may choose to adopt, among other topics. On December 18, 2023 the OECD announced plans to release additional guidance on model rules and to start the peer review process in 2024. Many jurisdictions in which the Company operates have adopted the global minimum tax provision of the OECD pillar two effective for tax years beginning in January 2024. It is possible that the implementation of the Inclusive Framework agreement, including the global minimum corporate tax rate, could have a material effect on our liability for corporate taxes and our consolidated effective tax rate in the future.
Our provision for income taxes is based on certain estimates and assumptions made by management. Our consolidated income tax rate is affected by the amount of pre-tax income earned in our various operating jurisdictions, the availability of benefits under tax treaties and the rates of taxes payable in respect of that income. We enter into many transactions and arrangements in the ordinary course of business in respect of which the tax treatment is not entirely certain. We therefore make estimates and judgments based on our knowledge and understanding of applicable tax laws and tax treaties and the application of those tax laws and tax treaties to our business, in determining our consolidated tax provision. For example, certain countries could seek to tax a greater share of income than we will allocate to our business in such countries. The final outcome of any audits by taxation authorities may differ from the estimates and assumptions that we may use in determining our consolidated tax provisions and accruals. This could result in a material adverse effect on our consolidated income tax provision, financial condition and the net income for the period in which such determinations are made.
Our deferred tax liabilities, deferred tax assets and any related valuation allowances are affected by events and transactions arising in the ordinary course of business, acquisitions of assets and businesses and non-recurring items. The assessment of the appropriate amount of a valuation allowance against the deferred tax assets is dependent upon several factors, including estimates of the realization of deferred income tax assets, which realization will be primarily based on future taxable income, including the reversal of existing taxable temporary differences. Significant judgment is applied to determine the appropriate amount of valuation allowance to record. Changes in the amount of any valuation allowance required could materially increase or decrease our provision for income taxes in a given period.
We have significant goodwill and other intangible assets and potential impairment of goodwill and other intangibles may have a significant adverse impact on our profitability.
Goodwill and intangible assets represent a significant portion of our total assets. Finite-lived intangible assets are subject to an impairment analysis whenever events or changes in circumstances indicate the carrying amount of the asset may not be recoverable. Goodwill and indefinite-lived intangible assets are tested for impairment annually, or more frequently if events or changes in circumstances indicate that the asset may be impaired. If impairment exists, we would be required to take an impairment charge with respect to the impaired asset.
For example, in 2023, 2022 and 2021, we recognized impairments to finite-lived and indefinite-lived intangible assets of less than $1 million, $1 million and $12 million, respectively. These asset impairments were primarily attributable to the discontinuance of certain product lines.
The Company conducted its annual goodwill impairment test as of October 1, 2023. No impairment to the goodwill of any reporting unit was identified. If market conditions deteriorate, or if the Company is unable to execute its strategies, it may be necessary to record impairment charges in the future.
See Note 5, “FAIR VALUE MEASUREMENTS” and Note 8, “INTANGIBLE ASSETS AND GOODWILL” to our audited Consolidated Financial Statements included elsewhere in this 10-K for further information on these impairment charges.
Events giving rise to impairment are difficult to predict, including the uncertainties associated with the launch of new products, and are an inherent risk in the pharmaceutical and medical device industries. As a result of the significance of goodwill and intangible assets, our financial condition and results of operations in a future period could be negatively impacted should such an impairment of goodwill or intangible assets occur, which could cause the market value of our common shares and/or debt securities to decline. We may be required to take additional impairment charges in the future and such impairment charges may be material.
Legal and Reputational Risks
We are or may become subject to legal and governmental proceedings that are uncertain, costly and time-consuming and could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We are involved, or may become involved, from time to time in legal and governmental proceedings, which may be material. In addition, the Company has agreed with BHC to assume a portion of future liability or damages associated with certain legal and administrative proceedings that existed at the time of the B+L IPO. These legal and administrative proceedings will remain with BHC and will be controlled by BHC, but the Company will share in applicable future liabilities, should any result from these proceedings.
These proceedings are complex and extended and occupy the resources of our management and employees. These proceedings are also costly to prosecute and defend and may involve substantial awards or damages payable by us if not found in our favor. We may also be required to pay substantial amounts or grant certain rights on unfavorable terms in order to settle such proceedings. Defending against or settling such claims and any unfavorable legal decisions, settlements or orders could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline. See Note 20, “LEGAL PROCEEDINGS” to our audited Consolidated Financial Statements for additional information.
For example, the pharmaceutical industry, has been the focus of both private payor and governmental concern regarding pricing of pharmaceutical products. Related actions, including Congressional and other governmental investigations and litigation, are costly and time-consuming and adverse resolution of such actions or changes in our business practices, such as our approach to the pricing of our pharmaceutical products, could adversely affect our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
In addition, in the United States, it has become increasingly common for patent infringement actions to prompt claims that antitrust laws have been violated during the prosecution of the patent or during litigation involving the defense of that patent. Such claims by direct and indirect purchasers and other payers are typically filed as class actions. The relief sought may include treble damages and restitution claims. Similarly, antitrust claims may be brought by government entities or private parties following settlement of patent litigation, alleging that such settlements are anti-competitive and in violation of antitrust laws. In the United States and Europe, regulatory authorities have continued to challenge as anti-competitive so-called “reverse payment” settlements between branded and generic drug manufacturers. We may also be subject to other antitrust litigation involving competition claims unrelated to patent infringement and prosecution. A successful antitrust claim by a private party or government entity against us could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We depend on third parties to meet their contractual, legal, regulatory and other obligations.
We rely on distributors, suppliers, contract research organizations, vendors, service providers, business partners and other third parties to research, develop, manufacture, distribute, market and sell our products, as well as perform other services relating to our business. We rely on these third parties to meet their contractual, legal, regulatory and other obligations. A failure to maintain these relationships or poor performance by these third parties could negatively impact our
business. In addition, we cannot guarantee that the contractual terms and protections and compliance controls, policies and procedures we have put in place will be sufficient to ensure that such third parties will meet their legal, contractual and regulatory obligations or that these terms, controls, policies, procedures and other protections will protect us from acts committed by our agents, contractors, distributors, suppliers, service providers or business partners that violate contractual obligations or the laws or regulations of the jurisdictions in which we operate, including matters respecting anti-corruption, fraud, bribery and kickbacks and false claims, pricing, sales and marketing practices, privacy laws and other legal obligations. Any failure of such third parties to meet these legal, contractual and regulatory obligations or any improper actions by such third parties or even allegations of such non-compliance or actions could damage our reputation, adversely impact our ability to conduct business in certain markets and subject us to civil or criminal legal proceedings and regulatory investigations, monetary and non-monetary damages and penalties and could cause us to incur significant legal and investigatory fees and, as a result, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
If our products cause, or are alleged to cause, serious or widespread personal injury, we may have to withdraw those products from the market and/or incur significant costs, including payment of substantial sums in damages, and we may be subject to exposure relating to product liability claims. In addition, our product liability self-insurance program may not be adequate to cover future losses.
We face an inherent business risk of exposure to significant product liability and other claims in the event that the use of our products caused, or is alleged to have caused, adverse effects. Product liability proceedings may be costly to prosecute and defend and may involve substantial awards or damages payable by us if not found in our favor.
Furthermore, our products may cause, or may appear to have caused, adverse side effects (including death) or potentially dangerous drug interactions that we may not learn about or understand fully until the drug has been administered to patients for some time. The withdrawal of a product following complaints and/or incurring significant costs, including the requirement to pay substantial damages in personal injury cases or product liability cases, could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
In addition, we self-insure substantially all of our product liability risk, and will periodically evaluate and adjust our claims reserves to reflect trends in our own experience, as well as industry trends. However, historical loss trends may not be adequate to cover future losses, as historical trends may not be indicative of future losses. If ultimate results exceed our estimates, this would result in losses in excess of our reserved amounts. If we were required to pay a significant amount on account of these liabilities for which we self-insure, this could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Our marketing, promotional and business practices, as well as the manner in which sales forces interact with purchasers, prescribers and patients, are subject to extensive regulation and any material failure to comply could result in significant sanctions against us.
The marketing, promotional and business practices of pharmaceutical and medical device companies, as well as the manner in which companies’ in-house or third-party sales forces interact with purchasers, prescribers and patients, are subject to extensive regulation, enforcement of which may result in the imposition of civil, regulatory and/or criminal penalties, injunctions and/or limitations on marketing practice for some of our products and/or pricing restrictions or mandated price reductions for some of our products. Many companies, including us, have been the subject of claims related to these practices asserted by federal authorities. These claims have resulted in fines and other consequences, such as entering into corporate integrity agreements with the U.S. government. Companies may not promote drugs or devices for “off-label” uses—that is, uses that are not described in the product’s labeling and that differ from those approved by the FDA, Health Canada, EMA or other applicable regulatory agencies. A company that is found to have improperly promoted off-label uses may be subject to significant liability, including civil and administrative remedies (such as entering into corporate integrity agreements with the U.S. government), as well as criminal sanctions. In addition, management’s attention could be diverted from our business operations and our reputation could be damaged.
Risks Relating to Specific Legislation and Regulations
We are subject to various laws and regulations, including “fraud and abuse” laws, anti-bribery laws, environmental laws and privacy and security laws, and a failure to comply with such laws and related regulations or prevail in any litigation or investigation related to noncompliance could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
Pharmaceutical and medical device companies have faced lawsuits and investigations pertaining to violations of health care “fraud and abuse” laws, such as the federal False Claims Act, the federal Anti-Kickback Statute (“AKS”) and other state and federal laws and regulations. The AKS prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any health care item or service reimbursable under federally financed health care programs. This statute has been interpreted to apply to arrangements between pharmaceutical or medical device manufacturers, on the one hand, and prescribers, purchasers, formulary managers and other health care related professionals, on the other hand. More generally, the federal False Claims Act, among other things, prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government. Pharmaceutical and medical device companies have been prosecuted or faced civil liability under these laws for a variety of alleged promotional and marketing activities, including engaging in off-label promotion that caused claims to be submitted for non-covered off-label uses. If we are in violation of any of these requirements or any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, this could have a significant impact on our business, including the imposition of significant criminal and civil fines and penalties, exclusion from federal health care programs or other sanctions, including consent orders or corporate integrity agreements.
In addition, the U.S. Department of Health and Human Services Office of Inspector General recommends, and increasingly states require pharmaceutical companies to have comprehensive compliance programs. Moreover, the Physician Payment Sunshine Act enacted in 2010 imposes reporting and disclosure requirements on device and drug manufacturers for any “transfer of value” made or distributed to prescribers and other health care providers. Failure to submit this required information may result in significant civil monetary penalties. While we have developed corporate compliance programs based on what we believe to be current best practices, we cannot provide assurance that we or our employees or agents are or will be in compliance with all applicable federal, state or foreign regulations and laws. If we are in violation of any of these requirements or any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant criminal and civil fines and penalties, exclusion from federal health care programs or other sanctions, including consent orders or corporate integrity agreements.
The U.S. FCPA, the Canadian Corruption of Foreign Public Officials Act and similar worldwide anti- bribery laws generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. Our policies mandate compliance with these anti-bribery laws. We operate in many parts of the world that have experienced governmental corruption and in certain circumstances, strict compliance with anti-bribery laws may conflict with local customs and practices or may require us to interact with doctors and hospitals, some of which may be state controlled, in a manner that is different than in the United States and Canada. We cannot provide assurance that our internal control policies and procedures will protect us from reckless or criminal acts committed by our employees, consultants, distributors, third party contractors or agents. Violations of these laws, or allegations of such violations, could disrupt our business and result in criminal or civil penalties or remedial measures, any of which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We are also subject to various state, federal and international laws and regulations governing the collection, transmission, dissemination, use, privacy, confidentiality, security, retention, availability, integrity and other processing of health-related and other sensitive and personal information, including the HIPAA. Many states in which we operate have laws that protect the privacy and security of sensitive and personal information, including health-related information. Certain state laws may be more stringent or broader in scope, or offer greater individual rights, with respect to sensitive and personal information than federal, international or other state laws, and such laws may differ from each other, which may complicate compliance efforts. For example, the California Consumer Privacy Act of 2018, as amended by the California Privacy Rights Act (“CPRA,” and collectively, “CCPA”) imposes stringent data privacy and security requirements and obligations with respect to the personal information of California residents and provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal data that may increase the likelihood of, and risks associated with, data breach litigation. The effects on our business of the CCPA and other similar state laws are potentially significant. State laws are changing rapidly and there is discussion in Congress of a new federal data protection and privacy law to which we may be subject. For instance, the CPRA maintains the core framework of the CCPA while also making a number of substantive changes. Since these data security regimes are evolving, uncertain and complex, especially for a global business such as ours, we will need to update or enhance our compliance measures from time to time and these updates or enhancements will require further implementation costs. Any failure, or perceived failure, by us to comply with current and future regulatory or customer-driven privacy, data protection, and information security requirements, or to prevent or mitigate security breaches, cyber- attacks, or improper access to, use of, or disclosure of data, or any security issues or cyber-attacks affecting our business, could result in significant liability, costs (including the costs of mitigation and recovery), a material loss of revenue resulting from the adverse impact on its reputation and brand, loss of proprietary information and data, disruption to its business and relationships, and diminished ability to retain or attract customers and business partners. Such
events may result in governmental enforcement actions and prosecutions, private litigation, fines and penalties or adverse publicity, and could cause customers and business partners to lose trust in us, which could have an adverse effect on our reputation and business.
Internationally, laws and regulations in many jurisdictions apply broadly to the collection, transmission, dissemination, use, privacy, confidentiality, security, retention, availability, integrity and other processing of health-related and other sensitive and personal information. For example, the EU’s General Data Protection Regulation (“GDPR”), and the UK’s General Data Protection Regulation (“UK GDPR”) together with national legislation, regulations and guidelines of the EU member states and the UK governing the processing of personal data, impose strict obligations and restrictions on the ability to collect, analyze, store, transfer and otherwise process personal data, including health data from clinical trials and adverse event reporting. The GDPR authorizes fines for certain violations of up to 4% of global annual revenue or €20 million (or GBP 17.5 million under the UK GDPR), whichever is greater. European data protection authorities may interpret the GDPR and national laws differently and impose additional requirements, which contributes to the complexity of processing personal data in or from the European Economic Area or UK. Guidance on implementation and compliance practices is often updated or otherwise revised. These laws require data controllers to implement stringent operational requirements, including, for example, transparent and expanded disclosure to data subjects about how their personal data is collected and processed, grant rights for data subjects to access, delete or object to the processing of their data, mandatory data breach notification requirements (and in certain cases, affected individuals), set limitations on retention of information and outline significant documentary requirements to demonstrate compliance through policies, procedures, training and audits. The GDPR also provides that EU member states may introduce further conditions, including limitations, and make their own laws and regulations, further limiting the processing of ‘special categories of personal data,’ including personal data related to health, biometric data used for unique identification purposes and genetic information, which could limit our ability to collect, use and share EU data, and could cause our compliance costs to increase, ultimately having an adverse impact on our business, and harm our business and financial condition.
The withdrawal of the UK from the European Union also has created uncertainty with regard to the regulation of data protection in the UK. Since January 1, 2021, when the transitional period following Brexit expired, we have been required to comply with the GDPR as well as the UK GDPR (combining the GDPR and the UK’s Data Protection Act of 2018), which exposes us to two parallel regimes, each of which authorizes similar fines and may subject us to increased compliance risk based on differing, and potentially inconsistent or conflicting, interpretation and enforcement by regulators and authorities (particularly, if the laws are amended in the future in divergent ways). With respect to transfers of personal data from the EEA, on June 28, 2021, the European Commission issued an adequacy decision in respect of the UK’s data protection framework, enabling data transfers from EU member states to the UK to continue without requiring organizations to put in place contractual or other measures in order to lawfully transfer personal data between the territories. While it is intended to last for at least four years, the European Commission may unilaterally revoke the adequacy decision at any point, and if this occurs, it could lead to additional costs and increase our overall risk exposure.
We are also subject to Canada’s federal Personal Information Protection and Electronic Documents Act (“PIPEDA”) and substantially similar equivalents at the provincial level with respect to the collection, use and disclosure of personal information in Canada. Such federal and provincial legislation impose data privacy and security obligations on our processing of personal information of Canadian residents. The federal, Quebec and Alberta legislation include mandatory data breach notification requirements. Canada’s Anti-Spam Legislation (“CASL”) also applies to the extent that we send commercial electronic messages from Canada or to electronic addresses in Canada. CASL contains prescriptive consent, form, content and unsubscribe mechanism requirements. Penalties for non-compliance with CASL are up to CAD $10 million per violation. These laws and regulations may be interpreted and applied differently over time and from jurisdiction to jurisdiction, and it is possible they will be interpreted and applied in ways that will materially and adversely affect our business. The regulatory framework for data privacy, data security and data transfers worldwide is rapidly evolving and is likely to remain uncertain for the foreseeable future. Complying with all of these laws and regulations involves costs to our business, and failure to comply with these laws and regulations can result in the imposition of significant civil and criminal penalties, as well as litigation, all of which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We are also subject to U.S. federal laws regarding reporting and payment obligations with respect to our participation in federal health care programs, including Medicare and Medicaid. Because our processes for calculating applicable government prices and the judgments involved in making these calculations involve subjective decisions and complex methodologies, these calculations are subject to risk of errors and differing interpretations. In addition, they are subject to review and challenge by the applicable governmental agencies, and it is possible that such reviews could result in changes that could have material adverse legal, regulatory, or economic consequences.
Legislative or regulatory reform of the health care system may affect our ability to sell our products profitably and could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
In the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the health care system in ways that could impact our ability to sell our products profitably. The Patient Protection and Affordable Care Act, as amended by the Health Care Reform Act (as defined below) may affect the operational results of companies in the pharmaceutical and medical device industries, including the Company and other health care related industries, by imposing on them additional costs. Effective January 1, 2010, the Health Care Reform Act increased the minimum Medicaid drug rebates for pharmaceutical companies, expanded the 340B drug discount program, and made changes to affect the Medicare Part D coverage gap, or “donut hole.” The law also revised the definition of “average manufacturer price” for reporting purposes, which may affect the amount of our Medicaid drug rebates to states. Beginning in 2011, the law imposed a significant annual fee on companies that manufacture or import branded prescription drug products. More recently, the Bipartisan Budget Act of 2018 amended the Patient Protection and Affordable Care Act, effective January 1, 2019, to close the donut hole in most Medicare drug plans. In addition, in April 2018, the Centers for Medicare & Medicaid Services published a final rule that gives states greater flexibility in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the Patient Protection and Affordable Care Act for plans sold through such marketplaces.
Although efforts at replacing the Health Care Reform Act have stalled in Congress, there could still be changes to this legislation in the near term. We cannot predict what those changes will be or when they will take effect, and we could face additional risks arising from such changes or changed interpretations of our obligations under the legislation. Because of this continued uncertainty, including the potential for further legal challenges or repeal of that legislation, we cannot quantify or predict with any certainty the likely impact of the Health Care Reform Act or its repeal on our business model, prospects, financial condition or results of operations, in particular on the pricing, coverage or reimbursement of any of our product candidates that may receive marketing approval. Additionally, policy efforts designed specifically to reduce patient out-of-pocket costs for medicines could result in new mandatory rebates and discounts or other pricing restrictions. Legislative efforts relating to drug pricing, the cost of prescription drugs under Medicare, the relationship between pricing and manufacturer patient programs, and government program reimbursement methodologies for drugs have been proposed and considered at the U.S. federal and state level. At the federal level, the administration’s budget proposal for fiscal year 2019 contained further drug price control measures that could be enacted in future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid and to eliminate cost sharing for generic drugs for low-income patients. While any proposed measures will require authorization through additional legislation to become effective, Congress and the administration have each indicated an intent to continue to seek new legislative or administrative measures to control drug costs, such as the Inflation Reduction Act, which, among other things, enables the U.S. government to impose penalties if drug prices are increased at a rate faster than inflation. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. We also anticipate that Congress, state legislatures, and third-party payors may continue to review and assess alternative health care delivery and payment systems and may in the future propose and adopt legislation or policy changes or implementations effecting additional fundamental changes in the health care delivery system. We cannot provide assurance as to the ultimate content, timing, or effect of changes, nor is it possible at this time to estimate the impact of any such potential legislation.
In 2019, the U.S. Health and Human Services Administration announced a preliminary plan to allow for the importation of certain lower-cost drugs from Canada. The preliminary plan excludes insulin, biological drugs, controlled substances and intravenous drugs. The preliminary plan relies on individual states to develop proposals for safe importation of those drugs from Canada and submit those proposals to the federal government for approval. Although the preliminary plan has some support from the current administration, at this time, studies to evaluate the related costs and benefits, evaluate the reasonableness of the logistics and measure the public reaction of such a plan have not been performed. While we do not believe this will have a significant impact on our future cash flows, we cannot provide assurance as to the ultimate content, timing, effect or impact of such a plan.
In 2019, the Government of Canada (Health Canada) published in the Canada Gazette amendments to the pricing regulation for patented drugs. These regulations were scheduled to become effective on July 1, 2021, but were delayed until July 1, 2022. The new regulations, among other things, change the mechanics of establishing the pricing for products submitted for approval after August 21, 2019 and the number and composition of reference countries used to determine if a drug’s price is excessive. While we do not believe this will have a significant impact on our future cash flows, as additional facts materialize, we cannot provide assurance as to the ultimate content, timing, effect or impact of such regulations.
The Health Care Reform Act and further changes to health care laws or regulatory framework that reduce our revenues or increase our costs could also have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We are subject to a broad range of environmental laws and regulations and may be subject to environmental remediation obligations under such safety and related laws and regulations. The impact of these obligations and the Company’s ability to respond effectively to them may have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We are subject to a broad range of federal, state, provincial and local environmental laws and regulations concerning the environment, safety matters, regulation of chemicals and product safety in the countries where we manufacture and sell our products or otherwise operate our business. These requirements include, among other matters, regulation of the handling, manufacture, transportation, storage, use and disposal of materials, including the discharge of pollutants, hazardous substances and waste into the environment. Compliance with environmental, health and safety laws and regulations could require us to incur significant operating or capital expenditures or result in significant restrictions on our operations. If we fail to comply with these environmental, health and safety laws and regulations, including failing to obtain any necessary permits, we could incur substantial civil or criminal fines or penalties or enforcement actions, including regulatory or judicial orders enjoining or curtailing our operations or requiring us to conduct or fund remedial or corrective measures, install pollution control equipment, reformulate or cease the marketing of our products or perform other actions. In the normal course of our business, regulated substances and waste may be released into the environment, which could cause environmental or property damage or personal injuries and which could subject us to remediation obligations regarding contaminated soil and groundwater, potential liability for damage claims or to social or reputational harm and other similar adverse impacts. Under certain of these laws and regulations, we may be subject to joint and several liability for environmental investigations and cleanups, including at properties that we currently or previously owned or operated, or at sites at which waste we generated was disposed, even if the contamination was not caused by us or was legal at the time it occurred.
We are also subject to extensive and evolving regulations regarding the manufacturing, processing, distribution, importing, exporting and labeling of our products and their raw materials. These laws and regulations may materially affect our operations by subjecting our products or raw materials to testing or reporting requirements or restrictions, moratoria, phase outs or other limitations on their sale or use. In particular, some of our products might be characterized as nanomaterials and then be subject to evolving, new nanomaterial regulations.
In recent years, legislation and regulation related to environmental protection have become increasingly stringent. Such legislation and regulations are complex and constantly changing. In particular, legislation and regulation relating to global climate, sustainability and product stewardship including greenhouse gas emissions, are at various stages of consideration and implementation. Future events, such as changes in existing laws or regulations or the enforcement thereof or the discovery of contamination at our facilities may, among other things, require us to install additional controls for certain of our emission sources, undertake changes in our manufacturing processes, remediate soil or groundwater contamination at facilities where such cleanup is not currently required or to take action to address social expectations or concerns arising from or relating to such changes and our response to such changes. The cost of such additional compliance or remediation obligations or responding to such social expectations or concerns may be significant and could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares and/or debt securities to decline.
We are currently governed by the corporate laws of Canada that in some cases have a different effect on shareholders than the corporate laws of Delaware.
We are currently governed by the Canada Business Corporations Act (“CBCA”) and other relevant laws which may affect the rights of shareholders differently than those of a company governed by the laws of a U.S. jurisdiction. There are certain material differences between the CBCA and Delaware General Corporation Law (“DGCL”). These include, but are not limited to, the following: (i) for material corporate transactions (such as mergers and amalgamations, other extraordinary corporate transactions or amendments to the Company’s articles) the CBCA generally requires approval by 66 2/3% of the votes cast by shareholders who voted, or as set out in the articles, as applicable, whereas DGCL generally requires only a majority vote; (ii) under the CBCA, holders of 5% or more of the Company’s shares that carry the right to vote at a meeting of shareholders can requisition a special meeting of shareholders, whereas such right does not exist under the DGCL; and (iii) in an uncontested election of directors at a shareholder meeting, the directors must be elected on an individual basis by majority vote.
Risks Relating to our Common Shares
Our by-laws designate specific courts in Canada and the federal district courts of the United States as the exclusive forum for certain litigation that may be initiated by our shareholders, which could limit our shareholders’ ability to obtain a favorable judicial forum for disputes with us.
Pursuant to our by-laws, unless we consent in writing to the selection of an alternative forum, the Supreme Court of British Columbia and the appellate courts therefrom shall, to the fullest extent permitted by law, be the sole and exclusive forum for: (i) any derivative action or proceeding brought on our behalf; (ii) any action or proceeding asserting a claim of breach of fiduciary duty owed by any director, officer or other employee of ours to us; (iii) any action or proceeding asserting a claim arising out of any provision of the CBCA or our by-laws (as they may be amended from time to time); or (iv) any action or proceeding asserting a claim otherwise related to the relationships among the Company, its affiliates and their respective shareholders, directors and/or officers, other than claims related to the business carried on by the Company or such affiliates (such provision, the “Canadian Forum Provision”). The Canadian Forum Provision will not apply to any causes of action arising under the Securities Act, the Exchange Act or other federal securities laws of the United States for which there is exclusive federal or concurrent federal and state jurisdiction. Additionally, our by-laws further provide that unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the sole and exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act (such provision, the “U.S. Federal Forum Provision”). In addition, our by-laws provide that any person or entity purchasing or otherwise acquiring any interest in our share capital is deemed to have notice of and consented to the Canadian Forum Provision and the U.S. Federal Forum Provision; provided, however, that shareholders cannot and will not be deemed to have waived our compliance with the U.S. federal securities laws and the rules and regulations thereunder.
The Canadian Forum Provision and the U.S. Federal Forum Provision in our by-laws may impose additional litigation costs on shareholders in pursuing any such claims. Additionally, the forum selection clauses in our by-laws may limit our shareholders’ ability to bring a claim in a judicial forum that they find favorable for disputes with us or our directors, officers or employees, which may discourage the filing of lawsuits against us and our directors, officers and employees, even though an action, if successful, might benefit our shareholders. In the event a court finds either exclusive forum provision contained in our by-laws to be unenforceable or inapplicable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, operating results and financial condition. The courts of the Province of British Columbia and appellate courts therefrom and the federal district courts of the United States may also reach different judgments or results than would other courts, including courts where a shareholder considering an action may be located or would otherwise choose to bring the action, and such judgments may be more or less favorable to us than our shareholders.
We do not expect to pay dividends on our common shares for the foreseeable future. Instead, we anticipate that all of our earnings in the foreseeable future will be used for the operation and growth of our business. Even if we decide in the future to pay a quarterly cash dividend to the holders of our common shares, we may change our dividend policy at any time.
We do not expect to pay dividends on our common shares for the foreseeable future. Instead, we anticipate that all of our earnings in the foreseeable future will be used for the operation and growth of our business. As a result, returns on your investment will primarily depend on the appreciation, if any, in the price of our common shares. Even if we decide in the future to pay a quarterly cash dividend to the holders of our common shares, our dividend policy may change at any time. The declaration and payment of dividends to holders of our common shares will be at the discretion of our Board of Directors in accordance with applicable law after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, cash flows, impact on our effective tax rate, indebtedness, legal requirements and other factors that our Board of Directors deems relevant. Payment of dividends may be subject to withholding taxes.
General Risk Factors
Our operating results and financial condition may fluctuate.
Our operating results and financial condition may fluctuate from quarter to quarter for a number of reasons. In addition, the market price of our common shares and/or debt securities can be volatile. The following events or occurrences, among others, could cause fluctuations in our financial performance and/or the market price of our common shares and/or debt securities from period to period:
•development and launch of new competitive products;
•the timing and receipt of regulatory approvals or lack of approvals;
•costs related to business development transactions;
•changes in the amount we spend to promote our products;
•delays between our expenditures to acquire new products, technologies or businesses and the generation of revenues from those acquired products, technologies or businesses;
•changes in treatment practices of health care and eye care professionals that currently prescribe certain of our products;
•increases in the cost of raw materials used to manufacture our products;
•actions by the FDA or other regulatory bodies relating to our manufacturers;
•manufacturing and supply interruptions;
•our responses to price competition;
•new legislation that would control or regulate the prices of drugs;
•a protracted and wide-ranging trade conflict between the United States and China;
•expenditures as a result of legal actions (and settlements thereof), including the defense of our patents and other intellectual property;
•market acceptance of our products;
•the timing of wholesaler and distributor purchases and success of our wholesaler and distributor arrangements;
•general economic and industry conditions, including potential fluctuations in interest rates;
•changes in seasonality of demand for certain of our products;
•foreign currency exchange rate fluctuations;
•changes to, or the confidence in, our business strategy;
•changes to, or the confidence in, our management; and
•expectations for future growth.
As a result, quarter-to-quarter comparisons of results from operations, or any other similar period-to-period comparisons, may not be reliable indicators of our future performance. In any quarterly period, our results may be below the expectations of market analysts and investors, which could cause the market value of our common shares and/or debt securities to decline.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Risk Management and Strategy
Cybersecurity risk management is an integral part of our overall enterprise risk management program. In order to assess, identify, manage and address the risk of cybersecurity threats or incidents, the Company has established a Cybersecurity Risk Management Program, which uses a risk-based approach to implement multi-layered controls designed to enable the Company to maintain agility while protecting critical infrastructure and data. This program, which is an integral part of our overall enterprise risk management program, implements controls and frameworks designed to align with industry best practices, including those based on the National Institute of Standards and Technology Cybersecurity Framework, the Sarbanes-Oxley Act of 2002 and HIPPA.
The Cybersecurity Risk Management Program is designed to identify potential vulnerabilities and threats and develop strategies to mitigate and remediate them. To assess, identify, manage, address and minimize the effects of a cybersecurity threat or incident, or a series of related cybersecurity threats or incidents, the Company undertakes a range of activities, including monitoring of its systems and networks, incident response planning, and employee training. The Company also has business continuity and disaster recovery plans in place in the event of a cybersecurity incident, which are regularly reviewed and updated as needed. The Company also regularly engages third-party assessors, consultants, auditors, and other experts to help identify, assess and address potential threats or incidents.
The Cybersecurity and Risk Management Team, as described below, is responsible for the operationalization of the Company's cybersecurity practices, which consists of, but are not limited to: (i) updating and enhancing the Cybersecurity
Risk Management Program, (ii) overseeing third-party assessors, consultants, auditors, and other experts, and (iii) assessing, identifying, managing and addressing potential threats or incidents.
When a cybersecurity threat or incident is identified, the Cybersecurity and Risk Management Team will perform a technical investigation which typically consists of the following phases:
i.Detection, which includes identifying the threat or incident, gathering all available facts surrounding the matter and performing an initial analysis to determine its level of severity. If the incident is classified as “Severity 1,” the Materiality Committee, as defined below, is notified to further assess the matter.
ii.Containment and Eradication, which includes determining the cause and vulnerabilities so that the threat or incident can be isolated and eliminated.
iii.Recovery, which includes repairing the impacted systems, and if applicable, notifying and instructing impacted parties of next steps.
iv.Post-Incident, which includes issuing a report summarizing the threat or incident, and the steps taken in assessing and eliminating the threat, as well as steps to implement to attempt to prevent similar future incidents.
The Materiality Committee is responsible for assessing whether a threat or an incident has materially affected or is likely to materially affect the Company’s business strategy, results of operations or financial condition. The Materiality Committee considers both quantitative and qualitative factors. Once it is determined that a matter has had a material impact or it is reasonably likely to have a material impact on the Company, the Materiality Committee is required to immediately report the incident to the Disclosure Committee and Audit and Risk Committee (the "Audit Committee") of the Board of Directors (the “Board”).
The Company emphasizes continuous risk evaluation and mitigation to improve the Cybersecurity Risk Management Program’s resilience and instill a culture of vigilance across the Company's business. To promote employee awareness of best practices, the Company socializes policies and tips through its intranet site, sends regular phishing simulations, emails newsletters and hosts cybersecurity learning exercises, all in addition to standard company-wide cybersecurity awareness trainings. The Company also participates in various cybersecurity network memberships, including:
•H-ISAC: a global cybersecurity best practice-sharing and threat intelligence network for health care stakeholders and
•Domestic Security Alliance Council: a partnership between U.S. government agencies and private sector organizations that exchanges security and intelligence information.
The Company has also implemented a risk management process designed to mitigate cybersecurity risks that arise from utilizing third-party service providers. The Company’s control over and ability to monitor the security posture of third parties with whom it does business remains limited and there can be no assurance that the Company can prevent, mitigate or remediate the risk of any compromise or failure in the security infrastructure owned or controlled by such third parties. Additionally, any contractual protections with such third parties, including the Company’s right to indemnification, if any at all, may be limited or insufficient to prevent a negative impact on its business from any such compromise or failure.
Impact of cybersecurity risks on business strategy, results of operations or financial condition
Despite our efforts, we cannot eliminate all risks from cybersecurity threats, or provide assurances that we have not experienced an undetected cybersecurity incident. For more information about these risks, please see “Risk Factors—Risks Relating to Information Technology—We have become increasingly dependent on information technology systems and infrastructure and any breakdown, interruption, breach or other compromise of our information technology systems or those of our third party service providers could subject us to liability or interrupt the operation of our business, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares to decline” under Item 1A of this Annual Report Form 10-K.
Governance
Because cybersecurity and data privacy can affect all facets of the Company's business, the Company employs governance structures that facilitate cross-functional, proactive risk management. The Board is responsible for oversight of the Company’s risks from cybersecurity threats and incidents and the Audit Committee maintains primary responsibility related to monitoring this oversight. As noted above, the Company has in place the following teams who are responsible for maintaining various phases of the Cybersecurity Risk Management Program:
•Cybersecurity and Risk Management Team which is led by the VP, IT Security and Risk Management, who reports directly to the Chief Information Officer (“CIO”). The Cybersecurity and Risk Management Team is overseen by:
(i) the Executive Committee, which consists of, among others, the CEO, Chief Financial Officer, Chief Legal Officer and Chief Compliance and Privacy Officer, and (ii) the Audit Committee. The Cybersecurity and Risk Management Team is responsible for maintaining and carrying out the Cybersecurity Risk Management Program.
•Materiality Committee, which is led by the CIO, Controller and Chief Accounting Officer, and Deputy General Counsel. The Cybersecurity and Risk Management Team informs the Materiality Committee of Severity 1 incidents and the Materiality Committee is then responsible for assessing whether a threat or an incident has materially affected or is likely to materially affect the Company’s business strategy, results of operations or financial condition. Once it is determined that a matter has had a material impact or it is reasonably likely to have a material impact on the Company, the Materiality Committee is required to immediately report the incident to the Disclosure Committee and Audit Committee.
•Audit Committee, which is comprised of independent directors, oversees the Cybersecurity and Risk Management Team and the team’s implementation of its Cybersecurity Risk Management Program. The Audit Committee receives quarterly updates regarding cybersecurity risks and/or policy. In addition, the Materiality Committee updates the Audit Committee, as necessary, regarding any material cybersecurity incidents.
•Disclosure Committee, which is led by the Chief Legal Officer and Chief Financial Officer. The Disclosure Committee is informed of potentially material threats and incidents by the Cybersecurity and Risk Management Team and Materiality Committee and the Disclosure Committee is responsible for the preparation, review and filing of any disclosure required by applicable law.
The Company’s VP, IT Security and Risk Management and CIO who collectively lead our Cybersecurity and Risk Management Team have relevant degrees and certifications in Information Technology and Security and have extensive experience in their current and prior roles related to IT Security. Along with leading the Company’s cybersecurity learning and awareness trainings, they also regularly participate in various third-party industry conferences and trainings.
Item 2. Properties
We own and lease a number of important properties. Our headquarters are located in Vaughan, Ontario, Canada. We own several manufacturing facilities throughout the United States. We also own or have an interest in manufacturing plants or other properties outside the United States, including in Mexico, and certain countries in Europe, the Middle East, Asia and South America.
We consider our facilities to be in satisfactory condition and suitable for their intended use, although some limited investments to improve our manufacturing and other related facilities are contemplated, based on the needs and requirements of our business. Our administrative, marketing, research/laboratory, distribution and warehousing facilities are located in various parts of the world. We co-locate our R&D activities with our manufacturing at the plant level in a number of facilities. Our scientists, engineers, quality assurance/quality control professionals and manufacturing technicians work side-by-side in designing and manufacturing products that fit the needs and requirements of our customers, regulators and business units. We believe that we have sufficient facilities to conduct our operations. The following are our principal properties, including the segments of our business that use each property:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Location | | Purpose | | Segment | | Owned
or
Leased | | Approximate
Square
Footage |
| Vaughan, Ontario, Canada | | Corporate headquarters and distribution facility | | All Segments | | Leased | | 66,000 | |
| Bridgewater, New Jersey | | Administration shared with BHC | | All Segments | | Leased | | 310,000 | |
| Rochester, New York | | Offices, R&D and manufacturing facility | | Pharmaceuticals + Vision Care | | Owned | | 953,000 | |
| Waterford, Ireland | | R&D and manufacturing facility | | Vision Care | | Owned | | 500,000 | |
| Woodruff, South Carolina | | Distribution facility | | Vision Care | | Leased | | 432,000 | |
| Jinan, China | | Offices and manufacturing facility | | Pharmaceuticals + Vision Care | | Owned | | 418,000 | |
| Berlin, Germany | | R&D, manufacturing, distribution and office facility | | Pharmaceuticals | | Owned | | 339,000 | |
| Greenville, South Carolina | | Manufacturing facility | | Vision Care | | Owned | | 314,000 | |
| Lynchburg, Virginia | | Offices and distribution facility | | Vision Care | | Owned | | 224,000 | |
| Tampa, Florida | | R&D and manufacturing facility | | Pharmaceuticals | | Owned | | 171,000 | |
| Aubenas, France | | Offices, manufacturing and warehouse facility | | Pharmaceuticals | | Owned | | 148,000 | |
| St. Louis, Missouri | | Offices, R&D and manufacturing facility | | Surgical | | Owned | | 140,000 | |
| Macherio, Italy | | Offices, manufacturing and warehouse facility | | Vision Care | | Owned | | 119,000 | |
| Clearwater, Florida | | R&D and manufacturing facility | | Surgical | | Owned | | 102,000 | |
| Beijing, China | | Manufacturing facility | | Vision Care | | Owned | | 97,000 | |
Item 3. Legal Proceedings
See Note 20, “LEGAL PROCEEDINGS” to our audited Consolidated Financial Statements for details on legal proceedings.
Item 4. Mine Safety Disclosures
None.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common shares are traded on the New York Stock Exchange (“NYSE”) and on the Toronto Stock Exchange (“TSX”) under the symbol “BLCO”.
Holders
The approximate number of holders of record of our common shares as of February 16, 2024 was 4.
Performance Graph
The graph below matches the cumulative total return of holders of Bausch + Lomb Corporation's common shares with the cumulative total returns of the: (i) S&P 500 index, (ii) the S&P/TSX Composite index, (iii) a 2022 peer group of companies and (iv) a 2023 peer group of companies. The 2022 peer group consists of a customized peer group of fourteen companies that includes: Agilent Technologies Inc, Alcon Inc., Align Technology Inc, The Cooper Companies Inc, Dentsply Sirona Inc, Dexcom Inc, Edwards Lifesciences Corp, Hologic Inc, Jazz Pharmaceuticals Plc, Perrigo Company Plc, Resmed Inc, Teleflex Inc, Zimmer Biomet Holdings Inc and Zoetis Inc. In 2023, the Company completed an assessment to review its current peers and added Organon & Co. to its peer group in order to ensure a robust group of peer companies across the health care supplies, health care equipment, and pharmaceuticals industries. The 2023 peer group therefore consists of a customized peer group of fifteen companies that includes: Agilent Technologies Inc, Alcon Inc., Align Technology Inc, The Cooper Companies Inc, Dentsply Sirona Inc, Dexcom Inc, Edwards Lifesciences Corp, Hologic Inc, Jazz Pharmaceuticals Plc, Organon & Co., Perrigo Company Plc, Resmed Inc, Teleflex Inc, Zimmer Biomet Holdings Inc and Zoetis Inc. The graph assumes that the value of the investment in our common shares, in each index, and in the peer groups (including reinvestment of dividends) was $100 on May 6, 2022 and tracks it through December 31, 2023.
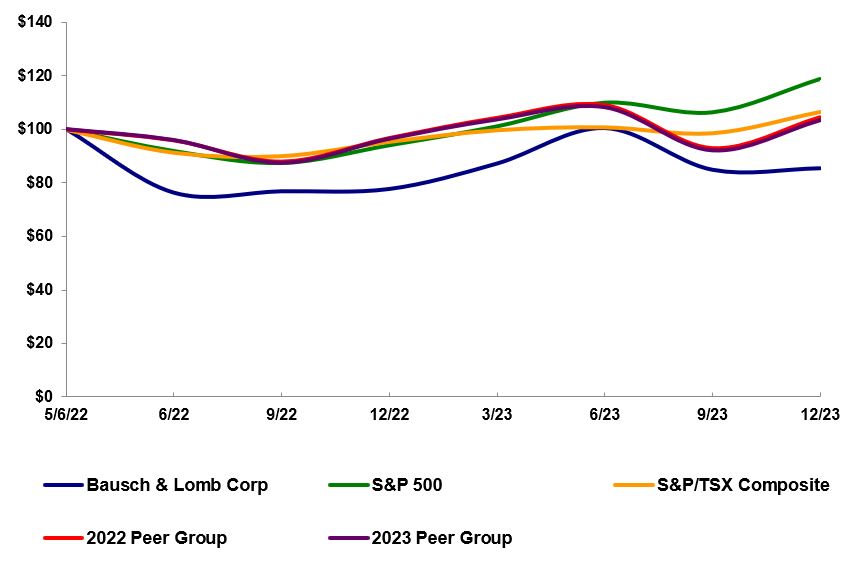
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| | | 5/6/22 | | 6/22 | | 9/22 | | 12/22 | | 3/23 | | 6/23 | | 9/23 | | 12/23 |
| Bausch + Lomb Corp | | $100.00 | | $76.20 | | $76.70 | | $77.55 | | $87.05 | | $100.35 | | $84.75 | | $85.30 |
| S&P 500 | | $100.00 | | $91.91 | | $87.43 | | $94.04 | | $101.09 | | $109.92 | | $106.33 | | $118.76 |
| S&P/TSX Composite | | $100.00 | | $91.35 | | $90.06 | | $95.43 | | $99.78 | | $100.87 | | $98.65 | | $106.64 |
| 2022 Peer Group | | $100.00 | | $95.90 | | $87.86 | | $96.72 | | $104.33 | | $109.18 | | $92.93 | | $104.57 |
| 2023 Peer Group | | $100.00 | | $95.94 | | $87.44 | | $96.41 | | $103.60 | | $108.18 | | $92.08 | | $103.30 |
Dividends
Since the B+L IPO, no dividends have been declared or paid. While our Board of Directors will review our dividend policy periodically, we currently do not intend to pay any cash dividends in the foreseeable future.
Restrictions on Share Ownership by Non-Canadians
There are no limitations under the laws of Canada or in our organizational documents on the right of foreigners to hold or vote securities of our Company, except that the Investment Canada Act (Canada) (the “Investment Canada Act”) may require review and approval by the Minister of Innovation, Science and Industry(Canada) (the “Minister”) of an acquisition of “control” of our Company by a “non-Canadian”.
Investment Canada Act
An acquisition of control of a Canadian business by a non-Canadian is either reviewable (a “Reviewable Transaction”), in which case it is subject to both a reporting obligation and an approval process, or notifiable, in which case it is subject to only a reporting obligation. In the case of a Reviewable Transaction, the non-Canadian acquirer must submit an application for review with the prescribed information. The Minister is then required to determine whether the Reviewable Transaction is likely to be of net benefit to Canada, taking into account the assessment factors specified in the Investment Canada Act and any written undertakings that may have been provided by the non-Canadian acquirer.
The Investment Canada Act also provides that any investment by a non-Canadian in a Canadian business, even where control has not been acquired, can be reviewed on grounds of whether it may be injurious to national security. Where an investment is determined to be injurious to national security, Cabinet can prohibit closing or, if closed, can order the investor to divest control. Short of a prohibition or divestment order, Cabinet can impose terms or conditions on the investment or can require the investor to provide binding undertakings to address the national security concern.
Competition Act
Part IX of the Competition Act (Canada) (the “Competition Act”) requires that a pre-merger notification filing be submitted to the Commissioner of Competition (the “Commissioner”) in respect of certain classes of merger transactions that exceed certain prescribed thresholds. If a proposed transaction exceeds such thresholds, subject to certain exceptions, the notification filing must be submitted to the Commissioner and the statutory waiting period must expire or be terminated early or waived by the Commissioner before the transaction can be completed.
All mergers, regardless of whether they are subject to Part IX of the Competition Act, are subject to the substantive mergers provisions under Section 92 of the Competition Act. In particular, the Commissioner may challenge a transaction before the Competition Tribunal where the transaction prevents or lessens, or is likely to prevent or lessen, competition substantially in a market. The Commissioner may not make an application to the Competition Tribunal under Section 92 of the Competition Act more than one year after the merger has been substantially completed.
Exchange Controls
Canada has no system of exchange controls. There are no Canadian exchange restrictions on the repatriation of capital or earnings of a Canadian public company to non-resident investors. There are no Canadian exchange restrictions affecting the remittance of dividends, profits, interest, royalties and other payments to non-resident holders of our securities.
Taxation
Canadian Federal Income Taxation
The following discussion is a summary of the principal Canadian federal income tax considerations generally applicable to a holder of our common shares who, at all relevant times, for purposes of the Income Tax Act (Canada) and the Income Tax Regulations (collectively, the “Canadian Tax Act”) deals at arm’s-length with, and is not affiliated with, our Company, beneficially owns its common shares as capital property, does not use or hold and is not deemed to use or hold such common shares in carrying on a business in Canada, does not with respect to common shares enter into a “derivative forward agreement” as defined in the Canadian Tax Act, and who, at all relevant times, for purposes of the application of the Canadian Tax Act and the Canada-U.S. Income Tax Convention (1980, as amended) (the “U.S. Treaty”), is resident in the United States, is not, and is not deemed to be, resident in Canada and is eligible for benefits under the U.S. Treaty (a “U.S. Holder”). Special rules, which are not discussed in the summary, may apply to a non-resident holder that is an insurer that carries on an insurance business in Canada and elsewhere or that is an “authorized foreign bank” as defined in the Canadian Tax Act.
The U.S. Treaty includes limitation on benefits rules that restrict the ability of certain persons who are resident in the United States to claim any or all benefits under the U.S. Treaty. Furthermore, limited liability companies (“LLCs”) that are not taxed as corporations pursuant to the provisions of the U.S. Internal Revenue Code of 1986, as amended do not generally qualify as resident in the United States for purposes of the U.S. Treaty. Under the U.S. Treaty, a resident of the United States who is a member of such an LLC and is otherwise eligible for benefits under the U.S. Treaty may generally be entitled
to claim benefits under the U.S. Treaty in respect of income, profits or gains derived through the LLC. Residents of the United States should consult their own tax advisors with respect to their eligibility for benefits under the U.S. Treaty.
This summary is based upon the current provisions of the U.S. Treaty and the Canadian Tax Act and our understanding of the current administrative policies and assessing practices of the Canada Revenue Agency published in writing prior to the date hereof. This summary takes into account all specific proposals to amend the U.S. Treaty and the Canadian Tax Act publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof. This summary does not otherwise take into account or anticipate changes in law or administrative policies and assessing practices, whether by judicial, regulatory, administrative or legislative decision or action, nor does it take into account provincial, territorial or foreign tax legislation or considerations, which may differ from those discussed herein.
This summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice generally or to any particular holder. Holders should consult their own tax advisors with respect to their own particular circumstances.
Gains on Disposition of Common Shares
In general, a U.S. Holder will not be subject to tax under the Canadian Tax Act on capital gains, or be entitled to deduct capital losses, arising on the disposition of such holder’s common shares unless the common shares are “taxable Canadian property” to the U.S. Holder and are not “treaty-protected property”.
As long as the common shares are then listed on a “designated stock exchange”, which currently includes the NYSE and TSX, the common shares generally will not constitute taxable Canadian property of a U.S. Holder, unless: (a) at any time during the 60-month period preceding the disposition, one or any combination of (i) the U.S. Holder, (ii) persons not dealing at arm’s length with such U.S. Holder, and (iii) partnerships in which the U.S. Holder or a person described in (ii) holds a membership interest (either directly or indirectly through one or more partnerships), owned 25% or more of the issued shares of any class or series of the capital stock of the Company and (b) more than 50% of the fair market value of the common shares was derived, directly or indirectly, from any combination of: (i) real or immoveable property situated in Canada, (ii) “Canadian resource property” (as such term is defined in the Canadian Tax Act), (iii) “timber resource property” (as such term is defined in the Canadian Tax Act) or (iv) options in respect of, or interests in, or for civil law rights in, any such properties whether or not the property exists, or the common shares are otherwise deemed to be taxable Canadian property.
Common shares will be treaty-protected property where the U.S. Holder is exempt from income tax under the Canadian Tax Act on the disposition of common shares because of the U.S. Treaty. Common shares owned by a U.S. Holder will generally be treaty-protected property where the value of the common shares is not derived principally from real property situated in Canada, as defined in the U.S. Treaty.
Dividends on Common Shares
Dividends paid or credited on the common shares or deemed to be paid or credited on the common shares to a U.S. Holder that is the beneficial owner of such dividends will generally be subject to non-resident withholding tax under the Canadian Tax Act and the U.S. Treaty at the rate of: (a) 5% of the amounts paid or credited if the U.S. Holder is a company that owns (or is deemed to own) at least 10% of our voting stock or (b) 15% of the amounts paid or credited in all other cases. The rate of withholding under the Canadian Tax Act in respect of dividends paid to non-residents of Canada is 25% where no tax treaty applies.
Securities Authorized for Issuance under Equity Compensation Plans
Information required under this Item will be included in our definitive proxy statement for the 2024 Annual Meeting of Shareholders expected to be filed with the SEC no later than 120 days after the end of the fiscal year covered by this Form 10-K (the “2024 Proxy Statement”), and such required information is incorporated herein by reference.
Purchases of Equity Securities by the Company and Affiliated Purchases
There were no purchases of equity securities by the Company during the fourth quarter of the year ended December 31, 2023.
Item 6. Reserved
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
INTRODUCTION
Unless the context otherwise indicates, as used in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” the terms “we,” “us,” “our,” “Bausch + Lomb,” the “Company,” and similar terms refer to Bausch + Lomb Corporation and its subsidiaries. This “Management’s Discussion and Analysis of Financial Condition and Results of Operations” has been updated through February 21, 2024 and should be read in conjunction with the audited Consolidated Financial Statements and the related notes thereto included elsewhere in this Annual Report on Form 10-K. The matters discussed in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contain certain forward-looking statements within the meaning of Section 27A of The Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of The Securities Exchange Act of 1934, as amended, and that may be forward-looking information within the meaning defined under applicable Canadian securities laws (collectively, “Forward-Looking Statements”). See “Forward-Looking Statements” at the end of this discussion. Additional Company information, including this Form 10-K, is available on SEDAR+ at www.sedarplus.ca and on the U.S. Securities and Exchange Commission (the “SEC”) website at www.sec.gov. All currency amounts are expressed in U.S. dollars, unless otherwise noted.
OVERVIEW
Bausch + Lomb is a leading global eye health company dedicated to protecting and enhancing the gift of sight for millions of people around the world—from the moment of birth through every phase of life. Our mission is simple, yet powerful: helping you see better, to live better. We develop, manufacture and market a range of products, primarily in the areas of eye health, which are marketed directly or indirectly in approximately 100 countries. As a fully integrated eye health business, Bausch + Lomb has an established line of contact lenses, intraocular lenses (“IOLs”) and other medical devices, surgical systems and devices, vitamin and mineral supplements, lens care products, prescription eye-medications and other consumer products that positions us to compete in all areas of the eye health market. Bausch + Lomb is a subsidiary of Bausch Health Companies Inc. (“BHC”), with BHC holding, directly or indirectly, approximately 88.4% of the issued and outstanding common shares of Bausch + Lomb, as of February 16, 2024. For additional discussion regarding the separation of Bausch + Lomb from BHC, refer to Item 1. "Business — Initial Public Offering and Separation of the Bausch + Lomb Eye Health Business".
Our comprehensive portfolio of approximately 400 products is built to serve our customers across the full spectrum of their eye health needs throughout their lives. Our iconic brand is built on the deep trust and loyalty of our customers established over our 170-year history. We have a significant global research, development, manufacturing and commercial footprint of approximately 13,300 employees and a presence in approximately 100 countries, extending our reach to billions of potential customers across the globe. We have long been associated with many of the most significant advances in eye health, and we believe we are well positioned to continue leading the advancement of eye health in the future.
Reportable Segments
Our portfolio of products falls into three operating and reportable segments: (i) Vision Care, (ii) Pharmaceuticals (formerly Ophthalmic Pharmaceuticals) and (iii) Surgical. We have found and continue to believe there is significant opportunity in these businesses and we believe our existing portfolio, commercial footprint and pipeline of product development projects position us to successfully compete in these markets and provide us with the greatest opportunity to build value for our shareholders. The following is a brief description of the Company’s segments:
The Vision Care segment—includes both our contact lens and consumer eye care businesses, and includes leading products such as our Biotrue® ONEday daily disposables and our Biotrue® multi-purpose solution.
Our contact lens portfolio spans the spectrum of wearing modalities, including daily disposable and frequently replaced contact lenses, and contact lenses that are indicated for therapeutic use and that can also provide optical correction during healing, if required. In particular, our Vision Care contact lens portfolio includes our Bausch + Lomb INFUSE® (silicone hydrogel (“SiHy”)) daily disposable contact lenses, Biotrue® ONEday daily disposables, PureVision® SiHy contact lenses, SofLens® daily disposables and Bausch + Lomb ULTRA® contact lenses.
Our consumer eye care business consists of contact lens care products, over-the-counter (“OTC”) eye drops that address various conditions, including eye allergies, conjunctivitis, dry eye and redness relief, and eye vitamins and mineral supplements. Within our consumer eye care business, our lens care product portfolio includes Biotrue® and Renu® multipurpose solutions and Boston® cleaning and conditioning solutions, our eye drops include Lumify®, Soothe®, Artelac®, Alaway® and Mioclear® and our eye vitamins include PreserVision® and Ocuvite®.
The Pharmaceuticals segment—consists of a broad line of proprietary and generic pharmaceutical products for post-operative treatments and treatments for a number of eye conditions, such as glaucoma, eye inflammation, ocular hypertension, dry eyes and retinal diseases. Key proprietary pharmaceutical brands are Vyzulta®, Lotemax®, Prolensa® and
Minims®. In addition, during September 2023, the Company acquired XIIDRA® (as further discussed below), which the Company expects will complement and grow its existing dry eye franchise. Effective June 30, 2023, the Company renamed its former Ophthalmic Pharmaceuticals segment to the Pharmaceuticals segment. Aside from the change in name, there were no other changes made to this segment at that time.
The Surgical segment— consists of medical device equipment, consumables and technologies for the treatment of cataracts, corneal, vitreous and retinal eye conditions, which includes IOLs and delivery systems, phacoemulsification equipment and other surgical instruments and devices necessary for cataract surgery. Key surgical brands include Akreos®, AMVISC®, IC-8® Apthera™, Crystalens® IOLs, enVista® IOLs, Millennium®, Stellaris Elite® vision enhancement system, Synergetics®, ClearVisc®, StableVisc™, Storz® ophthalmic instruments, VICTUS® femtosecond laser, Teneo™, Eyefill® and ZyoptixTM.
For additional discussion of our reportable segments, see the discussion in Item 1. "Business — Segment Information" and Note 22, “SEGMENT INFORMATION” to our audited Consolidated Financial Statements for further details on these reportable segments.
Positioning for Growth
Product Development
We continuously search for new product opportunities through internal development, strategic licensing agreements and acquisitions, that, if successful, will allow us to leverage our commercial footprint and supplement our existing product portfolio and address specific unmet needs in the market.
We are focused on bringing innovative products to market to serve doctors, patients and consumers in the pursuit of helping people see better to live better all over the world. We consistently look for key trends in the eye health market to meet changing doctor, patient and consumer needs and identify areas for investment to expand our market share and maintain our leading positions across business segments. Our leadership team actively manages our pipeline in order to identify what we believe are innovative and realizable projects that meet the unmet needs of consumers, patients and eye health professionals and are expected to provide incremental and sustainable revenues and growth into the future. We believe that our current pipeline is strong enough to meet these objectives and provide future sources of revenues, in our core businesses, sufficient enough to sustain our growth and corporate health.
We believe our eye health knowledge and insights allow us to capitalize on market trends by differentiating our approach to product development, with a pipeline focused on prioritizing customer needs and actively seeking external innovation to design, develop and advance creative, ethical eye health products across our portfolio, to address unmet and evolving needs of eye care professionals, patients and consumers. Our team of approximately 850 dedicated Research and Development (“R&D”) employees is focused on advancing our pipeline and identifying new product opportunities and we believe we have a significant innovation opportunity today. We plan to develop and, where applicable, commercialize our global pipeline of over 60 projects, many of which are global projects being developed in and for multiple countries. These global and individual projects are in various stages of pre-clinical and clinical development, including new contact lenses for myopia, next-generation cataract equipment, premium IOLs, investigational treatments for dry eye, novel formulation for eye vitamins and preservative free formulation of eye drops, among others, that are designed to grow our portfolio and accelerate future growth.
Our internal R&D organization focuses on the development of products through robust bench testing that is designed to comply with international standards and through clinical trials. Certain key near-term pipeline products that have received a significant portion of our R&D investment in current and prior periods are listed below.
•SiHy Daily - A silicone hydrogel daily disposable contact lens designed to provide outstanding comfort and clear vision throughout the day. To date SiHy Daily has been launched in approximately 50 countries, under the brand names INFUSE®, BAUSCH + LOMB ULTRA® ONE DAY and AQUALOX® ONE DAY. We continue to plan to launch our SiHy Daily lenses into additional countries throughout 2024. In addition, we launched our first silicone hydrogel daily disposable multifocal contact lens in May 2023, and plan to launch a toric lens in 2024.
•Lumify® (brimonidine tartrate ophthalmic solution, 0.025%) - An OTC redness reliever eye drop that significantly reduces redness to help eyes look whiter and brighter, revealing eyes’ natural beauty. To date, we have launched and acquired the right to launch Lumify® in various countries. We also have several innovative new line extension formulations that were recently launched or are under development, including Lumify Eye IlluminationsTM, three clinically proven products for the sensitive eye area which launched in the U.S. in September 2023, Lumify® Preservative Free, for which the New Drug Application (“NDA”) was submitted to the U.S. Food and Drug Administration (the “FDA”) in May 2023, and Lumify® Eye Allergy, for which we expect to submit an NDA during 2024.
•Biotrue® – We have expanded, and continue to expand, the Biotrue® brand. Biotrue® Hydration Plus Multi-Purpose Solution was launched in the U.S. and Canada (branded as Biotrue® Advanced MPS) in 2022 and we launched Biotrue® Advanced MPS in China in August 2023. In addition, certain Biotrue® branded dry eye line extensions have been developed or are currently under development, including Biotrue® Preservative Free Contact Lens Rehydrating Drops, which received FDA clearance in December 2022 and was launched in May 2023.
•MIEBO® (perfluorohexyloctane) (formerly known as NOV03) – In December 2019, we acquired an exclusive license from Novaliq GmbH (the “Novaliq License”) for the commercialization and development in the U.S. and Canada of MIEBO® for the treatment of the signs and symptoms of dry eye disease (“DED”). The NDA was filed with the FDA in June 2022, approved by the FDA on May 18, 2023 and launched in the U.S. in September 2023. We submitted the filing for Canadian approval of this product during the first quarter of 2023 and expect approval during 2024. MIEBO® is the first and only FDA-approved treatment for DED that directly targets tear evaporation and we believe the addition of MIEBO® will help build upon our strong portfolio of integrated eye health products.
•LuxLife® – We are expanding our portfolio of premium IOLs built on the “Lux” platform with the LuxLife® Trifocal IOL with two options, non-Toric and Toric for astigmatic patients. This product is expected to be launched in various European markets in 2024.
•enVista® – We are expanding our portfolio of premium IOLs built on the enVista® platform with enVista AspireTM (Monofocal Plus), enVista EnvyTM Trifocal and enVista BeyondTM (extended depth of focus (“EDOF”)) optical designs with two options: non-Toric and Toric for astigmatism patients. enVista AspireTM monofocal and toric IOLs with Intermediate Optimized optics launched in the U.S. during October 2023 and we anticipate launching the Trifocal and EDOF optical designs for presbyopia in the U.S. in 2024 and 2026, respectively.
Strategic Acquisitions and Licensing Agreements
To supplement our internal R&D initiatives and to build-out and refresh our product portfolio, we also search for opportunities to augment our pipeline through arrangements that allow us to gain access to unique products and investigational treatments, by strategically aligning ourselves with other innovative product solutions. In addition to licensing agreements, we selectively consider acquisitions that we believe align well with our current organization and strategic plan to help drive profitable growth and advance our mission of helping people see better to live better. Certain recent strategic acquisitions and licensing agreements that we have entered into include the following:
2023 Acquisitions
•Acquisition of XIIDRA® – In September 2023, the Company acquired XIIDRA®, the first and only non-steroid eye drop specifically approved to treat the signs and symptoms of dry eye disease focusing on inflammation associated with dry eye, which we expect to begin facing loss of exclusivity (“LOE”) in the second quarter of 2032, and certain other ophthalmology assets from Novartis Pharma AG and Novartis Finance Corporation (together with Novartis Pharma AG, “Novartis”) (the “XIIDRA Acquisition”). As part of the XIIDRA Acquisition, the Company also acquired libvatrep (also known as SAF312), an investigational compound being studied for the treatment of chronic ocular surface pain, AcuStream® technology, an investigational device that may have the potential to facilitate precise dosing and accurate delivery of certain topical ophthalmic medications to the eye, and OJL332, a non-competitive antagonist (inhibitor) of TRPV1 that is still in the pre-clinical stage. We believe the XIIDRA Acquisition will complement and grow our existing dry eye franchise.
•Acquisition of Blink® Product Line – In July 2023, we acquired the Blink® OTC product line of eye and contact lens drops from Johnson & Johnson Vision, which consists of Blink® Tears Lubricating Eye Drops, Blink® Tears Preservative Free Lubricating Eye Drops, Blink GelTears® Lubricating Eye Drops, Blink® Triple Care Lubricating Eye Drops, Blink Contacts® Lubricating Eye Drops and Blink-N-Clean® Lens Drops (collectively, the “Blink® Product Line”). We believe this acquisition will enable us to continue to grow our global OTC business.
•Acquisition of AcuFocus – During January 2023, we acquired AcuFocus, Inc. ("AcuFocus"). AcuFocus is an ophthalmic medical device company that has delivered breakthrough small aperture intraocular technology to address diverse unmet needs in eye care. The IC-8® Apthera™ IOL was approved by the FDA in July 2022 as the first and only small aperture non-toric EDOF IOL for certain cataract patients who have as much as 1.5 diopters of corneal astigmatism and wish to address presbyopia at the same time. We believe that the IC-8® AptheraTM EDOF IOL will bolster our surgical portfolio by enhancing our IOL offerings, which is a strategic area of focus for the Company.
2022 Licensing Agreement and Acquisitions
•During July 2022, we entered into an exclusive European distribution agreement with Sanoculis Ltd. ("Sanoculis") for Sanoculis' Minimally Invasive Micro Sclerostomy ("MIMS®"). MIMS® is an innovative minimally invasive surgical procedure for the treatment of glaucoma. We also made an equity investment in Sanoculis as part of a Series C round of funding and have an option to acquire all of the assets of Sanoculis.
•During September 2022, we entered into an exclusive distribution agreement with Alfa Instruments s.r.l., under which Bausch + Lomb will distribute and commercialize Alfa Instruments' line of surgical intraocular dyes, Vitreocare, globally with the exception of Italy, where Alfa Instruments is based.
•During November 2022, we acquired Paragon BioTeck, Inc. (“Paragon BioTeck”), an eye-care focused drug development company, having a primary emphasis on the early detection of ocular diseases. This acquisition allows us to maximize the revenues and margins associated with Paragon BioTeck’s products, for which Bausch + Lomb had previously had commercialization rights.
•During December 2022, we acquired Total Titanium Inc., an ophthalmic microsurgical instrument and machined parts manufacturing company. We believe that this acquisition is an important step in continuing to expand our surgical portfolio as it provides us with the opportunity to increase our manufacturing capacity and more specifically bolster our position in the ophthalmic microsurgical instrumentation market.
We regularly consider further strategic licensing and acquisition opportunities, some of which could be material in size.
Business Trends
In addition to the actions previously outlined, the events described below have affected and may affect our business trends. The matters discussed in this section contain Forward-Looking Statements. Please see “Forward-Looking Statements” for additional information.
Russia-Ukraine War
In February 2022, Russia invaded Ukraine. As military activity and sanctions against Russia, Belarus and specific areas of Ukraine have continued, the war has increasingly affected economic and global financial markets and exacerbated ongoing economic challenges, including issues such as high levels of inflation and global supply-chain disruption.
In May 2023, the Biden administration announced a round of U.S. sanctions and export controls against Russia and Belarus in response to the ongoing war. These sanctions temporarily impacted our ability to distribute our U.S. manufactured contact lenses and our U.S. surgical products to Russia and Belarus. However, in response to these sanctions, we applied for licenses with the U.S. Department of Commerce’s Bureau of Industry and Security for both Russia and Belarus. In Russia, we have now received all licenses necessary to allow us to resume the sale of the applicable currently sanctioned products in that country. In Belarus, certain of these licenses have been obtained and some remain pending and, as a result, we can resume the sale of substantially all of the applicable currently sanctioned products in that country and will resume sale of the remaining products once the pending licenses are obtained. The recent sanctions imposed by the U.S. Department of the Treasury’s Office of Foreign Assets Control (OFAC) in September 2023 do not require us to obtain any further licenses, other than those already in process. In addition, in June 2023, the EU agreed upon another round of sanctions against Russia that include, among other key elements, new targeted sanctions against individuals and entities, an expansion of the restrictions on the sale, export and transit of certain goods and technology and additional anti-circumvention measures. However, we were not required to obtain any additional licenses in response to these new EU sanctions, as we had previously obtained the requisite licenses from the applicable authorities in this region in response to sanctions previously imposed by the EU. To date, the challenges associated with the Russia-Ukraine War and related sanctions from the U.S., EU and elsewhere have not yet had a material impact on our operations.
Our revenues attributable to Russia, Ukraine and Belarus, in the aggregate, for 2023, 2022 and 2021 were approximately 3%, 4% and 4%, respectively, of our total revenues for such periods. In addition, we do not have any research or manufacturing facilities in Russia, Ukraine or Belarus. While we have been monitoring this conflict, and will continue to do so as this conflict continues to evolve, we are unable to predict the impact of this conflict on the Company’s business. See “Risk Factors—Risks Relating to the International Scope of our Business—As a result of the current conflict between Russia and Ukraine, the current and any future responses by the global community to such conflict and any counter responses by the Russian government or other entities or individuals, and the potential expansion of the conflict to other countries, we have experienced and may continue to experience an adverse impact on our business and operations in this region, as well as on our business and operations generally, which could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares to decline.”
Israel-Hamas Conflict
The conflict between Israel and Hamas began during October 2023. Our revenues attributable to Israel for 2023, 2022 and 2021 were less than 1% of our total revenues in each period. While we have been monitoring this conflict, and will continue to do so as this conflict continues to evolve, we are unable to predict the impact of this conflict on the Company’s business.
For a further discussion of these and other risks relating to our international business, see Item 1A. “Risk Factors" of this Form 10-K for additional information.
Supply Chain
As a result of global macroeconomic conditions, we have been experiencing certain supply chain challenges which have caused disruptions in availability and delays in shipping, which has led to challenges in meeting end market demand, primarily within our contact lens and surgical businesses.
The supply-chain challenges have impacted our revenues and resulting margins, despite our efforts to manage these impacts through strategic pricing actions and other initiatives. While we are unable to predict the duration and extent of these supply chain challenges, they could have an adverse impact on our results of operations. We will continue to monitor these supply chain challenges and are implementing actions to help mitigate these challenges, including strategically spot buying key components of inventory and securing multiple supply sources; however, we expect these supply constraints may build-up a higher cost of inventory and, therefore, put pressure on our future margins.
Global Minimum Corporate Tax Rate
On October 8, 2021, the Organisation for Economic Co-operation and Development (“OECD”)/G20 inclusive framework on Base Erosion and Profit Shifting (the “Inclusive Framework”) published a statement updating and finalizing the key components of a two-pillar plan on global tax reform originally agreed on July 1, 2021, and a timetable for implementation by 2023. The timetable for implementation has since been extended to 2024 or, with respect to certain components of the plan, to 2025. The Inclusive Framework plan has now been agreed to by 145 OECD members, including several countries which did not agree to the initial plan. Under pillar one, a portion of the residual profits of multinational businesses with global turnover above €20 billion and a profit margin above 10% will be allocated to market countries where such allocated profits would be taxed. Under pillar two, the Inclusive Framework has agreed on a global minimum corporate tax rate of 15% for companies with revenue above €750 million, calculated on a country-by-country basis. On October 30, 2021, the G20 formally endorsed the new global minimum corporate tax rate rules. The Inclusive Framework agreement must now be implemented by the OECD Members who have agreed to the plan, effective in 2024. Many members of the Inclusive Framework have either introduced or announced their intention to introduce certain components of the global minimum tax in line with the model rules for fiscal year beginning on or after December 31, 2023. For example, on December 15, 2022, the European Union member states unanimously adopted the directive to implement pillar two rules. According to the directive, the member states were expected to enact pillar two rules into domestic law in 2023, with certain elements becoming effective for fiscal years beginning on or after December 31, 2023. On August 4, 2023, Canada released draft legislation to enact certain components of the pillar two proposals into Canadian law as the Global Minimum Tax Act (“GMTA”). The GMTA is generally aligned with the model rules proposed by the OECD and is expected to become effective for fiscal years beginning on or after December 31, 2023. The United States did not announce plans to enact the tax measures under the two-pillar plan. On February 1, 2023, the U.S. Financial Accounting Standards Board indicated that they believe the minimum tax imposed under pillar two is an alternative minimum tax, and, accordingly, deferred tax assets and liabilities associated with the minimum tax would not be recognized or adjusted for the estimated future effects of the minimum tax but would be recognized in the period incurred. The OECD has published model rules and other guidance with respect to pillar two, which are generally consistent with the agreement reached by the Inclusive Framework in October 2021. On February 1, 2023, the Inclusive Framework released a package of technical and administrative guidance on the implementation of pillar two, including the scope of companies that will be subject to the Global Anti-Base Erosion Rules, transition rules, and guidance on domestic minimum taxes that countries may choose to adopt, among other topics. On December 18, 2023 the OECD announced plans to release additional guidance on model rules and to start the peer review process in 2024. Many jurisdictions in which the Company operates have adopted the global minimum tax provision of the OECD pillar two effective for tax years beginning in January 2024, and it is possible that the implementation of the Inclusive Framework agreement, including the global minimum corporate tax rate, could have a material effect on our liability for corporate taxes and our consolidated effective tax rate in the future.
Health Care Reform
The U.S. federal and state governments continue to propose and pass legislation designed to regulate the health care industry. Many of these changes focus on health care cost containment, which result in pricing pressures relating to the sales
and reimbursements of health care products. The Biden administration and Congress continue to focus on health care cost containment which could result in legislative and regulatory changes that may negatively impact our businesses.
In addition, we continue to face various proposed health care pricing changes and regulations from governments throughout the world in locations in which we operate our business. These proposed changes may also continue to result in pricing pressures relating to sales, promotions and reimbursement of our product portfolio.
We continually review newly enacted and proposed U.S. federal and state legislation, as well as proposed rulemaking and guidance published by the U.S. Department of Health and Human Services, the FDA and applicable foreign governments in locations in which we operate; however, at this time, it is unclear the effect these matters may have on our businesses.
For more information, see Item 1. “Business”.
Generic Competition and Loss of Exclusivity
Certain of our products face the expiration of their patent or regulatory exclusivity over the next five years, following which we anticipate generic competition of these products. Following a LOE of and/or generic competition for a product, we would anticipate that product sales for such product would decrease significantly shortly following the LOE or entry of a generic competitor. Where we have the rights, we may elect to launch an authorized generic (“AG”) of such product (either ourselves or through a third party) prior to, upon or following generic entry, which may mitigate the anticipated decrease in product sales.
Certain of our products have already been facing generic competition, such as Lotemax® Gel and Bepreve®, which began facing LOE in the U.S. during 2021 and in aggregate only accounted for less than 1% of our total revenues in 2021, and Prolensa®, which began facing LOE in the fourth quarter of 2023 and accounted for approximately 1% of our total revenues in 2023. While we expect our risk of LOE to be limited over the next five years, this could change based on, among other things, successful challenge to our patents, settlement of existing or future patent litigation and at-risk generic launches. We believe the entry into the market of generic competition generally would have an adverse impact on the volume and/or pricing of the affected products, however we are unable to predict the magnitude or timing of this impact.
In addition, in connection with our Lumify®, PreserVision®, Vyzulta® and Lotemax® SM products, we have commenced ongoing infringement proceedings against potential generic competitors or other potential infringers in the U.S. If we are not successful in these proceedings, we may face increased generic competition for these products.
In addition, the PreserVision® U.S. formulation patent expired in March 2021, but a patent covering methods of using the formulation remains in force into 2026. PreserVision® products accounted for approximately 7% and 7% of our total revenues in 2023 and 2022, respectively. PreserVision® is (or was) the subject of certain ongoing and past patent infringement proceedings. While the Company cannot predict the magnitude or timing of the impact from the PreserVision® patent expiry, this is an OTC product and thus, the impact is not expected to be as significant as the LOE of a branded pharmaceutical product.
See Note 20, “LEGAL PROCEEDINGS” to our audited Consolidated Financial Statements for further information.
RESULTS OF OPERATIONS
Our operating results for the years 2023, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change | | |
| (in millions) | 2023 | | 2022 | | 2021 | | 2022 to 2023 | | 2021 to 2022 | | | | | | |
| Revenues | | | | | | | | | | | | | | | |
| Product sales | $ | 4,131 | | | $ | 3,746 | | | $ | 3,737 | | | $ | 385 | | | $ | 9 | | | | | | | |
| Other revenues | 15 | | | 22 | | | 28 | | | (7) | | | (6) | | | | | | | |
| 4,146 | | | 3,768 | | | 3,765 | | | 378 | | | 3 | | | | | | | |
| Expenses | | | | | | | | | | | | | | | |
| Cost of goods sold (excluding amortization and impairments of intangible assets) (Note 3) | 1,640 | | | 1,511 | | | 1,458 | | | 129 | | | 53 | | | | | | | |
| Cost of other revenues | 2 | | | 8 | | | 9 | | | (6) | | | (1) | | | | | | | |
| Selling, general and administrative (Note 3) | 1,736 | | | 1,478 | | | 1,389 | | | 258 | | | 89 | | | | | | | |
| Research and development (Note 3) | 324 | | | 307 | | | 271 | | | 17 | | | 36 | | | | | | | |
| Amortization of intangible assets | 240 | | | 244 | | | 292 | | | (4) | | | (48) | | | | | | | |
| Other expense, net | 74 | | | 13 | | | 17 | | | 61 | | | (4) | | | | | | | |
| 4,016 | | | 3,561 | | | 3,436 | | | 455 | | | 125 | | | | | | | |
| Operating income | 130 | | | 207 | | | 329 | | | (77) | | | (122) | | | | | | | |
| Interest income | 15 | | | 6 | | | — | | | 9 | | | 6 | | | | | | | |
| Interest expense (Note 3) | (283) | | | (146) | | | — | | | (137) | | | (146) | | | | | | | |
| Foreign exchange and other | (28) | | | 6 | | | (11) | | | (34) | | | 17 | | | | | | | |
| (Loss) income before provision for income taxes | (166) | | | 73 | | | 318 | | | (239) | | | (245) | | | | | | | |
| Provision for income taxes | (82) | | | (58) | | | (125) | | | (24) | | | 67 | | | | | | | |
| Net (loss) income | (248) | | | 15 | | | 193 | | | (263) | | | (178) | | | | | | | |
| Net income attributable to noncontrolling interest | (12) | | | (9) | | | (11) | | | (3) | | | 2 | | | | | | | |
| Net (loss) income attributable to Bausch + Lomb Corporation | $ | (260) | | | $ | 6 | | | $ | 182 | | | $ | (266) | | | $ | (176) | | | | | | | |
A detailed discussion of the year-over-year changes of the Company’s 2023 results compared with that of 2022 can be found below. A detailed discussion of the year-over-year changes of the Company’s 2022 results compared with that of 2021 can be found under "Management’s Discussion and Analysis of Financial Condition and Results of Operations'' included in our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC and the CSA on February 22, 2023.
2023 Compared to 2022
Revenues
Our revenues are primarily generated from product sales in the therapeutic areas of eye health that consist of: (i) branded prescription eye-medications and pharmaceuticals, (ii) generic and branded generic prescription eye medications and pharmaceuticals, (iii) OTC vitamin and supplement products and (iv) medical devices (contact lenses, IOLs and ophthalmic surgical equipment). Other revenues include alliance and service revenue from the licensing and co-promotion of products and contract service revenue. Contract service revenue is derived primarily from contract manufacturing for third parties and is not material. See Note 22, “SEGMENT INFORMATION” to our audited Consolidated Financial Statements for the disaggregation of revenues which depicts how the nature, amount, timing and uncertainty of revenue and cash flows are affected by the economic factors of each category of customer contracts.
Our revenues were $4,146 million and $3,768 million for 2023 and 2022, respectively, an increase of $378 million, or 10%. The increase was attributable to: (i) an increase in volumes of $346 million across each of our segments and (ii) an increase in net realized pricing of $110 million, primarily driven by our Vision Care segment. The increases in revenue were partially offset by: (i) the unfavorable impact of foreign currencies across all of our international businesses of $68 million, primarily in Europe and Asia and (ii) the impact of divestitures and discontinuations of $10 million, related to the discontinuation of certain products within our Surgical and Vision Care segments.
The following table presents segment revenues, segment revenues as a percentage of total revenues and the period-over-period changes in segment revenues for 2023 and 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2023 | | 2022 | | Change |
| (in millions) | | Amount | | Pct. | | Amount | | Pct. | | Amount | | Pct. |
| Segment Revenues | | | | | | | | | | | | |
| Vision Care | | $ | 2,543 | | | 61 | % | | $ | 2,369 | | | 63 | % | | $ | 174 | | | 7 | % |
| Pharmaceuticals | | 836 | | | 20 | % | | 681 | | | 18 | % | | 155 | | | 23 | % |
| Surgical | | 767 | | | 19 | % | | 718 | | | 19 | % | | 49 | | | 7 | % |
| Total revenues | | $ | 4,146 | | | 100 | % | | $ | 3,768 | | | 100 | % | | $ | 378 | | | 10 | % |
Beginning in the first quarter of 2023, certain products historically included in the reported results of the Pharmaceuticals segment are now included in the reported results of the Vision Care segment and certain products included in the reported results of the Vision Care segment are now included in the reported results of the Pharmaceuticals segment. The net impact of these product movements were not material to the periods presented. Prior period presentations of segment revenues and profits have been conformed to the current segment reporting structure. See Note 22, “SEGMENT INFORMATION” to our audited Consolidated Financial Statements for additional information regarding these reportable segments.
Constant Currency Revenues and Constant Currency Revenue Growth (non-GAAP)
Constant Currency Revenue Growth, a non-GAAP measure, is defined as a change in Revenues (its most directly comparable GAAP financial measure) on a period-over-period basis adjusted for changes in foreign currency exchange rates (if applicable). The Company uses Constant Currency Revenues (non-GAAP) and Constant Currency Revenue Growth (non-GAAP) to assess performance of its reportable segments, and the Company in total, without the impact of foreign currency exchange fluctuations. The Company believes that such measures are useful to investors as they provide a supplemental period-to-period comparison.
Although changes in foreign currency exchange rates are part of our business, they are not within management’s control. Changes in foreign currency exchange rates, however, can mask positive or negative trends in the underlying business performance. The impact for changes in foreign currency exchange rates is determined as the difference in the current period reported revenues at their current period currency exchange rates and the current period reported revenues revalued using the monthly average currency exchange rates during the comparable prior period.
Non-GAAP financial measures and non-GAAP ratios are not prepared in accordance with GAAP nor do they have any standardized meaning under GAAP. In addition, other companies may use similarly titled non-GAAP financial measures and ratios that are calculated differently from the way we calculate such measures and ratios. Accordingly, the Company’s non-GAAP financial measures and ratios may not be comparable to such similarly titled non-GAAP financial measures and ratios used by other companies.
The following table presents a reconciliation of Revenues to constant currency revenues (non-GAAP) and the period-over-period changes in constant currency revenue (non-GAAP) for 2023 and 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | Change in
Constant Currency Revenue (Non-GAAP) |
| | Revenue
as
Reported | | Changes in Exchange Rates | | Constant Currency Revenue
(Non-GAAP) | | Revenue
as
Reported | | |
| (in millions) | | Amount | | Pct. |
| Vision Care | | $ | 2,543 | | | $ | 61 | | | $ | 2,604 | | | $ | 2,369 | | | | $ | 235 | | | 10 | % |
| Pharmaceuticals | | 836 | | | 6 | | | 842 | | | 681 | | | | 161 | | | 24 | % |
| Surgical | | 767 | | | 1 | | | 768 | | | 718 | | | | 50 | | | 7 | % |
| Total | | $ | 4,146 | | | $ | 68 | | | $ | 4,214 | | | $ | 3,768 | | | | $ | 446 | | | 12 | % |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Vision Care Segment Revenue
The Vision Care segment revenue was $2,543 million and $2,369 million for 2023 and 2022, respectively, an increase of $174 million, or 7%. The increase was driven by: (i) an increase in volumes of $133 million and (ii) an increase in net pricing of $105 million. The increases in volumes and pricing were primarily driven by higher sales from our Lumify®, dry eye, and eye vitamin product portfolios within our consumer eye care business and SiHy Daily lenses and Ultra® within our contact lens business. These increases were partially offset by: (i) the unfavorable impact of foreign currencies of $61 million, primarily in Asia and Europe and (ii) the impact of divestitures and discontinuations of $3 million, driven by the discontinuation of certain products.
Our change in volumes for 2023 within our contact lens business was unfavorably impacted by unfulfilled orders at our Lynchburg distribution facility. During the second quarter of 2023, we put into place a system upgrade; however, we incurred disruptions during the implementation of this upgrade, which resulted in the slower than normal processing of certain orders, thereby negatively impacting our revenues for 2023. We expect to substantially resolve the Lynchburg implementation disruptions during the first quarter of 2024.
Pharmaceuticals Segment Revenue
The Pharmaceuticals segment revenue was $836 million and $681 million for 2023 and 2022, respectively, an increase of $155 million, or 23%. The increase was primarily driven by: (i) incremental sales of $106 million, driven by the XIIDRA Acquisition and (ii) an increase in volumes of $61 million, primarily driven by opportunities from competitor supply issues within our generics business, the launch of MIEBO® and increased demand for Vyzulta®. These increases were partially offset by: (i) the unfavorable impact of foreign currencies of $6 million, primarily in Asia and (ii) a decrease in net realized pricing of $5 million.
Surgical Segment Revenue
The Surgical segment revenue was $767 million and $718 million for 2023 and 2022, respectively, an increase of $49 million, or 7%. The increase was driven by: (i) an increase in volumes of $36 million, primarily due to higher sales of consumables, equipment and premium IOLs, (ii) an increase in net realized pricing of $10 million primarily driven by strategic pricing increases taken in January 2023 across certain products and (iii) incremental sales attributable to acquisitions of $10 million. These increases were partially offset by: (i) the impact of divestitures and discontinuations of $6 million, related to the discontinuation of certain products and (ii) the unfavorable effect of foreign currencies of $1 million, primarily in Asia.
Cash Discounts and Allowances, Chargebacks and Distribution Fees
As is customary in the health care industry, gross product sales are subject to a variety of deductions in arriving at net product sales. Provisions for these deductions are recognized concurrently with the recognition of gross product sales. These provisions include cash discounts and allowances, chargebacks and distribution fees, which are paid or credited to direct customers, as well as rebates and returns, which can be paid or credited to direct and indirect customers. Provision balances relating to amounts payable to direct customers are netted against trade receivables and balances relating to indirect customers are included in accrued liabilities.
We actively manage these offerings, focusing on the incremental costs of our patient assistance programs, the level of discounting to non-retail accounts and identifying opportunities to minimize product returns. We also concentrate on managing our relationships with our payors and wholesalers, reviewing the ranges of our offerings and being disciplined as to the amount and type of incentives we negotiate. Provisions recorded to reduce gross product sales to net product sales and revenues for 2023 and 2022 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2023 | | 2022 |
| (in millions) | | Amount | | Pct. | | Amount | | Pct. |
| Gross product sales | | $ | 5,899 | | | 100.0 | % | | $ | 5,122 | | | 100.0 | % |
| Provisions to reduce gross product sales to net product sales | | | | | | | | |
| Discounts and allowances | | 368 | | | 6.20 | % | | 315 | | | 6.20 | % |
| Returns | | 84 | | | 1.40 | % | | 69 | | | 1.40 | % |
| Rebates | | 729 | | | 12.40 | % | | 528 | | | 10.30 | % |
| Chargebacks | | 559 | | | 9.50 | % | | 442 | | | 8.60 | % |
| Distribution fees | | 28 | | | 0.50 | % | | 22 | | | 0.40 | % |
| Total provisions | | 1,768 | | | 30.00 | % | | 1,376 | | | 26.90 | % |
| Net product sales | | 4,131 | | | 70.00 | % | | 3,746 | | | 73.10 | % |
| Other revenues | | 15 | | | | | 22 | | | |
| Revenues | | $ | 4,146 | | | | | $ | 3,768 | | | |
Cash discounts and allowances, returns, rebates, chargebacks and distribution fees as a percentage of gross product sales were 30.0% and 26.9% for 2023 and 2022, respectively, an increase of 3.1% percentage points, and is primarily attributable to increases in rebates and chargebacks as a percentage of revenues. The increase in rebates is primarily attributable to XIIDRA®. The increase in chargebacks is primarily attributable to our generics portfolio as a result of changes in product and customer mix, as well as growth in volume.
Operating Expenses
Cost of Goods Sold (exclusive of amortization and impairments of intangible assets)
Cost of goods sold primarily includes: manufacturing and packaging; the cost of products we purchase from third parties; royalty payments we make to third parties; depreciation of manufacturing facilities and equipment; and lower of cost or market adjustments to inventories. Cost of goods sold typically vary between periods as a result of product mix, volume, royalties, changes in foreign currency and inflation. Cost of goods sold excludes the amortization and impairments of intangible assets.
Cost of goods sold was $1,640 million and $1,511 million for 2023 and 2022, respectively, an increase of $129 million, or 9%. The increase was primarily driven by: (i) higher volumes, (ii) inflationary pressures, (iii) supply shortages resulting in increased costs, most notably in our Surgical products, (iv) higher manufacturing efficiency ramp-up costs of our Daily SiHy lenses during the first half of 2023 and (v) costs of sales associated with acquisitions entered into in 2023, which includes the amortization of an interim contract and inventory step-up. These increases were partially offset by the favorable impact of foreign currencies.
Contribution (product sales revenue less cost of goods sold, exclusive of amortization and impairments of intangible assets) increased by $256 million, primarily driven by: (i) the increase in net realized pricing and volumes, as previously discussed and (ii) contribution associated with acquisitions entered into in the current year, partially offset by: (i) the increase in cost of goods sold due to inflation and supply shortages, (ii) the Lynchburg system implementation disruptions (as further described above) and (iii) the unfavorable impact of foreign currencies.
Cost of goods sold as a percentage of Product sales was 39.7% and 40.3% for 2023 and 2022, respectively, a decrease of 0.6%, as the increased pricing and contribution from XIIDRA® was mostly able to offset the higher Cost of goods sold, as discussed above.
Selling, General and Administrative Expenses
Selling, general and administrative ("SG&A") expenses primarily include: employee compensation associated with sales and marketing, finance, legal, information technology, human resources and other administrative functions; certain outside legal fees and consultancy costs; product promotion expenses; overhead and occupancy costs; depreciation of corporate facilities and equipment; and other general and administrative costs.
SG&A expenses were $1,736 million and $1,478 million for 2023 and 2022, respectively, an increase of $258 million, or 17%. The increase was primarily attributable to: (i) higher selling and advertising and promotion costs, primarily due to XIIDRA® and the launch of MIEBO®, (ii) higher professional fees, primarily related to Business Transformation Costs (as defined below), (iii) higher compensation expenses, primarily related to dis-synergy costs associated with the Company
becoming a stand-alone entity and (iv) higher selling expense due to warehousing and distribution costs, mostly driven by inflation. These increases in SG&A expenses were partially offset by the favorable impact of foreign currencies.
As a result of the completion of the B+L IPO, and as the Company prepares for post-Separation operations, the Company is launching certain initiatives that may result in certain changes to, and investment in, its organizational structure and operations. The Company refers to the charges related to these initiatives as "Business Transformation Costs". These costs are recorded in SG&A in the audited Consolidated Statements of Operations and include third-party advisory costs, as well as certain compensation-related costs associated with changes in the Company's executive officers, such as severance-related costs associated with the departure of the Company's former executives and the costs associated with the appointment of the Company's new executives.
Research and Development Expenses
Included in R&D are costs related to our product development and quality assurance programs. Expenses related to product development include: employee compensation costs; overhead and occupancy costs; depreciation of research and development facilities and equipment; clinical trial costs; clinical manufacturing and scale-up costs; and other third-party development costs. Quality assurance are the costs incurred to meet evolving customer and regulatory standards and include: employee compensation costs; overhead and occupancy costs; amortization of software; and other third-party costs.
R&D expenses were $324 million and $307 million for 2023 and 2022, respectively, an increase of $17 million, or 6%, primarily due to certain products in development, as previously discussed. R&D expenses as a percentage of Product sales were approximately 7.8% and 8.2% for 2023 and 2022, respectively.
Amortization of Intangible Assets
Intangible assets with finite lives are amortized using the straight-line method over their estimated useful lives, generally 1 to 17 years. Management continually assesses the useful lives related to our long-lived assets to reflect the most current assumptions.
Amortization of Intangible assets was $240 million and $244 million for 2023 and 2022, respectively, a decrease of $4 million, or 2%, primarily due to fully amortized intangible assets no longer being amortized in 2023, partially offset by assets acquired through acquisitions, as previously discussed.
See Note 4, “ACQUISITIONS AND LICENSING AGREEMENTS” to our audited Consolidated Financial Statements for further details related to intangible assets acquired.
Other expense, net
Other expense, net for 2023 and 2022 consists of the following:
| | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 |
| Asset impairments | | $ | — | | | $ | 1 | |
| Restructuring, integration and separation costs | | 44 | | | 14 | |
| Litigation and other matters | | 3 | | | 1 | |
| Acquired in-process research and development costs | | — | | | 1 | |
| Acquisition-related costs | | 25 | | | 1 | |
| Acquisition-related contingent consideration | | 2 | | | (5) | |
| Other, net | | — | | | — | |
Other expense, net | | $ | 74 | | | $ | 13 | |
The Company evaluates opportunities to improve its operating results and implements cost savings programs to streamline its operations and eliminate redundant processes and expenses. Restructuring and integration costs are expenses associated with the implementation of cost savings programs and include expenses associated with reducing headcount and other cost reduction initiatives. See Note 16, “OTHER EXPENSE, NET” to our audited Consolidated Financial Statements for further details related to Restructuring, integration and separation costs.
Acquisition-related costs for 2023, primarily include transaction costs attributable to the XIIDRA Acquisition.
Operating Income
Operating income for 2023 and 2022 was $130 million and $207 million, respectively, a decrease of $77 million, or 37%, and primarily reflects the increase in SG&A and Other expense, partially offset by the increase in contribution, each as previously discussed.
Segment Profit
Segment profit is based on operating income after the elimination of intercompany transactions. Certain costs, such as Amortization of intangible assets and Other expense, net, are not included in the measure of segment profit, as management excludes these items in assessing segment financial performance. Segment profit is a measure of operating performance of our reportable segments and may not be comparable to similar measures reported by other companies. Segment profit is a performance metric utilized by the Company’s CEO, who is the Company’s Chief Operating Decision Maker, to allocate resources to and assess performance of the Company’s segments. See Note 22, “SEGMENT INFORMATION” to our audited Consolidated Financial Statements for a reconciliation of segment profit to Income before provision for income taxes.
The following table presents segment profits, segment profits as a percentage of segment revenues and the period-over-period changes in segment profits for 2023 and 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2023 | | 2022 | | Change |
| (in millions) | | Amount | | Pct. | | Amount | | Pct. | | Amount | | Pct. |
| Segment Profits / Segment Profit Margins | | | | | | | | | | | | |
| Vision Care | | $ | 689 | | | 27 | % | | $ | 636 | | | 27 | % | | $ | 53 | | | 8 | % |
| Pharmaceuticals | | 241 | | | 29 | % | | 203 | | | 30 | % | | 38 | | | 19 | % |
| Surgical | | 50 | | | 7 | % | | 42 | | | 6 | % | | 8 | | | 19 | % |
| | | | | | | | | | | | |
Vision Care Segment Profit
The Vision Care segment profit was $689 million and $636 million for 2023 and 2022, respectively, an increase of $53 million, or 8%. The increase was primarily driven by the increase in contribution, driven by the increase in revenues, as previously discussed. These increases in contribution were partially offset by: (i) higher selling expenses across each of our businesses, primarily attributable to increased distribution costs and (ii) higher advertising and promotional expenses driven by our Consumer business, primarily related to the launch of Lumify Eye IlluminationsTM.
Pharmaceuticals Segment Profit
The Pharmaceuticals segment profit was $241 million and $203 million for 2023 and 2022, respectively, an increase of $38 million, or 19%. The increase was primarily driven by increased contribution, driven by: (i) the XIIDRA Acquisition and
(ii) the increase in sales volume, as previously discussed. This increase was partially offset by selling and advertising and promotional expenses related to XIIDRA® and as a result of increased spend related to the launch of MIEBO®.
Surgical Segment Profit
The Surgical segment profit was $50 million and $42 million for 2023 and 2022, respectively, an increase of $8 million, or 19%. The increase was primarily driven by the increase in revenues, as previously discussed, partially offset by higher G&A and selling expenses, including higher warehousing and distribution costs.
Non-Operating Income and Expense
Interest Expense
Interest expense primarily consists of interest payments due, amortization of debt discounts and deferred issuance costs on indebtedness under our credit facilities.
Interest expense was $283 million and $146 million for 2023 and 2022, respectively, an increase of $137 million. The increase is primarily attributable to: (i) increased interest expense associated with the May 2027 Term Facility and interest expense related to our September 2028 Term Facility and October 2028 Secured Notes (each as defined and discussed in further detail, under Item “— Liquidity and Capital Resources — Liquidity and Debt — Long-term Debt”), (ii) certain upfront financing commitment costs incurred in connection with the XIIDRA Acquisition and (iii) interest expense related to the outstanding balance under our Revolving Credit Facility (as defined and discussed in further detail, under Item “— Liquidity and Capital Resources — Liquidity and Debt — Long-term Debt”). See Note 10, “FINANCING ARRANGEMENTS” to our audited Consolidated Financial Statements for further details regarding the October 2028 Secured Notes, the May 2027 Term Facility and the Revolving Credit Facility. For the year 2022, interest expense also included $47 million of interest attributed to the BHC Purchase Debt (as defined below).
On January 1, 2022, in anticipation of the B+L IPO, Bausch + Lomb issued a $2,200 million promissory note to BHC (the “BHC Purchase Debt”) in conjunction with a legal reorganization. The BHC Purchase Debt was repaid in full on May 10, 2022. See Note 3, “RELATED PARTIES” to our audited Consolidated Financial Statements for further details.
Foreign Exchange and Other
Foreign exchange and other primarily includes translation gains/losses on intercompany balances and third-party liabilities and the gain/loss due to the change in fair value of foreign currency exchange contracts. Foreign exchange and other was a net loss of $28 million and a gain of $6 million for 2023 and 2022, respectively.
Income Taxes
Provision for income taxes were $82 million and $58 million for 2023 and 2022, respectively, an increase of $24 million. The increase in income taxes was primarily related to: (i) a change in the jurisdictional mix of earnings and (ii) discrete tax effects of: (a) the valuation allowance established in Canada, (b) the reduction of certain tax attributes (c) the filings of certain tax returns and (d) disallowed interest deductions.
See Note 17, “INCOME TAXES” to our audited Consolidated Financial Statements for further details.
Net (loss) income attributable to Bausch + Lomb Corporation
Net loss attributable to Bausch + Lomb for 2023 was $260 million, as compared to Net income attributable to Bausch + Lomb for 2022 of $6 million, a decrease in our results of $266 million and was primarily due to: (i) an increase in interest expense of $137 million, (ii) the decrease in our operating results of $77 million, (iii) the unfavorable net change in Foreign exchange and other of $34 million and (iv) the increase in the Provision for income taxes of $24 million, each as previously discussed.
LIQUIDITY AND CAPITAL RESOURCES
Cash Flows
Summarized cash flow information for the years 2023, 2022 and 2021 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | Change |
| (in millions) | | 2023 | | 2022 | | 2021 | | 2022 to 2023 | | 2021 to 2022 |
| Net cash (used in) provided by operating activities | | $ | (17) | | | $ | 345 | | | $ | 873 | | | $ | (362) | | | $ | (528) | |
| Net cash used in investing activities | | (2,109) | | | (215) | | | (214) | | | (1,894) | | | (1) | |
| Net cash provided by (used in) financing activities | | 2,078 | | | 81 | | | (712) | | | 1,997 | | | 793 | |
| Effect of exchange rate changes on cash and cash equivalents and restricted cash | | 2 | | | (8) | | | (8) | | | 10 | | | — | |
| Net (decrease) increase in cash and cash equivalents and restricted cash | | (46) | | | 203 | | | (61) | | | (249) | | | 264 | |
| Cash and cash equivalents and restricted cash, beginning of period | | 380 | | | 177 | | | 238 | | | 203 | | | (61) | |
| Cash and cash equivalents and restricted cash, end of period | | $ | 334 | | | $ | 380 | | | $ | 177 | | | $ | (46) | | | $ | 203 | |
A detailed discussion of the year-over-year changes of the Company’s 2023 results compared with that of 2022 can be found below. A detailed discussion of the year-over-year changes of the Company’s 2022 results compared with that of 2021 can be found under "Management’s Discussion and Analysis of Financial Condition and Results of Operations'' included in our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC and the CSA on February 22, 2023.
Operating Activities
Net cash used in operating activities was $17 million for 2023, as compared to net cash provided by operating activities of $345 million for 2022, a decrease of $362 million. The decrease is primarily attributable to: (i) costs incurred in 2023 as a stand-alone entity, such as higher SG&A expense and increased interest payments, each as previously discussed and (ii) the change in our operating assets and liabilities, due to: (a) a strategic increase in inventories, (b) the timing of payments in the ordinary course of business and (c) growth in sales volumes, as previously discussed, which drove an increase in our trade receivables.
Investing Activities
Net cash used in investing activities was $2,109 million and $215 million for 2023 and 2022, respectively, an increase of $1,894 million and was primarily driven by payments related to acquisitions and other investments of $1,941 million primarily related to the XIIDRA Acquisition, the acquisition of the Blink® Product Line and the acquisition of AcuFocus, each as previously discussed, as well as a $45 million milestone payment related to the FDA's approval of the NDA for MIEBO®. This increase was partially offset by acquisition payments made in 2022 of $45 million related to the acquisitions of Total Titanium Inc. and Paragon BioTeck, Inc. and our agreement with Sanoculis, each as previously discussed.
Financing Activities
Net cash provided by financing activities was $2,078 million and $81 million for 2023 and 2022, respectively, an increase of $1,997 million. The increase is primarily attributable to the issuance of long-term debt, net of $2,276 million, related to the September 2028 Term Facility and the October 2028 Secured Notes and borrowings under the Revolving Credit Facility (each as defined below), during 2023, partially offset by repayments of debt of $161 million.
During 2022, Net cash provided by financing activities was driven by the issuance of long-term debt, net of $2,440 million, related to the issuance of the May 2027 Term Facility (defined below), partially offset by intercompany transactions between Bausch + Lomb and our parent company, BHC, which included Net transfers to BHC of $2,363 million. For further details regarding Net transfers to BHC, see Note 3, “RELATED PARTIES” to our audited Consolidated Financial Statements.
Liquidity and Debt
Future Sources of Liquidity
Our primary sources of liquidity are expected to be our cash and cash equivalents, cash collected from customers, funds as needed from our Revolving Credit Facility, and issuances of other long-term debt, additional equity and equity-linked
securities. We believe these sources will be sufficient to meet our current liquidity needs for the next twelve months, from the date of issuance of these financial statements, and be sufficient to support our future cash needs, however, we can provide no assurance that our liquidity and capital resources will meet future funding requirements.
The global financial markets recently have undergone and may continue to experience significant volatility and disruption. The timing and sustainability of an economic recovery is uncertain and additional macroeconomic, business and financial disruptions may arise. As markets change, there can be no assurance that the challenging economic environment or a further economic downturn would not impact our liquidity or our ability to obtain future financing on reasonable terms or at all.
We will regularly evaluate market conditions, our liquidity profile, and various financing alternatives for opportunities to enhance our capital structure. If opportunities are favorable, we may from time to time enter into new financing arrangements, refinance the Credit Facilities (as defined below) or repurchase debt, or issue additional equity and equity-linked securities.
Long-term Debt
Prior to the B+L IPO, we participated in BHC’s cash management arrangements, and generally all of our excess cash was transferred to BHC periodically. Cash disbursements for operations and/or investing activities were funded as needed by BHC. Cash and cash equivalents and restricted cash as presented in our audited Consolidated Financial Statements are amounts recorded on legal entities dedicated to Bausch + Lomb.
On May 10, 2022, in connection with the B+L IPO and in order to properly capitalize our business, Bausch + Lomb entered into a credit agreement (the “Credit Agreement”, and the credit facilities thereunder, the “Credit Facilities”). Prior to the September 2023 Credit Facility Amendment (as defined below), the Credit Agreement provided for a term loan of $2,500 million with a five-year term to maturity (the “May 2027 Term Facility”) and a five-year revolving credit facility of $500 million (the “Revolving Credit Facility”).
On September 29, 2023, Bausch + Lomb entered into an incremental term loan facility secured on a pari passu basis with the Company’s existing May 2027 Term Facility. This incremental term loan facility was entered into in the form of an incremental amendment (the "September 2023 Credit Facility Amendment") to the Company’s existing Credit Agreement (the Credit Agreement, as amended by the September 2023 Credit Facility Amendment, the “Amended Credit Agreement”) and consisted of borrowings of $500 million in new term B loans with a five-year term to maturity (the "September 2028 Term Facility" and, together with the May 2027 Term Facility and the Revolving Credit Facility, the “Senior Secured Credit Facilities”). A portion of the proceeds from the September 2028 Term Facility and October 2028 Secured Notes were used to finance the $1,750 million upfront payment related to the XIIDRA Acquisition and related acquisition and financing costs.
The Senior Secured Credit Facilities are secured by substantially all of the assets of Bausch + Lomb and its material, wholly-owned Canadian, U.S., Dutch and Irish subsidiaries, subject to certain exceptions. The May 2027 Term Facility and September 2028 Term Facility are denominated in U.S. dollars, and borrowings under the Revolving Credit Facility may be made available in U.S. dollars, euros, pounds sterling and Canadian dollars. As of December 31, 2023, the principal amounts outstanding under the May 2027 Term Facility and September 2028 Term Facility were $2,462 million and $499 million, respectively. As of December 31, 2023, the Company had $275 million of outstanding borrowings, $26 million of issued and outstanding letters of credit and remaining availability, subject to certain customary conditions, of $199 million under its Revolving Credit Facility.
Prior to November 29, 2022, Bausch + Lomb was a restricted subsidiary under the credit agreement of BHC (the "BHC Credit Agreement") and the senior notes indentures of BHC and Bausch Health Americas, Inc. (collectively the "BHC Indentures"), which meant that although neither we nor our subsidiaries were guarantors of BHC debt, our status as a restricted subsidiary meant that our ability to take certain actions, including the incurrence of debt, was restricted by the terms of the BHC Credit Agreement and BHC Indentures. On November 29, 2022, BHC designated Bausch + Lomb as an unrestricted subsidiary under the BHC Credit Agreement and the BHC Indentures. Following such designation, we are no longer restricted by the terms of the BHC Credit Agreement or BHC Indentures.
Description of Credit Facilities
Borrowings under the Revolving Credit Facility in: (i) U.S. dollars bear interest at a rate per annum equal to, at our option, either (a) a term Secured Overnight Financing Rate ("SOFR")-based rate or (b) a U.S. dollar base rate, (ii) Canadian dollars bear interest at a rate per annum equal to, at our option, either (a) Canadian Dollar Offered Rate ("CDOR") or (b) a Canadian dollar prime rate, (iii) euros bear interest at a rate per annum equal to EURIBOR and (iv) pounds sterling bear interest at a rate per annum equal to Sterling Overnight Index Average ("SONIA") (provided, however, that the term SOFR-based rate, CDOR, EURIBOR and SONIA shall be no less than 0.00% per annum at any time and the U.S. dollar base rate and the Canadian dollar prime rate shall be no less than 1.00% per annum at any time), in each case, plus an applicable margin. Term SOFR-based borrowings under the Revolving Credit Facility are subject to a credit spread adjustment of 0.10%.
The applicable interest rate margins for borrowings under the Revolving Credit Facility are (i) between 0.75% to 1.75% with respect to U.S. dollar base rate or Canadian dollar prime rate borrowings and between 1.75% to 2.75% with respect to SOFR, EURIBOR, SONIA or CDOR borrowings based on the Company’s total net leverage ratio and (ii) after (x) Bausch + Lomb’s senior unsecured non-credit-enhanced long-term indebtedness for borrowed money receives an investment grade rating from at least two of Standard & Poor’s (“S&P”), Moody’s and Fitch and (y) the May 2027 Term Facility and September 2028 Term Facility have been repaid in full in cash (the “IG Trigger”), between 0.015% to 0.475% with respect to U.S. dollar base rate or Canadian dollar prime rate borrowings and between 1.015% to 1.475% with respect to SOFR, EURIBOR, SONIA or CDOR borrowings based on the Company’s debt rating. The stated rate of interest for borrowings under the Revolving Credit Facility at December 31, 2023 ranges from 8.19% to 8.21% per annum. In addition, we are required to pay commitment fees of 0.25% per annum in respect of the unutilized commitments under the Revolving Credit Facility, payable quarterly in arrears until the IG Trigger and, thereafter, a facility fee between 0.110% to 0.275% of the total revolving commitments, whether used or unused, based on the Company’s debt rating and payable quarterly in arrears. We are also required to pay letter of credit fees on the maximum amount available to be drawn under all outstanding letters of credit in an amount equal to the applicable margin on SOFR borrowings under the Revolving Credit Facility on a per annum basis, payable quarterly in arrears, as well as customary fronting fees for the issuance of letters of credit and agency fees.
Borrowings under the May 2027 Term Facility bear interest at a rate per annum equal to, at our option, either: (i) a term SOFR-based rate, plus an applicable margin of 3.25% or (ii) a U.S. dollar base rate, plus an applicable margin of 2.25% (provided, however, that the term SOFR-based rate shall be no less than 0.50% per annum at any time and the U.S. dollar base rate shall not be lower than 1.50% per annum at any time). Term SOFR-based borrowings under the May 2027 Term Facility are subject to a credit spread adjustment of 0.10%. The stated rate of interest under the May 2027 Term Facility at December 31, 2023 was 8.71% per annum.
Borrowings under the September 2028 Term Facility bear interest at a rate per annum equal to, at our option, either: (i) a term SOFR-based rate, plus an applicable margin of 4.00%, or (ii) a U.S. dollar base rate, plus an applicable margin of 3.00% (provided, however, that the term SOFR-based rate shall be no less than 0.00% per annum at any time and the U.S. dollar base rate shall not be lower than 1.00% per annum at any time). Term SOFR-based borrowings under the September 2028 Term Facility are not subject to any credit spread adjustment. The stated rate of interest under the September 2028 Term Facility at December 31, 2023 was 9.36% per annum.
Subject to certain exceptions and customary baskets set forth in the Amended Credit Agreement, Bausch + Lomb is required to make mandatory prepayments of the loans under the May 2027 Term Facility and September 2028 Term Facility under certain circumstances, including from: (i) 100% of the net cash proceeds of insurance and condemnation proceeds for property or asset losses (subject to reinvestment rights, decrease based on leverage ratios and net proceeds threshold), (ii) 100% of the net cash proceeds from the incurrence of debt (other than permitted debt as described in the Amended Credit Agreement), (iii) 50% of Excess Cash Flow (as defined in the Amended Credit Agreement) subject to decrease based on leverage ratios and subject to a threshold amount and (iv) 100% of net cash proceeds from asset sales (subject to reinvestment rights, decrease based on leverage ratios and net proceeds threshold). These mandatory prepayments may be used to satisfy future amortization.
The amortization rate for the May 2027 Term Facility is 1.00% per annum, or $25 million, payable in quarterly installments, and the first installment was paid on September 30, 2022. Bausch + Lomb may direct that prepayments be
applied to such amortization payments in order of maturity. As of December 31, 2023, the remaining mandatory quarterly amortization payments for the May 2027 Term Facility were $81 million through March 2027, with the remaining term loan balance being due in May 2027.
The amortization rate for the September 2028 Term Facility is 1.00% per annum, or $5 million, payable in quarterly installments. Bausch + Lomb may direct that prepayments be applied to such amortization payments in order of maturity. As of December 31, 2023, the remaining mandatory quarterly amortization payments for the September 2028 Term Facility were $23 million through June 2028, with the remaining term loan balance being due in September 2028.
Description of Senior Secured Notes
On September 29, 2023, Bausch + Lomb issued $1,400 million aggregate principal amount of 8.375% Senior Secured Notes due October 2028 (the "October 2028 Secured Notes"). A portion of the proceeds from the October 2028 Secured Notes, along with the proceeds of September 2028 Term Facility, were used to finance the $1,750 million upfront payment related to the acquisition of XIIDRA® and certain other ophthalmology assets from Novartis and related acquisition-related transaction and financing costs. The October 2028 Secured Notes accrue interest at a rate of 8.375% per year, payable semi-annually in arrears on each April 1 and October 1, commencing on April 1, 2024.
The October 2028 Secured Notes are guaranteed by each of the Company’s subsidiaries that is a guarantor under the Amended Credit Agreement (the “Note Guarantors”). The October 2028 Secured Notes and the guarantees related thereto are senior obligations and are secured, subject to permitted liens and certain other exceptions, by the same first priority liens that secure the Company’s obligations under the Amended Credit Agreement under the terms of the indenture governing the October 2028 Secured Notes.
The October 2028 Secured Notes and the guarantees related thereto rank equally in right of repayment with all of the Company’s and Note Guarantors’ respective existing and future unsubordinated indebtedness and senior to the Company’s and Note Guarantors’ respective future subordinated indebtedness. The October 2028 Secured Notes and the guarantees related thereto are effectively pari passu with the Company’s and the Note Guarantors’ respective existing and future indebtedness secured by a first priority lien on the collateral securing the October 2028 Secured Notes and effectively senior to the Company’s and the Note Guarantors’ respective existing and future indebtedness that is unsecured, or that is secured by junior liens, in each case to the extent of the value of the collateral. In addition, the October 2028 Secured Notes are structurally subordinated to: (i) all liabilities of any of the Company’s subsidiaries that do not guarantee the October 2028 Secured Notes and (ii) any of the Company’s debt that is secured by assets that are not collateral for the October 2028 Secured Notes.
Upon the occurrence of a change in control (as defined in the indenture governing the October 2028 Secured Notes), unless the Company has exercised its right to redeem all of the notes of a series, holders of the October 2028 Secured Notes may require the Company to repurchase such holders' notes, in whole or in part, at a purchase price equal to 101% of the principal amount thereof plus accrued and unpaid interest, but not including, the date of redemption.
The October 2028 Secured Notes are redeemable at the option of the Company, in whole or in part, at any time on or after October 1, 2025, at the redemption prices set forth in the indenture. Prior to October 1, 2025, the Company may redeem the October 2028 Secured Notes in whole or in part at a redemption price equal to the principal amount of the Notes redeemed plus a make-whole premium. Prior to October 1, 2025, the Company may on any one or more occasions redeem up to 40% of the aggregate principal amount of the October 2028 Secured Notes at a redemption price of 108.375% of the principal amount thereof, redeemed plus accrued and unpaid interest to, but not including, the date of redemption with the proceeds of one of more equity offerings.
Weighted Average Stated Rate of Interest
The weighted average stated rate of interest for the Company’s outstanding debt obligations as of December 31, 2023 and December 31, 2022 was 8.65% and 7.84%, respectively.
Credit Ratings
As of the date of this filing, February 21, 2024, the credit ratings and outlook from Moody’s, S&P and Fitch for certain outstanding obligations of Bausch + Lomb were as follows:
| | | | | | | | | | | | | | | | | | | | |
| Rating Agency | | Corporate Rating | | Senior Secured Rating | | Outlook |
| Moody’s | | | | B1 | | Negative |
| Standard & Poor’s | | B- | | B- | | Positive |
| Fitch | | B- | | BB- | | Rating Watch Evolving |
Any downgrade in our corporate credit ratings or senior secured ratings may increase our cost of borrowing and may negatively impact our ability to raise additional debt capital.
Upon full Separation, we expect to refinance the Bausch + Lomb debt, and to transition to a longer-term capital structure.
OFF-BALANCE SHEET ARRANGEMENTS AND CONTRACTUAL OBLIGATIONS
We have no off-balance sheet arrangements that have a material current effect or that are reasonably likely to have a material future effect on our results of operations, financial condition, capital expenditures, liquidity, or capital resources.
Other Future Cash Requirements
Our other future cash requirements relate to working capital, capital expenditures, business development transactions (contingent consideration), restructuring and integration, benefit obligations and litigation settlements. In addition, we may use cash to enter into licensing arrangements and/or to make strategic acquisitions. We regularly consider further acquisition opportunities within our core therapeutic areas, some of which could be sizable.
In addition to our working capital requirements, as of December 31, 2023, we expect our primary cash requirements for 2024 to include:
•Debt repayments and interest—We expect to make interest payments of approximately $371 million and mandatory debt amortization payments of $30 million in 2024 under our Senior Secured Credit Facilities and may elect to make additional principal payments under certain circumstances. Further, in the ordinary course of business, we may borrow and repay amounts under our Revolving Credit Facility to meet business needs, see Item 1A. Risk Factors—Our indebtedness could adversely affect our business and our ability to meet our obligations;
•Capital expenditures—We expect to make payments of approximately $250 million for property, plant and equipment in 2024 and;
•Benefit obligations—We expect to make aggregate payments under our pension and postretirement obligations of $10 million in 2024. See Note 11, “PENSION AND POSTRETIREMENT EMPLOYEE BENEFIT PLANS” to our audited Consolidated Financial Statements for the year ended December 31, 2023, for additional information on pension and postretirement obligations included in this Form 10-K.
Acquisition of AcuFocus, Inc.
As previously discussed, on January 17, 2023, the Company acquired AcuFocus, Inc. (“AcuFocus”) for an up-front purchase price of $35 million. During January 2023, the Company paid approximately $31 million of the up-front purchase price, with the remaining purchase price to be paid within 18 months following the transaction, less any amounts that are the subject of any indemnification claims. If certain future sales-based milestones relating to the AcuFocus business are achieved between the closing date of the acquisition and December 31, 2027, additional payments by the Company will become due in future years.
Costs of Separation
In connection with the Separation, the Company has incurred and will continue to incur additional costs associated with activities taken to separate the Bausch + Lomb business from the remainder of BHC. Separation costs are incremental costs directly related to the Separation, and include but are not limited to: (i) legal, audit and advisory fees, (ii) talent acquisition costs and (iii) costs associated with establishing new boards of directors and related board committees for Bausch + Lomb. The Company has also incurred, and will continue to incur, separation-related costs which are incremental costs indirectly related to the Separation and include, but are not limited to: (i) IT infrastructure and software licensing costs, (ii) rebranding costs and (iii) costs associated with facility relocation and/or modification. The extent and timing of future charges for these costs cannot be reasonably estimated at this time and could be material.
Cost Savings Programs
As a result of the completion of the B+L IPO, and as the Company prepares for post-Separation operations, the Company is launching certain initiatives that may result in certain changes to, and investment in, its organizational structure and operations. The Company refers to the charges related to these initiatives as "Business Transformation Costs". These costs are recorded in SG&A in the audited Consolidated Statements of Operations and include third-party advisory costs, as well as certain compensation-related costs associated with changes in the Company's executive officers, such as severance-related costs associated with the departure of the Company's former executives and the costs associated with the appointment of the Company's new executives.
Further, in connection with the Separation and certain transformation initiatives, we continue to evaluate opportunities to improve our operating performance and may initiate cost savings programs to streamline our operations and eliminate redundant processes and expenses. These cost savings programs may include, but are not limited to: (i) reducing headcount, (ii) eliminating real estate costs associated with unused or under-utilized facilities and (iii) implementing contribution margin improvement and other cost reduction initiatives. Although a specific plan does not exist at this time, we may identify and take additional exit and cost-rationalization restructuring actions in the future, the costs of which could be material.
Future Litigation
In the ordinary course of business, we are involved in litigation, claims, government inquiries, investigations, charges and proceedings. See Note 20, “LEGAL PROCEEDINGS” to our audited Consolidated Financial Statements for further details of these matters. Our ability to successfully defend the Company against pending and future litigation may impact cash flows.
Future Licensing Payments
In the ordinary course of business, we may enter into select licensing and collaborative agreements for the commercialization and/or development of unique products. In connection with these agreements, the Company may pay an upfront fee to secure the agreement. See Note 21, “COMMITMENTS AND CONTINGENCIES” to our audited Consolidated Financial Statements for the year ended December 31, 2023, for additional information on these agreements included in this Form 10-K.
OUTSTANDING SHARE DATA
Our common shares are listed on the TSX and the NYSE under the ticker symbol “BLCO”.
At February 16, 2024, we had 350,992,243 issued and outstanding common shares. In addition, as of February 16, 2024, we had outstanding approximately 7,900,000 stock options and 5,300,000 restricted share units that each represent the right of a holder to receive one of Bausch + Lomb’s common shares and 1,200,000 performance-based restricted share units that represent the right of a holder to receive a number of the Company’s common shares up to a specified maximum. A maximum of approximately 3,100,000 common shares could be issued upon vesting of the performance-based restricted share units outstanding.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Critical accounting policies and estimates are those policies and estimates that are most important and material to the preparation of our audited Consolidated Financial Statements, and which require management’s most subjective and complex judgment due to the need to select policies from among alternatives available, and to make estimates about matters that are inherently uncertain. We base our estimates on historical experience and other factors that we believe to be reasonable under the circumstances. On an ongoing basis, we review our estimates to ensure that these estimates appropriately reflect changes in our business and new information as it becomes available. If historical experience and other factors we use to make these estimates do not reasonably reflect future activity, our results of operations and financial condition could be materially impacted.
Revenue Recognition
The Company's revenues are primarily generated from product sales in the therapeutic areas of eye health that consist of: (i) branded prescription eye-medications and pharmaceuticals, (ii) generic and branded generic prescription eye medications and pharmaceuticals, (iii) OTC vitamin and supplement products and (iv) medical devices (contact lenses, IOLs and ophthalmic surgical equipment). Other revenues include alliance and service revenue from the licensing and co-promotion of products and contract service revenue. Contract service revenue is derived primarily from contract manufacturing for third parties and is not material.
The Company recognizes revenue when the customer obtains control of promised goods or services and in an amount that reflects the consideration to which the Company expects to be entitled to receive in exchange for those goods or services. To achieve this core principle, the Company applies the five-step revenue model to contracts within its scope: (i) identify the contract(s) with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract and (v) recognize revenue when (or as) the entity satisfies a performance obligation.
The development and application of the critical accounting policies associated with the current revenue recognition guidance, including the policies associated with each of our product sales provisions and the table showing the activity and ending balances for our product sales provisions, are discussed in more detail in Note 2, “SIGNIFICANT ACCOUNTING POLICIES” to our audited Consolidated Financial Statements.
Acquisitions
To determine if an acquisition should be accounted for as a business combination or an asset acquisition, the Company first determines whether the set of assets acquired and/or liabilities assumed constitutes a business. If substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or a group of similar assets acquired, the set is not a business. To be considered a business, the set of assets acquired and/or liabilities assumed must include the minimum inputs and substantive processes necessary to significantly contribute to the ability to produce outputs.
If the set of assets acquired and/or liabilities assumed are deemed to constitute a business, the Company accounts for the acquisition as a business combination. Under a business combination, the Company measures the identifiable assets acquired, the liabilities assumed, and any non-controlling interest in the acquiree at their acquisition-date fair values.
•The fair value of the identifiable intangible assets is determined primarily using the “income approach,” which primarily consists of the following estimates and inputs: (i) a forecast of the expected future cash flows, which includes an estimated amount and timing of projected cash flows (including revenue growth rates, cost of goods sold, and operating expenses) and (ii) the risk-adjusted discount rate used to present value the cash flows. The fair value of acquired in-process research and development (“IPR&D”) is also recognized at fair value using an income approach and consists of the following estimates and inputs: (i) each asset’s probability-adjusted future cash flows, which reflect the different stages of development of each product and the associated probability of successful completion and (ii) the risk-adjusted discount rate used to present value the cash flows.
•Acquisition-related contingent consideration, which primarily consists of potential milestone payments, is determined in accordance with the acquisition method of accounting. The fair value of the acquisition-related contingent consideration is remeasured each reporting period, with changes in fair value recorded in the Consolidated Statements of Operations. The fair value measurement of contingent consideration obligations arising from business combinations is generally determined via a probability-weighted discounted cash flow analysis, using unobservable (Level 3) inputs. These inputs may include: (i) the estimated amount and timing of projected cash flows, (ii) the probability of the achievement of the factor(s) on which the contingency is based and (iii) the risk-adjusted discount rate used to present value the probability-weighted cash flows.
•Goodwill is recorded with the acquisition and is calculated as the difference between the acquisition date fair value of the consideration transferred and the values assigned to the assets acquired and liabilities assumed.
Transaction costs and costs to restructure the acquired company are expensed as incurred and the operating results of the acquired business are reflected in the Company's audited Consolidated Financial Statements from the date of acquisition.
If the set of assets acquired and/or liabilities assumed are deemed to not constitute a business, the transaction is accounted for as an asset acquisition. Under an asset acquisition, the cost accumulation model is used to recognize the assets acquired and liabilities assumed. In this model, the cost of acquisition, including certain transaction costs, is allocated to the assets acquired on the basis of relative fair values. Goodwill is not recognized in an asset acquisition. The amount allocated to acquired IPR&D with no alternative future use is charged to Other expense at the acquisition date. Additionally, any future contingent consideration is not recorded until it becomes probable and reasonably estimable.
Intangible Assets
We evaluate potential impairments of finite-lived intangible assets acquired through asset acquisitions or business combinations whenever events or changes in circumstances indicate that the carrying amounts of an asset group may not be recoverable. Our evaluation is based on an assessment of potential indicators of impairment, such as:
•an adverse change in legal factors or in the business climate that could affect the value of an asset. For example, a successful challenge of our patent rights resulting in earlier than expected generic competition;
•an adverse change in the extent or manner in which an asset is used or is expected to be used. For example, a decision not to pursue a product line-extension strategy to enhance an existing product due to changes in market conditions and/or technological advances; or
•current or forecasted reductions in revenue, operating income, or cash flows associated with the use of an asset. For example, the introduction of a competing product that results in a significant loss of market share.
If indicators of impairment are present, the asset group is tested for recoverability by comparing the carrying value of the asset group to the related estimated undiscounted future cash flows expected to be derived from the asset group. Impairment exists when the carrying value of the asset group exceeds the related estimated undiscounted future cash flows expected to be derived from the asset group, which include the amount and timing of the projected future cash flows. If impairment exists, the carrying value of the asset group is adjusted to its fair value. A discounted cash flow analysis is typically used to determine an asset group's fair value, using estimates and assumptions that market participants would apply. Some of the estimates and assumptions inherent in a discounted cash flow model include the amount and timing of the projected future cash flows, and the discount rate used to reflect the risks inherent in the future cash flows. A change in any of these estimates and assumptions could produce a different fair value, which could have a material impact on our results of operations. In addition, an intangible asset’s expected useful life can increase estimation risk, as longer-lived assets necessarily require longer-term cash flow forecasts, which for some of our intangible assets can be up to approximately 20 years. In connection with an impairment evaluation, we also reassess the remaining useful life of the intangible asset group and modify it, as appropriate.
Management continually assesses the useful lives of the Company's long-lived assets.
Indefinite-lived intangible assets, including acquired in-process research and development and the B&L corporate trademark, are tested for impairment annually, or more frequently if events or changes in circumstances between annual tests indicate that the asset may be impaired. Impairment losses on indefinite-lived intangible assets are recognized based solely on a comparison of their fair value to carrying value, without consideration of any recoverability test. In particular, we will continue to monitor closely the progression of our R&D programs as their likelihood of success is contingent upon the achievement of future milestones.
Goodwill
Goodwill is recorded with the acquisition of a business and is calculated as the difference between the acquisition date fair value of the consideration transferred and the values assigned to the assets acquired and liabilities assumed. A substantial portion of goodwill allocated to the Company is specific to the 2013 acquisition of the Company by BHC and has been allocated based on BHC's historical cost. Other goodwill amounts relate to other acquisitions by the Company. If a historical BHC acquisition contributed to both the Company and other BHC businesses, goodwill from the acquisition, based on BHC's historical cost, was allocated to the Company based on a relative fair value basis. Goodwill is not amortized but is tested for impairment at least annually as of October 1st at the reporting unit level. Goodwill impairment is measured as the amount by which a reporting unit's carrying value exceeds its fair value. A reporting unit is the same as, or one level below, an operating segment. An entity is permitted to first assess qualitatively whether it is necessary to perform a quantitative impairment test for any of its reporting units. The quantitative impairment test is required if the Company concludes that it is more likely than not that a reporting unit’s fair value is less than its carrying amount. In evaluating whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, the Company considers the totality of all relevant events or circumstances that affect the fair value or carrying amount of a reporting unit.
The fair value of a reporting unit refers to the price that would be received to sell the unit as a whole in an orderly transaction between market participants. The Company estimates the fair values of a reporting unit using a discounted cash flow model which utilizes Level 3 unobservable inputs. The discounted cash flow model relies on assumptions regarding revenue growth rates, gross profit, projected working capital needs, selling, general and administrative expenses, research and development expenses, capital expenditures, income tax rates, discount rates and terminal growth rates. To estimate fair value, the Company discounts the forecasted cash flows of each reporting unit. The discount rate the Company uses represents the estimated weighted average cost of capital, which reflects the overall level of inherent risk involved in its reporting unit operations and the rate of return a market participant would expect to earn. The quantitative fair value test is performed utilizing long-term growth rates and discount rates applied to the estimated cash flows in estimation of fair value. To estimate cash flows beyond the final year of its model, the Company estimates a terminal value by applying an in-perpetuity growth assumption and discount factor to determine the reporting unit's terminal value.
The Company forecasted cash flows for each of its reporting units and took into consideration economic conditions and trends, estimated future operating results, management's and a market participant's view of growth rates and product lives, and anticipated future economic conditions. Revenue growth rates inherent in these forecasts were based on input from internal and external market research that compare factors such as growth in global economies, recent industry trends and
product life-cycles. Macroeconomic factors such as changes in economies, changes in the competitive landscape including the unexpected loss of exclusivity to the Company's product portfolio, changes in government legislation, product life-cycles, industry consolidations and other changes beyond the Company's control could have a positive or negative impact on achieving its targets. Accordingly, if market conditions deteriorate, or if the Company is unable to execute its strategies, it may be necessary to record impairment charges in the future.
2021 Annual Goodwill Impairment Test
The Company conducted its annual goodwill impairment test as of October 1, 2021 by first assessing qualitative factors. In management's assessment, no qualitative factors were identified which suggested that it was more likely than not that the carrying amount of a reporting unit exceeded its fair value, and therefore concluded a quantitative fair value test for any of its reporting units was not required.
Second Quarter 2021 - Realignment of Segments
Bausch + Lomb has historically operated as part of BHC, reported under BHC’s segment structure and historically the Chief Operating Decision Maker (“CODM”) was the CODM of BHC. As Bausch + Lomb was transitioning into an independent, publicly traded company, BHC’s Chief Executive Officer (“CEO”), who was Bausch + Lomb’s CODM until the completion of the B+L IPO, evaluated how to view and measure Bausch + Lomb’s performance. This evaluation necessitated a realignment of Bausch + Lomb’s historical segment structure and, during the second quarter of 2021, Bausch + Lomb determined it is organized into three operating segments, which are also its reportable segments and reporting units. This realignment is consistent with how the CODM: (i) assesses operating performance on a regular basis, (ii) makes resource allocation decisions and (iii) designates responsibilities of his direct reports. Pursuant to these changes, effective in the second quarter of 2021, Bausch + Lomb operates in the following operating and reportable segments which are generally determined based on the decision-making structure of Bausch + Lomb and the grouping of similar products and services: (i) Vision Care (formerly named Vision Care/Consumer Health Care), (ii) Pharmaceuticals (formerly named Ophthalmic Pharmaceuticals) and (iii) Surgical.
This realignment in segment structure resulted in a change in the former Bausch + Lomb reporting units, which are now divided between the: (i) Vision Care, (ii) Pharmaceuticals and (iii) Surgical reporting units. As a result of this realignment, goodwill was reassigned to each of the aforementioned reporting units using a relative fair value approach.
Immediately prior to the change in reporting units, Bausch + Lomb performed a qualitative fair value assessment for its former Bausch + Lomb reporting units. Based on the qualitative fair value assessment performed, Management believed that it was more likely than not that the carrying value of its former Bausch + Lomb reporting units were less than their respective fair values and therefore, concluded a quantitative assessment was not required.
Immediately following the change in reporting units, as a result of the change in composition of the net assets for its current (i) Vision Care, (ii) Pharmaceuticals and (iii) Surgical reporting units, Bausch + Lomb performed a quantitative fair value assessment. The quantitative fair value test utilized long-term growth rates of 2.0% and 3.0% and a range of discount rates between 7.0% and 10.0%, in estimation of the fair value of the reporting units. After completing the testing, the fair value of each of these reporting units exceeded its carrying value by more than 45%, and, therefore, there was no impairment to goodwill.
2022 Annual Goodwill Impairment Test
The Company conducted its annual goodwill impairment test as of October 1, 2022 by performing a quantitative assessment for each of its reporting units. The quantitative assessment utilized long-term growth rates of 2.0% and 3.0% and discount rates of 9.5% and 12.25%, in estimation of the fair value of the reporting units. After completing the testing, the fair value of each of these reporting units exceeded its carrying value by more than 25%, and, therefore, there was no impairment to goodwill.
2023 Annual Goodwill Impairment Test
The Company conducted its annual goodwill impairment test as of October 1, 2023 by performing a quantitative assessment for each of its reporting units. The quantitative assessment utilized long-term growth rates of 2.0% and 3.0% and discount rates ranging from 10.25% and 11.50%, in estimation of the fair value of the reporting units. After completing the testing, the fair value of each of these reporting units exceeded its carrying value by more than 25%, and, therefore, there was no impairment to goodwill.
December 31, 2023 Goodwill Impairment Assessment
No events occurred or circumstances changed during the period from October 1, 2023 (the last time goodwill was tested for all reporting units) through December 31, 2023 that would indicate that the fair value of any reporting unit might be below its carrying value.
If market conditions deteriorate, or if the Company is unable to execute its strategies, it may be necessary to record impairment charges in the future.
There were no goodwill impairment charges through December 31, 2023.
See Note 8, “INTANGIBLE ASSETS AND GOODWILL” to our audited Consolidated Financial Statements for further details.
Contingencies
In the normal course of business, we are subject to loss contingencies, such as claims and assessments arising from litigation and other legal proceedings, contractual indemnities, product and environmental liabilities and tax matters. Other than loss contingencies that are assumed in business combinations for which we can reliably estimate the fair value, we are required to accrue for such loss contingencies if it is probable that the outcome will be unfavorable and if the amount of the loss can be reasonably estimated. We evaluate our exposure to loss based on the progress of each contingency, experience in similar contingencies and consultation with our legal counsel. We re-evaluate all contingencies as additional information becomes available. Given the uncertainties inherent in complex litigation and other contingencies, these evaluations can involve significant judgment about future events. The ultimate outcome of any litigation or other contingency may be material to our results of operations, financial condition and cash flows. See Note 20, “LEGAL PROCEEDINGS” to our audited Consolidated Financial Statements for further details regarding our current legal proceedings.
Income Taxes
We have operations in various countries that have differing tax laws and rates. Our tax structure is supported by current domestic tax laws in the countries in which we operate and the application of tax treaties between the various countries in which we operate. Our income tax reporting is subject to audit by domestic and foreign tax authorities. Our effective tax rate may change from year to year based on changes in the mix of activities and income earned under our intercompany arrangements among the different jurisdictions in which we operate, changes in tax laws in these jurisdictions, changes in tax treaties between various countries in which we operate, changes in our eligibility for benefits under those tax treaties and changes in the estimated values of deferred tax assets and liabilities. Such changes could result in an increase in the effective tax rate on all or a portion of our income and/or any of our subsidiaries.
Our provision for income taxes is based on a number of estimates and assumptions made by management. Our consolidated income tax rate is affected by the amount of income earned in our various operating jurisdictions, the availability of benefits under tax treaties and the rates of taxes payable in respect of that income. We enter into many transactions and arrangements in the ordinary course of business in which the tax treatment is not entirely certain. We must therefore make estimates and judgments based on our knowledge and understanding of applicable tax laws and tax treaties, and the application of those tax laws and tax treaties to our business, in determining our consolidated tax provision. For example, certain countries could seek to tax a greater share of income than has been provided for by us. The final outcome of any audits by taxation authorities may differ from the estimates and assumptions we have used in determining our consolidated income tax provisions and accruals. This could result in a material effect on our consolidated income tax provision, results of operations and financial condition for the period in which such determinations are made.
Our income tax returns are subject to audit in various jurisdictions. Existing and future audits by, or other disputes with, tax authorities may not be resolved favorably for us and could have a material adverse effect on our reported effective tax rate and after-tax cash flows. We record liabilities for uncertain tax positions, which involve significant management judgment. New laws and new interpretations of laws and rulings by tax authorities may affect the liability for uncertain tax positions. Due to the subjectivity and complex nature of the underlying issues, actual payments or assessments may differ from our estimates. To the extent that our estimates differ from amounts eventually assessed and paid our income and cash flows may be materially and adversely affected.
We assess whether it is more likely than not that we will realize the tax benefits associated with our deferred tax assets and establish a valuation allowance for assets that are not expected to result in a realized tax benefit. A significant amount of judgment is used in this process, including preparation of forecasts of future taxable income and evaluation of tax planning initiatives. If we revise these forecasts or determine that certain planning events will not occur, an adjustment to the valuation allowance will be made to tax expense in the period such determination is made.
NEW ACCOUNTING STANDARDS
Information regarding the recently issued new accounting guidance (adopted and not adopted as of December 31, 2023) is contained in Note 2, “SIGNIFICANT ACCOUNTING POLICIES” to our audited Consolidated Financial Statements.
FORWARD-LOOKING STATEMENTS
Caution regarding forward-looking information and statements and “Safe-Harbor” statements under the U.S. Private Securities Litigation Reform Act of 1995 and applicable Canadian securities laws:
To the extent any statements made in this Form 10-K contain information that is not historical, these statements are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and may be forward-looking information within the meaning defined under applicable Canadian securities laws (collectively, “forward-looking statements”).
These forward-looking statements relate to, among other things: our business strategy, business plans, business prospects and forecasts and changes thereto; product pipeline, prospective products and product approvals, expected launches of new products, product development and results of current and anticipated products; our recently consummated acquisition of XIIDRA® and certain other ophthalmology assets (the “XIIDRA Acquisition”); anticipated revenues for our products; expected R&D and marketing spend; our expected primary cash and working capital requirements for 2024 and beyond; our plans for continued improvement in operational efficiency and the anticipated impact of such plans; our expectations regarding the implementation of a system upgrade to our Lynchburg distribution facility; our liquidity and our ability to satisfy our debt maturities as they become due; our ability to comply with the covenants contained in our credit agreement, as recently amended, (the “Amended Credit Agreement”) and in the indenture governing our October 2028 Secured Notes; any proposed pricing actions; exposure to foreign currency exchange rate changes and interest rate changes; the outcome of contingencies, such as litigation, subpoenas, investigations, reviews, audits and regulatory proceedings; the anticipated impact of the adoption of new accounting standards; general market conditions and economic uncertainty; our expectations regarding our financial performance, including our future financial and operating performance, revenues, expenses, gross margins and income taxes; our impairment assessments, including the assumptions used therein and the results thereof; the anticipated effect of current market conditions and recessionary pressures in one or more of our markets; the anticipated effect of macroeconomic factors, including inflation; the anticipated impact from the ongoing conflicts between Russia and Ukraine and in the Middle East involving Israel and Hamas; and the anticipated separation from Bausch Health Companies Inc. (“BHC”), including the structure and expected timetable for completing such separation transaction.
Forward-looking statements can generally be identified by the use of words such as “believe,” “anticipate,” “expect,” “intend,” “estimate,” “plan,” “schedule,” “continue,” “future,” “will,” “may,” “can,” “might,” “could,” “would,” “should,” “target,” “potential,” “opportunity,” “designed,” “create,” “predict,” “project,” “timeline,” “forecast,” “outlook,” “guidance,” “seek,” “strive,” “suggest,” “prospective,” “strategy,” “indicative,” “intend,” “ongoing,” “decrease” or “increase” and positive and negative variations thereof or other similar expressions. In addition, any statements that refer to expectations, intentions, projections or other characterizations of future events or circumstances are forward-looking statements. These forward-looking statements may not be appropriate for other purposes. Although we have previously indicated certain of these statements set out herein, all of the statements in this Form 10-K that contain forward-looking statements are qualified by these cautionary statements. These statements are based upon the current expectations and beliefs of management. Although we believe that the expectations reflected in such forward-looking statements are reasonable, such statements involve risks and uncertainties, and undue reliance should not be placed on such statements. Certain material factors or assumptions are applied in making such forward-looking statements, including, but not limited to, factors and assumptions regarding the items previously outlined, those factors, risks and uncertainties outlined below and the assumption that none of these factors, risks and uncertainties will cause actual results or events to differ materially from those described in such forward-looking statements. Actual results may differ materially from those expressed or implied in such statements. Important factors, risks and uncertainties that could cause actual results to differ materially from these expectations include, among other things, the following:
•adverse economic conditions and other macroeconomic factors, including inflation, slower growth or a potential recession, which could adversely impact our revenues, expenses and resulting margins;
•the effect of current market conditions and recessionary pressures in one or more of our markets;
•the challenges the Company faces following its initial public offering (the “B+L IPO”), including the challenges and difficulties associated with managing an independent, complex business, the transitional services being provided by and to BHC, and any potential, actual or perceived conflict of interest of some of our directors and officers because of their equity ownership in BHC and/or because they also serve as directors of BHC;
•our status as a controlled company, and the possibility that BHC’s interest may conflict with our interests and the interests of our other shareholders and other stakeholders;
•the risks and uncertainties associated with the proposed plan to separate or spinoff Bausch + Lomb from BHC, which include, but are not limited to, the expected benefits and costs of the spinoff transaction, the expected timing of completion of the spinoff transaction and its terms (including the expectation that the spinoff transaction will be
completed following the achievement of targeted debt leverage ratios, subject to receipt of applicable shareholder and other necessary approvals and other factors), the ability to complete the spinoff transaction considering the various conditions to the completion of the spinoff transaction (some of which are outside the Company’s and BHC's control, including conditions related to regulatory matters and receipt of applicable shareholder approvals), the impact of any potential sales of our common shares by BHC, that market or other conditions are no longer favorable to completing the transaction, that applicable shareholder, stock exchange, regulatory or other approval is not obtained on the terms or timelines anticipated or at all, the impact on the spinoff transaction (and the timing thereof) of the filing by Norwich Pharmaceuticals Inc. ("Norwich") of its Abbreviated New Drug Application ("ANDA") for Xifaxan® (rifaxamin) 550 mg tablets and BHC's related lawsuit filed against Norwich in connection therewith (including BHC’s ability to successfully appeal the decision of the U.S. District Court for the District of Delaware in such lawsuit), business disruption during the pendency of, or following, the spinoff transaction, diversion of management time on spinoff transaction-related issues, retention of existing management team members, the reaction of customers and other parties to the spinoff transaction, the structure of the spinoff transaction and related distribution, the qualification of the spinoff transaction as a tax-free transaction for Canadian and/or U.S. federal income tax purposes (including whether or not an advance ruling from the Canada Revenue Agency and/or the Internal Revenue Service will be sought or obtained), the ability of the Company and BHC to satisfy the conditions required to maintain the tax-free status of the spinoff transaction (some of which are beyond their control), other potential tax or other liabilities that may arise as a result of the spinoff transaction, the potential dis-synergy costs resulting from the spinoff transaction, the impact of the spinoff transaction on relationships with customers, suppliers, employees and other business counterparties, general economic conditions, conditions in the markets the Company is engaged in, behavior of customers, suppliers and competitors, technological developments, as well as legal and regulatory rules affecting the Company’s business. In particular, the Company can offer no assurance that any spinoff transaction will occur at all, or that any such transaction will occur on the timelines or in the manner anticipated by the Company and BHC;
•ongoing litigation and potential additional litigation, claims, challenges and/or regulatory investigations challenging or otherwise relating to the B+L IPO and the proposed separation from BHC and the costs, expenses, use of resources, diversion of management time and efforts, liability and damages that may result therefrom;
•pricing decisions that we have implemented or may in the future elect to implement at the direction of our pricing committees or otherwise;
•legislative or policy efforts, including those that may be introduced and passed by the U.S. Congress, designed to reduce patient out-of-pocket costs for medicines and other products, which could result in new mandatory rebates and discounts or other pricing restrictions, controls or regulations (including mandatory price reductions);
•ongoing oversight and review of our products and facilities by regulatory and governmental agencies, including periodic audits by the U.S. Food and Drug Administration (the “FDA”) and equivalent agencies outside of the United States and the results thereof;
•actions by the FDA or other regulatory authorities with respect to our products or facilities;
•compliance with the legal and regulatory requirements of our marketed products;
•our ability to comply with the financial and other covenants contained in our Amended Credit Agreement, the indenture governing our October 2028 Secured Notes and other current or future debt agreements, including the limitations, restrictions and prohibitions such covenants may impose on the way we conduct our business, including prohibitions on incurring additional debt if certain financial covenants are not met, our ability to draw under the revolving credit facility under our Amended Credit Agreement (the “Revolving Credit Facility”) and restrictions on our ability to make certain investments and other restricted payments;
•any downgrade or additional downgrade by rating agencies in our or BHC's credit ratings, which may impact, among other things, our ability to raise debt and the cost of capital for additional debt issuances;
•changes in the assumptions used in connection with our impairment analyses or assessments, which would lead to a change in such impairment analyses and assessments and which could result in an impairment in the goodwill associated with any of our reporting units or impairment charges related to certain of our products or other intangible assets;
•the risks and uncertainties relating to the XIIDRA Acquisition, including our ability to effectively and efficiently integrate the acquired XIIDRA® product, pipeline products, transferred sales force and other assets into our existing business, risks that such integration efforts will potentially divert the efforts and attention of management and other employees away from our ongoing business operations, the effect of the transaction on our ability to maintain
relationships with customers, suppliers, and other business partners, risks relating to our increased levels of debt as a result of debt incurred to finance such acquisition and risks that we may not realize the expected benefits of the acquisition on a timely basis or at all;
•the possibility that the unaudited pro forma financial information included in this Form 10-K may not necessarily be indicative of what the consolidated results of operations would have been had the XIIDRA Acquisition been completed on January 1, 2022 and may differ materially from our actual results of operations;
•the uncertainties associated with the acquisition and launch of new products, assets and businesses (including the recently-acquired XIIDRA® product and Blink® product line and our recently launched MIEBO® product), including, but not limited to, our ability to provide the time, resources, expertise and funds required for the commercial launch of new products, the acceptance and demand for new products, the failure to obtain required regulatory approvals, clearances or authorizations, and the impact of competitive products and pricing, which could lead to material impairment charges;
•our ability or inability to extend the profitable life of our products, including through line extensions and other life-cycle programs;
•our ability to manage the transition to our new Chairman and Chief Executive Officer and other new executive officers and key employees, the success of such individuals in assuming their respective roles and the ability of such individuals to implement and achieve the strategies and goals of the Company as they develop;
•our ability to retain, motivate and recruit executives and other key employees;
•our ability to implement effective succession planning for our executives and other key employees;
•factors impacting our ability to achieve anticipated revenues for our products, including changes in anticipated marketing spend on such products and launch of competing products;
•factors impacting our ability to achieve anticipated market acceptance for our products, including the pricing of such products, effectiveness of promotional efforts, reputation of our products and launch of competing products;
•our ability to compete against companies that are larger and have greater financial, technical and human resources than we do, as well as other competitive factors, such as technological advances achieved, patents obtained and new products introduced by our competitors;
•the extent to which our products are reimbursed by government authorities, pharmacy benefit managers (“PBMs”) and other third-party payors; the impact our distribution, pricing and other practices may have on the decisions of such government authorities, PBMs and other third-party payors to reimburse our products; and the impact of obtaining or maintaining such reimbursement on the price and sales of our products;
•the inclusion of our products on formularies or our ability to achieve favorable formulary status, as well as the impact on the price and sales of our products in connection therewith;
•the consolidation of wholesalers, retail drug chains and other customer groups and the impact of such industry consolidation on our business;
•our ability to maintain strong relationships with physicians and other health care professionals;
•our eligibility for benefits under tax treaties and the continued availability of low effective tax rates for the business profits of certain of our subsidiaries;
•the implementation of the Organisation for Economic Co-operation and Development inclusive framework on Base Erosion and Profit Shifting, including the global minimum corporate tax rate, by the countries in which we operate;
•the actions of our third-party partners or service providers of research, development, manufacturing, marketing, distribution or other services, including their compliance with applicable laws and contracts, which actions may be beyond our control or influence, and the impact of such actions on us;
•the risks associated with the international scope of our operations, including our presence in emerging markets and the challenges we face when entering and operating in new and different geographic markets (including the challenges created by new and different regulatory regimes in such countries and the need to comply with applicable anti-bribery and economic sanctions, laws and regulations);
•adverse global economic conditions and credit markets and foreign currency exchange uncertainty and volatility in certain of the countries in which we do business;
•trade conflicts, including the trade conflict between the United States and China;
•risks associated with the ongoing conflict between Russia and Ukraine and the export controls, sanctions and other restrictive actions that have been or may be imposed by the United States, Canada, the EU and other countries against governmental and other entities and individuals in or associated with Russia, Belarus and parts of Ukraine, including its potential escalation and the potential impact on sales, earnings, market conditions and the ability of the Company to manage resources and historical investment in Russia;
•risks associated with the ongoing conflict in the Middle East involving Israel and Hamas, including its potential escalation and the potential impact on our operations, sale of products and revenues in this region;
•our ability to obtain, maintain and license sufficient intellectual property rights over our products and enforce and defend against challenges to such intellectual property;
•the introduction of generic, biosimilar or other competitors of our branded products and other products, including the introduction of products that compete against our products that do not have patent or data exclusivity rights;
•the expense, timing and outcome of pending or future legal and governmental proceedings, arbitrations, investigations, subpoenas, tax and other regulatory audits, examinations, reviews and regulatory proceedings against us or relating to us and settlements thereof;
•our ability to obtain components, raw materials or finished products supplied by third parties (some of which may be single-sourced) and other manufacturing and related supply difficulties, interruptions and delays;
•the disruption of delivery of our products and the routine flow of manufactured goods;
•potential work stoppages, slowdowns or other labor problems at our facilities and the resulting impact on our manufacturing, distribution and other operations;
•economic factors over which we have no control, including inflationary pressures as a result of historically high domestic and global inflation and otherwise, interest rates, foreign currency rates, and the potential effect of such factors on revenues, expenses and resulting margins;
•interest rate risks associated with our floating rate debt borrowings;
•our ability to effectively distribute our products and the effectiveness and success of our distribution arrangements;
•our ability to effectively promote our own products and those of our co-promotion partners;
•our ability to secure and maintain third-party research, development, manufacturing, licensing, marketing or distribution arrangements;
•the risk that our products could cause, or be alleged to cause, personal injury and adverse effects, leading to potential lawsuits, product liability claims and damages and/or recalls or withdrawals of products from the market;
•the mandatory or voluntary recall or withdrawal of our products from the market and the costs associated therewith;
•the availability of, and our ability to obtain and maintain, adequate insurance coverage and/or our ability to cover or insure against the total amount of the claims and liabilities we face, whether through third-party insurance or self-insurance;
•our indemnity agreements, which may result in an obligation to indemnify or reimburse the relevant counterparty, which amounts may be material;
•the difficulty in predicting the expense, timing and outcome within our legal and regulatory environment, including with respect to approvals by the FDA, Health Canada, the European Medicines Agency (“EMA”) and similar agencies in other jurisdictions, legal and regulatory proceedings and settlements thereof, the protection afforded by our patents and other intellectual and proprietary property, successful generic challenges to our products and infringement or alleged infringement of the intellectual property of others;
•the results of continuing safety and efficacy studies by industry and government agencies;
•the success of preclinical and clinical trials for our drug development pipeline or delays in clinical trials that adversely impact the timely commercialization of our pipeline products, as well as other factors impacting the commercial success of our products, which could lead to material impairment charges;
•uncertainties around the successful improvement and modification of our existing products and development of new products, which may require significant expenditures and efforts;
•the results of management reviews of our research and development portfolio (including following the receipt of clinical results or feedback from the FDA or other regulatory authorities), which could result in terminations of specific projects which, in turn, could lead to material impairment charges;
•the seasonality of sales of certain of our products;
•declines in the pricing and sales volume of certain of our products that are distributed or marketed by third parties, over which we have no or limited control;
•compliance by us or our third-party partners and service providers (over whom we may have limited influence), or the failure by us or these third parties to comply, with health care “fraud and abuse” laws and other extensive regulation of our marketing, promotional and business practices (including with respect to pricing), worldwide anti-bribery laws (including the U.S. Foreign Corrupt Practices Act and the Canadian Corruption of Foreign Public Officials Act), worldwide economic sanctions and/or export laws, worldwide environmental laws and regulation and privacy and security regulations;
•the impacts of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (the “Health Care Reform Act”) and any potential amendment thereof and other legislative and regulatory health care reforms in the countries in which we operate, including with respect to recent government inquiries on pricing;
•the impact of any changes in or reforms to the legislation, laws, rules, regulation and guidance that apply to us and our businesses and products or the enactment of any new or proposed legislation, laws, rules, regulations or guidance that will impact or apply to us or our businesses or products;
•the impact of changes in federal laws and policy that may be undertaken under the Biden administration;
•illegal distribution or sale of counterfeit versions of our products;
•interruptions, breakdowns or breaches in our information technology systems; and
•risks in Item 1A. “Risk Factors” in this Form 10-K.
Additional information about these factors and about the material factors or assumptions underlying such forward-looking statements may be found elsewhere in this Form 10-K, including under Item 1A. "Risk Factors", and in the Company's other filings with the U.S. Securities and Exchange Commission (the “SEC”) and the Canadian Securities Administrators (the “CSA”). When relying on our forward-looking statements to make decisions with respect to the Company, investors and others should carefully consider the foregoing factors and other uncertainties and potential events. These forward-looking statements speak only as of the date made. We undertake no obligation to update or revise any of these forward-looking statements to reflect events or circumstances after the date of this Form 10-K or to reflect actual outcomes, except as required by law. We caution that, as it is not possible to predict or identify all relevant factors that may impact forward-looking statements, the foregoing list of important factors that may affect future results is not exhaustive and should not be considered a complete statement of all potential risks and uncertainties.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Our business and financial results are affected by fluctuations in world financial markets, including the impacts of foreign currency exchange rate and interest rate movements. We evaluate our exposure to such risks on an ongoing basis, and seek ways to manage these risks to an acceptable level, based on management’s judgment of the appropriate trade-off between risk, opportunity and cost. We may use derivative financial instruments from time to time as a risk management tool and not for trading or speculative purposes.
Foreign Currency Risk
In the year ended December 31, 2023, a majority of our revenue and expense activities and capital expenditures were denominated in U.S. dollars. We have exposure to multiple foreign currencies, including, among others, the Euro, Chinese yuan, Russian ruble and Japanese yen. Our operations are subject to risks inherent in conducting business abroad, including price and currency exchange controls and fluctuations in the relative values of currencies. In addition, to the extent that we require, as a source of debt repayment, earnings and cash flows from some of our operations located in foreign countries, we are subject to risk of changes in the value of the U.S. dollar, relative to all other currencies in which we operate, which may materially affect our results of operations. Where possible, we manage foreign currency risk by managing same currency revenues in relation to same currency expenses. Further strengthening of the U.S. dollar and/or further devaluation of foreign currencies will have a negative impact on our reported revenue and reported results. As of December 31, 2023, a 1% change in foreign currency exchange rates would have impacted our shareholders’ equity by approximately $27 million.
Interest Rate Risk
During 2023, the Company became more susceptible to interest rate risk due to additional debt issuances. As of December 31, 2023, we had $3,236 million and $1,400 million in outstanding aggregate principal amount of issued variable rate and fixed rate debt, respectively. We are subject to interest rate risk on our variable rate debt as changes in interest rates could adversely affect earnings and cash flows. A 100 basis-points increase or decrease in interest rates would have an annualized pre-tax effect of approximately $32 million in our Consolidated Statements of Operations and Cash Flows, based on current outstanding borrowings and effective interest rates on our variable rate debt. While our variable-rate debt may impact earnings and cash flows as a result of changes in effective interest rates, it is not subject to changes in fair value. The estimated fair value of our issued fixed rate debt as of December 31, 2023 was $1,470 million. If interest rates were to increase by 100 basis-points, the fair value of our issued fixed rate debt would decrease by approximately $46 million. If interest rates were to decrease by 100 basis-points, the fair value of our issued fixed rate debt would increase by approximately $41 million.
Item 8. Financial Statements and Supplementary Data
The information required by this Item is contained in the financial statements set forth in Item 15. “Exhibits and Financial Statement Schedules” as part of this Form 10-K and is incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
The Company's management, with the participation of the Company's Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) as of December 31, 2023. Based on that evaluation, the Company's Chief Executive Officer and the Company's Chief Financial Officer have concluded that as of December 31, 2023, the Company’s disclosure controls and procedures were effective to provide reasonable assurance that the information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to management as appropriate to allow timely decisions regarding required disclosure.
Management's Annual Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of management, including the Company’s Chief Executive Officer and the Company’s Chief Financial Officer, the Company conducted an evaluation of the effectiveness of its internal control over financial reporting as of December 31, 2023 based on the framework described in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, management has concluded that the Company maintained effective internal control over financial reporting as of December 31, 2023. In accordance with SEC staff guidance, which allows companies to exclude acquisitions from management’s report on internal control over financial reporting for the first year after acquisition, management's evaluation and conclusion of internal control over financial reporting, as of December 31, 2023, did not include the internal controls related to the XIIDRA® Acquisition, which was acquired during September 2023. Total assets and revenues, attributable to the XIIDRA® Acquisition, represented approximately 2% and 3%, respectively, of the Company's consolidated assets and revenues as of, and for the year ended December 31, 2023.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2023 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Changes in Internal Control Over Financial Reporting
There have been no changes in the Company’s internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the quarter-ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Item 9B. Other Information
Outperformance Plan Performance Share Unit Award
On February 18, 2024, the Talent and Compensation Committee of the Board of Directors approved a Performance Share Unit (“PSU”) award for a limited number of key senior leaders (the “Executives”), including each of our current named executive officers (Brent Saunders, Sam Eldessouky and Yehia Hashad) whose compensation is required to be disclosed pursuant to SEC rules. This PSU award is designed to reward the Executives for achieving significant outperformance of performance goals that would ultimately deliver substantial value to our shareholders if achieved. The target grant date value of the awards for each of the Executives is $1,500,000.
The PSUs may be earned between 0% and 300% based on the level of achievement of: (i) a revenue metric (measured for fiscal year 2026) and (ii) a relative total shareholder return (“TSR”) metric measured over the three-year period ending December 31, 2026. In the event that the Company’s absolute TSR during such period is negative, then the maximum payout of the PSU award will be capped at 50%. Any PSUs that are earned will vest on February 28, 2027, subject generally to the Executive’s continued employment through such date, except in limited circumstances set forth in the applicable award agreement.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information required under this Item is incorporated herein by reference from information to be included in the 2024 Proxy Statement.
The Board of Directors has adopted a code of ethics (the "Code of Conduct") that applies to our Chief Executive Officer, Chief Financial Officer, the principal accounting officer, controller and all vice presidents and above in the finance department of the Company worldwide. A copy of the Code of Conduct can be found on our website at: www.bausch.com. We intend to satisfy the SEC and NYSE disclosure requirements regarding substantive amendments to, or waivers from, any provisions of our Code of Conduct on our website.
Item 11. Executive Compensation
Information required under this Item relating to executive compensation is incorporated herein by reference from information to be included in the 2024 Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information required under this Item relating to securities authorized for issuance under equity compensation plans and to security ownership of certain beneficial owners and management is incorporated herein by reference from information to be included in the 2024 Proxy Statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information required under this Item relating to certain relationships and transactions with related parties and about director independence is incorporated herein by reference from information to be included in the 2024 Proxy Statement.
Item 14. Principal Accounting Fees and Services
Information required under this Item relating to the fees for professional services rendered by our independent auditors in 2023 and 2022 is incorporated herein by reference from information to be included in the 2024 Proxy Statement.
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)Documents filed as a part of the report:
(1)The consolidated financial statements required to be filed in the Annual Report on Form 10-K are listed on page F-1 hereof.
(2)Exhibits
All schedules are omitted because they are not applicable or the required information is included in the financial statements or notes.
Item 16. Form 10-K Summary
None.
INDEX TO EXHIBITS
| | | | | |
Exhibit
Number | Exhibit Description |
|
|
| |
| |
| Indenture, dated as of September 29, 2023, by and among Bausch + Lomb Corporation, the guarantors party thereto and Citibank, N.A., acting through its agency and trust division, as trustee and as notes collateral agent thereto, originally filed as Exhibit 4.1 to Bausch + Lomb Corporation’s Form 8-K filed with the SEC on September 29, 2023, which is incorporated by reference herein. |
|
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| | | | | |
| |
| |
| Credit Agreement, dated as of May 10, 2022, as amended by the First Incremental Amendment, dated as of September 29, 2023, by and among Bausch + Lomb Corporation, certain subsidiaries of Bausch + Lomb Corporation as subsidiary guarantors, the lenders party thereto, Citibank, N.A., as collateral agent thereto, Goldman Sachs Bank USA, as term facility administrative agent thereto and JPMorgan Chase Bank, N.A., as first incremental term facility administrative agent thereto, originally filed as Exhibit 10.1 to Bausch + Lomb Corporation’s Form 8-K filed with the SEC on September 29, 2023, which is incorporated by reference herein.
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| | | | | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| 101.INS* | Inline XBRL Instance Document |
| 101.SCH* | Inline XBRL Taxonomy Extension Schema Document |
| 101.CAL* | Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.LAB* | Inline XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE* | Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| 101.DEF* | Inline XBRL Taxonomy Extension Definition Linkbase Document |
| 104* | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
_______________________________
* Filed herewith.
† Certain exhibits and schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The registrant hereby undertakes to furnish supplementally a copy of any omitted exhibit or schedule upon request by the Securities and Exchange Commission.
# Portions of this exhibit have been omitted because they are both (i) not material and (ii) would likely cause competitive harm to Bausch + Lomb Corporation if publicly disclosed.
†† Management contract or compensatory plan or arrangement.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | | | | | | | |
| | | BAUSCH + LOMB CORPORATION
(Registrant) |
| | | | | |
| Date: | February 21, 2024 | | By: | /s/ BRENTON L. SAUNDERS | |
| | | | | |
| | | | Brenton L. Saunders Chairman of the Board and Chief Executive Officer (Principal Executive Officer and Chairman of the Board) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | | | | |
| | Signature | | Title | | Date |
| | | | | | |
| | /s/ BRENTON L. SAUNDERS Brenton L. Saunders
| | Chairman of the Board and Chief Executive Officer | | February 21, 2024 |
| | /s/ SAM ELDESSOUKY Sam Eldessouky | | Executive Vice President and Chief Financial Officer (Principal Financial Officer) | | February 21, 2024 |
| | /s/ FREDERICK J. MUNSCH Frederick J. Munsch | | Senior Vice President, Controller and Chief Accounting Officer (Principal Accounting Officer) | | February 21, 2024 |
| /s/ THOMAS W. ROSS, SR. Thomas W. Ross, Sr. | | Director | | February 21, 2024 |
| /s/ RICHARD U. DESCHUTTER Richard U. DeSchutter | | Director | | February 21, 2024 |
| /s/ BRETT ICAHN Brett Icahn | | Director | | February 21, 2024 |
| /s/ NATHALIE BERNIER Nathalie Bernier | | Director | | February 21, 2024 |
| /s/ SARAH B. KAVANAGH Sarah B. Kavanagh | | Director | | February 21, 2024 |
| /s/ GARY HU Gary Hu | | Director | | February 21, 2024 |
| /s/ JOHN A. PAULSON John A. Paulson | | Director | | February 21, 2024 |
| /s/ RUSSEL C. ROBERTSON Russel C. Robertson | | Director | | February 21, 2024 |
| /s/ ANDREW C. VON ESCHENBACH Andrew C. von Eschenbach | | Director | | February 21, 2024 |
BAUSCH + LOMB CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | | | | | | | |
| | Page |
| | |
Report of Independent Registered Public Accounting Firm (PCAOB ID 238) | | |
Consolidated Balance Sheets as of December 31, 2023 and 2022 | | |
Consolidated Statements of Operations for the years ended December 31, 2023, 2022 and 2021 | | |
Consolidated Statements of Comprehensive (Loss) Income for the years ended December 31, 2023, 2022 and 2021 | | |
Consolidated Statements of Shareholders’ Equity for the years ended December 31, 2023, 2022 and 2021 | | |
Consolidated Statements of Cash Flows for the years ended December 31, 2023, 2022 and 2021 | | |
| Notes to Consolidated Financial Statements | | |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Bausch + Lomb Corporation
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Bausch + Lomb Corporation and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of operations, of comprehensive (loss) income, of shareholders' equity and of cash flows for each of the three years in the period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As described in Management’s Annual Report on Internal Control Over Financial Reporting, management has excluded the XIIDRA Acquisition (“XIIDRA”) from its assessment of internal control over financial reporting as of December 31, 2023, because it was acquired by the Company in a purchase business combination during 2023. We have also excluded XIIDRA from our audit of internal control over financial reporting. XIIDRA is a wholly-owned business whose total assets and total revenues excluded from management’s assessment and our audit of internal control over financial reporting represent approximately 2% and 3%, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2023.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and
expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that (i) relate to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
XIIDRA Acquisition - Valuation of Product Brand
As described in Note 4 to the consolidated financial statements, on September 29, 2023, the Company consummated the XIIDRA acquisition for an aggregate consideration of approximately $1,753 million. Of the intangible assets acquired, $1,595 million of product brand was recorded. The fair value of the identifiable intangible asset was determined using the income approach, which requires a forecast of the expected future cash flows, including revenue growth rates, cost of goods sold, operating expenses, and discount rate.
The principal considerations for our determination that performing procedures relating to the valuation of the product brand acquired in the XIIDRA acquisition is a critical audit matter are (i) the significant judgment by management when developing the fair value estimate of the acquired product brand; (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to revenue growth rates, cost of goods sold, operating expenses, and discount rate; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to acquisition accounting, including controls over management’s valuation of the product brand. These procedures also included, among others, (i) reading the stock and asset purchase agreement; (ii) testing management’s process for developing the fair value estimate of the product brand; (iii) evaluating the appropriateness of the income approach used by management; (iv) testing the completeness and accuracy of the underlying data used in the income approach; and (v) evaluating the reasonableness of the significant assumptions used by management related to revenue growth rates, cost of goods sold, operating expenses, and discount rate. Evaluating management’s assumptions related to revenue growth rates, cost of goods sold, and operating expenses involved considering (i) the current and past performance of XIIDRA; (ii) the consistency with external market and industry data; and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in evaluating (i) the appropriateness of the income approach and (ii) the reasonableness of the discount rate assumption.
Goodwill Impairment Assessments – Vision Care, Pharmaceuticals, and Surgical Reporting Units
As described in Notes 2 and 8 to the consolidated financial statements, the Company’s goodwill balance was $4,575 million as of December 31, 2023, and the goodwill associated with the Vision Care, Pharmaceuticals, and Surgical reporting units was $3,556 million, $693 million and $326 million, respectively. Goodwill is not amortized but is tested for impairment at least annually as of October 1st at the reporting unit level. A quantitative impairment test is required only when management concludes that it is more likely than not that a reporting unit’s fair value is less than its carrying amount. Goodwill impairment is measured by the amount the carrying value exceeds the fair value. Fair value of each reporting unit is estimated by management using a discounted cash flow model. Management conducted its annual goodwill impairment test by performing a quantitative assessment for each of its reporting units. The fair value of each of the reporting units exceeded their carrying value and therefore, management concluded there was no impairment to goodwill. Management’s discounted cash flow models rely on assumptions regarding revenue growth rates, gross profit, projected working capital needs, selling, general and administrative expenses, research and development expenses, capital expenditures, income tax rates, discount rates, and terminal growth rates.
The principal considerations for our determination that performing procedures relating to the goodwill impairment assessments of the Vision Care, Pharmaceuticals, and Surgical reporting units is a critical audit matter are (i) the significant judgment by management when developing the fair value estimate of the Vision Care, Pharmaceuticals and Surgical
reporting units; (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to revenue growth rates and discount rates for the Vision Care, Pharmaceuticals and Surgical reporting units; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s goodwill impairment assessments, including controls over the valuation of Vision Care, Pharmaceuticals, and Surgical reporting units. These procedures also included, among others (i) testing management’s process for developing the fair value estimate of the Vision Care, Pharmaceuticals, and Surgical reporting units; (ii) evaluating the appropriateness of the discounted cash flow models used by management; (iii) testing the completeness and accuracy of certain of the underlying data used in the discounted cash flow models; and (iv) evaluating the reasonableness of the significant assumptions used by management related to the revenue growth rates and discount rates. Evaluating management’s assumptions related to revenue growth rates involved evaluating whether the assumptions used by management were reasonable considering (i) the current and past performance of the related reporting units; (ii) the consistency with external market and industry data; and (iii) whether these assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in evaluating (i) the appropriateness of the discounted cash flow models and (ii) the reasonableness of the discount rate significant assumptions.
/s/ PricewaterhouseCoopers LLP
Florham Park, New Jersey
February 21, 2024
We have served as the Company’s auditor since 2020.
BAUSCH + LOMB CORPORATION
CONSOLIDATED BALANCE SHEETS
(in millions, except share amounts)
| | | | | | | | | | | | | | |
| | December 31, |
| | | 2023 | | 2022 |
| Assets | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | $ | 331 | | | $ | 354 | |
| Restricted cash | | 3 | | | 26 | |
Trade receivables, net (Note 3) | | 839 | | | 724 | |
| Inventories, net | | 1,028 | | | 628 | |
Prepaid expenses and other current assets (Note 3) | | 541 | | | 405 | |
| Total current assets | | 2,742 | | | 2,137 | |
| Property, plant and equipment, net | | 1,390 | | | 1,300 | |
| Intangible assets, net | | 3,589 | | | 2,058 | |
| Goodwill | | 4,575 | | | 4,507 | |
| Deferred tax assets, net | | 921 | | | 927 | |
Other non-current assets (Note 3) | | 225 | | | 215 | |
| Total assets | | $ | 13,442 | | | $ | 11,144 | |
| Liabilities | | | | |
| Current liabilities: | | | | |
Accounts payable (Note 3) | | $ | 522 | | | $ | 370 | |
| Accrued and other current liabilities | | 1,027 | | | 901 | |
| Current portion of long-term debt | | 30 | | | 25 | |
| Total current liabilities | | 1,579 | | | 1,296 | |
| Deferred tax liabilities, net | | 14 | | | 7 | |
| Other non-current liabilities | | 397 | | | 329 | |
| Long-term debt | | 4,532 | | | 2,411 | |
| Total liabilities | | 6,522 | | | 4,043 | |
Commitments and contingencies (Notes 20 and 21) | | | | |
| Equity | | | | |
Common shares, no par value, unlimited shares authorized, 350,913,804 and 350,000,749 issued and outstanding at December 31, 2023 and December 31, 2022, respectively | | — | | | — | |
Additional paid-in capital | | 8,349 | | | 8,285 | |
Accumulated (deficit) earnings | | (254) | | | 6 | |
| | | | |
| Accumulated other comprehensive loss | | (1,245) | | | (1,258) | |
Total Bausch + Lomb Corporation shareholders’ equity | | 6,850 | | | 7,033 | |
| Noncontrolling interest | | 70 | | | 68 | |
| Total equity | | 6,920 | | | 7,101 | |
| Total liabilities and equity | | $ | 13,442 | | | $ | 11,144 | |
On behalf of the Board:
| | | | | | | | |
| /s/ BRENTON L. SAUNDERS | | /s/ SARAH B. KAVANAGH |
| Brenton L. Saunders | | Sarah B. Kavanagh |
| Chairman of the Board and Chief Executive Officer | | Chairperson, Audit and Risk Committee |
The accompanying notes are an integral part of these consolidated financial statements.
BAUSCH + LOMB CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(in millions, except per share amounts)
| | | | | | | | | | | | | | | | | |
| | Years Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Revenues | | | | | |
| Product sales | $ | 4,131 | | | $ | 3,746 | | | $ | 3,737 | |
| Other revenues | 15 | | | 22 | | | 28 | |
| 4,146 | | | 3,768 | | | 3,765 | |
| Expenses | | | | | |
Cost of goods sold (excluding amortization and impairments of intangible assets) (Note 3) | 1,640 | | | 1,511 | | | 1,458 | |
| Cost of other revenues | 2 | | | 8 | | | 9 | |
Selling, general and administrative (Note 3) | 1,736 | | | 1,478 | | | 1,389 | |
Research and development (Note 3) | 324 | | | 307 | | | 271 | |
| Amortization of intangible assets | 240 | | | 244 | | | 292 | |
| Other expense, net | 74 | | | 13 | | | 17 | |
| 4,016 | | | 3,561 | | | 3,436 | |
| Operating income | 130 | | | 207 | | | 329 | |
| Interest income | 15 | | | 6 | | | — | |
| Interest expense (Note 3) | (283) | | | (146) | | | — | |
| Foreign exchange and other | (28) | | | 6 | | | (11) | |
| (Loss) income before provision for income taxes | (166) | | | 73 | | | 318 | |
| Provision for income taxes | (82) | | | (58) | | | (125) | |
| Net (loss) income | (248) | | | 15 | | | 193 | |
| Net income attributable to noncontrolling interest | (12) | | | (9) | | | (11) | |
| Net (loss) income attributable to Bausch + Lomb Corporation | $ | (260) | | | $ | 6 | | | $ | 182 | |
| | | | | |
| Basic and diluted (loss) income per share attributable to Bausch + Lomb Corporation | $ | (0.74) | | | $ | 0.02 | | | $ | 0.52 | |
| | | | | |
| Basic weighted-average common shares | 350.5 | | | 350.0 | | | 350.0 | |
| | | | | |
| Diluted weighted-average common shares | 350.5 | | | 350.2 | | | 350.0 | |
The accompanying notes are an integral part of these consolidated financial statements.
BAUSCH + LOMB CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE (LOSS) INCOME
(in millions)
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Net (loss) income | $ | (248) | | | $ | 15 | | | $ | 193 | |
| Other comprehensive income (loss) | | | | | |
| Pension and postretirement benefit plan adjustments: | | | | | |
| Net actuarial gain (loss) arising during the year | 1 | | | (4) | | | 24 | |
| Amortization of prior service credit | (3) | | | (3) | | | (4) | |
| Amortization of net loss and settlements | 2 | | | 10 | | | 10 | |
| Income tax (expense) benefit | (1) | | | (14) | | | 6 | |
| Foreign currency impact | — | | | 1 | | | 2 | |
| Net pension and postretirement benefit plan adjustments | (1) | | | (10) | | | 38 | |
| Foreign currency translation adjustment | 13 | | | (216) | | | (182) | |
| Other comprehensive income (loss) | 12 | | | (226) | | | (144) | |
| Comprehensive (loss) income | (236) | | | (211) | | | 49 | |
| Comprehensive income attributable to noncontrolling interest | (11) | | | (6) | | | (13) | |
| Comprehensive (loss) income attributable to Bausch + Lomb Corporation | $ | (247) | | | $ | (217) | | | $ | 36 | |
The accompanying notes are an integral part of these consolidated financial statements.
BAUSCH + LOMB CORPORATION
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY
(in millions)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | Bausch + Lomb Corporation Shareholders' Equity | | | | |
| | | Common Shares | | BHC Investment | | Additional Paid in Capital | | Accumulated Earnings | | Accumulated Other Comprehensive Loss | | Bausch + Lomb Corporation Shareholders’ Equity | | Non-controlling Interest | | Total Equity |
| | | | | | |
| | | Shares | | Amount | | | | | |
Balances, January 1, 2021 | | — | | | $ | — | | | $ | 10,807 | | | $ | — | | | $ | — | | | $ | (889) | | | $ | 9,918 | | | $ | 70 | | | $ | 9,988 | |
| Net decrease in BHC investment | | — | | | — | | | (625) | | | — | | | — | | | — | | | (625) | | | — | | | (625) | |
| Noncontrolling interest distributions | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (10) | | | (10) | |
| Net income | | — | | | — | | | 182 | | | — | | | — | | | — | | | 182 | | | 11 | | | 193 | |
| Other comprehensive (loss) income | | — | | | — | | | — | | | — | | | — | | | (146) | | | (146) | | | 2 | | | (144) | |
Balances, December 31, 2021 | | — | | | — | | | 10,364 | | | — | | | — | | | (1,035) | | | 9,329 | | | 73 | | | 9,402 | |
Issuance of common shares (Note 18) | | 350.0 | | | — | | | (8,164) | | | 8,164 | | | — | | | — | | | — | | | — | | | — | |
Issuance of BHC Purchase Debt (Note 3) | | — | | | — | | | (2,200) | | | — | | | — | | | — | | | (2,200) | | | — | | | (2,200) | |
Net distributions to BHC and affiliates (Note 3) | | — | | | — | | | — | | | 75 | | | — | | | — | | | 75 | | | — | | | 75 | |
| Noncontrolling interest distributions | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (11) | | | (11) | |
| Share-based compensation | | — | | | — | | | — | | | 46 | | | — | | | — | | | 46 | | | — | | | 46 | |
| Net income | | — | | | — | | | — | | | — | | | 6 | | | — | | | 6 | | | 9 | | | 15 | |
| Other comprehensive loss | | — | | | — | | | — | | | — | | | — | | | (223) | | | (223) | | | (3) | | | (226) | |
Balances, December 31, 2022 | | 350.0 | | | — | | | — | | | 8,285 | | | 6 | | | (1,258) | | | 7,033 | | | 68 | | | 7,101 | |
| Common shares issued under share-based compensation plans | | 0.9 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Share-based compensation | | — | | | — | | | — | | | 74 | | | — | | | — | | | 74 | | | — | | | 74 | |
| Employee withholding taxes related to share-based awards | | — | | | — | | | — | | | (10) | | | — | | | — | | | (10) | | | — | | | (10) | |
| Noncontrolling interest distributions | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (9) | | | (9) | |
| Net (loss) income | | — | | | — | | | — | | | — | | | (260) | | | — | | | (260) | | | 12 | | | (248) | |
| Other comprehensive income (loss) | | — | | | — | | | — | | | — | | | — | | | 13 | | | 13 | | | (1) | | | 12 | |
Balances, December 31, 2023 | | 350.9 | | | $ | — | | | $ | — | | | $ | 8,349 | | | $ | (254) | | | $ | (1,245) | | | $ | 6,850 | | | $ | 70 | | | $ | 6,920 | |
The accompanying notes are an integral part of these consolidated financial statements.
BAUSCH + LOMB CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in millions)
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | 2023 | | 2022 | | 2021 |
| Cash Flows From Operating Activities | | | | | | |
| Net (loss) income | | $ | (248) | | | $ | 15 | | | $ | 193 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | |
| Depreciation and amortization of intangible assets | | 382 | | | 379 | | | 415 | |
| Amortization and write-off of debt premiums, discounts and issuance costs | | 30 | | | 8 | | | — | |
| Asset impairments | | — | | | 1 | | | 12 | |
| Allowances for losses on trade receivables and inventories | | 21 | | | 25 | | | 37 | |
| Deferred income taxes | | (10) | | | (90) | | | 116 | |
| Additions (payments) to accrued legal settlements | | 2 | | | (4) | | | (1) | |
| Share-based compensation | | 74 | | | 62 | | | 62 | |
| Foreign exchange (loss) gain | | 12 | | | (7) | | | 12 | |
| Gain excluded from hedge effectiveness | | (13) | | | (6) | | | — | |
| Amortization of interim contract and inventory step-up resulting from acquisitions | | 23 | | | — | | | — | |
| Other | | (3) | | | (19) | | | (1) | |
| Changes in operating assets and liabilities: | | | | | | |
| Trade receivables | | (121) | | | (95) | | | (107) | |
| Inventories | | (264) | | | (106) | | | (15) | |
| Prepaid expenses and other current assets | | (147) | | | (7) | | | (13) | |
| Accounts payable, accrued and other liabilities | | 245 | | | 189 | | | 163 | |
| Net cash (used in) provided by operating activities | | (17) | | | 345 | | | 873 | |
| Cash Flows From Investing Activities | | | | | | |
| Acquisitions and other investments | | (1,941) | | | (45) | | | (16) | |
| Purchases of property, plant and equipment | | (181) | | | (175) | | | (193) | |
| Purchases of marketable securities | | (17) | | | (17) | | | (19) | |
| Proceeds from sale of marketable securities | | 16 | | | 22 | | | 14 | |
| Proceeds from sale of assets and businesses, net of costs to sell | | 1 | | | — | | | — | |
| Interest settlements from cross-currency swaps | | 13 | | | — | | | — | |
| Net cash used in investing activities | | (2,109) | | | (215) | | | (214) | |
| Cash Flows From Financing Activities | | | | | | |
| Issuance of long-term debt, net of discounts | | 2,276 | | | 2,440 | | | — | |
| Repayments of debt | | (161) | | | (13) | | | — | |
| Payment of employee withholding taxes related to share-based awards | | (10) | | | — | | | — | |
| Payments of financing costs | | (16) | | | (3) | | | — | |
| Payments of noncontrolling interest distributions | | (9) | | | (11) | | | (10) | |
| Net borrowings under BHC pooled financing arrangements (Note 3) | | — | | | 31 | | | 28 | |
| Net transfers to BHC (Note 3) | | — | | | (2,363) | | | (730) | |
| Other | | (2) | | | — | | | — | |
| Net cash provided by (used in) financing activities | | 2,078 | | | 81 | | | (712) | |
| Effect of exchange rate changes on cash and cash equivalents and restricted cash | | 2 | | | (8) | | | (8) | |
| Net (decrease) increase in cash and cash equivalents and restricted cash | | (46) | | | 203 | | | (61) | |
| Cash and cash equivalents and restricted cash, beginning of period | | 380 | | | 177 | | | 238 | |
| Cash and cash equivalents and restricted cash, end of period | | $ | 334 | | | $ | 380 | | | $ | 177 | |
| | | | | | |
| Non-cash Financing Activity | | | | | | |
| Accrued purchases of property, plant and equipment | | $ | 65 | | | $ | 38 | | | $ | 31 | |
| Issuance of BHC Purchase Debt (Note 3) | | $ | — | | | $ | 2,200 | | | $ | — | |
The accompanying notes are an integral part of these consolidated financial statements.
BAUSCH + LOMB CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1.DESCRIPTION OF BUSINESS
Overview
Bausch + Lomb Corporation (“Bausch + Lomb” or the “Company”) is a leading global eye health company dedicated to protecting and enhancing the gift of sight for millions of people around the world – from the moment of birth through every phase of life. The Company operates in three reportable segments: (i) Vision Care segment which includes both a contact lens business and a consumer eye care business that consists of contact lens care products, over-the-counter (“OTC”) eye drops and eye vitamins, (ii) Pharmaceuticals segment which consists of a broad line of proprietary and generic pharmaceutical products for post-operative treatments and treatments for a number of eye conditions, such as glaucoma, eye inflammation, ocular hypertension, dry eyes and retinal diseases and (iii) Surgical segment which consists of medical device equipment, consumables, instruments and technologies for the treatment of cataracts, corneal and vitreous and retinal eye conditions, which includes intraocular lenses (“IOLs”) and delivery systems, phacoemulsification equipment and other surgical instruments and devices necessary for ophthalmic surgery. Effective June 30, 2023, the Company renamed its former Ophthalmic Pharmaceuticals segment to the Pharmaceuticals segment. Aside from the change in name, there were no other changes made to this segment at that time. See Note 22, “SEGMENT INFORMATION” for additional information regarding these reportable segments. Bausch + Lomb is a subsidiary of Bausch Health Companies Inc. (“BHC”), with BHC holding, directly or indirectly, approximately 88.4% of the issued and outstanding common shares of Bausch + Lomb as of February 16, 2024.
Separation of Bausch + Lomb from BHC
On August 6, 2020, BHC announced its plan to separate Bausch + Lomb into an independent, publicly traded company, separate from the remainder of BHC (the “Separation”), commencing with an initial public offering of Bausch + Lomb's common shares (as further described below). Prior to January 1, 2022, Bausch + Lomb had nominal assets and liabilities. In connection with the B+L IPO (as defined below), BHC transferred to Bausch + Lomb, in a series of steps, all the entities, assets, liabilities and obligations that Bausch + Lomb held upon completion of the B+L IPO pursuant to a Master Separation Agreement (the “MSA”) with BHC.
The registration statement related to the initial public offering (the “IPO”) of Bausch + Lomb’s common shares (the “B+L IPO”) was declared effective on May 5, 2022, and Bausch + Lomb’s common shares began trading on the New York Stock Exchange and the Toronto Stock Exchange, in each case under the ticker symbol “BLCO”, on May 6, 2022. Bausch + Lomb also obtained a final receipt to its Canadian base PREP prospectus on May 5, 2022. Prior to the B+L IPO, Bausch + Lomb was a wholly-owned subsidiary of BHC. As of February 16, 2024, BHC directly or indirectly holds 310,449,643 Bausch + Lomb common shares, which represents approximately 88.4% of Bausch + Lomb common shares.
The completion of the full Separation of Bausch + Lomb, which includes the transfer of all or a portion of BHC’s remaining direct or indirect equity interest in Bausch + Lomb to its shareholders (the “Distribution”), is subject to the achievement of targeted debt leverage ratios and the receipt of applicable shareholder and other necessary approvals and other factors and is subject to various risk factors relating to the Separation. Bausch + Lomb understands that BHC continues to believe that completing the Separation makes strategic sense and that BHC continues to evaluate all relevant factors and considerations related to completing the Separation, including the effect of the lawsuit filed against Norwich Pharmaceuticals Inc.
2.SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
In connection with the B+L IPO, effective January 1, 2022, BHC transferred to Bausch + Lomb substantially all the entities, assets, liabilities and obligations related to the Bausch + Lomb business, such that the accompanying audited financial statements for all periods presented, including the historical results of the Company prior to January 1, 2022, are now referred to as “Consolidated Financial Statements”, and have been prepared pursuant to the rules and regulations for reporting on Form 10-K. Prior to January 1, 2022, the Company’s Consolidated Financial Statements were prepared on a combined basis and were derived from BHC’s historical consolidated financial statements.
Periods prior to the B+L IPO
Prior to the B+L IPO, Bausch + Lomb had historically operated as part of BHC; therefore, separate financial statements were not historically prepared. The accompanying audited Consolidated Financial Statements for periods prior to the B+L IPO were prepared from BHC’s historical accounting records.
Prior to the B+L IPO, Bausch + Lomb relied on BHC’s corporate and other support functions. Therefore, certain corporate and shared costs for periods prior to the B+L IPO were allocated to Bausch + Lomb, including expenses related to BHC support functions that were provided on a centralized basis, including expenses for executive oversight, treasury, accounting, legal, human resources, shared services, compliance, procurement, information technology and other corporate functions. The expenses associated with these services generally included all payroll and benefit costs, certain share-based compensation expenses related to BHC’s long-term incentive program for BHC employees who are providing corporate services to Bausch + Lomb, certain expenses associated with corporate insurance coverage and medical, pension, postretirement and other health plan costs for employees participating in BHC sponsored plans, as well as overhead costs related to the support functions. These expenses were allocated to Bausch + Lomb based on a specific identification basis or, when specific identification is not practicable, a proportional cost allocation method. Allocations were based on direct usage where identifiable as well a number of other utilization measures including headcount and relative revenues. See Note 3, “RELATED PARTIES” for further information regarding allocated expenses between Bausch + Lomb and BHC.
Management believes these cost allocations are a reasonable reflection of the utilization of services provided to, or the benefit derived by, the Company during the periods presented, though the allocations may not be indicative of the actual costs that would have been incurred had the Company operated as a standalone public company. Actual costs that may have been incurred if the Company had been a standalone company would depend on a number of factors, including the chosen organizational structure, whether functions were outsourced or performed by the Company employees, and strategic decisions made in areas such as research and development, information technology and infrastructure.
The Company's Consolidated Balance Sheets include all assets and liabilities directly attributable to Bausch + Lomb. To the extent that assets such as facilities are shared between Bausch + Lomb and other BHC owned businesses, the assets and any related lease liabilities are not included in the Company's Consolidated Balance Sheets, however a charge was allocated in the Company's Consolidated Statements of Operations for Bausch + Lomb’s utilization of these assets.
The Company's Consolidated Statements of Operations include all revenues and expenses directly attributable to Bausch + Lomb, including charges and allocations for facilities, functions and services used by Bausch + Lomb. All charges and allocations for facilities, functions and services performed by BHC have been recorded through BHC Investment by Bausch + Lomb to BHC in the period in which the cost was recorded in the Consolidated Statements of Operations. Prior to the B+L IPO, BHC’s cumulative interest in the assets and liabilities of the Company, inclusive of operating results, is presented as BHC investment on the Consolidated Balance Sheets. As part of the B+L IPO, BHC Investment was reclassified to Additional paid-in capital.
Current and deferred income taxes in the Consolidated Financial Statements were calculated on a separate return basis. However, because the Company filed as part of BHC’s tax group in certain jurisdictions, the Company’s actual tax balances may differ from those reported. The Company's portion of its domestic and certain income taxes for jurisdictions outside the U.S. are deemed to have been settled in the period the related tax expense was recorded.
Prior to the B+L IPO, BHC’s third-party debt and related interest expense were not attributed to the Company because the borrowings were not specifically identifiable to the Company. However, in connection with the B+L IPO, the Company incurred indebtedness directly attributable to the Company and has therefore recorded the related interest expense beginning in 2022.
BHC had entered into cross currency swaps and foreign currency exchange contracts to hedge certain foreign exchange exposures across BHC’s business. These instruments were attributed to the Company based on a specific identification basis or, when specific identification is not practicable, the related income or expense for these instruments was allocated based on relative net assets and revenues.
Periods subsequent to the B+L IPO
On May 10, 2022, Bausch + Lomb became an independent publicly traded company. The audited financial statements for all periods presented have been prepared by Bausch + Lomb in United States (“U.S.”) dollars and in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) for financial reporting and pursuant to the rules and regulations for reporting on Form 10-K. The Consolidated Financial Statements include the accounts of the Company and those of its subsidiaries and any variable interest entities for which the Company is the primary beneficiary. All intercompany transactions and balances have been eliminated.
Following the B+L IPO, certain functions that BHC provided to Bausch + Lomb prior to the B+L IPO were provided and, in some cases, continue to be provided to Bausch + Lomb by BHC under a Transition Services Agreement (the “TSA”) or are performed using Bausch + Lomb’s own resources or third-party service providers. Bausch + Lomb has incurred certain costs in its establishment as a standalone public company, and expects additional ongoing costs associated with operating as an independent, publicly traded company. See Note 3, “RELATED PARTIES” for further information regarding agreements between Bausch + Lomb and BHC.
Out of Period Adjustments
During the preparation of the Condensed Consolidated Financial Statements for the three months ended March 31, 2022, management identified immaterial prior period accounting misstatements related to the income tax impact of unrealized gains and losses of Bausch + Lomb’s pension and postretirement benefit plan, which are included in Other comprehensive loss in the Condensed Consolidated Statements of Comprehensive Income and related to the impact of deferred taxes on the Condensed Consolidated Statements of Cash Flows. The misstatements resulted in an overstatement of Other comprehensive loss and of Net cash provided by operating activities of $6 million and an overstatement of Net cash used in financing activities of $6 million for the year ended December 31, 2022 and in an understatement of $10 million of Accumulated other comprehensive loss in the Condensed Consolidated Balance Sheet as of December 31, 2021. Bausch + Lomb recorded out of period corrections for the misstatements during the year ended December 31, 2022, resulting in an out of period unrealized loss of $10 million, reflected in the Pension and postretirement benefit plan adjustments, net of income taxes caption of its Consolidated Statements of Comprehensive Income (Loss). The out of period correction also resulted in a decrease in the Deferred income taxes caption and an offsetting increase in the Net Transfers to BHC caption of its Consolidated Statements of Cash Flows of $10 million for the year ended December 31, 2022.
Management has evaluated this misstatement and related out of period correction and concluded that this misstatement is not material to the impacted periods.
Use of Estimates
In preparing the Company’s Consolidated Financial Statements, management is required to make estimates and assumptions. This includes estimates and assumptions regarding the nature, timing and extent of the impacts that certain global macroeconomic conditions, including, but not limited to, those related to inflation and supply chain, will have on the Company's operations and cash flows. The estimates and assumptions used by the Company affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Significant estimates made by management include: provisions for product returns, rebates, chargebacks, discounts and allowances and distribution fees paid to certain wholesalers; useful lives of finite-lived intangible assets and property, plant and equipment; expected future cash flows used in evaluating intangible assets for impairment, assessing compliance with debt covenants, reporting unit fair values for testing goodwill for impairment; acquisition-related contingent consideration liabilities; provisions for loss contingencies; provisions for income taxes, uncertain tax positions and realizability of deferred tax assets; the fair value of cross-currency swaps; the fair value of foreign currency exchange contracts; and the recognition of the fair value of assets and liabilities acquired in a business combination or asset acquisition. Prior to the B+L IPO, significant estimates made by management also included the related allocations described in the basis of presentation.
All estimates in these Consolidated Financial Statements are based on assumptions that management believes are reasonable. On an ongoing basis, management reviews its estimates to ensure that these estimates appropriately reflect changes in the Company's business and new information as it becomes available. If historical experience and other factors used by management to make these estimates do not reasonably reflect future activity, the Company's business, financial condition, cash flows and results of operations could be materially impacted.
The extent to which certain global macroeconomic conditions, including, but not limited to, those related to inflation and supply chain, may continue to impact the Company’s business, financial condition, cash flows and results of operations, in particular, will depend on future developments which are highly uncertain and many of which are outside the Company’s control. The Company has assessed the possible effects and outcomes of these macroeconomic conditions on, among other things, its supply chain, customers and distributors, discounts and rebates, employee base, product sustainability, research and development efforts, product pipeline and consumer demand and currently believes that its estimates are reasonable.
Reclassifications
Certain reclassifications have been made to prior year amounts to conform to the current year presentation.
Acquisitions
Acquired businesses are accounted for using the acquisition method of accounting, which requires that assets acquired and liabilities assumed be recorded at fair value, with limited exceptions. Transaction costs and costs to restructure the acquired company are expensed as incurred. The operating results of the acquired business are reflected in the Consolidated Financial Statements from the date of acquisition. Goodwill is recorded with the acquisition and is calculated as the difference between the acquisition date fair value of the consideration transferred and the values assigned to the assets acquired and liabilities assumed. Acquired in-process research and development (“IPR&D”) is recognized at fair value and initially characterized as an indefinite-lived intangible asset, irrespective of whether the acquired IPR&D has an alternative future use. If the acquired net assets do not constitute a business, the transaction is accounted for as an asset acquisition and no goodwill is recognized.
In an asset acquisition, the amount allocated to acquired IPR&D with no alternative future use is charged to Other expense at the acquisition date. Additionally, any future contingent consideration is not recorded until it becomes probable and reasonably estimable.
Fair Value of Financial Instruments
The estimated fair values of cash and cash equivalents, trade receivables, accounts payable and accrued liabilities approximate their carrying values due to their short maturity periods. The fair value of acquisition-related contingent consideration is based on estimated discounted future cash flows analyses and assessment of the probability of occurrence of potential future events.
Fair Value of Derivative Instruments
The accounting for changes in the fair value of a derivative instrument depends on whether the instrument has been designated and qualifies as part of a hedging relationship and on the type of hedging relationship. For derivative instruments designated and qualifying as hedging instruments, the hedging instrument must be designated, based upon the exposure being hedged, as a fair value hedge, cash flow hedge, or a hedge of the foreign currency exposure of a net investment in a foreign operation. For derivative instruments not designated as hedging instruments, the gain or loss is recognized in the Consolidated Statements of Operations during the current period.
The Company’s cross-currency swaps qualify for and have been designated as an accounting hedge of the foreign currency exposure of a net investment in a foreign operation and are remeasured at each reporting date to reflect changes in their fair values. The fair value is determined via a mark-to-market analysis, using observable (Level 2) inputs. These inputs may include: (i) the foreign currency exchange spot rate between the euro and U.S. dollar, (ii) the interest rate yield curves in the euro and U.S. dollar and (iii) the credit risk rating for each applicable counterparty. The net change in fair value of cross-currency swaps is reported as a gain or loss in the Consolidated Statements of Comprehensive (Loss) Income as part of Foreign currency translation adjustment to the extent they are effective, and remain in Accumulated other comprehensive (loss) income until either the sale or complete, or substantially complete, liquidation of the subsidiary. No portion of the cross-currency swaps was ineffective. The Company uses the spot method of assessing hedge effectiveness. The Company has elected to amortize amounts excluded from the assessment of effectiveness over the term of its cross-currency swaps as a reduction of Interest expense in the Consolidated Statements of Operations.
The Company uses foreign currency exchange contracts to economically hedge the foreign exchange exposure on certain of the Company's intercompany balances. The Company's foreign currency exchange contracts are remeasured at each reporting date to reflect changes in their fair values determined using forward rates, which are observable market inputs, multiplied by the notional amount. These contracts have not been designated as an accounting hedge, and therefore the net change in their fair value is reported as a gain or loss in the Consolidated Statements of Operations as part of Foreign exchange and other.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash in bank accounts and highly liquid investments with maturities of three months or less when purchased, and that is legally owned by the Company.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents, marketable securities, trade receivables, cross-currency swaps and foreign currency exchange contracts.
Cash deposited at banks may exceed the amount of insurance provided on such deposits. Generally, these cash deposits may be redeemed upon demand and are maintained with financial institutions with reputable credit and therefore bear minimal credit risk. The Company seeks to mitigate such risks by spreading its risk across multiple counterparties and monitoring the risk profiles of these counterparties.
Outside of the U.S., concentrations of credit risk with respect to trade receivables, which are typically unsecured, are limited due to the number of customers using the Company’s products, as well as their dispersion across many different geographic regions. The Company performs periodic credit evaluations of customers and does not require collateral. The Company monitors economic conditions, including volatility associated with international economies, and related impacts on the relevant financial markets and its business, especially in light of sovereign credit issues. The credit and economic conditions within Algeria, Argentina, Brazil, Belarus, Greece, Iran, Russia, Serbia, South Africa, Turkey, Ukraine and Venezuela have been weak in recent years. These conditions have increased, and may continue to increase, the average length of time that it takes to collect on the Company’s trade receivables outstanding in these countries.
As of December 31, 2023 and 2022, the Company's net trade receivable balance from Algeria, Argentina, Brazil, Belarus, Greece, Iran, Russia, Serbia, South Africa, Turkey, Ukraine and Venezuela amounted to $109 million and $101 million, respectively, the majority of which is current or less than 90 days past due. As of December 31, 2023 and 2022, the portion of the net trade receivable from these countries that is past due more than 90 days amounted to $1 million and $3 million, respectively.
Allowance for Credit Losses
An allowance is maintained for potential credit losses. The Company estimates the current expected credit loss on its receivables based on various factors, including historical credit loss experience, customer credit worthiness, value of collaterals (if any), and any relevant current and reasonably supportable future economic factors. Additionally, the Company generally estimates the expected credit loss on a pooled basis when customers are deemed to have similar risk characteristics. Trade receivable balances are written off against the allowance when it is deemed probable that the trade receivable will not be collected. Trade receivables, net are stated net of certain sales provisions and the allowance for credit losses.
The activity in the allowance for credit losses for trade receivables for the years 2023, 2022 and 2021 is as follows:
| | | | | | | | | | | | | | | | | |
| (in millions) | 2023 | | 2022 | | 2021 |
| Balance, beginning of period | $ | 22 | | | $ | 16 | | | $ | 17 | |
| Provision | 3 | | | 4 | | | 2 | |
| Write-offs | (3) | | | (2) | | | (2) | |
| Foreign exchange and other | (1) | | | 4 | | | (1) | |
| Balance, end of period | $ | 21 | | | $ | 22 | | | $ | 16 | |
Inventories
Inventories comprise raw materials, work in process and finished goods, which are valued at the lower of cost or net realizable value, on a first-in, first-out basis. The cost value for work in process and finished goods inventories includes materials, direct labor and an allocation of overheads.
The Company evaluates the carrying value of inventories on a regular basis, taking into account such factors as historical and anticipated future sales compared with quantities on hand, the price the Company expects to obtain for products in their respective markets compared with historical cost and the remaining shelf life of goods on hand.
Property, Plant and Equipment
Property, plant and equipment are reported at cost, less accumulated depreciation. Costs incurred on assets under construction are capitalized as construction in progress. Depreciation is calculated using the straight-line method, commencing when the assets become available for productive use, based on the following estimated useful lives:
| | | | | | | | |
| Land improvements | | 15 - 30 years |
| Buildings and improvements | | Up to 40 years |
| Machinery and equipment | | Up to 20 years |
| Other equipment | | 3 - 10 years |
| Leasehold improvements | | Lesser of term of lease or 10 years |
Intangible Assets
A substantial portion of the Intangible assets are specific to: (i) the 2013 acquisition of the Company by BHC and have been included based on BHC's historical cost and (ii) intangible assets acquired through various acquisitions. See Note 4, “ACQUISITIONS AND LICENSING AGREEMENTS” for further detail on these acquisitions. Intangible assets are reported at cost, less accumulated amortization and impairments. Intangible assets with finite lives are amortized over their estimated useful lives. Amortization is calculated primarily using the straight-line method based on the following estimated useful lives:
| | | | | | | | |
| Product brands | | 1 - 15 years |
| Corporate brands | | 5 - 17 years |
| Product rights | | 8 - 15 years |
| Out-licensed technology and other | | 8 years |
Acquired In-Process Research and Development
The fair value of in-process research and development ("IPR&D") acquired through a business combination is capitalized as an indefinite-lived intangible asset until the completion or abandonment of the related research and development activities. When the related research and development is completed, the asset will be assigned a useful life and amortized.
The fair value of an acquired IPR&D intangible asset is typically determined using an income approach. This approach starts with a forecast of the net cash flows expected to be generated by the asset over its estimated useful life. The net cash flows reflect the asset’s stage of completion, the probability of technical success, the projected costs to complete, expected market competition and an assessment of the asset’s life-cycle. The net cash flows are then adjusted to present value by applying an appropriate discount rate that reflects the risk factors associated with the expected cash flow streams.
Impairment of Long-Lived Assets
Long-lived assets with finite lives are tested for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. If indicators of impairment are present, the asset group is tested for recoverability by comparing the carrying value of the asset group to the related estimated undiscounted future cash flows expected to be derived from the asset group, which include the amount and timing of the projected future cash flows. If the expected undiscounted cash flows are less than the carrying value of the asset, then the asset is considered to be impaired and its carrying value is written down to fair value, based on the related estimated discounted future cash flows. Impairment losses are included in Other expense, net in the Consolidated Statements of Operations.
Indefinite-lived intangible assets, which includes acquired IPR&D and the corporate trademark acquired in 2013 as part of the acquisition of the Company (the ‘‘B&L Trademark’’), are tested for impairment annually or more frequently if events or changes in circumstances between annual tests indicate that the asset may be impaired. Impairment losses on indefinite-lived intangible assets are recognized based on a comparison of the fair value of the asset to its carrying value.
Goodwill
Goodwill is recorded with the acquisition of a business and is calculated as the difference between the acquisition date fair value of the consideration transferred and the values assigned to the assets acquired and liabilities assumed. A substantial portion of goodwill allocated to the Company is specific to the 2013 acquisition of the Company by BHC and has been allocated based on BHC's historical cost. Other goodwill amounts relate to other acquisitions by the Company. If a historical BHC acquisition contributed to both the Company and other BHC businesses, goodwill from the acquisition, based on BHC's historical cost, was allocated to the Company based on a relative fair value basis. Goodwill is not amortized but is tested for impairment at least annually as of October 1st at the reporting unit level. Goodwill impairment is measured as the amount by which a reporting unit's carrying value exceeds its fair value. A reporting unit is the same as, or one level below, an operating segment. An entity is permitted to first assess qualitatively whether it is necessary to perform a quantitative impairment test for any of its reporting units. The quantitative impairment test is required if the Company concludes that it is more likely than not that a reporting unit’s fair value is less than its carrying amount. In evaluating whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, the Company considers the totality of all relevant events or circumstances that affect the fair value or carrying amount of a reporting unit.
The fair value of a reporting unit refers to the price that would be received to sell the unit as a whole in an orderly transaction between market participants. Bausch + Lomb estimates the fair values of a reporting unit using a discounted cash flow model which utilizes Level 3 unobservable inputs. The discounted cash flow model relies on assumptions regarding revenue growth rates, gross profit, projected working capital needs, selling, general and administrative expenses, research and development expenses, capital expenditures, income tax rates, discount rates and terminal growth rates. To estimate fair value, Bausch + Lomb discounts the forecasted cash flows of each reporting unit. The discount rate Bausch + Lomb uses represents the
estimated weighted average cost of capital, which reflects the overall level of inherent risk involved in its reporting unit operations and the rate of return a market participant would expect to earn. The quantitative fair value test is performed utilizing long-term growth rates and discount rates applied to the estimated cash flows in estimation of fair value. To estimate cash flows beyond the final year of its model, Bausch + Lomb estimates a terminal value by applying an in-perpetuity growth assumption and discount factor to determine the reporting unit’s terminal value.
To forecast a reporting unit’s cash flows, Bausch + Lomb takes into consideration economic conditions and trends, estimated future operating results, management’s and a market participant’s view of growth rates and product lives and anticipates future economic conditions. Revenue growth rates inherent in these forecasts are based on input from internal and external market research that compare factors such as growth in global economies, recent industry trends and product life-cycles. Macroeconomic factors such as changes in economies, changes in the competitive landscape including the unexpected loss of exclusivity to Bausch + Lomb’s product portfolio, changes in government legislation, product life-cycles, industry consolidations and other changes beyond Bausch + Lomb’s control could have a positive or negative impact on achieving its targets. Accordingly, if market conditions deteriorate, or if Bausch + Lomb is unable to execute its strategies, it may be necessary to record impairment charges in the future.
An interim goodwill impairment test in advance of the annual impairment assessment may be required if adverse events occur that indicate an impairment might be present. For example, changes in reportable segments, unexpected adverse business conditions, economic factors and unanticipated competitive activities may signal that an interim impairment test is needed. Accordingly, among other factors, the Company monitors changes in its share price between annual impairment tests. The Company considers a decline in its share price that corresponds to an overall deterioration in stock market conditions to be less of an indicator of goodwill impairment than a unilateral decline in its share price reflecting adverse changes in its underlying operating performance, cash flows, financial condition and/or liquidity. In the event that the Company’s market capitalization does decline below its book value, the Company would consider the length and severity of the decline and the reason for the decline when assessing whether potential goodwill impairment exists. The Company believes that short-term fluctuations in share prices may not necessarily reflect underlying values.
Debt Discounts and Premiums, Issuance Costs and Deferred Financing Costs
Debt discounts, premiums and issuance costs are presented in the Consolidated Balance Sheets as a direct deduction from or addition to the carrying amount of the related debt and are amortized or accreted, using the effective interest method, as interest expense over the contractual lives of the related credit facilities or notes. Deferred financing costs associated with revolving credit facility arrangements are included in the balances of Prepaid expenses and other current assets and Other non-current assets in the Consolidated Balance Sheets and are amortized as interest expense over the contractual life of the related revolving credit facility.
Foreign Currency Translation
The assets and liabilities of the Company's foreign operations having a functional currency other than the U.S. dollar are translated into U.S. dollars at the exchange rate prevailing at the balance sheet date, and at the average exchange rate for the reporting period for revenue and expense accounts. The cumulative foreign currency translation adjustment is recorded as a component of accumulated other comprehensive loss in shareholders’ equity.
Foreign currency exchange gains and losses on transactions occurring in a currency other than an operation’s functional currency are recognized as a component of Foreign exchange and other in the Consolidated Statements of Operations. Foreign currency translation recorded in these Consolidated Financial Statements, is based on currency movements specific to the Company's Consolidated Financial Statements during the periods presented.
Revenue Recognition
The Company's revenues are primarily generated from product sales in the therapeutic areas of eye health that consist of: (i) branded prescription eye-medications and pharmaceuticals, (ii) generic and branded generic prescription eye medications and pharmaceuticals, (iii) OTC vitamin and supplement products and (iv) medical devices (contact lenses, IOLs and ophthalmic surgical equipment). Other revenues include alliance and service revenue from the licensing and co-promotion of products and contract service revenue. Contract service revenue is derived primarily from contract manufacturing for third parties and is not material. See Note 22, “SEGMENT INFORMATION” for the disaggregation of revenues.
The Company recognizes revenue when the customer obtains control of promised goods or services and in an amount that reflects the consideration to which the Company expects to be entitled to receive in exchange for those goods or services. To achieve this core principle, the Company applies the five-step revenue model to contracts within its scope: (i) identify the contract(s) with a customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract and (v) recognize revenue when (or as) the entity satisfies a performance obligation.
Product Sales
A contract with the Company’s customers exists for each product sale. Where a contract with a customer contains more than one performance obligation, the Company allocates the transaction price to each distinct performance obligation based on its relative standalone selling price. The transaction price is adjusted for variable consideration which is discussed further below. The Company recognizes revenue for product sales at a point in time, when the customer obtains control of the products in accordance with contracted delivery terms, which is generally upon shipment or customer receipt. Contracted delivery terms will vary by customer and geography. In the U.S., control is generally transferred to the customer upon receipt.
Revenue from sales of surgical equipment and related software is generally recognized upon delivery and installation of the equipment. IOLs and delivery systems, disposable surgical packs and other surgical instruments are distinct from the surgical equipment and may be sold together with the surgical equipment in a single contract or on a standalone basis. Revenue from the sale of delivery systems, disposable surgical packs and other surgical instruments is recognized in accordance with the contracted delivery terms, generally upon shipment or customer receipt. IOLs are sold primarily on a consignment basis and revenue is recognized upon notification of use, which typically occurs when a replacement order is placed.
When a sale transaction in the Surgical segment contains multiple performance obligations, the transaction price is allocated to each performance obligation based on the relative standalone sales price and revenue is recognized upon satisfaction of each performance obligation.
Product Sales Provisions
As is customary in the eye health industry, gross product sales of certain product categories are subject to a variety of deductions in arriving at reported net product sales. The transaction price for such product categories is typically adjusted for variable consideration, which may be in the form of cash discounts, allowances, returns, rebates, chargebacks and distribution fees paid to customers. Provisions for variable consideration are established to reflect the Company’s best estimates of the amount of consideration to which it is entitled based on the terms of the contract. The amount of variable consideration included in the transaction price may be constrained, and is included in the net sales price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in the future period.
Provisions for these deductions are recorded concurrently with the recognition of gross product sales revenue and include cash discounts and allowances, chargebacks and distribution fees, which are paid to direct customers, as well as rebates and returns, which can be paid to direct and indirect customers. Returns provision balances and volume discounts to direct customers are included in Accrued and other current liabilities. All other provisions related to direct customers are included in Trade receivables, net, while provision balances related to indirect customers are included in Accrued and other current liabilities.
The following tables present the activity and ending balances of the Company’s variable consideration provisions for years 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | | Discounts and Allowances | | Returns | | Rebates | | Chargebacks | | Distribution Fees | | Total |
Reserve balance, January 1, 2022 | | $ | 167 | | | $ | 60 | | | $ | 195 | | | $ | 29 | | | $ | 17 | | | $ | 468 | |
| Current period provision | | 315 | | | 69 | | | 528 | | | 442 | | | 22 | | | 1,376 | |
| Payments and credits | | (336) | | | (70) | | | (535) | | | (398) | | | (21) | | | (1,360) | |
Reserve balance, December 31, 2022 | | 146 | | | 59 | | | 188 | | | 73 | | | 18 | | | 484 | |
| Current period provision | | 368 | | | 84 | | | 729 | | | 559 | | | 28 | | | 1,768 | |
| Payments and credits | | (373) | | | (77) | | | (691) | | | (565) | | | (28) | | | (1,734) | |
Reserve balance, December 31, 2023 | | $ | 141 | | | $ | 66 | | | $ | 226 | | | $ | 67 | | | $ | 18 | | | $ | 518 | |
Included in Rebates in the table above are cooperative advertising credits due to customers of approximately $35 million and $35 million as of December 31, 2023 and 2022, respectively, which are reflected as a reduction of Trade accounts receivable, net in the Consolidated Balance Sheets. For 2023, included in Payments and credits in the table above, are payments made, or to be made, by Novartis, on behalf of the Company, in accordance with the agreements associated with the XIIDRA Acquisition.
The Company continually monitors its variable consideration provisions and evaluates the estimates used as additional information becomes available. Adjustments will be made to these provisions periodically to reflect new facts and circumstances that may indicate that historical experience may not be indicative of current and/or future results. The Company is required to make subjective judgments based primarily on its evaluation of current market conditions and trade inventory levels related to the Company's products. These judgments include the potential impact of macroeconomic factors
on, among other things, unemployment and related changes in customer health insurance levels and government stimulus bills that focus on ensuring availability and access to lifesaving drugs during a public health crisis. This evaluation may result in an increase or decrease in the experience rate that is applied to current and future sales, or require an adjustment related to past sales, or both. If the trend in actual amounts of variable consideration varies from the Company's prior estimates, the Company adjusts these estimates, when such trend is believed to be sustainable. At that time, the Company would record the necessary adjustments which would affect net product revenue and earnings reported in the current period. The Company applies this method consistently for contracts with similar characteristics. The following describes the major sources of variable consideration in the Company’s customer arrangements and the methodology, estimates and judgments applied to estimate each type of variable consideration.
Cash Discounts and Allowances
Cash discounts are offered for prompt payment and allowances for volume purchases. Provisions for cash discounts and allowances are estimated at the time of sale and recorded as direct reductions to trade receivables and revenue. Management estimates the provisions for cash discounts and allowances based on contractual sales terms with customers, an analysis of unpaid invoices and historical payment experience. Estimated cash discounts and allowances have historically been predictable and less subjective, due to the limited number of assumptions involved, the consistency of historical experience and the fact that these amounts are generally settled within one month of incurring the liability.
Returns
Consistent with industry practice, customers are generally allowed to return certain products, primarily of the Company's consumer and ophthalmic businesses, within a specified period of time before and after the product's expiration date. The returns provision is estimated utilizing historical sales and return rates over the period during which customers have a right of return, taking into account available information on competitive products and contract changes. The information utilized to estimate the returns provision includes: (i) historical return and exchange levels, (ii) external data with respect to inventory levels in the wholesale distribution channel, (iii) external data with respect to prescription demand for products, (iv) remaining shelf lives of products at the date of sale and (v) estimated returns liability to be processed by year of sale based on an analysis of lot information related to actual historical returns.
In determining the estimate for returns, management is required to make certain assumptions regarding the timing of the introduction of new products and the potential of these products to capture market share. In addition, certain assumptions with respect to the extent and pattern of decline associated with generic competition are necessary. These assumptions are formulated using market data for similar products, past experience and other available information. These assumptions are continually reassessed, and changes to the estimates and assumptions are made as new information becomes available.
Rebates and Chargebacks
Certain product sales, primarily proprietary and generic pharmaceutical products within the Pharmaceuticals segment, made under governmental and managed-care pricing programs in the U.S. are subject to rebates. The Company participates in state government-managed Medicaid programs, as well as certain other qualifying federal and state government programs whereby rebates are provided to participating government entities. Medicaid rebates are generally billed 45 days to 270 days after the quarter in which the product is dispensed to the Medicaid participant. As a result, the Medicaid rebate reserve includes an estimate of outstanding claims for end-customer sales that occurred, but for which the related claim has not been billed and/or paid, and an estimate for future claims that will be made when inventory in the distribution channel is sold through to plan participants. The calculation of the Medicaid rebate reserve also requires other estimates, such as estimates of sales mix, to determine which sales are subject to rebates and the amount of such rebates. Quarterly, the Medicaid rebate reserve is adjusted based on actual claims paid. Due to the delay in billing, adjustments to actual claims paid may incorporate revisions of that reserve for several periods.
Managed Care rebates relate to contractual agreements to sell products to managed care organizations and pharmacy benefit managers at contractual rebate percentages in exchange for volume and/or market share.
Chargebacks relate to contractual agreements to sell certain products, primarily proprietary and generic pharmaceutical products within the Pharmaceuticals segment to government agencies, group purchasing organizations and other indirect customers at contractual prices that are lower than the list prices the Company charges wholesalers. When these group purchasing organizations or other indirect customers purchase products through wholesalers at these reduced prices, the wholesaler charges the Company for the difference between the prices they paid the Company and the prices at which they sold the products to the indirect customers.
In estimating provisions for rebates and chargebacks, management considers relevant statutes with respect to governmental pricing programs and contractual sales terms with managed-care providers and group purchasing organizations. Management estimates the amount of product sales subject to these programs based on historical utilization levels. Changes in the level of
utilization of products through private or public benefit plans and group purchasing organizations will affect the amount of rebates and chargebacks that the Company is obligated to pay. Management continually updates these factors based on new contractual or statutory requirements, and any significant changes in sales trends that may impact the percentage of products subject to rebates or chargebacks.
The amount of Managed Care, Medicaid and other rebates and chargebacks as it relates to proprietary and generic pharmaceutical products within the Pharmaceuticals segment, has become more significant as a result of a combination of deeper discounts implemented in each of the last three years and increased Medicaid utilization due to expansion of government funding for these programs. Management’s estimate for rebates and chargebacks may be impacted by a number of factors, but the principal factor relates to the level of inventory in the distribution channel.
Rebate provisions are based on factors such as timing and terms of plans under contract, time to process rebates, product pricing, sales volumes, amount of inventory in the distribution channel and prescription trends. Adjustments to actual for the years 2023 and 2022 were not material to the Company's revenues or earnings.
Patient Co-Pay Assistance programs, Consumer Rebates and Loyalty Programs are rebates offered on a limited number of the Company’s products. Patient Co-Pay Assistance Programs are patient discount programs offered in the form of coupon cards or point of sale discounts, with which patients receive certain discounts off their prescription at participating pharmacies, as defined by the specific product program. An accrual for these programs is established, equal to management’s estimate of the discount, rebate and loyalty incentives attributable to a sale. That estimate is based on historical experience and other relevant factors. The accrual is adjusted throughout each quarter based on actual experience and changes in other factors, if any.
Distribution Fees
The Company sells products to certain wholesalers, and large pharmacy chains such as CVS and Walmart, usually under Distribution Services Agreements ("DSAs"). Under the DSAs, the wholesalers agree to provide services, and the Company pays the contracted DSA distribution service fees for these services based on product volumes. Additionally, price appreciation credits are generated when the Company increases a product’s wholesaler acquisition cost (“WAC”) under contracts with certain wholesalers. Under such contracts, the Company is entitled to credits from such wholesalers for the impact of that WAC increase on inventory currently on hand at the wholesalers. Such credits are offset against the total distribution service fees paid to each such wholesaler. The variable consideration associated with price appreciation credits is reflected in the transaction price of products sold when it is determined to be probable that a significant reversal will not occur.
Contract Assets and Contract Liabilities
There are no contract assets for any period presented. Contract liabilities consist of deferred revenue, the balance of which is not material to any period presented.
Sales Commissions
Sales commissions are generally attributed to periods shorter than one year and therefore are expensed when incurred. Sales commissions are included in selling, general and administrative expenses.
Financing Component
The Company has elected not to adjust consideration for the effects of a significant financing component when the period between the transfer of a promised good or service to the customer and when the customer pays for that good or service will be one year or less. The Company's global payment terms are generally between thirty to ninety days.
Leases
The Company leases certain facilities, vehicles and equipment principally under multi-year agreements generally having a lease term of one to twenty years, some of which include termination options and options to extend the lease term from one to five years or on a month-to-month basis. The Company includes options that are reasonably certain to be exercised as part of the lease term. The Company may negotiate termination clauses in anticipation of changes in market conditions but generally, these termination options are not exercised. Certain lease agreements also include variable payments that are dependent on usage or may vary month-to-month such as insurance, taxes and maintenance costs. None of the Company's lease agreements contain material residual value guarantees or material restrictive covenants.
The Company is required to record a right-of-use asset and corresponding lease liability, equal to the present value of the lease payments at the commencement date of each lease. For all asset classes, in determining future lease payments, the Company has elected to aggregate lease components, such as payments for rent, taxes and insurance costs with non-lease components such as maintenance costs, and account for these payments as a single lease component. In limited
circumstances, when the information necessary to determine the rate implicit in a lease is available, the present value of the lease payments is determined using the rate implicit in that lease. If the information necessary to determine the rate implicit in a lease is not available, the Company uses its incremental borrowing rate at the commencement of the lease, which represents the rate of interest that the Company would incur to borrow on a collateralized basis over a similar term.
All leases must be classified as either an operating lease or finance lease. The classification is determined based on whether substantive control has been transferred to the lessee. The classification governs the pattern of lease expense recognition. For leases classified as operating leases, total lease expense over the term of the lease is equal to the undiscounted payments due in accordance with the lease arrangement. Fixed lease expense is recognized periodically on a straight-line basis over the term of each lease and includes: (i) imputed interest during the period on the lease liability determined using the effective interest rate method plus (ii) amortization of the right-of-use asset for that period. Amortization of the right-of-use asset during the period is calculated as the difference between the straight-line expense and the imputed interest on the lease liability for that period. Variable lease expense is recognized when the achievement of the specific target is considered probable.
Research and Development Expenses
Costs related to internal research and development programs, including costs associated with the development of acquired IPR&D, are expensed as goods are delivered or services are performed. Under certain research and development arrangements with third parties, the Company may be required to make payments that are contingent on the achievement of specific developmental, regulatory and/or commercial milestones. Milestone payments made to third parties before a product receives regulatory approval, but after the milestone is determined to be probable, are expensed and included in Research and development expenses. Milestone payments made to third parties after regulatory approval is received are capitalized and amortized over the estimated useful life of the approved product.
Amounts due from third parties as reimbursement of development activities conducted under certain research and development arrangements are recognized as a reduction of Research and development expenses.
Legal Costs
Legal fees and other costs related to litigation and other legal proceedings or services are expensed as incurred and are included in Selling, general and administrative expenses. Certain legal costs associated with acquisitions are included in Acquisition-related costs and certain legal costs associated with divestitures, legal settlements and other business development activities are included in Litigation and other matters or Gain on investments, net within Other expense, net, as appropriate. Legal costs expensed are reported net of expected insurance recoveries. A claim for insurance recovery is recognized when realization becomes probable.
Advertising Costs
Advertising costs comprise product samples, print media, promotional materials and television advertising and are expensed on the first use of the advertisement. Included in Selling, general and administrative expenses are advertising costs of $375 million, $319 million and $335 million, for 2023, 2022 and 2021, respectively.
Share-Based Compensation
Prior to the B+L IPO, the Company participated in BHC’s long-term incentive program. Stock-based compensation expense reflected in the accompanying Consolidated Financial Statements for the year 2021 relates to stock plan awards of BHC awarded to Bausch + Lomb employees and not stock awards of Bausch + Lomb as Bausch + Lomb did not grant stock awards for any period presented prior to the B+L IPO. In addition to share-based compensation expense attributable to employees that are specific to the Bausch + Lomb business, share-based compensation expense also includes allocated charges from BHC, related to BHC employees providing corporate services to Bausch + Lomb. Accordingly, the amounts presented for the year 2021 are not necessarily indicative of future awards and do not necessarily reflect the results that Bausch + Lomb would have experienced as an independent company for the period presented.
Effective May 5, 2022, Bausch + Lomb established the Bausch + Lomb Corporation 2022 Omnibus Incentive Plan (as amended and restated, the “Omnibus Plan”). A total of 28,000,000 common shares of Bausch + Lomb were originally authorized for issuance under the Omnibus Plan. Effective April 24, 2023, Bausch + Lomb’s shareholders approved an amendment and restatement of the Omnibus Plan to increase the number of shares authorized for issuance thereunder by an additional 10,000,000 common shares, resulting in an aggregate 38,000,000 common shares of Bausch + Lomb authorized for issuance under the Plan (the “Plan Amendment”). The Omnibus Plan provides for the grant of various types of awards including restricted stock units (“RSUs”), stock appreciation rights, stock options, performance-based awards and cash awards. Under the Omnibus Plan, the exercise price of awards, if any, is set on the grant date and may not be less than the fair market value per share on that date. Generally, stock options have a term of ten years and a three-year vesting period, subject to limited exceptions.
The Company recognizes all share-based payments to employees of the Company, including grants of employee stock options and RSUs, at estimated fair value. The Company amortizes the fair value of stock option or RSU grants on a straight-line basis over the requisite service period of the individual stock option or RSU grant, which generally equals the vesting period. Stock option and RSU forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Share-based compensation is recorded in Research and development expenses and Selling, general and administrative expenses, as appropriate.
Acquisition-Related Contingent Consideration
Acquisition-related contingent consideration, which primarily consists of potential milestone payments and royalty obligations, is recorded in the Consolidated Balance Sheets at its acquisition date estimated fair value, in accordance with the acquisition method of accounting. The fair value of the acquisition-related contingent consideration is remeasured each reporting period, with changes in fair value recorded in the Consolidated Statements of Operations. The fair value measurement of contingent consideration obligations arising from business combinations is determined via a probability-weighted discounted cash flow analysis, using unobservable (Level 3) inputs. These inputs may include: (i) the estimated amount and timing of projected cash flows, (ii) the probability of the achievement of the factor(s) on which the contingency is based and (iii) the risk-adjusted discount rate used to present value the probability-weighted cash flows. Significant increases or decreases in any of those inputs in isolation could result in a significantly higher or lower fair value measurement.
Interest Expense
Interest expense includes interest on outstanding debt currently held by the Company, previous intercompany financing arrangements with BHC (refer to Note 3, “RELATED PARTIES”, for more detail), standby fees, the amortization of debt discounts and deferred financing costs, accretion of debt premiums and the amortization of amounts excluded from the assessment of effectiveness related to the Company's cross-currency swaps. Interest costs are expensed as incurred, except to the extent such interest is related to construction in progress, in which case interest is capitalized. Capitalized interest related to construction in progress as of December 31, 2023 and 2022 was $69 million and $63 million, respectively, and is included in Property, plant and equipment, net.
Income Taxes
Income taxes are accounted for under the liability method. Deferred tax assets and liabilities are recognized for the temporary differences between the financial statement and income tax bases of assets and liabilities, and for operating losses and tax credit carryforwards. A valuation allowance is provided for the portion of deferred tax assets that is more likely than not to remain unrealized. Deferred tax assets and liabilities are measured using enacted tax rates and laws. Deferred tax assets for outside basis differences in investments in subsidiaries are only recognized if the difference will be realized in the foreseeable future.
The tax benefit from an uncertain tax position is recognized only if it is more likely than not that the tax position will be sustained upon examination by the appropriate taxing authority, based on the technical merits of the position. The tax benefits recognized from such position are measured based on the amount for which there is a greater than 50% likelihood of being realized upon settlement. Liabilities associated with uncertain tax positions are classified as long-term unless expected to be paid within one year. Interest and penalties related to uncertain tax positions, if any, are recorded in the provision for income taxes and classified with the related liability on the Consolidated balance sheets.
Prior to the B+L IPO, income tax expense and deferred tax balances in the Consolidated Financial Statements were calculated on a separate tax return basis. The Company's operations were included in the tax returns of certain respective BHC entities of which the Company is a part.
(Loss) earnings Per Share Attributable to Bausch + Lomb Corporation
Basic (loss) earnings per share attributable to Bausch + Lomb Corporation is calculated by dividing Net (loss) income attributable to Bausch + Lomb Corporation by the weighted-average number of common shares outstanding during the reporting period. Diluted (loss) earnings per share attributable to Bausch + Lomb Corporation is calculated by dividing Net (loss) income attributable to Bausch + Lomb Corporation by the weighted-average number of common shares outstanding during the reporting period after giving effect to dilutive potential common shares for stock options and RSUs, determined using the treasury stock method.
Comprehensive (Loss) Income
Comprehensive (loss) income comprises Net (loss) income and Other comprehensive income (loss). Other comprehensive income (loss) includes items such as foreign currency translation adjustments and certain pension and other postretirement benefit plan adjustments. Accumulated other comprehensive loss is recorded as a component of equity.
Contingencies
In the normal course of business, the Company is subject to loss contingencies, such as claims and assessments arising from litigation and other legal proceedings, contractual indemnities, product and environmental liabilities and tax matters. Accruals for loss contingencies are recorded when the Company determines that it is both probable that a liability has been incurred and the amount of loss can be reasonably estimated. If the estimate of the amount of the loss is a range and some amount within the range appears to be a better estimate than any other amount within the range, that amount is accrued as a liability. If no amount within the range is a better estimate than any other amount, the minimum amount of the range is accrued as a liability. These accruals are adjusted periodically as assessments change or additional information becomes available.
If no accrual is made for a loss contingency because the amount of loss cannot be reasonably estimated, the Company will disclose contingent liabilities when there is at least a reasonable possibility that a loss or an additional loss may have been incurred.
Employee Benefit Plans
The Company sponsors various retirement and pension plans, including defined benefit pension plans, defined contribution plans and a participatory defined benefit postretirement plan. The determination of defined benefit pension and postretirement plan obligations and their associated expenses requires the use of actuarial valuations to estimate the benefits employees earn while working, as well as the present value of those benefits. Net actuarial gains and losses that exceed 10% of the greater of the plan’s projected benefit obligations or the market-related value of assets are amortized to earnings over the shorter of the estimated average future service period of the plan participants (or the estimated average future lifetime of the plan participants if the majority of plan participants are inactive) or the period until any anticipated final plan settlements.
In addition, BHC offers certain of its defined benefit plans, a participatory defined benefit postretirement medical and life insurance plans and defined contribution plan to be shared amongst its businesses, including the Company, and the participation of its employees and retirees in these plans is reflected as though the Company participated in a multiemployer plan with BHC. For the periods presented prior to the B+L IPO, a proportionate share of the cost associated with the multiemployer plan is reflected in the Consolidated Financial Statements, while any assets and liabilities associated with the multiemployer plan are retained by BHC and recorded on BHC’s balance sheet. Bausch + Lomb's proportionate share of these costs were not material for any period presented.
BHC Investment
Prior to the B+L IPO, BHC’s cumulative interest in the assets and liabilities of the Company, inclusive of operating results, is presented as BHC investment on the Consolidated Balance Sheets. The Consolidated Statements of Equity include net cash transfers and other transfers between BHC and the Company as well as related party receivables and payables between the Company and other BHC affiliates that were settled on a current basis. As part of the B+L IPO, BHC Investment was reclassified to Additional paid-in capital.
New Accounting Standards
There were no new accounting standards adopted during 2023.
In November 2023, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update ("ASU") 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. The ASU expands public entities’ segment disclosures by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment’s profit or loss and assets. The ASU
is effective for the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and subsequent interim periods, with early adoption permitted. Retrospective application is required for all periods presented in the financial statements. The Company is currently evaluating the impact of adopting this ASU on its disclosures.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components of the effective tax rate reconciliation, and modifies other income tax-related disclosures. The ASU is effective for the Company's Annual Report on Form 10-K for fiscal year ended December 31, 2025. Early adoption is permitted and may be applied prospectively or retrospectively. The Company is currently evaluating the impact of adoption of this ASU on its disclosures.
3.RELATED PARTIES
Prior to May 10, 2022, Bausch + Lomb had been managed and operated in the ordinary course of business with other affiliates of BHC. Accordingly, certain corporate and shared costs prior to May 10, 2022 were allocated to Bausch + Lomb and reflected as expenses in the Consolidated Financial Statements. On May 10, 2022, Bausch + Lomb became an independent publicly traded company. However, as of February 16, 2024 BHC directly or indirectly holds 310,449,643 Bausch + Lomb common shares, which represents approximately 88.4% of the issued and outstanding common shares of Bausch + Lomb.
Additionally, there have been no sales made to related parties for all periods presented.
Allocated Centralized Costs Prior to May 10, 2022
Prior to May 10, 2022, the Consolidated Financial Statements have been prepared on a standalone basis and were derived from the consolidated financial statements and accounting records of BHC. BHC incurred significant corporate costs for services it provided to Bausch + Lomb, as well as to other BHC businesses. The allocated corporate and shared costs to Bausch + Lomb for 2023, 2022 and 2021 were $0, $76 million and $390 million, respectively. The allocated corporate and shared costs to Bausch + Lomb are included in Cost of goods sold (excluding amortization and impairments of intangible assets), Selling, general and administrative ("SG&A") and Research and development in the Consolidated Statements of Operations. All such amounts have been deemed to have been incurred and settled by Bausch + Lomb in the period in which the costs were recorded and are included in Additional paid-in capital during 2022 and in BHC investment during 2021. See Note 2, “SIGNIFICANT ACCOUNTING POLICIES” for additional information on the allocation of functional service expenses and general corporate expenses.
In the opinion of management of BHC and Bausch + Lomb, the expense and cost allocations have been determined on a basis considered to be a reasonable reflection of the utilization of services provided or the benefit received by Bausch + Lomb during 2023, 2022 and 2021. The amounts that would have been, or will be, incurred on a standalone basis could differ from the amounts allocated due to economies of scale, difference in management judgment, a requirement for more or fewer employees or other factors. In addition, the future results of operations, financial position and cash flows could differ materially from the historical results presented herein.
Accounts Receivable and Payable
Certain related party transactions between Bausch + Lomb and BHC have been included in Additional paid-in capital during 2022 when the related party transactions were not settled in cash.
Certain transactions between Bausch + Lomb and BHC and affiliate businesses are cash-settled on a current basis and, therefore, are reflected in the Consolidated Balance Sheets. Amounts payable to BHC and its affiliates related to related party transactions were $43 million and $53 million as of December 31, 2023 and December 31, 2022 respectively, and are included within Accounts payable in the Consolidated Balance Sheets. Amounts due from BHC and its affiliates related to related party transactions were $55 million and $102 million as of December 31, 2023 and December 31, 2022, respectively, of which $36 million and $90 million are included within Prepaid expenses and other current assets and $19 million and $12 million are included within Other non-current assets on the Consolidated Balance Sheets as of December 31, 2023 and December 31, 2022, respectively. These amounts are inclusive of the receivables and payables associated with the separation agreements entered into in connection with the B+L IPO, as discussed below.
BHC Pooled Financing Arrangements
Prior to the B+L IPO, certain legal entities comprising Bausch + Lomb participated in BHC pooled financing arrangements, which allowed for individual legal entities participating in the arrangements to borrow from the sponsoring bank. Total borrowings by the BHC pool participants were limited to the aggregate cash maintained in accounts held by the sponsoring bank. Net borrowings under BHC pooled financing arrangements from legal entities comprising Bausch + Lomb were $0 as of December 31, 2023 and December 31, 2022. BHC held a net positive cash balance in this pool, as these borrowings were more than offset by cash held by other BHC owned legal entities, including legal entities which have commingled Bausch +
Lomb and non-Bausch + Lomb activities. Cash from these commingled legal entities has generally not been included in Bausch + Lomb’s Consolidated Balance Sheets as such cash is not specifically identifiable to Bausch + Lomb. These borrowings are presented on the Consolidated Balance Sheets within Accrued and other current liabilities and in the Cash Flows From Financing Activities section of the Consolidated Statements of Cash Flows as Net borrowings under BHC pooled financing arrangements. Interest incurred on such borrowings were not material for any period presented.
Net Transfers to BHC
The total effect of the settlement of related party transactions is reflected as a financing activity in the Consolidated Statements of Cash Flows. The components of the Net transfers to BHC for the periods prior to the B+L IPO are as follows:
| | | | | | | | | | | | | | |
| (in millions) | | 2022 | | 2021 |
| Cash pooling and general financing activities | | $ | (226) | | | $ | (1,317) | |
| Corporate allocations | | 76 | | | 390 | |
| Benefit from income taxes | | 225 | | | 302 | |
| Total net transfers to BHC (as reflected in the Consolidated Statements of Equity) | | 75 | | | (625) | |
| Payment of BHC Purchase Debt | | (2,200) | | | — | |
| Share-based compensation | | (16) | | | (62) | |
| Other, net | | (222) | | | (43) | |
| Net transfers to BHC (as reflected in the Consolidated Statements of Cash Flows) | | $ | (2,363) | | | $ | (730) | |
Repayment of BHC Purchase Debt and Return of Capital
On January 1, 2022, in anticipation of the B+L IPO, Bausch + Lomb issued a $2,200 million promissory note to BHC (the “BHC Purchase Debt”) in conjunction with a legal reorganization. On May 10, 2022, Bausch + Lomb made payments to BHC of: (i) $2,200 million in full satisfaction of the BHC Purchase Debt and (ii) $229 million in return of capital using the proceeds from the May 2027 Term Facility (as defined in Note 10, “FINANCING ARRANGEMENTS”) and cash on hand. Included in Interest expense in the Consolidated Statements of Operations for 2022 was $47 million of interest attributed to the BHC Purchase Debt.
Separation Agreement with BHC
In connection with the completion of the B+L IPO, the Company entered into the MSA, that, together with the other agreements summarized herein, govern the relationship between BHC and the Company following the completion of the B+L IPO.
Other agreements that the Company entered into with BHC that govern aspects of Bausch + Lomb’s relationship with BHC following the B+L IPO include:
•Transition Services Agreement - In connection with the completion of the B+L IPO, Bausch + Lomb has entered into the TSA with BHC to provide each other, on a transitional basis and certain administrative support services, for a limited time to help ensure an orderly transition following the B+L IPO. The TSA specifies the calculation of Bausch + Lomb costs and receipts for these services. Under the TSA, Bausch + Lomb has received certain services from BHC, including information technology services, technical and engineering support, application support for operations, legal, payroll, finance, tax and accounting, general administrative services and other support services, and has also provided certain similar services to BHC. Individual services provided under the TSA have been scheduled for a specific period, generally ranging from six to twelve months, depending on the nature of the services. As of the date of this filing, a number of these transitional services have either expired or been terminated; however, certain transitional services are still being provided by the parties.
•Tax Matters Agreement - In connection with the completion of the B+L IPO, Bausch + Lomb has entered into a Tax Matters Agreement with BHC that governs the parties’ respective rights, responsibilities and obligations with respect to tax liabilities and benefits, tax attributes, the preparation and filing of tax returns, the control of audits and other tax proceedings and other matters regarding taxes following the B+L IPO.
•Employee Matters Agreement - In connection with the completion of the B+L IPO, Bausch + Lomb has entered into an Employee Matters Agreement with BHC that governs, among other things, the allocation of employee-related liabilities, the mechanics for the transfer of Bausch + Lomb employees, the treatment of outstanding equity awards and the treatment of Bausch + Lomb employees’ participation in BHC’s retirement and health and welfare plans.
In addition to the previously discussed agreements, Bausch + Lomb has entered into certain other agreements with BHC including, but not limited to, the Intellectual Property Matters Agreement and the Real Estate Matters Agreement that provide a framework for the ongoing relationship with BHC.
Charges incurred related to the above agreements were $2 million and $8 million for 2023 and 2022, respectively, and are primarily reflected within SG&A in the Consolidated Statements of Operations.
4.ACQUISITIONS AND LICENSING AGREEMENTS
2023 Acquisitions
Acquisition of XIIDRA®
On June 30, 2023, a wholly owned subsidiary of the Company, Bausch + Lomb Ireland Limited, entered into a Stock and Asset Purchase Agreement (the “Acquisition Agreement”) with Novartis Pharma AG and Novartis Finance Corporation (together with Novartis Pharma AG, “Novartis”) and, solely for purposes of guaranteeing certain obligations of the acquiring entity under the Acquisition Agreement, the Company, to acquire XIIDRA® (lifitegrast ophthalmic solution) and certain other ophthalmology assets (the “XIIDRA Acquisition”).
On September 29, 2023, under the terms of the Acquisition Agreement, the Company, through its affiliate, consummated the XIIDRA Acquisition for: (i) an up-front cash payment of $1,750 million, (ii) the assumption of certain pre-existing milestone payments and (iii) potential future milestone obligations of up to $750 million, as discussed below. The strategic XIIDRA Acquisition is expected to complement Bausch + Lomb’s existing dry eye franchise that includes eye and contact lens drops from the Company’s consumer brand franchises and novel treatments within its pharmaceutical business, such as MIEBO® (perfluorohexyloctane ophthalmic solution). The assets acquired and liabilities assumed are included within the Company's Pharmaceuticals segment.
The XIIDRA Acquisition has been accounted for as a business combination under the acquisition method of accounting. The estimated aggregate acquisition consideration of approximately $1,753 million is calculated as follows:
| | | | | | | | |
| (in millions) | | |
| Cash consideration paid to Novartis at closing, per Acquisition Agreement | | $ | 1,750 | |
| Estimated fair value of contingent consideration | | 3 | |
| Aggregate purchase consideration | | $ | 1,753 | |
The up-front cash payment of $1,750 million was paid on September 29, 2023, using the proceeds received from the issuance of the October 2028 Secured Notes and the establishment of the September 2028 Term Facility, each as defined and further discussed in Note 10, “FINANCING ARRANGEMENTS”.
Contingent consideration included as part of the consideration relates to potential future milestone obligations of up to $750 million, including: (i) up to $475 million in cash payable upon the achievement of specified commercialization and sales milestones for certain pipeline products and (ii) up to $275 million in cash payable upon the achievement of specified sales milestones for XIIDRA®. The fair value of the contingent consideration recognized on the acquisition date of $3 million was estimated by using the inputs disclosed in Note 5, “FAIR VALUE MEASUREMENTS”. The Company reassesses its acquisition-related contingent consideration liabilities each quarter for changes in fair value.
Assets Acquired and Liabilities Assumed
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed related to the XIIDRA Acquisition as of the acquisition date, inclusive of measurement period adjustments:
| | | | | | | | |
| (in millions) | | |
| Intangible assets, net | | $ | 1,600 | |
| Prepaid expenses and other current assets | | 162 | |
| Accrued and other current liabilities | | (1) | |
| Other non-current liabilities | | (31) | |
| Total identifiable net assets | | 1,730 | |
| Goodwill | | 23 | |
| Total fair value of consideration transferred | | $ | 1,753 | |
Since the date of acquisition, adjustments made during the measurement period have included an increase of $5 million to Intangible assets, net with an offset to Prepaid expenses and other current assets, which is reflected in the table above.
The fair value of the identifiable intangible assets is determined primarily using the “income approach,” which requires a forecast of the expected future cash flows (including revenue growth rates, cost of goods sold, operating expenses and discount rate). The intangible assets acquired, as well as their fair values and estimated useful life consist of the following:
| | | | | | | | | | | | | | |
| (in millions) | | Fair Value | | Estimated Useful Life
(In Years) |
Product brand | | $ | 1,595 | | | 8.75 |
| Acquired in-process research and development intangible asset | | 5 | | | N/A |
| Total Intangible assets, net | | $ | 1,600 | | | |
Prepaid expenses and other current assets associated with the XIIDRA Acquisition represents the terms of an interim contract to purchase inventory, as embedded within the agreements associated with the XIIDRA Acquisition. The terms of the interim contract allowed the Company to acquire the remaining XIIDRA® inventory from Novartis at the end of the contractual term. The remaining inventory was acquired during December 2023, and the prepaid expenses and other current assets recognized were reclassified into Inventories, net as of December 31, 2023. The balance of this interim contract will be released to Cost of goods sold (excluding amortization and impairments of intangible assets) as the Company sells the acquired inventory, over an assumed inventory turnover cycle of approximately two years. Cost of goods sold for the year ended 2023 includes $20 million related to the release of this interim contract.
Other non-current liabilities associated with the XIIDRA Acquisition represent the fair value of the historical contingent consideration liability assumed from Novartis by the Company as a part of the XIIDRA Acquisition. The fair value of the assumed contingent consideration recognized on the acquisition date was $31 million and was estimated by using a discount rate of 11%. The Company reassesses its acquisition-related contingent consideration liabilities each quarter for changes in fair value. See Note 5, “FAIR VALUE MEASUREMENTS” for additional information regarding the fair value assessment of the acquisition-related contingent consideration liabilities.
Goodwill associated with the XIIDRA Acquisition represents the workforce acquired, as well as future operating efficiencies and cost savings. Substantially all of the goodwill associated with the XIIDRA Acquisition is deductible for income tax purposes.
The valuation of the assets acquired and liabilities assumed, as part of the XIIDRA Acquisition, has not yet been finalized as of December 31, 2023. The areas that could be subject to change primarily relate to income tax matters. The Company will finalize these amounts no later than one year from the acquisition date.
Revenue and Operating Results
Net revenues and earnings, attributable to the XIIDRA Acquisition, from the date of acquisition through December 31, 2023, were $106 million and $17 million, respectively.
Pro Forma Financial Information
The following table presents the unaudited pro forma combined results of the Company and the acquired assets for the years ended December 31, 2023 and 2022, as if the XIIDRA Acquisition, and the related financing, had occurred on January 1, 2022:
| | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 |
| Revenues | | $ | 4,395 | | | $ | 4,255 | |
| Net loss attributable to Bausch + Lomb Corporation | | $ | (471) | | | $ | (289) | |
The unaudited pro forma combined financial information was prepared using the acquisition method of accounting and was based on the historical financial information of the Company and the acquired assets. In order to reflect the occurrence of the acquisition on January 1, 2022 as required, the unaudited pro forma financial information includes adjustments to reflect incremental amortization expense incurred based on the fair values of the identifiable intangible assets acquired, the incremental cost of products sold related to the release of an interim contract to purchase inventory, as embedded within the agreements associated with the XIIDRA Acquisition, elimination of historical impairments and accretion expenses related to historical contingent considerations recorded by Novartis, the recording of new/assumed contingent consideration accretion expense, the additional interest expense associated with the issuance of debt to finance the acquisition and the tax impact of each of the aforementioned adjustments. Included in the Bausch + Lomb Consolidated Statements of Operations for 2023 are:
(i) acquisition-related transaction costs, included within Other expense, net, of $20 million, which are directly related to the XIIDRA Acquisition, and include expenditures for representation and warranty insurance premiums, legal, valuation, accounting and other similar professional services and (ii) acquisition-related financing costs, included within Interest expense, of $16 million, which are directly related to the XIIDRA Acquisition, and include expenditures for certain upfront financing commitment costs related to debt financing commitments in place prior to the XIIDRA Acquisition, the issuance of the October 2028 Secured Notes and the establishment of the September 2028 Term Facility, each as defined and further discussed in Note 10, “FINANCING ARRANGEMENTS”. These acquisition-related transaction and financing costs are reflected in pro forma Net loss attributable to Bausch + Lomb Corporation, in the table above, for 2022.
The unaudited pro forma financial information is not necessarily indicative of what the consolidated results of operations would have been had the XIIDRA Acquisition been completed on January 1, 2022. In addition, the unaudited pro forma financial information is not a projection of future results of operations of the combined company nor does it reflect the expected realization of any synergies or cost savings associated with the acquisition.
Acquisition of Blink® Product Line
On July 6, 2023, the Company announced that it had consummated a transaction with Johnson & Johnson Vision, pursuant to which the Company, through an affiliate, had acquired the Blink® product line of eye and contact lens drops, which consists of Blink® Tears Lubricating Eye Drops, Blink® Tears Preservative Free Lubricating Eye Drops, Blink GelTears® Lubricating Eye Drops, Blink® Triple Care Lubricating Eye Drops, Blink Contacts® Lubricating Eye Drops and Blink-N-Clean® Lens Drops. This acquisition was made by the Company to continue to grow its global over-the-counter business. Under the terms of the purchase agreement, the Company, through an affiliate, acquired the Blink® product line of eye and contact lens drops for an up-front cash payment of $107 million, which was paid on the closing of the transaction. The acquired assets are included within the Company's Vision Care segment.
The Company accounted for the transaction as an asset acquisition. The acquired assets consist of inventory and intangible assets. The intangible assets acquired, as well as their estimated useful lives consist of the following:
| | | | | | | | | | | | | | |
| (in millions) | | | | Estimated Useful Life
(In Years) |
Corporate brands | | $ | 73 | | | 12 |
| Product brands | | 12 | | | 10 |
| Technology and other | | 6 | | | 9 |
| Total Intangible assets, net | | $ | 91 | | | |
Since the date of acquisition, the Company has recorded certain non-material working capital adjustments, which are reflected in the table above.
Acquisition of AcuFocus, Inc.
On January 17, 2023, the Company acquired AcuFocus, Inc. ("AcuFocus") for an up-front payment of $35 million, $31 million of which was paid in January 2023 with the remaining purchase price to be paid within 18 months following the date of the transaction, less any amounts that are the subject of any indemnification claims. AcuFocus is an ophthalmic medical device company. The acquisition was made by the Company to acquire breakthrough small aperture intraocular technology for certain cataract patients. The AcuFocus business is included within the Surgical segment. Supplemental pro forma information related to revenue and earnings for 2023 are not provided as they did not have a material impact on the Company's operations. Additional contingent payments may become due upon achievement of future sales milestones. At the time of acquisition, the acquisition-related contingent consideration liability related to this transaction was approximately $5 million, which the Company reassesses each quarter for changes in fair value. See Note 5, “FAIR VALUE MEASUREMENTS” for additional information regarding the fair value assessment of the acquisition-related contingent consideration liabilities.
The acquisition of AcuFocus has been accounted for as a business combination under the acquisition method of accounting. As a result of this transaction, recorded within the Consolidated Balance Sheets are Inventories, net of $4 million, Prepaid expenses and other current assets of $4 million, Intangible assets, net of $28 million, Goodwill of $2 million, Deferred tax assets, net of $2 million, Property, plant and equipment, net of $1 million, Accounts payable of $1 million and Accrued and other current liabilities of $1 million. Since the date of acquisition, adjustments made during the measurement process have included a decrease of $6 million to Deferred tax assets, net with an offset to Goodwill.
2022 Licensing Agreement and Acquisitions
As described below, during 2022, the Company entered a strategic licensing agreement and completed the following acquisitions for an aggregate up-front payment of $45 million.
On July 28, 2022, the Company entered into an exclusive five year European distribution agreement with Sanoculis Ltd. ("Sanoculis") for Sanoculis' Minimally Invasive Micro Sclerostomy ("MIMS®"). MIMS® is an innovative minimally invasive surgical procedure for the treatment of glaucoma and is expected to complement existing Bausch + Lomb products within this market. As a part of the agreement, the Company agreed to purchase the MIMS® product from Sanoculis for distribution in various European countries.
On November 21, 2022, the Company acquired Paragon BioTeck, Inc. (“Paragon BioTeck”), an eye-care focused drug development company, having a primary emphasis on the early detection of ocular diseases. The acquisition of Paragon BioTeck has been accounted for by the Company as an asset acquisition. The primary asset in the transaction, the trademarks, represented substantially all of the fair value of the gross assets acquired. There are no future sales milestones associated with this transaction.
On December 12, 2022, the Company acquired Total Titanium, Inc. (“Total Titanium”), a privately held ophthalmic microsurgical instrument and machined parts manufacturing company. The transaction was completed to assist in driving revenue growth as well as increasing manufacturing capacity. The fair value of the acquisition of Total Titanium has been accounted for as a business combination and included in the Surgical segment. Supplemental pro forma information related to revenue and earnings for 2022 are not provided as they did not have a material impact on the Company's operations. Additional contingent payments may be payable upon reaching key future milestone achievements related to sales and employee retention. Refer to Note 21, “COMMITMENTS AND CONTINGENCIES” for further detail regarding potential future milestone payments related to previously entered transactions and agreements.
As a result of these transactions, recorded within the Consolidated Balance Sheet are Trade receivables, net of $1 million, Inventories, net of $1 million, Property, plant and equipment of $2 million, Intangibles, net of $43 million, Goodwill of $5 million and Deferred tax liabilities, net of $11 million.
5.FAIR VALUE MEASUREMENTS
Fair value measurements are estimated based on valuation techniques and inputs categorized as follows:
•Level 1 — Quoted prices in active markets for identical assets or liabilities;
•Level 2 — Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and
•Level 3 — Unobservable inputs that are supported by little or no market activity and that are financial instruments whose values are determined using discounted cash flow methodologies, pricing models, or similar techniques, as well as instruments for which the determination of fair value requires significant judgment or estimation.
If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following fair value hierarchy table presents the components and classification of the Company's financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2023 | | December 31, 2022 |
| (in millions) | | Carrying Value | | Level 1 | | Level 2 | | Level 3 | | Carrying Value | | Level 1 | | Level 2 | | Level 3 |
| Assets: | | | | | | | | | | | | | | | | |
| Cash equivalents | | $ | 44 | | | $ | 36 | | | $ | 8 | | | $ | — | | | $ | 81 | | | $ | 72 | | | $ | 9 | | | $ | — | |
| Foreign currency exchange contracts | | $ | 1 | | | $ | — | | | $ | 1 | | | $ | — | | | $ | 5 | | | $ | — | | | $ | 5 | | | $ | — | |
| Liabilities: | | | | | | | | | | | | | | | | |
| Acquisition-related contingent consideration | | $ | 44 | | | $ | — | | | $ | — | | | $ | 44 | | | $ | 4 | | | $ | — | | | $ | — | | | $ | 4 | |
| Foreign currency exchange contracts | | $ | 4 | | | $ | — | | | $ | 4 | | | $ | — | | | $ | 2 | | | $ | — | | | $ | 2 | | | $ | — | |
| Cross-currency swaps | | $ | 84 | | | $ | — | | | $ | 84 | | | $ | — | | | $ | 39 | | | $ | — | | | $ | 39 | | | $ | — | |
Cash equivalents consist of highly liquid investments, primarily money market funds, with maturities of three months or less when purchased, and are reflected in the Consolidated Balance Sheets at carrying value, which approximates fair value due to their short-term nature.
There were no transfers into or out of Level 3 during 2023 and 2022.
Cross-currency Swaps
During the third quarter of 2022, the Company entered into cross-currency swaps, with an aggregate notional value of $1,000 million, to mitigate fluctuation in the value of a portion of its euro-denominated net investment in its Consolidated Financial Statements from fluctuation in exchange rates. The euro-denominated net investment being hedged is the Company’s investment in certain euro-denominated subsidiaries.
The assets and liabilities associated with the Company's cross-currency swaps as included in the Consolidated Balance Sheets are as follows:
| | | | | | | | | | | |
| (in millions) | December 31, 2023 | | December 31, 2022 |
| Other non-current liabilities | $ | 90 | | | $ | 45 | |
| Prepaid expenses and other current assets | $ | 6 | | | $ | 6 | |
| Net fair value | $ | 84 | | | $ | 39 | |
The following table presents the effect of hedging instruments on the Consolidated Statements of Comprehensive Loss and the Consolidated Statements of Operations as of December 31, 2023 and 2022 :
| | | | | | | | | | | |
| (in millions) | 2023 | | 2022 |
| Loss recognized in Other comprehensive loss | $ | 45 | | | $ | 45 | |
| Gain excluded from assessment of hedge effectiveness | $ | 13 | | | $ | 6 | |
| Location of gain of excluded component | Interest Expense | | Interest Expense |
No portion of the cross-currency swaps were ineffective for 2023 and 2022. Interest settlement of the Company's cross-currency swaps occurs in January and July each year, with the first settlement having occurred in January 2023. During 2023, the Company received $13 million in interest settlements, which are reported as investing activities in the Consolidated Statements of Cash Flows.
Foreign Currency Exchange Contracts
The Company enters into foreign currency exchange contracts to economically hedge the foreign exchange exposure on certain of the Company's intercompany balances. As of December 31, 2023, these contracts had an aggregate notional amount of $331 million.
The assets and liabilities associated with the Company’s foreign exchange contracts as included in the Consolidated Balance Sheets December 31, 2023 and December 31, 2022 are as follows:
| | | | | | | | | | | |
| (in millions) | December 31,
2023 | | December 31,
2022 |
| Accrued and other current liabilities | $ | (4) | | | $ | (2) | |
| Prepaid expenses and other current assets | $ | 1 | | | $ | 5 | |
| Net fair value | $ | (3) | | | $ | 3 | |
The following table presents the effect of the Company’s foreign exchange contracts on the Consolidated Statements of Operations and the Consolidated Statements of Cash Flows for 2023 and 2022:
| | | | | | | | | | | |
| (in millions) | 2023 | | 2022 |
| (Loss) gain related to changes in fair value | $ | (6) | | | $ | 3 | |
| Gain (loss) related to settlements | $ | 2 | | | $ | (8) | |
Acquisition-related Contingent Consideration Obligations
Acquisition-related contingent consideration, which primarily consists of potential milestone payments, is recorded in the Consolidated Balance Sheets at its acquisition date estimated fair value, in accordance with the acquisition method of accounting. The fair value of the acquisition-related contingent consideration is remeasured each reporting period, with changes in fair value recorded in the Consolidated Statements of Operations. The fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement as defined in fair value measurement accounting.
The fair value measurement of contingent consideration obligations arising from business combinations is determined via a probability-weighted discounted cash flow analysis, using unobservable (Level 3) inputs. These inputs may include: (i) the estimated amount and timing of projected cash flows, (ii) the probability of the achievement of the factor(s) on which the contingency is based and (iii) the risk-adjusted discount rate used to present value the probability-weighted cash flows. Significant increases or decreases in any of those inputs in isolation could result in a significantly higher or lower fair value measurement. At December 31, 2023, the fair value measurements of acquisition-related contingent consideration were determined using risk-adjusted discount rates ranging from 11% to 28%, and a weighted average risk-adjusted discount rate of 12%. The weighted average risk-adjusted discount rate was calculated by weighting each contract’s relative fair value at December 31, 2023.
The following table presents a reconciliation of contingent consideration obligations measured on a recurring basis using significant unobservable inputs (Level 3) for the years 2023 and 2022:
| | | | | | | | | | | | | | | | | |
| (in millions) | 2023 | | 2022 |
| Balance, beginning of period | | $ | 4 | | | | $ | 9 | |
| Adjustments to Acquisition-related contingent consideration: | | | | | |
| Accretion for the time value of money | $ | 3 | | | | $ | — | | |
| Fair value adjustments due to changes in estimates of future payments | (1) | | | | (5) | | |
| Acquisition-related contingent consideration adjustments | | 2 | | | | (5) | |
| Additions (Note 4) | | 38 | | | | — | |
| Payments/Settlements | | — | | | | — | |
| Balance, end of period | | 44 | | | | 4 | |
| Current portion included in Accrued and other current liabilities | | 5 | | | | 4 | |
| Non-current portion | | $ | 39 | | | | $ | — | |
Fair Value of Long-term Debt
The fair value of long-term debt as of December 31, 2023 and 2022 were $4,668 million and $2,354 million, respectively, and was estimated using the quoted market prices for similar debt issuances (Level 2).
6.INVENTORIES
Inventories, net consist of:
| | | | | | | | | | | | | | |
| (in millions) | | December 31, 2023 | | December 31, 2022 |
| Raw materials | | $ | 261 | | | $ | 163 | |
| Work in process | | 100 | | | 44 | |
| Finished goods | | 667 | | | 421 | |
| | $ | 1,028 | | | $ | 628 | |
Inventory write-offs were $18 million, $21 million and $35 million for 2023, 2022 and 2021, respectively.
7.PROPERTY, PLANT AND EQUIPMENT
The major components of property, plant and equipment consist of:
| | | | | | | | | | | | | | |
| (in millions) | | December 31, 2023 | | December 31, 2022 |
| Land | | $ | 45 | | | $ | 44 | |
| Buildings | | 624 | | | 614 | |
| Machinery and equipment | | 1,657 | | | 1,585 | |
| Other equipment and leasehold improvements | | 347 | | | 335 | |
| Construction in progress | | 347 | | | 237 | |
| | 3,020 | | | 2,815 | |
| Less accumulated depreciation | | (1,630) | | | (1,515) | |
| | $ | 1,390 | | | $ | 1,300 | |
Depreciation expense was $142 million, $135 million and $123 million for 2023, 2022 and 2021, respectively.
8.INTANGIBLE ASSETS AND GOODWILL
Intangible Assets
The major components of intangible assets consist of:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Weighted-Average Remaining Useful Lives (Years) | | December 31, 2023 | | December 31, 2022 |
| (in millions) | | Gross Carrying Amount | | Accumulated Amortization and Impairments | | Net Carrying Amount | | Gross Carrying Amount | | Accumulated Amortization and Impairments | | Net Carrying Amount |
Finite-lived intangible assets: | | | | | | | | | | | | | |
| Product brands | 8 | | $ | 4,342 | | | $ | (2,581) | | | $ | 1,761 | | | $ | 2,650 | | | $ | (2,373) | | | $ | 277 | |
| Corporate brands | 11 | | 85 | | | (11) | | | 74 | | | 12 | | | (7) | | | 5 | |
| Product rights/patents | 4 | | 993 | | | (954) | | | 39 | | | 992 | | | (919) | | | 73 | |
| Technology and other | 7 | | 75 | | | (63) | | | 12 | | | 66 | | | (61) | | | 5 | |
| Total finite-lived intangible assets | | | 5,495 | | | (3,609) | | | 1,886 | | | 3,720 | | | (3,360) | | | 360 | |
| Acquired in-process research and development intangible asset | N/A | | 5 | | | — | | | 5 | | | — | | | — | | | — | |
| B&L Trademark | N/A | | 1,698 | | | — | | | 1,698 | | | 1,698 | | | — | | | 1,698 | |
| | | $ | 7,198 | | | $ | (3,609) | | | $ | 3,589 | | | $ | 5,418 | | | $ | (3,360) | | | $ | 2,058 | |
Long-lived assets with finite lives are tested for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. Impairment charges associated with these assets are included in Other expense, net in the Consolidated Statements of Operations. The Company continues to monitor the recoverability of its finite-lived intangible assets and tests the intangible assets for impairment if indicators of impairment are present.
Asset impairments for 2023, 2022 and 2021 were less than $1 million, $1 million and $12 million, respectively, related to the discontinuance of certain product lines.
Estimated amortization expense of finite-lived intangible assets for the five years ending December 31 and thereafter are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2024 | | 2025 | | 2026 | | 2027 | | 2028 | | Thereafter | | Total |
| Amortization | | $ | 288 | | | $ | 242 | | | $ | 209 | | | $ | 207 | | | $ | 207 | | | $ | 733 | | | $ | 1,886 | |
Goodwill
The changes in the carrying amounts of goodwill during the years ended 2023, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions) | | Bausch + Lomb | | Vision Care | | Pharmaceuticals | | Surgical | | Total |
Balance, January 1, 2021 | | $ | 4,685 | | | $ | — | | | $ | — | | | $ | — | | | $ | 4,685 | |
| Realignment of segment goodwill | | (4,685) | | | 3,674 | | | 689 | | | 322 | | | — | |
| Foreign exchange and other | | — | | | (78) | | | (14) | | | (7) | | | (99) | |
Balance, December 31, 2021 | | — | | | 3,596 | | | 675 | | | 315 | | | 4,586 | |
| Acquisitions (Note 4) | | — | | | — | | | — | | | 5 | | | 5 | |
| Foreign exchange and other | | — | | | (47) | | | (30) | | | (7) | | | (84) | |
Balance, December 31, 2022 | | — | | | 3,549 | | | 645 | | | 313 | | | 4,507 | |
| Acquisitions (Note 4) | | — | | | — | | | 23 | | | 8 | | | 31 | |
| Foreign exchange and other | | — | | | 7 | | | 25 | | | 5 | | | 37 | |
Balance, December 31, 2023 | | $ | — | | | $ | 3,556 | | | $ | 693 | | | $ | 326 | | | $ | 4,575 | |
Goodwill is not amortized but is tested for impairment at least annually as of October 1st at the reporting unit level. Refer below for results of the Company's recent goodwill impairment tests and impact of segment realignment on goodwill.
Refer to Note 2, “SIGNIFICANT ACCOUNTING POLICIES” for further detail regarding the Company's policies and testing approach in relation to goodwill impairment testing.
Second Quarter 2021 - Realignment of Segments
Bausch + Lomb has historically operated as part of BHC, reported under BHC’s segment structure and historically the Chief Operating Decision Maker (“CODM”) was the CODM of BHC. As Bausch + Lomb was transitioning into an independent, publicly traded company, BHC’s Chief Executive Officer (“CEO”), who was Bausch + Lomb’s CODM until the completion of the B+L IPO, evaluated how to view and measure Bausch + Lomb’s performance. This evaluation necessitated a realignment of Bausch + Lomb’s historical segment structure and, during the second quarter of 2021, Bausch + Lomb determined it is organized into three operating segments, which are also its reportable segments and reporting units. This realignment is consistent with how the CODM: (i) assesses operating performance on a regular basis, (ii) makes resource allocation decisions and (iii) designates responsibilities of his direct reports. Pursuant to these changes, effective in the second quarter of 2021, Bausch + Lomb operates in the following operating and reportable segments which are generally determined based on the decision-making structure of Bausch + Lomb and the grouping of similar products and services: (i) Vision Care (formerly named Vision Care/Consumer Health Care), (ii) Pharmaceuticals (formerly named Ophthalmic Pharmaceuticals) and (iii) Surgical.
This realignment in segment structure resulted in a change in the former Bausch + Lomb reporting unit, which is now divided between the: (i) Vision Care, (ii) Pharmaceuticals and (iii) Surgical reporting units. As a result of this realignment, goodwill was reassigned to each of the aforementioned reporting units using a relative fair value approach.
Immediately prior to the change in reporting units, Bausch + Lomb performed a qualitative fair value assessment for its former Bausch + Lomb reporting units. Based on the qualitative fair value assessment performed, Management believed that it was more likely than not that the carrying value of its former Bausch + Lomb reporting units were less than their respective fair values and therefore, concluded a quantitative assessment was not required.
Immediately following the change in reporting units, as a result of the change in composition of the net assets for its current (i) Vision Care, (ii) Pharmaceuticals and (iii) Surgical reporting units, Bausch + Lomb performed a quantitative fair value assessment. The quantitative fair value test utilized long-term growth rates of 2.0% and 3.0% and a range of discount rates between 7.0% and 10.0%, in estimation of the fair value of the reporting units. After completing the testing, the fair value of each of these reporting units exceeded its carrying value by more than 45%, and, therefore, there was no impairment to goodwill.
Annual Goodwill Impairment Tests
The Company conducted its annual goodwill impairment test as of October 1, 2021 by first assessing qualitative factors. In management's assessment, no qualitative factors were identified which suggested that it was more likely than not that the carrying amount of a reporting unit exceeded its fair value, and therefore concluded a quantitative fair value test for any of its reporting units was not required.
The Company conducted its annual goodwill impairment test as of October 1, 2022 by performing a quantitative assessment for each of its reporting units. The quantitative assessment utilized long-term growth rates of 2.0% and 3.0% and discount rates of 9.5% and 12.25%, in estimation of the fair value of the reporting units. After completing the testing, the fair value of each of these reporting units exceeded its carrying value by more than 25%, and, therefore, there was no impairment to goodwill.
The Company conducted its annual goodwill impairment test as of October 1, 2023 by performing a quantitative assessment for each of its reporting units. The quantitative assessment utilized long-term growth rates of 2.0% and 3.0% and discount rates ranging from 10.25% and 11.50%, in estimation of the fair value of the reporting units. After completing the testing, the fair value of each of these reporting units exceeded its carrying value by more than 25%, and, therefore, there was no impairment to goodwill.
December 31, 2023 Goodwill Impairment Assessment
No events occurred or circumstances changed during the period from October 1, 2023 (the last time goodwill was tested for all reporting units) through December 31, 2023 that would indicate that the fair value of any reporting unit might be below its carrying value.
If market conditions deteriorate, or if the Company is unable to execute its strategies, it may be necessary to record impairment charges in the future.
There were no goodwill impairment charges through December 31, 2023.
9.ACCRUED AND OTHER CURRENT LIABILITIES
Accrued and other current liabilities consist of:
| | | | | | | | | | | | | | |
| (in millions) | | December 31, 2023 | | December 31, 2022 |
| Employee compensation and benefit costs | | $ | 233 | | | $ | 196 | |
| Product rebates | | 191 | | | 153 | |
| Discounts and allowances | | 84 | | | 85 | |
| Product returns | | 66 | | | 59 | |
| Professional fees | | 53 | | | 66 | |
| Other | | 400 | | | 342 | |
| | $ | 1,027 | | | $ | 901 | |
10.FINANCING ARRANGEMENTS
Principal amounts of debt obligations and principal amounts of debt obligations net of issuance costs consist of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | December 31, 2023 | | December 31, 2022 |
| (in millions) | | Maturity | | Principal Amount | | Net of Issuance Costs | | Principal Amount | | Net of Issuance Costs |
| Senior Secured Credit Facilities | | | | | | | | | | |
| Revolving Credit Facility | | May 2027 | | $ | 275 | | | $ | 275 | | | $ | — | | | $ | — | |
| May 2027 Term Facility | | May 2027 | | 2,462 | | | 2,423 | | | 2,488 | | | 2,436 | |
| September 2028 Term Facility | | September 2028 | | 499 | | | 487 | | | — | | | — | |
| Senior Secured Notes | | | | | | | | | | |
8.375% Secured Notes | | October 2028 | | 1,400 | | | 1,377 | | | — | | | — | |
| Total long-term debt | | | | $ | 4,636 | | | 4,562 | | | $ | 2,488 | | | 2,436 | |
| Less: Current portion of long-term debt | | | | | | 30 | | | | | 25 | |
| Non-current portion of long-term debt | | | | | | $ | 4,532 | | | | | $ | 2,411 | |
Senior Secured Credit Facilities
On May 10, 2022, Bausch + Lomb entered into a credit agreement (the “Credit Agreement”, and the credit facilities thereunder, the “Credit Facilities”). Prior to the September 2023 Credit Facility Amendment (as defined below), the Credit Agreement provided for a term loan of $2,500 million with a five-year term to maturity (the “May 2027 Term Facility”) and a five-year revolving credit facility of $500 million (the “Revolving Credit Facility”).
On September 29, 2023, Bausch + Lomb entered into an incremental term loan facility secured on a pari passu basis with the Company’s existing May 2027 Term Facility. This incremental term loan facility was entered into in the form of an incremental amendment (the "September 2023 Credit Facility Amendment") to the Company’s existing Credit Agreement (the Credit Agreement, as amended by the September 2023 Credit Facility Amendment, the “Amended Credit Agreement”) and consisted of borrowings of $500 million in new term B loans with a five-year term to maturity (the "September 2028 Term Facility", and, together with the May 2027 Term Facility and the Revolving Credit Facility, the “Senior Secured Credit Facilities”). A portion of the proceeds from the September 2028 Term Facility and October 2028 Secured Notes (as defined below) were used to finance the $1,750 million upfront payment related to the XIIDRA Acquisition (as discussed further in Note 4, “ACQUISITIONS AND LICENSING AGREEMENTS”) and related acquisition and financing costs.
The Senior Secured Credit Facilities are secured by substantially all of the assets of Bausch + Lomb and its material, wholly-owned Canadian, U.S., Dutch and Irish subsidiaries, subject to certain exceptions. The May 2027 Term Facility and September 2028 Term Facility are denominated in U.S. dollars, and borrowings under the Revolving Credit Facility may be made available in U.S. dollars, euros, pounds sterling and Canadian dollars. As of December 31, 2023, the principal amounts outstanding under the May 2027 Term Facility and September 2028 Term Facility were $2,462 million and $499 million, respectively. As of December 31, 2023, the Company had $275 million of outstanding borrowings, $26 million of issued and outstanding letters of credit and remaining availability, subject to certain customary conditions, of $199 million under its Revolving Credit Facility.
Borrowings under the Revolving Credit Facility in: (i) U.S. dollars bear interest at a rate per annum equal to, at Bausch + Lomb’s option, either: (a) a term Secured Overnight Financing Rate ("SOFR")-based rate or (b) a U.S. dollar base rate, (ii) Canadian dollars bear interest at a rate per annum equal to, at Bausch + Lomb’s option, either: (a) Canadian Dollar Offered Rate ("CDOR") or (b) a Canadian dollar prime rate, (iii) euros bear interest at a rate per annum equal to EURIBOR and (iv) pounds sterling bear interest at a rate per annum equal to Sterling Overnight Index Average ("SONIA") (provided, however, that the term SOFR-based rate, CDOR, EURIBOR and SONIA shall be no less than 0.00% per annum at any time and the U.S. dollar base rate and the Canadian dollar prime rate shall be no less than 1.00% per annum at any time), in each case, plus an applicable margin. Term SOFR-based borrowings under the Revolving Credit Facility are subject to a credit spread adjustment of 0.10%.
The applicable interest rate margins for borrowings under the Revolving Credit Facility are: (i) between 0.75% to 1.75% with respect to U.S. dollar base rate or Canadian dollar prime rate borrowings and between 1.75% to 2.75% with respect to SOFR, EURIBOR, SONIA or CDOR borrowings based on Bausch + Lomb’s total net leverage ratio and (ii) after: (x) Bausch + Lomb’s senior unsecured non-credit-enhanced long-term indebtedness for borrowed money receives an investment grade rating from at least two of Standard & Poor’s (“S&P”), Moody’s and Fitch and (y) the May 2027 Term Facility and
September 2028 Term Facility have been repaid in full in cash (the “IG Trigger”), between 0.015% to 0.475% with respect to U.S. dollar base rate or Canadian dollar prime rate borrowings and between 1.015% to 1.475% with respect to SOFR, EURIBOR, SONIA or CDOR borrowings based on Bausch + Lomb’s debt rating. The stated rate of interest for borrowings under the Revolving Credit Facility at December 31, 2023 ranges from 8.19% to 8.21% per annum. In addition, Bausch + Lomb is required to pay commitment fees of 0.25% per annum in respect of the unutilized commitments under the Revolving Credit Facility, payable quarterly in arrears until the IG Trigger and, thereafter, a facility fee between 0.110% to 0.275% of the total revolving commitments, whether used or unused, based on Bausch + Lomb’s debt rating and payable quarterly in arrears. Bausch + Lomb is also required to pay letter of credit fees on the maximum amount available to be drawn under all outstanding letters of credit in an amount equal to the applicable margin on SOFR borrowings under the Revolving Credit Facility on a per annum basis, payable quarterly in arrears, as well as customary fronting fees for the issuance of letters of credit and agency fees.
Borrowings under the May 2027 Term Facility bear interest at a rate per annum equal to, at Bausch + Lomb’s option, either: (i) a term SOFR-based rate, plus an applicable margin of 3.25% or (ii) a U.S. dollar base rate, plus an applicable margin of 2.25% (provided, however, that the term SOFR-based rate shall be no less than 0.50% per annum at any time and the U.S. dollar base rate shall not be lower than 1.50% per annum at any time). Term SOFR-based borrowings under the May 2027 Term Facility are subject to a credit spread adjustment of 0.10%. The stated rate of interest under the May 2027 Term Facility at December 31, 2023 was 8.71% per annum.
Borrowings under the September 2028 Term Facility bear interest at a rate per annum equal to, at Bausch + Lomb’s option, either: (i) a term SOFR-based rate, plus an applicable margin of 4.00%, or (ii) a U.S. dollar base rate, plus an applicable margin of 3.00% (provided, however, that the term SOFR-based rate shall be no less than 0.00% per annum at any time and the U.S. dollar base rate shall not be lower than 1.00% per annum at any time). Term SOFR-based borrowings under the September 2028 Term Facility are not subject to any credit spread adjustment. The stated rate of interest under the September 2028 Term Facility at December 31, 2023 was 9.36% per annum.
Subject to certain exceptions and customary baskets set forth in the Amended Credit Agreement, Bausch + Lomb is required to make mandatory prepayments of the loans under the May 2027 Term Facility and September 2028 Term Facility under certain circumstances, including from: (i) 100% of the net cash proceeds of insurance and condemnation proceeds for property or asset losses (subject to reinvestment rights, decrease based on leverage ratios and net proceeds threshold), (ii) 100% of the net cash proceeds from the incurrence of debt (other than permitted debt as described in the Amended Credit Agreement), (iii) 50% of Excess Cash Flow (as defined in the Amended Credit Agreement) subject to decrease based on leverage ratios and subject to a threshold amount and (iv) 100% of net cash proceeds from asset sales (subject to reinvestment rights, decrease based on leverage ratios and net proceeds threshold). These mandatory prepayments may be used to satisfy future amortization.
The amortization rate for the May 2027 Term Facility is 1.00% per annum, or $25 million, payable in quarterly installments, and the first installment was paid on September 30, 2022. Bausch + Lomb may direct that prepayments be applied to such amortization payments in order of maturity. As of December 31, 2023, the remaining mandatory quarterly amortization payments for the May 2027 Term Facility were $81 million through March 2027, with the remaining term loan balance being due in May 2027.
The amortization rate for the September 2028 Term Facility is 1.00% per annum, or $5 million, payable in quarterly installments. Bausch + Lomb may direct that prepayments be applied to such amortization payments in order of maturity. As of December 31, 2023, the remaining mandatory quarterly amortization payments for the September 2028 Term Facility were $23 million through June 2028, with the remaining term loan balance being due in September 2028.
Senior Secured Notes
On September 29, 2023, Bausch + Lomb issued $1,400 million aggregate principal amount of 8.375% Senior Secured Notes due October 2028 (the "October 2028 Secured Notes"). A portion of the proceeds from the October 2028 Secured Notes, along with the proceeds of September 2028 Term Facility, were used to finance the $1,750 million upfront payment related to the acquisition of XIIDRA® and certain other ophthalmology assets from Novartis (as discussed further in Note 4, “ACQUISITIONS AND LICENSING AGREEMENTS”) and related acquisition-related transaction and financing costs. The October 2028 Secured Notes accrue interest at a rate of 8.375% per year, payable semi-annually in arrears on each April 1 and October 1, commencing on April 1, 2024.
The October 2028 Secured Notes are guaranteed by each of the Company’s subsidiaries that is a guarantor under the Amended Credit Agreement (the “Note Guarantors”). The October 2028 Secured Notes and the guarantees related thereto are senior obligations and are secured, subject to permitted liens and certain other exceptions, by the same first priority liens that secure the Company’s obligations under the Amended Credit Agreement under the terms of the indentures governing the October 2028 Secured Notes.
The October 2028 Secured Notes and the guarantees related thereto rank equally in right of repayment with all of the Company’s and Note Guarantors’ respective existing and future unsubordinated indebtedness and senior to the Company’s and Note Guarantors’ respective future subordinated indebtedness. The October 2028 Secured Notes and the guarantees related thereto are effectively pari passu with the Company’s and the Note Guarantors’ respective existing and future indebtedness secured by a first priority lien on the collateral securing the October 2028 Secured Notes and effectively senior to the Company’s and the Note Guarantors’ respective existing and future indebtedness that is unsecured, or that is secured by junior liens, in each case to the extent of the value of the collateral. In addition, the October 2028 Secured Notes are structurally subordinated to: (i) all liabilities of any of the Company’s subsidiaries that do not guarantee the October 2028 Secured Notes and (ii) any of the Company’s debt that is secured by assets that are not collateral.
Upon the occurrence of a change in control (as defined in the indenture governing the October 2028 Secured Notes), unless the Company has exercised its right to redeem all of the notes of a series, holders of the October 2028 Secured Notes may require the Company to repurchase such holder’s notes, in whole or in part, at a purchase price equal to 101% of the principal amount thereof plus accrued and unpaid interest, but not including, the date of purchase.
The October 2028 Secured Notes are redeemable at the option of the Company, in whole or in part, at any time on or after October 1, 2025, at the redemption prices set forth in the indenture. Prior to October 1, 2025, the Company may redeem the October 2028 Secured Notes in whole or in part at a redemption price equal to the principal amount of the Notes redeemed plus a make-whole premium. Prior to October 1, 2025, the Company may on any one or more occasions redeem up to 40% of the aggregate principal amount of the October 2028 Secured Notes at a redemption price of 108.375% of the principal amount thereof, redeemed plus accrued and unpaid interest to, but not including, the date of redemption with the proceeds of one of more equity offerings.
Weighted Average Stated Rate of Interest
The weighted average stated rate of interest for the Company’s outstanding debt obligations as of December 31, 2023 and December 31, 2022 was 8.65% and 7.84%, respectively.
Maturities and Mandatory Payments
Maturities and mandatory payments of debt obligations for the five succeeding years ending December 31 and thereafter are as follows:
| | | | | | | | |
| (in millions) | | |
| 2024 | | $ | 30 | |
| 2025 | | 30 | |
| 2026 | | 30 | |
| 2027 | | 2,667 | |
| 2028 | | 1,879 | |
| Thereafter | | — | |
| Total gross maturities | | 4,636 | |
| Unamortized discounts | | (74) | |
| Total long-term debt and other | | $ | 4,562 | |
Covenant Compliance
The Credit Facilities contain customary affirmative and negative covenants and specified events of default. These affirmative and negative covenants include, among other things, and subject to certain qualifications and exceptions, covenants that restrict Bausch + Lomb’s ability and the ability of its subsidiaries to: incur or guarantee additional indebtedness; create or permit liens on assets; pay dividends on capital stock or redeem, repurchase or retire capital stock or subordinated indebtedness; make certain investments and other restricted payments; engage in mergers, acquisitions, consolidations and amalgamations; transfer and sell certain assets; and engage in transactions with affiliates. The Revolving Credit Facility also contains financial covenants that: (1) prior to the IG Trigger, require Bausch + Lomb to, if, as of the last day of any fiscal quarter of Bausch + Lomb (commencing with the fiscal quarter ending December 31, 2022), loans under the Revolving Credit Facility and swingline loans are outstanding in an aggregate amount greater than 40% of the total commitments in respect of the Revolving Credit Facility at such time, maintain a maximum first lien net leverage ratio of not greater than 4.50:1.00 and (2) after the IG Trigger, require Bausch + Lomb to, as of the last day of each fiscal quarter ending after the IG Trigger, (a) maintain a total leverage ratio of not greater than 4.00:1.00 (provided that such ratio will increase to 4.50:1.00 in connection with certain acquisitions for the four fiscal quarter period commencing with the quarter in which such acquisition
is consummated) and (b) maintain an interest coverage ratio of not less than 3.00:1.00. The financial covenant in effect prior to the IG Trigger may be waived or amended without the consent of the term loan facility lenders and contains a customary term loan facility standstill and customary cure rights. The indenture governing the October 2028 Secured Notes also contains negative covenants and events of default that are similar to those contained in the Credit Facilities.
As of December 31, 2023, the Company was in compliance with its financial covenants related to its debt obligations. Bausch + Lomb, based on its current forecast for the next twelve months from the date of issuance of these financial statements, expects to remain in compliance with its financial covenants and meet its debt service obligations over that same period.
11.PENSION AND POSTRETIREMENT EMPLOYEE BENEFIT PLANS
Bausch + Lomb has defined benefit plans and a participatory defined benefit postretirement medical and life insurance plan, which covers a closed grandfathered group of legacy U.S. employees and employees in certain other countries. The U.S. defined benefit accruals were frozen as of December 31, 2004 and benefits that were earned up to December 31, 2004 were preserved. Participants continue to earn interest credits on their cash balance at an interest crediting rate that is equal to the greater of: i) the average annual yield on 10-year Treasury bonds in effect for the November preceding the plan year or ii) 4.50%. The most significant non-U.S. plans are two defined benefit plans in Ireland. In 2011, both Ireland defined benefit plans were closed to future service benefit accruals; however, additional accruals related to annual salary increases continued. In December 2014, one of the Ireland defined benefit plans was amended effective August 2014 to eliminate future benefit accruals related to salary increases. All of the pension benefits accrued through the plan amendment date were preserved. As a result of the plan amendment, there are no active plan participants accruing benefits under the amended Ireland defined benefit plan. The U.S. postretirement benefit plan was amended effective January 1, 2005 to eliminate employer contributions after age 65 for participants who did not meet the minimum requirements of age and service on that date. The employer contributions for medical and prescription drug benefits for participants retiring after March 1, 1989 were frozen effective January 1, 2010. Effective January 1, 2014, the Company no longer offers medical and life insurance coverage to new retirees.
In addition to the legacy benefit plans, outside of the U.S., a limited group of the Company's' employees are covered by defined benefit pension plans.
The Company uses December 31 as the year-end measurement date for all of its defined benefit pension plans and the postretirement benefit plan.
Accounting for Pension Benefit Plans and Postretirement Benefit Plan
The Company recognizes in its Consolidated Balance Sheets an asset or liability equal to the over- or under-funded benefit obligation of each defined benefit pension plan and postretirement benefit plan. Actuarial gains or losses and prior service costs or credits that arise during the period but are not recognized as components of net periodic benefit cost are recognized, net of tax, as a component of other comprehensive income (loss).
The amounts included in Accumulated other comprehensive loss as of December 31, 2023 and 2022 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Pension Benefit Plans | | U.S. Postretirement
Benefit Plan |
| U.S. Plan | | Non-U.S. Plans | |
| (in millions) | | 2023 | | 2022 | | 2023 | | 2022 | | 2023 | | 2022 |
| Unrecognized actuarial (losses) gains | | $ | (31) | | | $ | (35) | | | $ | (26) | | | $ | (23) | | | $ | 3 | | | $ | 3 | |
| Unrecognized prior service credits | | $ | — | | | $ | — | | | $ | 23 | | | $ | 23 | | | $ | 3 | | | $ | 6 | |
Net Periodic (Benefit) Cost
The following tables provides the components of net periodic (benefit) cost for Bausch + Lomb’s defined benefit pension plans and postretirement benefit plan for the years 2023, 2022 and 2021:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Pension Benefit Plans | | U.S. Postretirement Benefit Plan |
| U.S. Plan | | Non-U.S. Plans | |
| (in millions) | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| Service cost | | $ | 2 | | | $ | 1 | | | $ | 1 | | | $ | 2 | | | $ | 3 | | | $ | 2 | | | $ | — | | | $ | — | | | $ | — | |
| Interest cost | | 9 | | | 5 | | | 4 | | | 4 | | | 3 | | | 3 | | | 1 | | | 1 | | | 1 | |
| Expected return on plan assets | | (9) | | | (10) | | | (11) | | | (3) | | | (4) | | | (5) | | | — | | | — | | | — | |
| Amortization of prior service credit | | — | | | — | | | — | | | (1) | | | (1) | | | (1) | | | (2) | | | (2) | | | (3) | |
| Amortization of net loss | | 1 | | | — | | | — | | | — | | | 1 | | | 2 | | | — | | | — | | | — | |
| Settlement loss recognized | | — | | | 1 | | | — | | | 1 | | | 8 | | | 8 | | | — | | | — | | | — | |
| Net periodic (benefit) cost | | $ | 3 | | | $ | (3) | | | $ | (6) | | | $ | 3 | | | $ | 10 | | | $ | 9 | | | $ | (1) | | | $ | (1) | | | $ | (2) | |
Benefit Obligation, Change in Plan Assets and Funded Status
The table below presents components of the change in projected benefit obligation, change in plan assets and funded status for 2023 and 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Pension Benefit Plans | | U.S. Postretirement
Benefit Plan |
| U.S. Plan | | Non-U.S. Plans | |
| (in millions) | | 2023 | | 2022 | | 2023 | | 2022 | | 2023 | | 2022 |
| Change in Projected Benefit Obligation | | | | | | | | | | | | |
| Projected benefit obligation, beginning of year | | $ | 172 | | | $ | 220 | | | $ | 102 | | | $ | 218 | | | $ | 27 | | | $ | 35 | |
| Service cost | | 2 | | | 1 | | | 2 | | | 3 | | | — | | | — | |
| Interest cost | | 9 | | | 5 | | | 4 | | | 3 | | | 1 | | | 1 | |
| Employee contributions | | — | | | — | | | — | | | — | | | — | | | — | |
| Settlements | | — | | | (7) | | | (5) | | | (50) | | | — | | | — | |
| Benefits paid | | (16) | | | (11) | | | (3) | | | (4) | | | (3) | | | (4) | |
| Actuarial losses (gains) | | 3 | | | (36) | | | 8 | | | (53) | | | — | | | (5) | |
| Currency translation adjustments | | — | | | — | | | 3 | | | (15) | | | — | | | — | |
| Projected benefit obligation, end of year | | 170 | | | 172 | | | 111 | | | 102 | | | 25 | | | 27 | |
| | | | | | | | | | | | |
| Change in Plan Assets | | | | | | | | | | | | |
| Fair value of plan assets, beginning of year | | 162 | | | 224 | | | 92 | | | 171 | | | — | | | — | |
| Actual return on plan assets | | 16 | | | (44) | | | 9 | | | (40) | | | — | | | — | |
| Employee contributions | | — | | | — | | | — | | | — | | | — | | | — | |
| Company contributions | | — | | | — | | | 2 | | | 25 | | | 3 | | | 4 | |
| Settlements | | — | | | (7) | | | (5) | | | (50) | | | — | | | — | |
| Benefits paid | | (16) | | | (11) | | | (3) | | | (4) | | | (3) | | | (4) | |
| Currency translation adjustments | | — | | | — | | | 3 | | | (10) | | | — | | | — | |
| Fair value of plan assets, end of year | | 162 | | | 162 | | | 98 | | | 92 | | | — | | | — | |
| Funded Status at end of year | | $ | (8) | | | $ | (10) | | | $ | (13) | | | $ | (10) | | | $ | (25) | | | $ | (27) | |
| | | | | | | | | | | | |
| Recognized as: | | | | | | | | | | | | |
| Other non-current assets | | $ | — | | | $ | — | | | $ | 20 | | | $ | 22 | | | $ | — | | | $ | — | |
| Accrued and other current liabilities | | $ | — | | | $ | — | | | $ | 2 | | | $ | 2 | | | $ | 3 | | | $ | 4 | |
| Other non-current liabilities | | $ | 8 | | | $ | 10 | | | $ | 31 | | | $ | 30 | | | $ | 22 | | | $ | 23 | |
Included in Settlement loss recognized and Settlements in the tables above are the costs and payments associated with the conversion of a portion of the Company's defined benefit plan in Ireland to a defined contribution plan.
A number of the Company's pension benefit plans were underfunded as of December 31, 2023 and 2022, having accumulated benefit obligations exceeding the fair value of plan assets. Information for the underfunded pension benefit plans is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. Plan | | Non-U.S. Plans |
| (in millions) | | 2023 | | 2022 | | 2023 | | 2022 |
| Projected benefit obligation | | $ | 170 | | | $ | 172 | | | $ | 38 | | | $ | 37 | |
| Accumulated benefit obligation | | 170 | | | 172 | | | 32 | | | 32 | |
| Fair value of plan assets | | 162 | | | 162 | | | 5 | | | 6 | |
The Company's policy for funding its pension benefit plans is to make contributions that meet or exceed the minimum statutory funding requirements. These contributions are determined based upon recommendations made by the actuary under accepted actuarial principles. In 2024, the Company expects to contribute $4 million, $3 million and $3 million to the U.S. pension benefit plan, the non-U.S. pension benefit plans and the U.S. postretirement benefit plan, respectively. The Company plans to use postretirement benefit plan assets and cash on hand, as necessary, to fund the U.S. postretirement benefit plan benefit payments in 2024.
Estimated Future Benefit Payments
Future benefit payments over the next 10 years for the pension benefit plans and the postretirement benefit plan, which reflect expected future service, as appropriate, are expected to be paid as follows:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | Pension Benefit Plans | | U.S. Postretirement
Benefit Plan |
| U.S. Plan | | Non-U.S. Plans | |
| 2024 | | $ | 14 | | | $ | 5 | | | $ | 3 | |
| 2025 | | 19 | | | 4 | | | 3 | |
| 2026 | | 17 | | | 4 | | | 3 | |
| 2027 | | 17 | | | 5 | | | 3 | |
| 2028 | | 16 | | | 5 | | | 2 | |
| 2029 - 2033 | | 68 | | | 28 | | | 10 | |
Assumptions
The weighted-average assumptions used to determine net periodic benefit costs and benefit obligations for 2023, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Pension Benefit Plans | | U.S Postretirement Benefit Plan |
| | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| For Determining Net Periodic (Benefit) Cost | | | | | | | | | | | | |
| U.S. Plans: | | | | | | | | | | | | |
| Discount rate | | 5.41 | % | | 2.69 | % | | 2.25 | % | | 5.39 | % | | 2.57 | % | | 2.09 | % |
| Expected rate of return on plan assets | | 6.00 | % | | 4.50 | % | | 5.00 | % | | — | | | — | | | — | |
| Rate of compensation increase | | — | | | — | | | — | | | — | | | — | | | — | |
| Interest crediting rate | | 4.75 | % | | 4.75 | % | | 4.75 | % | | | | | | |
| Non-U.S. Plans: | | | | | | | | | | | | |
| Discount rate | | 3.83 | % | | 1.44 | % | | 1.14 | % | | | | | | |
| Expected rate of return on plan assets | | 4.10 | % | | 2.70 | % | | 2.73 | % | | | | | | |
| Rate of compensation increase | | 2.92 | % | | 2.55 | % | | 2.49 | % | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Pension Benefit Plans | | U.S. Postretirement Benefit Plan |
|
| | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| For Determining Benefit Obligation | | | | | | | | | | | | |
| U.S. Plans: | | | | | | | | | | | | |
| Discount rate | | 5.11 | % | | 5.41 | % | | 2.69 | % | | 5.08 | % | | 5.39 | % | | 2.57 | % |
| Rate of compensation increase | | — | | | — | | | — | | | — | | | — | | | — | |
| Interest crediting rate | | 4.75 | % | | 4.75 | % | | 4.75 | % | | | | | | |
| Non-U.S. Plans: | | | | | | | | | | | | |
| Discount rate | | 3.60 | % | | 3.83 | % | | 1.60 | % | | | | | | |
| Rate of compensation increase | | 2.94 | % | | 2.92 | % | | 2.60 | % | | | | | | |
The expected long-term rate of return on plan assets was developed based on a capital markets model that uses expected asset class returns, variance and correlation assumptions. The expected asset class returns were developed starting with current Treasury (for the U.S. pension plan) or Eurozone (for the Ireland pension plans) government yields and then adding corporate bond spreads and equity risk premiums to develop the return expectations for each asset class. The expected asset class returns are forward-looking. The variance and correlation assumptions are also forward-looking. They take into account historical relationships but are adjusted to reflect expected capital market trends.
The discount rate used to determine benefit obligations represents the current rate at which the benefit plan liabilities could be effectively settled considering the timing of expected payments for plan participants.
The 2024 expected rate of return for the U.S. pension benefit plan will be 6.00%. The 2024 expected rate of return for the Ireland pension benefit plans will be 4.50%.
Pension Benefit Plans Assets
Pension benefit plan assets are invested in several asset categories. The following presents the actual asset allocation as of December 31, 2023 and 2022:
| | | | | | | | | | | | | | |
| | 2023 | | 2022 |
| U.S. Plan | | | | |
| Cash and cash equivalents | | 1 | % | | 1 | % |
| Equity securities | | 39 | % | | 40 | % |
| Fixed income securities | | 60 | % | | 59 | % |
| Non-U.S. Plans | | | | |
| Cash and cash equivalents | | 9 | % | | 4 | % |
| Equity securities | | 25 | % | | 24 | % |
| Fixed income securities | | 48 | % | | 46 | % |
| Other | | 18 | % | | 26 | % |
The investment strategy underlying pension plan asset allocation is to manage the assets of the plan to provide for the non-current liabilities while maintaining sufficient liquidity to pay current benefits. Pension plan assets are diversified to protect against large investment losses and to reduce the probability of excessive performance volatility. Diversification of assets is achieved by allocating funds to various asset classes and investment styles within asset classes, and retaining investment management firm(s) with complementary investment philosophies, styles and approaches.
The Company's pension plan assets are managed by outside investment managers using a total return investment approach, whereby a mix of equity and debt securities investments are used to maximize the long-term rate of return on plan assets. A significant portion of the assets of the U.S. and Ireland pension plans have been invested in equity securities, as equity portfolios have historically provided higher returns than debt and other asset classes over extended time horizons. Correspondingly, equity investments also entail greater risks than other investments. Equity risks are balanced by investing a significant portion of plan assets in broadly diversified fixed income securities.
Fair Value of Plan Assets
The Company measured the fair value of plan assets based on the prices that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. See Note 5, “FAIR VALUE MEASUREMENTS” for details on the Company's' fair value measurements based on a three-tier hierarchy.
The table below presents total plan assets by investment category as of December 31, 2023 and 2022 and the classification of each investment category within the fair value hierarchy with respect to the inputs used to measure fair value. There were no transfers between Level 1, Level 2 or Level 3 during 2023 and 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Pension Benefit Plans - U.S. Plans |
| | December 31, 2023 | | December 31, 2022 |
| (in millions) | | Level 1 | | Level 2 | | Total | | Level 1 | | Level 2 | | Total |
| Cash and cash equivalents | | $ | 1 | | | $ | — | | | $ | 1 | | | $ | 2 | | | $ | — | | | $ | 2 | |
| Commingled funds: | | | | | | | | | | | | |
| Equity securities: | | | | | | | | | | | | |
| U.S. broad market | | — | | | 34 | | | 34 | | | — | | | 34 | | | 34 | |
| Emerging markets | | — | | | 6 | | | 6 | | | — | | | 7 | | | 7 | |
| Worldwide developed markets | | — | | | 13 | | | 13 | | | — | | | 14 | | | 14 | |
| Other assets | | — | | | 10 | | | 10 | | | — | | | 10 | | | 10 | |
| Fixed income securities: | | | | | | | | | | | | |
| Investment grade | | — | | | 98 | | | 98 | | | — | | | 95 | | | 95 | |
| | $ | 1 | | | $ | 161 | | | $ | 162 | | | $ | 2 | | | $ | 160 | | | $ | 162 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Pension Benefit Plans - Non-U.S. Plans |
| | December 31, 2023 | | December 31, 2022 |
| (in millions) | | Level 1 | | Level 2 | | Level 3 | | Total | | Level 1 | | Level 2 | | Level 3 | | Total |
| Cash equivalents | | $ | — | | | $ | 9 | | | $ | — | | | $ | 9 | | | $ | — | | | $ | 4 | | | $ | — | | | $ | 4 | |
| Commingled funds: | | | | | | | | | | | | | | | | |
| Equity securities: | | | | | | | | | | | | | | | | |
| Emerging markets | | — | | | 1 | | | — | | | 1 | | | — | | | 1 | | | — | | | 1 | |
| Developed markets | | — | | | 23 | | | — | | | 23 | | | — | | | 21 | | | — | | | 21 | |
| Fixed income securities: | | | | | | | | | | | | | | | | |
| Investment grade | | — | | | 2 | | | — | | | 2 | | | — | | | 2 | | | — | | | 2 | |
| | | | | | | | | | | | | | | | |
| Government bond funds | | 1 | | | 44 | | | — | | | 45 | | | 1 | | | 39 | | | — | | | 40 | |
| Other assets | | — | | | 5 | | | 13 | | | 18 | | | — | | | 12 | | | 12 | | | 24 | |
| | $ | 1 | | | $ | 84 | | | $ | 13 | | | $ | 98 | | | $ | 1 | | | $ | 79 | | | $ | 12 | | | $ | 92 | |
Cash equivalents consisted primarily of term deposits and money market instruments. The fair value of the term deposits approximates their carrying amounts due to their short-term maturities. The money market instruments also have short maturities and are valued using a market approach based on the quoted market prices of identical instruments.
Commingled funds are not publicly traded. The underlying assets in these funds are publicly traded on the exchanges and have readily available price quotes. The Ireland pension plans held approximately 93% and 92% of the non-U.S. commingled funds in 2023 and 2022, respectively. The commingled funds held by the U.S. and Ireland pension plans are primarily invested in index funds.
The underlying assets in the fixed income funds are generally valued using the net asset value per fund share, which is derived using a market approach with inputs that include broker quotes, benchmark yields, base spreads and reported trades.
Defined Contribution Plans
The Company sponsors defined contribution plans in the U.S., Ireland and certain other countries. Under these plans, employees are allowed to contribute a portion of their salaries to the plans, and the sponsor matches a portion of the employee contributions. Prior to the B+L IPO, the Company participated in BHC sponsored defined contribution plans. The Company and BHC (prior to the B+L IPO) contributed $34 million, $33 million and $36 million to these plans during the years 2023, 2022 and 2021, respectively.
12.LEASES
Right-of-use assets and lease liabilities associated with the Company's operating leases are included in the Consolidated Balance Sheets as follows:
| | | | | | | | | | | |
| (in millions) | December 31, 2023 | | December 31, 2022 |
| Right-of-use assets included in: | | | |
| Other non-current assets | $ | 114 | | | $ | 119 | |
| Lease liabilities included in: | | | |
| Accrued and other current liabilities | $ | 27 | | | $ | 26 | |
| Other non-current liabilities | 87 | | | 92 | |
| Total lease liabilities | $ | 114 | | | $ | 118 | |
As of December 31, 2023 and 2022, the Company's finance leases were not material and for 2023 and 2022 sub-lease income and short-term lease expense were not material. Lease expense for 2023 and 2022 includes:
| | | | | | | | | | | |
| (in millions) | 2023 | | 2022 |
| Operating lease costs | $ | 40 | | | $ | 37 | |
| Variable operating lease costs | $ | 7 | | | $ | 7 | |
Other information related to operating leases for 2023 and 2022 is as follows:
| | | | | | | | | | | |
| (dollars in millions) | 2023 | | 2022 |
| Cash paid from operating cash flows for amounts included in the measurement of lease liabilities | $ | 38 | | | $ | 35 | |
| Right-of-use assets obtained in exchange for new operating lease liabilities | $ | 24 | | | $ | 28 | |
| Weighted-average remaining lease term | 6.7 years | | 7.5 years |
| Weighted-average discount rate | 6.7 | % | | 6.4 | % |
As of December 31, 2023, future payments under noncancellable operating leases for each of the five succeeding years ending December 31 and thereafter are as follows:
| | | | | | |
| (in millions) | | |
| 2024 | | $ | 35 | |
| 2025 | | 27 | |
| 2026 | | 20 | |
| 2027 | | 16 | |
| 2028 | | 11 | |
| Thereafter | | 35 | |
| Total | | 144 | |
| Less: Imputed interest | | 30 | |
| Present value of remaining lease payments | | 114 | |
| Less: Current portion | | 27 | |
| Non-current portion | | $ | 87 | |
13.SHARE-BASED COMPENSATION
BHC Long-term Incentive Program
Prior to May 5, 2022, Bausch + Lomb employees participated in BHC’s long-term incentive program. Therefore, prior to May 5, 2022, share-based compensation expense attributable to Bausch + Lomb was derived from: (i) the specific identification of Bausch + Lomb employees and (ii) an allocation of charges from BHC, related to BHC employees providing corporate services to Bausch + Lomb. Accordingly, the amounts presented are not necessarily indicative of future awards and do not necessarily reflect the results that Bausch + Lomb would have experienced as an independent company for the periods presented. Subsequent to May 5, 2022, share-based compensation expense attributable to Bausch + Lomb employees participating in BHC’s long-term incentive program for grants made prior to May 5, 2022 is recognized as expense by Bausch + Lomb over the remaining vesting period.
Bausch + Lomb 2022 Omnibus Incentive Plan
Effective May 5, 2022, Bausch + Lomb established the Omnibus Plan. A total of 28,000,000 common shares of Bausch + Lomb are authorized for issuance under the Omnibus Plan. Effective April 24, 2023, Bausch + Lomb’s shareholders approved an amendment and restatement of the Plan to increase the number of shares authorized for issuance thereunder by an additional 10,000,000 common shares, resulting in an aggregate 38,000,000 common shares of Bausch + Lomb authorized for issuance. The Omnibus Plan provides for the grant of various types of awards, including RSUs, restricted stock, stock appreciation rights, stock options, performance-based awards and cash awards. Under the Omnibus Plan, the exercise price of awards, if any, is set on the grant date and may not be less than the fair market value per share on that date. Generally, stock options have a term of ten years and a three-year vesting period, subject to limited exceptions.
Share-based awards granted to senior management align with the Company’s focus on enhancing its revenue growth while maintaining focus on total shareholder return over the long-term. The share-based awards granted under this long-term incentive program consist of time-based stock options, time-based RSUs and performance-based RSUs (“PSUs”). The PSUs are comprised of awards that vest upon: (i) achievement of certain share price appreciation conditions, including absolute and relative total shareholder return (“TSR”) (the “TSR PSUs”) and (ii) attainment of certain performance targets that are based on the Company’s Organic Revenue Growth (the “Organic Revenue Growth PSUs”). If the Company’s performance is below a specified performance level, no common shares will be paid. Each vested PSU represents the right of a holder to receive a number of the Company’s common shares up to a specified maximum.
On May 5, 2022, in connection with the B+L IPO, Bausch + Lomb granted certain awards to certain eligible recipients (the "IPO Founder Grants"). Eligible recipients are individuals employed by Bausch + Lomb or employed by an affiliate of Bausch + Lomb. Approximately 3,900,000 IPO Founder Grants were issued to Bausch + Lomb executive officers and were awarded 50% in the form of stock options and 50% in the form of RSUs. Additionally, Bausch + Lomb granted approximately 5,700,000 stock options and RSUs to non-executive eligible recipients, of which approximately 4,300,000 were IPO Founder Grants. The IPO Founder Grants in the form of stock options have a three-year graded vesting period and the IPO Founder RSUs vest 50% in the second year and 50% in the third year after the grant. As discussed below, vesting of the IPO Founder Grants are linked to the completion of the Distribution and expense recognition will begin near the time of the Distribution.
During the third quarter of 2022, the Talent and Compensation Committee of the Board approved a retention program that includes the Company’s named executive officers (other than the CEO) and certain other employees. This program provides these executive officers (other than the CEO) for, among other benefits, pro-rata vesting of the IPO Founder Grants previously issued to these named executives, subject to certain restrictions, in the event of an involuntary termination of employment by the Company without "cause" or the employee's resignation for "good reason", in each case within the one-year following the Company’s appointment of the successor to the CEO (pro-rated based on the period of service relative to the original three-year vesting period associated with such grants). However, the IPO Founder Grants in the form of RSUs (while settled in connection with the termination of employment) will not be transferrable until, and the IPO Founder Grants in the form of stock options will not be exercisable until, the earliest to occur of: (i) the date BHC completes the spinoff distribution of the Company, (ii) a “change in control” (as defined in the applicable retention award letter), (iii) the date the Board of Directors of BHC determines that BHC will no longer pursue the spinoff distribution of the Company and (iv) the two-year anniversary of the executive’s termination of employment and the IPO Founder Grants in the form of stock options will be exercisable for two years following the later of this date and the termination date. Additionally, these named executive officers (other than the CEO) and certain other employees were granted a one-time award of approximately 850,000 RSUs in the aggregate under the retention program pursuant to the Omnibus Plan. The retention grant will generally vest in 1/3 installments on each of the first three anniversaries of the grant date based on continuous employment with the Company.
On February 15, 2023, Bausch + Lomb announced the appointment of Brent Saunders as CEO and Chair of the Board of Directors of the Company, effective as of March 6, 2023. Pursuant to Mr. Saunders' employment agreement, on February 23, 2023, Mr. Saunders was granted the following equity grants under the Omnibus Plan: 750,000 PSUs, 1,318,681 stock options and 375,000 RSUs. The RSUs are scheduled to vest 50% on the second anniversary of the grant date and the remaining 50% on the third anniversary of the grant date. The stock options are scheduled to vest in equal one-third installments on each of the first three anniversaries of the grant date. The PSUs vest on the fourth anniversary of the grant date based on the Company’s achievement of absolute share price hurdles, or upon achievement of absolute and relative TSR hurdles in relation to the S&P 500 Index during the four-year performance period.
Approximately 19,300,000 common shares were available for future grants as of December 31, 2023. Bausch + Lomb uses reserved and unissued common shares to satisfy its obligations under its share-based compensation plans.
The components and classification of share-based compensation expense related to stock options, PSUs and RSUs directly attributable to those employees specifically identified as Bausch + Lomb employees for both the BHC Long-term Incentive Program and the Plan for the years 2023, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Stock options | | $ | 11 | | | $ | 4 | | | $ | 3 | |
| PSUs/RSUs | | 63 | | | 52 | | | 35 | |
| Share-based compensation expense | | $ | 74 | | | $ | 56 | | | $ | 38 | |
| | | | | | |
| Research and development expenses | | $ | 5 | | | $ | 6 | | | $ | 6 | |
| Selling, general and administrative expenses | | 69 | | | 50 | | | 32 | |
| Share-based compensation expense | | $ | 74 | | | $ | 56 | | | $ | 38 | |
In addition to share-based compensation expense attributable to employees that are specific to Bausch + Lomb's business, share-based compensation expense also includes $0, $6 million and $24 million for the years 2023, 2022 and 2021 respectively, of allocated charges from BHC, based on revenues, related to BHC employees providing corporate services to Bausch + Lomb.
Stock Options
Stock options granted under the Plan generally expire on the tenth anniversary of the grant date. The exercise price of any stock option granted under the Plan will not be less than the closing price per common share preceding the date of grant. Stock options generally vest 33% each year over a three-year period, on the anniversary of the date of grant.
The fair values of all stock options granted under the Plan for the years 2023 and 2022 were estimated as of the date of grant using the Black-Scholes option-pricing model with the following weighted-average assumptions:
| | | | | | | | | | | | | | |
| | 2023 | | 2022 |
| Expected stock option life (years) | | 3.0 | | 3.0 |
| Expected volatility | | 35.3 | % | | 31.5 | % |
| Risk-free interest rate | | 4.6 | % | | 3.1 | % |
| Expected dividend yield | | — | % | | — | % |
The expected stock option life was determined based on historical exercise and forfeiture patterns associated with historical stock options granted to Bausch + Lomb employees under BHC’s long-term incentive plan. The expected volatility was determined based on implied and historical volatility of Bausch + Lomb’s selected peer companies. Bausch + Lomb will continue to leverage BHC’s historical stock option experience and peer company data until it has sufficient experience with its own equity awards and market data. The risk-free interest rate was determined based on the rate at the time of grant for zero-coupon U.S. government bonds with maturity dates equal to the expected life of the stock option. The expected dividend yield was determined based on the stock option’s exercise price and expected Bausch + Lomb annual dividend rate at the time of grant.
The Black-Scholes option-pricing model used by the Company to calculate stock option values was developed to estimate the fair value of freely tradable, fully transferable stock options without vesting restrictions, which significantly differ from Bausch + Lomb's stock option awards. This model also requires highly subjective assumptions, including future stock price volatility and expected time until exercise, which greatly affect the calculated values.
The following table summarizes stock option activity under the Plan during 2023:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (in millions, except per share amounts) | | Options | | Weighted-
Average
Exercise
Price Per Share | | Weighted-
Average
Remaining
Contractual
Term
(Years) | | Aggregate
Intrinsic
Value |
Outstanding, January 1, 2023 | | 6.3 | | $ | 18.00 | | | | | |
| Granted | | 3.5 | | | $ | 18.15 | | | | | |
| Exercised | | — | | | $ | — | | | | | |
| Expired or forfeited | | (1.8) | | | $ | 17.99 | | | | | |
Outstanding, December 31, 2023 | | 8.0 | | | $ | 18.07 | | | 7.7 | | $ | — | |
Vested and expected to vest, December 31, 2023 | | 4.7 | | | $ | 18.12 | | | 7.0 | | $ | — | |
Vested and exercisable, December 31, 2023 | | — | | | $ | — | | | — | | | $ | — | |
The weighted-average fair values of stock options granted to Bausch + Lomb employees in 2023 and 2022 were $5.33 and $3.84, respectively. There were no stock options exercised in 2023 or 2022.
As of December 31, 2023, the total remaining unrecognized compensation expense related to non-vested stock options amounted to $12 million, which will be amortized over the weighted-average remaining requisite service period of approximately 2.2 years. Unrecognized compensation does not include IPO Founder Grants as they are linked to the completion of the Distribution and expense recognition will begin near the time of the Distribution. The total fair value of stock options that vested during 2023 was $5 million. There were no stock options that vested during 2022.
Time-Based RSUs
RSUs under the Omnibus Plan generally vest 33% a year over a three-year period with the exception of IPO Founder RSUs and the RSUs granted to Mr. Saunders in connection with his appointment, which vest in two equal installments, such that 50% vest on the second anniversary and 50% vest on the third anniversary of the grant date. RSUs are credited with dividend equivalents, in the form of additional RSUs, when dividends are paid on the Bausch + Lomb’s common shares. Such additional RSUs will have the same vesting dates and will vest under the same terms as the RSUs in respect of which such additional RSUs are credited.
To the extent provided for in a RSU agreement, Bausch + Lomb may, in lieu of all or a portion of the common shares which would otherwise be provided to a holder, elect to pay a cash amount equivalent to the market price of the Company's common shares on the vesting date for each vested RSU. The amount of cash payment will be determined based on the average market price of the Company's common shares on the vesting date. The Company's current intent is to settle vested RSUs through the issuance of common shares.
Each vested RSU represents the right of a holder to receive one of the Company's common shares. The fair value of each RSU granted is estimated based on the trading price of the Company's common shares on the date of grant.
The following table summarizes non-vested RSU activity under the Plan during 2023:
| | | | | | | | | | | | | | |
| (in millions, except per share amounts) | |
Restricted Stock Units (RSUs) | | Weighted-
Average
Grant-Date
Fair Value Per Share |
Non-vested, January 1, 2023 | | 4.2 | | | $ | 16.67 | |
| Granted | | 3.4 | | | $ | 17.91 | |
| Vested | | (1.6) | | | $ | 16.86 | |
| Forfeited | | (0.7) | | | $ | 17.91 | |
Non-vested, December 31, 2023 | | 5.3 | | | $ | 17.25 | |
As of December 31, 2023, the total remaining unrecognized compensation expense related to non-vested RSUs amounted to $46 million, which will be amortized over the weighted-average remaining requisite service period of approximately 1.8 years. Unrecognized compensation does not include IPO Founder Grants as they are linked to the completion of the Distribution and expense recognition will begin near the time of the Distribution. The total fair value of RSUs vested in 2023 was $27 million. The total fair value of RSUs vested in 2022 was not material.
Performance-Based RSUs
Each vested PSU represents the right of a holder to receive a number of the Company's common shares up to a specified maximum. The performance-based PSUs are comprised of awards that vest upon: (i) achievement of certain share price appreciation conditions, including absolute and relative total shareholder return and (ii) attainment of certain performance targets that are based on the Company’s Organic Revenue Growth. If the Company's performance is below a specified performance level, no common shares will be paid. The maximum level of achievement of the performance goals is 200% of the target.
The fair value of each TSR PSU granted during 2023 was estimated using a Monte Carlo Simulation model, which utilizes multiple input variables to estimate the probability that the performance condition will be achieved. The fair value of the Organic Revenue Growth PSUs is estimated based on the trading price of the Company’s common shares on the date of grant. Expense recognized for the Organic Revenue Growth PSUs in each reporting period reflects the Company’s latest estimate of Organic Revenue Growth in determining the number of PSUs that are expected to vest. If the Organic Revenue Growth PSUs do not ultimately vest due to the Organic Revenue Growth targets not being met, no compensation expense is recognized and any previously recognized compensation expense is reversed.
There were no TSR PSUs granted in 2022 and 2021. The fair values of TSR PSUs granted during 2023 were estimated with the following assumptions:
| | | | | | | | |
| | 2023 |
| Contractual term (years) | | 3.6 |
| Expected volatility | | 35.4% |
| Risk-free interest rate | | 4.5% |
The expected volatility was determined based on implied and historical volatility of Bausch + Lomb’s selected peer companies. The risk-free interest rate was determined based on the rate at the time of grant for zero-coupon U.S. government bonds with maturity dates equal to the contractual term of the TSR PSU.
The following table summarizes the performance-based PSU activity during 2023:
| | | | | | | | | | | | | | |
| (in millions, except per share amounts) | |
Performance-based RSUs | | Weighted-
Average
Grant-Date
Fair Value Per Share |
Non-vested, January 1, 2023 | | — | | | $ | — | |
| Granted | | 1.3 | | | $ | 26.61 | |
| Vested | | — | | | $ | — | |
| Forfeited | | (0.1) | | | $ | 24.96 | |
Non-vested, December 31, 2023 | | 1.2 | | | $ | 26.82 | |
During 2023, the Company granted approximately 1,300,000 performance-based RSUs, consisting of i) approximately 1,200,000 TSR PSUs with an average grant date fair value of $27.65 per RSU and ii) approximately 100,000 Organic Revenue Growth PSUs with a weighted-average grant date fair value of $17.96 per RSU.
As of December 31, 2023, the total remaining unrecognized compensation expense related to non-vested performance-based RSUs amounted to $26 million, which will be amortized over the weighted-average remaining requisite service period of approximately 2.8 years. A maximum of approximately 3,100,000 common shares could be issued upon vesting of the performance-based RSUs outstanding December 31, 2023. There were no performance-based RSUs that vested during 2023.
In addition, while Bausch + Lomb did not grant performance-based RSUs during 2022, certain Bausch + Lomb employees continued to participate in BHC’s performance-based RSUs granted prior to May 5, 2022. As of December 31, 2023, the total remaining unrecognized compensation expense related to BHC non-vested performance-based RSUs was $0.
14.ACCUMULATED OTHER COMPREHENSIVE LOSS
Accumulated other comprehensive loss consists of:
| | | | | | | | | | | | | | |
| (in millions) | | December 31, 2023 | | December 31, 2022 |
| Foreign currency translation adjustment | | $ | (1,217) | | | $ | (1,231) | |
| Pension adjustment, net of tax | | (28) | | | (27) | |
| | $ | (1,245) | | | $ | (1,258) | |
Income taxes are not provided for foreign currency translation adjustments arising on the translation of Bausch + Lomb’s operations having a functional currency other than the U.S. dollar, except to the extent of translation adjustments related to Bausch + Lomb’s retained earnings for foreign jurisdictions in which Bausch + Lomb is not considered to be permanently reinvested.
15.RESEARCH AND DEVELOPMENT
Included in Research and development are costs related to product development and quality assurance programs. Quality assurance are the costs incurred to meet evolving customer and regulatory standards. Research and development costs for the years 2023, 2022 and 2021 consist of:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Product related research and development | | $ | 302 | | | $ | 284 | | | $ | 254 | |
| Quality assurance | | 22 | | | 23 | | | 17 | |
| Research and development | | $ | 324 | | | $ | 307 | | | $ | 271 | |
16.OTHER EXPENSE, NET
Other expense, net for the years 2023, 2022 and 2021 consist of:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Asset impairments | | $ | — | | | $ | 1 | | | $ | 12 | |
| Restructuring, integration and separation costs | | 44 | | | 14 | | | 2 | |
| Litigation and other matters | | 3 | | | 1 | | | (1) | |
| Acquired in-process research and development costs | | — | | | 1 | | | 5 | |
| Acquisition-related costs | | 25 | | | 1 | | | — | |
| Acquisition-related contingent consideration | | 2 | | | (5) | | | — | |
| Other, net | | — | | | — | | | (1) | |
Other expense, net | | $ | 74 | | | $ | 13 | | | $ | 17 | |
The Company evaluates opportunities to improve its operating results and implements cost savings programs to streamline its operations and eliminate redundant processes and expenses. Restructuring and integration costs are expenses associated with the implementation of these cost savings programs and include expenses associated with reducing headcount and other cost reduction initiatives. Restructuring and integration costs for the years 2023, 2022 and 2021 were $43 million, $5 million and, $2 million, respectively, and primarily consist of employee severance costs. These severance costs were provided under an ongoing benefit arrangement and were therefore recorded once they were both probable and reasonably estimable in accordance with the provisions of ASC 712-10, “Nonretirement Postemployment Benefits”.
In connection with the Separation, the Company has incurred and will continue to incur additional costs associated with activities taken to separate the Bausch + Lomb business from the remainder of BHC. Separation costs are incremental costs directly related to the Separation, and include but are not limited to: (i) legal, audit and advisory fees, (ii) talent acquisition costs and (iii) costs associated with establishing new boards of directors and related board committees for Bausch + Lomb. Included in Other expense for the years 2023, 2022 and 2021 are Separation costs of $1 million, $9 million and $0, respectively. The Company has also incurred, and will continue to incur, separation-related costs which are incremental costs indirectly related to the Separation and include, but are not limited to: (i) IT infrastructure and software licensing costs, (ii) rebranding costs and (iii) costs associated with facility relocation and/or modification. The extent and timing of future charges for these costs cannot be reasonably estimated at this time and could be material. Included within SG&A and R&D for the years 2023, 2022 and 2021 are Separation-related costs of $9 million, $26 million and $3 million, respectively.
As a result of the completion of the B+L IPO, and as the Company prepares for post-Separation operations, the Company is launching certain initiatives that may result in certain changes to, and investment in, its organizational structure and operations. The Company refers to the charges related to these initiatives as "Business Transformation Costs". These costs are recorded in SG&A in the audited Consolidated Statements of Operations and include third-party advisory costs, as well as certain compensation-related costs associated with changes in the Company's executive officers, such as severance-related costs associated with the departure of the Company's former executives and the costs associated with the appointment of the Company's new executives. Further, in connection with the Separation, the Company continues to evaluate opportunities to improve its operating performance and may initiate cost savings programs to streamline the Company's operations and eliminate redundant processes and expenses. These cost savings programs may include, but are not limited to: (i) reducing headcount, (ii) eliminating real estate costs associated with unused or under-utilized facilities and (iii) implementing contribution margin improvement and other cost reduction initiatives. Although a specific plan does not exist at this time, the Company may identify and take additional exit and cost-rationalization restructuring actions in the future, the costs of which could be material.
17.INCOME TAXES
The components of (Loss) income before provision for income taxes for 2023, 2022 and 2021 consist of:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Domestic | | $ | (194) | | | $ | (59) | | | $ | 365 | |
| Foreign | | 28 | | | 132 | | | (47) | |
| | $ | (166) | | | $ | 73 | | | $ | 318 | |
The components of Provision for income taxes for 2023, 2022 and 2021 consist of:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Current: | | | | | | |
| Domestic | | $ | — | | | $ | (3) | | | $ | (109) | |
| Foreign | | (71) | | | (68) | | | (90) | |
| | (71) | | | (71) | | | (199) | |
| Deferred: | | | | | | |
| Domestic | | (20) | | | 1 | | | 2 | |
| Foreign | | 9 | | | 12 | | | 72 | |
| | (11) | | | 13 | | | 74 | |
| | $ | (82) | | | $ | (58) | | | $ | (125) | |
The Provision for income taxes differs from the expected amount calculated by applying the Company's Canadian statutory rate of 26.5% to (Loss) income before provision for income taxes for 2023, 2022 and 2021 as follows:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| (Loss) income before provision for income taxes | | $ | (166) | | | $ | 73 | | | $ | 318 | |
| Provision for income taxes | | | | | | |
| Expected provision for income taxes at Canadian statutory rate | | $ | 44 | | | $ | (19) | | | $ | (86) | |
| Adjustments to tax attributes | | 1 | | | (1) | | | 6 | |
| Non-deductible amount of share-based compensation | | (7) | | | (8) | | | 2 | |
| Change in valuation allowance | | (42) | | | 3 | | | (2) | |
| Change in uncertain tax positions | | 2 | | | 5 | | | 15 | |
| Withholding tax | | (5) | | | (6) | | | 1 | |
| Return to provision | | (1) | | | 1 | | | 5 | |
| Foreign tax rate differences | | (57) | | | (34) | | | (56) | |
| Disallowed interest | | (14) | | | — | | | — | |
| Other | | (3) | | | 1 | | | (10) | |
| | $ | (82) | | | $ | (58) | | | $ | (125) | |
Deferred tax assets and liabilities consist of:
| | | | | | | | | | | | | | |
| (in millions) | | December 31, 2023 | | December 31, 2022 |
| Deferred tax assets: | | | | |
| Tax loss and credit carryforwards | | $ | 834 | | | $ | 686 | |
| Intangible assets | | 122 | | | 210 | |
| Provisions | | 177 | | | 157 | |
| Share-based compensation | | 11 | | | 10 | |
| Other | | 19 | | | 28 | |
| Total deferred tax assets | | 1,163 | | | 1,091 | |
| Less valuation allowance | | (150) | | | (54) | |
| Net deferred tax assets | | 1,013 | | | 1,037 | |
| Deferred tax liabilities: | | | | |
| Plant, equipment and technology | | 74 | | | 89 | |
| Outside basis differences | | 32 | | | 28 | |
| Total deferred tax liabilities | | 106 | | | 117 | |
| Net deferred tax asset | | $ | 907 | | | $ | 920 | |
The following table presents a reconciliation of the deferred tax asset valuation allowance for 2023, 2022 and 2021:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Balance, beginning of year | | $ | 54 | | | $ | 17 | | | $ | 15 | |
| Charged to Benefit from income taxes | | 42 | | | (3) | | | 2 | |
| Other | | 54 | | | 40 | | | — | |
| Balance, end of year | | $ | 150 | | | $ | 54 | | | $ | 17 | |
The realization of deferred tax assets is dependent on the Company generating sufficient domestic and foreign taxable income in the years that the temporary differences become deductible. A valuation allowance has been provided for the portion of the deferred tax assets that the Company determined is more likely than not to remain unrealized based on estimated future taxable income and tax planning strategies. The valuation allowance increased by $96 million during 2023 primarily due to the valuation allowance established against the prior deferred tax assets in Canada, additional current year loss in Canada as well as certain attributes the Company acquired during the year that are expected to expire prior to their utilization.
As of December 31, 2023 the Company had accumulated taxable losses available to offset future years' federal taxable income in the U.S. of approximately $36 million and expire from 2024 to 2036. These taxable losses are subject to annual loss limitations as a result of previous ownership changes. As of December 31, 2023, the Company U.S. research and development credits available to offset future years’ federal income taxes in the U.S. were approximately $28 million, which includes acquired research and development credits and which expire in years 2024 through 2042. As of December 31, 2023 the Company had accumulated taxable losses available to offset future years taxable income in Ireland of approximately $5,104 million. These taxable losses do not expire.
The Company provides for withholding tax on the unremitted earnings of its direct foreign affiliates except for its direct U.S. subsidiaries. The Company continues to assert that the unremitted earnings of its U.S. subsidiaries will be permanently reinvested and not repatriated. The Company provides for withholding tax on the unremitted earnings of its direct foreign affiliates except for its direct U.S. subsidiaries. The Company continues to assert that the unremitted earnings of its U.S. subsidiaries will be permanently reinvested and not repatriated. As of December 31, 2023, the Company estimates that there will be no tax liability attributable to unremitted earnings of its U.S. subsidiaries. However, future distributions could be subject to U.S. withholding tax.
As of December 31, 2023, unrecognized tax benefits (including interest and penalties) were $68 million, of which $60 million would affect the effective income tax rate if recognized.
The Company provides for interest and penalties related to unrecognized tax benefits in the provision for income taxes. As of December 31, 2023 and 2022, accrued interest and penalties related to unrecognized tax benefits were approximately $9 million and $9 million, respectively. In 2023, the Company did not recognize a net change in interest and penalties. In 2022 and 2021, the Company recognized a net increase of approximately $3 million and net decrease of approximately $1 million of interest and penalties, respectively.
The Company and one or more of its subsidiaries file federal income tax returns in Canada, the U.S. and other foreign jurisdictions, as well as various provinces and states in Canada and the U.S. The Company and its subsidiaries have open tax years, primarily from 2006 to 2022, with significant taxing jurisdictions listed in the table below, respectively, including Canada and the U.S. These open years contain certain matters that could be subject to differing interpretations of applicable tax laws and regulations and tax treaties, as they relate to the amount, timing, or inclusion of revenues and expenses, or the sustainability of income tax positions of the Company and its subsidiaries. Certain of these tax years are expected to remain open indefinitely.
The Company’s subsidiaries in Germany are under audit for tax years 2014 through 2019. During 2023, the Company received a preliminary assessment from the German taxing authority that would disallow certain transfer pricing adjustments. The Company intends to contest this alleged tax deficiency through the appropriate appeals process, and if necessary, intends to continue to contest any alleged tax deficiency through appropriate litigation. Accordingly, no income tax provision has been recorded as of December 31, 2023. The Company continues to believe any liability to arise from this audit would be indemnified by BHC pursuant to the Tax Matters Agreement.
| | | | | | | | |
| Jurisdiction: | | Open Years |
| United States - Federal | | 2015 - 2022 |
| Canada | | 2021 - 2022 |
| Germany | | 2014 - 2022 |
| France | | 2013 - 2022 |
| Ireland | | 2019 - 2022 |
| China | | 2013 - 2022 |
The following table presents a reconciliation of the unrecognized tax benefits for 2023, 2022 and 2021:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Balance, beginning of year | | $ | 70 | | | $ | 74 | | | $ | 62 | |
| Additions based on tax positions related to the current year | | — | | | 8 | | | 1 | |
| Additions for tax positions of prior years | | 3 | | | 1 | | | 48 | |
| Reductions for tax positions of prior years | | (1) | | | (11) | | | (7) | |
| Lapse of statute of limitations | | (4) | | | (2) | | | (30) | |
| Balance, end of year | | $ | 68 | | | $ | 70 | | | $ | 74 | |
The Company believes that it is reasonably possible that the total amount of unrecognized tax benefits at December 31, 2023 could decrease by $2 million in the next 12 months as a result of the resolution of certain tax audits and other events.
18.EARNINGS PER SHARE
On April 28, 2022, Bausch + Lomb effected a share consolidation as a result of which it had 350,000,000 issued and outstanding common shares. These common shares are treated as issued and outstanding at January 1, 2021 for purposes of calculating Basic and diluted income (loss) per share attributable to Bausch + Lomb Corporation.
(Loss) income per share attributable to Bausch + Lomb Corporation for 2023, 2022 and 2021 were calculated as follows:
| | | | | | | | | | | | | | | | | | | | |
| (in millions, except per share amounts) | | 2023 | | 2022 | | 2021 |
| Net (loss) income attributable to Bausch + Lomb Corporation | | $ | (260) | | | $ | 6 | | | $ | 182 | |
| Basic weighted-average common shares outstanding | | 350.5 | | | 350.0 | | | 350.0 | |
| Diluted effect of stock options and RSUs | | — | | | 0.2 | | | — | |
| Diluted weighted-average common shares outstanding | | $ | 350.5 | | | $ | 350.2 | | | $ | 350.0 | |
| (Loss) earnings per share attributable to Bausch + Lomb Corporation | | | | | | |
| Basic | | $ | (0.74) | | | $ | 0.02 | | | $ | 0.52 | |
| Diluted | | $ | (0.74) | | | $ | 0.02 | | | $ | 0.52 | |
There were no dilutive equity instruments or equity awards outstanding prior to the B+L IPO.
In 2023, all potential common shares issuable for RSUs, performance-based RSUs and stock options were excluded from the calculation of diluted loss per share, as the effect of including them would have been anti-dilutive. The dilutive effect of potential common shares issuable for RSUs, performance-based RSUs and stock options on the weighted-average number of common shares outstanding would have been approximately 1,539,000 common shares.
In 2023, RSUs, performance-based RSUs and stock options to purchase approximately 5,305,000 common shares were not included in the computation of diluted earnings per share because the effect would have been anti-dilutive under the treasury stock method. In 2023, an additional 4,041,000 IPO Founders Grants in the form of stock options and RSUs, which were granted to certain eligible recipients in connection with the B+L IPO, and an additional 750,000 PSUs, were not included in the computation of diluted earnings per share as they are either linked to the completion of the Distribution or the required performance conditions had not yet been met.
In 2022, RSUs and stock options to purchase approximately 2,068,000 common shares were not included in the computation of diluted earnings per share because the effect would have been anti-dilutive under the treasury stock method. In 2022, an additional 5,207,000 IPO Founders Grants in the form of stock options and RSUs were not included in the computation of diluted earnings per share as they are linked to the completion of the Distribution.
19.SUPPLEMENTAL CASH FLOW DISCLOSURES
Supplemental cash flow disclosures for the years 2023, 2022 and 2021 are as follows:
| | | | | | | | | | | | | | | | | | | | |
| (in millions) | | 2023 | | 2022 | | 2021 |
| Other Payments | | | | | | |
| Interest paid (Note 3) | | $ | 238 | | | $ | 132 | | | $ | — | |
| Income taxes paid | | $ | 64 | | | $ | 83 | | | $ | 53 | |
Interest paid during 2022 includes $47 million of interest attributed to the BHC Purchase Debt. Refer to Note 3, “RELATED PARTIES” for further detail regarding the BHC Purchase Debt.
20.LEGAL PROCEEDINGS
Bausch + Lomb is involved, and, from time to time, may become involved, in various legal and administrative proceedings, which include or may include product liability, intellectual property, commercial, tax, antitrust, governmental and regulatory investigations, related private litigation and ordinary course employment-related issues. From time to time, Bausch + Lomb also initiates or may initiate actions or file counterclaims. Bausch + Lomb could be subject to counterclaims or other suits in response to actions it may initiate. Bausch + Lomb believes that the prosecution of these actions and counterclaims is important to preserve and protect Bausch + Lomb, its reputation and its assets.
On a quarterly basis, Bausch + Lomb evaluates developments in legal proceedings, potential settlements and other matters that could increase or decrease the amount of the liability accrued. As of December 31, 2023, Bausch + Lomb’s Consolidated Balance Sheets includes accrued current loss contingencies of $5 million related to matters which are both probable and reasonably estimable. For all other matters, unless otherwise indicated, Bausch + Lomb cannot reasonably predict the outcome of these legal proceedings, nor can it estimate the amount of loss, or range of loss, if any, that may result from these proceedings. An adverse outcome in certain of these proceedings could have a material adverse effect on Bausch + Lomb’s business, financial condition and results of operations, and could cause the market value of its common shares and/or debt securities to decline.
Antitrust
Generic Pricing Antitrust Litigation
BHC’s subsidiaries, Oceanside Pharmaceuticals, Inc., Bausch Health US, LLC (formerly Valeant Pharmaceuticals North America LLC) (“Bausch Health US”), and Bausch Health Americas, Inc. (formerly Valeant Pharmaceuticals International) (“Bausch Health Americas”) (for the purposes of this paragraph, collectively, the “Company”), are defendants in multidistrict antitrust litigation (“MDL”) entitled In re: Generic Pharmaceuticals Pricing Antitrust Litigation, pending in the U.S. District Court for the Eastern District of Pennsylvania (MDL 2724, 16 MD-2724). The lawsuits seek damages under federal and state antitrust laws, state consumer protection and unjust enrichment laws and allege that the Company’s subsidiaries entered into a conspiracy to fix, stabilize, and raise prices, rig bids and engage in market and customer allocation for generic pharmaceuticals. The lawsuits, which have been brought as putative class actions by direct purchasers, end payers, and indirect resellers, and as direct actions by direct purchasers, end payers, insurers, hospitals, pharmacies, States, and various Counties, Cities, and Towns, have been or will be consolidated into the MDL. There are also additional, separate complaints which have been consolidated in the same MDL that do not name the Company or any of its subsidiaries as a defendant. On January 31, 2024, the United States Judicial Panel on Multidistrict Litigation entered a Remand Order remanding to the District of Connecticut, among other cases, State of Connecticut, et al. v. Sandoz, Inc., et al., C.A. No. 2:20-03539 (D. CT, C.A. No. 3:20-00802), in which the Company is a defendant. There are cases pending in the Court of Common Pleas of Philadelphia County against the Company and other defendants related to the multidistrict litigation, but no complaint has been filed in these cases. The cases have been put in deferred status. The Company disputes the claims against it and these cases will be defended vigorously.
Additionally, BHC and certain U.S. and Canadian subsidiaries (for the purposes of this paragraph, collectively “the Company”) have been named as defendants in a proposed class proceeding entitled Kathryn Eaton v. Teva Canada Limited, et al. in the Federal Court in Toronto, Ontario, Canada (Court File No. T-607-20). The plaintiff seeks to certify a proposed class action on behalf of persons in Canada who purchased generic drugs in the private sector, alleging that the Company and other defendants violated the Competition Act by conspiring to allocate the market, fix prices, and maintain the supply of generic drugs, and seeking damages under federal law. The proposed class action contains similar allegations to the In re: Generic Pharmaceuticals Pricing Antitrust Litigation pending in the United States Court for the Eastern District of Pennsylvania. The Company disputes the claims against it and this case will be defended vigorously.
These lawsuits cover products of both Bausch + Lomb and BHC’s other businesses. It is anticipated that Bausch + Lomb and BHC will split the fees and expenses associated with defending these claims, as well as any potential damages or other liabilities awarded in or otherwise arising from these claims, in the manner set forth in the MSA.
Product Liability
Shower to Shower® Products Liability Litigation
Since 2016, BHC and its affiliates, including Bausch + Lomb, have been named in a number of product liability lawsuits involving the Shower to Shower® body powder product acquired in September 2012 from Johnson & Johnson; due to dismissals, twenty-seven (27) of such product liability suits currently remain pending. In three (3) cases pending in the Atlantic County, New Jersey Multi-County Litigation, agreed stipulations of dismissal have been entered by the Court, thus dismissing the Company from those cases. Potential liability (including its attorneys’ fees and costs) arising out of these remaining suits is subject to full indemnification obligations of Johnson & Johnson owed to BHC and its affiliates, including Bausch + Lomb, and legal fees and costs will be paid by Johnson & Johnson. Twenty-six (26) of these lawsuits filed by individual plaintiffs allege that the use of Shower to Shower® caused the plaintiffs to develop ovarian cancer, mesothelioma or breast cancer. The allegations in these cases include failure to warn, design defect, manufacturing defect, negligence, gross negligence, breach of express and implied warranties, civil conspiracy concert in action, negligent misrepresentation, wrongful death, loss of consortium and/or punitive damages. The damages sought include compensatory damages, including medical expenses, lost wages or earning capacity, loss of consortium and/or compensation for pain and suffering, mental anguish anxiety and discomfort, physical impairment and loss of enjoyment of life. Plaintiffs also seek pre- and post-judgment interest, exemplary and punitive damages, and attorneys’ fees. Additionally, two proposed class actions were filed in Canada against BHC and various Johnson & Johnson entities (one in the Supreme Court of British Columbia and one in the Superior Court of Quebec), on behalf of persons who have purchased or used Johnson & Johnson’s Baby Powder or Shower to Shower®. The class actions allege the use of the product increases certain health risks (British Columbia) or negligence in failing to properly test, failing to warn of health risks, and failing to remove the products from the market in a timely manner (Quebec). The plaintiffs in these actions are seeking awards of general, special, compensatory and punitive damages. On November 17, 2020, the British Columbia court issued a judgment declining to certify a class as to BHC or Shower to Shower®, and at this time no appeal of that judgment has been filed. On December 16, 2021, the plaintiff in the British Columbia class action filed a Second Amended Notice of Civil Claim and Application for Certification, removing BHC as a defendant; as a result, the British Columbia class action is concluded as to BHC.
In October 2021, Johnson & Johnson, through one or more subsidiaries purported to complete a Texas divisional merger with respect to any talc liabilities at Johnson & Johnson Consumer, Inc. (“JJCI”). LTL Management, LLC (“LTL”), the resulting entity of the divisional merger, assumed JJCI’s talc liabilities and thereafter filed for Chapter 11 bankruptcy protection in the United States Bankruptcy Court for the Western District of North Carolina, which in November 2021 was transferred to the United States District Court for the District of New Jersey (the “Bankruptcy Court”). The first bankruptcy case was dismissed on April 4, 2023, after a decision by the Third Circuit Court of Appeals, and LTL re-filed a new chapter 11 case in the Bankruptcy Court on the same day. Several motions to dismiss were again filed, and on August 11, 2023, the Bankruptcy Court dismissed the second chapter 11 case. On August 24, 2023, LTL and certain supporting creditors and tort claimants filed notices of appeal of the dismissal order. On October 20, 2023, the Third Circuit accepted the appeal, which remains pending. During the pendency of LTL’s bankruptcy cases, the Bankruptcy Court extended a preliminary injunction that had stayed substantially all cases subject to the indemnification agreement related to Johnson & Johnson’s talc liability, which injunction was terminated in connection with the bankruptcy case dismissal.
After the dismissal of the Chapter 11 case, BHC’s and Bausch + Lomb’s position vis a vis Johnson & Johnson returned to the status quo prior to the filing. The litigation against BHC, Bausch + Lomb and other defendants is no longer stayed, and LTL and Johnson & Johnson continues to have indemnification obligations running to BHC and its affiliates, including Bausch + Lomb, for Shower to Shower® related product liability litigation.
Notwithstanding the divisional merger and LTL’s bankruptcy cases, BHC and Bausch + Lomb continue to have indemnification claims and rights against Johnson & Johnson and LTL pursuant to the terms of the indemnification agreement entered into between JJCI and its affiliates and BHC and its affiliates, which indemnification agreement remains in effect. As a result, it is Bausch + Lomb’s current expectation that BHC and Bausch + Lomb will not incur any material impairments with respect to its indemnification claims as a result of the divisional merger or the bankruptcy.
General Civil Actions
California Proposition 65 Related Matter
On June 19, 2019, plaintiffs filed a proposed class action in California state court against Bausch Health US and Johnson & Johnson (Gutierrez, et al. v. Johnson & Johnson, et al., Case No. 37-2019-00025810-CU-NP-CTL), asserting claims for purported violations of the California Consumer Legal Remedies Act, False Advertising Law and Unfair Competition Law in connection with their sale of talcum powder products that the plaintiffs allege violated Proposition 65 and/or the California Safe Cosmetics Act. This lawsuit was served on Bausch Health US in June 2019 and was subsequently removed to the United States District Court for the Southern District of California, where it is currently pending. Plaintiffs seek damages, disgorgement of profits, injunctive relief, and reimbursement/restitution. Bausch Health US filed a motion to dismiss Plaintiffs’ claims, which was granted in April 2020 without prejudice. In May 2020, Plaintiffs filed an amended complaint and in June 2020, filed a motion for leave to amend the complaint further, which was granted. In August 2020, Plaintiffs filed the Fifth Amended Complaint. On January 22, 2021, the Court granted the motion to dismiss with prejudice. On February 19, 2021, Plaintiffs filed a Notice of Appeal with the Ninth Circuit Court of Appeals. On July 1, 2021, Appellants (Plaintiffs) filed their opening brief; Appellees’ response briefs were filed October 8, 2021. This matter was stayed by the Ninth Circuit on December 7, 2021, due to the preliminary injunction entered by the Bankruptcy Court in the LTL bankruptcy proceeding. This stay included Appellants’ reply brief deadline, which was previously due to be filed on or before December 2, 2021. On March 9, 2022, the Ninth Circuit issued an order extending the stay through July 29, 2022. On July 29, 2022, Johnson & Johnson filed a status report in the Gutierrez appeal, outlining the developments since the last status report and the imposition of the stay. Johnson & Johnson noted that following a July 26, 2022, hearing, the Bankruptcy Court left the preliminary injunction in place, and asked the Ninth Circuit to continue to stay this action while the bankruptcy preliminary injunction remained in place. On January 20, 2023, the Ninth Circuit extended the stay until February 17, 2023. On February 17, 2023, Johnson & Johnson requested that the court afford it 60 days – until April 18, 2023, or seven (7) days following any lifting of the LTL Bankruptcy Court’s preliminary injunction, whichever comes earliest – to provide an additional status report about the bankruptcy proceeding and the Third Circuit dismissal for which the LTL has requested a rehearing. On April 7, 2023, Johnson & Johnson Consumer Inc. filed a status report regarding the bankruptcy proceeding advising the Court of the dismissal of the prior bankruptcy proceeding and the filing of the second bankruptcy proceeding, as well as the preliminary injunction and stay order, and requesting the stay of the appeal remain in place until May 10, 2023, which was granted. Following the entry of a preliminary injunction applicable to this case, which was extended until August 26, 2023, the Ninth Circuit extended the stay to June 15, 2023. On June 22, 2023, Johnson & Johnson/LTL filed a status report requesting the stay be extended to August 26, 2023, consistent with the extension of the preliminary injunction by the bankruptcy court. On August 15, 2023, Johnson & Johnson filed a supplemental status report notifying the Ninth Circuit that the second bankruptcy proceeding was dismissed on August 11, 2023 so the stay could be lifted and briefing could proceed to conclusion and setting of oral argument. On September 13, 2023, the Ninth Circuit lifted the stay. On January 28, 2024, the Ninth Circuit issued a notice of oral argument setting the argument for Monday, April 8, 2024.
Bausch Health US disputes the claims in this lawsuit and will defend it vigorously.
New Mexico Attorney General Consumer Protection Action
BHC and Bausch Health US were named in an action brought by State of New Mexico ex rel. Hector H. Balderas, Attorney General of New Mexico, in the County of Santa Fe New Mexico First Judicial District Court (New Mexico ex rel. Balderas v. Johnson & Johnson, et al., Civil Action No. D-101-CV-2020-00013, filed on January 2, 2020), alleging consumer protection claims against Johnson & Johnson and Johnson & Johnson Consumer, Inc., BHC and Bausch Health US related to Shower to Shower® and its alleged causal link to mesothelioma and other cancers. In April 2020, Bausch Health US filed a motion to dismiss, which in September 2020, the Court granted in part as to the New Mexico Medicaid Fraud Act and New Mexico Fraud Against Taxpayers Act claims and denied as to all other claims. The State of New Mexico brings claims against all defendants under the New Mexico Unfair Practices Act and other common law and equitable causes of action, alleging defendants engaged in wrongful marketing, sale and promotion of talcum powder products. The lawsuit seeks to recover the cost of the talcum powder products as well as the cost of treating asbestos-related cancers allegedly caused by those products. Bausch Health US filed its answer on November 16, 2020. On December 30, 2020, Johnson & Johnson filed a Motion for Partial Judgment on the Pleadings and on January 4, 2021, Bausch Health US filed a joinder to that motion, which was denied on March 8, 2021. Trial was scheduled to begin on May 30, 2023, until the case was stayed by an interlocutory appeal to the New Mexico Supreme Court by Johnson & Johnson.
On July 14, 2022, LTL filed an adversary proceeding in the Bankruptcy Court (Case No. 21-30589, Adv. Pro. No. 22-01231) against the State of New Mexico ex rel. Hector H. Balderas, Attorney General, and obtained an injunction from the Bankruptcy Court barring the New Mexico Attorney General from continuing to prosecute the action while the bankruptcy case was pending. Because the Bankruptcy Court has ultimately dismissed both LTL’s first and second bankruptcy cases, this suit has returned to its status quo prior to LTL’s filing.
BHC and Bausch Health US dispute the claims against them, and this lawsuit will be defended vigorously.
California Consumer Protection Action
On October 31, 2023, Plaintiff County of Los Angeles filed an action on behalf of the state of California against the Company and Johnson & Johnson, seeking injunctive relief, restitution and damages in California state court (People of the State of California, by and through County of Los Angeles v. Johnson & Johnson, et al., Case No. 23STCV27015). The lawsuit asserts claims for purported violations of the California False Advertising Law, Unfair Competition Law, and public nuisance claims, against multiple manufacturers of talcum powder products, including Shower to Shower®, that the plaintiffs allege caused or contributed to development of ovarian cancer and mesothelioma in residents of California. The lawsuit seeks injunctive relief, restitution, statutory penalties and damages. The Company and its affiliates dispute the claims against them, and this lawsuit will be defended vigorously.
U.S. Securities Litigation - New Jersey Declaratory Judgment Lawsuit
On March 24, 2022, BHC and Bausch + Lomb were named in a declaratory judgment action in the Superior Court of New Jersey, Somerset County, Chancery Division, brought by certain individual investors in BHC’s common shares and debt securities who are also maintaining individual securities fraud claims against BHC and certain current or former officers and directors as part of the U.S. Securities Litigation. This action seeks a declaratory judgment that alleged transfers of certain BHC assets to Bausch + Lomb would constitute a voidable transfer under the New Jersey Voidable Transactions Act and that Bausch + Lomb would be liable for damages, if any, awarded against BHC in the individual opt-out actions. The declaratory judgment action also alleges that the potential future separation of Bausch + Lomb from BHC by distribution of Bausch + Lomb stock to BHC’s shareholders would leave BHC with inadequate financial resources to satisfy these plaintiffs’ alleged securities fraud damages in the underlying individual opt-out actions. None of the plaintiffs in this declaratory judgment action have obtained a judgment against BHC in the underlying individual opt-out actions and BHC disputes the claims against it in those underlying actions. The underlying individual opt-out actions assert claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 (the "Exchange Act"), and certain actions assert claims under Section 18 of the Exchange Act. The allegations in those underlying individual opt-out actions are made against BHC and several of its former officers and directors only and relate to, among other things, allegedly false and misleading statements made during the 2013-2016 time period by BHC and/or failures to disclose information about BHC’s business and prospects, including relating to drug pricing and the use of specialty pharmacies. On March 31, 2022, BHC and Bausch + Lomb removed the declaratory judgment action to the U.S. District Court for the District of New Jersey. On April 29, 2022, Plaintiffs filed a motion to remand. On November 29, 2022, the District Court granted Plaintiffs’ remand motion and the case was remanded to the New Jersey Superior Court Chancery Division. On December 8, 2022, Plaintiffs filed a proposed Order to Show Cause and motion for a preliminary injunction and sought interim relief including expedited discovery. On December 13, 2022, the Court denied Plaintiffs’ proposed Order to Show Cause and stayed discovery pending the resolution of BHC’s and Bausch + Lomb’s forthcoming motions to dismiss, while instructing BHC to provide certain notice to Plaintiffs of the intended completion of a potential future distribution referenced above under certain circumstances. On December 22, 2022, Plaintiffs filed an amended complaint which, among other things, added claims seeking injunctive relief. On January 11, 2023, BHC and Bausch + Lomb moved to dismiss the amended complaint. Briefing was complete on February 24, 2023, and the motion to dismiss was heard on March 3, 2023. On April 3, 2023, the Court issued a decision granting in part and denying in part the motion to dismiss.
Both BHC and Bausch + Lomb dispute the claims in this declaratory judgment action and intend to vigorously defend this matter.
Doctors Allergy Formula Lawsuit
In April 2018, Doctors Allergy Formula, LLC (“Doctors Allergy”), filed a lawsuit against Bausch Health Americas in the Supreme Court of the State of New York, County of New York, asserting breach of contract and related claims under a 2015 Asset Purchase Agreement, which purports to include milestone payments that Doctors Allergy alleges should have been paid by Bausch Health Americas. Doctors Allergy claims its damages are not less than $23 million. Bausch Health Americas has asserted counterclaims against Doctors Allergy. Bausch Health Americas filed a motion seeking an order granting Bausch Health Americas' summary judgment on its counterclaims against Plaintiff and dismissing Plaintiff’s claims against Bausch Health Americas. The motion was fully briefed as of May 2021.The Court held a hearing on the motion on January 25, 2022. On May 12, 2023, the Court issued a Decision and Order denying Bausch Health Americas’ motion. On June 14, 2023, Bausch Health Americas filed a Notice of Appeal as to the Decision and Order. Bausch Health Americas disputes the claims against it and this lawsuit will be defended vigorously.
Intellectual Property Matters
PreserVision® AREDS Patent Litigation
PreserVision® AREDS and PreserVision® AREDS 2 are OTC eye vitamin formulas for those with moderate-to-advanced AMD. The PreserVision® U.S. formulation patent expired in March 2021, but a patent covering methods of using the formulation remains in force into 2026. Bausch & Lomb Incorporated (“B&L Inc.”) has filed patent infringement proceedings against 19 named defendants in 16 proceedings claiming infringement of these patents and, in certain circumstances, related unfair competition and false advertising causes of action. Thirteen of these proceedings were subsequently settled; two resulted in a default. As of the date of this filing, there is one ongoing action: Bausch & Lomb Inc. & PF Consumer Healthcare 1 LLC v. SBH Holdings LLC, C.A. No. 20-cv-01463-GBW-CJB (D. Del.). Bausch + Lomb remains confident in the strength of these patents and B&L Inc. will continue to vigorously pursue this matter and defend its intellectual property.
Lumify® Paragraph IV Proceedings - DRL
On August 16, 2021, B&L Inc. received a Notice of Paragraph IV Certification from Slayback Pharma LLC (“Slayback”), in which Slayback asserted that certain U.S. patents, each of which is listed in the FDA’s Orange Book for Lumify® (brimonidine tartrate solution) drops (the “Lumify Patents”), are either invalid, unenforceable and/or will not be infringed by the commercial manufacture, use or sale of Slayback’s generic drops, for which an Abbreviated New Drug Application (“ANDA”) has been filed by Slayback. B&L Inc., through its affiliate Bausch + Lomb Ireland Limited, exclusively licenses the Lumify Patents from Eye Therapies, LLC (“Eye Therapies”). On September 10, 2021, B&L Inc., Bausch + Lomb Ireland Limited and Eye Therapies filed suit against Slayback pursuant to the Hatch-Waxman Act, alleging infringement by Slayback of one or more claims of the Lumify Patents, thereby triggering a 30-month stay of the approval of the Slayback ANDA. Since then, U.S. Patent No. 9,259,425 has been dismissed from the case.
On May 15, 2023, the United States Patent & Trademark Office’s Patent Trial and Appeal Board issued a Final Written Decision, finding all claims of U.S. Patent No. 8,293,742 unpatentable. This decision has been appealed to the United States Court of Appeals for the Federal Circuit and the appeal is ongoing. Furthermore, two additional patents (US. Patent Nos. 11,596,600 and 11,833,245) have issued and been listed in the Orange Book as related to Lumify®. Lawsuits alleging infringement of these patents were filed against Slayback and its licensee, Dr. Reddy’s Laboratories S.A. and Dr. Reddy’s Laboratories, Inc. (collectively, “DRL”). Subsequently, on December 15, 2023, B&L Inc., Bausch + Lomb Ireland Limited, and Eye Therapies filed a Motion for a Preliminary Injunction requesting the court to enjoin any infringing activities by DRL.
Additionally, on December 18, 2023, B&L Inc., Bausch + Lomb Ireland Limited, and Eye Therapies amended its complaint to add claims for copyright infringement, as well as claims under the Lanham Act, including trademark and trade dress infringement.
The lawsuit against DRL is ongoing in the District of New Jersey, with no trial date set. Bausch + Lomb remains confident in the strength of the Lumify® related patents and intends to vigorously defend its intellectual property.
In addition to the intellectual property matters described above, in connection with the Vyzulta® and Lotemax® SM products, the Company has commenced ongoing infringement proceedings against a potential generic competitor in the U.S.
Completed or Inactive Matters
The following matters have concluded, have settled, are the subject of an agreement to settle or have otherwise been closed since January 1, 2023, have been inactive from the Company’s perspective for several fiscal quarters or the Company anticipates that no further material activity will take place with respect thereto. Due to the closure, settlement, inactivity or change in status of the matters referenced below, these matters will no longer appear in the Company's next public reports and disclosures, unless required. With respect to inactive matters, to the extent material activity takes place in subsequent quarters with respect thereto, the Company will provide updates as required or as deemed appropriate.
PreserVision® AREDS 2 Antitrust Litigation
B&L Inc. was a defendant in an antitrust suit filed by a competitor, ZeaVision, LLC (“ZeaVision”), on December 20, 2021, in the United States District Court for the Eastern District of Missouri (ZeaVision, LLC v. Bausch & Lomb Incorporated, et al., Civil Action No. 4:21-cv-01487), alleging various antitrust and Lanham act claims, including that B&L Inc.’s efforts to enforce its patents constitutes sham litigation, that certain B&L Inc. advertising is false and violates antitrust laws and the Lanham Act and that certain conduct by B&L Inc. constitutes monopolization. In November, 2022, the action was dismissed for lack of personal jurisdiction. ZeaVision appealed this decision. On August 14, 2023, B&L Inc. and ZeaVision entered into a settlement agreement, resolving all claims in this action. Shortly thereafter, ZeaVision filed a Stipulation of Dismissal in the Eighth Circuit, dismissing its appeal with prejudice.
Patent Litigation against Certain Ocuvite® and PreserVision®
In June and November, 2021, ZeaVision filed complaints for patent infringement (asserting three patents) against certain of the Ocuvite® and PreserVision® products in the Eastern District of Missouri (Case No. 4:21-cv-00739-RWS; Case No. 4:21-
cv-01352-RWS). The cases were subsequently consolidated. On August 14, 2023, the Parties entered into a settlement agreement, resolving all claims in this action. Shortly thereafter, the court dismissed the case with prejudice.
Lumify® Paragraph IV Proceedings - Lupin
A Notice of Paragraph IV Certification was received from Lupin Ltd. (“Lupin”) in January 2022 making similar assertions against the Lumify Patents, in connection with Lupin’s generic brimonidine tartrate solution for which ANDA No. 216716 had been filed by Lupin. Subsequently, in February 2022, B&L Inc., Bausch + Lomb Ireland Limited and Eye Therapies filed suit against Lupin pursuant to the Hatch-Waxman Act, alleging patent infringement of one or more of the Lumify Patents. On September 27, 2023, the Parties entered into a settlement agreement, pursuant to which all claims against Lupin were voluntarily dismissed by the Company. Shortly thereafter, on October 2, 2023, the court signed and entered the stipulated dismissal with respect to Lupin.
21.COMMITMENTS AND CONTINGENCIES
The Company has commitments related to capital expenditures of approximately $95 million as of December 31, 2023.
Under certain agreements, the Company may be required to make payments contingent upon the achievement of specific developmental, regulatory, or commercial milestones. As of December 31, 2023, the Company believes it is reasonably possible that it may potentially make milestone and license fee payments, including sales-based milestone payments, of approximately $63 million over time, in the aggregate, to third parties for products currently under development or being marketed, primarily consisting of the following:
•Under the terms of a December 2019 agreement with Novaliq GmbH, the Company has acquired an exclusive license for the commercialization and development in the U.S. and Canada of MIEBO® (perfluorohexyloctane), formerly known as NOV03, for the treatment of the signs and symptoms of dry eye disease and may be required to make sales-based milestone payments. The Company believes it is reasonably possible that these future sales-based payments over time may approximate $38 million, in the aggregate.
Due to the nature of these arrangements, the future potential payments related to the attainment of the specified milestones over a period of several years are inherently uncertain. As of December 31, 2023, no accruals related to the aforementioned agreements exist because the milestone targets are not yet probable of being achieved.
Indemnification Provisions
In the normal course of operations, the Company enters into agreements that include indemnification provisions for product liability and other matters. These provisions are generally subject to maximum amounts, specified claim periods and other conditions and limits. In addition, the Company is obligated to indemnify its officers and directors in respect of any legal claims or actions initiated against them in their capacity as officers and directors of the Company in accordance with applicable law and the Company has entered into indemnification agreements with its directors and certain officers with respect to such matters. Pursuant to such indemnities, the Company is indemnifying certain former officers and directors in respect of certain litigation and regulatory matters. As of December 31, 2023 and 2022, no material amounts were accrued for the Company obligations under these indemnification provisions.
22.SEGMENT INFORMATION
Reportable Segments
The Company’s CEO, who is the Company’s CODM, manages the business through operating and reportable segments consistent with how the Company’s CEO: (i) assesses operating performance on a regular basis, (ii) makes resource allocation decisions and (iii) designates responsibilities of his direct reports. Pursuant to these changes, effective in the second quarter of 2021, Bausch + Lomb operates in the following reportable segments which are generally determined based on the decision-making structure of Bausch + Lomb and the grouping of similar products and services: (i) Vision Care, (ii) Pharmaceuticals and (iii) Surgical. As of March 31, 2022, the Vision Care/Consumer Health Care segment name was changed to Vision Care. Effective June 30, 2023, the Company renamed its former Ophthalmic Pharmaceuticals segment to the Pharmaceuticals segment.
•The Vision Care segment consists of: (i) sales of contact lenses that span the spectrum of wearing modalities, including daily disposable and frequently replaced contact lenses, and (ii) sales of contact lens care products, OTC eye drops that address various conditions, including eye allergies, conjunctivitis, dry eye and redness relief, and eye vitamin and mineral supplements.
•The Pharmaceuticals segment consists of sales of a broad line of proprietary and generic pharmaceutical products for post-operative treatments and the treatment of a number of eye conditions, such as glaucoma, eye inflammation, ocular hypertension, dry eyes and retinal diseases.
•The Surgical segment consists of sales of medical device equipment, consumables and technologies for the treatment of cataracts, corneal, vitreous and retinal eye conditions, which includes IOLs and delivery systems, phacoemulsification equipment and other surgical instruments and devices necessary for cataract surgery.
Effective in the first quarter of 2023, certain products historically included in the reported results of the Pharmaceuticals segment are now included in the reported results of the Vision Care segment and certain products included in the reported results of the Vision Care segment are now included in the reported results of the Pharmaceuticals segment. Management believes these movements are necessary in order to better align these products with the groupings of similar products. The net impact of these product movements were not material to the periods presented. Prior period presentations of segment revenues and profits have been conformed to the current segment reporting structure.
Segment profit is based on operating income after the elimination of intercompany transactions. Certain costs, such as Amortization of intangible assets, and Other expense (income), net, are not included in the measure of segment profit, as management excludes these items in assessing segment financial performance.
Corporate includes the finance, treasury, certain research and development programs, tax and legal operations of Bausch + Lomb’s businesses and incurs certain expenses, gains and losses related to the overall management of Bausch + Lomb, which are not allocated to the other business segments. In assessing segment performance and managing operations, management does not review segment assets. Furthermore, a portion of share-based compensation is considered a corporate cost, since the amount of such expense depends on company-wide performance rather than the operating performance of any single segment.
Segment Revenues and Profit
Segment revenues and profits for the years 2023, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | | | |
| (in millions) | 2023 | | 2022 | | 2021 | | |
| Revenues: | | | | | | | |
| Vision Care | $ | 2,543 | | | $ | 2,369 | | | $ | 2,327 | | | |
| Pharmaceuticals | 836 | | | 681 | | | 720 | | | |
| Surgical | 767 | | | 718 | | | 718 | | | |
| Total revenues | 4,146 | | | 3,768 | | | 3,765 | | | |
| Segment profit: | | | | | | | |
| Vision Care | 689 | | | 636 | | | 576 | | | |
| Pharmaceuticals | 241 | | | 203 | | | 301 | | | |
| Surgical | 50 | | | 42 | | | 75 | | | |
| Total segment profit | 980 | | | 881 | | | 952 | | | |
| Corporate | (536) | | | (417) | | | (314) | | | |
| Amortization of intangible assets | (240) | | | (244) | | | (292) | | | |
| Other expense, net | (74) | | | (13) | | | (17) | | | |
| Operating income | 130 | | | 207 | | | 329 | | | |
| Interest income | 15 | | | 6 | | | — | | | |
Interest expense (Note 3) | (283) | | | (146) | | | — | | | |
| Foreign exchange and other | (28) | | | 6 | | | (11) | | | |
| Income before provision for income taxes | $ | (166) | | | $ | 73 | | | $ | 318 | | | |
Capital Expenditures
Capital expenditures paid by segment for the years 2023, 2022 and 2021 were as follows:
| | | | | | | | | | | | | | | | | |
| (in millions) | 2023 | | 2022 | | 2021 |
| Vision Care | $ | 116 | | | $ | 115 | | | $ | 137 | |
| Pharmaceuticals | 24 | | | 27 | | | 35 | |
| Surgical | 26 | | | 24 | | | 21 | |
| 166 | | | 166 | | | 193 | |
| Corporate | 15 | | | 9 | | | — | |
| $ | 181 | | | $ | 175 | | | $ | 193 | |
Revenues by Segment and by Product Category
Revenues by segment and product category were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Vision Care | | Pharmaceuticals | | Surgical | | Total |
| (in millions) | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| Pharmaceuticals | $ | 4 | | | $ | 4 | | | $ | 3 | | | $ | 614 | | | $ | 464 | | | $ | 490 | | | $ | — | | | $ | — | | | $ | — | | | $ | 618 | | | $ | 468 | | | $ | 493 | |
| Devices | 888 | | | 866 | | | 890 | | | — | | | — | | | — | | | 762 | | | 706 | | | 706 | | | 1,650 | | | 1,572 | | | 1,596 | |
| OTC | 1,611 | | | 1,461 | | | 1,394 | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,611 | | | 1,461 | | | 1,394 | |
| Branded and Other Generics | 32 | | | 31 | | | 31 | | | 220 | | | 214 | | | 223 | | | — | | | — | | | — | | | 252 | | | 245 | | | 254 | |
| Other revenues | 8 | | | 7 | | | 9 | | | 2 | | | 3 | | | 7 | | | 5 | | | 12 | | | 12 | | | 15 | | | 22 | | | 28 | |
| $ | 2,543 | | | $ | 2,369 | | | $ | 2,327 | | | $ | 836 | | | $ | 681 | | | $ | 720 | | | $ | 767 | | | $ | 718 | | | $ | 718 | | | $ | 4,146 | | | $ | 3,768 | | | $ | 3,765 | |
Certain reclassifications to product categories have been made and are reflected in the table above. These reclassifications are not material.
The top ten products/franchises represented 56%, 58% and 57% of total revenues for the years 2023, 2022 and 2021, respectively.
Geographic Information
Revenues are attributed to a geographic region based on the location of the customer for the years 2023, 2022 and 2021 and were as follows:
| | | | | | | | | | | | | | | | | |
| (in millions) | 2023 | | 2022 | | 2021 |
| U.S. and Puerto Rico | $ | 1,934 | | | $ | 1,695 | | | $ | 1,618 | |
| China | 344 | | | 343 | | | 390 | |
| France | 210 | | | 195 | | | 201 | |
| Japan | 187 | | | 192 | | | 224 | |
| Germany | 147 | | | 138 | | | 149 | |
| United Kingdom | 121 | | | 108 | | | 111 | |
| Canada | 110 | | | 101 | | | 101 | |
| Russia | 106 | | | 132 | | | 116 | |
| Spain | 85 | | | 77 | | | 80 | |
| Italy | 82 | | | 72 | | | 75 | |
| Mexico | 68 | | | 55 | | | 40 | |
| Poland | 51 | | | 44 | | | 42 | |
| South Korea | 46 | | | 44 | | | 46 | |
| Other | 655 | | | 572 | | | 572 | |
| $ | 4,146 | | | $ | 3,768 | | | $ | 3,765 | |
Certain reclassifications have been made and are reflected in the table above.
Long-lived assets consisting of property, plant and equipment, net of accumulated depreciation, are attributed to geographic regions based on their physical location as of December 31, 2023 and 2022 and were as follows:
| | | | | | | | | | | |
| (in millions) | 2023 | | 2022 |
| U.S. and Puerto Rico | $ | 666 | | | $ | 639 | |
| Ireland | 394 | | | 356 | |
| Germany | 98 | | | 89 | |
| Canada | 73 | | | 67 | |
| France | 52 | | | 44 | |
| China | 23 | | | 26 | |
| Italy | 23 | | | 20 | |
| Spain | 14 | | | 13 | |
| Other | 47 | | | 46 | |
| $ | 1,390 | | | $ | 1,300 | |
Major Customers
No individual customer accounted for 10% or more of total revenues.
