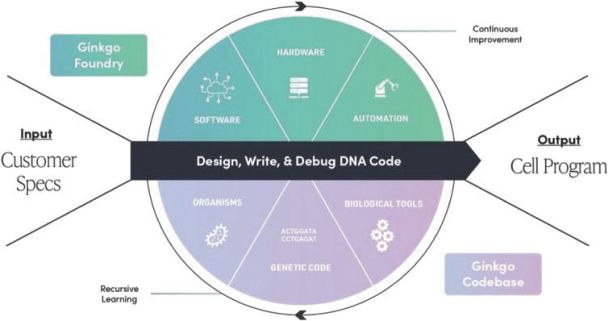
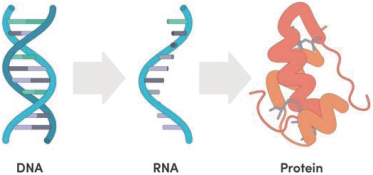
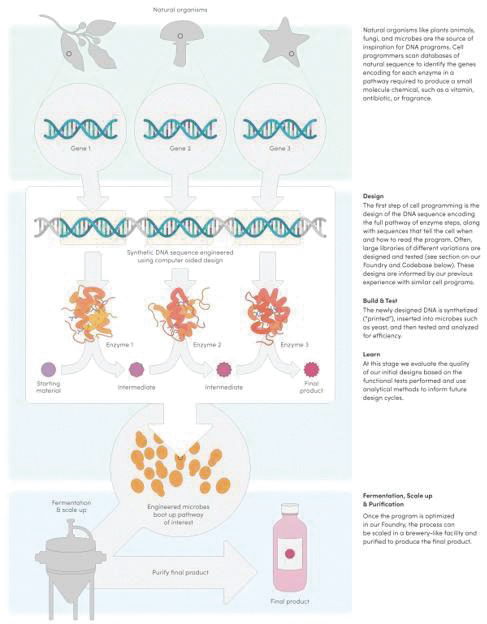
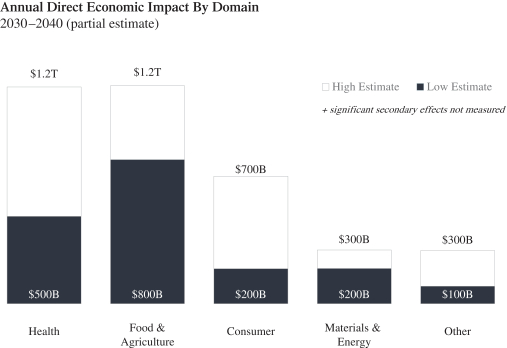
The information contained in the accompanying proxy statement/prospectus is not complete and may be changed. Ginkgo Bioworks Holdings, Inc. may not distribute and issue the shares of Ginkgo Class A Common Stock until the registration statement filed with the Securities and Exchange Commission, of which the accompanying document is a part, is declared effective.
PRELIMINARY—SUBJECT TO COMPLETION, DATED September 1, 2022

|

| |
| PROXY STATEMENT OF ZYMERGEN INC. | PROSPECTUS OF GINKGO BIOWORKS HOLDINGS, INC. | |
PROPOSED MERGER—YOUR VOTE IS VERY IMPORTANT
Dear Zymergen Stockholders:
You are cordially invited to attend a special meeting of the stockholders of Zymergen Inc., a Delaware public benefit corporation (“Zymergen”), which will be held at [●] Pacific Daylight Time, on [●], 2022. In light of the continuing effects of the coronavirus/COVID-19 outbreak and in the best interests of public health and the health and safety of the board of directors of Zymergen (the “Zymergen Board”), employees and stockholders, Zymergen is holding a virtual-only meeting (the “Special Meeting”). Stockholders can attend the meeting via the Internet at www.virtualshareholdermeeting.com/ZY2022SM by using the 16-digit control number which appears on your proxy card (printed in the box and marked by the arrow) and the instructions that accompanied your proxy materials.
As previously announced, Zymergen, Ginkgo Bioworks Holdings, Inc., a Delaware corporation (“Ginkgo”), and Pepper Merger Subsidiary Inc., a Delaware corporation and an indirect wholly owned subsidiary of Ginkgo (“Merger Sub”), have entered into an Agreement and Plan of Merger, dated as of July 24, 2022 (the “Merger Agreement”), pursuant to which, among other things, Merger Sub will merge with and into Zymergen, with Zymergen continuing as the surviving corporation (the “Merger”). The Zymergen Board and the board of directors of Ginkgo each unanimously approved the Merger Agreement and related transactions.
If the Merger is completed, at the effective time of the Merger (the “Effective Time”), each share of common stock, par value $0.001, of Zymergen (“Zymergen Common Stock”) that is issued and outstanding immediately prior to the Effective Time will be automatically cancelled and converted into the right to receive a number of shares of Class A common stock, par value $0.0001, of Ginkgo (“Ginkgo Class A Common Stock”) equal to the product of the number of shares of Zymergen Common Stock multiplied by 0.9179 (the “Exchange Ratio”). The Exchange Ratio is fixed and will not be adjusted to reflect changes in the price of Zymergen Common Stock or Ginkgo Class A Common Stock prior to the closing of the Merger. No fractional shares will be issued in the Merger. Instead, Zymergen stockholders will receive cash in lieu of any fractional shares. Based on the estimated number of shares of Zymergen Common Stock and Ginkgo Class A Common Stock outstanding on July 22, 2022, the last trading day before the public announcement of the Merger Agreement, Zymergen and Ginkgo estimate that, upon completion of the Merger, former Zymergen stockholders and certain other Zymergen equityholders will own approximately 5.25% of Ginkgo on a fully diluted basis, determined using the treasury stock method.
At the Special Meeting, Zymergen stockholders will be asked to vote on proposals to (i) adopt the Merger Agreement (the “Merger Proposal”) and (ii) approve adjournments of the Special Meeting, if necessary or appropriate, to solicit additional proxies if sufficient votes to approve the Merger Proposal have not been obtained by Zymergen (the “Adjournment Proposal”). Information about the Special Meeting, the Merger and other related business to be considered by Zymergen stockholders at the Special Meeting is included in the accompanying proxy statement/prospectus. We urge all Zymergen stockholders to read the accompanying proxy statement/prospectus, including the annexes. In particular, we urge you to read carefully “Risk Factors” beginning on page 24 of the accompanying proxy statement/prospectus.
Your vote is very important regardless of the number of shares of Zymergen Common Stock that you own. The Merger cannot be completed without the approval of the Merger Proposal by the affirmative vote of a majority of the outstanding shares of Zymergen Common Stock.