As filed with the Securities and Exchange Commission on February 14, 2022
Registration Statement No. 333-262002
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-4
(
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
(Exact Name of Registrant as Specified in Its Charter)
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Robb Knie
Chief Executive Officer
FoxWayne Enterprises Acquisition Corp.
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
| Richard
A. Friedman, Esq. Kandace Watson, Esq. Nazia J. Khan, Esq. Sheppard Mullin Richter & Hampton LLP 30 Rockefeller Plaza New York, NY 10112-0015 Telephone: (212) 653-8700 |
Amy M. Batten, Esq. Alexander M. Bowling, Esq. Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, LLP 150 Fayetteville Street, Suite 2300 Raleigh, NC 27601 Telephone: (919) 821-1220 |
Approximate date of commencement of proposed sale of the securities to the public: As soon as practicable after this Registration Statement becomes effective and on completion of the business combination described in the enclosed proxy statement/prospectus.
If the securities being registered on this Form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| ☒ | Smaller reporting company | |||||
| Emerging growth company |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
| Exchange Act Rule 13e-4(i) (Cross-Border Issuer Tender Offer) ☐ |
| Exchange Act Rule 14d-1(d) (Cross-Border Third-Party Tender Offer) ☐ |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until this Registration Statement shall become effective on such date as the U.S. Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary proxy statement/prospectus is not complete and may be changed. The securities described herein may not be sold until the registration statement filed with the U.S. Securities and Exchange Commission is declared effective. This preliminary proxy statement/prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROXY STATEMENT AND PROSPECTUS
SUBJECT TO COMPLETION, DATED FEBRUARY 14, 2022
FOXWAYNE ENTERPRISES ACQUISITION CORP.
Dear Stockholders of FoxWayne Enterprises Acquisition Corp.:
FoxWayne Enterprises Acquisition Corp., a Delaware corporation (“FoxWayne” “we,” “our,” or “us”), Gotham Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of FoxWayne (“Merger Sub”), Aerami Therapeutics Holdings, Inc., a Delaware corporation (“Aerami”), and the stockholders’ representative have entered into an Agreement and Plan of Merger dated December 7, 2021 (the “Merger Agreement”). Subject to the terms and conditions of the Merger Agreement, upon the closing of the transactions contemplated by the Merger Agreement, Merger Sub will merge with and into Aerami (the “Merger”), with Aerami surviving the Merger as a wholly owned subsidiary of FoxWayne (FoxWayne, after the Merger, the “Combined Company”). The Merger and the other transactions contemplated by the Merger Agreement are collectively referred to in this proxy statement/prospectus as the “Business Combination,” and the combined companies (FoxWayne and its wholly owned subsidiary, Aerami) after the Business Combination to be named Aerami Therapeutics Holdings, Inc. are referred to as the “Combined Company.”
FoxWayne is holding a special meeting (“special meeting”) of its stockholders to obtain the stockholder approvals necessary to complete the Business Combination. At the special meeting, which will be held on , 2022, at , Eastern time, via live webcast at , FoxWayne will ask its stockholders to approve and adopt the Merger Agreement and the Business Combination and to approve the other proposals described in this proxy statement/prospectus.
At the effective time of the Merger (“Effective Time”), by virtue of the Business Combination and without any action on the part of FoxWayne, Merger Sub, Aerami or the holders of any of Aerami’s securities:
| ● | each share of Aerami common stock, par value $0.0001 per share (“Aerami Common Stock”) issued and outstanding immediately prior to the Effective Time (including shares of Aerami Common Stock resulting from the conversion of Aerami senior preferred stock and the automatic cashless exercise of warrants granted to Aerami’s former placement agent (such warrants the “Placement Agent Warrants”), but excluding any cancelled shares and certain dissenting shares of Aerami Common Stock and Aerami preferred stock (Aerami Common Stock and Aerami Preferred Stock collectively, “Aerami Capital Stock”), will be cancelled and converted into the right to receive the number of shares of the Combined Company’s common stock, par value $0.0001 per share (“Combined Company common stock”) allocable from the Aggregate Consideration as set forth in the Consideration Schedule of the Merger Agreement. “Aggregate Consideration” means 25,000,000 shares of Combined Company common stock; | |
| ● | all shares of Aerami Capital Stock held in treasury by Aerami will be cancelled without any conversion thereof and no payment or distribution will be made with respect thereto; | |
| ● | each share of Aerami royalty preferred stock issued and outstanding immediately prior to the Effective Time will be cancelled and converted into the right to receive the number of shares of Combined Company common stock allocable from the Aggregate Consideration as set forth in the Consideration Schedule; | |
| ● | each share of Merger Sub issued and outstanding immediately prior to the Effective Time will be converted into one share of Aerami, as the wholly owned subsidiary of the Combined Company; |
| P-1 |
| ● | each warrant (other than the Placement Agent Warrants) to acquire Aerami Capital Stock (each, an “Aerami Warrant”) that is outstanding and unexercised immediately prior to the Effective Time, whether vested or unvested, which does not terminate by its terms in connection with the Merger, will be assumed by FoxWayne (each, an “Assumed Warrant”) and converted into a warrant to purchase such number of whole shares of Combined Company common stock equal to the product of the number of shares of Aerami Common Stock subject to such Assumed Warrant and the allocable per share consideration with respect to Aerami common stock (the “Per Share Common Consideration”) (rounded up to the nearest whole number), and the per share exercise price will be ratably adjusted (rounded up to the nearest whole cent). Immediately prior to the Effective Time, the Placement Agent Warrants will automatically net exercise in full into applicable shares of Aerami securities pursuant to the terms of the respective warrant agreements; | |
| ● | each outstanding and unexercised option to acquire shares of Aerami Common Stock that has not been exercised immediately prior to the Effective Time (the “Aerami Options”) will cease to represent the right to purchase Aerami Common Stock and will be assumed by FoxWayne and substituted and converted into an option to purchase such number of whole shares of Combined Company common stock (rounded down) pursuant to the Combined Company’s equity incentive plan (“Exchanged Options”) equal to the product of the number of shares of Aerami Common Stock subject to such Aerami Option and the Per Share Common Consideration, and at an exercise price (rounded up to the nearest whole cent) equal to the quotient of the exercise price of such Aerami Option and the Per Share Common Consideration; | |
| ● | each restricted stock unit award covering shares of Aerami Common Stock that is outstanding immediately prior to the Effective Time will be converted into the right to receive a restricted stock unit award for whole shares of Combined Company common stock (rounded down) pursuant to the Combined Company’s equity incentive plan (the “Exchanged RSU Award”) in an amount equal to the product of the number of shares of Aerami Common Stock subject to such Exchanged RSU Award and the Per Share Common Consideration; and | |
| ● | no fractional shares of Combined Company common stock will be issued by virtue of the Merger, and any fractional shares otherwise issuable to a holder of Aerami’s securities (after aggregating all fractional shares of the Combined Company common stock that otherwise would be received by such holder) will be rounded down to the nearest whole share of the Combined Company common stock. |
In addition, at the Effective Time up to shares of Combined Company Common Stock from the Aggregate Consideration may be issued to certain Aerami Equityholders in satisfaction of certain contractual arrangements as provided in the Consideration Schedule.
See the section titled “The Business Combination” of this proxy statement/prospectus for further information on the consideration being paid to the security holders of Aerami.
As of February 11, 2022, there was approximately $58.1 million in FoxWayne’s trust account (the “Trust Account”). On , 2022, the record date for the special meeting of stockholders, the last sale price of FoxWayne Class A Common Stock, par value $0.0001 per share (“Class A Common Stock”), was $ . Holders of FoxWayne securities should obtain current market quotations for their securities. The market price of FoxWayne’s securities could vary at any time before the Business Combination.
FoxWayne’s units, Class A Common Stock and warrants are currently listed on The Nasdaq Capital Market under the symbols “FOXWU,” “FOXW” and “FOXWW,” respectively. In connection with the closing of the Business Combination, FoxWayne intends to change its name to “Aerami Therapeutics Holdings, Inc.” and has applied to list the shares of common stock of the Combined Company on The Nasdaq Capital Market under the symbol “ .”
It is anticipated that, upon completion of the Business Combination, (i) the Aerami Equityholders will own, collectively, approximately 78% of the outstanding Combined Company common stock, and (ii) FoxWayne’s pre-closing stockholders will own approximately 22% of the outstanding Combined Company common stock, in each case assuming that none of FoxWayne’s outstanding public shares are redeemed in connection with the Business Combination, or approximately 88% and 12% respectively, assuming redemption of the maximum number of outstanding shares of FoxWayne capital stock that may be redeemed in connection with the Business Combination before the minimum cash condition under the Merger Agreement is not met.
This proxy statement/prospectus covers 25,000,000 shares of the Combined Company common stock, which consists of 25,000,000 shares issuable in connection with the Business Combination. This proxy statement/prospectus also covers shares of Combined Company common stock that may be issued to holders of shares of Aerami Options, Aerami restricted stock unit awards and Assumed Warrants assumed by FoxWayne in connection with the Business Combination (as more fully described in this proxy statement/prospectus).
| P-2 |
As described in this proxy statement/prospectus, FoxWayne Enterprises Acquisition Sponsor LLC, a Delaware limited liability company (the “Sponsor”), and FoxWayne’s officers and directors (together with the Sponsor, the “Insiders”) have agreed to (a) vote all of their shares of FoxWayne Class B common stock, par value $0.0001 per share (the “Founder Shares” or “Class B Common Stock”), and all of their shares of FoxWayne Class A Common Stock in favor of the Business Combination and (b) certain restrictions on their shares of FoxWayne Class A Common Stock and Founder Shares.
As described in this proxy statement/prospectus, certain officers and directors of Aerami are parties to a support agreement with FoxWayne whereby such officers and directors have agreed to vote all of their shares of Aerami Capital Stock in favor of approving the Business Combination and other proposed transactions contemplated by the Merger Agreement. In addition, Aerami will seek the written consent of Aerami’s stockholders as required to approve and adopt the Merger Agreement and the transactions contemplated thereunder.
After careful consideration, the FoxWayne and Aerami boards of directors have unanimously approved the Merger Agreement and related transactions, and the board of directors of FoxWayne has approved the other proposals described in this proxy statement/prospectus. Each of the FoxWayne and Aerami boards of directors has determined that it is advisable to consummate the Business Combination. The board of directors of FoxWayne recommends that its stockholders vote “FOR” the approval and adoption of the Merger Agreement and approval of the transactions contemplated thereby, including the Business Combination, and “FOR” all other proposals described in this proxy statement/prospectus. When you consider the recommendation of the proposals by the board of directors of FoxWayne, you should keep in mind that FoxWayne’s directors and officers have interests in the Business Combination that may conflict with your interests as a stockholder. See the section titled “The Business Combination Interests of Certain Persons in the Business Combination - Interests of Sponsor and FoxWayne Directors and Officers” in this proxy statement/prospectus for a further discussion of these considerations.
More information about FoxWayne, Aerami and the proposed transactions are included in this proxy statement/prospectus. FoxWayne urges you to read this proxy statement/prospectus, including the financial statements and annexes and other documents referred to herein, carefully and in their entirety. IN PARTICULAR, YOU SHOULD CAREFULLY CONSIDER THE MATTERS DISCUSSED UNDER “RISK FACTORS” BEGINNING ON PAGE 43 OF THIS PROXY STATEMENT/PROSPECTUS.
Your vote is very important, regardless of the number of shares of FoxWayne capital stock you own. To ensure your representation at the special meeting, please complete, sign, date and return the enclosed proxy card in the postage-paid envelope provided. If you hold your shares in “street name,” which means your shares are held of record by a broker, bank or other nominee, you should follow the instructions provided by your broker, bank or nominee to ensure that votes related to the shares you beneficially own are properly counted. Please submit your proxy promptly, whether or not you expect to attend the special meeting.
On behalf of our board of directors, I thank you for your support and look forward to the successful completion of the Business Combination.
| Sincerely, | |
Robb Knie Chairman, Chief Executive Officer and Chief Financial Officer |
NEITHER THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES REGULATORY AGENCY HAS APPROVED OR DISAPPROVED OF THE TRANSACTIONS DESCRIBED IN THIS PROXY STATEMENT/PROSPECTUS OR ANY OF THE SECURITIES TO BE ISSUED IN THE BUSINESS COMBINATION OR THE OTHER TRANSACTIONS DESCRIBED IN THIS PROXY STATEMENT/PROSPECTUS, PASSED UPON THE MERITS OR FAIRNESS OF THE BUSINESS COMBINATION OR RELATED TRANSACTIONS OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THIS PROXY STATEMENT/PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY CONSTITUTES A CRIMINAL OFFENSE.
This proxy statement/prospectus is dated , 2022 and is first being mailed to the stockholders of FoxWayne on or about that date.
| P-3 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
1 Rockefeller Plaza, Suite 1039
New York, NY 10020
NOTICE
OF SPECIAL MEETING OF STOCKHOLDERS
OF FOXWAYNE ENTERPRISES ACQUISITION CORP.
To Be Held On , 2022
To the Stockholders of FoxWayne Enterprises Acquisition Corp.:
NOTICE IS HEREBY GIVEN that the special meeting of stockholders of FoxWayne Enterprises Acquisition Corp will be held at , Eastern time, on , 2022, via live webcast at the following address: . At the special meeting, FoxWayne stockholders will be asked to consider and vote upon the following proposals:
| ● | The Transaction Proposal — To consider and vote upon a proposal to (a) approve and adopt the Agreement and Plan of Merger, dated as of December 7, 2021 by and among FoxWayne, Gotham Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of FoxWayne, Aerami Therapeutics Holdings, Inc., a Delaware corporation, and the stockholders’ representative, pursuant to which Merger Sub will merge with and into Aerami, with Aerami surviving the Merger as a wholly owned subsidiary of FoxWayne and (b) approve the Merger and the other transactions contemplated by the Merger Agreement (collectively, the “Business Combination” and such proposal, the “Transaction Proposal”) (Proposal 1). A copy of the Merger Agreement is attached to the accompanying proxy statement/prospectus as Annex A. | |
| ● | The Charter Proposal — To consider and vote upon a proposal to approve and adopt the Third Amended and Restated Certificate of Incorporation of FoxWayne, to, among other things, (a) change the corporate name of FoxWayne to “Aerami Therapeutics Holdings, Inc.;” (b) increase the number of authorized shares of the Combined Company’s capital stock; (c) amend the votes required to amend the Combined Company’s bylaws and certain provisions of the Combined Company’s Third Amended and Restated Certificate of Incorporation; (d) provide that the Combined Company’s board of directors (the “Combined Company Board”) or any individual director may be removed only for cause and amend the votes required to remove the Combined Company Board or any individual director; (e) eliminate provisions in FoxWayne’s Second Amended and Restated Certificate of Incorporation (the “Charter”) relating to FoxWayne’s Initial Business Combination that will no longer be applicable to the Combined Company following the effective time of the Merger and the closing of the Business Combination; and (f) make certain other changes that the Combined Company Board deems appropriate for a public operating company (collectively, the “Charter Proposal”) (Proposal 2). A copy of the proposed Third Amended and Restated Certificate of Incorporation is attached to the accompanying proxy statement/prospectus as Annex B. | |
| ● | The Bylaw Proposal — To consider and vote upon a proposal to amend and restate FoxWayne’s bylaws to, among other things, (a) expand the disclosure requirements of stockholders seeking to bring business before a meeting or nominate directors; (b) eliminate the ability of stockholders to take action by written consent; (c) increase the number of votes required to amend the bylaws; and (d) lock-up holders of Combined Company common stock issued as consideration pursuant to the Merger Agreement or upon the exercise, exchange, conversion, or settlement of any Exchanged Options, Exchanged RSU Awards or Assumed Warrants until 180 days after the date that the Form S-1 Shelf (as defined in the Amended and Restated Bylaws) is declared effective by the SEC, subject to certain exceptions (the “Bylaw Proposal”) (Proposal 3). A copy of the proposed Amended and Restated Bylaws is attached to the accompanying proxy statement/prospectus as Annex C. |
| P-4 |
| ● | The Nasdaq Proposal — To consider and vote upon a proposal to approve the issuance of more than 20% of the outstanding capital stock and voting power of FoxWayne, in connection with the terms of the Merger Agreement and the Business Combination and the New Equity Incentive Plan (as defined below), which will result in a change of control of FoxWayne, as required by applicable listing rules of the Nasdaq Stock Market LLC (the “Nasdaq Proposal”) (Proposal 4). In connection with the Merger, we intend to issue an aggregate of approximately 25,000,000 shares of the Combined Company common stock, and we will reserve shares of the Combined Company common stock for issuance upon the exercise of Exchanged Options, Exchanged RSU Awards and Assumed Warrants. In addition, we will reserve a number of shares that approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination for issuance under the New Equity Incentive Plan (which number will include the shares of Combined Company common stock reserved for issuance upon exercise of the Exchanged Options and Exchanged RSU Awards). | |
| ● | The New Equity Incentive Plan Proposal — To consider and vote upon a proposal to approve and adopt a 2022 incentive award plan (the “New Equity Incentive Plan), including the reservation of such number of shares of the Combined Company common stock as approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination and the other material terms thereunder (the “New Equity Incentive Plan Proposal”) (Proposal 5). A copy of the New Equity Incentive Plan is attached to the accompanying proxy statement/prospectus as Annex D. | |
| ● | The Additional Proposal — To consider and vote upon any other proposals determined by the FoxWayne Board to be necessary or appropriate in connection with the transactions contemplated by the Business Combination or the other proposals (the “Additional Proposal”) (Proposal 6). | |
| ● | The Adjournment Proposal — To consider and vote upon a proposal to approve the adjournment of the special meeting to a later date or dates, if necessary or appropriate, to permit further solicitation and vote of proxies in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal or the Additional Proposal (the “Adjournment Proposal” and, together with the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal and the Additional Proposal, the “Proposals”) (Proposal 7). |
The special meeting will be completely virtual. There will be no physical meeting location, and the special meeting will only be conducted via live webcast at the following address: .
Only holders of record of shares of Class A common stock, par value $0.0001 per share (“Class A Common Stock”) and Class B common stock, par value $0.0001 per share (“Class B Common Stock”) of FoxWayne at the close of business on , 2022 are entitled to notice of the virtual special meeting and to vote at the virtual special meeting and any adjournments or postponements thereof. A complete list of FoxWayne’s stockholders of record entitled to vote at the virtual special meeting will be available at the virtual special meeting and for ten days before the virtual special meeting (a) at FoxWayne’s principal executive offices for inspection by stockholders during ordinary business hours for any purpose germane to the virtual special meeting or (b) on a reasonably accessible electronic network by using the information provided with the notice of the meeting to gain access.
Pursuant to the Charter, we will provide the holders of shares of our Class A Common Stock originally sold as part of the units issued in our initial public offering (such holders, the “public stockholders”) with the opportunity to redeem, upon the consummation of the Business Combination, shares of Class A Common Stock then held by them for cash equal to their pro rata share of the aggregate amount on deposit in the Trust Account, which holds the proceeds from the initial public offering (“IPO”), as of two business days prior to the consummation of the Business Combination, including interest net of taxes payable by FoxWayne. For illustrative purposes, based on the fair value of cash and marketable securities held in the Trust Account as of February 11, 2022 of approximately $58.1 million and 5,750,000 shares of Class A Common Stock outstanding and redeemable as of February 11, 2022, the estimated per share redemption price would have been approximately $10.10. Notwithstanding the foregoing redemption rights, a public stockholder, together with any of his, her or its affiliates or any other person with whom he, she or it is acting in concert or as a “group” (as defined under Section 13(d)(3) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming in the aggregate his, her or its shares or, if part of such a group, the group’s shares, in excess of 15% of the outstanding shares of Class A Common Stock sold in the IPO. Holders of FoxWayne’s outstanding warrants sold in the IPO, which are exercisable for shares of Class A Common Stock under certain circumstances, do not have redemption rights in connection with the Business Combination. At the time of our IPO, our Sponsor, officers and directors agreed to waive their redemption rights in connection with the consummation of the Business Combination with respect to any shares of our common stock they may hold, and shares of Class B Common Stock will be excluded from the pro rata calculation used to determine the per share redemption price. None of our Sponsor, directors or officers received separate consideration for their waiver of redemption rights. As of the record date, our Sponsor, officers and directors and their affiliates own approximately % the outstanding shares of Class B Common Stock. Our Sponsor, officers and directors have agreed to vote any shares of Class A Common Stock and Class B Common Stock owned by them in favor of the Business Combination.
| P-5 |
We may not consummate the Business Combination if the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal are not approved at the special meeting of stockholders. The Bylaw Proposal, the New Equity Incentive Plan Proposal and the Additional Proposal are conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal. The Adjournment Proposal is not conditioned on the approval of any other Proposal set forth in the accompanying proxy statement/prospectus. The approval of the Transaction Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal and the Adjournment Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon at the special meeting, voting as a single class. The approval of the Charter Proposal and the Bylaw Proposal requires the affirmative vote of a majority of the issued and outstanding shares of Class A Common Stock and Class B Common Stock, voting as a single class.
As of February 11, 2022, there was approximately $58.1 million in the Trust Account, which FoxWayne intends to use for the purpose of consummating the Business Combination. Each redemption of shares by public stockholders will decrease the amount in the Trust Account. FoxWayne will not consummate the Business Combination if the redemption of shares would result in FoxWayne’s failure to have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act). In addition, the Merger Agreement includes a condition to closing that, unless waived by Aerami, requires FoxWayne have at least $15,000,000 in available cash immediately prior to the Effective Time of the Merger, after taking into account payments required to satisfy redemptions of shares by public stockholders and the payment of FoxWayne’s transaction costs.
YOUR VOTE IS VERY IMPORTANT, REGARDLESS OF THE NUMBER OF SHARES OF CAPITAL STOCK OF FOXWAYNE YOU OWN. To ensure your representation at the special meeting, please complete and return the enclosed proxy card or submit your proxy by following the instructions maintained in the proxy statement/prospectus and on your proxy card. Please submit your proxy promptly, whether or not you expect to attend the special meeting. If you hold your shares in “street name,” you should instruct your broker, bank or other nominee how to vote in accordance with the voting instruction form you received from your broker, bank or other nominee.
The board of directors of FoxWayne has unanimously approved the Merger Agreement and the transactions contemplated thereby and recommends that you vote “FOR” the Transaction Proposal, “FOR” the Charter Proposal, “FOR” the Nasdaq Proposal, “FOR” the Bylaw Proposal, “FOR” the New Equity Incentive Plan Proposal, “FOR” the Additional Proposal, and “FOR” the Adjournment Proposal.
Your attention is directed to the proxy statement/prospectus accompanying this notice (including the annexes thereto) for a more complete description of the proposed Business Combination and related transactions and each of the Proposals. We encourage you to read the accompanying proxy statement/prospectus carefully. If you have any questions or need assistance voting your shares, please call our proxy solicitor, , at (banks and brokers call collect at ).
, 2022
| By Order of the Board of Directors | |
Robb Knie Chairman, Chief Executive Officer and Chief Financial Officer |
| P-6 |
TABLE OF CONTENTS
| -i- |
| -ii- |
ABOUT THIS PROXY STATEMENT/PROSPECTUS
This document, which forms part of a registration statement on Form S-4 filed with the SEC by FoxWayne (File No. 333-2620002) (the “Registration Statement”), constitutes a prospectus of FoxWayne under Section 5 of the Securities Act of 1933, as amended (the “Securities Act”), with respect to the shares of Combined Company common stock to be issued if the Business Combination described below is consummated. This document also constitutes a notice of meeting and a proxy statement under Section 14(a) of the Exchange Act with respect to the special meeting of FoxWayne stockholders at which FoxWayne stockholders will be asked to consider and vote upon a proposal to approve the Business Combination by the approval and adoption of the Merger Agreement and other Proposals.
This proxy statement/prospectus incorporates important business and financial information about FoxWayne that is not included in or delivered with the document.
This information is available without charge to you upon written or oral request. To make this request, you should contact our proxy solicitor at: .
To obtain timely delivery of requested materials, you must request the information no later than five business days prior to the date of the special meeting.
You may also obtain additional information about us from documents filed with the SEC by following the instruction in the section titled “Where You Can Find Additional Information.”
MARKET AND INDUSTRY DATA
We are responsible for the disclosure contained in this proxy statement/ prospectus. However, certain information contained in this proxy statement/prospectus concerning the market and the industry in which Aerami operates and competes, including its market position, general expectations of market opportunity, size and growth rates is based on studies, publications, surveys and other data obtained from third-party sources and FoxWayne’s and Aerami’s own internal estimates and research. While we believe these sources to be reliable as of the date of this proxy statement/prospectus, we have not independently verified the market and industry data contained in this proxy statement/prospectus or the underlying assumptions relied on therein. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. The industry in which Aerami operates is subject to a high degree of uncertainty and risk. As a result, the estimates and market and industry information provided in this proxy statement/prospectus are subject to change based on various factors, including those described in the sections entitled “Cautionary Note Regarding Forward-Looking Statements” beginning on page 103 of this proxy statement/prospectus and “Risk Factors — Risks Related to Aerami’s Business” beginning on page 43 of this proxy statement/prospectus and elsewhere in this proxy statement/prospectus.
TRADEMARKS
This document contains references to trademarks, trade names and service marks belonging to other entities. Solely for convenience, trademarks, trade names and service marks referred to in this proxy statement/prospectus may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that the applicable owner or licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
| -3- |
CERTAIN DEFINED TERMS
Unless the context otherwise requires, references in this proxy statement/prospectus to:
| ● | “Acquired Aerami Entities” means, collectively, Aerami and its Subsidiaries. | |
| ● | “Additional Proposal” or “Additional Proposals” means any other proposals to be considered and voted upon as determined by the FoxWayne Board to be necessary or appropriate in connection with the transactions contemplated by the Business Combination or the other proposals. | |
| ● | “Aerami” means Aerami Therapeutics Holdings, Inc., a Delaware corporation. | |
| ● | “Aerami Board” means the board of directors of Aerami. | |
| ● | “Aerami Capital Stock” means Aerami Common Stock and Aerami Preferred Stock. | |
| ● | “Aerami Charter” means the Certificate of Incorporation of Aerami dated December 20, 2013, as the same may be amended, supplemented or modified from time to time. | |
| ● | “Aerami Common Stock” means shares of Aerami’s Common Stock, par value $0.0001 per share. | |
| ● | “Aerami Dissenting Shares” means shares of Aerami Capital Stock that are outstanding immediately prior to the Effective Time and that are held by Aerami stockholders who shall have not voted in favor of the Merger, consented thereto in writing or waived their respective appraisal or dissenters’ rights, and who shall have demanded properly in writing appraisal or dissenters’ rights for such Aerami Capital Stock in accordance with Section 262 of the DGCL, and otherwise complied with all of the provisions of the DGCL relevant to the exercise and perfection of appraisal rights. | |
| ● | “Aerami Equityholder” means each holder of any Aerami Capital Stock, Aerami Options, Aerami RSUs, Aerami Warrants, or Placement Agent Warrants. | |
| ● | “Aerami Fundamental Representations” means the representations and warranties of Aerami contained in Section 3.1 (Organization, Existence and Power), Section 3.2 (Authorization), Section 3.5 (Capitalization), and Section 3.24 (No Brokers) of the Merger Agreement. | |
| ● | “Aerami Option” means an option entitling the holder thereof to acquire shares of Aerami Common Stock from Aerami. | |
| ● | “Aerami Preferred Stock” means the Aerami Series A Preferred Stock, Aerami Series A-1 Preferred Stock, and Aerami Royalty Stock. | |
| ● | “Aerami Royalty Stock” means the Royalty Series Convertible Preferred Stock of Aerami, par value $0.0001 per share. | |
| ● | “Aerami RSUs” means a restricted stock units covering shares of Aerami Common Stock. | |
| ● | “Aerami Senior Preferred Stock” means the Aerami Series A Preferred Stock and the Aerami Series A-1 Preferred Stock. | |
| ● | “Aerami Series A Preferred Stock” means the Series A Preferred Stock of Aerami, par value $0.0001 per share. | |
| ● | “Aerami Series A-1 Preferred Stock” means the Series A-1 Preferred Stock of Aerami, par value $0.0001 per share. | |
| ● | “Aerami Stockholders” means holders of Aerami Common Stock and Aerami Preferred Stock. | |
| ● | “Aerami Warrants” means unexpired warrants to purchase Aerami Common Stock (which, for the avoidance of doubt, (i) includes any warrants to purchase Aerami Preferred Stock that convert into warrants to purchase Aerami Common Stock following the Conversion and (ii) excludes the Placement Agent Warrants). |
| -4- |
| ● | “Affiliate” means, when used with reference to any specified Person, any other Person directly or indirectly controlling, controlled by, or under direct or indirect common control with, such specified Person. For purposes of this definition, “control,” when used with respect to any specified Person, means the power to direct or cause the direction of management or policies of such Person, directly or indirectly, whether through the ownership of voting securities, by contract or otherwise. | |
| ● | “Aggregate Consideration” means 25,000,000 shares of Combined Company common stock. | |
| ● | “Ancillary Documents” means all of the agreements contemplated pursuant to the Merger Agreement. | |
| ● | “Business Combination” means the Merger and the other transactions contemplated by the Merger Agreement. | |
| ● | “Business Day” means a day other than Saturday, Sunday or any day on which banks located in the State of New York are authorized or obligated to close. | |
| ● | “Cancelled Shares” means shares of Aerami Capital Stock issued and outstanding immediately prior to the Effective Time that are owned or held in treasury by FoxWayne, Aerami, or any wholly owned subsidiary of FoxWayne or Aerami that will automatically be cancelled and extinguished at the Effective Time without any conversion thereof or payment of any cash or other property or consideration therefor and shall cease to exist. | |
| ● | “Charter” means FoxWayne’s Second Amended and Restated Certificate of Incorporation. | |
| ● | “Class A Common Stock” means shares of FoxWayne’s Class A common stock, par value $0.0001 per share. | |
| ● | “Class B Common Stock” means shares of FoxWayne’s Class B common stock, par value $0.0001 per share. | |
| ● | “Closing” means the effective time of the Merger and the closing of the Business Combination. | |
| ● | “Closing Date” means the date on which the Closing occurs. | |
| ● | “Code” means the Internal Revenue Code of 1986, as amended. | |
| ● | “Combined Company” means FoxWayne, as combined with its wholly owned subsidiaries (including Aerami) after the Business Combination. | |
| ● | “Combined Company common stock” means FoxWayne’s common stock, par value $0.0001 per share, after the filing of the Proposed Charter in connection with the Closing of the Business Combination. | |
| ● | “Combined Company preferred stock” means FoxWayne’s preferred stock, par value $0.0001 per share, after the filing of the Proposed Charter in connection with the Closing of the Business Combination. | |
| ● | “Consideration Schedule” means that certain schedule in spreadsheet format to be prepared and delivered by Aerami to FoxWayne, in form and substance reasonably satisfactory to FoxWayne and certified as complete and correct by Aerami’s chief executive officer, setting forth all of the following information as of immediately such date: (i) (A) the names of all of the Aerami Stockholders and their respective addresses, e-mail addresses and social security numbers or tax identification numbers (as applicable), (B) the number and type of shares of Aerami Capital Stock held by such Aerami Stockholders and the respective certificate numbers representing such shares, (C) the date of acquisition of shares of Aerami Capital Stock, (D) the calculation of the aggregate number of shares of Combined Company common stock issuable to each Aerami Stockholder at Closing pursuant to Section 1.6 of the Merger Agreement, assuming each Aerami Stockholder is issued a number of shares of Combined Company common stock pursuant to Section 1.6 of the Merger Agreement, a funds flow memorandum setting forth applicable wire transfer instructions and other information reasonably requested by FoxWayne and (E) such other information relevant thereto which FoxWayne may reasonably request with respect to any Transaction Expenses of Aerami to be paid in cash. | |
| ● | “Contract” means any contract, agreement, deed, indenture, note, bond, loan, license, instrument, lease, commitment, plan or other arrangement or understanding, whether oral or written, including all amendments, supplements, exhibits and schedules thereto. | |
| ● | “Conversion” means the automatic conversion of each share of Aerami Senior Preferred Stock into a number of shares of Aerami Common Stock at the then-effective conversion rate as calculated pursuant to the Aerami Charter. |
| -5- |
| ● | “COVID-19” means SARS-CoV-2 or COVID-19, and any evolutions or mutations thereof or related or associated epidemics, pandemics or disease outbreaks. | |
| ● | “COVID-19 Measures” means any quarantine, “shelter in place,” “stay at home,” workforce reduction, social distancing, shut down, closure, sequester, safety or similar Law, directive, guidelines or recommendations promulgated by any Governmental Authority, including the Centers for Disease Control and Prevention, the World Health Organization and the Occupational Safety and Health Administration, in each case, in connection with or in response to COVID-19. | |
| ● | “Cure Period” means, when a non-breaching party notifies in writing to the breaching party of the existence of an inaccuracy or breach, and if such inaccuracy or breach is curable, the 30-calendar day period following such written notification. | |
| ● | “DGCL” means General Corporation Law of the State of Delaware. | |
| ● | “EF Hutton” means EF Hutton (formerly Kingswood Capital Markets), a division of Benchmark Investments, Inc. | |
| ● | “Effective Time” means the date and time the Merger becomes effective. | |
| ● | “Encumbrance” means any lien, pledge, charge, community property interest, equitable interest, easement, security interest, deed of trust, mortgage, pledge, hypothecation, right-of-way, encroachment, building or use restriction, conditional sales agreement, encumbrance or other right of third parties, whether voluntarily incurred or arising by operation of law, and includes any agreement to give any of the foregoing in the future, and any contingent sale or other title retention agreement or lease in the nature thereof. | |
| ● | “Equity Interests” means, with respect to any Person, any capital stock, or other ownership, membership, partnership, joint venture or equity interest in, such Person or any indebtedness, securities, options, warrants, call, subscription or other rights or entitlements of, or granted by, such Person or any of its Affiliates that are convertible into, or are exercisable or exchangeable for, or giving any Person any right or entitlement to acquire any such capital stock or other ownership, partnership, joint venture or equity interest, in all cases, whether vested or unvested. | |
| ● | “ERISA” means the Employee Retirement Income Security Act of 1974, as amended, and the regulations promulgated thereunder. | |
| ● | “ERISA Affiliate” means any Person or entity (whether or not incorporated) that would be treated as a “single employer” or under common control with any of the Acquired Aerami Entities within the meaning of Section 414 of the Code. | |
| ● | “Exchange Act” means the Securities and Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder. | |
| ● | “Founder Shares” means the outstanding shares of Class B Common Stock. | |
| ● | “FoxWayne,” “we,” “our,” or “us” means FoxWayne Enterprises Acquisition Corp., a Delaware corporation. | |
| ● | “FoxWayne Board” means the board of directors of FoxWayne. | |
| ● | “FoxWayne Board Recommendation” means the recommendation of the board of directors of FoxWayne to approve the Proposals. | |
| ● | “FoxWayne Common Stock” means FoxWayne common stock, par value $0.0001 per share. | |
| ● | “FoxWayne Fundamental Representations” means the representations and warranties of FoxWayne and Merger Sub contained in Section 4.1 (Organization, Existence and Power), Section 4.2 (Authorization), Section 4.6 (Capitalization), and Section 4.26 (No Brokers) of the Merger Agreement. | |
| ● | “FoxWayne Preferred Stock” means FoxWayne’s Preferred Stock, par value $0.0001 per share. | |
| ● | “FoxWayne Stockholders” means any holder of FoxWayne Common Stock. |
| -6- |
| ● | “FoxWayne Support Agreements” means those agreements delivered by certain officers and directors of FoxWayne, pursuant to which, among other things, such FoxWayne stockholders agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement. | |
| ● | “FoxWayne Transaction Costs” means all out-of-pocket fees, costs and expenses of FoxWayne or Merger Sub incurred prior to and on the Closing Date in connection with the negotiation, preparation and execution of the Merger Agreement, the other transaction documents and the consummation of the transactions contemplated by the Merger Agreement and the other transaction documents, including, without duplication, (a) the sum of all outstanding deferred, unpaid or contingent underwriting, transaction, deal, brokerage, financial, accounting or legal advisory, auditor or SEC filing fees or any similar fees, commissions or expenses owed by FoxWayne or Merger Sub (to the extent FoxWayne or Merger Sub is responsible for or obligated to reimburse or repay any such amounts) to financial advisors, investment banks, data room administrators, financial printers, attorneys, accountants and other similar advisors, service providers and the SEC and (b) the cash portion of any loan payable to our Sponsor, the proceeds from which are used by FoxWayne to pay any of the fees, costs or expenses set forth in clause (a), but excluding, for the avoidance of doubt, (w) any accounting, legal or other advisory or any similar fees, commissions or expenses incurred in the ordinary course of business consistent with past practice and not in connection with the negotiation, preparation and execution of the Merger Agreement, the other transaction documents or the consummation of the transactions contemplated by the Merger Agreement and related transaction documents, (x) the portion of the filing fee for the notification and report forms filed under the HSR Act payable by Aerami, (y) certain other items mutually agreed upon by FoxWayne and Aerami and (z) the cash portion of any loan payable to the Sponsor, the proceeds from which are used by FoxWayne to pay any of the fees, costs or expenses set forth in clauses (w), (x) and (y). | |
| ● | “FoxWayne Transaction Expenses Cap” means an amount equal to $2,900,000. | |
| ● | “FoxWayne Warrants” means the public warrants and the Private Placement Warrants. | |
| ● | “Funded Indebtedness” means Indebtedness of the types described in clauses (a) and (b) of the definition of Indebtedness. | |
| ● | “GAAP” means generally accepted accounting principles in the United States. | |
| ● | “Governmental Authority” means any United States, foreign, supra-national, federal, state, provincial, local or self-regulatory governmental, regulatory or administrative authority, agency, division, body, organization or commission or any judicial or arbitral body. | |
| ● | “HSR Act” means the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended. | |
| ● | “Indebtedness” means, without duplication, (a) all obligations for borrowed money (including all obligations for principal, interest, penalties, fees and premiums, expenses and breakage costs) or extensions of credit (including under credit cards, bank overdrafts, and advances), (b) all obligations evidenced by bonds, debentures, notes, or other similar instruments (including all obligations for principal, interest, penalties, fees and premiums, expenses and breakage costs), (c) all obligations to pay the deferred purchase price of property or services, except trade accounts payable arising in the Ordinary Course of Business, (d) all obligations of others secured by an Encumbrance on any asset of such Person, (e) all obligations, contingent or otherwise, directly or indirectly guaranteeing any obligations of any other Person, (f) all obligations to reimburse the issuer in respect of letters of credit or under performance or surety bonds, or other similar obligations, and (g) all obligations in respect of bankers’ acceptances and under reverse repurchase agreements. | |
| ● | “Initial Business Combination” means FoxWayne’s initial merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. | |
| ● | “initial public offering” or “IPO” means FoxWayne’s initial public offering of units, which closed on January 22, 2021. | |
| ● | “initial stockholders” means the holders of Founder Shares, which includes the Sponsor and FoxWayne’s independent directors. |
| -7- |
| ● | “Intellectual Property” means all worldwide intellectual property and intellectual property rights, and all right, title and interest therein, including, without limitation, (a) all patents, patent applications, utility models, design registrations and certificates of invention and other governmental grants for the protection of inventions or industrial designs (including all related continuations, continuations-in-part, divisionals, reissues and reexaminations), invention disclosures, inventions and discoveries, whether or not patented or patentable and whether or not reduced to practice, improvements thereto, and other rights of invention; (b) brand marks, brand names, registered and unregistered trademarks, service marks, trade names, trade dress, logos, product names and slogans, including any common law rights, registrations and applications for the foregoing, and all goodwill associated with any of the foregoing; (c) copyrightable works, website content, all registered and unregistered copyrights in both published works and unpublished works, other rights of authorship and exploitation, and any applications, registrations and renewals in connection therewith; (d) all rights in mask works; (e) all know-how, trade secrets, confidential or proprietary information, customer lists, financial information, business information, technical information, data, process technology, plans, drawings and blue prints (the items in clause (e), collectively, “Trade Secrets”); (f) all Software; (g) all URLs, social media accounts, short codes, hash tags in internet web sites and internet domain names; (h) rights of attribution and integrity and other moral rights; (i) rights to exclude others from appropriating any of such Intellectual Property, including the right to sue for and remedies against past, present and future infringements of any or all of the foregoing and rights of priority and protection of interests therein, and any other proprietary, intellectual property; and (j) other rights relating to any or all of the foregoing anywhere in the world. | |
| ● | “IRS” means the Internal Revenue Service. | |
| ● | “JOBS Act” means the Jumpstart Our Business Startups Act of 2012. | |
| ● | “Law” means any federal, state, local or foreign law, statute, ordinance, code, decree, treaty, rule, rule of common law, policy, guidance, directive or regulation or Order of any Governmental Authority and all other provisions having the force or effect of law. | |
| ● | “Liabilities” means all debts, liabilities, commitments and obligations, whether accrued or fixed, absolute or contingent, matured or unmatured, determined or determinable, liquidated or unliquidated, asserted or unasserted, known or unknown, whenever or however arising, including those arising under applicable Law or any Proceeding or order of a Governmental Authority and those arising under any Contract, regardless of whether such debt, liability, commitment or obligation would be required to be reflected on a balance sheet prepared in accordance with GAAP or disclosed in the notes thereto. | |
| ● | “Lock-Up Agreement” means that certain letter agreement, dated as of January 19, 2021, by and between Sponsor and certain Insiders (as defined therein) delivered to FoxWayne. | |
| ● | “Material Adverse Effect” means with respect to any Person, any event, circumstance, change or effect (an “Effect”) that, individually or in the aggregate with any other Effect, (a) has materially and adversely impaired, or would reasonably be expected to materially and adversely impair, the ability of such Person to consummate the transactions contemplated by the Merger Agreement in accordance with terms of the Merger Agreement and applicable Law or in a timely manner or (b) has had, or would reasonably be expected to have, individually or in the aggregate, a material adverse effect on the business, condition (financial or otherwise), assets (including intangible assets), Liabilities or results of operations of such Person, taken as a whole; provided, that in the case of clause (b) only, in no event shall any Effect resulting or arising from any of the following, alone or in combination, be taken into account in determining whether there has been, a Material Adverse Effect: (i) any change in general economic conditions, (ii) any changes after the date of the Merger Agreement in applicable Law or the interpretation thereof or any COVID-19 Measures or any changes after the date of the Merger Agreement in such COVID-19 Measures or interpretations thereof, (iii) any change after the date of the Merger Agreement in accounting requirements or principles required by GAAP, (iv) any change in the industry generally in which such Person operates, (v) any outbreak, escalation or acts of terrorism, armed hostility or war, sabotage or military actions, or any weather-related event, earthquake, hurricane, tornado, fire or other natural disaster or any material worsening of such conditions, or (vi) any epidemics, pandemics or other outbreak of disease or illness or public health event (including COVID-19, or any COVID-19 Measures or any change in such COVID-19 Measures or interpretations following the date of the Merger Agreement); provided, further, that the exceptions set forth in clauses (i), (ii), (iii), (iv), (v) and (vi) shall only apply to the extent that such Effect does not have a materially disproportionate impact on such Person, taken as a whole, compared to other companies of similar size that operate in the industries in which such Person operates. |
| -8- |
| ● | “Merger” means the merger of Merger Sub with and into Aerami, with Aerami surviving the merger as a wholly owned subsidiary of FoxWayne. | |
| ● | “Merger Agreement” means that certain Agreement and Plan of Merger, dated as of December 7, 2021, by and among FoxWayne, Merger Sub, Aerami and the stockholders’ representative, including all schedules and exhibits thereto. | |
| ● | “Merger Sub” means Gotham Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of FoxWayne. | |
| ● | “Merger Sub Common Stock” means shares of Merger Sub’s common stock, par value $0.0001 per share. | |
| ● | “Nasdaq” means the Nasdaq Stock Market LLC. | |
| ● | “New Equity Incentive Plan” means the proposed 2022 incentive award plan of the Combined Company. | |
| ● | “Nominating Person” means (a) the stockholder nominating any person for election to the Board at an annual meeting or special meeting (but only if the election of directors is a matter specified in the notice of meeting given by or at the direction of the person calling such special meeting), (b) the beneficial owner or beneficial owners, if different, on whose behalf the notice of the business proposed to be brought before the annual meeting is made, and (c) any participant (as defined in paragraphs (a)(ii)-(vi) of Instruction 3 to Item 4 of Schedule 14A) with such stockholder in such solicitation. | |
| ● | “Order” means judgments, writs, decrees, directives, rulings, compliance agreements, injunctions, awards, assessments, writs, stipulations, determination of awards, settlement agreements or orders of any Governmental Authority or arbitrator. | |
| ● | “Ordinary Course of Business” means an action taken, or omitted to be taken, by any Person in the ordinary course of such Person’s business consistent with past practice (including any actions taken, or omitted to be taken, in good faith in light of COVID-19 and actions to respond to COVID-19 Measures). | |
| ● | “Organizational Documents” means (a) the articles or certificate of incorporation, all certificates of determination and designation, and the bylaws of a corporation; (b) the partnership agreement and any statement of partnership of a general partnership; (c) the limited partnership agreement and the certificate or articles of limited partnership of a limited partnership; (d) the operating agreement, limited liability company agreement and the certificate or articles of organization or formation of a limited liability company; (e) any charter or similar document adopted or filed in connection with the creation, formation or organization of any other Person; and (f) any amendment to any of the foregoing. | |
| ● | “Permits” means all licenses, permits, franchises, approvals, authorizations, consents or Orders of, or filings with, any Governmental Authority, whether foreign, federal, state or local, or any other Person. | |
| ● | “Permitted Encumbrances” means (a) any restriction on transfer arising under applicable securities laws; (b) Encumbrances for Taxes not yet due and payable; (c) mechanics’, carriers’, workers’, repairers’ and similar Encumbrances arising or incurred in the Ordinary Course of Business that are not yet due and payable and which are not, individually or in the aggregate, material to the business, operations and financial condition of the assets so encumbered of any Acquired Aerami Entities; and (d) zoning laws and other land use restrictions that do not, individually or in the aggregate, materially impair the present or anticipated use or occupancy of the property subject thereto. | |
| ● | “Per Share Common Consideration” means the number of shares of common stock of the Combined Company allocable from the Aggregate Consideration to each share of Aerami Common Stock that is issued and outstanding as of immediately prior to the Effective Time, as set forth on the Consideration Schedule. | |
| ● | “Per Share Consideration” means (a) with respect to shares of Aerami Common Stock, the Per Share Common Consideration, (b) with respect to shares of Aerami Royalty Stock, the Per Share Royalty Series Consideration, (c) with respect to shares of Aerami Series A Preferred Stock, the Per Share Series A Consideration, and (d) with respect to shares of Aerami Series A-1 Preferred Stock, the Per Share Series A-1 Consideration. |
| -9- |
| ● | “Per Share Royalty Series Consideration” means the number of shares of Combined Company common stock allocable from the Aggregate Consideration to each share of Aerami Royalty Stock that is issued and outstanding as of immediately prior to the Effective Time, as set forth on the Consideration Schedule. | |
| ● | “Per Share Series A Consideration” means the number of shares of Combined Company common stock allocable from the Aggregate Consideration to each share of Aerami Series A Preferred Stock that is issued and outstanding as of immediately prior to the Effective Time, as set forth on the Consideration Schedule. | |
| ● | “Per Share Series A-1 Consideration” means the number of shares of Combined Company common stock allocable from the Aggregate Consideration to each share of Aerami Series A-1 Preferred Stock that is issued and outstanding as of immediately prior to the Effective Time, as set forth on the Consideration Schedule. | |
| ● | “Person” means any individual, corporation (including any non-profit corporation), general or limited partnership, limited liability company, joint venture, estate, trust, association, organization, labor union, or other entity or governmental body. | |
| ● | “Placement Agent Warrants” means the warrants granted to Aerami’s former placement agent for the purchase of Senior Preferred Stock and Aerami Common Stock in connection with Aerami’s private placement of Series A preferred stock in 2020 and private placement of common stock in 2021. | |
| ● | “Preferred Stock” means (a) prior to giving effect to the Business Combination, FoxWayne’s Preferred Stock, par value $0.0001 per share and (b) after giving effect to the Business Combination, the Combined Company’s Preferred Stock, par value $0.0001 per share. | |
| ● | “Private Placement” means a private placement or private placements pursuant to which certain investors may purchase shares of FoxWayne Common Stock at $10.00 per share for an aggregate subscription amount of up to $30,000,000, to be consummated, if at all, immediately prior to or substantially concurrently with the Closing. | |
| ● | “Private Placement Warrants” means the warrants issued to the Sponsor in a private placement simultaneously with the closing of FoxWayne’s initial public offering. | |
| ● | “Proceeding” means any action, suit, litigation, complaint, dispute, arbitration, mediation, proceeding (including any civil, criminal, administrative, investigative or appellate proceeding), hearing, audit, inquiry, examination or investigation commenced, brought, conducted or heard by or before, or otherwise involving, any court or other Governmental Authority. | |
| ● | “Proposals” means the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal, the Additional Proposal and the Adjournment Proposal. | |
| ● | “Proposed Bylaws” means the Amended and Restated Bylaws of FoxWayne. | |
| ● | “Proposed Charter” means the Third Amended and Restated Certificate of Incorporation of FoxWayne. | |
| ● | “Proposing Person” means (a) the stockholder providing the notice of business proposed to be brought before an annual meeting, (b) the beneficial owner or beneficial owners, if different, on whose behalf the notice of the business proposed to be brought before the annual meeting is made, and (c) any participant (as defined in paragraphs (a)(ii)-(vi) of Instruction 3 to Item 4 of Schedule 14A) with such stockholder in such solicitation. | |
| ● | “public shares” means shares of FoxWayne Class A Common Stock sold as part of the units in the IPO (whether they were purchased in the IPO or thereafter in the open market). | |
| ● | “public stockholders” means the holders of our public shares. | |
| ● | “public warrants” means the warrants sold as part of the units in the IPO (whether they were purchased in the IPO or thereafter in the open market). | |
| ● | “Redemption Offer” means that opportunity given by FoxWayne to holders of Class A Common Stock (other than the Sponsor or any other party to any FoxWayne Stockholder Support Agreement) to have their shares of Class A Common Stock redeemed for the consideration, and on the terms and subject to the conditions and limitations, set forth in the Merger Agreement, the organizational documents of FoxWayne and that certain Trust Agreement, dated as of January 22, 2021, by and between FoxWayne and Continental Stock Transfer & Trust Company in conjunction with, inter alia, obtaining approval from holders of Class A Common Stock for the Business Combination. |
| -10- |
| ● | “Registration Statement” means this Registration Statement S-4. | |
| ● | “Representative” means any officer, director, manager, principal, attorney, financial advisor, agent, employee or other representative. | |
| ● | “Sarbanes-Oxley Act” means the Sarbanes-Oxley Act of 2002, as amended. | |
| ● | “SEC” means the United States Securities and Exchange Commission. | |
| ● | “Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder. | |
| ● | “Software” means any and all (a) computer programs, including any and all software implementation of algorithms, models and methodologies, whether in source code, object code, human readable form or other form, (b) databases and compilations, including any and all data and collections of data, whether machine readable or otherwise, (c) descriptions, flow charts and other work products used to design, plan, organize and develop any of the foregoing, (d) screens, user interfaces, report formats, firmware, development tools, templates, menus, buttons and icons and (e) documentation, including user manuals and training materials, relating to any of the foregoing. | |
| ● | “special meeting” means the special meeting of stockholders of FoxWayne that is the subject of this proxy statement/prospectus and any adjournments or postponements thereof. | |
| ● | “Sponsor” means FoxWayne Enterprises Acquisition Sponsor LLC, a Delaware limited liability company, of which Robb Knie, our Chairman, Chief Executive Officer and Chief Financial Officer, is the managing member. | |
| ● | “Sponsor Support Agreement” means that certain Sponsor Support Agreement, dated as of the Merger Agreement, delivered by the Sponsor and pursuant to which, among other things, the Sponsor agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement. | |
| ● | “stockholders’ representative” initially means Steve Thornton. | |
| ● | “Subsidiary” means when used in reference to any Person, any corporation or other entity of which such Person owns, directly or indirectly, (a) 50% or more of the outstanding shares of stock, other Equity Interests or voting securities, or (b) outstanding securities having ordinary voting power to elect the majority of the board of directors or other managing body of such corporation or entity. | |
| ● | “Tax” means any and all taxes, including any net income, alternative or add-on minimum, gross income, gross receipts, sales, use, ad valorem, value added, transfer, franchise, profits, license, registration, recording, documentary, conveyancing, gains, withholding, payroll, employment, excise, severance, stamp, occupation, premium, property, environmental or windfall profit, escheat, custom duty or other tax, governmental fee or other like assessment or charge in the nature of a tax, together with any interest, penalty, addition to tax or additional amount imposed by any Governmental Authority, whether disputed or not, or in respect of any failure to comply with any requirement regarding Tax Returns, including any such amounts payable pursuant to any tax-sharing agreement or other agreement relating to the payment of any such Tax, levy, impost, duty, assessment, charge or withholding, whether imposed directly, under Treasury Regulation Section 1.1502-6 (or any similar provision of state, local, or foreign Law), as a result of being a transferee, successor, or member of an affiliated, consolidated, unitary or combined group, by contract, or otherwise. | |
| ● | “Tax Return” means any return, report, declaration, claim for refund, information return or other document (including schedules thereto, other attachments thereto, amendments thereof, or any related or supporting information) filed or required to be filed with a Tax authority relating to any Tax. | |
| ● | “Transaction Expenses” means, without duplication, the aggregate amount of all fees, costs and expenses incurred by or on behalf of FoxWayne, Merger Sub, or any Acquired Aerami Entities arising from, incurred in connection with or related to the negotiation, preparation, execution and performance of the Merger Agreement and the transactions contemplated thereby, including (a) third-party fees, expenses and costs (including legal, accounting, brokers’, investment bankers’, consultants’, advisors’ and finders’ fees, costs and expenses) arising from, incurred in connection with or related to the Merger Agreement or the transactions contemplated thereby (whether or not such amounts have been billed as of or prior to the Closing Date), (b) all bonuses, incentive compensation, termination payments, severance, or other change-in-control, separation or other transaction-related payments solely payable in connection with the Merger or any of the other transactions contemplated thereby (whether paid or provided on or following the Closing Date), (c) the employer portion of any payroll, employment or similar Taxes incurred or to be incurred by FoxWayne, the Combined Company or any Acquired Aerami Entities in connection with the items described in clause (b), and (d) all other miscellaneous out-of-pocket expenses or costs incurred by or on behalf of FoxWayne, the Combined Company, or any Acquired Aerami Entities incurred in connection with, arising from or related to the Merger Agreement. | |
| ● | “Treasury Regulations” means the United States Treasury regulations promulgated under the Code. | |
| ● | “Trust Account” means the trust account that holds the proceeds (including interest not previously released to FoxWayne to pay its franchise and income taxes) from the IPO and a concurrent private placement of Private Placement Warrants to the Sponsor. | |
| ● | “Trustee” means Continental Stock Transfer & Trust Company. | |
| ● | “units” means the FoxWayne units sold in the IPO, each of which consists of one share of Class A Common Stock and one public warrant. |
| -11- |
SUMMARY OF KEY TERMS
This summary, together with the sections titled “Questions and Answers” and “Summary of the Proxy Statement/Prospectus,” highlights certain information included in this proxy statement/prospectus but does not include all of the information that is important to you. You should read carefully this entire proxy statement/prospectus, including the attached annexes, for a more complete understanding of the matters to be considered at the special meeting.
| ● | FoxWayne is a blank check company incorporated in Delaware on September 17, 2020 for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. FoxWayne is an emerging growth company and, as such, is subject to all of the risks associated with emerging growth companies. For more information about FoxWayne, see the section titled “Information About FoxWayne.” When you consider the recommendation of the Proposals by the FoxWayne Board, you should keep in mind that FoxWayne’s directors and officers have interests in the Business Combination that may conflict with your interests as a stockholder. Stockholders should take these interests into account in deciding whether to approve the Business Combination. See the subsection titled “The Business Combination - Interests of Certain Persons in the Business Combination - Interests of Sponsor and FoxWayne Directors and Officers” for additional information. The FoxWayne Board was aware of and considered these interests, among other matters, in evaluating the Business Combination and the other Proposals, and in recommending that FoxWayne stockholders vote “FOR” the Transaction Proposal and each of the other Proposals. | |
| ● | As of February 11, 2022, there are 5,800,000 shares of FoxWayne’s Class A Common Stock and 1,437,500 shares of FoxWayne’s Class B Common Stock issued and outstanding. In addition, as of February 11, 2022, there are 8,550,000 warrants of FoxWayne outstanding, consisting of 5,750,000 public warrants and 2,800,000 Private Placement Warrants. Each whole warrant entitles the holder to purchase one whole share of Class A Common Stock for $11.50 per share, subject to adjustment. The warrants will become exercisable on the later of (i) the completion of an Initial Business Combination or (ii) January 22, 2022. The warrants will expire five years after the completion of FoxWayne’s Initial Business Combination or earlier upon redemption or liquidation. Once the warrants become exercisable, FoxWayne may redeem the outstanding warrants, in whole and not in part, at a price of $0.01 per warrant in accordance with the terms of the warrant agreement; provided, however, that the Private Placement Warrants are non-redeemable so long as they are held by our Sponsor or its permitted transferees. For more information about the terms of the warrants, see the subsection titled “Description of New Securities — Warrants.” | |
| ● | Aerami, a Delaware corporation, is a clinical stage biopharmaceutical company dedicated to developing differentiated inhaled therapies for the treatment of severe respiratory and other chronic diseases where delivery of therapeutic agents via inhalation can provide an important treatment option. Aerami aims to address markets with high unmet needs where Aerami believes inhaled therapies can offer meaningful differentiation over current standards of care and improve patient experience and outcomes. For more information about Aerami, see the sections titled “Information About Aerami” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Aerami.” | |
| ● | On December 7, 2021, we and our wholly owned subsidiary, Merger Sub, entered into the Merger Agreement with Aerami and the stockholders’ representative. A copy of the Merger Agreement is attached to this proxy statement/prospectus as Annex A. | |
| ● | Pursuant to the Merger Agreement, and subject to the terms and conditions contained therein, Merger Sub will merge with and into Aerami, with Aerami surviving the Merger as a wholly owned subsidiary of the Combined Company. For more information about the Merger Agreement and the Business Combination, see the section titled “The Business Combination.” | |
| ● | In connection with the Business Combination, an aggregate of 25,000,000 shares of the Combined Company common stock will be issued to Aerami Equityholders and shares of the Combined Company common stock will be reserved for issuance upon the exercise of Exchanged Options, Exchanged RSU Awards and Assumed Warrants assumed by the Combined Company in the Merger. For more information about the Merger Agreement and the Business Combination, see the section titled “The Business Combination.” For more information about the New Equity Incentive Plan, see the subsection titled “Proposal 5 – The New Equity Incentive Plan Proposal.” |
| -12- |
| ● | Unless lawfully waived by the parties to the Merger Agreement, the Closing is subject to a number of conditions set forth in the Merger Agreement, including, among others, receipt of the requisite FoxWayne and Aerami stockholder approvals of the Merger Agreement, the Business Combination, the Charter Proposal, the Nasdaq Proposal and certain other Proposals as contemplated by this proxy statement/prospectus. For more information about the closing conditions to the Business Combination, see the subsection titled “The Business Combination — Conditions to Closing of the Merger Agreement.” | |
| ● | The Merger Agreement may be terminated at any time prior to the consummation of the Business Combination upon agreement of the parties thereto, or for other reasons in specified circumstances. For more information about the termination rights under the Merger Agreement, see the subsection titled “The Business Combination — Termination.” | |
| ● | The proposed Business Combination involves numerous risks. For more information about these risks, please see the section titled “Risk Factors.” | |
| ● | Under FoxWayne’s Charter, in connection with the Business Combination, our public stockholders may elect to have their shares redeemed for cash at the applicable redemption price per share calculated in accordance with FoxWayne’s Charter. As of February 11, 2022, this would have amounted to approximately $10.10 per share. If a holder exercises its redemption rights, then such holder will be exchanging its public shares for cash and will no longer own shares of FoxWayne following the completion of the Business Combination and will not participate in any future growth of the Combined Company. Such a holder will be entitled to receive cash for its public shares only if the stockholder properly demands redemption and delivers such stockholder’s shares (either physically or electronically) to our transfer agent at least two business days prior to the special meeting. For more information regarding these procedures, see the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights.” | |
| ● | We anticipate that, upon the Closing, the ownership of the Combined Company will be as follows: |
● the Aerami Equityholders will own 25,000,000 shares, or approximately 78%, of the outstanding common stock of the Combined Company;
● the public stockholders will own 5,800,000 shares, or approximately 18%, of the outstanding common stock of the Combined Company; and
● the initial stockholders will own 1,437,500 shares, or approximately 4%, of the outstanding common stock of the Combined Company.
The ownership of the Combined Company does not take into account the effect of (i) the redemption of any shares by FoxWayne’s public stockholders, (ii) the exercise of any FoxWayne Warrants or (iii) the assumption of Aerami Options, Aerami RSUs and Aerami Warrants in the Business Combination. Please see the sections titled “Summary of the Proxy Statement/Prospectus — Ownership of the Combined Company After the Closing” and “Unaudited Pro Forma Condensed Combined Financial Information” for further information.
The FoxWayne Board considered various factors in determining whether to approve the Merger Agreement and the Business Combination. For more information about the FoxWayne Board’s decision-making process, see the subsections titled “The Business Combination —FoxWayne Board’s Reasons for the Approval of the Business Combination.”
In addition to voting on the Transaction Proposal at the special meeting, FoxWayne’s stockholders will also be asked to vote on the approval of:
| ● | The Charter Proposal — To consider and vote upon a proposal to approve and adopt the Proposed Charter, to, among other things: | ||
| ● | change the corporate name of FoxWayne to “Aerami Therapeutics Holdings, Inc.”; | ||
| -13- |
| ● | increase the number of authorized shares of the Combined Company’s capital stock, par value $0.0001 per share, from 53,000,000 shares, consisting of (i) 52,000,000 shares of common stock, including 50,000,000 shares of Class A Common Stock and 2,000,000 shares of Class B Common Stock and (ii) 1,000,000 shares of Preferred Stock, to 210,000,000 shares, consisting of (i) 200,000,000 shares of Combined Company common stock and (ii) 10,000,000 shares of preferred stock, par value $0.0001 per share (“Combined Company preferred stock”); | ||
| ● | require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of Combined Company capital stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal, or adopt any provision inconsistent with Article V pertaining to stockholders; Article VI pertaining to directors; and Article VII pertaining to limitation of liability of the Combined Company’s Proposed Charter; | ||
| ● | provide that any director or the entire Combined Company Board may be removed from office at any time, but only for cause and only by the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon; | ||
| ● | require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend or repeal any provision of the Combined Company’s bylaws; and | ||
| ● | eliminate provisions in the Charter relating to FoxWayne’s Initial Business Combination that will no longer be applicable to the Combined Company following the Effective Time of the Merger and the Closing of the Business Combination and make certain other changes that the FoxWayne Board deems appropriate for a public operating company. | ||
| The full text of the Proposed Charter reflecting each of the proposed amendments pursuant to the Charter Proposal is attached to this proxy statement/prospectus as Annex B. | |||
| ● | The Bylaw Proposal — To consider and vote upon a proposal to approve and adopt the Proposed Bylaws to, among other things: | ||
| ● | require stockholders seeking to bring business before a meeting or to nominate candidates for election as directors to provide additional disclosures; | ||
| ● | prohibit stockholder action by written consent in lieu of a meeting; | ||
| ● | require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend or repeal any provision of the Combined Company’s bylaws; and | ||
| ● | lock-up holders of Combined Company common stock issued as consideration pursuant to the Merger Agreement or upon the exercise, exchange, conversion, or settlement of any Exchanged Options, Exchanged RSU Awards or Assumed Warrants until 180 days after the date that the Form S-1 Shelf is declared effective by the SEC, subject to certain exceptions. | ||
| The full text of the Proposed Bylaws reflecting each of the proposed amendments pursuant to the Bylaw Proposal is attached to this proxy statement/prospectus as Annex C. | |||
| ● | The Nasdaq Proposal — To consider and vote upon a proposal to approve the issuance of more than 20% of the outstanding capital stock and voting power of FoxWayne, in connection with the terms of the Merger Agreement and the Business Combination and the New Equity Incentive Plan, which will result in a change of control of FoxWayne, as required by applicable listing rules of Nasdaq. In connection with the Merger, we intend to issue an aggregate of approximately 25,000,000 shares of the Combined Company common stock, and we will reserve shares of the Combined Company common stock for issuance upon the exercise of Exchanged Options, Exchanged RSU Awards and Assumed Warrants assumed in the Merger. In addition, we will reserve a number of shares that approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination for issuance under the New Equity Incentive Plan (which number will include the shares of Combined Company common stock reserved for issuance upon exercise of the Exchanged Options and Exchanged RSU Awards). | ||
| ● | The New Equity Incentive Plan Proposal — To consider and vote upon a proposal to approve and adopt the New Equity Incentive Plan, including the reservation of such number of shares of the Combined Company common stock as approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination and the other material terms thereunder. A copy of the New Equity Incentive Plan is attached to this proxy statement/prospectus as Annex D. | |
| ● | The Additional Proposal — To consider and vote upon any other proposals determined by the FoxWayne Board to be necessary or appropriate in connection with the transactions contemplated by the Business Combination or the other Proposals (Proposal 6). | |
| ● | The Adjournment Proposal — To consider and vote upon a proposal to approve the adjournment of the special meeting to a later date or dates, if necessary or appropriate, to permit further solicitation and vote of proxies in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal or the Additional Proposal (Proposal 7). |
For more information, see the sections titled “Proposal 1 – The Transaction Proposal,” “Proposal 2 — The Charter Proposal,” “Proposal 3 — The Bylaw Proposal,” “Proposal 4 — The Nasdaq Proposal,” “Proposal 5 — The New Equity Incentive Plan Proposal,” “Proposal 6 — The Additional Proposal,” and “Proposal 7 — The Adjournment Proposal.”
| -14- |
QUESTIONS AND ANSWERS
The following questions and answers briefly address some commonly asked questions about the Proposals to be presented at the special meeting of stockholders of FoxWayne, including the proposed Business Combination. The following questions and answers do not include all the information that is important to stockholders. We urge stockholders to carefully read this entire proxy statement/prospectus, including the annexes and other documents referred to herein.
In this section, unless otherwise stated or the context requires otherwise, the terms “FoxWayne,” “we,” “us” and “our” refer to FoxWayne Enterprises Acquisition Corp. prior to the Closing and the Combined Company and its subsidiaries following the Closing.
| Q: | Why am I receiving this proxy statement/prospectus? |
| A: | FoxWayne is sending this proxy statement/prospectus to its stockholders to help them decide how to vote their shares of FoxWayne capital stock with respect to the matters to be considered at the special meeting. FoxWayne and Aerami have agreed to the Business Combination under the terms of the Merger Agreement that is attached to this proxy statement/prospectus as Annex A and is incorporated into this proxy statement/prospectus by reference. FoxWayne stockholders are being asked to consider and vote upon and approve the Transaction Proposal for the Business Combination and certain other Proposals, including the Charter Proposal, the Nasdaq Proposal, the Bylaw Proposal, the New Equity Incentive Plan Proposal, the Additional Proposal and the Adjournment Proposal. The Business Combination cannot be completed unless FoxWayne stockholders approve the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal. |
This proxy statement/prospectus and its annexes include important information about the proposed Business Combination and the other matters to be acted upon at the special meeting. You should read this proxy statement/prospectus and its annexes carefully and in their entirety.
Your vote is important. You are encouraged to submit your proxy as soon as possible after carefully reviewing this proxy statement/prospectus and its annexes.
| Q: | What is being voted on at the special meeting? |
| A: | FoxWayne stockholders will vote on the following proposals at the special meeting. |
| ● | The Transaction Proposal — To consider and vote upon a proposal to (a) approve and adopt the Agreement and Plan of Merger, dated as of December 7, 2021, by and among FoxWayne, Gotham Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of FoxWayne, Aerami Therapeutics Holdings, Inc., a Delaware corporation, and the stockholders’ representative, pursuant to which Merger Sub will merge with and into Aerami, with Aerami surviving the Merger as a wholly owned subsidiary of FoxWayne and (b) approve the Merger and the other transactions contemplated by the Merger Agreement (Proposal 1). A copy of the Merger Agreement is attached to this proxy statement/prospectus as Annex A. | |
| ● | The Charter Proposal — To consider and vote upon a proposal to approve and adopt the Proposed Charter, to, among other things: |
| ● | change the corporate name of FoxWayne to “Aerami Therapeutics Holdings, Inc.”; | |
| ● | increase the number of authorized shares of the Combined Company’s capital stock, par value $0.0001 per share, from 53,000,000 shares, consisting of (i) 52,000,000 shares of common stock, including 50,000,000 shares of Class A Common Stock and 2,000,000 shares of Class B Common Stock and (ii) 1,000,000 shares of Preferred Stock, to 210,000,000 shares, consisting of (i) 200,000,000 shares of Combined Company common stock and (ii) 10,000,000 shares of Combined Company preferred stock; | |
| ● | require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal, or adopt any provision inconsistent with Article V pertaining to stockholders; Article VI pertaining to directors; and Article VII pertaining to limitation of liability of the Proposed Charter; |
| -15- |
| ● | provide that any director or the entire Combined Company Board may be removed from office at any time, but only for cause and only by the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon; | |
| ● | require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend or repeal any provision of the Combined Company’s bylaws; and | |
| ● | eliminate provisions in the Charter relating to FoxWayne’s Initial Business Combination that will no longer be applicable to the Combined Company following the Effective Time of the Merger and the Closing of the Business Combination and make certain other changes that the FoxWayne Board deems appropriate for a public operating company. |
The full text of the Proposed Charter reflecting each of the proposed amendments pursuant to the Charter Proposal is attached to this proxy statement/prospectus as Annex B.
| ● | The Bylaw Proposal —To consider and vote upon a proposal to approve and adopt the Proposed Bylaws to, among other things: | ||
| ● | require stockholders seeking to bring business before a meeting or to nominate candidates for election as directors to provide additional disclosures; | ||
| ● | prohibit stockholder action by written consent in lieu of a meeting; | ||
| ● | require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend or repeal any provision of the Combined Company’s bylaws; and | ||
| ● | lock-up holders of Combined Company common stock issued as consideration pursuant to the Merger Agreement or upon the exercise, exchange, conversion, or settlement of any Exchanged Options, Exchanged RSU Awards or Assumed Warrants until 180 days after the date that the Form S-1 Shelf is declared effective by the SEC, subject to certain exceptions. | ||
| The full text of the Proposed Bylaws reflecting each of the proposed amendments pursuant to the Bylaw Proposal is attached to this proxy statement/prospectus as Annex C. | |||
| ● | The Nasdaq Proposal — To consider and vote upon a proposal to approve the issuance of more than 20% of the outstanding capital stock and voting power of FoxWayne, in connection with the terms of the Merger Agreement and the Business Combination and the New Equity Incentive Plan, which will result in a change of control of FoxWayne, as required by applicable listing rules of Nasdaq. In connection with the Merger, we intend to issue an aggregate of approximately 25,000,000 shares of Combined Company common stock, and we will reserve shares of Combined Company common stock for issuance upon the exercise of Exchanged Options, Exchanged RSU Awards and Assumed Warrants assumed in the Merger. In addition, we will reserve a number of shares that approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination for issuance under the New Equity Incentive Plan (which number will include the shares of Combined Company common stock reserved for issuance upon exercise of the Exchanged Options and Exchanged RSU Awards). | ||
| ● | The New Equity Incentive Plan Proposal — To consider and vote upon a proposal to approve and adopt the New Equity Incentive Plan, including the reservation of such number of shares of the Combined Company common stock as approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination and the other material terms thereunder. A copy of the New Equity Incentive Plan is attached to this proxy statement/prospectus as Annex D. | ||
| -16- |
| ● | The Additional Proposal — To consider and vote upon any other proposals determined by the FoxWayne Board to be necessary or appropriate in connection with the transactions contemplated by the Business Combination or the other Proposals (Proposal 6). | |
| ● | The Adjournment Proposal — To consider and vote upon a proposal to approve the adjournment of the special meeting to a later date or dates, if necessary or appropriate, to permit further solicitation and vote of proxies in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal or the Additional Proposal (Proposal 7). |
For more information, see the sections titled “Proposal 1 – The Transaction Proposal,” “Proposal 2 — The Charter Proposal,” “Proposal 3 — The Bylaw Proposal,” “Proposal 4 — The Nasdaq Proposal,” “Proposal 5 — The New Equity Incentive Plan Proposal,” “Proposal 6 — The Additional Proposal,” and “Proposal 7 — The Adjournment Proposal.”
| Q: | Are the Proposals conditioned on one another? |
| A: | We may not consummate the Business Combination unless the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal are approved at the special meeting. The Bylaw Proposal, the New Equity Incentive Plan Proposal and the Additional Proposal are conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal. The Adjournment Proposal is not conditioned on the approval of any other Proposal set forth in this proxy statement/prospectus. |
| Q: | What will happen in the Business Combination? |
| A: | On December 7, 2021, FoxWayne and Merger Sub entered into the Merger Agreement with Aerami and the stockholders’ representative. Pursuant to the Merger Agreement, and subject to the terms and conditions contained therein, Merger Sub will merge with and into Aerami, with Aerami surviving the Merger. After giving effect to the Merger, Aerami will become a wholly owned subsidiary of the Combined Company. At the Closing, it is anticipated that 25,000,000 shares of the Combined Company common stock will be issued to the Aerami Equityholders in the Business Combination. It is also anticipated that we will reserve for issuance up to shares of Combined Company common stock in respect of (i) Combined Company Options issued in exchange for outstanding, unexercised Aerami Options, (ii) Combined Company warrants issued in exchange for outstanding, unexercised Aerami Warrants, and (iii) outstanding Aerami RSUs exchanged for Combined Company restricted stock award units pursuant to the New Equity Incentive Plan. For more information about the Merger Agreement and the Business Combination, see the section titled “The Business Combination.” |
| Q: | Why is FoxWayne proposing the Business Combination? |
| A: | FoxWayne was formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination involving FoxWayne and one or more businesses. |
On January 19, 2021, FoxWayne completed its initial public offering of 5,000,000 units, raising gross proceeds of approximately $50 million. The underwriters of the initial public offering were granted a 45-day option from the date of the final prospectus relating to the initial public offering to purchase up to 750,000 additional units to cover overallotments, if any, at $10.00 per unit, less underwriting discounts and commissions. On February 22, 2021, the underwriters exercised the overallotment option and, on February 22, 2021, the underwriters purchased 750,000 additional units (the “overallotment units”), generating gross proceeds of approximately $7.5 million.
Simultaneously with the closing of the initial public offering, FoxWayne consummated the sale of 2,500,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant in a private placement to our Sponsor, generating gross proceeds of approximately $2,500,000. Simultaneously with the closing of the sale of the overallotment units, our Sponsor purchased an additional 300,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant, generating gross proceeds of approximately $300,000.
| -17- |
Following the closing of the initial public offering, FoxWayne management commenced an active search for businesses or assets to acquire for the purpose of consummating FoxWayne’s Initial Business Combination. FoxWayne management reviewed self-generated ideas, explored ideas with the underwriters from the initial public offering, considered transactions through various investment banking and advisory firms and contacted, and were contacted by, a number of individuals and entities with respect to hundreds of business combination opportunities. As part of this process, between January 22, 2021, the date of the closing of the initial public offering, and August 3, 2021, the date on which FoxWayne and Aerami entered into a letter of intent (as further described herein), FoxWayne and its representatives evaluated approximately 25 potential targets. These potential targets were in various categories of the transportation, security and artificial intelligence, autonomous agriculture and biopharmaceutical industries. FoxWayne and its representatives met with members of management, board members or other investors of approximately 12 different potential acquisition targets. FoxWayne then conducted additional due diligence under confidentiality or nondisclosure agreements with respect to 8 potential targets. FoxWayne conducted due diligence to varying degrees on the potential targets, including review of, among other things, the potential targets’ management team, stockholders, business model, valuation, balance sheet and historical and projected financials, in each case to the extent made available, and FoxWayne submitted indications of interest to 4 potential targets, including Aerami.
The FoxWayne Board considered a wide variety of factors in connection with its evaluation of the Business Combination. In light of the complexity of those factors, the FoxWayne Board, as a whole, did not consider it practicable to, nor did it attempt to, quantify or otherwise assign relative weights to the specific factors it took into account in reaching its decision. The FoxWayne Board viewed its decision as being based on all of the information available and the factors presented to and considered by it. In addition, individual members of the FoxWayne Board may have given different weight to different factors. This explanation of the reasons for the FoxWayne Board’s approval of the Business Combination, and all other information presented in this section, is forward-looking in nature and, therefore, should be read in light of the factors discussed in the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Before reaching its decision, the FoxWayne Board reviewed the results of the due diligence conducted by FoxWayne’s management and FoxWayne’s advisors, which included:
● meetings and calls with Aerami’s management regarding business model, operations and forecasts;
● a legal due diligence review conducted by Sheppard, Mullin, Richter & Hampton LLP which included, among other things, a review of material contracts, intellectual property matters and other legal documents posted to a virtual data room, conference calls with Aerami and its attorneys and certain public record searches of Aerami;
● a tax due diligence review conducted by WithumSmith+Brown, PC;
● an accounting due diligence review conducted by Chord Advisors, LLC;
● consultation with legal and financial advisors, primarily EF Hutton, and industry experts;
● financial and valuation analysis of Aerami and the Business Combination; and
● the financial statements of Aerami.
In approving the Business Combination, the FoxWayne Board determined not to obtain a fairness opinion. The officers and directors of FoxWayne have substantial experience in evaluating the operating and financial merits of companies from a wide range of industries and concluded that their experience and background enabled them to make the necessary analyses and determinations regarding the Business Combination.
The factors considered by the FoxWayne Board included, but were not limited to, the following:
● Market Opportunity. The FoxWayne Board noted that public markets have recently assigned premium values to companies developing inhaled therapy products. The FoxWayne Board determined that Aerami has an opportunity to capture this public investor momentum in the inhaled therapy space.
● Market Focus. The FoxWayne Board considered the fact that the products being developed by Aerami serve a broad and underserved market for inhaled therapies for severe respiratory and chronic diseases.
| -18- |
● Unique Products. The FoxWayne Board explored how Aerami’s potential product pipeline combines novel formulations of existing drugs with Aerami’s own proprietary and in-licensed inhalation technology and Aerami’s intellectual property portfolio.
● Operating History and Management Team. The FoxWayne Board considered Aerami’s operating history and strong management team, network of consultants, and experienced board of directors, the core of which is expected to remain with the post-combination company and seek to execute Aerami’s strategy.
● Terms of the Merger Agreement. The FoxWayne Board reviewed the financial and other terms of the Merger Agreement and determined that they were the product of arm’s-length negotiations among the parties.
In addition, the FoxWayne Board determined that the Business Combination satisfies the investment criteria that the FoxWayne Board identified in connection with the initial public offering. For more information, see the subsection titled “The Business Combination — Background of the Business Combination.”
In the course of its deliberations, the FoxWayne Board also considered a variety of uncertainties, risks and other potentially negative factors relevant to the Business Combination, including the following:
● Early Stage Company Risk. The risk that Aerami is an early stage company with a history of losses, and the risk that Aerami expects to incur significant expenses and continuing losses for the near term.
● Growth Risk. The risk that Aerami expects to invest in growth for the foreseeable future, and the risk that Aerami may fail to manage that growth effectively.
● Competitive Risk. The risk that Aerami currently faces competition from a number of companies and expects to face significant competition in the future.
● Supplier and Manufacturer Risk. The risk that Aerami is still developing relationships with suppliers and manufacturers for its products.
● Public Company Risk. The risks that are associated with being a publicly traded company that is in its early development stage.
● Benefits May Not Be Achieved Risk. The risk that the potential benefits of the Business Combination may not be fully achieved or may not be achieved within the expected timeframe.
● Redemption Risk. The risk that a significant number of FoxWayne’s stockholders elect to redeem their shares prior to the consummation of the Business Combination and pursuant to FoxWayne’s existing Charter, which would potentially make the Business Combination more difficult to complete or reduce the amount of cash available to the post-combination company to accelerate its business plan following the Closing.
● Stockholder Vote Risk. The risk that FoxWayne’s stockholders may fail to provide the votes necessary to effect the Business Combination.
● Litigation Risk. The risk of the possibility of litigation challenging the Business Combination or that an adverse judgment granting permanent injunctive relief could indefinitely enjoin consummation of the Business Combination.
● Closing Conditions Risk. The risk that completion of the Business Combination is conditioned on the satisfaction of certain closing conditions that are not within FoxWayne’s control.
● No Third-Party Valuation Risk. The risk that FoxWayne did not obtain a third-party valuation or fairness opinion in connection with the Business Combination.
● Fees, Expenses and Time Risk. The risk of incurring significant fees and expenses associated with completing the Business Combination and the substantial time and effort of management required to complete the Business Combination.
● Other Risks. Various other risk factors associated with Aerami’s business, as described in the section titled “Risk Factors.”
| -19- |
In addition to considering the factors described above, the FoxWayne Board also considered that the officers and directors of FoxWayne may have interests in the Business Combination as individuals that are in addition to, and that may be different from, the interests of FoxWayne’s stockholders. For more information, see the subsection titled “— The Business Combination - Interests of Certain Persons in the Business Combination - Interests of Sponsor and FoxWayne Directors and Officers.”
The FoxWayne Board concluded that the potential benefits that it expects FoxWayne and its stockholders to achieve as a result of the Business Combination outweigh the potentially negative factors associated with the Business Combination. Accordingly, the FoxWayne Board, based on its consideration of the specific factors listed above, unanimously (a) determined that the Business Combination and the other transactions contemplated by the Merger Agreement are fair to, and in the best interests of, FoxWayne’s stockholders, (b) approved, adopted and declared advisable the Merger Agreement and the transactions contemplated thereby and (c) recommended that the stockholders of FoxWayne approve each of the Proposals.
The above discussion of the material factors considered by the FoxWayne Board is not intended to be exhaustive but does set forth the principal factors considered by the FoxWayne Board.
| Q: | What conditions must be satisfied to complete the Business Combination? |
| A: | There are a number of closing conditions in the Merger Agreement, including obtaining any required approvals of certain Proposals by our stockholders and Aerami stockholders. For a summary of the conditions that must be satisfied or waived prior to completion of the Business Combination, see the subsection titled “The Business Combination — Conditions to Closing of the Merger Agreement.” |
| Q: | How will we be managed and governed following the Business Combination? |
| A: | Immediately after the Closing, the board of directors of the Combined Company will consist of 7 members, all of which will be designated by Aerami, and will be divided into three separate classes, designated as follows: |
| ● | the Class I directors will be and and their terms will expire at the first annual meeting of stockholders following the Effective Time; | |
| ● | the Class II directors will be and and their terms will expire at the second annual meeting of stockholders following the Effective Time; and | |
| ● | the Class III directors will be , and and their terms will expire at the third annual meeting of stockholders following the Effective Time. |
It is anticipated that will be designated Chairman of the Combined Company Board immediately after the Closing.
Please see the section titled “Management After the Business Combination.”
| Q: | What equity stake will our current stockholders and the holders of our Founder Shares hold in Combined Company following the consummation of the Business Combination? |
| A: | The following table sets forth the anticipated ownership of the Combined Company by our public stockholders and the holders of our Founder Shares upon the Closing, assuming the following redemption scenarios: |
| ● | Assuming no redemption: This presentation assumes that no public stockholders exercise redemption rights with respect to their public shares. | |
| ● | Assuming interim redemption: This presentation assumes that FoxWayne public stockholders holding approximately 1,885,941 shares of Class A Common Stock, or approximately 33%, out of the 5,750,000 outstanding and redeemable shares of FoxWayne Class A Common Stock will exercise their redemption rights upon consummation of the Business Combination at a redemption price of approximately $10.10 per share (aggregate $19.0 million). The estimated per share redemption value of $10.10 was calculated by dividing the amount of $58.1 million in the FoxWayne trust account as of September 30, 2021 by the total FoxWayne Class A Common Stock outstanding and redeemable of 5,750,000. | |
| ● | Assuming maximum redemption: The Merger Agreement includes a $15 million minimum cash condition. This presentation assumes that FoxWayne public stockholders holding approximately 3,771,881 shares of FoxWayne Class A Common Stock will exercise their redemption rights upon consummation of the Business Combination at a redemption price of approximately $10.10 per share, such that the $15 million cash condition is met after giving effect to transaction expenses to be paid upon consummation of the Business Combination. This leads to a total maximum redemption value of $38.1 million. Such amount represents the maximum number of FoxWayne Class A Common Stock redemptions that could occur before the minimum cash condition would not be met. |
| No Redemption Scenario |
Interim Redemption |
Max Redemption Scenario |
||||||||||||||||||||||
| Shares | % | Shares | % | Shares | % | |||||||||||||||||||
| Aerami Equityholders | 25,000,000 | 78 | 25,000,000 | 82 | 25,000,000 | 88 | ||||||||||||||||||
| FoxWayne public stockholders | 5,800,000 | 18 | 3,914,059 | 13 | 2,028,119 | 7 | ||||||||||||||||||
| FoxWayne Sponsor and Directors | 1,437,500 | 4 | 1,437,500 | 5 | 1,437,500 | 5 | ||||||||||||||||||
| Combined Company common stock | 32,237,500 | 100 | % | 30,351,559 | 100 | % | 28,465,619 | 100 | % | |||||||||||||||
The share ownership of the Combined Company common stock set forth above illustrates the dilution our public stockholders will face from the Founder Shares, which will automatically convert upon consummation of the Business Combination into shares of our Class A Common Stock on a one-for-one basis, resulting in the issuance of 1,437,500 shares of common stock of the Combined Company; however, the table does not take into account other outstanding securities that are potentially dilutive if converted into shares of Class A Common Stock in the future, including:
| ● | 291,943 shares of Aerami unvested restricted stock units; | |
| ● | 12,604,309 outstanding Aerami stock options; | |
| ● | 992,992 outstanding Aerami common stock warrants; | |
| ● | 2,800,000 outstanding Private Placement Warrants held by our Sponsor; | |
| ● | 5,750,000 outstanding public warrants held by FoxWayne public stockholders; and | |
| ● | 504,083 outstanding units held by FoxWayne public stockholders. |
Aerami securities are as of September 30, 2021 and do not reflect the effect of conversion as a result of the Business Combination.
Please see the sections titled “Summary of the Proxy Statement/Prospectus — Ownership of the Combined Company After the Closing” and “Unaudited Pro Forma Condensed Combined Financial Information” for further information on the dilutive impact of the Business Combination on our Class A Common Stock.
See the section titled “Comparative Share Information” for information on the impact of redemptions on the value of the shares owned by non-redeeming stockholders.
Deferred Underwriting Fees
The deferred IPO underwriting fee of $0.35 per unit, or approximately $2.0 million in the aggregate, will become payable to the underwriters from the amounts held in the Trust Account solely in the event that we complete a Business Combination pursuant to the terms of the underwriting agreement. Below is a summary of the total deferred underwriting commission to be paid upon closing of the Business Combination, assuming (i) no redemptions, (ii) 33% of the maximum redemptions and (iii) maximum redemptions that may occur and still satisfy the Merger Agreement requirement that FoxWayne have at least $15 million, after payment of underwriting fees and other transaction costs, at the Closing.
| Underwriting Fee (In thousands, except for number of shares) | ||||||||||||
| No Redemptions | Interim Redemptions | Maximum Redemptions | ||||||||||
| Redemptions ($) assuming $10.10 per share | $ | — | $ | 19,048 | $ | 38,096 | ||||||
| Redemptions (Shares) | — | 1,885,941 | 3,771,881 | |||||||||
| Effective Underwriting (Total Underwriting less redemptions) | $ | 57,500 | $ | 38,61 | 19,781 | |||||||
| Total Deferred Fee (%) | 3.5 | % | 3.5 | % | 3.5 | % | ||||||
| Total Deferred Underwriting Fee ($) | $ | 2,013 | $ | 1,352 | $ | 692 | ||||||
| Effective Deferred Underwriting Fee (as a percentage of cash left in Trust Account post redemptions) | 3.5 | % | 2.4 | % | 1.2 | % | ||||||
In connection with the identification and due diligence for potential targets for an Initial Business Combination, the FoxWayne Board consulted EF Hutton, the representative of our underwriters for the IPO. We issued EF Hutton, and/or its designees, 50,000 shares of our Class A Common Stock upon the consummation of our initial public offering. EF Hutton agreed not to transfer such shares until the completion of an Initial Business Combination. In addition, EF Hutton agreed (i) to waive its redemption rights with respect to such shares in connection with the completion of an Initial Business Combination and (ii) to waive its rights to liquidating distributions from our Trust Account with respect to such shares if we fail to complete our Initial Business Combination within a specified period.
| -20- |
| Q: | Why is FoxWayne proposing the amendments to the Charter set forth in the Charter Proposal? |
| A: | FoxWayne is proposing amendments to the Charter to approve certain items required to effectuate the Business Combination and other matters the FoxWayne Board believes are appropriate for the operation of the Combined Company, including provisions for, among other things: (i) increasing the number of authorized shares of the Combined Company’s capital stock, par value $0.0001 per share, from 53,000,000 shares, consisting of (A) 52,000,000 shares of common stock, including 50,000,000 shares of Class A Common Stock and 2,000,000 shares of Class B Common Stock and (B) 1,000,000 shares of Preferred Stock, to 210,000,000 shares, consisting of (1) 200,000,000 shares of Combined Company common stock and (2) 10,000,000 shares of Combined Company preferred stock; (ii) requiring the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal, or adopt any provision inconsistent with Article V pertaining to stockholders; Article VI pertaining to directors; Article VII pertaining to limitation of liability and Article IX pertaining to an amendment to FoxWayne’s bylaws; (iii) providing that any director or the entire FoxWayne Board may be removed from office at any time, but only for cause and only by the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of FoxWayne’s capital stock entitled to vote thereon; (iv) requiring the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend or repeal any provision of the Combined Company’s bylaws; and (v) to make certain other changes that the FoxWayne Board deems appropriate for a public operating company, including (A) eliminating certain provisions relating to an Initial Business Combination that will no longer be applicable to the Combined Company following the Closing, including provisions relating to (1) the Class B Common Stock, (2) redemption rights with respect to Class A Common Stock, (3) the Trust Account, (4) share issuances prior to the consummation of the Initial Business Combination, (5) transactions with affiliates and other blank check companies and (6) the minimum value of the target in the Initial Business Combination and (B) to change the post-combination company’s name to “Aerami Therapeutics Holdings, Inc.” Under the Charter and Delaware law, stockholder approval is required in order to effect the Charter Proposal. See the section titled “Proposal 2— The Charter Proposal” for additional information. |
| Q: | Why is FoxWayne proposing the Nasdaq Proposal? |
| A: | FoxWayne is proposing the Nasdaq Proposal in order to comply with Nasdaq Listing Rules, which require stockholder approval of certain transactions that result in the issuance of 20% or more of a company’s outstanding voting power or shares of common stock outstanding before the issuance of stock or securities. In connection with the Business Combination, we intend to issue an aggregate of approximately 25,000,000 shares of the Combined Company common stock to eligible Aerami security holders and we will reserve shares of the Combined Company common stock for issuance upon the exercise of Aerami options, restricted stock awards and warrants assumed in the Merger. In addition, we will reserve a number of shares that approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination for issuance under the New Equity Incentive Plan. Because FoxWayne expects to issue more than 20% of its outstanding voting power and outstanding common stock in connection with the Business Combination and the New Equity Incentive Plan, which will result in a change in control, FoxWayne is required to obtain stockholder approval of such issuances pursuant to Nasdaq Listing Rules. See the section titled “Proposal 4 — The Nasdaq Proposal” for additional information. |
| Q: | Did the FoxWayne Board obtain a third-party valuation or fairness opinion in determining whether or not to proceed with the Business Combination? |
| A: | No. The FoxWayne Board did not obtain a third-party valuation or fairness opinion in connection with its determination to approve the Business Combination. FoxWayne’s officers and directors have substantial experience in evaluating the operating and financial merits of companies from a wide range of industries and concluded that their experience and backgrounds enabled them to make the necessary analyses and determinations regarding the Business Combination. In addition, FoxWayne’s officers, directors and advisors have substantial experience with mergers and acquisitions. Accordingly, investors will be relying solely on the judgment of the FoxWayne Board in valuing Aerami and assuming the risk that the FoxWayne Board may not have properly valued the business. |
| -21- |
| Q: | What happens if I sell my shares of Class A Common Stock before the special meeting? |
| A: | The record date for the special meeting is earlier than the date that the Business Combination is expected to be completed. If you transfer your shares of Class A Common Stock after the record date, but before the special meeting, unless the transferee obtains from you a proxy to vote those shares, you will retain your right to vote at the special meeting. However, you will not be able to seek redemption of your shares of Class A Common Stock because you will no longer be able to deliver them for cancellation upon consummation of the Business Combination in accordance with the provisions described in this proxy statement/prospectus. If you transfer your shares of Class A Common Stock prior to the record date, you will have no right to vote those shares at the special meeting or seek redemption of those shares. |
| Q: | How has the announcement of the Business Combination affected the trading price of FoxWayne’s units, Class A Common Stock and warrants? |
| A: | On December 6, 2021, the last trading date prior to the publication of the articles speculating about the Business Combination, FoxWayne’s public units, Class A Common Stock and public warrants closed at $10.39, $9.98 and $0.4895, respectively. On February 11, 2022, the trading date immediately prior to the date of this proxy statement/prospectus, FoxWayne’s public units, Class A Common Stock and warrants closed at $10.18, $10.01 and $0.21, respectively. |
| Q: | Following the Business Combination, will FoxWayne’s securities continue to trade on a stock exchange? |
| A: | Yes. We anticipate that, following the Business Combination, our common stock and public warrants will continue trading on Nasdaq under the new symbols “ ” and “ ,” respectively. |
| Q: | What vote is required to approve the Proposals presented at the special meeting? |
| A: | Approval of each of the Transaction Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal, the Additional Proposal and the Adjournment Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon, voting as a single class. The approval of the Charter Proposal and the Bylaw Proposal requires the affirmative vote of a majority of the issued and outstanding shares of Class A Common Stock and Class B Common Stock, voting as a single class. |
| Q: | May the Sponsor or FoxWayne’s directors, officers or any of their respective affiliates purchase public shares in connection with the Business Combination? |
| A: | In connection with the stockholder vote to approve the proposed Business Combination, our Sponsor, directors, officers or any of their respective affiliates may privately negotiate transactions to purchase public shares from stockholders who would have otherwise elected to have their shares redeemed in conjunction with a proxy solicitation pursuant to the proxy rules for a per share pro rata portion of the Trust Account. There is no limit on the number of public shares our Sponsor, directors, officers or any of their respective affiliates may purchase in such transactions, subject to compliance with applicable law and the rules of Nasdaq. Any such privately negotiated purchases may be effected at purchase prices that are in excess of the per share pro rata portion of the Trust Account. However, our Sponsor, directors and officers have no current commitments, plans or intentions to engage in such transactions and have not formulated any terms or conditions for any such transactions. None of the funds in the Trust Account will be used to purchase public shares in such transactions. Our Sponsor, directors or officers will not make any such purchases when they are in possession of any material non-public information not disclosed to the seller of such public shares or during a restricted period under Regulation M under the Exchange Act. Such a purchase could include a contractual acknowledgement that such stockholder, although still the record holder of such public shares, is no longer the beneficial owner thereof and therefore agrees not to exercise its redemption rights, and could include a contractual provision that directs such stockholder to vote such shares in a manner directed by the purchaser. If our Sponsor, directors or officers purchase public shares in privately negotiated transactions from public stockholders who have already elected to exercise their redemption rights, such selling stockholders would be required to revoke their prior elections to redeem their shares. Any such privately negotiated purchases may be effected at purchase prices that are in excess of the per share pro rata portion of the Trust Account. |
| -22- |
| Q: | How many votes do I have at the special meeting? |
| A: | Our stockholders are entitled to one vote at the special meeting for each share of Class A Common Stock or Class B Common Stock held of record as of , 2022, the record date for the special meeting. As of the close of business on the record date, there were outstanding shares of Class A Common Stock, which are held by our public stockholders, and outstanding shares of Class B Common Stock, which are held by our initial stockholders. |
| Q: | What constitutes a quorum at the special meeting? |
| A: | Holders of a majority in voting power of Class A Common Stock and Class B Common Stock issued and outstanding and entitled to vote at the special meeting, virtually present or represented by proxy, constitute a quorum. In the absence of a quorum, the chairman of the meeting has the power to adjourn the special meeting. As of the record date for the special meeting, shares of Class A Common Stock and Class B Common Stock would be required to achieve a quorum. Abstentions will count as present for the purposes of establishing a quorum with respect to each Proposal. |
| Q: | How will the Sponsor and FoxWayne’s directors and officers vote? |
| A: | Our Sponsor, directors and officers have agreed to vote any shares of Class A Common Stock and Class B Common Stock owned by them in favor of the Business Combination. They own approximately % of our issued and outstanding shares of Class A Common Stock and Class B Common Stock, in the aggregate, as of the record date. Please see the subsection titled “The Business Combination — Related Agreements — Support Agreements.” |
| Q: | What interests do the current officers and directors have in the Business Combination? |
| A: | When you consider the FoxWayne Board’s recommendation of the Proposals, you should keep in mind that our directors and officers have interests in the Business Combination that are different from, or in addition to, those of other stockholders generally. Our directors were aware of and considered these interests, among other matters, in evaluating the Business Combination, and in recommending to stockholders that they approve the Business Combination. Stockholders should take these interests into account in deciding whether to approve the Business Combination. See the subsection titled “The Business Combination - Interests of Certain Persons in the Business Combination - Interests of Sponsor and FoxWayne Directors and Officers” for additional information. The FoxWayne Board was aware of and considered these interests, among other matters, in recommending that FoxWayne stockholders vote “FOR” each of the Proposals. These interests include: |
| ● | the fact that our Sponsor is expected to benefit from the completion of an Initial Business Combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to stockholders rather than liquidate; | |
| ● | the fact that our Sponsor paid $2.8 million for its 2,800,000 Private Placement Warrants (valued at $1,148,000, based on a valuation as of December 31, 2021, the most recent date for which a valuation is available) that would expire worthless if an Initial Business Combination is not consummated by April 22, 2022 (which may be extended to July 22, 2022); | |
| ● | the fact that, in order to induce FoxWayne and the underwriters of its initial public offering to enter into an underwriting agreement and to proceed with FoxWayne’s initial public offering, our Sponsor, officers and directors have agreed not to redeem any of the shares of our common stock held by them in connection with a stockholder vote to approve the Business Combination; | |
| ● | the fact that our Sponsor paid an aggregate of $25,000 for the Founder Shares, including 100,000 Founder Shares which were subsequently transferred to our independent directors, and that such securities will have a significantly higher value at the time of the Business Combination, which if unrestricted and freely tradable would be valued at approximately $ , based on the closing price of our Class A Common Stock of $ per share on , 2022, the record date of the special meeting of stockholders | |
| ● | the fact that given the difference in the purchase price our Sponsor paid for the Founder Shares as compared to the price of the units sold in FoxWayne’s initial public offering and the substantial number of shares of the Combined Company Common Stock that our Sponsor will receive upon conversion of the Founder Shares in connection with the Business Combination, our Sponsor and its affiliates may earn a positive rate of return on their investment even if the Combined Company Common Stock trades below the price paid for the units in FoxWayne’s initial public offering and even if FoxWayne’s public stockholders experience a negative rate of return following the closing of the Business Combination; | |
| ● | if the Trust Account is liquidated, including in the event we are unable to complete an Initial Business Combination within the required time period, our Sponsor has agreed to indemnify us to ensure that the proceeds in the Trust Account are not reduced below $10.00 per public share, or such lesser amount per public share as is in the Trust Account on the liquidation date, by the claims of (a) any third party (other than our independent registered public accounting firm) for services rendered or products sold to us or (b) a prospective target business with which we have entered into a letter of intent, confidentiality or other similar agreement or Merger Agreement, but only if such a third party or target business has not executed a waiver of all rights to seek access to the Trust Account; |
| -23- |
| ● | the fact that our independent directors Michael Reavey, Jeff Pavell, Jonathan Hale Zippin, and Sundeep Agrawal, each own 25,000 Founder Shares that were transferred from our Sponsor for their services rendered as directors, which if unrestricted and freely tradeable would be valued at approximately $ , based on the closing price of our Class A Common Stock of $ per share on , 2022 , all of which will become worthless if an Initial Business Combination is not consummated by April 22, 2022 (which may be extended to July 22, 2022); | |
| ● | the fact that our Sponsor, officers and directors will be reimbursed for out-of-pocket expenses incurred in connection with activities on our behalf, such as identifying potential target businesses and performing due diligence on suitable business combinations; and | |
| ● | the fact that we have agreed to pay the Sponsor $10,000 per month for office space, utilities and secretarial and administrative services and such arrangement will terminate upon the Closing; | |
| ● | the fact that Robb Knie, our Chief Executive Officer, Chief Financial Officer and director has made multiple loans to us that total approximately $560,000, and that if an Initial Business Combination is not consummated any loan amounts owed by us will be forgiven except to the extent that we have funds available to pay the loan outside of our Trust Account; | |
| ● | the fact that our Sponsor, officers and directors will lose their entire investment in us if an Initial Business Combination is not completed by April 22, 2022 (which may be extended to July 22, 2022). |
| Q: | What happens if I vote against the Transaction Proposal? |
| A: | Under the FoxWayne Charter, if the Transaction Proposal is not approved and we do not otherwise consummate an alternative business combination by April 22, 2022 (which may be extended to July 22, 2022), we will be required to dissolve and liquidate the Trust Account by returning the then-remaining funds in such account to our public stockholders. Effective January 14, 2022, the FoxWayne Board approved an extension of the time to consummate a Business Combination until April 22, 2022. In the event the Transaction Proposal is not approved by April 22, 2022, we intend to further extend the period of time we have to consummate an Initial Business Combination to July 22, 2022. See the section titled “Does FoxWayne intend to extend the period of time it has to consummate an Initial Business Combination?” |
| Q: | Do I have redemption rights? |
| A: | If you are a holder of public shares, you may elect to have your public shares redeemed for cash at the applicable redemption price per share equal to the quotient obtained by dividing (a) the aggregate amount on deposit in the Trust Account as of two business days prior to the consummation of the Business Combination, including interest not previously released to us to pay our franchise and income taxes, by (b) the total number of then outstanding shares of Class A Common Stock included as part of the units sold in the initial public offering; provided that we will not redeem any public shares to the extent that such redemption would result in FoxWayne having net tangible assets of less than $5,000,001 (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act). A public stockholder, together with any of his, her or its affiliates or any other person with whom it is acting in concert or as a “group” (as defined under Section 13(d)(3) of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its shares or, if part of such a group, the group’s shares, in excess of 15% of the public shares (the “15% threshold”). Unlike some other blank check companies, other than the net tangible asset requirement and the 15% threshold described above, we have no specified maximum redemption threshold and there is no other limit on the amount of public shares that you can redeem. Holders of our outstanding public warrants do not have redemption rights in connection with the Business Combination. At the time of our IPO, our Sponsor, officers and directors agreed to waive their redemption rights with respect to any shares of our common stock they may hold in connection with the consummation of the Business Combination. None of our Sponsor, directors or officers received separate consideration for their waiver of redemption rights. For illustrative purposes, based on the fair value of cash and marketable securities held in the Trust Account as of February 11, 2022 of approximately $58.1 million and 5,800,000 shares of Class A Common Stock outstanding and redeemable as of February 11, 2022, the estimated per share redemption price would have been approximately $10.10. Additionally, shares properly tendered for redemption will only be redeemed if the Business Combination is consummated; otherwise holders of such shares will only be entitled to a pro rata portion of the Trust Account (including interest but net of franchise and income taxes payable) (a) in connection with a stockholder vote to approve an amendment to FoxWayne’s Charter that would affect the substance or timing of our obligation to redeem 100% of our public shares if we have not consummated an Initial Business Combination by April 22, 2022 (which may be extended to July 22, 2022), (b) in connection with the liquidation of the Trust Account or (c) if we subsequently complete a different business combination on or before April 22, 2022 (which may be extended to July 22, 2022). Effective January 14, 2022, the FoxWayne Board approved an extension of the time to consummate a Business Combination until April 22, 2022. In the event the Transaction Proposal is not approved by April 22, 2022, we intend to extend the period of time we have to consummate an Initial Business Combination until July 22, 2022. See the section titled “Does FoxWayne intend to extend the period of time it has to consummate an Initial Business Combination?” |
| Q: | Will how I vote affect my ability to exercise redemption rights? |
| A: | No. You may exercise your redemption rights whether you vote your shares of Class A Common Stock for or against or abstain from voting on the Transaction Proposal or any other Proposal described in this proxy statement/prospectus. As a result, the Business Combination can be approved by stockholders who will redeem their shares and no longer remain stockholders. |
| -24- |
| Q: | How do I exercise my redemption rights? |
| A: | Pursuant to the FoxWayne Charter, we will provide the public holders of public shares with the opportunity to redeem, upon the consummation of the Business Combination, public shares then held by them for cash equal to their pro rata share of the aggregate amount on deposit in the Trust Account that holds the proceeds from our initial public offering, as of two business days prior to the consummation of the Business Combination, including interest net of taxes payable by FoxWayne. Notwithstanding the foregoing redemption rights, a public stockholder, together with any of his, her or its affiliates or any other person with whom he, she or it is acting in concert or as a “group” (as defined under Section 13(d)(3) of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its public shares or, if part of such a group, the group’s public shares, in excess of 15% of the outstanding public shares. In order to determine whether a stockholder is acting in concert or as a “group” (as defined in Section 13(d)(3) of the Exchange Act) with any other stockholder, FoxWayne will require each public stockholder seeking to exercise redemption rights to certify to FoxWayne whether such stockholder is acting in concert or as a group with any other stockholder. Further, the FoxWayne Charter provides that we will only redeem our public shares so long as (after such redemption) our net tangible assets will be at least $5,000,001 (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) either immediately prior to or upon consummation of our Initial Business Combination and after payment of underwriters’ fees and commissions. In addition, the Merger Agreement includes a condition to closing that, unless waived by Aerami, requires FoxWayne have at least $15,000,000 in available cash immediately prior to the Effective Time of the Merger, after taking into account payments required to satisfy redemptions of public shares by public stockholders and the payment of FoxWayne’s transaction costs. |
In order to exercise your redemption rights, you must (a) submit a written request for FoxWayne to redeem all or a portion of your public shares for cash to Continental Stock Transfer & Trust Company, FoxWayne’s transfer agent; and (b) deliver your public shares to Continental Stock Transfer & Trust Company, physically or electronically through DTC prior to 5:00 p.m., Eastern time, on , 2022 (two business days before the special meeting), and submit a request in writing that we redeem your public shares for cash to Continental Stock Transfer & Trust Company, our transfer agent, at the following address:
Continental Stock Transfer & Trust Company
1 State Street, 30th Floor
New York, New York 10004-1561
Attention: Mark Zimkind
Email: mzimkind@continentalstock.com
For illustrative purposes, based on the fair value of cash and marketable securities held in the Trust Account as of February 11, 2022 of approximately $58.1 million and 5,800,000 shares of Class A Common Stock outstanding and redeemable as of February 11, 2022, the estimated per share redemption price would have been approximately $10.10.
Holders of FoxWayne’s outstanding warrants sold in the initial public offering, which are exercisable for shares of Class A Common Stock under certain circumstances, do not have redemption rights in connection with the Business Combination.
At the time of our IPO, our Sponsor, officers and directors agreed to waive their redemption rights for no additional consideration in connection with the consummation of the Business Combination with respect to any shares of our common stock they may hold, and our shares of Class B Common Stock will be excluded from the pro rata calculation used to determine the per share redemption price. As of the record date, our Sponsor, officers and directors and their affiliates own approximately % of our outstanding shares of Class B Common Stock.
If a holder exercises its redemption rights, then such holder will be exchanging its shares of Class A Common Stock for cash and will no longer own shares of Class A Common Stock and will not participate in our future growth, if any. Such a holder will be entitled to receive cash for its public shares only if it properly demands redemption and delivers its shares (either physically or electronically) to our transfer agent in accordance with the procedures described herein. See the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights” for the procedures to be followed if you wish to redeem your shares for cash.
| -25- |
| Q: | What are the U.S. federal income tax consequences of exercising my redemption rights? |
| A: | The U.S. federal income tax consequences of a redemption depend on a holder’s particular facts and circumstances. See the subsection titled “The Business Combination — Material U.S. Federal Income Tax Consequences.” Tax matters are complicated, and the tax consequences of exercising your redemption rights will depend on the facts of your own situation, and we urge you to consult your tax advisors regarding the tax consequences of exercising your redemption rights and to rely solely upon their advice. |
| Q: | If I am a FoxWayne Warrant holder, can I exercise redemption rights with respect to my FoxWayne Warrants? |
| A: | No. The holders of FoxWayne Warrants have no redemption rights with respect to such warrants. |
| Q: | Do I have appraisal rights if I object to the proposed Business Combination? |
| A: | No. There are no appraisal rights available to holders of Class A Common Stock or Class B Common Stock in connection with the Business Combination. |
| Q: | What happens to the funds deposited in the Trust Account after consummation of the Business Combination? |
| A: | If the Transaction Proposal is approved, we intend to use a portion of the funds held in the Trust Account to pay (a) any Transaction Expenses of FoxWayne up to the FoxWayne Transaction Expenses Cap, (b) tax obligations and deferred underwriting discounts and commissions from our initial public offering and (C) for any redemptions of public shares. The remaining balance in the Trust Account will be used for general corporate purposes of the Combined Company. See the section titled “The Business Combination” for additional information. |
| Q: | What happens if the Business Combination is not consummated or is terminated? |
| A: | There are certain circumstances under which the Merger Agreement may be terminated. See the subsection titled “The Business Combination — Termination” for additional information regarding the parties’ specific termination rights. Effective January 14, 2022, the FoxWayne Board approved an extension of the time to consummate a Business Combination until April 22, 2022. In the event the Transaction Proposal is not approved by April 22, 2022 (which may be extended to July 22, 2022), pursuant to FoxWayne’s Charter, we will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter subject to lawfully available funds therefor, redeem 100% of the public shares in consideration of a per-share price, payable in cash, equal to the quotient obtained by dividing (A) the aggregate amount then on deposit in the Trust Account, including interest (net of taxes payable, less up to $50,000 of such net interest to pay dissolution expenses), by (B) the total number of then outstanding public shares, which redemption will completely extinguish rights of the public stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining stockholders and the FoxWayne Board in accordance with applicable law, dissolve and liquidate, subject in each case to FoxWayne’s obligations under the DGCL to provide for claims of creditors and other requirements of applicable law. |
We expect that the amount of any distribution our public stockholders will be entitled to receive upon our dissolution will be approximately the same as the amount they would have received if they had redeemed their shares in connection with the Business Combination, subject in each case to our obligations under the DGCL to provide for claims of creditors and other requirements of applicable law. Holders of our Founder Shares have waived any right to any liquidating distributions with respect to those shares.
In the event of liquidation, there will be no distribution with respect to our outstanding warrants. Accordingly, the warrants will expire worthless.
In the event an Initial Business Combination is not consummated by April 22, 2022, we intend to further extend the period of time we have to consummate an Initial Business Combination. See the section titled “Does FoxWayne intend to extend the period of time it has to consummate an Initial Business Combination?”
| Q: | Does FoxWayne intend to extend the period of time it has to consummate an Initial Business Combination? |
| A: | Our Charter initially required us to consummate an Initial Business Combination by January 22, 2022; however, the Charter provided a right to extend the period of time to consummate an Initial Business Combination up to two times, each by an additional three months (for a total of up to 18 months from the date of the consummation of our initial public offering, or until July 22, 2022), subject to our Sponsor depositing additional funds into the Trust Account. Specifically, our Sponsor or its affiliates or designees, upon five business days advance notice prior to the applicable deadline, must deposit into the Trust Account $143,750 ($0.025 per unit), on or prior to the date of the applicable deadline, for each of the available three month extensions, providing a total possible business combination period of 18 months at a total payment value of $287,500 ($0.025 per unit). Effective January 14, 2022, the FoxWayne Board approved an extension of the time to consummate a Business Combination until April 22, 2022. In the event the Initial Business Combination is not approved by April 22, 2022, we intend to further extend the period of time we have to consummate an Initial Business Combination. |
| -26- |
| Q: | When is the Business Combination expected to be consummated? |
| A: | It is currently anticipated that the Business Combination will be consummated promptly following the special meeting of our stockholders to be held on , 2022, provided that all the requisite stockholder approvals are obtained and other conditions to the consummation of the Business Combination have been satisfied or waived. For a description of the conditions for the completion of the Business Combination, see the subsection titled “The Business Combination — Conditions to Closing of the Merger Agreement.” |
| Q: | What do I need to do now? |
| A: | You are urged to read carefully and consider the information included in this proxy statement/prospectus, including the section titled “Risk Factors” and the annexes attached to this proxy statement/prospectus, and to consider how the Business Combination will affect you as a stockholder. You should then vote as soon as possible in accordance with the instructions provided in this proxy statement/prospectus and on the enclosed proxy card or, if you hold your shares through a brokerage firm, bank or other nominee, on the voting instruction form provided by the broker, bank or nominee. |
| Q: | How do I vote? |
| A: | If you were a holder of record of Class A Common Stock or Class B Common Stock on , 2022, the record date for the special meeting, you may vote with respect to the Proposals online at the virtual special meeting or by completing, signing, dating and returning the enclosed proxy card in the postage-paid envelope provided. If you hold your shares in “street name,” which means your shares are held of record by a broker, bank or other nominee, you should follow the instructions provided by your broker, bank or nominee to ensure that votes related to the shares you beneficially own are properly counted. In this regard, you must provide the record holder of your shares with instructions on how to vote your shares or, if you wish to virtually attend the special meeting and vote online, obtain a proxy from your broker, bank or nominee. |
| Q: | What will happen if I abstain from voting or fail to vote at the special meeting? |
| A: | At the special meeting, we will count a properly executed proxy marked “ABSTAIN” with respect to a particular proposal as present for purposes of determining whether a quorum is present. For purposes of approval, failure to vote or an abstention will have no effect on the Transaction Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal or the Adjournment Proposal. However, a stockholder’s failure to vote (online or by proxy) at the special meeting will have the same effect as a vote AGAINST the Charter Proposal and the Bylaw Proposal. |
| Q: | What will happen if I sign and submit my proxy card without indicating how I wish to vote? |
| A: | Signed and dated proxies received by us without an indication of how the stockholder intends to vote on a proposal will be voted “FOR” each Proposal being submitted to a vote of the stockholders at the special meeting. |
| Q: | If I am not going to attend the virtual special meeting online, should I submit my proxy card instead? |
| A: | Yes. Whether you plan to attend the special meeting or not, please read the enclosed proxy statement/prospectus carefully, and vote your shares by completing, signing, dating and returning the enclosed proxy card in the postage-paid envelope provided. |
| Q: | If my shares are held in “street name,” will my broker, bank or nominee automatically vote my shares for me? |
| A: | No. Under the rules of various national and regional securities exchanges, your broker, bank or nominee cannot vote your shares with respect to non-discretionary matters unless you provide instructions on how to vote in accordance with the information and procedures provided to you by your broker, bank or nominee. We believe the Proposals presented to our stockholders will be considered non-discretionary and therefore your broker, bank or nominee cannot vote your shares without your instruction. Your bank, broker or other nominee can vote your shares only if you provide instructions on how to vote. You should instruct your broker to vote your shares in accordance with directions you provide. |
| -27- |
| Q: | May I change my vote after I have submitted my executed proxy card? |
| A: | Yes. You may change your vote by sending a later-dated, signed proxy card to us at the address listed below so that it is received by us prior to the special meeting or by attending the virtual special meeting online and voting there. You also may revoke your proxy by sending a notice of revocation to us, which must be received prior to the special meeting. |
| Q: | What should I do if I receive more than one set of voting materials? |
| A: | You may receive more than one set of voting materials, including multiple copies of this proxy statement/prospectus and multiple proxy cards or voting instruction forms. For example, if you hold your shares in more than one brokerage account, you will receive a separate voting instruction form for each brokerage account in which you hold shares. If you are a holder of record and your shares are registered in more than one name, you will receive more than one proxy card. Please complete, sign, date and return each proxy card and voting instruction form that you receive in order to cast your vote with respect to all of your shares. |
| Q: | Who can help answer my questions? |
| A: | If you have questions about the Proposals or if you need additional copies of the proxy statement/prospectus or the enclosed proxy card you, should contact our proxy solicitor at: . |
To obtain timely delivery, our stockholders must request the materials no later than five business days prior to the special meeting.
You may also obtain additional information about us from documents filed with the SEC by following the instructions in the section titled “Where You Can Find Additional Information.”
If you intend to seek redemption of your public shares, you will need to send a letter demanding redemption and deliver your shares (either physically or electronically) to our transfer agent at least two business days prior to the special meeting in accordance with the procedures detailed under the question “How do I exercise my redemption rights?”. If you have questions regarding the certification of your position or delivery of your shares, please contact:
Continental Stock Transfer & Trust Company
1 State Street, 30th Floor
New York, New York 10004-1561
Attention: Mark Zimkind
Email: mzimkind@continentalstock.com
| Q: | Who will solicit and pay the cost of soliciting proxies? |
| A: | The FoxWayne Board is soliciting your proxy to vote your shares of Class A Common Stock and Class B Common Stock on all matters scheduled to come before the special meeting. We will pay the cost of soliciting proxies for the special meeting. We have engaged to assist in the solicitation of proxies for the special meeting. We have agreed to pay a fee of $ , plus disbursements. We will reimburse for reasonable out-of-pocket expenses and will indemnify and its affiliates against certain claims, liabilities, losses, damages and expenses. We will also reimburse banks, brokers and other custodians, nominees and fiduciaries representing beneficial owners of shares of Class A Common Stock and Class B Common Stock for their expenses in forwarding soliciting materials to beneficial owners of Class A Common Stock and Class B Common Stock and in obtaining voting instructions from those owners. Our directors and officers may also solicit proxies by telephone, by facsimile, by mail, on the Internet or in person. They will not be paid any additional amounts for soliciting proxies. |
| -28- |
SUMMARY OF THE PROXY STATEMENT/PROSPECTUS
This summary highlights selected information from this proxy statement/prospectus and does not include all of the information that is important to you. To better understand the Business Combination and the Proposals to be considered at the special meeting, you should read this entire proxy statement/prospectus carefully, including the annexes. See also the section titled “Where You Can Find Additional Information.”
Parties to the Business Combination
FoxWayne Enterprises Acquisition Corp.
FoxWayne is a Delaware corporation formed on September 17, 2020 for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination involving FoxWayne and one or more businesses.
FoxWayne’s units, Class A Common Stock and warrants are currently listed on The Nasdaq Capital Market under the symbols “FOXWU,” “FOXW” and “FOXWW,” respectively. In connection with the Closing of the Business Combination, FoxWayne intends to change its name to “Aerami Therapeutics Holdings, Inc.” and has applied to list the shares of common stock of the post-combination company on The Nasdaq Capital Market under the symbol “ .”
The mailing address of FoxWayne’s principal executive office is 1 Rockefeller Plaza, Suite 1039, New York, NY 10020, and its telephone number is (917) 284-8938.
For more information about FoxWayne, see the sections titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations of FoxWayne,” “Information About FoxWayne” and the financial statements of FoxWayne included herein.
Gotham Merger Sub, Inc.
Gotham Merger Sub, Inc. is a Delaware corporation and a wholly owned subsidiary of FoxWayne formed for the purpose of effecting the Business Combination. Merger Sub owns no material assets and does not operate any business.
Aerami Therapeutics Holdings, Inc.
Aerami, a Delaware corporation, is a clinical stage biopharmaceutical company dedicated to developing differentiated inhaled therapies for the treatment of severe respiratory and other chronic diseases where delivery of therapeutic agents via inhalation can provide an important treatment option. Aerami aims to address markets with high unmet needs where it believes inhaled therapies can offer meaningful differentiation over current standards of care and improve patient experience and outcomes. Aerami is developing multiple drug-device combination products which consist of inhalable formulations of drugs and biologics that are currently approved for the same or different indications combined with either its own proprietary inhaler or commercially available inhalers.
Aerami’s lead development program, AER-901, is an inhaled formulation of imatinib delivered via the FOX® high performing handheld nebulizer which we are developing for the treatment of pulmonary arterial hypertension. AER-901 is in a Phase 1 clinical trial, and in 2022 Aerami is targeting initiating the Phase 2 portion of a planned combined Phase 2/3 clinical trial.
Aerami also has a proprietary inhaler as the delivery device for its two other current programs, both of which address diabetes-related conditions. The AFINA™ inhaler, which is powered by a vibrating mesh technology, delivers approximately 80% of the drug to the lungs, which Aerami believes is 2-4 times higher than currently marketed inhalers – enabling efficient delivery of the dose of drug or biologic in a short amount of time. These results are based on silico modeling with computational fluid dynamics (“CFD”) and in vitro testing of device components assessed using an Alberta idealized adult airway to optimize mouthpiece and aerosol path design for dose delivered distal to the trachea, In conducting this study, human factors use testing was designed to determine the ability to perform inspiratory maneuvers with LED guidance within target flow limits. In vivo testing with healthy normal subjects of radiolabeled aerosol compared two breathing patterns for lung deposition efficiency, distribution and subject preference. Aerami believes AFINA™ can be successfully positioned as an alternative to currently marketed inhalers to treat lung diseases and to injectable or oral therapy for the treatment of systemic diseases. Aerami plans to file in 2022 for 510(k) clearance for AFINA™ for use as a general purpose nebulizer in the United States, which Aerami believes will be reviewed as a Class 2 device with no clinical data required based on substantial equivalence to several predicate devices such as iNeb AAD, Innospire Go, AireHealth nebulizer, and Microbase Portable Nebulizer.
AER-501 (soft mist inhaled insulin) is a patent protected, preservative-free, high purity liquid formulation of recombinant human insulin delivered via AFINATM. Aerami believes that clinical results across five Phase 1/2 clinical studies demonstrating the delivery of consistent doses of insulin, dose-linearity, and intra- and inter-subject variability similar to injections support advancing AER-501 to an end-of-Phase 2 meeting with the FDA and into global Phase 3 clinical trials for the treatment of Type 1 diabetes (“T1D”) and Type 2 diabetes (“T2D”). AER-601 (soft mist inhaled exenatide, a leading short-acting glucagon-like peptide analog, or GLP-1) is directed at the treatment of postprandial hyperglycemia. Aerami has completed preclinical development of AER-601, and this program is Phase 1-ready.
Due to the significant size of the diabetes market and the need for a large commercial presence, Aerami plans to seek partners for both of these programs at the appropriate time. Until such time and depending on available funding, Aerami plans to continue advancing the clinical development of AER-501 and AER-601.
Aerami plans to evaluate additional opportunities for combining our AFINATM soft mist inhaler with other drugs or biologics in order to grow our own severe respiratory and chronic disease product pipeline. Aerami also plans to seek out-licensing opportunities to combine our AFINATM device with other companies’ proprietary drugs or biologics for which inhalation could provide significant patient benefits.
The mailing address of Aerami’s principal executive office is 2520 Meridian Parkway, Suite 400, Durham, North Carolina 27713, and its telephone number is 919-589-7495.
For more information about Aerami, see the sections titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Aerami,” “Information About Aerami” and the financial statements of Aerami included herein.
The Business Combination
On December 7, 2021, we entered into the Merger Agreement with Merger Sub, Aerami and the stockholders’ representative. Pursuant to the Merger Agreement, and subject to the terms and conditions contained therein, Merger Sub will merge with and into Aerami, with Aerami surviving the Merger. After giving effect to the Merger, Aerami will become a wholly owned subsidiary of FoxWayne.
Immediately prior to the Effective Time and subject to receipt of the approval of the holders of Aerami Senior Preferred Stock, each share of Aerami Senior Preferred Stock that is issued and outstanding immediately prior to the Effective Time will be converted into shares of Aerami Common Stock at the then-effective conversion rate in accordance with the terms of the Aerami Charter. Following the Conversion, there will be no outstanding shares of Aerami Senior Preferred Stock and each holder of Aerami Senior Preferred Stock will thereafter cease to have any rights with respect to such securities.
| -29- |
At the Effective Time, by virtue of the Merger and without any action on the part of FoxWayne, Merger Sub, Aerami or the holders of any of Aerami’s securities:
| ● | each share of Aerami Common Stock issued and outstanding immediately prior to the Effective Time (including shares of Aerami Common Stock resulting from the Conversion and the automatic cashless exercise of the Placement Agent Warrants), excluding any Cancelled Shares or Aerami Dissenting Shares, issued and outstanding immediately prior to the Effective Time will be cancelled and converted into the right to receive the number of shares of Combined Company common stock equal to the Per Share Common Consideration; |
| ● | each share of Aerami Royalty Stock issued and outstanding immediately prior to the Effective Time will be cancelled and converted into the right to receive the number of shares of Combined Company common stock equal to the Per Share Royalty Series Consideration; |
| ● | all shares of Aerami Capital Stock held in treasury by Aerami will be cancelled without any conversion thereof and no payment or distribution will be made with respect thereto; |
| ● | each share of Merger Sub issued and outstanding immediately prior to the Effective Time will be converted into one share of Aerami, as the wholly owned subsidiary of the Combined Company; |
| ● | each Aerami Warrant that is outstanding and unexercised immediately prior to the Effective Time, whether vested or unvested, which is not terminated by its terms in connection with the Merger, will be assumed by FoxWayne and converted into a warrant to purchase such number of whole shares of Combined Company common stock (rounded up) at the per share exercise price (rounded up to the nearest whole cent) as set forth in the Consideration Schedule. The number of shares of Combined Company common stock into which an Assumed Warrant is exercisable shall be based on the Per Share Common Consideration into which such Assumed Warrant is exercisable, and the per share exercise price shall be ratably adjusted. Immediately prior to the Effective Time, the Placement Agent Warrants will automatically net exercise in full into applicable shares of Aerami Capital Stock pursuant to the terms of the respective warrant agreements; |
| ● | each Aerami Option that is outstanding and unexercised prior to the Effective Time will be assumed by FoxWayne and substituted and converted into an option to purchase such number of whole shares of Combined Company common stock (rounded down) pursuant to the Combined Company’s equity incentive plan equal to the product of the number of shares of Aerami Common Stock subject to such Aerami Option and the Per Share Common Consideration and at an exercise price (rounded up to the nearest whole cent) equal to the quotient of the exercise price of such Aerami Option and the Per Share Common Consideration; |
| ● | each Aerami RSU that is outstanding immediately prior to the Effective Time will be converted into the right to receive a restricted stock unit award for whole shares of Combined Company common stock (rounded down) pursuant to the Combined Company’s equity incentive plan in an amount equal to the product of the number of shares of Aerami Common Stock subject to such Aerami RSUs and the Per Share Common Consideration; and |
| ● | no fractional shares of Combined Company common stock will be issued by virtue of the Merger, and any fractional shares otherwise issuable to a holder of Aerami’s securities (after aggregating all fractional shares of Combined Company common stock that otherwise would be received by such holder) will be rounded down to the nearest whole share of Combined Company common stock. |
At the Effective Time, up to shares of Class A Common Stock from the Aggregate Consideration will be issued to certain Aerami Equityholders in satisfaction of obligations under agreements entered prior to or in connection with the Business Combination as provided in the Consideration Schedule. See “The Business Combination — Payments of Merger Consideration in Satisfaction of Obligations Under Other Agreements” below for more information.
Pursuant to the terms of the Charter, each outstanding share of Class B Common Stock will be converted into one share of Combined Company common stock at the Effective Time and will no longer be outstanding and will cease to exist.
For more information about the Merger Agreement and the Business Combination and other transactions contemplated thereby, see the section titled “The Business Combination.”
| -30- |
Representations and Warranties
The Merger Agreement contains customary representations and warranties of the parties thereto with respect to, among other things, (a) entity organization and qualification, (b) capital structure, (c) authorization to enter into the Merger Agreement and all other agreements contemplated by the Merger Agreement, (d) compliance with Laws and Permits, (e) financial statements and internal controls, (f) absence of certain changes and liabilities, (g) litigation, (h) labor and employee benefit matters, (i) environmental and safety matters, (j) tax matters, (k) property, (l) Intellectual Property, (m) insurance, (n) material contracts, (o) brokers, (p) regulatory compliance and (q) transactions with Affiliates.
Covenants
The Merger Agreement includes customary covenants of the parties with respect to operation of their respective businesses prior to consummation of the Merger and efforts to satisfy conditions to consummation of the Merger. The Merger Agreement also contains additional covenants of the parties, including, among others, covenants providing for FoxWayne and Aerami to cooperate in the preparation of this Registration Statement/proxy statement required to be filed in connection with the Merger and to obtain all requisite approvals of their respective stockholders including, in the case of FoxWayne, approval of the Business Combination, including the Merger, and the adoption and approval of the Merger Agreement, the Proposed Charter and the Proposed Bylaws, the issuance of the Aggregate Consideration under Nasdaq rules and the approval of the New Equity Incentive Plan. FoxWayne has also agreed to include in this proxy statement/prospectus the recommendation of the FoxWayne Board that stockholders approve all of the Proposals to be presented at the special meeting.
Conditions to Closing of the Merger Agreement
Mutual
The respective obligations of each of Aerami, FoxWayne and Merger Sub to consummate the Merger are subject to the satisfaction or waiver (where permissible), at or prior to the Closing of each of the following conditions:
| (i) | the requisite FoxWayne and Aerami stockholder consents shall have been obtained; | |
| (ii) | all consents, registrations, approvals, clearances, Permits and authorizations from Governmental Authorities that are set forth in the Merger Agreement shall have been obtained; | |
| (iii) | no Governmental Authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any Law or Order (whether temporary, preliminary or permanent) that is in effect and restrains, enjoins, makes illegal or otherwise prohibits the consummation of the transactions contemplated by the Merger Agreement and the Ancillary Documents; and | |
| (iv) | this Registration Statement and proxy statement shall have become effective in accordance with the provisions of the Securities Act. No stop order suspending the effectiveness of this Registration Statement or the proxy statement shall have been issued and remain in effect, and no Proceedings for that purpose shall have commenced or be threatened by the SEC. |
FoxWayne and Merger Sub
The obligations of FoxWayne and Merger Sub to consummate the Merger are subject to the satisfaction or waiver, at or prior to the Closing, of additional conditions, including, but not limited to, the following:
| (i) | Aerami’s representations and warranties being true and correct to the extent required pursuant to the Merger Agreement; | |
| (ii) | Aerami having complied with or performed in all material respects with all covenants and obligations required by the Merger Agreement to be complied with or performed by it on or prior to the Closing; |
| -31- |
| (iii) | no Material Adverse Effect having occurred on the Acquired Companies between the date of the Merger Agreement and the Closing; | |
| (iv) | there not being more than 25% of dissenting shares of Aerami Capital Stock; and | |
| (v) | payment of all consulting fees (not to exceed, in the aggregate, $500,000) incurred by FoxWayne in connection with the consummation of the transactions contemplated by the Merger Agreement. |
Aerami
The obligation of Aerami to consummate the Merger is subject to the satisfaction or waiver, at or prior to the Closing of additional conditions, including, but not limited to, the following:
| (i) | the representations and warranties of FoxWayne and Merger Sub being true and correct to the extent required pursuant to the Merger Agreement; | |
| (ii) | each of the covenants and obligations that FoxWayne and Merger Sub is required to comply with or to perform at or prior to the Closing shall have been complied with and performed in all material respects; |
| (iii) | since the date of the Merger Agreement, there shall not have occurred a Material Adverse Effect on FoxWayne; | |
| (iv) | at or prior to the Closing, FoxWayne shall deliver (or cause to be delivered) to Aerami a certificate executed on behalf of FoxWayne by its Chief Executive Officer containing the representation and warranty of FoxWayne that certain conditions set forth in the Merger Agreement have been duly satisfied; | |
| (v) | following payment by FoxWayne to its stockholders who have validly elected to have their shares of Combined Company common stock redeemed for cash pursuant to FoxWayne’s organizational documents as part of the Redemption Offer and after giving effect to the payment of the Transaction Expenses incurred by or on behalf of FoxWayne in accordance with the Merger Agreement, FoxWayne shall have an aggregate amount of cash of at least $15,000,000; | |
| (vi) | FoxWayne shall have made all necessary arrangements with the Trustee to have the funds contained in the Trust Account disbursed or available to FoxWayne, in accordance with the Trust Agreement and the Merger Agreement, contemporaneously with the Closing, and all such funds released from the Trust Account to FoxWayne shall be available to FoxWayne (and, following the Merger, the Combined Company); | |
| (vii) | FoxWayne shall have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act); | |
| (viii) | the Combined Company common stock to be issued pursuant to the Merger Agreement shall be listed or have been approved for listing on Nasdaq, subject only to official notice of issuance thereof; and
| |
| (ix) | the directors and executive officers of FoxWayne set forth in the Merger Agreement shall have been removed from their respective positions or tendered their irrevocable resignations, in each case effective as of the Effective Time. |
Closing
The Closing will occur as soon as reasonably practicable (and, in any event, within three Business Days) after satisfaction or (to the extent permitted by applicable Law) waiver of the conditions set forth in the Merger Agreement or at such other time, date and location FoxWayne and Aerami may agree in writing.
| -32- |
Termination
The Merger Agreement may be terminated at any time prior to the consummation of the Merger by mutual written consent of Aerami and FoxWayne and in certain other limited circumstances, including if the Merger has not been consummated by April 30, 2022 (as such date may be extended pursuant to the Merger Agreement).
Either FoxWayne or Aerami may also terminate the Merger Agreement if (i) certain Proposals fail to receive the requisite vote for approval at the special meeting; (ii) a Governmental Authority issues an Order or takes any other action which restrains, enjoins or otherwise prohibits the Merger or (iii) if (A) any representation or warranty of FoxWayne, Merger Sub or Aerami contained in the Merger Agreement shall fail to be accurate to the degree required by the Merger Agreement or (B) the covenants or obligations of FoxWayne, Merger Sub or Aerami contained in the Merger Agreement shall have been breached in any material respect; provided, however, that if an inaccuracy or breach is curable by the breaching party during the Cure Period, then the non-breaching party may not terminate the Merger Agreement as a result of such inaccuracy or breach prior to the expiration of the Cure Period unless the breaching party is no longer continuing to exercise reasonable best efforts to cure such inaccuracy or breach. FoxWayne may also terminate the Merger Agreement if a Material Adverse Effect shall have occurred with respect to the Acquired Companies, and Aerami may terminate the Merger Agreement if a Material Adverse Effect shall have occurred with respect to FoxWayne and its Subsidiaries, taken as a whole.
Effect of Termination
If the Merger Agreement is terminated, the Merger Agreement will become void, and there will be no liability under the Merger Agreement on the part of any party thereto, except as set forth in the Merger Agreement.
Regulatory Matters
Neither FoxWayne nor Aerami is aware of any material regulatory approvals or actions that are required for completion of the Business Combination. It is presently contemplated that if any additional regulatory approvals or actions are required, those approvals or actions will be sought. There can be no assurance, however, that any such additional approvals or actions will be obtained.
Related Agreements
Aerami Stockholder Support Agreement
Contemporaneously with the execution of the Merger Agreement, certain officers and directors of Aerami delivered Stockholder Support Agreements to FoxWayne. Under the Stockholder Support Agreement terms, such Aerami stockholders agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement. In addition, Aerami agreed to obtain additional Stockholder Support Agreements from certain of its larger stockholders.
Sponsor Support Agreement
Contemporaneously with the execution of the Merger Agreement, the Sponsor delivered the Sponsor Support Agreement pursuant to which, among other things, the Sponsor agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement.
FoxWayne Stockholder Support Agreement
Contemporaneously with the execution of the Merger Agreement, certain officers and directors of FoxWayne delivered FoxWayne Support Agreements, pursuant to which, among other things, such FoxWayne stockholders agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement. In addition, FoxWayne agreed to use its best efforts to obtain additional FoxWayne Support Agreements from certain of its larger stockholders.
| -33- |
Registration Rights Agreement
In connection with the Closing, Aerami, FoxWayne, and certain of their respective stockholders will enter into a Registration Rights Agreement (the “Registration Rights Agreement”). Following the Business Combination, pursuant to the Registration Rights Agreement, the Combined Company will be required to file a registration statement covering the resale of registrable securities held by the stockholders thereto.
Proposed Charter
Pursuant to the terms of the Merger Agreement, immediately prior to the consummation of the Business Combination, FoxWayne shall amend and restate its Charter to, among other things, (i) increase the total number of authorized shares of all classes of capital stock from 53,000,000 shares, consisting of (A) 52,000,000 shares of common stock, par value $0.0001 per share, including (1) 50,000,000 shares of Class A Common Stock and (2) 2,000,000 shares of Class B Common Stock and (B) 1,000,000 shares of Preferred Stock, par value $0.0001 per share, to 210,000,000 shares, consisting of (1) 200,000,000 shares of Combined Company common stock and (2) 10,000,000 shares of Combined Company preferred stock; (ii) require an affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal, or adopt any provisions inconsistent with Article V pertaining to stockholders. Article VI pertaining to directors; Article VII pertaining to limitation of liability and Article IX pertaining to an amendment to the Combined Company’s bylaws; (iii) provide that, subject to the rights of holders of the Combined Company’s preferred stock, the Combined Company Board or any individual directors may be removed only for cause and only by affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote at an election of directors; (iv) make certain other changes that the FoxWayne Board deems appropriate for a public operating company, including (A) eliminating certain provisions relating to an Initial Business Combination that will no longer be applicable to the Combined Company following the Closing, including provisions relating to (1) the Class B Common Stock, (2) redemption rights with respect to Class A Common Stock, (3) the Trust Account, (4) share issuances prior to the consummation of the Initial Business Combination, (5) transactions with affiliates and other blank check companies and (6) the minimum value of the target in the Initial Business Combination and (B) changing the post-combination company’s name to “Aerami Therapeutics Holdings, Inc.”
For more information about the amendments to FoxWayne’s Charter, see the section titled “Proposal 2 — The Charter Proposal.”
Management
Effective immediately after the consummation of the Business Combination, the business and affairs of the Combined Company will be managed by or under the direction of the Combined Company Board. The management team of the post-combination company is expected to be composed of the management team of Aerami. The Combined Company Board will have seven directors, all of which will be designated by Aerami. At the Closing, the executive officers of FoxWayne will resign and the individuals serving as executive officers of the Combined Company immediately after the Closing are expected to be the same individuals (in the same offices) as those of Aerami immediately prior to the Closing. See “Management After the Business Combination” for additional information.
Interests of Certain Persons in the Business Combination
Interests of Sponsor and FoxWayne Directors and Officers
In considering the recommendation of the FoxWayne Board to vote in favor of the Business Combination, stockholders should be aware that, aside from their interests as stockholders, our Sponsor and certain of our directors and officers have interests in the Business Combination that are different from, or in addition to, those of other stockholders generally. These interests may influence FoxWayne’s directors in making their recommendation that you vote in favor of the Business Combination and the transactions contemplated thereby. Our directors were aware of and considered these interests, among other matters, in evaluating the Business Combination and in recommending to stockholders that they approve the Business Combination. The existence of financial and personal interests of one or more of FoxWayne’s directors may result in a conflict of interest on the part of such director(s) between what he, she or they may believe is in the best interests of FoxWayne and its stockholders and what he, she or they may believe is best for himself, herself or themselves in determining to recommend that stockholders vote for the proposals. Stockholders should take these interests into account in deciding whether to approve the Business Combination. These interests include, among other things:
| ● | the fact that our Sponsor paid $2.8 million for its 2,800,000 Private Placement Warrants (valued at $1,148,000, based on a valuation as of December 31, 2021, the most recent date for which a valuation is available) that would expire worthless if a Business Combination is not consummated by April 22, 2022; (which may be extended to July 22, 2022) |
| -34- |
| ● | the fact that our Sponsor, officers and directors have agreed not to redeem any of the shares of our common stock held by them in connection with a stockholder vote to approve the Business Combination; | |
| ● | the fact that our Sponsor paid an aggregate of $25,000 for the Founder Shares, including 100,000 Founder Shares which were subsequently transferred to our independent directors, and that such securities will have a significantly higher value at the time of the Business Combination, which if unrestricted and freely tradable would be valued at approximately $ , based on the closing price of our Class A Common Stock of $ per share on , 2022, the record date of the special meeting of stockholders; | |
| ● | if the Trust Account is liquidated, including in the event we are unable to complete an Initial Business Combination within the required time period, our Sponsor has agreed to indemnify us to ensure that the proceeds in the Trust Account are not reduced below $10.00 per public share, or such lesser amount per public share as is in the Trust Account on the liquidation date, by the claims of (a) any third-party (other than our independent registered public accounting firm) for services rendered or products sold to us or (b) a prospective target business with which we have entered into a letter of intent, confidentiality or other similar agreement or Merger Agreement, but only if such a third party or target business has not executed a waiver of all rights to seek access to the Trust Account; | |
| ● | the fact that our independent directors own an aggregate of 100,000 Founder Shares that were transferred from our Sponsor, which if unrestricted and freely tradeable would be valued at approximately $ , based on the closing price of our Class A Common Stock of $ per share on , 2022; | |
| ● | the fact that the Sponsor will benefit from the completion of a business combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to stockholders rather than liquidate; | |
| ● | the fact that our initial stockholders, including our Sponsor and its affiliates, who purchased Class B Common Stock and Private Placement Warrants at the time of our IPO may experience a positive rate of return on their investment, even if our public stockholders experience a negative rate of return on their investment; | |
| ● | the current FoxWayne charter provides that FoxWayne renounces its interest in any corporate opportunity offered to any director or officer unless such opportunity is expressly offered to such person solely in his or her capacity as a director or officer of FoxWayne and such opportunity is one FoxWayne is legally and contractually permitted to undertake and would otherwise be reasonable for FoxWayne to pursue, and to the extent the director or officer is permitted to refer that opportunity to FoxWayne without violating another legal obligation. We do not believe, however, that the pre-existing fiduciary duties or contractual obligations of our officers and directors will materially undermine our ability to complete the Business Combination, and such pre-existing fiduciary duties and contractual obligations did not materially affect our search for an acquisition target; | |
| ● | the fact that our Sponsor, officers and directors will be reimbursed for out-of-pocket expenses incurred in connection with activities on our behalf, such as identifying potential target businesses and performing due diligence on suitable business combinations; and | |
| ● | the fact that our Sponsor, officers and directors will lose their entire investment in us if an Initial Business Combination is not completed by July 22, 2022. |
Interests of Aerami Directors and Officers
Aerami’s officers and the members of the Aerami Board who will be officers of the Combined Company and members of the Combined Company Board, respectively, have interests in the Business Combination that are different from, or in addition to, those of other Aerami Stockholders generally.
These interests include, among other things, the fact that:
| ● | The following executive officers of Aerami are expected to be appointed as executive officers of the Combined Company and enter into new employment agreements with the Combined Company: Steven Thornton (Chief Executive Officer), Barry Deutsch (Chief Financial Officer), and Timm Crowder (President and Chief Operating Officer); | |
| ● | The following members of the Aerami Board are expected to be appointed as directors of the Combined Company: Steven Thornton, Anne Whitaker, Darlene Deptula-Hicks, John Patton, and Bill Welch; | |
| ● | Mr. Adam Stern, a member of Aerami’s board of directors, is the Head of Private Equity Banking at Aegis Capital Corp., and Aegis Capital Corp. will receive a significant fee upon the consummation of the Business Combination pursuant to the terms of the Financial Advisory Agreement (the “Advisory Agreement”); and | |
| ● | The executive officers of Aerami and members of the Aerami Board are holders of, or are affiliated with entities that are holders of, Aerami equity interests and in such capacity will be entitled to receive the consideration payable in the Business Combination to all holders of such equity interests. |
Interests of EF Hutton
In connection with the identification and due diligence of potential targets for an Initial Business Combination, the FoxWayne Board consulted EF Hutton, the representative of our underwriters for the IPO. EF Hutton will be entitled to receive a deferred underwriting fee as described above under the subsection titled “Deferred Underwriting Fees.”
Reasons for the Approval of the Business Combination
After careful consideration, the FoxWayne Board recommends that our stockholders vote “FOR” the approval of the Transaction Proposal.
For a more complete description of our reasons for the approval of the Business Combination and the recommendation of the FoxWayne Board, see the subsection titled “The Business Combination —FoxWayne Board’s Reasons for the Approval of the Business Combination.”
| -35- |
Redemption Rights
Pursuant to the FoxWayne Charter, we will provide the public holders of public shares with the opportunity to redeem, upon the consummation of the Business Combination, public shares then held by them for cash equal to their pro rata share of the aggregate amount on deposit in the Trust Account that holds the proceeds from our initial public offering, as of two business days prior to the consummation of the Business Combination, including interest net of taxes payable by FoxWayne. Notwithstanding the foregoing redemption rights, a public stockholder, together with any of his, her or its affiliates or any other person with whom he, she or it is acting in concert or as a “group” (as defined under Section 13(d)(3) of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its public shares or, if part of such a group, the group’s public shares, in excess of 15% of the outstanding public shares. In order to determine whether a stockholder is acting in concert or as a “group” (as defined in Section 13(d)(3) of the Exchange Act) with any other stockholder, FoxWayne will require each public stockholder seeking to exercise redemption rights to certify to FoxWayne whether such stockholder is acting in concert or as a group with any other stockholder. Further, the FoxWayne Charter provides that we will only redeem our public shares so long as (after such redemption) our net tangible assets will be at least $5,000,001 (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) either immediately prior to or upon consummation of our Initial Business Combination and after payment of underwriters’ fees and commissions. In addition, the Merger Agreement includes a condition to closing that FoxWayne have at least $15,000,000 in available cash immediately prior to the Effective Time of the Merger, after taking into account payments required to satisfy redemptions of public shares by public stockholders and the payment of FoxWayne’s Transaction Expenses.
Public holders will be entitled to receive cash for redeemable public shares only if you:
| ● | submit a written request for FoxWayne to redeem all or a portion of your public shares for cash to Continental Stock Transfer & Trust Company, FoxWayne’s transfer agent; and | |
| ● | deliver your public shares to Continental Stock Transfer & Trust Company, physically or electronically through DTC. |
For illustrative purposes, based on the fair value of cash and marketable securities held in the Trust Account as of February 11, 2022 of approximately $58.1 million and 5,800,000 shares of Class A Common Stock outstanding and redeemable as of February 11, 2022, the estimated per share redemption price would have been approximately $10.10.
Holders of FoxWayne’s outstanding warrants sold in the initial public offering, which are exercisable for shares of Class A Common Stock under certain circumstances, do not have redemption rights in connection with the Business Combination.
At the time of our IPO our Sponsor, officers and directors agreed to waive their redemption rights for no additional consideration in connection with the consummation of the Business Combination with respect to any shares of our common stock they may hold, and our shares of Class B Common Stock will be excluded from the pro rata calculation used to determine the per share redemption price. As of the record date, our Sponsor, officers and directors and their affiliates own approximately % of our outstanding shares of Class B Common Stock.
If a holder exercises its redemption rights, then such holder will be exchanging its shares of Class A Common Stock for cash and will no longer own shares of Class A Common Stock and will not participate in our future growth, if any. Such a holder will be entitled to receive cash for its public shares only if it properly demands redemption and delivers its shares (either physically or electronically) to our transfer agent in accordance with the procedures described herein. See the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights” for the procedures to be followed if you wish to redeem your shares for cash.
Ownership of the Combined Company After the Closing
We anticipate that, upon completion of the Business Combination, the ownership of the Combined Company will be as follows:
| ● | the Aerami Equityholders will own 25,000,000 shares, or approximately 78%, of the outstanding common stock of the Combined Company; |
| -36- |
| ● | the public stockholders will own 5,800,000 shares, or approximately 18%, of the outstanding common stock of the Combined Company; and | |
| ● | the initial stockholders will own 1,437,500 shares, or approximately 4%, of the outstanding common stock of the Combined Company. |
The number of shares and the interests set forth above (a) assume (i) that no public stockholders elect to have their public shares redeemed and (ii) that there are no other issuances of equity interests of FoxWayne or Aerami, (b) do not take into account FoxWayne Warrants that will remain outstanding following the Business Combination and may be exercised at a later date and (c) do not take into account Aerami Options, Aerami RSUs and Aerami Warrants to be assumed in the Business Combination and that may be exercised at a later date. As a result of the Business Combination, the economic and voting interests of our public stockholders will decrease.
If we assume the interim redemption scenario described under the section titled “Unaudited Pro Forma Condensed Combined Financial Information — Basis of Presentation,” i.e., 1,885,941 shares of Class A Common Stock are redeemed, and the assumptions set forth in the foregoing clauses (a)(ii)–(iv) and (b) remain true, the ownership of the Combined Company upon the Closing will be as follows:
| ● | the Aerami Equityholders will own 25,000,000 shares, or approximately 82% of the outstanding Combined Company common stock; | |
| ● | the public stockholders will own 3,914,059 shares, or approximately 13% of the outstanding Combined Company common stock; and | |
| ● | the initial stockholders will own 1,437,500 shares, or approximately 5% of the outstanding Combined Company common stock. |
If we assume the maximum redemptions scenario described under the section titled “Unaudited Pro Forma Condensed Combined Financial Information — Basis of Presentation,” i.e., 3,771,881 shares of Class A Common Stock are redeemed, and the assumptions set forth in the foregoing clauses (a)(ii), (b) and (c) remain true, the ownership of Combined Company upon the Closing will be as follows:
| ● | the Aerami Equityholders will own 25,000,000 shares of our Class A Common Stock, or approximately 88% of the outstanding Combined Company common stock; | |
| ● | the public stockholders will own 2,028,119 shares of our Class A Common Stock, or approximately 7% of the outstanding Combined Company common stock; and | |
| ● | the initial stockholders will own 1,437,500 shares of our Class A Common Stock, or approximately 5% of the outstanding Combined Company common stock. |
Please see the section titled “Unaudited Pro Forma Condensed Combined Financial Information” for further information.
Expected Accounting Treatment
The Business Combination will be accounted for as a reverse recapitalization under GAAP. Under this method of accounting, FoxWayne will be treated as the “acquired” company for financial reporting purposes. See the subsection titled “The Business Combination — Expected Accounting Treatment.”
Appraisal Rights
Appraisal Rights of FoxWayne Stockholders
Appraisal rights are not available to holders of shares of Class A Common Stock and Class B Common Stock in connection with the Business Combination.
Other FoxWayne Proposals
In addition to the proposal to approve and adopt the Merger Agreement and the Business Combination, our stockholders are being asked to vote on proposals to amend and restate FoxWayne’s Charter to, among other things, (i) increase the number of authorized shares of FoxWayne’s capital stock, par value $0.0001 per share, from 53,000,000 shares, consisting of (a) 52,000,000 shares of common stock, including 50,000,000 shares of Class A Common Stock and 2,000,000 shares of Class B Common Stock and (b) 1,000,000 shares of Preferred Stock, to 210,000,000 shares, consisting of (1) 200,000,000 shares of Combined Company common stock and (2) 10,000,000 shares of Combined Company preferred stock, (ii) make certain other changes that the FoxWayne Board deems appropriate for a public operating company, including (a) eliminating certain provisions relating to an Initial Business Combination that will no longer be applicable to the Combined Company following the Closing, including provisions relating to (1) the Class B Common Stock, (2) redemption rights with respect to Class A Common Stock, (3) the Trust Account, (4) share issuances prior to the consummation of the Initial Business Combination, (5) transactions with affiliates and other blank check companies and (6) the minimum value of the target in the Initial Business Combination and (iii) change the post-combination company’s name to “Aerami Therapeutics Holdings, Inc.” A copy of the Proposed Charter reflecting the proposed amendments is attached to this proxy statement/prospectus as Annex B. For more information about the Charter Proposal, see the section titled “Proposal 2 — The Charter Proposal.”
| -37- |
Our stockholders are also being asked to approve the Proposed Bylaws to, among other things, (i) require stockholders seeking to bring business before a meeting or to nominate candidates for election as directors to provide additional disclosures, (ii) prohibit stockholder action by written consent in lieu of a meeting, (iii) require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend or repeal any provision of the Combined Company’s bylaws; and (iv) lock-up holders of Combined Company common stock issued as consideration pursuant to the Merger Agreement or upon the exercise, exchange, conversion, or settlement of any Exchanged Options, Exchanged RSU Awards or Assumed Warrants until 180 days after the date that the Form S-1 Shelf is declared effective by the SEC, subject to certain exceptions. A copy of the Proposed Bylaws is attached to this proxy statement/prospectus as Annex C. For more information about the Bylaw Proposal, see the section titled “Proposal 3 — The Bylaw Proposal.”
In addition, our stockholders are being asked to vote on a proposal to approve, as required by applicable listing rules of Nasdaq, the issuance of more than 20% of the outstanding capital stock of FoxWayne that represents more than 20% of our outstanding voting power, in connection with the terms of the Merger Agreement and the Business Combination and the New Equity Incentive Plan, which will result in a change of control of FoxWayne. We intend to issue an aggregate of approximately 25,000,000 shares of the Combined Company common stock in connection with the Merger. In addition, we will reserve shares of the Combined Company common stock for issuance upon the exercise of Aerami warrants assumed in the Merger, and we will reserve a number of shares that approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination for issuance under the New Equity Incentive Plan (which number will include the shares of Combined Company common stock to be reserved for issuance upon exercise of the Exchanged Options and Exchanged RSU Awards). For more information about the Nasdaq Proposal, see the section titled “Proposal 4 — The Nasdaq Proposal.”
Moreover, our stockholders are being asked to vote upon and approve (a) a proposal to approve and adopt the New Equity Incentive Plan, (b) a proposal to approve and adopt any other proposals determined by the FoxWayne Board to be necessary or appropriate in connection with the Business Combination or the other Proposals, and (c) a proposal to approve the adjournment of the special meeting to a later date or dates, if necessary or appropriate, to permit further solicitation and vote of proxies in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal or the Additional Proposal. See the sections titled “Proposal 5 — The New Equity Incentive Plan Proposal,” “Proposal 6 — The Additional Proposal,” and “Proposal 7 — The Adjournment Proposal” for more information.
Date, Time and Place of Special Meeting
The special meeting will be held at , Eastern time, on , 2022, via live webcast at the following address: , or such other date, time and place to which such meeting may be adjourned or postponed, to consider and vote upon the Proposals.
Voting Power; Record Date
You will be entitled to vote or direct votes to be cast at the virtual special meeting if you owned shares of Class A Common Stock or Class B Common Stock at the close of business on , 2022, which is the record date for the special meeting. You are entitled to one vote for each share of Class A Common Stock or Class B Common Stock that you owned as of the close of business on the record date. If your shares are held in “street name” or are in a margin or similar account, you should contact your broker, bank or other nominee to ensure that votes related to the shares you beneficially own are properly counted. On the record date, there were shares of Class A Common Stock and Class B Common Stock outstanding, of which were public shares and were Founder Shares, respectively, held by the initial stockholders.
| -38- |
Proxy Solicitation
Proxies may be solicited by mail. We have engaged to assist in the solicitation of proxies. If a stockholder grants a proxy, it may still vote its shares online if it revokes its proxy before the special meeting. A stockholder may also change its vote by submitting a later-dated proxy as described in the subsection titled “Special Meeting of FoxWayne Stockholders — Revoking Your Proxy.”
Quorum and Required Vote for Proposals for the Special Meeting
A quorum of our stockholders is necessary to hold a valid meeting. A quorum will be present at the special meeting if holders of a majority of the shares of our Class A Common Stock and Class B Common Stock entitled to vote thereat attend virtually or are represented by proxy at the special meeting. Abstentions will count as present for the purposes of establishing a quorum.
The approval of the Transaction Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal, and the Adjournment Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon at the special meeting, voting as a single class. The approval of the Charter Proposal and Bylaw Proposal requires the affirmative vote (online or by proxy) of a majority of the issued and outstanding shares of Class A Common Stock and Class B Common Stock, voting as a single class. Accordingly, a stockholder’s failure to vote (online or by proxy) at the special meeting will have no effect on the outcome of any vote on the Transaction Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal or the Adjournment Proposal but will have the same effect as a vote AGAINST the Charter Proposal and the Bylaw Proposal.
The Closing is conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal at the special meeting. The Bylaw Proposal, the New Equity Incentive Plan Proposal and the Additional Proposal are conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal. The Adjournment Proposal is not conditioned on the approval of any other Proposal set forth in this proxy statement/prospectus.
Recommendation to FoxWayne Stockholders
The FoxWayne Board believes that each of the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal, the Additional Proposal and the Adjournment Proposal is in the best interests of FoxWayne and its stockholders and recommends that its stockholders vote “FOR” each Proposal being submitted to a vote of the stockholders at the special meeting. For more information, see the sections titled “Proposal 1 – The Transaction Proposal,” “Proposal 2 — The Charter Proposal,” “Proposal 3 — The Bylaw Proposal,” “Proposal 4 — The Nasdaq Proposal,” “Proposal 5 — The New Equity Incentive Plan Proposal,” “Proposal 6 — The Additional Proposal,” and “Proposal 7 — The Adjournment Proposal.”
When you consider the recommendation of the FoxWayne Board in favor of approval of these Proposals, you should keep in mind that, aside from their interests as stockholders, our Sponsor and certain of our directors and officers have interests in the Business Combination that are different from, or in addition to, your interests as a stockholder. Please see the subsection titled “The Business Combination — Interests of Certain Persons in the Business Combination - Interests of Sponsor and FoxWayne Directors and Officers.”
Summary of Risk Factors
An investment in our securities involves a high degree of risk. In evaluating the Proposals set forth in this proxy statement/prospectus, you should carefully read this proxy statement/prospectus, including the annexes, and especially consider the factors discussed in the section titled “Risk Factors.” This summary should be read in conjunction with the “Risk Factors” section and should not be relied upon as an exhaustive summary of the material risks facing our business and the proposed Business Combination. The occurrence of one or more of the events or circumstances described in the section titled “Risk Factors,” alone or in combination with other events or circumstances, may materially adversely affect our business, financial condition and operating results. Such risks include, but are not limited to:
Risks Related to Aerami’s Business
| ● | Aerami has a limited operating history, has incurred significant operating losses since its inception and expects to incur significant losses for the foreseeable future. Aerami may never generate any revenue or become profitable or, if it achieves profitability, it may not be able to sustain such profitability. |
| -39- |
| ● | Aerami will require substantial additional capital to finance its operations, and a failure to obtain this necessary capital when needed on acceptable terms, or at all, could force Aerami to delay, limit, reduce or terminate its product development, commercialization efforts or other operations. | |
| ● | Aerami depends on the successful development, regulatory approval and commercialization of its product candidates, including, AER-901, AER-501 and AER-601. If Aerami or its collaborators are unable to successfully develop, obtain regulatory approval for and commercialize its product candidates or experience significant delays in doing so, its business will be materially harmed. | |
| ● | Clinical and preclinical development involves a lengthy and expensive process with uncertain outcome, and the results of preclinical studies and early clinical trials are not necessarily predictive of future results. Any of Aerami’s product candidates may not have favorable results in later clinical trials, if any, or receive regulatory approval on a timely basis, if at all. | |
| ● | Any difficulties or delays in the commencement or completion, or termination or suspension, of Aerami’s current or planned clinical trials could result in increased costs to Aerami, delay or limit its ability to generate revenue and adversely affect its commercial prospects. | |
| ● | Aerami may find it difficult to enroll patients in its clinical trials. If Aerami encounters difficulties enrolling subjects in its clinical trials, including as a result of the impact of COVID-19, its clinical development activities could be delayed or otherwise adversely affected. | |
| ● | Aerami relies on third parties to conduct its clinical trials and preclinical studies and to manufacture its product candidates. If these third parties do not successfully carry out their contractual duties, comply with applicable regulatory requirements or meet expected deadlines, Aerami’s development programs and its ability to seek or obtain regulatory approval for its product candidates or commercialize its products may be delayed. | |
| ● | Aerami faces significant competition, and its failure to effectively compete may prevent Aerami from achieving significant market penetration for its product candidates, if approved. | |
| ● | Aerami’s success depends on its ability to protect its intellectual property and its proprietary technologies. |
Risks Related to Becoming a Public Company
| ● | Following consummation of the Business Combination, the Combined Company will incur significant increased costs as a result of operating as a public company, and its management will be required to devote substantial time to new compliance initiatives. |
Risks Related to Combined Company Common Stock
| ● | The market price of Combined Company common stock is likely to be highly volatile, and you may lose some or all of your investment. | |
| ● | Volatility in the Combined Company’s share price could subject the Combined Company to securities class action litigation. |
Risks Related to FoxWayne’s Business and the Business Combination
| ● | FoxWayne’s Sponsor, directors and officers have interests in the Business Combination which may be different from or in addition to (and which may conflict with) the interests of its stockholders, including the fact that they may experience a positive rate of return on their investment, even if our public stockholders experience a negative rate of return on their investment and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to stockholders rather than liquidate. | |
| ● | Our Sponsor is expected to benefit from the completion of a business combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to stockholders rather than liquidate. | |
| ● | FoxWayne will be forced to liquidate the Trust Account if it cannot consummate a business combination by April 22, 2022 (which may be extended to July 22, 2022). In the event of a liquidation, FoxWayne’s public stockholders may receive $10.10 per share and the FoxWayne Warrants will expire worthless. | |
| ● | The fact that given the difference in the purchase price our Sponsor paid for the Founder Shares as compared to the price of the units sold in FoxWayne’s initial public offering and the substantial number of shares of the Combined Company Common Stock that our Sponsor will receive upon conversion of the Founder Shares in connection with the Business Combination, our Sponsor and its affiliates may earn a positive rate of return on their investment even if the Combined Company Common Stock trades below the price paid for the units in FoxWayne’s initial public offering and even if FoxWayne’s public stockholders experience a negative rate of return following the closing of the Business Combination; | |
| ● | If third parties bring claims against FoxWayne, the proceeds held in trust could be reduced and the per-share liquidation price received by FoxWayne stockholders may be less than $10.10. | |
| ● | The FoxWayne Board did not obtain a third-party valuation or fairness opinion in determining whether or not to proceed with the Business Combination. | |
| ● | If FoxWayne’s due diligence investigation of Aerami was inadequate, then stockholders of FoxWayne following the Business Combination could lose some or all of their investment. | |
| ● | The consummation of the Business Combination is subject to a number of conditions; if such conditions are not waived or satisfied, the Merger Agreement could be terminated and the Business Combination would not be completed. | |
| ● | Investors may not have the same benefits as an investor in an underwritten public offering. | |
| ● | The representations and warranties in the Merger Agreement will not survive the completion of the Business Combination, and FoxWayne will have limited recourse in the event the representations and warranties made by Aerami in the Merger Agreement prove to be inaccurate or incorrect. |
Risks Related to the Redemption
| ● | There is no guarantee that a stockholder’s decision whether to redeem their shares for a pro rata portion of the Trust Account will put the stockholder in a better future economic position. | |
| ● | If FoxWayne’s stockholders fail to comply with the redemption requirements specified in this proxy statement/prospectus, they will not be entitled to redeem their shares of Class A Common Stock for a pro rata portion of the funds held in the Trust Account. |
| -40- |
SUMMARY HISTORICAL FINANCIAL INFORMATION OF AERAMI
The summary historical financial information of Aerami as of and for the years ended December 31, 2020 and 2019 was derived from the audited financial statements of Aerami included elsewhere in this proxy statement/prospectus. The summary historical interim financial information of Aerami as of September 30, 2021, and for the nine months ended September 30, 2021 and 2020 was derived from the unaudited financial statements of Aerami included elsewhere in this proxy statement/prospectus and has been prepared on a basis consistent with the audited financial statements.
The following summary historical financial information should be read together with Aerami’s financial statements and accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Aerami” appearing elsewhere in this proxy statement/prospectus. The summary historical financial information in this section is not intended to replace Aerami’s financial statements and the related notes thereto. Aerami’s historical results are not necessarily indicative of the results that may be expected in the future, and Aerami’s results as of and for the nine months ended September 30, 2021 are not necessarily indicative of the results that may be expected for the year ending December 31, 2021, or any other period.
| Nine Months Ended September 30, | Year Ended December 31, | |||||||||||||||
| Statements of Operations | 2021 | 2020 | 2020 | 2019 | ||||||||||||
| (amounts in thousands except share and per share data) | ||||||||||||||||
| Revenues: | ||||||||||||||||
| License | $ | 1,500 | $ | - | $ | - | $ | - | ||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 3,982 | 2,582 | 3,461 | 11,953 | ||||||||||||
| General and administrative | 3,825 | 6,247 | 6,932 | 8,538 | ||||||||||||
| Total operating expenses | 7,807 | 8,829 | 10,393 | 20,491 | ||||||||||||
| Loss from operations | (6,307 | ) | (8,829 | ) | (10,393 | ) | (20,491 | ) | ||||||||
| Other (expense) income: | ||||||||||||||||
| Change in fair value of Harmony contingent settlement liability | 2,053 | 385 | 385 | (551 | ) | |||||||||||
| Change in fair value of Harmony purchase option agreement | 33 | (167 | ) | (201 | ) | (369 | ) | |||||||||
| Change in fair value of warrant liabilities | (545 | ) | - | - | - | |||||||||||
| Inducement charge from exercise of warrants | - | - | - | (2,551 | ) | |||||||||||
| Interest (expense) income, net | (89 | ) | (91 | ) | (100 | ) | 18 | |||||||||
| Loss on foreign exchange | (28 | ) | - | - | - | |||||||||||
| Gain on disposal of equipment | - | 10 | - | - | ||||||||||||
| Total other (expense) income | 1,424 | 137 | 84 | (3,453 | ) | |||||||||||
| Net loss | (4,883 | ) | (8,692 | ) | (10,309 | ) | (23,944 | ) | ||||||||
| Accretion of Series A preferred stock to redemption value | - | - | (221 | ) | - | |||||||||||
| Net loss applicable to common stockholders | $ | (4,883 | ) | $ | (8,692 | ) | $ | (10,530 | ) | $ | (23,944 | ) | ||||
| Per share information: | ||||||||||||||||
| Net loss per share of common stock, basic and diluted | $ | (0.14 | ) | $ | (0.25 | ) | $ | (0.30 | ) | $ | (0.76 | ) | ||||
| Weighted average shares of common stock outstanding, basic and diluted | 34,799,293 | 34,638,020 | 34,638,486 | 31,499,138 | ||||||||||||
| Balance Sheet Data | September 30, 2021 | December 31, 2020 | December 31, 2019 | |||||||||
| Total assets | $ | 15,499 | $ | 4,819 | $ | 7,095 | ||||||
| Total liabilities | 8,690 | 8,180 | 10,590 | |||||||||
| Total Series A and Series A-1 convertible preferred stock | 9,919 | 8,103 | - | |||||||||
| Total stockholders’ deficit | (3,110 | ) | (11,464 | ) | (3,495 | ) | ||||||
| -41- |
SUMMARY HISTORICAL FINANCIAL INFORMATION OF FOXWAYNE
FoxWayne’s balance sheet data as of December 31, 2020 and statement of operations data for the period from September 17, 2020 (inception) through December 31, 2020 are derived from FoxWayne’s audited financial statements included elsewhere in this proxy statement/prospectus. FoxWayne’s balance sheet data as of September 30, 2021 and statement of operations data for the nine months ended September 30, 2021 are derived from FoxWayne’s unaudited condensed financial statements included elsewhere in this proxy statement/prospectus and has been prepared on a consistent basis as the audited financial statements.
The following summary historical financial information should be read together with FoxWayne’s financial statements and accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations of FoxWayne” appearing elsewhere in this proxy statement/prospectus. The summary historical financial information in this section is not intended to replace FoxWayne’s financial statements and the related notes thereto. FoxWayne’s historical results are not necessarily indicative of the results that may be expected in the future.
| As of September 30, 2021 | As of December 31, 2020 | |||||||
| Balance Sheet Data: | ||||||||
| Working capital (deficit) | $ | (352,995 | ) | $ | (131,533 | ) | ||
| Trust account | 58,078,962 | — | ||||||
| Total assets | 58,270,753 | 153,142 | ||||||
| Total liabilities | 6,708,986 | 134.499 | ||||||
| Value of common stock subject to redemption | 58,075,000 | — | ||||||
| Stockholders’ equity (deficit) | (6,513,233 | ) | 18,643 | |||||
| Statement of Operations | For the Period from September 17, 2020 (Inception) through December 31, 2020 | |||
| General and administrative expenses | $ | 5,617 | ||
| Franchise tax expense | 740 | |||
| Net loss | (6,357 | ) | ||
| Weighted average shares outstanding, basic and diluted(1) | 1,250,000 | |||
| Basic and diluted net loss per common share | (0.01 | ) | ||
| (1) | Excludes up to 187,500 shares of Class B common stock subject to forfeiture in the event that the over-allotment option was not exercised in full or in part by the underwriters. Due to the exercise of the over-allotment on January 22, 2021, these shares are no longer subject to forfeiture. |
Statement of Operations
| Nine Months Ended September 30, 2021 | ||||
| Revenues | $ | — | ||
| Loss from operations | (946,119 | ) | ||
| Change in fair value of derivative warrant liabilities | 485,000 | |||
| Financing costs – derivative warrant liabilities | (212,494 | ) | ||
| Income from investments held in Trust Account | 3,962 | |||
| Net income (loss) | $ | (669,651 | ) | |
| Weighted average shares outstanding of Class A common stock | 5,353,846 | |||
| Basic and diluted net income (loss) per share, Class A common stock | (0.10 | ) | ||
| Weighted average shares outstanding of Class B common stock | 1,423,077 | |||
| Basic and diluted net income (loss) per share, Class B common stock | (0.10 | ) | ||
SUMMARY UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION OF FOXWAYNE AND AERAMI
The following summary unaudited pro forma condensed combined financial information for the nine months ended September 30, 2021 and for the year ended December 31, 2020 combines the historical statements of operations of FoxWayne and the historical statements of operations of Aerami, giving effect to the Business Combination and the completion of the private placement of Aerami Common Stock and other events contemplated by the Merger Agreement, as if they had occurred on January 1, 2020. The summary unaudited pro forma condensed combined balance sheet as of September 30, 2021 combines the historical balance sheets of FoxWayne and Aerami, giving effect to the completion of the private placement of Aerami Common Stock in October and November 2021 and the Business Combination as if they had occurred on September 30, 2021. The summary unaudited pro forma condensed combined financial information has been derived from and should be read in conjunction with the unaudited pro forma condensed combined financial information, including the notes thereto, which is included in this proxy statement/prospectus under the section titled “Unaudited Pro Forma Condensed Combined Financial Information.”
The unaudited pro forma condensed combined financial information has been prepared to illustrate the effect of the Business Combination and the completion of the private placement of Aerami Common Stock and other events contemplated by the Merger Agreement and has been prepared for informational purposes only. The unaudited pro forma condensed combined statements of operations are not necessarily indicative of what the actual results of operations would have been had the Business Combination and the completion of the private placement of Aerami Common Stock and other events contemplated by the Merger Agreement taken place on the dates indicated, nor are they indicative of the future consolidated results of operations of the Combined Company. The pro forma adjustments are based on information currently available. Actual results may differ materially from the assumptions within the accompanying unaudited pro forma condensed combined financial information.
The historical financial information has been adjusted to give pro forma effect to the following events that are related and/or directly attributable to the Business Combination and the completion of the private placement of Aerami Common Stock and other events contemplated by the Merger Agreement. The unaudited pro forma condensed combined financial information has been prepared using the assumptions below with respect to the potential redemption of FoxWayne Class A Common Stock into cash:
| ● | Assuming no redemption: This presentation assumes that no public stockholders exercise redemption rights with respect to their public shares. | |
| ● | Assuming interim redemption: This presentation assumes that FoxWayne public stockholders holding approximately 1,885,941 shares of FoxWayne Class A Common Stock, or approximately 33%, out of the 5,750,000 outstanding and redeemable shares of FoxWayne Class A Common Stock will exercise their redemption rights upon consummation of the Business Combination at a redemption price of approximately $10.10 per share (aggregate $19.0 million) to be redeemed out of the FoxWayne trust account. The estimated per share redemption value of $10.10 was calculated by dividing the amount of $58.1 million in the FoxWayne trust account as of September 30, 2021 by the total FoxWayne Class A Common Stock outstanding and redeemable of 5,750,000. | |
| ● | Assuming maximum redemption: The Merger Agreement includes a $15 million minimum cash condition. This presentation assumes that FoxWayne public stockholders holding approximately 3,771,881 shares of FoxWayne Class A Common Stock will exercise their redemption rights upon consummation of the Business Combination at a redemption price of approximately $10.10 per share, such that the $15 million cash condition is met after giving effect to transaction expenses to be paid upon consummation of the Business Combination. This leads to a total maximum redemption value of $38.1 million. Such amount represents the maximum number of FoxWayne Class A Common Stock redemptions that could occur before the minimum cash condition would not be met. |
| Pro Forma | Pro Forma | |||||||||||
| Pro Forma | Combined | Combined | ||||||||||
| Combined | (Assumes | (Assumes | ||||||||||
| (Assumes No | Interim | Maximum | ||||||||||
| (amounts in thousands except share and per share data) | Redemption) | Redemption) | Redemption) | |||||||||
| Summary Unaudited Pro Forma Condensed Combined | ||||||||||||
| Statement of Operations Data for the Nine Months Ended September 30, 2021 | ||||||||||||
| Total revenues | $ | 1,500 | $ | 1,500 | $ | 1,500 | ||||||
| Total operating expenses | $ | 8,753 | $ | 8,753 | $ | 8,753 | ||||||
| Net loss | $ | (5,012 | ) | $ | (5,012 | ) | $ | (5,012 | ) | |||
| Basic and diluted loss per share | $ | (0.05 | ) | $ | (0.05 | ) | $ | (0.05 | ) | |||
| Basic and diluted weighted-average shares outstanding | 101,295,709 | 99,409,768 | 97,523,828 | |||||||||
| Summary Unaudited Pro Forma Condensed Combined | ||||||||||||
| Statement of Operations Data for the Year Ended December 31, 2020 | ||||||||||||
| Total operating expenses | $ | 10,399 | $ | 10,399 | $ | 10,399 | ||||||
| Net loss | $ | (10,315 | ) | $ | (10,315 | ) | $ | (10,315 | ) | |||
| Basic and diluted loss per share | $ | (0.10 | ) | $ | (0.10 | ) | $ | (0.11 | ) | |||
| Basic and diluted weighted-average shares outstanding | 101,294,372 | 99,408,431 | 97,522,491 | |||||||||
| Summary Unaudited Pro Forma Condensed Combined | ||||||||||||
| Balance Sheet Data as of September 30, 2021 | ||||||||||||
| Total assets | $ | 73,665 | $ | 54,617 | $ | 35,569 | ||||||
| Total liabilities | $ | 7,941 | $ | 7,941 | $ | 7,941 | ||||||
| Total equity | $ | 65,724 | $ | 46,676 | $ | 27,628 | ||||||
| -42- |
RISK FACTORS
The following risk factors will apply to our business and operations following the completion of the Business Combination. These risk factors are not exhaustive and investors are encouraged to perform their own investigation with respect to the business, financial condition and prospects of Aerami and our business, financial condition and prospects following the completion of the Business Combination. You should carefully consider the following risk factors in addition to the other information included in this proxy statement/prospectus, including matters addressed in the section titled “Cautionary Note Regarding Forward-Looking Statements.” We may face additional risks and uncertainties that are not presently known to us, or that we currently deem immaterial, which may also impair our business or financial condition. The following discussion should be read in conjunction with the “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Aerami,” the financial statements of Aerami and notes to the financial statements included herein.
Risks Related to Aerami
Risks Related to Aerami’s Business
In this section, unless otherwise stated or the context requires otherwise, the terms “Aerami,” “we,” “us” and “our” refer to Aerami Therapeutics Holdings, Inc. and its subsidiaries prior to the Closing and the Combined Company and its subsidiaries following the Closing.
Risks Related to Our Limited Operating History, Our Financial Position and Capital Requirements
We have incurred significant losses since our inception, and we anticipate that we will continue to incur significant losses for the foreseeable future, which makes it difficult to assess our prospects. We may never achieve or maintain profitability.
We have incurred significant operating losses since our inception in February 2009, do not expect to become profitable in the near future, and may never achieve profitability. Our net losses were approximately $10.3 million and $23.9 million for the years ended December 31, 2020 and 2019, respectively and approximately $4.9 million for the nine months ended September 30, 2021. As of September 30, 2021, we had an accumulated deficit of approximately $130.2 million. Our cash and cash equivalents at September 30, 2021 were approximately $14.4 million. The majority of our operating losses have resulted from expenses incurred in connection with the development of our product candidates and general and administrative costs associated with our operations. Our product candidates will require substantial additional development time and resources before we would be able to apply for or receive regulatory approvals and begin generating revenue from product sales. We also do not yet have a sales organization or commercial infrastructure and, accordingly, we will incur significant expenses to develop a sales organization or commercial infrastructure in advance of generating any commercial product sales. As a result, we expect to continue to incur losses for the foreseeable future, and we anticipate these losses will increase as we continue to develop our product candidates through clinical trials and regulatory submissions. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are unable to predict the extent of any future losses or when we will become profitable, if at all. Even if we do become profitable, we may not be able to sustain or increase our profitability. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ deficit and working capital.
To become and remain profitable, we must succeed in developing and eventually commercializing products that generate significant revenue. This will require us to be successful in a range of challenging activities, including completing clinical trials and preclinical studies of our product candidates, obtaining regulatory approval for these product candidates, and manufacturing, marketing, and selling any products for which we may obtain regulatory approval. In addition, we plan to pursue a 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for our AFINA™ device, which we believe will be reviewed as a Class 2 device with no clinical data required based on substantial equivalence to several predicate devices such as iNeb AAD, Innospire Go, AireHealth nebulizer, and Microbase Portable Nebulizer. We are only in the preliminary stages of most of these activities. We may never succeed in these activities and, even if we do, may never generate revenue that is significant enough to achieve profitability. In addition, we have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical industry. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or if we will be able to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable may have an adverse effect on the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our product candidates or even continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
| -43- |
We are a clinical stage biopharmaceutical company with a limited operating history and no products approved for commercial sale.
We are a clinical stage biopharmaceutical company, and we have only a limited operating history upon which you can evaluate our business and prospects. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We have no products approved for commercial sale at this time and therefore have never generated any revenue from product sales, and we do not expect to generate such sales in the foreseeable future. In addition, we have incurred losses in each year since our inception. We have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical industry. In 2019, we completed our Phase 1/2a clinical trials for AER-501, and we commenced in 2021 a Phase 1 trial for AER-901, our inhaled imatinib program. We target initiating activities on the Phase 2 portion of our planned combined Phase 2/3 trial on AER-901 in 2022, pending the findings from our ongoing Phase 1 trial. We have limited experience as a company conducting clinical trials, submitting applications for regulatory approvals, such as a new drug application (“NDA”), a biologics license application (“BLA”) or a 510(k) clearance, or commercializing any products.
We will require substantial additional capital to finance our operations, and a failure to obtain this necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our development programs or other operations.
As a clinical stage company solely engaged in research and development activities, our operations have consumed substantial amounts of cash since our inception. We expect our expenses to increase in connection with our ongoing activities, particularly as we conduct our ongoing and planned clinical trials of AER-901 and pursue a 510(k) regulatory submission for our AFINA™ inhaler. If we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution. Because the outcome of any clinical trial or preclinical study is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our product candidates. Furthermore, following the completion of the Business Combination, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
Based upon our current operational plan and prior to the Business Combination, we believe our existing cash and cash equivalents as of September 30, 2021 and the $6.4 million in net proceeds received in October and November from our private placement of Aerami Common Stock will be sufficient to fund our operations though at least August 2022. We have based this estimate on assumptions that may prove to be imprecise, and we may exhaust our available capital resources sooner than we currently expect. For example, we may have adverse results if we move our product candidates through the regulatory and development processes. These events may increase our development costs more than we expect and could have a material adverse effect on our financial condition, business and results of operations. We plan to pursue additional financing to fund the continued development of our product candidates, including, but not limited to, cash proceeds resulting from the proposed Business Combination. Our operating plans and other demands on our cash resources may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings or other capital sources, including potential collaborations, licenses and other similar arrangements. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. Attempting to secure additional financing may divert our management from our day-to-day activities, which may adversely affect our ability to develop our product candidates.
| -44- |
Our future funding requirements, both short and long-term, depend on many factors, including:
| ● | the type, number, scope, progress, expansions, results, costs and timing of our clinical trials and preclinical studies of our product candidates which we are pursuing or may choose to pursue in the future; | |
| ● | our ability to in-license additional product candidates; | |
| ● | our ability to obtain 510(k) clearance and/or a CE mark for our AFINA™ device; | |
| ● | our ability to enter into partnering arrangements with respect to certain of our product candidates; | |
| ● | the outcome, timing and cost of seeking and obtaining regulatory approvals from the FDA, and/or any foreign regulatory authority, including the potential for such authorities to require that we perform more studies than those that we currently expect, or to make our product candidates satisfy higher specification standards than we currently plan to achieve; | |
| ● | the costs and timing of manufacturing for our product candidates, including commercial manufacturing if any of our product candidates is approved; | |
| ● | the cost and timing of establishing sales, marketing and distribution capabilities for our product candidates, if we receive regulatory approvals; | |
| ● | the cost to establish, maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make in connection with licensing, preparing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; | |
| ● | our efforts to enhance operational systems and hire additional personnel to satisfy our obligations as a public company, including enhanced internal controls over financial reporting; | |
| ● | the costs associated with hiring additional personnel and consultants as our clinical and preclinical activities increase; | |
| ● | our ability to achieve sufficient market acceptance, coverage and adequate reimbursement from third-party payors and adequate market share and revenue for any approved products; | |
| ● | patients’ willingness to pay out-of-pocket for any approved products in the absence of coverage and/or adequate reimbursement from third-party payors; | |
| ● | any delays and cost increases that result from the COVID-19 pandemic or future epidemic diseases; | |
| ● | the terms and timing of establishing and maintaining collaborations, licenses and other similar arrangements; and | |
| ● | costs associated with any products or technologies that we may in-license or acquire. |
Conducting clinical trials and preclinical studies is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain regulatory approval and commercialize our product candidates. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenue, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through equity offerings, debt financings, or other capital sources, including potential additional collaborations, licenses and other similar arrangements. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Any future debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Such restrictions could adversely impact our ability to conduct our operations and execute our business plan.
| -45- |
If we raise funds through additional collaborations, licenses and other similar arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us and/or that may reduce the value of our common stock. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed or on terms acceptable to us, we would be required to delay, limit, reduce, or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Our auditor has expressed substantial doubt about our ability to continue as a “going concern.”
The audit report on our financial statements for the year ended December 31, 2020 included an emphasis of matter paragraph related to recurring losses and cash outflows from operations and our dependence on additional financing to continue as a going concern. For the year ended December 31, 2020, we incurred a net loss of $10.3 million and, as of December 31, 2020, we had an accumulated deficit of $125.3 million. As of September 30, 2021, we incurred a net loss of $4.9 million and an accumulated deficit of $130.2 million. Further, we have no products approved for sale and we have incurred and expect to continue to incur significant costs in pursuit of financing and in connection with the proposed Business Combination. These factors, among others, raise substantial doubt about our ability to continue as a going concern. In light of these matters, our ability to continue as a going concern is dependent upon our ability to raise additional debt or equity financing, including through the Business Combination, or to enter into strategic partnerships. However, we may not be able to secure additional financing or enter strategic partnerships in a timely manner or on favorable terms, if at all.
If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements, and it is likely that our stockholders will lose all or a part of their investment in us. Future financial statements may also include statements expressing substantial doubt about our ability to continue as a going concern. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at all.
We have identified material weaknesses in our internal control over financial reporting and may identify additional material weaknesses or significant deficiencies in the future or otherwise fail to maintain an effective system of internal control, which may result in material misstatements of our consolidated financial statements and impair our ability to produce accurate financial statements or prevent fraud.
We have previously identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. In connection with the preparation of our audited financial statements for the years ended December 31, 2019 and December 31, 2020, management identified material weaknesses that existed in internal controls over financial reporting. For the year ended December 31, 2019, there were certain adjustments identified during the audit, specifically around accounting for stock-based compensation and accounting for a warrant tender transaction, which were required in order for the 2019 financial statements to be presented in accordance with GAAP. We identified the related control findings to be, in the aggregate, a material weakness that was caused by a historical lack of personnel with requisite skills to perform adequate technical reviews over complex or significant non-routine accounting matters, including derivative accounting. We also identified that certain personnel had full “administrative” rights within our accounting system, while also serving as the payroll administrator for all payroll functions. These types of rights and duties are not appropriate for individuals that have financial reporting responsibilities and should be restricted. As such, we have evaluated the related segregation of duties finding as a material weakness and these segregation of duties findings continued into 2020, resulting in a material weakness at December 31, 2020. In an effort to remediate the material weaknesses, in 2020, we shifted the administrative rights to the accounting system to an employee who does not have financial reporting responsibilities. While these actions represent important steps toward remediation, we will need to hire additional staff to fully address this material weakness, which we will not be able to do absent additional financial resources. Although we have made efforts to remediate the material weaknesses, we cannot assure you that our efforts will be successful or that additional material weaknesses or significant deficiencies will not be identified in the future. If we are unable to maintain proper and effective internal controls, we may not be able to produce timely and accurate financial statements and prevent fraud.
| -46- |
We are and have been past due on certain of our obligations, and we plan to settle certain outstanding obligations using merger consideration such that our stockholders’ interest in the Combined Company would be diluted. If not settled, certain of these obligations will result in continuing rights to acquire shares in Combined Company’s wholly owned subsidiary.
Pursuant to the Registration Rights Agreement dated November 30, 2017 (the “2017 Registration Rights Agreement”) between us and certain of our investors, we have accrued liabilities to such investors as a result of our failure to file a registration statement within 180 days of the final closing of a private placement offering commenced in 2017 (the “2017 Private Placement”). This liability accrued at the rate of 0.5% per month of the purchase price per unit held by the investors that are parties to the agreement, up to a maximum of 6.0%, plus interest at a rate of 2% per annum, accruing daily, for any payments not timely made. As of February 11, 2022, we have calculated this liability to be approximately $2.7 million. We plan to satisfy this obligation in connection with the Business Combination such that the parties to the 2017 Registration Rights Agreement would receive merger consideration with an aggregate value equal to the amount of the liability. However, the parties to the 2017 Registration Rights Agreement holding a majority of our shares subject to that agreement must consent to payment in the form of merger consideration and termination of the agreement, and no assurance can be given that the requisite consent will be obtained. In addition, we have entered into a Financial Advisory Agreement (the “Advisory Agreement”) with Aegis Capital Corp. (“Aegis”) under which a fee will be due in connection with the Business Combination. As permitted under that agreement, we plan to satisfy that obligation by directing the issuance of a portion of the Aggregate Consideration to Aegis.
We also plan to settle certain obligations to Aegis under the placement agent agreement entered in connection with our 2017 Private Placement relating to the payment of future royalties with an allocation of a portion of the Aggregate Consideration. We will need to obtain Aegis’ agreement to this arrangement.
In each instance, the allocation of a portion of the Aggregate Consideration to settle these obligations allows us to preserve our limited cash resources for the conduct of our product development and operational activities.
We may also seek to allocate a portion of the Aggregate Consideration (or issue shares of Aerami Common Stock prior to the effectiveness of the Business Combination) to address other contractual commitments relating to our stock that are not triggered by the Business Combination, but that we may deem to be in the best interests of our stockholders to terminate in connection with the Business Combination. For example, we have entered into certain agreements with Harmony Plus Holdings Limited and certain related stockholders (collectively, “Harmony Plus”), that obligate us to issue shares of Aerami Common Stock valued at $8.0 million upon the earlier of our initial public offering or a change of control, as well as to pay certain royalties and fees and provide board observation rights. While the Business Combination does not trigger the issuance of shares of Aerami Common Stock under these agreements, Harmony Plus would still be entitled to the issuance of the shares in the event of an initial public offering or change of control in Aerami as a subsidiary of the Combined Company and would be entitled to the continuation of other rights pursuant to those agreements. To address potential future uncertainty as to the capital structure of Aerami following the Closing, we are currently in discussions with Harmony Plus to pursue a mutually-agreeable resolution that would eliminate some or all of these obligations following the Business Combination in exchange for an allocation of a portion of the Aggregate Consideration, but there can be no assurance that we will reach agreement, in which case Aerami’s obligations to Harmony Plus would remain outstanding after the Closing.
The issuance of shares of Aerami Common Stock or allocation of Aggregate Consideration in any of these instances will reduce the amount of Aggregate Consideration payable to our current holders of capital stock and dilute their interests in the Combined Company, as the amount of Aggregate Consideration to be issued in the Business Combination is fixed at 25,000,000 shares of Combined Company common stock.
| -47- |
Risks Related to our Business Operations and Commercialization of our Product Candidates
A pandemic, epidemic, or outbreak of an infectious disease, such as COVID-19, may materially and adversely affect our business and our financial results and could cause a disruption to the development of our product candidates.
Public health crises such as pandemics or similar outbreaks could adversely impact our business. In December 2019, a novel strain of a virus named SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), or coronavirus, which causes COVID-19, surfaced in Wuhan, China and has spread worldwide. The COVID-19 pandemic continues to evolve and, to date, has led to the implementation of various responses, including government imposed quarantines, travel restrictions and other public health safety measures. The continued spread of COVID-19 and its variants globally could adversely impact our clinical trial activities, including our ability to recruit and retain patients and subjects, principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19. Similar to other biopharmaceutical companies, we have experienced delays in the recruitment of subjects in our Phase 1 clinical trial for AER-901 in Australia related to COVID-19 and we may experience further delays in enrolling participants in our clinical trials in the future. The extent to which COVID-19 (including its variants) and the global efforts to contain its spread will impact our business, including our operations, preclinical studies, clinical trials, and financial condition, will depend on future developments, which are highly uncertain and cannot be predicted at this time, and include the duration, severity, and scope of the pandemic and the actions taken by other parties, such as governmental authorities, to contain and treat COVID-19. If we or any future third parties with whom we partner (including manufacturers, vendors, strategic partners, clinical trial sites, and contract research organizations (“CROs”)), or the FDA or other health authorities, experience delays or other disruptions associated with the COVID-19 pandemic, our ability to conduct our business and operations could be materially and adversely affected, which could prevent or delay our ability to continue development of our product candidates and ultimately of reviews and approvals of our product candidates.
We have entered, and may in the future seek to enter, into collaborations, licenses and other similar arrangements and may not be successful in doing so, and even if we are, we may not realize the benefits of such relationships. If we are unable to establish or maintain licensing and collaboration arrangements with third-party commercial partners on acceptable terms, or at all, we may not be able to develop and commercialize our inhalation technology or our product candidates.
We have entered, and may in the future seek to enter, into collaborations, joint ventures, licenses and other similar arrangements for the development or commercialization of our inhalation technology and product candidates, due to capital costs required to develop or commercialize the inhalation technology and/or product candidate. For example, we are pursuing strategic partners to develop and commercialize AER-501 and AER-601 in key markets and intend to explore the out-license of AFINA™ to third parties for use with their own products, which may be dependent on our ability to have 510(k) clearance and a CE Mark. We have also signed an exclusive license and development agreement with Hangzhou Chance Pharmaceuticals Co., Ltd. (“Chance”) to develop and commercialize AER-901 for the treatment of pulmonary arterial hypertension (“PAH”) in mainland China, Hong Kong, Macau and Taiwan, but there is no guarantee that their development program will be successful or that they will achieve regulatory approval to commercialize it. We may not be successful in our efforts to establish future collaborations for our inhalation technology and product candidates because our research and development pipeline may be insufficient, our product candidates may be deemed to be at an inappropriate stage of development for collaborative effort or third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy or significant commercial opportunity. In addition, we face significant competition in seeking appropriate strategic partners, and the negotiation process can be time consuming and complex. Further, any future collaboration agreements may restrict us from entering into additional agreements with other potential collaborators. For example, under our amended and restated joint development agreement (the “Phillips JDA”) with Phillips-Medisize Corporation (“Phillips”) dated June 5, 2020, we have agreed to work exclusively with Phillips and its affiliates for an initial five-year term for the development and manufacturing of our AFINA™ device and the development of integrated connected health technologies for our AFINA™ device, as well as any other device we may acquire and any device that we may in-license that would require material development or refinement to be usable or commercially feasible for use with our formulations, other than the devices in-licensed from Vectura Limited (“Vectura”). We cannot be certain that, following a strategic transaction or license, we will achieve an economic benefit that justifies the transaction.
The terms of any additional collaborations or other arrangements that we may establish may not be favorable to us. For example, potential future collaborations may be terminable by our strategic partners, and we may not be able to adequately protect our rights under these agreements. In addition, strategic partners may negotiate for certain rights to control decisions regarding the development and commercialization of our product candidates, if approved, and may not conduct those activities in the same manner as we do. Termination of any collaborations or any delay in entering into collaborations related to our product candidates could delay the development and commercialization of our product candidates and reduce their competitiveness if they reach the market, which could have a material adverse effect on our business, financial condition and results of operations.
| -48- |
Our collaboration and licensing arrangements may not be successful.
Our collaboration and licensing arrangements, as well as any future collaboration and licensing arrangements that we may enter into, may not be successful. The success of our collaboration and licensing arrangements will depend heavily on the efforts and activities of our collaborators, which are not within our control. We may, in the course of our collaboration and licensing arrangements, be subject to numerous risks, including, but not limited to, the following:
| ● | our collaborators may have significant discretion in determining the efforts and resources that they will contribute; | |
| ● | our collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial, abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; | |
| ● | our collaborators may independently, or in conjunction with others, develop products that compete directly or indirectly with our product candidates; | |
| ● | we may grant exclusive rights to our collaborators that would restrict us from collaborating with others; | |
| ● | our collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability; | |
| ● | disputes may arise between us and our collaborators, which may cause a delay in or the termination of our research, development or commercialization activities; | |
| ● | our collaboration and licensing arrangements may be terminated, and if terminated, may result in our need for additional capital to pursue further product candidate development or commercialization; | |
| ● | our collaborators may own or co-own certain intellectual property arising from our collaboration and licensing arrangements with them, which may restrict our ability to develop or commercialize such intellectual property; and | |
| ● | our collaborators may alter the strategic direction of their business or may undergo a change of control or management, which may affect the success of our collaboration arrangements with them. |
We may be unable to continually develop or in-license a pipeline of product candidates, which could affect our business and prospects.
A key element of our long-term strategy is to continually develop or in-license a pipeline of proprietary inhaled drug-device combination product candidates. If we are unable to identify new biologics and small molecules with established proof of concept trials for our targeted indications that we can reformulate for use with our inhalation technology or otherwise expand our product candidate pipeline, whether through licensed or co-development opportunities, and obtain marketing approval for such product candidates within the timeframes that we anticipate, or at all, our business and prospects may be materially and adversely affected.
Because we have multiple product candidates in our pipeline, we may expend our limited resources to pursue a particular product candidate and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we have focused on specific product candidates and development programs, including the development of AER-901. However, we have multiple product candidates for which we may be able to conduct clinical trials over the next several years, which may make our decision as to which product candidates to focus on more difficult. As a result, we may forgo or delay pursuit of opportunities with other product candidates that could have had greater commercial potential. For example, we plan to seek to out-license our product candidates for diabetes. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through future collaborations, licenses and other similar arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
| -49- |
Additionally, we may pursue additional in-licenses or acquisitions of development-stage assets or programs, which entails additional risk to us. Identifying, selecting and acquiring promising product candidates requires substantial technical, financial and human resources expertise. Efforts to do so may not result in the actual acquisition or license of a particular product candidate, potentially resulting in a diversion of our management’s time and the expenditure of our resources with no resulting benefit. For example, if we are unable to identify programs that ultimately result in approved products, we may spend material amounts of our capital and other resources evaluating, acquiring and developing products that ultimately do not provide a return on our investment.
If the market opportunities for our products are smaller than we believe they are, our revenue may be adversely affected, and our business may suffer.
The precise incidence and prevalence for the conditions we aim to address with our product candidates are unknown. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations or market research, and may prove to be incorrect. Further, new trials may change the estimated incidence or prevalence of these diseases. The total addressable market across all of our product candidates will ultimately depend upon, among other things, the diagnosis criteria included in the final label for each of our product candidates approved for sale for these indications, the availability of alternative treatments and the safety, convenience, cost and efficacy of our product candidates relative to such alternative treatments, acceptance by the medical community and patient access, drug pricing and reimbursement. The number of patients in the United States and other major markets and elsewhere may turn out to be lower than expected, patients may not be otherwise amenable to treatment with our products or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business.
We may not succeed in establishing and maintaining collaborative relationships for AER-501, AER-601 and AER-901, which may significantly limit our ability to develop and commercialize them successfully, if at all.
In October 2017, we entered into a joint venture agreement with Tonghua Dongbao Pharmaceutical Co., Ltd. (“Dongbao”) and Shanghai Dongbao Biological Pharmaceutical Co., Ltd. (“Shanghai Dongbao”) for the purpose of (i) completing clinical trials in China for AER-501, (ii) gaining The National Medical Products Administration (“NMPA”) approval for AER-501 and (iii) commercializing AER-501 in China. All parties agreed not to carry out any business that would compete with this joint venture or invest into or act on behalf of a third party directly or directly competing with the joint venture. However, the joint venture was not formed or funded and never commenced operations. We may seek to progress the development of AER-501 by partnering with Dongbao and/or other potential third parties. In January 2021, we signed an exclusive license and development agreement with Chance to develop and commercialize AER-901 for the treatment of PAH in mainland China, Hong Kong, Macau and Taiwan. We plan to seek to progress the development of AER-601 by partnering with one or more potential third parties. There can be no assurance that we will be able to reach an agreement with any third party with respect to any of our product candidates or activate the joint venture with Dongbao with respect to AER-501 to pursue development of these product candidates.
We have entered into agreements with certain of our stockholders, which contain provisions that could restrict our operations, require us to make substantial royalty payments, and issue additional shares of Aerami Common Stock.
We have entered into certain agreements with Harmony Plus that obligate us to issue shares of Aerami Common Stock under certain circumstances, as well as to pay certain royalties and fees and provide board observation rights. The Business Combination does not trigger the issuance of any shares of Aerami Common Stock under these agreements; however, following the Business Combination, Harmony Plus would still be entitled to the issuance of shares of Aerami, as a subsidiary of the Combined Company, in the event of its initial public offering or a change of control. While we are currently negotiating with Harmony Plus to seek the termination of these obligations in connection with the Business Combination, we cannot assure you that we will be able to terminate these obligations, in which case they would remain in place with the wholly-owned subsidiary of the Combined Company after the Closing.
| -50- |
In addition, under our license, supply and manufacturing agreement with Hikma Pharmaceuticals LLC (“Hikma”), we granted an exclusive license to Hikma to register and commercialize AER-501 in all disease indications in the Middle East and Africa, excluding Turkey and certain other countries (the “Hikma Territory”). In addition, we granted Hikma a right of first refusal to license new inhaled insulin products in the Hikma Territory. However, since we failed to meet the agreed-upon timeframe for regulatory approval of AER-501, Hikma is entitled to receive an additional exclusive license in the Hikma Territory to another one of our products in development, of Hikma’s choosing, with identical terms to the current license (excluding the upfront license fee). In addition, Hikma has the right to convert an amount equal to the original upfront license fee under that agreement into shares of Aerami’s capital stock on terms equal to our most recent or current private or public financing, which Hikma has elected to exercise effective immediately prior to the Effective Time of the Business Combination.
We have also entered into a side letter agreement with Molex Ventures (the “Molex Side Letter”) in connection with its investment in Aerami Series A Preferred Stock in June 2020. Pursuant to the Molex Side Letter, we agreed to use the proceeds from Molex’s investment to acquire a global license to develop and commercialize inhaled imatinib for the treatment of PAH and to support a 510(k) regulatory submission in the United States seeking premarket clearance for our AFINA™ soft mist inhaler pursuant to an agreed upon timeline. Since we failed to meet certain timing requirements set forth in the Molex Side Letter with respect to the 510(k) submission, pursuant to the Molex Side Letter, Molex Ventures has the right to cause us to repurchase the 1,000 shares of Aerami Series A Preferred Stock purchased by Molex Ventures for the amount paid by Molex for such shares ($1 million). Under the Molex Side Letter, Molex Ventures also has a put option pursuant to which it can require us to, at any time and without condition, immediately repurchase all of our securities then owned by Molex Ventures for aggregate consideration of $1.00. In addition, the Molex Side Letter provides Molex Ventures preemptive rights in connection with certain new issuances of additional securities by us and certain registration rights with respect to our securities that it owns. The Molex Side Letter also requires the approval of Molex Ventures before we can undertake certain material corporate actions. We are in discussions with Molex Ventures to amend the Molex side letter in connection with the Business Combination to eliminate these rights upon completion of the Business Combination; however, we cannot assure you that we will be successful in eliminating these rights, in which case Molex would hold certain rights over Aerami, as an otherwise wholly owned subsidiary of the Combined Company.
Further, the placement agency agreement with Aegis in connection with our 2017 private placement provides for the payment of certain royalty rights, based on the payment of royalties to the holders of Aerami Royalty Stock. We plan to amend this agreement in connection with the Business Combination to eliminate these royalty payment obligations in exchange for the right to receive merger consideration in the Business Combination, however, we cannot assure you that we will be successful in eliminating these rights, in which case Aegis would continue to have certain royalty rights.
The terms of these agreements impose restrictions on, and any agreements entered into in the future may impose additional restrictions on, our freedom to operate our business. If we are obligated to pay royalty fees or repurchase previously issued shares in connection with these agreements, it would negatively impact our results of operations.
We depend on skilled labor, and our business and prospects may be adversely affected if we lose the services of our skilled personnel, including our senior management, or are unable to attract new skilled personnel.
Our ability to continue our operations and manage our potential future growth depends on our ability to hire and retain suitably skilled and qualified employees, including our senior management, in the long-term. Due to the specialized nature of our work, there is a limited supply of suitable candidates. We compete with other medical device and pharmaceutical companies, educational and research institutions and government entities, among others, for research, technical and clinical personnel. If we are unable to attract and retain skilled personnel, including in particular Steven Thornton, our Chief Executive Officer, Timm Crowder, our President and Chief Operating Officer, and Barry Deutsch, our Chief Financial Officer, our business and prospects may be materially and adversely affected.
| -51- |
We may be exposed to claims and may not be able to obtain or maintain adequate product liability insurance, which may limit commercialization of our product candidates.
Our business is exposed to the risk of product liability and other liability risks that are inherent in the development, manufacture, clinical testing and marketing of pharmaceutical and medical device products, and we will face an even greater risk if we commercialize our product candidates. These risks exist even if a product is approved for commercial sale by the FDA or regulatory authorities in other countries and manufactured in licensed facilities. Any side effects from our product candidates, manufacturing defects, misuse or abuse associated with our product candidates could result in injury to a patient or even death. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranty. Claims could also be asserted under state consumer protection acts.
Claims that are successfully brought against us could have a material and adverse effect on our financial condition and results of operations and may require us to limit commercialization of our product candidates. Further, even if we are successful in defending claims brought against us, our reputation could suffer and a successful defense against any such claims would require significant financial and management resources. Regardless of merit or eventual outcome, product liability claims may also result in, among others:
| ● | a decreased demand for our products; | |
| ● | a withdrawal or recall of our products from the market; | |
| ● | a withdrawal of participants from our ongoing clinical trials; | |
| ● | the distraction of our management’s attention from our core business activities to defend such claims; | |
| ● | additional costs to us to defend the related litigation; | |
| ● | substantial monetary awards to trial participants and patients; | |
| ● | regulatory investigation, product recalls, withdrawals or labeling, marketing or promotional restrictions | |
| ● | a loss of revenue; and | |
| ● | an inability to commercialize our product candidates. |
Our current insurance may not provide adequate coverage against our potential liabilities. Furthermore, we, and any of our commercial partners, licensors and licensees may not be able to obtain or maintain insurance on acceptable terms, or at all. In addition, our commercial partners, licensors and licensees may not be willing to indemnify us against these types of liabilities and may not themselves be sufficiently insured or have sufficient assets to satisfy any product liability claims. To the extent that they are uninsured or uninsurable, claims or losses that may be suffered by us, our commercial partners, licensors and licensees may have a material and adverse effect on our financial condition and results of operations.
Our business and operations would suffer in the event of third-party computer system failures, cyberattacks on third-party systems or deficiency in our cyber security.
We rely on information technology (“IT”) systems, including third-party “cloud based” service providers, to keep financial records, maintain laboratory data, clinical data and corporate records, to communicate with staff and external parties and to operate other critical functions. This includes critical systems such as email, other communication tools, electronic document repositories and archives. If any of these third-party information technology providers are compromised due to computer viruses, unauthorized access, malware, natural disasters, fire, terrorism, war and telecommunication failures, electrical failures, cyberattacks or cyber-intrusions over the internet, then sensitive emails or documents could be exposed or deleted. Similarly, we could incur business disruption if our access to the internet is compromised and we are unable to connect with third-party IT providers. A successful cyberattack could result in the theft or destruction of intellectual property, data or other misappropriation of assets, or otherwise compromise our confidential or proprietary information and disrupt our operations. The risk of a security breach or disruption, particularly through cyberattacks or cyber intrusion, including by computer hackers, foreign governments and cyber terrorists, has generally increased as the number, level of persistence, intensity and sophistication of attempted attacks and intrusions from around the world have increased. Such attacks are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. Cyberattacks could include wrongful conduct by hostile foreign governments, industrial espionage, wire fraud and other forms of cyber fraud, the deployment of harmful malware, denial-of-service, social engineering fraud or other means to threaten data security, confidentiality, integrity and availability. Furthermore, because the techniques used to obtain unauthorized access to, or to sabotage, systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures. We may also experience security breaches that may remain undetected for an extended period. A successful cyberattack could cause serious negative consequences for us, including, without limitation, the disruption of operations, the misappropriation of confidential business information, including financial information, trade secrets, financial loss and the disclosure of corporate strategic plans. Although we devote resources to protect our information systems, we realize that cyberattacks are a threat, and there can be no assurance that our efforts will prevent information security breaches that would result in business, legal, financial or reputational harm to us, or would have a material adverse effect on our results of operations and financial condition. Any failure to prevent or mitigate security breaches or improper access to, use of, or disclosure of our clinical data or patients’ personal data could result in significant liability under state (e.g., state breach notification laws), federal, and international law and may cause a material adverse impact to our reputation, affect our ability to conduct new studies and potentially disrupt our business.
| -52- |
We rely on those third parties to implement effective security measures and to safeguard important confidential personal data regarding our employees and our business operations. If a disruption event were to occur and cause interruptions in a third-party IT provider’s operations, it could result in a disruption in the development of our product candidates. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. If we are unable to prevent or mitigate the impact of such security or data privacy breaches, we could be exposed to litigation and governmental investigations, which could lead to a potential disruption to our business. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur significant liability and development of our product candidates could be delayed or could fail.
Certain technology features of the FOX® nebulizer and our AFINATM inhaler rely on wireless transmission technologies such as Wi-Fi and Bluetooth and may create security risks.
The FOX® nebulizer and our AFINATM inhaler are designed with smart technology and are Bluetooth-enabled with real time data collection capabilities. While this technology is intended to help optimize breathing and ensure precise delivery of the drug through the lungs, it may also create heightened security risks. Certain features of the device rely on the wireless transmission of data through Wi-Fi and/or Bluetooth sensors. These networks are often deemed less secure than a hard-wired network and are more susceptible to being compromised. The security of any particular wireless network is often outside of our control. Any breach of security could cause the FOX® nebulizer or our AFINATM Inhaler to not function properly.
If we or any of our CROs, contract manufacturers or suppliers fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business.
We and any third party CROs, contract manufacturers and suppliers we may engage are subject to numerous environmental, health and safety laws and regulations and permitting requirements, including those governing laboratory procedures and the generation, handling, use, storage, treatment and disposal of hazardous materials and wastes; the emission and discharge of hazardous materials into the ground, air and water; and employee health and safety. From time to time and in the future, our operations and the operations of our CROs, contract manufacturers and suppliers may involve the use of hazardous and flammable materials, including chemicals and biological materials, and may also produce hazardous waste products. In the event of contamination or injury resulting from the use or disposal of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
We may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. Current or future environmental laws and regulations may impair our research, development or production efforts. In addition, failure to comply with these laws and regulations may result in substantial fines, penalties or other sanctions. Any third-party CROs, contract manufacturers and suppliers we engage will also be subject to these and other environmental, health and safety laws and regulations. Liabilities they incur pursuant to these laws and regulations could result in significant costs or an interruption in operations, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
| -53- |
Our employees and our independent contractors, principal investigators, CROs, consultants or commercial partners, as well as their respective sub-contractors, if any, may engage in misconduct or fail to comply with certain regulatory standards and requirements, which could expose us to liability and adversely affect our reputation.
Our employees and our independent contractors, principal investigators, CROs, consultants or commercial partners, as well as their respective sub-contractors, if any, may engage in fraudulent conduct or other illegal activity, which may include intentional, reckless or negligent conduct that violates, among others, (a) FDA laws and regulations, or those of regulatory authorities in other countries, including those laws that require the reporting of true, complete and accurate information to the FDA, (b) manufacturing standards, (c) healthcare fraud and abuse laws or (d) laws that require the true, complete and accurate reporting of financial information or data. For example, such persons may improperly use or misrepresent information obtained in the course of our clinical trials, create fraudulent data in our preclinical studies or clinical trials or misappropriate our product candidates, which could result in regulatory sanctions being imposed on us and cause serious harm to our reputation. It is not always possible for us to identify or deter misconduct by our employees and third parties, and any precautions we may take to detect or prevent such misconduct may not be effective. Any misconduct or failure by our employees and our independent contractors, principal investigators, CROs, consultants or commercial partners, as well as their respective sub-contractors, if any, to comply with the applicable laws or regulations may subject us to enforcement action or otherwise expose us to liability or compliance costs, which, depending on the nature of the violation, may include but not necessarily be limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid or other government healthcare programs, injunctions, private qui tam actions brought by individual whistleblowers in the name of the government and the curtailment or restructuring of our operations as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws.
If any action is instituted against us as a result of the alleged misconduct of our employees or other third parties, regardless of the final outcome, our reputation may be adversely affected and our business may suffer as a result. If we are unsuccessful in defending against any such action, we may also be liable to significant fines or other sanctions, which could have a material and adverse effect on us.
We may engage in strategic transactions that could impact our liquidity, increase our expenses and present significant distractions to our management.
From time to time, we may consider strategic transactions, such as acquisitions of companies, asset purchases and out-licensing or in-licensing of intellectual property, products or technologies. Potential transactions that we may consider in the future include a variety of business arrangements, including spin-offs, strategic partnerships, joint ventures, restructurings, divestitures, business combinations and investments. Any future transactions could increase our near and long-term expenditures, result in potentially dilutive issuances of our equity securities, including our common stock, or the incurrence of debt, contingent liabilities, amortization expenses or acquired in-process research and development expenses, any of which could affect our financial condition, liquidity and results of operations. Future acquisitions may also require us to obtain additional financing, which may not be available on favorable terms or at all. These transactions may never be successful and may require significant time and attention of our management. In addition, the integration of any business that we may acquire in the future may disrupt our existing business and may be a complex, risky and costly endeavor for which we may never realize the full benefits of the acquisition. Accordingly, although there can be no assurance that we will undertake or successfully complete any additional transactions of the nature described above, any additional transactions that we do complete could have a material adverse effect on our business, results of operations, financial condition and prospects.
| -54- |
Our ability to use net operating loss carryforwards and other tax attributes may be limited in connection with this offering or other ownership changes.
We have incurred substantial losses during our history, do not expect to become profitable in the near future and may never achieve profitability. To the extent that we continue to generate taxable losses, unused losses will carry forward to offset future taxable income, if any, until such unused losses expire (if at all). As of December 31, 2020, we had federal and state net operating loss (“NOL”) carryforwards of approximately $83.0 million and $83.4 million, respectively, and federal and state research and development credit carryforwards of approximately $1.9 million and $1.5 million, respectively.
Under the legislation enacted in 2017, titled the Tax Cuts and Jobs Act (the “Tax Act”), federal NOL carryforwards arising in tax years beginning after December 31, 2017, may be carried forward indefinitely, but the deductibility of such federal NOLs in tax years beginning after December 31, 2020, is limited. Under the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”), federal NOL carryforwards arising in tax years beginning after December 31, 2017 and before January 1, 2021 may be carried back to each of the five tax years preceding the tax year of such loss. It is uncertain if and to what extent various states will conform to the Tax Act or the CARES Act. In addition, our NOL carryforwards are subject to review and possible adjustment by the IRS and state tax authorities. Under Section 382 of the Internal Revenue Code (the “Code”), our federal NOL carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership of our company. An “ownership change” pursuant to Section 382 of the Code generally occurs if one or more stockholders or groups of stockholders who own at least 5% of a company’s stock increase their ownership by more than 50 percentage points over their lowest ownership percentage within a rolling three-year period. Similar rules may apply under state tax laws. We have not yet formally determined the amount of the cumulative change in our ownership resulting from our recent private placement transaction, the Business Combination or other transactions, or any resulting limitations on our ability to utilize our NOL carryforwards and other tax attributes. However, we believe that our ability to utilize our NOL carryforwards and other tax attributes to offset future taxable income or tax liabilities is likely to be limited as a result of ownership changes, including potential changes in connection with the Business Combination. If we earn taxable income, such limitations could result in increased future income tax liability to us and our future cash flows could be adversely affected. We have recorded a full valuation allowance related to our NOL carryforwards and other deferred tax assets due to the uncertainty of the ultimate realization of the future benefits of those assets.
Risks Related to the Development and Regulatory Approval of our Product Candidates
Our preclinical studies and clinical trials may not be successful and delays to such preclinical studies or clinical trials may cause our costs to increase and significantly impair our ability to commercialize our product candidates. Results of previous clinical trials or interim results of ongoing clinical trials may not be predictive of future results.
Before we are able to commercialize our product candidates, we are required to undertake extensive preclinical studies and clinical trials to demonstrate that our product candidates are safe and effective for their intended uses. However, we cannot assure you that our product candidates will, in preclinical studies and clinical trials, demonstrate the safety and efficacy traits necessary to obtain marketing approval. Due to the nature of drug product development, many product candidates, especially those in early stages of development, may be terminated during development. We have successfully completed five Phase 1/2a studies for AER-501.We have commenced a Phase 1 clinical trial AER-901 and have completed the preclinical development of AER-601. However, we have not successfully completed the clinical development of any of our product candidates and, accordingly, do not have a track record of successfully bringing product candidates to market. Additionally, the outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and preliminary and interim results of a clinical trial do not necessarily predict final results.
Preclinical studies and clinical trials may fail due to factors such as flaws in trial design, dose selection and patient enrollment criteria. The results of preclinical studies and early clinical trials may not be indicative of the results of subsequent clinical trials. Product candidates may, in later stages of clinical testing, fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and earlier clinical trials. Moreover, there may be significant variability in safety or efficacy results between different trials of the same product candidate due to factors including, but not limited to, changes in trial protocols, differences in the composition of the patient population, adherence to the dosing regimen and other trial protocols and amendments to protocols and the rate of drop-out among patients in a clinical trial. If our preclinical studies or clinical trials are not successful and we are unable to bring our product candidates to market as a result, our business and prospects may be materially and adversely affected.
| -55- |
Furthermore, conducting preclinical studies and clinical trials is a costly and time-consuming process. The length of time required to conduct the required studies and trials may vary substantially according to the type, complexity, novelty and intended use of the product candidate. A single clinical trial may take up to several years to complete. Moreover, our preclinical studies and clinical trials may be delayed or halted due to various factors, including, among others:
| ● | delays due to the impact of the COVID-19 pandemic on our clinical development timelines; | |
| ● | delays in raising the funding necessary to initiate or continue a clinical trial; | |
| ● | delays in manufacturing sufficient quantities of clinical trial materials; | |
| ● | delays in reaching agreement on acceptable terms with prospective CROs and clinical trial sites; | |
| ● | delays in obtaining institutional review board approval at clinical trial sites; | |
| ● | delays in recruiting suitable patients to participate in a clinical trial; | |
| ● | delays in patients’ completion of clinical trials or their post-treatment follow-up; | |
| ● | regulatory authorities’ interpretation of our preclinical and clinical data; and | |
| ● | unforeseen safety issues, including a high and unacceptable severity, or prevalence, of undesirable side effects or adverse events caused by our product candidates or similar drug products or product candidates. |
If our preclinical studies or clinical trials are delayed, the potential regulatory approval and commercialization of our product candidates will be delayed and as a result, we may incur substantial additional costs or not be able to recoup our investment in the development of our product candidates, which would have a material and adverse effect on our business.
We have only recently begun testing of AER-901 (inhaled imatinib) delivered via the FOX® handheld nebulizer for the treatment of PAH to assess its safety and tolerability. Although we believe that AER-901 has therapeutic potential for PAH based on oral imatinib’s results in the Phase 3 IMPRES trial, we are utilizing a formulation which may not achieve better or similar levels of clinical activity or may have similar tolerability challenges as oral imatinib.
We are currently conducting our Phase 1 clinical trial for AER-901 delivered via the FOX® handheld nebulizer for the treatment of PAH. Our belief that AER-901 has a potential therapeutic benefit for PAH patients is based in part on the Phase 3 IMPRES trial conducted by Novartis AG (“Novartis”), which showed that oral administration of imatinib, marketed as Gleevec for multiple cancers, led to statistically significant improvements across both primary and secondary endpoints in PAH patients combined with PAH standard of care therapies. Despite statistically significant improvements in six-minute walk distance (“6MWD”), the primary endpoint, and multiple secondary hemodynamic endpoints, there was no difference between oral imatinib and placebo in time to clinical worsening, a composite endpoint consisting of death, hospitalization due to worsening PAH, worsening functional class, and a 15% reduction in 6MWD. However, oral imatinib was associated with significant adverse events that precluded its development as a therapy for PAH. We cannot be certain that AER-901 will show tolerability, and we may not be able to demonstrate to the satisfaction of the FDA the safety, efficacy and acceptable risk-benefit profile of AER-901 for PAH. A number of companies in the pharmaceutical and biotechnology industries, including Novartis in the IMPRES trial of oral imatinib, have suffered significant setbacks in Phase 3 clinical trials, even after positive results in earlier clinical trials. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway and safety or efficacy observations made in clinical trials, including previously unreported adverse events. We cannot be certain that we will not face similar setbacks. As a result of the foregoing, even if we are able to complete any planned and future clinical trials of AER-901, the results may not be sufficient to obtain regulatory approval.
| -56- |
We are planning to pursue the FDA 505(b)(2) pathway for certain of our product candidates. If we are unable to rely on the 505(b)(2) regulatory pathway to apply for marketing approval of such products in the United States, seeking approval through the 505(b)(1) NDA or 351(a) BLA pathway would require full reports of investigations of safety and effectiveness, and the process of obtaining marketing approval for such products would likely be significantly longer and more costly. Even if we are able to pursue the 505(b)(2) pathway, we could be subject to legal challenges and regulatory changes which might result in extensive delays or result in our 505(b)(2) being unsuccessful.
We plan to seek approval of certain of our product candidates under abbreviated regulatory pathways in the United States, such as the 505(b)(2) regulatory pathway. Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act (“FDCA”) was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Amendments, and permits the submission of an NDA where at least some of the information required for approval comes from preclinical studies or clinical trials not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The FDA interprets Section 505(b)(2) of the FDCA to permit the applicant to rely upon the FDA’s previous findings of safety and efficacy for an approved product. Section 505(b)(2), if applicable to us, would allow an NDA we submit to the FDA to rely in part on data in the public domain or the FDA’s prior conclusions regarding the safety and effectiveness of approved compounds, which could expedite the development program for a product candidate by potentially decreasing the amount of clinical data that we would need to generate in order to obtain FDA approval. We plan to pursue this pathway for AER-901, and we intend to rely upon the FDA’s previous findings of safety and efficacy for GLEEVEC® (imatinib mesylate) tablets. However, even if the FDA allows us to rely on the 505(b)(2) regulatory pathway, we cannot assure you that such marketing approval will be obtained in a timely manner, or at all. In the event the FDA determines that AER-901 does not qualify for a 505(b)(2) regulatory pathway, we would need to reconsider our plans, and the development pathway could be elongated by the requirement for a separate Phase 2 and 3 trial, which could jeopardize our ability to commercialize such product candidate in a cost-efficient manner, or at all.
The FDA may require us to perform additional clinical trials to support any change from the reference listed drug, which could be time-consuming and substantially delay our receipt of marketing approval. Also, as has been the experience of others in our industry, our competitors may file citizen petitions with the FDA to contest approval of our NDA, which may delay or even prevent the FDA from approving any NDA that we submit under the 505(b)(2) regulatory pathway. If an FDA decision or action relative to our product candidate, or the FDA’s interpretation of Section 505(b)(2) more generally, is successfully challenged, it could result in delays or even prevent the FDA from approving a 505(b)(2) application for our product candidates. Even if we are able to utilize the 505(b)(2) regulatory pathway, a drug approved via this pathway may be subject to the same post-approval limitations, conditions and requirements as any other drug.
In addition, we may face regulatory exclusivity delays or patent infringement lawsuits in relation to our NDAs submitted under the 505(b)(2) regulatory pathway, which may further delay or prevent the review or approval of our product candidates. The pharmaceutical industry is highly competitive, and 505(b)(2) NDAs are subject to special requirements designed to protect the patent rights of sponsors of previously approved drugs that are referenced in a 505(b)(2) NDA. If the previously approved drugs referenced in an applicant’s 505(b)(2) NDA are protected by a patent(s) listed in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations publication (the “Orange Book”), the 505(b)(2) applicant is required to certify when submitting their NDA whether, for each listed patent, they agree not to seek approval until such patent has expired or, instead, whether they believe each such patent is invalid or unenforceable or will not be infringed and therefore they seek approval before the patent would expire. If the applicant is seeking approval prior to the expiration of the patent, the applicant must give notice of its application to the sponsors of the listed drug and to the patent holder, and the sponsor or the patent holder may thereafter bring suit for patent infringement, which will trigger a mandatory 30-month delay (or the shorter of dismissal of the lawsuit or expiration of the patent(s)) in approval of the 505(b)(2) application. It is not uncommon for a manufacturer of an approved product to file a citizen petition with the FDA seeking to delay approval of, or impose additional approval requirements for, pending competing products. If successful, such petitions can significantly delay, or even prevent, the approval of the new product. However, even if the FDA ultimately denies such a petition, the FDA may substantially delay approval while it considers and responds to the petition.
If the FDA determines that AER-901 or any of our other product candidates do not qualify for the 505(b)(2) regulatory pathway, we would need to reconsider our plans and might not be able to commercialize such product candidates in a cost-efficient manner, or at all. For example, the 505(b)(2) pathway is not available for AER-501, our inhaled insulin product candidate, because FDA transitioned insulin products approved under NDAs to BLAs on March 23, 2020. Although there is an abbreviated 351(k) BLA pathway for biologics, FDA did not transition the reference product for AER-501 from an NDA to a BLA because it is currently withdrawn. We will therefore be required to submit an original 351(a) BLA for AER-501, which is subject to more extensive requirements and risks than a 505(b)(2) NDA.
If we were to pursue approval under the 505(b)(1) NDA pathway or the 351(a) BLA pathway for our other candidates, we would be subject to more extensive requirements and risks such as conducting additional clinical trials, providing additional data and information or meeting additional standards for marketing approval. As a result, the time and financial resources required to obtain marketing approval for our product candidates would likely increase substantially and further complications and risks associated with our product candidates may arise. Also, new competing products may reach the market faster than ours, which may materially and adversely affect our competitive position, business and prospects.
| -57- |
We intend to seek to market our AFINA™ inhalation device and will be required to obtain regulatory clearance(s) or approval(s). Any such regulatory process would be expensive, time-consuming and uncertain both in timing and in outcome.
Our AFINA™ soft mist inhalation device, and any other inhalation devices we may develop in the future, will be subject to regulation by the FDA as a medical device, including requirements for regulatory clearance or approval of such products before they can be marketed. Accordingly, we will be required to obtain FDA 510(k) clearance or premarket approval (“PMA”) in order to sell our AFINA™ device in a manner consistent with FDA laws and regulations. We also intend to apply for a CE mark for our AFINA™ device in Europe. Such regulatory approval processes or clearances are expensive, time-consuming and uncertain. Our efforts may never result in any 510(k) clearance, PMA or other regulatory clearance for AFINA™ or any other medical device products, and failure by us to obtain or comply with such approvals and clearances could have an adverse effect on our business, financial condition or operating results.
If we successfully obtain 510(k) clearance for AFINA™, we will be subject to a substantial number of additional requirements for medical devices, including establishment registration, device listing, and Quality System Regulation (“QSR”), which covers the design, testing, production, control, quality assurance, labeling, packaging, servicing, sterilization (if required), and storage and shipping of medical devices (among other activities), product labeling, advertising, recordkeeping, post-market surveillance, post-approval studies, adverse event reporting, and correction and removal (recall) regulations. We may be required to expend significant resources to ensure ongoing compliance with the FDA regulations and/or take satisfactory corrective action in response to enforcement action, which may have a material adverse effect on the ability to design, develop, and commercialize products using our technology as planned. Failure to comply with these requirements may subject us to a range of enforcement actions, such as warning letters, injunctions, civil monetary penalties, criminal prosecution, recall and/or seizure of products, and revocation of marketing authorization, as well as significant adverse publicity. If we fail to obtain, or experience significant delays in obtaining, regulatory approvals for AFINA™ or any other device products, such products may not be able to be launched or successfully commercialized in a timely manner, or at all.
The FDA will consider our product candidates to be combination products, which may result in additional regulatory risks and delays in the product development and regulatory approval process.
Our product candidates are delivered using inhalation devices and may be considered by the FDA to be drug-device combination products or biologic-device combination products. Accordingly, the inhalation devices will be evaluated as part of our NDA or BLA filing. When evaluating products that utilize a specific delivery system or device, the FDA will evaluate the characteristics of that delivery system and its functionality, as well as the potential for undesirable interactions between the product and the delivery system, including the potential to negatively impact the safety or effectiveness of the drug or biologic. The FDA review process can be more complicated for combination products, and may result in delays, particularly if novel delivery systems are involved. We rely on third parties for the design and manufacture of the inhalation technology used with our products, and in some cases for the right to refer to their data on file with the FDA or other regulators. Quality or design concerns with the inhalation devices, or commercial disputes with these third parties, could delay or prevent regulatory approval and commercialization of our product candidates.
We may encounter difficulties in enrolling patients in our clinical trials which could delay or otherwise adversely affect our clinical development activities.
We may not be able to commence or complete clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials. AER-901 is now in a Phase 1 clinical trial, and we target initiating activities on the Phase 2 portion of our planned combined Phase 2/3 trial in 2022. In addition, AER-501 could progress to Phase 3 clinical trials and we have completed the preclinical development of AER-601, which is now ready to progress to Phase 1 human clinical trials.
| -58- |
Patient enrollment may be affected by, among other reasons:
| ● | the severity of the disease under investigation; | |
| ● | the design of the clinical trial protocol and amendments to a protocol; | |
| ● | the size and nature of the patient population required for analysis of the trial’s primary endpoint; | |
| ● | eligibility criteria for the clinical trial in question; | |
| ● | the perceived risks and benefits of the product candidate under clinical testing, including a high and unacceptable severity, or prevalence, of undesirable side effects or adverse events caused by our product candidates or similar products or product candidates; | |
| ● | the availability and efficacy of approved drugs for the disease under investigation; | |
| ● | our ability to recruit clinical trial investigators with the appropriate competencies and experience, and to obtain investigational review board (“IRB”) approval to conduct trials at U.S. sites and similar approvals at any sites outside the U.S.; | |
| ● | competition for patients from other investigational clinical trials being conducted at the same time; | |
| ● | the clinical site’s ability to obtain and maintain patient consents; | |
| ● | the existing body of safety and efficacy data in respect of the product candidate under clinical testing; | |
| ● | movement restrictions, health reasons or otherwise resulting from the COVID-19 pandemic; | |
| ● | delays in or temporary suspension of the enrollment of patients in any planned clinical trial due to the COVID-19 pandemic, which we have experienced during our Phase 1 trial for AER-901 in Australia as a result of government imposed shutdowns; | |
| ● | the risk that patients enrolled in any clinical trials will drop out of the trials before completion, including as a result of contracting COVID-19 or other health conditions or being forced to quarantine; | |
| ● | the proximity of patients to clinical trial sites; and | |
| ● | the number and nature of competing therapies and clinical trials. |
Any negative results we may report in clinical trials of our product candidates may also make it difficult or impossible to recruit and retain patients in other clinical trials of that same product candidate.
In particular, we will be required to identify and enroll a sufficient number of patients with PAH for our clinical trials and studies of AER-901. PAH is a rare disease with a relatively small patient population, and our enrollment of clinical trial participants may be slow as a result. In addition, PAH patients may be less willing to participate in a clinical trial as a result of risks or restrictions arising from COVID-19, such as the risk of contracting the virus from increased social contact necessitated by participation in clinical trials. Furthermore, we are aware of a number of therapies for PAH that are being developed or that are already available on the market, and we expect to face competition from these investigational drugs or approved drugs for potential subjects in our clinical trials, which may delay enrollment in our planned clinical trials.
Delays or failures in planned patient enrollment or retention may result in increased costs, program delays, or both. We may, as a result of such delays or failures, be unable to carry out our clinical trials as planned or within the timeframe that we expect or at all, and our business and prospects may be materially and adversely affected as a result.
In July 2021, we received orphan drug designation from the FDA for imatinib use in the treatment of PAH and plan to apply for the equivalent designation in Europe once data from our Phase 1 trial is available; however, we may be unable to obtain such designations or to maintain the benefits associated with orphan drug status, including the potential for market exclusivity.
We have obtained orphan drug designation for AER-901 in the United States and plan to apply for orphan drug designation from the European Medicines Agency’s (“EMA’s”) Committee for Orphan Medicinal Products in the European Union once data from our Phase 1 trial is available. However, an orphan drug designation does not necessarily lead to a faster development process or expedite the regulatory review and does not increase the likelihood that a product candidate will receive approval from the FDA. We may not be able to obtain or maintain the benefits associated with orphan drug designation, including the potential for market exclusivity. Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a drug or biologic as an orphan drug if it is a product intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States, or a patient population of 200,000 or more in the United States where there is no reasonable expectation that the cost of developing the product will be recovered from sales in the United States. In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers.
| -59- |
Similarly, in the European Union, the European Commission, upon the recommendation of the EMA’s Committee for Orphan Medicinal Products, grants orphan drug designation to promote the development of drugs that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions and either (i) such condition affects not more than five in 10,000 persons in the European Union or (ii) without incentives, it is unlikely that the marketing of the drug in the European Union would be sufficient to justify the necessary investment in its development, and, in each case, for which no satisfactory method of diagnosis, prevention, or treatment has been authorized (or the product would be a significant benefit to those affected). In the European Union, orphan drug designation entitles a party to financial incentives such as reduction of fees or fee waivers.
Generally, if a product with an orphan drug designation subsequently receives the first regulatory approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA or the EMA from approving another marketing application for the same product and indication for that time period, except in limited circumstances. The applicable period is seven years in the United States and ten years in Europe. The European exclusivity period can be reduced to six years if, at the end of the fifth year, it is established that a product no longer meets the criteria for orphan drug designation, including if the product is sufficiently profitable so that market exclusivity is no longer justified.
We are aware that the FDA has granted orphan disease designation for imatinib for the treatment of PAH to two other companies, Aerovate and Tenax. While we believe that AER-901 offers significant advantages due to the delivery of liquid imatinib via a high performing handheld nebulizer that is currently used with another PAH treatment on the market in Europe, we may not be able to achieve the benefits of orphan designation if we are not the first to achieve regulatory approval or cannot show that AER-901 is superior to our competitors’ products.
Even if we obtain orphan drug exclusivity for AER-901, that exclusivity may not effectively protect AER-901 from competition because different products can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve a different product with the same active ingredient for the same condition if the FDA concludes that the later product is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. Therefore, there is no guarantee that we would be able to enjoy the benefits of such designation.
If a competitor has obtained orphan drug designation from the FDA for the same drug and same indication as we are seeking for a product candidate, and then obtains approval of that drug for that condition before we do, the resulting FDA exclusivity would significantly delay our ability to commercialize our product candidate.
Under the Orphan Drug Act, the first applicant to receive FDA approval for a particular active ingredient to treat a particular disease or condition with orphan drug designation is entitled to a seven-year exclusive marketing period in the United States for that product in that indication. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
During the exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease or condition, except in limited circumstances, such as if the second applicant demonstrates clinical superiority of its product to the product with orphan drug exclusivity through a demonstration of superior safety, superior efficacy or a major contribution to patient care, or if the manufacturer of the product with orphan exclusivity is not able to assure sufficient quantities of the product. “Same drug” means a drug that contains the same identity of the active moiety if it is a drug composed of small molecules, or of the principal molecular structural features if it is composed of macromolecules and is intended for the same use as a previously approved drug, except that if the subsequent drug can be shown to be clinically superior to the first drug, it will not be considered to be the same drug. Drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. We are aware that there are other companies working on an inhaled version of imatinib for treatment of PAH who have also received orphan drug designation and one such competitor has completed its own Phase 1 trial. Should those competitors receive FDA approval for that drug in advance of AER-901 receiving approval, the resulting FDA exclusivity would significantly delay our ability to commercialize inhaled imatinib for treatment of PAH unless we can show that AER-901 is “superior” to that drug.
| -60- |
We may conduct clinical trials for our product candidates outside the United States and the FDA may not accept data from such trials.
Although the FDA may accept data from clinical trials conducted outside the United States in support of safety and efficacy claims for our product candidates, if not conducted under an investigational new drug (“IND”), the acceptance of such data is subject to certain conditions set out in 21 C.F.R. § 312.120. For example, in order for the FDA to accept data from such a foreign clinical trial, the study must have been conducted in accordance with Good Clinical Practice (“GCP”), including review and approval by an independent ethics committee and obtaining the informed consent from subjects of the clinical trials. The FDA must also be able to validate the data from the study through an onsite inspection if the agency deems it necessary. In addition, foreign clinical data submitted to support FDA applications should be applicable to the U.S. population and U.S. medical practice. Other factors that may affect the acceptance of foreign clinical data include differences in clinical conditions, study populations or regulatory requirements between the United States and the foreign country.
We are currently conducting our Phase 1 trial for AER-901 in Australia and may conduct other clinical trials of our product candidates outside the United States. The FDA may not accept such foreign clinical data, and in such event, we may be required to re-conduct relevant clinical trials within the United States, which would be costly and time-consuming and which could have a material and adverse effect on our ability to carry out our business plans.
We rely on third parties to conduct our preclinical studies and clinical trials.
We currently rely on, and plan to continue to rely on, third-party CROs to monitor and manage data for our preclinical studies and clinical trials. However, we are and will be responsible for ensuring that each of our trials is conducted in accordance with the applicable regulatory standards and our reliance on CROs does not relieve us of our regulatory responsibilities.
The CROs on which we currently rely and who we will need to rely on in the future are required to comply with FDA regulations (and the regulations of comparable regulatory authorities in other countries) regarding GCP. Regulatory authorities enforce GCP standards through periodic inspections. If any of the CROs on which we rely fail to comply with the applicable GCP standards, the clinical data generated in our clinical trials may be deemed unreliable. While we have entered and expect to enter into additional contractual agreements with these CROs, we will have limited influence over their actual performance and cannot control whether or not they devote sufficient time and resources to our preclinical studies and clinical trials. A failure to comply with the applicable regulations in the conduct of the preclinical studies and clinical trials for our product candidates may require us to repeat such studies or trials, which would delay the process of obtaining marketing approval for our product candidates and have a material and adverse effect on our business and prospects.
CROs may have the ability to terminate their respective agreements with us if, among other things, it can be reasonably demonstrated that the safety of the patients participating in our clinical trials warrants such termination. If any of our agreements with a CRO is terminated, and if we are not able to enter into agreements with an alternative CRO on acceptable terms or in a timely manner, or at all, the clinical development of our product candidates may be delayed and our development expenses could increase.
We rely on third parties for the manufacture of our product candidates for clinical and preclinical development and expect to continue to do so for the foreseeable future. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We do not own or operate manufacturing facilities and have no plans to build our own clinical or commercial scale manufacturing capabilities. We rely, and expect to continue to rely, on third parties for the manufacture of our product candidates and related raw materials for clinical and preclinical development, as well as for commercial manufacture if any of our product candidates receive marketing approval. We have exclusive arrangements with certain of our licensors to purchase drug product, devices and/or device components for our product candidates. In addition, we have an exclusive arrangement with Phillips-Medisize for the joint development and manufacture of our AFINA™ device. If any of these suppliers and/or manufacturers fail to perform for any reason under our arrangements, we may face long delays and difficulties in securing alternate sources of supply and manufacture.
| -61- |
The facilities used by third-party manufacturers to manufacture our product candidates must be approved by the FDA pursuant to inspections that will be conducted after we submit an NDA or BLA to the FDA. We do not control the manufacturing process of, and are completely dependent on, third-party manufacturers for compliance with current Good Manufacturing Practice (“cGMP”) requirements for manufacture of drug products. If these third-party manufacturers cannot successfully manufacture our product candidates in a manner that conforms to the strict regulatory requirements of the FDA or comparable foreign regulatory authorities, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. In addition, we have no control over the ability of third-party manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect the supply of our products.
Our or a third party’s failure to execute on our manufacturing requirements, to do so on commercially reasonable terms and comply with cGMP could adversely affect our business in a number of ways, including:
| ● | an inability to initiate or continue clinical trials for our product candidates; | |
| ● | delay in submitting regulatory applications or receiving marketing approvals for our product candidates; | |
| ● | subjecting third-party manufacturing facilities or our manufacturing facilities to additional inspections by regulatory authorities; | |
| ● | requirements to cease development or to recall batches of our product candidates; and | |
| ● | in the event of approval to market and commercialize our product candidates, an inability to meet commercial demands for our product candidates or any other future product candidates. |
In addition, we may be unable to establish any agreements with third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| ● | failure of third-party manufacturers to comply with regulatory requirements and maintain quality assurance; | |
| ● | breach of the manufacturing agreement by the third party; | |
| ● | failure to manufacture our product according to our specifications; | |
| ● | failure to manufacture our product according to our schedule or at all; | |
| ● | misappropriation of our proprietary information, including our trade secrets and know-how; and | |
| ● | termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. |
Our product candidates and any products that we may develop in the future may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us. Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval, and any related remedial measures may be costly or time consuming to implement. We do not currently have arrangements in place for redundant supply or a second source for all required raw materials used in the manufacture of our product candidates. If our current or future third-party manufacturers cannot perform as agreed, we may be required to replace such manufacturers and we may be unable to replace them on a timely basis or at all.
Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to commercialize any products that receive marketing approval on a timely and competitive basis.
| -62- |
Our current pipeline product candidates require extensive clinical data analysis, regulatory review and additional testing. Clinical trials and data analysis can be very expensive, time-consuming and difficult to design and implement. If we are unsuccessful in obtaining regulatory approval for our product candidates, or any of our product candidates do not provide positive results, we may be required to delay or abandon development of such product, which would have a material adverse impact on our business.
Continuing product development requires additional and extensive clinical testing. We have completed the preclinical development of AER-601 and are seeking a partner to progress to Phase 1 human clinical trials. We are currently conducting a Phase 1 clinical trial with AER-901, and AER-501 is ready to progress to Phase 3 clinical trials. Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The clinical trial process is also time-consuming. We cannot provide any assurance or certainty regarding when we might receive regulatory approval for any of our product candidates. Furthermore, failure can occur at any stage of the process, and we could encounter problems that cause us to abandon an NDA or BLA filed with the FDA or repeat clinical trials. The commencement and completion of clinical trials for any current or future development product candidate may be delayed by several factors, including:
| ● | unforeseen safety issues; | |
| ● | determination of dosing issues; | |
| ● | lack of effectiveness during clinical trials; | |
| ● | slower than expected rates of patient recruitment; | |
| ● | inability to monitor patients adequately during or after treatment; and | |
| ● | inability or unwillingness of medical investigators to follow our clinical protocols or amendments to our protocols. |
In addition, the FDA or an IRB may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the FDA finds deficiencies in our IND submissions or the conduct of these trials. Therefore, we cannot provide any assurance or predict with certainty the schedule for future clinical trials. In the event we do not ultimately receive regulatory approval for our product candidates, we may be required to terminate development of our only product candidates.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial potential or result in significant negative consequences following any potential marketing approval.
If our product candidates are associated with undesirable side effects or have characteristics that are unexpected, we may need to abandon our development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Any serious adverse or undesirable side effects identified during the development of our product candidates could interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications, and in turn prevent us from commercializing our product candidates and generating revenues from their sale. In addition, if any of our product candidates receive regulatory approval and we or others later identify undesirable adverse effects caused by the product, we could face one or more of the following consequences:
| ● | regulatory authorities may require the addition of labeling statements, such as a boxed warning or a contraindication, or other safety labeling changes; | |
| ● | regulatory authorities may require a risk evaluation and mitigation strategy (“REMS”); | |
| ● | regulatory authorities may withdraw their approval of the product; | |
| ● | regulatory authorities may seize the product; | |
| ● | we may be required to change the way that the product is administered or conduct additional clinical trials, or we may need to recall the product; | |
| ● | we may be subject to litigation or product liability claims, fines, injunctions or criminal penalties; and | |
| ● | our reputation may suffer. |
| -63- |
In light of widely publicized events concerning the safety risk of certain drug products, regulatory authorities, members of Congress, the Government Accounting Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling or boxed warnings that further limit use of the drug products and establishment of risk management programs that may, for instance, restrict distribution of drug products. The increased attention to drug safety issues may result in a more cautious approach by the FDA to clinical trials. Data from clinical trials may receive greater scrutiny with respect to safety, which may make the FDA or other regulatory authorities more likely to terminate clinical trials before completion or require longer or additional clinical trials that may result in substantial additional expense and a delay or failure in obtaining approval or approval for a more limited indication than originally sought.
Any interim, topline or preliminary data from our preclinical studies and clinical trials that may be announced or published from time to time may change as more data becomes available and will remain subject to audit and verification procedures that could result in material changes in the final data.
In the future, we may publicly disclose preliminary, interim or topline data from our preclinical studies and clinical trials. These interim updates will be based on a preliminary analysis of then-available data, and the results and related findings and conclusions will be subject to change following a more comprehensive review of the data related to the particular study or trial. We will be required to make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the topline results that we may report might differ from future results of the same studies or trials, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. Topline data will remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, any topline data should be viewed with caution until the final data are available. In addition, we may report interim analyses of only certain endpoints rather than all endpoints. Interim data from clinical trials that we may complete will be subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data becomes available.
The marketing approval processes of the FDA and comparable regulatory authorities in other countries are unpredictable, and our product candidates may be subject to multiple rounds of review or may not receive marketing approval.
We have not previously submitted an NDA or BLA to the FDA or similar drug approval filings to comparable regulatory authorities in other countries for any product candidate, and we cannot assure you that any of our product candidates will receive marketing approval. Filing an application and obtaining marketing approval for a pharmaceutical product candidate is an extensive, lengthy, expensive and inherently uncertain process, and regulatory authorities may delay, limit or deny approval of our product candidates for many reasons, including, but not limited to, the following:
| ● | the data collected from our clinical trials may not be sufficient to support the submission of an NDA, BLA, or similar drug approval filing to the FDA or comparable regulatory authorities in other countries; | |
| ● | the FDA or comparable regulatory authorities in other countries may refuse to accept for filing an NDA, BLA, or similar drug approval filing if they deem the application to be incomplete; | |
| ● | the FDA or comparable regulatory authorities in other countries may disagree with the design, scope or implementation of our clinical trials; | |
| ● | we may be unable to demonstrate to the satisfaction of the FDA or comparable regulatory authorities in other countries that our product candidates are safe and effective for their proposed indication or that their clinical and other benefits outweigh their safety risks; | |
| ● | the results of our clinical trials may not meet the level of statistical significance required by the FDA or comparable regulatory authorities in other countries; |
| -64- |
| ● | the FDA or comparable regulatory authorities in other countries may disagree with the number, design, size, conduct or statistical analysis of one or more of our clinical trials; | |
| ● | the FDA or comparable regulatory authorities in other countries may disagree with our interpretation of data from our preclinical studies or clinical trials; | |
| ● | the FDA or comparable regulatory authorities in other countries may find deficiencies in the manufacturing processes or facilities of our third-party manufacturers with which we contract for clinical and commercial supplies; | |
| ● | our product candidates may not meet the level of quality and control required by the FDA or comparable regulatory authorities in other countries; | |
| ● | the FDA or comparable regulatory authorities in other countries may not approve our manufacturing processes or facilities or those of our third-party manufacturers, which would be required to be corrected prior to marketing approval; | |
| ● | the FDA or comparable regulatory authorities in other countries may require development of a costly and extensive REMS as a condition of approval; | |
| ● | the success or further approval of competing products approved in indications similar to those of our product candidates may change the standards for approval of our product candidates in their proposed indications; and | |
| ● | the approval policies of the FDA or comparable regulatory authorities in other countries may change in a manner that renders our clinical data insufficient for approval. |
In addition, the FDA or comparable regulatory authorities in other countries may, in their sole discretion, change their views in respect of regulatory pathways they had previously affirmed or clinical trial protocols they were previously not opposed to. In the event that this occurs, the clinical development and commercialization of our product candidates may be delayed or even derailed. In addition, we have not consulted with the FDA on the appropriate regulatory pathway and clinical trial protocols with respect to our planned development of inhaled imatinib for treatment of PAH or any of our other product candidates other than AER-501, and the determination of regulatory pathways and development of clinical trial protocols will be an extensive, lengthy, expensive and inherently uncertain process.
Even if we obtain marketing approval for any of our product candidates, the FDA or comparable regulatory authorities in other countries may approve our product candidates for fewer or more limited indications than what we requested approval for or may include safety warnings or other restrictions that may negatively impact the commercial viability of our product candidates. Likewise, regulatory authorities may grant approval contingent on the performance of costly post-marketing clinical trials or the conduct of an expensive REMS, which could significantly reduce the potential for commercial success or viability of our product candidates. We also may not be able to find acceptable collaborators to manufacture our drug products, if and when approved, in commercial quantities and at acceptable prices, or at all.
Even if we obtain marketing approval for our product candidates in the United States, we or our collaborators may not obtain marketing approval for the same product candidates elsewhere.
We have entered, and may in the future enter, into strategic collaboration arrangements with third parties to develop and commercialize our product candidates outside of the United States. For example, in January 2021, we entered into an exclusive license and development agreement with Chance to develop and commercialize AER-901 for the treatment of PAH in mainland China, Hong Kong, Macau and Taiwan. We have also entered into a license, supply and manufacturing agreement with Hikma, under which we granted an exclusive license to register and commercialize AER-501 in all disease indications in the Middle East and Africa, excluding Turkey and certain other countries, and we entered into a joint venture agreement with Dongbao for the purpose of (i) completing clinical trials in China for AER-501, (ii) gaining NMPA approval for AER-501 and (iii) commercializing AER-501 in China. In order to market any product candidate outside of the United States, we or our collaborators will be required to comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Clinical trials conducted in one country may not be recognized or accepted by regulatory authorities in other countries, and obtaining marketing approval in one country does not mean that marketing approval will be obtained in any other country. Approval processes vary among countries, and additional product testing and validation or additional administrative review periods may be required from one country to the next.
| -65- |
Seeking marketing approval in countries other than the United States could be costly and time-consuming, especially if additional preclinical studies or clinical trials are required to be conducted. We currently do not have any product candidates approved for sale in any jurisdiction, including non-U.S. markets, and we do not have experience in obtaining marketing approval in non-U.S. markets. We cannot assure you that such collaborators will be able to successfully obtain marketing approval for our product candidates outside of the United States. If we or our collaborators fail to obtain marketing approval in non-U.S. markets, or if such approval is delayed, our target market may be reduced, and our ability to realize the full market potential of our products will be adversely affected.
The terms of approvals, ongoing regulations and post-marketing restrictions for our products may limit how we manufacture and market our products, which could materially impair our ability to generate revenue.
Once marketing approval has been granted, an approved product and its manufacturer and marketer are subject to ongoing review and extensive regulation. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling and regulatory requirements. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use, and if we do not restrict the marketing of our products only to their approved indications, we may be subject to enforcement action for off-label marketing.
The FDA applies a heightened level of scrutiny to comparative claims when applying its statutory standards for advertising and promotion, including with regard to its requirement that promotional labeling be truthful and not misleading. Any claim of effectiveness made in prescription drug promotion, including comparative effectiveness, must be supported by substantial evidence or substantial clinical experience.
In addition, making comparative claims may draw concerns from our competitors. Where a company makes a claim in advertising or promotion that its product is superior to the product of a competitor (or that the competitor’s product is inferior), this creates a risk of a lawsuit by the competitor under federal and state false advertising or unfair and deceptive trade practices law, and possibly also state libel law. Such a suit may seek injunctive relief against further advertising, a court order directing corrective advertising, and compensatory and punitive damages where permitted by law.
We, and any potential collaborators we may have in the future, must therefore comply with requirements concerning advertising and promotion for any of our products for which we or our collaborators obtain marketing approval. Thus, if any of our current product candidates receive marketing approval, the accompanying label may limit the approved use of our product, which could limit sales of the product.
In addition, manufacturers of approved products and those manufacturers’ facilities are required to comply with extensive FDA requirements, such as ensuring that quality control and manufacturing procedures conform to cGMP applicable to drug manufacturers, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We, any contract manufacturers we may engage in the future, our future collaborators, licensees and their contract manufacturers will also be subject to other regulatory requirements, including submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements regarding the distribution of samples to clinicians, recordkeeping and costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product such as the requirement to implement a REMS.
Even if any of our product candidates obtain marketing approval, our products may not achieve market acceptance.
Our business model is to reformulate biologics and small molecules with established proof of concept trials in targeted indications for delivery using inhalation devices. Currently, each of our product candidates other than AER-901 is expected to utilize our AFINA™ inhalation technology. Part of our current strategy includes developing drug products that can be approved under abbreviated regulatory pathways in the United States, such as the 505(b)(2) regulatory pathway, which allows us to rely on existing knowledge of the safety and efficacy of the relevant reference listed drugs to support our applications for approval in the United States. While we believe that it will be less difficult for us to convince physicians, patients and other members of the medical community to accept and use these product candidates as compared to entirely new drugs, these or any of product candidates may nonetheless fail to gain sufficient market acceptance by physicians, patients, other healthcare providers and third-party payors. If any of our product candidates fail to achieve sufficient market acceptance, we may not be able to generate sufficient revenue to become profitable. The degree of market acceptance of our product candidates, if and when they are approved for commercial sale, will depend on a number of factors, including but not limited to:
| ● | the timing of our receipt of marketing approvals, the terms of such approvals and the countries in which such approvals are obtained; |
| -66- |
| ● | the safety, efficacy, reliability and ease of administration of our product candidates; | |
| ● | the prevalence and severity of undesirable side effects and adverse events; | |
| ● | the extent of the limitations or warnings required by the FDA or comparable regulatory authorities in other countries to be contained in the labeling of our product candidates; | |
| ● | the clinical indications for which our product candidates are approved; | |
| ● | the availability and perceived advantages of alternative therapies; | |
| ● | any publicity related to our product candidates or those of our competitors; | |
| ● | the quality and price of competing drug products; | |
| ● | our ability to obtain third-party payor coverage and sufficient reimbursement; | |
| ● | potential product liability claims; | |
| ● | the willingness of patients to pay out of pocket in the absence of third-party payor coverage; and | |
| ● | the selling efforts and commitment of our commercialization collaborators. |
If our product candidates, if and when approved, fail to receive a sufficient level of market acceptance, our ability to generate revenue from sales of our product candidates will be limited, and our business and results of operations may be materially and adversely affected.
The commercial success of our product candidates depends in part on the extent to which governmental authorities, private health insurers, and other third-party payors provide coverage and adequate reimbursements. Failure to obtain or maintain coverage and adequate reimbursements for our product candidates, if approved, could limit our ability to market our product and decrease our ability to generate revenue.
Patients in the United States and in other countries generally rely on third-party payors to be able to afford medical services and pharmaceutical products that receive FDA approval. Accordingly, market acceptance of our product candidates is dependent on the extent to which third-party coverage and adequate reimbursement is available from government health administration authorities (including in connection with government healthcare programs, such as Medicare and Medicaid in the United States), private healthcare insurers and other healthcare funding organizations.
Significant uncertainty exists as to the coverage and reimbursement status of any of our product candidates for which we may obtain regulatory approval. Coverage decisions may not favor new drug products when more established or lower-cost therapeutic alternatives are already available. Even if we obtain coverage for a given product candidate, the associated reimbursement rate may not be adequate to cover our costs, including research, development, intellectual property, manufacture, sale and distribution expenses, or may require co-payments that patients find unacceptably high. Patients are unlikely to use our products unless reimbursement is adequate to cover all or a significant portion of the cost of our product candidates.
Coverage and reimbursement policies for drug products can differ significantly from payor to payor as there is no uniform policy of coverage and reimbursement for drug products among third-party payors in the United States. There may be significant delays in obtaining coverage and reimbursement as the process of determining coverage and reimbursement is often time-consuming and costly, which will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage or adequate reimbursement will be obtained. It is difficult to predict at this time what government authorities and third-party payors will decide with respect to coverage and reimbursement for our product candidates.
The market for our product candidates will depend significantly on access to third-party payors’ drug formularies or lists of medications for which third-party payors provide coverage and reimbursement. Competition to be included in such formularies often leads to downward pricing pressures. In particular, third-party payors may refuse to include a particular reference listed drug in their formularies or otherwise restrict patient access to a reference listed drug when a less costly generic equivalent or other alternative is available.
| -67- |
The U.S. government, state legislatures and foreign governmental entities have shown significant interest in implementing cost containment programs to limit the growth of government-paid healthcare costs, including price controls, restrictions on reimbursement and coverage and requirements for substitution of generic products for branded prescription drugs. Adoption of government controls and measures and tightening of restrictive policies in jurisdictions with existing controls and measures, could exclude or limit our product candidates from coverage and limit payments for pharmaceuticals. Further, many third-party payors may refuse to provide coverage and adequate reimbursement for particular drugs when an equivalent generic drug, biosimilar or a less expensive therapy is available.
In addition, we expect that the increased emphasis on managed care and cost containment measures in the United States by third-party payors and government authorities will continue and will place pressure on pharmaceutical pricing and coverage. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more drug products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
If we are unable to obtain and maintain sufficient third-party coverage and adequate reimbursement for our product candidates, the commercial success of our product candidates may be greatly hindered and our financial condition and results of operations may be materially and adversely affected.
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program or other governmental pricing programs once our products come to market, we could be subject to additional reimbursement requirements, penalties, sanctions, and fines, which could have a material adverse effect on our business, financial condition, results of operations, and future prospects.
Pricing and rebate calculations vary across products and programs, are complex, and are often subject to interpretation by manufacturers, governmental or regulatory agencies, and the courts. Such interpretation can change and evolve over time. In the case of Medicaid pricing data, if a manufacturer becomes aware that its reporting for a prior quarter was incorrect or has changed as a result of recalculation of the pricing data, the manufacturer is obligated to resubmit the corrected data for up to three years after those data originally were due. Such restatements and recalculations increase costs for complying with the laws and regulations governing the Medicaid Drug Rebate Program and could result in an overage or underage in rebate liability for past quarters. Price recalculations also may affect the ceiling price at which a manufacturer is required to offer its products under the 340B program.
A failure to comply with reporting and payment obligations under the Medicaid Drug Rebate program and other governmental programs could negatively affect financial results. Centers for Medicare & Medicaid Services (“CMS”) issued a final regulation, which became effective on April 1, 2016, to implement the changes under the Patient Protection and Affordable Care Act, as amended (“ACA”) to the Medicaid Drug Rebate Program. The final regulation has increased and will continue to increase costs and the complexity of compliance, has been and will continue to be time-consuming to implement, and could have a material adverse effect on the results of operations, particularly if CMS challenges the approach a manufacturer has taken in the implementation of the final regulation. Other regulations and coverage expansion by various governmental agencies relating to the Medicaid Drug Rebate Program may have a similar impact.
Manufacturers have obligations to report the average sales price for certain drugs to the Medicare program as a part of the agreement to participate in the Medicaid Drug Rebate program. For calendar quarters beginning January 1, 2022, manufacturers will need to report the average sales price for certain drugs under the Medicare program regardless of whether they participate in the Medicaid Drug Rebate program. Statutory or regulatory changes or CMS guidance could affect the average sales price calculations for products and the resulting Medicare payment rate and could negatively affect results of operations.
| -68- |
The Health Resources and Services Administration (the “HRSA”) issued a final regulation regarding the calculation of the 340B ceiling price and the imposition of civil monetary penalties on manufacturers that knowingly and intentionally overcharge covered entities, which became effective on January 1, 2019. Implementation of this regulation has affected manufacturer obligations and potential liability under the 340B program. Manufacturers are also required to report the 340B ceiling prices for covered outpatient drugs to HRSA, which then publishes them to 340B covered entities. Any charge by HRSA that a manufacturer has violated the requirements of the program or the regulation could negatively affect financial results. Moreover, under a final regulation effective January 13, 2021, HRSA newly established an administrative dispute resolution (“ADR”) process for claims by covered entities that a manufacturer has engaged in overcharging, and by manufacturers that a covered entity violated the prohibitions against diversion or duplicate discounts. Such claims are to be resolved through an ADR panel of government officials rendering a decision that can be appealed to a federal court. An ADR proceeding could subject a manufacturer to onerous procedural requirements and could result in additional liability. Further, any additional future changes to the definition of average manufacturer price and the Medicaid rebate amount under the ACA or otherwise could affect our 340B ceiling price calculations and negatively affect results of operations. Civil monetary penalties can be applied if a manufacturer is (1) found to have knowingly submitted any false price or product information to the government, (2) found to have made a misrepresentation in the reporting of its average sales price, (3) fails to submit the required price data on a timely basis, or (4) found to have charged 340B covered entities more than the statutorily mandated ceiling price. CMS could also decide to terminate the Medicaid drug rebate agreement, or HRSA could decide to terminate the 340B program participation agreement, in which case federal payments may not be available under Medicaid or Medicare Part B for the manufacturer’s covered outpatient drugs.
In addition, manufacturers are required to provide to CMS a 70% discount on brand name prescription drugs utilized by Medicare Part D beneficiaries when those beneficiaries are in the coverage gap phase of the Part D benefit design. Congress could enact legislation that sunsets this discount program and replaces it with a new manufacturer discount program. Civil monetary penalties can be applied if a manufacturer fails to provide these discounts in the amount of 125% of the discount that was due. Congress further could enact a Medicare Part D inflation rebate, under which manufacturers would owe additional rebates if the average manufacturer price of a drug were to increase faster than the pace of inflation.
Pursuant to applicable law, knowing provision of false information in connection with price reporting or contract-based requirements under the U.S. Department of Veterans Affairs (“VA”) Federal Supply Schedule (“FSS”) and/or Tricare programs can subject a manufacturer to civil monetary penalties. These program and contract-based obligations also contain extensive disclosure and certification requirements. If a manufacturer overcharges the government in connection with its arrangements with FSS or Tricare, the manufacturer may be required to refund the difference to the government. Failure to make necessary disclosures or to identify contract overcharges can result in allegations against us under the False Claims Act and other laws and regulations. Unexpected refunds to the government, and/or response to a government investigation or enforcement action, would be expensive and time-consuming and could have a material adverse effect on our business, financial condition, results of operations, and future prospects.
We may not be able to build our marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates.
In order to market and sell any of our product candidates, if and when approved, we will be required to build marketing and sales capabilities. We cannot assure you that we will be successful in doing so or be able to do so in a cost-effective manner. In addition, we may enter into arrangements with third parties to market our product candidates. We may face significant competition for such partners. In addition, marketing and sales arrangements may be time-consuming to negotiate and document. We cannot assure you that we will be able to negotiate arrangements for the marketing and sales of our product candidates on acceptable terms, or at all. Even if we do enter into such arrangements, we cannot assure you that our partners will be successful in commercializing our products. If we or our partners are unable to successfully commercialize our product candidates whether in the United States or elsewhere, our business and results of operations may be materially and adversely affected.
| -69- |
The off-label use or misuse of our products may harm our image in the marketplace, result in injuries that lead to costly product liability suits, or result in costly investigations and regulatory agency sanctions under certain circumstances if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
If any of our product candidates are cleared by the FDA for a specific indication, we may only promote or market that product candidate for its specifically approved indication and make promotional claims consistent with the FDA-required product labeling. We will train our marketing and sales force, or require that any third-party partners train their marketing and sales force, against promoting our product candidates for “off-label uses” that would be inconsistent with FDA law and guidance. With respect to whether communications are consistent with the FDA-required product labeling, we cannot predict whether the FDA will agree with our assessment. We also cannot prevent a physician from using our products off-label, when in the physician’s independent professional medical judgment, he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use our products for uses for which they are not approved. Furthermore, the use of our products for indications other than those approved by the FDA may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients.
If the FDA determines that our promotional materials or training constitute promotion of an off-label or other improper use, it could request that we modify our training or promotional materials, or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil or administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs, mandatory compliance programs under corporate integrity agreements, debarment, refusal of government contracts, and the curtailment of our operations.
These regulations or codes may limit our ability to effectively market our products, or we could run afoul of the requirements imposed by these regulations, causing reputational harm. These regulations or codes may also impose potentially substantial costs on us.
Even if we obtain regulatory approval for a product candidate, our products and business will remain subject to ongoing regulatory obligations and review.
If our product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies and submission of safety, efficacy and other post-market information, including both federal and state requirements in the United States and comparable requirements outside of the United States. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, quality control, promotional and advertising compliance, distribution and supply chain traceability requirements (for example, under the U.S. Drug Supply Chain Security Act), drug sample accountability (under the Prescription Drug Marketing Act (“PDMA”) and similar state laws), complaint handling and pharmacovigilance requirements, and recalls if and when necessary. Any regulatory approvals that we may receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a REMS as a condition of approval of our product candidates, which could include requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. We will also be required to report certain adverse reactions and production problems, if any, to the FDA or other regulatory agencies and to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved label. As such, we may not promote our products for indications or uses for which they do not have FDA or other regulatory agency approval. The holder of an approved NDA or BLA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling, or manufacturing process. We could also be asked to conduct post-marketing clinical studies to verify the safety and efficacy of our product candidates in general or in specific patient subsets. An unsuccessful post-marketing study or failure to complete such a clinical study could result in the withdrawal of marketing approval. Furthermore, any new legislation addressing drug safety issues could result in delays in product development or commercialization or increased costs to assure compliance. Foreign regulatory authorities impose similar requirements. If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or disagrees with the promotion, marketing or labeling of a product, such regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
| ● | issue warning letters asserting that we are in violation of the law; |
| -70- |
| ● | seek an injunction or impose civil or criminal penalties or monetary fines; | |
| ● | suspend or withdraw regulatory approval; | |
| ● | suspend any of our ongoing clinical trials; | |
| ● | refuse to approve pending applications or supplements to approved applications submitted by us or our strategic partners; | |
| ● | restrict the marketing or manufacturing of our products; | |
| ● | seize or detain products, or require a product recall; | |
| ● | refuse to permit the import or export of our product candidates; or | |
| ● | refuse to allow us to enter into government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our product candidates. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our company and our operating results will be adversely affected.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business, prospects, financial condition and results of operations.
If our product candidates are approved for commercialization outside of the United States, we may be exposed to a number of risks associated with international business operations.
If our product candidates are approved for commercialization outside of the United States, we intend to enter into agreements with third parties to market the aforesaid drug products outside of the United States. In such event, we may be subject to risks related to international business operations, including, but not limited to:
| ● | varying levels of protection for intellectual property rights; | |
| ● | changes in tariffs and the imposition of trade barriers; | |
| ● | economic weakness, including inflation or political instability in particular foreign economies and markets; | |
| ● | differing payor reimbursement regimes, governmental payors or patient self-pay systems and price controls; | |
| ● | compliance with tax, employment, immigration and labor laws in respect of employees living or traveling abroad; | |
| ● | foreign tax laws; | |
| ● | currency fluctuations; and | |
| ● | business interruptions resulting from geopolitical actions, such as wars and terrorist attacks, among others, or natural disasters, such as fires, floods, earthquakes and hurricanes, among others. |
| -71- |
Our product candidates may be subject to recalls, withdrawals, seizures or other enforcement actions by the FDA or comparable regulatory authorities in other countries if we fail to comply with regulatory requirements or previously unknown problems with our product candidates are discovered after they reach the market.
The FDA or comparable regulatory authorities in other countries may withdraw approval of our product candidates if we fail to maintain compliance with regulatory requirements or if problems occur after our product candidates reach the market. The discovery of previously unknown problems with a drug product, including adverse events of unanticipated severity or frequency, problems with manufacturing processes or failure to comply with regulatory requirements, including the requirement to promote a drug product only for its approved indications and in accordance with the provisions of its approved label, may result in, among others:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; | |
| ● | warning letters or holds on post-approval clinical trials; | |
| ● | refusal of the FDA to approve pending NDAs or BLAs or supplements to approved NDAs or BLAs, or suspension or revocation of product license approvals; | |
| ● | product seizure or detention, or refusal to permit the import or export of the product; or | |
| ● | injunctions or the imposition of civil or criminal penalties. |
In the event that our drug products are subject to recalls, withdrawals, seizures or other enforcement actions by the FDA or comparable regulatory authorities, our reputation and demand for our drug products could be materially and adversely affected. In addition, we may incur significant and unexpected expenditures and management attention may be diverted in connection with any such recall, withdrawal, seizure or other enforcement action or any corrective action required to be taken, which could have a material and adverse impact on our business and financial condition.
We may not be able to engage third-party contract manufacturing organizations, or CMOs, to manufacture our drug products, if and when approved, on a commercial scale to meet commercial demand for our drug products.
We expect to rely on third-party CMOs or enter into contractual arrangements with third parties to manufacture our product candidates, if and when approved, on a commercial scale. However, we cannot assure you that we will be able to contract with such third parties on acceptable terms, if at all, or that such third parties will satisfy our quality standards or meet our supply requirements in a timely manner, if at all. In addition, only a limited number of manufacturers are capable of supplying pharmaceutical products. The manufacturing process for our product candidates will be highly regulated, and we will need to contract with manufacturers that can meet the relevant regulatory requirements on an ongoing basis. If the third-party manufacturers with whom we contract fail to perform their obligations, we may not be able to meet commercial demand for our product candidates, which would have a material and adverse impact on our business.
If we receive regulatory approval to market any of our product candidates, our relationships with healthcare providers, customers and third-party payors, as well as our general business operations, may be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, and failure to comply with such regulations could expose us to penalties including criminal sanctions, civil penalties, exclusion from government healthcare programs, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, customers and third-party payors will play a primary role in the recommendation and prescription of any product candidates for which we, or a potential future partner, may obtain marketing approval. Future arrangements with third-party payors, healthcare providers and customers and general operations may expose us, or a potential future partner, to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we, or a potential future partner, market, sell and distribute any product candidates for which we, or a potential future partner, obtain marketing approval. There are significant costs involved in complying with these laws and regulations. If we or potential future partners are found to have violated any applicable laws or regulations, significant civil or criminal damages, fines, sanctions or penalties could be imposed, including exclusion from participation in government healthcare programs, such as Medicare, and we may be required to change our method of operations and business strategy. A federal, state, local or foreign government could determine that we or a potential future partner are not operating in accordance with the law, or whether, when or how the laws, or the interpretation thereof, will change in the future and impact our business, financial condition, cash flows and results of operations. Any of these possibilities, if they occur, could adversely affect us.
| -72- |
Restrictions under applicable federal and state healthcare laws and regulations may include the following:
| ● | federal and state healthcare program anti-kickback laws (including the federal Anti-Kickback Statute and Civil Monetary Penalties Law) prohibit, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual for an item or service or the purchasing or ordering of a good or service, the federal and state anti-kickback statutes, which prohibit, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service. While the federal anti-referral laws cover referrals for items or services for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid, some state laws also cover items or services reimbursed by any and all payors. Such anti-kickback laws can be implicated by, among other activities, marketing arrangements with ordering providers, discount or rebate programs or other inducements to purchase our products. Violation of these laws can result in criminal prosecution and imposition of criminal penalties and fines, as well civil monetary penalties and multiple damage judgments, and exclusion from participation in federal healthcare programs; | |
| ● | the federal civil False Claims Act, which imposes liability, including through civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment of government funds or knowingly making, using, or causing to be made or used a false statement or record material to an obligation to pay money to the government, or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. Claims which include items or services resulting from a violation of the federal Anti-Kickback Statute constitute a false or fraudulent claim for purposes of the False Claims Act. There are also criminal penalties possible for making or presenting a false or fictitious or fraudulent claim to the federal government; | |
| ● | the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which imposes criminal and civil liability for, among other things, executing or attempting to execute a scheme or artifice to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; | |
| ● | the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the CMS information related to payments or other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other healthcare professionals beginning in 2022, and teaching hospitals, and requires applicable manufacturers to report annually to the government ownership and investment interests held by the physicians described above and their immediate family members and payments or other “transfers of value” to such physician owners; and | |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; and state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or report marketing expenditures and pricing information. |
| -73- |
Efforts to ensure that our internal operations and business arrangements with third parties comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our current and expected future business activities, including relationships with physicians and other healthcare providers, some of whom we expect will recommend, purchase or prescribe our products, could be subject to challenge under one or more of such laws.
If our operations are found to be in violation of any of these laws or any other governmental laws and regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, disgorgement, contractual damages, reputational harm, diminished profits and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs, which would adversely impact our statement of operations and cash flows.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
For example, in March 2010, President Obama signed into law the ACA. The ACA is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the health and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms.
Certain provisions of the ACA have been subject to judicial challenges as well as efforts to repeal or replace them or to alter their interpretation or implementation. For example, the Tax Act eliminated the shared responsibility payment for individuals who fail to maintain minimum essential coverage under section 5000A of the Code, commonly referred to as the individual mandate, effective January 1, 2019. Additional legislative changes to and regulatory changes under the ACA remain possible, but the nature and extent of such potential additional changes are uncertain at this time. We expect that the ACA, its implementation, efforts to repeal or replace, or invalidate the ACA, or portions thereof, and other healthcare reform measures that may be adopted in the future could have a material adverse effect on our industry generally and on our ability to maintain or increase sales of existing products or to successfully commercialize product candidates, if approved.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals for spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction, triggering the legislation’s automatic reduction to several government programs. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which began in 2013, and due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022. On December 10, 2021, President Biden signed a law that provides for 1% Medicare sequestration in the second quarter of 2022 and allows the full 2% sequestration thereafter until 2030. To offset the temporary suspension during the COVID-19 pandemic, in 2030, the sequestration will be 2.25% for the first half of the year and 3% in the second half of the year.
| -74- |
Further, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. In addition, in May 2019, the CMS issued a final rule to allow Medicare Advantage Plans the option of using step therapy, a type of prior authorization, for Part B drugs beginning January 1, 2020. This final rule codified CMS’ policy change that was effective January 1, 2019. On March 11, 2021, the President signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. At the state level, individual states have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
We expect that additional state and federal healthcare reform measures will be adopted in the future. Such reform measures may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements. It is also possible that additional governmental action could be taken in response to the COVID-19 pandemic.
Changes in and failures to comply with U.S. and foreign privacy and data protection laws, regulations and standards may adversely affect our business, operations and financial performance.
We are subject to or affected by numerous federal, state and foreign laws and regulations, as well as regulatory guidance, governing the collection, use, disclosure, retention, and security of personal data, such as information that we collect about patients and healthcare providers in connection with clinical trials in the United States and abroad. The global data protection landscape is ever evolving, and implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future. This evolution may create uncertainty in our business, affect our or our collaborators’, service providers’ and contractors’ ability to operate in certain jurisdictions or to collect, store, transfer, use and share personal information, necessitate the acceptance of more onerous obligations in our contracts, result in liability or impose additional costs on us. The cost of compliance with these laws, regulations and standards is high and is likely to increase in the future. Any failure or perceived failure by us or our collaborators, service providers and contractors to comply with federal, state or foreign laws or regulation, our internal policies and procedures or our contracts governing processing of personal information could result in negative publicity, diversion of management time and effort and proceedings against us by governmental entities or others. In many jurisdictions, enforcement actions and consequences for noncompliance are rising.
In the United States, HIPAA imposes privacy, security and breach reporting obligations upon “covered entities” (health plans, health care clearinghouses and certain health care providers) and their respective business associates that create, receive, maintain or transmit protected health information in connection with providing a specified service or performing a function for or on behalf of a covered entity. HIPAA mandates the reporting of certain breaches of protected health information to the Department of Health and Human Services (“HHS”), affected individuals and, if the breach is large enough, the media. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, or PHI, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. Even when HIPAA does not apply, according to the Federal Trade Commission (“FTC”), failing to take appropriate steps to keep consumers’ personal information secure, or failing to provide a level of security commensurate to promises made to individuals about the security of their personal information (such as in a privacy notice), may constitute unfair or deceptive acts or practices in violation of Section 5(a) of the Federal Trade Commission Act (“FTC Act”). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule. Enforcement by the FTC under the FTC Act can result in civil penalties or enforcement actions.
| -75- |
In addition, certain state laws govern the privacy and security of health information in certain circumstances, some of which are more stringent, broader in scope or offer greater individual rights with respect to PHI than HIPAA and many of which may differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, California enacted the California Consumer Privacy Act (“CCPA”) which took effect on January 1, 2020 and granted California residents new rights with regard to their personal information. The CCPA gives California residents privacy rights, including rights to access and delete their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Further, in November 2020, California voters approved the California Privacy Rights Act (the “CPRA”) ballot initiative, which significantly amends the CCPA, and imposes additional data protection obligations on covered businesses that collect personal information from California residents, including additional consumer rights processes, limitations on data uses, new audit requirements for high risk data, and opt outs for certain uses of sensitive data. It also creates a new California data protection agency specifically tasked to enforce the law, which would likely result in increased regulatory scrutiny of covered businesses in the areas of data protection and security. The majority of the provisions will go into effect on January 1, 2023, and additional compliance investment and potential business may be required. The CCPA, the CPRA or other domestic privacy and data protection laws and regulations may increase our compliance costs and potential liability.
Any operations we may have abroad may also be subject to increased scrutiny or attention from data protection authorities. Many countries have established or are in the process of establishing privacy and data security legal frameworks with which we, our collaborators, service providers, CROs, and contractors must comply. For example, the EU has adopted the EU General Data Protection Regulation (EU) 2016/679, or GDPR, which increases our obligations and liability for processing the personal information of individuals located in the European Economic Area (“EEA”) and United Kingdom, including clinical trial data. The GDPR has and will continue to increase compliance burdens on us to the extent we are operating in the applicable areas, including by mandating potentially burdensome documentation requirements and granting certain rights to individuals to control how we collect, use, disclose, retain and process information about them.
The processing of sensitive personal data, such as physical health condition, may impose heightened compliance burdens under the GDPR and is a topic of active interest among foreign regulators. In addition, the GDPR provides for more robust regulatory enforcement of data protection requirements and potential fines of up to 20 million Euros or 4% of the annual global revenue of the noncompliant company, whichever is greater. In addition, the GDPR increases the scrutiny of transfers of personal data from clinical trial sites located in the EEA to the United States and other jurisdictions that the European Commission does not recognize as having “adequate” data protection laws; in July 2020, the Court of Justice of the European Union limited how organizations could lawfully transfer personal data from the EEA to the United States by invalidating the EU-US Privacy Shield and imposing further restrictions on use of the standard contractual clauses, which could increase our costs and our ability to efficiently process personal data from the EEA. Additionally, following the United Kingdom’s withdrawal from the EEA and the EU, and the expiry of the transition period, companies will have to comply with the GDPR and the GDPR as incorporated into United Kingdom national law, the latter regime having the ability to separately fine up to the greater of 17.5 million pounds or 4% of global turnover. If we expand into other foreign countries and jurisdictions, we may be subject to additional laws and regulations that may affect how we conduct business. Additionally, our use of connected devices increases security risks to personal information, including by increasing the number of third-party device components, broadening the device’s attack surface, and relying on internet connections that we cannot control, and failure to secure personal data may be actionable under these or other local laws. In addition to the threat of unauthorized access or acquisition of sensitive or personal information, other threats include the deployment of harmful malware, ransomware attacks, denial-of-service (“DoS”) attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information.
| -76- |
The landscape of laws regulating personal data is constantly evolving, and compliance with these laws requires a flexible privacy framework and substantial resources, and compliance efforts will likely be an increasing and substantial cost in the future.
Risks Related to our Intellectual Property
Our commercial success depends largely on our ability to adequately protect our intellectual property.
Our commercial success depends, in large part, on our ability to obtain and maintain patent protection and trade secret protection in the United States and elsewhere in respect of our product candidates and inhalation technology. If we fail to adequately protect our intellectual property rights, our competitors may be able to erode, negate or preempt any competitive advantage we may have. To protect our competitive position, we have filed and will continue to file for patents in the United States and elsewhere in respect of our product candidates and inhalation technology. We own a number of both granted and pending domestic and international patents and applications and have a license to additional patents and applications. The process of identifying patentable subject matter and filing a patent application is expensive and time-consuming. We cannot assure you that we will be able to file the necessary or desirable patent applications at a reasonable cost, in a timely manner, or at all. Further, since certain patent applications are confidential until patents are issued or published, third parties may have filed patent applications for subject matters covered by our pending patent applications without us being aware of such applications, and our patent applications may not have priority over patent applications of others. In addition, we cannot assure you that our pending patent applications will result in patents being obtained. For example, an anonymous party filed a pre-grant opposition to one of our patent applications in India in January 2019, which is set for a hearing in January 2022. While we believe we have strong arguments to support grant of the patent, we may be unsuccessful, in which case that patent would not be granted. The standards that patent offices in different jurisdictions use to grant patents are not always applied predictably or uniformly and may change from time to time.
Even if we have been or are able to obtain patent protection for our product candidates or inhalation technology, if the scope of such patent protection is not sufficiently broad, we may not be able to rely on such patent protection to prevent third parties from developing or commercializing product candidates or technology that may copy or closely approach our product candidates or technology. The enforceability of patents in the pharmaceutical industry involves complex legal and scientific questions and can be uncertain. Accordingly, we cannot assure you that third parties will not successfully challenge the validity, enforceability or scope of our patents. A successful challenge to our patents may lead to generic versions of our drug products being launched before the expiry of our patents or otherwise limit our ability to stop others from using or commercializing similar or identical products and technology. A successful challenge to our patents may also reduce the duration of the patent protection of our drug products or technology. If any of our patents are narrowed or invalidated, our business and prospects may be materially and adversely affected. In addition, we cannot assure you that we will be able to detect unauthorized use or take appropriate, adequate and timely actions to enforce our intellectual property rights. If we are unable to adequately protect our intellectual property, our business, competitive position and prospects may be materially and adversely affected.
Even if our patents or patent applications are unchallenged, they may not adequately protect our intellectual property or prevent third parties from designing around our patents or other intellectual property rights. If the patent applications we file or may file do not lead to patents being granted or if the scope of any of our patent applications is challenged, we may face difficulties in developing our product candidates, companies may be dissuaded from collaborating with us, and our ability to commercialize our product candidates may be materially and adversely affected. We are unable to predict which of our patent applications will lead to patents or assure you that any of our patents will not be found invalid or unenforceable or challenged by third parties. The patents of others may prevent the commercialization of product candidates incorporating our technology. In addition, given the amount of time required for the development, clinical testing and regulatory review of new product candidates, any patents protecting our product candidates may expire before or shortly after such product candidates might become approved for commercialization.
| -77- |
Moreover, the issuance of a patent is not conclusive as to the inventorship of the patented subject matter, or its scope, validity or enforceability. We cannot assure you that all of the potentially relevant prior art, that is, any evidence that an invention is already known, relating to our patents and patent applications has been found. If such prior art exists, it may be used to invalidate a patent or may prevent a patent from being issued.
In addition, we, our collaborators, our licensors, or our licensees may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. As a result, we may miss potential opportunities to seek patent protection or strengthen our patent position.
If we are unable to protect our trade secrets, the value of our product candidates and inhalation technology may be negatively impacted, which would have a material and adverse effect on our competitive position and prospects.
In addition to patent protection, we rely on trade secret protection to protect certain aspects of our intellectual property. While we require parties who have access to any portion of our trade secrets, such as our employees, consultants, advisers, CROs, CMOs, collaborators and other third parties, to enter into non-disclosure and confidentiality agreements with us, we cannot assure you that these parties will not disclose our proprietary information, including our trade secrets, in breach of their contractual obligations. Enforcing a claim that a party has illegally disclosed or misappropriated a trade secret is difficult, costly and time-consuming, and we may not be successful in doing so. If the steps we have taken to protect our trade secrets are deemed by the adjudicating court to be inadequate, we may not be able to obtain adequate recourse against a party for misappropriating our trade secrets.
Trade secrets can be difficult to protect as they may, over time, be independently discovered by our competitors or otherwise become known despite our trade secret protection. If any of our trade secrets were to be lawfully obtained or independently developed by our competitors, we would have no right to prevent such competitors, or those to whom they communicate such technology or information, from using that technology or information to compete with us. Such competitors could attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights.
If our trade secrets were to be disclosed to or independently developed by our competitors, our competitors may be able to exploit our inhalation technology to develop competing product candidates, and the value of our inhalation technology and our product candidates may be negatively impacted. This would have a material and adverse effect on our competitive position and prospects.
We rely on licenses to intellectual property that are owned by third parties.
We have entered, and may in the future enter, into license agreements with third parties to license the rights to use their technologies in our research, development and commercialization activities, including but not limited to the Vectura License. License agreements generally impose various diligence, milestone payments, royalty, insurance and other obligations on us, and if we fail to comply with these obligations, our licensors may have the right to terminate these license agreements. Termination of these license agreements or the reduction or elimination of our licensed rights or the exclusivity of our licensed rights may have an adverse impact on, among others, our ability to develop and commercialize our product candidates. We cannot assure you that we will be able to negotiate new or reinstated licenses on commercially acceptable terms, or at all.
| -78- |
In addition, we license certain rights to develop and commercialize insulin and GLP-1 solution delivery devices containing the vibrating mesh technology used in our AFINA™ inhalation device pursuant to a license agreement with Philips Electronics UK. If our agreement with PEUK were to terminate, it would be difficult to replace because it is the sole supplier of our vibrating mesh technology. Under our agreement, we have an exclusive license to the vibrating mesh technology for use with insulin inhalers for the treatment of diabetes, excluding insulin delivery in an acute care hospital environment, and for the development and commercialization of GLP-1 solutions formulated for use with delivery devices containing the vibrating mesh aerosolization technology. If the PEUK agreement is terminated under certain circumstances, then we have the ability to seek other suppliers and PEUK is obligated to provide assistance to the alternative supplier so as to enable it to manufacture the components currently being manufactured by PEUK. However, there is no assurance that PEUK would be willing or able to perform in such circumstances. If our license with PEUK were to terminate, we would no longer have the right to use the PEUK vibrating mesh in our AFINA™ device in connection with AER-501 and AER-601. In addition, there can be no assurance that we will be able to license the vibrating mesh technology for use with any product candidates other than AER-501 and AER-601 that we may develop or in-license in the future. Furthermore, the license agreement provides that technology jointly developed by PEUK and us relating to the AFINA™ device is owned by PEUK, with an exclusive license to us in the fields of insulin and GLP-1 solutions. We would have to utilize an alternative aerosol generator component, which may require us to enter into a license agreement with one of a limited number of other suppliers. There are a limited number of other vibrating mesh suppliers in the world and there is no assurance that we would be able to execute an agreement with an alternative supplier. Even if we do enter into an arrangement with an alternative supplier to license such supplier’s technology or manufacture our vibrating mesh technology, such supplier may fail to provide aerosol generator components of adequate quality, quantity and/or pricing acceptable to us. In addition, any such substitution of the aerosol generator component in our product candidates may result in regulatory authorities requiring us to conduct additional studies to generate bridging or validating clinical performance data in order to ensure that the safety and efficacy of the product is not adversely affected. Such a requirement could result in regulatory and development delays.
Also, the agreements under which we license patent rights may not give us control over patent prosecution or maintenance, so that we may not be able to control which claims or arguments are presented and may not be able to secure, maintain or successfully enforce necessary or desirable patent protection from those patent rights. We do not have primary control over patent prosecution and maintenance for certain of the patents we license, and therefore cannot assure you that these patents and applications will be prosecuted or maintained in a manner consistent with the best interests of our business. We also cannot assure you that patent prosecution and maintenance activities by our licensors, if any, will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents.
Pursuant to the terms of some of our license agreements with third parties, some of our third-party licensors have the right, but not the obligation, in certain circumstances, to control the enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents. Even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our licensors, and we cannot assure you that we will receive such cooperation on commercially acceptable terms, or at all. We also cannot assure you that our licensors will allocate sufficient resources or prioritize their or our enforcement of these patents or defense of these claims to protect our interests in the licensed patents. If we cannot obtain patent protection, or enforce existing or future patents against third parties, our competitive position, business and prospects may be materially and adversely affected.
Further, licenses to intellectual property may not always be available to us on commercially acceptable terms, or at all. In the event that the licenses we rely on are not available to us on commercially acceptable terms, or at all, our ability to commercialize our product candidates or inhalation technology, and our business and prospects, may be materially and adversely affected.
We may become involved in litigation to protect our intellectual property or enforce our intellectual property rights, which could be expensive, time-consuming and may not be successful.
Competitors may infringe our patents or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, we may engage in litigation to, among others, enforce or defend our intellectual property rights, determine the validity or scope of our intellectual property rights and those of third parties, and protect our trade secrets. Such actions may be time-consuming and costly and may divert our management’s attention from our core business and reduce the resources available for our product development and other operating activities, and consequently have a material and adverse effect on our business and prospects, regardless of the outcome.
In addition, in an infringement proceeding, a court may decide that a patent owned by, or licensed to, us is invalid or unenforceable, or may refuse to stop the other party from using the technology in question on the ground that our patents do not cover such technology. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that our confidential information may be compromised by disclosure.
| -79- |
We may be subject to claims that our employees or consultants have wrongfully used or disclosed to us alleged trade secrets of their former employers or other clients.
We employ and in the future may employ individuals who were previously employed at universities or other diagnostic, life sciences or device companies, including our competitors or potential competitors, and who may have entered into proprietary rights, non-disclosure and non-competition agreements or similar agreements, in connection with such previous employment. Moreover, we engage the services of scientific advisers and consultants to assist us in the development of our products, many of whom were previously employed at or may have previously been or are currently providing consulting or advisory services to, other biotechnology or pharmaceutical companies, and who may have also entered into proprietary rights, non-disclosure and non-competition (or similar) agreements with such other companies.
While we require that our employees, scientific advisers and consultants do not use the proprietary information or know-how of others in their work for us, we cannot assure you that we will not be subject to claims that we or these employees, scientific advisers, consultants or independent contractors have inadvertently or otherwise used or disclosed the trade secrets or proprietary information of their former employers or former or present clients in their work for us, especially where such former employers or former or present clients are our competitors or potential competitors. Claims brought against us could cause us to incur unexpected and substantial costs, as well as divert our management’s attention from our core business and reduce the resources available for our clinical development, manufacturing and marketing activities. Consequently, our business may be materially and adversely affected.
We may be subject to claims from third parties that our products infringe their intellectual property rights.
The pharmaceutical industry has experienced rapid technological change and obsolescence in the past, and our competitors have strong incentives to stop or delay any introduction of new drug products or related technologies by, among others, establishing intellectual property rights over their drug products or technologies and aggressively enforcing these rights against potential new entrants into the market. We expect that we and other industry participants will be increasingly subject to infringement claims as the number of competitors and drug products grows.
Our commercial success depends in large part upon our ability to develop, manufacture, market and sell our product candidates without infringing on the patents or other proprietary rights of third parties. It is not always clear to industry participants, including us, what the scope of a patent covers. Due to the large number of patents in issue and patent applications filed in our industry, there is a risk that third parties will claim that our product candidates or technologies infringe their intellectual property rights.
Claims for infringement of intellectual property which are brought against us, whether with or without merit, and which are generally uninsurable, could result in time-consuming and costly litigation, diverting our management’s attention from our core business and reducing the resources available for our product development and other operating activities, and consequently have a material and adverse effect on our business and prospects, regardless of the outcome. Moreover, such proceedings could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not being issued. We also may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Uncertainties resulting from the initiation and continuation of litigation or other proceedings could also have a material and adverse effect on our ability to compete in the market. Third parties making claims against us could obtain injunctive or other equitable relief against us, which could prevent us from further developing or commercializing our product candidates.
In particular, we may be required to include a certification of patent invalidity or non-infringement, or a paragraph IV certification, in an NDA submitted under the 505(b)(2) regulatory pathway, to certify that a patent over a reference listed drug is invalid, unenforceable or will not be infringed by the manufacture, use or sale of our product candidate. The holder of such patent may file a patent infringement lawsuit against us after receiving notice of the paragraph IV certification. Any such patent infringement lawsuit, if filed, will trigger a one-time, automatic, 30-month stay of the FDA’s ability to approve our application, unless the patent litigation is resolved in our favor or the patent expires before that time. Accordingly, we may invest a significant amount of time and expense in the development of a product candidate only to be subject to significant delay and incur substantial costs in litigation before such product candidate may be commercialized, if at all. Companies that produce reference listed drugs routinely bring claims for patent infringement against applicants under the 505(b)(2) regulatory pathway that are seeking regulatory approval to manufacture and market generic or reformulated forms of their reference listed drugs.
| -80- |
In the event of a successful infringement claim against us, including an infringement claim filed in response to a paragraph IV certification, we may be required to pay damages, cease the development or commercialization of our drug products or product candidates, re-engineer or redevelop our drug products or product candidates or enter into royalty or licensing agreements, any of which could have a material and adverse impact on our business, financial condition and results of operations. Any effort to re-engineer or redevelop our products would require additional monies and time to be expended and may not ultimately be successful.
Infringement claims may be brought against us in the future, and we cannot assure you that we will prevail in any ensuing litigation given the complex technical issues and inherent uncertainties involved in intellectual property litigation. Our competitors may have substantially greater resources than we do and may be able to sustain the costs of such litigation more effectively than we can.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after its earliest claimed priority date. While various extensions may be available, the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized.
We intend to seek extensions of patent terms in the United States and, if available, in other countries where we prosecute patents. In the United States, the U.S. Drug Price Competition and Patent Term Restoration Act of 1984, as amended, or the Hatch-Waxman Act, permits patent owners to request a patent term extension, based on regulatory review period for a product, of up to five years beyond the normal expiration of the patent, which is limited to one patent claiming the approved drug product or use in an indication (or any additional indications approved during the period of extension). However, the applicable authorities, including the FDA and the U.S. Patent and Trademark Office (the “USPTO”) in the United States and comparable regulatory authorities in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or grant more limited extensions than we had requested. In such event, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our preclinical and clinical data in their marketing approval applications with the FDA to launch their drug product earlier than might otherwise be the case.
If we fail to comply with various procedural, document submission, fee payment or other requirements imposed by the USPTO or comparable patent agencies in other countries, our patent protection could be reduced or eliminated.
We are required, over the lifetime of an issued patent, to pay periodic maintenance fees to the USPTO and comparable patent agencies in other countries. We are also required by such patent agencies to comply with a number of procedural, documentary, fee payment and other conditions during the patent application process. While an inadvertent lapse can, in many cases, be cured by payment of a late fee or other means in accordance with the applicable rules, there are situations in which non-compliance can result in abandonment or lapse of a patent or patent application, resulting in the partial or complete loss of patent rights in the relevant jurisdiction. Such situations include, but are not limited to:
| ● | a failure to respond to official actions within the prescribed time limits; | |
| ● | the non-payment of fees; and | |
| ● | a failure to properly legalize and submit formal documents. |
If we or our licensors, which control the prosecution and maintenance of patents which we license, fail to maintain the patents or patent applications covering our product candidates or technology, such rights would be reduced or eliminated and, consequently, our competitive position, business and prospects may be materially and adversely affected.
| -81- |
Changes in patent laws or interpretations of patent laws in the United States or elsewhere may diminish the value of our intellectual property or narrow the scope of protection of our patents.
Changes to patent laws in the United States or other countries could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. For example, changes to the United States patent system have come into force under the Leahy-Smith America Invents Act, or the Leahy-Smith Act, which was signed into law in September 2011. Under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first to file” system in which the first inventor to file a patent application will be entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the USPTO, and may become involved in opposition, derivation, reexamination, inter partes review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, which could adversely affect our competitive position. While we cannot predict with certainty the impact the Leahy-Smith Act or any potential future changes to the United States or foreign patent systems will have on the operation of our business, the Leahy-Smith Act and such future changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, results of operations, financial condition and prospects. Additionally, the first to file system under the Leahy-Smith Act may incentivize companies like us in the pharmaceutical industry to file patent applications as soon as possible, and filing applications as soon as possible runs the risk that the application will not have the supporting data to claim the broadest protection possible in the United States.
In addition, recent court rulings in the United States have narrowed the scope of patent protection available and weakened the rights of patent owners, particularly in the pharmaceutical industry. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances, modifying some legal standards applied by the USPTO in examination of patent applications or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents, increase the likelihood of challenges to patents we obtain or license or weaken our ability to enforce patents that we have licensed or that we might obtain in the future.
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting, enforcing and defending patents on our inhalation technology and our product candidates throughout the world may be prohibitively expensive and may not be financially or commercially feasible. In countries where we have not obtained patent protection, our competitors may be able to use our proprietary technologies to develop competing product candidates.
Also, the legal systems of non-U.S. jurisdictions may not protect intellectual property rights to the same extent or in the same manner as the laws of the United States, and we may face significant difficulty in enforcing our intellectual property rights in these jurisdictions. The legal systems of certain developing countries may not favor the enforcement of patents and other intellectual property rights. We may therefore face difficulty in stopping the infringement or misappropriation of our patents or other intellectual property rights in those countries.
We need to protect our trademark, trade name and service mark rights to prevent competitors from taking advantage of our goodwill.
We have filed certain and expect to file additional trademark registrations for potential trade names for our product candidates and our AFINA™ device in relevant jurisdictions. We may not be granted registration of any potential trade names for our product candidates, and the use of any of our trademarks may not confer a competitive advantage in the marketplace. Furthermore, even if we are successful in our trademark registrations, the FDA has its own process for drug nomenclature and its own views concerning appropriate proprietary names. The FDA also has the power, even after granting market approval, to request a company to reconsider the name for a product because of evidence of confusion in the marketplace. The FDA and any other regulatory authorities may not approve of our trademarks or may request reconsideration of our trademarks at some time in the future. We believe that the protection of our trademark, trade name and service mark rights is an important factor in product recognition, protecting our brand, maintaining goodwill and maintaining or increasing market share. We may expend substantial cost and effort in an attempt to register new trademarks, trade names and service marks and maintain and enforce our trademark, trade name and service mark rights. If we do not adequately protect our rights in our trademarks, trade names and service marks from infringement, any goodwill that we have developed in those trademarks could be lost or impaired.
| -82- |
Third parties may claim that the sale or promotion of our products, when and if approved, may infringe on the trademark, trade name and service mark rights of others. Trademark, trade name and service mark infringement problems occur frequently in connection with the sale and marketing of pharmaceutical products. If we become involved in any dispute regarding our trademark, trade name and service mark rights, regardless of whether we prevail, we could be required to engage in costly, distracting and time-consuming litigation that could harm our business. If the trademarks, trade names and service marks we use are found to infringe upon the trademarks, trade names or service marks of another company, we could be liable for damages and be forced to stop using those trademarks, trade names or service marks, and as result, we could lose all the goodwill that has been developed in those trademarks, trade names or service marks.
Risks Related to Becoming a Public Company
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. We will be subject to the reporting requirements of the Exchange Act, which will require, among other things, that we file with the SEC annual, quarterly and current reports with respect to our business and financial condition. In addition, Sarbanes-Oxley, as well as rules subsequently adopted by the SEC and Nasdaq to implement provisions of Sarbanes-Oxley, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Further, pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, the SEC has adopted additional rules and regulations in these areas, such as mandatory “say on pay” voting requirements that will apply to us when we cease to be an emerging growth company. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
We expect the rules and regulations applicable to public companies to substantially increase our legal and financial compliance costs and to make some activities more time consuming and costly. If these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations. The increased costs increase our net loss and may require us to reduce costs in other areas of our business. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors and our board committees or as executive officers.
Risks Related to Combined Company Common Stock
The market price of Combined Company common stock is likely to be highly volatile, and you may lose some or all of your investment.
Following the Business Combination, the market price of Combined Company common stock is likely to be highly volatile and may be subject to wide fluctuations in response to a variety of factors, including the following:
| ● | the impact of the COVID-19 pandemic on Aerami’s business; |
| -83- |
| ● | the inability to obtain or maintain the listing of the Combined Company’s shares of common stock on Nasdaq; | |
| ● | the inability to recognize the anticipated benefits of the Business Combination, which may be affected by, among other things, competition and Aerami’s ability to grow and manage growth profitably and retain its key employees; | |
| ● | changes in applicable laws or regulations; | |
| ● | risks relating to the uncertainty of Aerami’s projected financial information; | |
| ● | the success of Aerami’s clinical trials and preclinical studies for its product candidates; | |
| ● | unexpected adverse side effects or inadequate efficacy of Aerami’s product candidates that may limit their development, regulatory approval and/or commercialization or may result in recalls or product liability claims; | |
| ● | risks related to the organic and inorganic growth of Aerami’s business and the timing of expected business milestones; and | |
| ● | the amount of redemption requests made by FoxWayne’s stockholders. |
In addition, the stock markets have experienced extreme price and volume fluctuations that affected and continue to affect the market prices of equity securities of many companies. These fluctuations have often been unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors, as well as general economic, political, regulatory and market conditions, may negatively affect the market price of Combined Company common stock, regardless of the Combined Company’s actual operating performance.
Volatility in the Combined Company’s share price could subject the Combined Company to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. Shareholder activism, which could take many forms or arise in a variety of situations, has been increasing recently. Volatility in the stock price of the Combined Company’s Common Stock or other reasons may in the future cause it to become the target of securities litigation or shareholder activism. Securities litigation and shareholder activism, including potential proxy contests, could result in substantial costs and divert management’s and the attention of the Combined Company’s Board and resources from the Combined Company’s business. Additionally, such securities litigation and shareholder activism could give rise to perceived uncertainties as to the Combined Company’s future, adversely affect its relationships with service providers and make it more difficult to attract and retain qualified personnel. Also, the Combined Company may be required to incur significant legal fees and other expenses related to any securities litigation and activist shareholder matters. If the Combined Company faces such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm its business.
If securities or industry analysts do not publish research or reports about the Combined Company, or publish negative reports, the Combined Company’s stock price and trading volume could decline.
The trading market for Combined Company common stock will depend, in part, on the research and reports that securities or industry analysts publish about the Combined Company. The Combined Company does not have any control over these analysts. If the Combined Company’s financial performance fails to meet analyst estimates or one or more of the analysts who cover the Combined Company downgrade its common stock or change their opinion, or provide more favorable relative recommendations about our competitors, the Combined Company’s stock price would likely decline. If one or more of these analysts cease coverage of the Combined Company or fail to regularly publish reports on the Combined Company, it could lose visibility in the financial markets, which could cause the Combined Company’s stock price or trading volume to decline.
Because the Combined Company does not anticipate paying any cash dividends in the foreseeable future, capital appreciation, if any, would be your sole source of gain.
The Combined Company currently anticipates that it will retain future earnings for the development, operation and expansion of its business and does not anticipate declaring or paying any cash dividends for the foreseeable future. As a result, capital appreciation, if any, of the Combined Company’s shares of common stock would be your sole source of gain on an investment in such shares for the foreseeable future.
Future sales of shares of Combined Company common stock may depress its stock price.
Pursuant to the Registration Rights Agreement and the Proposed Bylaws, after the consummation of the Business Combination and subject to certain exceptions, the Sponsor, those receiving shares of Combined Company common stock pursuant to the Merger Agreement, directors, officers and employees of Aerami receiving shares of Combined Company common stock upon the settlement or exercise of warrants, stock options or other equity awards, and warrant holders of Aerami receiving shares of Combined Company common stock upon the settlement or exercise of such warrants (other than holders of warrants that are currently listed) will be contractually restricted from selling or transferring any of their shares of common stock. Such restrictions begin at Closing and end, in the case of the shares that are restricted pursuant to the Proposed Bylaws, on the date that is 180 days after the date that the Form S-1 Shelf (as that term is defined in the Registration Rights Agreement) is declared effective by the SEC, and, in the case of the shares restricted pursuant to the Lock-up Agreement, on such dates as are described in the section titled “Proposal 1 - The Transaction Proposal — Related Agreements.”
| -84- |
However, following the expiration of the applicable lock-up period, such equityholders will not be restricted from selling shares of Combined Company common stock held by them, other than by applicable securities laws. Further, because the Combined Company is not expected to generate revenue in the near future, there is a likelihood that it will need to continue to raise capital through one or more equity financings in order to continue developing its product candidates. As such, sales of a substantial number of shares of Combined Company common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of Combined Company common stock. As restrictions on resale end and registration statements (filed after the Closing to provide for the resale of such shares from time to time) are available for use, the sale or possibility of sale of these shares could have the effect of increasing the volatility in the Combined Company’s share price or the market price of Combined Company common stock could decline if the holders of currently restricted shares sell them or are perceived by the market as intending to sell them.
The Proposed Charter and Proposed Bylaws that will be in effect immediately prior to the Effective Time will contain provisions that could significantly reduce the value of our shares to a potential acquiror or delay or prevent changes in control or changes in our management without the consent of our board of directors. The provisions in the Proposed Charter and Proposed Bylaws will include the following:
| ● | a classified board of directors with three-year staggered terms, which may delay the ability of stockholders to change the membership of a majority of our board of directors; | |
| ● | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; | |
| ● | the exclusive right of our board of directors, unless the board of directors grants such right to the stockholders, to elect a director to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; | |
| ● | the required approval of at least two-thirds of the shares entitled to vote to remove a director for cause, and the prohibition on removal of directors without cause; | |
| ● | the ability of our board of directors to authorize the issuance of shares of preferred stock and to determine the price and other terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquiror; | |
| ● | the ability of our board of directors to alter the Proposed Bylaws without obtaining stockholder approval; | |
| ● | the required approval of at least two-thirds of the shares entitled to vote to adopt, amend or repeal the Proposed Bylaws or repeal the provisions of the Proposed Charter regarding the election and removal of directors; | |
| ● | a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders; | |
| ● | an exclusive forum provision providing that the Court of Chancery of the State of Delaware will be the exclusive forum for certain actions and proceedings; | |
| ● | the requirement that a special meeting of stockholders may be called only by the board of directors or the chief executive officer, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors; and | |
| ● | advance notice procedures that stockholders must comply with in order to nominate candidates to our board of directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of us. |
We have opted out of the anti-takeover provisions contained in Section 203 of the Delaware General Corporation Law.
| -85- |
Our Proposed Charter will provide that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between us and our stockholders and that the federal district courts shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees or the underwriters of any offering giving rise to such claim.
The Proposed Charter will provide that (a) the Court of Chancery (the “Chancery Court”) of the State of Delaware (or, in the event that the Chancery Court does not have jurisdiction, the federal district court for the District of Delaware or other state courts of the State of Delaware) will be the sole and exclusive forum for (i) any derivative action, suit or proceeding brought on behalf of the Combined Company, (ii) any action, suit or proceeding asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, other employee or stockholder of the Combined Company to the Combined Company or to the Combined Company stockholders, (iii) any action, suit or proceeding arising pursuant to any provision of the DGCL or the Proposed Charter or Proposed Bylaws (as may be amended from time to time) or as to which the DGCL confers jurisdiction on the Chancery Court or (iv) any action, suit or proceeding asserting a claim against the Combined Company governed by the internal affairs doctrine; and (b) the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended. If any action the subject matter of which is within the scope of clause (a) of the immediately preceding sentence is filed in a court other than the courts in the State of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented to (x) the personal jurisdiction of the state and federal courts in the State of Delaware in connection with any action brought in any such court to enforce the provisions of clause (a) of the immediately preceding sentence and (y) having service of process made upon such stockholder in any such action by service upon such stockholder’s counsel in the Foreign Action as agent for such stockholder.
For the avoidance of doubt, this provision is intended to benefit and may be enforced by us, our officers and directors, the underwriters to any offering giving rise to such complaint, and any other professional entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying the offering. These choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees and result in increased costs for investors to bring a claim. By agreeing to this provision, however, stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder. Furthermore, the enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable. If a court were to find the choice of forum provisions in the Proposed Charter to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition.
The Combined Company is an emerging growth company and smaller reporting company, and the Combined Company cannot be certain if the reduced reporting requirements applicable to emerging growth companies and smaller reporting companies will make its shares less attractive to investors.
After the completion of the Business Combination, the Combined Company will be an emerging growth company, as defined in the JOBS Act. For as long as the Combined Company continues to be an emerging growth company, it may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including exemption from compliance with the auditor attestation requirements under Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. The Combined Company will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the closing of the initial public offering (December 31, 2026), (b) in which the Combined Company has total annual gross revenue of at least $1.07 billion or (c) in which the Combined Company is deemed to be a large accelerated filer, which means the market value of shares of Combined Company common stock that are held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which the Combined Company has issued more than $1.0 billion in non-convertible debt during the prior three-year period.
| -86- |
In addition, under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. The Combined Company has elected not to use this extended transition period for complying with new or revised accounting standards and, therefore, the Combined Company will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Following the Business Combination, we will also be a smaller reporting company as defined in the Exchange Act. Even after the Combined Company no longer qualifies as an emerging growth company, it may still qualify as a “smaller reporting company,” which would allow it to take advantage of many of the same exemptions from disclosure requirements including exemption from compliance with the auditor attestation requirements of Section 404 and reduced disclosure obligations regarding executive compensation in this proxy statement/prospectus and the Combined Company’s periodic reports and proxy statements. The Combined Company will be able to take advantage of these scaled disclosures for so long as its voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of its second fiscal quarter, or its annual revenue is less than $100.0 million during the most recently completed fiscal year for which audited financials are available and its voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of its second fiscal quarter.
The Combined Company cannot predict if investors will find its common stock less attractive because the Combined Company may rely on these exemptions. If some investors find Combined Company common stock less attractive as a result, there may be a less active trading market for the common stock and its market price may be more volatile.
General Risk Factors
Changes in laws or regulations, or a failure to comply with any laws or regulations, may adversely affect our business, investments and results of operations.
We are subject to laws and regulations enacted by national, regional and local governments. In particular, we are required to comply with certain SEC and other legal requirements. Compliance with, and monitoring of, applicable laws and regulations may be difficult, time consuming and costly. Those laws and regulations and their interpretation and application may also change from time to time, and those changes could have a material adverse effect on our business, investments and results of operations. In addition, a failure to comply with applicable laws or regulations, as interpreted and applied, could have a material adverse effect on our business, including our ability to complete the Business Combination, investments and results of operations.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws and anti-money laundering laws and regulations. We could face criminal liability and other serious consequences for violations, which could harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, and various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, and anti-corruption and anti-money laundering laws and regulations, including the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, CROs, contractors and other collaborators and partners from authorizing, promising, offering, providing, soliciting or receiving, directly or indirectly, improper payments or anything else of value to or from recipients in the public or private sector. We may engage third parties for clinical trials outside of the United States, to sell our products abroad once we enter a commercialization phase, and/or to obtain necessary permits, licenses, patent registrations and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, CROs, contractors and other collaborators and partners, even if we do not explicitly authorize or have actual knowledge of such activities. We are also subject to other U.S. laws and regulations governing export controls, as well as economic sanctions and embargoes on certain countries and persons.
| -87- |
Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or manmade disasters or business interruptions, for which we are predominantly self-insured. We rely on third-party manufacturers to produce our product candidates. Our ability to obtain clinical supplies of our product candidates could be disrupted if the operations of these suppliers were affected by a man-made or natural disaster or other business interruption. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price.
From time to time, the global credit and financial markets have experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. There can be no assurance that future deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, it may make any necessary debt or equity financing more difficult, more costly and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service providers, manufacturers and other partners may not survive an economic downturn, which could directly affect our ability to attain our operating goals on schedule and on budget.
Changes in U.S. tax law may materially adversely affect our financial condition, results of operations and cash flows.
Changes in laws and policy relating to taxes may have an adverse effect on our financial condition, results of operations and cash flows. For example, the Tax Act significantly changed the U.S. federal income taxation of U.S. corporations. The Tax Act remains unclear in various respects and has been, and may continue to be, the subject of amendments and technical corrections, as well as interpretations and implementing regulations by the Treasury and Internal Revenue Service (“IRS”), which have lessened or increased certain adverse impacts of the Tax Act and may continue to do so in the future. In addition, it is unclear how these U.S. federal income tax changes will affect state and local taxation, which often uses federal taxable income as a starting point for computing state and local tax liabilities. On March 27, 2020, the CARES Act was signed into law to address the COVID-19 crisis. The CARES Act is an approximately $2 trillion emergency economic stimulus package that includes numerous U.S. federal income tax provisions, including the modification of: (i) NOL rules (as discussed above), (ii) the alternative minimum tax refund and (iii) business interest deduction limitations under Section 163(j) of the Code. We continue to work with our tax advisors and auditors to determine the full impact the Tax Act and the CARES Act will have on us. We urge our investors to consult with their legal and tax advisors with respect to any changes in tax law and the potential tax consequences of investing in our common stock.
| -88- |
Risks Related to FoxWayne
In this section, unless otherwise stated or the context requires otherwise, the terms “FoxWayne,” “we,” “us” and “our” refer to FoxWayne Enterprises Acquisition Corp. prior to the Closing and the Combined Company and its subsidiaries following the Closing.
Risks Related to FoxWayne and the Business Combination
Subsequent to the consummation of the Business Combination, we may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on our financial condition, results of operations and stock price, which could cause you to lose some or all of your investment.
Although we have conducted due diligence on Aerami, we cannot assure you that this diligence revealed all material issues that may be present in Aerami, that it would be possible to uncover all material issues through a customary amount of due diligence, or that factors outside of our control will not later arise. As a result, we may be forced to later write-down or write-off assets, restructure our operations or incur impairment or other charges that could result in losses. Even if our due diligence successfully identifies certain risks, unexpected risks may arise and previously known risks may materialize in a manner not consistent with our preliminary risk analysis. Even though these charges may be non-cash items and may not have an immediate impact on our liquidity, the fact that we report charges of this nature could contribute to negative market perceptions about us following the completion of the Business Combination or our securities. In addition, charges of this nature may cause us to be unable to obtain future financing on favorable terms or at all. Accordingly, any stockholders who choose to remain stockholders following the Business Combination could suffer a reduction in the value of their securities.
Our initial stockholders have agreed to vote in favor of the Business Combination, regardless of how our public stockholders vote.
Unlike many other blank check companies in which the founders agree to vote their Founder Shares in accordance with the majority of the votes cast by public stockholders in connection with an Initial Business Combination, our initial stockholders have agreed to vote any shares of Class A Common Stock and Class B Common Stock owned by them in favor of the Business Combination. As of the record date, our initial stockholders own shares equal to approximately % of our issued and outstanding shares of Class A Common Stock and % of our issued and outstanding shares of Class B Common Stock. Accordingly, it is more likely that the necessary stockholder approval will be received for the Business Combination than would be the case if the initial stockholders agreed to vote any shares of Class A Common Stock and Class B Common Stock owned by them in accordance with the majority of the votes cast by our public stockholders.
Our Sponsor, members of the FoxWayne Board and our officers have interests in the Business Combination that are different from or are in addition to other stockholders in recommending that stockholders vote in favor of approval of the Transaction Proposal.
When considering the FoxWayne Board’s recommendation that our stockholders vote in favor of the approval of the Transaction Proposal, our stockholders should be aware that our Sponsor, directors and officers have interests in the Business Combination that may be different from, or in addition to, the interests of our stockholders. These interests include:
| ● | the fact that our Sponsor holds 2,800,000 Private Placement Warrants (valued at $1,148,000, based on a valuation as of December 31, 2021, the most recent date for which a valuation is available) that would expire worthless if an Initial Business Combination is not consummated; | |
| ● | the fact that our Sponsor, officers and directors have agreed not to redeem any of the shares of our common stock held by them in connection with a stockholder vote to approve the Business Combination; | |
| ● | the fact that our Sponsor paid an aggregate of $25,000 for the Founder Shares, including 100,000 Founder Shares which were subsequently transferred to our independent directors, and that such securities will have a significantly higher value at the time of the Business Combination, which if unrestricted and freely tradable would be valued at approximately $ , based on the closing price of our Class A Common Stock of $ per share on , 2022, the record date of the special meeting of stockholders; |
| -89- |
| ● | if the Trust Account is liquidated, including in the event we are unable to complete an Initial Business Combination within the required time period, our Sponsor has agreed to indemnify us to ensure that the proceeds in the Trust Account are not reduced below $10.10 per public share, or such lesser amount per public share as is in the Trust Account on the liquidation date, by the claims of (a) any third party (other than our independent registered public accounting firm) for services rendered or products sold to us or (b) a prospective target business with which we have entered into a letter of intent, confidentiality or other similar agreement or Merger Agreement, but only if such a third party or target business has not executed a waiver of all rights to seek access to the Trust Account; | |
| ● | the fact that our independent directors own an aggregate of 100,000 Founder Shares that were transferred from our Sponsor, which if unrestricted and freely tradeable would be valued at approximately $ , based on the closing price of our Class A Common Stock of $ per share on , 2022; | |
| ● | the fact that our Sponsor, officers and directors will be reimbursed for out-of-pocket expenses incurred in connection with activities on our behalf, such as identifying, investigating and completing an Initial Business Combination; | |
| ● | the fact that our Sponsor will benefit from the completion of a business combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to stockholders rather than liquidate; | |
| ● | the fact that our initial stockholders, including our Sponsor and its affiliates, who purchased Class B Common Stock and Private Placement Warrants at the time of our IPO may experience a positive rate of return on their investment, even if our public stockholders experience a negative rate of return on their investment; | |
| ● | the fact that our Sponsor, officers and directors will lose their entire investment in us if an Initial Business Combination is not completed by July 22, 2022. |
The FoxWayne Board was aware of and considered these interests, among other matters, in reaching the determination to approve the Business Combination and the Merger Agreement and in recommending that the holders of Combined Company common stock vote to approve the Business Combination and adopt the Merger Agreement. For additional information please see the section titled “The Business Combination — Interests of Certain Persons in the Business Combination — Interests of Sponsor and FoxWayne Directors and Officers.”
Our initial stockholders and Sponsor hold a significant number of shares of our common stock, and our Sponsor holds a significant number of our warrants. They will lose their entire investment in us if we do not complete an Initial Business Combination.
Our Sponsor and initial stockholders, including our officers and directors, hold all of the 1,437,500 shares of the Class B Common Stock. The Founder Shares will be worthless if we do not complete an Initial Business Combination by April 22, 2022 (which we intend to extend up to July 22, 2022 - See the section titled “Does FoxWayne intend to extend the period of time it has to consummate an Initial Business Combination?”). In addition, our Sponsor holds an aggregate of 2,800,000 Private Placement Warrants that will also be worthless if we do not complete an Initial Business Combination by April 22, 2022 (which we intend to extend up to July 22, 2022).
The Founder Shares are identical to the shares of Class A Common Stock included in the units, except that (i) the Founder Shares are automatically convertible into shares of our Class A Common Stock at the time of the closing of an Initial Business Combination on a one-for-one basis, subject to adjustment; (ii) the Founder Shares and the shares of Class A Common Stock into which the Founder Shares convert upon an Initial Business Combination are subject to certain transfer restrictions; (iii) our Sponsor, officers, directors and initial stockholders have entered into a letter agreement with us, pursuant to which they have agreed (A) to waive their redemption rights with respect to their Founder Shares and any public shares they own in connection with the completion of an Initial Business Combination, (B) waive their rights to liquidating distributions from the Trust Account with respect to their Founder Shares if we fail to complete an Initial Business Combination by April 22, 2022 (which we intend to extend up to July 22, 2022) (although they will be entitled to liquidating distributions from the Trust Account with respect to any public shares they hold if we fail to complete an Initial Business Combination by April 22, 2022 (which we intend to extend up to July 22, 2022)) and (C) waive their redemption rights with respect to their Founder Shares and public shares in connection with a stockholder vote to approve an amendment to our amended and restated certificate of incorporation (a) to modify the substance or timing of our obligation to allow redemption in connection with our Initial Business Combination or to redeem 100% of our public shares if we do not complete our Initial Business Combination by April 22, 2022 (which we intend to extend up to July 22, 2022) or (B) with respect to any other provision relating to stockholders’ rights or pre-Initial Business Combination activity.
The personal and financial interests of our Sponsor, officers and directors may have influenced their motivation in identifying and selecting the Business Combination, completing the Business Combination and influencing our operation following the Business Combination.
| -90- |
Our stockholders will have limited protection in the event that any of the representation and warranties made by Aerami in the Merger Agreement proves to be inaccurate or incorrect.
The representations, warranties and covenants made by Aerami to FoxWayne in the Merger Agreement will not survive the completion of the Business Combination. As a result, after the completion of the Business Combination, FoxWayne and its stockholders will not have the protection of any indemnification, if any representation or warranty made by Aerami in the Merger Agreement proves to be inaccurate or incorrect or there is a breach of a pre-closing covenant unless there is an intended fraud. Accordingly, to the extent such representations or warranties are incorrect or there is a breach of a pre-closing covenant by Aerami, FoxWayne would have limited, if any, indemnification claims with respect thereto and its financial condition or results of operations could be adversely affected.
Our officers and directors may have conflicts of interest in determining whether to present business opportunities to us or another entity with which they are, or may become, affiliated.
FoxWayne was created for the purpose of identifying and attempting to combine with one or more businesses. Our Sponsor, officers and directors are, or may in the future become, affiliated with entities (such as operating companies or investment vehicles) that are engaged in a similar business.
Our officers and directors also may become aware of business opportunities which may be appropriate for presentation to us and the other entities to which they owe certain fiduciary or contractual duties. Our organizational documents provide that we renounce our interest in any corporate opportunity offered to any director or officer unless such opportunity is expressly offered to such person solely in his or her capacity as our director or officer and such opportunity is one we are legally and contractually permitted to undertake and would otherwise be reasonable for us to pursue.
In the absence of the “corporate opportunity” waiver in our organizational documents, certain candidates may not be able to serve as an officer or director for us. We believe we substantially benefit from having representatives who bring significant, relevant and valuable experience to our management, and, as a result, the inclusion of the “corporate opportunity” waiver in our organizational documents provides us with greater flexibility to attract and retain the officers and directors that we believe are the best candidates.
However, the personal and financial interests of our directors and officers may influence their motivation in timely identifying and selecting a target business and completing a business combination. The different timelines of competing business combinations could cause our directors and officers to prioritize a different business combination over finding a suitable acquisition target for our business combination. Consequently, our directors’ and officers’ discretion in identifying and selecting a suitable target business may result in a conflict of interest when determining whether the terms, conditions and timing of a particular business combination are appropriate and in our shareholders’ best interest, which could negatively impact the timing for a business combination.
We have and will continue to incur significant transaction costs in connection with the Business Combination.
We have and expect to incur significant, non-recurring costs in connection with consummating the Business Combination. All expenses incurred in connection with the Merger Agreement and the Business Combination, including all legal, accounting, consulting, investment banking and other fees, expenses and costs, will be for the account of the party incurring such fees, expenses and costs. Our transaction expenses as a result of the Business Combination are currently estimated at approximately $4.9 million, including approximately $2.0 million in deferred underwriting discounts and commissions to the underwriters of our initial public offering.
We may be subject to business uncertainties while the Business Combination is pending.
Uncertainty about the effect of the Business Combination on employees and third parties may have an adverse effect on Aerami and consequently on FoxWayne. These uncertainties may impair Aerami’s ability to attract, retain and motivate key personnel and could cause third parties that deal with Aerami to defer entering into contracts or making other decisions or seek to change existing business relationships. If key employees depart because of issues relating to such uncertainty or a desire not to remain with the business, Aerami’s business following the Business Combination could be negatively impacted. In addition, the Merger Agreement restricts Aerami from making certain expenditures and taking other specified actions without the consent of FoxWayne until the Business Combination occurs. These restrictions may prevent Aerami from pursuing attractive business opportunities that may arise prior to the Closing. For additional information please see the subsection titled “The Business Combination — Conduct of Business Pending the Merger.”
The unaudited pro forma condensed combined financial information included in this proxy statement/prospectus may not be indicative of what our actual financial position or results of operations would have been.
The unaudited pro forma condensed combined financial information for FoxWayne and Aerami following the Business Combination in this proxy statement/prospectus is presented for illustrative purposes only and is not necessarily indicative of what our actual financial position or results of operations would have been had the Business Combination been completed on the dates indicated. See the section titled “Unaudited Pro Forma Condensed Combined Financial Information” for more information.
The consummation of the Business Combination is subject to a number of conditions, and if those conditions are not satisfied or waived, the Merger Agreement may be terminated in accordance with its terms and the Business Combination may not be completed.
The Merger Agreement is subject to a number of conditions which must be fulfilled in order to complete the Business Combination. Those conditions include, among other things: (a) approval by FoxWayne’s stockholders and Aerami’s stockholders, (b) FoxWayne having at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) as of the effective time of the consummation of the Business Combination, (c) the listing of the Combined Company common stock to be issued pursuant to the Merger Agreement on Nasdaq and the effectiveness of this proxy statement/Registration Statement and (d) FoxWayne having aggregate of at least $15,000,000 in cash, following payment by FoxWayne to its stockholders who have validly elected to have their shares of Combined Company common stock redeemed for cash and after giving effect to the payment of the Transaction Expenses of FoxWayne. In addition, the parties can mutually decide to terminate the Merger Agreement at any time, before or after stockholder approval but prior to the Effective Time of the Merger, or FoxWayne or Aerami may elect to terminate the Merger Agreement in certain other circumstances. For additional information please see the subsection titled “The Business Combination — Termination.”
| -91- |
Each of Aerami and FoxWayne may waive one or more of the conditions to the Business Combination.
Each of Aerami and FoxWayne may agree to waive, in whole or in part, certain conditions to their respective obligations to complete the Business Combination, to the extent permitted by their respective charters and applicable law, either unilaterally or by agreement of FoxWayne and Aerami. For example, it is a condition to each party’s obligation to close the Business Combination that certain representations and warranties made by the other party be true and correct to the extent required pursuant to the Merger Agreement as of the date of the Merger Agreement and the Closing Date. Neither FoxWayne nor Aerami is able to waive the condition that their respective stockholders approve the Business Combination. In addition, the following closing conditions may not be waived: (i) after giving effect to the completion of the redemption, the Company having net tangible assets of at least $5,000,001; (ii) the effectiveness of the Registration Statement on Form S-4 of which this proxy statement/prospectus forms a part and (iii) the absence of any law or order that would prohibit the consummation of the Merger or other transactions contemplated by the Merger Agreement. Other conditions are subject to waiver either unilaterally or by agreement of FoxWayne and Aerami. In the event of a waiver of a condition, the FoxWayne Board will evaluate the materiality of any such waiver to determine whether amendment of this proxy statement/prospectus and re-solicitation of proxies is necessary. In the event that the FoxWayne Board determines any such waiver is not significant enough to require re-solicitation of its stockholders, it will have the discretion to complete the Business Combination without seeking further stockholder approval. For additional information please see the subsection titled “The Business Combination — Conditions to Closing of the Merger Agreement.
The exercise of discretion by FoxWayne’s directors and officers in agreeing to changes to the terms of or waivers of closing conditions in the Merger Agreement may result in a conflict of interest when determining whether such changes to the terms of the Merger Agreement or waivers of closing conditions are appropriate and in the best interests of FoxWayne’s stockholders.
In the period leading up to the consummation of the Business Combination, other events may occur that, pursuant to the Merger Agreement, would require FoxWayne to agree to amend the Merger Agreement, to consent to certain actions or to waive rights that it is entitled to under the Merger Agreement. Such events could arise because of changes in the course of the business of Aerami, a request by Aerami and its management to undertake actions that would otherwise be prohibited by the terms of the Merger Agreement or the occurrence of other events that would have a Material Adverse Effect on the business of Aerami and could entitle FoxWayne to terminate the Merger Agreement. In any such circumstance, it would be in the discretion of FoxWayne, acting through the FoxWayne Board, to grant its consent or waive its rights. The existence of the financial and personal interests of the FoxWayne directors described elsewhere in this proxy statement/prospectus may result in a conflict of interest on the part of one or more of the FoxWayne directors between what he may believe is best for FoxWayne and FoxWayne stockholders and what he may believe is best for himself or his affiliates in determining whether or not to take the requested action. As of the date of this proxy statement/prospectus, FoxWayne does not believe there will be any changes or waivers that FoxWayne’s directors and officers would be likely to make after FoxWayne stockholder approval of the Business Combination has been obtained. While certain changes could be made without further stockholder approval, if there is a change to the terms of the Business Combination that would have a material impact on the stockholders, FoxWayne will be required to circulate a new or amended proxy statement/prospectus or supplement thereto and resolicit the vote of FoxWayne’s stockholders with respect to the Transaction Proposal.
If we are unable to complete an Initial Business Combination on or prior to April 22, 2022 (which may be further extended to July 22, 2022), our public stockholders may receive only $10.10 per share on the liquidation of our Trust Account (or less than $10.10 per share in certain circumstances where a third party brings a claim against us that our Sponsor, under certain circumstances, is unable to indemnify), and our warrants will expire worthless.
The FoxWayne Charter initially required that we consummate a business combination by January 22, 2022 (which could be extended up to two times, each by an additional three months, until July 22, 2022). Effective January 14, 2022, the FoxWayne Board approved an extension of the time to consummate a Business Combination until April 22, 2022. If we are unable to complete an Initial Business Combination on or prior to April 22, 2022 (which we intend to further extend up to July 22, 2022, our public stockholders may receive only $10.10 per share on the liquidation of our Trust Account as of February 11, 2022 (or less than $10.10 per share in certain circumstances where a third party brings a claim against us that, under certain circumstances, our Sponsor is unable to indemnify (as described below)), and our warrants will expire worthless. See the section titled “Does FoxWayne intend to extend the period of time it has to consummate an Initial Business Combination?” for additional information.
If third parties bring claims against us, the proceeds held in our Trust Account could be reduced and the per share redemption amount received by stockholders may be less than $10.10 per share.
Our placing of funds in the Trust Account may not protect those funds from third-party claims against us. Although we will seek to have all vendors, service providers (other than our independent registered public accounting firm), prospective target businesses and other entities with which we do business execute agreements with us waiving any right, title, interest or claim of any kind in or to any monies held in the Trust Account for the benefit of our public stockholders, such parties may not execute such agreements, or even if they execute such agreements, they may not be prevented from bringing claims against the Trust Account, including, but not limited to, fraudulent inducement, breach of fiduciary responsibility or other similar claims, as well as claims challenging the enforceability of the waiver, in each case in order to gain advantage with respect to a claim against our assets, including the funds held in the Trust Account. Although, except as set forth herein, no third parties have refused to execute an agreement waiving such claims to the monies held in the Trust Account to date, if any third party refuses to execute such an agreement in the future, our management will perform an analysis of the alternatives available to it and will only enter into an agreement with a third party that has not executed a waiver if management believes that such third party’s engagement would be significantly more beneficial to us than any alternative. WithumSmith+Brown, PC, our independent registered public accounting firm, will not execute agreements with us waiving such claims to the monies held in the Trust Account.
| -92- |
Examples of possible instances where we may engage a third party that refuses to execute a waiver include the engagement of a third-party consultant whose particular expertise or skills are believed by management to be significantly superior to those of other consultants that would agree to execute a waiver or in cases where management is unable to find a service provider willing to execute a waiver. In addition, there is no guarantee that such entities will agree to waive any claims they may have in the future as a result of, or arising out of, any negotiations, contracts or agreements with us and will not seek recourse against the Trust Account for any reason. Upon redemption of our public shares, if we are unable to complete our Business Combination within the prescribed timeframe, or upon the exercise of a redemption right in connection with our Business Combination, we will be required to provide for payment of claims of creditors that were not waived that may be brought against us within the ten years following redemption. Accordingly, the per-share redemption amount received by public stockholders could be less than the $10.10 per public share held in the Trust Account as of February 11, 2022, due to claims of such creditors. Our Sponsor has agreed that it will be liable to us if and to the extent any claims by a vendor for services rendered or products sold to us, or a prospective target business with which we have discussed entering into a transaction agreement, reduce the amount of funds in the Trust Account to below (i) $10.10 per public share or (ii) such lesser amount per public share held in the Trust Account as of the date of the liquidation of the Trust Account due to reductions in the value of the trust assets, in each case net of the interest which may be withdrawn to pay taxes. This liability will not apply with respect to any claims by a third party who executed a waiver of any and all rights to seek access to the Trust Account and except as to any claims under our indemnity of the underwriters of our initial public offering against certain liabilities, including liabilities under the Securities Act. Moreover, in the event that an executed waiver is deemed to be unenforceable against a third party, then our Sponsor will not be responsible to the extent of any liability for such third-party claims. We have not independently verified whether our Sponsor has sufficient funds to satisfy its indemnity obligations and believe that our Sponsor’s only assets are securities of our Company. We have not asked our Sponsor to reserve for such indemnification obligations. Therefore, we cannot assure you that our Sponsor would be able to satisfy those obligations. As a result, if any such claims were successfully made against the Trust Account, the funds available for our Initial Business Combination and redemptions could be reduced to less than $10.10 per public share. In such event, we may not be able to complete our Initial Business Combination, and you would receive such lesser amount per share in connection with any redemption of your public shares. None of our officers will indemnify us for claims by third parties including, without limitation, claims by vendors and prospective target businesses.
Our independent directors may decide not to enforce the indemnification obligations of our Sponsor, resulting in a reduction in the amount of funds in the Trust Account available for distribution to our public stockholders.
In the event that the proceeds in the Trust Account are reduced below the lesser of (a) $10.10 per public share or (ii) such lesser amount per share held in the Trust Account as of the date of the liquidation of the Trust Account due to reductions in the value of the trust assets, in each case net of the interest which may be withdrawn to pay taxes, and our Sponsor asserts that it is unable to satisfy its obligations or that it has no indemnification obligations related to a particular claim, our independent directors would determine whether to take legal action against our Sponsor to enforce its indemnification obligations.
While we currently expect that our independent directors would take legal action on our behalf against our Sponsor to enforce its indemnification obligations to us, it is possible that our independent directors in exercising their business judgment and subject to their fiduciary duties may not choose to do so if, for example, the cost of such legal action is deemed by the independent directors to be too high relative to the amount recoverable or if the independent directors determine that a favorable outcome is not likely. If our independent directors choose not to enforce these indemnification obligations, the amount of funds in the Trust Account available for distribution to our public stockholders may be reduced below the $10.10 per share which the Sponsor has agreed that it will be liable to us for.
| -93- |
If, after we distribute the proceeds in the Trust Account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, a bankruptcy court may seek to recover such proceeds, and the members of the FoxWayne Board may be viewed as having breached their fiduciary duties to our creditors, thereby exposing the members of the FoxWayne Board and us to claims of punitive damages.
If, after we distribute the proceeds in the Trust Account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, any distributions received by stockholders could be viewed under applicable debtor/creditor and/or bankruptcy laws as either a “preferential transfer” or a “fraudulent conveyance.” As a result, a bankruptcy court could seek to recover some or all amounts received by our stockholders. In addition, the FoxWayne Board may be viewed as having breached its fiduciary duty to our creditors and/or having acted in bad faith, thereby exposing itself and us to claims of punitive damages, by paying public stockholders from the Trust Account prior to addressing the claims of creditors.
If, before distributing the proceeds in the Trust Account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, the claims of creditors in such proceeding may have priority over the claims of our stockholders and the per-share amount that would otherwise be received by our stockholders in connection with our liquidation may be reduced.
If, before distributing the proceeds in the Trust Account to our public stockholders, we file a bankruptcy petition or an involuntary bankruptcy petition is filed against us that is not dismissed, the proceeds held in the Trust Account could be subject to applicable bankruptcy law and may be included in our bankruptcy estate and subject to the claims of third parties with priority over the claims of our stockholders. To the extent any bankruptcy claims deplete the Trust Account, the per-share amount that would otherwise be received by our stockholders in connection with our liquidation may be reduced.
Even if we consummate the Business Combination, there is no guarantee that the public warrants will be in the money at the time they become exercisable, and they may expire worthless.
The exercise price for our warrants is $11.50 per share of Class A Common Stock. There is no guarantee that the public warrants will be in the money following the time they become exercisable and prior to their expiration, and as such, the warrants may expire worthless.
We may amend the terms of the warrants in a manner that may be adverse to holders of public warrants with the approval by the holders of at least 50% of the then outstanding public warrants. As a result, the exercise price of the warrants could be increased, the exercise period could be shortened and the number of shares of our Class A Common Stock purchasable upon exercise of a warrant could be decreased, all without a holder’s approval.
Our warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and us. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of at least 50% of the then outstanding public warrants to make any change that adversely affects the interests of the registered holders of public warrants. Accordingly, we may amend the terms of the public warrants in a manner adverse to a holder if holders of at least 50% of the then-outstanding public warrants approve such amendment. Although our ability to amend the terms of the public warrants with the consent of at least 50% of the then outstanding public warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the warrants, convert the warrants into cash, shorten the exercise period or decrease the number of shares of our Class A Common Stock purchasable upon exercise of a warrant.
We may redeem unexpired warrants prior to their exercise at a time that is disadvantageous to warrant holders, thereby making their warrants worthless.
We have the ability to redeem outstanding warrants at any time after they become exercisable and prior to their expiration, at a price of $0.01 per warrant, provided that the last reported sales price of our Class A Common Stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30 trading-day period ending on the third trading day prior to the date on which we give proper notice of such redemption and provided certain other conditions are met. If and when the warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws. Redemption of the outstanding warrants could force our warrant holders (a) to exercise their warrants and pay the exercise price therefor at a time when it may be disadvantageous for such warrant holder to do so, (b) to sell the warrants at the then-current market price when you might otherwise wish to hold the warrants or (c) to accept the nominal redemption price which, at the time the outstanding warrants are called for redemption, is likely to be substantially less than the market value of the warrants. None of the Private Placement Warrants will be redeemable by us for cash so long as they are held by our Sponsor or its permitted transferees.
| -94- |
We may issue additional common stock or preferred stock to complete the Business Combination or under an employee incentive plan after completion of the Business Combination. We may also issue shares of Class A Common Stock upon the conversion of the Class B Common Stock at a ratio greater than one-to-one at the Closing of the Business Combination as a result of the anti-dilution provisions contained in the FoxWayne Charter. Any such issuances would dilute the interest of our stockholders and likely present other risks.
The FoxWayne Charter authorizes the issuance of up to 50,000,000 shares of Class A Common Stock, 2,000,000 shares of Class B Common Stock and 1,000,000 shares of Preferred Stock, par value $0.0001 per share. As of the record date for the special meeting, we have and authorized but unissued shares of Class A Common Stock and Class B Common Stock, respectively, available for issuance, which amount does not take into account the shares of Class A Common Stock reserved for issuance upon exercise of any outstanding warrants or the shares of Class A Common Stock issuable upon conversion of Class B Common Stock. As of the record date there are shares of Preferred Stock issued and outstanding. Shares of Class B Common Stock are convertible into shares of our Class A Common Stock initially at a one-for-one ratio but subject to adjustment, including in certain circumstances in which we issue Class A Common Stock or equity-linked securities related to our Initial Business Combination.
We may issue a substantial number of additional shares of common or preferred stock to complete the Business Combination or under an employee incentive plan after completion of the Business Combination. We may also issue shares of Class A Common Stock upon conversion of the Class B Common Stock at a ratio greater than one-to-one at the closing of the Business Combination as a result of the anti-dilution provisions contained in the FoxWayne Charter.
The issuance of additional shares of common or preferred stock:
| ● | may significantly dilute the equity interests of our investors; | |
| ● | may subordinate the rights of holders of common stock if preferred stock is issued with rights senior to those afforded our common stock; | |
| ● | could cause a change in control if a substantial number of shares of our common stock are issued, which may affect, among other things, our ability to use our net operating loss carryforwards, if any, and could result in the resignation or removal of our present officers and directors; and | |
| ● | may adversely affect prevailing market prices for our units, Class A Common Stock and/or warrants. |
Nasdaq may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
Our units, Class A Common Stock and public warrants are listed on Nasdaq. We cannot assure you that our securities will continue to be listed on Nasdaq after the consummation of the Business Combination. In connection with the Business Combination, we will be required to demonstrate compliance with Nasdaq’s initial listing requirements, which are more rigorous than Nasdaq’s continued listing requirements, in order to continue to maintain the listing of our securities on Nasdaq. We cannot assure you that we will be able to meet those initial listing requirements at that time. Our continued eligibility for listing may depend on, among other things, the number of our shares that are redeemed.
If Nasdaq delists our securities from trading on its exchange and we are not able to list our securities on another national securities exchange, we expect our securities could be quoted on an over-the-counter market. If this were to occur, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our securities; | |
| ● | reduced liquidity for our securities; |
| -95- |
| ● | a determination that our Class A Common Stock is a “penny stock” which will require brokers trading in our Class A Common Stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; | |
| ● | a limited amount of news and analyst coverage; and | |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or pre-empts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Because our units, Class A Common Stock and public warrants are listed on Nasdaq, our units, Class A Common Stock and public warrants qualify as “covered securities.” Although the states are pre-empted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. While we are not aware of a state having used these powers to prohibit or restrict the sale of securities issued by blank check companies, other than the state of Idaho, certain state securities regulators view blank check companies unfavorably and might use these powers, or threaten to use these powers, to hinder the sale of securities of blank check companies in their states. Further, if we were no longer listed on Nasdaq, our securities would not be “covered securities” and we would be subject to regulation in each state in which we offer our securities.
The FoxWayne Board did not obtain a third-party valuation or fairness opinion in determining whether or not to proceed with the Business Combination.
The FoxWayne Board did not obtain a third-party valuation or fairness opinion in connection with its determination to approve the Business Combination. FoxWayne’s officers and directors have substantial experience in evaluating the operating and financial merits of companies from a wide range of industries and concluded that their experience and backgrounds enabled them to make the necessary analyses and determinations regarding the Business Combination. Accordingly, investors will be relying solely on the judgment of the FoxWayne Board in valuing Aerami and assuming the risk that the FoxWayne Board may not have properly valued the business. The lack of a third-party valuation or fairness opinion may also lead an increased number of stockholders to vote against the proposed Business Combination or demand redemption of their shares for cash, which could potentially impact FoxWayne’s ability to consummate the Business Combination.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our Class A Common Stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of Class A Common Stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our Class A Common Stock. After the Business Combination:
| ● | assuming no redemptions by our public stockholders of public shares, our Sponsor and our current officers and directors will hold approximately 4% of our Class A Common Stock; |
| ● | assuming interim redemption by our public stockholders of public shares, our Sponsor and our current officers and directors will hold approximately 5% of our Class A Common Stock; and |
| ● | assuming maximum redemptions by our public stockholders of public shares, our Sponsor and our current officers and directors will hold approximately 5% of our Class A Common Stock, including the shares of Class A Common Stock into which the Founder Shares will convert (assuming redemption by our public stockholders of the maximum number of shares of Class A Common Stock that could occur before the $15 million minimum cash condition in the Merger Agreement would not be met). Our Sponsor, directors, officers and initial stockholders have agreed not to transfer, assign or sell their Founder Shares immediately following the consummation of the Business Combination. However, the terms of any restrictive agreements can be renegotiated at any time. |
If the Business Combination’s benefits do not meet the expectations of investors, stockholders or financial analysts, the market price of our securities may decline.
If the benefits of the Business Combination do not meet the expectations of investors or securities analysts, the market price of our securities prior to the Closing may decline. The market values of our securities at the time of the Business Combination may vary significantly from their prices on the date the Merger Agreement was executed, the date of this proxy statement/prospectus or the date on which our stockholders vote on the Business Combination.
| -96- |
Our Sponsor, directors, officers, advisors or any of their respective affiliates may elect to purchase public shares and/or warrants from public stockholders, which may influence the vote on the Transaction Proposal and reduce the public “float” of our Class A Common Stock and/or warrants.
Our Sponsor, directors, officers or any of their respective affiliates may purchase public shares and/or public warrants, or a combination thereof in privately negotiated transactions or in the open market either prior to or following the completion of the Business Combination, although they are under no obligation to do so. There is no limit on the number of public shares and/or public warrants our Sponsor, directors, officers or any of their respective affiliates may purchase in such transactions, subject to compliance with applicable law and the rules of Nasdaq. Any such privately negotiated purchases may be effected at purchase prices that are in excess of the per share pro rata portion of the Trust Account. However, our Sponsor, directors, officers and their respective affiliates have no current commitments, plans or intentions to engage in such transactions and have not formulated any terms or conditions for any such transactions. None of the funds in the Trust Account will be used to purchase public shares in such transactions. None of our Sponsor, directors, officers or any of their respective affiliates will make any such purchases when they are in possession of any material non-public information not disclosed to the seller of such public shares or during a restricted period under Regulation M under the Exchange Act. Such a purchase may include a contractual acknowledgement that such stockholder, although still the record holder of such public shares, is no longer the beneficial owner thereof and therefore agrees not to exercise its redemption rights. In the event that our Sponsor, directors, officers or any of their respective affiliates purchase shares in privately negotiated transactions from public stockholders who have already elected to exercise their redemption rights, such selling stockholders would be required to revoke their prior elections to redeem their shares.
The purpose of any such purchases could be to vote such shares in favor of the Business Combination and thereby increase the likelihood of obtaining stockholder approval of the Business Combination or to satisfy a closing condition in the Merger Agreement, where it appears that such requirement would otherwise not be met. Any such purchases of our public shares may result in the completion of the Business Combination that may not otherwise have been possible.
In addition, if such purchases are made, the public “float” of our Class A Common Stock and/or public warrants may be reduced and the number of beneficial holders of our securities may be reduced, which may make it difficult to maintain or obtain the quotation, listing or trading of our securities on a national securities exchange. See the subsection titled “The Business Combination — Potential Purchases of Public Shares” for a description of how our Sponsor, directors, officers or any of their respective affiliates may select which stockholders or warrant holders to purchase securities from in any private transaction.
Our warrants and Founder Shares may have an adverse effect on the market price of our Class A Common Stock and make it more difficult to effectuate our Business Combination.
We issued warrants to purchase 2,800,000 shares of our Class A Common Stock as part of the units offered in our initial public offering and, simultaneously with the closing of our initial public offering, we issued Private Placement Warrants to purchase an aggregate of 2,800,000 shares of Class A Common Stock at $11.50 per share.
Our initial stockholders currently own an aggregate of 1,437,500 Founder Shares. The Founder Shares are automatically convertible into shares of Class A Common Stock on a one-for-one basis, subject to adjustment, at the closing of our Initial Business Combination. In addition, if our Sponsor makes any working capital loans, up to $1,500,000 of such loans may be converted into warrants, at the price of $1.00 per warrant at the option of the lender. Any issuance of a substantial number of additional shares of Class A Common Stock upon exercise of these warrants will increase the number of issued and outstanding shares of Class A Common Stock and reduce the value of the Class A Common Stock issued to complete the Business Combination. Therefore, our warrants and Founder Shares may make it more difficult to effectuate the Business Combination or increase the cost of acquiring Aerami.
| -97- |
We do not have a specified maximum redemption threshold. The absence of such a redemption threshold may make it possible for us to complete the Business Combination even if a substantial majority of our stockholders do not agree.
The FoxWayne Charter does not provide a specified maximum redemption threshold, except that we will only redeem our public shares so long as (after such redemption) our net tangible assets will be at least $5,000,001 either immediately prior to or upon consummation of the Business Combination and after payment of underwriters’ fees and commissions (such that we are not subject to the SEC’s “penny stock” rules). As a result, we may be able to complete the Business Combination even though a substantial majority of our public stockholders do not agree with the transaction and have redeemed their shares or, if we seek stockholder approval of the Business Combination and do not conduct redemptions in connection with the Business Combination pursuant to the tender offer rules, have entered into privately negotiated agreements to sell their shares to our Sponsor, officers, directors or their affiliates. In the event the aggregate cash consideration we would be required to pay for all shares of Class A Common Stock that are validly submitted for redemption plus any amount required to satisfy the minimum cash conditions pursuant to the terms of the Merger Agreement exceed the aggregate amount of cash available to us, we will not complete the Business Combination or redeem any shares, all shares of Class A Common Stock submitted for redemption will be returned to the holders thereof unless the minimum cash condition in the Merger Agreement is waived by Aerami.
Our stockholders will have a reduced ownership and voting interests after the Business Combination and will exercise less influence over management.
In connection with the Business Combination, an aggregate of 25,000,000 shares of the Combined Company common stock will be issued to Aerami Equityholders and shares of the Combined Company common stock will be reserved for issuance upon the exercise of Exchanged Options, Exchanged RSU Awards and Assumed Warrants assumed by the Combined Company in the Merger. Upon the issuance of the shares of the Combined Company common stock to the Aerami Equityholders at the Closing, current FoxWayne stockholders will be diluted immediately and may be subject to further dilution by the future exercise of Assumed Warrants, Exchanged Options or Exchanged RSU Awards. Following the consummation of the Business Combination, current holders of FoxWayne common stock would own between approximately 12% and 22% of the Combined Company (subject to the assumptions described in “Unaudited Pro Forma Condensed Combined Financial Information – Notes to Unaudited Pro Forma Condensed Combined Financial Information – 1. Description of the Business Combination” below).
The market price of shares of Class A Common Stock after the Business Combination may be affected by factors different from those currently affecting the price of shares of Class A Common Stock.
Upon completion of the Business Combination, Aerami Equityholders will become holders of our Class A Common Stock. Prior to the Business Combination, FoxWayne has limited operations. Upon completion of the Business Combination, FoxWayne’s results of operations will depend upon the performance of the Aerami business, which is affected by factors that are different from those currently affecting the results of operations of FoxWayne.
The Business Combination may be materially adversely affected by the COVID-19 outbreak.
In December 2019, COVID-19 was reported to have surfaced in Wuhan, China, which has and is continuing to spread throughout China and other parts of the world, including the United States. On January 30, 2020, the World Health Organization declared the outbreak of COVID-19 a “Public Health Emergency of International Concern.” On January 31, 2020, U.S. Health and Human Services Secretary Alex M. Azar II declared a public health emergency for the United States to aid the U.S. healthcare community in responding to COVID-19, and on March 11, 2020, the World Health Organization characterized the outbreak as a “pandemic.” The COVID-19 outbreak has resulted, and a significant outbreak of other infectious diseases could result, in a widespread health crisis that could adversely affect the economies and financial markets worldwide. Additionally, our ability to consummate the Business Combination may be materially adversely affected due to significant governmental measures being implemented to contain the outbreak of COVID-19 or its impact, including travel restrictions, the shutdown of businesses and quarantines, among others, which may limit our ability to have meetings with potential investors or affect the ability of Aerami’s personnel, vendors and service providers to negotiate and consummate the Business Combination in a timely manner. The extent to which COVID-19 impacts the Business Combination will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others. If the disruptions posed by COVID-19 or other matters of global concern continue for an extensive period of time, our ability to consummate the Business Combination may be materially adversely affected.
Stockholder litigation and regulatory inquiries and investigations are expensive and could harm FoxWayne’s business, financial condition and operating results and could divert management attention.
In the past, securities class action litigation and/or stockholder derivative litigation and inquiries or investigations by regulatory authorities have often followed certain significant business transactions, such as the sale of a company or announcement of any other strategic transaction, such as the Business Combination. Any stockholder litigation and/or regulatory investigations against FoxWayne, whether or not resolved in FoxWayne’s favor, could result in substantial costs and divert FoxWayne’s management’s attention from other business concerns, which could adversely affect FoxWayne’s business and cash resources and the ultimate value FoxWayne’s stockholders receive as a result of the Business Combination.
| -98- |
In the event that a significant number of public shares are redeemed, our Class A Common Stock may become less liquid following the Business Combination.
If a significant number of public shares are redeemed, FoxWayne may be left with a significantly smaller number of stockholders. As a result, trading in the shares of the Combined Company may be limited and your ability to sell your shares in the market could be adversely affected.
Because the Combined Company does not anticipate paying any cash dividends in the foreseeable future, capital appreciation, if any, would be your sole source of gain.
The Combined Company currently anticipates that it will retain future earnings for the development, operation and expansion of its business and does not anticipate declaring or paying any cash dividends for the foreseeable future. As a result, capital appreciation, if any, of the Combined Company’s securities would be your sole source of gain on an investment in such securities for the foreseeable future.
FoxWayne’s warrant agreement designates the courts of the State of New York or the United States District Court for the Southern District of New York as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by holders of FoxWayne’s warrants, which could limit the ability of warrant holders to obtain a favorable judicial forum for disputes with FoxWayne.
FoxWayne’s warrant agreement provides that, subject to applicable law, (i) any action, proceeding or claim against FoxWayne arising out of or relating in any way to the warrant agreement, including under the Securities Act, will be brought and enforced in the courts of the State of New York or the United States District Court for the Southern District of New York, and (ii) that FoxWayne irrevocably submits to such jurisdiction, which jurisdiction shall be the exclusive forum for any such action, proceeding or claim. FoxWayne will waive any objection to such exclusive jurisdiction and that such courts represent an inconvenient forum.
Notwithstanding the foregoing, these provisions of the warrant agreement will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal district courts of the United States of America are the sole and exclusive forum. Any person or entity purchasing or otherwise acquiring any interest in any of FoxWayne’s warrants shall be deemed to have notice of and to have consented to the forum provisions in FoxWayne’s warrant agreement. If any action, the subject matter of which is within the scope the forum provisions of the warrant agreement, is filed in a court other than a court of the State of New York or the United States District Court for the Southern District of New York (a “foreign action”) in the name of any holder of FoxWayne’s warrants, such holder shall be deemed to have consented to: (x) the personal jurisdiction of the state and federal courts located in the State of New York in connection with any action brought in any such court to enforce the forum provisions (an “enforcement action”), and (y) having service of process made upon such warrant holder in any such enforcement action by service upon such warrant holder’s counsel in the foreign action as agent for such warrant holder.
This choice-of-forum provision may limit a warrant holder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with FoxWayne, which may discourage such lawsuits. Alternatively, if a court were to find this provision of FoxWayne’s warrant agreement inapplicable or unenforceable with respect to one or more of the specified types of actions or proceedings, FoxWayne may incur additional costs associated with resolving such matters in other jurisdictions, which could materially and adversely affect FoxWayne’s business, financial condition and results of operations and result in a diversion of the time and resources of FoxWayne’s management and board of directors.
Because the Combined Company will be a publicly traded company by virtue of a merger as opposed to an underwritten initial public offering, the process does not use the services of one or more underwriters, which could result in less diligence being conducted.
The Combined Company will be a publicly listed company upon the completion of the Business Combination. However, the Business Combination is not an underwritten initial public offering of the Combined Company’s securities and differs from an underwritten initial public offering in several significant ways. In an underwritten initial public offering, underwriters have liability under the U.S. securities laws for material misstatements or omissions in a registration statement pursuant to which an issuer sells securities. Because the underwriters have a “due diligence” defense to any such liability by, among other things, conducting a reasonable investigation, the underwriters and their counsel conduct a due diligence investigation of the issuer. Due diligence entails engaging legal, financial and/or other experts to perform an investigation as to the accuracy of an issuer’s disclosure regarding, among other things, its business and financial results. Because FoxWayne is already a publicly traded company, an underwriter has not been engaged. While the Sponsor may have an inherent conflict of interest because its shares and warrants will be worthless if a business combination is not completed, management and the board of directors of the acquirer undertake a certain level of due diligence. However, this due diligence is not necessarily the same level of due diligence undertaken by an underwriter in a traditional initial public offering. If such investigation had occurred, certain information in this proxy statement/prospectus may have been presented in a different manner or additional information may have been presented at the request of such underwriter.
In addition, because there are no underwriters engaged in connection with the Business Combination, prior to the opening of trading on the trading day immediately following the closing, there will be no traditional “roadshow” or book building process, and no price at which underwriters initially sold shares to the public to help inform efficient and sufficient price discovery with respect to the initial post-closing trades. Therefore, buy and sell orders submitted prior to and at the opening of initial post-closing trading of the Combined Company’s securities will not have the benefit of being informed by a published price range or a price at which the underwriters initially sold shares to the public, as would be the case in an underwritten initial public offering. There will be no underwriters assuming risk in connection with an initial resale of the Combined Company’s securities or helping to stabilize, maintain or affect the public price of the Combined Company’s securities following the Closing. Moreover, the Combined Company will not engage in, and have not and will not, directly or indirectly, request financial advisors to engage in, any special selling efforts or stabilization or price support activities in connection with the Combined Company’s securities that will be outstanding immediately following the Closing. In addition, since the Combined Company will become public through a merger, securities analysts of major brokerage firms may not provide coverage of Aerami since there is no incentive to brokerage firms to recommend the purchase of its shares of common stock. No assurance can be given that brokerage firms will, in the future, want to conduct any offerings on the Combined Company’s behalf. All of these differences from an underwritten public offering of the Combined Company’s securities could result in a more volatile price for the Combined Company’s securities.
In addition, the Sponsor, certain members of the FoxWayne board of directors and its officers, as well as their respective affiliates and permitted transferees, have interests in the Business Combination that are different from or are in addition to those of holders of the Combined Company’s securities following completion of the Business Combination, and that would not be present in an underwritten public offering of the Combined Company’s securities. Such interests may have influenced the FoxWayne Board in making their recommendation that the FoxWayne stockholders vote in favor of the approval of the Business Combination Proposal and the other proposals described in this proxy statement/prospectus.
Such differences from an underwritten public offering may present material risks to unaffiliated investors that would not exist if the Combined Company became a publicly listed company through an underwritten initial public offering instead of upon completion of the Business Combination.
| -99- |
FoxWayne has identified a material weakness in its internal control over financial reporting as of June 30, 2021, and as of September 30, 2021. If FoxWayne is unable to develop and maintain an effective system of internal control over financial reporting, it may not be able to accurately report its financial results in a timely manner, which may adversely affect investor confidence in FoxWayne and materially and adversely affect its business and operating results.
In connection with FoxWayne’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2021, after consultation with its independent registered public accounting firm, FoxWayne’s management concluded that, in light of such report, FoxWayne had identified a material weakness in its internal controls over financial reporting related to the accounting for a significant and unusual transaction related to the warrants FoxWayne issued in connection with its initial public offering.
FoxWayne identified a material weakness in its internal control over financial reporting related to FoxWayne’s application of ASC 480-10-S99-3A to its accounting classification of the public shares of Class A Common Stock issued in its initial public offering. As a result of this material weakness, FoxWayne’s management has concluded that its internal control over financial reporting was not effective as of September 30, 2021. Historically, a portion of such public shares was classified as permanent equity to maintain stockholders’ equity greater than $5 million on the basis that FoxWayne would not redeem such public shares in an amount that would cause its net tangible assets to be less than $5,000,001, as described in the Charter. Pursuant to FoxWayne’s re-evaluation of FoxWayne’s application of ASC 480-10-S99-3A to its accounting classification of the public shares, FoxWayne’s management has determined that such public shares include certain provisions that require classification of all of the public shares as temporary equity regardless of the net tangible assets redemption limitation contained in the Charter. For a discussion of management’s consideration of the material weakness identified related to FoxWayne’s application of ASC 480-10-S99-3A to its accounting classification of the public shares, see “Note 2” to the unaudited condensed financial statements for the quarter ended September 30, 2021.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of FoxWayne’s annual or interim financial statements will not be prevented or detected and corrected on a timely basis.
Effective internal controls are necessary for FoxWayne to provide reliable financial reports and prevent fraud. FoxWayne continues to evaluate steps to remediate the material weakness. These remediation measures may be time consuming and costly and there is no assurance that these initiatives will ultimately have the intended effects.
If FoxWayne identifies any new material weaknesses in the future, any such newly identified material weakness could limit FoxWayne’s ability to prevent or detect a misstatement of FoxWayne’s accounts or disclosures that could result in a material misstatement of FoxWayne’s annual or interim financial statements. In such case, FoxWayne may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to applicable stock exchange listing requirements, investors may lose confidence in FoxWayne’s financial reporting and FoxWayne’s stock price may decline as a result. FoxWayne cannot assure you that the measures FoxWayne has taken to date, or any measures FoxWayne may take in the future, will be sufficient to avoid potential future material weaknesses.
FoxWayne may face litigation and other risks as a result of the material weakness in its internal control over financial reporting.
As a result of the material weakness FoxWayne identified related to the change in accounting for the Warrants and public shares issued in FoxWayne’s initial public offering, and other matters raised or that may in the future be raised by the SEC, FoxWayne faces potential for litigation or other disputes which may include, among others, claims invoking the federal and state securities laws, contractual claims or other claims arising from the material weaknesses in FoxWayne’s internal control over financial reporting and the preparation of its financial statements. Any such litigation or dispute, whether successful or not, could have a material adverse effect on FoxWayne’s business, results of operations and financial condition or our ability to complete a business combination.
| -100- |
Risks Related to the Redemption
There is no guarantee that a stockholder’s decision whether to redeem its shares for a pro rata portion of the Trust Account will put the stockholder in a better future economic position.
We can give no assurance as to the price at which a stockholder may be able to sell its public shares in the future following the completion of the Business Combination or any alternative business combination. Certain events following the consummation of the Business Combination may cause an increase in our share price and may result in a lower value realized now than a stockholder might realize in the future had the stockholder redeemed their shares. Similarly, if a stockholder does not redeem their shares, the stockholder will bear the risk of ownership of the public shares after the consummation of the Business Combination, and there can be no assurance that a stockholder can sell its shares in the future for a greater amount than the redemption price set forth in this proxy statement/prospectus. A stockholder should consult, and rely solely upon, the stockholder’s own tax and/or financial advisor for assistance on how this may affect his, her or its individual situation.
If our stockholders fail to comply with the redemption requirements specified in this proxy statement/prospectus, they will not be entitled to redeem their shares of Class A Common Stock for a pro rata portion of the funds held in the Trust Account.
In order to exercise their redemption rights, holders of public shares are required to submit a request in writing and deliver their shares (either physically or electronically) to our transfer agent at least two business days prior to the special meeting. Stockholders electing to redeem their shares will receive their pro rata portion of the Trust Account, including interest not previously released to us to pay our franchise and income taxes, calculated as of two business days prior to the anticipated consummation of the Business Combination. See the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights” for additional information on how to exercise your redemption rights.
Stockholders who wish to redeem their shares for a pro rata portion of the Trust Account must comply with specific requirements for redemption that may make it more difficult for them to exercise their redemption rights prior to the deadline.
Public stockholders who wish to redeem their shares for a pro rata portion of the Trust Account must, among other things, as more fully described in the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights,” tender their certificates to our transfer agent or deliver their shares to the transfer agent electronically through DTC prior to 5:00 p.m., Eastern time, on , 2022. In order to obtain a physical stock certificate, a stockholder’s broker and/or clearing broker, DTC and our transfer agent will need to act to facilitate this request. It is our understanding that stockholders should generally allot at least two weeks to obtain physical certificates from the transfer agent. However, because we do not have any control over this process or over the brokers, it may take significantly longer than two weeks to obtain a physical stock certificate. If it takes longer than anticipated to obtain a physical certificate, stockholders who wish to redeem their shares may be unable to obtain physical certificates by the deadline for exercising their redemption rights and thus will be unable to redeem their shares.
In addition, holders of outstanding units of FoxWayne must separate the underlying public shares and public warrants prior to exercising redemption rights with respect to the public shares. If you hold units registered in your own name, you must deliver the certificate for such units or deliver such units electronically to Continental Stock Transfer & Trust Company with written instructions to separate such units into public shares and public warrants. This must be completed far enough in advance to permit the mailing of the public share certificates or electronic delivery of the public shares back to you so that you may then exercise your redemption rights with respect to the public shares following the separation of such public shares from the units.
| -101- |
If a broker, dealer, commercial bank, trust company or other nominee holds your units, you must instruct such nominee to separate your units. Your nominee must send written instructions by facsimile to Continental Stock Transfer & Trust Company. Such written instructions must include the number of units to be split and the nominee holding such units. Your nominee must also initiate electronically, using DTC’s DWAC system, a withdrawal of the relevant units and a deposit of the corresponding number of public shares and public warrants. This must be completed far enough in advance to permit your nominee to exercise your redemption rights with respect to the public shares following the separation of such public shares from the units. While this is typically done electronically on the same business day, you should allow at least one full business day to accomplish the separation. If you fail to cause your public shares to be separated in a timely manner, you will likely not be able to exercise your redemption rights.
If a public stockholder fails to receive notice of FoxWayne’s offer to redeem its public shares in connection with the Business Combination or fails to comply with the procedures for tendering its shares, such shares may not be redeemed.
Despite FoxWayne’s compliance with the U.S. federal proxy rules in connection with the Business Combination, if a public stockholder fails to receive this proxy statement/prospectus, such stockholder may not become aware of the opportunity to redeem its shares. This proxy statement/prospectus describes the various procedures that must be complied with in order to validly redeem public shares. In the event that a stockholder fails to comply with these or any other procedures, its shares may not be redeemed.
If FoxWayne is unable to consummate the Business Combination or any other Initial Business Combination by April 22, 2022 (which may be extended to July 22, 2022), the public stockholders may be forced to wait beyond such date before redemption from the Trust Account.
If FoxWayne is unable to complete an Initial Business Combination by April 22, 2022 (which may be extended to July 22, 2022) for any reason, compliance with Delaware law may require that FoxWayne submit a plan of dissolution to its then-existing stockholders for approval prior to the distribution of the proceeds held in the Trust Account. In that case, public stockholders may be forced to wait beyond April 22, 2022 (or July 22, 2022 if such date is extended) before they receive funds from the Trust Account. FoxWayne intends to extend the period of time it has to consummate an Initial Business Combination. See the section titled “Does FoxWayne intend to extend the period of time it has to consummate an Initial Business Combination?”
The U.S. federal income tax treatment of the redemption of our Class A Common Stock as a sale of such Class A Common Stock depends on a stockholder’s specific facts.
The U.S. federal income tax treatment of a redemption of our Class A Common Stock will depend on whether the redemption qualifies as a sale of such Class A Common Stock under Section 302 of the Code, which will depend largely on the total number of shares of our stock treated as held by the stockholder electing to redeem its Class A Common Stock (including any stock constructively owned by the holder including as a result of owning Private Placement Warrants or public warrants and any of our stock that a holder would directly or indirectly acquire pursuant to the Business Combination) relative to all of our shares of stock outstanding before and after the redemption. If such redemption is not treated as a sale of Class A Common Stock for U.S. federal income tax purposes, the redemption will instead be treated as a corporate distribution. See “– The Business Combination - Material U.S. Federal Income Tax Consequences” for a more detailed discussion of the U.S. federal income tax treatment of a redemption of Class A Common Stock.
| -102- |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This proxy statement/prospectus contains certain forward-looking statements within the meaning of the “safe harbor” provisions under the United States Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this proxy statement/prospectus, including statements regarding the parties’ ability to consummate the Business Combination, the anticipated benefits of the Business Combination, the Combined Company’s future financial performance or financial position following the Business Combination, the amount of cash expected to be available to the Combined Company following the Business Combination, the effect of any redemptions by FoxWayne stockholders, the Combined Company’s business strategy and development plans, the anticipated timing, costs, design and conduct of its ongoing and planned clinical trials for its product candidates, the timing and likelihood of regulatory filings and approvals for its product candidates, its ability to commercialize its product candidates, if approved, the impact of the COVID-19 pandemic on its business, the pricing and reimbursement of its product candidates, if approved, its intent to enter into any strategic arrangements, the timing and likelihood of success, plans and objectives of management for future operations, future operating results of anticipated product development efforts and expected use of proceeds from the Business Combination, and statements for the period following consummation of the Business Combination may contain forward-looking statements. Forward-looking statements appear in a number of places in this proxy statement/prospectus including, without limitation, in the sections titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Aerami” and “About Aerami.” In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. Forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “anticipate,” “estimate,” “target,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions, but the absence of these words does not mean that a statement is not forward-looking.
These forward-looking statements are based on various assumptions, whether or not identified in this proxy statement/prospectus, and on the current expectations of the respective management teams of Aerami and FoxWayne as applicable and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by a stockholder or other investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Many actual events and circumstances are beyond the control of Aerami and FoxWayne. These forward-looking statements are subject to a number of risks and uncertainties including, but not limited to, those factors described in “Risk Factors,” discussed and identified in public filings made with the SEC by FoxWayne and the following: (1) the risk that the Business Combination may not be completed in a timely manner or at all, which may adversely affect the price of FoxWayne’s securities; (2) the failure to satisfy the conditions to closing the Business Combination, including the approval of certain proposals by the stockholders of FoxWayne and Aerami; (3) the risk that some or all of FoxWayne’s stockholders may redeem their shares at the closing of the Business Combination; (4) the effect of the announcement or pendency of the Business Combination on Aerami’s business relationships and business generally; (5) the outcome of any legal proceedings that may be instituted related to the Business Combination; (6) the ability to realize the anticipated benefits of the Business Combination; (7) the risk that the Combined Company may use its capital resources sooner than it expects; (8) the risk that the product candidates that Aerami is developing may not progress through clinical development or receive regulatory approvals within expected timelines or at all; (9) the risk that Aerami’s clinical trials, including, without limitation, the Phase 1 trial currently underway for AER-901, may not confirm any safety, efficacy or other product characteristics that would enable subsequent clinical trials, such as the Phase 2/3 trial planned for AER-901, or filing for regulatory approval; (10) the risk that Aerami will be unable to successfully market or gain market acceptance of its product candidates, if approved; (11) the risk that Aerami’s product candidates may not be beneficial to patients or successfully commercialized, if approved; (12) the risk that Aerami has overestimated the size of the target patient population, their willingness to try new therapies and the willingness of physicians to prescribe these therapies; (13) the effects of competition on Aerami’s business; (14) the risk that third parties on which Aerami depends for clinical trials and other critical services will fail to perform satisfactorily; (15) the risk that Aerami may not be able to recruit and retain qualified personnel; (16) the risk that Aerami’s business, operations, clinical development plans and timelines, and supply chain could be adversely affected by the effects of health epidemics, including the ongoing COVID-19 pandemic; (17) the risk that Aerami will be unable to obtain and maintain sufficient intellectual property protection for its drug products and devices or will infringe the intellectual property protection of others; (18) the risk the Combined Company will fail to realize the anticipated benefits of the Business Combination; (19) costs and expenses related to the proposed transaction; (20) the possibility of third-party claims against the Trust Account; (21) changes in applicable law; and (22) other risks and uncertainties indicated in this proxy statement/prospectus, including those under the section titled “Risk Factors.”
There may be additional risks that neither FoxWayne nor Aerami presently know, or that FoxWayne or Aerami currently believe are immaterial, which could also cause actual results to differ from those contained in the forward-looking statements. If any of these risks materialize or FoxWayne’s and Aerami’s assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements.
| -103- |
In addition, forward-looking statements reflect FoxWayne’s and Aerami’s expectations, plans or forecasts of future events and views as of the date of this proxy statement/prospectus. FoxWayne and Aerami anticipate that subsequent events and developments will cause FoxWayne’s and Aerami’s assessments to change. However, while FoxWayne and Aerami may elect to update these forward-looking statements at some point in the future, FoxWayne and Aerami specifically disclaim any obligation to do so, except as otherwise required by law. These forward-looking statements should not be relied upon as representing FoxWayne’s and Aerami’s assessments of any date subsequent to the date of this proxy statement/prospectus. Accordingly, undue reliance should not be placed upon the forward-looking statements.
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X, as amended, and presents the combination of the historical financial information of FoxWayne and Aerami adjusted to give effect to the completion of Aerami’s private placement of Aerami Common Stock in October and November 2021 and the Business Combination.
The unaudited pro forma condensed combined balance sheet as of September 30, 2021 combines the historical unaudited condensed balance sheet of FoxWayne as of September 30, 2021 and the historical unaudited condensed consolidated balance sheet of Aerami as of September 30, 2021 on a pro forma basis as if the Business Combination, completion of the Aerami private placement of Aerami Common Stock, and the other events contemplated by the Merger Agreement, summarized below, had been consummated on September 30, 2021.
The unaudited pro forma condensed combined statement of operations for the nine months ended September 30, 2021 combines the historical unaudited condensed statement of operations of FoxWayne for the nine months ended September 30, 2021 and the historical unaudited consolidated statement of operations of Aerami for the nine months ended September 30, 2021 on a pro forma basis as if the Business Combination and the other events contemplated by the Merger Agreement, as summarized below, had been consummated on January 1, 2020. The unaudited pro forma condensed combined statement of operations for the year ended December 31, 2020 combines the historical statement of operations of FoxWayne for the period from September 17, 2020 (inception) through December 31, 2020 and historical consolidated statement of operations of Aerami for the year ended December 31, 2020 on a pro forma basis as if the Business Combination and the other events contemplated by the Merger Agreement, as summarized below, had been consummated on January 1, 2020.
The unaudited pro forma condensed combined financial statements have been presented for illustrative purposes only and do not necessarily reflect what the Combined Company’s financial condition or results of operations would have been had the Business Combination and the completion of the private placement of Aerami Common Stock and other events contemplated by the Merger Agreement occurred on the dates indicated. Further, the unaudited pro forma condensed combined financial information also may not be useful in predicting the future financial condition and results of operations of the Combined Company. The actual financial position and results of operations may differ significantly from the pro forma amounts reflected herein due to a variety of factors. The unaudited pro forma adjustments represent management’s estimates based on information available as of the date of these unaudited pro forma condensed combined financial statements and are subject to change as additional information becomes available and analyses are performed.
The unaudited pro forma condensed combined financial information should also be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations of FoxWayne,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Aerami” and other financial information included elsewhere in this proxy statement/prospectus.
On December 7, 2021, FoxWayne entered into the Merger Agreement with Merger Sub, Aerami and the stockholders’ representative, pursuant to which Merger Sub will merge with and into Aerami, with Aerami surviving the merger as a wholly owned subsidiary of FoxWayne. Upon the Closing, FoxWayne will then change its name to “Aerami Therapeutics Holdings, Inc.” The aggregate consideration to be paid by FoxWayne pursuant to the Merger Agreement will be 25,000,000 shares of Combined Company common stock.
| -104- |
The unaudited pro forma condensed combined financial information contained herein assumes that FoxWayne stockholders approve the Business Combination. FoxWayne’s public stockholders may elect to redeem their public shares for cash even if they approve the Business Combination. FoxWayne cannot predict how many of its public stockholders will exercise their right to have their public shares redeemed for cash. As a result, we have elected to provide the unaudited pro forma condensed combined financial information under three different redemption scenarios, which produce different allocations of total Combined Company equity between holders of the public FoxWayne shares. As described in greater detail in Note 2, Basis of Presentation, of the unaudited pro forma condensed combined financial information, the first scenario, or “no redemption scenario,” assumes that none of FoxWayne’s public stockholders will exercise their right to have their public shares redeemed for cash, the second scenario, or “interim redemption scenario,” assumes the holders of approximately 33% of outstanding and redeemable public FoxWayne shares will exercise their redemption rights upon consummation of the Business Combination, the third scenario, or “maximum redemption scenario,” assumes that holders of the maximum number of public shares that could be redeemed for cash while still leaving sufficient cash available to consummate the Business Combination (which approximates 66% of outstanding and redeemable public FoxWayne shares) will exercise their right to have their public shares redeemed for cash. The actual results will be within the parameters described by the scenarios. However, there can be no assurance regarding which scenario will be closest to the actual results. Under all three scenarios, Aerami is considered the accounting acquirer, as further discussed in Note 2, Basis of Presentation, of the unaudited pro forma condensed combined financial information.
Unaudited Pro Forma Condensed Combined Balance Sheet
As of September 30, 2021
(in thousands)
| Assuming no redemption | Assuming interim redemption | Assuming maximum redemption | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FoxWayne Enterprises Acquisition Corp. (historical) | Aerami
Therapeutics Holdings, Inc. (historical) | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Assets | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Current assets: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cash and cash equivalents | $ | 25 | $ | 14,355 | $ | 6,362 | A | $ | 51,612 | B | $ | 72,354 | $ | 6,362 | A | $ | 32,564 | B | $ | 53,306 | $ | 6,362 | A | $ | 13,516 | B | $ | 34,258 | ||||||||||||||||||||||||||||||||||||||||
| Accounts receivable | - | 251 | - | - | 251 | - | - | 251 | - | - | 251 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prepaid expenses and other current assets | 167 | 854 | - | - | 1,021 | - | - | 1,021 | - | - | 1,021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total current assets | 192 | 15,460 | 6,362 | 51,612 | 73,626 | 6,362 | 32,564 | 54,578 | 6,362 | 13,516 | 35,530 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Investments held in trust | 58,079 | - | - | (58,079 | ) | B | - | - | (58,079 | ) | B | - | - | (58,079 | ) | B | - | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Deposits and other assets | - | 39 | - | - | 39 | - | - | 39 | - | - | 39 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total assets | $ | 58,271 | $ | 15,499 | $ | 6,362 | $ | (6,467 | ) | $ | 73,665 | $ | 6,362 | $ | (25,515 | ) | $ | 54,617 | $ | 6,362 | $ | (44,563 | ) | $ | 35,569 | |||||||||||||||||||||||||||||||||||||||||||
| Liabilities, redeemable stock and stockholders’ equity (deficit) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Current liabilities | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accounts payable | $ | 88 | $ | 889 | $ | - | $ | - | $ | 977 | $ | - | $ | - | $ | 977 | $ | - | $ | - | $ | 977 | ||||||||||||||||||||||||||||||||||||||||||||||
| Accrued expenses | 238 | 3,776 | - | (2,930 | ) | C | 1,084 | - | (2,930 | ) | C | 1,084 | - | (2,930 | ) | C | 1,084 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Due to related party | 90 | - | - | (90 | ) | B | - | - | (90 | ) | B | - | - | (90 | ) | B | - | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Franchise tax payable | 128 | - | - | - | 128 | - | - | 128 | - | - | 128 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total current liabilities | 544 | 4,665 | - | (3,020 | ) | 2,189 | - | (3,020 | ) | 2,189 | - | (3,020 | ) | 2,189 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Harmony contingent settlement liability | - | 925 | - | - | 925 | - | - | 925 | - | - | 925 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Promissory note with related party | 48 | - | - | (48 | ) | B | - | - | (48 | ) | B | - | - | (48 | ) | B | - | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Deferred underwriting commissions | 2,013 | - | - | (2,013 | ) | B | - | - | (2,013 | ) | B | - | - | (2,013 | ) | B | - | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Warrant liability | 4,104 | 2,377 | - | (2,377 | ) | D | 4,104 | - | (2,377 | ) | D | 4,104 | - | (2,377 | ) | D | 4,104 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Customer advances and other liabilities | - | 723 | - | - | 723 | - | - | 723 | - | - | 723 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total liabilities | 6,709 | 8,690 | - | (7,458 | ) | 7,941 | - | (7,458 | ) | 7,941 | - | (7,458 | ) | 7,941 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Commitments and contingencies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Redeemable stock: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class A common stock subject to possible redemption | 58,075 | - | - | (58,075 | ) | E | - | - | (58,075 | ) | E | - | - | (58,075 | ) | E | - | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Class B common stock subject to possible redemption | - | - | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A convertible preferred stock | - | 2,410 | - | (2,410 | ) | E | - | - | (2,410 | ) | E | - | - | (2,410 | ) | E | - | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A-1 convertible preferred stock | - | 7,509 | - | (7,509 | ) | E | - | - | (7,509 | ) | E | - | - | (7,509 | ) | E | - | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Total redeemable stock | 58,075 | 9,919 | - | (67,994 | ) | - | - | (67,994 | ) | - | - | (67,994 | ) | - | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stockholders’ equity (deficit): | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Royalty series convertible preferred stock | - | 6,956 | - | (6,956 | ) | E | - | - | (6,956 | ) | E | - | - | (6,956 | ) | E | - | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock | - | 5 | 1 | A | 4 | F | 10 | 1 | A | 4 | F | 10 | 1 | A | 4 | F | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Additional paid-in capital | - | 120,131 | 6,361 | A | 69,424 | G | 195,916 | 6,361 | A | 50,376 | G | 176,868 | 6,361 | A | 31,328 | G | 157,820 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Accumulated deficit | (6,513 | ) | (130,202 | ) | - | 6,513 | H | (130,202 | ) | - | 6,513 | H | (130,202 | ) | - | 6,513 | H | (130,202 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Total stockholders’ equity (deficit) | (6,513 | ) | (3,110 | ) | 6,362 | 68,985 | 65,724 | 6,362 | 49,937 | 46,676 | 6,362 | 30,889 | 27,628 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total liabilities, redeemable stock and stockholder’s equity (deficit) | $ | 58,271 | $ | 15,499 | $ | 6,362 | $ | (6,467 | ) | $ | 73,665 | $ | 6,362 | $ | (25,515 | ) | $ | 54,617 | $ | 6,362 | $ | (44,563 | ) | $ | 35,569 | |||||||||||||||||||||||||||||||||||||||||||
| -105- |
Unaudited
Pro Forma Condensed Combined Statement of Operations
For the Nine Months Ended September 30, 2021
(in thousands, except share and per share data)
| Assuming no redemption | Assuming interim redemption | Assuming maximum redemption | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FoxWayne Enterprises Acquisition Corp. (historical) | Aerami
Therapeutics Holdings, Inc. (historical) | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | ||||||||||||||||||||||||||||||||||||||||
| Revenues: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| License | $ | - | $ | 1,500 | $ | - | $ | - | $ | 1,500 | $ | - | $ | - | $ | 1,500 | $ | - | $ | - | $ | 1,500 | ||||||||||||||||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Research and development | - | 3,982 | - | - | 3,982 | - | - | 3,982 | - | - | 3,982 | |||||||||||||||||||||||||||||||||||||||||||||
| General and administrative | 728 | 3,825 | - | - | 4,553 | - | - | 4,553 | - | - | 4,553 | |||||||||||||||||||||||||||||||||||||||||||||
| General and administrative with related party | 90 | - | - | - | 90 | - | - | 90 | - | - | 90 | |||||||||||||||||||||||||||||||||||||||||||||
| Franchise tax expense | 128 | - | - | - | 128 | - | - | 128 | - | - | 128 | |||||||||||||||||||||||||||||||||||||||||||||
| Total operating expenses | 946 | 7,807 | - | - | 8,753 | - | - | 8,753 | - | - | 8,753 | |||||||||||||||||||||||||||||||||||||||||||||
| Loss from operations | (946 | ) | (6,307 | ) | - | - | (7,253 | ) | - | - | (7,253 | ) | - | - | (7,253 | ) | ||||||||||||||||||||||||||||||||||||||||
| Other (expense) income: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Change in fair value of Harmony contingent settlement liability | - | 2,053 | - | - | 2,053 | - | - | 2,053 | - | - | 2,053 | |||||||||||||||||||||||||||||||||||||||||||||
| Change in fair value of purchase option agreement | - | 33 | - | - | 33 | - | - | 33 | - | - | 33 | |||||||||||||||||||||||||||||||||||||||||||||
| Change in fair value of warrant liabilities | 485 | (545 | ) | - | 545 | J | 485 | - | 545 | J | 485 | - | 545 | J | 485 | |||||||||||||||||||||||||||||||||||||||||
| Financing costs for warrant liabilities | (213 | ) | - | - | - | (213 | ) | - | - | (213 | ) | - | - | (213 | ) | |||||||||||||||||||||||||||||||||||||||||
| Income from investments held in trust account | 4 | - | - | (4 | ) | K | - | - | (4 | ) | K | - | - | (4 | ) | K | - | |||||||||||||||||||||||||||||||||||||||
| Interest expense, net | - | (89 | ) | - | - | (89 | ) | - | - | (89 | ) | - | - | (89 | ) | |||||||||||||||||||||||||||||||||||||||||
| Other expense, net | - | (28 | ) | - | - | (28 | ) | - | - | (28 | ) | - | - | (28 | ) | |||||||||||||||||||||||||||||||||||||||||
| Other income, net | 276 | 1,424 | - | 541 | 2,241 | - | 541 | 2,241 | - | 541 | 2,241 | |||||||||||||||||||||||||||||||||||||||||||||
| Net loss | $ | (670 | ) | $ | (4,883 | ) | $ | - | $ | 541 | $ | (5,012 | ) | $ | - | $ | 541 | $ | (5,012 | ) | $ | - | $ | 541 | $ | (5,012 | ) | |||||||||||||||||||||||||||||
| Per share information: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net loss per share of common stock - basic and diluted | $ | (0.10 | ) | $ | (0.14 | ) | $ | (0.05 | ) | $ | (0.05 | ) | $ | (0.05 | ) | |||||||||||||||||||||||||||||||||||||||||
| Weighted-average shares of common stock outstanding - basic and diluted | 6,776,923 | 34,799,293 | 101,295,709 | 99,409,768 | 97,523,828 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| -106- |
Unaudited
Pro Forma Condensed Combined Statement of Operations
For the Year Ended December 31, 2020
(in thousands, except share and per share data)
| Assuming no redemption | Assuming interim redemption | Assuming maximum redemption | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FoxWayne Enterprises Acquisition Corp. (historical) | Aerami Therapeutics Holdings, Inc. (historical) | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | Aerami Private Placement pro forma adjustments | Note | Pro forma transaction accounting adjustments | Note | Pro forma combined | ||||||||||||||||||||||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Research and development | $ | - | $ | 3,461 | $ | - | $ | - | $ | 3,461 | $ | - | $ | - | $ | 3,461 | $ | - | $ | - | $ | 3,461 | ||||||||||||||||||||||||||||||||||
| General and administrative | 5 | 6,932 | - | - | 6,937 | - | - | 6,937 | - | - | 6,937 | |||||||||||||||||||||||||||||||||||||||||||||
| Franchise tax expense | 1 | - | - | - | 1 | - | - | 1 | - | - | 1 | |||||||||||||||||||||||||||||||||||||||||||||
| Total operating expenses and loss from operations | (6 | ) | (10,393 | ) | - | - | (10,399 | ) | - | - | (10,399 | ) | - | - | (10,399 | ) | ||||||||||||||||||||||||||||||||||||||||
| Other (expense) income: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Change in fair value of Harmony contingent settlement liability | - | 385 | - | - | 385 | - | - | 385 | - | - | 385 | |||||||||||||||||||||||||||||||||||||||||||||
| Change in fair value of purchase option agreement | - | (201 | ) | - | - | (201 | ) | - | - | (201 | ) | - | - | (201 | ) | |||||||||||||||||||||||||||||||||||||||||
| Interest expense, net | - | (100 | ) | - | - | (100 | ) | - | - | (100 | ) | - | - | (100 | ) | |||||||||||||||||||||||||||||||||||||||||
| Other income, net | - | 84 | - | - | 84 | - | - | 84 | - | - | 84 | |||||||||||||||||||||||||||||||||||||||||||||
| Net loss | (6 | ) | (10,309 | ) | - | - | (10,315 | ) | - | - | (10,315 | ) | - | - | (10,315 | ) | ||||||||||||||||||||||||||||||||||||||||
| Accretion to redemption value | - | (221 | ) | - | 221 | I | - | - | 221 | I | - | - | 221 | I | - | |||||||||||||||||||||||||||||||||||||||||
| Net loss attributable to common stockholders | $ | (6 | ) | $ | (10,530 | ) | $ | - | $ | 221 | $ | (10,315 | ) | $ | - | $ | 221 | $ | (10,315 | ) | $ | - | $ | 221 | $ | (10,315 | ) | |||||||||||||||||||||||||||||
| Per share information: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net loss per share of common stock - basic and diluted | $ | (0.01 | ) | $ | (0.30 | ) | $ | (0.10 | ) | $ | (0.10 | ) | $ | (0.11 | ) | |||||||||||||||||||||||||||||||||||||||||
| Weighted-average shares of common stock outstanding - basic and diluted | 1,250,000 | 34,638,486 | 101,294,372 | 99,408,431 | 97,522,491 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| -107- |
Notes to Unaudited Pro Forma Condensed Combined Financial Information
1. Description of the Business Combination
On December 7, 2021, FoxWayne entered into the Merger Agreement with Merger Sub, Aerami and the stockholders’ representative, pursuant to which Merger Sub will merge with and into Aerami, with Aerami as the surviving company in the Merger and, after giving effect to such Merger, Aerami shall become a wholly owned subsidiary of FoxWayne. Upon the closing of the Business Combination, it is anticipated that FoxWayne will change its name to “Aerami Therapeutics Holdings, Inc.”
Subject to the terms and conditions of the Merger Agreement, FoxWayne will issue 25,000,000 shares of Combined Company common stock as Aggregate Consideration in the Merger.
The following represents the Aggregate Consideration under the no redemption, interim redemption and maximum redemption scenarios:
| No Redemption, Interim Redemption and Maximum Redemption | ||||||||
| Purchase price | Shares issued | |||||||
| Share consideration to Aerami Equityholders(a)(b) | $ | 250,000,000 | 25,000,000 | |||||
| (a) | The estimated fair market value of Class A Common Stock issued to Aerami Equityholders included in the consideration is $10 per share for pro forma presentation purposes. | |
| (b) | The total of 25 million consideration shares includes shares to be issued for all issued and outstanding Aerami Common Stock and Aerami Preferred Stock, plus shares to be issued in satisfaction of certain agreements entered prior to or in connection with the Business Combination that are contingent upon the Business Combination, including, but not limited to, shares to be issued in payment of financial advisory fees, in settlement of certain royalty obligations to Aerami’s placement agent and in settlement of penalties due under the 2017 Registration Rights Agreement (assuming receipt of required approvals under that agreement). |
The following summarizes the unaudited pro forma Class A Common Stock shares outstanding under the no redemption, interim redemption and maximum redemption scenarios:
| Assuming No Redemption | Assuming Interim Redemption | Assuming Maximum Redemption | ||||||||||||||||||||||
| Shares | % | Shares | % | Shares | % | |||||||||||||||||||
| FoxWayne public stockholders | 5,800,000 | 18 | % | 3,914,059 | 13 | % | 2,028,119 | 7 | % | |||||||||||||||
| FoxWayne Sponsor and Directors | 1,437,500 | 4 | % | 1,437,500 | 5 | % | 1,437,500 | 5 | % | |||||||||||||||
| Total FoxWayne | 7,237,500 | 22 | % | 5,351,559 | 18 | % | 3,465,619 | 12 | % | |||||||||||||||
| Aerami(a) | 25,000,000 | 78 | % | 25,000,000 | 82 | % | 25,000,000 | 88 | % | |||||||||||||||
| Total shares at Closing | 32,237,500 | 100 | % | 30,351,559 | 100 | % | 28,465,619 | 100 | % | |||||||||||||||
| (a) | Aggregate consideration to be issued in the Merger is $250.0 million or 25 million shares of Class A Common Stock. The estimated fair market value of common stock issued to Aerami Equityholders included in the consideration is $10 per share for pro forma presentation purposes. | |
| (b) | The Total Shares at Closing excludes outstanding securities that are potentially dilutive if converted into shares of Class A Common Stock in the future including: |
| ● | 291,943 shares of Aerami unvested restricted stock units; | |
| ● | 12,604,309 outstanding Aerami stock options; | |
| ● | 992,992 outstanding Aerami common stock warrants; | |
| ● | 2,800,000 outstanding Private Placement Warrants held by our Sponsor; | |
| ● | 5,750,000 outstanding warrants held by FoxWayne public stockholders; and | |
| ● | 504,083 outstanding units held by FoxWayne public stockholders. |
Aerami securities are as of September 30, 2021 and do not reflect the effect of conversion as a result of the Business Combination.
| -108- |
2. Basis of Presentation
The unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X, as amended. The adjustments in the unaudited pro forma condensed combined financial information have been identified and presented to provide relevant information necessary for an accurate understanding of the Combined Company following completion of Aerami’s private placement of Aerami Common Stock and consummation of the Business Combination. Assumptions and estimates underlying the unaudited pro forma adjustments set forth in the unaudited pro forma condensed combined financial information are described in the accompanying notes.
The Business Combination will be accounted for as a reverse recapitalization under GAAP. Under this method of accounting, FoxWayne will be treated as the “acquired” company for financial reporting purposes. This determination is primarily based on Aerami stockholders comprising a relative majority of the voting power of the Combined Company and having the ability to nominate the members of the Combined Company Board, Aerami’s operations prior to the acquisition comprising the only ongoing operations of the Combined Company, and Aerami’s senior management comprising all of the senior management of the Combined Company. Accordingly, for accounting purposes, the financial statements of the Combined Company will represent a continuation of the financial statements of Aerami with the Business Combination being treated as the equivalent of Aerami issuing stock for the net assets of FoxWayne, accompanied by a recapitalization. The net assets of FoxWayne will be stated at historical costs, with no goodwill or other intangible assets recorded. Operations prior to the Business Combination will be presented as those of Aerami in future reports of the Combined Company.
The unaudited pro forma condensed combined financial information has been prepared using the assumptions below with respect to the potential redemption of publicly held shares of FoxWayne:
| ● | Assuming No Redemptions: This scenario assumes that no FoxWayne stockholders exercise redemption rights with respect to their public shares. | |
| ● | Assuming interim redemption: This scenario assumes that FoxWayne public stockholders holding approximately 1,885,941 shares of Class A Common Stock, or approximately 33%, out of the 5,750,000 outstanding and redeemable shares of FoxWayne Class A Common Stock will exercise their redemption rights upon consummation of the Business Combination at a redemption price of approximately $10.10 per share (aggregate $19.0 million). The estimated per share redemption value of $10.10 was calculated by dividing the amount of $58.1 million in the FoxWayne trust account as of September 30, 2021 by the total FoxWayne Class A Common Stock outstanding and redeemable of 5,750,000. | |
| ● | Assuming Maximum Redemptions: This scenario assumes that 3,771,881 shares of Class A Common Stock are redeemed for an aggregate redemption payment of approximately $38.1 million. This maximum redemption scenario is based on the maximum number of redemptions which may occur but which would still provide FoxWayne with net tangible assets of $15.0 million, after giving effect to FoxWayne’s unpaid transaction expenses. |
If the actual facts are different from these assumptions, then the amounts and shares outstanding in the unaudited pro forma condensed combined financial information will be different.
The Combined Company expects to enter into new equity awards with certain of its employees and to make grants to equity awards to certain of its non-employee directors shortly following the consummation of the Business Combination. The terms of these new equity awards have not been finalized and remain subject to change. Accordingly, no effect has been given to the unaudited pro forma condensed combined financial information for the new awards.
The unaudited pro forma condensed combined financial information does not reflect the income tax effects of the pro forma adjustments as any change in the deferred tax balance would be offset by an increase in the valuation allowance given that Aerami incurred significant losses during the historical periods presented.
| -109- |
3. Adjustments to Unaudited Pro Forma Condensed Combined Financial Information
Adjustments to Unaudited Pro Forma Condensed Combined Balance Sheet
The adjustments included in the unaudited pro forma condensed combined balance sheet as of September 30, 2021 are as follows:
| (A) | Reflects the sale of 7,310,560 shares of Aerami Common Stock sold after September 30, 2021 that provided net proceeds of $6.4 million to Aerami. | |
| (B) | Represents the impact of the Business Combination on the cash balance of the Combined Company. The table below represents the sources and uses of funds related to the Business Combination (in thousands): |
| No | Interim | Max | ||||||||||||||
| Item | Note | Redemption | Redemption | Redemption | ||||||||||||
| Procceeds from cash held in Trust Account | 1 | $ | 58,079 | $ | 58,079 | $ | 58,079 | |||||||||
| Payment to redeeming FoxWayne stockholders | 2 | - | (19,048 | ) | (38,096 | ) | ||||||||||
| Payment of FoxWayne deferred underwriter commissions | 3 | (2,013 | ) | (2,013 | ) | (2,013 | ) | |||||||||
| Payment of FoxWayne transaction costs | 4 | (4,100 | ) | (4,100 | ) | (4,100 | ) | |||||||||
| Payment of accrued expenses | 5 | (216 | ) | (216 | ) | (216 | ) | |||||||||
| Repayment of related party debt and other obligations | 6 | (138 | ) | (138 | ) | (138 | ) | |||||||||
| Adjustment to cash and cash equivalents | $ | 51,612 | $ | 32,564 | $ | 13,516 | ||||||||||
| 1. | Represents the amount of cash held in the trust of FoxWayne at September 30, 2021. | |
| 2. | Represents the redemption of 1,885,941 shares of Class A Common Stock and 3,771,881 shares of Class A Common Stock at $10.10 per share paid to FoxWayne stockholders who are assumed to exercise their redemption rights under the interim and maximum redemption scenarios, respectively. | |
| 3. | Represents deferred commissions paid to FoxWayne’s underwriters in connection with FoxWayne’s initial public offering completed prior to September 30, 2021. | |
| 4. | Represents the estimated transaction costs incurred by FoxWayne and Aerami of $2.9 million and $1.2 million, respectively, and not accrued for as of September 30, 2021. | |
| 5. | Represents the payment of offering and transaction costs of FoxWayne and Aerami of $70 and $146, respectively, that are included in accrued expenses as of September 30, 2021. | |
| 6. | Reflects the repayment of FoxWayne’s promissory note with a related party and FoxWayne’s due to related party liability as of September 30, 2021. |
| (C) | Represents the payment of offering and transaction expenses of FoxWayne and Aerami of $70 and $146, respectively, that are included in accrued expenses as of September 30, 2021, and the issuance of 273,306 shares of Class A Common Stock to settle the Aerami registration rights obligation of $2.7 million as of September 30, 2021. | |
| (D) | Represents the settlement of Aerami liability-classified warrants through the automatic cashless exercise and issuance of Aerami Common Stock upon completion of the Business Combination. | |
| (E) | Represents the conversion into common stock or redemption of outstanding potentially redeemable securities and Aerami Royalty Stock upon completion of the Business Combination. |
| -110- |
| (F) | Represents the adjustment to the par value of Class A Common stock outstanding upon completion of the Business Combination (in thousands): |
| No | Interim | Max | ||||||||||
| Redemption | Redemption | Redemption | ||||||||||
| Combined pre-adjusted common stock | $ | 5 | $ | 5 | $ | 5 | ||||||
| Shares of Aerami common stock outstanding | 49,579,806 | 49,579,806 | 49,579,806 | |||||||||
| Conversion of FoxWayne Class A common stock | 5,800,000 | 3,914,059 | 2,028,119 | |||||||||
| Conversion of FoxWayne Class B common stock | 1,437,500 | 1,437,500 | 1,437,500 | |||||||||
| Conversion of Aerami Series A preferred stock | 1,990,000 | 1,990,000 | 1,990,000 | |||||||||
| Conversion of Aerami Series A-1 preferred stock | 20,920,000 | 20,920,000 | 20,920,000 | |||||||||
| Conversion of Aerami Royalty Series preferred stock | 5,082,918 | 5,082,918 | 5,082,918 | |||||||||
| Cashless exercise of Aerami Series A warrants for common stock | 92,080 | 92,080 | 92,080 | |||||||||
| Cashless exercise of Aerami Series A-1 warrants for common stock | 1,693,534 | 1,693,534 | 1,693,534 | |||||||||
| Cashless exercise of Aerami common stock warrants for common stock | 1,382,670 | 1,382,670 | 1,382,670 | |||||||||
| Issuance of common stock in connection with Aerami placement agent fee | 4,725,399 | 4,725,399 | 4,725,399 | |||||||||
| Issuance of common stock in connection with Aerami registration rights and royalty payments | 1,281,242 | 1,281,242 | 1,281,242 | |||||||||
| Total shares of common stock issued upon conversion | 93,985,149 | 92,099,208 | 90,213,268 | |||||||||
| Allocation to common stock at par value $0.0001 per share | $ | 9 | $ | 9 | $ | 9 | ||||||
| Adjustment to common stock | $ | 4 | $ | 4 | $ | 4 | ||||||
| (G) | Reflects the change in additional paid-in capital upon completion of the Business Combination as follows (in thousands): |
| No | Interim | Max | ||||||||||
| Redemption | Redemption | Redemption | ||||||||||
| Combined pre-adjusted additional paid-in capital balance | $ | 120,131 | $ | 120,131 | $ | 120,131 | ||||||
| Issuance of common stock upon settlement of Aerami registration rights obligation | 2,714 | 2,714 | 2,714 | |||||||||
| Cashless exercise of Aerami liability classified warrants | 2,377 | 2,377 | 2,377 | |||||||||
| Conversion of outstanding potentially redeemable securities into common stock | 67,994 | 67,994 | 67,994 | |||||||||
| Conversion of Aerami Royalty Series Convertible Preferred Stock | 6,956 | 6,956 | 6,956 | |||||||||
| Redemption of FoxWayne Class A common stock under a maximum redemption scenario | - | (19,048 | ) | (38,096 | ) | |||||||
| Adjustment to common stock | (4 | ) | (4 | ) | (4 | ) | ||||||
| Elimination of FoxWayne’s historical accumulated deficit upon completion of the Business Combination. | (6,513 | ) | (6,513 | ) | (6,513 | ) | ||||||
| Transaction costs paid upon completion of Business Combination | (4,100 | ) | (4,100 | ) | (4,100 | ) | ||||||
| Adjustment to additional paid-in capital | $ | 69,424 | $ | 50,376 | $ | 31,328 | ||||||
| (H) | Elimination of FoxWayne’s historical accumulated deficit upon completion of the Business Combination. |
Adjustments to Unaudited Pro Forma Condensed Combined Statements of Operations
| (I) | Reflects the elimination of accretion to redemption value for certain Aerami Series A Preferred Stock that contained a put feature outside Aerami’s control. | |
| (J) | Reflects the elimination of the change in fair value of Aerami liability-classified warrants to be cashless exercised in connection with the Business Combination. | |
| (K) | Reflects the elimination of interest income for FoxWayne cash held in trust account upon completion of the Business Combination. |
4. Net Loss per Share
Represents the net loss per share calculated using the historical weighted average shares outstanding and (i) Aerami’s private placement of Aerami Common Stock in October and November 2021 and (ii) the issuance of additional shares in connection with the Business Combination assuming such additional shares (i.e., from (i) and (ii)) were outstanding since January 1, 2020. As the Business Combination is being reflected as if they had occurred as of January 1, 2020, the calculation of weighted average shares outstanding for basic and diluted net loss per share assumes the shares issued in connection with the Business Combination have been outstanding for the entire periods presented. Under the maximum redemptions scenario, the shares of Class A Common Stock assumed to be redeemed by FoxWayne public stockholders are eliminated as of January 1, 2020.
| -111- |
Net loss per share also assumes the sale of Aerami’s Series A Preferred Stock from June 2020 through January 2021, assuming such shares were issued, outstanding and converted into Aerami Common Stock on January 1, 2020, and giving effect to the conversion of a portion of the Series A Preferred Stock into shares of Aerami Series A-1 Preferred Stock as a result of the participation of the holders of such shares in the 2021 private placement of Aerami’s Common Stock, all of which together have an impact on the calculation of the number of shares outstanding.
The unaudited pro forma condensed combined financial information has been prepared assuming the no redemptions, interim redemptions and maximum redemptions scenarios:
| Nine Months Ended September 30, 2021 | Year Ended December 31, 2020 | |||||||||||||||||||||||
| (in thousands, except share and per share data) | No Redemptions | Interim Redemptions | Maximum Redemptions | No Redemptions | Interim Redemptions | Maximum Redemptions | ||||||||||||||||||
| Pro forma net loss | $ | (5,012 | ) | (5,012 | ) | $ | (5,012 | ) | $ | (10,315 | ) | $ | (10,315 | ) | $ | (10,315 | ) | |||||||
| Pro forma weighted average shares outstanding - basic and diluted | 101,295,709 | 99,409,768 | 97,523,828 | 101,294,372 | 99,408,431 | 97,522,491 | ||||||||||||||||||
| Pro forma net loss per share of common stock - basic and diluted | $ | (0.05 | ) | $ | (0.05 | ) | $ | (0.05 | ) | $ | (0.10 | ) | $ | (0.10 | ) | $ | (0.11 | ) | ||||||
| Pro forma weighted average shares outstanding - basic and diluted(1): | ||||||||||||||||||||||||
| FoxWayne public stockholders | 5,800,000 | 3,914,059 | 2,028,119 | 5,800,000 | 3,914,059 | 2,028,119 | ||||||||||||||||||
| FoxWayne Sponsor and Directors | 1,437,500 | 1,437,500 | 1,437,500 | 1,437,500 | 1,437,500 | 1,437,500 | ||||||||||||||||||
| Total FoxWayne | 7,237,500 | 5,351,559 | 3,465,619 | 7,237,500 | 5,351,559 | 3,465,619 | ||||||||||||||||||
| Aerami Series A preferred stock | 1,990,000 | 1,990,000 | 1,990,000 | 1,990,000 | 1,990,000 | 1,990,000 | ||||||||||||||||||
| Aerami Series A-1 preferred stock | 20,920,000 | 20,920,000 | 20,920,000 | 20,920,000 | 20,920,000 | 20,920,000 | ||||||||||||||||||
| Aerami Private Placement | 22,250,560 | 22,250,560 | 22,250,560 | 22,250,560 | 22,250,560 | 22,250,560 | ||||||||||||||||||
| Aerami common stock | 34,639,806 | 34,639,806 | 34,639,806 | 34,638,469 | 34,638,469 | 34,638,469 | ||||||||||||||||||
| Aerami royalty preferred stock | 5,082,918 | 5,082,918 | 5,082,918 | 5,082,918 | 5,082,918 | 5,082,918 | ||||||||||||||||||
| Aerami cashless exercise warrants | 3,168,284 | 3,168,284 | 3,168,284 | 3,168,284 | 3,168,284 | 3,168,284 | ||||||||||||||||||
| Aerami placement agent and registration rights obligation settlement | 6,006,641 | 6,006,641 | 6,006,641 | 6,006,641 | 6,006,641 | 6,006,641 | ||||||||||||||||||
| Pro forma weighted average shares outstanding - basic and diluted | 101,295,709 | 99,409,768 | 97,523,828 | 101,294,372 | 99,408,431 | 97,522,491 | ||||||||||||||||||
| (1) | Outstanding options, unvested restricted stock units and warrants are anti-dilutive and, therefore, are not included in the calculation of diluted net loss per share. |
| -112- |
COMPARATIVE SHARE INFORMATION
The following tables set forth:
| ● | Historical per share information of FoxWayne for the nine months ended September 30, 2021 and for the period from inception (September 17, 2020) through December 31, 2020; | |
| ● | Historical per share information of Aerami for the nine months ended September 30, 2021 and for the year ended December 31, 2020; and | |
| ● | Unaudited pro forma per share information of the Combined Company for the nine months ended September 30, 2021 and for the year ended December 31, 2020, after giving effect to the completion of the private placement of Aerami Common Stock in October and November 2021 and the Business Combination, assuming three redemption scenarios as follows: |
| ● | Assuming no redemption: This scenario assumes that no public stockholders exercise redemption rights with respect to their public shares. | |
| ● | Assuming interim redemption: This presentation assumes that FoxWayne public stockholders holding approximately 1,885,941 shares of Class A Common Stock, or approximately 33%, out of the 5,750,000 outstanding and redeemable shares of FoxWayne Class A Common Stock will exercise their redemption rights upon consummation of the Business Combination at a redemption price of approximately $10.10 per share (aggregate $19.0 million) The estimated per share redemption value of $10.10 was calculated by dividing the amount of $58.1 million in the FoxWayne trust account as of September 30, 2021 by the total number of shares of FoxWayne Class A Common Stock outstanding and redeemable of 5,750,000. | |
| ● | Assuming maximum redemption: The Merger Agreement includes a $15 million minimum cash condition. This presentation assumes that FoxWayne public stockholders holding approximately 3,771,881 shares of FoxWayne Class A Common Stock will exercise their redemption rights upon consummation of the Business Combination at a redemption price of approximately $10.10 per share, such that the $15 million cash condition is met after giving effect to the transaction costs to be paid upon consummation of the Business Combination. This leads to a total maximum redemption value of $38.1 million. Such amount represents the maximum number of redemptions of shares of FoxWayne Class A Common Stock that could occur before the minimum cash condition would not be met. |
The following tables should be read in conjunction with the summary historical financial information included elsewhere in this proxy statement/prospectus, and the historical financial statements of FoxWayne and Aerami and the related notes thereto that are included elsewhere in this proxy statement/prospectus. The unaudited FoxWayne and Aerami pro forma combined per share information is derived from, and should be read in conjunction with, the unaudited pro forma condensed combined financial statements and the related notes thereto included elsewhere in this proxy statement/prospectus.
The unaudited pro forma combined net loss per share information below does not purport to represent the actual results of operations that would have occurred had the companies been combined during the periods presented, nor does it purport to represent the actual results of operations for any future date or period. The unaudited pro forma combined book value per share information below does not purport to represent what the value of FoxWayne and Aerami would have been had the companies been combined during the periods presented.
| -113- |
| Historical | Pro Forma Combined | |||||||||||||||||||
| (amounts in thousands except share and per share data) | FoxWayne Enterprises Acquisition Corp. (historical) | Aerami Therapeutics Holdings, Inc. (historical) | Assuming No Redemption | Assuming Interim Redemption | Assuming Maximum Redemption | |||||||||||||||
| For the Nine Months Ended September 30, 2021 | ||||||||||||||||||||
Book value per share - Class A redeemable common stock (1a) | $ | (1.13 | ) | $ | (0.06 | ) | $ | 0.65 | $ | 0.47 | $ | 0.28 | ||||||||
| Book value per share - basic and diluted Class A and Class B non-redeemable common stock (1b) | $ | (4.38 | ) | $ | - | $ | - | $ | - | - | ||||||||||
| Net loss per share - basic and diluted (2a) | $ | (0.13 | ) | $ | (0.14 | ) | $ | (0.05 | ) | $ | (0.05 | ) | (0.05 | ) | ||||||
| Net loss per share - basic and diluted Class A non-redeemable common stock (2b) | $ | (0.45 | ) | |||||||||||||||||
| Weighted-average shares outstanding - basic and diluted Class A redeemable common stock | 5,303,846 | 34,799,293 | 101,295,709 | 99,409,768 | 97,523,828 | |||||||||||||||
| Weighted-average shares outstanding - basic and diluted Class A and Class B non-redeemable common stock | 1,473,077 | |||||||||||||||||||
| (1a) | Book value per share is calculated as total equity divided by the number of shares of FoxWayne Class A Redeemable Common Stock outstanding at September 30, 2021 and Aerami Common Stock outstanding at September 30, 2021. |
| (1b) | Book value per share is calculated as total equity divided by the number of shares of FoxWayne Class A and Class B Non-Redeemable Common Stock outstanding at September 30, 2021. |
| (2a) | Net loss per share is based on the weighted average number of shares of FoxWayne Class A Redeemable Common Stock outstanding for the nine-month period ended September 30, 2021, the weighted average number of shares of Aerami Common Stock outstanding for the nine-month period ended September 30, 2021, and the pro forma information. |
| (2b) | Net loss per share is based on the weighted average number of shares of FoxWayne Class A and Class B Non-Redeemable Common Stock outstanding for the nine-month period ended September 30, 2021. |
TRADING MARKET AND DIVIDEND POLICY
FoxWayne’s units, Class A Common Stock and warrants are currently listed on The Nasdaq Capital Market under the symbols “FOXWU,” “FOXW” and “FOXWW,” respectively. On , 2022, the record date for the special meeting of stockholders, the last sale price of FoxWayne Class A Common Stock was $ . Holders of FoxWayne securities should obtain current market quotations for their securities. The market price of FoxWayne’s securities could vary at any time before the Business Combination. In connection with the closing of the Business Combination, FoxWayne intends to change its name to “Aerami Therapeutics Holdings, Inc.” and has applied to list the shares of common stock of the Combined Company on The Nasdaq Capital Market under the symbol “ .”
FoxWayne Dividend Policy
FoxWayne has not paid any cash dividends on its shares of common stock to date and does not intend to pay cash dividends prior to the completion of a business combination. The payment of cash dividends in the future will be dependent upon FoxWayne’s revenues and earnings, if any, capital requirements and general financial condition subsequent to completion of a business combination. The payment of any dividends subsequent to a business combination will be within the discretion of the Combined Company Board. It is the present intention of the FoxWayne Board to retain all earnings, if any, for use in its business operations and, accordingly, the FoxWayne Board does not anticipate declaring any dividends in the foreseeable future.
Aerami Dividend Policy
Information regarding Aerami’s dividend policy is not provided because there is no public market for Aerami’s securities.
Combined Company Dividend Policy
Following consummation of the Business Combination, the Combined Company Board will consider whether or not to institute a dividend policy. Currently, the Combined Company is expected to retain its earnings for use in business operations. Accordingly, we do not anticipate that the Combined Company Board will declare any dividends in the foreseeable future.
| -114- |
SPECIAL MEETING OF FOXWAYNE STOCKHOLDERS
In this section, unless otherwise stated or the context requires otherwise, the terms “FoxWayne,” “we,” “us” and “our” refer to FoxWayne Enterprises Acquisition Corp. prior to the Closing and the Combined Company and its subsidiaries following the Closing
General
We are furnishing this proxy statement/prospectus to our stockholders as part of the solicitation of proxies by the FoxWayne Board for use at the special meeting of stockholders to be held on , 2022, and at any adjournment or postponement thereof. This proxy statement/prospectus is first being furnished to our stockholders on or about , 2022. This proxy statement/prospectus provides you with information you need to know to be able to vote or instruct your vote to be cast at the special meeting.
All stockholders as of the record date, or their duly appointed proxies, may attend the special meeting, which will be a completely virtual meeting. There will be no physical meeting locations and the special meeting will only be conducted via live webcast. Stockholders may attend the special meeting online, including to vote and submit questions, at the following address: .
We are utilizing a virtual stockholder meeting format for the special meeting in light of the health risks associated with the ongoing outbreak of COVID-19. It is currently our intent to resume in-person meetings at our first annual meeting of stockholders after 2022, and thereafter, assuming normal circumstances. Our virtual stockholder meeting format uses technology designed to increase stockholder access, save us and our stockholders time and money and provide our stockholders rights and opportunities to participate in the virtual special meeting similar to those they would have at an in-person special meeting, at no cost. In addition to online attendance, we provide stockholders with an opportunity to hear all portions of the official special meeting as conducted by the FoxWayne Board, submit written questions and comments during the special meeting and vote online during the open poll portion of the special meeting. We welcome your suggestions on how we can make our virtual special meeting more effective and efficient.
Stockholders will have multiple opportunities to submit questions to us for the special meeting. Stockholders who wish to submit a question in advance may do so at . Stockholders also may submit questions live during the meeting. Questions pertinent to special meeting matters may be recognized and answered during the special meeting in our discretion, subject to time constraints. We reserve the right to edit or reject questions that are inappropriate for special meeting matters. In addition, we will offer live technical support for all stockholders attending the special meeting.
To attend online and participate in the special meeting, stockholders of record will need to .
Date, Time and Place
The special meeting will be held at , Eastern time, on , 2022, via live webcast at , or such other date, time and place to which such meeting may be adjourned or postponed, to consider and vote upon the Proposals.
Voting Power; Record Date
You will be entitled to vote or direct votes to be cast at the virtual special meeting if you owned shares of our Class A Common Stock or Class B Common Stock, at the close of business on , 2022, which is the record date for the special meeting. You are entitled to one vote for each share of our Class A Common Stock and Class B Common Stock that you owned as of the close of business on the record date. If your shares are held in “street name” or are in a margin or similar account, you should contact your broker, bank or other nominee to ensure that votes related to the shares you beneficially own are properly counted. On the record date, there were shares of Class A Common Stock and Class B Common Stock outstanding, of which were public shares and were Founder Shares, respectively, held by the initial stockholders.
Vote of our Sponsor and the Directors and Officers of FoxWayne
Our Sponsor, directors and officers have agreed to vote any shares of Class A Common Stock and Class B Common Stock owned by them in favor of the Business Combination.
Our Sponsor, directors and officers have entered into a letter agreement with us, pursuant to which they have waived their redemption rights with respect to any Class B Common Stock held by them and any Class A Common Stock they acquired in the initial public offering or after the initial public offering, in connection with the completion of the Business Combination. The Founder Shares held by our Sponsor and our directors and their respective affiliates have no redemption rights upon our liquidation and will be worthless if no business combination is effected by us by April 22, 2022 (which may be extended to July 22, 2022). However, our Sponsor, directors and officers are entitled to redemption rights upon our liquidation with respect to any shares of Class A Common Stock they may own.
| -115- |
Quorum and Required Vote for Proposals for the Special Meeting
A quorum of our stockholders is necessary to hold a valid meeting. A quorum will be present at the special meeting if holders of a majority of the shares of our Class A Common Stock and Class B Common Stock entitled to vote thereat attend virtually or are represented by proxy at the special meeting. Abstentions will count as present for the purposes of establishing a quorum.
The approval of the Transaction Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal, and the Adjournment Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon at the special meeting, voting as a single class. Approval of the Charter Proposal and the Bylaw Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote thereon at the special meeting, voting as a single class. Accordingly, a stockholder’s failure to vote by proxy or to vote online at the special meeting will have no effect on the outcome of any vote on the Transaction Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal, or the Adjournment Proposal, but will have the same effect as a vote AGAINST the Charter Proposal and the Bylaw Proposal.
The Closing is conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal at the special meeting. The Bylaw Proposal, the New Equity Incentive Plan Proposal, and the Additional Proposal are conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal. The Adjournment Proposal is not conditioned on the approval of any other Proposal set forth in this proxy statement/prospectus.
Recommendation to FoxWayne Stockholders
After careful consideration, the FoxWayne Board recommends that our stockholders vote “FOR” each Proposal being submitted to a vote of the stockholders at the special meeting.
For a more complete description of our reasons for the approval of the Business Combination and the recommendation of the FoxWayne Board, see the subsection titled “The Business Combination —FoxWayne Board’s Reasons for the Approval of the Business Combination.”
Voting Your Shares
Each share of Class A Common Stock and each share of Class B Common Stock that you own in your name entitles you to one vote on each of the Proposals for the special meeting. Your one or more proxy cards show the number of shares of Class A Common Stock and Class B Common Stock that you own. There are several ways to vote your shares of Class A Common Stock and Class B Common Stock:
| ● | You can vote your shares by completing, signing, dating and returning the enclosed proxy card in the postage-paid envelope provided. If you hold your shares in “street name” through a bank, broker or other nominee, you will need to follow the instructions provided to you by your bank, broker or other nominee to ensure that your shares are represented and voted at the special meeting. If you vote by proxy card, your “proxy,” whose name is listed on the proxy card, will vote your shares as you instruct on the proxy card. If you sign and return the proxy card but do not give instructions on how to vote your shares, your shares of Class A Common Stock and/or Class B Common Stock will be voted “FOR” the Transaction Proposal, “FOR” the Charter Proposal, “FOR” the Bylaw Proposal, “FOR” the Nasdaq Proposal, “FOR” the New Equity Incentive Plan Proposal, “FOR” the Additional Proposal and “FOR” the Adjournment Proposal. | |
| ● | You can attend the special meeting virtually and vote online even if you have previously voted by submitting a proxy pursuant to any of the methods noted above. However, if your shares of Class A Common Stock or Class B Common Stock are held in the name of your broker, bank or other nominee, you must get a proxy from the broker, bank or other nominee. That is the only way we can be sure that the broker, bank or nominee has not already voted your shares of Class A Common Stock or Class B Common Stock. |
| -116- |
Revoking Your Proxy
If you give a proxy, you may revoke it at any time before the special meeting or at such meeting by doing any one of the following:
| ● | you may send another proxy card with a later date; | |
| ● | you may notify our Secretary, in writing, before the special meeting that you have revoked your proxy; or | |
| ● | you may attend the special meeting virtually, revoke your proxy and vote online, as indicated above. |
No Additional Matters May Be Presented at the Special Meeting
The special meeting has been called to consider only the approval of the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal, the New Equity Incentive Plan Proposal, the Additional Proposal and the Adjournment Proposal. Under our bylaws, other than procedural matters incident to the conduct of the special meeting, except as set forth herein, no other matters may be considered at the special meeting if they are not included in this proxy statement/prospectus, which serves as the notice of the special meeting.
Who Can Answer Your Questions About Voting Your Shares or Warrants
If you have any questions about how to vote or direct a vote in respect of your shares of Class A Common Stock or Class B Common Stock, you may call, our proxy solicitor, at (banks and brokerage firms, please call collect at ).
Redemption Rights
Pursuant to the FoxWayne Charter, we will provide the public holders of public shares with the opportunity to redeem, upon the consummation of the Business Combination, public shares then held by them for cash equal to their pro rata share of the aggregate amount on deposit in the Trust Account that holds the proceeds from our initial public offering, as of two business days prior to the consummation of the Business Combination, including interest net of taxes payable by FoxWayne. Notwithstanding the foregoing redemption rights, a public stockholder, together with any of his, her or its affiliates or any other person with whom he, she or it is acting in concert or as a “group” (as defined under Section 13(d)(3) of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its public shares or, if part of such a group, the group’s public shares, in excess of 15% of the outstanding public shares. In order to determine whether a stockholder is acting in concert or as a “group” (as defined in Section 13(d)(3) of the Exchange Act) with any other stockholder, FoxWayne will require each public stockholder seeking to exercise redemption rights to certify to FoxWayne whether such stockholder is acting in concert or as a group with any other stockholder. Further, the FoxWayne Charter provides that we will only redeem our public shares so long as (after such redemption) our net tangible assets will be at least $5,000,001 either immediately prior to or upon consummation of our Initial Business Combination and after payment of underwriters’ fees and commissions. In addition, the Merger Agreement includes a condition to Closing that FoxWayne have at least $15,000,000 in available cash immediately prior to the Effective Time of the Merger, after taking into account payments required to satisfy redemptions of public shares by public stockholders and the payment of the Transaction Expenses of FoxWayne.
Public holders will be entitled to receive cash for redeemable public shares only if you:
| ● | submit a written request for FoxWayne to redeem all or a portion of your public shares for cash to Continental Stock Transfer & Trust Company, FoxWayne’s transfer agent; and | |
| ● | deliver your public shares to Continental Stock Transfer & Trust Company, physically or electronically through DTC prior to 5:00 p.m., Eastern time, on , 2022 (two business days before the special meeting), tender your shares physically or electronically and submit a request in writing that we redeem your public shares for cash to Continental Stock Transfer & Trust Company, our transfer agent, to the attention of Mark Zimkind at 1 State Street, 30th Floor, New York, New York 10004, by email at mzimkind@continentalstock.com. |
For illustrative purposes, based on the fair value of cash and marketable securities held in the Trust Account as of February 11, 2022 of approximately $58.1 million and 5,800,000 shares of Class A Common Stock outstanding and redeemable as of February 11, 2022, the estimated per share redemption price would have been approximately $10.10.
Holders of FoxWayne’s outstanding warrants sold in the initial public offering, which are exercisable for shares of Class A Common Stock under certain circumstances, do not have redemption rights in connection with the Business Combination.
| -117- |
Our Sponsor, officers and directors have agreed to waive their redemption rights in connection with the consummation of the Business Combination with respect to any shares of Class B Common Stock they may hold, and our shares of Class B Common Stock will be excluded from the pro rata calculation used to determine the per share redemption price. As of the record date, our Sponsor, officers and directors and their affiliates own approximately % of our outstanding shares of Class B Common Stock.
If a holder exercises its redemption rights, then such holder will be exchanging its shares of Class A Common Stock for cash and will no longer own shares of Class A Common Stock and will not participate in our future growth, if any. Such a holder will be entitled to receive cash for its public shares only if it properly demands redemption and delivers its shares (either physically or electronically) to our transfer agent in accordance with the procedures described herein. See the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights” for the procedures to be followed if you wish to redeem your shares for cash.
Any demand for redemption, once made, may be withdrawn at any time until the deadline for exercising redemption requests (and submitting shares to the transfer agent) and thereafter, with our consent, until the vote is taken with respect to the Business Combination. If you delivered your shares for redemption to the transfer agent and decide within the required timeframe not to exercise your redemption rights, you may request that the transfer agent return the shares (physically or electronically). You may make such request by contacting the transfer agent at the phone number or address listed above.
Holders of outstanding units of FoxWayne must separate the underlying public shares and public warrants prior to exercising redemption rights with respect to the public shares. If you hold units registered in your own name, you must deliver the certificate for such units or deliver such units electronically to Continental Stock Transfer & Trust Company with written instructions to separate such units into public shares and public warrants. This must be completed far enough in advance to permit the mailing of the public share certificates or electronic delivery of the public shares back to you so that you may then exercise your redemption rights with respect to the public shares following the separation of such public shares from the units.
If a broker, dealer, commercial bank, trust company or other nominee holds your units, you must instruct such nominee to separate your units. Your nominee must send written instructions by facsimile to Continental Stock Transfer & Trust Company. Such written instructions must include the number of units to be split and the nominee holding such units. Your nominee must also initiate electronically, using DTC’s DWAC system, a withdrawal of the relevant units and a deposit of the corresponding number of public shares and public warrants. This must be completed far enough in advance to permit your nominee to exercise your redemption rights with respect to the public shares following the separation of such public shares from the units. While this is typically done electronically on the same business day, you should allow at least one full business day to accomplish the separation. If you fail to cause your public shares to be separated in a timely manner, you will likely not be able to exercise your redemption rights.
Prior to exercising redemption rights, stockholders should verify the market price of our Class A Common Stock as they may receive higher proceeds from the sale of their Class A Common Stock in the public market than from exercising their redemption rights if the market price per share is higher than the redemption price. We cannot assure you that you will be able to sell your shares of Class A Common Stock in the open market, even if the market price per share is higher than the redemption price stated above, as there may not be sufficient liquidity in the Class A Common Stock when you wish to sell your shares.
If you exercise your redemption rights, your shares of Class A Common Stock will cease to be outstanding immediately prior to the Business Combination and will only represent the right to receive a pro rata share of the aggregate amount on deposit in the Trust Account. You will no longer own those shares and will have no right to participate in, or have any interest in, our future growth following the Business Combination, if any. You will be entitled to receive cash for these shares only if you properly and timely demand redemption.
If the Business Combination is not approved and we do not consummate a “Business Combination” (as defined in the Charter) by April 22, 2022 (which may be extended to July 22, 2022), we will be required to dissolve and liquidate our Trust Account by returning the then-remaining funds in such account to the public stockholders and our warrants will expire worthless. We intend to extend the period of time we have to consummate an Initial Business Combination. See the section titled “Does FoxWayne intend to extend the period of time it has to consummate an Initial Business Combination?”
| -118- |
Appraisal Rights
Appraisal rights are not available to holders of shares of Class A Common Stock and Class B Common Stock in connection with the Business Combination.
Proxy Solicitation Costs
We are soliciting proxies on behalf of the FoxWayne Board. This solicitation is being made by mail but also may be made by telephone or in person. FoxWayne and its directors, officers and employees may also solicit proxies in person. We will file with the SEC all scripts and other electronic communications as proxy soliciting materials. FoxWayne will bear the cost of the solicitation.
We have engaged to assist in the proxy solicitation process. We will pay that firm a fee of $ , plus disbursements. We will reimburse for reasonable out-of-pocket expenses and will indemnify and its affiliates against certain claims, liabilities, losses, damages and expenses. We will ask banks, brokers and other institutions, nominees and fiduciaries to forward the proxy materials to their principals and to obtain their authority to execute proxies and voting instructions. We will reimburse them for their reasonable expenses.
Share Ownership
As of , 2022, the record date of FoxWayne’s special meeting of stockholders, the Sponsor and FoxWayne’s directors, officers and other affiliates owned of record and were entitled to vote an aggregate of 1,437,500 Founder Shares that were issued prior to FoxWayne’s initial public offering. Such shares currently constitute approximately % of FoxWayne’s outstanding capital stock entitled to vote at the special meeting. The Sponsor and certain of FoxWayne’s directors, officers and other affiliates have agreed to vote the Founder Shares, as well as any other FoxWayne securities acquired in the aftermarket, in favor of each of the Proposals being presented at the special meeting. The Founder Shares have no right to participate in any redemption distribution and will be worthless if a business combination is not effected by FoxWayne.
| -119- |
PROPOSAL 1 — THE TRANSACTION PROPOSAL
Overview
We are asking our stockholders to approve and adopt the Merger Agreement and the Business Combination. Our stockholders should carefully read this proxy statement/prospectus in its entirety for more detailed information concerning the Merger Agreement, a copy of which is attached as Annex A to this proxy statement/prospectus. Please see the section above titled “The Business Combination” for additional information and a summary of certain terms of the Merger Agreement. You are urged to read carefully the Merger Agreement in its entirety before voting on this proposal.
Because we are holding a special meeting of stockholders to vote on the Business Combination, we may consummate the Business Combination only if it is approved by the affirmative vote (online or by proxy) of the holders of a majority of the outstanding shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon at the special meeting, voting as a single class.
THE BUSINESS COMBINATION
This section of the proxy statement/prospectus describes the material provisions of the Merger Agreement and the transactions contemplated thereby, but does not purport to describe all of the terms of the Merger Agreement. The following summary is qualified in its entirety by reference to the complete text of the Merger Agreement, a copy of which is attached as Annex A hereto. You are urged to read the Merger Agreement in its entirety because it is the primary legal document that governs the Business Combination.
The Merger Agreement contains representations, warranties and covenants that the respective parties made to each other as of the date of the Merger Agreement or other specific dates. The assertions embodied in those representations, warranties and covenants were made and will be made for purposes of the contract among the respective parties and are subject to important qualifications and limitations agreed to by the parties in connection with negotiating the Merger Agreement. The representations, warranties and covenants in the Merger Agreement are also modified in important part by the underlying disclosure letters, which we refer to as the “Schedules,” which are not filed publicly and which are subject to a contractual standard of materiality different from that generally applicable to stockholders and were used for the purpose of allocating risk among the parties rather than establishing matters as facts. Accordingly, you should not rely on the representations and warranties in the Merger Agreement as characterizations of the actual state of facts about the respective parties. Moreover, information concerning the subject matter of the representations and warranties may change after the date of the Merger Agreement, which subsequent information may or may not be fully reflected in our public disclosures.
Capitalized terms used but not defined herein shall have the meanings ascribed to such terms in the Merger Agreements.
General: Structure of the Business Combination
Subject to the terms and conditions of the Merger Agreement, upon the closing of the transactions contemplated by the Merger Agreement, Merger Sub will merge with and into Aerami, with Aerami surviving the Merger as a wholly owned subsidiary of the Combined Company.
Conversion of Securities
Immediately prior to the Effective Time and subject to the consent of the holders of Aerami Senior Preferred Stock, each share of Aerami Senior Preferred Stock will be converted into shares of Aerami Common Stock at the then-applicable conversion rate in accordance with the terms of the Aerami Charter.
Following the Conversion, there will be no outstanding shares of Aerami Senior Preferred Stock and each holder of Aerami Senior Preferred Stock will thereafter cease to have any rights with respect to such securities.
| -120- |
At the Effective Time, by virtue of the Business Combination and without any action on the part of FoxWayne, Merger Sub, Aerami or the Aerami Equityholders:
| ● | each share of Aerami Common Stock issued and outstanding immediately prior to the Effective Time (including shares of Aerami Common Stock resulting from the Conversion and the automatic cashless exercise of the Placement Agent Warrants, but excluding any Cancelled Shares or Aerami Dissenting Shares will be cancelled and converted into the right to receive the number of shares of Combined Company common stock equal to the Per Share Common Consideration; | |
| ● | all shares of Aerami Capital Stock held in treasury by Aerami will be cancelled without any conversion thereof and no payment or distribution will be made with respect thereto; | |
| ● | each share of Aerami Royalty Stock issued and outstanding immediately prior to the Effective Time will be cancelled and converted into the right to receive the number of shares of Combined Company common stock equal to Per Share Royalty Series Consideration; | |
| ● | each share of Merger Sub issued and outstanding immediately prior to the Effective Time will be converted into and exchanged for one share of Aerami, as the wholly owned subsidiary of the Combined Company; | |
| ● | each Aerami Warrant that is outstanding and unexercised immediately prior to the Effective Time, whether vested or unvested, which does not terminate by its terms in connection with the Merger, will be assumed by FoxWayne and converted into a warrant to purchase such number of whole shares of Combined Company common stock equal to the product of the number of shares of Aerami Common Stock subject to such Assumed Warrant and the Per Share Common Consideration (rounded up to the nearest whole number), and the per share exercise price will be ratably adjusted (rounded up to the nearest whole cent). Immediately prior to the Effective Time, the Placement Agent Warrants will automatically net exercise in full into applicable shares of Aerami securities pursuant to the terms of the respective warrant agreements; | |
| ● | each Aerami Option that is outstanding and unexercised immediately prior to the Effective Time will cease to represent the right to purchase Aerami Common Stock and will be assumed by FoxWayne and substituted and converted into an option to purchase such number of whole shares of Combined Company common stock (rounded down) pursuant to the Combined Company’s equity incentive plan equal to the product of the number of shares of Aerami Common Stock subject to such Aerami Option and the Per Share Common Consideration, and at an exercise price (rounded up to the nearest whole cent) equal to the quotient of the exercise price of such Aerami Option and the Per Share Common Consideration; | |
| ● | each Aerami RSU that is outstanding immediately prior to the Effective Time will be converted into the right to receive a restricted stock unit award for whole shares of Combined Company common stock (rounded down) pursuant to the Combined Company’s equity incentive plan in an amount equal to the product of the number of shares of Aerami Common Stock subject to such Exchanged RSU Award and the Per Share Common Consideration, and | |
| ● | no fractional shares of Combined Company common stock will be issued by virtue of the Merger and any fractional shares otherwise issuable to an Aerami Equityholder (after aggregating all fractional shares of the Combined Company common stock that otherwise would be received by such holder) will be rounded down to the nearest whole share of the Combined Company common stock. |
Except as specifically provided above, each Exchanged Option and each Exchanged RSU shall continue to be governed by the same terms and conditions (including vesting and exercisability terms) as were applicable to the corresponding former Aerami Option or former Aerami RSU, as applicable, immediately prior to the Effective Time, except to the extent such terms or conditions are rendered inoperative by the Business Combination.
Pursuant to the terms of the Charter, each outstanding share of Class B Common Stock will be converted into one share of Class A Common Stock and will no longer be outstanding and will cease to exist and each holder of Founder Shares will thereafter cease to have any rights with respect to such securities.
| -121- |
Payments of a Portion of Aggregate Consideration in Satisfaction of Obligations Under Other Agreements
At the Effective Time, up to shares of Combined Company common stock from the Aggregate Consideration will be issued to certain Aerami Equityholders in satisfaction of obligations under agreements entered prior to or in connection with the Business Combination, including (1) payment of the financial advisory fee to Aegis under the Advisory Agreement, (2) settlement of the obligation to pay royalties to Aegis under Aerami’s placement agent agreement with Aegis relating to its 2017 private placement in connection with the elimination of royalty payments to the Aerami Royalty Stock as a result of the Business Combination, and (3) assuming approval of the requisite holders of Aerami Capital Stock party to the 2017 Registration Rights Agreement, payment of the aggregate amount of the registration rights penalty and accrued interest.
In addition, Aerami may seek to allocate a portion of the Aggregate Consideration (or issue shares of Aerami Common Stock prior to effectiveness of the Business Combination) to address certain other contractual commitments relating to Aerami stock that are not triggered by the Business Combination, but that Aerami may deem to be in the best interests of its stockholders to terminate in connection with the Business Combination. Any such agreement would reduce the amount of Aggregate Consideration payable to Aerami stockholders.
Representations, Warranties and Covenants
The Merger Agreement contains customary representations, warranties and covenants of FoxWayne, Merger Sub and Aerami relating to, among other things, their ability to enter into the Merger Agreement and their respective outstanding capitalization. These representations and warranties are subject to materiality, knowledge and other similar qualifications in many respects and will not survive the Closing.
The Merger Agreement contains representations and warranties made by Aerami to FoxWayne and Merger Sub relating to a number of matters, including the following:
| ● | organization and qualification to do business; | |
| ● | Subsidiaries; | |
| ● | authority to enter into the Merger Agreement and the Ancillary Documents; | |
| ● | governmental consents and filings; | |
| ● | absence of conflicts with organizational documents, applicable laws and certain other agreements; | |
| ● | capitalization; | |
| ● | absence of litigation; | |
| ● | Intellectual Property; | |
| ● | material contracts; | |
| ● | certain Affiliate transactions; | |
| ● | property; | |
| ● | financial statements; | |
| ● | absence of certain liabilities; | |
| ● | absence of certain changes or events since September 30, 2021; | |
| ● | employee benefit matters; | |
| ● | labor matters; | |
| ● | Tax matters; | |
| ● | insurance; | |
| ● | compliance with Laws and Permits; |
| -122- |
| ● | environmental and safety laws; | |
| ● | FDA regulation; | |
| ● | Foreign Corrupt Practices Act; | |
| ● | data privacy; | |
| ● | takeover statutes; | |
| ● | brokers; and | |
| ● | information supplied. |
The Merger Agreement contains representations and warranties made by FoxWayne and Merger Sub to Aerami relating to a number of matters, including the following:
| ● | organization and qualification to do business; | |
| ● | Subsidiaries; | |
| ● | authority to enter into the Merger Agreement and the Ancillary Documents; | |
| ● | governmental consents and filings; | |
| ● | absence of conflicts with organizational documents, applicable laws and certain other agreements; | |
| ● | absence of prior Merger Sub operations; | |
| ● | capitalization; | |
| ● | absence of litigation; | |
| ● | internal controls, compliance with Nasdaq requirements and financial statements; | |
| ● | investment company and emerging growth company status; | |
| ● | business activities and liabilities; | |
| ● | certain Affiliate transactions; | |
| ● | property; | |
| ● | absence of certain liabilities; | |
| ● | absence of certain changes or events since FoxWayne’s formation; | |
| ● | employee matters and benefits; | |
| ● | Tax matters; | |
| ● | compliance with Laws and Permits; | |
| ● | Foreign Corrupt Practices Act; | |
| ● | SEC filings; | |
| ● | Trust Account; | |
| ● | Nasdaq listing; | |
| ● | issuance of Combined Company common stock pursuant to the terms of the Merger Agreement; | |
| ● | takeover statutes and charter provisions; | |
| ● | brokers; and | |
| ● | this proxy statement/Registration Statement. |
| -123- |
No Survival
The representations, warranties, covenants and agreements of Aerami, FoxWayne and Merger Sub contained in the Merger Agreement or in any instrument, document or certificate delivered pursuant to the Merger Agreement will terminate upon the occurrence of the Closing, and only those covenants and agreements contained in the Merger Agreement which by their terms expressly apply or are to be performed, in whole or in part, after the Closing will survive the Closing.
Closing
The Closing will occur as soon as reasonably practicable (and, in any event, within three Business Days) after satisfaction or (to the extent permitted by applicable Law) waiver of the conditions set forth in the Merger Agreement or at such other time, date and location FoxWayne and Aerami may agree in writing.
Conduct of Business Pending the Merger
From and after the date of the Merger Agreement until the earlier of (A) the termination of the Merger Agreement pursuant to its terms or (B) the Effective Time (such period, the “Interim Period”), except as expressly contemplated by the Merger Agreement, Aerami has agreed that it will, and will cause each other Acquired Company to, conduct its business in the Ordinary Course of Business. Without limiting the generality of the foregoing, during the Interim Period, except as expressly contemplated by the Merger Agreement, as required by applicable Law (including COVID-19 Measures) or pursuant to the written consent of FoxWayne, not to be unreasonably withheld, conditioned, or delayed, Aerami will not, and will cause each of the other Acquired Companies not to:
| ● | amend its certificate of incorporation, bylaws or other Organizational Documents (whether by merger, consolidation or otherwise); | |
| ● | declare, set aside or pay any dividend or other distribution (whether in cash, stock, debt or property or any combination thereof) in respect of any equity securities of any Acquired Company, or redeem, repurchase or otherwise acquire or offer to redeem, repurchase, or otherwise acquire any equity securities of any Acquired Company; | |
| ● | split, combine, subdivide or reclassify any shares of capital stock, membership interests or other equity interests; | |
| ● | issue, transfer, deliver, sell, pledge or otherwise encumber any Equity Interests of any Acquired Company; | |
| ● | make any material capital expenditures or incur any obligations or liabilities in respect thereof, other than agreements to advance the clinical development programs, provided that Aerami shall consult with FoxWayne and consider its recommendations relating thereto prior to entering into any such agreements; | |
| ● | directly or indirectly acquire by merger or consolidate with, or purchase substantially all of the assets of, any corporation, partnership, association, or other business organization or division thereof; | |
| ● | sell, lease, license or otherwise transfer, or create, incur, assume or suffer to exist any Encumbrance (other than Permitted Encumbrances) on, any of the assets, securities, properties, interests or businesses of the Acquired Companies, other than out-licenses of AER-501, AER-601, AER-901, or other products to third parties outside the United States; | |
| ● | make any loans, advances or capital contributions to, or investments in, any other Person; | |
| ● | make any payments constituting an Affiliate Transaction (as defined in the Merger Agreement) (other than salary payments in the Ordinary Course of Business consistent with past practice); |
| -124- |
| ● | create, incur, assume or otherwise become liable with respect to any Funded Indebtedness; | |
| ● | modify, amend, cancel, terminate or waive any rights under any Material Contract (as defined in the Merger Agreement), enter into any Contract that would have been a Material Contract had it been entered into prior to the date of the Merger Agreement, or otherwise waive, release or assign any material rights, claims or benefits of any Acquired Company; | |
| ● | (i) grant or agree to grant any severance or termination pay policies or increase any form of compensation or benefits payable to any director, officer, advisor, consultant, or employee of any Acquired Company or any of its ERISA Affiliates, including pursuant to any Employee Plan (as defined in the Merger Agreement); (ii) adopt, enter into, modify or terminate any Employee Plan; (iii) accelerate the vesting or payment of any compensation or benefits under any Employee Plan; (iv) grant any equity or equity-linked awards or other bonus, commission or other incentive compensation or provide any loan to any director, officer, advisor, consultant or employee of any Acquired Company or any of its ERISA Affiliates or (v) hire, demote, promote, change the title of, or terminate any employee, officer, director or consultant of any Acquired Company or any of its ERISA Affiliates or materially change the management structure of any Acquired Company; | |
| ● | implement or announce any employee layoffs, furloughs, reductions in force, reductions in compensation, hour or benefits, work schedule changes or similar actions that could implicate the WARN Act; | |
| ● | hire any employees with an annual base compensation of over $350,000, or terminate the employment of any employees with an annual base compensation of over $100,000, other than for cause; | |
| ● | fail to maintain, or allow to lapse, dispose of or abandon, including by failure to pay the required fees in any jurisdiction, any Acquired Company Intellectual Property, or grant permission to enter into the public domain any trade secrets included in the Acquired Company Intellectual Property; | |
| ● | transfer or grant to any third party any rights with respect to any Intellectual Property; | |
| ● | modify or amend in any material respect, terminate, permit to lapse, waive, or fail to enforce any material right or remedy under any Material Contract, or enter into any Contract that, if entered prior to the date of the Merger Agreement, would constitute a Material Contract; | |
| ● | change any Acquired Company’s methods of accounting or accounting practices, except as required by concurrent changes in GAAP as agreed to by such Acquired Company’s independent public accountants, or in connection with Aerami’s preparation for transition to public company accounting; | |
| ● | make any change to their cash management practices, policies or procedures with respect to collection of accounts receivable, establishment of reserves for uncollectible accounts, accrual of accounts receivable, inventory control, prepayment of expenses, payment of trade accounts payable, accrual of other expenses, deferral of revenue and acceptance of customer deposits; | |
| ● | commence, settle, or offer or propose to settle, (i) any Proceeding involving or against any Acquired Company, (ii) any equityholder litigation or dispute against any Acquired Company or any of its officers or directors or (iii) any Proceeding that relates to the transactions contemplated by the Merger Agreement; | |
| ● | cancel, settle or forgive any third-party Indebtedness owed to Aerami greater than $50,000 individually or $100,000 in the aggregate; | |
| ● | (i) make or change any material Tax election, settle or compromise any claim, notice, audit report or assessment in respect of Taxes, (ii) enter into any Tax allocation agreement, Tax sharing agreement, Tax indemnity agreement, pre-filing agreement, advance pricing agreement, cost sharing agreement or closing agreement relating to any Tax, (iii) file any federal or state income Tax Return or any other material Tax Return, (iv) amend any Tax Return, (v) surrender or forfeit any right to claim a Tax refund or (vi) consent to any extension or waiver of the statute of limitations period applicable to any Tax claim or assessment; | |
| ● | form or acquire any Subsidiaries; |
| -125- |
| ● | file any prospectus supplement or registration statement or consummate any offering of securities that requires registration under the Securities Act or that includes any actual or contingent commitment to register such securities under the Securities Act in the future; | |
| ● | liquidate, dissolve or effect a recapitalization or reorganization in any form of transaction; or | |
| ● | authorize or agree, resolve or commit to do any of the foregoing. |
During the Interim Period, except as expressly contemplated by the Merger Agreement, FoxWayne shall operate its business in the Ordinary Course of Business. Except as expressly contemplated by the Merger Agreement, as required by applicable Law (including COVID-19 Measures) or pursuant to the written consent of Aerami, FoxWayne shall not:
| ● | amend the Trust Agreement, its certificate of incorporation, bylaws or other Organizational Documents (whether by merger, consolidation or otherwise); | |
| ● | declare, set aside or pay any dividend or other distribution (whether in cash, stock, debt or property or any combination thereof) in respect of any equity securities of FoxWayne, or redeem, repurchase or otherwise acquire or offer to redeem, repurchase, or otherwise acquire any equity securities of FoxWayne, except for (i) transactions that would require an adjustment pursuant to the Merger Agreement, and for which the proper adjustment is made, (ii) in connection with a Private Placement conducted in compliance with applicable securities Laws, and (iii) the redemption of any shares of Combined Company common stock required by the Redemption Offer or as otherwise required by FoxWayne’s Organizational Documents in order to consummate the Transactions, repurchase, redeem or otherwise acquire, or offer to repurchase, redeem or otherwise acquire, any capital stock of, or other equity interests in, FoxWayne; | |
| ● | (i) issue, transfer, deliver, sell, pledge or otherwise encumber any shares of any Equity Interests of FoxWayne or Merger Sub, other than (A) in connection with the exercise of any FoxWayne Warrants outstanding on the date of the Merger Agreement (B) the Transactions, or (C) as otherwise permitted by the Merger Agreement (ii) amend, modify or waive any of the terms or rights set forth in any FoxWayne Warrant or the FoxWayne warrant agreement or (iii) amend any term of any equity securities of FoxWayne or Merger Sub (whether by merger, consolidation or otherwise); | |
| ● | acquire (by merger, consolidation, acquisition of stock or assets or otherwise), directly or indirectly, any assets, securities, properties, interests or businesses; | |
| ● | create, incur, assume or otherwise become liable with respect to any Indebtedness; | |
| ● | liquidate, dissolve or effect a recapitalization or reorganization in any form of transaction; | |
| ● | amend or modify the Lock-Up Agreement in a manner that would have the effect of decreasing the Founder Share Lock-up Periods (as defined therein) with respect to any of the Combined Company common stock restricted thereby to a period ending (x) in the event that a Private Placement is consummated in connection with the Closing, less than 90 days following the effectiveness of the Form S-1 Shelf (other than with respect to any of the Combined Company common stock transferred to the investor or investors participating in such Private Placement in connection with the completion thereof, as to which no such limitation shall apply), and (y) otherwise, less than 180 days after the Closing; or | |
| ● | agree, commit or authorize, in writing or otherwise, to take any of the foregoing actions. |
Additional Agreements
Registration Statement; Proxy Statement
As promptly as practicable after the execution of the Merger Agreement and delivery of certain financial statements by Aerami, FoxWayne agreed to prepare, with the assistance of Aerami, and file with the SEC this Registration Statement in connection with the registration under the Securities Act of the shares of Combined Company common stock pursuant to the Merger Agreement, which Registration Statement includes a proxy statement relating to the meeting of FoxWayne’s stockholders (including any adjournment or postponement thereof) to be held to consider the Proposals.
| -126- |
Written Consent
Aerami agreed that, as soon as reasonably practicable after this Registration Statement becomes effective, it will solicit the consent of its stockholders and use commercially reasonable efforts to obtain the same, including by the Aerami Board making a recommendation to the Aerami Stockholders to adopt the Merger Agreement.
FoxWayne Stockholders’ Meeting
FoxWayne agreed to call and hold the special meeting for the purpose of voting solely upon the Proposals. FoxWayne has agreed to use commercially reasonable efforts to, as promptly as practicable after this Registration Statement has been declared effective under the Securities Act, solicit proxies from the holders of Combined Company common stock to vote in accordance with the recommendation of the FoxWayne Board with respect to each of the Proposals. FoxWayne agreed, through the FoxWayne Board, to recommend to its stockholders that they approve the Proposals and to include the FoxWayne Board Recommendation in this proxy statement/prospectus. FoxWayne’s obligations to establish a record date, or duly call, give notice of, convene and hold the special meeting shall not be affected by any change to, withdrawal, withholding, qualification or modification of, or public proposal to change, withdraw, withhold, qualify or modify, the FoxWayne Board Recommendation.
Exclusivity
From the date of the Merger Agreement until the Closing, Aerami will not, and will cause its Subsidiaries, officers, employees, managers, directors, and agents and shall direct its representatives, accountants, consultants, investment bankers, legal counsel and advisors not to, directly or indirectly, (a) solicit, initiate, knowingly encourage or discuss any offer, inquiry, proposal or indication of interest, written or oral, (whether binding or non-binding) from any Person (other than FoxWayne or its Affiliates in connection with the transactions contemplated by the Merger Agreement) relating to an Alternative Company Transaction (an “Alternative Transaction Proposal”), (b) initiate any discussions or negotiations with any Person with respect to, or provide any non-public information or data concerning Aerami or any of its Subsidiaries to any Person relating to, an Alternative Transaction Proposal or afford to any Person access to the business, properties, assets or personnel of Aerami or any of its Subsidiaries in connection with an Alternative Transaction Proposal, (c) enter into any acquisition agreement, business combination, merger agreement or similar definitive agreement, or any letter of intent, memorandum of understanding or agreement in principle, or any other Contract relating to an Alternative Transaction Proposal or accept any offer relating to an Alternative Transaction Proposal, (d) approve, endorse or recommend, or propose publicly to approve, endorse or recommend any Alternative Transaction Proposal or (e) otherwise furnish any information with respect to, assist or knowingly participate in or facilitate in any other manner any effort or attempt by any Person (other than FoxWayne or its Affiliates) to do or seek to do any of the foregoing. Aerami shall notify FoxWayne promptly if any Person makes any Alternative Transaction Proposal to Aerami, which notice shall include a copy of such Alternative Transaction Proposal (or, where such Alternative Transaction Proposal is not submitted by such Person in writing, a reasonably detailed description of the material terms and conditions of such Alternative Transaction Proposal).
From and after the date of the Merger Agreement until the Closing Date, FoxWayne shall not take, nor shall it permit any of its Affiliates to take, and shall not authorize and will instruct its Representatives not to, whether directly or indirectly, any action to solicit, initiate, continue or engage in discussions or negotiations with, or enter into any agreement, letter of intent, memorandum of understanding or agreement in principle with, or encourage, respond, provide information to or commence due diligence with respect to, any Person (other than Aerami, its stockholders or any of their Affiliates or Representatives), concerning, relating to or which is intended or is reasonably likely to give rise to or result in, any offer, inquiry, proposal or indication of interest, written or oral relating to any business combination other than with Aerami, its stockholders and their respective Affiliates and Representatives.
Stock Exchange Listing
FoxWayne agreed to use its reasonable best efforts to cause the common stock of the Combined Company to be issued in connection with the Business Combination to be approved for listing on Nasdaq, subject to official notice of issuance, prior to the Effective Time.
| -127- |
Claims Against the Trust Account
Aerami agreed and acknowledged that the Trust Account has been established for the benefit of FoxWayne’s public stockholders and that, in the event the Business Combination is not consummated, FoxWayne may be obligated to return the funds contained therein to such public stockholders. As a result, Aerami agreed to waive any past, present, or future claims against the Trust Account and agreed not to seek recourse against the Trust Account.
Other Covenants and Agreements
The Merger Agreement contains other covenants and agreements, including covenants related to:
| ● | FoxWayne and Aerami providing access to books and records and furnishing relevant information to the other party, subject to certain limitations and confidentiality provisions; | |
| ● | director and officer indemnification; | |
| ● | prompt notification of certain matters; | |
| ● | FoxWayne and Aerami using reasonable best efforts to consummate the Business Combination; | |
| ● | public announcements relating to the Business Combination; | |
| ● | the intended tax treatment of the Business Combination; | |
| ● | FoxWayne using its reasonable best efforts to keep current and timely filing all reports required to be filed or furnished with the SEC and otherwise complying in all material respects with its reporting obligations under applicable securities law; and | |
| ● | Aerami using reasonable best efforts to deliver to FoxWayne by January 1, 2022 audited financial statements, including consolidated balance sheets, statements of operations and comprehensive income (loss), statements of changes in equity (deficiency) and statements of cash flows of Aerami and its Subsidiaries as of and for the years ended December 31, 2020 and December 31, 2019. |
In addition, Aerami has agreed to grant, following the Closing, certain options to its Chief Financial Officer, who will serve as the Chief Financial Officer of the Combined Company, and to the persons who will serve as directors of the Combined Company – see the section titled “Executive Compensation – Non-Employee Director Compensation Policy” below for further information
Conditions to Closing of the Merger Agreement
Mutual Conditions
The obligations of Aerami, FoxWayne and Merger Sub to consummate the Business Combination are subject to the satisfaction or waiver at or prior to the Closing of the following conditions:
| ● | the requisite FoxWayne and Aerami stockholder consents shall have been obtained; | |
| ● | all consents, registrations, approvals, clearances, Permits and authorizations from Governmental Authorities that are set forth in the Merger Agreement shall have been obtained; | |
| ● | no Governmental Authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any Law or Order (whether temporary, preliminary or permanent) that is in effect and restrains, enjoins, makes illegal or otherwise prohibits the consummation of the transactions contemplated by the Merger Agreement and the Ancillary Documents; and | |
| ● | this Registration Statement and proxy statement shall have become effective in accordance with the provisions of the Securities Act. No stop order suspending the effectiveness of this Registration Statement or the proxy statement shall have been issued and remain in effect, and no Proceedings for that purpose shall have commenced or be threatened by the SEC. |
| -128- |
FoxWayne and Merger Sub Conditions
The obligations of FoxWayne and Merger Sub to consummate the Business Combination are subject to the satisfaction or waiver at or prior to the Closing of the following additional conditions:
| ● | Each of (i) the Aerami Fundamental Representations shall be true and correct in all material respects (other than the representations contained in Section 3.5 (Capitalization) of the Merger Agreement, which shall be true and correct in all but de minimis respects) as of the Effective Date of the Merger Agreement and as of the Closing Date as if made on the Closing Date (except for Aerami Fundamental Representations that speak as of a particular date, which shall be true and correct in all material respects (other than the representations contained in Section 3.5 (Capitalization) of the Merger Agreement, which shall be true and correct in all but de minimis respects) as of such date), and (ii) the other representations and warranties made by Aerami in the Merger Agreement shall be true and correct in all respects as of the Effective Date of the Merger Agreement and as of the Closing Date as if made on the Closing Date (except for representations and warranties that speak as of a particular date, which shall be true and correct in all respects as of such date), except in each case of the items referenced in clause (ii) where the failure of such representations and warranties to be true and correct, taken as a whole, would not be reasonably likely to cause a Material Adverse Effect; | |
| ● | Each of the covenants and obligations that Aerami is required to comply with or to perform at or prior to the Closing shall have been complied with and performed in all material respects; | |
| ● | Since the date of the Merger Agreement, there shall not have occurred a Material Adverse Effect on the Acquired Companies; | |
| ● | The Combined Company shall cause to be paid all consulting fees of FoxWayne incurred in connection with the consummation of the transactions contemplated by the Merger Agreement first using the funds in the Trust Account Amount, if any; provided that such consulting fees shall not exceed, in the aggregate, $500,000; | |
| ● | There shall not be more than 25% of dissenting shares of Aerami Capital Stock; and | |
| ● | Aerami shall have delivered (or cause to be delivered) to FoxWayne and Merger Sub each of the following: (i) a certificate executed on behalf of Aerami by its Chief Executive Officer containing the representation and warranty of Aerami that the conditions noted above regarding accuracy of representations and warranties, performance of covenants, and absence of a Material Adverse Effect have been duly satisfied; (ii) executed copies of each agreement with the holders of certain contingent obligations reflecting the settlement of each such contingent obligation through the issuance of Combined Company common stock, as set forth in the Consideration Schedule; (iii) the Consideration Schedule completed to include all of the information specified in the Merger Agreement in a form reasonably satisfactory to FoxWayne and a certificate executed by Aerami’s Chief Executive Officer or Chief Financial Officer, dated as of the Closing Date, certifying on behalf of Aerami (and not in his or her individual capacity) that the Consideration Schedule is complete and correct; (iv) a certificate, meeting the requirements of Section 1445 of the Code and the Treasury Regulations thereunder, to the effect that the shares of Aerami Common Stock do not constitute a U.S. real property interest; and (v) evidence satisfactory to FoxWayne that each of (A) the Aerami 2015 Equity Incentive Plan (f/k/a the Dance Biopharm Holdings, Inc. 2015 Equity Incentive Plan), (B) the Aerami 2009 Equity Incentive Plan (f/k/a the Dance Pharmaceuticals, Inc. 2009 Equity Incentive Plan) and (C) Aerami’s equity incentive plan created for Anne Whitaker, the former Chief Executive Officer of Aerami, have been terminated. |
| -129- |
Aerami Conditions
The obligations of Aerami to consummate the Business Combination are subject to the satisfaction or waiver at or prior to Closing of the following additional conditions:
| ● | Each of (i) FoxWayne Fundamental Representations shall be true and correct in all but de minimis respects as of the Effective Date of Merger and as of the Closing Date as if made on the Closing Date (except for FoxWayne Fundamental Representations that speak as of a particular date, which shall be true and correct in all respects as of such date), and (ii) the other representations and warranties made by FoxWayne and Merger Sub in the Merger Agreement shall be true and correct in all material respects as of the Effective Date of the Merger Agreement and as of the Closing Date as if made on the Closing Date (except for representations and warranties that speak as of a particular date, which shall be true and correct in all material respects as of such date); | |
| ● | Each of the covenants and obligations that FoxWayne and Merger Sub is required to comply with or to perform at or prior to the Closing shall have been complied with and performed in all material respects; | |
| ● | Since the date of the Merger Agreement, there shall not have occurred a Material Adverse Effect on FoxWayne; | |
| ● | At or prior to the Closing, FoxWayne shall deliver (or cause to be delivered) to Aerami a certificate executed on behalf of FoxWayne by its Chief Executive Officer containing the representation and warranty of FoxWayne that the conditions noted above regarding accuracy of representations and warranties, performance of covenants, and absence of a Material Adverse Effect have been duly satisfied; | |
| ● | Following payment by FoxWayne to its stockholders who have validly elected to have their shares of Combined Company common stock redeemed for cash pursuant to FoxWayne’s Organizational Documents as part of the Redemption Offer and after giving effect to the payment of the Transaction Expenses incurred by or on behalf of FoxWayne in accordance with the Merger Agreement, FoxWayne shall have an aggregate amount of cash of at least $15,000,000; | |
| ● | FoxWayne shall have made all necessary arrangements with the Trustee to have the funds contained in the Trust Account disbursed or available to FoxWayne, in accordance with that certain Trust Agreement, dated as of January 22, 2021, between FoxWayne and the Trustee and the Merger Agreement, contemporaneously with the Closing, and all such funds released from the Trust Account to FoxWayne shall be available to FoxWayne (and, following the Merger, the Combined Company); | |
| ● | FoxWayne shall have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act); | |
| ● | Combined Company common stock to be issued pursuant to the Merger Agreement shall be listed or have been approved for listing on Nasdaq, subject only to official notice of issuance thereof; and | |
| ● | The directors and executive officers of FoxWayne set forth in the Merger Agreement shall have been removed from their respective positions or tendered their irrevocable resignations, in each case effective as of the Effective Time. |
| -130- |
Termination
The Merger Agreement may be terminated and the Merger and the other transactions contemplated by the Merger Agreement may be abandoned at any time prior to the Effective Time, notwithstanding any requisite approval and adoption of the Merger Agreement and the transactions contemplated thereby, as follows:
| ● | by mutual written agreement of FoxWayne and Aerami; | |
| ● | by either FoxWayne or Aerami if the Merger has not been consummated on or before April 30, 2022 (the “Outside Date”); provided that the right to terminate the Merger Agreement on or before the Outside Date shall not be available to any party whose breach of any provision of Merger Agreement results in the failure of the Merger to be consummated by such time; and provided, further, that the Outside Date shall be automatically extended to May 31, 2022 in the event that this Registration Statement has been filed, but not declared effective, on or before February 14, 2022; | |
| ● | by either FoxWayne or Aerami if the FoxWayne stockholder consent shall not have been obtained by reason of the failure to obtain the required vote at the special meeting; | |
| ● | by either FoxWayne or Aerami, if a Governmental Authority shall have issued any Order or taken any other action, in each case, which has become final and non-appealable and which restrains, enjoins or otherwise prohibits the Merger; | |
| ● | by FoxWayne if there shall have occurred a Material Adverse Effect on the Acquired Companies after the date of the Merger Agreement; | |
| ● | by Aerami if there shall have occurred a Material Adverse Effect on FoxWayne and its subsidiaries, taken as a whole, after the date of the Merger Agreement; | |
| ● | by FoxWayne, if (i) any representation or warranty of Aerami contained in the Merger Agreement shall be inaccurate such that Aerami’s conditions to Closing set forth in the Merger Agreement would not be satisfied, or (ii) the covenants or obligations of Aerami contained in the Merger Agreement shall have been breached in any material respect such that Aerami’s conditions to Closing set forth in the Merger Agreement would not be satisfied; provided, however, that if an inaccuracy or breach is curable by Aerami during the Cure Period, then FoxWayne may not terminate the Merger Agreement as a result of such inaccuracy or breach prior to the expiration of the Cure Period unless Aerami is no longer continuing to exercise reasonable best efforts to cure such inaccuracy or breach; or | |
| ● | by Aerami, if (i) any representation or warranty of FoxWayne contained in the Merger Agreement shall be inaccurate such that FoxWayne’s conditions to Closing set forth in the Merger Agreement would not be satisfied, or (ii) the covenants or obligations of FoxWayne or Merger Sub contained in the Merger Agreement shall have been breached in any material respect such that FoxWayne’s and Merger Sub’s conditions to Closing set forth in the Merger Agreement would not be satisfied; provided, however, that if an inaccuracy or breach is curable by FoxWayne or Merger Sub during the Cure Period, then Aerami may not terminate the Merger Agreement as a result of such inaccuracy or breach prior to the expiration of the Cure Period unless FoxWayne is no longer continuing to exercise reasonable best efforts to cure such inaccuracy or breach. |
Effect of Termination
If the Merger Agreement is terminated, the Merger Agreement will become void, and there will be no liability under the Merger Agreement on the part of any party thereto (or any representative of such party), except as set forth in the Merger Agreement or in the case of termination subsequent to fraud or a willful material breach of the Merger Agreement by a party thereto.
Related Agreements
This section describes the material provisions of certain additional agreements entered into or to be entered into pursuant to the Merger Agreement, which we refer to as the “Related Agreements,” but does not purport to describe all of the terms thereof. The Related Agreements have been or will be filed with the SEC at a future date. Stockholders and other interested parties are urged to read such Related Agreements in their entirety.
Support Agreements
Contemporaneously with the execution of the Merger Agreement, certain officers and directors of Aerami delivered Stockholder Support Agreements to FoxWayne. Under the Stockholder Support Agreement terms, such Aerami stockholders agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement. In addition, Aerami agreed to use its best efforts to obtain additional Stockholder Support Agreements from certain of its larger stockholders.
| -131- |
Contemporaneously with the execution of the Merger Agreement the Sponsor delivered the Sponsor Support Agreement, pursuant to which, among other things, the Sponsor agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement.
Contemporaneously with the execution of the Merger Agreement, certain officers and directors of FoxWayne delivered FoxWayne Support Agreements, pursuant to which, among other things, such FoxWayne stockholders agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement. In addition, FoxWayne agreed to use its best efforts to obtain additional FoxWayne Support Agreements from certain of its larger stockholders.
Registration Rights Agreement
In connection with the Closing, Aerami, FoxWayne, and certain of their respective stockholders will enter into the Registration Rights Agreement. Following the Business Combination, pursuant to the Registration Rights Agreement, the Combined Company will be required to file a registration statement covering the resale of registrable securities held by the stockholders party thereto.
Proposed Charter
Pursuant to the terms of the Merger Agreement, immediately prior to the consummation of the Business Combination, FoxWayne shall amend and restate its Charter to, among other things, (i) increase the total number of authorized shares of all classes of capital stock from 53,000,000 shares, consisting of (A) 52,000,000 shares of common stock, par value $0.0001 per share, including (1) 50,000,000 shares of Class A Common Stock and (2) 2,000,000 shares of Class B Common Stock and (B) 1,000,000 shares of Preferred Stock, par value $0.0001 per share, to 210,000,000 shares, consisting of (1) 200,000,000 shares of Combined Company common stock and (2) 10,000,000 shares of Combined Company preferred stock; (ii) require an affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal, or adopt any provisions inconsistent with Article V pertaining to stockholders; Article VI pertaining to directors. Article VII pertaining to limitation of liability and Article IX pertaining to an amendment to FoxWayne’s bylaws; (iii) provide that, subject to the rights of holders of FoxWayne’s preferred stock, the FoxWayne Board or any individual directors may be removed only for cause and only by affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of FoxWayne’s capital stock entitled to vote at an election of directors; and (iv) make certain other changes that the FoxWayne Board deems appropriate for a public operating company, including (A) eliminating certain provisions relating to an Initial Business Combination that will no longer be applicable to the Combined Company following the Closing, including provisions relating to (1) the Class B Common Stock, (2) redemption rights with respect to Class A Common Stock, (3) the Trust Account, (4) share issuances prior to the consummation of the Initial Business Combination, (5) transactions with affiliates and other blank check companies and (6) the minimum value of the target in the Initial Business Combination and (B) changing the post-combination company’s name to “Aerami Therapeutics Holdings, Inc.”
For more information about the amendments to FoxWayne’s Charter, see the section titled “Proposal 2 — The Charter Proposal.”
Lock-up Agreement
The Founder Shares and Private Placement Warrants and any shares of Class A Common Stock issued upon conversion or exercise thereof are subject to transfer restrictions pursuant to lock-up provisions in a letter agreement dated January 19, 2021 entered into by the Sponsor and FoxWayne’s officers and directors. Those lock-up provisions provide that such securities are not transferable or salable (i) in the case of the Founder Shares, (a) with respect to 50% of Founder Shares, for a period ending on the six-month anniversary of the date of the consummation of the Initial Business Combination and (b) with respect to the remaining 50% of such shares, for a period ending on the one-year anniversary of the date of the consummation of the Initial Business Combination, or earlier, in either case, if, subsequent to the Initial Business Combination FoxWayne completes a liquidation, merger, capital stock exchange or other similar transaction that results in all of the public stockholders having the right to exchange their shares of common stock for cash, securities or other property, and (ii) in the case of the Private Placement Warrants, the warrants that may be issued upon conversion of Working Capital Loans or Extension Loans, if any, and the Class A Common Stock underlying such warrants, until 30 days after the completion of the Initial Business Combination, except in each case (a) to FoxWayne’s officers or directors, any affiliates or family members of any of FoxWayne’s officers or directors, any affiliate of the Sponsor, any members of the Sponsor, or any of its affiliates, officers, directors, and direct and indirect equityholders, (b) in the case of an individual, by gift to a member of the individual’s immediate family, to a trust, the beneficiary of which is a member of the individual’s immediate family or an affiliate of such person, or to a charitable organization; (c) in the case of an individual, by virtue of laws of descent and distribution upon death of the individual; (d) in the case of an individual, pursuant to a qualified domestic relations order; (e) by private sales or transfers made in connection with the consummation of a business combination at prices no greater than the price at which the securities were originally purchased; (f) in the event of our liquidation prior to the completion of the Initial Business Combination; or (g) by virtue of the laws of Delaware or the Sponsor’s limited liability company agreement upon dissolution of the Sponsor, provided, however, that in the case of clauses (a) through (e), or (g) these permitted transferees must enter into a written agreement agreeing to be bound by these transfer restrictions.
Background of the Business Combination
FoxWayne is a Delaware corporation formed on September 17, 2020 for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. Although FoxWayne is not limited to a particular industry or geographic region for purposes of consummating an Initial Business Combination, its stated intention is to focus its search for a target business in the biotechnology and/or telemedicine sector of the healthcare industry or the technology industry in North America. FoxWayne’s business strategy is to identify and acquire a business with strong management and a strong business track record. The ultimate goal of this business strategy is to maximize stockholder value. The proposed Business Combination was the result of an extensive search for a potential transaction utilizing the broad network of contacts and corporate relationships developed by FoxWayne management. The terms of the Business Combination were the result of extensive negotiations between the FoxWayne management team and Aerami. The following is a brief description of the material background of these negotiations, the Business Combination and related transactions.
| -132- |
On January 19, 2021, FoxWayne completed its initial public offering of 5,000,000 units, raising gross proceeds of approximately $50 million. The underwriters of the initial public offering were granted a 45-day option from the date of the final prospectus relating to the initial public offering to purchase up to 750,000 additional units to cover overallotments, if any, at $10.00 per unit, less underwriting discounts and commissions. On February 22, 2021, the underwriters exercised the overallotment option and, on February 22, 2021, the underwriters purchased 750,000 additional units, generating gross proceeds of approximately $7.5 million.
Simultaneously with the closing of the initial public offering, FoxWayne consummated the sale of 2,500,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant in a private placement to our Sponsor, generating gross proceeds of approximately $2,500,000. Simultaneously with the closing of the sale of the overallotment units, our Sponsor purchased an additional 300,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant, generating gross proceeds of approximately $300,000.
Prior to the consummation of the initial public offering, neither FoxWayne, nor anyone on its behalf, contacted any prospective target business or had any substantive discussions, formal or otherwise, with respect to a business combination with FoxWayne.
Between January 22, 2021, the date of the closing of the initial public offering, and August 3, 2021, the date on which FoxWayne and Aerami entered into a letter of intent (as further described below), FoxWayne representatives, including the FoxWayne Board, evaluated approximately 25 potential targets and engaged with EF Hutton and Revere Securities LLC to seek potential private financing. These potential targets were in various categories of the transportation, security and artificial intelligence, autonomous agriculture and biopharmaceutical industries. FoxWayne representatives, including the FoxWayne Board, met with members of management, board members or other investors of approximately 12 different potential acquisition targets. FoxWayne representatives, including the FoxWayne Board, then conducted additional due diligence under confidentiality or nondisclosure agreements with respect to 8 potential targets (the “Potential Targets”).
Following our initial public offering, FoxWayne had discussions with EF Hutton, the representative of the underwriters in FoxWayne’s IPO, about engaging EF Hutton to act as a non-exclusive placement agent in connection with a proposed private placement. EF Hutton had multiple calls with the FoxWayne Board and provided advice to FoxWayne about Potential Targets in connection with the Business Combination.
FoxWayne representatives, including the FoxWayne Board, conducted due diligence to varying degrees on the Potential Targets, including review of, among other things, the Potential Targets’ management team, stockholders, business model, valuation, balance sheet and historical and projected financials, in each case to the extent made available, and FoxWayne submitted indications of interest to 4 Potential Targets, including Aerami. Following such reviews, and at various points in time, these discussions with the Potential Targets (other than Aerami) were discontinued for one or various reasons, including maturity of the business, a near-term path to profitability, valuation, state of financial systems and/or controls, impact of COVID-19, or growth potential, among other reasons.
FoxWayne representatives, including the FoxWayne Board, engaged in conversations and conducted meetings with representatives of a Potential Target identified by EF Hutton in the transportation, security and artificial intelligence industry (“Potential Target A”) from January 2021 to June 2021. In April 2021, the FoxWayne Board presented an initial indication of interest to representatives of Potential Target A, and the FoxWayne Board and representatives of EF Hutton, received access to Potential Target A’s data room and its investment information deck after executing a non-disclosure agreement. Draft letters of intent were exchanged between FoxWayne and Potential Target A in May and June 2021. In addition, FoxWayne representatives, including the FoxWayne Board and representatives of EF Hutton and Potential Target A discussed the potential terms of a transaction between FoxWayne and Potential Target A, including FoxWayne’s proposed $600 million valuation for Potential Target A, with the continuing stockholders of Potential Target A owning approximately 89% of the outstanding stock of FoxWayne after giving effect to a private placement financing and assuming no redemptions, and a minimum $75 million cash closing condition for FoxWayne’s trust account net of deferred underwriting fees and other transaction expenses. Potential Target A eventually discontinued negotiations stating its board of directors wanted to continue as a private company.
FoxWayne representatives, including the FoxWayne Board, engaged in conversations and conducted meetings with representatives of a Potential Target identified by Revere Securities LLC in the autonomous farming industry (“Potential Target B”) from April 2021 to July 2021. During this period, representatives of FoxWayne and Potential Target B discussed the potential terms of a transaction between FoxWayne and Potential Target B, including FoxWayne’s proposed valuation for Potential Target B. Potential Target B provided its investment information deck to the FoxWayne Board, and the parties signed a non-disclosure agreement in June 2021, and shared data room access in July 2021. The FoxWayne Board met with Potential Target B’s representatives and personnel, and spoke to its customers, including farmers, and had multiple conversations with its senior management. However, such discussions were eventually discontinued, and Potential Target B determined to pursue other funding opportunities.
FoxWayne Board member, Dr. Jonathan Zippin, and the chief executive officer and representatives of FoxWayne attended management meetings with representatives of a Potential Target in the biotechnology industry (“Potential Target C”) from May to July 2021. Potential Target C provided an investment information deck to the FoxWayne Board in June 2021 and shared data room information in July 2021 after FoxWayne signed a non-disclosure agreement. Dr. Zippin led the due diligence. In addition, representatives of FoxWayne, including Dr. Zippin and the chief executive officer, and Potential Target C discussed the potential terms of a transaction between FoxWayne and Potential Target C, including FoxWayne’s proposed valuation for Potential Target C. Such discussions were eventually discontinued, and Potential Target C determined to pursue an initial public offering.
| -133- |
Over the past two years, Aerami regularly engaged from time to time in a review of potential strategic alternatives, including additional fundraising from debt or equity sources, possible business combinations or other strategic opportunities or an initial public offering, with the assistance of Aerami’s strategic advisor, Aegis. Aerami management kept the Aerami Board informed on its strategic process through monthly updates during the same period. During this period, management of Aerami also became familiar with the general opportunities of potential business combinations with special purpose acquisition companies and consulted with Aegis regarding the structuring and potential benefits and risks associated with a transaction of that nature.
The chief executive officer of FoxWayne identified Aerami as a potential acquisition target following the initial public offering of a competitor of Aerami and initiated discussions with the chief executive officer of Aerami and Aerami Board member, Adam Stern. Between July 11, 2021 and July 27, 2021 the chief executive officer of FoxWayne, the chief executive officer of Aerami, Aerami Board member, Adam Stern, and Aegis representatives engaged in a series of discussions and information exchanges where Aerami management provided an overview of its current business, its product candidates and timelines and a summary of its financial highlights, which led to the parties entering a Mutual Non-Disclosure Agreement on July 22, 2021 at which time Aerami granted access to FoxWayne to its virtual data room.
The virtual data room included, among other materials, Aerami’s financial statements, books and records, clinical and regulatory information related to Aerami’s product candidates, business and sales documents, information related to Aerami’s intellectual property and other property documentation, and Aerami’s material contracts. Based on their review of the materials in the virtual data room, the chief executive officer and representatives of FoxWayne and Sheppard, Mullin, Richter & Hampton LLP (“Sheppard Mullin”), FoxWayne’s counsel, sent multiple supplemental due diligence requests to Aerami and Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan LLP (“Smith Anderson”), Aerami’s counsel, and Aerami and Smith Anderson provided written and oral responses to FoxWayne’s various diligence requests. In addition, representatives of FoxWayne, including the FoxWayne Board, and Aerami held telephonic conferences and virtual meetings to discuss due diligence materials and commercial and legal elements of Aerami’s business to assist FoxWayne in its diligence efforts. They discussed market trends with respect to companies developing inhaled therapies. As part of the due diligence process, the chief executive officers of FoxWayne and Aerami discussed, among other things, Aerami’s limited operating history, its history of losses and its capital requirements, risks associated with the development, regulatory approval and commercialization of Aerami’s product candidates, and risks related to the biotechnology industry generally. The chief executive officer of Aerami informed the chief executive officer of FoxWayne on July 27, 2021 that Aerami had obtained orphan drug status for AER-901.
FoxWayne Board members Sundeep Agrawal and Jonathan Zippin met with Aerami representatives to review Aerami’s technology, product offerings and opportunity. The chief executive officer of FoxWayne conducted due diligence based on publicly available information on Aerami’s competitors, including Aerovate Therapeutics, Inc., a biopharmaceutical company focused on developing inhaled therapies with securities that trade on The Nasdaq Global Market under the trading symbol AVTE, and MannKind Corporation, a biopharmaceutical company focused on the development and commercialization of inhaled therapeutic products with securities that trade on The Nasdaq Global Market under the trading symbol MNKD. Further due diligence on Aerami by FoxWayne representatives, including the chief executive officer and Drs. Agrawal and Zippin, included telephonic conferences and virtual meetings with Aerami representatives to discuss due diligence materials and commercial and legal elements of Aerami’s business, industry due diligence by speaking with scientific community members and market analysts, and evaluating events that may materially affect Aerami’s prospects and financial projections for future performance by reviewing Aerami’s clinical plan, finances and anticipated expenses for the next 24 months, speaking with clinical investigators about Aerami’s current studies, plans for standard blinded and unblinded studies, and plans for funding and clinical study enrollment.
In connection with the due diligence, FoxWayne assessed the market opportunities for Aerami’s product pipeline. To aid in this analysis, the FoxWayne Board analyzed publicly-traded companies that are developing and commercializing transformative therapies that are reshaping how patients are treated, eventually narrowing the group down to selected companies with publicly available information and with businesses similar or adjacent to Aerami’s business. The FoxWayne Board reviewed data from the selected comparable companies based on various metrics, including but not limited to enterprise value, market capitalization, total debt, available cash and cash equivalents, unique product positioning, status of product development, mechanism of action, lead indications, revenue projections, competition, rapidly evolving market needs, and path to revenue generation, margins and profitability, including the comparative and competitive information set forth below in the table.
| Company Information | Financial Information | IPO Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Company | Ticker | Product(s) | Product Description | Mechanism of Action | Lead Indication(s) | Current Status | Price 8/19 | Shares | Market Cap. ($M) | Total Debt ($M) | Cash & Equiv. ($M) | Enterprise Value ($M) | 2021E Rev. ($M) | 2022E Rev. ($M) | IPO Year | IPO Amount ($M) | Pre-IPO Cash ($M) | Pre-Money EV ($M) | Revenue Producing @ IPO | Yrs until Revenue Event from IPO | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Acceleron Pharma Inc. | XLRN | Sotatercept (IV), Various | Activin type IIa mAb, various | Fusion protein | PAH, PH, others | Pivotal Phase 3 late 2020; data by 2023 | 8,120.0 | 20.7 | 623.4 | 7,517.4 | 132.3 | 202.6 | 2013 | 83.7 | 28.4 | 314.5 | n/a | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Insmed Incorporated | INSM | TPIP, Brensocatib | Inhaled treprostinil palmitil | Prostacyclin agonist | NTM, PAH, PH | Phase 2a 24hr PVR data 2H 2021 | 2,905.7 | 593.4 | 928.3 | 2,570.7 | 190.2 | 329.3 | 2000 | n/a | n/a | n/a | n/a | n/a | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mannkind Corporation | MNKD | Afrezza, Tyvaso DPI | Inhaled insulin, others | Various | Diabetes, orphan lung | Commercial | 1,020.3 | 331.5 | 276.8 | 1,075.0 | 77.9 | 95.0 | 2004 | 87.5 | 56.1 | 365.9 | X | 12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gossamer Bio, Inc. | GOSS | Seralutinib, GB004 | Dry powder inhaled PDGFR, c-Kit inhibitor, HIF-1a stabilizer | Tyrosine kinase inhibitor | PAH, IBD | Phase 2 results in 1H 2022 | 607.0 | 184.5 | 453.3 | 338.2 | n/a | n/a | 2019 | 276.0 | 141.1 | 736.4 | X | 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aerovate Therapeutics Inc. | AVTE | AV-101 | Inhaled dry powder imatinib | Tyrosine kinase inhibitor | PAH | Phase 2b to initiate 2H 2021 | $ | 12.69 | 24.4 | 309.8 | 0.0 | 186.2 | 123.6 | n/a | n/a | 2021 | 121.6 | 4.6 | 202.0 | X | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Beyond Air Inc. | XAIR | LungFit | Nitric oxide from ambient air | Nitric oxide synthase endothelial activator | PPHN, pneumonia, bronchiolitis, NTM | PMA pending, US launch 2Q 2021 | 219.6 | 6.9 | 34.6 | 191.9 | 1.0 | 2.2 | n/a | n/a | n/a | n/a | n/a | n/a | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquida Corporation | LQDA | LIQ861; Treprostinil Injection | Inhaled dry powder / Injectable treprostinil | Prostacyclin agonist | PAH | PDUFA 4Q 2021 / Marketable | 123.9 | 16.6 | 53.6 | 86.9 | 8.9 | 11.6 | 2018 | 50.0 | 3.4 | 116.2 | n/a | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tenax Therapeutics Inc. | TENX | Imatinib; Levosimendan | Delayed release oral imatinib | Tyrosine kinase inhibitor | PAH, PH | Licensed 01/2021; Phase 1 PK study | 49.6 | 0.3 | 4.0 | 45.9 | n/a | 25.0 | n/a | n/a | n/a | n/a | n/a | n/a | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aerami Therapeutics | Private | AER-901, AER-501, AER-601 | Nebulized imatinib, soft mist insulin, inhaled GLP-1 | Tyrosine kinase inhibitor | PAH, Diabetes | Phase 1 results late 2021; AER-501 Phase 3 | n/a | — | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Low | 49.6 | 0.0 | 4.0 | 45.9 | 1.0 | 2.2 | 50.0 | 3.4 | 116.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Median | 458.4 | 18.7 | 231.5 | 265.0 | 77.9 | 60.0 | 87.5 | 28.4 | 314.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average | 1,669.5 | 144.2 | 320.0 | 1,493.7 | 82.1 | 110.9 | 123.8 | 46.7 | 347.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| High | 8,120.0 | 593.4 | 928.3 | 7,517.4 | 190.2 | 329.3 | 276.0 | 141.1 | 736.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Based in part on the above comparables with due regard for varying stages of product development and revenue generation, Aerami’s currently available cash balance and projected cash needs over the succeeding 24 months, the value that the public markets would likely ascribe to the post-combination entity, and other information that the FoxWayne Board deemed appropriate based on their experience, the FoxWayne Board initially estimated an aggregate enterprise valuation of Aerami in excess of $150.0 million, which lands in the low to median range of the selected comparable companies.
On July 27, 2021, FoxWayne provided a draft letter of intent to Aerami. From July 27, 2021 to August 3, 2021, the chief executive officers of FoxWayne and Aerami and representatives of Aegis held several conversations regarding terms of a potential business combination transaction including, among other things, valuation, transaction structure and consideration, potential sources and uses of capital, governance of the post-transaction combined company, transaction approval requirements and the scope and duration of exclusivity between the parties.
On July 30, 2021, the Aerami Board met to discuss the proposed letter of intent and authorized management to continue discussions with FoxWayne to finalize its terms, following which Aerami provided FoxWayne with a proposed revised draft. The chief executive officers of FoxWayne and Aerami continued to have discussions to finalize the letter of intent.
On August 3, 2021, the Aerami Board met to review what was proposed to be the final draft of the letter of intent. The chief executive officer of FoxWayne attended a portion of that meeting and provided background on the proposed transaction.
Following that meeting, on August 3, 2021, FoxWayne and Aerami executed a final letter of intent (the “Letter of Intent”) regarding the proposed Business Combination. Although Aerami had initially requested an enterprise valuation of approximately $400.00 million, the FoxWayne Board had considered competitive and comparative market data to determine Aerami’s enterprise valuation, including Aerami’s technology and market opportunity, valuations of competitive companies, publicly available filings with the Securities and Exchange Commission, analyst reports, and models, and the Letter of Intent proposed an enterprise valuation of Aerami of $157.00 million, on a no cash no debt basis, with the shares of FoxWayne Common Stock to be received valued at $10.00 per share, and exclusivity through October 31, 2021.
On August 5, 2021, Sheppard Mullin and Smith Anderson had an initial call discussing responsibilities and potential timeline issues.
On August 13, 2021, Sheppard Mullin and Smith Anderson had a follow up call regarding drafting responsibilities. Subsequent to that call, Sheppard Mullin delivered an initial due diligence request list to Aerami and Smith Anderson. In the weeks thereafter, Aerami and Smith Anderson responded to the due diligence requests, and based on those responses, FoxWayne and Sheppard Mullin asked additional questions and requested additional diligence materials.
On August 18, 2021, the chief executive officer of FoxWayne, Sheppard Mullin and EF Hutton conducted a conference call to discuss a potential private financing.
Between August 3, 2021 and August 20, 2021, the chief executive officers of FoxWayne and Aerami continued discussions regarding certain terms of the Letter of Intent that the parties had agreed to finalize within two weeks of executing the Letter of Intent, including, without limitation, proposed sources and uses of proceeds from the Business Combination, as well as certain matters impacting pro forma capitalization, board composition and the size of the proposed equity pool for the Combined Company. On August 20, 2021, the parties finalized these terms.
On August 20, 2021, EF Hutton led a call with the chief executive officers of FoxWayne and Aerami and representatives from Sheppard Mullin and Smith Anderson to discuss the various aspects to the transaction, including the Business Combination, a potential private financing by Aerami and a potential private financing by FoxWayne. These calls occurred weekly on a semi-regular basis through the months of August and September.
On August 26, 2021, Sheppard Mullin and Smith Anderson had a conference call to discuss the timing of Aerami’s proposed private financing and FoxWayne’s proposed private financing.
On September 2, 2021, Aerami commenced a private offering of Aerami Common Stock at a price of $1.00 per share. Aegis served as placement agent for the private financing.
On September 9, 2021, Smith Anderson delivered an initial draft of the Merger Agreement to Sheppard Mullin.
| -134- |
On September 20, 2021, Sheppard Mullin and Smith Anderson held a conference call to discuss the state of Aerami’s private financing and FoxWayne’s proposed private financing, and the participation by EF Hutton in both financings.
On September 21, 2021, Sheppard Mullin distributed to all parties a revised draft of the Merger Agreement, marked to show its comments from the draft distributed by Smith Anderson on September 9, 2021.
On September 23, 2021, a call was held with representatives from EF Hutton and Aerami’s placement agent, Aegis, as well as their respective counsel and Sheppard Mullin and Smith Anderson, to discuss Aerami’s private financing and the roles the two investment banks would play in connection therewith. A follow up call regarding this matter was held between the same parties on October 4, 2021.
On September 30, 2021, Smith Anderson sent a revised draft of the Merger Agreement responding to the draft circulated by Sheppard Mullin on September 21, 2021.
During the period between September 30, 2021 and October 21, 2021, FoxWayne and Sheppard Mullin continued to conduct in depth due diligence on Aerami including full access to a virtual data room, document review and questions put directly to and answered by Aerami management and their advisors, with correspondence taking place both through email and through live discussions.
During the same period, Aerami continued to conduct its private financing.
On October 21, 2021, Sheppard Mullin and Smith Anderson had a conference call to discuss various open items on the latest draft of the Merger Agreement and the other Ancillary Documents, including forms of a stockholder support agreement, sponsor support agreement, and parent support agreement, (the Ancillary Documents and the Merger Agreement are hereinafter collectively referred to as the “Transaction Documents”).
Between October 21, 2021 and November 4, 2021, the chief executive officers of FoxWayne and Aerami and representatives of Aegis continued discussions related to the expiring exclusivity period under the Letter of Intent, the progress of Aerami’s private financing and developments impacting valuation.
On November 2, 2021, Sheppard Mullin sent a revised draft of the Merger Agreement responding to the draft circulated by Smith Anderson on September 30, 2021.
On November 3, 2021, Aerami completed its private financing. Aerami sold a total of 22,250,000 shares of Aerami Common Stock in the private financing and received aggregate net proceeds of $19.2 million. In connection with the private financing, Aerami issued placement agent warrants to purchase 2,223,056 shares of Aerami Common Stock at an initial exercise price of $1.00 per share, exercisable for a period of five years. Shortly after Aerami completed its private financing, EF Hutton began the process of coordinating a proposed private financing.
On November 4, 2021, based on discussions between the chief executive officers of FoxWayne and Aerami and representatives of Aegis and with due consideration for Aerami’s anticipated capital needs following Aerami’s private financing, Aerami circulated an amendment to the Letter of Intent to FoxWayne and Sheppard Mullin. Prior to the date of the amendment to the Letter of Intent, FoxWayne had not identified any private financing opportunity on acceptable terms and, as a result, Aerami and FoxWayne agreed to adjust the minimum cash condition in the Merger Agreement from approximately $30 million to $15 million to close the Business Combination and to adjust the enterprise valuation for Aerami for purposes of the Merger Agreement. This amendment provided exclusivity through December 3, 2021 and increased the enterprise valuation of Aerami for purposes of the Merger Agreement to $250.00 million. This amendment was executed by FoxWayne and Aerami later that day.
On November 4, 2021, representatives of Sheppard Mullin delivered to FoxWayne an initial report summarizing the results of due diligence conducted up to that date. An updated diligence report was delivered by Sheppard Mullin on November 18, 2021, November 23, 2021 and on November 26, 2021, reflecting additional diligence material received up through those dates.
On November 5, 2021, Sheppard Mullin and Smith Anderson had two calls to discuss the proposed allocation of consideration to the various Aerami stockholders and others under the Merger Agreement, as well as the treatment of holders of options, restricted stock units, warrants and other outstanding rights to acquire equity of Aerami, as well as certain other obligations.
On November 9, 2021, Sheppard Mullin and Smith Anderson discussed by telephone the various open items in the Merger Agreement and the other Ancillary Documents.
On November 15, 2021, Sheppard Mullin and Smith Anderson had an initial call regarding this Registration Statement/proxy statement to be filed in connection with the Business Combination, which included the allocation of drafting responsibilities and proposed timelines. Follow up calls between Sheppard Mullin and Smith Anderson on this Registration Statement/proxy statement occurred on November 29, 2021 and December 3, 2021.
| -135- |
On November 18, 2021, Sheppard Mullin and Smith Anderson held a conference call to discuss signing and timing issues relating to the Merger Agreement and the other Ancillary Documents to be signed at the time the Merger Agreement was to be executed.
On November 22, 2021, Sheppard Mullin and Smith Anderson discussed that FoxWayne’s proposed private financing would not occur prior to the signing of the Merger Agreement and the resulting impact on the Transaction Documents, including the Registration Rights Agreement. We intend to engage one or more placement agents to help us seek private financing if needed to meet the $15 million minimum cash condition in the Merger Agreement to close the Business Combination. However, we may not be able to engage any such placement agent or obtain any proposed private financing on acceptable terms, if at all.
On November 23, 2021, the FoxWayne Board held a special meeting by teleconference with representatives of Sheppard Mullin and FoxWayne management participating. During this meeting, Sheppard Mullin provided to the FoxWayne Board a review of fiduciary duties under Delaware law in the context of consideration of the proposed Business Combination transaction. Sheppard Mullin also reviewed with the FoxWayne Board the scope of the due diligence review and the terms of the Business Combination, including the current drafts of the Merger Agreement and the Ancillary Documents. A question-and-answer session followed, during which the FoxWayne directors discussed the matters presented and asked questions of FoxWayne management and the representatives of Sheppard Mullin. Following discussion, the FoxWayne Board directed FoxWayne management and FoxWayne’s advisors to continue to negotiate with Aerami to resolve all open points.
During the period from November 23, 2021 to December 3, 2021, Sheppard Mullin and Smith Anderson negotiated and resolved the open points on all of the Transaction Documents.
On December 3, 2021, Sheppard Mullin received final copies of all of the Transaction Documents from Smith Anderson which were marked against the version which the FoxWayne Board reviewed in connection with its meeting on November 23, 2021. These documents were distributed to the members of the FoxWayne Board for their review.
On December 6, 2021, the FoxWayne Board held a special meeting to review the changes to the Transaction Documents since its meeting on November 23, 2021. After discussing the changes, the FoxWayne Board confirmed the execution of the Unanimous Written Consent and authorized the execution of the Merger Agreement.
On December 7, 2021, the parties executed the Merger Agreement pursuant to which a newly-formed, wholly owned subsidiary of FoxWayne will merge with and into Aerami, with Aerami surviving the Merger as a wholly owned subsidiary of FoxWayne.
After the execution of the Merger Agreement, and before the market opened on December 7, 2021, Aerami, FoxWayne and Merger Sub announced the Business Combination together with the execution of the Merger Agreement.
FoxWayne Board’s Reasons for the Approval of the Business Combination
The FoxWayne Board considered a wide variety of factors in connection with its evaluation of the Business Combination. In light of the complexity of those factors, the FoxWayne Board, as a whole, did not consider it practicable to, nor did it attempt to, quantify or otherwise assign relative weights to the specific factors it took into account in reaching its decision. The FoxWayne Board viewed its decision as being based on all of the information available and the factors presented to and considered by it. In addition, individual members of the FoxWayne Board may have given different weight to different factors. This explanation of the reasons for the FoxWayne Board’s approval of the Business Combination, and all other information presented in this section, is forward-looking in nature and, therefore, should be read in light of the factors discussed in the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Before reaching its decision, the FoxWayne Board reviewed the results of the due diligence conducted by FoxWayne’s management and FoxWayne’s advisors, which included:
| ● | meetings and calls with Aerami’s management regarding business model, operations and forecasts; | |
| ● | a legal due diligence review conducted by Sheppard Mullin which included, among other things, a review of material contracts, intellectual property matters and other legal documents posted to a virtual data room, conference calls with Aerami and its attorneys and certain public record searches of Aerami; | |
| ● | a tax due diligence review conducted by WithumSmith+Brown, PC; | |
| ● | an accounting due diligence review conducted by Chord Advisors, LLC; | |
| ● | consultation with legal and financial advisors, primarily EF Hutton, and industry experts; | |
| ● | financial and valuation analysis of Aerami and the Business Combination; and | |
| ● | the financial statements of Aerami. |
| -136- |
In approving the Business Combination, the FoxWayne Board determined not to obtain a fairness opinion. The officers and directors of FoxWayne have substantial experience in evaluating the operating and financial merits of companies from a wide range of industries and concluded that their experience and background enabled them to make the necessary analyses and determinations regarding the Business Combination.
The factors considered by the FoxWayne Board included, but were not limited to, the following:
| ● | Market Opportunity. The FoxWayne Board noted that public markets have recently assigned premium values to companies developing inhaled therapy products. The FoxWayne Board determined that Aerami has an opportunity to capture this public investor momentum in the inhaled therapy space. | |
| ● | Market Focus. The FoxWayne Board considered the fact that the products being developed by Aerami serve a broad and underserved market for inhaled therapies for severe respiratory and chronic diseases. | |
| ● | Unique Products. The FoxWayne Board explored how Aerami’s potential product pipeline combines novel formulations of existing drugs with Aerami’s own proprietary and in-licensed inhalation technology and Aerami’s intellectual property portfolio. | |
| ● | Operating History and Management Team. The FoxWayne Board considered Aerami’s operating history and strong management team, network of consultants, and experienced board of directors, the core of which is expected to remain with the post-combination company and seek to execute Aerami’s strategy. | |
| ● | Terms of the Merger Agreement. The FoxWayne Board reviewed the financial and other terms of the Merger Agreement and determined that they were the product of arm’s-length negotiations among the parties. |
In addition, the FoxWayne Board determined that the Business Combination satisfies the investment criteria that the FoxWayne Board identified in connection with the initial public offering. For more information, see the subsection titled “The Business Combination — Background of the Business Combination.”
In the course of its deliberations, the FoxWayne Board also considered a variety of uncertainties, risks and other potentially negative factors relevant to the Business Combination, including the following:
| ● | Early Stage Company Risk. The risk that Aerami is an early stage company with a history of losses, and the risk that Aerami expects to incur significant expenses and continuing losses for the near term. | |
| ● | Growth Risk. The risk that Aerami expects to invest in growth for the foreseeable future, and the risk that Aerami may fail to manage that growth effectively. | |
| ● | Competitive Risk. The risk that Aerami currently faces competition from a number of companies and expects to face significant competition in the future. | |
| ● | Supplier and Manufacturer Risk. The risk that Aerami is still developing relationships with suppliers and manufacturers for its product candidates. | |
| ● | Public Company Risk. The risks that are associated with being a publicly traded company that is in its early development stage. | |
| ● | Benefits May Not Be Achieved Risk. The risk that the potential benefits of the Business Combination may not be fully achieved or may not be achieved within the expected timeframe. | |
| ● | Redemption Risk. The risk that a significant number of FoxWayne’s stockholders elect to redeem their shares prior to the consummation of the Business Combination and pursuant to FoxWayne’s existing Charter, which would potentially make the Business Combination more difficult to complete or reduce the amount of cash available to the post-combination company to accelerate its business plan following the Closing. |
| -137- |
| ● | Stockholder Vote Risk. The risk that FoxWayne’s stockholders may fail to provide the votes necessary to effect the Business Combination. | |
| ● | Litigation Risk. The risk of the possibility of litigation challenging the Business Combination or that an adverse judgment granting permanent injunctive relief could indefinitely enjoin consummation of the Business Combination. | |
| ● | Closing Conditions Risk. The risk that completion of the Business Combination is conditioned on the satisfaction of certain closing conditions that are not within FoxWayne’s control. | |
| ● | No Third-Party Valuation Risk. The risk that FoxWayne did not obtain a third-party valuation or fairness opinion in connection with the Business Combination. | |
| ● | Fees, Expenses and Time Risk. The risk of incurring significant fees and expenses associated with completing the Business Combination and the substantial time and effort of management required to complete the Business Combination. | |
| ● | Other Risks. Various other risk factors associated with Aerami’s business, as described in the section titled “Risk Factors.” |
In addition to considering the factors described above, the FoxWayne Board also considered that the officers and directors of FoxWayne may have interests in the Business Combination as individuals that are in addition to, and that may be different from, the interests of FoxWayne’s stockholders. For more information, see the subsection titled “The Business Combination - Interests of Certain Persons in the Business Combination - Interests of Sponsor and FoxWayne Directors and Officers.”
The FoxWayne Board concluded that the potential benefits that it expects FoxWayne and its stockholders to achieve as a result of the Business Combination outweigh the potentially negative factors associated with the Business Combination. Accordingly, the FoxWayne Board, based on its consideration of the specific factors listed above, unanimously (a) determined that the Business Combination and the other transactions contemplated by the Merger Agreement are fair to, and in the best interests of, FoxWayne’s stockholders, (b) approved, adopted and declared advisable the Merger Agreement and the transactions contemplated thereby and (c) recommended that the stockholders of FoxWayne approve each of the Proposals.
The above discussion of the material factors considered by the FoxWayne Board is not intended to be exhaustive but does set forth the principal factors considered by the FoxWayne Board.
Satisfaction of 80% Test
It is a requirement under FoxWayne’s Charter and the Nasdaq listing requirements that the business or assets acquired in an Initial Business Combination have a fair market value equal to at least 80% of the balance of the funds in the Trust Account (excluding the deferred underwriting discounts and commissions and taxes payable on the income earned on the Trust Account) at the time of the execution of a definitive agreement for an Initial Business Combination. In connection with its evaluation and approval of the Business Combination, the FoxWayne Board determined that the fair market value of Aerami exceeded $46 million at the time the Merger Agreement was signed.
Interests of Certain Persons in the Business Combination
Interests of Sponsor and FoxWayne Directors and Officers
In considering the recommendation of the FoxWayne Board to vote in favor of the Business Combination, stockholders should be aware that, aside from their interests as stockholders, our Sponsor and certain of our directors and officers have interests in the Business Combination that are different from, or in addition to, those of other stockholders generally. Our directors were aware of and considered these interests, among other matters, in evaluating the Business Combination, and in recommending to stockholders that they approve the Business Combination. Stockholders should take these interests into account in deciding whether to approve the Business Combination. These interests include, among other things:
| ● | the fact that our Sponsor holds 2,800,000 Private Placement Warrants (valued at $1,148,000, based on a valuation as of December 31, 2021, the most recent date for which a valuation is available) that would expire worthless if a Business Combination is not consummated; |
| -138- |
| ● | the fact that our Sponsor, officers and directors have agreed not to redeem any of the shares of our common stock held by them in connection with a stockholder vote to approve the Business Combination; | |
| ● | the fact that our Sponsor paid an aggregate of $25,000 for the Founder Shares, including 100,000 Founder Shares which were subsequently transferred to our independent directors, and that such securities will have a significantly higher value at the time of the Business Combination, which if unrestricted and freely tradable would be valued at approximately $ , based on the closing price of our Class A Common Stock of $ per share on , 2022, the record date of the special meeting of stockholders | |
| ● | if the Trust Account is liquidated, including in the event we are unable to complete an Initial Business Combination within the required time period, our Sponsor has agreed to indemnify us to ensure that the proceeds in the Trust Account are not reduced below $10.00 per public share, or such lesser amount per public share as is in the Trust Account on the liquidation date, by the claims of (a) any third party (other than our independent registered public accounting firm) for services rendered or products sold to us or (b) a prospective target business with which we have entered into a letter of intent, confidentiality or other similar agreement or Merger Agreement, but only if such a third party or target business has not executed a waiver of all rights to seek access to the Trust Account; | |
| ● | the fact that our independent directors own an aggregate of 100,000 Founder Shares that were transferred from our Sponsor, which if unrestricted and freely tradeable would be valued at approximately $ , based on the closing price of our Class A Common Stock of $ per share on , 2022; | |
| ● | the fact that our Sponsor, officers and directors will be reimbursed for out-of-pocket expenses incurred in connection with activities on our behalf, such as identifying potential target businesses and performing due diligence on suitable business combinations; | |
| ● | the fact that our Sponsor will benefit from the completion of a business combination and may be incentivized to complete an acquisition of a less favorable target company or on terms less favorable to stockholders rather than liquidate; | |
| ● | the fact that our initial stockholders, including our Sponsor and its affiliates, who purchased Class B Common Stock and Private Placement Warrants at the time of our IPO may experience a positive rate of return on their investment, even if our public stockholders experience a negative rate of return on their investment; and | |
| ● | the fact that our Sponsor, officers and directors will lose their entire investment in us if an Initial Business Combination is not completed by July 22, 2022. |
Interests of Aerami Directors and Officers
Aerami’s officers and the members of the Aerami Board who are expected to be officers of the Combined Company or members of the Combined Company Board, respectively, have interests in the Business Combination that are different from, or in addition to, those of other Aerami Stockholders generally.
These interests include, among other things, the fact that:
| ● | The following executive officers of Aerami are expected to be appointed as executive officers of the Combined Company and enter into new employment agreements with the Combined Company: Steven Thornton (Chief Executive Officer), Barry Deutsch (Chief Financial Officer), and Timm Crowder (President and Chief Operating Officer); | |
| ● | The following members of the Aerami Board are expected to be appointed as directors of the Combined Company: Steven Thornton, Anne Whitaker, Darlene Deptula-Hicks, John Patton, and Bill Welch; | |
| ● | Mr. Adam Stern, a member of Aerami’s board of directors, is the Head of Private Equity Banking at Aegis Capital Corp., and Aegis Capital Corp. will receive a significant fee upon the consummation of the Business Combination pursuant to the terms of the Financial Advisory Agreement (the “Advisory Agreement”); and | |
| ● | The executive officers of Aerami and members of the Aerami Board are holders of, or affiliated with entities that are holders of, Aerami equity interests and in such capacity will be entitled to receive the consideration payable in the Business Combination to all holders of such equity interests. |
Interests of EF Hutton
In connection with the identification and due diligence of potential targets for an Initial Business Combination, the FoxWayne Board consulted EF Hutton, the representative of our underwriters for the IPO. EF Hutton will be entitled to receive a deferred underwriting fee as described above under the subsection titled “Deferred Underwriting Fees.”
| -139- |
Potential Purchases of Public Shares
In connection with the stockholder vote to approve the Business Combination, our Sponsor, directors, officers, initial stockholders or any of their respective affiliates may privately negotiate transactions to purchase public shares from stockholders who would have otherwise elected to have their shares redeemed in conjunction with the Business Combination for a per share pro rata portion of the Trust Account. There is no limit on the number of public shares our Sponsor, directors, officers, or any of their respective affiliates may purchase in such transactions, subject to compliance with applicable law and the rules of Nasdaq. Any such privately negotiated purchases may be effected at purchase prices that are in excess of the per share pro rata portion of the Trust Account. However, our Sponsor, directors, officers, and their respective affiliates have no current commitments, plans or intentions to engage in such transactions and have not formulated any terms or conditions for any such transactions. None of the funds in the Trust Account will be used to purchase public shares in such transactions. None of our Sponsor, directors, officers, or any of their respective affiliates will make any such purchases when they are in possession of any material non-public information not disclosed to the seller of such public shares or during a restricted period under Regulation M under the Exchange Act. Such a purchase could include a contractual acknowledgement that such stockholder, although still the record holder of such public shares, is no longer the beneficial owner thereof and therefore agrees not to exercise its redemption rights and could include a contractual provision that directs such stockholder to vote such shares in a manner directed by the purchaser.
In the event that our Sponsor, directors, officers or any of their respective affiliates purchase shares in privately negotiated transactions from public stockholders who have already elected to exercise their redemption rights, such selling stockholders would be required to revoke their prior elections to redeem their shares.
The purpose of any such purchases of public shares could be to (a) vote such shares in favor of the Business Combination and thereby increase the likelihood of obtaining stockholder approval of the Business Combination or (b) to satisfy a Closing condition in the Merger Agreement, where it appears that such requirement would otherwise not be met. Any such purchases of our public shares may result in the completion of the Business Combination that may not otherwise have been possible. Any such purchases will be reported pursuant to Section 13 and Section 16 of the Exchange Act to the extent the purchasers are subject to such reporting requirements.
In addition, if such purchases are made, the public “float” of our Class A Common Stock may be reduced and the number of beneficial holders of our securities may be reduced, which may make it difficult to maintain or obtain the quotation, listing or trading of our securities on a national securities exchange.
Our Sponsor, officers, directors or any of their respective affiliates anticipate that they may identify the stockholders with whom our Sponsor, officers, directors or any of their respective affiliates may pursue privately negotiated purchases by either the stockholders contacting us directly or by our receipt of redemption requests submitted by stockholders following our mailing of proxy materials in connection with the Business Combination. To the extent that our Sponsor, officers, directors or any of their respective affiliates enter into a privately negotiated purchase, they would identify and contact only potential selling stockholders who have expressed their election to redeem their shares for a pro rata share of the Trust Account or vote against the Business Combination, whether or not such stockholder has already submitted a proxy with respect to the Business Combination but only if such shares have not already been voted at the stockholder meeting related to the Business Combination. Our Sponsor, officers, directors or any of their respective affiliates will select which stockholders to purchase shares from based on the negotiated price and number of shares and any other factors that they may deem relevant and will only purchase public shares if such purchases comply with Regulation M under the Exchange Act and the other federal securities laws.
Any purchases by our Sponsor, officers, directors or any of their respective affiliates who are affiliated purchasers under Rule 10b-18 under the Exchange Act will only be made to the extent such purchases are able to be made in compliance with Rule 10b-18, which is a safe harbor from liability for manipulation under Section 9(a)(2) of and Rule 10b-5 under the Exchange Act. Rule 10b-18 has certain technical requirements that must be complied with in order for the safe harbor to be available to the purchaser. Our Sponsor, officers, directors and any of their respective affiliates will not make purchases of Class A Common Stock if the purchases would violate Section 9(a)(2) of or Rule 10b-5 under the Exchange Act.
Total Combined Company Shares to be issued in the Business Combination
We anticipate that, upon the Closing, the ownership of the Combined Company will be as follows:
| ● | the Aerami Equityholders will own 25,000,000 shares, or approximately 78%, of the outstanding Combined Company common stock; |
| -140- |
| ● | the public stockholders will own 5,800,000 shares, or approximately 18%, of the outstanding Combined Company common stock; and | |
| ● | the initial stockholders will own 1,437,500 shares, or approximately 4%, of the outstanding Combined Company common stock. |
The number of shares and the interests set forth above (a) assume (i) that no public stockholders elect to have their public shares redeemed and (ii) that there are no other issuances of equity interests of FoxWayne or Aerami, (b) do not take into account FoxWayne Warrants that will remain outstanding following the Business Combination and may be exercised at a later date and (c) do not take into account the Aerami Options, Aerami RSUs or Aerami Warrants that are assumed in the Business Combination. As a result of the Business Combination, the economic and voting interests of our public stockholders will decrease.
If we assume the interim redemption scenario described under the section titled “Unaudited Pro Forma Condensed Combined Financial Information — Basis of Presentation,” i.e., 1,885,941 shares of Class A Common Stock are redeemed, and the assumptions set forth in the foregoing clauses (a)(ii)–(iv) and (b) remain true, the ownership of the Combined Company upon the Closing will be as follows:
| ● | the Aerami Equityholders will own 25,000,000 shares, or approximately 82% of the outstanding Combined Company common stock; | |
| ● | the public stockholders will own 3,914,059 shares, or approximately 13% of the outstanding Combined Company common stock; and | |
| ● | the initial stockholders will own 1,437,500 shares, or approximately 5% of the outstanding Combined Company common stock. |
If we assume the maximum redemptions scenario described under the section titled “Unaudited Pro Forma Condensed Combined Financial Information — Basis of Presentation,” i.e., 3,771,881 shares of Class A Common Stock are redeemed, and the assumptions set forth in the foregoing clauses (a)(ii)–(iv) and (b) remain true, the ownership of the Combined Company upon the Closing will be as follows:
| ● | the Aerami Equityholders will own 25,000,000 shares, or approximately 88% of the outstanding Combined Company common stock; | |
| ● | the public stockholders will own 2,028,119 shares, or approximately 7% of the outstanding Combined Company common stock; and | |
| ● | the initial stockholders will own 1,437,500 shares, or approximately 5% of the outstanding Combined Company common stock. |
Please see the section titled “Unaudited Pro Forma Condensed Combined Financial Information” for further information.
Board of Directors of Combined Company Following the Business Combination
Prior to the Closing, FoxWayne will take all action to cause the individuals set forth below to be appointed to the Board of Directors of the Combined Company effective as of immediately after the Effective Time.
| Name | Age | Position | ||
| Steve Thornton | 64 | Chief Executive Officer and Director | ||
| Anne Whitaker | 54 | Director and Chairwoman | ||
| Darlene Deptula-Hicks | 64 | Director | ||
| John Patton | 75 | Director | ||
| Theodore Reiss | 64 | Director | ||
| Renee Tannenbaum | 69 | Director | ||
| Bill Welch | 55 | Director |
Appraisal Rights
Appraisal Rights of FoxWayne Stockholders
There are no appraisal rights available to holders of shares of Class A Common Stock and Class B Common Stock in connection with the Business Combination.
| -141- |
Expected Accounting Treatment
The Business Combination will be accounted for as a reverse recapitalization under GAAP. Under this method of accounting, FoxWayne will be treated as the “acquired” company for financial reporting purposes. This determination is primarily based on Aerami stockholders comprising a relative majority of the voting power of the Combined Company and having the ability to nominate the members of the Combined Company Board, Aerami’s operations prior to the acquisition comprising the only ongoing operations of the Combined Company and Aerami’s senior management comprising all of the senior management of the Combined Company. Accordingly, for accounting purposes, the financial statements of the Combined Company will represent a continuation of the financial statements of Aerami with the Business Combination treated as the equivalent of Aerami issuing stock for the net assets of FoxWayne, accompanied by a recapitalization. The net assets of FoxWayne will be stated at historical costs, with no goodwill or other intangible assets recorded. Operations prior to the Business Combination will be presented as those of Aerami in future reports of the Combined Company.
Material U.S. Federal Income Tax Consequences
The following is a general discussion of the material U.S. federal income tax consequences of (i) the exercise of redemption rights by holders of Class A Common Stock that elect to have their Class A Common Stock redeemed for cash if the Business Combination is completed, and (ii) the Business Combination to U.S. Holders of Aerami Common Stock. This discussion is limited to considerations relevant to holders of FoxWayne Class A Common Stock or Aerami common stock and, after the completion of the Business Combination, common stock of the Combined Company, as applicable. This discussion applies only to shares of Class A Common Stock and Aerami Common Stock held as a “capital asset” within the meaning of Section 1221 of the Code (generally property held for investment). This discussion does not address any tax treatment of other transactions occurring in connection with the Business Combination, including, but not limited to, the conversion of the Class B Common Stock to Class A Common Stock. This summary is based on the provisions of the Code, U.S. Treasury regulations promulgated thereunder (whether final, temporary, or proposed), administrative rules and judicial decisions, all as in effect on the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect holders to which this section applies. We have not sought and will not seek any rulings from the IRS or formal opinions of tax advisors with respect to the statements made and the conclusions reached in the following discussion. The statements and conclusions herein are not free from doubt, and there can be no assurance that your tax adviser, the IRS or a court will agree with such statements and conclusions or that, if challenged, such treatment will be sustained by a court.
The following does not purport to be a complete analysis of all potential tax effects resulting from the completion of the Business Combination and does not address all aspects of U.S. federal income taxation that may be relevant to particular holders in light of their personal circumstances, and accordingly, is not intended to be, and should not be construed as, tax advice. In addition, this summary does not address the U.S. federal 3.8% Medicare tax imposed on certain investment income, or any aspects of U.S. federal taxation other than those pertaining to the income tax, such as U.S. federal estate or gift tax laws, any state, local or non-U.S. tax laws or any tax treaties. This summary also does not address tax consequences applicable to investors that may be subject to special treatment under the U.S. federal income tax laws, such as:
| ● | banks, insurance companies or other financial institutions; | |
| ● | tax-exempt or governmental organizations; | |
| ● | qualified foreign pension funds (or any entities all of the interests of which are held by a qualified foreign pension fund); | |
| ● | dealers in securities or foreign currencies; | |
| ● | persons whose functional currency is not the U.S. dollar; | |
| ● | “controlled foreign corporations,” “passive foreign investment companies” and corporations that accumulate earnings to avoid U.S. federal income tax; | |
| ● | traders in securities that use the mark-to-market method of accounting for U.S. federal income tax purposes; | |
| ● | persons subject to the alternative minimum tax; | |
| ● | partnerships or other pass-through entities for U.S. federal income tax purposes or holders of interests therein; | |
| ● | persons deemed to sell Class A Common Stock under the constructive sale provisions of the Code; | |
| ● | persons that acquired Class A Common Stock through the exercise of employee stock options or otherwise as compensation or through a tax-qualified retirement plan; | |
| ● | real estate investment trusts; |
| -142- |
| ● | regulated investment companies; | |
| ● | certain former citizens or long-term residents of the United States; | |
| ● | persons that hold Class A Common Stock as part of a straddle, appreciated financial position, synthetic security, hedge, conversion transaction or other integrated investment or risk reduction transaction; and | |
| ● | the initial stockholders, the Sponsor or FoxWayne’s officers or directors. |
If a partnership (including an entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds Class A Common Stock or Aerami Common Stock, and, after the completion of the Business Combination, common stock of the Combined Company, the U.S. federal income tax treatment of a partner in the partnership generally will depend upon the status of the partner, upon the activities of the partnership and upon certain determinations made at the partner level. Accordingly, we urge partners in partnerships (including entities or arrangements treated as partnerships for U.S. federal income tax purposes) to consult, and rely solely upon, their tax advisors regarding the U.S. federal income tax consequences to them relating to the matters discussed below.
For purposes of this discussion, a “U.S. holder” is a beneficial owner of shares of Class A Common Stock or Aerami Common Stock, and, after the Business Combination, common stock of the Combined Company, that is, for U.S. federal income tax purposes:
| ● | an individual who is a citizen or resident of the United States; | |
| ● | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; | |
| ● | an estate the income of which is subject to U.S. federal income tax regardless of its source; or | |
| ● | a trust (a) the administration of which is subject to the primary supervision of a U.S. court and which has one or more United States persons (within the meaning of the Code) who have the authority to control all substantial decisions of the trust or (b) which has made a valid election under applicable U.S. Treasury regulations to be treated as a United States person for U.S. federal income tax purposes. |
Also, for purposes of this discussion, a “Non-U.S. holder” is any beneficial owner of Class A Common Stock Aerami Common Stock, and, after the Business Combination, common stock of the Combined Company that is neither a U.S. holder nor an entity treated as a partnership for U.S. federal income tax purposes.
THIS DISCUSSION IS FOR INFORMATIONAL PURPOSES ONLY AND IS NOT TAX ADVICE. INVESTORS SHOULD CONSULT, AND RELY SOLELY UPON, THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
The following discussion, “U.S. Federal Income Tax Consequences in Respect of the Redemption of Class A Common Stock”, constitutes the opinion of Sheppard Mullin, counsel to FoxWayne, as to the material U.S. federal income tax consequences to holders of Class A Common Stock of exercising redemption rights, subject to the limitations, exceptions, beliefs, assumptions, and qualifications described in such opinion and otherwise herein.
U.S. Federal Income Tax Consequences in Respect of the Redemption of Class A Common Stock
U.S. Holders
Tax Characterization of Redemption. In the event that a U.S. holder elects to redeem its Class A Common Stock is redeemed pursuant to the redemption provisions described in the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights,” the treatment of the transaction for U.S. federal income tax purposes will depend on whether the redemption qualifies as a sale or exchange of the Class A Common Stock under Section 302 of the Code or is treated as a distribution under Section 301 of the Code with respect to the U.S. Holder. If the redemption qualifies as a sale or exchange of Class A Common Stock, the U.S. holder will be treated as described under “— U.S. Federal Income Tax Consequences in Respect of the Redemption of Class A Common Stock — U.S. Holders — Gain or Loss on Redemption Treated as a Sale of Class A Common Stock” below. If the redemption does not qualify as a sale or exchange of Class A Common Stock, the U.S. holder will be treated as receiving a corporate distribution with the tax consequences described below under “— U.S. Federal Income Tax Consequences in Respect of the Redemption of Class A Common Stock — U.S. Holders — Taxation of Redemption Treated as a Distribution.”
Whether a redemption qualifies for sale or exchange treatment will depend largely on the total number of shares of Class A Common Stock treated as held by the U.S. holder (including any stock constructively owned by the holder including as a result of owning Private Placement Warrants or public warrants and any of our stock that a holder would directly or indirectly acquire pursuant to the Business Combination) relative to all shares of Class A Common Stock outstanding both before and after the redemption. The redemption of a U.S. holder’s Class A Common Stock generally will be treated as a sale or exchange of such Class A Common Stock (rather than as a corporate distribution) if the redemption (a) is “substantially disproportionate” with respect to the U.S. holder, (b) results in a “complete termination” of the U.S. holder’s interest in us or (c) is “not essentially equivalent to a dividend” with respect to the U.S. holder. These tests are explained more fully below.
| -143- |
In determining whether any of the foregoing tests is satisfied, a U.S. holder takes into account not only stock owned directly by the U.S. holder, but also shares of Class A Common Stock that are treated as constructively owned by the U.S. holder. In addition to stock actually owned by a U.S. holder, such U.S. holder may also be treated as constructively owning stock owned by certain related individuals and entities in which the U.S. holder has an interest or that have an interest in such U.S. holder, as well as any stock that the U.S. holder has a right to acquire by exercise of an option, which would generally include Class A Common Stock which could be acquired pursuant to the exercise of the warrants. In order to meet the substantially disproportionate test, the percentage of our outstanding voting stock actually and constructively owned by the U.S. holder immediately following the redemption of Class A Common Stock must, among other requirements, be less than 80% of the percentage of our outstanding voting stock actually and constructively owned by the U.S. holder immediately before the redemption. There will be a complete termination of a U.S. holder’s interest if either (a) all of the shares of Class A Common Stock actually and constructively owned by the U.S. holder are redeemed or (b) all of the shares of Class A Common Stock actually owned by the U.S. holder are redeemed and the U.S. holder is eligible to waive, and effectively waives in accordance with specific rules, the attribution of stock owned by certain family members, and the U.S. holder does not constructively own any other stock. Finally, the redemption of a U.S. holder’s Class A Common Stock will not be essentially equivalent to a dividend if such redemption results in a “meaningful reduction” of the U.S. holder’s proportionate interest in FoxWayne. Whether the redemption will result in a meaningful reduction in a U.S. holder’s proportionate interest in FoxWayne will depend on the particular facts and circumstances. However, the IRS has indicated in a published ruling that even a small reduction in the proportionate interest of a small minority stockholder in a publicly held corporation who exercises no control over corporate affairs may constitute such a “meaningful reduction.” A U.S. holder should consult with, and rely solely upon, its own tax advisors as to the tax consequences of electing to have Class A Common Stock redeemed for cash.
If none of the foregoing tests is satisfied, then the redemption will be treated as a corporate distribution, and the tax effects will be as described under “— Taxation of Redemption Treated as a Distribution” below. After the application of those rules, any remaining tax basis of the U.S. holder in the redeemed Class A Common Stock will be added to the U.S. holder’s adjusted tax basis in its remaining stock, or, if it has none, possibly to the U.S. holder’s adjusted tax basis in its warrants or in other stock constructively owned by it.
Gain or Loss on Redemption Treated as a Sale of Class A Common Stock. If the redemption qualifies as a sale of Class A Common Stock, a U.S. holder generally will recognize capital gain or loss in an amount equal to the difference between (a) the amount of cash received in such redemption and (b) the U.S. holder’s adjusted tax basis in its Class A Common Stock redeemed. A U.S. holder’s adjusted tax basis in its Class A Common Stock generally will equal the U.S. holder’s acquisition cost less any prior distributions paid to such U.S. holder that were treated as a return of capital for U.S. federal income tax purposes. Any capital gain or loss recognized with respect to a redemption generally will be long-term capital gain or loss if the U.S. holder held the Class A Common Stock redeemed for more than one year. Long-term capital gains recognized by non-corporate U.S. holders will be eligible to be taxed at reduced rates. The deductibility of capital losses is subject to limitations.
Taxation of Redemption Treated as a Distribution. If the redemption does not qualify as a sale of Class A Common Stock, the U.S. holder will generally be treated as receiving a distribution of cash from FoxWayne. Such distribution generally will constitute a dividend for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the U.S. holder’s adjusted tax basis in its Class A Common Stock. Any remaining excess will be treated as gain realized on the sale of the Class A Common Stock and will be treated as described under “— U.S. Federal Income Tax Consequences in Respect of the Redemption of Class A Common Stock — U.S. Holders — Gain or Loss on Redemption Treated as a Sale of Class A Common Stock” above.
| -144- |
Dividends FoxWayne pays to a U.S. holder that is a corporation for U.S. federal income tax purposes (i) generally will qualify for the dividends received deduction if the requisite holding period is satisfied and (ii) generally may be subject to the “extraordinary dividends” provisions of the Code (which could cause a reduction in the tax basis of such U.S. holder’s shares and cause such U.S. holders to recognize capital gain). Corporate U.S. holders are urged to consult their tax advisors concerning the availability of the dividends received deduction and the application of the “extraordinary dividend” provisions of the Code in their particular circumstances. With certain exceptions (including, but not limited to, dividends treated as investment income for purposes of investment interest deduction limitations), and provided certain holding period requirements are met, dividends FoxWayne pays to a non-corporate U.S. holder generally will constitute “qualified dividends” that will be subject to tax at the maximum tax rate accorded to long-term capital gains.
It is unclear whether the redemption rights with respect to the Class A Common Stock described in this proxy statement/prospectus may prevent a U.S. holder from satisfying the applicable holding period requirements with respect to the dividends received deduction, long-term capital gains treatment or the preferential tax rate on qualified dividend income, as the case may be.
The rules governing the U.S. federal income tax treatment of redemptions are complex and the determination of whether a redemption will be treated as a sale of the Class A Common Stock or as a distribution with respect to such stock is made on a holder-by-holder basis. U.S. holders of Class A Common Stock considering the exercise of their redemption rights should consult, and rely solely upon, their own tax advisors as to whether the redemption of their Class A Common Stock will be treated as a sale or as a distribution under the Code and any resultant tax consequences.
Information Withholding and Backup Withholding. Payments received by a U.S. holder as a result of the exercise of redemption rights may be subject, under certain circumstances, to information reporting and backup withholding. Backup withholding will not apply, however, to a U.S. holder that (a) is a corporation or entity that is otherwise exempt from backup withholding (which, when required, certifies as to its exempt status) or (b) furnishes a correct taxpayer identification number and makes any other required certification on IRS Form W-9. Backup withholding is not an additional tax. Rather, the U.S. income tax liability (if any) of persons subject to backup withholding will be reduced by the amount of tax withheld. If backup withholding results in an overpayment of taxes, a refund may be obtained, provided that the required information is timely furnished to the IRS.
Non-U.S. Holders
Tax Characterization of Redemption. The characterization of the redemption of a Non-U.S. holder’s Class A Common Stock pursuant to the redemption provisions described in the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights” as a sale or distribution for U.S. federal income tax purposes generally will be the same as the tax characterization of such a redemption of a U.S. holder’s Class A Common Stock, as described under “— U.S. Federal Income Tax Consequences in Respect of the Redemption of Class A Common Stock” above. However, the tax consequences of such characterization for Non-U.S. holders will generally differ from the consequences for U.S. holders as described below.
Gain on Redemption Treated as a Sale of Class A Common Stock. If the redemption qualifies as a sale of Class A Common Stock with respect to a Non-U.S. holder, such Non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized upon the redemption of its Class A Common Stock unless:
| ● | the gain is effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the Non-U.S. holder maintains a permanent establishment in the United States to which such gain is attributable); | |
| ● | the Non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the redemption and certain other requirements are met; or | |
| ● | FoxWayne is or has been a “United States real property holding corporation” for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of the redemption or the period that the Non-U.S. holder held Class A Common Stock. |
Gain described in the first bullet point above generally will be subject to U.S. federal income on a net income basis at the regular rates applicable to U.S. holders. A Non-U.S. holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items.
| -145- |
Gain described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty), which may be offset by U.S. source capital losses of the Non-U.S. holder (even though the individual is not considered a resident of the United States) provided that the Non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses.
If the third bullet point above applied to a Non-U.S. holder, any gain recognized by such holder with respect to such holder’s Class A Common Stock as a result of the redemption would be subject to tax at generally applicable U.S. federal income tax rates and a U.S. federal withholding tax could apply. However, FoxWayne believes that it is not, and has not been at any time during the five-year testing period, a United States real property holding corporation.
Taxation of Redemption Treated as a Distribution. If the redemption does not qualify as a sale of Class A Common Stock, a Non-U.S. holder will generally be treated as receiving a distribution of cash from FoxWayne. The determination of the extent to which such distribution will be treated as a dividend, return of capital, or gain realized on the sale of the Class A Common Stock will generally be the same as for U.S. holders of Class A Common Stock, as described in “— U.S. Federal Income Tax Consequences in Respect of the Redemption of Class A Common Stock — U.S. Holders” above. However, any portion of a distribution that is treated as gain realized on the sale of the Class A Common Stock will be treated as described under “— U.S. Federal Income Tax Consequences in Respect of the Redemption of Class A Common Stock — Non-U.S. Holders — Gain on Redemption Treated as a Sale of Class A Common Stock” above.
Subject to the withholding requirements under Sections 1471 through 1474 of the Code and the U.S. Treasury regulations and administrative guidance issued thereunder (“FATCA”) and provided such dividend is not effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States, each of which is discussed below, any portion of such distribution treated as dividend made to a Non-U.S. holder on our Class A Common Stock generally will be subject to U.S. withholding tax at a rate of 30% of the gross amount of the dividend unless an applicable income tax treaty provides for a lower rate. To receive the benefit of a reduced treaty rate, a Non-U.S. holder must provide the applicable withholding agent with an IRS Form W-8BEN or IRS Form W-8BEN-E (or other applicable or successor form) certifying qualification for the reduced rate. Because we generally cannot determine at the time we make a distribution whether or not the distribution will exceed our current and accumulated earnings and profits, we normally will withhold tax on the entire amount of any distribution at the 30% rate (subject to reduction by an applicable income tax treaty). However, some or all of any amounts thus withheld may be refundable to the Non-U.S. holder if it is subsequently determined that such distribution was, in fact, in excess of our current and accumulated earnings and profits.
Dividends paid to a Non-U.S. holder that are effectively connected with a trade or business conducted by the Non-U.S. holder within the United States (and, if required by an applicable income tax treaty, are treated as attributable to a permanent establishment maintained by the Non-U.S. holder in the United States) generally will be taxed on a net income basis at the rates and in the manner generally applicable to United States persons (as defined under the Code). Such effectively connected dividends will not be subject to U.S. withholding tax if the Non-U.S. holder satisfies certain certification requirements by providing the applicable withholding agent with a properly executed IRS Form W-8ECI certifying eligibility for exemption. If the Non-U.S. holder is a corporation for U.S. federal income tax purposes, it may also be subject to a branch profits tax (at a 30% rate or such lower rate as specified by an applicable income tax treaty) on its effectively connected earnings and profits (as adjusted for certain items), which will include effectively connected dividends.
The rules governing the U.S. federal income tax treatment of redemptions are complex, and the determination of whether a redemption will be treated as a sale of Class A Common Stock or as a distribution with respect to such stock is made on a holder-by-holder basis. As a result, a withholding agent may require a Non-U.S. holder to provide certain information regarding its ownership in order to determine whether the redemption proceeds should be treated as sale proceeds or as a distribution subject to withholding. Non-U.S. holders of Class A Common Stock considering the exercise of their redemption rights should consult, and rely solely upon, their own tax advisors as to whether the redemption of their Class A Common Stock will be treated as a sale or as a distribution under the Code and any resultant tax consequences.
Information Reporting and Backup Withholding. Any dividends paid to a Non-U.S. holder (including constructive dividends received pursuant to a redemption of Class A Common Stock) must be reported annually to the IRS and to the Non-U.S. holder. Copies of these information returns may be made available to the tax authorities in the country in which the Non-U.S. holder resides or is established. Any dividends paid to a Non-U.S. holder (including constructive dividends received pursuant to a redemption of our Class A Common Stock) generally will not be subject to backup withholding if the Non-U.S. holder establishes an exemption by properly certifying its non-U.S. status on an IRS Form W-8BEN or IRS Form W-8BEN-E (or other applicable or successor form).
| -146- |
Payments of the proceeds of the sale or other disposition by a Non-U.S. holder of Class A Common Stock effected by or through a U.S. office of a broker generally will be subject to information reporting and backup withholding (at the applicable rate) unless the Non-U.S. holder establishes an exemption by properly certifying its non-U.S. status on an IRS Form W-8BEN or IRS Form W-8BEN-E (or other applicable or successor form) and certain other conditions are met. Information reporting and backup withholding generally will not apply to any payment of the proceeds from a sale or other disposition of Class A Common Stock effected outside the United States by a non-U.S. office of a broker. However, unless such broker has documentary evidence in its records that the Non-U.S. holder is not a United States person and certain other conditions are met, or the Non-U.S. holder otherwise establishes an exemption, information reporting will apply to a payment of the proceeds of the disposition of Class A Common Stock effected outside the United States by such a broker if it has certain relationships within the United States.
Backup withholding is not an additional tax. Rather, the U.S. federal income tax liability (if any) of persons subject to backup withholding will be reduced by the amount of tax withheld. If backup withholding results in an overpayment of taxes, a refund may be obtained, provided that the required information is timely furnished to the IRS.
Additional Withholding Requirements under FATCA
FATCA imposes a 30% withholding tax on any dividends paid on Class A Common Stock, and subject to the discussion of certain proposed Treasury Regulations below, on the gross proceeds from a disposition of Class A Common Stock, in each case if paid to a “foreign financial institution” or a “non-financial foreign entity” (each as defined in the Code) (including, in some cases, when such foreign financial institution or non-financial foreign entity is acting as an intermediary), unless (a) in the case of a foreign financial institution, such institution enters into an agreement with the U.S. government to withhold on certain payments, and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are non-U.S. entities with U.S. owners), (b) in the case of a non-financial foreign entity, such entity certifies that it does not have any “substantial United States owners” (as defined in the Code) or provides the applicable withholding agent with a certification identifying the direct and indirect substantial United States owners of the entity (in either case, generally on an IRS Form W-8BEN-E), or (c) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules and provides appropriate documentation (such as an IRS Form W-8BEN-E). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing these rules may be subject to different rules. Under certain circumstances, a holder might be eligible for refunds or credits of such taxes.
The U.S. Treasury released proposed Treasury Regulations which, if finalized in their present form, would eliminate the federal withholding tax of 30% applicable to the gross proceeds of a sale or other disposition of our Class A Common Stock. In its preamble to such proposed Treasury Regulations, the U.S. Treasury stated that taxpayers may generally rely on the proposed regulations until final regulations are issued.
HOLDERS OF CLASS A COMMON STOCK CONTEMPLATING EXERCISE OF THEIR REDEMPTION RIGHTS SHOULD CONSULT, AND RELY SOLELY UPON, THEIR TAX ADVISORS TO DETERMINE THE SPECIFIC TAX CONSEQUENCES TO THEM OF SUCH A REDEMPTION, INCLUDING THE EFFECT OF ANY U.S. FEDERAL, STATE, LOCAL, NON-U.S. OR OTHER TAX LAWS.
U.S. Federal Income Tax Consequences of the Business Combination for the Holders of Aerami Common Stock
It is the opinion of Smith Anderson that the Merger contemplated by the Business Combination will qualify as a “reorganization” for U.S. federal income tax purposes within the meaning of Section 368(a) of the Code. This opinion is and will be subject to customary qualifications and assumptions, including assumptions regarding the absence of changes in existing facts and law and the completion of the Merger in the manner contemplated by the Merger Agreement. In rendering its tax opinion, counsel will rely upon representations and covenants, including those contained in representation letters from Aerami and FoxWayne reasonably satisfactory in form and substance to counsel, and will assume that these representations are and will be true, correct and complete without regard to any knowledge limitation, and that these covenants will be complied with. If any of those representations, covenants or assumptions is inaccurate, the tax consequences of the Merger could differ from those discussed here. An opinion of counsel represents counsel’s best legal judgment and is not binding on the IRS or any court, nor does it preclude the IRS from adopting a contrary position. In addition, we have not requested nor do we intend to request a ruling from the Internal Revenue Service as to the U.S. federal income tax consequences of the Merger. Accordingly, there can be no assurances that the IRS will not assert, or that a court will not sustain, a position contrary to any of the tax consequences set forth below or any of the tax consequences described in the tax opinion.
The obligations of FoxWayne, Aerami and the Merger Sub to complete the Merger are not conditioned on the receipt of opinions from Sheppard Mullin or Smith Anderson to the effect that the Merger qualifies for such treatment, and the Merger may occur even if it does not so qualify. Neither FoxWayne nor Aerami has requested, and neither intends to request, a ruling from the IRS as to the U.S. federal income tax consequences of the Merger. Consequently, no assurance can be given that the IRS will not assert, or that a court would not sustain, a position contrary to any of those set forth below. Accordingly, each holder of Aerami common stock is urged to consult its tax advisor with respect to the particular tax consequences of the Merger to such holder.
If the Merger qualifies as a “reorganization” within the meaning of Section 368(a) of the Code, each U.S. Holder of Aerami common stock generally will not recognize gain or loss upon exchanging its Aerami common stock for common stock of the Combined Company. The tax basis of the common stock of the Combined Company received by a holder of Aerami common stock will be the same as the tax basis of the Aerami common stock surrendered in exchange for the common stock of the Combined Company. The holder’s holding period for the shares of common stock of the Combined Company that it receives pursuant to the Merger will include its holding period for the shares of the Aerami common stock it surrenders. U.S. Holders who acquired their different blocks of Aerami Common Stock at different times or at different prices should consult their tax advisers regarding the identification of the bases or holding periods of the particular shares of common stock of the Combined Company received in the Merger.
U.S. Federal Income Tax Consequences if the Merger Fails to Qualify as a Reorganization within the Meaning of Section 368(a) of the Code and/or as a Transaction Governed by Section 351 of the Code
If the Merger contemplated by the Merger Agreement does not qualify as a “reorganization” within the meaning of Section 368(a) of the Code, then, for U.S. federal income tax purposes, a holder holding Aerami common stock generally would be treated as selling its Aerami common stock in exchange for common stock of the Combined Company in a taxable transaction.
A U.S. Holder who receives the merger consideration pursuant to the Merger would generally recognize capital gain or loss equal to the difference, if any, between (i) the sum of the fair market values of the common stock of the Combined Company, as determined for U.S. federal income tax purposes and (ii) such U.S. Holder’s adjusted tax basis in the Aerami common stock surrendered. Such gain or loss generally will be long-term capital gain or loss provided the U.S. Holder’s holding period for the Aerami common stock surrendered in the Merger exceeds one year as of the Closing Date. Long-term capital gain of certain non-corporate holders (including individuals) is currently eligible for U.S. federal income taxation at preferential rates (currently at a maximum rate of 20%). The deductibility of capital losses is subject to limitations under the Code. U.S. Holders that realize a loss should consult their tax advisors regarding the allowance of this loss.
A holder’s initial tax basis in the common stock of the Combined Company received in the Merger would equal the fair market value of such stock upon receipt, and the holding period for such stock will begin on the day following the Closing Date of the Merger.
THE FOREGOING DISCUSSION IS NOT TAX ADVICE OR A COMPREHENSIVE DISCUSSION OF ALL U.S. FEDERAL INCOME TAX CONSEQUENCES TO YOU IN CONNECTION WITH THE BUSINESS COMBINATION. YOU SHOULD CONSULT, AND RELY SOLELY UPON, YOUR TAX ADVISORS TO DETERMINE THE SPECIFIC TAX CONSEQUENCES TO THEM OF THE BUSINESS COMBINATION, INCLUDING THE EFFECT OF ANY U.S. FEDERAL, STATE, LOCAL, NON-U.S. OR OTHER TAX LAWS.
| -147- |
Regulatory Matters
Neither FoxWayne nor Aerami is aware of any material regulatory approvals or actions that are required for completion of the Business Combination. It is presently contemplated that if any additional regulatory approvals or actions are required, those approvals or actions will be sought. There can be no assurance, however, that any such additional approvals or actions will be obtained.
Vote Required for Approval
The Closing is conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal at the special meeting.
The Transaction Proposal (and consequently, the Merger Agreement and the Business Combination) will be approved and adopted only if we obtain the affirmative vote (online or by proxy) of holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon at the special meeting, voting as a single class. Failure to vote by proxy or to vote online at the special meeting or an abstention from voting will have no effect on the outcome of the vote on the Transaction Proposal.
Our Sponsor, directors and officers have agreed to vote any shares of Class A Common Stock and Class B Common Stock owned by them in favor of the Business Combination.
Recommendation of the FoxWayne Board
THE FOXWAYNE BOARD RECOMMENDS THAT FOXWAYNE STOCKHOLDERS VOTE “FOR” THE APPROVAL OF THE TRANSACTION PROPOSAL.
PROPOSAL 2 — THE CHARTER PROPOSAL
Overview
Assuming the Transaction Proposal and the Nasdaq Proposal are approved, FoxWayne will replace its current Charter with the Proposed Charter in the form attached to this proxy statement/prospectus as Annex B, which, in the judgment of the FoxWayne Board, is necessary to provide sufficient authorized capital to effect the Merger and reservation of shares for issuance subsequent to the Merger and adequately address the needs of the Combined Company following the Closing.
The following table sets forth a summary of the principal proposed changes between the Charter and the Proposed Charter. This summary is qualified by reference to the complete text of the Proposed Charter, a copy of which is attached to this proxy statement/prospectus as Annex B. All FoxWayne stockholders are encouraged to read the Proposed Charter in its entirety for a more complete description of its terms. Please also see the section titled “Description of New Securities” for a summary comparison of the principal differences between the Charter and the Proposed Charter.
| -148- |
| Existing Charter | Proposed Charter | |||
| Name Change | Under the existing Charter, the name of the corporation is FoxWayne Enterprises Acquisition Corp. | The Proposed Charter will provide that the name of the corporation will become Aerami Therapeutics Holdings, Inc. | ||
| Authorized Capital Stock | Under the existing Charter, the total number of authorized shares of all classes of capital stock is 53,000,000 shares, consisting of (a) 52,000,000 shares of common stock, par value $0.0001 per share, including (i) 50,000,000 shares of Class A Common Stock and (ii) 2,000,000 shares of Class B Common Stock and (b) 1,000,000 shares of Preferred Stock, par value $0.0001 per share. | The Proposed Charter would increase the total number of authorized shares of all classes of capital stock to 210,000,000 shares, consisting of (i) 200,000,000 shares of Combined Company common stock and (ii) 10,000,000 shares of Combined Company preferred stock.
In addition, the Proposed Charter provides for the elimination of Class B Common Stock and any rights of holders thereof. Upon the adoption of the Proposed Charter, each share of Class A Common Stock issued and outstanding or held in treasury immediately prior to the Effective Time will be automatically reclassified as and converted into one share of common stock. | ||
| Supermajority Voting for Amendments to Certain Provisions of FoxWayne’s Certificate of Incorporation | Under the existing Charter, the affirmative vote of a majority of the holders of the voting power of all then outstanding shares of FoxWayne’s capital stock is required to amend, alter, change or repeal, or adopt any provisions inconsistent with Article IV pertaining to the powers, preferences and relative, participating, optional and other or special rights of the Class B Common Stock; and Article VI pertaining to the bylaws. | The Proposed Charter will require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal, or adopt any provisions inconsistent with Article V pertaining to stockholders; and Article VI pertaining to directors. | ||
| Director Removal | Under the existing Charter, any or all of the directors may be removed from office at any time, but only for cause and only by the affirmative vote of holders of a majority of the voting power of all then outstanding shares of capital stock of FoxWayne entitled to vote generally in the election of directors, voting together as a single class. | The Proposed Charter will provide that, subject to the rights of holders of FoxWayne’s preferred stock, the FoxWayne Board or any individual directors may be removed only for cause and only by affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote at an election of directors. | ||
| Bylaw Amendment | Under the existing Charter, in addition to any vote of the holders of any class or series of capital stock of FoxWayne required by law or the existing Charter, the affirmative vote of the holders of at least a majority of the voting power of all then outstanding shares of capital stock of the FoxWayne entitled to vote generally in the election of directors, voting together as a single class, shall be required for the stockholders to adopt, amend, alter or repeal the By-Laws. | The Proposed Charter will require the affirmative vote of the holders of not less than two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend or repeal any provision of the Combined Company’s bylaws. | ||
| Purpose | Under the existing Charter, the purpose of FoxWayne is to consummate the Initial Business Combination. | The Proposed Charter will provide that the purpose of the Combined Company is to engage in any lawful act or activity for which corporations may be organized under the DGCL. |
| -149- |
In addition to the foregoing, the Proposed Charter will eliminate various provisions applicable only to blank check companies, including business combination requirements.
Reasons for the Amendments to FoxWayne’s Existing Charter
In the judgment of the FoxWayne Board, the Proposed Charter is necessary to address the needs of the Combined Company following the Closing. In particular:
| ● | The increase in authorized capital is intended to provide adequate authorized share capital to (a) accommodate the issuance of shares of Combined Company common stock pursuant to the Merger Agreement, the New Equity Incentive Plan, and the future conversion of outstanding warrants into shares of Class A Common Stock and (b) provide flexibility for future issuances of common stock and preferred stock if determined by the Combined Company to be in the best interests of the post-combination company without incurring the risk, delay and potential expense incident to obtaining stockholder approval for a particular issuance. | |
| ● | The supermajority voting amendment, the director removal amendment and the bylaw amendment are desirable to enhance the continuity and stability of the Combined Company Board. The supermajority voting requirements contained in the Proposed Charter, and the requirement that directors be removed only for cause and only by affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote at an election of directors, are appropriate at this time to protect all stockholders against the potential self-interested actions by one or a few large stockholders. In reaching this conclusion, the FoxWayne Board was cognizant of the potential for certain stockholders to hold a substantial beneficial ownership of its common stock following the Business Combination. FoxWayne further believes that going forward, a supermajority voting requirement encourages the person seeking control of the Combined Company to negotiate with the Combined Company Board to reach terms that are appropriate for all stockholders. | |
| ● | The amendment to FoxWayne’s purpose is appropriate to adequately update the Charter for the post-combination company, because it will eliminate obsolete language that will no longer be applicable following the consummation of the Business Combination and make such other changes that are more appropriate for a public operating company. |
Vote Required for Approval
The Charter Proposal is conditioned on the approval of the Transaction Proposal and the Nasdaq Proposal at the special meeting. If either the Transaction Proposal or the Nasdaq Proposal is not approved, Proposal 2 will have no effect, even if approved by our stockholders.
The approval of the Charter Proposal requires the affirmative vote (online or by proxy) of holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote thereon at the special meeting, voting as a single class. Failure to vote by proxy or to vote online at the special meeting or an abstention from voting will have the same effect as a vote “AGAINST” any of the Proposals.
| -150- |
Recommendation of the FoxWayne Board
THE FOXWAYNE BOARD RECOMMENDS THAT FOXWAYNE STOCKHOLDERS VOTE “FOR” THE CHARTER PROPOSAL
PROPOSAL 3 – THE BYLAW PROPOSAL
Overview
Assuming the Transaction Proposal and the Nasdaq Proposal are approved, FoxWayne will replace its current bylaws with the Proposed Bylaws in the form attached to this proxy statement/prospectus as Annex C, which, in the judgment of the FoxWayne Board, is necessary to adequately address the needs of the Combined Company following the Closing.
The following table sets forth a summary of the principal proposed changes between FoxWayne’s existing bylaws (the “Bylaws”) and the Proposed Bylaws. This summary is qualified by reference to the complete text of the Proposed Bylaws, a copy of which is attached to this proxy statement/prospectus as Annex C. All FoxWayne stockholders are encouraged to read the Proposed Bylaws in its entirety for a more complete description of its terms. Please also see the section titled “Description of New Securities” for a summary comparison of the principal differences between the Bylaws and the Proposed Bylaws.
| Existing Bylaws | Proposed Bylaws | |||
| Advance Notice Requirements for Stockholder Proposals and Director Nominations | Under the existing Bylaws, stockholders seeking to bring business before a meeting or to nominate candidates for election as directors must provide timely notice of their intent in writing subject to certain exceptions as set forth in Article II, Section 2.7 and Article III, Section 3.2. | The Proposed Bylaws will provide that stockholders seeking to bring business before a meeting of stockholders, or to nominate candidates for election as directors at a meeting of the stockholders, must provide timely notice of their intent in writing subject to certain exceptions and provide additional disclosures as required of any “Proposing Person” or “Nominating Person.” These additional disclosures will include (A) disclosure of any underlying derivative securities that constitute a “call equivalent position,” pursuant to Rule 16a-1(c) of the Exchange Act, (B) disclosure of any rights to dividends on the shares of any class or series of shares of the Combined Company, (C) disclosure of any material pending or threatened legal proceeding the Proposing Person is a party to that involves the Combined Company any of its officers directors, or affiliates, (D) disclosure of any other material relationship between such Proposing Person, and the Combined Company or its affiliates, (E) disclosure of any direct or indirect material interest in any material contract with the Combined Company or an affiliate, (F) a representation that such Proposing Person intends to deliver a proxy statement required to approve or adopt the proposal or otherwise solicit proxies from stockholders in support of such proposal and (G) disclosure of any other information relating to such Proposing Person that would be required to be disclosed in a proxy statement required to be made pursuant to Section 14(a) of the Exchange Act. |
| -151- |
| Action by Written Consent | Under the Existing Bylaws, any action required to be taken at any annual or special meeting of the stockholders may be taken with written consent. | The Proposed Bylaws prohibit stockholder action by written consent in lieu of a meeting. Notwithstanding the foregoing, certain actions by the holders of preferred stock voting separately as a class may be taken without a meeting without prior notice and without a vote, to the extent expressly so provided by the applicable certificate of designation. | ||
| Lock-up | Under the Existing Bylaws, stockholders are not subject to any lock-up restrictions. | Under the Proposed Bylaws, stockholders will be subject to a lock up provision pursuant to which holders of Combined Company common stock issued as consideration pursuant to the Merger Agreement or upon the exercise, exchange, conversion, or settlement of any Exchanged Options, Exchanged RSU Awards, or Assumed Warrants may not transfer such shares until 180 days after the date that the Form S-1 Shelf is declared effective by the SEC, subject to certain exceptions. | ||
| Supermajority Voting to Amend the Bylaws | Under the Existing Bylaws, the affirmative vote of a majority of the holders of the voting power of all then outstanding shares of FoxWayne’s capital stock is required to amend, alter, change or repeal the Bylaws. | The Proposed Bylaws will require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of Combined Company common stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal the Proposed Bylaws. |
Reasons for Amendment to FoxWayne’s Bylaws
The supermajority voting amendment, the advance notice and disclosure amendments, and the no action by written consent amendment are desirable to enhance the continuity and stability of the Combined Company Board. Furthermore, these amendments are necessary to protect all stockholders against the potential self-interested actions by one or a few large stockholders. In reaching this conclusion, the FoxWayne Board was cognizant of the potential for certain stockholders to hold a substantial beneficial ownership of its common stock following the Business Combination. FoxWayne further believes that going forward, a supermajority voting requirement encourages the person seeking control of the Combined Company to negotiate with the Combined Company Board to reach terms that are appropriate for all stockholders.
The Bylaw lock-up amendment is intended to protect all stockholders against the potential self-interested actions by one or a few large stockholders and prevent stockholders from unloading shares immediately, or soon after the business combination is completed which could be harmful to other stockholders and the Combined Company.
| -152- |
Vote Required for Approval
The Bylaw Proposal is conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal at the special meeting. If the Transaction Proposal, the Nasdaq Proposal or the Charter Proposal is not approved, this Proposal 3 will have no effect, even if approved by our stockholders.
The approval of the Bylaw Proposal requires the affirmative vote (online or by proxy) of holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote thereon at the special meeting, voting as a single class. Failure to vote by proxy or to vote online at the special meeting or an abstention from voting will have the same effect as a vote “AGAINST” any of these proposals.
Recommendation
of the FoxWayne Board
THE FOXWAYNE BOARD RECOMMENDS THAT FOXWAYNE STOCKHOLDERS VOTE “FOR” THE BYLAW PROPOSAL
PROPOSAL 4 — THE NASDAQ PROPOSAL
Overview
We are proposing the Nasdaq Proposal in order to comply with Nasdaq Listing Rules 5635(a) and (b). Under Nasdaq Listing Rule 5635(a), stockholder approval is required prior to the issuance of securities in connection with the acquisition of another company if such securities are not issued in a public offering and (A) have, or will have upon issuance, voting power equal to or in excess of 20% of the voting power outstanding before the issuance of common stock (or securities convertible into or exercisable for common stock); or (B) the number of shares of common stock to be issued is or will be equal to or in excess of 20% of the number of shares of common stock outstanding before the issuance of the stock or securities. Under Nasdaq Listing Rule 5635(b), stockholder approval is required prior to the issuance of securities when the issuance or potential issuance will result in a change of control.
Pursuant to the Merger Agreement, based on Aerami’s current capitalization, we anticipate that we will issue to Aerami Equityholders as consideration in the Merger, 25,000,000 shares of the Combined Company common stock, and we will reserve shares of the Combined Company common stock for issuance upon the exercise of Exchanged Options, the Assumed Warrants assumed in the Merger and the vesting of Exchanged RSU Awards. In addition, we will reserve a number of shares that approximates 20% of the fully-diluted Combined Company common stock as of immediately following the Business Combination for issuance under the New Equity Incentive Plan (which number will include the shares of Combined Company common stock reserved for issuance upon exercise of the Exchanged Options and Exchanged RSU Awards). Because the number of shares of common stock we anticipate issuing as consideration in the Merger and pursuant to the New Equity Incentive Plan (1) will constitute more than 20% of our outstanding common stock and voting power prior to such issuance and (2) will result in a change of control of FoxWayne, we are required to obtain stockholder approval of such issuance pursuant to Nasdaq Listing Rules 5635(a) and (b).
Effect of Proposal on Current Stockholders
If the Nasdaq Proposal is adopted, FoxWayne would issue shares representing more than 20% of the outstanding shares of Combined Company common stock in connection with the Business Combination. The issuance of such shares would result in significant dilution to FoxWayne’s stockholders and would afford such stockholders a smaller percentage interest in the voting power, liquidation value and aggregate book value of the Combined Company. If the Nasdaq Proposal is approved, assuming that 25,000,000 shares of Combined Company common stock are issued to Aerami Equityholders as consideration in the Merger, we anticipate that the Aerami Equityholders will hold 78% of the outstanding shares of the Combined Company common stock and the current FoxWayne stockholders will hold 22% of the outstanding shares of the Combined Company common stock immediately following completion of the Business Combination. This percentage assumes that no shares of FoxWayne common stock are redeemed in connection with the Merger, does not take into account any Exchanged Options, Exchanged RSU Awards and Assumed Warrants assumed in the Merger or other warrants or rights to purchase the Combined Company common stock that will be outstanding following the Merger or any equity awards that may be issued pursuant to the New Equity Incentive Plan Proposal following the Merger.
| -153- |
If the Nasdaq Proposal is not approved and we consummate the Business Combination on its current terms, FoxWayne would be in violation of Nasdaq Listing Rule 5635(a) and (b), which could result in the delisting of our securities from The Nasdaq Capital Market. If Nasdaq delists our securities from trading on its exchange, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our securities; | |
| ● | reduced liquidity with respect to our securities; | |
| ● | a determination that our shares are a “penny stock,” which will require brokers trading in our securities to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for our securities; | |
| ● | a limited amount of news and analyst coverage for the Combined Company; and | |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
It is a condition to the obligations of FoxWayne and Aerami to close the Business Combination that our common stock remain listed on The Nasdaq Capital Market. As a result, if the Nasdaq Proposal is not adopted, the Business Combination may not be completed unless this condition is waived.
Vote Required for Approval
The Closing is conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal at the special meeting.
Approval of the Nasdaq Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon at the special meeting, voting as a single class. Failure to vote by proxy or to vote online at the special meeting or an abstention from voting will have no effect on the outcome of the vote on the Nasdaq Proposal.
Recommendation of the FoxWayne Board
THE FOXWAYNE BOARD RECOMMENDS THAT FOXWAYNE STOCKHOLDERS VOTE “FOR” THE NASDAQ PROPOSAL
PROPOSAL 5 — THE NEW EQUITY INCENTIVE PLAN PROPOSAL
Overview
On , 2022, the FoxWayne Board adopted the New Equity Incentive Plan, subject to FoxWayne stockholder approval. If the New Equity Incentive Plan is approved by stockholders, the post-Combined Company will be authorized to grant equity awards to eligible service providers upon consummation of the Business Combination. A copy of the New Equity Incentive Plan is attached to this proxy statement/prospectus as Annex D. The New Equity Incentive Plan will become effective, if at all, on the date that it is approved by the FoxWayne stockholders and the Effective Time.
| -154- |
Under the New Equity Incentive Plan, a total of shares of Combined Company common stock will initially be available for grant to eligible participants—namely, any employee, consultant, or director of the Combined Company or any of its subsidiaries. This amount includes shares of Combined Company common stock underlying Exchanged Options and shares of Combined Company common stock underlying Exchanged RSU Awards to be issued under the New Equity Incentive Plan in connection with the Closing. The administrator of the New Equity Incentive Plan may grant incentive stock options (“ISOs”), non-qualified stock options (“NSOs”), stock appreciation rights (“SARs”), restricted stock, restricted stock units (“RSUs”) or other stock or cash based awards. No more than shares of Combined Company common stock may be issued pursuant to the exercise of ISOs. As of the record date, the chart below indicates the number of individuals who are eligible to receive awards under the New Equity Incentive Plan.
| Class | Potential Participants (Pre-Merger) | Potential Participants (Post-Merger) | ||||||
| Employees | — | 4 | ||||||
| Consultants | — | 14 | ||||||
| Non-Employee Directors | — | 6 | ||||||
Purpose of the New Equity Incentive Plan
The purpose of the New Equity Incentive Plan is to attract, retain, incentivize and reward service providers who will contribute to the Combined Company’s success. FoxWayne believes that equity compensation is critical in attracting and retaining top talent and will help focus them on the creation of long-term value consistent with the interests of FoxWayne’s stockholders.
Reasons for the Approval of the New Equity Incentive Plan Proposal
Stockholder approval of the New Equity Incentive Plan is necessary in order for FoxWayne to (a) meet the stockholder approval requirements of Nasdaq and (b) grant ISOs. Stockholders are also being asked to approve an annual limitation on the compensation paid to non-employee directors.
Consequences if the New Equity Incentive Plan Proposal is Not Approved
If the New Equity Incentive Plan Proposal is not approved by FoxWayne’s stockholders, the New Equity Incentive Plan will not become effective and the Combined Company will not be able to grant equity awards under the New Equity Incentive Plan. FoxWayne believes the Combined Company’s ability to recruit, retain and incentivize top talent will be adversely affected if the New Equity Incentive Plan Proposal is not approved.
Material Terms of the New Equity Incentive Plan
The following summarizes the material features of the New Equity Incentive Plan. This summary is qualified in its entirety by the full text of the New Equity Incentive Plan, a copy of which is attached as Annex D to this proxy statement/prospectus.
Types of Awards.
The New Equity Incentive Plan provides for the issuance of stock options, SARs, RSUs, and other stock or cash based awards to eligible participants, namely, employees, consultants and directors of the Combined Company and its subsidiaries.
If all or any part of an award expires, lapses or is terminated, exchanged for or settled in cash, surrendered, repurchased, cancelled without having been fully exercised or forfeited, in any case, in a manner that results in the Combined Company acquiring shares covered by the award at a price not greater than the price paid by the participant for such shares or not issuing any shares covered by the award, the unused shares covered by the award will, as applicable, become or again be available for award grants under the New Equity Incentive Plan. Further, shares delivered to the Combined Company by a participant to satisfy the applicable exercise or purchase price of an award and/or to satisfy any applicable tax withholding obligation with respect to an award (including shares retained by the Combined Company from the award being exercised or purchased and/or creating the tax obligation) will, as applicable, become or again be available for award grants under the New Equity Incentive Plan. The payment of dividend equivalents in cash in conjunction with any outstanding awards shall not count against the number of shares available for award grants. Notwithstanding anything to the contrary contained in the New Equity Incentive Plan, the following shares will not be added to the shares authorized for grant under the New Equity Incentive Plan and shall not be available for future grants of awards: (i) shares subject to an SAR that are not issued in connection with the stock settlement of the SAR on exercise thereof; and (ii) shares purchased on the open market with the cash proceeds from the exercise of stock options.
| -155- |
Administration.
The New Equity Incentive Plan will be administered by the Combined Company Board or a committee or subcommittee thereof (the “Administrator”). The Administrator may determine which eligible participants receive awards, grant awards and set award terms and conditions, subject to the conditions and limitations in the New Equity Incentive Plan. The Administrator has the authority to take all actions and make all determinations under the New Equity Incentive Plan, to interpret the New Equity Incentive Plan and award agreements, and to adopt, amend and repeal the New Equity Incentive Plan administrative rules, guidelines and practices as it deems advisable. The Administrator may correct defects and ambiguities, supply omissions and reconcile inconsistencies in the New Equity Incentive Plan or any award as it deems necessary or appropriate to administer the New Equity Incentive Plan and any awards. The Administrator’s determinations under the New Equity Incentive Plan are in its sole discretion and will be final and binding on all persons having or claiming any interest in the plan or any award. To the extent permissible under applicable law, the Combined Company Board or the Administrator may delegate any or all of its powers to one or more committees or officers of the Combined Company or any of its subsidiaries. The Combined Company Board or the Administrator may abolish any committee or re-vest in itself any previously delegated authority at any time.
Options.
FoxWayne may grant non-qualified stock options and ISOs (within the meaning of Section 422 of the Code) under the New Equity Incentive Plan. The vesting and other terms and conditions of any options granted to an eligible participant will be set forth in an award agreement, and subject to the provisions of the New Equity Incentive Plan, as will be determined by the Administrator. The exercise price of any option granted under the New Equity Incentive Plan must be at least equal to the fair market value of Combined Company common stock on the date the option is granted (or 110% of that fair market value in the case of ISOs granted to stockholders who beneficially own ten percent or more of the Combined Company’s outstanding shares). The maximum term of an option granted under the New Equity Incentive Plan is ten years (or five years for ISOs in the case of a stockholder holding 10% or more of the shares of the Combined Company). The amount of ISOs that become exercisable for the first time in a particular year cannot exceed a value of $100,000, determined using the fair market value of the shares on the date of grant, per participant.
Restricted Stock and RSUs.
The Combined Company may also grant restricted stock and RSUs under the New Equity Incentive Plan. The Administrator will determine the purchase price, vesting schedule and performance goals, if any, applicable to the grant of restricted stock or RSUs. Unless otherwise determined by the Administrator, if these restrictions, performance goals or other conditions are not satisfied, the restricted stock and RSUs will be forfeited. Subject to the provisions of the New Equity Incentive Plan, and the applicable individual award agreement, the Administrator has the sole discretion to provide for the accelerated vesting or the removal of any or all of the restrictions with respect to any award of restricted stock or RSUs.
Unless otherwise determined by the Administrator, participants with restricted stock will generally have all of the rights of a stockholder during the restricted period, including the right to receive dividends declared with respect to such shares. During the restricted period, participants with RSUs will generally not have any rights of a stockholder, unless the Administrator determines otherwise. If the Administrator provides, a grant of RSUs may provide a participant with the right to receive dividend equivalents, on the terms and conditions set forth in the award agreement.
Stock Appreciation Rights.
The Combined Company may also grant SARs under the New Equity Incentive Plan. An SAR granted under the New Equity Incentive Plan entitles its holder to receive, at the time of exercise, an amount in cash determined by multiplying the difference obtained by subtracting the exercise price per share of the SAR from the fair market value of Combined Company common stock on the date of exercise of the SAR by the number of shares of Combined Company common stock with respect to which the SAR was exercised. Each SAR will be granted with an exercise price no less than the fair market value of Combined Company common stock on the date of grant, unless otherwise determined by the Administrator. The maximum term of all SARs granted under the New Equity Incentive Plan will be determined by the Administrator but may not exceed ten years. The Administrator may determine to issue payment with respect to SARs in shares of common stock or cash, or any combination thereof.
| -156- |
Other Stock or Cash Based Awards.
The Administrator may grant other stock or cash based awards under the New Equity Incentive Plan, valued in whole or in part by reference to, or otherwise based on, shares of Combined Company common stock. The Administrator will determine the terms and conditions of these awards, including any purchase price, performance goals, transfer restrictions and vesting conditions. Such other stock or cash based awards may be paid in shares of Combined Company common stock, cash or other property, as determined by the Administrator.
Equitable Adjustments and Treatment of Outstanding Awards Upon a Corporate Transaction.
In the event of any nonreciprocal transaction between the Combined Company and its stockholders, such as a stock dividend, stock split, spin-off or recapitalization through a large, nonrecurring cash dividend, that affects the number or kind of shares (or other of the Combined Company’s securities) or the share price of Combined Company common stock (or other of the Combined Company’s securities) and causes a change in the per share value of Combined Company common stock underlying outstanding awards (each, an “Equity Restructuring”), the Administrator will equitably adjust each outstanding award as it deems appropriate to reflect such Equity Restructuring, which may include adjusting the number and type of securities subject to each award and/or the award’s exercise price or grant price (if applicable), granting new awards to participants, and making a cash payment to participants.
In the event of any dividend or other distribution (whether in the form of cash, Combined Company common stock, other securities, or other property), reorganization, merger, consolidation, combination, amalgamation, repurchase, recapitalization, liquidation, dissolution, or sale, transfer, exchange or other disposition of all or substantially all of the assets of the Combined Company, or sale or exchange of Combined Company common stock or other securities of the Combined Company, Change in Control (as defined below), issuance of warrants or other rights to purchase Combined Company common stock or other securities of the Combined Company, other similar corporate transaction or event, other unusual or nonrecurring transaction or event affecting the Combined Company or its financial statements or any change in any applicable laws or accounting principles, the Administrator may take any one or more of the following actions whenever the Administrator determines that such action is appropriate in order to (x) prevent dilution or enlargement of the benefits or potential benefits intended by the Combined Company to be made available under the New Equity Incentive Plan or with respect to any award granted or issued under the New Equity Incentive Plan, (y) to facilitate such transaction or event or (z) give effect to such changes in applicable laws or accounting principles (A) to provide for the cancellation of any such award in exchange for either an amount of cash or other property with a value equal to the amount that could have been obtained upon the exercise or settlement of the vested portion of such award or realization of the participant’s rights under the vested portion of such award, as applicable; provided that, if the amount that could have been obtained upon the exercise or settlement of the vested portion of such award or realization of the participant’s rights, in any case, is equal to or less than zero, then the award may be terminated without payment; provided, further, that awards held by members of the Combined Company’s Board will be settled in shares on or immediately prior to the applicable event if the Administrator takes such action; (B) to provide that such award shall vest and, to the extent applicable, be exercisable as to all shares covered thereby, notwithstanding anything to the contrary in the New Equity Incentive Plan or the provisions of such award; (C) to provide that such award be assumed by the successor or survivor corporation, or a parent or subsidiary thereof, or shall be substituted for by awards covering the stock of the successor or survivor corporation, or a parent or subsidiary thereof, with appropriate adjustments as to the number and kind of shares and/or applicable exercise or purchase price, in all cases, as determined by the Administrator; (D) to make adjustments in the number and type of shares of Combined Company common stock (or other securities or property) subject to outstanding awards and/or with respect to which awards may be granted under the New Equity Incentive Plan and/or in the terms and conditions of (including the grant or exercise price), and the criteria included in, outstanding awards; (E) to replace such award with other rights or property selected by the Administrator; and/or (F) to provide that the award will terminate and cannot vest, be exercised or become payable after the applicable event.
| -157- |
Definition of Change in Control.
For purposes of the New Equity Incentive Plan, a “Change in Control” will mean each of the following:
| ● | A transaction or series of transactions (other than an offering of Combined Company common stock to the general public through a registration statement filed with the SEC or a transaction or series of transactions that meets the requirements of clauses (i) and (ii) of the third bullet below) whereby any “person” or related “group” of “persons” (as such terms are used in Sections 13(d) and 14(d)(2) of the Exchange Act) (other than the Combined Company, any of its subsidiaries, an employee benefit plan maintained by the Combined Company or any of its subsidiaries or a “person” that, prior to such transaction, directly or indirectly controls, is controlled by, or is under common control with, the Combined Company) directly or indirectly acquires beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act) of securities of the Combined Company possessing more than 50% of the total combined voting power of the Combined Company’s securities outstanding immediately after such acquisition; | |
| ● | During any period of two consecutive years, individuals who, at the beginning of such period, constitute the Combined Company Board together with any new director(s) (other than a director designated by a person who shall have entered into an agreement with the Combined Company to effect a transaction described the first bullet above or the third bullet below) whose election by the Combined Company Board or nomination for election by the Combined Company’s stockholders was approved by a vote of at least two-thirds of the directors then still in office who either were directors at the beginning of the two year period or whose election or nomination for election was previously so approved, cease for any reason to constitute a majority thereof; or | |
| ● | The consummation by the Combined Company (whether directly involving the Combined Company or indirectly involving the Combined Company through one or more intermediaries) of (x) a merger, consolidation, reorganization, or business combination or (y) a sale or other disposition of all or substantially all of the Combined Company’s assets in any single transaction or series of related transactions or (z) the acquisition of assets or stock of another entity, in each case other than a transaction: (i) which results in the Combined Company’s voting securities outstanding immediately before the transaction continuing to represent (either by remaining outstanding or by being converted into voting securities of the Combined Company or the person that, as a result of the transaction, controls, directly or indirectly, the Combined Company or owns, directly or indirectly, all or substantially all of the Combined Company’s assets or otherwise succeeds to the business of the Combined Company (the Combined Company or such person, the “Successor Entity”)) directly or indirectly, at least a majority of the combined voting power of the Successor Entity’s outstanding voting securities immediately after the transaction, and (ii) after which no person or group beneficially owns voting securities representing 50% or more of the combined voting power of the Successor Entity; provided, however, that no person or group shall be treated for purposes of this clause (ii) as beneficially owning 50% or more of the combined voting power of the Successor Entity solely as a result of the voting power held in the Combined Company prior to the consummation of the transaction. |
Notwithstanding the foregoing, in no event shall the Business Combination or the transactions occurring in connection with the Merger Agreement constitute a Change in Control and, if a Change in Control constitutes a payment event with respect to any award (or portion of any award) that provides for the deferral of compensation that is subject to Section 409A, to the extent required to avoid the imposition of additional taxes under Section 409A, the transaction or event described in subsection (a), (b), or (c) with respect to such award (or portion thereof) shall only constitute a Change in Control for purposes of the payment timing of such award if such transaction also constitutes a “change in control event,” as defined in Treasury Regulation Section 1.409A-3(i)(5).
The Administrator shall have full and final authority, which shall be exercised in its discretion, to determine conclusively whether a Change in Control has occurred pursuant to the above definition, the date of the occurrence of such Change in Control and any incidental matters relating thereto; provided that any exercise of authority in conjunction with a determination of whether a Change in Control is a “change in control event” as defined in Treasury Regulation Section 1.409A-3(i)(5) shall be consistent with such regulation.
Tax Withholding.
The Combined Company or any subsidiary has the authority to deduct or withhold, or require a holder of an award to remit to the Combined Company, an amount sufficient to satisfy federal, state, local and foreign taxes required by law to be withheld with respect to any taxable event concerning the holder arising under the New Equity Incentive Plan. The Administrator will determine the methods by which a holder will make payments with respect to tax withholding obligations with respect to any awards under the New Equity Incentive Plan. Without limiting the foregoing, the Administrator may, in its discretion, withhold or allow a holder to elect to have the Combined Company withhold, shares of common stock otherwise issuable under an award (or allow the surrender of shares of common stock).
| -158- |
Amendment and Termination of the New Equity Incentive Plan.
The Administrator may amend, suspend or terminate the New Equity Incentive Plan at any time; provided that no amendment, other than an increase to the overall share limit, may materially and adversely affect any award outstanding at the time of such amendment without the affected participant’s consent. No awards may be granted under the New Equity Incentive Plan during any suspension period or after such termination. Awards outstanding at the time of any suspension or termination will continue to be governed by the New Equity Incentive Plan and the applicable award agreement, as in effect before such suspension or termination. The FoxWayne Board will obtain stockholder approval of any plan amendment to the extent necessary to comply with applicable laws.
New Equity Incentive Plan Term.
The New Equity Incentive Plan will expire on , the tenth anniversary of the date the FoxWayne Board adopted the New Equity Incentive Plan. No awards may be granted after that date under the New Equity Incentive Plan, although awards granted before that time will remain outstanding in accordance with their terms.
Claw-back.
All awards (including any proceeds, gains or other economic benefit a participant actually or constructively receives upon receipt or exercise of any award or the receipt or resale of any shares underlying the award) will be subject to any claw-back policy of the Combined Company, including any claw-back policy adopted to comply with applicable laws (including the Dodd-Frank Wall Street Reform and Consumer Protection Act and any rules or regulations promulgated thereunder).
United States Federal Income Tax Consequences
The following summarizes certain United States federal income tax consequences of awards granted under the New Equity Incentive Plan. It does not purport to be a complete description of all applicable rules, and those rules (including those summarized here) are subject to change.
Non-Qualified Stock Options.
A participant who has been granted a non-qualified stock option will not recognize taxable income upon the grant of a non-qualified stock option. Rather, at the time of exercise of such non-qualified stock option, the participant will recognize ordinary income for income tax purposes in an amount equal to the excess of the fair market value of the shares of common stock purchased over the exercise price. The Combined Company generally will be entitled to a tax deduction at such time and in the same amount that the participant recognizes ordinary income. If shares of common stock acquired upon exercise of a non-qualified stock option are later sold or exchanged, then the difference between the amount received upon such sale or exchange and the fair market value of such shares on the date of such exercise will generally be taxable as long-term or short-term capital gain or loss (if the shares are a capital asset of the participant) depending upon the length of time such shares were held by the participant.
Incentive Stock Options.
A participant who has been granted an incentive stock option will not realize taxable income at the time of grant, and the Combined Company will not be entitled to a tax deduction at that time. The exercise of an incentive stock option will not result in taxable income to the participant provided that the participant was, without a break in service, an employee of the Combined Company or a subsidiary during the period beginning on the date of the grant of the option and ending on the date three months prior to the date of exercise (one year prior to the date of exercise if the participant is disabled). The excess of the fair market value of the shares of common stock at the time of the exercise of an incentive stock option over the exercise price is included in calculating the participant’s alternative minimum taxable income for the tax year in which the incentive stock option is exercised unless the participant disposes of the shares in the year of exercise. If the participant does not sell or otherwise dispose of the shares within two years from the date of the grant of the incentive stock option or within one year after the transfer of such shares to the participant, then, upon disposition of such shares, any amount realized in excess of the exercise price will be taxed to the participant as capital gain and the Combined Company will not be entitled to a corresponding tax deduction. The participant will generally recognize a capital loss to the extent that the amount realized is less than the exercise price. If the foregoing holding period requirements are not met, the participant will generally realize ordinary income at the time of the disposition of the shares in an amount equal to the lesser of (i) the excess of the fair market value of the shares on the date of exercise over the exercise price or (ii) the excess, if any, of the amount realized upon disposition of the shares over the exercise price, and the Combined Company will be entitled to a corresponding tax deduction. Any amount realized in excess of the value of the shares on the date of exercise will be capital gain. If the amount realized is less than the exercise price, the participant will not recognize ordinary income, and the participant will generally recognize a capital loss equal to the excess of the exercise price over the amount realized upon the disposition of the shares.
| -159- |
Stock Appreciation Rights.
A participant who is granted an SAR generally will not recognize ordinary income upon receipt of the SAR. Rather, at the time of exercise of such SAR, the participant will recognize ordinary income for income tax purposes in an amount equal to the value of any cash received and the fair market value on the date of exercise of any shares of common stock received. The Combined Company generally will be entitled to a tax deduction at such time and in the same amount, if any, that the participant recognizes as ordinary income. The participant’s tax basis in any shares of common stock received upon exercise of an SAR will be the fair market value of the shares of common stock on the date of exercise, and if the shares are later sold or exchanged, then the difference between the amount received upon such sale or exchange and the fair market value of such shares on the date of exercise will generally be taxable as long-term or short-term capital gain or loss (if the shares are a capital asset of the participant) depending upon the length of time such shares were held by the participant.
Restricted Stock.
A participant generally will not be taxed upon the grant of restricted stock, but rather will recognize ordinary income in an amount equal to the fair market value of the shares of common stock at the earlier of the time the shares become transferable or are no longer subject to a substantial risk of forfeiture (within the meaning of the Code). The Combined Company generally will be entitled to a deduction at the time when, and in the amount that, the participant recognizes ordinary income on account of the lapse of the restrictions. A participant’s tax basis in the shares of common stock will equal their fair market value at the time the restrictions lapse, and the participant’s holding period for capital gains purposes will begin at that time. Any cash dividends paid on the shares of common stock before the restrictions lapse will be taxable to the participant as additional compensation and not as dividend income, unless the individual has made an election under Section 83(b) of the Code. Under Section 83(b) of the Code, a participant may elect to recognize ordinary income at the time the restricted shares are awarded in an amount equal to their fair market value at that time, notwithstanding the fact that such stock is subject to restrictions or transfer and a substantial risk of forfeiture. If such an election is made, no additional taxable income will be recognized by such participant at the time the restrictions lapse, the participant will have a tax basis in the shares of common stock equal to their fair market value on the date of their award, and the participant’s holding period for capital gains purposes will begin at that time. We generally will be entitled to a tax deduction at the time when, and to the extent that, ordinary income is recognized by such participant.
Restricted Stock Units.
In general, the grant of RSUs will not result in income for the participant or in a tax deduction for us. Upon the settlement of such an award in cash or shares of common stock, the participant will recognize ordinary income equal to the aggregate value of the payment received, and we generally will be entitled to a tax deduction at the same time and in the same amount.
Other Awards.
With respect to other stock-based awards, generally when the participant receives payment in respect of the award, the amount of cash and/or the fair market value of any shares of common stock or other property received will be ordinary income to the participant, and we generally will be entitled to a tax deduction at the same time and in the same amount.
| -160- |
New Equity Incentive Plan Benefits
No awards will be made under the New Equity Incentive Plan until after the Effective Time. Because the New Equity Incentive Plan is discretionary, benefits to be received by individual participants are not determinable. However, assuming stockholder approval of the New Equity Incentive Plan, the post-Combined Company intends to grant awards to certain non-employee directors and executive officers as set forth below:
| Name | Dollar Value ($) | Number of Options | ||||||
| Barry Deutsch | ||||||||
| Chief Financial Officer | — | (1) | ||||||
| Darlene Deptula Hicks | — | 100,000 | ||||||
| Theodore Reiss | — | 100,000 | ||||||
| Renee Tannenbaum | — | 100,000 | ||||||
| All Executive Officers as a Group | — | (1) | ||||||
| Non-Executive Director Group | — | 300,000 | ||||||
| Non-Executive Officer Employee Group (4 persons) | — | |||||||
| Total | — | (1) | ||||||
(1) Pursuant to Mr. Deutsch’s Employment Agreement with Aerami, if the Business Combination is consummated, Mr. Deutsch will be entitled to a grant of options to purchase shares of Combined Company common stock representing 1% of the post-Closing shares outstanding on a fully diluted basis.
Registration with the SEC
If our stockholders approve the New Equity Incentive Plan, the Combined Company intends to file a registration statement on Form S-8 registering the shares reserved for issuance under the New Equity Incentive Plan as soon as reasonably practicable after the Combined Company becomes eligible to use such form.
Vote Required for Approval
The New Equity Incentive Plan Proposal is conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal at the special meeting. If the Transaction Proposal, the Nasdaq Proposal or the Charter Proposal are not approved, this Proposal 5 will have no effect, even if approved by the FoxWayne stockholders.
The approval of the New Equity Incentive Plan Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon, voting as a single class. Failure to vote by proxy or to vote online at the special meeting or an abstention from voting will have no effect on the outcome of the vote on the New Equity Incentive Plan Proposal.
Recommendation of FoxWayne’s Board of Directors
FOXWAYNE’S BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” THE APPROVAL OF THE NEW EQUITY INCENTIVE PLAN PROPOSAL.
PROPOSAL 6 — THE ADDITIONAL PROPOSAL
Overview
The Additional Proposal, if adopted, will allow the stockholders to consider and vote upon any other proposals determined by the FoxWayne Board to be necessary or appropriate in connection with the transactions contemplated by the Business Combination or the other proposals at the special meeting.
| -161- |
Vote Required for Approval
The Additional Proposal is conditioned on the approval of the Transaction Proposal, the Nasdaq Proposal and the Charter Proposal at the special meeting. If the Transaction Proposal, the Nasdaq Proposal or the Charter Proposal are not approved, this Proposal 6 will have no effect, even if approved by our stockholders.
The approval of the Additional Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon, voting as a single class. Failure to vote by proxy or to vote online at the special meeting or an abstention from voting will have no effect on the outcome of the vote on the Additional Proposal.
Recommendation of the FoxWayne Board
THE FOXWAYNE BOARD RECOMMENDS THAT FOXWAYNE STOCKHOLDERS VOTE “FOR” THE APPROVAL OF THE ADDITIONAL PROPOSAL
PROPOSAL 7 — THE ADJOURNMENT PROPOSAL
Overview
The Adjournment Proposal, if adopted, will allow the FoxWayne Board to adjourn the special meeting to a later date or dates, if necessary or appropriate, to permit further solicitation and vote of proxies. The Adjournment Proposal will only be presented to our stockholders in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal or the New Equity Incentive Plan Proposal. If our stockholders approve the Adjournment Proposal, we may adjourn the special meeting and any adjourned session of the special meeting and use the additional time to solicit additional proxies, including the solicitation of proxies from our stockholders who have voted previously.
Consequences if the Adjournment Proposal is Not Approved
If the Adjournment Proposal is not approved by FoxWayne stockholders, FoxWayne may not be able to adjourn the special meeting to a later date in the event that there are insufficient votes for, or otherwise in connection with, the approval of the Transaction Proposal, the Charter Proposal, the Bylaw Proposal, the Nasdaq Proposal or the New Equity Incentive Plan Proposal.
Vote Required for Approval
The Adjournment Proposal is not conditioned on the approval of any other Proposal at the special meeting.
The approval of the Adjournment Proposal requires the affirmative vote (online or by proxy) of the holders of a majority of the shares of Class A Common Stock and Class B Common Stock entitled to vote and actually cast thereon at the special meeting, voting as a single class. Failure to vote by proxy or to vote online at the special meeting or an abstention from voting will have no effect on the outcome of the vote on the Adjournment Proposal.
Recommendation of the FoxWayne Board
THE FOXWAYNE BOARD RECOMMENDS THAT FOXWAYNE STOCKHOLDERS VOTE “FOR” THE APPROVAL OF THE ADJOURNMENT PROPOSAL.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF AERAMI
In this section, unless otherwise stated or the context requires otherwise, the terms “Aerami,” “we,” “us” and “our” refer to Aerami Therapeutics Holdings, Inc. and its subsidiaries prior to the Closing, which will be the business of the Combined Company and its subsidiaries following the Closing.
| -162- |
You should read the following discussion and analysis of our financial condition and results of operations together with “Summary Historical Financial Information of Aerami” and our consolidated financial statements and the related notes included elsewhere in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this prospectus, our actual results could differ materially from the results described in or implied by these forward-looking statements. You should carefully read the “Risk Factors” section of this prospectus to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Overview
We are a clinical stage biopharmaceutical company dedicated to developing differentiated inhaled therapies for the treatment of severe respiratory and other chronic diseases where delivery of therapeutic agents via inhalation can provide an important treatment option. We aim to address markets with high unmet needs where we believe inhaled therapies can offer meaningful differentiation over current standards of care and improve patient experience and outcomes. We are developing multiple drug-device combination products which consist of inhalable formulations of drugs and biologics that are currently approved for the same or different indications combined with either our own proprietary inhaler or commercially available inhalers.
Our lead development program, AER-901, is an inhaled formulation of imatinib delivered via the FOX® high performing handheld nebulizer which we are developing for the treatment of pulmonary arterial hypertension (“PAH”). AER-901 is in a Phase 1 clinical trial, and in 2022 we are targeting initiating the Phase 2 portion of a planned combined Phase 2/3 clinical trial.
We also have a proprietary inhaler as the delivery device for our two other current programs, both of which address diabetes-related conditions. The AFINA™ inhaler, which is powered by a vibrating mesh technology, delivers approximately 80% of the drug to the lungs, which we believe is 2-4 times higher than currently marketed inhalers – enabling efficient delivery of the dose of drug or biologic in a short amount of time. We believe AFINA™ can be successfully positioned as an alternative to currently marketed inhalers to treat lung diseases and to injectable or oral therapy for the treatment of systemic diseases. We plan to file in 2022 for 510(k) clearance for AFINA™ for use as a general purpose nebulizer in the United States, which we believe will be reviewed as a Class 2 device with no clinical data required based on substantial equivalence to several predicate devices such as iNeb AAD, Innospire Go, AireHealth nebulizer, and Microbase Portable Nebulizer.
AER-501 (soft mist inhaled insulin) is a patent protected, preservative-free, high purity liquid formulation of recombinant human insulin delivered via AFINATM. We believe that clinical results across five Phase 1/2 clinical studies demonstrating the delivery of consistent doses of insulin, dose-linearity, and intra- and inter-subject variability similar to injections support advancing AER-501 to an end-of-Phase 2 meeting with the FDA and into global Phase 3 clinical trials for the treatment of TID and T2D. AER-601 (soft mist inhaled exenatide, a leading short-acting glucagon-like peptide analog, or GLP-1) is directed at the treatment of postprandial hyperglycemia (“PPG”). We have completed preclinical development and this program is Phase 1-ready.
Due to the significant size of the diabetes market and the need for a large commercial presence, we plan to seek partners for both of these programs at the appropriate time. Until such time and depending on available funding, we plan to continue advancing the clinical development of AER-501 and AER-601.
We plan to evaluate additional opportunities for combining our AFINATM soft mist inhaler with other drugs or biologics in order to grow our own severe respiratory and chronic disease product pipeline. We also plan to seek out-licensing opportunities to combine our AFINATM device with other companies’ proprietary drugs or biologics for which inhalation could provide significant patient benefits.
Since our inception in 2009, we have devoted substantially all of our resources to identifying potential product candidates, raising capital, building our intellectual property portfolio, conducting preclinical studies and clinical trials, pursuing partnering arrangements and providing general and administrative support for these operations. We do not have any products approved for sale and have not generated any revenue from product sales. To date, we have financed our operations primarily through the sale of shares of equity securities, although we have also entered into license and collaboration arrangements that have provided limited upfront payments to date. During the nine months ended September 30, 2021, we received net cash proceeds of $14.7 million through private placements of Aerami Series A Preferred Stock and Aerami Common Stock. Between September 30, 2021 and November 3, 2021, we completed the private placement of Aerami Common Stock and received additional net proceeds of $6.4 million.
We have incurred significant operating losses since our inception. Our net losses for the nine months ended September 30, 2021 and the year ended December 31, 2020 were $4.9 million and $10.3 million, respectively. We had an accumulated deficit of $130.2 million as of September 30, 2021. Substantially all of our operating losses resulted from expenses incurred in connection with the development of our product candidates and general and administrative costs associated with our operations.
| -163- |
We expect to continue to incur significant expenses and losses for the foreseeable future. We anticipate our expenses will increase substantially as we continue our development of, seek regulatory approval for and potentially commercialize product candidates, seek to invest in adding to our pipeline, hire additional personnel, protect our intellectual property and incur additional costs associated with being a public company. Our net losses may fluctuate significantly from quarter-to-quarter and year-to-year, depending on the timing and scope of our clinical trials and preclinical studies and expenditures on pursuit of other development candidates or licensing activities. As of September 30, 2021, we had cash and cash equivalents of $14.4 million. To fund further operations, we will need to raise additional capital. Accordingly, we expect to finance our cash needs through a combination of equity offerings, debt financings or other capital sources, including potential additional collaborations, licenses, and other similar arrangements. Adequate funding may not be available to us on acceptable terms, if at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the existing ownership interests of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of holders of our common stock. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or drug candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. We may be unable to raise additional funds or enter into such other agreements when needed on favorable terms or at all. The inability to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy.
We currently have no sources of product revenue, and our ability to continue as a going concern is dependent on our ability to raise capital to fund our future business plans. There is inherent uncertainly associated with these fundraising activities. Volatility in the capital markets and general economic conditions in the United States may be a significant obstacle to raising the required funds. These factors raise substantial doubt about our ability to continue as a going concern. The financial statements included in this filing do not include any adjustments that might be necessary should we be unable to continue as a going concern. See Note 1 to our audited consolidated financial statements and Note 1 to our unaudited interim consolidated financial statements appearing elsewhere in this prospectus for additional information on our assessment of our ability to continue as a going concern.
We believe that our cash and cash equivalents as of September 30, 2021 of $14.4 million and the $6.4 million in net proceeds received in October and November from our private placement of common stock will be sufficient to fund our operations through at least August 2022. We have based these estimates on assumptions that may prove to be imprecise, and we may exhaust our available capital resources sooner than we currently expect. See “— Liquidity and capital resources.” Due to the numerous risks and uncertainties associated with the development of our programs, it is difficult to estimate the amounts of increased capital outlays and operating expenses associated with completing the research and development of our product candidates.
COVID-19 Pandemic
We are carefully monitoring the COVID-19 pandemic which continues to evolve worldwide. The continued spread of COVID-19 and the measures taken by governmental authorities, and any future epidemic disease outbreaks, could disrupt the supply chain and the manufacture or shipment of preclinical and clinical trial materials, delay, limit or prevent our employees and CROs from continuing research and development activities, and impede our clinical trial initiation and recruitment and the ability of patients to continue in clinical trials, impede testing, monitoring, data collection, analysis and other related activities, any of which could delay our clinical trials and preclinical studies, and increase our development costs and/or have a material adverse effect on our business, financial condition and results of operations. While we have experienced delays in the recruitment of subjects for our AER-901 Phase 1 trial due to government regulations in Australia related to COVID-19, we have continued to target initiating in 2022 activities on the Phase 2 portion of our planned combined Phase 2/3 trial. The effect of the COVID-19 pandemic on our development timelines is difficult to assess or predict. The future impact of the COVID-19 pandemic on our industry, the healthcare system and our current and future operations and financial condition will depend on future developments, which are highly uncertain and cannot be predicted with confidence.
| -164- |
Proposed Business Combination Transaction
On December 7, 2021, we entered into the Merger Agreement with FoxWayne and Merger Sub, pursuant to which, Aerami will be the surviving entity after the consummation of the Merger and become a wholly owned subsidiary of FoxWayne. The Business Combination will be accounted for as a reverse recapitalization in accordance with GAAP. Under the reverse recapitalization method, FoxWayne will be treated as the acquired company (Accounting Acquiree) for financial reporting purposes, and Aerami, the Accounting Acquirer, will be assumed to have issued shares of stock for the net assets of FoxWayne, with no goodwill or other intangible assets recorded. This determination is primarily based on the following predominant factors: (i) post-Closing, our stockholders are expected to have a majority of the voting power of the Combined Company and ability to elect the members of the Combined Company Board; (ii) the on-going operations post-Merger will comprise those of Aerami; and (iii) all of the senior management of the Combined Company will be those of Aerami. As a result of the Business Combination, FoxWayne will be renamed Aerami Therapeutics Holdings, Inc. The FoxWayne Board and Aerami Board have each approved the Business Combination. The completion of the Business Combination, which is expected to occur in the first quarter of 2022, is subject to approval of the stockholders of the respective entities and the satisfaction or waiver of certain other customary closing conditions.
FoxWayne is expected to receive net proceeds of approximately $58 million upon the Closing from the Trust Account, assuming no redemptions, $39 million assuming interim redemptions, and $15 million assuming maximum redemptions are effected by stockholders of FoxWayne. The Combined Company’s cash on hand after giving effect to the Business Combination, together with Aerami’s existing cash and cash equivalents, will be used to fund our development programs, working capital and general corporate purposes. We may also use a portion of the remaining net proceeds from the Business Combination and our existing cash and cash equivalents to in-license, acquire or invest in complementary businesses, technologies, products or assets. However, we have no current commitments or obligations to do so.
Financial Overview
Revenues
License revenues
To date, we have not generated any revenues from product sales. Substantially all of our revenue to date has been derived from the license agreement we entered into with Chance in January 2021 (the “Chance License Agreement”). Pursuant to the terms of the Chance License Agreement, we received a non-refundable upfront payment of $1.5 million and are eligible to receive up to $10.8 million in future development, regulatory, and commercial milestone payments.
Operating expenses
Research and development expenses
Research and development expenses consist of costs associated with the preclinical and clinical development of our product candidates, which include:
| ● | personnel-related expenses, including salaries, bonuses, benefits and stock-based compensation for employees engaged in research and development functions; | |
| ● | expenses incurred in connection with the clinical development and regulatory approval of our product candidates, including under agreements with third parties, such as consultants, contractors and CROs; | |
| ● | license fees with no alternative use; and | |
| ● | other expenses related to research and development. |
We expense research and development costs as incurred. Advance payments that we make for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. The prepaid amounts are expensed as the benefits are consumed.
Research and development activities are central to our business model. We expect that our research and development expenses will increase substantially for the foreseeable future in connection with our planned clinical development activities.
| -165- |
General and administrative expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation and benefits for our personnel. General and administrative expenses also include legal fees relating to intellectual property and corporate matters, professional fees for accounting, auditing, tax and consulting services, insurance costs, travel, direct and allocated facility related expenses and other operating costs.
We anticipate that our general and administrative expenses will increase substantially for the foreseeable future as we increase our administrative headcount to operate as a public company and as we advance our product candidates through clinical development. We also will incur additional expenses as a result of operating as a public company, including expenses related to compliance with the rules and regulations of the SEC and the Nasdaq listing rules, additional insurance expenses, investor relations activities and other administrative and professional services. In addition, if we obtain regulatory approval for any of our product candidates, we expect to incur expenses associated with building a sales and marketing team if we choose to commercialize such product candidates on our own.
Other income (expenses)
Interest expense, net
Interest expense, net consists of interest expense related to an agreement with Harmony Plus, offset by interest income earned on our cash and cash equivalents.
We entered into this agreement in connection with a stock purchase agreement with Harmony in 2017 (the “SPAR”), as described below. Under the consulting agreement, Harmony Plus will provide consulting services to Aerami for a period of five years commencing in August 2017. The total consideration for the consulting services is $1.5 million, payable in equal annual installments through August 2022 and recorded as a liability (under the Harmony contingent settlement liability) based on its present value, net of unamortized discount. The amortization of discount is reported as part of interest expense.
Inducement charge from exercise of warrants
Inducement charge from exercise of warrants consists of the charge recognized for the difference between the fair value before and after modification of warrants to purchase Aerami Common Stock related to the 3-for-1 warrant tender offer we conducted in 2019 with respect to outstanding warrants to purchase Aerami Common Stock (the “2019 Warrant Tender Offer”).
Change in fair value of Harmony contingent settlement liability
We entered into the SPAR with Harmony in 2017 in order to reacquire certain rights previously granted to Harmony to develop and commercialize AER-501 in China. As part of the SPAR, we agreed to issue to Harmony shares of Aerami Common Stock valued at $8 million upon the earlier of an initial public offering (“Aerami IPO”) or a change in control in which our stockholders would hold less than a majority of the outstanding shares of the surviving company. The requirement to issue Aerami Common Stock to Harmony represents a contingent settlement obligation and is accounted for as a liability. The contingent settlement liability was recorded at fair value and is subject to remeasurement at each balance sheet date until settled. The change in fair value of the Harmony contingent settlement liability reflects a non-cash charge primarily driven by the likelihood of an Aerami IPO or change in control and the underlying common stock. Changes in the contingent settlement liability fair value estimates result in an increase or decrease in our obligation and a corresponding charge or reduction to operating results.
Change in fair value of warrant liabilities
Our outstanding warrants to purchase shares of our convertible preferred stock are classified as liabilities, recorded at fair value and are subject to remeasurement at each balance sheet date until they are exercised, expired or are otherwise settled. The change in fair value of our warrant liabilities reflects a non-cash charge primarily driven by changes in the fair value of our underlying Aerami Series A Preferred Stock.
| -166- |
Change in fair value of purchase option agreement
As part of the SPAR, we also agreed to pay a royalty to Harmony on gross profits related to the sale of our products in China, which royalties would terminate under certain circumstances. Additionally, Harmony-related holders of Aerami Common Stock also granted us the option to reacquire the Aerami Common Stock held by them at any time prior to the fifth anniversary of an Aerami IPO. We account for the purchase option, which represents a royalty termination event, at fair value and remeasure it at each balance sheet date until settled. The change in fair value of purchase option agreement reflects a non-cash charge.
Results of operations
Comparison of the nine months ended September 30, 2021 and 2020
| Nine Months Ended September 30, | ||||||||||||
| 2021 | 2020 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Revenues: | ||||||||||||
| License | $ | 1,500 | $ | - | $ | 1,500 | ||||||
| Total revenues | 1,500 | - | 1,500 | |||||||||
| Operating expenses: | ||||||||||||
| Research and development | 3,982 | 2,582 | 1,400 | |||||||||
| General and administrative | 3,825 | 6,247 | (2,422 | ) | ||||||||
| Total operating expenses | 7,807 | 8,829 | (1,022 | ) | ||||||||
| Loss from operations | (6,307 | ) | (8,829 | ) | 2,522 | |||||||
| Other income (expenses): | ||||||||||||
| Interest expense, net | (89 | ) | (91 | ) | 2 | |||||||
| Loss on foreign exchange | (28 | ) | - | (28 | ) | |||||||
| Gain on disposal of equipment | - | 10 | (10 | ) | ||||||||
| Change in fair value of Harmony contingent settlement liability | 2,053 | 385 | 1,668 | |||||||||
| Change in fair value of warrant liabilities | (545 | ) | - | (545 | ) | |||||||
| Change in fair value of Harmony purchase option agreement | 33 | (167 | ) | 200 | ||||||||
| Total other income | 1,424 | 137 | 1,287 | |||||||||
| Net loss | $ | (4,883 | ) | $ | (8,692 | ) | 3,809 | |||||
| -167- |
Revenues
License revenues
To date, we have not generated any revenues from product sales. We do not expect to generate significant product revenue unless and until we commercialize our product candidates, if approved. All of our revenue for the nine months ended September 30, 2021 has been derived from the Chance License Agreement we entered into in January 2021. Under the terms of the Chance License Agreement, Chance paid us a $1.5 million upfront payment in 2021, which was fully recognized in January 2021 as the Supply Agreement did not represent a material right. We are eligible to receive $10.8 million in additional development and sales-based milestones as well as high-single-digit royalties on net sales of products under the Chance License Agreement.
Operating expenses
Research and development expenses
Research and development expenses increased by $1.4 million from $2.6 million for the nine months ended September 30, 2020 to $4.0 million for the nine months ended September 30, 2021. The increase in research and development expenses was primarily due to an increase in clinical trial and other development expenses of $2.7 million as we initiated our Phase 1 clinical trial for AER-901 and continued to pursue development of our other product candidates, offset by a $0.4 million decrease in research and development costs primarily associated with AER-601 as we substantially completed our preclinical activities in fiscal 2020 and a $0.6 million decrease in salaries and related benefits as we reduced employee headcount.
General and administrative expenses
General and administrative expenses decreased by $2.4 million from $6.2 million for the nine months ended September 30, 2020 to $3.8 million for the nine months ended September 30, 2021. The decrease was due to a decrease in salaries and related benefits of $1.2 million as we reduced employee headcount and a decrease in professional fees of $1.2 million.
Other income (expense)
Interest expense, net
Interest expense, net consists of interest expense related to our agreement with Harmony Plus, offset by interest income earned on our cash and cash equivalents.
Change in fair value of Harmony contingent settlement liability
For the nine months ended September 30, 2021 and 2020, we recognized non-cash income of $2.1 million and $0.4 million, respectively, due to the change in the fair value of the Harmony contingent settlement liability.
Change in fair value of warrant liabilities
For the nine months ended September 30, 2021, we recognized a non-cash charge of $0.5 million due to the change in the fair value of the convertible preferred stock warrant liability. The change in fair value of the warrant liabilities was de minimis for the nine months ended September 30, 2020.
Change in fair value of Harmony purchase option agreement
For the nine months ended September 30, 2021 and 2020, we recognized non-cash income and non-cash charges of $0.03 million and $0.2 million, respectively, due to the change in the fair value of the Harmony purchase option agreement.
| -168- |
Comparison of the years ended December 31, 2020 and 2019
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Operating expenses: | ||||||||||||
| Research and development | $ | 3,461 | $ | 11,953 | $ | (8,492 | ) | |||||
| General and administrative | 6,932 | 8,538 | (1,606 | ) | ||||||||
| Total operating expenses | 10,393 | 20,491 | (10,098 | ) | ||||||||
| Loss from operations | (10,393 | ) | (20,491 | ) | (10,098 | ) | ||||||
| Other income (expenses): | ||||||||||||
| Interest (expense) income, net | (100 | ) | 18 | (118 | ) | |||||||
| Inducement charge from exercise of warrants | - | (2,551 | ) | 2,551 | ||||||||
| Change in fair value of Harmony contingent settlement liability | 385 | (551 | ) | 936 | ||||||||
| Change in fair value of Harmony purchase option agreement | (201 | ) | (369 | ) | 168 | |||||||
| Total other income (expenses) | 84 | (3,453 | ) | 3,537 | ||||||||
| Net loss | $ | (10,309 | ) | $ | (23,944 | ) | 13,635 | |||||
Operating expenses
Research and development expenses
Research and development expenses decreased by $8.5 million from $12.0 million for the year ended December 31, 2019 to $3.5 million for the year ended December 31, 2020. The decrease in research and development expense was primarily due to a decrease in clinical trial and other development expenses of $4.8 million as we substantially completed our existing clinical development activities associated with AER-501. In addition, we had decreases in salaries and related benefits and facility costs of $3.2 million and $0.4 million, respectively, as we reduced our research and development employee headcount. These decreases were offset by a $1.1 million increase in research and development expenses associated with AER-901, of which $0.9 million was related to obtaining the licensing rights to develop and commercialize AER-901 during the year ended December 31, 2020 and $0.2 million related to our development efforts for this product candidate.
General and administrative expenses
General and administrative expenses decreased by $1.6 million from $8.5 million for the year ended December 31, 2019 to $6.9 million for the year ended December 31, 2020. The decrease was primarily due to decreases in salaries and related benefits of $1.6 million as we reduced employee headcount.
Other income (expenses)
Interest (expense) income, net
Interest (expense) income, net consists of interest expense related to our agreement with Harmony, offset by interest income earned on our cash and cash equivalents.
Inducement charge from exercise of warrants
We considered the 2019 Warrant Tender Offer to be analogous to an equity award modification under the guidance of ASC 718-20-35-3. Accordingly, for the year ended December 31, 2019, we recognized a non-cash charge of $2.6 million due to the change in the fair value before and after the modification of the warrants to purchase Aerami common stock.
| -169- |
Change in fair value of Harmony contingent settlement liability
For the years ended December 31, 2020 and 2019, we recognized non-cash income and a non-cash charge of $0.4 million and $0.6 million, respectively, due to the change in the fair value of the Harmony contingent settlement liability.
Change in fair value of purchase option agreement
For the years ended December 31, 2020 and 2019, we recognized non-cash charges of $0.2 million and $0.4 million, respectively, due to the change in the fair value of the purchase option agreement asset.
Liquidity and capital resources
Since our inception, we have incurred significant operating losses. We expect to incur significant expenses and operating losses for the foreseeable future as we advance the clinical development of our programs and pursue 510(k) regulatory clearance for the AFINA™ inhaler. We have funded our operations to date primarily with proceeds from the sales of our equity securities. For the nine-month period ended September 30, 2021, we received net cash proceeds of $14.7 million from the sale of our equity securities. If there are any net proceeds from the Business Combination then those funds will provide a further source of liquidity unless FoxWayne public stockholders holding more than approximately 3,771,881 shares of FoxWayne Class A Common Stock exercise their redemption rights upon consummation of the Business Combination at a redemption price of approximately $10.10 per share, which leads to a total maximum redemption value of more than $38.1 million, and the $15 million minimum cash condition set forth in the Merger Agreement is waived or reduced.
Private Placement of Aerami Series A Preferred Stock
From June 2020 through January 2021, we sold 12,450 shares of Aerami Series A Preferred Stock and received aggregate net proceeds of $9.7 million. If a holder of Aerami Series A Preferred Stock participated in our next follow-on financing in which we raised gross proceeds of at least $10 million, some or all of the holder’s Aerami Series A Preferred Stock would automatically convert into Aerami Series A-1 Preferred Stock on a one-for-one ratio, depending on how much the holder invested in the follow-on offering relative to the amount the holder invested in Aerami Series A Preferred Stock. Shares of Aerami Series A-1 Preferred Stock have the same rights and preferences of Aerami Series A Preferred Stock except for the conversion price, which is initially set at 50% per share of Aerami Series A-1 Preferred Stock. Holders of 10,460 shares of Aerami Series A Preferred Stock participated in our private placement of Aerami Common Stock from September 2021 through the final closing on November 3, 2021, resulting in the conversion of 10,460 shares of Aerami Series A Preferred Stock into the same number of shares of Aerami Series A-1 Preferred Stock as of November 4, 2021 (including 9,640 shares of Aerami Series A Preferred Stock deemed converted to Aerami Series A-1 Preferred Stock as of September 30, 2021). In connection with the Aerami Series A Preferred Stock offering, we issued to our placement agent in November 2021 230 warrants to purchase shares of Aerami Series A Preferred Stock and 962 warrants to purchase shares of Aerami Series A-1 Preferred Stock, both at an initial exercise price of $1,000 per share and exercisable for a period of five years.
Private Placement of Aerami Common Stock
In September 2021, we commenced a private placement offering of Aerami Common Stock at an offering price of $1.00 per share. We sold 14,940,000 shares of Aerami Common Stock in this private placement in September 2021 and received net proceeds of $12.8 million, and we sold an additional 7,310,560 shares of Aerami Common Stock thereafter through November 3, 2021 and received aggregate net proceeds of $6.4 million. In connection with the private placement, we issued in November 2021 to the placement agent 2,223,056 warrants to purchase shares of Aerami Common Stock at an initial exercise price of $1.00 per share and exercisable for a period of five years. This private offering terminated on November 3, 2021.
Funding requirements
Our operating expenses are expected to increase substantially as we continue to advance our portfolio of programs, particularly if and as we:
| ● | advance the development of our pipeline, including continuing the clinical development of our lead program, AER-901, and commencing preclinical studies and clinical trials for our other product candidates; | |
| ● | scale up our clinical and regulatory capabilities and pursue regulatory approvals for our product candidates; |
| -170- |
| ● | obtain, maintain, expand and protect our intellectual property portfolio; | |
| ● | hire additional research and development, clinical and commercial personnel; and | |
| ● | add operational, financial and management information systems and personnel to support our R&D efforts and conduct commercialization activities as our programs get closer to filing for regulatory approval. |
Based upon our current operational plan and prior to the Business Combination, we believe our existing cash and cash equivalents as of September 30, 2021 and the $6.4 million in net proceeds received in October and November from our private placement of Aerami Common Stock will be sufficient to fund our operations through at least August 2022. We have based this estimate on assumptions that may prove to be imprecise, and we may exhaust our available capital resources sooner than we currently expect. See “–Liquidity and capital resources.” Due to the numerous risks and uncertainties associated with the development of our programs, it is difficult to estimate the amounts of increased capital outlays and operating expenses associated with completing the research and development of our product candidates.
As mentioned above, we plan to pursue additional financing to fund the continued development of our product candidates, including, but not limited to, cash proceeds resulting from the proposed Business Combination. There can be no assurance that new funding will be available or available on acceptable terms, and there can be no guarantee that the Business Combination will successfully close or provide a particular level of proceeds.
Our future capital requirements are difficult to forecast and will depend on many factors, including but not limited to:
| ● | the type, number, scope, progress, expansions, results of and timing of clinical trials and preclinical studies of our product candidates which we are pursuing or may choose to pursue in the future; | |
| ● | the costs of acquiring licenses for the expansion of product development; | |
| ● | the costs, timing and outcome of regulatory review of our product candidates; | |
| ● | the costs of obtaining, maintaining and enforcing our patents and other intellectual property rights; | |
| ● | the costs and timing of manufacturing for our product candidates, including commercial manufacturing if any product candidate is approved; | |
| ● | our efforts to enhance operational systems and hire additional personnel to satisfy our obligations as a public company, including enhanced internal controls over financial reporting; | |
| ● | the costs associated with hiring additional personnel and consultants as our clinical and preclinical activities increase; | |
| ● | the costs and timing of establishing or securing sales and marketing capabilities if any product candidate is approved; | |
| ● | our ability to achieve sufficient market acceptance, coverage and adequate reimbursement from third-party payors and adequate market share and revenue for any approved products; | |
| ● | any delays and cost increases that result from the COVID-19 pandemic or future epidemic diseases; | |
| ● | the terms and timing of establishing and maintaining additional collaborations, licenses and other similar arrangements; | |
| ● | the achievement of milestones or occurrence of other developments that trigger payments under any additional collaboration agreements we obtain; and | |
| ● | the costs associated with any products or technologies that we may in-license or acquire. |
Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of holders of our common stock. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or drug candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay or limit product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
| -171- |
Cash flows
The
following table summarizes our sources and uses of cash for the nine months ended September 30, 2021 and 2020.
| Nine Months Ended September 30, | ||||||||||||
| 2021 | 2020 | Change | ||||||||||
| In thousands | ||||||||||||
| Cash used in operating activities | $ | (4,874 | ) | $ | (9,609 | ) | $ | 4,735 | ||||
| Cash provided by financing activities | 14,735 | 5,261 | 9,474 | |||||||||
| Net increase (decrease) in cash and cash equivalents | $ | 9,861 | $ | (4,348) | $ | 14,209 | ||||||
Operating activities
Net cash used in operating activities for the nine months ended September 30, 2021 was $4.9 million, consisting primarily of our net loss of $4.9 million and the non-cash gain in the change in fair value of the Harmony contingent settlement liability of $2.1 million. These decreases were offset by non-cash charges primarily attributable to the change in the fair value of the warrant liabilities and stock-based compensation expense of $0.5 million and $1.5 million, respectively.
Net cash used in operating activities for the nine months ended September 30, 2020 was $9.6 million and was primarily attributable to our net loss of $8.7 million, a change in the fair value of our contingent liability with Harmony of $0.4 million and the net change in our net operating assets of $2.6 million. These decreases were offset by $2.1 million in non-cash charges primarily attributable to stock-based compensation expense and the change in fair value of the Harmony purchase option of $1.8 million and $0.2 million, respectively.
Financing activities
During the nine months ended September 30, 2021, financing activities provided $17.2 million comprising gross proceeds from the sale of the Aerami Series A Preferred Stock in January 2021 and the private placement of Aerami Common Stock in September 2021, offset by $2.5 million in issuance costs related to these private placements, primarily consisting of placement agent fees and expenses.
During the nine months ended September 30, 2020, financing activities provided $6.4 million in gross proceeds from the sale of the Aerami Series A Preferred Stock during the period, offset by $1.1 million in issuance costs related to that financing.
| -172- |
The
following table summarizes our sources and uses of cash for the years ended December 31, 2020 and 2019:
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | Change | ||||||||||
| In thousands | ||||||||||||
| Cash used in operating activities | $ | (10,711 | ) | $ | (16,052 | ) | $ | 5,341 | ||||
| Cash provided by financing activities | 8,546 | 19,125 | (10,579 | ) | ||||||||
| Net (decrease) increase in cash and cash equivalents | $ | (2,165 | ) | $ | 3,073 | $ | (5,238 | ) | ||||
Operating activities
Net cash used in operating activities for the year ended December 31, 2020 was $10.7 million, consisting primarily of our net loss of $10.3 million, a decrease in accounts payable and accrued expenses of $2.7 million and non-cash income of $0.4 million relating to the change in fair value of the Harmony contingent settlement liability, offset primarily by non-cash charge of $2.6 million for stock-based compensation.
Net cash used in operating activities for the year ended December 31, 2019 was $16.1 million, consisting primarily of our net loss of $23.9 million, offset primarily by non-cash charges of $3.8 million and $2.6 million for stock-based compensation and the inducement charge relating to the exercise of warrants and depreciation expense, respectively.
Financing activities
During the year ended December 31, 2020, financing activities provided gross proceeds of $10.2 million from the sale of the Aerami Series A Preferred Stock, offset by $1.6 million for the payment of associated issuance costs.
During the year ended December 31, 2019, financing activities provided gross proceeds of $20.5 million from the 2019 Warrant Tender Offer, offset by $1.4 million for the payment of associated issuance costs.
Contractual obligations and other commitments
As of September 30, 2021, we had no non-cancellable commitments for purchase of clinical materials, contract manufacturing, maintenance and committed funding which we expect to pay within one year.
Off-balance sheet arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules.
Critical accounting policies and significant judgements
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, and expenses and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions.
| -173- |
While our significant accounting policies are described in more detail in Note 2 to our audited consolidated financial statements and Note 2 to our unaudited consolidated financial statements appearing at the end of this prospectus, we believe that the following accounting policies are the most critical to the judgments and estimates used in the preparation of our financial statements.
Change in fair value of Harmony contingent settlement liability
We have a contingent settlement liability relating to shares of our common stock we must issue to Harmony under the SPAR upon the earlier of an Aerami IPO or a change in control (each defined in the SPAR) which was recorded in the consolidated balance sheets at its estimated fair value. We remeasure the fair value of the liability each reporting period, with changes recorded in the consolidated statements of operations. The determination of fair value requires the exercise of significant judgment and estimates by management. These include estimates and assumptions regarding the achievement of an Aerami IPO or change of control event, forecasted price of our common stock and assumptions utilized in calculating a discount rate. If management’s assumptions prove to be inaccurate, it could result in changes to the contingent settlement liability and have a material effect on our results of operations.
Share-based compensation
We recognize the grant-date fair value of share-based awards issued as compensation expense on a straight-line basis over the requisite service period for time vested awards, which is generally the vesting period of the award, or when the achievement of the milestone becomes probable for performance or market conditions awards. The fair value of stock options is estimated at the time of grant using the Black-Scholes option pricing model, which requires the use of inputs and assumptions such as the estimated fair value of the underlying common stock, exercise price of the option, expected term, risk-free interest rate, expected volatility and dividend yield, the most critical of which is the estimated fair value of our common stock.
The inputs and assumptions used to estimate the fair value of share-based payment awards represent management’s best estimates and involve inherent uncertainties and the application of management’s judgment. As a result, if factors change and management uses different inputs and assumptions, our share-based compensation expense could be materially different for future awards.
Because Aerami Common Stock is not currently publicly traded, the fair value of the Aerami Common Stock underlying stock-based awards has been determined on each grant date by our board of directors, with inputs from management, and a number of subjective and objective factors, including our most recently available third-party valuation of common shares. All options to purchase shares of Aerami Common Stock are intended to be granted with an exercise price per share no less than the fair value per share of Aerami Common Stock underlying those options on the date of grant, based on the information known to us on the date of grant. The various objective and subjective factors used by our board of directors to determine the fair value of our common stock, also include the following:
| ● | the price of our preferred stock sold to investors in arm’s length transactions; | |
| ● | the rights, preferences and privileges of our preferred stock as compared to those of Aerami Common Stock, including the liquidation preferences of our preferred stock; | |
| ● | our results of operations and financial position; | |
| ● | the status of our development efforts; | |
| ● | the composition of, and changes to, our management team and board of directors; | |
| ● | the lack of liquidity of our common stock as a private company; |
| -174- |
| ● | our stage of development and business strategy and the material risks related to our business and industry; | |
| ● | external market conditions affecting the life sciences and biopharmaceutical industry sectors; | |
| ● | U.S. and global economic conditions; | |
| ● | the likelihood of achieving a liquidity event for the holders of our common stock, such as an Aerami IPO or a sale of our company, given prevailing market conditions; and | |
| ● | the market value and volatility of comparable companies. |
We considered the various methods for allocating the enterprise value across our classes and series of capital stock to determine the fair value of our common stock at each valuation date. In valuing our common and preferred stock, we determined the equity value of our business by using a net asset approach. The net asset approach is predicated on the assumption that a prudent buyer would pay no more than it would cost to purchase the assets (tangible and intangible) of a company at current market prices. This approach requires estimating the individual market values of our assets and liabilities to derive an adjusted enterprise value. The enterprise values determined by the net asset approach were then allocated to our common stock using the Option Pricing Method (“OPM”). The OPM treats common stock and preferred stock as call options on a company’s enterprise value, with exercise prices based on the liquidation preferences of the preferred stock. Therefore, the common stock has value only if the funds available for distribution to the stockholders exceed the value of the liquidation preference at the time of an assumed liquidity event such as a merger, sale or initial public offering. The common stock is modeled as a call option with a claim on the enterprise at an exercise price equal to the remaining value immediately after the preferred stock is liquidated. The OPM uses the Black-Scholes option-pricing model to determine the price of the call option. The OPM is appropriate to use when the range of possible future outcomes is so difficult to predict that forecasts would be highly speculative.
There are significant judgments and estimates inherent in the determination of the fair value of Aerami Common Stock. These judgments and estimates include assumptions regarding our future operating performance, the time to completing an Aerami IPO or other liquidity event and the determination of the appropriate valuation methods. If we had made different assumptions, our stock-based compensation expense, net loss and net loss per common share could have been significantly different.
As Combined Company common stock will be publicly traded following the completion of the Business Combination, it will not be necessary to use estimates to determine the fair value of the common stock.
Warrant liabilities
We issued warrants to purchase shares of Aerami Series A Preferred Stock in connection with our Series A Preferred Stock financing during 2020 and 2021. A portion of these warrants became exercisable for shares of Aerami Series A-1 Preferred Stock as a result of the participation of holders of Aerami Series A Preferred Stock in our 2021 private placement of Aerami Common Stock. The warrants were classified as liabilities on the consolidated balance sheet at September 30, 2021 and December 31, 2020 as the underlying Aerami Series A Preferred Stock and Aerami Series A-1 Preferred Stock are contingently redeemable and outside of Aerami’s control. The fair value of the warrants on the date of issuance was recorded as a long-term liability in the consolidated balance sheet and subsequently remeasured to fair value at each balance sheet date. Changes in the fair values of the warrants are recognized as other income or expense in the statements of operations.
In connection with our 2021 private placement of Aerami Common Stock, we also issued Placement Agent Warrants to purchase shares of Aerami Common Stock. These Placement Agent Warrants are liability-classified as they include settlement features that are not indexed to the Aerami Common Stock.
We used the Black-Scholes option pricing model, which incorporated assumptions and estimates, to value these warrants. Estimates and assumptions impacting the fair value measurement of the warrants included the fair value per share of the underlying Aerami Series A Preferred Stock, the Aerami Series A-1 Preferred Stock and the Aerami Common Stock, the remaining contractual term of the warrants, risk-free interest rate, expected dividend yield and expected volatility of the price of the underlying Aerami Series A Preferred Stock, Aerami Series A-1 Preferred Stock and the Aerami Common Stock. We determined the fair value per share of the underlying Aerami Series A Preferred Stock, the Aerami Series A-1 Preferred Stock and the Aerami Common Stock by taking into consideration the most recent sales of Aerami Series A Preferred Stock and the Aerami Common Stock, results obtained from third-party valuations and additional factors that were deemed relevant including an interpolation to the estimated fair value of the Business Combination with FoxWayne. The estimated fair value of a share of Aerami Common Stock used to determine the fair value of the common stock liability warrants was based on the sale of Aerami Common Stock in September 2021. We are a private company and lack company-specific historical and implied volatility information of our stock. Therefore, we estimated the expected stock volatility based on the historical volatility of publicly traded peer companies for a term equal to the remaining contractual term of the warrants at the time. The risk-free interest rate was determined by reference to the U.S. Treasury yield curve for time periods approximately equal to the remaining contractual term of the warrants. Expected dividend yield was determined based on the fact that we had never paid cash dividends and did not expect to pay any cash dividends in the foreseeable future.
| -175- |
License revenue
We account for revenue in accordance with Accounting Standards Codification (ASC) Topic 606, Revenue from Contracts with Customers. We perform the following five steps to recognize revenue under ASC Topic 606: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. We only recognize revenue when it is probable that we will collect the consideration to which we are entitled in exchange for the goods or services that will be transferred to the customer.
The Chance License Agreement provides for exclusive licensed rights to certain intellectual property, a non-refundable up-front payment, potential milestone payment(s) and potential royalty payment(s).
We evaluated the agreements under ASC 606 and determined that there were two promises: (i) an exclusive license to develop and commercialize AER-901 in the Territory and (ii) manufacture and supply of certain amounts of AER-901. The Company determined that these two promises represent distinct performance obligations for purposes of recognizing revenue and recognized $1.5 million as license revenue during the nine months ended September 30, 2021. At the time of the license signing, the Company evaluated the option to enter into the Supply Agreement and concluded no material right existed at such time.
Research and development expenses
Research and development expenses consist primarily of costs incurred in connection with development and regulatory approval of our lead product candidates. We expense research and development costs as incurred.
At the end of each reporting period, we compare payments made to third-party service providers to the estimated progress toward completion of the applicable research or development objectives. Such estimates are subject to change as additional information becomes available. Depending on the timing of payments to the service providers and the progress that we estimate has been made as a result of the service provided, we may record net prepaid or accrued expenses relating to these costs. To date, we have not made any material adjustments to our prior estimates of accrued research and development expenses.
Recent accounting pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 2 to our audited consolidated financial statements and Note 2 to our unaudited consolidated financial statements appearing elsewhere in this proxy statement/prospectus.
Qualitative and quantitative disclosures about market risk
We are exposed to market risk related to changes in interest rates. As of September 30, 2021, we had cash and cash equivalents of $14.4 million. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. Due to the low interest rates available on cash investments, we believe an immediate 10% change in interest rates would not have a material effect on our operating results until maturity, and therefore, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investment portfolio.
We are not currently exposed to significant market risk related to changes in foreign currency exchange rates; however, we do contract with vendors that are located outside of the United States and may be subject to fluctuations in foreign currency rates. We may enter into additional contracts with vendors located outside the United States in the future, which may increase our foreign currency exchange risk.
JOBS Act
Following the Business Combination, we will qualify as an “emerging growth company” as defined in the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies, including reduced disclosure about our executive compensation arrangements, exemption from the requirements to hold non-binding advisory votes on executive compensation and golden parachute payments and exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting.
We may take advantage of these exemptions until December 31, 2026 or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company earlier if we have more than $1.07 billion in annual revenue, we have more than $700.0 million in market value of our stock held by non-affiliates (and we have been a public company for at least 12 months and have filed one annual report on Form 10-K) or we issue more than $1.0 billion of non-convertible debt securities over a three-year period. For so long as we remain an emerging growth company, we are permitted, and intend, to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. We may choose to take advantage of some, but not all, of the available exemptions.
| -176- |
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We do not expect to “opt out” of such extended transition period.
We are also a smaller reporting company as defined in the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as our voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter, or our annual revenue is less than $100.0 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
INFORMATION ABOUT AERAMI
In this section, unless otherwise stated or the context requires otherwise, the terms “Aerami,” “we,” “us” and “our” refer to Aerami Therapeutics Holdings, Inc. and its subsidiaries prior to the Closing, which will be the business of the Combined Company and its subsidiaries following the Closing.
Overview
We are a clinical stage biopharmaceutical company dedicated to developing differentiated inhaled therapies for the treatment of severe respiratory and other chronic diseases where delivery of therapeutic agents via inhalation can provide an important treatment option. We aim to address markets with high unmet needs where we believe inhaled therapies can offer meaningful differentiation over current standards of care and improve patient experience and outcomes. We are developing multiple drug-device combination products which consist of inhalable formulations of drugs and biologics that are currently approved for the same or different indications combined with either our own proprietary inhaler or commercially available inhalers.
Our lead development program, AER-901, is an inhaled formulation of imatinib delivered via the FOX® high performing handheld nebulizer which we are developing for the treatment of pulmonary arterial hypertension (“PAH”). AER-901 is in a Phase 1 clinical trial, and in 2022 we are targeting initiating the Phase 2 portion of a planned combined Phase 2/3 clinical trial.
We also have a proprietary inhaler as the delivery device for our two other current programs, both of which address diabetes-related conditions. The AFINA™ inhaler, which is powered by a vibrating mesh technology, delivers approximately 80% of the drug to the lungs, which we believe is 2-4 times higher than currently marketed inhalers – enabling efficient delivery of the dose of drug or biologic in a short amount of time. We believe AFINA™ can be successfully positioned as an alternative to currently marketed inhalers to treat lung diseases and to injectable or oral therapy for the treatment of systemic diseases. We plan to file in 2022 for 510(k) clearance for AFINA™ for use as a general purpose nebulizer in the United States which we believe will be reviewed as a Class 2 device with no clinical data required based on substantial equivalence to several predicate devices such as iNeb AAD, Innospire Go, AireHealth nebulizer, and Microbase Portable Nebulizer.
AER-501 (soft mist inhaled insulin) is a patent protected, preservative-free, high purity liquid formulation of recombinant human insulin delivered via AFINATM. We believe that clinical results across five Phase 1/2 clinical studies demonstrating the delivery of consistent doses of insulin, dose-linearity, and intra- and inter-subject variability similar to injections support advancing AER-501 to an end-of-Phase 2 meeting with the FDA and into global Phase 3 clinical trials for the treatment of T1D and T2D diabetes. AER-601 (soft mist inhaled exenatide, a leading short-acting glucagon-like peptide analog, or GLP-1) is directed at the treatment of postprandial hyperglycemia (“PPG”). We have completed preclinical development and this program is Phase 1-ready.
| -177- |
Due to the significant size of the diabetes market and the need for a large commercial presence, we plan to seek partners for both of these programs at the appropriate time. Until such time and depending on available funding, we plan to continue advancing the clinical development of AER-501 and AER-601.
We plan to evaluate additional opportunities for combining our AFINATM soft mist inhaler with other drugs or biologics in order to grow our own severe respiratory and chronic disease product pipeline. We also plan to seek out-licensing opportunities to combine our AFINATM device with other companies’ proprietary drugs or biologics for which inhalation could provide significant patient benefits. The following table summarizes our current programs:

AER-901 (inhaled imatinib) for the treatment of PAH
PAH is a form of pulmonary hypertension, which is high blood pressure in the lungs caused by a narrowing of the small blood vessels that go to the lungs driven by excessive growth of the cells lining those blood vessels. Imatinib, which targets an underlying cause of PAH by neutralizing uncontrolled growth of arterial smooth muscle cells, demonstrated statistically significant improvement in 6MWD, and multiple secondary hemodynamic endpoints in PAH patients in an international Phase 3 trial conducted by Novartis using an oral formulation but was poorly tolerated due to adverse events and was never approved for the treatment of PAH. In June 2020, we entered into a global license and development agreement with Vectura under which we acquired a worldwide exclusive license to develop and commercialize Vectura’s proprietary FOX® device containing a formulation of the active ingredient imatinib (“AER-901”) for the treatment of PAH (the “Vectura License”). AER-901 is designed to deliver imatinib directly to the diseased tissue in the lungs using the FOX® high performing handheld nebulizer. We believe using the FOX® device, which is 510(k) cleared in the US and has a CE-mark in Europe, enhances the risk/benefit profile of treatment for PAH by enabling the delivery of a lower dose of the molecule precisely targeted deep in the lungs. We are currently dosing subjects in a Phase 1 clinical trial for inhaled imatinib and target initiating activities on the Phase 2 portion of our planned combined Phase 2/3 trial on our inhaled imatinib program in 2022.
AER-501 (soft mist inhaled insulin) for the treatment of T1D and T2D
AER-501 consists of a patent protected, preservative-free, high purity liquid formulation of recombinant human insulin, delivered via AFINATM. We believe that clinical results across five Phase 1/2 clinical studies, demonstrating the delivery of consistent doses of insulin, dose-linearity, and intra- and inter-subject variability similar to injections, we believe support advancing AER-501 to an end-of-Phase 2 meeting with the FDA and into global Phase 3 clinical trials. In a June 2021 Type C meeting we had with the FDA, the FDA confirmed that one Phase 3 Trial in T1D and one in T2D would be required to seek approval in the United States. Injectable insulin therapy is the cornerstone of care for all T1D and a significant percentage of T2D patients, but patient behavior suggests an interest in non-injectable administration, with a significant percentage of T2D patients delaying insulin therapy despite demonstrating poor glycemic control. We believe AFINA™ can be successfully positioned as an alternative to injectable therapy, with AFINA™ targeted to provide enhanced lung delivery and efficiency relative to conventional inhalers.
AER-601 (soft mist inhaled exenatide) for the treatment of postprandial hyperglycemia (“PPG”)
Exenatide and other short-acting glucagon-like peptide analogs (“GLP-1s”) have been clinically demonstrated to significantly reduce PPG associated with T2D when dosed pre-meal, but currently approved GLP-1s are only available in fixed-dose formulations and have been associated with a high rate of gastrointestinal side effects. Designed to be delivered via our proprietary AFINATM soft mist inhaler, AER-601 enables pulsatile delivery of a GLP-1 agonist, facilitating a lower and titratable dose, which we believe can offer a meaningful competitive advantage over currently available GLP-1 products. We have completed preclinical development and are seeking a partner to progress to Phase 1 human clinical trials.
| -178- |
AFINATM inhaler

Our AFINATM inhaler uses smart technology designed to ensure precise delivery of the drug through the lungs for local or systemic therapeutic effect. It is breath actuated with a visual indicator that lights up with constant green to indicate optimal breathing, or flashes if the patient inhales too fast or slow. It is Bluetooth-enabled with real time data collection capabilities.
The AFINA™ inhaler is powered by a vibrating mesh technology. The design of the inhaler allows the aerosol flow to be laminar, instead of turbulent, so more particles are delivered to the lungs rather than to the back of the throat. As a result, there is little to no coughing associated with inhalation of the gentle mist. The AFINA™ inhaler delivers approximately 80% of the drug to the lungs, which we believe is 2-4 times higher than conventional inhalers – enabling efficient delivery of the dose of drug in a short amount of time. AFINA’s functionality has been demonstrated through the development of AER-501, where it demonstrated rapid onset of activity, linear dose response and intra and inter-subject variability similar to injections. We believe AFINA™ can be successfully positioned as an alternative to conventional inhalers to treat lung diseases and to injectable or oral therapy for the treatment of systemic diseases. We plan to pursue a 510(k) clearance for use as a general purpose nebulizer in the United States in 2022. We also plan to pursue a CE-mark in Europe.
Our Strategy
Our goal is to become a leading biopharmaceutical company focused on the treatment of severe respiratory and other chronic diseases with significant unmet needs where delivery of therapeutic agents via inhalation can provide an important treatment option. The key elements of our strategy to achieve this goal include:
| ● | Progressing our inhaled imatinib product candidate (AER-901) through clinical development, regulatory approval and commercialization; | |
● |
Seeking strategic partners to advance our product candidates to treat diabetes, including progressing AER-501 into Phase 3 registration studies and AER-601 into the clinic; | |
| ● | Submitting our AFINA™ inhaler for a 510 (k) clearance by the FDA and a CE-mark in Europe as a general purpose nebulizer and developing a second generation device with an increased chamber size to allow for larger volumes to be dispensed and other modifications to increase the range of therapeutics the device could deliver; | |
| ● | Continuing to expand our internal pipeline via in-licensing early stage product candidates that target severe respiratory or other diseases with unmet needs where we believe applying inhalation technology can improve the efficacy, safety and patient experience of current therapies; and | |
| ● | Pursuing strategic collaborations or business combinations to expand the applications for our technology, including collaborations with biopharmaceutical and digital health companies. |
Our Development Programs
Lead Product Candidate: AER-901 – Inhaled Imatinib for Pulmonary Arterial Hypertension (PAH)
AER-901, inhaled imatinib, for the treatment of PAH is our lead product candidate. PAH is a form of pulmonary hypertension, which is high blood pressure in the lungs caused by a narrowing of the small blood vessels that go to the lungs driven by excessive growth of the cells lining those blood vessels. When the blood vessels become too narrow or blocked, a strain is put on the heart requiring it to work harder, often leading to limited physical activity, heart failure and death. PAH is a rare, progressive disease affecting more than 100,000 people in major markets and carries a median survival of five to seven years depending on the type of PAH involved.
| -179- |
PAH is classified into four functional classes, ranging from least severe (Class I) to most severe (Class IV). Treatment is focused on patients in classes II to IV and due to the progressive nature of PAH.
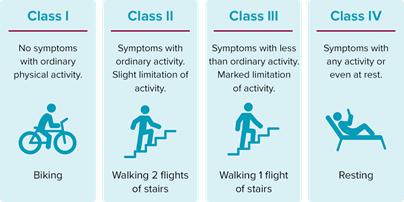
While physicians currently have many treatment options for patients with PAH, we are not aware of any disease modifying therapies available on the market as of the date hereof that effectively address the major underlying cause of PAH. Current PAH treatments such as prostacyclin analogs, endothelin receptor antagonists (“ERAs”), and drugs that target the nitric oxide pathway are used to help blood to flow freely through the blood vessels in the lung, but primarily offer symptomatic relief and only result in a modest improvement in mortality. The pathology of the disease demonstrates that the major abnormality is a progressive remodeling of the small blood vessels in the lungs driven by excessive growth in the cells lining those vessels. We are not aware of any disease modifying therapies that effectively address this major underlying cause of PAH that are currently available on the market. While the exact prevalence of PAH worldwide is not known, the prevalence has been estimated to range from 10 to 52 cases per million in the United States and Europe. In recent years, the global PAH market has grown to be an estimated $5 billion market and is projected to expand to $7.7 billion by 2028, assuming an anticipated compound annual growth rate of 4.9%. We believe the increase in the global PAH market is largely due to increasing incidence and improved physician awareness and diagnosis of the disease.
While advances in the treatment of PAH using vasodilatory agents over the last two decades have markedly improved median survival, PAH patients still face significant disease burden and premature death. The five-year survival rate for newly diagnosed and prevalent patients is between 61% and 65%, respectively.
The current standard of care for PAH is a regimen of two or more treatments, including prostanoids available in oral form or other products using different routes of administration. If approved, we believe that physicians may choose to use AER-901 prior to or in combination with any of these existing front-line agents, as well as oral PDE5 inhibitors or a sGC stimulator, which target the nitric oxide pathway, or oral ERAs. Due to the unmet need for a product that addresses the underlying disease, there are a number of therapies in early stage development of imatinib for use in treatment of PAH. We are aware of another company working on an inhaled version of imatinib for treatment of PAH, Aerovate Therapeutics, Inc. (“Aerovate”), which has completed a Phase 1 clinical trial of its product and initiated a Phase 2b/3 trial in December 2021. In addition, we believe that Tenax Therapeutics, Inc. (“Tenax”) is developing a delayed release oral formulation of imatinib for treatment of PAH. Despite the existence of competitors, we believe that AER-901 offers significant advantages due to the delivery of imatinib via a high performing handheld nebulizer that is currently used with another PAH treatment on the market in Europe.
Our vision is to establish AER-901 as the preferred add-on treatment to standard-of-care for PAH patients in classes II or III or those with slight or marked limitation of physical activity. We believe there is a large unmet need for new treatments that target the underlying pathophysiology of PAH, particularly a disease modifying drug that can address the progressive remodeling of pulmonary blood vessels caused by increased proliferation of cells in the blood vessels of the lung. Platelet derived growth factor and c-KIT signaling have been shown to be important in blood vessel proliferation and overgrowth in PAH and have provided potential targets for new treatments. Imatinib is a potent inhibitor of the platelet-derived growth factor receptor (“PDGFR”), a type of receptor tyrosine kinase, which is strongly upregulated in the small pulmonary arteries and causes pulmonary vascular remodeling. We believe imatinib as a potentially disease modifying agent with a novel mechanism targeting PDGFR would be highly complementary to the traditional therapies currently on the market targeting vasoconstriction. Since 2003, PDGFR antagonists have been thought to have therapeutic potential for PAH. Several preclinical animal studies have been conducted providing evidence that imatinib may prevent pulmonary blood vessel remodeling, pulmonary hypertension and right ventricle hypertrophy induced by chronic hypoxia.
| -180- |
In addition to the underlying pathologic rationale demonstrated by the preclinical models, the efficacy of imatinib as an add-on therapy for patients with PAH has been demonstrated in a large scale clinical trial. In a Phase 3 clinical trial, IMPRES, conducted by Novartis International AG, oral imatinib met its primary efficacy endpoint with a statistically significant improvement in exercise capacity, as measured by 6MWD, and demonstrated improved pulmonary hemodynamics among PAH patients who had failed two or three prior therapies.
Primary Endpoint: Change in Six Minute Walk Distance
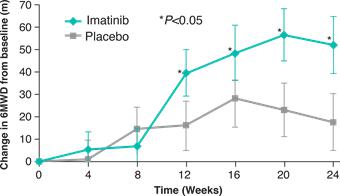
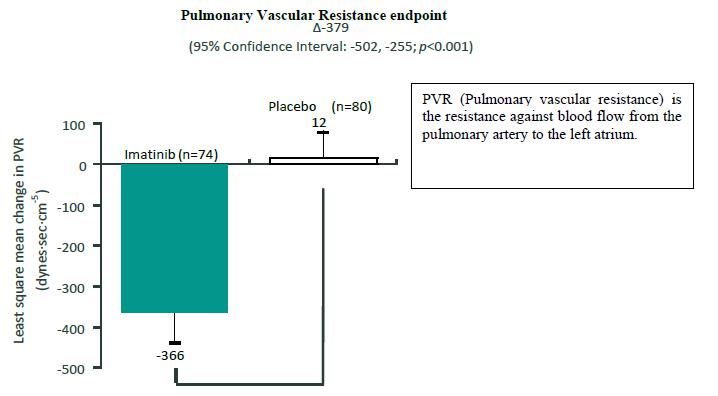
| -181- |
A high dropout rate due to elevated adverse events and an unexpectedly high rate of subdural hematoma in patients receiving oral imatinib and anticoagulation with vitamin K antagonists resulted in Novartis abandoning the development of oral imatinib for PAH.
We believe delivery of imatinib via inhalation represents an opportunity to achieve an enhanced risk-benefit profile over oral imatinib. Inhaled imatinib can enable the delivery of a lower dose of the molecule, with efficacy to be provided by more precisely targeting the deep lungs, to improve the side-effect profile while maximizing efficacy and convenience for the patient. Our planned development program leverages the combination of a liquid formulation of imatinib delivered via the FOX® nebulizer device that is used commercially with another approved PAH product in markets in Europe.
We are currently conducting a Phase 1 clinical trial of AER-901 in up to 72 healthy volunteers. The AER-901 Phase 1 clinical trial is a Single Ascending Dose (“SAD”) escalation study followed by a Multiple Ascending Dose (“MAD”) study. There is an optional crossover component of the study. In the SAD portion of the study, up to five cohorts are planned with doses ranging from 5mg to 80mg. Each cohort consists of eight healthy volunteers, six who are given inhaled imatinib delivered by the FOX® nebulizer and two subjects receiving placebo. Subjects and clinicians are blinded. A sentinel group of one active and one placebo subject is used in each dose escalation. A safety review committee reviews the safety data and provides approval for escalation. The MAD portion consists of up to four cohorts with doses determined by the SAD portion. The first MAD group may begin concurrently with the SAD, with the limitation that the first MAD dose can be no greater than 25% of the highest dose tested to date in the SAD. The MAD also consists of eight subjects (six active: two placebo) in each group. The MAD dosing is conducted over seven days with once daily dosing, though the dose may be changed to twice a day at our and principal investigator’s discretion. Subjects are confined to the Phase 1 unit for the dosing period and four days after dosing is complete. The optional crossover cohort compares a single dose of oral imatinib to a single dose of AER-901. Seven days of washout are utilized prior to crossing over from one route of administration to the other. Six subjects are planned to be dosed in this portion of the study. The purpose of the trial is to establish safety and tolerability of AER-901 and, in the optional cohort, to demonstrate that systemic levels of AER-901 were lower than oral imatinib.
Our clinical strategy for AER-901 is to complete a Phase 1 program to provide the pharmacokinetics data necessary for progressing into registration studies that would include a planned combined Phase 2/3 trial in 2022. We believe that this condensed pathway is possible due to inhaled imatinib’s potential as a disease modifying therapy, which could result in the product candidate being granted breakthrough status by the FDA. We plan to meet with the FDA following submission of our completed Phase 1 trial findings. We cannot provide assurance that AER-901 will receive breakthrough status or qualify for a 505(b)(2) regulatory pathway; however, we plan to pursue this pathway for AER-901, and we intend to rely upon the FDA’s previous findings of safety and efficacy for GLEEVEC® (imatinib mesylate) tablets. Even if the FDA allows us to rely on the 505(b)(2) regulatory pathway, we cannot assure you that such marketing approval will be obtained in a timely manner, or at all. In the event the FDA determines that AER-901 does not qualify for a 505(b)(2) regulatory pathway, we would need to reconsider our plans, and the development pathway could be elongated by the requirement for a separate Phase 2 and 3 trial, which could jeopardize our ability to commercialize such product candidate in a cost-efficient manner, or at all.
In July 2021, the FDA granted us orphan disease designation for imatinib for the treatment of PAH in the U.S. We plan to apply for the equivalent designation in Europe once we have our Phase 1 data. The FDA’s Office of Orphan Products Development grants orphan drug designation to a drug intended to treat a rare disease or condition, generally a disease or condition with either a patient population that affects fewer than 200,000 individuals in the United States or a patient population greater than 200,000 individuals in the United States where there is no reasonable expectation that the cost of developing and making available the drug will be recovered from sales of the drug in the United States. Orphan drug designation provides certain benefits, including the potential for a seven-year market exclusivity period upon regulatory approval, exemption from FDA application fees and tax credits for qualified clinical trials; however, an orphan drug designation does not necessarily lead to a faster development process or expedite the regulatory review and does not increase the likelihood that a product candidate will receive approval from the FDA.
We have entered into the Vectura License to develop and commercialize inhaled imatinib delivered via the FOX® device for the treatment of PAH. The FOX® device is a small, handheld, breath-activated, battery-powered inhalation system that delivers nebulized liquid drugs with high performance using a vibrating mesh technology. The device is CE-marked and has 510(k) clearance. FOX® is suitable for the delivery of small molecules and biologics, formulated as solutions or nano-suspensions. A version of the FOX® device was launched by Bayer in several EU and non-EU countries as Breelib® for the delivery of Ventavis® (iloprost) nebulization solution for the treatment of adult patients with primary pulmonary hypertension. We are responsible for the overall development and commercialization of inhaled imatinib, with Vectura providing a combination of its development services expertise and a license to its FOX® device. In return for the license, we will be obligated to pay Vectura certain regulatory and net sales-based milestones, a mid-single digit royalty on global net sales and will purchase FOX® devices from Vectura.
| -182- |
In January 2021 we entered into an exclusive license and development agreement with Chance to develop and commercialize AER-901 for the treatment of PAH in mainland China, Hong Kong, Macau and Taiwan. Under the agreement Chance will be responsible for the overall development and commercialization of AER-901 for PAH in the applicable territory.
We have received an upfront license fee from Chance and will receive development milestone payments up until market authorization of AER-901 in China. We will supply the product to Chance at an agreed transfer price and receive a high single digit royalty on net sales and sales milestone payments. Chance will be responsible for conducting and financing local clinical trials required to obtain market authorization in China and for the costs of commercialization in those countries for which they have rights.
AFINATM Inhaler
Our proprietary AFINATM inhaler is designed with smart technology to optimize the precise delivery of therapeutics to the lungs in seconds. The AFINATM inhaler is breath actuated with a visual indicator that lights up with constant green to indicate optimal breathing, or flashes if the patient inhales too fast or slow – designed to ensure precise delivery of the drug through the lungs for local or systemic therapeutic effect. It is Bluetooth-enabled with real time data collection capabilities.
The AFINA™ inhaler is powered by a vibrating mesh technology. It has a palladium-nickel coated mesh with thousands of tiny funnel-like holes. When the mesh vibrates 128,000 times per second, the liquid drug formulation gets pushed through the funnel, turning it into a fine mist of tiny droplets which are an appropriate size to breathe in.

The design of the inhaler allows the aerosol flow to be laminar, instead of turbulent, so more particles are delivered to the lungs rather than to the back of the throat. As a result, there is minimal to no coughing associated with inhalation of the gentle mist. The AFINA™ inhaler delivers approximately 80% of the drug to the lungs which we believe is 2-4 times higher than conventional inhalers – enabling efficient delivery of the right dose of drug in a short amount of time. These results are based on silico modeling with CFD and in vitro testing of device components assessed using an Alberta idealized adult airway to optimize mouthpiece and aerosol path design for dose delivered distal to the trachea, In conducting this study, human factors use testing was designed to determine the ability to perform inspiratory maneuvers with LED guidance within target flow limits. In vivo testing with healthy normal subjects of radiolabeled aerosol compared two breathing patterns for lung deposition efficiency, distribution and subject preference.
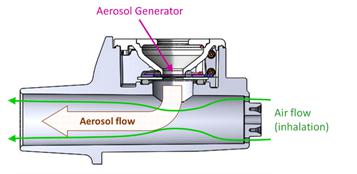
| -183- |
The vibrating mesh within the AFINA™ inhaler, which we license from PEUK, has been shown to be compatible with a wide range of small molecule drugs as well as multiple biologics of different molecular weights and particle size, with no changes to their activity or related impurities, including in our experience with our AER-501 and AER-601 product candidates. There is significant growth in the development of biologics expected to treat respiratory diseases, therefore we believe AFINA™ is well positioned to be a potential delivery option for these future products.
AFINA’s functionality has been demonstrated through the development of AER-501, our soft-mist inhaled insulin product candidate, where it demonstrated rapid onset of activity, linear dose response, and intra and inter-subject variability similar to injections. AFINATM is protected by a robust intellectual property portfolio with four U.S. patents and seven pending patent applications for the device, as well as an exclusive license from PEUK to its patent rights (9 U.S. patents) relating to AFINA’s vibrating mesh technology for use with insulin and GLP-1s. We intend to pursue additional rights from PEUK for use with other product formulations and indications for our own future in-licensed product candidates and collaborations or supply agreements with partners, although there can be no assurance we will be able to secure such rights in a timely manner or at all. We believe AFINA™ can be successfully positioned as an alternative to conventional inhalers to treat lung diseases and to injectable or oral therapy for the treatment of systemic diseases. We plan to pursue a 510(k) clearance in the United States for AFINA™ for use as a general purpose nebulizer in 2022. We believe that the product will be reviewed as a Class 2 device with no clinical data required based on substantial equivalence to several predicate devices such as iNeb AAD, Innospire Go, AireHealth nebulizer, and Microbase Portable Nebulizer, which we plan to confirm in our submission. We also plan to pursue a CE-mark in Europe.
AER-501 – Soft Mist Inhaled Insulin for T1D and T2D
Diabetes has continued to grow to pandemic levels across the world despite the availability of multiple new therapies and insulin devices to treat T1D and T2D. In its 2019 report, the International Diabetes Federation (“IDF”) estimated that approximately 463 million people worldwide suffer from diabetes. The IDF further estimated that global health expenditure attributed to the treatment of patients with diabetes was $760 billion in 2019, or 10% of global health expenditure. T2D accounts for 87% to 91% of all diabetes in high-income countries. According to the IDF, the number of patients with diabetes is estimated to grow to approximately 700 million by 2045. For most patients with diabetes, the disease leads to serious medical complications.
AER-501 is a biologic / device combination product designed to deliver a high purity liquid insulin formulation through our proprietary AFINATM soft mist inhaler to reduce or eliminate the need for daily insulin injections for T1D and T2D patients. We believe there is high unmet need for an alternative insulin therapy in T1D and T2D resulting from patient aversion to injections, with one study showing that 73% of patients with T2D indicating a reluctance to initiate insulin therapy and another study showing that 25% of patients would refuse insulin despite their physician’s recommendation. We believe there is potential for AER-501 to improve compliance and adherence, which represents a significant market opportunity for patients in the United States, Europe and China who have failed multiple oral adjunctive therapies.
We believe our clinical results to date for AER-501, which include five completed Phase 1/2 clinical studies, support progressing the product into Phase 3 development in the US, China and Europe. In these studies, AER-501 demonstrated the delivery of consistent doses of insulin, with faster onset than the injected comparator insulins, as well as dose linearity and, intra- and inter-subject variability similar to injections.
In a June 2021 Type C meeting we had with the FDA, the FDA confirmed that one Phase 3 Trial in T1D and one in T2D would be required to seek regulatory approval in the US. We intend to confirm the requirements for regulatory approval in other regions of the world as we develop our Phase 3 trial program.
AER-501 Phase 1/2 Clinical Studies
Aerami has completed five pharmacokinetic-pharmacodynamic (“PK/PD”) studies, consisting of three studies in subjects with T1D and two studies in subjects with T2D. In all five studies, the pharmacokinetics (“PK”) and pharmacodynamics (“PD”) of the AER-501 insulin inhalation solution were compared to subcutaneous rapid-acting insulin to determine the relative biopotency, efficiency and dose response of AER-501 (see Table 1 below). The relative biopotency and efficiency, and the dose response, were established with AER-501 insulin inhalation solution manufactured from rh-insulin sourced from Sanofi and formulated with HCL and NaOH. Two dosage strengths, 300 Units (“U”)/mL and 900 U/mL, were tested in the studies, with the solutions administered using the AFINA™ (previously referred to as Adagio-01) clinical inhaler.
In response to the FDA’s September 26, 2014 Type C written correspondence, Aerami completed three additional PK/PD studies: (1) a PK/PD dose response study in T1D patients (Samba-03); (2) a separate PK/PD dose response study in T2D patients with doses up to 48 inhaled units (“IU”)/dose (Samba-04); and (3) a variability single dose study at 12 IU/dose in T1D patients. All three PK/PD studies investigated the dose response of AER-501 with the (1) commercial rh-insulin supplier (Tonghua Dongbao Pharmaceutical, Co., Ltd.); (2) expected commercial insulin inhalation solution formulation containing 6mM sodium citrate; and (3) two strengths (280 U/mL and 840 U/mL) in the dosage form dispenser, administered with the AFINA™ device.
Table 1 Inhaled AER-501 vs Injected Humalog: 5 pharmacokinetic/pharmacodynamic studies
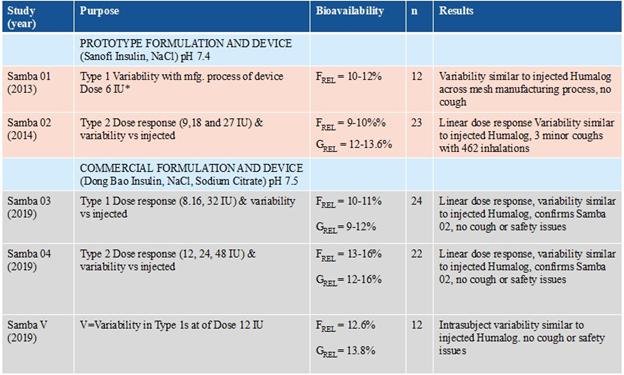
* 1 Inhaled Unit (IU) of Aer 501 = ~7.7 Units of Injected Humalog based on the relative bioavailability (FREL) ) of inhaled of ~13%)
To compare intra- and inter-subject variability of inhaled versus injected insulin, an additional PK/PD study (Samba V for variability) was successfully completed in T1D patients. The results of Samba 03, 04 and V each were positive and confirmed the relative potency, dose equivalence and variability of AER-501 compared with injected Humalog® (Table 1). In response to the FDA’s request, Aerami attempted to conduct a study in asthma/COPD patients (Samba A/C). Despite intensive efforts and substantial costs, subjects could not be recruited.
In our Phase 2a Samba V trial, we studied the inter- and intra-subject variability (i.e., what is happening in the same patient and how it compares to different patients) of inhaled human insulin with subcutaneously injected insulin (Humalog®, insulin lispro). Based on the results of this trial, AER-501 shows similar intra-subject variability as subcutaneously administered Humalog®. The inter-subject variability was higher with AER-501 than with Humalog®. AER-501 administered with the AFINA™ inhaler was safe and well tolerated in subjects with T1D in the study. There was no clinically important difference in the PK response (i.e., the drug absorption, distribution, metabolism and excretion) between the two treatments with AER-501 compared with Humalog®. There was no difference in the PD response (i.e., the biochemical and physiologic effects) between AER-501 and Humalog®. The data from this trial support investigating AER-501 human insulin inhalation solution using the AFINA™ inhaler in a larger population of subjects with T1D.
Faster Onset Compared to Humalog Injection
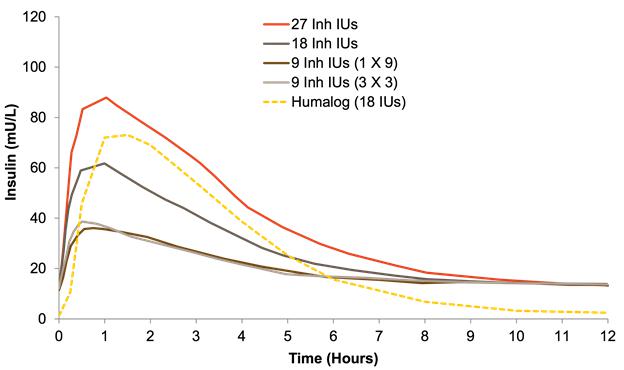
| ● | Aerami PK curves do not return to baseline because of endogenous human insulin. | |
| Humalog is not naturally present in human blood so curve returns to baseline |
The table above shows the mean baseline-adjusted insulin profiles after inhalation of 92.2 IU AER-501 INH and subcutaneous administration of 12 U Humalog® in linear scale.
We are pursuing strategic partners to develop and commercialize AER-501 in key markets. In return for the license(s) to AER-501, Aerami would seek upfront payments, certain regulatory and net sales-based milestone payments, a royalty on global sales and a supply agreement for the AFINA™ device. We have previously entered into a joint venture with Dongbao for the purpose of (i) completing clinical trials in China for AER-501, (ii) gaining NMPA approval for AER-501 and (iii) commercializing AER-501 in China. However, as of the date hereof, the joint venture has not been formed or funded and has never commenced operations. We may seek to progress the development of AER-501 by partnering with Dongbao and/or other potential third parties.
| -184- |
AER-601 – Soft Mist Inhaled Exenatide (GLP-1) for T2D
AER-601 is our proprietary inhalable formulation of exenatide, a fast acting GLP-1 analog delivered via our AFINATM inhaler for treatment of postprandial (i.e., after eating) hyperglycemia associated with T2D. Advances in diabetes treatment over the past 30 years have yielded substantial benefits for T2D patients but have largely addressed the management of basal glucose levels, while significant treatment gaps remain for postprandial hyperglycemia, or elevated blood glucose after meals. Recently, considerable attention has been focused on PPG since it has been shown to be an independent cardiovascular risk factor and it appears to be the rate limiting factor for achieving optimal glycemic control in T2D patients. Several studies have shown that PPG accounts for two thirds of the hyperglycemic burden in T2D patients who are using basal (long acting) insulin. PPG is defined as a plasma glucose level exceeding 140mg/dL following a meal. HbA1c is the current metric of choice for assessing the efficacy of new diabetes medicine, however diabetes treatment guidelines have recently added time in range as a critical metric to determine glycemic control due to HbA1c’s limitation in identifying hypoglycemia and PPG excursions. We believe the adoption of new diagnostic tools like continuous glucose monitors and apps that track daily patterns and optimize therapy and management of the disease are likely to increase the use of therapies to control PPG. This increase in awareness and measurement of PPG has created an interest in new therapies that target treating this component of glycemic control in T2D patients.
We believe AER-601, an inhaled formulation of exenatide, which is a synthetic version of a protein called exendin-4 from the Gila monster (lizard), delivered via our AFINATM inhaler has the potential to be a first-in-class inhalable GLP-1 analog that can provide meaningful benefits for T2D patients over currently available GLP-1s. In addition to offering patient convenience and potentially improved compliance over injectable GLP-1 analogs, we believe this delivery mechanism would facilitate a lower and titratable dose which in turn allows for flexibility in patients’ mealtimes and portion sizes as well as a lower incidence of nausea and other adverse gastrointestinal side effects.
GLP-1 analogs, also known as GLP-1 agonists, act as a replacement for the GLP-1 hormone, which stimulates insulin secretion, reduces glucagon secretion, slows gastric emptying and decreases appetite. As an adjunctive therapy to insulin, short acting GLP-1 analogs have been shown to be as effective in managing PPG as adding prandial insulin, with additional benefits including weight loss (as opposed to the unfavorable effect of weight gain with prandial insulin) and a decrease in cardiometabolic risk factors. Systematic reviews and meta-analyses of clinical studies demonstrate that GLP-1 receptor agonists added to basal insulin decrease postprandial glucose levels, HbA1c levels, body weight, and basal insulin requirements without increasing the risk of major hypoglycemic events. Injectable GLP’s have had significant uptake due to the benefit of weight loss and less risk of hypoglycemia versus insulin treatment and we believe that AER-601 could also prove to be effective in promoting weight loss.
Randomized studies that evaluate the effect of GLP-1 receptor agonists combined with basal insulin confirm these findings. One study found that the addition of injectable exenatide to basal insulin led to a reduction in HbA1c that was significantly greater than with placebo (−1.74 vs −1.04%; p < 0.001), with a lower increase in the dose of insulin required. We completed a seven day and twenty eight day toxicology program in two animal species in 2019 and the first quarter of 2020 that provides the data necessary to progress to a targeted first-in-human trial that could begin as soon as the second half of 2022. Data from these toxicology programs revealed no adverse findings up to the highest dose tested in both mice and monkey specimens. In mice, there were no treatment-related findings at exposures greater than 800 times that associated with 10ug BID Beta (No Observed Adverse Effect Level (“NOAEL”) = 9 mg/kg/day). The NOAEL denotes the level of exposure of an organism, found by experiment or observation, at which there is no biologically or statistically significant increase in the frequency or severity of any adverse effects of the tested protocol. In drug development, the NOAEL of a new drug is assessed in laboratory animals prior to initiation of human trials in order to establish a safe clinical starting dose in humans. In monkeys, there were no treatment-related findings at exposures approximately 100 times that associated with 10ug BID Beta (NOAEL = 1 mg/kg/day).
The inhalation administration of AER-601 (formally Dance 601) for 7 consecutive days to monkeys at doses up to 1.271 mg/kg/day was well tolerated with no AER-601-related mortalities or toxicologically significant effects. Based on the parameters examined in this study, the NOAEL was considered to be at the mean achieved inhaled dose level of 1.271 mg/kg/day for the monkeys when dosed for 7 consecutive days by inhalation for 15 minutes per day.
AER-601 was also administered to mice and observations made in all treated mice on Day 1 and Day 28 following inhalation administration. Overall, the Tmax values appeared to be independent of dose. In general, the Cmax values (i.e., the maximum serum concentration that a drug achieves in a specified compartment or test area of the body after the drug has been administered and before the administration of the second dose) appeared to increase in a less than dose proportional to dose proportional manner on both study days. The AUClast values (i.e., the area under the curve from dosing to the time of the last measured concentration) increased in a greater than dose proportional manner on both study days. AER-601 plasma exposure (as assessed by Cmax and AUClast values) generally increased following multiple administrations of AER-601 relative to single administrations. There appeared to be no differences in exposure between the sexes. No anti-drug antibodies were detected in the animals tested, suggesting no impact on toxicokinetic analysis.
We are seeking to license AER-601 to a global partner or multiple regional partners who would pursue approval for AER-601. In return for the license(s) to AER-601, Aerami would seek upfront payments, certain regulatory and net sales-based milestone payments, a royalty on global sales and a supply agreement for the AFINA™ device.
Commercial Strategy
We intend to focus our commercial efforts on AER-901, inhaled imatinib for treatment of PAH, in the U.S. market, subject to receipt of regulatory approval. In addition, we are seeking collaborations with established pharmaceutical companies to develop and commercialize AER-901 in foreign markets following applicable regulatory approvals.
| -185- |
For example, in January 2021 we executed an exclusive license and development agreement with Chance to develop and commercialize AER-901 for the treatment of PAH in mainland China, Hong Kong, Macau and Taiwan. Under the agreement Chance is responsible for the overall development and commercialization of AER-901 for PAH in the applicable territory. We have received an upfront license fee and will receive development milestone payments up until market authorization of AER-901 in China. We will supply the product to Chance at an agreed transfer price and receive a high single digit royalty on net sales and sales milestone payments. Chance will be responsible for conducting and financing local clinical trials required to obtain market authorization for AER-901 in China.
We intend to pursue strategic collaborations for AER-501 and AER-601 as a primary commercialization strategy. Currently, we have the commercial rights for AER-501 in all markets outside of MENA (Middle East and Northern Africa). Hikma acquired the commercial rights to AER-501 in MENA from us in 2013. We have previously entered into a joint venture with Dongbao for the purpose of (i) completing clinical trials in China for AER-501, (ii) gaining NMPA approval for AER-501 and (iii) commercializing AER-501 in China. However, as of the date hereof, the joint venture has not been formed or funded and has never commenced operations. We may seek to progress the development of AER-501 by partnering with Dongbao and/or other potential third parties. There can be no assurance that we will be able to reach an agreement with any third party or activate the joint venture with Dongbao with respect to AER-501, to pursue development of these product candidates. We have global commercial rights for AER-601.
Manufacturing
We use third-party manufacturers for the production of our product candidates for preclinical and clinical testing. We intend to continue to rely on third-party contract manufacturers for commercial manufacturing in the event our product candidates receive marketing approval. We believe there are multiple sources for all of the materials required for the manufacture of our product candidates. Our manufacturing strategy enables us to more efficiently direct financial resources to the research, development and commercialization of product candidates rather than diverting resources to internally develop manufacturing facilities. As our product candidates advance through development, we expect to enter into longer-term commercial supply agreements with key suppliers and manufacturers to fulfill and secure our production needs.
We have an agreement with Phillips to supply our AFINATM inhaler device. In connection with the Vectura License, we have an agreement with Vectura for the use and supply of the FOX® device in connection with AER-901.
Intellectual Property
We strive to protect the proprietary technology, inventions and improvements that are commercially important to our business, including seeking, maintaining, and defending patent rights, whether developed internally or licensed from third parties. We also rely on trade secrets and know-how relating to our proprietary technology and product candidates and continuing innovation to develop, strengthen and maintain our proprietary position. We also plan to rely on data exclusivity, marketing exclusivity and patent term extensions when available. Our commercial success will depend in part on our ability to obtain and maintain patent and other proprietary protection for our technology, inventions and improvements; to preserve the confidentiality of our trade secrets; to defend and enforce our proprietary rights, including any patents that we may own in the future; and to operate without infringing on the valid and enforceable patents and other proprietary rights of third parties. Intellectual property rights may not address all potential threats to our competitive advantage.
We have filed patent applications on certain important technological developments that relate to our AFINATM inhaler device and AER-501 and a Patent Cooperation Treaty (“PCT”) patent application on AER-601. We are in the process of nationalizing this PCT application in the United States, Europe, China, and Brazil. We have sought intellectual property protection in the United States and, where applicable, have sought or plan to seek intellectual property protection in selected other jurisdictions for significant inventions. For instance, for inventions related to the AFINATM inhaler device, we have granted patents or pending applications in the following foreign jurisdictions: Australia, Brazil, Canada, China, Germany, France, U.K., Spain, Italy, Hong Kong, India and Korea. We also have a pending PCT application. For inventions related to AER-501, we have granted patents or pending applications in the following foreign jurisdictions: Australia, Brazil, Canada, China, Germany, France, U.K., Spain, Italy, Hong Kong, India, Korea, Mexico, and Russia. We have obtained, are seeking, and will continue to seek patent protection on the methods, devices and formulations flowing from our research and development efforts.
| -186- |
The term of individual patents depends upon the legal term for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest filing date of a non-provisional patent application to which the patent claims priority in the applicable country. In the United States, a patent’s term may, in certain cases, be lengthened by patent term adjustment, or PTA, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office, or the USPTO, in examining and granting a patent, or may be shortened if a terminal disclaimer is filed in connection with a patent commonly owned or a patent naming a common inventor and having an earlier expiration date. The Patent Term Restoration Act of 1984, as amended, or the Hatch-Waxman Act, permits a patent term extension, or PTE, of up to five years beyond the expiration date of a U.S. patent as partial compensation for the length of time the drug is under regulatory review while the patent is in force. A PTE cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, and only one patent applicable to each regulatory review period may be extended. Further, only those claims covering the approved drug, a method for using it or a method for manufacturing it may be extended and the extension only applies to the approved drug, method for using it or method for manufacturing it for which the extension was obtained. Similar provisions are available in Europe and certain other foreign jurisdictions to extend the term of a patent that covers an approved drug.
As of February 11, 2022, we are the record owner of fourteen United States patents and eleven United States patent applications. Two of the United States patent applications were filed as PCT applications designating the United States. Below is a summary of some of our patent filings.
Inhalation Devices
Four of the granted United States patents and one of the pending United States applications relate to a single dose inhaler. These all have an expiration date of 2031. The four patents are U.S. 8,950,394, issued on February 10, 2015; 9,004,061 issued on April 14, 2015; 9,545,488 issued on January 17, 2017; and 10,525,214 issued on January 7, 2020. This family of applications has also been filed in multiple foreign jurisdictions. One of these applications was filed in India where an unknown third -party filed a pre-grant opposition to the Indian application. The Indian Patent Office scheduled a hearing to hear arguments regarding the opposition. However, the unknown third -party indicated that for financial reasons they would be unable to attend the hearing and the judge canceled the hearing. Written Submissions in response to the pre-grant hearing notice were filed by us with the Indian Patent Office (IPO) on January 25, 2022. We are awaiting a further communication from the Indian Patent Office as to whether the patent application will be passed onto allowance based on our Written Submissions.
Other United States applications were filed over the past five years and relate to the configuration of inhalers to enhance drug delivery efficiency, among other features.
Finally, we anticipate filing multiple other patent applications to cover several recent innovations relating to the AFINA™ device. We have not yet determined how many patent applications will be filed on these innovations.
Insulin Dispensing
We have filed multiple United States patent applications relating to the dispensing of liquid insulin. One United States application was filed in 2013 (with a claim of priority to a provisional application filed in 2012) and relates to a pump mechanism and methods for metered dose loading of an inhaler. This application was also filed in many foreign jurisdictions. Several patents have now issued in this family.
Another United States patent application was filed in 2014 (with a claim of priority to a provisional application filed in 2013) and relates to liquid dispensers for unit dose dispensing. This application has also been filed in multiple foreign jurisdictions. The original filing has issued as U.S. Patent No. 10,569,033, and a continuation patent application is still pending. We also filed two provisional United States patent applications in 2014 that relate to a self-puncturing liquid drug cartridge. These have since been converted to nonprovisional applications and have been filed in multiple foreign jurisdictions. Patents issuing in this family include U.S. Patent Nos. 10,307,550 and 10,471,222. Continuation applications are still pending.
Insulin Formulations
We have two families of patent applications covering various aspects of our preservative-free formulations. In the first family, we have three granted United States patents. These are U.S. Patent No. 9,180,261 (issued November 10, 2015), U.S. Patent No. 10,076,613 (issued on September 18, 2018), and U.S. Patent No. 10,744,282 (issued on August 18, 2020), each having an expiration date in 2031. The second family of patent applications on our insulin formulation includes U.S. Patent No. 10,842,951 (issued on November 24, 2020) having an expiration date in 2031 and U.S. Patent No. 11,096,990 (issued on August 24, 2021) having an expiration date in 2035. Appropriate foreign filings for each of these formulation applications have also been made. In addition, we have a continuation application pending to cover various aspects of the formulation, including uses for aerosolization and as an injectable.
| -187- |
Exenatide Formulations (AER-601)
In July 2019 we filed a provisional patent application on certain exenatide formulations. In July 2020, we filed a PCT application that claimed priority to this provisional application. We have filed a U.S. application and are in the process of filing appropriate foreign applications based on this PCT application.
Licensed Technology
We have an exclusive sub-license from PEUK (which sub-licenses the patents from Stamford Devices Limited, which in turn licenses the patents from Novartis Pharma AG or was assigned the patents from Novartis) to seven United States patents. The exclusive sub-license is in the field of inhaled insulin and GLP-1 delivery for treatment of diabetes, excluding delivery in an acute care hospital environment. These United States patents will expire between 2023 and 2035. The United States patents cover various aspects of the aerosolization device, including the vibrating mesh (including its manufacture and use), vibration control, fluid feed, and dosage introduction. Many of these have also been filed in foreign jurisdictions.
We also have an exclusive worldwide license from Vectura. The exclusive license is in the field of inhaled imatinib for the treatment of PAH using their proprietary FOX® device. The United States patents we licensed from Vectura expire between 2028 and 2034 and cover various aspects of the inhalation device.
Collaboration and Licensing Agreements
Vectura License
On June 7, 2020, we entered into the Vectura License with Vectura, pursuant to which we obtained from Vectura a worldwide, exclusive license to develop and commercialize Vectura’s proprietary FOX® device containing a formulation of the active ingredient imatinib (the “Product”) for the treatment of PAH. We also received the right of first negotiation to obtain rights for use of the Product in additional indications concerning respiratory and pulmonary diseases.
Under the Vectura License, we are obligated to use commercially reasonable efforts to develop and commercialize the Product and to pay milestone payments for the achievement of certain development, regulatory and sales milestones in amounts up to $161 million in the aggregate. We are also obligated to pay a mid-single digit royalty on sales of the Product. We must obtain supply of Vectura’s FOX® device from Vectura at a transfer price equal to Vectura’s costs of manufacture plus a specified percentage. The Vectura License may be terminated by either party for a material breach if such breach is not cured within sixty days’ notice of such breach of either party. Vectura will also have rights to terminate following certain triggering events.
In addition, on May 25, 2020, we entered into a development agreement with Vectura and made a development payment to Vectura of approximately $1.1 million to begin work to progress inhaled imatinib into Phase 1 human studies as soon as possible after execution of the Vectura License. The license includes provisions for a Device Supply Agreement for us in both development and commercialization of AER-901. We have entered into a clinical supply agreement with Vectura for the FOX® device and at an appropriate time to enter into one or more commercial supply agreements for the FOX® device. We have entered into a similar development agreement with Vectura to provide clinical supply for the Phase 1 clinical trials in China.
Amended and Restated Joint Development Agreement with Phillips-Medisize, LLC
On June 5, 2020, we entered into the Phillips JDA with Phillips, a subsidiary of Molex Ventures, for the continued development of our inhaler device products using the connectivity technology and manufacturing expertise of Phillips and its affiliates. Under the Phillips JDA, we have agreed to work exclusively with Phillips and its affiliates for the term of the agreement, which is initially for a minimum of five (5) years, for the development and manufacturing of our AFINA™ device, and the development of integrated connected health technologies for our AFINA™ device, as well as any other device we may acquire and any device that we may in-license that would require material development or refinement to be usable or commercially feasible for use with our formulations, other than devices in-licensed from Vectura under the Vectura License reflected above. Aerami and Phillips will agree at later date on commercially reasonable terms for manufacturing and supply of any products under the Phillips JDA.
| -188- |
License Agreement with Aerogen/Philips Electronics UK Limited
In November 2010, we entered into a license agreement with Aerogen Limited (“Aerogen”), pursuant to which Aerogen granted us an exclusive worldwide license, with sublicensing rights, to develop and commercialize insulin delivery devices containing Aerogen’s vibrating mesh aerosolization technology. Under this agreement, Aerogen is obligated to use commercially reasonable efforts to collaborate with us to exclusively develop and manufacture related devices, and we are responsible for establishing product requirements for the device. Further, we are responsible for obtaining and maintaining all regulatory approvals for the drug/device combination globally, as well as for all commercialization activities relating to the drug/device combination, whereas Aerogen is obligated to provide regulatory assistance on documents pertaining to the device. A joint development committee was formed to facilitate and oversee the development of the device. In addition, the license agreement provides that technology jointly developed by Aerogen and us relating to the device is owned by Aerogen with an exclusive license to us in the field of insulin.
In December 2014 we entered into a novation agreement with Aerogen and PEUK, in which Aerogen granted its rights under the above license agreement to PEUK (subject to Aerogen retaining certain of the rights and responsibilities with respect to the licensed intellectual property) and PEUK assumed the obligation to perform under the license agreement and be bound by the terms of the license agreement in every way as if a party in place of Aerogen. Additionally, in connection with the novation agreement, we have undertaken to perform under the agreement for the benefit of PEUK. Aerami and Aerogen mutually released and discharged each other from their novated liabilities under the agreement.
We are obligated to fund all device development activities and upon commercialization are obligated to pay PEUK tiered low single-digit royalties on net sales of the product, subject to an annual minimum payment commencing as of the second year of commercial sale, on a country-by-country basis for the longer of the life of certain patents covering the product or 10 years after the first commercial sale of the product in the respective country.
In August 2019, we entered into an addendum with PEUK to the license agreement to extend the scope of the license agreement to include the development and commercialization of GLP-1 solutions formulated for use with delivery devices containing the vibrating mesh aerosolization technology. We paid PEUK a fee of $150,000 upon execution of the addendum, with additional milestone-based payments due upon achievement of certain clinical and regulatory events.
PEUK will supply the device for clinical and commercial purposes based on stated pricing per the agreement under which we will pay PEUK a price equal to PEUK’s cost of the device plus a stated margin, which is subject to future negotiation. The license agreement will remain in force on a country-by-country basis until the later of (i) the expiration of the Aerogen patents covering the device on August 17, 2035 or (ii) 10 years after the first commercial sale of the product. In the case of material breach, the license may be prematurely terminated by either party by providing 90 days written notice to the breaching party and such breaching party has not cured the breach within the 90-day period.
License Agreement with Hikma Pharmaceuticals LLC
In January 2013, we entered into a license, supply and manufacturing agreement with Hikma Pharmaceuticals LLC (“Hikma”), under which we granted Hikma an exclusive license to register and commercialize AER-501 in all disease indications in the Middle East and Africa, excluding Turkey and certain other countries (collectively referred to as the “Hikma Territory”). Under this agreement, we are responsible for the global clinical development of AER-501 in any and all indications and for assembly of the registration materials submitted to the European Medicines Agency (the “EMA”) or FDA for approval. Hikma is responsible for registration and commercialization in the Hikma Territory. Additionally, we granted Hikma the right of first refusal to license new inhaled insulin products in the Hikma Territory, as well as the Palestinian Territories and Turkey, should such regions become available for inclusion in the agreement.
| -189- |
Hikma paid us a non-refundable upfront license fee of $450,000 in January 2013 and a non-refundable milestone of $300,000 in January 2014 upon achievement of a clinical milestone. We are also entitled to receive additional payments totaling $2.0 million if certain commercial and sales-based events are successfully achieved. The agreement has a fifteen-year term from the date of the launch of AER-501 in one of the countries in the Hikma Territory and will automatically renew for an additional twelve-month term prior to the expiration of the then-current term. The agreement may be terminated by either party by providing at least twelve months’ written notice prior to the expiration of the then-current term or for a material breach if such breach is not cured within two months’ notice of such breach, insolvency of either party, or in the event of a change of control.
A timeline for development of AER-501 was accepted by Hikma. Pursuant to the agreement, because AER-501 has not gained approval by the FDA or EMA in the accepted timeframe, Hikma is entitled to receive an additional exclusive license in the Hikma Territory to one other product of ours in development, of Hikma’s choosing, the terms of which will be identical to the current license, excluding the upfront license fee. As of the date hereof, Hikma has not exercised this right. In addition, Hikma also has the right to convert an amount equal to the upfront license fee to shares of our equity on terms equal to our most recent or current private or public financing, which Hikma has indicated its intent to exercise immediately prior to the Effective Time of the Business Combination.
Joint Venture Agreement with Dongbao and Shanghai Dongbao
In October 2017, we entered into a joint venture agreement (the “Dongbao Joint Venture Agreement”) with Dongbao and Shanghai Dongbao pursuant to which a joint venture (the “China JV”) would be formed with the purpose of (i) completing clinical trials in China for AER-501, (ii) gaining NMPA approval for AER-501, and (iii) commercializing AER-501 in China. We were to provide a capital contribution of RMB 46,170,000 for a 45% ownership interest in the China JV. All parties agreed that they would not carry out business competing with the China JV’s business or invest into or act on behalf of a third -party directly or indirectly competing with the China JV’s business. However, as of the date hereof, the China JV has not been formed or funded, and has never commenced operations. We may seek to progress the development of AER-501 by partnering with Dongbao and/or other potential third parties. There can be no assurance that we will be able to reach an agreement to pursue development of AER-501 with any third -party or activate the China JV with Dongbao. If we are not able to reach such an agreement, we do not currently have plans to continue to pursue development of AER-501 independently in China.
License Agreement with Hangzhou Chance Pharmaceuticals Co. Ltd.
In January 2021 we entered into an exclusive license and development agreement with Chance to develop and commercialize AER-901 for the treatment of PAH in mainland China, Hong Kong, Macau and Taiwan. Under the agreement Chance is responsible for the overall development and commercialization of AER-901 for PAH in the applicable territory.
We received an upfront license fee of $1,500,000 and will be entitled to receive development milestone payments up until market authorization of AER-901 in China. We will supply the product to Chance at an agreed transfer price and receive a high single digit royalty on net sales and sales milestone payments. Chance will be responsible for conducting and financing local clinical trials required to obtain market authorization in China and for the costs of commercialization in the countries for which they have rights.
Government Regulation
Government authorities in the United States at the federal, state and local level and in other countries, extensively regulate, among other things, the research, development, testing, manufacture, (including manufacturing changes), quality control, safety, effectiveness, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, sampling, marketing, and export and import of products such as those we are developing. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
| -190- |
U.S. Drug Development Process
In the United States, the FDA regulates drugs and biological products under the FDCA, the Public Health Service Act (the “PHSA”), and the FDA’s implementing regulations.
Failure to comply with the applicable U.S. requirements at any time during the product development process, or approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, imposition of a clinical hold, untitled or warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. The process required by the FDA before a drug or biological product may be marketed in the United States generally involves the following:
| ● | completion of preclinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s Good Laboratory Practice regulations; | |
| ● | submission to the FDA of an investigational new drug (“IND”) application, which must become effective before human clinical trials may begin; | |
| ● | approval by an independent institutional review board (“IRB”) at each clinical site before each trial may be initiated; | |
| ● | performance of adequate and well-controlled human clinical studies according to Good Clinical Practice(“GCP”) regulations, to establish the safety and efficacy of the proposed drug for each indication; | |
| ● | preparation and submission to the FDA of an NDA for a drug product or a BLA for a biological product, containing the results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the drug product, proposed labeling and other relevant information, to request approval to market the drug or biological product; | |
| ● | satisfactory completion of an FDA advisory committee review, if applicable; | |
| ● | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug product is, or components thereof are, produced to assess compliance with current Good Manufacturing Practice (“cGMP”) requirements and to assure that the facilities, methods and controls are adequate to preserve the drug or biologic’s identity, strength, quality and purity; | |
| ● | satisfactory completion of FDA audits of clinical trial sites to assure compliance with GCPs and the integrity of clinical data; | |
| ● | FDA review and approval of the NDA or BLA to permit marketing of the product for particular indications for use in the United States; | |
| ● | payment of fees, including annual program fees for each drug product on the market; and | |
| ● | ongoing compliance with any post-approval requirements, such as risk evaluation and mitigation strategies (“REMS”) and post-approval trials required by the FDA. |
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all.
Preclinical Studies
Once a pharmaceutical or biological product candidate is identified for development, it enters the preclinical testing stage. Preclinical studies include laboratory evaluations of product chemistry, toxicity, formulation and stability, as well as animal studies to assess potential safety and efficacy. When a sponsor wants to proceed to test the product candidate in humans, it must submit an IND application in order to conduct clinical trials.
| -191- |
An IND sponsor must submit the results of the preclinical studies, together with manufacturing information, analytical data and any available clinical data or literature, among other things, to the FDA as part of the IND. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions related to a proposed clinical trial and places the trial on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during clinical trials due to safety concerns or non-compliance and may be imposed on all product candidates within a certain therapeutic class. The FDA also can impose partial clinical holds, for example, prohibiting the initiation of clinical trials of a certain duration or for a certain dose.
Clinical Trials
Clinical trials involve the administration of the IND to human subjects and must be conducted under the supervision of one or more qualified investigators in accordance with GCP regulations. These regulations include the requirement that all research subjects provide informed consent in writing before their participation in any clinical trial. Further, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical study before it commences at that institution, and the IRB must conduct continuing review and reapprove the study at least annually. An IRB considers, among other things, whether the risks to individuals participating in the clinical trial are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the information regarding the clinical trial and the consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed.
Each new clinical protocol and any amendments to the protocol must be submitted for FDA review and to the IRBs for approval. Protocols detail, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria and the parameters to be used to monitor subject safety.
Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on their ClinicalTrials.gov website.
Human clinical trials are typically conducted in three or four sequential phases that may overlap or be combined:
| ● | Phase 1. The product is initially introduced into a small number of healthy human subjects or patients with the target disease and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, especially when the product is suspected or known to be unavoidably toxic, the initial human testing may be conducted in patients. | |
| ● | Phase 2. Involves clinical trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage and schedule. | |
| ● | Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population, generally at geographically dispersed clinical study sites and in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval. These clinical trials are intended to establish the overall risk/benefit relationship of the product and provide an adequate basis for product labeling. | |
| ● | Phase 4. In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved to gain more information about the product. |
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, Phase 2 and Phase 3 testing may not be completed successfully within any specified period, if at all, and favorable results in earlier preclinical studies or clinical trials may not predict the outcomes of subsequent trials. Clinical trials may be delayed for a variety of reasons including unexpected safety or efficacy concerns, slow enrollment of subjects, unexpected shortages in the drug product, or other reasons. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
| -192- |
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the product and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
Assuming successful completion of the required clinical testing in accordance with all applicable regulatory requirements, the results of product development, preclinical studies and clinical trials, along with detailed information relating to the product’s chemistry, the manufacturing process, analytical tests conducted on the product, proposed labeling and other relevant information, are submitted to the FDA under section 505 of the FDCA as part of an NDA for a new drug, or under section 351 of the PHSA as part of a BLA for a biological product, requesting approval to market the product for one or more indications for use. Under the Prescription Drug User Fee Act, or PDUFA, guidelines that are currently in effect, the FDA has a goal of ten months from the date of “filing” of a standard NDA for a new molecular entity or an original BLA to review and act on the submission. This review typically takes twelve months from the date the NDA or BLA is submitted to the FDA because the FDA has approximately two months to make a “filing” decision as to whether it will accept the application for filing. The actual review time may be significantly longer, depending on the complexity of the review, FDA requests for additional information and the sponsor’s submission of additional information.
The submission of an NDA or BLA is subject to the payment of a substantial application user fee, although a waiver of such fee may be obtained under certain limited circumstances. For example, the agency will waive the application fee for the first human drug application, including a biologics license application, that a small business or its affiliate submits for review. The sponsors of certain approved NDAs or BLAs may also be subject to annual program user fees.
In addition, under the Pediatric Research Equity Act of 2003, as amended and reauthorized (“PREA”), an NDA or BLA (or a supplement to an application) for a new active ingredient or new biological substance, new indication, new dosage form, new dosing regimen or new route of administration must contain data that are adequate to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective, unless the applicant has obtained a waiver or deferral.
A sponsor who is planning to submit a marketing application for a drug or biological product that includes a new active ingredient or new biological substance, new indication, new dosage form, new dosing regimen or new route of administration must submit an initial Pediatric Study Plan (“PSP”) within 60 days of an End-of-Phase 2 meeting or as may be agreed between the sponsor and the FDA. The initial PSP must include an outline of the pediatric trial or trials that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric trials along with supporting information. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of data or full or partial waivers. The FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from preclinical studies, early phase clinical trials or other clinical development program.
The FDA also may require submission of a REMS to mitigate any identified or suspected serious risks. The REMS could include medication guides, physician communication plans, assessment plans or elements to assure safe use, such as restricted distribution methods, patient registries or other risk minimization tools.
The FDA conducts a preliminary review of all NDAs and BLAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an application for filing. In this event, the application must be re-submitted with the additional information. The re-submitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review.
| -193- |
The FDA reviews an NDA or BLA to determine, among other things, whether a product is safe and effective for its intended use and whether the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, quality and purity. During its review, the FDA may raise additional issues or request additional data or information. This can delay, sometimes substantially, the FDA’s review and potential approval of an application.
Before approving an NDA or BLA, the FDA often will inspect the facility or facilities where the product is or will be manufactured.
The FDA may refer the NDA or BLA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. An advisory committee is a panel of experts, including clinicians and other scientific experts, who provide advice and recommendations when requested by the FDA. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Additionally, before approving an NDA or BLA, the FDA will typically inspect one or more clinical sites to assure clinical data supporting the submission were developed in compliance with GCP.
The approval process is lengthy and difficult, and the FDA may refuse to approve an NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical data or other data and information. Even if such data and information are submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive, and the FDA may interpret data differently than an applicant interprets the same data.
After evaluating the NDA or BLA and all related information, including the advisory committee’s recommendations, if any, and inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter or a complete response letter to indicate that the review cycle is complete, and that the application is not ready for approval. A complete response letter generally contains a statement of specific conditions that must be met to secure final approval of the application and may require additional clinical or preclinical testing for the FDA to reconsider the application. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the application, addressing all of the deficiencies identified in the letter, or withdraw the application or request an opportunity for a hearing.
Even with submission of additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications.
Even if a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may require post-approval studies, including Phase 4 clinical trials, be conducted to further assess safety and effectiveness after approval and testing and surveillance programs to monitor the safety of approved products that have been commercialized, or impose other conditions, including distribution and use restrictions or other risk management mechanisms under a REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs or if unexpected safety or efficacy concerns arise. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could impact the timeline for regulatory approval or otherwise impact ongoing development programs.
| -194- |
Abbreviated and 505(b)(2) New Drug Applications
Most drug products obtain FDA marketing approval pursuant to an NDA (described above) for innovator products, or an abbreviated new drug application, or ANDA, for generic products. Relevant to ANDAs, the Hatch-Waxman Act amendments to the FDCA established a statutory procedure for submission and FDA review and approval of ANDAs for generic versions of branded drugs previously approved by the FDA (such previously approved drugs are also referred to as listed drugs). Because the safety and efficacy of listed drugs have already been established by the brand company (sometimes referred to as the innovator), the FDA does not require a demonstration of safety and efficacy of generic products. However, a generic manufacturer is typically required to conduct bioequivalence studies of its test product against the listed drug. The bioequivalence studies for orally administered, systemically available drug products assess the rate and extent to which the active pharmaceutical ingredient is absorbed into the bloodstream from the drug product and becomes available at the site of action. Bioequivalence is established when there is an absence of a significant difference in the rate and extent for absorption of the generic product and the listed drug. For some drugs, other means of demonstrating bioequivalence may be required by the FDA, especially where rate and/or extent of absorption are difficult or impossible to measure. In addition to the bioequivalence data, an ANDA must contain patent certifications (indicating whether the applicant is seeking approval prior to the expiration of any patents that the innovator has identified as claiming the approved listed drug product) and chemistry, manufacturing, labeling and stability data.
A third alternative is a special type of NDA, commonly referred to as a 505(b)(2) NDA, which enables the applicant to rely, in part, on the FDA’s findings of safety and efficacy of an existing product, or published literature, in support of its application. 505(b)(2) NDAs often provide an alternate path to FDA approval for new or improved formulations or new uses of previously approved products. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely upon the FDA’s findings with respect to certain preclinical studies or clinical trials conducted for an approved product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the 505(b)(2) applicant.
In seeking approval for a drug through an NDA, including a 505(b)(2) NDA, applicants are required to list with the FDA certain patents of the applicant or that are held by third parties whose claims cover the applicant’s product. Upon approval of an NDA, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, or the Orange Book. Any subsequent applicant who files an ANDA seeking approval of a generic equivalent version of a drug listed in the Orange Book or a 505(b)(2) NDA referencing a drug listed in the Orange Book must make one of the following certifications to the FDA concerning patents: (1) the patent information concerning the reference listed drug product has not been submitted to the FDA; (2) any such patent that was filed has expired; (3) the date on which such patent will expire; or (4) such patent is invalid or will not be infringed upon by the manufacture, use or sale of the drug product for which the application is submitted. This last certification is known as a paragraph IV certification. A notice of the paragraph IV certification must be provided to each owner of the patent that is the subject of the certification and to the holder of the approved NDA to which the ANDA or 505(b)(2) application refers. The applicant may also elect to submit a “section viii” statement certifying that its proposed label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent.
If the reference NDA holder or patent owners assert a patent challenge directed to one of the Orange Book listed patents within 45 days of the receipt of the paragraph IV certification notice, the FDA is prohibited from approving the application until the earlier of 30 months from the receipt of the paragraph IV certification expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the applicant. The ANDA or 505(b)(2) application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the branded reference drug has expired as described in further detail below. Thus approval of a 505(b)(2) NDA or ANDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA or 505(b)(2) applicant.
| -195- |
Biosimilar and Interchangeable Biologics Applications
The Patient Protection and Affordable Care Act, signed into law on March 23, 2010, includes a subtitle called the Biologics Price Competition and Innovation Act of 2009, or BPCIA, which amended the PHSA and created an abbreviated approval pathway for biological products shown to be highly similar to an FDA-licensed reference biological product. An application for licensure of a biosimilar product under section 351(k) of the PHSA must include information demonstrating biosimilarity based upon the following, unless the FDA determines otherwise:
| ● | analytical studies demonstrating that the proposed biosimilar product is highly similar to the approved product notwithstanding minor differences in clinically inactive components; | |
| ● | animal studies (including the assessment of toxicity); and | |
| ● | a clinical study or studies (including the assessment of immunogenicity and pharmacokinetics or pharmacodynamics) sufficient to demonstrate safety, purity and potency in one or more conditions for which the reference biologic product is licensed and intended to be used. |
In addition, an application submitted under the 351(k) pathway must include information demonstrating that:
| ● | the proposed biosimilar product and reference product utilize the same mechanism of action for the condition(s) of use prescribed, recommended, or suggested in the proposed labeling, but only to the extent the mechanism(s) of action are known for the reference product; | |
| ● | the condition or conditions of use prescribed, recommended, or suggested in the labeling for the proposed biosimilar product have been previously approved for the reference product; | |
| ● | the route of administration, the dosage form, and the strength of the proposed biosimilar product are the same as those for the reference product; and | |
| ● | the facility in which the biological product is manufactured, processed, packed and held meets standards designed to assure that the biological product continues to be safe, pure, and potent. |
Biosimilarity, as defined in PHSA 351(i), means that the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components, and that there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product.
In addition, section 351(k)(4) of the PHSA provides for a designation of “interchangeability” between the reference and biosimilar products, whereby the biosimilar may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product. For a product that is administered more than once to an individual, the request for a designation of interchangeability must show that the risk to the patient in terms of safety or diminished efficacy of alternating or switching between the biosimilar and the reference product is no greater than the risk of using the reference product without such alternation or switch.
The submission of an application via the 351(k) pathway does not guarantee that the FDA will accept the application for filing and review, as the FDA may refuse to accept applications that it finds are insufficiently complete. In addition, the FDA may accept an application for filing but deny approval on the basis that the sponsor has not demonstrated biosimilarity, in which case the sponsor may choose to conduct further analytical, preclinical or clinical studies and submit a BLA for licensure as a new biological product under section 351(a) of the PHSA.
Special FDA Expedited Review and Approval Programs
The FDA has various programs, including fast track designation, accelerated approval, priority review and breakthrough therapy designation, which are intended to expedite or simplify the process for the development and FDA review of drugs and biologics that are intended for the treatment of serious or life-threatening diseases or conditions and demonstrate the potential to address unmet medical needs. The purpose of these programs is to provide important new drugs and biologics to patients earlier than under standard FDA review procedures.
To be eligible for a fast track designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address an unmet medical need. The FDA will determine that a product will fill an unmet medical need if it will provide a therapy where none exists or provide a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. The FDA may review sections of the marketing application for a fast track product on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the marketing application, the FDA agrees to accept sections of the marketing application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the marketing application.
| -196- |
The FDA may give a priority review designation to products that offer major advances in treatment or provide a treatment where no adequate therapy exists. A priority review means that the goal for the FDA to review an application is six months, rather than the standard review of ten months under current PDUFA guidelines. Under the new PDUFA agreement, these six and ten-month review periods are measured from the “filing” date rather than the receipt date for NDAs for new molecular entities and BLAs, which typically adds approximately two months to the timeline for review and decision from the date of submission. The review period may be suspended if the FDA requests additional information which may extend the timeline for review. Many products that are eligible for fast track designation are also likely to be considered appropriate to receive a priority review.
In addition, product candidates studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may be eligible for accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require a sponsor of a product receiving accelerated approval to perform post-marketing studies to verify and describe the predicted effect on irreversible morbidity or mortality or other clinical endpoint, and the product may be subject to accelerated withdrawal procedures in certain instances based on these studies.
Moreover, under the provisions of the Food and Drug Administration Safety and Innovation Act (“FDASIA”) passed in July 2012, a sponsor can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a product that is intended, alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Products designated as breakthrough therapies are also eligible for accelerated approval. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Fast track designation, priority review and breakthrough therapy designation do not change the standards for approval and approval is not guaranteed. Such designation may, however, expedite the development or approval process. Even if a product candidate qualifies for one or more of these programs, the FDA may later decide that the product candidate no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. We may explore some of these opportunities for our product candidates as appropriate.
Combination Products
Medical products containing a combination of new drugs, biological products, or medical devices are regulated as “combination products” in the United States. A combination product generally is defined as a product comprised of components from two or more regulatory categories, such as drug/device, device/biologic or drug/biologic. The term combination product includes: (i) a product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic or drug/device/biologic, that are physically, chemically or otherwise combined or mixed and produced as a single entity); (ii) two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, biological and device products or drug and biological products; (iii) a drug, device or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device or biological product where both are required to achieve the intended use, indication or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, such as to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or (iv) any investigational drug, device or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication or effect.
| -197- |
Each constituent part of a combination product is subject to the requirements established by the FDA for that type of constituent part, whether a new drug, biologic or device. In order to facilitate pre-market review of combination products, the FDA designates one of its centers to have primary jurisdiction for the pre-market review and regulation of the overall product based upon a determination by FDA of the primary mode of action of the combination product, and typically one application, such as for a drug/device combination product assigned to the FDA’s Center for Drug Evaluation and Research (“CDER”), an NDA, will be made.
A device with the primary purpose of delivering or aiding in the delivery of a drug and distributed containing a drug (i.e., a “prefilled delivery system”) is typically evaluated by CDER using drug authorities and device authorities, as necessary.
A device with the primary purpose of delivering or aiding in the delivery of a drug and that is distributed without the drug (i.e., unfilled) is typically evaluated by the FDA’s Center for Devices and Radiological Health and CDER, respectively, unless the intended use of the two products, through labeling, creates a combination product.
The FDA has indicated that nebulized and soft mist inhaled products, including all of our product candidates are combination products with a device component. Depending on whether the product being inhaled is a drug or biologic, FDA will consider the combination product to be either a drug/device combination product or a biologic/device combination product. Notably, on March 23, 2020, the FDA transitioned biological products which were approved under NDAs for historical reasons – which included insulin products – to BLAs. Therefore, FDA will consider our inhaled insulin product candidate to be a biologic/device combination product. Although there is an abbreviated pathway under section 351(k) of the PHSA for biologics, FDA did not transition the reference product for AER-501 from an NDA to a BLA because it is currently withdrawn. We will therefore be required to submit an original BLA under section 351(a) of the PHSA for AER-501, which is subject to more extensive requirements and risks than a 505(b)(2) NDA.
Post-Approval Requirements
Drugs and biologics manufactured or distributed pursuant to FDA approvals are subject to extensive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping (including certain electronic record and signature requirements), periodic reporting, product sampling and distribution, advertising and promotion and reporting of certain adverse experiences, deviations and other problems with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing annual product fees for certain marketed prescription drug and biologic products.
The FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed on the market. Products may be promoted only for the approved indications and in accordance with the provisions of the approved label. Further, manufacturers must continue to comply with cGMP requirements, which are extensive and require considerable time, resources and ongoing investment to ensure compliance. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
Manufacturers and certain other entities involved in the manufacturing and distribution of approved products are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. The cGMP requirements apply to all stages of the manufacturing process, including the production, processing, sterilization, packaging, labeling, storage and shipment of the product. Manufacturers must establish validated systems to ensure that products meet specifications and regulatory standards and test each product batch or lot prior to its release. Combination products are subject to FDA regulation to ensure the quality of both the constituent parts and the finished product.
Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance.
| -198- |
The FDA may impose a number of post-approval requirements as a condition of approval of an application. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. The FDA may also limit the indications for use or may impose labeling or other requirements on the product.
The FDA may withdraw product approval if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, problems with manufacturing processes, or failure to comply with regulatory requirements, may result in restrictions on the product or even complete withdrawal of the product from the market.
Potential implications also include required revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; | |
| ● | fines, warning letters or holds on post-approval clinical trials; | |
| ● | refusal of the FDA to approve pending marketing applications or supplements to approved marketing applications; | |
| ● | product seizure or detention, or refusal to permit the import or export of products; or | |
| ● | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, the distribution of prescription drugs is subject to the PDMA, which regulates the distribution of the products and product samples at the federal level and sets minimum standards for the registration and regulation of distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations, guidance and policies are often revised or reinterpreted by the agency in ways that may significantly affect our business and our product candidates. It is impossible to predict whether further legislative or FDA regulation or policy changes will be enacted or implemented and what the impact of such changes, if any, may be.
Patent Term Restoration
Depending upon the timing, duration and specifics of FDA approval of the use of our product candidates, some of our U.S. patents may be eligible for limited patent term extension (“PTE”) under the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to five years as compensation for patent term effectively lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved drug is eligible for the extension. Extensions are not granted as a matter of right and the extension must be applied for prior to expiration of the patent and within a sixty-day period from the date the product is first approved for commercial marketing. The USPTO, in consultation with the FDA, reviews and approves the application for any PTE or restoration. In the future, we may apply for PTEs, defined as the length of the regulatory review of products covered by our granted patents, for some of our currently owned or licensed applications and patents to add patent life beyond their current expiration dates. Such extensions will depend on the length of the regulatory review; however, there can be no assurance that any such extension will be granted to us.
| -199- |
Regulatory Exclusivity
Regulatory exclusivity provisions under the FDCA can also delay the submission or the approval of certain applications. The specific scope varies, but fundamentally the FDCA provides a five-year period of non-patent exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving applications for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Pediatric exclusivity is another type of exclusivity in the United States. Pediatric exclusivity, if granted, provides an additional six months to the term of any existing regulatory exclusivity, including the non-patent exclusivity periods described above, as well as 6-months added to the regulatory exclusivity period associated with any patent that is listed in FDA’s Orange Book for the listed drug product. This six-month exclusivity may be granted based on the voluntary completion of a pediatric clinical study in accordance with an FDA-issued “Written Request” for such a clinical study.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug or biologic intended to treat a rare disease or condition, generally a disease or condition with either a patient population that affects fewer than 200,000 individuals in the United States or a patient population greater than 200,000 individuals in the United States where there is no reasonable expectation that the cost of developing and making available the drug will be recovered from sales of the drug in the United States. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the generic identity of the product and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
The first applicant to receive FDA approval for a particular active ingredient to treat a particular disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the United States for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same product for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of the patients with the disease or condition for which the product was designated. Orphan drug exclusivity does not prevent the FDA from approving a different product for the same disease or condition, or the same product for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan drug designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
| -200- |
Medical Devices
Under the FDCA, the FDA has jurisdiction over medical devices, which include inhalation devices, such as our AFINA™ inhaler and Vectura’s FOX device. The FDA regulates the research, design, development, preclinical and clinical testing, manufacturing, safety, effectiveness, packaging, labeling, storage, recordkeeping, pre-market clearance or approval, adverse event reporting, marketing, promotion, sales, distribution and import and export of medical devices. Unless an exemption applies, each new or significantly modified medical device we seek to commercially distribute in the United States could require either a premarket notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, or approval from the FDA of a premarket approval (“PMA”) application. Both the 510(k) clearance and PMA processes can be resource intensive, expensive, and lengthy and require payment of significant user fees.
Device classification
Under the FDCA, medical devices are classified into one of three classes (Class I, Class II or Class III) depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurances with respect to safety and effectiveness. Class I includes devices with the lowest risk to the patient and are those for which safety and effectiveness can be reasonably assured by adherence to General Controls for Medical Devices, which require compliance with the applicable portions of the FDA’s Quality System Regulation, facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful and non-misleading labeling and promotional materials. While some Class I devices also require premarket clearance by the FDA through the 510(k) premarket notification process described below, most Class I products are exempt from the premarket notification requirements.
Class II devices are those that are subject to the General Controls, as well as Special Controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These Special Controls can include performance standards, patient registries, FDA guidance documents and post-market surveillance. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process.
Class III devices include devices deemed by the FDA to pose the greatest risk, such as life-supporting, life-sustaining devices, or implantable devices, in addition to those deemed novel and not substantially equivalent following the 510(k) process. The safety and effectiveness of Class III devices cannot be reasonably assured solely by the General Controls and Special Controls described above. Therefore, these devices are subject to the PMA process, which is generally more costly and time-consuming than the 510(k) process. Through the PMA process, the applicant must submit data and information demonstrating reasonable assurance of the safety and effectiveness of the device for its intended use to the FDA’s satisfaction. Accordingly, a PMA typically includes, but is not limited to, extensive technical information regarding device design and development, preclinical and clinical trial data, manufacturing information, labeling and financial disclosure information for the clinical investigators in device studies. The PMA application must provide valid scientific evidence that demonstrates to the FDA’s satisfaction a reasonable assurance of the safety and effectiveness of the device for its intended use.
The 510(k) clearance process
Under the 510(k) clearance process, the manufacturer must submit to the FDA a premarket notification, demonstrating that the device is “substantially equivalent” to a legally marketed predicate device. A predicate device is a legally marketed device that is not subject to a PMA, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, a device, that has been reclassified from Class III to Class II or I, or a device that was previously found substantially equivalent through the 510(k) process. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support substantial equivalence.
| -201- |
After a 510(k) premarket notification is submitted, the FDA determines whether to accept it for substantive review. If it lacks necessary information for substantive review, the FDA will refuse to accept the 510(k) premarket notification. If it is accepted for filing, the FDA begins a substantive review. By statute, the FDA is required to complete its review of a 510(k) notification within ninety (90) days of receiving the 510(k) notification. As a practical matter, clearance often takes longer, and clearance is never assured. Although many 510(k) premarket notifications are cleared without clinical data, the FDA may require further information, including data from samples collected in a clinical setting, to make a determination regarding substantial equivalence, which may significantly prolong the review process. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device.
If the FDA determines that the device is not “substantially equivalent” to a predicate device, or if the device is automatically classified into Class III, the device sponsor must then fulfill the much more rigorous premarketing requirements of the PMA approval process or seek reclassification of the device through the de novo classification process. The de novo classification process is an alternate pathway to classify medical devices that are automatically classified into Class III but which are low to moderate risk. A manufacturer can submit a petition for direct de novo review if the manufacturer is unable to identify an appropriate predicate device and the new device or new use of the device presents a moderate or low risk. De novo classification may also be available after receipt of a “not substantially equivalent” letter following submission of a 510(k) to FDA.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA application. The FDA requires each manufacturer to determine whether the proposed change requires a new submission in the first instance, but the FDA can review any such decision and disagree with a manufacturer’s determination. Many minor modifications are accomplished by an internal letter-to-file in which the manufacturer documents its reasoning for why a change does not require premarket submission to the FDA. The letter-to-file is in lieu of submitting a new 510(k) to obtain clearance for such change. The FDA can always review these letters to file in an inspection. If the FDA disagrees with a manufacturer’s determination regarding whether a new premarket submission is required for the modification of an existing 510(k)-cleared device, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or approval of a PMA application is obtained. In addition, in these circumstances, the FDA can impose significant regulatory fines or penalties for failure to submit the requisite application(s).
The PMA approval process
Following receipt of a PMA application, the FDA conducts an administrative review to determine whether the application is sufficiently complete to permit a substantive review. If it is not, the agency will refuse to file the PMA. If it is, the FDA will accept the application for filing and begin the review. The FDA has 180 days to review a filed PMA application, although the review of an application more often occurs over a significantly longer period of time. During this review period, the FDA may request additional information or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant’s response to deficiencies communicated by the FDA.
Before approving or denying a PMA, an FDA advisory committee may review the PMA at a public meeting and provide the FDA with the committee’s recommendation on whether the FDA should approve the submission, approve it with specific conditions, or not approve it. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Prior to approval of a PMA, the FDA may conduct inspections of the clinical trial data and clinical trial sites, as well as inspections of the manufacturing facility and processes. Overall, the FDA review of a PMA application generally takes between one and three years but may take significantly longer. The FDA can delay, limit or deny approval of a PMA application for many reasons, including:
| ● | the device may not be shown safe or effective to the FDA’s satisfaction; | |
| ● | the data from preclinical studies and/or clinical trials may be found unreliable or insufficient to support approval; | |
| ● | the manufacturing process or facilities may not meet applicable requirements; and | |
| ● | changes in FDA clearance or approval policies or adoption of new regulations may require additional data. |
| -202- |
If the FDA evaluation of a PMA is favorable, the FDA will issue either an approval letter, or an approvable letter, the latter of which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been fulfilled to the satisfaction of the FDA, the agency will issue a PMA approval letter authorizing commercial marketing of the device, subject to the conditions of approval and the limitations established in the approval letter. If the FDA’s evaluation of a PMA application or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. The FDA also may determine that additional tests or clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted and data is submitted in an amendment to the PMA, or the PMA is withdrawn and resubmitted when the data are available. The PMA process can be expensive, uncertain and lengthy and a number of devices for which the FDA approval has been sought by other companies have never been approved by the FDA for marketing.
New PMA applications or PMA supplements are required for modification to the manufacturing process, equipment or facility, quality control procedures, sterilization, packaging, expiration date, labeling, device specifications, ingredients, materials or design of a device that has been approved through the PMA process. PMA supplements often require submission of the same type of information as an initial PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the approved PMA application and may or may not require as extensive technical or clinical data or the convening of an advisory panel, depending on the nature of the proposed change.
In approving a PMA application, as a condition of approval, the FDA may also require some form of post- approval study or post-market surveillance, whereby the applicant conducts a follow-up study or follows certain patient groups for a number of years and makes periodic reports to the FDA on the clinical status of those patients when necessary to protect the public health or to provide additional or longer term safety and effectiveness data for the device. The FDA may also approve a PMA application with other post-approval conditions intended to ensure the safety and effectiveness of the device, such as, among other things, restrictions on labeling, promotion, sale, distribution and use. New PMA applications or PMA supplements may also be required for modifications to any approved diagnostic tests, including modifications to manufacturing processes, device labeling and device design, based on the findings of post-approval studies.
The investigational device process
In the United States, absent certain limited exceptions, human clinical trials intended to support medical device clearance or approval require an investigational device exemption, or IDE application. Some types of studies deemed to present “non-significant risk” are deemed to have an approved IDE-without affirmative submission of an IDE application to the FDA-once certain requirements are addressed and IRB approval is obtained. If the device presents a “significant risk” to human health, as defined by the FDA, the sponsor must submit an IDE application to the FDA and obtain IDE approval prior to commencing the human clinical trials. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. Generally, clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the study protocol and informed consent are approved by appropriate IRBs at the clinical trial sites. Submission of an IDE will not necessarily result in the ability to commence clinical trials, and although the FDA’s approval of an IDE allows clinical testing to go forward for a specified number of subjects, it does not bind the FDA to accept the results of the trial as sufficient to prove the product’s safety and efficacy, even if the trial meets its intended success criteria.
Such clinical trials must be conducted in accordance with the FDA’s IDE regulations that govern investigational device labeling, prohibit promotion and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. Clinical trials must further comply with good clinical practice regulations for IRB approval and for informed consent and other human subject protections. Required records and reports are subject to inspection by the FDA for any clinical trials subject to FDA oversight. The results of clinical testing may be unfavorable, or, even if the intended safety and efficacy success criteria are achieved, may not be considered sufficient for the FDA to grant marketing approval or clearance of a product. The commencement or completion of any clinical trial may be delayed or halted, or be inadequate to support approval of a PMA application or clearance of a 510(k) premarket notification, for numerous reasons.
| -203- |
The Breakthrough Devices Program is a voluntary program intended to expedite the review, development, assessment and review of certain medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions for which no approved or cleared treatment exists or that offer significant advantages over existing approved or cleared alternatives. All submissions for devices designated as Breakthrough Devices will receive priority review, meaning that the review of the submission is placed at the top of the appropriate review queue and receives additional review resources, as needed. Although Breakthrough Device designation or access to any other expedited program may expedite the development or approval process, it does not change the standards for approval. Access to an expedited program may also be withdrawn by the FDA if it believes that the designation is no longer supported by data from the clinical development program. Additionally, qualification for any expedited review procedure does not ensure that regulatory clearance or approval for such product will ultimately be obtained.
Post-market regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
| ● | establishment registration and device listing with the FDA; | |
| ● | QSR requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; | |
| ● | labeling regulations and FDA prohibitions against the promotion of investigational products, or the promotion of “off-label” uses of cleared or approved products; | |
| ● | requirements related to promotional activities; | |
| ● | clearance or approval of product modifications to 510(k)-cleared devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of a cleared device, or approval of certain modifications to PMA-approved devices; | |
| ● | medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur; | |
| ● | correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; | |
| ● | the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and | |
| ● | post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
Device manufacturing processes are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. Manufacturers are subject to periodic scheduled or unscheduled inspections by the FDA. A failure to maintain compliance with the QSR requirements could result in the shut-down of, or restrictions on, manufacturing operations and the recall or seizure of products. The discovery of previously unknown problems with products, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its clearance or off-label by a physician in the practice of medicine, could result in restrictions on the device, including the removal of the product from the market or voluntary or mandatory device recalls.
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that a manufacturer has failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, including the following:
| ● | issuance of warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties; | |
| ● | requesting or requiring recalls, withdrawals, or administrative detention or seizure of our products; | |
| ● | imposing operating restrictions or partial suspension or total shutdown of production; | |
| ● | refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products; | |
| ● | withdrawing 510(k) clearances or PMA approvals that have already been granted; | |
| ● | refusal to grant export approvals for our products; or | |
| ● | criminal prosecution. |
| -204- |
Other Health Care Laws
In addition to FDA restrictions on marketing of pharmaceutical products, other U.S. federal and state healthcare regulatory laws restrict business practices in the pharmaceutical industry, which include, but are not limited to, state and federal anti-kickback, false claims, physician payment and drug pricing transparency laws.
The federal Anti-Kickback Statute prohibits, among other things, any person or entity from knowingly and willfully offering, paying, soliciting, receiving or providing any remuneration, directly or indirectly, overtly or covertly, to induce or in return for purchasing, leasing, ordering, or arranging for or recommending the purchase, lease, or order of any item or service reimbursable, in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. There are a number of statutory exceptions and regulatory safe harbors protecting from prosecution some common activities like discounts, or engaging health care professionals as speakers or consultants; however, those exceptions and safe harbors are drawn narrowly, and there is no exception or safe harbor for many common business activities. The majority of states also have anti-kickback laws, which establish similar prohibitions and in some cases may apply to items or services reimbursed by any third-party payor, including commercial insurers.
The federal civil False Claims Act prohibits any person or entity from, among other things, knowingly presenting, or causing to be presented, a false, fictitious or fraudulent claim for payment of government funds or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the government. Claims that include items or services resulting from a violation of the federal Anti-Kickback Statute are deemed false or fraudulent claims for purposes of the federal civil False Claims Act. Actions under the civil False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Violations of the civil False Claims Act can result in very significant monetary penalties and treble damages.
In addition, the civil monetary penalties statute imposes penalties against any person who, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Many states also have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Given the significant size of actual and potential settlements, it is expected that the government authorities will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws.
The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) prohibits, among other actions, knowingly and willfully executing, or attempting to execute, a scheme or artifice to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
| -205- |
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and certain other healthcare providers. The Physician Payments Sunshine Act and its implementing regulations require that certain manufacturers of drugs, devices, biologicals or medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report information related to certain payments and “transfers of value” provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other health care professionals beginning in 2022, and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. In addition, certain states require implementation of compliance programs and compliance with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, impose restrictions on marketing practices, require tracking and reporting of pricing information and marketing expenditure as well as gifts, compensation and other remuneration or items of value provided to physicians and other healthcare professionals and entities.
Violation of any of such laws or any other governmental regulations that may apply to us can result in penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and individual imprisonment.
Pharmaceutical Coverage, Pricing and Reimbursement
Sales of our product candidates, if approved, by us or any potential commercial partners will depend, in part, on the extent to which such products will be covered by third-party payors, such as government healthcare programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly limiting coverage or reducing reimbursements for medical products and services. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Sales of any products for which we receive regulatory approval for commercial sale will therefore depend, in part, on the availability of coverage and adequate reimbursement from third-party payors. Third-party payors include government authorities, managed care plans, private health insurers and other organizations.
The process for determining whether a third-party payor will provide coverage for a drug typically is separate from the process for setting the price of such product or for establishing the reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the FDA-approved products for a particular indication. A decision by a third -party payor not to cover our product candidates could reduce a physician’s willingness to prescribe our products once approved and have a material adverse effect on our sales, results of operations and financial condition. Moreover, a third-party payor’s decision to provide coverage for a drug does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Additionally, coverage and reimbursement for products can differ significantly from payor to payor. One third-party payor’s decision to cover a particular medical product or service does not ensure that other payors will also provide coverage for the medical product or service or will provide coverage at an adequate reimbursement rate.
In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payor to not cover our product candidates could reduce physician usage of our products, once approved, and have a material adverse effect on our sales, results of operations and financial condition. Third-party payors are increasingly challenging the prices charged for medical products and services, examining the medical necessity and reviewing the cost-effectiveness of drugs, in addition to questioning safety and efficacy. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after FDA approval or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit.
Government Price Reporting
Once a product is launched, a manufacturer may participate in, and have certain price reporting obligations under, the Medicaid Drug Rebate Program, state Medicaid supplemental rebate program(s), and other governmental pricing programs. For calendar quarters beginning January 1, 2022, manufacturers will be required to report the average sales price for certain drugs under the Medicare program regardless of whether the manufacturer participates in the Medicaid Drug Rebate Program. Previously, this reporting obligation extended only to manufacturers participating in the Medicaid Drug Rebate Program. Under this Program, manufacturers are required to pay a rebate to each state Medicaid program for covered outpatient drugs that are dispensed to Medicaid beneficiaries and paid for by a state Medicaid program as a condition of having federal funds being made available for their drugs under Medicaid and Part B of the Medicare program.
| -206- |
Medicaid is a joint federal and state program that is administered by the states for low-income and disabled beneficiaries. Medicaid rebates are based on pricing data reported by manufacturers on a monthly and quarterly basis to the Centers for Medicare & Medicaid Services (“CMS”), the federal agency that administers the Medicaid and Medicare programs. These data include the average manufacturer price and, in the case of innovator products, the best price for each drug, which, in general, represents the lowest price available from the manufacturer to any entity in the U.S. in any pricing structure, calculated to include all sales and associated rebates, discounts, and other price concessions. The amount of the rebate is adjusted upward if the average manufacturer price increases more than inflation (measured by reference to the Consumer Price Index - Urban). Currently, the rebate is capped at 100% of the average manufacturer price, but, effective January 1, 2024, this cap on the rebate will be removed, and our rebate liability could increase accordingly.
If a manufacturer becomes aware that its reporting for a prior quarter was incorrect or has changed as a result of recalculating the pricing data, the manufacturer is obligated to resubmit the corrected data for up to three years after those data originally were due, which revisions could affect rebate liability for prior quarters. The federal Patient Protection and Affordable Care Act (“ACA”) made significant changes to the Medicaid Drug Rebate Program, and CMS issued a final regulation, which became effective on April 1, 2016, to implement the changes to the Medicaid Drug Rebate Program under the ACA. On December 21, 2020, CMS issued a final rule that modified Medicaid Drug Rebate Program regulations to permit reporting multiple best price figures with regard to value-based purchasing arrangements (beginning in 2022); provide definitions for “line extension,” “new formulation,” and related terms with the practical effect of expanding the scope of drugs considered to be line extensions (beginning in 2022); and revise best price and average manufacturer price exclusions of manufacturer-sponsored patient benefit programs, particularly regarding potential inapplicability of such exclusions in the context of pharmacy benefit manager “accumulator” programs (beginning in 2023).
Medicare is a federal program that is administered by the federal government that covers individuals age 65 and over or that are disabled as well as those with certain health conditions. Medicare Part B generally covers drugs that must be administered by physicians or other health care practitioners; among others. Medicare Part B generally pays for such drugs under a payment methodology based on the average sales price of the drugs. Manufacturers are required to report average sales price information to CMS on a quarterly basis. The manufacturer-submitted information is used by CMS to calculate Medicare payment rates.
Congress could enact additional changes that affect our overall rebate liability and the information manufacturers report to the government as part of price reporting calculations. For example, Congress is currently considering a Medicare Part B inflation rebate, under which manufacturers would owe additional rebates if the average sales price of a drug were to increase faster than the pace of inflation.
Civil monetary penalties can be applied if a manufacturer is (1) found to have knowingly submitted any false pricing or other information to the government, (2) found to have made a misrepresentation in the reporting of its average sales price, or (3) fails to submit the required data on a timely basis. Such conduct also could be grounds for CMS to terminate a Medicaid Drug Rebate Program agreement, in which case federal payments may not be available under Medicaid or Medicare Part B for the manufacturer’s covered outpatient drugs.
Federal law requires that any company that participates in the Medicaid Drug Rebate Program also participate in the Public Health Service’s 340B drug pricing program (the “340B program”) in order for federal funds to be available for the manufacturer’s drugs under Medicaid and Medicare Part B. The 340B program, which is administered by the Health Resources and Services Administration, or HRSA, requires participating manufacturers to agree to charge statutorily defined covered entities no more than the 340B “ceiling price” for the manufacturer’s covered outpatient drugs. Covered entities include hospitals that serve a disproportionate share of financially needy patients, community health clinics, and other entities that receive certain types of grants under the Public Health Service Act.
The ACA expanded the list of covered entities to include certain free-standing cancer hospitals, critical access hospitals, rural referral centers, and sole community hospitals, but exempts “orphan drugs” from the ceiling price requirements for these covered entities. The 340B ceiling price is calculated using a statutory formula, which is based on the average manufacturer price and Medicaid rebate amount for the covered outpatient drug as calculated under the Medicaid Drug Rebate Program. In general, products subject to Medicaid price reporting and rebate liability are also subject to the 340B ceiling price calculation and discount requirement.
| -207- |
HRSA issued a final regulation regarding the calculation of the 340B ceiling price and the imposition of civil monetary penalties on manufacturers that knowingly and intentionally overcharge covered entities, which became effective on January 1, 2019. It is currently unclear how HRSA will apply its enforcement authority under this regulation. Any charge by HRSA that a manufacturer has violated the requirements of the regulation could result in civil monetary penalties. Moreover, under a final regulation effective January 13, 2021, HRSA established a new administrative dispute resolution (“ADR”) process for claims by covered entities that a manufacturer has engaged in overcharging, and by manufacturers that a covered entity violated the prohibitions against diversion or duplicate discounts. Such claims are to be resolved through an ADR panel of government officials rendering a decision that can be appealed to a federal court. An ADR proceeding could subject a manufacturer to onerous procedural requirements and could result in additional liability. HRSA also implemented a price reporting system under which manufacturers are required to report 340B ceiling prices on a quarterly basis to HRSA, which then publishes those prices to 340B covered entities. In addition, legislation could be passed, that would further expand the 340B program to additional covered entities, or participating manufacturers could be required to agree to provide 340B discounted pricing on drugs used in an inpatient setting.
In order to be eligible to have their products paid for with federal funds under the Medicaid and Medicare Part B programs and purchased by certain federal agencies (VA, Department of Defense (“DoD”), Coast Guard, and Public Health Service (“PHS”)) and grantees, manufacturers must participate in the VA FSS pricing program. Prices for innovator drugs purchased by the VA, DoD, Coast Guard, and PHS are subject to a cap (known as the “Federal Ceiling Price”) equal to 76% of the annual non-federal average manufacturer price (“non-FAMP”) minus, if applicable, an additional discount. The additional discount applies if non-FAMP increases more than inflation (measured by reference to the Consumer Price Index - Urban). Manufacturers must also participate in the Tricare Retail Pharmacy Program, under which they pay quarterly rebates to DoD for prescriptions of innovator drugs dispensed to Tricare beneficiaries through Tricare Retail network pharmacies. The governing statute provides for civil monetary penalties for failure to provide information timely or for knowing submission of false information to the government.
Medicare Part D generally provides coverage to enrolled Medicare patients for self-administered drugs (i.e., drugs that are not administered by a physician). Medicare Part D is administered by private prescription drug plans approved by the U.S. government and, subject to detailed program rules and government oversight, each drug plan establishes its own Medicare Part D formulary for prescription drug coverage and pricing, which the drug plan may modify from time to time. The prescription drug plans negotiate pricing with manufacturers and pharmacies and may condition formulary placement on the availability of manufacturer discounts. In addition, manufacturers are required to provide to CMS a 70% discount on brand name prescription drugs utilized by Medicare Part D beneficiaries when those beneficiaries are in the coverage gap phase of the Part D benefit design. Civil monetary penalties can be applied if a manufacturer fails to provide these discounts in the amount of 125% of the discount that was due. Congress could enact legislation that sunsets this discount program and replaces it with a new manufacturer discount program. Congress further could enact a Medicare Part D inflation rebate, under which manufacturers would owe additional rebates if the average manufacturer price of a drug were to increase faster than the pace of inflation.
Congress also could enact a drug price negotiation program under which the prices for certain high Medicare spend single source drugs would be capped by reference to the non-federal average manufacturer price. This or any other legislative change could impact the market conditions for our products. We further expect continued scrutiny on government price reporting from Congress, agencies, and other bodies.
Group health plans, health insurance issuers, health maintenance organizations, other healthcare payors, and pharmacy benefit managers in the United States are adopting more aggressive utilization management techniques and are increasingly requiring significant discounts and rebates from manufacturers as a condition to including products on formulary with favorable coverage and cost-sharing. These payors may not cover or adequately reimburse for use of our products or may do so at levels that disadvantage them relative to competitive products. Outside the United States, within the EU, our products are paid for by a variety of payors, with governments being the primary source of payment. Government health authorities in the EU determine or influence reimbursement of products and set prices or otherwise regulate pricing. Negotiating prices with governmental authorities can delay commercialization of our products. Governments may use a variety of cost-containment measures to control the cost of products, including price cuts, mandatory rebates, value-based pricing, and reference pricing (i.e., referencing prices in other countries or prices of competitive products and using those reference prices to set a price). Budgetary pressures in many EU countries are continuing to cause governments to consider or implement various cost-containment measures, such as price freezes, increased price cuts and rebates, and expanded generic substitution and patient cost-sharing.
| -208- |
Healthcare Reform
The United States and many foreign jurisdictions have enacted or proposed legislative and regulatory changes affecting the healthcare system that could prevent or delay marketing approval of our product candidates or any future product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell a product for which we obtain marketing approval. Changes in regulations, statutes or the interpretation of existing regulations could impact our business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to product labeling; (iii) the recall or discontinuation of our products; or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended (collectively, the “ACA”) was passed, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly impacted the U.S. pharmaceutical industry. The ACA, among other things, subjected biological products to potential competition by lower-cost biosimilars, addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increases the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care organizations, establishes annual fees and taxes on manufacturers of certain branded prescription drugs, and creates a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 70% (increased pursuant to the Bipartisan Budget Act of 2018, effective as of 2019) point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D.
Certain provisions of the ACA have been subject to judicial challenges as well as efforts to repeal or replace them or to alter their interpretation or implementation. There have been legislative and judicial efforts to repeal, replace, or change some or all of the ACA, including judicial challenges in the Fifth Circuit Court and the United States Supreme Court. While Congress has not passed repeal legislation to date, the Tax Cuts and Jobs Act, or Tax Act, repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Complying with any new legislation or changes in healthcare regulation could be time-intensive and expensive, resulting in a material adverse effect on our business. Additional legislative changes to and regulatory changes under the ACA remain possible, but the nature and extent of such potential additional changes are uncertain at this time. We expect that the ACA, its implementation, efforts to repeal or replace, or invalidate the ACA, or portions thereof, and other healthcare reform measures that may be adopted in the future, could have a material adverse effect on our industry generally and on our ability to maintain or increase sales of existing products or to successfully commercialize product candidates, if approved.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. For example, the American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Other legislative changes include aggregate reductions to Medicare payments to providers of 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013 and will remain in effect through 2030, with the exception of a temporary suspension implemented under various COVID-19 relief legislation from May 1, 2020 through March 31, 2022. On December 10, 2021, President Biden signed a law that provides for 1% Medicare sequestration in the second quarter of 2022 and allows the full 2% sequestration thereafter until 2030. To offset the temporary suspension during the COVID-19 pandemic, in 2030, the sequestration will be 2.25% for the first half of the year, and 3% in the second half of the year.
| -209- |
There has been increasing legislative, regulatory and enforcement interest in the United States with respect to specialty drug pricing practices. For example, there have been several recent U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare and state Medicaid programs, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. As another example, in May 2019, the Centers for Medicare & Medicaid Services (“CMS”) issued a final rule to allow Medicare Advantage Plans the option of using step therapy, a type of prior authorization, for Part B drugs beginning January 1, 2020. This final rule codified CMS’ policy change that was effective January 1, 2019. Further, under the American Rescue Plan Act of 2021, effective January 1, 2024, the statutory cap on Medicaid Drug Rebate Program rebates that manufacturers pay to state Medicaid programs will be eliminated. Elimination of this cap may require pharmaceutical manufacturers to pay more in rebates than it receives on the sale of products, which could have a material impact on our business.
At the state level, individual states are increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional health care authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other health care programs. These measures could reduce the ultimate demand for our products, once approved, or put pressure on our product pricing.
We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates once approved or additional pricing pressures. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our drugs.
Our revenue prospects could be affected by changes in healthcare spending and policy in the United States and abroad. We operate in a highly regulated industry and new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to healthcare availability, the method of delivery or payment for healthcare products and services could negatively impact our business, operations and financial condition.
There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls may adversely affect:
| ● | the demand for our product candidates, if we obtain regulatory approval; | |
| ● | our ability to set a price that we believe is fair for our products; | |
| ● | our ability to obtain coverage and reimbursement approval for a product; | |
| ● | our ability to generate revenue and achieve or maintain profitability; | |
| ● | the level of taxes that we are required to pay; and | |
| ● | the availability of capital. |
Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors, which may adversely affect our future profitability.
| -210- |
Data Privacy and Security
Pharmaceutical companies may be subject to U.S. federal and state and foreign health information privacy, security and data breach notification laws, which may govern the collection, use, disclosure and protection of health-related and other personal information. In the U.S., HIPAA imposes privacy, security and breach reporting obligations upon “covered entities” (health plans, health care clearinghouses and certain health care providers), and their respective business associates, that create, receive, maintain or transmit protected health information in connection with providing a specified service performing a function for or on behalf of a covered entity. HIPAA mandates the reporting of certain breaches of protected health information to HHS, affected individuals and if the breach is large enough, the media. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information, or subject to a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. Even when HIPAA does not apply, according to the Federal Trade Commission (the “FTC”), failing to take appropriate steps to keep consumers’ personal information secure, or failing to provide a level of security commensurate to promises made to individual about the security of their personal information (such as in a privacy notice) may constitute unfair or deceptive acts or practices in violation of Section 5(a) of the Federal Trade Commission Act (the “FTC Act”). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards. The FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule. Enforcement by the FTC under the FTC Act can result in civil penalties or enforcement actions.
In addition, certain state and non-U.S. laws, such as the European Union General Data Protection Regulation (the “GDPR”), govern the privacy and security of personal information, including health-related information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, the California Consumer Privacy Act (the “CCPA”), went into effect January 1, 2020, which, among other things, granted California residents new rights with regard to their personal information and created new transparency requirements and other data privacy obligations for covered businesses and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Although the law includes limited exceptions for health-related information, including for “protected health information” maintained by a covered entity or business associate and personal information collected as part of a clinical trial, it may regulate or impact our processing of personal information depending on the context. Further, in November 2020, California voters approved the California Privacy Rights Act (the “CPRA”) ballot initiative, which introduced significant amendments to the CCPA—including additional individual rights, limitations on data uses, audit requirements for higher-risk data—and established and funded a dedicated California privacy regulator, the California Privacy Protection Agency. The CPRA will impose additional data protection obligations on covered companies that collect personal information from California residents, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. The majority of the provisions will go into effect on January 1, 2023, and additional compliance investment and potential business process changes may be required. In Europe, the GDPR increases our obligations and liability for processing the personal data of individuals located in the European Union. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to 20 million Euros or 4% of the annual global revenues of the noncompliant company, whichever is greater. Relatedly, following the United Kingdom’s withdrawal from the EEA and the European Union, and the expiry of the transition period, companies have to comply with the GDPR and the GDPR as incorporated into United Kingdom national law, the latter regime having the ability to separately fine up to the greater of 17.5 million pounds or 4% of global turnover. Additionally, our use of connected devices increases security risks to personal information, including by increasing the number of third-party device components, broadening the device’s attack surface, and relying on internet connections that we cannot control, and failure to secure personal data may be actionable under these or other local laws. In addition to the threat of unauthorized access or acquisition of sensitive or personal information, other threats include the deployment of harmful malware, ransomware attacks, DoS attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information.
The landscape of laws regulating personal data is constantly evolving, and compliance with these laws requires a flexible privacy framework and substantial resources, and compliance efforts will likely be an increasing and substantial cost in the future.
| -211- |
Foreign Regulation of Drugs
In order to market any product outside of the United States, we will need to comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding development, approval, commercial sales and distribution of our products, and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products, if approved. Whether or not we obtain FDA approval for a product, we must obtain the necessary approvals from the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies among countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, and a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Competition
The pharmaceutical industry is intensely competitive, subject to rapid and significant technological change and places emphasis on the value of proprietary products. While we believe that our technologies and experience provide us with a competitive advantage, our competitors include major multinational pharmaceutical companies, specialty pharmaceutical companies, established biotechnology companies, biopharmaceutical companies, academic research institutions, public and private research institutions and generic drug companies. Many of our competitors have greater financial and other resources than we have, including larger research and development staff and greater commercialization and marketing resources. As a result, these companies may obtain marketing approval more rapidly than we are able and may be more effective in selling and marketing their products. Smaller or early stage companies may also prove to be significant competitors, particularly through collaboration arrangements with large, established companies.
AER-901 (inhaled imatinib)
We intend to develop and seek approval of AER- 901 for the treatment of PAH in patients taking two or more approved PAH therapies. Physicians currently have many treatment options for patients with PAH and the standard of care is a regimen of two or more treatments, including prostanoids available in oral form as Orenitram (United Therapeutics Corporation, or United Therapeutics) and Uptravi (Janssen Pharmaceuticals, Inc., or Janssen). Other products using different routes of administration are also available such as Tyvaso (United Therapeutics), by inhalation and by infusion as Remodulin (United Therapeutics). If approved, we believe that physicians may choose to use AER-901 prior to or in combination with any of these existing front-line agents as well as the oral PDE5 inhibitors, including Revatio (Pfizer) and Adcirca (United Therapeutics); the sGC stimulator Adempas (Bayer AG); and oral ERAs, including Tracleer (Janssen), Letairis (Gilead) and Opsumit (Janssen). We may also face competition from or be used in combination with investigational new drugs with novel mechanisms of action like sotatercept (Acceleron Pharma, Inc.), seralutinib (Gossamer Bio, Inc.), rodatristat (Altavant Sciences, Inc.) and/or PF-06842874 (Pfizer) if they are approved. To our knowledge, Tenax (oral) and Aerovate (inhaled) are also developing imatinib for PAH, and both companies are at a clinical stage of development.
AER-501 (soft mist inhaled insulin) for the treatment of T1D and T2D
Subject to available funding, we intend to continue advancing AER-501 to an end-of-Phase 2 meeting with the FDA and into global Phase 3 clinical trials. Pending results from these trials, we intend to seek approval of AER–501 for the treatment of T1D and T2D. Current treatment for T1D involves insulin injections or the use of an insulin pump, frequent blood sugar checks, and carbohydrate counting. Treatment of T2D primarily involves lifestyle changes, monitoring of blood sugar levels, along with diabetes medications, insulin or both. If approved, we believe that physicians may choose to use AER-501 in combination with or instead of other forms of insulin because of its route of administration (which avoids needles), ease of titration and PK profile. Many types of insulin are available, including short-acting (such as Novolin and Velosulin), rapid-acting insulin (such as Lispro (Humalog), Aspart (Novolog) and Glulisine (Apidra)), long-acting insulin (such as insulin glargine (Basaglar, Lantus, Toujeo) and insulin detemir (Levemir)) and intermediate options (such as NPH(N)). Depending on the needs of the patient, a doctor may prescribe a mixture of insulin types to use throughout the day and night.
As insulin cannot be taken orally to lower blood sugar because stomach enzymes interfere with insulin’s action, insulin is often injected using a fine needle and syringe or an insulin pen. An insulin pump may also be an option. The pump is a device about the size of a small cellphone worn on the outside of your body. A tube connects the reservoir of insulin to a catheter that is inserted under the skin of your abdomen. A tubeless pump that works wirelessly is also now available.
In September 2016, the FDA approved the first artificial pancreas for people with type 1 diabetes who are age 14 and older. A second artificial pancreas was approved in December 2019. Since then, systems have been approved for children older than 2 years old. An artificial pancreas is also called closed-loop insulin delivery. The implanted device links a continuous glucose monitor, which checks blood sugar levels every five minutes, to an insulin pump. The device automatically delivers the correct amount of insulin when the monitor indicates it is needed. There are more artificial pancreas (closed loop) systems currently in clinical trials.
Other oral or injected medications are sometimes prescribed as well. Some diabetes medications stimulate the pancreas to produce and release more insulin. Others inhibit the production and release of glucose from the liver, which means the person needs less insulin to transport sugar into his or her cells. Still others block the action of stomach or intestinal enzymes that break down carbohydrates or make tissues more sensitive to insulin. Metformin (Glumetza, Fortamet, others) is generally the first medication prescribed for T2D.
AER-601 (soft mist inhaled exenatide) for the treatment of PPG
Subject to available funding, we plan to advance AER-601 to Phase 1 trials. Pending results of the requisite trials, we plan to seek approval of AER–601 to improve glycemic control in patients with T2D in combination with metformin or insulin glargine (with or without metformin) when maximally tolerated doses of these products in addition to diet and exercise do not provide adequate glycemic control.
Both basal and postprandial elevations contribute to the hyperglycemic exposure of diabetes, but current therapies are mainly effective in controlling the basal component. Inability to control postprandial (following meals) hyperglycemia limits success in maintaining overall glycemic control beyond the first 5 to 10 years after diagnosis, and it is also related to the weight gain that is common during insulin therapy. The “prandial problem” comprising abnormalities of glucose and other metabolites, weight gain, and risk of hypoglycemia. Several approaches to prandial abnormalities have recently been studied, but the patient populations for which they are best suited and the best ways of using them remain incompletely defined. Encouragingly, several proof-of-concept studies suggest that short-acting glucagon- like peptide 1 agonists (GLP1) can be very effective in controlling postprandial hyperglycemia. We anticipate that AER 601, if approved, will compete with several GLP1 products which are now available to be used in combination with a long acting insulin to improve glycemic control including RYBELSUS® (Novo Nordisk), Trulicity® (Lilly) and Soliqua® (Sanofi), which are administered via an injection, and Ozempic® (Novo Nordisk), which is given orally.
Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. Our competitors may succeed in developing, acquiring or licensing, on an exclusive basis, technologies and drug products that are more effective or less costly than our product candidates, which could render our product candidates obsolete and non-competitive. We expect any products that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price and the availability of reimbursement from government and other third-party payors. We also expect to face competition in our efforts in recruiting and retaining qualified personnel and establishing clinical trial sites and patient enrollment in clinical trials.
Employees
As of February 11, 2022, we had four full-time employees. None of our employees are represented by labor unions or covered by collective bargaining agreements. We consider the relationship with our employees to be good.
Facilities
Our facilities consist of an office space of approximately 100 square feet in Durham, North Carolina under a lease that expires on October 31, 2022 and office space of approximately 150 square feet in Deerfield, Illinois under a lease which expires in June, 2022. We believe that our current facilities are sufficient for our current needs.
Legal Proceedings
We are not currently subject to any material legal proceedings.
| -212- |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF FOXWAYNE
In this section, unless otherwise stated or the context requires otherwise, the terms “FoxWayne,” “we,” “us” and “our” refer to FoxWayne prior to the Closing.
The following discussion and analysis should be read in conjunction with the financial statements and related notes of FoxWayne included elsewhere in this proxy statement/prospectus. This discussion includes forward-looking statements reflecting our current expectations, estimates and assumptions concerning events and financial trends that may affect our future operating results or financial position. Actual results and the timing of events may differ materially from those included in these forward-looking statements due to a number of factors, including those discussed in the sections titled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements.”
Overview
We are a blank check company incorporated in Delaware on September 17, 2020. We were formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. We are an emerging growth company and, as such, we are subject to all of the risks associated with emerging growth companies.
Our sponsor is FoxWayne Enterprises Acquisition Sponsor LLC, a Delaware limited liability company. The registration statement for our initial public offering was declared effective on January 19, 2021. On January 22, 2021, we consummated our initial public offering of 5,750,000 units, including 750,000 additional units to cover over-allotments, at $10.00 per unit, generating gross proceeds of $57.5 million, and incurring offering costs of approximately $4.2 million, of which approximately $2.0 million was for deferred underwriting commissions.
Simultaneously with the closing of the initial public offering, we consummated the private placement of 2,800,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant to the Sponsor, generating proceeds of $2.8 million.
Upon the closing of the initial public offering and the private placement, approximately $58.1 million ($10.10 per unit) of the net proceeds of the initial public offering and certain of the proceeds of the private placement was placed in the Trust Account located in the United States with Continental Stock Transfer & Trust Company acting as Trustee, and invested only in U.S. government treasury bills with a maturity of 185 days or less or in money market funds investing solely in U.S. Treasuries and meeting certain conditions under Rule 2a-7 under the Investment Company Act of 1940, as amended (the “Investment Company Act”), as determined by us, until the earlier of: (i) the completion of a business combination and (ii) the distribution of the Trust Account as described below.
Our management has broad discretion with respect to the specific application of the net proceeds of the initial public offering and the sale of the Private Placement Warrants, although substantially all of the net proceeds are intended to be applied generally toward consummating a business combination. There is no assurance that we will be able to complete a business combination successfully. We must complete one or more Initial Business Combinations having an aggregate fair market value of at least 80% of the net assets held in the Trust Account (excluding the deferred underwriting commissions and taxes payable) at the time of the agreement to enter into the Initial Business Combination. However, we will only complete a business combination if the post-business combination company owns or acquires 50% or more of the voting securities of the target or otherwise acquires a controlling interest in the target sufficient for it not to be required to register as an investment company under the Investment Company Act.
If we are unable to complete a business combination within 12 months from the closing of the initial public offering, or April 22, 2022, (or up to 18 months from the consummation of the initial public offering, or July 22, 2022, if we extend the period of time to consummate a business combination) (the “Combination Period”), we will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account including interest earned on the funds held in the Trust Account and not previously released to us to pay our taxes (less up to $50,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the remaining stockholders and the board of directors, dissolve and liquidate, subject in each case to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law.
| -213- |
Liquidity and Capital Resources
As of September 30, 2021, we had approximately $25,000 in cash and working capital deficit of approximately $353,000. As of December 31, 2020, we had approximately $3,000 in cash and a working capital deficiency of approximately $131,000 (not taking into account tax obligations of approximately $1,000 that may be paid using investment income earned in Trust Account).
On January 22, 2021, we consummated the initial public offering of 5,750,000 units, generating gross proceeds of $57,500,000. Simultaneously with the closing of the initial public offering, we consummated the sale of 2,800,000 Private Placement Warrants, at a purchase price of $1.00 per Private Placement Warrant, generating gross proceeds of approximately $2,800,000. Following the closing of the initial public offering, $58,075,000 of the net proceeds of the sale of the units and the Private Placement Warrants were placed in the Trust Account maintained by Continental Stock Transfer & Trust Company, acting as Trustee.
Our liquidity needs prior to the consummation of the initial public offering were satisfied through the payment of $25,000 from the Sponsor to purchase 1,437,500 shares of our Class B Common Stock and loan proceeds from the Robb Knie, our Chief Executive Officer, Chief Financial Officer and director, pursuant to a promissory note (“Note”). We repaid $1,615 of the outstanding Note balance on December 31, 2020 and repaid the remaining amount of $40,510 in full on January 26, 2021. Subsequent to the consummation of the Initial Public Offering, our liquidity has been satisfied through the net proceeds from the consummation of the initial public offering and the private placement held outside of the Trust Account and a promissory note from our Chief Executive Officer. In addition, in order to finance transaction costs in connection with a business combination, the Sponsor or an affiliate of the Sponsor, or certain of our officers and directors may, but are not obligated to, provide us with funds as may be required (“Working Capital Loans”). As of September 30, 2021, and December 31, 2020, there were no amounts outstanding under any Working Capital Loans.
On September 21, 2021, Robb Knie, our Chief Executive Officer, Chief Financial Officer and director loaned $100,000 to us. The loan was evidenced by a promissory note (“Promissory Note”) which is non-interest bearing, non-convertible, and payable upon the consummation of our initial merger, share exchange, asset acquisition or other similar business combination with one or more businesses or entities. If an initial merger, share exchange, asset acquisition or other similar business combination is not consummated, the Promissory Note will not be repaid by us, and all amounts owed thereunder by us will be forgiven except to the extent that we have funds available to pay it outside of our Trust Account. As of September 30, 2021, there was approximately $48,000 outstanding under the Promissory Note.
In connection with our assessment of going concern considerations in accordance with Financial Accounting Standard Board’s (“FASB’s”) Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the mandatory liquidation and subsequent dissolution raises substantial doubt about our ability to continue as a going concern, and management has determined it may be probable that we would be unable to meet our obligations as they become due within one year raising substantial doubt about our ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities should we be required to liquidate after April 22, 2022.
Management continues to evaluate the impact of the COVID-19 pandemic and has concluded that the specific impact is not readily determinable as of the date of the unaudited condensed financial statements. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Results of Operations
Our entire activity since inception up to September 30, 2021, has been in preparation for our formation and the initial public offering, and since the initial public offering, our search for a prospective target for a business combination. We will not be generating any operating revenues until, at the earliest, the closing and completion of our Initial Business Combination.
| -214- |
For the three months ended September 30, 2021, we had net income of approximately $1.1 million, which consisted of change in fair value of derivative liabilities of $1.5 million, income from investment held in the Trust Account of approximately $1,000, partially offset by general and administrative expenses of approximately $347,000, general and administrative expenses to related party of $30,000 and franchise tax expense of approximately $43,000.
For the nine months ended September 30, 2021, we had net loss of approximately $670,000, which consisted of general and administrative expenses of approximately $728,000, general and administrative expenses to related party of $90,000, franchise tax expense of approximately $128,000, financing costs to derivative warrant liabilities of approximately $212,000, partially offset by change in fair value of derivative liabilities of $485,000 and income from investment held in the Trust Account of approximately $4,000.
Related Party Transactions
Founder Shares
On October 15, 2020, our Sponsor purchased 1,437,500 shares of our Class B Common Stock, for an aggregate price of $25,000. In October 2020, our Sponsor transferred 25,000 Founder Shares to each of Messrs. Reavey, Pavell, Zippin and Agrawal and 180,000 Founder Shares to certain other initial stockholders. The per share purchase price of the Founder Shares was determined by dividing the amount of cash contributed to us by the aggregate number of Founder Shares issued. The initial stockholders agreed to forfeit up to 187,500 Founder Shares to the extent that the over-allotment option was not exercised in full by the underwriters, so that the Founder Shares would represent 20.0% of our issued and outstanding shares after the initial public offering (excluding the Representative’s Shares (as defined herein)). The underwriter exercised its over-allotment option in full on January 22, 2021; thus, these 187,500 Founder Shares are no longer subject to forfeiture.
The initial stockholders agreed, subject to limited exceptions, not to transfer, assign or sell (i) with respect to 50% of Founder Shares, for a period ending on the six-month anniversary of the date of the consummation of the Initial Business Combination and (ii) with respect to the remaining 50% of such shares, for a period ending on the one-year anniversary of the date of the consummation of the Initial Business Combination, or earlier, in either case, if, subsequent to the Initial Business Combination, we complete a liquidation, merger, capital stock exchange or other similar transaction that results in all of the public stockholders having the right to exchange their shares of common stock for cash, securities or other property.
Private Placement Warrants
Simultaneously with the closing of the initial public offering, we consummated the private placement of 2,800,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant to our Sponsor, generating proceeds of $2.8 million.
Each whole Private Placement Warrant is exercisable for one whole share of Class A Common Stock at a price of $11.50 per share. A portion of the proceeds from the sale of the Private Placement Warrants to our Sponsor was added to the proceeds from the initial public offering held in the Trust Account. If we do not complete a business combination within the Combination Period, the Private Placement Warrants will expire worthless. The Private Placement Warrants will be non-redeemable for cash and exercisable on a cashless basis so long as they are held by our Sponsor or its permitted transferees.
Our Sponsor and our officers and directors agreed, subject to limited exceptions, not to transfer, assign or sell any of their Private Placement Warrants until 30 days after the completion of the Initial Business Combination.
Related Party Loans
On September 30, 2020, Robb Knie, our Chief Executive Officer, Chief Financial Officer and director, agreed to loan us an aggregate of up to $150,000 pursuant to a promissory note. This loan was non-interest bearing and payable upon the completion of the initial public offering. We borrowed $42,125 under the note. We repaid $1,615 of the outstanding note balance on December 31, 2020 and repaid the remaining amount of $40,510 in full on January 26, 2021.
| -215- |
In addition, in order to finance transaction costs in connection with a business combination, our Sponsor or an affiliate of our Sponsor, or certain of our officers and directors may, but are not obligated to, provide Working Capital Loans. If we complete a business combination, we would repay the Working Capital Loans out of the proceeds of the Trust Account released to us. Otherwise, the Working Capital Loans would be repaid only out of funds held outside the Trust Account. Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. The Working Capital Loans would either be repaid upon consummation of a business combination or, at the lenders’ discretion, up to $1.5 million of such Working Capital Loans may be convertible into warrants of the post business combination entity at a price of $1.00 per warrant. The warrants would be identical to the Private Placement Warrants. As of December 31, 2020, we had no borrowings under the Working Capital Loans.
We may extend the period of time to consummate a business combination up to two times, each by an additional three months (for a total of 18 months to complete a business combination). In order to extend the time available for us to consummate a business combination, our Sponsor or our affiliates or designees must deposit into the Trust Account $143,750 ($0.10 per public share), on or prior to the date of the applicable deadline, for each three-month extension (the “Extension Loans”). Any such payments would be made in the form of a non-interest bearing, unsecured promissory note. Such notes would either be paid upon consummation of a business combination, or, at the relevant insiders’ discretion, converted upon consummation of a business combination into additional Private Placement Warrants at a price of $1.00 per Private Placement Warrant. Our Sponsor and our affiliates or designees are not obligated to fund the Trust Account to extend the time for us to complete a business combination.
Administrative Services Agreement
Commencing on the date that our securities were first listed on Nasdaq through the earlier of consummation of the Initial Business Combination and our liquidation, we agreed to pay our Sponsor a total of $10,000 per month for office space, utilities and secretarial and administrative services.
Our officers or directors will be reimbursed for any out-of-pocket expenses incurred in connection with activities on our behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. The audit committee will review on a quarterly basis all payments that were made to our Sponsor, officers, directors or their affiliates and will determine which expenses and the amount of expenses that will be reimbursed. There is no cap or ceiling on the reimbursement of out-of-pocket expenses incurred by such persons in connection with activities on our behalf.
Contractual Obligations
Registration and Stockholder Rights
The holders of Founder Shares, Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans or Extension Loans, if any, (and any shares of Class A Common Stock issuable upon the exercise of the Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans and upon conversion of the Founder Shares) are entitled to registration rights pursuant to a registration rights agreement signed upon the consummation of the initial public offering. The holders of these securities are entitled to make up to three demands, excluding short form demands, that we register such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to the completion of the Initial Business Combination. We will bear the expenses incurred in connection with the filing of any such registration statements.
| -216- |
Underwriting Agreement
We granted the underwriters a 45-day option from the date of initial public offering to purchase up to 750,000 additional units to cover over-allotments, if any, at the initial public offering price less the underwriting discounts and commissions. The underwriter exercised its over-allotment option in full on January 22, 2021.
The underwriters were entitled to an underwriting discount of $0.20 per unit, or approximately $1.2 million in the aggregate, paid upon the closing of the initial public offering. In addition, the underwriters will be entitled to a deferred fee of $0.35 per unit, or approximately $2.0 million in the aggregate. The deferred fee will become payable to the underwriters from the amounts held in the Trust Account solely in the event that we complete a business combination, subject to the terms of the underwriting agreement.
We issued EF Hutton (formerly Kingswood Capital Markets), a division of Benchmark Investments, Inc. (“EF Hutton”), the representative of the underwriters, and/or its designees, 50,000 shares of Class A Common Stock (the “Representative’s Shares”) upon the consummation of the initial public offering. EF Hutton agreed not to transfer, assign or sell any such shares until the completion of the Initial Business Combination. In addition, EF Hutton agreed (i) to waive its redemption rights with respect to such shares in connection with the completion of the Initial Business Combination and (ii) to waive its rights to liquidating distributions from the Trust Account with respect to such shares if we fail to complete our Initial Business Combination within the Combination Period. We recorded the fair value of the 50,000 Representative’s Shares, $500,000, charged as an offering cost to stockholders’ equity (deficit).
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic on the industry and has concluded that while it is reasonably possible that the virus could have a negative effect on our Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of the unaudited condensed financial statements. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Critical Accounting Policies
Derivative Warrant Liabilities
We do not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. We evaluate all of our financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to Accounting Standards Codification (“ASC”) 480 and FASB ASC Topic 815, “Derivatives and Hedging” (“ASC 815”). The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
The public warrants issued in connection with the initial public offering and the Private Placement Warrants are recognized as derivative liabilities in accordance with ASC 815. Accordingly, we recognize the warrant instruments as liabilities at fair value and adjusts the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in our unaudited condensed statement of operations. The fair value of the public warrants issued in connection with the initial public offering and the Private Placement Warrants were initially measured at fair value using a Monte Carlo simulation model and subsequently, the fair value of the Private Placement Warrants have been estimated using a Monte Carlo simulation model each measurement date. The fair value of public warrants issued in connection with the initial public offering have subsequently been measured based on the listed market price of such warrants. The determination of the fair value of the warrant liability may be subject to change as more current information becomes available and accordingly the actual results could differ significantly. Derivative warrant liabilities are classified as non-current liabilities as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
Offering Costs Associated with the Initial Public Offering
Offering costs consisted of legal, accounting, underwriting fees and other costs incurred through the initial public offering that were directly related to the initial public offering. Offering costs are allocated to the separable financial instruments issued in the initial public offering based on a relative fair value basis, compared to total proceeds received. Offering costs associated with warrant liabilities are expensed as incurred, presented as non-operating expenses in the statement of operations. Offering costs associated with the Class A Common Stock are charged against their carrying value upon the completion of the initial public offering. For the nine months ended September 30, 2021, of the total offering costs of the initial public offering, approximately $213,000 is included in financing cost - derivative warrant liabilities in the unaudited condensed statement of operations and approximately $4.0 million was charged against the carrying value of the Class A Common Stock subject to possible redemption. We classify deferred underwriting commissions as non-current liabilities since their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
| -217- |
Class A Common Stock Subject to Possible Redemption
We account for our Class A Common Stock subject to possible redemption in accordance with the guidance in ASC 480. Class A Common Stock subject to mandatory redemption (if any) is classified as a liability instrument and is measured at fair value. Conditionally redeemable Class A Common Stock (including shares of Class A Common Stock that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within our control) is classified as temporary equity. At all other times, Class A Common Stock is classified as stockholders’ equity. Our Class A Common Stock features certain redemption rights that are considered to be outside of our control and subject to occurrence of uncertain future events. Accordingly, as of September 30, 2021, 5,750,000 shares of Class A Common Stock subject to possible redemption at the redemption amount were presented at redemption value as temporary equity, outside of the stockholders’ equity section of our unaudited condensed balance sheet.
Under ASC 480-10-S99, we have elected to recognize changes in the redemption value immediately as they occur and adjust the carrying value of the security to equal the redemption value at the end of each reporting period. This method would view the end of the reporting period as if it were also the redemption date for the security. Effective with the closing of the initial public offering (including exercise of the over-allotment option), we recognized the accretion from initial book value to redemption amount, which resulted in charges against additional paid-in capital (to the extent available) and accumulated deficit.
Net Income (Loss) Per Share of Common Stock
We comply with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” We have two classes of shares, which are referred to as Class A Common Stock and Class B Common Stock. Income and losses are shared pro rata between the two classes of shares. Net income (loss) per common share is calculated by dividing the net income (loss) by the weighted average shares of common stock outstanding for the respective period.
The calculation of diluted net income (loss) per common stock does not consider the effect of the warrants issued in connection with the initial public offering (including exercise of the over-allotment option) and the private placement to purchase an aggregate of 8,550,000 shares of common stock in the calculation of diluted income (loss) per share, because their exercise is contingent upon future events. As of September 30, 2021, we did not have any dilutive securities or other contracts that could, potentially, be exercised or converted into common shares and then share in our earnings. As a result, diluted net income (loss) per share is the same as basic net income (loss) per share for the three and nine months ended September 30, 2021. Accretion associated with the redeemable Class A Common Stock is excluded from earnings per share as the redemption value approximates fair value.
Recent Accounting Pronouncements
Our management does not believe that any recently issued, but not yet effective, accounting standards updates, if currently adopted, would have a material effect on our unaudited condensed financial statements.
Off-Balance Sheet Arrangements
As of September 30, 2021 and December 31, 2020, we did not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, revenues, expenses, results of operations, liquidity, capital expenditures or capital resources, nor did we have any commitments or contractual obligations.
| -218- |
JOBS Act
The JOBS Act contains provisions that, among other things, relax certain reporting requirements for qualifying public companies. We qualify as an “emerging growth company” and under the JOBS Act are allowed to comply with new or revised accounting pronouncements based on the effective date for private (not publicly traded) companies. We are electing to delay the adoption of new or revised accounting standards, and as a result, we may not comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result, the unaudited condensed financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
Additionally, we are in the process of evaluating the benefits of relying on the other reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if, as an “emerging growth company,” we choose to rely on such exemptions we may not be required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis) and (iv) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive Officer’s compensation to median employee compensation. These exemptions will apply for a period of five years following the completion of our initial public offering or until we are no longer an “emerging growth company,” whichever is earlier.
| -219- |
INFORMATION ABOUT FOXWAYNE
In this section, unless otherwise stated or the context requires otherwise, the terms “FoxWayne,” “we,” “us” and “our” refer to FoxWayne Enterprises Acquisition Corp. prior to the Closing.
Overview
FoxWayne is a Delaware corporation formed on September 17, 2020 for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. The proposed Business Combination was the result of an extensive search for a potential transaction utilizing the broad network of contacts and corporate relationships developed by FoxWayne management. The terms of the Business Combination were the result of extensive negotiations between the FoxWayne management team and Aerami. The following is a brief description of the material background of these negotiations, the Business Combination and related transactions.
On January 19, 2021, FoxWayne completed its initial public offering of 5,000,000 units, raising gross proceeds of approximately $50 million. The underwriters of the initial public offering were granted a 45-day option from the date of the final prospectus relating to the initial public offering to purchase up to 750,000 additional units to cover overallotments, if any, at $10.00 per unit, less underwriting discounts and commissions. On February 22, 2021, the underwriters exercised the overallotment option and, on February 22, 2021, the underwriters purchased 750,000 additional units, generating gross proceeds of approximately $7.5 million.
Simultaneously with the closing of the initial public offering, FoxWayne consummated the sale of 2,500,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant in a private placement to our Sponsor, generating gross proceeds of approximately $2,500,000. Simultaneously with the closing of the sale of the overallotment units, our Sponsor purchased an additional 300,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant, generating gross proceeds of approximately $300,000.
Prior to the consummation of the initial public offering, neither FoxWayne, nor anyone on its behalf, contacted any prospective target business or had any substantive discussions, formal or otherwise, with respect to a business combination with FoxWayne.
Initial Business Combination
Our Initial Business Combination must occur with one or more target businesses that together have an aggregate fair market value of at least 80% of the assets held in the Trust Account (excluding the deferred underwriting commissions and taxes payable) at the time of the agreement to enter into the Initial Business Combination. If the FoxWayne Board is not able to independently determine the fair market value of the target business or businesses, we will obtain an opinion from an independent investment banking firm that is a member of FINRA, or an independent accounting firm with respect to the satisfaction of such criteria. Our stockholders may not be provided with a copy of such opinion, nor will they be able to rely on such opinion.
The net proceeds of our initial public offering and the sale of the Private Placement Warrants released to us from the Trust Account upon the closing of our Initial Business Combination may be used as consideration to pay the sellers of a target business with which we complete our Initial Business Combination. If our Initial Business Combination is paid for using equity or debt securities, or not all of the funds released from the Trust Account are used for payment of the consideration in connection with our Initial Business Combination or used for redemption of our public shares, we may use the balance of the cash released to us from the Trust Account following the closing for general corporate purposes, including for maintenance or expansion of operations of the post-transaction businesses, the payment of principal or interest due on indebtedness incurred in completing our Initial Business Combination, to fund the purchase of other companies or for working capital.
| -220- |
In addition, we may be required to obtain additional financing in connection with the closing of our Initial Business Combination to be used following the closing for general corporate purposes as described above. There is no limitation on our ability to raise funds through the issuance of equity or equity-linked securities or through loans, advances or other indebtedness in connection with our Initial Business Combination, including pursuant to forward purchase agreements or backstop agreements we may enter into following consummation of our initial public offering. Subject to compliance with applicable securities laws, we would only complete such financing simultaneously with the completion of our Initial Business Combination. At this time, we are not a party to any arrangement or understanding with any third -party with respect to raising any additional funds through the sale of securities or otherwise. None of our sponsors, officers, directors or stockholders is required to provide any financing to us in connection with or after our Initial Business Combination. We may also obtain financing prior to the closing of our Initial Business Combination to fund our working capital needs and transaction costs in connection with our search for and completion of our Initial Business Combination. The FoxWayne Charter provides that, following our initial public offering and prior to the consummation of our Initial Business Combination, we will be prohibited from issuing additional securities that would entitle the holders thereof to (i) receive funds from the Trust Account or (ii) vote as a class with our public shares (a) on any Initial Business Combination or (b) to approve an amendment to the FoxWayne Charter to (x) extend the time we have to consummate a business combination beyond 12 months from the closing of our initial public offering (or up to 18 months from the consummation of our initial public offering if we extend the period of time to consummate a business combination) or (y) amend the foregoing provisions, unless (in connection with any such amendment to the FoxWayne Charter) we offer our public stockholders the opportunity to redeem their public shares.
Redemption Rights for Holders of Public Shares
Redemption Rights
Pursuant to the FoxWayne Charter, we will provide the public holders of public shares with the opportunity to redeem, upon the consummation of the Business Combination, public shares then held by them for cash equal to their pro rata share of the aggregate amount on deposit in the Trust Account that holds the proceeds from our initial public offering, as of two business days prior to the consummation of the Business Combination, including interest net of taxes payable by FoxWayne. Notwithstanding the foregoing redemption rights, a public stockholder, together with any of his, her or its Affiliates or any other person with whom he, she or it is acting in concert or as a “group” (as defined under Section 13(d)(3) of the Exchange Act), will be restricted from redeeming in the aggregate his, her or its public shares or, if part of such a group, the group’s public shares, in excess of 15% of the outstanding public shares. In order to determine whether a stockholder is acting in concert or as a “group” (as defined in Section 13(d)(3) of the Exchange Act) with any other stockholder, FoxWayne may require each public stockholder seeking to exercise redemption rights to certify to FoxWayne whether such stockholder is acting in concert or as a group with any other stockholder. Further, the FoxWayne Charter provides that we will only redeem our public shares so long as (after such redemption) our net tangible assets will be at least $5,000,001 either immediately prior to or upon consummation of our Initial Business Combination and after payment of underwriters’ fees and commissions or any greater net tangible asset or cash requirement which may be contained in the agreement relating to our Initial Business Combination. In addition, the Merger Agreement includes a condition to closing that FoxWayne have at least $15,000,000 in available cash immediately prior to the Effective Time of the Merger, after taking into account payments required to satisfy redemptions of public shares by public stockholders and the payment of Transaction Expenses of FoxWayne.
Public holders will be entitled to receive cash for redeemable public shares only if you:
| ● | submit a written request for FoxWayne to redeem all or a portion of your public shares for cash to Continental Stock Transfer & Trust Company, FoxWayne’s transfer agent; and | |
| ● | deliver your public shares to Continental Stock Transfer & Trust Company, physically or electronically through DTC prior to 5:00 p.m., Eastern time, on , 2022 (two business days before the special meeting), tender your shares physically or electronically and submit a request in writing that we redeem your public shares for cash to Continental Stock Transfer & Trust Company, our transfer agent, to the attention of Mark Zimkind at 1 State Street, 30th Floor, New York, New York 10004, by email at mzimkind@continentalstock.com. |
For illustrative purposes, based on the fair value of cash and marketable securities held in the Trust Account as of February 11, 2022 of approximately $58.1 million and 5,800,000 shares of Class A Common Stock outstanding and redeemable as of February 11, 2022, the estimated per share redemption price would have been approximately $10.10.
| -221- |
Holders of FoxWayne’s outstanding warrants sold in the initial public offering, which are exercisable for shares of Class A Common Stock under certain circumstances, do not have redemption rights in connection with the Business Combination.
At the time of our IPO our Sponsor, officers and directors agreed to waive their redemption rights for no additional consideration in connection with the consummation of the Business Combination with respect to any shares of our common stock they may hold, and our shares of Class B Common Stock will be excluded from the pro rata calculation used to determine the per share redemption price. As of the record date, our Sponsor, officers and directors and their affiliates own approximately % of our outstanding shares of Class B Common Stock.
If a holder exercises its redemption rights, then such holder will be exchanging its shares of Class A Common Stock for cash and will no longer own shares of Class A Common Stock and will not participate in our future growth, if any. Such a holder will be entitled to receive cash for its public shares only if it properly demands redemption and delivers its shares (either physically or electronically) to our transfer agent in accordance with the procedures described herein. See the subsection titled “Special Meeting of FoxWayne Stockholders — Redemption Rights” for the procedures to be followed if you wish to redeem your shares for cash.
Submission of Business Combination to a Stockholder Vote
The special meeting of FoxWayne stockholders to which this proxy statement/prospectus relates is being held to solicit your approval of, among other things, the Business Combination. Unlike many other blank check companies, FoxWayne public stockholders are not required to vote against the Business Combination in order to exercise their redemption rights. If the Business Combination is not completed, then public stockholders electing to exercise their redemption rights will not be entitled to receive such payments. Our Sponsor, directors and officers have agreed to vote any shares of our Class A Common Stock and Class B Common Stock owned by them in favor of the Business Combination.
Directors; Management
The FoxWayne Board is currently comprised of five directors and is divided into three classes with only one class of directors being elected in each year and each class (except for those directors appointed prior to our first annual meeting of stockholders) serving a three-year term.
Our founder, Robb Knie, has served as President, Chief Executive Officer and as Chairman of Hoth Therapeutics, Inc. (Nasdaq: HOTH), a biopharmaceutical company, since May 2017. From 2002 to 2010, Mr. Knie was a Semiconductor Analyst for PAW Partners. From 1993 until 1995, Mr. Knie served as Northeast Regional Manager of American Express Financial Advisors, a financial services company. He has been featured on Bloomberg, The Wall Street Journal and Forbes Magazine as an Independent Equity Analyst. Mr. Knie has over 20 years of equity markets experience. Mr. Knie has been a member of the American Chemical Society, Institute of Electrical and Electronics Engineers, as well as The National Alliance for Youth Sports.
Michael Reavey, our director, is an established security executive with over 20 years-experience in security leadership spanning product engineering, incident response, security assurance, and risk management. At Electronic Arts (“EA”), a gaming company, where he is the Vice President-Enterprise Security since October 2017, he is responsible for the security of EA’s global enterprise. Since February 2020, he also serves as a technical advisor to Change Healthcare, Inc., a provider of revenue and payment cycle management and clinical information exchange solutions, connecting payers, providers, and patients in the United States healthcare system. Prior to joining EA, Mr. Reavey was a Partner at Microsoft (where he worked from 2003 to October 2017) and head of Program Management for Microsoft Azure’s core security services. He was in charge of developing, building and running services that provided Azure’s authorization, authentication, encryption, data protection and systems security. During this time, Mr. Reavey also brought several advanced security products to market as part of Office 365 and Azure Security offerings, which have saved countless customers from zero-day attacks. Before joining the Cloud and Enterprise division Mr. Reavey was a General Manager in the Trustworthy Computing Group at Microsoft Corporation. Most notably he led Microsoft’s response as to sophisticated cyber events such as the Snowden revelations, cyberattacks like Flame and Stuxnet and developed new ways to help Microsoft’s enterprise customers get secure and stay secure with mature programs built around a predictable product servicing and data protection. Prior to joining Microsoft, Mr. Reavey served as a Captain in the U.S. Air Force, where he led mobile technical teams that secured and optimized Air Force networks at installations world-wide and launched red-team efforts to validate Air Force cyber defenses as part of the 92nd Information Warfare Squadron at Kelly Air Base, TX.
| -222- |
Jeff Pavell, M.D., our director, has over twenty years’ medical experience. Presently, Dr. Pavell acts as an off-campus site proctor for Rusk Institute residents training for Rehabilitation Medicine. He is board certified in physical medicine and rehabilitation, pain medicine, and sports medicine, as well as acupuncture, which he uses as complementary care for his patients. Dr. Pavell is currently the Chief of Rehabilitation Medicine at Englewood Hospital and Medical Center. Dr. Pavell acts as Race Director & President of the Haworth 5K (since 2009) as well as President of the Vikings travel soccer & football clubs (since 2011). He also teaches students and residents at Columbia University, where he is on the teaching staff. He has, along with two colleagues, co-authored a chapter, “History and Past Medical History,” in The Low Back Pain Handbooks First and Second Editions, both well-received texts for primary care practitioners. Dr. Pavell graduated from the New York College of Osteopathic Medicine with honors and went on to do his residency and Chief Residency at the New York University Medical Center’s Rusk Institute of Rehabilitation and the Bellevue Hospital. He then completed a fellowship in Buffalo in the use of fluoroscopic injections for management of pain and immobility.
Jonathan Hale Zippin M.D., Ph.D., our director, is an Associate Professor of Dermatology and Pharmacology and an Associate Attending Dermatologist at Weill Medical College of Cornell University since 2010, where he is the current Vice Chair of Research in the Department of Dermatology and is the Director of the Contact, Occupational, and Photo Dermatitis Unit. Following completion of his post-doctoral studies in 2010, Dr. Zippin joined the faculty of the Department of Dermatology at Weill Cornell Medical College. Dr. Zippin founded and is the Director of the Contact, Occupational, and Photodermatitis Service which provides comprehensive dermatologic allergy care for hundreds of patients a year. Dr. Zippin also serves as the Director of the dermatology course for the medical school. Over the past ten years, he has served on the general faculty council, which is responsible for approving medical college policy and promotions. Dr. Zippin is a fellow of the American Academy of Dermatology and member of the Society for Investigative Dermatology and the American Contact Dermatitis Society. He has served on the Board of Directors for the American Contact Dermatitis Society and multiple committees within the organization. He is currently on the Council for the Pan-American Society for Pigment Cell Research. For the past 18 years, he has helped to establish the role of distinct cAMP microdomains in mammalian cell biology and revealed new mechanisms in insulin release and cancer. Dr. Zippin has published numerous peer-reviewed papers, been awarded multiple patents, and written book chapters. He serves as a reviewer for multiple journals such as Cell Reports Medicine, Dermatitis, Journal of American Academy of Dermatology, Pigment Cell and Melanoma Research, and Molecular Carcinogenesis. Zippin has served as a consultant for numerous pharmaceutical companies including Pfizer and Celgene. He is also the founder of CEP Biotech that is developing antibody based diagnostics for cancer. Finally, Dr. Zippin has been involved in the design and/or execution of multiple clinical trials testing both devices and pharmaceutical interventions.
Sundeep Agrawal, M.D., our director, is currently a Managing Director at Colt Ventures, a family office focused on investing in private and public life science companies since 2019. He has experience across venture capital and public equity, healthcare investment banking, clinical medicine and research. Previously, he was a Vice President at Longitude Capital from 2017 to 2019, an approximately $2.0 billion healthcare investment firm focused on public and private investments in life sciences. Dr. Agrawal has served as a Board Observer at Recode Therapeutics since March 2020 and served as a Board Observer at Axonics Modulation Tech (Nasdaq: AXNX) from April 2018 to December 2018 and as a Board Observer at Venus Concept (Nasdaq: VERO) from July 2017 to December 2018. Prior to Longitude Capital, he was an Executive Director in Healthcare Investment Banking at Oppenheimer & Co. from 2010-2017 where he worked on public and private capital markets transactions in healthcare. Dr. Agrawal completed clinical training at Lenox Hill Hospital from 2013 to 2014. He has clinical and basic science research experience with publications in leading journals and has been the recipient of several national research awards and grants. Dr. Agrawal currently sits on the advisory board of APN Health (since 2016), a medical device company, and on the board of IndoUSrare, an independent non-profit organization focused on helping patients of Indian origin with rare diseases in the USA, India, and globally, since 2020. Dr. Agrawal holds an M.D. from the George Washington School of Medicine and a B.A. in Biology from George Washington University.
| -223- |
Director Independence
Nasdaq listing standards require that a majority of our board of directors be independent. An “independent director” is defined generally as a person other than an officer or employee of the company or its subsidiaries or any other individual having a relationship which in the opinion of the company’s board of directors, would interfere with the director’s exercise of independent judgment in carrying out the responsibilities of a director. The FoxWayne Board has determined that Messrs. Reavey, Pavell, Zippin and Agrawal are “independent directors” as defined in the Nasdaq listing standards and applicable SEC rules. Our audit committee is entirely composed of independent directors meeting Nasdaq’s additional requirements applicable to members of the audit committee.
Committees of the FoxWayne Board
FoxWayne’s Board has two standing committees: an audit committee and a compensation committee. Subject to phase-in rules and a limited exception, the rules of Nasdaq and Rule 10A-3 of the Exchange Act require that the audit committee of a listed company be comprised solely of independent directors, and the rules of Nasdaq require that the compensation committee of a listed company be comprised solely of independent directors.
Audit Committee
FoxWayne has established an audit committee of the FoxWayne Board. Messrs. Pavell, Reavey and Zippin serve as members of our audit committee, with Mr. Pavell serving as the Chairman of the audit committee. Under the Nasdaq listing standards and applicable SEC rules, we are required to have at least three members of the audit committee, all of whom must be independent, subject to certain phase-in provisions. Each such person meets the independent director standard under Nasdaq listing standards and under Rule 10-A-3(b)(1) of the Exchange Act.
Each member of the audit committee is financially literate, and our board of directors has determined that Mr. Reavey qualifies as an “audit committee financial expert” as defined in applicable SEC rules.
FoxWayne has adopted an audit committee charter, which details the principal functions of the audit committee, including:
| ● | the appointment, compensation, retention, replacement, and oversight of the work of the independent registered public accounting firm and any other independent registered public accounting firm engaged by us; | |
| ● | pre-approving all audit and permitted non-audit services to be provided by the independent registered public accounting firm or any other registered public accounting firm engaged by us, and establishing pre-approval policies and procedures; | |
| ● | reviewing and discussing with the independent registered public accounting firm all relationships the independent registered public accounting firm have with us in order to evaluate their continued independence; | |
| ● | setting clear hiring policies for employees or former employees of the independent registered public accounting firm; | |
| ● | setting clear policies for audit partner rotation in compliance with applicable laws and regulations; | |
| ● | obtaining and reviewing a report, at least annually, from the independent registered public accounting firm describing (i) the independent registered public accounting firm’s internal quality-control procedures and (ii) any material issues raised by the most recent internal quality-control review, or peer review, of the audit firm, or by any inquiry or investigation by governmental or professional authorities within the preceding five years respecting one or more independent audits carried out by the firm and any steps taken to deal with such issues; | |
| ● | reviewing and approving any related party transaction required to be disclosed pursuant to Item 404 of Regulation S-K promulgated by the SEC prior to us entering into such transaction; and | |
| ● | reviewing with management, the independent registered public accounting firm, and our legal advisors, as appropriate, any legal, regulatory or compliance matters, including any correspondence with regulators or government agencies and any employee complaints or published reports that raise material issues regarding our financial statements or accounting policies and any significant changes in accounting standards or rules promulgated by the FASB, the SEC or other regulatory authorities. |
| -224- |
Compensation Committee
FoxWayne has established a compensation committee of the FoxWayne Board. Messrs. Agrawal, Reavey and Pavell serve as members of our compensation committee, with Mr. Agrawal serving as the chairman of the compensation committee. Under the Nasdaq listing standards and applicable SEC rules, we are required to have at least two members of the compensation committee, all of whom must be independent, subject to certain phase-in provisions. Each such person meets the independent director standard under Nasdaq listing standards applicable to members of the compensation committee.
FoxWayne has adopted a compensation committee charter, which details the principal functions of the compensation committee, including:
| ● | reviewing and approving on an annual basis the corporate goals and objectives relevant to our Chief Executive Officer’s compensation, evaluating our Chief Executive Officer’s performance in light of such goals and objectives and determining and approving the remuneration (if any) of our Chief Executive Officer based on such evaluation; | |
| ● | reviewing and approving on an annual basis the compensation of all of our other officers; | |
| ● | reviewing on an annual basis our executive compensation policies and plans; | |
| ● | implementing and administering our incentive compensation equity-based remuneration plans; | |
| ● | assisting management in complying with our proxy statement and annual report disclosure requirements; | |
| ● | approving all special perquisites, special cash payments and other special compensation and benefit arrangements for our officers and employees; | |
| ● | if required, producing a report on executive compensation to be included in our annual proxy statement; and | |
| ● | reviewing, evaluating and recommending changes, if appropriate, to the remuneration for directors. |
Notwithstanding the foregoing, as indicated above, other than reimbursement of expenses, no compensation of any kind, including finders, consulting or other similar fees, will be paid to any of our existing stockholders, officers, directors or any of their respective affiliates, prior to, or for any services they render in order to complete the consummation of a business combination although we may consider cash or other compensation to officers or advisors we may hire. Accordingly, it is likely that prior to the consummation of an Initial Business Combination, the compensation committee will only be responsible for the review and recommendation of any compensation arrangements to be entered into in connection with such Initial Business Combination.
The charter also provides that the compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, legal counsel or other adviser and will be directly responsible for the appointment, compensation and oversight of the work of any such adviser. However, before engaging or receiving advice from a compensation consultant, external legal counsel or any other adviser, the compensation committee will consider the independence of each such adviser, including the factors required by Nasdaq and the SEC.
Director Nominations
FoxWayne does not have a standing nominating committee. In accordance with Rule 5605(e)(2) of the Nasdaq Rules, a majority of the independent directors may recommend a director nominee for selection by the FoxWayne Board. The FoxWayne Board believes that the independent directors can satisfactorily carry out the responsibility of properly selecting or approving director nominees without the formation of a standing nominating committee. As there is no standing nominating committee, we do not have a nominating committee charter in place.
The FoxWayne Board will also consider director candidates recommended for nomination by our stockholders during such times as they are seeking proposed nominees to stand for election at the next annual meeting of stockholders (or, if applicable, a special meeting of stockholders). Our stockholders that wish to nominate a director for election to the FoxWayne Board should follow the procedures set forth in our Bylaws.
FoxWayne has not formally established any specific, minimum qualifications that must be met or skills that are necessary for directors to possess. In general, in identifying and evaluating nominees for director, the FoxWayne Board considers educational background, diversity of professional experience, knowledge of our business, integrity, professional reputation, independence, wisdom, and the ability to represent the best interests of our stockholders.
| -225- |
Compensation Committee Interlocks and Insider Participation
None of our officers currently serves, and in the past year have not served, as a member of the compensation committee of any entity that has one or more officers serving on the FoxWayne Board.
Committee Membership, Meetings and Attendance
During the fiscal year ended December 31, 2021, the FoxWayne Board held eight meetings, our audit committee held five meetings, and our compensation committee held one meeting. We encourage all of our directors to attend our annual meetings of stockholders.
Code of Ethics and Committee Charters
We have adopted a Code of Ethics applicable to our directors, officers and employees. We have filed a copy of our Code of Ethics and our audit and compensation committee charters as exhibits to the registration statement relating to our initial public offering. You are able to review these documents by accessing our public filings at the SEC’s web site at www.sec.gov. In addition, a copy of the Code of Ethics will be provided without charge upon request from us. We intend to disclose any amendments to or waivers of certain provisions of our Code of Ethics in a Current Report on Form 8-K. See the section of this prospectus titled “Where You Can Find Additional Information.”
Limitation on Liability and Indemnification of Officers and Directors
The FoxWayne Charter provides that our officers and directors will be indemnified by us to the fullest extent authorized by Delaware law, as it now exists or may in the future be amended. In addition, the FoxWayne Charter provides that our directors will not be personally liable for monetary damages to us or our stockholders for breaches of their fiduciary duty as directors, unless they violated their duty of loyalty to us or our stockholders, acted in bad faith, knowingly or intentionally violated the law, authorized unlawful payments of dividends, unlawful stock purchases or unlawful redemptions, or derived an improper personal benefit from their actions as directors.
We have entered into agreements with our officers and directors to provide contractual indemnification in addition to the indemnification provided for in the FoxWayne Charter. Our Bylaws also permit us to secure insurance on behalf of any officer, director or employee for any liability arising out of his or her actions, regardless of whether Delaware law would permit such indemnification.
Our officers and directors have agreed, and any persons who may become officers or directors prior to the Initial Business Combination will agree, to waive any right, title, interest or claim of any kind in or to any monies in the Trust Account, and to waive any right, title, interest or claim of any kind they may have in the future as a result of, or arising out of, any services provided to us and will not seek recourse against the Trust Account for any reason whatsoever. Accordingly, any indemnification provided will only be able to be satisfied by us if (a) we have sufficient funds outside of the Trust Account or (b) we consummate an Initial Business Combination.
Our indemnification obligations may discourage stockholders from bringing a lawsuit against our officers or directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against our officers and directors, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against our officers and directors pursuant to these indemnification provisions.
We believe that these provisions, the insurance and the indemnity agreements are necessary to attract and retain talented and experienced officers and directors.
Status as a Public Company
We are an “emerging growth company,” as defined in Section 2(a)(19) of the Securities Act, as modified by the JOBS Act. As such, we are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. If some investors find our securities less attractive as a result, there may be a less active trading market for our securities and the prices of our securities may be more volatile.
| -226- |
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We intend to take advantage of the benefits of this extended transition period.
We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of our initial public offering, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our Class A common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
Lack of Business Diversification
For an indefinite period of time after the completion of the proposed Business Combination, the prospects for our success may depend entirely on the future performance of the Combined Company. Unlike other entities that have the resources to complete business combinations with multiple entities in one or several industries, it is probable that we will not have the resources to diversify our operations and mitigate the risks of being in a single line of business. By completing the Business Combination with only a single entity, our lack of diversification may:
| ● | subject us to negative economic, competitive and regulatory developments, any or all of which may have a substantial adverse impact on the particular industry in which we operate after our Initial Business Combination; and | |
| ● | cause us to depend on the marketing and sale of a single product or limited number of products or services. |
Competition
In identifying, evaluating and selecting a target business for our business combination, we may encounter intense competition from other entities having a business objective similar to ours, including other blank check companies, private equity groups and leveraged buyout funds, and operating businesses seeking strategic acquisitions. Many of these entities are well established and have extensive experience identifying and effecting business combinations directly or through affiliates. Moreover, many of these competitors possess greater financial, technical, human and other resources than we do. Our ability to acquire larger target businesses will be limited by our available financial resources. This inherent limitation gives others an advantage in pursuing the acquisition of a target business. Furthermore, our obligation to pay cash in connection with our public stockholders who exercise their redemption rights may reduce the resources available to us for our Initial Business Combination and our outstanding warrants, and the future dilution they potentially represent, may not be viewed favorably by certain target businesses. Either of these factors may place us at a competitive disadvantage in successfully negotiating an Initial Business Combination.
Facilities
Our executive offices are located at 1 Rockefeller Plaza, Suite 1039, New York, NY 10020. The cost for our use of this space is included in the $10,000 per month fee we will pay to our Sponsor for office space, administrative and support services. We consider our current office space adequate for our current operations.
Employees and Human Capital Resources
We currently have one individual, Robb Knie, who serves as both our Chief Executive Officer and Chief Financial Officer. Mr. Knie is not obligated to devote any specific number of hours to our matters, but he intends to devote as much of his time as he deems necessary to our affairs until we have completed our Initial Business Combination. The amount of time Mr. Knie will devote in any time period will vary based on whether a target business has been selected for our Initial Business Combination and the stage of the Initial Business Combination process we are in. We do not intend to have any full time employees prior to the completion of our Initial Business Combination.
| -227- |
Periodic Reporting and Financial Information
We have registered our units, Class A Common Stock and warrants under the Exchange Act and have reporting obligations, including the requirement that we file annual, quarterly and current reports with the SEC. In accordance with the requirements of the Exchange Act, our annual reports will contain financial statements audited and reported on by our independent registered public accountants.
We will be required to evaluate our internal control procedures for the fiscal year ending December 31, 2021 as required by the Sarbanes-Oxley Act. Only in the event we are deemed to be a large accelerated filer or an accelerated filer will we be required to have our internal control procedures audited. A target company may not be in compliance with the provisions of the Sarbanes-Oxley Act regarding adequacy of their internal controls. The development of the internal controls of any such entity to achieve compliance with the Sarbanes-Oxley Act may increase the time and costs necessary to complete any such acquisition.
Legal Proceedings
There is no material litigation, arbitration or governmental proceeding currently pending against us or any members of our management team in their capacity as such.
| -228- |
EXECUTIVE COMPENSATION
FoxWayne
In October 2020, the Sponsor transferred 25,000 Founder Shares to each of Messrs. Reavey, Pavell, Zippin and Agrawal. Upon completion of the Business Combination, FoxWayne will reimburse its Sponsor, directors and officers, or any of their respective affiliates, for any out-of-pocket expenses incurred in connection with activities on our behalf such as identifying potential target businesses and performing the due diligence.
Upon completion of the Business Combination, directors or members of FoxWayne’s management team who remain with us may be paid consulting or management fees from the Combined Company. All of these fees will be fully disclosed to stockholders, to the extent then known, in the offer materials or proxy solicitation materials furnished to FoxWayne’s stockholders in connection with the Business Combination. FoxWayne has not established any limit on the amount of such fees that may be paid by the Combined Company to our directors or members of management. Any compensation paid to FoxWayne’s officers will be determined, or recommended to the FoxWayne Board for determination, either by a compensation committee constituted solely by independent directors or by a majority of the independent directors on the FoxWayne Board.
FoxWayne does not intend to take any action to ensure that members of its management team maintain their positions with the Combined Company after the consummation of the Business Combination, and it is not intended that FoxWayne’s directors and officers will remain with the Combined Company after the Business Combination. FoxWayne is not party to any agreements with its directors or officers that provide for benefits upon termination of employment.
For more information about the interests of the Sponsor, directors and officers in the Business Combination, see the subsection titled “The Business Combination – Interests of Certain Persons in the Business Combination - Interests of Sponsor and FoxWayne Directors and Officers.”
Aerami
This section discusses the material components of the executive compensation program for Aerami executive officers who are named in the “Summary Compensation Table” below. In 2021, Aerami’s “named executive officers” and their positions with Aerami were as follows:
| ● | Steven Thornton, who serves as Chief Executive Officer; and | |
| ● | Timm Crowder, who serves as President and Chief Operating Officer. |
Following the Closing, the currently serving named executive officers will continue in their current positions with the Combined Company.
This discussion may contain forward-looking statements that are based on current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that the Combined Company adopts following the Closing may differ materially from the currently planned programs summarized in this discussion.
| -229- |
Summary Compensation Table
The following table presents summary information regarding the total compensation that was awarded to, earned by or paid to the named executive officers for services rendered to Aerami during the year ended December 31, 2021.
| Name and Principal Position | Year | Salary ($) | Bonus ($)(1) | Option Awards ($)(2) | All Other Compensation ($)(3) | Total ($) | ||||||||||||||||||
| Steven Thornton Chief Executive Officer | 2021 | 480,000 | — | 797,459 | — | 1,277,459 | ||||||||||||||||||
| Timm Crowder President and Chief Operating Officer | 2021 | 375,000 | — | 49,880 | 11,600 | 436,480 | ||||||||||||||||||
| (1) | As of the date of this filing, annual bonus amounts have not been determined by the compensation committee of the Aerami Board. | |
| (2) | In accordance with SEC rules, this column reflects the aggregate grant-date fair value of the Aerami Options granted during 2021 computed in accordance with ASC Topic 718 for stock-based compensation transactions. Assumptions used in the calculation of these amounts are included in Note 2 to Aerami’s financial statements appearing elsewhere in this proxy statement/prospectus. These amounts do not reflect the actual economic value that will be realized by the named executive officer upon the vesting of the stock options, the exercise of the stock options, or the sale of the common stock underlying such stock options. | |
| (3) | Amounts reported include matching contributions to the 401(k) retirement plan. |
Narrative Disclosure to Compensation Tables
Base Salary
The compensation of the named executive officers is generally determined in connection with the commencement of employment of the executive by the Aerami Board or the compensation committee. The following represent the base salaries that were effective for 2021 for the named executive officers.
| Name | 2021
Annual Base Salary ($) | |||
| Steven Thornton | 480,000 | |||
| Timm Crowder | 375,000 | |||
| -230- |
Bonus Compensation
Aerami considers annual performance bonuses to be an important component of its total compensation program and provides incentives necessary to retain executive officers. Each of the named executive officers is eligible to receive an annual performance-based cash bonus based on a specified target annual bonus award amount, expressed as a percentage of the named executive officer’s base salary. In 2021, Mr. Thornton and Mr. Crowder participated in Aerami’s annual performance bonus program at the following target percentages of base salary:
| Name | Target Percentage (%) | |||
| Steven Thornton | 75 | (1) | ||
| Timm Crowder | 50 | |||
| (1) | Pursuant to Mr. Thornton’s Employment Agreement with Aerami, he may elect to receive up to 50% of his annual performance bonus in the form of fully vested RSUs. |
For 2021, the annual bonus for the current serving named executive officers will be determined by the compensation committee of the Aerami Board based on the achievement of operational milestones established by each named executive officer and the compensation committee (or, in the case of Mr. Crowder, Mr. Thornton). As of the date of this filing, the board of directors has not approved annual performance bonuses for 2021. We will provide the amounts of such annual performance bonuses once determined in an amendment to this Registration Statement on Form S-4, of which this proxy statement /prospectus forms a part, prior to its being declared effective.
Mr. Thornton was also eligible to receive a one-time bonus (a “Special Bonus”) equal in the aggregate up to 25% of Mr. Thornton’s base salary in the year to which the Special Bonus relates, in the following amounts and based upon achievement of goals corresponding, on or before April 1, 2021: (1) Mr. Thornton was eligible for a Special Bonus in the amount of 10% of his base salary upon completion of an offering of Aerami Series A Preferred Stock resulting in either $15 million total investment in Aerami or, if the Aerami Board decides to close the round at a lesser amount; (2) Mr. Thornton was eligible for a Special Bonus in the amount of 5% of his base salary upon raising at least $3 million in funds for a convertible note; and (3) Mr. Thornton was eligible for a Special Bonus in the amount of 10% of his base salary upon Aerami’s initiation of Phase 1 human clinical trials (SAD and MAD) and a six month single species (dog) toxicology program for AER-901, inhaled imatinib. The compensation committee determined that none of these goals had been satisfied on or before April 1, 2021 and accordingly no Special Bonus was paid.
Equity-Based Awards
Aerami’s equity-based awards are designed to align Aerami’s interests and the interests of its stockholders with those of its employees and consultants, including the named executive officers. The Aerami Board or the compensation committee thereunder is responsible for approving equity grants.
Aerami currently maintains the Aerami Therapeutics, Inc. 2015 Equity Incentive Plan (“2015 Plan”). The terms of the 2015 Plan are described below under “—Incentive Award Plans.” Aerami offers awards of stock options to purchase shares of its common stock to eligible service providers, including the named executive officers, pursuant to the 2015 Plan. As mentioned below, in connection with the completion of the Business Combination and the adoption of the New Equity Incentive Plan, no further awards will be granted under the 2015 Plan.
All options are granted with an exercise price per share that is no less than the fair market value of Aerami’s common stock on the date of grant of each award. Aerami’s stock option awards generally vest over a three-year or four-year period and may be subject to acceleration of vesting and exercisability under certain termination and change in control events.
| -231- |
In February 2021, the Aerami Board in its discretion granted an option to purchase 200,000 shares of Aerami Common Stock to Mr. Crowder at an exercise price of $0.35. The option award vests over four years with one fourth vesting on the first anniversary of the vesting commencement date and the remainder vesting in equal monthly installments thereafter.
In April 2021, the Aerami Board granted an option to purchase 3,203,932 shares of Aerami Common Stock to Mr. Thornton in accordance with his employment agreement with an exercise price of $0.35. The option award vests over three years with one third vesting on the first anniversary of the vesting commencement date and the remainder vesting in equal monthly installments thereafter.
No other equity-based incentive awards were granted to the named executive officers during 2021.
Employment Arrangements with the Named Executive Officers
Aerami is a party to employment agreements with each of the currently serving named executive officers. The arrangements generally provide for at-will employment without any specific term and set forth the named executive officer’s initial base salary, bonus potential, eligibility for employee benefits and severance benefits upon a qualifying termination of employment.
Employment Agreement with Steven Thornton
Aerami has entered into an employment agreement with Mr. Thornton, its Chief Executive Officer. Pursuant to his agreement, Mr. Thornton is entitled to an annual base salary of $480,000 and is eligible to receive a target annual incentive bonus of 75% of his base salary.
If Mr. Thornton’s employment is terminated by Aerami without “cause” or by Mr. Thornton with “good reason” before or more than 12 months following a “change in control” (in each case as defined in his employment agreement), he is entitled to: (1) a monthly payment equal to his monthly base salary and one-twelfth of his annual targeted bonus for 12 months, (2) full acceleration of the vesting of all his outstanding equity awards that would have vested during the three year period following the termination date, and (3) a lump-sum payment equal to the monthly cost for providing medical, dental, and vision insurance coverage as in effect for Mr. Thornton at the time of his termination multiplied by 12.
If Mr. Thornton’s employment is terminated by Aerami without “cause” or by Mr. Thornton with “good reason” within a period of 12 months following a “change in control,” he is entitled to: (1) a lump-sum payment equal to his annual base salary and annual targeted bonus, (2) full acceleration of the vesting of all his outstanding equity awards, and (3) a lump-sum payment equal to the monthly cost for providing medical, dental, and vision insurance coverage as in effect for Mr. Thornton at the time of his termination multiplied by 12.
Mr. Thornton’s severance benefits are conditioned, among other things, on his complying with his post-termination obligations under his agreements with Aerami, including a one-year non-competition obligation, and timely signing a general release of claims in Aerami’s favor.
| -232- |
We intend to enter into an amended and restated employment agreement with Mr. Thornton in connection with the consummation of the Business Combination. We will provide the principal terms of such agreement in an amendment to this Registration Statement prior to its being declared effective.
Employment Agreement with Timm Crowder
Aerami has entered into an employment agreement with Mr. Crowder, its President and Chief Operating Officer. Pursuant to his agreement, Mr. Crowder is entitled to an annual base salary of $375,000 and is eligible to receive a target annual incentive bonus of 50% of his base salary.
If Mr. Crowder’s employment is terminated by Aerami without “cause” or by Mr. Crowder with “good reason” before or more than 12 months following a “change in control” (in each case as defined in his employment agreement), he is entitled to: (1) a monthly payment equal to his monthly base salary and one-twelfth of his annual targeted bonus for 12 months, (2) full acceleration of the vesting of all his outstanding equity awards that would have vested during the three year period following the termination date, and (3) a lump-sum payment equal to the monthly cost for providing medical, dental, and vision insurance coverage as in effect for Mr. Crowder at the time of his termination multiplied by 12.
If Mr. Crowder’s employment is terminated by Aerami without “cause” or by Mr. Crowder with “good reason” within a period of 12 months following a “change in control,” he is entitled to: (1) a lump-sum payment equal to his annual base salary and annual targeted bonus, (2) full acceleration of the vesting of all his outstanding equity awards, and (3) a lump-sum payment equal to the monthly cost for providing medical, dental, and vision insurance coverage as in effect for Mr. Crowder at the time of his termination multiplied by 12.
Mr. Crowder’s severance benefits are conditioned, among other things, on his complying with his post-termination obligations under his agreements with Aerami, including a one-year non-competition obligation, and timely signing a general release of claims in Aerami’s favor.
We intend to enter into an amended and restated employment agreement with Mr. Crowder in connection with the consummation of the Business Combination. We will provide the principal terms of such agreement in an amendment to this Registration Statement/proxy statement prior to its being declared effective.
| -233- |
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information regarding Aerami equity awards granted to the named executive officers that remain outstanding as of December 31, 2021.
| Option Awards(1) | ||||||||||||||||
| Name | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | |||||||||||
| Steven Thornton | 7/7/2018 | 30,000 | — | 2.78 | 7/6/2028 | |||||||||||
| Chief Executive Officer | 7/7/2018 | 100,000 | — | 2.78 | 8/6/2028 | |||||||||||
| 11/20/2020 | (2) | 950,000 | 870,833 | 0.35 | 11/21/2030 | |||||||||||
| 4/28/2021 | (3) | — | 3,203,932 | 0.35 | 4/28/2023 | |||||||||||
| Timm Crowder | 9/20/2020 | (5)(6) | 45,833 | 54,167 | 0.35 | 2/3/2030 | ||||||||||
| President and Chief Operating Officer | 10/27/2020 | (5)(6) | 130,625 | 154,375 | 0.35 | 2/3/2030 | ||||||||||
| 2/2/2021 | — | 200,000 | 0.35 | 2/2/2031 | ||||||||||||
| (1) | Except as otherwise described below, all of the outstanding equity awards are stock options granted under and subject to the terms of the 2015 Plan, described below under “—Incentive Award Plans.” The vesting of each equity award is subject to the executive’s continuous service with us through the applicable vesting dates. Each of the named executive officers’ employment agreements entitles them to accelerated vesting of all outstanding equity awards upon a qualifying termination in connection with or following a change in control of Aerami. For additional discussion, please see “—Employment Arrangements with the Named Executive Officers” above. | |
| (2) | The option awards vests over one year in equal monthly installments. | |
| (3) | Each option award vests over three years with one third vesting on the first anniversary of the vesting commencement date and the remainder vesting in equal monthly installments thereafter. | |
| (4) | The option award was granted outside the 2015 Plan; however, the terms and conditions of the award are consistent with the 2015 Plan and option awards thereunder. | |
| (5) | Each option award vests over four years with one quarter vesting on the first anniversary of the vesting commencement date and the remainder vesting in equal monthly installments thereafter. | |
| (6) | Each option award was originally granted on August 19, 2020 with an exercise price of $1.86. In September and October 2020, the Aerami Board approved an option repricing program whereby certain previously granted and unexercised options held by current employees with exercise prices above $0.35 per share were repriced on a one-for-one basis to $0.35 per share, which represented the per share fair market value of the Aerami Common Stock as of the date of the repricing. Aerami treated the repricing as a modification of the original awards and calculated additional compensation costs for the difference between the fair value of the modified award and the fair value of the original award on the modification date. The exercise price for option awards held by Mr. Crowder originally granted in August 2020 were adjusted in connection with the option repricing program. |
Narrative Disclosure to Outstanding Equity Awards at Fiscal Year-End Table
The named executive officers did not exercise any stock option awards during the fiscal year ended December 31, 2021. Aerami did not engage in any re-pricings or other modifications or cancellations to any of the named executive officers’ outstanding equity awards during the year ended December 31, 2021.
| -234- |
Other Elements of Compensation
Perquisites, Health and Welfare Benefits
The named executive officers are eligible to participate in Aerami’s employee benefit plans, including medical, dental, vision, group life, disability and accidental death and dismemberment insurance plans, in each case on the same basis as all of Aerami’s other employees.
Aerami generally does not provide perquisites or personal benefits to the named executive officers, except in limited circumstances. Aerami does, however, pay the premiums for term life insurance and disability insurance for all of Aerami’s employees, including the named executive officers. The Aerami Board may elect to adopt qualified or non-qualified benefit plans in the future if it determines that doing so is in its best interests.
401(k) Plan
Aerami provides a 401(k) plan that provides eligible U.S. employees with an opportunity to save for retirement on a tax advantaged basis. Eligible employees are able to defer eligible compensation up to certain Code limits, which are updated annually. The 401(k) plan is intended to be qualified under Section 401(a) of the Code, with the related trust intended to be tax exempt under Section 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan are deductible by us when made, and contributions and earnings on those amounts are not generally taxable to the employees until withdrawn or distributed from the 401(k) plan. Aerami believes that providing a vehicle for tax-deferred retirement savings through Aerami’s 401(k) plan adds to the overall desirability of its executive compensation package and further incentivizes its employees, including the named executive officers, in accordance with Aerami’s compensation policies.
Nonqualified Deferred Compensation
Aerami does not maintain nonqualified defined contribution plans or other nonqualified deferred compensation plans. Aerami may elect to provide officers and other employees with non-qualified defined contribution or other nonqualified deferred compensation benefits in the future if it determines that doing so is in the company’s best interests.
No Tax Gross Ups
Aerami does not make gross-up payments to cover the named executive officers’ personal income taxes that may pertain to any of the compensation or benefits paid or provided by us.
Incentive Award Plans
2015 Equity Incentive Plan
In June 2015, the Aerami Board approved the 2015 Plan.
A total of 12,848,069 shares of Aerami Common Stock are currently reserved for issuance under the 2015 Plan. As of September 30, 2021, 10,560,045 shares of Aerami Common Stock were subject to outstanding option and restricted stock unit awards and 2,288,024 shares of Aerami Common Stock remained available for future issuance. A number of shares of Aerami Common Stock available for issuance under the 2015 Plan is automatically increased on January 1st of each year for a period of ten years commencing on January 1, 2016 and ending on (and including) January 1, 2025, in an amount equal to 6% of the total number of shares of Aerami Common Stock outstanding on December 31st of the preceding calendar year, unless the Aerami Board acts prior to the first day of any calendar year, to provide that there shall be no increase in the share reserve for such calendar year or that the increase in the share reserve for such calendar year will be a lesser number of shares of common stock than would otherwise occur pursuant to the preceding sentence. None of the shares of common stock available for issuance pursuant to the preceding sentence will be issued in respect of ISOs. After the effective date of the New Equity Incentive Plan, no additional awards will be granted under the 2015 Plan.
| -235- |
Administration. The compensation committee of the Aerami Board administers the 2015 Plan, provided that the entire Aerami Board may act in lieu of the compensation committee subject to certain limitations. Subject to the terms and conditions of the 2015 Plan, the compensation committee has the authority in its discretion to determine the eligible participants to whom, and the time or times at which, awards may be granted, the number of shares, units or other rights subject to each award, the exercise, base or purchase price of an award (if any), the time or times at which an award will become vested, exercisable or payable, the performance criteria, performance goals and other conditions of an award, the duration of the award, and all other terms of the award. Subject to the terms of the 2015 Plan, the compensation committee has the authority to amend the terms of an award in any manner that is not inconsistent with the 2015 Plan, provided that no such action shall adversely affect the rights of a participant with respect to an outstanding award without the participant’s consent. The compensation committee shall also have discretionary authority to interpret the 2015 Plan, to make all factual determinations under the 2015 Plan, and to make all other determinations necessary or advisable for 2015 Plan administration, including, without limitation, to correct any defect, to supply any omission or to reconcile any inconsistency in the 2015 Plan or any award agreement thereunder. The compensation committee may prescribe, amend, and rescind rules and regulations relating to the 2015 Plan.
Eligibility. The compensation committee has the authority to designate participants under the 2015 Plan from individuals who are employees, officers, directors, consultants and other service providers of Aerami. The compensation committee will determine the terms and conditions of all awards granted to such participants.
Awards. The 2015 Plan provides that the compensation committee may grant or issue stock options, SARs, restricted stock, RSUs, performance shares, performance units, and other cash based or other equity-based awards, or any combination thereof. Each award is set forth in a separate agreement with the person receiving the award and indicates the type, terms and conditions of the award.
| ● | Non-qualified stock options (“NQSOs”) provide for the right to purchase shares of Aerami Common Stock at a specified price which may not be less than 100% of the fair market value of a share of stock on the date of grant, and usually will become exercisable in one or more installments after the grant date, subject to the participant’s continued employment or service with Aerami and/or subject to the attainment of any specified performance goals established by the compensation committee. NQSOs may be granted for any term specified by the compensation committee, but the term may not exceed 10 years. |
| ● | SARs may be awarded entitling the participant to receive a payment, upon the exercise of such right, in such amount and at such time, and subject to such conditions, as determined by the compensation committee and set forth in the applicable share agreement, including the time period at which the SARs become vested and/or exercisable. Subject to such terms and conditions as shall be specified in an award agreement, a vested SAR may be exercised in whole or in part at any time during the term thereof and, upon such exercise and payment of any applicable exercise price, the participating will be entitled to receive an amount determined by multiplying: (i) the excess of the fair market value of a share of Aerami Common Stock on the date of exercise of the SAR over the base price of such SAR by (ii) the number of shares as to which such SAR is exercised. |
| ● | Restricted stock may be granted to participants and made subject to such restrictions as may be determined by the compensation committee. Shares granted under any restricted stock award may not be transferred, assigned or subject to any encumbrance, pledge, or charge until all applicable restrictions are removed or have expired, unless otherwise allowed by the compensation committee. Recipients of restricted stock, unlike recipients of options, may have voting rights and may receive dividends, if any, prior to when the restrictions lapse. |
| ● | Stock units may be awarded to participants, typically without payment of consideration or for a nominal purchase price, but subject to vesting conditions including continued employment established by the compensation committee. Like restricted stock, restricted stock units may not be transferred, assigned or subject to any encumbrance, pledge, or charge until all applicable restrictions are removed or have expired, unless otherwise allowed by the compensation committee. Unlike restricted stock, stock underlying restricted stock units will not be issued until sometime after the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied and the shares have been issued. | |
| ● | Performance shares may be granted to participants and are contractual representing a notional unit interest equal in value to a share of Aerami Common Stock to be paid, subject to such conditions including the achievement of performance goals and the time period for such achievement as established by the compensation committee and set forth in the award agreement. After the applicable time period has ended, the number of performance Shares earned by the participant over such time period shall be determined as a function of the extent to which the applicable corresponding performance goals have been achieved. This determination shall be made solely by the Committee. The compensation committee may, in its discretion, waive any performance or vesting conditions relating to a performance share award. |
| ● | Performance units may be awarded to participants having an initial notional value equal to a dollar amount determined by the compensation committee to be earned based on the achievement of set performance goals over a specified time period. After the applicable time period has ended, the number of performance units earned by the participant, and the amount payable in cash, in shares or in a combination thereof, over such time period shall be determined as a function of the extent to which the applicable corresponding performance goals have been achieved. |
| ● | Incentive bonus awards may be awarded by the compensation committee for a given year or years entitling the participating to payment in cash or shares of common stock based upon the attainment of specified levels of performance as measured by pre-established, objective performance criteria determined at the discretion of the compensation committee. The amount of an incentive bonus award to be paid upon the attainment of each targeted level of performance will equal a percentage of a participant’s base salary for the fiscal year, a fixed dollar amount, or such other formula, as determined by the compensation committee. | |
| ● | Other cash-based and other stock-based awards may entitle participants to receive shares of Aerami Common Stock or cash payments in the future in the amounts and subject to the terms and conditions that the compensation committee will determine. |
Change in Control. The compensation committee may, at the time of the grant of an award and as set forth in an award agreement, provide for the effect of a “Change in Control” (as defined below) on an award. Except to the extent provided by an award agreement, upon or in anticipation of any Change in Control, the compensation committee has broad authority to make modifications, adjustments or amendments to outstanding awards or the 2015 Plan as the compensation committee deems necessary or appropriate.
Under the 2015 Plan, a “Change in Control” is generally defined as the occurrence of any one of the following events:
| ● | The acquisition by a person or group of securities of Aerami representing 50% or more of either (A) the combined voting power of Aerami’s then outstanding securities or (B) the then outstanding shares of Aerami Common Stock (in either such case other than as a result of an acquisition of securities directly from Aerami); | |
| ● | any consolidation or merger of Aerami where the stockholders of Aerami, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own shares representing in the aggregate 50% or more of the combined voting power of the securities of the corporation issuing cash or securities in the consolidation or merger; | |
| ● | there shall occur (A) any sale, lease, exchange or other transfer of all or substantially all of the assets of Aerami, subject to certain restructurings, or (B) the approval by Aerami’s stockholders of any plan or proposal for the liquidation or dissolution of Aerami; or | |
| ● | the members of the Aerami Board at the beginning of any consecutive 24-calendar-month period cease for any reason other than due to death to constitute at least a majority of the members of the Aerami Board, subject to an exception for new members approved by the legacy Aerami Board. |
| -236- |
Notwithstanding the foregoing, no event or condition shall constitute a Change in Control to the extent that, if it were, a 20% tax would be imposed under Section 409A of the Code; provided that, in such a case, the event or condition shall continue to constitute a Change in Control to the maximum extent possible (e.g., if applicable, in respect of vesting without an acceleration of distribution) without causing the imposition of such 20% tax.
Amendment and Termination of the 2015 Plan. The Aerami Board may suspend or terminate the 2015 Plan and may amend the 2015 Plan at any time. However, (a) no such amendment, suspension or termination will materially and adversely affect the rights of any participant under any outstanding awards and (b) stockholder approval of any amendment to the 2015 Plan must be obtained to the extent necessary and desirable to comply with any applicable law, regulation or stock exchange rule, or for any amendment to the 2015 Plan that increases the number of shares available under the 2015 Plan. If not terminated earlier by the compensation committee or the Aerami Board, no further awards will be granted under the 2015 Plan on or after the tenth anniversary of the adoption of the 2015 Plan by the Aerami Board.
Director Compensation
The following table summarizes the total compensation of each of the non-employee directors of Aerami who served during 2021.
| Name and Principal Position | Year | Fees earned or paid in cash ($) | All Other Compensation ($) | Total ($) | ||||||||||
| Darlene Deptula-Hicks | 2021 | 14,348 | — | 14,348 | ||||||||||
| Ken Lee | 2021 | 25,000 | — | 25,000 | ||||||||||
| John Patton | 2021 | 34,678 | 63,100 | (1) | 97,778 | |||||||||
| Adam Stern | 2021 | 52,242 | — | 52,242 | ||||||||||
| Anne Whitaker | 2021 | 130,829 | — | 130,829 | ||||||||||
| Bill Welch | 2021 | 55,829 | — | 55,829 | (2) | |||||||||
| (1) | Amount reported includes $63,100 paid to Mr. Patton in 2021 pursuant to the Consulting Agreement entered into with Aerami on September 19, 2019. See “Certain Relationships and Related Party Transactions — Aerami’s Related Party Transactions — Separation and Release Agreement with Dr. Patton” for additional information on these arrangements. | |
| (2) | Amounts paid to Molex, Mr. Welch’s employer during 2021. |
During December 2021, each of Renee Tannenbaum and Theodore Reiss, who are expected to be appointed as directors of the Combined Company, served as advisors to the Aerami Board pursuant to separate Board Advisor Agreements. Aerami has accrued $3,940 for the service of Dr. Tannebaum and $2,446 for the service of Dr. Reiss for advisory services during December 2021.
Non-Employee director compensation policy
The Aerami Board has adopted a non-employee director compensation policy that is designed to enable us to attract and retain, on a long-term basis, highly qualified non-employee directors. Under the policy, each director who is not an employee is paid cash compensation, as set forth below:
Annual Retainer | |||||
| ● | Board of Directors | $ | 50,000 | ||
| ● | Board of Directors Chair | $ | 75,000 | ||
| ● | Audit Committee Chair | $ | 20,000 | ||
| ● | Audit Committee Member | $ | 10,000 | ||
| ● | Compensation Committee Chair | $ | 15,000 | ||
| ● | Compensation Committee Member | $ | 7,500 | ||
| ● | Nominating and Corporate Governance Committee Chair | $ | 10,000 | ||
| ● | Nominating and Corporate Governance Committee Member | $ | 5,000 | ||
| -237- |
In addition, in connection with the Closing, each non-employee director who has joined or joins the Board since November 2021 will be entitled to a grant of options to purchase 100,000 shares of the Combined Company common stock to be made on March 1, 2022 or such later date following completion of the Closing as determined by the compensation committee if the Closing has not occurred by March 1, 2022. The option grant will vest with respect to 33.3% on the first anniversary of the grant and in equal monthly instalments thereafter until the award is fully vested on the third anniversary of the grant.
Aerami expects to review director compensation periodically to ensure that director compensation remains competitive such that the company is able to recruit and retain qualified directors. Following the consummation of the Business Combination, the Combined Company intends to develop a director compensation program that is designed to align compensation with its business objectives and the creation of stockholder value, while enabling the Combined Company to attract, retain, incentivize and reward directors who contribute to the long-term success of the Combined Company.
MANAGEMENT AFTER THE BUSINESS COMBINATION
Information about Directors Expected to be Appointed to the Board Upon the Closing of the Business Combination
Effective immediately after the consummation of the Business Combination, the business and affairs of the post-combination company will be managed by or under the direction of the Combined Company Board. The management team of the post-combination company is expected to be composed of the management team of Aerami. The following table lists the names, ages as of February 11, 2022, and positions of the individuals who are expected to serve as directors and executive officers of the post-combination company upon consummation of the Business Combination:
| Name | Age | Position | ||||
| Executive Officers | ||||||
| Steven Thornton | 64 | Chief Executive Officer and Director | ||||
| Barry Deutsch | 58 | Chief Financial Officer | ||||
| Timm Crowder | 52 | President and Chief Operating Officer | ||||
| Non-Employee Directors | ||||||
| Anne Whitaker | 54 | Chairwoman and Director | ||||
| Darlene Deptula-Hicks | 64 | Director | ||||
| John S. Patton | 75 | Director | ||||
| Theodore F. Reiss | 64 | Director | ||||
| Renee P. Tannenbaum | 69 | Director | ||||
| William (“Bill”) P. Welch | 55 | Director | ||||
| -238- |
Executive Officers
Steven Thornton, Chief Executive Officer and Director. Mr. Thornton has served as Chief Executive Officer of Aerami since November 2020 and has served on the Aerami Board since April 2017, including serving as the Chairman of the Aerami Board from January 2020 to November 2021. Mr. Thornton also served as our Chief Executive Officer from July 2018 to September 2018. In 2011, he became President and Chief Executive Officer of Excelsior Medical LLC, a supplier of saline and heparin pre-filled syringes and disinfection caps in the U.S., until its sale to ICU Medical and Medline Industries in late 2015. From May 2007 to September 2010, he was President and Chief Executive Officer of Bioniche Pharma Inc., a company specialized in the development, manufacture and sale of both proprietary and generic injectable pharmaceuticals. In 2002, Mr. Thornton joined SkyePharma Inc., a company developing drug delivery technology for the sustained release of injectable drugs, where he served as President until its sale in 2007, at which time he was asked to join its board of directors. Mr. Thornton has held a number of board of director positions and has acted in advisory capacities for both private and public companies. He is currently the Chairman of the board of directors for XBolt Orthopaedics, an Irish based company focused on the marketing and sale of a novel device for hip fixation. Mr. Thornton received a B.A. in applied social sciences from Edge Hill College.
FoxWayne and Aerami believe that Mr. Thornton’s extensive experience in the pharmaceutical and medical device industries, including both executive and board roles, and his service as the Chief Executive Officer, qualify him to serve as a member of the Combined Company Board.
Barry Deutsch, Chief Financial Officer. Mr. Deutsch has served as Chief Financial Officer of Aerami since November 2021. Prior to joining Aerami, Mr. Deutsch served as the Chief Financial Officer and, prior to that, Vice President of Business Development at Xeris Pharmaceuticals, Inc. (Nasdaq: XERS) from July 2017 to October 2021. Previously, from 2007 to 2017, Mr. Deutsch was a Vice President for the BioScience Division of Baxter Healthcare Corporation, Baxalta Incorporated following its spinoff from Baxter, and Shire plc following its acquisition of Baxalta. Mr. Deutsch’s roles included serving as Vice President of Business Development at Baxter BioScience and Baxalta and head of business development and public-private partnerships for the intercontinental region at Baxalta and Shire. Mr. Deutsch also has held chief financial officer and vice president of business and corporate development roles at Ovation Pharmaceuticals, Inc. and TLContact, Inc. and investment banking positions at healthcare-focused Vector Securities International, Inc. and Salomon Brothers. Mr. Deutsch received a B.S. in Economics degree in finance and accounting from The Wharton School of the University of Pennsylvania and an M.B.A. from the Kellogg School of Management at Northwestern University.
Timm Crowder, Ph.D., President and Chief Operating Officer. Dr. Crowder has served as Aerami’s Chief Operating Officer since February 2020 and as President since October 2020. Prior to Aerami, Dr. Crowder served as Chief Operating Officer at Spyryx Biosciences, a biopharmaceutical company developing inhaled therapeutics for severe respiratory diseases from 2015 until 2019. Previously, he served as the director of Advanced Manufacturing Technology at GlaxoSmithKline, PLC (GSK: NYSE), where he led innovation in the areas of aerosol and dry powder inhaler release testing, technology enhanced manufacturing, and electronic inhaler and particle engineering technologies. Earlier in his tenure at GSK, he contributed to the development of manufacturing systems and quality-by-design for GSK’s current asthma and COPD products. Prior to GSK, Dr. Crowder was Co-Founder and Chief Technology Officer of Oriel Therapeutics Inc., an inhaler technology company launched to commercialize a dry powder inhaler he developed during his graduate research. Oriel was subsequently acquired by Sandoz Pharmaceuticals. Dr. Crowder earned his Ph.D. in biomedical engineering from UNC-Chapel Hill, a master’s degree in physics from North Carolina State University and a B.S. from Davidson College.
Directors
Anne Whitaker, Chairwoman and Director. Ms. Whitaker has served as a director since 2018 and has served as the Chairwoman of the Aerami Board since November 2020. She also served as Aerami’s Chief Executive Officer from October 2018 to November 2020. Ms. Whitaker started her healthcare career in 1991 as a sales representative with The Upjohn Company, now Pfizer, Inc. (NYSE: PFE). She subsequently transitioned to GlaxoSmithKline PLC (NYSE: GSK) in 1992 where she rose in the leadership ranks to become a member of the global management team as the Global Head of the Leadership and Organization Development Centre of Excellence and ultimately served as the Senior Vice President and Business Unit Head for the Cardiovascular, Metabolic, and Urology franchises from September 2009 to September 2011. Ms. Whitaker joined Sanofi SA (Euronext Paris: SAN-FR) in 2011 where she served through August 2014 as the President of the North America Region and CEO of Sanofi US, LLC. Ms. Whitaker served as the CEO and President of Synta Pharmaceuticals, Inc. (Nasdaq: SNTA) an oncology focused late stage pharmaceutical development company, which is now Madrigal Pharmaceuticals (Nasdaq: MDGL), from September 2014 to April 2015. She joined Bausch Healthcare Companies (NYSE: BHC) in May 2015 and served through January 2017 as Executive Vice President and Company Group Chairman for the global branded pharmaceutical segment where she notably led the successful integration of two multi-billion dollar businesses, Salix Pharmaceuticals and Dendreon. In 2017, Ms. Whitaker transitioned to the early stage biotech sector and helped build Novoclem Therapeutics, Inc., a subsidiary of KNOW Bio, LLC, focused on developing therapies to treat people living with severe, chronic respiratory diseases. Prior to joining Aerami, starting in April 2018, Ms. Whitaker served as Managing Partner for Anne Whitaker Group, LLC, a consultant and professional service firm focused primarily on advising biotech and specialty pharmaceutical companies with commercial and product development strategy. Ms. Whitaker received a B.S. in Chemistry from the University of North Alabama.
| -239- |
She serves as an independent director on the board of four public companies including OraSure (OSUR: Nasdaq), a diagnostic company; Faron Pharmaceuticals (FARN: LSE), a development stage pharma company; Caladrius (CLBS: Nasdaq), a development stage biotech company; and Mallinckrodt (MKN: NYSE), a specialty pharmaceutical company. She also serves on the board of Curio Digital Therapeutics, Inc., a privately held women’s health focused digital therapeutics company; Bryn Pharma, a privately held specialty pharma company; Trinity Life Science Partners, a privately held pharma services company; and the Board of Trustees for the University of North Alabama, her alma mater. She has previously served on the board of UDG Healthcare (UDGH: LSE), Wolfspeed, Inc. (formerly known as Cree, Inc.) (WOLF:NYSE), Vectura, Group Plc (VEC-GB: London Exchange), Synta Pharmaceuticals, Inc. (SNTA: Nasdaq) now Madrigal Pharmaceuticals, Inc. (MDGL: Nasdaq), Novoclem Therapeutics, Inc., and the Foundation Board for the North Carolina School of Mathematics and Science. In addition to her board work, she is an active industry advisor to private equity and venture capital funds in the US and Europe.
FoxWayne and Aerami believe that Ms. Whitaker’s experience in the life sciences industry, including in both executive and board roles, as well as experience she provides as Aerami’s former Chief Executive Officer qualify her to serve as a member of the Combined Company Board.
Darlene Deptula-Hicks, Director. Ms. Deptula-Hicks has served on the Aerami Board since October 2021. Ms. Hicks has served as the Chief Financial Officer of F-star Therapeutics, Inc. (Nasdaq: FSTX) since May 2019. Since January 2018, Ms. Deptula-Hicks has operated Crimson Consulting LLC, a strategic and financial consulting services company, and previously served as acting Chief Financial Officer for Northern Biologics, Inc. From May 2017 to January 2018, she served as Senior Vice President and Chief Financial Officer of T2 Biosystems, Inc., (Nasdaq:TTOO) and from December 2014 to February 2017, Ms. Deptula-Hicks was Senior Vice President and Chief Financial Officer of Pieris Pharmaceuticals, Inc. (Nasdaq:PIRS) From 2012 until November 2014, she served as Vice President and Chief Financial Officer of Microline Surgical, Inc. Ms. Deptula-Hicks received an M.B.A. from Rivier University and a B.S. in Accounting from Southern New Hampshire University.
Ms. Deptula-Hicks currently serves on the board of directors of Abcuro, Inc. and previously served on the board of directors and as audit committee chair of Xentic Biosciences (Nasdaq:XBIO), US Falcon, Inc., Technest Holdings, Inc. (AMEX:TCNH), and IMCOR Pharmaceuticals (Nasdaq:IMPH).
FoxWayne and Aerami believe that Ms. Deptula-Hicks’s extensive experience as a financial executive at public companies in the life sciences industry, in addition to her prior service on public company boards, qualify her to serve as a member of the Combined Company Board.
John S. Patton, Ph.D., Director. Dr. Patton has served on the Aerami Board since 2009, and is the founder, former Chief Executive Officer and former Chairman of the Board of Aerami. He is Executive Chairman of iPharma Limited, an inhalation contract development research organization and beginning in February 2020 became part-time Chief Executive Officer of Tesio Pharmaceuticals, an injectable osteoarthritis company. Dr. Patton cofounded and served as a member of the board of directors of InCarda Therapeutics, Inc., an inhalation focused cardiovascular company, from 2009 until 2015. In 1998, Dr. Patton was the founding investor of Halozyme Therapeutics, Inc., a biopharmaceutical company, and served as a member of its board of directors from 2004 until 2015. From 1990 to 2008, Dr. Patton served in a variety of roles at Inhale/Nektar Therapeutics, which he co-founded, focusing on inhalation and PEGylation technologies, including as Vice President of Research from 1991 to 2001, Chief Scientific Officer from November 2001 to March 2008, and a member of the board of directors from 1990 to 2008. From 1985 to 1990, Dr. Patton served as the head of the drug delivery group of Genentech, Inc., a biotechnology company. From 1979 to 1985, Dr. Patton served as a professor of microbiology and of marine sciences at the University of Georgia. Dr. Patton also previously served as a member of the board of directors of Activaero GmbH, a drug delivery company, from 2007 until its acquisition by Vectura Group plc in March 2014. Dr. Patton serves on the advisory board of the Eberly College of Science at Pennsylvania State University and is a member of the Scripps Institution of Oceanography Advisory Counsel. Dr. Patton earned a B.S. in Zoology from Pennsylvania State University, an M.S. in Oceanography from the University of Rhode Island, and a Ph.D. in Biology from the University of California, San Diego. Dr. Patton also has held post doctorate positions in Biomedicine at Harvard Medical School and the University of Lund in Sweden.
| -240- |
FoxWayne and Aerami believe that Dr. Patton is qualified to serve on the Combined Company Board because of the perspective and experience he provides as Aerami’s founder and former Chief Executive Officer, as well as his many years of experience with inhalation technology and within the life sciences and pharmaceutical industries.
Theodore Reiss, MD, MBE, Director. Dr. Reiss has served as an advisor to the Aerami Board since 2021. He currently serves as Executive Vice President and Chief Medical Officer at Repertoire Immune Medicines, where he is responsible for developing, accelerating and executing the company’s clinical development portfolio, and providing medical expertise in decisions affecting the company’s clinical development programs. Dr. Reiss was previously a Venture Partner at Novo Ventures, the venture investing arm of Novo Holdings A/S, from January 2020 to November 2020. From September 2015 to December 2019, Dr. Reiss served as the Head, Clinical Research and Development, Inflammation & Immunology, at Celgene Corporation, prior to which Dr. Reiss was Vice President and Clinical Development Head, Primary Care Franchise, for Novartis. Dr. Reiss previously held management positions at Covance (now a division of Labcorp) and Merck & Co., Inc. Dr. Reiss currently serves as a member of the Board of Directors of the American Thoracic Society and the Commissioner’s Science Board at the FDA. Dr. Reiss received a M.D. from the Vanderbilt University School of Medicine, a Master of Bioethics from the University of Pennsylvania, and a B.S. in Biology and History from the University of Pennsylvania.
FoxWayne and Aerami believe that Dr. Reiss’ extensive experience in pharmaceutical development, translation science, inhaled therapy, and respiratory development, in addition to executive leadership experience in the pharmaceutical and life science industries, provides him with the qualifications and skills to serve as a director of the Combined Company Board.
Renee P. Tannenbaum, Pharm.D., Director. Dr. Tannenbaum has served as an advisor to the Aerami Board since 2021. Currently, she serves as a strategic advisor to several early stage biopharmaceutical companies, including Viracta Therapeutics and Glyscend Therapeutics. Most recently she served as Vice President of Global Partnering at Halozyme, Inc. from August 2016 to July 2021, where she was responsible for leading the team that executes business development activities and the company’s alliances through partnerships and collaborations. Dr. Tannenbaum was previously Head of Global Customer Excellence at AbbVie from October 2012 to January 2016, where she was responsible for building commercial capabilities for the organization. Previously, Dr. Tannenbaum served as President of Myrtle Potter & Company, LLC, a global life sciences consulting and advisory firm from April 2011 to October 2012 and Executive Vice President and Chief Commercial Officer at Elan Pharmaceuticals, Inc., from May 2009 to January 2011, where she was responsible for revenue generation for Elan’s marketed products, preparing for the commercialization of the company’s pipeline, including its Alzheimer’s portfolio, and strengthening the company’s overall commercial capabilities. Prior to her role at Elan, Dr. Tannenbaum was at Novartis Pharma AG for three years, where she led the Global Commercial Operations organization. Prior to that, Dr. Tannenbaum spent nine years at Bristol Myers Squibb and 16 years at Merck & Co., Inc. where she held a variety of leadership positions in operations and general management. Dr. Tannenbaum has served as a director of Zogenix, Inc. (Nasdaq: ZGNX) since February 2015 and Cardiff Oncology (Nasdaq: CRDF) since June 2021. Dr. Tannenbaum served as a director to Nordic Nanovector ASA, a publicly-traded company in Norway, and Cipher Pharmaceuticals, Inc. a Canadian publicly-traded company, from April to August 2016, Sharps Compliance Inc. from November 2012 to November 2014 and Immune Pharmaceuticals, Inc., a publicly-traded company, from August 2011 to October 2012. Dr. Tannenbaum retains a faculty position at the University of the Sciences’ Mayes College of Healthcare Business and Policy and serves as the Dean’s Professor. Dr. Tannenbaum received a Doctor of Pharmacy degree from the Philadelphia College of Pharmacy and Sciences, an M.B.A. from Temple University, and a B.S. in Pharmacy from the University of Connecticut.
FoxWayne and Aerami believe that Dr. Tannenbaum’s extensive experience in the biopharmaceutical industry, including providing strong executive leadership to numerous biopharmaceutical companies provides her with the qualifications and skills to serve as a director of the Combined Company Board.
Bill Welch, Director. Mr. Welch has served on the Aerami Board since January 2020. He served as Chief Technology Officer of Phillips-Medisize, a Molex Company, until his retirement in December 2021. Phillips-Medisize is a leading, global contract development and manufacturing organization focused on drug delivery devices, combination products, and connected health. Mr. Welch led their global pre-production services organization, a 900-person team spanning front-end innovation, development, and new product introduction. Mr. Welch joined Phillips-Medisize in February 2002, and held roles in operations, development, engineering, and sales during his tenure. He was active in the company’s merger and acquisition activity, including leading the search, acquisition, and integration of locations in China and Denmark. Prior to joining Phillips-Medisize, Mr. Welch worked 12 years in the Tier 1 automotive and electronics industries, in technical, supervisory, and business unit leadership roles. Mr. Welch received a B.S. in Industrial Engineering from University of Minnesota Duluth. Mr. Welch also serves on the board of Cadence, Inc., a metals-focused contract manufacturer serving the medical, automotive, defense, and industrial markets.
| -241- |
FoxWayne and Aerami believe that Mr. Welch’s experience as an executive in the pharmaceutical and medical device industries, particularly with respect to development and manufacturing of drug delivery and combination products, qualify him to serve as a member of the Combined Company Board.
Family Relationships
There are no family relationships between the Combined Company Board and any of its executive officers.
Board of Directors
Board Composition
FoxWayne’s Board is currently authorized to have five members and currently consists of five members including Robb Knie, Michael Reavey, Jeff Pavell, Jonathan Hale Zippin, and Sundeep Agrawal. Pursuant to the Merger Agreement, except as otherwise determined by Aerami, the directors of Aerami immediately prior to the Effective Time will be the directors of the Combined Company, in addition to Renee Tannenbaum and Theodore Reiss, until their respective successors are duly elected or appointed and qualified, and FoxWayne will take all action to cause such persons to be appointed to the Combined Company Board effective as of the Effective Time. We expect that the Combined Company Board will be Steven Thornton, Anne Whitaker, Darlene Deptula-Hicks, John Patton, Theodore Reiss, Renee Tannenbaum, and Bill Welch.
In accordance with the Combined Company’s amended and restated certificate of incorporation and amended and restated bylaws that will be in effect upon the consummation of the Business Combination, the Combined Company’s Board will be divided into three classes with staggered three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. The Combined Company’s directors will be divided among the three classes as follows:
● the Class I directors will be and and their terms will expire at the first annual meeting of stockholders following the Effective Time;
● the Class II directors will be and and their terms will expire at the second annual meeting of stockholders following the Effective Time; and
● the Class III directors will be , and and their terms will expire at the third annual meeting of stockholders following the Effective Time.
Directors in a particular class will be elected for three-year terms at the annual meeting of stockholders in the year in which their terms expire. As a result, only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Each director shall hold office until his or her successor is duly elected and qualified or until his or her earlier death, resignation, disqualification or removal.
The Combined Company’s Proposed Charter and Proposed Bylaws that will be in effect upon the consummation of the Business Combination provide that only the Combined Company Board can fill vacant directorships, including newly-created seats. When the number of directors is increased or decreased, the Combined Company Board will determine the class or classes to which the increased or decreased number of directors shall be apportioned; provided, however, that no decrease in the number of directors shall shorten the term of any incumbent director.
| -242- |
Director Independence
Nasdaq listing rules require that a majority of the board of directors of a company listed on Nasdaq be composed of “independent directors,” which is defined generally as a person other than an officer or employee of the company or its subsidiaries or any other individual having a relationship, which, in the opinion of the company’s board of directors, would interfere with the director’s exercise of independent judgment in carrying out the responsibilities of a director. The FoxWayne Board has determined that, upon the consummation of the Business Combination, each of Ms. Deptula-Hicks, Dr. Reiss, Dr. Tannenbaum, and Mr. Welch will be an independent director under the Nasdaq listing rules. In making these determinations, the board of directors considered the current and prior relationships that each non-employee director has with Aerami and will have with the Combined Company and all other facts and circumstances that the board of directors deemed relevant in determining independence, including the beneficial ownership of Combined Company common stock by each non-employee director after the Business Combination, and the transactions involving them described in the section titled “Certain Relationships and Related Transactions.”
Committees of the Board of Directors
The standing committees of Combined Company Board will consist of an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee. The expected composition of each committee following the Business Combination is set forth below.
Audit Committee
The Audit Committee of the Combined Company will be established in accordance with Section 3(a)(58)(A) of the Exchange Act and following the Business Combination will consist of , and , each of whom are independent directors under Nasdaq listing standards and Rule 10A-3 of the Exchange Act and are “financially literate” as defined under the Nasdaq listing standards. will serve as chairman of the Audit Committee. The Board has determined that qualifies as an “audit committee financial expert,” as defined under rules and regulations of the SEC.
The Audit Committee’s duties will be specified in the Audit Committee Charter to be adopted at the Effective Time.
Compensation Committee
Following the Business Combination, the Compensation Committee will consist of , and , each of whom is an independent director. will serve as chairman of the Compensation Committee. The functions of the Compensation Committee will be set forth in a Compensation Committee Charter to be adopted at the Effective Time.
Nominating and Corporate Governance Committee
Following the Business Combination, our Nominating and Corporate Governance Committee (the “Nominating Committee”) will consist of , and , each of whom is an independent director under Nasdaq’s listing standards. is the chair the Nominating Committee. The Nominating Committee is responsible for overseeing the selection of persons to be nominated to serve on our board of directors. The Nominating Committee will consider persons identified by its members, management, stockholders, investment bankers and others.
The guidelines for selecting nominees, will be specified in the Nominating and Corporate Governance Committee Charter to be adopted at the Effective Time.
Code of Conduct and Ethics
Upon the consummation of the Business Combination, the Combined Company Board will adopt a new code of conduct and ethics (the “Combined Company Code of Ethics”) for the Combined Company’s directors, officers, employees and certain affiliates in accordance with applicable federal securities laws, a copy of which will be available on the Combined Company’s website at .
If we amend or grant a waiver of one or more of the provisions of the Combined Company Code of Ethics, we intend to satisfy the requirements under Item 5.05 of Form 8-K regarding the disclosure of amendments to or waivers from provisions of our Combined Company Code of Ethics that apply to our principal executive officer, principal financial officer and principal accounting officer by posting the required information on the Combined Company’s website at . The information on this website is not part of this proxy statement/prospectus.
| -243- |
COMPARISON OF CORPORATE GOVERNANCE AND STOCKHOLDER RIGHTS
Set forth below is a summary comparison of material differences between the rights of FoxWayne stockholders under the existing Charter and existing Bylaws (left column) and under the Proposed Charter and Proposed Bylaws (right column). The summary set forth below is not intended to be complete or to provide a comprehensive discussion of the governing documents described herein. The summary below is subject to, and qualified in its entirety by reference to, the full text of the existing Charter and existing Bylaws as well as the Proposed Charter a copy of which is attached as Annex B to this proxy statement/prospectus, and the Proposed Bylaws, a copy of which is attached as Annex C to this proxy statement/prospectus, as well as the relevant provisions of the DGCL. You should carefully read this entire document and the other referenced documents, including the governing corporate instruments, for a more complete understanding of the differences between being a FoxWayne stockholder before the Business Combination and being a Combined Company stockholder following the completion of the Business Combination.
For information on the Charter Amendment Proposal, see the section titled “Proposal 2 - The Charter Proposal.” For information on the Bylaw Amendment Proposal, see the section titled “Proposal 3 - The Bylaw Proposal.”
| Current Governance | Proposed Governance | |
| Name Change | ||
| Under the existing Charter, the name of the corporation is FoxWayne Enterprises Acquisition Corp. | The Proposed Charter will provide that the name of the corporation will become Aerami Therapeutics Holdings, Inc. | |
| Purpose | ||
| Under the existing Charter, the purpose of FoxWayne is to consummate the Initial Business Combination. | The Proposed Charter will provide that the purpose of the Combined Company to engage in any lawful act or activity for which corporations may be organized under the DGCL. | |
| Authorized Capital Stock | ||
| Under the existing Charter, the total number of authorized shares of all classes of capital stock is 53,000,000 shares, consisting of (a) 52,000,000 shares of common stock, par value $0.0001 per share, including (i) 50,000,000 shares of Class A Common Stock and (ii) 2,000,000 shares of Class B Common Stock and (b) 1,000,000 shares of Preferred Stock, par value $0.0001 per share. | The Proposed Charter would increase the total number of authorized shares of all classes of capital stock to 210,000,000 shares, consisting of (i) 200,000,000 shares of Combined Company common stock and (ii)10,000,000 shares of Combined Company preferred stock. | |
| In addition, the Proposed Charter provides for the elimination of Class B Common Stock and any rights of holders thereof. Upon the adoption of the Proposed Charter, each share of Class A Common Stock issued and outstanding or held in treasury immediately prior to the Effective Time will be automatically reclassified as and converted into one share of common stock. | ||
| Supermajority Voting Provisions | ||
| Under the existing Charter, the affirmative vote of a majority of the holders of the voting power of all then outstanding shares of FoxWayne’s capital stock is required to amend, alter, change or repeal, or adopt any provisions inconsistent with Article IV pertaining to the powers, preferences and relative, participating, optional and other or special rights of the Class B Common Stock; Article VI pertaining to the bylaws. | The Proposed Charter will require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal, or adopt any provisions inconsistent with Article V pertaining to stockholders; Article VI pertaining to directors; and Article VII pertaining to limitation of liability and Article IX pertaining to an amendment to the Combined Company’s bylaws. | |
| -244- |
| Under the existing Bylaws, the affirmative vote of a majority of the holders of the voting power of all then outstanding shares of FoxWayne’s capital stock is required to amend, alter, change or repeal the Bylaws. | The Proposed Bylaws will require the affirmative vote of the holders of at least two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend, alter, change or repeal the Proposed Bylaws. | |
| Director Removal | ||
| Under the existing Charter, any or all of the directors may be removed from office at any time, but only for cause and only by the affirmative vote of holders of a majority of the voting power of all then outstanding shares of capital stock of FoxWayne entitled to vote generally in the election of directors, voting together as a single class. | The Proposed Charter will provide that, subject to the rights of holders of the Combined Company’s preferred stock, the Combined Company Board or any individual directors may be removed only for cause and only by affirmative vote of the holders of at least two-thirds of the voting power of all then-outstanding shares of the Combined Company’s capital stock entitled to vote at an election of directors. | |
| Bylaw Amendment | ||
| Under the existing Charter, in addition to any vote of the holders of any class or series of capital stock of FoxWayne required by law or the existing Charter, the affirmative vote of the holders of at least a majority of the voting power of all then outstanding shares of capital stock of FoxWayne entitled to vote generally in the election of directors, voting together as a single class, shall be required for the stockholders to adopt, amend, alter or repeal the By-Laws. | The Proposed Charter will require the affirmative vote of the holders of not less than two-thirds of the voting power of all then outstanding shares of the Combined Company’s capital stock entitled to vote thereon, voting together as a single class, to amend or repeal any provision of the Combined Company’s bylaws. | |
| Notice Requirements for Stockholder Proposals and Director Nominations | ||
| Under the existing Bylaws, stockholders seeking to bring business before a meeting or to nominate candidates for election as directors must provide timely notice of their intent in writing subject to certain exceptions as set forth in Article II, Section 2.7 and Article III, Section 3.2 | The Proposed Bylaws will provide that stockholders seeking to bring business before a meeting of stockholders, or to nominate candidates for election as directors at a meeting of the stockholders, must provide timely notice of their intent in writing subject to certain exceptions and provide additional disclosures as required of any “Proposing Person” or “Nominating Person.” These additional disclosures will include (A) disclosure of any underlying derivative securities that constitute a “call equivalent position,” pursuant to Rule 16a-1(c) of the Exchange Act, (B) disclosure of any rights to dividends on the shares of any class or series of shares of the Combined Company, (C) disclosure of any material pending or threatened legal proceeding the Proposing Person is a party to that involves the Combined Company or any of its officers or directors, affiliates, (D) disclosure of any other material relationship between such Proposing Person, and the Combined Company its affiliates, (E) disclosure of any direct or indirect material interest in any material contract with the Combined Company or an affiliate, (F) a representation that such Proposing Person intends to deliver a proxy statement required to approve or adopt the proposal or otherwise solicit proxies from stockholders in support of such proposal and (G) disclosure of any other information relating to such Proposing Person that would be required to be disclosed in a proxy statement required to be made pursuant to Section 14(a) of the Exchange Act. | |
| Action by Written Consent | ||
| Under the existing Bylaws, any action required to be taken at any annual or special meeting of the stockholders may be taken with written consent. | The Proposed Bylaws prohibit stockholder action by written consent in lieu of a meeting. Notwithstanding the foregoing, certain actions by the holders of preferred stock voting separately as a class may be taken to the extent expressly so provided by the applicable certificate of designation. | |
| Lock-up | ||
| Under the existing Bylaws, stockholders are not subject to any lock-up restrictions. | Under the Proposed Bylaws, stockholder will be subject to lock up provision pursuant to which holders of Combined Company common stock issued as consideration pursuant to the Merger Agreement or upon the exercise, exchange, conversion, or settlement of any Exchanged Options, Exchanged RSU Awards, or Assumed Warrants may not Transfer such shares until 180 days after the date that the Form S-1 Shelf is declared effective by the SEC, subject to certain exceptions. | |
| -245- |
DESCRIPTION OF NEW SECURITIES
The following summary of certain provisions of Combined Company securities does not purport to be complete and is subject to the Proposed Charter, the Proposed Bylaws and the provisions of applicable law. A copy of the Proposed Charter is attached as Annex B to this proxy statement/prospectus and a copy of the Proposed Bylaws is attached as Annex C to this proxy statement/prospectus. In this section, “we,” “our,” the “Company” or “Combined Company” generally refers to the Combined Company from and after the Business Combination.
Authorized and Outstanding Stock
The total amount of our authorized stock will consist of 210,000,000 shares, consisting of 200,000,000 shares of common stock and 10,000,000 shares of preferred stock. We expect to have approximately shares of Combined Company common stock outstanding immediately after the consummation of the Business Combination and related transactions, assuming that none of the outstanding shares of FoxWayne Class A Common Stock are redeemed in connection with the Business Combination. Upon the adoption of the Proposed Charter, all Class B Common Stock, and any rights of the holders thereof will be eliminated. Each share of Class A Common Stock issued and outstanding or held in treasury immediately prior to the Effective Time will be reclassified as Combined Company common stock. As of the Record Date, there were shares of FoxWayne Class A Common Stock, shares of FoxWayne Class B Common Stock and shares of FoxWayne Preferred Stock outstanding. We do not expect any shares of Combined Company preferred stock will be issued or outstanding immediately after the Business Combination.
The following summary describes all material provisions of our capital stock. We urge you to read the Proposed Charter and the Proposed Bylaws (copies of which are attached to this proxy statement/prospectus as Annex B and Annex C, respectively).
Combined Company Common Stock
Voting Rights
Each holder of Combined Company common stock will be entitled to one vote for each share of Combined Company common stock held of record by such holder as of the record date for determining stockholders entitled to vote on such matters. Except as otherwise required by law, holders of Combined Company common stock shall not be entitled to vote on any amendment to the Proposed Charter (including any certificate of designation) that relates solely to the rights, powers, preferences (or the qualifications, limitations or restrictions thereof) or other terms of one or more outstanding series of Preferred Stock if the holders of such affected series are entitled, either separately or together with the holders of one or more other such series, to vote thereon pursuant to the Proposed Charter (including any certificate of designation) or pursuant to the DGCL.
Dividend Rights
Subject to any other provisions of the Proposed Charter, as it may be amended from time to time, applicable law, and the rights and preferences of any holders of outstanding Preferred Stock, holders of shares of Combined Company common stock will be entitled to the payment of dividends on the Combined Company common stock when, as and if declared by the Combined Company Board in accordance with applicable law.
Rights Upon Liquidation
Subject to any other provisions of the Proposed Charter, as it may be amended from time to time, applicable law, and the rights and preferences of any holders of any shares of any outstanding series of Preferred Stock, in the event of any liquidation, dissolution or winding up of the Combined Company, whether voluntary or involuntary, the funds and assets of the Combined Company that may be legally distributed to the Combined Company’s stockholders shall be distributed among the holders of the then outstanding Combined Company common stock pro rata in accordance with the number of shares of Combined Company common stock held by each such holder.
| -246- |
Other Rights
Lock-Up
Under the Proposed Charter and Proposed Bylaws, stockholders will be subject to a lock up provision pursuant to which holders of the Combined Company common stock issued as consideration pursuant to the Merger Agreement or upon the exercise, exchange, conversion, or settlement of any Exchanged Options, Exchanged RSU Awards, or Assumed Warrants may not Transfer such shares until 180 days after the date that the Form S-1 Shelf is declared effective by the SEC, subject to certain exceptions.
Combined Company Preferred Stock
The Combined Company Board has the authority to issue shares of Preferred Stock from time to time in one or more series, on terms it may determine, to divide shares of Preferred Stock into one or more series and to fix the designations, preferences, privileges, and restrictions of Preferred Stock, including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preference and the number of shares constituting any series or the designation of any series to the fullest extent permitted by the DGCL. The issuance of Combined Company preferred stock could have the effect of decreasing the trading price of Combined Company common stock, restricting dividends on the capital stock of Combined Company, diluting the voting power of the Combined Company common stock, impairing the liquidation rights of the capital stock of the Combined Company, or delaying or preventing a change in control of the Combined Company.
Election and Directors Vacancies
Subject to any other provisions of the Proposed Charter, as it may be amended from time to time, applicable law, and the rights and preferences of any holders of any shares of any outstanding series of Preferred Stock, the number of directors of the Combined Company Board shall be fixed solely and exclusively by resolution duly adopted from time to time by the Combined Company Board. The Combined Company Board will be divided into three classes, designated Class I, II and III. The initial Class I directors shall serve for a term expiring at the first annual meeting of the stockholders following the Effective Time; the initial Class II directors shall serve for a term expiring at the second annual meeting of the stockholders following the Effective Time; and the initial Class III directors shall serve for a term expiring at the third annual meeting of the stockholders following the Effective Time.
Except as the DGCL may otherwise require and subject to the rights, if any, of the holders of any series of Combined Company preferred stock, in the interim between annual meetings of stockholders or special meetings of stockholders called for the election of directors and/or the removal of one or more directors and the filling of any vacancy in that connection, newly created directorships and any vacancies on the Combined Company Board, including unfilled vacancies resulting from the removal of directors, may be filled only by the affirmative vote of a majority of the remaining directors then in office, even though less than a quorum, or by a sole remaining director. All directors will hold office until the expiration of their respective terms of office and until their successors will have been elected and qualified. A director elected or appointed to fill a vacancy resulting from the death, resignation or removal of a director or a newly created directorship will serve for the remainder of the full term of the class of directors in which the new directorship was created or the vacancy occurred and until his or her successor will have been elected and qualified.
Subject to the rights, of the holders of any series of Combined Company preferred stock, the Combined Company Board or any individual director may be removed from office at any time, but only for cause and only by the affirmative vote of the holders of at least two-thirds of the voting power of all of the then outstanding shares of voting stock of the Combined Company entitled to vote at an election of directors.
| -247- |
Quorum
Unless otherwise provided by law, the Proposed Charter or Proposed Bylaws, the holders of a majority in voting power of the stock issued and outstanding and entitled to vote, present in person, or by remote communication, if applicable, or represented by proxy, shall constitute a quorum for the transaction of business at all meetings of the stockholders. A quorum, once established at a meeting, shall not be broken by the withdrawal of enough votes to leave less than a quorum. If, however, a quorum is not present or represented at any meeting of the stockholders, then the directors present thereat may adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum is present.
Anti-takeover Effects of the Proposed Charter and the Proposed Bylaws
The Proposed Charter and the Proposed Bylaws contain provisions that may delay, defer or discourage another party from acquiring control of us. We expect that these provisions, which are summarized below, will discourage coercive takeover practices or inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with the Combined Company Board, which we believe may result in an improvement of the terms of any such acquisition in favor of our stockholders. However, they also give the Combined Company Board the power to discourage acquisitions that some stockholders may favor.
Classified Combined Company Board
As indicated above, the Proposed Charter provides that the Combined Company Board will be divided into three classes of directors, with each class of directors being elected by the Combined Company stockholders every three years. The classification of directors will have the effect of making it more difficult for stockholders to change the composition of the Combined Company Board.
Authorized but Unissued Capital Stock
Delaware law does not require stockholder approval for any issuance of authorized shares. However, the listing requirements of Nasdaq, which would apply if and so long as the Combined Company common stock remains listed on Nasdaq, require stockholder approval of certain issuances equal to or exceeding 20% of the then outstanding voting power or then outstanding number of shares of Combined Company common stock. Additional shares that may be issued in the future may be used for a variety of corporate purposes, including, but not limited to, future public offerings, to raise additional capital or to facilitate acquisitions.
One of the effects of the existence of unissued and unreserved Combined Company common stock may be to enable the Combined Company Board to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of the Combined Company by means of a merger, tender offer, proxy contest or otherwise and thereby protect the continuity of management and possibly deprive stockholders of opportunities to sell their shares of Combined Company common stock at prices higher than prevailing market prices.
Special Meeting, Action by Written Consent and Advance Notice Requirements for Stockholder Proposal
Unless otherwise required by law, and subject to the rights, if any, of the holders of any series of the Combined Company preferred stock, special meetings of the stockholders of Combined Company, for any purpose or purposes, may be called only by or at the direction of (i) a majority of the Combined Company Board, (ii) the Chairman of the Combined Company, (iii) or the Chief Executive Officer of the Combined Company. Unless otherwise required by law, written notice of a special meeting of stockholders, stating the time, place and purpose or purposes thereof, shall be given to each stockholder entitled to vote at such meeting, not less than 10 or more than 60 days before the date fixed for the meeting. Business transacted at any special meeting of stockholders will be limited to the purposes stated in the notice.
In addition, the Proposed Bylaws require advance notice procedures for stockholder proposals to be brought before an annual meeting of the stockholders, including the nomination of directors. Stockholders at an annual meeting may only consider the proposals specified in the notice of meeting or brought before the meeting by or at the direction of the Combined Company Board, or by a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has delivered a timely written notice in proper form to the Combined Company’s secretary, of the stockholder’s intention to bring such business before the meeting.
| -248- |
These provisions could have the effect of delaying until the next stockholder meeting any stockholder actions, even if such actions are favored by the holders of a majority of the Combined Company’s outstanding voting securities.
Amendment to Charter and Bylaws
The DGCL provides generally that the affirmative vote of a majority of the outstanding stock entitled to vote on amendments to a corporation’s certificate of incorporation or bylaws is required to approve such amendment, unless a corporation’s certificate of incorporation or bylaws, as the case may be, requires a greater percentage.
The Proposed Charter will provide that the following provisions therein may be amended, altered, repealed or rescinded only by the affirmative vote of the holders of at least two-thirds in voting power of all the then outstanding shares of Combined Company’s stock entitled to vote thereon as a class:
| ● | the provisions regarding the size, classification, appointment, removal and authority of the Combined Company Board; | |
| ● | the provisions prohibiting stockholder actions without a meeting; | |
| ● | the provisions regarding calling special meetings of stockholders; | |
| ● | the provisions regarding the limited liability of directors of the Combined Company. |
The Proposed Bylaws may be amended or repealed (A) by the affirmative vote of a majority of the entire Combined Company Board then in office (subject to any bylaw requiring the affirmative vote of a larger percentage of the members of the Combined Company Board) or (B) without the approval of the Combined Company Board, by the affirmative vote of the holders of two-thirds of the outstanding voting stock of the Combined Company entitled to vote generally in an election of directors, voting together as a single class.
Limitations on Liability and Indemnification of Officers and Directors
The Proposed Charter limits the liability of the directors of the Combined Company to the fullest extent permitted by the DGCL, and the Proposed Bylaws provide that the Combined Company will indemnify them to the fullest extent permitted by such law. The Combined Company expects to enter into agreements to indemnify its directors, executive officers and other employees as determined by the Combined Company Board. Under the terms of such indemnification agreements, the Combined Company will be required to indemnify each of its directors and officers, to the fullest extent permitted by the laws of the State of Delaware, if the indemnitee acted in good faith and in a manner the indemnitee reasonably believed to be in or not opposed to the best interests of the Combined Company. The Combined Company must indemnify its officers and directors against all reasonable fees, expenses, charges and other costs of any type or nature whatsoever, including any and all expenses and obligations paid or incurred in connection with investigating, defending, being a witness in, participating in (including on appeal), or preparing to defend, be a witness or participating in any completed, actual, pending or threatened action, suit, claim or proceeding, whether civil, criminal, administrative or investigative, or establishing or enforcing a right to indemnification under the indemnification agreement. The indemnification agreements will also require the Combined Company, if so requested, to advance all reasonable fees, expenses, charges and other costs that such director or officer incurred, provided that such person will return any such advance if it is ultimately determined that such person is not entitled to indemnification by the Combined Company. Any claims for indemnification by the Combined Company’s directors and officers may reduce the Combined Company’s available funds to satisfy successful third-party claims against it and may reduce the amount of money available to it.
| -249- |
UNITS
FoxWayne’s initial public offering consisted of units, each of which, had an offering price of $10.00 and consisted of one share of Class A Common Stock and one redeemable warrant. Each warrant entitles the registered holder to purchase one share of Class A Common Stock at a price of $11.50 per share, subject to adjustment. On or about February 26, 2021, FoxWayne’s Class A Common Stock and warrants commenced separate trading on Nasdaq, and unit holders were given the opportunity to continue to hold units or separate their units into the component securities. Units not separated are currently listed under the symbol “FOXWU.”
WARRANTS
Public Stockholders’ Warrants
FoxWayne’s initial public offering consisted of units, each of which, had an offering price of $10.00 and consisted of one share of Class A Common Stock and one redeemable warrant. Each warrant entitles the registered holder to purchase one share of our Class A Common Stock at a price of $11.50 per share, subject to adjustment as discussed below, at any time commencing on the later of 12 months from the closing of the initial public offering or the completion of the Initial Business Combination. The warrants will expire five years after the completion of the Initial Business Combination, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
FoxWayne will not be obligated to deliver any shares of Class A Common Stock pursuant to the exercise of a warrant and will have no obligation to settle such warrant exercise unless a registration statement under the Securities Act with respect to the shares of Class A Common Stock underlying the warrants is then effective and a prospectus relating thereto is current, subject to satisfying our obligations described below with respect to registration. No warrant will be exercisable and FoxWayne will not be obligated to issue shares of Class A Common Stock upon exercise of a warrant unless Class A Common Stock issuable upon such warrant exercise has been registered, qualified or deemed to be exempt under the securities laws of the state of residence of the registered holder of the warrants. In the event that the conditions in the two immediately preceding sentences are not satisfied with respect to a warrant, the holder of such warrant will not be entitled to exercise such warrant and such warrant may have no value and expire worthless. In no event will FoxWayne be required to net cash settle any warrant. In the event that a registration statement is not effective for the exercised warrants, the purchaser of a unit containing such warrant will have paid the full purchase price for the unit solely for the share of Class A Common Stock underlying such unit.
FoxWayne has agreed that as soon as practicable, but in no event later than 15 business days, after the closing of the Initial Business Combination, FoxWayne will use reasonable best efforts to file, and within 60 business days following the Initial Business Combination to have declared effective, a registration statement for the registration, under the Securities Act, of the shares of Class A Common Stock issuable upon exercise of the warrants. FoxWayne will use reasonable best efforts to maintain the effectiveness of such registration statement, and a current prospectus relating thereto, until the expiration of the warrants in accordance with the provisions of the warrant agreement. Notwithstanding the above, if FoxWayne’s Class A Common Stock is at the time of any exercise of a warrant not listed on a national securities exchange such that it satisfies the definition of a “covered security” under Section 18(b)(1) of the Securities Act, we may, at our option, require holders of public warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and, in the event we so elect, we will not be required to file or maintain in effect a registration statement, but we will be required to use our best efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available.
Redemption of Warrants Public Stockholders’ Warrants. Once the warrants become exercisable, FoxWayne may call the warrants for redemption:
| ● | in whole and not in part; | |
| ● | at a price of $0.01 per warrant; | |
| ● | upon not less than 30 days’ prior written notice of redemption to each warrant holder; and | |
| ● | if, and only if, the reported last sale price of the Class A Common Stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending three business days before we send the notice of redemption to the warrant holders. |
| -250- |
If and when the warrants become redeemable by us, we may exercise our redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws. FoxWayne will use its best efforts to register or qualify such shares of Class A Common Stock under the blue sky laws of the state of residence in those states in which the warrants were offered.
FoxWayne has established the last of the redemption criterion discussed above to prevent a redemption call unless there is at the time of the call a significant premium to the warrant exercise price. If the foregoing conditions are satisfied and we issue a notice of redemption of the warrants, each warrant holder will be entitled to exercise its warrant prior to the scheduled redemption date. However, the price of the Class A Common Stock may fall below the $18.00 redemption trigger price (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) as well as the $11.50 warrant exercise price after the redemption notice is issued.
Redemption Procedures and Cashless Exercise. If FoxWayne calls the warrants for redemption as described above, FoxWayne’s management will have the option to require any holder that wishes to exercise its warrant to do so on a “cashless basis.” In determining whether to require all holders to exercise their warrants on a “cashless basis,” our management will consider, among other factors, our cash position, the number of warrants that are outstanding and the dilutive effect on our stockholders of issuing the maximum number of shares of Class A Common Stock issuable upon the exercise of our warrants. If our management takes advantage of this option, all holders of warrants would pay the exercise price by surrendering their warrants for that number of shares of Class A Common Stock equal to the quotient obtained by dividing (x) the product of the number of shares of Class A Common Stock underlying the warrants, multiplied by the excess of the “fair market value” (as defined herein) over the exercise price of the warrants by (y) the fair market value. The “fair market value” shall mean the average last reported sale price of the Class A Common Stock for the ten trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants. If our management takes advantage of this option, the notice of redemption will contain the information necessary to calculate the number of shares of Class A Common Stock to be received upon exercise of the warrants, including the “fair market value” in such case. Requiring a cashless exercise in this manner will reduce the number of shares to be issued and thereby lessen the dilutive effect of a warrant redemption. We believe this feature is an attractive option to us if we do not need the cash from the exercise of the warrants after our Initial Business Combination. If we call our warrants for redemption and our management does not take advantage of this option, our Sponsor and its permitted transferees would still be entitled to exercise their Private Placement Warrants for cash or on a cashless basis using the same formula described above that other warrant holders would have been required to use had all warrant holders been required to exercise their warrants on a cashless basis, as described in more detail below.
A holder of a warrant may notify FoxWayne in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the warrant agent’s actual knowledge, would beneficially own in excess of 4.8% or 9.8% (or such other amount as a holder may specify) of the shares of Class A Common Stock outstanding immediately after giving effect to such exercise.
If the number of outstanding shares of Class A Common Stock is increased by a stock dividend payable in shares of Class A Common Stock, or by a split-up of shares of Class A Common Stock or other similar event, then, on the effective date of such stock dividend, split-up or similar event, the number of shares of Class A Common Stock issuable on exercise of each warrant will be increased in proportion to such increase in the outstanding shares of Class A Common Stock. A rights offering to holders of Class A Common Stock entitling holders to purchase shares of Class A Common Stock at a price less than the fair market value will be deemed a stock dividend of a number of shares of Class A Common Stock equal to the product of (i) the number of shares of Class A Common Stock actually sold in such rights offering (or issuable under any other equity securities sold in such rights offering that are convertible into or exercisable for Class A Common Stock) multiplied by (ii) one minus the quotient of (x) the price per share of Class A Common Stock paid in such rights offering divided by (y) the fair market value. For these purposes (i) if the rights offering is for securities convertible into or exercisable for Class A Common Stock, in determining the price payable for Class A Common Stock, there will be taken into account any consideration received for such rights, as well as any additional amount payable upon exercise or conversion and (ii) fair market value means the volume weighted average price of Class A Common Stock as reported during the ten trading day period ending on the trading day prior to the first date on which the shares of Class A Common Stock trade on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
| -251- |
In addition, if FoxWayne, at any time while the warrants are outstanding and unexpired, pays a dividend or makes a distribution in cash, securities or other assets to the holders of Class A Common Stock on account of such shares of Class A Common Stock (or other shares of our capital stock into which the warrants are convertible), other than (a) as described above, (b) certain ordinary cash dividends, (c) to satisfy the redemption rights of the holders of Class A Common Stock in connection with the Initial Business Combination, (d) to satisfy the redemption rights of the holders of Class A Common Stock in connection with a stockholder vote to amend FoxWayne’s Charter (i) to modify the substance or timing of our obligation to allow redemption in connection with the Initial Business Combination or to redeem 100% of our public shares if we do not complete the Initial Business Combination within 12 months from the closing of the initial public offering (or up to 18 months from the consummation of the initial public offering if we extend the period of time to consummate an Initial Business Combination), or (ii) with respect to any other provision relating to stockholders’ rights or pre- Initial Business Combination activity, or (e) in connection with the redemption of our public shares upon our failure to complete the Initial Business Combination, then the warrant exercise price will be decreased, effective immediately after the effective date of such event, by the amount of cash and/or the fair market value of any securities or other assets paid on each share of Class A Common Stock in respect of such event.
If the number of outstanding shares of our Class A Common Stock is decreased by a consolidation, combination, reverse stock split or reclassification of shares of Class A Common Stock or other similar event, then, on the effective date of such consolidation, combination, reverse stock split, reclassification or similar event, the number of shares of Class A Common Stock issuable on exercise of each warrant will be decreased in proportion to such decrease in outstanding shares of Class A Common Stock.
Whenever the number of shares of Class A Common Stock purchasable upon the exercise of the warrants is adjusted, as described above, the warrant exercise price will be adjusted by multiplying the warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of shares of Class A Common Stock purchasable upon the exercise of the warrants immediately prior to such adjustment, and (y) the denominator of which will be the number of shares of Class A Common Stock so purchasable immediately thereafter.
Public Warrant Holders’ Rights Under the Initial Business Combination
In case of any reclassification or reorganization of the outstanding shares of Class A Common Stock (other than those described above or that solely affects the par value of such shares of Class A Common Stock), or in the case of any merger or consolidation of FoxWayne with or into another corporation (other than a consolidation or merger in which we are the continuing corporation and that does not result in any reclassification or reorganization of our outstanding shares of Class A Common Stock), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of us as an entirety or substantially as an entirety in connection with which we are dissolved, the holders of the warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the warrants and in lieu of the shares of Class A Common Stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the warrants would have received if such holder had exercised their warrants immediately prior to such event. If less than 70% of the consideration receivable by the holders of Class A Common Stock in such a transaction is payable in the form of common stock in the combined company that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the warrant properly exercises the warrant within 30 days following public disclosure of such transaction, the warrant exercise price will be reduced as specified in the warrant agreement based on the Black-Scholes value (as defined in the warrant agreement) of the warrant. The warrants are issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and us. You should review a copy of the warrant agreement, which has been filed as an exhibit to the registration statement of which this prospectus is a part, for a complete description of the terms and conditions applicable to the warrants. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of at least 50% of the then outstanding public warrants to make any change that adversely affects the interests of the registered holders of public warrants.
| -252- |
In addition, if (x) FoxWayne issues additional shares of Class A Common Stock or equity-linked securities for capital raising purposes in connection with the closing of the Initial Business Combination at an issue price or effective issue price of less than $9.20 per share of Class A Common Stock (with such issue price or effective issue price to be determined in good faith by the FoxWayne Board and, in the case of any such issuance to our Sponsor or its affiliates, without taking into account any Founder Shares held by our sponsor or such affiliates, as applicable, prior to such issuance) (the “Newly Issued Price”), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the Initial Business Combination on the date of the consummation of the Initial Business Combination (net of redemptions), and (z) the volume weighted average trading price of Class A Common Stock during the 20 trading day period starting on the trading day prior to the day on which we consummate the Initial Business Combination (such price, the “Market Value”) is below $9.20 per share, then the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the greater of the Market Value and the Newly Issued Price, and the $18.00 per share redemption trigger price described above will be adjusted (to the nearest cent) to be equal to 180% of the greater of the Market Value and the Newly Issued Price.
The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price (or on a cashless basis, if applicable), by certified or official bank check payable to us, for the number of warrants being exercised. The warrant holders do not have the rights or privileges of holders of Class A Common Stock or any voting rights until they exercise their warrants and receive shares of Class A Common Stock. After the issuance of shares of Class A Common Stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, round down to the nearest whole number of shares of Class A Common Stock to be issued to the warrant holder.
We have agreed that, subject to applicable law, any action, proceeding or claim against us arising out of or relating in any way to the warrant agreement will be brought and enforced in the courts of the State of New York or the United States District Court for the Southern District of New York, and we irrevocably submit to such jurisdiction, which jurisdiction will be the exclusive forum for any such action, proceeding or claim. This provision applies to claims under the Securities Act but does not apply to claims under the Exchange Act or any claim for which the federal district courts of the United States of America are the sole and exclusive forum.
Private Placement Warrants
Simultaneously with the closing of the initial public offering, FoxWayne consummated the sale of 2,500,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant in a private placement to our Sponsor, generating gross proceeds of approximately $2,500,000. Simultaneously with the closing of the sale of the overallotment units, our Sponsor purchased an additional 300,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant, generating gross proceeds of approximately $300,000.
The Private Placement Warrants (including the warrants that may be issued upon conversion of Working Capital Loans and Extension Loans and the Class A Common Stock issuable upon exercise of the Private Placement Warrant) will not be transferable, assignable or salable until 30 days following the completion of the Initial Business Combination (except, among other limited exceptions restricting the transfers of Founder Shares and Private Placement Warrants to our officers, directors and other persons or entities affiliated with or related to our Sponsor, each of which will be subject to the same transfer restrictions) and they will not be redeemable by FoxWayne so long as they are held by our Sponsor or its permitted transferees. Otherwise, the Private Placement Warrants have terms and provisions that are identical to those of the warrants sold as part of the units in the initial public offering, including as to exercise price, exercisability and exercise period. If the Private Placement Warrants are held by holders other than the Sponsor or its permitted transferees, the Private Placement Warrants will be redeemable by us and exercisable by the holders on the same basis as the warrants included in the units sold in the initial public offering. Each of the warrants that may be issued upon conversion of Working Capital Loans and Extension Loans shall be identical to the Private Placement Warrants.
| -253- |
If holders of the Private Placement Warrant elect to exercise them on a cashless basis, they would pay the exercise price by surrendering their warrants for that number of shares of Class A Common Stock equal to the quotient obtained by dividing (x) the product of the number of shares of Class A Common Stock underlying the warrants, multiplied by the excess of the “fair market value” (defined below) over the exercise price of the warrants by (y) the fair market value. “Fair market value” means the average last reported sale price of the Class A Common Stock for the 10 trading days ending on the third trading day prior to the date on which the notice of warrant exercise is sent to the warrant agent. The reason that we have agreed that these warrants will be exercisable on a cashless basis so long as they are held by the Sponsor or its permitted transferees is because it is not known at this time whether they will be affiliated with us following an Initial Business Combination. If they remain affiliated with us, their ability to sell our securities in the open market will be significantly limited. We expect to have policies in place that prohibit insiders from selling our securities except during specific periods of time. Even during such periods of time when insiders will be permitted to sell our securities, an insider cannot trade in our securities if he or she is in possession of material non-public information. Accordingly, unlike public stockholders who could sell the shares of Class A Common Stock issuable upon exercise of the warrants freely in the open market, the insiders could be significantly restricted from doing so. As a result, we believe that allowing the holders to exercise such warrants on a cashless basis is appropriate.
In order to finance transaction costs in connection with an Initial Business Combination, our Sponsor or an affiliate of our Sponsor or certain of our officers and directors may, but are not obligated to, loan us funds as may be required. Up to $1,500,000 of such Working Capital Loans may be convertible into warrants at a price of $1.00 per warrant at the option of the lender. Such warrants would be identical to the Private Placement Warrants, including as to exercise price, exercisability and exercise period.
Our Sponsor has agreed not to transfer, assign or sell any of the Private Placement Warrants (including the Class A Common Stock issuable upon exercise of any of these warrants) until we complete our Initial Business Combination pursuant to the Lock-Up Agreement., except that, among other limited exceptions as described under the section of this prospectus titled “Principal Stockholders — Restrictions on Transfers of Founder Shares and Private Placement Warrants” made to our officers and directors and other persons or entities affiliated with or related to our Sponsor, each of which will be subject to the same transfer restrictions.
Aerami Warrants and Placement Agent Warrants
At the Effective Time, each Aerami Warrant that is outstanding and unexercised immediately prior to the Effective Time, whether vested or unvested, which does not terminate by its terms in connection with the Business Combination, will be assumed by FoxWayne and converted into a warrant to purchase shares of the Combined Company common stock on substantially the same terms and subject to substantially the same conditions (including as to vesting and exercisability) as are in effect with respect to such Aerami Warrants immediately prior to the Effective Time, with appropriate adjustments to the number of shares of Combined Company common stock underlying such warrant and the exercise price applicable thereto to account for the Merger.
Immediately prior to the Effective Time, the Placement Agent Warrants will automatically net exercise in full into applicable shares of Aerami securities pursuant to the terms of the respective warrant agreement.
LISTING OF SECURITIES
FoxWayne’s units, Class A Common Stock and warrants are currently listed on The Nasdaq Capital Market under the symbols “FOXWU,” “FOXW” and “FOXWW,” respectively. In connection with the closing of the Business Combination, FoxWayne intends to change its name to “Aerami Therapeutics Holdings, Inc.” and has submitted an application to list the shares of common stock of the Combined Company on The Nasdaq Capital Market under the symbol “ .”
| -254- |
CERTAIN ANTI-TAKEOVER PROVISIONS OF DELAWARE LAW AND OUR PROPOSED CHARTER AND BYLAWS
We have opted out of Section 203 of the DGCL under the existing Charter, and we will opt out of Section 203 of the DGCL under the Proposed Charter. Under Section 203 of the DGCL, Combined Company will be prohibited from engaging in any Business Combination with any stockholder for a period of three years following the time that such stockholder (the “interested stockholder”) came to own at least 15% of the outstanding voting stock of Combined Company (the “acquisition”), except if:
| ● | the Combined Company Board approved the acquisition prior to its consummation; | |
| ● | the interested stockholder owned at least 85% of the outstanding voting stock upon consummation of the acquisition; or | |
| ● | the Business Combination is approved by the Combined Company Board, and by a two-thirds majority vote of the other stockholders in a meeting. |
Generally, a “business combination” includes any merger, consolidation, asset or stock sale or certain other transactions resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who, together with that person’s affiliates and associates, owns, or within the previous three years owned, 15% or more of our voting stock.
Under certain circumstances, declining to opt out of Section 203 of the DGCL will make it more difficult for a person who would be an “interested stockholder” to effect various business combinations with Combined Company for a three-year period. This may encourage companies interested in acquiring Combined Company to negotiate in advance with the Combined Company Board because the stockholder approval requirement would be avoided if the Combined Company Board approves the acquisition which results in the stockholder becoming an interested stockholder. This may also have the effect of preventing changes in the Combined Company Board and may make it more difficult to accomplish transactions which stockholders may otherwise deem to be in their best interests.
Written Consent by Stockholders
Under the Proposed Charter, subject to the rights of any series of Preferred Stock then outstanding, any action required or permitted to be taken by the stockholders of Combined Company must be effected at a duly called annual or special meeting of stockholders of Combined Company and may not be effected by any consent in writing by such stockholders.
Special Meeting of Stockholders
Under the Proposed Charter, special meetings of stockholders of Combined Company may be called only by the chairperson of the Combined Company Board, the chief executive officer of Combined Company or the Combined Company Board acting pursuant to a resolution adopted by a majority of the total number of authorized directors whether or not there exist any vacancies in previously authorized directorships, and may not be called by any other person or persons. Only such business shall be considered at a special meeting of stockholders as shall have been stated in the notice for such meeting.
Advance Notice Requirements for Stockholder Proposals and Director Nominations
Under the Proposed Charter, advance notice of stockholder nominations for the election of directors and of business to be brought by stockholders before any meeting of the stockholders of Combined Company shall be given in the manner and to the extent provided in Combined Company’s bylaws.
| -255- |
SECURITIES ACT RESTRICTIONS ON RESALE OF NEW COMBINED COMPANY SECURITIES
Rule 144—General
Pursuant to Rule 144, a person who has beneficially owned restricted shares of our common stock or warrants for at least six months would be entitled to sell their securities provided that (a) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, a sale; (b) we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale and have filed all required reports under Section 13 or 15(d) of the Exchange Act during the 12 months preceding the sale (or such shorter period as we were required to file reports) other than Form 8-K reports, and (c) we have submitted electronically every Interactive Data File required to be submitted pursuant to SEC Rule 405 of the Securities Act, during the 12 months preceding such sale (or for such shorter period as we were required to submit such files). Persons who have beneficially owned restricted shares of Combined Company common stock or Aerami Warrants for at least six months but who are affiliates at the time of, or at any time during the 90 days preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three month period only a number of securities that does not exceed the greater of:
| ● | 1% of the total number of shares of such Combined Company securities then outstanding; or | |
| ● | the average reported weekly trading volume of such Combined Company securities during the four weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales by our affiliates under Rule 144 are also limited by manner of sale provisions and notice requirements and to the availability of current public information about us.
Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies
Rule 144 is not available for the resale of securities initially issued by shell companies or issuers that have been at any time previously a shell company. However, Rule 144 also includes an important exception to this prohibition if the following conditions are met:
| ● | the issuer of the securities that was formerly a shell company has ceased to be a shell company; | |
| ● | the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; | |
| ● | the issuer of the securities has filed all Exchange Act reports and materials required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports; and | |
| ● | at least one year has elapsed from the time that the issuer filed current Form 10 type information with the SEC reflecting its status as an entity that is not a shell company. |
As a result, FoxWayne’s initial stockholders will be able to sell their Founder Shares pursuant to Rule 144 without registration one year after FoxWayne has completed the Business Combination, although these shares may be sold sooner to the extent the resale of such shares has been registered on a registration statement that has been declared effective by the SEC.
We anticipate that following the consummation of the Business Combination, the Combined Company will not be a shell company, and so, once the conditions set forth in the exceptions listed above are satisfied, Rule 144 will become available for resale of the above noted restricted securities.
Registration Rights
In connection with the Closing, Aerami, FoxWayne, and certain of their respective stockholders will enter into a registration rights agreement, pursuant to which the Combined Company will be required to subsequently file a registration statement covering the resale of registrable securities held by the stockholders party thereto.
| -256- |
BENEFICIAL OWNERSHIP OF SECURITIES
The following table sets forth information known to FoxWayne regarding (a) the actual beneficial ownership of FoxWayne’s Class A Common Stock and Class B Common Stock, as of the record date prior to the Business Combination, and (b) the expected beneficial ownership of Combined Company common stock, assuming that no public shares of FoxWayne are redeemed, and alternatively the maximum redemptions scenario, which assumes that 3,771,881 shares of Class A Common Stock are redeemed as further described in the section titled “Unaudited Pro Forma Condensed Combined Financial Information — Basis of Pro Forma Presentation” by:
| ● | each of FoxWayne’s named executive officers and directors (pre-Business Combination); | |
| ● | each person who will become an executive officer or director of the Combined Company, post-Business Combination; | |
| ● | all current executive officers and directors of FoxWayne as a group, pre-Business Combination and all executive officers and directors of the Combined Company, post-Business Combination; and | |
| ● | each person who is, or is expected to be, the beneficial owner of more than 5% of the outstanding securities, pre-Business Combination, or Combined Company securities, post-Business Combination. |
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days of the record date.
The beneficial ownership of prior to the Business Combination is based on shares of Class A Common Stock and shares of Class B Common Stock (including Founder Shares) issued and outstanding in the aggregate as of the record date.
The expected beneficial ownership of shares of Combined Company common stock immediately following consummation of the Business Combination, assuming none of our public shares are redeemed, (a) assumes (i) that none of FoxWayne’s initial stockholders or the Aerami Equityholders purchase shares of Class A Common Stock in the open market, (ii) that are no other issuances of equity interests of FoxWayne and (iii) that there are no exercises of Aerami Options or Aerami Warrants and (b) does not take into account FoxWayne Warrants that will remain outstanding following the Business Combination and may be exercised at a later date.
The expected beneficial ownership of shares of Combined Company common stock immediately following consummation of the Business Combination, assuming the maximum redemptions scenario where 1,885,941 public shares have been redeemed, (a) assumes (i) that none of FoxWayne’s initial stockholders or the Aerami Equityholders purchase shares of Class A Common Stock in the open market, (ii) that there are no other issuances of equity interests of FoxWayne and (iii) that there are no exercises of Aerami Options or Aerami Warrants and (b) does not take into account FoxWayne Warrants that will remain outstanding following the Business Combination and may be exercised at a later date.
The expected beneficial ownership of shares of Combined Company common stock immediately following consummation of the Business Combination, assuming the interim redemptions scenario where 3,771,881 public shares have been redeemed, (a) assumes (i) that none of FoxWayne’s initial stockholders or the Aerami Equityholders purchase shares of Class A Common Stock in the open market, (ii) that there are no other issuances of equity interests of FoxWayne and (iii) that there are no exercises of Aerami Options or Aerami Warrants and (b) does not take into account FoxWayne Warrants that will remain outstanding following the Business Combination and may be exercised at a later date.
Unless otherwise indicated, we believe that all persons named in the table below have sole voting and investment power with respect to all shares of voting common stock beneficially owned by them.
| -257- |
| After the Business Combination | ||||||||||||||||||||||||
| Prior to the Business Combination | Assuming No Redemptions Scenario | Assuming
Interim Redemptions Scenario | Assuming Maximum Redemptions Scenario | |||||||||||||||||||||
| Name and Address of Beneficial Owners(1) | Number
of shares of Common Stock | % | Number of shares of Common Stock | % | Number of shares of Common Stock | % | ||||||||||||||||||
| Directors and Named Executive Officers of FoxWayne | ||||||||||||||||||||||||
| Robb Knie | ||||||||||||||||||||||||
| Jeff Pavell | ||||||||||||||||||||||||
| Sundeep Agrawal | ||||||||||||||||||||||||
| Michael Reavey | ||||||||||||||||||||||||
| Jonathan Hale Zippin | ||||||||||||||||||||||||
| All Directors and Named Executive Officers of FoxWayne as a Group (5 Individuals) | ||||||||||||||||||||||||
| Directors and Executive Officers of Combined Company | ||||||||||||||||||||||||
| Steven Thornton | ||||||||||||||||||||||||
| Barry Deutsch | ||||||||||||||||||||||||
| Timm Crowder | ||||||||||||||||||||||||
| Anne Whitaker | ||||||||||||||||||||||||
| Darlene Deptula-Hicks | ||||||||||||||||||||||||
| John S. Patton | ||||||||||||||||||||||||
| Theodore Reiss | ||||||||||||||||||||||||
| Renee P. Tannenbaum | ||||||||||||||||||||||||
| Bill Welch | ||||||||||||||||||||||||
| All Directors and Executive Officers of Combined Company as a Group (9 Individuals) | ||||||||||||||||||||||||
| 5% or Greater Stockholders | ||||||||||||||||||||||||
| Basso Capital Management, L.P.; Basso SPAC Fund LLC; Basso GP, LLC, Basso Management, LLC, Howard I. Fischer | ||||||||||||||||||||||||
| FoxWayne Enterprises Acquisition Sponsor LLC | ||||||||||||||||||||||||
| First Riverside Investors LP | ||||||||||||||||||||||||
| Hudson Bay Capital Management LP | ||||||||||||||||||||||||
| John S. Patton | ||||||||||||||||||||||||
| Karpus Investment Management | ||||||||||||||||||||||||
| Molex Ventures LLC | ||||||||||||||||||||||||
Polar Asset Management Partners Inc. | ||||||||||||||||||||||||
| Shaolin Capital Management LLC | ||||||||||||||||||||||||
* Less than 1%.
| (1) | Unless otherwise noted, the business address of each of the entities, directors and executive officers affiliated with FoxWayne in this table is 1 Rockefeller Plaza, Suite 1039, New York, NY 10020 and the business address of each of the entities, directors and executive officers affiliated with Aerami in this table is 2520 Meridian Parkway Suite 400, Durham, North Carolina 27713. |
| -258- |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
FoxWayne Related Party Transactions
Founder Shares
On October 15, 2020, the Sponsor paid $25,000 to cover certain of our offering and formation costs in consideration for 1,437,500 Founder Shares. In October 2020, the Sponsor transferred 25,000 Founder Shares to each of our independent directors, Messrs. Reavey, Pavell, Zippin and Agrawal, and 180,000 Founder Shares to certain other initial stockholders.
The Founder Shares included an aggregate of up to 187,500 shares that were subject to forfeiture depending on the extent to which the underwriters’ over-allotment option was exercised, so that the number of Founder Shares would equal, on an as-converted basis, approximately 20% of our issued and outstanding shares after the initial public offering. As a result of the underwriters’ election to fully exercise their over-allotment option, the 187,500 Founder Shares are no longer subject to forfeiture.
The initial stockholders agreed, subject to limited exceptions, not to transfer, assign or sell (i) with respect to 50% of Founder Shares, for a period ending on the six-month anniversary of the date of the consummation of the Initial Business Combination and (ii) with respect to the remaining 50% of such shares, for a period ending on the one-year anniversary of the date of the consummation of the Initial Business Combination, or earlier, in either case, if, subsequent to the Initial Business Combination, we complete a liquidation, merger, capital stock exchange or other similar transaction that results in all of the public stockholders having the right to exchange their shares of common stock for cash, securities or other property. However, if the initial stockholders acquire public shares in or after the initial public offering, they will be entitled to liquidating distributions from the Trust Account with respect to such shares if we fail to complete a Business Combination within the Combination Period.
Private Placement Warrants
Simultaneously with the closing of the initial public offering, the Sponsor purchased an aggregate of 2,800,000 Private Placement Warrants at a price of $1.00 per Private Placement Warrant, for an aggregate purchase price of $2,800,000, in a private placement. Each Private Placement Warrant is exercisable for one share of Class A Common Stock at a price of $11.50 per share, subject to adjustment. A portion of the proceeds from the Private Placement Warrants were added to the proceeds from the initial public offering held in the Trust Account. If we do not complete a Business Combination within the Combination Period, the proceeds from the sale of the Private Placement Warrants will be used to fund the redemption of the public shares (subject to the requirements of applicable law) and the Private Placement Warrants will expire worthless.
Related Party Loans
In order to finance transaction costs in connection with a Business Combination, our Sponsor or an affiliate of our Sponsor, or certain of our officers and directors may, but are not obligated to, loan us funds as may be required. If we complete a Business Combination, we would repay the Working Capital Loans out of the proceeds of the Trust Account released to us. Otherwise, the Working Capital Loans would be repaid only out of funds held outside the Trust Account. In the event that a Business Combination does not close, we may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans, but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with respect to such loans. The Working Capital Loans would either be repaid upon consummation of a Business Combination, without interest, or, at the lender’s discretion, up to $1,500,000 of such Working Capital Loans may be convertible into warrants of the post-Business Combination entity at a price of $1.00 per warrant. The warrants would be identical to the Private Placement Warrants. As of December 31, 2020 and September 30, 2021, we had no outstanding borrowings under the Working Capital Loans, respectively.
| -259- |
Promissory Note — Related Party
On September 30, 2020, Robb Knie, our Chairman, Chief Executive Officer and Chief Financial Officer, agreed to loan us an aggregate of up to $150,000 pursuant to a promissory note. This loan was non-interest bearing and payable upon the completion of the initial public offering. We borrowed $42,125 under the note. We repaid $1,615 of the outstanding note balance on December 31, 2020 and repaid the remaining amount of $40,510 in full on January 26, 2021.
Administrative Services Agreement
We have agreed to pay the Sponsor $10,000 per month for office space, utilities and secretarial and administrative services. Upon completion of our Initial Business Combination or our liquidation, we will cease paying these monthly fees.
Registration Rights
Pursuant to a registration rights agreement entered into on January 19, 2021, the holders of the Founder Shares, Private Placement Warrants and any warrants that may be issued upon conversion of Working Capital Loans (and any shares of Class A Common Stock issuable upon the exercise of the Private Placement Warrants and warrants that may be issued upon conversion of the Working Capital Loans) will have registration rights to require us to register a sale of any of our securities held by them. The holders of these securities are entitled to make up to three demands, excluding short form demands, that we register such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to the completion of the Initial Business Combination and rights to require us to register for resale such securities pursuant to Rule 415 under the Securities Act. The registration rights agreement does not contain liquidating damages or other cash settlement provisions resulting from delays in registering our securities. We will bear the expenses incurred in connection with the filing of any such registration statements.
Support Agreements
Sponsor Support Agreement
Contemporaneously with the execution of the Merger Agreement, the Sponsor entered into the Sponsor Support Agreement, pursuant to which, among other things, the Sponsor agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement.
FoxWayne Stockholder Support Agreement
Contemporaneously with the execution of the Merger Agreement, certain officers and directors of FoxWayne entered into FoxWayne Support Agreements, pursuant to which, among other things, such FoxWayne stockholders agreed to vote in favor of the Merger and the transactions contemplated by the Merger Agreement.
For more information regarding the Support Agreements, see the section titled “Proposal 1 — The Transaction Proposal — Related Agreements —Support Agreements.”
Policies and Procedures for FoxWayne’s Related Party Transactions
We have not yet adopted a formal policy for the review, approval or ratification of related party transactions; however, our audit committee, pursuant to a written charter that we adopted prior to the consummation of our initial public offering, is responsible for reviewing and approving related party transactions to the extent that we enter into such transactions. An affirmative vote of a majority of the members of the audit committee present at a meeting at which a quorum is present is required in order to approve a related party transaction. A majority of the members of the entire audit committee constitutes a quorum. Without a meeting, the unanimous written consent of all of the members of the audit committee is required to approve a related party transaction. A form of the audit committee charter that we adopted prior to the consummation of our initial public offering is filed as an exhibit to the registration statement relating to our initial public offering. We also require each of our directors and officers to complete a directors’ and officers’ questionnaire that elicits information about related party transactions.
| -260- |
These procedures are intended to determine whether any such related party transaction impairs the independence of a director or presents a conflict of interest on the part of a director, employee or officer.
No finder’s fees, reimbursements or cash payments will be made to our Sponsor, existing officers, directors, or our or their affiliates, for services rendered to us prior to or in connection with the completion of our Initial Business Combination although we may consider cash or other compensation to officers or advisors we may hire subsequent to our initial public offering to be paid either prior to or in connection with our Initial Business Combination. In addition, the following payments will be made to our Sponsor, officers or directors, or our or their affiliates, none of which will be made from the proceeds of our initial public offering held in the Trust Account prior to the completion of our Initial Business Combination:
| ● | Repayment to an aggregate of up to $150,000 in loans made to us by an affiliate of our Sponsor; | |
| ● | Reimbursement for any out-of-pocket expenses related to identifying, investigating and completing an Initial Business Combination; and | |
| ● | Repayment of loans which may be made by our Sponsor or an affiliate of our Sponsor or certain of our officers and directors to finance transaction costs in connection with an intended Initial Business Combination, the terms of which have not been determined nor have any written agreements been executed with respect thereto. Up to $1,500,000 of such loans may be convertible into warrants, at a price of $1.00 per warrant at the option of the lender. |
Our audit committee will review on a quarterly basis all payments that were made to our sponsor, officers or directors or our or their affiliates.
Aerami’s Related Party Transactions
In addition to the compensation arrangements, including employment, termination of employment, and change in control arrangements and indemnification arrangements, discussed, when required, in the sections titled “Management After the Business Combination” and “Executive Compensation” and the registration rights described in the section titled “Description of New Securities,” the following is a description of each transaction since February 1, 2017 and each currently proposed transaction in which:
| ● | Aerami has been or is to be a participant; | |
| ● | the amount involved exceeded or exceeds $120,000; and | |
| ● | any of Aerami’s directors, executive officers or holders of more than 5% of its capital stock prior to the Business Combination, or any immediate family member of, or person sharing the household with, any of these individuals, had or will have a direct or indirect material interest. |
2017 Private Placement Offering
From
November 2017 through June 2018, Aerami raised approximately $20.8 million in gross proceeds (the “2017 Private Placement”)
by issuing units consisting of one share of Aerami Common Stock, one half of one share of Aerami Royalty Stock, and a warrant to purchase
one half of one share of Aerami Common Stock to investors. Aerami engaged SternAegis Ventures (“SternAegis”) through Aegis
as its exclusive placement agent to assist in the offering. In connection with the 2017 Private Placement, Aegis received compensation
from Aerami as described below under “—Aegis Agreements.” Aegis also received rights to receive royalty payments if
and when paid to the holders of Aerami’s royalty stock. The following table sets forth the aggregate number of shares issued to
Aerami’s related parties in the 2017 Private Placement:
| -261- |
| Aggregate Purchase Price | ||||||||||||||||||||
| Participants |
Aerami Common Stock |
Aerami Royalty Stock |
Warrants to Purchase Shares of Aerami Common Stock |
Cash | Conversion of Bridge Notes | |||||||||||||||
| Molex Ventures LLC(1) | 1,400,000 | 700,000 | 700,000 | $ | 7,000,000 | $ | - | |||||||||||||
| John S. Patton(2) | 156,572 | 78,286 | 78,286 | $ | - | $ | 626,289 | |||||||||||||
| Adam K. Stern(3) | 50,644 | 25,322 | 25,322 | $ | 120,000 | $ | 106,583 | |||||||||||||
| Steven Thornton(4) | 36,645 | 18,322 | 18,322 | $ | 50,000 | $ | 106,583 | |||||||||||||
| (1) | Molex Ventures LLC has the right to designate an individual for election to the Aerami Board and is a beneficial holder of more than 5% of outstanding Aerami Common Stock. Molex Ventures tendered their warrants in the 2019 Warrant Tender Offer. | |
| (2) | Dr. Patton, a member of the Aerami Board, is a beneficial holder of more than 5% of outstanding Aerami Common Stock. | |
| (3) | Mr. Stern is a member of the Aerami Board and Chief Executive Officer of SternAegis Ventures and Head of Private Equity Banking at Aegis. | |
| (4) | Mr. Thornton is the Chief Executive Officer of Aerami and a member of the Aerami Board. Mr. Thornton tendered his warrants in the 2019 Warrant Tender Offer. |
2019 Warrant Tender Offering Affiliate Participation
From January through March 2019, Aerami raised approximately $20.5 million in gross proceeds (the “2019 Warrant Tender Offer”) by issuing shares of Aerami Common Stock to investors in a 3-for-1 warrant tender offer. Aerami engaged Aegis to serve as warrant agent in connection with the 2019 Warrant Tender Offer, and Aegis received compensation from Aerami as described below under “—Aegis Agreements.” The following table sets forth the aggregate number of shares of Aerami Common Stock issued to Aerami related parties in the 2019 Warrant Tender Offer:
| Participants | Aerami Common Stock | Purchase Price Cash | ||||||
| Molex Ventures LLC(1) | 2,100,000 | $ | 3,500,000 | |||||
| Adam K. Stern(2) | 381,249 | $ | 635,415 | |||||
| John S. Patton(3) | 60,000 | $ | 100,000 | |||||
| Steven Thornton(4) | 54,966 | $ | 91,610 | |||||
| (1) | Molex Ventures has the right to designate an individual for election to the Aerami Board and is a beneficial holder of more than 5% of outstanding Aerami Common Stock. | |
| (2) | Mr. Stern is a member of the Aerami Board and Chief Executive Officer of SternAegis and Head of Private Equity Banking at Aegis. | |
| (3) | Mr. Patton is a member of the Aerami Board. | |
| (4) | Mr. Thornton is the Chief Executive Officer of Aerami and a member of the Aerami Board. |
Series A Preferred Stock Offering
From March 2020 through March 2021, Aerami raised aggregate gross proceeds of $12,450,000 by issuing shares of Aerami Series A Preferred Stock to investors in a private placement (the “Series A Offering”). In connection with the Series A Offering, Aegis received compensation from Aerami as described below under “—Aegis Agreements.” The following table sets forth the aggregate number of shares of Aerami Series A Preferred Stock issued to Aerami related parties in the Series A Offering:
| -262- |
| Participants | Series A Preferred Stock | Purchase Price Cash | ||||||
| Molex Ventures LLC(1) | 1,000 | $ | 1,000,000 | |||||
| Adam K. Stern(2) | 100 | $ | 100,000 | |||||
| John S. Patton(3) | 10 | $ | 10,000 | |||||
| Anne Whitaker (4) | 20 | $ | 20,000 | |||||
| Steven Thornton(5) | 20 | $ | 20,000 | |||||
| Kenneth B. Lee, Jr. (6) | 5 | $ | 5,000 | |||||
| (1) | Molex Ventures has the right to designate an individual for election to the Aerami Board and is a beneficial holder of more than 5% of outstanding Aerami Common Stock. | |
| (2) | Mr. Stern is a member of the Aerami Board and Chief Executive Officer of SternAegis and Head of Private Equity Banking at Aegis. | |
| (3) | Mr. Patton is a member of the Aerami Board. | |
| (4) | Ms. Whitaker is a member of the Aerami Board and a former Chief Executive Officer of Aerami. | |
| (5) | Mr. Thornton is the Chief Executive Officer of Aerami and a member of the Aerami Board. | |
| (6) | Mr. Lee was a member of the Aerami Board until June 2021. |
2021 Private Placement Offering
From September 2021 until November 3, 2021, Aerami raised aggregate gross proceeds of $22,250,560 by issuing shares of Aerami Common Stock to investors in a private placement (the “2021 Private Placement”). In connection with the 2021 Private Placement, Aegis received compensation from Aerami as described below under “—Aegis Agreements.” The following table sets forth the aggregate number of shares of Aerami Common Stock issued to Aerami related parties in the 2021 Private Placement.
| Participants | Aerami Common Stock | Purchase Price Cash | ||||||
| Adam K. Stern(1) | 100,000 | $ | 100,000 | |||||
| Anne Whitaker (2) | 20,000 | $ | 20,000 | |||||
| Steven Thornton(3) | 20,000 | $ | 20,000 | |||||
| (1) | Mr. Stern is a member of the Aerami Board and Chief Executive Officer of SternAegis and Head of Private Equity Banking at Aegis. | |
| (2) | Ms. Whitaker is a member of the Aerami Board and a former Chief Executive Officer of Aerami. | |
| (3) | Mr. Thornton is the Chief Executive Officer of Aerami and a member of the Aerami Board. |
| -263- |
Holders of Aerami Series A Preferred Stock who participated in the 2021 Private Placement had some or all of their shares of Aerami Series A Preferred Stock automatically convert into shares of Aerami Series A-1 Preferred Stock at a one-for-one ratio depending on the amount each holder invested in the 2021 Private Placement. The following table sets forth the aggregate number of shares of Aerami Series A Preferred Stock converted into shares of Aerami Series A-1 Preferred Stock by Aerami related parties:
| Participants | Series A Preferred Stock Converted into Series A-1 Preferred Stock | |||
| Adam K. Stern(1) | 100 | |||
| Anne Whitaker (2) | 20 | |||
| Steven Thornton(3) | 20 | |||
| (1) | Mr. Stern is a member of the Aerami Board and Chief Executive Officer of SternAegis and Head of Private Equity Banking at Aegis. | |
| (2) | Ms. Whitaker is a member of the Aerami Board. | |
| (3) | Mr. Thornton is the Chief Executive Officer of Aerami and a member of the Aerami Board. |
2017 Registration Rights Agreement
In connection with the initial closing of Aerami’s 2017 Private Placement, Aerami entered into the 2017 Registration Rights Agreement with certain of Aerami’s stockholders (the “Existing Holders”), including John Patton and Steve Thornton, and its then 5% stockholders, pursuant to which the stockholders were granted certain registration rights with respect to Aerami Common Stock and the shares of Aerami Common Stock underlying the Aerami Royalty Stock and applicable Aerami Warrants. Pursuant to the terms of the 2017 Registration Rights Agreement, Aerami has accrued liabilities to the Existing Holders as a result of Aerami’s failure to file a registration statement within 180 days of the final closing of the 2017 Private Placement. As of February 11, 2022, Aerami has calculated this liability to be approximately $2.7 million. Aerami plans to satisfy this obligation in connection with the Business Combination such that the parties to the 2017 Registration Rights Agreement would receive merger consideration with an aggregate value equal to the amount of the liability and the agreement would terminate. However, the parties to the 2017 Registration Rights Agreement holding a majority of Aerami shares subject to that agreement must consent to payment in the form of merger consideration and termination of the agreement, which may not be obtained.
Voting Agreement
Aerami has entered into a Voting Agreement (the “Voting Agreement”) with certain of its investors, pursuant to which certain holders of Aerami stock, including Kenneth Lee, John Patton, Adam Stern, Steve Thornton, Bill Welch, Anne Whitaker and its 5% stockholders, including, but not limited to, Molex Ventures, agreed to vote their shares of Aerami Common Stock for the election of directors. Aerami plans to seek consent of the parties to the Voting Agreement to terminate the Voting Agreement upon the effectiveness of the Business Combination, which will require the consent of the holders of a majority of the shares of Aerami Common Stock, on an as-converted basis, subject to the Voting Agreement, which may not be obtained.
Amended and Restated Joint Development Agreement with Phillips-Medisize, LLC
On June 5, 2020, Aerami entered into the Phillips JDA with Phillips, which is an affiliate of Molex Ventures, for the development and manufacturing of Aerami’s AFINA™ device, and the development of integrated connected health technologies for Aerami’s AFINA™ device, as well as any other device Aerami may acquire and any device that Aerami may in-license that would require material development or refinement to be usable or commercially feasible for use with Aerami’s formulations, other than the devices in-licensed from Vectura under the Vectura License. Molex Ventures has the right to designate an individual for election to the Aerami Board and is a beneficial holder of more than 5% of the outstanding Aerami Common Stock. During the years ended 2019 and 2020, Aerami paid Phillips $1.2 million and $0.2 million, respectively, for device development and manufacturing services.
| -264- |
Side Letter Agreement with Molex Ventures
On June 5, 2020, Aerami entered into the Molex Side Letter with Molex Ventures in connection with Molex Ventures’ investment of $1 million to purchase 1,000 shares of Aerami Series A Preferred Stock in the Series A Offering. Pursuant to the Molex Side Letter, Aerami agreed to provide Molex Ventures with certain additional rights to those being provided to investors in the Series A Offering, including information rights and rights to inspection, the right to appoint a director to the Aerami Board, the right to have an observer attend the meetings of the Aerami Board, the right of first offer with respect to future issuances of securities by Aerami (subject to certain exceptions), the right to approve certain material actions of Aerami and additional registration rights to those provided in the 2017 Registration Rights Agreement. In addition, pursuant to the Molex Side Letter, Aerami is required to obtain the approval of Molex Ventures (or in certain instances its representative on the Aerami Board) to take certain actions, including to issue any senior securities, borrow amounts in excess of $500,000, enter into certain related party transactions, or initiate a public offering other than a fully-committed underwritten public offering with gross proceeds of at least $50 million and pursuant to which Aerami Common Stock is listed on the NYSE or one of the Nasdaq stock markets. Further, Aerami agreed that if it sells securities for an aggregate gross purchase price of $25 million or more, Aerami would pay off any then-outstanding indebtedness and obtain releases of any encumbrances on Aerami’s assets. Under the Molex Side Letter, Aerami agreed to use the proceeds of Molex Ventures’ investment in the Series A Offering to support the Vectura License (as defined below) and to support a 510(k) regulatory submission in the United States seeking premarket clearance for Aerami’s AFINA™ soft mist inhalation device, including for the fees and expenses payable pursuant to the Phillips JDA, and for any expenses incurred in the performance of Aerami’s obligations thereunder, including employee related expenses, lease expenses and expenses related to the Molex Ventures investment. Because Aerami failed to meet certain timing requirements set forth in the Molex Side Letter with respect to the 510(k) submission (subject to certain carve-outs and cure period), Molex Ventures has the right to cause Aerami to repurchase the 1,000 shares of Aerami Series A Preferred Stock purchased by Molex Ventures for the amount paid by Molex for such shares. Under the Molex Side Letter, Molex Ventures also has a put option pursuant to which it can require Aerami to, at any time and without condition, immediately repurchase all of Aerami’s securities then owned by Molex Ventures for aggregate consideration of $1.00. Aerami is currently in discussions with Molex Ventures to terminate the rights and obligations under the Molex Side Letter in connection with completion of the Business Combination.
Harmony
In September 2015, Aerami entered into an exclusive license agreement (the “Harmony License”) with Harmony Biopharm Limited, a BVI Business Company incorporated under the law of the British Virgin Islands (“Harmony Biopharm”), under which Aerami granted Harmony Biopharm the right to develop and commercialize AER-501 in China, India and various other countries in Asia.
On August 24, 2017, Aerami entered into a Stock Purchase Agreement and Release (the “Harmony SPA”) with Harmony Biopharm’s parent, Harmony Plus, under which Aerami reacquired the license previously granted to Harmony Biopharm by acquiring 100% of the stock in Harmony Biopharm. As part of the consideration under the Harmony SPA, Aerami agreed to: (i) issue to Harmony Plus a number of shares of Aerami Common Stock equal to $8.0 million to be issued upon the earlier of an Aerami IPO and/or a change of control; (ii) forgive the repayment of $300,000 that was owed to Aerami under the Harmony License; and (iii) pay to Harmony Plus a running royalty equal to 7.5% of gross profits from the sale of Aerami’s products in China until August 24, 2027 (subject to certain early termination events).
As part of the Harmony SPA, Aerami also entered into a consulting agreement with Harmony Plus, under which Harmony Plus agreed to provide consulting services to Aerami for a five-year term commencing in August 2017. Total compensation for the services is $1.5 million, payable in annual installments of $300,000 each through August 2022. Aerami has paid Harmony Plus a total of $1.2 million to date under the consulting agreement and remains obligated to pay $300,000. On August 24, 2017, Aerami also entered into a Market Development Arrangement with Harmony Plus under which Aerami is obligated to pay Harmony Plus a fee equal to 5% of any and all revenue that Aerami receives in China, India and various other countries in Asia from certain qualified partners introduced to Aerami by Harmony Plus.
In September 2017, Aerami entered into an option to repurchase agreement (the “Option Agreement”) with certain of Aerami’s stockholders that are affiliated with Harmony Plus (the “Harmony Investors”). The Option Agreement provides Aerami, among other things, the right to repurchase all of the shares of Aerami Common Stock held by the Harmony Investors (the “Repurchase Option”). Aerami may exercise the Repurchase Option in whole or in part, one or more times, at any time prior to the fifth anniversary of an Aerami IPO. If Aerami exercises the Repurchase Option prior to an Aerami IPO or a change of control, the purchase price for the shares subject to the Repurchase Option will be equal to the greater of (i) $5.00 per share, or (ii) 90% of the Fair Value (as defined in the Option Agreement) of a share. If Aerami exercises the Repurchase Option after an Aerami IPO or a change of control, the purchase price for the shares subject to the Repurchase Option will be equal to the greater of (x) $10.00 per share, or (y) 90% of the Fair Value of a share. Pursuant to the Option Agreement, Aerami has a right of first refusal with respect to a proposed transfer of any of the shares held by the Harmony Investors that are subject to the Option Agreement.
| -265- |
In connection with the Option Agreement, Aerami entered into an amendment to the Harmony SPA on September 25, 2017, which provides that Aerami’s obligation to pay the running royalty under the Harmony SPA would terminate if Aerami or any other person, at any time prior to the fifth anniversary of an Aerami IPO, purchases at least the lesser of: (a) one-half of the 2,299,844 shares of Aerami Common Stock owned by the Harmony Investors, or (b) a number of shares of Aerami Common Stock equal to 2,299,844 less the number of shares sold or transferred by the Harmony Investors after August 24, 2017, at a price per share of at least the greater of (i) $5.00 per share or (ii) 90% of the fair value of a share, if such purchase occurs before an Aerami IPO or change of control, or the greater of (x) $10.00 per share or (y) 90% of the fair value of share, if such purchase occurs after the Aerami IPO.
On February 22, 2018, Aerami entered into a letter agreement with Gwynneth Gold Limited (“Gwynneth Gold”), an affiliate of Harmony Plus, which provides Gwynneth Gold the right to have an observer attend the meetings of the Aerami Board. The Aerami Board observer designated by Gwynneth Gold is currently Vivian Yan, a former member of the Aerami Board.
To address potential future uncertainty as to the capital structure of the Combined Company, Aerami is in discussions with Harmony Plus to pursue a mutually-agreeable resolution that would eliminate some or all of these obligations following the Business Combination in exchange for an allocation of merger consideration, but there can be no assurance that Aerami will reach such an agreement, in which case Aerami’s obligations to Harmony Plus and its affiliates would remain outstanding after the Closing.
Vectura License
On June 7, 2020, Aerami entered into the Vectura License with Vectura, pursuant to which Aerami obtained from Vectura a worldwide, exclusive license to develop and commercialize Vectura’s proprietary FOX® containing a formulation of the active ingredient imatinib for the treatment of PAH. Ms. Whitaker was a member of Vectura’s board of directors at the time of entering into the transaction. Aerami also received the right of first negotiation to obtain rights for use of the Product in additional indications concerning respiratory and pulmonary diseases.
Under the Vectura License, Aerami is obligated to use commercially reasonable efforts to develop and commercialize the Product and to pay milestone payments for the achievement of certain development, regulatory and sales milestones in amounts up to $161 million in the aggregate. Aerami is also obligated to pay a mid-single digit royalty on sales of the Product. Aerami must obtain supply of Vectura’s FOX® device from Vectura at a transfer price equal to Vectura’s costs of manufacture plus a specified percentage.
In addition, on May 25, 2020, Aerami entered into a development agreement with Vectura and made a development payment to Vectura of approximately $1.1 million to begin work to progress inhaled imatinib into Phase 1 human studies as soon as possible after execution of the Vectura License. The license included provisions for a Device Supply Agreement for Aerami in both development and commercialization of AER-901. Aerami has entered into a clinical supply agreement with Vectura for the FOX® device and at an appropriate time to enter into one or more commercial supply agreements for the FOX® device. Aerami has entered into a similar development agreement with Vectura to provide clinical supply for the Phase 1 clinical trials in China.
Separation and Release Agreement with Dr. Patton
Aerami entered into a Separation and Release Agreement with Dr. Patton, a former Chief Executive Officer of Aerami and former Chairman of the Aerami Board, in connection with the termination of his employment on August 31, 2019, pursuant to which Aerami agreed to pay Dr. Patton severance in the amount of $675,000, payable in equal installments over the 12-month period ending August 31, 2020 in accordance with Aerami’s regular payroll period.
In the event that Aerami completes a financing transaction resulting in proceeds to Aerami of at least $15 million, any unpaid installments of the severance owed to Dr. Patton will become due and payable within 30 days of the closing date of such transaction. In addition, under the terms of this agreement, upon the treatment of the first patient in a Phase 3 clinical trial of AER-501 and also in the event Aerami receives final approval from the FDA, EMA or NMPA for AER-501, Aerami is required to grant Dr. Patton an option to purchase a number of shares of Aerami Common Stock equal to 1% of Aerami’s fully-diluted capital stock calculated as of a designated date.
| -266- |
On September 19, 2019 Aerami entered into a Consulting Agreement with Dr. Patton pursuant to which Dr. Patton agreed to provide certain consulting services as requested by Aerami for a period of nine years following the effective date. In return for those services, Dr. Patton received a monthly fee of $12,000 per month for the first 12 months and is now compensated for actual time spent on assigned projects at an hourly rate of $400.
Sublease Agreement with iPharma
From July 2017 through August 2019, iPharma, an affiliate of Dr. Patton, subleased lab space from Aerami for which iPharma paid Aerami approximately $63,000 and $110,000 during the years ended December 31, 2018 and 2019, respectively.
Aegis Agreements
Aerami has entered into several transactions involving Aegis. Since December 2014, Aegis has had the right to designate one member of the Aerami Board, who is currently Adam Stern (the “Aegis Nominee”). Mr. Stern’s successor, if any, will be chosen by Aegis, subject to the reasonable approval of Aerami. This right will terminate at the Effective Time, and Mr. Stern will not serve as a director of the Combined Company. These transactions include the engagement of Aegis as the placement agent for certain of Aerami’s prior offerings, the engagement of Aegis as the warrant agent for the 2019 Warrant Tender Offer, and the engagement of Aegis as a financial advisor, as further described below.
| ● | From November 2017 through June 2018, Aegis served as placement agent for the 2017 Private Placement for which Aerami paid Aegis fees and expense reimbursement in an aggregate amount of approximately $2 million and granted Aegis placement agent warrants to purchase 693,444 shares of Aerami Common Stock at an exercise price of $5.00 per share. Aegis also received rights to receive royalty payments if and when paid to the holders of Aerami Royalty Stock. | |
| ● | From January 2019 through June 2019, Aegis provided services as Aerami’s warrant agent in connection with the 2019 Warrant Tender Offer. In connection with the 2019 Warrant Tender Offer, Aerami offered holders of outstanding placement agent warrants the right to cashlessly exercise each such warrant in exchange for two-thirds of one share of Aerami Common Stock. A total of 1,971,219 placement agent warrants were cashlessly tendered and approximately 1,314,847 shares were issued to affiliates of Aegis. In addition, Aerami paid Aegis warrant solicitation fees and expense reimbursement in an aggregate amount of $1,424,164. | |
| ● | From March 2020 through March 2021, Aegis served as placement agent for the Series A Offering for which Aerami paid Aegis fees and expense reimbursement in an aggregate amount of approximately $1.5 million and agreed to grant Aegis Placement Agent Warrants to purchase 1,192 shares of Series A Preferred Stock at an exercise price of $1,000 per share. Following the 2021 Private Placement, Aerami issued the Placement Agent Warrants relating to the Series A Private Placement, reflecting the conversion of a portion of the warrants to purchase shares of Aerami Series A-1 Preferred Stock as a result of the participation of holders of Series A Preferred Stock in the 2021 Private Placement. As a result, Aerami issued to Aegis Placement Agent Warrants to purchase 148 shares of Aerami Series A Preferred Stock and Placement Agent Warrants to purchase 1,044 shares of Aerami Series A-1 Preferred Stock, each at an exercise price of $1,000 per share. These Placement Agent Warrants will exercise automatically on a cashless basis in connection with the Business Combination. | |
| ● | From September 2021 through November 3, 2021, Aegis served as placement agent for the 2021 Private Placement for which Aerami paid Aegis fees and expense reimbursement in an aggregate amount of approximately $2.9 million and granted Aegis Placement Agent Warrants to purchase 2,223,056 shares of Aerami Common Stock at an exercise price of $1.00 per share. These Placement Agent Warrants will exercise automatically on a cashless basis in connection with the Business Combination. |
| -267- |
In addition, in April 2021, Aerami entered into the Advisory Agreement with Aegis pursuant to which Aegis agreed to provide Aerami with certain customary financial advisory services that may be reasonably requested by Aerami from time to time during the 12 month period following the execution of the Advisory Agreement. During the term of the Advisory Agreement, Aegis may introduce Aerami to third parties who may be interested in pursuing strategic transactions. In the event Aerami consummates a merger, acquisition or certain other change in control transactions (an “M&A Transaction”) with a third -party introduced to Aerami by Aegis, Aegis will be entitled to a fee equal to 5% of the consideration paid in the M&A transaction. In the event Aerami consummates the Business Combination, Aegis will be entitled to compensation under the terms of the Advisory Agreement. Aegis is also entitled to compensation if Aerami enters into any joint-venture, partnership, strategic collaboration or investment, licensing transaction, sale or purchase of specific assets, consulting agreement, co-promotion or distribution agreement or other profit or revenue sharing or other business arrangement with a third -party introduced to Aerami by Aegis (such transactions, collectively, “Business Development Transactions”). Aegis is entitled to fees consisting of between 5% and 6.5% of the consideration paid in any such Business Development Transaction, depending on Aegis’ level of involvement in concluding the Business Development Transaction. Under this agreement, Aerami has agreed to pay Aegis 5% of all revenues received under Aerami’s January 2021 agreements with Chance. In addition, Aerami has agreed to pay Aegis 5% of the Aggregate Consideration to be paid in the Business Combination, which Aegis has agreed Aerami may elect to pay in an allocation of Aggregate Consideration. Aerami has also agreed to reimburse Aegis for certain customary expenses.
Other Transactions
Chad Whitaker, the son of Anne Whitaker, who is the Chairwoman of the Aerami Board and former Chief Executive Officer of Aerami, serves as the Aerami Associate Director of Operations. Mr. Whitaker was paid a base salary of $22,705 in 2018, $115,000 in 2019, and $132,901 in 2020, and is entitled to a base salary of $132,825 in 2021 and holds options to purchase 25,000 shares of Aerami Common Stock that were granted in 2018, 30,000 shares of Aerami Common Stock that were granted in 2019, and 12,000 shares of Aerami Common Stock that were granted in 2021. Mr. Whitaker also participates in other employee benefit plans and arrangements that are made generally available to other employees.
LEGAL MATTERS
Sheppard, Mullin, Richter & Hampton LLP will pass upon the validity of the Combined Company common stock issued in connection with the Business Combination and upon certain U.S. federal income tax consequences to FoxWayne stockholders as a result of the Business Combination and certain tax matters will be passed upon by Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan LLP.
EXPERTS
The financial statements of FoxWayne Enterprises Acquisition Corp. as of December 31, 2020, and for the period from September 17, 2020 (inception) through December 31, 2020, appearing in this proxy statement/prospectus have been audited by WithumSmith+Brown, PC, independent registered public accounting firm, as set forth in their report thereon, appearing elsewhere in this prospectus, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The audited financial statements of Aerami Therapeutics Holdings, Inc. and Subsidiaries as of and for the years ended December 31, 2020 and 2019 included in this proxy statement/prospectus and elsewhere in this Registration Statement have been so included in reliance upon the report of Grant Thornton LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
| -268- |
HOUSEHOLDING INFORMATION
Unless FoxWayne has received contrary instructions, FoxWayne may send a single copy of this proxy statement/prospectus to any household at which two or more stockholders reside if we believe the stockholders are members of the same family. This process, known as “householding,” reduces the volume of duplicate information received at any one household and helps to reduce our expenses. However, if stockholders prefer to receive multiple sets of FoxWayne’s disclosure documents at the same address this year, the stockholders should follow the instructions described below. Similarly, if an address is shared with another stockholder and together both of the stockholders would like to receive only a single set of FoxWayne’s disclosure documents, the stockholders should follow these instructions:
| ● | If the shares are registered in the name of the stockholder, the stockholder should contact FoxWayne at its offices at 1 Rockefeller Plaza, Suite 1039, New York, NY 10020 or its telephone number at (214) 368-0821 or send an email to Hayley@foxwayne.com to inform FoxWayne of his or her request; or | |
| ● | If a bank, broker or other nominee holds the shares, the stockholder should contact the bank, broker or other nominee directly. |
| -269- |
TRANSFER AGENT, WARRANT AGENT AND REGISTRAR
The transfer agent, warrant agent and registrar for our securities is Continental Stock Transfer & Trust Company.
SUBMISSION OF STOCKHOLDER PROPOSALS
The FoxWayne Board is aware of no other matter that may be brought before the special meeting. Under Delaware law, only business that is specified in the notice of special meeting to stockholders may be transacted at the special meeting.
FUTURE STOCKHOLDER PROPOSALS AND NOMINATIONS
We anticipate that the 2022 annual meeting of stockholders will be held no later than , 2022. For any proposal to be considered for inclusion in our proxy statement and form of proxy for submission to the stockholders at our 2022 annual meeting of stockholders, it must be submitted in writing and comply with the requirements of Rule 14a-8 of the Exchange Act and our bylaws. Assuming the meeting is held on or about , 2022, such proposals must be received by the Combined Company at its offices at 2525 Meridian Parkway, Suite 400, Durham, North Carolina 27713, within a reasonable time before the Combined Company begins to print and send its proxy materials for the meeting.
In addition, the Combined Company’s amended and restated bylaws, which will be effective upon the consummation of the Business Combination, provide notice procedures for stockholders to propose business to be considered by stockholders at a meeting or to nominate any person for election to the Board. To be timely, a stockholder’s notice must be delivered to, or mailed and received at, the principal executive offices of the Combined Company not less than 90 days nor more than 120 days prior to the one-year anniversary of the preceding year’s annual meeting; provided, however, that if no annual meeting was held in the preceding year, to be timely, a stockholder’s notice must be so delivered, or mailed and received, not earlier than the close of business on the 120th day prior to such annual meeting and not later than the close of business on the later of the 90th day prior to such annual meeting or, if later, the 10th day following the day on which public disclosure of the date of such annual meeting was first made by the Combined Company; provided, further, that if the date of the annual meeting is more than 30 days before or more than 60 days after such anniversary date, to be timely, a stockholder’s notice must be so delivered, or mailed and received, not later than the 90th day prior to such annual meeting or, if later, the 10th day following the day on which public disclosure of the date of such annual meeting was first made by the Combined Company. Thus, for our 2022 annual meeting of stockholders, notice of a proposal or a notice of nomination for election to the Board must be delivered to our Secretary no later than , 2022 and no earlier than , 2022. The Chairman of the Combined Company Board may refuse to acknowledge the introduction of any stockholder proposal not made in compliance with the foregoing procedures.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
FoxWayne files reports, proxy statements and other information with the SEC as required by the Exchange Act. You can read FoxWayne’s SEC filings, including this proxy statement/prospectus, over the Internet at the SEC’s website at http://www.sec.gov.
If you would like additional copies of this proxy statement/prospectus or if you have questions about the Business Combination or the Proposals to be presented at the special meeting, you should contact FoxWayne’s proxy solicitation agent at the following address and telephone number:
If you are a FoxWayne stockholder and would like to request documents, please do so no later than five business days before the special meeting, or no later than , 2022, in order to receive them before the special meeting. If you request any documents from FoxWayne, FoxWayne will mail them to you by first class mail, or another equally prompt means.
| -270- |
All information included in this proxy statement/prospectus relating to FoxWayne has been supplied by FoxWayne, and all such information relating to Aerami has been supplied by Aerami. Information provided by either FoxWayne or Aerami does not constitute any representation, estimate or projection of the other, or any other party. Information and statements contained in this proxy statement/prospectus or any annex are qualified in all respects by reference to the copies of the relevant agreements or other annexes filed as exhibits to this proxy statement/prospectus.
Neither FoxWayne nor Aerami has authorized anyone to give any information or make any representation about the Business Combination or their companies that is different from, or in addition to, that included in this proxy statement/prospectus or in any of the materials that have been incorporated in this proxy statement/prospectus. Therefore, if anyone does give you information of this sort, you should not rely on it. If you are in a jurisdiction where offers to exchange or sell, or solicitations of offers to exchange or purchase, the securities offered by this proxy statement/prospectus or the solicitation of proxies is unlawful, or if you are a person to whom it is unlawful to direct these types of activities, then the offer presented in this proxy statement/prospectus does not extend to you. The information included in this proxy statement/prospectus speaks only as of the date of this proxy statement/prospectus unless the information specifically indicates that another date applies.
STOCKHOLDER COMMUNICATIONS
The FoxWayne Board welcomes communications from our stockholders. Our stockholders may send communications to the FoxWayne Board, any committee of the FoxWayne Board or any other director in particular to:
FoxWayne Enterprises Acquisition Corp,
1 Rockefeller Plaza, Suite 1039
New York, NY 10020
Our stockholders should mark the envelope containing each communication as “Stockholder Communication with Directors” and clearly identify the intended recipient(s) of the communication. FoxWayne’s Chief Executive Officer will review each communication received from our stockholders and will forward the communication, as expeditiously as reasonably practicable, to the addressees if: (a) the communication complies with the requirements of any applicable policy adopted by the FoxWayne Board relating to the subject matter of the communication; and (b) the communication falls within the scope of matters generally considered by the FoxWayne Board. To the extent the subject matter of a communication relates to matters that have been delegated by the FoxWayne Board to a committee or to an executive officer of FoxWayne, then FoxWayne’s Chief Executive Officer may forward the communication to the executive officer or chairman of the committee to which the matter has been delegated. The acceptance and forwarding of communications to the members of the FoxWayne Board or an executive officer does not imply or create any fiduciary duty of any FoxWayne Board member or executive officer to the person submitting the communications.
DELIVERY OF DOCUMENTS TO STOCKHOLDERS
Pursuant to the rules of the SEC, FoxWayne and the services that it employs to deliver communications to its stockholders are permitted to deliver to two or more stockholders sharing the same address a single copy of each of FoxWayne’s annual report to stockholders and FoxWayne’s proxy statement/prospectus. Upon written or oral request, FoxWayne will deliver a separate copy of its annual report to stockholders and/or proxy statement/prospectus to any stockholder at a shared address to which a single copy of each document was delivered and who wishes to receive separate copies of such documents. Stockholders receiving multiple copies of such documents may likewise request that FoxWayne deliver single copies of such documents in the future. Stockholders receiving multiple copies of such documents may request that FoxWayne deliver single copies of such documents in the future. Stockholders may notify FoxWayne of their requests by calling or writing at 1 Rockefeller Plaza, Suite 1039, New York, New York 10020, or at (917) 284-8938, or at 2525 Meridian Parkway, Suite 400, Durham, North Carolina 27713 (if after the Business Combination).
| -271- |
INDEX TO FINANCIAL STATEMENTS
| -272- |
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of
FoxWayne Enterprises Acquisition Corp.
Opinion on the Financial Statements
We have audited the accompanying balance sheet of FoxWayne Enterprises Acquisition Corp. (the “Company”) as of December 31, 2020, the related statements of operations, changes in stockholder’s equity and cash flows for the period from September 17, 2020 (inception) through December 31, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020, and the results of its operations and its cash flows for the period from September 17, 2020 (inception) through December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, if the Company is unable to complete a business combination by January 22, 2022, then the Company will cease all operations except for the purpose of liquidating. The dates for mandatory liquidation and subsequent dissolution raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ WithumSmith+Brown, PC
We have served as the Company’s auditor since 2020.
New York, New York
March 29, 2021
| F-1 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
BALANCE SHEET
December 31, 2020
| Assets: | ||||
| Current assets: | ||||
| Cash | $ | |||
| Total Current Assets | ||||
| Deferred offering costs associated with initial public offering | ||||
| Total Assets | $ | |||
| Liabilities and Stockholders’ Equity: | ||||
| Current liabilities: | ||||
| Accounts payable | $ | |||
| Accrued expenses | ||||
| Franchise tax payable | ||||
| Note payable - related party | ||||
| Total Current Liabilities | ||||
| Commitments and Contingencies (Note 5) | ||||
| Stockholders’ Equity: | ||||
| Preferred stock, $ par value; shares authorized; issued and outstanding | ||||
| Class A common stock, $ par value; shares authorized; issued and outstanding | ||||
| Class B common stock, $ par value; shares authorized; shares issued and outstanding (1) | ||||
| Additional paid-in capital | ||||
| Accumulated deficit | ( | ) | ||
| Total Stockholders’ Equity | ||||
| Total Liabilities and Stockholders’ Equity | $ | |||
| (1) |
The accompanying notes are an integral part of these financial statements.
| F-2 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
STATEMENT OF OPERATIONS
FOR THE PERIOD FROM SEPTEMBER 17, 2020 (INCEPTION) THROUGH DECEMBER 31, 2020
| General and administrative expenses | $ | |||
| Franchise tax expense | ||||
| Net loss | $ | ( | ) | |
| Weighted average shares outstanding, basic and diluted (1) | ||||
| Basic and diluted net loss per share | $ | ( | ) |
| (1) |
The accompanying notes are an integral part of these financial statements.
| F-3 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE PERIOD FROM SEPTEMBER 17, 2020 (INCEPTION) THROUGH DECEMBER 31, 2020
| Common Stock | Additional | Total | ||||||||||||||||||||||||||
| Class A | Class B | Paid-In | Accumulated | Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Equity | ||||||||||||||||||||||
| Balance - September 17, 2020 (inception) | $ | - | $ | $ | $ | $ | ||||||||||||||||||||||
| Issuance of Class B common stock to Sponsor (1) | - | |||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance - December 31, 2020 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||
| (1) |
The accompanying notes are an integral part of these financial statements.
| F-4 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
STATEMENT OF CASH FLOWS
FOR THE PERIOD FROM SEPTEMBER 17, 2020 (INCEPTION) THROUGH DECEMBER 31, 2020
| Cash Flows from Operating Activities: | ||||
| Net loss | $ | ( | ) | |
| Changes in operating assets and liabilities: | ||||
| Accounts payable | ||||
| Franchise tax payable | ||||
| Net cash used in operating activities | ( | ) | ||
| Cash Flows from Financing Activities: | ||||
| Proceeds from note payable to related party | ||||
| Repayment of note payable to related party | ( | ) | ||
| Proceeds from issuance of Class B common stock to Sponsor | ||||
| Offering costs paid | ( | ) | ||
| Net cash provided by financing activities | ||||
| Net increase in cash | ||||
| Cash - beginning of the period | ||||
| Cash - end of the period | $ | |||
| Supplemental disclosure of noncash activities: | ||||
| Deferred offering costs included in accounts payable | $ | |||
| Deferred offering costs included in accrued expenses | $ | |||
The accompanying notes are an integral part of these financial statements.
| F-5 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
Note 1 — Description of Organization and Business Operations
FoxWayne Enterprises Acquisition Corp. (the “Company”) is a blank check company incorporated in Delaware on September 17, 2020, for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses (the “Business Combination”). The Company is an emerging growth company and, as such, the Company is subject to all of the risks associated with emerging growth companies.
As of December 31, 2020, the Company had not commenced any operations. All activity for the period from September 17, 2020 (inception) through December 31, 2020 relates to the Company’s formation and the initial public offering (the “Initial Public Offering”) described below. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company will generate non-operating income in the form of interest income on cash and cash equivalents from the proceeds derived from the Initial Public Offering. The Company has selected December 31 as its fiscal year end.
The
Company’s sponsor is FoxWayne Enterprises Acquisition Sponsor LLC, a Delaware limited liability company (the “Sponsor”).
The registration statement for the Company’s Initial Public Offering was declared effective on January 19, 2021. On January
22, 2021, the Company consummated its Initial Public Offering of units (the “Units” and, with respect to
the Class A common stock included in the Units being offered, the “Public Shares”), including additional Units
to cover over-allotments (the “Over-Allotment Units”), at $ per Unit, generating gross proceeds of $
Simultaneously
with the closing of the Initial Public Offering, the Company consummated the private placement (“Private Placement”)
of
Upon
the closing of the Initial Public Offering and the Private Placement, approximately $
The
Company’s management has broad discretion with respect to the specific application of the net proceeds of the Initial Public
Offering and the sale of the Private Placement Warrants, although substantially all of the net proceeds are intended to be applied
generally toward consummating a Business Combination. There is no assurance that the Company will be able to complete a Business
Combination successfully.
| F-6 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
The
Company will provide the holders (the “Public Stockholders”) of the Public Shares with the opportunity to redeem all
or a portion of their Public Shares upon the completion of a Business Combination either (i) in connection with a stockholder
meeting called to approve the Business Combination or (ii) by means of a tender offer. The decision as to whether the Company
will seek stockholder approval of a Business Combination or conduct a tender offer will be made by the Company, solely in its
discretion. The Public Stockholders will be entitled to redeem their Public Shares for a pro rata portion of the amount then held
in the Trust Account (initially anticipated to be $ per Public Share). The per-share amount to be distributed to Public Stockholders
who redeem their Public Shares will not be reduced by the deferred underwriting commissions the Company will pay to the underwriters
(as discussed in Note 5). These Public Shares will be recorded at a redemption value and classified as temporary equity upon the
completion of the Initial Public Offering in accordance with the Financial Accounting Standards Board’s (“FASB”)
Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing Liabilities from Equity.” The Company
will proceed with a Business Combination if a majority of the shares voted are voted in favor of the Business Combination. The
Company will not redeem the Public Shares in an amount that would cause its net tangible assets to be less than $
The Amended and Restated Certificate of Incorporation will provide that a public stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming its shares with respect to more than an aggregate of 15% or more of the Public Shares, without the prior consent of the Company.
The Sponsor and the Company’s officers and directors (the “Initial Stockholders”) agreed not to propose an amendment to the Amended and Restated Certificate of Incorporation to modify the substance or timing of the Company’s obligation to redeem 100% of the Public Shares if the Company does not complete a Business Combination within 12 months from the closing of the Initial Public Offering, or January 22, 2022, (or up to 18 months from the consummation of the Initial Public Offering, or July 22, 2022, if the Company extends the period of time to consummate a Business Combination) (the “Combination Period”), or with respect to any other material provisions relating to stockholders’ rights or pre-initial Business Combination activity, unless the Company provides the Public Stockholders with the opportunity to redeem their Public Shares in conjunction with any such amendment.
If
the Company anticipates that it may not be able to consummate the initial Business Combination within 12 months, the Company may,
by resolution of the board if requested by the Sponsor, extend the period of time to consummate a Business Combination up to two
times, each by an additional three months (for a total of up to 18 months to complete a Business Combination), subject to the
Sponsor depositing additional funds into the trust account as set out below. Pursuant to the terms of the Amended and Restated
Certificate of Incorporation and the trust agreement to be entered into between the Company and Continental Stock Transfer &
Trust Company, in order for the time available for the Company to consummate the initial Business Combination to be extended,
the Sponsor or its affiliates or designees, upon five business days advance notice prior to the applicable deadline, must deposit
into the Trust Account $
| F-7 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
If
the Company is unable to complete a Business Combination within the Combination Period, the Company will (i) cease all operations
except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter,
redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account
including interest earned on the funds held in the Trust Account and not previously released to the Company to pay its taxes (less
up to $
The Initial Stockholders agreed to waive their rights to liquidating distributions from the Trust Account with respect to the Founder Shares if the Company fails to complete a Business Combination within the Combination Period. However, if the Initial Stockholders acquire Public Shares in or after the Initial Public Offering, they will be entitled to liquidating distributions from the Trust Account with respect to such Public Shares if the Company fails to complete a Business Combination within the Combination Period. The underwriters agreed to waive their rights to the deferred underwriting commission (see Note 5) held in the Trust Account in the event the Company does not complete a Business Combination within in the Combination Period and, in such event, such amounts will be included with the other funds held in the Trust Account that will be available to fund the redemption of the Public Shares. , as amended (the “Securities Act”). The Company will seek to reduce the possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have all vendors, service providers (except the Company’s independent registered public accounting firm), prospective target businesses or other entities with which the Company does business, execute agreements with the Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account.
Liquidity and Capital Resources
As
of December 31, 2020, the Company had cash of approximately $
The
Company’s liquidity needs prior to the consummation of the Initial Public Offering were satisfied through the payment of
$
| F-8 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
Going Concern Consideration
As
of December 31, 2020, the Company had cash of approximately $
Through
December 31, 2020, the Company’s liquidity needs have been satisfied through receipt of a $
In addition, in order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company Working Capital Loans (Note 4).
In connection with the Company’s assessment of going concern considerations in accordance with Financial Accounting Standard Board’s Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the mandatory liquidation and subsequent dissolution raises substantial doubt about the Company’s ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities should the Company be required to liquidate after January 22, 2022.
Note 2 — Summary of Significant Accounting Policies
Basis of Presentation
The accompanying financial statements are presented in U.S. dollars in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the SEC.
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a)(19) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that an emerging growth company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company that is neither an emerging growth company nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Concentration of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit risk consist of a cash account in a financial institution,
which, at times, may exceed the Federal depository insurance coverage of $
Cash and Cash Equivalents
The
Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents.
The Company had
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under FASB ASC Topic 820, “Fair Value Measurements”, approximates the carrying amounts represented in the balance sheet, primarily due to their short-term nature.
| F-9 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. Actual results could differ from those estimates.
Deferred Offering Costs Associated with the Initial Public Offering
Deferred
offering costs consist of legal, accounting, underwriting fees and other costs incurred that were directly related to the Initial
Public Offering and that were charged to stockholders’ equity upon the completion of the Initial Public Offering on January
22, 2021. As of December 31, 2020, the Company incurred approximately $
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” Net loss per share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the period, excluding common stock subject to forfeiture. Weighted average shares at December 31, 2020 were reduced for the effect of an aggregate of shares of common stock that are subject to forfeiture if the over-allotment option was not exercised in full or in part by the underwriters (see Note 6). At December 31, 2020, the Company did not have any dilutive securities and other contracts that could, potentially, be exercised or converted into shares of common stock and then share in the earnings of the Company. As a result, diluted loss per share is the same as basic loss per share for the period presented.
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under FASB ASC Topic 740, “Income Taxes.” Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. Deferred tax assets were deemed immaterial as of December 31, 2020.
FASB ASC Topic 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. There were no unrecognized tax benefits as of December 31, 2020. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2020. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
The provision for income taxes was deemed to be de minimis for the period from September 17, 2020 (inception) through December 31, 2020.
Recent Accounting Pronouncements
The Company’s management does not believe that any recently issued, but not yet effective, accounting standards if currently adopted would have a material effect on the accompanying financial statements.
Note 3 — Initial Public Offering
On
January 22, 2021, the Company consummated its Initial Public Offering of Units, including Over-Allotment Units,
at $ per Unit, generating gross proceeds of $
| F-10 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
Each Unit consists of one share of Class A common stock and one redeemable warrant (each, a “Public Warrant”). Each whole Public Warrant entitles the holder to purchase one share of Class A common stock at a price of $ per share, subject to adjustment (see Note 6).
Note 4 — Related Party Transactions
Founder Shares
On
October 15, 2020, the Sponsor purchased shares of the Company’s Class B common stock, par value $ per share
(the “Founder Shares”), for an aggregate price of $. In October 2020, the Sponsor transferred 25,000 Founder
Shares to each of Messrs. Reavey, Pavell, Zippin and Agrawal and Founder Shares to certain other Initial Stockholders.
The per share purchase price of the Founder Shares was determined by dividing the amount of cash contributed to the Company by
the aggregate number of Founder Shares issued.
Private Placement Warrants
Simultaneously
with the closing of the Initial Public Offering, the Company consummated the Private Placement of
Each whole Private Placement Warrant is exercisable for one whole share of Class A common stock at a price of $ per share. A portion of the proceeds from the sale of the Private Placement Warrants to the Sponsor was added to the proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination within the Combination Period, the Private Placement Warrants will expire worthless. The Private Placement Warrants will be non-redeemable for cash and exercisable on a cashless basis so long as they are held by the Sponsor or its permitted transferees.
The Sponsor and the Company’s officers and directors agreed, subject to limited exceptions, not to transfer, assign or sell any of their Private Placement Warrants until 30 days after the completion of the initial Business Combination.
Related Party Loans
On
September 30, 2020, Robb Knie, CEO, agreed to loan the Company an aggregate of up to $
| F-11 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
In
addition, in order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the
Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may
be required (“Working Capital Loans”). If the Company completes a Business Combination, the Company would repay the
Working Capital Loans out of the proceeds of the Trust Account released to the Company. Otherwise, the Working Capital Loans would
be repaid only out of funds held outside the Trust Account. In the event that a Business Combination does not close, the Company
may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans but no proceeds held in the Trust
Account would be used to repay the Working Capital Loans. Except for the foregoing, the terms of such Working Capital Loans, if
any, have not been determined and no written agreements exist with respect to such loans.
As
discussed in Note 1, the Company may extend the period of time to consummate a Business Combination up to two times, each by an
additional three months (for a total of 18 months to complete a Business Combination). In order to extend the time available for
the Company to consummate a Business Combination, the Sponsor or its affiliates or designees must deposit into the Trust Account
$
Administrative Services Agreement
Commencing
on the date that the Company’s securities were first listed on Nasdaq through the earlier of consummation of the initial
Business Combination and the Company’s liquidation, the Company agreed to pay the Sponsor a total of $
The Company’s officers or directors will be reimbursed for any out-of-pocket expenses incurred in connection with activities on the Company’s behalf such as identifying potential target businesses and performing due diligence on suitable Business Combinations. The audit committee will review on a quarterly basis all payments that were made to the Sponsor, officers, directors or their affiliates and will determine which expenses and the amount of expenses that will be reimbursed. There is no cap or ceiling on the reimbursement of out-of-pocket expenses incurred by such persons in connection with activities on the Company’s behalf.
Note 5 — Commitments and Contingencies
Registration and Stockholder Rights
The holders of Founder Shares, Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans or Extension Loans, if any, (and any shares of Class A common stock issuable upon the exercise of the Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans and upon conversion of the Founder Shares) were entitled to registration rights pursuant to a registration rights agreement signed upon the consummation of the Initial Public Offering. The holders of these securities were entitled to make up to three demands, excluding short form demands, that the Company register such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to the completion of the initial Business Combination. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
The
underwriters were entitled to an underwriting discount of $
| F-12 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
The
Company issued Kingswood Capital Markets, a division of Benchmark Investments, Inc. (“Kingswood”), the Representative
of the underwriters (the “Representative”), and/or its designees, shares of Class A common stock (the “Representative’s
Shares”) upon the consummation of the Initial Public Offering. Kingswood agreed not to transfer, assign or sell any such shares
until the completion of the initial Business Combination. In addition, Kingswood agreed (i) to waive its redemption rights with respect
to such shares in connection with the completion of the initial Business Combination and (ii) to waive its rights to liquidating distributions
from the Trust Account with respect to such shares if the Company fails to complete its initial Business Combination within the Combination
Period. The Company recorded the fair value of the Representative Shares, $
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic on the industry and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 6 — Stockholders’ Equity
Class A Common Stock — The Company is authorized to issue shares of Class A common stock with a par value of $ per share. As of December 31, 2020, there were shares of Class A common stock issued or outstanding.
Class
B Common Stock — The Company is authorized to issue shares of Class B common stock with a par value of
$ per share. At December 31, 2020, there were shares of Class B common stock issued and outstanding, of which
an aggregate of up to shares of Class B common stock are subject to forfeiture to the extent that the underwriters’
over-allotment option was not exercised in full or in part, so that the Initial Stockholders will collectively own
Holders of the Class A common stock and holders of the Class B common stock will vote together as a single class on all matters submitted to a vote of the stockholders, except as required by law. Each share of common stock will have one vote on all such matters. However, the holders of the Founder Shares have the right to elect all of the Company’s directors prior to the initial Business Combination.
The Class B common stock will automatically convert into Class A common stock at the closing of the initial business Combination on a one-for-one basis, subject to adjustment for stock splits, stock dividends, reorganizations, recapitalizations and the like, and subject to further adjustment as provided herein. In the case that additional shares of Class A common stock or equity-linked securities are issued or deemed issued in connection with the initial Business Combination, the number of shares of Class A common stock issuable upon conversion of all Founder Shares will equal, in the aggregate, on an as-converted basis, 20% of the sum of the total number of all shares of common stock outstanding upon the completion of the Initial Public Offering, plus the total number of shares of Class A common stock issued, or deemed issued or issuable upon conversion or exercise of any equity-linked securities or rights issued or deemed issued, by the Company in connection with or in relation to the consummation of the initial Business Combination, excluding any shares of Class A common stock or equity-linked securities exercisable for or convertible into shares of Class A common stock issued, or to be issued, to any seller in the initial Business Combination and any private placement-equivalent warrants issued to the Sponsor, officers or directors upon conversion of Working Capital Loans or Extension Loans; provided that such conversion of Founder Shares will never occur on a less than one for one basis.
Preferred Stock — The Company is authorized to issue shares of preferred stock, par value $ per share, with such designations, voting and other rights and preferences as may be determined from time to time by the Company’s board of directors. As of December 31, 2020, there were shares of preferred stock issued or outstanding.
| F-13 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
Warrants — Public Warrants may only be exercised for a whole number of shares. No fractional Public Warrants will be issued upon separation of the Units and only whole Public Warrants will trade. The Public Warrants will become exercisable on the later of (a) the completion of a Business Combination or (b) 12 months from the closing of the Initial Public Offering; provided in each case that the Company has an effective registration statement under the Securities Act covering the shares of Class A common stock issuable upon exercise of the Public Warrants and a current prospectus relating to them is available (or the Company permits holders to exercise their Public Warrants on a cashless basis and such cashless exercise is exempt from registration under the Securities Act). The Company agreed that as soon as practicable, but in no event later than 15 business days after the closing of the initial Business Combination, it will use its commercially reasonable efforts to file with the SEC and have an effective registration statement covering the shares of the Class A common stock issuable upon exercise of the warrants and to maintain a current prospectus relating to those shares of the Class A common stock until the warrants expire or are redeemed. If a registration statement covering the shares of the Class A common stock issuable upon exercise of the warrants is not effective by the 60th business day after the closing of the initial Business Combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption.
The Private Placement Warrants are identical to the Public Warrants, except that the Private Placement Warrants and the shares of Class A common stock issuable upon exercise of the Private Placement Warrants will not be transferable, assignable or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions. Additionally, the Private Placement Warrants will be non-redeemable so long as they are held by the Sponsor or its permitted transferees. If the Private Placement Warrants are held by someone other than the Sponsor or its permitted transferees, the Private Placement Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
Once the warrants become exercisable, the Company may redeem the outstanding warrants (except as described herein with respect to the Private Placement Warrants):
| ● | in whole and not in part; | |
| ● | at a price of $0.01 per warrant; | |
| ● | upon a minimum of 30 days’ prior written notice of redemption; and | |
| ● | if, and only if, the last sale price of Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third day prior to the date on which the Company sends the notice of redemption to the warrant holders. |
| F-14 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
NOTES TO FINANCIAL STATEMENTS
The Company will not redeem the warrants as described above unless a registration statement under the Securities Act covering the shares of Class A common stock issuable upon exercise of the warrants is effective and a current prospectus relating to those shares of Class A common stock is available throughout the 30-day redemption period, except if the warrants may be exercised on a cashless basis and such cashless exercise is exempt from registration under the Securities Act.
In no event will the Company be required to net cash settle any warrant. If the Company is unable to complete a Business Combination within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of warrants will not receive any of such funds with respect to their warrants, nor will they receive any distribution from the Company’s assets held outside of the Trust Account with the respect to such warrants. Accordingly, the warrants may expire worthless.
Note 7 — Subsequent Events
The Company evaluated subsequent events and transactions that occurred after the balance sheet date through the date that the financial statements were available to be issued. Based on this review, the Company did not identify any subsequent events, except as noted above in Note 1, 3, 4, 5 and 6, that would have required adjustment or disclosure in the financial statements.
| F-15 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
CONDENSED BALANCE SHEETS
| September 30, | December 31, | |||||||
| 2021 | 2020 | |||||||
| (Unaudited) | ||||||||
| Assets: | ||||||||
| Current assets: | ||||||||
| Cash | $ | $ | ||||||
| Prepaid expenses | - | |||||||
| Total Current Assets | ||||||||
| Investments held in Trust Account | - | |||||||
| Deferred offering costs associated with initial public offering | - | |||||||
| Total Assets | $ | $ | ||||||
| Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders’ Equity (Deficit): | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Due to related party | - | |||||||
| Franchise tax payable | ||||||||
| Note payable - related party | - | |||||||
| Total Current Liabilities | ||||||||
| Promissory note - related party | - | |||||||
| Deferred underwriting commissions | - | |||||||
| Derivative warrant liabilities | - | |||||||
| Total Liabilities | ||||||||
| Commitments and Contingencies | ||||||||
| Class A common stock subject to possible redemption, $ par value; and -- shares issued and outstanding at $ per share at September 30, 2021 and December 31, 2020, respectively | - | |||||||
| Stockholders’ Equity (Deficit): | ||||||||
| Preferred stock, $ par value; shares authorized; issued and outstanding | - | - | ||||||
| Class A common stock, $ par value; shares authorized; and -- shares issued and outstanding at September 30, 2021 and December 31, 2020, respectively | - | |||||||
| Class B common stock, $ par value; shares authorized; shares issued and outstanding at September 30, 2021 and December 31, 2020 (1) | ||||||||
| Additional paid-in capital | - | |||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total Stockholders’ Equity (Deficit) | ( | ) | ||||||
| Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders’ Equity (Deficit) | $ | $ | ||||||
| (1) |
The accompanying notes are an integral part of these unaudited condensed financial statements.
| F-16 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
UNAUDITED CONDENSED STATEMENTS OF OPERATIONS
| For
the Three Months Ended September 30, 2021 | For
the Nine Months Ended September 30, 2021 | |||||||
| General and administrative expenses | $ | $ | ||||||
| General and administrative expenses - related party | ||||||||
| Franchise tax expense | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Other income (expense) | ||||||||
| Change in fair value of derivative warrant liabilities | ||||||||
| Financing costs - derivative warrant liabilities | - | ( | ) | |||||
| Income from investments held in Trust Account | ||||||||
| Income (loss) before income tax | ( | ) | ||||||
| Income tax expense | - | - | ||||||
| Net income (loss) | $ | $ | ( | ) | ||||
| Weighted average shares outstanding of Class A common stock | ||||||||
| Basic and diluted net income (loss) per share, Class A common stock | $ | $ | ( | ) | ||||
| Weighted average shares outstanding of Class B common stock | ||||||||
| Basic and diluted net income (loss) per share, Class B common stock | $ | $ | ( | ) | ||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| F-17 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
UNAUDITED CONDENSED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2021
| Common Stock | ||||||||||||||||||||||||||||
| Class A | Class B | Additional | Accumulated | Total Stockholders’ Equity | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Paid-In Capital | Deficit | (Deficit) | ||||||||||||||||||||||
| Balance - December 31, 2020 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||
| Issuance of Representative’s Shares | - | - | - | |||||||||||||||||||||||||
| Excess of cash received over fair value of the private placement warrants | - | - | - | - | - | |||||||||||||||||||||||
| Accretion of Class A common stock subject to redemption amount | - | - | - | - | ( | ) | ( | ) | ( | ) | ||||||||||||||||||
| Net loss | - | - | - | - | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance - March 31, 2021 (unaudited) (as restated) | $ | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Net loss | - | - | - | - | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance - June 30, 2021 (unaudited) (as restated) | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||
| Net income | - | - | - | - | - | |||||||||||||||||||||||
| Balance - September 30, 2021 (unaudited) | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| F-18 |
FOXWAYNE ENTERPRISES ACQUISITION CORP.
UNAUDITED CONDENSED STATEME1NTS OF CASH FLOWS
| For
the Nine Months Ended September 30, 2021 | ||||
| Cash Flows from Operating Activities: | ||||
| Net loss | $ | ( | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||
| Change in fair value of derivative warrant liabilities | ( | ) | ||
| Financing costs - derivative warrant liabilities | ||||
| Income from investments held in Trust Account | ( | ) | ||
| Changes in operating assets and liabilities: | ||||
| Prepaid expenses | ( | ) | ||
| Accounts payable | ||||
| Accrued expenses | ||||
| Due to related party | ||||
| Franchise tax payable | ||||
| Net cash used in operating activities | ( | ) | ||
| Cash Flows from Investing Activities | ||||
| Cash deposited in Trust Account | ( | ) | ||
| Net cash used in investing activities | ( | ) | ||
| Cash Flows from Financing Activities: | ||||
| Repayment of note payable to related party | ( | ) | ||
| Proceeds from promissory note to related party | ||||
| Proceeds received from initial public offering, gross | ||||
| Proceeds received from private placement | ||||
| Offering costs paid | ( | ) | ||
| Net cash provided by financing activities | ||||
| Net increase in cash | ||||
| Cash - beginning of the period | ||||
| Cash - end of the period | $ | |||
| Supplemental disclosure of noncash activities: | ||||
| Reversal of offering costs included in accrued expenses in prior year | $ | ( | ) | |
| Offering costs included in accrued expenses | $ | |||
| Issuance of Representative’s Shares at the fair value of offering costs | $ | |||
| Deferred underwriting commissions in connection with the initial public offering | $ | |||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| F-19 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 1—Description of Organization and Business Operations
FoxWayne Enterprises Acquisition Corp. (the “Company”) is a blank check company incorporated in Delaware on September 17, 2020, for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses (the “Business Combination”). The Company is an emerging growth company and, as such, the Company is subject to all of the risks associated with emerging growth companies.
As of September 30, 2021, the Company had not commenced any operations. All activity for the period from September 17, 2020 (inception) through September 30, 2021, relates to the Company’s formation and the initial public offering (the “Initial Public Offering”) described below, and, subsequent to the Initial Public Offering, identifying a target company for a Business Combination. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company generates non-operating income in the form of interest income on its investments held in the trust account from the proceeds of its Initial Public Offering.
The
Company’s sponsor is FoxWayne Enterprises Acquisition Sponsor LLC, a Delaware limited liability company (the “Sponsor”).
The registration statement for the Company’s Initial Public Offering was declared effective on January 19, 2021. On January 22,
2021, the Company consummated its Initial Public Offering of units (the “Units” and, with respect to the Class
A common stock included in the Units being offered, the “Public Shares”), including additional Units to cover over-allotments
(the “Over-Allotment Units”), at $ per Unit, generating gross proceeds of $
Simultaneously
with the closing of the Initial Public Offering, the Company consummated the private placement (“Private Placement”) of
Upon
the closing of the Initial Public Offering and the Private Placement, approximately $
The
Company’s management has broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering
and the sale of the Private Placement Warrants, although substantially all of the net proceeds are intended to be applied generally toward
consummating a Business Combination. There is no assurance that the Company will be able to complete a Business Combination successfully.
| F-20 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
The
Company will provide its holders of the Public Shares (the “Public Stockholders”) with the opportunity to redeem all or a
portion of their Public Shares upon the completion of a Business Combination either (i) in connection with a stockholder meeting called
to approve the Business Combination or (ii) by means of a tender offer. The decision as to whether the Company will seek stockholder
approval of a Business Combination or conduct a tender offer will be made by the Company, solely in its discretion. The Public Stockholders
will be entitled to redeem their Public Shares for a pro rata portion of the amount then held in the Trust Account (initially anticipated
to be $ per Public Share). The per-share amount to be distributed to Public Stockholders who redeem their Public Shares will not
be reduced by the deferred underwriting commissions the Company will pay to the underwriters (as discussed in Note 5). These Public Shares
are recorded at a redemption value and classified as temporary equity upon the completion of the Initial Public Offering in accordance
with the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 480 “Distinguishing
Liabilities from Equity” (“ASC 480”). The Company will proceed with a Business Combination if a majority of the shares
voted are voted in favor of the Business Combination. The Company will not redeem the Public Shares in an amount that would cause its
net tangible assets to be less than $
The Amended and Restated Certificate of Incorporation provides that a public stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming its shares with respect to more than an aggregate of 15% or more of the Public Shares, without the prior consent of the Company.
The Sponsor and the Company’s officers and directors (the “Initial Stockholders”) agreed not to propose an amendment to the Amended and Restated Certificate of Incorporation to modify the substance or timing of the Company’s obligation to redeem 100% of the Public Shares if the Company does not complete a Business Combination within 12 months from the closing of the Initial Public Offering, or January 22, 2022, (or up to 18 months from the consummation of the Initial Public Offering, or July 22, 2022, if the Company extends the period of time to consummate a Business Combination) (the “Combination Period”), or with respect to any other material provisions relating to stockholders’ rights or pre-initial Business Combination activity, unless the Company provides the Public Stockholders with the opportunity to redeem their Public Shares in conjunction with any such amendment.
If
the Company anticipates that it may not be able to consummate the initial Business Combination within 12 months, the Company may, by
resolution of the board if requested by the Sponsor, extend the period of time to consummate a Business Combination up to two times,
each by an additional three months (for a total of up to 18 months to complete a Business Combination), subject to the Sponsor depositing
additional funds into the trust account as set out below. Pursuant to the terms of the Amended and Restated Certificate of Incorporation
and the trust agreement to be entered into between the Company and Continental Stock Transfer & Trust Company, in order for the time
available for the Company to consummate the initial Business Combination to be extended, the Sponsor or its affiliates or designees,
upon five business days advance notice prior to the applicable deadline, must deposit into the Trust Account $
If
the Company is unable to complete a Business Combination within the Combination Period, the Company will (i) cease all operations except
for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the Public
Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account including interest
earned on the funds held in the Trust Account and not previously released to the Company to pay its taxes (less up to $
| F-21 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
The Initial Stockholders agreed to waive their rights to liquidating distributions from the Trust Account with respect to the Founder Shares if the Company fails to complete a Business Combination within the Combination Period. However, if the Initial Stockholders acquire Public Shares in or after the Initial Public Offering, they will be entitled to liquidating distributions from the Trust Account with respect to such Public Shares if the Company fails to complete a Business Combination within the Combination Period. The underwriters agreed to waive their rights to the deferred underwriting commission (see Note 5) held in the Trust Account in the event the Company does not complete a Business Combination within in the Combination Period and, in such event, such amounts will be included with the other funds held in the Trust Account that will be available to fund the redemption of the Public Shares. The Company will seek to reduce the possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have all vendors, service providers (except the Company’s independent registered public accounting firm), prospective target businesses or other entities with which the Company does business, execute agreements with the Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account.
Proposed Business Combination
On August 3, 2021, the Company executed a non-binding letter of intent with Aerami Therapeutics Holdings, LLC.
Liquidity and Going Concern Consideration
As
of September 30, 2021, the Company had cash of approximately $
The
Company’s liquidity needs prior to the consummation of the Initial Public Offering were satisfied through the payment of $
On
September 21, 2021, Robb Knie, the Chief Executive Officer (CEO) of the Company loaned $
| F-22 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
In connection with the Company’s assessment of going concern considerations in accordance with Financial Accounting Standard Board’s Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the mandatory liquidation and subsequent dissolution raises substantial doubt about the Company’s ability to continue as a going concern, and management has determined it may be probable that the Company would be unable to meet its obligations as they become due within one year raising substantial doubt about the Company’s ability to continue as a going concern. No adjustments have been made to the carrying amounts of assets or liabilities should the Company be required to liquidate after January 22, 2022.
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 global pandemic on the industry and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations, and/or search for a target company, the specific impact is not readily determinable as of the date of these unaudited condensed financial statement. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 2— Basis of Presentation and Summary of Significant Accounting Policies (restated)
Basis of Presentation
The accompanying unaudited condensed financial statements are presented in U.S. dollars in conformity with accounting principles generally accepted in the United States of America (“GAAP”) for financial information and pursuant to the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”). Accordingly, they do not include all of the information and footnotes required by GAAP. In the opinion of management, the unaudited condensed financial statements reflect all adjustments, which include normal recurring adjustments, necessary for the fair statement of the balances and results for the period presented. Operating results for the three and nine months ended September 30, 2021, are not necessarily indicative of the results that may be expected through December 31, 2021 or for any period after that.
The accompanying unaudited condensed financial statements should be read in conjunction with the audited financial statements and notes thereto included in the Annual Report on Form 10-K filed by the Company with the SEC on March 29, 2021.
Restatement of Previously Reported Financial Statements (restated)
In
preparation of the Company’s unaudited condensed financial statements as of and for quarterly period ended September 30, 2021,
the Company concluded it should restate its financial statements to classify all Class A common stock subject to possible redemption
in temporary equity. In accordance with the SEC and its staff’s guidance on redeemable equity instruments, ASC 480, paragraph 10-S99,
redemption provisions not solely within the control of the Company require common stock subject to redemption to be classified outside
of permanent equity. The Company had previously classified a portion of its Class A common stock in permanent equity, or total stockholders’
equity. Although the Company did not specify a maximum redemption threshold, its charter currently provides that, the Company will not
redeem its public shares in an amount that would cause its net tangible assets to be less than $
In accordance with SEC Staff Accounting Bulletin No. 99, “Materiality,” and SEC Staff Accounting Bulletin No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements,” the Company evaluated the corrections and has determined that the related impact was material to the previously filed financial statements that contained the error, reported in the Company’s Form 8-K filed with the SEC on January 28, 2021, and the Company’s Form 10-Qs for the quarterly periods ended March 31, 2021, and June 30, 2021 (the “Affected Quarterly Periods”). Therefore, the Company, in consultation with its Audit Committee, concluded that the IPO Balance Sheet and the Affected Quarterly Periods should be restated to present all Class A common stock subject to possible redemption as temporary equity and to recognize accretion from the initial book value to redemption value at the time of its Initial Public Offering. As such, the Company is reporting these restatements to those periods in this Quarterly Report on Form 10-Q/A.
The table below presents the effect of the financial statement adjustments related to the restatement discussed above of the Company’s previously reported IPO Balance Sheet as of January 22, 2021:
| As of January 22, 2021 | As Previously Restated | Adjustment | As Restated | |||||||||
| Total assets | $ | $ | $ | |||||||||
| Total liabilities | $ | $ | $ | |||||||||
| Class A common stock subject to possible redemption | ||||||||||||
| Preferred stock | - | - | - | |||||||||
| Class A common stock | ( | ) | ||||||||||
| Class B common stock | - | |||||||||||
| Additional paid-in capital | ( | ) | - | |||||||||
| Accumulated deficit | ( | ) | ( | ) | ( | ) | ||||||
| Total stockholders’ equity (deficit) | $ | $ | ( | ) | $ | ( | ) | |||||
| Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders’ Equity (Deficit) | $ | $ | ( | ) | $ | |||||||
| Shares of Class A common stock subject to possible redemption | ||||||||||||
| Shares of Class A non-redeemable common stock | ( | ) | ||||||||||
The impact of the restatement on the financial statements for the unaudited Affected Quarterly Periods is presented below. There is no impact to the reported amounts for total assets, total liabilities, cash flows and net income (loss).
| F-23 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
The table below presents the effect of the financial statement adjustments related to the restatement discussed above of the Company’s previously reported unaudited condensed balance sheet as of March 31, 2021:
| As of March 31, 2021 (unaudited) | As Previously Reported | Adjustment | As Restated | |||||||||
| Total assets | $ | $ | $ | |||||||||
| Total liabilities | $ | $ | $ | |||||||||
| Class A common stock subject to possible redemption | ||||||||||||
| Preferred stock | - | - | - | |||||||||
| Class A common stock | ( | ) | ||||||||||
| Class B common stock | - | |||||||||||
| Additional paid-in capital | ( | ) | - | |||||||||
| Accumulated deficit | ( | ) | ( | ) | ( | ) | ||||||
| Total stockholders’ equity (deficit) | $ | $ | ( | ) | $ | ( | ) | |||||
| Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders’ Equity (Deficit) | $ | $ | $ | |||||||||
| Shares of Class A common stock subject to possible redemption | ||||||||||||
| Shares of Class A non-redeemable common stock | ( | ) | ||||||||||
The Company’s unaudited condensed statement of stockholders’ equity has been restated to reflect the changes to the impacted stockholders’ equity accounts described above.
The table below presents the effect of the financial statement adjustments related to the restatement discussed above of the Company’s previously reported unaudited condensed statement of cash flows for the three months ended March 31, 2021:
| Three Months Ended March 31, 2021 (unaudited) | ||||||||||||
| Supplemental Disclosure of Noncash Financing Activities: | As Reported | Adjustment | As Restated | |||||||||
| Initial value of Class A common stock subject to possible redemption | $ | $ | ( | ) | $ | - | ||||||
| Change in value of Class A common stock subject to possible redemption | $ | $ | ( | ) | $ | |||||||
The table below presents the effect of the financial statement adjustments related to the restatement discussed above of the Company’s previously reported unaudited condensed balance sheet as of June 30, 2021:
| As of June 30, 2021 (unaudited) | As Previously Reported | Adjustment | As Restated | |||||||||
| Total assets | $ | $ | $ | |||||||||
| Total liabilities | $ | $ | $ | |||||||||
| Class A common stock subject to possible redemption | ||||||||||||
| Preferred stock | - | - | - | |||||||||
| Class A common stock | ( | ) | ||||||||||
| Class B common stock | - | |||||||||||
| Additional paid-in capital | ( | ) | - | |||||||||
| Accumulated deficit | ( | ) | ( | ) | ( | ) | ||||||
| Total stockholders’ equity (deficit) | $ | $ | ( | ) | $ | ( | ) | |||||
| Total Liabilities, Class A Common Stock Subject to Possible Redemption and Stockholders’ Equity (Deficit) | $ | $ | $ | |||||||||
| Shares of Class A common stock subject to possible redemption | ||||||||||||
| Shares of Class A non-redeemable common stock | ( | ) | ||||||||||
The Company’s unaudited condensed statement of stockholders’ equity has been restated to reflect the changes to the impacted stockholders’ equity accounts described above.
The table below presents the effect of the financial statement adjustments related to the restatement discussed above of the Company’s previously reported unaudited condensed statement of cash flows for the six months ended June 30, 2021:
| Six Months Ended June 30, 2021 (unaudited) | ||||||||||||
| Supplemental Disclosure of Noncash Financing Activities: | As Reported | Adjustment | As Restated | |||||||||
| Initial value of Class A common stock subject to possible redemption | $ | $ | ( | ) | $ | - | ||||||
| Change in value of Class A common stock subject to possible redemption | $ | ( | ) | $ | $ | |||||||
In connection with the change in presentation for the Class A common stock subject to possible redemption, the Company has revised its earnings per share calculation to allocate income and losses shared pro rata between the two classes of shares. This presentation contemplates a Business Combination as the most likely outcome, in which case, both classes of shares participate pro rata in the income and losses of the Company. The impact to the reported amounts of weighted average shares outstanding and basic and diluted loss per common stock is presented below for the unaudited Affected Quarterly Periods:
| Loss Per Common Stock | ||||||||||||
| As Reported | Adjustment | As Restated | ||||||||||
| For the three months ended March 31, 2021 (unaudited) | ||||||||||||
| Net loss | $ | ( | ) | $ | $ | ( | ) | |||||
| Weighted average shares outstanding - Class A common stock | ( | ) | ||||||||||
| Basic and diluted earnings per common stock - Class A common stock | $ | $ | ( | ) | $ | ( | ) | |||||
| Weighted average shares outstanding - Class B common stock | ( | ) | ||||||||||
| Basic and diluted loss per common stock - Class B common stock | $ | ( | ) | $ | $ | ( | ) | |||||
| For three months ended June 30, 2021 (unaudited) | ||||||||||||
| Net loss | $ | ( | ) | $ | $ | ( | ) | |||||
| Weighted average shares outstanding - Class A common stock | - | |||||||||||
| Basic and diluted earnings per common stock - Class A common stock | $ | $ | ( | ) | $ | ( | ) | |||||
| Weighted average shares outstanding - Class B common stock | - | |||||||||||
| Basic and diluted loss per common stock - Class B common stock | $ | ( | ) | $ | $ | ( | ) | |||||
| For the six months ended June 30, 2021 (unaudited) | ||||||||||||
| Net loss | $ | ( | ) | $ | $ | ( | ) | |||||
| Weighted average shares outstanding - Class A common stock | ( | ) | ||||||||||
| Basic and diluted earnings per common stock - Class A common stock | $ | $ | ( | ) | $ | ( | ) | |||||
| Weighted average shares outstanding - Class B common stock | - | |||||||||||
| Basic and diluted loss per common stock - Class B common stock | $ | ( | ) | $ | $ | ( | ) | |||||
| F-24 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a)(19) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that an emerging growth company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard.
This may make comparison of the Company’s unaudited condensed financial statements with another public company that is neither an emerging growth company nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Use of Estimates
The preparation of unaudited condensed financial statements in conformity with GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the unaudited condensed financial statements and the reported amounts of revenues and expenses during the reporting period. Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the unaudited condensed financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates.
Cash and Cash Equivalents
The
Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents.
The Company had
Investments Held in Trust Account
The Company’s portfolio of investments is comprised of U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or investments in money market funds that invest in U.S. government securities and generally have a readily determinable fair value, or a combination thereof. When the Company’s investments held in the Trust Account are comprised of U.S. government securities, the investments are classified as trading securities. When the Company’s investments held in the Trust Account are comprised of money market funds, the investments are recognized at fair value. Trading securities and investments in money market funds are presented on the balance sheets at fair value at the end of each reporting period. Gains and losses resulting from the change in fair value of these securities is included in income from investments held in the Trust Account in the accompanying unaudited condensed statements of operations. The estimated fair values of investments held in the Trust Account are determined using available market information.
| F-25 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
Concentration of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit risk consist of cash accounts in a financial institution,
which, at times, may exceed the Federal Depository Insurance Corporation coverage limit of $
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under the FASB ASC Topic 820, “Fair Value Measurements,” equal or approximates the carrying amounts represented in the condensed balance sheets.
Fair Value Measurements
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value.
The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers consist of:
| ● | Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets; |
| ● | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and | |
| ● | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
Derivative Warrant Liabilities
The Company does not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. The Company evaluates all of its financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives, pursuant to ASC 480 and FASB ASC Topic 815, “Derivatives and Hedging” (“ASC 815”). The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period.
The warrants issued in connection with the Initial Public Offering (the “Public Warrants”) and the Private Placement Warrants are recognized as derivative liabilities in accordance with ASC 815. Accordingly, the Company recognizes the warrant instruments as liabilities at fair value and adjusts the instruments to fair value at each reporting period. The liabilities are subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in the Company’s unaudited condensed statement of operations. The fair value of the Public Warrants issued in connection with the Public Offering and Private Placement Warrants were initially measured at fair value using a Monte Carlo simulation model and subsequently, the fair value of the Private Placement Warrants have been estimated using a Monte Carlo simulation model each measurement date. The fair value of Public Warrants issued in connection with the Initial Public Offering have subsequently been measured based on the listed market price of such warrants. The determination of the fair value of the warrant liability may be subject to change as more current information becomes available and accordingly the actual results could differ significantly. Derivative warrant liabilities are classified as non-current liabilities as their liquidation is not reasonably expected to require the use of current assets or require the creation of current liabilities.
| F-26 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
Offering Costs Associated with the Initial Public Offering
Offering
costs consisted of legal, accounting, underwriting fees and other costs incurred through the Initial Public Offering that were directly
related to the Initial Public Offering. Offering costs are allocated to the separable financial instruments issued in the Initial Public
Offering based on a relative fair value basis, compared to total proceeds received. Offering costs associated with warrant liabilities
are expensed as incurred, presented as non-operating expenses in the statement of operations. Offering costs associated with the Class
A common stock are charged against their carrying value upon the completion of the Initial Public Offering. For the nine months ended
September 30, 2021, of the total offering costs of the Initial Public Offering, approximately $
Class A Common Stock Subject to Possible Redemption
The
Company accounts for its Class A common stock subject to possible redemption in accordance with the guidance in ASC 480. Class A common
stock subject to mandatory redemption (if any) is classified as a liability instrument and is measured at fair value. Conditionally redeemable
Class A common stock (including shares of Class A common stock that feature redemption rights that are either within the control of the
holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) is classified
as temporary equity. At all other times, Class A common stock is classified as stockholders’ equity. The Company’s Class
A common stock features certain redemption rights that are considered to be outside of the Company’s control and subject to occurrence
of uncertain future events. Accordingly, as of September 30, 2021,
Under ASC 480-10-S99, the Company has elected to recognize changes in the redemption value immediately as they occur and adjust the carrying value of the security to equal the redemption value at the end of each reporting period. This method would view the end of the reporting period as if it were also the redemption date for the security. Effective with the closing of the Initial Public Offering (including exercise of the over-allotment option), the Company recognized the accretion from initial book value to redemption amount, which resulted in charges against additional paid-in capital (to the extent available) and accumulated deficit.
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share.” The Company has two classes of shares, which are referred to as Class A common stock and Class B common stock. Income and losses are shared pro rata between the two classes of shares. Net income (loss) per common share is calculated by dividing the net income (loss) by the weighted average shares of common stock outstanding for the respective period.
The calculation of diluted net income (loss) per common stock does not consider the effect of the warrants issued in connection with the Initial Public Offering (including exercise of the over-allotment option) and the Private Placement to purchase an aggregate of 8,550,000 shares of common stock in the calculation of diluted income (loss) per share, because their exercise is contingent upon future events. As of September 30, 2021, the Company did not have any dilutive securities or other contracts that could, potentially, be exercised or converted into common shares and then share in the earnings of the Company. As a result, diluted net income (loss) per share is the same as basic net income (loss) per share for the three and nine months ended September 30, 2021. Accretion associated with the redeemable Class A common stock is excluded from earnings per share as the redemption value approximates fair value.
| F-27 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
| For the Three Months Ended September 30, 2021 | For the Nine Months Ended September 30, 2021 | |||||||||||||||
| Class A | Class B | Class A | Class B | |||||||||||||
| Basic and diluted net income (loss) per common stock: | ||||||||||||||||
| Numerator: | ||||||||||||||||
| Allocation of net income (loss) | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||
| Denominator: | ||||||||||||||||
| Basic and diluted weighted average common stock outstanding | ||||||||||||||||
| Basic and diluted net income (loss) per common stock | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under FASB ASC Topic 740, “Income Taxes” (“ASC 740”). Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. As of September 30, 2021, the Company had deferred tax assets with a full valuation allowance against them. Deferred tax assets were deemed immaterial as of December 31, 2020.
FASB 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. There were no unrecognized tax benefits as of September 30, 2021, and December 31, 2020. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of September 30, 2021, and December 31, 2020. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
Recent Accounting Pronouncements
The Company’s management does not believe that any recently issued, but not yet effective, accounting standards updates, if currently adopted, would have a material effect on the Company’s unaudited condensed financial statements.
Note 3—Initial Public Offering
On
January 22, 2021, the Company consummated its Initial Public Offering of Units, including Over-Allotment Units, at
$ per Unit, generating gross proceeds of $
Each Unit consists of one share of Class A common stock and one redeemable warrant (each, a “Public Warrant”). Each whole Public Warrant entitles the holder to purchase one share of Class A common stock at a price of $ per share, subject to adjustment (see Note 8).
| F-28 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 4—Related Party Transactions
Founder Shares
On
October 15, 2020, the Sponsor purchased shares of the Company’s Class B common stock, par value $ per share (the
“Founder Shares”), for an aggregate price of $
Private Placement Warrants
Simultaneously
with the closing of the Initial Public Offering, the Company consummated the Private Placement of
Each whole Private Placement Warrant is exercisable for one whole share of Class A common stock at a price of $ per share. A portion of the proceeds from the sale of the Private Placement Warrants to the Sponsor was added to the proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination within the Combination Period, the Private Placement Warrants will expire worthless. The Private Placement Warrants will be non-redeemable for cash and exercisable on a cashless basis so long as they are held by the Sponsor or its permitted transferees.
The Sponsor and the Company’s officers and directors agreed, subject to limited exceptions, not to transfer, assign or sell any of their Private Placement Warrants until 30 days after the completion of the initial Business Combination.
Related Party Loans
On
September 30, 2020, Robb Knie, CEO, agreed to loan the Company an aggregate of up to $
On
September 21, 2021, Robb Knie, CEO, loaned $
In
addition, in order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor,
or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working
Capital Loans”). If the Company completes a Business Combination, the Company will repay the Working Capital Loans out of the proceeds
of the Trust Account released to the Company. Otherwise, the Working Capital Loans would be repaid only out of funds held outside the
Trust Account. In the event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust
Account to repay the Working Capital Loans, but no proceeds held in the Trust Account would be used to repay the Working Capital Loans.
Except for the foregoing, the terms of such Working Capital Loans, if any, have not been determined and no written agreements exist with
respect to such loans. The Working Capital Loans would either be repaid upon consummation of a Business Combination or, at the lenders’
discretion, up to $
| F-29 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
As
discussed in Note 1, the Company may extend the period of time to consummate a Business Combination up to two times, each by an additional
three months (for a total of 18 months to complete a Business Combination). In order to extend the time available for the Company to
consummate a Business Combination, the Sponsor or its affiliates or designees must deposit into the Trust Account $
Administrative Services Agreement
Commencing
on the date that the Company’s securities were first listed on Nasdaq through the earlier of consummation of the initial Business
Combination and the Company’s liquidation, the Company agreed to pay the Sponsor a total of $
The Company’s officers or directors will be reimbursed for any out-of-pocket expenses incurred in connection with activities on the Company’s behalf such as identifying potential target businesses and performing due diligence on suitable Business Combinations. The audit committee of the Company’s Board of Directors will review on a quarterly basis all payments that were made to the Sponsor, officers, directors or their affiliates and will determine which expenses and the amount of expenses that will be reimbursed. There is no cap or ceiling on the reimbursement of out-of-pocket expenses incurred by such persons in connection with activities on the Company’s behalf.
Note 5—Commitments & Contingencies
Registration and Stockholder Rights
The holders of Founder Shares, Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans or Extension Loans, if any, (and any shares of Class A common stock issuable upon the exercise of the Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans and upon conversion of the Founder Shares) are entitled to registration rights pursuant to a registration rights agreement signed upon the consummation of the Initial Public Offering. The holders of these securities are entitled to make up to three demands, excluding short form demands, that the Company register such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to the completion of the initial Business Combination. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
The
underwriters were entitled to an underwriting discount of $
| F-30 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
The
Company issued EF Hutton (formerly Kingswood Capital Markets), a division of Benchmark Investments, Inc. (“EF Hutton”),
the Representative of the underwriters (the “Representative”), and/or its designees, shares of Class A common stock (the “Representative’s
Shares”) upon the consummation of the Initial Public Offering. EF Hutton agreed not to transfer, assign or sell any such shares
until the completion of the initial Business Combination. In addition, EF Hutton agreed (i) to waive its redemption rights with respect
to such shares in connection with the completion of the initial Business Combination and (ii) to waive its rights to liquidating distributions
from the Trust Account with respect to such shares if the Company fails to complete its initial Business Combination within the Combination
Period. The Company recorded the fair value of the Representative Shares, $
Risks and Uncertainties
Management continues to evaluate the impact of the COVID-19 pandemic on the industry and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations and/or search for a target company, the specific impact is not readily determinable as of the date of these unaudited condensed financial statements. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 6—Derivative Warrant Liabilities
As
of September 30, 2021, the Company has
Public Warrants may only be exercised for a whole number of shares. No fractional Public Warrants will be issued upon separation of the Units and only whole Public Warrants will trade. The Public Warrants will become exercisable on the later of (a) the completion of a Business Combination or (b) 12 months from the closing of the Initial Public Offering; provided in each case that the Company has an effective registration statement under the Securities Act covering the shares of Class A common stock issuable upon exercise of the Public Warrants and a current prospectus relating to them is available (or the Company permits holders to exercise their Public Warrants on a cashless basis and such cashless exercise is exempt from registration under the Securities Act). The Company agreed that as soon as practicable, but in no event later than 15 business days after the closing of the initial Business Combination, it will use its commercially reasonable efforts to file with the SEC and have an effective registration statement covering the shares of the Class A common stock issuable upon exercise of the warrants and to maintain a current prospectus relating to those shares of the Class A common stock until the warrants expire or are redeemed. If a registration statement covering the shares of the Class A common stock issuable upon exercise of the warrants is not effective by the 60th business day after the closing of the initial Business Combination, warrant holders may, until such time as there is an effective registration statement and during any period when the Company will have failed to maintain an effective registration statement, exercise warrants on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act or another exemption.
The
warrants have an exercise price of $
The Private Placement Warrants are identical to the Public Warrants, except that the Private Placement Warrants and the shares of Class A common stock issuable upon exercise of the Private Placement Warrants will not be transferable, assignable or salable until 30 days after the completion of a Business Combination, subject to certain limited exceptions. Additionally, the Private Placement Warrants will be non-redeemable so long as they are held by the Sponsor or its permitted transferees. If the Private Placement Warrants are held by someone other than the Sponsor or its permitted transferees, the Private Placement Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
| F-31 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
Once the warrants become exercisable, the Company may redeem the outstanding warrants (except as described herein with respect to the Private Placement Warrants):
| ● | in whole and not in part; | |
| ● | at a price of $0.01 per warrant; | |
| ● | upon a minimum of 30 days’ prior written notice of redemption; and | |
| ● | if, and only if, the last sale price of Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third day prior to the date on which the Company sends the notice of redemption to the warrant holders. |
The Company will not redeem the warrants as described above unless a registration statement under the Securities Act covering the shares of Class A common stock issuable upon exercise of the warrants is effective and a current prospectus relating to those shares of Class A common stock is available throughout the 30-day redemption period, except if the warrants may be exercised on a cashless basis and such cashless exercise is exempt from registration under the Securities Act.
If the Company is unable to complete a Business Combination within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of warrants will not receive any of such funds with respect to their warrants, nor will they receive any distribution from the Company’s assets held outside of the Trust Account with the respect to such warrants. Accordingly, the warrants may expire worthless.
Note 7—Class A Common Stock Subject to Possible Redemption
The Company’s Class A common stock feature certain redemption rights that are considered to be outside of the Company’s control and subject to the occurrence of future events. The Company is authorized to issue shares of Class A common stock with a par value of $ per share. Holders of the Company’s Class A common stock are entitled to one vote for each share. As of September 30, 2021, there were shares of Class A common stock outstanding, shares of which were subject to possible redemption and are classified outside of permanent equity in the unaudited condensed balance sheet. As of December 31, 2020, there were no shares of Class A common stock issued or outstanding.
The Class A common stock subject to possible redemption reflected on the unaudited condensed balance sheet is reconciled on the following table:
| Gross proceeds | $ | |||
| Less: | ||||
| Proceeds allocated to Public Warrants | ( | ) | ||
| Class A common stock issuance costs | ( | ) | ||
| Plus: | ||||
| Accretion of carrying value to redemption value | ||||
| Class A common stock subject to possible redemption | $ |
Note 8—Stockholders’ Equity (Deficit)
Preferred Stock — The Company is authorized to issue shares of preferred stock, par value $ per share, with such designations, voting and other rights and preferences as may be determined from time to time by the Company’s board of directors. As of September 30, 2021, and December 31, 2020, there were shares of preferred stock issued or outstanding.
Class A Common Stock — The Company is authorized to issue shares of Class A common stock with a par value of $ per share. As of September 30, 2021, there were shares of Class A common stock issued or outstanding, shares of which were subject to possible redemption and are classified outside of permanent equity in the unaudited condensed balance sheet (see Note 7). As of December 31, 2020, there were shares of Class A common stock issued or outstanding.
| F-32 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
Class B Common Stock — The Company is authorized to issue shares of Class B common stock with a par value of $ per share. As of September 30, 2021 and December 31, 2020, there were shares of Class B common stock issued and outstanding, of which an aggregate of up to shares of Class B common stock were subject to forfeiture to the extent that the underwriters’ over-allotment option was not exercised in full or in part, so that the Initial Stockholders will collectively own 20% of the Company’s issued and outstanding common stock after the Initial Public Offering (excluding the Representative’s Shares). The underwriter exercised its over-allotment option in full on January 22, 2021; thus, these Founder Shares are no longer subject to forfeiture.
Holders of the Class A common stock and holders of the Class B common stock will vote together as a single class on all matters submitted to a vote of the stockholders, except as required by law. Each share of common stock will have one vote on all such matters. However, the holders of the Founder Shares have the right to elect all of the Company’s directors prior to the initial Business Combination.
The Class B common stock will automatically convert into Class A common stock at the closing of the initial Business Combination on a one-for-one basis, subject to adjustment for stock splits, stock dividends, reorganizations, recapitalizations and the like, and subject to further adjustment as provided herein. In the case that additional shares of Class A common stock or equity-linked securities are issued or deemed issued in connection with the initial Business Combination, the number of shares of Class A common stock issuable upon conversion of all Founder Shares will equal, in the aggregate, on an as-converted basis, 20% of the sum of the total number of all shares of common stock outstanding upon the completion of the Initial Public Offering, plus the total number of shares of Class A common stock issued, or deemed issued or issuable upon conversion or exercise of any equity-linked securities or rights issued or deemed issued, by the Company in connection with or in relation to the consummation of the initial Business Combination, excluding any shares of Class A common stock or equity-linked securities exercisable for or convertible into shares of Class A common stock issued, or to be issued, to any seller in the initial Business Combination and any private placement-equivalent warrants issued to the Sponsor, officers or directors upon conversion of Working Capital Loans or Extension Loans; provided that such conversion of Founder Shares will never occur on a less than one for one basis.
Note 9—Fair Value Measurements
The following table presents information about the Company’s financial assets and liabilities that are measured at fair value on a recurring basis as of September 30, 2021.
| Description | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Other Unobservable Inputs (Level 3) | |||||||||
| Assets: | ||||||||||||
| Investments held in Trust Account | $ | $ | $ | |||||||||
| Liabilities: | ||||||||||||
| Derivative warrant liabilities - Public Warrants | $ | $ | $ | |||||||||
| Derivative warrant liabilities - Private Placement Warrants | $ | $ | $ | |||||||||
Transfers to/from Levels 1, 2, and 3 are recognized at the beginning of the reporting period. The estimated fair value of the Public Warrants transferred from a Level 3 fair value measurement to a Level 1 fair value measurement, when the Public Warrants were separately listed and traded in February 2021.
Level 1 assets include investments in U.S. Treasury securities. The Company uses inputs such as actual trade data, quoted market prices from dealers or brokers, and other similar sources to determine the fair value of its investments.
Subsequent to the Public Warrants being separately listed and traded, their value is based on their observable listed trading price, a Level 1 measurement.
| F-33 |
FOXWAYNE
ENTERPRISES ACQUISITION CORP.
NOTES TO THE UNAUDITED CONDENSED FINANCIAL STATEMENTS
Level 3 instruments are comprised of derivative warrant liabilities measured at fair value using a Monte Carlo simulation model. The estimated fair value of the Private Placement Warrants and the Public Warrants, prior to the Public Warrants being traded in an active market, was determined using Level 3 inputs. Inherent in a Monte Carlo simulation model are assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates the volatility of its common stock warrants based on implied volatility from the Company’s traded warrants and from historical volatility of select peer company’s common stock that matches the expected remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which the Company anticipates remaining at zero.
The following table provides quantitative information regarding Level 3 fair value measurements inputs at their measurement dates:
| As of January 22, 2021 | September 30, 2021 | |||||||
| Option term (in years) | ||||||||
| Volatility | % | % | ||||||
| Risk-free interest rate | % | % | ||||||
| Expected dividends | % | % | ||||||
| Stock price | $ | $ | ||||||
The change in the fair value of the derivative warrant liabilities, measured using Level 3 inputs, for the three and nine months ended September 30, 2021, is summarized as follows:
| Derivative warrant liabilities at January 1, 2021 | $ | |||
| Issuance of Public and Private Warrants | ||||
| Transfer of Public Warrants to a Level 1 measurement | ( | ) | ||
| Change in fair value of derivative warrant liabilities | ( | ) | ||
| Derivative warrant liabilities at March 31, 2021 | $ | |||
| Change in fair value of derivative warrant liabilities | ||||
| Derivative warrant liabilities at June 30, 2021 | $ | |||
| Change in fair value of derivative warrant liabilities | ( | ) | ||
| Derivative warrant liabilities at September 30, 2021 | $ |
Note 10—Subsequent Events
The Company evaluated subsequent events and transactions that occurred up to the date unaudited condensed financial statements were available to be issued. Based upon this review the Company did not identify any subsequent events that would have required adjustment or disclosure in the unaudited condensed financial statements, except for the restatement discussed in Note 2.
| F-34 |
Consolidated Financial Statements and Report of Independent Registered Public Accounting Firm | |
| Aerami Therapeutics Holdings, Inc. and Subsidiaries | |
| December 31, 2020 and 2019 | |
| F-35 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders Aerami Therapeutics Holdings, Inc.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheets of Aerami Therapeutics Holdings, Inc. (a Delaware corporation) and subsidiaries (the “Company”) as of December 31, 2020 and 2019, the related consolidated statements of operations, convertible preferred stock and stockholders’ deficit and cash flows for the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company incurred a net loss of $10.3 million for the year ended December 31, 2020 and does not have a commercial product that can generate revenue or support its current cost structure. These conditions, along with other matters as set forth in Note 1, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Grant Thornton LLP
We have served as the Company’s auditor since 2019.
Iselin, New Jersey
May 3, 2021 (except for Note 2 - Preferred Stock and Net Loss Per Share, as to which the date is January 4, 2022)
| F-36 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
CONSOLIDATED BALANCE SHEETS
(in thousands of U.S. dollars, except share and per share data)
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Assets: | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 4,494 | $ | 6,659 | ||||
| Prepaid expenses and other current assets | 319 | 141 | ||||||
| Total current assets | 4,813 | 6,800 | ||||||
| Other non-current assets | 6 | 214 | ||||||
| Property, equipment and right-of-use assets, net | — | 81 | ||||||
| Total assets | $ | 4,819 | $ | 7,095 | ||||
| Liabilities, convertible preferred stock, and stockholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 245 | $ | 586 | ||||
| Accrued expenses and other current liabilities | 3,316 | 5,442 | ||||||
| Total current liabilities | 3,561 | 6,028 | ||||||
| Harmony contingent settlement liability | 2,978 | 3,363 | ||||||
| Warrant liability | 664 | - | ||||||
| Customer advances and other liabilities | 977 | 1,199 | ||||||
| Total liabilities | 8,180 | 10,590 | ||||||
| Commitments and contingencies (Note 10) | ||||||||
| Convertible preferred stock: | ||||||||
| Series A preferred stock, $0.0001 par value; 50,000 shares authorized; 10,168 and 0 shares issued and outstanding as of December 31, 2020 and 2019, respectively | 8,103 | - | ||||||
| Series A-1 preferred stock, $0.0001 par value; 50,000 shares authorized; no shares issued or outstanding | - | - | ||||||
| Total convertible preferred stock | 8,103 | - | ||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, $0.0001 par value; 5,500,000 shares authorized; no shares issued or outstanding | - | - | ||||||
| Royalty Series convertible preferred stock, $0.0001 par value; 4,400,000 shares authorized; 2,541,459 shares issued and outstanding as of December 31, 2020 and 2019 | 6,956 | 6,956 | ||||||
| Common stock, $0.0001 par value; 150,000,000 shares authorized; 34,639,806 and 34,633,690 shares issued and outstanding as of December 31, 2020 and 2019, respectively | 3 | 3 | ||||||
| Additional paid-in capital | 106,896 | 104,556 | ||||||
| Accumulated deficit | (125,319 | ) | (115,010 | ) | ||||
| Total stockholders’ deficit | (11,464 | ) | (3,495 | ) | ||||
| Total liabilities, convertible preferred stock, and stockholders’ deficit | $ | 4,819 | $ | 7,095 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-37 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands of U.S. dollars, except share and per share data)
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | 3,461 | $ | 11,953 | ||||
| General and administrative | 6,932 | 8,538 | ||||||
| Total operating expenses | 10,393 | 20,491 | ||||||
| Other income (expenses): | ||||||||
| Change in fair value of Harmony contingent settlement liability | 385 | (551 | ) | |||||
| Interest (expense) income, net | (100 | ) | 18 | |||||
| Change in fair value of Harmony purchase option agreement | (201 | ) | (369 | ) | ||||
| Inducement charge from exercise of warrants | - | (2,551 | ) | |||||
| Total other income (expenses) | 84 | (3,453 | ) | |||||
| Net loss | (10,309 | ) | (23,944 | ) | ||||
| Accretion of Series A preferred stock to redemption value | (221 | ) | - | |||||
| Net loss attributable to common stockholders | $ | (10,530 | ) | $ | (23,944 | ) | ||
| Per share information: | ||||||||
| Net loss per share of common stock, basic and diluted | $ | (0.30 | ) | $ | (0.76 | ) | ||
| Weighted average common shares outstanding, basic and diluted | 34,638,486 | 31,499,138 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-38 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT
(in thousands of U.S. dollars, except share data)
| Convertible Preferred Stock | Stockholders’ Deficit | |||||||||||||||||||||||||||||||||||||||||||
| Royalty Series | ||||||||||||||||||||||||||||||||||||||||||||
| Series A | Convertible | Additional | Total | |||||||||||||||||||||||||||||||||||||||||
| Preferred Stock | Preferred Stock | Common Stock | Warrants | Paid-In | Accumulated | Stockholders’ | ||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||||||||||||||
| Balances at December 31, 2018 | - | $ | - | 2,541,459 | $ | 6,956 | 20,895,242 | $ | 2 | - | $ | - | $ | 78,936 | $ | (91,066 | ) | $ | (5,172 | ) | ||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options | - | - | - | - | 60,041 | - | - | - | 7 | - | 7 | |||||||||||||||||||||||||||||||||
| Issuance of common stock as payment for services | - | - | - | - | 35,876 | - | - | - | 119 | - | 119 | |||||||||||||||||||||||||||||||||
| Repurchase of warrants | - | - | - | - | - | - | - | (495 | ) | - | - | (495 | ) | |||||||||||||||||||||||||||||||
| Reissuance of warrants | - | - | - | - | - | - | - | 495 | - | - | 495 | |||||||||||||||||||||||||||||||||
| Issuance of common stock upon cashless exercise of warrants | - | - | - | - | 1,314,847 | - | - | - | 929 | - | 929 | |||||||||||||||||||||||||||||||||
| Issuance of common stock upon exercise of warrants, less issuance costs of $2,357 | - | - | - | - | 12,327,684 | 1 | - | - | 20,739 | - | 20,740 | |||||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | - | - | - | - | 3,826 | - | 3,826 | |||||||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | - | - | (23,944 | ) | (23,944 | ) | |||||||||||||||||||||||||||||||
| Balances at December 31, 2019 | - | - | 2,541,459 | 6,956 | 34,633,690 | 3 | - | - | 104,556 | (115,010 | ) | (3,495 | ) | |||||||||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options | - | - | - | - | 6,116 | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||
| Issuance of Series A preferred stock, less issuance costs of $1,622 | 10,168 | 7,882 | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||
| Accretion of Series A preferred stock to redemption value | - | 221 | - | - | - | - | - | - | (221 | ) | - | (221 | ) | |||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | - | - | - | - | 2,561 | - | 2,561 | |||||||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | - | - | (10,309 | ) | (10,309 | ) | |||||||||||||||||||||||||||||||
| Balances at December 31, 2020 | 10,168 | $ | 8,103 | 2,541,459 | $ | 6,956 | 34,639,806 | $ | 3 | - | $ | - | $ | 106,896 | $ | (125,319 | ) | $ | (11,464 | ) | ||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-39 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands of U.S. dollars)
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (10,309 | ) | $ | (23,944 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | - | 210 | ||||||
| Change in fair value of Harmony contingent settlement liability | (385 | ) | 551 | |||||
| Change in fair value of Harmony purchase option agreement | 201 | 369 | ||||||
| Amortization of debt discount | 78 | 102 | ||||||
| Stock-based compensation | 2,561 | 3,826 | ||||||
| Loss on disposal of fixed assets | - | 15 | ||||||
| Issuance of common stock for services | - | 119 | ||||||
| Inducement charge from exercise of warrants | - | 2,551 | ||||||
| Net changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (171 | ) | (8 | ) | ||||
| Accounts payable and accrued expenses | (2,686 | ) | 157 | |||||
| Net cash used in operating activities | (10,711 | ) | (16,052 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from issuance of Series A preferred stock | 10,168 | - | ||||||
| Issuance costs from issuance of Series A preferred stock | (1,622 | ) | - | |||||
| Repurchase of warrants in connection with 2019 Warrant Tender Offer | - | (495 | ) | |||||
| Reissuance of warrants in connection with 2019 Warrant Tender Offer | - | 495 | ||||||
| Proceeds from 2019 Warrant Tender Offer | - | 20,546 | ||||||
| Issuance costs from 2019 Warrant Tender Offer | - | (1,428 | ) | |||||
| Proceeds from stock option exercises | - | 7 | ||||||
| Net cash provided by financing activities | 8,546 | 19,125 | ||||||
| Net (decrease) increase in cash and cash equivalents | (2,165 | ) | 3,073 | |||||
| Cash and cash equivalents at beginning of year | 6,659 | 3,586 | ||||||
| Cash and cash equivalents at end of year | $ | 4,494 | $ | 6,659 | ||||
| Non-cash transactions and supplemental cash flow information: | ||||||||
| Non-cash issuance costs to placement agent | $ | 1,011 | $ | 929 | ||||
| Accretion of Series A preferred stock to redemption value | $ | 221 | $ | - | ||||
| Recognition of warrant liability in connection with issuance of Series A preferred stock | $ | 664 | $ | - | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-40 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
1. Organization and Basis of Presentation
Aerami Therapeutics Holdings, Inc., (“Aerami Holdings”), incorporated on December 20, 2013 in the State of Delaware, together with its wholly owned subsidiaries, Aerami Therapeutics, Inc. (“Aerami Opco”) and Aerami Therapeutics Australia Pty, Ltd. (“Aerami Australia”) (and collectively with Aerami Holdings and Aerami Opco, the “Company”), is a clinical stage biopharmaceutical company dedicated to developing inhaled therapies for the treatment of severe respiratory and chronic diseases.
Aerami Opco, which was incorporated in the State of Delaware on February 9, 2009, has devoted all its efforts to developing its technology, raising capital, and hiring employees. Aerami Australia was incorporated in Australia on October 7, 2020 and has been engaged in conducting a Phase 1 clinical trial for the Company’s lead development program.
Going Concern
The Company has no products approved for sale and has incurred significant operating losses since inception. The Company has never been profitable and has incurred net losses of $10.3 million and $23.9 million for the years ended December 31, 2020 and 2019, respectively. As of December 31, 2020, the Company had an accumulated deficit of $125.3 million. Achieving profitability is dependent upon the successful development, approval, and commercialization of the Company’s product candidates and achieving a level of revenues adequate to support the Company’s cost structure. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to raise additional capital. Management intends to fund future operations through additional financing which could include private and/or public debt offerings and equity offerings. In addition, the Company may seek additional capital through arrangements with strategic partners or from other sources. There can be no assurances, however, that additional funding will be available on terms acceptable to the Company, or at all. These conditions, among other factors, raise substantial doubt about the Company’s ability to continue as a going concern.
The accompanying consolidated financial statements have been prepared assuming the Company will continue to operate as a going concern, which contemplates the realization of assets and the settlement of liabilities in the normal course of business. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty related to the Company’s ability to continue as a going concern.
COVID-19
On March 11, 2020, the World Health Organization declared a global pandemic related to COVID-19, which continues to spread throughout the United States and globally. The pandemic is creating disruption in the economy including supply chains, production, and sales across various industries. The impact on the Company’s operations and financial performance will depend on the duration and extent of the pandemic, which includes the impact on the Company’s vendors, and employees. Therefore, the extent to which the COVID-19 pandemic may impact the Company’s financial condition or results of operations is uncertain at this time.
Risks and Uncertainties
The Company is subject to risks and uncertainties common to early-stage companies in the biopharmaceutical industry, including, but not limited to, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with governmental regulations, dependency on key suppliers including for certain active pharmaceutical ingredients and the ability to secure additional capital to fund operations. Drug candidates currently under development will require extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts will require significant amounts of additional capital, adequate personnel, and infrastructure and extensive compliance and reporting capabilities. Even if the Company’s drug development efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales.
| F-41 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
2. Summary of Significant Accounting Policies
Basis of Presentation
The consolidated financial statements, which include Aerami Holdings and its wholly owned subsidiaries, are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). All significant intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates and changes in estimates may occur. The most significant matters involving management’s estimates include recoverability of long-lived assets, research and development accruals, valuation of the Harmony contingent settlement liability, and the valuations of common stock to calculate stock-based compensation and the inducement charge from exercise of warrants.
Cash and cash equivalents
Cash and cash equivalents consist principally of cash held in commercial bank accounts and money market funds. The Company considers all highly liquid investments with maturities of three months or less at the date of acquisition to be cash equivalents.
Concentration of Credit Risk
The Company’s financial instruments that are exposed to concentration of credit risk consist primarily of cash and cash equivalents. The Company maintains its cash in bank accounts, which generally exceed federally insured limits. The Company maintains its cash equivalents in investments in interest-bearing money market funds. The Company has never experienced any losses related to these balances.
Property, Equipment, and Right-of-Use Assets
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization are recognized using the straight-line method over the estimated useful lives the assets as follows:
| Asset category | Estimated useful life | |
| Lab equipment | 5 years | |
| Leasehold improvements | Lesser of lease term or 5 years | |
| Computer equipment | 3 years | |
| Office equipment | 5 years |
Estimated useful lives are periodically assessed to determine if changes are appropriate. Maintenance and repairs are charged to expense as incurred. When assets are retired or otherwise disposed of, the cost of these assets and related accumulated depreciation or amortization are eliminated from the consolidated balance sheet and any resulting gains or losses are included in the consolidated statement of operations in the period of disposal.
| F-42 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Long-Lived Assets
The Company regularly reviews the carrying values and estimated lives of its long-lived assets, including property and equipment, to determine whether indicators of impairment may exist which warrant adjustments to carrying values or estimated useful lives. The determinants used for this evaluation include management’s estimate of the asset’s ability to generate positive income from operations and positive cash flow in future periods as well as the strategic significance of the assets to the Company’s business objective. Should an impairment occur, the impairment loss would be measured based on the excess of the carrying amount over the asset’s fair value. No impairment charge was recorded during the years ended December 31, 2020 or 2019.
Leases
The Company determines if a contract is, or contains, a lease at contract inception. The Company’s right-of-use (“ROU”) assets are related to operating leases for lab and office space and are included in property, equipment, and right-of-use assets in the consolidated balance sheet as of December 31, 2019. The Company had no ROU assets as of December 31, 2020.
ROU assets represent the right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make lease payments arising from the lease. ROU assets and lease liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The incremental borrowing rate is determined by using the rate of interest that the Company would pay to borrow on a collateralized basis an amount equal to the lease payments for a similar term and in a similar economic environment. Lease terms include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option.
The Company excludes variable lease payments in measuring ROU assets and lease liabilities, other than those that depend on an index or a rate or are in-substance fixed payments. Lease and non-lease components are accounted for separately.
Accrued Research and Development Expenses
As part of the process of preparing its consolidated financial statements, the Company is required to estimate its accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf and estimating the level of service performed and the associated cost incurred for the service when the Company has not yet been invoiced. Most of the Company’s service providers invoice monthly in arrears for services performed or when contractual milestones are met. Estimates of accrued expenses as of each balance sheet date in the consolidated financial statements are based on facts and circumstances known at that time. The Company periodically confirms the accuracy of estimates with the service providers and adjusts if necessary. The significant estimates in accrued research and development expenses are related to expenses incurred with respect to contract research organizations (“CROs”), contract manufacturing organizations (“CMOs”) and other vendors in connection with research and development and manufacturing activities.
The Company bases its expenses related to CROs and CMOs on estimates of the services received and efforts expended pursuant to quotations and contracts with such vendors that conduct research and development and manufacturing activities on the Company’s behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to vendors will exceed the level of services provided and result in a prepayment of the applicable research and development or manufacturing expense. In accruing service fees, the Company estimates the time over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from estimates, the accrual or prepaid expense is adjusted accordingly. Although estimates are not expected to be materially different from amounts actually incurred, the Company’s understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and could result in amounts that are too high or too low in any particular period. There have been no material changes in estimates for the periods presented.
| F-43 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Preferred Stock
In order to comply with the Securities and Exchange Commission (“SEC”) reporting requirements, the Company has reissued its previously issued consolidated financial statements to classify the Series A preferred stock and the Series A-1 preferred stock as temporary equity in the accompanying consolidated balance sheets at December 31, 2020 and 2019 and the consolidated statements of convertible preferred stock and stockholders’ deficit for the years ended December 31, 2020 and 2019. The Series A preferred stock and Series A-1 preferred stock become redeemable due to certain deemed liquidation events, as defined in the Company’s certificate of incorporation, which are outside of the Company’s control. In addition, the put option attached to the shares of Series A preferred stock issued to a related party was accreted to its redemption value by $0.2 million for the year ended December 31, 2020, as management determined that the contingency relating to the Company’s completion of certain research and development activities as stated within the side letter agreement with the related party was not probable (see Note 7).
Warrants
Warrants that are considered freestanding equity-classified instruments due to their detachable and separately exercisable features and that met the indexation criteria in Accounting Standards Codification (“ASC”) 815-40-15-5 through 15-8 (indexation criteria) are presented as a component of the stockholders’ deficit in accordance with equity-classification guidance in ASC 815-40-25. Warrants that have features of or are indexed to shares that are redeemable or contingently redeemable and accounted for in temporary equity are presented as warrant liability in accordance with the provisions of ASC 480. These warrants are initially recognized at their fair value and remeasured at each reporting period, with changes in fair value recognized as other income/expense in the consolidated statements of operations. Contingently issuable warrants arising from a contract that is outstanding and in scope of the indexation criteria are considered issued for accounting purposes in accordance with ASC 815-40-15-6.
Equity Issuance Costs
Issuance costs are allocated between the individual freestanding instruments identified on the same basis as proceeds were allocated. Issuance costs associated with the issuance of stock or equity contracts (i.e., equity-classified warrants and Royalty stock (as defined below)) are recorded as a charge against the gross proceeds of the offering.
Revenue Recognition
The Company adopted Accounting Standard Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASC 606” or “New Revenue Recognition Standard”), which supersedes ASC Topic 605, Revenue Recognition (“ASC 605”), effective January 1, 2018 using the modified retrospective approach and applied this approach to contracts with customers that were not completed as of January 1, 2018. ASC 606, as amended, defines a five-step process to achieve this core principle: (1) identify the contract with the customer, (2) identify the performance obligations in the contract, (3) determine the transaction price, (4) allocate the transaction price to the performance obligations in the contract, and (5) recognize revenue when (or as) the entity satisfies a performance obligation. As part of the accounting for these arrangements, the Company must use significant judgment to determine the transaction price and timing of revenue recognition.
This adoption primarily affected the recognition of non-refundable up-front fees and milestone payments. The new revenue standard generally requires licenses that are not considered distinct performance obligations from other goods or services within a contract to be bundled with those goods or services as a combined performance obligation. Revenue associated with the combined performance obligation is recognized over time as the customer benefits from the license. See Note 6 for further information.
| F-44 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Research and Development Expenses
Research and development expenses primarily consist of costs associated with the preclinical and clinical development of the Company’s product candidates, including the following:
| ● | external research and development expenses incurred under arrangements with third parties, such as CROs and other vendors and CMOs to produce drug substance and drug product; and | |
| ● | employee-related expenses, including salaries, benefits, and stock-based compensation expenses. |
All research and development expenses are charged to operations as incurred in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 730, Research and Development. The Company accounts for non-refundable advance payments for goods and services that will be used in future research and development activities as expenses when the service has been performed or when the goods have been received, rather than when the payment is made.
Stock-Based Compensation Expense
The Company follows the provisions of ASC 718, Compensation—Stock Compensation, which requires the measurement and recognition of compensation expense for all stock-based payment awards made to employees and non-employee directors, including employee stock options. Stock-based compensation expense is based on the grant-date fair value estimated in accordance with the provisions of ASC 718 and is recognized as an expense over the requisite service period. For grants containing performance- based vesting provisions, the grant-date fair value of the milestone-based stock options is recognized as compensation expense once it is probable that the condition will be achieved. The Company accounts for actual forfeitures in the period the forfeitures occur.
The Company complies with ASU 2017-09, Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting, which clarifies when a change to terms or conditions of a share-based payment award must be accounted for as a modification. This guidance requires modification accounting if the vesting condition, fair value, or the award classification is not the same both before and after a change to the terms and conditions of the award.
The Company also complies with ASU 2018-07, Improvements to Nonemployee Share-Based Payment Accounting, (“ASU 2018-07”) which supersedes ASC 505-50 and expands the scope of ASC 718 to include all share-based payments arrangements related to the acquisition of goods and services from both employees and non-employees. The measurement date for non-employee awards is the date of grant. Compensation expense for non-employees is recognized, without changes to the fair value of the award, over the requisite service period, which is the vesting period of the respective award.
The Company uses the Black-Scholes option-pricing model to estimate the grant-date fair value of awards. The assumptions management made with respect to valuation model inputs for awards are summarized as follows:
Risk-free interest rate: The risk-free interest rate is based on U.S. Treasury, zero-coupon issues with a remaining term equal to the expected term assumed at the date of grant.
Expected dividend yield: The Company uses a dividend yield of zero since the Company has never paid cash dividends to stockholders and has no current intentions to pay cash dividends.
Expected volatility: Due to the lack of trading history, the Company’s computation of stock-price volatility is based on the volatility rates of comparable publicly held companies.
Expected term: The Company’s computation of expected term for awards to employees is determined using the “simplified” method, which is the midpoint between the vesting date and the end of the contractual term. The Company believes that it does not have sufficient reliable exercise data to justify the use of a method other than the “simplified” method of estimating the expected exercise term of employee stock option grants.
| F-45 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
The range of assumptions used in the Black-Scholes option-pricing model for awards is as follows:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Risk-free interest rate | 0.35 - 0.41 | % | 1.41 - 2.80 | % | ||||
| Expected dividend yield | 0 | % | 0 | % | ||||
| Expected volatility | 83.1 - 84.9 | % | 60.6 - 78.3 | % | ||||
| Expected term (in years) | 5 | 5-10 | ||||||
Income Taxes
The Company accounts for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to be recovered or settled. Realization of deferred tax assets is dependent upon future taxable income. A valuation allowance is recognized if it is more likely than not that some portion or all of a deferred tax asset will not be realized based on the weight of available evidence, including expected future earnings. The Company recognizes an uncertain tax position in its financial statements when it concludes that a tax position is more likely than not to be sustained upon examination based solely on its technical merits. Only after a tax position passes the first step of recognition will measurement be required. Under the measurement step, the tax benefit is measured as the largest amount of benefit that is more likely than not to be realized upon effective settlement. This is determined on a cumulative probability basis. The full impact of any change in recognition or measurement is reflected in the period in which such change occurs. The Company elected to record any interest or penalties related to income taxes as part of its income tax expense.
Fair Value of Financial Instruments
The Company determines the fair value of financial and nonfinancial assets and liabilities using the fair value hierarchy, which describes three levels of input that may be used to measure fair value, as follows:
| Level 1: | Quoted prices in active markets for identical assets or liabilities. | |
| Level 2: | Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. For marketable securities, the Company reviews trading activity and pricing as of the measurement date; and | |
| Level 3: | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The Company’s Level 1 assets consist of investments in money market funds, which are reflected in cash and cash equivalents in the accompanying consolidated balance sheets. The Company does not hold any assets or liabilities that are measured using Level 2 inputs. The Company’s Level 3 assets consist of the Harmony purchase option agreement and the Company’s level 3 liabilities consist of the Harmony contingent settlement liability and the warrant liability (See Note 6). Level 3 assets and liabilities are valued based on various estimates and inputs, including probability of success of product development, discount rates and amount of time until an exit scenario. Each reporting period, the Harmony option, the Harmony contingent settlement liability and the warrant liability are adjusted to fair value with the changes in fair value recognized in other income and expense. Changes in fair value reflect changes in information about the probability and timing of realization.
| F-46 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Net Loss Per Share
In order to comply with the SEC reporting requirements, the Company has reissued its previously issued consolidated financial statements to include net loss per share. Basic net loss per share of common stock is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during each period. Diluted net loss per share of common stock includes the effect, if any, from the potential exercise or conversion of securities, such as convertible preferred stock, stock options and warrants which would result in the issuance of incremental shares of common stock. For diluted net loss per share, the weighted-average number of shares of common stock is the same for basic net loss per share since, when a net loss exists, dilutive securities are not included in the calculation as the impact is anti-dilutive.
The following potentially dilutive securities have been excluded from the computation of diluted weighted-average shares of common stock outstanding, prior to the use of the two-class method, as described below, as they would be anti-dilutive (in thousands):
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Convertible preferred stock | 12,709 | 2,541 | ||||||
| Stock options | 10,259 | 9,146 | ||||||
| Unvested restricted stock units | 449 | 434 | ||||||
| Warrants | 1,957 | 5,778 | ||||||
| 25,374 | 17,899 | |||||||
The Company’s Series A Preferred Stock contractually entitles the holders of such shares to participate in dividends but does not contractually require the holders of such shares to participate in losses of the Company. Accordingly, in periods in which the Company reports a net loss, such losses were not allocated to participating securities. The Company reported a net loss per share of common stock for the years ended December 31, 2020 and 2019.
Segment Information
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker or decision-making group in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business as one operating and reporting segment.
Recently Adopted Accounting Pronouncements
In November 2018, the FASB issued ASU 2018-18, Collaborative Arrangements (Topic 808), which clarifies the interaction between the guidance for collaborative arrangements (Topic 808) and the new revenue recognition standard (Topic 606). For public companies, the amendments are effective for annual reporting periods beginning after December 15, 2019, including interim periods within those annual periods, with early adoption permitted. The Company adopted Topic 808 effective January 1, 2020, and it did not have a material impact on the consolidated financial statements.
| F-47 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820), which modifies the disclosure requirements on fair value measurements in Topic 820, Fair Value Measurement, including, among other changes, the consideration of costs and benefits when evaluating disclosure requirements. For public companies, the amendments are effective for annual reporting periods beginning after December 15, 2019, including interim periods within those annual periods, with early adoption permitted. The Company adopted Topic 820 effective January 1, 2020, and it did not have a material impact on the consolidated financial statements.
Recently Issued Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, “(Subtopic 470-20): Debt—Debt with Conversion and Other Options” (“ASU 2020-06”) to address the complexity associated with applying GAAP to certain financial instruments with characteristics of liabilities and equity. ASU 2020-06 includes amendments to the guidance on convertible instruments and the derivative scope exception for contracts in an entity’s own equity and simplifies the accounting for convertible instruments which include beneficial conversion features or cash conversion features by removing certain separation models in Subtopic 470-20.
Additionally, ASU 2020-06 will require entities to use the “if-converted” method when calculating diluted earnings per share for convertible instruments. ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. The Company is currently evaluating the impact of ASU 2020-06 on the consolidated financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. This guidance removes certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences. This guidance also clarifies and simplifies other areas of ASC 740. The guidance will be effective for fiscal years and interim periods beginning after December 15, 2021, and early adoption is permitted. The adoption of this guidance is not expected to have a material impact on the consolidated financial statements.
3. Fair Value of Financial Assets and Liabilities
The following table summarizes assets and liabilities recorded at fair value, and the classification by level of input within the fair value hierarchy (in thousands):
| December 31, 2020 | ||||||||||||||||
| Basis of Fair Value Measurements | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: | ||||||||||||||||
| Investment in money market funds | $ | 1,488 | $ | 1,488 | $ | - | $ | - | ||||||||
| Harmony purchase option agreement | $ | 6 | $ | - | $ | - | $ | 6 | ||||||||
| Liabilities: | ||||||||||||||||
| Harmony contingent settlement liability | $ | 2,978 | $ | - | $ | - | $ | 2,978 | ||||||||
| Warrant liability | $ | 664 | $ | - | $ | - | $ | 664 | ||||||||
| F-48 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
| December 31, 2019 | ||||||||||||||||
| Basis of Fair Value Measurements | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: | ||||||||||||||||
| Investment in money market funds | $ | 6,509 | $ | 6,509 | $ | - | $ | - | ||||||||
| Harmony purchase option agreement | $ | 207 | $ | - | $ | - | $ | 207 | ||||||||
| Liabilities: | ||||||||||||||||
| Harmony contingent settlement liability | $ | 3,363 | $ | - | $ | - | $ | 3,363 | ||||||||
The following table summarizes the changes in the fair value of assets and liabilities classified as Level 3 in the fair value hierarchy (in thousands):
Harmony Option | Harmony Contingent Settlement Liability | Warrant Liability | ||||||||||
| Balance at December 31, 2018 | $ | 576 | $ | 2,812 | $ | - | ||||||
| Change in fair value | (369 | ) | 551 | - | ||||||||
| Balance at December 31, 2019 | 207 | 3,363 | - | |||||||||
| Issuance | - | - | 664 | |||||||||
| Change in fair value | (201 | ) | (385 | ) | - | |||||||
| Balance at December 31, 2020 | $ | 6 | $ | 2,978 | $ | 664 | ||||||
4. Property, Equipment and Right-of-use Assets
Property, equipment, and right-of-use assets consist of the following (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Lab equipment | $ | 1,087 | $ | 1,787 | ||||
| Leasehold improvements | - | 11 | ||||||
| Computer and office equipment | - | 131 | ||||||
| Right-of-use assets | - | 112 | ||||||
| Total property, equipment, and right-of-use assets | 1,087 | 2,041 | ||||||
| Less accumulated depreciation and amortization | (1,087 | ) | (1,960 | ) | ||||
| Property, equipment, and right-of-use assets, net | $ | - | $ | 81 | ||||
Depreciation expense was $0.2 million for the year ended December 31, 2019. No depreciation expense was recorded during the year ended December 31, 2020.
ROU assets are related to operating leases for office space and lab space. Pursuant to the adoption of ASC 842, both leases were classified as operating leases. The discount rate used in the calculation of lease liabilities was 12%, which is the estimate of the rate of interest that the Company would have to pay to borrow on a collateralized basis over a similar term. Total rent expense for all leases was less than $0.1 million for the years ended December 31, 2020 and 2019, net of cash received from the Company’s sublessee of $0.1 million during 2019. Cash paid from operating cash flows for amounts included in the measurement of lease liabilities was $0.1 million per year during the years ended December 31, 2020 and 2019. At December 31, 2019, the Company’s operating leases had a weighted-average remaining lease term of 0.92 years. The Company had no long-term leases as of December 31, 2020.
| F-49 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Right-of-use assets and lease liabilities are recorded on the consolidated balance sheets as follows (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Assets: | ||||||||
| Property, equipment and right-of-use assets, net | $ | - | $ | 81 | ||||
| Liabilities: | ||||||||
| Accrued expenses and other current liabilities | $ | - | $ | 82 | ||||
5. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Accrued research and development expenses | $ | 99 | $ | 221 | ||||
| Accrued general and administrative expenses | - | 284 | ||||||
| Accrued employee-related expenses | 243 | 1,915 | ||||||
| Lease liabilities | - | 82 | ||||||
| Harmony Plus payable, current portion (Note 6) | 300 | 300 | ||||||
| Registration rights penalty (Note 10) | 2,674 | 2,640 | ||||||
| Accrued expenses and other current liabilities | $ | 3,316 | $ | 5,442 | ||||
6. Licenses and Collaborations
License and Development Agreements with Vectura Limited
In June 2020, the Company entered into an exclusive license agreement with Vectura Limited (“Vectura”) to acquire a worldwide, exclusive license to develop and commercialize Vectura’s proprietary FOX® device containing a formulation of the active ingredient imatinib (“AER-901”) for the treatment of pulmonary arterial hypertension (“PAH”) (the “Vectura License”). The Company also received the right of first negotiation to obtain rights for the use of AER-901 in additional indications concerning respiratory and pulmonary diseases. In addition, in May 2020, the Company entered into a development agreement with Vectura and made a payment of approximately $1.1 million to begin work to progress inhaled imatinib into a Phase 1 human clinical study as soon as possible after execution of the Vectura License. The Company capitalized the $1.1 million payment as a prepaid asset and recognized $0.9 million as research and development expense for the year ended December 31, 2020.
Under the Vectura License, the Company is obligated to use commercially reasonable efforts to develop and commercialize AER-901 and to pay milestone payments for the achievement of certain development, regulatory and sales milestones in amounts up to $161 million in the aggregate. The Company is also obligated to pay a mid- single-digit royalty on sales of AER-901. The Company must obtain supplies of Vectura’s FOX® device from Vectura at a transfer price equal to Vectura’s costs of manufacture plus a specified percentage. The Vectura License may be terminated by either party for a material breach if such breach is not cured within two months’ notice of such breach or insolvency of either party. Vectura will also have rights to terminate following certain triggering events.
| F-50 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Amended and Restated Joint Development Agreement with Phillips-Medisize, LLC (“Phillips”)
In June 2020, the Company entered into an amended and restated joint development agreement (the “Amended Phillips JDA”) with Phillips. Under the Amended Phillips JDA, the Company agreed to work exclusively with Phillips and its affiliates for the term of the agreement, which shall initially be for a minimum of five (5) years, for the development and manufacturing of the Company’s AFINA™ device and the development of integrated connected health technologies for the AFINA™ device, as well as any other device the Company may acquire and any device that it may in-license that would require material development or refinement to be usable or commercially feasible for use with the Company’s formulations, other than the devices in-licensed from the licensor under the Vectura license agreement. The Company and Phillips will negotiate at a later date on commercially reasonable terms for manufacturing and supply of any products under the Amended Phillips JDA. The Amended Phillips JDA otherwise amends and restates the Company’s then existing Phillips JDA on substantially the same terms.
License Agreement with Aerogen Limited/Philips Electronics UK
In November 2010, the Company entered into a license agreement with Aerogen Limited (“Aerogen”), pursuant to which Aerogen granted the Company an exclusive worldwide license, with sublicensing rights, to develop and commercialize insulin delivery devices containing Aerogen’s vibrating mesh aerosolization technology. Under the agreement, Aerogen is obligated to use commercially reasonable efforts to collaborate with the Company to exclusively develop and manufacture related devices, and the Company is responsible for establishing product requirements for the device. Further, the Company is responsible for obtaining and maintaining all regulatory approvals for the drug/device combination globally, as well as for all commercialization activities relating to the drug/device combination, whereas Aerogen is obligated to provide regulatory assistance on documents pertaining to the device. A joint development committee was formed to facilitate and oversee the development of the device. In addition, the license agreement provides that technology jointly developed by Aerogen and the Company relating to the device is owned by Aerogen with an exclusive license to the Company in the field of insulin.
In December 2014, the Company entered into a novation agreement with Aerogen and Philips Electronics UK (“PEUK”), in which Aerogen granted its rights under the above license agreement to PEUK (subject to Aerogen retaining certain of the rights and responsibilities with respect to the licensed intellectual property) and PEUK assumed the obligation to perform under the license agreement and be bound by the terms of the license agreement in every way as if a party in place of Aerogen. Additionally, in connection with the novation agreement, the Company has undertaken to perform under the agreement for the benefit of PEUK. Aerami and Aerogen mutually released and discharged each other from their novated liabilities under the agreement.
The Company is obligated to fund all device development activities and upon commercialization is obligated to pay Philips tiered low single-digit royalties on net sales of the product, subject to an annual minimum payment commencing as of the second year of commercial sale, on a country-by-country basis for the longer of the life of certain patents covering the product or 10 years after the first commercial sale of the product in the respective country.
In August 2019, the Company entered into an addendum with PEUK to the license agreement to extend the scope of the license agreement to include the development and commercialization of glucagon-like peptide 1 (“GLP-1”) solutions formulated for use with the delivery devices containing the vibrating mesh aerosolization technology. The Company paid $0.2 million to PEUK upon execution of the addendum. The Company is obligated to use commercially reasonable efforts to develop and commercialize insulin and GLP-1 solution delivery devices containing the vibrating mesh technology and to pay milestone payments for the achievement of certain development and regulatory milestones in amounts up to $2.2 million in the aggregate. In the event of termination of the GLP-1 license by the Company not related to clinical or technical failure, the Company is obligated to pay PEUK a termination payment of $0.5 million if such termination arises prior to completion of Phase IIb clinical trials or $1.3 million if termination occurs after completion of Phase IIb clinical trials.
| F-51 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
PEUK will supply the device for clinical and commercial purposes based on stated pricing per the agreement under which the Company will pay PEUK a price equal to PEUK’s cost of the device plus a stated margin, which is subject to future negotiation. The license agreement will remain in force on a country-by-country basis until the later of (i) the expiration of the Aerogen patents covering the device on August 8, 2034 or (ii) 10 years after the first commercial sale of the product. In the case of material breach, the license may be prematurely terminated by either party by providing 90 days written notice to the breaching party and such breaching party has not cured the breach within the 90-day period.
License Agreement with Hikma Pharmaceuticals LLC (“Hikma”)
In January 2013, the Company entered into a license, supply, and manufacturing agreement with Hikma, under which the Company granted an exclusive license to register and commercialize its inhaled human insulin and device combination (“AER-501”) in all disease indications in the Middle East and Africa, excluding Turkey and certain other countries (collectively referred to as the “Hikma Territory”). Under the agreement, the Company is responsible for the global clinical development of AER-501 in any and all indications and for assembly of the registration materials submitted to the European Medicines Agency (the “EMA”) or the U.S. Food and Drug Administration (“FDA”) for approval. Hikma is responsible for registration and commercialization in the Hikma Territory. Additionally, the Company granted Hikma the right of first refusal to license new inhaled insulin products in the Hikma Territory as well as the Palestinian Territories and Turkey should such regions become available for inclusion in the agreement.
Under the terms of the agreement, the Company received a non-refundable upfront license fee of $0.5 million in January 2013 and a non-refundable milestone of $0.3 million in January 2014 upon achievement of a clinical milestone. The Company is also entitled to receive up to $2.0 million in potential future milestone payments if certain commercial and sales-based events are successfully achieved. The terms of the agreement also provide for Hikma to pay the Company a percent of net sales of the licensed product in the Hikma Territory.
The agreement has a 15-year term from the date of the launch of AER-501 in one of the countries in the Hikma Territory and will automatically renew for an additional 12-month term prior to the expiration of the then-current term. The agreement may be terminated by either party by providing at least twelve months’ written notice prior to the expiration of the then-current term or for a material breach if such breach is not cured within two-months’ notice of such breach, insolvency of either party, or a change of control.
A timeline for development of AER-501 has been accepted by Hikma. Pursuant to the terms of the agreement with Hikma, if (i) AER-501 fails to gain approval by the FDA or EMA or (ii) AER-501 does not obtain at least one of the foregoing approvals within two years after the applicable timeframe in the agreement, Hikma will receive an additional exclusive license to one other Company product in development, of Hikma’s choosing, the terms of which will be identical to the current license excluding the upfront license fee. In addition, Hikma would have the right to convert an amount equal to the upfront license fee to shares of the Company’s equity, on terms equal to the Company’s most recent or current private or public financing. We did not meet the agreed-upon timeframe for regulatory approval of AER-501, and, as such, Hikma is entitled to receive the additional exclusive license in the Hikma Territory to another one of our products in development and the right to convert an amount equal to the original upfront license fee under that agreement into shares of Aerami’s capital stock. As of December 31, 2020, Hikma has not exercised these rights, but we cannot assure you that Hikma will not exercise these rights at a future time.
The Company evaluated the agreement under ASC 606 and determined that there were four promises: (i) an exclusive license to register and commercialize in the Hikma Territory; (ii) performance of global clinical development of AER-501 in any and all indications; (iii) assembly of the registration materials submitted to EMA or the FDA; and (iv) manufacture and supply of certain amounts of AER-501. The Company determined none of the promises are distinct since each is highly interrelated and dependent on the other and the license is largely symbolic and has no stand-alone functionality or value. Therefore, all promises are combined as a single performance obligation.
| F-52 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
The Company determined that the transaction price consisted of the upfront payment of $0.5 million and the $0.3 million clinical milestone. Future potential milestones payments under the agreement were excluded from the transaction price under the sales-based royalty exception to the commercial and sales- based milestone payments. Since Hikma does not benefit from the license until commercial launch in the Hikma Territory, revenue from the initial non-refundable payment and clinical milestone will be recognized over the period beginning upon commercial launch in the Hikma Territory through the end of the license term. The timing of such a commercial launch is uncertain. The commercial and sales-based milestone payments will be recognized as the related sales occur.
Contract liabilities of $0.8 million related to consideration received for which the performance obligation has not yet been completed are included within customer advances and other liabilities.
Agreements with Harmony Biopharm Limited and Harmony Plus Holdings Limited
In September 2015, the Company entered into an exclusive license agreement with Harmony Biopharm Limited (“Harmony Licensee”), under which the Company granted Harmony Licensee the right to develop and commercialize AER-501 in China, India, and various other countries in Asia.
On August 24, 2017 the Company and Harmony Licensee’s parent, Harmony Plus Holdings Limited (“Harmony Plus”), entered into a Stock Purchase Agreement and Release (the “SPAR”) under which the Company reacquired the license previously granted to Harmony Licensee by acquiring 100% of the stock in Harmony Licensee for the following consideration: (i) a number of shares of the Company’s common stock equal to $8.0 million issued upon the earlier of an initial public offering (“IPO”) and/or a change of control, as defined in the SPAR; (ii) forgiveness of repayment of the $0.3 million that was owed to the Company under a previous agreement with Harmony Plus; and (iii) royalties payable equal to 7.5% of gross profits from the sale of products in China for the period ending on the earlier of (a) the tenth anniversary of the SPAR and (b) a Royalty Termination Event.
A Royalty Termination Event would occur if the Company or any other person at any time prior to the fifth anniversary of the Company’s IPO purchases at least the lesser of: a) one-half of the 2,299,844 shares, or 1,149,922 shares, owned by certain investors that are affiliated with Harmony Plus (the “Harmony Investors”); or b) 2,299,844 shares less any shares sold/transferred by the Harmony Investors after August 24, 2017 at a price per share of at least the greater of (1) $5.00 per share or (2) 90% of the fair value of a share (as defined in the SPAR), if such purchase occurs before an IPO or change of control (as defined in the SPAR); or the greater of (1) $10.00 per share or (2) 90% of the fair value of a share (as defined in the SPAR), if such purchase occurs after an IPO.
As part of the SPAR, the Company and Harmony Plus also entered into a consulting agreement under which Harmony Plus is to provide consulting services for a five-year term commencing in August 2017. Total compensation for the services is $1.5 million payable in annual installments of $0.3 million each through August 2022. Upon termination of the consulting agreement, the Company would be obligated to pay the remaining amounts due irrespective of whether services are provided and irrespective of which party terminates the consulting agreement. The Company therefore concluded that the amounts owed under the consulting agreement represented additional consideration under the SPAR.
On August 24, 2017, the Company also entered into a Market Development Arrangement with Harmony Plus under which the Company is obligated to pay Harmony Plus a fee equal to 5% of all revenue received from certain qualified partners in China, India, and various other countries in Asia introduced to Aerami by Harmony Plus.
The Company determined that the reacquisition of rights previously granted to Harmony Licensee did not meet the definition of a business under ASC 805 Business Combinations. The transaction was determined to be a cancellation or rescission and not a business combination, and, as such, the accounting is in accordance with the requirements of ASC 952 Franchisors, with all consideration being recognized in operating expenses.
| F-53 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
The components of consideration associated with the above settlement and the accounting treatment are as follows:
| 1) | Common stock equal to $8.0 million to be issued upon the earlier of an IPO and/or Change of Control represents a conditional obligation that the Company must settle by issuing a variable number of its equity shares equal to a fixed monetary amount known at inception and therefore must be recognized as a liability under ASC 480-10-25-14(a). ASC 480-30-7 requires obligations that can be settled in shares with a fixed monetary value at settlement to be carried at fair value. This liability had a fair value of $3.0 million and $3.4 million at December 31, 2020 and 2019, respectively, and is recorded as the Harmony contingent settlement liability on the consolidated balance sheets. | |
| 2) | Forgiveness of repayment of $0.3 million – this receivable was written off during a prior year and there was no impact on the consolidated financial statements at the time of settlement. | |
| 3) | Consulting agreement – as of December 31, 2020 and 2019, the face value of amounts payable to Harmony Plus was $0.6 million and $0.9 million, respectively. As of December 31, 2020 and 2019, the present value of amounts payable to Harmony Plus was $0.5 million, net of a $0.1 million unamortized discount, and $0.7 million, net of a $0.2 million unamortized discount, respectively. The discount is based on an imputed interest rate of 12% and is being amortized as interest expense over the term of the consulting agreement. $0.1 million of debt discount per year was amortized as interest expense during the years ended December 31, 2020 and 2019. |
The Harmony Plus payable is recorded on the consolidated balance sheets as follows (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Accrued expenses and other current liabilities | $ | 300 | $ | 300 | ||||
| Customer advances and other liabilities | 227 | 449 | ||||||
| Harmony Plus payable, total | $ | 527 | $ | 749 | ||||
| 4) | 7.5% royalties and 5% marketing fee – since the potential royalties and fees are not exchange- traded and the underlying is a specified volume of sales in various countries, they are eligible for the scope exception in ASC 815 and will be recorded when payments become due or payable. | |
| 5) | Concurrent with the execution of the SPAR, the Company entered into separate agreements with the individual Harmony Investors under which the Company could acquire the shares held by the Harmony Investors under terms consistent with the SPAR. This right represents an asset to the Company as it can reacquire the shares at a discount and terminate future royalty payment obligations. The right had an underlying fair value of $0.2 million as of December 31, 2019 and is included in other non-current assets on the consolidated balance sheets. The underlying fair value of the right was de minimis as of December 31, 2020. |
Joint Venture Agreement with Dongbao and Shanghai Dongbao
In October 2017, the Company entered into a joint venture agreement (the “Dongbao Joint Venture Agreement”) with Dongbao and Shanghai Dongbao Biological Pharmaceutical Co., Ltd. (“Shanghai Dongbao”) pursuant to which a joint venture (the “China JV”) would be formed with the purpose of (i) completing clinical trials in China for AER-501, (ii) gaining China Food and Drug Administration approval for AER-501, and (iii) commercializing AER-501 in China. The Company was to provide a capital contribution of RMB 46,170,000 for a 45% ownership interest in the China JV. All parties agreed that they would not carry out business competing with the China JV’s business or invest into or act on behalf of a third party directly or indirectly competing with the China JV’s business. However, as of the date of these consolidated financial statements, the China JV has not been formed or funded and has never commenced operations.
| F-54 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
7. Convertible Preferred Stock and Stockholders’ Deficit
During the year ended December 31, 2019, the Company issued 35,876 shares of the Company’s common stock as payment for services valued at $0.1 million.
2020 Series A Preferred Stock Financing and Warrants
From June 2020 through December 2020, the Company sold 10,168 shares of its Series A preferred stock and received net proceeds of $7.9 million (the “2020 Series A Preferred Stock Financing”).
In connection with the financing, the Company is also obligated to issue placement agent warrants to purchase 964 shares of Series A preferred stock to its placement agent, Aegis Capital Corp. (the “Placement Agent” or “Aegis”), a related party. The placement agent warrants will have an exercise price of $1,000 per share and will be exercisable for five years from the date of issuance. The warrants are liability-classified as the underlying Series A preferred stock is contingently redeemable upon certain liquidation events which are deemed to be outside the Company’s control. The warrants will be remeasured at each reporting period until exercised, reclassified, expired, or otherwise settled. Changes in fair value of the warrant liability will be recorded within the consolidated statements of operations. The Company allocated $0.7 million of the net proceeds to the warrant liability based on the estimated fair value of the warrants. A warrant liability was recorded in the balance sheet upon the deemed issuance. The change in fair value of the warrants was de minimis during the year ended December 31, 2020.
In connection with the Company’s initial offering of its Series A preferred stock, a future follow-on offering right (the “Future Follow-on Right”) was included providing the holders of Series A preferred stock a right of first offer to purchase securities sold in a follow-on offering, in such amount up to one hundred percent of the original amount of each such Series A preferred stock in the initial offering.
Concurrent with the Company’s initial offering of its Series A preferred stock, the Company entered into a side letter agreement (the “Put Option”) whereby a related party investor may, at its option, elect to put the Series A preferred stock back to the Company for $1.0 million in the event the Company does not complete specific research and development activities that lead to a 510(k) regulatory submission to the FDA by June 30, 2021.
The Company determined that the Future Follow-on Right and the Put Option did not meet the definition of a freestanding financial instrument as they are not legally detachable. The Future Follow-on Right and Put Option were also evaluated as an embedded derivative and the Company determined they did not meet the definition of a derivative instrument for which bifurcation would be required.
2020 Warrants
At December 31, 2020, there are 964 warrants outstanding to acquire shares of the Company’s Series A preferred stock. The warrants were deemed issued in connection with the 2020 sales of the Company’s Series A preferred stock and entitle the holders to acquire shares of the Company’s Series A preferred stock. These warrants are liability-classified as the underlying Series A preferred stock is contingently redeemable and outside the Company’s control. The warrants had a grant-date fair value of $0.7 million, and a warrant liability was recorded in the balance sheet upon issuance.
The range of assumptions used in the Black-Scholes option-pricing model to fair value the warrants at issuance are as follows:
| Expected volatility rate | 90.0 | % | ||
| Risk-free interest rate | 0.28 – 0.45 | % | ||
| Expected term (in years) | 5.0 | |||
| Exercise price (actual amount per share) | $ | 1,000 | ||
| Fair value of Series A preferred stock (actual amount per share) | $ | 1,000 |
| F-55 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
2019 Warrant Tender Offer
From January 2019 through March 2019, the Company raised $20.5 million in gross proceeds ($19.1 million in net proceeds) by issuing 12,327,684 shares of common stock upon the exercise of 4,109,228 warrants as part of a 3-for-1 warrant tender offer (the “2019 Warrant Tender Offer”). During the 2019 Warrant Tender Offer, the Company also facilitated the transfer of 495,201 warrants from existing warrant holders to other investors who immediately exercised them per the terms of the 2019 Warrant Tender Offer for a transfer price of $1 per share. The Company considered this transaction to be a repurchase and immediate resale of the warrants and accounted for it in accordance with ASC 718-20-35-7, concluding that the amount paid was equal to the fair value of the warrants at the repurchase date.
The Company considered the 2019 Warrant Tender Offer to be analogous to an equity award modification under the guidance of ASC 718-20-35-3. Therefore, the Company recognized an inducement charge equal to the difference between the fair value of the warrants before and after modification of $2.6 million.
The Company retained Aegis to act as its warrant agent for the 2019 Warrant Tender Offer. Aegis received cash fees and reimbursement of expenses totaling $1.4 million in connection with the 2019 Warrant Tender Offer. In addition, as additional compensation, the Company offered Aegis the ability to settle previously outstanding Placement Agent Warrants through a cashless inducement offer whereby the Warrant Agent would receive 0.67 shares for each 1 Placement Agent Warrant exercised under the 2019 Warrant Tender Offer. An additional 1,314,847 shares of common stock were issued upon the cashless exercise of 1,971,219 Placement Agent Warrants during the warrant tender. The Company recorded an amount equal to the fair value of the incremental common stock issued because of the 2019 Warrant Tender Offer of $0.9 million through equity as non-cash issuance costs.
Common Stock
Liquidation
Upon liquidation or dissolution, holders of the Company’s common stock are entitled to share ratably in all net assets available for distribution to stockholders after the Company has paid, or provided for payment of, all of its debts and liabilities, and after payment of any liquidation preferences to holders of the Royalty Series convertible preferred stock (“Royalty stock”).
Voting
Holders of the Company’s common stock are entitled to one vote per share in the election of directors and on all other matters on which stockholders are entitled or permitted to vote. Holders of common stock are not entitled to cumulative voting rights.
| F-56 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Shares of common stock reserved for future issuance are shown below (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Common stock warrants | 993 | 5,778 | ||||||
| Series A preferred stock warrants | 964 | - | ||||||
| Series A preferred stock | 10,168 | - | ||||||
| Royalty stock | 2,541 | 2,541 | ||||||
| Stock options issued and outstanding | 10,259 | 9,146 | ||||||
| Unvested restricted stock units | 449 | 434 | ||||||
| Future equity awards | 2,612 | 2,131 | ||||||
| Total | 27,986 | 20,030 | ||||||
Series A Preferred Stock
Conversion
Each share of Series A preferred stock may be converted at the option of the holder thereof into shares of common stock at any time and from time to time at the then-applicable conversion rate described below. Upon either (i) an IPO of the Company’s common stock in which the Company receives at least $10 million in gross proceeds (or a lesser amount if holders of a majority of the Series A preferred stock (voting on an as-converted basis) approve such lesser amount) and shares of the Company’s common stock are listed on the Nasdaq Stock Market (“Nasdaq”), the New York Stock Exchange (the “NYSE”) or other exchange approved by the Board or (ii) a public or private capital raising transaction in which the Company receives gross proceeds of at least $10 million (or a lesser amount if holders of a majority of the shares of Series A preferred stock approve such lesser amount) that is concurrent with a merger of the Company with a public company with the resulting shares being listed on Nasdaq, the NYSE or other exchange or marketplace approved by the Board (each, a “QIPO”), each share of Series A preferred stock will automatically be converted into shares of common stock at the then-applicable conversion rate described below, following which the holder will cease to be entitled to any liquidation preferences.
Conversion Rate
If a holder of Series A preferred stock voluntarily converts its shares of Series A preferred stock into common stock prior to a QIPO, the number of shares of common stock into which each share of Series A preferred stock will be converted is determined by dividing the Stated Value of $1,000 plus declared and unpaid dividends thereon by $1.00 (as adjusted for stock splits, stock dividends, combinations, and similar events with respect to the common stock and certain fundamental events). If the shares of Series A preferred stock are automatically converted into shares of common stock in connection with a QIPO, the number of shares of common stock into which each share of Series A preferred stock will be converted is determined by dividing the Stated Value of $1,000 plus declared and unpaid dividends thereon by the lesser of $1.00 (as adjusted for stock splits, stock dividends, combinations and similar events with respect to the common stock and certain fundamental events) or 75% of either the per share closing sale price of the common stock to the public or the per share price of the shares sold in the capital raising transaction that is concurrent with the merger of the Company with a public company, as applicable. The conversion rate for the Series A-1 preferred stock that is issued to holders of Series A preferred stock that invest in a Follow-On Offering as described below will be 50% of the applicable conversion rate of the Series A preferred stock.
| F-57 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
If a holder of Series A preferred stock participates in the Company’s next follow-on financing in which the Company raises gross proceeds of at least $10 million in a private placement (subject to customary exclusions) or $10 million (or such lesser amount as is approved by holders of a majority of the shares of Series A preferred stock) in the QIPO (the “Follow-On Offering”), some or all (depending on the amount the holder invests in the Follow-On Offering) of the holder’s shares of Series A preferred stock will automatically convert into Series A-1 preferred stock at a 1:1 ratio. If a holder of Series A preferred stock invests the same amount or more in the Follow-On Offering that the holder invested in, all of such holder’s shares of Series A preferred stock will automatically convert into shares of Series A-1 preferred stock upon the closing of the Follow-On Offering (or immediately prior to the QIPO if the Follow-On Offering is the QIPO). If a holder of Series A preferred stock invests in the Follow-On Offering in an amount less than such holder’s investment amount in this offering, a portion of the holder’s Series A preferred stock will convert into Series A-1 preferred stock determined by multiplying the number of shares of Series A preferred stock held by the holder prior to the Follow-On Offering by a fraction equal to the amount the holder invested in the Follow-On Offering divided by the amount the holder invested in the 2020 Series A Preferred Stock Financing.
Liquidation
In the event of liquidation of the Company or a merger or consolidation of the Company or the sale of all or substantially all of its assets on a consolidated basis, the holders of Series A preferred stock will be entitled to receive in priority from the holders of common stock and Royalty stock an amount equal to $1,000 per share of Series A preferred stock plus declared and unpaid dividends plus amounts that holders of Series A preferred stock would have been entitled to receive had all preferences of the Series A preferred stock, Royalty stock and any other series of preferred stock designated from time to time been paid in full and all holders of Series A preferred stock, Royalty stock, common stock and other designated series of preferred stock participated in the distribution of residual proceeds on a pro rata as-converted basis (the “Preferential Amount”). After the payment of the Preferential Amount to the holders of Series A preferred stock, the Series A preferred stock will not participate in the distribution of any remaining amounts.
Voting
Holders of Series A preferred stock will vote together with the common stock and Royalty stock on an as-converted basis, and not as a separate class, except as described below.
Holders of Series A preferred stock will vote together as a single class on an as-converted basis with respect to:
| (i) | amending, altering, or repealing any provision of the Series A preferred stock Certificate of Designations in a manner that adversely affects the powers, preferences, or rights of the Series A preferred stock. | |
| (ii) | the liquidation, dissolution or winding up of the Company, or effecting any merger or consolidation of the Company. | |
| (iii) | creating any additional class or series of capital stock that is senior to the Series A preferred stock with respect to liquidation preferences, dividends or redemption or increase the authorized shares of Series A preferred stock or of any additional class of capital stock of the Company unless they rank junior or on parity with the Series A preferred stock. | |
| (iv) | reclassifying any capital stock that is on parity with the Series A preferred stock with respect to liquidation preferences, dividends or redemption into a senior class or series of securities or reclassifying any capital stock that is junior to the Series A preferred stock with respect to liquidation preferences, dividends or redemption into a parity or senior class or series of securities. | |
| (v) | making or paying redemptions, dividends, or other distributions on any shares of capital stock other than those authorized under the Series A preferred stock Certificate of Designations or the Certificate of Designations of the Royalty stock or on any other series of preferred stock designated from time to time that ranks on parity with or senior to the Series A preferred stock, repurchases of stock issued as equity compensation or as approved by the Board of Directors of the Company; and | |
| (vi) | issuing Series A-1 preferred stock other than upon conversion of the Series A preferred stock as described above. |
| F-58 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Royalty Series Convertible Preferred Stock
The holders of Royalty stock are entitled, in the aggregate, for each calendar year, a royalty payment in an amount equal to the lesser of: (1) $50 million or (2) (i) 3% of net sales plus (ii) 5% of licensing proceeds commencing on the date following the last day of the fiscal quarter as of which the Company’s net sales and licensing proceeds for four consecutive quarters is equal to at least $50 million and ending on the tenth anniversary of such date or earlier in the case of an acquisition (“Royalty Period”).
The rights to royalty payments will terminate upon the earliest of (i) the occurrence of an acquisition, so long as the holders of Royalty stock have received, in the aggregate, an amount equal to at least $50.00 per share of Royalty stock held by them, (ii) with respect to a specific holder, the conversion by such holder of Royalty stock into shares of the Company’s common stock and (iii) the expiration of the Royalty Period. The rights to royalty payments are unsecured obligations of the Company.
Conversion
Royalty stock is convertible into the Company’s common stock at the option of the holder at any time after the one-year anniversary of the date on which the Company becomes a reporting company under the Securities Exchange Act of 1934. If at any time while the Royalty stock is outstanding, the Company effects any merger with or into another entity, the Company effects any sale of all or substantially all of its assets in one transaction or a series of related transactions, or the Company effects any reclassification of the common stock pursuant to which the common stock is effectively converted into or exchanged for other securities, cash or property, (collectively, “Fundamental Transaction”), then, upon any subsequent conversion of the Royalty stock, the holders have the right to receive, for each share of Royalty stock that would have been issuable upon such conversion immediately prior to the occurrence of such Fundamental Transaction, the same kind and amount of securities, cash or property as it would have been entitled to receive upon the occurrence of such Fundamental Transaction if it had been, immediately prior to such Fundamental Transaction, the holder of one share of common stock.
Liquidation
In the event of liquidation of the Company, royalty payments are due and to be paid prior to any other payments to holders of Royalty stock or common stock. Any remaining amounts would be allocated to the holders of the Royalty stock and common stock on a pro-rata basis.
Voting
The holders of Royalty stock vote together with the common stock on an as-converted basis, and not as a separate class, except those holders of Royalty stock vote as a separate class with respect to amending, altering, or repealing any provision of the Certificate of Designations in a manner that adversely affects the powers, preferences, or rights of the Royalty stock.
As the holders of the of Royalty stock do not control the board of directors or the stockholders’ vote and, therefore, do not have the ability to control a liquidation of the Company or a Fundamental Transaction that could result in a redemption of the Royalty stock, the Royalty stock is classified as equity.
| F-59 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Warrants
The following table summarizes common stock warrant activity during the years ended December 31, 2020 and 2019:
| Underlying Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||||
Outstanding at December 31, 2018 | 11,858,203 | $ | 3.38 | 2.40 years | $ | - | ||||||||
| Issued | - | |||||||||||||
| Exercised | (6,080,447 | ) | $ | 5.00 | ||||||||||
| Forfeited or Expired | - | |||||||||||||
| Outstanding at December 31, 2019 | 5,777,756 | $ | 4.99 | 1.14 years | $ | - | ||||||||
| Issued | - | |||||||||||||
| Exercised | - | |||||||||||||
| Forfeited or Expired | (4,784,764 | ) | $ | 5.00 | ||||||||||
| Outstanding at December 31, 2020 | 992,992 | $ | 4.95 | 1.99 years | $ | - | ||||||||
8. Stock-Based Awards
Total stock-based compensation expense recognized was as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Research and development | $ | 549 | $ | 1,303 | ||||
| General and administrative | 2,012 | 2,523 | ||||||
| Total stock-based compensation expense | $ | 2,561 | $ | 3,826 | ||||
At December 31, 2020, the Company had approximately $1.2 million of unrecognized compensation cost related to unvested awards with milestone-based vesting criteria. This expense will be recognized when achievement of the milestone becomes probable. In addition, as of December 31, 2020, there was $1.7 million of unrecognized compensation cost related to unvested awards with time-based vesting which will be recognized over a period of 2.5 years.
The 2009 Plan
In 2009, the Company adopted the 2009 Equity Incentive Plan (the “2009 Plan”) under which ten million shares of common stock were reserved for issuance to employees, non-employee directors and consultants of the Company. Awards granted under the 2009 Plan are either non-statutory stock options or restricted stock units. The 2009 Plan expired in September 2015, and no further awards will be made under it.
| F-60 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
2009 Plan - Stock Options
A summary of stock option activity for the 2009 Plan for the years ended December 31, 2020 and 2019 is presented below:
| Underlying Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value (in thousands) | |||||||||||
| Outstanding at December 31, 2018 | 962,199 | $ | 0.91 | 1.83 years | $ | 1,802 | ||||||||
| Exercised | (60,041 | ) | $ | 0.11 | ||||||||||
| Forfeited or Expired | (296,707 | ) | $ | 0.08 | ||||||||||
| Outstanding at December 31, 2019 | 605,451 | $ | 1.39 | 1.67 years | $ | 285 | ||||||||
| Exercised | (6,116 | ) | $ | 1.84 | ||||||||||
| Forfeited or Expired | (411,788 | ) | $ | 1.24 | ||||||||||
| Outstanding at December 31, 2020 | 187,547 | $ | 1.77 | 0.91 years | $ | - | ||||||||
| Exercisable at December 31, 2020 | 187,547 | $ | 1.77 | 0.91 years | $ | - | ||||||||
| Vested and expected to vest at December 31, 2020 | 187,547 | $ | 1.77 | 0.91 years | $ | - | ||||||||
2009 Plan – Restricted Stock Units (“RSUs”)
RSUs convert into shares of common stock upon vesting on a one-for-one basis. RSU vesting only occurs upon an initial public offering or a change in control. No stock-based compensation expense has been recorded in relation to these RSUs as the events were not deemed probable at December 31, 2020 or 2019.
A summary of RSU activity for the 2009 Plan is presented below:
| Weighted Average | ||||||||
| Restricted | Grant-Date | |||||||
| Stock Units | Fair Value | |||||||
| Outstanding at December 31, 2018 | 251,436 | $ | 3.39 | |||||
| Granted | - | |||||||
| Forfeited or Expired | (7,535 | ) | $ | 3.15 | ||||
| Outstanding at December 31, 2019 | 243,901 | $ | 3.39 | |||||
| Granted | - | |||||||
| Forfeited or Expired | (55,253 | ) | $ | 3.39 | ||||
| Outstanding at December 31, 2020 | 188,648 | $ | 3.39 | |||||
The 2015 Plan
The Company adopted 2015 Equity Incentive Plan (the “2015 Plan”) under which 4,347,559 shares of common stock were reserved for issuance to eligible employees, officers, non-employee directors and other individual service providers of the Company. Under the 2015 Plan, the number of shares available for issuance automatically increases annually for a period of 10 years by an amount equal to 6% of the total number of shares of common stock outstanding on December 31 of the previous year. In connection with this provision, the number of shares available for issuance under the 2015 Plan increased by 2,081,621 shares effective January 1, 2020. As of January 1, 2021, there were approximately 2,611,550 shares available for future grants.
| F-61 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
The 2015 Plan permits the grant of nonqualified stock options, incentive stock options, stock appreciation rights, restricted stock, stock units, performance shares, performance units, incentive bonus awards, other cash-based awards, and other stock-based awards to eligible recipients. Recipients of stock options are eligible to purchase shares of the Company’s common stock at an exercise price equal to no less than the estimated fair market value of such stock on the date of grant. The maximum term of options granted under the 2015 Plan is ten years.
The Company grants options with a service-based vesting requirement and granted RSUs with the same vesting requirements discussed under the 2009 Plan. Options granted under the plan have a variety of vesting schedules, however, they generally vest 25% on the grant date, 25% on the one-year anniversary of the grant date, and the remaining 50% vest in equal monthly installments on the next twenty-four monthly anniversary dates thereafter.
2015 Plan - Stock Options
A summary of stock option activity for the 2015 Plan for the years ended December 31, 2020 and 2019 is presented below:
| Underlying Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||||
| Outstanding at December 31, 2018 | 5,641,733 | $ | 3.75 | 8.43 years | - | |||||||||
| Granted | 1,050,000 | $ | 2.00 | |||||||||||
| Forfeited or Expired | (324,737 | ) | $ | 3.61 | ||||||||||
| Outstanding at December 31, 2019 | 6,366,996 | $ | 3.47 | 7.78 years | - | |||||||||
| Granted | 2,275,000 | $ | 0.35 | |||||||||||
| Forfeited or Expired | (743,864 | ) | $ | 2.53 | ||||||||||
| Outstanding at December 31, 2020 | 7,898,132 | $ | 2.69 | 7.50 years | - | |||||||||
| Exercisable at December 31, 2020 | 5,208,712 | $ | 3.61 | 6.60 years | - | |||||||||
| Vested and expected to vest at December 31, 2020 | 7,898,132 | $ | 2.58 | 7.56 years | ||||||||||
2015 Plan - Restricted Stock Units
A summary of RSU activity for the 2015 Plan for the years ended December 31, 2020 and 2019 is presented below:
| Weighted | ||||||||
| Restricted Stock Units | Average Grant-Date Fair Value | |||||||
Outstanding at December 31, 2018 | 190,000 | $ | 3.15 | |||||
| Granted | - | |||||||
| Forfeited or Expired | - | |||||||
| Outstanding at December 31, 2019 | 190,000 | $ | 3.15 | |||||
| Granted | 90,000 | $ | 1.86 | |||||
| Forfeited or Expired | (20,000 | ) | $ | 3.15 | ||||
| Outstanding at December 31, 2020 | 260,000 | $ | 2.52 | |||||
| F-62 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
The Whitaker Plan
In October 2018, the Company adopted an equity incentive plan (the “Whitaker Plan”) under which 2,173,107 shares of common stock were reserved for issuance to its newly hired Chief Executive Officer. The terms of options granted under the Whitaker Plan are identical to those granted under the 2015 Plan. 2,173,107 options with a weighted-average grant-date fair value of $3.8 million were granted on December 7, 2018 and vest 33% on October 1, 2019 with the remaining 67% vesting in 24 equal monthly installments thereafter. The options have an exercise price of $2.78 and are exercisable for 10 years from the date of issuance.
A summary of stock option activity for the Whitaker Plan for the years ended December 31, 2020 and 2019 is presented below:
| Underlying Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic | |||||||||||
| Outstanding at December 31, 2018 | - | |||||||||||||
| Granted | 2,173,107 | $ | 2.78 | |||||||||||
| Forfeited or Expired | - | |||||||||||||
| Outstanding at December 31, 2019 | 2,173,107 | |||||||||||||
| Granted | - | $ | 2.78 | 8.94 years | $ | - | ||||||||
| Forfeited or Expired | - | |||||||||||||
| Outstanding at December 31, 2020 | 2,173,107 | $ | 2.78 | 7.94 years | $ | - | ||||||||
| Exercisable at December 31, 2020 | 1,569,164 | $ | 2.78 | 7.94 years | $ | - | ||||||||
| Vested and expected to vest as December 31, 2020 | 2,173,107 | $ | 2.78 | 7.94 years | ||||||||||
9. Income Taxes
The reconciliation of the federal statutory income tax rate to the effective income tax rate is as follows:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Statutory Federal income tax rate | 21.0 | % | 21.0 | % | ||||
| Permanent items | (0.8 | )% | (3.9 | )% | ||||
| Tax credits | 1.3 | % | 1.8 | % | ||||
| Other | (0.3 | )% | (0.2 | )% | ||||
| Valuation allowance | (21.2 | )% | (18.7 | )% | ||||
| Net | 0.0 | % | 0.0 | % | ||||
| F-63 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
Deferred income taxes reflect the net effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets at December 31, 2020 and 2019 are shown below (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Net operating loss carryforwards | $ | 19,076 | $ | 17,227 | ||||
| Research & development tax credits | 2,268 | 2,111 | ||||||
| Capitalized start-up costs | 519 | 585 | ||||||
| Stock-based compensation | 3,064 | 2,556 | ||||||
| Other | 179 | 184 | ||||||
| Total gross deferred income tax assets | 25,106 | 22,663 | ||||||
| Less: Valuation allowance | (25,106 | ) | (22,663 | ) | ||||
| Net deferred income tax assets | $ | - | $ | - | ||||
Valuation allowances require an assessment of both positive and negative evidence when determining whether it is more likely than not that deferred tax assets are recoverable. Such assessment is required on a jurisdiction-by-jurisdiction basis. Based on the available objective evidence, management believes it is more likely than not the U.S. and state deferred tax assets are not realizable as of December 31, 2020 and 2019. Accordingly, the Company has established a full valuation allowance against its deferred tax assets. For the years ended December 31, 2020 and 2019, the valuation allowance increased $2.4 million and $1.6 million, respectively.
Pursuant to the Internal Revenue Code (the “IRC”) Sections 382 and 383, annual use of the Company’s net operating loss and research and development credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. The Company has reviewed the impact of the IRC Sections 382 and 383 on its net operating loss and credit carryforwards and has concluded that the Company has experienced an ownership change that will result in an annual limit on the amount of allowed net operating losses and credit carryforwards. While the Company believes an ownership change has occurred, it has not yet completed its analysis to measure the extent of any potential limitation. The Company plans to finalize this analysis as the likelihood of tax attribute utilization becomes probable.
As of December 31, 2020, the Company had approximately $83.0 million and $83.4 million of Federal and state net operating losses, respectively. The losses will begin to expire in 2029 for Federal and state purposes.
As of December 31, 2020, the Company had Federal and state research and development credits of $1.9 million and $1.5 million, respectively. The Federal research and development credit carryovers will begin to expire in 2031, if not utilized, while the state research and development credit carryovers have no expiration date.
At both December 31, 2020 and 2019, the Company had approximately $0.8 million unrecognized tax benefits, all of which, if recognized, would not affect the effective tax rate.
A reconciliation of the beginning and ending amounts of unrecognized tax benefits in 2020 and 2019 is as follows (in thousands):
| Balance at December 31, 2018 | $ | 583 | ||
| Gross increase for tax positions of current year | 192 | |||
| Balance at December 31, 2019 | 775 | |||
| Gross increase for tax positions of current year | 58 | |||
| Balance at December 31, 2020 | $ | 833 |
| F-64 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
There are no liabilities from unrecognized tax benefits included in the Company’s consolidated balance sheets as of December 31, 2020 and 2019, and therefore the Company has not incurred any penalties or interest.
The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by the Federal and state jurisdictions where applicable. There are currently no pending income tax examinations. The Company’s tax years for 2009 and forward are subject to review by the Federal and state tax authorities due to the carryforward of unutilized net operating losses and research and development credits.
10. Commitments and Contingencies
Registration Rights Penalty
On November 30, 2017, the Company entered into a new registration rights agreement (the “2017 RRA”) with certain investors. Pursuant to the terms of the 2017 RRA, the Company was obligated to prepare and file a Registration Statement on Form S-1 with the SEC no later than 180 days following the last closing of the Company’s private placement offering in 2018 (“Registration Filing Deadline”). If the Company failed to file a Registration Statement prior to the Registration Filing Deadline, the Company would be subject to penalties of up to 6% of the proceeds received in the Company’s private placement offerings held from 2015 to 2018, plus interest of 2% per year following the Registration Filing Deadline. As of December 31, 2018, the Company expected to incur the maximum penalty and accrued $2.6 million related to the 2017 RRA. Interest of less than $0.1 million was accrued during each of the years ended December 31, 2020 and 2019.
Operating Leases
In August 2017, the Company entered into a lease with its then Chief Executive Officer, Dr. John S. Patton, for space to serve as its headquarters in San Francisco, California. The lease was terminated in April 2019.
In January 2019, the Company entered into a lease for office space in Union City, California. In August 2019, the Company amended the lease to reduce the number of square feet. During the year ended 2019, the Company subleased a portion of this space to a related party on a month-to-month basis. The Company ceased using the space in March 2020 and accelerated the amortization of the related right-of-use asset from the lease termination date, November 30, 2020, to the abandonment date, February 29, 2020.
In May 2019, the Company entered into a lease for space to serve as its headquarters in Durham, North Carolina. The lease agreement expired in November 2020 after several three-month automatic extensions. In December 2020, the Company renewed the lease for a three-month period and is accounting for the renewal as a short-term lease.
Employee Benefit Plans
In April 2019, the Company began sponsoring a defined contribution savings and investment plan (the “Plan”) as allowed under Section 401(k) of the IRC. Employees become eligible for participation upon the start of employment and may elect to have a portion of their salary deferred and contributed to the Plan up to the limit allowed under the IRC. The Company makes a matching contribution to the Plan for each participant who has elected to make tax-deferred contributions for the Plan year. These contributions vest immediately. During each of the years ended December 31, 2020 and 2019, the Company contributed $0.1 million to the Plan per year.
| F-65 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
11. Related Parties
Dr. Patton, a board member and a former Chief Executive Officer of the Company, leased office space to the Company (see Note 10) for use as its headquarters. This lease was terminated in April 2019. Rent paid to Dr. Patton was less than $0.1 million during the year ended December 31, 2019.
In August 2019, the Company entered into a separation agreement with Dr. Patton, pursuant to which the Company was obligated to pay severance of $0.7 million in equal semi-monthly installments through August 2020. However, if the Company closes $15 million in financing proceeds prior to August 2020, all unpaid amounts would be paid within 30 days of closing. In connection with the separation, Dr. Patton’s outstanding equity awards were immediately vested. This modification to terms did not result in any incremental stock- based compensation expense. Upon the treatment of the first patient in a Phase 3 clinical trial of AER-501, Dr. Patton will be granted an option to purchase a number of shares of the Company’s common stock equal to 1% of the fully diluted capital stock of the Company at the time of grant, provided that he remains a service provider to the Company as of the date of the grant. In addition, in the event the Company receives final approval from the FDA, EMA, or CFDA for AER-501, Dr. Patton will be granted an option to purchase a number of shares of the Company’s common stock equal to 1% of the fully diluted capital stock of the Company at the time of grant, provided that he remains a service provider to the Company as of the date of the grant. Following the separation, the Company entered into a consulting agreement with Dr. Patton, under which the Company paid $0.1 million per year during the years ended December 31, 2020 and 2019, respectively.
Dr. Patton’s son was employed by the Company through March 31, 2020. The son of Anne Whitaker, the Company’s former Chief Executive Officer and current Chairman of the Board of Directors, is employed by the Company.
iPharma Ltd., an inhalation development CRO which was cofounded by Dr. Patton and of which he is a shareholder, subleased lab space from the Company and paid the Company $0.1 million during the year ended December 31, 2019. During the year ended December 31, 2020, iPharma Ltd. purchased $8,000 worth of equipment from the Company in connection with the closure of its California facility.
Molex Ventures, LLC (“Molex”), a stockholder of the Company and who appointed a director to the Company’s Board of Directors, is the parent company of Phillips (See Note 6). The Company paid Phillips $0.2 million and $1.2 million during the years ended December 31, 2020 and 2019 for device development and manufacturing services, respectively.
A member of the Company’s Board of Directors and stockholder is also the head of Aegis’ private equity banking. Refer to the transactions in Note 7 with Aegis. During 2019, Aegis served as the Company’s warrant agent for the 2019 Warrant Tender Offer pursuant to which the Company paid Aegis $1.4 million cash issuance costs and allowed the placement agent to cashless exercise 1,971,219 warrants resulting in non-cash issuance costs of $0.9 million. During 2020, Aegis served as the Company’s placement agent for the 2020 Series A Preferred Stock Financing pursuant to which the Company paid Aegis $1.2 million cash issuance costs. The Company was obligated to issue to Aegis 964 placement agent warrants to purchase Series A preferred stock in connection with the 2020 Series A Preferred Stock Financing.
Ms. Whitaker served as a director on Vectura Limited’s Board of Directors through September 2020.
12. Subsequent Events
The Company has completed an evaluation of all subsequent events through May 3, 2021, the date the consolidated financial statements were available to be issued, to ensure that these financial statements include appropriate disclosure of events both recognized in the December 31, 2020 consolidated financial statements and events which have occurred but were not recognized in the consolidated financial statements. Except as described below, the Company is not aware of any additional subsequent events that have occurred that require disclosure.
Series A Financing
In January 2021, the Company sold an additional 2,282 shares of Series A preferred stock and received net proceeds of $2.0 million. Upon completion of the sale, the Company was obligated to issue an additional 228 warrants for Series A preferred stock to Aegis for acting as the placement agent.
License Agreement with Hangzhou Chance Pharmaceuticals Co. Ltd.
In January 2021, the Company signed an exclusive license and development agreement with Hangzhou Chance Pharmaceuticals Co. Ltd. (“Chance”) to develop and commercialize AER-901 for the treatment of PAH in mainland China, Hong Kong, Macau, and Taiwan.
The Company received an upfront license fee of $1.5 million and will receive additional milestone payments upon achievement of certain clinical and sales events. The Company will supply the product to Chance at an agreed transfer price and receive a high single-digit royalty on net sales. Chance is responsible for conducting and financing local clinical trials required to obtain market authorization in China.
| F-66 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
UNAUDITED CONSOLIDATED BALANCE SHEETS
(in thousands of U.S. dollars, except share and per share data)
| September 30, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 14,355 | $ | 4,494 | ||||
| Contract assets | 251 | — | ||||||
| Prepaid expenses and other current assets | 854 | 319 | ||||||
| Total current assets | 15,460 | 4,813 | ||||||
| Other non-current assets | 39 | 6 | ||||||
| Equipment, net | — | — | ||||||
| Total assets | $ | 15,499 | $ | 4,819 | ||||
| Liabilities, convertible preferred stock, and stockholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 889 | $ | 245 | ||||
| Accrued expenses and other current liabilities | 3,776 | 3,316 | ||||||
| Total current liabilities | 4,665 | 3,561 | ||||||
| Harmony contingent settlement liability | 925 | 2,978 | ||||||
| Warrant liabilities | 2,377 | 664 | ||||||
| Customer advances and other liabilities | 723 | 977 | ||||||
| Total liabilities | 8,690 | 8,180 | ||||||
| Commitments and contingencies (Note 10) | ||||||||
| Convertible preferred stock: | ||||||||
| Series A preferred stock, $0.0001 par value; 50,000 shares authorized; 2,810 shares and 10,168 shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | 2,410 | 8,103 | ||||||
| Series A-1 preferred stock, $0.0001 par value; 50,000 shares authorized; 9,640 and 0 shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | 7,509 | — | ||||||
| Total convertible preferred stock | 9,919 | 8,103 | ||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, $0.0001 par value; 5,500,000 shares authorized; no shares issued or outstanding | — | — | ||||||
| Royalty Series convertible preferred stock, $0.0001 par value; 4,400,000 shares authorized; 2,541,459 shares issued and outstanding as of September 30, 2021 and December 31, 2020 | 6,956 | 6,956 | ||||||
| Common stock, $0.0001 par value; 150,000,000 shares authorized; 49,579,806 shares and 34,639,806 shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | 5 | 3 | ||||||
| Additional paid-in capital | 120,131 | 106,896 | ||||||
| Accumulated deficit | (130,202 | ) | (125,319 | ) | ||||
| Total stockholders’ deficit | (3,110 | ) | (11,464 | ) | ||||
| Total liabilities, convertible preferred stock and stockholders’ deficit | $ | 15,499 | $ | 4,819 | ||||
The accompanying notes are an integral part of these unaudited consolidated financial statements.
| F-67 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
UNAUDITED CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands of U.S. dollars, except share and per share data)
| Nine Months Ended September 30, | ||||||||
| 2021 | 2020 | |||||||
| Revenues | ||||||||
| License | $ | 1,500 | $ | — | ||||
| Total revenues | 1,500 | — | ||||||
| Operating expenses | ||||||||
| Research and development | 3,982 | 2,582 | ||||||
| General and administrative | 3,825 | 6,247 | ||||||
| Total operating expenses | 7,807 | 8,829 | ||||||
| Loss from operations | (6,307 | ) | (8,829 | ) | ||||
| Other income (expenses) | ||||||||
| Change in fair value of Harmony contingent settlement liability | 2,053 | 385 | ||||||
| Change in fair value of Harmony purchase option agreement | 33 | (167 | ) | |||||
| Loss on foreign exchange | (28 | ) | - | |||||
| Interest expense, net | (89 | ) | (91 | ) | ||||
| Change in fair value of warrant liabilities | (545 | ) | — | |||||
| Gain on disposal of equipment | - | 10 | ||||||
| Total other income | 1,424 | 137 | ||||||
| Net loss | $ | (4,883 | ) | $ | (8,692 | ) | ||
| Per share information: | ||||||||
| Net loss per share of common stock, basic and diluted | $ | (0.14 | ) | $ | (0.25) | |||
| Weighted average common shares outstanding, basic and diluted | 34,799,293 | 34,638,020 | ||||||
The accompanying notes are an integral part of these unaudited consolidated financial statements.
| F-68 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
UNAUDITED CONSOLIDATED STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND
STOCKHOLDERS’ DEFICIT
(in thousands of U.S. dollars, except share data)
| Convertible Preferred Stock | Stockholders’ Deficit | |||||||||||||||||||||||||||||||||||||||||||
| Series A | Series A-1 | Royalty Series | ||||||||||||||||||||||||||||||||||||||||||
| Preferred | Preferred | Convertible | Additional | Total | ||||||||||||||||||||||||||||||||||||||||
| Stock | Stock | Preferred Stock | Common Stock | Paid-In | Accumulated | Stockholders’ | ||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||||||||||||||
| Balance at December 31, 2020 | 10,168 | $ | 8,103 | - | $ | - | 2,541,459 | $ | 6,956 | 34,639,806 | $ | 3 | $ | 106,896 | $ | (125,319 | ) | $ | (11,464 | ) | ||||||||||||||||||||||||
| Issuance of common stock
under private placement, less issuance costs of $3,189 | - | - | - | - | - | - | 14,940,000 | 2 | 11,749 | - | 11,751 | |||||||||||||||||||||||||||||||||
| Issuance of Series A preferred
stock, less issuance costs of $309 | 2,282 | 1,816 | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||
| Conversion of Series A preferred
stock to Series A-1 preferred stock | (9,640 | ) | (7,509 | ) | 9,640 | 7,509 | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | - | - | - | - | 1,486 | - | 1,486 | |||||||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | - | - | (4,883 | ) | (4,883 | ) | |||||||||||||||||||||||||||||||
| Balance at September 30, 2021 | 2,810 | $ | 2,410 | 9,640 | $ | 7,509 | 2,541,459 | $ | 6,956 | 49,579,806 | $ | 5 | $ | 120,131 | $ | (130,202 | ) | $ | (3,110 | ) | ||||||||||||||||||||||||
| Balance at December 31, 2019 | - | $ | - | - | $ | - | 2,541,459 | $ | 6,956 | 34,633,690 | $ | 3 | $ | 104,556 | $ | (115,010 | ) | $ | (3,495 | ) | ||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options | - | - | - | - | - | - | 6,116 | - | - | - | - | |||||||||||||||||||||||||||||||||
| Issuance of Series A Preferred
Stock, less issuance costs of $1,087 | 6,348 | 4,860 | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | - | - | - | - | 1,838 | - | 1,838 | |||||||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | - | - | - | (8,692 | ) | (8,692 | ) | |||||||||||||||||||||||||||||||
| Balance at September 30, 2020 | 6,348 | $ | 4,860 | - | $ | - | 2,541,459 | $ | 6,956 | 34,639,806 | $ | 3 | $ | 106,394 | $ | (123,702 | ) | $ | (10,349 | ) | ||||||||||||||||||||||||
The accompanying notes are an integral part of these unaudited consolidated financial statements.
| F-69 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
UNAUDITED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands of U.S. dollars)
| Nine Months Ended September 30, | ||||||||
| 2021 | 2020 | |||||||
| Cash flows from operating activities | ||||||||
| Net loss | $ | (4,883 | ) | $ | (8,692 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Change in fair value of Harmony contingent settlement liability | (2,053 | ) | (385 | ) | ||||
| Change in fair value of warrant liabilities | 545 | - | ||||||
| Change in fair value of Harmony purchase option agreement | (33 | ) | 167 | |||||
| Amortization of debt discount | 46 | 63 | ||||||
| Operating lease right-of-use asset amortization and lease liability accretion, net | - | 12 | ||||||
| Stock-based compensation | 1,486 | 1,838 | ||||||
| Net changes in operating assets and liabilities: | ||||||||
| Contract assets | (251 | ) | - | |||||
| Prepaid expenses and other current assets | (535 | ) | (638 | ) | ||||
| Accounts payable, accrued expenses and other liabilities | 804 | (1,974 | ) | |||||
| Net cash used in operating activities | (4,874 | ) | (9,609 | ) | ||||
Cash flows from financing activities | ||||||||
| Proceeds from issuance of common stock under private placement | 14,940 | - | ||||||
| Proceeds from issuance of Series A preferred stock | 2,282 | 6,348 | ||||||
| Issuance costs relating to Series A preferred stock offering | (309 | ) | (1,087 | ) | ||||
| Issuance costs relating to common stock offering under private placement | (2,178 | ) | - | |||||
| Net cash provided by financing activities | 14,735 | 5,261 | ||||||
| Net increase (decrease) in cash and cash equivalents | 9,861 | (4,348 | ) | |||||
| Cash and cash equivalents at beginning of period | 4,494 | 6,659 | ||||||
| Cash and cash equivalents at end of period | $ | 14,355 | $ | 2,311 | ||||
| Non-cash transactions and supplemental cash flow information: | ||||||||
| Recognition of warrant liabilities in connection with issuance of Series A preferred stock | $ | $ | 401 | |||||
| Issuance costs relating to common stock offering under private placement settled through issuance of common stock warrants | $ | 1,011 | $ | - | ||||
The accompanying notes are an integral part of these unaudited consolidated financial statements.
| F-70 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Basis of Presentation
Aerami Therapeutics Holdings, Inc., (“Aerami Holdings), incorporated on December 20, 2013 in the State of Delaware, together with its wholly owned subsidiaries, Aerami Therapeutics, Inc. (“Aerami Opco”) and Aerami Therapeutics Australia Pty, Ltd. (“Aerami Australia”) (and collectively with Aerami Holdings and Aerami Opco, the “Company”), is a clinical stage biopharmaceutical company dedicated to developing inhaled therapies for the treatment of severe respiratory and chronic diseases.
Aerami Opco, which was incorporated in the State of Delaware on February 9, 2009, has devoted all its efforts to developing its technology, raising capital and hiring employees. Aerami Australia was incorporated in Australia on October 7, 2020 and has been engaged in conducting a Phase 1 clinical trial for the Company’s lead development program.
Going Concern
The Company has no products approved for sale and has incurred significant operating losses since its inception. For the nine months ended September 30, 2021 and 2020, the Company has incurred net losses of $4.9 million and $8.7 million, respectively, and as of September 30, 2021, the Company had an accumulated deficit of $130.2 million. Achieving profitability is dependent upon the successful development, approval and commercialization of the Company’s product candidates and achieving a level of revenues adequate to support the Company’s cost structure. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to raise additional capital. Management intends to fund future operations through additional financing which could include private or public debt or equity offerings. In addition, the Company may seek additional capital through arrangements with strategic partners or from other sources. There can be no assurances, however, that additional funding will be available on terms acceptable to the Company, or at all. These conditions, among other factors, raise substantial doubt about the Company’s ability to continue as a going concern.
The accompanying unaudited consolidated financial statements have been prepared assuming the Company will continue to operate as a going concern, which contemplates the realization of assets and the settlement of liabilities in the normal course of business. The unaudited consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty related to the Company’s ability to continue as a going concern.
COVID-19 PANDEMIC
The COVID-19 pandemic continues to significantly impact the United States and the rest of the world, including creating disruption in the economy such as the supply chains, production, and sales across various industries. The impact on the Company’s operations and financial performance will depend on the duration and extent of the pandemic, which includes the impact on the Company’s customers, vendors, and employees. The extent to which this pandemic could adversely impact the Company’s future business, financial condition and results of operations is dependent upon various factors, many of which are highly uncertain and outside the control of the Company. While the Company experienced delays in the recruitment of subjects for the AER-901 Phase 1 trial due to government regulations in Australia related to COVID-19, the Company continues to target initiating in 2022 activities on the Phase 2 portion of the Company’s planned combined Phase 2/3 trial for its inhaled imatinib program.
Risks and Uncertainties
The Company is subject to risks and uncertainties common to early-stage companies in the biopharmaceutical industry, including but not limited to development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with governmental regulations, dependency on key suppliers including for certain active pharmaceutical ingredients and the ability to secure additional capital to fund operations. Drug candidates currently under development will require extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts will require significant amounts of additional capital, adequate personnel, and infrastructure, and extensive compliance and reporting capabilities. Even if the Company’s drug development efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales.
| F-71 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
2. Summary of Significant Accounting Policies
Basis of Presentation
The unaudited consolidated financial statements, which include Aerami Holdings and its wholly owned subsidiaries, are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). All significant intercompany accounts and transactions have been eliminated in consolidation.
Unaudited Interim Results
These unaudited consolidated financial statements and accompanying notes should be read in conjunction with the Company’s annual financial statements as of and for the year ended December 31, 2020. The accompanying unaudited consolidated financial statements as of September 30, 2021 and for the nine months ended September 30, 2021 and 2020 are unaudited but include all adjustments that management believes are necessary for a fair presentation of the periods presented. Interim results are not necessarily indicative of results for a full year. Balance sheet amounts as of December 31, 2020 have been derived from the audited financial statements as of that date.
Use of Estimates
The preparation of the unaudited consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates, and changes in estimates may occur. The most significant matters involving management’s estimates include research and development accruals, valuation of the Harmony contingent settlement liability, valuation of warrant liabilities, and the valuation of common stock to calculate stock-based compensation.
Cash and cash equivalents
Cash and cash equivalents consist principally of cash held in commercial bank accounts and money market funds. The Company considers all highly liquid investments with maturities of three months or less at the date of acquisition to be cash equivalents.
Concentration of Credit Risk
The Company’s financial instruments that are exposed to concentration of credit risk consist primarily of cash and cash equivalents. The Company maintains its cash in bank accounts, which generally exceed federally insured limits. The Company maintains its cash equivalents in investments in interest-bearing money market funds. The Company has never experienced any losses related to these balances.
| F-72 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Equipment
Equipment is stated at cost less accumulated depreciation and amortization. Depreciation and amortization are recognized using the straight-line method over the estimated useful lives of the assets as follows:
| Asset category | Estimated useful life | |
| Lab equipment | 5 years | |
| Computer equipment | 3 years | |
| Office equipment | 5 years |
Estimated useful lives are periodically assessed to determine if changes are appropriate. Maintenance and repairs are charged to expense as incurred. When assets are retired or otherwise disposed of, the cost of these assets and related accumulated depreciation or amortization are eliminated from the consolidated balance sheet and any resulting gains or losses are included in the consolidated statement of operations in the period of disposal.
Long-Lived Assets
The Company regularly reviews the carrying values and estimated lives of its long-lived assets, including property, equipment and right-of-use (“ROU”) assets, to determine whether indicators of impairment may exist which warrant adjustments to carrying values or estimated useful lives. The determinants used for this evaluation include management’s estimate of the asset’s ability to generate positive income from operations and positive cash flow in future periods as well as the strategic significance of the assets to the Company’s business objective. Should an impairment occur, the impairment loss would be measured based on the excess of the carrying amount over the asset’s fair value. No impairment charge was recorded during the nine months ended September 30, 2021 or 2020.
Leases
The Company determines if a contract is or contains a lease at contract inception. The Company’s ROU assets were related to operating leases for lab and office space. As of September 30, 2021 and December 31, 2020, the Company’s ROU assets have been fully depreciated and there were no outstanding lease liabilities.
ROU assets represent the right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make lease payments arising from the lease. ROU assets and lease liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The incremental borrowing rate is determined by using the rate of interest that the Company would pay to borrow on a collateralized basis an amount equal to the lease payments for a similar term and in a similar economic environment. Lease terms include options to extend or terminate the lease when it is reasonably certain that the Company will exercise that option.
The Company excludes variable lease payments in measuring ROU assets and lease liabilities, other than those that depend on an index or a rate or are in-substance fixed payments. Lease and non-lease components are generally accounted for separately.
Accrued Research and Development Expenses
As part of the process of preparing its consolidated financial statements, the Company is required to estimate its accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf and estimating the level of service performed and the associated cost incurred for the service when the Company has not yet been invoiced. Most of the Company’s service providers invoice monthly in arrears for services performed or when contractual milestones are met. Estimates of accrued expenses as of each balance sheet date in the unaudited consolidated financial statements are based on facts and circumstances known at that time. The Company periodically confirms the accuracy of estimates with the service providers and makes adjustments if necessary. The significant estimates in accrued research and development expenses are related to expenses incurred with respect to contract research organizations (“CROs”), contract manufacturing organizations (“CMOs”) and other vendors in connection with research and development and manufacturing activities.
| F-73 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
The Company bases its expenses related to CROs and CMOs on estimates of the services received and efforts expended pursuant to quotations and contracts with such vendors that conduct research and development and manufacturing activities on the Company’s behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to vendors will exceed the level of services provided and result in a prepayment of the applicable research and development or manufacturing expense. In accruing service fees, the Company estimates the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from estimates, the accrual or prepaid expense is adjusted accordingly. Although estimates are not expected to be materially different from amounts actually incurred, the Company’s understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and could result in amounts that are too high or too low in any particular period. There have been no material changes in estimates for the periods presented.
Warrants
Warrants that are considered freestanding equity-classified instruments due to their detachable and separately exercisable features and that met the indexation criteria in Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) ASC 815-40-15-5 through 15-8 (indexation criteria) are presented as a component of the stockholders’ deficit in accordance with equity-classification guidance in ASC 815-40-25. Warrants that have features of or are indexed to shares that are redeemable or contingently redeemable and accounted for in temporary equity are presented as warrant liability in accordance with the provisions of ASC 480. These warrants are initially recognized at their fair value and remeasured at each reporting period, with changes in fair value recognized as other income/expense in the consolidated statements of operations. Contingently issuable warrants arising from a contract that is outstanding and in scope of the indexation criteria are considered issued for accounting purposes in accordance with ASCS 815-40-15-6 and presented as component of the stockholders’ deficit.
Preferred Stock
The Company classifies Series A preferred stock and Series A-1 preferred stock as temporary equity in the accompanying consolidated balance sheets at September 30, 2021 and December 31, 2020 since these shares of preferred stock become redeemable due to certain deemed liquidation events, as defined in the Company’s certificate of incorporation, which are outside of the Company’s control. No accretion has been recorded relating to the Series A preferred stock as deemed liquidation events are not probable, except for the put option attached to the shares of preferred stock issued to a related party which were accreted to its redemption value at December 31, 2020, as management determined that the contingency relating to the Company’s completion of certain research and development activities as stated within the side letter agreement with the related party (the “Put Option”) (see Note 7) is not probable. No accretion was recognized for the periods ended September 30, 2021 and 2020.
Equity Issuance Costs
Issuance costs are allocated between the individual freestanding instruments identified on the same basis as proceeds were allocated. Issuance costs associated with the issuance of stock or equity contracts (i.e., equity-classified warrants and Royalty stock (as defined below)) are recorded as a charge against the gross proceeds of the offering.
| F-74 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Revenue Recognition
The Company adopted Accounting Standard Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASC 606” or “New Revenue Recognition Standard”), which supersedes ASC Topic 605, Revenue Recognition (“ASC 605”), effective January 1, 2018 using the modified retrospective approach and applied this approach to contracts with customers that were not completed as of January 1, 2018. ASC 606, as amended, defines a five-step process to achieve this core principle: (1) identify the contract with the customer, (2) identify the performance obligations in the contract, (3) determine the transaction price, (4) allocate the transaction price to the performance obligations in the contract, and (5) recognize revenue when (or as) the entity satisfies a performance obligation. As part of the accounting for these arrangements, the Company must use significant judgment to determine the transaction price and timing of revenue recognition.
This adoption primarily affected the recognition of non-refundable up-front fees and milestone payments. The new revenue standard generally requires licenses that are not considered distinct performance obligations from other goods or services within a contract to be bundled with those goods or services as a combined performance obligation. Revenue associated with the combined performance obligation is recognized over time as the customer benefits from the license. See Note 6 for further information.
Advance billings under the supply agreement with Chance Pharmaceuticals Co. Ltd. are recognized as contract assets with the corresponding credit to deferred revenue which is included in customer advances and other liabilities in the accompanying consolidated balance sheets (see Note 6). Deferred revenues are recognized as income upon satisfactory completion of the related performance obligations.
Research and Development Expenses
Research and development expenses primarily consist of costs associated with the preclinical and clinical development of the Company’s product candidates, including the following:
| ● | external research and development expenses incurred under arrangements with third parties, such as CROs and other vendors and CMOs to produce drug substance and drug product; and | |
| ● | employee-related expenses, including salaries, benefits and stock-based compensation expense. |
All research and development expenses are charged to operations as incurred in accordance with FASB ASC Topic 730, Research and Development. The Company accounts for non-refundable advance payments for goods and services that will be used in future research and development activities as expenses when the service has been performed or when the goods have been received, rather than when the payment is made.
Stock-Based Compensation Expense
The Company follows the provisions of ASC 718, Compensation—Stock Compensation, which requires the measurement and recognition of compensation expense for all stock-based payment awards made to employees and non-employee directors, including employee stock options. Stock-based compensation expense is based on the grant-date fair value estimated in accordance with the provisions of ASC 718 and is generally recognized as an expense over the requisite service period. For grants containing performance- based vesting provisions, the grant-date fair value of the milestone-based stock options is recognized as compensation expense once it is probable that the condition will be achieved. The Company accounts for actual forfeitures in the period the forfeitures occur.
The Company complies with ASU 2017-09, Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting, which clarifies when a change to terms or conditions of a share-based payment award must be accounted for as a modification. This guidance requires modification accounting if the vesting condition, fair value or the award classification is not the same both before and after a change to the terms and conditions of the award.
| F-75 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
The Company also complies with ASU 2018-07, Improvements to Nonemployee Share-Based Payment Accounting, (“ASU 2018-07”), which supersedes ASC 505-50 and expands the scope of ASC 718 to include all share-based payments arrangements related to the acquisition of goods and services from both employees and non-employees. The measurement date for non-employee awards is the date of grant. Compensation expense for non-employees is recognized, without changes to the fair value of the award, over the requisite service period, which is the vesting period of the respective award.
The Company uses the Black-Scholes option-pricing model to estimate the grant-date fair value of awards using the following assumptions and/or valuation model inputs for awards:
| ○ | Risk-free interest rate: The risk-free interest rate is based on U.S. Treasury, zero-coupon issues with a remaining term equal to the expected term assumed at the date of grant. | |
| ○ | Expected dividend yield: The Company uses a dividend yield of zero since the Company has never paid cash dividends to stockholders and has no current intentions to pay cash dividends. | |
| ○ | Expected volatility: Due to the lack of trading history, the Company’s computation of stock-price volatility is based on the volatility rates of comparable publicly held companies. | |
| ○ | Expected term: The Company’s computation of expected term for awards to employees is determined using the “simplified” method, which is the midpoint between the vesting date and the end of the contractual term. The Company believes that it does not have sufficient reliable exercise data in order to justify the use of a method other than the “simplified” method of estimating the expected exercise term of employee stock option grants. | |
| ○ | The assumptions used in the Black-Scholes option-pricing model for awards are as follows: |
| Nine Months Ended September 30, | ||||||||
| 2021 | 2020 | |||||||
| Risk-free interest rate | 1.0 | % | 0.4 | % | ||||
| Expected volatility | 86.1 | % | 83.1 | % | ||||
| Expected term (in years) | 5.87 | 5.83 | ||||||
Income Taxes
The Company accounts for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to be recovered or settled. Realization of deferred tax assets is dependent upon future taxable income. A valuation allowance is recognized if it is more likely than not that some portion or all of a deferred tax asset will not be realized based on the weight of available evidence, including expected future earnings. The Company recognizes an uncertain tax position in its financial statements when it concludes that a tax position is more likely than not to be sustained upon examination based solely on its technical merits. Only after a tax position passes the first step of recognition will measurement be required. Under the measurement step, the tax benefit is measured as the largest amount of benefit that is more likely than not to be realized upon effective settlement. This is determined on a cumulative probability basis. The full impact of any change in recognition or measurement is reflected in the period in which such change occurs. The Company elected to record any interest or penalties related to income taxes as part of its income tax expense.
Fair Value of Financial Instruments
The Company determines the fair value of financial and nonfinancial assets and liabilities using the fair value hierarchy, which describes three levels of inputs that may be used to measure fair value, as follows:
| Level 1: | Quoted prices in active markets for identical assets or liabilities. |
| F-76 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
| Level 2: | Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. For marketable securities, the Company reviews trading activity and pricing as of the measurement date; and | |
| Level 3: | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The Company’s Level 1 assets consist of investments in money market funds, which are reflected in cash and cash equivalents in the accompanying consolidated balance sheets. The Company does not hold any assets or liabilities that are measured using Level 2 inputs. The Company’s Level 3 assets consist of the Harmony purchase option agreement while the Level 3 liabilities consist of the Harmony contingent settlement liability and warrant liabilities. Level 3 assets and liabilities are valued based on various estimates and inputs, including probability of success of product development, discount rates and amount of time until an exit scenario. Each reporting period, Level 2 and Level 3 assets and liabilities are adjusted to fair value with the changes in fair value recognized in other income and expense. Changes in fair value reflect changes in information about the probability and timing of realization.
Net Loss Per Share
Basic net loss per share of common stock is computed by dividing net loss by the weighted average number of shares of common stock outstanding during each period. Diluted net loss per share of common stock includes the effect, if any, from the potential exercise or conversion of securities, such as redeemable convertible preferred stock, stock options and warrants which would result in the issuance of incremental shares of common stock. For diluted net loss per share, the weighted average number of shares of common stock is the same for basic net loss per share due to the fact that, when a net loss exists, dilutive securities are not included in the calculation as the impact is anti-dilutive.
The following potentially dilutive securities have been excluded from the computation of diluted weighted average shares of common stock outstanding, prior to the use of the two-class method, as they would be anti-dilutive (in thousands):
| Nine Months Ended September 30, | ||||||||
| 2021 | 2020 | |||||||
| Convertible preferred stock | 24,631 | 8,889 | ||||||
| Stock options | 12,604 | 9,682 | ||||||
| Unvested restricted stock units | 292 | 449 | ||||||
| Warrants | 4,640 | 1,575 | ||||||
| 42,167 | 20,595 | |||||||
The Company’s Series A preferred stock contractually entitles the holders of such shares to participate in dividends but does not contractually require the holders of such shares to participate in losses of the Company. Accordingly, in periods in which the Company reports a net loss, such losses were not allocated to participating securities. The Company reported a net loss per share of common stock for the nine-month periods ended September 30, 2021 and 2020.
| F-77 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Segment Information
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker or decision-making group in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business as one operating and reporting segment.
Recently Issued Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, “(Subtopic 470-20): Debt—Debt with Conversion and Other Options” (“ASU 2020-06”) to address the complexity associated with applying GAAP to certain financial instruments with characteristics of liabilities and equity. ASU 2020-06 includes amendments to the guidance on convertible instruments and the derivative scope exception for contracts in an entity’s own equity and simplifies the accounting for convertible instruments which include beneficial conversion features or cash conversion features by removing certain separation models in Subtopic 470-20. Additionally, ASU 2020-06 will require entities to use the “if-converted” method when calculating diluted earnings per share for convertible instruments. ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. The Company is currently evaluating the impact of ASU 2020-06 on the consolidated financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. This guidance removes certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences. This guidance also clarifies and simplifies other areas of ASC 740. The guidance will be effective for fiscal years and interim periods beginning after December 15, 2021, and early adoption is permitted. The adoption of this guidance is not expected to have a material impact on the consolidated financial statements.
3. Fair Value of Financial Assets and Liabilities
The following table summarizes assets and liabilities recorded at fair value, and the classification by level of input within the fair value hierarchy (in thousands):
| September 30, 2021 | ||||||||||||||||
| Basis of Fair Value Measurements | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: | ||||||||||||||||
| Investment in money market funds | $ | 1,589 | $ | 1,589 | $ | - | $ | - | ||||||||
| Harmony purchase option agreement | $ | 39 | $ | - | $ | - | $ | 39 | ||||||||
| Liabilities: | ||||||||||||||||
| Harmony contingent settlement liability | $ | 925 | $ | - | $ | - | $ | 925 | ||||||||
| Warrant liabilities – Series A preferred stock | $ | 1,366 | $ | - | $ | - | $ | 1,366 | ||||||||
| Warrant liabilities – common stock | $ | 1,011 | $ | - | $ | - | $ | 1,011 | ||||||||
| F-78 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
| December 31, 2020 | ||||||||||||||||
| Basis of Fair Value Measurements | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: | ||||||||||||||||
| Investment in money market funds | $ | 1,488 | $ | 1,488 | $ | - | $ | - | ||||||||
| Harmony purchase option agreement | $ | 6 | $ | - | $ | - | $ | 6 | ||||||||
| Liabilities: | ||||||||||||||||
| Harmony contingent settlement liability | $ | 2,978 | $ | - | $ | - | $ | 2,978 | ||||||||
| Warrant liabilities – Series A preferred stock | $ | 664 | $ | - | $ | - | $ | 664 | ||||||||
The following table summarizes the changes in the fair value of assets and liabilities classified as Level 3 in the fair value hierarchy (in thousands):
Harmony Option | Harmony Contingent Settlement Liability | Warrant Liabilities Series A | Warrant Liabilities Common Stock | |||||||||||||
| Balance at December 31, 2020 | $ | 6 | $ | 2,978 | $ | 664 | $ | - | ||||||||
| Additions during the period | - | - | 157 | 1,011 | ||||||||||||
| Change in fair value | 33 | (2,053 | ) | 545 | - | |||||||||||
| Balance at September 30, 2021 | $ | 39 | $ | 925 | $ | 1,366 | $ | 1,011 | ||||||||
4. Equipment, net
For the periods ended September 30, 2021 and December 31, 2020, this account consists of lab equipment with total costs of $1.1 million less accumulated depreciation of $1.1 million. No depreciation expense was recorded during the nine months ended September 30, 2021 or 2020 as these assets have been fully depreciated.
5. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
| September 30, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Registration rights penalty (Note 10) | $ | 2,714 | $ | 2,674 | ||||
| Accrued employee-related expenses | 499 | 243 | ||||||
| Harmony Plus payable, current portion | 300 | 300 | ||||||
| Deferred revenue | 251 | - | ||||||
| Accrued research and development expenses | 12 | 99 | ||||||
| Total Accrued Expenses and Other Current Liabilities | $ | 3,776 | $ | 3,316 | ||||
| F-79 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
6. Licenses and Collaborations
License Agreement with Hangzhou Chance Pharmaceuticals Co. Ltd.
In January 2021, the Company signed an exclusive license agreement (the “License Agreement”) with Hangzhou Chance Pharmaceuticals Co. Ltd. (“Chance”) to develop and commercialize AER-901 for the treatment of pulmonary arterial hypertension (“PAH”) in mainland China, Hong Kong, Macau, and Taiwan (the “Territory”). In accordance with the License Agreement, Chance is responsible for conducting and financing local clinical trials required to obtain market authorization in the Territory. In September 2021, the Company executed a supply agreement (the “Supply Agreement”) with Chance, whereby the Company will be responsible for supplying drug product and devices to Chance for use in their clinical trials.
Under the terms of the License Agreement, the Company received a non-refundable upfront license fee of $1.5 million in March 2021. The Company is also entitled to receive up to $10.8 million in potential future milestone payments if certain development, regulatory, and sales-based events are successfully achieved. The terms of the License Agreement also provide for Chance to pay the Company a percent of net sales of the licensed product in the Territory. The Supply Agreement is structured such that the Company will supply Chance product and devices at cost plus a margin.
The License Agreement will continue in full force for 15 years following the first commercial sale of the licensed products. The License Agreement may be terminated by either party with written notice for a material breach, if such breach is not cured within two months’ notice, or for the insolvency of either party. The License agreement may also be terminated by the Company in the event Chance fails to pay undisputed amounts in a timely manner or Chance does not use commercially reasonable efforts to progress the development and commercialization of the licensed product or for Chance failing to achieve any development milestone.
The Company evaluated the agreements under ASC 606 and determined that there were two promises: (i) an exclusive license to develop and commercialize AER-901 in the Territory and (ii) manufacture and supply of certain amounts of AER-901. The Company determined that these two promises represent distinct performance obligations for purposes of recognizing revenue and recognized $1.5 million as license revenue during the nine months ended September 30, 2021. At the time of the license signing, the Company evaluated the option to enter into the Supply Agreement and concluded no material right existed at such time.
The Company determined that the upfront payment of $1.5 million constitutes the transaction price as of the outset of the License Agreement. Future potential regulatory and development milestone payments were fully constrained as the risk of significant revenue reversal related to these amounts has not yet been resolved. The achievement of the future milestones is not within the Company’s control and is subject to certain research and development success or regulatory approvals and therefore carry significant uncertainty. The Company will reevaluate the likelihood of achieving these milestones at the end of each reporting period and adjust the transaction price in the period the risk is resolved. In addition, the Company will recognize any consideration related to sales-based milestones and royalties when the subsequent sales occur. Consideration related to the Supply Agreement will be allocated to the supply performance obligation and will be recognized at a point in time as the Company delivers supplies and satisfies its obligation.
License and Development Agreements with Vectura Limited
In June 2020, the Company entered into an exclusive license agreement with Vectura Limited (“Vectura”) to acquire a worldwide, exclusive license to develop and commercialize Vectura’s proprietary FOX® device containing a formulation of the active ingredient imatinib (“AER-901”) for the treatment of PAH (the “Vectura License”). The Company also received the right of first negotiation to obtain rights for the use of AER-901 in additional indications concerning respiratory and pulmonary diseases. In addition, in May 2020, the Company entered into a development agreement with Vectura and made a payment of approximately $1.1 million to begin work to progress inhaled imatinib into a Phase 1 human clinical study as soon as possible after execution of the Vectura License. The Company capitalized the $1.1 million payment as a prepaid asset and recognized $0.2 million and $0.5 million as research and development expense for the nine months ended September 30, 2021 and 2020, respectively.
| F-80 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Under the Vectura License, the Company is obligated to use commercially reasonable efforts to develop and commercialize AER-901 and to pay milestone payments for the achievement of certain development, regulatory and sales milestones in amounts up to $161 million in the aggregate. The Company is also obligated to pay a mid-single-digit royalty on sales of AER-901. The Company must obtain supply of Vectura’s FOX® device from Vectura at a transfer price equal to Vectura’s costs of manufacture plus a specified percentage. The Vectura License may be terminated by either party for a material breach if such breach is not cured within sixty days’ notice of such breach of either party. Vectura will also have rights to terminate following certain triggering events.
Amended and Restated Joint Development Agreement with Phillips-Medisize, LLC.
In June 2020, the Company entered into an amended and restated joint development agreement (the “Amended Phillips JDA”) with Phillips-Medisize, LLC (“Phillips”). Under the Amended Phillips JDA, the Company agreed to work exclusively with Phillips and its affiliates for the term of the agreement, which shall initially be for a minimum of five (5) years, for the development and manufacturing of the Company’s AFINA™ device and the development of integrated connected health technologies for the AFINA™ device, as well as any other device the Company may acquire and any device that it may in-license that would require material development or refinement to be usable or commercially feasible for use with the Company’s formulations, other than the devices in-licensed from the licensor under the Vectura License. The Company and Phillips will negotiate at a later date on commercially reasonable terms for manufacturing and supply of any products under the Amended Phillips JDA. The Amended Phillips JDA otherwise amends and restates the Company’s existing Phillips JDA on substantially the same terms.
License Agreement with Aerogen/Philips Electronics UK
In November 2010, the Company entered into a license agreement with Aerogen Limited (“Aerogen”), pursuant to which Aerogen granted the Company an exclusive worldwide license, with sublicensing rights, to develop and commercialize insulin delivery devices containing Aerogen’s vibrating mesh aerosolization technology. Under the agreement, Aerogen is obligated to use commercially reasonable efforts to collaborate with the Company to exclusively develop and manufacture related devices, and the Company is responsible for establishing product requirements for the device. Further, the Company is responsible for obtaining and maintaining all regulatory approvals for the drug/device combination globally as well as for all commercialization activities relating to the drug/device combination, whereas Aerogen is obligated to provide regulatory assistance on documents pertaining to the device. A joint development committee was formed to facilitate and oversee the development of the device. In addition, the license agreement provides that technology jointly developed by Aerogen and the Company relating to the device is owned by Aerogen with an exclusive license to the Company in the field of insulin.
In December 2014, the Company entered into a novation agreement with Aerogen and Philips Electronics UK (“PEUK”), in which Aerogen granted its rights under the above license agreement to PEUK (subject to Aerogen retaining certain of the rights and responsibilities with respect to the licensed intellectual property) and PEUK assumed the obligation to perform under the license agreement and be bound by the terms of the license agreement in every way as if a party in place of Aerogen. Additionally, in connection with the novation agreement, the Company has undertaken to perform under the agreement for the benefit of PEUK. Aerami and Aerogen mutually released and discharged each other from their novated liabilities under the agreement.
The Company is obligated to fund all device development activities and upon commercialization is obligated to pay PEUK tiered low single-digit royalties on net sales of the product, subject to an annual minimum payment commencing as of the second year of commercial sale, on a country-by-country basis for the longer of the life of certain patents covering the product or 10 years after the first commercial sale of the product in the respective country.
| F-81 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
In August 2019, the Company entered into an addendum with PEUK to the license agreement to extend the scope of the license agreement to include the development and commercialization of glucagon-like peptide 1 (“GLP-1”) solutions formulated for use with the delivery devices containing the vibrating mesh aerosolization technology. The Company paid $0.2 million to PEUK upon execution of the addendum. The Company is obligated to use commercially reasonable efforts to develop and commercialize insulin and GLP-1 solution delivery devices containing the vibrating mesh technology and to pay milestone payments for the achievement of certain development and regulatory milestones in amounts up to $2.2 million in the aggregate. In the event of termination of the GLP-1 license by the Company not related to clinical or technical failure, the Company is obligated to pay PEUK a termination payment of $0.5 million if such termination arises prior to completion of Phase IIb clinical trials or $1.3 million if termination occurs after completion of Phase IIb clinical trials.
PEUK will supply proprietary components for the device for clinical and commercial purposes based on stated pricing per the agreement under which the Company will pay PEUK a price equal to PEUK’s cost of the components plus a stated margin, which is subject to future negotiation. The license agreement will remain in force on a country-by-country basis until the later of (i) the expiration of the Aerogen patents covering the device on August 8, 2034 or (ii) 10 years after the first commercial sale of the product. In the case of material breach, the license may be prematurely terminated by either party by providing 90 days written notice to the breaching party and such breaching party has not cured the breach within the 90-day period.
License Agreement with Hikma Pharmaceuticals LLC (“Hikma”)
In January 2013, the Company entered into a license, supply, and manufacturing agreement with Hikma, under which the Company granted an exclusive license to register and commercialize its inhaled human insulin and device combination (“AER-501”) in all disease indications in the Middle East and Africa, excluding Turkey and certain other countries (collectively referred to as the “Hikma Territory”). Under the agreement, the Company is responsible for the global clinical development of AER-501 in any and all indications and for assembly of the registration materials submitted to the European Medicines Agency (the “EMA”) or U.S. Food and Drug Administration (the “FDA”) for approval. Hikma is responsible for registration and commercialization in the Hikma Territory. Additionally, the Company granted Hikma the right of first refusal to license new inhaled insulin products in the Hikma Territory as well as the Palestinian Territories and Turkey should such regions become available for inclusion in the agreement.
Under the terms of the agreement, the Company received a non-refundable upfront license fee of $0.5 million in January 2013 and a non-refundable milestone of $0.3 million in January 2014 upon achievement of a clinical milestone. The Company is also entitled to receive up to $2.0 million in potential future milestone payments if certain commercial and sales-based events are successfully achieved. The terms of the agreement also provide for Hikma to pay the Company a percent of net sales of the licensed product in the Hikma Territory.
The agreement has a 15-year term from the date of the launch of AER-501 in one of the countries in the Hikma Territory and will automatically renew for an additional 12-month term prior to the expiration of the then-current term. The agreement may be terminated by either party by providing at least twelve months’ written notice prior to the expiration of the then-current term or for a material breach if such breach is not cured within two-months’ notice of such breach, insolvency of either party, or a change of control.
A timeline for development of AER-501 has been accepted by Hikma. Pursuant to the terms of the agreement with Hikma, if (i) AER-501 fails to gain approval by the FDA or EMA or (ii) AER-501 does not obtain at least one of the foregoing approvals within two years after the applicable timeframe in the agreement, Hikma will receive an additional exclusive license to one other Company product in development, of Hikma’s choosing, the terms of which will be identical to the current license excluding the upfront license fee. In addition, Hikma would also have the right to convert an amount equal to the upfront license fee to shares of the Company’s equity, on terms equal to the Company’s most recent or current private or public financing. We did not meet the agreed-upon timeframe for regulatory approval of AER-501, and, as such, Hikma is entitled to receive the additional exclusive license in the Hikma Territory to another one of our products in development and the right to convert an amount equal to the original upfront license fee under that agreement into shares of Aerami’s capital stock. As of the date hereof, Hikma has not exercised these rights, but we cannot assure you that Hikma will not exercise these rights at a future time.
| F-82 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
The Company evaluated the agreement under ASC 606 and determined that there were four promises: (i) an exclusive license to register and commercialize in the Hikma Territory; (ii) performance of global clinical development of AER-501 in any and all indications; (iii) assembly of the registration materials submitted to EMA or the FDA; and (iv) manufacture and supply of certain amounts of AER-501. The Company determined none of the promises are distinct since each is highly interrelated and dependent on the other and the license is largely symbolic and has no stand-alone functionality or value. Therefore, all promises are combined as a single performance obligation.
The Company determined that the transaction price consisted of the upfront payment of $0.5 million and the $0.3 million clinical milestone. Future potential milestones payments under the agreement were excluded from the transaction price under the sales-based royalty exception to the commercial and sales- based milestone payments. Since Hikma does not benefit from the license until commercial launch in the Hikma Territory, revenue from the initial non-refundable payment and clinical milestone will be recognized over the period beginning upon commercial launch in the Hikma Territory through the end of the license term. The timing of such a commercial launch is uncertain. The commercial and sales-based milestone payments will be recognized as the related sales occur.
Contract liabilities of $0.8 million related to consideration received for which the performance obligation has not yet been completed are included within customer advances and other liabilities as of September 30, 2021 and December 31, 2020.
Agreements with Harmony Biopharm Limited and Harmony Plus Holdings Limited
In September 2015, the Company entered into an exclusive license agreement with Harmony Biopharm Limited (“Harmony Licensee”), under which the Company granted Harmony Licensee the right to develop and commercialize AER-501 in China, India, and various other countries in Asia.
On August 24, 2017 the Company and Harmony Licensee’s parent, Harmony Plus Holdings Limited (“Harmony Plus”), entered into a Stock Purchase Agreement and Release (the “SPAR”) under which the Company reacquired the license previously granted to Harmony Licensee by acquiring 100% of the stock in Harmony Licensee for the following consideration: (i) a number of shares of the Company’s common stock equal to $8.0 million issued upon the earlier of an initial public offering (“IPO”) and/or change of control, as defined in the SPAR; (ii) forgiveness of repayment of the $0.3 million that was owed to the Company under a previous agreement with Harmony Plus; and (iii) royalties payable equal to 7.5% of gross profits from the sale of products in China for the period ending on the earlier of (i) the 10th anniversary of the SPAR and (ii) a Royalty Termination Event.
A Royalty Termination Event would occur if the Company or any other person at any time prior to the fifth anniversary of the Company’s IPO purchases at least the lesser of: a) one-half of the 2,299,844 shares, or 1,149,922 shares, owned by certain investors that are affiliated with Harmony Plus (the “Harmony Investors”) or b) 2,299,844 shares less any shares sold/transferred by the Harmony Investors after August 24, 2017 at a price per share of at least the greater of (1) $5.00 per share or (2) 90% of the fair value of a share (as defined in the SPAR), if such purchase occurs before an IPO or change of control (as defined in the SPAR); or the greater of (1) $10.00 per share or (2) 90% of the fair value of a share (as defined in the SPAR), if such purchase occurs after an IPO.
As part of the SPAR, the Company and Harmony Plus also entered into a consulting agreement under which Harmony Plus is to provide consulting services for a five-year term commencing in August 2017. Total compensation for the services is $1.5 million payable in annual installments of $0.3 million each through August 2022. Upon termination of the consulting agreement, the Company would be obligated to pay the remaining amounts due irrespective of whether services are provided and irrespective of which party terminates the consulting agreement. The Company therefore concluded that the amounts owed under the consulting agreement represented additional consideration under the SPAR.
| F-83 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
On August 24, 2017, the Company also entered into a Market Development Arrangement with Harmony Plus under which the Company is obligated to pay Harmony Plus a fee equal to 5% of any and all revenue received from certain qualified partners in China, India and various other countries in Asia introduced to Aerami by Harmony Plus.
The Company determined that the reacquisition of rights previously granted to Harmony Licensee did not meet the definition of a business under ASC 805 Business Combinations. The transaction was determined to be a cancellation or rescission and not a business combination, and, as such, the accounting is in accordance with the requirements of ASC 952 Franchisors, with all consideration being recognized in operating expenses.
The components of consideration associated with the above settlement and the accounting treatment are as follows:
| 1) | Common stock equal to $8.0 million to be issued upon the earlier of an IPO and/or Change of Control represents a conditional obligation that the Company must settle by issuing a variable number of its equity shares equal to a fixed monetary amount known at inception and therefore must be recognized as a liability under ASC 480-10-25-14(a). ASC 480-30-7 requires obligations that can be settled in shares with a fixed monetary value at settlement to be carried at fair value. This liability had a fair value of $0.9 million and $3.0 million at September 30, 2021 and December 31, 2020, respectively, and is recorded as the Harmony contingent settlement liability on the consolidated balance sheets. | |
| 2) | Forgiveness of repayment of $0.3 million – this receivable was written off during a prior year and there was no impact to the consolidated financial statements at the time of settlement. | |
| 3) | Consulting agreement – as of September 30, 2021 and December 31, 2020, the face value of amounts payable to Harmony Plus was $0.3 million and $0.6 million, respectively. As of September 30, 2021 and December 31, 2020, the present value of amounts payable to Harmony Plus was $0.3 million net of a $27,000 unamortized discount and $0.5 million net of a $0.1 million unamortized discount, respectively. The discount is based on an imputed interest rate of 12% and is being amortized as interest expense over the term of the consulting agreement. $0.1 million of debt discount was amortized as interest expense during the nine-month periods ended September 30, 2021 and 2020. |
The Harmony Plus payable is recorded on the consolidated balance sheets as follows (in thousands):
| September 30, 2021 | December 31, 2020 | |||||||
| Accrued expenses and other current liabilities | $ | 246 | $ | 300 | ||||
| Customer advances and other liabilities | 27 | 227 | ||||||
| Harmony Plus payable, total | $ | 273 | $ | 527 | ||||
| 4) | 7.5% royalties and 5% marketing fee – since the potential royalties and fees are not exchange- traded and the underlying is a specified volume of sales in various countries, they are eligible for the scope exception in ASC 815 and will be recorded when payments become due or payable. | |
| 5) | Concurrent with the execution of the SPAR, the Company entered into separate agreements with the individual Harmony Investors under which the Company could acquire the shares held by the Harmony Investors under terms consistent with the SPAR. This right represents an asset to the Company as it can reacquire the shares at a discount and terminate future royalty payment obligations. The underlying fair value of the right was de minimis as of September 30, 2021 and December 31, 2020. |
| F-84 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Joint Venture Agreement with Dongbao and Shanghai Dongbao
In October 2017, the Company entered into a joint venture agreement (the “Dongbao Joint Venture Agreement”) with Dongbao and Shanghai Dongbao Biological Pharmaceutical Co., Ltd. (“Shanghai Dongbao”) pursuant to which a joint venture (the “China JV”) would be formed with the purpose of (i) completing clinical trials in China for AER-501, (ii) gaining China Food and Drug Administration approval for AER-501, and (iii) commercializing AER-501 in China. The Company was to provide a capital contribution of RMB 46,170,000 for a 45% ownership interest in the China JV. All parties agreed that they would not carry out business competing with the China JV’s business or invest into or act on behalf of a third party directly or indirectly competing with the China JV’s business. However, as of the date of these consolidated financial statements, the China JV has not been formed or funded and has never commenced operations.
7. Convertible Preferred Stock and Stockholders’ Deficit
| a. | 2021 Common Stock Offering |
Pursuant to a private placement offering of its common stock commenced in September 2021 (the “2021 Common Stock Offering”), the Company sold 14,940,000 shares of common stock in the first two closings held in September 2021 with net cash proceeds of $12.8 million. In connection with the 2021 Common Stock Offering, the Company paid cash fees and reimbursement of expenses totaling approximately $2.2 million and was obligated to issue placement agent warrants to purchase shares of common stock of the Company with value equal to 10% of the common stock sold in the 2021 Common Stock Offering (5% of sales to Company affiliates) (“Common Stock Warrants”) to its placement agent, Aegis Capital Corp. (the “Placement Agent” or “Aegis”). The Common Stock Warrants will be exercisable over five years from the date of issuance with an exercise price of $1 per share of common stock. The Common Stock Warrants are liability-classified as they include settlement features that are not indexed to the Company’s common stock.
| b. | 2020 Series A Preferred Stock Financing |
From June 2020 through December 2020, the Company sold 10,168 shares of its Series A preferred stock and received net proceeds of $7.9 million (the “2020 Series A Preferred Stock Financing”).
In connection with the financing, the Company became obligated to issue placement agent warrants to purchase 964 shares of Series A preferred stock to its placement agent, Aegis (“Series A Warrants”). In January 2021, the Company sold an additional 2,282 shares of Series A preferred stock for net proceeds of $1.8 million (together with the offer and sale of shares of Series A preferred stock from June 2020 through December 2020, the “Series A Preferred Stock Financing”) and became obligated to issue an additional 228 Series A Warrants to Aegis. The Series A Warrants will have an exercise price of $1,000 per share and will be exercisable for five years from the date of issuance. The Series A Warrants are liability-classified as the underlying Series A preferred stock is contingently redeemable upon certain deemed liquidation events which are deemed to be outside of the Company’s control and will be remeasured at each reporting period until exercised, reclassified, expired, or otherwise settled. Changes in fair value of the Series A Warrant liability will be recorded within the consolidated statements of operations. The Company allocated $0.7 million of the net proceeds to the Series A Warrant liability based on the estimated fair value of the warrants. The change in fair value of the Series A Warrants was $0.5 million for the nine months ended September 30, 2021.
The terms of the Series A preferred stock provide the holders of Series A preferred stock a right of first offer to purchase securities sold in a future follow-on offering (the “Future Follow-on Right”), in such amount up to one hundred percent of the original amount of each such Series A preferred stockholder paid in the initial offering. See Note 7.e. below for details of the conversion process.
| F-85 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Concurrent with the Company’s initial offering of its Series A preferred stock, the Company entered into a side letter agreement (the “Put Option”) whereby a related party investor may, at its option, elect to put the Series A preferred stock back to the Company for $1.0 million in the event the Company did not complete specific research and development activities leading to a 510(k) regulatory submission to the FDA by June 30, 2021. As of September 30, 2021, the Company had not completed the requirements set forth in the Put Option, and the related party investor has not exercised the Put Option right.
The Company determined that the Future Follow-on Right and the Put Option did not meet the definition of freestanding financial instrument as they are not legally detachable. The Future Follow-on Right and Put Option were also evaluated as an embedded derivative, and the Company determined they did not meet the definition of a derivative instrument for which bifurcation would be required.
| c. | Common Stock Warrants and Series A Preferred Stock Warrants |
As of September 30, 2021, there were 1,492,691 warrants with a fair value of $1.0 million deemed outstanding in connection with the 2021 Common Stock Offering that entitle the holder (Aegis) to acquire shares of the Company’s common stock. These Common Stock Warrants were recognized as warrant liabilities as non-cash issuance costs (see Note 7.a.).
As of September 30, 2021 and December 31, 2020, there were 1,192 and 964 warrants outstanding, respectively to acquire shares of the Company’s Series A preferred stock which were issued in connection with the 2020 Series A Preferred Stock Financing (see Note 7.b. above) and entitle the holders to acquire shares of the Company’s Series A preferred stock. These warrants are liability-classified as the underlying Series A preferred stock is contingently redeemable and outside of the Company’s control. The warrants had a grant-date fair value of $0.8 million and warrant liabilities was recorded in the balance sheet upon issuance. As of September 30, 2021, the fair value of the warrants is $1.4 million.
The fair values of the Common Stock Warrants and the Series A Warrants at issuance were estimated using the Black-Scholes option-pricing model using the following assumptions:
| Common Stock Warrants | Series A Warrants | |||||||
| Risk-free interest rate | 0.98 | % | 0.28% - 0.45 | % | ||||
| Expected volatility | 87.00 | % | 90.00 | % | ||||
| Expected term (in years) | 5.00 | 5.00 | ||||||
| Exercise price per share (actual amount per share) | $ | 1.00 | $ | 1,000.00 | ||||
| Fair value of common stock (actual amount per share) | $ | 1.00 | $ | 1,000.00 | ||||
As of September 30, 2021, the fair values of the Common Stock Warrants and the Series A Warrants are estimated using the Black-Scholes option-pricing model using the following assumptions:
| Common Stock Warrants | Series A Warrants | |||||||
| Risk-free interest rate | 0.98 | % | 0.98 | % | ||||
| Expected volatility | 87.00 | % | 87.00 | % | ||||
| Expected term (in years) | 5.00 | 5.00 | ||||||
| Exercise price per share (actual amount per share) | $ | 1.00 | $ | 1,000.00 | ||||
| Fair value of common stock (actual amount per share) | $ | 1.00 | $ | 1,000.00 | ||||
| F-86 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
| d. | Common Stock |
Liquidation
Upon liquidation or dissolution, holders of the Company’s common stock are entitled to share ratably in all net assets available for distribution to stockholders after the Company has paid, or provided for payment of, all of its debts and liabilities, and after payment of any liquidation preferences to holders of the Royalty Series convertible preferred stock (“Royalty stock”) and other class of capital stock having preference over the common stock.
Voting
Holders of the Company’s common stock are entitled to one vote per share in the election of directors and on all other matters on which stockholders are entitled or permitted to vote. Holders of common stock are not entitled to cumulative voting rights.
Shares of common stock reserved for future issuance are shown below (in thousands):
| September 30, 2021 | December 31, 2020 | |||||||
| Common stock warrants | 2,486 | 993 | ||||||
| Series A preferred stock warrants | 230 | 964 | ||||||
| Series A-1 preferred stock warrants | 1,924 | - | ||||||
| Series A preferred stock | 2,810 | 10,168 | ||||||
| Series A-1 preferred stock | 19,280 | - | ||||||
| Royalty stock | 2,541 | 2,541 | ||||||
| Stock options issued and outstanding | 12,604 | 10,259 | ||||||
| Unvested restricted stock units | 292 | 449 | ||||||
| Future equity awards | 2,288 | 2,612 | ||||||
| Total | 44,455 | 27,986 | ||||||
| e. | Series A Preferred Stock |
Conversion
Each share of Series A preferred stock and Series A-1 preferred stock (“Series A-1 preferred stock” and collectively with the Series A preferred stock, “Series A preferred stock”) may be converted at the option of the holder thereof into shares of common stock at any time and from time to time at the then-applicable conversion rate described below. Upon either (i) an IPO of the Company’s common stock in which the Company receives at least $10 million in gross proceeds (or a lesser amount if holders of a majority of the Series A preferred stock (voting on an as-converted basis) approve such lesser amount) and shares of the Company’s common stock are listed on Nasdaq Stock Market (“Nasdaq”), the New York Stock Exchange (the “NYSE”) or other exchange approved by the Board or (ii) a public or private capital raising transaction in which the Company receives gross proceeds of at least $10 million (or a lesser amount if holders of a majority of the shares of Series A preferred stock approve such lesser amount) that is concurrent with a merger of the Company with a public company with the resulting shares being listed on Nasdaq, the NYSE or other exchange or marketplace approved by the Board (each, a “QIPO”), each share of Series A preferred stock will automatically be converted into shares of common stock at the then-applicable conversion rate described below, following which the holder will cease to be entitled to any liquidation preferences.
| F-87 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Conversion Rate
If a holder of Series A preferred stock voluntarily converts its shares of Series A preferred stock into common stock prior to a QIPO, the number of shares of common stock into which each share of Series A preferred stock will be converted is determined by dividing the Stated Value of $1,000 plus declared and unpaid dividends thereon by $1.00 (as adjusted for stock splits, stock dividends, combinations, and similar events with respect to the common stock and certain fundamental events). If the shares of Series A preferred stock are automatically converted into shares of common stock in connection with a QIPO, the number of shares of common stock into which each share of Series A preferred stock will be converted is determined by dividing the Stated Value of $1,000 plus declared and unpaid dividends thereon by the lesser of $1.00 (as adjusted for stock splits, stock dividends, combinations and similar events with respect to the common stock and certain fundamental events) or 75% of either the per share closing sale price of the common stock to the public or the per share price of the shares sold in the capital raising transaction that is concurrent with the merger of the Company with a public company, as applicable. The conversion rate for the Series A-1 preferred stock that is issued to holders of Series A preferred stock that invest in a Follow-On Offering as described below will be 50% of the applicable conversion rate of the Series A preferred stock.
If a holder of Series A preferred stock participates in the Company’s next follow-on financing in which the Company raises gross proceeds of at least $10 million in a private placement (subject to customary exclusions) or $10 million (or such lesser amount as is approved by holders of a majority of the shares of Series A Stock) in the QIPO (the “Follow-On Offering”), some or all (depending on the amount the holder invests in the Follow-On Offering) of the holder’s shares of Series A preferred stock will automatically convert into Series A-1 preferred stock at a 1:1 ratio. If a holder of Series A preferred stock invests the same amount or more in the Follow-On Offering that the holder invested in, all of such holder’s shares of Series A preferred stock will automatically convert into shares of Series A-1 preferred stock upon the closing of the Follow-On Offering (or immediately prior to the QIPO if the Follow-On Offering is the QIPO). If a holder of Series A preferred stock invests in the Follow-On Offering in an amount less than such holder’s investment amount in this offering, a portion of the holder’s Series A preferred stock will convert into Series A-1 preferred stock determined by multiplying the number of shares of Series A preferred stock held by the holder prior to the Follow-On Offering by a fraction equal to the amount the holder invested in the Follow-On Offering divided by the amount the holder invested in the Series A Preferred Stock Financing.
As of September 30, 2021, 9,640 shares of Series A preferred stock were converted into Series A-1 preferred stock following the occurrence of the trigger event (sale of shares of common stock with gross proceeds of at least $10 million).
Liquidation
In the event of liquidation of the Company or a merger or consolidation of the Company or the sale of all or substantially all of its assets on a consolidated basis, the holders of Series A preferred stock will be entitled to receive in priority to the holders of common stock and Royalty stock an amount equal to $1,000 per share of Series A preferred stock plus declared and unpaid dividends plus amounts that holders of Series A preferred stock would have been entitled to receive had all preferences of the Series A preferred stock, Royalty stock and any other series of preferred stock designated from time to time been paid in full and all holders of Series A preferred stock, Royalty stock, common stock and other designated series of preferred stock participated in the distribution of residual proceeds on a pro rata as-converted basis (the “Preferential Amount”). After the payment of the Preferential Amount to the holders of Series A preferred stock, the Series A preferred stock will not participate in the distribution of any remaining amounts.
Voting
Holders of Series A preferred stock will vote together with the common stock and Royalty stock on an as-converted basis, and not as a separate class, except as described below.
| F-88 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Holders of Series A preferred stock will vote together as a single class on an as-converted basis with respect to:
| (i) | amending, altering, or repealing any provision of the Series A preferred stock Certificate of Designations in a manner that adversely affects the powers, preferences, or rights of the Series A preferred stock. | |
| (ii) | the liquidation, dissolution or winding up of the Company, or effecting any merger or consolidation of the Company. | |
| (iii) | creating any additional class or series of capital stock that is senior to the Series A preferred stock with respect to liquidation preferences, dividends or redemption or increase the authorized shares of Series A preferred stock or of any additional class of capital stock of the Company unless they rank junior or on parity with the Series A preferred stock. | |
| (iv) | reclassifying any capital stock that is on parity with the Series A preferred stock with respect to liquidation preferences, dividends or redemption into a senior class or series of securities or reclassifying any capital stock that is junior to the Series A preferred stock with respect to liquidation preferences, dividends or redemption into a parity or senior class or series of securities. | |
| (v) | making or paying redemptions, dividends, or other distributions on any shares of capital stock other than those authorized under the Series A preferred stock Certificate of Designations or the Certificate of Designations of the Royalty stock or on any other series of preferred stock designated from time to time that ranks on parity with or senior to the Series A preferred stock and repurchases of stock issued as equity compensation or as approved by the Board of Directors of the Company; or | |
| (vi) | issuing Series A-1 preferred stock other than upon conversion of the Series A preferred stock as described above. |
The holders of Royalty stock are entitled, in the aggregate, for each calendar year, a royalty payment in an amount equal to the lesser of: (1) $50 million or (2) (i) 3% of net sales plus (ii) 5% of licensing proceeds commencing on the date following the last day of the fiscal quarter as of which the Company’s net sales and licensing proceeds for four consecutive quarters is equal to at least $50 million and ending on the tenth anniversary of such date or earlier in the case of an acquisition (“Royalty Period”).
The rights to royalty payments will terminate upon the earliest of (i) the occurrence of an acquisition, so long as the holders of Royalty stock have received, in the aggregate, an amount equal to at least $50.00 per share of Royalty stock held by them, (ii) with respect to a specific holder, the conversion by such holder of Royalty stock into shares of the Company’s common stock and (iii) the expiration of the Royalty Period. The rights to royalty payments are unsecured obligations of the Company.
Conversion
Royalty stock is convertible into the Company’s common stock at the option of the holder at any time after the one-year anniversary of the date on which the Company becomes a reporting company under the Exchange Act. If at any time while the Royalty stock is outstanding, the Company effects any merger with or into another entity, the Company effects any sale of all or substantially all of its assets in one transaction or a series of related transactions, or the Company effects any reclassification of the common stock pursuant to which the common stock is effectively converted into or exchanged for other securities, cash or property (collectively, “Fundamental Transaction”), then, upon any subsequent conversion of the Royalty stock, the holders have the right to receive, for each share of Royalty stock that would have been issuable upon such conversion immediately prior to the occurrence of such Fundamental Transaction, the same kind and amount of securities, cash or property as it would have been entitled to receive upon the occurrence of such Fundamental Transaction if it had been, immediately prior to such Fundamental Transaction, the holder of one share of common stock.
| F-89 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Liquidation
In the event of liquidation of the Company, royalty payments are due and to be paid prior to any other payments to holders of Royalty stock or common stock. Any remaining amounts would be allocated to the holders of the Royalty stock and common stock on a pro-rata basis.
Voting
The holders of Royalty stock vote together with the common stock on an as-converted basis, and not as a separate class, except that holders of Royalty stock vote as a separate class with respect to amending, altering, or repealing any provision of the certificate of designation in a manner that adversely affects the powers, preferences, or rights of the Royalty stock.
As the holders of the of Royalty stock do not control the board of directors or stockholder vote and, therefore, do not have the ability to control a liquidation of the Company or a Fundamental Transaction that could result in a redemption of the Royalty stock, the Royalty stock is classified as equity.
| f. | Warrants |
The following table summarizes common stock warrant activity for the nine months ended September 30, 2021:
| Underlying Shares | Weighted Average Exercise Price (US$) | Weighted Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands | |||||||||||||
| Outstanding at December 31, 2020 | 992,992 | $ | 4.95 | 1.20 | - | |||||||||||
| Issued (Note 7.a.) | 1,493,000 | $ | 1.00 | 5.00 | - | |||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Forfeited or Expired | - | - | - | - | ||||||||||||
| Outstanding at September 30, 2021 | 2,485,992 | |||||||||||||||
| 8. | Stock-Based Awards |
Total stock-based compensation expense recognized as follows (in thousands):
| Nine Months Ended September 30, | ||||||||
| 2021 | 2020 | |||||||
| General and administrative | $ | 1,361 | $ | 1,429 | ||||
| Research and development | 125 | 409 | ||||||
| Total stock-based compensation expense | $ | 1,486 | $ | 1,838 | ||||
As of September 30, 2021, there was $1.0 million of unrecognized compensation cost related to unvested awards with time-based vesting which will be recognized over a period of 3.43 years.
| F-90 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
The 2009 and 2015 Equity Incentive Plans
In 2009, the Company adopted the 2009 Equity Incentive Plan (the “2009 Plan”) under which ten million shares of common stock were reserved for issuance to employees, non-employee directors and consultants of the Company. The 2009 Plan expired in September 2015, and no further awards will be made under that plan. The Company adopted the 2015 Equity Incentive Plan (the “2015 Plan” and collectively with the 2009 Plan, the “Plans”) under which, as amended, 12,848,069 shares of common stock were reserved for issuance to eligible employees, officers, non-employee directors and other individual service providers of the Company. The Plans permit the grant of nonqualified stock options, incentive stock options, stock appreciation rights, restricted stock, stock units, performance shares, performance units, incentive bonus awards, other cash-based awards, and other stock-based awards to eligible recipients. Recipients of stock options are eligible to purchase shares of the Company’s common stock at an exercise price equal to no less than the estimated fair market value of such stock on the date of grant. The maximum term of options granted under the 2015 Plan is ten years. Options granted under the Plans have a variety of vesting schedules, however, they generally vest 25% on the grant date, 25% on the one-year anniversary of the grant date, and the remaining 50% vest in equal monthly installments on the next twenty-four monthly anniversary dates thereafter. Awards granted under the Plans were either non-statutory stock options or restricted stock units (“RSUs”). RSUs convert into shares of common stock upon vesting on a one-for-one basis. RSU vesting only occurs upon an initial public offering or a change in control. No stock-based compensation expense has been recorded in relation to these RSUs as the events were not deemed probable as of September 30, 2021 or 2020. As of September 30, 2021, the Company had approximately $0.7 million unrecognized compensation cost related to unvested RSUs.
Under the 2015 Plan, the number of shares available for issuance automatically increases annually for a period of 10 years by an amount equal to 6% of the total number of shares of common stock outstanding on December 31 of the previous year. As of September 30, 2021, there were 2,288,024 shares available for future grants.
The Whitaker Plan
In October 2018, the Company adopted an equity incentive plan (the “Whitaker Plan”) under which 2,173,107 shares of common stock were reserved for issuance to its newly hired Chief Executive Officer. The terms of options granted under the Whitaker Plan are identical to those granted under the 2015 Plan. 2,173,107 options with a weighted average grant-date fair value of $3.8 million were granted on December 7, 2018 and vest 33% on October 1, 2019 with the remaining 67% vesting in 24 equal monthly installments thereafter. The options have an exercise price of $2.78 and are exercisable for 10 years from the date of issuance.
| F-91 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
A summary of stock option activity for all outstanding plans for the nine months ended September 30, 2021, is as follows:
| Underlying Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value (in thousands) | |||||||||||||
| Outstanding at December 31, 2020 | 10,258,786 | $ | 2.61 | 6.84 years | $ | - | ||||||||||
| Granted | 3,425,932 | $ | 0.35 | |||||||||||||
| Expired | (966,240 | ) | $ | 3.94 | ||||||||||||
| Forfeited | (114,169 | ) | $ | 2.59 | ||||||||||||
| Outstanding at September 30, 2021 | 12,604,039 | $ | 1.89 | 7.70 years | $ | - | ||||||||||
| Exercisable at September 30, 2021 | 8,185,942 | $ | 2.70 | 6.78 years | $ | - | ||||||||||
| Vested and expected to vest at September 30, 2021 | 12,604,039 | $ | 1.89 | 7.70 years | $ | - | ||||||||||
A summary of RSU activity for all outstanding Plans for the nine months ended September 30, 2021, is as follows:
| Weighted | ||||||||
| Average | ||||||||
| Restricted | Grant-Date | |||||||
| Stock Units | Fair Value | |||||||
| Outstanding at December 31, 2020 | 448,648 | $ | 2.75 | |||||
| Forfeited | (156,705 | ) | $ | 3.21 | ||||
| Outstanding at September 30, 2021 | 291,943 | $ | 2.50 | |||||
| 9. | Income Taxes |
The reconciliation of the federal statutory income tax rate to the effective income tax rate is as follows:
| Nine Months Ended September 30, | ||||||||
| 2021 | 2020 | |||||||
| Statutory Federal income tax rate | 21.0 | % | 21.0 | % | ||||
| Permanent items | (6.6 | )% | (0.6 | )% | ||||
| Tax credits | 1.2 | % | 1.1 | % | ||||
| Other | (0.3 | )% | (0.3 | )% | ||||
| Valuation allowance | (15.3 | )% | (21.3 | )% | ||||
| Net | 0.0 | % | 0.1 | % | ||||
| F-92 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
Deferred income taxes reflect the net effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets as of September 30, 2021 and December 31, 2020, are shown below (in thousands):
| September 30, 2021 | December 31, 2020 | |||||||
| Net operating loss carryforwards | $ | 19,997 | $ | 19,076 | ||||
| Research & development tax credits | 2,368 | 2,268 | ||||||
| Capitalized start-up costs | 469 | 519 | ||||||
| Stock-based compensation | 3,404 | 3,064 | ||||||
| Other | 289 | 179 | ||||||
| Total gross deferred income tax assets | 26,527 | 25,106 | ||||||
| Less: Valuation allowance | (26,527 | ) | (25,106 | ) | ||||
| Net deferred income tax assets | $ | - | $ | - | ||||
Valuation allowances require an assessment of both positive and negative evidence when determining whether it is more likely than not that deferred tax assets are recoverable. Such assessment is required on a jurisdiction-by-jurisdiction basis. Based on the available objective evidence, management believes it is more likely than not the U.S. and state deferred tax assets are not realizable as of September 30, 2021 and December 31, 2020. Accordingly, the Company has established a full valuation allowance against its deferred tax assets. For the nine months ended September 30, 2021 and 2020, the valuation allowance increased $1.4 million and $2.1 million, respectively.
Pursuant to the Internal Revenue Code (the “IRC”) Sections 382 and 383, annual use of the Company’s net operating loss and research and development credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. The Company has reviewed the impact of the IRC Sections 382 and 383 on its net operating loss and credit carryforwards and has concluded that the Company has experienced an ownership change that will result in an annual limit on the amount of allowed net operating losses and credit carryforwards. While the Company believes an ownership change has occurred, it has not yet completed its analysis to measure the extent of any potential limitation. The Company plans to finalize this analysis as the likelihood of tax attribute utilization becomes probable.
As of September 30, 2021, the Company had approximately $87.0 million and $87.4 million of Federal and state net operating losses, respectively. These losses will begin to expire in 2029.
As of September 30, 2021, the Company had Federal and state research and development credits of $2.0 million and $1.5 million, respectively. The Federal research and development credit carryovers will begin to expire in 2031, if not utilized, while the state research and development credit carryovers predominately have no expiration date.
As of September 30, 2021 and December 31, 2020, the Company had approximately $0.9 and $0.8 million of unrecognized tax benefits, respectively, all of which, if recognized, would not affect the effective tax rate.
A reconciliation of the unrecognized tax benefits during the nine months ended September 30, 2021, is as follows (in thousands):
| Balance at December 31, 2020 | $ | 832 | ||
| Gross increase for tax positions of current year | 43 | |||
| Balance at September 30, 2021 | $ | 875 |
There are no liabilities from unrecognized tax benefits included in the Company’s consolidated balance sheets as of September 30, 2021 and December 31, 2020, and therefore the Company has not incurred any penalties or interest.
| F-93 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
The Company files tax returns as prescribed by the tax laws of the jurisdictions in which it operates. In the normal course of business, the Company is subject to examination by the Federal and state jurisdictions where applicable. There are currently no pending income tax examinations. The Company’s tax years for 2009 and forward are subject to review by the Federal and state tax authorities due to the carryforward of unutilized net operating losses and research and development credits.
| 10. | Commitments and Contingencies |
Registration Rights Penalty
On November 30, 2017, the Company entered into a new registration rights agreement (the “2017 RRA”) with certain investors. Pursuant to the terms of the 2017 RRA, the Company was obligated to prepare and file a Registration Statement on Form S-1 with the SEC no later than 180 days following the last closing of the Company’s private placement offering in 2018 (the “Registration Filing Deadline”). If the Company failed to file a Registration Statement prior to the Registration Filing Deadline, the Company would be subject to penalties of up to 6% of the proceeds received in the Company’s private placement offerings held from 2015 to 2018, plus interest of 2% per year following the Registration Filing Deadline. As of December 31, 2018, the Company expected to incur the maximum penalty and accrued $2.6 million related to the 2017 RRA. Interest of less than $0.1 million was accrued during each of the nine months ended September 30, 2021 and 2020. Accruals for penalty and related interest of $2.7 million are presented as part of accrued expenses and other current liabilities as of September 30, 2021 and December 31, 2020.
Operating Leases
In January 2019, the Company entered into a lease for office space in Union City, California. In August 2019, the Company amended the lease to reduce the number of square feet. During the year ended 2019, the Company subleased a portion of this space to a related party on a month-to-month basis. The Company ceased using the space in March 2020 and accelerated the amortization of the related right-of-use asset from the lease termination date, November 30, 2020, to the abandonment date, February 29, 2020.
In May 2019, the Company entered into a lease for space to serve as its headquarters in Durham, North Carolina. The lease agreement expired in November 2020 after several three-month automatic extensions. In December 2020, the Company renewed the lease for a three-month period and is accounting for the renewal as a short-term lease.
Employee Benefit Plans
In April 2019, the Company began sponsoring a defined contribution savings and investment plan (the “Plan”) as allowed under Section 401(k) of the IRC. Employees become eligible for participation upon the start of employment and may elect to have a portion of their salary deferred and contributed to the Plan up to the limit allowed under the IRC. The Company makes a matching contribution to the Plan for each participant who elected to make tax-deferred contributions for the Plan year. These contributions vest immediately. During each of the nine months ended September 30, 2021 and 2020, the Company contributed $0.1 million to the Plan per year.
Legal Proceedings
The Company is not a party to any material litigation and does not have contingency reserves established for any litigation liabilities. At each reporting date, the Company evaluates whether a potential loss amount or a potential range of loss is probable and reasonably estimable under the provisions of the authoritative guidance that addresses accounting for contingencies. The Company expenses as incurred the costs related to such legal proceedings.
| F-94 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
| 11. | Related Parties |
In August 2019, the Company entered into a separation agreement with Dr. John S. Patton, a board member and former Chief Executive Officer of the Company, pursuant to which the Company was obligated to pay severance of $0.7 million in equal semi-monthly installments through August 2020. If the Company closes $15 million in financing proceeds prior to August 2020, all unpaid amounts would be paid within 30 days of closing. In connection with the separation, Dr. Patton’s outstanding equity awards were immediately vested. This modification to terms did not result in any incremental stock- based compensation expense. Upon the treatment of the first patient in a Phase 3 clinical trial of AER-501, Dr. Patton will be granted an option to purchase shares of the Company’s common stock equal to 1% of the fully diluted capital stock of the Company at the time of grant, provided that he remains a service provider to the Company as of the date of the grant. In addition, in the event the Company receives final approval from the FDA, EMA, or CFDA for AER-501, Dr. Patton will be granted an option to purchase shares of the Company’s common stock equal to 1% of the fully diluted capital stock of the Company at the time of grant, provided he remains a service provider to the Company as of the date of the grant. Following the separation, the Company entered into a consulting agreement with Dr. Patton, under which the Company paid less than $0.1 million during each of the nine months ended September 30, 2021 and 2020.
iPharma Ltd., an inhalation development CRO which was cofounded by Dr. Patton and of which he is a shareholder, subleased lab space from the Company and paid the Company $0.1 million during the year ended December 31, 2019. During the nine months ended September 30, 2020, iPharma Ltd. purchased $8,000 of equipment from the Company in connection with the closure of its California facility. No purchases were made during the nine months ended September 30, 2021.
Dr. Patton’s son was employed by the Company through March 31, 2020. The son of Anne Whitaker, the Company’s former Chief Executive Officer and current Chairman of the Board of Directors, is employed by the Company.
Molex Ventures, LLC (“Molex”), a stockholder of the Company and who appointed a director to the Company’s Board of Directors, is the parent company of Phillips, with whom the Company has a joint development agreement. The Company paid Phillips $7,000 and $0.1 million during the nine-month periods ended September 30, 2021 and 2020, respectively, for device development and manufacturing services.
A member of the Company’s Board of Directors and stockholder is also the head of Aegis’ private equity banking. Refer to the transactions in Note 7 with Aegis. For the nine months ended September 30, 2021, the Company paid approximately $1.9 million cash in issuance fees to Aegis and recognized a non-cash issuance fee of 1,493,000 common stock warrants with a fair value of approximately $1.0 million. Aegis also served as the Company’s placement agent for the 2020 Series A Preferred Stock Financing. For the nine-month periods ended September 30, 2021 and 2020, the Company paid Aegis $0.3 million and $1.1 million, respectively, in cash issuance costs and granted 228 and 964 placement agent warrants, respectively, to purchase Series A preferred stock in connection with the issuance of the Series A preferred stock (see Note 7).
Ms. Whitaker served as a director on Vectura Limited’s Board of Directors through September 2020.
| 12. | Subsequent Events |
The Company has completed an evaluation of all subsequent events through January 4, 2022, the date the consolidated financial statements were available to be issued, to ensure that these financial statements include appropriate disclosure of events both recognized in the September 30, 2021 consolidated financial statements and events which have occurred but were not recognized in the consolidated financial statements. Except as described below, the Company is not aware of any additional subsequent events that have occurred that require disclosure.
| F-95 |
Aerami Therapeutics Holdings, Inc. and Subsidiaries
NOTES TO THE UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
| a. | Proposed Business Combination with FoxWayne Enterprises Acquisition Corp. |
On December 7, 2021, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with FoxWayne Enterprises Acquisition Corp., a special purpose acquisition company (the “SPAC’), Gotham Merger Sub, Inc. (“Merger Sub”, a wholly owned subsidiary of the SPAC) and a stockholders’ representative of the Company (the Stockholders’ Representative”), pursuant to which, among other things, the parties will consummate a business combination whereby Merger Sub will be merged with and into the Company with the Company surviving the Merger as a wholly owned subsidiary of the SPAC (the “Proposed Transaction”). In connection with the Proposed Transaction, with certain exceptions, the outstanding and issued shares of the Company’s common stock, Series A preferred stock, Series A-1 preferred stock, and Royalty stock (collectively, the “Company Capital Stock”) will be converted into the right to receive shares of the SPAC’s common stock. Also, except for the placement agent warrants as discussed below, all warrants to acquire the Company’s Capital Stock prior to the effective date of the Proposed Transaction will be assumed by the SPAC and will be exercisable for such number of whole shares of the SPAC’s common stock at a per share exercise price as set forth in the Merger Agreement. The placement agent warrants issued in connection with the Series A Preferred Stock Financing and 2021 Common Stock Offering will be automatically exercised on a cashless basis immediately prior to the completion of the Proposed Transaction.
| b. | Additional Closings for 2021 Common Stock Offering |
In connection with the 2021 Common Stock Offering as discussed in Note 7, the Company held four additional closings between October 1, 2021 to November 3, 2021 and sold a total of 7,310,560 shares of common stock with net proceeds of $6.4 million during that period.
| c. | Warrant Issuances |
Pursuant to Placement Agency Agreements with Aegis dated March 23, 2020 with respect to the 2020 Series A Offering and dated September 2, 2021 with respect to the 2021 Common Stock Offering as discussed in Note 7 on November 22, 2021, the Company issued to Aegis Common Stock Warrants to purchase 2,223,056 shares of the Company’s common stock (including 1,493,000 warrants granted to Aegis for the common shares sold in the 1st and 2nd Closings in September 2021) and Series A preferred stock warrants to purchase 230 shares of the Company’s Series A preferred stock and 962 of the Company’s Series A-1 preferred stock.
| d. | Conversion of Series A Preferred Stock to Series A-1 Preferred Stock |
As discussed in Note 7.b. with respect to the Follow-on Offering, since the trigger event (sale of shares of common stock with gross proceeds of at least $10 million) occurred as of September 30, 2021, 9,640 and 820 shares of Series A preferred stock were converted automatically into Series A-1 preferred stock as of September 30, 2021 and subsequently through November 4, 2021, respectively. As of November 4, 2021, 10,460 shares of Series A preferred stock had been converted into Series A-1 preferred stock, reflecting the investment of holders of Series A Preferred Stock in the 2021 Common Stock Offering, reducing the balance of outstanding issued Series A Preferred Stock to 1,990 shares.
| F-96 |
Annex A
AGREEMENT AND PLAN OF MERGER
by and among
FOXWAYNE ENTERPRISES ACQUISITION CORP.,
GOTHAM MERGER SUB, INC.,
AERAMI THERAPEUTICS HOLDINGS, INC.
and
STEVE THORNTON, solely in his capacity as the Stockholders’ Representative
Dated as of December 7, 2021
| -273- |
TABLE OF CONTENTS
| Page | ||
| ARTICLE I. THE MERGER | 278 | |
| 1.1 | The Merger | 278 |
| 1.2 | Effective Time | 279 |
| 1.3 | Organizational Documents | 279 |
| 1.4 | Officers and Directors of Parent and the Surviving Corporation | 279 |
| 1.5 | Subsequent Actions | 280 |
| 1.6 | Treatment of Company Capital Shares | 280 |
| 1.7 | Company Dissenting Shares | 282 |
| 1.8 | Treatment of Merger Sub Shares. | 283 |
| 1.9 | Exchange Procedures | 283 |
| 1.10 | Withholding | 285 |
| 1.11 | Equitable Adjustments | 285 |
| ARTICLE II. CLOSING | 286 | |
| 2.1. | Closing and Closing Deliverables | 286 |
| 2.2. | Closing Statement | 286 |
| 2.3. | Pre-Closing Deliveries | 286 |
| ARTICLE III. REPRESENTATIONS AND WARRANTIES OF THE COMPANY | 287 | |
| 3.1. | Organization, Existence and Power | 287 |
| 3.2. | Authorization | 288 |
| 3.3. | Governmental Consents and Filings | 288 |
| 3.4. | No Conflict or Violation | 288 |
| 3.5. | Capitalization | 289 |
| 3.6. | Litigation | 290 |
| 3.7. | Intellectual Property | 290 |
| 3.8. | Agreements; Actions | 294 |
| 3.9. | Certain Transactions | 295 |
| 3.10. | Property | 296 |
| 3.11. | Financial Statements | 296 |
| 3.12. | Undisclosed Liabilities | 296 |
| 3.13. | Absence of Changes | 296 |
| 3.14. | Employee Benefits Matters | 297 |
| 3.15. | Labor Matters. | 298 |
| 3.16. | Tax Matters | 299 |
| 3.17. | Insurance | 301 |
| 3.18. | Compliance with Laws; Permits | 301 |
| 3.19. | Environmental and Safety Laws | 301 |
| 3.20. | FDA Regulation | 302 |
| 3.21. | Foreign Corrupt Practices Act | 303 |
| 3.22. | Data Privacy | 303 |
| 3.23. | Takeover Statutes | 303 |
| 3.24. | No Brokers | 304 |
| 3.25. | Information Supplied. | 304 |
| 3.26. | No Additional Representations or Warranties. | 304 |
| -274- |
| ARTICLE IV. REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB | 304 | |
| 4.1. | Organization, Existence and Power | 305 |
| 4.2. | Authorization | 305 |
| 4.3. | Governmental Consents and Filings | 306 |
| 4.4. | No Conflict or Violation | 306 |
| 4.5. | No Prior Merger Sub Operations | 306 |
| 4.6. | Capitalization | 306 |
| 4.7. | Litigation | 307 |
| 4.8. | [Reserved]. | 307 |
| 4.9. | Internal Controls; Listing; Financial Statements. | 307 |
| 4.10. | Investment Company Act; JOBS Act. | 308 |
| 4.11. | Business Activities; Liabilities | 308 |
| 4.12. | Certain Transactions | 309 |
| 4.13. | Property | 309 |
| 4.14. | Undisclosed Liabilities. | 309 |
| 4.15. | Absence of Changes. | 309 |
| 4.16 | Employee Matters; Benefits. | 309 |
| 4.17 | Tax Matters | 310 |
| 4.18 | Compliance with Laws; Permits | 311 |
| 4.19. | Foreign Corrupt Practices Act | 311 |
| 4.20. | SEC Filings. | 312 |
| 4.21. | Trust Account. | 312 |
| 4.22. | Nasdaq Listing. | 312 |
| 4.23. | [Reserved]. | 313 |
| 4.24. | Valid Issuance. | 313 |
| 4.25. | Takeover Statutes and Charter Provisions. | 313 |
| 4.26. | No Brokers | 313 |
| 4.27. | Registration Statement and Proxy Statement. | 313 |
| 4.28. | No Additional Representations or Warranties. | 313 |
| ARTICLE V. COVENANTS OF THE COMPANY | 314 | |
| 5.1. | Interim Operations | 314 |
| 5.2. | Access and Information | 316 |
| 5.3. | No Claim Against the Trust Account. | 316 |
| 5.4. | Exclusivity. | 317 |
| 5.5. | Registration Statement and Proxy Statement Filing; Information Supplied. | 317 |
| 5.6. | Amendments to Third Party Contracts. | 318 |
| ARTICLE VI. COVENANTS OF PARENT | 318 | |
| 6.1. | Interim Operations. | 318 |
| 6.2. | Trust Account. | 319 |
| 6.3. | Access and Information. | 319 |
| 6.4. | Exclusivity. | 320 |
| 6.5. | Indemnification; Directors’ and Officers’ Insurance. | 320 |
| 6.6. | Parent Nasdaq Listing. | 321 |
| 6.7. | Parent Public Filings. | 321 |
| 6.8. | Private Placements. | 321 |
| 6.9. | Post-Closing Board of Directors and Officers of Parent. | 322 |
| 6.10. | Stockholder Litigation. | 322 |
| 6.11. | Extension of SPAC. | 322 |
| -275- |
| ARTICLE VII. JOINT COVENANTS | 322 | |
| 7.1. | Preparation of Registration Statement. | 322 |
| 7.2. | Parent Special Meeting. | 323 |
| 7.3. | Company Requisite Stockholder Consent. | 324 |
| 7.4. | Cooperation; Efforts to Consummate. | 324 |
| 7.5. | Status; Notifications. | 325 |
| 7.6. | Publicity. | 325 |
| 7.7. | Section 16 Matters. | 325 |
| 7.8. | Tax Matters. | 325 |
| 7.9. | Parent Equity Incentive Plan. | 326 |
| 7.10. | Registration Rights Agreement. | 326 |
| 7.11. | 280G Matters | 326 |
| 7.12. | Further Assurances | 327 |
| ARTICLE VIII. CONDITIONS TO CLOSING | 327 | |
| 8.1. | Mutual Conditions to Obligations of Each Party | 327 |
| 8.2. | Conditions to Obligations of the Company | 327 |
| 8.3. | Conditions to Obligations of Parent and Merger Sub | 328 |
| ARTICLE IX. TERMINATION | 329 | |
| 9.1. | Termination | 329 |
| 9.2. | Effect of Termination | 330 |
| 9.3. | Fees and Expenses | 331 |
| ARTICLE X. MISCELLANEOUS | 331 | |
| 10.1. | Defined Terms | 331 |
| 10.2. | Notices | 341 |
| 10.3. | Rules of Construction | 341 |
| 10.4. | References | 342 |
| 10.5. | Entire Agreement | 342 |
| 10.6. | Assignment | 342 |
| 10.7. | Amendment; Modification | 343 |
| 10.8. | Rights of Third Parties. | 343 |
| 10.9. | Waiver | 343 |
| 10.10. | Severability | 343 |
| 10.11. | Burden and Benefit | 343 |
| 10.12. | Governing Law | 343 |
| 10.13. | Consent to Jurisdiction | 343 |
| 10.14. | Waiver of Trial by Jury | 344 |
| 10.15. | No Survival of Representations, Warranties and Covenants. | 344 |
| 10.16. | Specific Performance | 344 |
| 10.17. | Cumulative Remedies | 344 |
| 10.18. | Expenses | 344 |
| 10.19. | Representation by Counsel | 344 |
| 10.20. | Execution and Counterparts | 345 |
| 10.21. | Stockholders’ Representative | 345 |
| 10.22. | Company Representation | 345 |
| Exhibits | |
| Exhibit A-1 | Key Stockholders |
| Exhibit A-2 | Form of Stockholder Support Agreement |
| Exhibit B | Form of Sponsor Support Agreement |
| Exhibit C | Form of Parent Support Agreements |
| Exhibit D | Form of Registration Rights Agreement |
| Exhibit E | Form of Restated Parent Certificate of Incorporation |
| Exhibit F | Form of Restated Parent Bylaws |
| Exhibit G | Form of Parent Equity Incentive Plan |
| Exhibit H | Form of Certificate of Merger |
| Exhibit I | Form of Restated Company Bylaws |
| Schedules | |
| Company Disclosure Letter | |
| Parent Disclosure Letter | |
| -276- |
AGREEMENT AND PLAN OF MERGER
This AGREEMENT AND PLAN OF MERGER (this “Agreement”), dated as of December 7, 2021, is by and among FOXWAYNE ENTERPRISES ACQUISITION CORP., a Delaware corporation (“Parent”), GOTHAM MERGER SUB, INC., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), Aerami Therapeutics Holdings, Inc., a Delaware corporation (the “Company”), and STEVE THORNTON, solely in his capacity as the Stockholders’ Representative (the “Stockholders’ Representative”).
RECITALS
WHEREAS, Parent is a special purpose acquisition company formed to acquire one or more operating businesses through a merger, capital stock exchange, asset acquisition, stock purchase, reorganization, recapitalization, exchangeable share transaction or other similar business transaction (a “Business Combination”).
WHEREAS, Merger Sub is a newly formed, wholly owned, direct Subsidiary of Parent, and was formed for the sole purpose of consummating the transactions contemplated by this Agreement and by the Ancillary Documents (collectively, the “Transactions”).
WHEREAS, each of Parent, Merger Sub and the Company desire to effect the acquisition of the Company by Parent through the merger of Merger Sub with and into the Company (the “Merger”);
WHEREAS, the parties intend that immediately following the Merger, the Company shall be the Surviving Corporation of the Merger, all pursuant to the terms and subject to the conditions hereinafter set forth and in accordance with the General Corporation Law of the State of Delaware (the “DGCL”);
WHEREAS, the board of directors of the Company (the “Company Board”) has carefully considered the terms of this Agreement and has (i) determined that the Transactions are fair to, advisable and in the best interests of the Company and the Company Stockholders, (ii) approved and declared advisable this Agreement, the Ancillary Documents, and the Transactions, and (iii) adopted a resolution directing that the adoption of this Agreement and the Ancillary Documents be submitted to the Company Stockholders for consideration and recommending that all of the Company Stockholders adopt this Agreement and the Ancillary Documents and approve the Transactions;
WHEREAS, the board of directors of Merger Sub has carefully considered the terms of this Agreement and has (i) determined that the Transactions are fair to, advisable and in the best interests of Merger Sub and its sole stockholder, (ii) approved and declared advisable this Agreement, the Ancillary Documents, and the Transactions, and (iii) adopted a resolution directing that the adoption of this Agreement and the Ancillary Documents be submitted to Parent, as the sole stockholder of Merger Sub, for consideration and recommending that Parent adopt this Agreement and the Ancillary Documents and approve the Merger;
WHEREAS, the board of directors of Parent (the “Parent Board”) has unanimously (i) determined that the Transactions are fair to, advisable and in the best interests of Parent and its stockholders and (ii) approved and declared advisable this Agreement, the Ancillary Documents, and the Transactions, including the issuance of Parent Common Stock to the Company Stockholders pursuant to the terms of this Agreement, and (iii) adopted a resolution directing that the adoption of this Agreement and the Ancillary Documents be submitted to the Parent Stockholders for consideration and recommending that all of the Parent Stockholders adopt this Agreement and the Ancillary Documents and approve the Merger;
| -277- |
WHEREAS, contemporaneously with the execution and delivery of this Agreement, and as a condition and inducement to Parent’s and Merger Sub’s willingness to enter this Agreement, each Company Stockholder identified on Exhibit A-1 (each a “Key Stockholder”) are entering into and delivering support agreements, substantially in the form attached hereto as Exhibit A-2 (the “Stockholder Support Agreement”), pursuant to which such Key Stockholders have, subject to the terms and conditions set forth therein and among other things, agreed to vote all of their shares of Company Capital Stock in favor of the adoption of this Agreement and thereby approve the transactions contemplated hereby, including the Merger; provided that, notwithstanding the foregoing, with respect to those Key Stockholders who are not officers or directors of the Company, the Company shall exercise best efforts to obtain such Stockholder Support Agreements as soon as practicable after the date of this Agreement.
WHEREAS, contemporaneously with the execution and delivery of this Agreement, in connection with the Transactions, FoxWayne Enterprises Acquisition Sponsor LLC (the “Sponsor”) has entered into a Support Agreement, dated as of the date hereof (the “Sponsor Support Agreement”), with the Company and Parent, in the form set forth on Exhibit B, pursuant to which, among other things, the Sponsor has agreed to vote in favor of the adoption of this Agreement and thereby approve the transactions contemplated hereby, including the Merger;
WHEREAS, contemporaneously with the execution and delivery of this Agreement, in connection with the Transactions, certain other Parent Stockholders (other than the Sponsor) have entered into Support Agreements, dated as of the date hereof (collectively with the Sponsor Support Agreement, the “Parent Support Agreements”), with the Company and Parent, in the form set forth on Exhibit C, pursuant to which, among other things, such Parent Stockholders have agreed to vote in favor of the adoption of this Agreement and thereby approve the transactions contemplated hereby, including the Merger; provided that, notwithstanding the foregoing, with respect to those Key Stockholders who are not officers or directors of Parent, Parent shall exercise best efforts to obtain such Parent Support Agreements as soon as practicable after the date of this Agreement.
WHEREAS, the Sponsor and certain Insiders (as defined therein) have delivered to the Parent a certain letter agreement, dated as of January 19, 2021 (the “Lock-Up Agreement”), pursuant to which the Sponsor and each such Insider has agreed, among other things, to certain restrictions on the transfer of the shares Parent Common Stock held by each such Person;
WHEREAS, contemporaneously with the Closing, in connection with the Transactions, Parent, the Company, certain Parent Stockholders, and certain Company Stockholders will enter into that certain Registration Rights Agreement (the “Registration Rights Agreement”), in the form set forth on Exhibit D (with such modifications or amendments thereto as may be reasonably agreed by the Company to be necessary in the event of a Private Placement), to be effective upon the Closing;
WHEREAS, pursuant to Parent’s Organizational Documents, Parent shall provide an opportunity to Parent Stockholders (other than the Sponsor or any other party to any Parent Support Agreement) to have their shares of Parent Common Stock redeemed for the consideration, and on the terms and subject to the conditions and limitations, set forth in this Agreement, Parent’s Organizational Documents and the Trust Agreement in conjunction with, inter alia, obtaining approval from Parent Stockholders for the Business Combination (the “Redemption Offer”);
WHEREAS, immediately prior to the consummation of the Transactions and the effectiveness of the filing of the Parent Restated Charter (as defined below), the Company shall cause its Organizational Documents to be amended to change its name from “Aerami Therapeutics Holdings, Inc.” to “Aerami, Inc.”, including by filing a certificate of amendment to its certificate of incorporation with the Delaware Secretary of State in accordance with the DGCL (the “Name Change Amendment”);
| -278- |
WHEREAS, immediately prior to the consummation of the Transactions, Parent shall, subject to the filing and effectiveness of the Name Change Amendment and obtaining Parent Requisite Stockholder Consent, cause the amended and restated certificate of incorporation in the form set forth on Exhibit E (the “Parent Restated Charter”) to be filed with the Delaware Secretary of State, which Parent Restated Charter shall be the certificate of incorporation of Parent, until thereafter supplemented or amended in accordance with its terms and the DGCL;
WHEREAS, immediately prior to the consummation of the Transactions, Parent shall, subject to the filing and effectiveness of the Name Change Amendment and obtaining Parent Requisite Stockholder Consent, cause the bylaws in the form set forth on Exhibit F (the “Parent Restated Bylaws”) to be adopted, which shall become effective immediately upon the effectiveness of the Parent Restated Charter and shall be the bylaws of Parent, until thereafter supplemented or amended in accordance with the Parent Restated Charter, such bylaws and the DGCL;
WHEREAS, upon or shortly following the consummation of the Transactions, Parent shall, subject to obtaining Parent Requisite Stockholder Consent, adopt an equity incentive plan (the “Parent Equity Incentive Plan”), in the form set forth on Exhibit G;
WHEREAS, at the Effective Time, each of Steve Thornton, Barry Deutsch, and Timm Crowder will enter into employment agreements with the Parent on terms not less favorable than those in existence with the Company as of the Effective Date;
WHEREAS, Parent and the Company intend that, for U.S. federal income tax purposes (and for purposes of any applicable state or local income tax law that follows U.S. federal income tax treatment), (i) the Merger shall constitute a “reorganization” within the meaning of Section 368(a) of the Internal Revenue Code of 1986 (the “Code”) to which Parent and the Company are to be parties under Section 368(b) of the Code and (ii) this Agreement shall constitute a “plan of reorganization” within the meaning of Section 368 of the Code and the Treasury Regulations promulgated thereunder, and this Agreement is hereby adopted as a “plan of reorganization” within the meaning of U.S. Treasury Regulations Section 1.368-2(g) and 1.368-3(a) (the “Intended Tax Treatment”);
WHEREAS, in connection with the Closing, Parent shall be renamed “Aerami Therapeutics Holdings, Inc.” and shall trade publicly on the Nasdaq Capital Market LLC under a new ticker symbol selected by the Company with the consent of Parent (such consent not to be unreasonably withheld, conditioned, or delayed); and
WHEREAS, the parties desire to make certain representations, warranties, covenants and agreements in connection with the Merger.
AGREEMENT
NOW THEREFORE, in consideration of the respective covenants and promises contained herein and for other good and valuable consideration, the receipt and adequacy of which are hereby acknowledged, the parties hereto agree as follows:
Article I.
THE MERGER
1.1 The Merger. Pursuant to the terms and subject to the conditions of this Agreement, and in accordance with the DGCL, at the Effective Time (as defined below), (a) Merger Sub shall be merged with and into the Company; (b) the separate corporate existence of the Merger Sub shall thereupon cease, and the Company shall be the successor or surviving corporation in the Merger (sometimes hereinafter referred to as the “Surviving Corporation”); and (c) the Surviving Corporation shall become a wholly-owned Subsidiary of Parent, and the separate corporate existence of the Company with all of its rights, privileges, immunities, powers and franchises shall continue unaffected by the Merger as provided in the DGCL.
| -279- |
1.2 Effective Time. Immediately following the Closing on the Closing Date, the parties shall file a Certificate of Merger substantially in the form attached hereto as Exhibit H (the “Certificate of Merger”) with the Secretary of State of the State of Delaware in accordance with the relevant provisions of the DGCL and make any other filings, recording or publications required to be made by the Company or Merger Sub under the DGCL in connection with the Merger. The Merger shall become effective upon the filing and acceptance by the Secretary of State of the State of Delaware of the Certificate of Merger or at such later time as is agreed to by Parent and the Company and specified in the Certificate of Merger (the time at which the Merger becomes effective is herein referred to as the “Effective Time”).
1.3 Organizational Documents.
(a) Immediately prior to the filing and effectiveness of the Parent Restated Charter, the Company shall file the Name Change Amendment with the Delaware Secretary of State.
(b) Immediately prior to the Effective Time, but subject to the filing and effectiveness of the Name Change Amendment and the Parent Requisite Stockholder Consent, Parent shall take all action necessary to cause the certificate of incorporation of the Parent as then in effect to be amended and restated to read in its entirety in the form of the Parent Restated Charter, which shall be the certificate of incorporation of the Parent until thereafter amended as provided therein or by applicable Law;
(c) Immediately prior to the Effective Time, but subject to the filing and effectiveness of the Name Change Amendment and the Parent Requisite Stockholder Consent, Parent shall take all action necessary to cause the bylaws of the Parent as then in effect to be amended and restated to read in their entirety in the form of the Parent Restated Bylaws, which shall become effective immediately upon the effectiveness of the Parent Restated Charter and shall be the bylaws of the Parent until thereafter amended as provided in the Parent Restated Charter, such bylaws and applicable Law;
(d) At the Effective Time, by virtue of the Merger and without any additional action on the part of the Company or its stockholders, the certificate of incorporation of the Company as in effect immediately prior to the Effective Time shall be amended and restated to read in its entirety in the form included as Exhibit A to the Certificate of Merger attached hereto and, as so amended and restated, shall be the certificate of incorporation of the Surviving Corporation, until thereafter amended in accordance with its terms and the DGCL; and
(e) At the Effective Time, the bylaws of the Company as in effect immediately prior to the Effective Time shall be amended and restated to read in their entirety in the form of Exhibit I attached hereto and, as so amended and restated, shall be the bylaws of the Surviving Corporation, until thereafter amended in accordance with the terms of the certificate of incorporation of the Surviving Corporation, such bylaws and the DGCL.
1.4 Officers and Directors of Parent and the Surviving Corporation. Unless otherwise determined by the Company prior to the Effective Time, the officers and directors of the Company immediately prior to the Effective Time, from and after the Effective Time, shall be the officers and directors, respectively, of the Surviving Corporation, until their respective successors are duly elected or appointed and qualified. Parent shall take all action to cause the persons set forth in Section 1.4 of the Company Disclosure Letter to be appointed to the Board of Directors of Parent effective as of immediately following the Effective Time, such that, as of such time, such persons shall be the only directors of Parent (the “Post-Closing Board of Directors”). Each person appointed as a director of Parent pursuant to the preceding sentence shall remain in office as a director of Parent until his or her successor is elected or appointed and qualified or until his or her earlier death, resignation or removal in accordance with the Parent Organizational Documents. The Parties shall take all necessary actions so that the officers of the Company at the Effective Time shall, from and after the Effective Time, be the officers of the Parent until their successors have been duly elected or appointed and qualified or until their earlier death, resignation or removal in accordance with the Parent’s Organizational Documents.
| -280- |
1.5 Subsequent Actions. If at any time after the Effective Time the Surviving Corporation shall determine, in its sole discretion, or shall be advised, that any deeds, bills of sale, instruments of conveyance, assignments, assurances or any other actions or things are necessary or desirable to vest, perfect or confirm of record or otherwise in the Surviving Corporation its right, title or interest in, to or under any of the rights, properties or assets of the Company and Merger Sub acquired or to be acquired by the Surviving Corporation as a result of, or in connection with, the Merger or otherwise to carry out this Agreement, then the officers of the Surviving Corporation shall be authorized to execute and deliver, in the name and on behalf of the Company and Merger Sub, all such deeds, bills of sale, instruments of conveyance, assignments and assurances and to take and do, in the name and on behalf of the Company and Merger Sub or otherwise, all such other actions and things as may be necessary or desirable to vest, perfect or confirm any and all right, title or interest in, to and under such rights, properties or assets in the Surviving Corporation or otherwise to carry out this Agreement.
1.6 Treatment of Company Capital Shares.
(i) Company Capital Stock. Upon the terms and subject to the conditions of this Section 1.6 and elsewhere in this Agreement, at the Effective Time, by virtue of the Merger and without any action on the part of Parent, Merger Sub, the Company or the Company Stockholders, each share of Company Capital Stock (other than Cancelled Shares and Company Dissenting Shares) issued and outstanding immediately prior to the Effective Time will be cancelled and will be converted automatically into the non-transferable right to receive the Per Share Consideration, in each case as set forth in the Consideration Schedule; and
(ii) Company Dissenting Share. Each of the Company Dissenting Shares issued and outstanding immediately prior to the Effective Time shall be cancelled and cease to exist in accordance with Section 1.7(a) and shall thereafter represent only the right to receive the applicable payments set forth in Section 1.7(a).
(b) Company Options. Upon the terms and subject to the conditions of this Section 1.6(b) and elsewhere in this Agreement, at the Effective Time, by virtue of the Merger and pursuant to the terms of the applicable Company Plans (or other instrument pursuant to which the applicable Company Options have been issued) and at the direction of the Company’s Board of Directors but without further action by Parent, Merger Sub, or any holder of Company Options, each outstanding and unexercised Company Option that has not been exercised prior to the Effective Time in accordance with the option exercise instructions provided by the Company to holders of vested Company Options, shall cease to represent the right to purchase Company Common Stock and shall be assumed by Parent and substituted and converted into an option to purchase Parent Common Stock pursuant to the Parent Equity Incentive Plan (each, an “Exchanged Option”) for an amount of Parent Common Stock (rounded down to the whole share Parent Common Stock) equal to the product of the number of Company Common Stock subject to such Company Option and the Per Share Common Consideration, and at an exercise price (rounded up to the nearest whole cent) equal to the quotient of the exercise price of such Company Option and the Per Share Common Consideration and subject to such terms and conditions, in each case, as set forth on the Consideration Schedule pursuant to Section 1.6(d). By way of example, if the Per Share Common Consideration for the Company Common Stock into which such Exchanged Option is exercisable is 0.1, the number of shares of Parent Common Stock into which such Exchanged Option would be exercisable would be 1/10th of the number of shares of Company Common Stock into which it is currently exercisable and the per share exercise price would be multiplied by 10. In addition, each Exchanged Option shall otherwise be subject to the same vesting, expiration and forfeiture provisions and other similar terms applicable to the corresponding Company Option immediately prior to the Effective Time, except for such other immaterial administrative or ministerial changes as the Parent Board (or the compensation committee of the Parent Board) may determine in good faith are appropriate to effectuate the administration of the Exchanged Options. Such assumption and conversion shall occur in a manner intended to comply with the requirements of Section 409A and 424 of the Code, as applicable.
| -281- |
(c) Company RSUs. Each Company RSU Award, to the extent not already vested or automatically vested by virtue of the Merger, shall be assumed by Parent and substituted and converted into an RSU Award for Parent Common Stock pursuant to the Parent Equity Incentive Plan (each an “Exchanged RSU Award”) for an amount of Parent Common Stock (rounded down to the whole share Parent Common Stock) equal to the product of the number of Company Common subject to such Company RSU Award and the Per Share Common Consideration, in each case, as set forth on the Consideration Schedule pursuant to Section 1.6(d). By way of example, if the Per Share Common Consideration for the Company Common Stock into which such Exchanged RSU Award is exercisable is 0.1, the number of shares of Parent Common Stock into which such Exchanged RSU Award would be exercisable would be 1/10th of the number of shares of Company Capital Stock into which it is currently exercisable. In addition, each Exchanged RSU Award shall be subject to the same vesting, expiration and forfeiture provisions and other similar terms applicable to the corresponding Company RSU Award immediately prior to the Effective Time, subject to the adjustments required by this Section 1.6 after giving effect to the Merger.
(d) Termination of Company Plans. Prior to the Effective Time, Parent shall approve and adopt the Parent Equity Incentive Plan in accordance with Section 7.9 in order to issue all of the Exchanged RSU Awards and Exchanged Options as well as make all future equity compensation awards. In conjunction with Parent’s timely adoption and approval of the Parent Equity Incentive Plan as set forth herein, the Company shall cause all Company Plans to be terminated and all outstanding awards thereunder to be converted into awards under the Parent Equity Incentive Plan at the time of the Closing (with no Person retaining any rights under the Company Plans and no Person retaining any rights or interests in awards under the Company Plans following Closing). Prior to the Effective Time, and subject to the review, comment, and approval of Parent (not to be unreasonably withheld, delayed or conditioned), the Company shall take all actions necessary to effect the transactions contemplated by Sections 1.6(b) and 1.6(c), including, but not limited to, any actions as may be required under the applicable Company Plans and all Company Options and/or Company RSU Award agreements, including delivering a notice of the terms of this Agreement to all holders of Company Options and Company RSU Awards. Materials to be submitted to holders of Company Options and Company RSU Awards in connection with any notice required under this Section 1.6(d) shall be subject to review and approval by Parent, which approval shall not be unreasonably withheld, delayed or conditioned.
(e) Company Warrants. At the Effective Time, each Company Warrant outstanding immediately prior to the Effective Time, whether vested or unvested, that does not terminate by its terms in connection with the Merger, shall be assumed by Parent (each an “Assumed Warrant”). Each Assumed Warrant shall continue to have, and be subject to, the same terms and conditions applicable to such Company Warrant immediately prior to the Closing Date; provided, that such Assumed Warrant shall be exercisable for that number of whole shares of Parent Common Stock (rounded up to the nearest whole number) and at the per share exercise price (rounded up to the nearest whole cent) and subject to such terms and conditions, in each case, as set forth on the Consideration Schedule. Prior to the Closing, Parent will reserve a sufficient number of shares of Parent Common Stock to permit the exercise of the Assumed Warrants. The number of shares of Parent Common Stock into which an Assumed Warrant is exercisable shall be based on the Per Share Consideration for the relevant Company Capital Stock into which such Assumed Warrant is exercisable, and the per share exercise price shall be ratably adjusted. By way of example, if the Per Share Consideration for the Company Capital Stock into which such Assumed Warrant is exercisable is 0.1, the number of shares of Parent Common Stock into which such Assumed Warrant would be exercisable would be 1/10th of the number of shares of Company Capital Stock into which it is currently exercisable and the per share exercise price would be multiplied by 10 In addition, prior to the Effective Time, and subject to the review, comment and approval of Parent (not to be unreasonably withheld, delayed or conditioned), the Company shall take all actions necessary to effect the transactions contemplated by this Section 1.6(e), including, but not limited to, any actions as may be required under the applicable Company Warrants.
| -282- |
(f) No Further Ownership Rights in Company Securities. At the Effective Time, each holder of issued and outstanding Company Capital Stock immediately prior to the Effective Time shall cease to have any rights as a holder of securities of the Company. After the Effective Time, there shall be no further registration of transfers on the transfer books of the Surviving Corporation of the Company Capital Stock outstanding immediately prior to the Effective Time. If, after the Effective Time, a valid certificate previously representing any of such shares of Company Capital Stock (a “Company Stock Certificate”) is presented to the Surviving Corporation or Parent, such Company Stock Certificate shall be canceled (as applicable) and shall be exchanged as provided in Section 1.8.
(g) Certain Company Common Stock. At the Effective Time, each share of Company Capital Stock issued and outstanding immediately prior to the Effective Time that is owned or held in treasury by Parent, the Company, or any wholly owned Subsidiary of Parent or the Company shall automatically be cancelled and extinguished without any conversion thereof or payment of any cash or other property or consideration therefor and shall cease to exist (collectively, the “Cancelled Shares”).
(h) Contingent Obligations. Schedule 1.6(h) to the Company Disclosure Letter sets forth those certain contingent obligations or commitments (the “Contingent Obligations”) with respect to which the settlement of such contingent obligations or commitments is to be made out of the Aggregate Consideration (to the extent not otherwise addressed prior to the Closing).
1.7 Company Dissenting Shares.
(a) Notwithstanding any provision of this Agreement to the contrary and to the extent available under the DGCL, shares of Company Stock that are outstanding immediately prior to the Effective Time and that are held by Company Stockholders who shall have not voted in favor of the Merger, consented thereto in writing or waived their respective appraisal or dissenters’ rights under the Company Stockholders Agreements or otherwise, and who shall have demanded properly in writing appraisal or dissenters’ rights for such Company Stock in accordance with Section 262 of the DGCL, and otherwise complied with all of the provisions of the DGCL relevant to the exercise and perfection of appraisal rights, shall not be converted into, and such Company Stockholders shall have no right to receive, the applicable Per Share Merger Consideration, unless and until such stockholder fails to perfect, withdraws or otherwise loses his, her or its right to appraisal and payment under the DGCL. Any Company Stockholder who fails to perfect, effectively withdraws or otherwise loses his, her or its rights to appraisal with respect to such shares of Company Stock under Section 262 of the DGCL shall thereupon be deemed to have been converted into, and to have become exchangeable, as of the Effective Time, for the right to receive the applicable Per Share Merger Consideration, without any interest thereon, upon surrender, if applicable, in the manner provided in Section 1.9(b), of the Certificate or Certificates that formerly evidenced such shares of Company Stock, and such shares of Company Stock shall cease to be “Company Dissenting Shares” for purposes of this Agreement.
| -283- |
(b) Prior to the Closing, the Company shall give Parent prompt notice (and in any event within two Business Days) of any demands received by the Company for appraisal of shares of Company Stock, attempted withdrawals of such demands and any other instruments served pursuant to the DGCL and received by the Company relating to rights to be paid the fair value of Company Dissenting Shares, and Parent shall have the right to participate in, at its sole cost and expense, but not control, all negotiations and proceedings with respect to such demands. Prior to the Effective Time, the Company shall not, except with the prior written consent of Parent (which shall not be unreasonably withheld, conditioned or delayed), make any payment with respect to, or settle or compromise or offer to settle or compromise, any such demands or waive any failure to timely deliver a written demand for appraisal or otherwise comply with the provisions under Section 262 of the DGCL, or agree or commit to do any of the foregoing.
1.8 Treatment of Merger Sub Shares. Each share of Merger Sub that is issued and outstanding immediately prior to the Effective Time will, by virtue of the Merger and without further action on the part of the sole shareholder of Merger Sub, be converted into and become one share of the Surviving Corporation (and the shares of Surviving Corporation into which the shares of Merger Sub are so converted shall be the only shares of the Surviving Corporation that are issued and outstanding immediately after the Effective Time). Each certificate evidencing ownership of shares of Merger Sub will, as of the Effective Time, be deemed to evidence ownership of such shares of the Surviving Corporation.
1.9 Exchange Procedures.
(a) Exchange Agent. Prior to the Effective Time, Parent shall deposit or cause to be deposited with a bank or trust company selected by Parent and consented to by the Company (consent not to be unreasonably withheld, conditioned, or delayed), to serve as the exchange agent (the “Exchange Agent”), for the benefit of the Company Stockholders, an aggregate number of shares of Parent Common Stock to be issued in non-certificated book-entry form comprising the amounts required to be delivered in respect of the Aggregate Consideration pursuant to Section 1.6 (and as set forth on the Consideration Schedule). In addition, Parent shall deposit or cause to be deposited with the Exchange Agent, as necessary from time to time after the Effective Time, any dividends or other distributions, if any, to which the holders of Company Stock may be entitled pursuant to Section 1.9(c) with both a record and payment date after the Effective Time and prior to the surrender of such Company Capital Stock. Such shares of Parent Common Stock and the amount of any dividends or other distributions deposited with the Exchange Agent pursuant to this Section 1.9 shall be the “Exchange Fund.” The Exchange Fund shall not be used for any purpose other than a purpose expressly provided for in this Agreement. For the avoidance of doubt, references to “Company Capital Stock” in this Section 1.9(a) shall exclude Company Dissenting Shares.
(b) Procedures for Surrender. Prior to the Effective Time, Parent shall cause the Exchange Agent to mail to each record holder of Company Capital Stock entitled to receive the applicable Per Share Consideration pursuant to Section 1.6 a letter of transmittal (the “Letter of Transmittal”), which shall be in a form reasonably acceptable to Parent and the Company and shall specify (i) that, with respect to shares of Company Capital Stock evidenced by Company Stock Certificates, delivery shall be effected, and risk of loss and title to the Company Stock Certificates shall pass, only upon proper delivery of the Letter of Transmittal to the Exchange Agent, and (ii) instructions for use in effecting the surrender of the Company Stock Certificates or non-certificated shares of Company Common Stock represented by book-entry (“Book-Entry Shares”) in exchange for the applicable Per Share Consideration payable in respect of the shares of Company Common Stock evidenced by such Company Stock Certificates or Book-Entry Shares, as applicable, pursuant to the Letter of Transmittal. Within two (2) Business Days (but in no event prior to the Effective Time) after the surrender of Company Stock Certificates, if any (or affidavits in lieu thereof in accordance with Section 1.9(i)), for cancellation to the Exchange Agent and delivery of a Letter of Transmittal with respect to all Certificates or Book-Entry Shares held by such holder for cancellation, duly completed and validly executed in accordance with the instructions thereto, and such other documents as may be required pursuant to such instructions (the “Transmittal Documents”), the holder of such shares of Company Capital Stock shall be entitled to receive in exchange therefor and Parent shall cause the Exchange Agent to deliver, the applicable Per Share Consideration in accordance with the provisions of Section 1.6 and as set forth in the Consideration Schedule, and the Company Stock Certificates and Book-Entry Shares so surrendered shall forthwith be cancelled. Until surrendered as contemplated by this Section 1.9(b), each Company Stock Certificate and Book-Entry Share entitled to receive the applicable Per Share Consideration in accordance with Section 1.6 shall be deemed at all times after the Effective Time to represent only the right to receive upon such surrender the applicable Per Share Consideration that such holder is entitled to receive in accordance with the provisions of Section 1.6.
| -284- |
(c) Delivery of Consideration to Other Persons. If any Per Share Consideration is to be delivered or issued to a Person other than the Person in whose name the surrendered Company Stock Certificate or Book-Entry Share is registered immediately prior to the Effective Time, it shall be a condition to such delivery that (i) the transfer of such Company Capital Stock shall have been permitted in accordance with the terms of the Organizational Documents of the Company as in effect immediately prior to the Effective Time, (ii) such Company Stock Certificate shall be properly endorsed or shall otherwise be in proper form for transfer, (iii) the recipient of such Per Share Consideration, or the Person in whose name such Per Share Consideration is delivered or issued, shall have already executed and delivered such other Transmittal Documents as are reasonably deemed necessary by the Exchange Agent or Parent and (iv) the Person requesting such delivery shall pay to the Exchange Agent any transfer or other similar Taxes required as a result of such delivery to a Person other than the registered holder of such Company Stock Certificate or establish to the satisfaction of the Exchange Agent that such Tax has been paid or is not payable.
(d) Stop Transfer. After the Effective Time, there shall be no further registration of transfers of Company Stock. If, after the Effective Time, Company Stock Certificates are presented to the Surviving Corporation, Parent or the Exchange Agent, they shall be canceled and exchanged for the Per Share Consideration in accordance with, the procedures set forth in this Section 1.9.
(e) Distributions with Respect to Un-surrendered Certificates. All shares of Parent Common Stock to be issued pursuant to the Merger shall be deemed issued and outstanding as of the Effective Time and whenever a dividend or other distribution is declared by Parent in respect of Parent Common Stock, the record date for which is at or after the Effective Time, that declaration shall include dividends or other distributions in respect of all shares issuable pursuant to this Agreement. No dividends or other distributions in respect of shares of Parent Common Stock shall be paid to any holder of any un-surrendered Company Stock Certificate until the Company Stock Certificate (or affidavit of loss in lieu of a Certificate as provided in Section 1.9(i)) is surrendered for exchange in accordance with this Section 1.9. Subject to applicable Law (including the effects of escheat and Tax Laws), following such surrender, there shall be issued or paid to the holder of record of the whole shares of Parent Common Stock issued in exchange for Company Capital Stock (other than Company Dissenting Shares) in accordance with this Section 1.9, (i) at the time of such surrender, the dividends or other distributions with a record date after the Effective Time theretofore payable with respect to such whole shares of Parent Common Stock and not paid and (ii) at the appropriate payment date, the dividends or other distributions payable with respect to such whole shares of Parent Common Stock with a record date after the Effective Time and prior to surrender, but with a payment date subsequent to surrender.
(f) Fractional Shares. Notwithstanding anything to the contrary contained herein, no fraction of a share of Parent Common Stock will be issued by virtue of the Merger or the other Transactions, and each Person who would otherwise be entitled to a fraction of a share of Parent Common Stock (after aggregating all fractional shares of Parent Common Stock that otherwise would be received by such holder) shall instead have the number of shares of Parent Common Stock issued to such Person rounded down to the nearest whole share of Parent Common Stock.
| -285- |
(g) No Interest. No interest will be paid or accrued on any amount payable for shares of Parent Common Stock pursuant to this Section 1.9.
(h) Termination of Exchange Fund. Any portion of the Exchange Fund (including the proceeds of any deposit of the Exchange Fund and any shares of Parent Common Stock) that remains unclaimed by the 180th day after the Effective Time (or, if sooner, as of a date which is immediately prior to such time as such amounts would otherwise escheat to or become property of any government entity) shall be delivered to Parent. Any holder of Company Capital Stock (other than Company Dissenting Shares) who has not theretofore complied with this Section 1.9 shall thereafter look only to Parent for delivery of the Per Share Consideration and any unpaid non-stock dividends and any other dividends or other distributions, in each case, that such holder has the right to receive pursuant to this Section 1.9.
(i) Lost, Stolen or Destroyed Certificates. In the event that any Company Stock Certificate shall have been lost, stolen or destroyed, upon the making of an affidavit of that fact by the Person claiming such Company Stock Certificate to be lost, stolen or destroyed and, if required by Parent, the posting by such Person of a bond in customary amount and upon such terms as may reasonably be required as indemnity against any claim that may be made against it with respect to such Company Stock Certificate, the Exchange Agent will issue in exchange for such lost, stolen or destroyed Company Stock Certificate the Per Share Consideration and any unpaid non-stock dividends and any other dividends or other distributions, in each case, payable or issuable pursuant to this Section 1.9, had such lost, stolen or destroyed Company Stock Certificate been surrendered.
(j) No Liability. None of the Exchange Agent, Parent or the Surviving Corporation shall be liable to any holder of Company Common Stock for any Parent Common Stock (or dividends or distributions with respect thereto) or cash delivered to a public official pursuant to any abandoned property, escheat or similar Law in accordance with this Section 1.9.
1.10 Withholding. Each of Parent, Merger Sub, the Surviving Corporation, the Exchange Agent and any other applicable withholding agent shall be entitled to deduct or withhold from the amounts payable or issuable (including shares of Parent Common Stock deliverable) under this Agreement such amounts as such Person is required to deduct or withhold in accordance with the Code and any other applicable Law. Any such withheld or deducted amount shall be timely paid over to the appropriate Governmental Authority and treated as though such amount had been paid to the Person in respect of whom such withholding was determined to be necessary. Any compensatory payments contemplated to be made hereunder shall be made through the payroll procedures of the applicable Person.
1.11 Equitable Adjustments. In the event of any stock split, reverse stock split, stock dividend (including any dividend or distribution of securities convertible into capital stock), reorganization, reclassification, combination, recapitalization or other like change with respect to the Company Capital Stock or Parent Common Stock occurring after the date hereof and prior to the Effective Time, all references herein to specified numbers of shares of any class or series affected thereby, and all calculations provided for that are based upon numbers of shares of any class or series (or prices therefor) affected thereby, shall be equitably adjusted to the extent necessary to provide the parties the same economic effect as contemplated by this Agreement prior to such stock split, reverse stock split, stock dividend, reorganization, reclassification, combination, capitalization or other like change. Nothing in this Section 1.11 shall be construed to permit the Company or Parent to take any action with respect to its securities that is prohibited by the terms of this Agreement.
| -286- |
Article II.
CLOSING
2.1. Closing and Closing Deliverables. The closing of the Merger (the “Closing”) shall take place at the offices of Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P. in Raleigh, North Carolina at 9:00 a.m. Eastern Time as soon as reasonably practicable (and, in any event, within three (3) Business Days) after satisfaction or (to the extent permitted by applicable Law) waiver of the conditions set forth in Article VIII hereof, or at such other time, date and location (or by electronic exchange of signatures) as Parent and the Company may agree in writing (the date of the Closing, the “Closing Date”).
2.2. Closing Statement. No later than five (5) Business Days prior to the Closing Date, each of Parent and the Company shall provide to the other party a written report setting forth the aggregate amount of all Transaction Expenses to be paid in cash by or on behalf of such party to third parties and such Transaction Expenses shall be paid by Parent or the Surviving Corporation to the applicable third parties immediately after Closing, as directed in such written reports by Parent in the sole discretion of Parent (in the case of Parent’s Transaction Expenses), and as directed by the Company (in the case of the Company’s Transaction Expenses); provided, however, that the aggregate amount of such Transaction Expenses to be paid on behalf of Parent shall not cause the amount of Parent’s available cash at Closing to be reduced below $15,000,000 (after accounting for the payment in cash of such Transaction Expenses even if not paid prior to the Closing), and (b) the aggregate amount of Parent’s Transaction Expenses shall not, without the prior written consent of the Company, exceed $2,900,000 in the aggregate (the “Parent Transaction Fee Cap”).
2.3. Pre-Closing Deliveries.
(a) No later than five (5) Business Days prior to the date the Registration Statement becomes effective under the Securities Act, the Company shall prepare and deliver to Parent a schedule in spreadsheet format (the “Consideration Schedule”), in form and substance reasonably satisfactory to Parent and certified as complete and correct by the Company’s chief executive officer, setting forth all of the following information as of immediately such date:
(i) (A) the names of all of the Company Stockholders and their respective addresses, e-mail addresses and social security numbers or tax identification numbers (as applicable), (B) the number and type of shares of Company Capital Stock held by such Company Stockholders and the respective certificate numbers representing such shares, (C) the date of acquisition of shares of Company Capital Stock, (D) the calculation of the aggregate number of shares of Parent Common Stock issuable to each Company Stockholder at Closing pursuant to Section 1.6, assuming each Company Stockholder is issued a number of shares of Parent Common Stock pursuant to Section 1.6, a funds flow memorandum setting forth applicable wire transfer instructions and other information reasonably requested by Parent and (E) such other information relevant thereto which Parent may reasonably request with respect to any Transaction Expenses of the Company to be paid in cash. All amounts and allocations set forth in the Consideration Schedule shall be conclusive and binding upon the Company and the Company Stockholders and neither Parent or Merger Sub, nor, after Closing, the Surviving Corporation shall have any obligation to verify the accuracy of the Consideration Schedule; and
| -287- |
(ii) (A) the names of the holders of the Contingent Obligations, together with the respective addresses, e-mail addresses and social security numbers or tax identification numbers (as applicable), and (B) the calculation of the aggregate number of shares of Parent Common Stock issuable to each holder of such Contingent Obligations at Closing in settlement thereof out of the Aggregate Consideration.
Notwithstanding anything to the contrary herein, the parties acknowledge and agree that Parent and each of its Affiliates shall be entitled to rely on the Consideration Schedule as setting forth a true, correct and complete listing of the items set forth therein, and neither Parent nor any of its Affiliates shall have any Liability or obligation to any Person, including the Company Stockholders, for any Liabilities arising from or relating to errors, omissions or inaccuracies in calculating the portion of the Aggregate Consideration to be received by each Company Stockholder or any other errors, omissions or inaccuracies in the Consideration Schedule.
(b) The Company shall deliver a preliminary Consideration Schedule on or prior to five (5) Business Days prior to the date of the initial filing of the Registration Statement (which date shall not be earlier than January 1, 2022 (unless the Company shall have otherwise agreed in writing)), setting forth its reasonable best estimates as of such date of the information to be contained therein.
Article III.
REPRESENTATIONS AND WARRANTIES OF the company
The Company hereby represents and warrants to Parent and Merger Sub, as of the date hereof, as follows, except as otherwise set forth on the Company Disclosure Letter, which exceptions shall apply to (a) the representations and warranties or covenants contained in the Section of this Agreement to which the applicable Section of the Company Disclosure Letter corresponds in number, (b) any representation and warranty or covenant to which it is referred by cross reference and (c) any other representation or warranty or covenant to the extent it is reasonably apparent on the face of such disclosure that such disclosure is applicable to such representation or warranty or covenant:
3.1. Organization, Existence and Power.
(a) Each of the Acquired Companies is a corporation duly incorporated, validly existing and in good standing under the Laws of its respective jurisdiction of incorporation and has all requisite corporate or similar power and authority required to carry on its business as now conducted, to own or use the properties and assets that it purports to own or use. Each of the Acquired Companies is duly qualified to do business as a foreign corporation and is in good standing in each jurisdiction where such qualification is necessary, except in those jurisdictions where the failure to be so qualified or in good standing, when taken together with all other failures of any of the Acquired Companies to be so qualified or in good standing, would not reasonably be expected to have a Material Adverse Effect on the Acquired Companies. The Company has made available to Parent prior to the date hereof complete and accurate copies of its Organizational Documents and the Organizational Documents of each Subsidiary of the Company, each as currently in effect.
(b) All of the issued and outstanding shares of capital stock of, or other Equity Interests in, each Subsidiary of the Company (i) have been validly issued and are fully paid and nonassessable and are wholly owned, directly or indirectly, by the Company free and clear of all Encumbrances, other than Permitted Encumbrances, (ii) were issued in compliance with all applicable Laws and (iii) were not issued in violation of any preemptive rights or rights of first refusal created by statute, the Company’s Organizational Documents, or any agreement to which the Company is a party. Section 3.1(b) of the Company Disclosure Letter sets forth an accurate and complete list as of the date hereof of each Subsidiary of the Company and each Person in which the Company or any of its Subsidiaries owns an Equity Interest or other voting interest, together with (i) the jurisdiction of incorporation or organization, as the case may be, of each Subsidiary of the Company or such other Person and (ii) the type and percentage of interest held, directly or indirectly, by the Company in each of its Subsidiaries or in each such other Person.
| -288- |
3.2. Authorization. The Company has all requisite corporate power and authority to execute and deliver this Agreement and all agreements contemplated by this Agreement (the “Ancillary Documents”) to be executed and delivered by the Company, as the case may be, pursuant hereto, to consummate the transactions contemplated hereby and thereby and to perform its obligations hereunder and thereunder. The execution and delivery by the Company of this Agreement and the Ancillary Documents and the consummation by the Company of the transactions contemplated hereby and thereby have been duly authorized by all necessary action on the part of the Company and the Company Board. No other corporate proceedings on the part of the Company are necessary to authorize this Agreement and the transactions contemplated hereby (other than the Company Requisite Stockholder Consent). The Company Requisite Stockholder Consent is the only vote or consent of the holders of any class or series of Company Capital Stock necessary to adopt this Agreement and approve the terms of the Merger and the consummation of the transactions contemplated hereby. This Agreement has been, and the Ancillary Documents will be, duly executed and delivered by the Company and is, and the Ancillary Documents will be, the legal, valid and binding obligations of the Company, enforceable against the Company in accordance with their terms, in each case, except as such enforceability may be limited by (a) bankruptcy, insolvency, moratorium, reorganization or other similar Laws affecting creditors’ rights generally and (b) the general principles of equity, regardless of whether asserted in a Proceeding in equity or at Law (collectively, the “Enforceability Exceptions”).
3.3. Governmental Consents and Filings. Assuming the accuracy of the representations made by Parent and Merger Sub in Article IV, no consent, approval, order or authorization of, or registration, qualification, designation, declaration or filing with, any Governmental Authority is required on the part of the Company in connection with the consummation of the transactions contemplated by this Agreement, except for (a) the filing of the Certificate of Merger with the Secretary of State of the State of Delaware, and (b) compliance with any applicable requirements of the Securities Act, the Exchange Act and any other applicable U.S. state or federal securities laws.
3.4. No Conflict or Violation. Except as set forth on Section 3.4 of the Company Disclosure Letter, the execution, delivery and performance by the Company of this Agreement and the consummation by the Company of the transactions contemplated hereby, including the Merger, do not and will not, (a) contravene, conflict with, or result in any violation or breach of any provision of the Organizational Documents of any Acquired Company, (b) contravene, conflict with or result in a violation or breach of any provision of any applicable Law, to the extent that such breach or violation would be reasonably expected to have a Material Adverse Effect on the Acquired Companies, (c) require any consent or other action by any Person under, result in a breach of, constitute a default, or an event that, with or without notice or lapse of time or both, would result in a breach of, or constitute a default under, or cause or permit the termination, cancellation, acceleration or other change of any right or obligation or the loss of any benefit to which any Acquired Company is entitled under any provision of any material Contract binding upon any Acquired Company, or under which any of the assets of any Acquired Company is bound or affected, or any material license, franchise, permit, certificate, approval or other similar authorization affecting, or relating in any way to, the assets or business of any Acquired Company or (d) result in the creation or imposition of any Encumbrance on any asset of any Acquired Company (except for Permitted Encumbrances).
| -289- |
3.5. Capitalization.
(a) As of the date hereof, the authorized capital stock of the Company consists of (i) 150,000,000 shares of Company Common Stock, 56,890,366 shares of which are issued and outstanding as of the date hereof and (ii) 10,000,000 shares of Company Preferred Stock, (A) 4,400,000 shares of which have been designated Company Royalty Series Convertible Preferred Stock, 2,541,459 shares of which are issued and outstanding as of the date hereof; (B) 50,000 shares of which have been designated Company Series A Preferred Stock, 1,990 shares of which are issued and outstanding as of the date hereof; (C) 50,000 shares of which have been designated Company Series A-1 Preferred Stock, 10,460 shares of which are issued and outstanding as of the date hereof; and (D) 5,500,000 shares of which have not been designated to any specific series of Company Preferred Stock as of the date hereof. Section 3.5(a) of the Company Disclosure Letter contains a complete and correct list of each outstanding share of Company Capital Stock as of the date hereof, including the holder thereof.
(b) The outstanding shares of Company Capital Stock (i) are duly authorized and validly issued, (ii) are free of any Encumbrances, (iii) were issued in compliance with all applicable Laws and (iv) were not issued in violation of any preemptive rights or rights of first refusal created by statute, the Company’s Organizational Documents, or any agreement to which the Company is a party. Except as set forth in Section 3.5(b) of the Company Disclosure Letter, (A) there are no Contracts to which the Company or any Subsidiary of the Company is a party relating to the issuance, sale, transfer or voting of any Equity Interests of any Acquired Company or any of its Subsidiaries and (B) there are no options, warrants, calls, rights, commitments or agreements obligating any Acquired Company to issue, deliver, sell, repurchase or redeem, or cause to be issued, delivered, sold, repurchased or redeemed, any equity securities or obligating any Acquired Company to grant, or enter into any option, warrant, call, right, commitment or agreement relating to any Equity Interests of any Acquired Company. Except as set forth in Section 3.5(b) of the Company Disclosure Letter, there are no bonds, debentures, notes or other instrument of Indebtedness to which the Company or any Subsidiary of the Company is a party giving or purporting to give the holder thereof the right to vote or consent (or convertible into or exchangeable for securities of any Acquired Company having the right to vote or consent) on any matters on which the security holders of any Acquired Company may vote.
(c) Section 3.5(c) of the Company Disclosure Letter sets forth accurate and complete information with respect to (x) the holder, the grant date, the vesting schedule, the exercise price (if applicable), the expiration date (if applicable), the shares underlying and the tax status (if applicable) of each Company Option, Company RSU Award and Company Warrant outstanding as of the date of this Agreement and (y) the intended holder, the vesting, the shares underlying and the tax status (if applicable) of each Company Option, Company RSU Award and/or Company Warrant promised by the Company but not yet granted. All outstanding Company Options and/or Company RSU Awards were granted pursuant to the terms of the applicable Company Plan. Each Company Option and Company RSU Award is exempt from Section 409A of the Code. Each Company Option characterized by the Company as an “incentive stock option” within the meaning of Section 422 of the Code complies with all of the applicable requirements of Section 422 of the Code.
(d) Except as set forth in Section 3.5(d) or Section 3.15(m) of the Company Disclosure Letter, no agreement evidencing Company Options, Company RSU Awards or Company Warrants contains a provision for acceleration of vesting (or lapse of a repurchase right) or other changes in the vesting provisions or other terms of such agreement or understanding upon the occurrence of any event or combination of events, including in the case where the Company Plans or underlying awards are not assumed in an acquisition. The Company has no obligation (contingent or otherwise) to purchase or redeem any of its capital stock.
(e) Other than Acquired Companies (other than the Company), of which Company is the sole stockholder, none of the Acquired Companies owns or controls, directly or indirectly, any interest in any other corporation, partnership, trust, joint venture, limited liability company, association, or other business entity. Each Acquired Company is not a participant in any joint venture, partnership or similar arrangement.
| -290- |
3.6. Litigation.
(a) As of the date hereof, there are no Proceedings pending, or to the Knowledge of the Company, threatened in writing against the Company or any of its Subsidiaries or any of their predecessors or against any officer, director, shareholder, employee or agent of the Company or any of its Subsidiaries in their capacity as such or relating to their employment services or relationship with the Company, its Subsidiaries, or any of their Affiliates, except as would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect or prevent, delay or impair the ability of the Company to consummate the Transactions.
(b) As of the date hereof, neither the Company nor any of its Subsidiaries is a party to or subject to the provisions of any Governmental Order that restricts the manner in which the Company or any of its Subsidiaries conducts its business, except as would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect or prevent, delay or impair the ability of the Company to consummate the Transactions.
3.7. Intellectual Property.
(a) Section 3.7(a) of the Company Disclosure Letter sets forth an accurate and complete list as of the date of this Agreement of (i) each item of Company Registered Intellectual Property, (ii) the jurisdiction in which such item of Company Registered Intellectual Property has been registered or filed, the applicable application, registration, or serial or other similar identification number, the filing date or registration date and issuance or grant date, (iii) the record owner thereof, (iv) all registration, maintenance or renewal fees that are due or filings that must be made within one hundred and twenty (120) days of the date hereof for the purposes of maintaining, perfecting, preserving or renewing any registrations for such Company Registered Intellectual Property and (v) any other Person that has, or to the Company’s Knowledge, purports to have, an ownership interest in such item of Company Registered Intellectual Property and the nature of such ownership interest. The Company has provided to Parent complete and accurate copies of all invention disclosures, applications, material correspondence with any Governmental Authority, and other material documents related to the prosecution and maintenance of each such item of Company Registered Intellectual Property.
(b) The Acquired Companies exclusively own all right, title and interest to and in the Company Intellectual Property (other than (i) Company Intellectual Property exclusively and non-exclusively licensed to any Acquired Company, as identified in Section 3.7(b)(i) of the Company Disclosure Letter and (ii) commercially available software products licensed to any Acquired Company under standard end-user object code license agreements (the “Off-the-Shelf Software Licenses”)), in each case, free and clear of any Encumbrances (other than Permitted Encumbrances). Without limiting the generality of the foregoing: (1) all documents and instruments necessary to register or apply for or renew registration of Company Registered Intellectual Property have been validly executed, delivered and filed in a timely manner with the appropriate Governmental Authority; (2) each item of Company Registered Intellectual Property is and at all times has been filed and maintained in compliance with all applicable Laws (including without limitation all applicable duties of candor and good faith in dealing with any applicable patent office) and all filings, payments and other actions required to be made or taken to maintain such item of Company Registered Intellectual Property in full force and effect have been made by the applicable deadline; (3) except as set forth in Section 3.7(b)(i) of the Company Disclosure Letter, no funding, facilities, or personnel of any Governmental Authority or academic institution, were used, directly or indirectly, to develop or create, in whole or in part, any Company Intellectual Property by, for or on behalf of any Acquired Company; and (4) except as set forth in Section 3.7(b)(i), no Acquired Company has assigned or otherwise transferred ownership of, or agreed to assign or otherwise transfer ownership of, any Company Intellectual Property to any other Person. Section 3.7(b)(i) of the Company Disclosure Letter sets forth an accurate and complete list as of the date of this Agreement of each Contract pursuant to which any Person granted to any Acquired Company any license or right or interest in any Intellectual Property (other than Off-the-Shelf Software Licenses). The Company has delivered or made available to Parent, a complete and accurate copy of all Contracts listed on Section 3.7(b)(i) of the Company Disclosure Letter. With respect to each of such Contracts: (x) such Contract is valid and binding on the Acquired Companies, as applicable, and in full force and effect; (y) no Acquired Company has received any written notice of termination or cancellation under such Contract, or received any written notice of breach or default under such Contract, and there are no outstanding or, to the Company’s Knowledge, threatened, Proceeding with respect to any such Contracts; and (z) to the Company’s Knowledge, neither any Acquired Company, nor any other party to any such Contract, is in breach or default thereof in any material respect, and to the Company’s Knowledge, there does not exist any event that, with the giving of notice or the lapse of time or both, would constitute such a breach or default. Each Acquired Company has obtained and possesses valid licenses to use all Software present on the computers and other software-enabled electronic devices that it owns or leases or that it has otherwise provided to its employees for their own use in connection with the business of the Acquired Companies. All Software necessary for the operation of the business of an Acquired Company is owned by the appropriate Acquired Company or has been properly licensed from the owner of such Software and the appropriate Acquired Company has sufficient licenses to cover every site, seat, copy, installation, and user of all such Software. No Contract to which an Acquired Company is a party contains a covenant not to compete or otherwise limits the ability of such Acquired Company to use or exploit fully any Company Intellectual Property.
| -291- |
(c) Section 3.7(c) of the Company Disclosure Letter sets forth an accurate and complete list as of the date of this Agreement of each Contract pursuant to which any Person has been granted by any Acquired Company any license, option, distribution right, or covenant not to sue under, or otherwise has received or acquired any right (whether or not currently exercisable) or interest in, any Company Intellectual Property, and except as set forth on Section 3.7(c) of the Company Disclosure Letter, there exists no obligation by any Acquired Company to assign, license or otherwise transfer any of the Company Intellectual Property to any Person. Except as set forth on Section 3.7(c) of the Company Disclosure Letter, the Company is not bound by, and no Company Intellectual Property is subject to, any Contract containing any covenant or other provision that in any way limits or restricts the ability of any Acquired Company to use, exploit, assert or enforce any Company Intellectual Property anywhere in the world. The Company has delivered or made available to Parent, a complete and accurate copy of all Contracts listed on Section 3.7(c) of the Company Disclosure Letter.
(d) The consummation of the transactions contemplated by this Agreement will neither result in the modification, cancellation, termination, suspension of, or acceleration of any payments with respect to any Contract set forth on Section 3.7(b)(i) or Section 3.7(c) of the Company Disclosure Letter, nor give any third party to any such Contract the right to do any of the foregoing. Following the Closing, Parent, the Surviving Corporation or the applicable Acquired Company will be permitted to exercise all of the rights of such Person under such Contracts to the same extent such Person would have been able to had the transactions contemplated by this Agreement not occurred and without the payment of any additional amounts or consideration other than ongoing fees, royalties or payments that any Acquired Company would otherwise be required to pay pursuant to such Contracts. Other than with respect to Off-the-Shelf Software Licenses, and the Contracts set forth on Section 3.7(b)(i) and Section 3.7(c) of the Company Disclosure Letter, there are no outstanding Contracts, options, licenses, agreements, claims, Encumbrances or shared ownership interests of any kind relating to (i) any Company Intellectual Property in which any Acquired Company has an ownership interest, or (ii) to the Company’s Knowledge, any other Company Intellectual Property.
| -292- |
(e) The registered Intellectual Property rights within the Company Registered Intellectual Property are to the Company’s Knowledge valid and enforceable. There is no pending or, to the Company’s Knowledge threatened: inter partes review, opposition, cancellation, interference, reexamination, post grant review, or other Proceeding, in each case before a court or other Governmental Authority (collectively, “Disputes”) challenging the legality, validity, enforceability, inventorship, ownership, or right to use, sell, license or dispose of any of the Company Intellectual Property or alleging any misuse of any of the Company Intellectual Property (provided the foregoing representation is made to the Company’s Knowledge with respect to Intellectual Property licensed to any Acquired Company). The Company Intellectual Property is not subject to any outstanding Order, settlement or other disposition as a result of a Proceeding (provided the foregoing representation is made to the Company’s Knowledge with respect to Intellectual Property licensed to any Acquired Company), and no Acquired Company has received any written notice asserting that any Company Intellectual Property or the proposed use, sale, license or disposition thereof conflicts with or infringes or misappropriates or will conflict with or infringe or misappropriate the rights of any other Person.
(f) The Acquired Companies have taken commercially reasonable steps to maintain the confidentiality of and otherwise protect and enforce its rights in all Trade Secrets included in the Company Intellectual Property. To the Company’s Knowledge, there have been no unauthorized disclosures of any Trade Secrets of any Acquired Company. To the Company’s Knowledge, no current or former Company Stockholder, officer, director or employee of any Acquired Company has any claim, right (whether or not currently exercisable), or interest to or in any Company Intellectual Property. To the Company’s Knowledge, no employee of or consultant to any Acquired Company is (i) bound by or otherwise subject to any Contract restricting him or her from performing his or her duties for any Acquired Company or (ii) in breach of any Contract with any former employer or other Person concerning, or confidentiality provisions protecting trade secrets and confidential information comprising, Company Intellectual Property. To the Company’s Knowledge, there are no inventions of any employees or consultants of the Acquired Companies that are necessary to the operations of the Acquired Companies that were made prior to their employment by any Acquired Company.
(g) The Company Intellectual Property constitutes all Intellectual Property necessary for the Acquired Companies to conduct the business of the Acquired Companies as currently conducted. The operation by the Acquired Companies of their businesses as currently conducted and as currently proposed to be conducted does not and will not infringe upon, misappropriate, use without authorization, or otherwise violate, nor has the operation of such businesses ever infringed upon, misappropriated, used without authorization or otherwise violated, the Intellectual Property rights of any Person. There is no Proceeding pending, or to the Company’s Knowledge threatened, against an Acquired Company relating to any Intellectual Property used or allegedly used in the conduct of the business of an Acquired Company as currently conducted and as currently proposed to be conducted. The Acquired Companies own or possess sufficient legal rights to, and have the right to bring actions for the infringement of, all Company Intellectual Property without any conflict with, or infringement of, the rights of others (but subject to any rights or obligations with respect to Company Intellectual Property licensed to any Acquired Company pursuant to any Contract set forth on Section 3.7(b)(i) of the Company Disclosure Letter). No Acquired Company has received any communications alleging that any Acquired Company has, or by conducting its business, would infringe upon, misappropriate, use without authorization, or otherwise violate any of the Intellectual Property of any other Person (including any request or demand that an Acquired Company license or refrain from using any Intellectual Property rights of any Person). No formal, written legal opinion concerning or with respect to any third party Intellectual Property relating to any technology or process or product developed or proposed to be developed, marketed or sold by any Acquired Company, including without limitation any freedom-to-operate, product clearance, or right-to-use opinion, has been conducted by or on behalf of, or delivered to any Acquired Company. To the Company’s Knowledge, no third party is infringing upon, or violating any license or agreement with any Acquired Company relating to any Company Intellectual Property. To the Company’s Knowledge, no Person is (or has in the past been) infringing upon, misappropriating, using without authorization or otherwise violating any Company Intellectual Property owned by an Acquired Company. No Acquired Company has brought any Proceeding against any Person alleging infringement, misappropriation, unauthorized use or other violation of any such Intellectual Property. No Acquired Company has made, sent or otherwise delivered to any Person any charge, complaint, claim, demand or notice alleging any past, present or future infringement, misappropriation, unauthorized use or other violation of any Company Intellectual Property (including any request or demand that such Person license or refrain from using any Company Intellectual Property).
| -293- |
(h) Except as set forth in Section 3.7(h) of the Company Disclosure Letter, (i) no Acquired Company is bound by any Contract to indemnify, defend, hold harmless or reimburse any other Person with respect to any Intellectual Property infringement, misappropriation or similar claim, and (ii) to the Company’s Knowledge, no Acquired Company has ever assumed, or agreed to discharge or otherwise take responsibility for, any existing or potential liability of another Person for infringement, misappropriation, or violation of any Intellectual Property, which assumption, agreement or responsibility remains in force as of the date of this Agreement.
(i) No current or former Employee or contractor of any Acquired Company who has authored, co-authored or otherwise contributed to the conception or development of any Intellectual Property on behalf of an Acquired Company has filed, asserted in writing, or threatened in writing any claim against an Acquired Company in connection with his or her involvement with any such Intellectual Property, and no such current or former Employee or contractor has any Intellectual Property rights of any kind now used or necessary for the Company which Intellectual Property rights have not been assigned to the Company.
(j) Section 3.7(j) of the Company Disclosure Letter separately lists and identifies all Software necessary for the business of an Acquired Company (“Company Software”) except for Open Source Software. To the Company’s Knowledge, the Software contained in the Company Intellectual Property substantially performs in accordance with the documentation and other written materials related to such Software, and to the Company’s Knowledge, is free from all viruses, worms, Trojan horses, and other material defects in programming and operations. Each Acquired Company has taken commercially reasonable steps to safeguard the confidentiality for such Software. The Company has not disclosed or licensed any source code to any Person (including to a third party escrow agent) other than to employees or contractors performing services on the Company’s behalf who are bound by obligations of confidentiality. No Person has any right to access or use any source code for any software that is owned by an Acquired Company for any purpose other than the conduct of the Business, and to the Knowledge of the Company, no event has occurred, and no circumstance or condition exists, that (with or without notice or lapse of time, or both) will, or would reasonably be expected to, nor will this Agreement or the transactions contemplated hereby, result in the disclosure or release of such source code by an Acquired Company, the escrow agent(s) (if any), or any other Person to any Person.
(k) The Acquired Companies have sufficient rights to use all Software, including middleware, databases, and systems, information technology equipment necessary to the operation of the business of the Acquired Companies (the “IT Assets”) all of which rights shall survive in all material respects by the consummation of the transactions contemplated hereby. The Acquired Companies do not use, rely on or contract with any Person to provide services bureau, outsourcing or other computer processing services to the Acquired Companies except as described in Section 3.7(k)(i) of the Company Disclosure Letter. The IT Assets have not materially malfunctioned or failed within the past three years and to the Company’s Knowledge, do not contain any viruses, bugs, faults or other devices or effects that (i) enable or assist any Person to access without authorization or disable, erase or otherwise impair in any way the IT Assets, or (ii) otherwise materially adversely affect the functionality of the IT Assets. The Acquired Companies have taken commercially reasonable steps to provide for the remote-site back-up of data and information necessary for the conduct of the Business and have in place commercially reasonable disaster recovery and business continuity plans, procedures and facilities, as described in Section 3.7(k)(ii) of the Company Disclosure Letter. To the Knowledge of the Company, no Person has gained unauthorized access to any IT Assets during the past three years. The Acquired Companies have taken commercially reasonable efforts to maintain safeguards, security measures and procedures against the unauthorized access, disclosure, destruction, loss, or alteration of customer data or information (including any personal or device-specific information) in its possession or control that comply with any applicable contractual and legal requirements.
| -294- |
(l) Section 3.7(l)(i) of the Company Disclosure Letter identifies (i) each item of Open Source Software that is contained in, linked to or distributed with any of the Software in the Company Intellectual Property, (ii) the applicable Open Source Software license and (iii) the products, technology or services of Company to which each such item of Open Source Software relates. Except as set forth in Section 3.7(l)(ii) of the Company Disclosure Letter, no Open Source Software is incorporated (either directly by any Acquired Company, or indirectly, by the incorporation of Third Party software that itself incorporates Open Source Software) into or distributed with any of the Company Intellectual Property, and no product, technology or service of any Acquired Company is intermingled or bundled with or otherwise derived from or contains part of any Open Source Software. No Acquired Company has used any Open Source Software that (y) creates or imposes, or purports to create or impose, any obligation on any Acquired Company with respect to any product, service or technology of the Company Group with respect to the Company Intellectual Property, or (z) grants, or purports to grant, to any Person, any rights or immunities under any of the Company Intellectual Property (including by using any Open Source Software that requires, as a condition of use, modification and/or distribution of such Open Source Software that other Software incorporated into, derived from or distributed with such Open Source Software be (A) disclosed or distributed in source code form, (B) licensed for the purpose of making derivative works, or (C) be redistributable at no charge).
(m) The Acquired Companies have complied in all material respects with all Internet domain name registration and other requirements of Internet domain administration authorities concerning all domain names that are Company Owned Intellectual Property, and operated all websites associated with such domain names in accordance with all applicable Laws. The appropriate Acquired Company is the owner of, or has sufficient rights to display or make available, all content, data, and other information displayed or made available, as applicable, on all websites associated with any domain name included in the Company Intellectual Property.
3.8. Agreements; Actions.
(a) Except for this Agreement and the Contracts listed in Section 3.8(a) of the Company Disclosure Letter (any such Contract listed or required to be listed on Section 3.8(a) of the Company Disclosure Letter, a “Material Contract”), there are no Contracts to which any Acquired Company is a party or by which it is bound that involve (i) obligations (contingent or otherwise) of, or payments to, the Acquired Companies in excess of $1,000,000 on an annual basis, (ii) the license of any patent, copyright, trademark, trade secret or other proprietary right to or from any Acquired Company (other than Off-the-Shelf Software Licenses) which such license is material to the Acquired Companies, (iii) the grant of rights to manufacture, produce, assemble, license, market, or sell its products to any other Person that limit the Acquired Companies’ exclusive right to develop, manufacture, assemble, distribute, market or sell its products, (iv) the sharing of revenues, profits, losses, costs or Liabilities or for joint research, development, marketing or distribution, including any partnership, joint venture or similar Contract, (v) any employment agreements and consulting agreements (except for any Acquired Company’s standard form consulting agreements) which involve payments by any Acquired Company in excess of $1,000,000 on an annual basis, (vi) any agreement under which any Acquired Company is restricted from carrying on any business anywhere in the world (other than non-disclosure agreements and customer contracts entered into in the Ordinary Course of Business that contain non-solicitation obligations), (vii) any agreement with any Governmental Authority, (viii) any agreement that grants exclusive or preferential rights or “most favored nations” status to any person or other similar restrictions, rights or obligations, binding on the Company or any of its Subsidiaries, (ix) any agreement that prohibits the Company or any of its Subsidiaries from soliciting any customers or strategic partners, (x) any agreement evidencing any Indebtedness of any Acquired Company, granting any Lien on any asset of any Acquired Company, extending credit to any Person, or granting a material performance bond, letter of credit or any other similar instrument in excess of $500,000, (xi) any agreement that requires the Company or any of its Subsidiaries, or any successor to or acquirer of the Company, to make any payment to another person as a result (in whole or in part) of the transactions contemplated by this Agreement (a “Change in Control Payment”) or gives another Person a right to receive or elect to receive a Change in Control Payment, (xii) any agreement which grants any person a right of first refusal, right of first offer or similar right with respect to any properties, assets or businesses of the Company or any of its Subsidiaries, (xiii) any agreement for the disposition of a material portion of any Acquired Company’s assets or (xiv) any agreement for the acquisition by any Acquired Company of the business or securities or other ownership interests of another Person.
| -295- |
(b) (i) Each Material Contract is a valid and binding agreement of the applicable Acquired Company and is in full force and effect, subject to the Enforceability Exceptions, (ii) each Acquired Company has performed, in all material respects, all obligations required to be performed by it under each of the Material Contracts to which it is a party, (iii) each Acquired Company is not, and, to the Company’s Knowledge, no other party is, in default or breach in any material respect under the terms of any Material Contract, and no event has occurred, and no circumstance or condition exists, that (with or without notice or lapse of time) will, or would reasonably be expected to, (A) result in a material violation or breach of any of the provisions of any Material Contract, (B) give any Person the right to declare a material default under any Material Contract, (C) give any Person the right to accelerate the maturity or performance of any grant or rights or other obligation under a Material Contract or (D) give any Person the right to cancel, terminate or modify any Material Contract and (iv) as of the date of this Agreement, no Acquired Company nor any Affiliate has received any written notice or other written communication regarding a material violation or breach of, or material default under, or the cancellation or termination of any Material Contract or regarding any counterparty’s intent to cancel or terminate any Material Contract.
(c) Except for this Agreement and the Transactions, each of the Acquired Companies has no interests, rights, obligations or liabilities with respect to, and is not party to, bound by or has its assets or property subject to, in each case whether directly or indirectly, any Contract or transaction which is, or could reasonably be interpreted as constituting, a Business Combination.
3.9. Certain Transactions. Except as set forth in Section 3.9 of the Company Disclosure Letter, there are no transactions, Contracts, agreements, arrangements or understandings or series of related transactions, Contracts, agreements, arrangements or understandings (each, an “Affiliate Transaction”), nor are there any of the foregoing currently proposed, that (if proposed but not having been consummated or executed, if consummated or executed) would be required to be disclosed under Item 404 of Regulation S-K promulgated under the Securities Act other than, in any such case, compensation paid to and/or benefits provided in the ordinary course to an Affiliate who is an employee, independent contractor or director. The Company has made available to Parent copies of each Contract or other relevant documentation (including any amendments or modifications thereto) available as of the date of this Agreement with respect to each Affiliate Transaction.
| -296- |
3.10. Property. All material property and assets reflected on the books of any Acquired Company as owned by an Acquired Company is owned by such Acquired Company, free and clear of all Encumbrances, except for Permitted Encumbrances. With respect to the property and assets it leases, each Acquired Company is in compliance in all material respects with such leases and, to the Company’s Knowledge, holds a valid leasehold interest free of any Encumbrances other than those of the lessors of such property or assets. No Acquired Company owns, or has ever owned, any real property. The material property and assets of the Company are sufficient for it to conduct its Business as currently conducted and as proposed to be conducted and are in good condition and working order.
3.11. Financial Statements.
(a) The Company has delivered to Parent (a) its audited consolidated financial statements as of and for the years ended December 31, 2019 and December 31, 2020 and (b) its unaudited consolidated financial statements (including balance sheet, income statement, statement of stockholders’ equity and statement of cash flows) as of June 30, 2021 (the “Company Balance Sheet Date”) and for the six (6) month period ended on the Company Balance Sheet Date (collectively, the “Company Financial Statements”). The Company Financial Statements have been prepared in accordance with GAAP applied on a consistent basis throughout the periods indicated and in accordance with the requirements of the Public Company Accounting Oversight Board for public companies. The Company Financial Statements fairly present in all material respects, the financial condition and operating results of the Company as of the dates, and for the periods, indicated therein. Since June 30, 2021, except as required by applicable Law or U.S. GAAP, there has been no change in any accounting principle, procedure or practice by the Company or in the method of applying any such principle, procedure or practice.
(b) The Company maintains a system of internal accounting controls designed to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain asset accountability; (iii) access to property is permitted only in accordance with management’s general or specific authorization; and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences.
3.12. Undisclosed Liabilities. As of the date of this Agreement, no Acquired Company has any Liabilities, other than: (a) Liabilities reserved for or disclosed on the Company Balance Sheet or in the notes thereto; (b) Liabilities incurred in the Ordinary Course of Business since the Company Balance Sheet Date; (c) Liabilities under executory Contracts identified on the Company Disclosure Letter; (d) Liabilities arising under this Agreement, (e) as set forth on Section 3.12 of the Company Disclosure Letter, or (f) that would not, individually or in the aggregate, reasonably be expected to be material to the Acquired Companies, taken as a whole.
3.13. Absence of Changes. From September 30, 2021 until the date of this Agreement, (a) the Acquired Companies have conducted in all material respects their respective businesses in the ordinary course and in a manner consistent with past practice; (b) there has not been any Material Adverse Effect; and (c) the Company has not taken any action that, if taken after the date of this Agreement and prior to the consummation of the Merger, would require the consent of Parent pursuant to Section 5.1.
| -297- |
3.14. Employee Benefits Matters.
(a) Section 3.14(a) of the Company Disclosure Letter sets forth a list of each “employee benefit plan,” as defined in Section 3(3) of ERISA, and each employment, severance or similar Contract and each other plan, agreement, offer letter, policy, program, commitment or arrangement (written or oral) providing for compensation, bonuses, commission, profit-sharing, retention, equity or other equity-related rights, incentive or deferred compensation, vacation benefits, insurance (including any self-insured arrangements), health or medical benefits, welfare benefits, life, accident, dental or vision benefits, tuition benefits, vacation or paid-time-off, employee assistance program, disability or sick leave benefits, workers’ compensation, supplemental unemployment benefits, severance benefits, change of control payments, post-employment or retirement benefits and other employee benefits, in any case, which is maintained, administered or contributed to by the Acquired Companies or any ERISA Affiliate thereof and covers any employee or former employee of any Acquired Company, or with respect to which any Acquired Company has or may have any liability (whether actual or contingent) (collectively, the “Employee Plans”).
(b) With respect to each Employee Plan, the Company has made available to Parent, to the extent applicable, accurate and complete copies of (i) the Employee Plan document, including any amendments thereto, and all related trust documents, insurance contracts or other funding vehicles, (ii) a written description of such Employee Plan if such plan is not set forth in a written document, (iii) non-discrimination testing results for the three most recently completed plan years, (iv) the most recently prepared actuarial report for any Employee Plan that is funded, and (v) all material and non-routine correspondence to or from any Governmental Authority received in the last three years with respect to any Employee Plan.
(c) Each Employee Plan has been established, operated and administered in accordance with its terms and in compliance in all material respects with applicable Law, including any applicable provisions of ERISA and the Code. All contributions or other amounts payable by the Company or any of its Subsidiaries with respect to each Employee Plan in respect of current or prior plan years have been paid or accrued in accordance with GAAP. There are no Proceedings (other than routine claims for benefits) pending, or to the Knowledge of the Company, threatened in writing by, on behalf of or against any Employee Plan that could reasonably be expected to result in any material liability to the Company or any of its Subsidiaries.
(d) With respect to each Employee Plan, the Company has made available to Parent, to the extent applicable, accurate and complete copies of (i) the most recent summary plan description together with any summaries of all material modifications thereto, (ii) the most recent IRS determination or opinion letter issued with respect to such plan, and (iii) the three most recently filed annual reports (Form 5500 or 990 series and all schedules and financial statements attached thereto).
(e) Each Employee Plan that is intended to be qualified under Section 401(a) of the Code has been determined by the IRS to be qualified under Section 401(a) of the Code (or time is remaining to apply for such determination), and to the Knowledge of the Company, nothing has occurred that would reasonably be expected to adversely affect the qualification of any such Employee Plan. With respect to any Employee Plan, except as would not reasonably be expected to have a Material Adverse Effect, neither the Company nor any of its Subsidiaries has engaged in a transaction in connection with which the Company or any of its Subsidiaries reasonably could be expected to be subject to either a civil penalty assessed pursuant to Section 409 or 502(i) of ERISA or a Tax imposed pursuant to Section 4975 or 4976 of the Code.
(f) Neither the Company nor any ERISA Affiliate has maintained, established, sponsored, or contributed to (or had any obligation to contribute) in the last six years to a plan that is: (i) a “pension plan” within the meaning of Section 3(2) of ERISA that is subject to Section 412 of the Code or the minimum funding standards under Section 302 or Title IV of ERISA; (ii) a “multiple employer plan” within the meaning of Section 413(c) of the Code; or (iii) a “multiple employer welfare arrangement” within the meaning of Section 3(40) of ERISA.
| -298- |
(g) Neither the Company nor any ERISA Affiliate has maintained, established, participated in or contributed to, or is or has been obligated to contribute to, or has otherwise incurred any obligation or liability (including any contingent liability) under, any Multiemployer Plan in the last six years.
(h) Except for group health plan continuation coverage as required by applicable Law and for which the covered individual pays the full cost of coverage, no Employee Plan provides retiree or post-employment medical, disability, life insurance or other welfare benefits coverage to any Person, and none of the Company or any of its Subsidiaries has any obligation to provide such benefits.
(i) Each Employee Plan that is a “nonqualified deferred compensation plan” (within the meaning of Section 409A of the Code) is in documentary compliance with, and has been operated and administered in all material respects in compliance with, Section 409A of the Code and the guidance issued by the IRS provided thereunder. All Company Options have an exercise price at least equal to the fair market value of Company Common Stock on the date of grant of such Company Options.
(j) Neither the execution and delivery of this Agreement nor the consummation of the Transactions could, either alone or in combination with another event, (i) entitle any Company Employee to severance pay or any material increase in severance pay, (ii) accelerate the time of payment or vesting, or materially increase the amount of compensation due to any such Company Employee, (iii) result in the requirement or obligation to fund any Employee Plan, or (iv) result in the payment of any amount that could individually or in combination with any other such payment, constitute an “excess parachute payment” as defined in Section 280G(b)(1) of the Code.
(k) Neither the Company nor any Subsidiary has any obligation to provide, and no Employee Plan or other agreement provides, any individual with the right to, a gross up, indemnification or reimbursement payment for any excise or additional taxes, interest or penalties incurred pursuant to Section 409A or Section 4999 of the Code.
(l) No Employee Plan is subject to the laws outside of the United States.
(m) The Company has not materially modified, terminated or created any material Employee Plan since December 31, 2020
(n) Each Employee Plan that is subject to the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (the “Affordable Care Act”) has been established, maintained and administered in compliance with the requirements of the Affordable Care Act. No event has occurred nor circumstances exist that would cause the Acquired Companies to be subject to any Taxes assessable under Sections 4980H(a) and 4980H(b) of the Code. The Acquired Companies have complied in all material respects with the annual health insurance coverage reporting requirements under Code Sections 6055 and 6056.
3.15. Labor Matters.
(a) Neither the Company nor any of its Subsidiaries is a party to any collective bargaining agreement or other agreement with a labor union or like organization, and to the Knowledge of the Company, there are no activities or Proceedings by any individual or group of individuals, including representatives of any labor organizations or labor unions, to organize any employees of the Company or any of its Subsidiaries.
| -299- |
(b) As of the date of this Agreement and in the last three (3) years, there is no, and has not been any, strike, lockout, slowdown, work stoppage, unfair labor practice or other material labor dispute, or material arbitration or grievance pending, or to the Knowledge of the Company, threatened in writing that would reasonably be expected to interfere in any material respect with the respective business activities of the Company or any of its Subsidiaries or prevent, materially delay or materially impair the ability of the Company to consummate the Transactions. Each of the Company and its Subsidiaries has been, and is, in compliance in all material respects with all applicable Law respecting labor, employment and employment practices, terms and conditions of employment, wages and hours, discrimination, harassment, retaliation, worker classification, leaves of absence, and occupational safety and health. The Company and its Subsidiaries are not aware of any allegations relating to officers, directors or employees of the Company or its Subsidiaries, that, if known to the public, would bring the Company or its Subsidiaries into material disrepute. Neither the Company nor any of its Subsidiaries has retained or engaged any worker as an independent contractor or consultant. There has never been any suit, charge, claim or litigation against the Company or its Subsidiaries relating to any labor, employment or health and safety matters. Neither the Company nor its Subsidiaries have been subject to any audit or investigation by the U.S. Department of Labor, the Occupational Safety and Health Administration and/or their respective state and local analogues. All employees of the Company and its Subsidiaries are legally entitled to work in the United States, and the Company maintains accurate and complete Form I-9s for all current employees. Neither the Company nor its Subsidiaries are delinquent in any payments to employees, and neither the Company nor its Subsidiaries are liable for any arrears to any employees.
3.16. Tax Matters.
(a) Except as set forth on Section 3.16 of the Company Disclosure Letter, each Acquired Company has duly and timely filed with the appropriate Tax authorities all Tax Returns required to be filed. All such Tax Returns are complete and accurate in all material respects. All Taxes required to be paid and for which any Acquired Company is liable (whether or not shown on any Tax Returns) have been paid. No Acquired Company is currently the beneficiary of any extension of time within which to file any Tax Return (other than automatic extensions obtained in the Ordinary Course of Business). No written claim has ever been made by a Tax authority or other Governmental Authority in a jurisdiction where any Acquired Company does not file Tax Returns that such Acquired Company is or may be subject to taxation by that jurisdiction.
(b) Since the Company Balance Sheet Date, no Acquired Company has incurred any liability for Taxes outside the Ordinary Course of Business or otherwise inconsistent with past custom and practice.
(c) No deficiencies for Taxes with respect to any Acquired Company have been claimed, proposed or assessed in writing by any Tax authority or other Governmental Authority. There are no pending or, to the Knowledge of the Company, threatened audits, assessments or other actions for or relating to any liability in respect of Taxes of any Acquired Company. No Acquired Company (or any predecessor thereof) has waived any statute of limitations in respect of Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency that is still in effect, nor has any request been made in writing for any such extension or waiver that is still pending.
(d) There are no liens for Taxes upon any property or asset of the Company (other than statutory liens for current taxes not yet due and payable).
| -300- |
(e) The Company will not be required to include any item of income in, or exclude any item of deduction from, taxable income for any period (or any portion thereof) beginning after the Closing Date as a result of any installment sale or other transaction on or prior to the Closing Date, any accounting method change made or required to be made on or prior to the Closing Date or any agreement with any Tax authority entered into on or prior to the Closing Date, the use of an improper method of accounting for any period or portion thereof ending prior to the Closing Date, any private letter ruling obtained by the Company prior to the Closing, any prepaid amount received on or prior to the Closing, an election under Section 108(i) of the Code or election under Section 965(h) of the Code, or any intercompany transaction or excess loss account described in the Treasury Regulations promulgated pursuant to Section 1502 of the Code (or any corresponding or similar provision of state, local or foreign law) in respect of taxable periods (or portions thereof) effected or existing prior to the Closing.
(f) No Acquired Company is a partner for Tax purposes with respect to any joint venture, partnership, or other arrangement or Contract which is treated as a partnership for Tax purposes and with respect to which it treats itself as a partner for Tax purposes.
(g) No Acquired Company is a party to or bound by any Tax indemnity agreement, Tax sharing agreement, Tax allocation agreement or similar Contract (other than customary provisions in Contracts not primarily relating to Taxes).
(h) No Acquired Company has been a party to a transaction that is or is substantially similar to a “listed transaction,” as such term is defined in Treasury Regulations Section 1.6011-4(b)(2), or any other transaction requiring disclosure under analogous provisions of state, local or foreign Tax law.
(i) No Acquired Company has ever been a member of an affiliated group filing a consolidated federal income Tax Return (other than a group of which the Company is the common parent) or a combined, consolidated, unitary or other affiliated group Tax Return for state, local or foreign Tax purposes.
(j) Each Acquired Company has timely withheld and paid all Taxes required to have been withheld and paid in connection with amounts paid or owing to any employee, independent contractor, creditor, and equityholders of such Acquired Company or other Person. Each Acquired Company has properly classified all individuals providing services to it as employees or non-employees for all relevant Tax purposes.
(k) In the last three years, no Acquired Company has been a party to any distribution that the parties to which treated as satisfying the requirements of Section 355 of the Code.
(l) The Company operates at least one significant historic business line, or owns at least a significant portion of its historic business assets, in each case within the meaning of Treas. Reg. §1.368-1(d).
(m) The Company has not taken or agreed to take any action that would reasonably be expected to prevent the Merger from qualifying for the Intended Tax Treatment. To the Knowledge of the Company, there are no facts, circumstances or plans that, either alone or in combination, could reasonably be expected to prevent the Transaction from qualifying for the Intended Tax Treatment.
(n) No Acquired Company has requested, applied for, or sought any relief, assistance or benefit with respect to Taxes from any Governmental Authority under any COVID-19 Measures, including the deferral of any Taxes from a taxable period (or portion thereof) ending on the Closing Date to a taxable period (or portion thereof) beginning after the Closing Date.
| -301- |
3.17. Insurance. The Company has made available to Parent a list of, and accurate and complete copies of, all material insurance policies and fidelity bonds relating to the assets, business, operations, employees, officers or directors of each Acquired Company as of the date of this Agreement, each of which is in full force and effect. Other than claims made in the ordinary course, there are no pending claims under any such policies or bonds, including any claims for loss or damage to the properties, assets or business of the Acquired Companies. All premiums payable under all such policies and bonds have been timely paid and each Acquired Company has otherwise complied in all material respects with the terms and conditions of all such policies and bonds. There has not been any actual, or to the Company’s Knowledge, threatened termination of, premium increase with respect to, or material alteration of coverage under, any of such policies or bonds. No Acquired Company has any self-insurance or co-insurance programs.
3.18. Compliance with Laws; Permits.
(a) Each Acquired Company is, and for the past three (3) years has been, in compliance, in all material respects, with any applicable Law. Currently and for the past three (3) years, each Acquired Company has had all material Permits, and each Acquired Company is and has been in compliance with each such Permit. No Acquired Company has received any written notice or other written communication regarding any violation of or failure to comply with any term or requirement of any material Permit held as of the date hereof by such Acquired Company or any revocation, withdrawal, suspension, cancellation, termination or modification of any such Permit. Section 3.18 of the Company Disclosure Letter sets forth (a) an accurate and complete list of all material Permits issued to each Acquired Company and (b) an accurate and complete list of all material Permits for which each Acquired Company has applied or has taken the steps necessary to secure or maintain or that each Acquired Company otherwise intends to obtain (in each case, with respect to material Permits that have not yet been obtained by the applicable Acquired Company). Each such material Permit has that been validly issued or obtained is, and immediately following the consummation of the transaction contemplated hereby will be, in full force and effect.
(b) To the Company’s Knowledge, no officer, director, employee, or representative or agent of the Company has entered into any contract, agreement or arrangement, or made any financial or affiliation arrangements with any Person with whom the Company has or hopes to have a business relationship that is illegal or in non-compliance with any Laws.
3.19. Environmental and Safety Laws. To the Company’s Knowledge, (a) each Acquired Company is and has been in compliance, in all material respects, with all Environmental Laws; (b) there has been no release or to the Company’s Knowledge threatened release of any pollutant, contaminant or toxic or hazardous material, substance or waste or petroleum or any fraction thereof (each a “Hazardous Substance”), on, upon, into or from any site currently or heretofore owned, leased or otherwise used by any Acquired Company; (c) there have been no Hazardous Substances generated by any Acquired Company that have been disposed of or come to rest at any site that has been included in any published U.S. federal, state or local “superfund” site list or any other similar list of hazardous or toxic waste sites published by any governmental authority in the United States; and (d) there are no underground storage tanks located on, no polychlorinated biphenyls (“PCBs”) or PCB-containing equipment used or stored on, and no hazardous waste as defined by the Resource Conservation and Recovery Act, as amended, stored on, any site owned or operated by any Acquired Company, except for the storage of hazardous waste in compliance with Environmental Laws.
| -302- |
3.20. FDA Regulation.
(a) Each Acquired Company is and has been in material compliance with all applicable Laws administered or issued by the U.S. Food and Drug Administration (the “FDA”) or any similar Governmental Authority, including the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 201 et seq.) and the Public Health Service Act (42 U.S.C. §§ 262-263), the regulations promulgated thereunder, and all other Laws regarding developing, testing, manufacturing, labeling, transportation, importing, exporting, marketing, distributing or promoting the products of the Acquired Companies (“FDA Laws”). To the Knowledge of each Acquired Company, there are no facts or circumstances that reasonably would be expected to give rise to any material liability under any FDA Laws.
(b) No Acquired Company nor any of its facilities has been subject to a FDA or other Governmental Authority shutdown or import or export prohibition, nor received any FDA Form 483 or other Governmental Authority notice of inspectional observations, warning letter, untitled letter, or other notice or correspondence from the FDA or other Governmental Authority alleging or asserting noncompliance with any FDA Law, Regulatory Permit, or any request or requirement of the FDA or other Governmental Authority, and, to the Company’s Knowledge, neither the FDA nor any other Governmental Authority is considering such action.
(c) All preclinical and clinical investigations sponsored or conducted by or on behalf of any Acquired Company are being and have been conducted in material compliance with applicable research protocols and FDA Laws, and neither the FDA nor any other Governmental Authority has initiated, or, to the Company’s Knowledge, threatened to initiate any action to place a clinical hold on, or otherwise terminate, materially delay, limit, or suspend any clinical investigation conducted or proposed to be conducted by or on behalf of any Acquired Company.
(d) All applications, notifications, submissions, and information submitted to the FDA or other Governmental Entity relating to products that are regulated under FDA Laws, including product candidates being researched, tested, and developed, when submitted to the FDA or other Governmental Entity were true, complete and correct in all material respects as of the date of submission (or were corrected in or supplemented by a subsequent submission) and any necessary or required material updates, changes, corrections or modification to such applications, submissions, information and data have been submitted to the FDA or other Governmental Entity. To the Knowledge of the Company there are no facts or circumstances that would be reasonably likely to lead the revocation, suspension, limitation, or cancellation of a Regulatory Permit required under FDA Laws. Neither the FDA nor any other Governmental Authority has recommended any action be taken by any Acquired Company during the course of a clinical trial that materially delayed, limited, modified or suspended it.
(e) Neither of any Acquired Company, nor, to the Knowledge of any Acquired Company, any officer, employee, or agent of any Acquired Company has made an untrue statement of a material fact or fraudulent or misleading statement to a Governmental Entity, failed to disclose a material fact required to be disclosed to a Governmental Entity, or committed an act, made a statement, or failed to make a statement that would reasonably be expected to provide a basis for the FDA to invoke its policy respecting “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto (the “FDA Ethics Policy”). To the Knowledge of the Company, none of the aforementioned is or has been under investigation resulting from any allegedly untrue, fraudulent, misleading, or false statement or omission, including data fraud, or had any action pending or threatened relating to the FDA Ethics Policy.
(f) No Acquired Company, nor to any Acquired Company’s knowledge, any officer, employee or agent of an Acquired Company has been convicted of any crime or engaged in any conduct that would reasonably be expected to result in (i) debarment under 21 U.S.C. Section 335a or any similar Law, or (ii) exclusion under 42 U.S.C. Section 1320a-7 or any similar Law.
| -303- |
3.21. Foreign Corrupt Practices Act. No Acquired Company nor any of its directors, officers, employees or, to the Knowledge of the Company, agents have, directly or indirectly, made, offered, promised or authorized any payment or gift of any money or anything of value to or for the benefit of any “foreign official” (as such term is defined in the U.S. Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”)), foreign political party or official thereof or candidate for foreign political office for the purpose of (a) influencing any official act or decision of such official, party or candidate, (b) inducing such official, party or candidate to use his, her or its influence to affect any act or decision of a foreign Governmental Authority, or (c) securing any improper advantage, in the case of (a), (b) and (c) above in order to assist any Acquired Company or any of its Affiliates in obtaining or retaining business for or with, or directing business to, any Person. No Acquired Company nor any of its directors, officers, employees or, to the Knowledge of the Company, agents have made or authorized any bribe, rebate, payoff, influence payment, kickback or other unlawful payment of funds or received or retained any funds in violation of any law, rule or regulation. Neither the Acquired Companies, nor to the Knowledge of the Company, any director, officer, agent, employee, Affiliate or Person acting on behalf of the Company is currently subject to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Treasury Department (“OFAC”).
3.22. Data Privacy. In connection with its collection, storage, transfer (including any transfer across national borders) and/or use of any Personal Information, each Acquired Company is and has been, to the Company’s Knowledge, in material compliance with all applicable Laws in all relevant jurisdictions, such Acquired Company’s privacy policies and the requirements of any contract or codes of conduct to which such Acquired Company is a party. Each Acquired Company has commercially reasonable physical, technical, organizational and administrative security measures and policies in place designed to protect all Personal Information collected by it or on its behalf from and against unauthorized access, acquisition, modification, use and/or disclosure. To the extent an Acquired Company maintains or transmits protected health information, as defined under 45 C.F.R. § 160.103, such Acquired Company is in material compliance with the applicable requirements of the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, including all rules and regulations promulgated thereunder. Each Acquired Company is and has been, to the Company’s Knowledge, in material compliance in all material respects with all Laws relating to data loss, theft and breach of security notification obligations. No Acquired Company has received any written claim, notice, inquiry, or demand from any Person, Governmental Authority, consumer advocacy groups, or industry or trade organizations, and no action, suit or proceeding has been asserted or commenced, alleging that an Acquired Company is in violation of Privacy and Security Laws. No Acquired Company has had any breaches or lapses of security, denial of service attacks, nor any unauthorized access to, use, modification, acquisition, disclosure of, or other misuse of any Personal Information or confidential information. To the Company’s Knowledge, the execution, delivery, or performance of this Agreement by the Acquired Companies and the consummation of the transactions contemplated by this agreement will not (i) violate in any material respect any Privacy and Data Security Laws or contracts that are applicable to the processing of Personal Information by the Acquired Companies; (ii) require Acquired Companies to provide any notice to, or seek any consent from any employee, customer, service provider or other third party thereunder as it relates to Personal Information; or (iii) under Privacy and Data Security Laws or applicable contracts, restrict, impair, or limit the ability of the Acquired Companies or Parent or Merger Sub to use Personal Information after the Closing.
3.23. Takeover Statutes. The Company Board has taken all actions necessary so that the restrictions on take-over bids, equity acquisitions, business combinations and equityholder vote and any other “moratorium”, “control share acquisition”, “business combination”, “fair price” or other similar anti-takeover laws or regulations that are or may purport to be applicable will not apply with respect to or as a result of the Merger or the other transactions contemplated by this Agreement.
| -304- |
3.24. No Brokers. Except for the fees payable to Persons set forth on Section 3.24 of the Company Disclosure Letter (which fees shall be the sole responsibility of the Company), there is no investment banker, broker, finder or other intermediary that has been retained by or is authorized to act on behalf of any Acquired Company or who is or may be entitled to any fee or commission from the Company or any of its Affiliates in connection with the transactions contemplated by this Agreement.
3.25. Information Supplied. None of the information relating to the Acquired Companies supplied by or on behalf of the Company in writing specifically for inclusion or incorporation by reference in the Registration Statement or the Proxy Statement will, as of (i) the time the Registration Statement becomes effective under the Securities Act, (ii) the date of mailing of the Proxy Statement to the holders of Parent Common Stock, (iii) the time of the Special Meeting or (iv) the Closing, contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements therein, in light of the circumstances under which they were made, not misleading, except for any change disclosed in writing by or on behalf of the Company to Parent or its counsel prior to the filing thereof pursuant to Section 7.1 hereof. Notwithstanding the foregoing provisions of this Section 3.25, no representation, warranty or covenant is made by the Company with respect to information or statements made or incorporated by reference in the Registration Statement or the Proxy Statement that were not furnished by or on behalf of the Company in writing for inclusion or incorporation by reference therein, including any information furnished by or on behalf of Parent in writing specifically for inclusion or incorporation by reference therein.
3.26. No Additional Representations or Warranties. Except as provided in this Article III or in the Company Closing Certificate, neither any Acquired Company, nor any of their respective Affiliates, nor any of their respective directors, officers, employees, shareholders, partners, members or representatives has made, or is making, any representation or warranty whatsoever to Parent or its Affiliates and no such party shall be liable in respect of the accuracy or completeness of any information provided to Parent or its Affiliates. Without limiting the foregoing, Parent acknowledges that Parent, together with its advisors, has made its own investigation of Acquired Companies and is not relying on any implied warranties or upon any representation or warranty whatsoever as to the prospects (financial or otherwise) or the viability or likelihood of success of the business of the Acquired Companies as conducted after the Closing, as contained in any materials provided by the Company or any of its Affiliates or any of their respective directors, officers, employees, shareholders, partners, members or representatives or otherwise. For the purposes herein, any information provided to, or made available to, Parent by the Acquired Companies shall include any and all information that may be contained or posted prior to 5:00 p.m. (Eastern Time) three (3) Business Days prior to the execution of this Agreement in the electronic data room established by the Company or its representatives in connection with the Transactions.
Article
IV.
REPRESENTATIONS AND WARRANTIES OF parent and merger sub
Except as disclosed in the Parent SEC Documents filed with or furnished to the SEC prior to the date of this Agreement (other than any risk factor disclosures or other similar cautionary or predictive statements therein), Parent and Merger Sub hereby jointly and severally represent and warrant to the Company, as of the date hereof, as follows, except as otherwise set forth on the Parent Disclosure Letter, which exceptions shall apply to (a) the representations and warranties or covenants contained in the Section of this Agreement to which the applicable Section of the Parent Disclosure Letter corresponds in number, (b) any representation and warranty or covenant to which it is referred by cross reference and (c) any other representation or warranty or covenant to the extent it is reasonably apparent on the face of such disclosure that such disclosure is applicable to such representation or warranty or covenant:
| -305- |
4.1. Organization, Existence and Power.
(a) Each of Parent and Merger Sub is a corporation duly incorporated, validly existing and in good standing under the laws of the State of Delaware and has all requisite corporate power and authority required to carry on its business as now conducted, to own or use the properties and assets that it purports to own or use and to perform all of its obligations under all Contracts. Each of Parent and Merger Sub is duly qualified to do business as a foreign corporation and is in good standing in each jurisdiction where the failure to be so qualified or in good standing would not reasonably be expected to have a Material Adverse Effect on Parent. Parent has made available to the Company prior to the date hereof complete and accurate copies of its Organizational Documents and the Organizational Documents of each Subsidiary of Parent, each as currently in effect.
(b) All of the issued and outstanding shares of capital stock of, or other Equity Interests in, each Subsidiary of Parent have been validly issued and are fully paid and nonassessable and are wholly owned, directly or indirectly, by Parent free and clear of all Encumbrances, other than Permitted Encumbrances. Section 4.1(b) of the Parent Disclosure Letter sets forth an accurate and complete list as of the date hereof of each Subsidiary of Parent and each Person in which Parent or any of its Subsidiaries owns an Equity Interest or other voting interest, together with (i) the jurisdiction of incorporation or organization, as the case may be, of each Subsidiary of Parent or such other Person and (ii) the type and percentage of interest held, directly or indirectly, by Parent in each of its Subsidiaries or in each such other Person.
4.2. Authorization.
(a) Each of Parent and Merger Sub has all requisite corporate power and authority to execute and deliver this Agreement and the Ancillary Documents to be executed and delivered by Parent or Merger Sub, as the case may be, pursuant hereto, to consummate the transactions contemplated hereby and thereby and to perform their obligations hereunder and thereunder. The execution and delivery by Parent and Merger Sub of this Agreement and the Ancillary Documents, as applicable, and the consummation by Parent and Merger Sub of the transactions contemplated hereby and thereby have been duly authorized by all necessary action on the part of Parent, Parent Board, Merger Sub and the board of directors of Merger Sub. No other corporate proceedings on the part of Parent or Merger Sub are necessary to authorize this Agreement and the transactions contemplated hereby (other than Parent Requisite Stockholder Consent and approval of Parent, as the sole stockholder of Merger Sub). This Agreement has been, and the Ancillary Documents will be, duly executed and delivered by each of Parent and Merger Sub and is, and the Ancillary Documents will be, the legal, valid and binding obligations of Parent and Merger Sub, enforceable against Parent and Merger Sub in accordance with their terms, in each case, except as such enforceability may be limited by the Enforceability Exceptions.
(b) At a meeting duly called and held, the Parent Board has: (i) determined that this Agreement and the transactions contemplated hereby are fair to, advisable and in the best interests of Parent and its stockholders; (ii) determined that the fair market value of the Company is equal to at least 80% of the amount held in the Trust Account (excluding any deferred underwriting commissions and taxes payable on interest earned) as of the date hereof; (iii) approved the transactions contemplated by this Agreement as a Business Combination; and (iv) resolved to recommend to the stockholders of Parent approval of each of the matters requiring Parent Requisite Stockholder Consent.
| -306- |
4.3. Governmental Consents and Filings. Assuming the accuracy of the representations made by the Company in Article III, no consent, approval, order or authorization of, or registration, qualification, designation, declaration or filing with, any federal, state or local Governmental Authority is required on the part of Parent in connection with the consummation of the transactions contemplated by this Agreement, except for (a) the filing of Parent Restated Certificate, which will have been filed as of the Closing, (b) compliance with any applicable requirements of the Securities Act, the Exchange Act and any other applicable U.S. state or federal securities laws, (c) the filing of the Certificate of Merger, (d) such filings with and approvals of Nasdaq to permit the Parent Common Stock to be issued in connection with the transactions contemplated by this Agreement and the other Ancillary Documents to be listed on Nasdaq, and (e) the filing with the SEC of (1) the Registration Statement and the Proxy Statement and the declaration of the effectiveness thereof by the SEC and (2) such reports under Section 13(a) or 15(d) of the Exchange Act as may be required in connection with this Agreement.
4.4. No Conflict or Violation. The execution, delivery and performance by Parent and Merger Sub of this Agreement and the consummation by Parent and Merger Sub of the transactions contemplated hereby, including the Merger, do not and will not (a) contravene, conflict with, or result in any violation or breach of any provision of the Organizational Documents of Parent or Merger Sub, (b) subject to the exceptions set forth in Section 4.3, contravene, conflict with or result in a violation or breach of any provision of any applicable Law, to the extent that such breach or violation would be reasonably expected to have a Material Adverse Effect on Parent, (c) require any consent or other action by any Person under, result in a breach of, constitute a default, or an event that, with or without notice or lapse of time or both, would result in a breach of, or constitute a default under, or cause or permit the termination, cancellation, acceleration or other change of any right or obligation or the loss of any benefit to which Parent is entitled under any provision of any Contract binding upon Parent, or under which any of the assets of Parent is bound or affected, or any material license, franchise, permit, certificate, approval or other similar authorization affecting, or relating in any way to, the assets or business of Parent or (d) result in the creation or imposition of any Encumbrance on any asset of Parent (except for Permitted Encumbrances).
4.5. No Prior Merger Sub Operations. Merger Sub was formed solely for the purpose of effecting the Merger and has not engaged in any business activities or conducted any operations other than in connection with the transactions contemplated hereby. Parent is the sole stockholder of Merger Sub.
4.6. Capitalization.
(a) Parent Stock. As of the date hereof, the authorized capital stock of Parent consists of (i) 50,000,000 shares of Parent Class A Common Stock, 5,800,000 shares of which are issued and outstanding as of the date hereof, (ii) 2,000,000 shares of Parent Class B Common Stock, 1,437,500 shares of which are issued and outstanding as of the date hereof, (iii) 1,000,000 of preferred stock, none of which is issued and outstanding as of the date hereof.
(b) Parent Warrants. As of the date hereof and without taking into effect the Private Placements, Parent has issued and outstanding 5,750,000 public warrants (the “Parent Public Warrants”) and 2,800,000 private placement warrants (the “Parent Private Placement Warrants”, and together with the Parent Public Warrants, the “Parent Warrants”) entitling the holder thereof to purchase one share of Parent Common Stock at an exercise price of $11.50 per share of Parent Common Stock pursuant to, and subject to adjustments as provided by, the terms of the Parent Warrant Agreement. Subject to the terms of conditions of the Parent Warrant Agreement, the Parent Warrants will be exercisable after giving effect to the Merger for one share of Parent Common Stock at an exercise price of $11.50 per share.
| -307- |
(c) The outstanding shares of Parent Common Stock (i) are duly authorized and validly issued, (ii) are free of any Encumbrances, (iii) were issued in compliance with all applicable Laws and (iv) were not issued in violation of any preemptive rights or rights of first refusal created by statute, Parent’s Organization Documents, or otherwise. Except as set forth in Section 4.6(c) of the Parent Disclosure Letter, (A) there are no Contracts relating to the issuance, sale, transfer or voting of any Equity Interests of Parent and (B) there are no options, warrants, calls, rights, commitments or agreements obligating Parent to issue, deliver, sell, repurchase or redeem, or cause to be issued, delivered, sold, repurchased or redeemed, any equity securities or obligating Parent to grant, or enter into any option, warrant, call, right, commitment or agreement relating to any Equity Interests of Parent. Except as set forth in Section 4.6(c) of the Parent Disclosure Letter, there are no bonds, debentures, notes or other instrument of Indebtedness to which Parent is a party giving or purporting to give the holder thereof the right to vote or consent (or convertible into or exchangeable for securities of Parent having the right to vote or consent) on any matters on which the security holders of Parent may vote.
(d) Other than Merger Sub, of which Parent is the sole stockholder, Parent does not own or have a right to acquire, directly or indirectly, any interest or investment (whether equity or debt) in any corporation, partnership, joint venture, business, trust or other entity. Except for this Agreement and the Transactions, Parent has no interests, rights, obligations or liabilities with respect to, and is not party to, bound by or has its assets or property subject to, in each case whether directly or indirectly, any Contract or transaction which is, or could reasonably be interpreted as constituting, a Business Combination.
4.7. Litigation.
(a) As of the date hereof, there are no Proceedings pending, or to the Knowledge of Parent, threatened in writing against Parent or any of its Subsidiaries or any of their predecessors or against any officer, director, shareholder, employee or agent of Parent or any of its Subsidiaries in their capacity as such or relating to their employment services or relationship with Parent, its Subsidiaries, or any of their Affiliates, except as would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect or prevent, materially delay or materially impair the ability of Parent or Merger Sub to consummate the Transactions.
(b) As of the date hereof, neither Parent nor any of its Subsidiaries is a party to or subject to the provisions of any Governmental Order that restricts the manner in which Parent or any of its Subsidiaries conducts its business, except as would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect or prevent, materially delay or materially impair the ability of Parent or Merger Sub to consummate the Transactions.
4.8. [Reserved].
4.9. Internal Controls; Listing; Financial Statements.
(a) Except as is not required in reliance on exemptions from various reporting requirements by virtue of Parent’s status as an “emerging growth company” within the meaning of the Securities Act, as modified by the JOBS Act, or “smaller reporting company” within the meaning of the Exchange Act, since its initial public offering, (i) Parent has established and maintained a system of internal controls over financial reporting (as defined in Rule 13a-15 and Rule 15d-15 under the Exchange Act) sufficient to provide reasonable assurance regarding the reliability of Parent’s financial reporting and the preparation of Parent’s financial statements for external purposes in accordance with GAAP and (ii) Parent has established and maintained disclosure controls and procedures (as defined in Rule 13a-15 and Rule 15d-15 under the Exchange Act) designed to ensure that material information relating to Parent is made known to Parent’s principal executive officer and principal financial officer by others within Parent.
| -308- |
(b) Each director and executive officer of Parent has filed with the SEC on a timely basis all statements required by Section 16(a) of the Exchange Act and the rules and regulations promulgated thereunder. There are no outstanding loans or other extensions of credit made by Parent to any executive officer (as defined in Rule 3b-7 under the Exchange Act) or director of Parent. Parent has not taken any action prohibited by Section 402 of the Sarbanes-Oxley Act.
(c) Since its initial public offering, Parent has complied in all material respects with the applicable listing and corporate governance rules and regulations of Nasdaq. The issued and outstanding shares of Parent Common Stock are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on Nasdaq. There is no proceeding pending or, to the Knowledge of Parent, threatened against Parent by Nasdaq or the SEC with respect to any intention by such entity to deregister Parent Common Stock or prohibit or terminate the listing of Parent Common Stock on Nasdaq. Parent has taken no action that is designed to terminate the registration of Parent Common Stock under the Exchange Act.
(d) The Parent SEC Reports contain true and complete copies of the applicable Parent Financial Statements. Except as disclosed in the Parent SEC Reports, Parent Financial Statements (i) fairly present in all material respects the consolidated financial position of Parent and its consolidated Subsidiaries, as at the respective dates thereof, and the consolidated results of their operations and their consolidated cash flows for the respective periods then ended, (ii) were prepared in conformity with GAAP applied on a consistent basis during the periods involved (except as may be indicated therein or in the notes thereto), and (iii) comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC, the Exchange Act and the Securities Act in effect as of the respective dates thereof.
4.10. Investment Company Act; JOBS Act. Parent is not an “investment company” or a Person directly or indirectly “controlled” by or acting on behalf of an “investment company”, in each case within the meaning of the Investment Company Act. Parent constitutes an “emerging growth company” within the meaning of the JOBS Act.
4.11. Business Activities; Liabilities.
(a) Since its date of incorporation, neither Parent nor Merger Sub has carried on any business or conducted any material operations other than: (i) directed towards the accomplishment of a Business Combination and (ii) the execution of this Agreement and the Ancillary Documents to which it is a party, the performance of its obligations hereunder and thereunder and matters ancillary thereto. Except as set forth in Section 4.11 of the Parent Disclosure Letter and other than under this Agreement and the Ancillary Documents or pursuant to the performance of its obligations thereunder, neither Parent nor Merger Sub has any liabilities.
(b) Except as set forth in Parent’s Organizational Documents or as otherwise contemplated by this Agreement or the Ancillary Documents and the Transactions, there is no Contract, commitment, or Governmental Order binding upon Parent or Merger Sub or to which Parent or Merger Sub is a party which has or would reasonably be expected to have the effect of prohibiting or impairing any business practice of Parent or Merger Sub or any acquisition of property by Parent or Merger Sub or the conduct of business by Parent or Merger Sub as currently conducted or as contemplated to be conducted as of the Closing, other than such effects, individually or in the aggregate, which have not been and would not reasonably be expected to be material to Parent or Merger Sub.
(c) Except for this Agreement and the Ancillary Documents and the Transactions, Parent has no material interests, rights, obligations or liabilities with respect to, and is not party to, bound by or has its assets or property subject to, in each case whether directly or indirectly, any Contract or transaction which is, or would reasonably be interpreted as constituting, a Business Combination.
| -309- |
(d) Except for this Agreement and the agreements expressly contemplated hereby or with respect to advisors and consultants in connection with the Transactions (including any agreements permitted by Section 6.1) or Parent’s initial public offering and contemporaneous private placement, neither Parent nor Merger Sub is and at no time has been, party to any Contract with any Person that would require payments by Parent or Merger Sub in excess of $50,000 in the aggregate with respect to any individual Contract or more than $250,000 in the aggregate when taken together with all other Contracts (other than this Agreement and the agreements expressly contemplated hereby (including any agreements permitted by Section 6.1)).
4.12. Certain Transactions. Except as described in the Parent SEC Reports, set forth on Schedule 4.12 of the Parent Disclosure Letter or as relate to (a) reimbursement for expenses incurred on behalf of Parent or (b) with respect to any Person’s ownership of Parent Common Stock, there are no material Contracts between Parent, on the one hand, and, on the other hand, (i) any present or former manager, employee, officer or director of Parent or its Affiliates or (ii) any record or beneficial owner of more than five percent (5%) of the outstanding shares of Parent Common Stock as of the date hereof or its or his Affiliates.
4.13. Property. Subject to the restrictions on the Trust Account set forth in the Trust Agreement (each as defined below), the material property and assets that Parent owns are free and clear of all Encumbrances, except for Permitted Encumbrances. With respect to the material property and assets it leases, Parent is in compliance in all material respects with such leases and, to its Knowledge, holds a valid leasehold interest free of any Encumbrances other than those of the lessors of such property or assets. Parent does not own, and has not ever owned, any real property.
4.14. Undisclosed Liabilities. There is no liability, debt or obligation of or claim or judgment against Parent or any of its Subsidiaries, (whether direct or indirect, absolute or contingent, accrued or unaccrued, known or unknown, liquidated or unliquidated, or due or to become due), except for liabilities and obligations (a) reflected or reserved for on the financial statements or disclosed in the notes thereto included in the Parent SEC Reports, (b) that have arisen since the date of the most recent balance sheet included in the Parent SEC Reports in the ordinary course of the operation of business of Parent, (c) which would not have, or would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect on Parent, (d) liabilities under this Agreement, or (e) as set forth on Section 4.14 of the Parent Disclosure Letter.
4.15. Absence of Changes. Since the date of Parent’s incorporation through the date of this Agreement:
(a) there has not been any event that has had or would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect on Parent; and
(b) except as set forth on Section 4.15 of the Parent Disclosure Letter, Parent has conducted its business and operated its properties in the Ordinary Course of Business consistent with past practice in all material respects.
4.16 Employee Matters; Benefits. Parent does not have and has not ever had any employees. Other than reimbursement of any out-of-pocket expenses incurred by Parent’s officers and directors in connection with activities on Parent’s behalf in an aggregate amount not in excess of the amount of cash held by Parent outside of the Trust Account, Parent does not have any unsatisfied liability with respect to any director, officer, individual consultant or employee. Parent does not maintain, sponsor or have any liability (contingent or otherwise) with respect to, any “employee benefit plan” as defined in Section 3(3) of ERISA or any other plan, policy, program or agreement providing compensation or other benefits to any current or former director, officer, individual consultant or employee, which are maintained, sponsored or contributed to by Parent, any Subsidiary or any other trade or business that would be treated as a single employer with Parent under Title IV of ERISA.
| -310- |
4.17 Tax Matters.
(a) Parent has timely filed all Tax Returns required to have been filed by it and timely paid all Taxes required to be paid by it (whether or not shown on such Tax Returns). Parent is not currently the beneficiary of any extension of time within which to file any Tax Return (other than automatic extensions obtained in the Ordinary Course of Business). No written claim has ever been made by a Tax authority or other Governmental Authority in a jurisdiction where Parent does not file Tax Returns that Parent is or may be subject to taxation by that jurisdiction.
(b) No deficiencies for Taxes with respect to Parent have been claimed, proposed or assessed in writing by any Tax authority or other Governmental Authority. There are no pending or, to Parent’s Knowledge, threatened audits, assessments or other actions for or relating to any liability in respect of Taxes of Parent. Parent (and any predecessor thereof) has not waived any statute of limitations in respect of Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency that is still in effect, nor has any request been made in writing for any such extension or waiver that is still pending.
(c) There are no liens for Taxes upon any property or asset of Parent (other than statutory liens for current taxes not yet due and payable).
(d) Parent will not be required to include any item of income in, or exclude any item of deduction from, taxable income for any period (or any portion thereof) beginning after the Closing Date as a result of any installment sale or other transaction on or prior to the Closing Date, any accounting method change made or required to be made on or prior to the Closing Date or any agreement with any Tax authority entered into on or prior to the Closing Date, the use of an improper method of accounting for any period or portion thereof ending prior to the Closing Date, any private letter ruling obtained by Parent prior to the Closing, any prepaid amount received on or prior to the Closing, an election under Section 108(i) of the Code or election under Section 965(h) of the Code, or any intercompany transaction or excess loss account described in the Treasury Regulations promulgated pursuant to Section 1502 of the Code (or any corresponding or similar provision of state, local or foreign law) in respect of taxable periods (or portions thereof) effected or existing prior to the Closing.
(e) Parent is not a partner for Tax purposes with respect to any joint venture, partnership, or other arrangement or Contract which is treated as a partnership for Tax purposes and with respect to which it treats itself as a partner for Tax purposes.
(f) Parent is not a party to or bound by any Tax indemnity agreement, Tax sharing agreement, Tax allocation agreement or similar Contract (other than customary provisions in Contracts not primarily relating to Taxes).
(g) Parent has not been a party to a transaction that is or is substantially similar to a “listed transaction,” as such term is defined in Treasury Regulations Section 1.6011-4(b)(2), or any other transaction requiring disclosure under analogous provisions of state, local or foreign Tax law.
| -311- |
(h) Parent has never been a member of an affiliated group filing a consolidated federal income Tax Return (other than a group of which Parent is the common parent) or a combined, consolidated, unitary or other affiliated group Tax Return for state, local or foreign Tax purposes.
(i) Parent has timely withheld and paid all Taxes required to have been withheld and paid in connection with amounts paid or owing to any employee, independent contractor, creditor, equityholders of Parent. Parent has properly classified all individuals providing services to it as employees or non-employees for all relevant Tax purposes.
(j) In the last three years, Parent has not been a party to any distribution that the parties to which treated as satisfying the requirements of Section 355 of the Code.
(k) It is the present intention of Parent to continue at least one significant historic business line of the Company or to use at least a significant portion of the Company’s historic business assets in a business, in each case within the meaning of Treasury Regulation §1.368-1(d).
(l) Merger Sub was formed solely for the purpose of effectuating the Transaction and has not undertaken any business activities other than matters incidental to such purpose.
(m) To the Knowledge of Parent, there are no facts, circumstances or plans that, either alone or in combination, could reasonably be expected to prevent the Transaction from qualifying for the Intended Tax Treatment.
4.18 Compliance with Laws; Permits. Parent is, and at all times since the date of its formation has been, in compliance, in all material respects, with any applicable Law. Parent has all material Permits, and Parent is in compliance in all material respects with such Permits. Parent has not received any written notice or other written communication regarding any violation of or failure to comply with any term or requirement of any material Permit held as of the date hereof by Parent or any revocation, withdrawal, suspension, cancellation, termination or modification of any such Permit. Section 4.18 of the Parent Disclosure Letter sets forth (a) an accurate and complete list of all material Permits issued to Parent and (b) an accurate and complete list of all material Permits for which Parent has applied or has taken the steps necessary to secure or maintain or that Parent otherwise intends to obtain (in each case, with respect to material Permits that have not yet been obtained by Parent). Each such material Permit has that been validly issued or obtained is, and immediately following the consummation of the transaction contemplated hereby will be, in full force and effect.
4.19. Foreign Corrupt Practices Act. Neither Parent nor any of its directors, officers, employees or, to the Knowledge of Parent, agents have, directly or indirectly, made, offered, promised or authorized any payment or gift of any money or anything of value to or for the benefit of any “foreign official” (as such term is defined in the FCPA), foreign political party or official thereof or candidate for foreign political office for the purpose of (a) influencing any official act or decision of such official, party or candidate, (b) inducing such official, party or candidate to use his, her or its influence to affect any act or decision of a foreign Governmental Authority, or (c) securing any improper advantage, in the case of (a), (b) and (c) above in order to assist Parent or any of its Affiliates in obtaining or retaining business for or with, or directing business to, any Person. Neither Parent nor any of its directors, officers, employees or, to the Knowledge of Parent, agents have made or authorized any bribe, rebate, payoff, influence payment, kickback or other unlawful payment of funds or received or retained any funds in violation of any law, rule or regulation. Neither the Parent, nor to the Knowledge of the Parent, any director, officer, agent, employee, Affiliate or Person acting on behalf of the Parent is currently subject to any U.S. sanctions administrated by OFAC.
| -312- |
4.20. SEC Filings. Parent has timely filed or furnished all statements, prospectuses, registration statements, forms, reports and documents required to be filed by it with the SEC since its initial public offering pursuant to the Exchange Act or the Securities Act (collectively, and together with any exhibits and schedules thereto and other information incorporated therein, and as they have been supplemented, modified or amended since the time of filing, the “Parent SEC Reports”). Each of the Parent SEC Reports, as of the respective date of its filing, and as of the date of any amendment, complied in all material respects with the applicable requirements of the Securities Act, the Exchange Act, the Sarbanes-Oxley Act and any rules and regulations promulgated thereunder applicable to the Parent SEC Reports. As of the respective date of its filing, the Parent SEC Reports did not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading. As of the date hereof, there are no outstanding or unresolved comments in comment letters received from the SEC with respect to the Parent SEC Reports.
4.21. Trust Account. As of the date hereof, Parent has $58,079,455.21 in the account established by Parent for the benefit of its public stockholders at the Trustee (the “Trust Account”), such monies invested in United States Government securities within the meaning of Section 2(a)(16) of the Investment Company Act of 1940, having a maturity of 185 days or less, or in money market funds meeting the conditions of paragraphs (d)(2), (d)(3), (d)(4) and (d)(5) of Rule 2a-7 promulgated under the Investment Company Act of 1940 and held in trust by Continental Stock Transfer & Trust Company (the “Trustee”) pursuant to that certain Trust Agreement, dated as of January 22, 2021, between Parent and the Trustee (the “Trust Agreement”). The Trust Agreement is valid and in full force and effect and enforceable in accordance with its terms (subject to the Enforceability Exception) and has not been amended or modified. There are no separate Contracts, side letters or other arrangements or understandings (whether written or unwritten, express or implied) that would cause the description of the Trust Agreement in the Parent SEC Reports to be inaccurate or that would entitle any Person (other than stockholders of Parent holding Parent Common Stock sold in Parent’s initial public offering who shall have elected to redeem their shares of Parent Common Stock pursuant to Parent Governing Documents) to any portion of the proceeds in the Trust Account. Prior to the Closing and except as permitted by the Trust Agreement, none of the funds held in the Trust Account may be released. There are no Legal Proceedings pending or, to the Knowledge of Parent, threatened with respect to the Trust Account. Parent has performed all material obligations required to be performed by it to date under, and is not in default, breach or delinquent in performance or any other respect (claimed or actual) in connection with, the Trust Agreement, and no event has occurred which, with due notice or lapse of time or both, would constitute such a default or breach thereunder. As of the Effective Time, the obligations of Parent to dissolve or liquidate pursuant to Parent’s Organizational Documents shall terminate, and as of the Effective Time, Parent shall have no obligation whatsoever pursuant to Parent’s Organizational Documents to dissolve and liquidate the assets of Parent by reason of the consummation of the transactions contemplated hereby. To the Knowledge of Parent, as of the date hereof, following the Effective Time, no Parent Stockholder shall be entitled to receive any amount from the Trust Account, except to the extent such Parent Stockholder validly elects to redeem their shares of Parent Common Stock in connection with the Redemption Offer. As of the date hereof, assuming the accuracy of the representations and warranties of the Company contained herein and the compliance by the Company with its obligations hereunder, neither Parent or Merger Sub have any reason to believe that any of the conditions to the use of funds in the Trust Account will not be satisfied or funds available in the Trust Account will not be available to Parent and Merger Sub on the Closing Date.
4.22. Nasdaq Listing. The issued and outstanding shares of Parent Common Stock are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on Nasdaq under the symbol “FOXW.” The Parent Warrants are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on Nasdaq under the symbol “FOXWW”. Parent is in compliance in all material respects with the rules of Nasdaq, and there is no action or proceeding pending, or to the Knowledge of Parent, threatened in writing against Parent by Nasdaq, the Financial Industry Regulatory Authority or the SEC with respect to any intention by such entity to deregister the Parent Common Stock or terminate the listing of Parent Common Stock on Nasdaq. None of Parent or its Affiliates has taken any action in an attempt to terminate the registration of the Parent Common Stock or Parent Warrants under the Exchange Act except as contemplated by this Agreement.
| -313- |
4.23. [Reserved].
4.24. Valid Issuance. The shares of Parent Common Stock issuable as Per Share Consideration, when issued, sold and delivered in accordance with the terms of this Agreement, will be duly authorized and validly issued, fully paid and nonassessable and will be issued free and clear of any Encumbrances (other than transfer restrictions under applicable securities Laws) or any preemptive rights.
4.25. Takeover Statutes and Charter Provisions. Each of the board of directors of Parent and Merger Sub has taken all action necessary so that the restrictions on a “business combination” (as such term is used in Section 203 of the DGCL) contained in Section 203 of the DGCL or any similar restrictions under any applicable foreign Laws will be inapplicable to this Agreement and the Merger. As of the date of this Agreement, no “fair price,” “moratorium,” “control share acquisition” or other applicable antitakeover Law or similar domestic or foreign Law applies with respect to Parent or Merger Sub in connection with this Agreement or the Merger. As of the date of this Agreement, there is no stockholder rights plan, “poison pill” or similar antitakeover agreement or plan in effect to which Parent or Merger Sub is subject, party or otherwise bound.
4.26. No Brokers. Except as set forth on Section 4.26 of the Parent Disclosure Letter, there is no investment banker, broker, finder or other intermediary that has been retained by or is authorized to act on behalf of Parent or who is or may be entitled to any fee or commission from Parent or any of its Affiliates in connection with the transactions contemplated by this Agreement.
4.27. Registration Statement and Proxy Statement. On the effective date of the Registration Statement, the Registration Statement, and when first filed in accordance with Rule 424(b) and/or filed pursuant to Section 14A, the Proxy Statement (or any amendment or supplement thereto), shall comply in all material respects with the applicable requirements of the Securities Act and the Exchange Act. On the date of any filing pursuant to Rule 424(b) and/or Section 14A, the date the Proxy Statement, as applicable, is first mailed to the Parent Stockholders, at the time of the Special Meeting, and at the Effective Time, the Proxy Statement, as applicable (together with any amendments or supplements thereto) will not include any untrue statement of a material fact or omit to state a material fact necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading; provided, however, that Parent makes no representations or warranties as to the information contained in or omitted from the Registration Statement or the Proxy Statement in reliance upon and in conformity with information furnished in writing to Parent by or on behalf of the Company specifically for inclusion in the Registration Statement or the Proxy Statement
4.28. No Additional Representations or Warranties. Except as provided in this Article IV or in the Parent Closing Certificate, neither Parent nor any of its respective Affiliates, nor any of their respective directors, officers, employees, shareholders, partners, members or representatives has made, or is making, any representation or warranty whatsoever to the Company or its Affiliates and no such party shall be liable in respect of the accuracy or completeness of any information provided to the Company or its Affiliates. Without limiting the foregoing, the Company acknowledges that the Company, together with its advisors, has made its own investigation of Parent and is not relying on any implied warranties or upon any representation or warranty whatsoever as to the prospects (financial or otherwise) or the viability or likelihood of success of the business of Parent as conducted after the Closing, as contained in any materials provided by Parent or any of its Affiliates or any of their respective directors, officers, employees, shareholders, partners, members or representatives or otherwise.
| -314- |
Article V.
COVENANTS OF THE COMPANY
5.1. Interim Operations. From and after the date of this Agreement until the earlier of (A) the termination of this Agreement in accordance with the provisions of Section 9.1 or (B) the Effective Time (such period, the “Interim Period”), except as expressly contemplated by this Agreement, the Company shall, and shall cause each other Acquired Company to, conduct its business in the Ordinary Course of Business. Without limiting the generality of the foregoing, during the Interim Period, except as expressly contemplated by this Agreement, as set forth on Section 5.1 of the Company Disclosure Letter, as required by applicable Law (including COVID-19 Measures) or pursuant to the written consent of Parent, not to be unreasonably withheld, conditioned, or delayed, the Company shall not, and shall cause each of the other Acquired Companies not to:
(a) amend its certificate of incorporation, bylaws or other Organizational Documents (whether by merger, consolidation or otherwise);
(b) declare, set aside or pay any dividend or other distribution (whether in cash, stock, debt or property or any combination thereof) in respect of any equity securities of any Acquired Company, or redeem, repurchase or otherwise acquire or offer to redeem, repurchase, or otherwise acquire any equity securities of any Acquired Company;
(c) split, combine, subdivide or reclassify any shares of capital stock, membership interests or other equity interests;
(d) (i) issue, transfer, deliver, sell, pledge or otherwise encumber any Equity Interests of any Acquired Company;
(e) make any material capital expenditures or incur any obligations or liabilities in respect thereof, other than agreements to advance the clinical development programs, provided that the Company shall consult with Parent and consider its recommendations relating thereto prior to entering into any such agreements;
(f) directly or indirectly acquire by merger or consolidate with, or purchase substantially all of the assets of, any corporation, partnership, association, or other business organization or division thereof;
(g) sell, lease, license or otherwise transfer, or create, incur, assume or suffer to exist any Encumbrance (other than Permitted Encumbrances) on, any of the assets, securities, properties, interests or businesses of the Acquired Companies, other than out-licenses of AER-501, AER-601, AER-901, or other products to third parties outside the United States;
(h) make any loans, advances or capital contributions to, or investments in, any other Person;
(i) make any payments constituting an Affiliate Transaction (other than salary payments in the Ordinary Course of Business consistent with past practice);
| -315- |
(j) create, incur, assume or otherwise become liable with respect to any Funded Indebtedness;
(k) modify, amend, cancel, terminate or waive any rights under any Material Contract, enter into any Contract that would have been a Material Contract had it been entered into prior to the date of this Agreement, or otherwise waive, release or assign any material rights, claims or benefits of any Acquired Company;
(l) (i) grant or agree to grant any severance or termination pay policies or increase any form of compensation or benefits payable to any director, officer, advisor, consultant, or employee of any Acquired Company or any of its ERISA Affiliates, including pursuant to any Employee Plan; (ii) adopt, enter into, modify or terminate any Employee Plan; (iii) accelerate the vesting or payment of any compensation or benefits under any Employee Plan; (iv) grant any equity or equity-linked awards or other bonus, commission or other incentive compensation or provide any loan to any director, officer, advisor, consultant or employee of any Acquired Company or any of its ERISA Affiliates or (v) hire, demote, promote, change the title of, or terminate any employee, officer, director or consultant of any Acquired Company or any of its ERISA Affiliates or materially change the management structure of any Acquired Company;
(m) implement or announce any employee layoffs, furloughs, reductions in force, reductions in compensation, hour or benefits, work schedule changes or similar actions that could implicate the WARN Act;
(n) hire any employees with an annual base compensation of over $350,000, or terminate the employment of any employees with an annual base compensation of over $100,000, other than for cause;
(o) fail to maintain, or allow to lapse, dispose of or abandon, including by failure to pay the required fees in any jurisdiction, any Company Intellectual Property, or grant permission to enter into the public domain any trade secrets included in the Company Intellectual Property;
(p) transfer or grant to any third party any rights with respect to any Intellectual Property;
(q) modify or amend in any material respect, terminate, permit to lapse, waive, or fail to enforce any material right or remedy under any Material Contract, or enter into any Contract that, if entered prior to the date of this Agreement, would constitute a Material Contract;
(r) change any Acquired Company’s methods of accounting or accounting practices, except as required by concurrent changes in GAAP as agreed to by such Acquired Company’s independent public accountants, or in connection with the Company’s preparation for transition to public company accounting;
(s) make any change to their cash management practices, policies or procedures with respect to collection of accounts receivable, establishment of reserves for uncollectible accounts, accrual of accounts receivable, inventory control, prepayment of expenses, payment of trade accounts payable, accrual of other expenses, deferral of revenue and acceptance of customer deposits;
(t) commence, settle, or offer or propose to settle, (i) any Proceeding involving or against any Acquired Company, (ii) any equityholder litigation or dispute against any Acquired Company or any of its officers or directors or (iii) any Proceeding that relates to the transactions contemplated by this Agreement;
| -316- |
(u) cancel, settle or forgive any third party Indebtedness owed to the Company greater than $50,000 individually or $100,000 in the aggregate;
(v) (i) make or change any material Tax election, settle or compromise any claim, notice, audit report or assessment in respect of Taxes, (ii) enter into any Tax allocation agreement, Tax sharing agreement, Tax indemnity agreement, pre-filing agreement, advance pricing agreement, cost sharing agreement or closing agreement relating to any Tax, (iii) file any federal or state income Tax Return or any other material Tax Return, (iv) amend any Tax Return, (v) surrender or forfeit any right to claim a Tax refund or (vi) consent to any extension or waiver of the statute of limitations period applicable to any Tax claim or assessment;
(w) form or acquire any Subsidiaries;
(x) file any prospectus supplement or registration statement or consummate any offering of securities that requires registration under the Securities Act or that includes any actual or contingent commitment to register such securities under the Securities Act in the future;
(y) liquidate, dissolve or effect a recapitalization or reorganization in any form of transaction; or
(z) authorize or agree, resolve or commit to do any of the foregoing.
5.2. Access and Information.
(a) During the Interim Period, the Company shall, and shall cause each other Acquired Company to, (i) give Parent and its Representatives reasonable access to the offices, properties, books and records of the Acquired Companies, (ii) furnish to Parent and its Representatives such financial and operating data, information related to the Acquired Companies as such Persons may reasonably request and (iii) instruct the Company’s Representatives to cooperate with Parent in its investigation of the Acquired Companies. Any investigation pursuant to this Section 5.2(a) shall be conducted in such manner as not to interfere unreasonably with the conduct of the business of the Acquired Companies.
(b) Notwithstanding anything herein to the contrary in this Section 5.2, no access or examination contemplated by this Section 5.2 shall be permitted to the extent that it would require any Acquired Company to waive the attorney-client privilege or attorney work product privilege, or violate any applicable Law; provided, that each Acquired Company (i) shall be entitled to withhold only such information that may not be provided without causing such violation or waiver, (ii) shall provide to Parent all related information that may be provided without causing such violation or waiver (including, to the extent permitted, redacted versions of any such information), and (iii) shall enter into such effective and appropriate joint-defense agreements or other protective arrangements as may be reasonably requested by Parent in order that all such information may be provided to Parent without causing such violation or waiver.
5.3. No Claim Against the Trust Account. The Company acknowledges that Parent has established the Trust Account for the benefit of Parent’s public stockholders and that disbursements from the Trust Account are available only in the limited circumstances set forth in the Parent SEC Reports, Parent’s Organizational Documents, and the Trust Agreement. The Company further acknowledges that Parent’s sole assets consist of the cash proceeds of Parent’s initial public offering and private placements of its securities, and that substantially all of these proceeds have been deposited in the Trust Account for the benefit of its public stockholders. The Company further acknowledges that, if the transactions contemplated by this Agreement, or in the event of termination of this Agreement, another Business Combination, are or is not consummated by April 22, 2022 (as may be extended pursuant to Section 6.11 below, the “SPAC Termination Date”) or such later date as approved by the stockholders of Parent to complete a Business Combination, Parent will be obligated to return to its stockholders the amounts being held in the Trust Account. Accordingly, the Company (on behalf of itself and its Affiliates) hereby waives any past, present or future claim of any kind against, and any right to access, the Trust Account, any trustee of the Trust Account and Parent to collect from the Trust Account any monies that may be owed to them by Parent or any of its Affiliates for any reason whatsoever, and will not seek recourse against the Trust Account at any time for any reason whatsoever, including, without limitation, for any willful breach of this Agreement. This Section 5.3 shall survive the termination of this Agreement for any reason.
| -317- |
5.4. Exclusivity. From the date hereof until the Closing, the Company shall not, and shall cause its Subsidiaries, officers, employees, managers, directors, and agents and shall direct its representatives, accountants, consultants, investment bankers, legal counsel and advisors not to, directly or indirectly, (a) solicit, initiate, knowingly encourage or discuss any offer, inquiry, proposal or indication of interest, written or oral, (whether binding or non-binding) from any Person (other than Parent or its Affiliates in connection with the transactions contemplated hereby) relating to an Alternative Company Transaction (an “Alternative Transaction Proposal”), (b) initiate any discussions or negotiations with any Person with respect to, or provide any non-public information or data concerning the Company or any of its Subsidiaries to any Person relating to, an Alternative Transaction Proposal or afford to any Person access to the business, properties, assets or personnel of the Company or any of its Subsidiaries in connection with an Alternative Transaction Proposal, (c) enter into any acquisition agreement, business combination, merger agreement or similar definitive agreement, or any letter of intent, memorandum of understanding or agreement in principle, or any other Contract relating to an Alternative Transaction Proposal or accept any offer relating to an Alternative Transaction Proposal, (d) approve, endorse or recommend, or propose publicly to approve, endorse or recommend any Alternative Transaction Proposal or (e) otherwise furnish any information with respect to, assist or knowingly participate in or facilitate in any other manner any effort or attempt by any Person (other than Parent or its Affiliates) to do or seek to do any of the foregoing. The Company shall notify Parent promptly if any Person makes to the Company any Alternative Transaction Proposal, which notice shall include a copy of such Alternative Transaction Proposal (or, where such Alternative Transaction Proposal is not submitted by such Person in writing, a reasonably detailed description of the material terms and conditions of such Alternative Transaction Proposal). Upon the effectiveness of this Agreement, the Company shall immediately terminate all discussions and negotiations with any Persons related to an Alternative Company Transaction, and as promptly as practicable thereafter request that each such Person promptly return or destroy all confidential information concerning the Company and its Subsidiaries and the Company shall take all reasonable necessary actions to secure its rights and ensure the performance of any such Person’s obligations under any applicable confidentiality agreement.
5.5. Registration Statement and Proxy Statement Filing; Information Supplied.
(a) The Company shall use reasonable best efforts to deliver to Parent by January 1, 2022, audited financial statements, including consolidated balance sheets, statements of operations and comprehensive income (loss), statements of changes in equity (deficiency) and statements of cash flows of the Company and its Subsidiaries as of and for the years ended December 31, 2020 and December 31, 2019 in each case, prepared in accordance with GAAP and Regulation S-X, and in compliance with the Securities Act and the Exchange Act, for inclusion in the Registration Statement and the Proxy Statement (the “Company S-4 Financial Statements”). To the extent required under the Securities Act or the Exchange Act, the Company shall deliver to Parent any additional required audited or interim unaudited financial statements, including, without limitation, unaudited pro forma financial statements that comply with the requirements of Regulation S-X under the rules and regulations of the SEC (as interpreted by the staff of the SEC). The Company shall be available to, and the Company and its Subsidiaries shall use reasonable best efforts to make their officers and employees available to, in each case, during normal business hours and upon reasonable advance notice, Parent and its counsel in connection with (i) the drafting of the Registration Statement and the Proxy Statement and (ii) responding in a timely manner to comments on the Registration Statement and the Proxy Statement from the SEC. Parent shall reasonably cooperate with the Company in connection with the preparation for inclusion in the Proxy Statement of the pro forma financial statements described above.
| -318- |
(b) From and after the date on which the Proxy Statement is mailed to Parent’s stockholders, the Company will give Parent prompt written notice of any action taken or not taken by the Company or its Subsidiaries or of any development regarding the Company or its Subsidiaries, in any such case which is known by the Company, that would cause the Proxy Statement to contain an untrue statement of a material fact or omit to state a material fact necessary in order to make the statements, in light of the circumstances under which they were made, not misleading; provided, that, if any such action shall be taken or fail to be taken or such development shall otherwise occur, Parent and the Company shall cooperate fully to cause an amendment or supplement to be made promptly to the Proxy Statement, such that the Proxy Statement no longer contains an untrue statement of a material fact or omits to state to state a material fact necessary in order to make the statements, in light of the circumstances under which they were made, not misleading.
5.6. Amendments to Third Party Contracts. Prior to the Closing Date, the Company shall use commercially reasonable efforts to enter into an amendment with respect to each of the third-party Contracts set forth in Section 5.6 of the Company Disclosure Letter.
Article VI.
COVENANTS of Parent
6.1. Interim Operations. During the Interim Period, except as expressly contemplated by this Agreement, Parent shall operate its business in the Ordinary Course of Business. Without limiting the generality of the foregoing, except as expressly contemplated by this Agreement, as set forth on Section 6.1 of the Parent Disclosure Letter, as required by applicable Law (including COVID-19 Measures) or pursuant to the written consent of the Company, Parent shall not:
(a) amend the Trust Agreement, its certificate of incorporation, bylaws or other Organizational Documents (whether by merger, consolidation or otherwise);
(b) declare, set aside or pay any dividend or other distribution (whether in cash, stock, debt or property or any combination thereof) in respect of any equity securities of Parent, or redeem, repurchase or otherwise acquire or offer to redeem, repurchase, or otherwise acquire any equity securities of Parent, except for (i) transactions that would require an adjustment pursuant to Section 1.11, and for which the proper adjustment is made, (ii) in connection with a Private Placement conducted in compliance with applicable securities Laws, and (iii) the redemption of any shares of Parent Common Stock required by the Redemption Offer or as otherwise required by Parent’s Organizational Documents in order to consummate the Transactions, repurchase, redeem or otherwise acquire, or offer to repurchase, redeem or otherwise acquire, any capital stock of, or other equity interests in, Parent;
(c) (i) issue, transfer, deliver, sell, pledge or otherwise encumber any shares of any Equity Interests of Parent or Merger Sub, other than (A) in connection with the exercise of any Parent Warrants outstanding on the date hereof or (B) the Transactions, or (C) as otherwise permitted by Section 6.1(b) or (ii) amend, modify or waive any of the terms or rights set forth in any Parent Warrant or the Parent Warrant Agreement or (ii) amend any term of any equity securities of Parent or Merger Sub (whether by merger, consolidation or otherwise);
| -319- |
(d) acquire (by merger, consolidation, acquisition of stock or assets or otherwise), directly or indirectly, any assets, securities, properties, interests or businesses;
(e) create, incur, assume or otherwise become liable with respect to any Indebtedness;
(f) liquidate, dissolve or effect a recapitalization or reorganization in any form of transaction;
(g) amend or modify the Lock-Up Agreement in a manner that would have the effect of decreasing the Founder Share Lock-up Periods (as defined therein) with respect to any of the Parent Common Stock restricted thereby to a period ending (x) in the event that a Private Placement is consummated in connection with the Closing, less than ninety (90) days following the effectiveness of the Form S-1 Shelf (as defined in the Registration Rights Agreement) (other than with respect to any of the Parent Common Stock transferred to the investor or investors participating in such Private Placement in connection with the completion thereof, as to which no such limitation shall apply), and (y) otherwise, less than one hundred and eighty (180) days after the Closing,
(h) agree, commit or authorize, in writing or otherwise, to take any of the foregoing actions.
6.2. Trust Account. Prior to or at the Closing (subject to the satisfaction or waiver of the conditions set forth in Article X), Parent shall make appropriate arrangements to cause the funds in the Trust Account to be disbursed in accordance with the Trust Agreement for the following: (a) the redemption of shares of Parent Common Stock required pursuant to the Redemption Offer; and (b) the balance of the assets in the Trust Account, if any, after payment of the amounts required under the foregoing clause (a) (such balance, the “Trust Account Amount”), to be disbursed to Parent or as Parent may direct with the prior written consent of the Company.
6.3. Access and Information.
(a) During the Interim Period, Parent shall (i) give Company and its Representatives reasonable access to the offices, properties, books and records of Parent, (ii) furnish to Company and its Representatives such financial and operating data, information related to Parent as such Persons may reasonably request and (iii) instruct its Representatives to cooperate with Company in its investigation of Parent. Any investigation pursuant to this Section 6.3(a) shall be conducted in such manner as not to interfere unreasonably with the conduct of the business of Parent.
(b) Notwithstanding anything herein to the contrary in this Section 6.3, no access or examination contemplated by this Section 6.3 shall be permitted to the extent that it would require Parent to waive the attorney-client privilege or attorney work product privilege, or violate any applicable Law; provided, that Parent (i) shall be entitled to withhold only such information that may not be provided without causing such violation or waiver, (ii) shall provide to Company all related information that may be provided without causing such violation or waiver (including, to the extent permitted, redacted versions of any such information), and (iii) shall enter into such effective and appropriate joint-defense agreements or other protective arrangements as may be reasonably requested by Company in order that all such information may be provided to Company without causing such violation or waiver.
| -320- |
6.4. Exclusivity. From and after the date of this Agreement until the Closing Date, Parent shall not take, nor shall it permit any of its Affiliates to take, and shall not authorize and will instruct its Representatives not to, whether directly or indirectly, any action to solicit, initiate, continue or engage in discussions or negotiations with, or enter into any agreement, letter of intent, memorandum of understanding or agreement in principle with, or encourage, respond, provide information to or commence due diligence with respect to, any Person (other than the Company, its stockholders or any of their Affiliates or Representatives), concerning, relating to or which is intended or is reasonably likely to give rise to or result in, any offer, inquiry, proposal or indication of interest, written or oral relating to any Business Combination (a “Business Combination Proposal”) other than with the Company, its stockholders and their respective Affiliates and Representatives. Parent shall, and shall cause its Affiliates to, and shall authorize and instruct its Representatives to, immediately cease any and all existing discussions or negotiations with any Person conducted prior to the date hereof with respect to, or which is reasonably likely to give rise to or result in, a Business Combination Proposal. Parent shall be liable for any breach of this Section 6.4 by any of its Representatives.
6.5. Indemnification; Directors’ and Officers’ Insurance.
(a) For a period of six (6) years from and after the Effective Time, Parent and the Surviving Corporation agree that they will indemnify and hold harmless, to the fullest extent Parent, Merger Sub or the Company would be permitted to do so under applicable Law or their respective Organizational Documents in effect as of the date of this Agreement (excluding, for avoidance of doubt, any indemnification for gross negligence, fraud, or willful misconduct), each present and former (determined as of the Effective Time) director and officer of Parent, Merger Sub and the Company and each of their respective Subsidiaries, in each case, when acting in such capacity (collectively, the “Indemnified Parties”), against any costs or expenses (including reasonable attorneys’ fees), judgments, fines, losses, claims, damages or liabilities (collectively, “Costs”) incurred in connection with, arising out of or otherwise related to any Proceeding, in connection with, arising out of or otherwise related to matters existing or occurring at or prior to the Effective Time, whether asserted or claimed prior to, at or after the Effective Time, including in connection with (i) the Transactions, and (ii) actions to enforce this provision or any other indemnification or advancement right of any Indemnified Party, and Parent or the Surviving Corporation shall also advance expenses as incurred to the fullest extent that the Company, Parent or Merger Sub, as applicable, would have been permitted to do so under applicable Law and its respective Organizational Documents in effect as of the date of this Agreement; provided that any Person to whom expenses are advanced provides an undertaking to repay such advances if it is ultimately determined by final adjudication that such Person is not entitled to indemnification.
(b) Parent shall cause the Surviving Corporation as of the Effective Time to obtain and fully pay, as a portion of the Company’s Transaction Expenses, the premium for “tail” insurance policies for the extension of (i) the directors’ and officers’ liability coverage of the Company’s existing directors’ and officers’ insurance policies, and (ii) the Company’s existing fiduciary liability insurance policies, in each case, for a claims reporting or discovery period of the lesser of six (6) years from and after the Effective Time or the maximum permitted under such policy (the “Tail Period”) from one or more insurance carriers with the same or better credit rating as the Company’s insurance carrier as of the date of this Agreement with respect to directors’ and officers’ liability insurance and fiduciary liability insurance (collectively, “D&O Insurance”) with terms, conditions, retentions and limits of liability that are at least as favorable to the insureds as the Company’s existing policies with respect to matters existing or occurring at or prior to the Effective Time (including in connection with this Agreement or the Transactions).
(c) Parent shall, as of the Effective Time, obtain and fully pay, as a portion of Parent’s Transaction Expenses, the premium for “tail” insurance policies for the extension of Parent’s existing D&O Insurance, in each case, for the Tail Period, with terms, conditions, retentions and limits of liability that are at least as favorable to the insureds as Parent’s existing policies with respect to matters existing or occurring at or prior to the Effective Time (including in connection with this Agreement or the Transactions).
| -321- |
(d) If Parent or the Surviving Corporation or any of their respective successors or assigns (i) shall consolidate with or merge into any other Person and shall not be the continuing or surviving Person of such consolidation or merger or (ii) shall transfer all or substantially all of its properties and assets to any Person, then, and in each such case, proper provisions shall be made so that the successors and assigns of Parent or the Surviving Corporation shall assume all of the obligations set forth in this Section 6.5.
(e) Prior to the Closing, Parent shall use commercially reasonable efforts to obtain D&O Insurance reasonably satisfactory to the Company and that shall be effective as of Closing and will cover those Persons who will be the directors and officers of Parent and its Subsidiaries (including the directors and officers of the Company and its Subsidiaries) at and after the Closing on terms not less favorable than the better of (a) the terms of the current directors’ and officers’ liability insurance in place for the Company’s and its Subsidiaries’ directors and officers and (b) the terms of a typical directors’ and officers’ liability insurance policy for a company whose equity is listed on Nasdaq which policy has a scope and amount of coverage that is reasonably appropriate for a company of similar characteristics (including the line of business and revenues) as Parent and its Subsidiaries (including the Company and its Subsidiaries).
(f) The rights of the Indemnified Parties under this Section 6.5 are in addition to any rights such Indemnified Parties may have under the Organizational Documents of Parent, Merger Sub, the Company or any of their respective Subsidiaries, or under any applicable Contracts or Laws, and nothing in this Agreement is intended to, shall be construed or shall release, waiver or impair any rights to directors’ and officers’ insurance claims under any policy that is or has been in existence with respect to Parent, Merger Sub, the Company or any of their respective Subsidiaries for any of their respective directors, officers or other employees (it being understood that the indemnification provided for in this Section 6.5 is not prior to or in substitution of any such claims under such policies).
(g) This Section 6.5 is intended to be for the benefit of, and from and after the Effective Time shall be enforceable by, each of the Indemnified Parties, who shall be third party beneficiaries of this Section 6.5.
6.6. Parent Nasdaq Listing. From the date hereof through the Closing, Parent shall (a) ensure that Parent remains listed as a public company on, and for shares of Parent Common Stock to be listed on, Nasdaq, and (b) use its reasonable best efforts to cause the Parent Common Stock to be issued pursuant to this Agreement to the Company’s stockholders to be approved for listing on Nasdaq, subject to official notice of issuance, prior to the Effective Time.
6.7. Parent Public Filings. From the date hereof through the Closing, Parent will use reasonable best efforts to keep current and timely file all reports required to be filed or furnished with the SEC and otherwise comply in all material respects with its reporting obligations under applicable securities Laws.
6.8. Private Placements. To the extent that Parent elects to pursue a Private Placement during the period beginning on the date hereof through the Closing, Parent and Merger Sub shall conduct such Private Placement solely in compliance with applicable securities Laws and shall keep the Company reasonably informed as to the status of and subscriptions received for such Private Placement.
| -322- |
6.9. Post-Closing Board of Directors and Officers of Parent. Parent shall take all such action within its power as may be necessary or appropriate such that immediately following the Effective Time the officers and Post-Closing Board of Directors of Parent shall be as set forth in Section 1.4 above.
6.10. Stockholder Litigation. In the event that any Proceeding related to this Agreement, any Transaction Document or the transactions contemplated hereby or thereby is brought, or to the knowledge of any party hereto, threatened in writing, against any party hereto or its respective Board of Directors by any stockholders of any party prior to the Closing (“Stockholder Litigation”), such party shall promptly notify and consult the other parties of any such Proceeding.
6.11. Extension of SPAC. Parent shall take such actions necessary to provide that, if the Closing has not occurred prior to the SPAC Termination Date then in effect, the SPAC Termination Date shall be extended to a date no earlier that the Outside Date (as defined below).
Article VII.
Joint Covenants
7.1. Preparation of Registration Statement.
(a) As promptly as practicable following the execution and delivery of this Agreement and the delivery of the Company S-4 Financial Statements, Parent shall prepare, with the assistance of the Company, and cause to be filed with the SEC a registration statement on Form S-4 (as amended or supplemented from time to time, and including the Proxy Statement contained therein, the “Registration Statement”) in connection with the registration under the Securities Act of Parent Common Stock to be issued under this Agreement, which Registration Statement will also contain the Proxy Statement. Each of Parent and the Company shall use its reasonable best efforts to cooperate in the preparation of the Registration Statement, the Proxy Statement and any other documents and to cause the Registration Statement and the Proxy Statement to comply with the rules and regulations promulgated by the SEC, to have the Registration Statement declared effective under the Securities Act as promptly as practicable after such filing and to keep the Registration Statement effective as long as is necessary to consummate the Merger. Each of Parent and the Company shall furnish all information concerning it as may reasonably be requested by the other Party in connection with such actions and the preparation of the Registration Statement and the Proxy Statement. Promptly after the Registration Statement has been declared effective under the Securities Act, Parent will cause the Proxy Statement to be mailed to stockholders of Parent.
(b) Each of Parent and the Company shall cooperate and mutually agree upon (such agreement not to be unreasonably withheld or delayed), any response to comments of the SEC or its staff with respect to the Registration Statement and any amendment to the Registration Statement filed in response thereto. If Parent or the Company becomes aware that any information contained in the Registration Statement shall have become false or misleading in any material respect or that the Registration Statement is required to be amended in order to comply with applicable Law, then (i) such Party shall promptly inform the other Parties and (ii) Parent, on the one hand, and the Company, on the other hand, and shall cooperate and mutually agree upon (such agreement not to be unreasonably withheld or delayed) an amendment or supplement to the Registration Statement. Parent and the Company shall use reasonable best efforts to cause the Registration Statement as so amended or supplemented, to be filed with the SEC and to be disseminated to the holders of shares of Parent Common Stock, as applicable, in each case, pursuant to applicable Law and subject to the terms and conditions of this Agreement and Parent Organizational Documents. Each of the Company and Parent shall provide the other Parties with copies of any written comments, and shall inform such other Parties of any oral comments, that Parent receives from the SEC or its staff with respect to the Registration Statement promptly after the receipt of such comments and shall give the other Parties a reasonable opportunity to review and comment on any proposed written or oral responses to such comments prior to responding to the SEC or its staff.
| -323- |
(c) Parent agrees to include provisions in the Proxy Statement and to take reasonable action related thereto, with respect to (i) approval of the Business Combination, including the Merger, and the adoption and approval of this Agreement in accordance with applicable Law and exchange rules and regulations (the “Transaction Proposal”), (ii) approval of the Parent Restated Charter and the restated bylaws of Parent (the “Amendment Proposal”) and each change to the Parent Restated Charter that is required to be separately approved, (iii) to the extent required by the Nasdaq listing rules, approval of the issuance of the Aggregate Consideration together with any Parent Common Stock being issued pursuant to a Private Placement (the “Nasdaq Proposal”), (iv) the approval and adoption of the Parent Equity Incentive Plan (the “Parent Equity Incentive Plan Proposal”), (v) adjournment of the Special Meeting, if necessary, to permit further solicitation of proxies because there are not sufficient votes to approve and adopt any of the foregoing proposals and (vi) approval of any other proposals reasonably agreed by Parent and the Company to be necessary or appropriate in connection with the transaction contemplated hereby (the “Additional Proposal” and together with the Transaction Proposal, the Amendment Proposal, the Nasdaq Proposal and Parent Equity Incentive Plan Proposal, the “Proposals”). Without the prior written consent of the Company, the Proposals shall be the only matters (other than procedural matters) which Parent shall propose to be acted on by Parent’s stockholders at the Special Meeting.
7.2. Parent Special Meeting.
(a) Parent shall use commercially reasonable efforts to, as promptly as practicable, (i) establish the record date (which record date shall be mutually agreed with the Company), or duly call, give notice of, convene and hold the Special Meeting in accordance with the DGCL, (ii) after the Registration Statement has been declared effective under the Securities Act, cause the Proxy Statement to be disseminated to Parent’s stockholders in compliance with applicable Law and (iii) after the Registration Statement has been declared effective under the Securities Act, solicit proxies from the holders of Parent Common Stock to vote in accordance with the recommendation of Parent Board with respect to each of the Proposals.
(b) Parent shall, through Parent Board, recommend to its stockholders that they approve the Proposals (the “Parent Board Recommendation”) and shall include Parent Board Recommendation in the Proxy Statement.
(c) To the fullest extent permitted by applicable Law, (x) Parent’s obligations to establish a record date, or duly call, give notice of, convene and hold the Special Meeting shall not be affected by any change to, withdrawal, withholding, qualification or modification of, or public proposal to change, withdraw, withhold, qualify or modify, the Parent Board Recommendation and (y) Parent agrees that if Parent Requisite Stockholder Consent shall not have been obtained at any such Special Meeting, then Parent shall promptly continue to take all such commercially reasonable actions, including the actions required by this Section 7.2, and hold such additional Special Meetings in order to obtain Parent Requisite Stockholder Consent. Parent may only adjourn the Special Meeting (i) to solicit additional proxies for the purpose of obtaining Parent Requisite Stockholder Consent, (ii) for the absence of a quorum and (iii) to allow reasonable additional time for the filing or mailing of any supplemental or amended disclosure that Parent has determined in good faith after consultation with outside legal counsel is required under applicable Law and for such supplemental or amended disclosure to be disseminated and reviewed by Parent Stockholders prior to the Special Meeting; provided, that, without the consent of the Company, the Special Meeting (x) may not be adjourned to a date that is more than fifteen (15) days after the date for which the Special Meeting was originally scheduled (excluding any adjournments required by applicable Law) and (y) shall not be held later than three (3) Business Days prior to the Outside Date.
| -324- |
7.3. Company Requisite Stockholder Consent.
(a) The Company shall solicit the Company Requisite Stockholder Consent via written consent as soon as promptly as practicable after the Registration Statement becomes effective and use commercially reasonable efforts to obtain the Company Requisite Stockholder Consent. In connection therewith, the Company shall use commercially reasonable efforts to, as promptly as practicable, (i) establish the record date (which record date shall be mutually agreed with Parent) for determining the Company Stockholders entitled to provide such written consent, (ii) cause the Consent Solicitation Statement to be disseminated to the Company Stockholders in compliance with applicable Law and (iii) solicit written consents from the Company Stockholders to give the Company Requisite Stockholder Consent.
(b) The Company shall, through the Company Board, recommend to the Company Stockholders that they adopt this Agreement (the “Company Board Recommendation”) and shall include the Company Board Recommendation in the Consent Solicitation Statement, subject to the provisions of this Section 7.3. The Company will provide Parent with copies of all stockholder consents it receives within one (1) Business Day of receipt. If the Company Requisite Stockholder Consent is obtained, then promptly following the receipt of the required written consents, the Company will prepare and deliver to its stockholders who have not consented the notice required by Section 228(e) of the DGCL. Unless this Agreement has been terminated in accordance with its terms, the Company’s obligation to solicit written consents from the Company Stockholders to give the Company Requisite Stockholder Consent in accordance with this Section 7.3 shall not be limited or otherwise affected by the making, commencement, disclosure, announcement or submission of any Alternative Transaction Proposal.
(c) The Company will give Parent and its counsel the opportunity to review drafts of the Consent Solicitation Statement and comment thereon. The Company agrees to correct promptly any information provided in the Consent Solicitation Statement that it obtains Knowledge of having become false or misleading in any material respect, and the Company further agrees to take all commercially reasonable steps to cause the Consent Solicitation Notice, as so corrected, to be disseminated to its stockholders.
7.4. Cooperation; Efforts to Consummate.
(a) On the terms and subject to the conditions set forth in this Agreement, the Company and Parent shall cooperate with each other and use (and shall cause their respective Subsidiaries and Affiliates to use) their respective reasonable best efforts to take or cause to be taken all actions, and do or cause to be done all things, reasonably necessary, proper or advisable on its part under this Agreement and applicable Law to consummate and make effective the Transactions as soon as reasonably practicable, including preparing and filing as promptly as reasonably practicable all documentation to effect all necessary notices, reports and other filings and to obtain as promptly as reasonably practicable all consents, registrations, approvals, clearances, Permits and authorizations necessary, proper or advisable to be obtained from any third party or any Governmental Authority in order to consummate the Transactions. Notwithstanding the foregoing or anything to the contrary in this Agreement, but subject to Parent’s obligations pursuant to Section 7.4(c), in no event shall either the Company or Parent or any of their respective Affiliates be required to pay any consideration to any third parties or give anything of value to obtain any such Person’s authorization, approval, consent or waiver to effectuate the Transactions, other than filing, recordation or similar fees. Notwithstanding anything to the contrary contained herein, no action taken by the Company or Parent under this Section 7.4 will constitute a breach of Section 5.1 or Section 6.1, respectively.
(b) Parent and the Company shall each have the right to review in advance, and to the extent reasonably practicable, each will consult with the other in connection with, all of the information relating to Parent or the Company, as applicable, and any of their respective Subsidiaries, that appears in any filing made with, or written materials submitted to, any third party or any Governmental Authority in connection with the Transactions (including the Registration Statement). Neither the Company nor Parent shall permit any of its officers or other Representatives to participate in any meeting or discussion with any Governmental Authority in respect of any filings, investigation or other inquiry relating to the Transactions unless, to the extent practicable, it consults with the other Party in advance.
| -325- |
7.5. Status; Notifications. Subject to applicable Law and as otherwise required by any Governmental Authority, the Company and Parent each shall keep the other apprised of the status of matters relating to the consummation of the Transactions, including promptly furnishing the other with copies of notices or other communications received by Parent or the Company, as applicable, or any of its Subsidiaries or Affiliates, from any third party or any Governmental Authority with respect to the Transactions.
7.6. Publicity. The initial press release with respect to the execution and delivery of this Agreement shall be a release mutually agreed to by the parties. Thereafter, each of Parent, Merger Sub, and the Company agrees that no public release or announcement or statement concerning this Agreement or the transactions contemplated hereby shall be issued by any party without the prior written consent of the other parties hereto (which consent shall not be unreasonably withheld, conditioned or delayed), except (i) as required by applicable law or the rules or regulations of any applicable Governmental Authority or stock exchange to which the relevant party is subject, in which case the party required to make the release or announcement shall consult with the other party about, and allow the other party reasonable time to comment on, such release or announcement in advance of such issuance or (ii) for such releases, announcements or statements that are consistent with other such releases, announcement or statements made after the date of this Agreement in compliance with this Section 7.6.
7.7. Section 16 Matters. Prior to the Closing, each of Parent, Merger Sub and the Company shall take all steps as may be required, to the extent permitted under applicable Law, to cause any dispositions of the shares of Company Common Stock or acquisitions of Parent Common Stock (including, in each case, securities deliverable upon exercise, vesting or settlement of any derivative securities) resulting from the Transactions by each individual who may become subject to the reporting requirements of Section 16(a) of the Exchange Act in connection with the Transactions to be exempt under SEC Rule 16b-3(d) promulgated under the Exchange Act.
7.8. Tax Matters.
(a) Notwithstanding anything to the contrary contained herein, Parent and the Company shall be responsible for and shall each pay one-half of the Transfer Taxes required to be paid by the Company, Parent or any of their Subsidiaries incurred in connection with the Transactions. Unless otherwise required by applicable Law, the Company shall file all necessary Tax Returns with respect to all such Transfer Taxes, and if required by applicable Law, Parent will join in the execution of any such Tax Returns. The Company and Parent agree to reasonably cooperate to reduce or eliminate the amount of any such Transfer Taxes.
(b) None of the Parties shall (and each Party shall cause its Subsidiaries and Affiliates not to) take or cause to be taken, or knowingly fail to take or knowingly cause to be failed to be taken, any action that would reasonably be expected to prevent the Merger from qualifying for the Intended Tax Treatment. Both prior to and following the Effective Time, each of the Parties shall, and shall cause their respective Subsidiaries and Affiliates to, use their reasonable best efforts to cause the Merger to qualify for the Intended Tax Treatment.
| -326- |
(c) The Parties shall, and shall cause their respective Affiliates to, unless otherwise required by a final determination within the meaning of Section 1313(a) of the Code, file all income Tax Returns to be filed on a basis consistent with the Intended Tax Treatment. Each of the Parties agrees to use reasonable best efforts to promptly notify all other Parties of any challenge to the Intended Tax Treatment by any Governmental Authority.
7.9. Parent Equity Incentive Plan. Parent shall, prior to the Effective Time, approve and adopt the Parent Equity Incentive Plan to be effective in connection with the Closing. The Parent Equity Incentive Plan shall provide for an initial aggregate share reserve thereunder equal to 20% of the number of shares of Parent Common Stock on a fully diluted basis at the Closing, and shall provide for the assumption and substitution thereunder pursuant to Section 1.6 of the Exchanged Options and Exchanged RSU Awards (which assumption shall not increase nor be in addition to the number of shares set forth above for the Parent Equity Incentive Plan). Parent shall file a Form S-8 Registration Statement with the SEC with respect to the shares of Parent Common Stock issuable under the Parent Equity Incentive Plan as soon as practicable following the filing by the Parent with the SEC of the Form 8-K required to be filed within four Business Days after the Closing.
7.10. Registration Rights Agreement. At the Closing, (a) Parent shall deliver to the Company a copy of the Registration Rights Agreement duly executed by Parent, and shall use reasonable best efforts to cause each applicable Parent Stockholder to deliver to the Company a copy of the Registration Rights Agreement duly executed by such Parent Stockholder, and (b) the Company shall deliver to Parent a copy of the Registration Rights Agreement duly executed by the Company.
7.11. 280G Matters. If a 280G Vote (as defined in clause (b) below) is applicable, as soon as practicable following the date of this Agreement, but in no event less than five (5) Business Days prior to the Effective Time, the Company shall (a) obtain and deliver to Parent, prior to the initiation of the Company Requisite Stockholder Consent procedure under clause (b) below from each Person who is, with respect to the Company, a “disqualified individual” (within the meaning of Section 280G of the Code) as of immediately prior to the initiation of such Company Requisite Stockholder Consent (each, a “Disqualified Individual”), and who might otherwise have, receive or have the right or entitlement to receive a “parachute payment” (within the meaning of Section 280G of the Code), a waiver (a “Parachute Payment Waiver”), in a form reviewed and approved by Parent, of such Disqualified Individual’s rights to all such payments and/or benefits applicable to such Disqualified Individual (the “Waived Parachute Payments”) so that all remaining payments and/or benefits applicable to such Disqualified Individual shall not be deemed to be “excess parachute payments” (within the meaning of Section 280G of the Code) and (b) submit to the Company Stockholders for approval (in a manner satisfactory to Parent) by such number of Company Stockholders in a manner that meets the requirements of Section 280G(b)(5)(B) of the Code, any payments and/or benefits that Parent and the Company reasonably determine may separately or in the aggregate, constitute “parachute payments” (within the meaning of Section 280G of the Code), such that such payments and benefits shall not be deemed to be “parachute payments” under Section 280G of the Code (the foregoing actions, a “280G Vote”). As soon as practicable following the date of this Agreement, if a 280G Vote is required (and in any event prior to Closing), the Company shall deliver to Parent evidence reasonably satisfactory to Parent, (i) that a 280G Vote was solicited in conformance with Section 280G of the Code, and the requisite stockholder approval was obtained with respect to any payments and/or benefits that were subject to the Company stockholder vote (the “Section 280G Approval”) or (ii) that the Section 280G Approval was not obtained and as a consequence, pursuant to the Parachute Payment Waiver, such “parachute payments” shall not be made or provided. The form of the Parachute Payment Waiver, the disclosure statement, any other materials to be submitted to the Company Stockholders in connection with the Section 280G Approval and the calculations related to the foregoing (the “Section 280G Soliciting Materials”) shall be subject to advance review and approval by Parent, which approval shall not be unreasonably withheld, delayed or conditioned.
| -327- |
7.12. Further Assurances. Upon the terms and subject to the conditions contained herein, the parties agree (a) to use reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things necessary, proper or advisable to consummate and make effective the transactions contemplated by this Agreement, (b) to execute any documents, instruments or conveyances of any kind which may be reasonably necessary or advisable to carry out any of the transactions contemplated hereunder or thereunder, and (c) to cooperate with each other in connection with the foregoing. Without limiting the foregoing, the parties agree to use their respective reasonable best efforts (i) to obtain all necessary waivers, consents and approvals necessary or desirable for the consummation of the transactions contemplated by this Agreement; (ii) to give all notices to, and make all registrations and filings with, third parties, including Governmental Authorities necessary or desirable for the consummation of the transactions contemplated by this Agreement; and (iii) to fulfill all conditions of the other party set forth in Article VIII. Each party shall provide the other parties, as applicable, with a reasonable opportunity to approve (which approval shall not be unreasonably withheld, delayed or conditioned) any waivers, consents, approvals, notices, Orders, registrations and filings to be made, given or used by any such party and shall, as promptly as reasonably practicable, deliver to the other parties, as applicable, a copy of any such registration or filing made, any such notice given or any such waiver, consent, approval or Order obtained by any such party prior to the Closing Date as the other parties may reasonably request.
Article
VIII.
CONDITIONS TO closing
8.1. Mutual Conditions to Obligations of Each Party. The respective obligations of each Party to consummate the Merger are subject to the satisfaction or waiver, at or prior to the Closing, of each of the following conditions:
(a) Stockholder Approval. (i) the Parent Requisite Stockholder Consent shall have been obtained, and (ii) the Company Requisite Stockholder Consent shall have been obtained.
(b) Regulatory Approvals. All consents, registrations, approvals, clearances, Permits and authorizations from Governmental Entities that are set forth in Section 8.1(b) of the Company Disclosure Letter shall have been obtained.
(c) No Laws or Orders. No Governmental Authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any Law or Order (whether temporary, preliminary or permanent) that is in effect and restrains, enjoins, makes illegal or otherwise prohibits the consummation of the Transactions.
(d) Registration Statement and Proxy Statement. The Registration Statement and Proxy Statement shall have become effective in accordance with the provisions of the Securities Act. No stop order suspending the effectiveness of the Registration Statement or the Proxy Statement shall have been issued and remain in effect, and no Proceedings for that purpose shall have commenced or be threatened by the SEC.
8.2. Conditions to Obligations of the Company. The obligations of the Company to consummate the Merger are subject to the satisfaction or waiver, at or prior to the Closing, of each of the following conditions:
(a) Representations and Warranties. Each of (i) Parent Fundamental Representations shall be true and correct in all but de minimis respects as of the date of this Agreement and as of the Closing Date as if made on the Closing Date (except for Parent Fundamental Representations that speak as of a particular date, which shall be true and correct in all respects as of such date), and (ii) the other representations and warranties made by Parent and Merger Sub in this Agreement shall be true and correct in all material respects as of the date of this Agreement and as of the Closing Date as if made on the Closing Date (except for representations and warranties that speak as of a particular date, which shall be true and correct in all material respects as of such date).
| -328- |
(b) Covenants. Each of the covenants and obligations that Parent and Merger Sub is required to comply with or to perform at or prior to the Closing shall have been complied with and performed in all material respects.
(c) Material Adverse Effect on Parent. Since the date of this Agreement, there shall not have occurred a Material Adverse Effect on Parent.
(d) Parent Closing Certificate. At or prior to the Closing, Parent shall deliver (or cause to be delivered) to the Company a certificate executed on behalf of Parent by its chief executive officer containing the representation and warranty of Parent that the conditions set forth in Sections 8.2(a), 8.2(b) and 8.2(c) have been duly satisfied (the “Parent Closing Certificate”).
(e) Minimum Cash Condition. Following payment by Parent to its stockholders who have validly elected to have their shares of Parent Common Stock redeemed for cash pursuant to Parent’s Organizational Documents as part of the Redemption Offer and after giving effect to the payment of the Transaction Expenses incurred by or on behalf of Parent in accordance with Section 2.2, Parent shall have an aggregate amount of cash of at least $15,000,000.
(f) Parent shall have made all necessary arrangements with the Trustee to have the funds contained in the Trust Account disbursed or available to Parent, in accordance with the Trust Agreement and this Agreement, contemporaneously with the Closing, and all such funds released from the Trust Account to Parent shall be available to Parent (and, following the Merger, the Surviving Corporation).
(g) Net Tangible Assets. Parent shall have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act).
(h) Nasdaq Listing. Parent Common Stock to be issued pursuant to this Agreement shall be listed or have been approved for listing on Nasdaq, subject only to official notice of issuance thereof.
(i) [Reserved].
(j) D&O Resignations. The directors and executive officers of Parent listed in Section 8.2(j) of the Parent Disclosure Letter shall have been removed from their respective positions or tendered their irrevocable resignations, in each case effective as of the Effective Time.
8.3. Conditions to Obligations of Parent and Merger Sub. The obligations of Parent and Merger Sub to consummate the Merger are subject to the satisfaction or waiver, at or prior to the Closing, of each of the following conditions:
(a) Representations and Warranties. Each of (i) the Company Fundamental Representations shall be true and correct in all material respects (other than the representations contained in Section 3.5, which shall be true and correct in all but de minimis respects) as of the date of this Agreement and as of the Closing Date as if made on the Closing Date (except for Company Fundamental Representations that speak as of a particular date, which shall be true and correct in all material respects (other than the representations contained in Section 3.5, which shall be true and correct in all but de minimis respects) as of such date), and (ii) the other representations and warranties made by the Company in this Agreement shall be true and correct in all respects as of the date of this Agreement and as of the Closing Date as if made on the Closing Date (except for representations and warranties that speak as of a particular date, which shall be true and correct in all respects as of such date), except in each case of this clause (ii) where the failure of such representations and warranties to be true and correct, taken as a whole, would not be reasonably likely to cause a Material Adverse Effect.
| -329- |
(b) Covenants. Each of the covenants and obligations that the Company is required to comply with or to perform at or prior to the Closing shall have been complied with and performed in all material respects.
(c) Material Adverse Effect on the Company. Since the date of this Agreement, there shall not have occurred a Material Adverse Effect on the Acquired Companies.
(d) Consulting Arrangements. The Surviving Corporation shall cause to be paid all consulting fees of Parent incurred in connection with the consummation of the transactions contemplated by this Agreement first using the funds in the Trust Account Amount, if any; provided that such consulting fees shall not exceed, in the aggregate, $500,000.
(e) Dissenting Shares. There shall not be more than twenty-five percent (25.0%) of Company Dissenting Shares.
(f) Other Deliveries. The Company shall have delivered (or cause to be delivered) to Parent and Merger Sub each of the following:
(i) a certificate executed on behalf of the Company by its chief executive officer containing the representation and warranty of the Company that the conditions set forth in Sections 8.3(a), 8.3(b) and 8.3(c) have been duly satisfied (the “Company Closing Certificate”);
(ii) executed copies of each agreement with the holders of the Contingent Obligations reflecting the settlement of each such Contingent Obligation through the issuance of Parent Common Stock, as set forth in the Consideration Schedule;
(iii) the Consideration Schedule completed to include all of the information specified in Section 2.3 in a form reasonably satisfactory to Parent and a certificate executed by the Company’s chief executive officer or chief financial officer, dated as of the Closing Date, certifying on behalf of the Company (and not in his or her individual capacity) that the Consideration Schedule is complete and correct;
(iv) a certificate, meeting the requirements of Section 1445 of the Code and the Treasury Regulations thereunder, to the effect that the Shares do not constitute a U.S. real property interest; and
(v) evidence satisfactory to Parent that all Company Plans have been terminated.
Article IX.
TERMiNATION
9.1. Termination. This Agreement may be terminated and the Merger may be abandoned at any time prior to the Effective Time only as follows:
(a) by mutual written agreement of the Company and Parent;
| -330- |
(b) by either the Company or Parent, if the Merger has not been consummated on or before April 30, 2022 (the “Outside Date”); provided that the right to terminate this Agreement pursuant to this Section 9.1(b) shall not be available to any party whose breach of any provision of this Agreement results in the failure of the Merger to be consummated by such time; and provided, further, that the Outside Date shall be automatically extended to May 31, 2022 in the event that the Registration Statement has been filed, but not declared effective, on or before February 14, 2022;
(c) by either the Company or Parent if the Parent Requisite Stockholder Consent shall not have been obtained by reason of the failure to obtain the required vote at the Special Meeting duly convened therefor or at any adjournment or postponement thereof;
(d) by either Parent or the Company, if a Governmental Authority shall have issued any Order or taken any other action, in each case, which has become final and non-appealable and which restrains, enjoins or otherwise prohibits the Merger;
(e) by Parent if there shall have occurred a Material Adverse Effect on the Acquired Companies after the date of this Agreement;
(f) by the Company if there shall have occurred a Material Adverse Effect on Parent and its Subsidiaries, taken as a whole, after the date of this Agreement;
(g) by Parent, if (i) any representation or warranty of the Company contained in this Agreement shall be inaccurate such that the condition set forth in Section 8.3(a) would not be satisfied, or (ii) the covenants or obligations of the Company contained in this Agreement shall have been breached in any material respect such that the condition set forth in Section 8.3(b) would not be satisfied; provided, however, that if an inaccuracy or breach is curable by the Company during the thirty (30) calendar day period after Parent notifies the Company in writing of the existence of such inaccuracy or breach (the “Company Cure Period”), then Parent may not terminate this Agreement under this Section 9.1(g) as a result of such inaccuracy or breach prior to the expiration of the Company Cure Period unless the Company is no longer continuing to exercise reasonable best efforts to cure such inaccuracy or breach;
(h) by the Company, if (i) any representation or warranty of Parent contained in this Agreement shall be inaccurate such that the condition set forth in Section 8.2(a) would not be satisfied, or (ii) the covenants or obligations of Parent or Merger Sub contained in this Agreement shall have been breached in any material respect such that the condition set forth in Section 8.2(b) would not be satisfied; provided, however, that if an inaccuracy or breach is curable by Parent or Merger Sub during the thirty (30) calendar day period after the Company notifies Parent in writing of the existence of such inaccuracy or breach (the “Parent Cure Period”), then the Company may not terminate this Agreement under this Section 9.1(h) as a result of such inaccuracy or breach prior to the expiration of Parent Cure Period unless Parent is no longer continuing to exercise reasonable best efforts to cure such inaccuracy or breach.
9.2. Effect of Termination. If this Agreement is terminated pursuant to Section 9.1, this Agreement shall become void and of no effect without liability of any party (or any Representative of such party) to any other party; provided that the parties shall, in all events, remain bound by and continue to be subject to the provisions set forth in Sections 9.3, 10.8, 10.12, 10.14, and 10.18, which shall survive any termination of this Agreement.
| -331- |
9.3. Fees and Expenses. Except as otherwise set forth in this Agreement or any Transaction Document, all expenses incurred in connection with this Agreement and the Transactions shall be paid by the party incurring such expenses, whether or not the Transactions are consummated.
Article
X.
MISCELLANEOUS
10.1. Defined Terms. As used herein, the terms below shall have the following meanings. Any such term, unless the context otherwise requires, may be used in the singular or plural, depending upon the reference.
“Acquired Companies” means, collectively, the Company and its Subsidiaries.
“Affiliate” means, when used with reference to any specified Person, any other Person directly or indirectly controlling, controlled by, or under direct or indirect common control with, such specified Person. For purposes of this definition, “control,” when used with respect to any specified Person, means the power to direct or cause the direction of management or policies of such Person, directly or indirectly, whether through the ownership of voting securities, by contract or otherwise.
“Aggregate Consideration” means 25,000,000 shares of Parent Common Stock.
“Alternative Company Transaction” means, other than the transactions contemplated by this Agreement and the Ancillary Documents (including the transactions with Parent contemplated hereby and any Financing transaction), any (i) reorganization, liquidation, refinancing, dissolution or recapitalization of the Company, (ii) merger, capital stock exchange, consolidation, exchangeable share transaction or other business combination involving the Company or its Subsidiaries, (iii) purchase or sale of all or substantially all of the Company Common Stock or other Equity Securities of the Company or its Subsidiaries (including any rights to acquire, or securities convertible into or exchange for, any such Equity Securities) or the assets used primarily in the business of the Company or its Subsidiaries, or (iv) any similar transaction or business combination involving the Company, its Subsidiaries or their respective assets.
“Antitrust Law” means the Sherman Antitrust Act of 1890, the Clayton Act of 1914, the HSR Act and all other United States or non-United States antitrust, competition, merger control or other Laws that are designed or intended to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade or lessening of competition through merger or acquisition.
“Business Day” means a day other than Saturday, Sunday or any day on which banks located in the State of New York are authorized or obligated to close.
“Code” means the Internal Revenue Code of 1986, as amended.
“Company Capital Stock” means Company Common Stock and Company Preferred Stock.
“Company Common Stock” means the Company’s Common Stock, $0.0001 par value per share.
“Company Disclosure Letter” means a letter delivered by the Company to Parent and Merger Sub as of the date hereof which sets forth the exceptions to the representations and warranties contained in Article III hereof and certain other information called for by this Agreement.
| -332- |
“Company Fundamental Representations” means the representations and warranties of the Company contained in Section 3.1 (Organization, Existence and Power), Section 3.2 (Authorization), Section 3.5 (Capitalization), and Section 3.24 (No Brokers).
“Company Intellectual Property” means all Intellectual Property that is owned or licensed by any Acquired Company.
“Company Option” means an option entitling the holder thereof to acquire shares of Company Common Stock from the Company.
“Company Plans” mean each of (i) the Aerami 2015 Equity Incentive Plan (f/k/a the Dance Biopharm Holdings, Inc. 2015 Equity Incentive Plan), (ii) the Aerami 2009 Equity Incentive Plan (f/k/a the Dance Pharmaceuticals, Inc. 2009 Equity Incentive Plan) and (iii) the Company’s equity incentive plan created for Anne Whitaker, the former Chief Executive Officer of the Company.
“Company Preferred Stock” means the Company Series A Preferred Stock, the Company Series A-1 Preferred Stock and the Company Royalty Series Convertible Preferred Stock.
“Company Registered Intellectual Property” means all applications, registrations and filings for Intellectual Property that have been registered, filed, certified or otherwise perfected or recorded or are the subject of a pending application for such, with or by any Governmental Authority or the Internet domain name registrar, by, on behalf of or in the name of any of the Acquired Companies (including all Internet domain names), in each case to the extent used or useful in the business of the Company.
“Company Requisite Stockholder Consent” means, with respect to this Agreement, approval by (a) holders of not less than fifty percent (50%) of all outstanding shares of Company Capital Stock, voting together as a single class and on an as-converted to Company Common Stock basis, and (b) holders of not less than fifty percent (50%) of the outstanding shares of Company Series A Preferred Stock and Company Series A-1 Preferred Stock, voting together as a single class on an as-converted basis.
“Company Royalty Series Convertible Preferred Stock” means the Royalty Series Convertible Preferred Stock of the Company, par value $0.0001 per share.
“Company RSU Award” means a restricted stock unit award covering shares of Company Common Stock.
“Company Series A Preferred Stock” means the Series A Preferred Stock of the Company, par value $0.0001 per share.
“Company Series A-1 Preferred Stock” means the Series A-1 Preferred Stock of the Company, par value $0.0001 per share.
“Company Stockholders” means any holder of Company Capital Stock.
“Company Warrant” means an outstanding warrant entitling the holder thereof to acquire Company Capital Stock from the Company.
“Consent Solicitation Statement” means the consent solicitation statement with respect to the solicitation by the Company of the Company Requisite Stockholder Consent.
| -333- |
“Continuing Service Provider” means each employee and independent contractor of any Acquired Company who continues his or her employment or other service with Parent or its Affiliates (including any Acquired Company) at the Closing.
“Contract” means any contract, agreement, deed, indenture, note, bond, loan, license, instrument, lease, commitment, plan or other arrangement or understanding, whether oral or written, including all amendments, supplements, exhibits and schedules thereto.
“COVID-19” means SARS-CoV-2 or COVID-19, and any evolutions or mutations thereof or related or associated epidemics, pandemics or disease outbreaks.
“COVID-19 Measures” means any quarantine, “shelter in place,” “stay at home,” workforce reduction, social distancing, shut down, closure, sequester, safety or similar Law, directive, guidelines or recommendations promulgated by any Governmental Authority, including the Centers for Disease Control and Prevention, the World Health Organization and the Occupational Safety and Health Administration, in each case, in connection with or in response to COVID-19.
“Encumbrance” means any lien, pledge, charge, community property interest, equitable interest, easement, security interest, deed of trust, mortgage, pledge, hypothecation, right-of-way, encroachment, building or use restriction, conditional sales agreement, encumbrance or other right of third parties, whether voluntarily incurred or arising by operation of law, and includes any agreement to give any of the foregoing in the future, and any contingent sale or other title retention agreement or lease in the nature thereof.
“Environmental Laws” means any applicable Law, regulation, or other applicable requirement relating to (a) releases or threatened release of Hazardous Substance; (b) pollution or protection of employee health or safety, public health or the environment; or (c) the manufacture, handling, transport, use, treatment, storage, or disposal of Hazardous Substances.
“Equity Interests” means, with respect to any Person, any capital stock, or other ownership, membership, partnership, joint venture or equity interest in, such Person or any indebtedness, securities, options, warrants, call, subscription or other rights or entitlements of, or granted by, such Person or any of its Affiliates that are convertible into, or are exercisable or exchangeable for, or giving any Person any right or entitlement to acquire any such capital stock or other ownership, partnership, joint venture or equity interest, in all cases, whether vested or unvested.
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended, and the regulations promulgated thereunder.
“ERISA Affiliate” means any Person or entity (whether or not incorporated) that would be treated as a “single employer” or under common control with any of the Acquired Companies within the meaning of Section 414 of the Code.
“Exchange Act” means the Securities and Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
“Funded Indebtedness” means Indebtedness of the types described in clauses (a) and (b) of the definition thereof.
“GAAP” means generally accepted accounting principles in the United States.
| -334- |
“Governmental Authority” means any United States, foreign, supra-national, federal, state, provincial, local or self-regulatory governmental, regulatory or administrative authority, agency, division, body, organization or commission or any judicial or arbitral body.
“HSR Act” means the Hart-Scott-Rodino Antitrust Improvements Act of 1976 and the rules and regulations promulgated thereunder.
“Indebtedness” means, without duplication, (a) all obligations for borrowed money (including all obligations for principal, interest, penalties, fees and premiums, expenses and breakage costs) or extensions of credit (including under credit cards, bank overdrafts, and advances), (b) all obligations evidenced by bonds, debentures, notes, or other similar instruments (including all obligations for principal, interest, penalties, fees and premiums, expenses and breakage costs), (c) all obligations to pay the deferred purchase price of property or services, except trade accounts payable arising in the Ordinary Course of Business, (d) all obligations of others secured by an Encumbrance on any asset of such Person, © all obligations, contingent or otherwise, directly or indirectly guaranteeing any obligations of any other Person, (f) all obligations to reimburse the issuer in respect of letters of credit or under performance or surety bonds, or other similar obligations, and (g) all obligations in respect of bankers’ acceptances and under reverse repurchase agreements.
“Intellectual Property” means all worldwide intellectual property and intellectual property rights, and all right title and interest therein, including, without limitation, (a) all patents, patent applications, utility models, design registrations and certificates of invention and other governmental grants for the protection of inventions or industrial designs (including all related continuations, continuations-in-part, divisionals, reissues and reexaminations), invention disclosures, inventions and discoveries, whether or not patented or patentable and whether or not reduced to practice, improvements thereto, and other rights of invention; (b) brand marks, brand names, registered and unregistered trademarks, service marks, trade names, trade dress, logos, product names and slogans, including any common law rights, registrations and applications for the foregoing, and all goodwill associated with any of the foregoing; (c) copyrightable works, website content, all registered and unregistered copyrights in both published works and unpublished works, other rights of authorship and exploitation, and any applications, registrations and renewals in connection therewith; (d) all rights in mask works; (e) all know-how, trade secrets, confidential or proprietary information, customer lists, financial information, business information, technical information, data, process technology, plans, drawings and blue prints (the items in clause (e), collectively, “Trade Secrets”); (f) all Software; (g) all URLs, social media accounts, short codes, hash tags in internet web sites and internet domain names; (h) rights of attribution and integrity and other moral rights; and (i) rights to exclude others from appropriating any of such Intellectual Property, including the right to sue for and remedies against past, present and future infringements of any or all of the foregoing and rights of priority and protection of interests therein, and any other proprietary, intellectual property; and (i) other rights relating to any or all of the foregoing anywhere in the world.
“IRS” means the U.S. Internal Revenue Service.
“JOBS Act” means the Jumpstart Our Business Startups Act of 2012.
“Knowledge” means (a) with respect to the Company, the actual knowledge of Steve Thornton and Timm Crowder, after reasonable investigation, and (b) with respect to Parent, the actual knowledge of Robb Knie, after reasonable investigation.
“Law” means any federal, state, local or foreign law, statute, ordinance, code, decree, treaty, rule, rule of common law, policy, guidance, directive or regulation or Order of any Governmental Authority and all other provisions having the force or effect of law.
| -335- |
“Liabilities” means all debts, liabilities, commitments and obligations, whether accrued or fixed, absolute or contingent, matured or unmatured, determined or determinable, liquidated or unliquidated, asserted or unasserted, known or unknown, whenever or however arising, including those arising under applicable Law or any Proceeding or order of a Governmental Authority and those arising under any Contract, regardless of whether such debt, liability, commitment or obligation would be required to be reflected on a balance sheet prepared in accordance with GAAP or disclosed in the notes thereto.
“Material Adverse Effect” means with respect to any Person, any event, circumstance, change or effect (an “Effect”) that, individually or in the aggregate with any other Effect, (a) has materially and adversely impaired, or would reasonably be expected to materially and adversely impair, the ability of such Person to consummate the transactions contemplated by this Agreement in accordance with terms of this Agreement and applicable Law or in a timely manner or (b) has had, or would reasonably be expected to have, individually or in the aggregate, a material adverse effect on the business, condition (financial or otherwise), assets (including intangible assets), Liabilities or results of operations of such Person, taken as a whole; provided, that in the case of clause (b) only, in no event shall any Effect resulting or arising from any of the following, alone or in combination, be taken into account in determining whether there has been, a Material Adverse Effect: (i) any change in general economic conditions, (ii) any changes after the date hereof in applicable Law or the interpretation thereof or any COVID-19 Measures or any changes after the date hereof in such COVID-19 Measures or interpretations thereof, (iii) any change after the date hereof in accounting requirements or principles required by GAAP, (iv) any change in the industry generally in which such Person operates, (v) any outbreak, escalation or acts of terrorism, armed hostility or war, sabotage or military actions, or any weather-related event, earthquake, hurricane, tornado, fire or other natural disaster or any material worsening of such conditions, or (vi) any epidemics, pandemics or other outbreak of disease or illness or public health event (including COVID-19, or any COVID-19 Measures or any change in such COVID-19 Measures or interpretations following the date of this Agreement); provided, further, that the exceptions set forth in clauses (i), (ii), (iii), (iv), (v) and (vi) shall only apply to the extent that such Effect does not have a materially disproportionate impact on such Person, taken as a whole, compared to other companies of similar size that operate in the industries in which Person operates.
“Order” means judgments, writs, decrees, directives, rulings, compliance agreements, injunctions, awards, assessments, writs, stipulations, determination of awards, settlement agreements or orders of any Governmental Authority or arbitrator.
“Ordinary Course of Business” means an action taken, or omitted to be taken, by any Person in the ordinary course of such Person’s business consistent with past practice (including any actions taken, or omitted to be taken, in good faith in light of COVID-19 and actions to respond to COVID-19 Measures).
“Organizational Documents” means (a) the articles or certificate of incorporation, all certificates of determination and designation, and the bylaws of a corporation; (b) the partnership agreement and any statement of partnership of a general partnership; (c) the limited partnership agreement and the certificate or articles of limited partnership of a limited partnership; (d) the operating agreement, limited liability company agreement and the certificate or articles of organization or formation of a limited liability company; (e) any charter or similar document adopted or filed in connection with the creation, formation or organization of any other Person; and (f) any amendment to any of the foregoing.
“Parent Class A Common Stock” means Parent’s Class A Common Stock, $0.0001 par value per share.
“Parent Class B Common Stock” means Parent’s Class B Common Stock, $0.0001 par value per share.
| -336- |
“Parent Common Stock” means the Parent Class A Common Stock and the Parent Class B Capital Stock.
“Parent Disclosure Letter” means a letter delivered by Parent to the Company as of the date hereof which sets forth the exceptions to the representations and warranties contained in Article IV hereof and certain other information called for by this Agreement.
“Parent Fundamental Representations” means the representations and warranties of Parent and Merger Sub contained in Section 4.1 (Organization, Existence and Power), Section 4.2 (Authorization), Section 4.6 (Capitalization), and Section 4.26 (No Brokers).
“Parent Restated Certificate” means the Amended and Restated Certificate of Incorporation of Parent in the form of Exhibit E attached hereto.
“Parent Requisite Stockholder Consent” means the approval of the holders of a majority in voting power of the outstanding Parent Common Stock, voting together as a single class and, if the Parent Board determines it is necessary or advisable, the holders of a majority of the outstanding Parent Class A Common Stock, voting as a separate class.
“Parent Stockholders” means any holder of Parent Common Stock.
“Permits” means all licenses, permits, franchises, approvals, authorizations, consents or Orders of, or filings with, any Governmental Authority, whether foreign, federal, state or local, or any other Person.
“Permitted Encumbrances” means (a) any restriction on transfer arising under applicable securities laws; (b) Encumbrances for Taxes not yet due and payable; (c) mechanics’, carriers’, workers’, repairers’ and similar Encumbrances arising or incurred in the Ordinary Course of Business that are not yet due and payable and which are not, individually or in the aggregate, material to the business, operations and financial condition of the assets so encumbered of any Acquired Company; and (d) zoning laws and other land use restrictions that do not, individually or in the aggregate, materially impair the present or anticipated use or occupancy of the property subject thereto.
“Per Share Common Consideration” means the number of shares of Parent Common Stock allocable from the Aggregate Consideration to each share of Company Common Stock that is issued and outstanding as of immediately prior to the Effective Time, as set forth on the Consideration Schedule.
“Per Share Consideration” means (a) with respect to shares of Company Common Stock, the Per Share Common Consideration, (b) with respect to shares of Company Royalty Series Convertible Stock, the Per Share Royalty Series Consideration, (c) with respect to shares of Company Series A Preferred Stock, the Per Share Series A Consideration, and (d) with respect to shares of Company Series A-1 Preferred Stock, the Per Share Series A-1 Consideration.
“Per Share Royalty Series Consideration” means the number of shares of Parent Common Stock allocable from the Aggregate Consideration to each share of Company Royalty Series Convertible Preferred Stock that is issued and outstanding as of immediately prior to the Effective Time, as set forth on the Consideration Schedule.
“Per Share Series A Consideration” means the number of shares of Parent Common Stock allocable from the Aggregate Consideration to each share of Company Series A Preferred Stock that is issued and outstanding as of immediately prior to the Effective Time, as set forth on the Consideration Schedule.
| -337- |
“Per Share Series A-1 Consideration” means the number of shares of Parent Common Stock allocable from the Aggregate Consideration to each share of Company Series A-1 Preferred Stock that is issued and outstanding as of immediately prior to the Effective Time, as set forth on the Consideration Schedule.
“Person” means any individual, corporation (including any non-profit corporation), general or limited partnership, limited liability company, joint venture, estate, trust, association, organization, labor union, or other entity or governmental body.
“Personal Information” means any information relating to an identified or identifiable natural person; an “identifiable person” is one who can be identified, directly or indirectly, in particular by reference to an identification number or to one or more factors specific to his physical, physiological, mental, economic, cultural or social identity, including unique device or browser identifiers, names, ages, addresses, telephone numbers, email addresses, social security numbers, passport numbers, alien registration numbers, medical history, employment history, and/or account information; and shall also mean “personal information”, “personal health information” and “personal financial information” each as defined by applicable Laws relating to the collection, use, sharing, handling, storage, retention, destruction, and/or disclosure of information about an identifiable individual.
“Privacy and Security Laws” means all applicable Laws concerning the collection, handling, receipt, use, processing, disclosure, storage, maintenance, transmission, encryption, access to, breach or breach notification of, or privacy or security or protection of Personal Information and all guidance and implementing regulations issued by any Governmental Authority (including staff reports) thereunder.
“Private Placement” means a private placement or private placements pursuant to which certain investors may purchase shares of Parent Common Stock at $10.00 per share for an aggregate subscription amount of up to $30,000,000, to be consummated, if at all, immediately prior to or substantially concurrently with the Closing;
“Proceeding” means any action, suit, litigation, complaint, dispute, arbitration, mediation, proceeding (including any civil, criminal, administrative, investigative or appellate proceeding), hearing, audit, inquiry, examination or investigation commenced, brought, conducted or heard by or before, or otherwise involving, any court or other Governmental Authority.
“Proxy Statement” means the proxy statement relating to Parent’s Special Meeting.
“Representative” means any officer, director, manager, principal, attorney, financial advisor, agent, employee or other representative.
“Sarbanes-Oxley Act” means the Sarbanes-Oxley Act of 2002.
“SEC” means the United States Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
| -338- |
“Software” means any and all (a) computer programs, including any and all software implementation of algorithms, models and methodologies, whether in source code, object code, human readable form or other form, (b) databases and compilations, including any and all data and collections of data, whether machine readable or otherwise, (c) descriptions, flow charts and other work products used to design, plan, organize and develop any of the foregoing, (d) screens, user interfaces, report formats, firmware, development tools, templates, menus, buttons and icons and (e) documentation, including user manuals and training materials, relating to any of the foregoing.
“Special Meeting” means a meeting of the holders of Parent Common Stock to be held for the purpose of approving the Proposals.
“Subsidiary” means when used in reference to any Person, any corporation or other entity of which such Person owns, directly or indirectly, (a) fifty percent (50%) or more of the outstanding shares of stock, other Equity Interests or voting securities, or (b) outstanding securities having ordinary voting power to elect the majority of the board of directors or other managing body of such corporation or entity.
“Tax” means any and all taxes, including any net income, alternative or add-on minimum, gross income, gross receipts, sales, use, ad valorem, value added, transfer, franchise, profits, license, registration, recording, documentary, conveyancing, gains, withholding, payroll, employment, excise, severance, stamp, occupation, premium, property, environmental or windfall profit, escheat, custom duty or other tax, governmental fee or other like assessment or charge in the nature of a tax, together with any interest, penalty, addition to tax or additional amount imposed by any Governmental Authority, whether disputed or not, or in respect of any failure to comply with any requirement regarding Tax Returns, including any such amounts payable pursuant to any tax-sharing agreement or other agreement relating to the payment of any such Tax, levy, impost, duty, assessment, charge or withholding, whether imposed directly, under Treasury Regulation Section 1.1502-6 (or any similar provision of state, local, or foreign Law), as a result of being a transferee, successor, or member of an affiliated, consolidated, unitary or combined group, by contract, or otherwise.
“Tax Return” means any return, report, declaration, claim for refund, information return or other document (including schedules thereto, other attachments thereto, amendments thereof, or any related or supporting information) filed or required to be filed with a Tax authority relating to any Tax.
“Transaction Expenses” without duplication, the aggregate amount of all fees, costs and expenses incurred by or on behalf of Parent, Merger Sub, or any Acquired Company arising from, incurred in connection with or related to the negotiation, preparation, execution and performance of this Agreement and the transactions contemplated hereby, including (a) third party fees, expenses and costs (including legal, accounting, broker’s, investment banker’s, consultant’s, advisor’s and finder’s fees, costs and expenses) arising from, incurred in connection with or related to this Agreement or the transactions contemplated hereby (whether or not such amounts have been billed as of or prior to the Closing Date), (b) all bonuses, incentive compensation, termination payments, severance, or other change-in-control, separation or other transaction-related payments solely payable in connection with the Merger or any of the other transactions contemplated hereby (whether paid or provided on or following the Closing Date), (c) the employer portion of any payroll, employment or similar Taxes incurred or to be incurred by Parent, the Surviving Corporation or any Acquired Company in connection with the items described in clause (b), and (d) all other miscellaneous out-of-pocket expenses or costs incurred by or on behalf of Parent, the Surviving Corporation, or any Acquired Company incurred in connection with, arising from or related to this Agreement.
“Transfer Taxes” means all transfer, documentary, sales, use, stamp, registration, property or other similar Taxes.
| -339- |
“Treasury Regulations” means the United States Treasury regulations promulgated under the Code.
The following terms shall have the meanings defined for such terms in the Sections set forth below:
| Defined Term | Section |
| 280G Vote | Section 7.11 |
| Additional Proposal | Section 7.1(c)(vi) |
| Affiliate Transaction | Section 3.9 |
| Agreement | Introductory Paragraph |
| Alternative Transaction Proposal | Section 5.4 |
| Amendment Proposal | Section 7.1(c)(ii) |
| Ancillary Documents | Section 3.2 |
| Book-Entry Shares | Section 1.9(b) |
| Business Combination | Recitals |
| Business Combination Proposal | Section 6.4 |
| Cancelled Shares | Section 1.6(h) |
| Certificate of Merger | Section 1.2 |
| Closing | Section 2.1 |
| Closing Date | Section 2.1 |
| Company | Introductory Paragraph |
| Company Balance Sheet Date | Section 3.12 |
| Company Board | Recitals |
| Company Board Recommendation | Section 7.3(b) |
| Company Cure Period | Section 9.1(g) |
| Company Financial Statements | Section 3.12 |
| Company Stock Certificate | Section 1.6(f) |
| Consideration Schedule | Section 2.3 |
| Costs | Section 6.5(a) |
| D&O Insurance | Section 6.5(b) |
| DGCL | Recitals |
| Disputes | Section 3.7(c) |
| Disqualified Individual | Section 7.11 |
| Company Dissenting Shares | Section 1.7 |
| Effective Time | Section 1.2 |
| Employee Plan(s) | Section 3.15(g) |
| Enforceability Exceptions | Section 3.2 |
| Exchange Agent | Section 1.9(a) |
| Exchange Fund | Section 1.9(a) |
| Exchanged Option | Section 1.6(c) |
| Exchanged RSU Award | Section 1.6(d) |
| FDA | Section 3.20 |
| FDA Ethics Policy | Section 3.20 |
| FDA Laws | Section 3.20 |
| Hazardous Substance | Section 3.22 |
| Indemnification Agreement | Section 6.10 |
| Indemnified Party/Parties | Section 6.5(a) |
| Intended Tax Treatment | Recitals |
| -340- |
| Interim Period | Section 5.1 |
| Inventions Assignment Agreement | Section 3.18 |
| Key Stockholder | Recitals |
| Letter of Transmittal | Section 1.9(b) |
| Lock-Up Agreement | Recitals |
| Material Contract | Section 3.8(a) |
| Merger | Recitals |
| Merger Sub | Introductory Paragraph |
| Merger Sub Certificate | Section 1.3(a) |
| Nasdaq Proposal | Section 7.1(c)(iii) |
| Off-the-Shelf Software Licenses | Section 3.7(b) |
| Outside Date | Section 9.1(b) |
| Redemption Offer | Recitals |
| Parachute Payment Waiver | Section 7.11 |
| Parent | Introductory Paragraph |
| Parent Board | Recitals |
| Parent Board Recommendation | Section 7.2(b) |
| Parent Cure Period | Section 9.1(h) |
| Parent Equity Incentive Plan | Recitals |
| Parent Equity Incentive Plan Proposal | Section 7.1(c)(iv) |
| Parent Financial Statements | Section 4.9(d) |
| Parent Private Placement Warrants | Section 4.6(b) |
| Parent Public Warrants | Section 4.6(b) |
| Parent Restated Charter | Recitals |
| Parent SEC Reports | Section 4.20 |
| Parent Support Agreements | Recitals |
| Parent Transaction Fee Cap | Section 2.2 |
| Parent Warrants | Section 4.6(b) |
| PCBs | Section 3.19 |
| Post-Closing Board of Directors | Section 6.9(a) |
| Proposals | Section 7.1(c)(vi) |
| Registration Rights Agreement | Recitals |
| Registration Statement | Section 7.1(a) |
| Section 280G Approval | Section 7.11 |
| Section 280G Soliciting Materials | Section 7.11 |
| Smith Anderson | Section 10.22 |
| Sponsor | Recitals |
| Sponsor Support Agreement | Recitals |
| Stockholder Support Agreement | Recitals |
| Stockholders’ Representative | Introductory Paragraph |
| Surviving Corporation | Section 1.1 |
| Tail Period | Section 6.5(b) |
| Transaction Proposal | Section 7.1(c)(i) |
| Transactions | Recitals |
| Transmittal Documents | Section 1.9(b) |
| Trust Account | Section 4.21 |
| Trust Account Amount | Section 6.2(b) |
| Trust Agreement | Section 4.21 |
| Trustee | Section 4.21 |
| Waived Parachute Payments | Section 7.11 |
| -341- |
10.2. Notices. All notices and other communications to be given or made hereunder shall, unless otherwise specified herein, be in writing and shall be deemed to have been duly given or made on the date of receipt by the recipient thereof if received prior to 5:00 p.m. Eastern Time in the place of receipt and such day is a Business Day (or otherwise on the next succeeding Business Day) if (a) served by personal delivery or by a nationally recognized overnight courier service upon the party for whom it is intended, (b) delivered by registered or certified mail, return receipt requested, or (c) sent by email, in each case, addressed as follows:
If to the Company (prior to the Closing) or to the Stockholders’ Representative, addressed to:
Aerami Therapeutics Holdings, Inc.
2520 Meridian Parkway, Suite 400
Durham, North Carolina, 27713
Attn: Steve Thornton
Email: sthornton@aerami.com
With a copy (which shall not constitute notice) to:
Smith,
Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P.
150 Fayetteville Street
Suite 2300
Raleigh, NC 27601
Attn:
Amy Batten and Peter Bosman
Email: abatten@smithlaw.com; pbosman@smithlaw.com
If to Parent, Merger Sub or the Surviving Corporation, addressed to:
FoxWayne Enterprises Acquisition Corp.
1 Rockefeller Plaza, Suite 1039
New York, New York 10020
Attn: Robb Knie
Email: robb@foxwayne.com
With a copy (which shall not constitute notice) to:
Sheppard,
Mullin, Richter & Hampton LLP
30 Rockefeller Plaza
New
York, New York 10112
Attn: Richard Friedman
Email: rafriedman@sheppardmullin.com
or to such other place and with such other copies as a party may designate as to itself by written notice to the others.
10.3. Rules of Construction.
(a) The parties agree that they have been represented by counsel during the negotiation and execution of this Agreement and, therefore, waive the application of any Law, regulation, holding or rule of construction providing that ambiguities in any agreement or other document will be construed against the party drafting such agreement or document.
| -342- |
(b) Any statement in this Agreement to the effect that any information, document or other material has been delivered, furnished or “made available” to the other party or any of such party’s Representatives means that such information, document or other material was posted to the electronic data room hosted by or on behalf of the Company with access provided to Parent in connection with the transactions contemplated by this Agreement no later than 12:01 a.m. Eastern Time on the date which is two (2) Business Days prior to the date of this Agreement and has been made available on a continuous basis by or on behalf of such party for review therein by the other party and its Representatives since such time.
10.4. References. The titles, captions or headings of the Articles and Sections herein are inserted for convenience of reference only and are not intended to be a part of or to affect the meaning or interpretation of this Agreement. All references to “days” or “months” shall be deemed references to calendar days or months. All references to “$” or “dollars” shall be deemed references to United States dollars. Unless the context otherwise requires, any reference to an “Article”, “Section,” “Exhibit,” or “Schedule” shall be deemed to refer to an article of this Agreement, Section of this Agreement, exhibit to this Agreement or a schedule to this Agreement, as applicable. All exhibits or schedules annexed hereto or referred to herein are hereby incorporated in and made a part of this Agreement as if set forth in full herein. Any capitalized terms used in any exhibit or schedule but not otherwise defined therein, shall have the meaning defined in this Agreement. Where a word or phrase is defined herein, each of its other grammatical forms shall have a corresponding meaning. The word “party” shall, unless the context otherwise requires, be construed to mean a party to this Agreement. Any reference to a party to this Agreement or any other agreement or document contemplated hereby shall include such successors and permitted assigns. Any reference to any federal, state, county, local or foreign statute or Law shall be deemed also to include any modification, amendment, re-enactment thereof, any provision substituted therefore and refer to all rules and regulations promulgated thereunder, unless the context requires otherwise. For all purposes of and under this Agreement, (a) the words “include”, “includes” and “including” shall be deemed to be immediately followed by the words “without limitation”; (b) words (including defined terms) in the singular shall be deemed to include the plural and vice versa; (c) words of one gender shall be deemed to include the other genders as the context requires; (d) “or” is not exclusive; (e) the word “will” shall be construed to have the same meaning and effect as the word “shall”, (f) unless otherwise stated, any reference herein to any Person shall be construed to include such Person’s successors and assigns, and (g) the terms “hereof,” “herein,” “hereto,” “herewith”, “hereunder” and any other words of similar import shall, unless otherwise stated, be construed to refer to this Agreement as a whole (including the exhibits and schedules hereto) and not to any particular term or provision of this Agreement, unless otherwise specified.
10.5. Entire Agreement. This Agreement, including the Exhibits hereto, the Company Disclosure Letter, the Parent Disclosure Letter and the other agreements, documents and written understandings referred to herein or otherwise entered into or delivered by the parties hereto pursuant to this Agreement (including each Letter of Transmittal), constitute the entire agreement and understanding of the parties hereto with respect to the subject matter hereof and supersede all other prior covenants, agreements (including any letters of intent between the parties), undertakings, obligations, promises, arrangements, communications, representations and warranties, whether oral or written, by any party hereto with respect to the subject matter hereof.
10.6. Assignment. No party may assign, delegate or otherwise transfer any of its rights or obligations under this Agreement without the consent of each other party hereto.
| -343- |
10.7. Amendment; Modification. This Agreement may not be amended or modified except in an instrument in writing signed by the parties hereto. No waiver of this Agreement shall be binding unless executed in writing by the party to be bound thereby.
10.8. Rights of Third Parties. Nothing expressed or implied in this Agreement is intended or shall be construed to confer upon or give any Person, other than the Parties hereto, any right or remedies under or by reason of this Agreement; provided, however, that, notwithstanding the foregoing, (a) the past, present and future directors, officers, employees, incorporators, members, partners, stockholders, Affiliates, agents, attorneys, advisors and representatives of the parties, and any Affiliate of any of the foregoing (and their successors, heirs and representatives), are intended third-party beneficiaries of, and may enforce, Section 10.12 and (b) the present and former officers and directors of Parent and/or the Company (and their respective successors, heirs and representatives) are intended third-party beneficiaries of, and may enforce, Section 6.5.
10.9. Waiver. Except where a specific period for action or inaction is provided herein, neither the failure nor any delay on the part of any party in exercising any right, power or privilege under this Agreement or the documents referred to in this Agreement shall operate as a waiver thereof, nor shall any waiver on the part of any party of any such right, power or privilege, nor any single or partial exercise of any such right, power or privilege, preclude any other or further exercise thereof or the exercise of any other such right, power or privilege. The failure of a party to exercise any right conferred herein within the time required shall cause such right to terminate with respect to the transaction or circumstances giving rise to such right, but not to any such right arising as a result of any other transactions or circumstances.
10.10. Severability. If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced as a result of any rule of Law or public policy, all other terms and other provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the transactions contemplated by this Agreement is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in an acceptable manner to the end that the transactions contemplated by this Agreement are fulfilled to the greatest extent possible.
10.11. Burden and Benefit. This Agreement shall be binding upon and shall inure to the benefit of, the parties hereto and their respective successors and permitted assigns. This Agreement and all of its conditions and provisions are for the sole and exclusive benefit of the parties hereto and their respective successors and permitted assigns, and nothing in this Agreement, express or implied, is intended to confer upon any Person other than the parties hereto any rights or remedies of any nature whatsoever under or by reason of this Agreement or any provision hereof, except as set forth in Section 10.8 above.
10.12. Governing Law. This Agreement shall be governed by and construed in accordance with the internal laws of the State of Delaware, without giving effect to principles of conflicts of laws that would require the application of the laws of any other jurisdiction.
10.13. Consent to Jurisdiction. The parties hereto agree that any Proceeding seeking to enforce any provision of, or based on any matter arising out of or in connection with, this Agreement or the transactions contemplated hereby shall be brought exclusively in any federal or state court located in the State of Delaware, and each of the parties hereby irrevocably consents to the jurisdiction of such courts (and of the appropriate appellate courts therefrom) in any such Proceeding and irrevocably waives, to the fullest extent permitted by law, any objection that it may now or hereafter have to the laying of the venue of any such Proceeding in any such court or that any such Proceeding brought in any such court has been brought in an inconvenient forum. Process in any such Proceeding may be served on any party anywhere in the world, whether within or without the jurisdiction of any such court. Without limiting the foregoing, each party agrees that service of process on such party as provided in Section 10.2 shall be deemed effective service of process on such party.
| -344- |
10.14. Waiver of Trial by Jury. EACH PARTY TO THIS AGREEMENT ACKNOWLEDGES AND AGREES THAT ANY CONTROVERSY WHICH MAY ARISE UNDER THIS AGREEMENT IS LIKELY TO INVOLVE COMPLICATED AND DIFFICULT ISSUES, AND THEREFORE IT HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THIS AGREEMENT AND ANY OF THE AGREEMENTS DELIVERED IN CONNECTION HEREWITH OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (A) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE EITHER OF SUCH WAIVERS, (B) IT UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF SUCH WAIVERS, (C) IT MAKES SUCH WAIVERS VOLUNTARILY, AND (D) IT HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 10.14.
10.15. No Survival of Representations, Warranties and Covenants. None of the representations, warranties, covenants and agreements in this Agreement or in any instrument, document or certificate delivered pursuant to this Agreement shall survive the Closing and all such representations, warranties, covenants, and agreements shall expire upon the occurrence of the Closing, except for those covenants and agreements contained herein and therein which by their terms expressly apply or are to be performed, in whole or in part, after the Closing and then only to such extent.
10.16. Specific Performance. The parties hereto agree that irreparable damage would occur if any provision of this Agreement were not performed in accordance with the terms hereof or were otherwise breached and that, irrespective of any other rights or remedies that may be available to the parties as provided herein or otherwise, the parties shall be entitled to an injunction or injunctions to prevent breaches of this Agreement or to enforce specifically the performance of the terms and provisions hereof in any federal or state court located in the State of Delaware. Each of the parties hereto agrees that it will not oppose the granting of an injunction, specific performance and other equitable relief on the basis that the other parties hereto have an adequate remedy at law or an award of specific performance is not an appropriate remedy for any reason at law or equity. The parties hereto acknowledge and agree that any party seeking an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement in accordance with this Section 10.16 shall not be required to provide any bond or other security in connection with any such order or injunction.
10.17. Cumulative Remedies. Except as otherwise expressly set forth in this Agreement, all rights and remedies of any party hereto are cumulative of each other and of every other right or remedy such party may otherwise have at Law or in equity, and the exercise of one or more rights or remedies shall not prejudice or impair the concurrent or subsequent exercise of other rights or remedies.
10.18. Expenses. Except as otherwise expressly set forth in this Agreement, all costs and expenses incurred in connection with this Agreement and the transactions contemplated hereby shall be paid by the party incurring such expenses.
10.19. Representation by Counsel. Each party hereto represents and agrees with each other that it has been represented by or had the opportunity to be represented by, independent counsel of its own choosing, and that it has had the full right and opportunity to consult with its respective attorney(s), that to the extent, if any, that it desired, it availed itself of this right and opportunity, that it or its authorized officers (as the case may be) have carefully read and fully understand this Agreement in its entirety and have had it fully explained to them by such party’s respective counsel, that each is fully aware of the contents thereof and its meaning, intent and legal effect, and that it or its authorized officer (as the case may be) is competent to execute this Agreement and has executed this Agreement free from coercion, duress or undue influence.
| -345- |
10.20. Execution and Counterparts. This Agreement may be executed in one or more counterparts, each of which when executed shall be deemed an original and all of which together shall constitute one and the same instrument. The parties agree that this Agreement shall be legally binding upon the electronic transmission, including by facsimile or email, by each party of a signed signature page to this Agreement to the other party.
10.21. Stockholders’ Representative.
(a) Appointment. By executing this Agreement, the Company (and, by virtue of the adoption of this Agreement and the approval of the Merger by the Company Requisite Stockholder Consent, each Company Stockholder) shall be deemed to have irrevocably constituted and appointed, effective from and after the Effective Time, Steve Thornton as the true, exclusive and lawful agent and attorney-in-fact for and on behalf of each Company Stockholder to act in the name, place and stead of the Company Stockholders in connection with the transactions contemplated by this Agreement, in accordance with the terms and provisions of this Agreement, and to act on behalf of the Company Stockholders in any Proceeding involving this Agreement, to do or refrain from doing all such further acts and things, and to execute all such documents as the Stockholders’ Representative shall deem necessary or appropriate in connection with the transactions contemplated by this Agreement, including in respect of the following matters:
(i) to execute and deliver all amendments, waivers, ancillary agreements, stock powers, certificates and documents that the Stockholders’ Representative deems necessary or appropriate in connection with the consummation of the transactions contemplated by this Agreement;
(ii) giving and receiving any notice or instruction permitted or required to be given to or received by any Company Stockholder under this Agreement;
(iii) dealing with Parent under this Agreement with respect to all matters arising under this Agreement; and
(iv) engaging counsel, accountants or other Stockholders’ Representatives in connection with the foregoing matters.
(b) Authorization. Each Company Stockholder authorizes the Stockholders’ Representative, on such Company Stockholder’s behalf, to:
(i) receive all notices or documents given or to be given to any of the Company Stockholders by Parent or the Surviving Corporation pursuant hereto or in connection herewith and to receive and accept service of legal process in connection with any suit or proceeding arising under this Agreement;
| -346- |
(ii) engage counsel, and such accountants and other advisors for any of the Company Stockholders and incur such other expenses on behalf of any of the Company Stockholders in connection with this Agreement and the transactions contemplated hereby or thereby as the Stockholders’ Representative may in its sole discretion deem appropriate; and
(iii) take such action on behalf of any of the Company Stockholders as the Stockholders’ Representative may in its sole discretion deem appropriate in respect of: (A) taking such other action as the Stockholders’ Representative is authorized to take under this Agreement; (B) receiving all documents or certificates and making all determinations, on behalf of any of the Company Stockholders, required under this Agreement; and (C) all such action as may be necessary after the Closing Date to carry out any of the transactions contemplated by this Agreement, including any waiver of any obligation of Parent or the Surviving Corporation.
(c) Decisions. All actions, decisions and instructions of the Stockholders’ Representative shall be conclusive and binding upon all of the Company Stockholders and no Company Stockholder shall have any claim or cause of action against the Stockholders’ Representative, and the Stockholders’ Representative shall have no liability to any Company Stockholder, for any action taken, decision made or instruction given by the Stockholders’ Representative in connection with this Agreement, except in the case of its own gross negligence or willful misconduct.
(d) Reliance. Parent, Merger Sub and the Surviving Corporation shall not be obliged to inquire into the authority of the Stockholders’ Representative, and Parent, Merger Sub and the Surviving Corporation shall be fully protected in dealing with the Stockholders’ Representative in good faith.
(e) Successor Stockholders’ Representative. If the Stockholders’ Representative shall die, become disabled, resign or otherwise be unable to fulfill its responsibilities hereunder, the Company Stockholders who in the aggregate held at least a majority of the Company Capital Stock immediately prior to the Effective Time shall appoint a new Stockholders’ Representative as soon as reasonably practicable by written consent by sending notice and a copy of the duly executed written consent appointing such new Stockholders’ Representative to Parent and the Surviving Corporation. Such appointment will be effective upon the later of the date indicated in the consent or the date such consent is received by Parent and the Surviving Corporation. Company Stockholders who in the aggregate held at least a majority of the Company Capital Stock immediately prior to the Effective Time shall have the right at any time to remove the then-acting Stockholders’ Representative and to appoint a successor Stockholders’ Representative; provided, however, that neither such removal of the then acting Stockholders’ Representative nor such appointment of a successor Stockholders’ Representative shall be effective until the delivery to Parent and Surviving Corporation of executed counterparts of a writing signed by each such Company Stockholder with respect to such removal and appointment, together with an acknowledgment signed by the successor Stockholders’ Representative appointed in such writing that it, he or she accepts the responsibility of successor Stockholders’ Representative and agrees to perform and be bound by all of the provisions of this Agreement applicable to the Stockholders’ Representative. Each successor Stockholders’ Representative shall have all of the power, authority, rights, privileges and obligations conferred by this Agreement upon the original Stockholders’ Representative, and the term “Stockholders’ Representative” as used herein shall be deemed to include any interim or successor Stockholders’ Representative.
| -347- |
10.22. Company Representation. Parent, for itself and on behalf of its Subsidiaries (including Merger Sub), and each Acquired Company, for itself and on behalf of its Subsidiaries and their respective directors, equityholders, members, partners, officers, employees and Affiliates, hereby agree that, in the event that a dispute arises after the Closing between Parent, the Surviving Corporation, its Subsidiaries or any of their Affiliates, on the one hand, and the Company Stockholders, Stockholder Representative, or any of their Affiliates, on the other hand, Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P. (“Smith Anderson”) may represent the Company Stockholders, Stockholder Representative, or such Affiliate in such dispute, even though the interests of the Company Stockholders, Stockholder Representative, or such Affiliate may be directly adverse to Parent, the Surviving Corporation, its Subsidiaries and/or their Affiliates and even though Smith Anderson may have represented the Company and/or its Subsidiaries in a matter substantially related to such dispute.
10.23 Parent Transaction Engagements. Notwithstanding anything to the contrary in this Agreement, from and after the Closing, (i) all communications between Parent or its subsidiaries or any of their respective directors, equityholders, members, partners, officers, employees and Affiliates, on one hand, and Sheppard, Mullin, Richter & Hampton LLP (“Parent’s Counsel”), on the other hand, in the connection with the transactions contemplated by this Agreement (collectively, the “Parent Transaction Engagements”) shall be deemed to be attorney-client confidences that belong solely to the members of the board of directors of Parent as of immediately prior to the Effective Time (the “Transaction Board Members”) and not Parent or its subsidiaries, (ii) neither the Company nor its subsidiaries nor any of their respective directors, equityholders, members, partners, officers, employees and Affiliates shall have access to any such communications, or to any of the files or other documents delivered or prepared in connection therewith, (iii) the Transaction Board Members shall be the sole holders of the attorney-client privilege with respect to each Parent Transaction Engagement, and neither the Parent nor its subsidiaries or any of their respective representatives nor the Company or its subsidiaries or any of their respective representatives shall be a holder thereof, (iv) to the extent that files of Parent’s Counsel in respect of any Parent Transaction Engagement constitute property of the client thereof, only Transaction Board Members shall hold such property rights thereto, and (v) unless directed to do so by the Transaction Board Members or by a court of competent jurisdiction or other Governmental Authority (and then in each case only to the extent of such direction), Parent’s Counsel shall not have any duty whatsoever to reveal or disclose any such attorney-client communications or files related to Parent or its subsidiaries or any of their respective representatives or the Company or its subsidiaries or any of their respective representatives by reason of any attorney-client relationship between Parent’s Counsel and Parent or any of its subsidiaries or otherwise.
(Signature Page Follows)
| -348- |
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed as of the day and year first set forth above.
| FOXWAYNE ENTERPRISES ACQUISITION CORP. | ||
| By: | ||
| Name: | ||
| Title: | ||
| GOTHAM MERGER SUB, INC. | ||
| By: | ||
| Name: | ||
| Title: | ||
| AERAMI THERAPEUTICS HOLDINGS, INC. | ||
| By: | ||
| Name: | ||
| Title: | ||
| STEVE THORNTON, as Stockholders Representative | ||
[Signature Page to Agreement and Plan of Merger]
| -349- |
Exhibit A-2
FORM OF STOCKHOLDER SUPPORT AGREEMENT
This STOCKHOLDER SUPPORT AGREEMENT (this “Agreement”), dated as of November [●], 2021, is by and among FOXWAYNE ENTERPRISES ACQUISITION CORP., a Delaware corporation (“Parent”), AERAMI THERAPEUTICS HOLDINGS, INC., a Delaware corporation (the “Company”), and the undersigned (“Stockholder”).
RECITALS
A. Parent, the Company, GOTHAM MERGER SUB, INC., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), and, STEVE THORNTON (solely in his capacity as Stockholders’ Representative) are contemporaneously entering into an Agreement and Plan of Merger (together with the Ancillary Documents, each as amended from time to time, the “Merger Agreement”), dated as of November [], 2021, pursuant to which, among other things, the Merger Sub is to merge with and into the Company, with the Company surviving as a wholly-owned subsidiary of Parent (the “Merger”).
B. As of the date hereof, Stockholder is the Beneficial Owner (as defined below) of that number of shares of (i) common stock, par value $0.0001 per share, of the Company (“Company Common Stock”), (ii) Series A Preferred Stock of the Company, par value $0.0001 per share (“Company Series A Preferred Stock”), (iii) Series A-1 Preferred Stock of the Company, par value $0.0001 per share (“Company Series A-1 Preferred Stock”), and (iv) Royalty Series Convertible Preferred Stock of the Company, par value $0.0001 per share (“Company Royalty Series Convertible Preferred Stock” and, together with the Company Common Stock, Company Series A Preferred Stock, and Company Series A-1 Preferred Stock, the “Company Capital Stock”), each as set forth beside Stockholder’s name on Schedule A hereto and has or will have the sole power to vote (or to direct the voting of) such shares (all such shares of Company Capital Stock Beneficially Owned by Stockholder as of the date hereof or during the term of this Agreement, the “Owned Shares”).
C. Parent and the Company, in consideration for the benefits to be received by each Stockholder under the terms of the Merger Agreement, have required that Stockholder enter into this Agreement as a condition and inducement to the willingness of Parent and the Company to enter into the Merger Agreement and consummate the Transactions.
Accordingly, and in consideration of the foregoing and the mutual representations, warranties, covenants and agreements contained herein, the parties hereto, intending to be legally bound, hereby agree as follows:
AGREEMENT
ARTICLE 1
VOTING AGREEMENT
1.1 Agreement to Vote.
(a) From the date of this Agreement until the Expiration Time (as defined below), Stockholder will (and, if applicable, will cause each of its Affiliates that has the right to vote or direct the voting of any Subject Shares (as defined below) to) (i) appear at any meeting of stockholders or otherwise cause any Subject Shares to be counted as present thereat for purposes of calculating a quorum, (ii) (A) vote in favor of, or (B) in the event that the Company seeks Stockholder’s approval via written consent, as promptly as reasonably practicable (and in any event within two (2) Business Days) following the delivery by the Company of the applicable Consent Solicitation Statement), duly execute and deliver to the Company and Parent the written approval solicited by the Company pursuant to such Consent Solicitation Statement under which Stockholder shall irrevocably and unconditionally consent to, the Company Stockholder Matters (as defined herein), and (iii) withhold its approval of or vote against any action, proposal, transaction or agreement that could reasonably be expected to (1) result in a breach of any covenant, representation or warranty or any other obligation or agreement under this Agreement or the Merger Agreement or (2) otherwise interfere with the Transactions.
| -350- |
(b) Stockholder will not enter into any agreement with any Person (other than the Company) prior to the Expiration Time (with respect to periods prior to the Expiration Time) directly or indirectly to vote, grant any proxy or give instructions with respect to the voting of the Subject Shares, the effect of which would be inconsistent with or violate any provision contained in herein. Any vote or consent (or withholding of a vote or consent or otherwise abstaining from voting or consenting) by Stockholder that is not in accordance with this Section 1.1 will be considered null and void.
(c) The Company may, in its sole discretion, waive the provisions of this Section 1.1 as to any matter brought to the stockholders of the Company for a vote (or consent pursuant to an action by written consent of the stockholders, if applicable).
1.2 Revocation of Prior Proxies; Grant of Irrevocable Proxy.
(a) Stockholder hereby represents and warrants that any proxies heretofore given in respect of the Owned Shares or the Subject Shares (other than the proxies granted pursuant to the Voting Agreement, which, for the avoidance of doubt shall not apply to the extent that Stockholder votes in favor of, or grants its written consent to, the Company Stockholder Matters pursuant to this Agreement) are not irrevocable, and Stockholder hereby revokes any and all prior proxies with respect to the Subject Shares (other than the proxies granted pursuant to the Voting Agreement, which, for the avoidance of doubt shall not apply to the extent that Stockholder votes in favor of, or grants its written consent to, the Company Stockholder Matters pursuant to this Agreement). Prior to the Expiration Time, Stockholder will not directly or indirectly grant any proxies or powers of attorney (other than to Parent), deposit any of the Owned Shares or the Subject Shares into a voting trust or enter into a voting agreement (other than this Agreement) with respect to any of the Owned Shares or the Subject Shares.
(b) Stockholder hereby appoints the Company and any designee of the Company, each of them individually, such Stockholder’s proxy and attorney in fact until the Expiration Time, with full power of substitution and re-substitution, to vote, direct the vote or act by written consent with respect to the Owned Shares (i) in accordance with Section 1.1 hereof and (ii) to sign his, her or its name (as a stockholder) to any consent, certificate or other document relating to the Company that the Law of any state or the rules of any bank, broker or depositary may permit or require in connection with any matter referred to in Section 1.1. This proxy is given to secure the performance of the duties of such Stockholder under this Agreement and its existence will not be deemed to relieve such Stockholder of his, her or its obligations under Section 1.1. Stockholder affirms that this proxy is coupled with an interest and is irrevocable until the Expiration Time, where upon such proxy and power of attorney will automatically terminate and be deemed null and void. Stockholder will take such further action or execute such other instruments as may be necessary to effectuate the intent of this proxy. The proxy granted herein is intended to comply with the requirements of Delaware General Corporation Law applicable to irrevocable proxies. The proxy granted herein shall not be revoked when the interest with which it is coupled is extinguished. The power of attorney granted by Stockholder herein is a durable power of attorney and shall survive the dissolution, bankruptcy, death or incapacity of Stockholder.
| -351- |
1.3 Merger Consideration. Stockholder hereby acknowledges that Stockholder agrees with and approves in all respects (i) the total amount, and the mechanics for the allocation, of the Aggregate Consideration and the Per Share Consideration pursuant to the Merger Agreement (including, for the avoidance of doubt, the schedules and exhibits to the Merger Agreement), (ii) the components of the Aggregate Consideration and the Per Share Consideration (including any amounts of the payments to holders of Company Capital Stock, Company Warrants, Company Options, and Company RSU Awards), in each case, determined in accordance with the Merger Agreement, and (iii) the allocation of various forms of consideration as set forth in the Merger Agreement.
1.4 Appraisal Rights Waiver. Stockholder hereby (i) forever waives all appraisal or dissenter’s rights under applicable Law with respect to the Merger (including Section 262 of the Delaware General Corporation Law) and (ii) withdraws all written objections to the Merger and/or demands for appraisal, if any, with respect to the Owned Shares or the Subject Shares by Stockholder.
1.5 Stockholders’ Representative. Stockholder hereby irrevocably nominates, constitutes and appoints the Stockholders’ Representative as its, his or her true and lawful agent, proxy and attorney in fact, with full power and authority (and power of substitution and re-substitution), to act in the name, place and stead of Stockholder for purposes of voting, taking any action by written consent, executing and delivering any documents, receiving any notice and taking any actions that the Stockholders’ Representative may, in its sole discretion, determine to be necessary, desirable or appropriate within the bounds of the Stockholders’ Representative’s authority under the express terms of the Merger Agreement.
1.6 Release. Effective upon the Closing and receipt by Stockholder of the right to receive the portion of the merger consideration to which Stockholder is entitled at Closing pursuant to the Merger Agreement, if any, Stockholder hereby generally releases, remises and forever discharges Parent, Merger Sub, the Company, the Stockholders’ Representative, the Surviving Corporation and their respective Agents (as herein defined) from and against any and all claims, demands, liens, actions, agreements, suits, causes of action, obligations, controversies, debts, costs, attorneys’ fees, expenses, damages, judgments, orders and liabilities of whatever kind or nature in law, equity or otherwise, whether or not now known or suspected, that have existed or may have existed, or that do exist or that hereafter shall or may exist, based on any facts, events or omissions occurring from any time on or prior to the execution and delivery of this Agreement that arise out of any rights Stockholder may have in his, her or its capacity as a holder of Company Capital Stock against the Company or any of its Affiliates; provided, however, that nothing in this Agreement shall be construed to release, remise, discharge or acquit: (a) any claims or rights Stockholder had, has or may have under the Merger Agreement or any other agreements or instruments executed and delivered in connection with the Merger Agreement to which Stockholder is a party or beneficiary or otherwise with respect to the Merger; (b) if Stockholder is or was a director or officer of the Company, any claim or right of Stockholder to be indemnified as a result of serving as a director or officer of the Company, including but not limited to any rights available to Stockholder for indemnification or insurance recoveries under the Company’s Organizational Documents, any agreement between Stockholder and the Company or any directors’ and officers’ insurance policy for Stockholder’s benefit or under applicable Law; (c) any claims arising out of actual and intentional fraud; and (d) if Stockholder is or was an employee of the Company, any rights with respect to earned but unpaid salary or other compensation or benefits that accrued prior to the Closing in the ordinary course of business. As used herein, an “Agent” of a party is each of its predecessors, its former or present officers, employees, directors, stockholders, parents, subsidiaries, Affiliates, partners, related corporate entities, agents, attorneys, members, heirs, executors, administrators, conservators, successors and assigns.
Stockholder waives all rights under any Law, rule, provision or statute of any jurisdiction that states in full (or otherwise in substance) as follows:
“A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.”
| -352- |
1.7 Directors and Officers. Notwithstanding any provision of this Agreement to the contrary, nothing in this Agreement shall (or shall require Stockholder to attempt to) limit or restrict any Stockholder in his or her capacity as a director or officer of the Company or any designee of the Stockholder who is a director or officer of the Company from acting in such capacity or voting in such person’s sole discretion on any matter (it being understood that this Agreement shall apply to Stockholder solely in Stockholder’s capacity as a stockholder of the Company).
ARTICLE 2
DEFINITIONS
Capitalized terms used but not defined in this Agreement are used in this Agreement with the meanings given to such terms in the Merger Agreement. In addition, for purposes of this Agreement:
“Affiliate” means, with respect to any specified Person, a Person who, at the time of determination, directly or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, such specified Person. For purposes of this Agreement, with respect to Stockholder, “Affiliate” does not include the Company and the Persons that directly or indirectly through one or more intermediaries are controlled by the Company.
“Beneficially Owned” or “Beneficial Ownership” with respect to any securities means having beneficial ownership of such securities (as determined pursuant to Rule 13d-3 under the Exchange Act, disregarding the phrase “within 60 days” in paragraph (d)(1)(i) thereof), including pursuant to any agreement, arrangement or understanding, whether or not in writing. Without duplicative counting of the same securities, securities Beneficially Owned by a Person include securities Beneficially Owned by (i) all Affiliates of such Person and (ii) all other Persons with whom such Person would constitute a “group” within the meaning of Section 13(d) of the Exchange Act and the rules promulgated thereunder. For the avoidance of doubt, “Beneficially Own” and “Beneficial Ownership” shall also include record ownership of securities.
“Beneficial Owner” with respect to any securities means a Person that has Beneficial Ownership of such securities.
“Company Stockholder Matters” means approval of the execution and delivery of the Merger Agreement, the Merger, and the consummation of the Transactions on the terms set forth in the Merger Agreement along with any approval that is required under the organizational documents of the Company or otherwise sought with respect to the Merger Agreement or the transactions contemplated thereby.
“Subject Shares” means, with respect to Stockholder, the Owned Shares set forth on Schedule A hereto (together with any other Company Capital Stock or other equity interests (including anything convertible into such equity interests) of the Company that each Stockholder acquires record or beneficial ownership of after the date hereof).
“Voting Agreement” means that certain Voting Agreement, dated as of November 30, 2017, among the Company and certain Stockholders of the Company.
| -353- |
ARTICLE 3
REPRESENTATIONS, WARRANTIES AND ADDITIONAL COVENANTS OF STOCKHOLDER
Stockholder represents, warrants and covenants to the Company that:
3.1 Ownership. Stockholder is the sole Beneficial Owner and the record and legal owner of the Company Capital Stock identified or required to be identified on Schedule A, subject (with respect to shares of Company Capital Stock underlying options and warrants) to the Company’s due authorization and valid issuance thereof, and such shares constitute all of the capital stock of the Company that are Beneficially Owned by Stockholder. Subject (with respect to shares of Company Capital Stock underlying options and warrants) to the Company’s due authorization and valid issuance thereof, Stockholder has (or, with respect to shares of Company Capital Stock underlying options or warrants, will have, following the exercise of such options or warrants) good and valid title to all of the Owned Shares, free and clear of all liens, claims, options, proxies, voting agreements, marital, community property and other spousal interests, and security interests and has the sole right to such Company Capital Stock and there are no restrictions on rights of disposition or other liens or encumbrances pertaining to such Company Capital Stock (other than pursuant to this Agreement and compliance with applicable securities laws) that could adversely affect the Merger, the Merger Agreement, or the exercise or fulfillment of the rights and obligations of Stockholder under this Agreement. None of the Owned Shares are or will be subject to any voting trust or other contract with respect to the voting thereof (other than the Voting Agreement, which is expected to be terminated in connection with the Closing, and this Agreement), and no proxy, power of attorney or other authorization has been granted with respect to any of such Company Capital Stock
3.2 Authority and Non-Contravention.
(a) Stockholder is either a natural person or a corporation, limited partnership, limited liability company or other entity duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is incorporated, organized or constituted. Stockholder has all necessary power and authority and legal capacity to execute and deliver this Agreement, to perform its obligations hereunder and to consummate the transactions contemplated hereby. The execution and delivery of this Agreement by Stockholder and the consummation by Stockholder of the transactions contemplated hereby have been duly and validly authorized by all necessary corporate or other action, and no other corporate or other proceedings on the part of Stockholder are necessary to authorize this Agreement or to consummate the transactions contemplated hereby.
(b) This Agreement has been duly and validly executed and delivered by Stockholder and constitutes the legal, valid and binding obligation of Stockholder, enforceable against Stockholder in accordance with its terms except (i) to the extent limited by applicable bankruptcy, insolvency or similar laws affecting creditors’ rights and (ii) the remedy of specific performance and injunctive and other forms of equitable relief may be subject to equitable defenses and to the discretion of the court before which any proceeding therefor may be brought.
(c) Stockholder is not nor will it be required to make any filing with or give any notice to, or to obtain any consent from, any Person in connection with the execution, delivery or performance of this Agreement or obtain any permit or approval from any government authority for any of the transactions contemplated hereby.
(d) Neither the execution and delivery of this Agreement by Stockholder nor the consummation of the transactions contemplated hereby will directly or indirectly (whether with notice or lapse of time or both) (i) conflict with, result in any violation of or constitute a default by Stockholder under any mortgage, bond, indenture, agreement, instrument or obligation to which Stockholder is a party or by which it or any of the Owned Shares are bound, or violate any permit of any government authority, or any applicable law or order to which Stockholder, or any of the Company Capital Stock, may be subject, or (ii) result in the imposition or creation of any lien or encumbrance upon or with respect to any of the Owned Shares.
| -354- |
(e) Stockholder has the requisite voting power and the requisite power, authority and capacity, as applicable, to issue instructions with respect to the matters set forth in Article 1 and the requisite power, authority and capacity, as applicable, to agree to all of the matters set forth in this Agreement, in each case with respect to all of the Owned Shares, with no limitations, qualifications or restrictions on such rights.
3.3 Total Shares. Except as set forth on Schedule A, Stockholder is not the Beneficial Owner of, and does not have (whether currently, upon lapse of time, following the satisfaction of any conditions, upon the occurrence of any event or any combination of the foregoing) any right to acquire, and has no other interest in or voting rights with respect to, any Company Capital Stock or any securities convertible into or exchangeable or exercisable for Company Capital Stock.
3.4 Merger Agreement. Stockholder has received a copy of the Merger Agreement (including, for the avoidance of doubt, the schedules and exhibits thereto, other than the Consideration Schedule, of which Stockholder acknowledges a final copy will be provided prior to the consummation of the Transactions) and has carefully read and understands the scope and effect of the provisions thereof and of this Agreement and has discussed the foregoing with Stockholder’s professional advisors to the extent Stockholder has deemed necessary.
3.5 Reliance. Stockholder understands and acknowledges that Parent and the Company are each entering into the Merger Agreement in reliance upon Stockholder’s execution, delivery and performance of this Agreement.
3.6 No Litigation. As of the date of this Agreement, there is no Proceeding pending or, to the knowledge of Stockholder, threatened against Stockholder or the Owned Shares that could reasonably be expected to impair the ability of Stockholder to perform its obligations hereunder or consummate the transactions contemplated hereby.
3.7 Brokers and Finders. No broker, finder, financial advisor, investment banker or other person is entitled to any brokerage, finder’s, financial advisor’s or other similar fee or commission in connection with the transactions contemplated hereby based upon arrangements made by or on behalf of Stockholder.
ARTICLE 4
REPRESENTATIONS AND WARRANTIES OF THE COMPANY AND PARENT
Each of the Company and Parent, severally and not jointly, represents and warrants to Stockholder that (i) this Agreement constitutes the legal, valid and binding obligation of such Person enforceable against such Person in accordance with its terms (assuming this Agreement constitutes the legal, valid and binding obligation of Stockholder), except (x) to the extent limited by applicable bankruptcy, insolvency or similar laws affecting creditors’ rights and (y) the remedy of specific performance and injunctive and other forms of equitable relief may be subject to equitable defenses and to the discretion of the court before which any proceeding therefor may be brought, (ii) each of the Company and Parent has the corporate power and authority to execute and deliver this Agreement and to perform its obligations hereunder, (iii) the execution and delivery by Company and Parent of this Agreement and the consummation by the Company and Parent of the transactions contemplated hereby have been duly and validly authorized by the Company and Parent and no other corporate proceedings on the part of the Company or Parent are necessary to authorize this Agreement or to consummate the transactions contemplated hereby, and (iv) this Agreement has been duly and validly executed and delivered by each of the Company and Parent.
| -355- |
ARTICLE 5
TERM AND TERMINATION
This Agreement will become effective as of the date hereof. This Agreement will terminate upon the earliest to occur of (i) the Effective Time, (ii) the date of termination of the Merger Agreement, or (iii) the mutual agreement of the Company and Stockholder to terminate this Agreement (any such time under clauses (i) through (iii) being referred to herein as the “Expiration Time.” The parties acknowledge that, upon termination of this Agreement as permitted under and in accordance with the terms of this Article 5, no party to this Agreement shall have the right to recover any claim with respect to any losses suffered by such party in connection with such termination, except that the termination of this Agreement will not relieve Stockholder from any liability for any inaccuracy in or breach of any representation, warranty or covenant contained in this Agreement. Notwithstanding anything to the contrary herein, Article 6 will survive any termination of this Agreement.
ARTICLE 6
GENERAL PROVISIONS
6.1 Action in Stockholder Capacity Only. Stockholder is entering into this Agreement solely in Stockholder’s capacity as a record holder and Beneficial Owner, as applicable, of the Company Capital Stock and not in Stockholder’s capacity as a director or officer of the Company. Nothing herein will limit or affect Stockholder’s ability to act as an officer or director of the Company.
6.2 No Ownership Interest. Nothing contained in this Agreement will be deemed to vest in Parent, the Company or any of their respective Affiliates any direct or indirect ownership or incidents of ownership of or with respect to the Owned Shares or the Subject Shares.
6.3 Notices. All notices and other communications hereunder must be in writing and will be deemed to have been duly given (a) when delivered or sent if delivered in Person or sent by email transmission (provided confirmation of email transmission is obtained) or (ii) on the next Business Day if transmitted by national overnight courier, in each case as follows:
If to the Company, addressed to:
Aerami Therapeutics Holdings, Inc.
2520 Meridian Parkway, Suite 400
Durham, North Carolina, 27713
Attn: Steve Thornton
Email: sthornton@aerami.com
With a copy (which shall not constitute notice) to:
Smith,
Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P.
150 Fayetteville Street
Suite 2300
Raleigh, NC 27601
Attn:
Amy Batten and Peter Bosman
Email: abatten@smithlaw.com; pbosman@smithlaw.com
| -356- |
If to Parent, addressed to:
FoxWayne Enterprises Acquisition Corp.
1 Rockefeller Plaza, Suite 1039
New York, New York 10020
Attn: Robb Knie
Email: robb@foxwayne.com
With a copy (which shall not constitute notice) to:
Sheppard,
Mullin, Richter & Hampton LLP
30 Rockefeller Plaza
New
York, New York 10112
Attn: Richard Friedman
Email: rafriedman@sheppardmullin.com
If to Stockholder, to Stockholder’s address set forth on Schedule A.
6.4 Publicity. Unless required by applicable law or permitted by the Merger Agreement, Stockholder will not, and will not authorize or direct any of its Affiliates or representatives to, make any press release or public announcement with respect to this Agreement or the Merger Agreement or the transactions contemplated hereby or thereby, without the prior written consent of Parent in each instance.
6.5 Further Actions. Upon the request of any party to this Agreement, each other party will (a) furnish to the requesting party any additional information, (b) execute and deliver, at their own expense, any other documents and (c) take any other actions as the requesting party may reasonably require to more effectively carry out the intent of this Agreement. Stockholder hereby agrees that either the Company or Parent may publish and disclose in any filing made by Parent or the Company with the SEC, the Nasdaq Stock Market or other applicable regulatory authority Stockholder’s identity and ownership of any Company Capital Stock and the nature of such Stockholder’s commitments, arrangements and understandings under this Agreement and may further file this Agreement as an exhibit to any filing made by Parent or the Company with the SEC. Stockholder agrees to (x) provide any information reasonably requested by the Company for any such regulatory application or filing and (y) notify the Company promptly of any additional shares of capital stock of the Company of which Stockholder becomes the Beneficial Owner after the date of this Agreement.
6.6 Transfer of Subject Shares. Except as expressly contemplated by the Merger Agreement or with the prior written consent of the Company (such consent to be given or withheld in its sole discretion), from and after the date hereof, each Stockholder agrees not to (a) Transfer any of the Subject Shares, (b) enter into (i) any option, warrant, purchase right, or other Contract that would (either alone or in connection with one or more events or developments (including the satisfaction or waiver of any conditions precedent)) require such Stockholder to Transfer the Subject Shares or (ii) any voting trust, proxy or other Contract with respect to the voting or Transfer of the Subject Company Shares, or (c) take any actions (i) having the effect of preventing or disabling such Stockholder from performing its obligations under this Agreement or (ii) in furtherance of any of the matters described in the foregoing clauses (a) or (b). For purposes of this Agreement, “Transfer” means any, direct or indirect, sale, transfer, assignment, pledge, mortgage, exchange, hypothecation, grant of a security interest in or disposition or encumbrance of an interest (whether with or without consideration, whether voluntarily or involuntarily or by operation of law or otherwise).
6.7 Miscellaneous. Sections 10.3 (Rules of Construction), 10.4 (References), 10.5 (Entire Agreement), 10.8 (Rights of Third Parties), 10.9 (Waiver), 10.10 (Severability), 10.12 (Governing Law), 10.13 (Consent to Jurisdiction), 10.14 (Waiver of Trial by Jury), 10.16 (Specific Performance), 10.17 (Cumulative Remedies), 10.18 (Expenses), 10.20 (Execution and Counterparts) of the Merger Agreement are incorporated herein by reference and shall apply to this Agreement, mutatis mutandis.
[remainder of page intentionally blank]
| -357- |
[Signature Page to Stockholder Support Agreement]
IN WITNESS WHEREOF, the parties hereto have caused this Stockholder Support Agreement to be duly executed as of the day and year first above written.
| PARENT: | FOXWAYNE ENTERPRISES ACQUISITION CORP. | |
| By: | ||
| Name: | ||
| Title: | ||
| COMPANY: | AERAMI THERAPEUTICS HOLDINGS, INC. | |
| By: | ||
| Name: | ||
| Title: | ||
| -358- |
| STOCKHOLDER: | [ENTITY NAME] | |
| By: | ||
| Name: | ||
Title: |
||
| [NATURAL PERSON NAME] | ||
| Additional Signature (if held jointly): | ||
| (If held jointly) | ||
| (Printed Full Name) | ||
| -359- |
SCHEDULE A
| NAME AND | COMPANY CAPITAL STOCK | |
ADDRESS OF STOCKHOLDER |
BENEFICIALLY OWNED | |
| [Name] | [●] shares of Company Common Stock | |
| [Address 1] | [●] shares of Company Series A Preferred Stock | |
| [Address 2] | [●] shares of Company Series A-1 Preferred Stock | |
| [E-mail] | [●] shares of Company Royalty Series Convertible Preferred Stock | |
| [●] shares of Company Common Stock underlying outstanding options | ||
| [●] shares of Company Common Stock underlying outstanding warrants |
| -360- |
Exhibit B
FORM OF SPONSOR SUPPORT AGREEMENT
This SPONSOR SUPPORT AGREEMENT (this “Agreement”), dated as of November [●], 2021, is by and among FOXWAYNE ENTERPRISES ACQUISITION SPONSOR LLC, a Delaware limited liability company (“Sponsor”), FOXWAYNE ENTERPRISES ACQUISITION CORP., a Delaware corporation (“Parent”), and AERAMI THERAPEUTICS HOLDINGS, INC., a Delaware corporation (the “Company”).
RECITALS
A. Parent, the Company, GOTHAM MERGER SUB, INC., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), and, STEVE THORNTON (solely in his capacity as Stockholders’ Representative) are contemporaneously entering into an Agreement and Plan of Merger (together with the Ancillary Documents, each as amended from time to time, the “Merger Agreement”), dated as of November [●], 2021, pursuant to which, among other things, the Merger Sub is to merge with and into the Company, with the Company surviving as a wholly-owned subsidiary of Parent (the “Merger”).
B. As of the date hereof, Sponsor is the Beneficial Owner (as defined below) of that number of shares of Class B Common Stock, par value $0.0001 per share, of the Parent (“Parent Common Stock”) set forth on Schedule A hereto and has or will have the sole power to vote (or to direct the voting of) such shares (all such shares as of the date hereof or during the term of this Agreement, the “Owned Shares”).
C. Parent and the Company, in consideration for the benefits to be delivered under the terms of the Merger Agreement, have required that Sponsor enter into this Agreement as a condition and inducement to the willingness of Parent and the Company to enter into the Merger Agreement and consummate the Transactions.
Accordingly, and in consideration of the foregoing and the mutual representations, warranties, covenants and agreements contained herein, the parties hereto, intending to be legally bound, hereby agree as follows:
AGREEMENT
ARTICLE 1
VOTING AGREEMENT
1.1 Agreement to Vote.
(a) From the date of this Agreement until the Expiration Time (as defined below), Sponsor will (and, if applicable, will cause each of its Affiliates that has the right to vote or direct the voting of any Subject Shares (as defined below) to) (i) appear at any meeting of stockholders or otherwise cause any Subject Shares to be counted as present thereat for purposes of calculating a quorum, (ii) (A) vote in favor of, or (B) in the event that the Parent seeks stockholder’s approval via written consent, as promptly as reasonably practicable (and in any event within two (2) Business Days) following the delivery by the Parent of the applicable requested written consent), duly execute and deliver to the Company and Parent the written approval solicited by the Parent pursuant to such written consent under which Sponsor shall irrevocably and unconditionally consent to, the Parent Stockholder Matters (as defined herein), and (iii) withhold its approval of or vote against any action, proposal, transaction or agreement that could reasonably be expected to (1) result in a breach of any covenant, representation or warranty or any other obligation or agreement under this Agreement or the Merger Agreement or (2) otherwise interfere with the Transactions.
| -361- |
(b) Sponsor will not enter into any agreement with any Person (other than the Parent) prior to the Expiration Time (with respect to periods prior to the Expiration Time) directly or indirectly to vote, grant any proxy or give instructions with respect to the voting of the Subject Shares, the effect of which would be inconsistent with or violate any provision contained in herein. Any vote or consent (or withholding of a vote or consent or otherwise abstaining from voting or consenting) by Sponsor that is not in accordance with this Section 1.1 will be considered null and void.
(c) Sponsor agrees that it shall not, directly or indirectly, prior to the Closing, redeem any of the Owned Shares.
(d) The Parent may, in its sole discretion, waive the provisions of this Section 1.1 as to any matter brought to the stockholders of the Parent for a vote (or consent pursuant to an action by written consent of the stockholders, if applicable).
1.2 Revocation of Prior Proxies; Grant of Irrevocable Proxy.
(a) Sponsor hereby represents and warrants that any proxies heretofore given in respect of the Owned Shares or the Subject Shares (other than the proxies granted pursuant to any voting agreement, which, for the avoidance of doubt shall not apply to the extent that Sponsor votes in favor of, or grants its written consent to, the Parent Stockholder Matters pursuant to this Agreement) are not irrevocable, and Sponsor hereby revokes any and all prior proxies with respect to the Subject Shares (other than the proxies granted pursuant to any voting agreement, which, for the avoidance of doubt shall not apply to the extent that Sponsor votes in favor of, or grants its written consent to, the Parent Stockholder Matters pursuant to this Agreement). Prior to the Expiration Time, Sponsor will not directly or indirectly grant any proxies or powers of attorney (other than to Parent), deposit any of the Owned Shares or the Subject Shares into a voting trust or enter into a voting agreement (other than this Agreement) with respect to any of the Owned Shares or the Subject Shares.
(b) Sponsor hereby appoints Parent and any designee of Parent, each of them individually, Sponsor’s proxy and attorney in fact until the Expiration Time, with full power of substitution and re-substitution, to vote, direct the vote or act by written consent with respect to the Owned Shares (i) in accordance with Section 1.1 hereof, (ii) to sign its name (as a stockholder) to any consent, certificate or other document relating to the Parent that the Law of any state or the rules of any bank, broker or depositary may permit or require in connection with any matter referred to in Section 1.1, and (iii) for purposes of voting, taking any action by written consent, executing and delivering any documents, receiving any notice and taking any actions that the Parent or an may, in its sole discretion, determine to be necessary, desirable or appropriate that are consistent with the express terms of the Merger Agreement. This proxy is given to secure the performance of the duties of Sponsor under this Agreement and its existence will not be deemed to relieve Sponsor of its obligations under Section 1.1. Sponsor affirms that this proxy is coupled with an interest and is irrevocable until the Expiration Time, where upon such proxy and power of attorney will automatically terminate and be deemed null and void. Sponsor will take such further action or execute such other instruments as may be necessary to effectuate the intent of this proxy. The proxy granted herein is intended to comply with the requirements of Laws applicable to irrevocable proxies. The proxy granted herein shall not be revoked when the interest with which it is coupled is extinguished. The power of attorney granted by Sponsor herein is a durable power of attorney and shall survive the dissolution, bankruptcy, cessation or suspension of Sponsor.
1.3 Appraisal Rights Waiver. Sponsor hereby (i) forever waives all appraisal or dissenter’s rights under applicable Law with respect to the Merger and (ii) withdraws all written objections to the Merger and/or demands for appraisal, if any, with respect to the Owned Shares or the Subject Shares by Sponsor.
| -362- |
1.4 Release. Effective upon the Closing and delivery to Company Stockholders of the right to receive the portion of the merger consideration to which stockholders are entitled at Closing pursuant to the Merger Agreement, if any, Sponsor hereby generally releases, remises and forever discharges Parent, Merger Sub, the Company, the Stockholders’ Representative, the Surviving Corporation and their respective Agents (as herein defined) from and against any and all claims, demands, liens, actions, agreements, suits, causes of action, obligations, controversies, debts, costs, attorneys’ fees, expenses, damages, judgments, orders and liabilities of whatever kind or nature in law, equity or otherwise, whether or not now known or suspected, that have existed or may have existed, or that do exist or that hereafter shall or may exist, based on any facts, events or omissions occurring from any time on or prior to the execution and delivery of this Agreement that arise out of any rights Sponsor may have in its capacity as a holder of Parent Capital Stock against the Parent or any of its Affiliates; provided, however, that nothing in this Agreement shall be construed to release, remise, discharge or acquit: (a) any claims or rights Sponsor had, has or may have under the Merger Agreement or any other agreements or instruments executed and delivered in connection with the Merger Agreement to which Sponsor is a party or beneficiary or otherwise with respect to the Merger; (b) any rights available to Sponsor for indemnification under any insurance policy for Sponsor’s benefit or as required under applicable Law; or (c) any claims arising out of actual and intentional fraud. As used herein, an “Agent” of a party is each of its predecessors, its former or present officers, employees, directors, stockholders, parents, subsidiaries, Affiliates, partners, related corporate entities, agents, attorneys, members, heirs, executors, administrators, conservators, successors and assigns.
Sponsor waives all rights under any Law, rule, provision or statute of any jurisdiction that states in full (or otherwise in substance) as follows:
“A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.”
ARTICLE 2
DEFINITIONS
Capitalized terms used but not defined in this Agreement are used in this Agreement with the meanings given to such terms in the Merger Agreement. In addition, for purposes of this Agreement:
“Affiliate” means, with respect to any specified Person, a Person who, at the time of determination, directly or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, such specified Person. For purposes of this Agreement, with respect to Sponsor, “Affiliate” does not include the Parent and the Persons that directly or indirectly through one or more intermediaries are controlled by the Parent.
“Beneficially Owned” or “Beneficial Ownership” with respect to any securities means having beneficial ownership of such securities (as determined pursuant to Rule 13d-3 under the Exchange Act, disregarding the phrase “within 60 days” in paragraph (d)(1)(i) thereof), including pursuant to any agreement, arrangement or understanding, whether or not in writing. Without duplicative counting of the same securities, securities Beneficially Owned by a Person include securities Beneficially Owned by (i) all Affiliates of such Person and (ii) all other Persons with whom such Person would constitute a “group” within the meaning of Section 13(d) of the Exchange Act and the rules promulgated thereunder. For the avoidance of doubt, “Beneficially Own” and “Beneficial Ownership” shall also include record ownership of securities.
| -363- |
“Beneficial Owner” with respect to any securities means a Person that has Beneficial Ownership of such securities.
“Parent Stockholder Matters” means approval of the execution and delivery of the Merger Agreement, the Merger, and the consummation of the Transactions on the terms set forth in the Merger Agreement along with any approval that is required under the organizational documents of the Parent or otherwise sought with respect to the Merger Agreement or the transactions contemplated thereby.
“Subject Shares” means, with respect to Sponsor, the Owned Shares set forth on Schedule A hereto (together with any other Parent Capital Stock or other equity interests (including anything convertible into such equity interests) of the Parent that Sponsor acquires record or beneficial ownership of after the date hereof).
ARTICLE 3
REPRESENTATIONS, WARRANTIES AND ADDITIONAL COVENANTS OF SPONSOR
Sponsor represents, warrants and covenants to the Parent that:
3.1 Ownership. Sponsor is the sole Beneficial Owner and the record and legal owner of the Parent Capital Stock identified or required to be identified on Schedule A, subject (with respect to shares of Parent Capital Stock underlying options and warrants) to the Parent’s due authorization and valid issuance thereof, and such shares constitute all of the capital stock of the Parent that are Beneficially Owned by Sponsor. Subject (with respect to shares of Parent Capital Stock underlying options and warrants) to the Parent’s due authorization and valid issuance thereof, Sponsor has (or, with respect to shares of Parent Capital Stock underlying options or warrants, will have, following the exercise of such options or warrants) good and valid title to all of the Owned Shares, free and clear of all liens, claims, options, proxies, voting agreements, marital, community property and other spousal interests, and security interests and has the sole right to such Parent Capital Stock and there are no restrictions on rights of disposition or other liens or encumbrances pertaining to such Parent Capital Stock (other than pursuant to this Agreement and compliance with applicable securities laws) that could adversely affect the Merger, the Merger Agreement, or the exercise or fulfillment of the rights and obligations of Sponsor under this Agreement. None of the Owned Shares are or will be subject to any voting trust or other contract with respect to the voting thereof, and no proxy, power of attorney or other authorization has been granted with respect to any of such Parent Capital Stock
3.2 Authority and Non-Contravention.
(a) Sponsor is duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is incorporated, organized or constituted. Sponsor has all necessary power and authority and legal capacity to execute and deliver this Agreement, to perform its obligations hereunder and to consummate the transactions contemplated hereby. The execution and delivery of this Agreement by Sponsor and the consummation by Sponsor of the transactions contemplated hereby have been duly and validly authorized by all necessary corporate or other action, and no other corporate or other proceedings on the part of Sponsor are necessary to authorize this Agreement or to consummate the transactions contemplated hereby.
(b) This Agreement has been duly and validly executed and delivered by Sponsor and constitutes the legal, valid and binding obligation of Sponsor, enforceable against Sponsor in accordance with its terms except (i) to the extent limited by applicable bankruptcy, insolvency or similar laws affecting creditors’ rights and (ii) the remedy of specific performance and injunctive and other forms of equitable relief may be subject to equitable defenses and to the discretion of the court before which any proceeding therefor may be brought.
| -364- |
(c) Sponsor is not nor will it be required to make any filing with or give any notice to, or to obtain any consent from, any Person in connection with the execution, delivery or performance of this Agreement or obtain any permit or approval from any government authority for any of the transactions contemplated hereby.
(d) Neither the execution and delivery of this Agreement by Sponsor nor the consummation of the transactions contemplated hereby will directly or indirectly (whether with notice or lapse of time or both) (i) conflict with, result in any violation of or constitute a default by Sponsor under any mortgage, bond, indenture, agreement, instrument or obligation to which Sponsor is a party or by which it or any of the Owned Shares are bound, or violate any permit of any government authority, or any applicable law or order to which Sponsor, or any of the Parent Capital Stock, may be subject, or (ii) result in the imposition or creation of any lien or encumbrance upon or with respect to any of the Owned Shares.
(e) Sponsor has the requisite voting power and the requisite power, authority and capacity, as applicable, to issue instructions with respect to the matters set forth in Article 1 and the requisite power, authority and capacity, as applicable, to agree to all of the matters set forth in this Agreement, in each case with respect to all of the Owned Shares, with no limitations, qualifications or restrictions on such rights.
3.3 Total Shares. Except as set forth on Schedule A, Sponsor is not the Beneficial Owner of, and does not have (whether currently, upon lapse of time, following the satisfaction of any conditions, upon the occurrence of any event or any combination of the foregoing) any right to acquire, and has no other interest in or voting rights with respect to, any Parent Capital Stock or any securities convertible into or exchangeable or exercisable for Parent Capital Stock.
3.4 Merger Agreement. Sponsor has received a copy of the Merger Agreement (including, for the avoidance of doubt, the schedules and exhibits thereto, other than the Consideration Schedule, of which Sponsor acknowledges a final copy will be provided prior to the consummation of the Transactions) and has carefully read and understands the scope and effect of the provisions thereof and of this Agreement and has discussed the foregoing with Sponsor’s professional advisors to the extent Sponsor has deemed necessary.
3.5 Reliance. Sponsor understands and acknowledges that Parent and the Company are each entering into the Merger Agreement in reliance upon Sponsor’s execution, delivery and performance of this Agreement.
3.6 No Litigation. As of the date of this Agreement, there is no Proceeding pending or, to the knowledge of Sponsor, threatened against Sponsor or the Owned Shares that could reasonably be expected to impair the ability of Sponsor to perform its obligations hereunder or consummate the transactions contemplated hereby.
3.7 Brokers and Finders. No broker, finder, financial advisor, investment banker or other person is entitled to any brokerage, finder’s, financial advisor’s or other similar fee or commission in connection with the transactions contemplated hereby based upon arrangements made by or on behalf of the Sponsor.
| -365- |
ARTICLE 4
REPRESENTATIONS AND WARRANTIES OF THE COMPANY AND PARENT
Each of the Company and Parent, severally and not jointly, represents and warrants to Sponsor that (i) this Agreement constitutes the legal, valid and binding obligation of such Person enforceable against such Person in accordance with its terms (assuming this Agreement constitutes the legal, valid and binding obligation of Sponsor), except (x) to the extent limited by applicable bankruptcy, insolvency or similar laws affecting creditors’ rights and (y) the remedy of specific performance and injunctive and other forms of equitable relief may be subject to equitable defenses and to the discretion of the court before which any proceeding therefor may be brought, (ii) each of the Company and Parent has the corporate power and authority to execute and deliver this Agreement and to perform its obligations hereunder, (iii) the execution and delivery by Company and Parent of this Agreement and the consummation by the Company and Parent of the transactions contemplated hereby have been duly and validly authorized by the Company and Parent and no other corporate proceedings on the part of the Company or Parent are necessary to authorize this Agreement or to consummate the transactions contemplated hereby, and (iv) this Agreement has been duly and validly executed and delivered by each of the Company and Parent.
ARTICLE 5
TERM AND TERMINATION
This Agreement will become effective as of the date hereof. This Agreement will terminate upon the earliest to occur of (i) the Effective Time, (ii) the date of termination of the Merger Agreement, or (iii) the mutual agreement of the Parent and Sponsor to terminate this Agreement (any such time under clauses (i) through (iii) being referred to herein as the “Expiration Time.” The parties acknowledge that, upon termination of this Agreement as permitted under and in accordance with the terms of this Article 5, no party to this Agreement shall have the right to recover any claim with respect to any losses suffered by such party in connection with such termination, except that the termination of this Agreement will not relieve Sponsor from any liability for any inaccuracy in or breach of any representation, warranty or covenant contained in this Agreement. Notwithstanding anything to the contrary herein, Article 6 will survive any termination of this Agreement.
ARTICLE 6
GENERAL PROVISIONS
6.1 Action in Stockholder Capacity Only. Sponsor is entering into this Agreement solely in Sponsor’s capacity as a Beneficial Owner, as applicable, of the Parent Capital Stock and not in any other capacity.
6.2 No Ownership Interest. Nothing contained in this Agreement will be deemed to vest in Parent, the Company or any of their respective Affiliates any direct or indirect ownership or incidents of ownership of or with respect to the Owned Shares or the Subject Shares.
6.3 Notices. All notices and other communications hereunder must be in writing and will be deemed to have been duly given (a) when delivered or sent if delivered in Person or sent by email transmission (provided confirmation of email transmission is obtained) or (ii) on the next Business Day if transmitted by national overnight courier, in each case as follows:
If to the Company, addressed to:
Aerami Therapeutics Holdings, Inc.
2520 Meridian Parkway, Suite 400
Durham, North Carolina, 27713
Attn: Steve Thornton
Email: sthornton@aerami.com
| -366- |
With a copy (which shall not constitute notice) to:
Smith,
Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P.
150 Fayetteville Street
Suite 2300
Raleigh, NC 27601
Attn:
Amy Batten and Peter Bosman
Email: abatten@smithlaw.com; pbosman@smithlaw.com
If to Parent, addressed to:
FoxWayne Enterprises Acquisition Corp.
1 Rockefeller Plaza, Suite 1039
New York, New York 10020
Attn: Robb Knie
Email: robb@foxwayne.com
With a copy (which shall not constitute notice) to:
Sheppard,
Mullin, Richter & Hampton LLP
30 Rockefeller Plaza
New
York, New York 10112
Attn: Richard Friedman
Email: rafriedman@sheppardmullin.com
If to Sponsor, to Sponsor’s address set forth on Schedule A.
6.4 Publicity. Unless required by applicable law or permitted by the Merger Agreement, Sponsor will not, and will not authorize or direct any of its Affiliates or representatives to, make any press release or public announcement with respect to this Agreement or the Merger Agreement or the transactions contemplated hereby or thereby, without the prior written consent of Parent in each instance.
6.5 Further Actions. Upon the request of any party to this Agreement, each other party will (a) furnish to the requesting party any additional information, (b) execute and deliver, at their own expense, any other documents and (c) take any other actions as the requesting party may reasonably require to more effectively carry out the intent of this Agreement. Sponsor hereby agrees that either the Company or Parent may publish and disclose in any filing made by Parent or the Company with the SEC, the Nasdaq Stock Market or other applicable regulatory authority Sponsor’s identity and ownership of any Company Capital Stock or Parent Capital Stock and the nature of Sponsor’s commitments, arrangements and understandings under this Agreement and may further file this Agreement as an exhibit to any filing made by Parent or the Company with the SEC. Sponsor agrees to (x) provide any information reasonably requested by the Company or Parent for any such regulatory application or filing and (y) notify the Company and Parent promptly of any additional shares of capital stock of the Company or Parent of which Sponsor becomes the Beneficial Owner after the date of this Agreement.
| -367- |
6.6 Transfer of Subject Shares. Except as expressly contemplated by the Merger Agreement or with the prior written consent of the Parent (such consent to be given or withheld in its sole discretion), from and after the date hereof, Sponsor agrees not to (a) Transfer any of the Subject Shares, (b) enter into (i) any option, warrant, purchase right, or other Contract that would (either alone or in connection with one or more events or developments (including the satisfaction or waiver of any conditions precedent)) require such Sponsor to Transfer the Subject Shares or (ii) any voting trust, proxy or other Contract with respect to the voting or Transfer of the Subject Shares, or (c) take any actions (i) having the effect of preventing or disabling Sponsor from performing its obligations under this Agreement or (ii) in furtherance of any of the matters described in the foregoing clauses (a) or (b). For purposes of this Agreement, “Transfer” means any, direct or indirect, sale, transfer, assignment, pledge, mortgage, exchange, hypothecation, grant of a security interest in or disposition or encumbrance of an interest (whether with or without consideration, whether voluntarily or involuntarily or by operation of law or otherwise).
6.7 Miscellaneous. Sections 10.3 (Rules of Construction), 10.4 (References), 10.5 (Entire Agreement), 10.8 (Rights of Third Parties), 10.9 (Waiver), 10.10 (Severability), 10.12 (Governing Law), 10.13 (Consent to Jurisdiction), 10.14 (Waiver of Trial by Jury), 10.16 (Specific Performance), 10.17 (Cumulative Remedies), 10.18 (Expenses), 10.20 (Execution and Counterparts) of the Merger Agreement are incorporated herein by reference and shall apply to this Agreement, mutatis mutandis.
[remainder of page intentionally blank]
| -368- |
[Signature Page to Sponsor Support Agreement]
IN WITNESS WHEREOF, the parties hereto have caused this Sponsor Support Agreement to be duly executed as of the day and year first above written.
| PARENT: | FOXWAYNE ENTERPRISES ACQUISITION CORP. | |
| By: |
| |
| Name: | ||
| Title: | ||
| COMPANY: | AERAMI THERAPEUTICS HOLDINGS, INC. | |
| By: |
| |
| Name: | ||
| Title: | ||
| -369- |
| SPONSOR: | FOXWAYNE ENTERPRISES ACQUISITION SPONSOR LLC | |
| By: |
| |
| Name: | ||
| Title: | ||
| -370- |
SCHEDULE A
| PARENT CAPITAL STOCK | ||
ADDRESS OF SPONSOR |
BENEFICIALLY OWNED | |
| [Attention to (Name & Title)] | [●] shares of Class B Common Stock of Parent | |
| [Address 1] | [●] shares of Class B Common Stock of Parent underlying outstanding warrants | |
| [Address 2] | ||
| [E-mail] |
| -371- |
Exhibit C
FORM OF PARENT SUPPORT AGREEMENT
This PARENT SUPPORT AGREEMENT (this “Agreement”), dated as of November [●], 2021, is by and among FOXWAYNE ENTERPRISES ACQUISITION CORP., a Delaware corporation (“Parent”), AERAMI THERAPEUTICS HOLDINGS, INC., a Delaware corporation (the “Company”) and the undersigned (“Stockholder”).
RECITALS
A. Parent, the Company, GOTHAM MERGER SUB, INC., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), and, STEVE THORNTON (solely in his capacity as Stockholders’ Representative) are contemporaneously entering into an Agreement and Plan of Merger (together with the Ancillary Documents, each as amended from time to time, the “Merger Agreement”), dated as of November [●], 2021, pursuant to which, among other things, the Merger Sub is to merge with and into the Company, with the Company surviving as a wholly-owned subsidiary of Parent (the “Merger”).
B. As of the date hereof, Stockholder is the Beneficial Owner (as defined below) of that number of shares of Class A Common Stock, par value $0.0001 per share, of the Parent (“Parent Common Stock”) set forth beside Stockholder’s name on Schedule A hereto and has or will have the sole power to vote (or to direct the voting of) such shares (all such shares of Parent Capital Stock Beneficially Owned by Stockholder as of the date hereof or during the term of this Agreement, the “Owned Shares”).
C. Parent and the Company, in consideration for the benefits to be delivered under the terms of the Merger Agreement, have required that Stockholder enter into this Agreement as a condition and inducement to the willingness of Parent and the Company to enter into the Merger Agreement and consummate the Transactions.
Accordingly, and in consideration of the foregoing and the mutual representations, warranties, covenants and agreements contained herein, the parties hereto, intending to be legally bound, hereby agree as follows:
AGREEMENT
ARTICLE 1
VOTING AGREEMENT
1.1 Agreement to Vote.
(a) From the date of this Agreement until the Expiration Time (as defined below), Stockholder will (and, if applicable, will cause each of its Affiliates that has the right to vote or direct the voting of any Subject Shares (as defined below) to) (i) appear at any meeting of stockholders or otherwise cause any Subject Shares to be counted as present thereat for purposes of calculating a quorum, (ii) (A) vote in favor of, or (B) in the event that the Parent seeks Stockholder’s approval via written consent, as promptly as reasonably practicable (and in any event within two (2) Business Days) following the delivery by the Parent of the applicable requested written consent), duly execute and deliver to the Company and Parent the written approval solicited by the Parent pursuant to such written consent under which Stockholder shall irrevocably and unconditionally consent to, the Parent Stockholder Matters (as defined herein), and (iii) withhold its approval of or vote against any action, proposal, transaction or agreement that could reasonably be expected to (1) result in a breach of any covenant, representation or warranty or any other obligation or agreement under this Agreement or the Merger Agreement or (2) otherwise interfere with the Transactions.
| -372- |
(b) Stockholder will not enter into any agreement with any Person (other than the Parent) prior to the Expiration Time (with respect to periods prior to the Expiration Time) directly or indirectly to vote, grant any proxy or give instructions with respect to the voting of the Subject Shares, the effect of which would be inconsistent with or violate any provision contained in herein. Any vote or consent (or withholding of a vote or consent or otherwise abstaining from voting or consenting) by Stockholder that is not in accordance with this Section 1.1 will be considered null and void.
(c) Stockholder agrees that he, she or it shall not, directly or indirectly, prior to the Closing, redeem any of the Owned Shares.
(d) The Parent may, in its sole discretion, waive the provisions of this Section 1.1 as to any matter brought to the stockholders of the Parent for a vote (or consent pursuant to an action by written consent of the stockholders, if applicable).
1.2 Revocation of Prior Proxies; Grant of Irrevocable Proxy.
(a) Stockholder hereby represents and warrants that any proxies heretofore given in respect of the Owned Shares or the Subject Shares (other than the proxies granted pursuant to any voting agreement, which, for the avoidance of doubt shall not apply to the extent that Stockholder votes in favor of, or grants its written consent to, the Parent Stockholder Matters pursuant to this Agreement) are not irrevocable, and Stockholder hereby revokes any and all prior proxies with respect to the Subject Shares (other than the proxies granted pursuant to any voting agreement, which, for the avoidance of doubt shall not apply to the extent that Stockholder votes in favor of, or grants its written consent to, the Parent Stockholder Matters pursuant to this Agreement). Prior to the Expiration Time, Stockholder will not directly or indirectly grant any proxies or powers of attorney (other than to Parent), deposit any of the Owned Shares or the Subject Shares into a voting trust or enter into a voting agreement (other than this Agreement) with respect to any of the Owned Shares or the Subject Shares.
(b) Stockholder hereby appoints Parent and any designee of Parent, each of them individually, such Stockholder’s proxy and attorney in fact until the Expiration Time, with full power of substitution and re-substitution, to vote, direct the vote or act by written consent with respect to the Owned Shares (i) in accordance with Section 1.1 hereof, (ii) to sign his, her or its name (as a stockholder) to any consent, certificate or other document relating to the Parent that the Law of any state or the rules of any bank, broker or depositary may permit or require in connection with any matter referred to in Section 1.1, and (iii) for purposes of voting, taking any action by written consent, executing and delivering any documents, receiving any notice and taking any actions that the Parent or an may, in its sole discretion, determine to be necessary, desirable or appropriate that are consistent with the express terms of the Merger Agreement. This proxy is given to secure the performance of the duties of such Stockholder under this Agreement and its existence will not be deemed to relieve such Stockholder of his, her or its obligations under Section 1.1. Stockholder affirms that this proxy is coupled with an interest and is irrevocable until the Expiration Time, where upon such proxy and power of attorney will automatically terminate and be deemed null and void. Stockholder will take such further action or execute such other instruments as may be necessary to effectuate the intent of this proxy. The proxy granted herein is intended to comply with the requirements of Laws applicable to irrevocable proxies. The proxy granted herein shall not be revoked when the interest with which it is coupled is extinguished. The power of attorney granted by Stockholder herein is a durable power of attorney and shall survive the dissolution, bankruptcy, death or incapacity of Stockholder.
1.3 Appraisal Rights Waiver. Stockholder hereby (i) forever waives all appraisal or dissenter’s rights under applicable Law with respect to the Merger and (ii) withdraws all written objections to the Merger and/or demands for appraisal, if any, with respect to the Owned Shares or the Subject Shares by Stockholder.
| -373- |
1.4 Release. Effective upon the Closing and delivery to Company Stockholders of the right to receive the portion of the merger consideration to which such stockholders are entitled at Closing pursuant to the Merger Agreement, if any, Stockholder hereby generally releases, remises and forever discharges Parent, Merger Sub, the Company, the Stockholders’ Representative, the Surviving Corporation and their respective Agents (as herein defined) from and against any and all claims, demands, liens, actions, agreements, suits, causes of action, obligations, controversies, debts, costs, attorneys’ fees, expenses, damages, judgments, orders and liabilities of whatever kind or nature in law, equity or otherwise, whether or not now known or suspected, that have existed or may have existed, or that do exist or that hereafter shall or may exist, based on any facts, events or omissions occurring from any time on or prior to the execution and delivery of this Agreement that arise out of any rights Stockholder may have in his, her or its capacity as a holder of Parent Capital Stock against the Parent or any of its Affiliates; provided, however, that nothing in this Agreement shall be construed to release, remise, discharge or acquit: (a) any claims or rights Stockholder had, has or may have under the Merger Agreement or any other agreements or instruments executed and delivered in connection with the Merger Agreement to which Stockholder is a party or beneficiary or otherwise with respect to the Merger; (b) if Stockholder is or was a director or officer of the Parent, any claim or right of Stockholder to be indemnified as a result of serving as a director or officer of the Parent, including but not limited to any rights available to Stockholder for indemnification or insurance recoveries under the Parent’s Organizational Documents, any agreement between Stockholder and the Parent or any directors’ and officers’ insurance policy for Stockholder’s benefit or under applicable Law; (c) any claims arising out of actual and intentional fraud; and (d) if Stockholder is or was an employee of the Parent, any rights with respect to earned but unpaid salary or other compensation or benefits that accrued prior to the Closing in the ordinary course of business. As used herein, an “Agent” of a party is each of its predecessors, its former or present officers, employees, directors, stockholders, parents, subsidiaries, Affiliates, partners, related corporate entities, agents, attorneys, members, heirs, executors, administrators, conservators, successors and assigns.
Stockholder waives all rights under any Law, rule, provision or statute of any jurisdiction that states in full (or otherwise in substance) as follows:
“A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.”
1.5 Directors and Officers. Notwithstanding any provision of this Agreement to the contrary, nothing in this Agreement shall (or shall require either Stockholder to attempt to) limit or restrict any Stockholder in his or her capacity as a director or officer of the Parent or any designee of the Parent who is a director or officer of the Parent from acting in such capacity or voting in such person’s sole discretion on any matter (it being understood that this Agreement shall apply to each Stockholder solely in such Stockholder’s capacity as a stockholder of the Parent).
ARTICLE 2
DEFINITIONS
Capitalized terms used but not defined in this Agreement are used in this Agreement with the meanings given to such terms in the Merger Agreement. In addition, for purposes of this Agreement:
“Affiliate” means, with respect to any specified Person, a Person who, at the time of determination, directly or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, such specified Person. For purposes of this Agreement, with respect to Stockholder, “Affiliate” does not include the Parent and the Persons that directly or indirectly through one or more intermediaries are controlled by the Parent.
| -374- |
“Beneficially Owned” or “Beneficial Ownership” with respect to any securities means having beneficial ownership of such securities (as determined pursuant to Rule 13d-3 under the Exchange Act, disregarding the phrase “within 60 days” in paragraph (d)(1)(i) thereof), including pursuant to any agreement, arrangement or understanding, whether or not in writing. Without duplicative counting of the same securities, securities Beneficially Owned by a Person include securities Beneficially Owned by (i) all Affiliates of such Person and (ii) all other Persons with whom such Person would constitute a “group” within the meaning of Section 13(d) of the Exchange Act and the rules promulgated thereunder. For the avoidance of doubt, “Beneficially Own” and “Beneficial Ownership” shall also include record ownership of securities.
“Beneficial Owner” with respect to any securities means a Person that has Beneficial Ownership of such securities.
“Parent Stockholder Matters” means approval of the execution and delivery of the Merger Agreement, the Merger, and the consummation of the Transactions on the terms set forth in the Merger Agreement along with any approval that is required under the organizational documents of the Parent or otherwise sought with respect to the Merger Agreement or the transactions contemplated thereby.
“Subject Shares” means, with respect to Stockholder, the Owned Shares set forth on Schedule A hereto (together with any other Parent Capital Stock or other equity interests (including anything convertible into such equity interests) of the Parent that Stockholder acquires record or beneficial ownership of after the date hereof).
ARTICLE 3
REPRESENTATIONS, WARRANTIES AND ADDITIONAL COVENANTS OF STOCKHOLDER
Stockholder represents, warrants and covenants to the Parent that:
3.1 Ownership. Stockholder is the sole Beneficial Owner and the record and legal owner of the Parent Capital Stock identified or required to be identified on Schedule A, subject (with respect to shares of Parent Capital Stock underlying options and warrants) to the Parent’s due authorization and valid issuance thereof, and such shares constitute all of the capital stock of the Parent that are Beneficially Owned by Stockholder. Subject (with respect to shares of Parent Capital Stock underlying options and warrants) to the Parent’s due authorization and valid issuance thereof, Stockholder has (or, with respect to shares of Parent Capital Stock underlying options or warrants, will have, following the exercise of such options or warrants) good and valid title to all of the Owned Shares, free and clear of all liens, claims, options, proxies, voting agreements, marital, community property and other spousal interests, and security interests and has the sole right to such Parent Capital Stock and there are no restrictions on rights of disposition or other liens or encumbrances pertaining to such Parent Capital Stock (other than pursuant to this Agreement and compliance with applicable securities laws) that could adversely affect the Merger, the Merger Agreement, or the exercise or fulfillment of the rights and obligations of Stockholder under this Agreement. None of the Owned Shares are or will be subject to any voting trust or other contract with respect to the voting thereof, and no proxy, power of attorney or other authorization has been granted with respect to any of such Parent Capital Stock
| -375- |
3.2 Authority and Non-Contravention.
(a) Stockholder is either a natural person or a corporation, limited partnership, limited liability company or other entity duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is incorporated, organized or constituted. Stockholder has all necessary power and authority and legal capacity to execute and deliver this Agreement, to perform its obligations hereunder and to consummate the transactions contemplated hereby. The execution and delivery of this Agreement by Stockholder and the consummation by Stockholder of the transactions contemplated hereby have been duly and validly authorized by all necessary corporate or other action, and no other corporate or other proceedings on the part of Stockholder are necessary to authorize this Agreement or to consummate the transactions contemplated hereby.
(b) This Agreement has been duly and validly executed and delivered by Stockholder and constitutes the legal, valid and binding obligation of Stockholder, enforceable against Stockholder in accordance with its terms except (i) to the extent limited by applicable bankruptcy, insolvency or similar laws affecting creditors’ rights and (ii) the remedy of specific performance and injunctive and other forms of equitable relief may be subject to equitable defenses and to the discretion of the court before which any proceeding therefor may be brought.
(c) Stockholder is not nor will it be required to make any filing with or give any notice to, or to obtain any consent from, any Person in connection with the execution, delivery or performance of this Agreement or obtain any permit or approval from any government authority for any of the transactions contemplated hereby.
(d) Neither the execution and delivery of this Agreement by Stockholder nor the consummation of the transactions contemplated hereby will directly or indirectly (whether with notice or lapse of time or both) (i) conflict with, result in any violation of or constitute a default by Stockholder under any mortgage, bond, indenture, agreement, instrument or obligation to which Stockholder is a party or by which it or any of the Owned Shares are bound, or violate any permit of any government authority, or any applicable law or order to which Stockholder, or any of the Parent Capital Stock, may be subject, or (ii) result in the imposition or creation of any lien or encumbrance upon or with respect to any of the Owned Shares.
(e) Stockholder has the requisite voting power and the requisite power, authority and capacity, as applicable, to issue instructions with respect to the matters set forth in Article 1 and the requisite power, authority and capacity, as applicable, to agree to all of the matters set forth in this Agreement, in each case with respect to all of the Owned Shares, with no limitations, qualifications or restrictions on such rights.
3.3 Total Shares. Except as set forth on Schedule A, Stockholder is not the Beneficial Owner of, and does not have (whether currently, upon lapse of time, following the satisfaction of any conditions, upon the occurrence of any event or any combination of the foregoing) any right to acquire, and has no other interest in or voting rights with respect to, any Parent Capital Stock or any securities convertible into or exchangeable or exercisable for Parent Capital Stock.
3.4 Merger Agreement. Stockholder has received a copy of the Merger Agreement (including, for the avoidance of doubt, the schedules and exhibits thereto, other than the Consideration Schedule, of which Stockholder acknowledges a final copy will be provided prior to the consummation of the Transactions) and has carefully read and understands the scope and effect of the provisions thereof and of this Agreement and has discussed the foregoing with Stockholder’s professional advisors to the extent Stockholder has deemed necessary.
3.5 Reliance. Stockholder understands and acknowledges that Parent and the Company are each entering into the Merger Agreement in reliance upon Stockholder’s execution, delivery and performance of this Agreement.
3.6 No Litigation. As of the date of this Agreement, there is no Proceeding pending or, to the knowledge of Stockholder, threatened against Stockholder or the Owned Shares that could reasonably be expected to impair the ability of Stockholder to perform its obligations hereunder or consummate the transactions contemplated hereby.
| -376- |
3.7 Brokers and Finders. No broker, finder, financial advisor, investment banker or other person is entitled to any brokerage, finder’s, financial advisor’s or other similar fee or commission in connection with the transactions contemplated hereby based upon arrangements made by or on behalf of Stockholder.
ARTICLE 4
REPRESENTATIONS AND WARRANTIES OF THE COMPANY AND PARENT
Each of the Company and Parent, severally and not jointly, represents and warrants to Stockholder that (i) this Agreement constitutes the legal, valid and binding obligation of such Person enforceable against such Person in accordance with its terms (assuming this Agreement constitutes the legal, valid and binding obligation of Stockholder), except (x) to the extent limited by applicable bankruptcy, insolvency or similar laws affecting creditors’ rights and (y) the remedy of specific performance and injunctive and other forms of equitable relief may be subject to equitable defenses and to the discretion of the court before which any proceeding therefor may be brought, (ii) each of the Company and Parent has the corporate power and authority to execute and deliver this Agreement and to perform its obligations hereunder, (iii) the execution and delivery by Company and Parent of this Agreement and the consummation by the Company and Parent of the transactions contemplated hereby have been duly and validly authorized by the Company and Parent and no other corporate proceedings on the part of the Company or Parent are necessary to authorize this Agreement or to consummate the transactions contemplated hereby, and (iv) this Agreement has been duly and validly executed and delivered by each of the Company and Parent.
ARTICLE 5
TERM AND TERMINATION
This Agreement will become effective as of the date hereof. This Agreement will terminate upon the earliest to occur of (i) the Effective Time, (ii) the date of termination of the Merger Agreement, or (iii) the mutual agreement of the Parent and Stockholder to terminate this Agreement (any such time under clauses (i) through (iii) being referred to herein as the “Expiration Time.” The parties acknowledge that, upon termination of this Agreement as permitted under and in accordance with the terms of this Article 5, no party to this Agreement shall have the right to recover any claim with respect to any losses suffered by such party in connection with such termination, except that the termination of this Agreement will not relieve Stockholder from any liability for any inaccuracy in or breach of any representation, warranty or covenant contained in this Agreement. Notwithstanding anything to the contrary herein, Article 6 will survive any termination of this Agreement.
| -377- |
ARTICLE 6
GENERAL PROVISIONS
6.1 Action in Stockholder Capacity Only. Stockholder is entering into this Agreement solely in Stockholder’s capacity as a Beneficial Owner, as applicable, of the Parent Capital Stock and not in Stockholder’s capacity as a director or officer of the Parent. Nothing herein will limit or affect Stockholder’s ability to act as an officer or director of the Parent.
6.2 No Ownership Interest. Nothing contained in this Agreement will be deemed to vest in Parent, the Company or any of their respective Affiliates any direct or indirect ownership or incidents of ownership of or with respect to the Owned Shares or the Subject Shares.
6.3 Notices. All notices and other communications hereunder must be in writing and will be deemed to have been duly given (a) when delivered or sent if delivered in Person or sent by email transmission (provided confirmation of email transmission is obtained) or (ii) on the next Business Day if transmitted by national overnight courier, in each case as follows:
If to the Company, addressed to:
Aerami Therapeutics Holdings, Inc.
2520 Meridian Parkway, Suite 400
Durham, North Carolina, 27713
Attn: Steve Thornton
Email: sthornton@aerami.com
With a copy (which shall not constitute notice) to:
Smith,
Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P.
150 Fayetteville Street
Suite 2300
Raleigh, NC 27601
Attn:
Amy Batten and Peter Bosman
Email: abatten@smithlaw.com; pbosman@smithlaw.com
If to Parent, addressed to:
FoxWayne Enterprises Acquisition Corp.
1 Rockefeller Plaza, Suite 1039
New York, New York 10020
Attn: Robb Knie
Email: robb@foxwayne.com
With a copy (which shall not constitute notice) to:
Sheppard,
Mullin, Richter & Hampton LLP
30 Rockefeller Plaza
New
York, New York 10112
Attn: Richard Friedman
Email: rafriedman@sheppardmullin.com
If to Stockholder, to Stockholder’s address set forth on Schedule A.
6.4 Publicity. Unless required by applicable law or permitted by the Merger Agreement, Stockholder will not, and will not authorize or direct any of its Affiliates or representatives to, make any press release or public announcement with respect to this Agreement or the Merger Agreement or the transactions contemplated hereby or thereby, without the prior written consent of Parent in each instance.
| -378- |
6.5 Further Actions. Upon the request of any party to this Agreement, each other party will (a) furnish to the requesting party any additional information, (b) execute and deliver, at their own expense, any other documents and (c) take any other actions as the requesting party may reasonably require to more effectively carry out the intent of this Agreement. Stockholder hereby agrees that either the Company or Parent may publish and disclose in any filing made by Parent or the Company with the SEC, the Nasdaq Stock Market or other applicable regulatory authority Stockholder’s identity and ownership of any Company Capital Stock or Parent Capital Stock and the nature of such Stockholder’s commitments, arrangements and understandings under this Agreement and may further file this Agreement as an exhibit to any filing made by Parent or the Company with the SEC. Stockholder agrees to (x) provide any information reasonably requested by the Company or Parent for any such regulatory application or filing and (y) notify the Company and Parent promptly of any additional shares of capital stock of the Company or Parent of which Stockholder becomes the Beneficial Owner after the date of this Agreement.
6.6 Transfer of Subject Shares. Except as expressly contemplated by the Merger Agreement or with the prior written consent of the Parent (such consent to be given or withheld in its sole discretion), from and after the date hereof, Stockholder agrees not to (a) Transfer any of the Subject Shares, (b) enter into (i) any option, warrant, purchase right, or other Contract that would (either alone or in connection with one or more events or developments (including the satisfaction or waiver of any conditions precedent)) require such Stockholder to Transfer the Subject Shares or (ii) any voting trust, proxy or other Contract with respect to the voting or Transfer of the Subject Shares, or (c) take any actions (i) having the effect of preventing or disabling such Stockholder from performing its obligations under this Agreement or (ii) in furtherance of any of the matters described in the foregoing clauses (a) or (b). For purposes of this Agreement, “Transfer” means any, direct or indirect, sale, transfer, assignment, pledge, mortgage, exchange, hypothecation, grant of a security interest in or disposition or encumbrance of an interest (whether with or without consideration, whether voluntarily or involuntarily or by operation of law or otherwise).
6.7 Miscellaneous. Sections 10.3 (Rules of Construction), 10.4 (References), 10.5 (Entire Agreement), 10.8 (Rights of Third Parties), 10.9 (Waiver), 10.10 (Severability), 10.12 (Governing Law), 10.13 (Consent to Jurisdiction), 10.14 (Waiver of Trial by Jury), 10.16 (Specific Performance), 10.17 (Cumulative Remedies), 10.18 (Expenses), 10.20 (Execution and Counterparts) of the Merger Agreement are incorporated herein by reference and shall apply to this Agreement, mutatis mutandis.
[remainder of page intentionally blank]
| -379- |
[Signature Page to Parent Support Agreement]
IN WITNESS WHEREOF, the parties hereto have caused this Parent Support Agreement to be duly executed as of the day and year first above written.
| PARENT: | FOXWAYNE ENTERPRISES ACQUISITION CORP. | |
| By: | ||
| Name: | ||
| Title: | ||
| COMPANY: | AERAMI THERAPEUTICS HOLDINGS, INC.
| |
| By: | ||
| Name: | ||
| Title: | ||
| -380- |
| STOCKHOLDER: | [ENTITY NAME] | |
| By: | ||
| Name: | ||
| Title: | ||
| [NATURAL PERSON NAME] | ||
| Additional Signature (if held jointly): | ||
| (If held jointly) | ||
| (Printed Full Name) | ||
| -381- |
SCHEDULE A
| NAME AND | PARENT CAPITAL STOCK | |
| ADDRESS OF STOCKHOLDER | BENEFICIALLY OWNED | |
| [Name] | [●] shares of Class A Common Stock of Parent | |
| [Address 1] | ||
| [Address 2] | [●] shares of Class A Common Stock of Parent underlying outstanding warrants | |
| [E-mail] |
| -382- |
EXHIBIT E
AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
FOXWAYNE ENTERPRISES ACQUISITION CORP.
[Attached as Annex B to this proxy statement/prospectus]
| -383- |
EXHIBIT F
Amended and Restated Bylaws of
Aerami Therapeutics Holdings, Inc.
[Attached as Annex C to this proxy statement/prospectus]
| -384- |
EXHIBIT G
AERAMI THERAPEUTICS HOLDINGS, INC.
2022 INCENTIVE AWARD PLAN
[Attached as Annex D to this proxy statement/prospectus]
| -385- |
EXHIBIT H
CERTIFICATE OF MERGER OF
GOTHAM MERGER SUB, INC.,
WITH AND INTO
AERAMI, INC.
Pursuant to the provisions of Title 8, Section 251(c) of the General Corporation Law of the State of Delaware (the “DGCL”), Aerami, Inc. (f/k/a Aerami Therapeutics Holdings, Inc.), a corporation organized and existing under the laws of Delaware (the “Company”), hereby certifies the following information relating to the merger (the “Merger”) of Gotham Merger Sub, Inc., a corporation organized and existing under the laws of Delaware (“Merger Sub”), with and into the Company, with the Company as the surviving corporation of the Merger:
FIRST: The names and states of incorporation of the constituent corporations to the Merger (the “Constituent Corporations”) are as follows:
| Name | State of Incorporation | |
| Aerami, Inc. | Delaware | |
| Gotham Merger Sub, Inc. | Delaware |
SECOND: The Agreement and Plan of Merger (the “Agreement”), dated December 7, 2021 by and among the Company, FoxWayne Enterprises Acquisition Corp. (n/k/a Aerami Therapeutics Holdings, Inc.), a Delaware corporation, Merger Sub, and Steve Thornton (solely in his capacity as the stockholder representative as set forth therein), has been approved, adopted, certified, executed and acknowledged by each of the Constituent Corporations pursuant to, and in accordance with, Section 251 of the DGCL (with the stockholders of each Constituent Corporation adopting the Agreement by written consent in lieu of a meeting of stockholders pursuant to Section 228 of the DGCL).
THIRD: The Company shall be the surviving corporation of the Merger and the name of the surviving corporation shall be Aerami, Inc.
FOURTH: The certificate of incorporation of the Company, as in effect prior to the filing of this Certificate of Merger, shall be amended and restated in its entirety in the Merger to read as set forth on Annex A attached hereto, and, as so amended and restated, shall be the certificate of incorporation of the surviving corporation.
FIFTH: This Certificate of Merger and the Merger shall become effective immediately upon the filing of this Certificate of Merger with the Secretary of State of the State of Delaware.
SIXTH: The executed Agreement is on file at the principal place of business of the surviving corporation. The address of the principal place of business of the surviving corporation at which the executed Agreement is on file is 2520 Meridian Parkway, Suite 400, Durham, North Carolina 27713.
SEVENTH: A copy of the Agreement will be furnished by the surviving corporation on request, without cost, to any stockholder of either of the Constituent Corporations.
| -386- |
IN WITNESS WHEREOF, said surviving corporation has caused this certificate to be signed by an authorized officer on the _____ day of _____, 2022.
| AERAMI, INC. | ||
| By: | ||
| Name: | ||
| Title: | ||
| -387- |
Exhibit A
AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
AERAMI INC.
1. The name of the corporation is Aerami, Inc. (hereinafter the “Corporation”).
2. The address of the Corporation’s registered office in the State of Delaware is [●]. The name of its registered agent at such address is [●].
3. The purpose of the Corporation is to engage in any lawful act or activity for which corporations may be organized under the General Corporation Law of the State of Delaware (the “DGCL”).
4. The total number of shares of capital stock which the Corporation shall have authority to issue is one (1) share of Common Stock, par value $0.0001 per share.
5. The number of directors of the Corporation may be fixed by the Bylaws.
6. In furtherance and not in limitation of the powers conferred by statute, the Board of Directors is expressly authorized to adopt, alter, amend and repeal the Bylaws of the Corporation.
7. No director of the Corporation shall have personal liability arising out of an action whether by or in the right of the Corporation or otherwise for monetary damages for breach of fiduciary duty as a director; provided, however, that the foregoing shall not limit or eliminate the liability of a director (i) for any breach of the director’s duty of loyalty to the Corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the DGCL or any successor provision, (iv) for any transaction from which such director derived an improper personal benefit, or (v) for acts or omissions occurring prior to the date of the effectiveness of this provision.
Furthermore, notwithstanding the foregoing provision, if the DGCL is amended or enacted to permit further limitation or elimination of the personal liability of the director, the personal liability of the Corporation’s directors shall be limited or eliminated to the fullest extent permitted by the applicable law.
This provision shall not affect any provision permitted under the DGCL in the Certificate of Incorporation, Bylaws or contract or resolution of the Corporation indemnifying or agreeing to indemnify a director against personal liability. Any repeal or modification of this provision shall not adversely affect any limitation hereunder on the personal liability of the director with respect to acts or omissions occurring prior to such repeal or modification.
8. Except as set forth herein, the Corporation reserves the right to amend, alter, change or repeal any provision contained in this Certificate of Incorporation, in the manner now or hereafter prescribed by statute, and all rights conferred on stockholders herein are granted subject to this reservation.
| -388- |
EXHIBIT I
AMENDED AND RESTATED BYLAWS
AERAMI, INC.
Aerami, Inc. (the “Corporation”), pursuant to the provisions of Section 109 of the Delaware General Corporation Law, hereby adopts these Amended and Restated Bylaws, which restate, amend and supersede the bylaws of the Corporation, as previously amended and restated, in their entirety as described below:
ARTICLE I
Offices
Section 1.1. Registered Office. The registered office shall be established and maintained at [Corporation Trust Center, 1209 Orange Street, City of Wilmington, County of New Castle, Delaware 19801], and [Corporation Trust Company] shall be the registered agent of the Corporation in charge thereof.
Section 1.2. Other Offices. The Corporation may have other offices, either within or without the State of Delaware, at such place or places as the Board of Directors may from time to time appoint or the business of the Corporation may require.
ARTICLE II
Stockholders
Section 2.1. Annual Meetings. An annual meeting of stockholders shall be held for the election of directors, and for the transaction of such other business as may properly come before the meeting, at such date, time and place, which date shall be within thirteen (13) months subsequent to the later of the date of incorporation or the last annual meeting, either within or without the State of Delaware, as may be designated by resolution of the Board of Directors from time to time.
Section 2.2. Special Meetings. Special meetings of stockholders for any purpose or purposes may be called at any time by the Board of Directors, the Chairman of the Board, if any, or by the President and shall be called by the President or the Secretary at the request in writing of stockholders owning at least a majority of the issued and outstanding shares of common stock of the Corporation entitled to vote at the meeting. Calls for such meeting shall specify the purposes thereof and no business other than that specified in the call shall be considered at any special meeting.
Section 2.3. Notice of Meetings. Whenever stockholders are required or permitted to take any action at a meeting, a notice of the meeting stating the place, date and hour of the meeting, the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting, and, in the case of a special meeting, the purpose or purposes for which the meeting is called shall be given in writing or by electronic transmission in a form consented to by the stockholders. Unless otherwise provided by law, the written notice of any meeting shall be given not less than ten (10) nor more than sixty (60) days before the date of the meeting to each stockholder entitled to vote at such meeting. If mailed, such notice shall be deemed to be given when deposited in the mail, postage prepaid, directed to the stockholder at his or her address as it appears on the records of the Corporation. If transmitted electronically, notice shall be deemed given: (1) by facsimile, when directed to a number at which the stockholder has consented to receive notice; (2) by electronic mail, when directed to an electronic mail address at which the stockholder has consented to receive notice; (3) by a posting on an electronic network together with separate notice to the stockholder of such specific posting, upon the later of (A) such posting or (B) the giving of such separate notice; and (4) by any other form of electronic transmission, when directed to the stockholder.
| -389- |
Section 2.4. Adjournments. Any meeting of stockholders, annual or special, may adjourn from time to time to reconvene at the same or some other place, and notice need not be given of any such adjourned meeting if the time and place, if any, thereof and the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such adjourned meeting are announced at the meeting at which the adjournment is taken. At the adjourned meeting the Corporation may transact any business which might have been transacted at the original meeting. If the adjournment is for more than thirty (30) days, or if after the adjournment a new record date is fixed for the adjourned meeting, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting.
Section 2.5. Quorum. At each meeting of stockholders, except where otherwise provided by law or the Certificate of Incorporation or these Bylaws, the holders of a majority of the outstanding shares of stock entitled to vote at the meeting, present in person or by proxy, shall constitute a quorum. With respect to any matter on which stockholders vote separately as a class, the holders of a majority of the outstanding shares of such class shall constitute a quorum for a meeting with respect to such matter. Two or more classes or series of stock shall be considered a single class for purposes of determining existence of a quorum entitled or required to vote together as a single class at the meeting on such matter. In the absence of a quorum, the stockholders so present may, by majority vote, adjourn the meeting from time to time in the manner provided in Section 2.4 of these Bylaws until a quorum shall attend. Shares of its own stock belonging to the Corporation or to another corporation, if a majority of the shares entitled to vote in the election of directors of such other corporation is held, directly or indirectly, by the Corporation, shall neither be entitled to vote nor be counted for quorum purposes; provided, however, that the foregoing shall not limit the right of any corporation to vote stock, including but not limited to its own stock, held by it in a fiduciary capacity.
Section 2.6. Organization. Meetings of stockholders shall be presided over by the Chairman of the Board, if any, or in their absence by the President, or in the absence of the foregoing persons, by a chairman designated by the Board of Directors, or in the absence of such designation, by a chairman chosen at the meeting. The Secretary shall act as secretary of the meeting, but in the Secretary’s absence the chairman of the meeting may appoint any person to act as secretary of the meeting.
Section 2.7. Voting; Proxies.
(A) Except as otherwise provided by law or the Certificate of Incorporation, at every meeting of stockholders, each stockholder of the Corporation entitled to vote shall have one (1) vote, in person or by proxy, for each share of stock held by such stockholder which has voting power upon the matter in question.
(B) Each stockholder entitled to vote at a meeting of stockholders or to express consent or dissent to corporate action in writing without a meeting may authorize another person or persons to act for such stockholder by proxy, but no such proxy shall be voted or acted upon after three (3) years from its date, unless the proxy provides for a longer period.
| -390- |
(C) Without limiting the manner in which a stockholder may authorize another person or persons to act for such stockholder as proxy pursuant to subsection (B) of this section, the following shall constitute a valid means by which a stockholder may grant such authority: (1) A stockholder may execute a writing authorizing another person or persons to act for such stockholder as proxy. Execution may be accomplished by the stockholder or such stockholder’s authorized officer, director, employee or agent signing such writing or causing such person’s signature to be affixed to such writing by any reasonable means including, but not limited to, by facsimile signature. (2) A stockholder may authorize another person or persons to act for such stockholder as proxy by transmitting or authorizing the transmission of a telegram, cablegram or other means of electronic transmission to the person who will be the holder of the proxy or to a proxy solicitation firm, proxy support service organization or like agent duly authorized by the person who will be the holder of the proxy to receive such transmission; provided, however, that any such telegram, cablegram or other means of electronic transmission must either set forth or be submitted with information from which it can be determined that the telegram, cablegram or other electronic transmission was authorized by the stockholder. If it is determined that such telegrams, cablegrams or other electronic transmissions are valid, the inspectors or, if there are no inspectors, such other persons making that determination shall specify the information upon which they relied.
(D) Any copy, facsimile telecommunication or other reliable reproduction of the writing or transmission created pursuant to subsection (C) of this section may be substituted or used in lieu of the original writing or transmission for any and all purposes for which the original writing or transmission could be used, provided that such copy, facsimile telecommunication or other reproduction shall be a complete reproduction of the entire original writing or transmission.
(E) A duly executed proxy shall be irrevocable if it states that it is irrevocable and if, and only as long as, it is coupled with an interest sufficient in law to support an irrevocable power. A stockholder may revoke any proxy which is not irrevocable by attending the meeting and voting in person or by filing an instrument in writing revoking the proxy or another duly executed proxy bearing a later date with the Secretary of the Corporation. Voting at meetings of stockholders need not be by written ballot unless the Board of Directors, or holders of a majority of the outstanding shares of all classes of stock entitled to vote thereon present in person or by proxy at such meeting shall so determine. At all meetings of stockholders for the election of directors, a plurality of the votes cast shall be sufficient to elect. All other elections and questions shall, unless otherwise provided by law or by the Certificate of Incorporation or these Bylaws, be decided by the vote of the holders of a majority of the shares of stock entitled to vote thereon present in person or by proxy at the meeting. Unless otherwise provided in the Certificate of Incorporation, voting at all elections for directors may not be cumulative.
Section 2.8. Fixing Date for Determination of Stockholders of Record.
(A) In order that the Corporation may determine the stockholders entitled to notice of or to vote at any meeting of stockholders or any adjournment thereof, or entitled to receive payment of any dividend or other distribution or allotment of any rights, or entitled to exercise any rights in respect of any change, conversion or exchange of stock or for the purpose of any other lawful action, the Board of Directors may fix, in advance, a record date, which shall not precede the date such record date is fixed and shall not be more than sixty (60) nor less than ten (10) days before the date of such meeting, nor more than sixty (60) days prior to any other action. If no record date is fixed, the record date for determining stockholders entitled to notice of or to vote at a meeting of stockholders shall be at the close of business on the day next preceding the day on which notice is given. The record date for any other purpose other than stockholder action by written consent shall be at the close of business on the day on which the Board of Directors adopts the resolution relating thereto. A determination of stockholders of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Board of Directors may fix a new record date for the adjourned meeting.
| -391- |
(B) In order that the Corporation may determine the stockholders entitled to consent to corporate action in writing without a meeting, the Board of Directors may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board of Directors, and which date shall not be more than ten (10) days after the date upon which the resolution fixing the record date is adopted by the Board of Directors. Any stockholder of record seeking to have the stockholders authorize or take corporate action by written consent shall, by written notice to the Secretary, request the Board of Directors to fix a record date. The Board of Directors shall promptly, but in all events within ten (10) days after the date on which such a request is received, adopt a resolution fixing the record date. If no record date has been fixed by the Board of Directors within ten (10) days of the date on which such a request is received, the record date for determining stockholders entitled to consent to corporate action in writing without a meeting, when no prior action by the Board of Directors is required by applicable law, shall be the first date on which a signed written consent setting forth the action taken or proposed to be taken is delivered to the Corporation by delivery to its registered office in the State of Delaware, its principal place of business or any officer or agent of the Corporation having custody of the book in which proceedings of meetings of stockholders are recorded. Delivery made to the Corporation’s registered office shall be by hand or by certified or registered mail, return receipt requested. If no record date has been fixed by the Board of Directors and prior action by the Board of Directors is required by applicable law, the record date for determining stockholders entitled to consent to corporate action in writing without a meeting shall be at the close of business on the date on which the Board of Directors adopts the resolution taking such prior action.
Section 2.9. List of Stockholders Entitled to Vote. The Secretary shall prepare and make, at least ten (10) days before every meeting of stockholders, a complete list of the stockholders entitled to vote at the meeting, arranged in alphabetical order, and showing the address of each stockholder and the number of shares registered in the name of each stockholder. Such list shall be open to the examination of any stockholder for any purpose germane to the meeting for a period of at least ten (10) days prior to the meeting, during ordinary business hours, at the principal place of business of the Corporation. The list shall also be produced and kept at the time and place of the meeting during the whole time thereof and may be inspected by any stockholder who is present. Upon the willful neglect or refusal of the directors to produce such a list at any meeting for the election of directors, they shall be ineligible for election to any office at such meeting. The stock ledger shall be the only evidence as to who are the stockholders entitled to examine the list of stockholders or to vote in person or by proxy at any meeting of stockholders.
Section 2.10. Inspectors of Elections; Opening and Closing the Polls.
(A) The Board of Directors by resolution shall appoint one or more inspectors, which inspector or inspectors may include individuals who serve the Corporation in other capacities, including, without limitation, as officers, employees, agents or representatives of the Corporation, to act at the meeting and make a written report thereof. One or more persons may be designated as alternate inspectors to replace any inspector who fails to act. If no inspector or alternate has been appointed to act, or if all inspectors or alternates who have been appointed are unable to act, at a meeting of stockholders, the chairman of the meeting shall appoint one or more inspectors to act at the meeting. The inspectors shall: (1) ascertain the number of shares outstanding and the voting power of each; (2) determine the shares represented at a meeting and the validity of proxies and ballots; (3) count all votes and ballots; (4) determine and retain for a reasonable period a record of the disposition of any challenges made to any determination by the inspectors; and (5) certify their determination of the number of shares represented at the meeting, and their count of all votes and ballots. Each inspector, before discharging his or her duties, shall take and sign an oath faithfully to execute the duties of inspector with strict impartiality and according to the best of his or her ability.
(B) The chairman of the meeting shall fix and announce at the meeting the date and time of the opening and the closing of the polls for each matter upon which the stockholders will vote at the meeting.
| -392- |
Section 2.11. Action by Consent of Stockholders. Unless otherwise restricted by the Certificate of Incorporation, any action required or permitted to be taken at any annual or special meeting of the stockholders may be taken without a meeting, without prior notice and without a vote, if a consent in writing, setting forth the action so taken, shall be signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted and shall be delivered to the Corporation by delivery to its registered office in the State of Delaware, its principal place of business or an officer or agent of the Corporation having custody of the book in which proceedings of meetings of stockholders are recorded. Delivery made to the Corporation’s registered office shall be by hand or by certified or registered mail, return receipt requested. A stockholder’s consent may be in an electronic form and delivered by electronic means. If transmitted electronically, such consent shall be deemed to have been signed on the date on which such consent was transmitted. No consent transmitted electronically shall be deemed to have been delivered until such consent is reproduced in paper form and until such paper form shall be delivered to the Corporation at its registered office in the State of Delaware, its principal place of business or an officer or agent of the Corporation having custody of the book in which proceedings of meetings of stockholders are recorded. Prompt notice of the taking of the corporate action without a meeting by less than unanimous written consent shall be given to those stockholders who have not consented in writing.
Section 2.12. Participating by Electronic Means. Any stockholder may participate in any meeting of the stockholders by means of telephone conference or similar communications equipment by which all persons participating in the meeting can hear each other at the same time. Such participation shall constitute presence in person at the meeting.
ARTICLE III
Board of Directors
Section 3.1. Power; Number; Qualifications. The business and affairs of the Corporation shall be managed by or under the direction of the Board of Directors, except as may be otherwise provided by law or the Certificate of Incorporation. The number of Directors shall not be less than one (1) nor more than three (3). The exact number of Directors shall be set within such limits from time to time by the affirmative vote of a majority of the stockholders or by resolution of the Board of Directors. Directors need not be stockholders.
| -393- |
Section 3.2. Election; Resignation; Removal; Vacancies. Except as otherwise provided in this Section 3.2, the directors shall be elected annually at the annual meeting of the stockholders. Each director (whenever elected) shall hold office until the annual meeting of stockholders or any special meeting called to elect directors next succeeding his or her election and until his or her successor is elected and qualified or until his or her earlier resignation or removal, except as provided in the Certificate of Incorporation. Directors may not be elected by written consent except by unanimous written consent, unless all of the directorships to which directors could be elected at an annual meeting are vacant and are filled by such action. Any Director may resign at any time upon notice in writing or by electronic transmission to the Board of Directors, to the Chairman of the Board, if any, or to the President of the Corporation. Such resignation shall take effect at the time specified therein, and unless otherwise specified therein, no acceptance of such resignation shall be necessary to make it effective. Unless otherwise provided in the Certificate of Incorporation or Bylaws, when one or more directors shall resign from the Board of Directors, effective at a future date, a majority of the directors then in office, including those who have so resigned, shall have power to fill such vacancy or vacancies, the vote thereon to take effect when such resignation or resignations shall become effective, and each director so chosen shall hold office as provided in this section in the filling of other vacancies. Any director may be removed with or without cause at any time upon the affirmative vote of the holders of a majority of the outstanding shares of stock of the Corporation entitled to vote for the election of such director given at a special meeting of such stockholders called for the purpose. If any vacancies shall occur in the Board of Directors by reason of death, resignation, removal or otherwise, or if the authorized number of directors shall be increased, the directors then in office shall continue to act, and such vacancies shall be filled by a majority of the directors then in office, though less than a quorum; provided, however, that whenever the holders of any class or classes of stock or series thereof are entitled to elect one or more directors by the provisions of the Certificate of Incorporation, vacancies and newly created directorships of such class or classes or series shall be filled by a majority of the directors elected by such class or classes or series thereof then in office though less than a quorum, by a sole remaining director so elected, or if there is no such director remaining, by the affirmative vote of the holders of a majority of the shares of that class or series. Any such vacancies or newly created directorships may also be filled upon the affirmative vote of the holders of a plurality of the outstanding shares of stock of the Corporation entitled to vote for the election of directors given at a special meeting of the stockholders called for the purpose.
Section 3.3. Regular Meetings. Regular meetings of the Board of Directors may be held at such places within or without the State of Delaware and at such times as the Board of Directors may from time to time determine, and, if so determined, notices thereof need not be given.
Section 3.4. Special Meetings. Special meetings of the Board of Directors may be held at any time or place within or without the State of Delaware whenever called by the Chairman of the Board, if any, the President, any Vice President, the Secretary, or by any two (2) members of the Board of Directors. Notice thereof shall be given by the person or persons calling the meeting, not later than the second day before the date of the special meeting.
Section 3.5. Telephonic Meetings Permitted. Members of the Board of Directors, or any committee designated by the Board of Directors, may participate in a meeting of such Board of Directors or committee by means of conference telephone or other communications equipment by which all persons participating in the meeting can hear each other, and participation in a meeting pursuant to this Bylaw shall constitute presence in person at such meeting.
Section 3.6. Quorum; Vote Required for Action. At all meetings of the Board of Directors a majority of the whole Board of Directors shall constitute a quorum for the transaction of business. Unless the Certificate of Incorporation or these Bylaws otherwise provide, the vote of a majority of the directors present shall be the act of the Board of Directors. In the absence of a quorum at any meeting of the Board of Directors, a majority of the directors present may adjourn the meeting from time to time until a quorum shall attend.
Section 3.7. Organization. Meetings of the Board of Directors shall be presided over by the Chairman of the Board, if any, or in their absence by the President, or in the President’s absence by a chairman chosen at the meeting. The Secretary shall act as secretary of the meeting, but in the Secretary’s absence the chairman of the meeting may appoint any person to act as secretary of the meeting.
| -394- |
Section 3.8. Informal Action by Directors. Unless otherwise restricted by the Certificate of Incorporation or these Bylaws, any action required or permitted to be taken at any meeting of the Board of Directors, or of any committee thereof, may be taken without a meeting if all members of the Board of Directors or such committee, as the case may be, consent thereto in writing or by electronic transmission, and the writing or writings or electronic transmission or transmissions are filed with the minutes of proceedings of the Board of Directors or committee.
Section 3.9. Compensation. The directors may be paid their expenses, if any, of attendance at each meeting of the Board of Directors and may be paid a fixed sum for attendance at each such meeting of the Board of Directors or a stated salary as director, as the Board of Directors may determine. No such payment shall preclude any director from serving the Corporation in any other capacity and receiving compensation therefor. Members of special or standing committees may be allowed like compensation for attending committee meetings.
ARTICLE IV
Committees
Section 4.1. Committees. The Board of Directors may designate one or more committees, each committee to consist of one or more of the directors of the Corporation. The Board of Directors may designate one or more directors as alternate members of any committee who may replace any absent or disqualified member at any meeting of the committee. In the absence or disqualification of a member of the committee, the member or members thereof present at any meeting and not disqualified from voting, whether or not such member or members constitute a quorum, may unanimously appoint another member of the Board of Directors to act at the meeting in place of any such absent or disqualified member. Any such committee, to the extent provided in the resolution of the Board of Directors, shall have and may exercise all the powers and authority of the Board of Directors in the management of the business and affairs of the Corporation and may authorize the seal of the Corporation to be affixed to all papers which may require it; but no such committee shall have power or authority in reference to amending the Certificate of Incorporation (except that a committee may, to the extent authorized in the resolution or resolutions providing for the issuance of shares of stock adopted by the Board of Directors as provided in Section 151(a) of the General Corporation Law of the State of Delaware, fix any of the preferences or rights of such shares, except voting rights of the shares), adopting an agreement of merger or consolidation, recommending to the stockholders the sale, lease or exchange of all or substantially all of the Corporation’s property and assets, recommending to the stockholders a dissolution of the Corporation or a revocation of dissolution, or amending these Bylaws; and, unless the resolution expressly so provides, no such committee shall have the power or authority to declare a dividend or to authorize the issuance of stock.
Section 4.2. Committee Rules. Unless the Board of Directors otherwise provides, each committee designated by the Board of Directors may make, alter and repeal rules for conduct of its business. In the absence of such rules each committee shall conduct its business in the same manner as the Board of Directors conducts its business pursuant to Article III of these Bylaws.
| -395- |
ARTICLE V
Officers
Section 5.1. Officers; Election. As soon as practicable after the annual meeting of stockholders in each year, the Board of Directors shall elect a President and a Secretary. The Board of Directors may also elect a Chairman of the Board, a Chief Executive Officer, a Chief Financial Officer, a Treasurer, one or more Vice Presidents and one or more Assistant Vice Presidents, one or more Assistant Secretaries and may give any of them such further designations or alternate titles as it considers desirable. Any number of offices may be held by the same person. The Board of Directors may also empower the President of the Corporation to appoint such other officers as the business of the Corporation may require.
Section 5.2. Term of Office; Resignation; Removal; Vacancies. Except as otherwise provided in the resolution of the Board of Directors electing any officer, each officer shall serve at the pleasure of the Board of Directors or until his earlier resignation or removal. Any officer may resign at any time upon written notice to the Board of Directors or to the President of the Corporation. Such resignation shall take effect at the time specified therein, and, unless otherwise specified therein, no acceptance of such resignation shall be necessary to make it effective. The Board of Directors may remove any officer with or without cause at any time, provided that such action by the Board of Directors shall require the vote of a majority of the whole Board of Directors. Any officer who has been appointed by the President pursuant to Section 5.1 above may be removed with or without cause by the President or any other officer upon whom such power of removal may be conferred by the Board of Directors. Any such removal shall be without prejudice to the contractual rights of such officer, if any, with the Corporation, but the election of an officer shall not of itself create contractual rights. Any vacancy occurring in any office of the Corporation by death, resignation, removal or otherwise may be filled for the unexpired portion of the term by the Board of Directors at any regular or special meeting or by the President in the manner provided in Section 5.1 for election of officers following the annual meeting of stockholders.
Section 5.3. Chairman of the Board of Directors. The Chairman of the Board of Directors, if there be such an officer, shall preside at all meetings of the Board of Directors and of the stockholders at which he or she shall be present. The Chairman of the Board shall have and may exercise such powers and perform such other duties as are, from time to time, assigned to him or her by the Board of Directors and as may be provided by law. If there is no Chief Executive Officer or President appointed, the Chairman of the Board shall be the general manager and chief executive officer of the Corporation and shall have general charge and supervision of the business of the Corporation.
Section 5.4. President. The general management and executive authority of the Corporation and the responsibility for carrying out the policies adopted by the Board of Directors shall be vested in the President of the Corporation who shall have general charge and supervision of the business of the Corporation. He or she shall have power to sign all stock certificates, contracts and other instruments of the Corporation which are authorized and shall have general supervision and direction of all of the other officers, employees, and agents of the Corporation.
Section 5.5. Vice Presidents. The Vice President or Vice Presidents, if any, at the request of the President or in the absence or disability of the President, shall perform the duties of the President, and when so acting shall have the powers of the President. If there be more than one Vice President, the Board of Directors may determine which one or more of the Vice Presidents shall perform any of such duties; or if such determination is not made by the Board of Directors, the President may make such determination; otherwise any of the Vice Presidents may perform any of such duties. The Vice President or Vice Presidents shall have such other powers and perform such other duties as may be assigned to such Vice President or Vice Presidents by the Board of Directors or the President or as may be provided by law.
| -396- |
Section 5.6. Secretary. The Secretary shall have the duty to record the proceedings of the meetings of the stockholders, the Board of Directors and any committees in a book to be kept for that purpose; he or she shall see that all notices are duly given in accordance with the provisions of these Bylaws or as required by law; he or she shall be custodian of the records of the Corporation; he or she may affix the corporate seal to any document the execution of which, on behalf of the Corporation, is duly authorized, and when so affixed may attest the same; and, in general, he or she shall perform all duties incident to the office of secretary of a corporation, and such other duties as, from time to time, may be assigned to him or her by the Board of Directors or the President or as may be provided by law.
Section 5.7. Chief Financial Officer. The Chief Financial Officer, if there be such an officer, shall have charge of and be responsible for all funds, securities, receipts and disbursements of the Corporation and shall deposit or cause to be deposited, in the name of the Corporation, all moneys or other valuable effects in such banks, trust companies or other depositories as shall, from time to time, be selected by or under authority of the Board of Directors; if required by the Board of Directors, he or she shall give a bond for the faithful discharge of his or her duties, with such surety or sureties as the Board of Directors may determine; he or she shall keep or cause to be kept full and accurate records of all receipts and disbursements in books of the Corporation and shall render to the Chairman of the Board, if any, and to the Board of Directors, whenever requested, an account of the financial condition of the Corporation; and, in general, he or she shall perform all the duties incident to the office of treasurer of a corporation and such other duties as may be assigned to him or her by the Board of Directors or the President or as may be provided by law.
Section 5.8. Other Officers. The other officers, if any, of the Corporation shall have such powers and duties in the management of the Corporation as shall be stated in a resolution adopted by the Board of Directors which is not inconsistent with these Bylaws and, to the extent not so stated, as generally pertain to their respective offices, subject to the control of the Board of Directors. The Board of Directors may require any officer, agent or employee to give security for the faithful performance of his duties.
Section 5.9. Compensation. The compensation of the officers shall be fixed from time to time by the Board of Directors, and no officers shall be prevented from receiving such compensation by reason of the fact that such officer is also a director of the Corporation.
ARTICLE VI
Stock
Section 6.1. Certificates. Every holder of stock shall be entitled to have a certificate signed by or in the name of the Corporation by the President (or by a Chairman of the Board, a Chief Executive Officer, or a Vice President, if any) and by the Secretary (or by a Chief Financial Officer, Treasurer, Assistant Secretary or Assistant Treasurer, if any) certifying the number of shares owned by him or her in the Corporation. Any of or all the signatures on the certificate may be a facsimile. In case any officer, transfer agent or registrar who has signed or whose facsimile signature has been placed upon a certificate shall have ceased to be such officer, transfer agent or registrar before such certificate is issued, it may be issued by the Corporation with the same effect as if such person were such officer, transfer agent or registrar at the date of issue.
Section 6.2. Transfers. Stock of the Corporation shall be transferable in the manner prescribed by law and in these Bylaws. Transfers of stock shall be made on the books of the Corporation only by the person named in the certificate or by his or her attorney lawfully constituted in writing and upon the surrender of the certificate thereunder, which shall be canceled before a new certificate shall be issued.
| -397- |
Section 6.3. Lost, Stolen or Destroyed Stock Certificates; Issuance of New Certificates. The Corporation may issue a new certificate of stock in the place of any certificate theretofore issued by it, alleged to have been lost, stolen or destroyed, and the Corporation may require the owner of the lost, stolen or destroyed certificate, or such owner’s legal representative, to give the Corporation a bond sufficient to indemnify it against any claim that may be made against it on account of the alleged loss, theft or destruction of any such certificate or the issuance of such new certificate.
Section 6.4. Transfer Agent and Registrar. The Board of Directors may appoint one or more transfer agents or one or more transfer clerks and one or more registrars and may require all stock certificates to bear the signature or signatures of any of them.
Section 6.5. Examination of Books by Stockholders. Any stockholder, in person or by attorney or other agent, shall, upon written demand under oath stating the purpose thereof, have the right during the usual hours for business to inspect for any proper purpose the Corporation’s stock ledger, a list of its stockholders, and its other books and records, and to make copies or extracts therefrom. A proper purpose shall mean a purpose reasonably related to such person’s interest as a stockholder. In every instance where an attorney or other agent shall be the person who seeks the right to inspection, the demand under oath shall be accompanied by a power of attorney or such other writing which authorizes the attorney or other agent to so act on behalf of the stockholder. The demand under oath shall be directed to the Corporation at its registered office in the State of Delaware or at its principal place of business.
ARTICLE VII
Indemnification
Section 7.1. Right to Indemnification. The Corporation shall indemnify and hold harmless, to the fullest extent permitted by applicable law as it presently exists or may hereafter be amended, any person (an “Indemnitee”) who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative (a “Proceeding”), by reason of the fact that the Indemnitee, or a person for whom the Indemnitee is the legal representative, is or was after the date on which these Bylaws are first adopted, a director or officer of the Corporation or, while a director or officer of the Corporation, is or was after the date on which these Bylaws are first adopted serving at the request of the Corporation as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust, enterprise or nonprofit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including attorneys’ fees) reasonably incurred by such Indemnitee. Notwithstanding the preceding sentence, except as otherwise provided in Section 7.3, the Corporation shall be required to indemnify an Indemnitee in connection with a Proceeding (or part thereof) commenced by such Indemnitee only if the commencement of such Proceeding (or part thereof) by the Indemnitee was authorized by the Board of Directors of the Corporation.
Section 7.2. Prepayment of Expenses. The Corporation may, in its discretion, pay the expenses (including attorneys’ fees) incurred by an Indemnitee in defending any Proceeding in advance of its final disposition; provided, however, that, to the extent required by law, such payment of expenses in advance of the final disposition of the Proceeding shall be made only upon receipt of an undertaking by the Indemnitee to repay all amounts advanced if it should ultimately be determined that the Indemnitee is not entitled to be indemnified under this Article VII or otherwise.
| -398- |
Section 7.3. Claims. If a claim for indemnification or payment of expenses under this Article VII is not paid in full within sixty (60) days after a written claim therefor by the Indemnitee has been received by the Corporation, the Indemnitee may file suit to recover the unpaid amount of such claim and, if successful in whole or in part, shall be entitled to be paid the expense of prosecuting such claim. In any such action the Corporation shall have the burden of proving that the Indemnitee is not entitled to the requested indemnification or payment of expenses under applicable law.
Section 7.4. Nonexclusivity of Rights. The rights conferred on any Indemnitee by this Article VII shall not be exclusive of any other rights which such Indemnitee may have or hereafter acquire under any statute, provision of the Certificate of Incorporation, these Bylaws, agreement, vote of stockholders or disinterested directors or otherwise.
Section 7.5. Other Sources. The Corporation’s obligation, if any, to indemnify or to advance expenses to any Indemnitee who was or is serving at its request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust, enterprise or nonprofit entity shall be reduced by any amount such Indemnitee may collect as indemnification or advancement of expenses from such other corporation, partnership, joint venture, trust, enterprise or nonprofit enterprise.
Section 7.6. Amendment or Repeal. Any repeal or modification of the foregoing provisions of this Article VII shall not adversely affect any right or protection hereunder of any Indemnitee in respect of any act or omission occurring prior to the time of such repeal or modification.
Section 7.7. Other Indemnification and Prepayment of Expenses. This Article VII shall not limit the right of the Corporation, to the extent and in the manner permitted by law, to indemnify and to advance expenses to persons other than Indemnitees when and as authorized by appropriate corporate action.
Section 7.8. Insurance. The Corporation may purchase and maintain insurance on behalf of any Indemnitee against any liability asserted against such Indemnitee and incurred by such Indemnitee in any such capacity, or arising out of the Indemnitee’s status as such, whether or not the Corporation would have the power or the obligation to indemnify such Indemnitee against such liability under the provisions of this Article VII.
Section 7.9. Limitation on Liability. No person shall be personally liable to the Corporation or its stockholders for monetary damages for breach of fiduciary duty as a director; provided, however, that the foregoing shall not eliminate or limit the liability of a director (i) for any breach of the director’s duty of loyalty, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the General Corporation Law of the State of Delaware, (iv) for any transaction from which the director derived an improper benefit or (v) for acts or omissions occurring prior to the date of the effectiveness of this provision; provided, further, that if the General Corporation Law of the State of Delaware is amended to authorize corporate action further eliminating or limiting the personal liability of directors, then the personal liability of a director of the Corporation shall be eliminated or limited to the fullest extent permitted by the General Corporation Law of the State of Delaware, as amended from time to time.
| -399- |
ARTICLE VIII
Contracts, Loans, Bank Accounts, Checks and Drafts
Section 8.1. Execution of Contracts and Other Instruments. Except as these Bylaws may otherwise provide, the Board of Directors or its duly appointed and authorized committee may authorize any officer or officers, or agent or agents, to enter into any contract or execute and deliver any instrument in the name of and on behalf of the Corporation, and such authorization may be general or confined to specific instances. Except as so authorized or otherwise expressly provided in these Bylaws, no officer, agent or employee shall have any power or authority to bind the Corporation by any contract or engagement or to pledge its credit or to render it liable for any purpose or in any amount.
Section 8.2. Loans. No loans shall be contracted on behalf of the Corporation and no negotiable paper shall be issued in its name, unless and except as authorized by the Board of Directors or its duly appointed and authorized committee. When so authorized by the Board of Directors or such committee, any officer or agent of the Corporation may effect loans and advances at any time for the Corporation from any bank, trust company or other institution, or from any firm, corporation or individual, and for such loans and advances may make, execute and deliver promissory notes, bonds or other evidences of indebtedness of the Corporation and, when authorized as aforesaid, may mortgage, pledge, hypothecate or transfer any and all stocks, securities and other property, real or personal, at any time held by the Corporation, and to that end endorse, assign and deliver the same as security for the payment of any and all loans, advances, indebtedness and liabilities of the Corporation. Such authorization may be general or confined to specific instances.
Section 8.3. Bank Accounts. The Board of Directors or its duly appointed and authorized committee from time to time may authorize the opening and keeping of general and/or special bank accounts with such banks, trust companies or other depositaries as may be selected by the Board of Directors, its duly appointed and authorized committee or by any officer or officers, or agent or agents, of the Corporation to whom such power may be delegated from time to time by the Board of Directors. The Board of Directors or its duly appointed and authorized committee may make such rules and regulations with respect to said bank accounts, not inconsistent with the provisions of these Bylaws, as are deemed advisable.
Section 8.4. Checks, Drafts, Etc. All checks, drafts or other orders for the payment of money, notes, acceptances or other evidences of indebtedness issued in the name of the Corporation shall be signed by such officer or officers, or agent or agents, of the Corporation, and in such manner as shall be determined from time to time by resolution of the Board of Directors or its duly appointed and authorized committee. Endorsements for deposit to the credit of the Corporation in any of its duly authorized depositories may be made, without counter-signature, by the President or any other officer or agent of the Corporation to whom the Board of Directors or its duly appointed and authorized committee, by resolution, shall have delegated such power or by hand-stamped impression in the name of the Corporation.
ARTICLE IX
Miscellaneous
Section 9.1. Fiscal Year. The fiscal year of the Corporation shall end on the 31st day of December, unless otherwise determined by resolution of the Board of Directors.
Section 9.2. Seal. The corporate seal shall have the name of the Corporation inscribed thereon and shall be in such form as may be approved from time to time by the Board of Directors.
Section 9.3. Waiver of Notice of Meetings of Stockholders, Directors and Committees. Any written waiver of notice, signed by the person entitled to notice, or a waiver by electronic transmission by the person entitled to notice, whether before or after the time stated therein, shall be deemed equivalent to notice. Attendance of a person at a meeting shall constitute a waiver of notice of such meeting, except when the person attends a meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Neither the business to be transacted at, nor the purpose of, any regular or special meeting of the stockholders, directors, or members of a committee of directors need be specified in any written waiver of notice or any waiver by electronic transmission.
| -400- |
Section 9.4. Interested Directors; Quorum. No contract or transaction between the Corporation and one or more of its directors or officers, or between the Corporation and any other corporation, partnership, association or other organization in which one or more of its directors or officers are directors or officers, or have a financial interest, shall be void or voidable solely for this reason, or solely because the director or officer is present at or participates in the meeting of the Board of Directors or committee thereof which authorizes the contract or transaction, or solely because any such director’s or officer’s votes are counted for such purpose, if: (1) the material facts as to his or her relationship or interest and as to the contract or transaction are disclosed or are known to the Board of Directors or the committee, and the Board of Directors or committee in good faith authorizes the contract or transaction by the affirmative votes of a majority of the disinterested directors, even though the disinterested directors constitute less than a quorum; or (2) the material facts as to his or her relationship or interest and as to the contract or transaction are disclosed or are known to the stockholders entitled to vote thereon, and the contract or transaction is specifically approved in good faith by vote of the stockholders; or (3) the contract or transaction is fair as to the Corporation as of the time it is authorized, approved or ratified by the Board of Directors, a committee thereof or the stockholders. Common or interested directors may be counted in determining the presence of a quorum at a meeting of the Board of Directors or of a committee which authorizes the contract or transaction.
Section 9.5. Form of Records. Any records maintained by the Corporation in the regular course of its business, including its stock ledger, books of account and minute books, may be kept on, or by means of or be in the form of, any information storage device or method, provided that the records so kept can be converted into clearly legible form within a reasonable time. The Corporation shall so convert any records so kept upon the request of any person entitled to inspect such records.
Section 9.6. Amendment of Bylaws. These Bylaws shall be subject to alteration, amendment or repeal, and new Bylaws, not inconsistent with any provision of law or the Certificate of Incorporation, may be made, either by the affirmative vote of a majority of the whole Board of Directors at any meeting thereof or, if the power to make, amend, alter or repeal the Bylaws shall not have been granted to the Board of Directors in the Certificate of Incorporation, by the affirmative vote of the holders of a majority of the shares of the Corporation present in person or by proxy at any annual or special meeting and entitled to vote thereat, a quorum being present. Notice of the proposal to make, alter, amend or repeal the Bylaws of the Corporation shall be included in the notice of such meeting of the Board of Directors or of the stockholders, as the case may be.
| -401- |
Annex B
AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
FOXWAYNE ENTERPRISES ACQUISITION CORP.
FoxWayne Enterprises Acquisition Corp., a corporation organized and existing under the laws of the State of Delaware (the “Corporation”), DOES HEREBY CERTIFY AS FOLLOWS:
1. The name of the Corporation is “FoxWayne Enterprises Acquisition Corp.”. The original certificate of incorporation of the Corporation was filed with the Secretary of State of the State of Delaware on September 17, 2020.
2. This Amended and Restated Certificate of Incorporation of the Corporation, which both restates and amends the provisions of the certificate of incorporation of the Corporation as heretofore in effect, was duly adopted in accordance with Sections 242 and 245 of the General Corporation Law of the State of Delaware.
3. The text of the certificate of incorporation of the Corporation as heretofore in effect is hereby amended and restated to read in its entirety as follows:
ARTICLE I
NAME
The name of the corporation is Aerami Therapeutics Holdings, Inc. (the “Corporation”).
ARTICLE II
REGISTERED OFFICE AND AGENT
The address of the Corporation’s registered office in the State of Delaware is [●]. The name of its registered agent at such address is [●].
ARTICLE III
PURPOSE
The purpose of the Corporation is to engage in any lawful act or activity for which corporations may be organized under the General Corporation Law of the State of Delaware, as it now exists or may hereafter be amended and supplemented (the “DGCL”).
ARTICLE IV
CAPITAL STOCK
The Corporation is authorized to issue two classes of stock to be designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares of capital stock which the Corporation shall have authority to issue is 210,000,000. The total number of shares of Common Stock that the Corporation is authorized to issue is 200,000,000, having a par value of $0.0001 per share, and the total number of Preferred Stock that the Corporation is authorized to issue is 10,000,000, having a par value $0.0001 per share.
Upon the filing and effectiveness of this Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware (the “Effective Time”), each share of Class A Common Stock, par value $0.0001 per share, issued and outstanding or held in treasury immediately prior to the Effective Time (including each share of Class A Common Stock, par value $0.0001 per share, of the Corporation issued upon the conversion of shares of Class B Common Stock, par value $0.0001 per share, of the Corporation) (“Old Common Stock”), shall be automatically reclassified as and converted into one share of Common Stock. Any stock certificate or book entry representing shares of Old Common Stock shall thereafter be deemed to represent a number of whole shares of Common Stock into which such shares of Old Common Stock shall have been reclassified pursuant to this paragraph, without the need for surrender or exchange.
The designations and the powers, privileges and rights, and the qualifications, limitations or restrictions thereof in respect of each class of capital stock of the Corporation are as follows:
| -402- |
A. COMMON STOCK
| 1. | General. The voting, dividend, liquidation, and other rights and powers of the Common Stock are subject to and qualified by the rights, powers and preferences of any series of Preferred Stock as may be designated by the Board of Directors of the Corporation (the “Board of Directors”) and outstanding from time to time. | |
| 2. | Voting. Except as otherwise provided herein or expressly required by law, each holder of Common Stock, as such, shall be entitled to vote on each matter submitted to a vote of stockholders and shall be entitled to one (1) vote for each share of Common Stock held of record by such holder as of the record date for determining stockholders entitled to vote on such matter. Except as otherwise required by law, holders of Common Stock, as such, shall not be entitled to vote on any amendment to this Amended and Restated Certificate of Incorporation (including any Certificate of Designation (as defined below)) that relates solely to the rights, powers, preferences (or the qualifications, limitations or restrictions thereof) or other terms of one or more outstanding series of Preferred Stock if the holders of such affected series are entitled, either separately or together with the holders of one or more other such series, to vote thereon pursuant to this Amended and Restated Certificate of Incorporation (including any Certificate of Designation) or pursuant to the DGCL. | |
| Subject to the rights of any holders of any outstanding series of Preferred Stock, the number of authorized shares of Common Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the stock of the Corporation entitled to vote, irrespective of the provisions of Section 242(b)(2) of the DGCL. | ||
| 3. | Dividends. Subject to applicable law and the rights and preferences of any holders of any outstanding series of Preferred Stock, the holders of Common Stock, as such, shall be entitled to the payment of dividends on the Common Stock when, as and if declared by the Board of Directors in accordance with applicable law. | |
| 4. | Liquidation. Subject to the rights and preferences of any holders of any shares of any outstanding series of Preferred Stock, in the event of any liquidation, dissolution or winding up of the Corporation, whether voluntary or involuntary, the funds and assets of the Corporation that may be legally distributed to the Corporation’s stockholders shall be distributed among the holders of the then outstanding Common Stock pro rata in accordance with the number of shares of Common Stock held by each such holder. |
B. PREFERRED STOCK
| 1. | Shares of Preferred Stock may be issued from time to time in one or more series, each of such series to have such terms as stated or expressed herein and in the resolution or resolutions providing for the creation and issuance of such series adopted by the Board of Directors as hereinafter provided. | |
| 2. | Authority is hereby expressly granted to the Board of Directors from time to time to issue the Preferred Stock in one or more series, and in connection with the creation of any such series, by adopting a resolution or resolutions providing for the issuance of the shares thereof and by filing a certificate of designation relating thereto in accordance with the DGCL (a “Certificate of Designation”), to determine and fix the number of shares of such series and such voting powers, full or limited, or no voting powers, and such designations, preferences and relative participating, optional or other special rights, and qualifications, limitations or restrictions thereof, including without limitation thereof, dividend rights, conversion rights, redemption privileges and liquidation preferences, and to increase or decrease (but not below the number of shares of such series then outstanding) the number of shares of any series as shall be stated and expressed in such resolutions, all to the fullest extent now or hereafter permitted by the DGCL. Without limiting the generality of the foregoing, the resolution or resolutions providing for the creation and issuance of any series of Preferred Stock may provide that such series shall be superior or rank equally or be junior to any other series of Preferred Stock to the extent permitted by law and this Amended and Restated Certificate of Incorporation (including any Certificate of Designation). Except as otherwise required by law, holders of any series of Preferred Stock shall be entitled only to such voting rights, if any, as shall expressly be granted thereto by this Amended and Restated Certificate of Incorporation (including any Certificate of Designation). |
| 3. | The number of authorized shares of Preferred Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the stock of the Corporation entitled to vote, irrespective of the provisions of Section 242(b)(2) of the DGCL. |
| -403- |
ARTICLE V
STOCKHOLDERS
1. No Action without Meeting. Any action required or permitted to be taken by the stockholders of the Corporation must be effected at an annual or special meeting of the stockholders of the Corporation, and shall not be taken by written consent in lieu of a meeting. Notwithstanding the foregoing, any action required or permitted to be taken by the holders of any series of Preferred Stock, voting separately as a series or separately as a class with one or more other such series, may be taken without a meeting, without prior notice and without a vote, to the extent expressly so provided by the applicable Certificate of Designation relating to such series of Preferred Stock, if a consent or consents in writing, setting forth the action so taken, shall be signed by the holders of outstanding shares of the relevant series of Preferred Stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted and shall be delivered to the Corporation in accordance with the applicable provisions of the DGCL. Notwithstanding anything herein to the contrary, the affirmative vote of not less than two thirds (2/3) of the outstanding shares of capital stock entitled to vote thereon, and the affirmative vote of not less than two thirds (2/3) of the outstanding shares of each class entitled to vote thereon as a class, shall be required to amend or repeal any provision of this Article V, Section 1.
2. Special Meetings. Except as otherwise required by statute and subject to the rights, if any, of the holders of any series of Preferred Stock, special meetings of the stockholders of the Corporation may be called, for any purpose or purposes, at any time only by or at the direction of the Board of Directors, the Chairperson of the Board of Directors or the Chief Executive Officer of the Corporation, and shall not be called by any other person or persons. Only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of stockholders of the Corporation. Notwithstanding anything herein to the contrary, the affirmative vote of not less than two thirds (2/3) of the outstanding shares of capital stock entitled to vote thereon, and the affirmative vote of not less than two thirds (2/3) of the outstanding shares of each class entitled to vote thereon as a class, shall be required to amend or repeal any provision of this Article V, Section 2.
ARTICLE VI
DIRECTORS
1. General. The business and affairs of the Corporation shall be managed by or under the direction of the Board of Directors except as otherwise provided herein or required by law.
2. Election of Directors. Election of Directors need not be by written ballot unless the Bylaws of the Corporation (the “Bylaws”) shall so provide.
3. Number of Directors; Term of Office. Subject to the special rights of the holders of one or more outstanding series of Preferred Stock to elect directors, the directors of the Corporation shall be classified with respect to the time for which they severally hold office into three classes, designated as Class I, Class II and Class III. The initial Class I directors shall serve for a term expiring at the first annual meeting of the stockholders following the date of this Amended and Restated Certificate of Incorporation; the initial Class II directors shall serve for a term expiring at the second annual meeting of the stockholders following the date of this Amended and Restated Certificate of Incorporation; and the initial Class III directors shall serve for a term expiring at the third annual meeting of the stockholders following the date of this Amended and Restated Certificate of Incorporation. At each annual meeting of the stockholders of the Corporation beginning with the first annual meeting of the stockholders following the date of this Amended and Restated Certificate of Incorporation, subject to the special rights of the holders of one or more outstanding series of Preferred Stock to elect directors, the successors of the class of directors whose term expires at that meeting shall be elected to hold office for a term expiring at the annual meeting of stockholders held in the third year following the year of their election. Each director shall hold office until his or her successor is duly elected and qualified or until his or her earlier death, resignation, disqualification or removal. No decrease in the number of directors shall shorten the term of any incumbent director. The Board of Directors is authorized to assign members of the Board of Directors already in office to Class I, Class II and Class III.
| -404- |
Except as otherwise expressly provided by the DGCL or this Amended and Restated Certificate of Incorporation, the business and affairs of the Corporation shall be managed by or under the direction of the Board of Directors. The number of directors which shall constitute the whole Board of Directors shall be fixed exclusively by one or more resolutions adopted from time to time by the Board of Directors.
Notwithstanding anything herein to the contrary, the affirmative vote of not less than two thirds (2/3) of the outstanding shares of capital stock entitled to vote thereon, and the affirmative vote of not less than two thirds (2/3) of the outstanding shares of each class entitled to vote thereon as a class, shall be required to amend or repeal any provision of this Article VI, Section 3.
4. Vacancies. Subject to the rights, if any, of the holders of any series of Preferred Stock to elect Directors and to fill vacancies in the Board of Directors relating thereto, any and all vacancies in the Board of Directors, however occurring, including, without limitation, by reason of an increase in the size of the Board of Directors, or the death, resignation, disqualification or removal of a Director, shall be filled solely and exclusively by the affirmative vote of a majority of the remaining Directors then in office, even if less than a quorum of the Board of Directors, and not by the stockholders. Any Director appointed in accordance with the preceding sentence shall hold office for the remainder of the full term of the class of Directors in which the new directorship was created or the vacancy occurred and until such Director’s successor shall have been duly elected and qualified or until his or her earlier resignation, death or removal. Subject to the rights, if any, of the holders of any series of Preferred Stock to elect Directors, when the number of Directors is increased or decreased, the Board of Directors shall, subject to Article VI, Section 3 hereof, determine the class or classes to which the increased or decreased number of Directors shall be apportioned; provided, however, that no decrease in the number of Directors shall shorten the term of any incumbent Director. In the event of a vacancy in the Board of Directors, the remaining Directors, except as otherwise provided by law, shall exercise the powers of the full Board of Directors until the vacancy is filled.
5. Removal. Subject to the special rights of the holders of one or more outstanding series of Preferred Stock to elect directors, the Board of Directors or any individual director may be removed from office at any time, but only for cause and only by the affirmative vote of the holders of at least two-thirds (66 and 2/3%) of the voting power of all of the then outstanding shares of voting stock of the Corporation entitled to vote at an election of directors.
ARTICLE VII
LIMITATION OF LIABILITY
1. Limitation of Director Liability. A Director of the Corporation shall not be personally liable to the Corporation or its stockholders for monetary damages for breach of his or her fiduciary duty as a Director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL as the same exists or hereafter may be amended. Any amendment, repeal or modification of this Article VII, or the adoption of any provision of the Amended and Restated Certificate of Incorporation inconsistent with this Article VII, shall not adversely affect any right or protection of a director of the Corporation with respect to any act or omission occurring prior to such amendment, repeal, modification or adoption.
2. Any amendment, repeal or modification of this Article VII by either of (i) the stockholders of the Corporation or (ii) an amendment to the DGCL, shall not adversely affect any right or protection existing at the time of such amendment, repeal or modification with respect to any acts or omissions occurring before such amendment, repeal or modification of a person serving as a Director at the time of such amendment, repeal or modification.
3. Notwithstanding anything herein to the contrary, the affirmative vote of not less than two thirds (2/3) of the outstanding shares of capital stock entitled to vote thereon, and the affirmative vote of not less than two thirds (2/3) of the outstanding shares of each class entitled to vote thereon as a class, shall be required to amend or repeal any provision of this Article VII.
ARTICLE VIII
INDEMNIFICATION
The Corporation shall have the power to provide rights to indemnification and advancement of expenses to its current and former officers, directors, employees and agents and to any person who is or was serving at the request of the Corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise.
| -405- |
ARTICLE IX
AMENDMENT OF BYLAWS
1. Amendment by Directors. Except as otherwise provided by law, the Bylaws of the Corporation may be amended or repealed by the Board of Directors.
2. Amendment by Stockholders. Except as otherwise provided therein, the Bylaws of the Corporation may be amended or repealed at any annual meeting of stockholders, or special meeting of stockholders called for such purpose, by the affirmative vote of not less than two thirds (2/3) of the outstanding shares of capital stock entitled to vote on such amendment or repeal, voting together as a single class.
ARTICLE X
AMENDMENT OF CERTIFICATE OF INCORPORATION
The Corporation reserves the right to amend or repeal this Certificate in the manner now or hereafter prescribed by statute and this Certificate, and all rights conferred upon stockholders herein are granted subject to this reservation. Except as otherwise required by this Certificate or by law, whenever any vote of the holders of capital stock of the Corporation is required to amend or repeal any provision of this Certificate, such amendment or repeal shall require the affirmative vote of the majority of the outstanding shares of capital stock entitled to vote on such amendment or repeal, and the affirmative vote of the majority of the outstanding shares of each class entitled to vote thereon as a class, at a duly constituted meeting of stockholders called expressly for such purpose.
If any provision or provisions of this Amended and Restated Certificate of Incorporation shall be held to be invalid, illegal or unenforceable as applied to any circumstance for any reason whatsoever: (i) the validity, legality and enforceability of such provisions in any other circumstance and of the remaining provisions of this Amended and Restated Certificate of Incorporation (including, without limitation, each portion of any paragraph of this Amended and Restated Certificate of Incorporation containing any such provision held to be invalid, illegal or unenforceable that is not itself held to be invalid, illegal or unenforceable) shall not, to the fullest extent permitted by applicable law, in any way be affected or impaired thereby and (ii) to the fullest extent permitted by applicable law, the provisions of this Amended and Restated Certificate of Incorporation (including, without limitation, each such portion of any paragraph of this Amended and Restated Certificate containing any such provision held to be invalid, illegal or unenforceable) shall be construed so as to permit the Corporation to protect its directors, officers, employees and agents from personal liability in respect of their good faith service to or for the benefit of the Corporation to the fullest extent permitted by law.
ARTICLE XI
FORUM SELECTION
Unless the Corporation consents in writing to the selection of an alternative forum, (a) the Court of Chancery (the “Chancery Court”) of the State of Delaware (or, in the event that the Chancery Court does not have jurisdiction, the federal district court for the District of Delaware or other state courts of the State of Delaware) shall, to the fullest extent permitted by law, be the sole and exclusive forum for (i) any derivative action, suit or proceeding brought on behalf of the Corporation, (ii) any action, suit or proceeding asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, other employee or stockholder of the Corporation to the Corporation or to the Corporation’s stockholders, (iii) any action, suit or proceeding arising pursuant to any provision of the DGCL or the bylaws of the Corporation or this Restated Certificate (as either may be amended from time to time) or as to which the DGCL confers jurisdiction on the Chancery Court or (iv) any action, suit or proceeding asserting a claim against the Corporation governed by the internal affairs doctrine; and (b) the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended. If any action the subject matter of which is within the scope of clause (a) of the immediately preceding sentence is filed in a court other than the courts in the State of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented to (x) the personal jurisdiction of the state and federal courts in the State of Delaware in connection with any action brought in any such court to enforce the provisions of clause (a) of the immediately preceding sentence and (y) having service of process made upon such stockholder in any such action by service upon such stockholder’s counsel in the Foreign Action as agent for such stockholder.
Any person or entity purchasing or otherwise acquiring any interest in any security of the Corporation shall be deemed to have notice of and consented to this Article XI. Notwithstanding the foregoing, the provisions of this Article XI shall not apply to suits brought to enforce any liability or duty created by the Securities Exchange Act of 1934, as amended, or any other claim for which the federal courts of the United States have exclusive jurisdiction.
If any provision or provisions of this Article XI shall be held to be invalid, illegal or unenforceable as applied to any circumstance for any reason whatsoever, (a) the validity, legality and enforceability of such provisions in any other circumstance and of the remaining provisions of this Article XI (including, without limitation, each portion of any paragraph of this Article XI containing any such provision held to be invalid, illegal or unenforceable that is not itself held to be invalid, illegal or unenforceable) shall not in any way be affected or impaired thereby and (b) the application of such provision to other persons or entities and circumstances shall not in any way be affected or impaired thereby.
ARTICLE XII
BUSINESS COMBINATIONS
1. Opt Out of DGCL 203. The Corporation shall not be governed by Section 203 of the DGCL.
[End of Text]
| -406- |
THIS AMENDED AND RESTATED CERTIFICATE OF INCORPORATION is executed as of this ____ day of __________, 2022.
| AERAMI THERAPEUTICS HOLDINGS, INC. | ||
| By: | ||
| Name: | ||
| Title: | ||
| -407- |
Annex C
AMENDED
AND RESTATED BYLAWS OF
AERAMI THERAPEUTICS HOLDINGS, INC.
Article I - Corporate Offices
1.1 Registered Office. The address of the registered office of Aerami Therapeutics Holdings, Inc. (the “Corporation”) in the State of Delaware, and the name of its registered agent at such address, shall be as set forth in the Corporation’s certificate of incorporation, as the same may be amended and/or restated from time to time (the “Certificate of Incorporation”).
1.2 Other Offices. The Corporation may have additional offices at any place or places, within or outside the State of Delaware, as the Corporation’s board of directors (the “Board”) may from time to time establish or as the business of the Corporation may require.
Article II - Meetings of Stockholders
2.1 Place of Meetings. Meetings of stockholders shall be held at any place, within or outside the State of Delaware, designated by the Board. The Board may, in its sole discretion, determine that a meeting of stockholders shall not be held at any place, but may instead be held solely by means of remote communication as authorized by Section 211(a) of the General Corporation Law of the State of Delaware (the “DGCL”). In the absence of any such designation or determination, stockholders’ meetings shall be held at the Corporation’s principal executive office.
2.2 Annual Meeting. The Board shall designate the date and time of the annual meeting. At the annual meeting, directors shall be elected and other proper business properly brought before the meeting in accordance with Section 2.4 may be transacted. The Board may postpone, reschedule or cancel any previously scheduled annual meeting of stockholders.
2.3 Special Meeting. Special meetings of the stockholders may be called only by such persons and only in such manner as set forth in the Certificate of Incorporation.
No business may be transacted at any special meeting of stockholders other than the business specified in the notice of such meeting. The Board may postpone, reschedule or cancel any previously scheduled special meeting of stockholders.
2.4 Notice of Business to be Brought before a Meeting.
(a) At an annual meeting of the stockholders, only such business shall be conducted as shall have been properly brought before the meeting. To be properly brought before an annual meeting, business must be (i) specified in a notice of meeting given by or at the direction of the Board, (ii) if not specified in a notice of meeting, otherwise brought before the meeting by the Board or the Chairperson of the Board or (iii) otherwise properly brought before the meeting by a stockholder present in person who (A) (1) was a record owner of shares of the Corporation both at the time of giving the notice provided for in this Section 2.4 and at the time of the meeting, (2) is entitled to vote at the meeting and (3) has complied with this Section 2.4 in all applicable respects or (B) properly made such proposal in accordance with Rule 14a-8 under the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder (as so amended and inclusive of such rules and regulations, the “Exchange Act”). The foregoing clause (iii) shall be the exclusive means for a stockholder to propose business to be brought before an annual meeting of the stockholders. The only matters that may be brought before a special meeting are the matters specified in the notice of meeting given by or at the direction of the person calling the meeting pursuant to Section 2.3, and stockholders shall not be permitted to propose business to be brought before a special meeting of the stockholders. For purposes of this Section 2.4, “present in person” shall mean that the stockholder proposing that the business be brought before the annual meeting of the Corporation, or a qualified representative of such proposing stockholder, appear at such annual meeting in person, or by remote communication, if applicable. A “qualified representative” of such proposing stockholder shall be a duly authorized officer, manager or partner of such stockholder or any other person authorized by a writing executed by such stockholder or an electronic transmission delivered by such stockholder to act for such stockholder as proxy at the meeting of stockholders and such person must produce such writing or electronic transmission, or a reliable reproduction of the writing or electronic transmission, at the meeting of stockholders. Stockholders seeking to nominate persons for election to the Board must comply with Section 2.5, and this Section 2.4 shall not be applicable to nominations except as expressly provided in Section 2.5.
| -408- |
(b) Without qualification, for business to be properly brought before an annual meeting by a stockholder, the stockholder must (i) provide Timely Notice (as defined below) thereof in writing and in proper form to the Secretary of the Corporation and (ii) provide any updates or supplements to such notice at the times and in the forms required by this Section 2.4. To be timely, a stockholder’s notice must be delivered to, or mailed and received at, the principal executive offices of the Corporation not less than ninety (90) days nor more than one hundred twenty (120) days prior to the one-year anniversary of the preceding year’s annual meeting; provided, however, that if no annual meeting was held in the preceding year, to be timely, a stockholder’s notice must be so delivered, or mailed and received, not earlier than the close of business on the one hundred and twentieth (120th) day prior to such annual meeting and not later than the close of business on the later of the ninetieth (90th) day prior to such annual meeting or, if later, the tenth (10th) day following the day on which public disclosure of the date of such annual meeting was first made by the Corporation; provided, further, that if the date of the annual meeting is more than thirty (30) days before or more than sixty (60) days after such anniversary date, to be timely, a stockholder’s notice must be so delivered, or mailed and received, not later than the ninetieth (90th) day prior to such annual meeting or, if later, the tenth (10th) day following the day on which public disclosure of the date of such annual meeting was first made by the Corporation (such notice within such time periods, “Timely Notice”). In no event shall any adjournment or postponement of an annual meeting or the announcement thereof commence a new time period for the giving of Timely Notice as described above.
(c) To be in proper form for purposes of this Section 2.4, a stockholder’s Timely Notice to the Secretary of the Corporation shall set forth:
(i) As to each Proposing Person (as defined below), (A) the name and address of such Proposing Person (including, if applicable, the name and address that appear on the Corporation’s books and records); and (B) the class or series and number of shares of the Corporation that are, directly or indirectly, owned of record or beneficially owned (within the meaning of Rule 13d-3 under the Exchange Act) by such Proposing Person, except that such Proposing Person shall in all events be deemed to beneficially own any shares of any class or series of the Corporation as to which such Proposing Person has a right to acquire beneficial ownership at any time in the future (the disclosures to be made pursuant to the foregoing clauses (A) and (B) are referred to as “Stockholder Information”);
(ii) As to each Proposing Person, (A) the full notional amount of any securities that, directly or indirectly, underlie any “derivative security” (as such term is defined in Rule 16a-1(c) under the Exchange Act) that constitutes a “call equivalent position” (as such term is defined in Rule 16a-1(b) under the Exchange Act) (“Synthetic Equity Position”) and that is, directly or indirectly, held or maintained by such Proposing Person with respect to any shares of any class or series of shares of the Corporation; provided that, for the purposes of the definition of “Synthetic Equity Position,” the term “derivative security” shall also include any security or instrument that would not otherwise constitute a “derivative security” as a result of any feature that would make any conversion, exercise or similar right or privilege of such security or instrument becoming determinable only at some future date or upon the happening of a future occurrence, in which case the determination of the amount of securities into which such security or instrument would be convertible or exercisable shall be made assuming that such security or instrument is immediately convertible or exercisable at the time of such determination; and, provided, further, that any Proposing Person satisfying the requirements of Rule 13d-1(b)(1) under the Exchange Act (other than a Proposing Person that so satisfies Rule 13d-1(b)(1) under the Exchange Act solely by reason of Rule 13d-1(b)(1)(ii)(E)) shall not be deemed to hold or maintain the notional amount of any securities that underlie a Synthetic Equity Position held by such Proposing Person as a hedge with respect to a bona fide derivatives trade or position of such Proposing Person arising in the ordinary course of such Proposing Person’s business as a derivatives dealer, (B) any rights to dividends on the shares of any class or series of shares of the Corporation owned beneficially by such Proposing Person that are separated or separable from the underlying shares of the Corporation, (C) any material pending or threatened legal proceeding in which such Proposing Person is a party or material participant involving the Corporation or any of its officers or directors, or any affiliate of the Corporation, (D) any other material relationship between such Proposing Person, on the one hand, and the Corporation or any affiliate of the Corporation, on the other hand, (E) any direct or indirect material interest in any material contract or agreement of such Proposing Person with the Corporation or any affiliate of the Corporation (including, in any such case, any employment agreement, collective bargaining agreement or consulting agreement), (F) a representation that such Proposing Person intends or is part of a group that intends to deliver a proxy statement or form of proxy to holders of at least the percentage of the Corporation’s outstanding capital stock required to approve or adopt the proposal or otherwise solicit proxies from stockholders in support of such proposal and (G) any other information relating to such Proposing Person that would be required to be disclosed in a proxy statement or other filing required to be made in connection with solicitations of proxies or consents by such Proposing Person in support of the business proposed to be brought before the meeting pursuant to Section 14(a) of the Exchange Act (the disclosures to be made pursuant to the foregoing clauses (A) through (G) are referred to as “Disclosable Interests”); provided, however, that Disclosable Interests shall not include any such disclosures with
| -409- |
(iii) As to each item of business that the stockholder proposes to bring before the annual meeting, (A) a brief description of the business desired to be brought before the annual meeting, the reasons for conducting such business at the annual meeting and any material interest in such business of each Proposing Person, (B) the text of the proposal or business (including the text of any resolutions proposed for consideration and in the event that such business includes a proposal to amend the bylaws, the language of the proposed amendment), (C) a reasonably detailed description of all agreements, arrangements and understandings (x) between or among any of the Proposing Persons or (y) between or among any Proposing Person and any other person or entity (including their names) in connection with the proposal of such business by such stockholder; and (D) any other information relating to such item of business that would be required to be disclosed in a proxy statement or other filing required to be made in connection with solicitations of proxies in support of the business proposed to be brought before the meeting pursuant to Section 14(a) of the Exchange Act; provided, however, that the disclosures required by this Section 2.4(c)(iii) shall not include any disclosures with respect to any broker, dealer, commercial bank, trust company or other nominee who is a Proposing Person solely as a result of being the stockholder directed to prepare and submit the notice required by these bylaws on behalf of a beneficial owner.
For purposes of this Section 2.4, the term “Proposing Person” shall mean (i) the stockholder providing the notice of business proposed to be brought before an annual meeting, (ii) the beneficial owner or beneficial owners, if different, on whose behalf the notice of the business proposed to be brought before the annual meeting is made, and (iii) any participant (as defined in paragraphs (a)(ii)-(vi) of Instruction 3 to Item 4 of Schedule 14A) with such stockholder in such solicitation.
(d) A Proposing Person shall update and supplement its notice to the Corporation of its intent to propose business at an annual meeting, if necessary, so that the information provided or required to be provided in such notice pursuant to this Section 2.4 shall be true and correct as of the record date for stockholders entitled to vote at the meeting and as of the date that is ten (10) business days prior to the meeting or any adjournment or postponement thereof, and such update and supplement shall be delivered to, or mailed and received by, the Secretary of the Corporation at the principal executive offices of the Corporation not later than five (5) business days after the record date for stockholders entitled to vote at the meeting (in the case of the update and supplement required to be made as of such record date), and not later than eight (8) business days prior to the date for the meeting or, if practicable, any adjournment or postponement thereof (and, if not practicable, on the first practicable date prior to the date to which the meeting has been adjourned or postponed) (in the case of the update and supplement required to be made as of ten (10) business days prior to the meeting or any adjournment or postponement thereof). For the avoidance of doubt, the obligation to update and supplement as set forth in this paragraph or any other Section of these bylaws shall not limit the Corporation’s rights with respect to any deficiencies in any notice provided by a stockholder, extend any applicable deadlines hereunder or enable or be deemed to permit a stockholder who has previously submitted notice hereunder to amend or update any proposal or to submit any new proposal, including by changing or adding matters, business or resolutions proposed to be brought before a meeting of the stockholders.
(e) Notwithstanding anything in these bylaws to the contrary, no business shall be conducted at an annual meeting that is not properly brought before the meeting in accordance with this Section 2.4. The presiding officer of the meeting shall, if the facts warrant, determine that the business was not properly brought before the meeting in accordance with this Section 2.4, and if he or she should so determine, he or she shall so declare to the meeting and any such business not properly brought before the meeting shall not be transacted.
(f) This Section 2.4 is expressly intended to apply to any business proposed to be brought before an annual meeting of stockholders other than any proposal made in accordance with Rule 14a-8 under the Exchange Act and included in the Corporation’s proxy statement. In addition to the requirements of this Section 2.4 with respect to any business proposed to be brought before an annual meeting, each Proposing Person shall comply with all applicable requirements of the Exchange Act with respect to any such business. Nothing in this Section 2.4 shall be deemed to affect the rights of stockholders to request inclusion of proposals in the Corporation’s proxy statement pursuant to Rule 14a-8 under the Exchange Act.
| -410- |
(g) For purposes of these bylaws, “public disclosure” shall mean disclosure in a press release reported by the Dow Jones News Service, Associated Press or comparable national news service or in a document publicly filed by the Corporation with the Securities and Exchange Commission pursuant to Sections 13, 14 or 15(d) of the Exchange Act.
2.5 Notice of Nominations for Election to the Board.
(a) Nominations of any person for election to the Board at an annual meeting or at a special meeting (but only if the election of directors is a matter specified in the notice of meeting given by or at the direction of the person calling such special meeting) may be made at such meeting only (i) by or at the direction of the Board, including by any committee or persons authorized to do so by the Board or these bylaws, or (ii) by a stockholder present in person (A) who was a record owner of shares of the Corporation both at the time of giving the notice provided for in this Section 2.5 and at the time of the meeting, (B) is entitled to vote at the meeting, and (C) has complied with this Section 2.5 as to such notice and nomination. For purposes of this Section 2.5, “present in person” shall mean that the stockholder proposing that the business be brought before the meeting of the Corporation, or a qualified representative of such stockholder, appear at such meeting in person, or by remote communication, if applicable. A “qualified representative” of such proposing stockholder shall be a duly authorized officer, manager or partner of such stockholder or any other person authorized by a writing executed by such stockholder or an electronic transmission delivered by such stockholder to act for such stockholder as proxy at the meeting of stockholders and such person must produce such writing or electronic transmission, or a reliable reproduction of the writing or electronic transmission, at the meeting of stockholders. The foregoing clause (ii) shall be the exclusive means for a stockholder to make any nomination of a person or persons for election to the Board at an annual meeting or special meeting.
(b) (i) For a stockholder to make any nomination of a person or persons for election to the Board at an annual meeting, the stockholder must (1) provide Timely Notice (as defined in Section 2.4) thereof in writing and in proper form to the Secretary of the Corporation, (2) provide the information, agreements and questionnaires with respect to such stockholder and its candidate for nomination as required to be set forth by this Section 2.5 and (3) provide any updates or supplements to such notice at the times and in the forms required by this Section 2.5.
(ii) If the election of directors is a matter specified in the notice of meeting given by or at the direction of the person calling a special meeting, then for a stockholder to make any nomination of a person or persons for election to the Board at a special meeting, the stockholder must (i) provide Timely Notice thereof in writing and in proper form to the Secretary of the Corporation at the principal executive offices of the Corporation, (ii) provide the information with respect to such stockholder and its candidate for nomination as required by this Section 2.5 and (iii) provide any updates or supplements to such notice at the times and in the forms required by this Section 2.5. To be timely, a stockholder’s notice for nominations to be made at a special meeting must be delivered to, or mailed and received at, the principal executive offices of the Corporation not earlier than the one hundred twentieth (120th) day prior to such special meeting and not later than the ninetieth (90th) day prior to such special meeting or, if later, the tenth (10th) day following the day on which public disclosure (as defined in Section 2.4) of the date of such special meeting was first made.
(iii) In no event shall any adjournment or postponement of an annual meeting or special meeting or the announcement thereof commence a new time period for the giving of a stockholder’s notice as described above.
(iv) In no event may a Nominating Person provide Timely Notice with respect to a greater number of director candidates than are subject to election by stockholders at the applicable meeting. If the Corporation shall, subsequent to such notice, increase the number of directors subject to election at the meeting, such notice as to any additional nominees shall be due on the later of (i) the conclusion of the time period for Timely Notice, (ii) the date set forth in Section 2.5(b)(ii) or (iii) the tenth day following the date of public disclosure (as defined in Section 2.4) of such increase.
(c) To be in proper form for purposes of this Section 2.5, a stockholder’s notice to the Secretary of the Corporation shall set forth:
(i) As to each Nominating Person (as defined below), the Stockholder Information (as defined in Section 2.4(c)(i), except that for purposes of this Section 2.5, the term “Nominating Person” shall be substituted for the term “Proposing Person” in all places it appears in Section 2.4(c)(i));
| -411- |
(ii) As to each Nominating Person, any Disclosable Interests (as defined in Section 2.4(c)(ii), except that for purposes of this Section 2.5, the term “Nominating Person” shall be substituted for the term “Proposing Person” in all places it appears in Section 2.4(c)(ii) and the disclosure with respect to the business to be brought before the meeting in Section 2.4(c)(ii) shall be made with respect to the election of directors at the meeting); and
(iii) As to each candidate whom a Nominating Person proposes to nominate for election as a director, (A) all information with respect to such candidate for nomination that would be required to be set forth in a stockholder’s notice pursuant to this Section 2.5 if such candidate for nomination were a Nominating Person, (B) all information relating to such candidate for nomination that is required to be disclosed in a proxy statement or other filings required to be made in connection with solicitations of proxies for election of directors in a contested election pursuant to Section 14(a) under the Exchange Act (including such candidate’s written consent to being named in the proxy statement as a nominee and to serving as a director if elected), (C) a description of any direct or indirect material interest in any material contract or agreement between or among any Nominating Person, on the one hand, and each candidate for nomination or his or her respective associates or any other participants in such solicitation, on the other hand, including, without limitation, all information that would be required to be disclosed pursuant to Item 404 under Regulation S-K if such Nominating Person were the “registrant” for purposes of such rule and the candidate for nomination were a director or executive officer of such registrant and (D) a completed and signed questionnaire, representation and agreement as provided in Section 2.5(f).
For purposes of this Section 2.5, the term “Nominating Person” shall mean (i) the stockholder providing the notice of the nomination proposed to be made at the meeting, (ii) the beneficial owner or beneficial owners, if different, on whose behalf the notice of the nomination proposed to be made at the meeting is made, and (iii) any other participant in such solicitation.
(d) A stockholder providing notice of any nomination proposed to be made at a meeting shall further update and supplement such notice, if necessary, so that the information provided or required to be provided in such notice pursuant to this Section 2.5 shall be true and correct as of the record date for stockholders entitled to vote at the meeting and as of the date that is ten (10) business days prior to the meeting or any adjournment or postponement thereof, and such update and supplement shall be delivered to, or mailed and received by, the Secretary of the Corporation at the principal executive offices of the Corporation not later than five (5) business days after the record date for stockholders entitled to vote at the meeting (in the case of the update and supplement required to be made as of such record date), and not later than eight (8) business days prior to the date for the meeting or, if practicable, any adjournment or postponement thereof (and, if not practicable, on the first practicable date prior to the date to which the meeting has been adjourned or postponed) (in the case of the update and supplement required to be made as of ten (10) business days prior to the meeting or any adjournment or postponement thereof). For the avoidance of doubt, the obligation to update and supplement as set forth in this paragraph or any other Section of these bylaws shall not limit the Corporation’s rights with respect to any deficiencies in any notice provided by a stockholder, extend any applicable deadlines hereunder or enable or be deemed to permit a stockholder who has previously submitted notice hereunder to amend or update any nomination or to submit any new nomination.
(e) In addition to the requirements of this Section 2.5 with respect to any nomination proposed to be made at a meeting, each Nominating Person shall comply with all applicable requirements of the Exchange Act with respect to any such nominations.
(f) To be eligible to be a candidate for election as a director of the Corporation at an annual or special meeting, a candidate must be nominated in the manner prescribed in Section 2.5 and the candidate for nomination, whether nominated by the Board or by a stockholder of record, must have previously delivered (in accordance with the time period prescribed for delivery in a notice to such candidate given by or on behalf of the Board), to the Secretary of the Corporation at the principal executive offices of the Corporation, (i) a completed written questionnaire (in a form provided by the Corporation) with respect to the background, qualifications, stock ownership and independence of such proposed nominee and (ii) a written representation and agreement (in form provided by the Corporation) that such candidate for nomination (A) is not and, if elected as a director during his or her term of office, will not become a party to (1) any agreement, arrangement or understanding with, and has not given and will not give any commitment or assurance to, any person or entity as to how such proposed nominee, if elected as a director of the Corporation, will act or vote on any issue or question (a “Voting Commitment”) or (2) any Voting Commitment that could limit or interfere with such proposed nominee’s ability to comply, if elected as a director of the Corporation, with such proposed nominee’s fiduciary duties under applicable law, (B) is not, and will not become a party to, any agreement, arrangement or understanding with any person or entity other than the Corporation with respect to any direct or indirect compensation or reimbursement for service as a director that has not been disclosed to the Corporation and (C) if elected as a director of the Corporation, will comply with all applicable corporate governance, conflict of interest, confidentiality, stock ownership and trading and other policies and guidelines of the Corporation applicable to directors and in effect during such person’s term in office as a director (and, if requested by any candidate for nomination, the Secretary of the Corporation shall provide to such candidate for nomination all such policies and guidelines then in effect).
| -412- |
(g) The Board may also require any proposed candidate for nomination as a Director to furnish such other information as may reasonably be requested by the Board in writing prior to the meeting of stockholders at which such candidate’s nomination is to be acted upon in order for the Board to determine the eligibility of such candidate for nomination to be an independent director of the Corporation in accordance with the Corporation’s corporate governance guidelines.
(h) A candidate for nomination as a director shall further update and supplement the materials delivered pursuant to this Section 2.5, if necessary, so that the information provided or required to be provided pursuant to this Section 2.5 shall be true and correct as of the record date for stockholders entitled to vote at the meeting and as of the date that is ten (10) business days prior to the meeting or any adjournment or postponement thereof, and such update and supplement shall be delivered to, or mailed and received by, the Secretary of the Corporation at the principal executive offices of the Corporation (or any other office specified by the Corporation in any public announcement) not later than five (5) business days after the record date for stockholders entitled to vote at the meeting (in the case of the update and supplement required to be made as of such record date), and not later than eight (8) business days prior to the date for the meeting or, if practicable, any adjournment or postponement thereof (and, if not practicable, on the first practicable date prior to the date to which the meeting has been adjourned or postponed) (in the case of the update and supplement required to be made as of ten (10) business days prior to the meeting or any adjournment or postponement thereof). For the avoidance of doubt, the obligation to update and supplement as set forth in this paragraph or any other Section of these bylaws shall not limit the Corporation’s rights with respect to any deficiencies in any materials delivered pursuant to this Section 2.5 by a candidate for director, extend any applicable deadlines hereunder or enable or be deemed to permit a stockholder who has previously submitted notice hereunder to amend or update any proposal or to submit any new proposal, including by changing or adding nominees, matters, business or resolutions proposed to amend or update any nomination or to submit any new nomination.
(i) No candidate shall be eligible for nomination as a director of the Corporation unless such candidate for nomination and the Nominating Person seeking to place such candidate’s name in nomination has complied with this Section 2.5. The presiding officer at the meeting shall, if the facts warrant, determine that a nomination was not properly made in accordance with Section 2.5, and if he or she should so determine, he or she shall so declare such determination to the meeting, the defective nomination shall be disregarded and any ballots cast for the candidate in question (but in the case of any form of ballot listing other qualified nominees, only the votes cast for the nominee in question) shall be void and of no force or effect.
(j) Notwithstanding anything in these bylaws to the contrary, no candidate for nomination shall be eligible to be seated as a director of the Corporation unless nominated and elected in accordance with Section 2.5.
2.6 Notice of Stockholders’ Meetings. Unless otherwise provided by law, the Certificate of Incorporation or these bylaws, the notice of any meeting of stockholders shall be sent or otherwise given in accordance with Section 8.1 not less than ten (10) nor more than sixty (60) days before the date of the meeting to each stockholder entitled to vote at such meeting. The notice shall specify the place, if any, date and time of the meeting, the means of remote communication, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting, and, in the case of a special meeting, the purpose or purposes for which the meeting is called.
2.7 Quorum; Adjournment. Unless otherwise provided by law, the Certificate of Incorporation or these bylaws, the holders of a majority in voting power of the stock issued and outstanding and entitled to vote, present in person, or by remote communication, if applicable, or represented by proxy, shall constitute a quorum for the transaction of business at all meetings of the stockholders. A quorum, once established at a meeting, shall not be broken by the withdrawal of enough votes to leave less than a quorum. If, however, a quorum is not present or represented at any meeting of the stockholders, then either (i) the person presiding over the meeting or (ii) a majority in voting power of the stockholders entitled to vote at the meeting, present in person, or by remote communication, if applicable, or represented by proxy, shall have power to recess the meeting or adjourn the meeting from time to time in the manner provided in Section 2.8 until a quorum is present or represented. At any recessed or adjourned meeting at which a quorum is present or represented, any business may be transacted that might have been transacted at the meeting as originally noticed.
| -413- |
2.8 Adjourned Meeting; Notice. When a meeting is adjourned to another time or place, unless these bylaws otherwise require, notice need not be given of the adjourned meeting if the time, place, if any, thereof, and the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such adjourned meeting are announced at the meeting at which the adjournment is taken. At any adjourned meeting, the Corporation may transact any business which might have been transacted at the original meeting. If the adjournment is for more than thirty (30) days, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting. If after the adjournment a new record date for determination of stockholders entitled to vote is fixed for the adjourned meeting, the Board shall fix as the record date for determining stockholders entitled to notice of such adjourned meeting the same or an earlier date as that fixed for determination of stockholders entitled to vote at the adjourned meeting, and shall give notice of the adjourned meeting to each stockholder of record entitled to vote at such meeting as of the record date so fixed for notice of such adjourned meeting.
2.9 Conduct of Business. The date and time of the opening and the closing of the polls for each matter upon which the stockholders will vote at a meeting shall be announced at the meeting by the person presiding over the meeting. The Board may adopt by resolution such rules and regulations for the conduct of the meeting of stockholders as it shall deem appropriate. At every meeting of the stockholders, the Chairperson of the Board, or in his or her absence or inability to act, the Chief Executive Officer, or in his or her absence or inability to act, the officer or director whom the Board shall appoint, shall act as chairperson of, and preside at the meeting. Except to the extent inconsistent with such rules and regulations as adopted by the Board, the person presiding over any meeting of stockholders shall have the right and authority to convene and (for any or no reason) to recess and/or adjourn the meeting, to prescribe such rules, regulations and procedures (which need not be in writing) and to do all such acts as, in the judgment of such presiding person, are appropriate for the proper conduct of the meeting. Such rules, regulations or procedures, whether adopted by the Board or prescribed by the person presiding over the meeting, may include, without limitation, the following: (i) the establishment of an agenda or order of business for the meeting; (ii) rules and procedures for maintaining order at the meeting and the safety of those present (including, without limitation, rules and procedures for removal of disruptive persons from the meeting); (iii) limitations on attendance at or participation in the meeting to stockholders entitled to vote at the meeting, their duly authorized and constituted proxies or such other persons as the person presiding over the meeting shall determine; (iv) restrictions on entry to the meeting after the time fixed for the commencement thereof; and (v) limitations on the time allotted to questions or comments by participants. The presiding person at any meeting of stockholders, in addition to making any other determinations that may be appropriate to the conduct of the meeting (including, without limitation, determinations with respect to the administration and/or interpretation of any of the rules, regulations or procedures of the meeting, whether adopted by the Board or prescribed by the person presiding over the meeting), shall, if the facts warrant, determine and declare to the meeting that a matter of business was not properly brought before the meeting and if such presiding person should so determine, such presiding person shall so declare to the meeting and any such matter or business not properly brought before the meeting shall not be transacted or considered. Unless and to the extent determined by the Board or the person presiding over the meeting, meetings of stockholders shall not be required to be held in accordance with the rules of parliamentary procedure.
2.10 Voting. Except as may be otherwise provided in the Certificate of Incorporation, these bylaws or the DGCL, each stockholder shall be entitled to one (1) vote for each share of capital stock held by such stockholder.
Except as otherwise provided by the Certificate of Incorporation, at all duly called or convened meetings of stockholders at which a quorum is present, for the election of directors, a plurality of the votes cast shall be sufficient to elect a director. Except as otherwise provided by the Certificate of Incorporation, these bylaws, the rules or regulations of any stock exchange applicable to the Corporation, or applicable law or pursuant to any regulation applicable to the Corporation or its securities, each other matter presented to the stockholders at a duly called or convened meeting at which a quorum is present shall be decided by the affirmative vote of the holders of a majority in voting power of the votes cast (excluding abstentions and broker non-votes) on such matter.
2.11 Record Date for Stockholder Meetings and Other Purposes. In order that the Corporation may determine the stockholders entitled to notice of or to vote at any meeting of stockholders or any adjournment thereof, the Board may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board, and which record date shall, unless otherwise required by law, not be more than sixty (60) days nor less than ten (10) days before the date of such meeting. If the Board so fixes a date, such date shall also be the record date for determining the stockholders entitled to vote at such meeting unless the Board determines, at the time it fixes such record date, that a later date on or before the date of the meeting shall be the date for making such determination. If no record date is fixed by the Board, the record date for determining stockholders entitled to notice of or to vote at a meeting of stockholders shall be the close of business on the next day preceding the day on which notice is first given, or, if notice is waived, at the close of business on the day next preceding the day on which the meeting is held. A determination of stockholders of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Board may fix a new record date for determination of stockholders entitled to vote at the adjourned meeting; and in such case shall also fix as the record date for stockholders entitled to notice of such adjourned meeting the same or an earlier date as that fixed for determination of stockholders entitled to vote in accordance herewith at the adjourned meeting.
| -414- |
In order that the Corporation may determine the stockholders entitled to receive payment of any dividend or other distribution or allotment or any rights or the stockholders entitled to exercise any rights in respect of any change, conversion or exchange of capital stock, or for the purposes of any other lawful action, the Board may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted, and which record date shall be not more than sixty (60) days prior to such action. If no record date is fixed, the record date for determining stockholders for any such purpose shall be at the close of business on the day on which the Board adopts the resolution relating thereto.
2.12 Proxies. Each stockholder entitled to vote at a meeting of stockholders may authorize another person or persons to act for such stockholder by proxy authorized by an instrument in writing or by a transmission permitted by law filed in accordance with the procedure established for the meeting, but no such proxy shall be voted or acted upon after three (3) years from its date, unless the proxy provides for a longer period. The revocability of a proxy that states on its face that it is irrevocable shall be governed by the provisions of Section 212 of the DGCL. A proxy may be in the form of an electronic transmission which sets forth or is submitted with information from which it can be determined that the transmission was authorized by the stockholder.
2.13 List of Stockholders Entitled to Vote. The Corporation shall prepare, at least ten (10) days before every meeting of stockholders, a complete list of the stockholders entitled to vote at the meeting (provided, however, that if the record date for determining the stockholders entitled to vote is less than ten (10) days before the date of the meeting, the list shall reflect the stockholders entitled to vote as of the tenth (10th) day before the meeting date), arranged in alphabetical order, and showing the address of each stockholder and the number of shares registered in the name of each stockholder. The Corporation shall not be required to include electronic mail addresses or other electronic contact information on such list. Such list shall be open to the examination of any stockholder, for any purpose germane to the meeting for a period of at least ten (10) days prior to the meeting: (i) on a reasonably accessible electronic network, provided that the information required to gain access to such list is provided with the notice of the meeting, or (ii) during ordinary business hours, at the Corporation’s principal executive office. In the event that the Corporation determines to make the list available on an electronic network, the Corporation may take reasonable steps to ensure that such information is available only to stockholders of the Corporation. If the meeting is to be held at a place, then the list shall be produced and kept at the time and place of the meeting during the whole time thereof, and may be inspected by any stockholder who is present. If the meeting is to be held solely by means of remote communication, then the list shall also be open to the examination of any stockholder during the whole time of the meeting on a reasonably accessible electronic network, and the information required to access such list shall be provided with the notice of the meeting. Such list shall presumptively determine the identity of the stockholders entitled to vote at the meeting and the number of shares held by each of them. Except as otherwise provided by law, the stock ledger shall be the only evidence as to who are the stockholders entitled to examine the list of stockholders required by this Section 2.13 or to vote in person or by proxy at any meeting of stockholders.
2.14 Inspectors of Election. Before any meeting of stockholders, the Corporation shall appoint an inspector or inspectors of election to act at the meeting or its adjournment and make a written report thereof. The Corporation may designate one or more persons as alternate inspectors to replace any inspector who fails to act. If any person appointed as inspector or any alternate fails to appear or fails or refuses to act, then the person presiding over the meeting shall appoint a person to fill that vacancy.
Such inspectors shall:
(i) determine the number of shares outstanding and the voting power of each, the number of shares represented at the meeting and the validity of any proxies and ballots;
(ii) count all votes or ballots;
(iii) count and tabulate all votes;
| -415- |
(iv) determine and retain for a reasonable period a record of the disposition of any challenges made to any determination by the inspector(s); and
(v) certify its or their determination of the number of shares represented at the meeting and its or their count of all votes and ballots.
Each inspector, before entering upon the discharge of the duties of inspector, shall take and sign an oath faithfully to execute the duties of inspection with strict impartiality and according to the best of such inspector’s ability. Any report or certificate made by the inspectors of election is prima facie evidence of the facts stated therein. The inspectors of election may appoint such persons to assist them in performing their duties as they determine.
2.15 Delivery to the Corporation. Whenever this Article II requires one or more persons (including a record or beneficial owner of stock) to deliver a document or information to the Corporation or any officer, employee or agent thereof (including any notice, request, questionnaire, revocation, representation or other document or agreement), such document or information shall be in writing exclusively (and not in an electronic transmission) and shall be delivered exclusively by hand (including, without limitation, overnight courier service) or by certified or registered mail, return receipt requested, and the Corporation shall not be required to accept delivery of any document not in such written form or so delivered. For the avoidance of doubt, the Corporation expressly opts out of Section 116 of the DGCL with respect to the delivery of information and documents to the Corporation required by this Article II.
2.16 No Stockholder Action by Written Consent Without a Meeting. Any action required or permitted to be taken by the stockholders of the Corporation must be effected at an annual or special meeting of stockholders of the Corporation, and may not be taken by written consent in lieu of a meeting. Notwithstanding the foregoing, any action required or permitted to be taken by the holders of any series of preferred stock of the Corporation, voting separately as a series or separately as a class with one or more other such series, may be taken without a meeting, without prior notice and without a vote, to the extent expressly so provided by the applicable certificate of designation relating to such series of preferred stock of the Corporation, if a consent or consents in writing, setting forth the action so taken, shall be signed by the holders of outstanding shares of the relevant series of preferred stock of the Corporation having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted and shall be delivered to the Corporation in accordance with the applicable provisions of the DGCL.
Article III - Directors
3.1 Powers. Except as otherwise provided by the Certificate of Incorporation or the DGCL, the business and affairs of the Corporation shall be managed by or under the direction of the Board.
3.2 Number of Directors. Subject to the Certificate of Incorporation, the total number of directors constituting the Board shall be determined from time to time by resolution of the Board. No reduction of the authorized number of directors shall have the effect of removing any director before that director’s term of office expires.
3.3 Election, Qualification and Term of Office of Directors. Except as provided in Section 3.4, and subject to the Certificate of Incorporation, each director, including a director elected to fill a vacancy or newly created directorship, shall hold office until the expiration of the term of the class, if any, for which elected and until such director’s successor is elected and qualified or until such director’s earlier death, resignation, disqualification or removal. Directors need not be stockholders. The Certificate of Incorporation or these bylaws may prescribe qualifications for directors.
3.4 Resignation and Vacancies. Any director may resign at any time upon notice given in writing or by electronic transmission to the Corporation. The resignation shall take effect at the time specified therein or upon the happening of an event specified therein, and if no time or event is specified, at the time of its receipt. When one or more directors so resigns and the resignation is effective at a future date or upon the happening of an event to occur on a future date, a majority of the directors then in office, including those who have so resigned, shall have power to fill such vacancy or vacancies, the vote thereon to take effect when such resignation or resignations shall become effective, and each director so chosen shall hold office as provided in Section 3.3.
| -416- |
Unless otherwise provided in the Certificate of Incorporation or these bylaws, vacancies resulting from the death, resignation, disqualification or removal of any director, and newly created directorships resulting from any increase in the authorized number of directors shall be filled only by a majority of the directors then in office, although less than a quorum, or by a sole remaining director. Any director elected in accordance with the preceding sentence shall hold office for the remainder of the full term of the director for which the vacancy was created or occurred and until such director’s successor shall have been elected and qualified.
3.5 Place of Meetings; Meetings by Telephone. The Board may hold meetings, both regular and special, either within or outside the State of Delaware.
Unless otherwise restricted by the Certificate of Incorporation or these bylaws, members of the Board, or any committee designated by the Board, may participate in a meeting of the Board, or any committee, by means of conference telephone or other communications equipment by means of which all persons participating in the meeting can hear each other, and such participation in a meeting pursuant to this bylaw shall constitute presence in person at the meeting.
3.6 Regular Meetings. Regular meetings of the Board may be held within or outside the State of Delaware and at such time and at such place as which has been designated by the Board and publicized among all directors, either orally or in writing, by telephone, including a voice-messaging system or other system designed to record and communicate messages, facsimile, telegraph or telex, or by electronic mail or other means of electronic transmission. No further notice shall be required for regular meetings of the Board.
3.7 Special Meetings; Notice. Special meetings of the Board for any purpose or purposes may be called at any time by the Chairperson of the Board, the Chief Executive Officer or a majority of the total number of directors constituting the Board.
Notice of the time and place of special meetings shall be:
(i) delivered personally by hand, by courier or by telephone;
(ii) sent by United States first-class mail, postage prepaid;
(iii) sent by facsimile or electronic mail; or
(iv) sent by other means of electronic transmission,
directed to each director at that director’s address, telephone number, facsimile number or electronic mail address, or other address for electronic transmission, as the case may be, as shown on the Corporation’s records.
If the notice is (i) delivered personally by hand, by courier or by telephone, (ii) sent by facsimile or electronic mail, or (iii) sent by other means of electronic transmission, it shall be delivered or sent at least twenty-four (24) hours before the time of the holding of the meeting. If the notice is sent by U.S. mail, it shall be deposited in the U.S. mail at least four (4) days before the time of the holding of the meeting. The notice need not specify the place of the meeting (if the meeting is to be held at the Corporation’s principal executive office) nor the purpose of the meeting.
3.8 Quorum. At all meetings of the Board, unless otherwise provided by the Certificate of Incorporation, a majority of the total number of directors shall constitute a quorum for the transaction of business. The vote of a majority of the directors present at any meeting at which a quorum is present shall be the act of the Board, except as may be otherwise specifically provided by statute, the Certificate of Incorporation or these bylaws. If a quorum is not present at any meeting of the Board, then the directors present thereat may adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum is present.
3.9 Board Action without a Meeting. Unless otherwise restricted by the Certificate of Incorporation or these bylaws, any action required or permitted to be taken at any meeting of the Board, or of any committee thereof, may be taken without a meeting if all members of the Board or committee, as the case may be, consent thereto in writing or by electronic transmission. After an action is taken, the consent or consents relating thereto shall be filed with the minutes of the proceedings of the Board, or the committee thereof, in the same paper or electronic form as the minutes are maintained. Such action by written consent or consent by electronic transmission shall have the same force and effect as a unanimous vote of the Board.
| -417- |
3.10 Fees and Compensation of Directors. Unless otherwise restricted by the Certificate of Incorporation or these bylaws, the Board, or a designated committee thereof, shall have the authority to fix the compensation, including fees and reimbursement of expenses, of directors for services to the Corporation, provided that directors who are serving the Corporation as employees and who receive compensation for their services as such shall not receive any salary or other compensation for their services as directors of the Corporation.
3.11 Removal of Directors. Subject to the special rights of the holders of one or more outstanding series of Preferred Stock to elect directors, the Board of Directors or any individual director may be removed from office at any time, but only for cause and only by the affirmative vote of the holders of at least two-thirds (66 and 2/3%) of the voting power of all the then outstanding shares of voting stock of the Corporation entitled to vote at an election of directors.
Article IV - Committees
4.1 Committees of Directors. The Board may designate one (1) or more committees, each committee to consist of one (1) or more of the directors of the Corporation. The Board may designate one (1) or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of the committee. In the absence or disqualification of a member of a committee, the member or members thereof present at any meeting and not disqualified from voting, whether or not such member or members constitute a quorum, may unanimously appoint another member of the Board to act at the meeting in the place of any such absent or disqualified member. Any such committee, to the extent provided in the resolution of the Board or in these bylaws, shall have and may exercise all the powers and authority of the Board in the management of the business and affairs of the Corporation, and may authorize the seal of the Corporation to be affixed to all papers that may require it; but no such committee shall have the power or authority to (i) approve or adopt, or recommend to the stockholders, any action or matter expressly required by the DGCL to be submitted to stockholders for approval, or (ii) adopt, amend or repeal any bylaw of the Corporation.
4.2 Committee Minutes. Each committee shall keep regular minutes of its meetings and report the same to the Board when required.
4.3 Meetings and Actions of Committees. Meetings and actions of committees shall be governed by, and held and taken in accordance with, the provisions of:
(i) Section 3.5 (Place of Meetings; Meetings by Telephone);
(ii) Section 3.6 (Regular Meetings);
(iii) Section 3.7 (Special Meetings; Notice);
(iv) Section 3.9 (Board Action Without a Meeting); and
(v) Section 7.13 (Waiver of Notice),
with such changes in the context of those bylaws as are necessary to substitute the committee and its members for the Board and its members; provided, however, that:
(i) the time of regular meetings of committees may be determined either by resolution of the Board or by resolution of the committee;
(ii) special meetings of committees may also be called by resolution of the Board or the chairperson of the applicable committee; and
(iii) the Board may adopt rules for the governance of any committee to override the provisions that would otherwise apply to the committee pursuant to this Section 4.3, provided that such rules do not violate the provisions of the Certificate of Incorporation or applicable law.
| -418- |
4.4 Subcommittees. Unless otherwise provided in the Certificate of Incorporation, these bylaws or the resolutions of the Board designating the committee, a committee may create one (1) or more subcommittees, each subcommittee to consist of one (1) or more members of the committee, and delegate to a subcommittee any or all of the powers and authority of the committee.
Article V - Officers
5.1 Officers. The officers of the Corporation shall include a Chief Executive Officer and a Secretary. The Corporation may also have, at the discretion of the Board, a Chairperson of the Board, a Vice Chairperson of the Board, a Chief Financial Officer, a Treasurer, one (1) or more Vice Presidents, one (1) or more Assistant Vice Presidents, one (1) or more Assistant Treasurers, one (1) or more Assistant Secretaries, and any such other officers as may be appointed in accordance with the provisions of these bylaws. Any number of offices may be held by the same person. No officer need be a stockholder or director of the Corporation.
5.2 Appointment of Officers. The Board shall appoint the officers of the Corporation, except such officers as may be appointed in accordance with the provisions of Section 5.3.
5.3 Subordinate Officers. The Board may appoint, or empower the Chief Executive Officer to appoint, such other officers and agents as the business of the Corporation may require. Each of such officers and agents shall hold office for such period, have such authority, and perform such duties as are provided in these bylaws or as the Board may from time to time determine.
5.4 Removal and Resignation of Officers. Subject to the rights, if any, of an officer under any contract of employment any officer may be removed, either with or without cause, by the Board or, except in the case of an officer chosen by the Board, by any officer upon whom such power of removal may be conferred by the Board.
Any
officer may resign at any time by giving written notice to the Corporation. Any resignation shall take effect at the date of the receipt
of that notice or at any later time specified in that notice. Unless otherwise specified in the notice of resignation, the acceptance
of the resignation shall not be necessary to make it effective. Any resignation is without prejudice to the rights, if any, of the Corporation
under any contract to which the officer is a party.
5.5 Vacancies in Offices. Any vacancy occurring in any office of the Corporation shall be filled by the Board or as provided in Section 5.2.
5.6 Representation of Shares of Other Corporations. The Chairperson of the Board, the Chief Executive Officer, or any other person authorized by the Board or the Chief Executive Officer, is authorized to vote, represent and exercise on behalf of this Corporation all rights incident to any and all shares or voting securities of any other corporation or other person standing in the name of this Corporation. The authority granted herein may be exercised either by such person directly or by any other person authorized to do so by proxy or power of attorney duly executed by such person having the authority.
5.7 Authority and Duties of Officers. All officers of the Corporation shall respectively have such authority and perform such duties in the management of the business of the Corporation as may be provided herein or designated from time to time by the Board and, to the extent not so provided, as generally pertain to their respective offices, subject to the control of the Board.
5.8 Compensation. The compensation of the officers of the Corporation for their services as such shall be fixed from time to time by or at the direction of the Board. An officer of the Corporation shall not be prevented from receiving compensation by reason of the fact that he or she is also a director of the Corporation.
Article VI - Records
A stock ledger consisting of one or more records in which the names of all of the Corporation’s stockholders of record, the address and number of shares registered in the name of each such stockholder, and all issuances and transfers of stock of the corporation are recorded in accordance with Section 224 of the DGCL shall be administered by or on behalf of the Corporation. Any records administered by or on behalf of the Corporation in the regular course of its business, including its stock ledger, books of account, and minute books, may be kept on, or by means of, or be in the form of, any information storage device, or method, or one or more electronic networks or databases (including one or more distributed electronic networks or databases), provided that the records so kept can be converted into clearly legible paper form within a reasonable time and, with respect to the stock ledger, that the records so kept (i) can be used to prepare the list of stockholders specified in Sections 219 and 220 of the DGCL, (ii) record the information specified in Sections 156, 159, 217(a) and 218 of the DGCL, and (iii) record transfers of stock as governed by Article 8 of the Uniform Commercial Code as adopted in the State of Delaware.
| -419- |
Article VII - General Matters
7.1 Execution of Corporate Contracts and Instruments. The Board, except as otherwise provided in these bylaws, may authorize any officer or officers, or agent or agents, to enter into any contract or execute any instrument in the name of and on behalf of the Corporation; such authority may be general or confined to specific instances.
7.2 Stock Certificates. The shares of the Corporation shall be represented by certificates or shall be uncertificated. Certificates for the shares of stock, if any, shall be in such form as is consistent with the Certificate of Incorporation and applicable law. Every holder of stock represented by a certificate shall be entitled to have a certificate signed by, or in the name of the Corporation by, any two officers authorized to sign stock certificates representing the number of shares registered in certificate form. The Chairperson of the Board, the Chief Executive Officer, the Treasurer, or the Secretary of the Corporation shall be specifically authorized to sign stock certificates. Any or all of the signatures on the certificate may be a facsimile. In case any officer, transfer agent or registrar who has signed or whose facsimile signature has been placed upon a certificate has ceased to be such officer, transfer agent or registrar before such certificate is issued, it may be issued by the Corporation with the same effect as if he or she were such officer, transfer agent or registrar at the date of issue.
7.3 Special Designation of Certificates. If the Corporation is authorized to issue more than one class of stock or more than one series of any class, then the powers, the designations, the preferences and the relative, participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights shall be set forth in full or summarized on the face or on the back of the certificate that the Corporation shall issue to represent such class or series of stock (or, in the case of uncertificated shares, set forth in a notice provided pursuant to Section 151 of the DGCL); provided, however, that except as otherwise provided in Section 202 of the DGCL, in lieu of the foregoing requirements, there may be set forth on the face of back of the certificate that the Corporation shall issue to represent such class or series of stock (or, in the case of any uncertificated shares, included in the aforementioned notice) a statement that the Corporation will furnish without charge to each stockholder who so requests the powers, the designations, the preferences and the relative, participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights.
7.4 Lost Certificates. Except as provided in this Section 7.4, no new certificates for shares shall be issued to replace a previously issued certificate unless the latter is surrendered to the Corporation and cancelled at the same time. The Corporation may issue a new certificate of stock or uncertificated shares in the place of any certificate theretofore issued by it, alleged to have been lost, stolen or destroyed, and the Corporation may require the owner of the lost, stolen or destroyed certificate, or such owner’s legal representative, to give the Corporation a bond sufficient to indemnify it against any claim that may be made against it on account of the alleged loss, theft or destruction of any such certificate or the issuance of such new certificate or uncertificated shares.
7.5 Shares Without Certificates. The Corporation may adopt a system of issuance, recordation and transfer of its shares of stock by electronic or other means not involving the issuance of certificates, provided the use of such system by the Corporation is permitted in accordance with applicable law.
7.6 Construction; Definitions. Unless the context requires otherwise, the general provisions, rules of construction and definitions in the DGCL shall govern the construction of these bylaws. Without limiting the generality of this provision, the singular number includes the plural and the plural number includes the singular.
7.7 Dividends. The Board, subject to any restrictions contained in either (i) the DGCL or (ii) the Certificate of Incorporation, may declare and pay dividends upon the shares of its capital stock. Dividends may be paid in cash, in property or in shares of the Corporation’s capital stock. The Board may set apart out of any of the funds of the Corporation available for dividends a reserve or reserves for any proper purpose and may abolish any such reserve. Such purposes shall include but not be limited to equalizing dividends, repairing or maintaining any property of the Corporation, and meeting contingencies.
7.8 Fiscal Year. The fiscal year of the Corporation shall be fixed by resolution of the Board and may be changed by the Board.
| -420- |
7.9 Seal. The Corporation may adopt a corporate seal, which shall be adopted and which may be altered by the Board. The Corporation may use the corporate seal by causing it or a facsimile thereof to be impressed or affixed or in any other manner reproduced.
7.10 Transfer of Stock. Shares of the stock of the Corporation shall be transferable in the manner prescribed by law and in these bylaws, subject to any restrictions on transfer and ownership (including any such restrictions imposed by these bylaws). Subject to compliance with any restrictions on transfer and ownership thereon (including any such restrictions imposed by these bylaws), shares of stock of the Corporation shall be transferred on the books of the Corporation only by the holder of record thereof or by such holder’s attorney duly authorized in writing, upon surrender to the Corporation of the certificate or certificates representing such shares endorsed by the appropriate person or persons (or by delivery of duly executed instructions with respect to uncertificated shares), with such evidence of the authenticity of such endorsement or execution, transfer, authorization and other matters as the Corporation may reasonably require, and accompanied by all necessary stock transfer stamps. No transfer of stock shall be valid as against the Corporation for any purpose until it shall have been entered in the stock records of the Corporation by an entry showing the names of the persons from and to whom it was transferred.
7.11 Registered Stockholders. The Corporation:
(i) shall be entitled to recognize the exclusive right of a person registered on its books as the owner of shares to receive dividends and to vote as such owner; and
(ii) shall not be bound to recognize any equitable or other claim to or interest in such share or shares on the part of another person, whether or not it shall have express or other notice thereof, except as otherwise provided by the laws of the State of Delaware.
7.12. Lockup.
(a) Subject to Section 7.12(b) and Section 7.12(f), the holders (the “Lockup Holders”) of shares of common stock, par value $0.0001 per share (the “common stock”), of the Corporation issued (i) as consideration pursuant to that certain Agreement and Plan of Merger, dated as of December 7, 2021 (the “Merger Agreement”), by and among Parent, Merger Sub, the Company and the Stockholders’ Representative (each as defined in the Merger Agreement) in connection with the business combination transaction contemplated thereby (the “Business Combination Transaction”), or (ii) upon the exercise, exchange, conversion, or settlement of any Exchanged Options, Exchanged RSU Awards, Assumed Warrants (such shares referred to in Section 7.12(a)(i), the “Merger Consideration Shares” and such shares referred to in Section 7.12(a)(ii), the “Assumption Shares”), may not Transfer any Merger Consideration Shares or Assumption Shares (and such Merger Consideration Shares and Assumption Shares shall be subject to the restrictions on Transfer as provided herein) until the end of the Lockup Period (the “Lockup”).
(b) The restrictions set forth in Section 7.12(a) shall not apply to:
(i) in the case of an entity, Transfers (A) to a stockholder, partner, member or affiliate of such entity, including, for the avoidance of doubt, any distributions by Aegis Capital Corp. (“Aegis”) of Merger Consideration Shares to various brokers; provided, however, that such Merger Consideration Shares shall continue to be governed by the terms of this Section 7.12 or (B) by virtue of the laws of the state of the entity’s organization and the entity’s organizational documents upon dissolution of the entity;
(ii) in the case of an individual, Transfers (A) by gift to members of the individual’s immediate family (as defined below) or to a trust, the beneficiary of which is a member of one of the individual’s immediate family, an affiliate of such person or to a charitable organization, (B) by virtue of laws of descent and distribution upon death of the individual, or (C) pursuant to a qualified domestic relations order or in connection with a divorce settlement;
(iii) the exercise of any options, warrants or other convertible securities to purchase shares of common stock (which exercises may be effected on a cashless basis to the extent the instruments representing such options or warrants permit exercises on a cashless basis); provided, that any shares of common stock issued upon such exercise shall be subject to the Lockup;
(iv) Transfers to the Corporation to satisfy tax withholding obligations pursuant to the Corporation’s equity incentive plans or arrangements;
| -421- |
(v) Transfers to the Corporation pursuant to any contractual arrangement in effect at the effective time of the Business Combination Transaction that provides for the repurchase by the Corporation or forfeiture of a Lockup Holder’s shares of common stock or options to purchase shares of common stock in connection with the termination of such Lockup Holder’s service to the Corporation;
(vi) the entry, by a Lockup Holder, at any time after the effective time of the Business Combination Transaction, of any trading plan providing for the sale of shares of common stock by such Lockup Holder, which trading plan meets the requirements of Rule 10b5-1(c) under the Exchange Act; provided, however, that such plan does not provide for, or permit, the sale of any shares of common stock during the Lockup and no public announcement or filing is voluntarily made or required regarding such plan during the Lockup;
(vii) transactions in the event of completion of a bona fide liquidation, dissolution, winding up, merger, consolidation, share exchange or other similar transaction that results in all shares of common stock being convertible for, or exchangeable into, cash, securities or other property; or
(viii) in connection with any bona fide mortgage, pledge or encumbrance to a financial institution in connection with any bona fide loan or debt transaction or enforcement thereunder, including foreclosure thereof.
(c) Notwithstanding the other provisions set forth in this Section 7.12, the Board may, by the vote of 80% of the total number of directors, determine to waive, amend, or repeal the Lockup obligations set forth herein.
(d) For purposes of this Section 7.12:
(i) the term “Lockup Period” means the period beginning on the closing date of the Business Combination Transaction and ending on the date that is 180 days after the date that the “Form S-1 Shelf” (as such term is defined in the form of Registration Rights Agreement attached as Exhibit D to the Merger Agreement) is declared effective by the Securities and Exchange Commission;
(ii) the term “Transfer” means, directly or indirectly, whether by merger, consolidation, division or otherwise, the (A) sale or assignment of, offer to sell, contract or agreement to sell, hypothecate, pledge, grant of any option to purchase or otherwise dispose of or agreement to dispose of, directly or indirectly, or establishment or increase of a put equivalent position or liquidation with respect to or decrease of a call equivalent position within the meaning of Section 16 of the Exchange Act with respect to, any security, (B) entry into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any security, whether any such transaction is to be settled by delivery of such securities, in cash or otherwise, or (C) public announcement of any intention to effect any transaction specified in clause (A) or (B).
(e) Until the expiration of the Lockup Period, in addition to any greater or additional vote that may be required by the Certificate of Incorporation, these bylaws, or applicable law, this Section 7.12 may not be altered, amended or repealed, and no provision inconsistent with this Section 7.12 may be adopted, (i) by the Board without the affirmative vote of 80% of the total number of directors, or (ii) by the stockholders without the affirmative vote of the holders of 80% in voting power of the outstanding shares of capital stock entitled to vote thereon.
(f) Notwithstanding anything to the contrary set forth in the other provisions of this Section 7.12, in no event shall any of the following be subject to the Lockup: (A) any shares of Class B common stock, par value $0.0001 per share (“Class B common stock”), of the Corporation, or any shares of any class or series of capital stock of the Corporation into which such shares of Class B common stock may be converted (including, without limitation, any shares of Class A common stock, par value $0.0001 per share (“Class A common stock”), into which such shares of Class B common stock may be converted in connection with the closing of the Business Combination Transaction (the “Conversion Shares”)) or any shares of common stock into which any such Conversion Shares may be reclassified, or (B) any shares of Class A common stock or common stock held by a holder who purchased such shares pursuant to a private placement in connection with the Business Combination Transaction, or (C) any warrants of the Corporation that are outstanding prior to the Business Combination Transaction (but not the Assumed Warrants) or any shares of capital stock of the Corporation into which they may be exercised or converted; provided, however, that for the avoidance of doubt, the securities identified in clauses (A) through (C) above may be subject to other contractual restrictions on transfer imposed in connection with the Merger Agreement and the Business Combination Transaction.
| -422- |
(g) These bylaws (including this Section 7.12) have been adopted prior to the Effective Time (as defined in the Merger Agreement), and the restrictions on Transfer set forth in this Section 7.12 shall apply in accordance with the terms hereof to all shares of capital stock of the Corporation subject hereto issued at or after the Effective Time (which shall include, for the avoidance of doubt, the Merger Consideration Shares and the Assumption Shares).
7.13 Waiver of Notice. Whenever notice is required to be given under any provision of the DGCL, the Certificate of Incorporation or these bylaws, a written waiver, signed by the person entitled to notice, or a waiver by electronic transmission by the person entitled to notice, whether before or after the time of the event for which notice is to be given, shall be deemed equivalent to notice. Attendance of a person at a meeting shall constitute a waiver of notice of such meeting, except when the person attends a meeting for the express purpose of objecting at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Neither the business to be transacted at, nor the purpose of, any regular or special meeting of the stockholders need be specified in any written waiver of notice or any waiver by electronic transmission unless so required by the Certificate of Incorporation or these bylaws.
Article VIII - Notice
8.1 Delivery of Notice; Notice by Electronic Transmission. Without limiting the manner by which notice otherwise may be given effectively to stockholders, any notice to stockholders given by the Corporation under any provisions of the DGCL, the Certificate of Incorporation, or these bylaws may be given in writing directed to the stockholder’s mailing address (or by electronic transmission directed to the stockholder’s electronic mail address, as applicable) as it appears on the records of the Corporation and shall be given (1) if mailed, when the notice is deposited in the U.S. mail, postage prepaid, (2) if delivered by courier service, the earlier of when the notice is received or left at such stockholder’s address or (3) if given by electronic mail, when directed to such stockholder’s electronic mail address unless the stockholder has notified the Corporation in writing or by electronic transmission of an objection to receiving notice by electronic mail. A notice by electronic mail must include a prominent legend that the communication is an important notice regarding the Corporation.
Without limiting the manner by which notice otherwise may be given effectively to stockholders, any notice to stockholders given by the Corporation under any provision of the DGCL, the Certificate of Incorporation or these bylaws shall be effective if given by a form of electronic transmission consented to by the stockholder to whom the notice is given. Any such consent shall be revocable by the stockholder by written notice or electronic transmission to the Corporation. Notwithstanding the provisions of this paragraph, the Corporation may give a notice by electronic mail in accordance with the first paragraph of this section without obtaining the consent required by this paragraph.
Any notice given pursuant to the preceding paragraph shall be deemed given:
(i) if by facsimile telecommunication, when directed to a number at which the stockholder has consented to receive notice;
(ii) if by a posting on an electronic network together with separate notice to the stockholder of such specific posting, upon the later of (A) such posting and (B) the giving of such separate notice; and
(iii) if by any other form of electronic transmission, when directed to the stockholder.
Notwithstanding the foregoing, a notice may not be given by an electronic transmission from and after the time that (1) the Corporation is unable to deliver by such electronic transmission two (2) consecutive notices given by the Corporation and (2) such inability becomes known to the Secretary or an Assistant Secretary of the Corporation or to the transfer agent, or other person responsible for the giving of notice; provided, however, that the inadvertent failure to discover such inability shall not invalidate any meeting or other action.
An affidavit of the Secretary or an Assistant Secretary of the Corporation or of the transfer agent or other agent of the Corporation that the notice has been given shall, in the absence of fraud, be prima facie evidence of the facts stated therein.
| -423- |
Article IX - Indemnification
9.1 Indemnification of Directors and Officers. The Corporation shall indemnify and hold harmless, to the fullest extent permitted by the DGCL as it presently exists or may hereafter be amended, any director or officer of the Corporation who was or is made or is threatened to be made a party or is otherwise involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative (a “Proceeding”) by reason of the fact that he or she, or a person for whom he or she is the legal representative, is or was a director or officer of the Corporation or, while serving as a director or officer of the Corporation, is or was serving at the request of the Corporation as a director, officer, employee or agent of another corporation or of a partnership (a “covered person”), joint venture, trust, enterprise or non-profit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses (including, without limitation, attorneys’ fees, judgments, fines, ERISA excise taxes or penalties, and amounts paid in settlement) reasonably incurred by such person in connection with any such Proceeding. Notwithstanding the preceding sentence, except as otherwise provided in Section 9.4, the Corporation shall be required to indemnify a person in connection with a Proceeding initiated by such person only if the Proceeding was authorized in the specific case by the Board.
9.2 Indemnification of Others. The Corporation shall also have the power to indemnify and hold harmless, to the fullest extent permitted by applicable law as it presently exists or may hereafter be amended, any employee or agent of the Corporation who was or is made or is threatened to be made a party or is otherwise involved in any Proceeding by reason of the fact that he or she, or a person for whom he or she is the legal representative, is or was an employee or agent of the Corporation or is or was serving at the request of the Corporation as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust, enterprise or non-profit entity, including service with respect to employee benefit plans, against all liability and loss suffered and expenses reasonably incurred by such person in connection with any such Proceeding.
9.3 Prepayment of Expenses. The Corporation shall to the fullest extent not prohibited by applicable law pay the expenses (including, without limitation, attorneys’ fees) incurred by any director or officer of the Corporation, and may also pay the expenses incurred by any employee or agent of the Corporation, in defending any Proceeding in advance of its final disposition; provided, however, that such payment of expenses in advance of the final disposition of the Proceeding shall be made only upon receipt of an undertaking by the person to repay all amounts advanced if it should be ultimately determined that the person is not entitled to be indemnified under this Article IX or otherwise.
9.4 Determination; Claim. If a claim for indemnification (following the final disposition of such Proceeding) under this Article IX is not paid in full within sixty (60) days, or a claim for advancement of expenses under this Article IX is not paid in full within thirty (30) days, after a written claim therefor has been received by the Corporation, the claimant may thereafter (but not before) file suit to recover the unpaid amount of such claim and, if successful in whole or in part, shall be entitled to be paid the expense of prosecuting such claim to the fullest extent permitted by law. In any such action the Corporation shall have the burden of proving that the claimant was not entitled to the requested indemnification or payment of expenses under applicable law.
9.5 Non-Exclusivity of Rights. The rights conferred on any person by this Article IX shall not be exclusive of any other rights which such person may have or hereafter acquire under any statute, provision of the Certificate of Incorporation, these bylaws, agreement, vote of stockholders or disinterested directors or otherwise.
9.6 Insurance. The Corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the Corporation, or is or was serving at the request of the Corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust enterprise or non-profit entity against any liability asserted against him or her and incurred by him or her in any such capacity, or arising out of his or her status as such, whether or not the Corporation would have the power to indemnify him or her against such liability under the provisions of the DGCL.
9.7 Other Indemnification. The Corporation’s obligation, if any, to indemnify or advance expenses to any person who was or is serving at its request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust, enterprise or non-profit entity shall be reduced by any amount such person actually collects as indemnification or advancement of expenses from such other corporation, partnership, joint venture, trust, enterprise or non-profit enterprise.
9.8 Continuation of Indemnification. The rights to indemnification and to prepayment of expenses provided by, or granted pursuant to, this Article X shall continue notwithstanding that the person has ceased to be a director or officer of the Corporation and shall inure to the benefit of the estate, heirs, executors, administrators, legatees and distributees of such person.
| -424- |
9.9 Amendment or Repeal; Interpretation. The provisions of this Article IX shall constitute a contract between the Corporation, on the one hand, and, on the other hand, each individual who serves or has served as a director or officer of the Corporation (whether before or after the adoption of these bylaws), in consideration of such person’s performance of such services, and pursuant to this Article IX the Corporation intends to be legally bound to each such current or former director or officer of the Corporation. With respect to current and former directors and officers of the Corporation, the rights conferred under this Article IX are present contractual rights and such rights are fully vested, and shall be deemed to have vested fully, immediately upon adoption of these bylaws. With respect to any directors or officers of the Corporation who commence service following adoption of these bylaws, the rights conferred under this provision shall be present contractual rights and such rights shall fully vest, and be deemed to have vested fully, immediately upon such director or officer commencing service as a director or officer of the Corporation. Any repeal or modification of the foregoing provisions of this Article IX shall not adversely affect any right or protection (i) hereunder of any person in respect of any act or omission occurring prior to the time of such repeal or modification or (ii) under any agreement providing for indemnification or advancement of expenses to an officer or director of the Corporation in effect prior to the time of such repeal or modification.
Any reference to an officer of the Corporation in this Article IX shall be deemed to refer exclusively to the Chief Executive Officer, and the Secretary of the Corporation, or other officer of the Corporation appointed by (x) the Board pursuant to Article V or (y) an officer to whom the Board has delegated the power to appoint officers pursuant to Article V, and any reference to an officer of any other corporation, partnership, joint venture, trust, employee benefit plan or other enterprise shall be deemed to refer exclusively to an officer appointed by the board of directors (or equivalent governing body) of such other entity pursuant to the certificate of incorporation and bylaws (or equivalent organizational documents) of such other corporation, partnership, joint venture, trust, employee benefit plan or other enterprise. The fact that any person who is or was an employee of the Corporation or an employee of any other corporation, partnership, joint venture, trust, employee benefit plan or other enterprise has been given or has used the title of “Vice President” or any other title that could be construed to suggest or imply that such person is or may be an officer of the Corporation or of such other corporation, partnership, joint venture, trust, employee benefit plan or other enterprise shall not result in such person being constituted as, or being deemed to be, an officer of the Corporation or of such other corporation, partnership, joint venture, trust, employee benefit plan or other enterprise for purposes of this Article IX.
Article XI- Amendments
The Board is expressly empowered to adopt, amend or repeal the bylaws of the Corporation. The stockholders also shall have power to adopt, amend or repeal the bylaws of the Corporation; provided, however, that such action by stockholders shall require, in addition to any other vote required by the Certificate of Incorporation, these bylaws or applicable law, the affirmative vote of the holders of at least two-thirds of the voting power of all the then-outstanding shares of voting stock of the Corporation with the power to vote generally in an election of directors, voting together as a single class.
Article XI- Definitions
As used in these bylaws, unless the context otherwise requires, the following terms shall have the following meanings:
An “electronic transmission” means any form of communication, not directly involving the physical transmission of paper, including the use of, or participation in, one or more electronic networks or databases (including one or more distributed electronic networks or databases), that creates a record that may be retained, retrieved and reviewed by a recipient thereof, and that may be directly reproduced in paper form by such a recipient through an automated process.
An “electronic mail” means an electronic transmission directed to a unique electronic mail address (which electronic mail shall be deemed to include any files attached thereto and any information hyperlinked to a website if such electronic mail includes the contact information of an officer or agent of the Corporation who is available to assist with accessing such files and information).
An “electronic mail address” means a destination, commonly expressed as a string of characters, consisting of a unique user name or mailbox (commonly referred to as the “local part” of the address) and a reference to an internet domain (commonly referred to as the “domain part” of the address), whether or not displayed, to which electronic mail can be sent or delivered.
The term “person” means any individual, general partnership, limited partnership, limited liability company, corporation, trust, business trust, joint stock company, joint venture, unincorporated association, cooperative or association or any other legal entity or organization of whatever nature, and shall include any successor (by merger or otherwise) of such entity.
The undersigned hereby certifies that he is the duly elected, qualified, and acting [Secretary] of Aerami Therapeutics Holdings, Inc., a Delaware corporation (the “Corporation”), and that the foregoing bylaws were approved on , 2022, effective as of , 2022, by the Corporation’s board of directors.
| -425- |
IN WITNESS WHEREOF, the undersigned has hereunto set his hand this _______________ day of _______________ , 2022.
| [Name] | |
| [Full Title of Secretary] |
| -426- |
Annex D
AERAMI THERAPEUTICS HOLDINGS, INC.
2022 INCENTIVE AWARD PLAN
ARTICLE I
PURPOSE
The Plan’s purpose is to enhance the Company’s ability to attract, retain and motivate persons who make (or are expected to make) important contributions to the Company by providing these individuals with equity ownership and/or equity-linked compensation opportunities. Capitalized terms used in the Plan are defined in Article XI.
ARTICLE II
ELIGIBILITY
Service Providers are eligible to be granted Awards under the Plan, subject to the limitations described herein.
ARTICLE III
ADMINISTRATION AND DELEGATION
3.1 Administration. The Plan is administered by the Administrator. The Administrator has authority to determine which Service Providers receive Awards, grant Awards and set Award terms and conditions, subject to the conditions and limitations in the Plan. The Administrator also has the authority to take all actions and make all determinations under the Plan, to interpret the Plan and Award Agreements, and to adopt, amend and repeal Plan administrative rules, guidelines and practices as it deems advisable. The Administrator may correct defects and ambiguities, supply omissions and reconcile inconsistencies in the Plan or any Award as it deems necessary or appropriate to administer the Plan and any Awards. The Administrator’s determinations under the Plan are in its sole discretion and will be final and binding on all persons having or claiming any interest in the Plan or any Award.
3.2 Appointment of Committees. To the extent Applicable Laws permit, the Board or the Administrator may delegate any or all of its powers under the Plan to one or more Committees or officers of the Company or any of its Subsidiaries. The Board or the Administrator may abolish any Committee or re-vest in itself any previously delegated authority at any time.
ARTICLE IV
STOCK AVAILABLE FOR AWARDS
4.1 Number of Shares. Subject to adjustment under Article VIII and the terms of this Article IV, Awards may be made under the Plan covering up to the Overall Share Limit. Shares issued under the Plan may consist of authorized but unissued Shares or Shares purchased on the open market or treasury Shares.
4.2 Share Recycling. If all or any part of an Award expires, lapses or is terminated, exchanged for or settled in cash, surrendered, repurchased, canceled without having been fully exercised or forfeited, in any case, in a manner that results in the Company acquiring Shares covered by the Award at a price not greater than the price (as adjusted to reflect any Equity Restructuring) paid by the Participant for such Shares or not issuing any Shares covered by the Award, the unused Shares covered by the Award will, as applicable, become or again be available for Award grants under the Plan. Further, Shares delivered (either by actual delivery or attestation) to the Company by a Participant to satisfy the applicable exercise or purchase price of an Award and/or to satisfy any applicable tax withholding obligation with respect to an Award (including Shares retained by the Company from the Award being exercised or purchased and/or creating the tax obligation) will, as applicable, become or again be available for Award grants under the Plan. The payment of Dividend Equivalents in cash in conjunction with any outstanding Awards shall not count against the Overall Share Limit. Notwithstanding anything to the contrary contained herein, the following Shares shall not be added to the Shares authorized for grant under Section 4.1 and shall not be available for future grants of Awards: (a) Shares subject to a Stock Appreciation Right that are not issued in connection with the stock settlement of the Stock Appreciation Right on exercise thereof; and (b) Shares purchased on the open market with the cash proceeds from the exercise of Options.
| -427- |
4.3 Incentive Stock Option Limitations. Notwithstanding anything to the contrary herein, no more than [ ] Shares may be issued pursuant to the exercise of Incentive Stock Options.
4.4 Substitute Awards. In connection with an entity’s merger or consolidation with the Company or the Company’s acquisition of an entity’s property or stock, the Administrator may grant Awards in substitution for any options or other stock or stock-based awards granted before such merger or consolidation by such entity or its affiliate. Substitute Awards may be granted on such terms as the Administrator deems appropriate, notwithstanding limitations on Awards in the Plan. Substitute Awards will not count against the Overall Share Limit (nor shall Shares subject to a Substitute Award be added to the Shares available for Awards under the Plan as provided above), except that Shares acquired by exercise of substitute Incentive Stock Options will count against the maximum number of Shares that may be issued pursuant to the exercise of Incentive Stock Options under the Plan. Additionally, in the event that a company acquired by the Company or any Subsidiary or with which the Company or any Subsidiary combines has shares available under a pre-existing plan approved by stockholders and not adopted in contemplation of such acquisition or combination, the shares available for grant pursuant to the terms of such pre-existing plan (as adjusted, to the extent appropriate, using the exchange ratio or other adjustment or valuation ratio or formula used in such acquisition or combination to determine the consideration payable to the holders of common stock of the entities party to such acquisition or combination) may be used for Awards under the Plan and shall not reduce the Shares authorized for grant under the Plan (and Shares subject to such Awards shall not be added to the Shares available for Awards under the Plan as provided above); provided that Awards using such available shares shall not be made after the date awards or grants could have been made under the terms of the pre-existing plan, absent the acquisition or combination, and shall only be made to individuals who were not Service Providers prior to such acquisition or combination.
4.5 Non-Employee Director Compensation. Notwithstanding any provision to the contrary in the Plan, the Administrator may establish compensation for Non-Employee Directors from time to time, subject to the limitations in the Plan. The Administrator will from time to time determine the terms, conditions and amounts of all such Non-Employee Director compensation in its discretion and pursuant to the exercise of its business judgment, taking into account such factors, circumstances and considerations as it shall deem relevant from time to time, provided that the sum of any cash compensation, or other compensation, and the value (determined as of the grant date in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718, or any successor thereto) of Awards granted to a Non-Employee Director as compensation for services as a Non-Employee Director during any fiscal year of the Company may not exceed $750,000, increased to $1,000,000 in the fiscal year in which the Plan’s effective date occurs or in the fiscal year of a Non-Employee Director’s initial service as a Non-Employee Director (in either case, the “NED Limit”), which limit will not apply to the compensation of a Non-Employee Director who serves in any capacity in addition to that of Non-Employee Director for which he or she receives additional compensation. For the avoidance of doubt, the value of any cash or equity-based compensation granted prior to the Plan’s effective date shall not count against the NED Limit. The Administrator may make exceptions to a NED Limit for individual Non-Employee Directors in extraordinary circumstances, as the Administrator may determine in its discretion, provided that the Non-Employee Director receiving such additional compensation may not participate in the decision to award such compensation or in other contemporaneous compensation decisions involving Non-Employee Directors.
ARTICLE V
STOCK OPTIONS AND STOCK APPRECIATION RIGHTS
5.1 General. The Administrator may grant Options or Stock Appreciation Rights to Service Providers subject to the limitations in the Plan, including any limitations in the Plan that apply to Incentive Stock Options. The Administrator will determine the number of Shares covered by each Option and Stock Appreciation Right, the exercise price of each Option and Stock Appreciation Right and the conditions and limitations applicable to the exercise of each Option and Stock Appreciation Right. A Stock Appreciation Right will entitle the Participant (or other person entitled to exercise the Stock Appreciation Right) to receive from the Company upon exercise of the exercisable portion of the Stock Appreciation Right an amount determined by multiplying the excess, if any, of the Fair Market Value of one Share on the date of exercise over the exercise price per Share of the Stock Appreciation Right by the number of Shares with respect to which the Stock Appreciation Right is exercised, subject to any limitations of the Plan or that the Administrator may impose and payable in cash, Shares valued at Fair Market Value, or a combination of the two as the Administrator may determine or provide in the Award Agreement.
| -428- |
5.2 Exercise Price. The Administrator will establish each Option’s and Stock Appreciation Right’s exercise price and specify the exercise price in the Award Agreement. The exercise price will not be less than 100% of the Fair Market Value on the grant date of the Option or Stock Appreciation Right. Notwithstanding the foregoing, in the case of an Option or a Stock Appreciation Right that is a Substitute Award, the exercise price per share of the Shares subject to such Option or Stock Appreciation Right, as applicable, may be less than the Fair Market Value per share on the date of grant; provided that the exercise price of any Substitute Award shall be determined in accordance with the applicable requirements of Sections 424 and 409A of the Code.
5.3 Duration. Each Option or Stock Appreciation Right will be exercisable at such times and as specified in the Award Agreement, provided that, unless otherwise determined and expressly provided by the Administrator, the term of an Option or Stock Appreciation Right will not exceed ten years. Notwithstanding the foregoing, to the extent permitted under Applicable Laws, if the Participant, prior to the end of the term of an Option or Stock Appreciation Right (i) violates the non-competition, non-solicitation, confidentiality or other similar restrictive covenant provisions of any employment contract, confidentiality and nondisclosure agreement or other agreement between the Participant and the Company or any of its Subsidiaries or (ii) commits a material violation or breach of a Company policy that relates to discrimination, harassment, retaliation or that is otherwise customarily punishable by termination of employment, the right of the Participant and the Participant’s transferees to exercise any Option or Stock Appreciation Right issued to the Participant shall terminate immediately upon such violation or breach, unless the Company otherwise determines.
5.4 Exercise. Options and Stock Appreciation Rights may be exercised by delivering to the Company a written notice of exercise, in a form the Administrator approves (which may be electronic), signed by the person authorized to exercise the Option or Stock Appreciation Right, together with, as applicable, payment in full (i) as specified in Section 5.5 for the number of Shares for which the Award is exercised and (ii) as specified in Section 9.5 for any applicable taxes. Unless the Administrator otherwise determines, an Option or Stock Appreciation Right may not be exercised for a fraction of a Share.
5.5 Payment Upon Exercise. Subject to Section 10.8, any Company insider trading policy (including blackout periods) and Applicable Laws, the exercise price of an Option must be paid by:
(a) cash, wire transfer of immediately available funds or by check payable to the order of the Company, provided that the Company may limit the use of one of the foregoing payment forms if one or more of the payment forms below is permitted;
(b) if there is a public market for Shares at the time of exercise, unless the Company otherwise determines, (A) delivery (including electronically or telephonically to the extent permitted by the Company) of an irrevocable and unconditional undertaking by a broker acceptable to the Company to deliver promptly to the Company sufficient funds to pay the exercise price, or (B) the Participant’s delivery to the Company of a copy of irrevocable and unconditional instructions to a broker acceptable to the Company to deliver promptly to the Company cash or a check sufficient to pay the exercise price; provided that such amount is paid to the Company at such time as may be required by the Administrator;
(c) to the extent permitted by the Administrator, delivery (either by actual delivery or attestation) of Shares owned by the Participant valued at their fair market value;
(d) to the extent permitted by the Administrator, surrendering Shares then issuable upon the Option’s exercise valued at their fair market value on the exercise date;
(e) to the extent permitted by the Administrator, delivery of a promissory note or any other property that the Administrator determines is good and valuable consideration; or
(f) to the extent permitted by the Company, any combination of the above payment forms approved by the Administrator.
| -429- |
ARTICLE VI
RESTRICTED STOCK; RESTRICTED STOCK UNITS
6.1 General. The Administrator may grant Restricted Stock, or the right to purchase Restricted Stock, to any Service Provider, subject to the Company’s right to repurchase all or part of such shares at their issue price or other stated or formula price from the Participant (or to require forfeiture of such shares) if conditions the Administrator specifies in the Award Agreement are not satisfied before the end of the applicable restriction period or periods that the Administrator establishes for such Award. In addition, the Administrator may grant Restricted Stock Units to Service Providers, which may be subject to vesting and forfeiture conditions during the applicable restriction period or periods, as set forth in an Award Agreement. The Administrator will determine and set forth in the Award Agreement the terms and conditions for each Restricted Stock and Restricted Stock Unit Award, subject to the conditions and limitations contained in the Plan.
6.2 Restricted Stock.
(a) Dividends. Participants holding shares of Restricted Stock will be entitled to all ordinary cash dividends paid with respect to such Shares, unless the Administrator provides otherwise in the Award Agreement. In addition, unless the Administrator provides otherwise, if any dividends or distributions are paid in Shares, or consist of a dividend or distribution to holders of Common Stock of property other than an ordinary cash dividend, the Shares or other property will be subject to the same restrictions on transferability and forfeitability as the shares of Restricted Stock with respect to which they were paid. Notwithstanding anything to the contrary contained herein, the following Shares shall not be added to the Shares authorized for grant under Section 4.1 and shall not be available for future grants of Awards: (a) Shares subject to a Stock Appreciation Right that are not issued in connection with the stock settlement of the Stock Appreciation Right on exercise thereof; and (b) Shares purchased on the open market with the cash proceeds from the exercise of Options.
(b) Stock Certificates. The Company may require that the Participant deposit in escrow with the Company (or its designee) any stock certificates issued in respect of shares of Restricted Stock, together with a stock power endorsed in blank.
6.3 Restricted Stock Units.
(a) Settlement. The Administrator may provide that settlement of Restricted Stock Units will occur upon or as soon as reasonably practicable after the Restricted Stock Units vest or will instead be deferred, on a mandatory basis or at the Participant’s election, in a manner intended to comply with Section 409A.
(b) Stockholder Rights. A Participant will have no rights of a stockholder with respect to Shares subject to any Restricted Stock Unit unless and until the Shares are delivered in settlement of the Restricted Stock Unit.
(c) Dividend Equivalents. If the Administrator provides, a grant of Restricted Stock Units may provide a Participant with the right to receive Dividend Equivalents. Dividend Equivalents may be paid currently or credited to an account for the Participant, settled in cash or Shares and subject to the same restrictions on transferability and forfeitability as the Restricted Stock Units with respect to which the Dividend Equivalents are granted and subject to other terms and conditions as set forth in the Award Agreement.
ARTICLE VII
OTHER STOCK OR CASH BASED AWARDS
Other Stock or Cash Based Awards may be granted to Participants, including Awards entitling Participants to receive Shares to be delivered in the future and including annual or other periodic or long-term cash bonus awards (whether based on specified Performance Criteria or otherwise), in each case subject to any conditions and limitations in the Plan. Such Other Stock or Cash Based Awards will also be available as a payment form in the settlement of other Awards, as standalone payments and as payment in lieu of compensation to which a Participant is otherwise entitled. Other Stock or Cash Based Awards may be paid in Shares, cash or other property, as the Administrator determines. Subject to the provisions of the Plan, the Administrator will determine the terms and conditions of each Other Stock or Cash Based Award, including any purchase price, performance goals (which may be based on the Performance Criteria), transfer restrictions, and vesting conditions, which will be set forth in the applicable Award Agreement.
| -430- |
ARTICLE VIII
ADJUSTMENTS FOR CHANGES IN COMMON STOCK
AND CERTAIN OTHER EVENTS
8.1 Equity Restructuring. In connection with any Equity Restructuring, notwithstanding anything to the contrary in this Article VIII, the Administrator will equitably adjust each outstanding Award as it deems appropriate to reflect the Equity Restructuring, which may include adjusting the number and type of securities subject to each outstanding Award and/or the Award’s exercise price or grant price (if applicable), granting new Awards to Participants, and making a cash payment to Participants. The adjustments provided under this Section 8.1 will be nondiscretionary and final and binding on the affected Participant and the Company; provided that the Administrator will determine whether an adjustment is equitable.
8.2 Corporate Transactions. In the event of any dividend or other distribution (whether in the form of cash, Common Stock, other securities, or other property), reorganization, merger, consolidation, combination, amalgamation, repurchase, recapitalization, liquidation, dissolution, or sale, transfer, exchange or other disposition of all or substantially all of the assets of the Company, or sale or exchange of Common Stock or other securities of the Company, Change in Control, issuance of warrants or other rights to purchase Common Stock or other securities of the Company, other similar corporate transaction or event, other unusual or nonrecurring transaction or event affecting the Company or its financial statements or any change in any Applicable Laws or accounting principles, the Administrator, on such terms and conditions as it deems appropriate, either by the terms of the Award or by action taken prior to the occurrence of such transaction or event (except that action to give effect to a change in Applicable Law or accounting principles may be made within a reasonable period of time after such change) and either automatically or upon the Participant’s request, is hereby authorized to take any one or more of the following actions whenever the Administrator determines that such action is appropriate in order to (x) prevent dilution or enlargement of the benefits or potential benefits intended by the Company to be made available under the Plan or with respect to any Award granted or issued under the Plan, (y) to facilitate such transaction or event or (z) give effect to such changes in Applicable Laws or accounting principles:
(a) To provide for the cancellation of any such Award in exchange for either an amount of cash or other property with a value equal to the amount that could have been obtained upon the exercise or settlement of the vested portion of such Award or realization of the Participant’s rights under the vested portion of such Award, as applicable; provided that, if the amount that could have been obtained upon the exercise or settlement of the vested portion of such Award or realization of the Participant’s rights, in any case, is equal to or less than zero, then the Award may be terminated without payment; provided, further, that Awards held by members of the Board will be settled in Shares on or immediately prior to the applicable event if the Administrator takes action under this clause (a).
(b) To provide that such Award shall vest and, to the extent applicable, be exercisable as to all shares covered thereby, notwithstanding anything to the contrary in the Plan or the provisions of such Award;
(c) To provide that such Award be assumed by the successor or survivor corporation, or a parent or subsidiary thereof, or shall be substituted for by awards covering the stock of the successor or survivor corporation, or a parent or subsidiary thereof, with appropriate adjustments as to the number and kind of shares and/or applicable exercise or purchase price, in all cases, as determined by the Administrator;
(d) To make adjustments in the number and type of shares of Common Stock (or other securities or property) subject to outstanding Awards and/or with respect to which Awards may be granted under the Plan (including, but not limited to, adjustments of the limitations in Article IV hereof on the maximum number and kind of shares which may be issued) and/or in the terms and conditions of (including the grant or exercise price), and the criteria included in, outstanding Awards;
(e) To replace such Award with other rights or property selected by the Administrator; and/or
(f) To provide that the Award will terminate and cannot vest, be exercised or become payable after the applicable event.
8.3 Administrative Stand Still. In the event of any pending stock dividend, stock split, combination or exchange of shares, merger, consolidation or other distribution (other than normal cash dividends) of Company assets to stockholders, or any other extraordinary transaction or change affecting the Shares or the share price of Common Stock, including any Equity Restructuring or any securities offering or other similar transaction, for administrative convenience, the Administrator may refuse to permit the exercise of any Award for up to sixty days before or after such transaction.
| -431- |
8.4 General. Except as expressly provided in the Plan or the Administrator’s action under the Plan, no Participant will have any rights due to any subdivision or consolidation of Shares of any class, dividend payment, increase or decrease in the number of Shares of any class or dissolution, liquidation, merger, or consolidation of the Company or other corporation. Except as expressly provided with respect to an Equity Restructuring under Section 8.1 above or the Administrator’s action under the Plan, no issuance by the Company of Shares of any class, or securities convertible into Shares of any class, will affect, and no adjustment will be made regarding, the number of Shares subject to an Award or the Award’s grant or exercise price. The existence of the Plan, any Award Agreements and the Awards granted hereunder will not affect or restrict in any way the Company’s right or power to make or authorize (i) any adjustment, recapitalization, reorganization or other change in the Company’s capital structure or its business, (ii) any merger, consolidation dissolution or liquidation of the Company or sale of Company assets or (iii) any sale or issuance of securities, including securities with rights superior to those of the Shares or securities convertible into or exchangeable for Shares. The Administrator may treat Participants and Awards (or portions thereof) differently under this Article VIII.
ARTICLE IX
GENERAL PROVISIONS APPLICABLE TO AWARDS
9.1 Transferability. Except as the Administrator may determine or provide in an Award Agreement or otherwise for Awards other than Incentive Stock Options, Awards may not be sold, assigned, transferred, pledged or otherwise encumbered, either voluntarily or by operation of law, except by will or the laws of descent and distribution, or, subject to the Administrator’s consent, pursuant to a domestic relations order, and, during the life of the Participant, will be exercisable only by the Participant. References to a Participant, to the extent relevant in the context, will include references to a Participant’s authorized transferee that the Administrator specifically approves.
9.2 Documentation. Each Award will be evidenced in an Award Agreement, which may be written or electronic, as the Administrator determines. Each Award may contain terms and conditions in addition to those set forth in the Plan.
9.3 Discretion. Except as the Plan otherwise provides, each Award may be made alone or in addition or in relation to any other Award. The terms of each Award to a Participant need not be identical, and the Administrator need not treat Participants or Awards (or portions thereof) uniformly.
9.4 Termination of Status. The Administrator will determine how the disability, death, retirement, authorized leave of absence, or any other change or purported change in a Participant’s Service Provider status affects an Award and the extent to which, and the period during which, the Participant, the Participant’s legal representative, conservator, guardian or Designated Beneficiary may exercise rights under the Award, if applicable.
9.5 Withholding. Each Participant must pay the Company, or make provision satisfactory to the Administrator for payment of, any taxes required by Applicable Law to be withheld in connection with such Participant’s Awards by the date of the event creating the tax liability. The Company may deduct an amount sufficient to satisfy such tax obligations based on the applicable statutory withholding rates (or such other rate as may be determined by the Company after considering any accounting consequences or costs) from any payment of any kind otherwise due to a Participant. In the absence of a contrary determination by the Company (or, with respect to withholding pursuant to clause (ii) below with respect to Awards held by individuals subject to Section 16 of the Exchange Act, a contrary determination by the Administrator), all tax withholding obligations will be calculated based on the minimum applicable statutory withholding rates. Subject to Section 10.8 and any Company insider trading policy (including blackout periods), Participants may satisfy such tax obligations (i) in cash, by wire transfer of immediately available funds, by check made payable to the order of the Company, provided that the Company may limit the use of the foregoing payment forms if one or more of the payment forms below is permitted, (ii) to the extent permitted by the Administrator, in whole or in part by delivery of Shares, including Shares retained from the Award creating the tax obligation, valued at their fair market value, (iii) if there is a public market for Shares at the time the tax obligations are satisfied, unless the Company otherwise determines, (A) delivery (including electronically or telephonically to the extent permitted by the Company) of an irrevocable and unconditional undertaking by a broker acceptable to the Company to deliver promptly to the Company sufficient funds to satisfy the tax obligations, or (B) delivery by the Participant to the Company of a copy of irrevocable and unconditional instructions to a broker acceptable to the Company to deliver promptly to the Company cash or a check sufficient to satisfy the tax withholding; provided that such amount is paid to the Company at such time as may be required by the Administrator, or (iv) to the extent permitted by the Company, any combination of the foregoing payment forms approved by the Administrator. Notwithstanding any other provision of the Plan, the number of Shares which may be so delivered or retained pursuant to clause (ii) of the immediately preceding sentence shall be limited to the number of Shares which have a fair market value on the date of delivery or retention no greater than the aggregate amount of such liabilities based on the maximum individual statutory tax rate in the applicable jurisdiction at the time of such withholding (or such other rate as may be required to avoid liability classification of the applicable award under generally accepted accounting principles in the United States of America); provided, however, to the extent such Shares were acquired by Participant from the Company as compensation, the Shares must have been held for the minimum period required by applicable accounting rules to avoid a charge to the Company’s earnings for financial reporting purposes; provided, further, that any such Shares delivered or retained shall be rounded up to the nearest whole Share to the extent rounding up to the nearest whole Share does not result in the liability classification of the applicable Award under generally accepted accounting principles in the United States of America. If any tax withholding obligation will be satisfied under clause (ii) of the immediately preceding sentence by the Company’s retention of Shares from the Award creating the tax obligation and there is a public market for Shares at the time the tax obligation is satisfied, the Company may elect to instruct any brokerage firm determined acceptable to the Company for such purpose to sell on the applicable Participant’s behalf some or all of the Shares retained and to remit the proceeds of the sale to the Company or its designee, and each Participant’s acceptance of an Award under the Plan will constitute the Participant’s authorization to the Company and instruction and authorization to such brokerage firm to complete the transactions described in this sentence.
| -432- |
9.6 Amendment of Award; Repricing. The Administrator may amend, modify, or terminate any outstanding Award, including by substituting another Award of the same or a different type, changing the exercise or settlement date, and converting an Incentive Stock Option to a Non-Qualified Stock Option. The Participant’s consent to such action will be required unless (i) the action, taking into account any related action, does not materially and adversely affect the Participant’s rights under the Award, or (ii) the change is permitted under Article VIII or pursuant to Section 10.6. Notwithstanding the foregoing or anything in the Plan to the contrary, the Administrator may not except pursuant to Article VIII, without the approval of the stockholders of the Company, reduce the exercise price or base price per share of outstanding Options or Stock Appreciation Rights or cancel outstanding Options or Stock Appreciation Rights that have an exercise price or base price in excess of Fair Market Value in exchange for cash, other Awards or Options or Stock Appreciation Rights with an exercise price or base price per share that is less than the exercise price or base price per share of the original Options or Stock Appreciation Rights.
9.7 Conditions on Delivery of Stock. The Company will not be obligated to deliver any Shares under the Plan or remove restrictions from Shares previously delivered under the Plan until (i) all Award conditions have been met or removed to the Company’s satisfaction, (ii) as determined by the Company, all other legal matters regarding the issuance and delivery of such Shares have been satisfied, including any applicable securities laws and stock exchange or stock market rules and regulations, and (iii) the Participant has executed and delivered to the Company such representations or agreements as the Administrator deems necessary or appropriate to satisfy any Applicable Laws. The Company’s inability to obtain authority from any regulatory body having jurisdiction, which the Administrator determines is necessary to the lawful issuance and sale of any securities, will relieve the Company of any liability for failing to issue or sell such Shares as to which such requisite authority has not been obtained.
9.8 Acceleration. The Administrator may at any time provide that any Award will become immediately vested and fully or partially exercisable, free of some or all restrictions or conditions, or otherwise fully or partially realizable.
9.9 Additional Terms of Incentive Stock Options. The Administrator may grant Incentive Stock Options only to employees of the Company, any of its present or future parent or subsidiary corporations, as defined in Sections 424(e) or (f) of the Code, respectively, and any other entities the employees of which are eligible to receive Incentive Stock Options under the Code. If an Incentive Stock Option is granted to a Greater Than 10% Stockholder, the exercise price will not be less than 110% of the Fair Market Value on the Option’s grant date, and the term of the Option will not exceed five years. All Incentive Stock Options will be subject to, and construed consistently with, Section 422 of the Code. By accepting an Incentive Stock Option, the Participant agrees to give prompt notice to the Company of dispositions or other transfers (other than in connection with a Change in Control) of Shares acquired under the Option made within (i) two years from the grant date of the Option or (ii) one year after the transfer of such Shares to the Participant, specifying the date of the disposition or other transfer and the amount the Participant realized, in cash, other property, assumption of indebtedness or other consideration, in such disposition or other transfer. Neither the Company nor the Administrator will be liable to a Participant, or any other party, if an Incentive Stock Option fails or ceases to qualify as an “incentive stock option” under Section 422 of the Code. Any Incentive Stock Option or portion thereof that fails to qualify as an “incentive stock option” under Section 422 of the Code for any reason, including becoming exercisable with respect to Shares having a fair market value exceeding the $100,000 limitation under Treasury Regulation Section 1.422-4, will be a Non-Qualified Stock Option.
| -433- |
ARTICLE X
MISCELLANEOUS
10.1 No Right to Employment or Other Status. No person will have any claim or right to be granted an Award, and the grant of an Award will not be construed as giving a Participant the right to continued employment or any other relationship with the Company or a Subsidiary. The Company and its Subsidiaries expressly reserve the right at any time to dismiss or otherwise terminate its relationship with a Participant free from any liability or claim under the Plan or any Award, except as expressly provided in an Award Agreement or other written agreement between the Participant and the Company or any Subsidiary.
10.2 No Rights as Stockholder; Certificates. Subject to the Award Agreement, no Participant or Designated Beneficiary will have any rights as a stockholder with respect to any Shares to be distributed under an Award until becoming the record holder of such Shares. Notwithstanding any other provision of the Plan, unless the Administrator otherwise determines or Applicable Laws require, the Company will not be required to deliver to any Participant certificates evidencing Shares issued in connection with any Award and instead such Shares may be recorded in the books of the Company (or, as applicable, its transfer agent or stock plan administrator). The Company may place legends on stock certificates issued under the Plan that the Administrator deems necessary or appropriate to comply with Applicable Laws.
10.3 Effective Date and Term of Plan. The Plan will become effective upon the consummation of the Merger (the “Effective Date”) and will remain in effect until the tenth anniversary of the Effective Date. Notwithstanding anything to the contrary in the Plan, Incentive Stock Options may not be granted under the Plan after the tenth anniversary of the earlier of (i) the date the Board adopted the Plan or (ii) the date the Company’s stockholders approved the Plan.
10.4 Amendment of Plan. The Administrator may amend, suspend or terminate the Plan at any time; provided that no amendment, other than an increase to the Overall Share Limit, may materially and adversely affect any Award outstanding at the time of such amendment without the affected Participant’s consent. No Awards may be granted under the Plan during any suspension period or after Plan termination. Awards outstanding at the time of any Plan suspension or termination will continue to be governed by the Plan and the Award Agreement, as in effect before such suspension or termination. The Board will obtain stockholder approval of any Plan amendment to the extent necessary to comply with Applicable Laws.
10.5 Provisions for Foreign Participants. The Administrator may modify Awards granted to Participants who are foreign nationals or employed outside the United States or establish sub-plans or procedures under the Plan to address differences in laws, rules, regulations or customs of such foreign jurisdictions with respect to tax, securities, currency, employee benefit or other matters.
10.6 Section 409A.
(a) General. The Company intends that all Awards be structured to comply with, or be exempt from, Section 409A, such that no adverse tax consequences, interest, or penalties under Section 409A apply. Notwithstanding anything in the Plan or any Award Agreement to the contrary, the Administrator may, without a Participant’s consent, amend this Plan or Awards, adopt policies and procedures, or take any other actions (including amendments, policies, procedures and retroactive actions) as are necessary or appropriate to preserve the intended tax treatment of Awards, including any such actions intended to (A) exempt this Plan or any Award from Section 409A, or (B) comply with Section 409A, including regulations, guidance, compliance programs and other interpretative authority that may be issued after an Award’s grant date. The Company makes no representations or warranties as to an Award’s tax treatment under Section 409A or otherwise. The Company will have no obligation under this Section 10.6 or otherwise to avoid the taxes, penalties or interest under Section 409A with respect to any Award and will have no liability to any Participant or any other person if any Award, compensation or other benefits under the Plan are determined to constitute noncompliant “nonqualified deferred compensation” subject to taxes, penalties or interest under Section 409A.
(b) Separation from Service. If an Award constitutes “nonqualified deferred compensation” under Section 409A, any payment or settlement of such Award upon a termination of a Participant’s Service Provider relationship will, to the extent necessary to avoid taxes under Section 409A, be made only upon the Participant’s “separation from service” (within the meaning of Section 409A), whether such “separation from service” occurs upon or after the termination of the Participant’s Service Provider relationship. For purposes of this Plan or any Award Agreement relating to any such payments or benefits, references to a “termination,” “termination of employment,” or like terms means a “separation from service.”
| -434- |
(c) Payments to Specified Employees. Notwithstanding any contrary provision in the Plan or any Award Agreement, any payment(s) of “nonqualified deferred compensation” required to be made under an Award to a “specified employee” (as defined under Section 409A and as the Administrator determines) due to his or her “separation from service” will, to the extent necessary to avoid taxes under Section 409A(a)(2)(B)(i) of the Code, be delayed for the six-month period immediately following such “separation from service” (or, if earlier, until the specified employee’s death) and will instead be paid (as set forth in the Award Agreement) on the day immediately following such six-month period or as soon as administratively practicable thereafter (without interest). Any payments of “nonqualified deferred compensation” under such Award payable more than six months following the Participant’s “separation from service” will be paid at the time or times the payments are otherwise scheduled to be made.
10.7 Limitations on Liability. Notwithstanding any other provisions of the Plan, no individual acting as a director, officer, other employee or agent of the Company or any Subsidiary will be liable to any Participant, former Participant, spouse, beneficiary, or any other person for any claim, loss, liability, or expense incurred in connection with the Plan or any Award, and such individual will not be personally liable with respect to the Plan because of any contract or other instrument executed in his or her capacity as an Administrator, director, officer, other employee or agent of the Company or any Subsidiary. The Company will indemnify and hold harmless each director, officer, other employee and agent of the Company or any Subsidiary that has been or will be granted or delegated any duty or power relating to the Plan’s administration or interpretation, against any cost or expense (including attorneys’ fees) or liability (including any sum paid in settlement of a claim with the Administrator’s approval) arising from any act or omission concerning this Plan unless arising from such person’s own fraud or bad faith.
10.8 Lock-Up Period. The Company may, at the request of any underwriter representative, the Company or otherwise, in connection with registering the offering of any Company securities under the Securities Act, prohibit Participants from, directly or indirectly, selling or otherwise transferring any Shares or other Company securities during a period of up to one hundred eighty (180) days following the effective date of a Company registration statement filed under the Securities Act, or such shorter or longer period as determined by the underwriter or the Company, and subject to such exceptions as may be determined by the underwriter or the Company.
10.9 Data Privacy. As a condition for receiving any Award, each Participant explicitly and unambiguously consents to the collection, use and transfer, in electronic or other form, of personal data as described in this section by and among the Company and its Subsidiaries and affiliates exclusively for implementing, administering and managing the Participant’s participation in the Plan. The Company and its Subsidiaries and affiliates may hold certain personal information about a Participant, including the Participant’s name, address and telephone number; birthdate; social security, insurance number or other identification number; salary; nationality; job title(s); any Shares held in the Company or its Subsidiaries and affiliates; and Award details, to implement, manage and administer the Plan and Awards (the “Data”). The Company and its Subsidiaries and affiliates may transfer the Data amongst themselves as necessary to implement, administer and manage a Participant’s participation in the Plan, and the Company and its Subsidiaries and affiliates may transfer the Data to third parties assisting the Company with Plan implementation, administration and management. These recipients may be located in the Participant’s country, or elsewhere, and the Participant’s country may have different data privacy laws and protections than the recipients’ country. By accepting an Award, each Participant authorizes such recipients to receive, possess, use, retain and transfer the Data, in electronic or other form, to implement, administer and manage the Participant’s participation in the Plan, including any required Data transfer to a broker or other third party with whom the Company or the Participant may elect to deposit any Shares. The Data related to a Participant will be held only as long as necessary to implement, administer, and manage the Participant’s participation in the Plan. A Participant may, at any time, view the Data that the Company holds regarding such Participant, request additional information about the storage and processing of the Data regarding such Participant, recommend any necessary corrections to the Data regarding the Participant or refuse or withdraw the consents in this Section 10.9 in writing, without cost, by contacting the local human resources representative. The Company may cancel Participant’s ability to participate in the Plan and, in the Administrator’s discretion, the Participant may forfeit any outstanding Awards if the Participant refuses or withdraws the consents in this Section 10.9. For more information on the consequences of refusing or withdrawing consent, Participants may contact their local human resources representative.
| -435- |
10.10 Severability. If any portion of the Plan or any action taken under it is held illegal or invalid for any reason, the illegality or invalidity will not affect the remaining parts of the Plan, and the Plan will be construed and enforced as if the illegal or invalid provisions had been excluded, and the illegal or invalid action will be null and void.
10.11 Governing Documents. If any contradiction occurs between the Plan and any Award Agreement or other written agreement between a Participant and the Company (or any Subsidiary) that the Administrator has approved, the Plan will govern, unless it is expressly specified in such Award Agreement or other written document that a specific provision of the Plan will not apply.
10.12 Governing Law. The Plan and all Awards will be governed by and interpreted in accordance with the laws of the State of Delaware, disregarding any state’s choice-of-law principles requiring the application of a jurisdiction’s laws other than the State of Delaware.
10.13 Claw-back Provisions. All Awards (including any proceeds, gains or other economic benefit the Participant actually or constructively receives upon receipt or exercise of any Award or the receipt or resale of any Shares underlying the Award) will be subject to any Company claw-back policy, including any claw-back policy adopted to comply with Applicable Laws (including the Dodd-Frank Wall Street Reform and Consumer Protection Act and any rules or regulations promulgated thereunder).
10.14 Titles and Headings. The titles and headings in the Plan are for convenience of reference only and, if any conflict, the Plan’s text, rather than such titles or headings, will control.
10.15 Conformity to Securities Laws. Participant acknowledges that the Plan is intended to conform to the extent necessary with Applicable Laws. Notwithstanding anything herein to the contrary, the Plan and all Awards will be administered only in conformance with Applicable Laws. To the extent Applicable Laws permit, the Plan and all Award Agreements will be deemed amended as necessary to conform to Applicable Laws.
10.16 Relationship to Other Benefits. No payment under the Plan will be taken into account in determining any benefits under any pension, retirement, savings, profit sharing, group insurance, welfare or other benefit plan of the Company or any Subsidiary except as expressly provided in writing in such other plan or an agreement thereunder.
10.17 Broker-Assisted Sales. In the event of a broker-assisted sale of Shares in connection with the payment of amounts owed by a Participant under or with respect to the Plan or Awards, including amounts to be paid under the final sentence of Section 9.5: (a) any Shares to be sold through the broker-assisted sale will be sold on the day the payment first becomes due, or as soon thereafter as practicable; (b) such Shares may be sold as part of a block trade with other Participants in the Plan in which all participants receive an average price; (c) the applicable Participant will be responsible for all broker’s fees and other costs of sale, and by accepting an Award, each Participant agrees to indemnify and hold the Company harmless from any losses, costs, damages, or expenses relating to any such sale; (d) to the extent the Company or its designee receives proceeds of such sale that exceed the amount owed, the Company will pay such excess in cash to the applicable Participant as soon as reasonably practicable; (e) the Company and its designees are under no obligation to arrange for such sale at any particular price; and (f) in the event the proceeds of such sale are insufficient to satisfy the Participant’s applicable obligation, the Participant may be required to pay immediately upon demand to the Company or its designee an amount in cash sufficient to satisfy any remaining portion of the Participant’s obligation.
ARTICLE XI
DEFINITIONS
As used in the Plan, the following words and phrases will have the following meanings:
11.1 “Administrator” means the Board or a Committee to the extent that the Board’s powers or authority under the Plan have been delegated to such Committee.
11.2 “Applicable Laws” means the requirements relating to the administration of equity incentive plans under U.S. federal and state securities, tax and other applicable laws, rules and regulations, the applicable rules of any stock exchange or quotation system on which the Common Stock is listed or quoted and the applicable laws and rules of any foreign country or other jurisdiction where Awards are granted.
| -436- |
11.3 “Award” means, individually or collectively, a grant under the Plan of Options, Stock Appreciation Rights, Restricted Stock, Restricted Stock Units or Other Stock or Cash Based Awards.
11.4 “Award Agreement” means a written agreement evidencing an Award, which may be electronic, that contains such terms and conditions as the Administrator determines, consistent with and subject to the terms and conditions of the Plan.
11.5 “Board” means the Board of Directors of the Company.
11.6 “Cause” means (i) if a Participant is a party to a written employment, severance or consulting agreement with the Company or any of its Subsidiaries or an Award Agreement in which the term “cause” is defined (a “Relevant Agreement”), “Cause” as defined in the Relevant Agreement, and (ii) if no Relevant Agreement exists, (A) the Administrator’s determination that the Participant failed to substantially perform the Participant’s duties (other than a failure resulting from the Participant’s Disability); (B) the Administrator’s determination that the Participant failed to carry out, or comply with any lawful and reasonable directive of the Board or the Participant’s immediate supervisor; (C) the Participant’s unauthorized use or disclosure of confidential information or trade secrets of the Company or any of its Subsidiaries or any material breach of a written agreement between the Participant and the Company; (D) the occurrence of any act or omission by the Participant that could reasonably be expected to result in (or has resulted in) the Participant’s conviction, plea of no contest, plea of nolo contendere, or imposition of un-adjudicated probation for any felony or indictable offense or crime involving moral turpitude; (E) the Participant’s unlawful use (including being under the influence) or possession of illegal drugs on the premises of the Company or any of its Subsidiaries or while performing the Participant’s duties and responsibilities for the Company or any of its Subsidiaries; or (F) the Participant’s commission of an act of fraud, embezzlement, misappropriation, misconduct, or breach of fiduciary duty against the Company or any of its Subsidiaries.
11.7 “Change in Control” means and includes each of the following:
(a) A transaction or series of transactions (other than an offering of Common Stock to the general public through a registration statement filed with the Securities and Exchange Commission or a transaction or series of transactions that meets the requirements of clauses (i) and (ii) of subsection (b) below) whereby any “person” or related “group” of “persons” (as such terms are used in Sections 13(d) and 14(d)(2) of the Exchange Act) (other than the Company, any of its Subsidiaries, an employee benefit plan maintained by the Company or any of its Subsidiaries or a “person” that, prior to such transaction, directly or indirectly controls, is controlled by, or is under common control with, the Company) directly or indirectly acquires beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act) of securities of the Company possessing more than 50% of the total combined voting power of the Company’s securities outstanding immediately after such acquisition;
(b) During any period of two consecutive years, individuals who, at the beginning of such period, constitute the Board together with any new Director(s) (other than a Director designated by a person who shall have entered into an agreement with the Company to effect a transaction described in subsections (a) or (c)) whose election by the Board or nomination for election by the Company’s stockholders was approved by a vote of at least two-thirds of the Directors then still in office who either were Directors at the beginning of the two year period or whose election or nomination for election was previously so approved, cease for any reason to constitute a majority thereof; or
(c) The consummation by the Company (whether directly involving the Company or indirectly involving the Company through one or more intermediaries) of (x) a merger, consolidation, reorganization, or business combination or (y) a sale or other disposition of all or substantially all of the Company’s assets in any single transaction or series of related transactions or (z) the acquisition of assets or stock of another entity, in each case other than a transaction:
(i) which results in the Company’s voting securities outstanding immediately before the transaction continuing to represent (either by remaining outstanding or by being converted into voting securities of the Company or the person that, as a result of the transaction, controls, directly or indirectly, the Company or owns, directly or indirectly, all or substantially all of the Company’s assets or otherwise succeeds to the business of the Company (the Company or such person, the “Successor Entity”)) directly or indirectly, at least a majority of the combined voting power of the Successor Entity’s outstanding voting securities immediately after the transaction, and
(ii) after which no person or group beneficially owns voting securities representing 50% or more of the combined voting power of the Successor Entity; provided, however, that no person or group shall be treated for purposes of this clause (ii) as beneficially owning 50% or more of the combined voting power of the Successor Entity solely as a result of the voting power held in the Company prior to the consummation of the transaction.
| -437- |
Notwithstanding the foregoing, in no event shall the Merger or the transactions occurring in connection with the Merger Agreement constitute a Change in Control and, if a Change in Control constitutes a payment event with respect to any Award (or portion of any Award) that provides for the deferral of compensation that is subject to Section 409A, to the extent required to avoid the imposition of additional taxes under Section 409A, the transaction or event described in subsection (a), (b), or (c) with respect to such Award (or portion thereof) shall only constitute a Change in Control for purposes of the payment timing of such Award if such transaction also constitutes a “change in control event,” as defined in Treasury Regulation Section 1.409A-3(i)(5).
The Administrator shall have full and final authority, which shall be exercised in its discretion, to determine conclusively whether a Change in Control has occurred pursuant to the above definition, the date of the occurrence of such Change in Control and any incidental matters relating thereto; provided that any exercise of authority in conjunction with a determination of whether a Change in Control is a “change in control event” as defined in Treasury Regulation Section 1.409A-3(i)(5) shall be consistent with such regulation.
11.8 “Code” means the Internal Revenue Code of 1986, as amended, and the regulations issued thereunder.
11.9 “Committee” means one or more committees or subcommittees of the Board, which may include one or more Company directors or executive officers, to the extent Applicable Laws permit. To the extent required to comply with the provisions of Rule 16b-3, it is intended that each member of the Committee will be, at the time the Committee takes any action with respect to an Award that is subject to Rule 16b-3, a “non-employee director” within the meaning of Rule 16b-3; however, a Committee member’s failure to qualify as a “non-employee director” within the meaning of Rule 16b-3 will not invalidate any Award granted by the Committee that is otherwise validly granted under the Plan.
11.10 “Common Stock” means the common stock of the Company.
11.11 “Company” means Aerami Therapeutics Holdings, Inc., a Delaware corporation, or any successor.
11.12 “Consultant” means any person, including any adviser, engaged by the Company or a Subsidiary to render services to such entity if the consultant or adviser: (i) renders bona fide services to the Company; (ii) renders services not in connection with the offer or sale of securities in a capital-raising transaction and does not directly or indirectly promote or maintain a market for the Company’s securities; and (iii) is a natural person.
11.13 “Designated Beneficiary” means the beneficiary or beneficiaries the Participant designates, in a manner the Administrator determines, to receive amounts due or exercise the Participant’s rights if the Participant dies or becomes incapacitated. Without a Participant’s effective designation, “Designated Beneficiary” will mean the Participant’s estate.
11.14 “Director” means a Board member.
11.15 “Disability” means a permanent and total disability under Section 22(e)(3) of the Code, as amended.
11.16 “Dividend Equivalents” means a right granted to a Participant under the Plan to receive the equivalent value (in cash or Shares) of dividends paid on Shares.
11.17 “Employee” means any employee of the Company or its Subsidiaries.
11.18 “Equity Restructuring” means a nonreciprocal transaction between the Company and its stockholders, such as a stock dividend, stock split, spin-off or recapitalization through a large, nonrecurring cash dividend, that affects the number or kind of Shares (or other Company securities) or the share price of Common Stock (or other Company securities) and causes a change in the per share value of the Common Stock underlying outstanding Awards.
11.19 “Exchange Act” means the Securities Exchange Act of 1934, as amended.
| -438- |
11.20 “Fair Market Value” means, as of any date, the value of Common Stock determined as follows: (i) if the Common Stock is listed on any established stock exchange, its Fair Market Value will be the closing sales price for such Common Stock as quoted on such exchange for the last market trading day prior to such date, as reported in The Wall Street Journal or another source the Administrator deems reliable; (ii) if the Common Stock is not traded on a stock exchange but is quoted on a national market or other quotation system, the closing sales price on such date, or if no sales occurred on such date, then on the last date preceding such date during which a sale occurred, as reported in The Wall Street Journal or another source the Administrator deems reliable; or (iii) if the Common Stock is not listed on any established stock exchange or quoted on a national market or other quotation system, the Administrator may determine the Fair Market Value in its discretion.
11.21 “Greater Than 10% Stockholder” means an individual then owning (within the meaning of Section 424(d) of the Code) more than 10% of the total combined voting power of all classes of stock of the Company or its parent or subsidiary corporation, as defined in Section 424(e) and (f) of the Code, respectively.
11.22 “Incentive Stock Option” means an Option intended to qualify as an “incentive stock option” as defined in Section 422 of the Code.
11.23 “Merger” means the transactions contemplated by the Merger Agreement.
11.24 “Merger Agreement” means the Agreement and Plan of Merger, dated as of December 7, 2021, by and among FoxWayne Enterprises Acquisition Corp., Gotham Merger Sub, Inc., Aerami Therapeutics Holdings, Inc., and Steven Thornton.
11.25 “Non-Employee Director” means a Director who is not an Employee.
11.26 “Non-Qualified Stock Option” means an Option not intended or not qualifying as an Incentive Stock Option.
11.27 “Option” means an option to purchase Shares.
11.28 “Other Stock or Cash Based Awards” means cash awards, awards of Shares, and other awards valued wholly or partially by referring to, or are otherwise based on, Shares or other property.
11.29 “Overall Share Limit” means [ ] Shares which equals twenty percent (20%) of the number of shares of Common Stock on a fully diluted basis as of the closing of the Merger as set forth in Section 7.9 of the Merger Agreement.
11.30 “Participant” means a Service Provider who has been granted an Award.
11.31 “Performance Criteria” mean the criteria (and adjustments) that the Administrator may select for an Award to establish performance goals for a performance period, which may include the following: net earnings or losses (either before or after one or more of interest, taxes, depreciation, amortization, and non-cash equity-based compensation expense); gross or net sales or revenue or sales or revenue growth; net income (either before or after taxes) or adjusted net income; profits (including but not limited to gross profits, net profits, profit growth, net operation profit or economic profit), profit return ratios or operating margin; budget or operating earnings (either before or after taxes or before or after allocation of corporate overhead and bonus); cash flow (including operating cash flow and free cash flow or cash flow return on capital); return on assets; return on capital or invested capital; cost of capital; return on stockholders’ equity; total stockholder return; return on sales; costs, reductions in costs and cost control measures; expenses; working capital; earnings or loss per share; adjusted earnings or loss per share; price per share or dividends per share (or appreciation in or maintenance of such price or dividends); regulatory achievements or compliance; implementation, completion or attainment of objectives relating to research, development, regulatory, commercial, or strategic milestones or developments; market share; economic value or economic value added models; division, group or corporate financial goals; customer satisfaction/growth; customer service; employee satisfaction; recruitment and maintenance of personnel; human resources management; supervision of litigation and other legal matters; strategic partnerships and transactions; financial ratios (including those measuring liquidity, activity, profitability or leverage); debt levels or reductions; sales-related goals; financing and other capital raising transactions; cash on hand; acquisition activity; investment sourcing activity; and marketing initiatives, any of which may be measured in absolute terms or as compared to any incremental increase or decrease. Such performance goals also may be based solely by reference to the Company’s performance or the performance of a Subsidiary, division, business segment or business unit of the Company or a Subsidiary, or based upon performance relative to performance of other companies or upon comparisons of any of the indicators of performance relative to performance of other companies. The Committee may provide for exclusion of the impact of an event or occurrence which the Committee determines should appropriately be excluded, including (a) restructurings, discontinued operations, extraordinary items, and other unusual, infrequently occurring or non-recurring charges or events, (b) asset write-downs, (c) litigation or claim judgments or settlements, (d) acquisitions or divestitures, (e) reorganization or change in the corporate structure or capital structure of the Company, (f) an event either not directly related to the operations of the Company, Subsidiary, division, business segment or business unit or not within the reasonable control of management, (g) foreign exchange gains and losses, (h) a change in the fiscal year of the Company, (i) the refinancing or repurchase of bank loans or debt securities, (j) unbudgeted capital expenditures, (k) the issuance or repurchase of equity securities and other changes in the number of outstanding shares, (l) conversion of some or all of convertible securities to Common Stock, (m) any business interruption event (n) the cumulative effects of tax or accounting changes in accordance with U.S. generally accepted accounting principles, or (o) the effect of changes in other laws or regulatory rules affecting reported results.
| -439- |
11.32 “Plan” means this 2022 Incentive Award Plan.
11.33 “Restricted Stock” means Shares awarded to a Participant under Article VI subject to certain vesting conditions and other restrictions.
11.34 “Restricted Stock Unit” means an unfunded, unsecured right to receive, on the applicable settlement date, one Share or an amount in cash or other consideration determined by the Administrator to be of equal value as of such settlement date, subject to certain vesting conditions and other restrictions.
11.35 “Rule 16b-3” means Rule 16b-3 promulgated under the Exchange Act.
11.36 “Section 409A” means Section 409A of the Code and all regulations, guidance, compliance programs and other interpretative authority thereunder.
11.37 “Securities Act” means the Securities Act of 1933, as amended.
11.38 “Service Provider” means an Employee, Consultant, or Director.
11.39 “Shares” means shares of Common Stock.
11.40 “Stock Appreciation Right” means a stock appreciation right granted under Article V.
11.41 “Subsidiary” means any entity (other than the Company), whether domestic or foreign, in an unbroken chain of entities beginning with the Company if each of the entities other than the last entity in the unbroken chain beneficially owns, at the time of the determination, securities or interests representing at least 50% of the total combined voting power of all classes of securities or interests in one of the other entities in such chain.
11.42 “Substitute Awards” shall mean Awards granted or Shares issued by the Company in assumption of, or in substitution or exchange for, awards previously granted, or the right or obligation to make future awards, in each case by a company acquired by the Company or any Subsidiary or with which the Company or any Subsidiary combines.
11.43 “Termination of Service” means the date the Participant ceases to be a Service Provider.
| -440- |
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Indemnification of Directors and Officers
Section 145(a) of the DGCL provides, in general, that a corporation may indemnify any person who was or is a party to or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation), by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding, if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe that the person’s conduct was unlawful.
Section 145(b) of the DGCL provides, in general, that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor because the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made with respect to any claim, issue or matter as to which he or she shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses that the Court of Chancery or other adjudicating court shall deem proper.
Section 145(g) of the DGCL provides, in general, that a corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person and incurred by such person in any such capacity, or arising out of such person’s status as such, whether or not the corporation would have the power to indemnify the person against such liability under Section 145 of the DGCL.
In connection with the Business Combination, FoxWayne will enter into indemnification agreements with each of its directors and executive officers. These agreements will provide that FoxWayne will indemnify each of its directors and such officers to the fullest extent permitted by law and its charter and its bylaws.
FoxWayne will also maintain a general liability insurance policy, which will cover certain liabilities of directors and officers of FoxWayne arising out of claims based on acts or omissions in their capacities as directors or officers.
Item 21. Exhibits and Financial Statement Schedules.
(a) Exhibits:
| -441- |
| -442- |
* Previously filed
# To be filed by amendment.
† Indicates a management contract or compensatory plan.
Undertakings.
The undersigned Registrant hereby undertakes:
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement: |
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; | ||
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of this Registration Statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in this Registration Statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
| -443- |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. | |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. | |
| (4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. | |
| (5) | The undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424; | ||
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant; | ||
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned Registrant; and | ||
| (iv) | Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser. |
| (6) | That, prior to any public reoffering of the securities registered hereunder through use of a prospectus which is a part of this Registration Statement, by any person or party who is deemed to be an underwriter within the meaning of Rule 145(c), the Registrant undertakes that such reoffering prospectus will contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other items of the applicable form. | |
| (7) | That every prospectus: (i) that is filed pursuant to the immediately preceding paragraph, or (ii) that purports to meet the requirements of Section 10(a)(3) of the Securities Act of 1933 and is used in connection with an offering of securities subject to Rule 415, will be filed as a part of an amendment to the registration statement and will not be used until such amendment is effective, and that, for purposes of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. | |
| (8) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. | |
| (9) | The undersigned Registrant hereby undertakes to respond to requests for information that is incorporated by reference into the prospectus pursuant to Items 4, 10(b), 11, or 13 of this Form, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request. | |
| (10) | The undersigned Registrant hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective. |
| -444- |
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New York, State of New York, on February 14, 2022.
| FoxWayne Enterprises Acquisition Corp. | ||
| By: | /s/ Robb Knie | |
| Name: | Robb Knie | |
| Title: | Chief Executive Officer | |
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Robb Knie | Chairman, Chief Executive Officer, Chief Financial Officer and Director | February 14, 2022 | ||
| Robb Knie | ||||
| * | Director | February 14, 2022 | ||
| Sundeep Agrawal, M.D. | ||||
| * | Director | February 14, 2022 | ||
| Jonathan Hale Zippin M.D., Ph.D. | ||||
| * | Director | February 14, 2022 | ||
| Michael Reavey | ||||
| * | Director | February 14, 2022 | ||
| Jeff Pavell, M.D. |
| *By: | /s/ Robb Knie | |
| Name: | Robb Knie | |
| Title: | Attorney-in-fact |
| -445- |