Exhibit 99.2
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS FOR THE THREE MONTHS ENDED MARCH 31, 2023 AND 2022
This management discussion and analysis (“MD&A”) of the financial condition and results of operations of Cresco Labs Inc. (the “Company,” “Cresco Labs,” “we,” or “our”) is dated May 24, 2023 and has been prepared for the three months ended March 31, 2023 and 2022. It is supplemental to, and should be read in conjunction with, the Company’s audited Consolidated Financial Statements and accompanying notes as of and for the years ended December 31, 2022 and 2021, which were previously filed on SEDAR, and the Company’s unaudited condensed interim consolidated financial statements and accompanying notes as of and for the three months ended March 31, 2023 and 2022. The Company’s financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). Financial information presented in this MD&A is presented in United States (“U.S.”) dollars (“USD” or “$”) unless otherwise indicated. The three months ended data presented below is unaudited.
The Company has provided certain supplemental non-GAAP financial measures in this MD&A. Where the Company has provided such non-GAAP financial measures, we have also provided a reconciliation to the most comparable GAAP financial measure. Please see the information under the heading “Non-GAAP Financial Measures” for additional information on the Company’s use of non-GAAP financial measures.
This MD&A contains certain “forward-looking statements” and certain “forward-looking information” as defined under applicable U.S. securities laws and Canadian securities laws. Please refer to the discussion of forward-looking statements and information set out under the heading “Cautionary Note Regarding Forward-Looking Information,” located at the beginning of the Company’s Annual Information Form for the year ended December 31, 2022, filed on SEDAR. As a result of many factors, the Company’s actual results may differ materially from those anticipated in these forward-looking statements and information. Please refer to the discussion of risks and uncertainties set out under the heading “Risk Factors,” located within the Company’s Annual Information Form for the year ended December 31, 2022, filed on SEDAR.
OVERVIEW OF THE COMPANY
Cresco Labs was incorporated in the Province of British Columbia and is licensed to cultivate, manufacture and sell cannabis and cannabis-based products. The Company operates in and/or has ownership interests in Arizona, California, Florida, Illinois, Maryland, Massachusetts, Michigan, New York, Ohio and Pennsylvania.
Cresco Labs is primarily engaged in the business of cultivating medical-grade cannabis, manufacturing medical- grade products derived from cannabis cultivation and distributing such products to medical or adult-use consumers in legalized cannabis markets. Cresco Labs exists to provide high-quality and consistent cannabis-based products to consumers. Cresco Labs’ business focuses on regulatory compliance while working to develop condition-specific strains of cannabis and non-invasive delivery methods (alternatives to smoke inhalation) to provide controlled-dosage medicinal cannabis relief to qualified patients and consumers in legalized cannabis markets. As of March 31, 2023, the Company was operating one (1) adult-use and medical cannabis cultivation center, two (2) adult-use and medical cannabis manufacturing centers, five (5) adult-use and medical dispensary locations and five (5) adult-use dispensary locations in Illinois; one (1) medical cannabis cultivation and manufacturing center and eleven (11) medical dispensary locations in Pennsylvania; one (1) medical cannabis cultivation and processing center and five (5) medical dispensary locations in Ohio; two (2) adult-use and medical cannabis cultivation centers, one (1) adult-use and medical cannabis cultivation and distribution facility and one (1) adult-use and medical cannabis distribution facility in California; one
1
(1) adult-use and medical cannabis cultivation and manufacturing center and one (1) adult-use and medical dispensary location in Arizona; one (1) medical processing center in Maryland; three (3) adult-use and medical cannabis cultivation and manufacturing centers, one (1) medical dispensary location, one (1) adult-use dispensary location and two (2) adult-use and medical dispensary locations in Massachusetts; one (1) medical cannabis manufacturing center and four (4) medical dispensary locations in New York; one (1) adult-use and medical cannabis cultivation and processing center in Michigan; and one (1) medical cannabis cultivation and manufacturing center and twenty-eight (28) medical dispensary locations in Florida. For additional information on wholly-owned or effectively controlled subsidiaries and affiliates of Cresco Labs, refer to Note 2 under the heading “Basis of Consolidation” of the Company’s unaudited condensed interim consolidated financial statements for the three months ended March 31, 2023 and 2022.
During 2019, the Company announced a new dispensary brand, Sunnyside*®1. Sunnyside* was created to accelerate industry growth by shifting consumer expectations and perceptions around shopping for cannabis from intimidation and doubt to curiosity and acceptance through a new trial and marketing approach. During the first quarter of 2023, the Company opened one (1) Sunnyside* dispensary in Pennsylvania and seven (7) Sunnyside* dispensaries in Florida. As of March 31, 2023, the Company operated ten (10) Sunnyside* dispensaries in Illinois, eleven (11) dispensaries in Pennsylvania, five (5) dispensaries in Ohio, one (1) dispensary in Arizona, four (4) dispensaries in Massachusetts, four (4) dispensaries in New York and twenty-eight (28) dispensaries in Florida. In April of 2023, the Company opened one (1) additional Sunnyside* location in Miami, Florida, bringing the total number of dispensaries in the state to twenty-nine (29). Cresco Labs’ portfolio of owned cannabis consumer-packaged goods includes Cresco®1, High Supply®2, Mindy’sTM, Good News®2, RemediTM, Wonder Wellness Co.®2 and FloraCal® Farms2. The Company distributes and markets these products both to third-party licensed retail cannabis stores across the U.S. and to Cresco Labs’ owned retail stores.
Cresco Labs’ corporate headquarters is located at Suite 110, 400 W. Erie St, Chicago, IL 60654 and the registered office is located at Suite 2500, 666 Burrard Street, Vancouver, BC V6C 2X8. The Company employs approximately 3,200 people across the organization as of March 31, 2023.
Issuing IPO, Reverse Takeover & Corporate Structure
The Company (then Randsburg Gold Corporation) was incorporated in the Province of British Columbia under the Company Act (British Columbia) on July 6, 1990. On December 30, 1997, the Company changed its name from Randsburg Gold Corporation to Randsburg International Gold Corp. (“Randsburg”) and consolidated its common shares on a five (5) old for one (1) new basis. On November 30, 2018, in connection with a reverse takeover (the “Transaction”), the Company, (i) consolidated its outstanding Randsburg common shares on an 812.63 old for one (1) new basis and (ii) filed an alteration to its Notice of Articles with the British Columbia Registrar of Companies to (a) change its name from Randsburg International Gold Corp to Cresco Labs Inc., (b) amend the rights and restrictions of its existing class of common shares and redesignate such class as the class of Subordinate Voting Shares (“SVS”) and (c) create the Proportionate Voting Shares (“PVS”) and the Super Voting Shares (“MVS”).
Pursuant to the Transaction, the Company (then Randsburg) and Cresco Labs, LLC, completed a series of transactions on November 30, 2018, resulting in a reorganization of Cresco Labs, LLC and Randsburg in which Randsburg became the indirect parent and sole voting unitholder of Cresco Labs, LLC. The Transaction constituted a reverse takeover of Randsburg by Cresco Labs, LLC under applicable securities laws. Cresco Labs, LLC was formed as a limited liability company under the laws of the State of Illinois on October 8, 2013 and is governed by an amended and restated limited liability company agreement.
| 1 | The Sunnyside*® (inclusive of the stand-alone asterisk mark) and Cresco® brands maintain federal trademark registrations for websites pertaining to medical cannabis and cannabis educational services, as well as multiple state trademark registrations. |
| 2 | The High Supply®, Good News®, Wonder Wellness Co.® and FloraCal® Farms brands maintain federal trademark registrations for apparel and multiple state trademark registrations. |
2
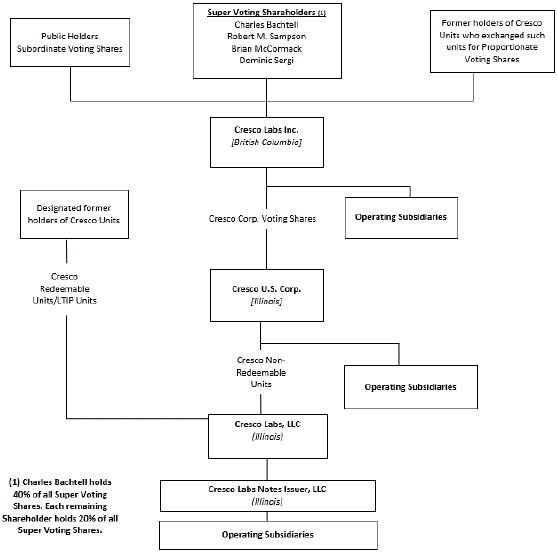
Set forth below is the condensed organization chart of the Company.
Recent Developments
On March 23, 2022, the Company announced it had entered into a definitive arrangement agreement (the “Arrangement Agreement”) with Columbia Care Inc. (“Columbia Care”) to acquire all of the issued and outstanding shares of Columbia Care pursuant to a statutory plan of arrangement (the “Arrangement”) in an all-share transaction (the “Columbia Care Transaction”). Under the terms of the Arrangement Agreement, holders of common shares of Columbia Care will receive 0.5579 SVS of Cresco Labs for each Columbia Care share, subject to adjustments. On July 8, 2022, the shareholders of Columbia Care voted to approve the Arrangement. On July 15, 2022, Columbia Care obtained the final order from the Supreme Court of British Columbia approving the Arrangement. The Company continues to collaborate closely with Columbia Care on the required divestiture transactions to find a path forward that makes both strategic and financial sense. The Company has no update on the timing for execution of agreements relating to outstanding divestiture transactions.
3
In the fourth quarter of 2022, Management committed to a plan to restructure certain operations and activities within the California reporting unit. The plan was effective as of May 15, 2023. In conjunction with the termination of manufacturing and distribution activities at these locations, the Company entered into an agreement with a third-party distribution company to purchase and distribute the on-hand inventory as of that date. Related to that plan, during the first quarter of 2023, the Company recognized a $1.1 million gain on lease termination related to the impacted facilities and additional depreciation expense taken on leasehold improvements at those locations in the amount of $1.1 million. Further, $1.0 million of accounts receivable was reserved for and the Company recorded a $0.7 million severance accrual for one-time involuntary termination benefits.
Components of Our Results of Operations
Revenue
For the three months ended March 31, 2023 and 2022, approximately 57.6% and 55.6% of our revenue was derived from Company-owned retail dispensary locations. Retail revenue includes medical and adult-use cannabis sales in the U.S. Revenue from the wholesale of cannabis products represents the remaining 42.4% and 44.4% for the same periods.
Gross profit
Gross profit is calculated as revenue less cost of goods sold (“COGS”). COGS includes the direct costs attributable to the cultivation and production of the products sold and is comprised of the following:
| • | Direct labor costs: These expenses include all salaries, benefits and taxes for all employees at the cultivation and manufacturing facilities. |
| • | Direct supplies: The direct material cost for maintenance of the plants, the supplies and nutrients, the production expenses, packaging costs and equipment used to process marijuana. |
| • | Facility expenses: The facility expense for the cultivation operations is the cost for the facility, utilities, property taxes, maintenance and costs associated with monitoring the security systems. |
| • | Other operating expenses: These expenses include all costs associated with the facility itself including insurance, community benefit fees, professional services related to licenses and compliance, uniforms, employee training programs, tracking and inventory management systems, product testing, business development, information technology, license renewal fees and certain excise taxes. |
In addition to market fluctuations, cannabis costs are affected by various state regulations that limit the sourcing and procurement of cannabis products. The changes in regulatory environments may create fluctuations in gross profit over comparative periods. Additionally, gross profit may include the cost of inventory required to be marked to fair value as part of purchase accounting in a business combination.
Selling, general and administrative expenses (“SG&A”)
SG&A consist mainly of salary and benefit costs of executive and back-office employees, consulting and professional fees, advertising and marketing, office and retail operation costs, share-based compensation, certain excise taxes, technology, insurance, security, travel and entertainment, rent expense and business expansion costs.
4
Selling costs generally correlate to revenue. As a percentage of sales, we expect SG&A to generally decrease as our revenue increases due to efficiencies associated with scaling the business, while market conditions and investments in growing the business may contribute to increases as a percentage of sales in some periods.
For the three months ended March 31, 2023 and 2022, SG&A was comprised of the following:
| Three Months Ended | ||||||||
| March 31, | ||||||||
| ($ in thousands) |
2023 | 2022 | ||||||
| Payroll and employee costs |
$ | 40,234 | $ | 39,487 | ||||
| Selling and marketing expenses |
2,492 | 3,025 | ||||||
| Share-based compensation |
6,124 | 6,506 | ||||||
| Depreciation and amortization |
4,273 | 4,552 | ||||||
| Excise taxes |
3,712 | 4,912 | ||||||
| Facility expenses |
5,802 | 7,750 | ||||||
| Consulting and professional fees |
2,012 | 4,412 | ||||||
| Computer and software expense |
2,480 | 2,650 | ||||||
| Business insurance |
2,510 | 2,443 | ||||||
| Rental fees |
3,436 | 1,962 | ||||||
| Accounting |
2,392 | 2,540 | ||||||
| Legal |
2,110 | 3,843 | ||||||
| Travel and employee expenses |
864 | 1,120 | ||||||
| Litigation accrual adjustment |
380 | 513 | ||||||
| Other expenses |
3,473 | 1,391 | ||||||
|
|
|
|
|
|||||
| Total SG&A |
$ | 82,294 | $ | 87,106 | ||||
|
|
|
|
|
|||||
Other income (expense), net
Other income (expense), net consists mainly of reoccurring gains (losses) on derivative instruments, foreign currency and derivative liabilities related to warrants as well as ad hoc expenses such as gain (loss) on lease termination and gain (loss) on disposition of assets. These gains (losses) do not generally correlate to revenue and do not include interest expense, net, which when added to Other income, net, sum to Total other expense, net discussed in the “Selected Financial Information” section below.
5
For the three months ended March 31, 2023 and 2022, Other income (expense), net consisted of the following:
| Three Months Ended | ||||||||
| March 31, | ||||||||
| ($ in thousands) |
2023 | 2022 | ||||||
| Unrealized gain on derivative liabilities - warrants |
$ | — | $ | 375 | ||||
| Loss on derivative instruments |
— | (5,667 | ) | |||||
| (Loss) gain on provision - loan receivable |
(59 | ) | 738 | |||||
| Unrealized loss on investments held at fair value |
(37 | ) | (1,670 | ) | ||||
| Loss on disposal of assets |
(66 | ) | — | |||||
| (Loss) gain on foreign currency |
(31 | ) | 68 | |||||
| Gain on lease termination |
1,135 | — | ||||||
| Other income (loss), net |
17 | (616 | ) | |||||
|
|
|
|
|
|||||
| Total Other income (expense), net |
$ | 959 | $ | (6,772 | ) | |||
|
|
|
|
|
|||||
Income Taxes
The Company is classified for U.S. federal income tax purposes as a U.S. corporation under Section 7874 of the Internal Revenue Code (“IRC”). The Company is subject to income taxes in the jurisdictions in which it operates and, consequently, income tax expense is a function of the allocation of taxable income by jurisdiction and the various activities that impact the timing of taxable events. As the Company operates in the cannabis industry, the Company is subject to the limits of IRC Section 280E for U.S. federal income tax purposes as well as state income tax purposes. Under IRC Section 280E, the Company is only allowed to deduct expenses directly related to sales of product. This results in permanent differences between ordinary and necessary business expenses deemed non-allowable under IRC Section 280E. However, certain states do not conform to IRC Section 280E and, accordingly, the Company deducts all operating expenses on its state income tax returns in these states.
SELECTED FINANCIAL INFORMATION
The Company reports results of operations of its affiliates from the date that control commences, either through the purchase of the business, through a management agreement or through other arrangements that grant such control. The following selected financial information includes only the results of operations after the Company established control of its affiliates. Accordingly, the information included below may not be representative of the results of operations if such affiliates had included their results of operations for the entire reporting period.
Summary of Quarterly Results
| ($ in thousands) |
2023 | 2022 | 2021 | |||||||||||||||||||||||||||||
| Q1 | Q4 | Q3 | Q2 | Q1 | Q4 | Q3 | Q2 | |||||||||||||||||||||||||
| Revenues, net |
$ | 194,202 | $ | 199,580 | $ | 210,484 | $ | 218,226 | $ | 214,391 | $ | 217,787 | $ | 215,483 | $ | 209,975 | ||||||||||||||||
| Income (loss) from operations |
3,586 | (143,479 | ) | 16,240 | 22,677 | 20,267 | 15,557 | (264,018 | ) | 14,872 | ||||||||||||||||||||||
| Net loss attributable to Cresco Labs Inc. |
(26,051 | ) | (161,337 | ) | (9,788 | ) | (13,541 | ) | (27,381 | ) | (14,732 | ) | (270,645 | ) | (4,827 | ) | ||||||||||||||||
| Basic and Diluted EPS |
$ | (0.09 | ) | $ | (0.54 | ) | $ | (0.03 | ) | $ | (0.05 | ) | $ | (0.09 | ) | $ | (0.08 | ) | $ | (1.00 | ) | $ | (0.02 | ) | ||||||||
6
Three Months Ended March 31, 2023 Compared to the Three Months Ended March 31, 2022
The following tables set forth selected consolidated financial information for the periods indicated that are derived from our unaudited condensed interim consolidated financial statements and the respective accompanying notes prepared in accordance with GAAP.
The selected consolidated financial information set out below may not be indicative of the Company’s future performance:
| Three Months Ended March 31, | ||||||||||||||||
| ($ in thousands) |
2023 | 2022 | $ Change | % Change | ||||||||||||
| Revenues, net |
$ | 194,202 | $ | 214,391 | $ | (20,189 | ) | (9.4 | )% | |||||||
| Cost of goods sold |
108,322 | 107,018 | 1,304 | 1.2 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
85,880 | 107,373 | (21,493 | ) | (20.0 | )% | ||||||||||
| Total operating expenses |
82,294 | 87,106 | (4,812 | ) | (5.5 | )% | ||||||||||
| Total other expense, net |
(14,589 | ) | (21,135 | ) | 6,546 | (31.0 | )% | |||||||||
| Income tax expense |
(16,809 | ) | (22,807 | ) | 5,998 | (26.3 | )% | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss1 |
$ | (27,812 | ) | $ | (23,675 | ) | $ | (4,137 | ) | 17.5 | % | |||||
|
|
|
|
|
|
|
|||||||||||
| 1 | Net loss includes amounts attributable to non-controlling interests. |
Revenues, net
Revenue for the three months ended March 31, 2023 decreased $20.2 million, or 9.4%, compared to the three months ended March 31, 2022. The decrease in revenue was primarily driven by lower sales volumes and price compression in Illinois and Pennsylvania due to increased market competition. The decrease was partially offset by an increase in revenue generated in Michigan as a result of stronger flower supply during the first quarter of 2023.
COGS and Gross profit
COGS for the three months ended March 31, 2023, increased $1.3 million, or 1.2%, compared to the three months ended March 31, 2022. The increase was primarily attributable to increased production to accommodate for the increased wholesale activity in Michigan as well as increased inventory write-offs in 2023.
Gross profit decreased by $21.5 million, or 20.0%, for the three months ended March 31, 2023, compared to the three months ended March 31, 2022. As a percentage of Revenue, net, Gross profit was 44.2% and 50.1% for the three months ended March 31, 2023 and 2022, respectively. The decline in gross profit as a percentage of revenue, net was driven by the combination of price compression, increased fixed cost absorption on lower revenue in 2023 and a decrease in sales from higher margin states resulting in higher percentage of total sales generated from lower margin states.
Total operating expenses
Total operating expenses for the three months ended March 31, 2023 decreased $4.8 million, or 5.5%, compared to the three months ended March 31, 2022. The decrease was primarily attributable to reductions in legal, consulting and professional fees and excise taxes.
7
Total other expense, net
Total other expense, net for the three months ended March 31, 2023 decreased $6.5 million, or 31.0%, compared to the three months ended March 31, 2022, primarily due to a loss on derivative instruments, unrealized loss on investments held at fair value and a gain on lease termination in the first quarter of 2023.
Provision for income taxes
Income tax expense for the three months ended March 31, 2023, decreased $6.0 million, or 26.3%, compared to the three months ended March 31, 2022. The change was primarily due to the impact of lower pre-tax book income, additional states decoupling from IRC Section 280E and various state tax rate changes.
Net loss
Net loss for the three months ended March 31, 2023, increased $4.1 million, compared to the three months ended March 31, 2022. This was primarily driven by lower revenues and gross profit, partially offset by lower total operating expenses and lower income tax expense.
8
Non-GAAP Financial Measures
Earnings before interest, taxes, depreciation and amortization (“EBITDA”) and Adjusted EBITDA are non-GAAP financial measures and do not have standardized definitions under GAAP. The Company has provided the non-GAAP financial measures, which are not calculated or presented in accordance with GAAP, as supplemental information and in addition to the financial measures that are calculated and presented in accordance with GAAP and may not be comparable to similar measures presented by other issuers. These supplemental non-GAAP financial measures are presented because management has evaluated the financial results both including and excluding the adjusted items and believe that the supplemental non-GAAP financial measures presented provide additional perspectives and insights when analyzing the core operating performance of the business. These supplemental non-GAAP financial measures should not be considered superior to, as a substitute for, or as an alternative to and should only be considered in conjunction with, the GAAP financial measures presented herein. Accordingly, the Company has included below reconciliations of the supplemental non-GAAP financial measures to the most directly comparable financial measures calculated and presented in accordance with GAAP.
| Three Months Ended March 31, | ||||||||||||||||
| ($ in thousands) |
2023 | 2022 | $ Change | % Change | ||||||||||||
| Net loss1 |
$ | (27,812 | ) | $ | (23,675 | ) | $ | (4,137 | ) | 17.5 | % | |||||
| Depreciation and amortization |
12,961 | 10,960 | 2,001 | 18.3 | % | |||||||||||
| Interest expense, net |
15,548 | 14,363 | 1,185 | 8.3 | % | |||||||||||
| Income tax expense |
16,809 | 22,807 | (5,998 | ) | (26.3 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| EBITDA (non-GAAP) |
$ | 17,506 | $ | 24,455 | $ | (6,949 | ) | (28.4 | )% | |||||||
|
|
|
|
|
|
|
|||||||||||
| Other income, net |
(959 | ) | 6,772 | (7,731 | ) | (114.2 | )% | |||||||||
| Fair value mark-up for acquired inventory |
— | 5,322 | (5,322 | ) | (100.0 | )% | ||||||||||
| Adjustments for acquisition and other non-core costs |
5,671 | 6,694 | (1,023 | ) | (15.3 | )% | ||||||||||
| Share-based compensation |
7,062 | 7,506 | (444 | ) | (5.9 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Adjusted EBITDA (non-GAAP) |
$ | 29,280 | $ | 50,749 | $ | (21,469 | ) | (42.3 | )% | |||||||
|
|
|
|
|
|
|
|||||||||||
| 1 | Net loss includes amounts attributable to non-controlling interests. |
Adjusted EBITDA (non-GAAP)
Adjusted EBITDA, a non-GAAP financial measure, excludes depreciation and amortization; interest expense, net; income taxes; other income, net; share-based compensation; adjustments for acquisition and other non-core costs and adjustments for the fair value of mark-up for acquired inventory. Non-core costs include non-operating costs such as costs related to restructuring, loss on sale of assets, unique legal expenses and other expenses that are mostly one-time in nature. Adjusted EBITDA was $29.3 million for the three months ended March 31, 2023, compared to $50.7 million for the three months ended March 31, 2022. The decrease in adjusted EBITDA of $21.5 million is due to lower gross profit as well as slightly lower SG&A.
9
LIQUIDITY AND CAPITAL RESOURCES
Overview
Our primary sources of liquidity are cash and cash equivalents from the operations of our business and debt and equity offerings. Our principal uses of cash include working capital related items, capital expenditures, debt and tax related payments. Additionally, we may use cash for acquisitions and other investing or financing activities.
As of March 31, 2023, the Company held $88.8 million in cash and cash equivalents and $1.7 million in restricted cash compared to December 31, 2022, where the Company held $119.3 million in cash and cash equivalents and $2.2 million in restricted cash.
The Company is generally able to access private and/or public financing through, but not limited to, institutional lenders, such as the Senior Loan of $400.0 million, effective August 12, 2021, private loans through individual investors and private and public equity raises such as the equity distribution agreement that was announced on April 26, 2021 with Canaccord Genuity Corp.
The Company expects cash on hand and cash flows from operations, along with the private and/or public financing options discussed above, will be adequate to meet capital requirements and operational needs for the next twelve months.
We cannot guarantee this will be the case or that our assumptions regarding revenues and expenses underlying this belief will be accurate. If, in the future, we require more liquidity than contemplated, we may need to raise additional funds through debt and/or equity offerings. Adequate funds may not be available when needed or may not be available on terms favorable to us. If additional funds are raised by issuing equity securities, dilution to existing shareholders may result. If we raise additional funds by obtaining loans from third parties, the terms of those financing arrangements may include negative covenants or other restrictions on our business that could impair our operational flexibility and would also require us to fund additional interest expense. If funding is insufficient at any time in the future, we may be unable to develop or enhance our products or services, take advantage of business opportunities, or respond to competitive pressures, any of which could have a material adverse effect on our business, financial condition and results of operations. See the section entitled “Risk Factors” in the Company’s Annual Information Form for the year ended December 31, 2022, which is available on SEDAR under the Company’s issuer profile, for further information.
Cash Flows
Operating Activities
Net cash provided by operating activities was $3.3 million for the three months ended March 31, 2023, an increase of $6.7 million compared to $3.4 million of cash used in operating activities during the three months ended March 31, 2022. The $6.7 million increase was primarily attributable to favorable changes in working capital, including strategic purchases of certain raw materials inventory in the prior year, and timing of income tax payments.
As of March 31, 2023, the Company had working capital (defined as current assets less restricted cash, less current liabilities) of $12.1 million compared to $66.0 million as of March 31, 2022. The $53.9 million decrease in working capital was primarily attributable to decreases in inventory, partially offset by decreases in accounts payable and other accrued expenses.
10
Investing Activities
Net cash used in investing activities was $20.7 million for the three months ended March 31, 2023, a decrease of $13.6 million compared to $34.2 million used in the three months ended March 31, 2022. The decrease in net cash used in investing activities was primarily driven by a reduction from prior year of $15.0 million in purchases of property and equipment due to significant investments in the first quarter of 2022 related to our New York operations.
Financing Activities
Net cash used in financing activities was $13.6 million for the three months ended March 31, 2023, an increase in cash used of $7.3 million compared to cash used in financing activities of $6.4 million for the three months ended March 31, 2022. The increase was primarily driven by a $4.4 million increase in distributions to non-controlling interest redeemable unit holders and other members, along with a decrease in proceeds from exercise of stock options, warrants and sell-to-cover shares in the current period.
11
OFF-BALANCE SHEET ARRANGEMENTS AND PROPOSED TRANSACTIONS
| (a) | Off-Balance Sheet Arrangements |
The Company has no material undisclosed off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on its results of operations, financial condition, revenues or expenses, liquidity, capital expenditures or capital resources that is material to investors.
| (b) | Proposed Transactions |
On March 23, 2022, the Company announced that it had entered into the Arrangement Agreement with Columbia Care in respect of the Columbia Care Transaction. See “Overview of the Company – Recent Developments.” After giving effect to the Columbia Care Transaction, the Company would have pro forma revenue, without taking into consideration elimination of revenues related to divestitures, based on actual 2021 results, of $1.3 billion. While divestitures are anticipated to be required for state regulatory approval, the scope and financial impact of any divestitures cannot be quantified at this time. After giving effect to the Columbia Care Transaction, Columbia Care Shareholders will hold approximately 35% of the pro forma Cresco Labs Shares (on a fully diluted in-the-money basis).
The Columbia Care Transaction has been unanimously approved by the boards of directors of each of the Company and Columbia Care. The Columbia Care Transaction is subject to, among other things, receipt of the necessary approvals of the Supreme Court of British Columbia, the approval of two-thirds of the votes cast by shareholders of Columbia Care at a special meeting of shareholders to approve the Columbia Care Transaction, receipt of the required regulatory approvals, including, but not limited to, approval pursuant to the Hart Scott Rodino Antitrust Improvements Act (the “HSR Act”) and other customary closing conditions. As announced on May 16, 2022, the 30-day waiting period under the HSR Act expired, which marked a major step towards closing of the transaction. Approval of the shareholders of the Company is not required in connection with the Columbia Care Transaction.
On July 8, 2022, at the Columbia Care Meeting, the shareholders of Columbia Care voted in favor of a special resolution to approve the Arrangement.
On July 15, 2022, Columbia Care obtained the final order from the Supreme Court of British Columbia approving the Arrangement.
The Company continues to collaborate closely with Columbia Care on the required divestiture transactions to find a path forward that makes both strategic and financial sense. The Company has no update on the timing for execution of agreements relating to outstanding divestiture transactions.
On November 4, 2022, the Company announced that it had entered into a definitive agreement to divest certain assets of Cresco Labs and Columbia Care to entities owned and controlled by Sean “Diddy” Combs, for total consideration of $185.0 million (the “Combs Transaction”). The divestiture of the Assets is required for Cresco Labs to close its previously announced acquisition of Columbia Care and is expected to close concurrently with the closing of the Columbia Care Transaction. The purchasing entities will acquire certain Cresco Labs and Columbia Care assets in New York, Illinois, and Massachusetts. A portion of the purchase price is payable upon closing of the Combs Transaction, subject to adjustments contained in the definitive agreements, and will be comprised of approximately $110.0 million in cash and approximately $45.0 million of seller notes. The remaining portion of the purchase price is payable post-closing upon achievement of
12
certain short-term, objective, and market-based milestones. The following combination of Cresco Labs (“CL”) and Columbia Care (“CC”) assets will be divested in the Combs Transaction:
| • | New York: Brooklyn (CC), Manhattan (CC), New Hartford (CL) and Rochester (CC) retail assets and Rochester (CC) production asset. |
| • | Massachusetts: Greenfield (CC), Worcester (CL) and Leicester (CL) retail assets and Leicester (CL) production asset. |
| • | Illinois: Chicago – Jefferson Park (CC) and Villa Park (CC) retail assets and Aurora (CC) production asset. |
The closing of the Combs Transaction is subject to certain closing conditions in the definitive agreements, including the receipt of all required regulatory approvals; clearance under the HSR Act; and the closing of the Columbia Care Transaction.
CONTRACTUAL OBLIGATIONS
As of March 31, 2023, maturities of lease liabilities were as follows:
| ($ in thousands) |
Total | Operating Leases |
Finance Leases |
|||||||||
| 2023 |
$ | 25,187 | $ | 21,088 | $ | 4,099 | ||||||
| 2024 |
33,254 | 28,000 | 5,254 | |||||||||
| 2025 |
33,969 | 28,691 | 5,278 | |||||||||
| 2026 |
34,384 | 28,979 | 5,405 | |||||||||
| 2027 |
34,617 | 29,126 | 5,491 | |||||||||
| Thereafter |
212,684 | 188,661 | 24,023 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total lease payments |
$ | 374,095 | $ | 324,545 | $ | 49,550 | ||||||
|
|
|
|
|
|
|
|||||||
| Less: imputed interest |
(190,170 | ) | (167,873 | ) | (22,297 | ) | ||||||
| Less: tenant improvement allowance |
(5,298 | ) | (4,653 | ) | (645 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Present value of lease liabilities |
178,627 | 152,019 | 26,608 | |||||||||
|
|
|
|
|
|
|
|||||||
| Less: short-term lease liabilities |
(26,363 | ) | (22,265 | ) | (4,098 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Present value of long-term lease liabilities |
$ | 152,264 | $ | 129,754 | $ | 22,510 | ||||||
|
|
|
|
|
|
|
|||||||
In addition to the future minimum lease payments disclosed above, the Company is responsible for real estate taxes and common operating expenses incurred by the building or facility in which it leases space. Additionally, the Company will continue to invest in its facilities through construction and other capital expenditures as it expands its footprint in existing and new markets.
13
In addition to the lease commitments above, the Company has the following contractual obligations as of March 31, 2023:
| ($ in thousands) |
< 1 Year | 1 to 3 Years | 3 to 5 Years | Total | ||||||||||||
| Accounts payable & Accrued liabilities |
$ | 96,633 | $ | — | $ | — | $ | 96,633 | ||||||||
| Deferred consideration, contingent consideration and other payables, short-term |
41,046 | — | — | 41,046 | ||||||||||||
| Deferred consideration, long-term |
— | 6,112 | — | 6,112 | ||||||||||||
| Long-term notes payable and loans payable and Short-term borrowings |
28,112 | — | 470,895 | 499,007 | ||||||||||||
| Other long-term liabilities |
— | — | 7,000 | 7,000 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total obligations as of March 31, 2023 |
$ | 165,791 | $ | 6,112 | $ | 477,895 | $ | 649,798 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
14
RELATED PARTY TRANSACTIONS
| (a) | Transactions with Key Management Personnel |
Related parties, including key management personnel, hold 89.0 million Redeemable Units of Cresco Labs, LLC, which is equal to a deficit of $50.9 million of Non-controlling interests as of March 31, 2023. During the three months ended March 31, 2023 and 2022, 72.0% and 85.3% respectively, of required tax distribution payments in accordance with the tax receivable agreement to holders of Cresco Labs, LLC were made to related parties including to key management personnel.
| (b) | Related Parties - Leases |
For the three months ended March 31, 2022 the Company had lease liabilities for real estate lease agreements in which the lessors have a minority interest in SLO Cultivation, Inc. (“SLO”) and MedMar, Inc (“MedMar”). The lease liabilities were incurred in January 2019 and May 2020 and were to expire in 2027 through 2030, except for the leases associated with SLO minority interest holders (“SLO Leases”). During the second quarter of 2022, the Company exercised its early termination right to reduce the SLO Leases term to 180 days. This early termination resulted in a reduction in lease liability and right-of-use (“ROU”) assets. The remaining liability for the SLO Leases expired in the fourth quarter of 2022.
The Company has liabilities for real estate leases and other financing agreements in which the lessor is Clear Heights Properties where Dominic Sergi, MVS shareholder, is Chief Executive Officer. The liabilities were incurred by entering into operating leases, finance leases and other financing transactions with terms that will expire in 2030. During the three months ended March 31, 2023 and 2022, the Company received tenant improvement allowance reimbursements of $nil and $1.4 million, respectively. The Company expects to receive further reimbursements of $0.8 million within the next twelve months.
Below is a summary of the expense resulting from the related party lease liabilities for the three months ended March 31, 2023 and 2022:
| Three Months Ended | ||||||||||||
| March 31, | ||||||||||||
| ($ in thousands) |
Classification | 2023 | 2022 | |||||||||
| Operating Leases |
||||||||||||
| Lessor has minority interest in SLO |
Rent expense | $ | — | $ | 379 | |||||||
| Lessor has minority interest in MedMar |
Rent expense | 73 | 73 | |||||||||
| Lessor is an MVS shareholder |
Rent expense | 296 | 296 | |||||||||
| Finance Leases |
||||||||||||
| Lessor has minority interest in MedMar |
Depreciation expense | $ | 76 | $ | 76 | |||||||
| Lessor has minority interest in MedMar |
Interest expense | 63 | 69 | |||||||||
| Lessor is an MVS shareholder |
Depreciation expense | 22 | 19 | |||||||||
| Lessor is an MVS shareholder |
Interest expense | 19 | 34 | |||||||||
15
Additionally, below is a summary of the ROU assets and lease liabilities attributable to related party lease liabilities as of March 31, 2023 and December 31, 2022:
| As of March 31, 2023 | As of December 31, 2022 | |||||||||||||||
| ($ in thousands) |
ROU Asset | Lease Liability |
ROU Asset | Lease Liability |
||||||||||||
| Operating Leases |
||||||||||||||||
| Lessor has minority interest in MedMar |
$ | 1,386 | $ | 1,430 | $ | 1,415 | $ | 1,456 | ||||||||
| Lessor is an MVS shareholder |
5,740 | 5,814 | 5,849 | 5,907 | ||||||||||||
| Finance Leases |
||||||||||||||||
| Lessor has minority interest in MedMar |
$ | 1,958 | $ | 2,394 | $ | 2,034 | $ | 2,452 | ||||||||
| Lessor is an MVS shareholder |
619 | 570 | 596 | 555 | ||||||||||||
During the three months ended March 31, 2023 and 2022, the Company recorded interest expense on finance liabilities of $0.1 million. As of March 31, 2023 and December 31, 2022, the Company had finance liabilities totaling $1.5 million, respectively. All finance liabilities outstanding are due to an entity controlled by an MVS shareholder.
16
FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT
The Company’s financial instruments are held at amortized cost (adjusted for impairments or expected credit losses, as applicable) or fair value. The carrying values of financial instruments held at amortized cost approximate their fair values as of March 31, 2023 and December 31, 2022, due to their nature and relatively short maturity date. Financial assets and liabilities with embedded derivative features are carried at fair value.
Financial instruments recorded at fair value are classified using a fair value hierarchy that reflects the significance of the inputs to fair value measurements. The three levels of hierarchy are:
| • | Level 1 – Unadjusted quoted prices in active markets for identical assets or liabilities; |
| • | Level 2 – Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly; and |
| • | Level 3 – Inputs for the asset or liability that are not based on observable market data. |
There have been no transfers into or out of level 3 during the periods ended March 31, 2023 and December 31, 2022.
The following tables summarize the Company’s financial instruments as of March 31, 2023 and December 31, 2022:
| March 31, 2023 | ||||||||||||||||||||
| ($ in thousands) |
Amortized Cost |
Level 1 | Level 2 | Level 3 | Total | |||||||||||||||
| Financial Assets: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 88,799 | $ | — | $ | — | $ | — | $ | 88,799 | ||||||||||
| Restricted cash1 |
1,653 | — | — | — | 1,653 | |||||||||||||||
| Security deposits2 |
4,347 | — | — | — | 4,347 | |||||||||||||||
| Accounts receivable, net |
49,602 | — | — | — | 49,602 | |||||||||||||||
| Loans receivable, short-term |
458 | — | — | — | 458 | |||||||||||||||
| Loans receivable, long-term |
823 | — | — | — | 823 | |||||||||||||||
| Investments |
— | 99 | 432 | 660 | 1,191 | |||||||||||||||
| Financial Liabilities: |
||||||||||||||||||||
| Accounts payable |
$ | 20,134 | $ | — | $ | — | $ | — | $ | 20,134 | ||||||||||
| Accrued liabilities |
76,499 | — | — | — | 76,499 | |||||||||||||||
| Short-term borrowings |
28,112 | — | — | — | 28,112 | |||||||||||||||
| Current portion of lease liabilities |
26,363 | — | — | — | 26,363 | |||||||||||||||
| Deferred consideration and other payables, short-term |
6 | 6 | — | 41,034 | 41,046 | |||||||||||||||
| Lease liabilities |
152,264 | — | — | — | 152,264 | |||||||||||||||
| Deferred consideration, long-term |
— | — | — | 6,112 | 6,112 | |||||||||||||||
| Long-term notes payable and loans payable |
470,895 | — | — | — | 470,895 | |||||||||||||||
| Other long-term liabilities |
7,000 | — | — | — | 7,000 | |||||||||||||||
| 1 | Restricted cash balances include various escrow accounts related to investments, acquisition, and facility licensing requirements. |
| 2 | Security deposits are included in “Other non-current assets” on the Unaudited Condensed Interim Consolidated Balance Sheets. |
17
| December 31, 2022 | ||||||||||||||||||||
| ($ in thousands) |
Amortized Cost |
Level 1 | Level 2 | Level 3 | Total | |||||||||||||||
| Financial Assets: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 119,341 | $ | — | $ | — | $ | — | $ | 119,341 | ||||||||||
| Restricted cash1 |
2,169 | — | — | — | 2,169 | |||||||||||||||
| Security deposits2 |
4,367 | — | — | — | 4,367 | |||||||||||||||
| Accounts receivable, net |
56,492 | — | — | — | 56,492 | |||||||||||||||
| Loans receivable, short-term |
447 | — | — | — | 447 | |||||||||||||||
| Loans receivable, long-term |
823 | — | — | — | 823 | |||||||||||||||
| Investments |
— | 136 | 432 | 660 | 1,228 | |||||||||||||||
| Financial Liabilities: |
||||||||||||||||||||
| Accounts payable |
$ | 28,093 | $ | — | $ | — | $ | — | $ | 28,093 | ||||||||||
| Accrued liabilities |
65,161 | — | — | — | 65,161 | |||||||||||||||
| Short-term borrowings |
18,812 | — | — | — | 18,812 | |||||||||||||||
| Current portion of lease liabilities |
26,124 | — | — | — | 26,124 | |||||||||||||||
| Deferred consideration and other payables, short-term |
6 | 7 | — | 47,821 | 47,834 | |||||||||||||||
| Lease liabilities |
156,180 | — | — | — | 156,180 | |||||||||||||||
| Deferred consideration, long-term |
— | — | — | 7,770 | 7,770 | |||||||||||||||
| Long-term notes payable and loans payable |
469,055 | — | — | — | 469,055 | |||||||||||||||
| Other long-term liabilities |
7,000 | — | — | — | 7,000 | |||||||||||||||
| 1 | Restricted cash balances include various escrow accounts related to investments, acquisitions and facility licensing requirements. |
| 2 | Security deposits are included in “Other non-current assets” on the Unaudited Condensed Interim Consolidated Balance Sheets. |
18
Financial Risk Management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board of Directors and Company management mitigate these risks by assessing, monitoring and approving the Company’s risk management processes:
| (a) | Credit and Banking Risk |
Credit risk is the risk of a potential loss to the Company if a customer or a third-party to a financial instrument fails to meet its contractual obligations. The maximum credit exposure as of March 31, 2023 and December 31, 2022 is the carrying amount of cash, accounts receivable and loans receivable. The Company does not have significant credit risk with respect to its growth in its key retail markets, as payment is typically due upon transferring the goods to the customer at our dispensaries, which currently accept only cash and debit cards. Additionally, the Company does not have significant credit risk with respect to its loan counterparties as the interest rate on our Senior Loan is not variable and therefore, is not materially impacted by interest rate increases enacted by the Federal Reserve. Although all deposited cash is placed with U.S. financial institutions in good standing with regulatory authorities, changes in U.S. federal banking laws related to the deposit and holding of funds derived from activities related to the cannabis industry have passed the U.S. House of Representatives but were not voted on within the U.S. Senate, and would need to be reintroduced by Congress. Given that current U.S. federal law provides that the production and possession of cannabis is illegal, there is a strong argument that banks cannot accept or deposit funds from businesses involved with the cannabis industry, leading to an increased risk of legal actions against the Company and forfeitures of the Company’s assets.
| (b) | Asset Forfeiture Risk |
Because the cannabis industry remains illegal under U.S. federal law, any property owned by participants in the cannabis industry which are either used in the course of conducting such business, or are the proceeds of such business, could be subject to seizure by law enforcement and subsequent civil asset forfeiture. Even if the owner of the property was never charged with a crime, the property in question could still be seized and subject to an administrative proceeding by which, with minimal due process, it could be subject to forfeiture.
| (c) | Liquidity Risk |
The Company prepares its financial statements assuming that the Company will continue as a going concern. The Company has incurred historical losses from operations. Management has implemented strategies to expand its retail footprint, increase revenues, cut cost, improve margins and obtain additional financing if needed. There can be no assurances that Management’s plans would materialize as expected.
Liquidity risk is the risk that the Company will not be able to meet its financial obligations associated with financial liabilities. The Company primarily manages liquidity risk through the management of its capital structure by ensuring that it will have sufficient liquidity to settle obligations and liabilities when due. The Company expects to continue to raise capital to fund operations and the expansion of its business.
19
| (d) | Market Risk |
| (i) | Currency Risk |
The operating results and balance sheet of the Company are reported in USD. As of March 31, 2023 and December 31, 2022, the Company’s financial assets and liabilities are primarily in USD. However, from time to time some of the Company’s financial transactions are denominated in currencies other than USD. The results of the Company’s operations are subject to currency transaction and translation risks. For the three months ended March 31, 2023, the Company recorded a loss of $nil in foreign currency exchanges, compared to a $0.1 million gain in foreign currency exchanges for the three months ended March 31, 2022.
As of March 31, 2023 and December 31, 2022, the Company had no hedging agreements in place with respect to foreign exchange rates. The Company has not entered into any agreements or purchased any instruments to hedge possible currency risks at this time.
| (ii) | Interest Rate Risk |
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. An increase or decrease in the Company’s incremental borrowing rate would result in an associated increase or decrease in Deferred consideration, contingent consideration and other payables and Interest expense, net. The Company’s Senior Loan accrues at a rate of 9.5% per annum and has an effective interest rate of 11.0%.
| (iii) | Price Risk |
Price risk is the risk of variability in fair value due to movements in equity or market prices. The Company is subject to price risk related to derivative liabilities and contingent consideration that are valued based on the Company’s own stock price. An increase or decrease in stock price would result in an associated increase or decrease to Deferred consideration, contingent consideration and other payables, short-term and Derivative liabilities, short-term with a corresponding change to Other income (expense), net.
| (iv) | Tax Risk |
Tax risk is the risk of changes in the tax environment that would have a material adverse effect on the Company’s business, results of operations and financial condition. Currently, state-licensed marijuana businesses are assessed a comparatively high effective federal tax rate due to IRC Section 280E, which bars businesses from deducting all expenses except their cost of goods sold when calculating federal tax liability. Any increase in tax levies resulting from additional tax measures may have a further adverse effect on the operations of the Company, while any decrease in such tax levies will be beneficial to future operations.
20
| (v) | Regulatory Risk |
Regulatory risk pertains to the risk that the Company’s business objectives are contingent, in part, upon the compliance of regulatory requirements. Due to the nature of the industry, the Company recognizes that regulatory requirements are more stringent and punitive in nature. Any delays in obtaining, or failure to obtain regulatory approvals can significantly delay operational and product development and can have a material adverse effect on the Company’s business, results of operation and financial condition. The Company is cognizant of the advent of regulatory changes occurring in the cannabis industry on the city, state and national levels. Although the regulatory outlook on the cannabis industry has been moving in a positive trend, any unforeseen regulatory changes could have a material adverse impact on the goals and operations of the Company’s business.
| (vi) | Economic Risk |
The Company’s business, financial condition and operating results may be negatively impacted by challenging global economic conditions. A global economic slowdown would cause disruptions and extreme volatility in global financial markets, increased rates of default and bankruptcy and declining consumer and business confidence, which can lead to decreased levels of consumer spending. These macroeconomic developments could negatively impact the Company’s business, which depends on the general economic environment and levels of consumer spending. As a result, the Company may not be able to maintain its existing customers or attract new customers, or the Company may be forced to reduce the price of its products. The Company is unable to predict the likelihood of the occurrence, duration or severity of such disruptions in the credit and financial markets or adverse global economic conditions. Any general or market-specific economic downturn could have a material adverse effect on our business, financial condition and operating results.
| (vii) | Inflation Risk |
The Company has experienced increased inflationary pressures, including increased cultivation costs, distribution costs and operating expenses, which adversely has impacted our operating results. The Company expects these inflationary pressures to continue throughout 2023. The Company maintains strategies to mitigate the impact of higher raw material, energy and commodity costs, which include cost reduction, sourcing and other actions, which may help to offset a portion of the adverse impact.
21
SUMMARY OF OUTSTANDING SHARE AND SHARE-BASED DATA
Cresco has the following securities issued and outstanding, as of March 31, 2023:
| Securities |
Number of Shares (in thousands) |
|||
| Issued and Outstanding |
||||
| Super Voting Shares |
500 | |||
| Subordinate Voting Shares1 |
289,010 | |||
| Proportionate Voting Shares2 |
19,902 | |||
| Special Subordinate Voting Shares3 |
1 | |||
| Redeemable Shares |
104,306 | |||
| Stock Options |
27,708 | |||
| Restricted Stock Units |
8,375 | |||
| 1 | SVS includes shares pending issuance or cancellation |
| 2 | PVS presented on an “as-converted” basis to SVS (1-to-200) |
| 3 | SSVS presented on an “as-converted” basis to SVS (1-to-0.00001) |
22
Federal Regulatory Environment
Canadian-Securities Administrators Staff Notice 51-352 (Revised) – Issuers with U.S. Marijuana-Related Activities (“Staff Notice 51-352”) provides specific disclosure expectations for issuers that currently have, or are in the process of developing, cannabis-related activities in the U.S. as permitted within a particular state’s regulatory framework. All issuers with U.S. cannabis-related activities are expected to clearly and prominently disclose certain prescribed information in prospectus filings and other required disclosure documents.
In accordance with Staff Notice 51-352, Cresco Labs will evaluate, monitor and reassess the disclosures contained herein and any related risks, on an ongoing basis and the same will be supplemented, amended and communicated to investors in public filings, including in the event of government policy changes or the introduction of new or amended guidance, laws or regulations regarding marijuana regulation. As a result of the Company’s operations, it is subject to Staff Notice 51-352 and accordingly provides the following disclosure:
Cresco Labs currently directly derives a substantial portion of its revenues from the cannabis industry in certain U.S. states, which industry is illegal under U.S. Federal Law. As of March 31, 2023, the Company is directly involved (through licensed subsidiaries) in both the medical and adult-use cannabis industry in the states of Illinois, Pennsylvania, Ohio, California, Arizona, New York, Maryland, Massachusetts, Michigan and Florida as permitted within such states under applicable state law which states have regulated such industries.
The cultivation, sale and use of cannabis is illegal under federal law pursuant to the U.S. Controlled Substance Act of 1970 (“CSA”). Under the CSA, the policies and regulations of the U.S. Federal Government and its agencies are that cannabis has no medical benefit and a range of activities including cultivation and the personal use of cannabis is prohibited. The Supremacy Clause of the U.S. Constitution establishes that the U.S. Constitution and federal laws made pursuant to it are paramount and in case of conflict between federal and state law, the federal law shall apply.
On January 4, 2018, former U.S. Attorney General Jeff Sessions issued a memorandum to U.S. district attorneys which rescinded previous guidance from the U.S. Department of Justice specific to cannabis enforcement in the U.S., including the Cole Memo (the “Memo”). The Memo previously provided guidance to prioritize a limited scope of federal enforcement including the prevention of the distribution of marijuana to minors, revenue from the sale of marijuana from going to criminal enterprises, diversion of marijuana from states where it is legal under state law in some form to other states, state-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity, violence and the use of firearms in the cultivation and distribution of marijuana, drugged driving and the exacerbation of other adverse public health consequences associated with marijuana use, the growing of marijuana on public lands and marijuana possession or use on federal property. With the Memo rescinded, U.S. federal prosecutors have been given discretion in determining whether to prosecute cannabis-related violations of U.S. Federal Law. If the Department of Justice policy was to aggressively pursue financiers or equity owners of cannabis-related business and U.S. Attorneys followed such Department of Justice policies through pursuing prosecutions, then the Company could face, (i) seizure of its cash and other assets used to support or derived from its cannabis subsidiaries and (ii) the arrest of its employees, directors, officers, managers and investors, who could face charges of ancillary criminal violations of the CSA for aiding and abetting and conspiring to violate the CSA by virtue of providing financial support to state-licensed or permitted cultivators, processors, distributors and/or retailers of cannabis. Additionally, as has been affirmed by U.S. Customs and Border Protection, employees, directors, officers, managers and investors of the Company who are not U.S. citizens face the risk of being barred from entry into the U.S. for life. The Rohrabacher–Farr amendment (also known as the Rohrabacher–
23
Blumenauer amendment) prohibits the Department of Justice from spending funds to interfere with the implementation of state medical cannabis laws. It first passed the U.S. House of Representatives in May 2014 and became law in December 2014 as part of an omnibus spending bill. The passage of the amendment was the first time either chamber of Congress had voted to protect medical cannabis patients and is viewed as a historic victory for cannabis reform advocates at the federal level. The amendment does not change the legal status of cannabis and must be renewed each fiscal year in order to remain in effect. Since 2015, Congress has used a rider provision in the Consolidated Appropriations Acts (currently the Joyce Amendment, but previously called the Rohrabacher-Blumenauer Amendment and before that the Rohrabacher-Farr Amendment) to prevent the federal government from using congressional appropriated funds to enforce federal cannabis laws against state-compliant actors in jurisdictions that have legalized medical cannabis and cannabis-related activities. The Joyce Amendment was again included in the most recent annual appropriations bill. Additionally, the Blumenauer-McClintock-Norton-Lee amendment had been under consideration. This amendment would have extended the protections of the Joyce Amendment to adult-use businesses. However, the Blumenauer-McClintock-Norton-Lee amendment was not included in the appropriations bill that was passed by Congress on March 10, 2022 and signed by President Biden on March 15, 2022.
Unless and until the U.S. Congress amends the CSA with respect to medical and/or adult-use cannabis (and as to the timing or scope of any such potential amendments there can be no assurance), there is a significant risk that federal authorities may enforce current U.S. federal law. If the U.S. Federal Government begins to enforce U.S. federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing applicable state laws are repealed or curtailed, the Company’s business, results of operations, financial condition and prospects would be materially adversely affected.
Despite the current state of the federal law and the CSA, the states of Arizona, California, Connecticut, Nevada, Massachusetts, Maine, Michigan, New Jersey, New Mexico, New York, Illinois, Montana, Washington, Oregon, Colorado, Vermont, Virginia, Alaska, Rhode Island, Maryland, Missouri, Delaware and the District of Columbia, have legalized adult-use of cannabis. Adult-use sales have not yet begun in Maryland, Delaware and Virginia. Additionally, although the District of Columbia voters passed a ballot initiative in November 2014, no adult-use operations exist yet because of a prohibition on using funds for regulation within a federal appropriations amendment to local District spending powers.
There were several cannabis ballot initiatives considered by voters during the November 2022 elections. Voters in Maryland and Missouri voted in favor of legalizing adult-use cannabis.
In addition, over three quarters of the U.S. states have enacted legislation to legalize and regulate the sale and use of medical cannabis, provided that there are strict purchasing or possession limits. However, there is no guarantee that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local government authorities will not limit the applicability of state laws within their respective jurisdictions.
The Company’s objective is to capitalize on the opportunities presented as a result of the changing regulatory environment governing the cannabis industry in the U.S. Accordingly, there are significant risks associated with the business of the Company. Unless and until the U.S. Congress amends the CSA with respect to medical and/or adult-use cannabis (and as to the timing or scope of any such potential amendments there can be no assurance), there is a significant risk that federal authorities may enforce current federal law and the business of the Company may be deemed to be producing, cultivating, extracting, or dispensing cannabis or aiding or abetting or otherwise engaging in a conspiracy to commit such acts in violation of federal law.
24
For these reasons, the Company’s investments in the U.S. cannabis market may subject the Company to heightened scrutiny by regulators, stock exchanges, clearing agencies and other Canadian authorities. There are risks associated with the business of the Company. See sections “Risk Factors,” “General Development of the Business” and “Description of the Business” in the Annual Information Form for the year ended December 31, 2022, filed on SEDAR.
On November 20, 2019, the House Judiciary Committee approved the Marijuana Opportunity Reinvestment and Expungement Act of 2019 (the “MORE Act”) by a 24 to 10 vote. The MORE Act would decriminalize and remove Cannabis as a Schedule I controlled substance. In April 2021, days before a floor vote in the U.S. House of Representatives, the MORE Act was stalled due to a late added amendment. While the main thrust of the bill remained intact, including a tax to fund programs to repair the harms of the drug war, a provision was added requiring a federal permit to operate a “cannabis enterprise” along with restrictions that could ban people with prior marijuana convictions from being eligible. Advocates viewed the amendment as problematic as it allows for federal cannabis permits to be suspended or revoked if a person has a past or current legal proceeding related to a felony violation of any state or federal cannabis law. Following the Judiciary Committee approval in November, 2019, the MORE Act was passed by the House by a vote of 228-164 in December 2020. The bill did not advance in the U.S. Senate. The bill was reintroduced by Representative Nadler (D-NY) in May 2021. On September 30, 2021, the MORE Act passed the House Judiciary Committee by a vote of 26-15. Two Republicans joined all of the committee’s Democratic members to move the bill forward. On April 1, 2022, the U.S. House of Representatives passed the MORE Act once again. The bill was received in the Senate and Read twice and referred to the Committee on Finance; however, the bill was not brought to a vote in 2022 and would need to be reintroduced by the new Congress.
On April 19, 2021, the SAFE Banking Act of 2019 (the “SAFE Banking Act” or “SAFE”) again passed the U.S. House of Representatives by a 321 – 101 vote. The U.S. Senate opted to pursue comprehensive federal reform legislation rather than bring the SAFE Banking Act up for a regular order vote due to proposed comprehensive federal reform legislation led by Senate Majority Leader Chuck Schumer (D-NY), Senator Ron Wyden (D-OR) and Senator Cory Booker (D-NJ). On April 27, 2023, SAFE was reintroduced as a bipartisan and bicameral piece of legislation by Sen. Jeff Merkley (D-OR), Sen. Steve Daines (R-MT), Rep. Dave Joyce (R-OH), and Rep. Earl Blumenauer (D-OR). On May 11, 2023, the Senate Banking Committee met to discuss marijuana banking issues and bipartisan legislation to resolve the industry’s unique financial challenges. If a bill were to advance through the committee stage, it would be voted by the Senate for the first time.
On February 1, 2021, Leader Schumer and Senators Wyden and Booker issued a joint statement announcing the imminent release of comprehensive cannabis reform legislation which stated, “We will release a unified discussion draft on comprehensive reform to ensure restorative justice, protect public health and implement responsible taxes and regulations.” On July 14, 2021, Leader Schumer and Senators Wyden and Booker released the Cannabis Administration and Opportunity Act (the “CAO Act”), a 163-page discussion draft bill, alongside a 30-page summary document, which effectively deschedules cannabis, provides restorative justice for past cannabis-related convictions and establishes a federal regulatory system within the U.S. Food and Drug Administration (“FDA”) for cannabis products. In addition to the aforementioned provisions, the bill also maintains state authority to establish individual cannabis policies and establishes a federal tax on cannabis products. Stakeholder comments were submitted to the Sponsoring Offices on or before the requested deadline of September 1, 2021. The Sponsoring Offices spent significant time considering those comments and amended the discussion draft bill. On July 21, 2022, Leader Schumer and Senators Wyden and Booker formally filed the CAO Act. The bill was not brought to a vote in 2022 and would need to be reintroduced by the new Congress.
25
On November 15, 2021, Rep. Nancy Mace (R-SC) introduced the States Reform Act. The bill, if enacted, would legalize cannabis at the federal level by removing cannabis from the Controlled Substances Act and provide some deference to the states and state programs. The bill defers to the states to prohibit or commercially regulate adult-use cannabis within their borders. In addition to state regulation, cannabis would generally be regulated at the federal level in manner similar to alcohol, including by the U.S. FDA, the U.S. Department of Agriculture and the Alcohol and Tobacco Tax and Trade Bureau, which would be renamed the Bureau of Alcohol, Tobacco and Cannabis Tax and Trade Bureau. The States Reform Act was referred to the House Judiciary Committee and will be reported to several other committees and subcommittees before advancement. The bill was not brought to a vote in 2022 and would need to be reintroduced by the new Congress.
On June 23, 2022, U.S. Congressmen Troy A Carter, Sr. (D-LA) and co-sponsors Guy Reschenthaler (R-PA), David Joyce (R-OH), Dwight Evans (D-PA) and Patrick Ryan (D-NY) introduced bipartisan legislation, The Capital Lending and Investment for Marijuana Businesses Act, to allow state legal American cannabis companies, including small, minority and veteran-owned businesses the ability to access critical lending and investment opportunities currently available to other domestic and regulated industries. The bill was not brought to a vote in 2022 and would need to be reintroduced by the new Congress.
On October 6, 2022, President Joe Biden announced he will take executive action to pardon thousands of people convicted of marijuana possession under federal law. President Biden said he would also encourage state governors to take similar action with state offenses and asked the U.S. Department of Health and Human Services and the U.S. Department of Justice to review how marijuana is scheduled, or classified, under federal law.
On December 27, 2022, Congresswoman Rep. Nancy Mace (R-SC) filed a bill that would provide federal tax relief for cannabis businesses by amending the Internal Revenue Service’s 280E Code. The bill would allow state-legal cannabis operators to be able to deduct business expenses on their federal taxes, an option applicable to any other legal business. The bill did not receive a vote. On April 17, 2023, Rep. Earl Blumenauer (D-OR), refiled the bill, the Small Business Tax Equity Act, which would amend IRS code 280E to allow state-legal cannabis businesses to take federal tax deductions. Rep. Mace, together with Rep. Barbara Lee (D-CA) and Rep. Joyce, are cosponsors the refiled bill in addition to Rep. Blumenauer.
On January 16, 2023, Rep. Alex Mooney (R-WV) introduced a bill, the Second Amendment Protection Act, cosponsored by Rep. Brian Mast (R-FL) and Rep. Thomas Massie (R-KY), which would allow medical cannabis patients to purchase and possess firearms.
On April 14, 2023, Rep. Joyce and House Democratic Leader Hakeem Jeffries (D-NY) reintroduced bipartisan legislation, the Preparing Regulators Effectively for a Post-Prohibition Adult Use Regulated Environment Act (“PREPARE Act”), which would create a process for the federal government to establish regulations for cannabis upon legalization. The PREPARE Act directs the U.S. Attorney General to establish the “Commission on the Federal Regulation of Cannabis” to advise on a regulatory framework modeled after Federal and State regulatory frameworks with respect to alcohol.
On April 19, 2023, Rep. Joyce and Rep. Alexandria Ocasio-Cortez (D-NY) introduced the Harnessing Opportunities by Pursuing Expungement Act. This bipartisan bill would reduce the financial and administrative burden on states with respect to expunging cannabis offenses. Specifically, the bill would create a new grant program under the U.S. Department of Justice, the State Expungement Opportunity Grant Program, and authorize it to be funded up to $20 million over the span of Fiscal Years 2024-2033.
26
On April 20, 2023, Rep. Brian Mast (R-FL) filed the Gun Rights and Marijuana Act, which would allow medical cannabis patients and adult use consumers to purchase and possess firearms.
Rep. Barbara Lee (D-CA) introduced the Veterans Medical Marijuana Safe Harbor Act on April 19, 2023, with 12 cosponsors. Sen. Brian Schatz (D-HI) is leading a companion measure filed on April 20, 2023. The bills seek to legalize medical cannabis for military veterans. Physicians employed by the U.S. Department of Veterans Affairs would also be permitted to make recommendations for medical cannabis for the first time under the bill.
The States in Which We Operate, Their Legal Framework and How it Affects Our Business
Illinois Operations
The Compassionate Use of Medical Cannabis Pilot Program Act, which allows individuals diagnosed with a debilitating medical condition access to medical cannabis, became effective January 1, 2014. There were over forty-one (41) qualifying conditions as part of the initial medical program.
The Opioid Alternative Pilot Program launched on January 31, 2019 and allows patients that receive or are qualified to receive opioid prescriptions access to medical cannabis as an alternative in situations where an opioid could generally be prescribed. Under this program, patients with doctor approval can receive near-immediate access to cannabis products from an Illinois licensed dispensary. The Opioid Alternative Pilot Program eliminates the previously required fingerprinting and background checks that often delay patients’ access to medical cannabis by up to three months.
In January 2019, J.B. Pritzker was sworn into office as Governor of Illinois. Cresco Labs’ CEO and co-founder, Charles Bachtell, was appointed to the Cannabis Legalization Subcommittee of the Governor’s transition team. Cannabis Legalization was one of four subcommittees under the Governor’s Restorative Justice and Safe Communities Transition Committee. The primary goals of the Cannabis Legalization Subcommittee were to evaluate and develop implementation recommendations for the Governor’s platform on legalizing cannabis.
In June 2019, the Illinois House of Representatives and Senate passed Senate Bill (“SB”) 2023 which added eleven (11) additional debilitating illnesses such as chronic pain, migraines and irritable bowel syndrome to the list of qualifying medical conditions. This bill was signed into law in August 2019 by Governor J.B. Pritzker.
Additionally, in June 2019, Governor Pritzker signed the Cannabis Regulation and Taxation Act (“CRTA”) into law, making Illinois the 11th state to legalize recreational cannabis. Adult-use sales of cannabis in Illinois began on January 1, 2020.
Cresco Labs is licensed to operate in the State of Illinois as a medical and adult-use cultivator and product manufacturer. Phoenix Farms, LLC (“Phoenix”), PDI Medical III, LLC (“PDI”), FloraMedex, LLC (“FloraMedex”), MedMar Lakeview, LLC (“MedMar Lakeview”) and MedMar Rockford, LLC (“MedMar Rockford”) are each licensed to operate retail dispensaries in the State of Illinois. Further, each of these medical dispensary licenses allowed for one (1) additional adult-use dispensary license, for a total of ten (10) dispensary locations, which are all now open and branded as Sunnyside* dispensaries. In November 2021, the Company relocated its Sunnyside* dispensaries in Buffalo Grove and Lakeview (Chicago) to larger facilities. The new 10,000 square-foot Sunnyside* Lakeview location is approximately 400 feet from Wrigley Field, the home of the Chicago Cubs, making it the closest cannabis dispensary in the country to a national sports stadium. Under applicable laws, the licenses permit Cresco Labs and its subsidiaries to collectively cultivate, manufacture, process, package, sell and purchase cannabis pursuant to the terms of the
27
licenses, which are issued by the Illinois Department of Agriculture (“IDOA”) and the Illinois Department of Financial and Professional Regulation (“IDFPR”) under the provisions of the Illinois Revised Statutes 410 ILCS 130 and 410 ILCS 705. All licenses are, as of the date hereof, active with the State of Illinois, including three (3) transportation licenses. There are currently seven (7) categories of licenses in Illinois: (i) medical cultivation/processing; (ii) adult-use cultivation/processing; (iii) dual use (medical plus adult-use) dispensary; (iv) adult-use dispensary; (v) craft grower; (vi) infuser and (vii) transporting. The licenses are independently issued for each approved activity.
All cultivation/processing establishments and transporters must register with the IDOA and all dispensaries must register with the IDFPR. If applications contain all required information and after vetting by officers, establishments are issued a registration certificate. Registration certificates for medical cannabis operations are valid for a period of one (1) year and are subject to annual renewals after required fees are paid and the business remains in good standing. Registration certificates for adult-use operations are valid for a period of two (2) years and are subject to renewals after required fees are paid and the business remains in good standing. Renewal requests are typically communicated through email from the IDOA or IDFPR and include a renewal form. While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Illinois cannabis licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Illinois cannabis and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
The retail dispensary licenses held by Phoenix, PDI, FloraMedex, MedMar Lakeview and MedMar Rockford permit the Company to purchase cannabis and cannabis products from cultivation/processing facilities, craft growers and infusers and allows the sale of cannabis and cannabis products to registered patients and adult-use customers. As of March 31, 2023, the Company has opened ten (10) Sunnyside* dispensary locations in Illinois, the maximum allowed by the State of Illinois. Two (2) of the ten (10) are located within the City of Chicago.
The three (3) medical cultivation licenses held by Cresco Labs permit it to acquire, possess, cultivate, manufacture/process into edible medical cannabis products and/or cannabis marijuana-infused products, deliver, transfer, test, transport, supply or sell cannabis and related supplies to medical cannabis dispensaries. In September 2019, the three (3) cultivation facilities were approved for growing adult-use cannabis by the IDOA, for a total cultivation capacity of 630,000 square feet, the maximum allowed by law.
The CRTA mandates that the IDOA issue up to forty (40) craft grower licenses by July 1, 2020. The CRTA further required the IDOA to issue up to sixty (60) craft grower licenses by December 21, 2021. After January 1, 2022, the IDOA may by rule modify or raise the number of craft grower licenses. However, at no time may the number of craft grower licenses exceed one hundred fifty (150). Pursuant to the CRTA, the IDOA was also required to issue up to forty (40) infuser licenses by July 1, 2020 and then could issue up to sixty (60) additional infuser licenses by December 21, 2021. Prior to the issuance of these up to sixty (60) additional licenses, the CRTA permits the IDOA to adopt emergency rules to modify or raise the number of infuser licenses. After January 1, 2022, the IDOA may again modify or raise the number of infuser licenses by rule. The IDOA is also authorized under the CRTA to issue an unlimited amount of transporter licenses, starting, according to the CRTA, no later than July 1, 2020. On August 2, 2021, the IDOA announced that it had issued the first round of adult-use cannabis licenses under the CRTA. In total, on that day, it issued thirty-two (32) initial craft grower licenses, twenty-eight (28) infuser licenses and nine (9) transporter licenses. Since that time, the IDOA has issued additional licenses, as authorized under the CRTA. In total the IDOA has issued eighty-eight (88) craft grower licenses, fifty-four (54) infuser licenses and two hundred twenty-two (222) transporter licenses.
28
The Cannabis Regulation and Tax Act also requires the award of conditional adult-use dispensing licenses by the IDFPR. On September 3, 2021, the IDFPR announced the results of several lotteries to award one hundred eighty-five (185) conditional adult-use dispensing licenses that have been part of an application process since early 2020. However, as a result of a series of lawsuits, those licenses were not immediately formally awarded. On July 22, 2022, the IDFPR began issuing Conditional Adult Use Dispensing Organization Licenses, awarding one hundred forty-nine (149) Conditional Licenses initially. The IDFPR previously announced its intention to conduct an additional lottery to award conditional adult-use dispensing organization licenses and resolve pending litigation. With court approval, the IDFPR conducted fifty-one (51) corrective lotteries over three days. The Qualifying Applicant Lottery was held on June 21, 2022; the Social Equity Justice Involved Lottery was held on June 22, 2022 and the Tied Applicant Lottery was held on June 23, 2022.
In August 2022, following almost a year of delays, the State of Illinois resumed issuing social equity licenses, issuing one hundred seventy-seven (177) of the one hundred eighty-five (185) licenses it was supposed to issue as of late July 2022. On November 10, 2022, IDFPR announced the issuance of the first full adult-use cannabis dispensing organization licenses to social equity applicants. On that date IDFPR issued an update on the issuance of “conditional” licenses, stating that to date it had issued one hundred ninety-two (192) “conditional” adult-use cannabis dispensing organization licenses.
In January 2023, Illinois regulators implemented several modifications for social equity applicants vying for one of the state’s fifty-five (55) new adult-use retail licenses. Among the changes, license seekers will face a simpler application, related fees will drop from $2,500 to $250 and all winners will be selected via lottery. IDFPR said it will begin accepting applications January 30 after distributing them across the state’s seventeen (17) dedicated regions.
Other application modifications include:
| • | Eliminating residency requirements and bonus points for military veterans; |
| • | Removing an allowance for applicants to gain social equity status by hiring at least ten (10) employees who lived in disproportionate areas of marijuana arrests or were arrested or convicted of low-level marijuana offenses; and |
| • | Applicants can only apply for licenses in one (1) region and file only one (1) application. |
In late April 2023, IDFPR announced it received 2,693 applications for the upcoming lottery, which is expected to take place in May 2023.
Pennsylvania Operations
The Pennsylvania medical marijuana program was signed into law on April 17, 2016 under Act 16 and provided access to state residents with one (1) of twenty-one (21) qualifying conditions. The state, which consists of over 12 million U.S. citizens and qualifies as the fifth largest population in the U.S., operates as a high-barrier market with very limited market participation. The state originally awarded only twelve (12) licenses to cultivate/process and twenty-seven (27) licenses to operate retail dispensaries (which entitled holders up to three (3) medical dispensary locations). Out of the hundreds of applicants in each license category, Cresco Yeltrah, LLC (“Yeltrah”) was awarded one (1) medical cannabis grower/processor license in Pennsylvania and one (1) dispensary license allowing three (3) dispensary locations in Pennsylvania. Cresco Labs was awarded the second highest overall score during the application process. On June 30, 2021, Pennsylvania Governor Tom Wolf signed into law PA House Bill (“HB”) 1024, amending Act 16. HB 1024 implemented several changes to Act 16 including but not limited to the ability for grower/processors to
29
obtain and transport bulk post-harvest plant material between grower/processors to process medical marijuana. The amendatory legislation also expanded the list of qualifying conditions, permits limited remediation of cannabis flower, requires the Department of Agriculture to update its list of approved pesticides and expands the number of clinical registrants and affords clinical registrants with the same rights as grower/processors.
Retail sales commenced in February 2018 to a limited number of retail locations across the state. On February 15, 2018, Yeltrah was the first grower/processor to release product into the Pennsylvania market (approximately six (6) weeks ahead of any other producer) and its dispensary was the first to sell product to patients in the state.
On March 22, 2018, it was announced that the final phase of the Pennsylvania medical marijuana program would initiate its rollout, which would include thirteen (13) additional cultivation/processing licenses and twenty-three (23) additional dispensary licenses. The application period ran from April 2018 through May 2018. Yeltrah submitted additional dispensary applications and in December 2018 one (1) additional dispensary license was obtained to open three (3) additional dispensary locations, for a total of six (6) dispensary locations in the State of Pennsylvania. All six (6) dispensary locations are currently operational.
Under applicable laws, the licenses permit Yeltrah to cultivate, manufacture, process, package, sell and purchase medical marijuana pursuant to the terms of the licenses, which are issued by the Pennsylvania Department of Health (“PDOH”) under the provisions of Medical Marijuana Act (35 P.S. §10231.101 — 10231.2110) and Chapters 1141, 1151 and 1161 of the Pennsylvania regulations. In the latter half of 2022, the PDOH completed the process of revising its medical regulations, which were implemented in the first quarter of 2023.
There are three (3) categories of licenses in Pennsylvania: (i) grower/processer, (ii) dispensary and (iii) clinical registrant. The Yeltrah licenses are independently issued for each approved activity for use at Yeltrah facilities in Pennsylvania.
All grower/processor establishments and all dispensaries must register with the PDOH. Registration certificates are valid for a period of one (1) year and are subject to annual renewals after required fees are paid and the business remains in good standing. While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Pennsylvania cannabis licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Pennsylvania cannabis and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
The retail dispensary licenses permit Yeltrah to purchase marijuana and marijuana products from grower/processer facilities and allows the sale of marijuana and marijuana products to registered patients. The medical grower/processor license permits Yeltrah to acquire, possess, cultivate, manufacture/process into edible medical marijuana products and/or medical marijuana-infused products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
On November 24, 2021, Cresco Labs completed its acquisition of Bay, LLC d/b/a Cure Pennsylvania for aggregate consideration of $89.0 million. The acquisition added one (1) additional dispensary license, which allowed for three (3) additional dispensary locations in the State of Pennsylvania. All three (3) dispensary locations are operational and have been rebranded as Sunnyside* dispensaries.
On December 9, 2021, Cresco completed its acquisition of Laurel Harvest Labs, LLC (“Laurel Harvest”) for consideration equal to $136.7 million. Laurel Harvest’s permit is a Clinical Registrant permit license (“CR”). A CR
30
permittee is required to have a contractual relationship with an academic clinical research center under which the academic or clinical research center provides advice to the permit holder regarding patient health and safety, medical applications and dispensing and management of controlled substances, among other things. Laurel Harvest has a contractual relationship with Temple University, which has established one of the most sophisticated cannabis research programs in the country. A CR permittee is approved by the PDOH to hold a permit as both a grower/processor and a dispensary. The Laurel Harvest cultivation and processing facility is currently under construction in Mt. Joy. At the time of the acquisition, Laurel Harvest had one (1) operational dispensary in Montgomeryville. The CR permit entitled Laurel Harvest to an additional five (5) dispensary locations throughout the Commonwealth. In the first quarter of 2022, the dispensary was rebranded as a Sunnyside* dispensary. On February 8, 2023, the Company announced it had opened a second dispensary under the Laurel Harvest license in Erie, PA.
On February 4, 2022, the PDOH’s Office of Medical Marijuana released a statement announcing that it was ordering the recall of certain vape medical marijuana products containing some added ingredients that had not been approved for inhalation by the U.S. FDA. This recall effected three vape product formulations sold by Cresco entities in Pennsylvania. The Company previously reviewed the pertinent facts and completed its assessment of the potential impact of the recall, concluding no material impact to the consolidated financial position, results of operations, or cash flows. However, in June 2022, a Commonwealth Court stopped the recall, allowing the sale of products that had been taken off shelves to resume.
On September 1, 2022, the Company closed on a sale and leaseback transaction to sell its Brookville, Pennsylvania, facility to Aventine Property Group (“Aventine”). Concurrent with the closing of the sale, the Company entered into a long-term, triple-net lease agreement with Aventine regarding the property and will continue to operate the facility as a permitted cannabis cultivation and processing facility.
Newly elected Governor Josh Shapiro (D) frequently issued support for legalizing adult-use cannabis during his campaign. Democrats won enough seats in this past November’s midterm elections to control Pennsylvania’s House for the first time in over a decade. Governor Shapiro has said legalization efforts must include criminal justice reform, specifically mentioning expungement of non-violent marijuana convictions.
Ohio Operations
HB 523, effective on September 8, 2016, legalized medical marijuana in Ohio. The Ohio Medical Marijuana Control Program (“OMMCP”) allows people with certain medical conditions, upon the recommendation of an Ohio-licensed physician certified by the State Medical Board, to purchase and use medical marijuana. HB 523 required that the framework for the OMMCP become effective as of September 2018. This timeframe allowed for a deliberate process to ensure the safety of the public and to promote access to a safe product.
The three (3) following state government agencies are responsible for the operation of OMMCP: (1) the Ohio Department of Commerce is responsible for overseeing medical marijuana cultivators, processors and testing laboratories; (2) the State of Ohio Board of Pharmacy (“Ohio Pharmacy Board”) is responsible for overseeing medical marijuana retail dispensaries, the registration of medical marijuana patients and caregivers, the approval of new forms of medical marijuana and coordinating the Medical Marijuana Advisory Committee and (3) the State Medical Board of Ohio is responsible for certifying physicians to recommend medical marijuana and may add to the list of qualifying conditions for which medical marijuana can be recommended.
31
Several forms of medical marijuana are legal in Ohio, these include: inhalation of marijuana through a vaporizer (not direct smoking), oils, tinctures, plant material, edibles, patches and any other forms approved by the Ohio Pharmacy Board.
On June 4, 2018, the Ohio Pharmacy Board awarded fifty-six (56) medical marijuana provisional dispensary licenses. The licenses were awarded after an extensive review of three hundred seventy-six (376) submitted dispensary applications.
By rule, the Ohio Pharmacy Board was limited to issuing up to sixty (60) dispensary licenses across the state (58 were initially issued) but had the authority to increase the number of licenses. The Ohio Pharmacy Board opened up a new application period for dispensaries, increasing the potential number of dispensaries in the state to one hundred thirty one (131). A drawing was held on January 27, 2022, to ultimately award seventy-three (73) provisional dispensary licenses. The initial drawing simply determined the order in which each applicant was selected by region. Official winners were not announced at that time. On May 16, 2022, the State of Ohio Board of Pharmacy issued seventy (70) provisional dispensary licenses as part of the RFA II process (three (3) were held for further vetting and one (1) has since been awarded – seventy-one (71) in total). Currently, eighty-one (81) dispensaries are operational in Ohio, with only five (5) of those coming from RFA II. However, the Ohio Pharmacy Board left unchanged a regulation that limits the number of dispensary certificates of operation that a single owner can hold at five (5). Per the program rules, the Ohio Pharmacy Board will consider, on at least a biennial basis, whether enough medical marijuana dispensaries exist, considering the state population, the number of patients seeking to use medical marijuana and the geographic distribution of dispensary sites.
Cresco Labs Ohio, LLC (“Cresco Labs Ohio”) was awarded one (1) dispensary license located in Wintersville, Ohio. The dispensary license permits Cresco Labs Ohio to purchase marijuana and marijuana products from cultivation/processing facilities and allows the sale of marijuana and marijuana products to registered patients. Cresco Labs Ohio applied for and, on November 30, 2017, received one (1) cultivation license. Cresco Labs Ohio’s cultivation facility is a hybrid greenhouse structure located in Yellow Springs, Ohio. The medical cultivation license authorizes Cresco Labs Ohio to grow, harvest, package and transport medical marijuana products. On December 12, 2018, Cresco Labs Ohio was granted the first dispensary Certificate of Operation in the state. Retail sales commenced on January 16, 2019, with the first cannabis sale taking place at the Wintersville dispensary. This was the second state medical marijuana program in which the Company was first to market.
On June 8, 2020, Cresco Labs Ohio was granted a provisional processing license by the State of Ohio. This license allows Cresco Labs Ohio to extract oils and manufacture products from cannabis which will now provide the Company the ability to sell its entire brand portfolio in Ohio. Cresco Labs Ohio received its Certificate of Operation to begin processing activities on June 11, 2021.
Ohio cultivation and processor licenses are renewable annually by the Ohio Department of Commerce (“ODOC”). Renewal applications are due at least thirty (30) days prior to the expiration date of the Certificate of Operation. The ODOC shall grant a renewal if the renewal application was timely filed, the annual fee was timely paid, there are no reasons warranting denial of the renewal and the cultivator/processor passes inspection. Ohio dispensary licenses expire biennially on the date identified on the certificate. Renewal information, including a renewal fee, must be submitted at least forty-five (45) days prior to the date the existing certificate expires. If the dispensary is operated in compliance with Ohio dispensary regulations and the renewal fee is paid, the Ohio Pharmacy Board shall renew the Certificate of Operation within forty-five (45) days after the renewal application is received. While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Ohio cannabis licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Ohio cannabis and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
32
On February 16, 2021, the Company completed its acquisition of Verdant Creations, LLC for total consideration of $25.0 million. The acquisition added dispensaries in Cincinnati, Chillicothe, Newark and Marion, Ohio. This acquisition brought the Company’s dispensary presence in Ohio to five (5), the maximum allowed by the State of Ohio.
The Ohio Pharmacy Board approved employee discounts which took effect in July 2022. Previously, companies could not offer discounts to employees that are also patients/cardholders. SB 261, which includes changes to the medical cannabis program, passed in the Senate on December 15, 2021, and was sent to the Government Oversight Committee in the House.
The 2021 to 2022 adult-use ballot initiative, which had been in process, was pushed back to the 2023 November election cycle. On January 28, 2022, Ohio Secretary of State Frank LaRose announced that the Coalition to Regulate Marijuana Like Alcohol (the “Coalition”) had submitted enough valid signatures to trigger an “initiated statute” process, which places the group’s adult-use cannabis statute before the legislature. Lawmakers had four months to act on the bill. If the bill is amended or not acted upon, the Coalition could have accepted the legislature’s response or gather enough signatures to place the question of adult-use cannabis legalization on the general election ballot.
After conducting a signature gathering and ballot initiative campaign, the Coalition-led initiated statute was struck from last year’s ballot after disagreement with state lawmakers over the interpretation of a 10-day deadline related to ballot initiatives outlined by the Ohio Constitution. A lawsuit ensued, and the parties eventually settled: Secretary LaRose resubmitted the petition when a new slate of legislators convened in January 2023 and allowed the Coalition to reuse the initial signatures it collected in support of legalizing cannabis.
If the Republican Statehouse super majority fails to adopt the measure within four months, the question could come before Ohio voters in November 2023. The 34-page act would legalize the possession, purchase, and sale of marijuana by Ohioans ages twenty-one (21) and older, while implementing a 10% tax on the sale of all cannabis products.
Secretary LaRose’s reintroduction of the proposal abides by a May agreement reached with the Coalition shortly after the pro-legalization group accused Republican lawmakers of blocking the cannabis question from the November 2022 ballot.
California Operations
In 1996, California was the first state to legalize medical marijuana through Proposition 215, the Compassionate Use Act of 1996 (“CUA”). This legalized the use, possession and cultivation of medical marijuana by patients with a physician’s recommendation.
In 2003, SB 420 was signed into law establishing an optional identification card system for medical marijuana patients.
In September 2015, the California legislature passed three (3) bills collectively known as the “Medical Cannabis Regulation and Safety Act” (“MCRSA”). The MCRSA established a licensing and regulatory framework for medical marijuana businesses in California. The system created multiple license types for dispensaries, infused products manufacturers, cultivation facilities, testing laboratories, transportation companies and distributors. Edible infused
33
product manufacturers would require either volatile solvent or non-volatile solvent manufacturing licenses depending on their specific extraction methodology. Multiple agencies would oversee different aspects of the program and businesses would require a state license and local approval to operate. However, in November 2016, voters in California overwhelmingly passed Proposition 64, the “Adult-Use of Marijuana Act” (“AUMA”) creating an adult-use marijuana program for adults twenty-one years of age or older. AUMA had some conflicting provisions with MCRSA, so in June 2017, the California State Legislature passed SB 94, known as Medicinal and Adult-Use Cannabis Regulation and Safety Act (“MAUCRSA”), which amalgamates MCRSA and AUMA to provide a set of regulations to govern medical and adult-use licensing regime for cannabis businesses in the State of California. MAUCRSA went into effect on January 1, 2018. Previously, the four (4) agencies that regulated marijuana at the state level are the Bureau of Cannabis Control (“BCC”), the California Department of Food and Agriculture, the California Department of Public Health (“CDPH”) and the California Department of Tax and Fee Administration (“CDTFA”). On July 12, 2021, California Governor Gavin Newsom signed into law Assembly Bill 141, which established the Department of Cannabis Control (“DCC”). The DCC consolidates the BCC, CDFA’s CalCannabis Licensing Division and CDPH’s Manufactured Cannabis Safety Branch into a single department. The DCC is charged with licensing, inspecting and providing regulatory oversight over all cannabis businesses in California.
In order to legally operate a medical or adult-use cannabis business in California, the operator must have both a local and state license. This requirement limits license holders to operate only in cities with marijuana licensing programs. Therefore, cities in California are allowed to determine if they will have a marijuana licensing program and determine the number of licenses they will issue to marijuana operators.
California Operations — SLO and CannaRoyalty Corp. d/b/a Origin House (“Origin House”)
On June 7, 2018, Cresco Labs acquired a 60% ownership interest in SLO. On September 27, 2018, Cresco acquired a further 20% ownership interest in SLO bringing its total ownership to 80%. SLO operates cannabis facilities in the city of Mendota (Fresno County). SLO is licensed to manufacture and distribute medical and adult-use cannabis in the State of California pursuant to the terms of the licenses.
On January 8, 2020, Cresco Labs acquired all of the issued and outstanding shares of Origin House, a leading distributor and provider of brand support services in California.
California state and local licenses are renewed annually. Each year, licensees are required to submit a renewal application. While renewals are annual, there is no ultimate expiry after which no renewals are permitted. Additionally, in respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner and there are no material violations noted against the applicable license, the Company would expect to receive the applicable renewed license in the ordinary course of business. While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that the licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of the Company in California and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
The Company is licensed to cultivate, manufacture and distribute medical and adult-use cannabis and cannabis-related products:
West Sacramento (Yolo County)
| • | Origin House has been issued one (1) provisional Type 11 (Distribution) license. |
34
| • | Origin House submitted an annual application for the one (1) listed license type to the state regulator and is awaiting approval for this annual application. |
La Habra (Orange County)
| • | Origin House has been issued one (1) provisional Type 11 (Distribution) license. |
| • | Origin House submitted an annual application for the one (1) listed license type to the state regulator and is awaiting approval for this annual application. |
Unincorporated Sonoma (Sonoma County)
| • | Origin House has been issued one (1) provisional Cultivation, Medium Indoor license. |
| • | Origin House has been issued one (1) provisional Processor license. |
| • | Origin House has been issued one (1) provisional Type 11 (Distribution) license. |
| • | Origin House has been issued one (1) annual license for Type 11 (Distribution). |
| • | Origin House has been issued one (1) provisional Cultivation, Small Indoor license. |
| • | Origin House has been issued one (1) provisional Nursery license. |
| • | All provisional licenses have corresponding annual applications pending with the DCC. |
In March 2022, the DCC initiated a rule making process in which it promulgated a comprehensive regulatory proposal, including amendments to its current rules that would make permanent emergency rules that have been in effect since September 2021. Comments on the proposed rules were submitted by all interested parties by April 19, 2022. The DCC then considered the comments submitted and issued an updated rule set for a second comment period. A final version of the rules was filed by the DCC with the California Office of Administrative Law on September 26, 2022. The rules are now in effect. Among other rule making activities, the DCC is currently considering new rules related to the establishment of a standard cannabinoids test method, including standardized operating procedures which will be utilized by all licensed testing laboratories in California. With respect to legislation, the California legislature passed AB 195, which was signed into law by Governor Newsom on June 30, 2022. AB-195 eliminates the cannabis cultivation tax and serves to shift responsibility for collecting the cannabis excise tax from distributors to retailers.
During the second quarter of 2022, the Company initiated a plan to shut down a cultivation facility and production facility in California. As a result of this plan, the Company exercised its early termination right to reduce the existing lease terms to 180 days. All operations at the facilities ceased in the third quarter of 2022 and the corresponding licenses were surrendered in the fourth quarter of 2022.
During the fourth quarter of 2022, Management committed to a plan to restructure certain operations and activities within the California reporting unit. It was determined that the Company’s shift in strategy was an indicator of impairment for associated assets. $89.5 million in goodwill impairment and $1.0 million in impairment to ROU assets was recorded to the California reporting unit during 2022. During the first quarter of 2023, the Company adjusted the values of certain leases at the facilities impacted as a result of a change in the underlying assumptions regarding renewal options for those leases. Due to differences between the carrying amounts of the ROU assets and lease liabilities associated with these leases, a gain on lease termination of $1.1 million has been recorded for the three months ended March 31, 2023, and is included in Other income (expense), net, in the Unaudited Condensed Interim Consolidated Statements of Operations. Further, the Company accelerated depreciation on leasehold improvements related to those leases, with additional depreciation expense taken on these leasehold improvements in the amount of $1.1 million during the first quarter of 2023. Further, $1.0 million of accounts receivable was reserved for and the Company recorded a $0.7 million severance accrual for one-time involuntary termination benefits.
35
Arizona Operations
In 2010, Arizona passed Ballot Proposition 203, which amended Title 36 to the Arizona Revised Statutes. This amendment added Chapter 28.1, titled the Arizona Medical Marijuana Act (“AMMA”). The AMMA is codified in Arizona Revised Statutes §36-2801 et. seq. The AMMA also appointed the Arizona Department of Health Services (“ADHS”) as the regulator for the program and authorized ADHS to promulgate, adopt and enforce regulations for the AMMA. These ADHS regulations are embodied in the Arizona Administrative Code Title 9 Chapter 17. In order to qualify to use medical marijuana under the AMMA, a patient is required to have a “debilitating medical condition.”
The ADHS has established the Arizona Department of Health Services Medical Marijuana Program (“MMJ Program”), which includes a vertically-integrated license, meaning if allocated a Medical Marijuana Dispensary Registration Certificate (“AZ Dispensary License”), entities are authorized to dispense and cultivate medical cannabis. Each AZ Dispensary License allows the holding entity to operate one (1) on-site cultivation facility and one (1) off-site cultivation facility which can be located anywhere within the State of Arizona. An entity holding an AZ Dispensary License is required to file an application to renew with the ADHS on a biannual basis, which must also include audited annual financial statements. While an AZ Dispensary License may not be sold, transferred or otherwise conveyed, AZ Dispensary License holders typically contract with third parties to provide various services related to the ongoing operation, maintenance and governance of its dispensary and/or cultivation facility so long as such contracts do not violate the requirements of the AMMA or the MMJ Program.
On October 24, 2018, Cresco Labs obtained a 100% ownership interest in Arizona Facilities Supply, LLC which included a vertically-integrated cultivation, processing and dispensary operation in Arizona.
In November 2020, voters in Arizona passed an adult-use marijuana measure to allow for the sale of recreational marijuana in the state. During 2021, the Company received approval from the ADHS to serve adult-use customers at its Sunnyside* dispensary in Phoenix, Arizona. Adult-use sales launched in February of 2021. No cannabis reforms occurred in the 2022 Legislative Session. However, pursuant to the state’s Social Equity Ownership Program required under Proposition 207, twenty-six (26) new social equity licenses were awarded in April of 2022. License holders have eighteen months to become operational or potentially forfeit their license.
During the third quarter of 2022, the Company shut down a cultivation facility in Arizona. The Company is currently in the process of determining a disposal plan for the assets at this location.
During the fourth quarter of 2022, Management determined it is more likely than not that the Arizona reporting unit carrying values exceed their fair value due to updated forecasts and projections for the reporting unit. $10.1 million in goodwill impairment was recorded to the Arizona reporting unit during 2022.
The licenses in Arizona are renewed bi-annually. Before expiry, licensees are required to submit a renewal application. While renewals are granted bi-annually, there is no ultimate expiry after which no renewals are permitted. Additionally, with respect of the renewal process, provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner and there are no material violations noted against the applicable license, Cresco Labs would expect to receive the applicable renewed license in the ordinary course of business. While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Arizona cannabis licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Arizona cannabis and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
36
In January 2023, the Arizona Department of Revenue released a report highlighting that for eight straight months medical marijuana sales dropped from the month prior. By contrast, adult-use cannabis sales hit a new high in the same month.
New York Operations
The State of New York’s medical cannabis program was introduced in July 2014 when former Governor Andrew Cuomo signed the Compassionate Care Act, which legalized medical cannabis oils for patients with certain qualifying conditions. Under this program, five (5) registered organizations (“ROs”) were licensed to dispense cannabis oil to patients, with the first sale to a patient completed in January 2016. In December 2016, the New York State Department of Health (“NYSDOH”) added chronic pain as a qualifying condition and in the month-and-a-half following the addition of chronic pain, the number of registered patients increased by 18%. In August 2017, the NYSDOH granted licenses to five (5) additional ROs.
In July 2018, the NYSDOH added opioid replacement as a qualifying condition, meaning any condition for which an opioid could be prescribed is now a qualifying condition for medical cannabis. In August 2018, former Governor Cuomo, prompted by an NYSDOH study which concluded the “positive effects” of cannabis legalization “outweigh the potential negative impacts,” appointed a group to draft a bill for regulating legal adult-use cannabis sales in New York.
On October 8, 2019, the Company completed its acquisition of Gloucester Street Capital, the parent entity of Valley Agriceuticals, LLC (“Valley Ag”). Valley Ag is one (1) of the ten (10) holders of a vertically-integrated license from NYSDOH allowing for the cultivation and processing of medical cannabis as well as the establishment of four (4) medical cannabis dispensaries in the State of New York. While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that New York cannabis licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of New York cannabis and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
On January 6, 2021, former Governor Cuomo announced a proposal to legalize and create a comprehensive system to oversee and regulate adult-use cannabis in New York as part of the 2021 State of the State. Under the Governor’s proposal, a new Office of Cannabis Management (the “OCM”) would be created to oversee the new adult-use program, as well as the state’s existing medical and cannabinoid hemp programs. Additionally, an equitable structure for the adult-use market will be created by offering licensing opportunities and assistance to entrepreneurs in communities of color who have been disproportionately impacted by the war on drugs.
On February 16, 2021, former Governor Cuomo announced 30-day amendments to the Governor’s proposal to establish a comprehensive adult-use cannabis program in New York. Specifically, these amendments detailed how the $100.0 million in social equity funding will be allocated, enable the use of delivery services and refine which criminal charges will be enforced as it relates to the improper sale of cannabis to further reduce the impact on communities.
Former Governor Cuomo signed SB 854/AB 1248A on March 31, 2021, creating the Empire State’s adult-use cannabis program. This legislation expands Cresco Labs’ potential dispensary footprint to eight (8), with three (3) dispensaries reserved to be co-located adult-use, allows existing vertical ROs to wholesale branded products and creates a strong
37
social equity program with 50.0% of licenses dedicated to social equity applicants. The Cannabis Control Board (the “CCB”) oversees the rollout of the program was seated in summer/early fall 2021. The CCB held its first meeting on October 5, 2021. At that meeting the CCB announced changes to the state’s medical program that would go into effect immediately including that cannabis flower could be sold to patients. Since that initial meeting, the CCB has granted certifying healthcare providers wider discretion in recommending medical cannabis, increased the amount of medical cannabis a patient can purchase at one time, and implemented home cultivation rules as well as new cannabinoid hemp rules. In December 2022, the OCM promulgated a series of adult-use regulations that would govern, among other things, the licensing process for the adult-use cannabis program. Those rules are still undergoing a public comment and revision process. The initial public comment period on those proposed rules closes on February 13, 2023. OCM has also engaged in the development of other adult-use regulations, including those governing packaging, labeling, marketing, advertising and laboratories, which have all been finalized.
Previously, on March 30, 2022, proposed rules related to the issuance of conditional adult-use retail dispensary licenses were published by the OCM. Those rules underwent a public comment period and final rules were approved by the CCB on July 14, 2022. The regulations went into effect on August 3, 2022. In addition to the adoption of rules and ongoing rule makings, on February 22, 2022, the current governor of New York Kathy Hochul signed legislation that provided a path for New York’s existing hemp operators to obtain provisional cannabis cultivator and processor licenses. Under that law, hemp farmers that were licensed with the Department of Agriculture as of December 31, 2021 would be allowed to cultivate up to 43,500 square feet outdoors, 25,000 square feet in greenhouse facilities, or no more than 30,000 square feet comprising a combination of the outdoor and greenhouse space. The hemp businesses would be required to meet environmental sustainability, labor peace, and equity benchmarks to be allowed to cultivate and minimally process cannabis until June 2023. Hemp businesses issued provisional licenses are required to begin operations within six months of the license being issued. After June 2023, the hemp businesses are required to apply for a cultivator or processor license.
Through the aforementioned agreements and regulatory approval, Cresco Labs now has a license for a cultivation and manufacturing facility within the State of New York, as well as four (4) dispensary locations strategically located across the state. These four (4) dispensary locations are branded as Sunnyside* dispensaries. The Company has successfully renewed its initial licenses and all licenses are, as of the date hereof, active with the State of New York.
Further, New York State’s fiscal year 2022 to 2023 budget includes Section 280E Deductions, which permits tax deductions for commercial cannabis activity. This applies to taxable years beginning on January 1, 2023. The budget also includes a $200.0 million Social Equity Fund, which allows New York State to invest in a private fund to finance the leasing and equipping of up to one hundred and fifty (150) conditional adult-use retail dispensaries in New York State to be operated by individuals who have been impacted by the inequitable enforcement of marijuana laws.
Through the OCM, New York began issuing licenses for cannabis cultivation and processing in April and August of 2022, respectively. Approximately two hundred seventy-nine (279) conditional cultivation licenses have been granted and approximately forty (40) conditional processor licenses. The application period for Conditional Adult-Use Retail Dispensary (“CAURD”) licenses was open from August 25, 2022 to September 25, 2022 and the state received approximately nine hundred (900) applications, for one hundred seventy-five (175) available licenses. On April 3, 2023, the CCB provisionally approved ninety-nine (99) more CAURD licenses, increasing New York’s total provisional retail dispensary licenses to one hundred and sixty-five (165). The licenses included four (4) for Western New York, one (1) for Central New York, five (5) for Mid- Hudson and three (3) for Brooklyn. The CCB had previously been prevented from issuing provisional licenses in those regions because of a court injunction. The CCB has now granted at least one (1) CAURD provisional license in each region other than the Finger Lakes, which remains blocked by court injunction.
38
On December 29, 2022, New York officially opened retail cannabis sales to adults over age twenty-one (21). Under the law passed in March 2021, consumers are allowed to purchase up to three (3) ounces of cannabis and twenty-four (24) grams of cannabis concentrate. The state currently has eight (8) open adult-use dispensaries.
Massachusetts Operations
The Massachusetts medical cannabis market was established through “An Act for the Humanitarian Medical Use of Marijuana” in November 2012 when voters passed Ballot Question 3 “Massachusetts Medical Marijuana Initiative” with 63.0% of the vote. The first Massachusetts dispensary opened in June 2015 and by November 2016, Massachusetts voters legalized adult-use cannabis by passing ballot Question 4 – Legalize Marijuana with 54.0% of the vote. In July 2017, former Governor Baker signed legislation that would lay the groundwork for the state’s adult-use market. The Cannabis Control Commission (the “CCC”) (the state’s regulatory body which creates regulations for both the medical and adult-use market) aimed to officially launch adult-use sales on July 1, 2018 but stumbling blocks such as a lack of licensed testing labs and disagreements between officials and businesses slowed the rollout and sales for adult-use cannabis officially began in November 2018.
The CCC oversees the medical and adult-use cannabis programs. Each medical licensee must be vertically-integrated and may have up to two (2) locations. Licensed medical dispensaries are given priority in adult-use licensing. Adult-use cultivators will be grouped into eleven (11) tiers of production (ranging from up to 5,000 square feet to no larger than 100,000 square feet) and regulators will move a licensee down to a lower tier if that licensee has not shown an ability to sell at least 70% of what it produced. Medical dispensaries that wish to add the ability to sell cannabis products to non-patients will be required to reserve 35% of their inventory or the six-month average of their medical cannabis sales for medical cannabis patients. In order to achieve an adult-use license, a prospective licensee must first sign a “Host Community Agreement” with the town in which it wishes to locate. Roughly one-third of municipalities in the state have a ban or moratorium in place that prohibits cannabis businesses from operating within their jurisdiction. In both the medical and adult-use markets, extracted oils, edibles and flower products are permitted, as well as wholesaling.
On October 1, 2019, Cresco Labs acquired Hope Heal Health, Inc. (“HHH”) via certain agreements giving it operational control before cash consideration was settled. In August 2019, HHH entered into a Host Community Agreement with the municipality of Fall River. On February 7, 2020, the Company legally closed the acquisition and cash funding of $27.5 million. The closing coincided with state approval allowing recreational cannabis sales at the Company’s Fall River dispensary.
Registration certificates are valid for a period of one (1) year and are subject to annual renewals after required fees are paid and the business remains in good standing. Renewal requests are typically communicated through email from the Massachusetts CCC and include a renewal form. While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Massachusetts cannabis licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Massachusetts cannabis and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
On September 2, 2021, the Company completed the acquisition of 100% of the membership interests of Cultivate Licensing, LLC (“Cultivate”) for total consideration of $99.3 million. Cultivate owned and operated two (2) cultivation and manufacturing center locations, two (2) adult-use and medical dispensary locations and one (1) adult-use dispensary location. The closing of this acquisition was contingent upon the Company surrendering its adult-use retail license for the Fall River dispensary. After the closing of the acquisition, the Fall River dispensary location is medical only.
39
The Massachusetts Senate and House of Representatives debated and voted on bills SB 2823 and HB 4791 in April and June, respectively, and passed the bills in August of 2022. The bills address several cannabis related issues, including host community agreement reform, a social equity trust fund and the referendum process for social consumption licenses. On August 11, 2022, former Governor Baker signed both measures into law.
Starting in January 2023, Massachusetts adopted a curriculum designed to educate teens on the risks of driving while under the influence of cannabis. Under the program, as of January 1, 2023, Massachusetts became the first state that has legalized the recreational use of marijuana to adopt the curriculum designed by AAA Northeast, according to the state Registry of Motor Vehicles. The current driver education curriculum addressing impaired driving was updated to include information on cannabis, such as how tetrahydrocannabinol (“THC”) — the active chemical in marijuana — affects cognition, vision, reaction time, and perception of time and distance.
Michigan Operations
In November 2008, Michigan residents approved the Michigan Medical Marihuana Act (the “MMMA”) to provide a legal framework for a safe and effective medical marijuana program. In September 2016, the Michigan Senate passed the Medical Marihuana Facilities Licensing Act (the “MMFLA”) and the Marihuana Tracking Act (the “MTA”) and together with the MMMA, (the “Michigan Cannabis Regulations”) provides a comprehensive licensing and tracking scheme, respectively, for the medical marijuana program. Additionally, the Michigan Department of Licensing and Regulatory Affairs and its licensing board (“LARA”) has supplemented the Michigan Cannabis Regulations with “Emergency Rules” to further clarify the regulatory landscape surrounding the medical marijuana program. The scope of LARA’s remit has expanded in the last several years and LARA is now the Cannabis Regulatory Agency (“CRA”). LARA is the main regulatory authority for the licensing of marijuana businesses.
On November 6, 2018, Michigan voters approved Proposal 1, to make marijuana legal under state and local law for adults twenty-one years of age or older and to control the commercial production and distribution of marijuana under a system that licenses, regulates and taxes the businesses involved. The act would be known as the Michigan Regulation and Taxation of Marihuana Act. In accordance with Proposal 1, LARA began accepting applications for retail (recreational) dispensaries on November 1, 2019.
On March 25, 2019, an affiliate of the Company (the “Michigan Affiliate”) announced that it had completed the most comprehensive portion of Michigan’s application process, being pre-qualified for a cultivation and processing license in Michigan. The pre-qualification represents the authorization of the entity to move forward with the licensing process for its intended facilities.
On November 13, 2019, Michigan announced any existing medically licensed businesses would be allowed to sell recreational-use marijuana beginning December 1, 2019. On March 5, 2020, the Michigan Affiliate was issued a medical processing license to begin manufacturing and processing flower into edible medical marijuana products and/or medical marijuana-infused products.
In 2020, the Michigan Affiliate received approval to operate one (1) adult-use processor license and one (1) medical processor license. The Michigan Affiliate received its first medical and adult-use cultivation licenses in June 2021. Additional cultivation licenses have been added as production capacity continues to grow.
40
All Michigan licenses are renewed annually through the Cannabis Regulatory Agency after the required fees are paid and the business remains in good standing. In addition, a sworn statement is required that states that the business is in good standing and will uphold a continuing reporting duty. The renewal fees are to be determined by the amount of gross weight of marijuana products transferred during the past year. While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Michigan cannabis licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Michigan cannabis and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
On April 22, 2020, the Michigan Affiliate and related parties of the Company executed an amended and restated operating agreement which increased the Company’s related parties’ ownership from 50.0% to 85.0% in exchange for a capital commitment of $25.0 million. Provisions contained in the operating agreement entitle related parties of the Company to a majority of profit and gives the Company control of the Michigan Affiliate and rights and exposure to variable returns. The Company has the right to direct all the relevant activities of and has the full decision-making power over the Michigan Affiliate.
On April 23, 2020, the Company announced that it had completed the sale of its Marshall, MI facility to Innovative Industrial Properties. Inc. (“IIP”). Concurrent with the closing of the sale, Cresco Labs entered into a long-term, triple-net lease agreement with IIP and continues to operate the property as a licensed cannabis cultivation and processing facility upon completion of redevelopment. On October 4, 2021, the Company unveiled its Marshall facility while celebrating the first harvest at the property. Following the unveiling of its Marshall facility, the Michigan Affiliate expanded its licensure to fully realize the growth potential of the Marshall facility. In late 2021, the Michigan Affiliate was awarded eight (8) additional Medical Class C Grower licenses bringing its total medical grow licenses to ten (10) in addition to its one (1) existing Medical Processor license. With increased medical grow potential, the Michigan Affiliate was also able to acquire seven (7) Adult Use Excess Grower licenses in addition to its existing five (5) Adult Use Class C Grow licenses and one (1) Adult Use Processor license.
Florida Operations
In 2014, the Florida Legislature passed the Compassionate Use Act (the “CUA”) which was a low-THC (CBD) law, allowing cannabis containing not more than 0.8% THC to be sold to patients diagnosed with severe seizures or muscle spasms and cancer. The CUA created a competitive licensing structure and originally allowed for one (1) vertically-integrated license to be awarded in each of five (5) regions. The CUA set forth the criteria for applicants as well as the minimum qualifying criteria which included the requirement to hold a nursery certificate evidencing the capacity to cultivate a minimum of 400,000 plants, to be operated by a nurseryman and to be a registered nursery for at least thirty continuous years. The CUA also created a state registry to track dispensations. In 2016, the Florida Legislature passed the Right to Try Act (the “RTA”), which expanded the State’s medical cannabis program to allow for full potency THC products to be sold as “medical marijuana” to qualified patients.
In November of 2016, the Florida Medical Marijuana Legalization ballot initiative (the “Initiative”) to expand the medical cannabis program under the RTA was approved by 71.3% of voters, thereby amending the Florida constitution. The Initiative is now codified as Article X, Section 29 of the Florida Constitution.
The Initiative expanded the list of qualifying medical conditions to include cancer, epilepsy, glaucoma, HIV and AIDS, ALS, Crohn’s disease, Parkinson’s disease, multiple sclerosis, or other debilitating medical conditions of the same kind or class or comparable to those other qualifying conditions and for which a physician believes the benefits outweigh the risks to the patient. The Initiative also provided for the implementation of state-issued medical cannabis
41
identification cards. In 2017, the Florida Legislature passed legislation implementing the constitutional amendment and further codifying the changes set forth in the constitution into law (the “2017 Law”). The 2017 Law provides for the issuance of ten (10) licenses to specific entities and another four (4) licenses to be issued for every 100,000 active qualified patients added to the registry. The 2017 law also initially limited license holders to a maximum of twenty-five (25) dispensary locations with the ability to purchase additional dispensary locations from one another and for an additional five (5) locations to be allowed by the State for every 100,000 active qualified patients added to the registry. The 2017 legislation’s cap on dispensing facilities expired in April 2020.
On March 18, 2019, Governor Ron DeSantis signed SB 182 “Medical Use of Marijuana” into law. Among other provisions, SB 182 repealed the state’s smoking ban that had been in place. The medical program is currently administered by the Florida Department of Health’s (“FDOH”) Office of Medical Marijuana Use (“OMMU”). OMMU is responsible for crafting and implementing regulations governing the program, overseeing the Medical Marijuana Use Registry, licensing operators to cultivate, process and dispense medical marijuana and certifying testing laboratories. Governor DeSantis signed SB 768 into law on April 20, 2022, which includes the following provisions: FDOH will now collect samples of marijuana and marijuana delivery devices from a medical marijuana treatment center (“MMTC”) for specified testing, rather than only samples of edibles; FDOH is required to promulgate rules to allow for potency variations not to exceed 15% from labels and FDOH has the authority not to renew the license of a MMTC that has not begun to cultivate by their renewal date.
With regard to the potential for adult-use cannabis in the state, a group, Regulate Florida, sought to place the questions of whether to legalize adult-use cannabis on the November 2022 ballot but was not successful. The group has indicated it will target the 2024 ballot instead. Regulate Florida will need to gather more than 222,000 signatures to trigger judicial and fiscal review and then more than 890,000 signatures to make the 2024 ballot. On February 2, 2023, the “Smart & Safe Florida” political committee had submitted 294,037 petition signatures, according to the state Division of elections website. As of the end of April 2023, advocates had collected 841,130 signatures.
On April 14, 2021, the Company completed the acquisition of Bluma Wellness Inc. (“Bluma”) for total consideration of $238.1 million. Bluma owns and operates 3 Boys Farm, LLC dba One Plant Florida (“One Plant”), a vertically-integrated, licensed MMTC in the State of Florida. One Plant cultivates, processes, dispenses and retails medical cannabis to qualified patients in the State of Florida through multiple retail dispensaries and an innovative next-day door-to-door e-commerce home delivery service, thereby offering convenient access for its customers and meeting the demands of an evolving retail landscape. As of the acquisition date, Bluma, under One Plant, had eight (8) strategically located dispensaries. Since the acquisition, Cresco has rebranded these dispensaries as Sunnyside*® and opened an additional twenty (20) locations as of March 31, 2023.
While the Company’s compliance controls have been developed to mitigate the risk of any material violations of a license arising, there is no assurance that Florida cannabis licenses will be renewed in the future in a timely manner. Any unexpected delays or costs associated with the licensing renewal process could impede the ongoing or planned operations of Florida cannabis and could have a material adverse effect on the Company’s business, financial condition, results of operations or prospects.
42