UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number
October 12
(Exact name of Registrant as specified in its Charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
The |
||
|
|
The |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
☒ |
|
Smaller reporting company |
|
||
|
|
|
|
Emerging growth company |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, based on the closing price of the shares of common stock on The Nasdaq Stock Market on June 30, 2023, was $
The number of shares of the registrant’s common stock outstanding as of March 18, 2024 was
Auditor Firm Id: |
Auditor Name: |
Auditor Location: |
Table of Contents
|
|
Page |
PART I |
|
|
2 |
||
25 |
||
63 |
||
63 |
||
65 |
||
65 |
||
65 |
||
|
|
|
PART II |
|
|
66 |
||
66 |
||
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
67 |
|
78 |
||
78 |
||
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
78 |
|
79 |
||
80 |
||
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
80 |
|
|
|
|
PART III |
|
|
81 |
||
89 |
||
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
101 |
|
Certain Relationships and Related Transactions, and Director Independence |
104 |
|
105 |
||
|
|
|
PART IV |
|
|
107 |
||
109 |
i
Special Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K (“Annual Report”) contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These forward-looking statements are based on our management’s current beliefs and assumptions and on information currently available to our management, and are contained principally in the sections entitled “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “best in class,” “could,” “seeks,” “estimates,” “expects,” “first-in-class,” “focused,” “goal,” “intends,” “may,” “objective,” “opportunity,” “pipeline,” “plans,” “potential,” “predicts,” “projects,” “pursuing,” “should,” “target,” “treatment option,” “will,” “would,” “might,” “can,” “continue” or similar expressions and the negatives of those terms.
These forward-looking statements include, among other things, statements about:
1
PART I
Item 1. Business.
I. OVERVIEW
We are a clinical-stage biopharmaceutical company focused on the development of human polyclonal immunotherapeutic antibodies, or human immunoglobulins (hIgG), to address immune system disorders and infectious diseases. Our antibodies are both target-specific and polyclonal, meaning they are comprised of multiple hIgGs and can bind to multiple sites on specific immunogens, making them ideally suited to address the complexities associated with many immune-mediated disorders. Our lead candidate, SAB-142 is a human anti-thymocyte globulin (ATG) focused on preventing or delaying the progression of type 1 diabetes (T1D). We recently initiated a Phase 1 trial of SAB-142 to establish its safety and pharmacokinetic profiles in human subjects.
In addition to SAB-142, we also have clinical stage assets targeting infectious diseases that have significant mortality and morbidity in the general population and in high-risk patients. To date, we have conducted seven clinical trials, including Phase 1, Phase 2 and Phase 3, totaling more than 700 individuals dosed with our proprietary hIgGs. In May of 2023, we received Fast Track Designation and Breakthrough Therapy Designation from the Food and Drug Administration Center for Biologics Evaluation and Research (“CBER”) for our SAB-176 immunoglobulin targeting multiple strains of influenza based upon positive clinical data from a Phase 2a trial.
More broadly, we believe that our proprietary platform, referred to as DiversitAb,™ holds the potential to generate additional novel therapeutic candidates to expand our pipeline. DiversitAb utilizes the human immune response to generate the optimal repertoire of IgGs for drug targets of interest. We believe it is the only technology capable of producing disease-targeted, hIgG in large quantities without the need for human plasma donors. We have optimized genetic engineering in the development of transchromosomic cattle, or Tc Bovine, which produce hIgGs. Our engineering of the DiversitAb production system drives IgG1 production across our pipeline. As our lead program SAB-142 advances, we intend to expand our pipeline in complimentary indications through strategic utilization of our platform.
II. BUSINESS STRATEGY
In summary:
2
III. RECENT MILESTONES
We have achieved multiple recent milestones, including:
IV. KEY PRODUCT DIFFERENTIATORS
1. Multivalent product platform to address complex diseases
Our unique production system harnesses the natural advantages of polyclonal immunoglobulins to protect against evolving disease targets by activating our body’s immune system in a target-specific way. Our IgGs are engineered primarily to produce >90% of the IgG1 isotype with fully functional human antibody variable regions (or Fab domains) that specifically bind to target antigens, thus providing multivalent properties. This multi-epitope capability can address individual variability of epitopes associated with immunological diseases and neutralize highly mutating targets, thus preventing mutation escape in infectious diseases. There is a demonstrable and significant potential advantage of Tc Bovine-produced hIgGs characterized by their ability to bind to both foreign exogenous or human endogenous protein targets, ability to activate human effector cells, and do so in a way that does not cause serum sickness or anti-drug antibody formation.
Another key product differentiator of our platform is the ability to produce a multitarget product that addresses the complexity of disease in a single drug product vial. This is a particularly powerful multivalent combination when multiple antigen targets are combined with the muti-epitope targeting of a single antigen (described above), as the therapeutic advantage is expanded to address multiple disease modalities all within a single vial.
2. Rapid Product Development Capability with Proven Regulatory Pathway
Our polyclonal development approach leverages our production system to capture discovery and production efficiencies not available to mAb product development. Through the utilization of our Tc Bovine, we can simultaneously perform discovery and production functions of our polyclonal development, significantly improving the time of antibody discovery and production. This efficiency was demonstrated during the COVID-19 pandemic where SAB-185 product that is in compliance with Current Good Manufacturing Practice regulations enforced by the FDA (cGMP), was produced in 90 days from initial product concept. Our discovery process simply involves antigen design and production as the vaccinated Tc Bovine does the rest including antibody design, down selection, and scaled production all in one system.
Our regulatory pathway has also been established with the US FDA as well as MHRA in the United Kingdom and TGA in Australia. The FDA regulates polyclonal hIgGs and mAbs differently, as mAbs are regulated through the Center for Drug Evaluation and Research (“CDER”) while pAbs are regulated by CBER. CBER has approved over 36 IgG products from both human- and animal-derived plasma. Further, CBER is very familiar with our DiversitAb™ production system and pAb product. We have navigated three SAB drug products through seven clinical trials with one product having advanced to Phase 3.
3
V. PIPELINE PROGRAMS
1. SAB-142 HUMAN ANTI-THYMOCYTE GLOBULIN FOR TYPE 1 DIABETES
a. Summary of SAB 142 for T1D
SAB-142 is a first-in-class, human, multi-target anti-thymocyte globulin treatment designed to provide superior efficacy and safety in delaying the onset or progression of T1D.
The mechanism of action of SAB-142 has been clinically validated in numerous clinical trials with a rabbit anti-thymocyte globulin (rATG). Data from more than 700 human subjects treated with antibodies produced by our platform support expectation of a zero serum sickness rate and zero incidence of neutralizing anti-drug antibodies (ADA) within the upcoming SAB-142 trials. There is an established regulatory path for T1D indications using the SAB-142 modality. We initiated the Phase 1 clinical study with the first patient dosed November 2023. Finally, our next steps will be to file a clinical trial application (CTA) in the EU and an investigational new drug (IND) application in the United States to expand the clinical trials to global jurisdictions.
b. Type 1 Diabetes Background
T1D is a complex and life-threatening autoimmune disease in which the body mistakenly attacks the insulin-producing beta cells of the pancreas. Living with this disease requires daily, sometimes hourly, intensive insulin management with the potential for numerous complications. Despite improvements in glucose monitoring and insulin administrations, mortality amongst people with T1D remains up to 13 times higher compared to matched controls. From a drug development perspective, shifting away from chronic disease management and towards disease-modifying therapies has the potential to change and save millions of lives.
Based on birth cohorts from 1950 to 2040, 6.85 million lives will be lost by 2040 if people are unable to access interventions to diagnose and treat T1D. According to these estimates, T1D stands to become one of the world’s largest deadly chronic health conditions, similar in scale and impact to HIV.
c. Therapeutic Potential of the Polyclonal Modality in New-Onset Type 1 Diabetes
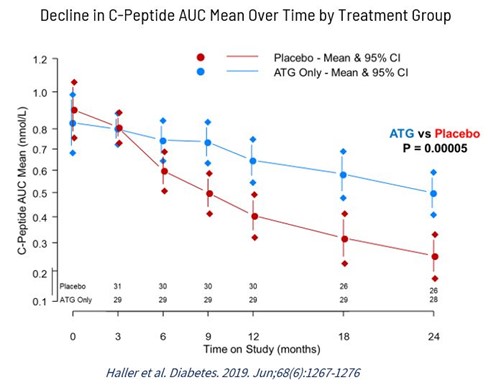
Maintenance of the level of connecting peptide, a short 31 amino acid polypeptide that connects insulin’s A chain to its B chain in the proinsulin molecule, commonly referred to as C-peptide, is a validated surrogate endpoint for endogenous insulin production, essential for the prevention of progression of T1D. Placebo controlled trials with low-dose rATG, defined as a single dose of 2.5 milligram per kilogram (mg/kg), have shown statistically significant maintenance of C-peptide levels and thus a delay in progression of recent onset T1D.
Based on the results of a Phase 2 clinical trial conducted at the University of Florida, a single dose of rATG showed sustained benefit in T1D over a two-year period by maintaining significantly higher C-peptide levels than a placebo control. However, more than 65% of treated patients in this study acquired serum sickness due to the infusion of a non-human antibody, with symptoms that included rash, malaise, fever, and joint swelling. The symptoms often required treatment with steroids that control serum sickness but impair diabetes management and reduces the capacity to re-dose rATG when C-peptide levels begin to drop as shown in Figure 2 below.
4

Figure 1: Rabbit ATG Study for Type 1 diabetes
d. Comparison Efficacy of SAB-142 to rATG
The journey for a polyclonal antibody began over 15 years ago when the first preclinical study demonstrating efficacy of rabbit anti-thymocyte globulin (rATG, Thymoglobulin™) in non-obese diabetic (NOD) mice was performed. Since then, groundbreaking clinical trials with rabbit ATG have been conducted in patients with a recent T1D onset diagnosis. Rabbit ATG, as shown in Figure 1 below, has shown potential therapeutic benefit in T1D as a multi-target polyclonal antibody known to bind multiple lymphocyte cell markers likely associated with this mechanism of action in the modulation of the immune system.

Figure 2: Multi-Target Binding of Thymoglobulin (rATG) IgG Polyclonal Immunoglobulin
All antibody products targeting human proteins have the potential for on-target or off-target adverse effects. Figure 3below summarizes an indicative safety assay, showing that compared to rATG, SAB-142 has a potentially better safety profile as much more of the SAB-142 antibody is required to have the same red blood cell binding activity as the FDA-approved rATG. We have also shown in the table to the right that SAB-142 has potentially higher potency relative to rATG as measured by complement-dependent cytotoxicity activity, using human complement.
5
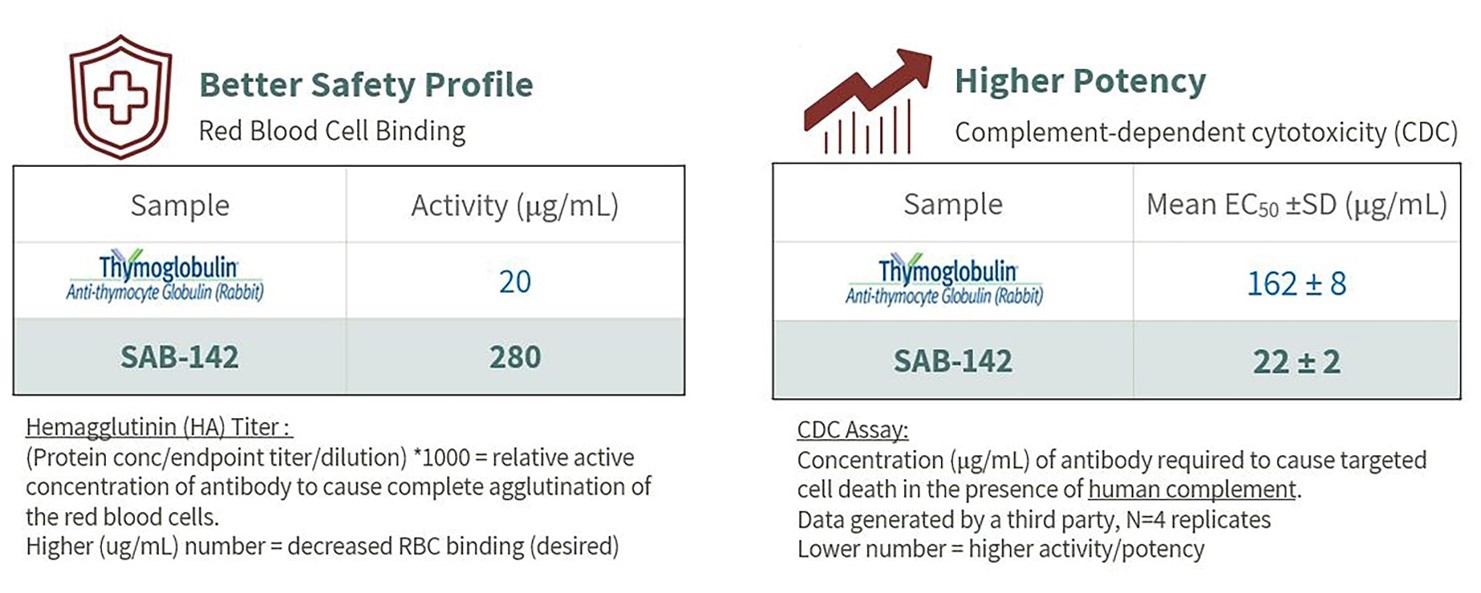
Figure 3: SAB-142 has Potential for a Best-in-Class Safety Profile with Higher Potency Compared to FDA Approved rATG (Thymoglobulin)
To characterize the multi-target binding profile of SAB-142, we compared the target-specific binding profile of SAB-142 to rATG against CD markers associated with human lymphocytes. The data shows similar targeted binding activity between SAB-142 and rATG to the CD2 cell marker associated with human lymphocytes.
Figure 4 below illustrates SAB-142’s similar binding profile to T-cell subsets compared to rATG. This in-vitro flow cytometry data shows SAB-142 on the X-axis binding to the exact same human T-lymphocyte, T helper cells and cytotoxic T-cell populations as rATG on the Y-axis.
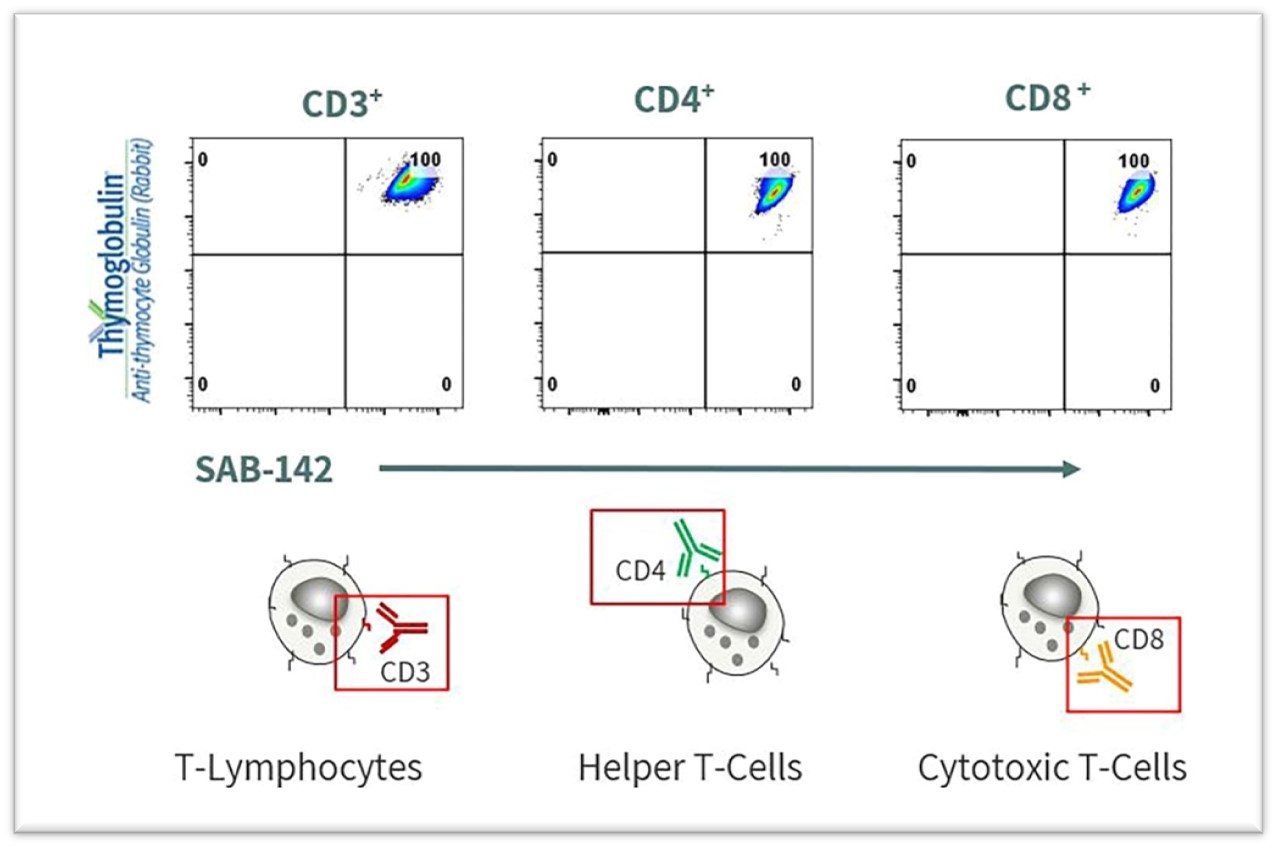
Figure 4: SAB-142 Demonstrates Similar T-Cell Subset Binding Profile as rATG
We further demonstrated that SAB-142 has a similar mechanism of action associated with T-cell subsets compared to rATG shown in Figure 5 below. Similar to rATG, SAB-142 induced a significant reduction of live cytotoxic CD8+T-cells in a dose-dependent manner compared to the non-target-specific human IgG control. Furthermore, SAB-142 activated the
6
conversion of naïve CD4+ cells to an activated state and most importantly, preserved T regulatory cells, again, similar to rATG. As the data shows, SAB-142 demonstrated a multi-target binding profile and mechanism of action similar to FDA-approved rATG with the potential of an improved safety profile and potency.
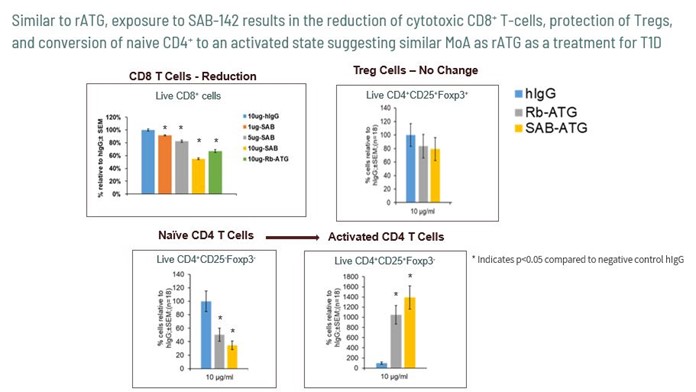
Figure 5: SAB-142 Demonstrates Similar T-Cell Subset Mechanism of Action as rATG
A review of safety parameters based on both short and long-term safety data (up to five years from three separate clinical trials conducted with low-dose rATG in Stage 3 T1D patients) highlights issues associated with dosing humans with rabbit-derived antibodies. Three studies were conducted with two doses of rATG: a single trial with a 6.5 mg/kg dose, and two studies using a low dose of 2.5 mg/kg. Each trial was adequately designed, randomized, double-blind, and placebo controlled. In all three trials, extensive safety assessments and a long-term safety follow-up showed no increase in infection versus placebo, no opportunistic infections or infections known to develop predominantly in immunosuppressed patients, and no difficulty in clearing infections. The 6.5 mg/kg trial investigated an immune response to either a recall or novel antigen as a representative of an immune response to vaccination or an infection. The findings demonstrated that the administration of a single dose of rATG did not result in decreased humoral response versus placebo. Finally, none of the three trials observed an increase in liquid cancers or solid malignancies. In summary, the overall long-term safety profile of a low-dose ATG is supportive of the vision to use SAB-142 as a lifelong disease-modifying treatment without a risk of immunosuppression associated with clinically significant effects such as infections, malignancies or suppressed humoral response.
e. Comparative MoA and Administration of rabbit ATG and SAB-142
Rabbit ATG shows therapeutic promise but offers problematic potential for adverse events that could inhibit long term disease modification and redosing; we believe those issues are resolved by SAB-142. SAB-142 represents an opportunity to offer a novel human alternative to rabbit- or equine-derived ATG IgGs with potential for safe and reliable re-dosing while avoiding the risk factors observed with currently available therapies.
While the mechanism of action of our compound closely resembles rATG, SAB-142 has clear advantages that are fundamental for safe and reliable re-dosing required to delay disease progression. It is well established that treatment with heterologous proteins such as rATG can result in serum sickness, which can trigger Grade 3 adverse events. Serum sickness is defined as a Type 3 hypersensitivity reaction. The heterologous nature of rATG also results in the production of neutralizing anti-drug antibodies (ADAs) in the majority of patients even after a single course of therapy. Neutralizing anti-drug antibodies, or NAbs, are a subset of ADAs that bind to the drug and inhibit its pharmacologic action or activity. Once pharmacological function is inhibited, beta cells are left unprotected from attacks by the cytotoxic CD8-positive T-cells or inflammatory mediators, and the disease continues to progress.
7
Data from preclinical studies and clinical trials suggest that commercially approved rATG has been shown to transiently restore immune-tolerance and reduce autoimmune attack on pancreatic beta cells in T1D patients. Following IV administration, both rATG and SAB-142 have been shown to target key circulating immune cell types involved in an autoimmune response in T1D. Both ATGs cause a dose-proportional transient reduction of CD4+ and CD8+ T-cells while sparing T regulatory (Treg) cells in addition to modulating other autoimmune pathways involved in T1D pathophysiology. These include macrophages and B cells. By reducing overreactive CD4+ and CD8+ T cells while preserving T reg cells, SAB-142 is expected to reduce autoimmune β-cell destruction and delay progression or onset of T1D in patients with Stage 3 or Stage 2 T1D respectively.
In addition to potentially preserving beta cell function in early T1D patients, SAB-142 offers the potential of re-dosing when examining clinically meaningful indicators such as C-peptide levels and glycosylated hemoglobin (HbA1c), without the potential risk of inducing major immune reactions of animal derived IgGs. A short-term safety signal observed in these clinical trials includes serum sickness (SS) and cytokine release syndrome (CRS). In all three trials with rATG, cytokine release syndrome (CRS) was observed. However, its incidence appears to be dose dependent.
f. Comparative Efficacy of teplizumab and SAB-142
Teplizumab, sold under the brand name Tzield, is a humanized anti-CD3 monoclonal antibody that is the first approved treatment indicated to delay the onset of 2 T1D. Teplizumab was approved by the FDA in November of 2022 and is the first approved disease-modifying drug for T1D.
To complete a comparative profile of the SAB-142, it is important to understand how it could be benchmarked against teplizumab, a humanized monoclonal antibody. Teplizumab exclusively binds to the CD3 receptor on T-cells, while the mode of action for SAB-142 is multifactorial, binding to multiple T cell receptors associated with various lymphocyte subsets. Teplizumab is currently approved in a single indication, Stage 2 T1D. With SAB-142, we are targeting both Stage 2 and Stage 3 T1D patients. (See Figures 6 and 8) It is anticipated that Sanofi may file a supplemental NDA with the US FDA for approval for use in patients with Stage 3 T1D.
Based on the published peer-reviewed analysis, teplizumab showed a 63% effect on C-peptide AUC after year two, whereas rATG showed 103% effect on C-peptide AUC at year two. Teplizumab also has immunogenicity liability. Of patients treated with teplizumab, 57% had ADAs, 46% of which were neutralizing ADAs. Due to the human nature of SAB-142, the probability of ADA or neutralizing ADA is very low, which is supported by the immunogenicity data from our clinical trials. Lastly, teplizumab is administered intravenously with a daily IV required over 12-14 days, while SAB-142 dosing is expected to be administered over one to two days.
g. Comparative MoA and Administration of teplizumab and SAB-142
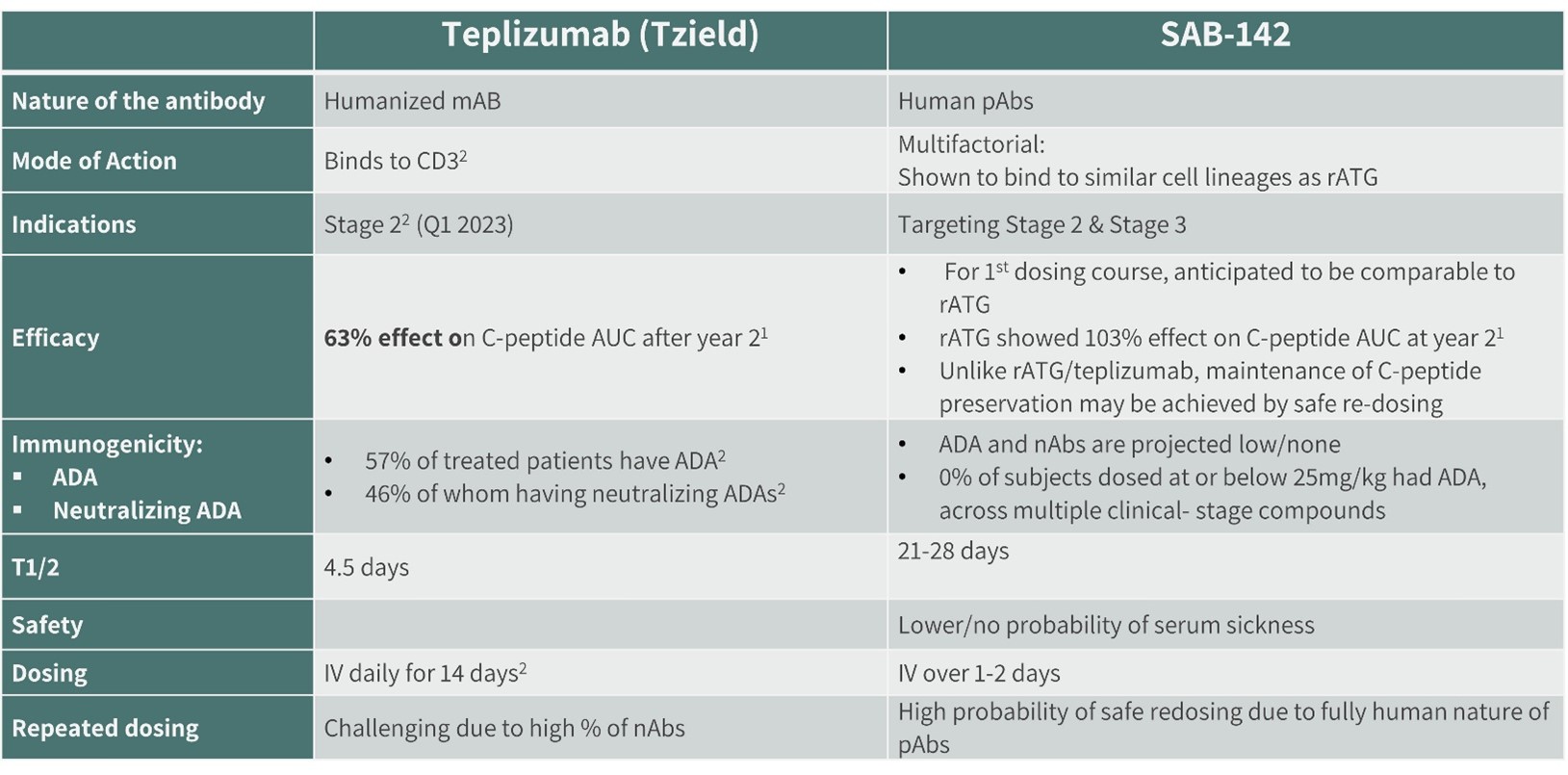
Figure 6: SAB-142 Comparison with Teplizumab (Tzield)
8
h. Manufacturing of SAB-142
To produce SAB-142, we strategically focused on making a best-in-class, human, anti-thymocyte globulin. To accomplish this, we focused our development on improving both the potency and safety profile of SAB-142 benchmarked against FDA-approved rATG or Thymoglobulin™. Recapitulating the rATG mechanism of action was anticipated as the production process of SAB-142 is nearly identical to rATG, wherein the wild-type rabbits are vaccinated with human thymocytes which mount a rabbit polyclonal response. These antibodies are subsequently purified from the plasma (Figure 7). SAB uses a similar production process where we also vaccinate our Tc Bovine with human thymocytes. However, instead of producing animal polyclonal antibodies, our production system produces human anti-thymocyte globulin, which elicits a mechanism of action similar to that of rATG. According to our estimates, between 15-20 Tc bovine could produce tens of thousands of doses of SAB-142.

Figure 7: SAB-142 Production Similar to FDA Approved rATG
i. Indication and Clinical Strategy for SAB-142
Immunological processes resulting in the breakdown of self-tolerance and gradual destruction of pancreatic beta cells by the patient’s own immune system preceding the clinical onset of disease oftentimes starts very early in patients’ lives, sometimes as early as in utero. The average age of clinical onset of T1D is 13 years old. Stage 1 is the start of T1D, marked by individuals having two or more diabetes-related autoantibodies and still normal blood sugar concentrations. In Stage 2, individuals have dysglycemia but without symptoms. Stage 3 is the time of a full clinical diagnosis. Unfortunately, when an individual is first diagnosed with clinical stage T1D, 50- 90% of pancreatic insulin-producing beta cells are already destroyed. Hence, it is critical to start therapy that preserves the remaining fully functional beta cells as soon as possible as it may provide the highest benefit throughout the patient’s lifetime (see Figure 8).
9
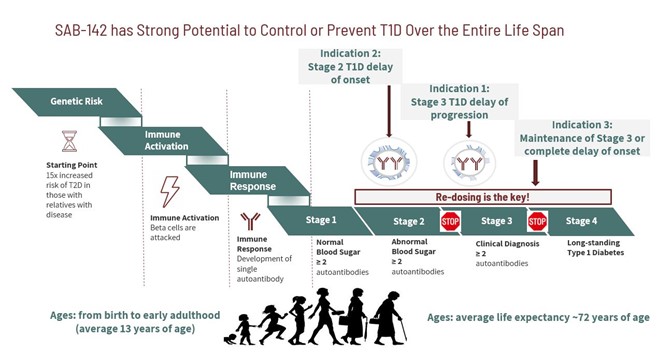
Figure 8: SAB-142 has Strong Potential to Control or Prevent T1D Over the Entire Life Span
One of the early proposed studies in our clinical development program will be in those patients with Stage 3 T1D. The trial participants will be patients who are recently diagnosed with the disease. Following the trials in Stage 3, we would progress into clinical trials in Stage 2 patients. Stage 2 patients are those who do not yet have a full clinical onset of T1D and have even more functional beta cells that can be further preserved. In this patient population, we will aim to delay the onset of full clinical T1D along with evaluation of the re-dosing potentially aimed at fully preventing clinical onset of disease. The ultimate vision for SAB-142 is founded on the potential ability to safely re-dose by delivering a consistent and effective dose of this medication only once per year to fully halt progression of established clinical disease or delay its onset indefinitely.
j. Preclinical Studies for SAB-142
We have completed the GLP toxicology study that enables filing an IND submission.
Objectives:
Results:
This data demonstrates the power of our multi-target approach, which is currently only possible with our production system, where SAB-142 directly impacts each individual immune cell subset. These in vivo results suggest that SAB-142 may have pharmacokinetic and pharmacodynamic attributes relevant for disease-modifying effects in T1D while having the impactful product advantage of an improved safety profile. IND/CTA filings are anticipated by fourth quarter 2024.
10
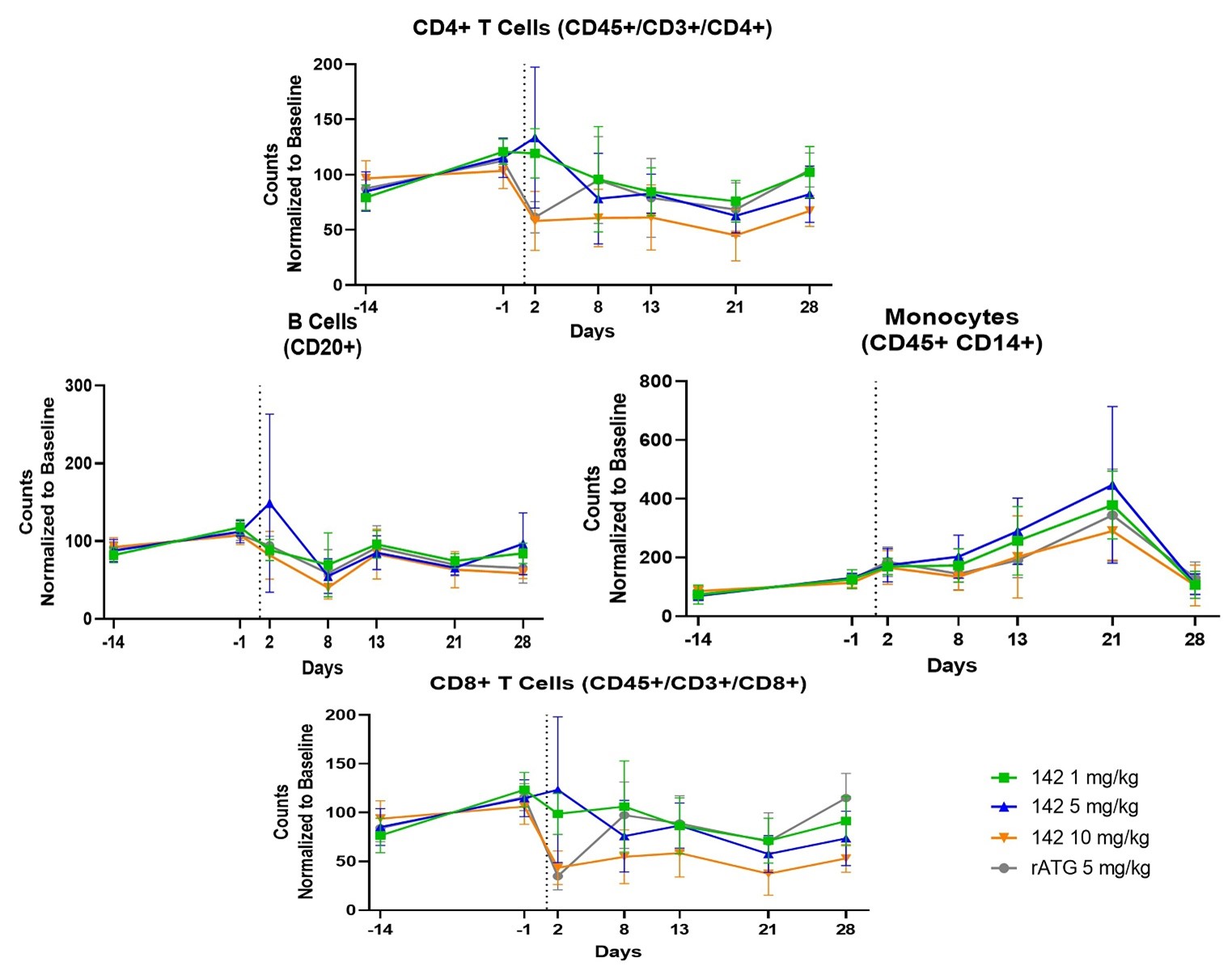
Figure 9: SAB-142 GLP Toxicology Study Results Shows Impacts on Major Relevant T-cell Subsets in-vivo Enables IND Submission
Additionally, we have started a GLP juvenile toxicology study in non-human primates that will read out in the second half of 2024 to enable inclusion of adolescent T1D patients in the future Phase 2b study of SAB-142.
k. Clinical Trial Design and Timelines
Our Phase 1 trial is a randomized, double-blind, single ascending dose trial to assess safety, tolerability, pharmacokinetics, pharmacodynamics, and proof of biological activity (POBA) of SAB-142 for T1D indications (See Figure 10). We commenced dosing in healthy volunteers in November of 2023. The proposed dose range for SAB-142 is projected to be a single infusion over two days ranging between 0.03 and 2.5 mg/kg. We believe major outcomes from this Phase 1 trial will include validating SAB-142 potential for safety superiority based on an anticipated 0% serum sickness and 0% neutralizing anti-drug antibody. We also believe the study will validate the mechanism of action of SAB-142 in humans and establish proof of biological activity based on the change versus baseline in the most important cell lineages such as CD3, CD4, CD8-positive T-cells and Tregs vs. those of rATG in a cross-study comparison.
11
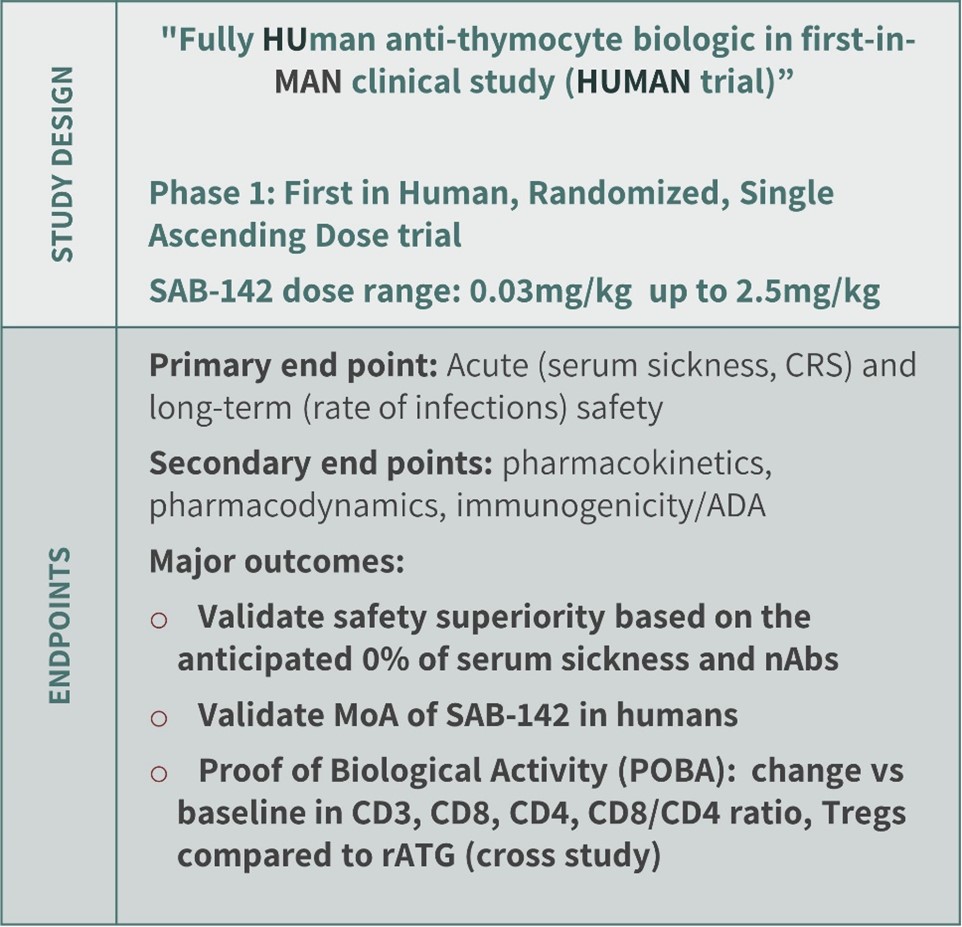
Figure 10: SAB-142 Phase 1 Clinical Plan
In addition to the currently approved and ongoing Phase 1, we plan to bring this program to IND and CTA filings with global regulatory authorities by the mid-2024. As there are unmet medical needs globally for disease-modifying treatments of T1D, we plan to work with global health authorities and file clinical trial applications and clinical trial notifications in other countries to have a global footprint and reach patients with T1D worldwide. We anticipate topline results by the end of 2024. Topline data will include the safety data to support re-dosing along with proof of biological activity. Topline data will further enable global Phase 2 clinical proof of concept and dose-range finding trials in adults and even more importantly, in adolescent T1D population, another critical milestone for 2024 as T1D onset most often occurs in pediatric and adolescent patient populations. Figure 11 shows a proposed timeline of our planned milestones in the next 24 months.
12

Figure 11: SAB-142 Timeline and Key Milestones
2. SAB-176 HUMAN ANTI-INFLUENZA GLOBULIN FOR HIGH-RISK INFLUENZA
a. Summary of SAB-176
SAB-176 is a multivalent, broadly neutralizing -human polyclonal IgG therapeutic candidate in development for the treatment or prevention of severe influenza. This novel, specifically targeted high-potency immunotherapy leverages the human immune response and is designed to bind and neutralize both Type A and Type B influenza, including emerging and mutating strains. It may also be modified to address annual strain changes when needed. Nonclinical and clinical data suggests that SAB-176 offers broad protection against diverse influenza strains, even those that were not specifically targeted, potentially because of its strong cross-reactive potencies to conserved epitopes.
We have completed multiple clinical and nonclinical studies to date, including a Phase 1 trial in healthy volunteers, and most recently a Phase 2a challenge study that was initiated in June 2021. SAB-176 has the potential to complement seasonal vaccine programs to achieve better efficacy than small molecule anti-influenza antivirals in the general population, avoid development of resistant strains, and serve as a protective prophylactic in high-risk populations. We believe that this promising therapy is well-suited to address highly mutating viruses that have significant annual health impacts as well as pandemic potential.
Intravenous, sub-cutaneous, and intra-muscular routes of administration are in development for this product.
b. Influenza Background
Despite numerous available vaccines and treatments, influenza remains a disease with high-unmet medical need, accounting for ~500,000 cases, ~30,000+ deaths, and $11.2 billion dollars per year in direct and indirect costs in the US alone. Only ~50% of high-risk patients vaccinate, and even when vaccines are used, CDC reports adjusted vaccine effectiveness (VE) ranged from only 10% to 60% from 2004 through 2022, with 60% reported only in a single season. Immunocompromised/immunosenescent patients show even lower levels of response to vaccines, with VE at ~24%. No approved treatment of influenza showed any efficacy in hospitalized patients and viral mutations result in reduced susceptibility or resistance to current treatments.
Seasonal influenza remains a meaningful burden for the healthcare system. While the influenza season differs each year, the CDC estimates there are on average 9 to 41 million cases of influenza each year, with 140,000-710,000 hospitalizations and
13
12,000-52,000 deaths per year (average 2010-2020). Oseltamivir phosphate (branded: Tamiflu®) is an effective therapy for treating the flu if used within two days of onset. However, some patients still develop severe disease and are resistant to treatment (estimates of resistance vary: 3-27%). As such, we see the potential for an additional treatment for flu, particularly in higher-risk patients.
c. Competition and SAB-176 Value Proposition
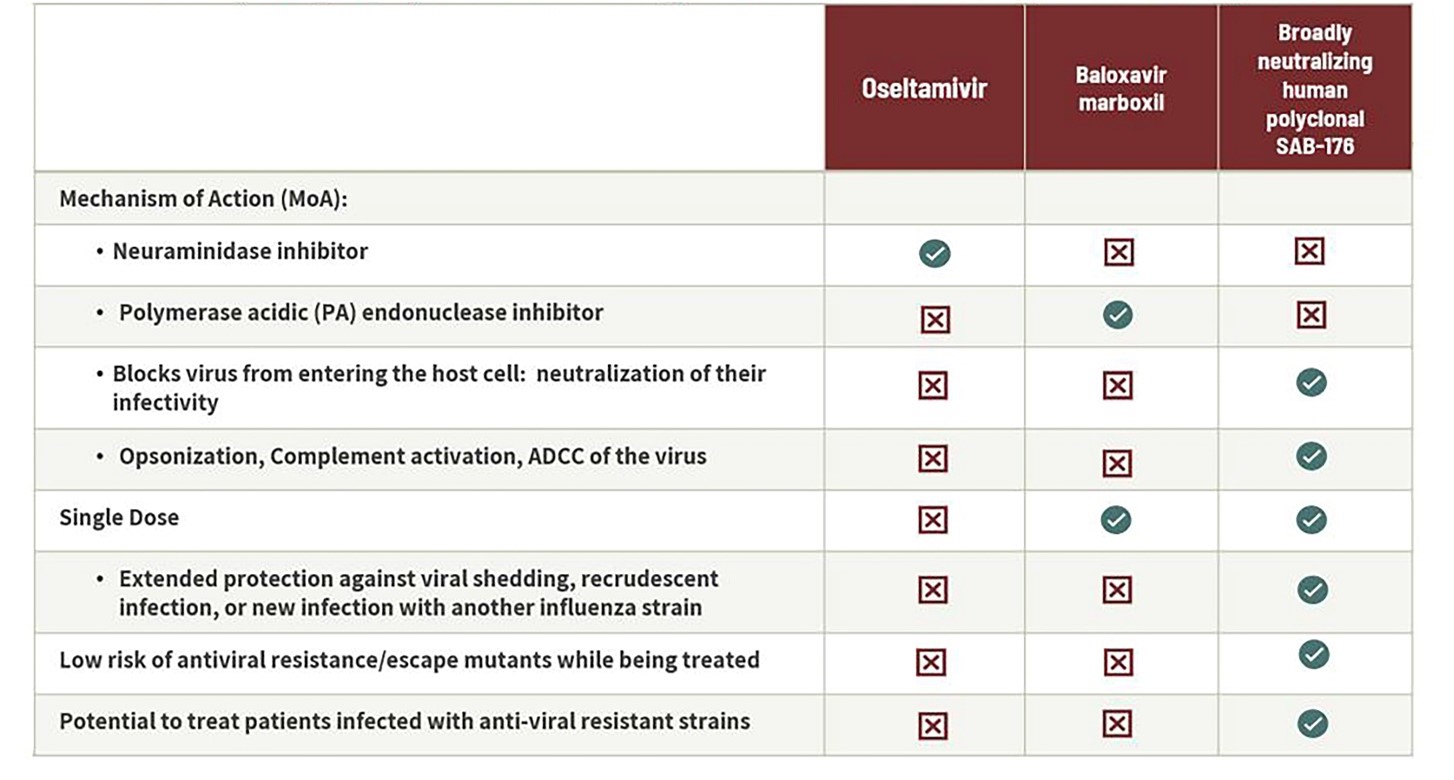
Figure 12: Only SAB-176 Provides Potential for Influenza Biologic with Low Risk of Escape Mutants
SAB-176 represents a comprehensive approach to treatment and prophylaxis (PreP and PEP) of high-risk patients with influenza as a broadly neutralizing -human polyclonal immunoglobulin therapeutic with several anti-viral mechanisms. To summarize, a few key differentiation aspects of this asset include a multi-pronged approach by neutralizing the virus directly and by inducing Antibody Dependent Cellular Cytotoxicity (ADCC), coupled with a long half-life aimed at providing an extended protection against viral shedding and recrudescent infection, low risk of antiviral resistance/escape mutants, and potential to treat patients infected with anti-viral resistant strains. (See Figure 12).
d. Phase 2a Challenge Trial
In December 2021, we announced topline data for a Phase 2a challenge trial that was initiated in June 2021. This was a randomized, double-blind, placebo-controlled study evaluating the safety and treatment efficacy of SAB-176 in 60 healthy adults challenged with a pandemic influenza virus strain (pH1N1). Participants were randomized to receive either SAB-176 (25 mg/kg dose) or placebo and were intranasally inoculated with pandemic H1N1 (2009/California) virus. Nasopharyngeal swabs were taken 8 days after inoculation.
The primary endpoint of the study was reduction of the nasopharyngeal viral load of subjects treated with SAB-176 (expressed as area under the curve, or AUC) compared to those receiving placebo over an 8-day timepoint as measured by qRT-PCR. SAB-176 met the primary endpoint of significantly reducing patient pH1N1 influenza viral load in the treated subjects (p = 0.026, one sided).
14
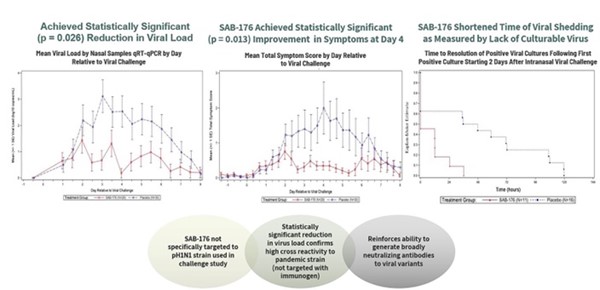
Figure 13: Phase 2a Double-Blind, Placebo-Controlled Study
Secondary endpoints produced similar results, showing separation of SAB-176 vs placebo. One of the secondary endpoints of the challenge study was reduction of clinical flu signs and symptoms in the subjects receiving active treatment (n=8) compared to placebo controls (n=12) for those who had signs and symptoms. SAB-176 achieved statistical significance in meeting the secondary endpoint at Day 4 (p = 0.013, one sided) in symptomatic patients. In this study, SAB-176 also appeared to be safe and well tolerated. No SAB-176-related serious adverse events (SAEs) were observed, and most adverse events were mild to moderate.
e. Phase 1 Trial
SAB-176 was evaluated in an ascending dose, double-blind, randomized, placebo-controlled Phase 1 safety trial in 27 healthy volunteers in 2020. The FDA allowed us to initiate a Phase 1 trial in healthy adults based on the safety profile in the preclinical data set. A Safety Review Committee (SRC) monitored adverse events after each cohort was infused and recommended that each later cohort could be infused with the next highest dose according to the study protocol. Although anticipated adverse events were noted among the SAB-176 and placebo participants, no drug related SAEs were identified by the SRC.
Proprietary DiversitAb™ Production System Overview
Our proprietary DiversitAb™ production system gives us the unique ability to generate targeted, human IgGs without the need for human donors or plasma. These diverse and high-potency hIgGs can be targeted to human immunogens for immune disorders or cancer, viruses, bacteria, and toxins. The production system relies on advanced genetic engineering that functionally replaces bovine IgGs with human hIgGs (resulting in our Tc Bovine) produced from the full germ-line repertoire of human antibody heavy chain and kappa light chain genes on an engineered human artificial chromosome (HAC). The human antibody genes have been further engineered to efficiently produce a diverse repertoire of human immunoglobulin G (which is referred to as hIgG) in bovine B-cells in response to specifically targeted immunogens as a result of the hyperimmunization of the Tc Bovine. Bovine were selected because they are large animals that produce large amounts of plasma, and as ruminants, have high concentrations of circulating hIgGs with a robust response to immunogen challenge that produces high potency, high avidity hIgGs.
Through our DiversitAb™ production system, we have engineered a targeted human immunoglobulin production system that emulates the way that the human immune system synergistically targets the complexity of human disease. The discovery, development and production process represent a “plug-and-play” approach:
15
Our DiversitAb™ production system is replicable and scalable given Tc Bovine are genetic clones. Animals can be produced through cloning technology and plasma fractionation to meet market demand through a well-established and scalable GMP process. We believe that targeted human IgGs can be produced against the same immunogen or multiple immunogens, depending on the disease target and indication, in as many Tc Bovine as necessary to generate sufficient doses to fully supply the target market. Human IgG consistency of product is achieved by testing the potency of IgGs contained in each plasma collection and then combining plasma collections in a manufacturing pool that generates specified potencies within a specified antibody protein concentration.
We believe that the speed with which we can deploy our DiversitAb™ production system to develop countermeasures for emerging diseases and pandemics represents a significant advantage relative to other antibody manufacturers. We have successfully utilized our DiversitAb™ production system technology to generate early proof of concept and initial clinical lots that address specified immunotherapy targets in as little as 128 days, including completion of IND-enabling studies, in response to the COVID-19 pandemic.
VI. Manufacturing Strategy
In support of our operations, we currently operate two plasma fractionation purification facilities in Sioux Falls, South Dakota: a 50L small batch scale cGMP suite that has produced clinical grade drug product to accommodate Pre-Clinical and Phase 1 studies, and a 200L scale larger batch cGMP suite that was completed in 2021 which can be used to produce clinical grade drug substance and drug product to accommodate larger sized advanced Phase 2 clinical studies or Emergency Use.
In addition, we maintain supportive laboratory facilities and operations in Sioux Falls, South Dakota, for drug discovery, product and process development, and clinical manufacturing. We have fully GLP and cGMP compliant quality control testing facilities and we have further developed our own internal antigen (immunogen) discovery and production capabilities to accommodate the Tc Bovine immunizations that improve our overall plasma production speed and efficiency further enhancing our drug discovery and clinical manufacturing timeline.
Our Tc Bovine are housed at dedicated specialty facilities, accredited by the American Association for Accreditation of Laboratory Animal Care (the “AAALAC”) that cater to the production, health, safety, and welfare of the animals, and provide plasma production. We recently completed an expansion of our research and development laboratory facilities to accommodate our discovery programs, support for our pre-clinical pipeline programs, and process development research for our product candidates. The upstream process is easily scalable. Animals donate plasma three times per month (2.1% of bodyweight each time). To produce more product, more animals are added to the program and immunized to the target.
VII. COMPETITION
The biopharmaceutical industry is highly competitive and subject to rapid and significant technological change as research provides a deeper understanding of the pathology of diseases and new technologies and treatments are developed. We believe our scientific knowledge, technology, and development capabilities provide us with substantial competitive advantages, but we face potential competition from multiple sources, major pharmaceutical, specialty pharmaceutical and existing or emerging biotechnology companies, academic research institutions, governmental agencies, and public and private research institutions worldwide.
Our competitors may have significantly greater financial resources, robust drug pipelines, established presence in the market and expertise in research and development, manufacturing, pre-clinical and clinical testing, obtaining regulatory approvals and reimbursement and marketing approved products than we do. These competitors also compete with us in recruiting and retaining qualified clinical, regulatory, scientific, sales, marketing, and management personnel, in establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
16
If any future product candidates identified through our current lead programs are eventually approved for sale, they will likely compete with a range of treatments that are either in development or currently marketed for use in those same disease indications. Our success will partially depend on our ability to obtain, maintain, enforce, and defend patents and other intellectual property rights with respect to our hIgGs that are proven to be safer or more effective or are less expensive than competing products. We could see a reduction or elimination in our commercial opportunity if our competitors develop and commercialize drugs that are safer, better tolerated, more effective, more convenient to administer, less expensive, more resistant to viral escape, or receive a more favorable label than our product candidates.
VIII. INTELLECTUAL PROPERTY
We actively seek to protect the intellectual property and proprietary technology production system that we believe is important to our business, which includes seeking and maintaining patents covering our technology production system and products, and any other inventions that are commercially or strategically important to the development of our business. We also seek to protect the confidentiality of trade secrets that may be important to the development of our business. Our ability to stop third parties from making, using, selling, offering to sell, or importing our products may depend on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities. For more information, please see “Risk Factors – Risks Related to Our Intellectual Property”.
The portfolio of intellectual property and trade secrets that we have developed includes patents related to the activity of our human artificial chromosome and methods that we expect to generate human IgGs at commercial scale. The patent portfolio includes composition and method patents. Our goal is to continue expansion of the breadth of claims and length of claim protections. Our technologies may be difficult to replicate, creating potential barriers to entry, as our genetic engineering know-how and suite of proprietary production system IP and trade secrets have been developed and optimized over nearly two decades.
We expect our global patent protection to extend to 2041 and beyond with respect to producing commercial-scale hIgGs using our chromosome engineering that generates high concentrations of hIgGs in ungulates. However, we recognize that patents and other intellectual property rights in biotechnology are constantly evolving with many risks and uncertainties, which may affect those rights.
As of March 2024, our patent portfolio includes over 40 issued patents or pending applications. We have made strategic filings in jurisdictions including the United States, Australia, Canada, China, Europe, Japan, Korea, and Mexico.
These patent families cover:
17
IX. U.S. PATENT SYSTEM
In most countries in which we file, including the United States, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent’s term may potentially be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. PTO in examining and granting a patent considering delays on the part of the patentee or may be shortened if a patent is terminally disclaimed over an earlier filed patent. In the United States, the patent term that covers an FDA-licensed biologic may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product licensure, only one patent applicable to a licensed biologic may be extended and only those claims covering the licensed biologic, a method for using it, or a method for manufacturing it may be extended. Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers a licensed biologic. In the future, if and when our product candidates receive FDA approval or licensure, we expect to apply for patent term extensions on patents covering those products. We expect to seek patent term extensions to any of our issued patents in any jurisdiction where these are available, however there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with our assessment of whether such extensions should be granted, and if granted, the length of such extensions. For more information regarding the risks related to our intellectual property, see the section titled “Risk Factors – Risks Related to Our Intellectual Property”.
X. U.S. Patent Term Restoration
Depending upon the timing, duration, and specifics of FDA approval of product candidates, some of a sponsor’s U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during the product development and FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval or licensure date. The patent term restoration period generally is- once the patent issues- one-half the time between the effective date of an IND and the submission date of a biologics license application (“BLA”) less any time the sponsor did not act with due diligence during the period, plus the time between the submission date of a BLA and the approval of that application less any time the sponsor did not act with due diligence during the period. Only one patent applicable to an approved biological product is eligible for the extension, only those claims covering the licensed biologic, a method for using it or a method for manufacturing it may be extended and the application for the extension must be submitted prior to the expiration of the patent. Moreover, a given patent may only be extended once based on a single product. The U.S. PTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
x1. Government Regulation
In the United States, we expect our hIgG product candidates to be regulated by the FDA as biological products. Additionally, in manufacturing our product candidates, we alter the genomic DNA in animals, and FDA considers such altered genomic DNA in an animal to be a new animal drug, which require submission and approval of a New Animal Drug Application (NADA) prior to being marketed in the United States.
18
1. Regulation of Transgenic Animals and New Animal Drugs
The U.S. Department of Agriculture (USDA) regulates the company’s Tc Bovine husbandry activities, including housing, healthcare, and general management of these specialized animals. This includes regulations and periodic facility inspections and reporting. We also are voluntarily accredited by the AAALAC. The AAALAC International accreditation program evaluates organizations that use animals in research, teaching or testing. Those that meet or exceed AAALAC standards are awarded accreditation. The accreditation process includes an extensive internal review conducted by the institution applying for accreditation.
The FDA considers, with limited exclusions, the altered genomic DNA in an animal to be a drug because such altered DNA is an article intended to affect the structure or function of the body of the animal, and, in some cases, intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in the animal. In the United States, new animal drugs are subject to regulation under the Federal Food, Drug, and Cosmetic (the “FDCA”), and under the FDCA, in general, a new animal drug is “deemed unsafe” and adulterated unless the FDA has approved a new animal drug application (NADA) for its intended use or unless the drug is only for investigational use and conforms to specified exemptions for such use under an investigational new animal drug (INAD) exemption. Further, early in the development process, FDA has allowed the submission of information to FDA’s Center for Veterinary Medicine (CVM), without the establishment of an INAD file, such as through creation of a veterinary master file (VMF), subject to certain conditions such as restrictions on introducing any food derived from such investigational animals into the food supply.
The requirements governing development and approval of a new animal drug are analogous to those for new human drugs. A NADA must generally be accompanied by payment of a substantial user fee and must contain substantial evidence of the safety and effectiveness of the new animal drug as well as detailed descriptions of the methods used in and the facilities and controls used for the manufacturing, processing and packaging of the new animal drug to enable FDA to reach a determination that such methods, facilities and controls are adequate to preserve the identify, strength, quality and purity of the new animal drug. Further, when FDA reviews and approves a NADA, FDA generally conducts a review of environmental risks pursuant to the requirements of the National Environmental Policy Act (NEPA), if any and where required.
2. U.S. Biological Products Development Process
In the United States, biologic products are licensed by the FDA for marketing under the Public Health Service Act, (PHS Act), and regulated under the FDCA. Both the FDCA and the PHS Act and their corresponding regulations govern, among other things, the testing, manufacturing, safety, purity, potency, efficacy, labeling, packaging, record keeping, storage, distribution, marketing, sales, import, export, reporting, advertising, and other promotional practices involving biologic products. FDA authorization is required prior to clinical testing of biologic products. FDA licensure also must be obtained prior to marketing of biologic products. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial financial resources and time.
3. Hybrid Process for a Biological Product Is Developed from Animals with Intentionally Altered Genomic DNA
The process required by the FDA before a biologic product may be marketed in the United States is generally well documented. In the case of a product that is developed from animals with intentionally altered genomic DNA as the donor material source, the process is more complex and involves both CVM, to oversee the intentionally altered genomic DNA in animals and the Office of Tissues and Advanced Therapies (“OTAT”) at CBER to oversee the immunoglobulin products.
During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical trial investigators. Annual progress reports detailing the results of the clinical trials must be submitted to the FDA. Written INAD and IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events, any findings from other studies, tests in laboratory animals or in vitro testing that suggest a significant risk for human subjects, or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receipt of the information. Phase 1, Phase 2, and Phase 3 clinical trials may not be completed successfully within any specified period, if at all. The FDA or the sponsor or its data safety monitoring board may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being
19
conducted in accordance with the IRB’s requirements or if the biologic has been associated with unexpected serious harm to patients.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the physical characteristics of the biologic as well as finalize a process for manufacturing the product in commercial quantities in accordance with GMP requirements. To help reduce the risk of the introduction of adventitious agents with the use of biologics, the PHS Act emphasizes the importance of manufacturing control for biologic products whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the sponsor must develop methods for testing the identity, strength, quality, potency, and purity of the final biological product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the biological product candidate does not undergo unacceptable deterioration over its shelf life.
There are also various laws and regulations regarding laboratory practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances in connection with the research. In each of these areas, the FDA and other regulatory authorities have broad regulatory and enforcement powers, including the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products and withdraw approvals.
4. U.S. Review and Approval Processes
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of a NADA requesting approval of the altered genomic DNA in donor animals and a BLA requesting approval to market the product for one or more indications. The BLA must include results of product development, laboratory and animal studies, human studies, information on the manufacture and composition of the product, proposed labeling and other relevant information. The testing and approval processes require substantial time and effort, and there can be no assurance that the FDA will accept the BLA for filing and, even if filed, that any approval will be granted on a timely basis, if at all.
Under the Prescription Drug User Fee Act (PDUFA), as amended, each BLA may be accompanied by a significant user fee. Under federal law, the submission of most applications for approval of drug and biologic products is subject to an application user fee. The sponsor of an approved application is also subject to an annual program fee. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business.
Within 60 days following submission of a BLA or within 30 days following submission of a NADA, the FDA reviews the submitted application to determine if it is substantially complete before the FDA accepts it for filing. The FDA may refuse to file any application that it deems incomplete or not properly reviewable at the time of submission and may request additional information. In this event, the application must be resubmitted with additional information. The resubmitted application also is subject to review to determine if it is substantially complete before the FDA accepts it for filing. In most cases, the submission of an application to FDA is subject to a substantial application user fee, although the fee may be waived under certain circumstances.
Under the performance goals and policies implemented by the FDA under the Animal Drug User Fee Act (ADUFA) for original NADAs, the FDA targets 180 days from the submission date in which to complete its initial review and act on a standard application. A NADA is considered incomplete if it requires additional data or information to enable the FDA to complete and reach a decision on issues presented in the NADA. Once the sponsor reactivates the NADA by addressing identified deficiencies, the FDA targets 135 to 180 days, depending in part on whether the deficiencies are identified as not substantial or substantial, respectively, to complete its review and respond to the applicant.
The sponsor of a new animal drug may voluntarily decide to utilize FDA’s “phased review” process to complete all technical sections required for approval of a new animal drug before submitting a NADA by submitting such information during the investigational phase of the animal drug development process. Utilizing this process, the sponsor may submit an administrative NADA, which is a NADA submitted after all technical sections necessary to fulfill the requirements for the approval of a new animal drug have been reviewed by the CVM and the CVM has issued a technical section complete letter for each of the required technical sections. The FDA targets 60 days from the filing date to complete its review and act on an administrative NADA.
Under the performance goals and policies implemented by the FDA under the PDUFA for original BLAs, the FDA targets ten months from the filing date in which to complete its initial review of a standard application and respond to the applicant, and six months from the filing date for an application with priority review. The FDA does not always meet its PDUFA goal dates, and the review process is often significantly extended by FDA requests for additional information or clarification.
20
Once the submission is accepted for filing, the FDA begins an in-depth substantive review of the NADA and BLA. The FDA reviews the applications to determine, among other things, whether the proposed product is safe, pure, and potent, for its intended use, and whether the product is being manufactured in accordance with cGMP to ensure its continued safety, purity, and potency. The FDA may refer applications for novel biological products or biological products that present difficult or novel questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation, and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. During the biological product approval process, the FDA also will determine whether a REMS is necessary to assure the safe use of the biological product. If the FDA concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the FDA will not approve the BLA without a REMS, if required.
5. Post-Approval Requirements
Maintaining substantial compliance with applicable federal, state, and local statutes and regulations requires the expenditure of substantial time and financial resources. Rigorous and extensive FDA regulation of biological products continues after approval, particularly with respect to GMP. We will rely, and expect to continue to rely, on third parties to produce clinical and commercial quantities of any products that we may commercialize. Manufacturers of our products are required to comply with applicable requirements in the GMP regulations, including quality control and quality assurance and maintenance of records and documentation.
Following approval, the manufacturing facilities are subject to periodic inspections by the FDA, and such inspections may result in an issuance of FDA Form 483 deficiency observations, an untitled letter, or a warning letter, which can lead to plant shutdown and other more serious penalties and fines. Prior to the institution of any manufacturing changes, a determination needs to be made whether FDA approval is required in advance. If not done in accordance with FDA expectations, the FDA may restrict supply and may take further enforcement action. Annual product reports are required to be submitted. Other post-approval requirements applicable to biological products include reporting of GMP deviations that may affect the identity, potency, purity and overall safety of a distributed product, record-keeping requirements, reporting of adverse events, reporting updated safety and efficacy information, and complying with electronic record and signature requirements.
Additionally, rigorous and extensive FDA regulation of new animal drugs continues after approval. Owners of approved NADAs continue to have ongoing responsibilities under the FDCA, including registration and listing, recordkeeping, filing supplements, and periodic reporting.
6. Expedited Review and Approval Programs
The FDA has various programs, including fast track designation, priority review, accelerated approval and breakthrough therapy designation, which are intended to expedite or simplify the process for the development and FDA review of biological products that are intended for the treatment of serious or life-threatening diseases or conditions and demonstrate the potential to address unmet medical needs. The purpose of these programs is to provide important new biological products to patients earlier than under standard FDA review procedures. To be eligible for a fast-track designation, the FDA must determine, based on the request of a sponsor, that a biological product is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address an unmet medical need. The FDA will determine that a product will fill an unmet medical need if it will provide a therapy where none exists or provide a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. In addition to other benefits, such as the ability to have greater interactions with the FDA, the FDA may initiate review of sections of a fast track BLA before the application is complete, a process known as rolling review.
The FDA may give a priority review designation, such as a rare pediatric disease designation, to biological products that treat a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. A priority review means that the goal for the FDA’s review of an application is six months, rather than the standard goal of ten months under current PDUFA guidelines. Most products that are eligible for fast-track designation may also be considered appropriate to receive a priority review. In addition, biological products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the biological product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments.
21
Under the FDA Safety and Innovation Act enacted in 2012, a sponsor can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug or biological product that is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug or biological product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Breakthrough therapy designation comes with all the benefits of fast-track designation, which means that the sponsor may file sections of the BLA for review on a rolling basis if certain conditions are satisfied, including an agreement with the FDA on the proposed schedule for submission of portions of the application and the payment of applicable user fees before the FDA may initiate a review. Drug and biological products designated as breakthrough therapies are also eligible for accelerated approval. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification and the time period for FDA review or approval will not be shortened. Furthermore, fast track designation, priority review, accelerated approval and breakthrough therapy designation do not change the standards for approval and may not ultimately expedite the development or approval process.
7. Emergency Use Authorizations
While, in most cases, a biologic must be approved by the FDA pursuant to a BLA before the product may be sold, when there is a public health emergency involving chemical, biological, radiological, or nuclear agents, including infectious diseases like COVID-19, new therapeutics may be distributed pursuant to an Emergency Use Authorization (EUA). Under an EUA, the FDA may authorize the emergency use of an unapproved medical product or an unapproved use of an approved product for certain emergency circumstances to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, and after the Secretary of the Department of Health and Human Services has issued a declaration of emergency or threat justifying emergency use. EUAs are intended to address serious or life-threatening diseases or conditions caused by a chemical, biological, radiological, or nuclear agent, including emerging infectious disease threats, such as the COVID-19 pandemic. To receive an EUA, the product sponsor must demonstrate that the product “may be effective” in the prevention, diagnosis, or treatment of an applicable disease or condition. Additionally, the FDA must determine that the product’s known and potential benefits outweigh the known and potential risks. Further there must be no adequate, approved, and available alternative product for the indication. Potential alternative products may be unavailable if there are insufficient supplies to meet the emergency need. The FDA may establish additional conditions on an EUA that are necessary to protect public health, including conditions related to information that must be disseminated to health care providers and patients, the monitoring and reporting of adverse events, and record keeping. Conditions may also relate to how a product is distributed and administered and how a product is advertised. Importantly, EUAs are not full marketing approvals. Rather, EUAs are only effective for the duration of the applicable EUA declaration. Full approval of the product under applicable standards established under the FDCA would be necessary to continue to distribute the product absent an EUA. EUAs may also be revised or revoked by FDA at any time.
8. Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the United States, or 200,000 or more individuals in the United States for which there is no reasonable expectation that the cost of developing and making available in the United States a drug or biologic for this type of disease or condition will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested before submitting a BLA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. The orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review or approval process.
9. Pediatric Trials
Under the Pediatric Research Equity Act (PREA), a BLA or supplement to a BLA must contain data to assess the safety and efficacy of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDCA requires that a sponsor who is planning to submit a marketing application for a drug or biologic product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan, or PSP, within sixty days of an end-of-Phase 2 meeting or as may be agreed between the sponsor and FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design,
22
age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from nonclinical studies, early phase clinical trials, and/or other clinical development programs. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements.
10. Marketing Exclusivity
Depending upon the timing, duration, and specifics of the FDA approval of the use of our product candidates, some of our United States patents may be eligible for limited patent term extension under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. In addition, a patent can only be extended once and only for a single product. The U.S. PTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. The Biologics Price Competition and Innovation Act of 2009 (“BPCIA”), which was enacted as part of the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (the “ACA”), created an abbreviated approval pathway for biological products that are demonstrated to be “biosimilar” or “interchangeable” with an FDA-licensed reference biological product via an approved BLA. Biosimilarity to an approved reference product requires that there be no differences in conditions of use, route of administration, dosage form and strength and no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency. Biosimilarity is demonstrated in steps beginning with rigorous analytical studies or “fingerprinting,” in vitro studies, in vivo animal studies and generally at least one clinical study, absent a waiver from the Secretary of the U.S. Department of Health and Human Services (“HHS”).
11. Additional Regulation
In addition to the foregoing, state and federal laws regarding environmental protection and hazardous substances, including the Occupational Safety and Health Act, the Resource Conservancy and Recovery Act and the Toxic Substances Control Act, affect our business. These and other laws govern our use, handling and disposal of various biological, chemical, and radioactive substances used in, and wastes generated by, our operations. If our operations result in contamination of the environment or expose individuals to hazardous substances, we could be liable for damages and governmental fines. We believe that we are in material compliance with applicable environmental laws and that continued compliance therewith will not have a material adverse effect on our business. We cannot predict, however, how changes in these laws may affect our future operations.
XII. Regulation Outside of the United States
In addition to regulations in the United States, we are, and will continue to be, subject to a variety of regulations in other jurisdictions governing, among other things, clinical studies and any commercial sales and distribution of our products. Because biologically sourced raw materials are subject to unique contamination risks, their use may be restricted in some countries. Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical studies or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical study application much like the IND prior to the commencement of human clinical studies.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, and criminal prosecution.
23
XIII. Our Corporate History
SAB Sciences, Inc. (formerly SAB Biotherapeutics, Inc.) was incorporated in April 2014 as a Delaware corporation (“Legacy SAB”). We acquired all the intellectual property rights to Tc Bovine and the DiversitAb™ production system from Sanford Applied Biosciences, a wholly owned subsidiary of Sanford Health, to develop targeted human IgGs to specific targets and advance clinical development and commercialization. The technology was originally contemplated in 1998 by professors at the University of Massachusetts Amherst and Amherst College who recognized a significant gap in immunotherapy applications, namely, using the way our bodies fight disease through a human immunoglobulin response. The technology founders established a biotech company called “Hematech” to develop the technology. This founding company was purchased and became a wholly owned subsidiary of Kirin in Tokyo, Japan in 2005. In 2007, the pharmaceutical division of Kirin became Kirin Pharma and in 2008 merged with Kyowa Hakko Kogyo to become Kyowa Hakko Kirin (“KHK”). The technology was developed through 2012 by Hematech as a wholly owned subsidiary of KHK. On December 31, 2012, KHK divested the technology and transferred ownership of all property, assets, and intellectual property of Hematech to Sanford Health and the technology was further developed by Sanford Applied Biosciences until we acquired it in its entirety in June 2014.
Since acquiring the technology in 2014, we have continued to develop intellectual property and specifically targeted human IgGs to multiple disease indications, and we have conducted or collaborated in eight clinical trials (six of which are in review), where we have demonstrated safety and efficacy in multiple Tc Bovine-derived human IgG product candidates. We have developed our rapid response capabilities and completed proof of concept using private resources as well as over $200 million of funds awarded from the U.S. Government emerging disease and medical countermeasures programs.
In October 2021, we completed our business combination with Big Cypress Acquisition Corp. (“BCYP”), pursuant to which we debuted as a publicly traded company (the “Business Combination”). BCYP was incorporated as a special purpose acquisition company in the State of Delaware on November 12, 2020. On January 14, 2021, BCYP completed its initial public offering. On October 22, 2021, BCYP consummated the Business Combination with Legacy SAB, which changed its name from SAB Biotherapeutics, Inc. to Legacy SAB. In connection with the closing of the Business Combination, BCYP changed its name to SAB Biotherapeutics, Inc. and Legacy SAB became a wholly-owned subsidiary of SAB Biotherapeutics, Inc.
XIV. Corporate Information
Our principal executive offices are located at 2100 East 54th Street North, Sioux Falls, South Dakota 57104, and our telephone number is (605)-679-6980. Our corporate website address is www.sab.bio. Our Annual Reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and proxy statements, and all amendments thereto, are available free of charge on our website. These reports are posted on our website as soon as reasonably practicable after they are electronically filed with the U.S. Securities and Exchange Commission (the “SEC”). The public may read and copy any materials that we file with the SEC electronically through the SEC website (www.sec.gov). The information contained on the SEC’s website is not incorporated by reference into this Annual Report on Form 10-K and should not be considered to be part thereof.
XV. Human Capital
As of December 31, 2023, we had 57 full-time employees, including 8 who hold advanced degrees. Of these employees, 35 were engaged in research and development activities, 7 were engaged in clinical activities and 14 were engaged in general and administrative activities. As of December 31, 2023, none of our employees were represented by labor unions or covered by collective bargaining agreements. We consider our relationship with our employees to be good. We emphasize several measures and objectives in managing its human capital assets, including, among others, (i) employee safety and wellness, (ii) talent acquisition and retention, (iii) employee engagement, development, and training, (iv) diversity and inclusion and (v) compensation. These targeted ideals may include annual bonuses, stock-based compensation awards, a 401(k) plan with employee matching opportunities, healthcare, and insurance benefits, health savings and flexible spending accounts, paid time off, family leave, family care resources, and/or employee assistance programs. We also provide our employees with access to various innovative, flexible, and convenient health and wellness programs. We designed these programs to support employees’ physical and mental health by providing tools and resources to improve or maintain their health status and encourage engagement in healthy behaviors.
24
Item 1A. Risk Factors.
Investing in our securities involves a high degree of risk. Before you make a decision to buy our securities, in addition to the risks and uncertainties discussed above under “Special Note Regarding Forward-Looking Statements,” you should carefully consider the risks and uncertainties described below together with all of the other information contained in this Form 10-K, including our financial statements and related notes included at the end of this Form 10-K and in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” If any of the events or developments described below were to occur, our business, prospects, operating results and financial condition could suffer materially, the trading price of our securities could decline, and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
Risk Factors Summary
Below is a summary of material factors that make an investment in our securities speculative or risky. Importantly, this summary does not address all of the risks and uncertainties that we face. You should carefully consider the full risk factor disclosure outlined in this Form 10-K, in addition to the other information herein, including the section of this report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes.
25
26
Risks Related to Our Business and Operations
We are a clinical-stage biopharmaceutical company and have incurred significant losses since our inception. We realized net losses in the fiscal year ended December 31, 2023 and 2022, we expect to continue to incur net losses for the foreseeable future, and we may never achieve or maintain profitability in the future.
We are a clinical-stage biopharmaceutical company. We expect to experience variability in revenue and expenses which makes it difficult to evaluate our business and prospects. As such, we have incurred and anticipate that we will continue to incur significant operating losses in the foreseeable future. Our historical losses resulted principally from costs incurred in research and development, preclinical testing, clinical development of product candidates as well as costs incurred for research programs and from general and administrative costs associated with these operations. In the future, we intend to continue to conduct research and development, preclinical testing, clinical trials and regulatory compliance activities that, together with anticipated general and administrative expenses, will result in incurring further significant losses for the next several years. We expect that our operating expenses will continue to increase significantly, including as we:
Biopharmaceutical product development entails substantial upfront capital expenditures and significant risk that any potential product candidate will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval, secure market access and reimbursement and become commercially viable, and therefore any investment in us is highly speculative. Accordingly, before making an investment in us, you should consider our prospects, factoring in the costs, uncertainties, delays and difficulties frequently encountered by companies in clinical development, especially clinical-stage biopharmaceutical companies such as ours. Any predictions you make about our future success or viability may not be as accurate as they would otherwise be if we had a longer operating history or a history of successfully developing and
27
commercializing pharmaceutical products. We may encounter unforeseen expenses, difficulties, complications, delays and other known or unknown factors in achieving our business objectives.
Our expenses could increase beyond expectations for a variety of reasons, including due to our growth strategy and the increase in the scope and complexity of our operations. In executing our strategy and plans to invest in enhancing and scaling our business, we will need to generate significant additional revenue to achieve and maintain future profitability. We may not be able to generate sufficient revenue to achieve profitability and our recent and historical growth should not be considered indicative of future performance.
Our limited operating history makes future forecasting difficult.
We commenced operations in April 2014 and became a public company in October 2021. As a result of our limited operating history, it is difficult to accurately forecast revenues or to predict operating expenses. Our current and future expense estimates are based, in large part, on our estimates of future revenue and on our research, development and commercialization plans. In particular, we plan to increase operating expenses significantly in order to expand our research, development and sales and marketing operations. To the extent that these expenses precede increased revenue, our business, results of operations and financial condition would be materially adversely affected. We may be unable to, or may elect not to, adjust spending quickly enough to offset any unexpected revenue shortfall. Therefore, any significant shortfall in revenue in relation to our expectations would also have a material adverse effect on our business, results of operations and financial condition.
The successful development of pharmaceutical products is highly uncertain.
We currently have no products approved for sale and are investing substantially all of our efforts and financial resources in the development of our immunotherapy platform and clinical development of our current lead programs. The success of our business, including our ability to finance our company and generate any revenue in the future, will primarily depend on the successful development, regulatory approval and commercialization of therapeutic biological product candidates. We will need to raise sufficient funds for, and successfully complete, our preclinical development programs and future clinical trials of product candidates for our lead programs.
There is no guarantee that any product candidate we develop will proceed into and through clinical development or achieve regulatory approval to allow such products to be commercialized. Successful development of therapeutic biological products is highly uncertain and is dependent on numerous factors, many of which are beyond our control. Product candidates that appear promising in the early phases of development may fail to reach the market for several reasons, including:
In addition, the length of time necessary to complete clinical trials and submit an application for marketing approval for a final decision by a regulatory authority varies significantly among product candidates, and any delay in receipt of marketing approval for a product candidate could negatively impact market acceptance of any resulting product. Even if we are successful in obtaining marketing approval, commercial success of any approved products will also depend in large part on the availability of coverage and adequate reimbursement from third-party payors, including government payors such as the Medicare and Medicaid programs and managed care organizations in the United States or country specific governmental organizations in foreign countries, which may be affected by existing and future healthcare reform measures designed to reduce the cost of healthcare. Third-party payors could require us to conduct additional studies, including post-marketing studies related to the cost effectiveness of a product, to qualify for reimbursement, which could be costly and divert our resources. If government and other healthcare payors were not to provide coverage and adequate reimbursement for our products once approved, market acceptance and commercial success would be reduced.
In addition, if any of our product candidates receive marketing approval, we will be subject to significant regulatory obligations regarding the submission of safety and other post-marketing information and reports and registration, and will need to continue to comply (ensure that our third-party providers comply) with current Good Manufacturing Practices (cGMPs), and good clinical practices (GCPs), for any clinical trials that we conduct post-approval. In addition, there is always the risk that we, a regulatory authority or a third party might identify previously unknown problems with a product post-approval, such as adverse events of unanticipated severity or frequency. Compliance with these requirements is costly,
28
and any failure to comply or other issues with our product candidates post-approval could adversely affect our business, financial condition and results of operations.
All of our product candidates are in preclinical or clinical development. Clinical drug development is expensive, time consuming and uncertain, and we may ultimately not be able to obtain regulatory approvals for the commercialization of some or all of our product candidates.
The research, testing, manufacturing, labeling, approval, selling, marketing and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities, which regulations differ from country to country. Our product candidates are in various stages of development and are subject to the risks of failure typical of drug development. The development and approval process is expensive and can take many years to complete, and its outcome is inherently uncertain. We have not submitted an application for, or received, marketing approval for any of our product candidates. We have limited experience in conducting and managing the later-stage clinical trials necessary to obtain regulatory approvals, including approval by the FDA. To receive regulatory approval, we must, among other things, demonstrate with substantial evidence from clinical trials that the product candidate is safe and effective for each indication for which approval is sought, and failure can occur in any stage of development. Satisfaction of the approval requirements typically takes several years, and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the pharmaceutical product. We cannot predict if or when we might receive regulatory approvals for any of our product candidates currently under development.
We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. The clinical development of our initial and potential additional product candidates is susceptible to the risk of failure inherent at any stage of development, including failure to demonstrate efficacy in a clinical trial or across a broad population of patients, the occurrence of adverse events that are severe or medically or commercially unacceptable, failure to comply with protocols or applicable regulatory requirements and determination by the FDA or any comparable foreign regulatory authority that a product candidate may not continue development or is not approvable. It is possible that even if any of our product candidates have a beneficial effect, that effect will not be detected during clinical evaluation as a result of one or more of a variety of factors, including the size, duration, design, measurements, conduct or analysis of our clinical trials. Conversely, as a result of the same factors, our clinical trials may indicate an apparent positive effect of such product candidate that is greater than the actual positive effect, if any. Similarly, in our clinical trials we may fail to detect toxicity of, or intolerability caused by, such product candidate, or mistakenly believe that our product candidates are toxic or not well tolerated when that is not in fact the case. Serious adverse events or other adverse events, as well as tolerability issues, could hinder or prevent market acceptance of the product candidate at issue.
The FDA and foreign regulatory authorities also have substantial discretion in the drug approval process. The number and types of preclinical studies and clinical trials that the FDA will require to establish substantial evidence of safety and effectiveness for regulatory approval varies depending on the product candidate, the disease or condition that the product candidate is designed to address, and the regulations applicable to any particular product candidate. Approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among countries and regulatory authorities, and there may be varying interpretations of data obtained from preclinical studies or clinical trials, either of which may cause delays or limitations in the approval or the decision not to approve an application. Regulatory agencies can delay, limit or deny approval of a product candidate for many reasons, including:
29
This lengthy approval process, as well as the unpredictability of future clinical trial results, may result in our failing to obtain regulatory approval to market our product candidates, which would significantly harm our business, results of operations and prospects. The FDA or a comparable foreign regulatory authority may require more information, including additional preclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or which we may lead us to decide to abandon the development program.
In addition, even if we were to obtain marketing approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request (including failing to approve the most commercially promising indications), may require a REMS that restricts prescribing or distribution of our therapeutic biological product candidates, may grant approval contingent on the performance of costly post-marketing clinical studies, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate.
Regulatory approval for the genetic modification of animals, including those from which antibodies are isolated for injection into human patients, requires the approval of a New Animal Drug Application (NADA), which can be a lengthy and expensive process with uncertain outcomes, delays to which could substantially harm our business.
We cannot commercialize our therapeutic biological product candidates in the United States without first obtaining a regulatory approval for our animal drug candidates, i.e., the genomic modifications to our Tc Bovine, in the form of a NADA. The requirements governing development and approval of a new animal drug are largely analogous to those for new human drugs, requiring a demonstration of the safety and efficacy of the drug for the target indication, a demonstration that the manufacturing facilities, processes and controls are adequate with respect to such product candidate to assure safety, purity and potency, and a review of potential environmental impacts from the altered genomic DNA and the transgenic animals pursuant to the requirements of the National Environmental Policy Act (NEPA).
The time required to obtain approval for a NADA by the FDA and comparable foreign regulatory authorities is unpredictable. Approval policies, regulations, or the type and amount of data necessary to gain approval is dependent on the specific product candidate and may change during the course of the product candidate’s preclinical and clinical development. Furthermore, we have not obtained regulatory approval for an animal drug, and it is possible that none of our existing animal drug candidates, or any future animal drug candidates, will ever obtain regulatory approval. The reasons our animal drug candidates could fail to receive regulatory approvals are generally the same as the reasons that human drug product candidates may fail to obtain approval. Our failure to obtain a regulatory approval for our animal drug candidates could significantly harm our business, the results of our operations and our prospects. Requests for additional information from a regulatory authority could delay or prevent approval or result in our decision to abandon the development program entirely.
If we do receive regulatory approval of our animal drug candidates, then we will have ongoing responsibilities including registration, recordkeeping, filing supplements, and periodic reporting, which could reveal additional complications and threaten the ongoing approval of our animal drug candidates. Further, as our polyclonal antibody product candidates are regulated as biological products, such product candidates will also require the submission and approval of a BLA prior to marketing. In general, to commercialize any of our product candidates, we must obtain marketing authorization for both the therapeutic antibody product and the altered animal genomic DNA that enables production of the polyclonal antibodies.
Any delay in obtaining or failure to obtain required approvals could materially adversely affect our ability to generate revenue from the particular product candidate, which likely would result in significant harm to our financial position and adversely impact our stock price.
We are not permitted to market our product candidates in the United States until we receive approval of a NADA and BLA from the FDA or in other countries until we receive similar marketing authorization from applicable regulatory authorities outside the United States. We are also not permitted to promote our product candidates as safe and effective therapies until after receiving approval. Obtaining approval of a NADA or BLA can be a lengthy, expensive and uncertain process. If we fail to obtain FDA approval to market our product candidates, we will be unable to sell our product candidates in the United States, which will significantly impair our ability to generate any revenue. In addition, failure to comply with FDA and non-U.S. regulatory requirements may, either before or after product approval, if any, subject our company to administrative or judicially imposed sanctions, including:
30
Even if we do receive regulatory approval to market a product candidate, any such approval may be subject to limitations on the indicated uses for which we may market the product. It is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain the appropriate regulatory approvals necessary for us to commence product sales. Any delay in obtaining, or an inability to obtain, applicable regulatory approvals would prevent us from commercializing our product candidates, generating revenue and achieving and sustaining profitability.
If we encounter difficulties enrolling patients in clinical trials, clinical trials of our product candidates may be delayed or otherwise adversely affected.
We may not be able to initiate or continue clinical trials for any product candidate we develop if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or comparable foreign regulatory authorities. The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until conclusion. We may experience difficulties in patient enrollment in clinical trials for a variety of reasons, including:
If we experience delays or difficulties in the enrollment of subjects in our anticipated clinical trials, such clinical trials may be delayed or terminated. Even if we are able to enroll a sufficient number of subjects in our future clinical trials, if the pace of enrollment is slower than we expect, the development costs for our product candidates may increase and the completion of such trials may be delayed, or the trials could become too expensive to complete. Our failure to timely complete our current and planned clinical trials would delay the approval and commercialization of our product candidates, impair the commercial performance of our product candidates, may decrease the period of commercial exclusivity and consequently harm our business and results of operations.
31
Our preclinical studies and clinical trials may fail to demonstrate substantial evidence of the safety and efficacy of our product candidates, or serious adverse or unacceptable side effects may be identified during the development of our product candidates, which could prevent, delay or limit the scope of regulatory approval of our product candidates, limit their commercialization, increase costs or necessitate the abandonment or limitation of the development of some of our product candidates.
To obtain the requisite regulatory approvals for the commercial sale of our product candidates, we must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that such product candidates are safe, pure and potent for use in each target indication. These trials are expensive and time consuming, and their outcomes are inherently uncertain. Failures can occur at any time during the development process. Preclinical studies and clinical trials often fail to demonstrate safety or efficacy of the product candidate studied for the target indication, and most product candidates that begin clinical trials are never approved.
Success in preclinical studies does not ensure that later clinical trials will generate adequate data to demonstrate the efficacy and safety of any product candidate we may develop. Likewise, a number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience than us, have suffered significant setbacks in clinical trials, even after seeing promising results in earlier preclinical studies or clinical trials. Despite the results reported in preclinical studies for our product candidates to date, results may not be replicated in subsequent studies, and we do not know whether the clinical trials we may conduct will demonstrate adequate efficacy and safety to support regulatory approval of any current or future product candidate we develop. Moreover, later audits of earlier preclinical data may reveal inaccuracies or deviations impacting the integrity of those data.
Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in such studies or trials nonetheless failed to obtain FDA or other necessary regulatory agency approval.
We may fail to demonstrate with substantial evidence from adequate and well-controlled trials, and to the satisfaction of the FDA or comparable foreign regulatory authorities, that our product candidates are safe and potent for their intended uses. If any future late-stage clinical trials we may conduct do not produce favorable results, our ability to achieve regulatory approval for any of our product candidates may be adversely impacted. Even if we believe that we have adequate data to support an application for regulatory approval to market any of our product candidates, the FDA or other regulatory authorities may not agree with our interpretation of the relevant data and may require that we conduct additional preclinical studies or clinical trials to support the regulatory approval of any product candidate that we develop. If we fail to obtain results in our planned and future preclinical and clinical activities and studies sufficient to meet the requirements of the relevant regulatory agencies, the development timeline and regulatory approval and commercialization prospects for any potential product candidate, and, correspondingly, our business and financial prospects, would be materially adversely affected.
Our business is highly dependent on the success of our product candidates. If we are unable to successfully complete clinical development, obtain regulatory approval for or commercialize one or more of our product candidates, or if we experience delays in doing so, our business will be materially harmed.
We have not completed the development of any product candidates. Our future success and ability to generate revenue from our product candidates, which we do not expect will occur for several years, if ever, is dependent on our ability to successfully develop, obtain regulatory approval for and commercialize one or more of our product candidates. All of our product candidates, including our lead product candidate SAB-142, are in early stages of development and require substantial additional investment for clinical development, regulatory review and approval in one or more jurisdictions. If any of our product candidates encounters safety or efficacy problems, development delays or regulatory issues or other problems, our development plans and business would be materially harmed.
All of our other product candidates are in earlier stages of development and will require substantial additional investment for clinical development, regulatory review and approval in one or more jurisdictions. If any of our product candidates encounters safety or efficacy problems, development delays or regulatory issues or other problems, our development plans and business would be materially harmed.
32
We may not have the financial resources to continue development of our product candidates if we experience any issues that delay or prevent regulatory approval of, or our ability to commercialize, our product candidates, including:
We conduct certain research and development operations through our Australian wholly-owned subsidiary. If we lose our ability to operate in Australia, or if our subsidiary is unable to receive the research and development tax credit allowed by Australian regulations, our business and results of operations could suffer.
Our wholly-owned Australian subsidiary, SAB Australia, was formed to conduct various preclinical and clinical activities for SAB-142 and other future drug candidates in Australia. Due to the geographical distance and lack of employees currently in Australia, as well as our lack of experience operating in Australia, we may not be able to efficiently or successfully monitor, develop and commercialize our lead products in Australia, including conducting clinical trials. Furthermore, we have no assurance that the results of any clinical trials that we conduct for our product candidates in Australia will be accepted by the FDA or applicable foreign authorities.
In addition, current Australian tax regulations provide for a refundable research and development tax credit equal to 39.5% of qualified expenditures. Although we have previously claimed a refundable research and development tax credit there is a possibility that we may not be able to claim such credit, or we might qualify for a lesser credit. If we lose our ability to operate SAB Australia, or if in the future we are ineligible or unable to receive the research and development tax credit or are required to refund any research and development tax credit previously received or have to reserve for such credit in our financial statements, or if the Australian government significantly reduces or eliminates the tax credit, our business and results of operation may be adversely affected.
The regulatory approval processes of the FDA are lengthy, time-consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
We are not permitted to commercialize, market, promote or sell any product candidate in the United States without obtaining regulatory approval from the FDA. The time required to obtain approval by the FDA and comparable foreign authorities is inherently unpredictable, but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. To date, we have not submitted a NADA or BLA to the FDA or similar drug or biological product approval submissions to comparable foreign regulatory authorities for any product candidate.
33
We may never obtain FDA approval for any product candidates in the United States, and even if we do, we may never obtain approval for or commercialize any product candidates in any other jurisdiction, which would limit our ability to realize their full market potential.
In addition to regulations in the United States, to market and sell our product candidates in the European Union, many Asian countries and other jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements, both from a clinical and manufacturing perspective. The approval procedure for complex therapeutic biological product candidates such as ours varies among countries and can involve additional testing and validation and additional administrative review periods. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. Clinical trials accepted in one country may not be accepted by regulatory authorities in other countries.
In addition, many countries outside the United States require that a product be approved for reimbursement before it can be approved for sale in that country. A product candidate that has been approved for sale in a particular country may not receive reimbursement approval in that country. We may not be able to obtain approvals from regulatory authorities or payor authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory or payor authorities in other countries or jurisdictions, and approval by one regulatory or payor authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for future regulatory approvals and may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain approval of any of our product candidates by regulatory or payor authorities in the European Union, Asia or elsewhere, the commercial prospects of that product candidate may be significantly diminished. We do not have any product candidates approved for sale in any jurisdiction, including in the United States or in international markets, and we do not have experience in obtaining regulatory approval in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of our products will be unrealized.
The FDA or comparable foreign regulatory authorities may disagree with our regulatory plan for our product candidates.
The general approach for FDA approval of a new drug is dispositive data from two or more well-controlled Phase 3 clinical trials of the product candidate in the relevant patient population. Phase 3 clinical trials typically involve a large number of patients, have significant costs and take years to complete. In addition, there is no assurance that the endpoints and trial designs that we intend to use for our planned clinical trials, including those that we have developed based on feedback from regulatory agencies or those that have been used for the approval of similar drugs, will be acceptable for future approvals. Our clinical trial results may not support approval of our product candidates. In addition, our product candidates could fail to receive regulatory approval, or regulatory approval could be delayed, for many reasons, including the following:
34
If our clinical trials fail to replicate positive results from earlier preclinical studies or clinical trials conducted by us or third parties, we may be unable to successfully develop, obtain regulatory approval for or commercialize our product candidates.
The results observed from preclinical studies or early-stage clinical trials of our product candidates may not necessarily be predictive of the results of later-stage clinical trials that we conduct. Similarly, positive results from such preclinical studies or early-stage clinical trials may not be replicated in our subsequent preclinical studies or clinical trials. There can be no assurance that any of our clinical trials will ultimately be successful or support further clinical development of any of our product candidates. There is a high failure rate for drugs proceeding through clinical trials. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in early-stage development, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in preclinical studies and clinical trials, including previously unreported adverse events.
We may incur unexpected costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
To obtain the requisite regulatory approvals to commercialize any of our product candidates, we must demonstrate through extensive preclinical studies and clinical trials that our product candidates are safe and effective in humans. We may experience delays in completing our clinical trials or preclinical studies and initiating or completing additional clinical trials or preclinical studies, including as a result of regulators not allowing or delay in allowing clinical trials to proceed under an INAD or IND, or not approving or delaying approval for any clinical trial grant or similar approval we need to initiate a clinical trial. We may also experience numerous unforeseen events during our clinical trials that could delay or prevent our ability to receive marketing approval or commercialize the product candidates we develop, including:
Regulators or IRBs of the institutions in which clinical trials are being conducted may suspend, limit or terminate a clinical trial, or data monitoring committees may recommend that we suspend or terminate a clinical trial, due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, safety issues or adverse side effects, failure to demonstrate a benefit from using an investigational product, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Negative or inconclusive results from our clinical trials or preclinical studies could mandate repeated or additional clinical trials and, to the extent we choose to conduct clinical trials in other indications, could result in changes to or delays in clinical trials of our
35
product candidates in such other indications. We do not know whether any clinical trials that we conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market our product candidates for the indications that we are pursuing. If later-stage clinical trials do not produce favorable results, our ability to obtain regulatory approval for our product candidates will be adversely impacted.
Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process for product candidates is expensive, time-consuming and uncertain, and may prevent us from obtaining approvals for the commercialization of our product candidates.
Any product candidate we develop, and the activities associated with its development and commercialization, including its design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale, and distribution, are subject to comprehensive regulation by the FDA and other regulatory authorities in the United States and by comparable authorities in other countries. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate in a given jurisdiction. We have not received approval to market any product candidates from regulatory authorities in any jurisdiction and it is possible that none of the product candidates we are developing or may seek to develop in the future will ever obtain regulatory approval.
We have no experience in submitting and supporting the applications necessary to gain marketing approvals and expect to rely on third-party CROs or regulatory consultants to assist us in this process. Securing regulatory approval requires the submission of extensive preclinical and clinical data and supporting information to the various regulatory authorities for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing regulatory approval also requires the submission of information about the product manufacturing process to, and successful inspection of manufacturing facilities by, the relevant regulatory authority. Any product candidates we develop may not be effective, may be only moderately effective, or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude its obtaining marketing approval or prevent or limit commercial use.
The process of obtaining marketing approvals, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity, and novelty of the product candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA and comparable authorities in other countries have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit, or prevent marketing approval of a product candidate. Any marketing approval that we may ultimately obtain could be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval or if we fail to obtain approval of any product candidates we may develop, the commercial prospects for those product candidates may be harmed, and our ability to generate revenues will be materially impaired.
Interim, topline and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data becomes available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim, topline or preliminary data from our clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary or topline data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim and preliminary data should be viewed with caution until the final data are available. Adverse differences between preliminary or interim data and final data could significantly harm our reputation and business prospects.
If we do not achieve our projected development and commercialization goals in the timeframes we announce and expect, the development and commercialization of our product candidates may be delayed, and our business and results of operations may be harmed.
For planning purposes, we sometimes estimate the timing of the accomplishment of various scientific, clinical, regulatory and other product development objectives. These milestones may include our expectations regarding the commencement or completion of scientific studies and clinical trials, the submission of regulatory filings or commercialization objectives. From time to time, we may publicly announce the expected timing of some of these milestones, such as the completion of an
36
ongoing clinical trial, the initiation of other clinical programs, receipt of marketing approval or a commercial launch of a product. The achievement of many of these milestones may be outside of our control. All of these milestones are based on a variety of assumptions which, if not realized as expected, may cause the timing of achievement of the milestones to vary considerably from our estimates, including:
If we fail to achieve announced milestones in the timeframes we expect, the development and commercialization of our product candidates may be delayed, and our business and results of operations may be harmed.
Changes in methods of product candidate manufacturing or formulation may result in additional costs or delays.
As product candidates proceed through preclinical studies to late-stage clinical trials towards potential approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods and formulation, are altered along the way in an effort to optimize processes and results. Such changes carry the risk that they will not achieve these intended objectives. Any of these changes could cause our product candidates to perform differently and affect the results of planned clinical trials or other future clinical trials conducted with the materials manufactured using altered processes. Such changes may also require additional testing, FDA notification or FDA approval. This could delay or prevent completion of clinical trials, require conducting bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay or prevent approval of our product candidates and jeopardize our ability to commence sales and generate revenue.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following regulatory approval, if obtained.
Undesirable side effects caused by any of our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or comparable foreign regulatory authorities or a more restrictive label for any of our product candidates that may receive regulatory approval. In our planned and future clinical trials of our product candidates, we may observe a more unfavorable safety and tolerability profile than was observed in earlier-stage testing of these candidates.
If unacceptable side effects arise in the development of our product candidates, we, the FDA or comparable foreign regulatory authorities, the IRBs, or independent ethics committees at the institutions in which our trials are conducted, could suspend, limit or terminate our clinical trials, or the independent safety monitoring committee could recommend that we suspend, limit or terminate our trials, or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all targeted indications. Treatment-emergent side effects that are deemed to be related to administration of our product candidates could delay recruitment of clinical trial subjects or may cause subjects that enroll in our clinical trials to discontinue participation in our clinical trials. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff. We may need to train medical personnel using our product candidates to understand the side effect profiles for our clinical trials and upon any commercialization of any of our product candidates. Inadequate training in recognizing or managing the potential side effects of our product candidates could result in harm to patients that are administered our product candidates.
Additionally, during the course of our product development programs, FDA or comparable foreign regulatory authority review teams may change, and new agency personnel may view the risk-benefit profile of any product candidates we may develop differently than prior agency review teams. Any negative views as to the risk-benefit profile of the product candidates we are developing for our lead programs or any product candidates we may develop in the future could lead FDA
37
or comparable foreign regulatory authorities to require that we conduct additional clinical trials or could require more onerous clinical trial designs for any then-ongoing or future clinical trials. The product-related side effects also could result in potential product liability claims being asserted against us. Furthermore, we or others may later identify undesirable side effects caused by our products, including during any long-term follow-up observation period.
If any of our product candidates receives regulatory approval, and we or others later identify undesirable side effects caused or risks exacerbated by such product, a number of potentially significant negative consequences could result. For example, the FDA could require us to adopt a REMS to ensure that the benefits of treatment with such product candidate outweigh the risks for each potential patient; a REMS may include, among other things, a communication plan to healthcare practitioners, patient education, extensive patient monitoring or distribution systems and processes that are highly controlled, restrictive and more costly than what is typical for the biopharmaceutical industry. Other potentially significant negative consequences include that:
Any of these occurrences could diminish the usage or otherwise limit the commercial success of our product candidates and prevent us from achieving or maintaining market acceptance of the affected product candidate, if approved by applicable regulatory authorities, and may adversely affect our business, financial condition and prospects significantly.
The future commercial success of our product candidates will depend on the degree of market acceptance of our potential products among physicians, patients, healthcare payers, and the medical community.
When available on the market, our products may not achieve an adequate level of acceptance by physicians, patients and the medical community, which may result in us failing to achieve profitability. In addition, efforts to educate the medical community and third-party payers on the benefits of our products may require significant resources and may never be successful, which would prevent us from generating significant revenues or becoming profitable.
Failure to successfully identify, develop and commercialize additional products or product candidates could impair our ability to grow.
Although a substantial amount of our efforts will focus on the continued preclinical and clinical testing and potential approval of product candidates in our current pipeline, a key element of long-term growth strategy is to develop and market additional products and product candidates. Because we have limited financial and managerial resources, research programs to identify product candidates will require substantial additional technical, financial and human resources, whether or not any product candidates are ultimately identified. The success of this strategy depends partly upon our ability to identify, select and develop promising product candidates and products. Our technology platforms may fail to discover and to generate additional product candidates that are suitable for further development. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate may not be suitable for clinical development as a result of its harmful side effects, limited efficacy or other characteristics that indicate that it is unlikely to be a product that will receive approval by the FDA and other comparable foreign regulatory authorities and achieve market acceptance. If we do not successfully develop and commercialize product candidates based upon its technological approach, we may not be able to obtain product or collaboration revenues in future periods, which would adversely affect our business, prospects, financial condition and results of operations.
Our long-term growth strategy to develop and market additional products and product candidates is heavily dependent on precise, accurate and reliable scientific data to identify, select and develop promising pharmaceutical product candidates and
38
products. Our business decisions may therefore be adversely influenced by improper or fraudulent scientific data sourced from third parties. Any irregularities in the scientific data used by us to determine our focus in research and development of product candidates and products could have a material adverse effect on our business, prospects, financial condition and results of operations.
If we are unable to develop our sales, marketing and distribution capability on our own or through collaborations with marketing partners, we will not be successful in commercializing our product candidates.
We currently have no marketing, sales or distribution capabilities. We intend to establish a sales and marketing organization, either on our own or in collaboration with third parties, with technical expertise and supporting distribution capabilities to commercialize SAB-142, and our other product candidates that may receive regulatory approval in key territories. These efforts will require substantial additional resources, some or all of which may be incurred in advance of any approval of the product candidate. Any failure or delay in the development of our or third parties’ internal sales, marketing and distribution capabilities would adversely impact the commercialization of SAB-142, and our other product candidates and other future product candidates.
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
With respect to our existing and future product candidates, we may choose to collaborate with third parties that have direct sales forces and established distribution systems to serve as an alternative to our own sales force and distribution systems. Our future product revenue may be lower than if we directly marketed or sold our product candidates, if approved. In addition, any revenue we receive will depend in whole or in part upon the efforts of these third parties, which may not be successful and are generally not within our control. If we are not successful in commercializing any approved products, our future product revenue will suffer, and we may incur significant additional losses.
If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
Product liability lawsuits against us or any of our future collaborators could divert our resources and attention, cause us to incur substantial liabilities and limit commercialization of our product candidates.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical products. Currently, we have no products that have been approved for commercial sale; however, the use of our product candidates by us and any collaborators in clinical trials, and the sale of these product candidates, if approved, in the future, may expose us to liability claims. We face an inherent risk of product liability lawsuits related to the use of our product candidates in patients, and will face an even greater risk if product candidates are approved by regulatory authorities and introduced commercially. Product liability claims may be brought against us by participants enrolled in our clinical trials, patients, health care providers, pharmaceutical companies, our collaborators or others using, administering or selling any of our future approved products. If we cannot successfully defend ourselves against any such claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Regardless of the merits or eventual outcome, liability claims may result in:
39
Although the clinical trial process is designed to identify and assess potential side effects, clinical development does not always fully characterize the safety and efficacy profile of a new medicine, and it is always possible that a drug, even after regulatory approval, may exhibit unforeseen side effects. If our product candidates were to cause adverse side effects during clinical trials or after approval, we may be exposed to substantial liabilities. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our product candidates. If any of our product candidates are approved for commercial sale, we will be highly dependent upon consumer perceptions of us and the safety and quality of our products. We could be adversely affected if we are subject to negative publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our products or any similar products distributed by other companies.
Although we maintain product liability insurance coverage consistent with industry norms, including clinical trial liability, this insurance may not fully cover potential liabilities that we may incur. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. We will need to increase our insurance coverage if we commercialize any product that receives regulatory approval. In addition, insurance coverage is becoming increasingly expensive. If we are unable to maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims, it could prevent or inhibit the development and commercial production and sale of our product candidates, which could harm our business, financial condition, results of operations and prospects.
Our current and future relationships with customers and third-party payors in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third party payors, distributors, retailers, marketers and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and similar state or foreign laws which may constrain the business or financial arrangements and relationships through which we sell, market and distribute any product candidates for which we obtain marketing approval. In addition, we may be subject to transparency laws and patient privacy regulation by U.S. federal and state governments and by governments in foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include, but are not necessarily limited to:
40
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, including, without limitation, damages, fines, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business, including our collaborators, is found not to be in compliance with applicable laws, it may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
Regulatory approval for any approved product is limited by the FDA to those specific indications and conditions for which clinical safety and efficacy have been demonstrated.
Any regulatory approval is limited to those specific diseases and indications for which a product is deemed to be safe and effective by the FDA. In addition to the FDA approval required for new formulations, any new indication for an approved product also requires FDA approval. If we are not able to obtain FDA approval for any desired future indications for our products, our ability to effectively market and sell our products may be reduced and our business may be adversely affected.
While our ability to promote the products is limited to those indications that are specifically approved by the FDA, physicians may choose to prescribe drugs for uses that are not described in the product’s approved labeling and for uses that differ from those tested in clinical studies and approved by the regulatory authorities. These “off-label” uses are common across medical specialties and may constitute an appropriate treatment for some patients in varied circumstances. Regulatory authorities in the United States generally do not regulate a physician’s use of professional judgment in prescribing treatments for patients. Regulatory authorities do, however, restrict communications by pharmaceutical companies on the subject of off-label use or off-label information. If our promotional activities fail to comply with these regulations or guidelines, we may be subject to warnings from, or enforcement action by, these authorities. In addition, our failure to follow FDA rules and guidelines relating to promotion and advertising may cause the FDA to suspend or withdraw an approved product from the market, require a recall or corrective advertising, institute fines, or could result in disgorgement of money, operating restrictions, injunctions or civil or criminal prosecution by the government, any of which could harm our reputation and business.
Current and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize any product candidates we or our collaborators develop and may adversely affect the prices for such product candidates.
In the United States and certain non-U.S. jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could, among other things, prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our or our collaborators’ ability to profitably sell any product candidates that obtain marketing approval.
41
For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively the Affordable Care Act, was enacted in the United States.
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the Affordable Care Act. However, following several years of litigation in the federal courts, in June 2021, the U.S. Supreme Court upheld the ACA when it dismissed a legal challenge to the ACA’s constitutionality. Further legislative and regulatory changes under the ACA remain possible, but it is unknown what form any such changes or any law would take or how or whether such changes may affect the biopharmaceutical industry as a whole or our business in the future.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, included reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Further, there has been heightened governmental scrutiny recently over pharmaceutical pricing practices in light of the rising cost of prescription drugs and biologics. Such scrutiny has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies, rebates and price negotiation for pharmaceutical products. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product and medical device pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and medical devices to purchase, and which suppliers will be included in their prescription drug and other healthcare programs.
We expect that other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria, new payment methodologies and in additional downward pressure on the price that we or our collaborators may receive for any approved or cleared product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we or our collaborators are slow or unable to adapt to new requirements or policies, or if we or our collaborators are not able to maintain regulatory compliance, any of our product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
Even if we obtain regulatory approval for a product candidates, our products will remain subject to regulatory scrutiny.
Even if we obtain regulatory approval in a jurisdiction for our product candidates, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, recordkeeping, and submission of safety and other post-market information. Any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product. For example, the holder of an approved BLA is obligated to monitor and report adverse events and any failure of a product to meet the specifications in the BLA. The holder of an approved BLA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials and claims must be consistent with approved labeling and be in compliance with FDA regulations as well as other potentially applicable federal and state laws. In addition, biological product advertising and promotional materials intended to be used during the first 120 days after approval must be submitted to the FDA during the BLA review period. After approval, advertising and promotional materials must be submitted to the FDA 30 days prior to their intended use.
In addition, product manufacturers are subject to payment of program fees and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with GMP requirements and adherence to commitments made in the BLA or foreign marketing application. If we or a regulatory agency discovers previously unknown problems with a product such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured or with the integrity or sufficiency of data, records, or documentation, or disagrees with the promotion, marketing or labeling of that product, a regulatory agency may impose restrictions relative to that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
42
If we or a regulatory agency later discovers previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or if we fail to comply with applicable regulatory requirements following approval of any of our product candidates, a regulatory agency may:
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenue.
Advertising and promotion of any human therapeutic biological product candidate that obtains approval in the United States will be heavily scrutinized by the FDA, the U.S. Federal Trade Commission, the Department of Justice (DOJ), the Office of Inspector General of the Department of Health and Human Services (HHS), state attorneys general, members of the U.S. Congress and the public. Additionally, advertising and promotion of any product candidate that obtains approval outside of the United States will be heavily scrutinized by comparable foreign entities and stakeholders. Violations, including actual or alleged promotion of our products for unapproved or off-label uses, are subject to enforcement letters, inquiries and investigations, and civil and criminal sanctions by the FDA, other U.S. governmental authorities, or comparable foreign bodies. Any actual or alleged failure to comply with labeling and promotion requirements may result in fines, warning letters, mandates to issue corrective information to healthcare practitioners and/or the general public, injunctions, or civil or criminal penalties.
In addition, the FDA’s policies may change, and additional government laws may be enacted and implementing regulations promulgated, which could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business, prospects, financial condition and results of operations.
Disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being developed, approved or commercialized in a timely manner or at all, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, statutory, regulatory and policy changes, the FDA’s ability to hire and retain key personnel and accept the payment of user fees, and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review times at the FDA have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at the FDA and other agencies may also slow the time necessary for new biologics or modifications to approved biologics to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, including for 35 days beginning on December 22,
43
2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities. Since that time, there have been several threatened “shutdowns” of the U.S. federal government. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
We must attract and retain highly skilled personnel and strategic partners, and we may be unable to effectively manage our growth with our limited resources.
We have limited human resources and our future success depends and will depend in part on our ability to attract, train, retain and motivate highly skilled executive level management, research and development, and sales personnel and to establish and maintain effective strategic alliances with key companies in our industry. Competition is intense for many of these types of personnel from other companies, consulting firms and more established organizations, many of which have significantly larger operations and greater financial, marketing, human, and other resources. We may not be successful in attracting and retaining qualified personnel on a timely basis, on competitive terms or at all. If we are not successful in attracting and retaining these personnel, our business, prospects, financial condition and results of operations may be materially adversely affected.
We anticipate adding new employees and we will have to integrate such new employees into our operations.
Our officers and directors may not possess all of the skills or experience necessary to successfully implement our business plan. Further, we anticipate hiring new employees. Failure to fully integrate new employees into our operations could have a material adverse effect on our business, prospects, financial condition and results of operations.
We depend on our senior management and senior scientific staff, and their loss or unavailability could put us at a competitive disadvantage.
Our success depends largely on the skills, experience and reputation of certain key management and personnel, in particular our directors, executive officers and senior scientific staff. The loss or unavailability of any of these individuals for any significant period of time could have a material adverse effect on our business, prospects, financial condition and results of operations.
Our employees and independent contractors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could negatively impact our business, prospects, financial condition and operating results.
We are exposed to the risk that our employees, independent contractors, consultants, commercial partners, suppliers and distributors may engage in fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless or negligent conduct or disclosure of unauthorized activities to us that violates: (i) the rules and regulations of the FDA and other similar foreign regulatory bodies, including those laws requiring the reporting of true, complete and accurate information to such regulators; (ii) manufacturing standards; (iii) healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws; or (iv) laws that require the true, complete and accurate reporting of financial information or data. These laws may impact, among other things, future sales, marketing and education programs. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commissions, certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials.
We have adopted a code of conduct, but it is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent these activities may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgement, imprisonment, additional integrity reporting and oversight obligations, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm,
44
diminished profits and future earnings and curtailment of operations, any of which could adversely affect our ability to operate our business and our results of operations. Whether or not we are successful in defending against any such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations, which could harm our business, financial condition and results of operations.
We rely on third parties to perform some of our research and preclinical studies, and we plan to rely on third parties to conduct our clinical trials. If these third parties do not satisfactorily carry out their contractual duties or fail to meet expected deadlines, our development programs may be delayed or subject to increased costs, each of which may have an adverse effect on our business and prospects.
We do not have the ability to conduct all aspects of our preclinical studies or future clinical trials ourselves. As a result, we are, and expect to remain, dependent on third parties to perform some of our research and preclinical studies and any future clinical trials of our product candidates, including but not limited to governmental agencies and university laboratories, contract manufacturers, contract research organizations (CROs), distribution and supply (logistics) services organizations, contract testing organizations (CTOs), consultants or consultant organization with specialized knowledge based expertise. The timing of the initiation and completion of our current and planned preclinical studies and clinical trials will therefore be partially controlled by such third parties and may result in delays to our development programs. Specifically, we expect CROs, clinical investigators, and consultants to play a significant role in the conduct of future clinical trials and the subsequent collection and analysis of data. However, we will not be able to control all aspects of their activities. Nevertheless, as the sponsor of the INADs, INDs and clinical protocols governing our future clinical trials, we will be responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the CROs, CTOs, and other third parties does not relieve us of our regulatory responsibilities. We, our CROs, CTOs, and clinical sites will be required to comply with GLP requirements for preclinical studies, as well as GCP requirements for clinical trials involving human subjects, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area, and comparable foreign regulatory authorities, for all of our current product candidates and any future product candidates in clinical development. Regulatory authorities enforce these GLP and GCP requirements through periodic inspections of trial sponsors, testing laboratories, clinical trial investigators, and clinical trial sites. If we or any of our CROs, CTOs, or clinical trial sites fail to adhere to our clinical trial protocols or to comply with applicable GLP or GCP requirements, as applicable, the data generated in our future preclinical studies or clinical trials may be deemed unreliable, and the FDA or comparable foreign regulatory authorities may require us to perform additional preclinical studies or clinical trials before accepting for review or approving our marketing applications. In addition, our clinical trials must be conducted with product candidates produced under GMP regulations. Our failure to comply with these regulations may require us to stop and/or repeat clinical trials, which would delay the marketing approval process.
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA or comparable foreign regulatory authorities. The FDA or comparable foreign regulatory authority may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the trial results or data. The FDA or comparable foreign regulatory authority may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA or comparable foreign regulatory authority, as the case may be, and may ultimately lead to the denial of marketing approval of our product candidates.
There is no guarantee that any such CROs, CTOs, clinical trial investigators or other third parties on which we plan to rely will devote adequate time and resources to our development activities or perform as contractually required. Further, the performance of our third parties on which we rely may be interrupted by the ongoing COVID-19 pandemic, including due to travel or quarantine policies, heightened exposure of CRO staff who are healthcare providers to COVID-19 or prioritization of resources toward the pandemic (similar public health emergencies that may arise in the future). If any of these third parties fails to meet expected deadlines, adhere to our clinical protocols or meet regulatory requirements, otherwise performs in a substandard manner, or terminates its engagement with us, the timelines for our development programs may be extended or delayed or our development activities may be suspended or terminated. If any of our future clinical trial sites terminates for any reason, we may experience the loss of follow-up information on subjects enrolled in such clinical trials unless we are able to transfer those subjects to another qualified clinical trial site, which may be difficult or impossible.
45
We are limited in our ability to manufacture pharmaceutical products.
To be successful, our products and the products of our partners must be manufactured in commercial quantities in compliance with regulatory requirements and at a commercially acceptable cost. We have not commercialized any pharmaceutical products, nor have we demonstrated an ability to manufacture commercial quantities of our or our partners’ product candidates in accordance with regulatory requirements. If we are unable to produce suitable quantities of our or our partners’ products, or contract third parties to do so, in accordance with regulatory standards at a commercially acceptable cost, our ability or the ability of our partners to conduct clinical trials, obtain regulatory approvals and market such products may be adversely affected, which could adversely affect our competitive position and our chances of achieving profitability. There can be no assurance that such products can be manufactured by us or any other party at a cost or in quantities which are commercially viable.
We intend to rely on third parties to produce commercial supplies of our product candidates.
We intend to rely on third-party manufacturers to supply us with sufficient quantities of our product candidates to be used, if approved, for commercialization. We do not yet have a commercial supply agreement for commercial quantities of drug substance or drug product. If we are not able to meet market demand for any approved product, it would negatively impact our ability to generate revenue, harm our reputation, and could have an adverse effect on our business and financial condition.
Further, our reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured product candidates ourselves, including:
In addition, if we enter into a strategic collaboration with a third party for the commercialization of our current or any future product candidates, we will not be able to control the amount of time or resources that they devote to such efforts. If any strategic collaborator does not commit adequate resources to the marketing and distribution of our product candidates, it could limit our potential revenues.
Any of these events could lead to clinical trial delays or failure to obtain regulatory approval, or impact our ability to successfully commercialize our current or any future product candidates once approved. Some of these events could be the basis for FDA action, including injunction, request for recall, seizure, or total or partial suspension of production.
46
If we fail to successfully operate our animal production facility, it may adversely affect our clinical trials and the commercial viability of our product candidates.
We operate our own animal production facility, where we produce supplies of our product candidates for our preclinical and clinical studies, and such facility is currently subject to certain regulatory requirements and inspections, including by the USDA to ensure compliance with the Animal Welfare Act and other regulations relating to the care and welfare of laboratory and research animals.
Before approving any of our product candidates for commercialization, the FDA must conduct a pre-approval inspection of our animal production and manufacturing facilities to determine whether the manufacturing processes and facilities comply with GMPs. If and when we obtain regulatory approval for any of our product candidates, we would need to register our animal production and manufacturing facilities with the FDA and list all licensed biological products manufactured at such facilities. Even if the FDA determines that our facilities are in substantial compliance with applicable regulations and standards, we would be subject to ongoing periodic unannounced inspection by the FDA, the USDA, corresponding state agencies and potentially third-party collaborators to ensure strict compliance with GMPs, animal welfare requirements, and other applicable laws and government regulations. Our license to manufacture such future approved product candidates will be subject to continued regulatory review.
In addition, our animal production facility maintains detailed standard operating procedures and other documentation necessary to comply with the Animal Welfare Act and applicable regulations for the humane treatment of the pigs and piglets in our custody. We also maintain an Institutional Animal Care and Use Committee (IACUC) to provide ongoing oversight and to conduct assessments of the care and use of the animals in our research and development programs. If the USDA determines that our current equipment, facilities, or processes relating to donor animal production do not comply with applicable Animal Welfare Act standards, it may issue an inspection report documenting the deficiencies and setting deadlines for any required corrective actions. For continued noncompliance, the USDA may impose fines, suspend, or revoke animal research licenses or confiscate research animals.
There can be no assurance that we will not encounter difficulties in scaling up our manufacturing processes. Significant scale-up of manufacturing may result in unanticipated technical challenges and may require additional inspections, permits, or other authorizations by the FDA, the USDA, or corresponding state agencies. We may encounter difficulties in scaling up production, including problems involving raw material suppliers, production yields, technical difficulties, scaled-up product characteristics, quality control and assurance, shortage of qualified personnel, capacity constraints, compliance with FDA and foreign regulations, environmental compliance, production costs and development of advanced manufacturing techniques and process controls. The actual cost to manufacture and process our product candidates could also be greater than we expect and could materially and adversely affect the commercial viability of any product candidates that we develop. Any of these difficulties, if they occur and are not resolved to the satisfaction of the FDA or other regulatory agency, could lead to significant delays and possibly the termination of the future development or commercial program for such product candidate. These risks become more acute as we scale-up for commercial quantities, where a reliable source of product becomes critical to commercial success. The commercial viability of any of our product candidates, if approved, will depend on our ability to produce our product candidates at a large scale. Failure to achieve this level of supply could jeopardize the successful commercialization of our therapeutic product candidates, should any be approved for marketing.
The manufacture of polyclonal antibodies from transgenic animals is complex and requires significant expertise, including the development of advanced manufacturing techniques and process controls. Manufacturers of polyclonal antibody products often encounter difficulties in production, particularly in scaling out up and validating initial production and ensuring the absence of contamination. These problems include difficulties with production costs and yields, quality control, including stability of the product candidate, quality assurance testing, operator error, shortages of qualified personnel, shortages of raw materials, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if contaminants are discovered in our animal production facility, it may need to be closed for an extended period of time to investigate and remedy the contamination. We cannot ensure provide assurance that any stability or other issues relating to the manufacture of our product candidates will not occur in the future.
Our manufacturing capabilities could be affected by cost-overruns, resource constraints, unexpected delays, equipment failures, labor shortages or disputes, natural disasters, power failures and numerous other factors that could prevent us from realizing the intended benefits of our manufacturing strategy, jeopardize our ability to produce our product candidates, and have a material adverse effect on our business, financial condition, results of operations and prospects.
47
Our product candidates are uniquely manufactured, and we may encounter difficulties in production, particularly with respect to scaling our manufacturing capabilities.
The manufacturing process used to produce Tc Bovine is novel and has not been validated for commercial production.
There is a risk that of we may experience manufacturing issues associated with the differences in donor starting materials, interruptions in the manufacturing process, contamination, equipment or reagent failure, improper installation or operation of equipment, vendor or operator error, and variability in product characteristics. Even minor deviations from our normal manufacturing processes could result in reduced production yields, lot failures, product defects, product delays, product recalls, product liability claims and other supply disruptions. Further, as product candidates advance through preclinical to later-stage clinical trials towards approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods, are altered in an effort to optimize processes and results. We may not achieve our intended objectives and any of these changes could cause our product candidates to perform differently than we expect, potentially affecting the results of future clinical trials.
Although we continually attempt to optimize our manufacturing process, doing so is a difficult and uncertain task and there are risks associated with scaling to the level required for future initial clinical trials, advanced late-stage clinical trials or commercialization, including, among others, cost overruns, potential problems with process scale-up, process reproducibility, stability issues, lot consistency and timely availability of reagents or raw materials. If we are unable to adequately validate or scale-up our manufacturing processes, we may encounter lengthy delays in commercializing our product candidates.
The manufacturing process for any products candidates that we may develop is subject to the FDA and foreign regulatory authority approval processes and, if we choose to outsource our commercial production, we will need to contract with third-party manufacturers who we believe can meet applicable FDA, USDA, and foreign regulatory authority requirements on an ongoing basis. If we are unable to reliably produce any product candidate to specifications acceptable to the FDA, the USDA, or other regulatory authorities, we may not obtain or maintain the approvals we need to commercialize our products. Even if we obtain regulatory approval for any of our product candidates, there is no assurance that either we or any third-party manufacturers we may contract with in the future will be able to manufacture the approved product to specifications and under GMPs acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product, or to meet potential future demand. Any of these challenges could delay completion of future clinical trials, require bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidates, impair commercialization efforts, increase our cost of goods and have an adverse effect on our business, financial condition, results of operations and growth prospects.
Our future success depends on our ability to manufacture our product candidates on a timely basis with acceptable manufacturing costs, while at the same time maintaining good quality control and complying with applicable regulatory requirements. Our inability to do so could have a material adverse effect on our business, financial condition, prospects and results of operations. In addition, we could incur higher manufacturing costs if manufacturing processes or standards change and we could need to replace, modify, design or build and install equipment, all of which would require additional capital expenditures.
We have not entered into long term manufacturing and supply agreements with any producers.
On October 26, 2022, we entered into a Manufacturing Option Agreement (the “Emergent Manufacturing Agreement”) and Right of First Refusal Agreement (the “Emergent RoFR Agreement,” and together with the Emergent Manufacturing Agreement, the “Emergent Agreements”) with Emergent BioSolutions Canada, Inc., a wholly-owned subsidiary of Emergent BioSolutions Inc. (“Emergent”). The Emergent Agreements contemplate that we will enter into one or more binding Master Manufacturing Services Agreements, whereby Emergent will provide contract development and manufacturing services to produce our fully-human polyclonal antibody products. Under the Emergent Manufacturing Agreement, we granted Emergent an exclusive option for the exclusive commercial manufacture of commercial stage product utilizing our humanized polyclonal antibodies. Pursuant to the terms of our arrangement, we will notify Emergent in advance of our first commercial manufacturing needs for any product and each additional product, and Emergent may then exercise the exclusive manufacturing option with respect to such product. Under the Emergent RoFR Agreement, we granted Emergent an exclusive right of first refusal to license and develop our products, developed using humanized polyclonal antibodies based on our platform to treat (i) botulism anti-toxin, (ii) pandemic influenza, or (iii) anti-fungal diseases. Any definitive manufacturing arrangement will be determined at the time any Master Manufacturing Services Agreement is entered into with Emergent, and there is no guarantee we will do so.
We intend to pursue agreements with contract manufacturers to produce the components and drug products that we will use in the future for the commercialization of products that make using of our technology, as well as for labeling and finishing services. We may not be able to enter into such arrangements on acceptable terms or at all. Components of our product
48
candidates are currently manufactured for us in small quantities for use in our preclinical and clinical studies. We will require significantly greater quantities to commercialize any given product. We may not be able to find alternate sources of comparable components. If we are unable to obtain adequate supplies of components from our existing suppliers or need to switch to an alternate supplier and obtain FDA or other regulatory agency approval of that supplier, commercialization of our product candidates may be delayed. If we are unable to obtain sufficient compounds and labeling services on acceptable terms, or if we should encounter delays or difficulties in our relationships with our current and future suppliers or if our current and future suppliers of each component do not comply with applicable regulations for the manufacturing and production of drugs, our business, financial condition, and results of operations may be materially harmed.
We are subject to manufacturing risks that could substantially increase the costs and limit supply of product candidates or prevent us from achieving a commercially viable production process.
The process of manufacturing our product candidates is complex, highly regulated and subject to several risks, including:
Any changes in our manufacturing processes as a result of scaling up may result in the need to obtain additional regulatory approvals. Difficulties in achieving commercial-scale production or the need for additional regulatory approvals as a result of scaling up could delay the development and regulatory approval of our product candidates and ultimately affect our success. We may not achieve the manufacturing productivity (“yield”) required to achieve a commercially viable cost of goods. Low productivities may result in a cost of goods which is too high to allow profitable commercialization, or give rise to the need for additional manufacturing process optimization which would require additional funding and time.
Additionally, the process of manufacturing biologics, such as our product candidates, is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, vendor or operator error, inconsistency in yields, variability in product characteristics and difficulties in scaling the production process. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination.
We and our contract manufacturers are subject to significant regulatory oversight with respect to manufacturing our products. The manufacturing facilities on which we rely may not continue to meet regulatory requirements and may have limited capacity.
All parties involved in the preparation of therapeutics for clinical trial or commercial sale are subject to extensive regulation. Components of a finished therapeutic product approved for commercial sale or used in late-stage clinical trials must be manufactured in accordance with GMP requirements. These regulations govern manufacturing processes and procedures (including recordkeeping) and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. In addition, due to our use of transgenic animals to manufacture our product candidates, we, and potentially our third-party manufacturers, are subject to animal welfare requirements as part of our production process. The FDA, the USDA, and comparable foreign regulatory agencies may also implement new standards at any time, or change their interpretations and enforcement of existing standards, including for the manufacture, packaging or testing of biological products or for the care and welfare of research animals.
Poor control of production processes can lead to the introduction of adventitious agents or other contaminants, or to inadvertent changes in the properties or stability of our product candidates that may not be detectable in final product testing. We or our contract manufacturers must supply all necessary documentation in support of a NADA and BLA on a timely basis and must adhere to the FDA’s GMP requirements and USDA animal welfare requirements enforced by each agency through its respective facilities inspection program. Our facilities and quality systems and the facilities and quality systems of some or all of our third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our product candidates. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or the associated quality systems for compliance with the regulations applicable to the activities being conducted. If these facilities do not pass a pre-approval plant inspection, FDA approval of the products will not be granted.
49
The regulatory authorities also may, at any time following approval of a product for sale, audit our manufacturing facilities or those of our third-party manufacturers. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time-consuming for us or our third-party manufacturers to implement and that may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a manufacturing facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
If we or any of our third-party manufacturers or testing contractors fail to maintain regulatory compliance, the FDA can impose regulatory sanctions including, among other things, warning or untitled letters, fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approvals, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions and criminal prosecutions. Such an occurrence may cause our business, financial condition and results of operations to be materially harmed.
The manufacturing facilities in which our product candidates are made could be adversely affected by equipment failures, labor shortages, natural disasters, power failures and numerous other factors.
We presently manufacture our product candidates at our lab facilities in South Dakota. If our lab facilities were to be damaged or destroyed by fire, flood, other natural disaster or other occurrences of any kind, it would have a material adverse effect on our ability to produce product candidates and on our business, financial condition and results of operations.
We must comply with applicable current Good Manufacturing Practice, or cGMP, regulations and guidelines. We may encounter difficulties in achieving quality control and quality assurance and may experience shortages in qualified personnel. We are subject to inspections by regulatory authorities to confirm compliance with applicable regulatory requirements. Any failure to follow cGMP or other regulatory requirements or delay, interruption or other issues that arise in the manufacture, fill-finish, packaging, or storage of our product candidates as a result of a failure of our facilities or the facilities or operations of third parties to comply with regulatory requirements or pass any regulatory authority inspection could significantly impair our ability to develop and commercialize our product candidates, leading to significant delays in the availability of therapeutic product for clinical studies or the termination or hold on a clinical study, or the delay or prevention of a filing or approval of marketing applications for our product candidates. Significant noncompliance could also result in the imposition of sanctions, including fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approvals for our product candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could damage our reputation. If we are not able to achieve and maintain regulatory compliance, we may not be permitted to market our product candidates and/or may be subject to product recalls, seizures, injunctions, or criminal prosecution.
Any adverse developments affecting manufacturing operations for our product candidates, if any are approved, may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, or other interruptions in the supply of product candidates. We may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives.
Our product candidates that have been produced and are stored for later use may degrade, become contaminated or suffer other quality defects, which may cause the affected product candidates to no longer be suitable for their intended use in clinical studies or other development activities. If the defective product candidates cannot be replaced in a timely fashion, we may incur significant delays in our development programs that could adversely affect the value of such product candidates.
Outbreaks of livestock diseases and other events affecting the health of our bovine herd can adversely impact our ability to conduct our operations and production of our product candidates.
Our product candidates are based on materials produced by genetically engineered bovines. We maintain a herd of approximately 80 genetically engineered production animals at a single location in South Dakota and a larger herd of recipient animals at other locations. Our ability to produce product candidates is dependent on the continued health and productivity of these animals. The supply of our product candidates can be adversely impacted by outbreaks of livestock diseases, which can have a significant adverse impact on our financial condition. Our animals produced by the recipient herd do not typically become productive until 18 months from the start of gestation. If all or a material number of the productive herd were to become diseased, injured or die as a result of bacterial, fungal or viral infections, such as foot and mouth disease, or natural disaster or other occurrences of any kind, it would have a material adverse effect on our ability to produce product candidates and on our business, financial condition and results of operations.
50
Extreme factors or forces beyond our control could negatively impact our business.
Natural disasters, fire, bioterrorism or other acts of terrorism or vandalism, animal activist activity or adverse public perception or media coverage or other public relations issues, pandemics or extreme weather, including droughts, floods, excessive cold or heat, hurricanes or other storms, could impair the health or growth of livestock or interfere with our operations due to power outages, fuel shortages, feed shortages, decrease in availability of water, damage to our production and manufacturing facilities or disruption of transportation channels which would delay the development, regulatory approval and manufacture of our product candidates and ultimately affect our success. Any of these factors could have an adverse effect on our financial condition and ability to operate.
Cyber-attacks or other failures in our telecommunications or information technology systems, or those of our collaborators, CROs, third-party logistics providers, distributors or other contractors or consultants, could result in information theft, data corruption and significant disruption of our business operations.
We, along with our collaborators, CROs, third-party logistics providers, distributors and other contractors and consultants, utilize information technology, or IT, systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including third parties gaining access to employee accounts using stolen or inferred credentials, computer malware, viruses, spamming, phishing attacks or other means, and deliberate attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our, our collaborators’, CROs’, third-party logistics providers’, distributors’ and other contractors’ and consultants’ systems and networks, and the confidentiality, availability and integrity of our data. There can be no assurance that we will be successful in preventing cyber-attacks or successfully mitigating their effects. Like other companies, we have on occasion experienced, and will continue to experience, threats to our data and systems, including malicious codes and viruses, phishing, business email compromise attacks or other cyber-attacks.
There can be no assurance that our collaborators, CROs, third-party logistics providers, distributors and other contractors and consultants will be successful in protecting our clinical and other data that is stored on their systems. Any cyber-attack, data breach or destruction or loss of data could result in a violation of applicable U.S. and international privacy, data protection and other laws and subject us to litigation and governmental investigations and proceedings by federal, state and local regulatory entities in the United States and by international regulatory entities, resulting in exposure to material civil and/or criminal liability. Further, our general liability insurance and corporate risk program may not cover all potential claims to which we are exposed and may not be adequate to indemnify us for all liability that may be imposed, which could have a material adverse effect on our business and prospects. In addition, we may suffer reputational harm or face litigation or adverse regulatory action as a result of cyber-attacks or other data security breaches and may incur significant additional expense to implement further data protection measures.
See Item 1C. “Cybersecurity”, of this Annual Report on Form 10-K for more information.
Collaborations with third parties may be important to our business. If these collaborations are not successful, our business could be adversely affected.
In addition to our current collaborations, we may in the future seek third-party collaborators for the development and commercialization of product candidates. If we enter into such collaborations, we will have limited control over the amount and timing of resources that our collaborators will dedicate to the development or commercialization of our product candidates. Our ability to generate revenue from any future collaboration or license agreement will depend on the collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. In addition, any collaborators may have the right to abandon research or development projects and terminate applicable agreements, including any funding obligations, prior to or upon the expiration of the agreed upon terms.
Any collaboration that we enter into in the future may pose a number of risks, including the following:
51
Additionally, subject to its contractual obligations to us, if one of our collaborators is involved in a business combination, the collaborator might deemphasize or terminate the development or commercialization of any product candidate licensed to it by us. If one of our collaborators terminates its agreement with us, we may find it more difficult to attract new collaborators and our perception in the business and financial communities could be adversely affected.
We operate in a highly competitive industry.
We are engaged in highly competitive industries. We compete with many public and private companies, including pharmaceutical companies, chemical companies, specialized biotechnology companies and academic institutions. Many of our competitors have substantially greater financial, scientific and technical resources, and manufacturing and marketing experience and capabilities than us. In addition, many of our competitors have significantly greater experience conducting preclinical studies and clinical trials of new pharmaceutical products, and in obtaining regulatory approvals for pharmaceutical products. Our competitors and competitors of our collaborators may develop and commercialize such products more rapidly than we and our collaborators do. Competition may increase further as a result of potential advances from the study of pharmaceutical products, and greater availability of capital for investment in this field. There can be no assurance that our competitors will not succeed in developing technologies and products that are more effective than any being developed by us or that would render our technology and products obsolete or noncompetitive. There can be no assurance that these and other efforts by potential competitors will not be successful, or that other methods will not be developed to compete with our technology. There are specific products and technologies that compete with our current product pipeline and that may outperform or be more competitive than our products. For example, there are multiple products that may be competitive with SAB-142 for T1D such as animal-derived polyclonal biologics ThymoglobulinTM (Sanofi), and AtgamTM (Pfizer), and monoclonal antibodies such as TzieldTM (Sanofi). Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis, drug products that are more effective, safer or less costly than any product candidate that we may develop. Our existing competitors and new market entrants may respond more quickly to or integrate new or emerging technologies such as artificial intelligence and machine learning, undertake more extensive marketing campaigns, have greater access to clinical information to support ongoing product position in the market, have greater financial, marketing and other resources or be more successful in attracting potential customers, employees and strategic partners.
We have no sales and marketing experience.
We have no experience in sales, marketing or distribution. Before we can market any of our product candidates directly, we must develop a substantial marketing and sales force with technical expertise and supporting distribution capability. Alternatively, we may obtain the assistance of a pharmaceutical company with a large distribution system and a large direct sales force. We do not have any existing distribution arrangements with any pharmaceutical company for our products. There can be no assurance that we will be able to establish sales and distribution capabilities or be successful in gaining market acceptance for our products.
52
We are subject to stringent environmental regulation and potentially subject to environmental litigation, proceedings, and investigations.
Our business operations and use of real property are subject to stringent federal, state, and local environmental laws and regulations pertaining to safe working conditions, ethical experimental use of animals, the discharge of materials into the environment, and the handling and disposition of wastes (including solid and hazardous wastes) or otherwise relating to protection of the environment. These laws include the Occupational Safety and Health Act, the Toxic Test Substances Control Act and the Resource Conservation and Recovery Act. Compliance with these laws and regulations, and the ability to comply with any modifications to these laws and regulations, is material to our business. New matters or sites may be identified in the future that will require additional investigation, assessment, or expenditures. In addition, some of our facilities have been in operation for some time and, over time, we and any other prior operators of these facilities may have generated and disposed of wastes that now may be considered hazardous. Future discovery of contamination of property underlying or in the vicinity of our present or former properties or manufacturing facilities and/or waste disposal sites could require us to incur additional expenses. In addition, claimants may sue us for injury or contamination that results from our use of or our handling of contaminants, and our liability may exceed our total assets. Compliance with environmental laws and regulations may be expensive, and current or future environmental regulations may impair our research, development or production efforts. The occurrence of any of these events, the implementation of new laws and regulations, or stricter interpretation of existing laws or regulations, could adversely affect our financial condition and ability to operate.
If we or any contract manufacturers and suppliers we engage fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our research and development activities involve the use of biological and hazardous materials and produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials, which could cause an interruption of our commercialization efforts, research and development efforts and business operations, environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. We do not carry specific biological waste or hazardous waste insurance coverage, workers compensation or property and casualty and general liability insurance policies that include coverage for damages and fines arising from biological or hazardous waste exposure or contamination.
Risks Related to Our Intellectual Property
Our success depends on our ability to maintain the proprietary nature of our technology.
Our success in large part depends on our ability to maintain the proprietary nature of our technology and other trade secrets. To do so, we must prosecute and maintain existing patents, obtain new patents and pursue trade secret and other intellectual property protection. We also must operate without infringing the proprietary rights of third-parties or allowing third-parties to infringe our rights. Patent issues relating to pharmaceuticals and biologics involve complex legal, scientific and factual questions. To date, no consistent policy has emerged regarding the breadth of biotechnology patent claims that are granted by the U.S. Patent and Trademark Office (“USPTO”) or enforced by the federal courts. Therefore, we do not know whether any particular patent applications will result in the issuance of patents, or that any patents issued to us will provide us with any competitive advantage. We also cannot be sure that we will develop additional proprietary products that are patentable.
53
Furthermore, there is a risk that others will independently develop or duplicate similar technology or products or circumvent the patents issued to us.
Third parties may claim we infringe their intellectual property rights.
Our research, development and commercialization activities may be found to infringe patents owned by third-parties from whom we do not hold licenses or other rights to use their intellectual properties. There may be rights we are not aware of, including applications that have been filed, but not published that, when issued, could be asserted against us. These third-parties could bring claims against us, and that may cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
As a result of potential patent infringement claims, or in order to avoid potential claims, we may choose or be required to seek a license from the third-party. These licenses may not be available on acceptable terms, or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us might be non-exclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. All of the issues described above could also impact our collaborators, which would also impact the success of the collaboration and therefore us.
We may become involved in litigation to protect or enforce our patents or the patents of our collaborators or licensors, which could be expensive and time-consuming.
Competitors may infringe our patents or the patents of our collaborators or licensors. As a result, we may be required to file suit to counter infringement for unauthorized use. This can be expensive, particularly for a company of our size, and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover our technology. An adverse determination of any litigation or defense proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at the risk of not issuing.
Even if we are successful, litigation may result in substantial costs and distraction to our management. Even with a broad portfolio, we may not be able, alone or with our collaborators and licensors, to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the U.S.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If investors perceive these results to be negative, the market price for our common stock could be significantly harmed.
If patent laws or the interpretation of patent laws change, our competitors may be able to develop and commercialize our discoveries.
Important legal issues remain to be resolved as to the extent and scope of available patent protection for biopharmaceutical products and processes in the U.S. and other important markets outside the U.S., such as Europe and Japan. In addition, foreign markets may not provide the same level of patent protection as provided under the U.S. patent system. Litigation or administrative proceedings may be necessary to determine the validity and scope of certain of our and others’ proprietary rights. Any such litigation or proceeding may result in a significant commitment of resources in the future and could force us to do one or more of the following: cease selling or using any of our products that incorporate the challenged intellectual property, which would adversely affect our revenue; obtain a license from the holder of the intellectual property right alleged to have been infringed, which license may not be available on reasonable terms, if at all; and redesign our products to avoid infringing the intellectual property rights of third-parties, which may be time-consuming or impossible to do. In addition, changes in, or different interpretations of, patent laws in the U.S. and other countries may result in patent laws that allow others to use our discoveries or develop and commercialize our products. We cannot provide assurance that the patents we obtain or the unpatented technology we hold will afford us significant commercial protection.
54
We have third party collaborators that might claim rights in or to our technology and/or assets.
We have extensive experience collaborating with multiple parties in Government and industry, and has agreements and collaborations that allow potential claims and actual rights, such as shared publication rights, shared inventions, access to assets, potential claims of co-inventorship, limited rights to data, general purpose rights to data, and other claims that may affect our business operations, intellectual property portfolio, interruption of operating assets or our ability to protect our own rights. There can be no assurance that our competitors, suppliers, service providers, collaborators or other parties will not succeed in asserting rights that are or become contrary to our interests.
Changes in patent law in the United States and in ex-U.S. jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing and proposing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents, particularly those directed to pharmaceutical and biopharmaceutical products and uses could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. We cannot predict how these decisions or any future decisions by the U.S. Congress, the federal courts or the USPTO may impact the value of our patents. Similarly, any adverse changes in the patent laws of other jurisdictions could have a material adverse effect on our business and financial condition.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world is expensive. While many of our licensed patents, including the patents covering our lead product candidates, have been issued in major markets and other countries, our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States where we have issued patents, or from selling or importing products made using our inventions in other jurisdictions. Competitors may also use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we do not have patent protection or where we have patent protection but where enforcement is not as strong as that in the United States. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent such competition.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to pharmaceutical and biopharmaceutical products, which could make it difficult for us or our licensors to stop the infringement of our patents or marketing of competing products against third parties in violation of our proprietary rights generally. The initiation of proceedings for infringement by third parties or by third parties to challenge the scope or validity of our patent rights in foreign jurisdictions could also result in substantial cost and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and any related patent applications at risk of not issuing and could provoke third parties to assert claims against us or our licensors. We may not prevail in any lawsuits that we initiate or are initiated against us, and the damages or other remedies awarded in lawsuits that we initiate, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
If we do not obtain patent term extension and data exclusivity for any product candidates we may develop, our business may be materially harmed.
Depending upon the timing, duration and specifics of any FDA marketing approval of any product candidates we may develop, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Action of 1984, or Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a
55
patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent per eligible drug may be extended and only those claims covering the approved drug, an approved method for using it or a method for manufacturing it may be extended. Patent term extensions tied to marketing approval in foreign jurisdictions may also be available for our patents. However, we may not be granted an extension because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our business, financial condition, results of operations and prospects could be materially harmed.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively, and our business may be adversely affected.
Risks Related to Being a Public Company
We incur increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could adversely affect our business, financial condition, and results of operations.
As a public company, we are and will continue to be subject to the reporting requirements of the Exchange Act, the listing standards of Nasdaq and other applicable securities rules and regulations. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting, and financial compliance costs, make some activities more difficult, time-consuming and costly, and place significant strain on our personnel, systems, and resources. For example, the Exchange Act requires, among other things, that we file annual, quarterly, and current reports with respect to our business and results of operations. As a result of the complexity involved in complying with the rules and regulations applicable to public companies, our management’s attention may be diverted from other business concerns, which could harm our business, financial condition, and results of operations, although we have already hired additional employees to assist us in complying with these requirements, we may need to hire more employees in the future or engage outside consultants, which will increase our operating expenses.
In addition, changing laws, regulations, and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs, and making some activities more time-consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest substantial resources to comply with evolving laws, regulations, and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from business operations to compliance activities. If our efforts to comply with new laws, regulations, and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us, and our business may be harmed.
We also expect that being a public company and these new rules and regulations will make it increasingly expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors (the “Board”), particularly to serve on our Audit Committee of the Board (the “Audit Committee”) and compensation committee of the Board (the “Compensation Committee”), and qualified executive officers.
As a result of disclosure of information in filings required of a public company, our business and financial condition are more visible, which may result in an increased risk of threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business, financial condition, and results of operations could be harmed, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and harm our business, financial condition, and results of operations.
56
We are an “emerging growth company,” and our election to comply with the reduced disclosure requirements as a public company may make our common stock less attractive to investors.
For so long as we remain an “emerging growth company” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart our Business Startups Act of 2012, (the “JOBS Act”), we may take advantage of certain exemptions from various requirements that are applicable to public companies that are not “emerging growth companies,” including not being required to comply with the independent auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, being required to provide fewer years of audited financial statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We may lose our emerging growth company status and become subject to the U.S. Securities and Exchange Commission’s (the “SEC”) internal control over financial reporting management and auditor attestation requirements. If we are unable to certify the effectiveness of our internal controls, or if our internal controls have a material weakness, we could be subject to regulatory scrutiny and a loss of confidence by stockholders, which could harm our business and adversely affect the market price of our common stock. We will cease to be an “emerging growth company” upon the earliest to occur of: (i) the last day of the fiscal year in which we have more than $1.235 billion in annual revenue; (ii) the date we qualify as a large accelerated filer, with at least $700 million of equity securities held by non-affiliates; (iii) the date on which we have, in any three-year period, issued more than $1.0 billion in non-convertible debt securities; and (iv) December 31, 2026 (the last day of the fiscal year following the fifth anniversary of becoming a public company).
As an emerging growth company, we may choose to take advantage of some but not all of these reduced reporting burdens. Accordingly, the information we provide to our stockholders may be different than the information you receive from other public companies in which you hold stock. In addition, the JOBS Act also provides that an “emerging growth company” can take advantage of an extended transition period for complying with new or revised accounting standards. We have elected to take advantage of this extended transition period under the JOBS Act. As a result, our operating results and financial statements may not be comparable to the operating results and financial statements of other companies who have adopted the new or revised accounting standards. It is possible that some investors will find our common stock less attractive as a result, which may result in a less active trading market for our common stock and higher volatility in our stock price.
Investors may find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile and may decline.
If we fail to maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable regulations could be impaired.
As a public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act and the rules and regulations of the applicable listing standards of Nasdaq. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting and financial compliance costs, make some activities more difficult, time-consuming and costly and place significant strain on our personnel, systems and resources.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we will file with the SEC is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms and that information required to be disclosed in reports under the Exchange Act is accumulated and communicated to our principal executive and financial officers. We are also continuing to improve our internal control over financial reporting, which includes hiring additional accounting and financial personnel to implement such processes and controls. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting, we have expended, and anticipate that we will continue to expend, significant resources, including accounting-related costs and significant management oversight. If any of these new or improved controls and systems do not perform as expected, we may experience material weaknesses in our controls.
Our current controls and any new controls that we develop may become inadequate because of changes in conditions in our business. Further, weaknesses in our disclosure controls and internal control over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls or any difficulties encountered in their implementation or improvement could harm our results of operations or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods. Any failure to implement and maintain effective internal control over financial reporting also could adversely affect the results of periodic management evaluations and annual independent
57
registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we will eventually be required to include in our periodic reports that will be filed with the SEC. Ineffective disclosure controls and procedures and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the trading price of our common stock. In addition, if we are unable to continue to meet these requirements, we may not be able to remain listed on Nasdaq. We are not currently required to comply with the SEC rules that implement Section 404 of the Sarbanes-Oxley Act and are therefore not required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. As a public company, we are required to provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our Form 10-K.
Our independent registered public accounting firm is not required to formally attest to the effectiveness of our internal control over financial reporting until after we are no longer an “emerging growth company” as defined in the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our internal control over financial reporting is documented, designed or operating. Any failure to maintain effective disclosure controls and internal control over financial reporting could have an adverse effect on our business and results of operations and could cause a decline in the price of our common stock.
We have identified a material weakness in our internal control over financial reporting and determined that our disclosure controls and procedures were ineffective as of December 31, 2023. In the future, we may identify additional material weaknesses or otherwise fail to maintain an effective system of internal control over financial reporting or adequate disclosure controls and procedures, which may result in material errors in our financial statements or cause us to fail to meet our period reporting obligations, and adversely affect the trading price of our common stock.
Under the supervision and with participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2023, and we have concluded that our internal controls over financial reporting were not effective as of December 31, 2023, due to the existence of material weaknesses in such controls. We have also concluded that our disclosure controls and procedures were not effective as of December 31, 2023, all as described in Item 9A, “Controls and Procedures,” of this Annual Report on Form 10-K. Management is actively engaged in the planning for, and implementation of, remediation efforts to address our material weaknesses. Continuing costs to remedy these material weaknesses and to address inquiries from regulators may be significant and may require significant attention from our management and other personnel, and we cannot assure you that we will be able to remedy the material weaknesses.
The incurrence of significant additional expense, or the requirement that management and other personnel devote significant time to these matters could reduce the time available to execute on our business strategies and could have a material adverse effect on our business, financial condition and results of operations. We also cannot assure you that additional material weaknesses in our internal control over financial reporting will not arise or be identified in the future. If our remediation efforts are insufficient to address the identified deficiencies, or if additional deficiencies in our internal control over financial reporting are discovered or occur in the future, our Consolidated financial statements may contain material misstatements and we could be required to restate our financial results and may be unable to make our filings with the SEC on a timely basis. Moreover, because of the inherent limitations of any control system, material misstatements due to error or fraud may not be prevented or detected on a timely basis, or at all.
If we are unable to provide reliable and timely financial reports in the future, our business and reputation may be further harmed. Failures in internal controls may negatively affect investor confidence in our management and the accuracy of our financial statements and disclosures or result in adverse publicity and concerns from investors and commercial customers, any of which could have a negative effect on the price of our shares, subject us to regulatory investigations and penalties and/or shareholder litigation, and materially adversely impact our business and financial condition.
Our warrants are accounted for as liabilities and changes in value of the warrants could have a material effect on our financial results.
In October 2021, the Company consummated the business combination contemplated by the agreement and plan of merger, dated as of June 21, 2021, as amended on August 12, 2021, made by and among Big Cypress Acquisition Corp., a Delaware corporation (“BCYP”), Big Cypress Merger Sub Inc., a Delaware corporation (“Merger Sub”), the Company, and Shareholder Representative Services LLC, a Colorado limited liability company, solely in its capacity as the representative, agent and attorney-in-fact of the SAB Stockholders (the “Business Combination”). Prior to the Business Combination, on April 12, 2021, the staff of the SEC issued a Staff Statement on Accounting and Reporting Considerations for Warrants Issued by Special Purpose Acquisition Companies (“SPACs”) (the “SEC Staff Statement”). The SEC Staff Statement focused on certain accounting and reporting considerations related to warrants of a kind similar to warrants that we issued prior to the
58
Business Combination at the time of our initial public offering and the exercises by the underwriters of their over-allotment options in January 2021. In response to the SEC Staff Statement, we determined to classify the warrants as derivative liabilities measured at fair value, with the initial valuation occurring on October 22, 2021, the “Closing Date” of the Business Combination, with changes in fair value each period reported in earnings.
On September 29, 2023, the Company entered into a securities purchase agreement with certain accredited investors (the “September 2023 Purchase Agreement”), pursuant to which the Company agreed to issue and sell shares of preferred stock and warrants, in a private placement. See Note 12, Warrants for further information about the private placement offering.
As a result, included on our balance sheet are derivative liabilities related to embedded features contained within the warrants. Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity provides for the remeasurement of the fair value of such derivatives at each balance sheet date, with a resulting non-cash gain or loss related to the change in the fair value being recognized in earnings in the statement of income. As a result of the recurring fair value measurement, our financial statements and results of operations may fluctuate quarterly based on factors which are outside of our control. Due to the recurring fair value measurement, we expect that we will recognize non-cash gains or losses on the warrants each reporting period and that the amount of such gains or losses could be material.
Our business, financial condition, and results of operations may fluctuate on a quarterly and annual basis, which may result in a decline in our stock price if such fluctuations result in a failure to meet the expectations of securities analysts or investors.
Our operating results have in the past and could in the future vary significantly from quarter-to-quarter and year-to-year and may fail to match our past performance, our projections or the expectations of securities analysts because of a variety of factors, many of which are outside of our control and, as a result, should not be relied upon as an indicator of future performance. As a result, we may not be able to accurately forecast our operating results and growth rate. Any of these events could cause the market price of our common stock to fluctuate. Factors that may contribute to the variability of our operating results include, but are not limited to: our ability to attract new clients and partners, retain existing clients and partners and maximize engagement and enrollment with existing and future clients; changes in our sales and implementation cycles, especially in the case of our large clients; new solution introductions and expansions, or challenges with such introductions; changes in our pricing or fee policies or those of our competitors; the timing and success of new solution introductions by us or our competitors or announcements by competitors or other third parties of significant new products or acquisitions or entrance into certain markets; any other change in the competitive landscape of our industry, including consolidation among our competitors; increases in operating expenses that we may incur to grow and expand our operations and to remain competitive; our ability to successfully expand our business, whether domestically or internationally; breaches of security or privacy; changes in stock-based compensation expenses; the amount and timing of operating costs and capital expenditures related to the expansion of our business; adverse litigation judgments, settlements, or other litigation-related costs; changes in the legislative or regulatory environment, including with respect to privacy or data protection, or enforcement by government regulators, including fines, orders, or consent decrees; the cost and potential outcomes of ongoing or future regulatory investigations or examinations, or of future litigation; changes in our effective tax rate; our ability to make accurate accounting estimates and appropriately recognize revenue for our solutions for which there are no relevant comparable products; changes in accounting standards, policies, guidance, interpretations, or principles; instability in the financial markets; general economic conditions, both domestic and international; volatility in the global financial markets; political, economic, and social instability, including terrorist activities and health epidemics (including the recent outbreak of COVID-19), and any disruption these events may cause to the global economy; and changes in business or macroeconomic conditions. The impact of one or more of the foregoing or other factors may cause our operating results to vary significantly.
Changes in accounting principles may cause previously unanticipated fluctuations in our financial results, and the implementation of such changes may impact our ability to meet our financial reporting obligations.
We prepare our financial statements in conformity with accounting principles generally accepted in the U.S. (“U.S. GAAP”), which are subject to interpretation or changes by the FASB, the SEC, and other various bodies formed to promulgate and interpret appropriate accounting principles. New accounting pronouncements and changes in accounting principles have occurred in the past and are expected to occur in the future which may have a significant effect on our financial results. Furthermore, any difficulties in implementation of changes in accounting principles, including the ability to modify our accounting systems, could cause us to fail to meet our financial reporting obligations, which could result in regulatory discipline and harm investors’ confidence in us.
59
If our estimates or judgments relating to our critical accounting policies prove to be incorrect, our business, financial condition, and results of operations could be adversely affected.
The preparation of financial statements in conformity with U.S. GAAP and our key metrics require management to make estimates and assumptions that affect the amounts reported in the Consolidated financial statements and accompanying notes and amounts reported in our key metrics. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, as provided in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” The results of these estimates form the basis for making judgments about the carrying values of assets, liabilities, and equity and the amount of revenue and expenses that are not readily apparent from other sources. Significant assumptions and estimates used in preparing our Consolidated financial statements include those related to allowance for doubtful accounts, assessment of the useful life and recoverability of long-lived assets, fair value of guarantees included in revenue arrangements and fair values of stock-based awards, warrants, contingent consideration, and income taxes. Our results of operations may be adversely affected if our assumptions change or if actual circumstances differ from those in our assumptions, which could cause our results of operations to fall below the expectations of securities analysts and investors, resulting in a decline in the trading price of our common stock.
Risks Related to our Common Stock
Anti-takeover provisions contained in our certificate of incorporation as well as provisions of Delaware law, could impair a takeover attempt.
Our certificate of incorporation contains provisions that may discourage unsolicited takeover proposals that stockholders may consider to be in their best interests. We are also subject to anti-takeover provisions under Delaware law, which could delay or prevent a change of control. Together these provisions may make more difficult the removal of management and may discourage transactions that otherwise could involve payment of a premium over prevailing market prices for our securities. These provisions include:
As a Delaware corporation, we are also subject to provisions of Delaware law, including Section 203 of the DGCL, which prevents some stockholders holding more than 15% of our outstanding common stock from engaging in certain business combinations without approval of the holders of substantially all of our common stock. Any provision of our certificate of incorporation, bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of common stock and could also affect the price that some investors are willing to pay for our common stock.
The market price of our securities may be volatile, which could cause the value of any investment in our securities to decline.
The price of our securities may fluctuate significantly due to general market and economic conditions. An active trading market for our securities may not develop or, if developed, it may not be sustained. In addition, fluctuations in the price of our securities could contribute to the loss of all or part of your investment. Even if an active market for our securities develops and continues, the trading price of our securities could be volatile and subject to wide fluctuations in response to various factors, some of which are beyond our control. Any of the factors listed below could have a material adverse effect on an investment in our securities and our securities may trade at prices significantly below the price paid for them. In such circumstances, the trading price of our securities may not recover and may experience a further decline. Factors affecting the trading price of our securities may include, but are not solely limited to, the risk factors identified herein.
60
The stock market in general, and Nasdaq and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation, if instituted, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results, or financial condition. These market and industry factors may materially reduce the market price of our common stock and warrants regardless of our operating performance.
An investment in our common stock is extremely speculative and there can be no assurance of any return on any such investment.
An investment in our common stock is extremely speculative and there is no assurance that investors will obtain any return on their investment. Investors will be subject to substantial risks involved in an investment in us, including the risk of losing their entire investment.
There can be no assurance that we will be able to comply with the continued listing standards of Nasdaq.
If Nasdaq delists our securities from trading on its exchange for failure to meet their continued listing standards, we and our stockholders could face significant negative consequences including:
Nasdaq previously notified the Company that, due to the average closing price of our common stock, it was below the trading price criteria of the exchange. In order to regain compliance, we effected a reverse stock split of our common stock at a ratio of 1-for-10, in January 2024 (the “Reverse Stock Split”). We are no longer considered below the minimum share price continued listing criterion. The Reverse Stock Split may adversely affect the liquidity of the shares of our common stock given the reduced number of shares outstanding following the reverse split, especially if the reverse split-adjusted market price of our common stock does not generate greater investor interest. Furthermore, there can be no assurance that such reverse split will continue to be sufficient to satisfy the minimum share price requirement.
Because we have no current plans to pay cash dividends on our common stock for the foreseeable future, investors may not receive any return on their investment unless they sell their common stock for a price greater than the price paid.
We may retain future earnings, if any, for future operations, expansion and debt repayment and have no current plans to pay any cash dividends for the foreseeable future. Any decision to declare and pay dividends as a public company in the future will be made at the discretion of our Board and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions and other factors that our Board may deem relevant. As a result, investors may not receive any return on an investment in our common stock unless they sell the common stock for a price greater than the price paid.
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock. Sales of significant number of shares of our common stock may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that it deems reasonable or appropriate and make it more difficult for you to sell shares of our common stock. Certain holders of our securities are entitled to rights with respect to the registration of the shares of our common stock under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
61
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital may be needed in the future to continue our planned operations, including conducting clinical trials, commercialization efforts, expanded research and development activities and costs associated with operating as a public company. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner it determines from time to time. We may also sell our common stock as part of entering into strategic alliances, creating joint ventures or collaborations or entering into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts. If we raise additional funds through further issuances of equity or convertible debt securities, our existing stockholders could suffer dilution, and any new equity securities we issue could have rights, preferences, and privileges superior to those of holders of our common stock. Any debt financing secured by us in the future could involve restrictive covenants relating to our capital raising activities and other financial and operational matters. In addition, we may not be able to obtain additional financing on terms favorable to us, if at all. If we are unable to obtain adequate financing or financing on terms satisfactory to us, when we require it, our ability to continue to support our business growth and to respond to business challenges could be significantly limited.
On January 26, 2024, we entered into a Controlled Equity Offering℠ Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”), relating to shares of our common stock. In accordance with the terms of the Sales Agreement, we may offer and sell shares of our common stock having an aggregate offering price of up to $20,000,000 from time to time through Cantor, acting as our sales agent. As of the date hereof, we have not offered or sold any shares of common stock pursuant to the Sales Agreement.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements or insufficient disclosures due to error or fraud may occur and not be detected.
We have a significant number of (i) warrants which are currently exercisable for shares of our common stock or shares of preferred stock convertible into shares of our common stock, and (ii) shares of preferred stock convertible into shares of common stock, and the exercise or conversion thereof would increase the number of shares eligible for future resale in the public market and result in dilution to our stockholders.
On January 15, 2022, outstanding warrants to purchase an aggregate of 5,958,600 shares of our common stock (595,860 shares following the Reverse Stock Split) became exercisable, in accordance with the terms of the warrant agreement governing those securities. The exercise price of these warrants is $115.00 per share following the Reverse Stock Split. To the extent such warrants are exercised, additional shares of our common stock will be issued, which will result in dilution to the holders of shares of our common stock and increase the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market or the fact that such warrants may be exercised could adversely affect the market price of our common stock.
On November 28, 2023, we registered up to 344,626,967 shares of our common stock (34,462,696 shares following the Reverse Stock Split), in connection with a private placement of securities consummated in October 2023. The shares of common stock offered for resale by these selling stockholders represented approximately 658.7% of our total common stock outstanding as of October 30, 2023, and represents approximately 373.6% of our total common stock outstanding as of March 15, 2024 (reflecting subsequent conversions of preferred stock and the Reverse Stock Split). Although each stockholder for whom the shares of common stock registered for resale is not permitted to convert their Preferred Stock into shares of common stock to the extent that after giving effect to such conversion, such holder would (together with such holder’s affiliates and related parties) beneficially own in excess of 4.99% (or 9.99% at the election of the holder) of the shares of common stock outstanding immediately after giving effect to such conversion, the market price of our common stock could decline if the holders of such shares sell them over time or are perceived by the market as intending to sell them.
62
Risks Related to Capital Markets
If securities or industry analysts do not publish research or reports about our business or publish negative reports, the market price of our common stock could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If regular publication of research reports ceases, we could lose visibility in the financial markets, which in turn could cause the market price or trading volume of our common stock to decline. Moreover, if one or more of the analysts who cover us downgrade our common stock or if reporting results do not meet their expectations, the market price of our securities could decline.
Reports published by analysts, including projections in those reports that differ from our actual results, could adversely affect the price and trading volume of our common stock.
Securities research analysts may establish and publish their own periodic projections for us. These projections may vary widely and may not accurately predict the results we actually achieve. The price of our common stock may decline if our actual results do not match the projections of these securities research analysts. Similarly, if one or more of the analysts who write reports on us downgrades our stock or publishes inaccurate or unfavorable research about our business, the price of our common stock could decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, the price or the trading volume of our common stock could decline.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our securities may be volatile and, in the past, companies that have experienced volatility in the market price of their securities have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert management’s attention from other business concerns, which could seriously harm our business.
Risks Related to Tax
Changes in legislation in U.S. and foreign taxation of international business activities or the adoption of other tax reform policies, as well as the application of such laws, could adversely impact our financial position and operating results.
As we expand the scale of our business activities, any changes in the U.S. or foreign taxation of such activities may increase our worldwide effective tax rate and harm our business, results of operations, and financial condition. For example, the Biden administration has proposed changes to federal income tax laws that would, among other things, impose a 15% minimum tax on corporate book income for certain taxpayers and strengthen the global intangible low-taxed income regime imposed by the Tax Cuts and Jobs Act of 2017 while eliminating related tax exemptions. The impact of future changes to U.S. and foreign tax law on our business is uncertain and could be adverse, and we will continue to monitor and assess the impact of any such changes.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
Risk management and strategy
We recognize the critical importance of developing, implementing, and maintaining cybersecurity measures to safeguard our information systems and protect the confidentiality, integrity, and availability of our data.
Managing Material Risks & Integrated Overall Risk Management
We have implemented tools, processes, and strategies to promote a company-wide culture of cybersecurity risk management. This ensures that cybersecurity considerations are integrated into our decision-making processes to monitor and manage risk. Our IT Department works closely with our leadership and key operating personnel to evaluate and address cybersecurity risks in alignment with our business objectives and operational needs.
63
Engage Third-parties on Risk Management
Due to the complexity and evolving nature of cybersecurity threats, we engaged with a cybersecurity assessment firm as an external expert, to evaluate and test our risk management systems. This partnership enables us to leverage specialized knowledge and insights, of dedicated cybersecurity firms. Our collaborations with this third-party include regular system audits, threat assessments, 24-hour monitoring, and consultation on security enhancements.
Oversee Third-party Risk
Because we are aware of the risks associated with third-party service providers, we conduct security assessments of all third-party providers before engagement to ensure compliance with industry cybersecurity standards and frameworks. This includes assessments performed by our Senior Director of IT, who oversees the Company’s cybersecurity function.
Risks from Cybersecurity Threats
We have not encountered cybersecurity challenges that have materially impaired our operations or financial standing. Although we have not experienced cybersecurity incidents, a significant cybersecurity incidence could reasonably have a material adverse effect as against us, such as malware or ransomware attacks or DoS attacks, which could lead to business disruptions, unplanned downtimes or outages, particularly in critical systems or services, may impact our ability to operate efficiently, affecting business continuity.
Governance
We have implemented standard operating procedures to define the channels by which cybersecurity threats are communicated to the Board. This ensures that Board has oversight and effective governance in managing risks associated with cybersecurity threats.
Board of Directors Oversight
The Audit Committee is central to the Board’s oversight of cybersecurity risks and bears the primary responsibility for this domain. The Audit Committee is composed of board members with diverse expertise including, risk management, and finance, which we believe equips them to oversee cybersecurity and other risks effectively.
Management’s Role Managing Risk
The Senior Director of IT plays a pivotal role in informing the Audit Committee on cybersecurity risks. This role provides briefings to the Audit Committee on a regular basis, with a minimum frequency of once per year. These briefings encompass a broad range of topics, including:
Risk Management Personnel
Primary responsibility for assessing, monitoring and managing our cybersecurity risks rests with the Senior Director of IT and department staff. Our IT team oversees our governance programs, tests our compliance with standards, remediates known risks, stays informed of significant developments in the cybersecurity domain, and leads our employee training program.
Monitor Cybersecurity Incidents
The Senior Director of IT is continually informed about the latest developments in cybersecurity, including potential threats and innovative risk management techniques. This ongoing knowledge acquisition is crucial for the effective prevention, detection, mitigation, and remediation of cybersecurity incidents. Under his direction, the IT department implements and oversees processes for the regular monitoring of our information systems. This includes the deployment of advanced security measures and regular system audits to identify potential vulnerabilities. In the event of a cybersecurity incident, the IT department is equipped with a well-defined written procedure. This plan includes immediate actions to mitigate the impact and long-term strategies for remediation and prevention of future incidents.
Reporting to Board of Directors
The Senior Director of IT consistently communicates with the Audit Committee regarding critical cybersecurity risks and incidents, ensuring that the organization's highest governance bodies remain well-informed about our cybersecurity status and
64
potential vulnerabilities. Moreover, matters of significant cybersecurity importance, along with strategic risk management decisions, are promptly escalated to the Board of Directors. This process ensures that the Board maintains thorough oversight and is equipped to offer informed guidance on critical cybersecurity issues.
Item 2. Properties.
Research Center
As of December 31, 2023, our facilities include current Good Manufacturing Practice (cGMP) operations where drug products are manufactured in the clinical manufacturing facility located within the 60,000 square foot laboratory bay at the Sanford Research Center (the “Research Center”) in Sioux Falls, South Dakota encompassing a 17,300 square foot manufacturing area that includes the clinical manufacturing facility, -20°C plasma storage, and a controlled warehouse.
The Research Center lease is currently set to expire in August 2024.
TC Cattle Facility
Transchromosomic (Tc) cattle used for hyperimmunization, and plasma collection are housed at our animal facilities which we refer to as the “Pharm”. The Pharm is a biosecure site dedicated to housing and rearing these animals. The physical surroundings are maintained in accordance with various governmental regulations. This site also includes surgical suite and plasma collection areas. Facilities are appropriate for cattle housing and give adequate protection from inclement weather conditions. Double barrier fencing (perimeter fencing and locked exterior gating) is designed to prevent Tc cattle from escaping or other unwanted animals from entering. Production animal pens consist of concrete feeding floors, water fountains and outdoor dirt lots. A biosecurity program is critical to the production of human pharmaceuticals from animals. The production herd is considered “closed” from a biosecurity perspective and inputs (feed, nutritional additives, medications, etc.) and outputs to the system are carefully monitored according to the appropriate regulations. A pest control program is instituted to control vermin. The biosecurity program is managed using a combination of procedural controls, facility design features (such as barriers, fencing and housing), controlled access and employee training into or out of the site. Tc Bovine plasma is collected from the animals in designated areas at the Pharm. The areas are cleaned and maintained per the rules and regulations of the FDA. The Fenwal Auto-C plasmapheresis machine (human device) is used to collect plasma. Plasma is collected aseptically under standard sanitary conditions using a closed system and sterile bags to avoid microbial contamination. Following plasmapheresis, the plasma bioprocessing bags are labeled and shipped to the Company’s manufacturing facility or to contract manufacturers.
The Pharm real property lease in Canton, South Dakota is currently set to expire in November 2038.
Corporate Headquarters
The Company leases its corporate headquarters located at 2100 East 54th Street North, Sioux Falls, SD 57104. The lease covers approximately 49,600 square feet of office and laboratory space, with approximately 18,400 square feet of space dedicated to research and development activities. The Company believes that its existing facilities and other available properties will be sufficient for its needs for the foreseeable future.
Item 3. Legal Proceedings.
We are not currently a party to any material litigation, nor are we aware of any pending or threatened litigation against us that we believe would materially affect our business, operating results, financial condition, or cash flows. Participants in our industry face frequent claims and litigation, including securities litigation, claims regarding patent and other intellectual property rights, and other liability claims. As a result, we may be involved in various legal proceedings from time to time in the future.
Item 4. Mine Safety Disclosures.
Not Applicable.
65
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock and warrants are listed on Nasdaq under the symbols “SABS” and “SABSW”, respectively. On March 18, 2024, the closing price of our common stock was $5.40 per share and the closing price of our warrants was $0.0301 per warrant.
Holders of Our Common Stock
As of March 18, 2024, we had 260 holders of record of our common stock. Certain shares are held in “street” name and accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We currently intend to retain all available funds and any future earnings to fund the growth and development of our business. We have never declared or paid any cash dividends on our capital stock. We do not intend to pay cash dividends to our stockholders in the foreseeable future. Investors should not purchase our common stock with the expectation of receiving cash dividends.
Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions, and other factors that our board of directors may deem relevant.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required by Item 5 of Form 10-K regarding equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Form 10-K.
Recent Sales of Unregistered Securities
All sales of unregistered securities by us during the year ended December 31, 2023 have been included previously in a Quarterly Report on Form 10-Q or in a Current Report on Form 8-K.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Item 6. [Reserved].
66
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this Annual Report. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below and those discussed in “Risk Factors” included elsewhere in this Annual Report. As used in this report, unless the context suggests otherwise, “we,” “us,” our” or “the Company” refer to SAB Biotherapeutics, Inc. and its subsidiaries.
Overview
We are a clinical-stage, biopharmaceutical company focused on the development of powerful and proprietary immunotherapeutic polyclonal human antibodies to treat and prevent infectious diseases and immune and autoimmune disorders, including infectious diseases resulting from outbreaks and pandemics as well as immunology, gastroenterology, and respiratory diseases that have significant mortality and health impacts on immunocompromised patients. We have applied advanced genetic engineering and antibody science to develop transchromosomic (Tc) Bovine™. Our novel immunotherapy platform that is developing fully-human hIgC for delaying the onset or progression of T1D.
We are advancing clinical programs in two indications, and preclinical development in three indications. In addition, we are executing on two research collaborations with global pharmaceutical companies, including CSL Behring and an undisclosed collaboration.
We formed SAB Australia, in order to qualify for the Australian government’s research and development tax credit for research and development dollars spend in Australia. The primary purpose of SAB Australia is to conduct clinical trials for SAB-142. We started phase 1 trials in the fourth quarter of 2023.
We generated total revenue of $2.2 million and $23.9 million for the years ended December 31, 2023 and 2022, respectively (90.6% decline). Our revenue to date has been primarily derived from government grants.
We plan to focus a substantial portion of our resources on continued research and development efforts towards deepening our technology and expertise with our platform and as well as indications in infectious disease and autoimmune indications. As a result, we expect to continue to make significant investments in these areas for the foreseeable future. We incurred research and development expenses of $16.5 million and $36.4 million for the years ended December 31, 2023 and 2022, respectively, and general and administrative expenses of $23.8 million and $16.4 million for the years ended December 31, 2023 and 2022, respectively. We expect to continue to incur significant expenses, and we expect such expenses to increase substantially in connection with our ongoing activities, including as we:
To date, we have primarily financed our operations from government agreements and the issuance and sale of common stock and preferred stock.
Our net loss for the year ended December 31, 2023, was $42.2 million and our net loss for the year ended December 31, 2022 was $18.7 million. As of December 31, 2023, we had an accumulated deficit of $90.1 million, and cash and cash equivalents totaling $56.6 million.
Recent Developments
Effective January 5, 2024, we filed articles of amendment to our articles of incorporation to affect a one-for-ten reverse split of our issued and outstanding shares of Common Stock. All references to common stock, warrants and options to purchase
67
common stock, including per share data and related information contained in the accompanying Consolidated Financial Statements have been retroactively adjusted to reflect the effect of the Reverse Stock Split for all periods presented.
Key Factors Affecting Our Results of Operations and Future Performance
We believe that our financial performance has been, and in the foreseeable future will continue to be, primarily driven by multiple factors as described below, each of which presents growth opportunities for our business. These factors also pose important challenges that we must successfully address in order to sustain our growth and improve our results of operations. Our ability to successfully address these challenges is subject to various risks and uncertainties, including those described in Part I, Item 1A of this Form 10-K.
Components of Results of Operations
Revenue
Our revenue has historically been generated through grants from government and other (non-government) organizations. We currently have no commercially-approved products.
Grant revenue is recognized for the period that the research and development services occur, as qualifying expenses are incurred or conditions of the grants are met. We concluded that payments received under these grants represent conditional, nonreciprocal contributions, as described in ASC 958, Not-for-Profit Entities, and that the grants are not within the scope of ASC 606, Revenue from Contracts with Customers, as the organizations providing the grants do not meet the definition of a customer. Expenses for grants are tracked by using a project code specific to the grant, and the employees also track hours worked by using the project code.
For the years ended December 31, 2023 and 2022, we received the following grants:
Government grants
The total revenue for government grants was approximately $2.2 million and $23.9 million, respectively, for the years ended December 31, 2023 and 2022.
National Institute of Health – National Institute of Allergy and Infectious Disease (“NIH-NIAID”) (Federal Award #1R44AI117976-01A1) – this grant was for $1.4 million and started in September 2019 through August 2021. This grant was subsequently amended to extend the end date to August 2022. No grant income was recognized for the year ended December 31, 2023. For the year ended December 31, 2022 there was approximately $182 thousand in grant income recognized from this grant. This grant was completed in 2022.
NIH-NIAID (Federal Award #1R41AI131823-02) – this grant was for approximately $1.5 million and started in April 2019 through March 2021. The grant was subsequently amended to extend the date through March 2023. For the years ended December 31, 2023 and 2022, approximately $192 thousand and $328 thousand, respectively, in grant income was recognized from this grant. This grant was completed as of June 30, 2023.
NIH-NIAID through Geneva Foundation (Federal Award #1R01AI132313-01, Subaward #S-10511-01) – this grant was for approximately $2.7 million and started in August 2017 through July 2021. The grant was subsequently amended to extend the end date to July 2023. For the years ended December 31, 2023 and 2022, there was approximately $273 thousand and $1.1 million, respectively, in grant income recognized from this grant. This grant was completed as of June 30, 2023.
Department of Defense (“DoD”), Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense Enabling Biotechnologies (“JPEO”) through Advanced Technology International – this grant was for a potential of $25 million, awarded in stages starting in August 2019 and with potential stages running through February 2023. Additional contract modifications were added to this contract in 2020 and 2021 for work on a COVID therapeutic, bringing the contract total to $203.6 million. For the years ended December 31, 2023 and 2022, there was approximately $1.8 million and $22.2 million, respectively, in grant income recognized from this grant. This grant was terminated in 2022.
The grants for the JPEO Rapid Response contract are cost reimbursement agreements, with reimbursement of qualified direct research and development expense (labor and consumables) with an overhead charge (based on actual, reviewed quarterly) and a fixed fee (9%).
On August 3, 2022, we received notice from the DoD terminating the JPEO Rapid Response contract (the “JPEO Rapid Response Contract Termination”). We engaged in negotiations with the DoD to compensate us for services provided prior to the JPEO Rapid Response Contract Termination and costs we would be expected to bear in future periods. A termination and settlement proposal was submitted to the DoD on September 9, 2022; we submitted a final invoice on December 15, 2022;
68
and received payment from the DoD on or about January 12, 2023. The terms of the arrangement provide for a cost-reimbursable structure, and state that the parties will work in good faith equitable reimbursement for work performed toward accomplishment of tasks provided in the agreement. At this time, other than certain deferred obligations (presented within deferred grant income within our consolidated balance sheet) potentially payable to the DoD solely due to subsequent negotiations with third-party vendors, we believe and have been advised there is a reasonable, good faith basis for the position that no present or future obligations exist. Revenue recognized subsequent to the JPEO Rapid Response Contract Termination relates to satisfaction of residual obligations under the termination and settlement agreement—see Note 2, Summary of Significant Accounting Policies for further information about our established revenue recognition process.
Operating Expenses
Research and Development Expenses
Research and development expenses primarily consist of salaries, benefits, incentive compensation, stock-based compensation, laboratory supplies and materials for employees and contractors engaged in research and product development, licensing fees to use certain technology in our research and development projects, fees paid to consultants and various entities that perform certain research and testing on our behalf. Research and development expenses are tracked by target/project code. Indirect general and administrative costs are allocated based upon a percentage of direct costs. We expense all research and development costs in the period in which they are incurred.
Research and development activities consist of discovery research for our platform development and the various indications we are working on. We have not historically tracked our research and development expenses on a product candidate-by-product candidate basis.
For the years ended December 31, 2023 and 2022, we had contracts with multiple CROs to conduct and complete clinical studies. In the case of SAB-185, the CRO has been contracted and paid by the US government. For SAB-176, PPD Development, LP, acting as CRO oversaw the Phase 1 safety study. The terms of that agreement are subject to confidentiality, and the status of the agreement is that it is current, in good standing and 100% of the contract has been paid as of December 31, 2023. SAB has also contracted with hVIVO Services Limited to conduct the Phase 2a influenza study on SAB-176. The terms of that agreement are subject to confidentiality, and the status of the agreement is that it is current, in good standing and 100% of the contract has been paid as of December 31, 2023. For SAB-142, Avance, acting as CRO oversaw Phase 1 safety study. This study started in December 2023 and the terms of that agreement are subject to confidentiality and the status of the agreement is that it is current.
We expect to continue to incur substantial research and development expenses as we conduct discovery research to enhance our platform and work on our indications. We expect to hire additional employees and continue research and development and manufacturing activities. As a result, we expect that our research and development expenses will continue to increase in future periods and vary from period to period as a percentage of revenue.
Major components within our research and development expenses are salaries and benefits (laboratory & farm), laboratory supplies, animal care, contract manufacturing, clinical trial expense, outside laboratory services, project consulting, and facility expense. Our platform allows us to work on multiple projects with the same resources, as the research and development process of each product is very similar (with minimal differences in the manufacturing process).
Research and development expenses by component for the years ended December 31, 2023 and 2022 were as follows:
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Salaries & benefits |
|
$ |
6,623,281 |
|
|
$ |
12,032,720 |
|
Laboratory supplies |
|
|
1,006,756 |
|
|
|
6,441,181 |
|
Animal care |
|
|
936,192 |
|
|
|
1,560,099 |
|
Contract manufacturing |
|
|
388,518 |
|
|
|
5,256,518 |
|
Clinical trial expense |
|
|
809,678 |
|
|
|
271,283 |
|
Outside laboratory services |
|
|
987,613 |
|
|
|
4,561,696 |
|
Project consulting |
|
|
371,235 |
|
|
|
805,994 |
|
Facility expense |
|
|
5,278,702 |
|
|
|
5,354,356 |
|
Other expenses |
|
|
113,030 |
|
|
|
154,666 |
|
Total research and development expenses |
|
$ |
16,515,005 |
|
|
$ |
36,438,513 |
|
69
General and Administrative Expenses
General and administrative expenses primarily consist of salaries, benefits and stock-based compensation costs for employees in our executive, accounting and finance, project management, corporate development, office administration, legal and human resources functions as well as professional services fees, such as consulting, audit, tax and legal fees, general corporate costs and allocated overhead expenses. General and administrative expenses also include rent and facilities expenses allocated based upon total direct costs. We expect that our general and administrative expenses will continue to increase in future periods, primarily due to increased headcount to support anticipated growth in the business and due to incremental costs associated with operating as a public company, including costs to comply with the rules and regulations applicable to companies listed on a securities exchange and costs related to compliance and reporting obligations pursuant to the rules and regulations of the SEC and stock exchange listing standards, public relations, insurance and professional services. We expect these expenses to vary from period to period in absolute terms and as a percentage of revenue.
Nonoperating (Expense) Income
Gain (loss) on change in fair value of warrant liabilities
Gain (loss) on change in fair value of warrant liabilities consists of the changes in the fair value of the warrant liabilities.
Other income
Other income primarily consists of income associated with the refundable portion of Australian research and development tax credits.
Interest income
Interest income consists of interest earned on cash balances in our bank accounts.
Interest expense
Interest expense consists primarily of interest related to borrowings under notes payable for equipment, abated rent, and insurance financing.
Results of Operations
The following tables set forth our results of operations for the years ended December 31, 2023 and 2022:
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Revenue |
|
|
|
|
|
|
||
Grant revenue |
|
$ |
2,238,991 |
|
|
$ |
23,904,181 |
|
Total revenue |
|
|
2,238,991 |
|
|
|
23,904,181 |
|
Operating expenses |
|
|
|
|
|
|
||
Research and development |
|
|
16,515,005 |
|
|
|
36,438,513 |
|
General and administrative |
|
|
23,799,306 |
|
|
|
16,383,285 |
|
Total operating expenses |
|
|
40,314,311 |
|
|
|
52,821,798 |
|
Loss from operations |
|
|
(38,075,320 |
) |
|
|
(28,917,617 |
) |
Other income (expense) |
|
|
|
|
|
|
||
Changes in fair value of warrant liabilities |
|
|
(4,823,237 |
) |
|
|
10,399,200 |
|
Interest expense |
|
|
(315,284 |
) |
|
|
(301,584 |
) |
Interest income |
|
|
584,966 |
|
|
|
71,072 |
|
Other income |
|
|
435,089 |
|
|
|
33,754 |
|
Total other income (expense) |
|
|
(4,118,466 |
) |
|
|
10,202,442 |
|
Loss before income taxes |
|
|
(42,193,786 |
) |
|
|
(18,715,175 |
) |
Income tax expense (benefit) |
|
|
— |
|
|
|
25,629 |
|
Net loss |
|
$ |
(42,193,786 |
) |
|
$ |
(18,740,804 |
) |
70
Comparison of the Years Ended December 31, 2023 and 2022
Revenue
|
|
Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Revenue |
|
$ |
2,238,991 |
|
|
$ |
23,904,181 |
|
|
$ |
(21,665,190 |
) |
|
|
(90.6 |
)% |
Total revenue |
|
$ |
2,238,991 |
|
|
$ |
23,904,181 |
|
|
|
|
|
|
|
||
Revenue decreased by $21.7 million, or 90.6%, in 2023, primarily due to the JPEO Rapid Response Contract Termination. Included in revenues for the year ended December 31, 2023, are amounts for billable costs related to closeout activities and charges of $0.1 million for labor, $0.8 million for supplies, and $1.3 million for outside research manufacturing services, as compared to $3.1 million for labor, $5.4 million for supplies, and $5.3 million for outside research manufacturing services for the year ended December 31, 2022.
Research and Development
|
|
Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Research and development |
|
$ |
16,515,005 |
|
|
$ |
36,438,513 |
|
|
$ |
(19,923,508 |
) |
|
|
(54.7 |
)% |
Total research and development expenses |
|
$ |
16,515,005 |
|
|
$ |
36,438,513 |
|
|
|
|
|
|
|
||
Research and development expenses decreased by $19.9 million, or 54.7%, for the year ended December 31, 2023 as compared to the year ended December 31, 2022, primarily due to decreases in laboratory supplies (year-over-year decrease of $5.4 million, 84.4%), contract manufacturing costs (year-over-year decrease of $4.9 million, 92.6%), salaries and benefits (year-over-year decrease of $5.4 million, 45.0%), outside lab services due to the JPEO Rapid Response Contract Termination (year-over-year decrease of $3.6 million, 78.3%), project consulting (year-over-year decrease of $0.4 million, 53.9%) and offset by overhead costs (year-over-year increase of $0.1 million, 1.7%).
General and Administrative
|
|
Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
General and administrative |
|
$ |
23,799,306 |
|
|
$ |
16,383,285 |
|
|
$ |
7,416,021 |
|
|
|
45.3 |
% |
Total general and administrative expenses |
|
$ |
23,799,306 |
|
|
$ |
16,383,285 |
|
|
|
|
|
|
|
||
General and administrative expenses increased by $7.4 million, or 45.3%, for the year ended December 31, 2023, as compared to the year ended December 31, 2022, primarily due to other administrative support fees relating to IT, human resources, and legal (year-over-year increase of $7.7 million, 131.5%), and salaries and benefits (year-over-year increase of $2.2 million, 35.5%), offset by insurance costs (year-over-year decrease of $1.4 million, 51.5%), project consulting (year-over-year decrease of $1.2 million, 73.6%). We anticipate that our general and administrative expenses will increase in the future as we increase our headcount to support our continued research activities and development of our product candidates. We also anticipate that we will incur increased accounting, audit, legal, regulatory, compliance, director and officer insurance costs as well as investor and public relations expenses associated with being a public company.
Non-operating (Expense) Income
|
|
Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Changes in fair value of warrant liabilities |
|
$ |
(4,823,237 |
) |
|
$ |
10,399,200 |
|
|
$ |
(15,222,437 |
) |
|
|
(146.4 |
)% |
Other income |
|
|
435,089 |
|
|
|
33,754 |
|
|
|
401,335 |
|
|
|
1,189.0 |
% |
Total non-operating income |
|
$ |
(4,388,148 |
) |
|
$ |
10,432,954 |
|
|
|
|
|
|
|
||
Total non-operating income decreased by $14.8 million, or 142.1% for the year ended December 31, 2023 as compared to the year ended December 31, 2022 primarily due to changes in the fair value of the warrant liabilities (year-over-year decrease of $15.2 million, 146.4%), primarily offset by the Australian research and development tax credit of $0.3 million.
71
Interest Expense
|
|
Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Interest expense |
|
$ |
315,284 |
|
|
$ |
301,584 |
|
|
$ |
13,700 |
|
|
|
4.5 |
% |
Total interest expense |
|
$ |
315,284 |
|
|
$ |
301,584 |
|
|
|
|
|
|
|
||
Interest expense increased for the year ended December 31, 2023 as compared to the year ended December 31, 2022, primarily due to the 8% Unsecured Convertible Note accrued interest payable realized over a full year.
Interest Income
|
|
Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Interest income |
|
$ |
584,966 |
|
|
$ |
71,072 |
|
|
$ |
513,894 |
|
|
|
723.1 |
% |
Total interest income |
|
$ |
584,966 |
|
|
$ |
71,072 |
|
|
|
|
|
|
|
||
Interest income increased by $514 thousand, or 723.1% for the year ended December 31, 2023 as compared to the year ended December 31, 2022, primarily due to higher interest rates and interest earning cash balances.
Liquidity and Capital Resources
As of December 31, 2023 and December 31, 2022, we had $56.6 million and $15.0 million, respectively, of cash and cash equivalents.
We intend to continue to invest in our business and, as a result, may incur operating losses in future periods. We expect to continue to invest in research and development efforts towards expanding our capabilities and expertise along our platform and the primary pipeline development targets we are working on, as well as building our business development team and marketing our solutions to partners in support of the growth of the business.
We anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of, and seek regulatory approvals for, our product candidates, and begin commercialization of our products. As a result, we will require additional capital to fund our operations in order to support our long-term plans.
We have incurred operating losses for the past several years. While we intend to continue to keep operating expenses at a reduced level there can be no assurance that our current level of operating expenses will not increase or that other uses of cash will not be necessary. Based on our current level of operating expenses, existing resources will be sufficient to cover operating cash needs through the twelve months following the date these financials are made available for issuance. We intend to seek additional capital through equity and/or debt financings, collaborative or other funding arrangements. Should we seek additional financing from outside sources, we may not be able to raise such financing on terms acceptable to us or at all. If we are unable to raise additional capital when required or on acceptable terms, we may be required to scale back or discontinue the advancement of product candidates, reduce headcount, liquidate our assets, file for bankruptcy, reorganize, merge with another entity, or cease operations.
Sources of Liquidity
Since our inception, we have financed our operations primarily from revenue in the form of government grants and from equity financings.
Equity Financings and Option Exercises
As of December 31, 2023, we have raised approximately $157.4 million since our inception from the issuance and sale of convertible preferred shares, net of issuance costs associated with such financings, the merger transaction in 2021, proceeds from private placements of securities, and exercises of employee stock options.
72
Notes payable
8% Unsecured Convertible Note
Pursuant to the Fourth Amendment to our lease with Sanford Health, we agreed to a period of abated rent (“Abated Rent”) from October 1, 2022 to September 30, 2023 pertaining to our leased laboratory bay at the Research Center. In exchange for the Abated Rent, effective as of October 1, 2022, we issued to Sanford Health an 8% unsecured, convertible promissory note (the “8% Unsecured Convertible Note”).
Pursuant to the 8% Unsecured Convertible Note, we shall pay the sum of approximately $542 thousand plus accrued and unpaid interest thereon on September 30, 2024. Simple interest shall accrue on the outstanding Principal from and after the date of the 8% Unsecured Convertible Note and shall be payable on September 31, 2024 (the “Maturity Date”). Sanford Health shall have the right, but not the obligation, to convert all or any part of the outstanding Principal of the 8% Unsecured Convertible Note, together with any accrued and unpaid interest thereon to the date of such conversion, into such number of fully paid and non-assessable shares of our common stock, at any time and from time to time, prior to the later of the Maturity Date and the date on which the 8% Unsecured Convertible Note is paid in full, subject to certain restrictions, at a conversion price per share of common stock equal to greater of (x) $15.00 and (y) the price at which the we sells shares of common stock in any bona fide private or public equity financing prior to the Maturity Date.
Insurance Financing
We obtained financing for certain Director & Officer liability insurance policy premiums. The agreement assigns First Insurance Funding (the “Lender”) a first priority lien on and security interest in the financed policies and any additional premium required in the financed policies including (a) all returned or unearned premiums, (b) all additional cash contributions or collateral amounts assessed by the insurance companies in relation to the financed policies and financed by Lender, (c) any credits generated by the financed policies, (d) dividend payments, and (e) loss payments which reduce unearned premiums. If any circumstances exist in which premiums related to any Financed Policy could become fully earned in the event of loss, Lender shall be named a loss-payee with respect to such policy.
The total premiums, taxes and fees financed is approximately $0.8 million with an annual interest rate of 7.96% and approximately $1.2 million with an annual interest rate 5.47% for the years ended December 31, 2023 and 2022, respectively. In consideration of the premium payment by Lender to the insurance companies or the agent or broker, we unconditionally promise to pay lender the amount financed plus interest and other charges permitted under the agreement. We paid the financing through installment payments with the last payment for the current note being September 22, 2023. At December 31, 2023 and 2022 we recognized approximately $509 thousand and $773 thousand, respectively as an insurance financing note payable in our consolidated financial statements. We will pay the insurance financing through installment payments with the last payment for the current note being on September 22, 2024.
Please refer to Note 9, Notes Payable, in our consolidated financial statements for additional information on our debt.
Cash Flows
The following table summarizes our cash flows for the years ended December 31, 2023 and 2022:
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Net cash used in operating activities |
|
$ |
(25,119,405 |
) |
|
$ |
(23,459,511 |
) |
Net cash used in investing activities |
|
|
(152,704 |
) |
|
|
(2,090,024 |
) |
Net cash provided by financing activities |
|
|
66,773,137 |
|
|
|
1,051,411 |
|
Effect of exchange rate changes on cash and cash equivalents |
|
|
18,144 |
|
|
|
— |
|
Net increase (decrease) in cash and cash equivalents |
|
$ |
41,519,172 |
|
|
$ |
(24,498,124 |
) |
Operating Activities
Net cash used by operating activities increased by $1.7 million in the year ended December 31, 2023 as compared to the year ended December 31, 2022, primarily due to an increase in our net loss adjusted for non-cash items of $4.2 million, offset by a decrease in cash used in operating activities related to change in our operating assets and liabilities of $2.5 million. Year-over-year changes in cash used by operating activities is explained by shifts in the working capital balances as we continue to advance our lead programs after the JPEO Rapid Response Contract Termination.
73
Investing Activities
Net cash used by investing activities decreased by $1.9 million for the year ended December 31, 2023 as compared to the year ended December 31, 2022, primarily due to a decrease in purchases of equipment. Capital asset purchases completed in 2022 relate substantially to leasehold improvements at the Company’s corporate headquarters and completion of the clinical manufacturing facility at the Research Center.
Financing Activities
Net cash provided by financing activities increased by $65.7 million for the year ended December 31, 2023 as compared to the year ended December 31, 2022, primarily due to increased proceeds from equity issuances of $59.7 million, a reduction in stock repurchases of $5.5 million, and reduced net payments on notes payable of $0.8 million.
Contractual Obligations and Commitments
We enter into contracts in the normal course of business with third parties, including CROs. These payments are not included in the table above, as the amount and timing of such payments are not known.
As of December 31, 2023, there were no material changes outside of the ordinary course of business to our commitments and contractual obligations.
Income Taxes
We had $40.8 million of federal net operating loss carryforwards as of December 31, 2023. Our carryforwards are subject to review and possible adjustment by the appropriate taxing authorities.
These carryforwards may generally be utilized in any future period but may be subject to limitations based upon changes in the ownership of our shares in a prior or future period. We have not quantified the amount of such limitations, if any.
Off-Balance Sheet Arrangements
We did not have, for the periods presented, and we do not currently have, any off-balance sheet financing arrangements or any relationships with unconsolidated entities or financial partnerships, including entities sometimes referred to as structured finance or special purpose entities, that were established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Critical Accounting Policies and Estimates
We have prepared our consolidated financial statements in accordance with U.S. GAAP. Our preparation of these consolidated financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenue, expenses and related disclosures. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 2, Summary of Significant Accounting Policies, in our consolidated financial statements, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Research and development expenses
Costs incurred in connection with research and development activities are expensed as incurred. These include licensing fees to use certain technology in our research and development projects, fees paid to consultants and various entities that perform certain research and testing on behalf of us, and expenses related to animal care, research-use equipment depreciation, salaries, benefits, and stock-based compensation granted to employees in research and development functions.
We had contracts with multiple CROs to complete studies as part of research grant agreements. These costs include upfront, milestone and monthly expenses as well as reimbursement for pass through costs. All research and development costs are expensed as incurred except when we are accounting for nonrefundable advance payments for goods or services to be used in
74
future research and development activities. In these cases, these payments are capitalized at the time of payment and expensed in the period the research and development activity is performed. As actual costs become known to us, we adjust our accrual; such changes in estimate may be a material change in our clinical study accrual, which could also materially affect our results of operations.
Revenue Recognition
Our revenue is primarily generated through grants from government and other (non-government) organizations.
Grant revenue is recognized for the period that the research and development services occur, as qualifying expenses are incurred or conditions of the grants are met. We concluded that payments received under these grants represent conditional, nonreciprocal contributions, as described in ASC 958, Not-for-Profit Entities, and that the grants are not within the scope of ASC 606, Revenue from Contracts with Customers, as the organizations providing the grants do not meet the definition of a customer. Expenses for grants are tracked by using a project code specific to the grant, and the employees also track hours worked by using the project code.
Stock-Based Compensation
We recognize compensation cost relating to stock-based payment transactions using a fair-value measurement method, which requires all stock-based payments to employees, directors, and non-employee consultants, including grants of stock options, to be recognized in operating results as compensation expense based on fair value over the requisite service period of the awards. The board of directors elected to determine the fair value of our common stock based on the closing market price at closing on the date of grant. In determining the fair value of our stock-based awards, we utilize the Black-Scholes option-pricing model, which uses both historical and current market data to estimate fair value. The Black-Scholes option-pricing model incorporates various assumptions, such as the value of the underlying common stock, the risk-free interest rate, expected volatility, expected dividend yield, and expected life of the options. For awards with performance-based vesting criteria, we estimate the probability of achievement of the performance criteria and recognize compensation expense related to those awards expected to vest. We recognized stock-based compensation expense over the expected term. Forfeitures are recorded when they occur. Stock-based compensation expense is classified in our consolidated statements of operations based on the function to which the related services are provided. We recognize stock-based compensation expense over the expected term.
See Note 11, Stock Option Plan, in our consolidated financial statements for information concerning certain specific assumptions we used in applying the Black-Scholes option pricing model to determine the estimated fair value of our stock options granted for the for the years ended December 31, 2023 and 2022.
Warrant Liabilities Valuations
Liability Classified Warrants
We are required to periodically estimate the fair value liability of our private placement warrants issued simultaneously with the closing of our initial public offering (the “Private Placement Warrant”) liabilities with the assistance of an independent third-party valuation firm. The assumptions underlying these valuations represented our best estimates, which involved inherent uncertainties and the application of significant levels of our judgment. The fair value liability of our public warrants issued upon the closing of our initial public offering (the “Public Warrants”) is determined by reference to the quoted market price.
The warrants are accounted for as liabilities in accordance with ASC 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity, and were presented within warrant liabilities on the consolidated balance sheets as of December 31, 2023 and 2022. The initial fair value of the warrant liabilities were measured at fair value on the issuance date, and changes in the fair value of the warrant liabilities were presented within changes in fair value of warrant liabilities in the consolidated statements of operations for the years ended December 31, 2023 and 2022.
Public Warrants and Private Placement Warrants
The fair value of the Private Placement Warrants was determined utilizing both the Black-Scholes Merton formula and a Monte Carlo Simulation (“MCS”) analysis. Specifically, we considered a MCS to derive the implied volatility in the publicly listed price of the Public Warrants We then considered this implied volatility in selecting the volatility for the application of a Black-Scholes Merton model for the Private Placement Warrants. We determined the fair value of the Public Warrants by reference to the quoted market price.
75
The Public Warrants were classified as a Level 1 fair value measurement, due to the use of the quoted market price, and the Private Placement Warrants held privately by assignees of Big Cypress Holdings LLC, were classified as a Level 3 fair value measurement, due to the use of unobservable inputs. See Note 12, Warrants, for further information regarding the Public Warrants and Private Placement Warrants.
The measurement as of December 31, 2023 and 2022 for the Private Placement Warrant liability was approximately $6 thousand and $10 thousand, respectively, and the change in fair value of the Private Placement Warrant liability was approximately $4 thousand and $417 thousand, for the years ended December 31, 2023 and 2022, respectively.
The key inputs into the valuations as of December 31, 2023 and 2022 were as follows:
|
|
December 31, |
|
|
December 31, |
|
||
Risk-free interest rate |
|
|
4.03 |
% |
|
|
4.00 |
% |
Expected term remaining (years) |
|
|
2.81 |
|
|
|
3.81 |
|
Implied volatility |
|
|
85.0 |
% |
|
|
82.0 |
% |
Closing common stock price on the measurement date |
|
$ |
0.69 |
|
|
$ |
0.59 |
|
September 2023 Purchase Agreement Warrants
We established fair value of the Preferred Warrants utilizing the Black-Scholes Merton formula. All tranches of the Preferred Warrants were classified as Level 3 fair value measurements, due to unobservable inputs. See Note 12, Warrants, for further information regarding the Preferred Warrants.
The initial measurement as of October 3, 2023 and the measurement of December 31, 2023 for the Preferred Warrant liability was approximately $10.9 million and $11.6 million, respectively. The change in fair value of the Preferred Warrant liability was approximately $0.7 million for the year ended December 31, 2023. In November 2023, 59,654 Tranche A Warrants with a value of $0.7 million were exercised and 10,486 Tranche A Warrants with a value of $0.1 million, 9,154 Tranche B Warrants with a value of $1.1 million and 22,885 Tranche C Warrants with a value of $2.4 million were forfeited.
The key inputs utilized in determining the fair value of each Tranche A Warrant as of the Initial Issuance Date was as follows:
|
|
October 3, 2023 |
|
|
|
Risk-free interest rate (1) |
|
|
5.58 |
% |
|
Expected term remaining (years) (1) |
|
|
0.16 |
|
|
Implied volatility |
|
|
65.0 |
% |
|
Underlying Stock Price (Preferred Series A) |
|
$ |
546.30 |
|
|
(1) |
Reflects a probability-weighted input derived from multiple Black-Scholes calculations. These calculations account for various potential dates for the public announcement of the comprehensive data set from the Sanofi S.A. Protect trial, spanning from mid-October to December 15, 2023. |
The key inputs utilized in determining the fair value of each Tranche B Warrants as of the Initial Issuance Date and December 31, 2023 were as follows:
|
|
October 3, 2023 |
|
|
December 31, |
|
||
Risk-free interest rate (1) |
|
|
2.68 |
% |
|
|
2.58 |
% |
Expected term remaining (years) (1) |
|
|
0.75 |
|
|
|
0.69 |
|
Implied volatility |
|
|
75.0 |
% |
|
|
85.0 |
% |
Underlying Stock Price (Preferred Series A) |
|
$ |
546.30 |
|
|
$ |
560.56 |
|
76
The key inputs utilized in determining the fair value of each Tranche C Warrants as of the Initial Issuance Date and December 31, 2023 were as follows:
|
|
October 3, 2023 |
|
|
December 31, |
|
||
Risk-free interest rate (1) |
|
|
4.80 |
% |
|
|
3.85 |
% |
Expected term remaining (years) (1) |
|
|
5.15 |
|
|
|
4.91 |
|
Implied volatility |
|
|
85.0 |
% |
|
|
85.0 |
% |
Underlying Stock Price (Preferred Series A) |
|
$ |
546.30 |
|
|
$ |
560.56 |
|
The initial fair value of each Preferred Placement Agent Warrant issued and exercisable at $6.30 has been determined using the Black-Scholes option-pricing model.
The key inputs into the valuations as of the October 3, 2023 initial measurement date were as follows:
|
|
Initial Measurement |
|
|
Risk-free interest rate |
|
|
4.80 |
% |
Expected term remaining (years) |
|
|
5.00 |
|
Implied volatility |
|
|
85.0 |
% |
Closing common stock price on the measurement date |
|
$ |
0.63 |
|
Upon initial measurement, the fair value of each Preferred Placement Agent Warrant was determined to be $4.40, per warrant for a value of approximately $3.7 million. The total fair value of the Preferred PIPE Placement Agent Warrants was recognized as a non-cash expense and allocated to additional paid-in capital within the consolidated statement of changes in stockholders’ equity and consolidated balance sheet.
Common Stock Valuations
Prior to becoming a public company, we were required to periodically estimate the fair value of our common stock with the assistance of an independent third-party valuation firm, as discussed above, when issuing stock options and computing our estimated stock-based compensation expense. The assumptions underlying these valuations represented our best estimates, which involved inherent uncertainties and the application of significant levels of our judgment. In order to determine the fair value of our common stock, we considered, among other items, previous transactions involving the sale of our securities, our business, financial condition and results of operations, economic and industry trends, the market performance of comparable publicly traded companies, and the lack of marketability of our common stock.
We determine the fair value of our common stock based on the closing market price at closing on the date of grant.
Compensation expense related to stock-based transactions is measured and recognized in the financial statements at fair value of our post-merger common stock based on the closing market price at closing on the date of grant. Stock-based compensation expense is measured at the grant date based on the fair value of the equity award and is recognized as expense over the requisite service period, which is generally the vesting period, on the straight-line method. We estimate the fair value of each stock option award on the date of grant using the Black-Scholes option-pricing model. Determining the fair value of stock option awards at the grant date requires judgment, including estimating the expected volatility, expected term, risk-free interest rate, and expected dividends.
Lease Liabilities and Right-of-Use Assets
We are party to certain contractual arrangements for equipment, lab space, and an animal facility, which meet the definition of leases under FASB ASC Topic 842, Leases (“ASC 842”). In accordance with ASC 842, we, as of January 1, 2018 (the date of adoption), recorded right-of-use assets and related lease liabilities for the present value of the lease payments over the lease terms. We utilized the practical expedient regarding lease and non-lease components and have combined such items into a single combined component. Our incremental borrowing rate was used in the calculation of our right-of-use assets and lease liabilities.
77
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 3, New Accounting Standards, in our consolidated financial statements.
JOBS Act Accounting Election
We qualify as an “emerging growth company” as defined in the JOBS Act. An emerging growth company may take advantage of reduced reporting requirements that are not otherwise applicable to public companies. These provisions include, but are not limited to:
We may use these provisions until the last day of our fiscal year in which the fifth anniversary of the completion of our initial public offering occurred. However, if certain events occur prior to the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenue exceeds $1.235 billion, or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period.
We have elected to take advantage of certain of the reduced disclosure obligations in this Form 10-K and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our shareholders may be different than the information you receive from other public companies in which you hold stock.
The JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards, until those standards apply to private companies. We have elected to take advantage of the benefits of this extended transition period and, therefore, we will not be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards. Until the date that we are no longer an emerging growth company or affirmatively and irrevocably opt out of the exemption provided by Section 7(a)(2)(B) of the Securities Act upon issuance of a new or revised accounting standard that applies to our financial statements and that has a different effective date for public and private companies, we will disclose the date on which we will adopt the recently issued accounting standard.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
Not Applicable.
Item 8. Financial Statements and Supplementary Data.
The consolidated financial statements required pursuant to this item are included in Part IV, Item 15 of this Annual Report, and are presented beginning on page F-1.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
78
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer has evaluated the effectiveness of our disclosure controls and procedures. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost benefit relationship of possible controls and procedures. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that the Company’s disclosure controls and procedures were not effective as of the end of the fiscal year covered by this Annual Report as a result of the material weaknesses in Internal Control over Financial Reporting described below.
Management’s Report on Internal Control over Financial Reporting
Management, including our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a- 15(f) and 15d-15(f) under the Exchange Act and based upon the criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“the COSO framework”). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external purposes in accordance with U.S. GAAP.
An effective internal control system, no matter how well designed, has inherent limitations, including the possibility of human error or overriding of controls, and therefore can provide only reasonable assurance with respect to reliable financial reporting. Because of its inherent limitations, our internal control over financial reporting may not prevent or detect all misstatements, including the possibility of human error, the circumvention or overriding of controls, or fraud. Effective internal controls can provide only reasonable assurance with respect to the preparation and fair presentation of financial statements.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we have conducted an evaluation of the effectiveness of our internal control over financial reporting based on the COSO framework. Based on evaluation under these criteria and based upon the existence of the material weakness described below, management determined, that we did not maintain effective internal control over financial reporting as of December 31, 2023.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that a reasonable possibility exists that a material misstatement of our annual or interim financial statements would not be prevented or detected on a timely basis.
We identified deficiencies in the control environment component of the COSO Framework that constitute a material weakness:
Management believes that the material weakness set forth above is the result of the scale of our operations, is intrinsic to our size, and intends to take remedial actions described below.
79
Plan for Remediation of Material Weakness
We continue to work to strengthen our internal control over financial reporting and are committed to ensuring that such controls are designed and operating effectively. We are implementing process and control improvements to address the above material weakness as follows:
We are committed to continuing to improve our internal control processes related to these matters and will continue to review our financial reporting controls and procedures. As we continue to evaluate and work to improve our internal control over financial reporting, we may take additional measures to address deficiencies or modify certain of the remediation measures described above.
Changes in Internal Control Over Financial Reporting
Other than as described above, there have been no changes in our internal control over financial reporting in connection with the evaluation required by Rules 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the fiscal quarter to which period covered by this Annual Report on Form 10-K relates that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
80
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Directors and Executive Officers
The following persons are serving as our executive officers and directors:
Name |
|
Age |
|
Position(s) |
Samuel J. Reich |
|
49 |
|
Class III Director, Chairman of the Board and Chief Executive Officer |
Christine Hamilton, MBA |
|
68 |
|
Class III Director |
Eddie J. Sullivan, PhD |
|
58 |
|
Class III Director and President |
Jeffrey G. Spragens |
|
82 |
|
Class II Director |
David Link, MBA |
|
68 |
|
Class II Director |
Katie Ellias |
|
45 |
|
Class II Director |
Andrew Moin |
|
40 |
|
Class II Director |
William Polvino, MD |
|
63 |
|
Class I Director |
Scott Giberson |
|
55 |
|
Class I Director |
Erick Lucera |
|
56 |
|
Class I Director |
Michael G. King, Jr. |
|
63 |
|
Chief Financial Officer |
Christoph Bausch, PhD |
|
53 |
|
Chief Operating Officer |
Alexandra Kropotova, MD |
|
51 |
|
Chief Medical Officer |
Family Relationships
There are no family relationships among any of our directors or executive officers. Edward Hamilton, our former Chairman, retired from such role as of the consummation of the Business Combination. Mr. Hamilton was named as a board observer in October 2021. Edward Hamilton is Christine Hamilton’s husband.
Executive Officers
Samuel J. Reich has served as a member of our board of directors from November 2020 and our CEO since January 2024 and was named chairman of our board of directors in October 2021, and was named Chief Executive Officer in January 2024. Mr. Reich served as our Chief Executive Officer and Chief Financial Officer from November 2020 until October 2020 prior to the closing of our Business Combination. Mr. Reich co-founded Biscayne Neurotherapeutics, Inc. in 2011 and served as its Executive Chairman until its sale to Supernus Pharmaceuticals (Nasdaq: SUPN) in October 2018. Biscayne Neurotherapeutics was focused on novel treatments for seizure disorders. Previously, Mr. Reich was the Executive Vice President of OPKO Ophthalmologics, a division of OPKO Health, Inc. (Nasdaq: OPK) from March 2007 to November 2008, where Mr. Reich served on the executive committee and lead the Ophthalmologics business division. Prior to his position at OPKO, Mr. Reich was the Founder and Executive Vice President of Acuity Pharmaceuticals, Inc., where he worked from July 2002 through March 2007, at which time Acuity Pharmaceuticals merged with OPKO Health. Mr. Reich was a doctoral candidate in the Department of Ophthalmology at the University of Pennsylvania Medical School. He left graduate school prior to the completion of his Ph.D. to establish Acuity. Prior to that, he was a graduate student at the University of Pennsylvania in the Biomedical Studies graduate program. He has authored six peer- reviewed scientific publications and is currently an inventor on sixteen issued U.S. patents and over 50 issued foreign patents. Mr. Reich holds a B.A. with High Honors in Biochemistry from Clark University, cum laude, Phi Beta Kappa.
Eddie J. Sullivan, PhD, is our co-founder and has served as our president since 2014 and our past CEO from 2014 until January 2024. Dr. Sullivan has served in biopharma leadership positions for more than 25 years. Prior to joining us, he held the CEO role or other leadership roles in our predecessor entities, including CEO of Hematech, a subsidiary of Kyowa Hakko Kirin. During that time, he led initiatives to develop infectious disease, cancer, and autoimmune immunotherapies. In addition to raising over $250 million in capital to develop biopharmaceutical platform technologies, he has also led several successful mergers and acquisitions. A recognized thought leader in antibodies and transgenic animals, Dr. Sullivan serves on the board of directors for the Biotechnology Innovation Organization (BIO) and has served on its executive committee. He has worked with industry committees and discussion groups that have focused on animal biotechnology, regulatory framework, human immunotherapies, and global health threats. Dr. Sullivan was governor-appointed to South Dakota’s Research Commercialization Council and is Chairman of the state’s National Science Foundation-EPSCoR committee. He
81
also founded, served as president, and remains an advisor to the state affiliate of BIO, South Dakota Biotech, and in 2014 was honored for his leadership, innovation, vision, and entrepreneurship with the inaugural LIVE award. He holds an undergraduate degree from the University of Arizona and graduate degrees from Brigham Young University, Kennedy-Western University, and Utah State University in both reproduction and business.
Michael G King, Jr., is our Chief Financial Officer as of October 2023. Mr. King is an award-winning biotechnology industry research analyst with over 25 years of experience advising investors and issuers. From June 2022 to May 2023, Mr. King was Co-Head of Healthcare Research at EF Hutton Group., where he provided coverage on 15 healthcare and biotechnology companies across a range of market capitalizations. From January 2021 to May 2022, he was Managing Director and Senior Biotechnology Analyst with H.C. Wainwright & Co., where he provided coverage on 21 healthcare and biotechnology companies From May 2018 to December 2020, Mr. King acted as Entrepreneur in Residence at Fortress Biotech, Inc., where he was responsible for identifying promising therapeutic molecules, securing rights to their development and commercialization, and forming and capitalization new companies around these molecules. Mr. King has previously held senior roles with prominent companies including JMP Securities LLC, Rodman and Renshaw LLC, Ziopharm Oncology, Inc. Wedbush PacGrow Life Sciences, Bank of America, Robertson Stephens, and Vector Securities. Mr. King’s extensive investment banking and public company advisory experience includes equity research, capital markets, corporate finance, and M&A advisory. He received his BA in Finance from the Bernard M. Baruch College of the City University of New York.
Christoph Bausch, PhD, MBA, is our Chief Operating Officer as of May 2022, overseeing all Research & Manufacturing operations of the company. Prior to his role as COO, he served as Chief Science Officer since joining SAB in April 2017, providing leadership in all areas of Research & Development, and functioned as drug development lead for a Stage 3 clinically advanced drug product. Dr. Bausch is an experienced research scientist, biotech entrepreneur and business development executive who has led the successful discovery, development, biomanufacturing, and commercialization of platform technologies in the life sciences. Previously, Dr. Bausch has served as founder and director of a molecular diagnostic company and has provided life science consulting for Keion Group, LLC. Dr. Bausch held several science-based business development positions prior to joining SAB, most recently for multi-billion-dollar global industrial biomanufacturing leader POET, LLC, where he structured strategic partnerships, prospected, and vetted new technologies and streamlined research and development activities. He also worked in both research and commercialization roles for Fortune 500 life science and high technology company Sigma-Aldrich, now MilliporeSigma. Dr. Bausch received his PhD in Microbiology at The Ohio State University, Columbus, Ohio, completed Post-Doctoral Training at the Stowers Institute for Medical Research, Kansas City, Missouri and earned an MBA from St. Louis University, St. Louis, Missouri, in addition to a BA in Biology from the University of Nebraska-Lincoln, Lincoln, Nebraska.
Alexandra Kropotova, M.D., is our Executive Vice President & Chief Medical Officer as of June, 2022, leading the strategy, direction, and execution of the company’s clinical development for the entire portfolio. Dr. Kropotova is a biopharmaceutical executive with expertise in all phases of global clinical development, translational medicine and medical affairs. Prior to joining SAB Biotherapeutics, as a Therapeutic Area Head of Global Specialty R&D at Teva Pharmaceuticals from April 2016 to June 2022, Alexandra led innovative drug development focused on delivering a broad portfolio of immunology, respiratory, and immuno-oncology assets spanning from pre-IND to BLA/NDA filing of biologics and complex drug-device combination products. Prior to Teva, Dr. Kropotova served in various roles at Sanofi, including Vice President, Strategy & Strategic Planning Head, North American Medical Affairs; Associate Vice President and subsequently Vice President, Immuno-Inflammation, Global R&D Clinical Development; and Senior Medical Director, Respiratory, Allergy & Anti-Infectives. She also served in various roles at Pfizer Inc., most recently as Director & Head of Global Clinical Respiratory and Analgesics. She continues to serve on the Board of Directors at iBio, a global leader in plant-based biologics manufacturing and development of novel biopharmaceuticals. Dr. Kropotova received her MBA from Ohio University Graduate School of Business, Athens, Ohio; and her M.D. in Internal Medicine from the Vladivostok State Medical University, Vladivostok, Russia.
Non-Employee Directors
Biographical information for Eddie J. Sullivan PhD, our President and Class III director, and Samuel J. Reich, our Chairman of the Board, Chief Executive Officer and Class III director, is set forth above in “Item 10. Executive Officers”.
Christine Hamilton, MBA, is our co-founder and has served as a member of our board of directors since 2014. Ms. Hamilton is the owner and managing partner of Christiansen Land and Cattle, Ltd., a fourth-generation diversified farming and ranching enterprise. She also owns Dakota Packing, Inc., a wholesale company based in Las Vegas that provides high-end, “center-of-the-plate” protein products to a national customer base. Ms. Hamilton has served on the board of directors for several financial and public companies including HF Financial Corporation, Home Federal Bank (now Great Western Bancorp, NYSE: GWB) and, in 2018, was recognized for her exemplary service as a board member of the Federal Reserve Bank (Ninth District) after a four-year term. She currently serves as a board member for publicly traded Titan Machinery,
82
Padlock Ranch, and Meadowlark Institute. Ms. Hamilton was a governor-appointed commissioner for South Dakota Game Fish & Parks and is a 2016 inductee to the South Dakota Hall of Fame for her contributions to the state and agribusiness. In 2000, Ms. Hamilton and her family formed the Matson Halverson Christiansen Hamilton Foundation (MHCH), a not-for-profit foundation with a mission to improve the quality of life and create opportunities for growth and enterprise development in South Dakota. Ms. Hamilton holds a philosophy degree from Smith College in Northampton, Massachusetts, and an MBA in entrepreneurship from the University of Arizona. Ms. Hamilton is well qualified to serve on our board of directors because of her extensive public company board experience.
Katie Ellias, joined the SAB board of directors in November 2023. Ms. Ellias serves as a Managing Director at the JDRF T1D Fund LLC, a venture philanthropy fund with approximately $200 million in assets (the “T1D Fund”), including an investment in the Company. Ms. Ellias joined the T1D Fund in 2018 where she has led a number of investments in companies developing T1D-oriented therapies, and served as a director on the board of several including, DiogenX, Veralox Therapeutics, i2O Therapeutics, and Capillary Biomedical. Ms. Ellias joined the T1D Fund from Endeavour Vision, a Geneva-based growth stage venture fund. She was previously Principal at Sofinnova Partners, Paris, a leading early-stage life sciences fund. Ms. Ellias has also held roles in business development with Medtronic and started her career at McKinsey & Company. She holds an M.B.A. in Healthcare Management from the Wharton School at the University of Pennsylvania and a B.A. in International Relations and Political Science from Yale University.
Scott Giberson, RPh, MPH, D.Sc., Rear Admiral (retired), joined the SAB board of directors in July 2022. He is currently the President of AMI Expeditionary Healthcare, a private global healthcare solutions company where he has fostered global client relations at the highest levels, since March 2021. Clients include senior leadership of multiple U.S. and foreign government entities, the WHO, UN and private industry partners such as the Gates Foundation. RADM Giberson retired after 27 years as two-star admiral and as an Assistant U.S. Surgeon General, serving in a variety of senior roles with the U.S. Department of Health and Human Services from March 2010 to March 2021. RADM (rert.) Giberson served as the acting Deputy Surgeon General of the United States (2013-2014), he was the Surgeon General's principal liaison with health leadership in multiple U.S. Departments. He also held executive positions as the Senior Advisor to the Office of Surgeon General, Director of Commissioned Corps Headquarters, Chief Pharmacist of the USPHS (2010-2014), Director of the IHS National HIV/AIDS Program and Senior Public Health Advisor for Pacific Command's Center of Excellence in Disaster Management and Humanitarian Assistance (2003-2006). He served as overall Commander of the Commissioned Corps' Ebola Response in West Africa. RADM Giberson has authored numerous articles and delivered well over 100 keynote lectures on leadership, global health, and public health at numerous venues both domestically and internationally. RADM Giberson has received many awards including the Presidential Unit Citation from President Obama in the Oval Office for leadership during the West African Ebola response. The Military Officers Association of America selected him as on the of the “Top 100 Veterans in the Last 100 Years You Need to Know”. RADM Giberson is a graduate of Temple University and U. of Massachusetts/Amherst, holds a Pharmacy degree and licensure, MPH, and graduate certificate in Health Emergencies in Large Populations from the International Committee of the Red Cross. He has received three honorary Doctoral degrees (one for his pioneering work in interprofessional practice). He is also a Fellow of Wharton Business School (U. of Pennsylvania) Executive Leadership Program. Mr. Giberson is well qualified to serve on our board of directors because of his extensive experience in the medical industry.
David Link, MBA, has served as a member of our board of directors since 2018 and is currently Vice-Chairman. Mr. Link is the former executive vice president and chief strategy office at Sanford Health with more than three decades of experience in strategy, planning and financial operations. During his tenure, Mr. Link contributed significantly to growing the organization from a regional health system into one of the nation’s largest non-profit, integrated health care delivery systems. He was also charged with overseeing Sanford Health Plan, Sanford Foundation and research and development, including Sanford Research. Under his leadership, the initial Sanford Clinic was created as well as the development of Sanford World Clinics, an initiative designed to provide communities around the world with permanent, sustainable health care infrastructure. Currently, Dave serves as an appointed program director in the President’s Office at Dakota State University, one of the nation’s leading programs in cyber security. Dave holds board or committee positions with Enterprise 605, the South Dakota REACH Committee, South Dakota Research and Commercialization Council and Sanford Research. In 2019, he was honored for his exemplary leadership and support of the state’s bioscience industry with the LIVE Award at the South Dakota Biotech. Dave holds a bachelor’s degree in data processing and computer science, an MBA from the University of South Dakota and a master’s in healthcare administration from the University of Minnesota. Mr. Link is well qualified to serve on our board of directors because of his extensive experience in the biotechnology industry and his extensive public company board experience.
Erick Lucera, joined the SAB board of directors in April 2023. From 2020 to February 2023, Mr. Lucera served as Chief Financial Officer of AVEO Oncology, a public biotech company, and subsequent to the close of its acquisition, worked on integration with LG Chem, Ltd. From 2016 to 2020, Mr. Lucera served as Chief Financial Officer, Treasurer and Secretary of VALERITAS, a publicly traded commercial-stage medical technology company where he led multiple successful public
83
offerings. From 2017 to the present, Mr. Lucera has served as a member of the Board of Directors and Audit Committee Chairman of Beyond Air, a publicly held commercial-stage medical device and biopharmaceutical company developing a platform of nitric oxide generators and delivery systems. From 2021 to the present, Mr. Lucera has served as a member of the Board of Directors and Audit Committee Chairman of Bone Biologics Corporation, a publicly held company focusing on regenerative medicine therapies to treat bone disorders. From 2015 to 2016, Mr. Lucera served as Chief Financial Officer, Treasurer and Secretary of VIVENTIA Bio, acquired by Eleven Biotherapeutics, Inc., now Sesen Bio, a biotechnology company focused on developing targeted protein therapeutics for the treatment of cancer. Early in his career, Mr. Lucera spent more than 15 years covering healthcare and the life sciences in investment management. Given Mr. Lucera’s extensive experience in strategic planning and finance, we believe that Mr. Lucera is well qualified to serve as a member of the Board of Directors.
Andrew Moin, joined the SAB board of directors in October 2023. Mr. Moin is a Partner and Analyst at Sessa Capital, a New York based investment advisor registered with the SEC. Mr. Moin has been with Sessa since 2012, where he works on idea generation, research, and investment implementation. Prior to Sessa, from 2008-2012, Mr. Moin was in the Tax Group at Sullivan & Cromwell LLP, where he advised corporate and other clients on a variety of transactions. In the non-profit realm, Andrew has served on the Young Leadership Committee of the New York City Chapter of the JDRF and was Chair of the Board of Trustees at the Great Neck Community School. Andrew received a B.A. in Economics, with distinction, from Amherst College and a J D., magna cum laude, from Harvard Law School.
Dr. William J. Polvino, MD, has served as a member of our board of directors since 2019, after having served as our business advisor for several years. Dr. Polvino is pharmaceutical entrepreneur with more than 25 years of experience in the healthcare arena. He is currently chief executive officer of Bridge Medicines, a pioneering drug discovery company focused on advancing promising early technologies from concept to clinic. Prior to Bridge Medicines, Dr. Polvino was president and chief executive officer of Veloxis Pharmaceuticals A/S (NASDAQ-OMX: VELO), a public biotechnology company that deployed proprietary formulation technology to develop and commercialize an innovative oral drug product for transplant patients. He also served as president and CEO of Helsinn Therapeutics (formerly Sapphire Therapeutics) and has held executive and senior-level positions in drug development at Merck, Wyeth and Theravance. Dr. Polvino earned his medical degree from Rutgers Medical School and a B.S. in Biology from Boston College. He trained in internal medicine at Massachusetts General Hospital and was a fellow in clinical pharmacology at the National Institutes of Health prior to entering the pharmaceutical and biotechnology industry. Dr. Polvino is well qualified to serve on our board of directors because of his extensive experience in the biotechnology industry and his extensive public company management experience.
Jeffrey G. Spragens has served as a member of our board of directors since November 2020. From 2005 through 2013, Mr. Spragens was a Co-Founder and the CEO of SafeStitch Medical, Inc., a medical device company that pioneered incisionless surgery techniques that helps to relieve GERD and obesity. In 2013, SafeStitch merged with TransEnterix, Inc. (NYSE: TRXC). In addition, Mr. Spragens was one of the three founding board members of North American Vaccine, which became a publicly traded company in 1990. At North American Vaccine, Mr. Spragens was responsible for securing initial financing and building a commercial manufacturing facility. Mr. Spragens was instrumental in North American Vaccine’s acquisition by Baxter International (NYSE: BAX) in 1999. Mr. Spragens has also been a successful real estate developer and entrepreneur. Mr. Spragens was President of FCH services from 1973 until 1986. FCH developed and managed units of coop and condo housing financed with HUD financing with offices in several major cities. In 1986, Mr. Spragens converted to condo ownership 1,000 apartment units in San Mateo, California, resulting in one of the largest residential projects in California at that time. Mr. Spragens was Managing Partner of Gateway Associates, Inc. from 1990 to 2000. In addition, Mr. Spragens is President and 50% owner of Mint Management Company, a residential property management company he co-founded in 1987, which develops, owns and operates apartment units in New Jersey, Michigan and Kansas. Mr. Spragens developed and continues to own and operate Inman Grove Shopping Center in Edison, New Jersey. Mr. Spragens is also a well-known and respected philanthropist. Mr. Spragens is a Founding Board Member and Treasurer of Foundation for Peace. Foundation for Peace provides healthcare, education, and clean water to those in need in Dominican Republic and Haiti. He is also a member of the Board of Directors and Finance Committee of Hernia Help, which provides free hernia surgery to underserved children and adults in developing countries. Mr. Spragens has a BA from the University of Cincinnati, a Law Degree from George Washington University, and an MA from American University. Mr. Spragens is well qualified to serve on our board of directors because of his extensive public company management and multi-sector investment experience, and his public company board experience.
Director Independence
The listing rules of Nasdaq require us to maintain a board of directors comprised of a majority of independent directors, as determined affirmatively by our board of directors. In addition, the Nasdaq listing rules require that, subject to specified exceptions, each member of our audit, compensation and nominating and corporate governance committees must be
84
independent. Audit committee members and compensation committee members must also satisfy the independence criteria set forth in Rule 10A-3 and Rule 10C-1, respectively, under the Exchange Act. Under the Nasdaq listing rules, a director will only qualify as an “independent director” if, in the opinion of our board of directors, the director does not have a relationship that would interfere with the exercise of independent judgment in carrying out his or her responsibilities.
Our board of directors has undertaken a review of the independence of our directors and considered whether any director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that none of Christine Hamilton, Jeffrey Spragens, William Polvino, David Link, Scott Giberson, Erick Lucera, Katie Ellias, and Andrew Moin (representing eight of our ten directors), has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that they each are an “independent director” as that term is defined under the Nasdaq listing rules.
In making these determinations, our board of directors considered the relationships that each nonemployee director has with us and all other facts and circumstances our board of directors deemed relevant in determining their independence, including consulting relationships, family relationships and the beneficial ownership of our capital stock by each non-employee director.
Board Composition
Our business and affairs are organized under the direction of our board of directors. Our board currently consists of ten (10) directors divided into three classes as follows:
or, in each case, until their respective successor is duly elected and qualified, or until their earlier resignation, removal or death.
Messrs. Lucera, Giberson, and Dr. Polvino currently serve as the Class I directors, Ms. Ellias, Messrs. Link, Spragens and Moin currently serve as the Class II directors, and Ms. Hamilton, and Messrs. Reich and Sullivan currently serve as Class III directors.
At each annual meeting of stockholders, the successors to directors whose terms then expire will serve until the third annual meeting following their election and until their successors are duly elected and qualified. The authorized size of the board of directors will be fixed exclusively by resolutions of the board of directors. The authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed between the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the board of directors may have the effect of delaying or preventing changes in its control or management. Our board of directors may be removed for cause by the affirmative vote of the holders of at least 66 2/3% of its voting stock.
Board Meetings
During 2023, our board of directors held six meetings, and each director attended at least 75% of the aggregate of (i) the total number of meetings of our board of directors held during the period for which he or she has been a director and (ii) the total number of meetings held by all committees of our board of directors on which he or she served during the periods that he or she served.
Committees of the Board of Directors
Our board of directors has three standing committees: an audit committee, a nominating and corporate governance committee (“nominating committee”) and a compensation committee. Subject to phase-in rules and a limited exception, Nasdaq rules and Rule 10A-3 of the Exchange Act require that the audit committee of a listed company be comprised solely of independent directors, and Nasdaq rules require that the compensation committee and nominating committee of a listed
85
company be comprised solely of independent directors. Each of our committees is comprised entirely of independent directors.
Audit Committee
On October 22, 2021, we established an audit committee of the board of directors. Erick Lucera, William Polvino, and Jeffrey Spragens serve as members of the audit committee, with Erick Lucera serving as the Chairman of the audit committee. Under the Nasdaq listing standards and applicable SEC rules, we are required to have at least three members of the audit committee, all of whom must be independent. Each of Mr. Lucera, Dr. Polvino, and Mr. Spragens meet the independent director standard under Nasdaq listing standards and under Rule 10A-3(b)(1) of the Exchange Act. The Audit Committee held five meetings during 2023.
Each member of the audit committee is financially literate, and our board of directors has determined that each Mr. Lucera and Mr. Spragens qualifies as an “audit committee financial expert” as defined in applicable SEC rules.
We adopted a restated audit committee charter on October 22, 2021 which details the principal functions of the audit committee, including:
Compensation Committee
On October 22, 2021, we established a compensation committee of the board of directors. Christine Hamilton, Eric Lucera and Katie Ellias serve as members of the compensation committee. Christine Hamilton serves as the Chairwoman of the compensation committee. Under the Nasdaq listing standards and applicable SEC rules, we are required to have at least two members of the compensation committee, all of whom must be independent. Each of Mr. Lucera, Ms. Ellias and Ms. Hamilton are independent. The Compensation Committee held two meetings during 2023.
We adopted a restated compensation committee charter on October 22, 2021, which details the principal functions of the compensation committee, including:
86
Notwithstanding the foregoing, other than as indicated in this Form 10-K, no compensation of any kind, including finders, consulting, or other similar fees, will be paid to any of our existing stockholders, officers, directors, or any of their respective affiliates, prior to, or for any services they render to effectuate the offering.
The charter also provides that the compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, legal counsel or other adviser and will be directly responsible for the appointment, compensation and oversight of the work of any such adviser. However, before engaging or receiving advice from a compensation consultant, external legal counsel or any other adviser, the compensation committee will consider the independence of each such adviser, including the factors required by Nasdaq and the SEC.
Compensation Committee Interlocks and Insider Participation
No person who served as a member of the compensation committee during the fiscal year ended December 31, 2023 was a current or former officer or employee of the Company or engaged in certain transactions with the Company required to be disclosed by regulations of the SEC. Additionally, there were no compensation committee “interlocks” during the fiscal year ended December 31, 2023, which generally means that no executive officer of the Company served as a director or member of the compensation committee of another entity, one of whose executive officers served as a director or member of the compensation committee of the Company.
Nominating Committee
On October 22, 2021, we established a nominating committee of the board of directors. David Link, Scott Giberson, and Andrew Moin currently serve as members of the Nominating and Governance Committee. David Link serves as the Chairman of the nominating committee. Under the Nasdaq listing standards and applicable SEC rules, we are required to have at least two members of the nominating committee, all of whom must be independent. Each of Mr. Link, Mr. Giberson, and Mr. Moin are independent. The Nominating Committee held two meetings during 2023.
We adopted a restated nominating committee charter on October 22, 2021, which details the purpose and responsibilities of the nominating committee, including:
The nominating committee will consider several qualifications relating to management and leadership experience, background and integrity and professionalism in evaluating a person’s candidacy for membership on the board of directors. The nominating committee may require certain skills or attributes, such as financial or accounting experience, to meet specific board needs that arise from time to time and will also consider the overall experience and makeup of its members to obtain a broad and diverse mix of board members. The nominating committee does not distinguish among nominees recommended by stockholders and other persons.
We have not formally established any specific, minimum qualifications that must be met or skills that are necessary for directors to possess. In general, in identifying and evaluating nominees for director, the board of directors considers educational background, diversity of professional experience, knowledge of our business, integrity, professional reputation, independence, wisdom, and the ability to represent the best interests of our stockholders.
Executive Sessions of Independent Directors
Independent directors are required to meet regularly without management participation. During 2023, there were six meetings of independent directors.
87
Director Nominations
The process of recommending director nominees for selection by the board of directors is undertaken by the nominating committee (see above).
The board of directors will also consider director candidates recommended for nomination by our stockholders during such times as they are seeking proposed nominees to stand for election at the next annual meeting of stockholders (or, if applicable, a special meeting of stockholders). Our stockholders that wish to nominate a director for election to our board of directors should follow the procedures set forth in our bylaws. In 2023, there were no material changes have been made to the procedures by which security holders may recommend nominees to our board of directors.
Section 16 Reporting Compliance
Section 16(a) of the Exchange Act requires certain of our officers and our directors, and persons who own more than 10 percent of a registered class of our equity securities, to file reports of ownership and changes in ownership with the SEC. Officers, directors, and greater than 10 percent stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.
Based solely on our review of copies of such forms received by us, we believe that during the year ended December 31, 2023, all filing requirements applicable to all of our officers, directors, and greater than 10% beneficial stockholders were timely complied with.
Code of Ethics
We adopted a restated Code of Ethics applicable to our directors, officers, and employees. A copy of our Code of Ethics and copies of our audit, nominating and compensation committee charters are available on our website at https://www.sabbiotherapeutics.com/.
In addition, a copy of the Code of Ethics will be provided without charge upon written request, addressed to:
SAB Biotherapeutics, Inc.
2100 East 54th Street North
Sioux Falls, South Dakota 57104
Attn: Corporate Secretary
We intend to disclose any amendments to or waivers of certain provisions of our Code of Ethics in a Current Report on Form 8-K. Please see “Where You Can Find Additional Information” for additional information.
Board Oversight of Risk
The Board’s Role
The Board’s role in the Company’s risk oversight process includes receipt and review of scheduled and ad hoc reports from members of the executive management team which relate to areas of actual or potential material risk to the Company, including but not limited to, operational, financial, legal, regulatory, strategic, transactional and reputational risks. The full Board receives these reports from the appropriate “risk owner” within the organization to enable each member of the Board to understand our risk identification, risk management and risk mitigation strategies.
Risk Assessment in Compensation Policies and Practices for Employees
The Compensation Committee reviewed the elements of our compensation policies and practices for all of our employees, including our named executive officers, to evaluate whether risks that may arise from such compensation policies and practices are reasonably likely to have a material adverse effect on our Company. The Compensation Committee has concluded that the following current features of our compensation programs guard against excessive risk-taking:
88
The Compensation Committee believes that, for all of our employees, including our named executive officers, our compensation programs do not lead to excessive risk-taking and instead encourage behavior that supports sustainable value creation. We believe that risks that may arise from our compensation policies and practices for our employees, including our named executive officers, are not reasonably likely to have a material adverse effect on our Company.
Item 11. Executive Compensation.
The following is a discussion and analysis of compensation arrangements of the Company’s named executive officers. This discussion may contain forward-looking statements that are based on the Company’s current plans, considerations, expectations and determinations regarding future compensation programs. The actual compensation programs that the Company adopts may differ materially from the currently planned programs that are summarized in this discussion. As an “emerging growth company” as defined in the JOBS Act, we are not required to include a Compensation Discussion and Analysis section and have elected to comply with the scaled disclosure requirements applicable to emerging growth companies.
Summary Executive Compensation Table
The following table sets forth information regarding the compensation awarded to, earned by or paid to Our named executive officers for the fiscal years ended December 31, 2023 and 2022.
|
|
|
|
Salary |
|
|
Option Awards (1) |
|
|
Stock Awards (2) |
|
|
Non-Equity Incentive Plan Compensation |
|
|
All Other Compensation |
|
|
Total |
|
||||||
Name and Principal Position |
|
Year |
|
($) |
|
|
($) |
|
|
($) |
|
|
($) |
|
|
($) |
|
|
($) |
|
||||||
Samuel J. Reich (3) |
|
2023 |
|
|
350,000 |
|
|
|
202,598 |
|
|
|
— |
|
|
|
— |
|
|
|
13,200 |
|
|
|
565,798 |
|
Chairman of the Board of Directors and Chief Executive Officer |
|
2022 |
|
|
350,000 |
|
|
|
304,600 |
|
|
|
— |
|
|
|
14,000 |
|
|
|
12,200 |
|
|
|
680,800 |
|
Eddie J. Sullivan, PhD. (4) |
|
2023 |
|
|
377,200 |
|
|
|
202,598 |
|
|
|
— |
|
|
|
— |
|
|
|
25,359 |
|
|
|
605,157 |
|
President |
|
2022 |
|
|
377,200 |
|
|
|
44,725 |
|
|
|
— |
|
|
|
42,435 |
|
|
|
10,982 |
|
|
|
475,342 |
|
Alexandra Kropotova, MD (5) |
|
2023 |
|
|
525,000 |
|
|
|
— |
|
|
|
147,125 |
|
|
|
236,250 |
|
|
|
13,200 |
|
|
|
921,575 |
|
EVP, Chief Medical Officer |
|
2022 |
|
|
282,692 |
|
|
|
10,344 |
|
|
|
567,000 |
|
|
|
— |
|
|
|
2,423 |
|
|
|
862,459 |
|
Michael G. King, Jr. (6) |
|
2023 |
|
|
60,577 |
|
|
|
661,411 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
721,988 |
|
EVP, Chief Financial Officer |
|
2022 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Christoph Bausch, PhD (7) |
|
2023 |
|
|
325,000 |
|
|
|
106,123 |
|
|
|
— |
|
|
|
105,000 |
|
|
|
13,200 |
|
|
|
549,323 |
|
EVP, Chief Operating Officer |
|
2022 |
|
|
308,855 |
|
|
|
184,551 |
|
|
|
— |
|
|
|
50,445 |
|
|
|
12,200 |
|
|
|
556,051 |
|
89
90
Outstanding Equity Awards at Fiscal 2023 Year-End
The following table sets forth information regarding outstanding equity awards held by our named executive officers as of December 31, 2023.
|
|
Option Awards |
|
Stock Awards |
|
|||||||||||||||||
Name |
|
Number of Securities Underlying Unexercised Options (#) Exercisable |
|
|
Number of Securities Underlying Unexercised Options (#) Unexercisable |
|
|
Option Exercise Price ($) |
|
|
Option Expiration Date |
|
Number of Shares or Units of Stock That Have Not Vested (#) Exercisable |
|
|
Market Value of Shares or Units of Stock That Have Not Vested ($) |
|
|||||
Samuel J. Reich |
|
|
25,277 |
|
|
|
9,723 |
|
(1) |
|
111.70 |
|
|
11/16/2031 |
|
|
— |
|
|
|
— |
|
|
|
|
700 |
|
|
|
— |
|
|
|
17.80 |
|
|
3/15/2032 |
|
|
— |
|
|
|
— |
|
|
|
|
16,406 |
|
|
|
36,094 |
|
(2) |
|
7.11 |
|
|
9/12/2032 |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
52,500 |
|
(3) |
|
5.35 |
|
|
3/13/2033 |
|
|
— |
|
|
|
— |
|
Eddie J. Sullivan, PhD. |
|
|
13,958 |
|
|
|
— |
|
|
|
5.40 |
|
|
8/4/2024 |
|
|
— |
|
|
|
— |
|
|
|
|
32,570 |
|
|
|
— |
|
|
|
5.40 |
|
|
12/11/2024 |
|
|
— |
|
|
|
— |
|
|
|
|
2,326 |
|
|
|
— |
|
|
|
26.90 |
|
|
4/26/2030 |
|
|
— |
|
|
|
— |
|
|
|
|
2,121 |
|
|
|
— |
|
|
|
17.80 |
|
|
3/15/2032 |
|
|
— |
|
|
|
— |
|
|
|
|
1,093 |
|
|
|
2,407 |
|
(4) |
|
7.11 |
|
|
9/12/2032 |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
52,500 |
|
(5) |
|
5.35 |
|
|
3/13/2033 |
|
|
— |
|
|
|
— |
|
Alexandra Kropotova, MD |
|
|
573 |
|
|
|
1,259 |
|
(6) |
|
7.11 |
|
|
9/12/2032 |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
18,750 |
|
(7) |
|
128,906 |
|
|
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
27,500 |
|
(7) |
|
189,063 |
|
|
Michael G. King, Jr. |
|
|
— |
|
|
|
85,000 |
|
(8) |
|
7.98 |
|
|
11/1/2033 |
|
|
— |
|
|
|
— |
|
Christoph Bausch, PhD |
|
|
10,468 |
|
|
|
— |
|
|
|
10.70 |
|
|
3/12/2027 |
|
|
— |
|
|
|
— |
|
|
|
|
8,142 |
|
|
|
— |
|
|
|
10.70 |
|
|
3/12/2027 |
|
|
— |
|
|
|
— |
|
|
|
|
6,979 |
|
|
|
— |
|
|
|
10.70 |
|
|
3/12/2028 |
|
|
— |
|
|
|
— |
|
|
|
|
1,163 |
|
|
|
— |
|
|
|
26.90 |
|
|
4/26/2030 |
|
|
— |
|
|
|
— |
|
|
|
|
2,497 |
|
|
|
— |
|
|
|
17.80 |
|
|
3/15/2032 |
|
|
— |
|
|
|
— |
|
|
|
|
8,589 |
|
|
|
18,898 |
|
(9) |
|
7.11 |
|
|
9/12/2032 |
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
27,500 |
|
(10) |
|
5.35 |
|
|
3/13/2033 |
|
|
— |
|
|
|
— |
|
91
Named Executive Officer Employment Arrangements
Below are descriptions of the current employment agreements with our named executive officers.
Samuel J. Reich
On November 17, 2021, we entered into an Executive Employment Agreement with Mr. Reich to serve as our Chairman of the Board of Directors. The agreement provides Mr. Reich an annual base salary of $350,000, and his eligibility to participate in the Company’s benefit plans generally. The agreement also subjects Mr. Reich to standard nondisclosure, invention assignment, and arbitration provisions. If Mr. Reich's employment is terminated by the Company without Cause (as defined in the employment agreement) (other than for death or disability) or the term of his employment is not renewed, Mr. Reich will receive (i) a severance payment equal to one year of his then base salary, payable in a lump sum five business days after his release becomes final, (ii) the applicable accrued but unpaid annual bonus, if any, for the fiscal year ended prior to his date of termination, payable at the same time annual bonuses for such fiscal year are paid to other key executives of the Company, (iii) one hundred percent of his outstanding unvested equity awards as of the date of termination will be fully vested and exercisable, and (iv) reimbursement of the COBRA premiums, if any, for continuation coverage for Mr. Reich, his spouse and dependents under the Company’s group health, dental and vision plans for a twelve month period from the date of termination.
Eddie J. Sullivan
On March 5, 2024, we entered into an Executive Employment Agreement with Dr. Sullivan to continue to serve as our President. The agreement provides Dr. Sullivan an annual base salary of $485,000, and his eligibility to participate in the Company’s benefit plans generally. The agreement also subjects Dr. Sullivan to standard nondisclosure, invention assignment, and arbitration provisions. If Dr. Sullivan’s employment is terminated by the Company without Cause (as defined in the employment agreement) (other than for death or disability) or the term of his employment is not renewed, Dr. Sullivan will receive: (i) a severance payment equal to one year of his then base salary, payable in a lump sum five business days after his release becomes final, (ii) the applicable accrued but unpaid annual bonus, if any, for the fiscal year ended prior to his date of termination, payable at the same time annual bonuses for such fiscal year are paid to other key executives of the Company, (iii) one hundred percent of his outstanding unvested equity awards as of the date of termination will be fully vested and exercisable, and (iv) reimbursement of the COBRA premiums, if any, for continuation coverage for Dr. Sullivan, his spouse and dependents under the Company’s group health, dental and vision plans for a twelve month period from the date of termination.
Alexandra Kropotova
On May 20, 2022, we entered into an Executive Employment Agreement with Dr. Kropotova to serve as our Executive Vice President – Chief Medical Officer. The agreement provides Dr. Kropotova an annual base salary of $525,000, and her eligibility to participate in the Company’s benefit plans generally. The agreement also subjects Dr. Kropotova to standard nondisclosure, invention assignment, and arbitration provisions. If Dr. Kropotova’s employment is terminated by the Company without Cause (as defined in the employment agreement) (other than for death or disability) or the term of her employment is not renewed, Dr. Kropotova will receive: (i) a severance payment equal to one year of her then base salary, payable in a lump sum five business days after his release becomes final, (ii) the applicable accrued but unpaid annual bonus, if any, for the fiscal year ended prior to her date of termination, payable at the same time annual bonuses for such fiscal year are paid to other key executives of the Company, (iii) one hundred percent of her outstanding unvested equity awards as of the date of termination will be fully vested and exercisable, and (iv) reimbursement of the COBRA premiums, if any, for continuation coverage for Dr. Kropotova, her spouse and dependents under the Company’s group health, dental and vision plans for a six month period from the date of termination.
Michael G. King, Jr.
On October 23, 2023, we entered into an Executive Employment Agreement with Mr. King to serve as our Executive Vice President – Chief Financial Officer. The agreement provides Mr. King an annual base salary of $450,000, and his eligibility to participate in the Company’s benefit plans generally. The agreement also subjects Mr. King to standard nondisclosure, invention assignment, and arbitration provisions. If Mr. King’s employment is terminated by the Company without Cause (as defined in the employment agreement) (other than for death or disability) or the term of his employment is not renewed, Mr. King will receive: (i) a severance payment equal to one year of his then base salary, payable in a lump sum five business days after his release becomes final, (ii) the applicable accrued but unpaid annual bonus, if any, for the fiscal year ended prior to her date of termination, payable at the same time annual bonuses for such fiscal year are paid to other key executives of the Company, (iii) fifty percent of his outstanding unvested equity awards as of the date of termination will be fully vested and
92
exercisable, and (iv) reimbursement of the COBRA premiums, if any, for continuation coverage for Mr. King, his spouse and dependents under the Company’s group health, dental and vision plans for a six month period from the date of termination.
Christoph Bausch
On March 5, 2024, we entered into an Executive Employment Agreement with Dr. Bausch to continue to serve as our Chief Operating Officer. The agreement provides Dr. Bausch an annual base salary of $425,000, and his eligibility to participate in the Company’s benefit plans generally. The agreement also subjects Dr. Bausch to standard nondisclosure, invention assignment, and arbitration provisions. If Dr. Bausch’s employment is terminated by the Company without Cause (as defined in the employment agreement) (other than for death or disability) or the term of his employment is not renewed, Dr. Bausch will receive: (i) a severance payment equal to one year of his then base salary, payable in a lump sum five business days after his release becomes final, (ii) the applicable accrued but unpaid annual bonus, if any, for the fiscal year ended prior to his date of termination, payable at the same time annual bonuses for such fiscal year are paid to other key executives of the Company, (iii) one hundred percent of his outstanding unvested equity awards as of the date of termination will be fully vested and exercisable, and (iv) reimbursement of the COBRA premiums, if any, for continuation coverage for Dr. Bausch, his spouse and dependents under the Company’s group health, dental and vision plans for a twelve month period from the date of termination.
Summary Director Compensation Table
The following table sets forth information regarding the compensation awarded to, earned by or paid to our directors for the fiscal year ended December 31, 2023.
|
|
Fees Earned or Paid in Cash |
|
|
Option Awards (1) |
|
|
Stock Awards (2) |
|
|
Total |
|
||||
Name |
|
($) |
|
|
($) |
|
|
($) |
|
|
($) |
|
||||
Samuel J. Reich |
|
|
— |
|
|
|
202,598 |
|
|
|
— |
|
|
|
202,598 |
|
Christine Hamilton, MBA |
|
|
25,000 |
|
|
|
— |
|
|
|
— |
|
|
|
25,000 |
|
Eddie J. Sullivan, PhD |
|
|
— |
|
|
|
202,598 |
|
|
|
— |
|
|
|
202,598 |
|
Jeffrey G. Spragens |
|
|
25,000 |
|
|
|
— |
|
|
|
— |
|
|
|
25,000 |
|
David Link, MBA |
|
|
25,000 |
|
|
|
— |
|
|
|
— |
|
|
|
25,000 |
|
Katie Ellias |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
William Polvino, MD |
|
|
25,000 |
|
|
|
— |
|
|
|
— |
|
|
|
25,000 |
|
Scott Giberson |
|
|
25,000 |
|
|
|
— |
|
|
|
— |
|
|
|
25,000 |
|
Erick Lucera |
|
|
18,750 |
|
|
|
8,178 |
|
|
|
— |
|
|
|
26,928 |
|
Andrew Moin |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Narrative to Director Compensation Table
Our director compensation policy is intended to provide a total compensation package that enables us to attract and retain qualified and experienced individuals to serve as directors and to align our directors’ interests with those of our stockholders.
Annual Cash Compensation
The annual retainers payable to non-employee directors for service on the Board and its committees are $25,000 for service on the Board.
93
Inaugural Equity Grants
Each non-employee director who joins the board receives an equity award of an option to purchase 25,000 shares of our common stock, which vests monthly over a three-year period beginning one month from the date of grant.
SAB Biotherapeutics 2021 Equity Incentive Plan
The SAB Biotherapeutics 2021 Equity Incentive Plan (the “Incentive Plan”) was adopted in connection with, and become effective at the closing of, the Business Combination.
Summary of the Incentive Plan
General
The Incentive Plan covers the grant of awards to our employees (including officers), non-employee consultants and non-employee directors and those of our affiliates. For purposes of the Incentive Plan, our affiliates include any corporation, partnership, limited liability company, joint venture or other entity, with respect to which we, directly or indirectly, own either (i) stock possessing more than fifty percent (50%) of the total combined voting power of all classes of stock entitled to vote, or more than fifty percent (50%) of the total value of all shares of all classes of stock of such corporation, or (ii) an aggregate of more than fifty percent (50%) of the profits interest or capital interest of any non-corporate entity.
The compensation committee administers the Incentive Plan. The full Board must approve all decisions regarding awards to non-employee directors.
Up to a maximum of 1,600,000 shares of our common stock (as adjusted following the Reverse Stock Split) may be delivered in settlement of awards granted under the Incentive Plan initially. The number of shares authorized for issuance will increase each fiscal year, beginning fiscal year 2022 and occurring each year thereafter through 2031, by 2.0% of the number of our shares of common stock issued and outstanding on a fully-diluted basis as of the last day of the preceding fiscal year (such lesser number of shares as determined by our board of directors in its sole discretion). In no event, however, shall the aggregate number of shares that may be issued pursuant to this annual increase under the Incentive Plan exceed 500,000.
Up to a maximum of 1,600,000 shares of our common stock (as adjusted following the Reverse Stock Split) may be issued under the Incentive Plan pursuant to the exercise of incentive stock options. The stock delivered to settle awards made under the Incentive Plan may be authorized and unissued shares or treasury shares, including shares repurchased by us for purposes of the Incentive Plan. If any shares subject to any award granted under the Incentive Plan (other than a substitute award as described below) is forfeited or otherwise terminated without delivery of such shares (if such shares are returned to us due to a forfeiture restriction under such award), the shares subject to such awards will again be available for issuance under the Incentive Plan. However, any shares that are withheld or applied as payment for shares issued upon exercise of an award or for the withholding or payment of taxes due upon exercise of an award will continue to be treated as having been delivered under the Incentive Plan and will not again be available for grant under the Incentive Plan. Upon settlement of any stock appreciation rights (“SARs”), the number of shares underlying the portion of the SARs that is exercised will be treated as having been delivered for purposes of determining the maximum number of shares available for grant under the Incentive Plan and shall not again be treated as available for issuance under the Incentive Plan.
If a dividend or other distribution (whether in cash, shares of common stock or other property), recapitalization, forward or reverse stock split, subdivision, consolidation or reduction of capital, reorganization, merger, consolidation, scheme of arrangement, split-up, spin-off or combination involving us or repurchase or exchange of our shares or other securities, or other rights to purchase shares of our securities or other similar transaction or event affects our common stock such that the compensation committee determines that an adjustment is appropriate in order to prevent dilution or enlargement of the benefits (potential benefits) provided to grantees under the Incentive Plan, the compensation committee will make an equitable change or adjustment as it deems appropriate to the number of type of securities with respect to which awards may be granted, (ii) the number and type of securities subject to outstanding awards, (iii) the exercise price with respect to any option or SAR or, if deemed appropriate, make provision for a cash payment to the holder of such outstanding award, and (iv) the number and kind of outstanding restricted shares, or the shares underlying any other form of award.
Types of Awards
The Incentive Plan permits the granting of any or all of the following types of awards to all grantees:
94
Generally, awards under the Incentive Plan are granted for no consideration other than prior and future services. Awards granted under the Incentive Plan may, in the discretion of the committee, be granted alone or in addition to, in tandem with or in substitution for, any other award under the Incentive Plan; provided, however, that if an SAR is granted in tandem with an ISO, the SAR and ISO must have the same grant date and term and the exercise price of the SAR may not be less than the exercise price of the ISO. The material terms of each award will be set forth in a written award agreement between the grantee and us.
Stock Options and SARs
The committee is authorized to grant SARs and stock options (including incentive stock options (ISOs) except that an ISO may only be granted to an employee of ours or one of our subsidiary corporations). A stock option allows a grantee to purchase a specified number of shares of our common stock at a predetermined price per share (the “exercise price”) during a fixed period measured from the date of grant. An SAR entitles the grantee to receive the excess of the fair market value of a specified number of shares on the date of exercise over a predetermined exercise price per share. The exercise price of an option or an SAR will be determined by the committee and set forth in the applicable award agreement, but the exercise price may not be less than the fair market value of a share of common stock on the grant date. The term of each option or SAR is determined by the committee and set forth in the applicable award agreement, except that the term may not exceed ten (10) years (five (5) years if the grantee holds more than 10% of the total combined voting power of all classes of our capital
stock).
Options may be exercised by payment of the purchase price through one or more of the following means: payment in cash (including personal check or wire transfer); delivering shares of our common stock previously owned by the grantee; or, with the approval of the compensation committee, (i) delivery of shares of our common stock acquired upon the exercise of such options, or (ii) the sale of shares acquired upon exercise of the options through a broker-dealer to whom the grantee has delivered irrevocable notice of exercise and instructions to deliver sales proceeds sufficient to pay us the exercise price.
Following shareholder approval of the Incentive Plan on October 20, 2021, ISOs may be granted pursuant to the terms of the Incentive Plan.
Restricted Shares
The committee may award restricted shares consisting of shares of our common stock which remain subject to a risk of forfeiture and may not be disposed of by grantees until certain restrictions established by the committee lapse. The vesting conditions may be service-based (i.e., requiring continuous service for a specified period) or performance-based (i.e., requiring achievement of certain specified performance objectives) or both. A grantee receiving restricted shares will have all of the rights of a stockholder, including the right to vote the shares and the right to receive any dividends, except as otherwise provided in the applicable award agreement. Upon termination of the grantee’s affiliation with us during the restriction period (or, if applicable, upon the failure to satisfy the specified performance objectives during the restriction period), the restricted shares will be forfeited as provided in the applicable award agreement.
Deferred Stock and Restricted Stock Units
The committee may also grant deferred stock awards and/or restricted stock unit awards. A deferred stock award is the grant of a right to receive a specified number of shares of our common stock at the end of specified deferral periods or upon the occurrence of a specified event, which satisfies the requirements of Section 409A of the Internal Revenue Code. A restricted stock unit award is the grant of a right to receive a specified number of shares of our common stock upon lapse of a specified forfeiture condition (such as completion of a specified period of service or achievement of certain specified performance objectives). If the service condition and/or specified performance objectives are not satisfied during the restriction period, the award will lapse without the issuance of the shares underlying such award.
Restricted stock units and deferred stock awards carry no voting or other rights associated with stock ownership until the shares underlying the award are delivered in settlement of the award. Unless otherwise determined by the compensation committee, grantees will have the rights to receive dividend equivalents in respect of deferred stock and/or restricted stock units, which dividend equivalents shall be deemed reinvested in additional shares of deferred stock or restricted stock units,
95
as applicable, which shall remain subject to the same forfeiture conditions applicable to the deferred stock or restricted stock units to which such dividend equivalents relate.
Performance Units
The committee may grant performance units, which entitle a grantee to cash or shares conditioned upon the fulfillment of certain performance conditions and other restrictions as specified by the committee and reflected in the applicable award agreement. The initial value of a performance unit will be determined by the committee at the time of grant. The committee will determine the terms and conditions of such awards, including performance and other restrictions placed on these awards, which will be reflected in the applicable award agreement.
Performance Shares
The committee may grant performance shares, which entitle a grantee to a certain number of shares of common stock, conditioned upon the fulfillment of certain performance conditions and other restrictions as specified by the committee and reflected in the applicable award agreement. The committee will determine the terms and conditions of such awards, including performance and other restrictions placed on these awards, which will be reflected in the applicable award agreement.
Bonus Shares
The committee may grant fully vested shares of our common stock as bonus shares on such terms and conditions as specified in the applicable award agreement.
Dividend Equivalents
The committee is authorized to grant dividend equivalents, which provide a grantee the right to receive payment equal to the dividends paid on a specified number of shares of our common stock. Dividend equivalents may be paid directly to grantees or may be deferred for later delivery under the Incentive Plan. If deferred, such dividend equivalents may be credited with interest or may be deemed to be invested in shares of our common stock, other awards under the Incentive Plan or in other property.
Other Stock-Based Awards
The Incentive Plan authorizes the committee to grant awards that are valued in whole or in part by reference to or otherwise based on certain other securities. The committee determines the terms and conditions of such awards, including whether awards are paid in shares or cash.
Business Combination, Consolidation or Similar Corporate Transaction
If there is a merger or consolidation of us with or into another corporation or a sale of substantially all of our stock (a “Corporate Transaction”), and the outstanding awards are not assumed by surviving company (its parent company) or replaced with equivalent awards granted by the surviving company(its parent company),the committee will cancel any outstanding awards that are not vested and nonforfeitable as of the consummation of such Corporate Transaction (unless the committee accelerates the vesting of any such awards) and with respect to any vested and nonforfeitable awards, the committee may either (i) allow all grantees to exercise options and SARs within a reasonable period prior to the consummation of the Corporate Transaction and cancel any outstanding options or SARs that remain unexercised upon consummation of the Corporate Transaction, or (ii) cancel any or all of such outstanding awards (including options and SARs) in exchange for a payment (in cash, or in securities or other property) in an amount equal to the amount that the grantee would have received (net of the exercise price with respect to any options or SARs) if the vested awards were settled or distributed or such vested options and SARs were exercised immediately prior to the consummation of the Corporate Transaction. If an exercise price of an option or SAR exceeds the fair market value of our common stock and the option or SAR is not assumed or replaced by the surviving company(its parent company),such options and SARs will be cancelled without any payment to the grantee.
Amendment to and Termination of the Incentive Plan
The Incentive Plan may be amended, altered, suspended, discontinued or terminated by our board of directors without further stockholder approval, unless such approval is required by law or regulation or under the rules of any stock exchange or automated quotation system on which our common stock is then listed or quoted. Thus, stockholder approval will not necessarily be required for amendments which might increase the cost of the Incentive Plan or broaden eligibility. Stockholder approval will not be deemed to be required under laws or regulations that condition favorable treatment of grantees on such approval, although our board of directors may, in its discretion, seek stockholder approval in any circumstance in which it deems such approval advisable.
96
In addition, subject to the terms of the Incentive Plan, no amendment or termination of the Incentive Plan may materially and adversely affect the right of a grantee under any award granted under the Incentive Plan.
Unless earlier terminated by our board of directors, the Incentive Plan will terminate when no shares remain reserved and available for issuance or, if earlier, on the tenth anniversary of the effective date of the Incentive Plan.
SAB Biotherapeutics 2021 Employee Stock Purchase Plan
The SAB Biotherapeutics 2021 Employee Stock Purchase Plan, (the “ESPP”) was adopted in connection with, and became effective at the closing of, the Business Combination. The ESPP provides eligible employees an opportunity to purchase shares of common stock at a discount through accumulated contributions of their earned compensation. The ESPP’s initial share reserve is one million shares of SAB Biotherapeutics common stock. Offering periods will not commence under the ESPP until determined by the board of directors or compensation committee.
Summary of the Employee Stock Purchase Plan
Administration
The ESPP is administered by the board of directors, or a committee appointed by the board of directors, which may be the compensation committee. The board of directors or committee administering the ESPP (the “Administrator”) has authority to construe and interpret the ESPP and to establish rules and regulations for the administration of the ESPP.
Eligibility
Eligible employees of the Company or a participating subsidiary may participate in the ESPP. One is an eligible employee for an accumulation period if he or she is an employee of the Company or a participating subsidiary both on the date determined by the ESPP administrator that enrollment forms must be received for an accumulation period and on the first day of the accumulation period. Notwithstanding the preceding sentences, an employee is not eligible to participate in the ESPP if on the first day of the accumulation period (1) such employee is a member of a collective bargaining unit whose benefits were the subject of good faith bargaining; (2) such employee is customarily employed 20 or less hours per week or five months or less per year; or (3) such employee is an employee of a participating subsidiary who is a resident of a foreign jurisdiction and
(i) participation is prohibited under the laws of such foreign jurisdiction or (ii) compliance with the laws of such foreign jurisdiction would violate Section 423 of the Code. An employee is also not eligible to participate if immediately after any purchase of shares under the ESPP, the employee would own capital stock of the Company and/or hold outstanding options to purchase such stock constituting five percent (5%) or more of the total combined voting power or value of all classes of the capital stock of the Company or of any subsidiary of the Company.
As of December 31, 2023, the Company had approximately 57 employees that would be eligible to participate in the ESPP.
Shares Available for Issuance
As noted above, the maximum aggregate number of shares of Company stock that may be issued under the ESPP is one million shares.
Enrollment Dates, Accumulation Periods and Purchase Dates
The accumulation periods under the ESPP will generally be a specified one-year period, or such other period, not to exceed twenty-seven (27) months, as determined by the Administrator. The first trading day of each accumulation period is the enrollment date, which is the date as of which eligible employees are granted contractual rights to purchase shares of Company stock under the ESPP. Payroll deductions may be made during the accumulation period by eligible employee selecting to participate as described below. The last trading day of each accumulation period will be the Company stock purchase date (unless the Administrator selects a different date) and on such date any contractual rights remaining outstanding will be deemed to be exercised and shares of Company stock will be purchased, as described below.
Participation in the ESPP
An eligible employee may become a participant in the ESPP by submitting an enrollment form, and payroll deductions for such employee will begin as soon as administratively feasible after such form is received in good order, subject to compliance with such policies, rules and procedures as we may establish in connection therewith.
As of each purchase date (which is the last trading day of an accumulation period as stated above), an employee’s payroll deductions made during the accumulation period and not withdrawn by the employee or otherwise paid to the employee are used to buy shares of Company stock. The per share purchase price on the purchase date is 85% of the lower of (1) the fair
97
market value of a share of Company stock on the purchase date, or (2) the fair market value of a share of Company stock on the first trading day of the accumulation period.
An employee will not be permitted to purchase more than 25,000 shares of Company stock on any purchase date, or such lower maximum number as may be determined by the Administrator. An employee’s right to purchase shares under the ESPP in any calendar year cannot exceed $25,000, as measured by the fair market value of such shares (determined for each accumulation period as of the first trading day of the accumulation period).
An employee can invest any amount from 1% to 15% of his or her base earnings in Company stock through payroll deductions under the ESPP. Payroll deductions are credited to recordkeeping accounts. No earnings are credited to the accounts.
Withdrawal from the ESPP, Cessation of Payroll Deductions, Mandatory Cessation of Participation
An employee may withdraw from the ESPP in full (but not in part) during any accumulation period by delivering a notice of withdrawal to us (in a manner prescribed by the Administrator) at any time prior to the first day of the last calendar month immediately preceding the purchase date for such accumulation period, or at such shorter time in advance of the purchase date as the Administrator may permit. If notice of withdrawal is timely received, all funds then accumulated in the employee’s account will not be used to purchase shares, but will instead be distributed to the employee as soon as administratively practical, and the employee’s payroll deductions will cease as soon as administratively practical.
An employee also may cease payroll deductions as of the last day of any month during an accumulation period by delivering a notice of cessation to us at the time and in the manner prescribed by the Administrator. Unless the employee also withdraws from the ESPP as described in the preceding paragraph, the employee’s accumulated payroll deductions will be applied to purchase shares of Company stock on the purchase date as described above.
Participation in the ESPP immediately terminates when an employee ceases to be an eligible employee for any reason, including voluntary or involuntary termination of employment. Upon the termination of an employee’s participation in the ESPP, all accumulated payroll deductions of the employee will be returned to the employee.
Amendment and Termination
The board of directors or the compensation committee may amend or alter any provision of the ESPP and may terminate the ESPP at any time. Under certain circumstances, an amendment to the ESPP may require the approval of our stockholders. In addition, if the ESPP is amended to change the aggregate number of shares issuable thereunder or the provisions regarding eligible employees, certain tax advantages under the Code as discussed below (see “Certain Federal Income Tax Consequences Relating to the ESPP”) will only continue if we obtain stockholder approval of such amendment. Certain amendments to the ESPP may be made by the Administrator without stockholder approval.
In the event of any Company reorganization, recapitalization, stock split, reverse stock split, stock dividend, combination of shares, merger, consolidation, acquisition of property or shares, separation, asset spin-off, stock rights offering, liquidation or other similar change in the capital structure of the Company, the shares subject to an employee’s election to purchase Company stock during an accumulation period will be adjusted and the aggregate number and kind of shares available under the ESPP and the purchase price of shares will also be adjusted, in each case to the extent deemed appropriate by the Administrator. Generally, if a dissolution or liquidation of the Company occurs during an accumulation period, any rights an employee has to acquire Company stock under the ESPP will be terminated, but an employee will have the right to acquire Company stock before the dissolution or liquidation.
Certain Federal Income Tax Consequences Relating to the ESPP
The following summary of the income tax consequences of the ESPP is based on current provisions of the Code and regulations thereunder. The summary does not address tax rates or state or local income taxes or taxes in jurisdictions other than the United States, nor does it address employment tax.
Enrollment or Purchase of Company Stock under the ESPP. No federal income tax consequences arise at the time of an employee’s enrollment in the ESPP or upon the purchase of Company stock under the ESPP. However, as discussed below, if an employee disposes of Company stock acquired under the ESPP, such employee will have the federal income tax consequences described below in the year such employee disposes of the stock. Amounts withheld by payroll deduction are subject to federal income tax as though those amounts had been paid in cash. Whenever an employee transfers any shares of Company stock in a manner which may constitute a disposition, such employee must promptly advise the Secretary of the Company of the facts concerning that transfer.
Early Dispositions. If an employee disposes of Company stock purchased under the ESPP within two years after the first trading day of an accumulation period or within one year after the shares of Company stock are transferred to such employee
98
or to an account in such employee’s name (the “Tax Holding Period”), such employee will recognize compensation income in the year of disposition in an amount equal to the excess of (A) the lesser of the fair market value of the Company stock on the purchase date or the proceeds from the sale or exchange of the shares over (B) the price such employee paid for the Company stock. The Company must report such compensation as taxable ordinary income to the Internal Revenue Service on such employee’s annual Form W-2.The amount, if any, that is taxable as ordinary income is added to the purchase price and becomes part of the cost basis for that Company stock for federal income tax purposes. If the disposition of the Company stock involves a sale or exchange, such employee generally may also realize a short-term capital gain or loss equal to the difference between such employee’s cost basis (calculated pursuant to the preceding sentence)and the proceeds from the sale or exchange of the shares.
Later Dispositions. If an employee disposes of Company stock purchased under the ESPP on a date after the Tax Holding Period, or if such employee dies at any time while owning Company stock, such employee (such employee’s estate) will have included in such employee’s compensation as taxable ordinary income in the year of disposition or death, an amount equal to the lesser of
The amount which is taxable as ordinary income is added to the cost basis of that Company stock for federal income tax purposes. The cost basis is therefore the sum of the purchase price of the Company stock and the ordinary income recognized from the formula above. If the disposition of the Company stock involves a sale or exchange, such employee will also realize a long-term capital gain or loss equal to the difference between such employee’s cost basis (calculated pursuant to the preceding sentence) and the proceeds from the sale or exchange of the shares.
The Company is not entitled to a deduction for amounts taxed as ordinary income or capital gain to an employee except to the extent of ordinary income recognized upon a sale or disposition during the Tax Holding Period (an early disposition).
SAB Biotherapeutics 2014 Equity Incentive Plan
The SAB Biotherapeutics 2014 Equity Incentive Plan (the “2014 Incentive Plan”) was adopted on June 27, 2014.
Summary of the 2014 Incentive Plan
General
The 2014 Incentive Plan covers the grant of awards to our employees (including officers), non-employee consultants and non-employee directors and those of our affiliates. For purposes of the 2014 Incentive Plan, our affiliates include any “parent” or “majority-owned subsidiary” of the Company, as such terms are defined in Rule 405 of the Securities Act.
The compensation committee administers the 2014 Incentive Plan. The full Board must approve all decisions regarding awards to non-employee directors.
Up to a maximum of 800,000 shares of our common stock (as adjusted following the Reverse Stock Split) may be delivered in settlement of awards granted under the 2014 Incentive Plan.
Types of Awards
The Incentive Plan permits the granting of any or all of the following types of awards to all grantees:
For a description of each of these types of awards, see “SAB Biotherapeutics 2021 Equity Incentive Plan”.
99
Business Combination, Consolidation or Similar Corporate Transaction
If there is a merger or consolidation of us with or into another corporation or a sale of substantially all of our stock (a “Corporate Transaction”), and the outstanding awards are not assumed by surviving company (its parent company) or replaced with equivalent awards granted by the surviving company(its parent company),the committee will cancel any outstanding awards that are not vested and nonforfeitable as of the consummation of such Corporate Transaction (unless the committee accelerates the vesting of any such awards) and with respect to any vested and nonforfeitable awards, the committee may either (i) allow all grantees to exercise options and SARs within a reasonable period prior to the consummation of the Corporate Transaction and cancel any outstanding options or SARs that remain unexercised upon consummation of the Corporate Transaction, or (ii) cancel any or all of such outstanding awards (including options and SARs) in exchange for a payment (in cash, or in securities or other property) in an amount equal to the amount that the grantee would have received (net of the exercise price with respect to any options or SARs) if the vested awards were settled or distributed or such vested options and SARs were exercised immediately prior to the consummation of the Corporate Transaction. If an exercise price of an option or SAR exceeds the fair market value of our common stock and the option or SAR is not assumed or replaced by the surviving company(its parent company),such options and SARs will be cancelled without any payment to the grantee.
Amendment to and Termination of the 2014 Incentive Plan
The 2014 Incentive Plan may be amended, altered, suspended, discontinued or terminated by our board of directors without further stockholder approval, unless such approval is required by law or regulation or under the rules of any stock exchange or automated quotation system on which our common stock is then listed or quoted. Thus, stockholder approval will not necessarily be required for amendments which might increase the cost of the 2014 Incentive Plan or broaden eligibility. Stockholder approval will not be deemed to be required under laws or regulations that condition favorable treatment of grantees on such approval, although our board of directors may, in its discretion, seek stockholder approval in any circumstance in which it deems such approval advisable.
In addition, subject to the terms of the 2014 Incentive Plan, no amendment or termination of the 2014 Incentive Plan may materially and adversely affect the right of a grantee under any award granted under the 2014 Incentive Plan.
Unless earlier terminated by our board of directors, the 2014 Incentive Plan will terminate when no shares remain reserved and available for issuance or, if earlier, on the tenth anniversary of the effective date of the 2014 Incentive Plan, which is June 27, 2024.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers. For more information, see “Certain Relationships and Related Transactions, and Director Independence - Indemnification Agreements.”
Potential Payments upon Termination or Change in Control
The table below reflects, as applicable, amounts payable to our current named executive officers in connection with a termination by the Company without cause, by the executive for good reason, or upon non-renewal by the Company in the event of a change in control. For purposes of our agreements with our named executive officers, “cause” means, in the judgement of the Company: (i) executive engages in any act or omission which is in bad faith and to the detriment of the Company; (ii) executive willfully and materially violates any of the Company’s then-current policies and procedures; (iii) executive’s willful failure to perform his or her duties under the employment agreement; (iv) executive exhibits unfitness for service, dishonesty, habitual neglect, persistent and serious deficiencies in performance, or incompetence; (v) executive is convicted of, or there is an entry of guilty (or a nolo contender) plea by executive to, a crime (other than a minor traffic violation); (vi) executive materially breaches provision of the agreement related to nondisclosure, assignment of inventions and/or non-solicitation; or (vii) executive refuses or fails to act on any reasonable or lawful directive or order from the Board or executive's supervisor.
100
A summary of the potential payments that each of our current named executive officers would have received upon the occurrence of these events, assuming that each triggering event occurred on December 31, 2023, is set forth below.
|
|
Salary |
|
|
Equity |
|
|
Perquisites / Benefits |
|
|
Other |
|
|
Total |
|
|||||
Name and Principal Position |
|
($) |
|
|
($) |
|
|
($) |
|
|
($) |
|
|
($) |
|
|||||
Samuel J. Reich |
|
|
350,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
350,000 |
|
Chairman of the Board of Directors and Chief Executive Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Eddie J. Sullivan, PhD. |
|
|
377,200 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
377,200 |
|
President |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Alexandra Kropotova, MD |
|
|
525,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
525,000 |
|
EVP, Chief Medical Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Michael G. King, Jr. |
|
|
450,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
450,000 |
|
EVP, Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Christoph Bausch, PhD |
|
|
325,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
325,000 |
|
EVP, Chief Operating Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth information regarding the beneficial ownership of our common stock as of March 18, 2024, by:
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security. Under those rules, beneficial ownership includes securities that the individual or entity has the right to acquire, such as through the exercise of stock options, within 60 days. Shares subject to options that are currently exercisable or exercisable within 60 days are considered outstanding and beneficially owned by the person holding such options for the purpose of computing the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the Company believes that the persons and entities named in the table below have sole voting and investment power with respect to all shares shown as beneficially owned by them. Unless otherwise noted, the business address of each of the directors and executive officers of the Company is 2100 East 54th Street North, Sioux Falls, SD 57104.
The percentage of beneficial ownership of the Company is calculated based on 9,225,494 shares of common stock outstanding as of March 18, 2024. Shares of common stock subject to warrants, options or rights currently exercisable, or exercisable within 60 days of March 18, 2024 are counted as beneficially owned.
101
Shares Beneficially Owned(1)
Beneficial Owner |
|
Common Stock |
|
|
Percent |
|
Series A-2 |
|
|
Percent |
|
Percent |
|||||||||||
Executive Officers and Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Christine Hamilton (2) |
|
|
881,242 |
|
|
|
9.48 |
|
% |
|
|
— |
|
|
* |
|
% |
|
5.51 |
|
% |
||
Eddie J. Sullivan, PhD (3) |
|
|
590,975 |
|
|
|
6.36 |
|
% |
|
|
— |
|
|
* |
|
% |
|
3.69 |
|
% |
||
Samuel J. Reich (4) |
|
|
144,718 |
|
|
|
1.56 |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
||
Jeffrey G. Spragens (5) |
|
|
49,789 |
|
|
* |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
|||
William Polvino, MD (6) |
|
|
13,958 |
|
|
* |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
|||
David Link, MBA (7) |
|
|
20,394 |
|
|
* |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
|||
Scott Giberson (8) |
|
|
1,534 |
|
|
* |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
|||
Erick Lucera (9) |
|
|
902 |
|
|
* |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
|||
Andrew Moin (10) |
|
|
458,457 |
|
|
|
4.97 |
|
% |
|
|
28,380 |
|
|
67.19 |
|
% |
|
24.29 |
|
% |
||
Katie Ellias (11) |
|
|
285,714 |
|
|
3.1 |
|
% |
|
|
— |
|
|
* |
|
% |
|
|
1.79 |
|
% |
||
Alexandra Kropotova (12) |
|
|
23,161 |
|
|
* |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
|||
Michael G. King, Jr. (13) |
|
|
500 |
|
|
* |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
|||
Christoph Bausch (14) |
|
|
48,722 |
|
|
* |
|
% |
|
|
— |
|
|
* |
|
% |
|
* |
|
% |
|||
All directors and executive officers |
|
|
2,520,066 |
|
|
|
26.40 |
|
% |
|
|
28,380 |
|
|
|
67.19 |
|
% |
|
|
33.85 |
|
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Other 5% Stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Entities affiliated with BVF |
|
|
917,826 |
|
|
|
9.95 |
|
% |
|
|
12,217 |
|
|
28.93 |
|
% |
|
15.99 |
|
% |
||
Entities Managed by RTW |
|
|
917,827 |
|
|
|
9.95 |
|
% |
|
|
217 |
|
|
* |
|
% |
|
5.97 |
|
% |
||
* Represents beneficial ownership of less than one percent (1%).
102
103
Equity Compensation Plan Information
We currently maintain the following equity compensation plans that provide for the issuance of shares of our common stock to our officers and other employees, directors and consultants, each of which has been approved by our stockholders: the Incentive Plan; and the ESPP. We also maintain the 2014 Incentive Plan, which was not approved by our securityholders and was in place prior to us being a public company.
The following table presents information as of December 31, 2023 with respect to compensation plans under which shares of our common stock may be issued:
|
|
(a) |
|
|
(b) |
|
|
(c) |
|
|||
|
|
Number of Securities |
|
|
Weighted-average exercise price of outstanding securities |
|
|
Number of securities remaining available for future issuance under equity compensation plans |
|
|||
Equity compensation plans approved by security holders (2) |
|
|
667,731 |
|
|
$ |
13.82 |
|
|
|
620,031 |
|
Equity compensation plans not approved by security holders (3) |
|
|
408,675 |
|
|
$ |
14.49 |
|
|
|
323,755 |
|
Total |
|
|
1,076,406 |
|
|
$ |
14.08 |
|
|
|
943,786 |
|
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The following includes a summary of transactions since January 1, 2023 to which we have been a party, in which the amount involved in the transaction exceeded the lesser of (i) $120,000 and (ii) 1% of the average of the Company’s total assets at year-end for the last two completed fiscal years, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive Compensation.”
October 2023 Private Placement
On September 29, 2023, we entered into a securities purchase agreement with certain accredited investors (the “September 2023 Purchase Agreement”), pursuant to which the Company agreed to issue and sell shares of preferred stock and warrants, in a private placement which provides for up to $110 million in proceeds across multiple tranches. Between October 2023 and November 2023, we received an aggregate of approximately $67.1 million for shares of preferred stock issued in this private placement offering. On September 29, 2023, we entered into a Board Designation Agreement, dated as of September 29, 2023, with Sessa Capital (Master), L.P. (“Sessa Capital”), pursuant to which Andrew Moin, who is a partner of Sessa Capital, was appointed as a member of the Board.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers. Each indemnification agreement provides for indemnification and advancements by us of certain expenses and costs relating to claims, suits or proceedings arising from his or her service to us or, at our request, service to other entities, as officers or directors to the maximum extent permitted by applicable law.
Policies and Procedures for Transactions with Related Parties
The Company has adopted a written Related Party Transaction Policy that set forth its procedures for the identification, review, consideration and approval or ratification of related person transactions. A related person includes directors, executive officers, beneficial owners of 5% or more of any class of the Company’s voting securities, immediate family members of any of the foregoing persons, and any entities in which any of the foregoing is an executive officer or is an owner
104
of 5% or more ownership interest. Under the Related Party Transaction Policy, if a transaction involving an amount in excess of $120,000 has been identified as a related person transaction, including any transaction that was not a related person transaction when originally consummated or any transaction that was not initially identified as a related person transaction prior to consummation, information regarding the related person transaction must be reviewed and approved by the Company’s audit committee.
In considering related person transactions, the Company’s audit committee will take into account the relevant available facts and circumstances including, but not limited to:
The Related Party Transaction Policy requires that, in determining whether to approve, ratify or reject a related person transaction, the audit committee must review all relevant information available to it about such transaction, and that it may approve or ratify the related person transaction only if it determines that, under all of the circumstances, the transaction is in, or is not inconsistent with, the best interests of the Company.
Item 14. Principal Accounting Fees and Services.
The following table represents aggregate fees billed to the Company for the fiscal year ended December 31, 2023 by EisnerAmper LLP (“EisnerAmper”), the Company’s independent registered public accounting firm.
(US Dollars) |
|
2023 |
|
|
2022 |
|
||
Audit fees |
|
$ |
299,687 |
|
|
$ |
— |
|
Audit-related fees |
|
|
— |
|
|
|
— |
|
Tax fees |
|
|
— |
|
|
|
— |
|
All other fees |
|
|
— |
|
|
|
— |
|
Total |
|
$ |
299,687 |
|
|
$ |
— |
|
Audit fees for the fiscal years ended December 31, 2023 rendered by EisnerAmper relate to professional services rendered for the audit of our financial statements, quarterly reviews, issuance of consents, and review of documents filed with the SEC.
The following table represents aggregate fees for professional services rendered for the Company by Mayer Hoffman McCann P.C. (“MHM”), its former independent registered public accounting firm for the years ended December 31, 2023 and 2022. Substantially all of MHM’s personnel, who work under the control of MHM shareholders, are employees of wholly-owned subsidiaries of CBIZ, Inc., which provides personnel and various services to MHM in an alternative practice structure.
(US Dollars) |
|
2023 |
|
|
2022 |
|
||
Audit fees |
|
$ |
318,388 |
|
|
$ |
710,644 |
|
Audit-related fees |
|
|
— |
|
|
|
— |
|
Tax fees |
|
|
— |
|
|
|
— |
|
All other fees |
|
|
— |
|
|
|
— |
|
Total |
|
$ |
318,388 |
|
|
$ |
710,644 |
|
Audit fees for the fiscal years ended December 31, 2023 and 2022 rendered by MHM relate to professional services rendered for the audits of our fiscal year 2022 financial statements, quarterly reviews prior to the Company’s change in independent registered public accounting firm, issuance of consents, and review of documents filed with the SEC.
105
Pre-Approval Policies and Procedures
The Audit Committee has adopted a policy that sets forth the procedures and conditions pursuant to which audit and non-audit services proposed to be performed by the independent auditor may be pre-approved. The policy generally provides that we will not engage our independent registered public accounting firm (EisnerAmper) to render any audit, audit-related, tax or permissible non-audit service unless the service is either (i) explicitly approved by the Audit Committee (“specific pre-approval”) or (ii) entered into pursuant to the pre-approval policies and procedures described in the policy (“general pre-approval”). Unless a type of service to be provided by our independent registered public accounting firm has received general pre-approval under the policy, it requires specific pre-approval by the Audit Committee or by a designated member of the Audit Committee to whom the committee has delegated the authority to grant pre-approvals. Any proposed services exceeding pre-approved cost levels or budgeted amounts will also require specific pre-approval. For both types of pre-approval, the Audit Committee will consider whether such services are consistent with the SEC’s rules on auditor independence.
106
PART IV
Item 15. Exhibits, Financial Statement Schedules.
|
|
|
|
|
|
Exhibit Number |
Description |
Schedule/ Form |
File No. |
Exhibit |
Filing Date |
1.1 |
8-K |
001-39871 |
1.1 |
January 26, 2024 |
|
2.1+ |
8-K |
001-39871 |
2.1+ |
October 28, 2021 |
|
2.2+ |
8-K |
001-39871 |
2.2 |
October 28, 2021 |
|
3.1 |
8-K |
001-39871 |
3.1 |
October 28, 2021 |
|
3.2 |
8-K |
001-39871 |
3.2 |
October 28, 2021 |
|
3.3 |
8-K |
001-39871 |
3.1 |
October 2, 2023 |
|
3.4 |
Certificate of Amendment to the Amended and Restated Certificate of Incorporation |
8-K |
001-39871 |
3.1 |
November 22, 2023 |
3.5 |
8-K |
001-39871 |
3.1 |
January 3, 2024 |
|
4.1 |
S-1/A |
333-258869 |
4.2 |
January 4, 2021 |
|
4.2 |
Specimen Warrant Certificate of Registrant (incorporated by reference to Exhibit 4.3 of Form S-1/A.) |
S-1/A |
333-258869 |
4.3 |
January 4, 2021 |
4.3 |
Form of Warrant Agreement between Registrant and Continental Stock Transfer & Trust Company. |
S-1/A |
333-258869 |
4.4 |
January 4, 2021 |
4.4 |
10-Q |
001-39871 |
4.1 |
May 15, 2023 |
|
4.5* |
|
|
|
|
|
4.6 |
8-K |
001-39871 |
4.1 |
October 2, 2023 |
|
4.7 |
8-K |
001-39871 |
4.2 |
October 2, 2023 |
|
4.8 |
8-K |
001-39871 |
4.3 |
October 2, 2023 |
|
10.1 |
8-K |
001-39871 |
10.1 |
October 28, 2021 |
|
10.2¥ |
8-K |
001-39871 |
10.2¥ |
October 28, 2021 |
|
10.3¥ |
8-K |
001-39871 |
10.1 |
November 19, 2021 |
107
10.4 |
8-K |
001-39871 |
10.6 |
October 28, 2021 |
|
10.6¥ |
SAB Biotherapeutics, Inc. 2021 Omnibus Equity Incentive Plan. |
8-K |
001-39871 |
10.7 |
October 28, 2021 |
10.6¥ |
SAB Biotherapeutics, Inc. 2021 Employee Stock Purchase Plan. |
8-K |
001-39871 |
10.8 |
October 28, 2021 |
10.7 |
S-4 |
333-258869 |
10.3 |
September 22, 2021 |
|
10.8 |
S-4 |
333-258869 |
10.4 |
September 22, 2021 |
|
10.9 |
S-4 |
333-258869 |
10.5 |
September 22, 2021 |
|
10.10 |
S-4 |
333-258869 |
10.7 |
September 22, 2021 |
|
10.11 |
S-4 |
333-258869 |
10.8 |
September 22, 2021 |
|
10.12¥ |
10-K |
001-39871 |
10.5 |
April 14, 2023 |
|
10.13 |
10-K |
001-39871 |
10.14 |
April 14, 2023 |
|
10.14 |
8-K |
001-39871 |
10.1 |
October 13, 2022 |
|
10.15+ |
8-K |
001-39871 |
10.1 |
November 1, 2022 |
|
10.16+ |
8-K |
001-39871 |
10.2 |
November 1, 2022 |
|
10.17 |
8-K |
001-39871 |
10.1 |
December 12, 2022 |
|
10.18 |
8-K |
001-39871 |
10.1 |
October 2, 2023 |
|
10.19¥ |
8-K |
001-39871 |
10.1 |
October 27, 2023 |
|
10.20¥ |
S-8 |
333-277314 |
99.2 |
February 23, 2024 |
|
10.21¥ |
8-K |
001-39871 |
10.1 |
March 8, 2024 |
|
10.22¥ |
8-K |
001-39871 |
10.2 |
March 8, 2024 |
|
16.1 |
Letter from Mayer Hoffman McCann P.C. to the Securities and Exchange Commission dated July 31, 2023 |
8-K |
001-39871 |
16.1 |
July 31, 2023 |
21.1* |
|
|
|
|
|
23.1* |
|
|
|
|
|
23.2* |
|
|
|
|
|
24.1* |
Power of Attorney (included on a signature page of the initial filing of this Annual Report) |
|
|
|
|
31.1* |
|
|
|
|
108
31.2* |
|
|
|
|
|
32.1* |
|
|
|
|
|
32.2* |
|
|
|
|
|
97.1* |
|
|
|
|
|
101.INS |
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
|
|
|
|
101.SCH |
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
|
|
|
|
104 |
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
|
|
|
* Filed herewith.
+ Schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request.
¥ Denotes management contract or any compensatory plan, contract or arrangement.
Item 16. Form 10-K Summary
None.
109
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
SAB BIOTHERAPEUTICS, INC. |
|
|
|
|
|
Date: March 28, 2024 |
|
By: |
/s/ Samuel J. Reich |
|
|
|
Samuel J. Reich |
|
|
|
Chair and Chief Executive Officer |
The undersigned officers and directors of SAB Biotherapeutics, Inc., hereby severally constitute and appoint Samuel J. Reich and Eddie J. Sullivan, and each of them individually, with full power of substitution and resubstitution, as their true and lawful attorneys and agents, to do any and all acts and things in their name and behalf in their capacities as directors and officers and to execute any and all instruments for them and in their names in the capacities indicated below, which said attorneys and agents, may deem necessary or advisable to enable said corporation to comply with the Securities Exchange Act of 1934, as amended, and any rules, regulations and requirements of the Securities and Exchange Commission, in connection with this Annual Report on Form 10-K, including specifically but without limitation, power and authority to sign for them or any of them in their names in the capacities indicated below, any and all amendments hereto, and they do hereby ratify and confirm all that said attorneys and agents, or either of them, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
Name |
|
Title |
|
Date |
|
|
|
|
|
/s/ Samuel J. Reich |
|
Chair and Chief Executive Officer |
|
March 28, 2024 |
Samuel J. Reich |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ Michael G. King, Jr. |
|
Chief Financial Officer |
|
March 28, 2024 |
Michael G King, Jr. |
|
(Principal Financial Officer and Principal Accounting Officer) |
|
|
|
|
|
|
|
/s/ Eddie J. Sullivan, PhD |
|
President and Director |
|
March 28, 2024 |
Eddie J. Sullivan, PhD |
|
|
|
|
|
|
|
|
|
/s/ Katie Ellias |
|
Director |
|
March 28, 2024 |
Katie Ellias |
|
|
|
|
|
|
|
|
|
/s/ Christine Hamilton, MBA |
|
Director |
|
March 28, 2024 |
Christine Hamilton, MBA |
|
|
|
|
|
|
|
|
|
/s/ Scott Giberson, RPh, MPH, D.Sc. |
|
Director |
|
March 28, 2024 |
Scott Giberson, RPh, MPH, D.Sc. |
|
|
|
|
|
|
|
|
|
/s/ David Link, MBA |
|
Director |
|
March 28, 2024 |
David Link, MBA |
|
|
|
|
|
|
|
|
|
/s/ Erick Lucera |
|
Director |
|
March 28, 2024 |
Erick Lucera |
|
|
|
|
|
|
|
|
|
/s/ Andrew Moin |
|
Director |
|
March 28, 2024 |
Andrew Moin |
|
|
|
|
|
|
|
|
|
/s/ William Polvino, MD |
|
Director |
|
March 28, 2024 |
William Polvino, MD |
|
|
|
|
|
|
|
|
|
/s/ Jeffrey G. Spragens |
|
Director |
|
March 28, 2024 |
Jeffrey G. Spragens |
|
|
|
|
|
|
|
|
|
1
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
SAb Biotherapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of SAB Biotherapeutics, Inc. and Subsidiaries (the “Company”) as of December 31, 2023, and the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows for the year then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2023, and the consolidated results of their operations and their cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
We also have audited the adjustments to the 2022 financial statements to retrospectively reflect the reverse stock split, as described in Note 2. In our opinion, such adjustments are appropriate and have been properly applied. We were not engaged to audit, review, or apply any procedures to the 2022 financial statements of the Company other than with respect to the adjustments and, accordingly, we do not express an opinion or any other form of assurance on the 2022 financial statements taken as a whole.
/s/ EisnerAmper LLP
We have served as the Company’s auditor since 2023.
EISNERAMPER LLP
Iselin, New Jersey
March 28, 2024
F-2
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
SAB Biotherapeutics, Inc. and Subsidiaries
Opinion on the Consolidated Financial Statements
We have audited, before the effects of the adjustments to retrospectively apply the reverse stock split described in Note 2, the accompanying consolidated balance sheet of SAB Biotherapeutics, Inc. and Subsidiaries (“Company”) as of December 31, 2022, and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for the year then ended, and the related notes (collectively referred to as the “financial statements”). The 2022 financial statements before the effects of the adjustments discussed in Note 2 are not presented herein. In our opinion, the financial statements, before the effects of the adjustments to retrospectively apply the reverse stock split described in Note 2, present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of their operations and their cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
We were not engaged to audit, review, or apply any procedures to the adjustments to retrospectively apply the reverse stock split described in Note 2 and, accordingly, we do not express an opinion or any other form of assurance about whether such adjustments are appropriate and have been properly applied. Those adjustments were audited by EisnerAmper L.L.P.
Going Concern Uncertainty
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company does not generate sufficient cash flows from operations to maintain operations and, therefore, is dependent on additional financing to fund operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1 to the financial statements. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Mayer Hoffman McCann P.C.
We served as the Company's auditor from 2019 to 2023
San Diego, California
April 14, 2023
F-3
SAB Biotherapeutics, Inc. and Subsidiaries
Consolidated Balance Sheets
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
|
|
|
|
||
Assets |
|
|
|
|
|
|
||
Current assets |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Accounts receivable, net |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
Long-term prepaid insurance |
|
|
|
|
|
|
||
Operating lease right-of-use assets |
|
|
|
|
|
|
||
Financing lease right-of-use assets |
|
|
|
|
|
|
||
Property, plant and equipment, net |
|
|
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
|
||
Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
||
Current liabilities |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
|
|
$ |
|
||
Notes payable |
|
|
|
|
|
|
||
Operating lease liabilities, current portion |
|
|
|
|
|
|
||
Finance lease liabilities, current portion |
|
|
|
|
|
|
||
Deferred grant income |
|
|
|
|
|
|
||
Accrued expenses and other current liabilities |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
Operating lease liabilities, noncurrent |
|
|
|
|
|
|
||
Finance lease liabilities, noncurrent |
|
|
|
|
|
|
||
Warrant liabilities |
|
|
|
|
|
|
||
Notes payable, noncurrent |
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
Stockholders’ equity |
|
|
|
|
|
|
||
Preferred stock; $ |
|
|
|
|
|
|
||
Common stock; $ |
|
|
|
|
|
|
||
Treasury stock, at cost; |
|
|
( |
) |
|
|
( |
) |
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated other comprehensive income |
|
|
|
|
|
— |
|
|
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Total stockholders’ equity |
|
|
|
|
|
|
||
Total liabilities and stockholders’ equity |
|
$ |
|
|
$ |
|
||
*The consolidated balance sheets' common stock share amounts have been retroactively adjusted to account for the Company's Reverse Stock Split, effective January 5, 2024.
See accompanying notes to the consolidated financial statements.
F-4
SAB Biotherapeutics, Inc. and Subsidiaries
Consolidated Statements of Operations and Comprehensive Loss
|
|
For The Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Revenue |
|
|
|
|
|
|
||
|
$ |
|
|
$ |
|
|||
Total revenue |
|
|
|
|
|
|
||
Operating expenses |
|
|
|
|
|
|
||
Research and development |
|
|
|
|
|
|
||
General and administrative |
|
|
|
|
|
|
||
Total operating expenses |
|
|
|
|
|
|
||
Loss from operations |
|
|
( |
) |
|
|
( |
) |
Other income (expense) |
|
|
|
|
|
|
||
Changes in fair value of warrant liabilities |
|
|
( |
) |
|
|
|
|
Interest expense |
|
|
( |
) |
|
|
( |
) |
Interest income |
|
|
|
|
|
|
||
Other income |
|
|
|
|
|
|
||
Total other income (expense) |
|
|
( |
) |
|
|
|
|
Loss before income taxes |
|
|
( |
) |
|
|
( |
) |
Income tax expense (benefit) |
|
|
— |
|
|
|
|
|
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Other comprehensive loss: |
|
|
|
|
|
|
||
Foreign currency translation |
|
$ |
|
|
|
— |
|
|
Total comprehensive loss |
|
$ |
( |
) |
|
$ |
( |
) |
Loss per common share attributable to the Company’s shareholders |
|
|
|
|
|
|
||
Basic and diluted loss per common share |
|
$ |
( |
) |
|
$ |
( |
) |
Weighted-average common shares outstanding – basic and diluted |
|
|
|
|
|
|
||
*The consolidated statements of operations and comprehensive loss's share and per share amounts have been retroactively adjusted to account for the Company's Reverse Stock Split, effective January 5, 2024.
See accompanying notes to the consolidated financial statements.
F-5
SAB Biotherapeutics, Inc. and Subsidiaries
Consolidated Statements of Changes In Stockholders’ Equity
For the years ended December 31, 2023 and 2022
|
|
|
Common stock |
|
|
Preferred Stock |
|
|
|
|
|
Treasury Stock |
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Additional |
|
|
Shares |
|
|
Amount |
|
|
Accumulated |
|
|
Accumulated Other Comprehensive Income |
|
|
Total Stockholders’ |
|
||||||||||
Balance at December 31, 2021 |
|
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
( |
) |
|
$ |
— |
|
|
$ |
|
||||||||
Forward Share Purchase Agreement, final settlement |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Repurchase of common stock pursuant to the Forward Share Purchase Agreement |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
— |
|
|
— |
|
|
|
— |
|
|||||
Stock-based compensation |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Issuance of common stock for exercise of stock options |
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Issuance of common stock and warrants under private placement offering, net of issuance costs of $ |
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Net Loss |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
( |
) |
|
— |
|
|
|
( |
) |
||||||
Balance at December 31, 2022 |
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
— |
|
|
|
|
||||
Issuance of common stock for exercise of stock options |
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Issuance of common stock for settlement of accrued liabilities and professional fees |
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|||||||
Professional fees settled with warrants |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Professional fees settled with shares |
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Issuance of Series A Preferred Stock and warrants under private placement offering |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Series A Preferred Stock warrant exercise |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|||||||||
Conversion of Series A Preferred Stock into common shares |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Stock-based compensation |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
||||||||
Net loss |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
|
( |
) |
|
— |
|
|
|
( |
) |
|||||
Foreign currency translation |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
||||||
Balance at December 31, 2023 |
|
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
|
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
|
$ |
|
|||||||
*The consolidated statements of stockholder's equity share amounts have been retroactively adjusted to account for the Company's Reverse Stock Split, effective January 5, 2024.
See accompanying notes to the consolidated financial statements.
F-6
SAB Biotherapeutics, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Cash flows from operating activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
|
|
|
|
||
Amortization of finance right-of-use assets |
|
|
|
|
|
|
||
Stock-based compensation expense |
|
|
|
|
|
|
||
Gain on sale of equipment |
|
|
|
|
|
( |
) |
|
Gain on partial lease termination |
|
|
— |
|
|
|
( |
) |
Loss on private placement issuance |
|
|
|
|
|
— |
|
|
Gain from private placement warrant termination |
|
|
( |
) |
|
|
— |
|
Changes in fair value of warrant liabilities |
|
|
|
|
|
( |
) |
|
Professional fees settled with equity instruments |
|
|
|
|
|
— |
|
|
Changes in operating assets and liabilities |
|
|
|
|
|
|
||
Accounts receivable |
|
|
|
|
|
|
||
Prepaid expenses |
|
|
( |
) |
|
|
|
|
Operating lease right-of-use assets |
|
|
|
|
|
|
||
Accounts payable |
|
|
( |
) |
|
|
( |
) |
Due to related party |
|
|
— |
|
|
|
( |
) |
Deferred grant income |
|
|
|
|
|
( |
) |
|
Accrued expense and other current liabilities |
|
|
( |
) |
|
|
( |
) |
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Cash flows from investing activities: |
|
|
|
|
|
|
||
Proceeds from the sale of equipment |
|
|
|
|
|
|
||
Purchases of equipment |
|
|
( |
) |
|
|
( |
) |
Net cash used in investing activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Cash flows from financing activities: |
|
|
|
|
|
|
||
Proceeds from issuance of common stock |
|
|
— |
|
|
|
|
|
Proceeds from private placement issuance of preferred stock and warrants |
|
|
|
|
|
— |
|
|
Proceeds from exercise of private placement preferred warrants |
|
|
|
|
|
— |
|
|
Payments related to the Forward Share Purchase Agreement |
|
|
— |
|
|
|
( |
) |
Proceeds from issuance of notes payable |
|
|
|
|
|
|
||
Payments of notes payable |
|
|
( |
) |
|
|
( |
) |
Principal payments on finance leases |
|
|
( |
) |
|
|
( |
) |
Proceeds from exercise of stock options |
|
|
|
|
|
|
||
Net cash provided by financing activities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Effect of exchange rate changes on cash and cash equivalents |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
||
Net increase (decrease) in cash and cash equivalents |
|
|
|
|
|
( |
) |
|
Cash and cash equivalents |
|
|
|
|
|
|
||
Beginning of year |
|
|
|
|
|
|
||
End of year |
|
$ |
|
|
$ |
|
||
Supplemental cash flow information: |
|
|
|
|
|
|
||
Cash paid for interest |
|
$ |
|
|
$ |
|
||
Cash paid for income taxes |
|
$ |
— |
|
|
$ |
|
|
|
|
|
|
|
|
|
||
Supplemental information on non-cash investing and finance activities: |
|
|
|
|
|
|
||
Right-of-use assets obtained in exchange for operating lease liabilities |
|
$ |
|
|
$ |
|
||
Right-of-use assets forfeited due to partial lease terminations |
|
$ |
— |
|
|
$ |
|
|
Operating lease liabilities eliminated due to partial lease terminations |
|
$ |
— |
|
|
$ |
|
|
Note payable issued in consideration for abated lease payments |
|
$ |
— |
|
|
$ |
|
|
Settlement of accrued liabilities through the issuance of common stock |
|
$ |
|
|
$ |
— |
|
|
Issuance of common stock for prepaid marketing and investor related consulting services |
|
$ |
|
|
$ |
— |
|
|
Fair value of private placement preferred warrant liability associated with warrant exercise |
|
$ |
|
|
$ |
— |
|
|
See accompanying notes to the consolidated financial statements.
F-7
SAb Biotherapeutics, Inc. and subsidiaries
Notes to conSOLIDATED financial statements
(1) Nature of Business
SAB Biotherapeutics, Inc., a Delaware corporation (“SAB” or “SAB Biotherapeutics”, and together with its subsidiaries, the “Company”), is a clinical-stage biopharmaceutical company focused on the development and commercialization of a portfolio of products from its proprietary immunotherapy platform to produce fully targeted human polyclonal antibodies, without using human plasma or serum. SAB’s novel immunotherapy platform that is developing fully-human hIgC for delaying onset or progression of T1D.
Australian Research and Development Tax Credit
In June 2023, the Company formed a new subsidiary in Australia, SAB BIO PTY LTD, a proprietary limited company (“SAB Australia”), primarily to conduct preclinical and clinical activities for product candidates. SAB Australia’s research and development activities qualify for the Australian government’s tax credit program, which provides a
Liquidity
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern, which contemplates continuity of operations, realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has experienced net losses, negative cash flows from operations and, as of December 31, 2023, had an accumulated deficit of $
On September 29, 2023, the Company entered into a securities purchase agreement with certain accredited investors (the “September 2023 Purchase Agreement”), pursuant to which the Company agreed to issue and sell shares of preferred stock and warrants, in a private placement which provides for up to $
Based on the Company’s current level of operating expenses, existing resources will be sufficient to cover operating cash needs through the twelve months following the date these financials are issued. The Company plans to seek additional funding through a combination of equity or debt financings, or other third-party financing, collaborative or other funding arrangements. Should the Company seek additional financing from outside sources, the Company may not be able to raise such financing on terms acceptable to the Company or at all. If the Company is unable to raise additional capital when required or on acceptable terms, the Company may be required to scale back or discontinue the advancement of product candidates, reduce headcount, liquidate assets, file for bankruptcy, reorganize, merge with another entity, or cease operations.
(2) Summary of Significant Accounting Policies
A summary of the significant accounting policies applied in preparation of the accompanying consolidated financial statements is set forth below.
Basis of presentation
The financial statements have been prepared in conformity with GAAP and include all adjustments necessary for the fair presentation of the Company’s financial position for the years presented.
Emerging growth company status
Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new
F-8
or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Principles of consolidation
The accompanying consolidated financial statements include the results of the Company and its wholly owned subsidiaries, SAB Sciences, Inc., SAB LLC, SAB Capra, LLC, Aurochs, LLC, and SAB Australia. Intercompany balances and transactions have been eliminated in consolidation.
Significant risks and uncertainties
The Company’s operations are subject to a number of factors that can affect its operating results and financial condition. Such factors include, but are not limited to, the results of research and development efforts, clinical trial activities of the Company’s product candidates, the Company’s ability to obtain regulatory approval to market its product candidates, competition from products manufactured and sold or being developed by other companies, and the Company’s ability to raise capital.
The Company currently has no commercially approved products and there can be no assurance that the Company’s research and development will be successfully commercialized. Developing and commercializing a product requires significant time and capital and is subject to regulatory review and approval as well as competition from other biotechnology and pharmaceutical companies. The Company operates in an environment of rapid change and is dependent upon the continued services of its employees and obtaining and protecting intellectual property.
Funding from government grants is not guaranteed to cover all costs, and additional funding may be needed to cover operational costs as the Company moves forward to with our efforts to develop a commercially approved product.
Use of estimates
Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The following fair value hierarchy classifies the inputs to valuation techniques that would be used to measure fair value into one of three levels:
Level 1: Unadjusted quoted prices in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs that reflect the reporting entity’s own assumptions.
Certain of the Company’s financial instruments are not measured at fair value on a recurring basis but are recorded at amounts that approximate their fair value due to the short-term nature of their maturities, such as cash and cash equivalents, accounts receivable, accounts payable and accrued expenses.
F-9
The Company accounts for warrants to purchase its preferred and common stock pursuant to ASC Topic 470, Debt, and ASC Topic 480, Distinguishing Liabilities from Equity, and classifies warrants for preferred and common stock as liabilities or equity. The warrants classified as liabilities are reported at their estimated fair value (see Note 13, Fair Value Measurements) and any changes in fair value are reflected in other income and expense. The warrants classified as equity are reported at their estimated relative fair value with no subsequent remeasurement. The Company’s outstanding warrants are discussed in more detail in Note 13, Fair Value Measurements.
Cash, cash equivalents, and restricted cash
Cash equivalents include short-term, highly liquid instruments, consisting of money market accounts and short-term investments with original maturities at the date of purchase of 90 days or less.
Accounts receivable
Accounts receivable are carried at original invoice amount, less an allowance for doubtful accounts. The Company estimates an allowance for doubtful accounts for potential credit losses that are expected to be incurred, based on management’s assessment of the collectability of specific accounts, the aging of the accounts receivable, historical information and other currently available evidence. Receivables are written off when deemed uncollectible. To date,
Concentration of credit risk
The Company maintains its cash and cash equivalent balances in the form of business checking accounts and money market accounts, the balances of which, at times, may exceed federally insured limits. Although the Company currently believes that the financial institutions with whom it does business will be able to fulfill their commitments to the Company, there is no assurance that those institutions will be able to continue to do so. The Company has not experienced any credit losses associated with its balances in such accounts for the year ended December 31, 2023 and 2022.
Lease liabilities and right-of-use assets
The Company is party to certain contractual arrangements for equipment, lab space, and an animal facility, which meet the definition of leases under ASC 842. In accordance with ASC 842, the Company recorded right-of-use assets and related lease liabilities for the present value of the lease payments over the lease terms. The Company’s IBR was used in the calculation of its right-of-use assets and lease liabilities.
The Company elected not to apply the recognition requirements of ASC 842 to short-term leases, which are deemed to be leases with a lease term of twelve months or less. Instead, the Company recognized lease payments in the Consolidated Statements of Operations on a straight-line basis over the lease term and variable payments in the period in which the obligation for these payments was incurred. The Company elected this policy for all classes of underlying assets.
Research and development expenses
Expenses incurred in connection with research and development activities are expensed as incurred. These include licensing fees to use certain technology in the Company’s research and development projects, fees paid to consultants and various entities that perform certain research and testing on behalf of the Company, and expenses related to animal care, research-use equipment depreciation, salaries, benefits, and stock-based compensation granted to employees in research and development functions.
During the years ended December 31, 2023 and 2022, the Company had contracts with multiple CROs to complete studies as part of research grant agreements. These costs include upfront, milestone and monthly expenses as well as reimbursement for pass through costs. All research and development costs are expensed as incurred except when the Company is accounting for nonrefundable advance payments for goods or services to be used in future research and development activities. In these cases, these payments are capitalized at the time of payment and expensed in the period the research and development activity is performed. As actual costs become known, the Company will adjust the accrual; such changes in estimate may be a material change in the Company’s clinical study accrual, which could also materially affect reported results of operations. For the years ended December 31, 2023 and 2022, there were no material adjustments to the Company’s prior period estimates of accrued expenses for clinical trial.
F-10
Property, Plant and Equipment
The Company records property, plant, and equipment at cost less depreciation and amortization. Depreciation is calculated using straight-line methods over the following estimated useful lives:
Animal facility equipment |
|
Laboratory equipment |
|
Leasehold improvements |
|
Office furniture and equipment |
|
Vehicles |
Repairs and maintenance expenses are expensed as incurred.
Impairment of long-lived assets
The Company reviews the recoverability of long-lived assets, including the related useful lives, whenever events or changes in circumstances indicate that the carrying amount of a long-lived asset may not be recoverable. If necessary, the Company compares the estimated undiscounted future net cash flows to the related asset’s carrying value to determine whether there has been an impairment. If an asset is considered impaired, the asset is written down to fair value, which is based either on discounted cash flows or appraised values in the period the impairment becomes known. The Company believes that long-lived assets are recoverable, and
Stock-based compensation
FASB ASC Topic 718, Compensation– Stock Compensation, prescribes accounting and reporting standards for all share-based payment transactions in which employee and non-employee services are acquired. The Company recognizes compensation cost relating to stock-based payment transactions using a fair-value measurement method, which requires all stock-based payments to employees, directors, and non-employee consultants, including grants of stock options, to be recognized in operating results as compensation expense based on fair value over the requisite service period of the awards. The Company determines the fair value of common stock based on the closing market price at closing on the date of the grant.
Income taxes
Deferred income taxes reflect future tax effects of temporary differences between the tax and financial reporting basis of the Company’s assets and liabilities measured using enacted tax laws and statutory tax rates applicable to the periods when the temporary differences will affect taxable income. When necessary, deferred tax assets are reduced by a valuation allowance, to reflect realizable value, and all deferred tax balances are reported as long-term on the consolidated balance sheet. Accruals are maintained for uncertain tax positions, as necessary.
The Company uses a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken, or expected to be taken, in a tax return. The Company has elected to treat interest and penalties related to income taxes, to the extent they arise, as a component of income taxes.
Revenue recognition
The Company’s revenue is primarily generated through grants from government and other (non-government) organizations.
Grant revenue is recognized during the period that the research and development services occur, as qualifying expenses are incurred or conditions of the grants are met. Deferred grant income represents grant proceeds received by the Company prior to the period in which the underlying research and development services have not yet been performed. The Company concluded that payments received under these grants represent conditional, nonreciprocal contributions, as described in ASC
F-11
Foreign Currency Translations and Transactions
Comprehensive income (loss)
Foreign currency translation adjustments of $
Litigation
From time to time, the Company is involved in legal proceedings, investigations and claims generally incidental to its normal business activities. In accordance with U.S. GAAP, the Company accrues for loss contingencies when it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Legal costs in connection with loss contingencies are expensed as incurred.
Earnings per share
In accordance with ASC 260, Earnings per Share(“ASC 260”), basic net income (loss) per share attributable to common stockholders is computed by dividing net income (loss) attributable to common stockholders by the weighted-average number of common stock outstanding during the period. Diluted net income (loss) per share attributable to common stockholders is computed by dividing the diluted net income (loss) attributable to common stockholders by the weighted-average number of common stock outstanding for the period including potential dilutive common shares such as stock options.
In accordance with ASC 280, Segment Reporting, the Company’s business activities are organized into
Australian Research and Development Tax Credit
The Company recognizes other income from Australian research and development incentives when there is reasonable assurance that the income will be received, the relevant expenditure has been incurred, and the consideration can be reliably measured. The research and development incentive is one of the key elements of the Australian Government’s support for Australia’s innovation system and is supported by legislative law primarily in the form of the Australian Income Tax Assessment Act 1997, as long as eligibility criteria are met. Under the program, a percentage of eligible research and development expenses incurred by the Company through its subsidiary in Australia are reimbursed.
Management has assessed the Company’s research and development activities and expenditures to determine which activities and expenditures are likely to be eligible under the research and development incentive regime described above. At each period end, management estimates the refundable tax offset available to the Company based on available information at the time and it is included in other income in the consolidated statements of operations.
Retroactive Adjustments for Common Stock Reverse Split
On January 5, 2024, the Company completed a reverse stock split of the Company’s Common Stock. As a result of the Reverse Stock Split, every ten of the Company’s issued shares of Common Stock were automatically combined into one
F-12
issued share of Common Stock, without any change to the par value per share. All share and per share numbers in this Annual Report on Form 10-K have been adjusted to reflect the Reverse Stock Split.
(3) New accounting standards
Recently-adopted standards
In July 2016, the FASB issued ASU No. 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”), which requires the measurement of all expected credit losses of financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. Financial institutions and other organizations will now use forward-looking information to better inform their credit loss estimates. In addition, the ASU amends the accounting for credit losses on available-for-sale debt securities and purchased financial assets with credit deterioration. ASU 2016-13 is effective for periods beginning after December 15, 2022, and interim periods within those fiscal years. The Company
Recently Issued Accounting Standards
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The amendments require (i) enhanced disclosures in connection with an entity's effective tax rate reconciliation and (ii) income taxes paid disaggregated by jurisdiction. The amendments are effective for annual periods beginning after December 15, 2024. The Company is currently evaluating the impact of adopting this ASU on its consolidated financial statements and disclosures.
(4) Revenue
During the years ended December 31, 2023 and 2022, the Company worked on the following grants:
Government grants
The total revenue for government grants was approximately $
NIH-NIAID (Federal Award #1R44AI117976-01A1) – this grant was for $
NIH-NIAID (Federal Award #1R41AI131823-02) – this grant was for approximately $
NIH-NIAID through Geneva Foundation (Federal Award #1R01AI132313-01, Subaward #S-10511-01) – this grant was for approximately $
DoD, JPEO through Advanced Technology International – this grant was for a potential of $
The grants for the JPEO Rapid Response contract are cost reimbursement agreements, with reimbursement of qualified direct research and development expense (labor and consumables) with an overhead charge (based on actual, reviewed quarterly) and a fixed fee (
On August 3, 2022, the Company received notice from the DoD terminating the JPEO Rapid Response contract. The Company engaged in negotiations with the DoD to compensate the Company for services provided prior to the JPEO Rapid
F-13
Response Contract Termination and costs the Company would be expected to bear in future periods. A termination and settlement proposal was submitted the DoD on September 9, 2022; the Company submitted a final invoice on December 15, 2022; and received payment from the DoD on or about January 12, 2023. The terms of the arrangement provide for a cost-reimbursable structure, and state that the parties will work in good faith equitable reimbursement for work performed toward accomplishment of the tasks provided in the agreement. At this time, other than certain deferred obligations (presented within deferred grant income within the Company's consolidated balance sheet) potentially payable to the DoD solely due to subsequent negotiations with third-party vendors, the Company believes and has been advised there is a reasonable, good faith basis for the position that no present or future obligations exist. Revenue recognized subsequent to the JPEO Rapid Response Contract Termination relates to satisfaction of residual obligations under the termination and settlement agreement—see Note 2, Summary of Significant Accounting Policies in the Company's consolidated financial statements for further information about the Company's established revenue recognition process.
(5) Earnings per share
Since the Company reported a net loss for the years ended December 31, 2023 and 2022, it was required by ASC 260 to use basic weighted-average shares outstanding when calculating diluted net loss per share for the years ended December 31, 2023 and 2022, as the potential dilutive securities are anti-dilutive.
|
|
For The Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Calculation of basic and diluted loss per share |
|
|
|
|
|
|
||
Net loss attributable to the Company’s shareholders |
|
$ |
( |
) |
|
$ |
( |
) |
Weighted-average common shares outstanding – |
|
|
|
|
|
|
||
Net loss per share, basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
The Company’s potentially dilutive securities, which include stock options, restricted stock awards, common stock warrants, earnout shares, and contingently issuable earnout shares have been excluded from the computation of diluted net loss per share as the effect would be to reduce the net loss per share. Therefore, the weighted average number of common shares outstanding used to calculate both basic and diluted net loss per share attributable to common stockholders is the same.
|
|
For The Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Stock options and awards |
|
|
|
|
|
|
||
Convertible Debt |
|
|
|
|
|
|
||
Common Stock Warrants (1) |
|
|
|
|
|
|
||
Earnout Shares (2) |
|
|
|
|
|
|
||
Series A Preferred Stock (3) |
|
|
|
|
|
— |
|
|
Preferred Stock Warrants (4) |
|
|
|
|
|
— |
|
|
Contingently issuable Earnout Shares from unexercised Rollover |
|
|
|
|
|
|
||
Total |
|
|
|
|
|
|
||
F-14
(6) Property, Plant and Equipment
As of December 31, 2023 and 2022, the Company’s equipment was as follows:
|
|
December 31, |
|
|
December 31, |
|
||
Laboratory equipment |
|
$ |
|
|
$ |
|
||
Animal facility leasehold improvements |
|
|
|
|
|
|
||
Animal facility equipment |
|
|
|
|
|
|
||
Construction-in-progress |
|
|
|
|
|
|
||
Leasehold improvements |
|
|
|
|
|
|
||
Vehicles |
|
|
|
|
|
|
||
Office furniture and equipment |
|
|
|
|
|
|
||
Total Property, plant and equipment, gross |
|
|
|
|
|
|
||
Less: accumulated depreciation and amortization |
|
|
( |
) |
|
|
( |
) |
Property, plant and equipment, net |
|
$ |
|
|
$ |
|
||
Depreciation and amortization expense for the years ended December 31, 2023 and 2022 was $
All tangible personal property with a useful life of at least
As of December 31, 2023 and 2022, the Company’s construction-in-progress was as follows:
|
|
December 31, |
|
|
December 31, |
|
||
New office space at Headquarters |
|
$ |
|
|
$ |
|
||
IT equipment at Headquarters |
|
|
|
|
|
|
||
Software |
|
|
|
|
|
|
||
Total construction-in-progress |
|
$ |
|
|
$ |
|
||
(7) Leases
The Company has an operating lease for lab space from Sanford Health, under a lease that started in June 2014 and initially ended in June 2019, at which time the lease was extended through August 2024. This lease can be terminated with
F-15
lease liability were remeasured at the modification date of October 1, 2022. The October 2022 lease amendment reduced the lease payment to approximately $
The Company entered into a lease for office, laboratory, and warehouse space in November 2020, which the Company amended in July 2022. This lease has a
The Company has the following finance leases:
The lease agreements do not require material variable lease payments, residual value guarantees or restrictive covenants.
The amortizable lives of the operating lease assets are limited by their expected lease terms. The amortizable lives of the finance lease assets are limited by their expected lives, as the Company intends to exercise the purchase options at the end of the leases.
Animal Facility |
|
Equipment |
|
Land |
Indefinite |
The Company’s weighted-average remaining lease term and weighted-average discount rate for operating and finance leases as of December 31, 2023 are:
|
|
Operating |
|
Finance |
|
|
Weighted-average remaining lease term |
|
|
|
|||
Weighted-average discount rate |
|
|
|
% |
||
F-16
The table below reconciles the undiscounted future minimum lease payments under non-cancelable leases with terms of more than one year to the total lease liabilities recognized on the consolidated balance sheet as of December 31, 2023:
|
|
Operating |
|
|
Finance |
|
||
2024 |
|
$ |
|
|
$ |
|
||
2025 |
|
|
|
|
|
|
||
2026 |
|
|
|
|
|
|
||
2027 |
|
|
— |
|
|
|
|
|
2028 |
|
|
— |
|
|
|
|
|
Thereafter |
|
|
— |
|
|
|
|
|
Undiscounted future minimum lease payments |
|
|
|
|
|
|
||
Less: Amount representing interest payments |
|
|
( |
) |
|
|
( |
) |
Total lease liabilities |
|
|
|
|
|
|
||
Less current portion |
|
|
( |
) |
|
|
( |
) |
Noncurrent lease liabilities |
|
$ |
|
|
$ |
|
||
Operating lease expense was approximately $
Finance lease costs for the years ended December 31, 2023 and 2022 included approximately $
Cash payments under operating and finance leases were approximately $
(8) Accrued Expenses and Other Current Liabilities
As of December 31, 2023 and 2022, accrued expenses and other current liabilities consisted of the following:
|
|
December 31, |
|
|
December 31, |
|
||
Accrued vacation |
|
$ |
|
|
$ |
|
||
Accrued payroll |
|
|
|
|
|
|
||
Accrued construction-in-progress |
|
|
|
|
|
|
||
Accrued consulting |
|
|
|
|
|
|
||
Accrued clinical trial expense |
|
|
|
|
|
|
||
Accrued outside laboratory services |
|
|
|
|
|
|
||
Accrued bonus & severance |
|
|
|
|
|
|
||
Accrued contract manufacturing |
|
|
|
|
|
|
||
Accrued legal |
|
|
|
|
|
|
||
Accrued financing fees payable |
|
|
|
|
|
|
||
Accrued franchise tax payable |
|
|
|
|
|
|
||
Accrued interest |
|
|
|
|
|
|
||
Other accrued expenses |
|
|
|
|
|
|
||
|
|
$ |
|
|
$ |
|
||
F-17
(9) Notes Payable
As of December 31, 2023 and 2022, notes payable was as follows:
|
|
December 31, |
|
|
December 31, |
|
||
Insurance financing note payable |
|
$ |
|
|
$ |
|
||
8% Unsecured Convertible Note |
|
|
|
|
|
|
||
Total notes payable |
|
|
|
|
|
|
||
Less: notes payable - current portion |
|
|
|
|
|
|
||
Notes payable, noncurrent |
|
$ |
|
|
$ |
|
||
8% Unsecured Convertible Note
Pursuant to the Fourth Amendment to the Company’s lease with Sanford Health, the Company and Sanford Health agreed to a period of Abated Rent from October 1, 2022 to September 30, 2023. In exchange for the Abated Rent, effective as of October 1, 2022, the Company issued to Sanford Health an
Pursuant to the 8% Unsecured Convertible Note, the Company shall pay the sum of approximately $
The Company evaluated the treatment of the 8% Unsecured Convertible Note under ASC 470 and determined the Principal in its entirety would be allocated to debt. The Company’s consolidated balance sheet as of December 31, 2023, includes accrued interest relating to the 8% Unsecured Convertible Note of approximately $
Insurance Financing
The Company obtained financing for certain Director & Officer liability insurance policy premiums. The agreement assigns First Insurance Funding (“Lender”) a first priority lien on and security interest in the financed policies and any additional premium required in the financed policies including (a) all returned or unearned premiums, (b) all additional cash contributions or collateral amounts assessed by the insurance companies in relation to the financed policies and financed by Lender, (c) any credits generated by the financed policies, (d) dividend payments, and (e) loss payments which reduce unearned premiums. If any circumstances exist in which premiums related to any Financed Policy could become fully earned in the event of loss, Lender shall be named a loss-payee with respect to such policy.
The total premiums, taxes and fees financed is approximately $
(10) Stockholder's Equity
Authorized Capital Stock
The total number of shares of the Company’s authorized capital stock is
F-18
Series A Preferred Stock
On September 29, 2023, the Company entered into a securities purchase agreement (the “September 2023 Purchase Agreement”) with certain accredited investors, pursuant to which the Company agreed to issue and sell, in a private placement (the “September 2023 Offering”), (i)
On October 3, 2023, the Company closed on the issuance of the
The Company recorded $
Subject to the terms and limitations contained in the Certificate of Designation:
Prior to the extended mandatory exercise time, certain investors informed the Company that they would not exercise their mandatorily exercisable Preferred Tranche A Warrants. Certain of the investors agreed to assume and exercise
F-19
Warrants and the remaining Tranche B Warrants and Tranche C Warrants issued to the Investors who failed to exercise their Tranche B Warrants were cancelled. Following these updates to the offering, the Company issued
Pursuant to the Certificate of Designation of Preferences, Rights and Limitations of the Series A Convertible Voting Preferred Stock, (the “Certificate of Designation”), all shares of Series A-1 Preferred Stock, subject to the Stockholder Approval obtained in November 2023, were automatically converted into an aggregate of
Following Shareholder Approval of the September 2023 Private Placement on November 22, 2023, the Company issued
For information pertaining to the Company’s outstanding warrants to purchase shares of the Company’s preferred stock, see Note 12, Warrants.
Earnout Shares
On October 22, 2021 (the “Closing Date”), the Company consummated the business combination contemplated by the agreement and plan of merger, dated as of June 21, 2021, as amended on August 12, 2021, made by and among Big Cypress Acquisition Corp., a Delaware corporation (“BCYP”), Big Cypress Merger Sub Inc., a Delaware corporation (“Merger Sub”), the Company, and Shareholder Representative Services LLC, a Colorado limited liability company, solely in its capacity as the representative, agent and attorney-in-fact of the SAB Stockholders (the “Business Combination”). Upon closing of the Business Combination, Merger Sub merged with SAB Biotherapeutics, with SAB Biotherapeutics as the surviving company of the merger. Upon closing of the Business Combination, BCYP changed its name to “SAB Biotherapeutics, Inc.”.
Additionally, the Business Combination Agreement included an earnout provision whereby the shareholders of SAB Biotherapeutics shall be entitled to receive additional consideration (“Earnout Shares”) if the Company meets certain Volume Weighted Average Price (“VWAP") thresholds, or a change in control with a per share price exceeding the VWAP thresholds within a five-year period immediately following the Closing.
The Earnout Shares shall be released in four equal increments as follows:
Pursuant to the terms of the Business Combination Agreement, SAB Biotherapeutics’ securityholders (including vested option holders) who own SAB Biotherapeutics securities immediately prior to the Closing Date will have the contingent right to receive their pro rata portion of (i) an aggregate of
The Earnout Shares are indexed to the Company’s equity and meet the criteria for equity classification. On the Closing Date, the fair value of the
F-20
dividend by reducing additional paid-in capital, which was offset by the increase in additional paid-in capital associated with the Business Combination.
Warrants
For information pertaining to the Company’s outstanding warrants to purchase shares of the Company’s common stock, see Note 12, Warrants.
(11) Stock Option Plans
On August 5, 2014, the Company approved a stock option grant plan (the “2014 Equity Incentive Plan”) for employees, directors, and non-employee consultants, which provides for the issuance of options to purchase common stock. As of December 31, 2023, there were
The Company adopted the 2021 Omnibus Equity Incentive Plan (the “2021 Equity Incentive Plan”, and collectively with the 2014 Equity Incentive Plan, the “Equity Compensation Plans”), which reserved
The expected term of the stock options was estimated using the “simplified” method, as defined by the SEC’s Staff Accounting Bulletin No. 107, Share-Based Payment. The volatility assumption was determined by examining the historical volatilities for industry peer companies, as the Company does not have sufficient trading history for its common stock. The risk-free interest rate assumption is based on the U.S. Treasury instruments whose term was consistent with the expected term of the options. The dividend assumption is based on the Company’s history and expectation of dividend payouts. The Company has never paid dividends on its common stock and does not anticipate paying dividends on its common stock in the foreseeable future. Therefore, the Company has assumed no dividend yield for purposes of estimating the fair value of the options.
Stock Options
Stock option activity for employees and non-employees under the Equity Compensation Plans for the years ended December 31, 2023 and 2022 was as follows:
|
|
Options |
|
|
Weighted |
|
|
Weighted Average Remaining Contractual Life (years) |
|
|
Aggregate Intrinsic Value |
|
||||
Outstanding options, December 31, 2022 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Granted |
|
|
|
|
$ |
|
|
|
|
|
|
|
||||
Forfeited |
|
|
( |
) |
|
$ |
|
|
|
|
|
|
|
|||
Exercised |
|
|
( |
) |
|
$ |
|
|
|
|
|
|
|
|||
Expired |
|
|
( |
) |
|
$ |
|
|
|
|
|
|
|
|||
Outstanding options, December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Options vested and exercisable, December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Total unrecognized compensation cost related to non-vested stock options as of December 31, 2023 was approximately $
The weighted average grant date fair value of options granted during the years ended December 31, 2023 and 2022, was $
F-21
The estimated fair value of stock options granted during to employees and consultants for the years ended December 31, 2023 and 2022, were calculated using the Black-Scholes option-pricing model using the following assumptions:
|
|
For The Year Ended December 31, |
||||||||
|
|
2023 |
|
2022 |
||||||
Expected volatility |
|
|
% |
|
|
% |
||||
Weighted-average volatility |
|
|
|
% |
|
|
|
% |
||
Expected dividends |
|
— |
|
% |
|
— |
|
% |
||
Expected term (in years) |
|
|
|
|
|
|
||||
Risk-free rate |
|
|
% |
|
|
% |
||||
Restricted Stock
Stock award activity for employees and non-employees under the Equity Compensation Plans for the year ended December 31, 2023 was as follows:
|
|
Number of shares |
|
|
Weighted |
|
||
Unvested as of December 31, 2022 |
|
|
|
|
$ |
|
||
Granted |
|
|
|
|
$ |
|
||
Vested |
|
|
( |
) |
|
$ |
|
|
Unvested as of December 31, 2023 |
|
|
|
|
$ |
|
||
At December 31, 2023, the Company had an aggregate of $
Stock-based compensation expense
Stock-based compensation expense for the December 31, 2023 and 2022 was as follows:
|
|
For The Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Research and development |
|
$ |
|
|
$ |
|
||
General and administrative |
|
|
|
|
|
|
||
Total |
|
$ |
|
|
$ |
|
||
(12) Warrants
Public Warrants
Each whole Public Warrant entitles the holder to purchase
Once the warrants become exercisable, the Company may call the warrants for redemption:
F-22
If the Company calls the warrants for redemption as described above, management will have the option to require any holder that wishes to exercise its warrant to do so on a “cashless basis.” If management takes advantage of this option, all holders of warrants would pay the exercise price by surrendering their warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the excess of the “fair market value” (defined below) over the exercise price of the warrants by (y) the fair market value. The “fair market value” shall mean the average reported last sale price of the common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants.
Private Placement Warrants
The Private Placement Warrants and the common stock issuable upon the exercise of the Private Placement Warrants were not transferable, assignable or saleable until after the completion of the Company's merger transaction in 2021. Additionally, the Private Placement Warrants will be exercisable on a cashless basis and be non-redeemable as long as they are held by the initial purchasers or their permitted transferees. If the Private Placement Warrants are held by someone other than the initial purchasers or their permitted transferees, the Private Placement Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
As of December 31, 2023,
PIPE Warrants and PIPE Placement Agent Warrants
In December 2022, the Company entered into a securities purchase agreement with certain institutional and accredited investors for the sale by the Company of
2023 Ladenburg Agreement Warrants
On March 21, 2023, the Company entered into a settlement agreement with Ladenburg Thalmann & Co. Inc. (“Ladenburg”), effective March 23, 2023 (the “2023 Ladenburg Agreement”, regarding the action brought by Ladenburg, the “Ladenburg Action”). In connection with the 2023 Ladenburg Agreement, on March 24, 2023, the Company (i) issued the Ladenburg Warrants to purchase up to
F-23
September 2023 Purchase Agreement Warrants
As of December 31, 2023, the Company now has outstanding
Both the Tranche B Warrants and Tranche C Warrants were classified as derivative liabilities because they are redeemable for cash upon occurrence of a Fundamental Transaction, as defined in the Forms for such warrants, which may be outside the control of the Company.
Preferred Placement Agent Warrant
On November 21, 2023 the Company issued to Chardan Capital Markets LLC, the placement agent for the Preferred Warrants, a warrant to purchase
The following table summarizes warrant activity for the year ended December 31, 2023:
|
|
Outstanding |
|
|
Warrants Issued |
|
|
Warrants Exercised |
|
|
Warrants Forfeited |
|
|
Outstanding |
|
|||||
Transaction |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
||||
Business Combination Public Warrants |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Private Placement Warrants |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
PIPE Warrants |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
PIPE Placement Agent Warrants |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Ladenburg Warrants |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Tranche A Warrants |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|||
Tranche B Warrants |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|||
Tranche C Warrants |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|||
Preferred PIPE Placement Agent Warrants |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Presentation and Valuation of the Warrants — Liability Classified Warrants
Public Warrants and Private Placement Warrants
The Public Warrants and Private Placement Warrants are accounted for as liabilities in accordance with ASC 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity and were presented within warrant liabilities on the consolidated balance sheets as of December 31, 2023 and 2022. The initial fair value of the warrant liabilities was measured at fair value at the Closing Date, and changes in the fair value of the warrant liabilities were presented within changes in fair value of warrant liabilities in the consolidated statements of operations for the years ended December 31, 2023 and 2022.
On the Closing Date, the Company established the fair value of the Private Placement Warrants utilizing both the Black-Scholes Merton formula and a MCS analysis. Specifically, the Company considered an MCS to derive the implied volatility in the publicly-listed price of the Public Warrants. The Company then considered this implied volatility in selecting the volatility for the application of a Black-Scholes Merton model for the Private Placement Warrants. The Company determined the fair value of the Public Warrants by reference to the quoted market price.
The Public Warrants were classified as a Level 1 fair value measurement, due to the use of the quoted market price, and the Private Placement Warrants held privately by assignees of Big Cypress Holdings LLC, were classified as a Level 3 fair value measurement, due to the use of unobservable inputs. See Note 13, Fair Value Measurements, for changes in fair value of the Private Placement Warrants.
F-24
The key inputs into the valuations as of the December 31, 2023 and 2022 were as follows:
|
|
December 31, |
|
|
December 31, |
|
||
Risk-free interest rate |
|
|
% |
|
|
% |
||
Expected term remaining (years) |
|
|
|
|
|
|
||
Implied volatility |
|
|
% |
|
|
% |
||
Closing common stock price on the measurement date |
|
$ |
|
|
$ |
|
||
Preferred Warrants
Should the Company enter into or be party to a fundamental transaction, the Company will be required to purchase all outstanding Warrants from the holders by paying cash in an amount equal to the Black Scholes Value of the unexercised portion of each Preferred Warrant. As a result, the Preferred Warrants are accounted for as derivative liabilities in accordance with ASC 480 and ASC 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity and were presented within warrant liabilities on the consolidated balance sheet as of December 31, 2023. The initial fair value of the warrant liabilities was measured at fair value at the Closing Date, and changes in the fair value of the warrant liabilities were presented within changes in fair value of warrant liabilities in the consolidated statement of operations for the year ended December 31, 2023.
On the Initial Issuance Date, the Company established the fair value of the Preferred Warrants utilizing the Black-Scholes Merton formula.
All tranches of the Preferred Warrants were classified as Level 3 fair value measurements, due to the use of unobservable inputs. See Note 13, Fair Value Measurements, for changes in fair value of the Preferred Warrants.
The key inputs utilized in determining the fair value of each Tranche A Warrant as of the Initial Issuance Date was as follows:
|
|
October 3, 2023 |
|
|
|
Risk-free interest rate (1) |
|
|
% |
|
|
Expected term remaining (years) (1) |
|
|
|
|
|
Implied volatility |
|
|
% |
|
|
Underlying Stock Price (Preferred Series A) |
|
$ |
|
|
|
(1) |
Reflects a probability-weighted input derived from multiple Black-Scholes calculations. These calculations account for various potential dates for the public announcement of the comprehensive data set from the Sanofi S.A. Protect trial, spanning from mid-October to December 15, 2023. |
The key inputs utilized in determining the fair value of each Tranche B Warrants as of the Initial Issuance Date and December 31, 2023 were as follows:
|
|
October 3, 2023 |
|
|
December 31, |
|
||
Risk-free interest rate (1) |
|
|
% |
|
|
% |
||
Expected term remaining (years) (1) |
|
|
|
|
|
|
||
Implied volatility |
|
|
% |
|
|
% |
||
Underlying Stock Price (Preferred Series A) |
|
$ |
|
|
$ |
|
||
F-25
The key inputs utilized in determining the fair value of each Tranche C Warrants as of the Initial Issuance Date and December 31, 2023 were as follows:
|
|
October 3, 2023 |
|
|
December 31, |
|
||
Risk-free interest rate (1) |
|
|
% |
|
|
% |
||
Expected term remaining (years) (1) |
|
|
|
|
|
|
||
Implied volatility |
|
|
% |
|
|
% |
||
Underlying Stock Price (Preferred Series A) |
|
$ |
|
|
$ |
|
||
Equity Classified Warrants
The Company determined the Ladenburg Warrants, PIPE Warrants, PIPE Placement Agent Warrants, and Preferred PIPE Placement Agent Warrants met all necessary criteria to be accounted for as equity in accordance with ASC 815-40, Derivatives and Hedging—Contracts in Entity’s Own Equity. As such, they are presented within additional paid-in capital within Company’s consolidated statements of changes in stockholders’ equity and consolidated balance sheets.
Warrants classified as equity are initially measured at fair value. Subsequent changes in fair value are not recognized as long as the warrants continue to be classified as equity.
The initial fair value of each PIPE Warrant and PIPE Placement Agent Warrant issued was determined using the Black-Scholes option-pricing model. All relevant terms and conditions for the PIPE Warrant and PIPE Placement Agent Warrant are identical with the exception of the exercise prices of $
The key inputs into the valuations as of the initial measurement date, December 7, 2022, were as follows:
|
|
Initial Measurement |
|
|
Risk-free interest rate |
|
|
% |
|
Expected term remaining (years) |
|
|
|
|
Implied volatility |
|
|
% |
|
Closing common stock price on the measurement date, less discount for lack of marketability (1) |
|
$ |
|
|
(1) |
As the underlying shares are restricted from sale for a period of 180 days from the date of the 2022 Private Placement, the fair value of the warrants was estimated using the Black-Scholes option pricing model that uses several inputs, including market price of the Company’s common shares at the end of each reporting period (a level one input), less a discount for lack of marketability (a level two input). The discount for lack of marketability was estimated upon consideration of volatility and the length of the lock-up period. |
Upon initial measurement, the fair value of the PIPE Warrants and PIPE Placement Agent Warrants were determined to be $
The initial fair value of each Ladenburg Warrant issued and exercisable at $
F-26
The key inputs into the valuations as of the 2023 Ladenburg Agreement initial measurement date, March 21, 2023, were as follows:
|
|
Initial Measurement |
|
|
Risk-free interest rate |
|
|
% |
|
Expected term remaining (years) |
|
|
|
|
Implied volatility |
|
|
% |
|
Closing common stock price on the measurement date |
|
$ |
|
|
Upon initial measurement, the fair value of each Ladenburg Warrant was determined to be $
The initial fair value of each Preferred Placement Agent Warrant issued and exercisable at $
The key inputs into the valuations as of the October 3, 2023 initial measurement date were as follows:
|
|
Initial Measurement |
|
|
Risk-free interest rate |
|
|
% |
|
Expected term remaining (years) |
|
|
|
|
Implied volatility |
|
|
% |
|
Closing common stock price on the measurement date |
|
$ |
|
|
Upon initial measurement, the fair value of each Preferred Placement Agent Warrant was determined to be $
(13) Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The following fair value hierarchy classifies the inputs to valuation techniques that would be used to measure fair value into one of three levels:
Level 1: Unadjusted quoted prices in active markets for identical assets or liabilities.
Level 2: Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active.
Level 3: Unobservable inputs that reflect the reporting entity’s own assumptions.
The following table presents information about the Company's assets and liabilities that are measured at fair value on a recurring basis at December 31, 2023 and 2022, and indicates the fair value hierarchy of the valuation inputs the Company utilized to determine such fair value:
F-27
|
|
As of December 31, 2023 |
|
|||||||||||||
|
|
Total |
|
|
Quoted |
|
|
Significant |
|
|
Significant |
|
||||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Public Warrant liability |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Private Placement Warrant liability |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Preferred Warrants |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
As of December 31, 2022 |
|
|||||||||||||
|
|
Total |
|
|
Quoted |
|
|
Significant |
|
|
Significant |
|
||||
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Public Warrant liability |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Private Placement Warrant liability |
|
$ |
|
|
|
|
|
|
|
|
|
|
||||
Total |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
The following table provides a summary of changes in Level 3 fair value measurements for the Private Placement Warrant Liability:
|
|
|
|
|
Balance, December 31, 2022 |
|
$ |
|
|
Change in fair value of Private Placement Warrant liability |
|
|
( |
) |
Balance, December 31, 2023 |
|
$ |
|
|
The following table provides a summary of the changes in Level 3 fair value measurements for the Preferred Warrant liabilities:
Fair Value as of October 3, 2023 |
|
$ |
|
|
Change in fair value (1) |
|
|
|
|
Fair Value as of December 31, 2023 |
|
$ |
|
(1) |
Includes the impact of a $ |
As of December 31, 2023 and 2022, the Company did not have any other assets or liabilities that are recorded at fair value on a recurring basis.
The Company believes that the carrying amounts of its cash and cash equivalents, accounts receivable, accounts payable, notes payable, accrued expenses and other current liabilities approximate their fair values due to their near-term maturities.
(14) Income Taxes
Net deferred tax assets as of December 31, 2023 and 2022 consisted of the following:
F-28
|
|
December 31, |
|
|
December 31, |
|
||
Deferred tax assets: |
|
|
|
|
|
|
||
Tax Carryforwards |
|
$ |
|
|
$ |
|
||
Compensation Accruals |
|
|
|
|
|
|
||
Amortizable R&D Intangibles |
|
|
|
|
|
|
||
Other Deferred Tax Assets |
|
|
|
|
|
|
||
Total deferred tax assets |
|
|
|
|
|
|
||
Less valuation allowance |
|
|
( |
) |
|
|
( |
) |
Total deferred tax assets |
|
|
|
|
|
|
||
Deferred tax liabilities: |
|
|
|
|
|
|
||
PPE |
|
$ |
|
|
$ |
|
||
Other Deferred Tax Liabilities |
|
|
|
|
|
|
||
Total deferred tax liabilities |
|
|
|
|
|
|
||
Net deferred tax asset (liability) |
|
$ |
|
|
$ |
|
||
The reconciliation between the Company’s effective tax rate and the statutory tax rate of
|
|
December 31, |
|
|
|
|
|
December 31, |
|
|
|
|
||||
Rate reconciliation: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Federal income tax at statutory |
|
$ |
( |
) |
|
|
% |
|
$ |
( |
) |
|
|
% |
||
Equity raise |
|
|
|
|
|
( |
)% |
|
|
( |
) |
|
|
% |
||
Research and development credit RTP |
|
|
( |
) |
|
|
% |
|
|
( |
) |
|
|
% |
||
Other permanent items |
|
|
( |
) |
|
|
% |
|
|
( |
) |
|
|
% |
||
Valuation allowance |
|
|
|
|
|
( |
)% |
|
|
|
|
|
( |
)% |
||
|
|
$ |
|
|
|
% |
|
$ |
|
|
|
( |
)% |
|||
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of the deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. Based upon the level of historical losses and the uncertainty of future taxable income over the periods which the Company will realize the benefits of its net deferred tax assets, management believes it is more likely than not that the Company will not fully realize the benefits on the balance of its net deferred tax asset and, accordingly, the Company has established a valuation allowance on its net deferred tax assets. The valuation allowance increased by approximately $
As of December 31, 2023, the Company had approximately $
Utilization of the Company’s net operating loss (and tax credit carryforwards) are subject to annual limitation(s) due to an ownership change that occurred as a result of the October 2023 Private Placement. In general, an “ownership change”, as defined by Section 382 of the Internal Revenue Code of 1986, as amended, results from a transaction or series of transaction over a three-year period resulting in an ownership change of more than 50 percentage points of the outstanding stock of a company by certain stockholders. However, because the Company was already in a full valuation allowance position, the effect of the ownership was insignificant.
Prior to 2022, taxpayers had the option under Section 174 of the Internal Revenue Code to either deduct their research and development costs or capitalize and amortize such costs over a period of not less than 60 months. As part of the tax law changes in the Tax Act enacted in 2017, starting with tax years beginning after December 31, 2021, Congress requires taxpayers to capitalize expenditures that qualify as Section 174 research and development costs and recover them over 5 years for expenditures attributed to domestic research and 15 years for expenditures attributed to foreign research. The 2022
F-29
effective income tax rate was impacted by the Section 174 capitalization requirement combined with the restriction on net operating losses to only reduce taxable income by 80%.
U.S. GAAP provides that the tax effects from uncertain tax positions can be recognized in the consolidated financial statements only if the position is more likely than not of being sustained on audit, based on the technical merits of the position. As of December 31, 2023 and 2022, there were
The Company files tax returns as prescribed by the laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by federal and state jurisdictions, where applicable. The Company’s tax years are still open under the statute from 2020 to present. However, to the extent allowed by law, the taxing authorities may have the right to examine the period from
(15) Related Party Transactions
For the years ended December 31, 2023 and 2022, there were no related party transactions with directors, executive officers, or beneficial owners of
(16) Employee Benefit Plan
The Company sponsors a defined contribution retirement plan. All the Company’s employees are eligible to be enrolled in the employer-sponsored contributory retirement savings plan, which include features under Section 401(k) of the Internal Revenue Code of 1986, as amended, and provides for Company matching contributions. The Company’s contributions to the plan are determined by its Board of Directors, subject to certain minimum requirements specified in the plan. The Company has historically made matching contributions of
(17) Commitments and Contingencies
The Company is not a party to any litigation, and, to its best knowledge, no action, suit or proceeding has been threatened against the Company which are expected to have a material adverse effect on its financial condition, results of operations or liquidity.
(18) Subsequent Events
On January 26, 2024, the Company entered into a Controlled Equity Offering Sales Agreement (the “Sales Agreement”) with Cantor Fitzgerald & Co. (“Cantor”), relating to shares of our common stock. In accordance with the terms of the Sales Agreement, the Company may offer and sell shares of our common stock having an aggregate offering price of up to $
F-30